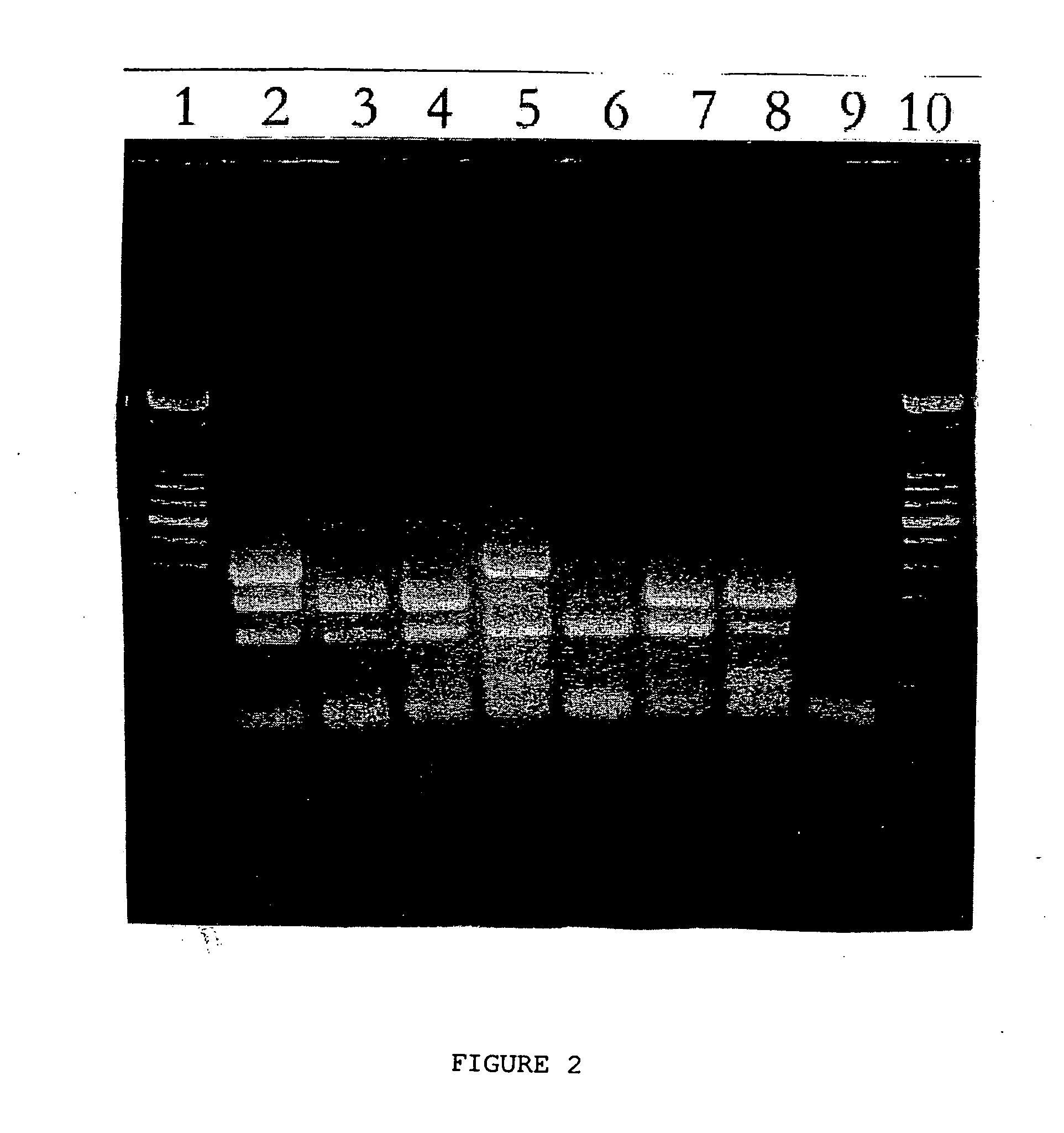
Patents
Literature
348 results about "Rotavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A contagious virus that cause inflammation of the stomach and intestines leading to diarrhea.
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
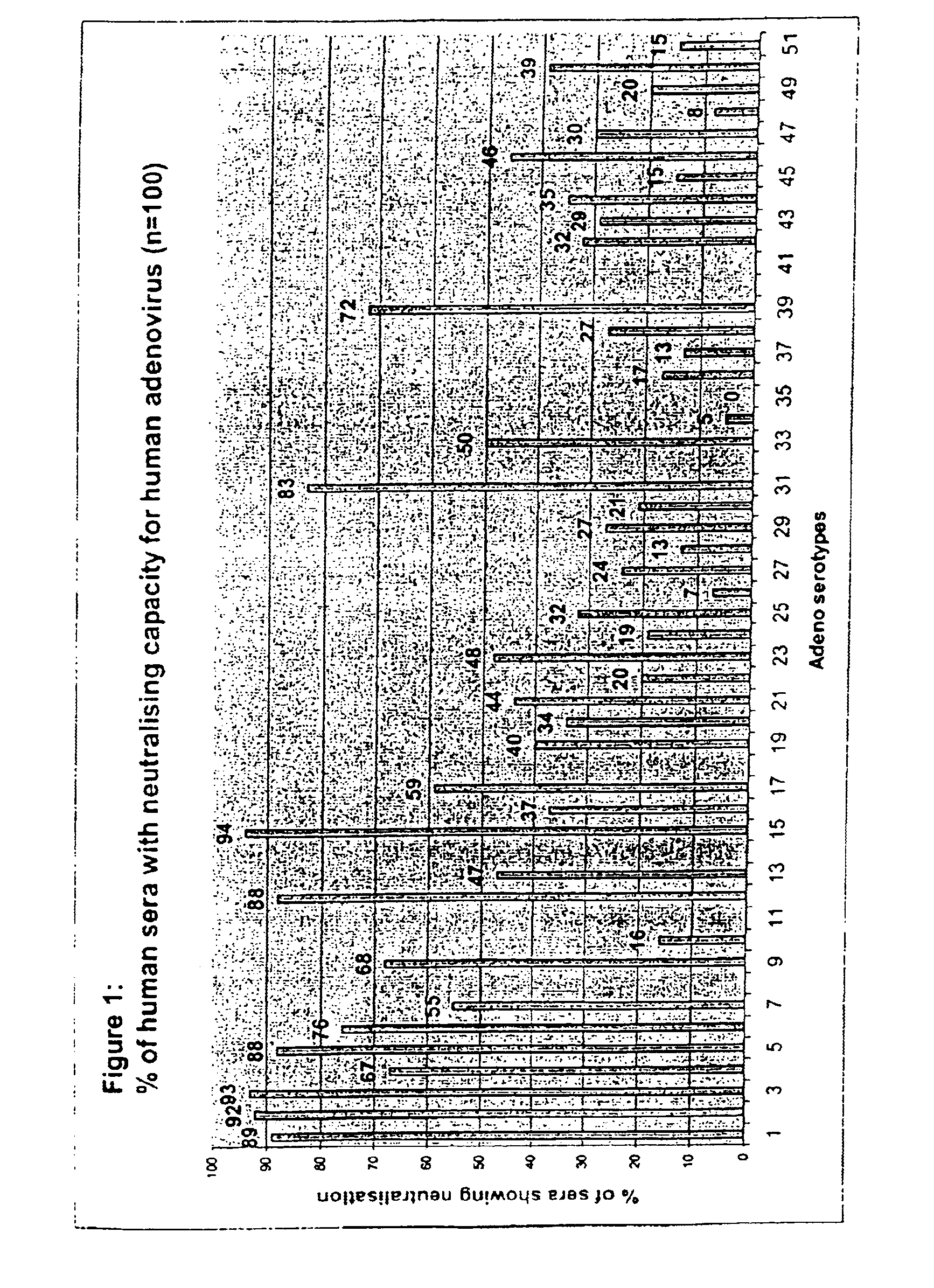
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
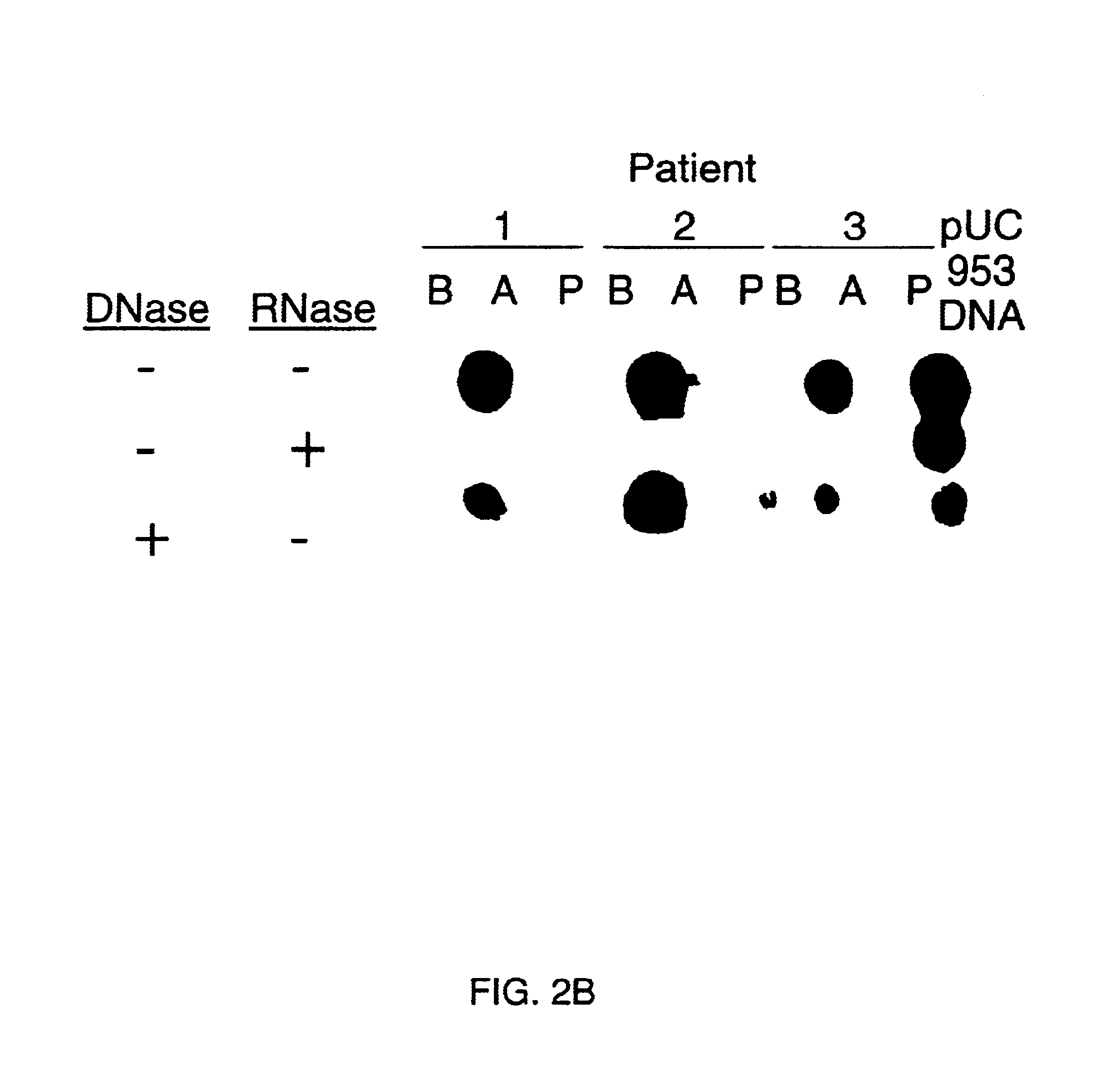
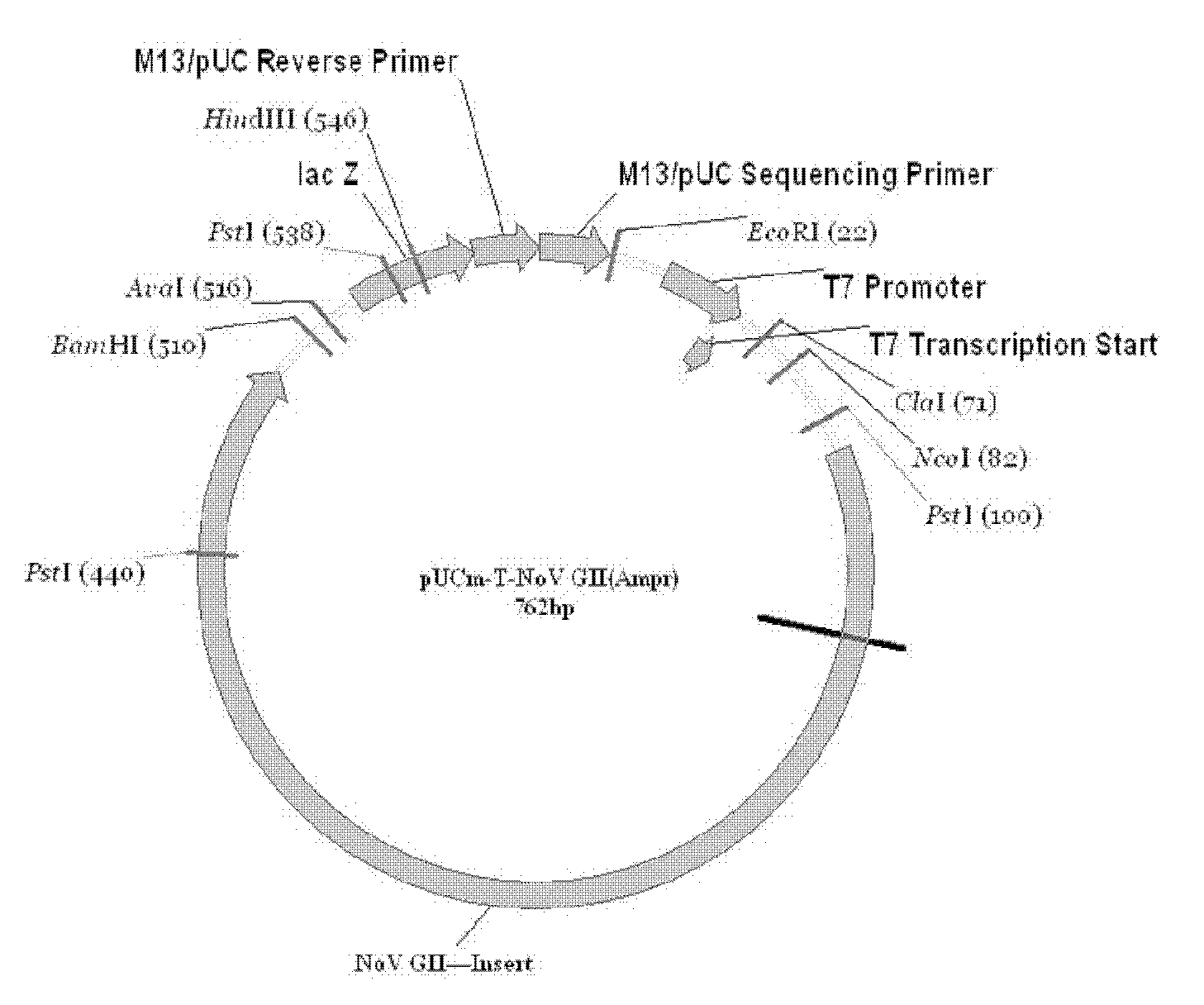
Methods and reagents to detect and characterize norwalk and related viruses
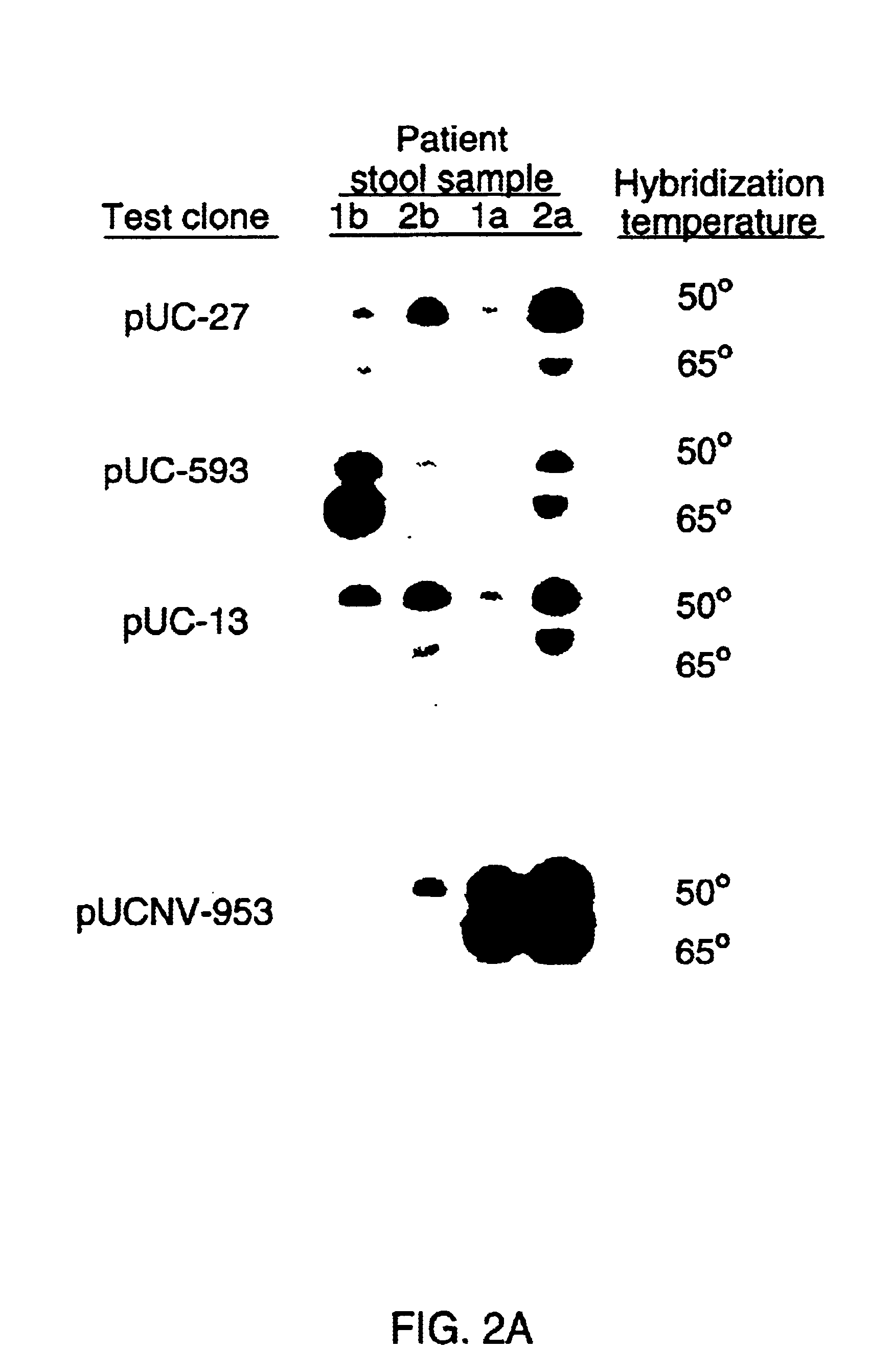
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
Infant milk powder added with biostime and preparation method thereof
ActiveCN101449708AAdvantage maintenanceColonization fastMilk preparationFood preparationTreatment effectVegetable oil
The invention discloses an infant milk power added with synbiotics, comprising the following components: fresh milk 40-68 gram; demineralized whey powder 15-34 gram; granulated sugar3-6 graml; vegetable oil 4-8 gram; compound prebiotics 0.4-20 gram; compound probiotic 1.0*10<5>-1.0*10<11> cfu. The inventive infant milk power can be ysed for building advantages of beneficial bacterium when a newborn infant starts colony valuation. Through adding probiotic, the number of probiotic is advanced to increase in a short time, through adding prebiotics having selectively increasing effect on probiotic, the added probiotic is performed with fast field setting, thereby maintaining advantages of probiotic in intestinal flora. The invention also markedly relieves diarrhoetic symptoms, and has definite treatment effect on diarrhea caused by rotavirus.
Owner:SHANDONG LONGLIVE BIO TECH CO LTD
Therapeutic antimicrobial compositions and methods
InactiveUS20050271711A1Applied to skinImmediate and residual effectivenessAntibacterial agentsBiocideOrganic acidRotavirus RNA
Therapeutic antimicrobial compositions and methods for providing enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respitory syricytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, therapeutic antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain, and therapeutic methods of use thereof.
Owner:THE PROCTER & GAMBLE COMPANY
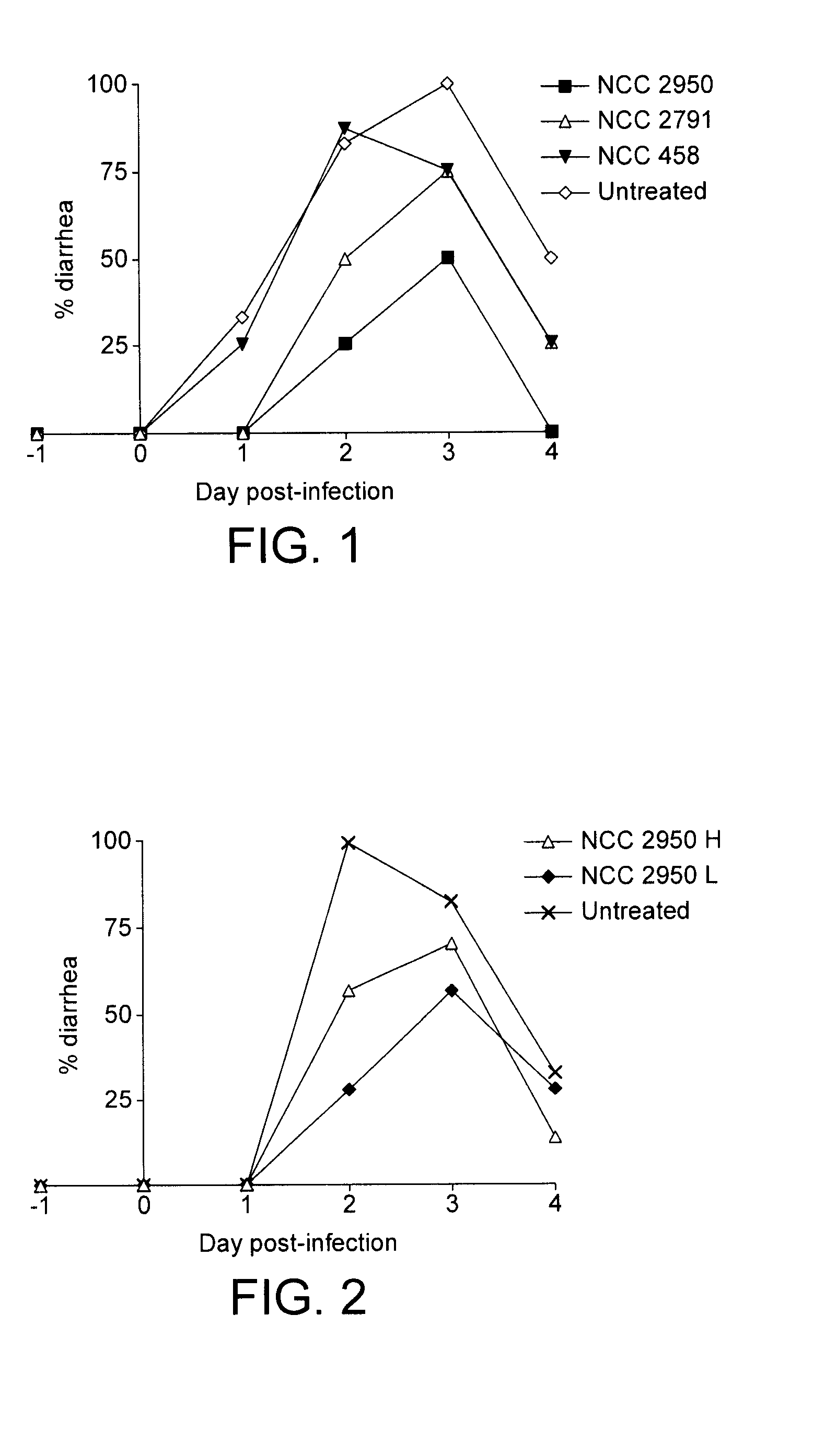
Prevention and treatment of rotavirus diarrhoea
InactiveUS20120107279A1Few infectionEffective preventionBiocideBacteriaBifidobacterium breveRotavirus
This invention relates to Bifidobacterium breve CNCM I-3865, to a composition comprising Bifidobacterium breve CNCM I-3865 and to the use of Bifidobacterium breve CNCM I-3865 in the prevention or treatment of rotavirus diarrhoea. prevention and treatment of rotavirus diarrhoea
Owner:NESTEC SA
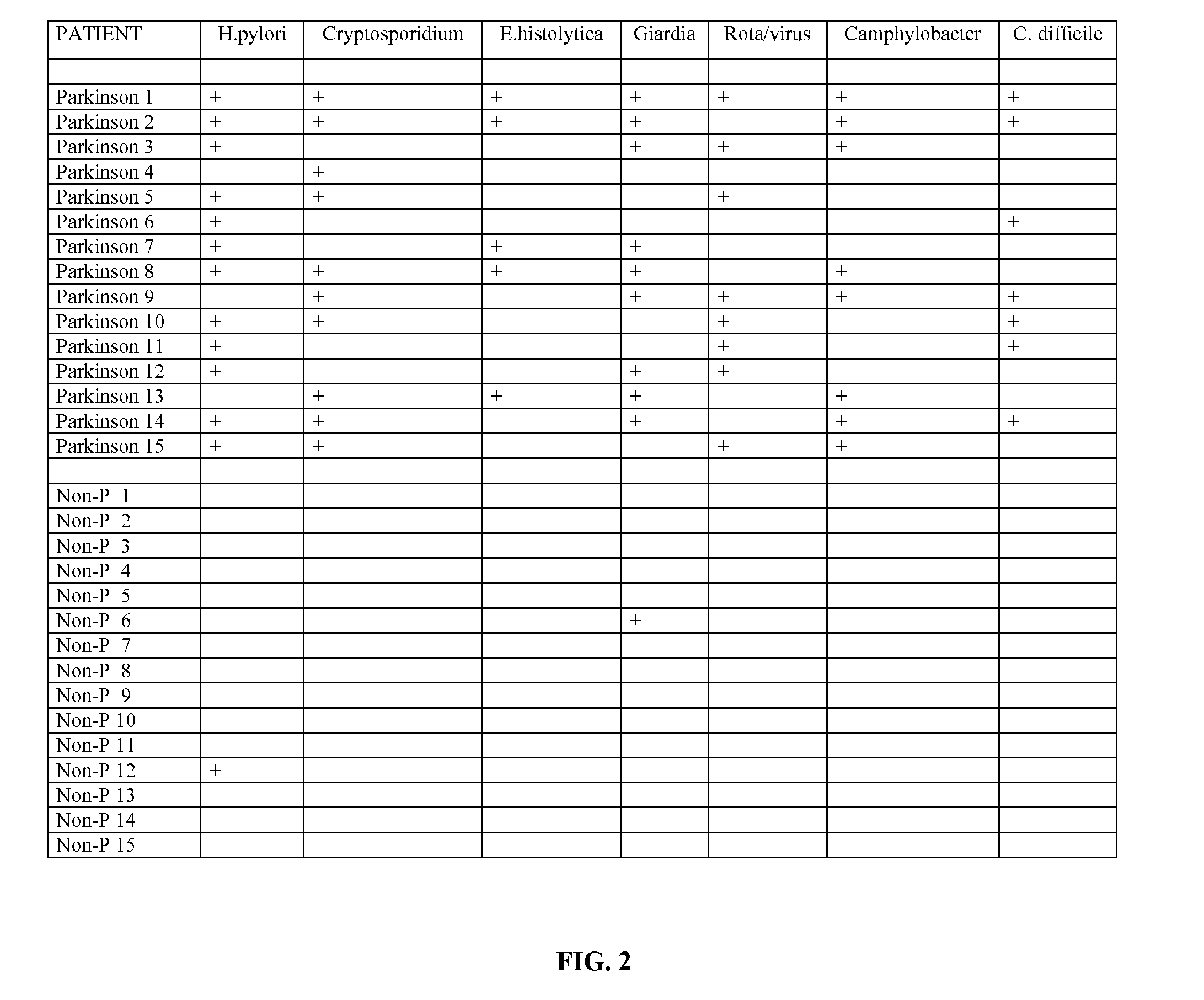
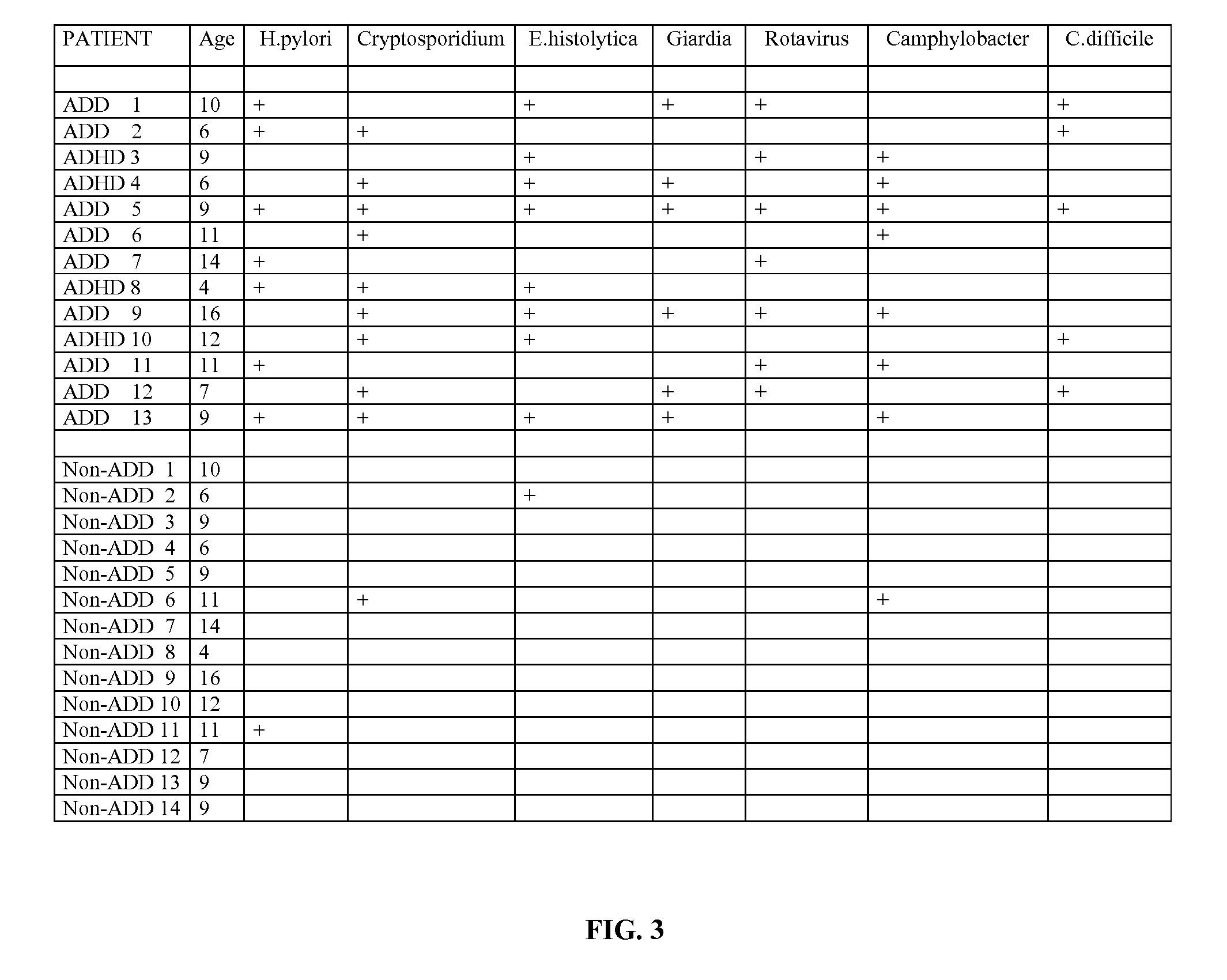
Methods for Diagnosing Pervasive Development Disorders, Dysautonomia and Other Neurological Conditions
Methods for aiding in the diagnosis of disorders including, but not limited to, PDDs (Pervasive Development Disorders), Dysautonomic disorders, Parkinson's disease and SIDS (Sudden Infant Death Syndrome). In one aspect, a diagnosis method comprises analyzing a stool sample of an individual for the presence of a biological marker (or marker compound) comprising one or more pathogens, which provides an indication of whether the individual has, or can develop, a disorder including, but not limited to, a PDD, Dysautonomia, Parkinsons disease and SIDS. Preferably, the presence of one or more pathogens is determined using a stool immunoassay to determine the presence of antigens in a stool sample, wherein such antigens are associated with one or more pathogens including, but not limited to, Giardia, Cryptosporidium, E. histolytica, C. difficile, Adenovirus, Rotavirus or H. pylori.
Owner:CUREMARK
Antimicrobial compositions, products and methods employing same
InactiveUS7569530B1Applied to skinImmediate and residual effectivenessAntibacterial agentsCosmetic preparationsOrganic acidGram-positive bacterium
Antimicrobial compositions that provide enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respiratory syncytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain. Further, products incorporating the antimicrobial compositions of the present invention and methods of using the antimicrobial compositions and products are disclosed herein.
Owner:THE PROCTER & GAMBLE COMPANY
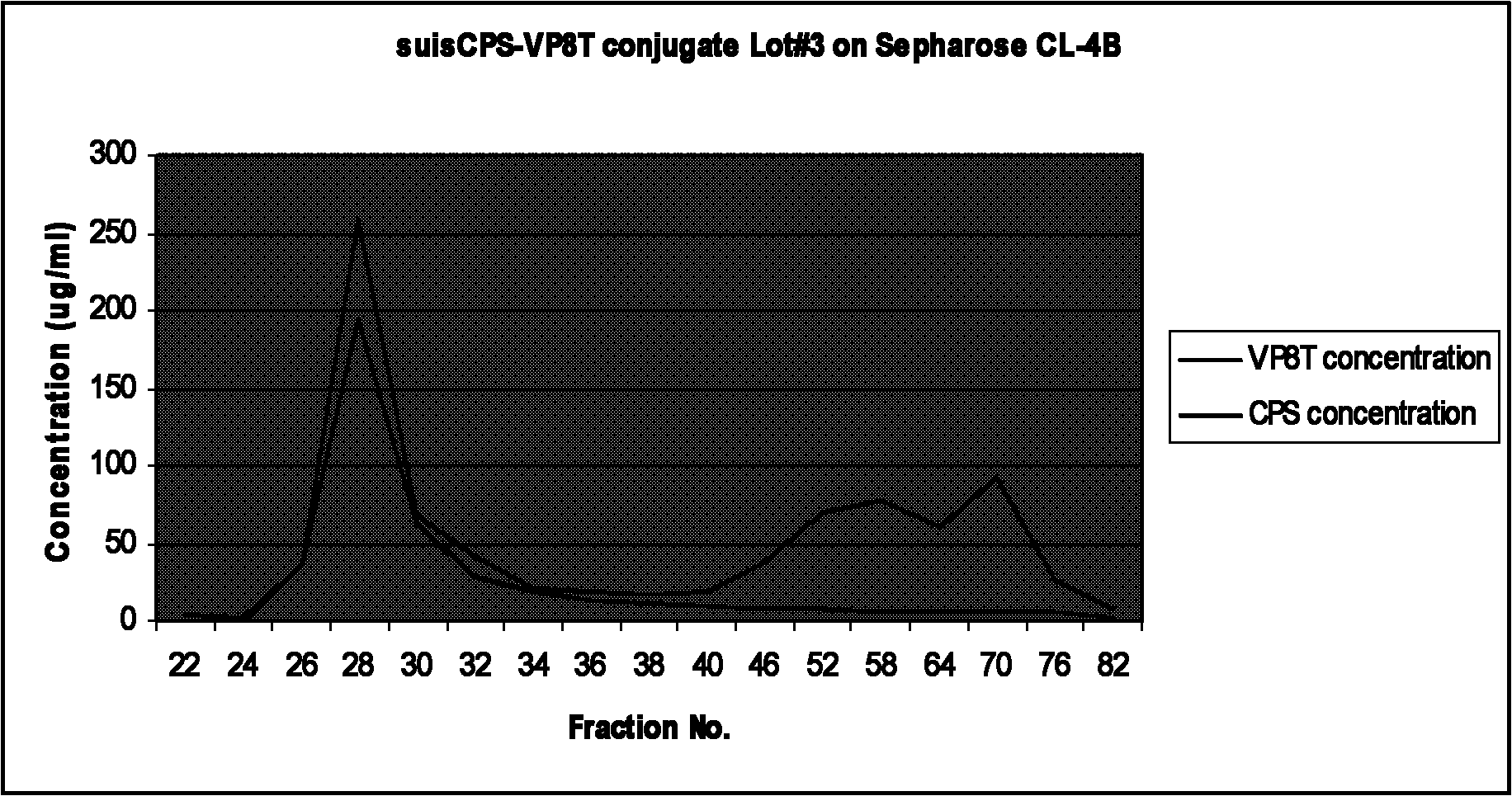
Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
The invention relates to a bacterial polysaccharide-protein conjugate vaccine with immunogenicity, in particular to a conjugate vaccine which is formed by connecting a recombinant rotavirus protein with a bacterial polysaccharide by using a covalent bond, a nucleotide sequence for coding the recombinant rotavirus protein, a recombinant expression system, a protein expressed by the recombinant expression system, a preparation method of the conjugate vaccine and a pneumococcus polysaccharide-recombinant rotavirus protein conjugate vaccine. The bacterial polysaccharide is connected with a recombinant rotavirus surface protein through a covalent bond. The recombinant rotavirus protein is selected from a partial or complete amino acid sequence of a P-gene rotavirus protein and a partial or complete amino acid sequence of a G-gene rotavirus protein.
Owner:普大生物科技(泰州)有限公司
Multiplex rt-pcr/pcr for simultaneous detection of bovine coronavirus, bovine rotavirus, cryptosporidium parvum, and escherichia coli
InactiveUS20050026144A1Rapid and sensitive and specificRapid and sensitive and detectionSugar derivativesMicrobiological testing/measurementBovine rotavirusEscherichia coli
The present invention provides a multiplex RT-PCR / PCR method, which enables in a single assay the simultaneous detection of any combination of bovine rotavirus, bovine coronavirus, Cryptosporidium parvum, and optionally, Escherichia coli strains producing K99 pili or heat-stable enterotoxin STa.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
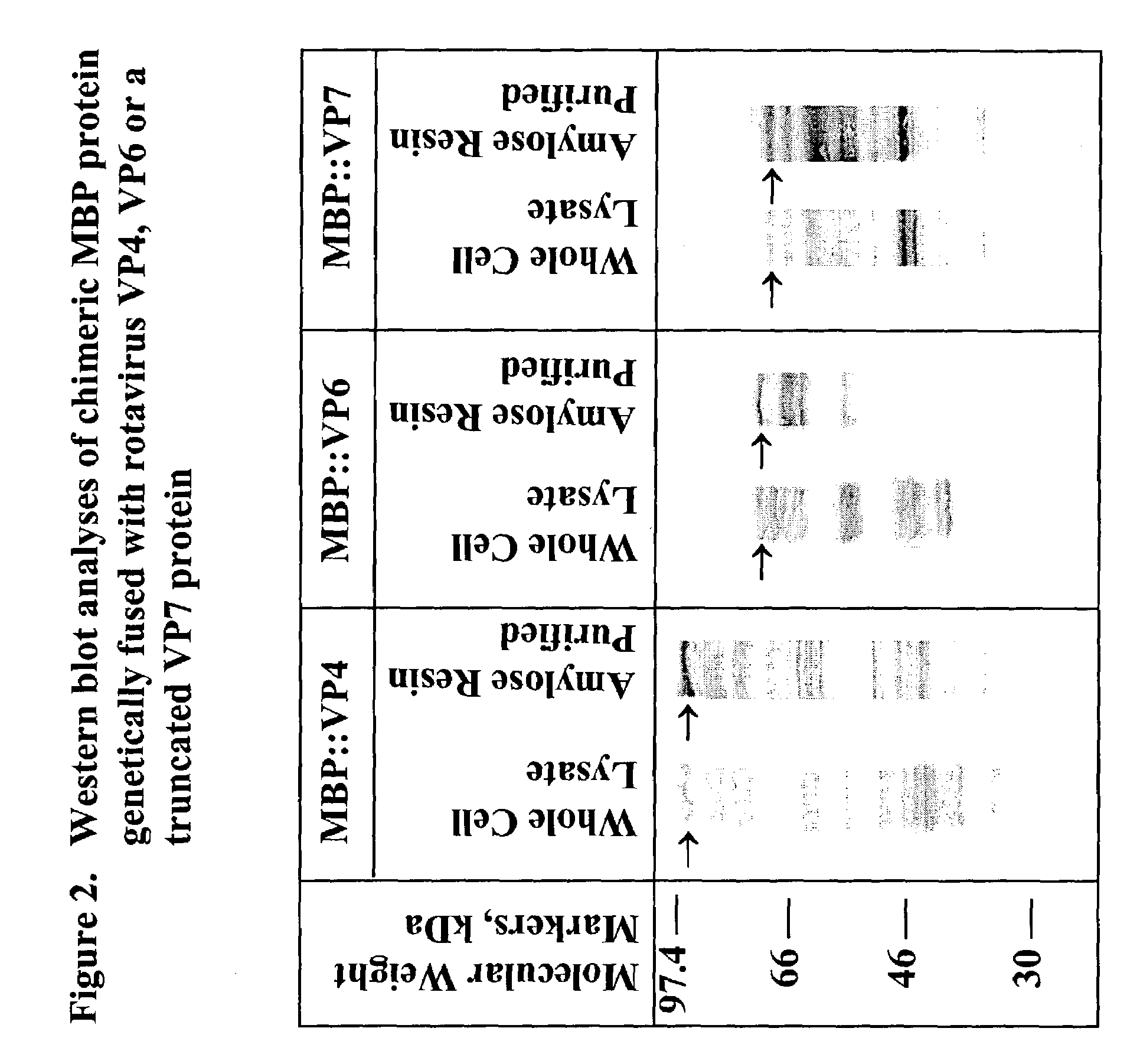
Rotavirus subunit vaccine
The present invention is directed to the generation and use of recombinant rotavirus fusion proteins as immunogens to produce a protective immune response from immunized individuals. In one embodiment, the present invention contemplates a recombinant rotavirus fusion protein vaccine composition comprising a rotavirus subunit protein or immunogenic fragment thereof, and an adjuvant in combination with the recombinant rotavirus subunit fusion protein. In one aspect of this embodiment, the recombinant rotavirus fusion protein comprises a rotavirus subunit protein and a fusion partner protein in genetic association with the rotavirus subunit protein, wherein the fusion partner protein does not interfere with expression and immunogenicity of the rotavirus subunit protein, the fusion partner protein prevents complex formation by the rotavirus subunit protein, and the fusion partner protein facilitates purification of the recombinant rotavirus fusion protein. In another aspect of this embodiment, the rotavirus subunit protein is selected from the group consisting of VP1, VP2, VP3, VP4, VP6, VP7, NSP1, NSP2, NSP3, NSP4 or NSP5. In yet another aspect of this embodiment, the rotavirus subunit protein is VP6.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
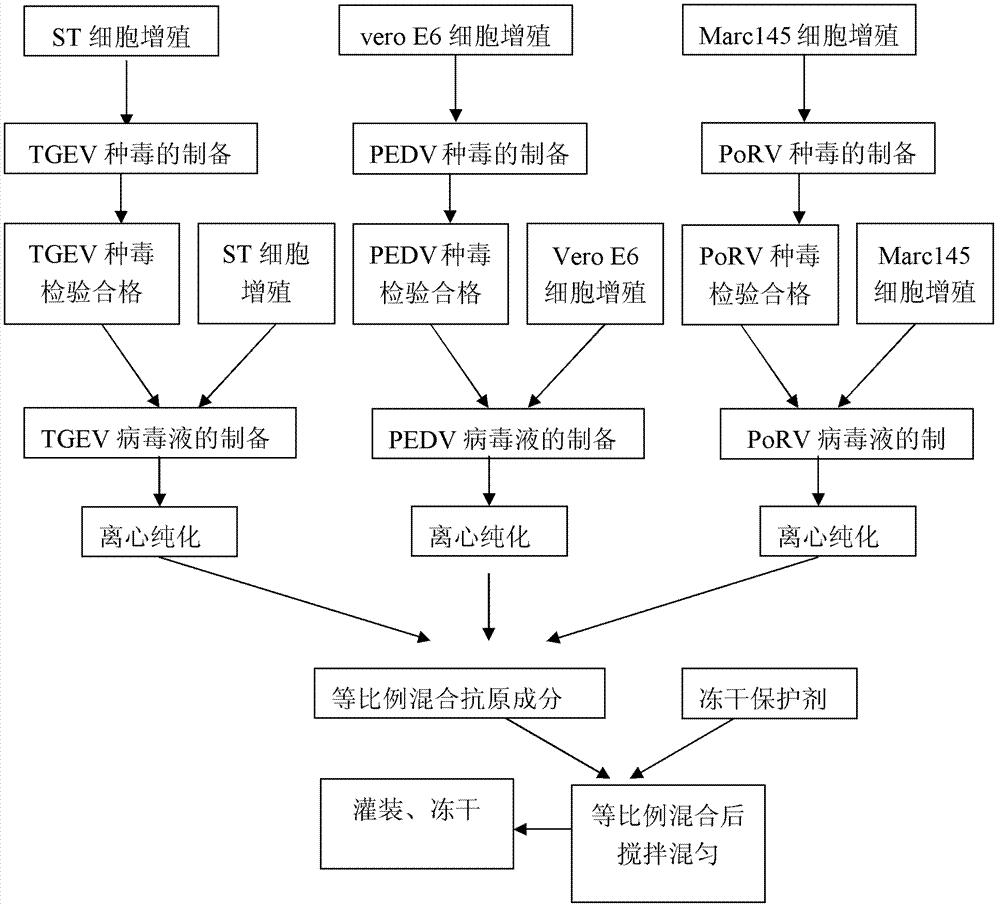
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Preparation method and application of phenylpropanoid compound in tobacco
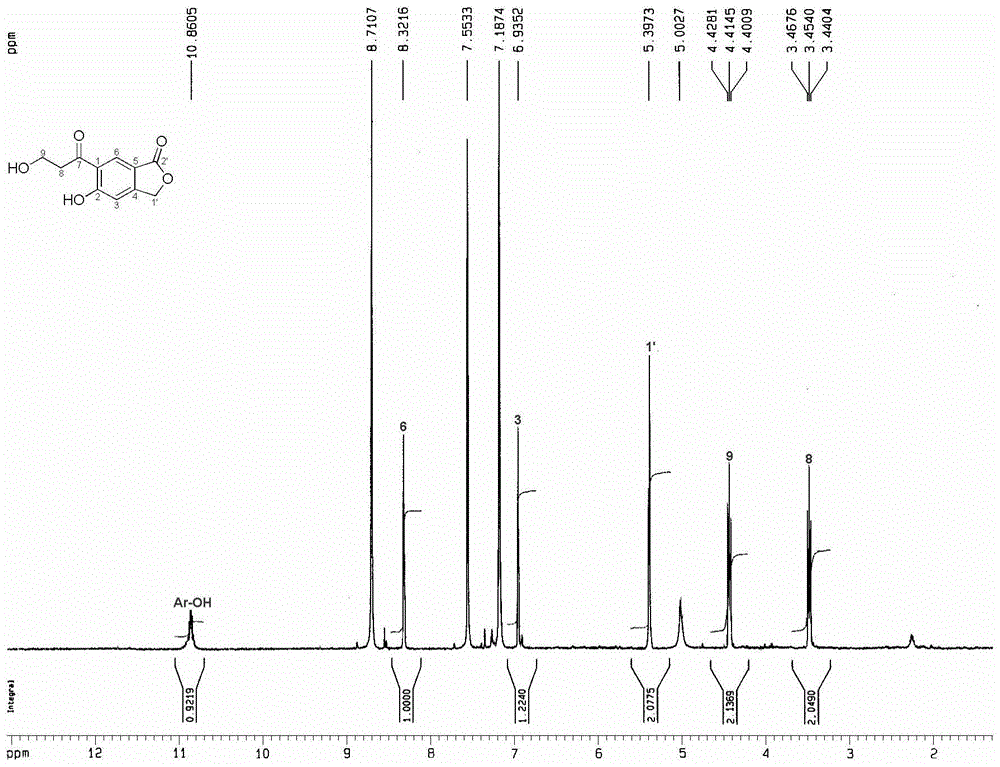
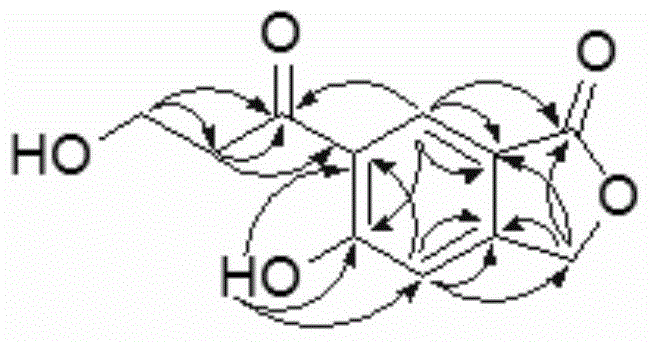
ActiveCN104945360ASimple structureGood anti-rotavirus activityOrganic chemistryAntiviralsBiotechnologyChromatographic separation
The invention discloses a of a phenylpropanoid compound showing as the first formula (Please see the formula in the instruction) and a preparation method and application thereof and belongs to the technical field of tobacco chemistry. Tobacco serves as a raw material, extraction is conducted with a high-concentration methanol aqueous solution or a high-concentration ethanol aqueous solution or a high-concentration acetone aqueous solution as an extraction solvent, and the extraction solutions are merged, filtered, and decompressed condensed to form extract; the extract is packed by the adoption of a silica gel to be conducted silica gel column chromatography; a mixing solvent of chloroform-acetone serves as an eluent to conduct gradient elution, and TLC monitors and merges same parts and condenses; separation and purification by use of high-pressure liquid chromatography are further conducted on the part obtained by eluting, collecting and condensing by the mixing solvent of the chloroform-acetone with a volume matching of 6:4, and the phenylpropanoid compound is obtained. The activity test indicates that the compound has a good inhibiting effect on rotavirus. The compound is simple in structure, good in activity and capable of being used as a guiding compound for resisting the rotavirus.
Owner:CHINA TOBACCO YUNNAN IND
Antimicrobial compositions, products and methods employing same
InactiveUS20090202463A1Immediate and residual effectivenessApplied to skinAntibacterial agentsBiocideRotavirus RNARotavirus
Antimicrobial compositions that provide enhanced immediate and residual anti-viral and antibacterial efficacy against rhinovirus, rotavirus, coronovirus, respiratory syncytial virus, Gram-positive bacteria, Gram-negative bacteria and combinations thereof. More specifically, antimicrobial compositions comprising an organic acid or organic acid mixture and a short-chain anionic surfactant having at least one of a large head group; a branched alkyl chain and an unsaturated alkyl chain. Further, products incorporating the antimicrobial compositions of the present invention and methods of using the antimicrobial compositions and products are disclosed herein.
Owner:PROCTER & GAMBLE CO
Kit and oligonucleotide sequences for detecting rotavirus A
InactiveCN101671746AHigh sensitivityHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesMagnesium pyrophosphateRotavirus RNA
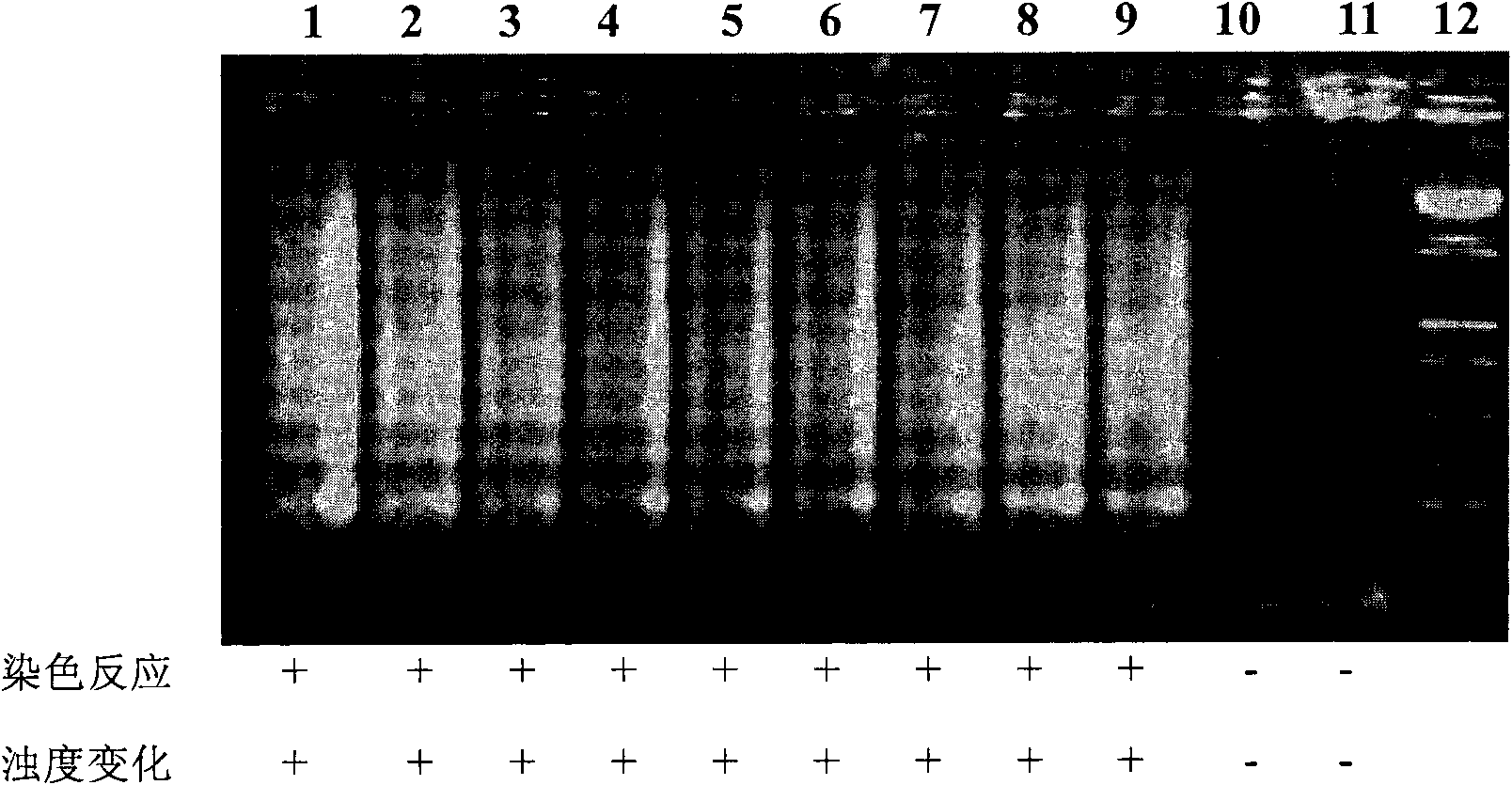
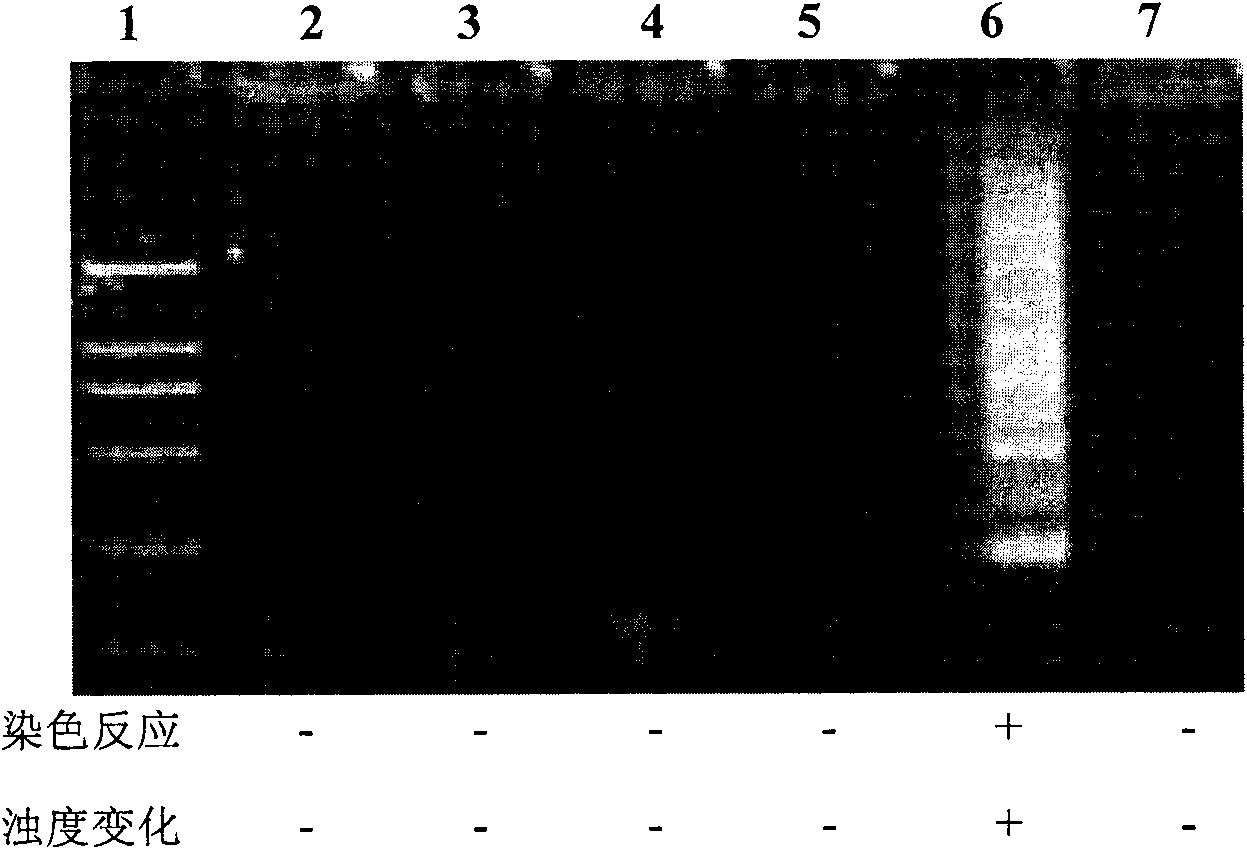
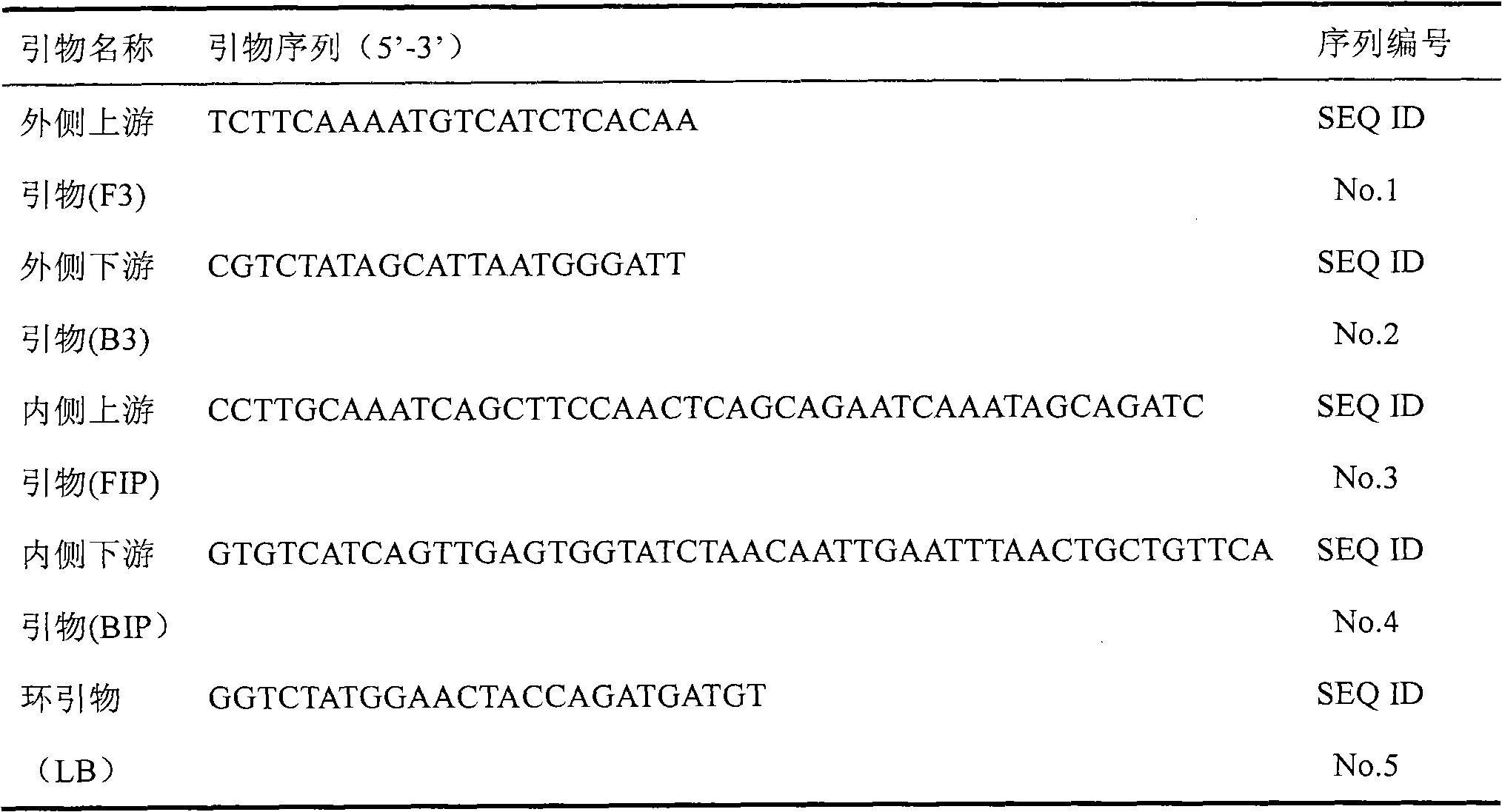
The invention discloses a kit and oligonucleotide sequences for detecting rotavirus A, in particular to a group of oligonucleotides which are used for detecting rotavirus A and have the oligonucleotide sequences shown from the sequence table SEQ ID No.1 to the sequence table SEQ ID No.5, the kit containing the oligonucleotides and a detection method thereof. The kit of the invention has high sensitivity, strong specificity, low cost and simple and convenient operation. During detection, when lots of nucleic acid is synthesized, a by-product magnesium pyrophosphate precipitate is generated, which has high specificity. Whether the products are amplified or not can be judged only by observing the turbidity of the products with naked eyes.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for simultaneously detecting multiple RT-PCR of GETV, PEDV, TGEV, PDCoV and PoRV
InactiveCN108950083AStrong specificityImprove efficiencyMicrobiological testing/measurementMicroorganism based processesRotavirus RNANucleotide
The invention discloses a multiple RT-PCR primer group for simultaneously detecting porcine gatahvirus (GETV), porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV) and porcine A rotaviruses (PoRV), which has a nucleotide sequence as shown in SEQ ID NO:1-SEQ ID NO:10. The invention further discloses a multiple RT-PCR detection method for detecting GETV, PEDV, TGEV, PDCoV and PoRV from a sample in one time by utilizing the multiple RT-PCR primer group. Compared with an existing conventional RT-PCR, the detection method has strong specificity and high sensitivity, can realizesimultaneous identification of five viruses including GETV, PEDV, TGEV, PDCoV and PoRV, and has accurate detection result and high detection efficiency.
Owner:HENAN AGRICULTURAL UNIVERSITY
Primer, probe and kit for detecting rotavirus and Norovirus liquid phase chips
InactiveCN102154528AAccurate detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationRotavirus RNAFluorescence
The invention provides a primer, a probe and a kit for detecting rotavirus and Norovirus liquid phase chips. The primer comprises three pairs of primers of a specific amplification A type rotavirus sequence, a GI type Norovirus sequence and a GII type Norovirus sequence. The probe comprises three specificity detection probes of A type rotavirus, GI type Norovirus and GII type Norovirus. The kit comprises the three pair of primers and a specificity detection microsphere mixture prepared by coupling the three specificity detection probes and a fluorescence coding microsphere. The experiment proves that the primer and the probe disclosed by the invention are characterized in that a method of combining multiple PCR (polymerase chain reaction) with liquid phase chip detection can simultaneously and accurately detect the A type rotavirus, the GI type Norovirus and the GII type Norovirus. The primer, the probe and the kit have the advantages of strong specificity and high sensitivity.
Owner:何雅青 +2
Employment of rotavirus proteins, derived proteins and peptides for the modulation of tisssue permeability
InactiveUS20060251663A1Improve drug deliveryImprove permeabilityOrganic active ingredientsVirusesCancer cellRotavirus RNA
The present invetnion refers to the use of rotavirus proteins VP4, VP8 and their derived fusion proteins and peptides, for enhancing the delivery of pharmaceutical agents through the paracellular pathway. These rotavirus proteins and derived peptides may additionally be employed to reduce unwanted cellular adhesion that can occur between cancerous cells, or between normal cells as a result of surgery, injury, chemotherapy, disease, inflammation or other pathological conditions.
Owner:CENT DE INVESTIGACION & DE ESTUDIOS AVANZADOS DEL INST POLITECNICO NACIONAL
Anti-diarrhea yelk antibody feed additive for swine and injection formulation, and preparation method
InactiveCN1484972ASimple preparation processLow costPharmaceutical delivery mechanismAnimal feeding stuffRotavirusAnti-diarrhea
A feed additive containing yolk antibodies against diarrhea in pigs. The antibodies are preparing from bacilluscoli, epidemic diarrhea or rotavirus, or immunizing non-immunitive hens laying eggs withcombined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens. A yolk antibody injecting agent, which is prepared from bacilluscoli, epidemic diarrheaor rotavirus, or immunizing non-immunitive hens laying eggs with combined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens.
Owner:HEBEI KEXING PHARMA
Chinese medicine preparation for treating infant rotavirus enteritis and preparation method thereof
InactiveCN102198262AEasy to manufactureLow costDigestive systemAntiviralsRotavirus RNAManufacturing technology
The invention discloses a Chinese medicine preparation for treating infant rotavirus enteritis. The Chinese medicine preparation comprises the following raw materials in parts by weight: 10-20 parts of Chinese pulsatilla root, 10-20 parts of purslane, 10-20 parts of yam, 10-20 parts of baical skullcap root, 10-20 parts of wrinkled gianthyssop herb, 10-20 parts of radix glehniae, 10-20 parts of bighead atractylodes rhizome, 10-20 parts of Indian buead, 10-20 parts of burnet, 10-20 parts of longstamen onion bulb, 10-20 parts of kudzu vine root, 10-20 parts of coptis, 10-20 parts of dried tangerine peel, 10-20 parts of nutmeg, 10-20 parts of pomegranate rind, 10-20 parts of malt, 10-20 parts of grifola, 10-20 parts of ginger and 10-20 parts of endothelium corneum gigeriae galli. The preparation has the effects of relieving pain, regulating vital energy, tonifying the spleen, nourishing the spleen, the lung and the kidney, tonifying middle-Jiao and Qi, ascending yang and stopping diarrhea, and is mainly used for treating diarrhea caused by invasion of exogenous evil and weakness of spleen and stomach. The Chinese medicine preparation has the advantages of simple manufacture technology, small toxic side effect, convenience for administration, easiness for manufacture and low cost. The preparation can directly act on the focus, requires short curative time and avoids relapse after rehabilitation.
Owner:南通市星期七旅游开发有限公司
Molecular kit for rapidly identifying three types of piglet virus diarrhea and application of molecular kit
ActiveCN104611466ANo mutual interferenceLow minimum detectable concentrationMicrobiological testing/measurementMicroorganism based processesRotavirus RNAAstrovirus gastroenteritis
The invention discloses a kit for detecting pig epidemic diarrhea viruses, pig transmissible gastroenteritis viruses and pig rotaviruses. The kit comprises primer pairs shown by SEQ ID NO:1-2, SEQ ID NO:3-4 and SEQ ID NO:5-6 for respectively performing specific amplification on the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The invention also discloses applications of the primer pairs shown by the SEQ ID NO:1-2, the SEQ ID NO:3-4 and the SEQ ID NO:5-6 in preparation of reagents for detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The detection kit disclosed by the invention can be used for accurately and effectively detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses, and is strong in specificity, high in sensitivity, short in time consumption, rapid in detection and good in application prospect.
Owner:SICHUAN AGRI UNIV
Formulations for preservation of rotavirus
This invention provides formulations and methods for stabilizing viruses in liquid and dried formulations. In particular, formulations are provided including Zn2+ cations that stabilize the viability of Rotaviruses. Methods of vaccination include neutralization of gastric contents and administration of the vaccine formulations of the invention.
Owner:ARIDIS PHARMA INC
Medication for preventing and treating diseases of pigs
InactiveCN1626106AEasy to prepareMedication convenienceAntibacterial agentsOrganic active ingredientsDiseaseMetritis
A veterinary medicine for preventing and treating the frequently encountered diseases of pig, such as metritis, mammitis, acyesis, sterility, dysentery, epidemic enteritis, paratyphoid, asthma, etc is prepared from 15 raw materials including florfenicol, amoxicillin, lucomycin, doxycycline, etc.
Owner:杨联华
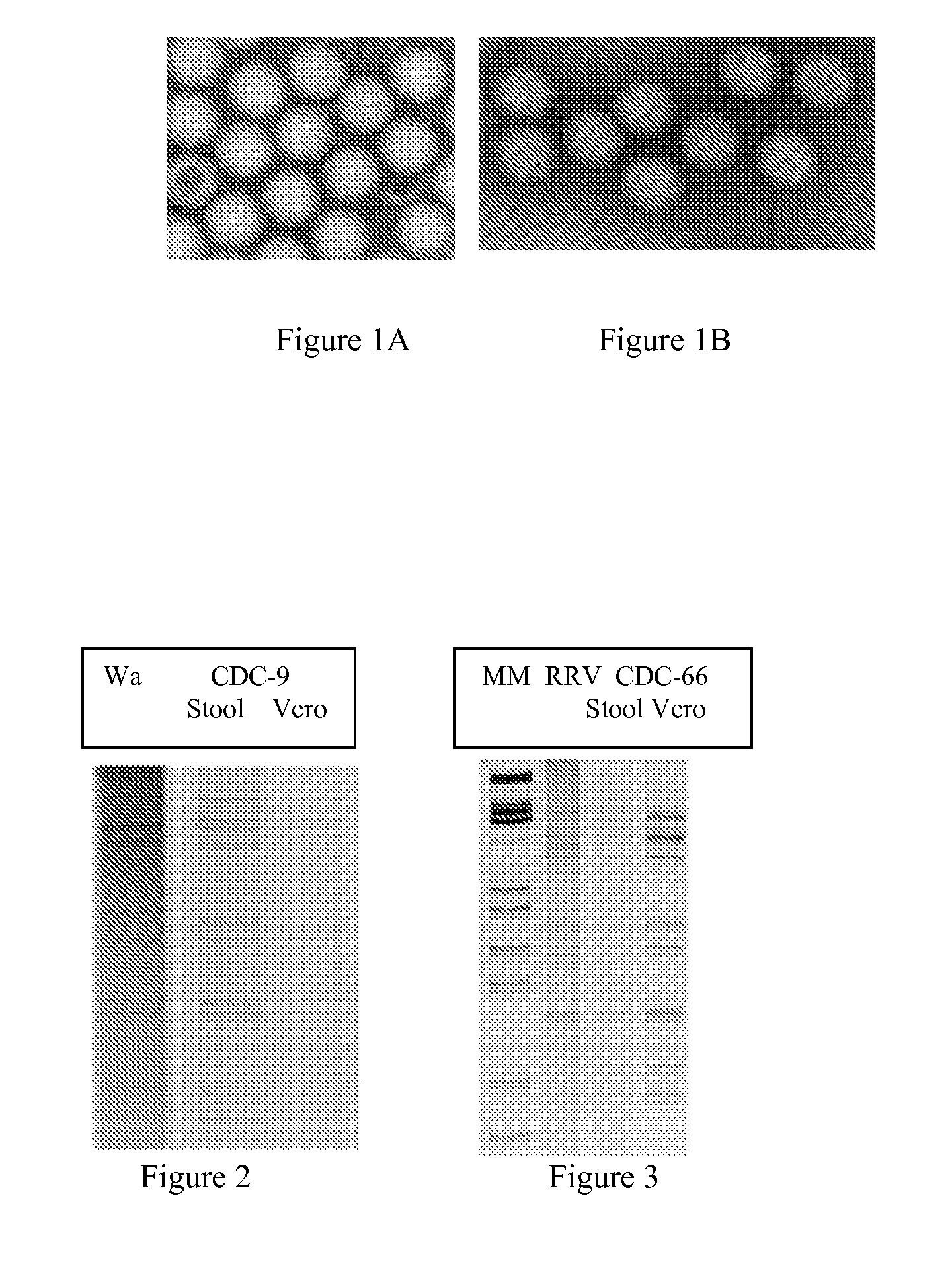
Human rotavirus vaccine strains and diagnostics
A vaccine composition and method of vaccination are provided useful for immunizing a subject against a rotavirus. The vaccines include rotavirus strains CDC-9 and CDC-66, fragments thereof, homologues thereof, or combinations thereof. Inventive vaccines may include a fragment of CDC-9, CDC-66, homologues thereof, or combinations thereof. Methods of inducing an immunological response are provided by administering an inventive vaccine.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICES CENT FOR DISEASE CONTROL & PREVENTION
Complementing cell lines
A packaging cell line that complements recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells that are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 that expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell lines can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. Also, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, and measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Primer combination for simultaneously identifying 8 kinds of cattle pathogens and GeXP detection method
InactiveCN106191309APromote healthy developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesVesicular StomatitisPathogen
The invention discloses a primer combination for simultaneously identifying 8 kinds of cattle pathogens and a GeXP detection method. The primer combination provided by the invention consists of a primer pair I, a primer pair II, a primer pair III, a primer pair IV, a primer pair V, a primer pair VI, a primer pair VII and a primer pair VIII. The invention also provides the GeXP detection method for simultaneously identifying foot and mouth disease viruses, blue tongue viruses, vesicular stomatitis viruses, bovine viral diarrhoea viruses, bovine rotaviruses, enterotoxigenic escherichia coli, infectious bovine rhinotracheitis viruses and peste des petits ruminants viruses. The GeXP detection method built by the invention can be used for simultaneously identifying 8 kinds of cattle infectious disease pathogens. The method has the characteristics of high flux, specificity and high sensitivity, and can be used for the cattle disease epidemiology monitoring and the emergent epidemic situation identification and diagnosis, and guarantees the healthy development of cattle rearing industry.
Owner:GUANGXI VETERINARY RES INST
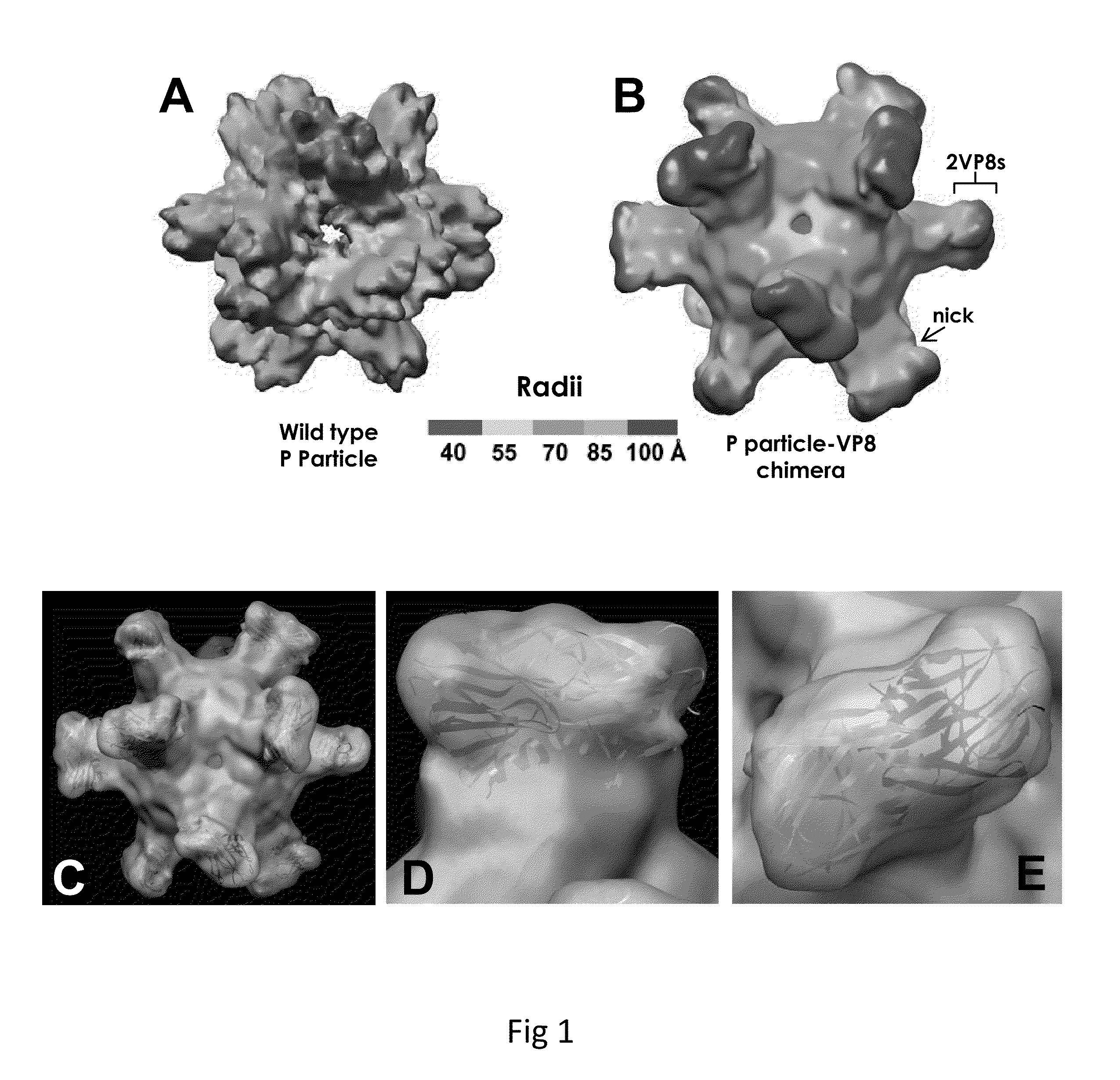
Antigen-norovirus P-domain monomers and dimers, antigen-norovirus P-particle molecules, and methods for their making and use
ActiveUS8486421B2Easy to produceImproving immunogenicitySsRNA viruses positive-sensePeptide/protein ingredientsDiseaseAntigen
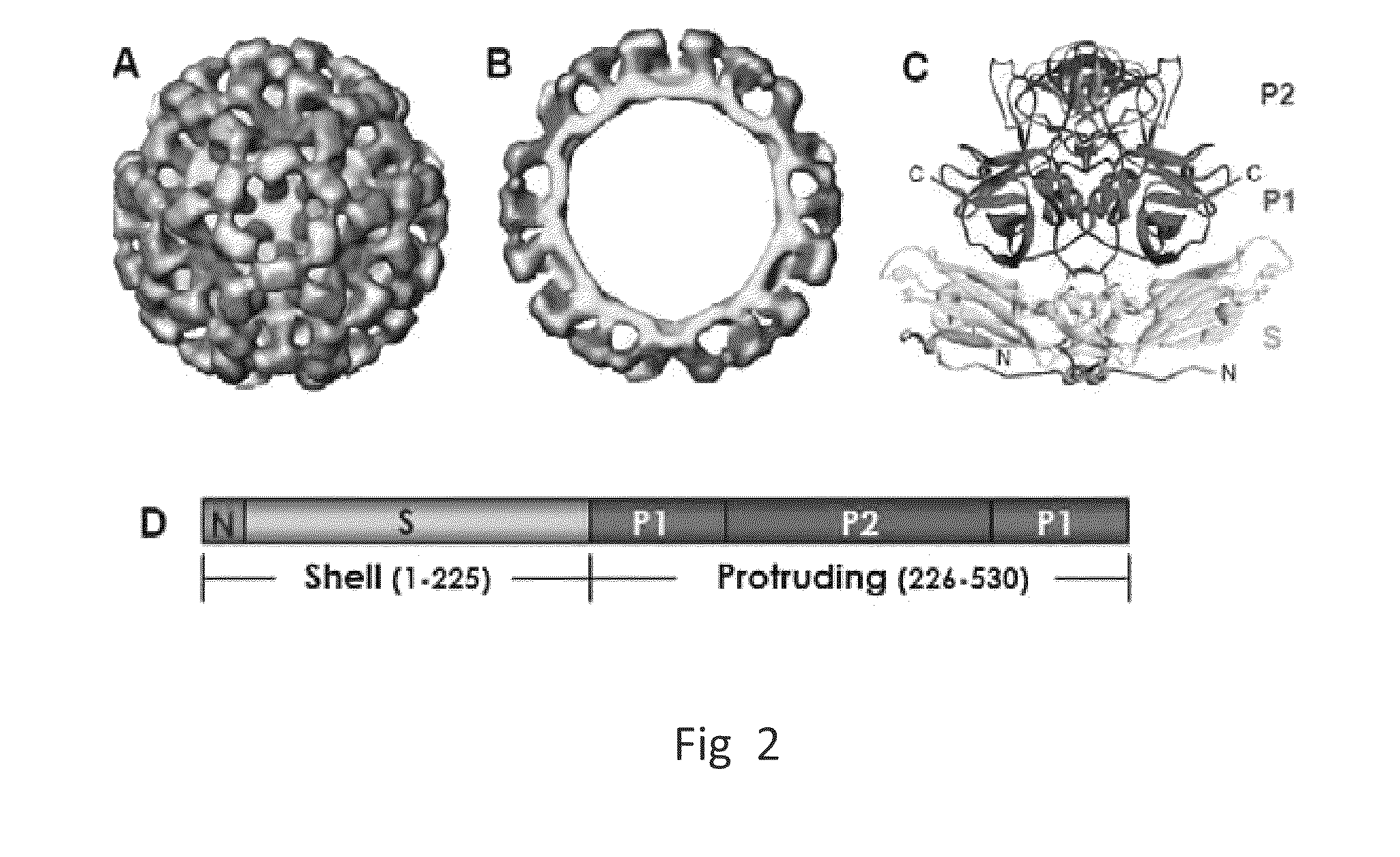
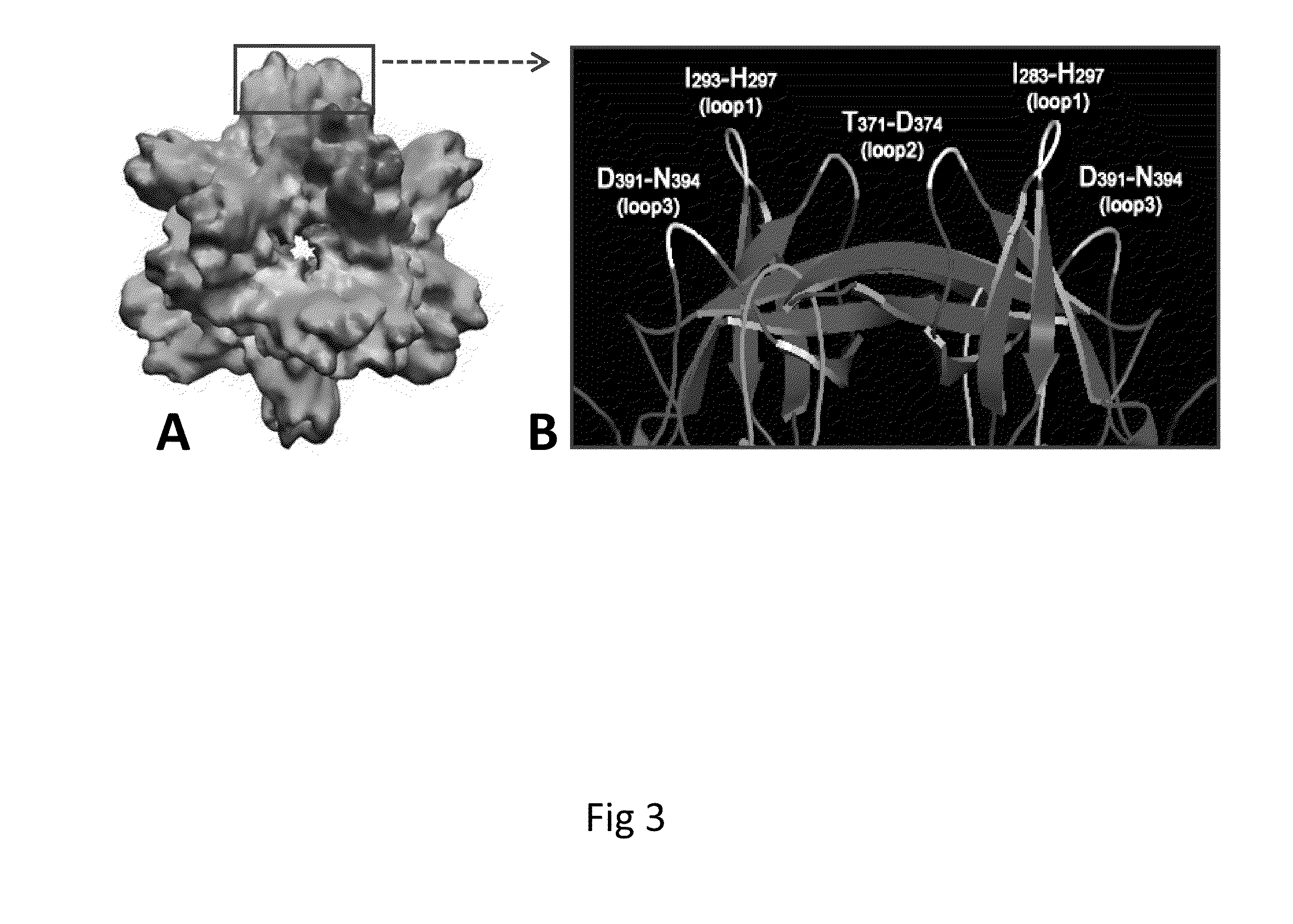
A substituted Norovirus capsid protein monomer, having only the P-domain and called an antigen-Norovirus P-domain monomer, includes a foreign antigen inserted into one or more of three surface loops present on each P-domain monomer by molecular cloning. The antigen-P-domain monomer can assemble spontaneously into an octahedral form, called an antigen-Norovirus P-particle, that is composed of 24 copies of the antigen-P-domain monomer. Each substituted P-domain monomer will contain one to three copies of the foreign antigen, for a total of 24-72 antigen copies on each antigen-P-particle. The antigen-P-particle is useful in methods for diagnosing, immunizing and treating individuals infected with a foreign virus, for example Rotavirus, and can serve as a carrier for presentation of foreign antigens for development of novel vaccines against many infectious and non-infectious diseases. The substituted Norovirus P-particles can be readily produced in E. coli and yeast, are highly stable and tolerate a wide range of physio-chemical conditions. A modified Norovirus P-domain monomer includes one or more restriction recognition sites inserted within one or more of the three loops of the P-domain monomers, to provide user-friendly cloning cassettes for conveniently inserting candidate foreign antigens into the surface loops. The P-particle-VP8 chimeras may also serve as a dual vaccine against both rotavirus and norovirus.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Rotavirus RT PCR detecting kit and its detecting method
InactiveCN1814790AHigh sensitivityHigh speedMicrobiological testing/measurementRotavirus RNATest sample
Thisa invention relates to a semi-set RT-PCR test reagent box for testing and verifying wheel-like virus including a MLV inverse transcriptase, Rnasin, DNA poly-enzyme, primers, dNTP, MgCl2 and 10 x buffer solution, in which, the upstream primer P1 is 5'-GTA TGG TAT TGA ATA TAC CAC-3', the downstream primer P2 is 5'-GAT CCT GTT GGC CAT CC-3. The RT-PCR test method includes: picking up a due test sample template, adding MLV inverse transcriptases, Rnasin, DNA poly-enzymes, primers, dNTP, MgCl2, 10 x buffer solution, the due test sample template and DECP water to be mixed uniformly to be enlarged on a PRC instrument, then the product is electrophoresis-enlarged in a device to get a result, which is recorded, analyzed and judged.
Owner:GUANGDONG INST OF MICROORGANISM +1
Dual RT-PCR method and special reagent case for checking cattle rotavirus and cattle virus diarrhea virus simultaneously
InactiveCN101063176ARapid diagnosisImprove featuresMicrobiological testing/measurementBovine Viral Diarrhea VirusesRotavirus RNA
Owner:SHANDONG OX LIVESTOCK BREEDING CO LTD
Triple high immunity immunoglobulin for anti transmissible gastroenteritis of pig, epidemic diarrhea of pig, and rotavirus of pig
InactiveCN1958070ASmall molecular weightImprove securityAntiviralsAntibody ingredientsAdjuvantRotavirus RNA
An immunoglobulin for preventing the infectious gastroenteritis, epidemic diarrhea and rotavirus of hog is prepared from their immunogens through cell culture, concentrating and proportionally mixing it with complete Frennd's adjuvant. It features that the glycoside-peptide injection is used as its immunopotentiator.
Owner:张中洋
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com