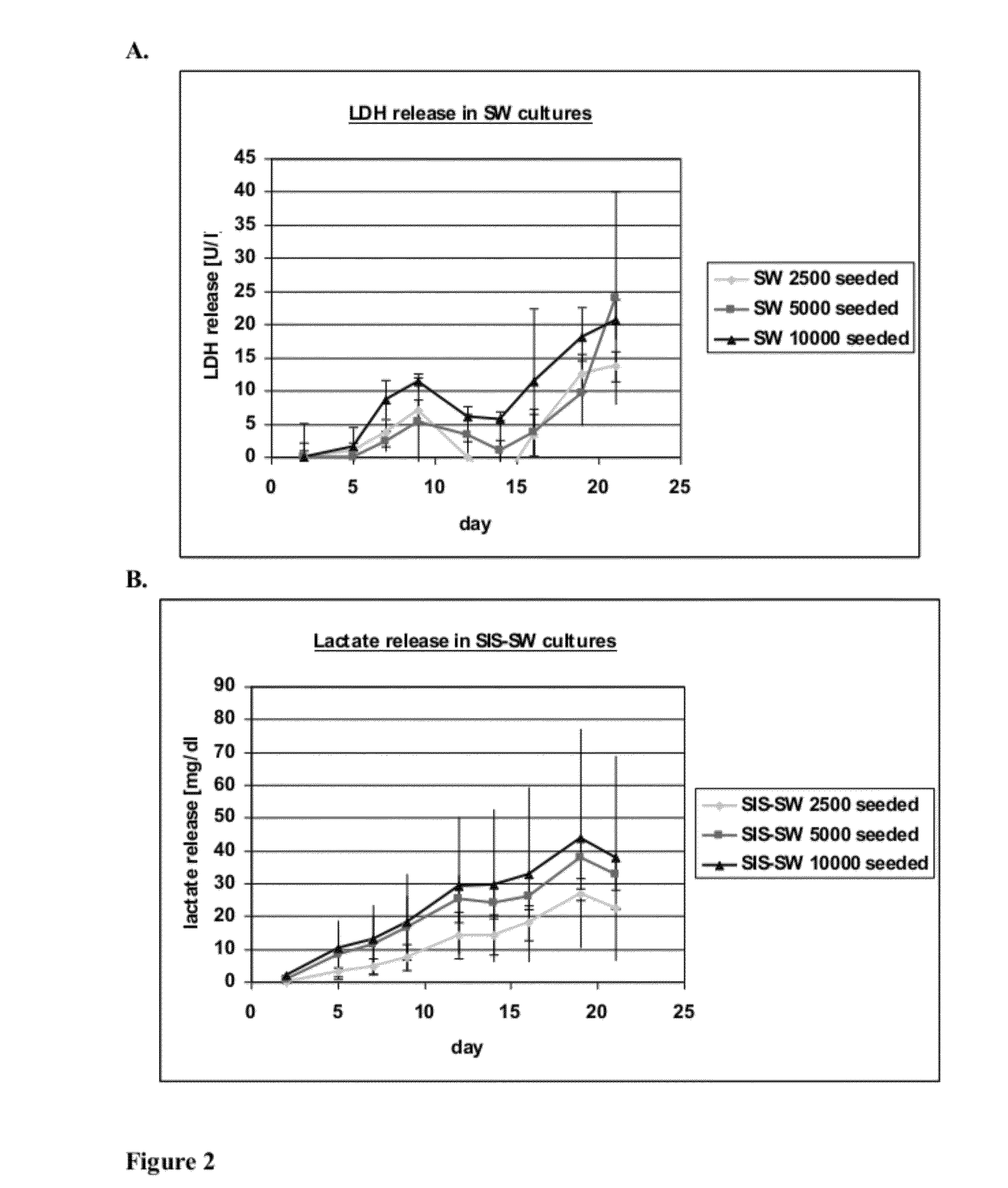
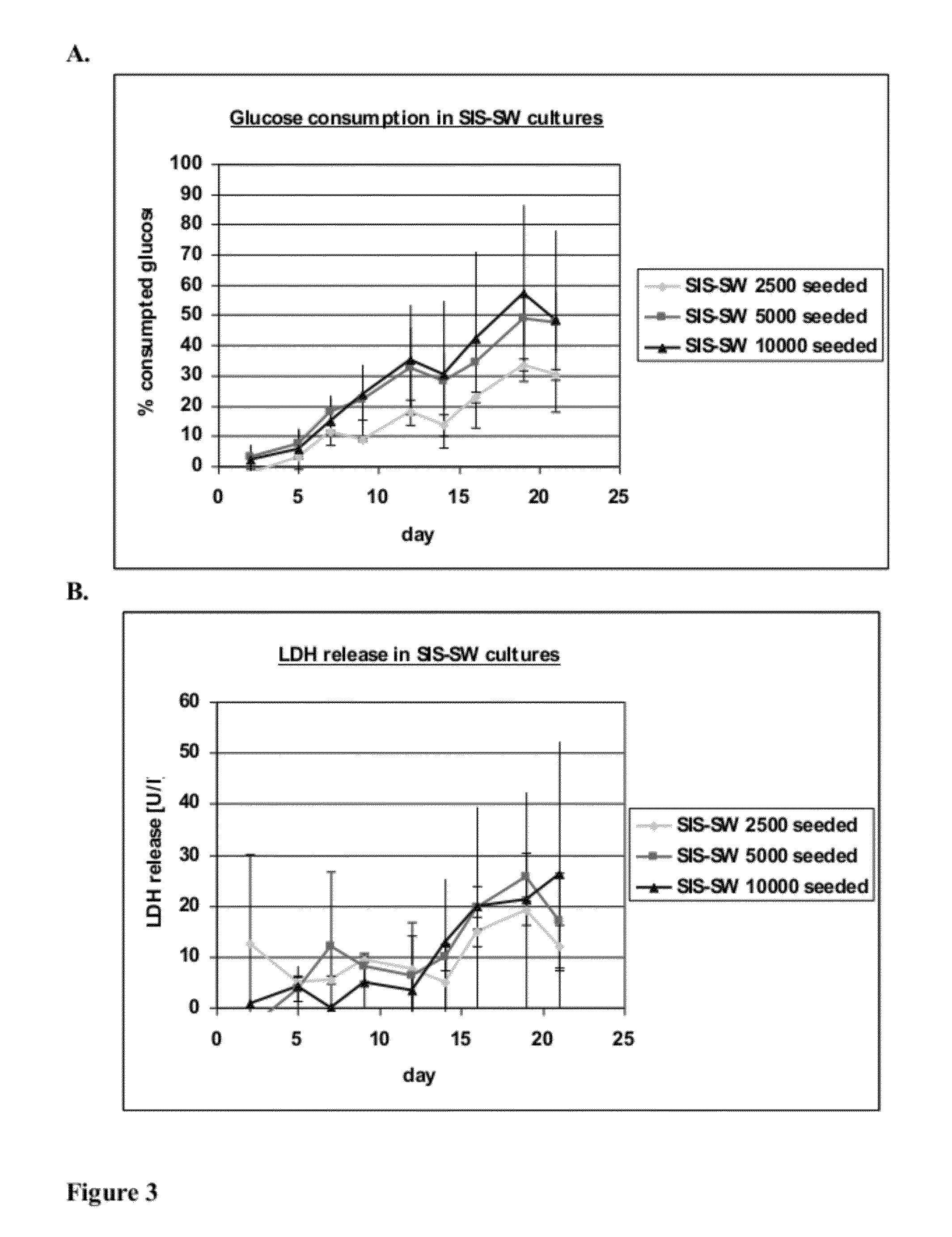
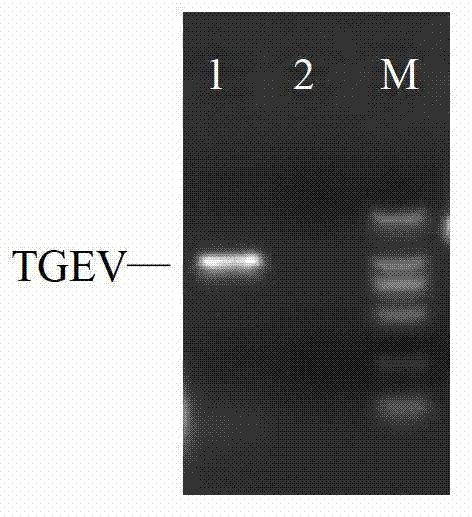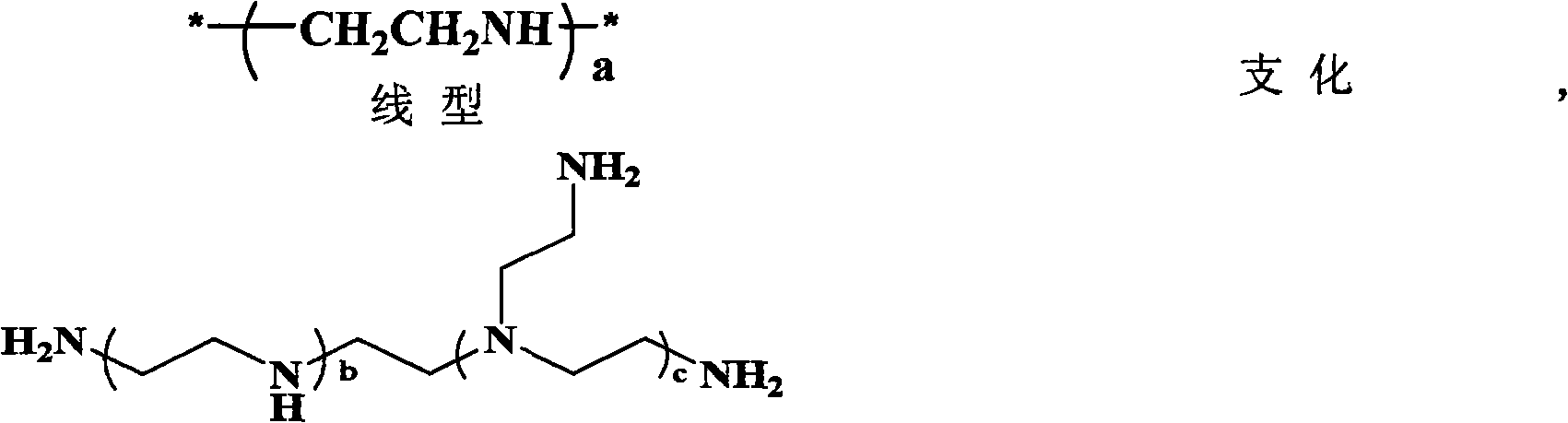Patents
Literature
316 results about "Kidney cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renal cell carcinoma (RCC) is also called hypernephroma, renal adenocarcinoma, or renal or kidney cancer. It's the most common kind of kidney cancer found in adults. The kidneys are organs in your body that help get rid of waste, while also regulating fluid balance. There are tiny tubes in the kidneys called tubules.
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
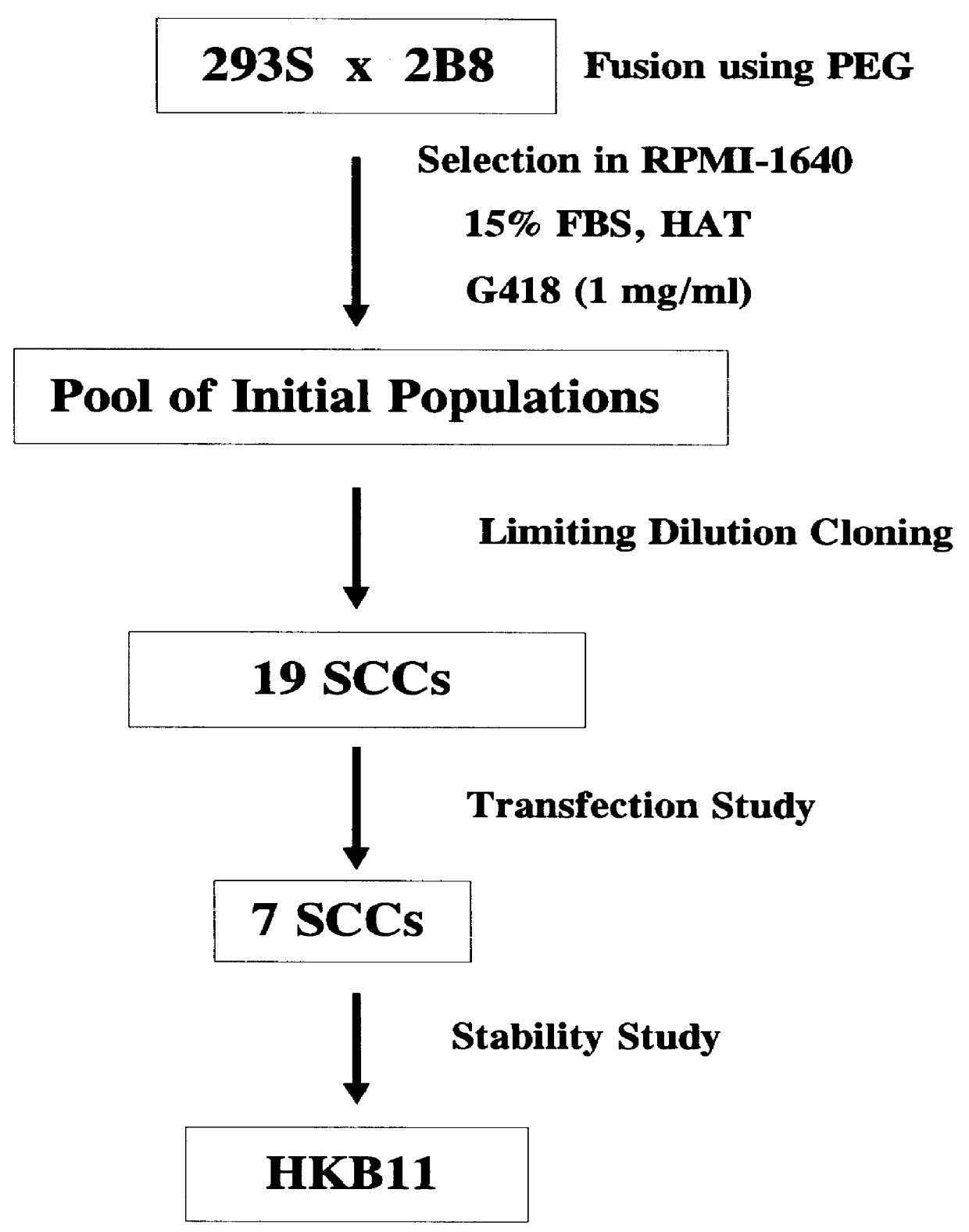
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
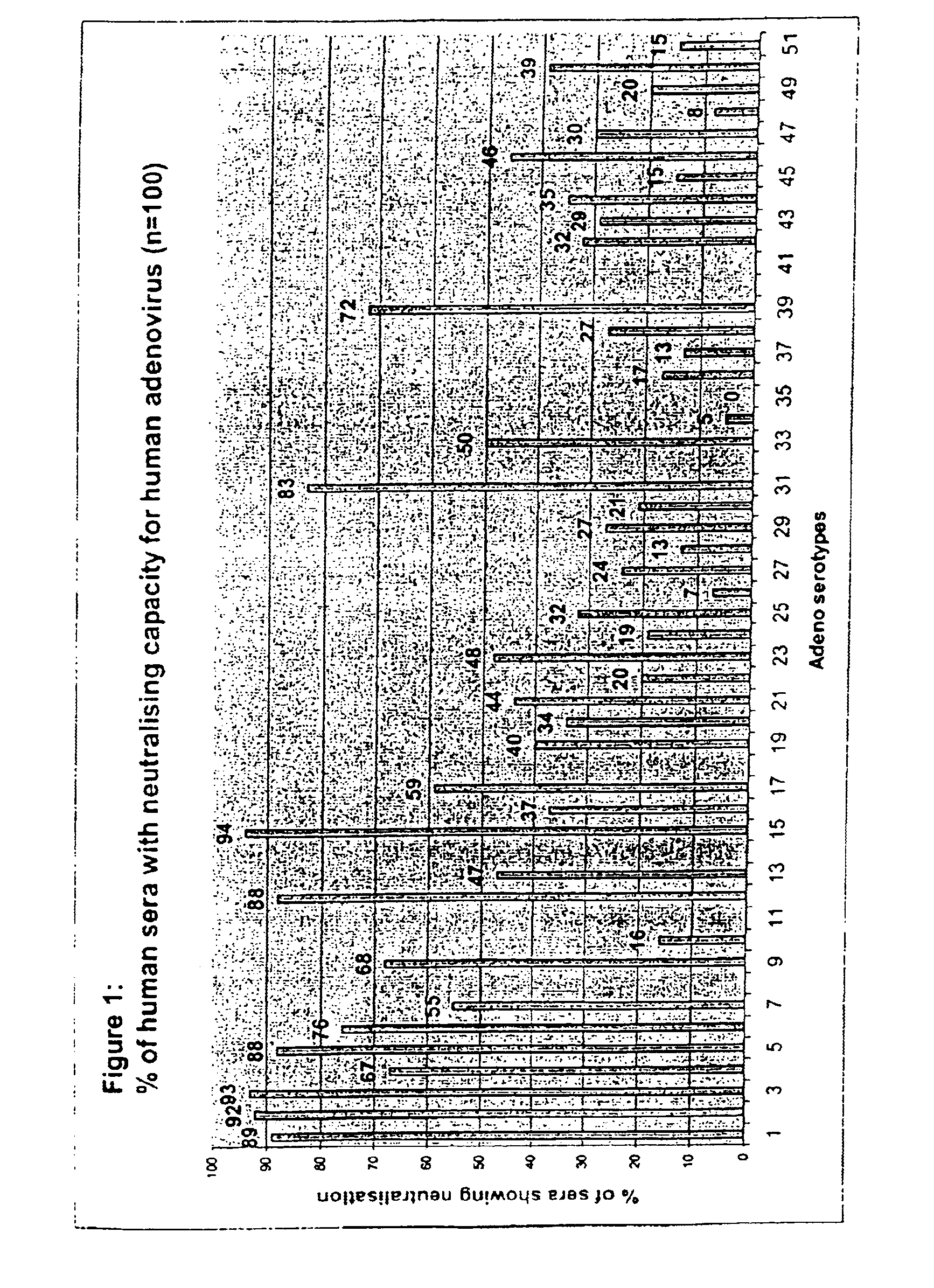
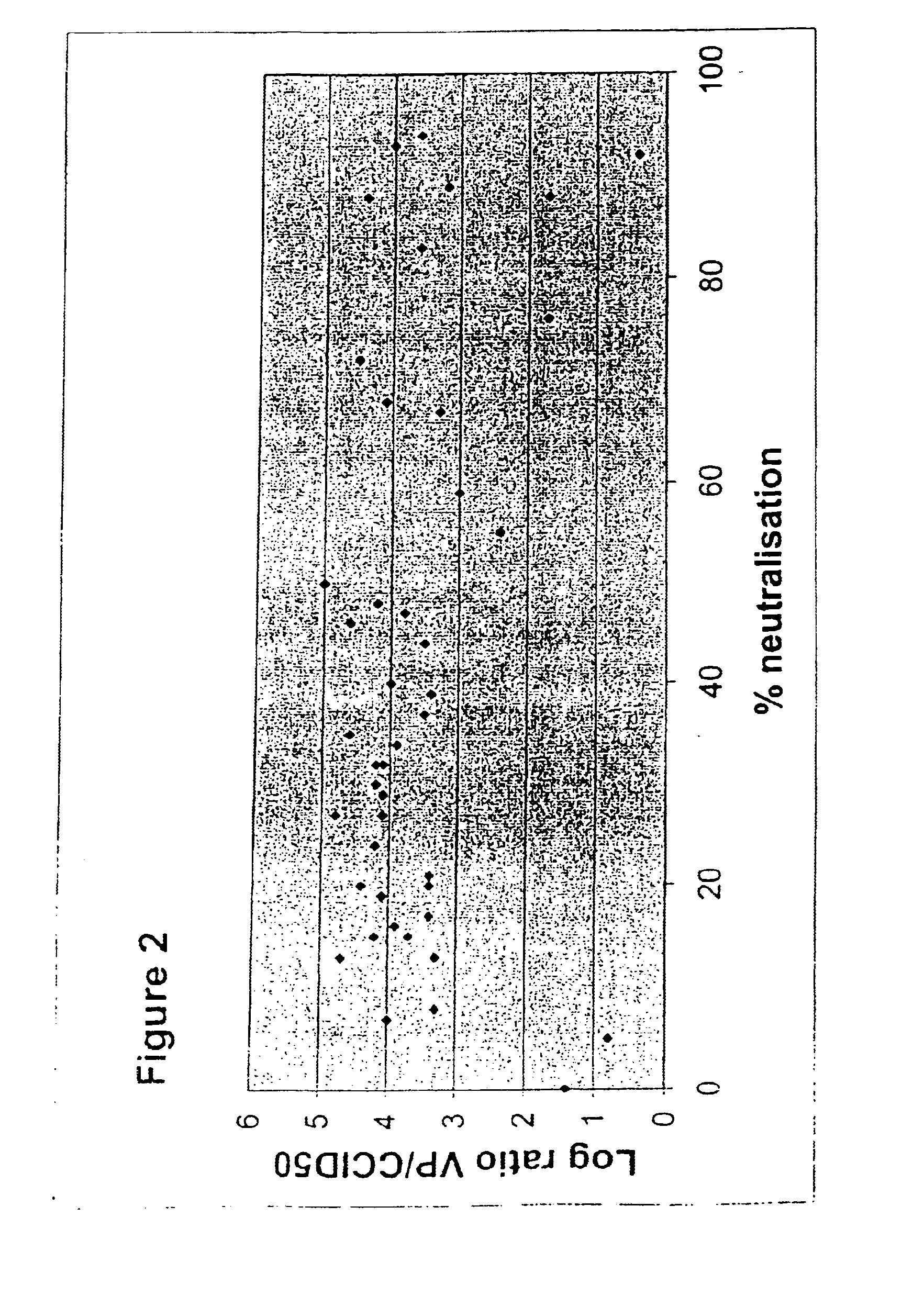
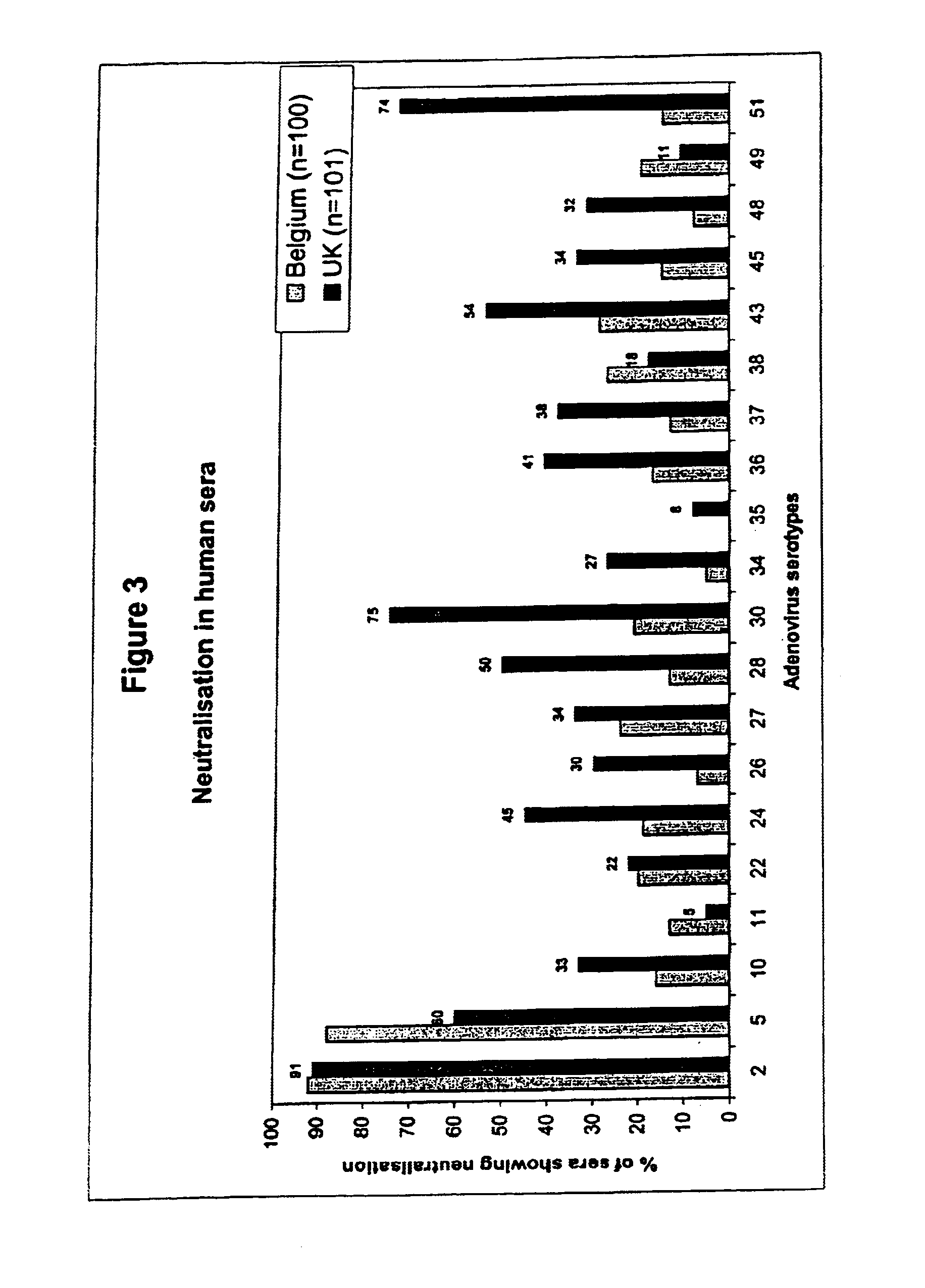
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Enhanced production of recombinant proteins by transient transfection of suspension-growing mammalian cells
ActiveUS20050170450A1Increase transcriptional activityIncreasing nuclear importGenetically modified cellsVirus peptidesEpstein–Barr virusGene Variant
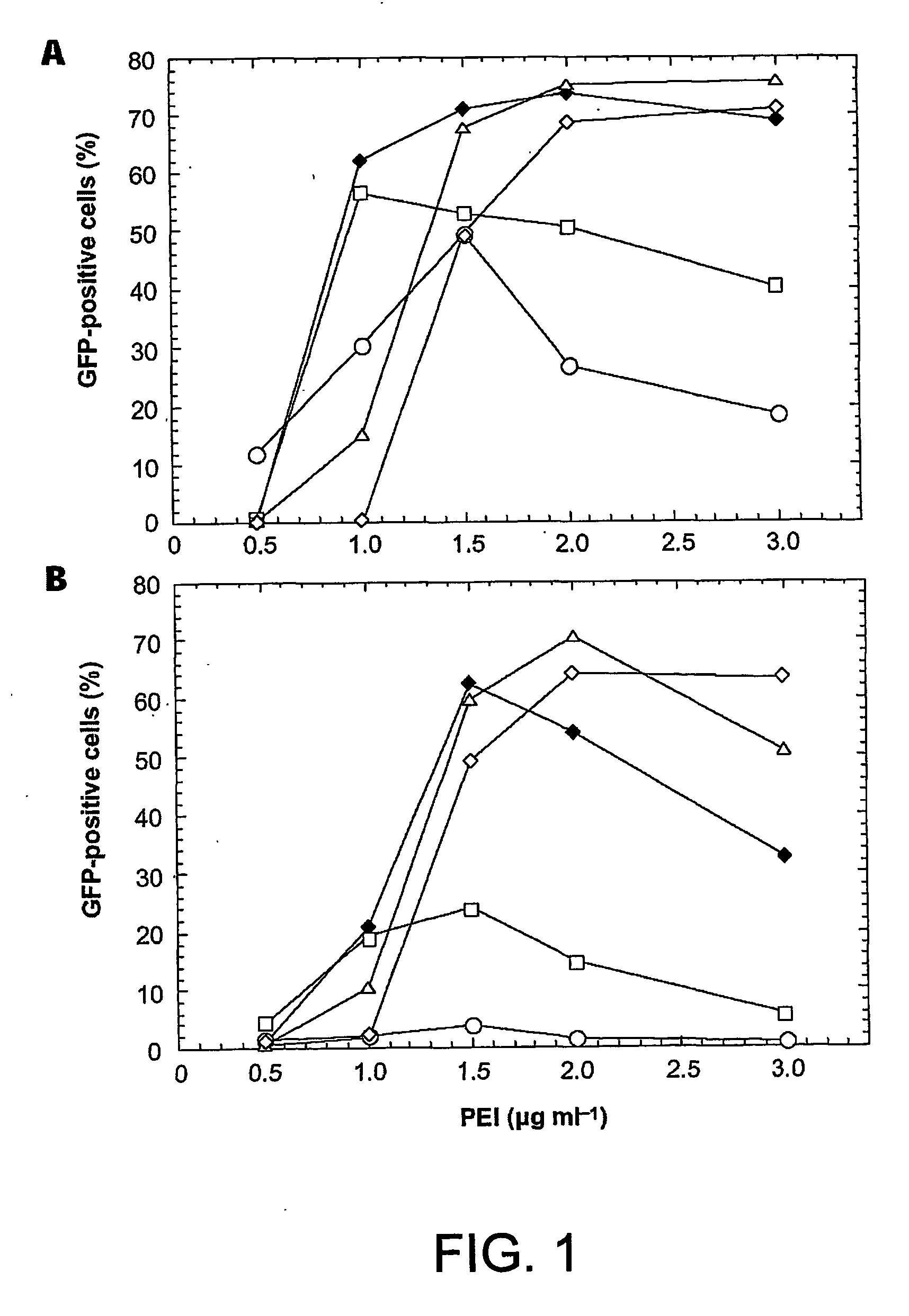
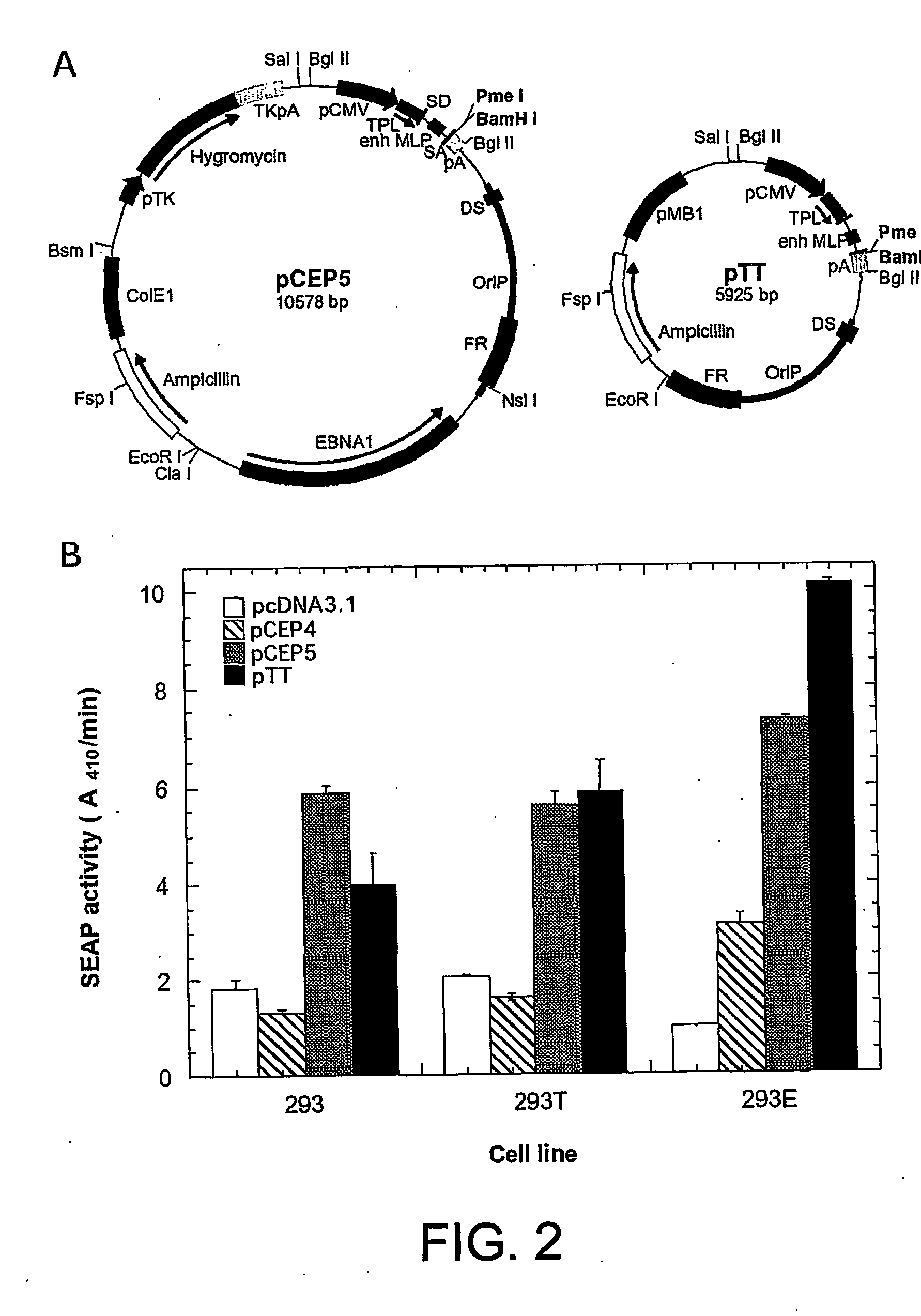
Disclosed is a new process for the production of recombinant proteins, by transient transfection of suspension-grown human embryonic kidney cells (293 cell line and its genetic variants) with an expression vector, using polyethylenimine (PEI) as a transfection reagent. In a preferred embodiment, the process uses 293E cells expressing the Epstein-Barr virus (EBV) EBNA 1 protein, in combination with an oriP-based episomal expression vector having an improved cytomegalovirus expression cassette comprising the CMV5 promoter. The process combines in a single step the cell growth, transfection and protein expression, is carried out without changing the culture medium, and allows to achieve high expression levels in a short period of time. The process may be carried out in a serum-free, low-protein culture medium, is easily scalable, compatible with continuous production processes, and fully adapted to high-throughput production of milligram quantities of recombinant proteins.
Owner:NAT RES COUNCIL OF CANADA
sgRNA sequence for knocking out human CYP2E1, construction method of deficiency cell strain of CYP2E1 and application thereof
ActiveCN106191057AAchieve silencingImproved silence is not completeGenetically modified cellsEnzymesHuman bodyDisease
The present invention provides an sgRNA sequence for knocking out the human CYP2E1 gene. The target DNA sequence of the sgRNA is at least one of the sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2. The present invention also provides a method for knocking out CYP2E1 gene of human embryonic kidney cells, which is adapted to transform CYP2E1 gene in human embryonic kidney cells by using CRISPR / Cas system. The invention also provides a CYP2E1 knockout cell strain. CYP2E1 involves in the important metabolism function of a human body. The CYP2E1 gene knock-out cell strain provided by the invention provides an effective platform for studying the metabolism function of exogenous chemical or exogenous poison in the body, and a powerful tool for studying chronic diseases (such as alcoholic liver disease and diabetes) and tumor-related diseases.
Owner:SUN YAT SEN UNIV
Human embryo kidney 293 cell amplifying protein-free medium
The invention relates to a culture medium and the usage thereof, which particularly relates to a human embryonic kidney 293 cell amplification culture medium and the usage thereof. The invention pertains to the field of biological products. The human embryonic kidney 293 cell amplification protein-free culture medium includes a basic culture medium and further includes a phosphatidylcholine and trace elements. The human embryonic kidney 293 cell protein-free culture medium does not contain any animal serum or protein, and consists of amino acids, vitamins, inorganic salts, sugars, phosphatidyl choline and trace elements. The human embryonic kidney 293 cell serum-free culture medium can directly replace the conventional culture medium which contains serum, and the human embryonic kidney 293 cell does not need an adaptation process.
Owner:天津百若克医药生物技术有限责任公司
Lonidamine analogues and treatment of polycystic kidney disease
Lonidamine derivatives can be useful in methods of treating, inhibiting, and / or preventing polycystic kidney disease (PKD). Accordingly, lonidamine derivatives can be administered in a therapeutically effective amount for inhibiting, and / or preventing polycystic kidney disease (PKD) in the subject. This can include administering a therapeutically effective amount of the lonidamine derivatives for inhibiting CFTR and / or Hsp90 or biological pathway thereof. Also, the method can include administering the lonidamine derivatives in a therapeutically effective amount for inhibiting ErbB2, Src, Raf-1, B-Raf, MEK, Cdk4, NKCC1, or combinations thereof. For example, the therapeutically effective amount of the lonidamine derivatives can be configured so as to provide a concentration in or adjacent to a kidney cell of about 0.25 uM or more or less.
Owner:UNIVERSITY OF KANSAS
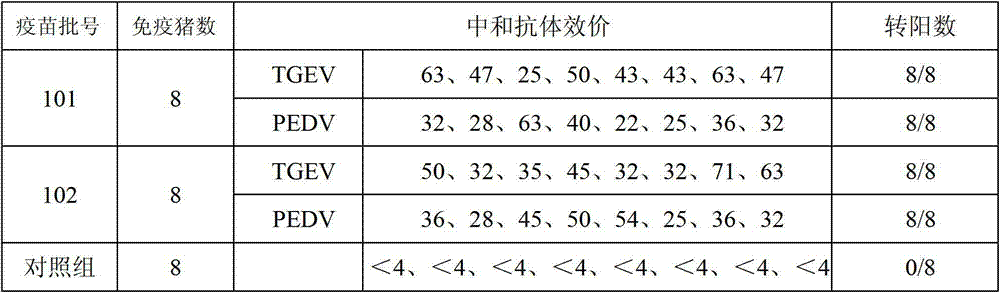
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
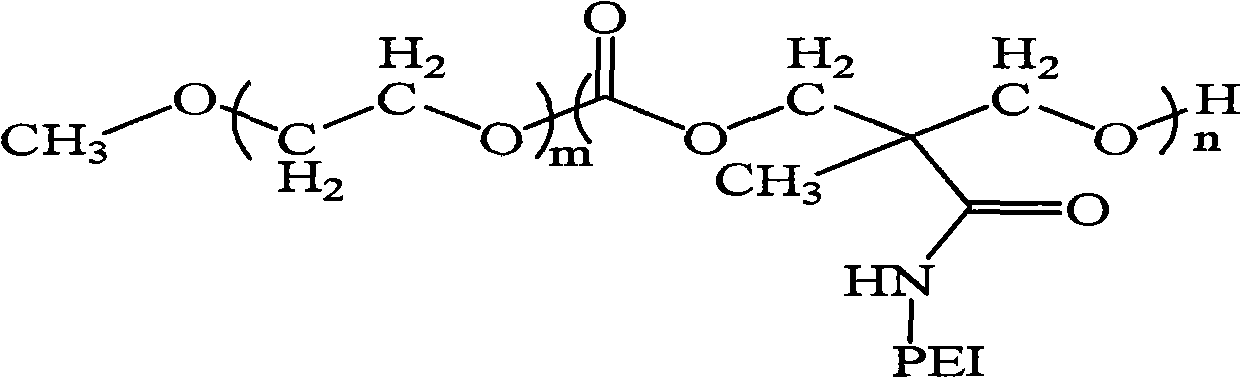
Polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine copolymer, preparation method thereof and application thereof
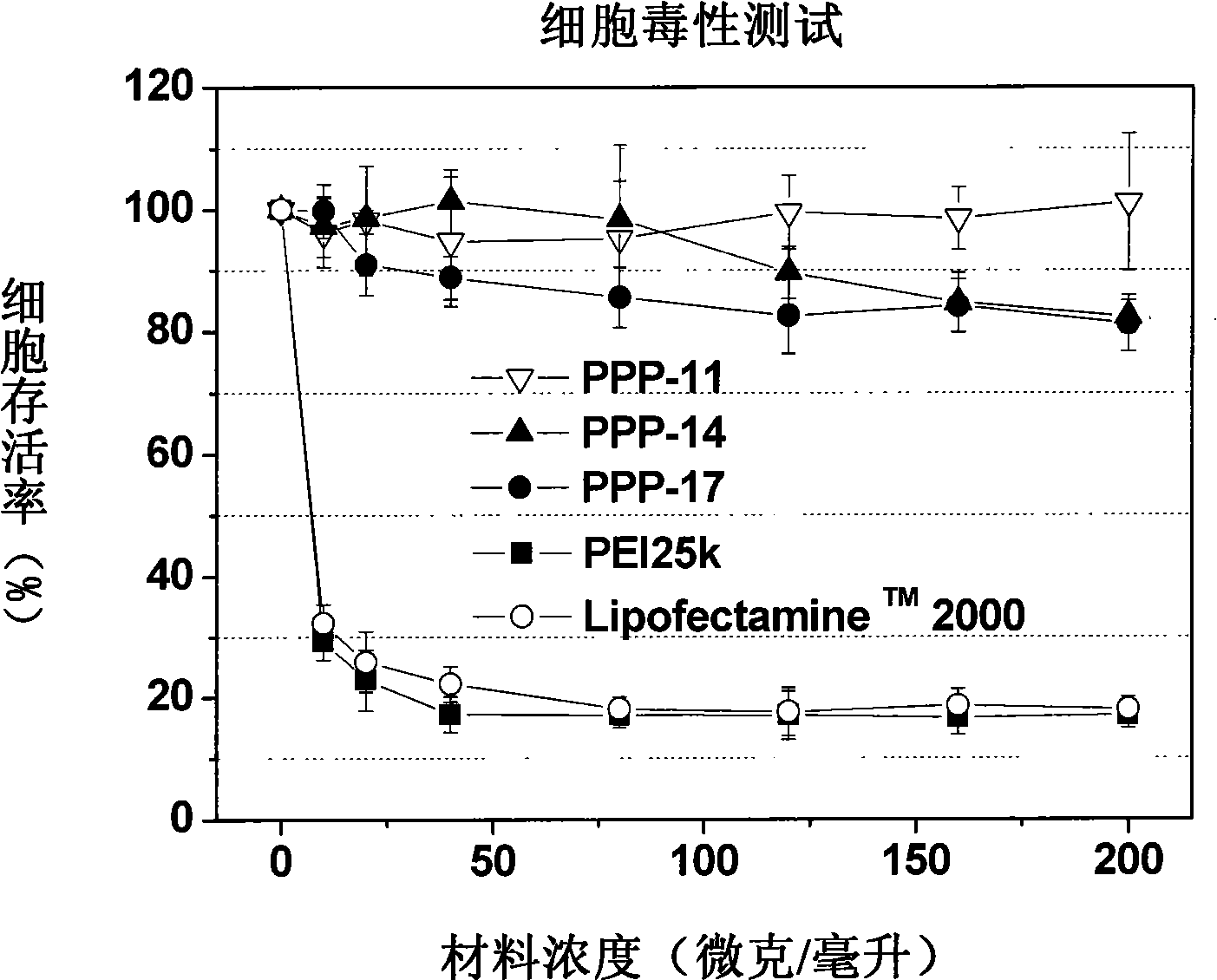
The invention relates to polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine copolymer, a preparation method thereof and application thereof. The preparation method comprises the step of directly condensing carboxyl in the polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine segmented copolymer with amino in polyethyleneimine to form the graft copolymer. The copolymer is a polycation gene carrier, integrates the advantages of polyethylene glycol, Makrolan and polyethyleneimine, and has high transfection efficiency, wherein the highest transfection efficiency to the medium luciferase plasmid of african green monkey kidney cell is 14 times of that of American Invitrogen biological company transfection reagent Lipofetamine<TM>2000, can effectively antagonize the inhibiting effect of blood serum to the transfection, and has less cell toxicity, wherein consistency thereof is not higher than 200 microgramme / ml and cell survival rate is more than 80%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
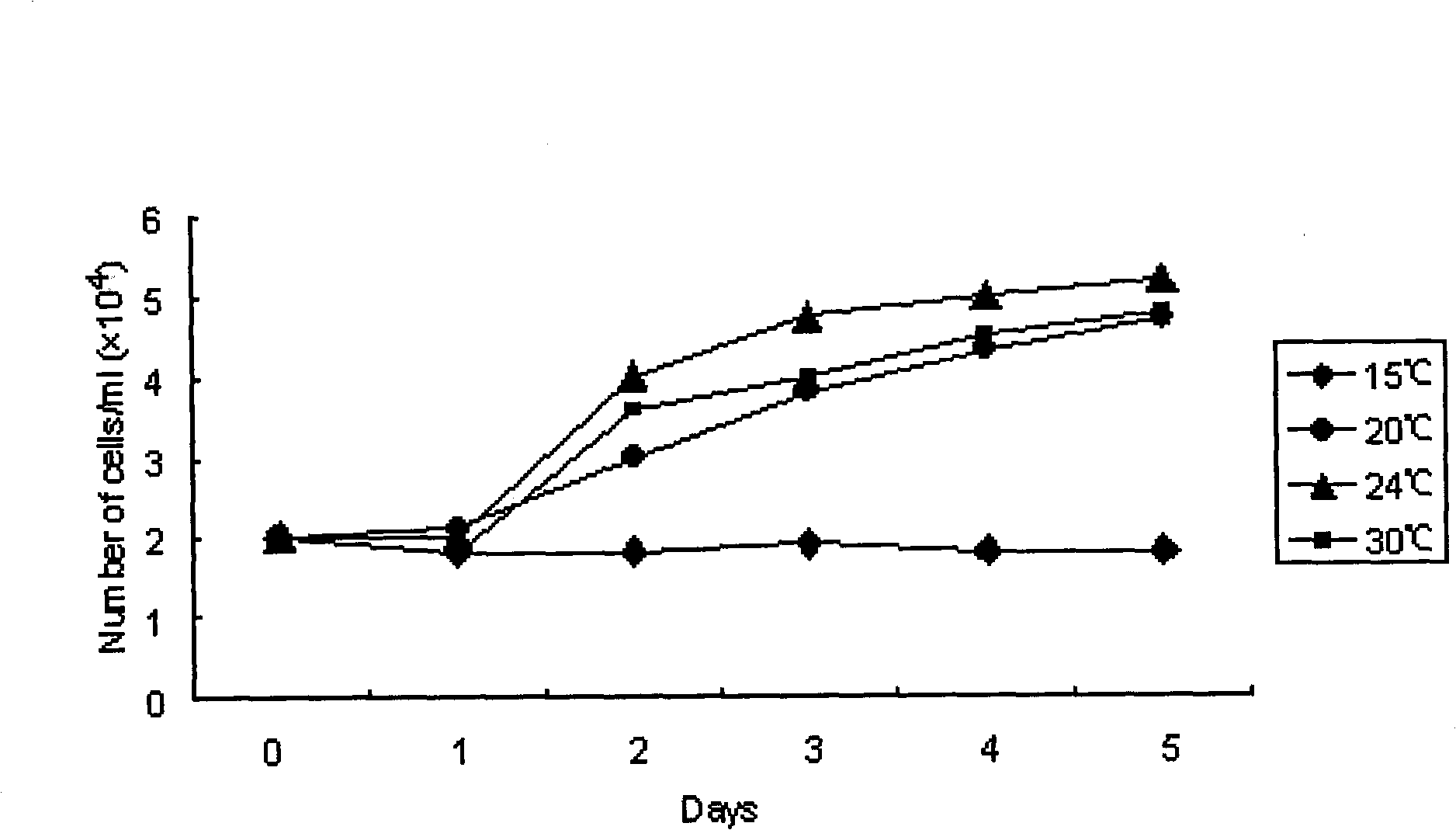
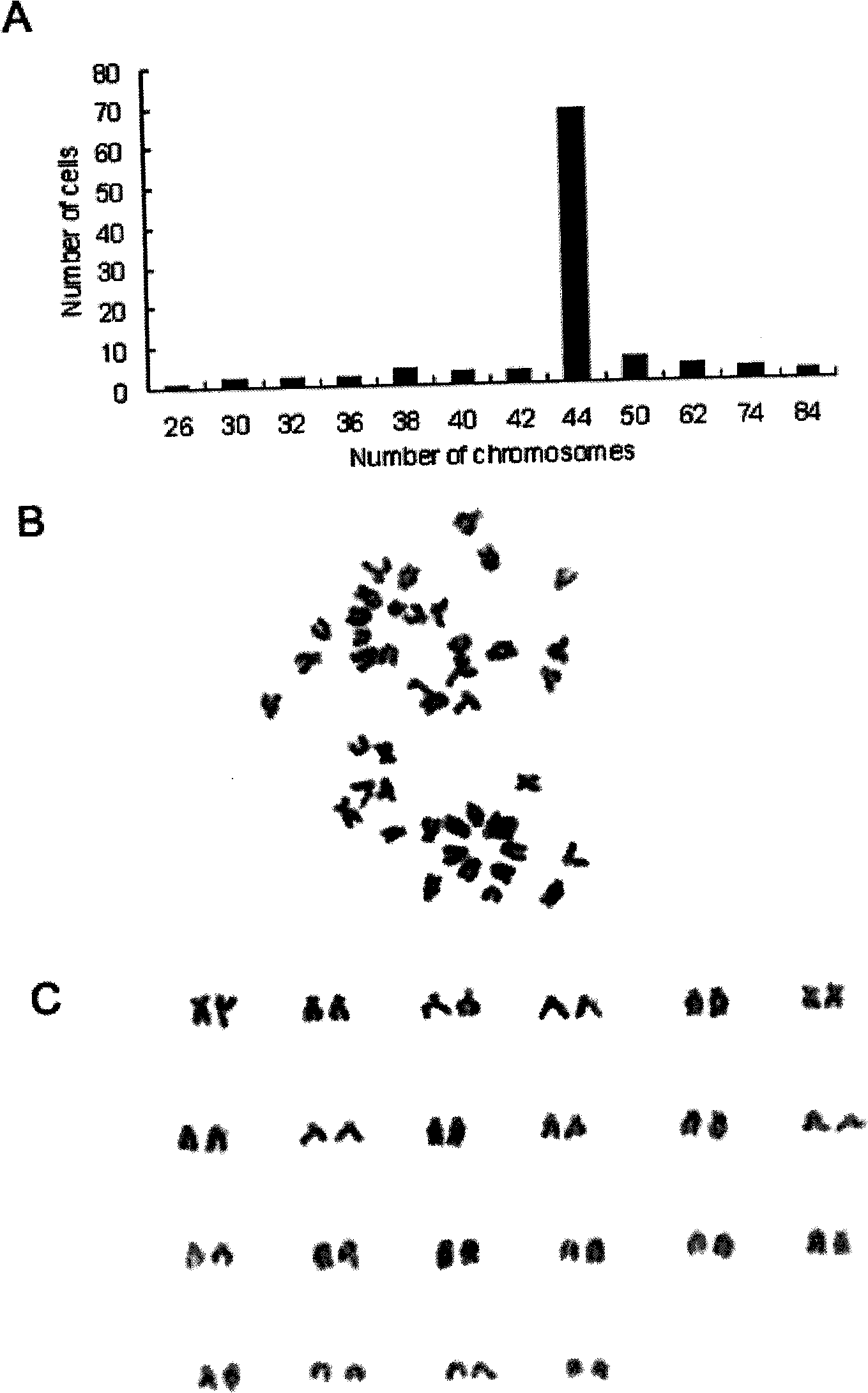
Method for constructing kidney cell line of scophthalmus maximus
ActiveCN101591638AGood repeatabilityThe identification method is credibleTissue culturePrimary cellBottle
The invention discloses a method for constructing a kidney cell line of scophthalmus maximus, which comprises the following steps of: preparing a cell culture fluid first, taking a kidney tissue of the scophthalmus maximus as a material, using 0.5 percent trypsinase to digest the kidney tissue for 30 minutes at room temperature after the rinsing and shearing, adopting an 200-mesh nylon gauze to filter and collect cells, split charging the cells with the quantity of 5-10*10 counts / bottle into culture bottles with the growth area of 25 cm, and placing the culture bottles at a temperature of 24 DEG C for culturing; replacing the cell culture fluid with half quantity each four days, and using 0.25 percent trypsinase digest the cells for passage when primary cells grow to be monolayered; and performing passage once each 8 to 10 days, wherein the content of serum in the cell culture fluid is reduced to 10 percent from 20 percent when the 8th generation is reached, and the cell line is successfully established at the moment. The kidney cell line of the scophthalmus maximus obtained by utilizing the method is fibroid and can be continuously transferred for more than 40 generations; the cell line can be directly applied to pathogeny characteristics research, vaccine development and functional gene research; and the construction method is suitable to construct kidney cell lines of other fishes.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Beta-cyclodextrin-modified polyamide-dendrimer and preparation method of gold nanoparticle compound of beta-cyclodextrin-modified polyamide-dendrimer
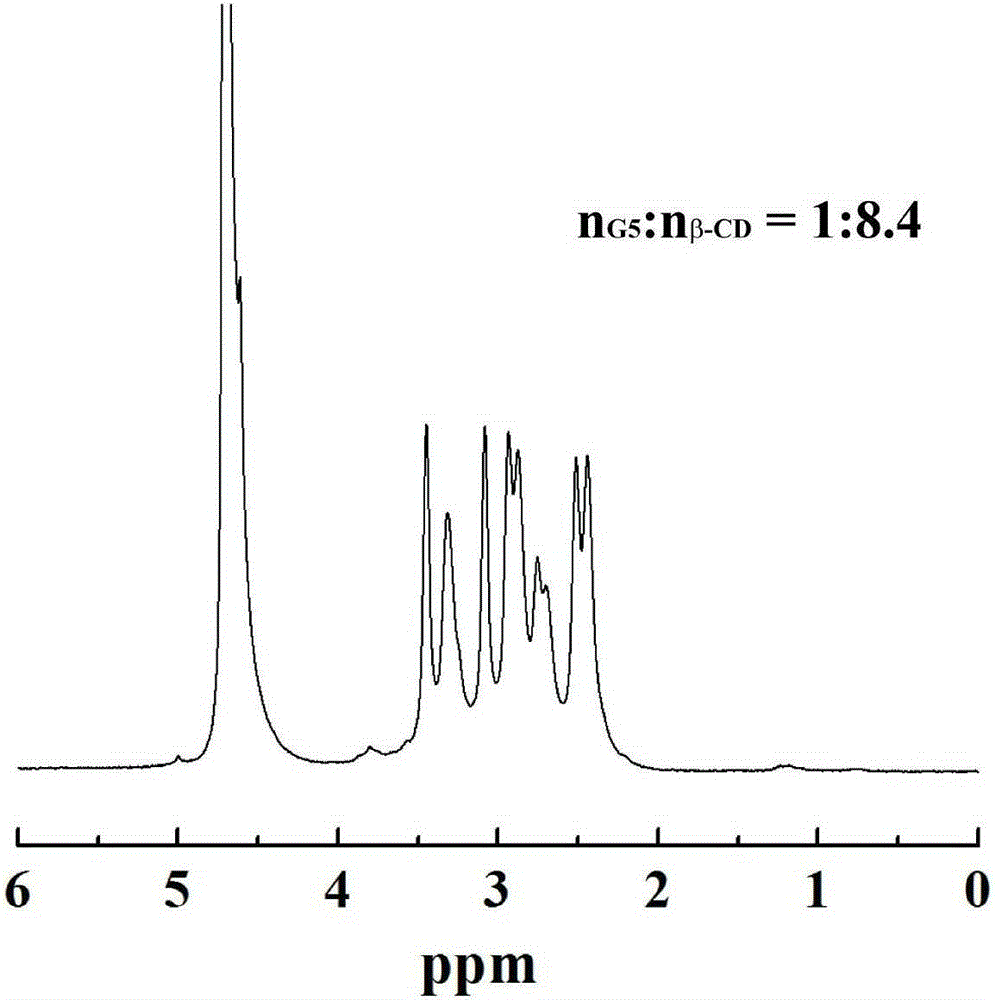
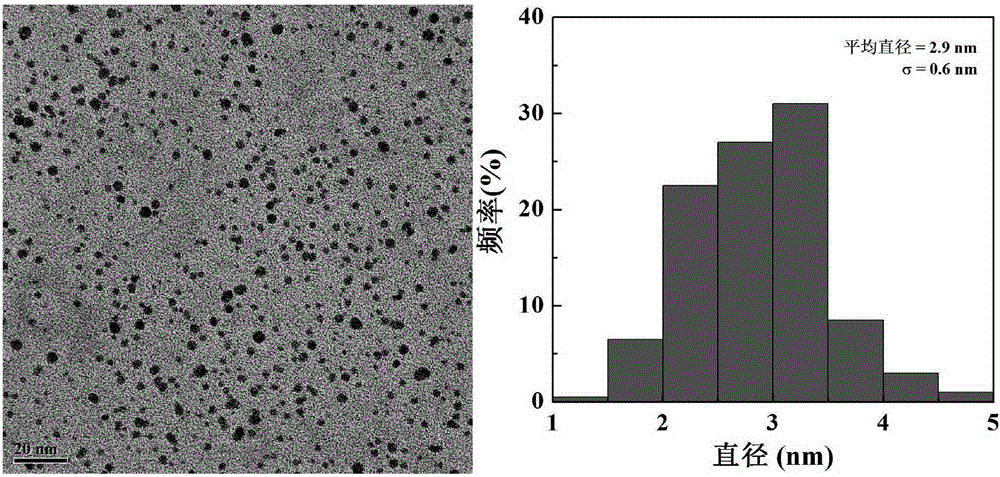
InactiveCN105199400ALow toxicityGood biocompatibilityOther foreign material introduction processesVector-based foreign material introductionDendrimerFreeze-drying
The invention relates to a beta-cyclodextrin-modified polyamide-dendrimer and a preparation method of a gold nanoparticle compound of the beta-cyclodextrin-modified polyamide-dendrimer. The preparation method includes: mixing DMSO (dimethyl sulfoxide) solutions in which beta-CD and CD I are dissolved, respectively, adding DMSO solution of G5PAMAM after reacting, performing stirring at room temperature for 3 d, and performing dialysis and freeze-drying to obtain G5.NH2-beta-CD; adding HauCl4.4H2O solution into the obtained G5.NH2-beta-CD solution, adding NaBH4 solution after stirring, performing stirring for 2-4 h, and performing dialysis and freeze-drying to obtain a target compound {(Au0)25-G5.NH2-beta-CD}. The prepared compound is a good gene carrier, capable of successfully carrying and expressing pDNA-transfected human embryonic kidney cells, transfection conditions are mild, operating is easy, transfection efficiency is high, and the compound has a promising application in terms of biomedicine such as gene therapy.
Owner:DONGHUA UNIV
Serum-free medium suitable for large-scale single-cell suspension culture of young hamster kidney cells
InactiveCN102268403AIncrease productivityEasy to separate and purifyArtificial cell constructsVertebrate cellsCulture mediumsAmino acid
The invention discloses a serum-free medium suitable for large-scale single-cell suspension culture of young hamster kidney cells (BHK cells), including 21 kinds of amino acids, 11 kinds of inorganic salts, 12 kinds of vitamins, 1 kind of protein hydrolyzate, 2 kinds of Lipids, 2 buffer components and 6 additives. The BHK cell serum-free medium of the present invention produces biological products by single-cell suspension culture, which can not only avoid various disadvantages of serum culture, but also eliminate the problem of difficult scale-up of roller bottle adherent culture, thereby improving the production efficiency of BHK cell culture And reduce production costs, while ensuring product quality, has good application value and huge market prospects.
Owner:上海米迪生物技术有限公司
Viral particles as immunogens against enterovirus infection and production thereof
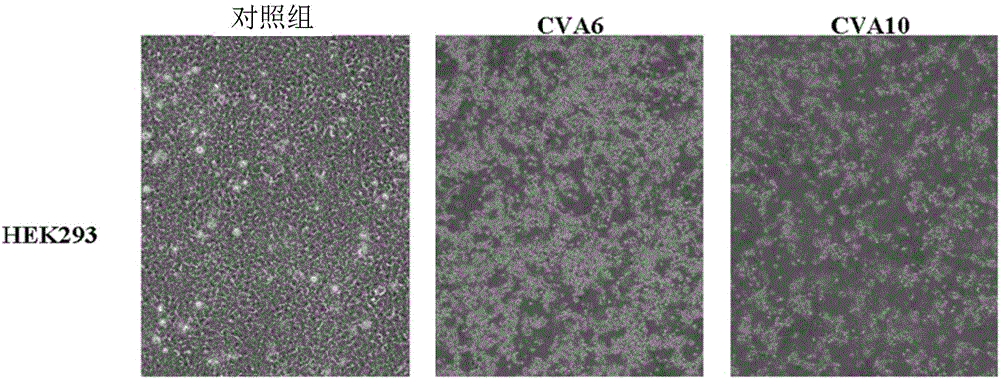
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
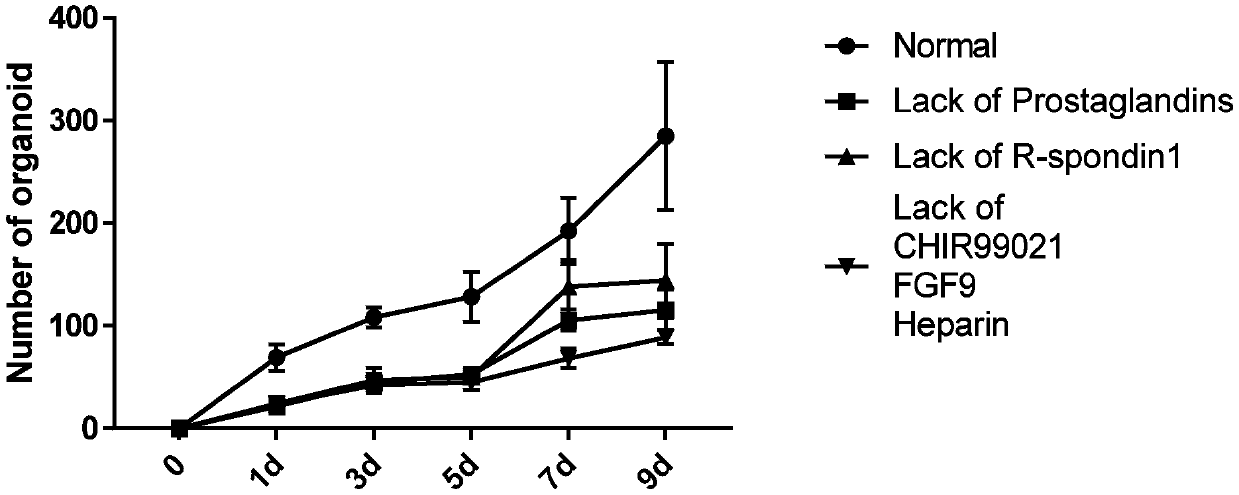
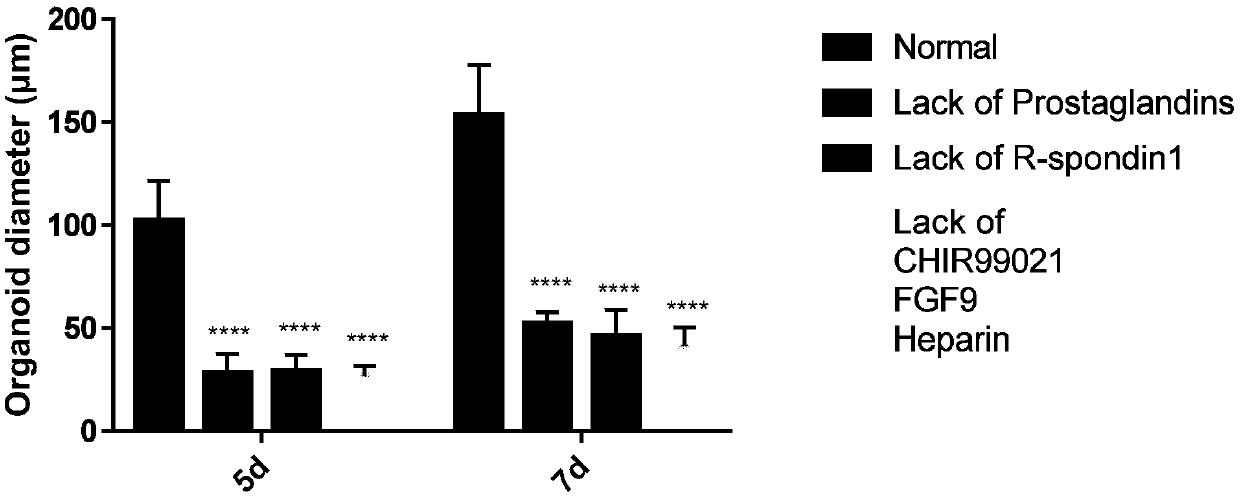
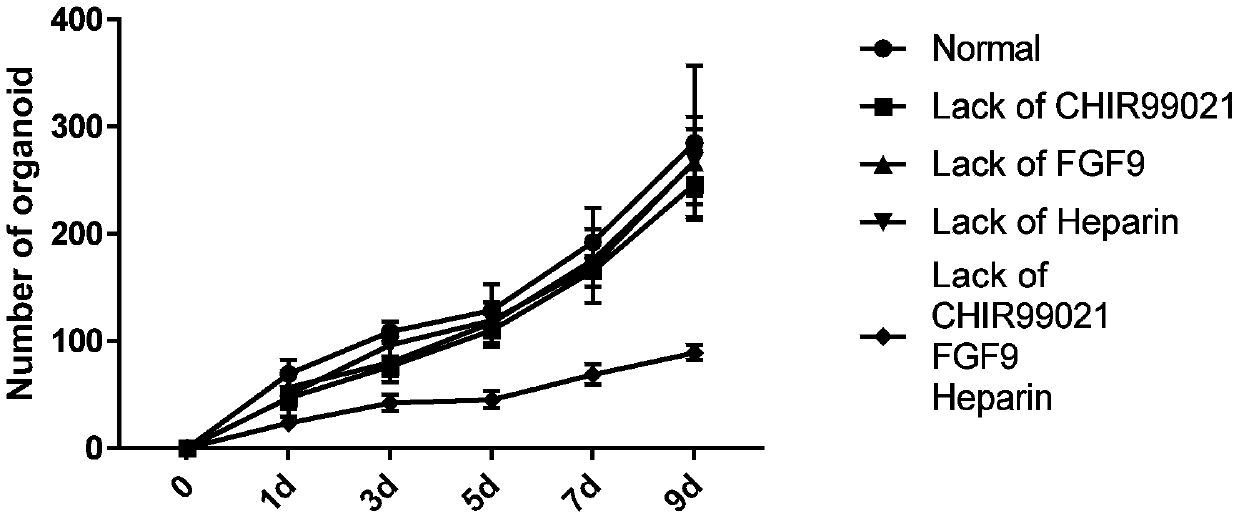
Organoids comprising isolated renal cells and use thereof
InactiveUS20160101133A1BiocidePharmaceutical delivery mechanismBlood Vessel EndotheliumErythroid cell
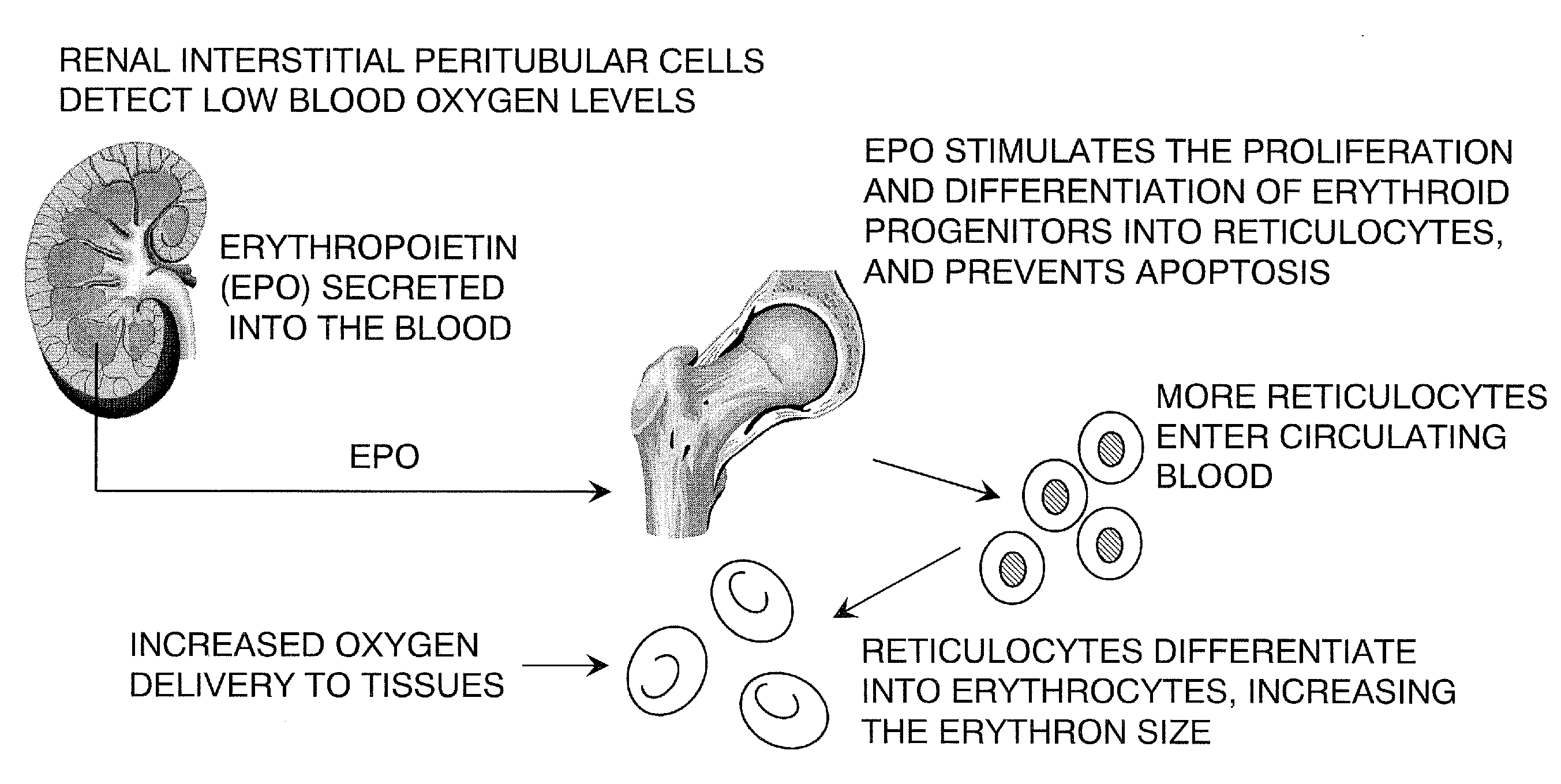
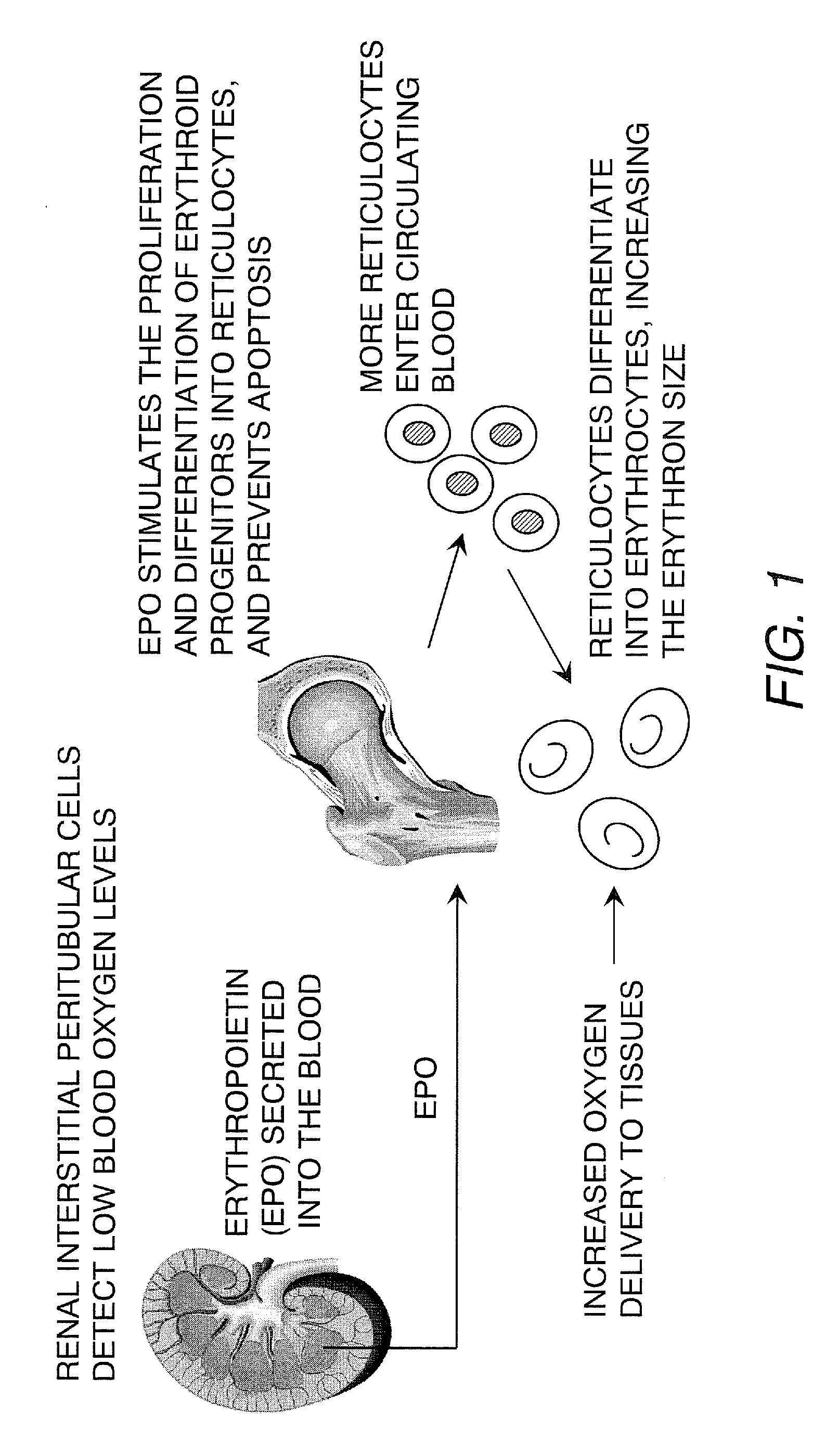
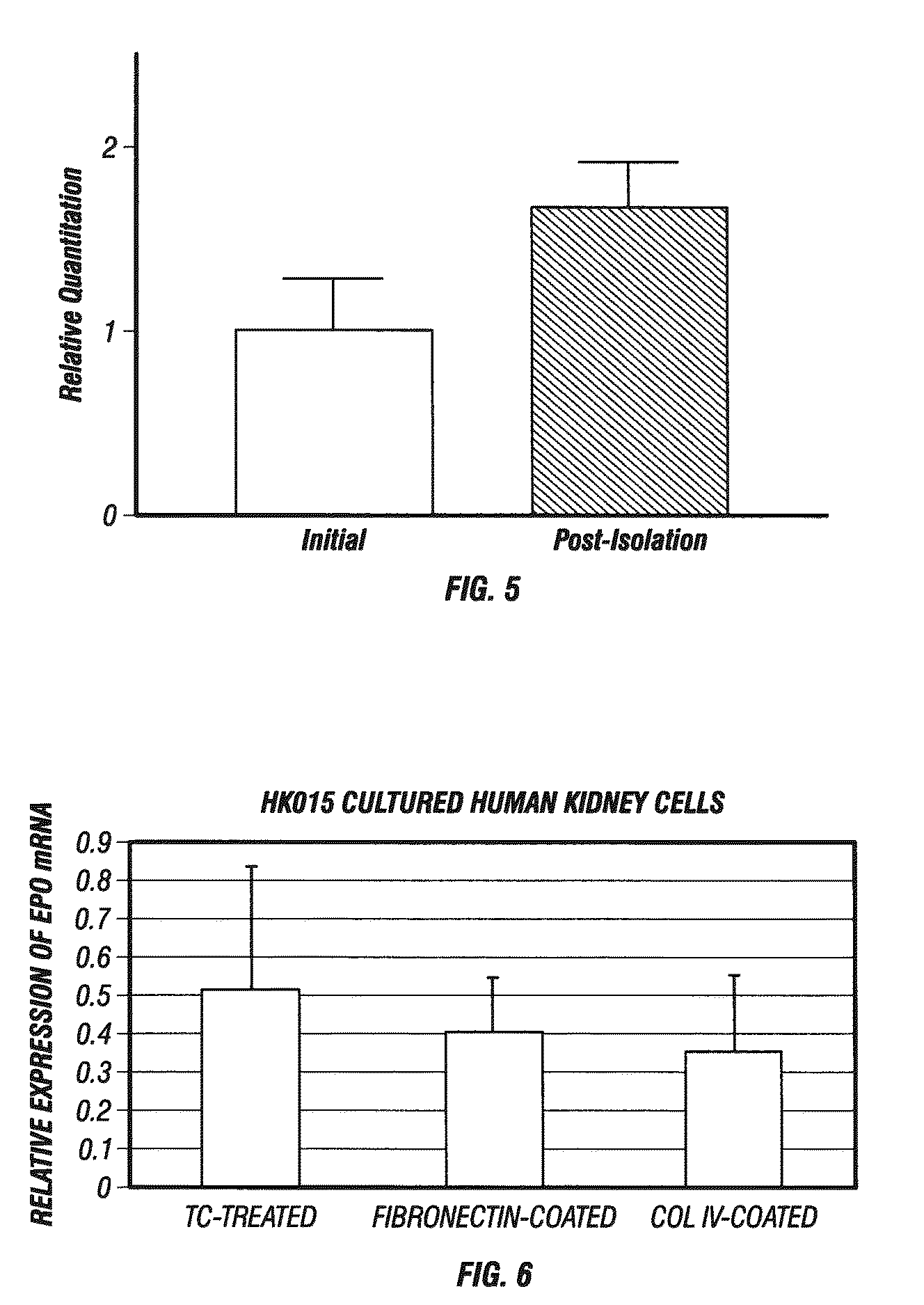
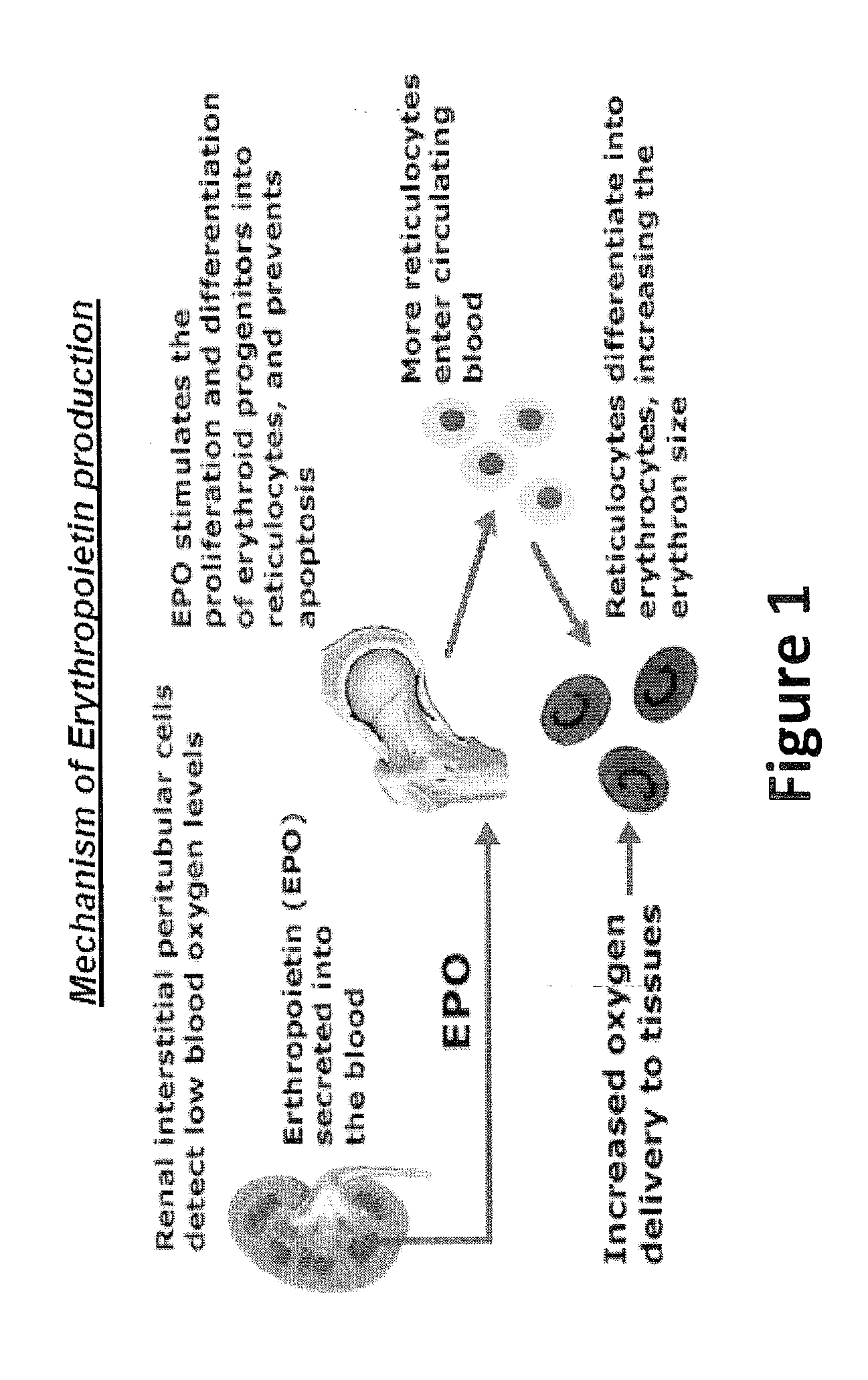
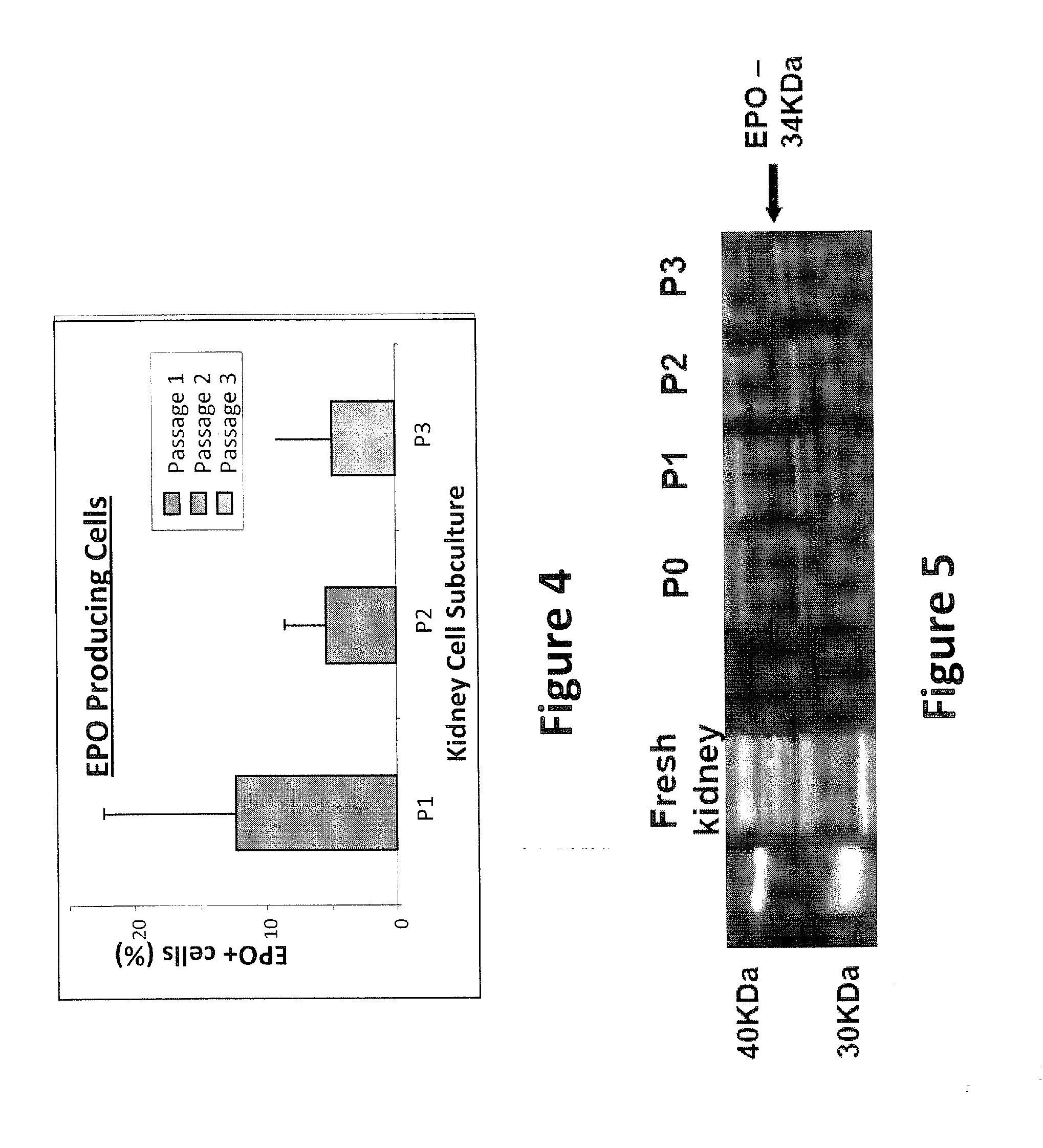
Described herein are organoids comprising admixtures of selected bioactive primary renal cells and a bioactive cell population, e.g., an endothelial cell populations, e.g. HUVEC cells, and methods of treating a subject in need thereof with such organoids. Further, the isolated renal cells, which may include tubular and erythropoietin {EPO}-producing kidney cell populations, and / or the endothelial cell populations may be of autologous, syngeneic, allogeneic or xenogeneic origin, or any combination thereof. Further provided are methods of treating a subject in need with the organoids.
Culture medium and organoid culture method for 3D culture of kidney tissue organoids
ActiveCN109609441AActive featuresImplement featuresArtificial cell constructsCell culture active agentsSignalling pathwaysMicrobiology
The invention discloses a culture medium and an organoid culture method for 3D culture of kidney tissue organoids. According to culture and growth characteristics of kidney-tissue-derived cells, the culture medium disclosed by the invention is prepared by blending a plurality of selected cytokine components in certain proportion; so that, signaling pathway regulatory factor content of the cytokines in the blended culture medium is appropriate. Thus, effective formation of organoids by kidney cells and renal cancer cells in 3D environment is allowed.
Owner:ACCURATE INT BIOTECHNOLOGY (GUANGZHOU) CO LTD
Kidney structures and methods of forming the same
Provided herein are isolated populations of kidney cells harvested from differentiated cells of the kidney, wherein cells have been expanded in vitro, and methods of use thereof. The cells may be provided in a three dimensional matrix for culturing in vitro and / or implanting in vivo. Methods of seeding cells onto the matrix are also provided.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Isolated renal cells and uses thereof
ActiveUS8318484B2Decrease the declinationRegeneration of kidney functionBiocideCell dissociation methodsErythropoietinKidney cell
The invention is directed to isolated renal cells, including tubular and erythropoietin (EPO)-producing kidney cell populations, and methods of isolating and culturing the same, as well as methods of treating a subject in need with the cell populations.
Owner:PROKIDNEY
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671AStable titerImproving immunogenicitySerum immunoglobulinsMicroorganism based processesSequence analysisImmunogenicity
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
Method for preparing double yolk antibody of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN101845095AStrong specificityStrong targetingEgg immunoglobulinsImmunoglobulins against virusesPig farmsYolk
The invention discloses a method for preparing a double yolk antibody of a porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The method comprises the following steps of: performing porcine transmissible gastroenteritis virus multiplication on porcine kidney cells (PK15); performing porcine epidemic diarrhea virus multiplication on African green monkey kidney cells (Vero); emulsifying the two cell cultures used as antigen with an oil emulsion adjuvant to prepare immunogen, namely, mixing the two kinds of viruses in a ratio of (1-3):(1-3) to prepare the immunogen; immunizing non-immunologic laying hens; and obtaining the double yolk antibody which can prevent and treat porcine transmissible gastroenteritis and porcine epidemic diarrhea based on the collection and purification of the yolk. When the double yolk antibody is used for curing experimental pigs, the clinical symptoms in the experiment are obviously reduced compared with a control group, and the death rate of the experimental group is obviously lower than that of the control group. The double yolk antibody has obvious preventing and treating functions when applied in a pig farm with high incidence rate of the porcine transmissible gastroenteritis and the porcine epidemic diarrhea.
Owner:PU LIKE BIO ENG
Selective cell therapy for the treatment of renal failure
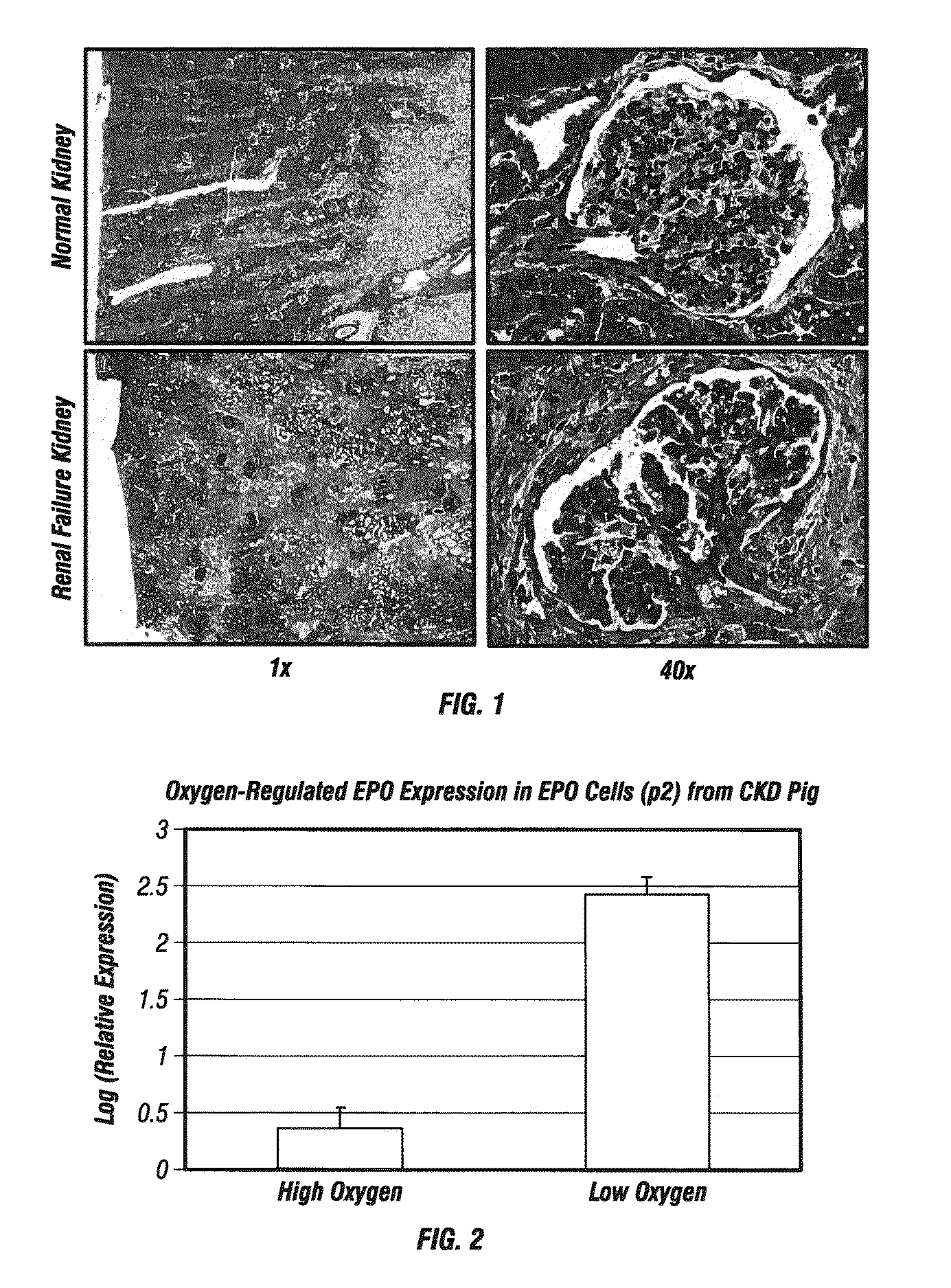
Provided herein are isolated populations of kidney cells harvested from differentiated cells of the kidney, wherein the cells have been expanded in vitro. The kidney cells preferably produce erythropoietin (EPO). The kidney cells may also be selected based upon EPO production. Methods of producing an isolated population of EPO producing cells are also provided, and methods of treating a kidney disease resulting in decreased EPO production in a patient in need thereof are provided, including administering the population to the patient, whereby the cells express EPO in vivo in an oxygen tension-dependent manner.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
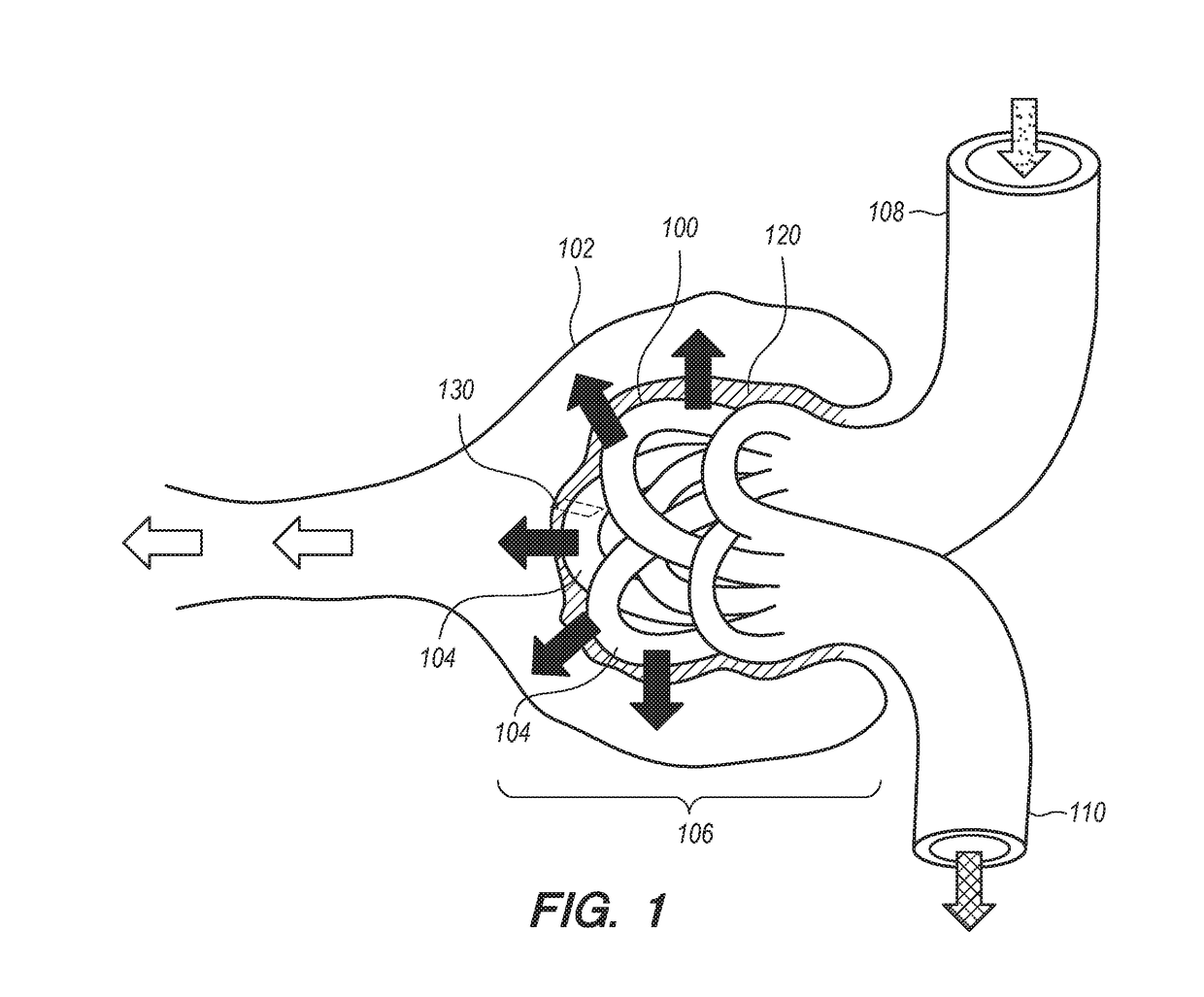
Compositions and methods for a three dimensional ex-vivo glomerular cell co-culture biological engineering model
InactiveUS20180298343A1Avoid traversalBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseVascular endothelium
The invention provides compositions, methods and systems for the ex vivo co-culture of renal cells, more specifically, for the co-culture of glomerulus-derived vascular endothelial cells and podocyte cells using apparatus that mimics the in vivo cellular architecture of the renal corpuscle. The invention described herein finds a variety of uses, for example, as a model system for the study of renal corpuscle function, including the filtration of the blood supply that occurs at the interface of the glomerulus and Bowman's capsule, normal physiology of those cell types, and as a model system for the study of renal disease, including the study of drug effects on the functioning of these cell types.
Owner:SIVAKUMARAN ARUNTHATHY
Cell culture method for duck flavivirus
InactiveCN102220290AStable proliferationObvious cytopathicViruses/bacteriophagesDiseaseCytopathic effect
The invention provides a cell culture method for duck flavivirus. The method comprises the following steps: inoculating to attach an anchorage-dependent kidney cell BHK21 of a baby hamster to the surface of a cell culture container to form a monolayer; inoculating the monolayer with virus; culturing the monolayer inoculated with the virus in an incubator with 5% CO2 at 37 DEG C; and continuously passing by taking a cell culture product as an inoculum for the next passage. According to the cell culture method provided by the invention, after the virus is continuously passed for 5-7 generations on the monolayer, stable multiplication can be obtained; and after the cell is inoculated for 3-4 days, an obvious cytopathic effect can be formed. The method provides a convenient and visual virus operating platform for studying the biological characteristics of the virus and the molecular basis of virus pathopoiesia and for the vaccine research developed for effectively preventing and controlling the disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
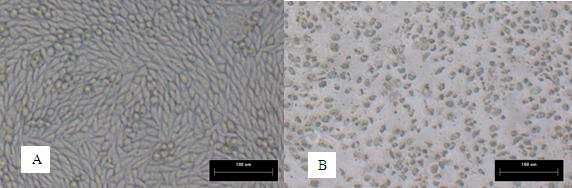
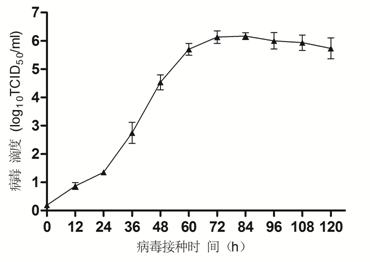
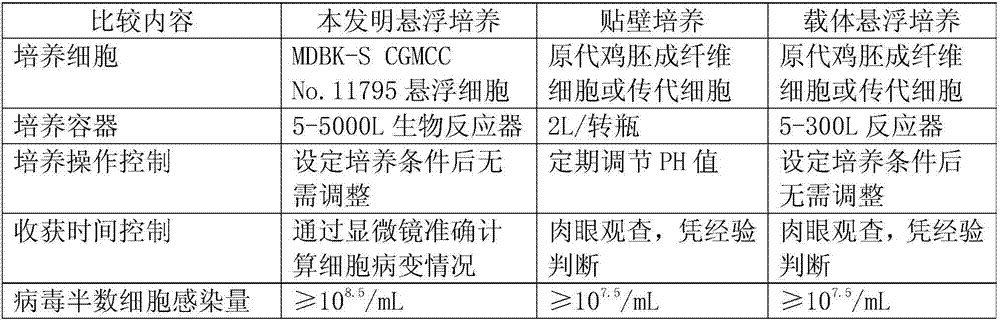
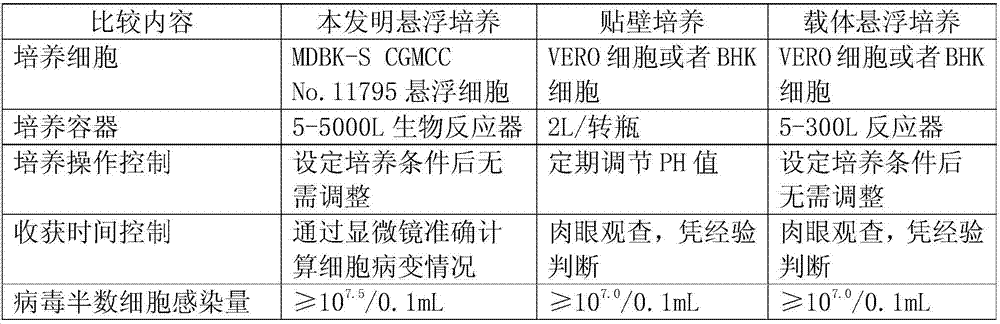
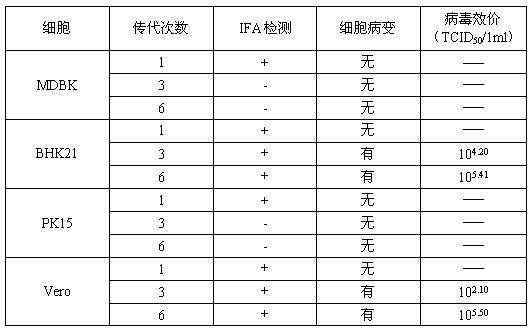
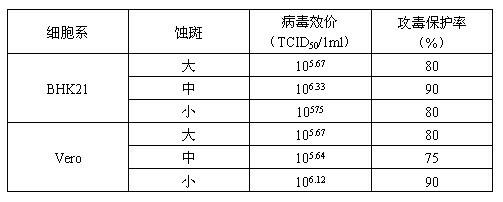
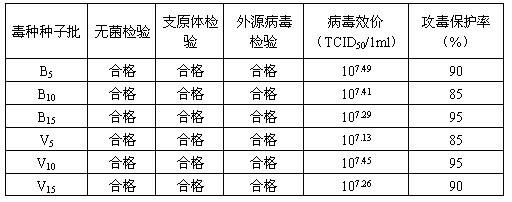
Bovine kidney cell capable of being subjected to suspension culture, suspension culture method for bovine kidney cell and application thereof
ActiveCN107201334AIncrease productionSolve technical problems with differencesSsRNA viruses negative-senseSsRNA viruses positive-senseAdherent CultureSuspension culture
The invention discloses a bovine kidney cell capable of being subjected to suspension culture, a suspension culture method for the bovine kidney cell and application thereof. The bovine kidney cell, the suspension culture method for the bovine kidney cell (MDBK) and the application have the advantages that a large quantity of manual operation for cell digestion, liquid change, frequent seed separation and the like can be omitted by the suspension culture method, the suspension culture method is simple, convenient and speedy, processes can be automated by the aid of reactor culture, the cell yield of the unit volume can be increased, and the technical difficult problem of difference between the traditional adherent culture cell batches can be solved; the bovine kidney cell (MDBK) and suspension culture cells of the bovine kidney cell can be used in the fields of culture of diversified sensitive viruses, production of antibody proteins, production of vaccine, examination of exogenous viruses of products and the like.
Owner:JINYUBAOLING BIO PHARM CO LTD
Duck hemorrhagic oophoritis inactivated vaccine production method by using cell line and product thereof
ActiveCN102488893AImprove adaptabilityHigh potencyViral antigen ingredientsInactivation/attenuationVaccine ProductionHamster
A duck hemorrhagic oophoritis inactivated vaccine production method by using a cell line and a product thereof belong to the field of biotechnology. According to the method provided by the invention, the cell line is selected from hamster kidney cell line or African green monkey kidney cell line, and the preparation of the vaccine is accomplished by the following steps of: (1) breeding strain, (2) establishing virus seed group, (3) preparing cell venom, (4) inactivating virus, (5) washing and concentrating cell venom, (6) emulsifying and the like. The method provided by the invention has characteristics of simple and stable production process, easy operation and high security. The prepared duck hemorrhagic oophoritis inactivated vaccine has advantages of high quality, high yield, low cost, small inter-batch difference, good safety and high immune efficacy.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
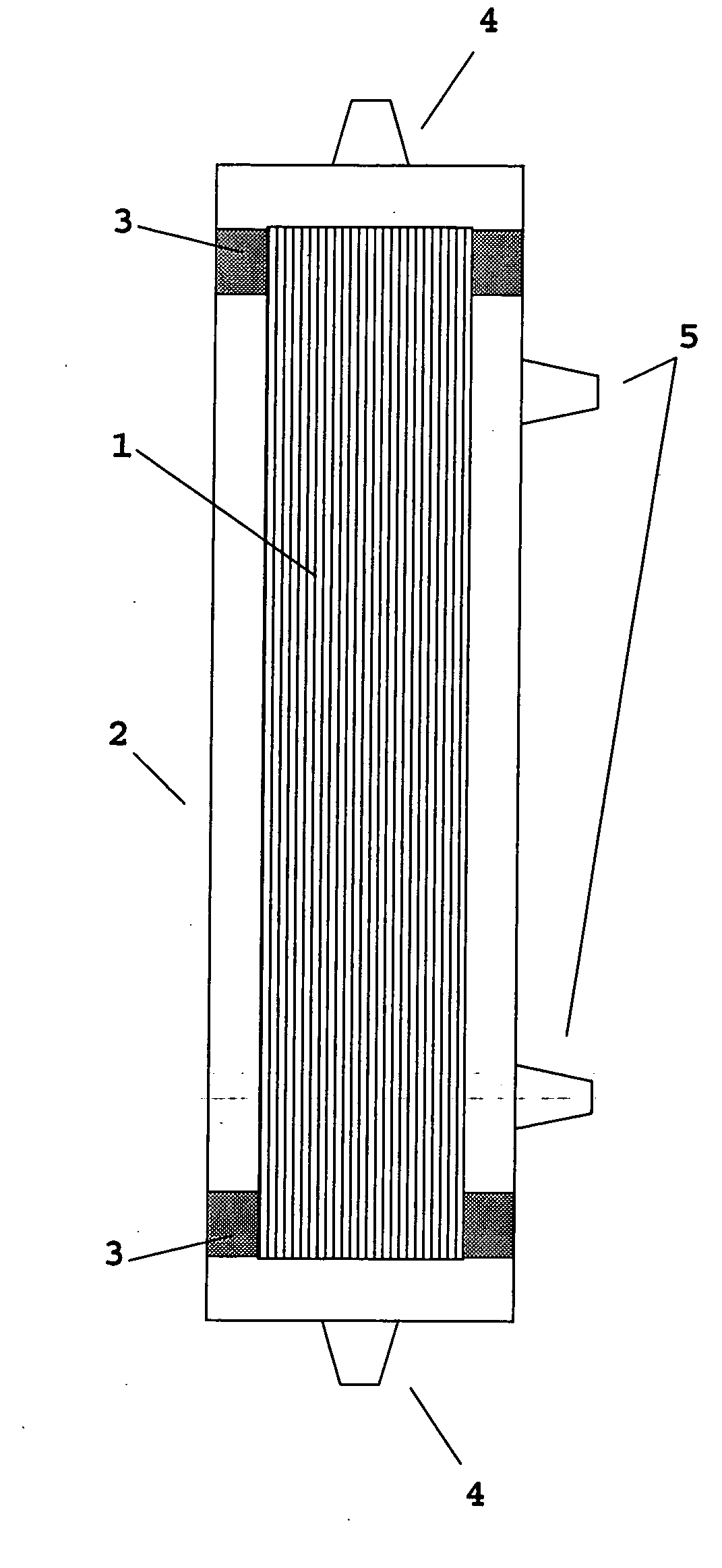
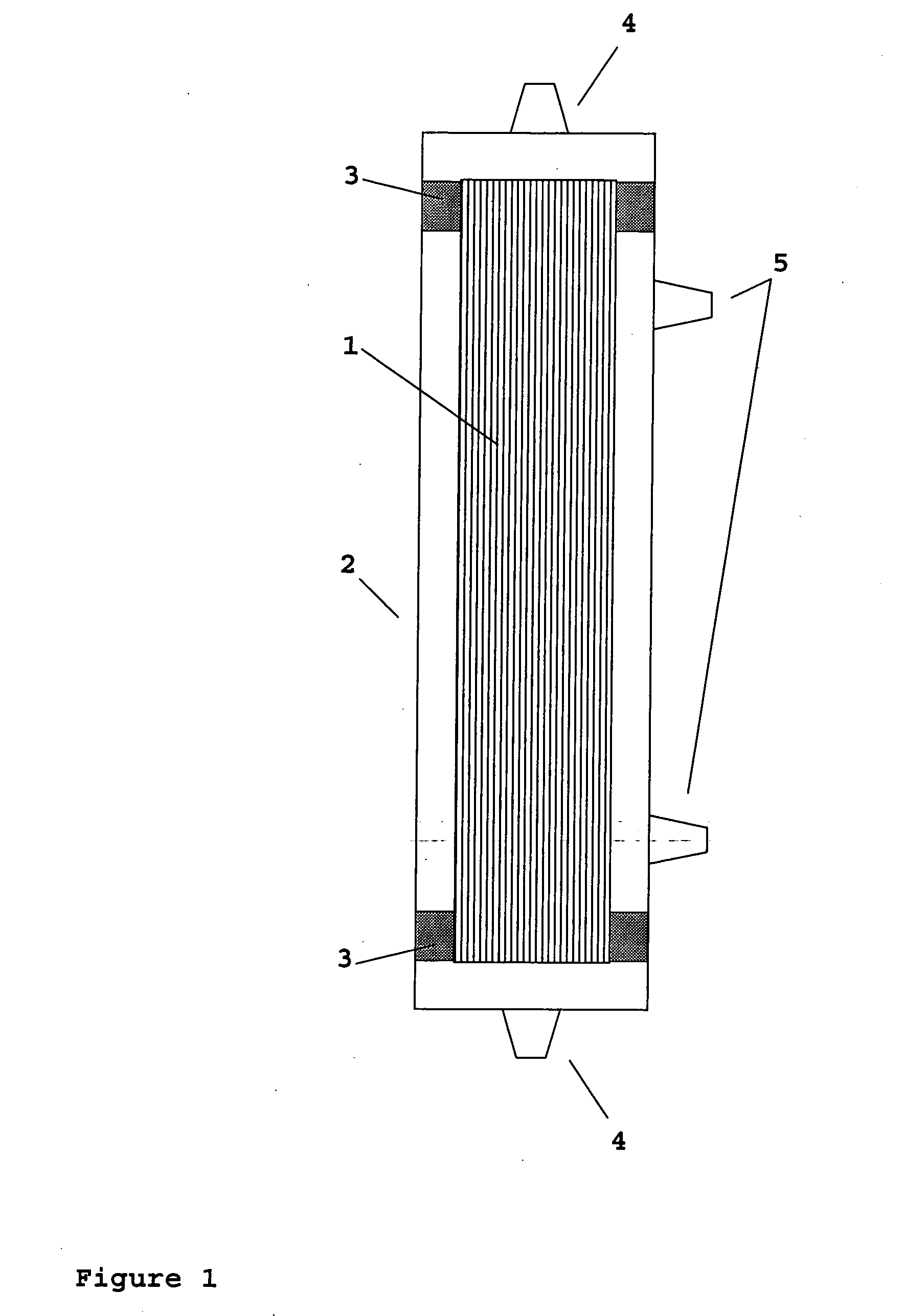
Device for renal cell expansion
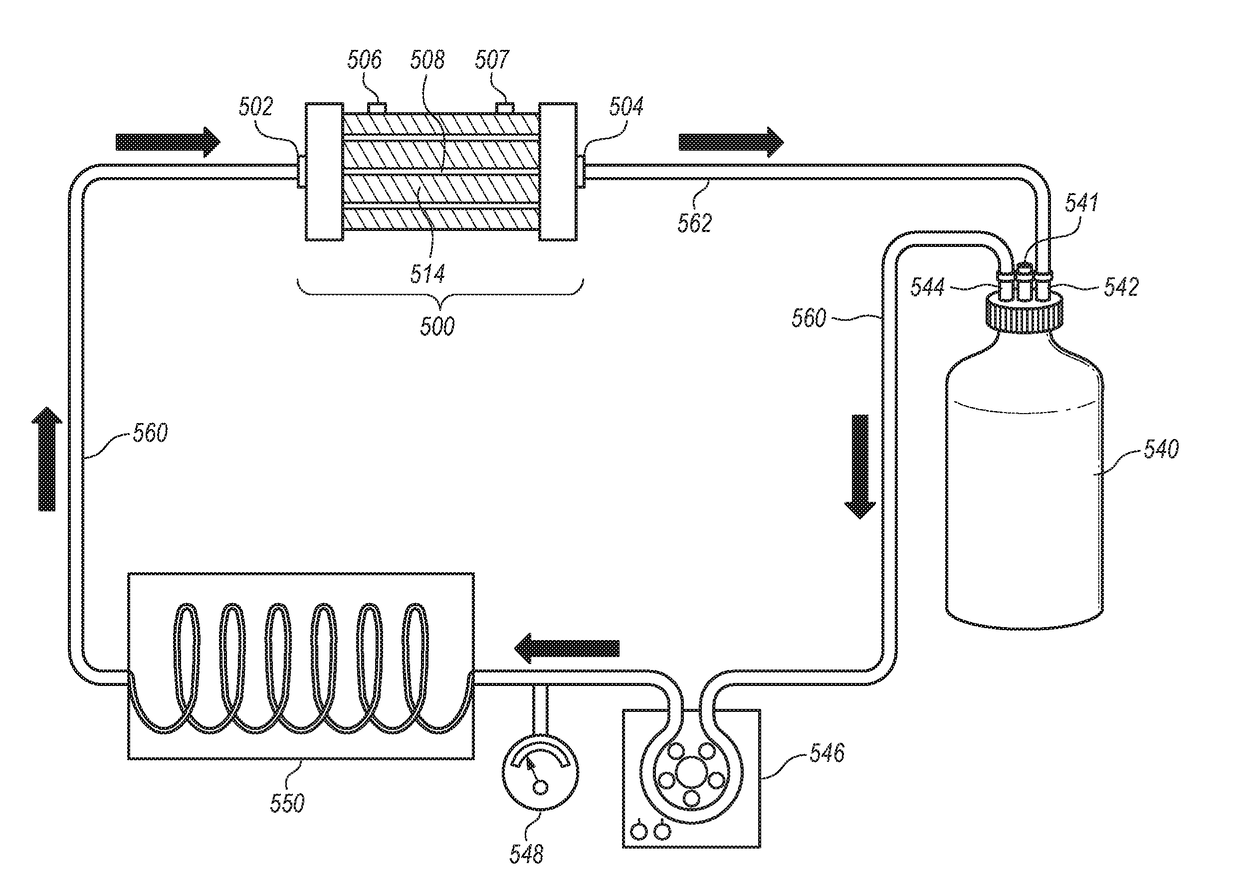
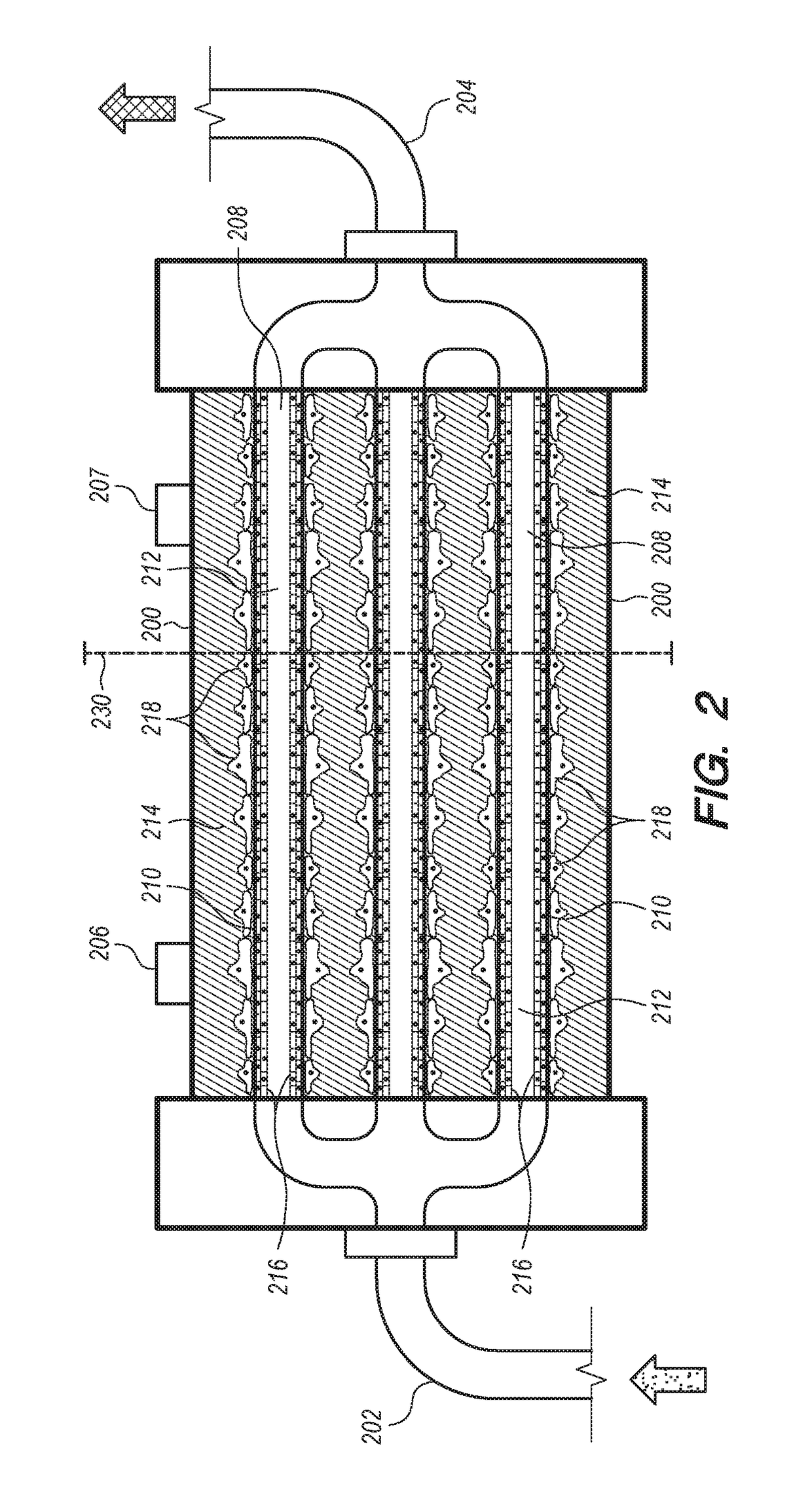
ActiveUS20120145626A1High performance featuresImprove performanceBioreactor/fermenter combinationsBiological substance pretreatmentsAcrylonitrileSodium methallyl sulfonate
A device for culturing, storing and expanding adherent renal cells. The device permits the adhesion and proliferation of the cells and the surface characteristics of the membrane material used in said device further permit the cellular matrix components (EMC). A method for expanding adherent renal cells and methods of using particular membranes for culturing, storing and expanding adherent renal cells. The membranes comprise a copolymer of acrylonitrile and sodium methallyl sulfonate as well as polyethylenimine.
Owner:GAMBRO LUNDIA AB
Bioartificial proximal tubule systems and methods of use
This application invention discloses bioartificial proximal tubule device, constructed by preparing a decellularized biological matrix, seeding the biological matrix with mammalian kidney-derived cells and optionally mammalian endothelial cells. The device may then be cultured statically or matured using bioreactor culture to allow differentiation of the kidney cells into functioning proximal tubule cells. The device is capable of carrying out proximal tubule functions. The application also describes various methods of making the proximal tubule devices. The application also further describes methods of use of bioartificial proximal tubule devices for e.g. in vitro studies of tubule cell transport, toxicity effects of various compounds or pharmaceutical compound screening.
Owner:DEPUY SYNTHES PROD INC
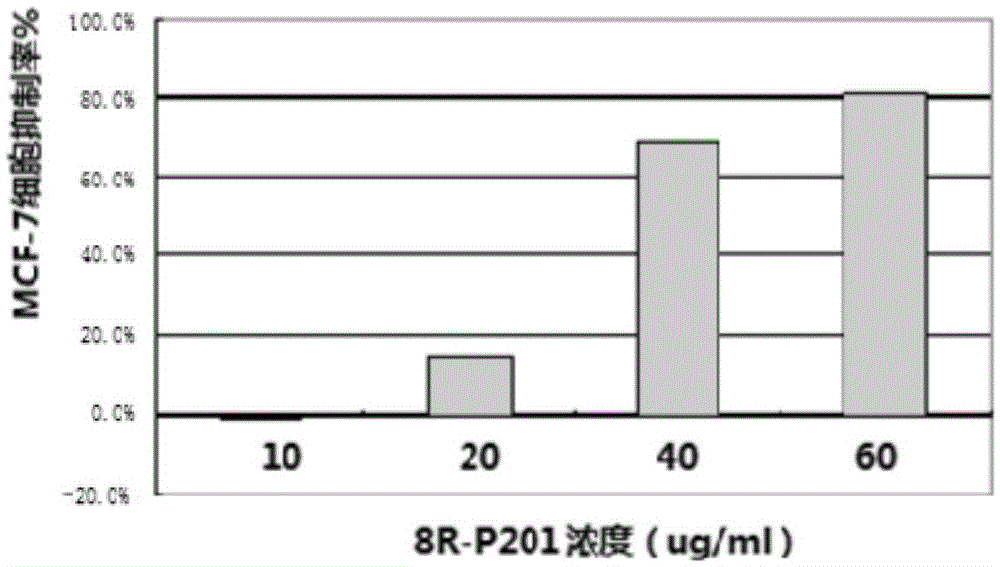
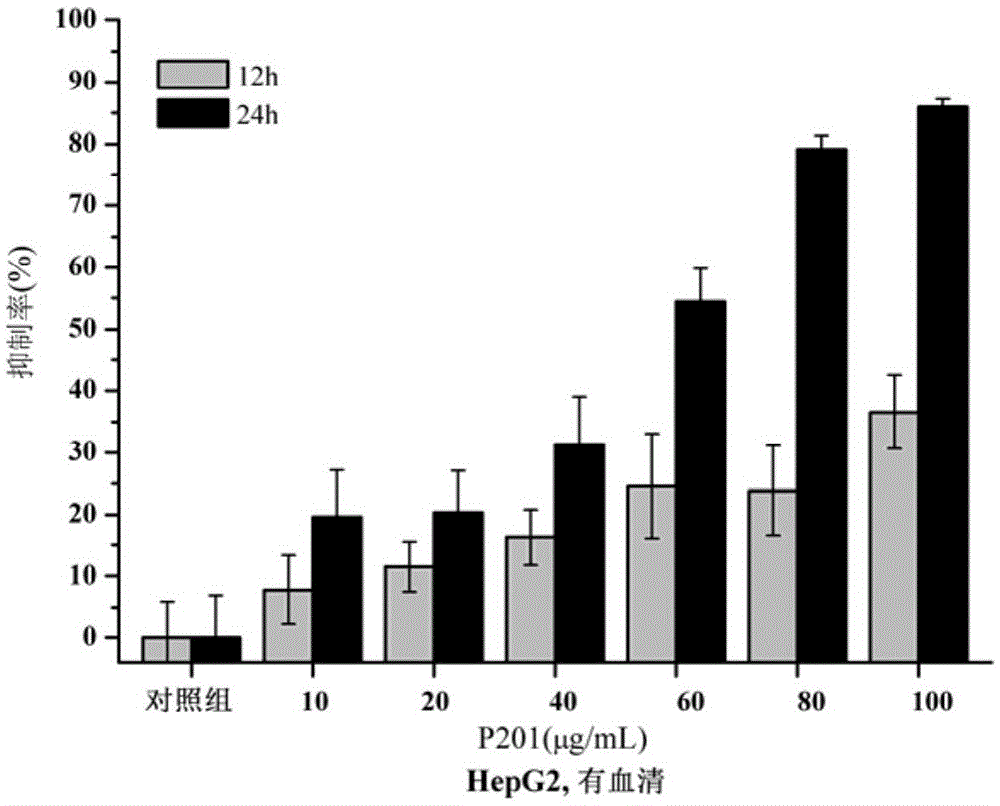
Polypeptide molecule exerting selective killing and migration inhibiting effect on cancer cells, and design method and application thereof
ActiveCN105524159AGood killing effectStrong migration inhibitionPeptide/protein ingredientsAntineoplastic agentsCancer cellProstate cancer
The invention discloses a polypeptide molecule exerting selective killing and migration inhibiting effect on cancer cells, and a design method and application thereof, belonging to the technical field of application of biopharmacy. The polypeptide molecule comprises a polypeptide amino acid sequence and constituent (SEQ ID No. 1) and contains altogether 12 amino acids. Through 9R or 8R coupling reconstruction and designing, the polypeptide molecule has strong killing effect on malignant cells of human liver cancer, prostatic cancer, breast cancer and the like and low killing effect on normal human hepatic cells, embryonic kidney cells and umbilical vein endothelial cells; in particular, the polypeptide molecule has strong selective killing effect on liver cancer cells and inhibitory effect on migration of human liver cancer and umbilical vein endothelial cells compared with normal human hepatic cells. The polypeptide molecule is mainly applied to preparation of drugs used for selective killing and migration inhibiting of malignant cells of human liver cancer.
Owner:SOUTHWEST JIAOTONG UNIV
Polypeptide hydrogel with slow-release exosomes and preparation method and application thereof
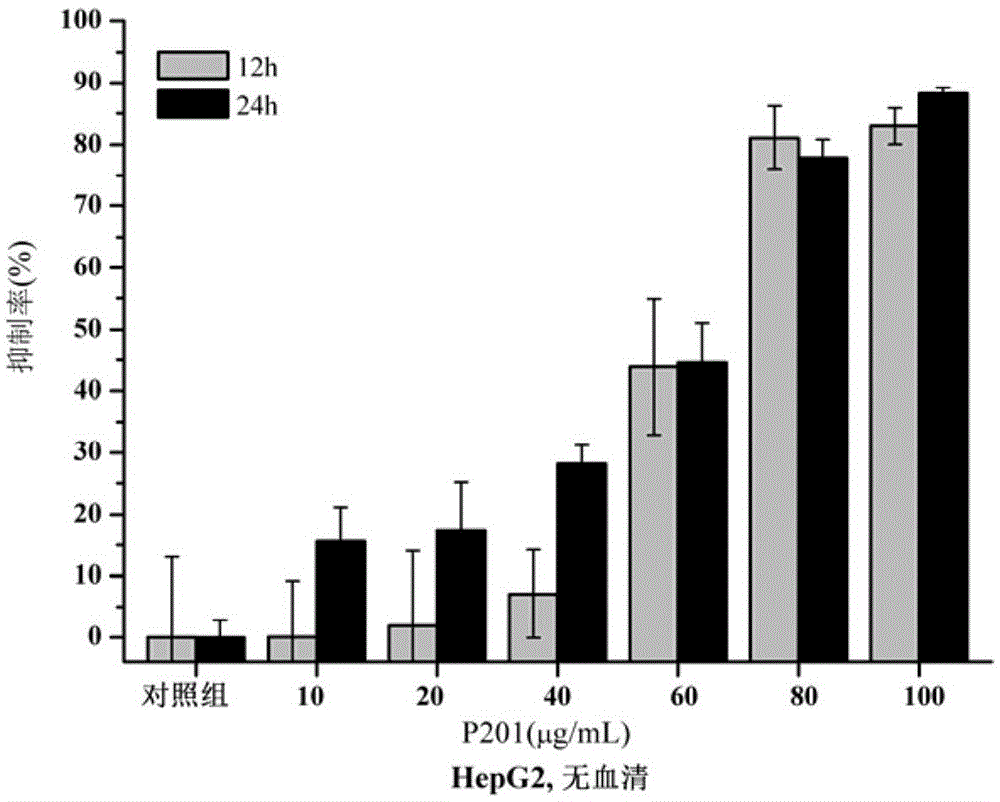
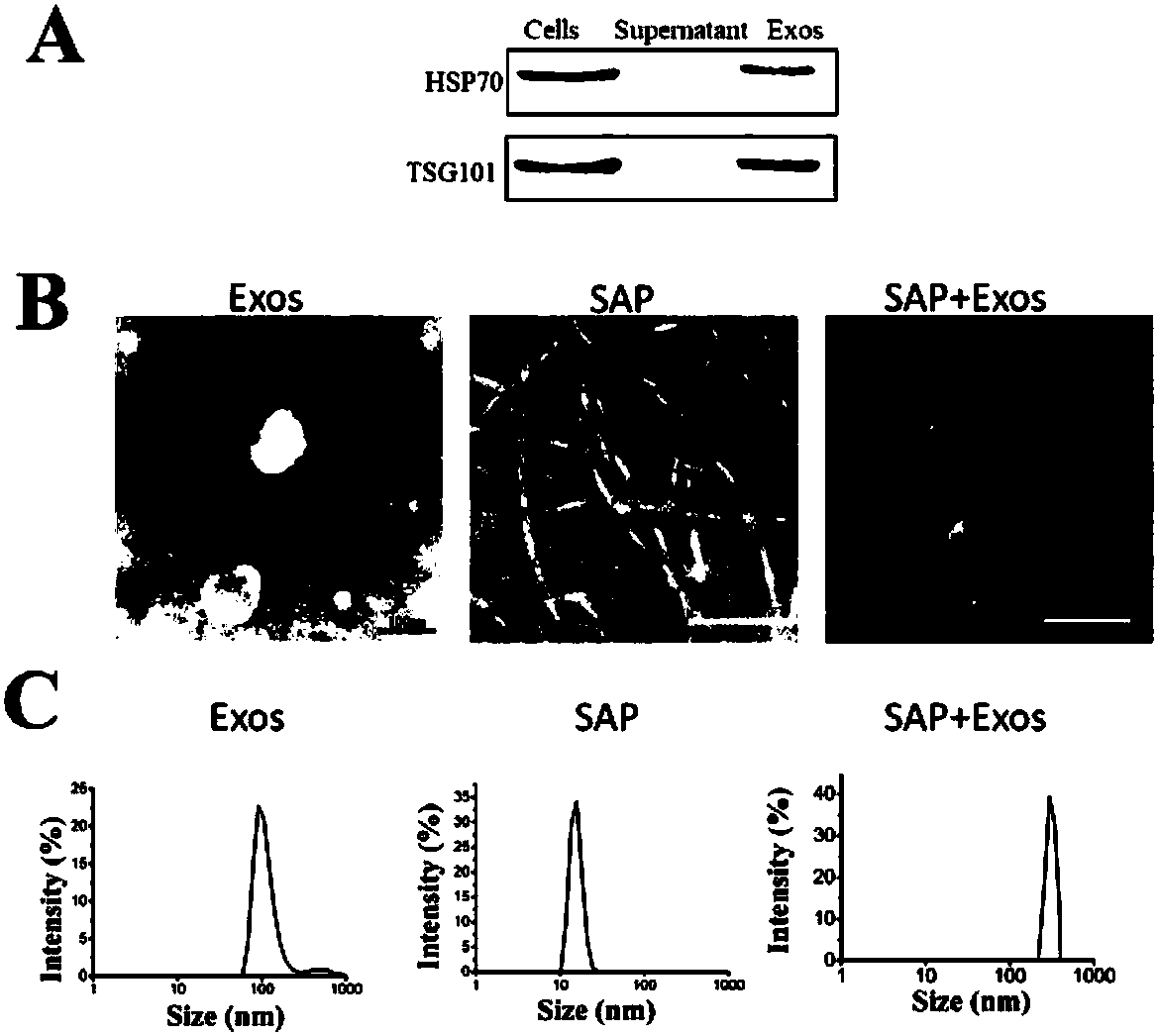
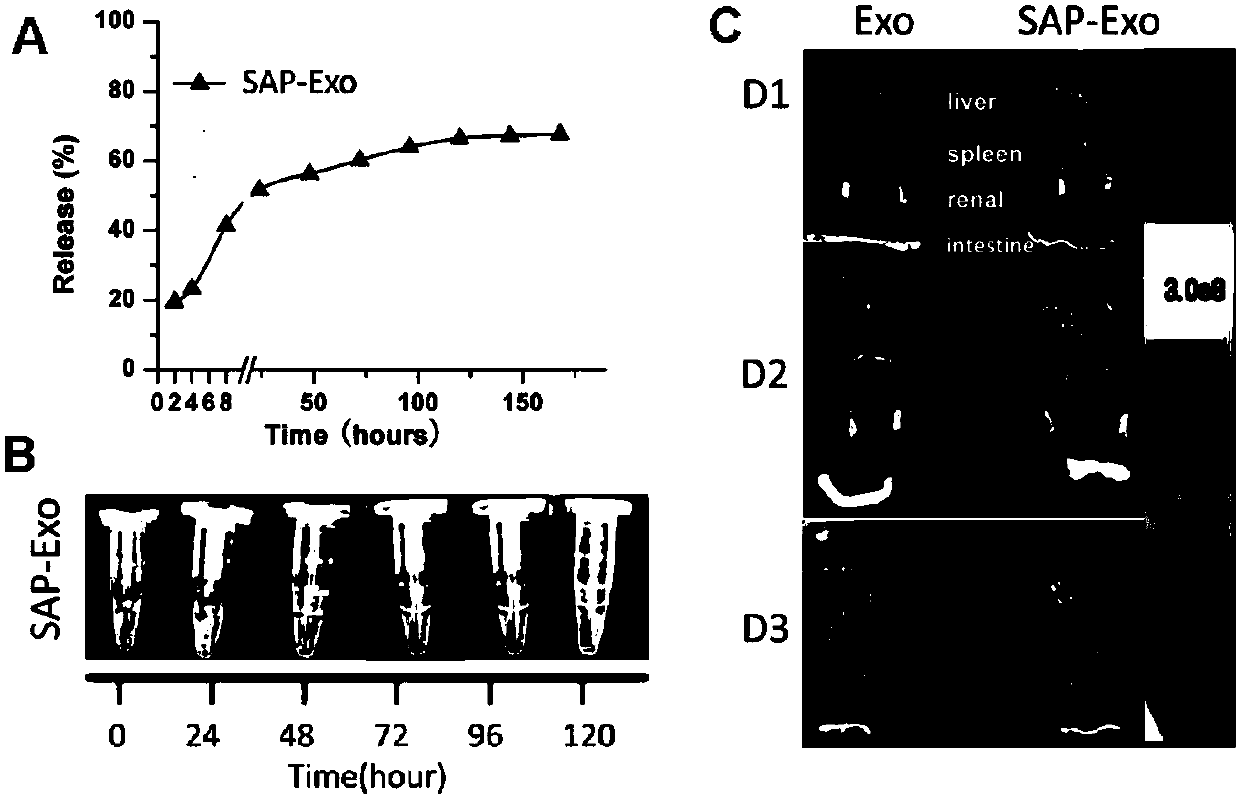
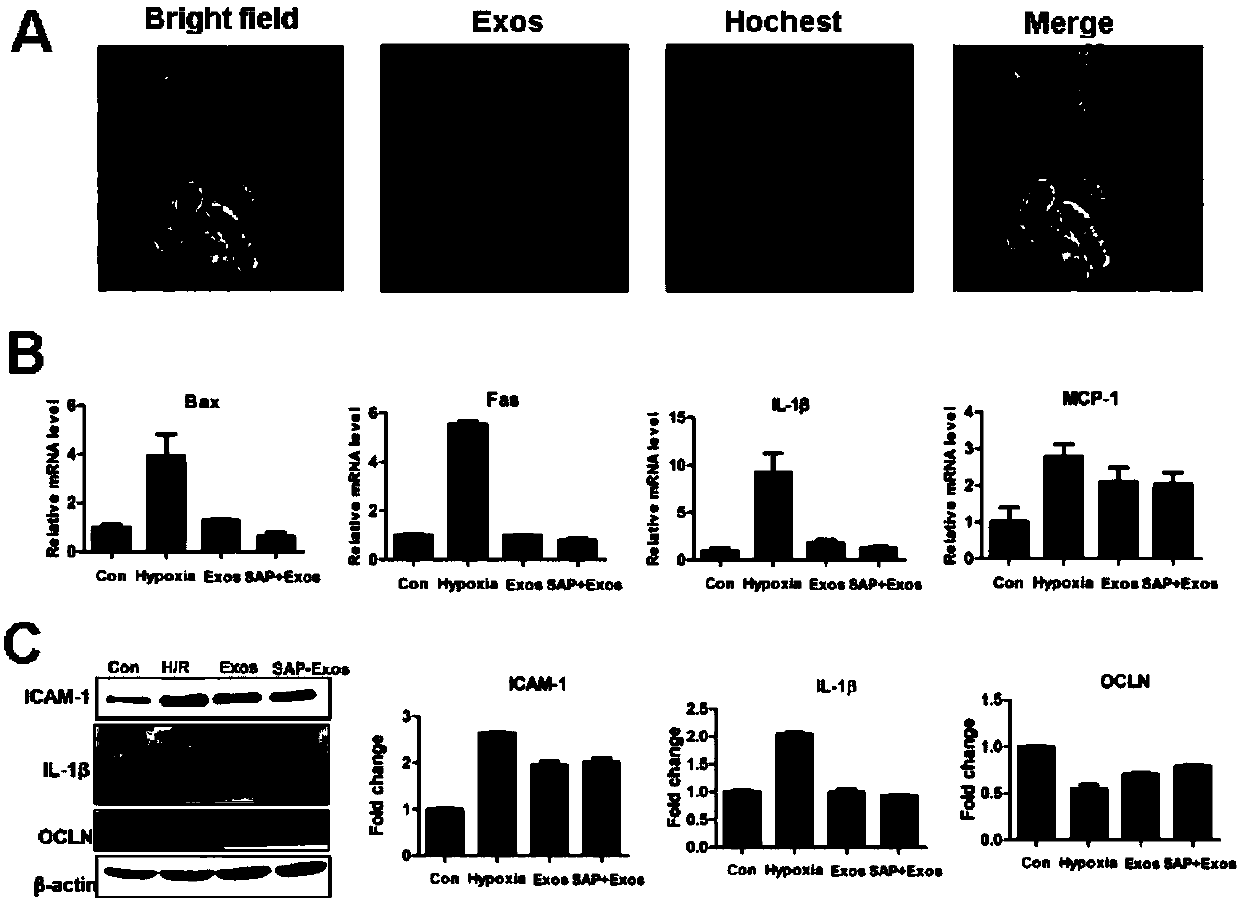
ActiveCN110229214AProlong and enhance efficacyReduce releaseAerosol deliveryOintment deliveryInflammatory factorsCell-Extracellular Matrix
The invention discloses a polypeptide hydrogel. The polypeptide hydrogel is prepared by mixing a self-assembled peptide (SAP) aqueous solution with exosomes (Exos). The present invention also discloses a preparation method and application of the polypeptide hydrogel. The polypeptide hydrogel provided by the invention can effectively slow down the release of the Exos and prolong the half-life of the Exos; and the released Exos have the activity similar to that of newly-separated Exos, and can effectively reduce HK2 cell injury caused by hypoxia and promote the recovery of vascular endothelial damage. The polypeptide hydrogel disclosed by the invention is capable of effectively reducing acute kidney injury and slowing down renal fibrosis by reducing kidney cell apoptosis and inflammatory factors, the expression amount of kidney injury marker protein-neutrophil gelatinase-related apolipoprotein (NGAL), and the synthesis and secretion of renal extracellular matrix (ECM), and has a good clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Vivo assay for anti angiogenic compounds
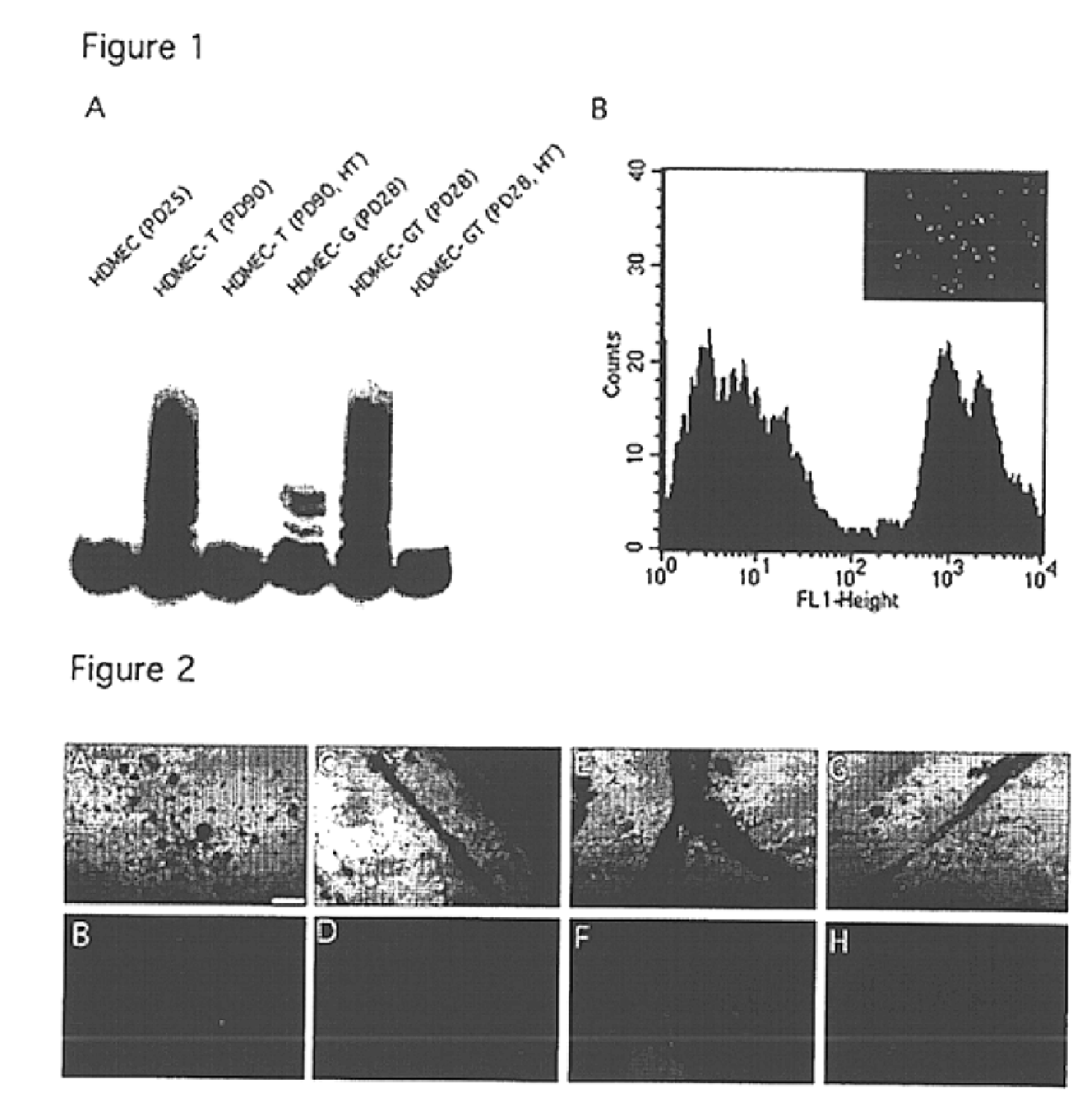
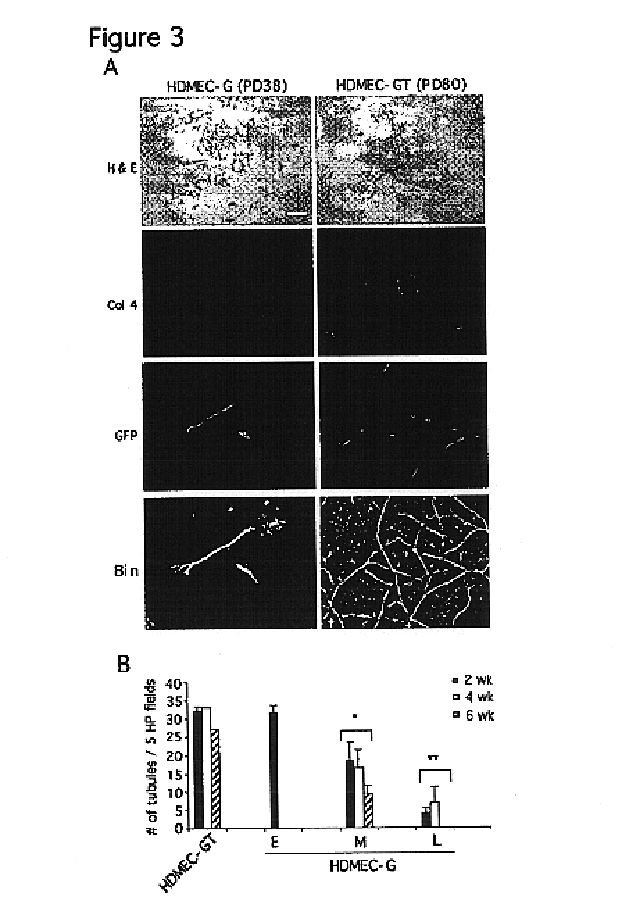
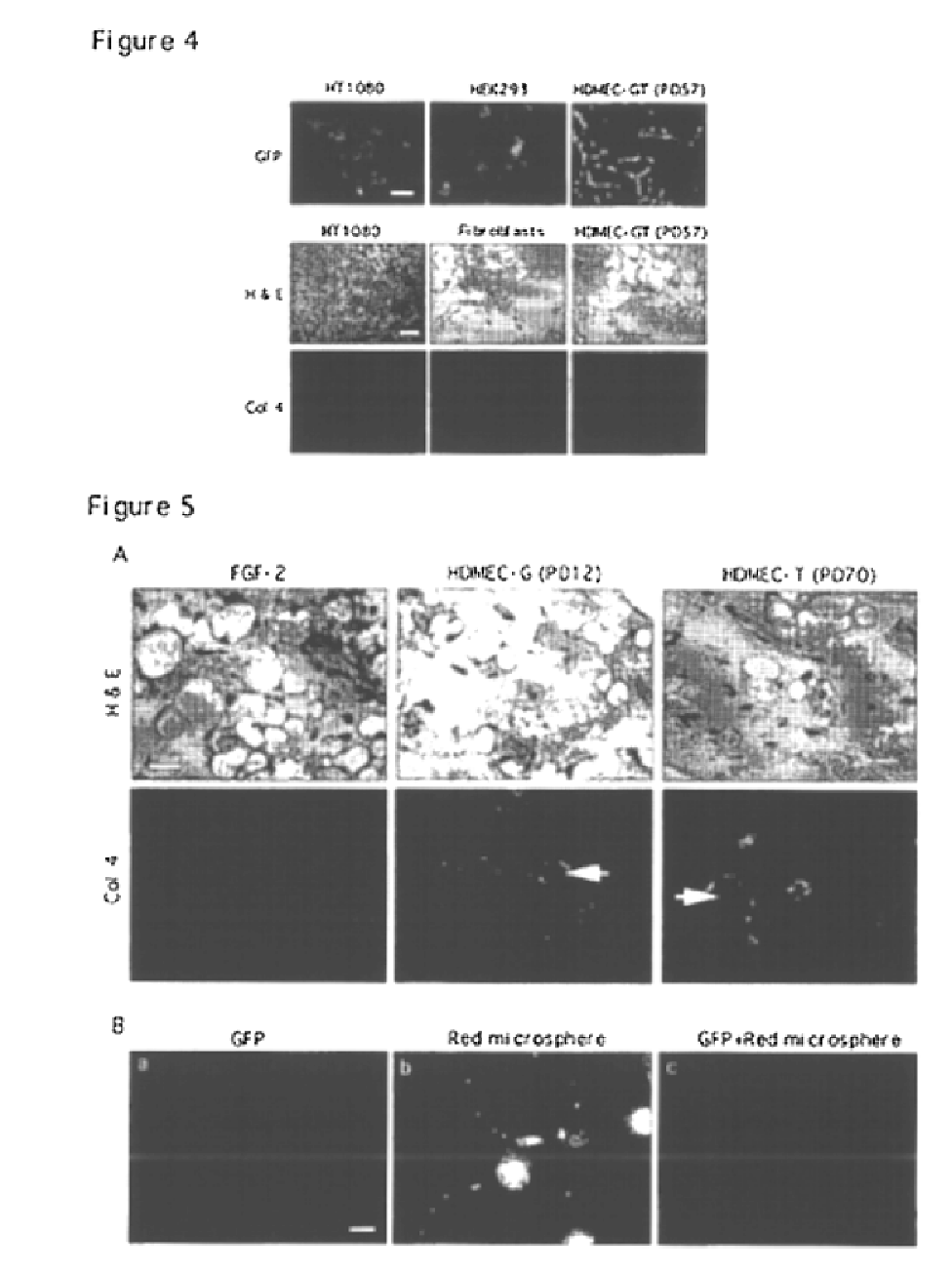
We report the use of telomerase-immortalized human microvascular endothelial cells in the formation of functional capillary blood vessels in vivo. Previously we showed the superior in vitro survival of human telomerase reverse transcriptase (hTERT)-transduced human endothelial cells. Here we show that retroviral-mediated transduction of hTERT in human dermal microvascular endothelial cells (HDMEC) results in cell lines that form microvascular structures when subcutaneously implanted in severe combined immunodeficiency (SCID) mice. The human origin of xenografted microvaculature was confirmed both by basement membrane immunoreactivity with anti-human type IV collagen staining and visualization of fluorescent vessels containing HDMEC that were co-transduced with hTERT and green fluorescent protein (eGFP). The lack of human vascular structures after implantation of HT1080 fibrosarcoma cells, 293 human embryonic kidney cells or human skin fibroblasts demonstrated the specificity of HDMEC at forming capillaries. Intravascular red fluorescent microspheres injected into the host circulation were found within green “telomerized” microvessels indicating functional murine-human vessel anastamoses. Whereas primary HDMEC-derived vessel density decreased steadily with time, telomerized HDMEC maintained durable vessels 6 weeks after xenografting. Modulation of implant vessel density by exposure to different angiogenic and angiostatic factors demonstrated the utility of this system for the study of human microvascular remodeling in vivo.
Owner:HERRON G SCOTT
Process for preparing subunit vaccine of reovirus antigen for grass carp
InactiveCN1616094AEasy to prepareEasy to operateViral antigen ingredientsAntiviralsInfected cellReovirus (antigen)
The preparation process of reovirus antigen subunit vaccine for grass carp includes the following steps: proliferation of GCRV873, which is the Hunan Shaoyang strain of reovirus, in grass carp kidney cell line; centrifugal purification and concentration of GCRV873 infected cell virus suspension; processing purified virus with surfactant to disintegrate complete virus structure to form viral nucleic acid and capsid protein subunit component; digesting with nuclease to degrade free virus genome dsRNA; dialysis to obtain reovirus protein subunit antigen preparation for grass carp containing no nuclease; and diluting with physiological saline to obtain the said vaccine. The vaccine of the present invention has the advantages of containing complete virus protein antigen component, containing no genetic virus matter RNA, simple preparation process, high product purity, etc.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com