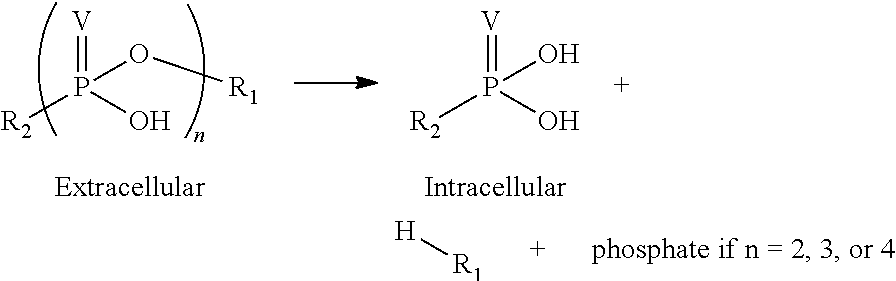
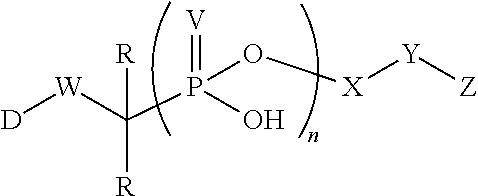
Patents
Literature
145 results about "Cell penetration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
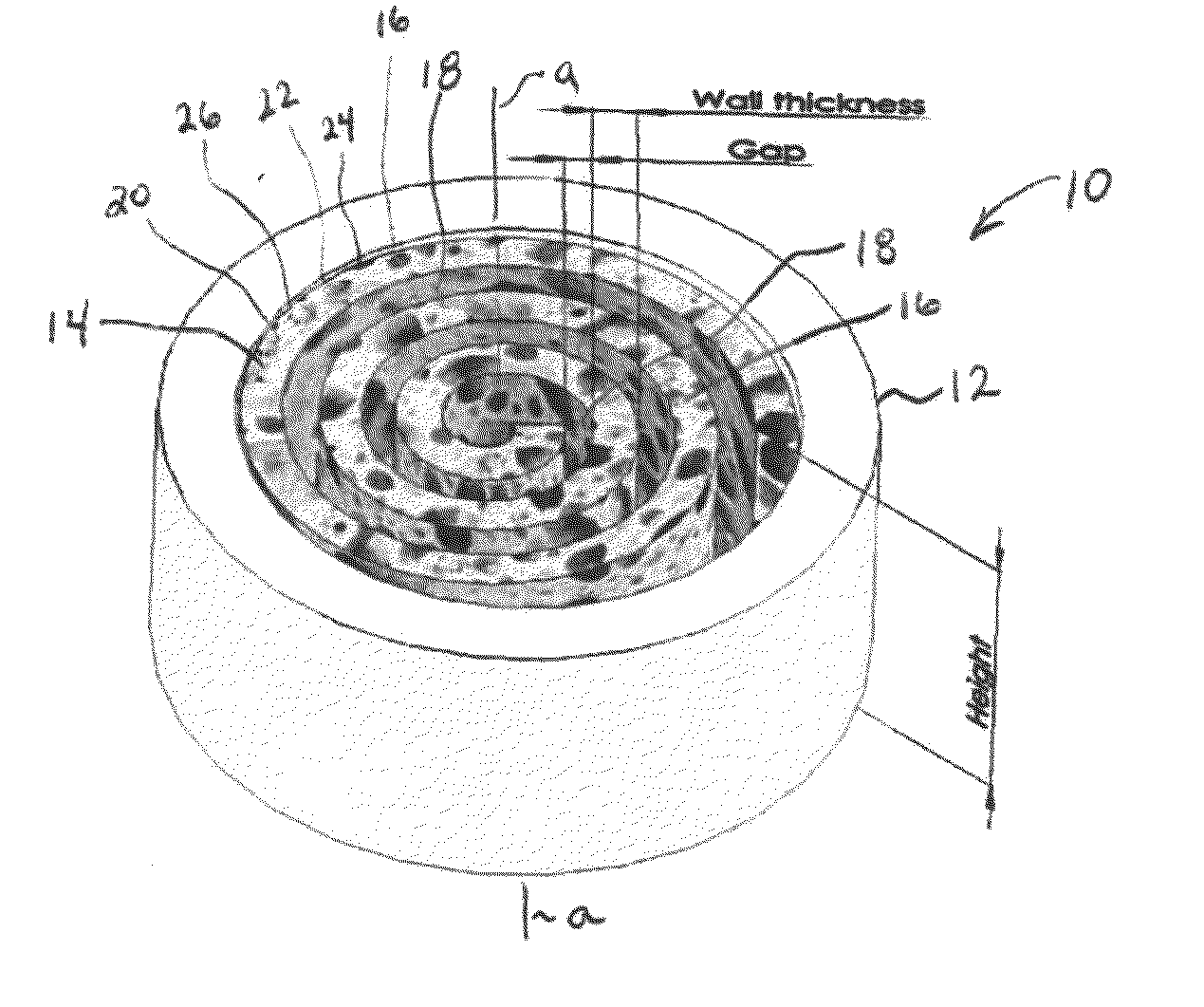
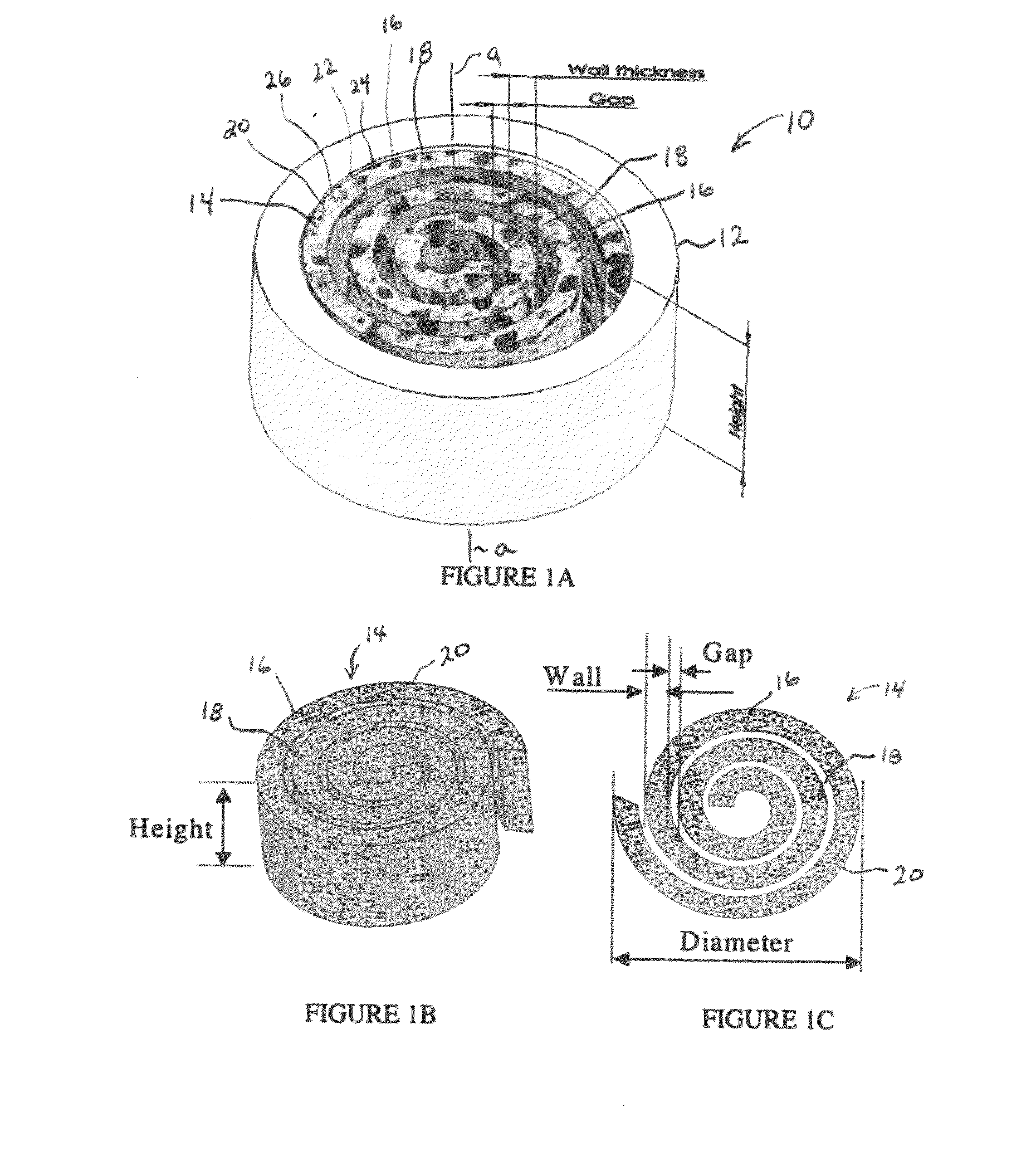
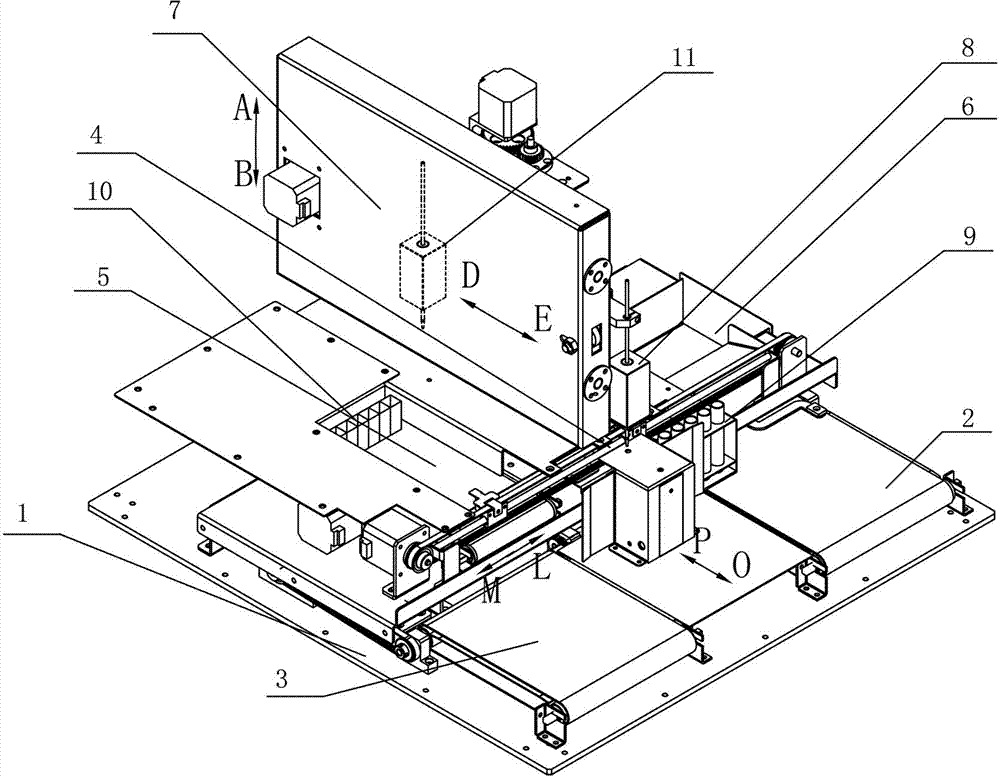
Synergetic functionalized spiral-in-tubular bone scaffolds
InactiveUS20100310623A1Increase the number ofIncrease alkaline phosphatase activityPeptide/protein ingredientsBone implantPorous sheetCell seeding
An integrated scaffold for bone tissue engineering has a tubular outer shell and a spiral scaffold made of a porous sheet. The spiral scaffold is formed such that the porous sheet defines a series of spiral coils with gaps of controlled width between the coils to provide an open geometry for enhanced cell growth. The spiral scaffold resides within the bore of the shell and is integrated with the shell to fix the geometry of the spiral scaffold. Nanofibers may be deposited on the porous sheet to enhance cell penetration into the spiral scaffold. The spiral scaffold may have alternating layers of polymer and ceramic on the porous sheet that have been built up using a layer-by-layer method. The spiral scaffold may be seeded with cells by growing a cell sheet and placing the cell sheet on the porous sheet before it is rolled.
Owner:UNIV OF CONNECTICUT
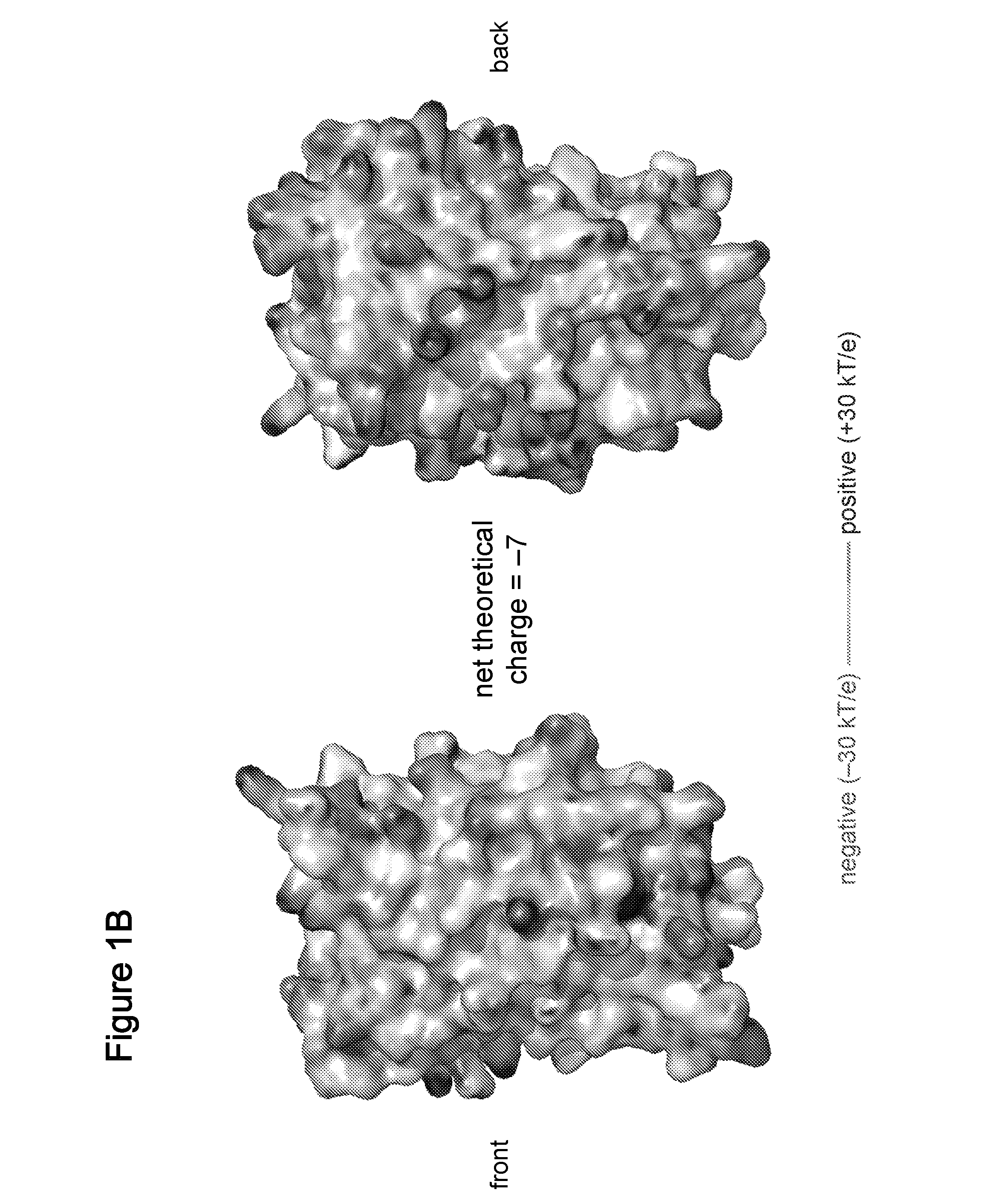
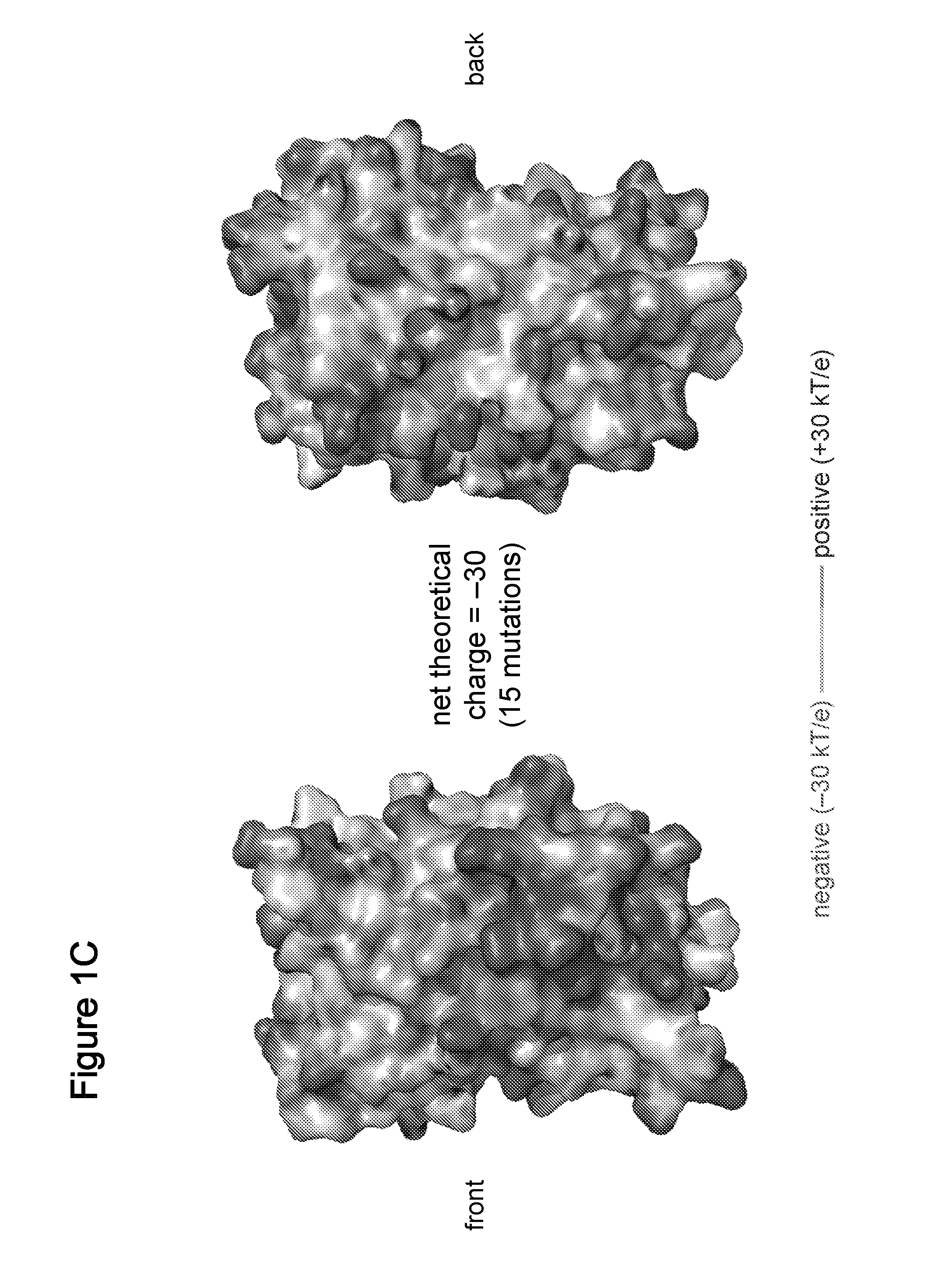
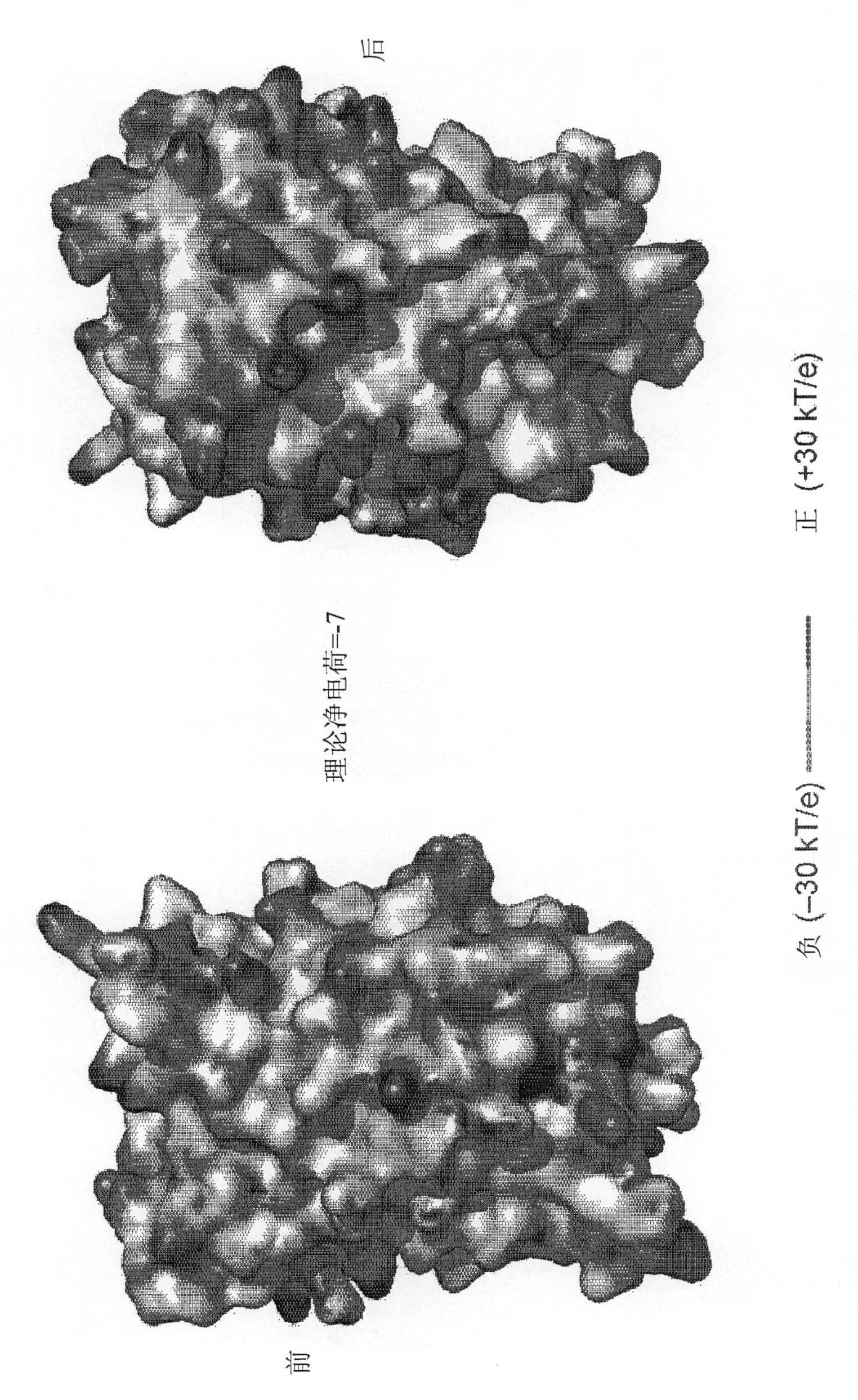
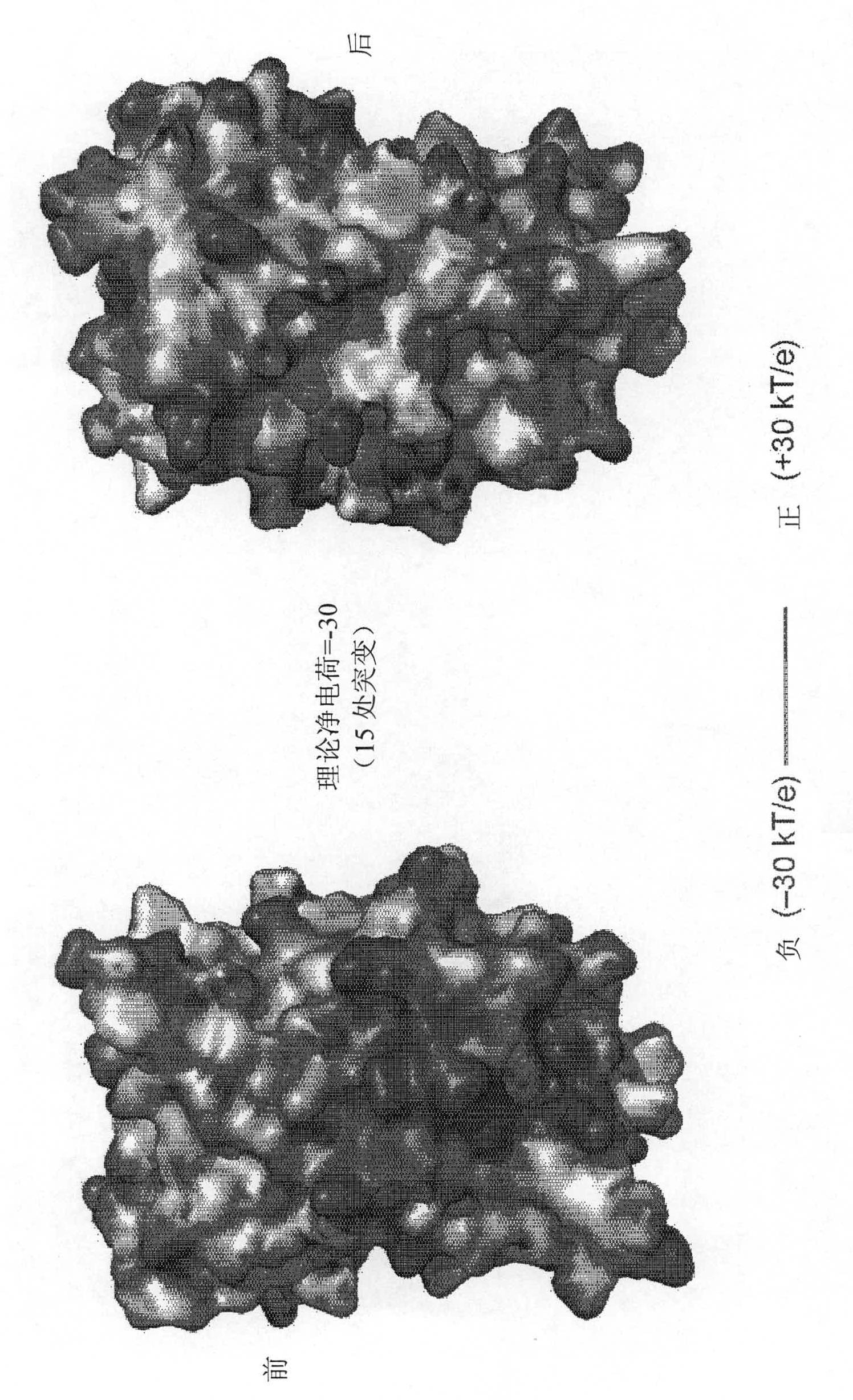
Supercharged proteins for cell penetration
InactiveUS20110112040A1Improved cell penetrationReduce biological activityMicrobiological testing/measurementSaccharide peptide ingredientsInfective disorderDisease cause
Compositions, systems and related methods for delivering a supercharged protein or a complex of a supercharged protein and therapeutic agent (e g, nucleic acid, peptide, small molecule) to cells are disclosed. Superpositively charged proteins may be associated with nucleic acids (which typically have a net negative charge) via electrostatic interactions. The systems and methods may involve altering the primary sequence of a protein in order to “supercharge” the protein (e g, to generate a superpositively-charged protein). The compositions may be used to treat proliferative diseases, infectious diseases, cardiovascular diseases, inborn errors in metabolism, genetic diseases, etc.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Peptidomimetic macrocycles
InactiveUS20100216688A1Low affinityEasy to transportPeptide/protein ingredientsAntipyreticHuman proteinsCell penetration
The present invention provides biologically active crosslinked polypeptides with improved properties relative to their corresponding precursor polypeptides, having good cell penetration properties and reduced binding to human proteins. The invention additionally provides methods of identifying and making such improved polypeptides.
Owner:AILERON THERAPEUTICS INC
Peptidomimetic macrocycles
The present invention provides biologically active crosslinked polypeptides with improved properties relative to their corresponding precursor polypeptides, having good cell penetration properties and reduced binding to human proteins. The invention additionally provides methods of identifying and making such improved polypeptides.
Owner:AILERON THERAPEUTICS INC
Circulating tumor cell detection kit and application thereof
InactiveCN105785005AAccurate detectionEfficient enrichmentMaterial analysisLymphatic SpreadFluorescence
The invention relates to a circulating tumor cell detection kit and application thereof. The circulating tumor cell detection kit comprises a chip, sample diluent, a cell separation fluid, a cell trapping agent, a cell cleaning solution, a cell fixing agent, antibody diluent, a cell penetration agent and a fluorescence dye. The application method of the circulating tumor cell detection kit mainly comprises the several steps of separation of circulating tumor cells, trapped antibody coating, trapping of the circulating tumor cells and immuno-fluorescent staining of the circulating tumor cells. The circulating tumor cell detection kit is mainly used for tumor prognosis, reappear, metastasis, curative effect monitoring, early diagnosis and early warning, monitoring of curative effect and tumor progression situation, and utilizes the molecular subtyping, screening specificity and other characteristics of the circulating tumor cells to conduct targeted research on circulating tumor cell sensitive drug so as to supplement tumor metastasis, relapse and drug resistance mechanisms and guide individualized medical treatment.
Owner:杭州华得森生物技术有限公司
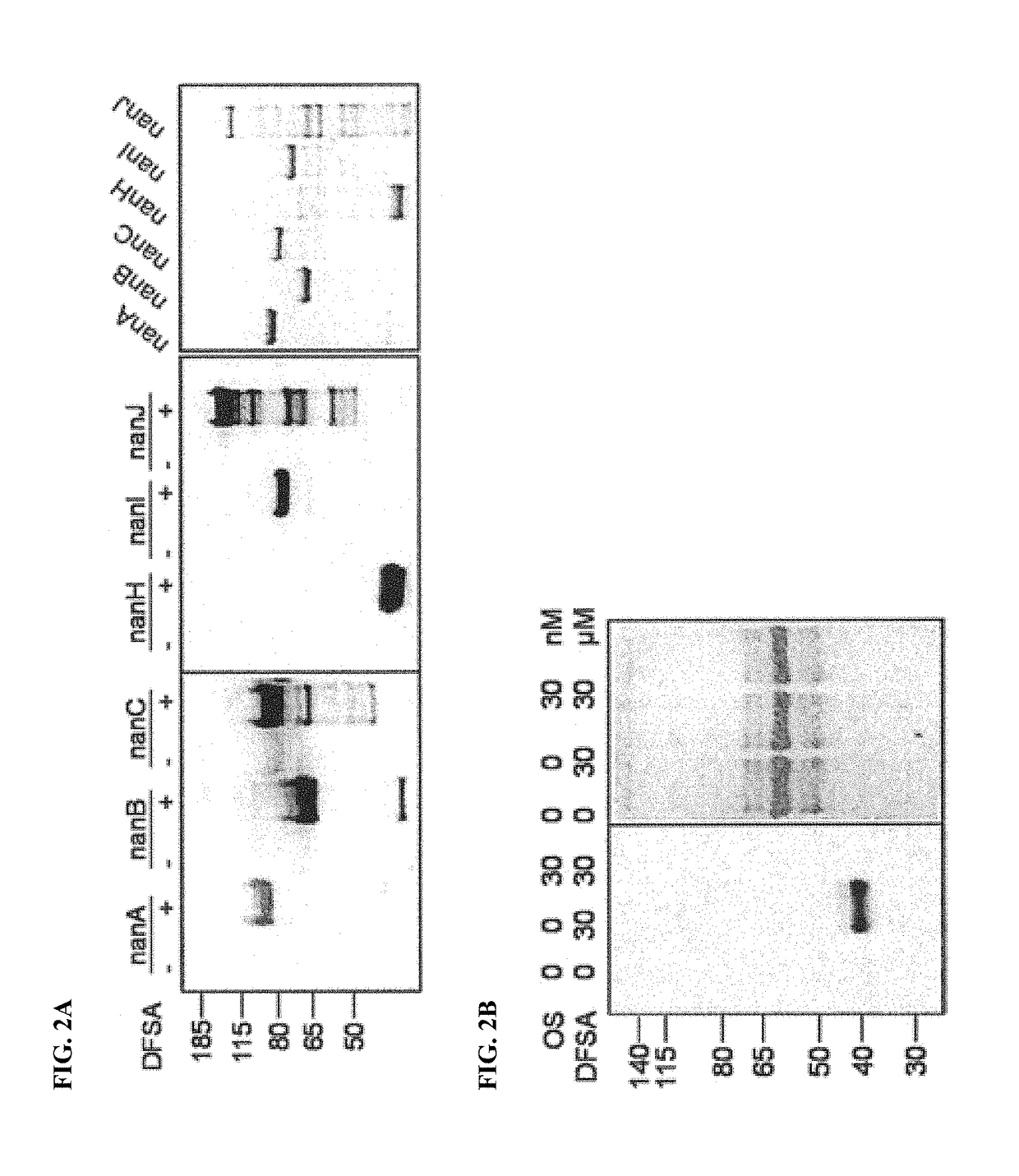
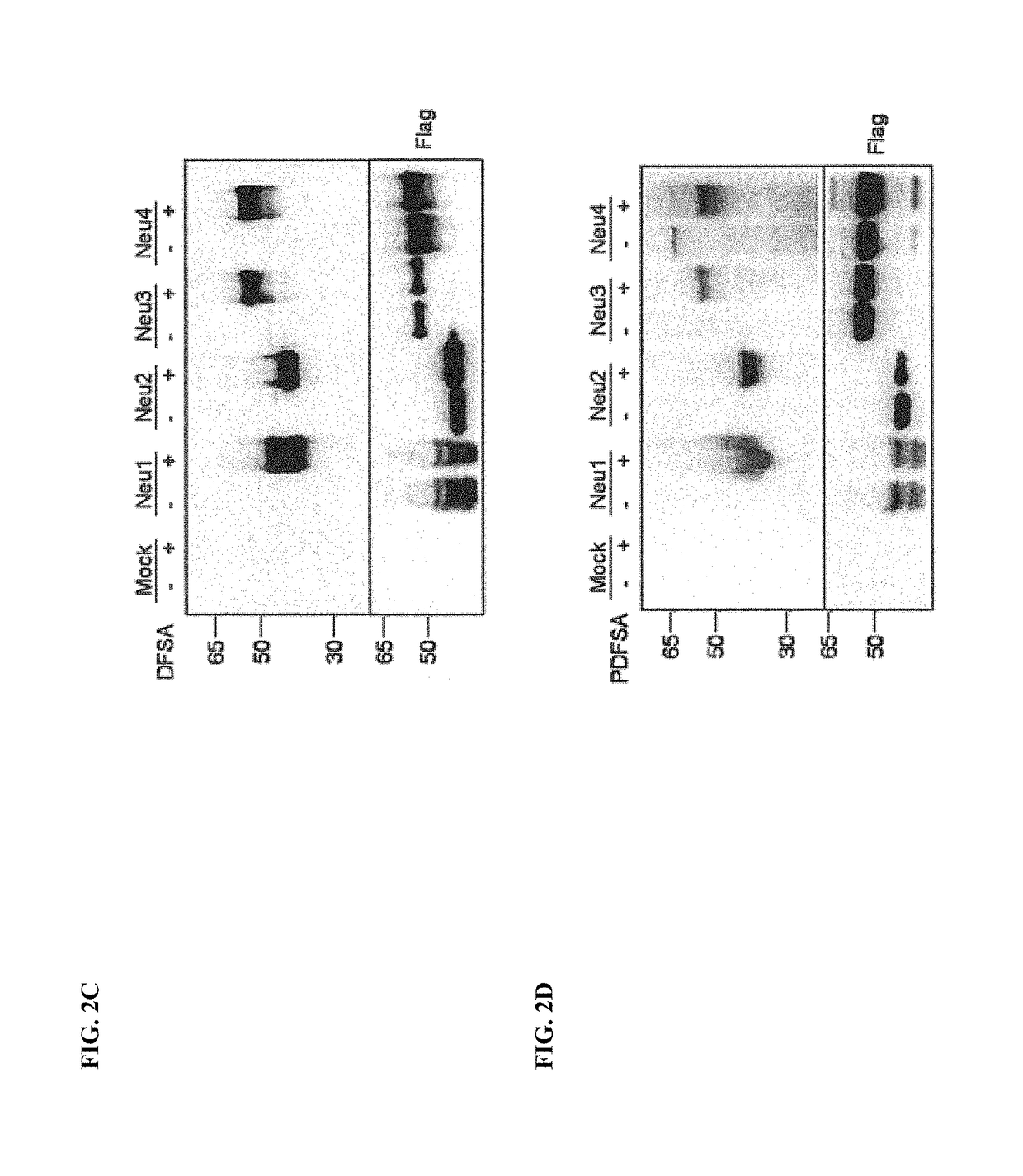
Cell-permeable probes for identification and imaging of sialidases
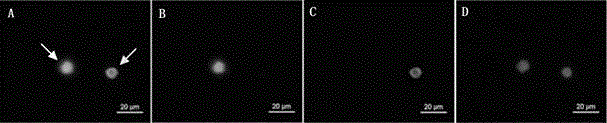
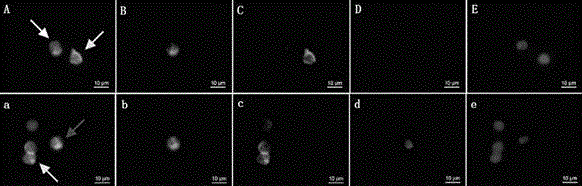
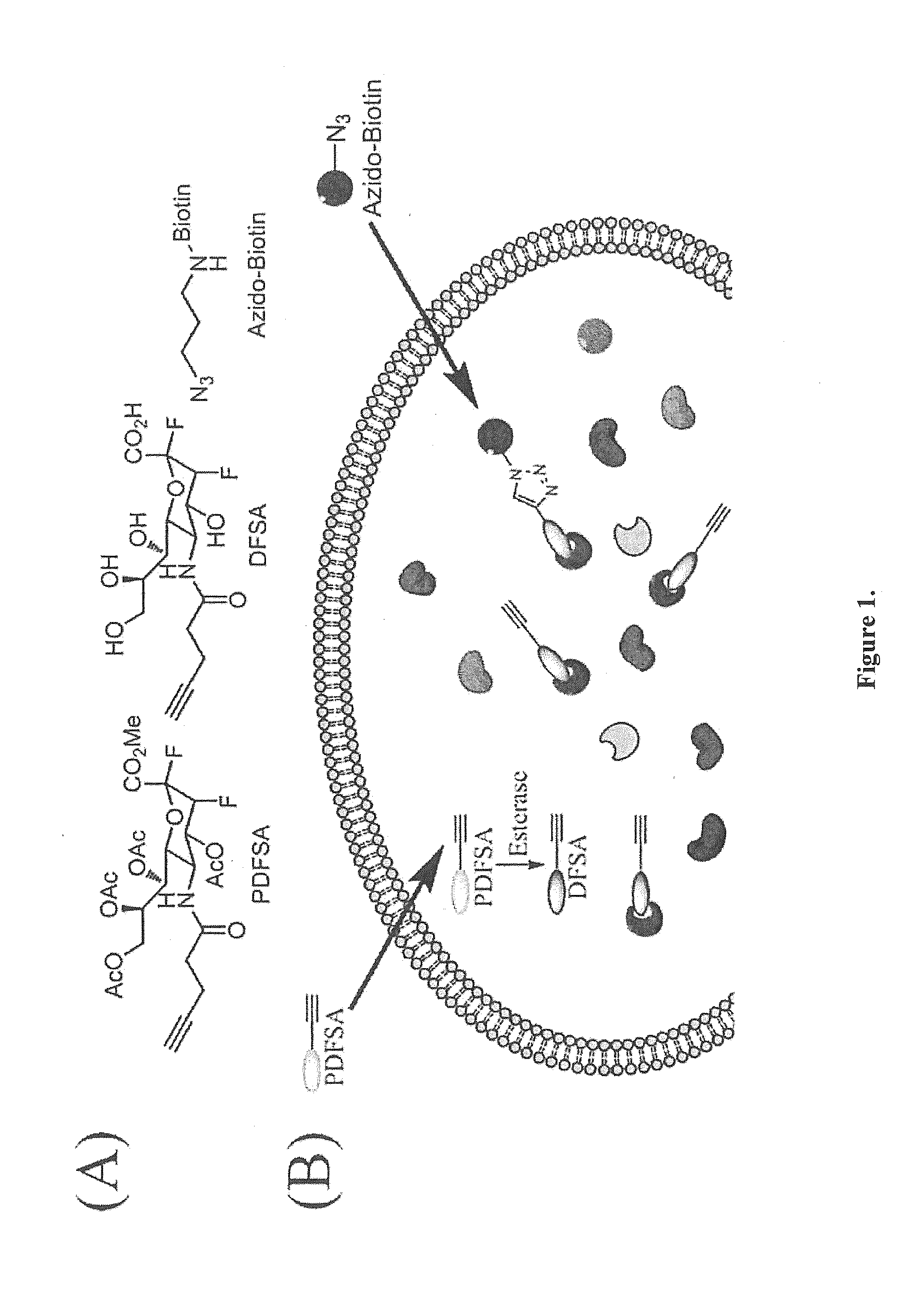
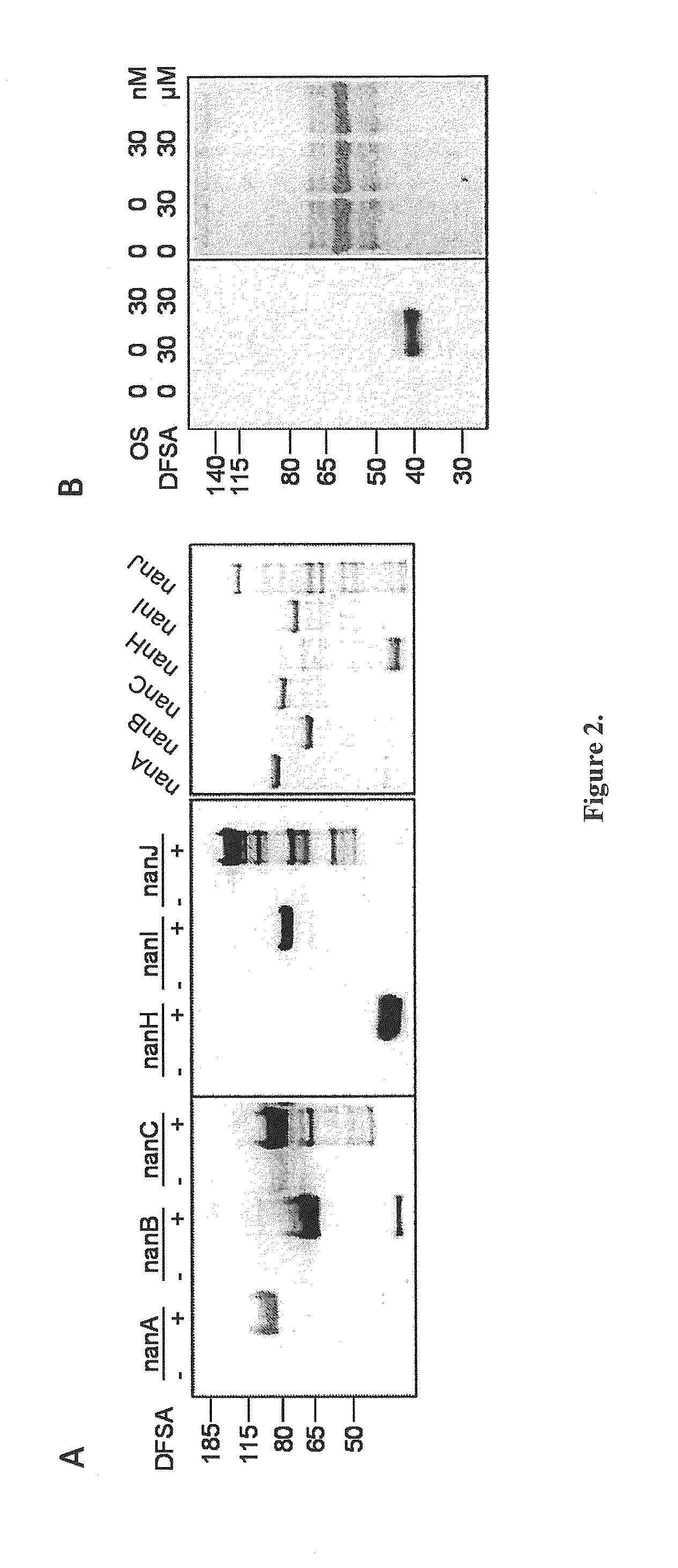
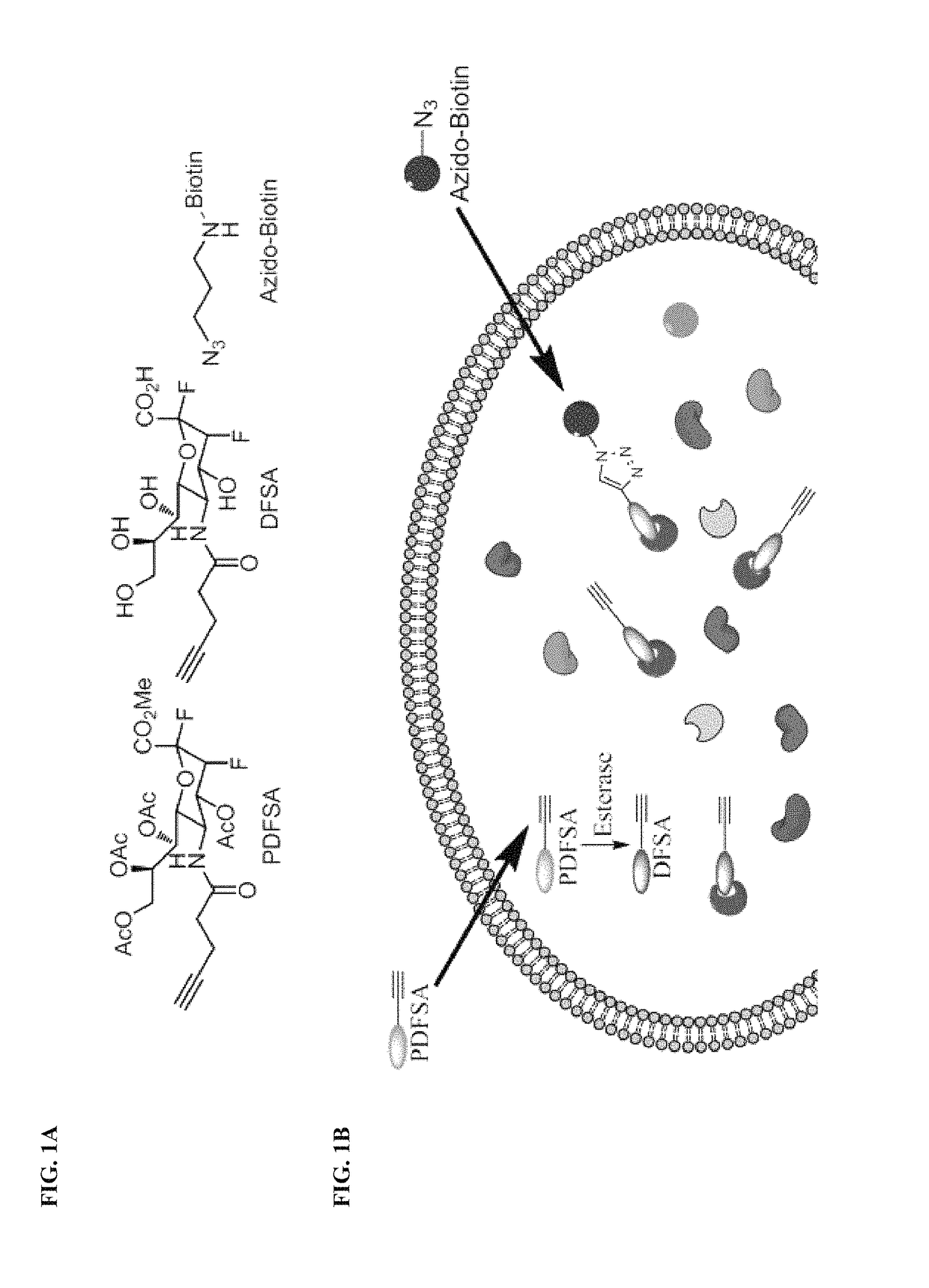
Provided herein are novel irreversible sialidase inhibitors. These compounds can be conjugated with a detectable tagging moiety such as azide-annexed biotin via CuAAC for isolation and identification of sialidases. The provided compounds and the corresponding detectable conjugates are useful for detecting sialidase-containing pathogens and imaging in situ sialidase activities under physiological conditions.
Owner:ACAD SINIC
Morpholino-based antisense agent
ActiveUS20120296087A1High activityHigh selectivitySilicon organic compoundsActivity regulationOligomerRegulation of gene expression
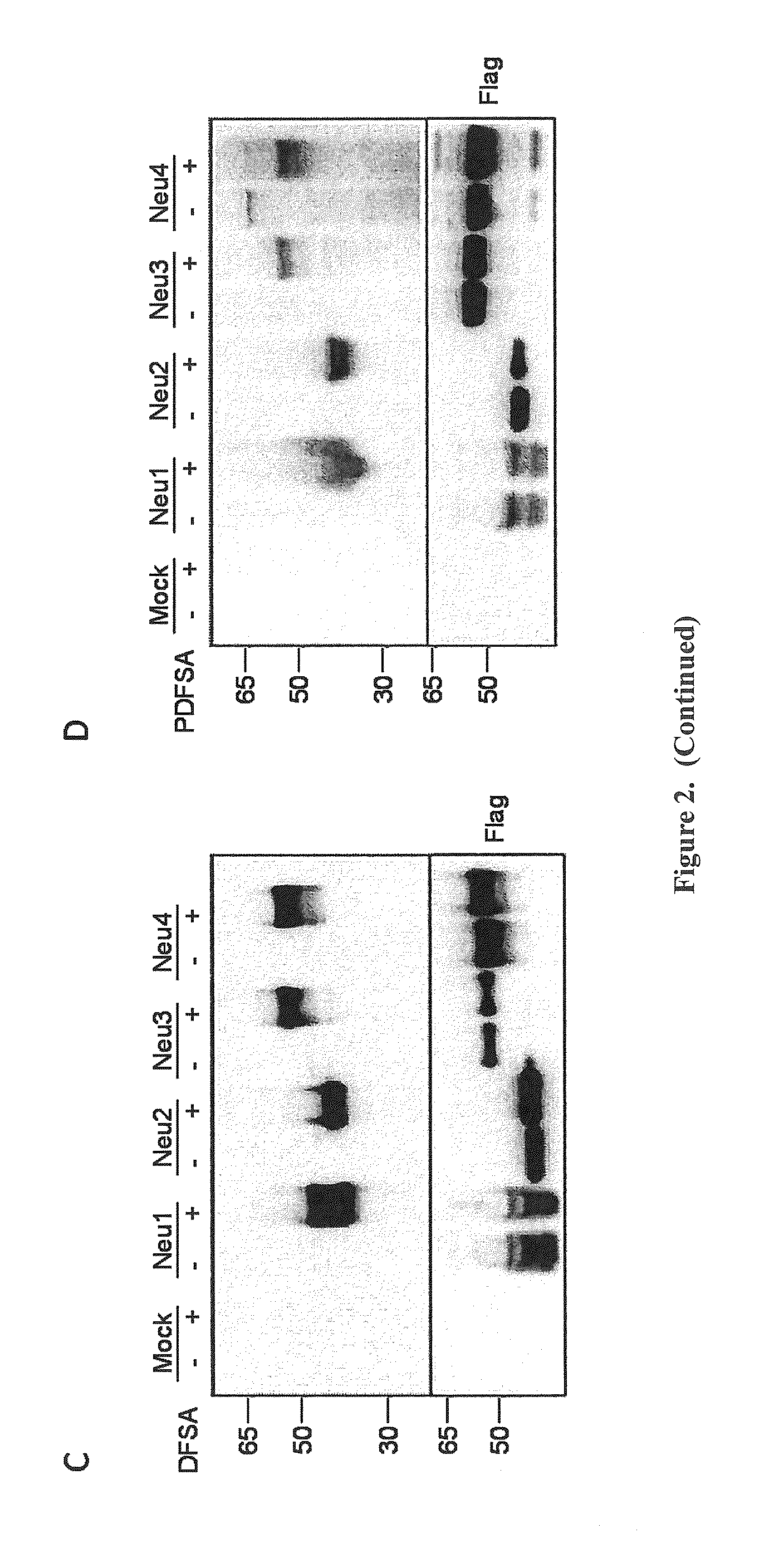
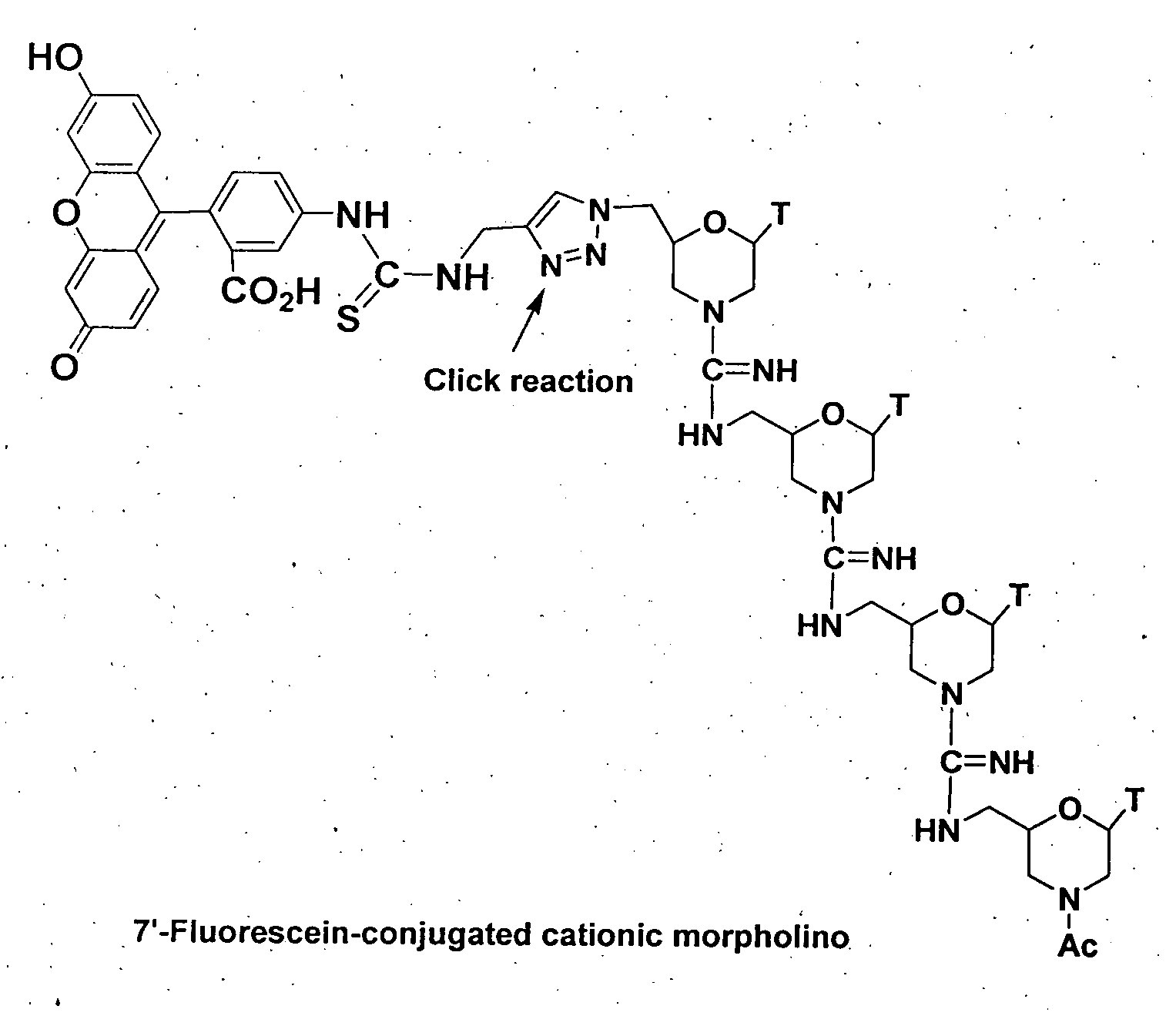
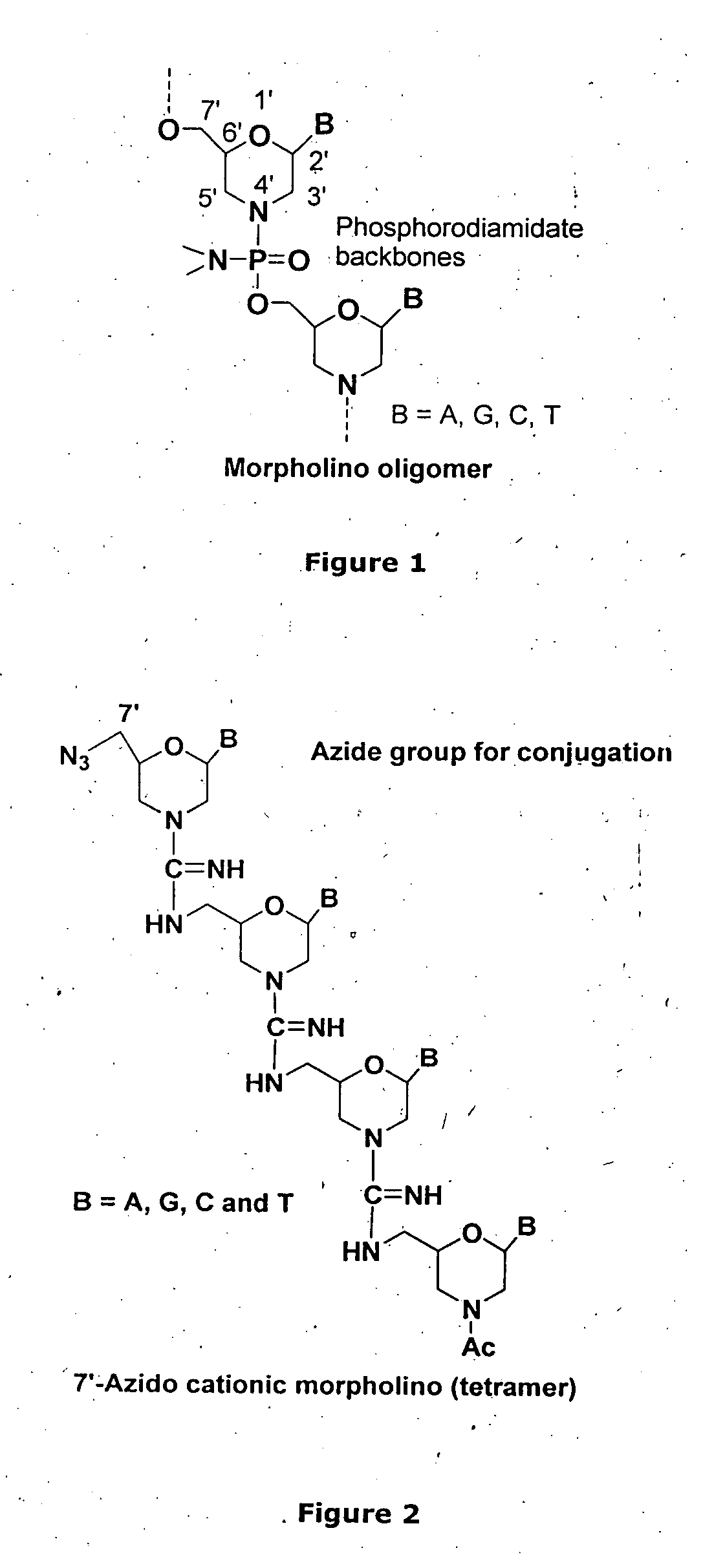
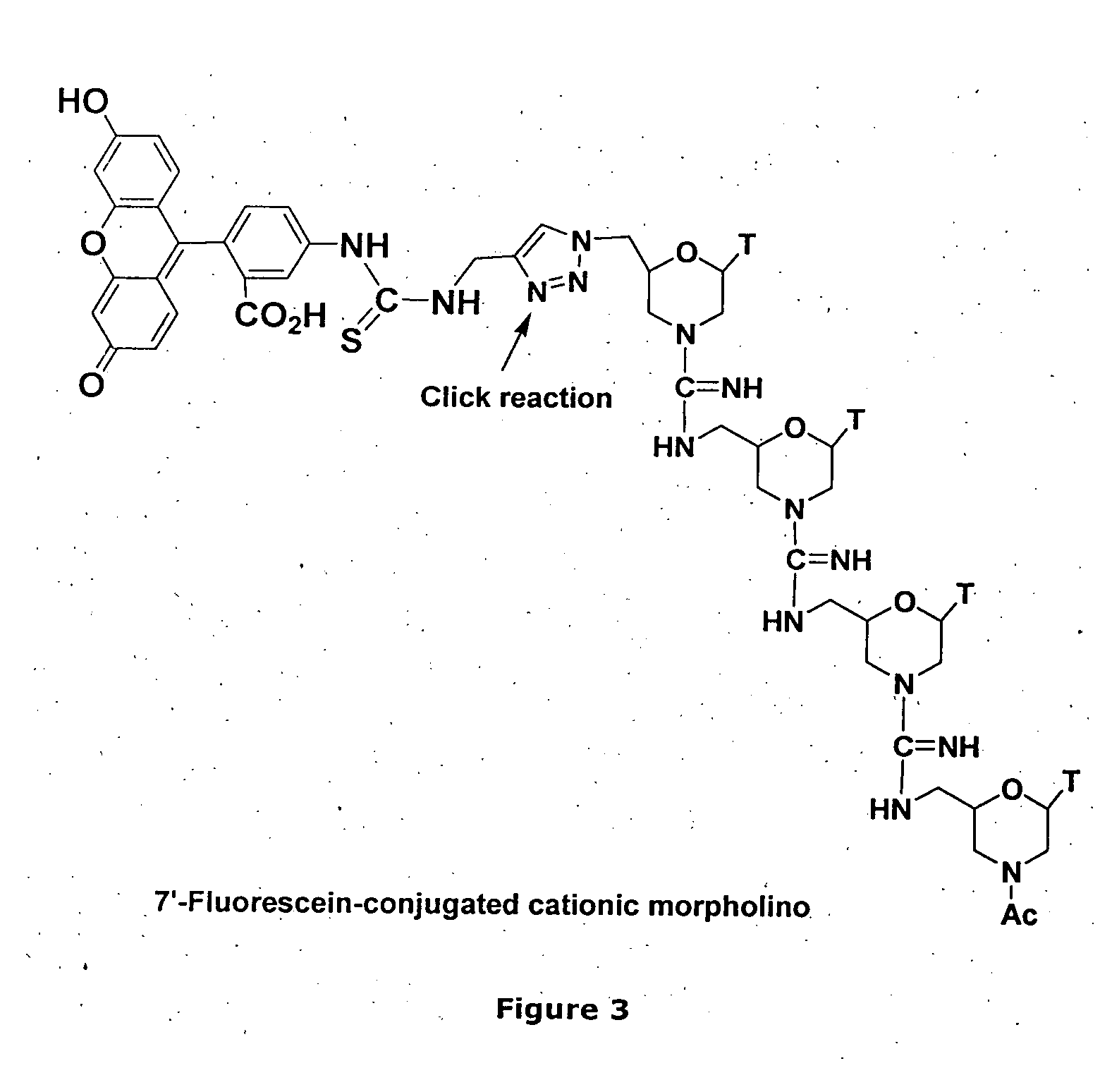
Morpholino-based oligomers suitable as antisense agent comprising modifications of phosphorodiamidate backbone or modification with 5-substituted pyrimidines of morpholino compound that is soluble in culture medium and sufficient for cell penetration thereby eliminating the need for injecting into the cells. Monomers comprising the said oligomers and its method of manufacture, method of manufacture of the said oligomers and its dye, flurophore, drug, biomolecule conjugate wherein the said oligomers find different end use but not limited to regulation of gene expression, tissue culture with improved transfection efficiency and related studies on cellular transfection.
Owner:INDIAN ASSOC FOR THE CULTIVATION OF SCI
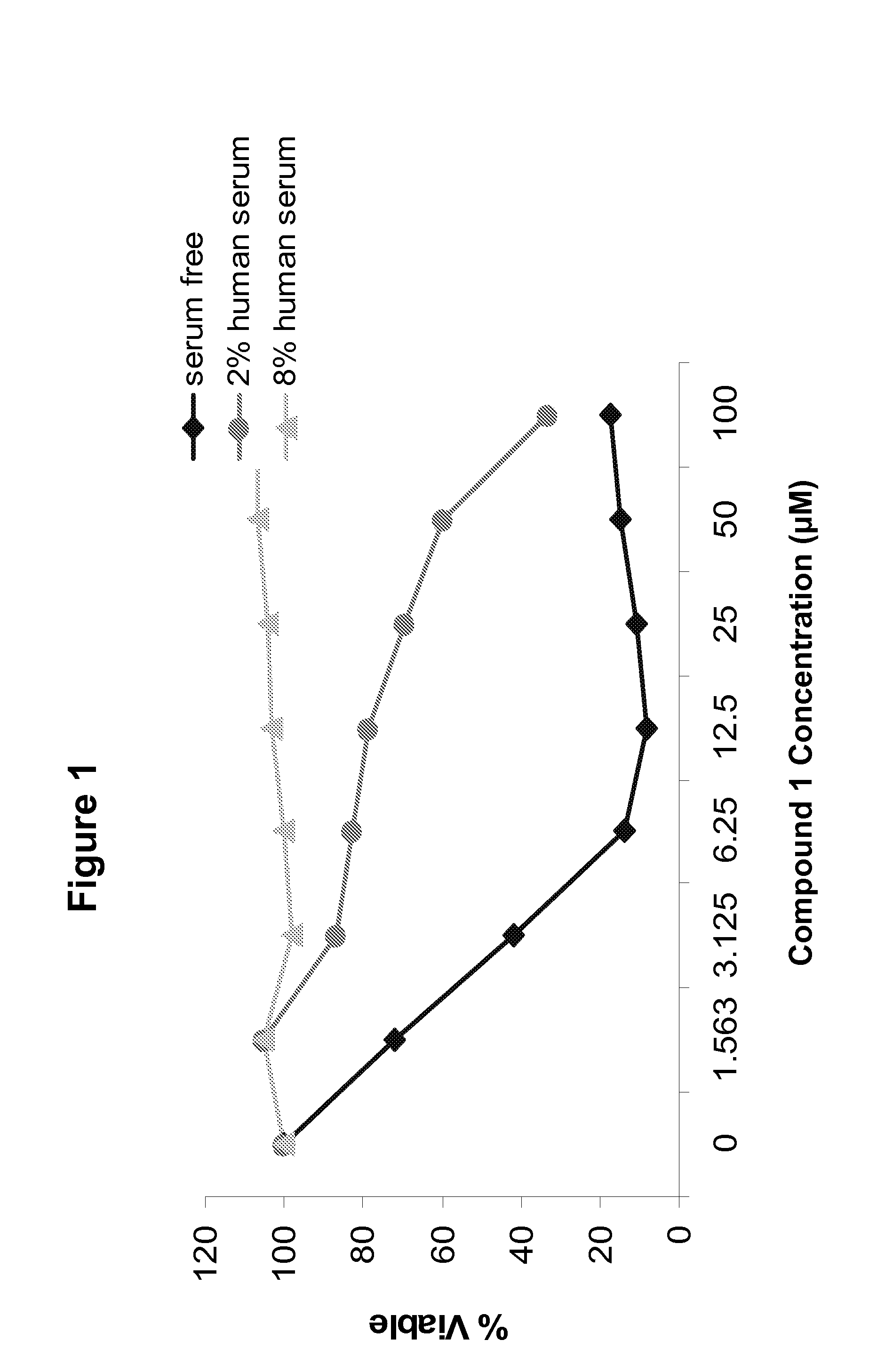
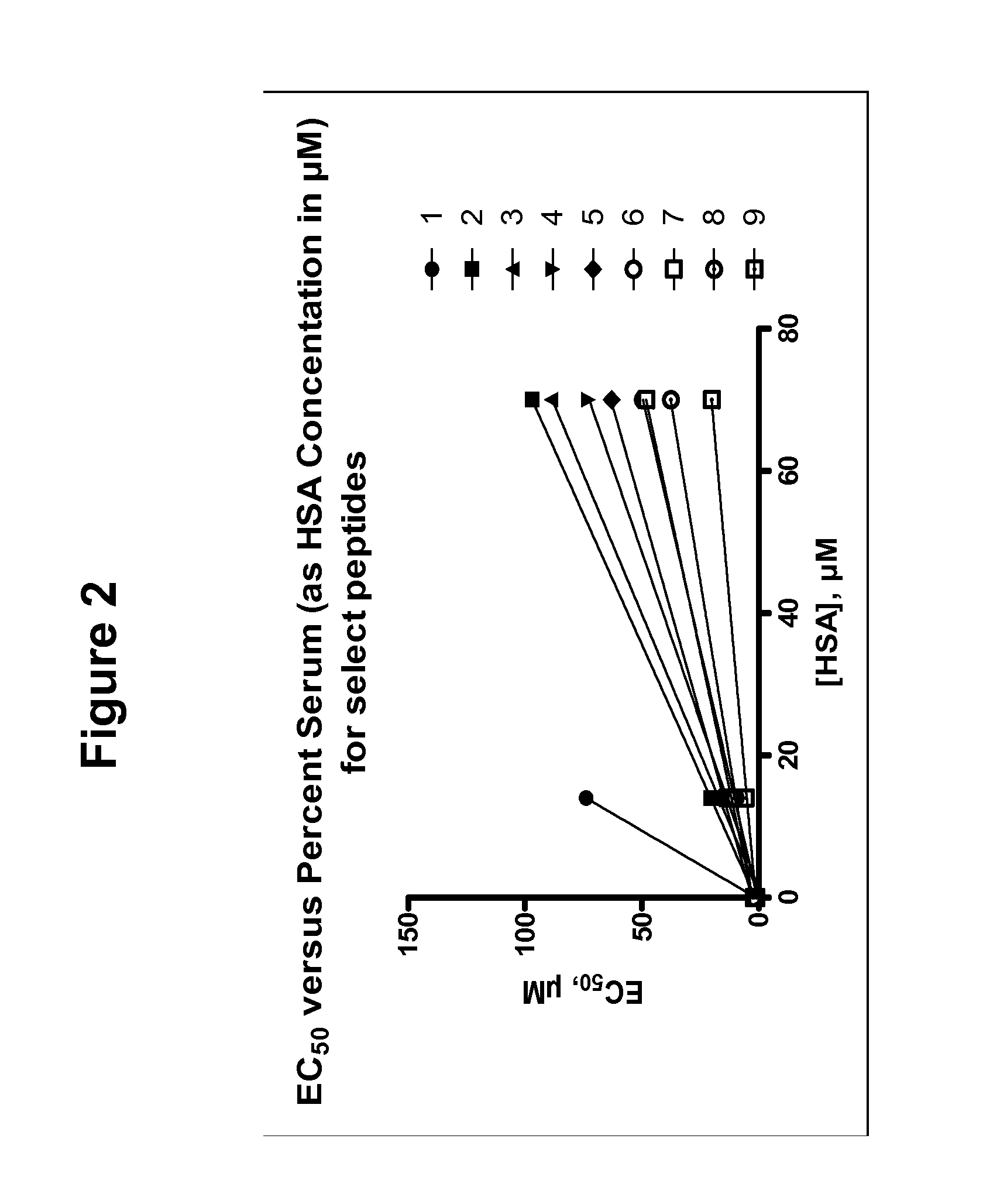
Cell permeable conjugates of peptides for inhibition of protein kinases
InactiveUS20070078092A1Reduce generationAccelerated deathSenses disorderNervous disorderPTK InhibitorsDisease cause
The present invention provides inhibitors of protein kinases comprising a molecule having at least a first moiety competent for penetration of the molecule into cells, and a second moiety for having a protein kinase inhibiting effect within the cells. The first moiety is joined to the second moiety through a linker or a spacer. The complex molecules are preferably peptide conjugates having improved cell-permeability, serum stability and kinase selectivity compared to known protein kinase inhibitors. Pharmaceutical compositions that include these protein kinase inhibitors, and methods of using such compositions for treatment of cancers and other diseases associated with protein kinase activity are also disclosed.
Owner:CUREGENICS
Cell-permeable probes for identification and imaging of sialidases
Provided herein are novel irreversible sialidase inhibitors. These compounds can be conjugated with a detectable tagging moiety such as azide-annexed biotin via CuAAC for isolation and identification of sialidases. The provided compounds and the corresponding detectable conjugates are useful for detecting sialidase-containing pathogens and imaging in situ sialidase activities under physiological conditions.
Owner:ACAD SINIC
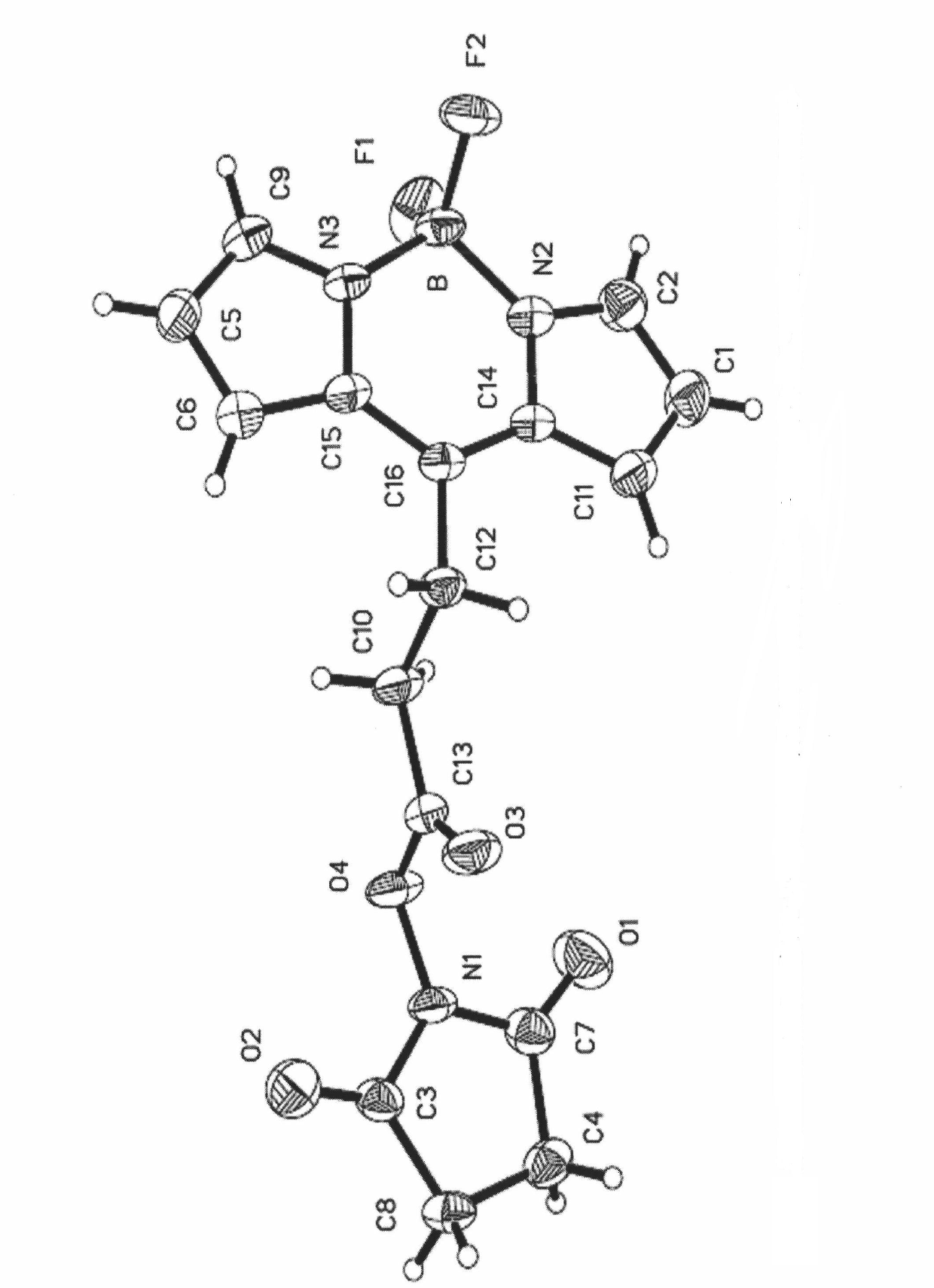
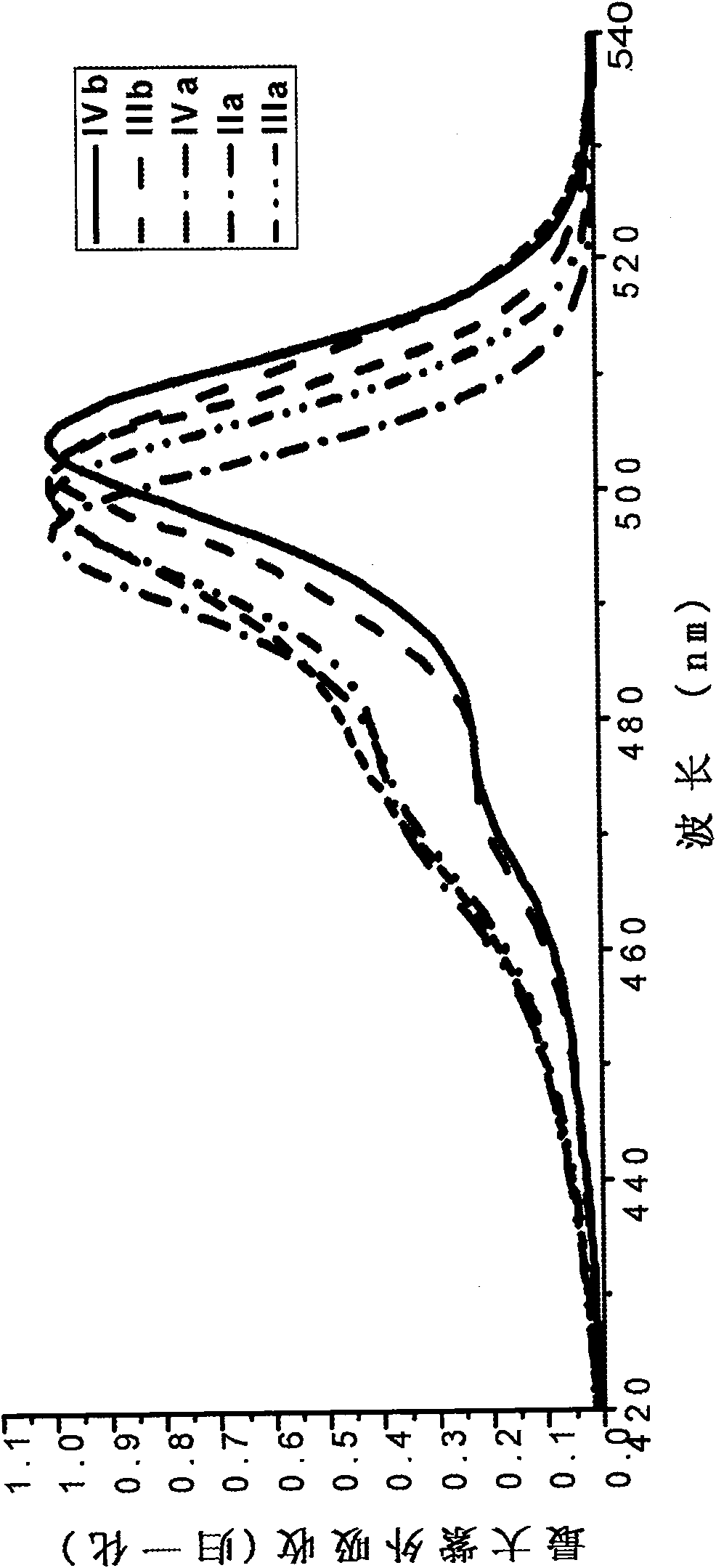
Type I boron fluoride complex dipyrromethene dye, and preparation method and application thereof
InactiveCN102061103AThe reaction steps are simpleMild conditionsMethine/polymethine dyesBiological testingCarboxyl radicalEther
The invention relates to a type I boron fluoride complex dipyrromethene fluorescent dye. The fluorescent dye is shown as a general formula I, wherein R1, R2, R3, R5, R6 and R7 are H or C1-C8 alkyl respectively; R8 is a radical of a structural formula X, Y, Z or P; M is H, Na, K, N (R9R10R11R12) or N-succinimido; and R9, R10, R11 and R12 are H, C1-C8 alkyl or C1-C8 alkyl with substituent group-OH, an ether bond or carboxyl. The dye is synthesized by directly reacting substituted pyrrole with anhydride, has a high photo-physical property, stability, cell penetration capability and intracellular dissolving capability and is particularly suitable for bioluminescence analysis and biological marking.
Owner:DALIAN UNIV OF TECH +1
Development of Protein-Based Biotherapeutics That Penetrates Cell-Membrane and Induces Anti-Hepatocellular Carcinoma Effect - Improved Cell-Permeable Suppressor of Cytokine Signaling (iCP-SOCS3) Proteins, Polynucleotides Encoding the Same, and Anti-Hepatocellular Carcinoma Compositions Comprising the Same
InactiveUS20160060310A1Good effectImprove efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityHepatocellular carcinoma
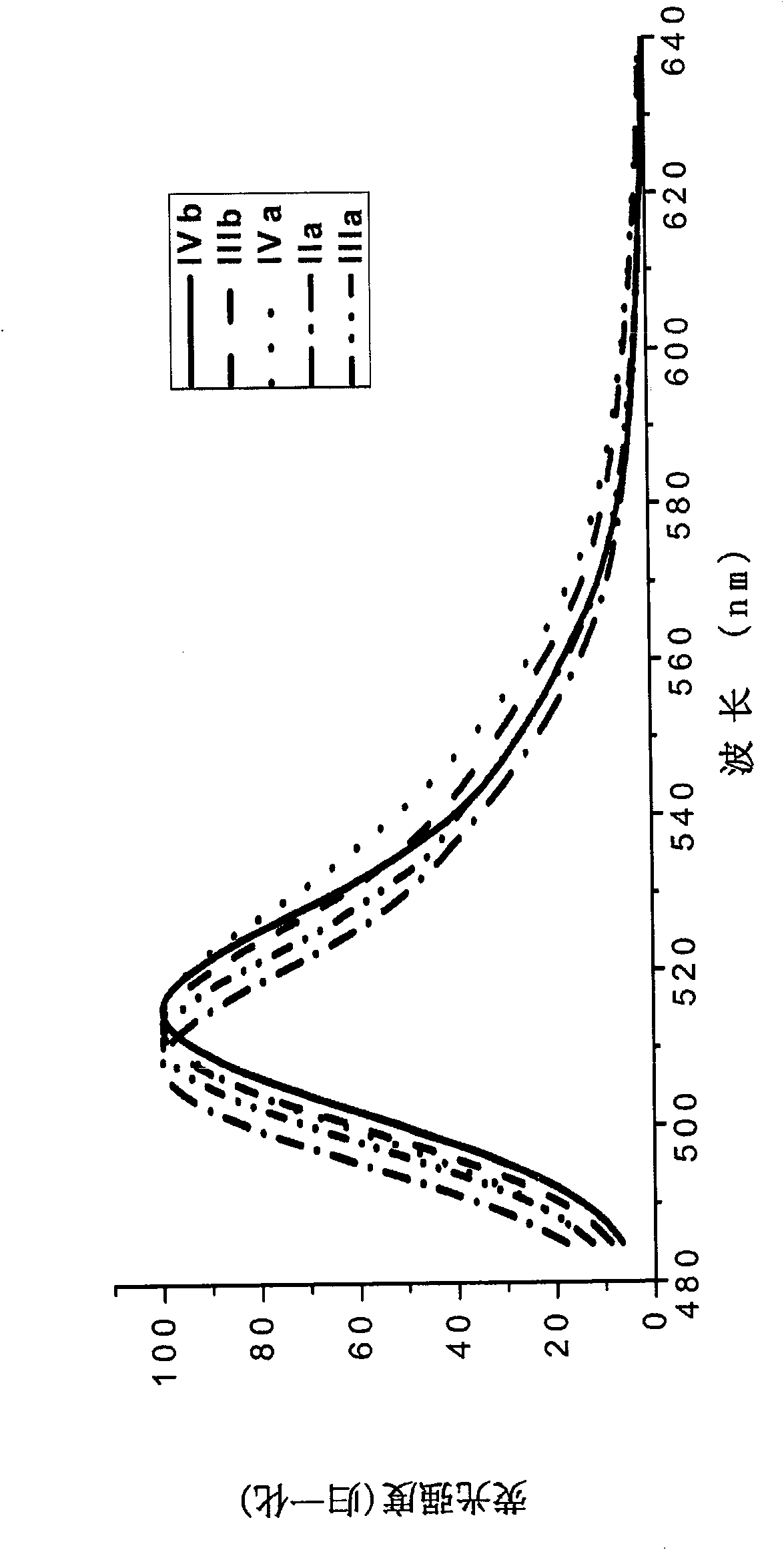
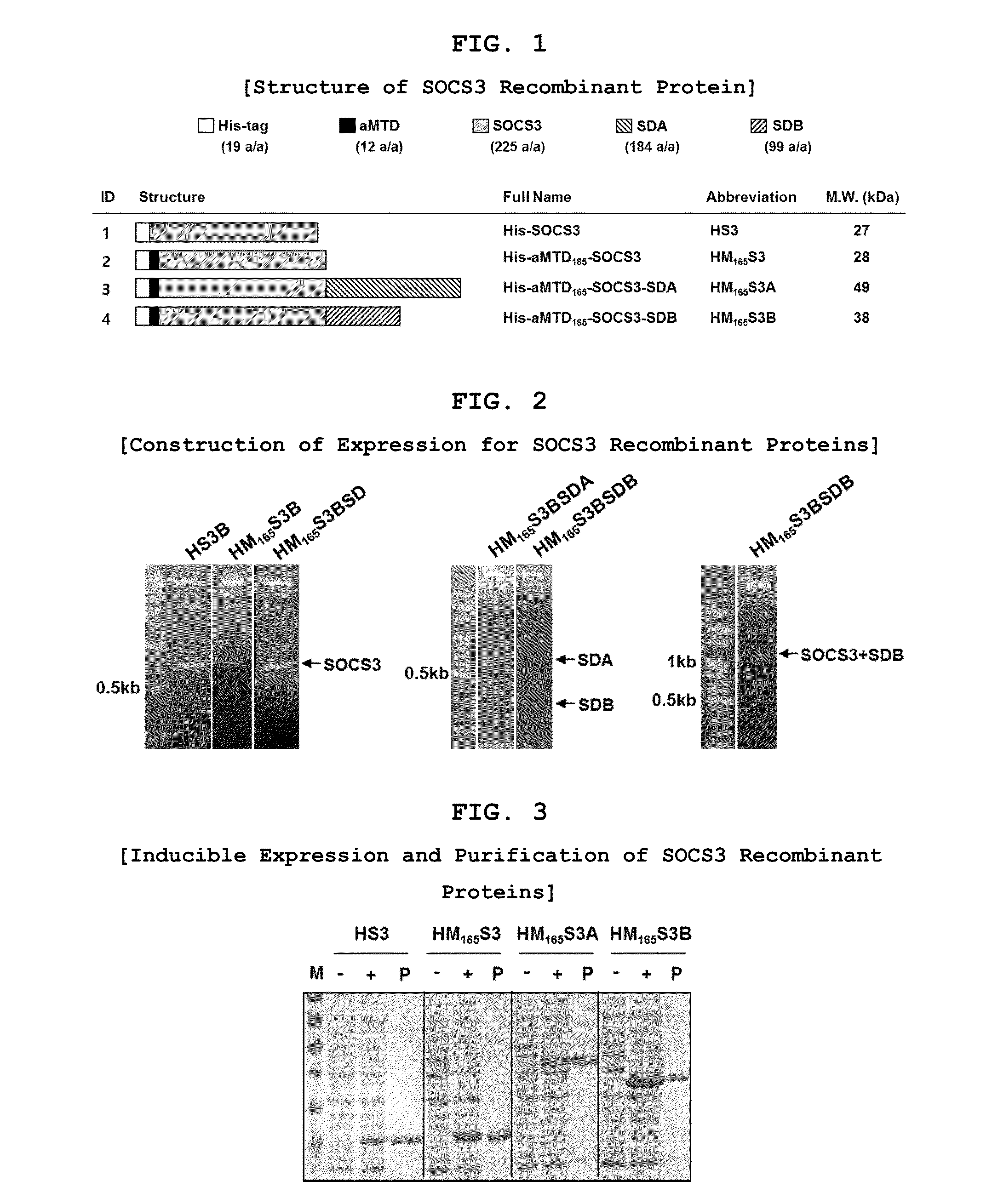
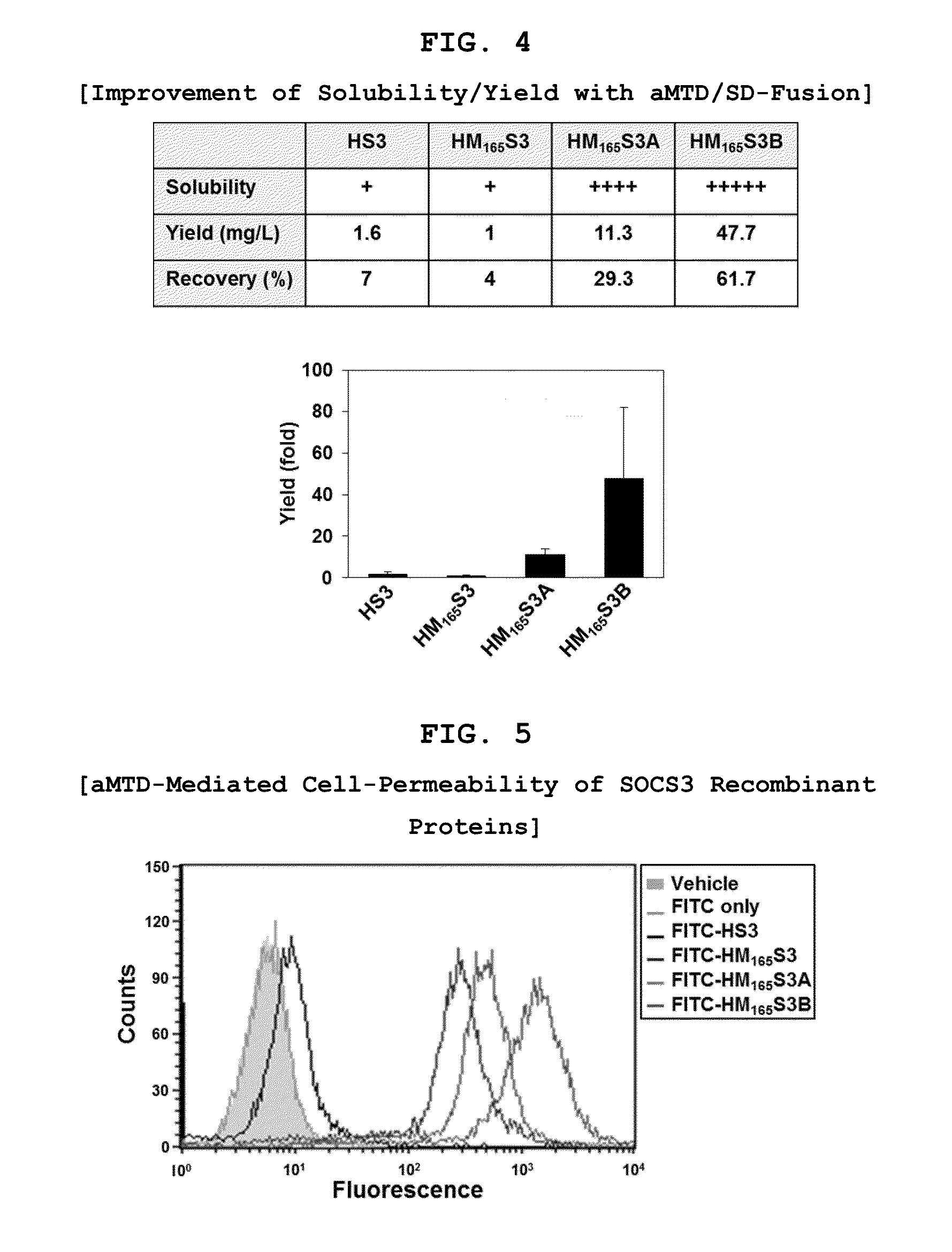
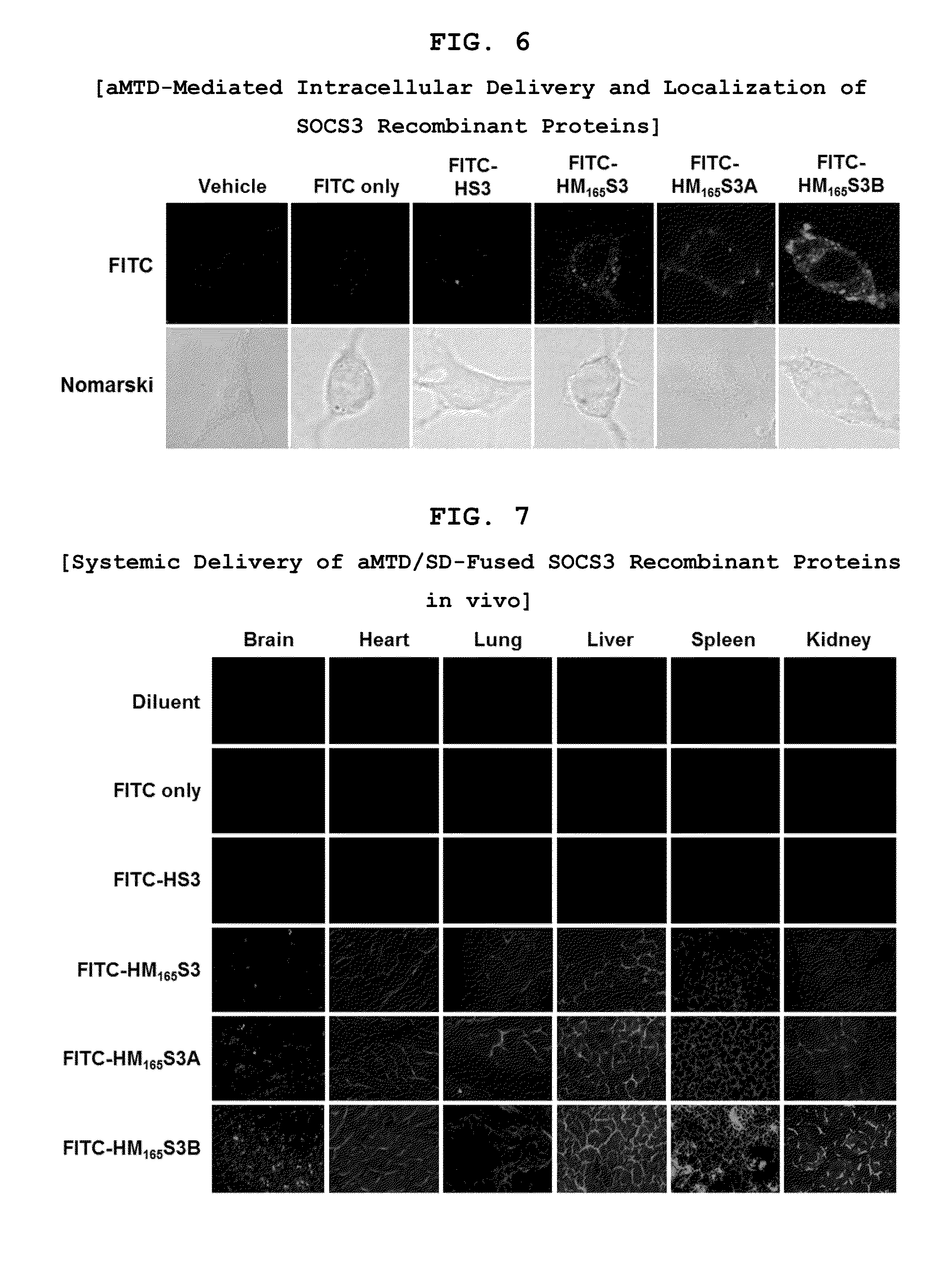
Protein transduction exploits the ability of some cell-penetrating peptide (CPP) sequences to enhance the uptake of proteins and other macromolecules by mammalian cells. Previously developed hydrophobic CPPs, named membrane translocating sequence (MTS), membrane translocating motif (MTM) and macromolecule transduction domain (MTD), are able to deliver biologically active proteins into a variety of cells and tissues. Various cargo proteins fused to these CPPs have been used to test the functional and / or therapeutic efficacy of protein transduction. For example, recombinant proteins consisting of suppressor of cytokine signaling 3 protein (CP-SOCS3) fused to the fibroblast growth factor (FGF) 4-derived MTM were developed to inhibit inflammation and apoptosis. However, CP-SOCS3 fusion proteins expressed in bacteria were hard to purify in soluble form. To address these critical limitations, CPP sequences called advanced MTDs (aMTD) have been developed in this art. This is accomplished by (i) analyzing previous developed hydrophobic CPP sequences to identify specific critical factors (CFs) that affect intracellular delivery potential and (ii) constructing artificial aMTD sequences satisfied for each critical factor. In addition, solubilization domains (SDs) have been incorporated into the aMTD-fused SOCS3 recombinant proteins to enhance solubility with corresponding increases in protein yield and cell- / tissue-permeability. These recombinant SOCS3 proteins fused to aMTD / SD having much higher solubility / yield and cell- / tissue-permeability have been named as improved cell-permeable SOCS3 (iCP-SOCS3) proteins. Previously developed CP-SOCS3 proteins fused to MTM were only tested or used as anti-inflammatory agents to treat acute liver injury. In the present art, iCP-SOCS3 proteins have been tested for use as anti-cancer agents in the treatment of hepatocellular carcinoma. Since SOCS3 is frequently deleted in and loss of SOCS3 in hepatocytes promotes resistance to apoptosis and proliferation, we reasoned that iCP-SOCS3 could be used as a protein-based intracellular replacement therapy for the treatment of hepatocellular carcinoma. The results support this reasoning: treatment of hepatocellular carcinoma cells with iCP-SOCS3 results in reduced cancer cell viability, enhanced apoptosis and loss of cell migration / invasion potential. Furthermore, iCP-SOCS3 inhibits the growth of hepatocellular carcinoma in a subcutaneous xenografts model. In the present invention with iCP-SOCS3 fused to an empirically determined combination of newly developed aMTD and customized SD, macromolecule intracellular transduction technology (MITT) enabled by the advanced MTD may provide novel protein therapy against hepatocellular carcinoma.
Owner:CELLIVERY THERAPEUTICS
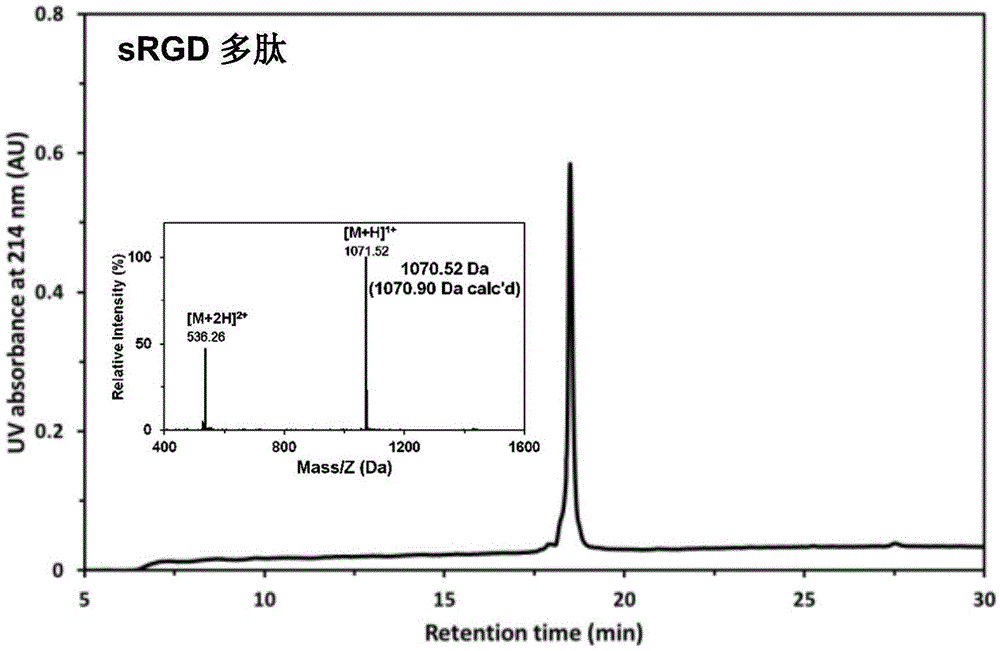
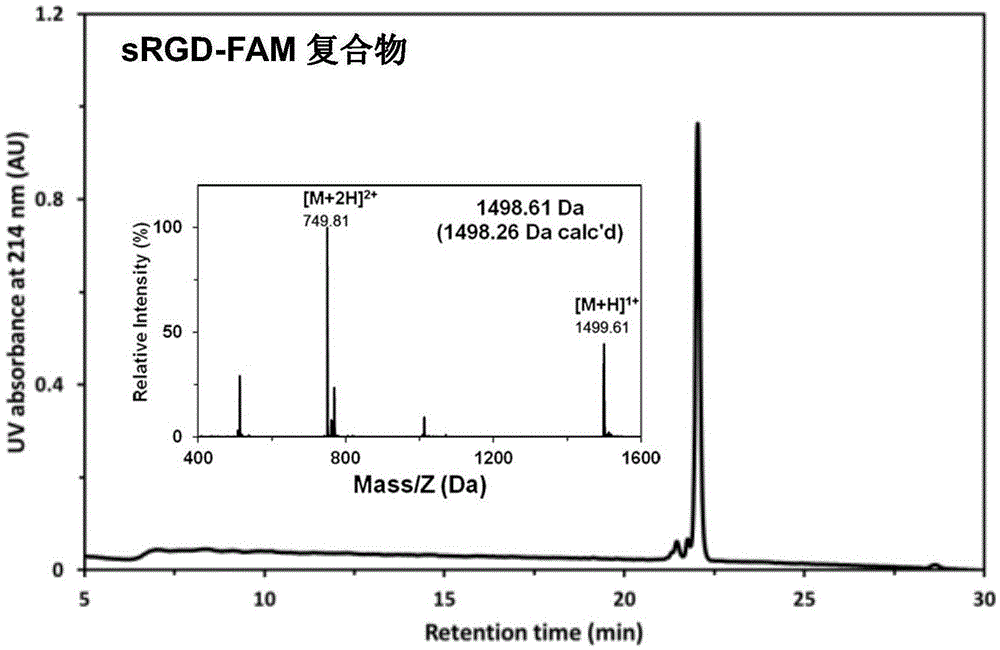
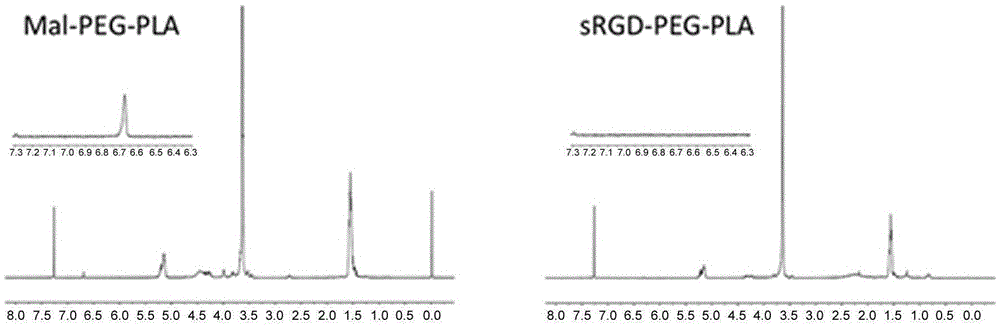
Stapled-RGD polypeptide, and applications thereof in tumor targeting delivery
InactiveCN106699845AStrong penetrating powerPowerfulOrganic active ingredientsPeptidesTumor targetBinding peptide
The invention belongs to the field of pharmacology, and relates to stapled-RGD polypeptide, and applications thereof in tumor targeting delivery. Multifunctional targeting polypeptide molecule stapled-RGD with high combination activity with integrin and biological membrane barrier penetration capacity is designed and prepared based on binding peptide cyclization technology; and preparation of fluorescein, drugs, and high molecule carriers modified by the multifunctional targeting polypeptide molecule stapled-RGD, and applications of the fluorescein, the drugs, and the high molecule carriers in tumor imaging and construction of targeted therapy targeted drug delivery systems are disclosed. It is shown by results that specific uptaking of model drugs carried by stapled-RGD by positive cells, tumor mimicry vessels, and tumor ball tissues of expressed integrin is realized, higher tumor targeting capacity, imaging functions, and membrane barrier cell penetration capacity are achieved; nano targeted drug delivery systems constructed by the high molecule carriers modified by stapled-RGD can be used for delivering carried model drugs to target tissues, antitumor drug effect is improved obviously; and stapled-RGD possesses a promising application prospect in mediating drugs, nano targeted drug delivery system membrane barrier penetration, active target searching, tumor diagnosis, and targeted therapy.
Owner:FUDAN UNIV
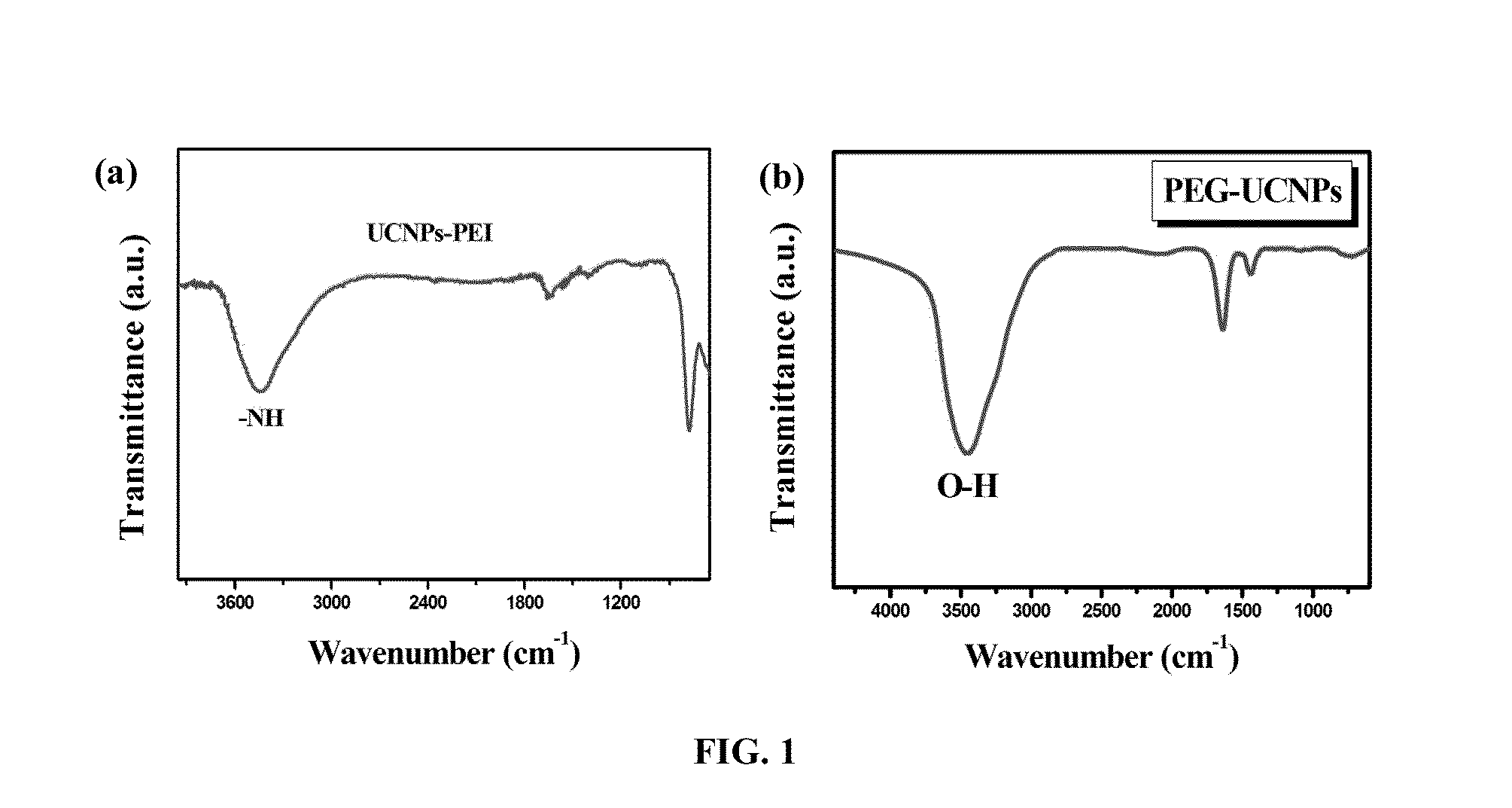
BIOPROBE BASED ON SINGLE-PHASE UPCONVERSION NANOPARTICLES (UCNPs) FOR MULTI-MODAL BIOIMAGING
InactiveUS20140147391A1Maintain good propertiesSignificant valueUltrasonic/sonic/infrasonic diagnosticsPowder deliveryDiagnostic Radiology ModalityCytotoxicity
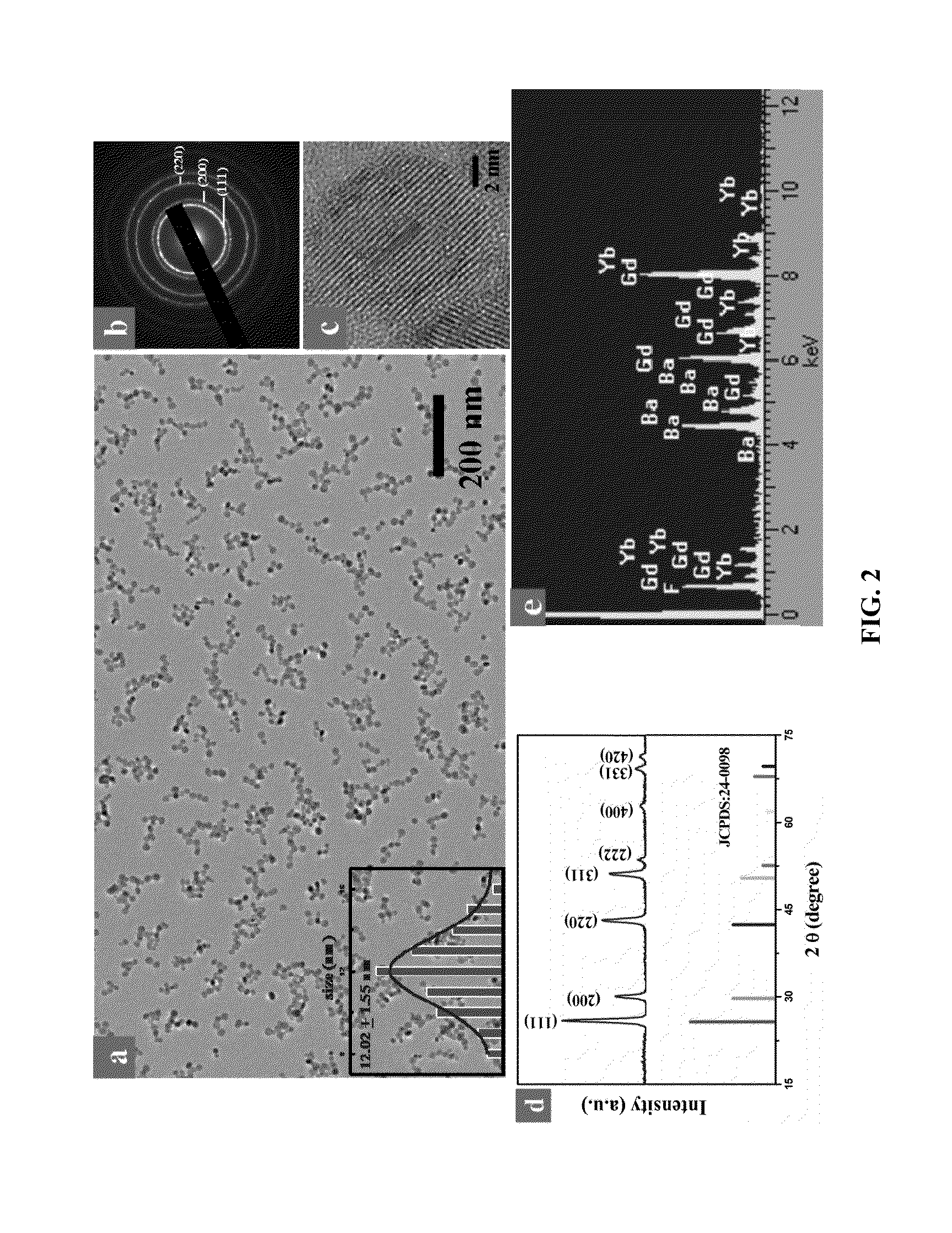
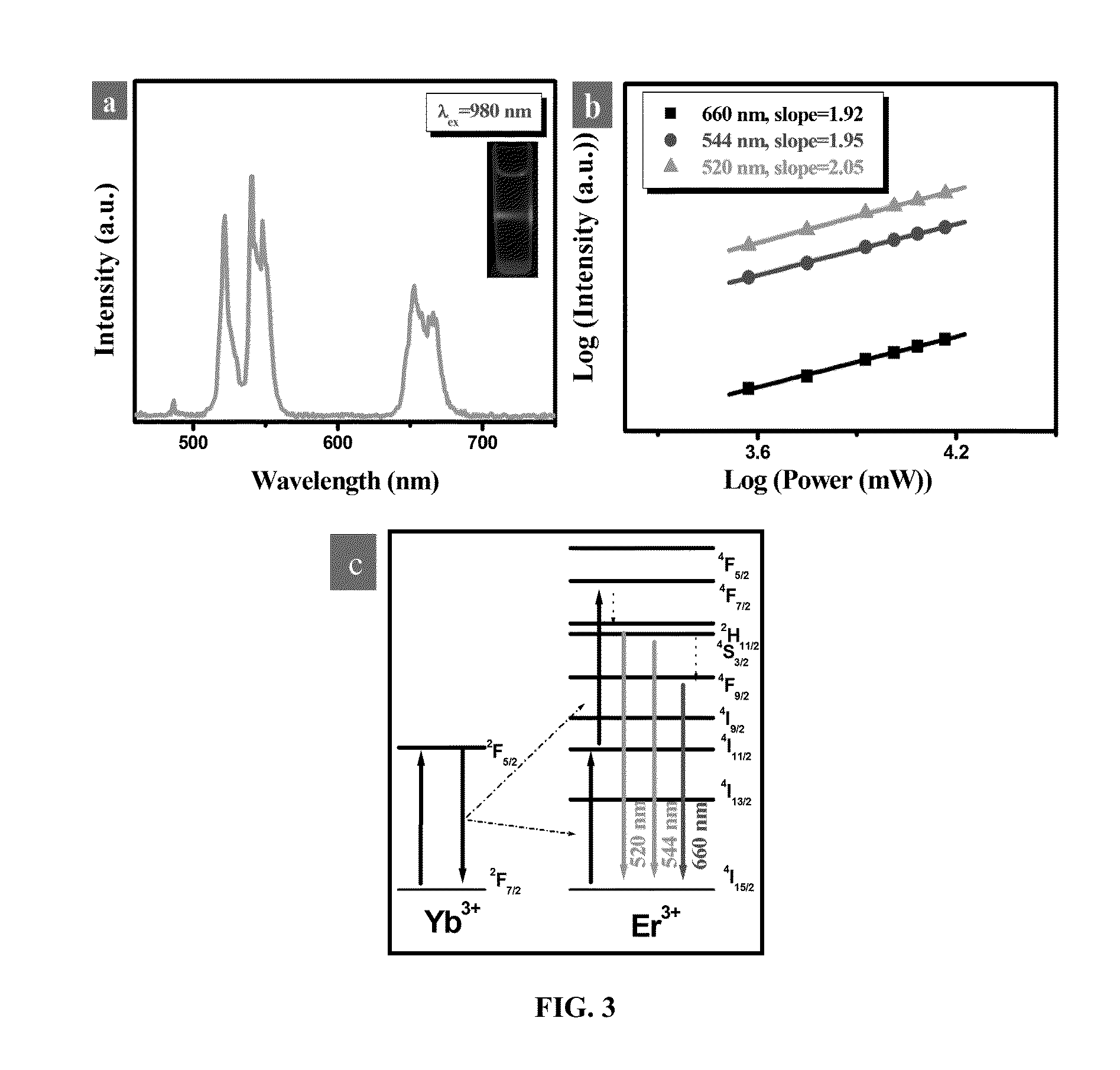
A bioprobe based on surface-modified single-phase BaGdF5:Yb / Er upconversion nanoparticles (UCNPs) for multi-modal bioimaging of fluorescent, magnetic resonance imaging (MRI) and computed X-ray tomography (CT) is disclosed herein. The modified UCNPs of the present invention are synthesized by a facile one-pot hydrothermal method with simultaneous surface modification of the nanoparticles. The surface-modified UCNPs of the present invention are useful in a variety of biomedical application fields due to their advantages in in vitro and in vivo multi-modal bioimaging such as small particle size up to 15 nm, substantially free of autofluorescence, low cytotoxicity, capable of being excited at near-infrared (NIR) wavelength, ability to deep cell penetration, long-lasting signal and long circulation time in vivo, different X-ray absorption coefficients at different photon energy levels between Ba and Gd, large magnetic moment, etc.
Owner:THE HONG KONG POLYTECHNIC UNIV
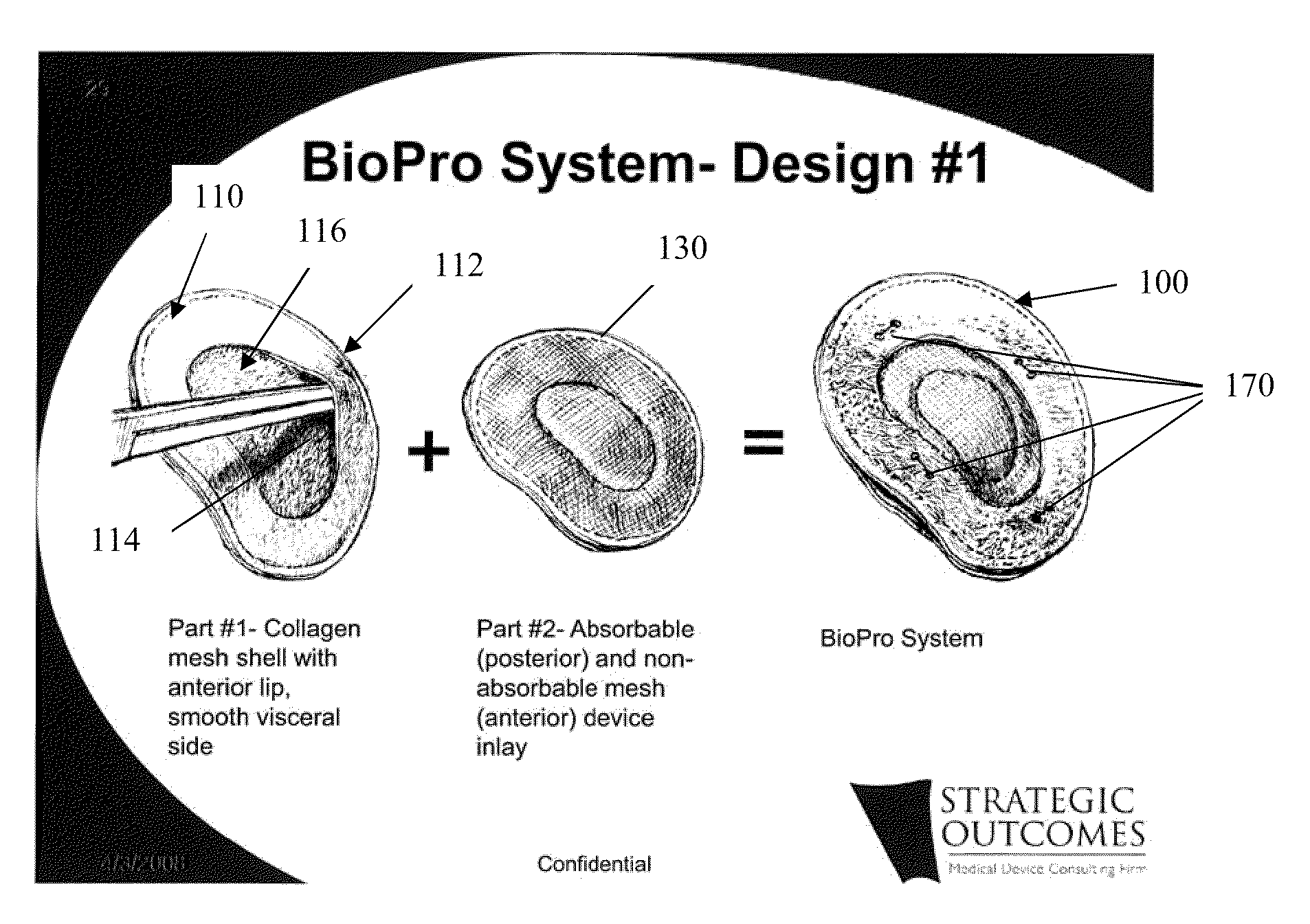
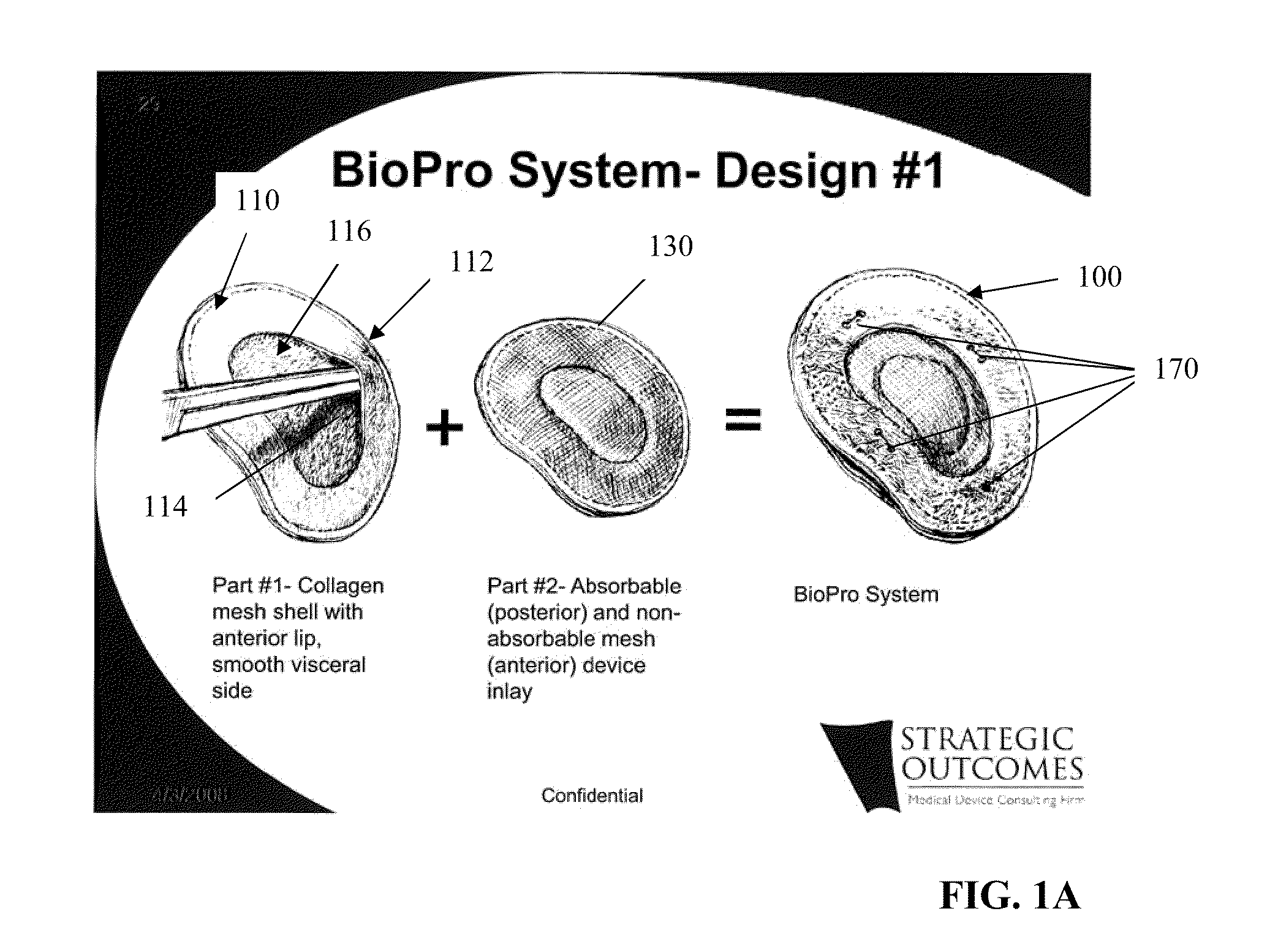
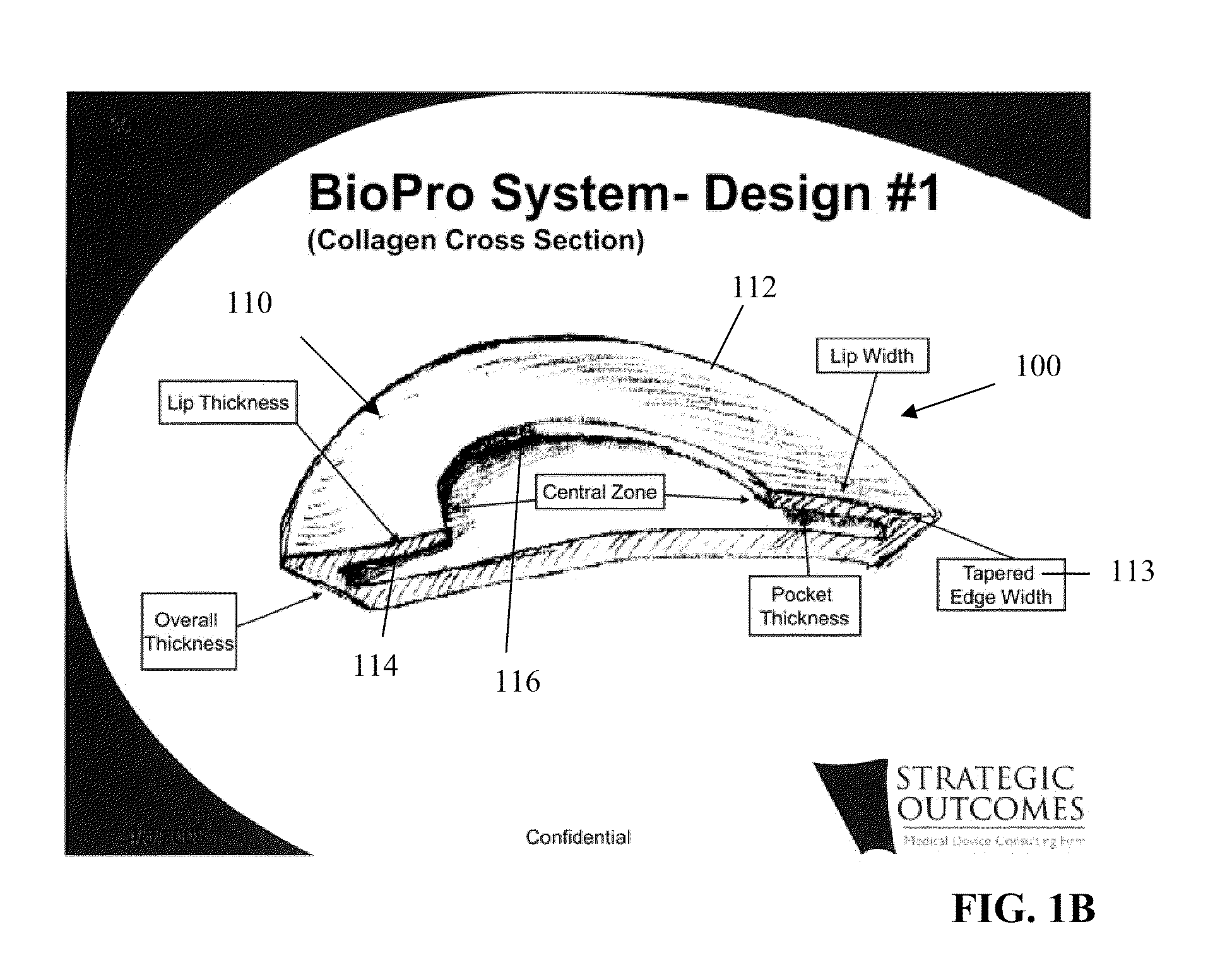
Tissue Reconstruction Devices and Methods
InactiveUS20090312843A1Reliable in-growthReduce chanceLaminationLamination apparatusPolypropylene meshTissue reconstruction
The present invention provides an implantable prosthesis comprising a viscera separating barrier that defines a shell having a pocket with an opening that receives an insert. It is contemplated that the insert comprises at least one of an absorbable mesh, a non-absorbable mesh, or an absorbable and non-absorbable mesh combination inserted into the pocket through the opening. In preferred embodiments the viscera separating barrier is acellular collagen, and the absorbable mesh is selected from at least one of a polyglactin, a polyglycolic acid, a polyglactin and a polylactic acid, and the non-absorbable mesh comprises a polypropylene mesh. It is contemplated that the viscera separating barrier acts to reduce or even eliminate attachment of viscera to the prosthesis to encourage rapid cell penetration and revascularization; the non-absorbable mesh will provide immediate, reliable in-growth; and the absorbable mesh will give stability to the prosthesis.
Owner:FORD STEVEN PALMER +1
Supercharged proteins for cell penetration
Compositions, systems and related methods for delivering a supercharged protein or a complex of a supercharged protein and therapeutic agent (e g, nucleic acid, peptide, small molecule) to cells are disclosed. Superpositively charged proteins may be associated with nucleic acids (which typically have a net negative charge) via electrostatic interactions. The systems and methods may involve altering the primary sequence of a protein in order to ''supercharge'' the protein (e g, to generate a superpositively-charged protein). The compositions may be used to treat proliferative diseases, infectious diseases, cardiovascular diseases, inborn errors in metabolism, genetic diseases, etc.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Full-automatic determining instrument of red blood cell osmotic fragility
ActiveCN103033482AFully automatedThe detection process is fastColor/spectral properties measurementsData displayPeristaltic pump
The invention discloses a full-automatic determining instrument of red blood cell osmotic fragility. The full-automatic determining instrument is based on the principle of light-proof turbidimetry and consists of a sample absorbing device, an automatic electromechanical device, a light detector and a circuit system, wherein the sample absorbing device is used for absorbing a blood sample through the instrument; the automatic electromechanical device is used for accomplishing various control actions in the automatic measurement of the instrument; the light detector is used for radiating reaction liquid to generate a permeable light signal; the circuit system is used for controlling various stepper motors, electromagnetic valves and peristaltic pumps, converting the light signal into an electric signal, amplifying the electric signal and converting the electric signal into a digital signal so as to carry out data processing analysis, data display and communication between the instrument and a PC (Personal Computer). The full-automatic determining instrument is convenient to operate, rapid in measuring speed, high in precision and good in repeatability, and the information of the red blood cell osmotic fragility of the blood sample can be obtained through full-automatic detection.
Owner:广东华赢医疗科技有限公司
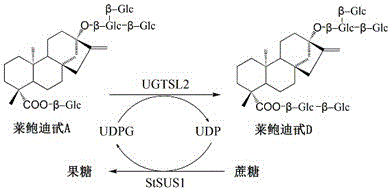
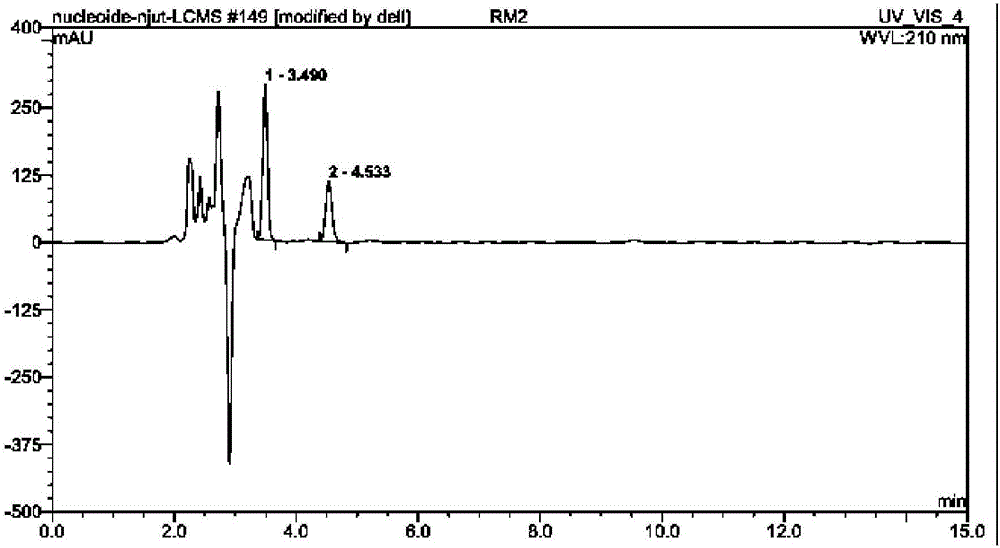
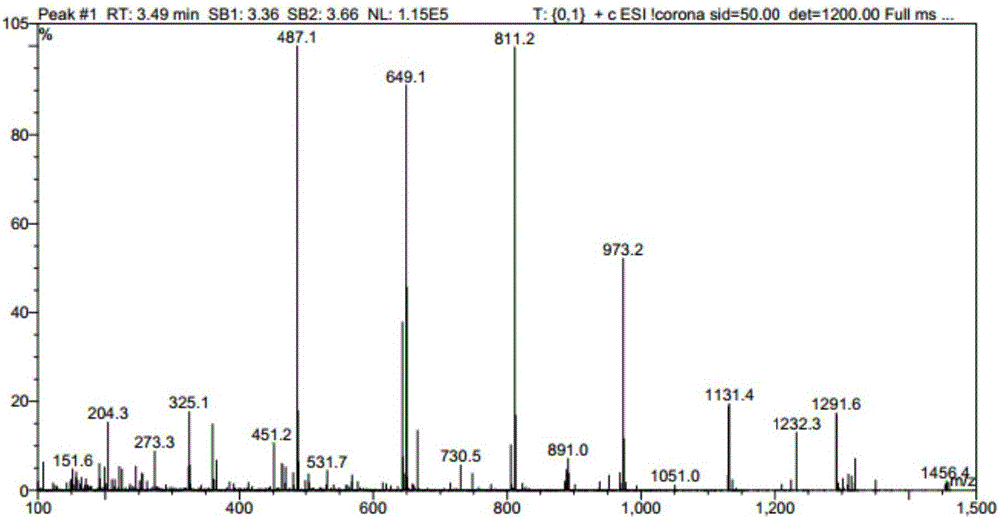
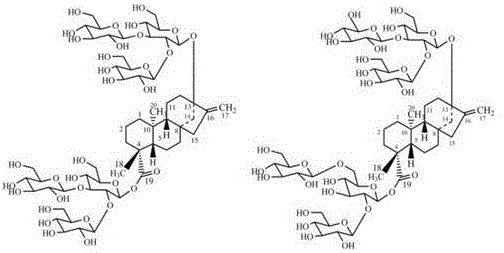
Recombinant bacterium and application of recombinant bacterium to generation of rebaudioside D by catalyzing rebaudioside A
ActiveCN106754595AAvoid separation and purificationHigh yieldBacteriaMicroorganism based processesSucrose synthetaseRebaudioside D
The invention discloses a recombinant bacterium and application of the recombinant bacterium to the generation of rebaudioside D by catalyzing rebaudioside A. The recombinant bacterium contains a tomato-derived glycosyltransferase UGTSL2 gene and a potato-derived sucrose synthase StSUS1 gene; the tomato-derived glycosyltransferase UGTSL2 gene is cloned between NdeI and XhoI sites of pRSFDuet-1 to construct a recombinant plasmid pRSFDuet-SL2; then the potato-derived sucrose synthase StSUS1 gene is cloned between NcoI and EcoRI sites of the pRSFDuet-SL2 to construct a recombinant plasmid pRSFDuet-SL2-SUS1; the recombinant plasmid pRSFDuet-SL2-SUS1 is transferred into a host cell to obtain the recombinant bacterium. After the recombinant bacterium is subjected to induction expression, the recombinant bacterium is added into a reaction mixture to catalyze the rebaudioside A to generate the rebaudioside D; in reaction, crude enzyme liquid obtained by crushing the recombinant bacterium is utilized and separation and purification of an enzyme are avoided; lyophilized powder does not need to be produced; UDP (Uridine Diphosphate) or UDP-glucose and any cell penetration agent or other chemical reagents do not need to be added into a reaction solution, so that the recombinant bacterium has a better environment-friendly property. The yield of the rebaudioside D can reach 10.8g / L.
Owner:XINGHUA GL STEVIA CO LTD
Thiadiazole compounds useful as inhibitors of H+/K+ atpase
Novel 3,5-disubstituted 1,2,4-thiadiazole compounds are provided, which are effective in treating peptic ulcers by the inhibition of H+ / K+-ATPase. The compounds of the present invention are 3,5-disubstituted 1,2,4-thiadiazole corresponding to the general formula (I): where R1 is a group with cell penetration properties for the inhibition of the enzyme in-vitro and in-vivo, and Y is a substituent that tunes the reactivity of the inhibitor towards the cysteine residue of H+ / K+-ATPase. The Y group may also serve in recognition.
Owner:APOTEX TECH INC
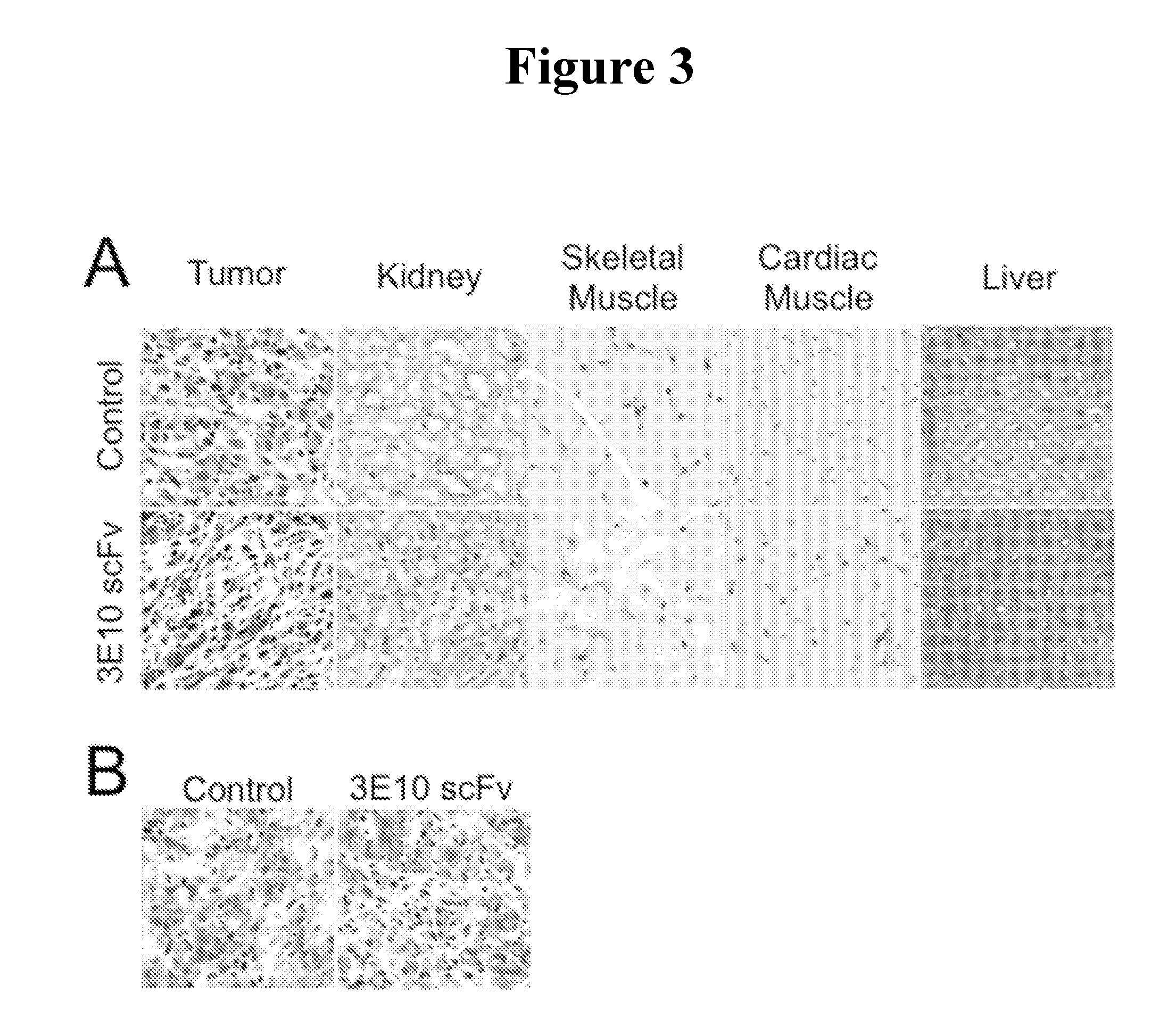
Methods for dna-dependent targeting of a cell permeant antibody
ActiveUS20160235859A1Peptide/protein ingredientsImmunoglobulins against animals/humansExtracellularCell penetration
The invention provides methods for selective targeting of live cells, which have undergone or are undergoing radiation or chemotherapy, at a site of interest with a cell-penetrating polypeptide. In one embodiment of the invention, the method comprises contacting the live cells with a cell-penetrating polypeptide comprising cell-penetrating determinants so that the cell-penetrating polypeptide binds extracellular DNA near or around the live cells so as to form a complex or association therewith such that the complex or associated polypeptide-DNA so bound bind the live cells and penetrates the live cells thereby selectively targeting live cells at a site of interest with a cell-penetrating polypeptide.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Method for preparing rebaudioside M2 by catalyzing rebaudioside A through recombinant bacterium
ActiveCN106834389AAvoid separation and purificationHigh yieldBacteriaMicroorganism based processesSucrose synthetaseRebaudioside D
The invention discloses a recombinant bacterium and application of the recombinant bacterium to preparation of rebaudioside M2 by catalyzing rebaudioside A. The recombinant bacterium contains a tomato-based glycosyltransferase UGTSL2 gene and a potato-based sucrose synthase StSUS1 gene at the same time; the tomato-based glycosyltransferase UGTSL2 gene is cloned between NdeI and XhoI sites of pRSFDuet-1 and is constructed to obtain a recombinant plasmid pRSFDuet-SL2; then the potato-based sucrose synthase StSUS1 gene is cloned between NcoI and EcoRI sites of the pRSFDuet-SL2 and is constructed to obtain a recombinant plasmid pRSFDuet-SL2-SUS1; the recombinant plasmid pRSFDuet-SL2-SUS1 is transferred into a host cell to obtain the recombinant bacterium. After the recombinant bacterium is subjected to induced expression, crude enzyme liquid is taken and is added into a reaction mixture to catalyze the rebaudioside A to generate the rebaudioside M2; in a reaction process, the crude enzyme liquid obtained by crushing the recombinant bacterium is utilized and separation and purification of an enzyme are avoided; lyophilized powder is also not needed and substrates including rebaudioside D and UDP or UDP-glucose and any cell penetration agent or other chemical reagents do not need to be added, so that the environmental friendliness is better. The yield of the rebaudioside M2 reaches 11.09g / L.
Owner:NANJING UNIV OF TECH
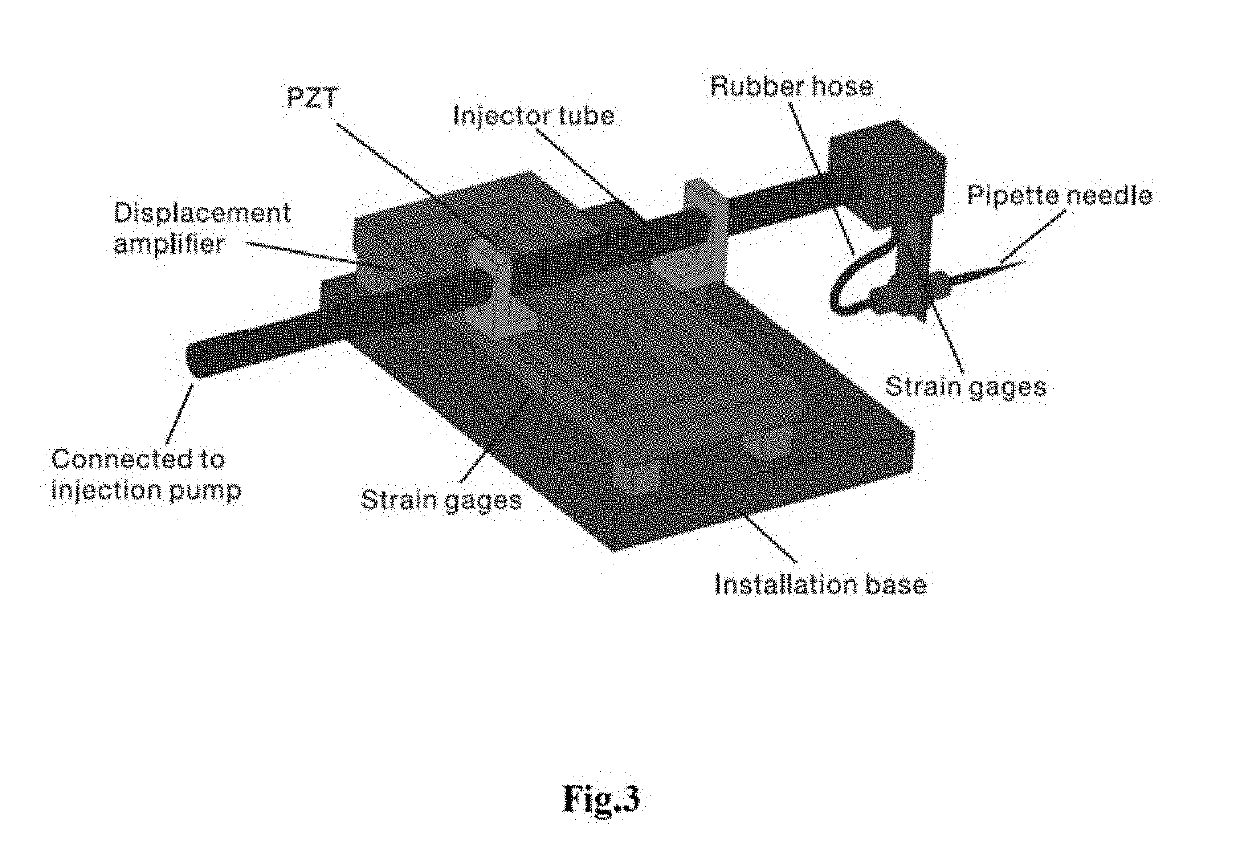
Cell microinjection system with force feedback
ActiveUS20190292567A1Low costEasy to installAnimal reproductionProgramme-controlled manipulatorElectricityEmbryo
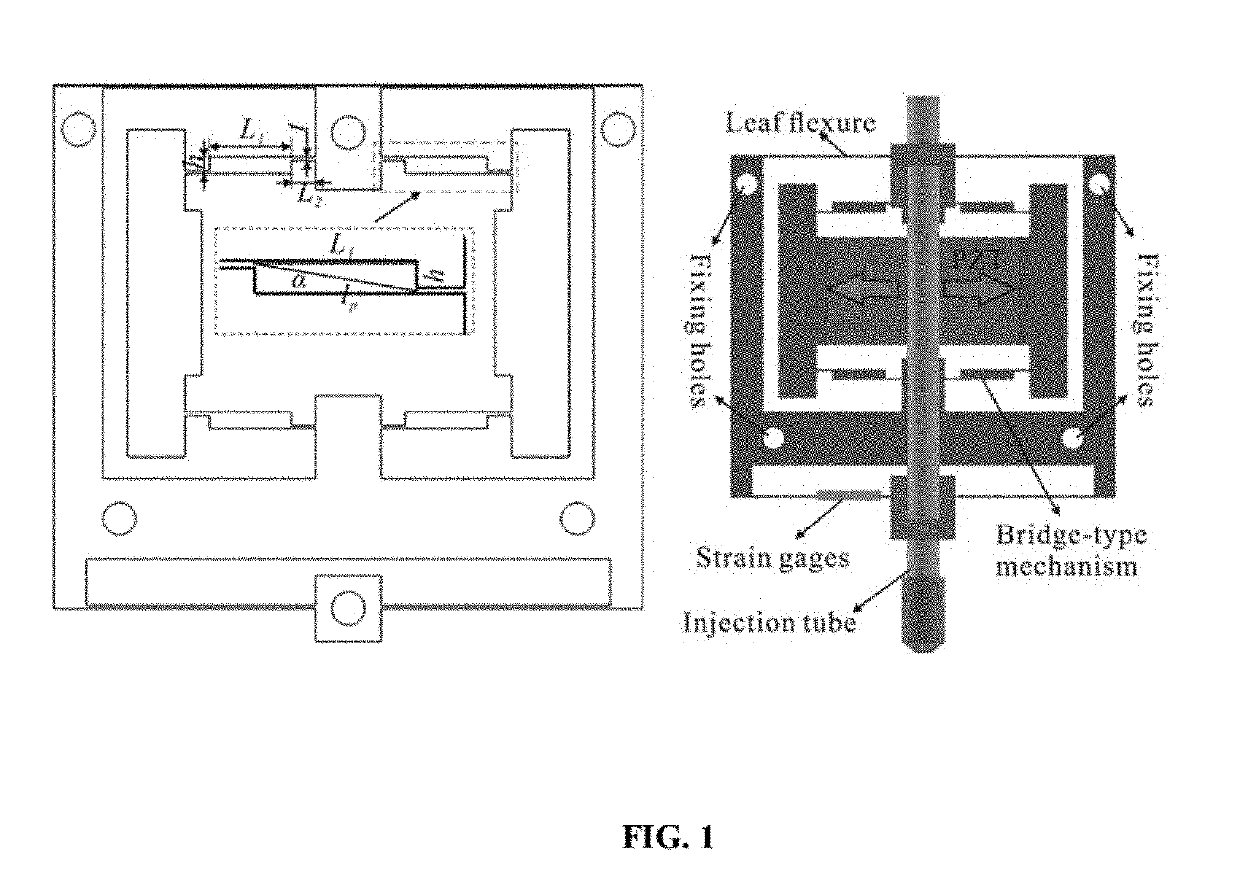
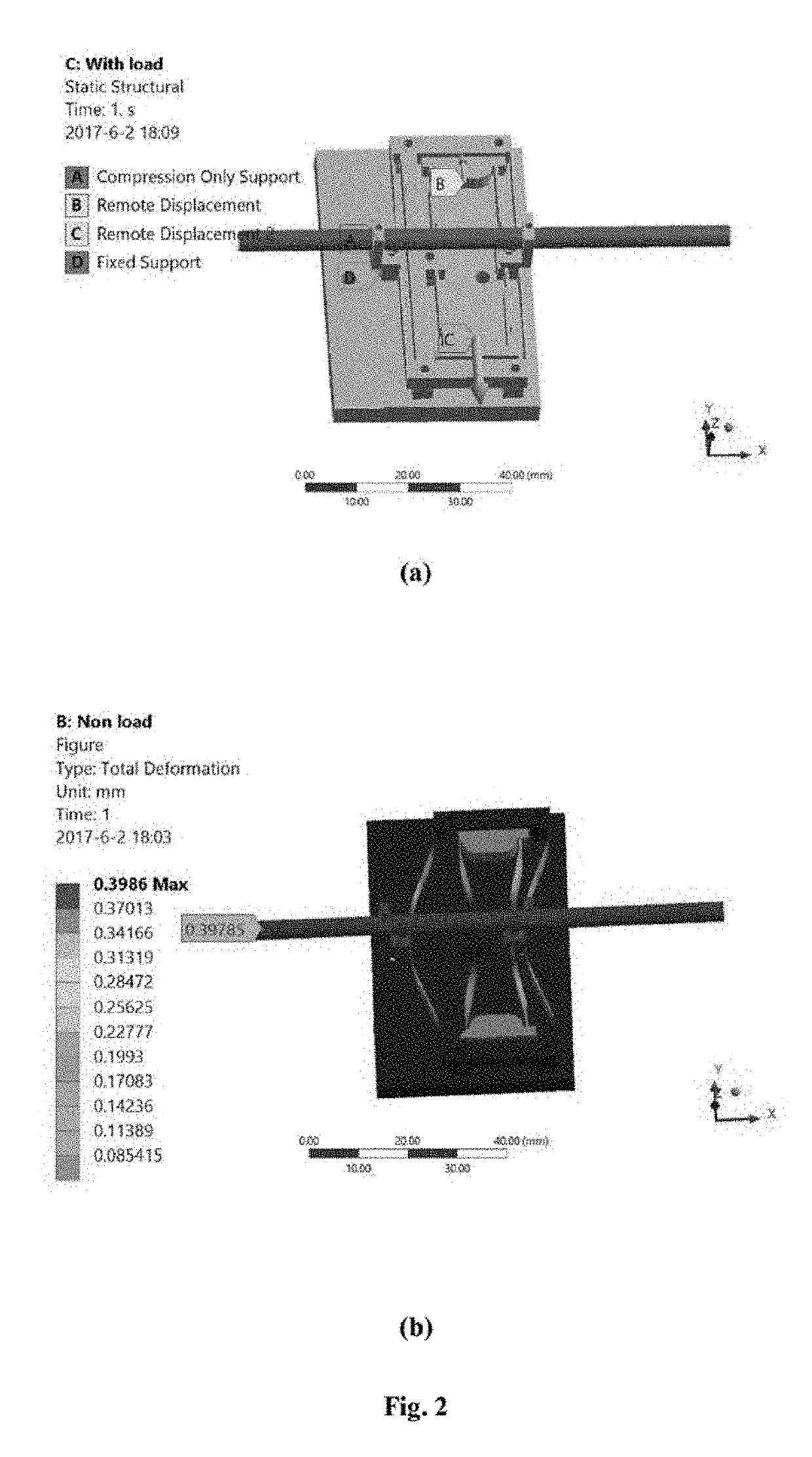
A novel piezo-driven cell injection system with force feedback overcomes the unsatisfied force interaction between the pipette needle and embryos in conventional position control. By integrating semiconductor strain-gage sensors for detecting the cell penetration force and the micropipette relative position in real time, the developed cell microinjection system features high operation speed, confident success rate, and high survival rate. The effectiveness of the developed cell injection system is experimentally verified by penetrating zebrafish embryos. The injection of 100 embryos are conducted with separate position control and force control. Results indicate that the force control enables a survival rate of 86%, which is higher than the survival rate of 82% produced by the position control in the same control environment. The experimental results quantitatively demonstrate the superiority of force control over conventional position control for the first time.
Owner:UNIVERSITY OF MACAU
Virus coated nanoparticles and uses thereof
The present invention discloses method to coat nanoparticles with viral envelope containing specific proteins. The present invention also discloses that such viral envelope coated nanoparticles can be targeted to specific cells and cellular entry pathway, thereby permitting their use as vaccines, in targeted delivery of therapeutic products and in the study of virus adsorption, cell penetration and viral entry pathways.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Device for analysis of cellular motility
InactiveUS20160067710A1Easy to operateMinimal effortBioreactor/fermenter combinationsBiological substance pretreatmentsCellular motilityEngineering
A mesoscale fluidic system comprises a substrate having a sample chamber and an analysis chamber. The sample chamber comprises a cell permeable filter defining a sample application compartment and a conditioning medium compartment. The sample chamber has a sample inlet port in the sample application compartment. The analysis chamber has an entry port and an exit port. The conditioning medium compartment is in fluid communication with the entry port of the analysis chamber via a channel. The sample application compartment is below the cell permeable filter and the conditioning medium compartment is above the cell permeable filter. The mesoscale fluidic system is suited for analysing cellular motility in a sample. Also disclosed is a method of estimating the quantity of motile cells in a sample and a method of extracting motile cells from non-motile cells.
Owner:MOTILITYCOUNT
Polypeptide-antibody immune conjugate and preparation method thereof
InactiveCN106822863AGrowth inhibitionReserved functionPeptide/protein ingredientsPharmaceutical non-active ingredientsMelanomaCell membrane
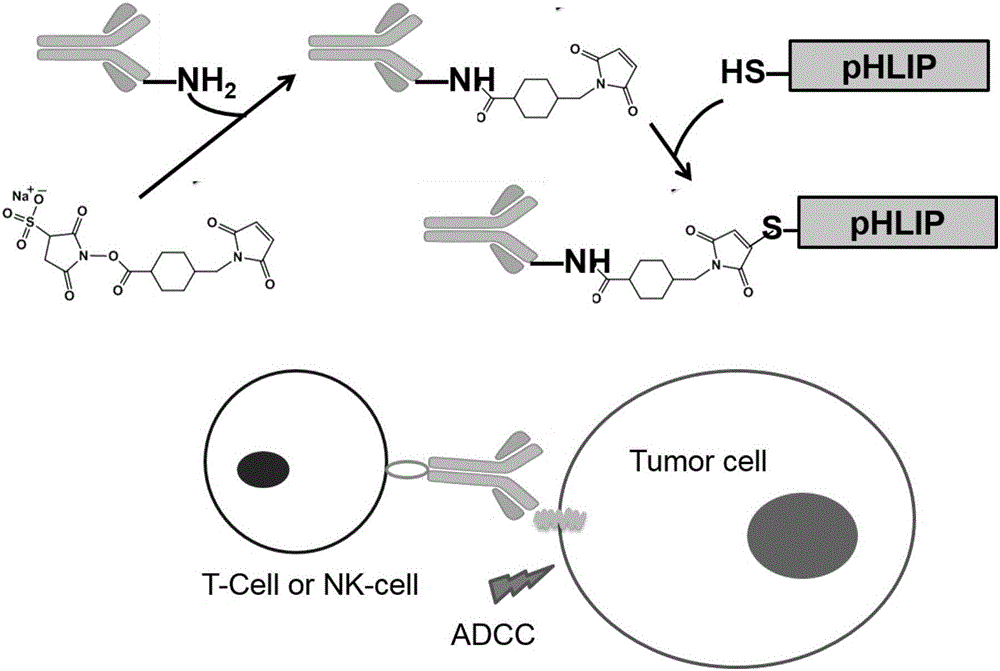
The invention provides a polypeptide-antibody immune conjugate and a preparation method thereof. The immune conjugate is formed by connecting a polypeptide and an antibody or an antibody FC segment through a covalent bond, wherein the polypeptide is pH-sensitive cell-penetrating peptide. According to the cell-penetrating peptide-antibody immune conjugate, the characteristic of pH response cell penetration of the cell-penetrating peptide is reserved, and the function of causing antibody dependent cell-mediated cytotoxin (ADCC) of the antibody is reserved. An in vitro experiment proves that acid response cell penetration can be achieved by the polypeptide-antibody immune conjugate and the protein of the antibody or the antibody Fc segment is anchored on a cell membrane to induce ADCC. An in vivo experiment proves that the polypeptide-antibody immune conjugate is capable of obviously inhibiting growth of murine melanoma and breast cancer and can be applied to immunotherapy of a clinical solid tumor.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
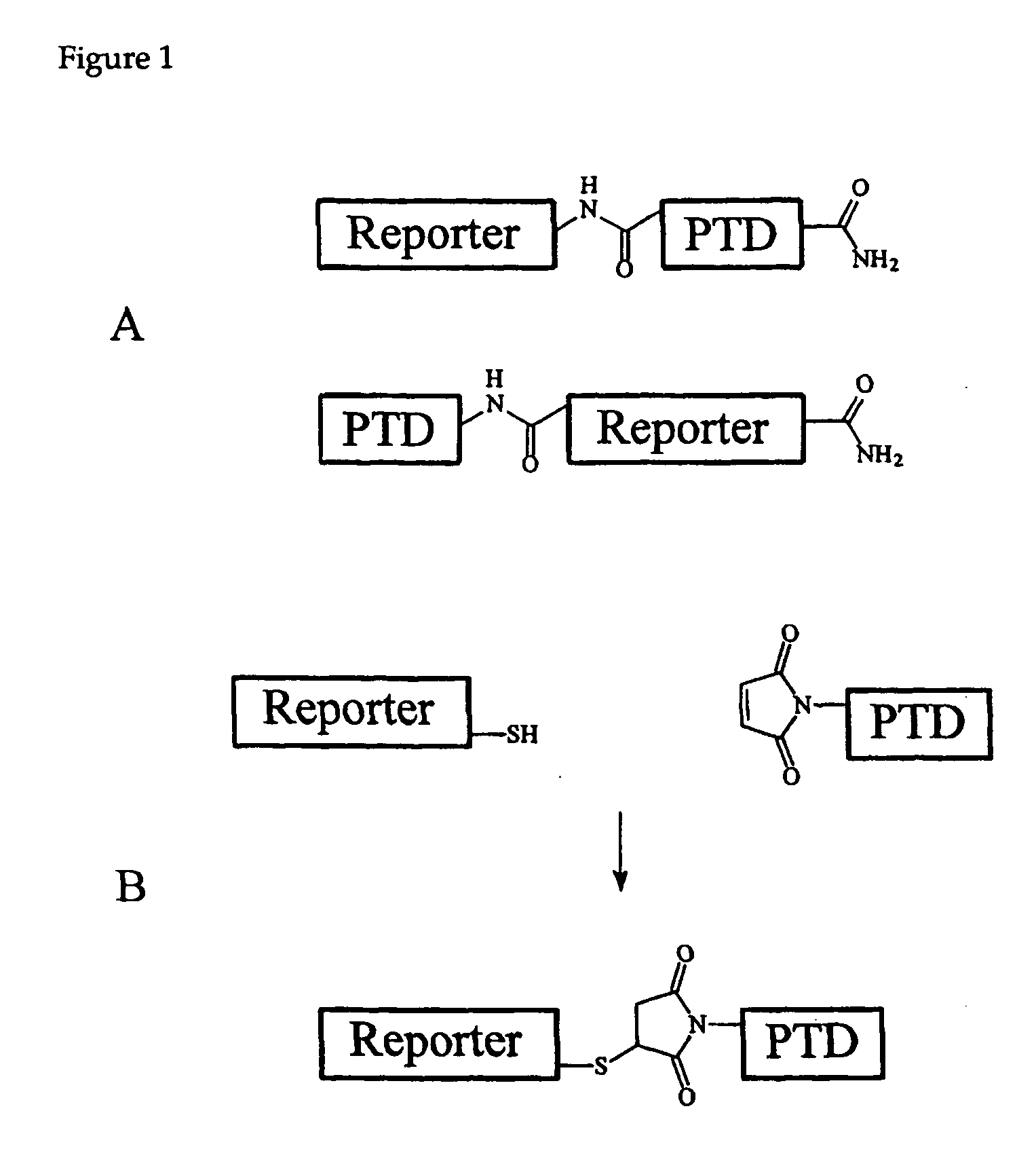
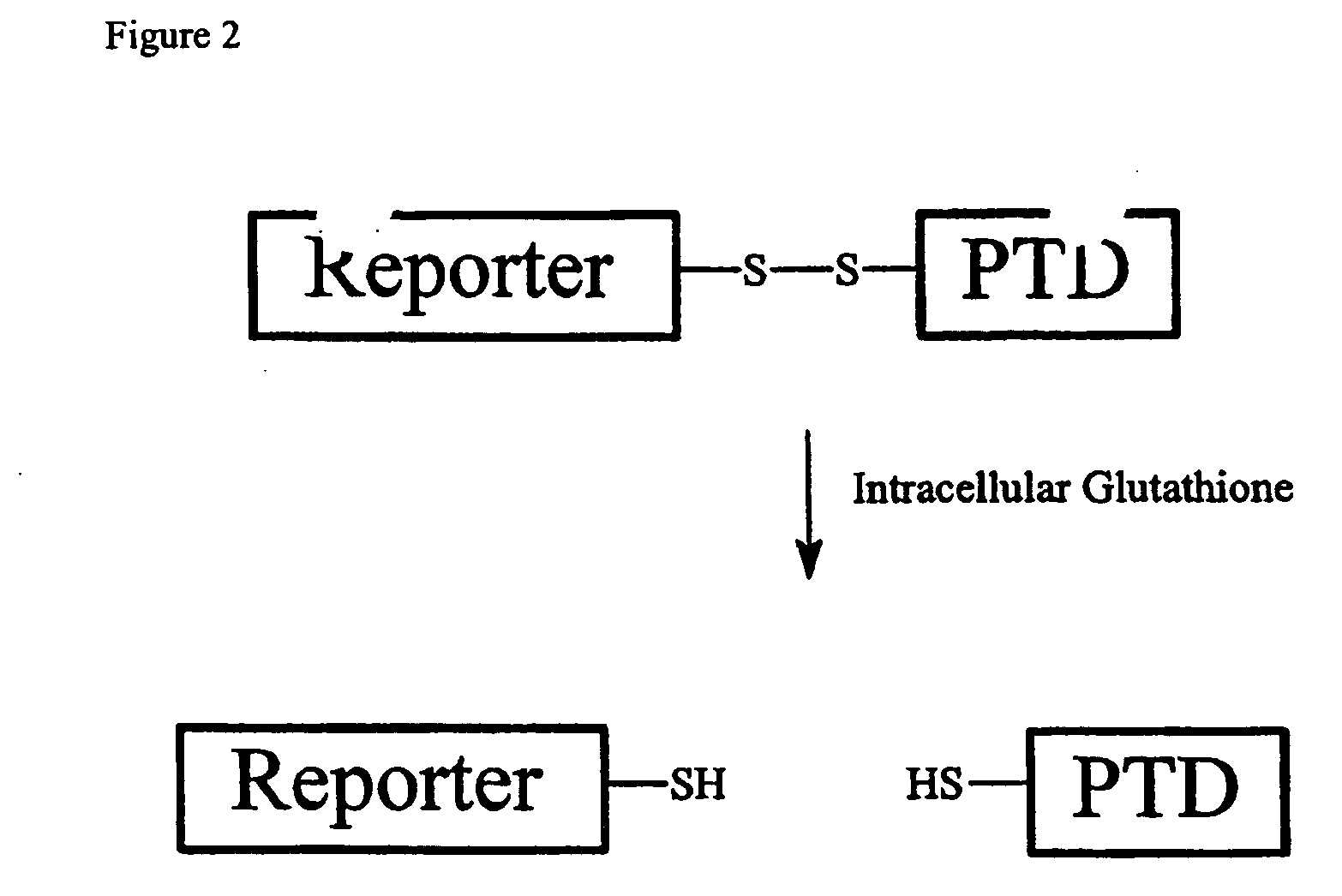
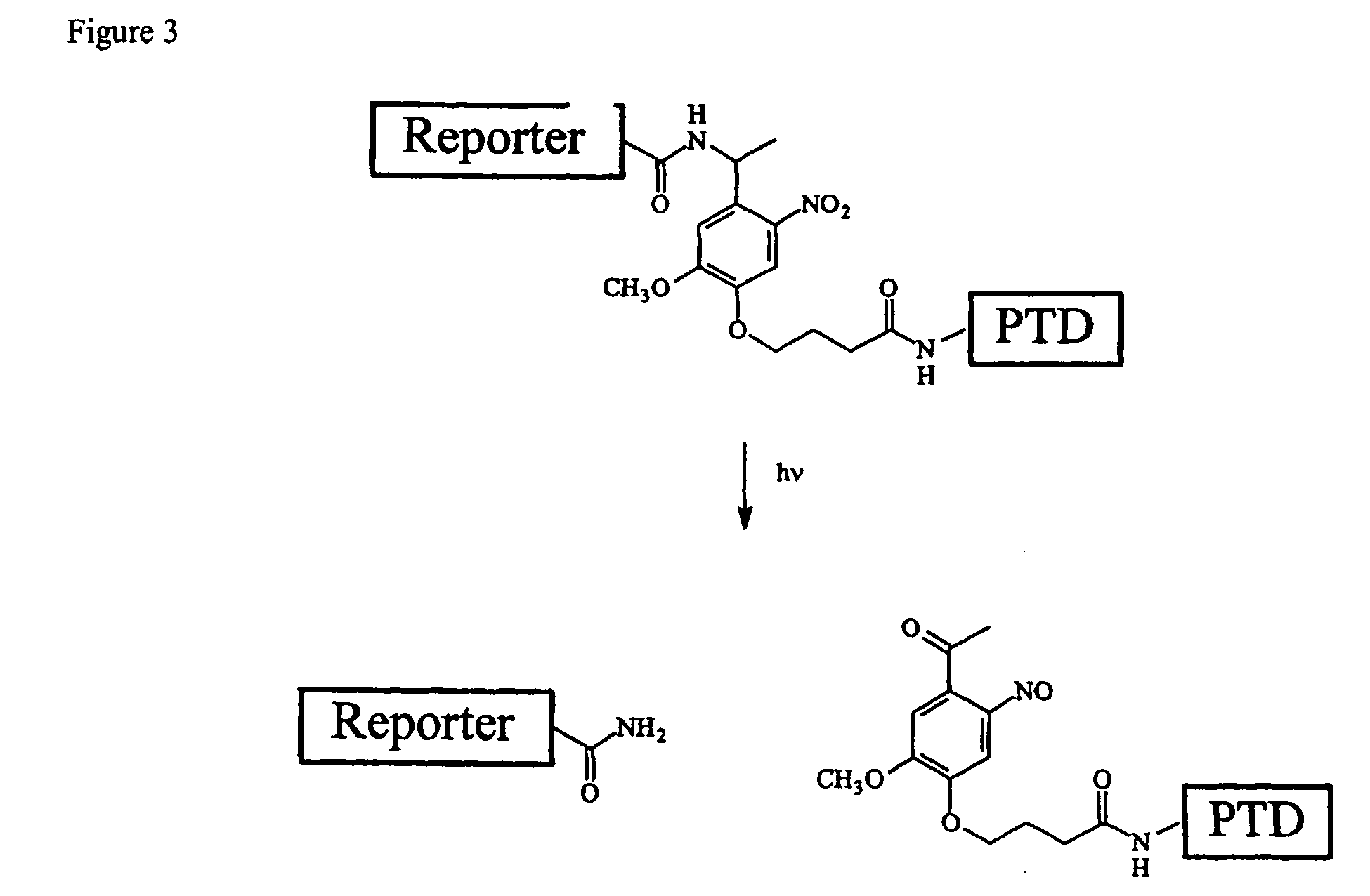
Cell-permeable enzyme activation reporter that can be loaded in a high throughput and gentle manner
InactiveUS20050282239A1Microbiological testing/measurementDepsipeptidesChemical reactionTarget peptide
A membrane traversing peptide conjugate comprises (i) a label, (ii) a target peptide, attached to the label, and (iii) a transduction domain, attached to the target peptide. The target peptide is a reactant for a chemical reaction occurring in a cell.
Owner:RGT UNIV OF CALIFORNIA
Nano composition with hair loss preventing, hair growing, hair fixing and hair blackening functions as well as preparation method and application thereof
PendingCN112516006ASingle anti-off mechanismPoor anti-shedding effectCosmetic preparationsHair cosmeticsCholesterolPolythylene glycol
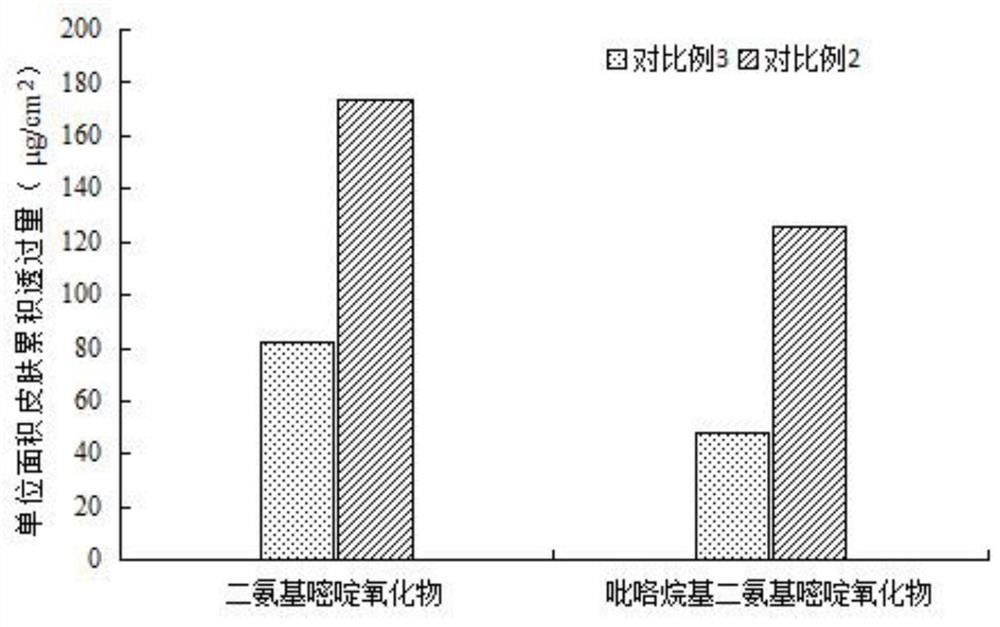
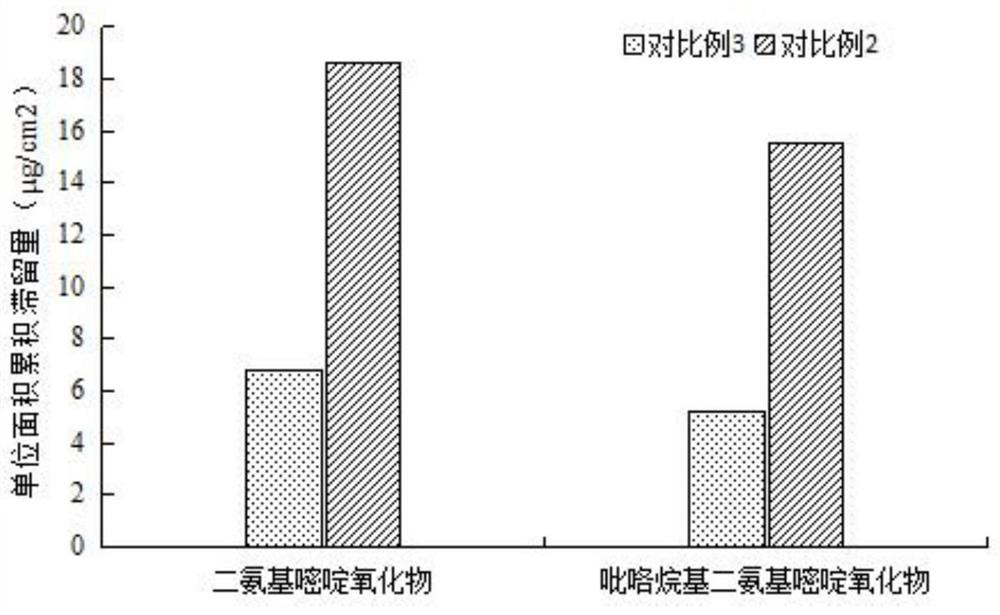
The invention provides a nano composition with hair loss preventing, hair growing, hair fixing and hair blackening functions as well as a preparation method and application thereof, and relates to thetechnical field of cosmetics with special functions. The nano composition comprises diaminopyrimidine oxide, pyrrolidinyl diaminopyrimidine oxide, hair growing peptide, hair fixing peptide, hair blackening peptide, a cell penetrating agent, vitamin E, polydiethylene glycol succinate, phospholipid, cholesterol, polyhydric alcohol and water. The nano composition is good in transdermal performance,improves the adsorption capacity of a preparation on scalp, deeply permeates into hair follicles, can be retained in the hair follicles for a long time, can be maintained at an effective concentrationfor a long time, improves the bioavailability, reduces the use amount of active ingredients, and enables low-content and anti-hair-loss active matter to play an excellent anti-hair-loss effect.
Owner:WUHAN BEST CARRIER NANO TECH
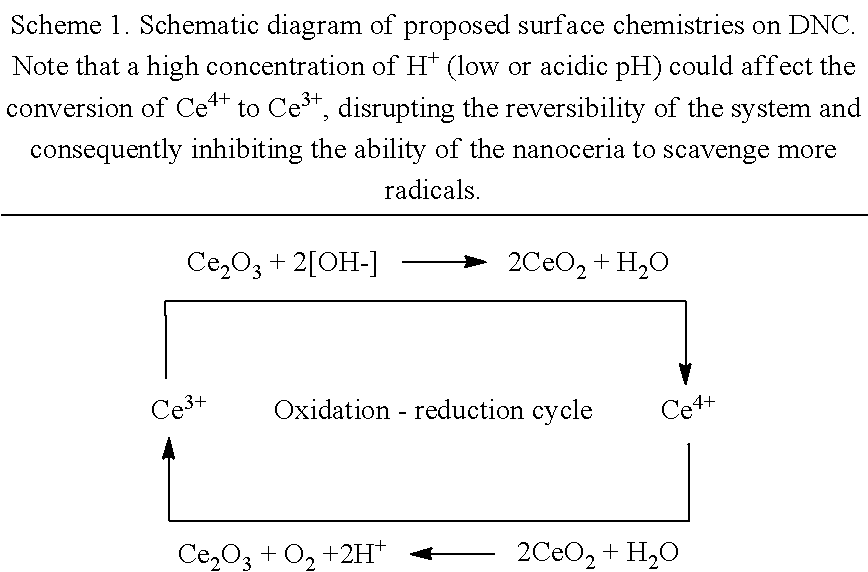
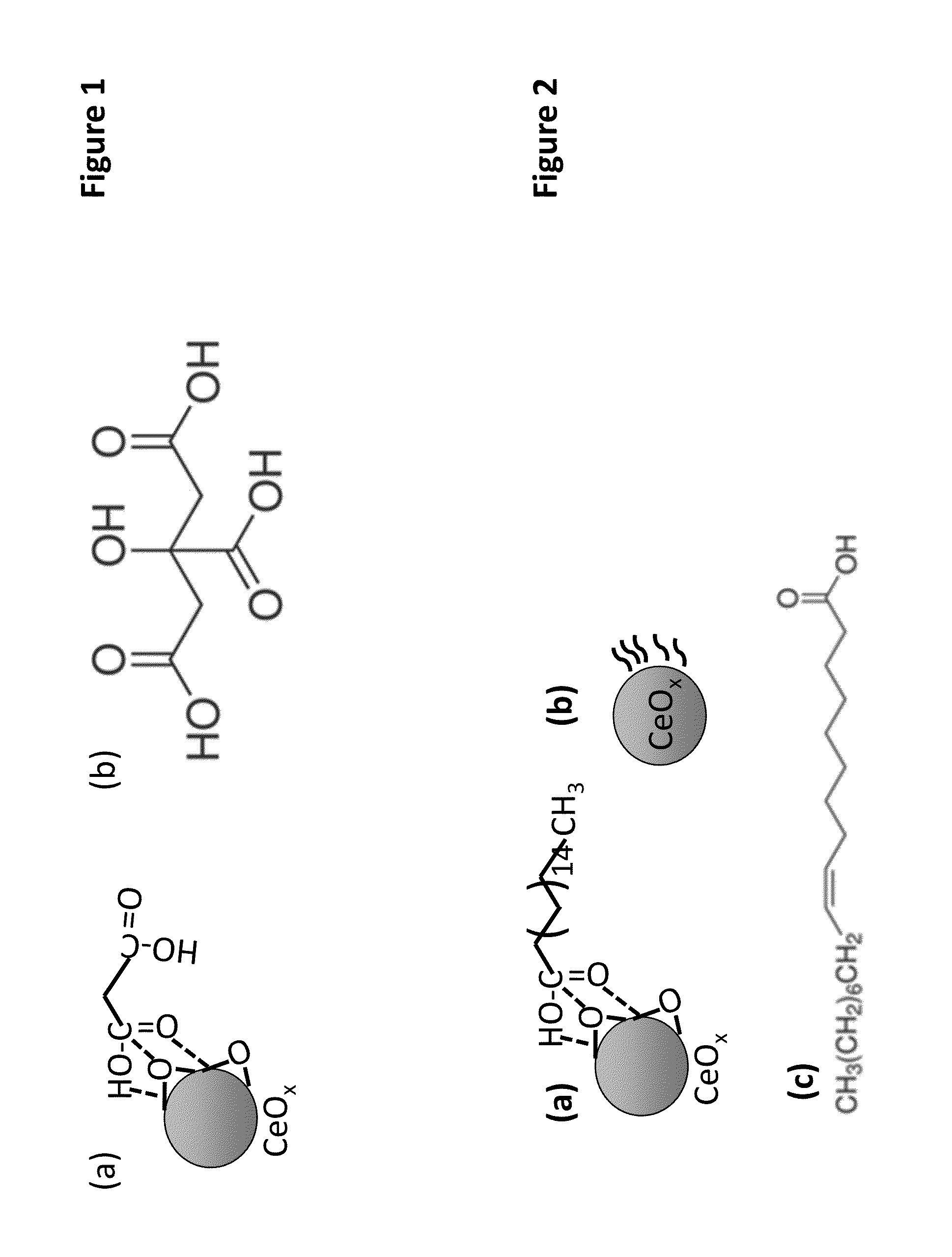
Method of enhancing the biodistribution and tissue targeting properties of therapeutic ceco2 particles via nano-encapsulation and coating
InactiveUS20140271899A1Enhancing their anti-oxidative activityMaximize biocompatibilityHeavy metal active ingredientsBiocideTissue targetingDisease
The present invention provides methods and liposomal compositions useful in therapeutics, and diagnosis, prognosis, testing, screening, treatment and / or prevention of various disease conditions. The present invention provides imaging methods for various conditions. The present invention is a multi-layered drug delivery pathway, inclusive of nanoparticle liposomal formulations and mechanisms of localized action via unzipping upon delivery to the affected tissue site. The nano-encapsulation methodology allows maximization of a potent antioxidant's biocompatibility, increased target cell penetration and uptake, reduced off-target effects and retention of high anti-oxidative activity for promising therapeutic potential.
Owner:PEROXYIUM INC DELAWARE C
Preparation method of alginate-hydrogel nanometer fiber scaffold
ActiveCN108159493AGood biocompatibilityGreat penetration depthMaterial nanotechnologyNanomedicineFiberFreeze-drying
The invention relates to a preparation method of an alginate-hydrogel nanometer fiber scaffold. The preparation method comprises following steps: a polycaprolactone solution is prepared; a sodium alginate solution is prepared; a double-nozzle electrostatic spinning system is adopted for electrostatic spinning so as to obtain polycaprolactone-sodium alginate nanometer fiber film; the polycaprolactone-sodium alginate nanometer fiber film is immersed in a calcium chloride solution, is washed fully with an ethanol aqueous solution, is subjected to freeze drying, is immersed and washed with chloroform, is washed with ethanol and distilled water successively, and is subjected to freeze drying so as to obtain the alginate-hydrogel nanometer fiber scaffold. The alginate-hydrogel nanometer fiber scaffold possesses a stable three dimensional structure and excellent biocompatibility, can be used for 3D cell culture, and is capable of optimizing cell penetration depth.
Owner:山东省日照市人民医院
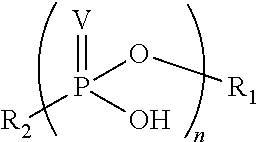
Phosponate linkers and their use to facilitate cellular retention of compounds
ActiveUS20190030171A1Facilitates intracellular retentionPhosphonate group is stableAntipyreticGroup 5/15 element organic compoundsCellular retentionCell permeability
Phosphonate linkers and their use for delivering compounds with passive cell permeability into a cell wherein the phosphonate group facilitates cellular retention of the compound are described.
Owner:MERCK SHARP & DOHME LLC
Cell Permeability Assay in a Living Array of Multiple Cell Types and Multiple Layers of a Porous Substrate
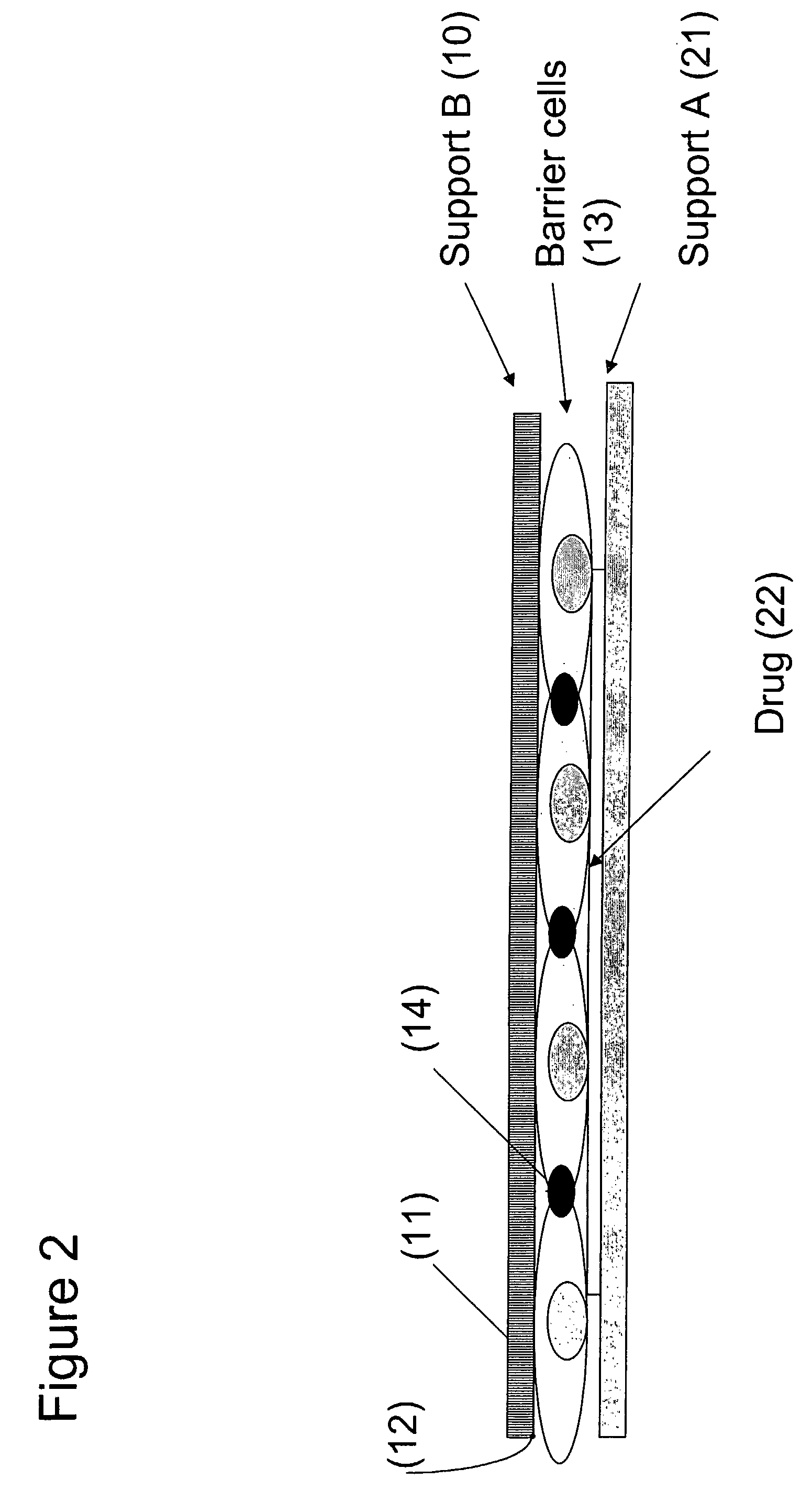
InactiveUS20090042215A1Efficacy of passing the barrier layer may be assessedSignal can be affectedBioreactor/fermenter combinationsBiological substance pretreatmentsPorous substrateCell type
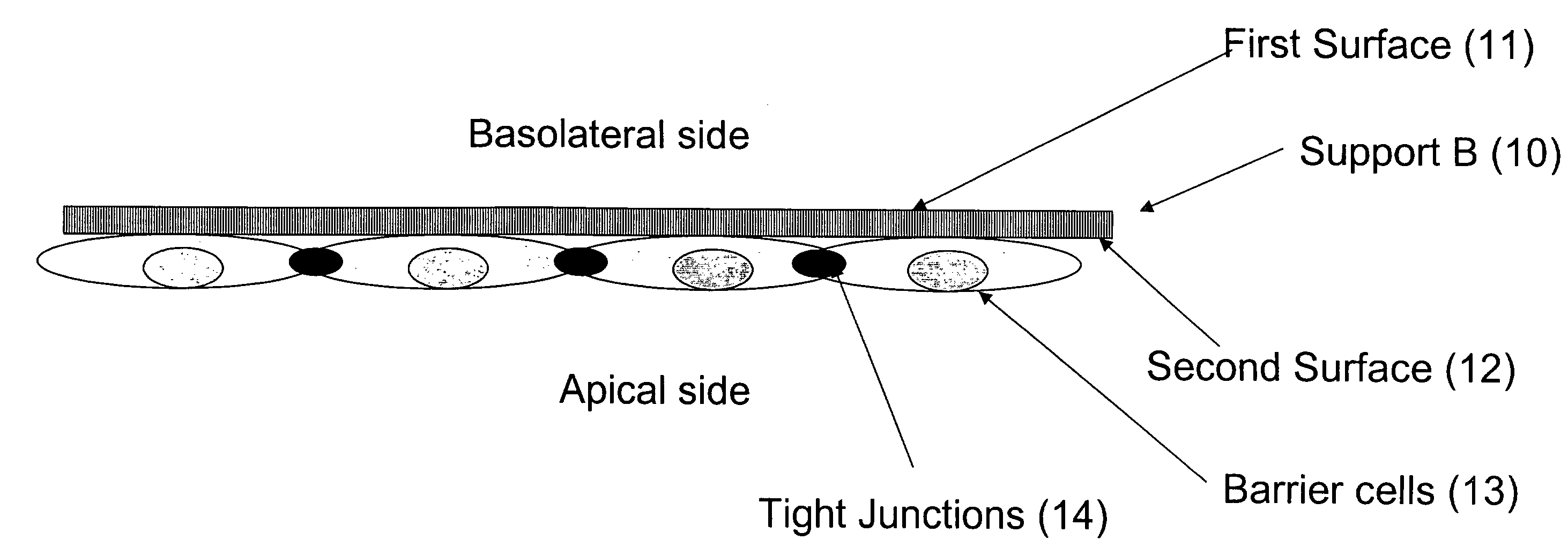
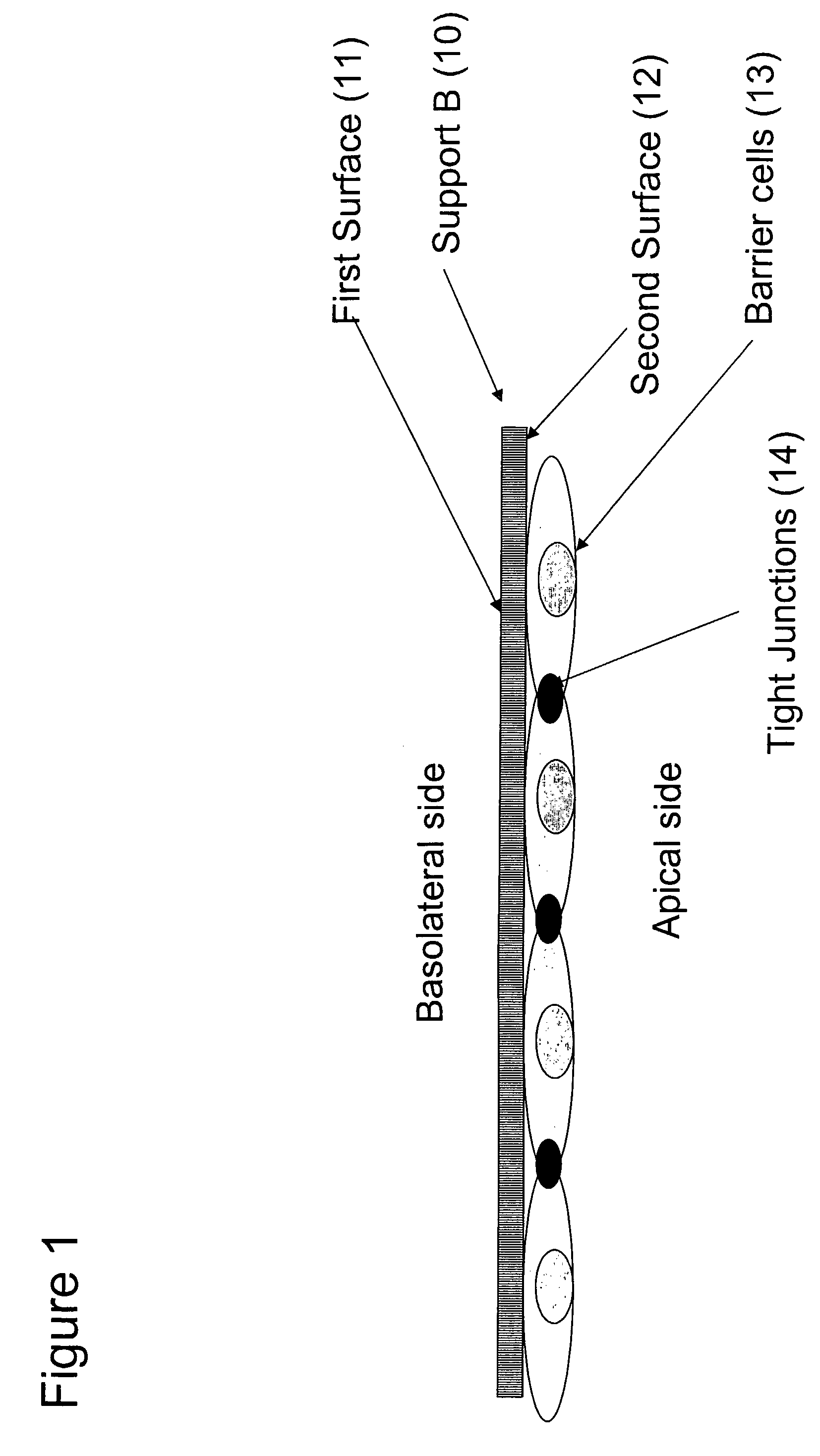
The present invention provides a device for determining drug efficacy on target cells comprising (1) a porous support (B) having first and second surfaces and at least one area with a plurality of through-going channels, wherein said area comprises on said first surface at least one region, which can be contacted with target cells, and said area comprises on said second surface at least one region comprising a layer of barrier cells presenting a significant barrier adhered to said second surface, wherein said region on the first surface is located opposite to said region on the second surface of the porous support (B), and wherein said layer of barrier cells provides a barrier for the passage of drugs, and (2) a support (A) comprising drugs, which can be contacted with the barrier cells of the porous support (B).
Owner:PAMGENE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com