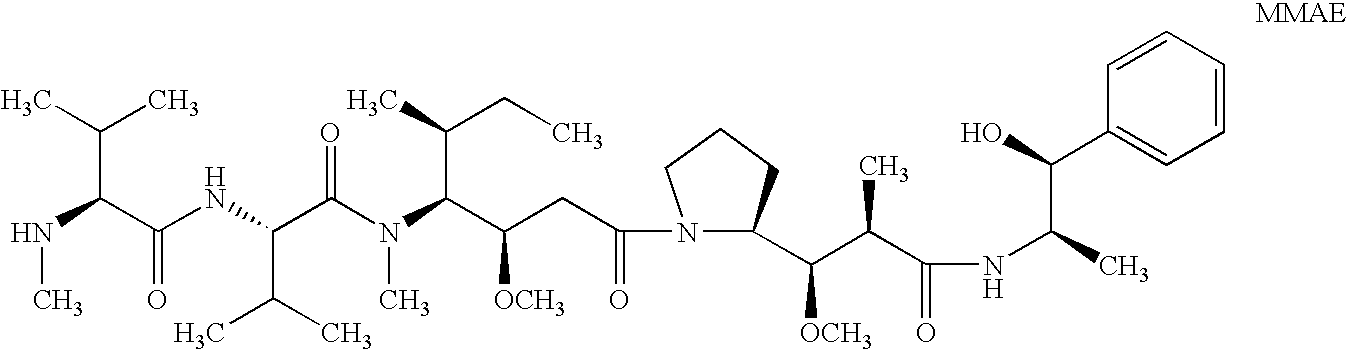
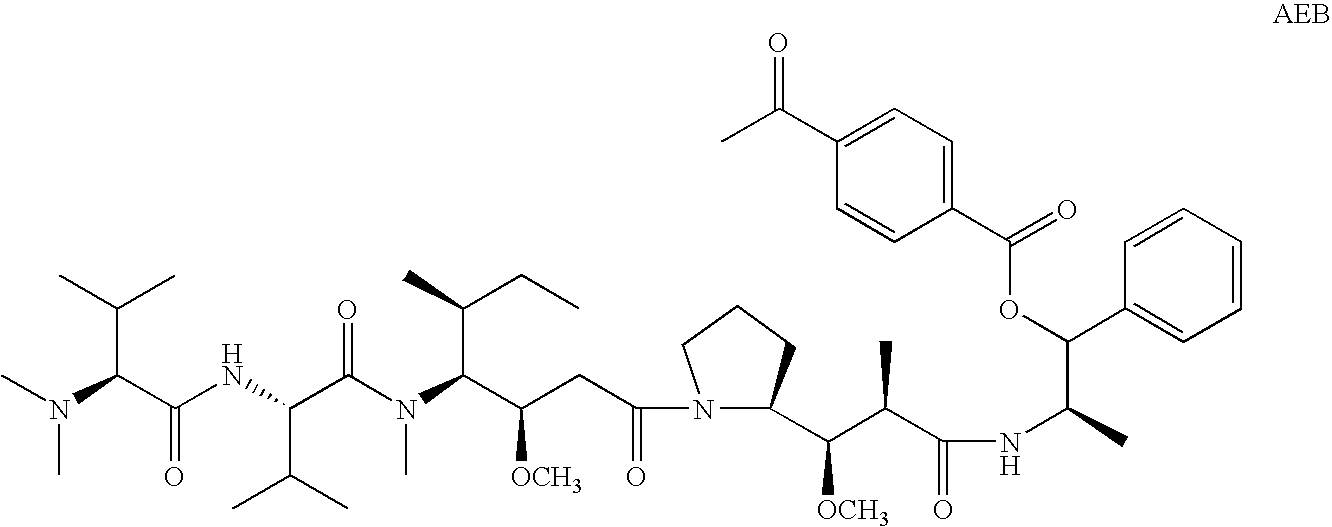
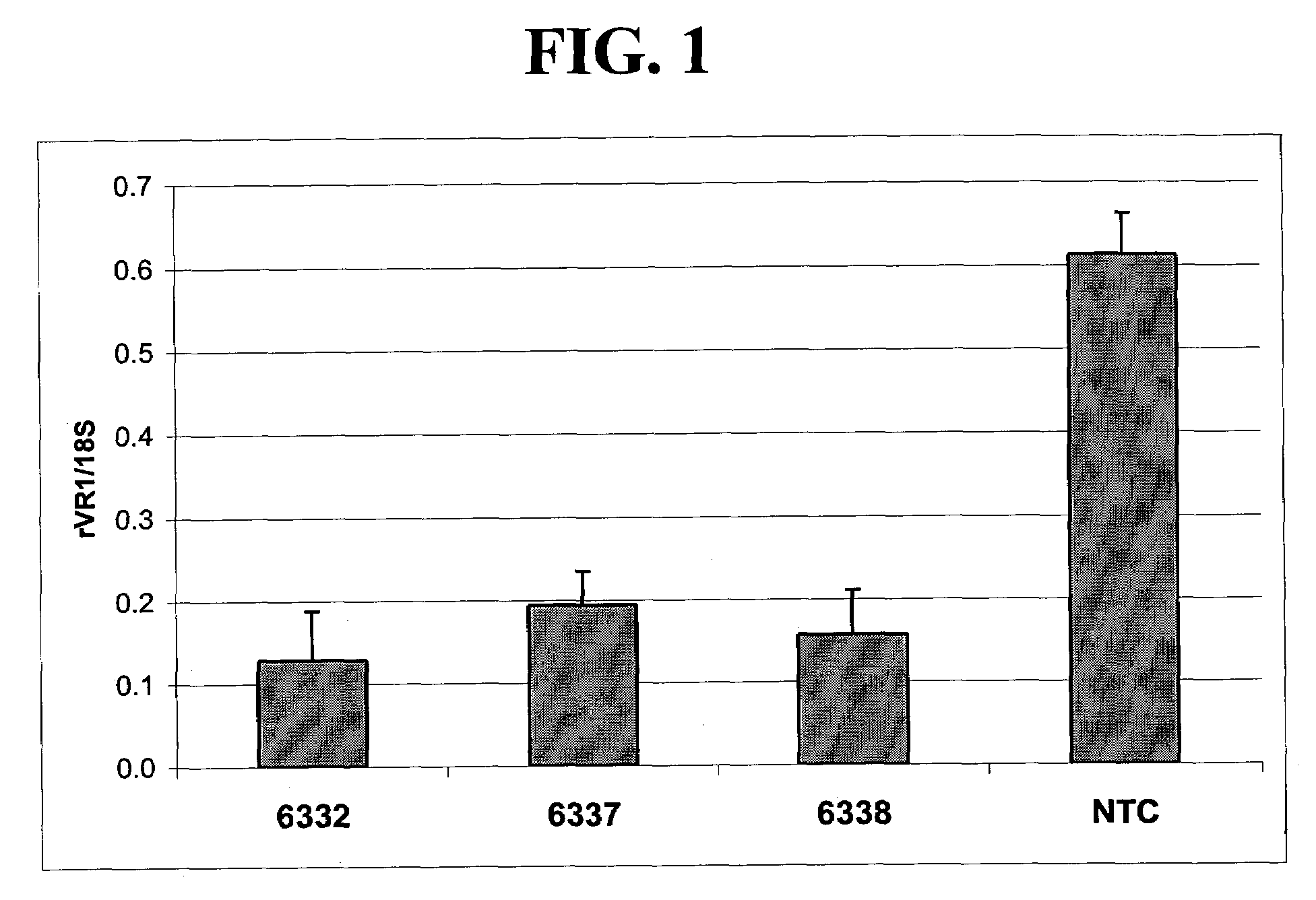
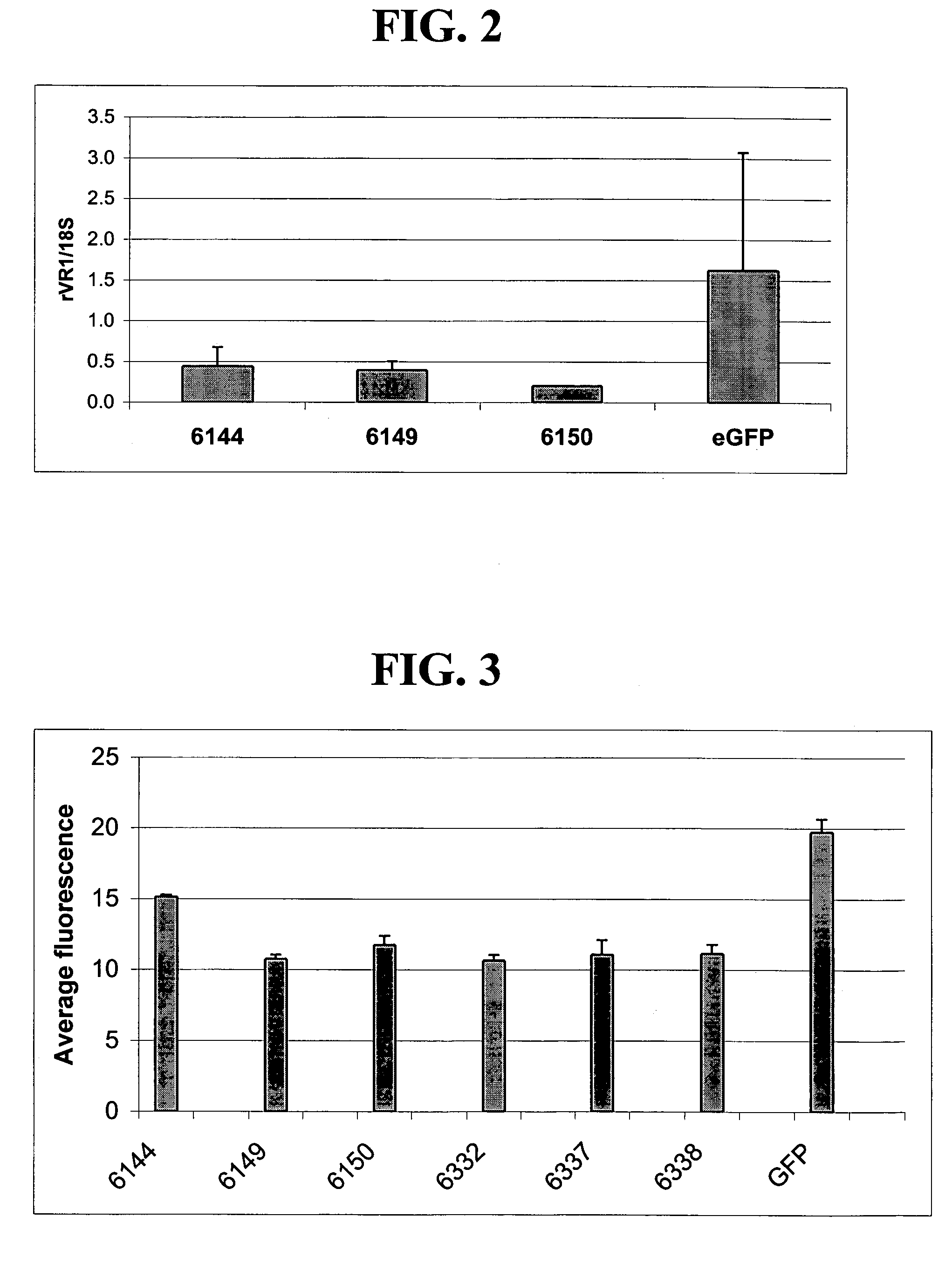
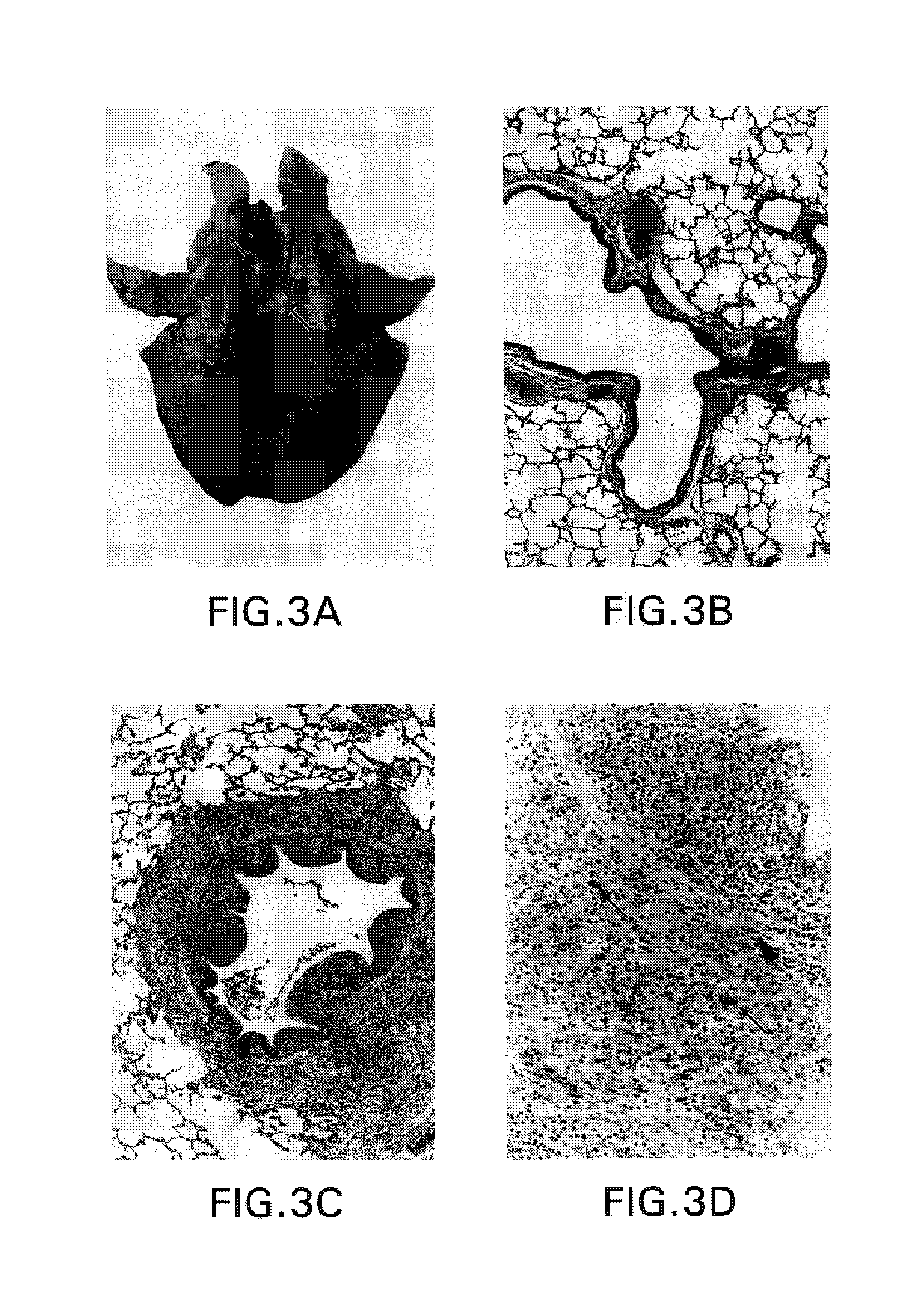
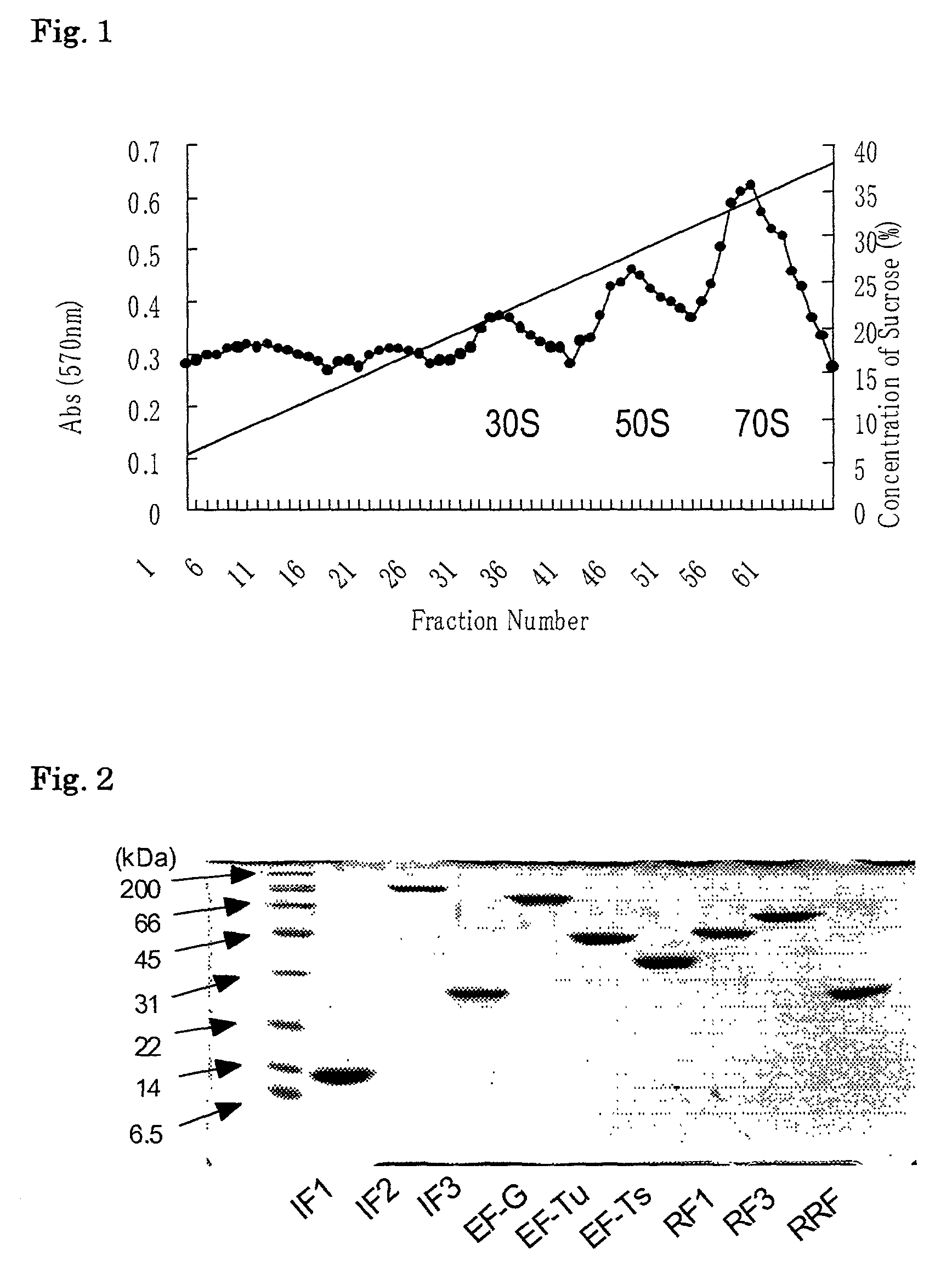
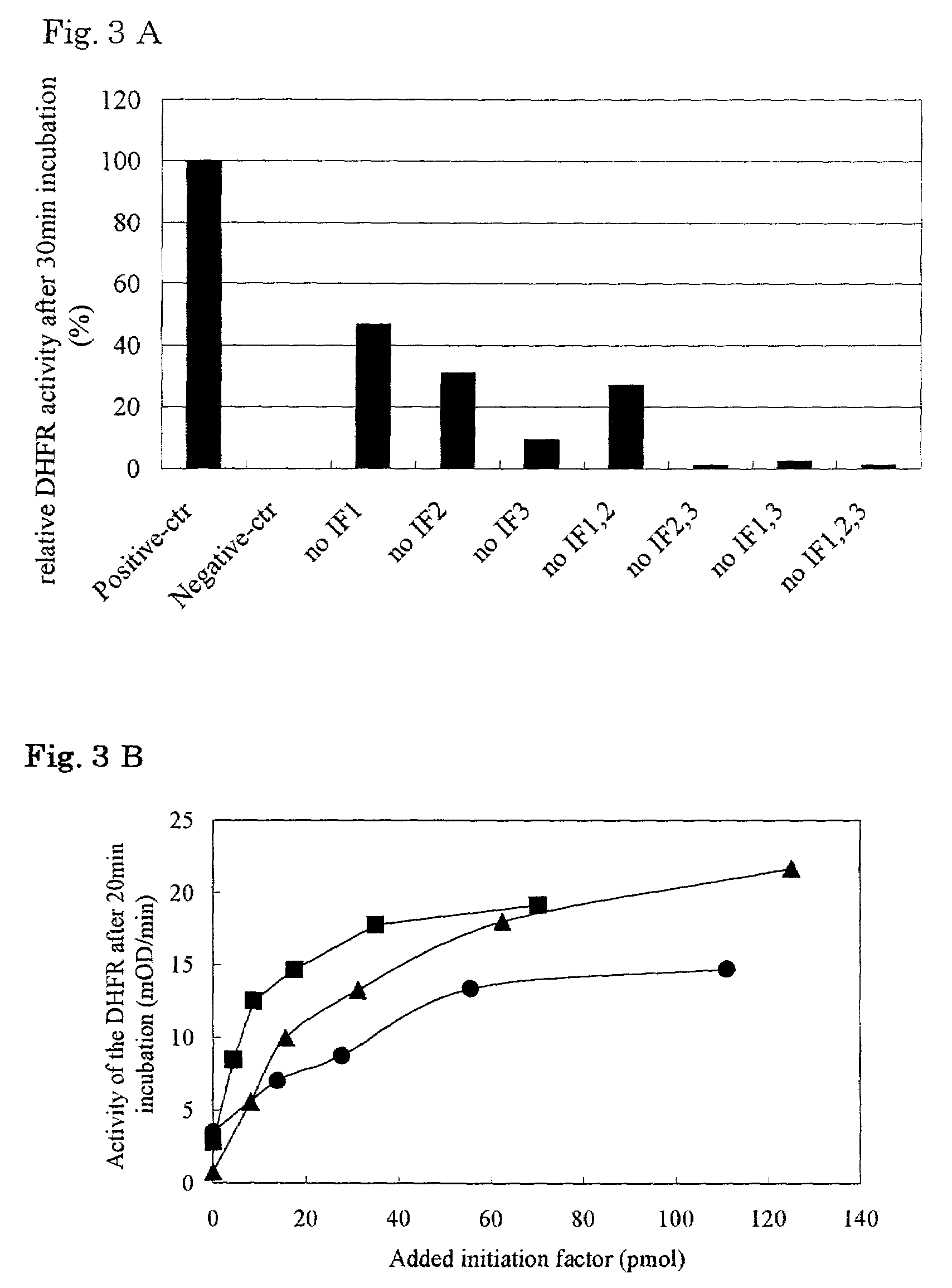
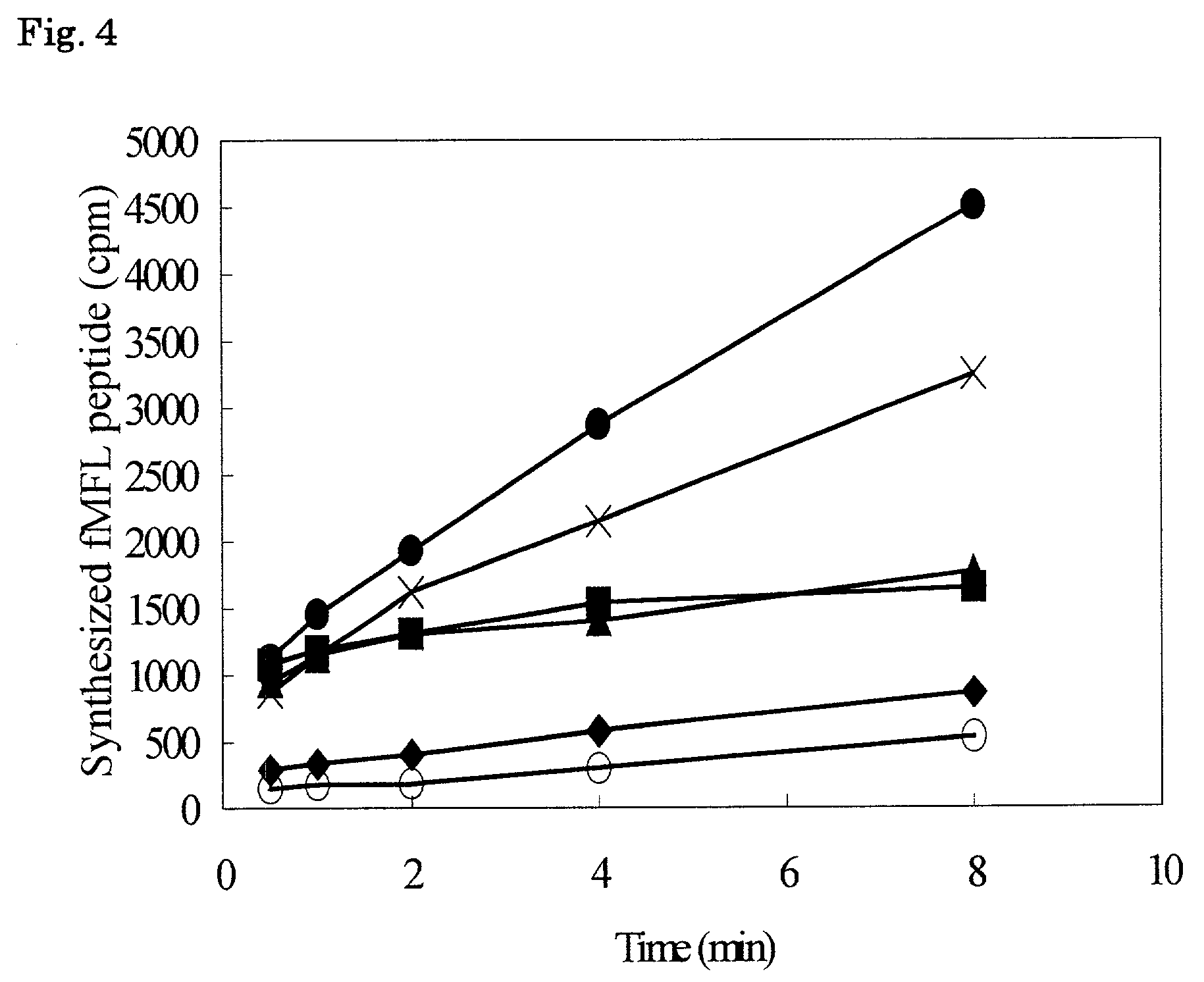
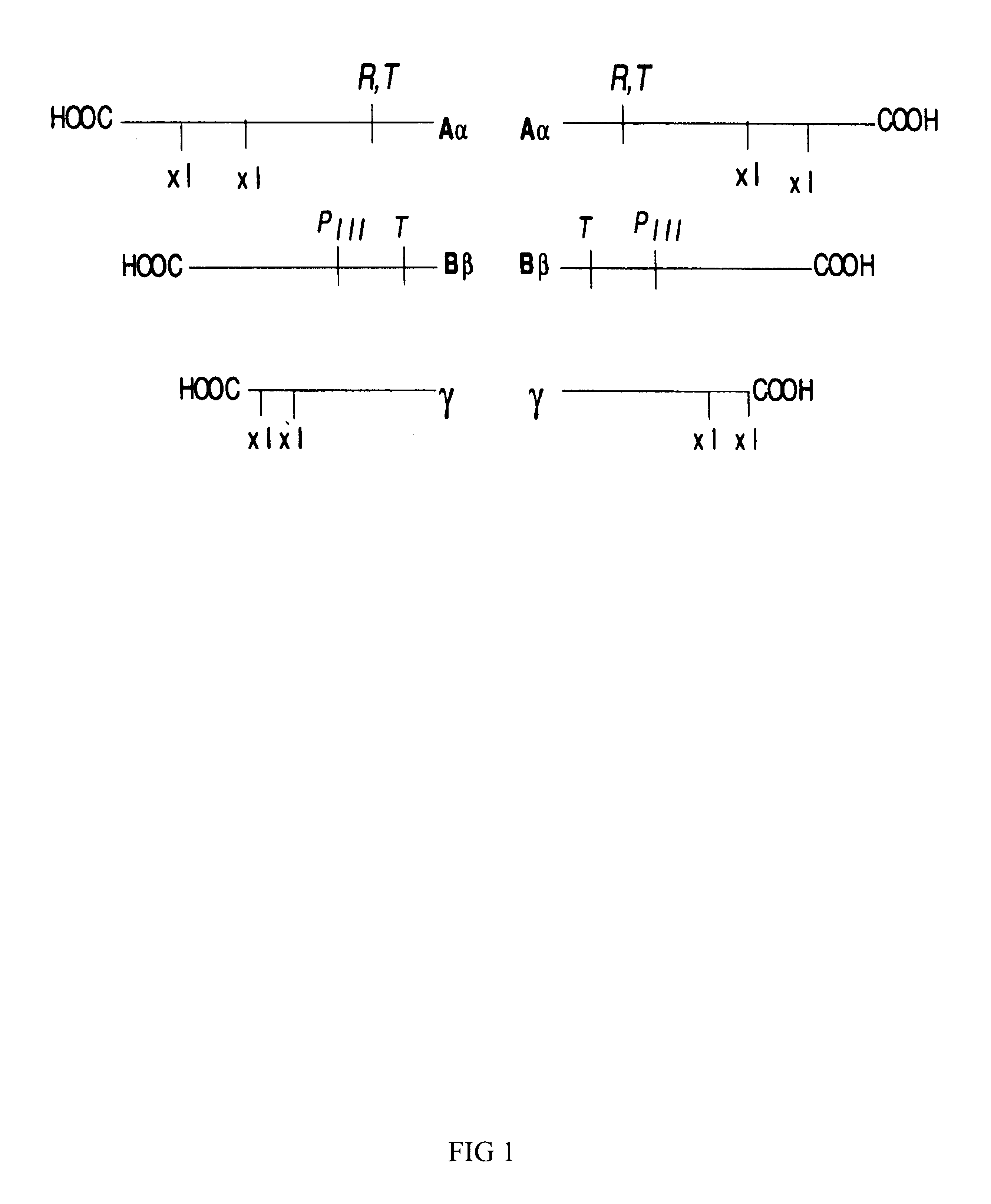Patents
Literature
1276 results about "Fadd protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
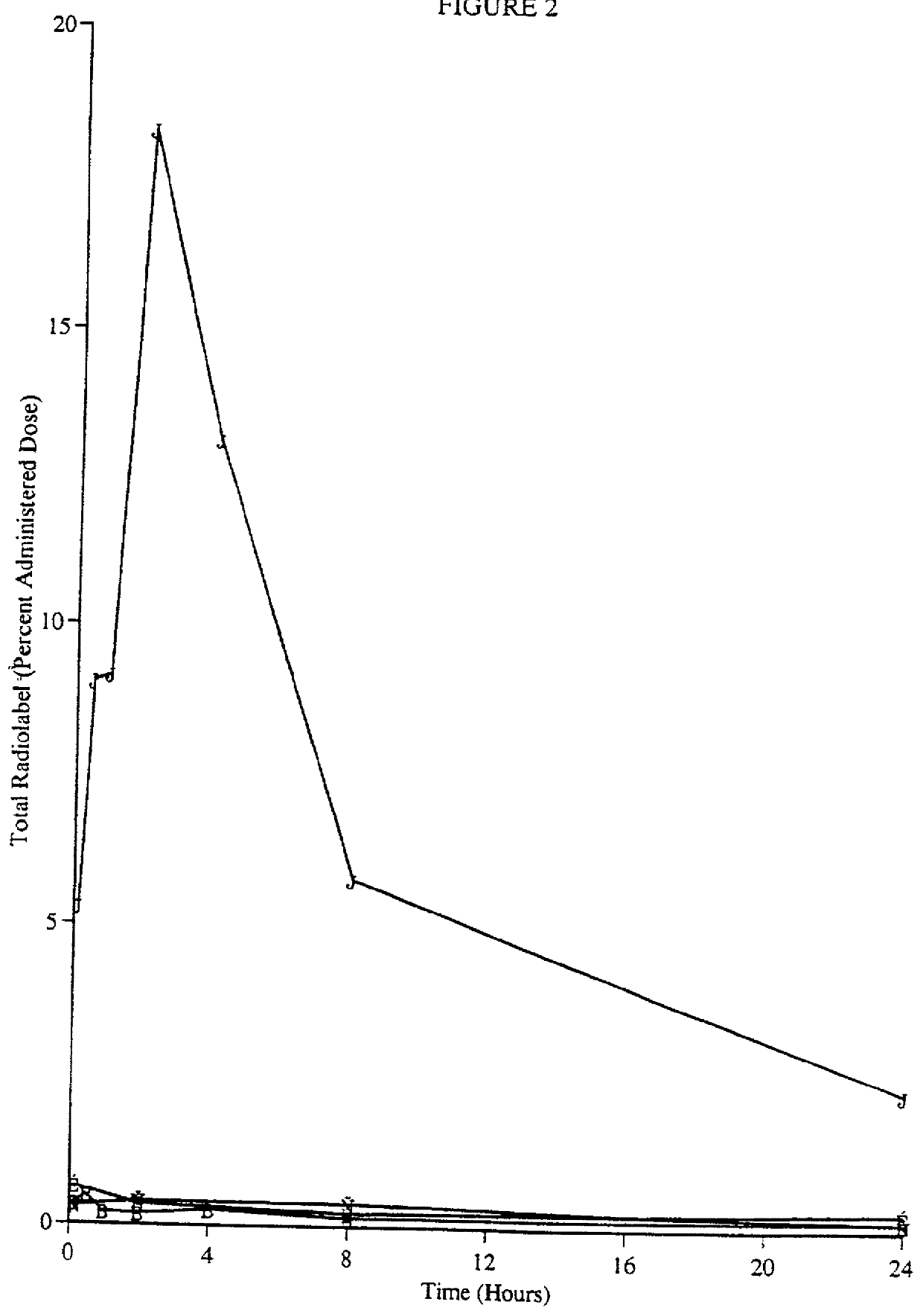
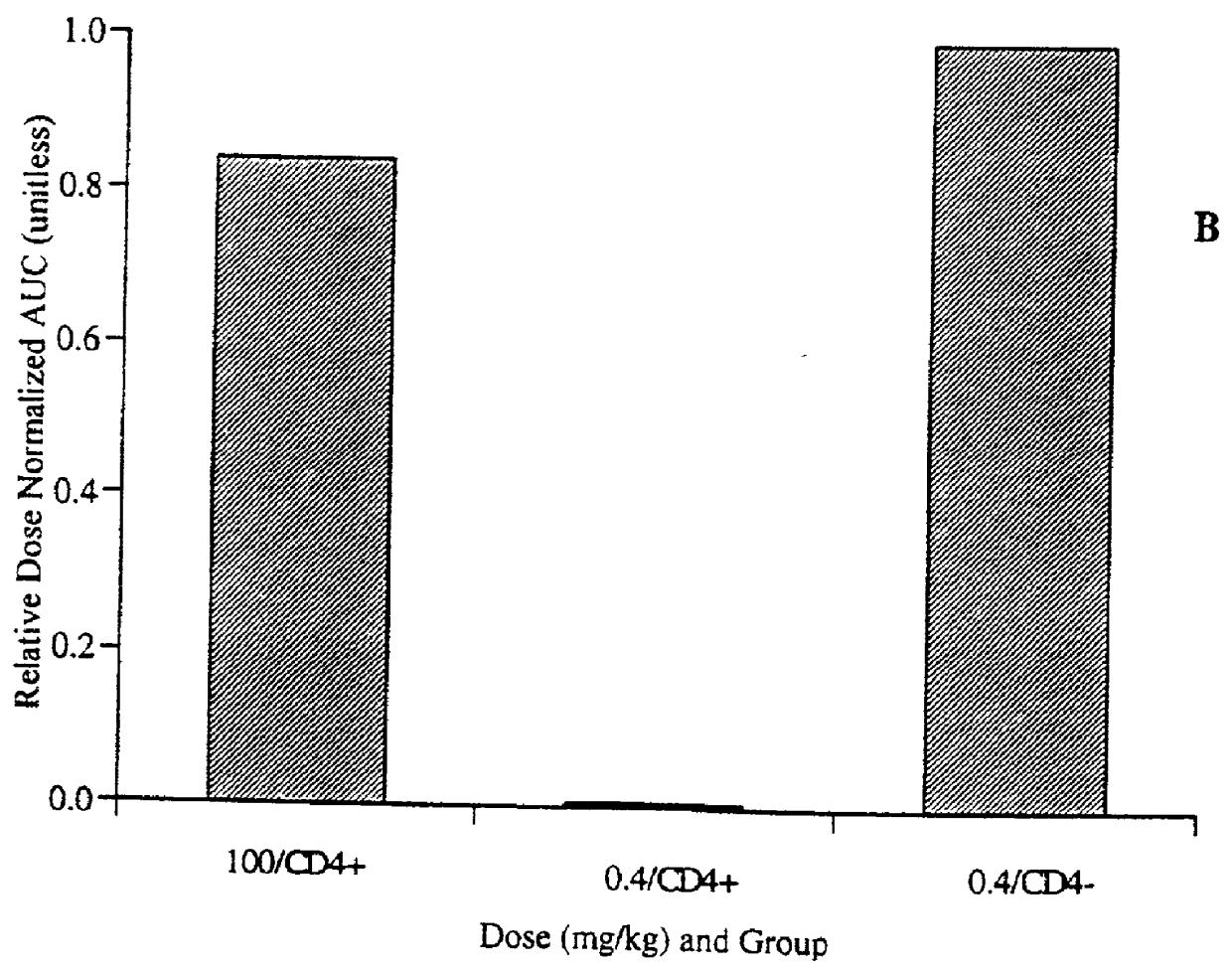
FADD (Fas-associated death domain) adaptor is a crucial protein involved in the induction of cell death but also mediates non-apoptotic actions via a phosphorylated form (p-Ser194-FADD).
Integrated active flux microfluidic devices and methods
InactiveUS6767706B2Rapid and complete exposureQuick and accurate and inexpensive analysisBioreactor/fermenter combinationsFlow mixersAntigenHybridization probe
The invention relates to a microfabricated device for the rapid detection of DNA, proteins or other molecules associated with a particular disease. The devices and methods of the invention can be used for the simultaneous diagnosis of multiple diseases by detecting molecules (e.g. amounts of molecules), such as polynucleotides (e.g., DNA) or proteins (e.g., antibodies), by measuring the signal of a detectable reporter associated with hybridized polynucleotides or antigen / antibody complex. In the microfabricated device according to the invention, detection of the presence of molecules (i.e., polynucleotides, proteins, or antigen / antibody complexes) are correlated to a hybridization signal from an optically-detectable (e.g. fluorescent) reporter associated with the bound molecules. These hybridization signals can be detected by any suitable means, for example optical, and can be stored for example in a computer as a representation of the presence of a particular gene. Hybridization probes can be immobilized on a substrate that forms part of or is exposed to a channel or channels of the device that form a closed loop, for circulation of sample to actively contact complementary probes. Universal chips according to the invention can be fabricated not only with DNA but also with other molecules such as RNA, proteins, peptide nucleic acid (PNA) and polyamide molecules.
Owner:CALIFORNIA INST OF TECH
Recombinant anti-CD30 antibodies and uses thereof
InactiveUS20040018194A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseChemotherapeutic drugs
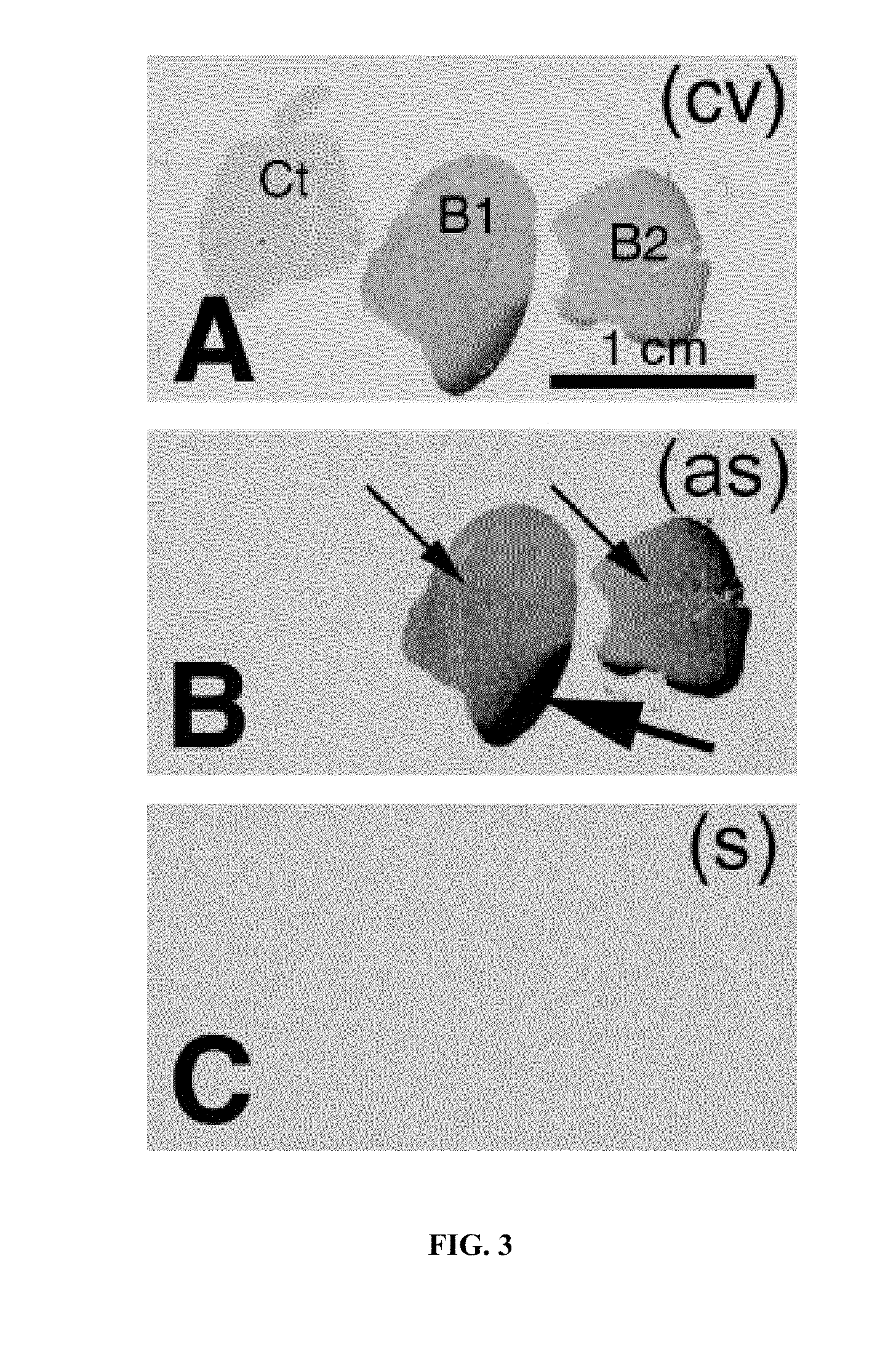
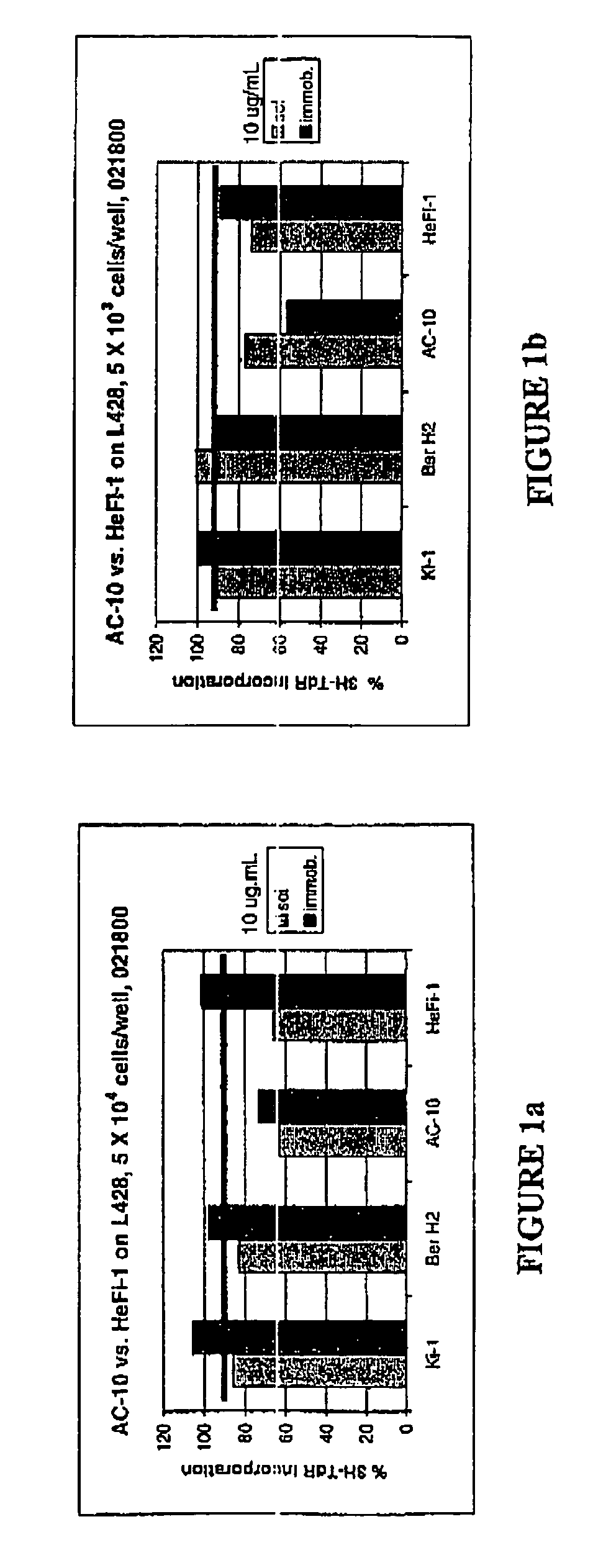
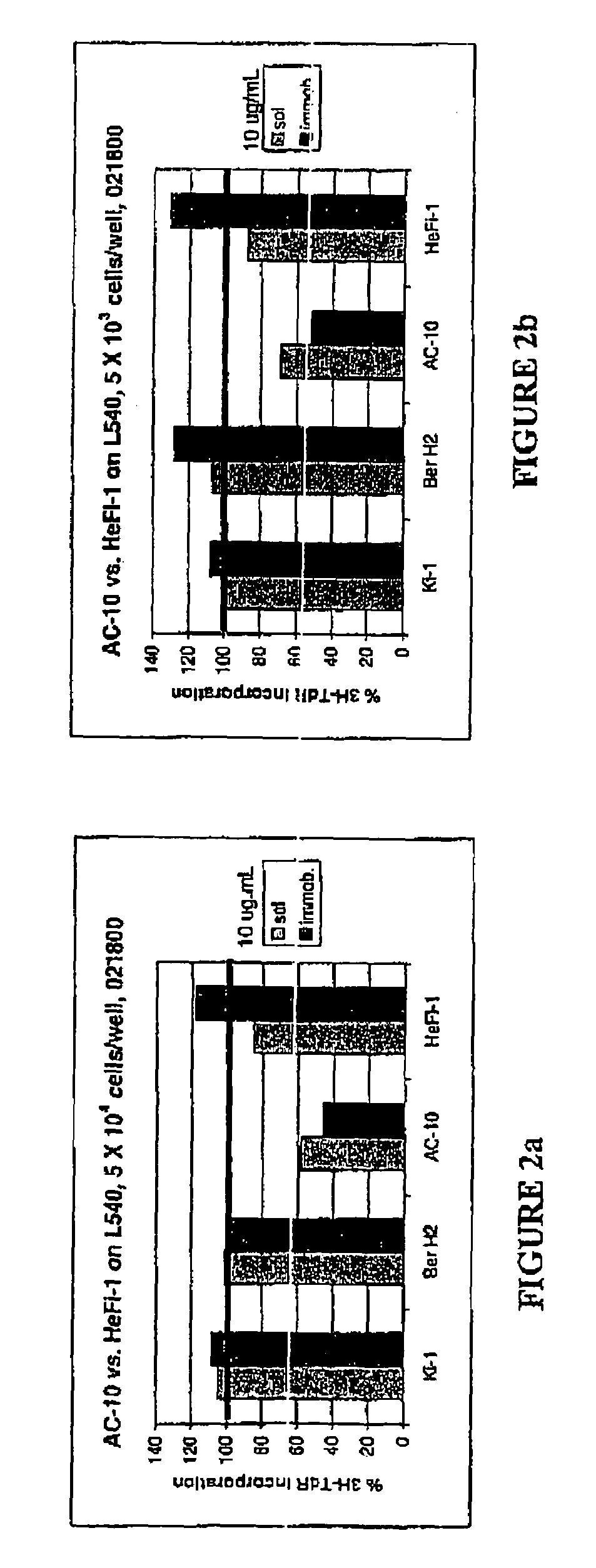
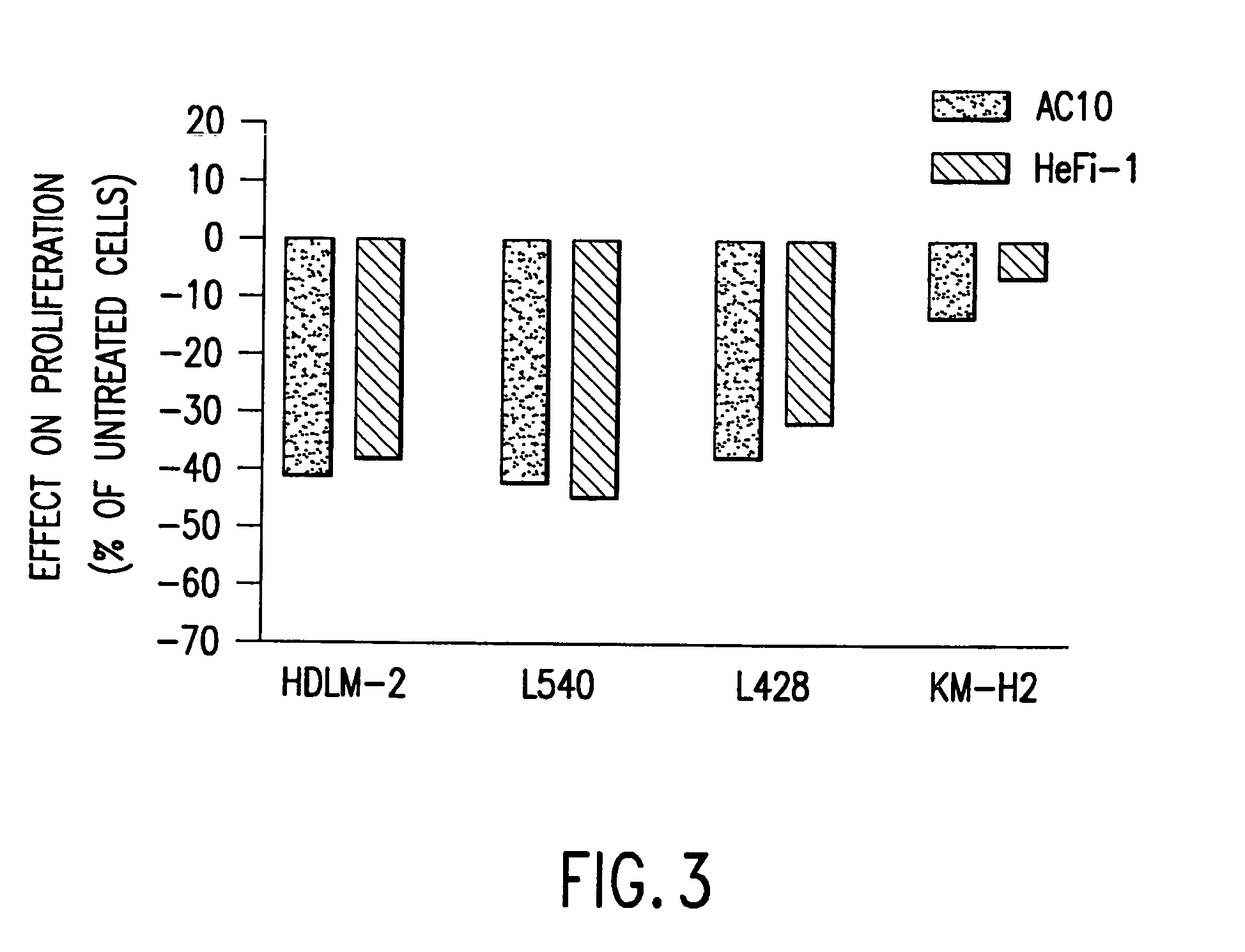
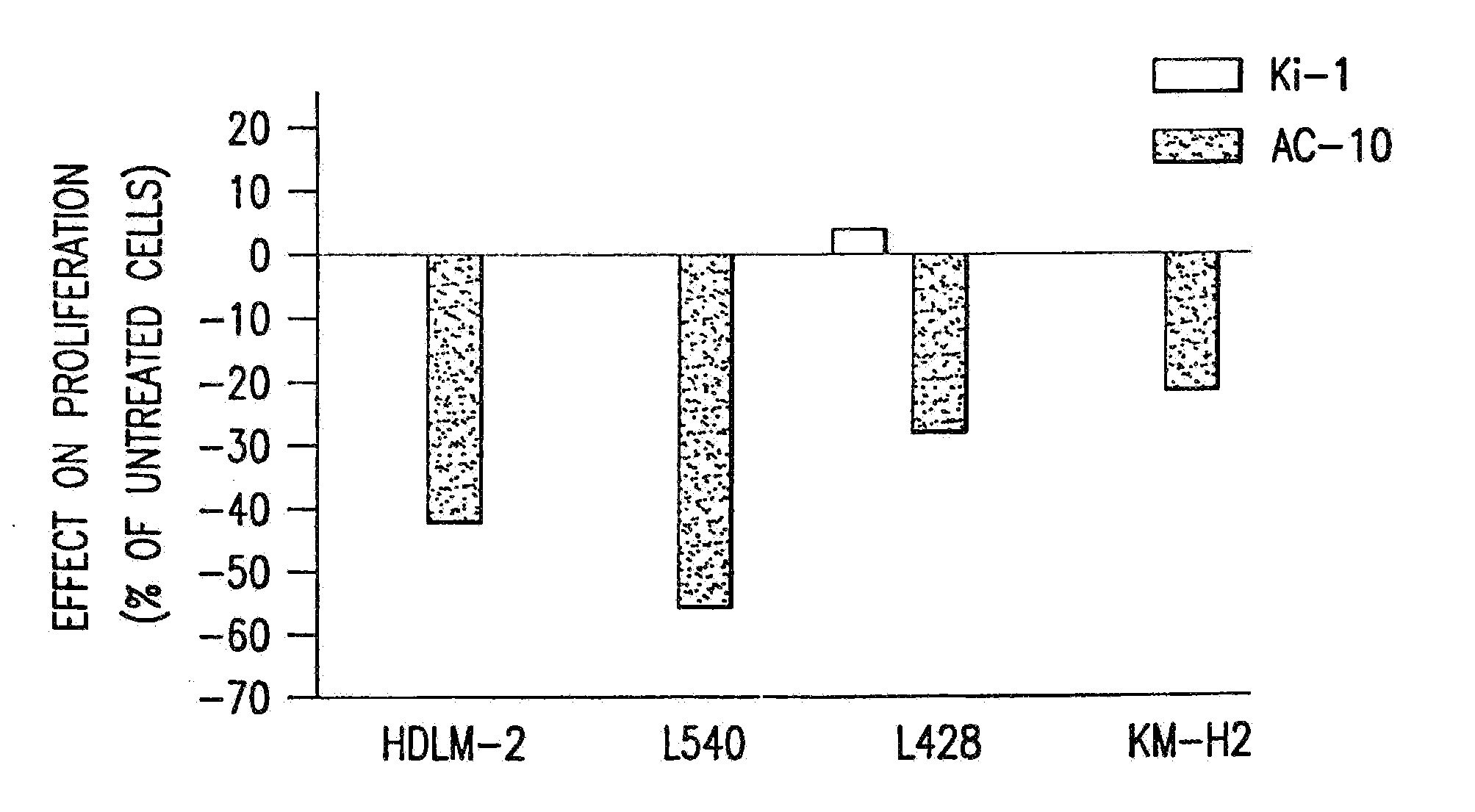
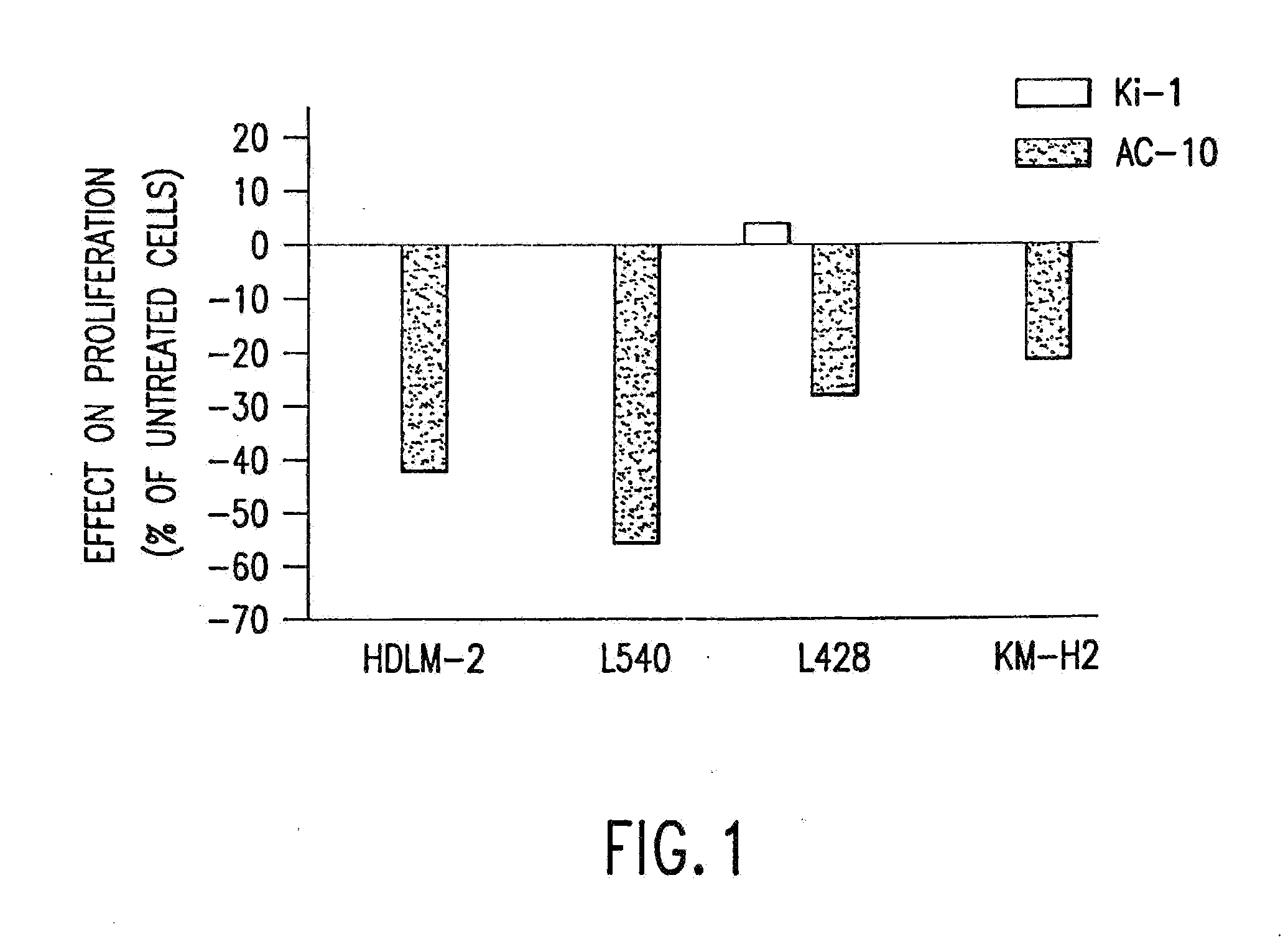
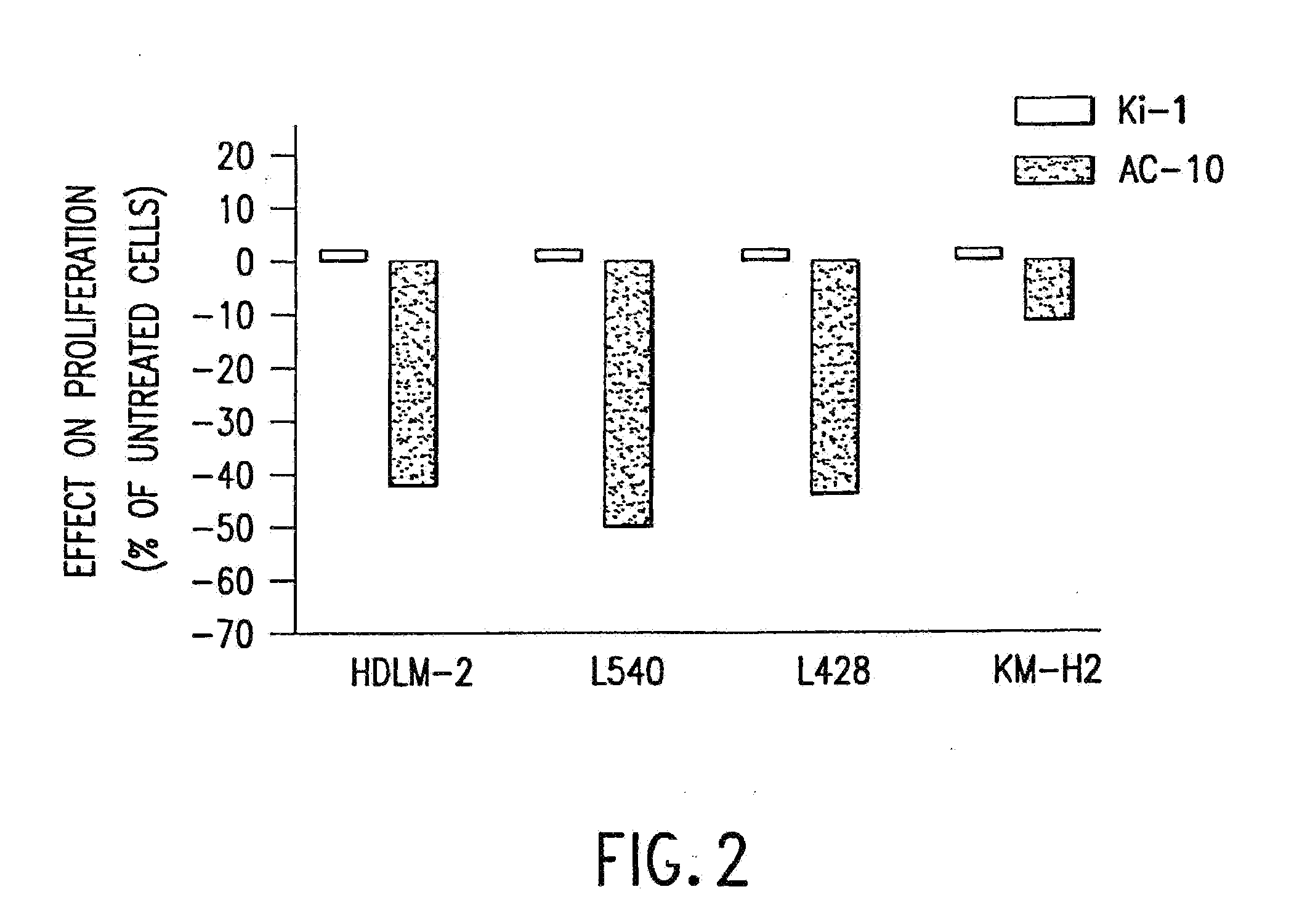
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
Treatment of neuropathic pain with zinc finger proteins
Owner:SANGAMO BIOSCIENCES INC
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
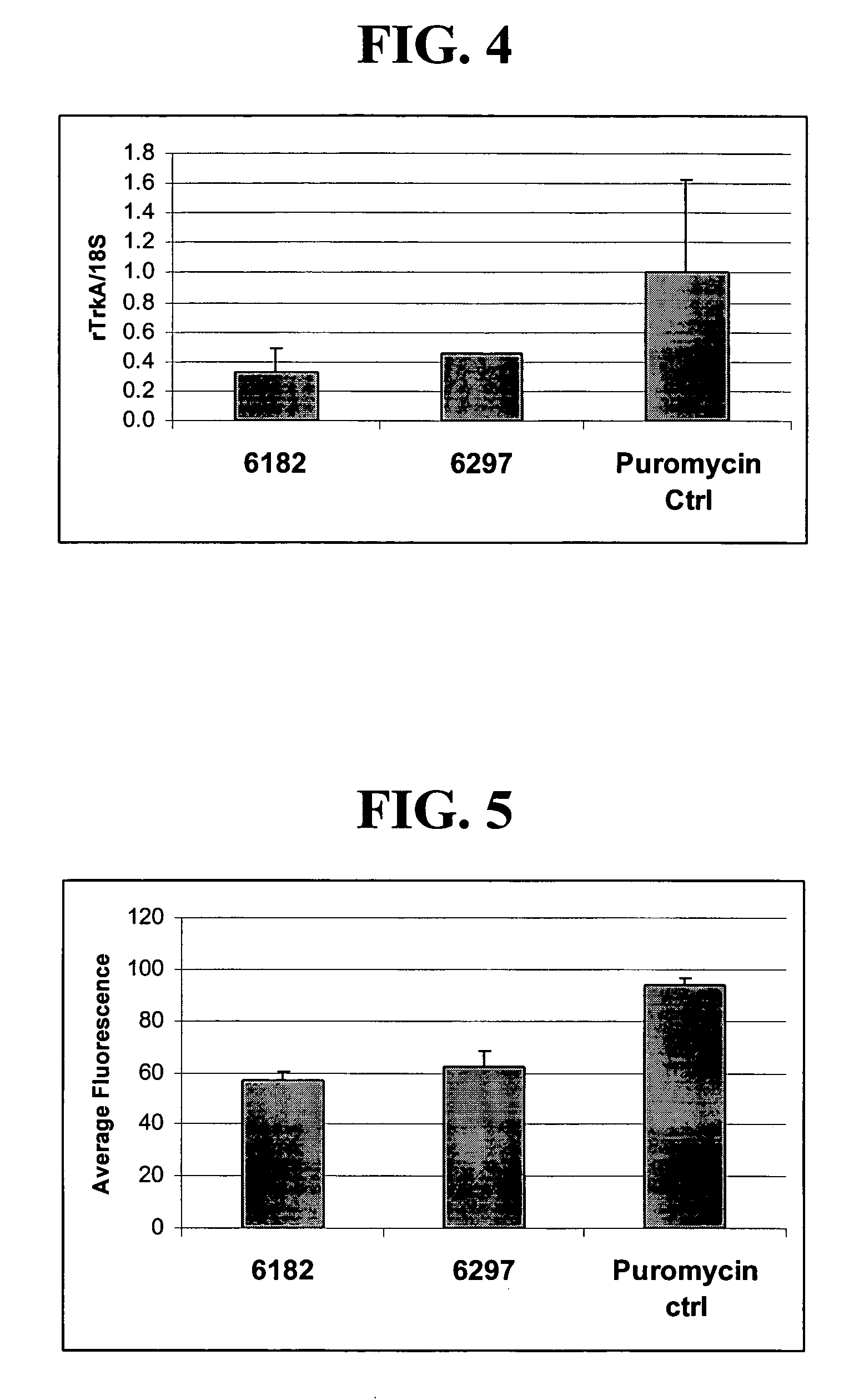
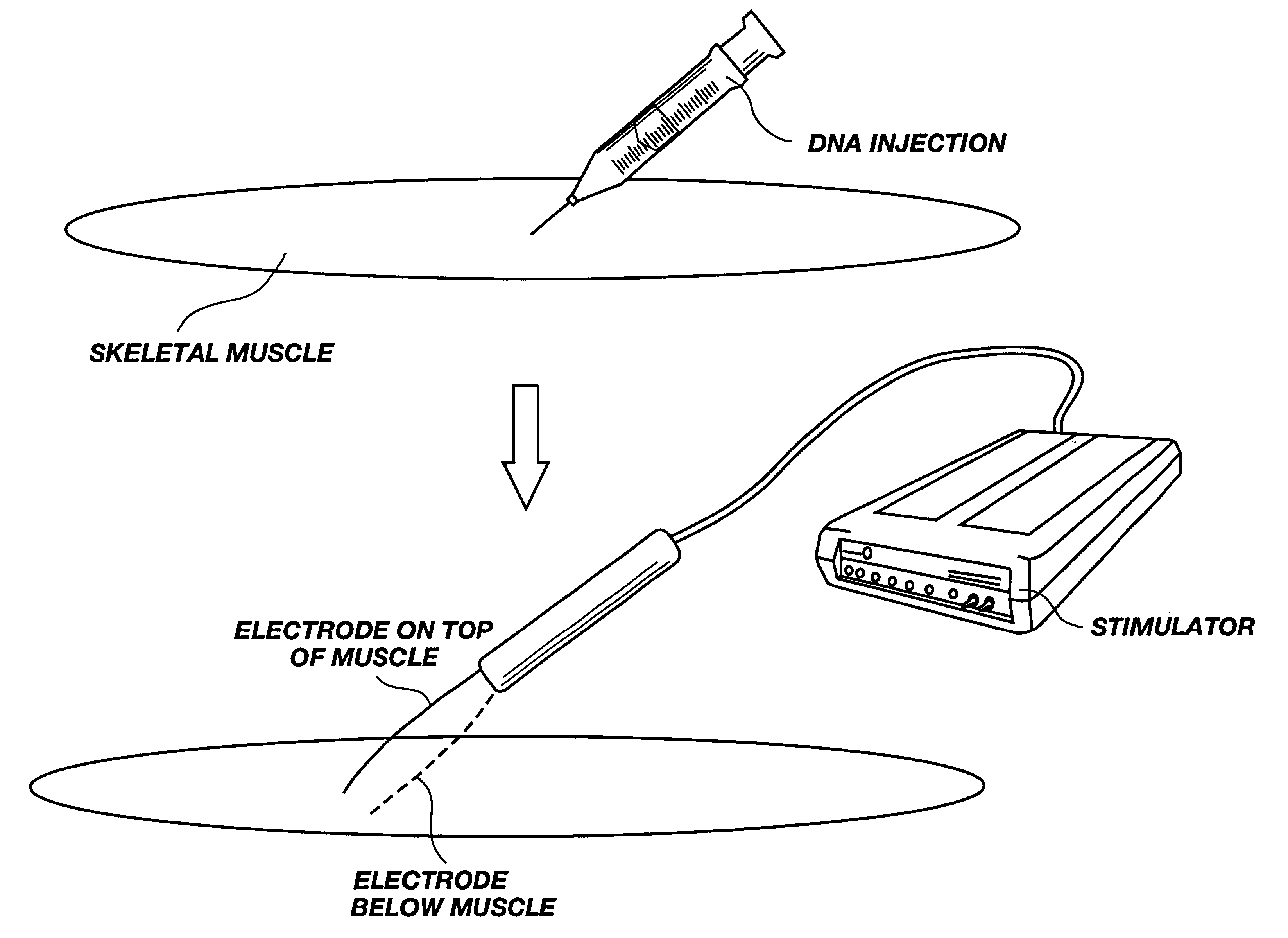
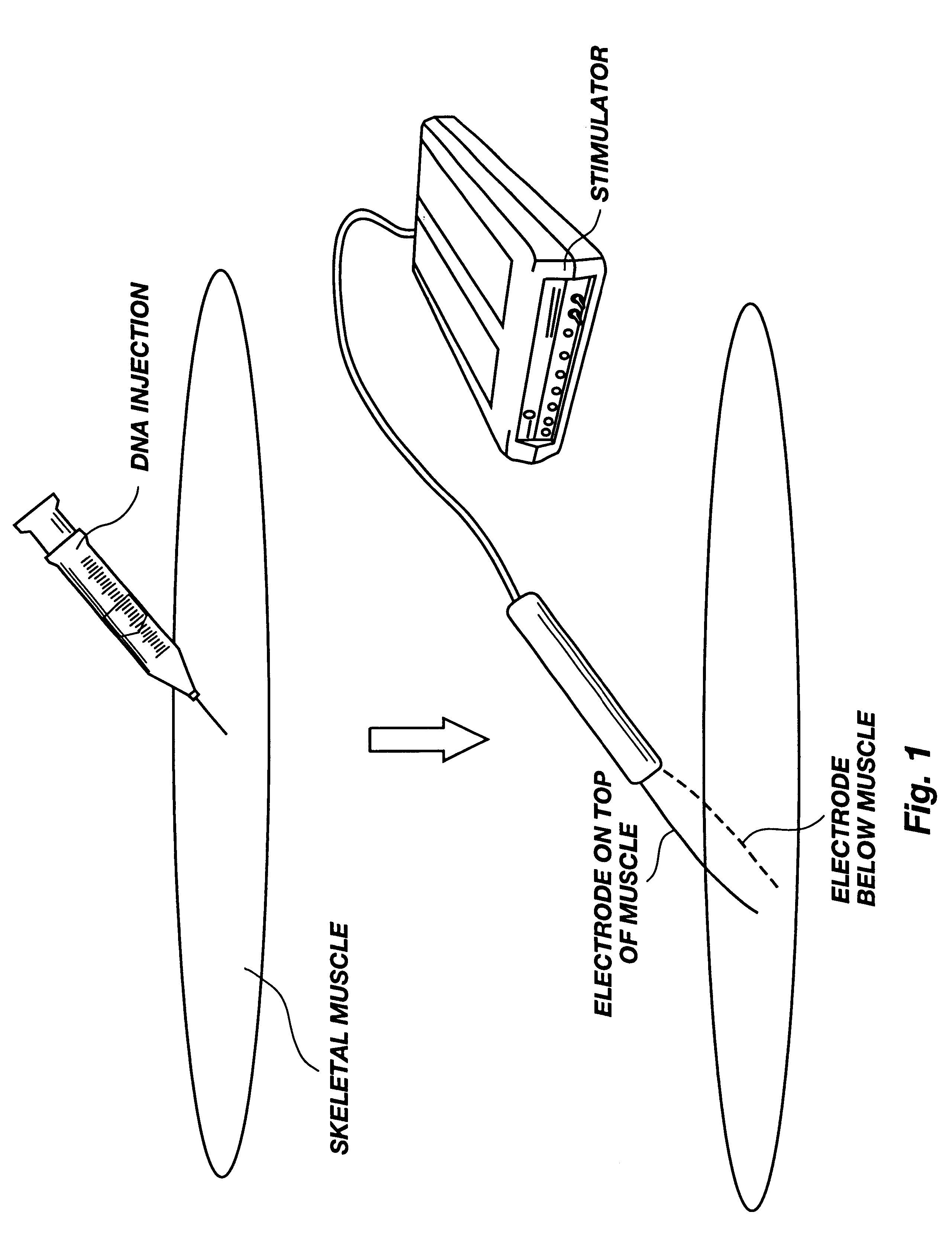
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
Delivery of mRNA for the augmentation of proteins and enzymes in human genetic diseases
InactiveUS20110244026A1Facilitating transfectionReduce deliveryOrganic active ingredientsDigestive systemDiseaseADAMTS Proteins
Disclosed herein are compositions and methods of modulating the expression of gene or the production of a protein by transfecting target cells with nucleic acids. The compositions disclosed herein demonstrate a high transfection efficacy and are capable of ameliorating diseases associated with protein or enzyme deficiencies.
Owner:TRANSLATE BIO INC
Recombinant anti-CD30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's Disease cells. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEAGEN INC
Mutated Tn5 transposase proteins and the use thereof
Transposase proteins that are modified relative to and have higher transposase activities than the wild-type Tn5 transposase are disclosed. A transposase protein of the present invention differs from the wild-type Tn5 transposase at amino acid position 41, 42, 450, or 454 and has greater avidity than the wild-type Tn5 transposase for at least one of a Tn5 outside end sequence as defined by SEQ ID NO:3, a Tn5 inside end sequence as defined by SEQ ID NO:4, and a modified Tn5 outside end sequence as defined by SEQ ID NO:5. Also disclosed are various systems and methods of using the transposase proteins of the present invention for in vitro or in vivo transposition.
Owner:WISCONSIN ALUMNI RES FOUND
Protein scaffolds for antibody mimics and other binding proteins
InactiveUS20050255548A1Easy to foldImprove stabilityAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsWAS PROTEINAntibody
Disclosed herein are proteins that include a fibronectin type III domain having at least one randomized loop. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
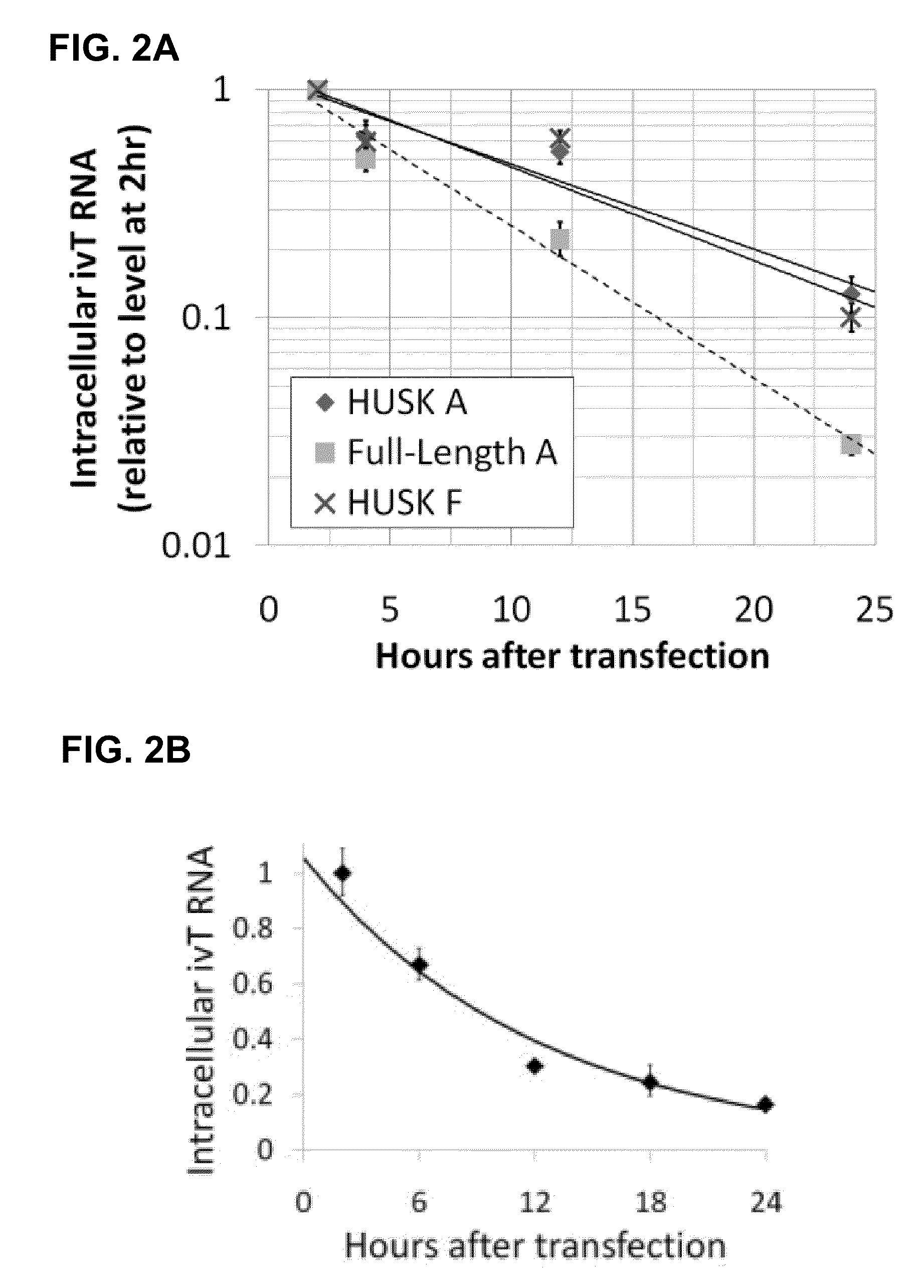
Innate immune suppression enables repeated delivery of long RNA molecules
InactiveUS20100273220A1Suppressing innate immune responseReduce expressionSugar derivativesArtificial cell constructsENCODETransient transfection
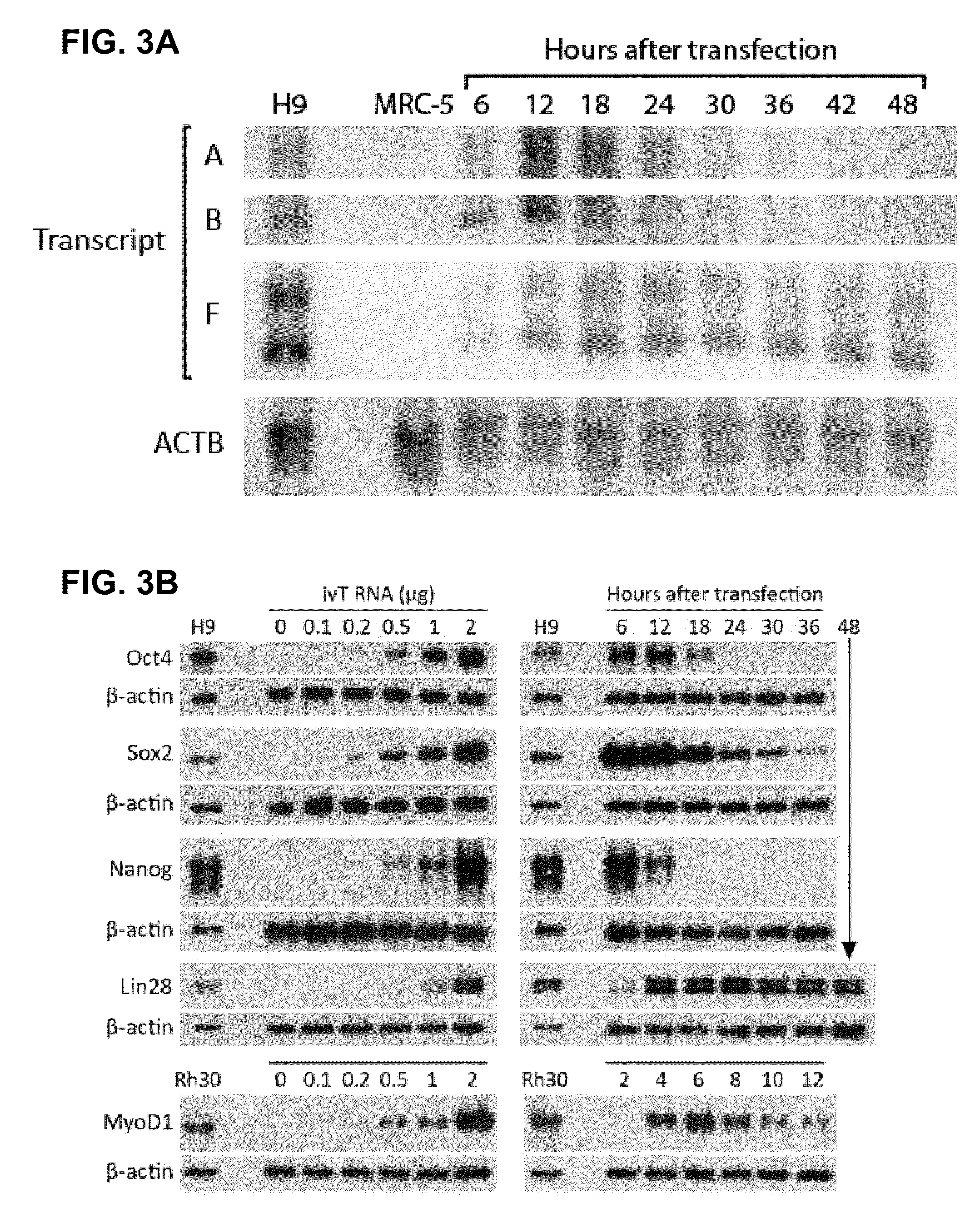
The present invention relates in part to methods for suppressing the innate immune response of a cell to transfection with an exogenous nucleic acid, to methods for increasing expression of a protein encoded by an exogenous nucleic acid by repeated delivery of the exogenous nucleic acid to a cell, and to methods of changing the phenotype of a cell by differentiating, transdifferentiating or dedifferentiating cells by repeatedly delivering one or more nucleic acids that encode defined proteins. A method is provided for extended transient transfection by repeated delivery of an in vitro-transcribed RNA (“ivT-RNA”) to a cell to achieve a high and sustained level of expression of a protein encoded by an ivT-RNA transcripts.
Owner:MASSACHUSETTS INST OF TECH
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Process for producing peptides by using in vitro transcription/translation system
The present invention relates to a reaction system whereby a peptide produced in an in vitro peptide synthesis system can be efficiently isolated at a high purity from the reaction system. Thus the present invention is a process for producing a peptide or a peptide derivative by using a reaction system of transcribing a DNA into an RNA and then translating the RNA produced or a reaction system of translating an RNA in vitro wherein at least one protein component of the reaction system is labeled with a first substance which adheres to a second substance, and said second substance is used as an adsorbent for capturing said labeled protein components after translating.
Owner:BIOCOMBER
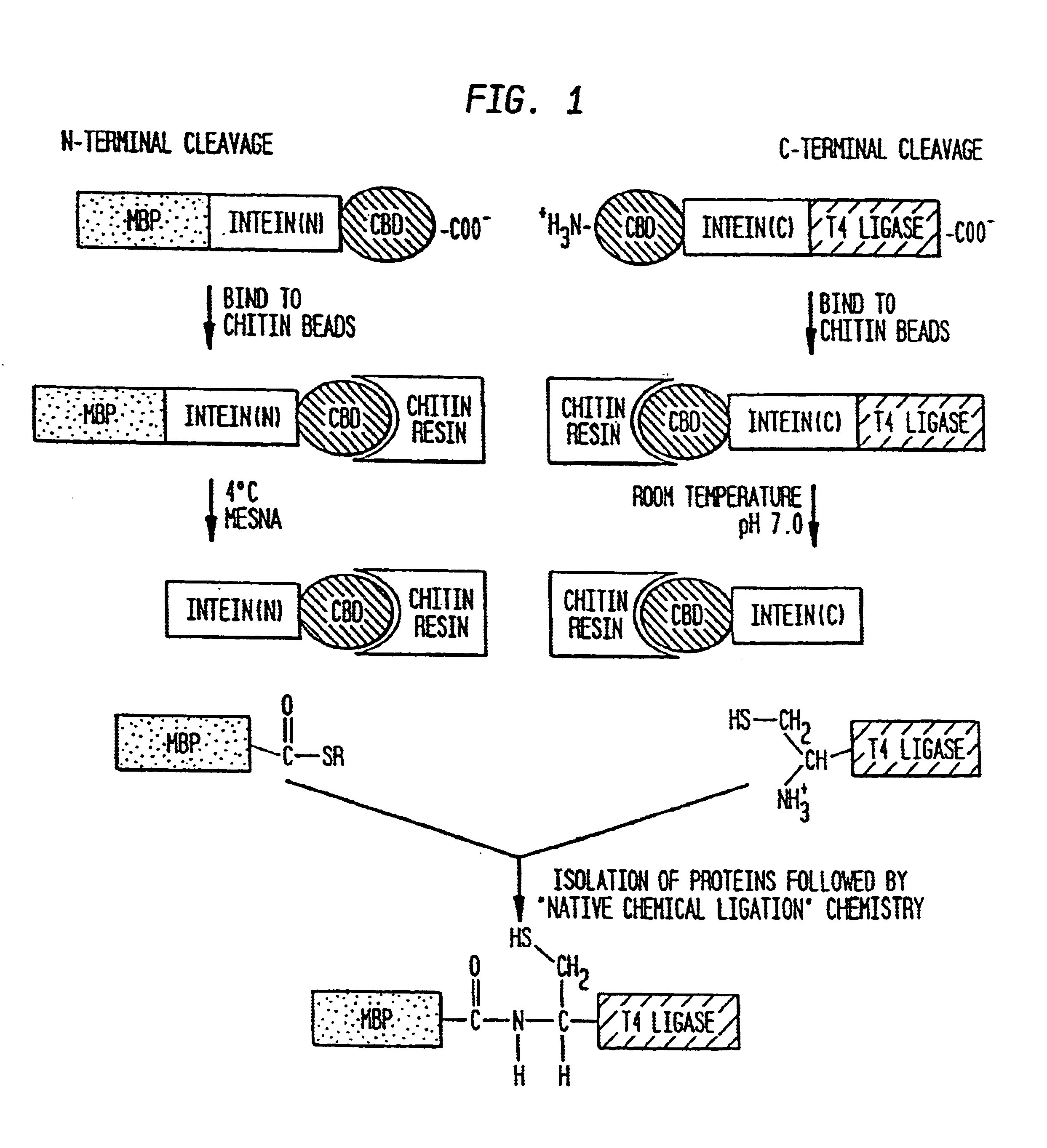
Intein-mediated protein ligation of expressed proteins
InactiveUS6849428B1Eliminate needBacteriaFusion with post-translational modification motifProtein targetIntein
A method for the ligation of expressed proteins which utilizes inteins, for example the RIR1 intein from Methanobacterium thermotrophicum, is provided. Constructs of the Mth RIR1 intein in which either the C-terminal asparagine or N-terminal cysteine of the intein are replaced with alanine enable the facile isolation of a protein with a specified N-terminal, for example, cysteine for use in the fusion of two or more expressed proteins. The method involves the steps of generating a C-terminal thioester-tagged target protein and a second target protein having a specified N-terminal via inteins, such as the modified Mth RIR1 intein, and ligating these proteins. A similar method for producing a cyclic or polymerized protein is provided. Modified inteins engineered to cleave at their C-terminus or N-terminus, respectively, and DNA and plasmids encoding these modified inteins are also provided.
Owner:NEW ENGLAND BIOLABS
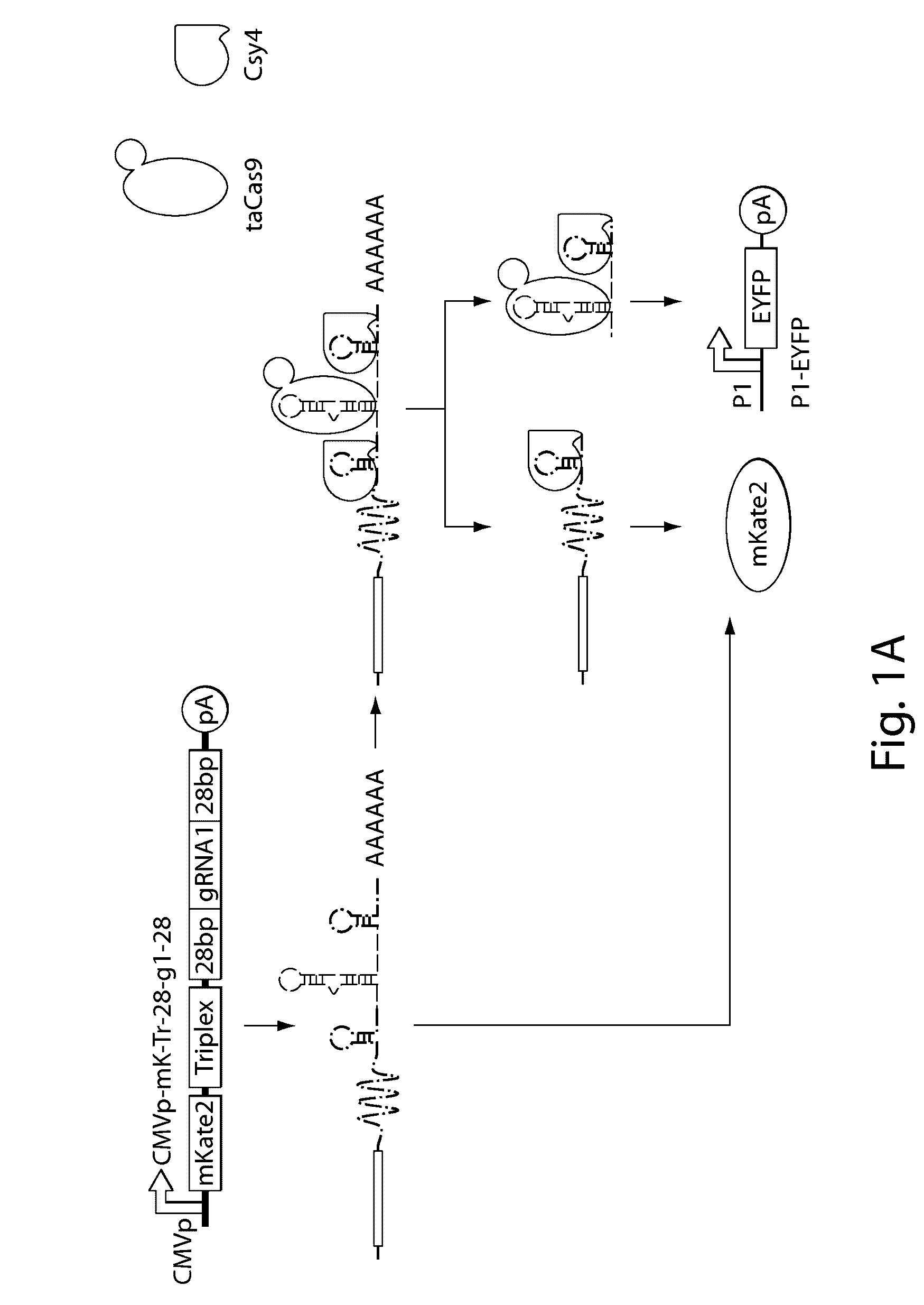
Methods and compositions for the production of guide RNA
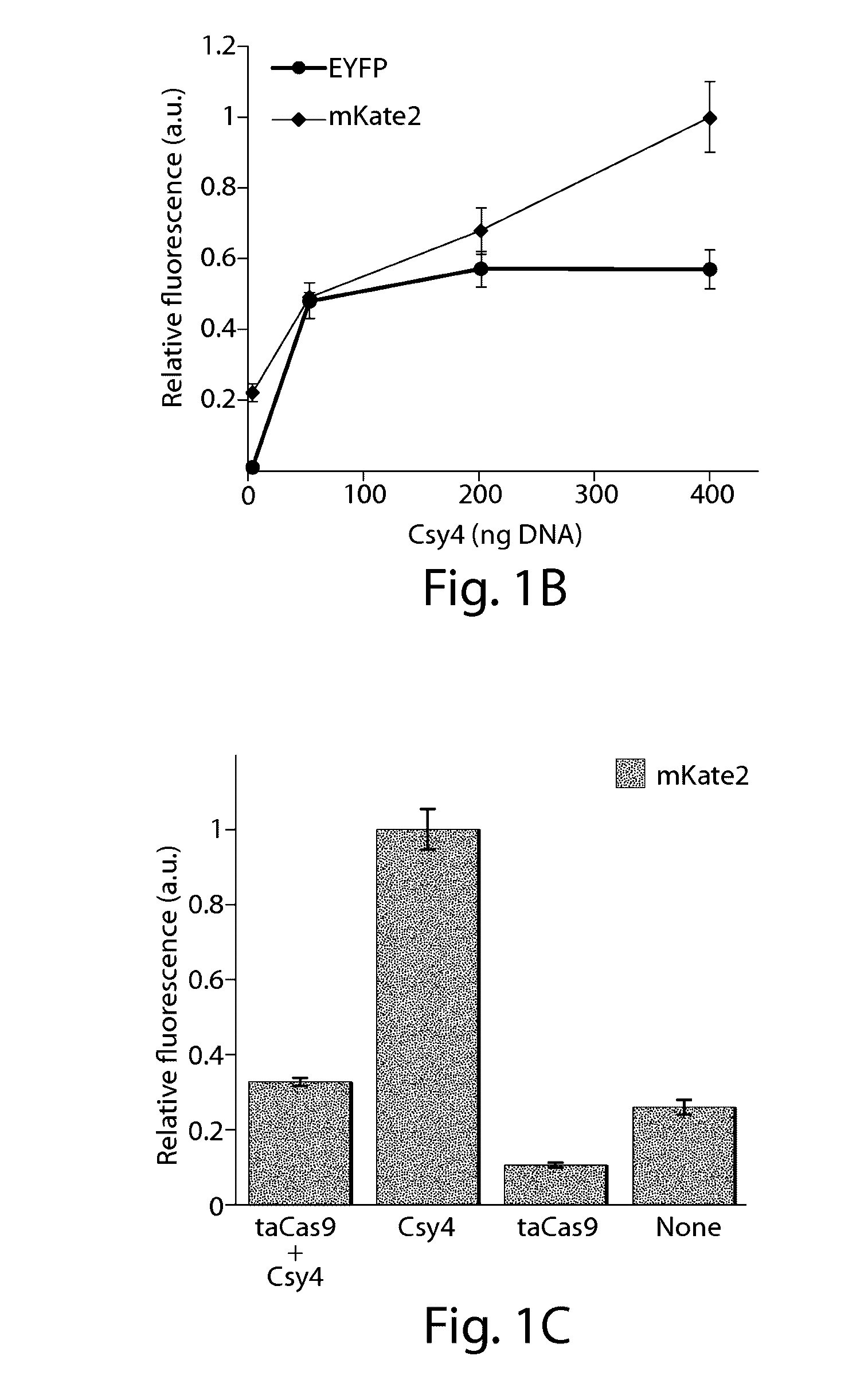
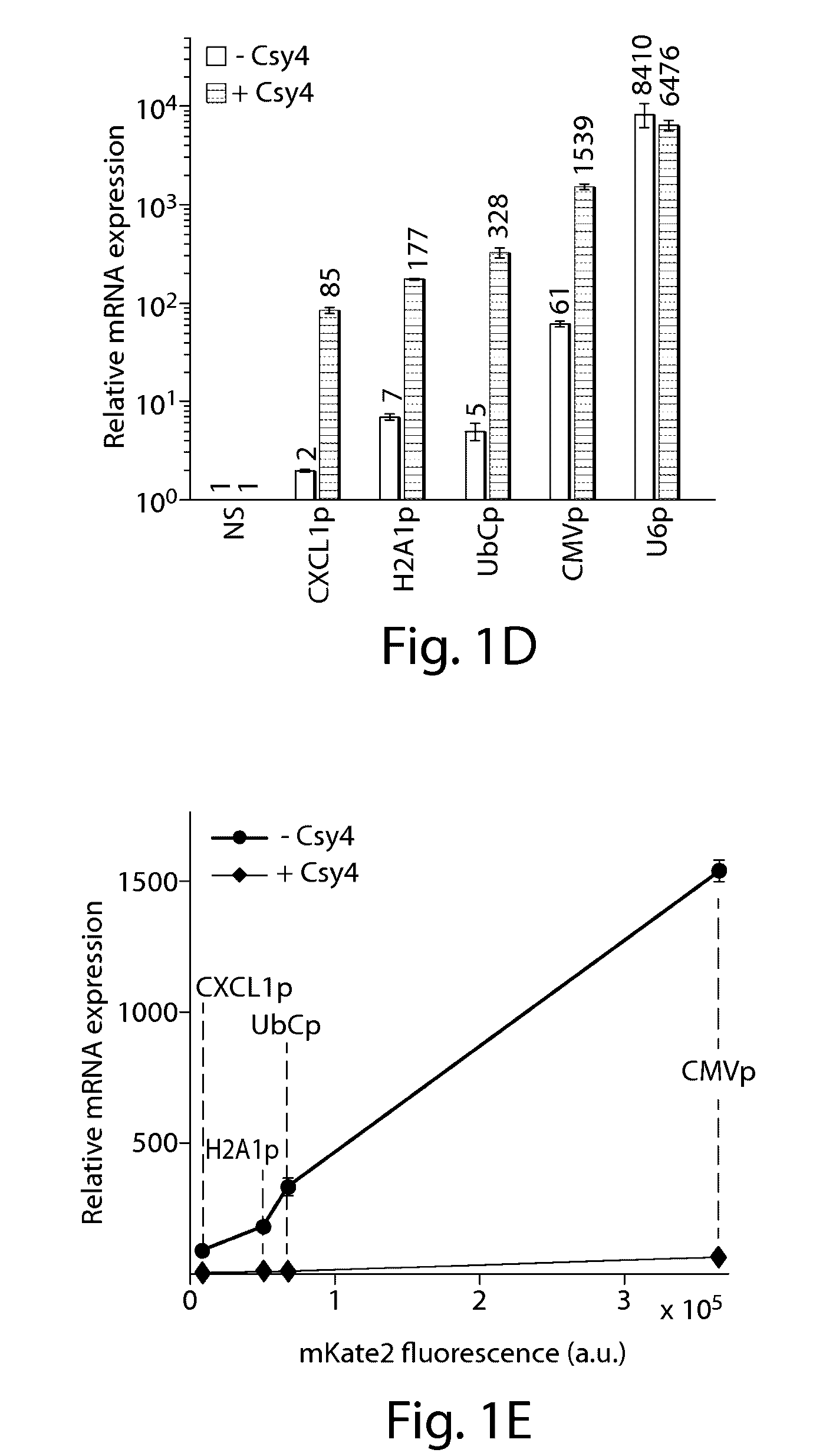
Various aspects and embodiments of the present disclosure relate to methods and compositions that combine multiple mammalian RNA regulatory strategies, including RNA triple helix structures, introns, microRNAs, and ribozymes with Cas-based CRISPR transcription factors and ribonuclease-based RNA processing in human cells. The methods and compositions of the present disclosure, in some embodiments, enable multiplexed production of proteins and multiple guide RNAs from a single compact RNA-polymerase-II-expressed transcript for efficient modulation of synthetic constructs and endogenous human promoters.
Owner:MASSACHUSETTS INST OF TECH
Recombinant Anti-cd30 antibodies and uses thereof
The present invention relates to methods and compositions for the treatment of Hodgkin's Disease, comprising administering proteins characterized by their ability to bind to CD30, or compete with monoclonal antibodies AC10 or HeFi-1 for binding to CD30, and exert a cytostatic or cytotoxic effect on Hodgkin's disease cells in the absence of effector cells or complement. Such proteins include derivatives of monoclonal antibodies AC10 and HeFi-1. The proteins of the invention can be human, humanized, or chimeric antibodies; further, they can be conjugated to cytotoxic agents such as chemotherapeutic drugs. The invention further relates to nucleic acids encoding the proteins of the invention. The invention yet further relates to a method for identifying an anti-CD30 antibody useful for the treatment or prevention of Hodgkin's Disease.
Owner:SEATTLE GENETICS INC
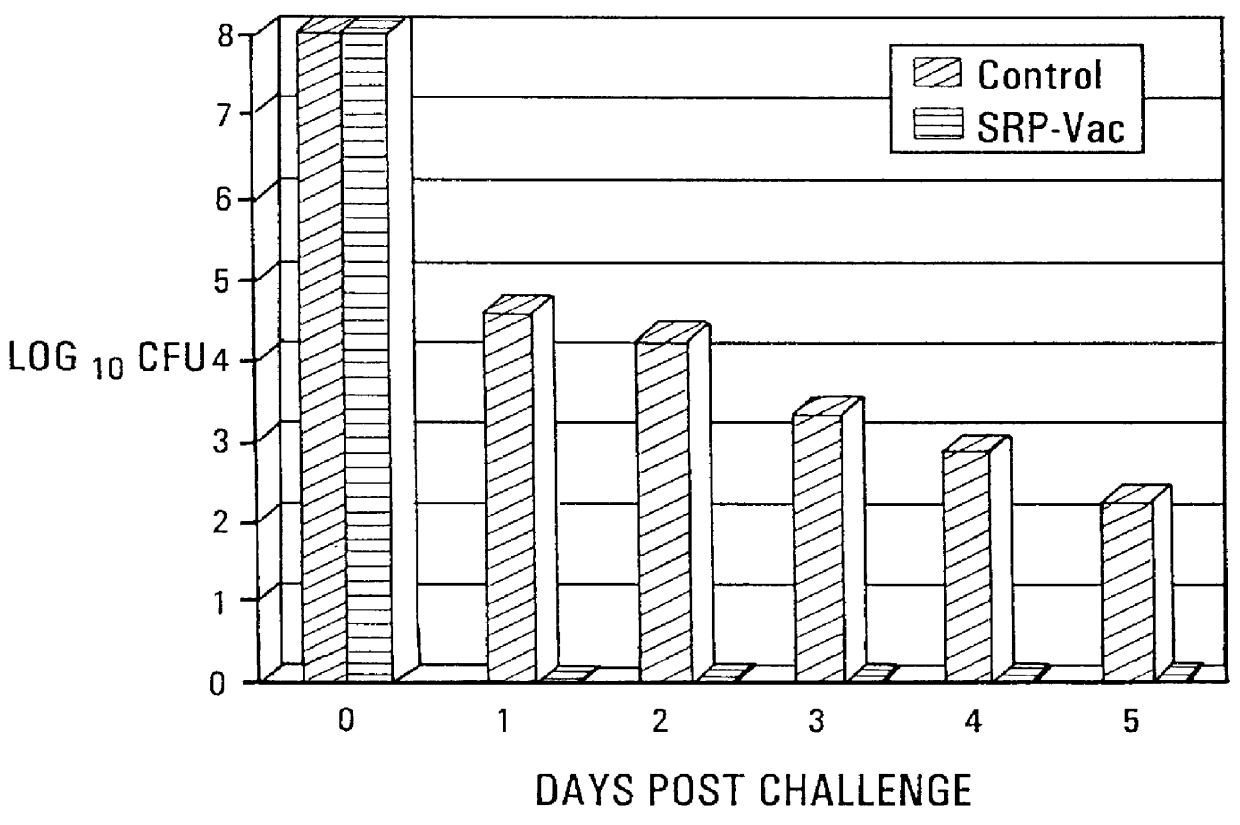
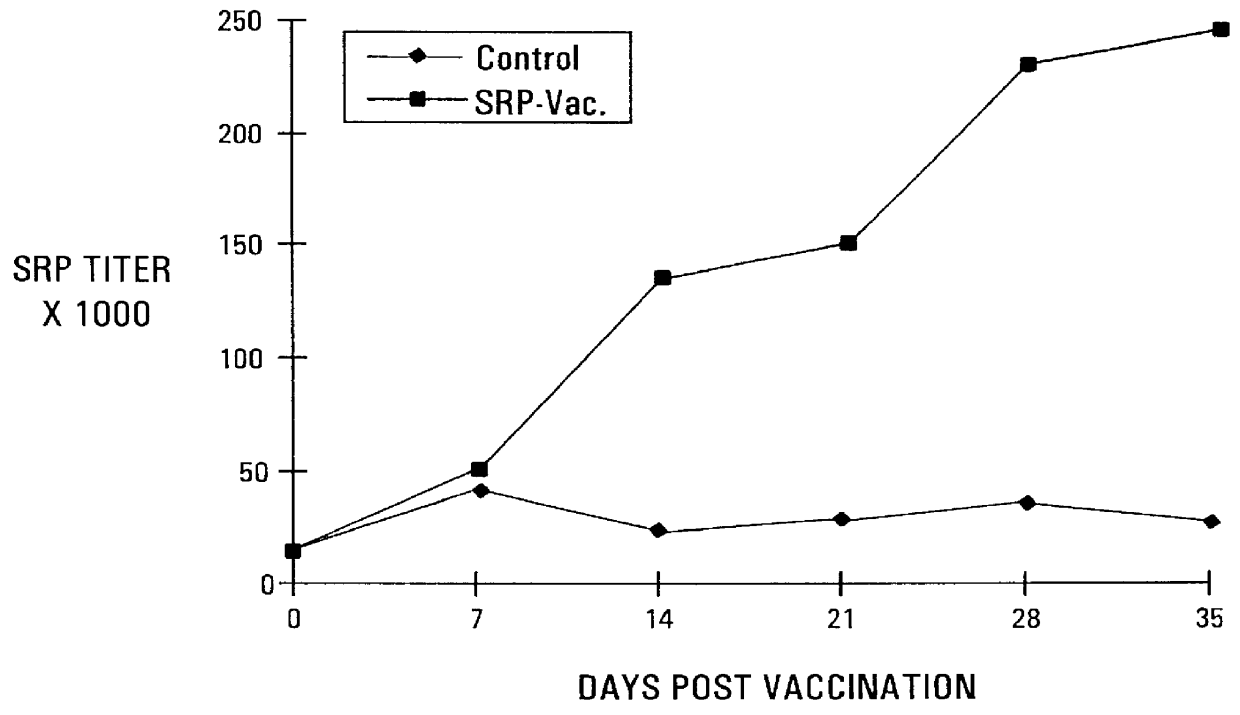
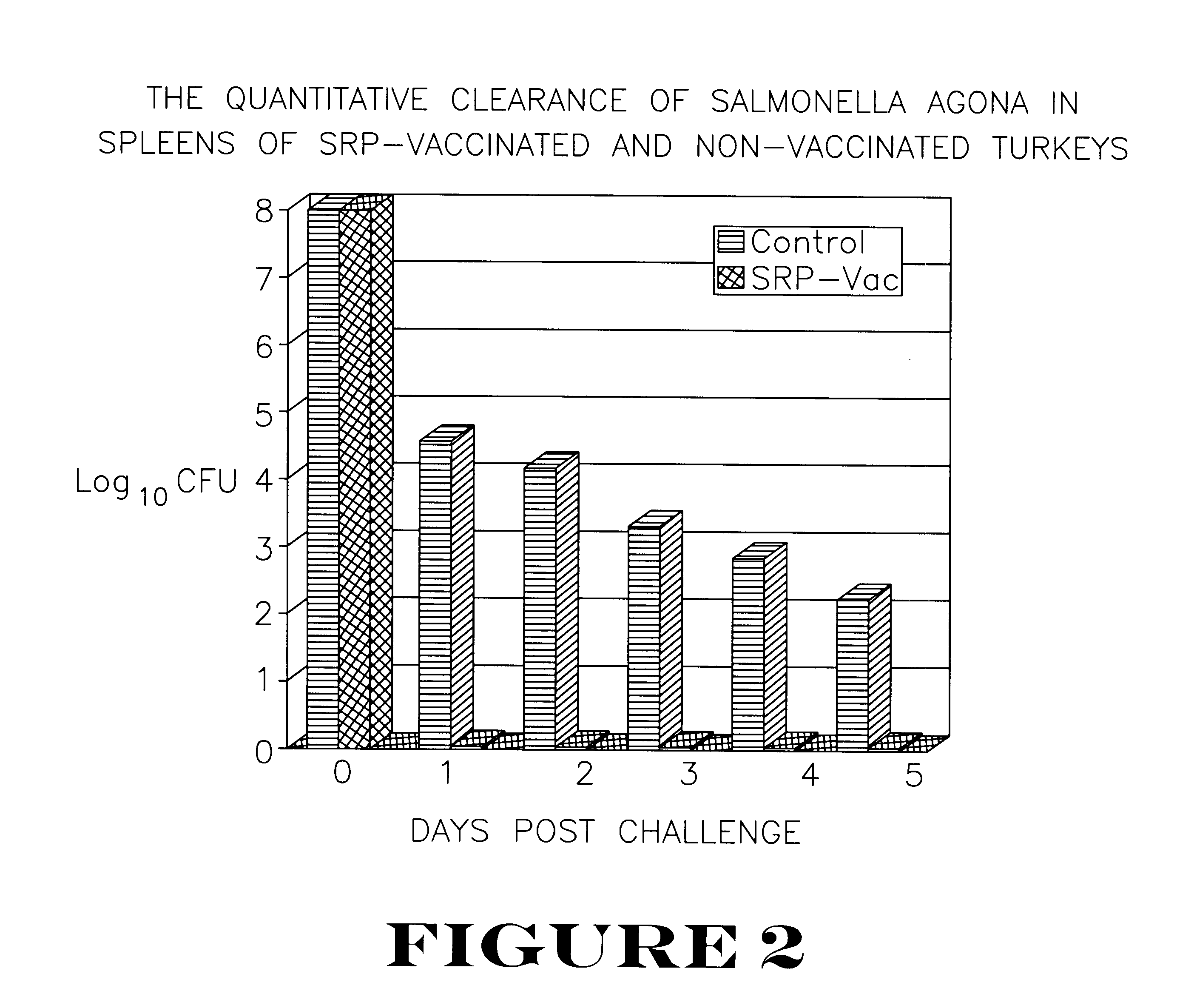
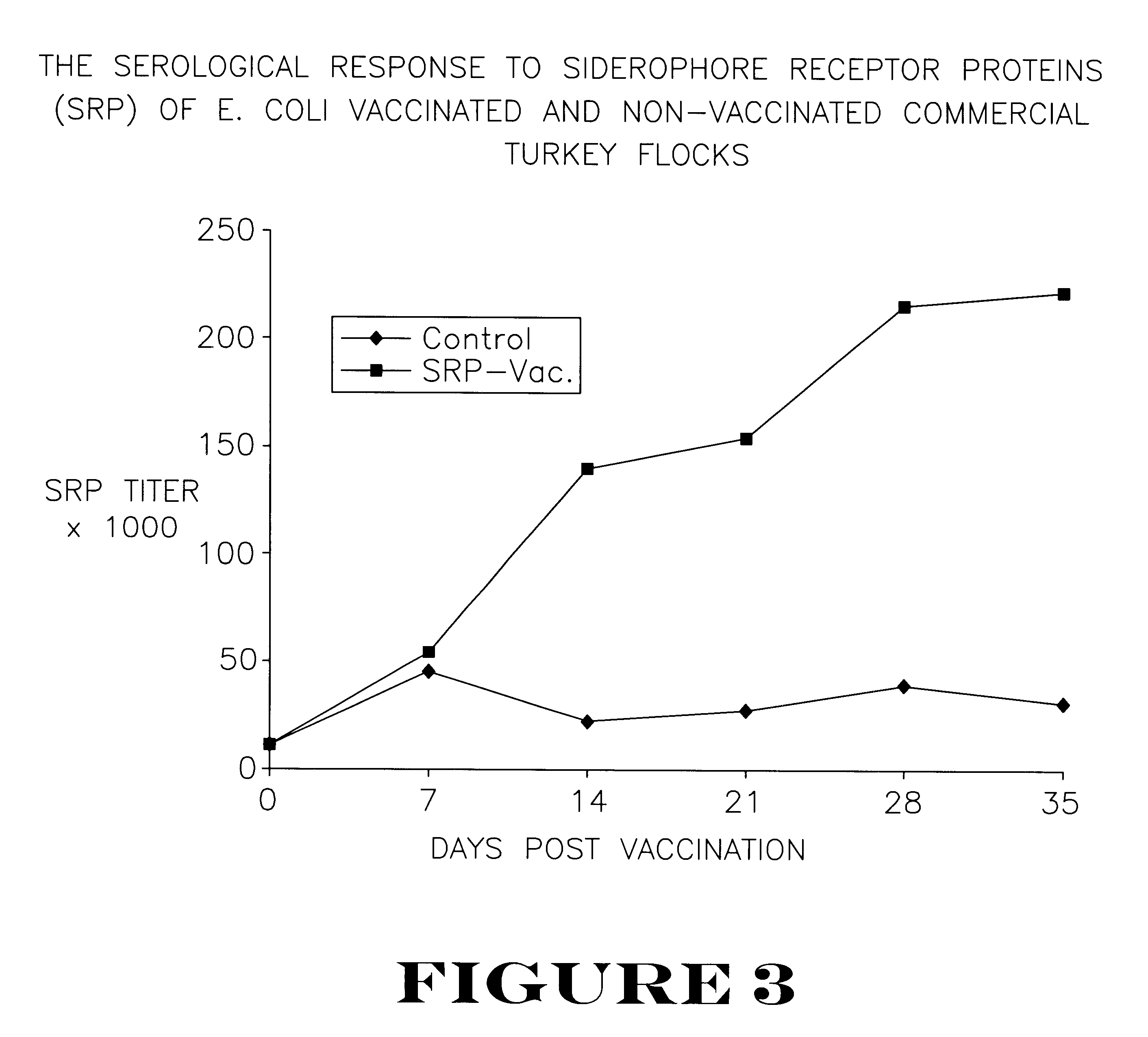
Active immunization using a siderophore receptor protein
InactiveUS6027736AEasy to produceInhibition capacityAntibacterial agentsBacterial antigen ingredientsADAMTS ProteinsMicrobiology
The invention provides a vaccine for immunizing poultry and other animals against infection by a gram-negative bacteria, and a method of immunizing an animal using the vaccine. The vaccine may contain purified siderophore receptor proteins derived from a single strain or species of gram-negative bacteria or other organism, which are cross-reactive with siderophores produced by two or more strains, species or genera of gram-negative bacteria. The invention further provides a process for isolating and purifying the siderophore receptor proteins, and for preparing a vaccine containing the proteins. Also provided is a method for diagnosing gram-negative sepsis.
Owner:EPITOPIX LLC
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS7067110B1Improving immunogenicityStrengthIn-vivo radioactive preparationsAntibody mimetics/scaffoldsPeptide antigenFc(alpha) receptor
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Method of treating immune cell mediated systemic diseases
InactiveUS20010056066A1Prolonged systemic exposureEnhance and create propertyPeptide/protein ingredientsAntipyreticAntigenWhole body
An improved method of treating immune cell mediated systemic diseases, particularly T and B cell mediated diseases, is provided by increasing the systemic exposure, or bioavailibility, of a therapeutic protein. Such therapeutic protein is selected from the group consisting of a monoclonal antibody, a soluble receptor and a soluble ligand which binds to an antigen expressed on the surface of an immunce cell.
Owner:SMITHKLINE BECKMAN CORP
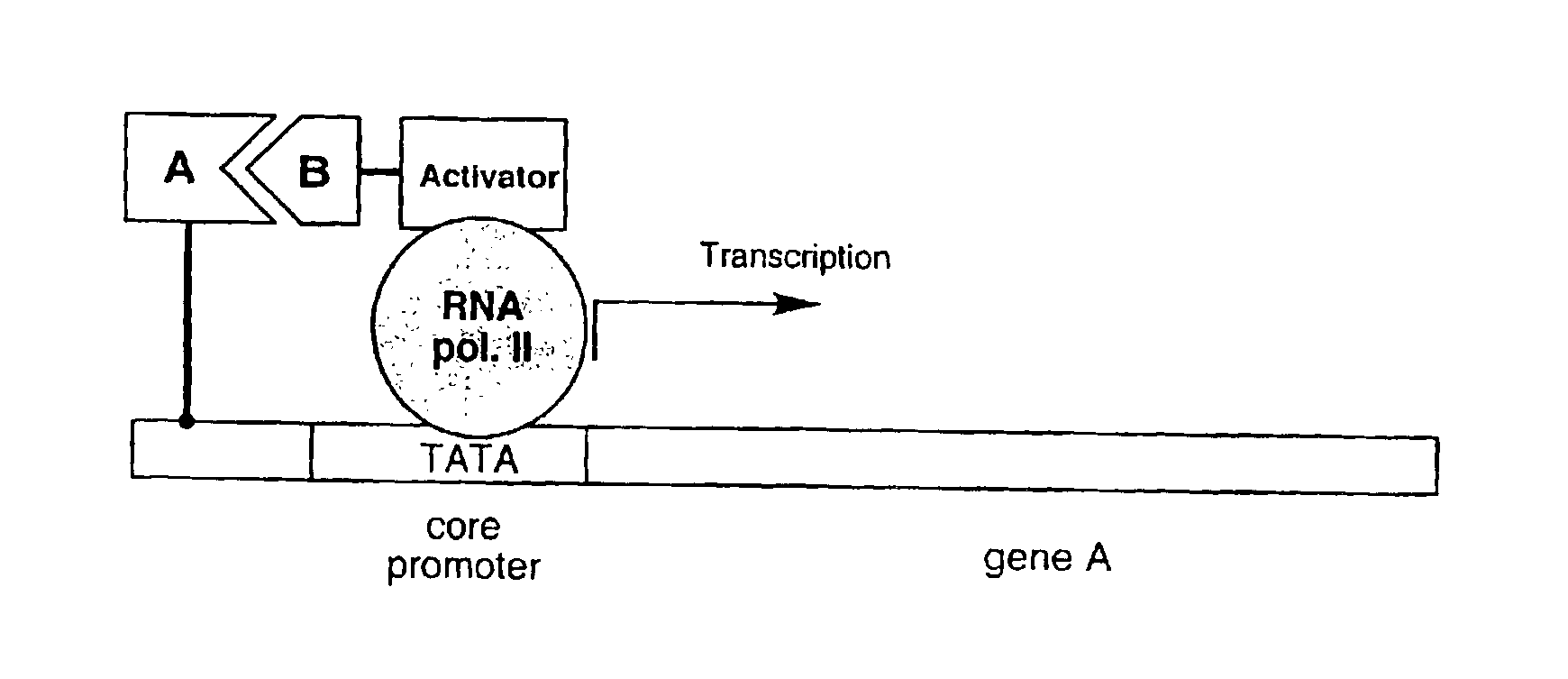
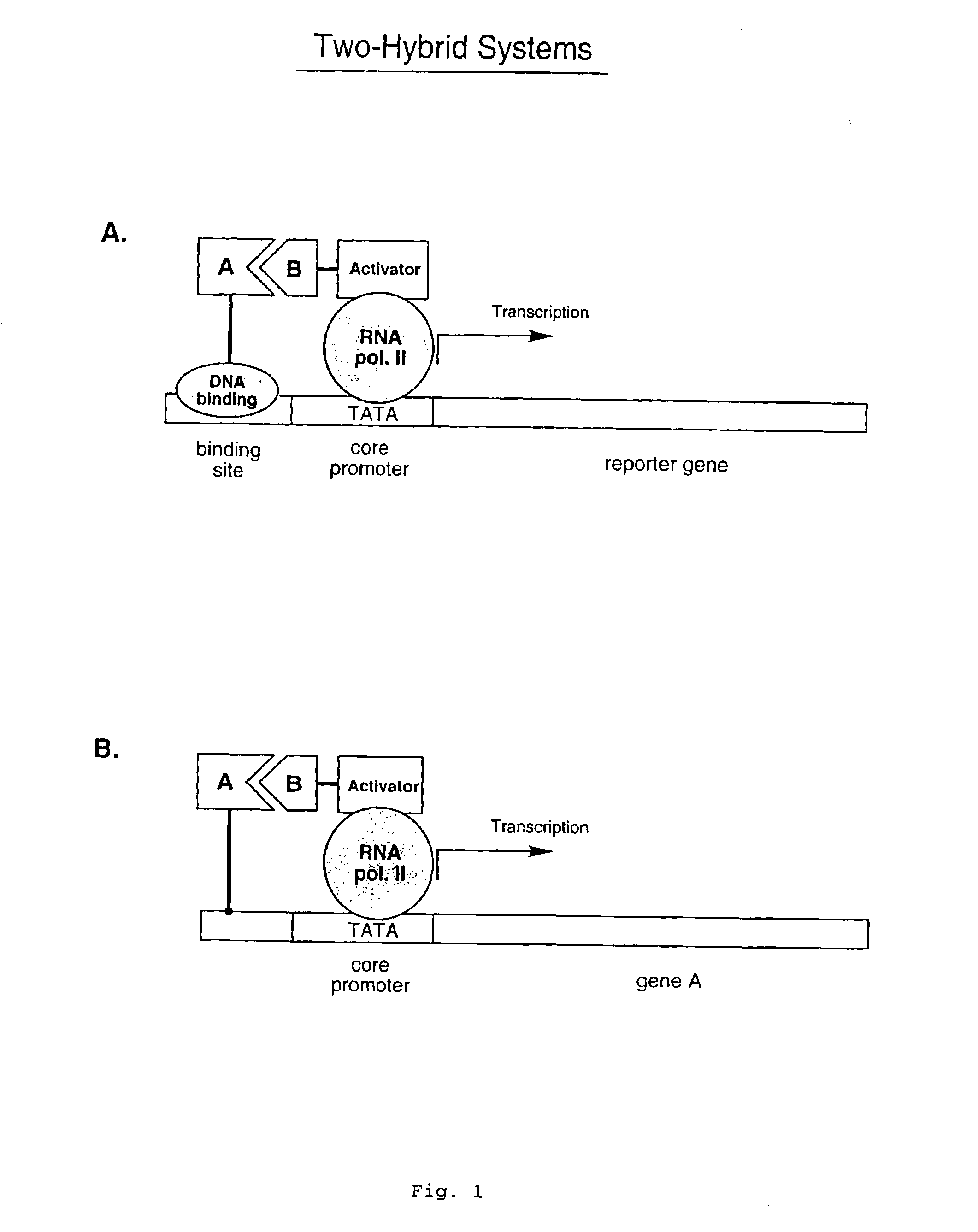
In vitro protein interaction detection systems
ActiveUS6951725B2Reduce transcriptionAvoid interactionMicrobiological testing/measurementDepsipeptidesADAMTS ProteinsArrestin
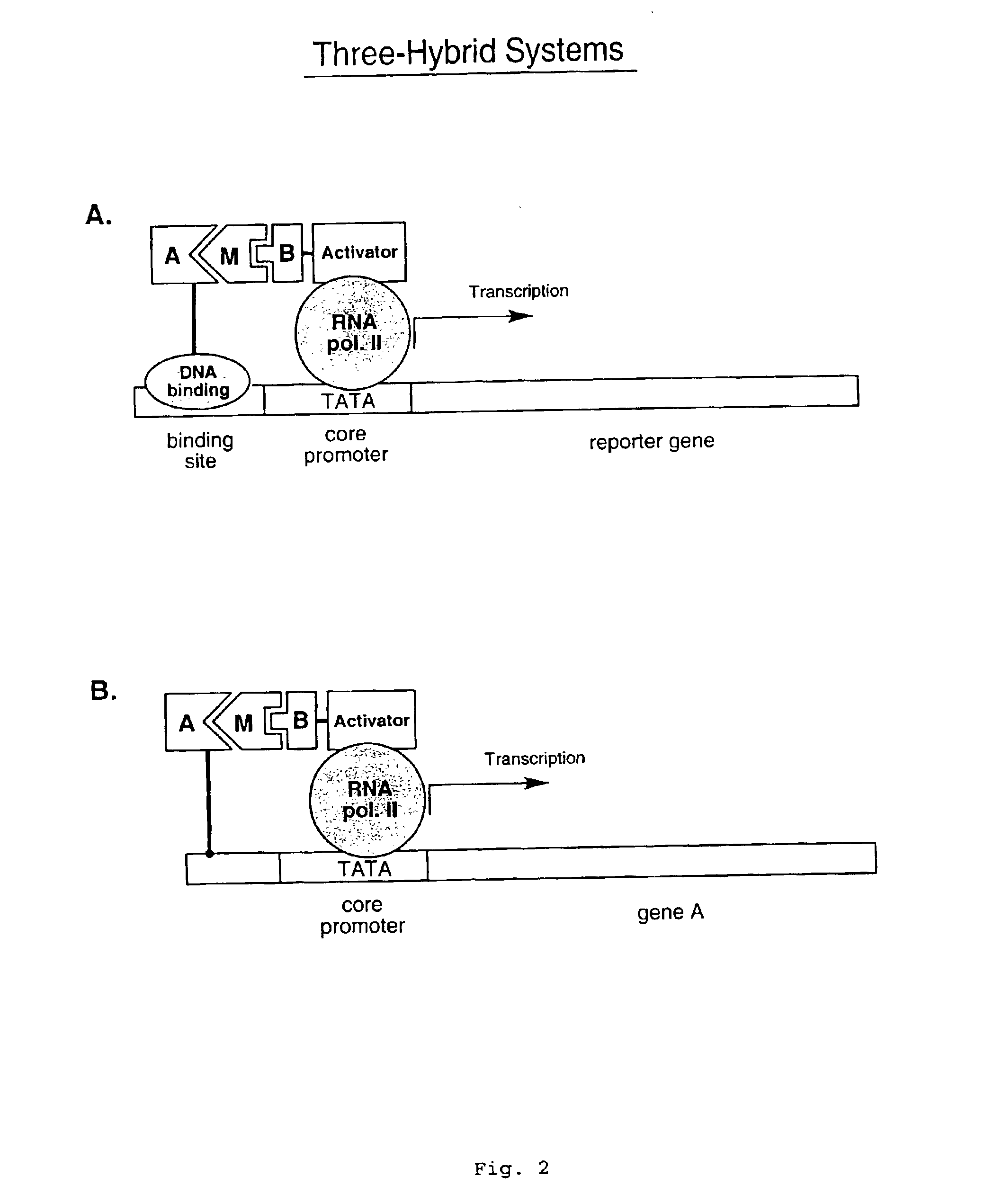
Disclosed herein are in vitro assays for the identification of interactions between proteins or other molecules, the identification of transcriptional activator proteins, and the detection of compounds that inhibit protein / protein or protein / compound interactions. Also disclosed herein are in vitro assays for the selection of interacting proteins and transcriptional activator proteins out of libraries.
Owner:BRISTOL MYERS SQUIBB CO
Modified RCA proteins
InactiveUS6897290B1Suppression of rejection of transplantsReduce tissue damagePeptide/protein ingredientsAntibody mimetics/scaffoldsShort Consensus RepeatADAMTS Proteins
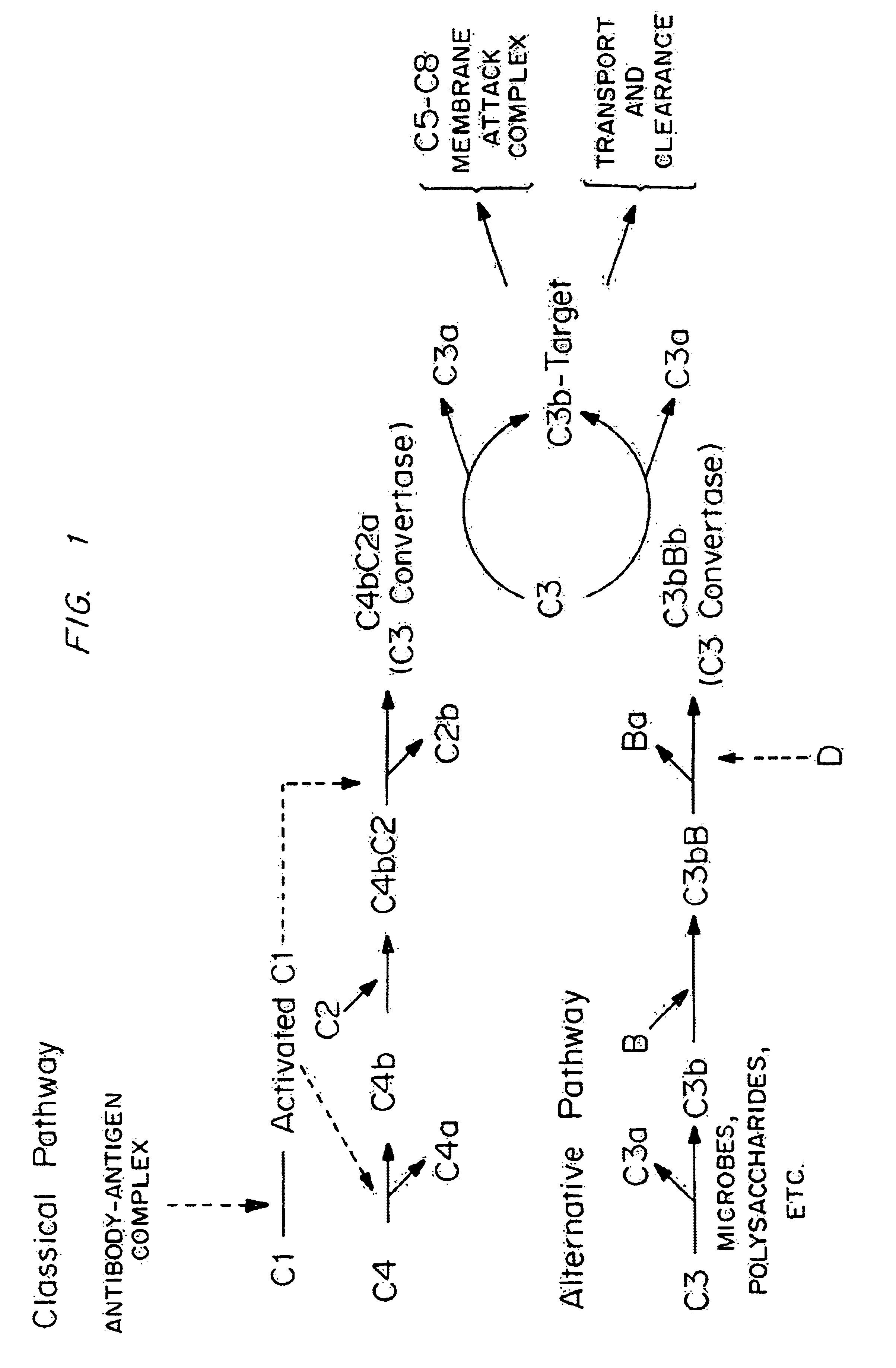
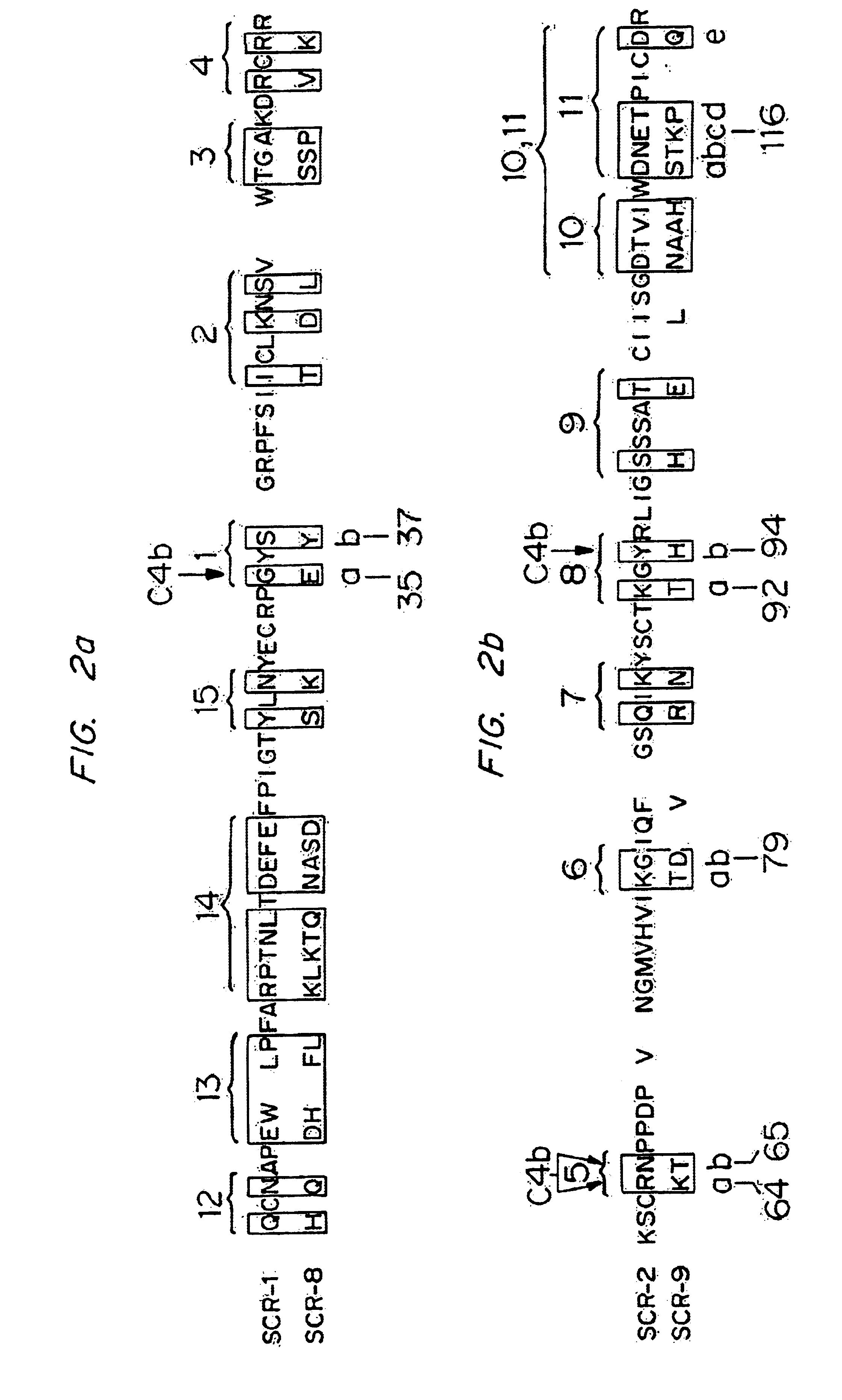
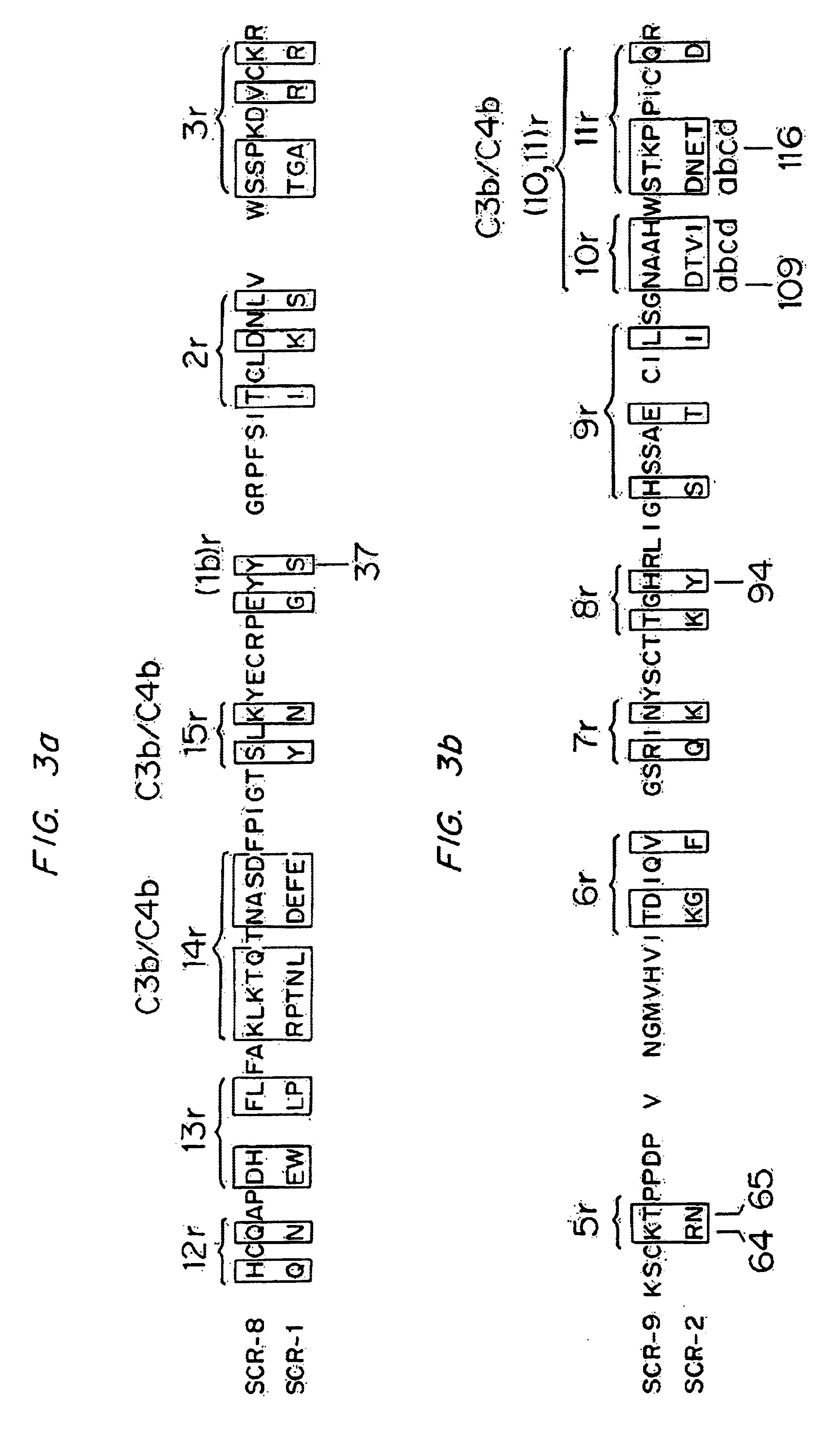
Analogs of regulators of complement activation (RCA) proteins which have altered specificities and affinities for the targets C3b and / or C4b are described. These analogs are obtained by substituting amino acids which affect the complement inhibitory activities of these proteins, substituting, rearranging or adding SCRs (short consensus repeats) or SCR regions to the proteins, deleting amino acid sequences, and combinations thereof.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Enzyme-mediated modification of fibrin for tissue engineering
The invention provides fibrin-based, biocompatible materials useful in promoting cell growth, wound healing, and tissue regeneration. These materials are provided as part of several cell and tissue scaffolding structures that provide particular application for use in wound-healing and tissue regenerating. Methods for preparing these compositions and using them are also disclosed as part of the invention. A variety of peptides may be used in conjunction with the practice of the invention, in particular, the peptide IKVAV, and variants thereof. Generally, the compositions may be described as comprising a protein network (e.g., fibrin) and a peptide having an amino acid sequence that comprises a transglutaminase substrate domain (e.g., a factor XIIIa substrate domain) and a bioactive factor (e.g., a peptide or protein, such as a polypeptide growth factor), the peptide being covalently bound to the protein network. Other applications of the technology include their use on implantable devices (e.g., vascular graphs), tissue and cell scaffolding. Other applications include use in surgical adhesive or sealant, as well as in peripheral nerve regeneration and angiogenesis.
Owner:CALIFORNIA INST OF TECH
Polymorphisms in known genes associated with inflammatory autoimmune disease, methods of detection and uses thereof
The present invention is based on the discovery of novel polymorphisms (SNPs) in the genes known in the art to contribute to inflammatory and autoimmune disorders. Such polymorphisms can lead to a variety of disorders that are mediated / modulated by a variant inflammatory and autoimmune disorders associated protein. The present invention provides reagents used for detecting and expressing the variant nucleic acid / protein sequence as well as methods of identifying and using these variants.
Owner:CELERA CORPORATION +1
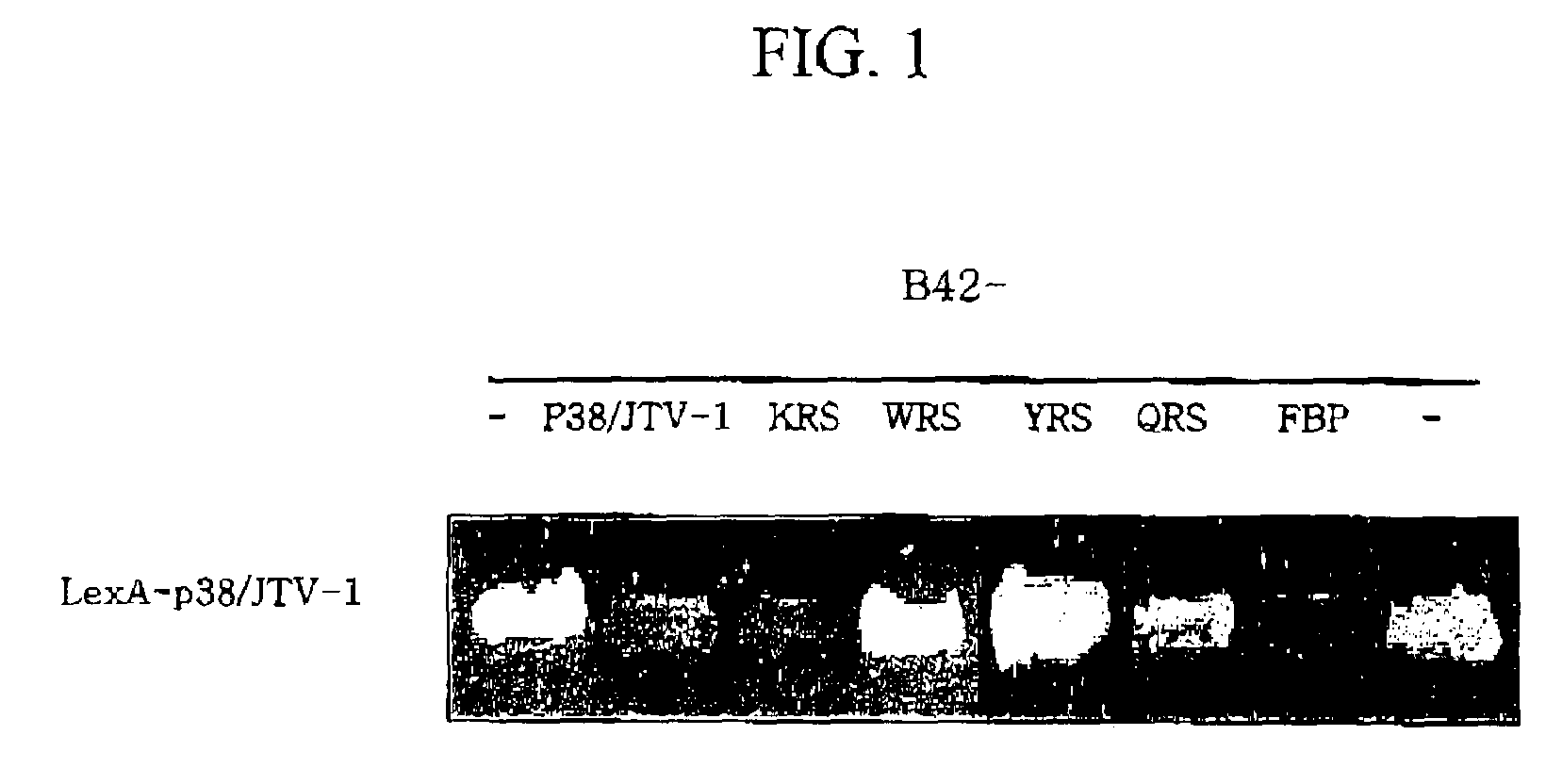
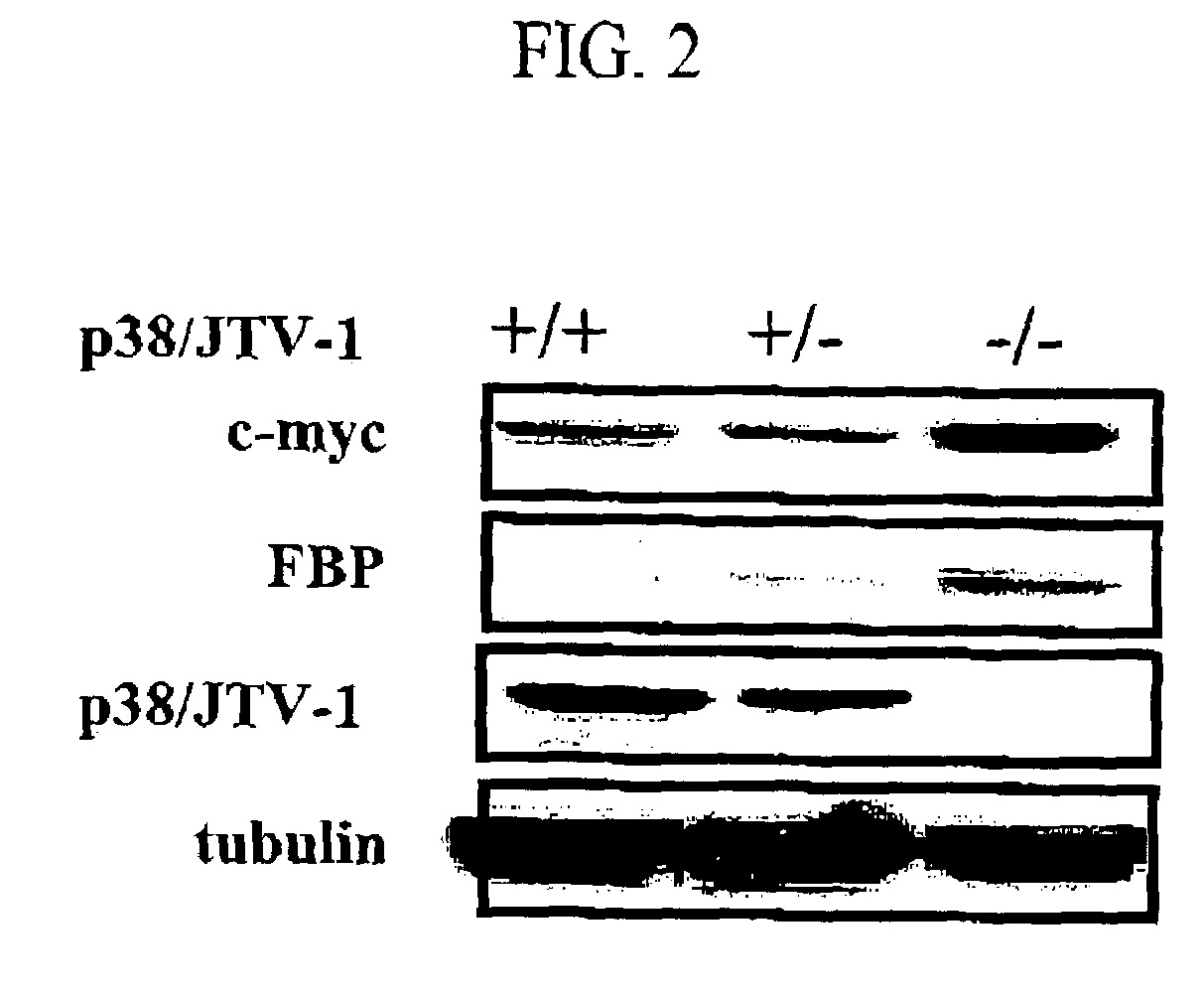
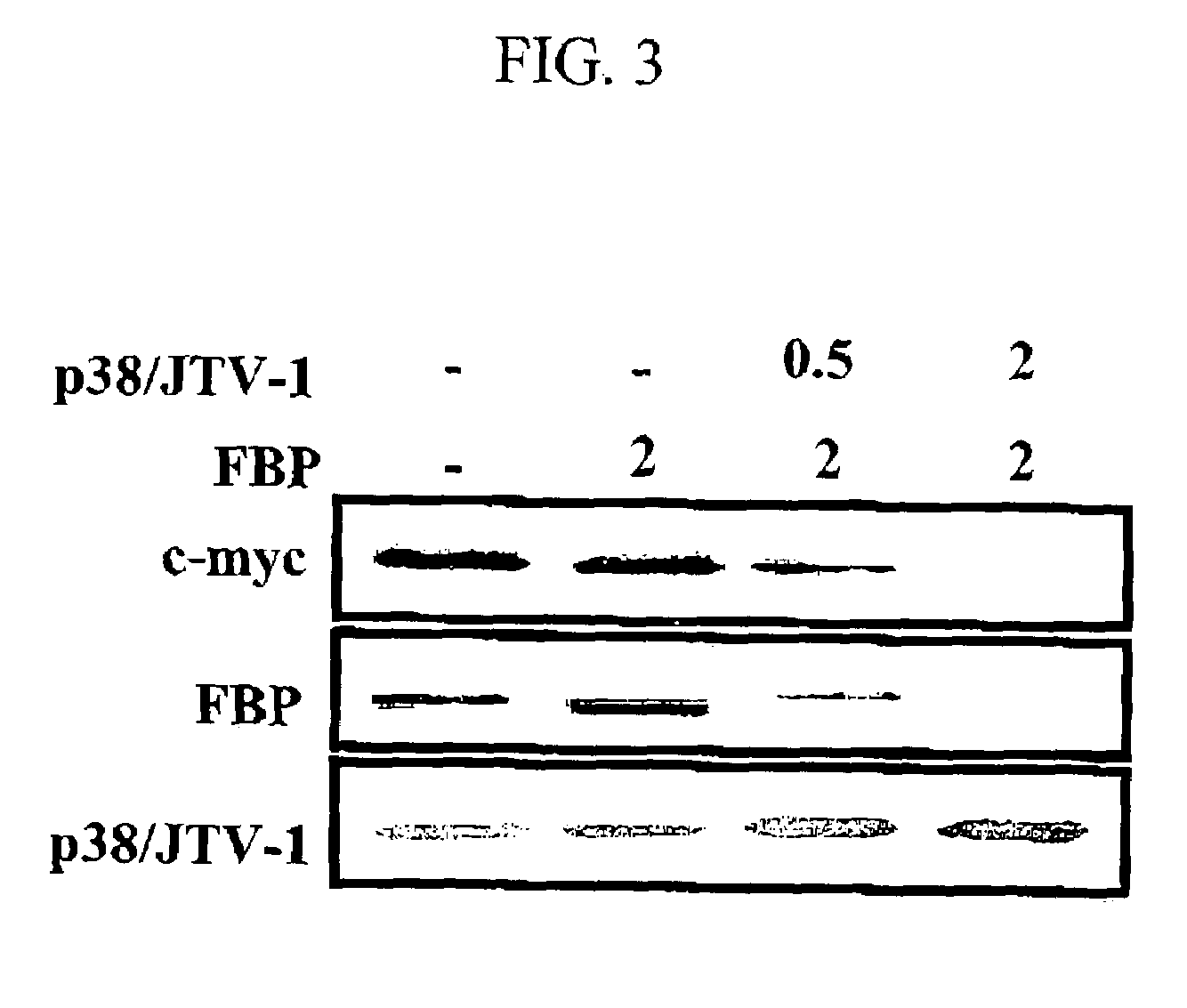
Method for treating cancer using p38/JTV-1 and method for screening pharmaceutical composition for treating cancer
The present invention relates to a method for treating cancer using p38 / JTV-1 and a method for screening pharmaceutical composition for treating cancer. More particularly, this invention relates to the method for treating cancer, which comprises administering the effective amount of p38 / JTV-1 protein or a nucleic acid encoding for said protein to the patient and the method for screening a pharmaceutical composition for treating cancer characterized by selecting a substance having an effect on the increase of the activity of the p38 / JTV-1 protein and the intracellular level thereof.The method according to the invention can be effectively used to treat cancer through the mechanism of suppressing the proliferation of cancer cells by binding to FBP (FUSE-binding protein) and thereby promoting the ubiquitination of FBP to downregulate c-myc gene, which is a proto-oncogene, and the mechanism of promoting the apoptosis of cells by binding to PDK-1 (phosphoinositide-dependent protein) and thereby inhibiting the phosphorylation of AKT (serine / threonine kinase). Also, p38 / JTV-1 can be used as a target for screening of new anticancer agents, by virtue of such regulation mechanisms of p38 / JTV-1.
Owner:MEDICINAL BIOCONVERGENCE RES CENT
Genomic editing of genes involved in autism spectrum disorders
The present invention provides genetically modified animals and cells comprising edited chromosomal sequences encoding proteins associated with ASD. In particular, the animals or cells are generated using a zinc finger nuclease-mediated editing process. Also provided are methods of using the genetically modified animals or cells disclosed herein to study ASD development and screen agents for assessing their effect on progression or symptoms of an ASD.
Owner:SIGMA ALDRICH CO LLC
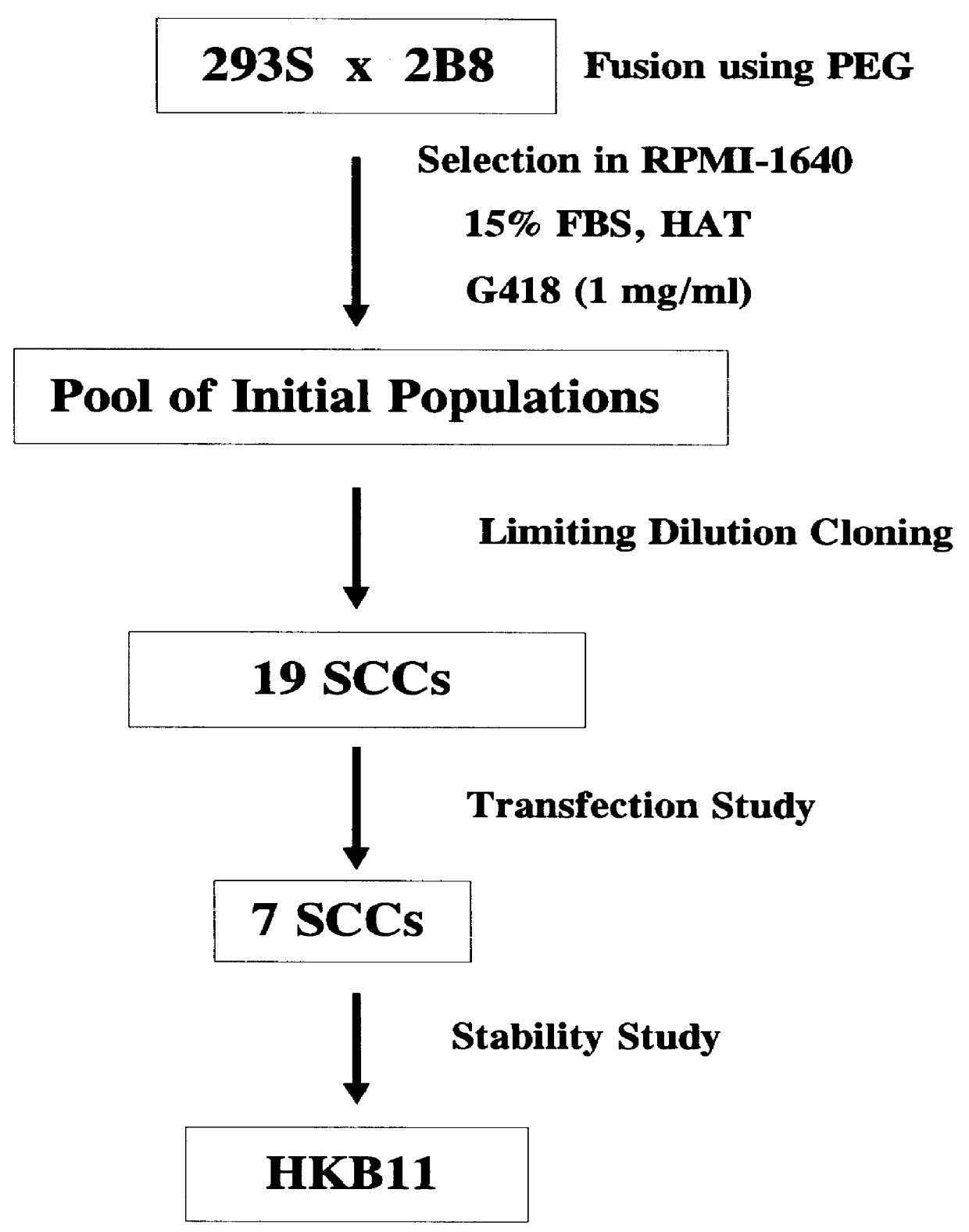
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
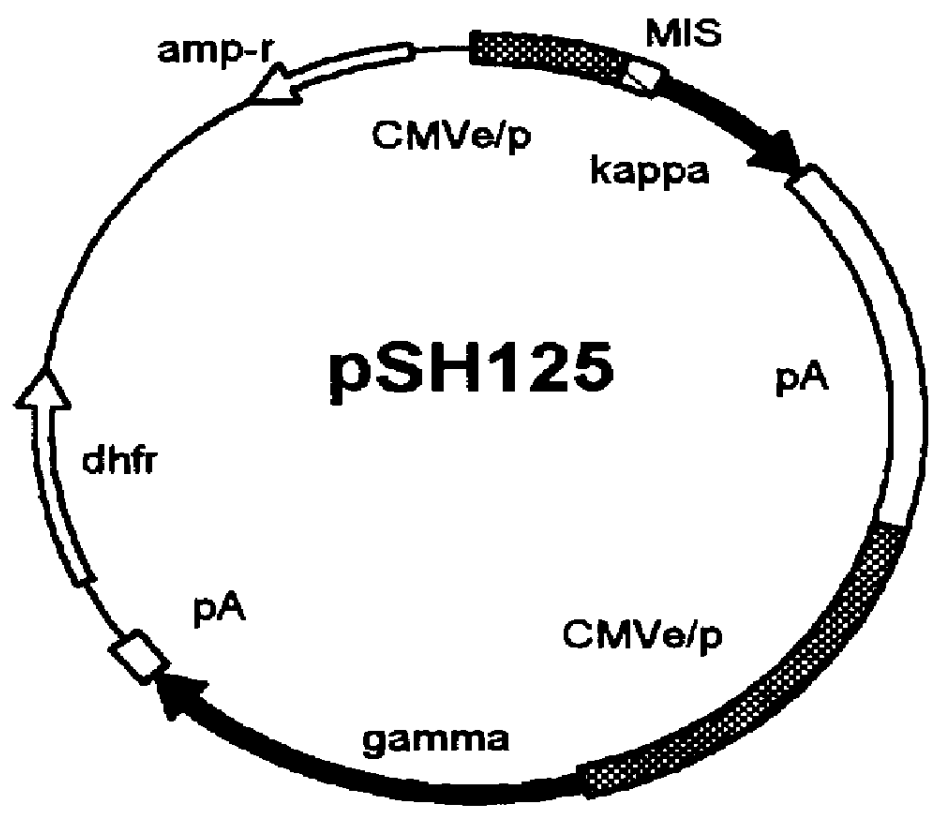
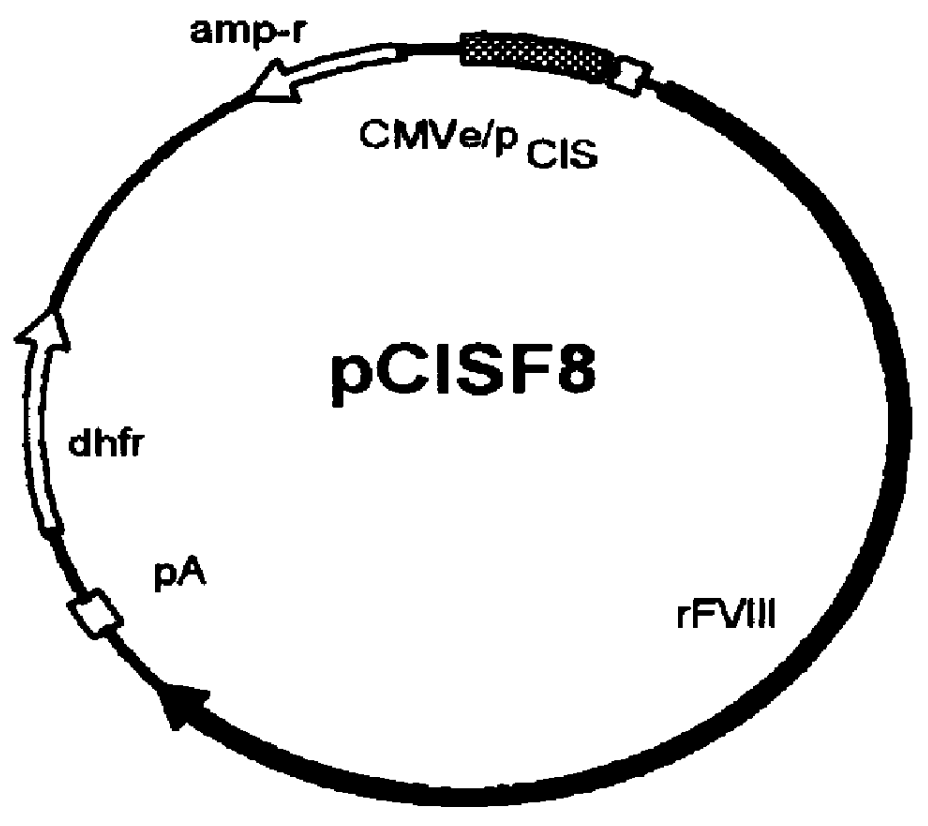
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
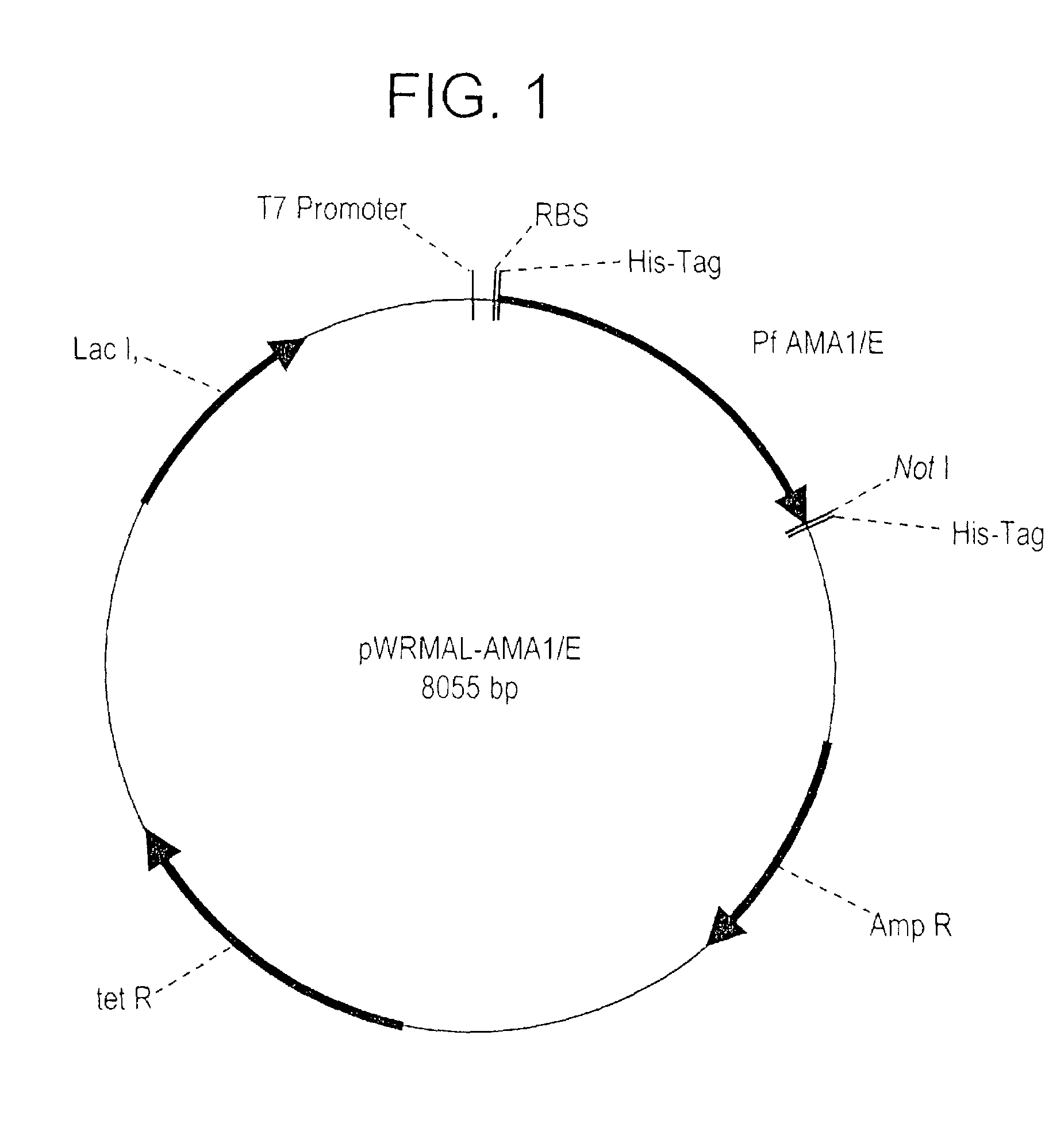
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7029685B2Eliminate the problemImprove responseProtozoaFermentationADAMTS ProteinsMalarial parasites
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
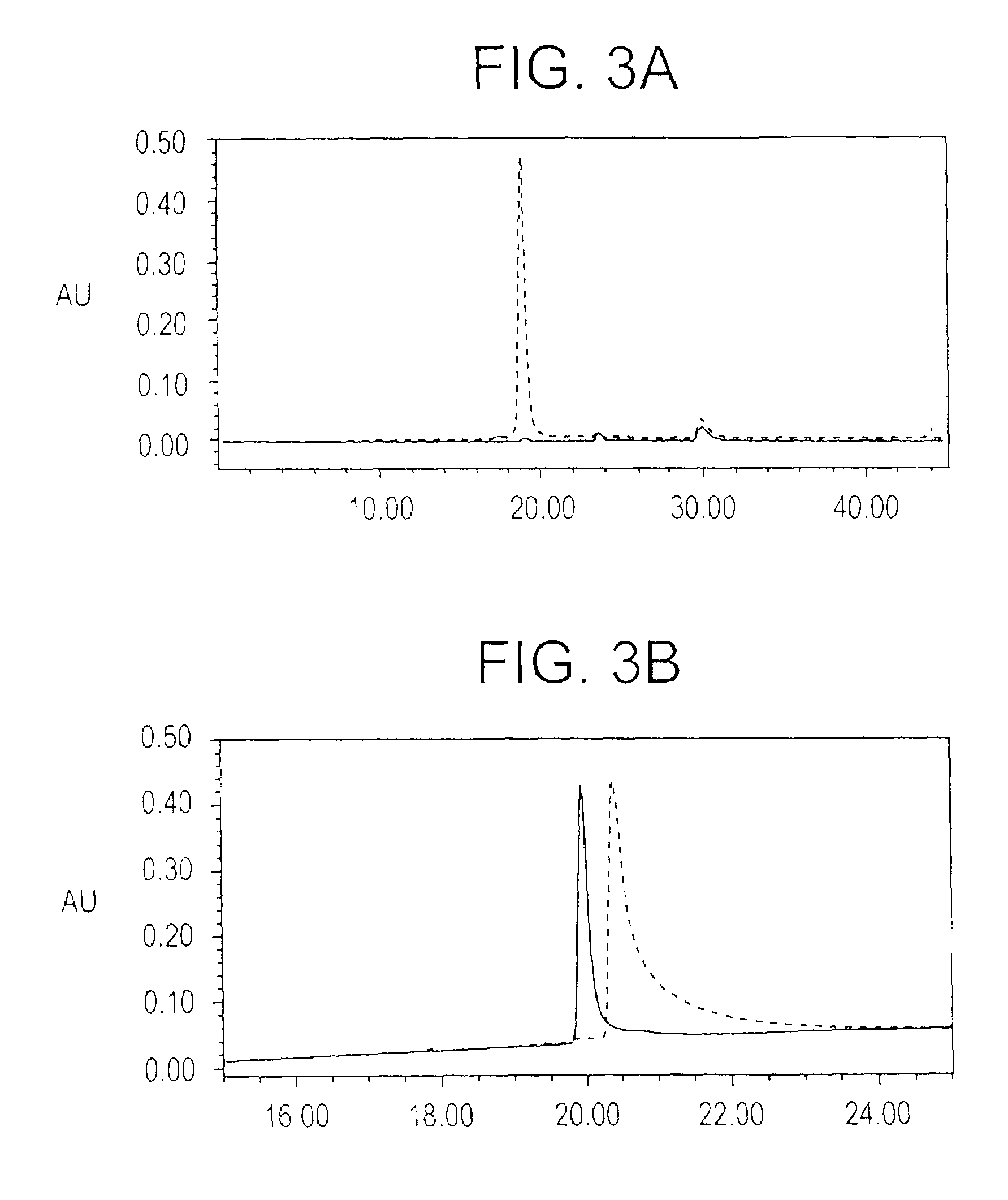
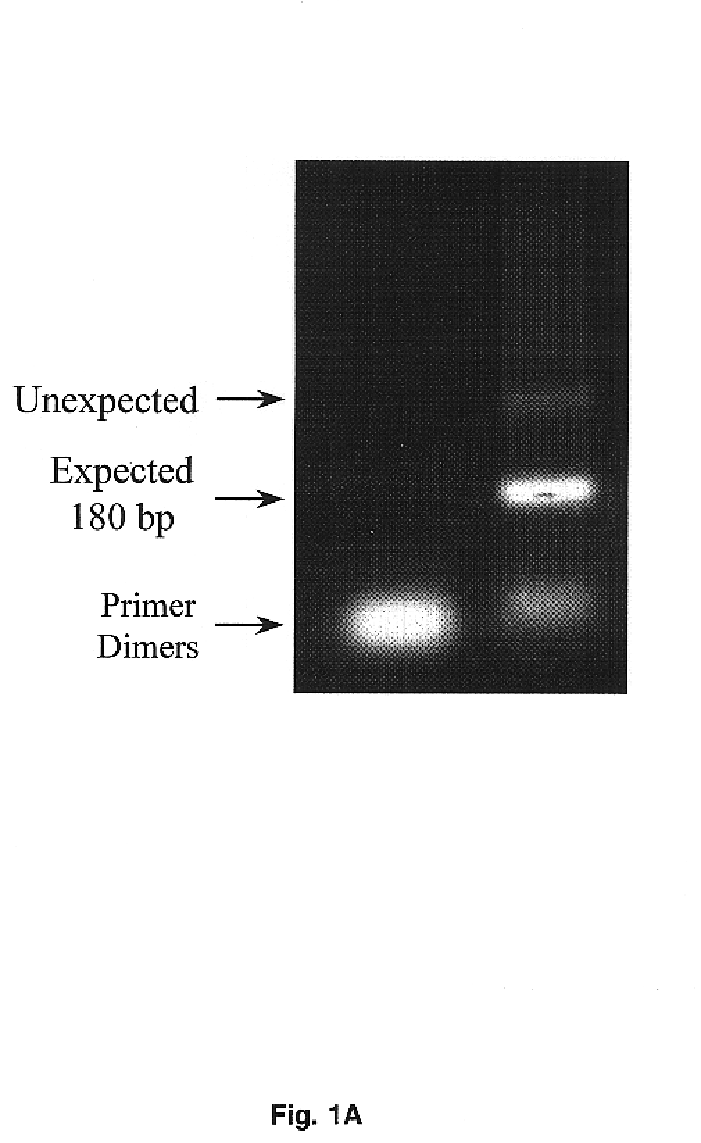
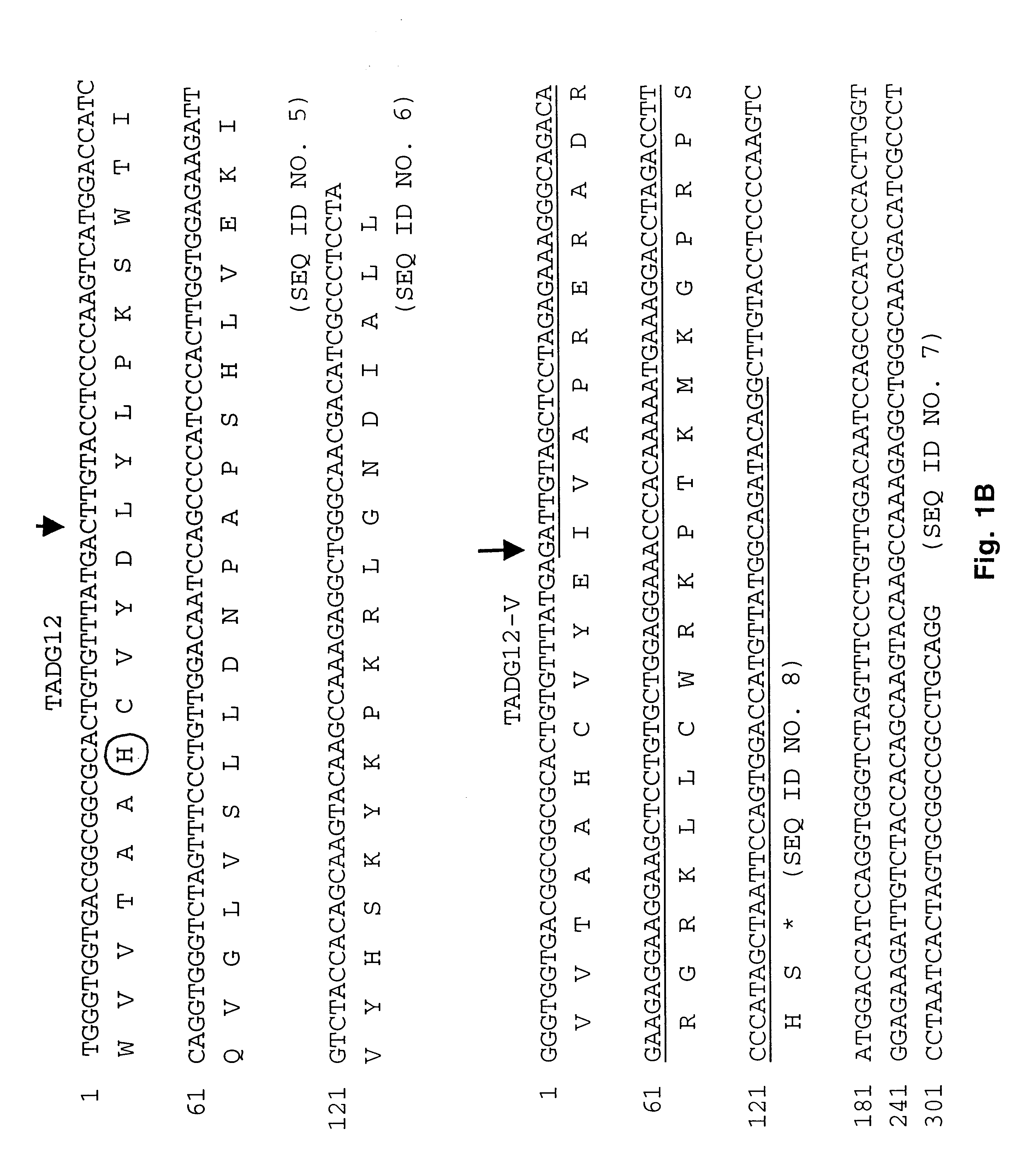
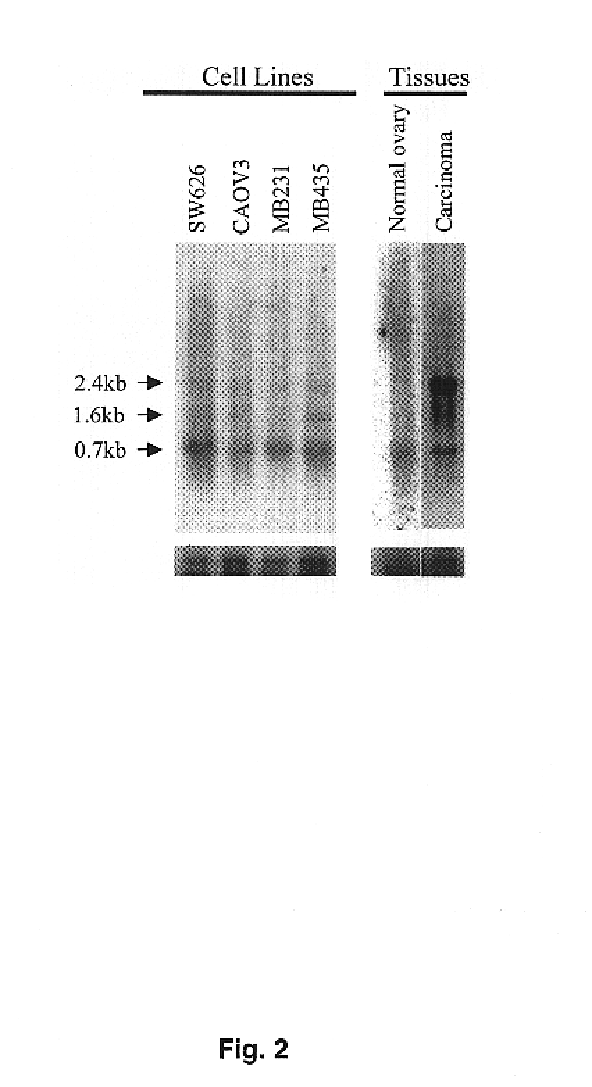
Transmembrane serine protease overexpressed in ovarian carcinoma and uses thereof
InactiveUS6942978B1Inhibit expressionTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsADAMTS ProteinsA-DNA
The present invention provides a TADG-12 protein and a DNA fragment encoding such protein. Also provided is a vector / host cell capable of expressing the DNA. The present invention further provides various methods of early detection of associated ovarian and other malignancies, and of interactive therapies for cancer treatment utilizing the DNA and / or protein disclosed herein.
Owner:BIOVENTURES LLC
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Active immunization using a siderophore receptor protein
InactiveUS6432412B1Easy to produceInhibition capacityAntibacterial agentsBiocideADAMTS ProteinsMicrobiology
Owner:EPITOPIX LLC
Targeting proteins to cells expressing mannose receptors via expression in insect cells
The present invention is based on the discovery that proteins produced in insect cell cultures are glycosylated in a unique manner that causes them to be selectively imported by cells that express mannose receptors on their membranes, particularly macrophages. Proteins that are selectively imported into cells containing mannose receptors are provided, as well as pharmaceutical compositions containing such proteins and methods for producing such proteins. Application of the present invention to produce proteins useful for treating lysosomal storage disorders is also disclosed. Engineering of cells to express mannose receptors so that they will selectively import proteins produced in insect cells is also taught, as well as a protein targeting system using such cells and proteins. Finally, an improved elution buffer for the purification of proteins produced in insect cells from a Concanavalin A column is provided.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Methods and compositions for increasing longevity and protein yield from a cell culture
ActiveUS7531327B2Increase longevity and recombinant protein yieldIncreases lifespan and viabilityPeptide/protein ingredientsApoptosis related proteinsApoptosisHormone
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com