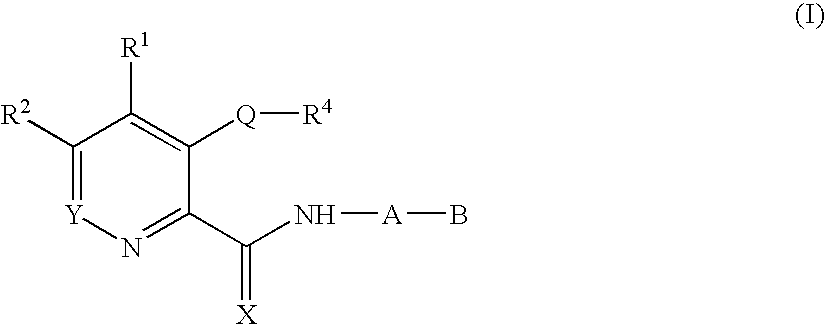Patents
Literature
161 results about "Tumor progression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor progression is the third and last phase in tumor development. This phase is characterised by increased growth speed and invasiveness of the tumor cells. As a result of the progression, phenotypical changes occur and the tumor becomes more aggressive and acquires greater malignant potential. Together with the progression, more and more aneuploidy occurs. This may be evident as nuclear polymorphism.
Antibodies that block receptor protein tyrosine kinase activation, methods of screening for and uses thereof
ActiveUS20050147612A1Reduce usageUseful in treatmentFungiSenses disorderProtein-Tyrosine KinasesAntibody fragments
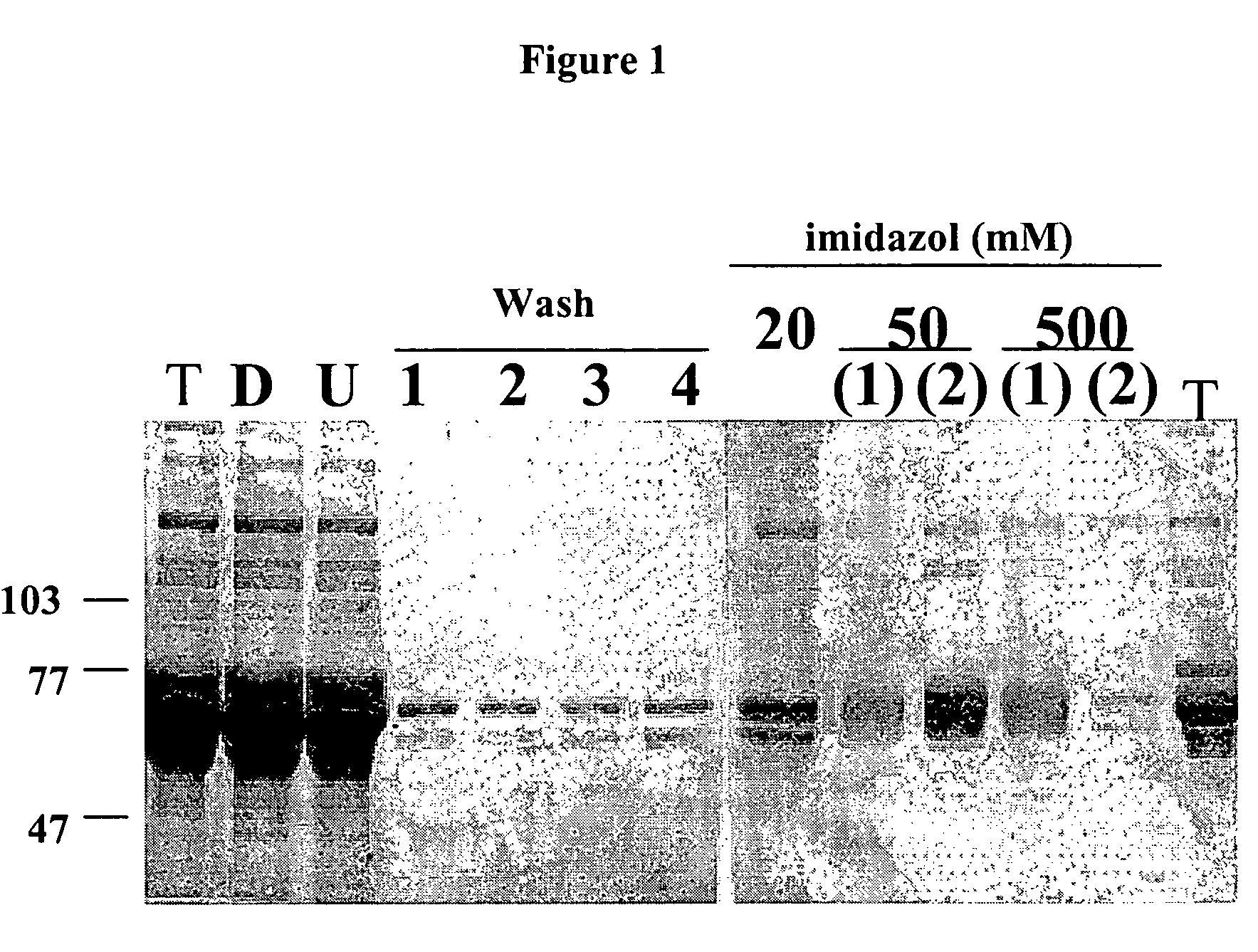
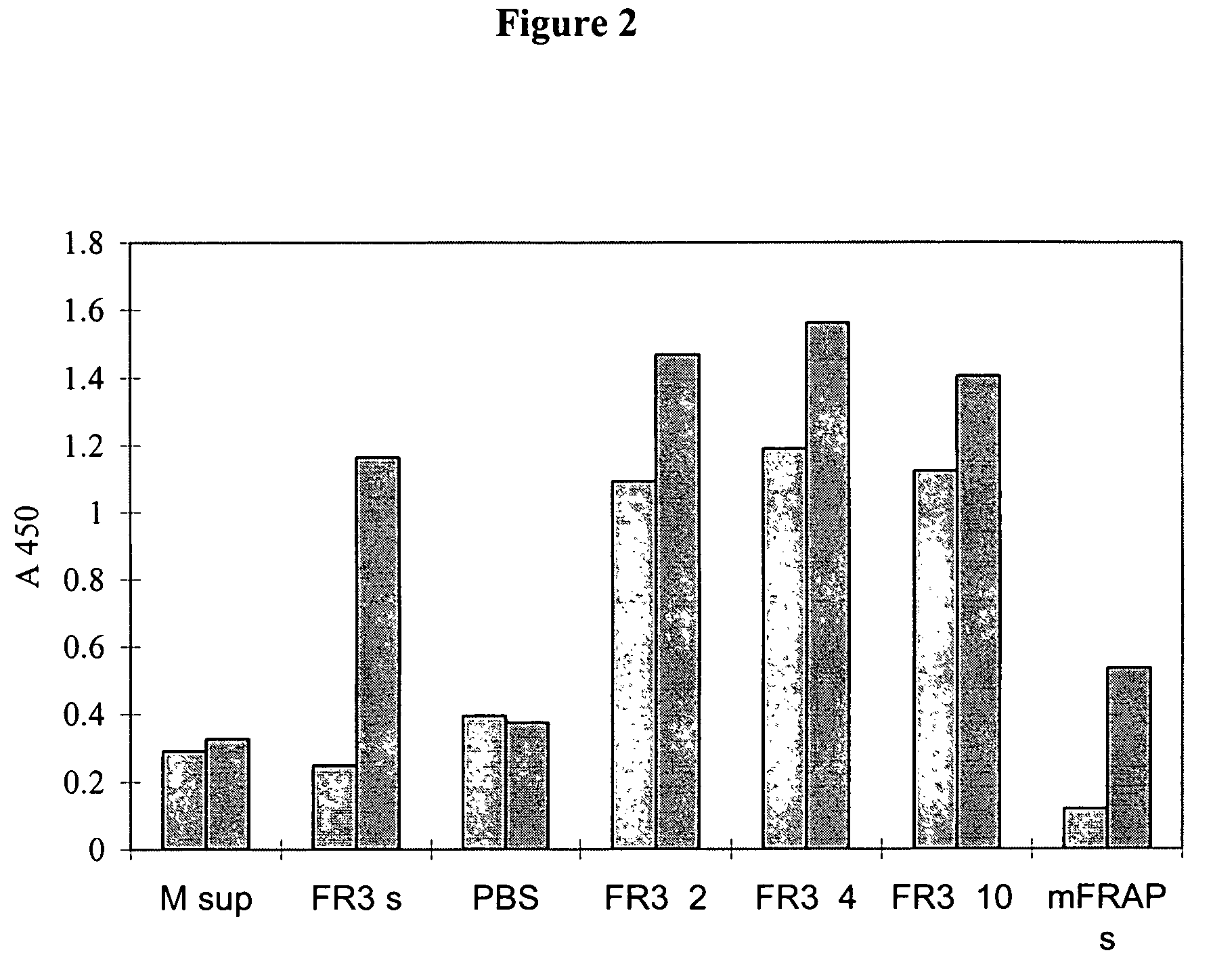
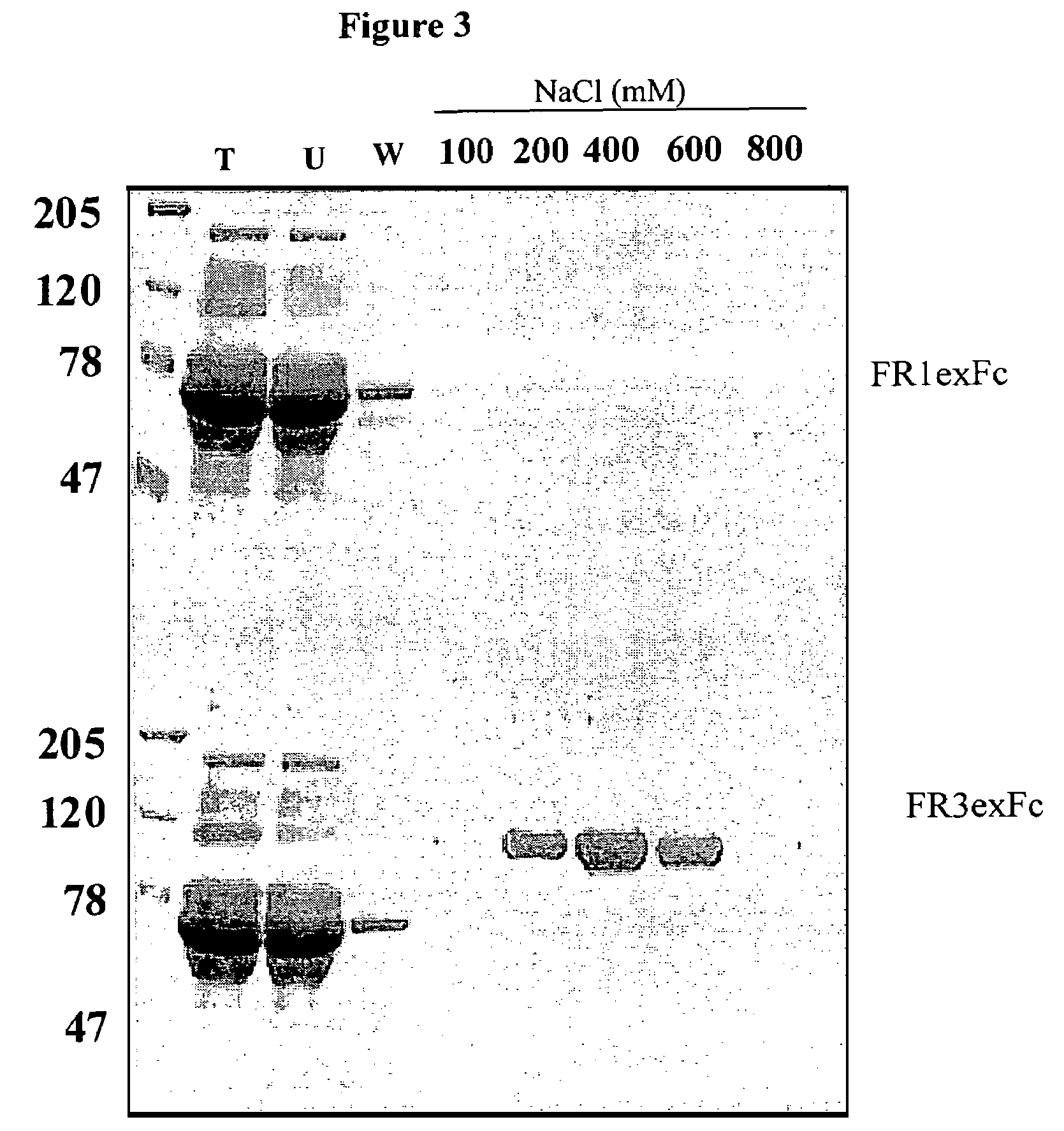
Molecules comprising the antigen-binding portion of antibodies that block constitutive and / or ligand-dependent activation of a receptor protein tyrosine kinase, such as fibroblast growth factor receptor 3 (FGFR3), are found through screening methods, where a soluble dimeric form of a receptor protein tyrosine kinase is used as target for screening a library of antibody fragments displayed on the surface of bacteriophage. The molecules of the present invention which block constitutive activation can be administered to treat or inhibit skeletal dysplasia, craniosynostosis disorders, cell proliferative diseases or disorders, or tumor progression associated with the constitutive activation of a receptor protein tyrosine kinase.
Owner:FIBRON
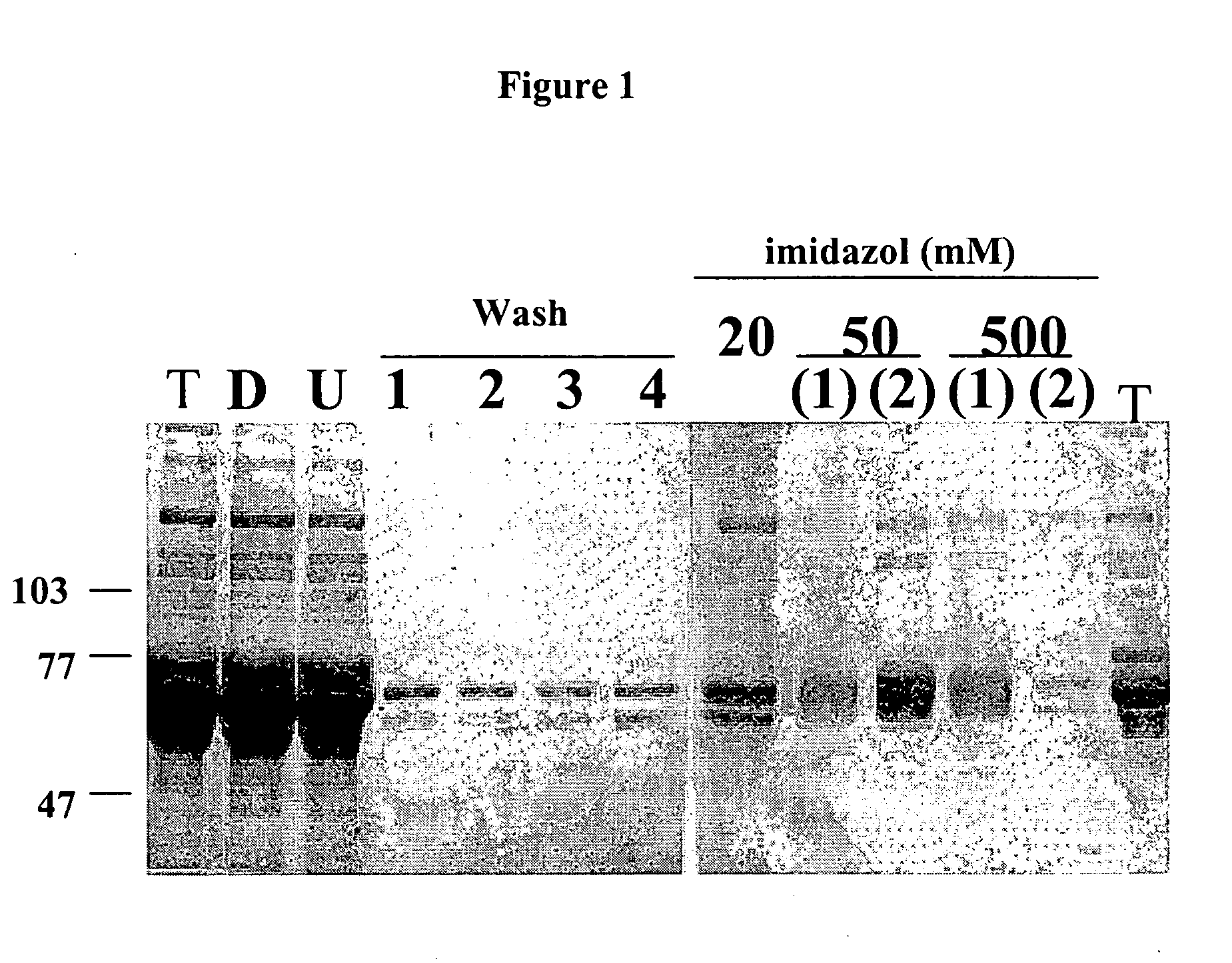
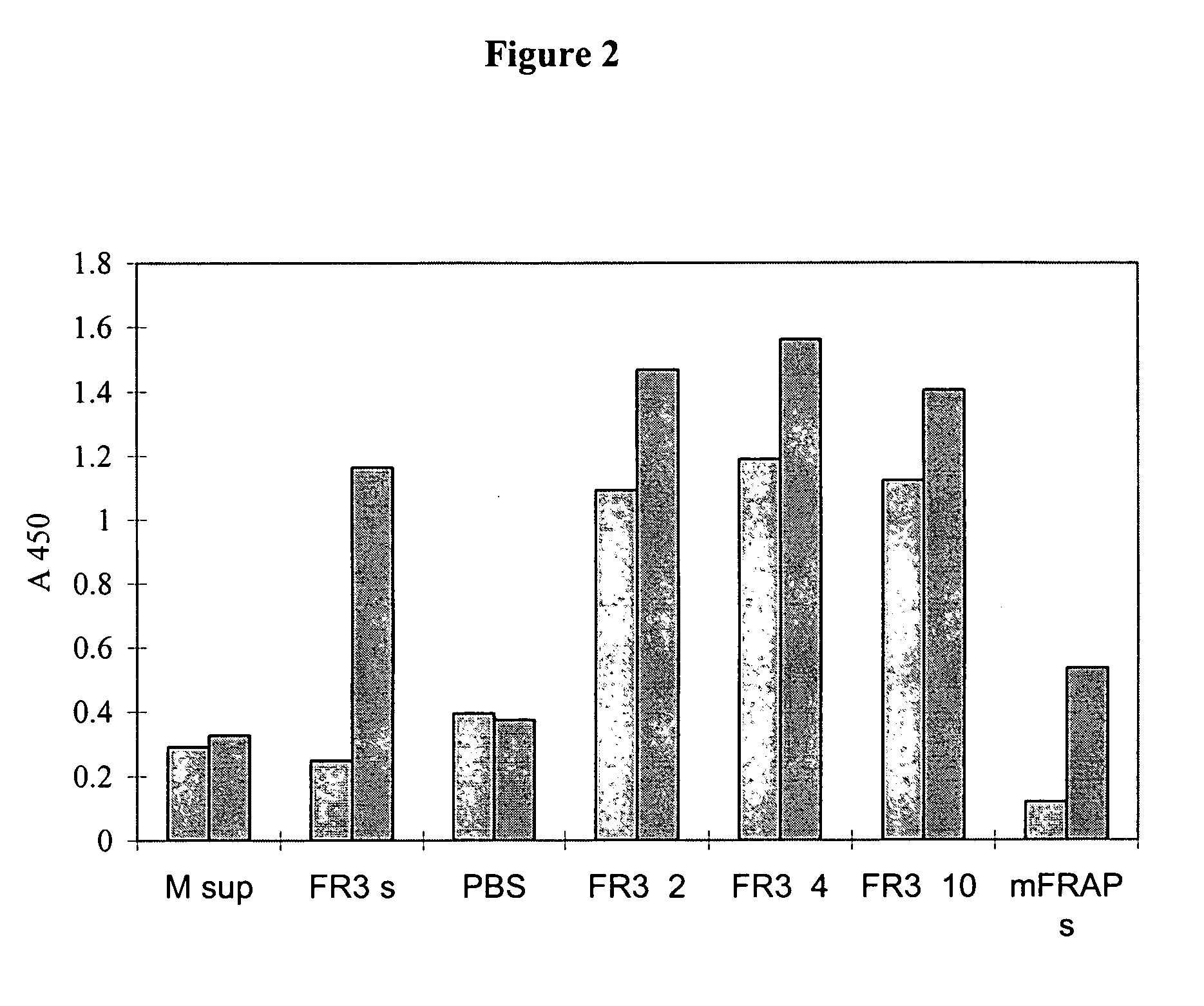
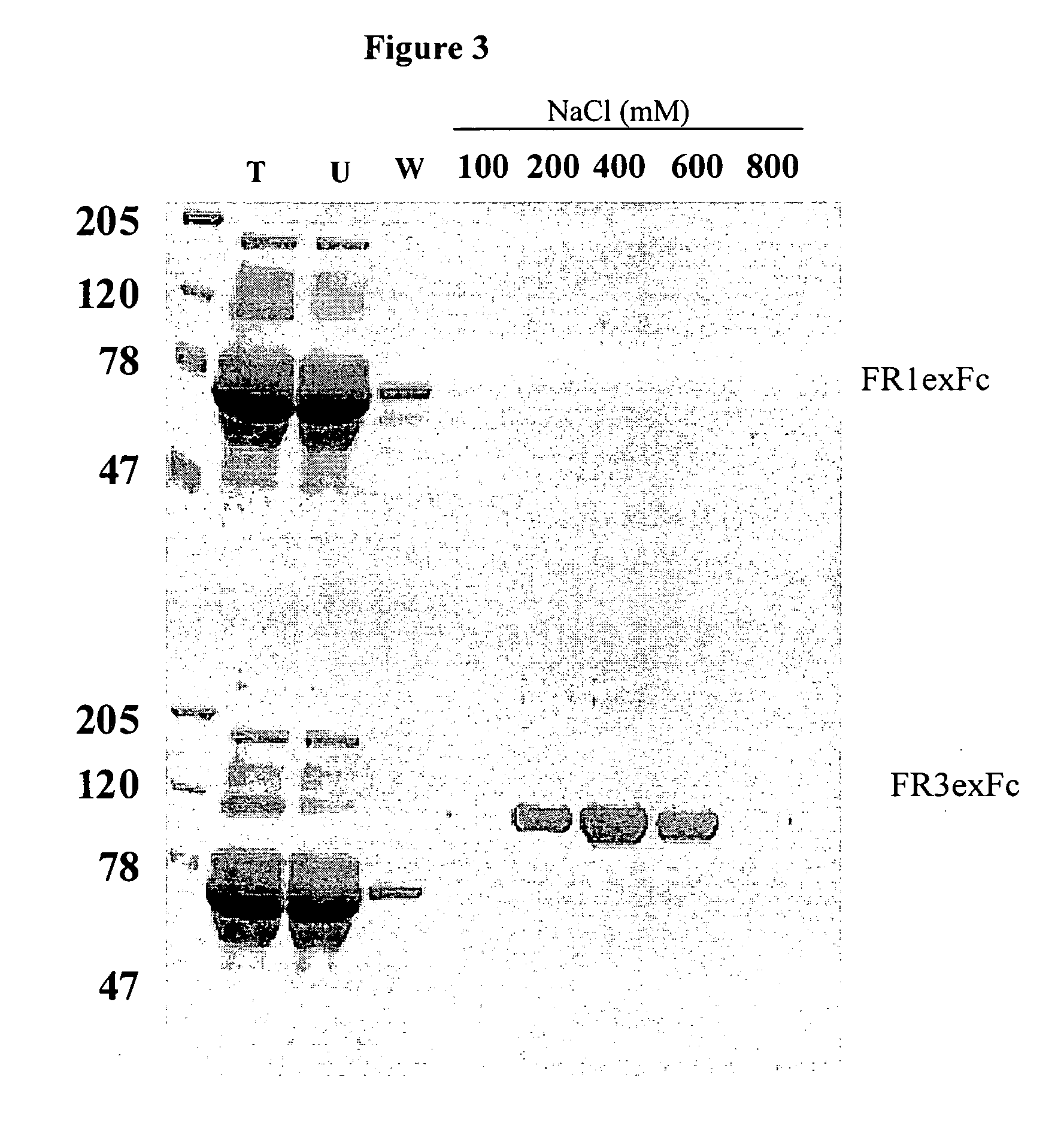
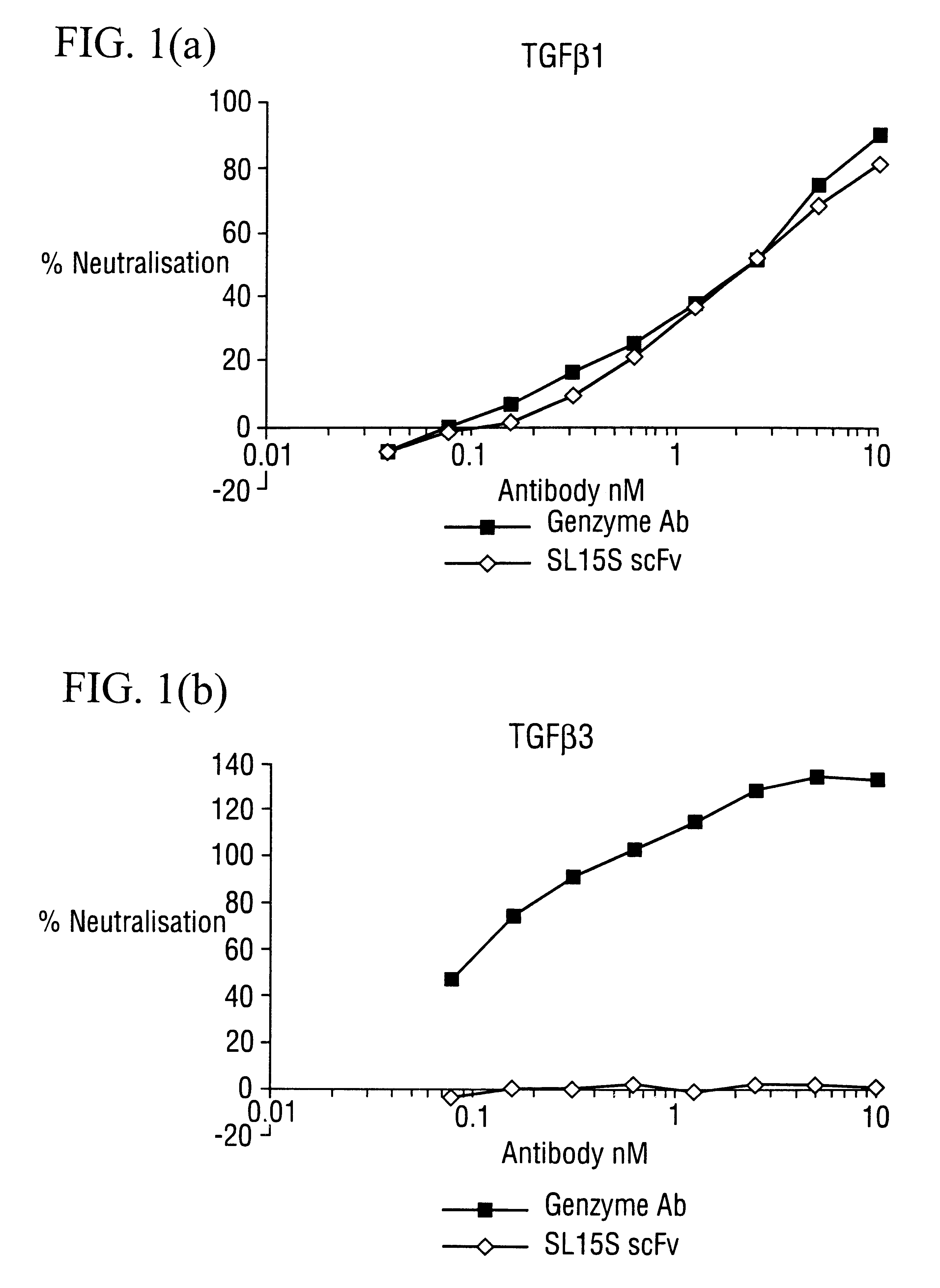
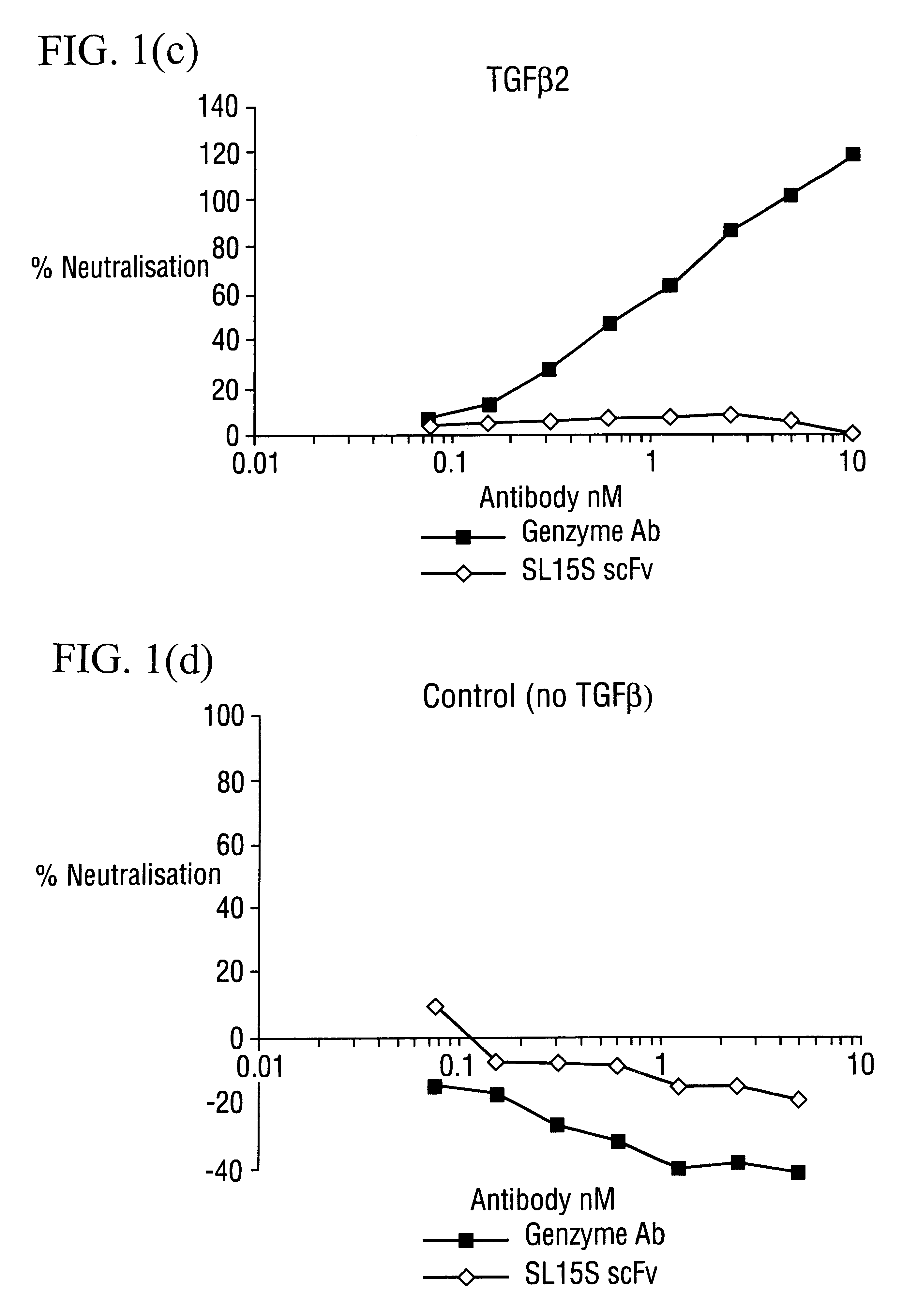
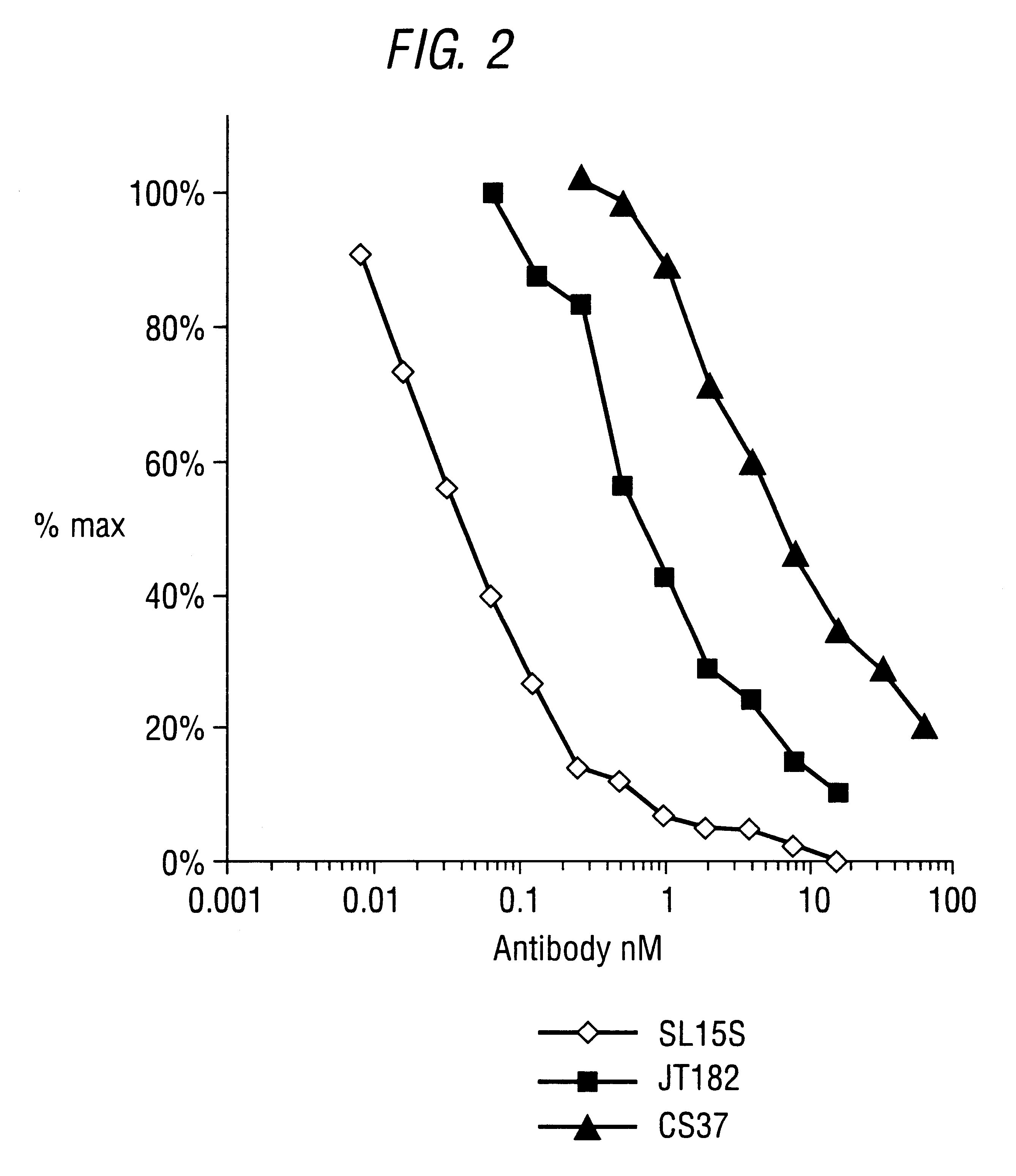
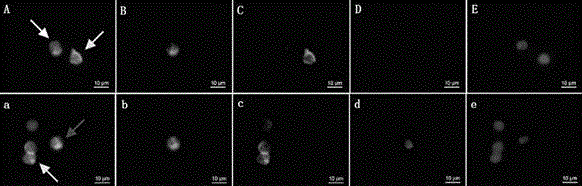
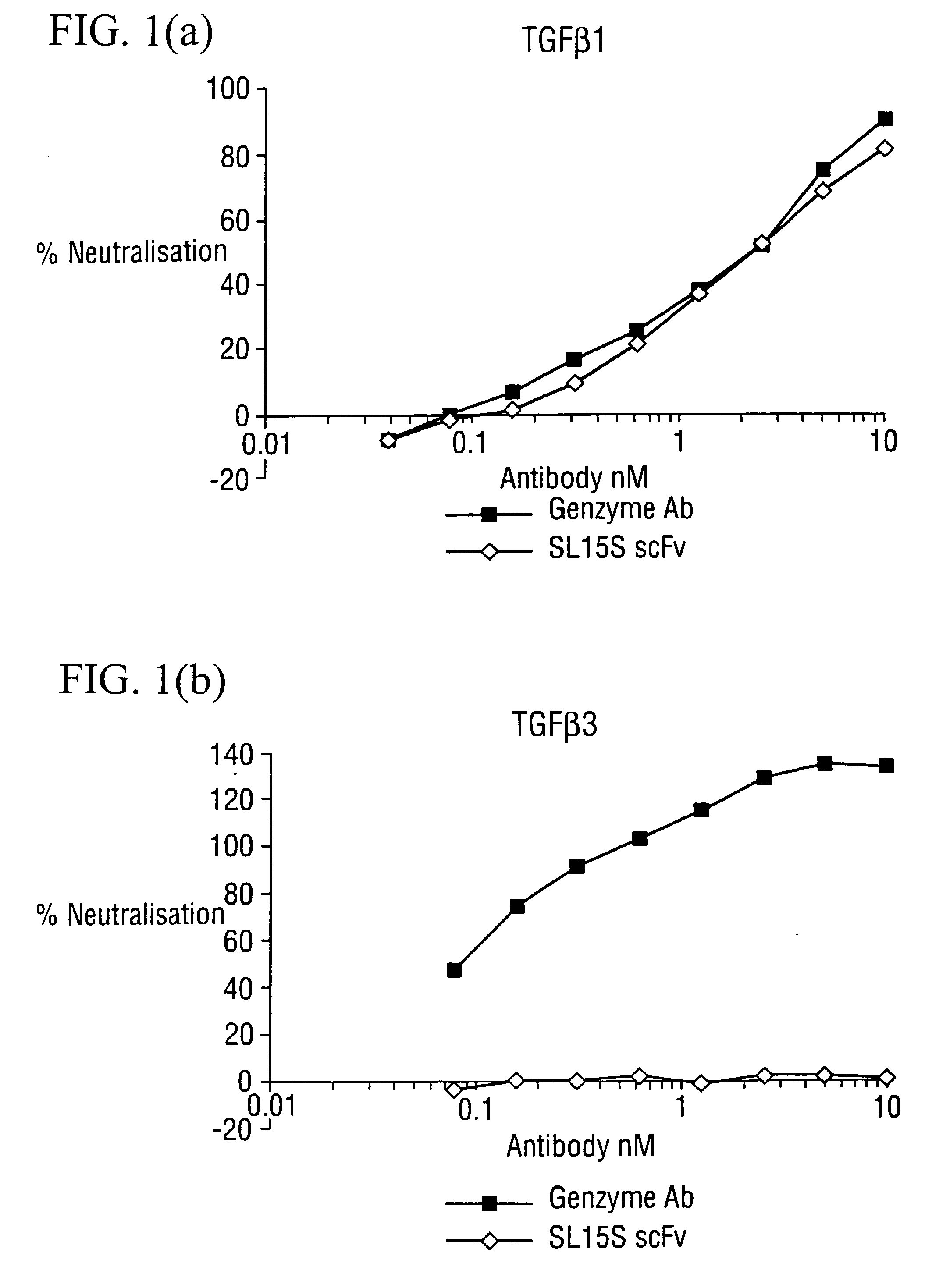
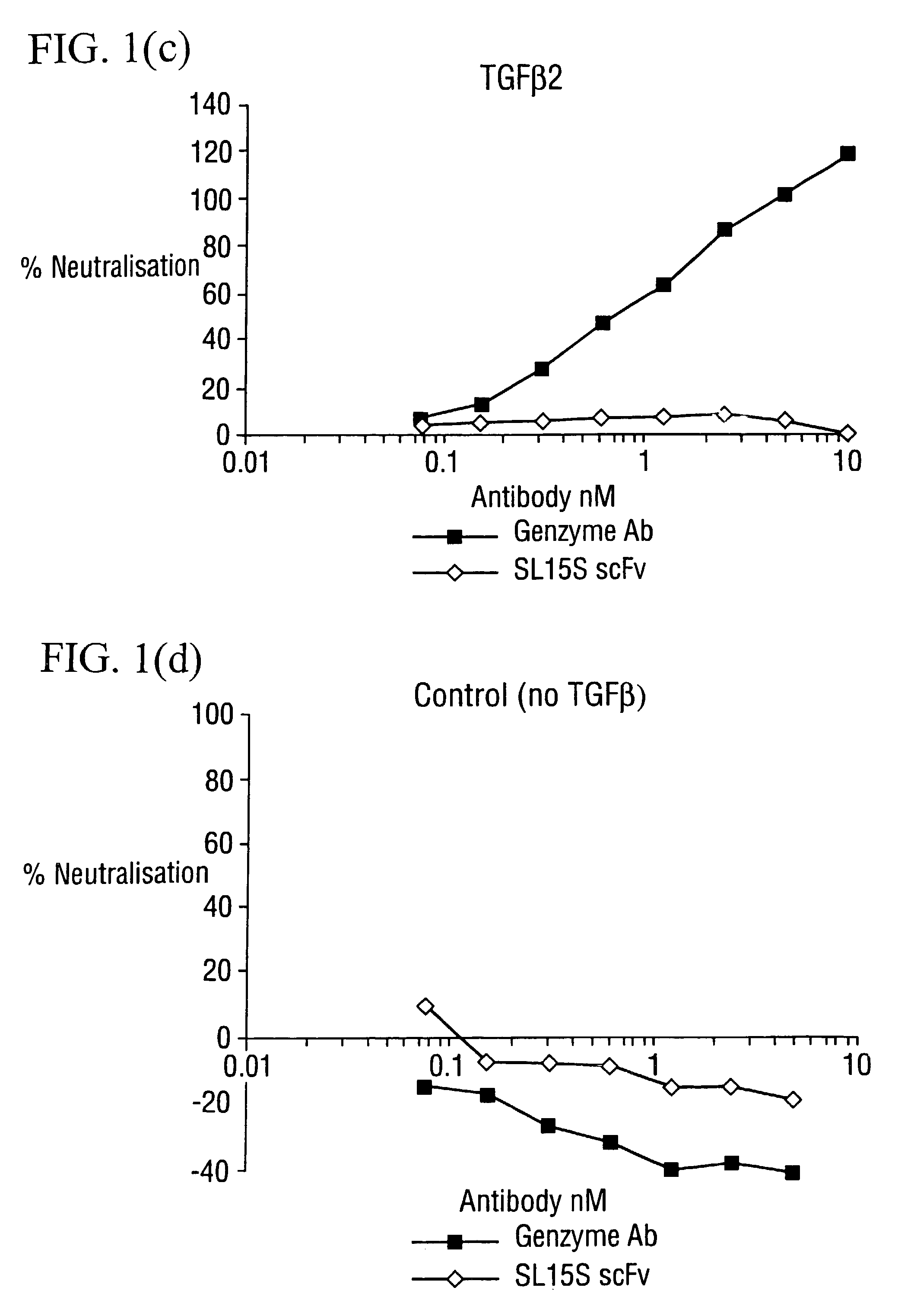
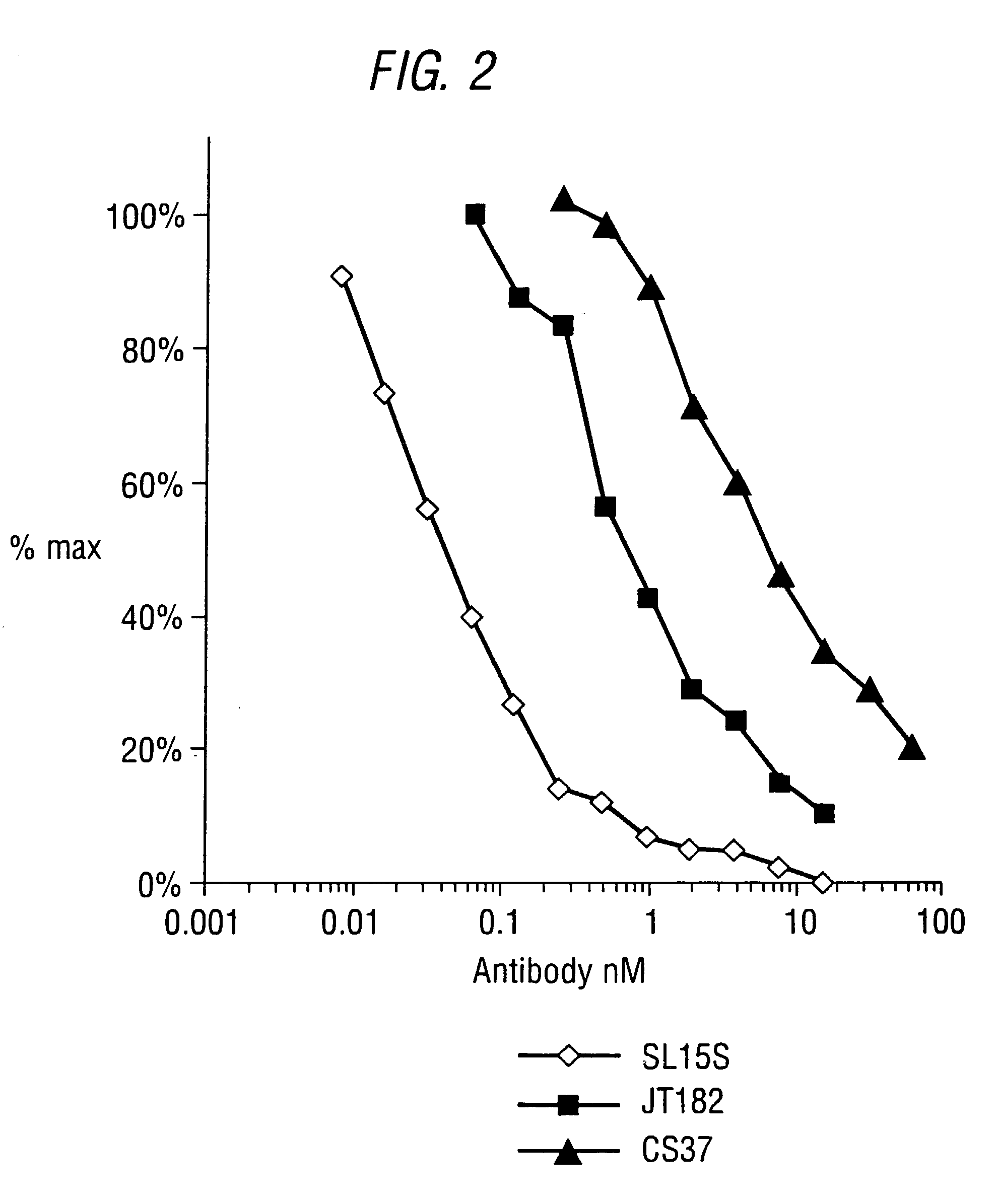
Specific binding members for TGFbeta1
The invention provides specific binding members, for example in the form of antibody variable domains, based on the CDR3 sequences of the antibody VH regions of SL15 (SEQ ID NO:4) and JT182 (SEQ ID NO:10). The antibodies have strong neutralizing activity for TGFbeta1 and are useful in treating conditions associated with excess TGFbeta1 activity, such as fibrosis, immune responses and tumor progression.
Owner:MEDIMMUNE LTD
Compounds and methods for treatment of cancer
ActiveUS20070004627A1Reduce tumor volumeInhibit tumor growthBiocidePeptide/protein ingredientsLymphatic SpreadImproved survival
The invention relates to methods and compounds for treating or preventing cancer. Methods for treating or preventing cancer, for inhibiting tumor growth, reducing tumor volume, inhibiting tumor progression, inhibiting metastasis, and improving survival are provided herein.
Owner:FIBROGEN INC
Method and apparatus for motion detection
Image analysis techniques may be employed to identify moving and / or static object within a sequence of spatial data frames (102, 300). Attributes of interest may be identified within a sequence of spatial data frames (102, 300). The attributes of interest may be clustered and examined across frames of the spatial data to detect motion vectors. A system (200) may derive information about these attributes of interest and their motion over time and identify moving and / or static objects, and the moving and / or static objects may be used to generate natural language messages describing the motion of the attributes of interest. Example uses include description of moving and / or static objects in data such as weather data, oil spills, cellular growth (e.g., tumor progression), atmospheric conditions (e.g., the size of a hole in the ozone layer), or any other implementation where it may be desirable to detect motion vectors in a sequence of spatial data frames.
Owner:ARRIA DATA2TEXT +1
Antibodies that block receptor protein tyrosine kinase activation, methods of screening for and uses thereof
Molecules comprising the antigen-binding portion of antibodies that block constitutive and / or ligand-dependent activation of a receptor protein tyrosine kinase, such as fibroblast growth factor receptor 3 (FGFR3), are found through screening methods, where a soluble dimeric form of a receptor protein tyrosine kinase is used as target for screening a library of antibody fragments displayed on the surface of bacteriophage. The molecules of the present invention which block constitutive activation can be administered to treat or inhibit skeletal dysplasia, craniosynostosis disorders, cell proliferative diseases or disorders, or tumor progression associated with the constitutive activation of a receptor protein tyrosine kinase.
Owner:FIBRON
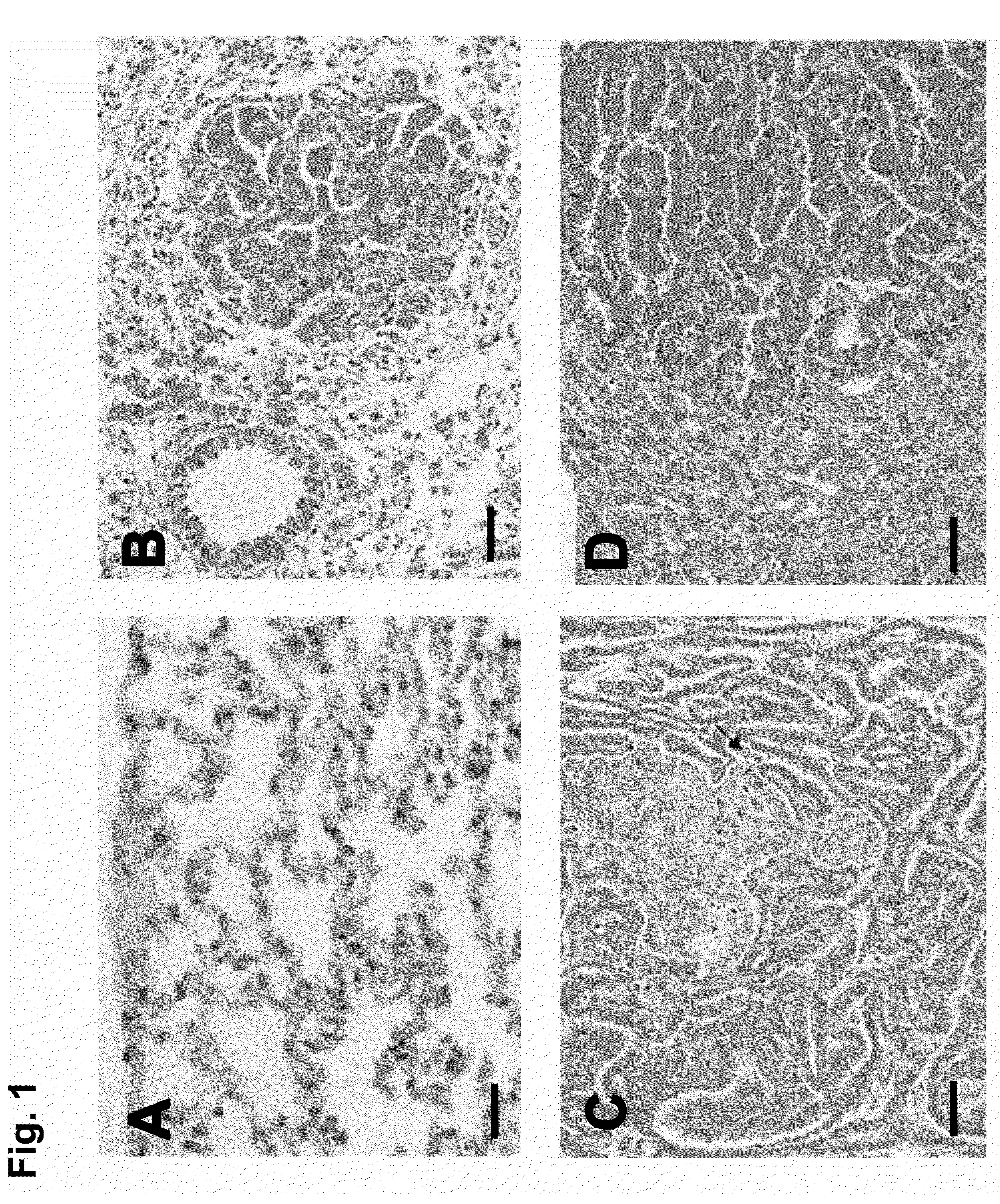
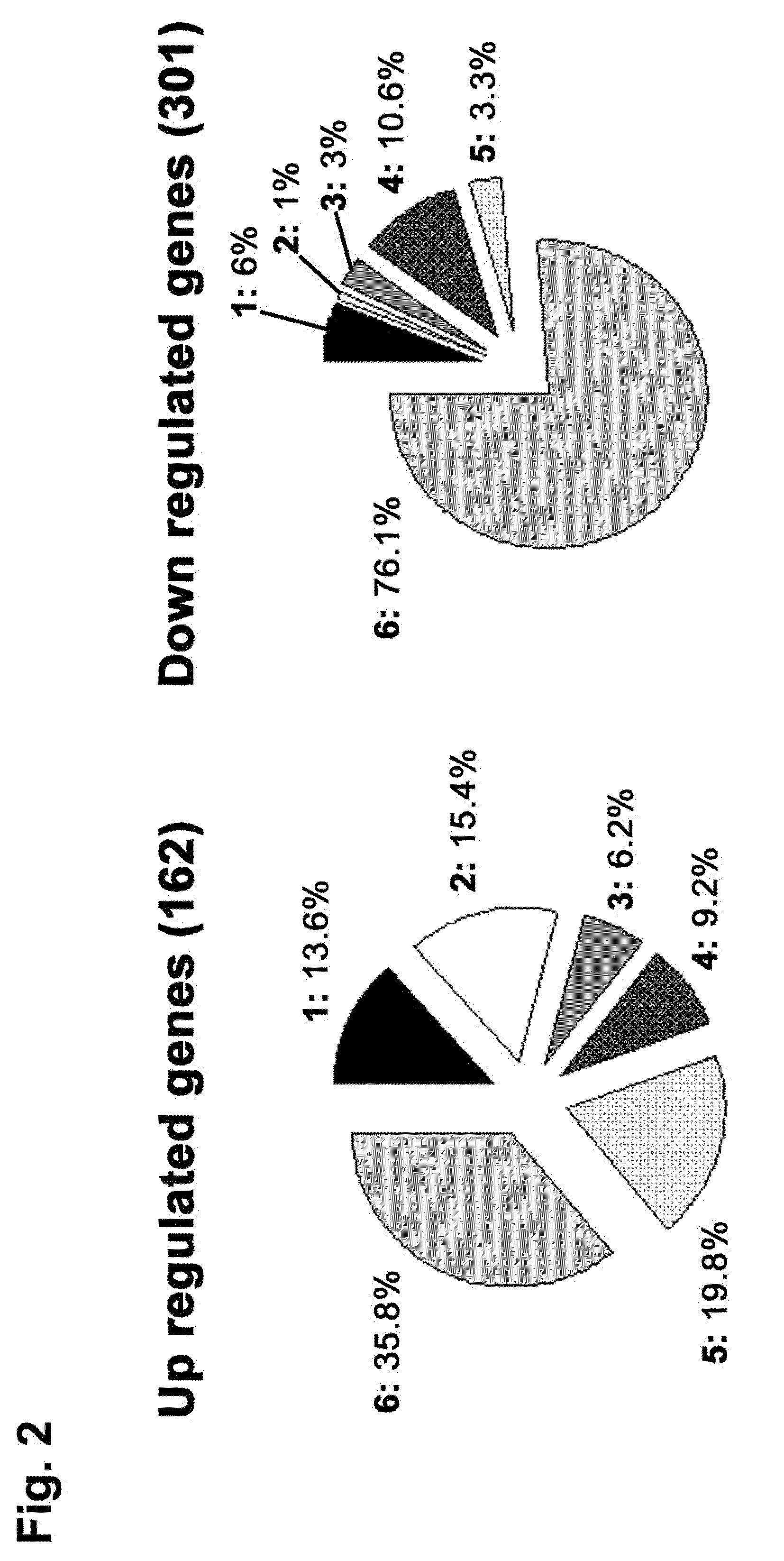
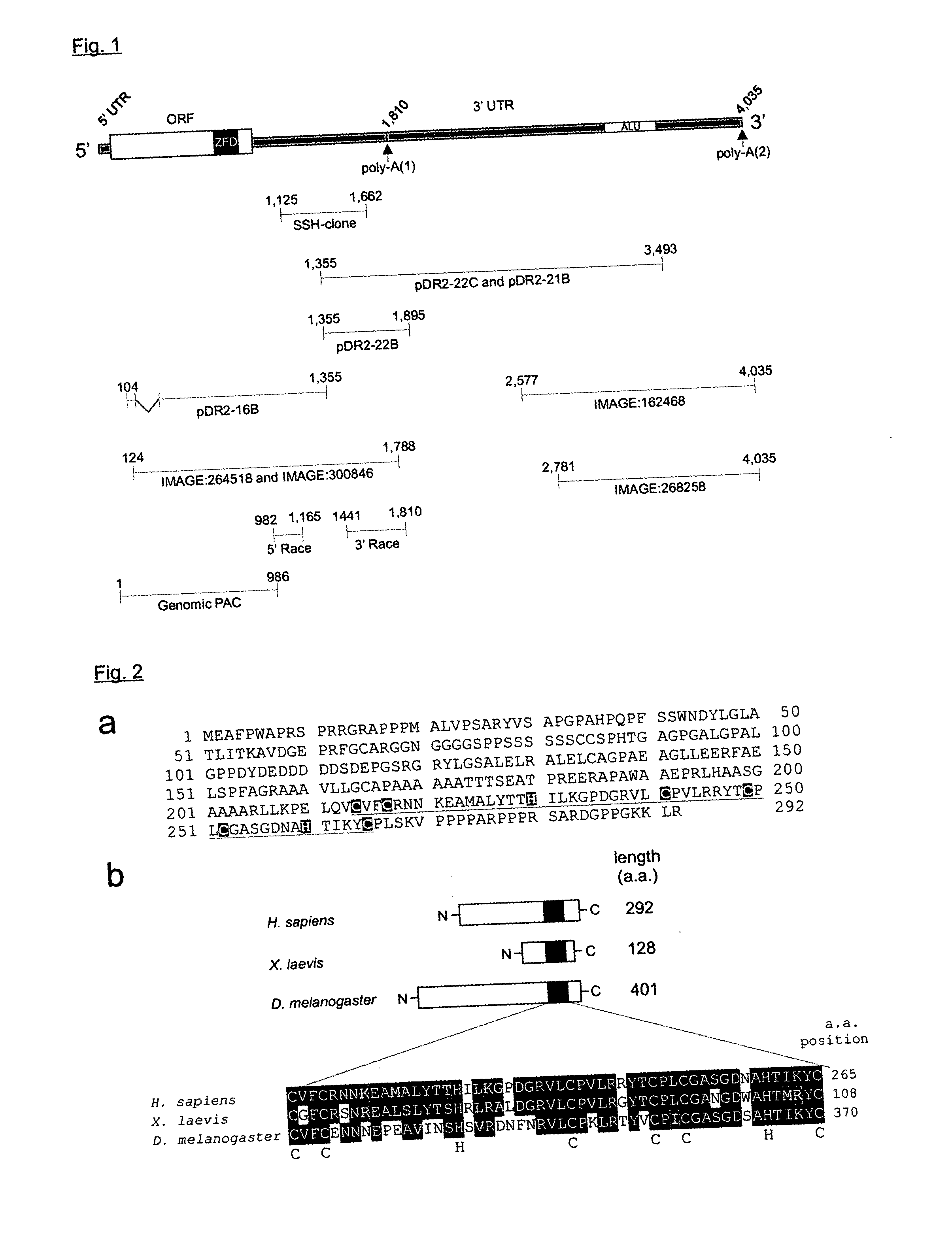
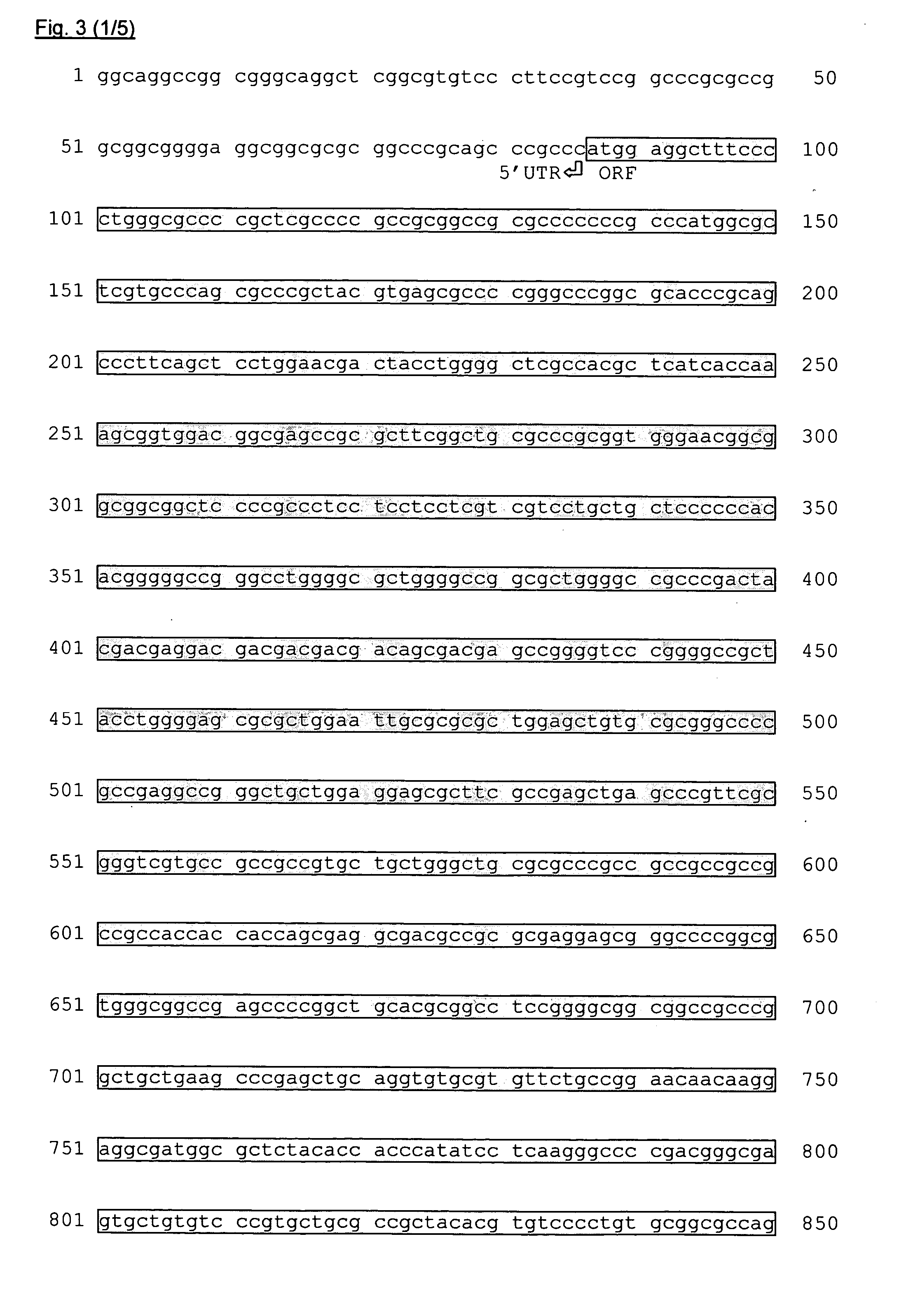
Compositions and methods for the diagnosis, prevention and treatment of tumor progression
The present invention relates to methods and compositions for the diagnosis, prevention, and treatment of tumor progression in cells involved in human tumors such as melanomas, breast, gastrointestinal, lung, and bone tumors, various types of skin cancers, and other neoplastic conditions such as leukemias and lymphomas. Genes are identified that are differentially expressed in benign (e.g., non-malignant) tumor cells relative to malignant tumor cells exhibiting a high metastatic potential. Genes are also identified via the ability of their gene products to interact with gene products involved in the progression to, and / or aggressiveness of, neoplastic tumor disease states. The genes and gene products identified can be used diagnostically or for therapeutic intervention.
Owner:MILLENNIUM PHARMA INC
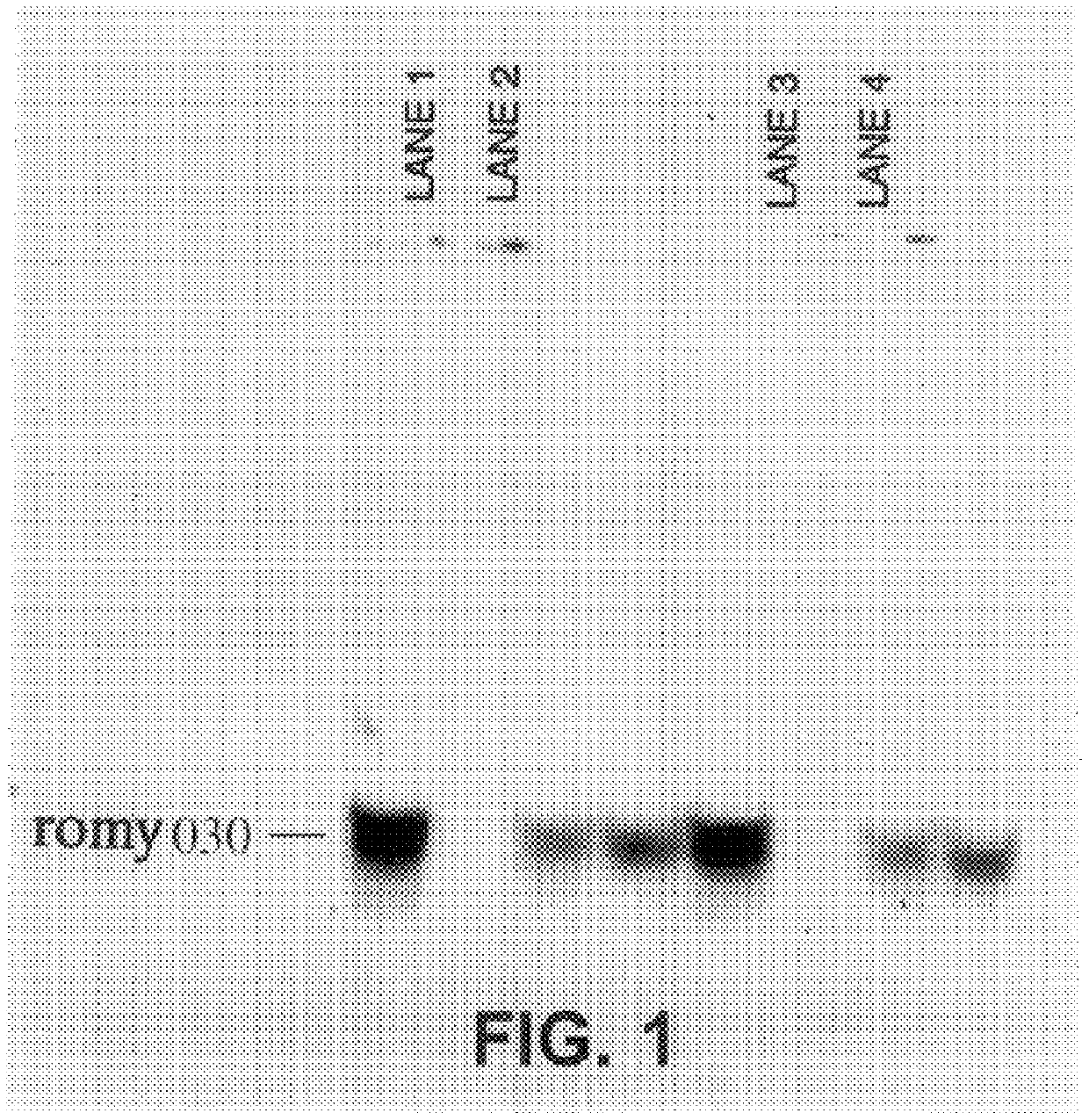
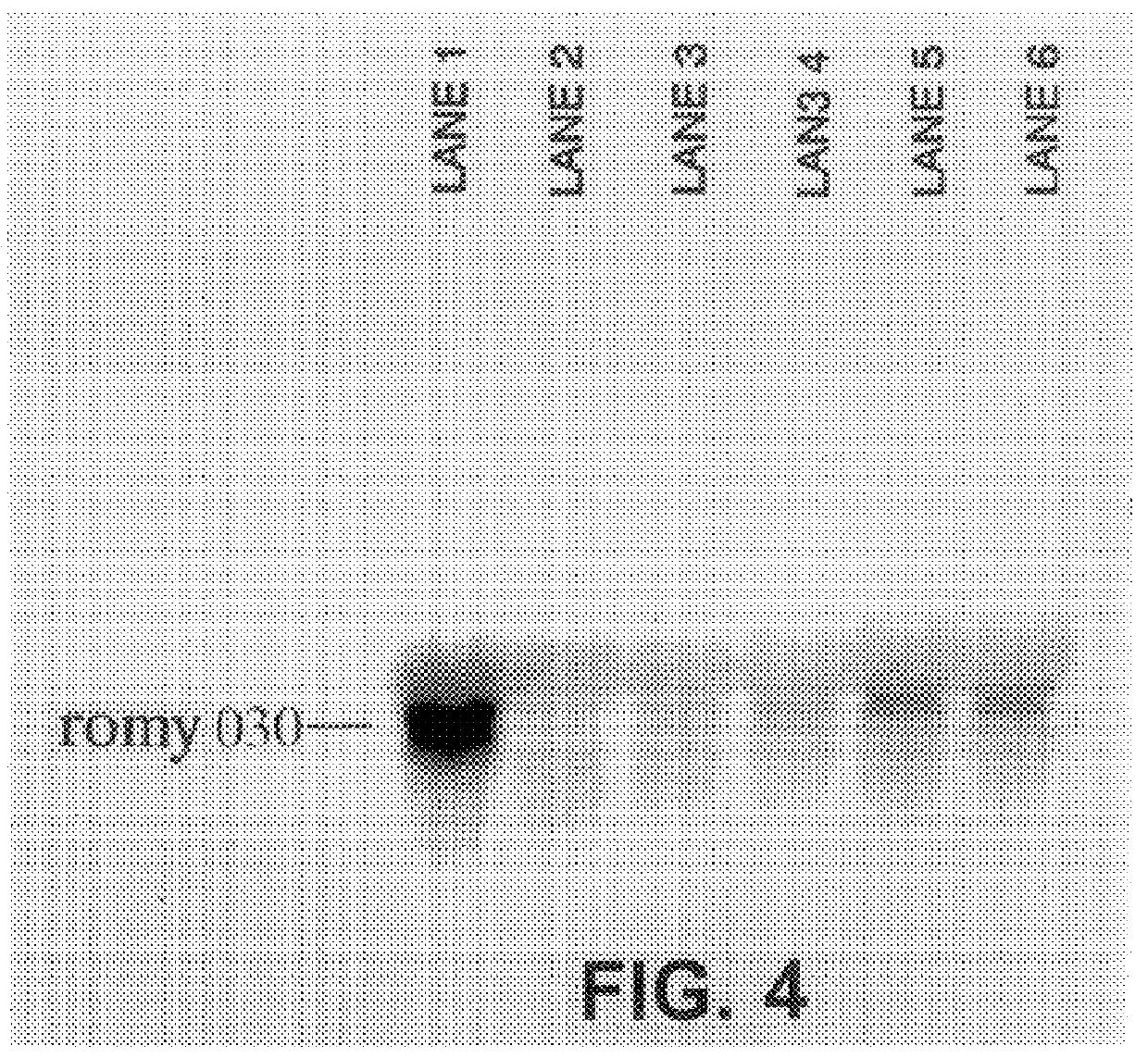
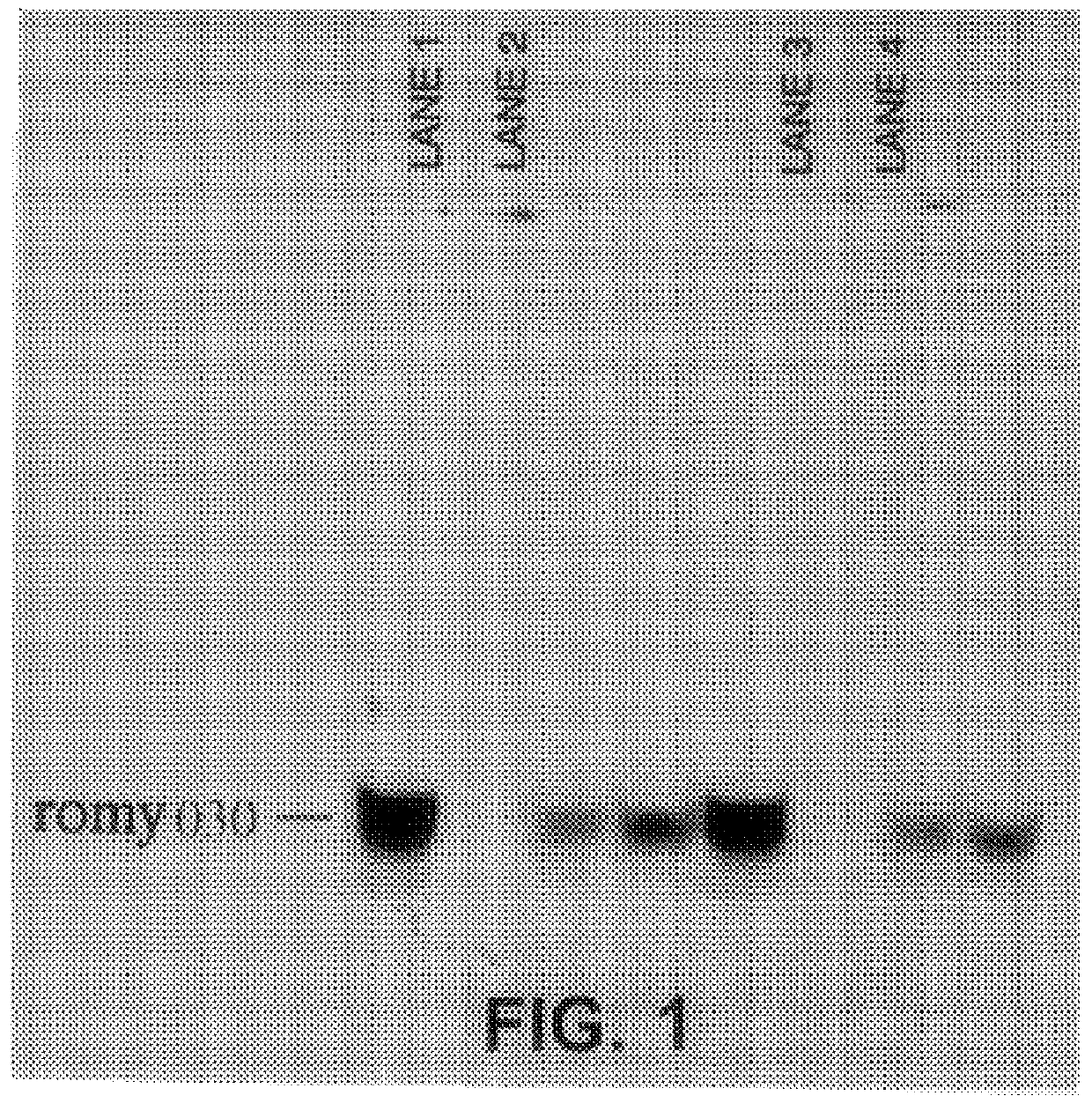
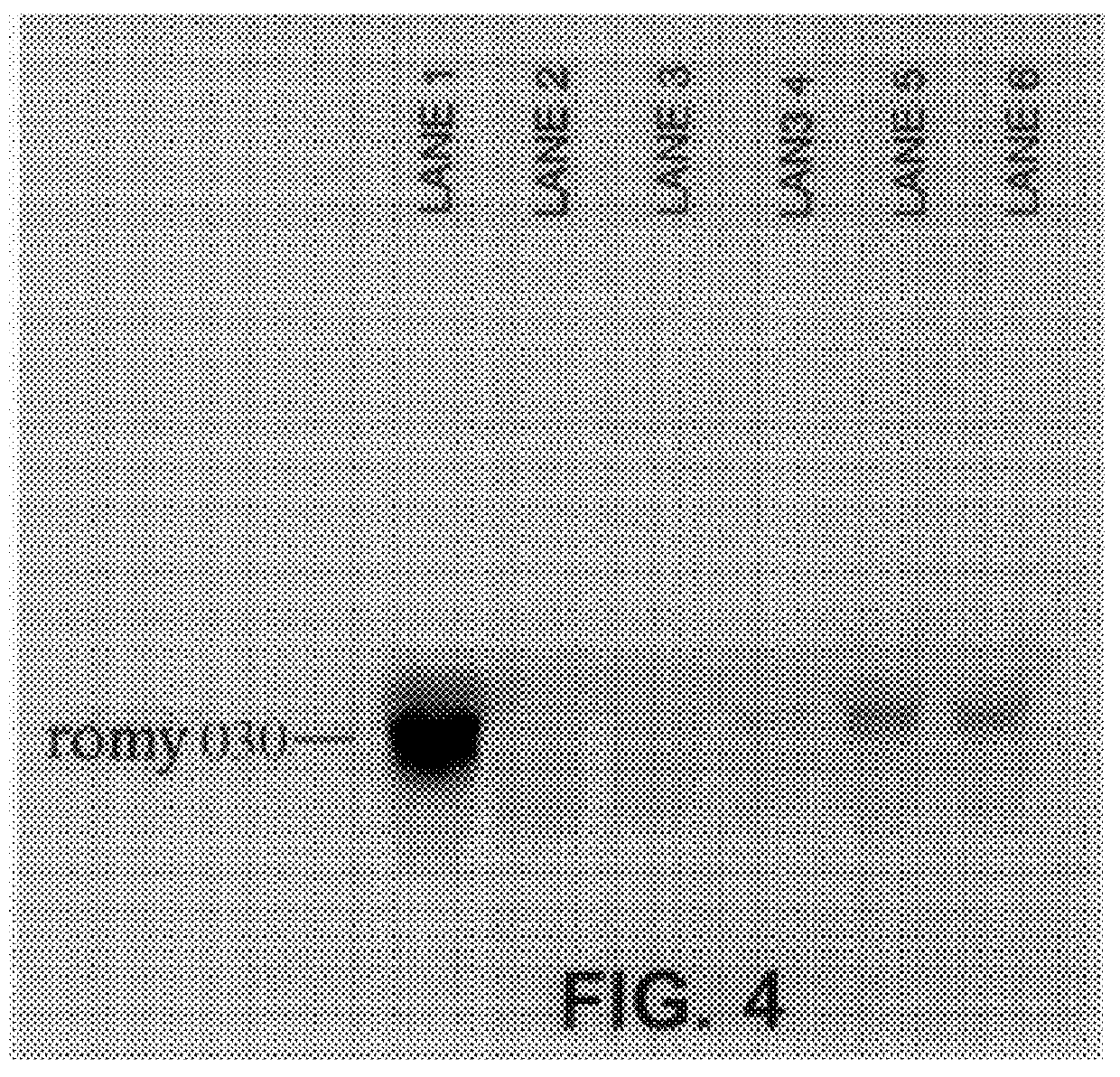
Methods for detecting fohy030
The present invention relates to methods and compositions for the diagnosis, prevention, and treatment of tumor progression in cells involved in human tumors such as melanomas, breast, gastrointestinal, lung, and bone tumors, various types of skin cancers, and other neoplastic conditions such as leukemias and lymphomas. Genes are identified that are differentially expressed in benign (e.g., non-malignant) tumor cells relative to malignant tumor cells exhibiting a high metastatic potential. Genes are also identified via the ability of their gene products to interact with gene products involved in the progression to, and / or aggressiveness of, neoplastic tumor disease states. The genes and gene products identified can be used diagnostically or for therapeutic intervention.
Owner:MILLENNIUM PHARMA INC
Circulating tumor cell detection kit and application thereof
InactiveCN105785005AAccurate detectionEfficient enrichmentMaterial analysisLymphatic SpreadFluorescence
The invention relates to a circulating tumor cell detection kit and application thereof. The circulating tumor cell detection kit comprises a chip, sample diluent, a cell separation fluid, a cell trapping agent, a cell cleaning solution, a cell fixing agent, antibody diluent, a cell penetration agent and a fluorescence dye. The application method of the circulating tumor cell detection kit mainly comprises the several steps of separation of circulating tumor cells, trapped antibody coating, trapping of the circulating tumor cells and immuno-fluorescent staining of the circulating tumor cells. The circulating tumor cell detection kit is mainly used for tumor prognosis, reappear, metastasis, curative effect monitoring, early diagnosis and early warning, monitoring of curative effect and tumor progression situation, and utilizes the molecular subtyping, screening specificity and other characteristics of the circulating tumor cells to conduct targeted research on circulating tumor cell sensitive drug so as to supplement tumor metastasis, relapse and drug resistance mechanisms and guide individualized medical treatment.
Owner:杭州华得森生物技术有限公司
Specific binding members for TGFbeta1
The invention provides specific binding members, for example in the form of antibody variable domains, based on the CDR3 sequences of the antibody VH regions of SL15 (SEQ ID NO:4) and JT182 (SEQ ID NO:10). The antibodies have strong neutralising activity for TGFbeta1 and are useful in treating conditions associated with excess TGFbeta1 activity, such as fibrosis, immune responses and tumor progression
Owner:MEDIMMUNE LTD
Inhibition Of Superoxide Dismutase By Tetrathiomolybdate: Identification Of New Anti-Angiogenic And Antitumor Agents
InactiveUS20080031817A1Prevent proliferationInhibit angiogenesisBiocideCompound screeningAnticarcinogenDepressant
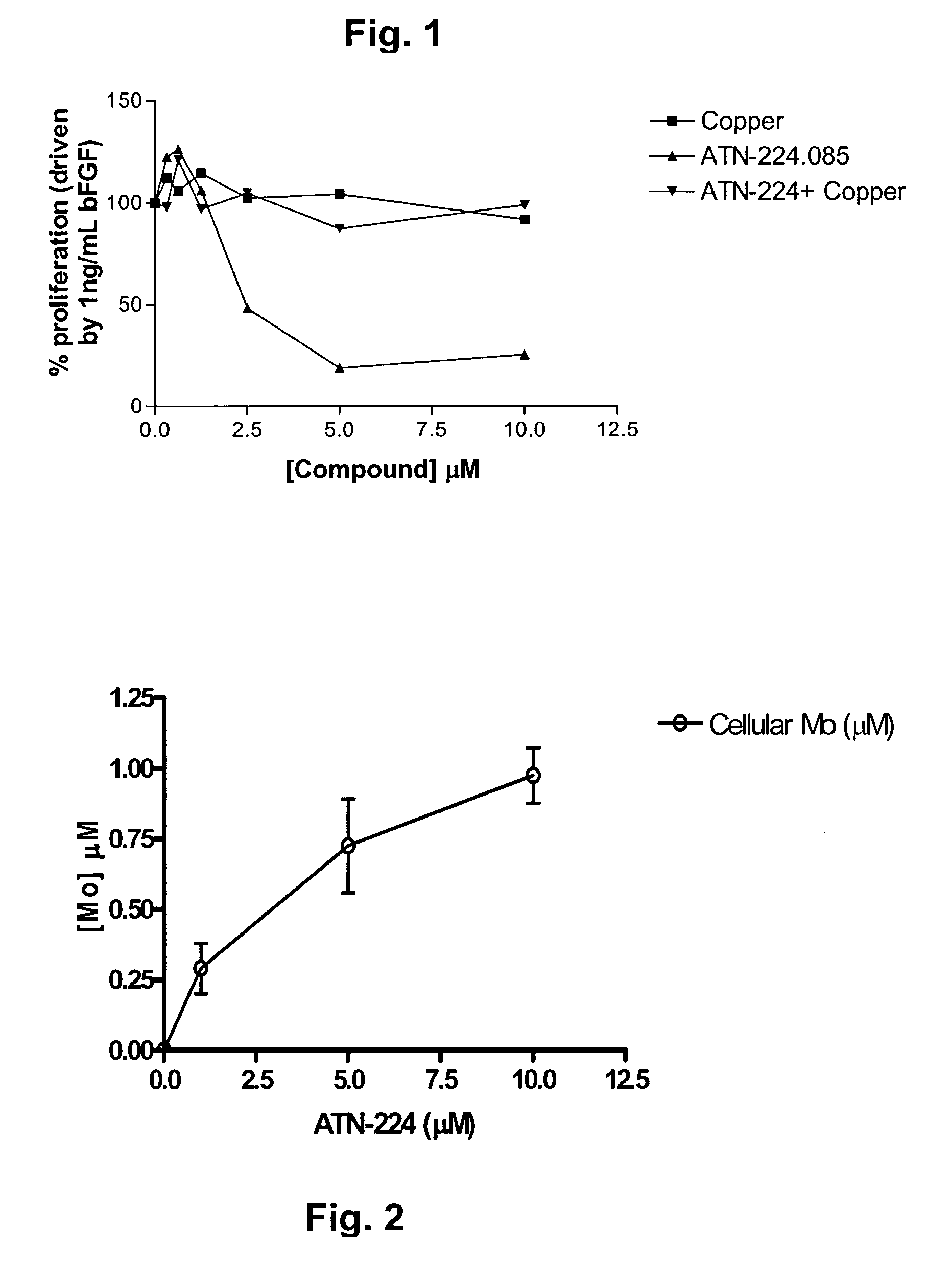
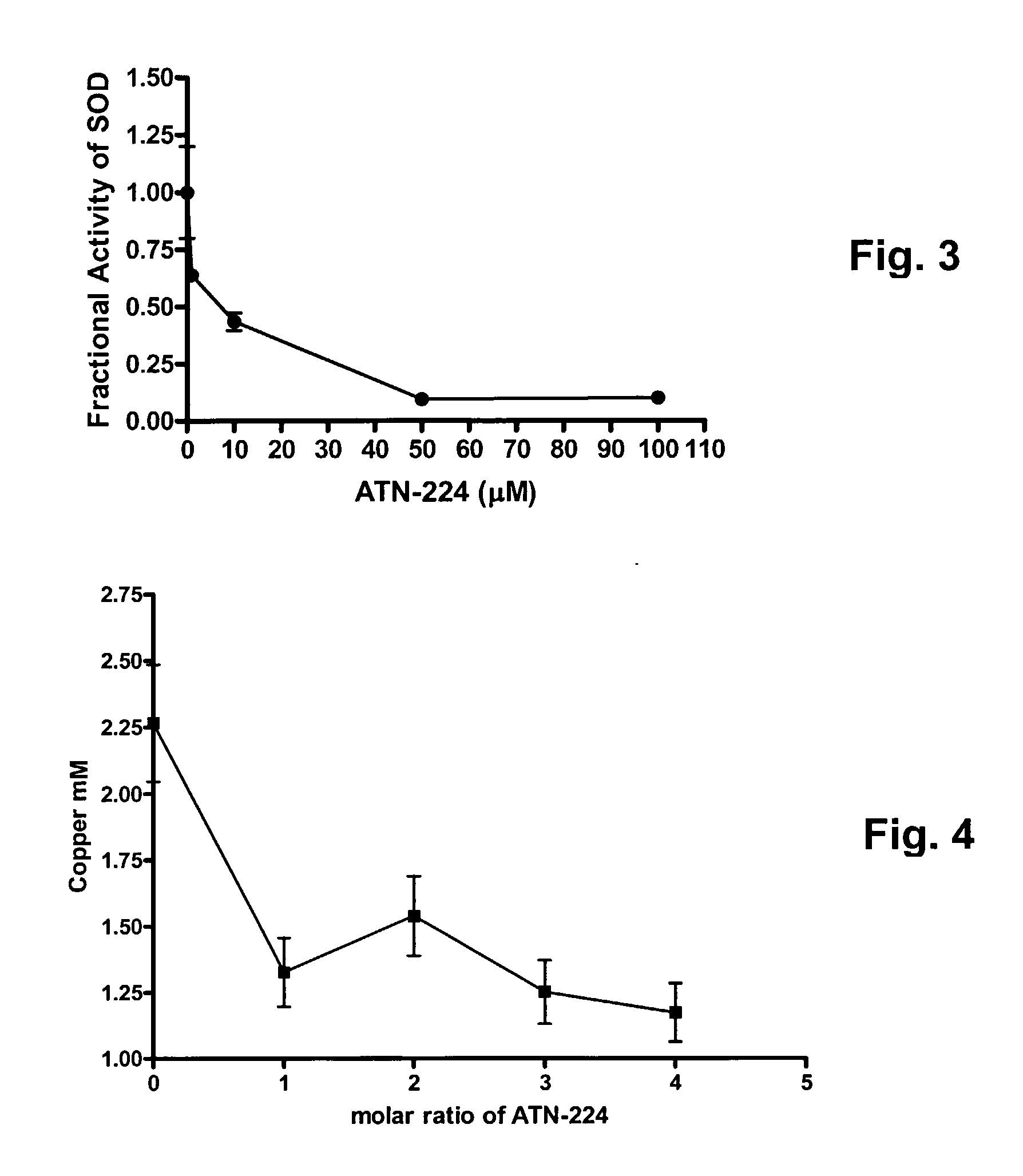
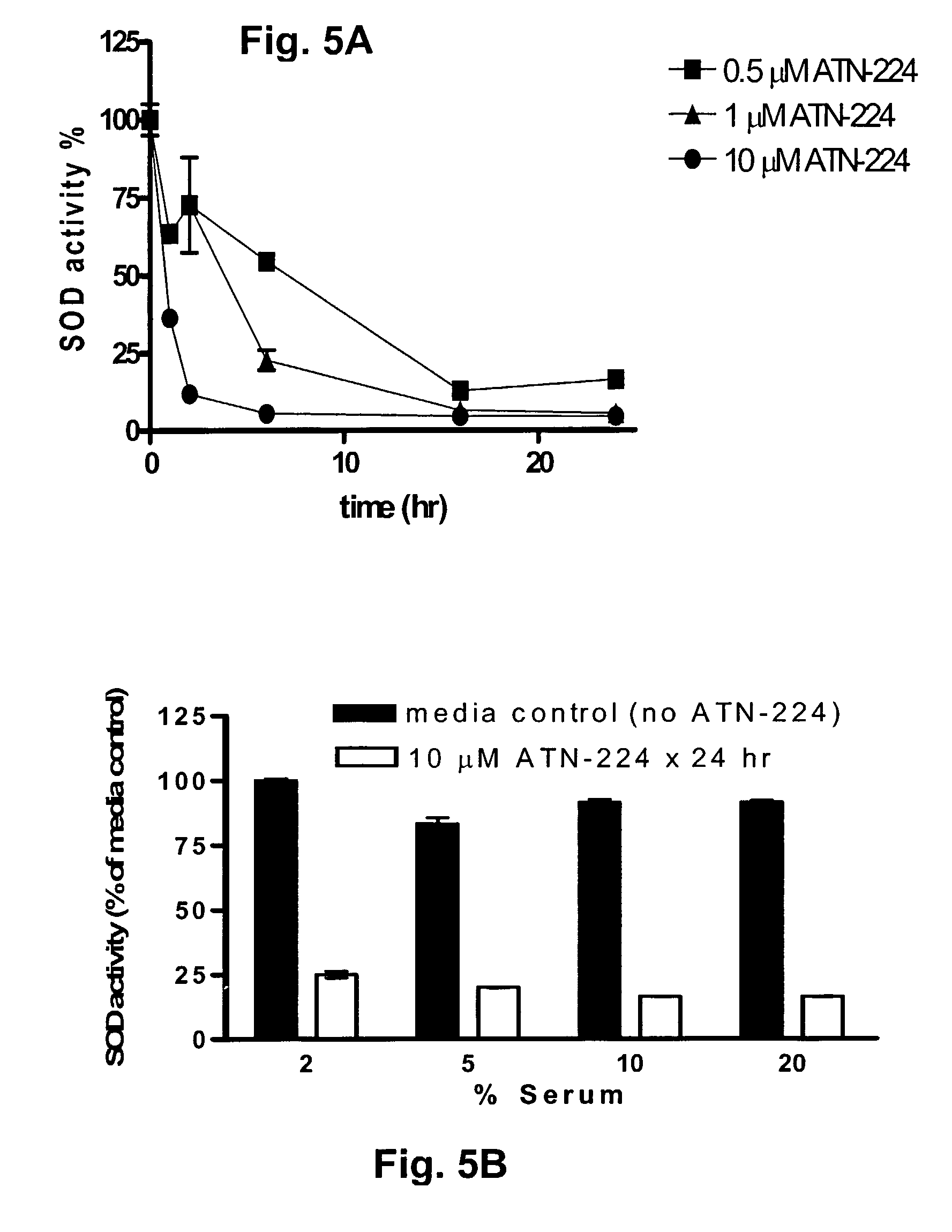
Though copper is elevated in the tumor tissue and plasma of patients with various malignancies, the molecular targets for copper binding agents in angiogenesis and tumor progression remain poorly understood. It is disclosed that one anti-angiogenic target for the copper binding agent tetrathiomolybdate is intracellular CuZn-superoxide dismutase (SOD1). A second generation tetrathiomolybdate analog, ATN-224, inhibits endothelial cell (EC) proliferation in vitro, binds to SOD1 and inhibits its activity without displacing bound copper ATN-224 can accumulate in ECs and inhibit CuZnSOD activity with an IC50 similar to the IC50 for EC proliferation, resulting in increased generation of intracellular reactive oxygen species. Inhibition of EC proliferation by ATN-224 in vitro is substantially reversed by a synthetic porphyrin SOD mimetic. Similar results were observed in vivo, where inhibition of angiogenesis by ATN-224 in a Matrigel plug model was also reversed by MnTBAP. Thus, a distinct molecular target for copper depletion therapy has been identified and SOD1 is now validated as a target for anti-angiogenesis. Methods for screening, or designing, such SOD1 inhibitors for use as angiogenesis inhibitors and anti-cancer agents are disclosed.
Owner:ATTENUON LLC
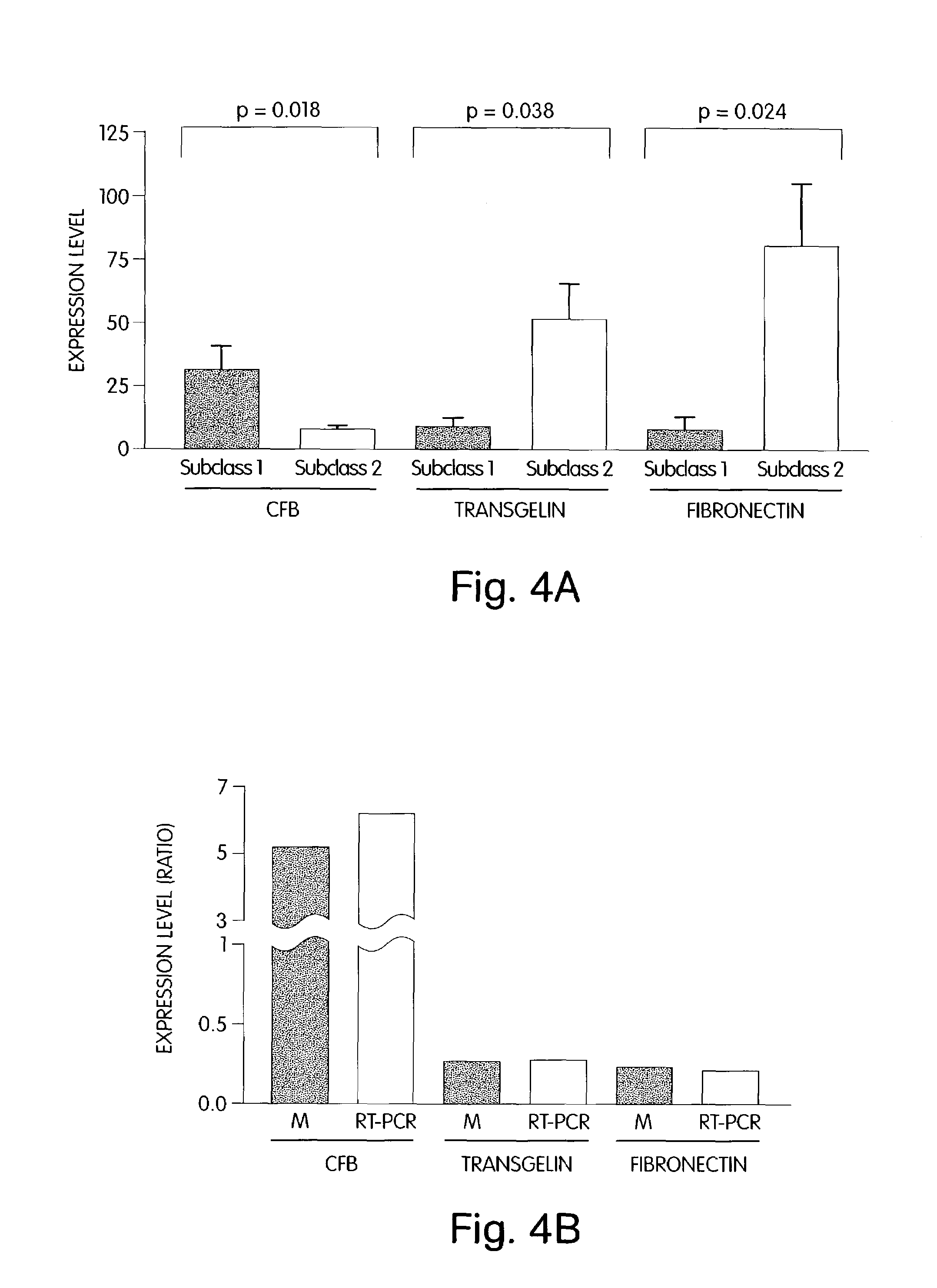
Diagnostic tests using gene expression ratios
InactiveUS20100028876A1Successfully diagnoseDifficult to assess clinicallyMicrobiological testing/measurementBiological testingCancer cellTissue sample
The invention provides methods for diagnosing biological states or conditions based on ratios of gene expression data from cell or tissue samples, such as cancer cell or tissue samples. The invention also provides sets of genes that are expressed differentially in normal and cancer lung cells and tissues. These sets of genes can be used to discriminate between normal and malignant cells or tissues, and between classes of malignant cells or tissues. Accordingly, diagnostic assays for classification of tumors, prediction of tumor outcome, selecting and monitoring treatment regimens and monitoring tumour progression / regression also are provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Diagnostic and Prognostic Tests
InactiveUS20090104617A1Simple powerEasy to adaptMicrobiological testing/measurementMaterial analysisTissue sampleNormal tissue
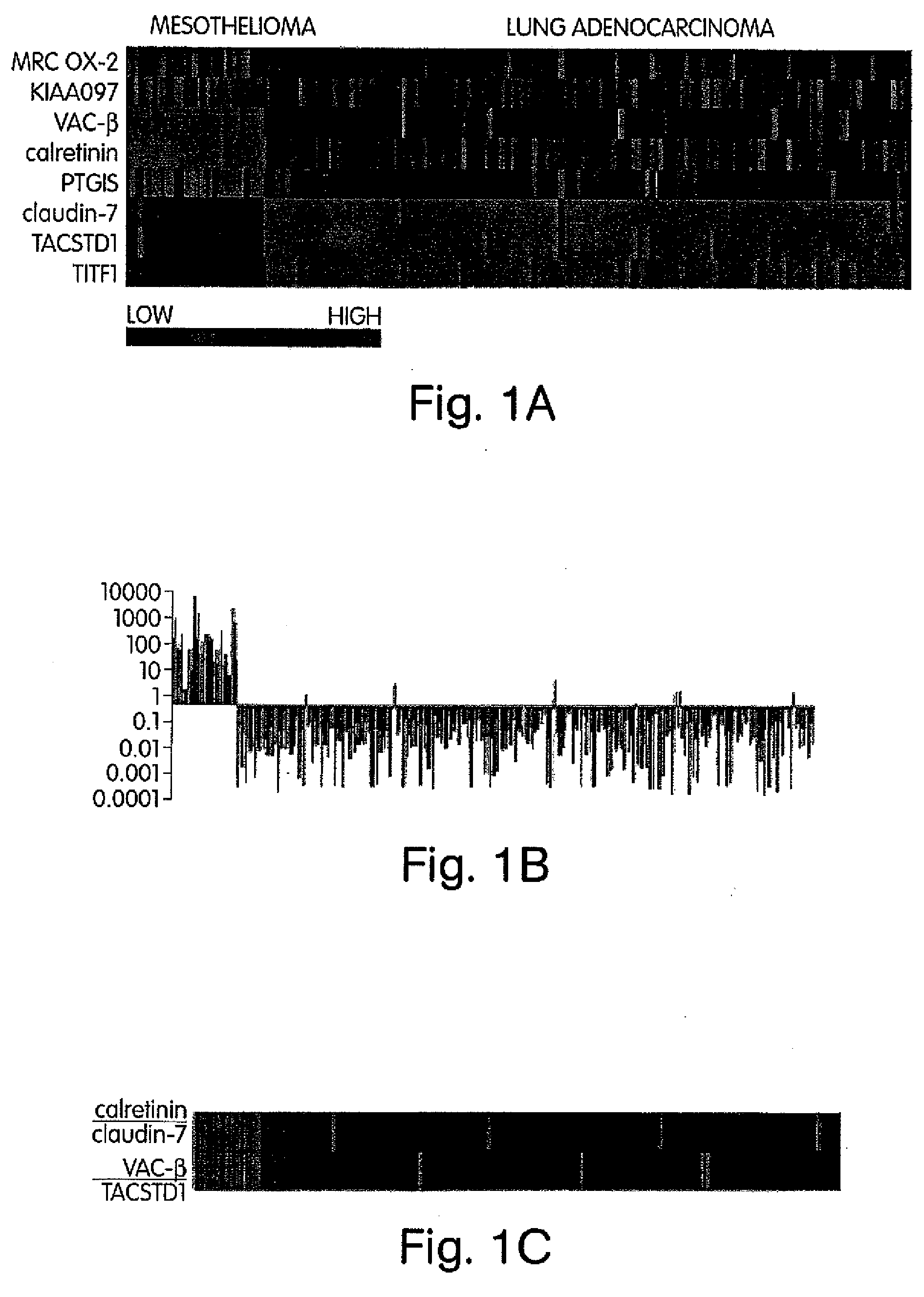
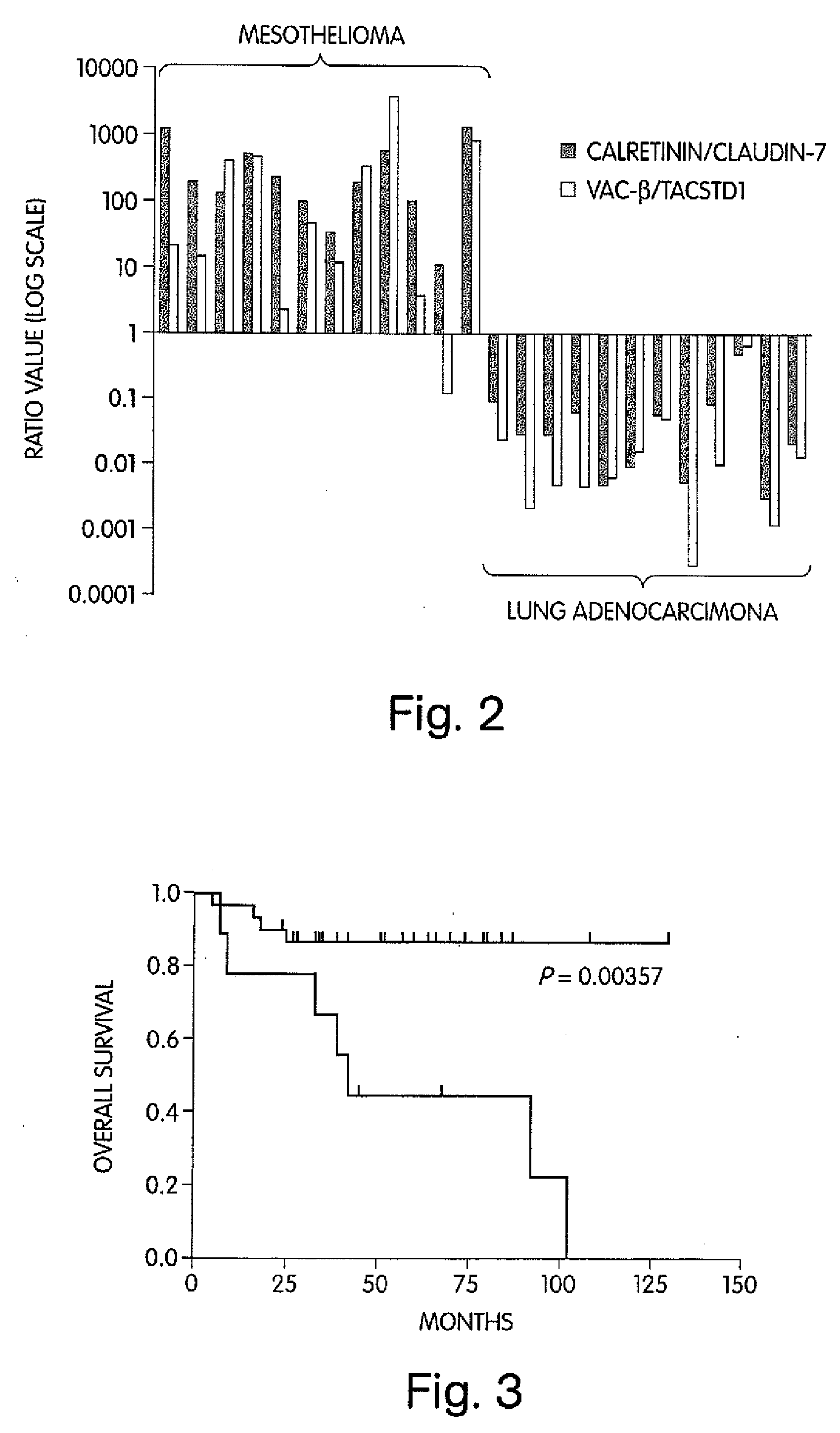
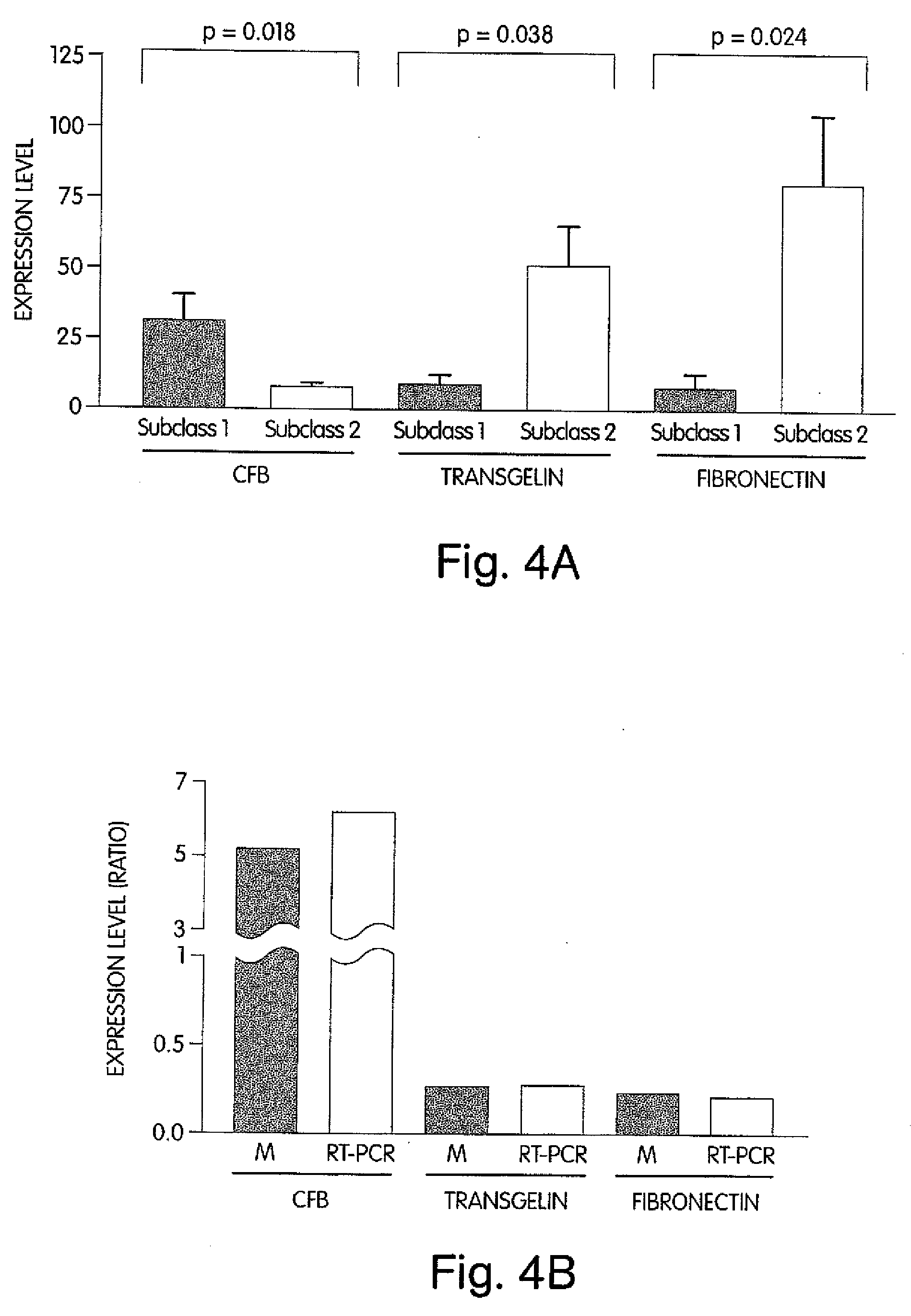
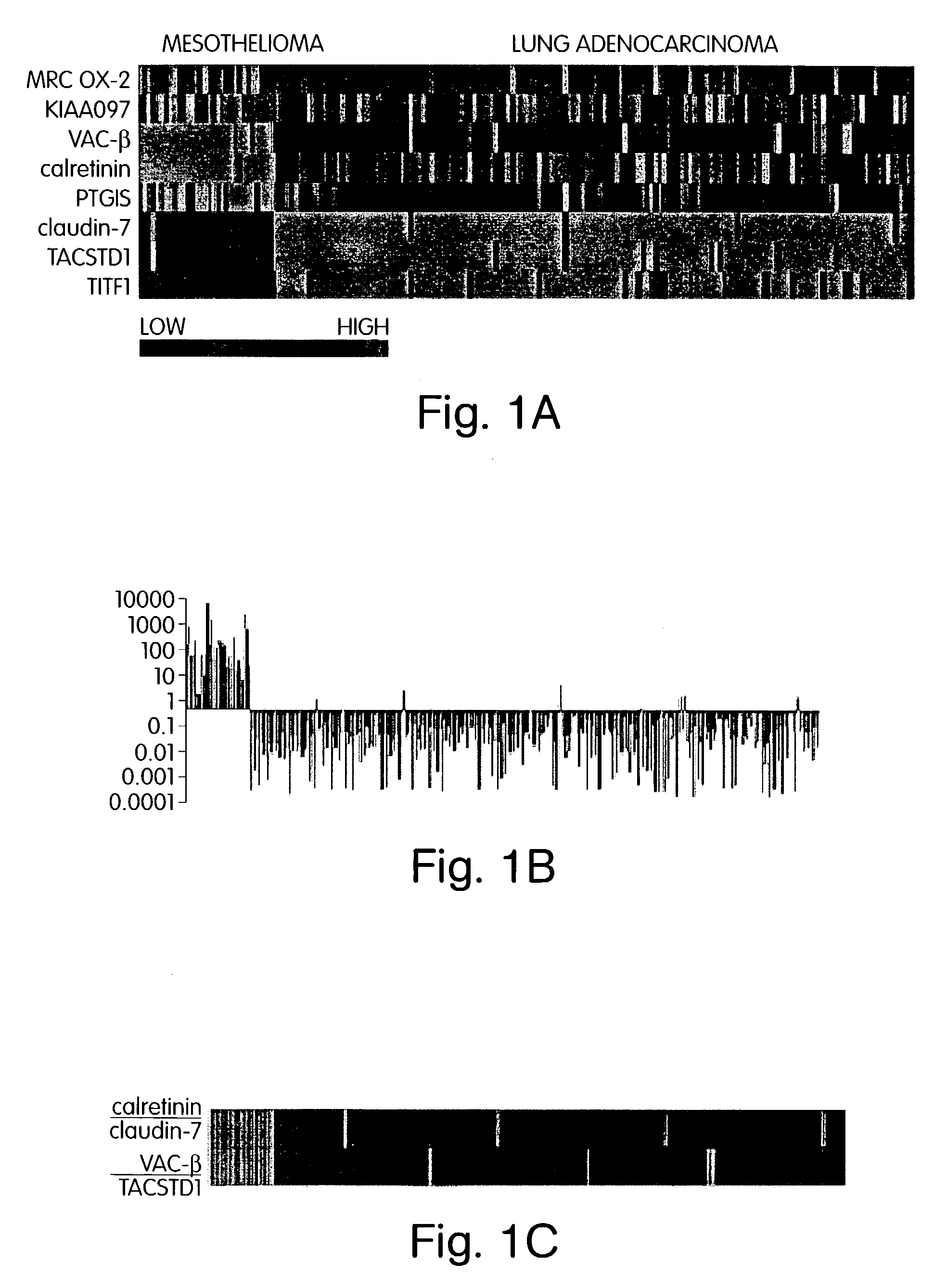
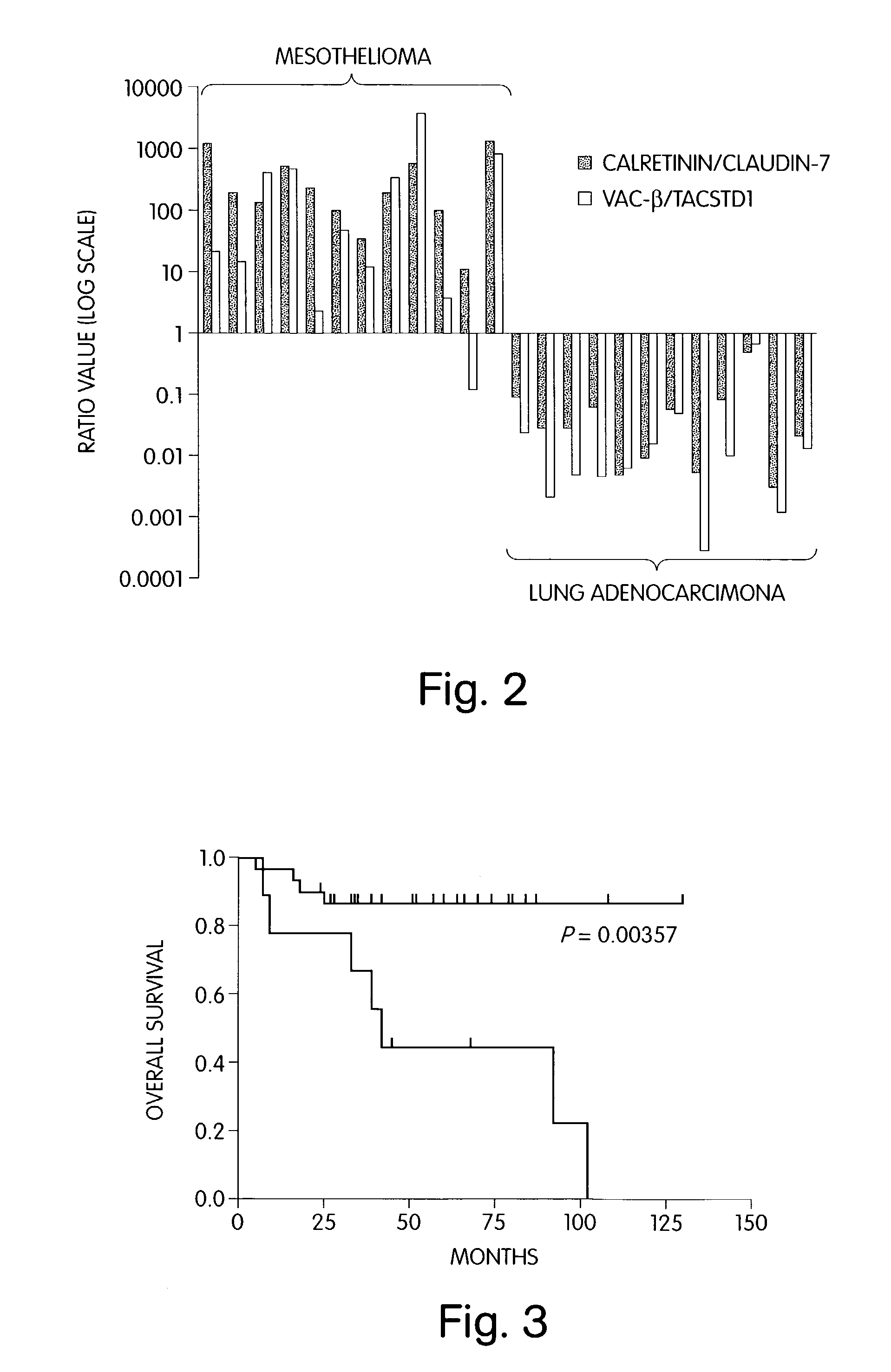
The invention provides methods for diagnosing biological states or conditions based on ratios of gene expression data from tissue samples, such as cancer tissue samples. The invention also provides sets of genes that are expressed differentially in malignant pleural mesothelioma. These sets of genes can be used to discriminate between normal and malignant tissues, and between classes of malignant tissues. Accordingly, diagnostic assays for classification of tumors, prediction of tumor outcome, selecting and monitoring treatment regimens and monitoring tumor progression / regression also are provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Compounds and methods for treatment of cancer
ActiveUS8530404B2Altering activity of tumorReduce tumor volumeBiocidePeptide/protein ingredientsCancer preventionLymphatic Spread
The invention relates to methods and compounds for treating or preventing cancer. Methods for treating or preventing cancer, for inhibiting tumor growth, reducing tumor volume, inhibiting tumor progression, inhibiting metastasis, and improving survival are provided herein.
Owner:FIBROGEN INC
Compositions and Methods for Inhibiting Growth of SMAD-4 Deficient Cancers
The present invention is in the fields of cell biology, immunology and oncology. The invention relates to the discovery that there is a relationship between the expression levels of the tumor suppressor gene smad4 (also known as dpc4) and integrin αvβ6, and the responsiveness of patient populations to αvβ6-active compounds and compositions (e.g., antibodies and other ligands that bind αvβ6), particularly in cancer cells from such patient populations, more particularly on carcinomas such as pancreatic carcinomas. The invention thus provides methods for determining the responsiveness of tumor cells (particularly those from pancreatic tumors) to such αvβ6-active compounds and compositions by examining the expression of αvβ6 and smad4 by the tumor cells, as well as methods of diagnosis and treatment / prevention of tumor progression using ligands, including antibodies and small molecule drugs, that bind to integrin αvβ6 on the surfaces of tumor cells and / or that block one or more components of the TGF-β pathway, particularly in smad4-deficient tumor cells.
Owner:BIOGEN MA INC
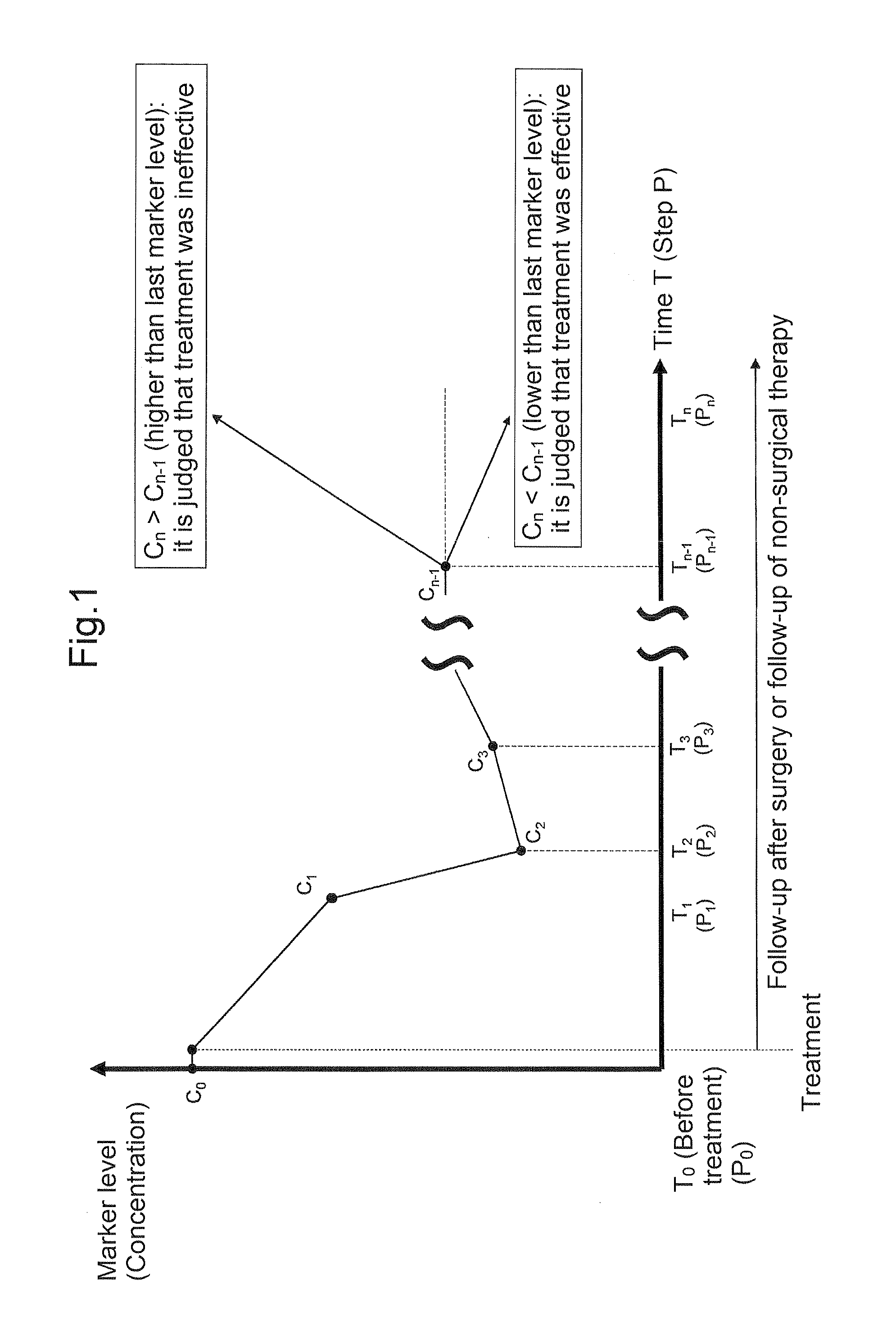
Reagents and methods for cancer prognosis and pathological staging
InactiveUS20070207489A1Microbiological testing/measurementMaterial analysisTumor progressionTumor cells
This invention provides reagents and methods for assessing tumor progression using tissue or tumor cell-containing samples from an individual. The invention also provides reagents and methods for assessing response to chemotherapy.
Owner:VENTANA MEDICAL SYST INC
Antibodies that block receptor protein tyrosine kinase activation, methods of screening for and uses thereof
Owner:FIBRON
Diagnostic and prognostic tests
InactiveUS7622260B2Simple powerEasy to adaptMicrobiological testing/measurementBiological testingTissue sampleExpression gene
The invention provides methods for diagnosing biological states or conditions based on ratios of gene expression data from tissue samples, such as cancer tissue samples. The invention also provides sets of genes that are expressed differentially in malignant pleural mesothelioma. These sets of genes can be used to discriminate between normal and malignant tissues, and between classes of malignant tissues. Accordingly, diagnostic assays for classification of tumors, prediction of tumor outcome, selecting and monitoring treatment regimens and monitoring tumor progression / regression also are provided.
Owner:WESLEYAN UNIVERSITY +1
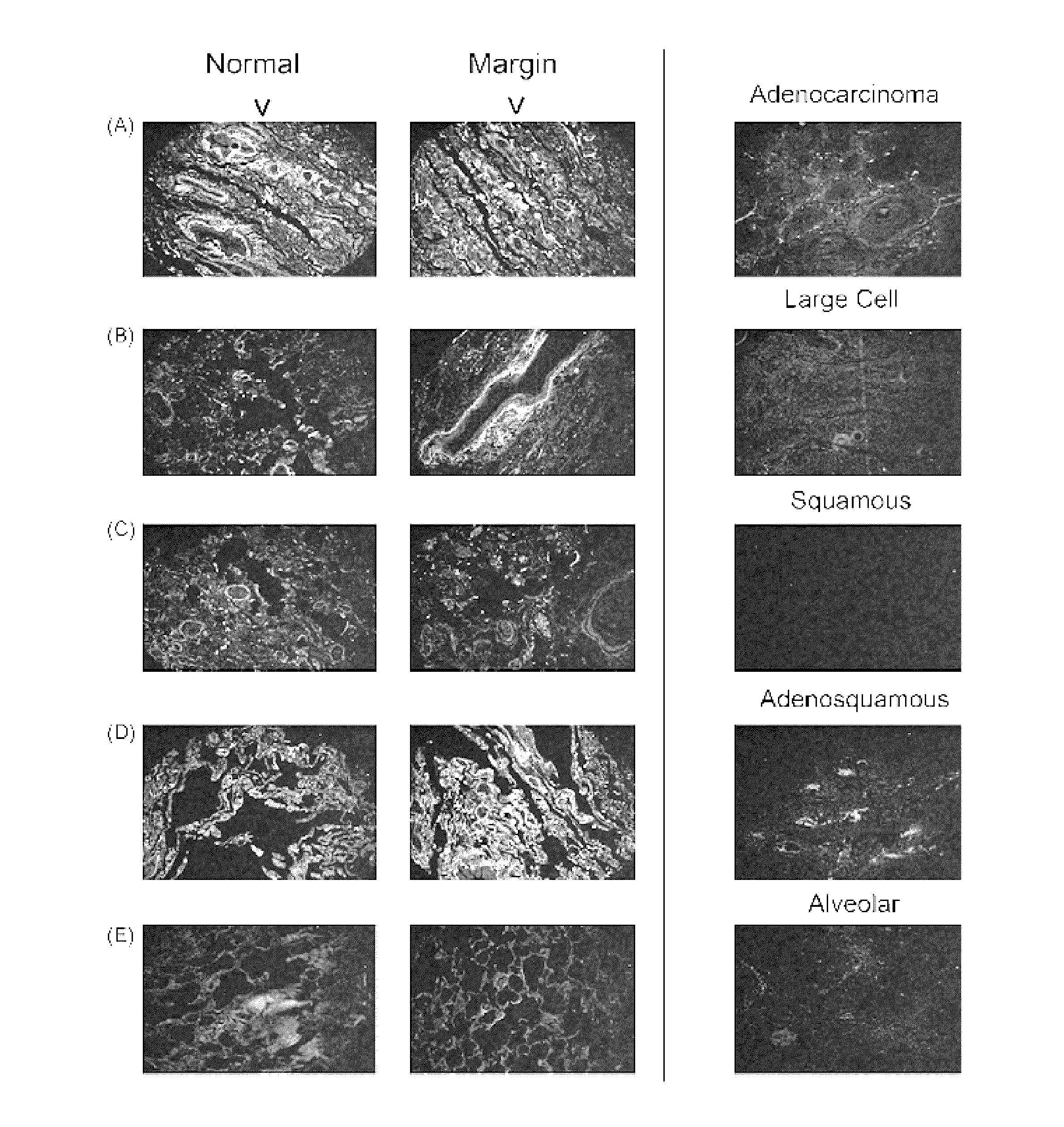
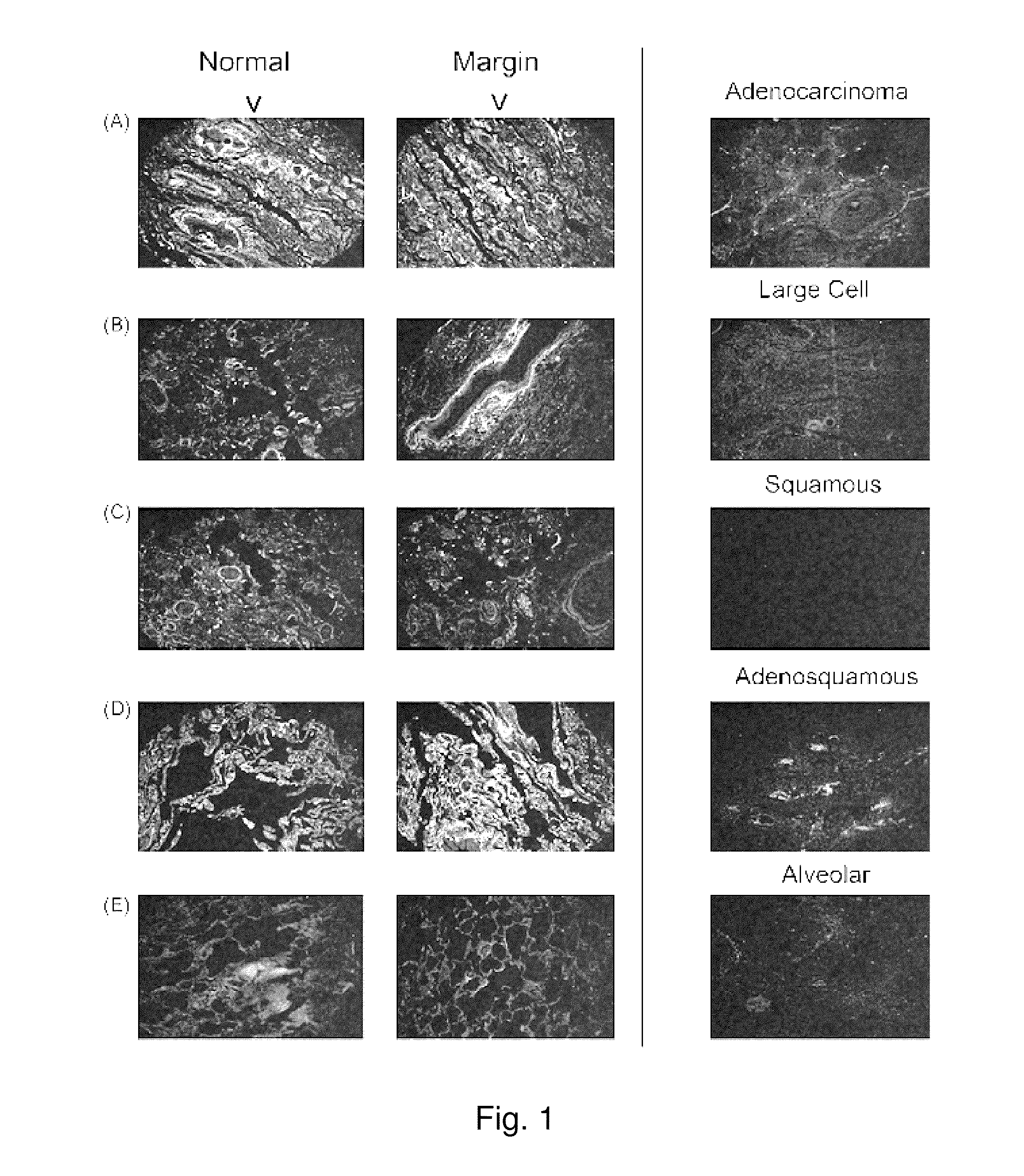
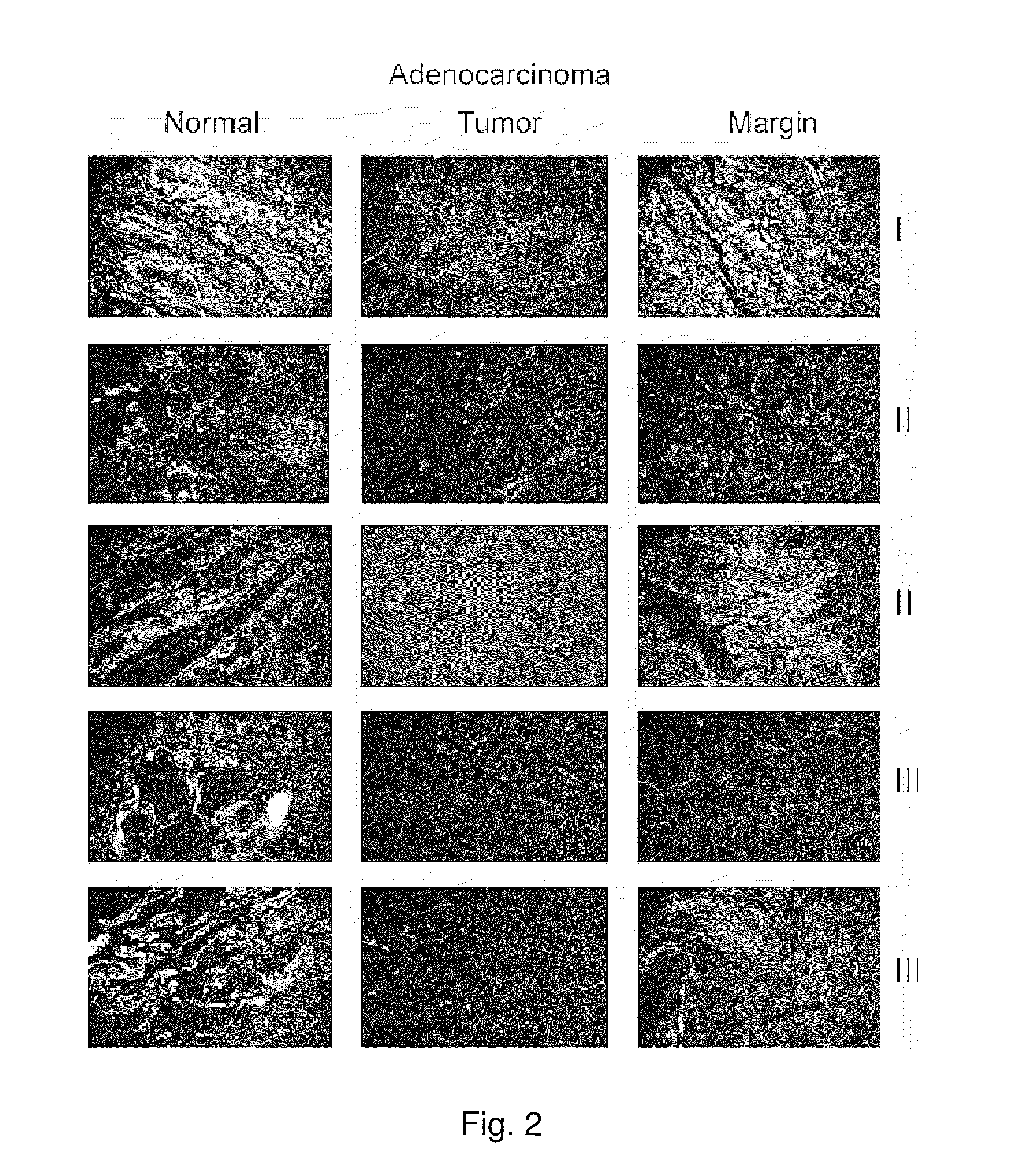
Lipocalin-type prostaglandin d2 synthase as a biomarker for lung cancer progression and prognosis
InactiveUS20110318308A1Small toxicityEffective treatmentOrganic active ingredientsBiocideTube formationLipocalin-type PGDS
A PGD(2) receptor (DP) deficiency enhances tumor progression accompanied by abnormal vascular expansion. In tumors, angiogenic endothelial cells highly express DP receptor, and its deficiency accelerates vascular leakage and angiogenesis. Administration of a synthetic DP agonist, BW245C, markedly suppresses tumor growth as well as tumor hyperpermeability in WT mice, but not in DP-deficient mice. In a corneal angiogenesis assay and a modified Miles assay, host DP deficiency potentiates angiogenesis and vascular hyperpermeability under COX-2-active situation, whereas exogenous administration of BW245C strongly inhibits both angiogenic properties in WT mice. In an in vitro assay, BW245C does not affect endothelial migration and tube formation, processes that are necessary for angiogenesis; however, it strongly improves endothelial barrier function via an increase in intracellular cAMP production. PGD(2) / DP receptor is a newly identified regulator of tumor vascular permeability, indicating DP agonism can be exploited as a therapy for the treatment of cancer.
Owner:WINTHROP UNIV HOSPITAL
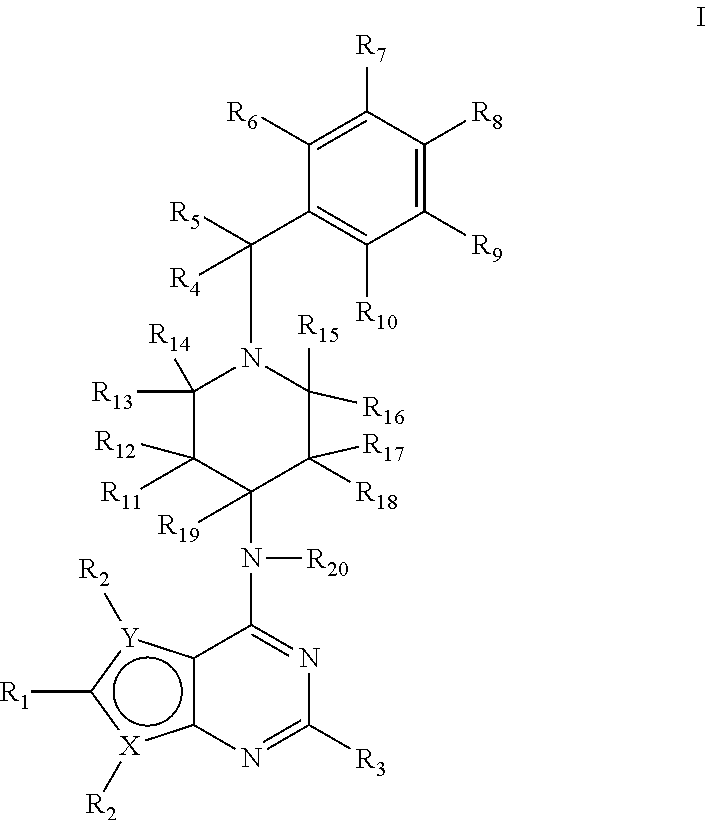
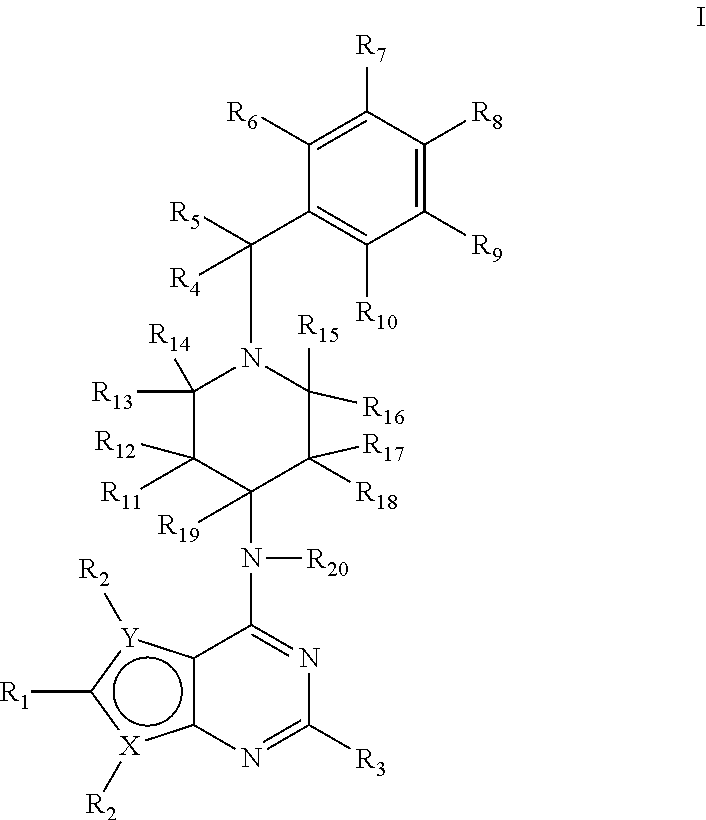
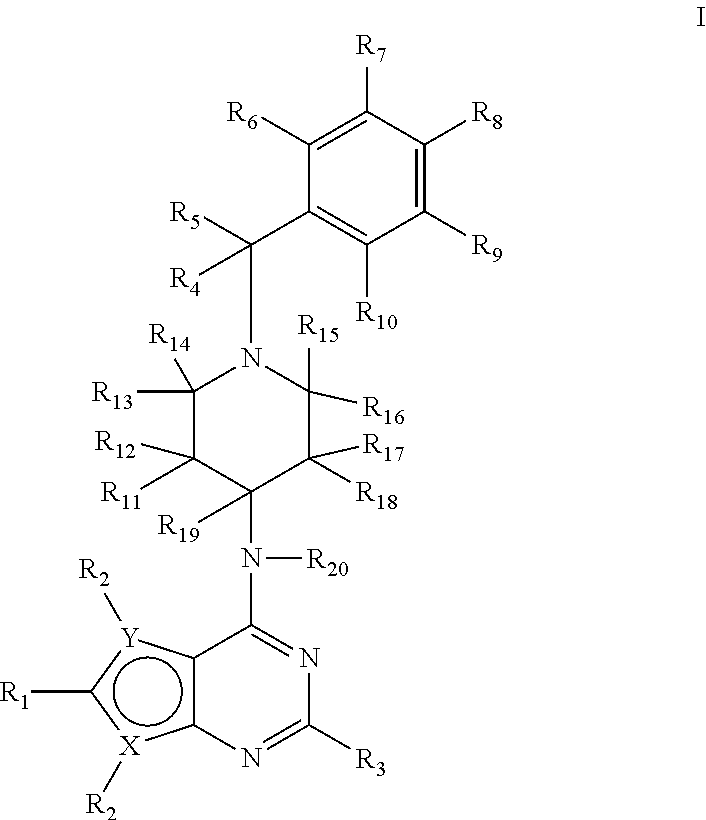
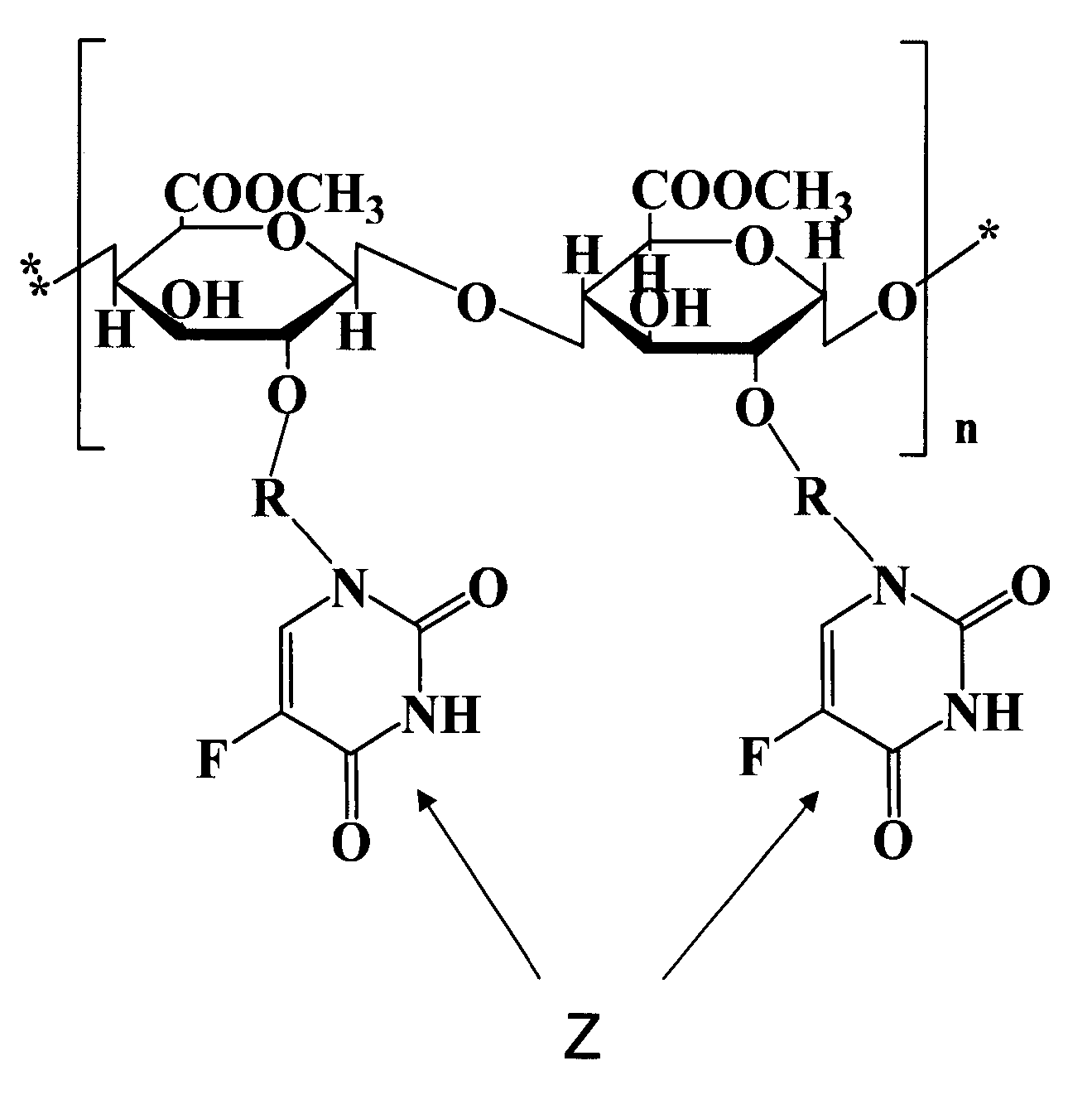
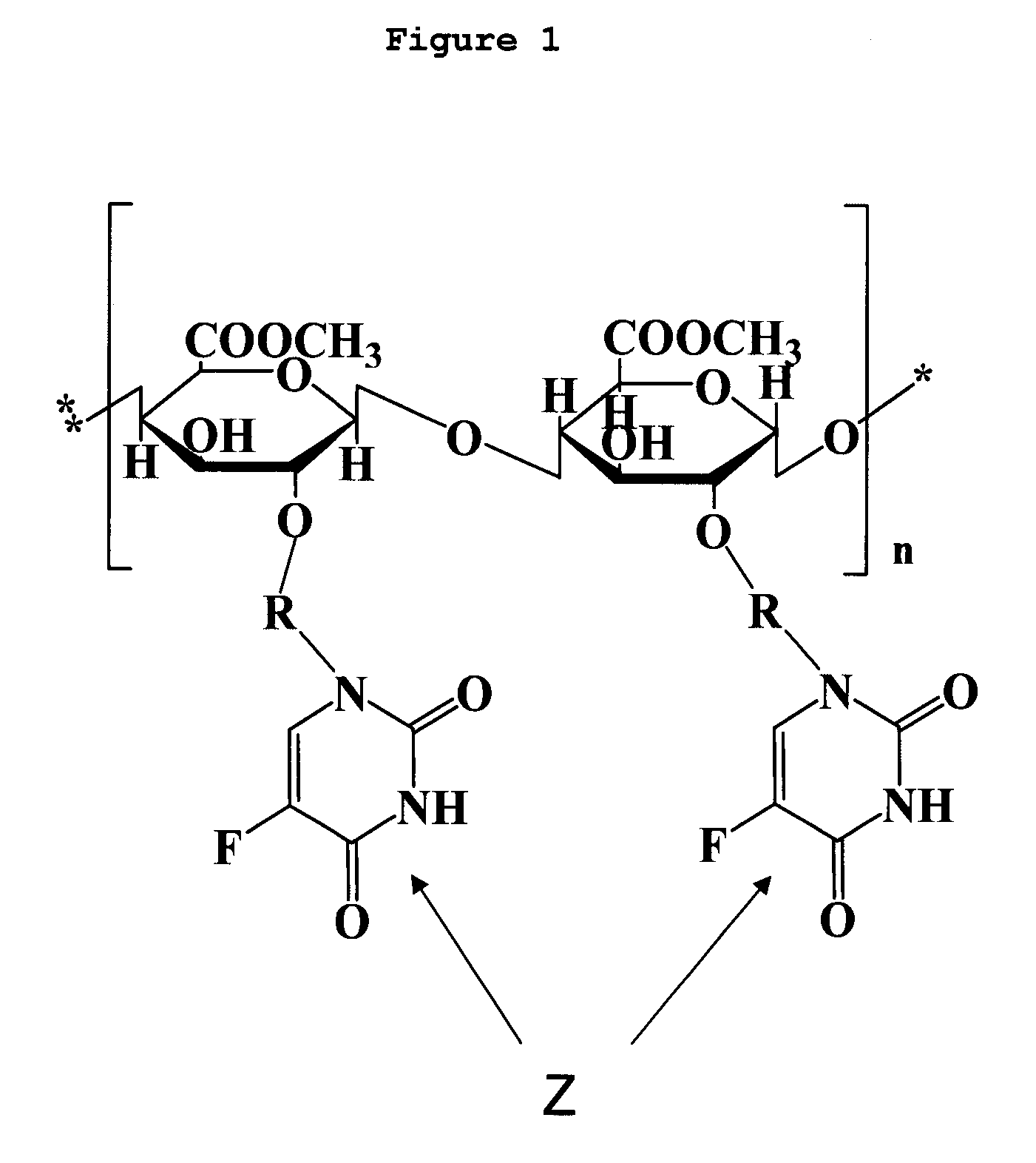
Deuterium-Enriched Pyrimidine Compounds and Derivatives
The present invention is concerned with deuterium-enriched pyrimidine compounds of formula I, their derivatives, enantiomers, diastereomers, solvates and pharmaceutical salts thereof,and their uses in the treatment, prevention and modulation of various diseases including chronic liver diseases, liver cirrhosis, liver fibrosis, hepatocellular carcinoma, liver cancer, renal cell carcinoma, kidney cancer, colorectal cancer, brain cancer, breast cancer, blood cancer, lung cancer, thyroid cancer, ovarian cancer, pancreas cancer, prostate cancer, stomach cancer, testicular cancer, uterus cancer, intestinal cancer, skin cancer, and other forms of cancer, carcinoid tumors, teratocarcinoma, tumor progression, metastasis and fibrosis in the neuroendocrine neoplasia, fibrotic processes as well as a disease state modulated directly or indirectly with 5-HT receptors, 5-HT1, 5-HT1A, 5-HT2 receptors, 5-HT2A and 5-HT2B receptors, dopamine receptors and multiple kinase pathways.
Owner:DHANOA DALJIT SINGH
Methods and compositions for treating cancer
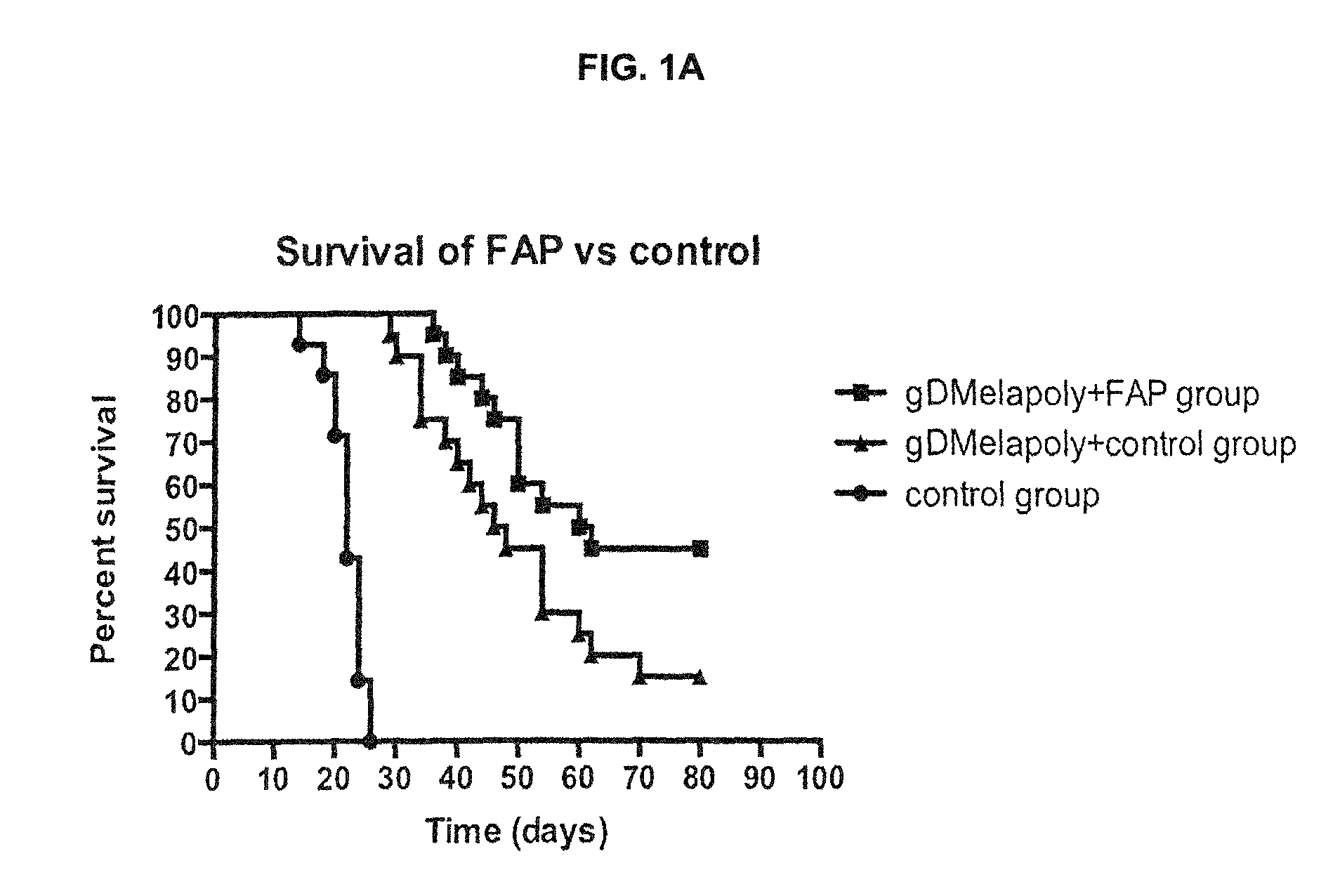
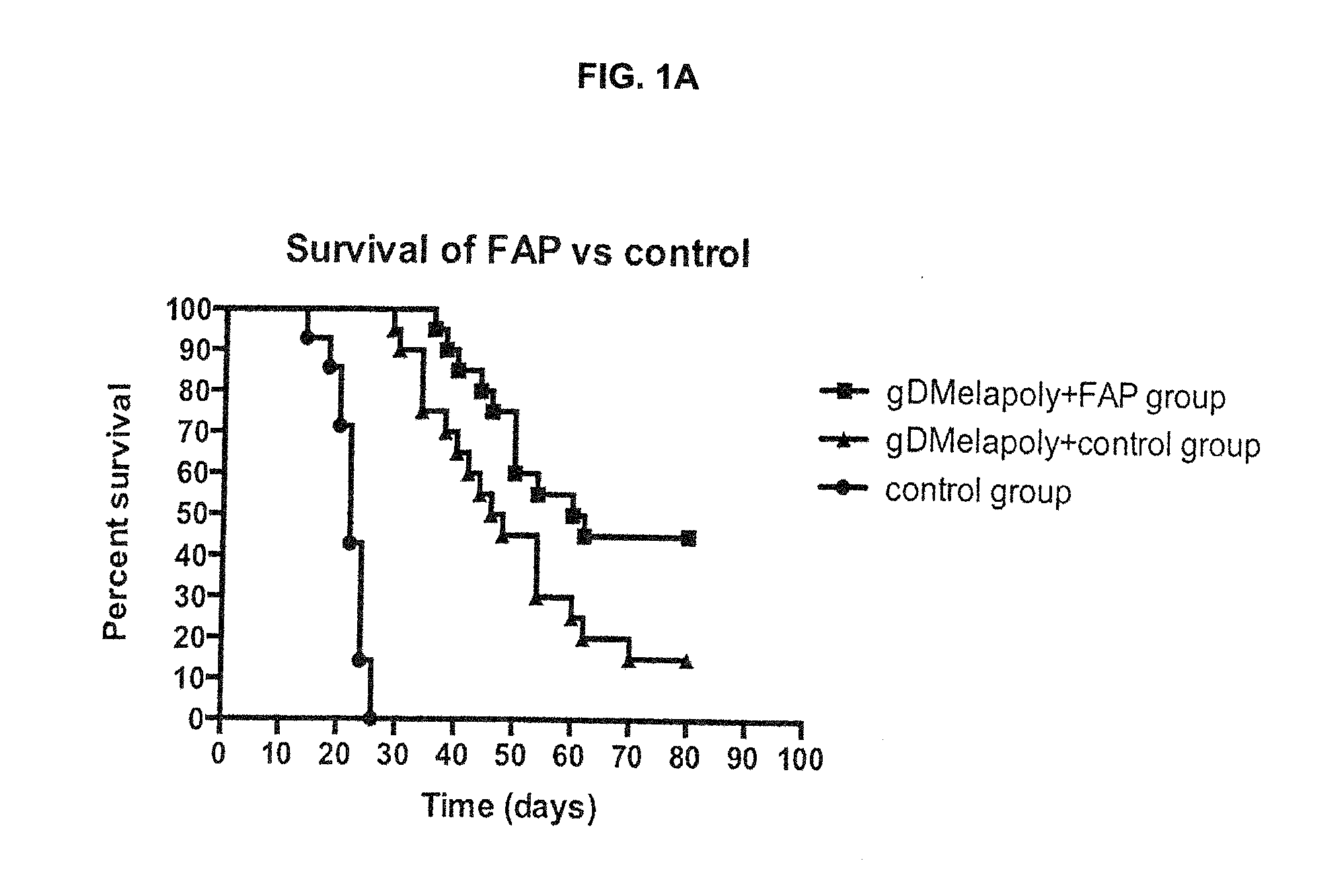
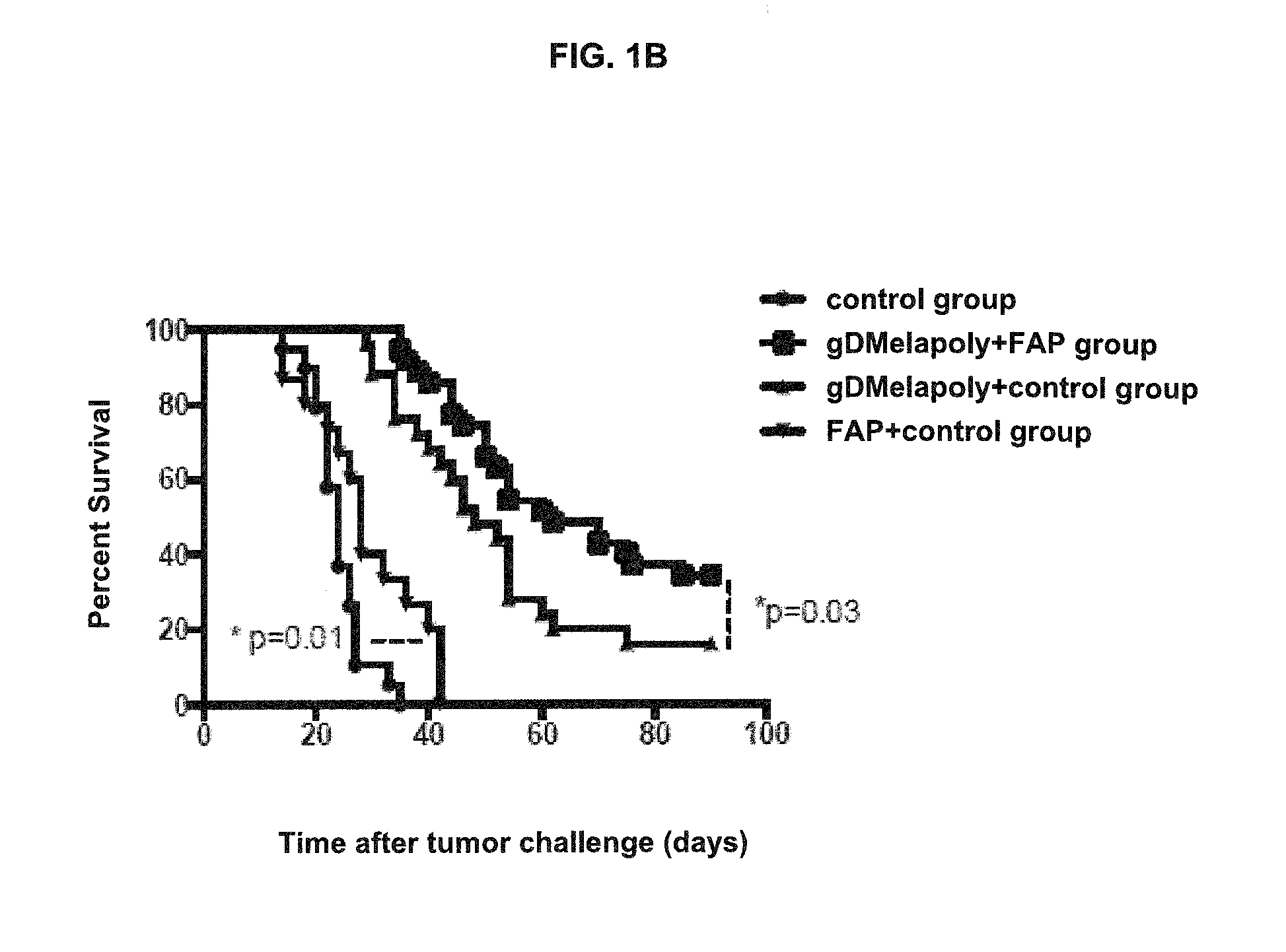
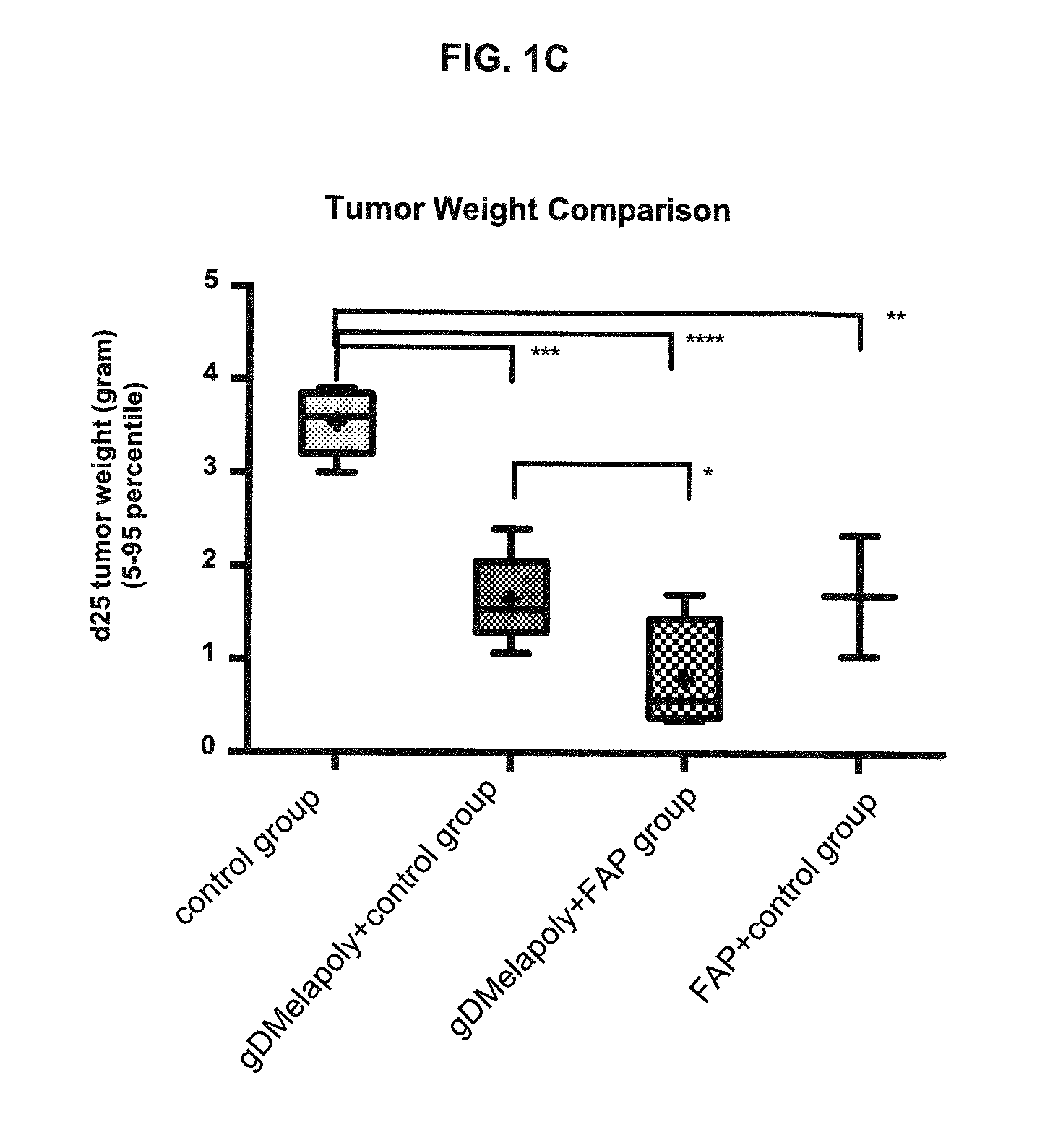
ActiveUS9402888B2Good curative effectReduce capacityPeptide/protein ingredientsViral antigen ingredientsCancer preventionCancer antigen
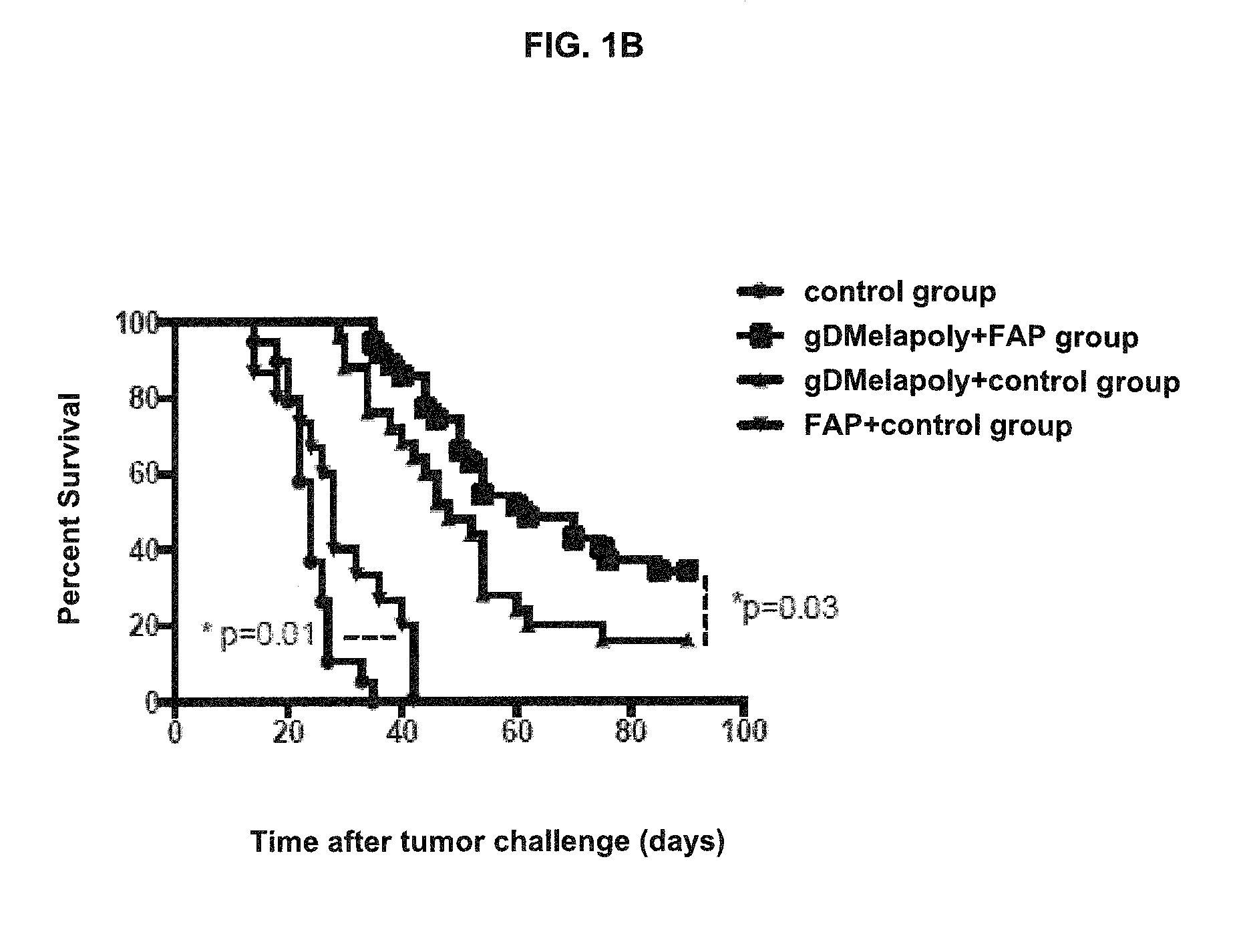
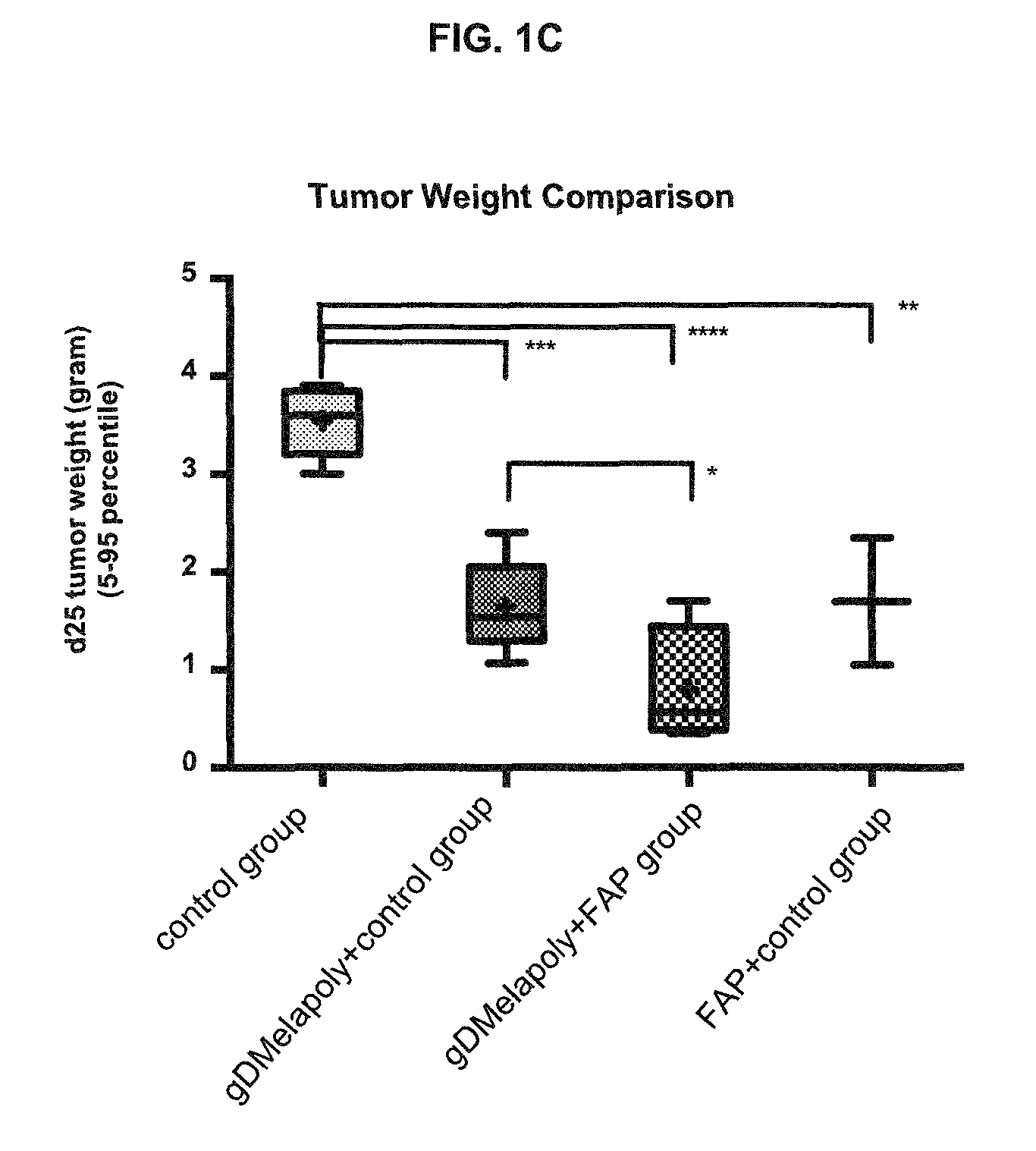
A immunogenic composition is provided for use in methods for treating or preventing the development of a cancer, comprising a nucleic acid sequence encoding a cancer antigen and a nucleic acid sequence encoding fibroblast activation protein (FAP). In one embodiment, the composition comprises a vector comprising a first expression cassette comprising a nucleic acid sequence encoding an antigen of a, operatively linked to an expression control sequence that directs the expression of the antigen in a mammalian host cell. The composition further contains a vector comprising a second expression cassette comprising a nucleic acid sequence encoding fibroblast activation protein (FAP) operatively linked to an expression control sequence directing the expression of FAP in a mammalian host cell. In one embodiment, the cancer is one in which tumor progression depends on the fibroblasts expressing fibroblast activation protein (FAP).
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
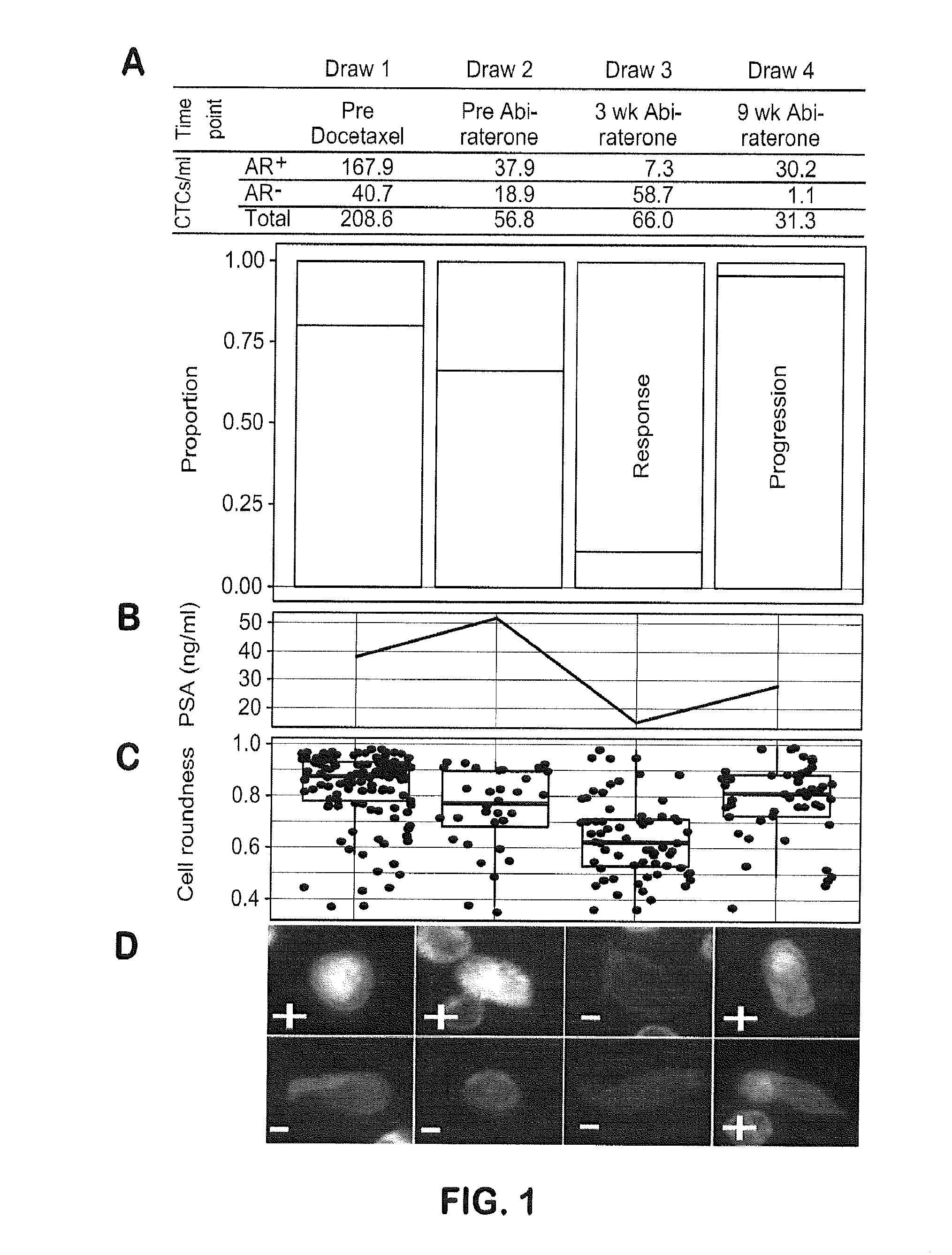
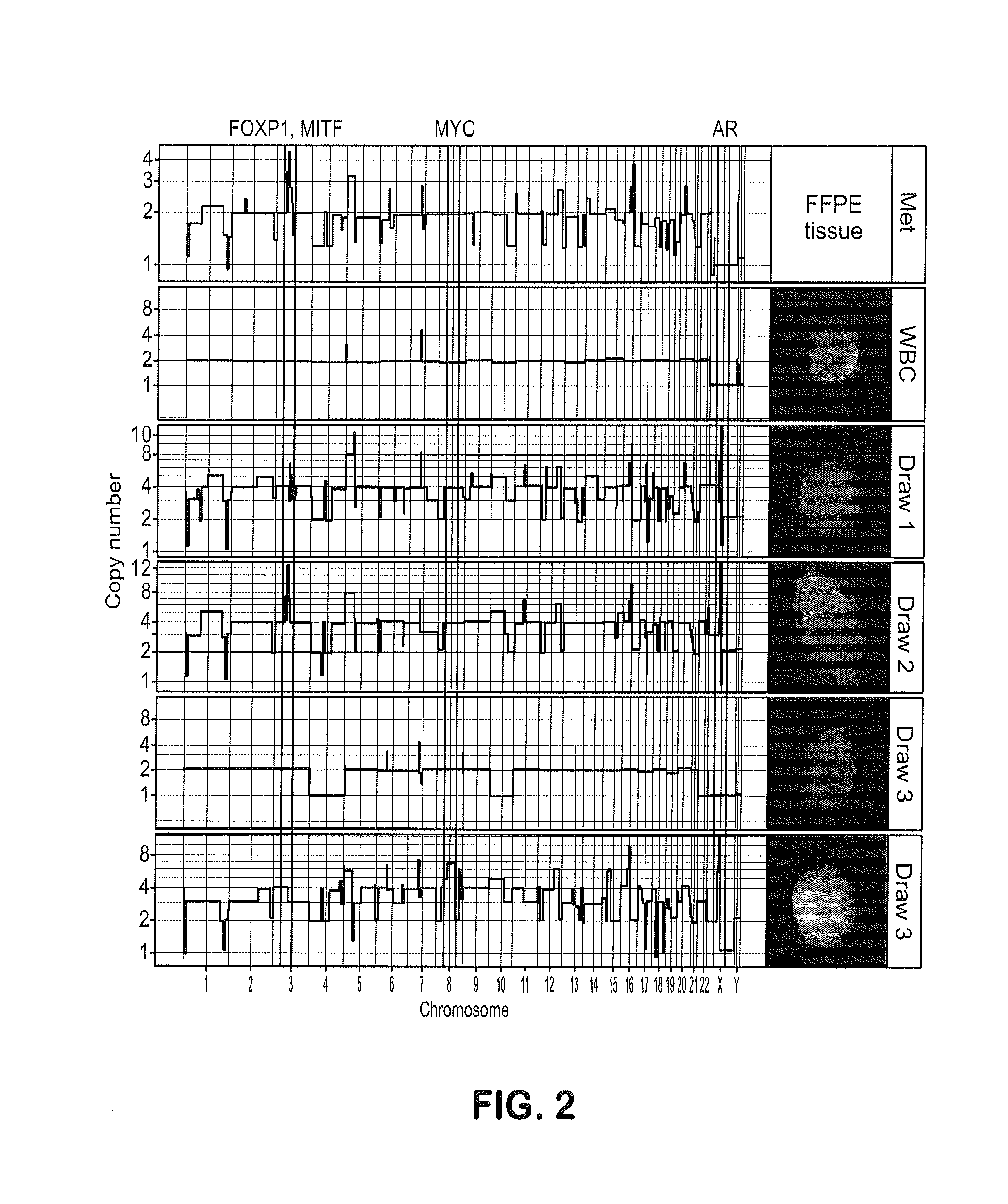
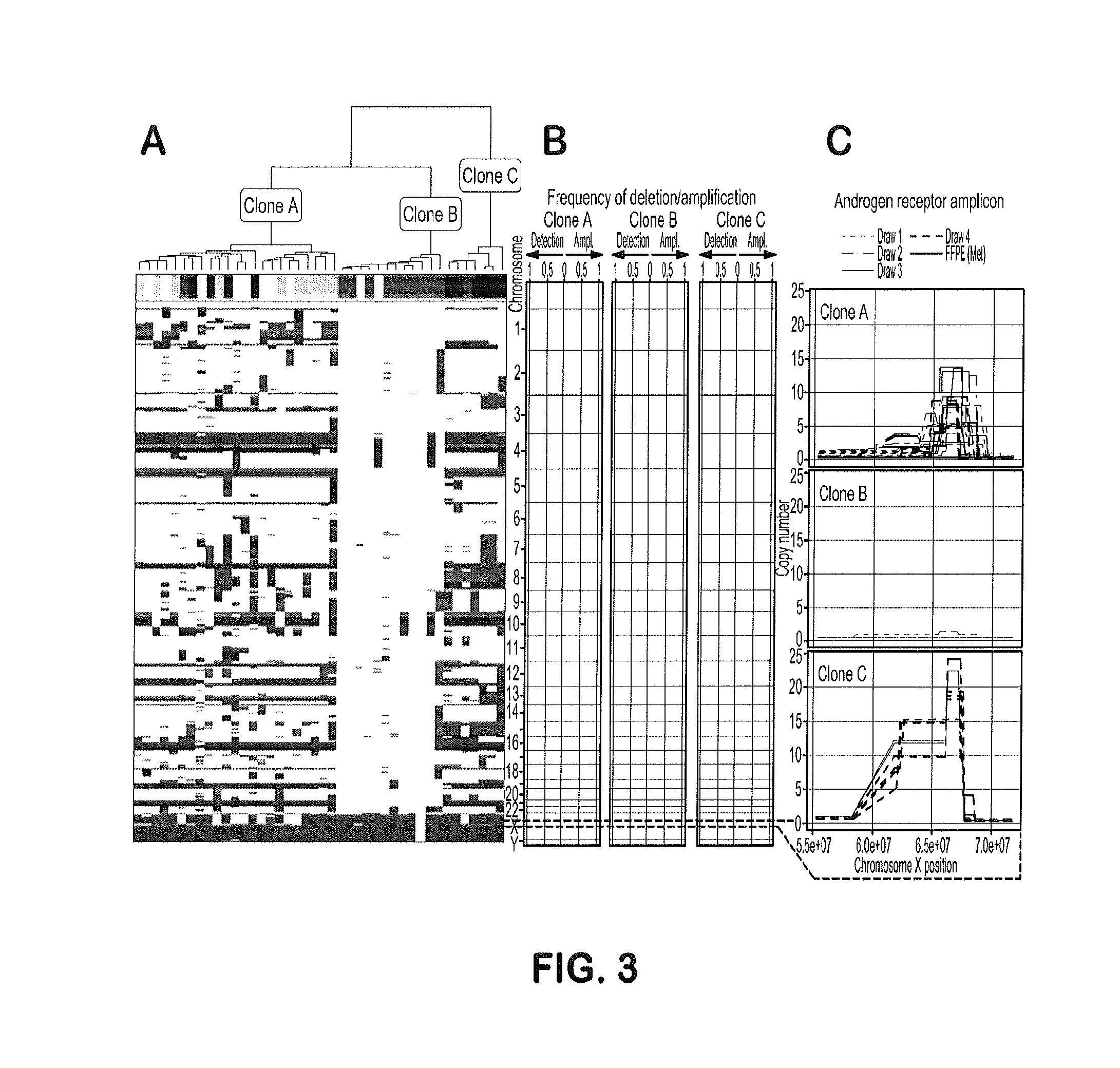
Genotypic and Phenotypic Analysis of Circulating Tumor Cells to Monitor Tumor Evolution in Prostate Cancer Patients
The present invention provides methods for predicting response to a hormone-directed therapy or chemotherapy in a prostate cancer (PCa) patient comprising (a) performing a direct analysis comprising immunofluorescent staining and morphological characterization of nucleated cells in a blood sample obtained from the patient to identify and enumerate circulating tumor cells (CTC); (b) individually characterizing genotypic, morphometric and protein expression parameters to generate a profile for each of the CTCs, and (c) predicting response to hormone-directed therapy in the prostate cancer PCa patient based on said profile. In some embodiments, the methods comprise repeating steps (a) through (c) at one or more timepoints after initial diagnosis of prostate cancer to sequentially monitor said genotypic, morphometric and protein expression parameters.
Owner:COLD SPRING HARBOR LAB INC +1
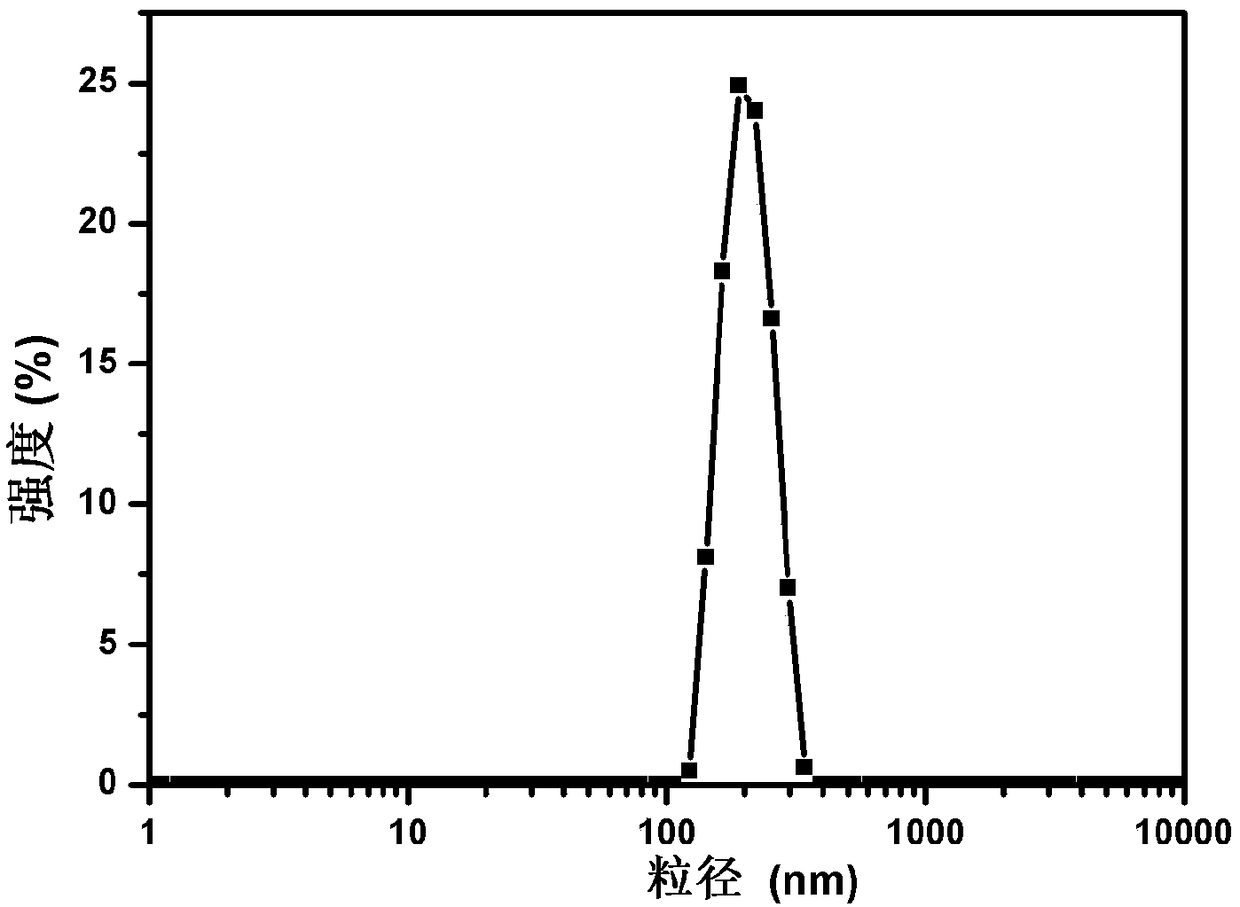
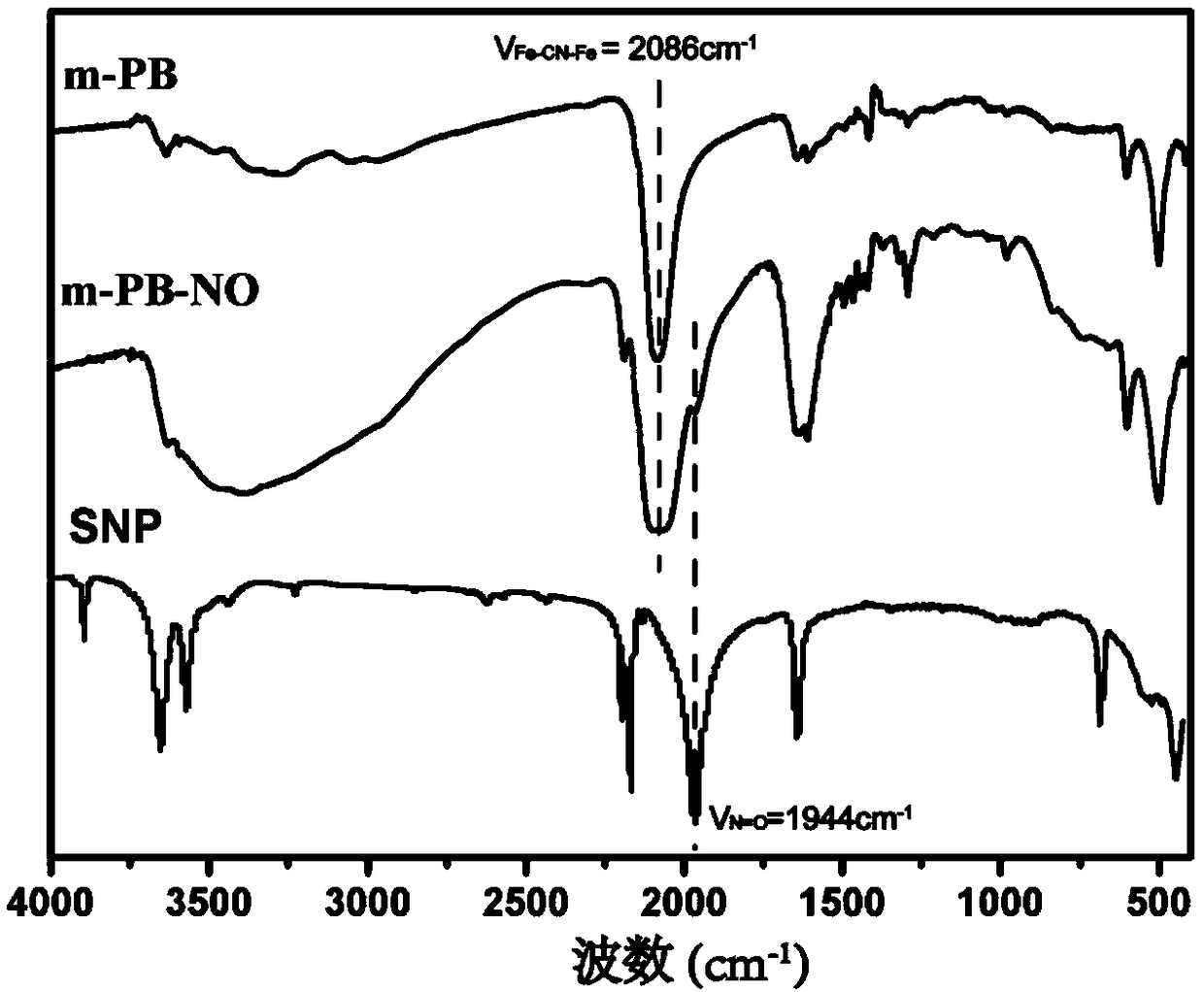
Sodium nitroprusside-conjugated medicine-carrying prussian blue analogue nano-photothermal therapeutic agent and preparation method thereof
ActiveCN108785673AUniform particle sizeGood biocompatibilityPowder deliveryOrganic active ingredientsLattice defectsPhotothermal ablation
The invention discloses a sodium nitroprusside-conjugated medicine-carrying prussian blue analogue nano-photothermal therapeutic agent and a preparation method thereof. The particle size of the sodiumnitroprusside-conjugated medicine-carrying prussian blue analogue nano-photothermal therapeutic agent prepared by a hydrothermal reaction method is 205.4nm, and passive targeting can be achieved by means of an enhanced permeability and retention (EPR) effect of a tumor site. Under the illumination of near-infrared laser, the nano-photothermal therapeutic agent can not only induce photothermal ablation of tumor cells through excellent photothermal conversion efficiency, but also can control NO release, thereby improving the EPR effect and increasing intratumoral delivery of nanoparticles. Furthermore, the NO can also inhibit tumor progression by inducing apoptosis of the tumor cells, preventing angiogenesis, reversing multidrug resistance, and the like. On the other hand, due to the structural difference between sodium nitroprusside and potassium ferricyanide, the lattice defects of the nanoparticles are caused, and the drug loading capacity of the nano-photothermal therapeutic agent is accordingly increased. Therefore, after chemotherapeutic drugs are carried, under the irradiation of near-infrared light, the nano-photothermal therapeutic agent can realize dose-controlled NO release and phototherapy and chemotherapy combined oncotherapy. In addition, the sodium nitroprusside-conjugated medicine-carrying prussian blue analogue nano-photothermal therapeutic agent provided by theinvention also has good photothermal stability and certain photoacoustic contrast properties.
Owner:CHONGQING MEDICAL UNIVERSITY
Medicament, compositions, and substances for treating and identifying adenocarcinoma of the lung
InactiveUS20110064739A1Reduced activityReduce expressionOrganic active ingredientsPeptide/protein ingredientsCell cycleBlood vessel
The invention is based on the finding that in mammalian lungs c-myc acts as a molecular switch, specifically inducing an expression pattern in vivo, which results in prototypical mammalian adenocarcinoma of the lung and liver metastasis. A set of factors essential for the processes of tumorigenesis and tumor progression, i.e. cell cycle and apoptosis, cell growth, extracellular signaling, angiogenesis and invasion, is identified, whose expression is significantly changed. In particular, the expression pattern found uncovers the network of molecules leading to mammalian papillary adenocarcinomas of the lung.
Owner:BORLAK JURGEN +2
Method to control tumor progression and invasiveness
InactiveUS20050089896A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsCell adhesionAdhesion process
Owner:ROY FRANS +2
Methods and compositions for treating or preventing cancer
ActiveUS20140271724A1Good curative effectReduce capacityPeptide/protein ingredientsGenetic material ingredientsCancer preventionCancer antigen
A immunogenic composition is provided for use in methods for treating or preventing the development of a cancer, comprising a nucleic acid sequence encoding a cancer antigen and a nucleic acid sequence encoding fibroblast activation protein (FAP). In one embodiment, the composition comprises a vector comprising a first expression cassette comprising a nucleic acid sequence encoding an antigen of a, operatively linked to an expression control sequence that directs the expression of the antigen in a mammalian host cell. The composition further contains a vector comprising a second expression cassette comprising a nucleic acid sequence encoding fibroblast activation protein (FAP) operatively linked to an expression control sequence directing the expression of FAP in a mammalian host cell. In one embodiment, the cancer is one in which tumor progression depends on the fibroblasts expressing fibroblast activation protein (FAP).
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
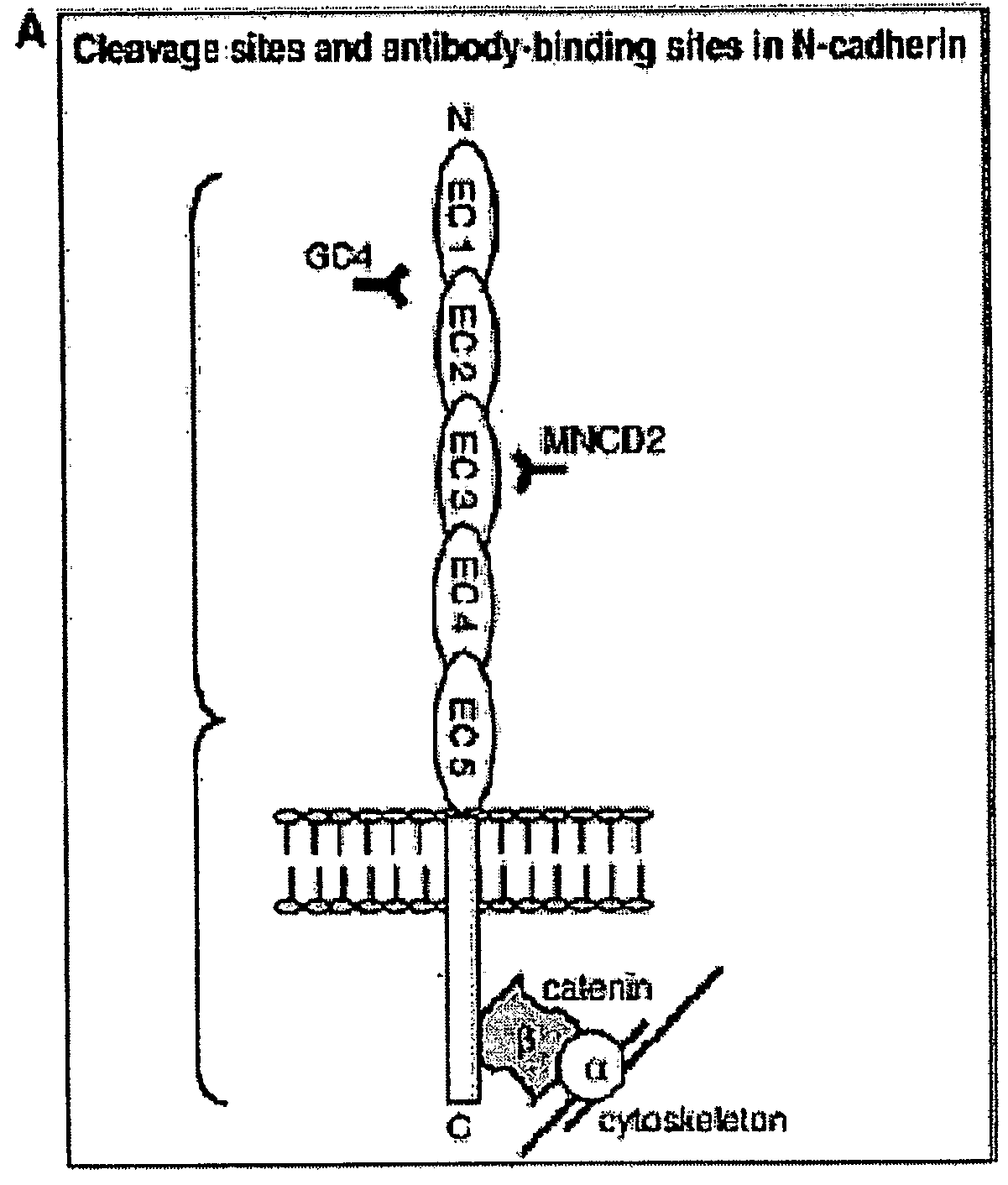
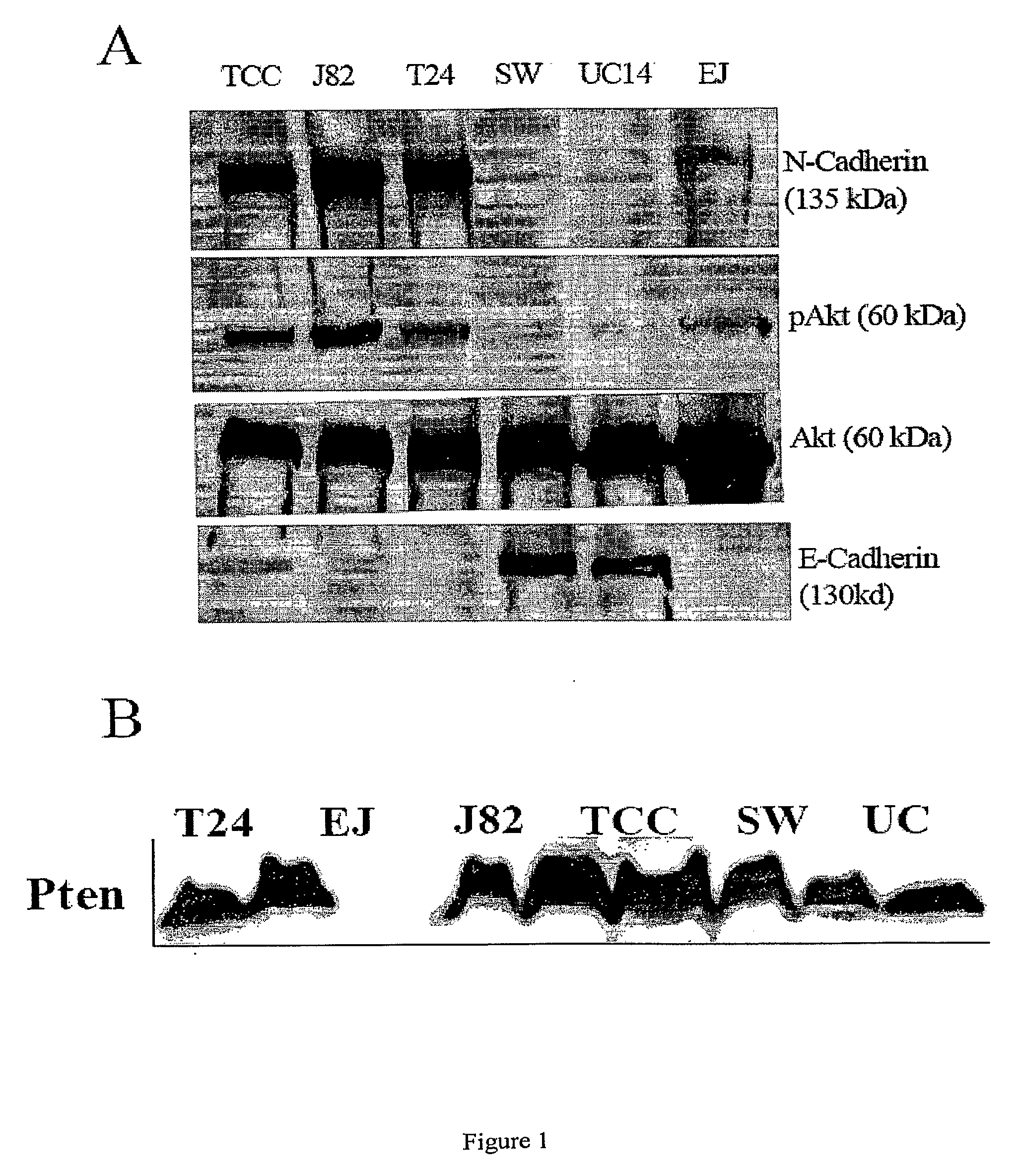
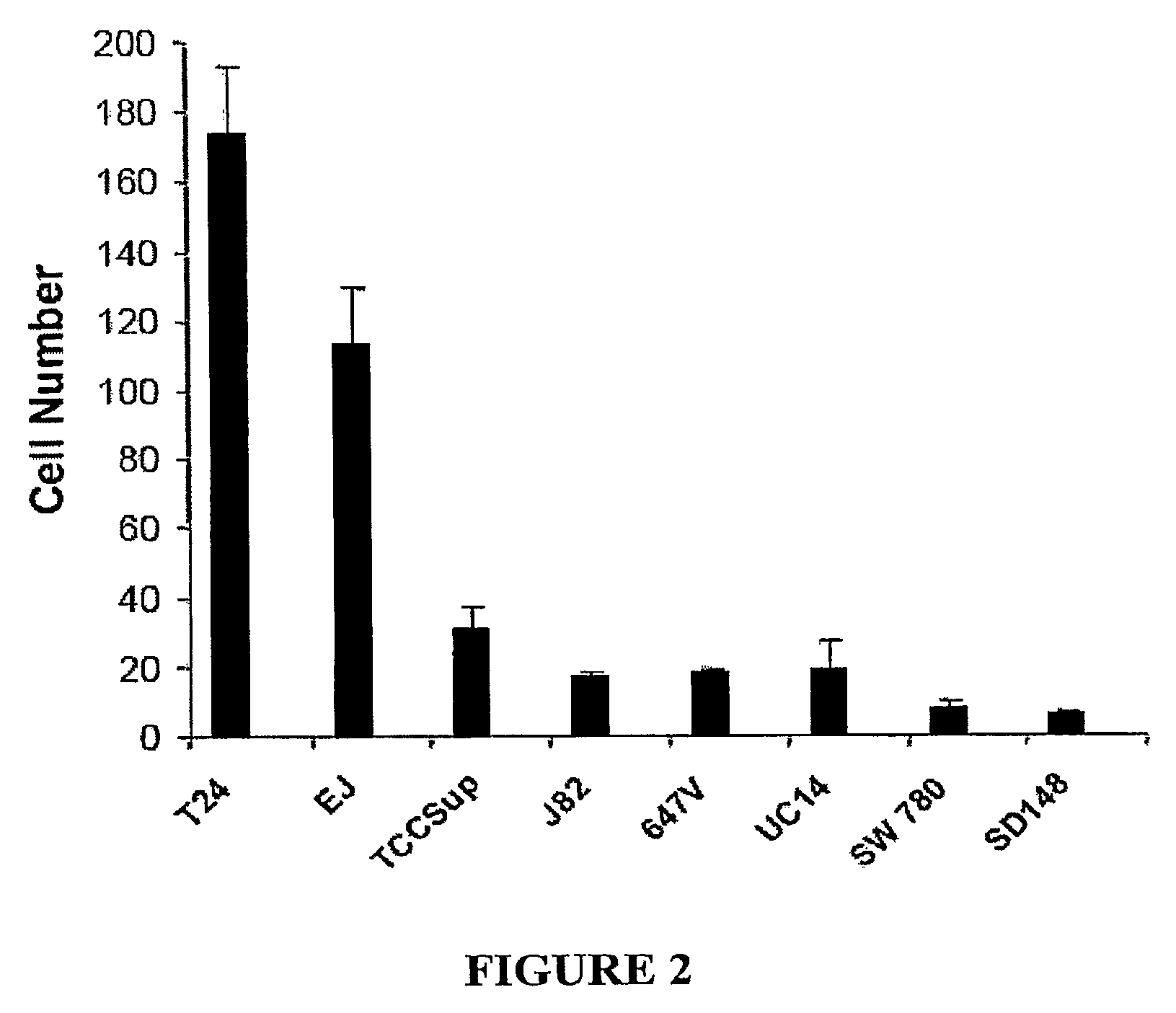
N-Cadherin and Ly6 E: Targets for Cancer Diagnosis and Therapy
InactiveUS20090130108A1Help in prognosisInhibiting and reducingOrganic active ingredientsMicrobiological testing/measurementLymphatic SpreadImmunotherapeutic agent
The present invention provides methods of diagnosis, providing a prognosis and a therapeutic target for the treatment of cancers that overexpress N-cadherin and Ly6-E, including prostrate and bladder cancers. The invention further provides methods of drug discovery to identify pharmaceutical agents that inhibit or prevent the binding of N-cadherin and Ly6-E to its receptor, which are useful when used alone or in combination with known chemotherapeutics, immunotherapeutics, and radiotherapy for the reversal of resistance, tumor progression, and metastasis of cancers associated with the overexpession of N-cadherin and Ly6-E.
Owner:RGT UNIV OF CALIFORNIA
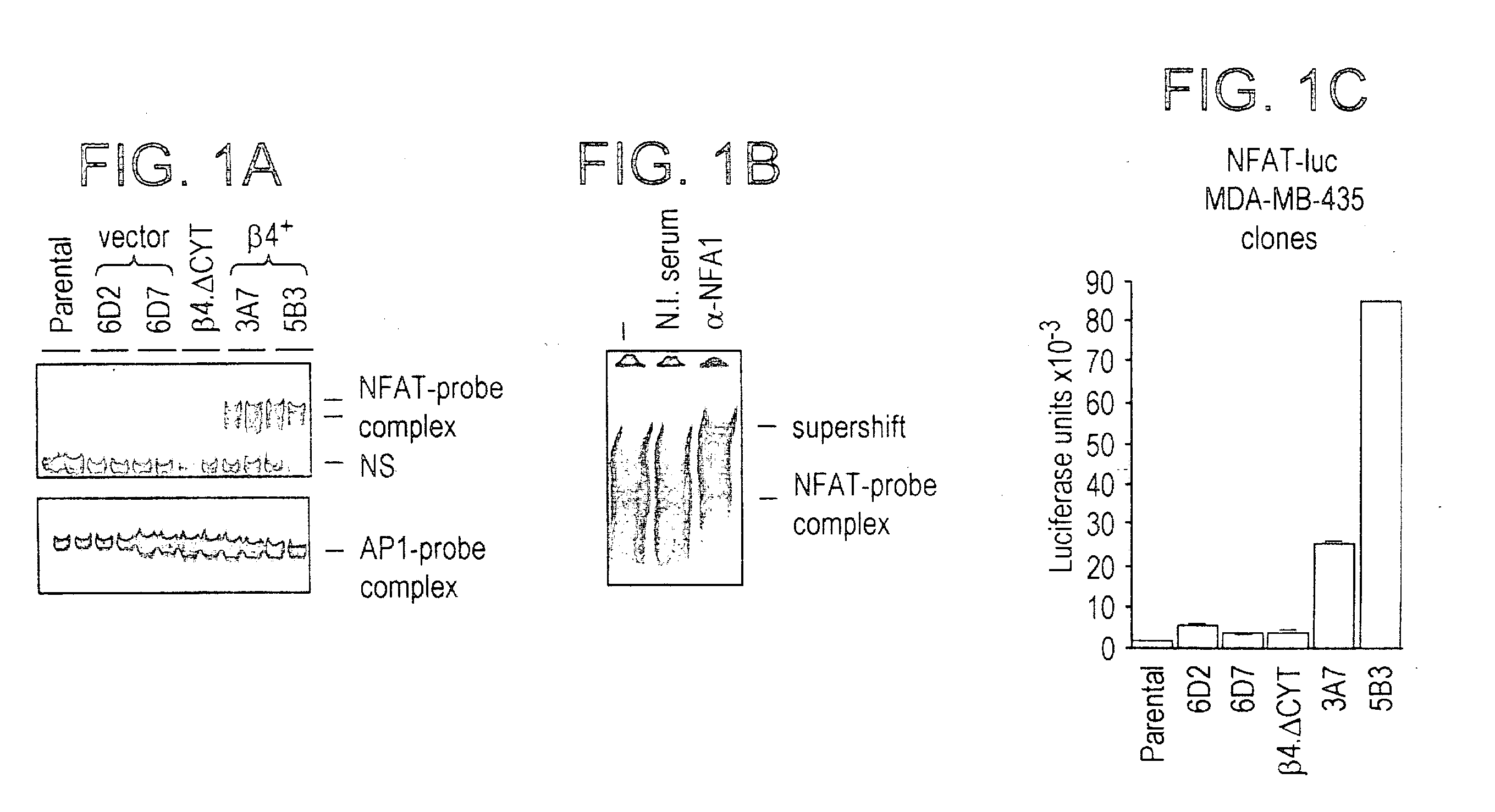
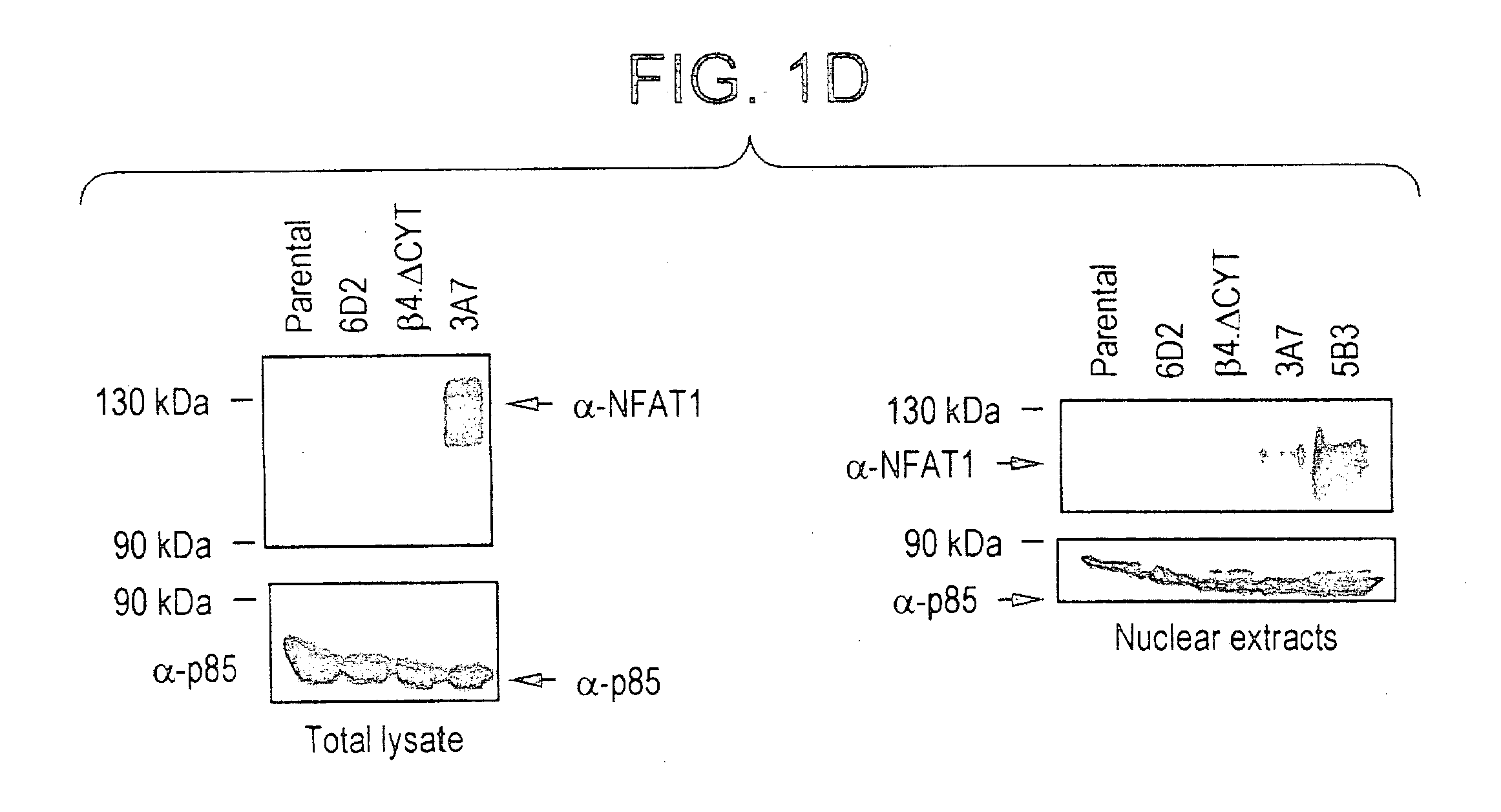
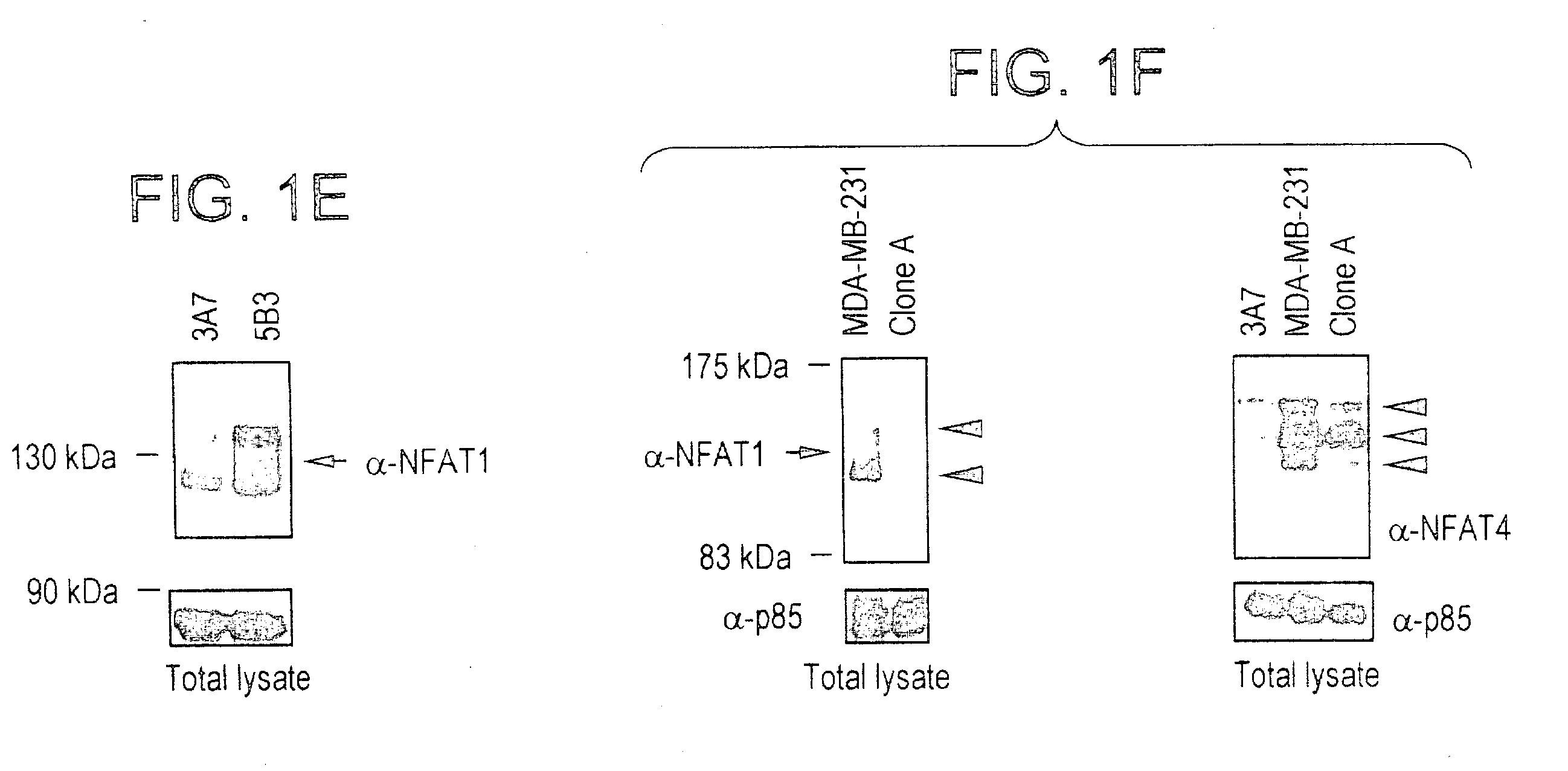
NFAT transcription factors in tumor progression
InactiveUS20050100897A1Easy to produceIncrease transcriptional activityMicrobiological testing/measurementTumor progressionBiology
The present invention provides therapeutic and diagnositic methods for neoplasia treatment. The invention also relates to determining the prognosis of a neoplasia or determining a treatment protocal. Further features of the invention are methods for identifying NFAT target genes that promote neoplasia progression.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of combined genome
PendingCN111575376AImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationRenal clear cell carcinomaClear cell renal cell carcinoma
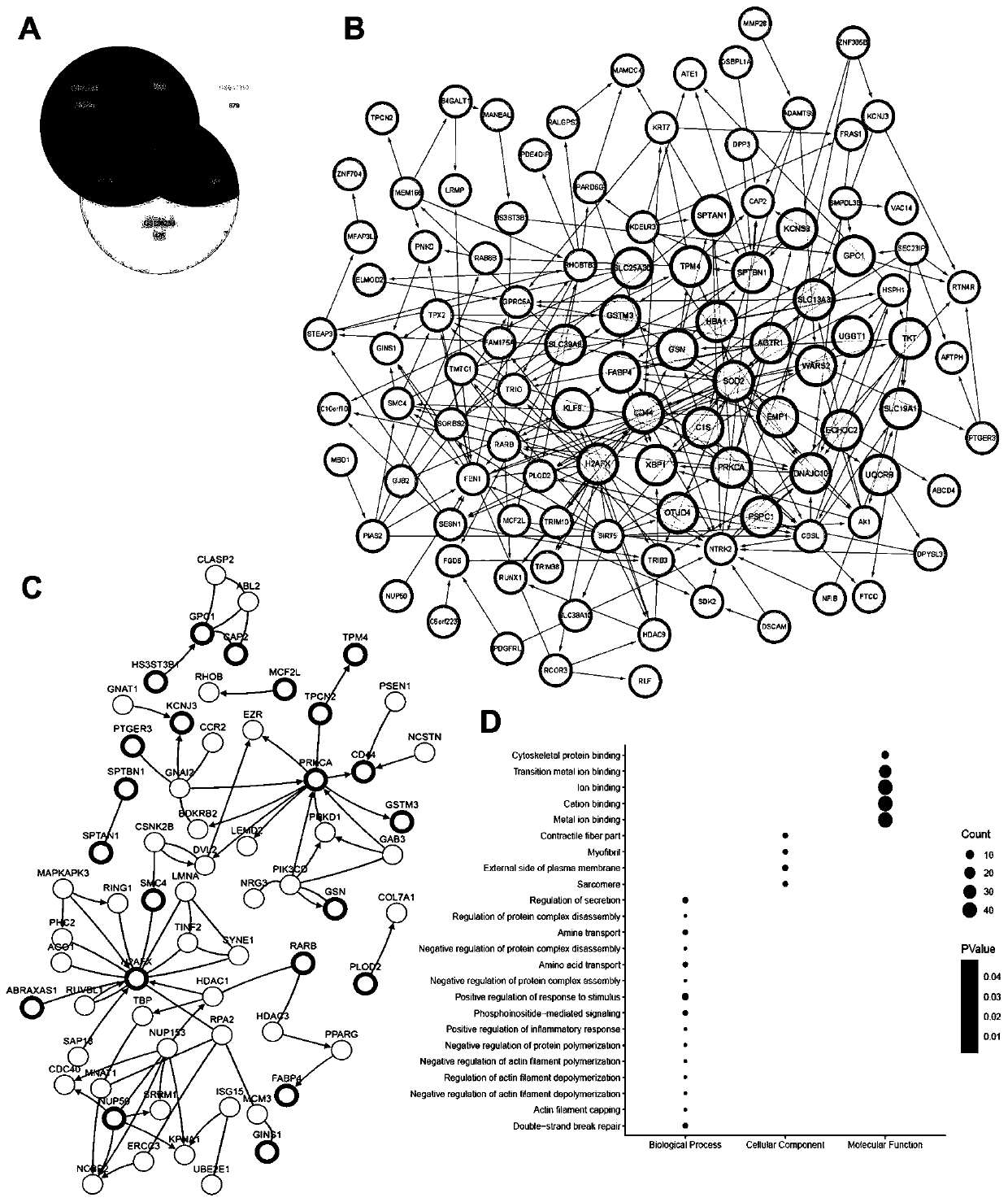
The invention discloses a combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of the combined genome, relates to the technical field of medical biological detection, and provides novel application of the combined genome of ADAMTS9, C1S, DPYSL3, H2AFX, MINA, PLOD2, RUNX1, SLC19A1, TPX2 and TRIB3, particularly application in preparation of a ccRCC prognosis evaluation reagent or kit. The genome is derived from a molecular marker significantly correlated with a ccRCC metastasis way, and the discovery of the genome model provides a new strategy for predicting the ccRCC recurrent risk and the long-term survival condition of a patient after operation, plays important roles in judging the prognosis of the ccRCC patient, can evaluate the risk level of tumor progression or death of the patient after ccRCC operation, contributes to guiding a clinician to perform an individualized precise therapeutic strategy, can increase the postoperative survivalrate of the patient, and has important guide significance postoperative follow-up monitoring and sequential therapy management of the ccRCC patient.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
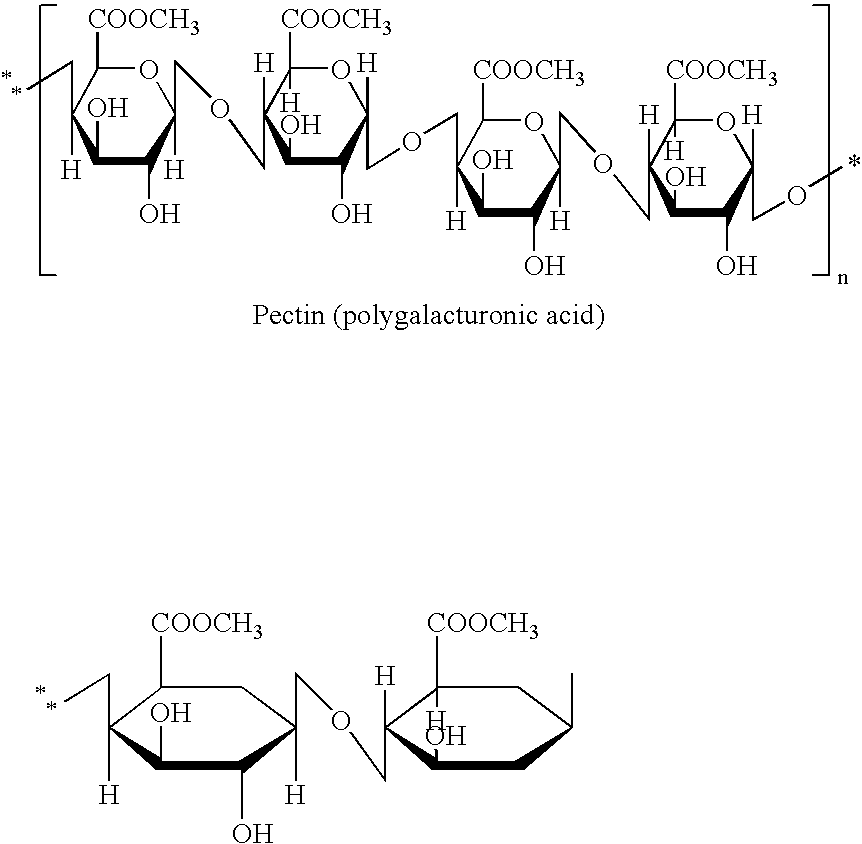
Novel polysaccharide pro-drug 5-fluorouracil (5-FU) with enhanced target specificity for colorectal cancer and its preparation methods
InactiveUS20080085871A1Good treatment effectHigh selectivityBiocideSugar derivativesUpper gastrointestinalActive agent
This invention describes a novel polysaccharide prodrug of 5-fluorouracil (5-FU) with enhanced target specificity for colorectal cancer treatment, and its preparation methods. The prodrug is synthesized by chemically linking anti-cancer drug 5-fluorouracil (5-FU) with a specially selected polysaccharide with molecular weight of 105˜107 Da containing galactose residues. Its distinctive characteristics are that it is a prodrug synthesized by chemically linking polysaccharides with 5-FU through different bridge links for the targeted treatment of colorectal cancer; that the polysaccharides in the chemical compound contain galactose residues; and that these polysaccharides are prepared from natural gums or plant materials. Due to these unique characteristics, as an oral preparation, the polysaccharide component of this novel prodrug can protect the active agent 5-FU from absorption (or metabolism) in the upper gastrointestinal tract and deliver a high concentration of the 5-FU to the colorectal area. Upon reaching the colorectal area, the 5-FU-galactose portion of the prodrug will bind to galectin-3, a-galactoside-binding protein implicated in tumor progression by interactions with its ligands, such as TF (Thomsen-Friedenreich, Galb3GalNAc), Tn (GalNAcaThr / Ser), and Sialy-Tn with galactose residues, which are highly expressed among colorectal cancer cells. Finally, the active 5-FU component will be released locally from the polysaccharide via enzymatic hydrolysis from the local bacterial flora, allowing it to actively kill the colorectal cancer cells. In summary, this novel target-specific prodrug can enhance the selectivity of 5-FU and increase its therapeutic effects in the treatment of colorectal cancer. In addition, with this enhanced target specificity, it is possible to maximize the 5-FU efficacy in cancer patients by having either less toxicity with the same or higher therapeutic dose, and / or administer a lower dosage (if so desired) to achieve the same therapeutic effects, but with much less toxicity. Multiple examples of various approaches to synthesize this novel prodrug are enclosed herein along with several animal model experiments to substantiate the claims as stated above.
Owner:TAM JOEMY C +3
Colorectal cancer marker galectin, method for analyzing galectin concentration in blood sample, and kit for detecting colorectal cancer marker galectin
InactiveUS20130065258A1High positive rateImprove patient capture rateBiological material analysisPeptide preparation methodsGalectin-3 AntibodyPrognostic prediction
The present invention provides a tumor screening marker that can be actually used in clinical practice to detect colorectal cancer, and a tumor progression marker that can complement CEA or CA19-9. Galectin-1 used as a tumor screening marker or a tumor progression marker for colorectal cancer. Galectin-3 used as a tumor screening marker. Galectin-4 used as a tumor progression marker, a tumor screening marker, or a prognostic prediction marker for colorectal cancer. A method of analyzing the galectin concentration in a collected blood sample using the galectin. A colorectal cancer marker detection kit comprising a detection antibody selected from the group consisting of a fluorescently labeled galectin-1 antibody, a fluorescently labeled galectin-3 antibody, and a fluorescently labeled galectin-4 antibody.
Owner:SHIMADZU CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com