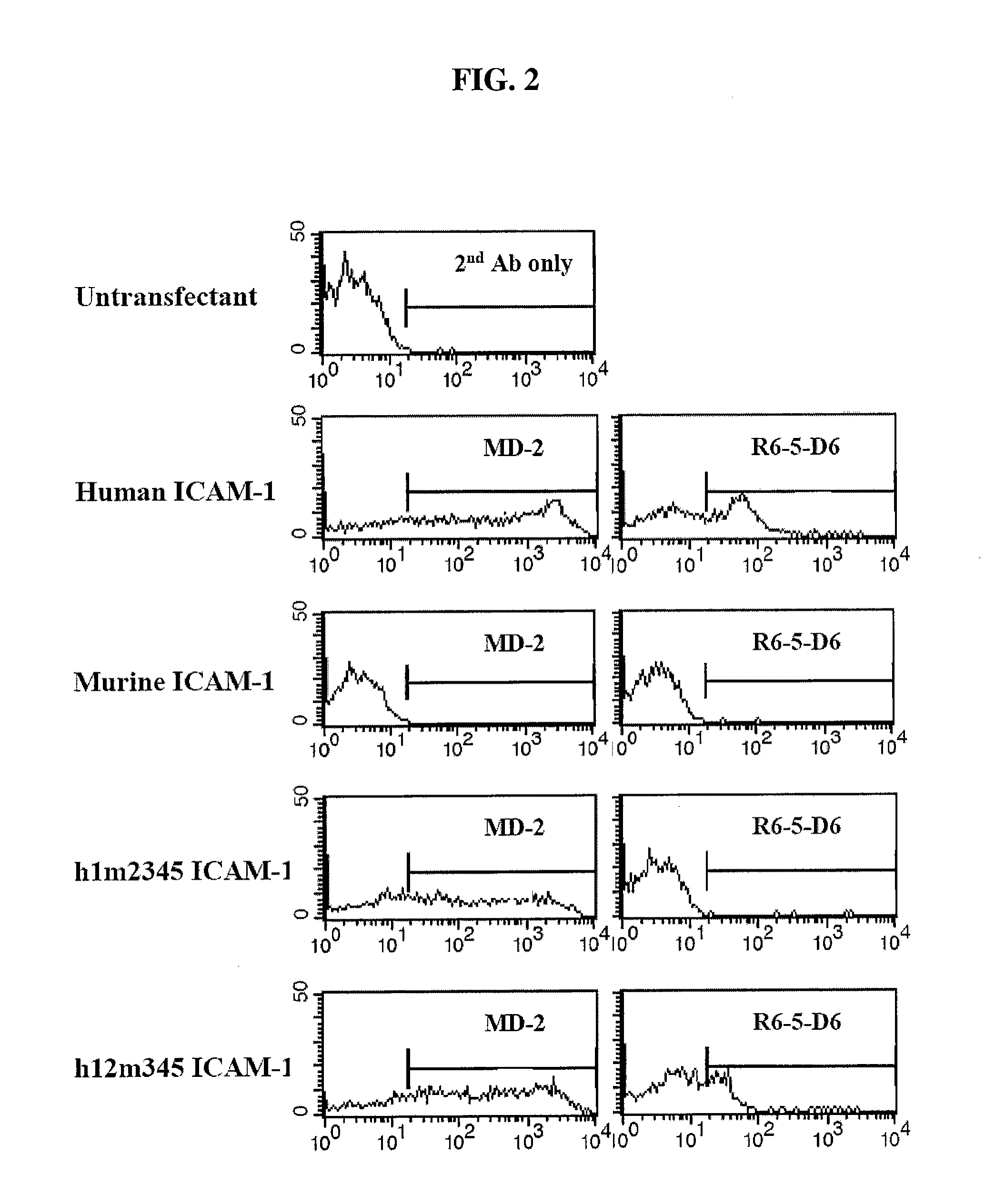
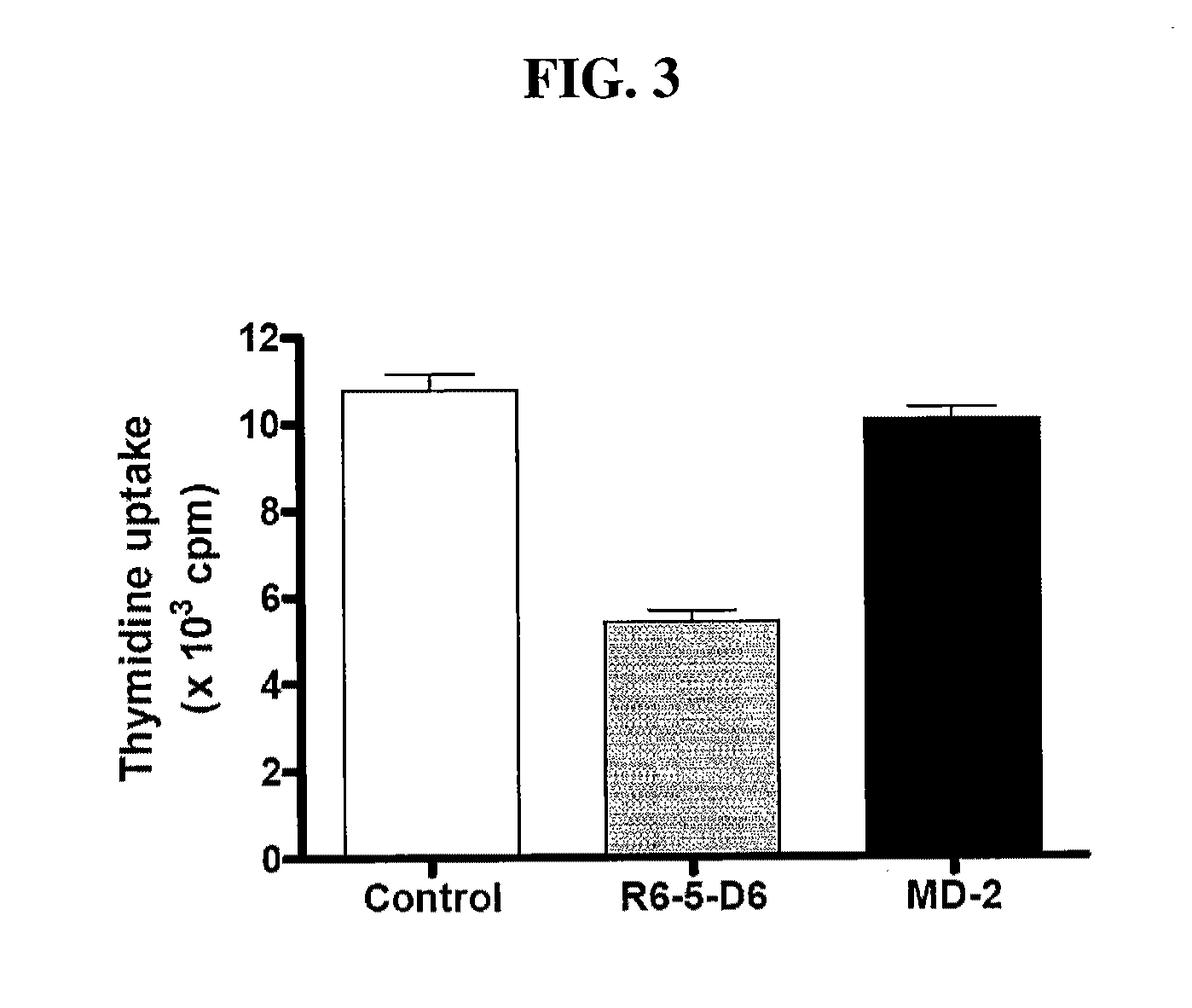
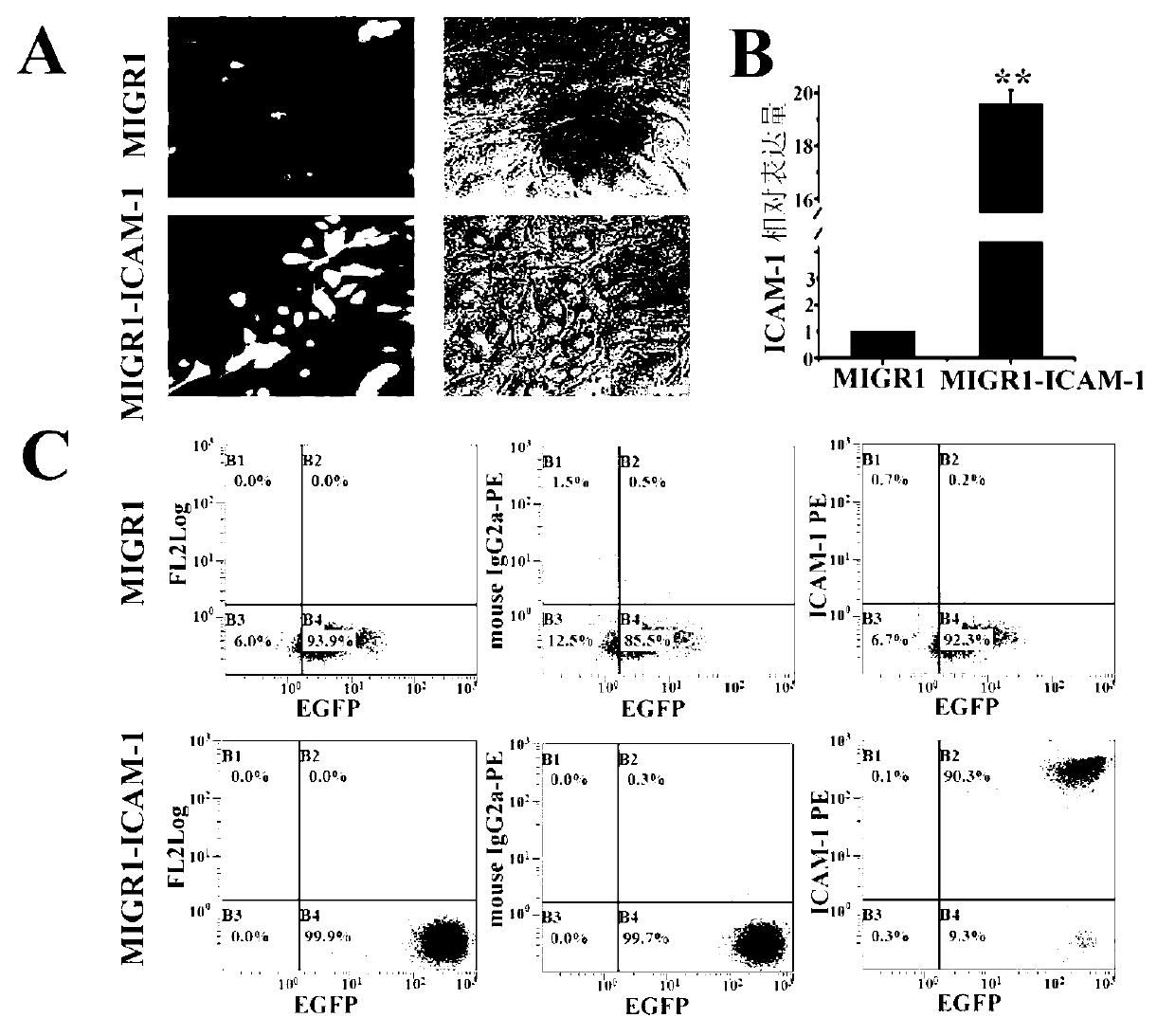
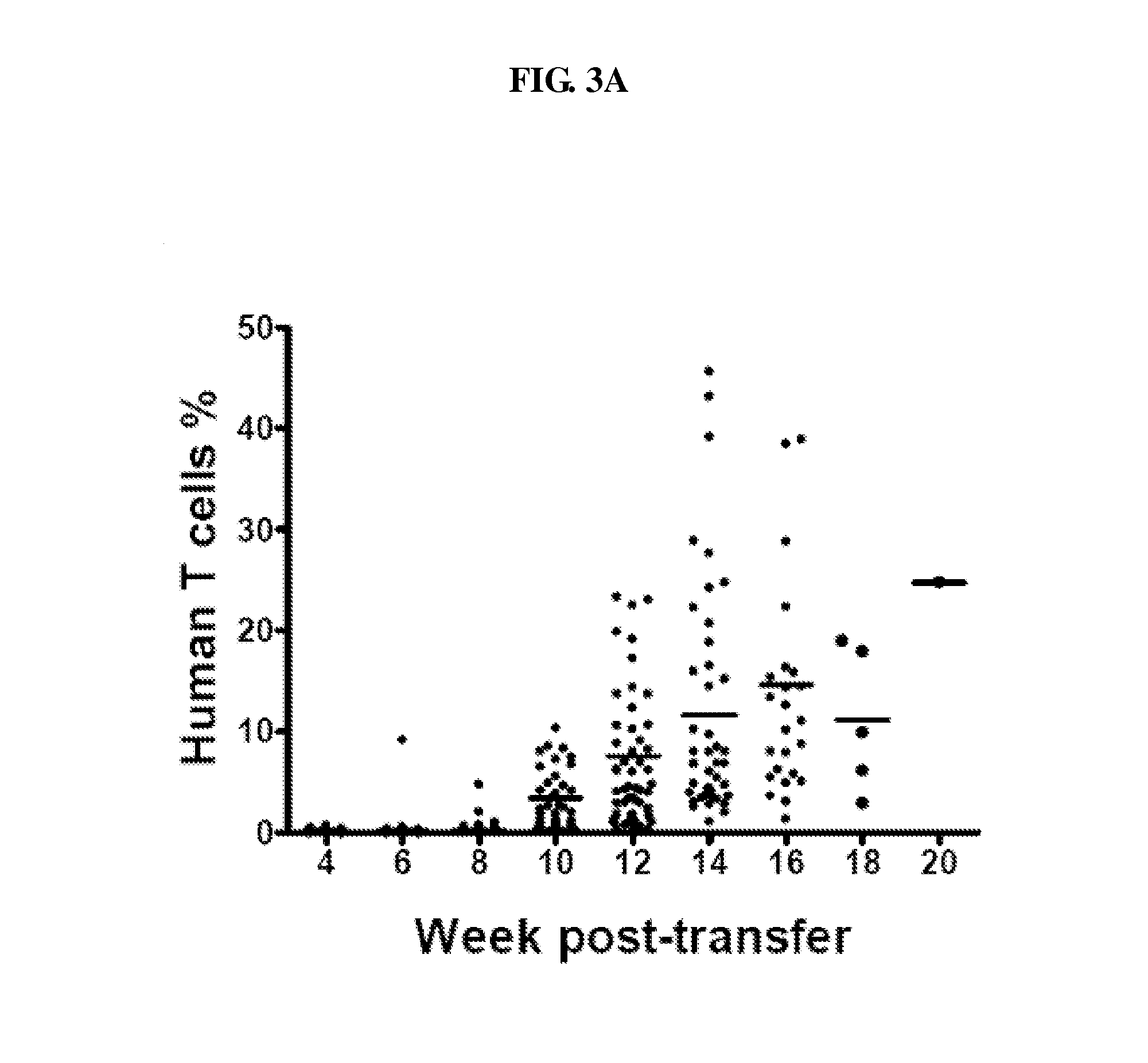
Patents
Literature
105 results about "ICAM-1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ICAM-1 (Intercellular Adhesion Molecule 1) also known as CD54 (Cluster of Differentiation 54) is a protein that in humans is encoded by the ICAM1 gene. This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by rhinovirus as a receptor for entry into respiratory epithelium.
Compositions for nasal drug delivery, methods of making same, and methods of removing residual solvent from pharmaceutical preparations
InactiveUS6391452B1Taller in heightSlow down the mixing speedLiquid surface applicatorsPeptide/protein ingredientsNasal cavityNose
The present invention relates to pharmaceutical compositions for delivery of drugs intended to reside in the nose, compositions for nasal administration of drugs, e.g., antiviral agents, and particularly antiviral agents comprising the human major rhinovirus receptor, also known as intercellular adhesion molecule-1 (ICAM-1); to methods of making said nasal drug compositions, and to an improved process for the removal of residual solvent from pharmaceutical matrices.
Owner:BAYER PHARMA CORP
Regulation of gene expression
InactiveUS6022863APrevention of fetal rejectionSugar derivativesGenetic material ingredientsMHC class IDisease
The present invention relates to utrons, RNA molecules which contain promoter regulatory motif(s) and DNA analogs thereof and DNA molecules that can be transcribed to produce the foregoing. In particular, the invention provides gene promoter suppressing nucleic acids which suppress transcription from a promoter of interest. In a preferred embodiment, the invention provides the TSU gene, nucleotide sequences of the TSU gene and RNA, as well as fragments, homologs and derivatives thereof. Methods of isolating TSU genes are also provided. Therapeutic and diagnostic methods and pharmaceutical compositions are also provided. In particular, the invention relates to methods for cell replacement therapy, gene therapy or organ transplantation wherein TSU nucleic acids suppress MHC class I and II gene expression, thus preventing immuno-rejection of non-autologous cells or organs. The invention also provides methods for treatment of diseases or disorders by suppression of MHC class I, MHC class II, ICAM-1, B7-1, B7-2, and / or Fc gamma R expression by provision of TSU function.
Owner:YALE UNIV
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
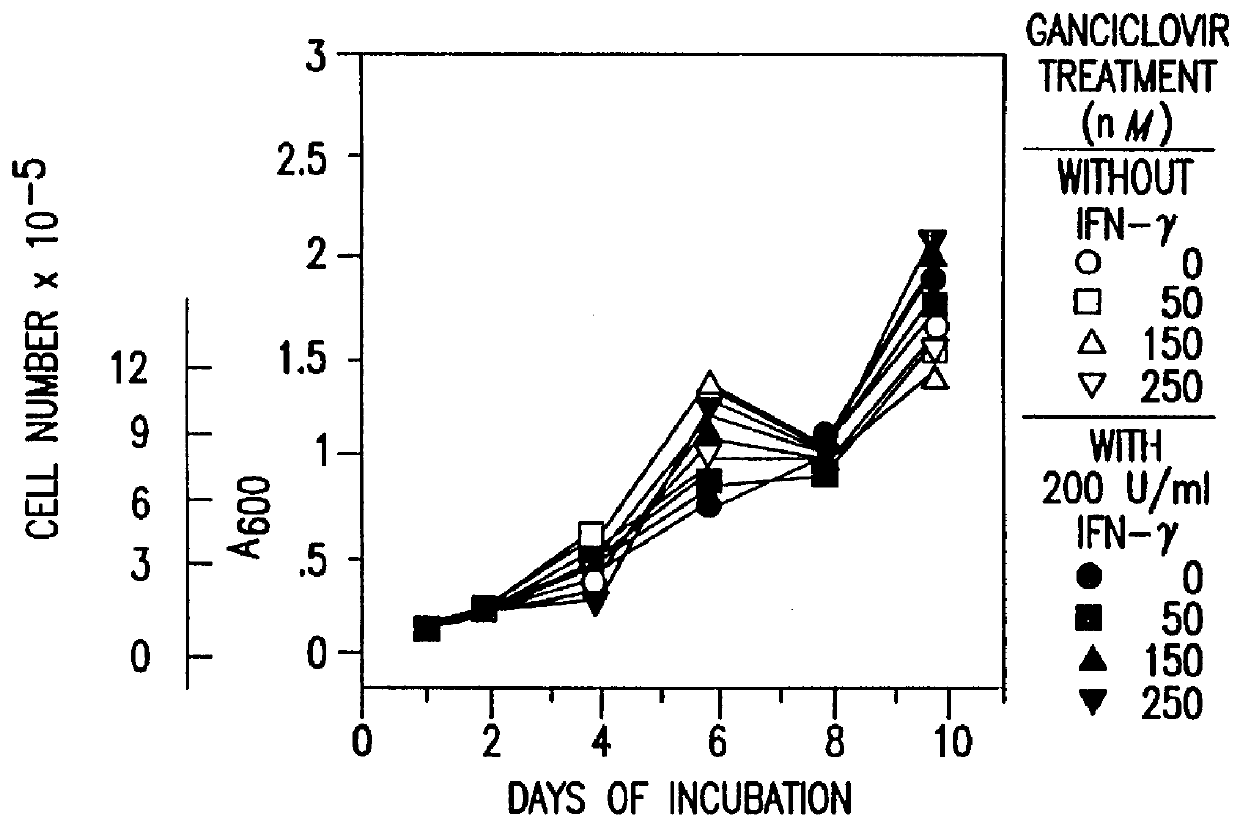
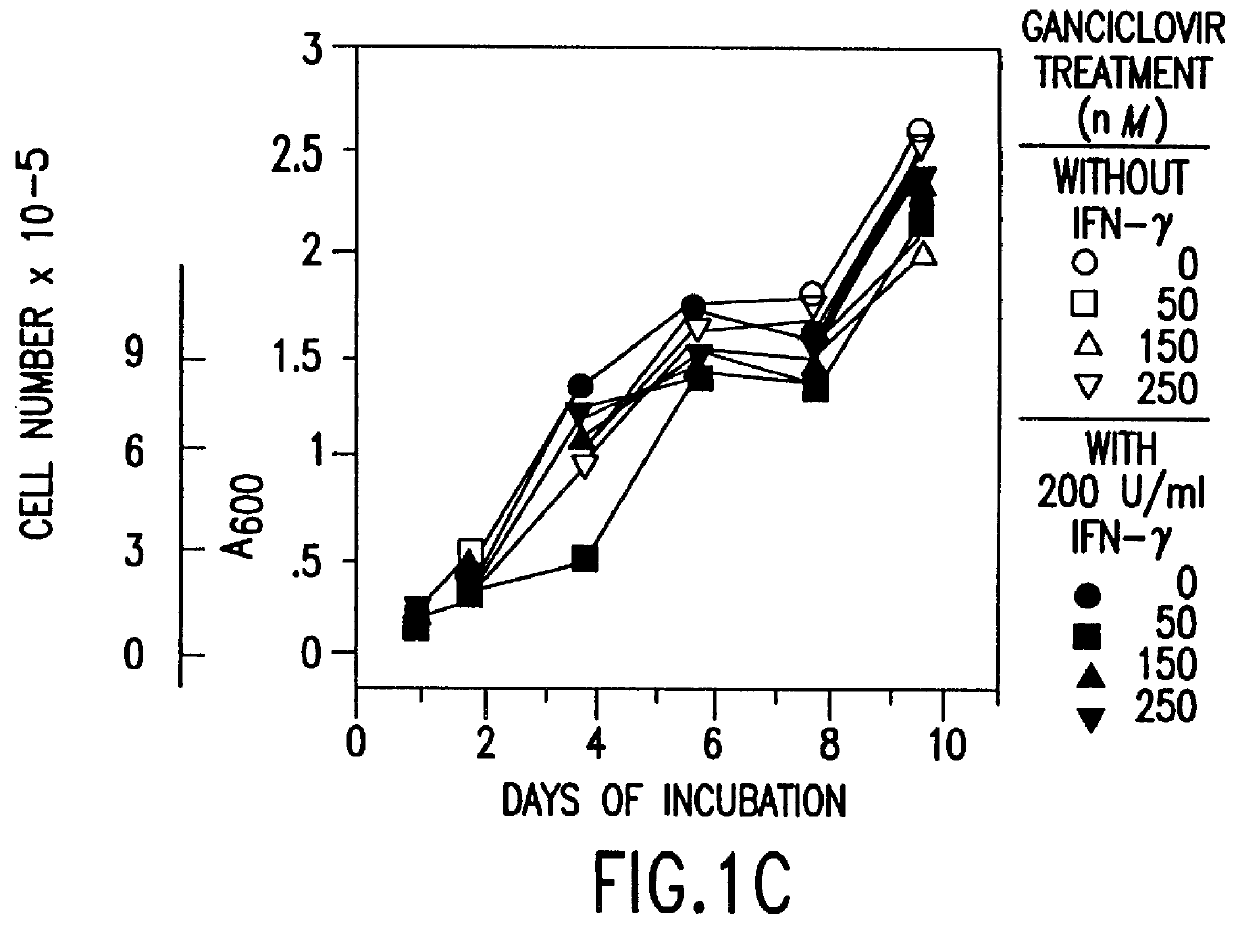
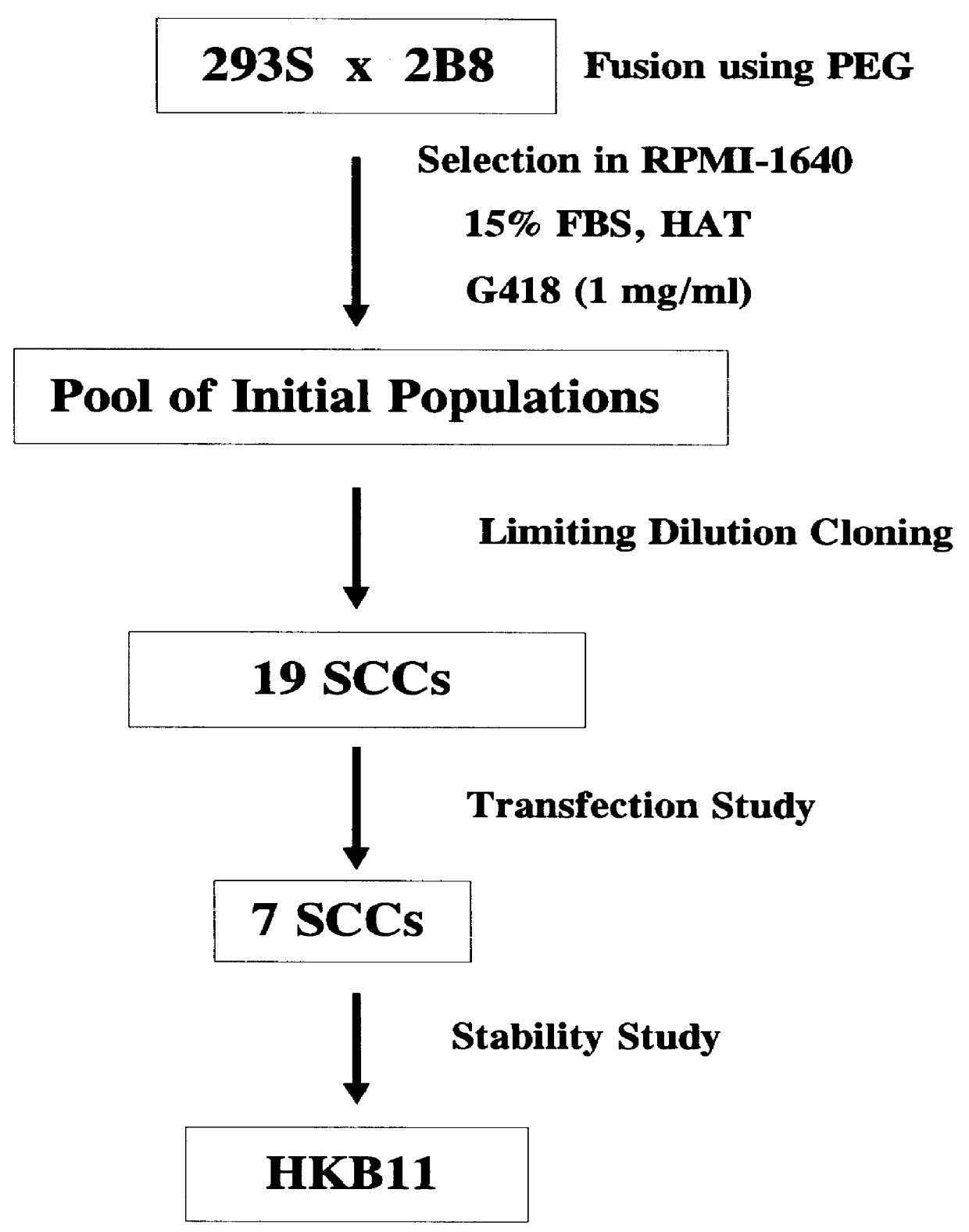
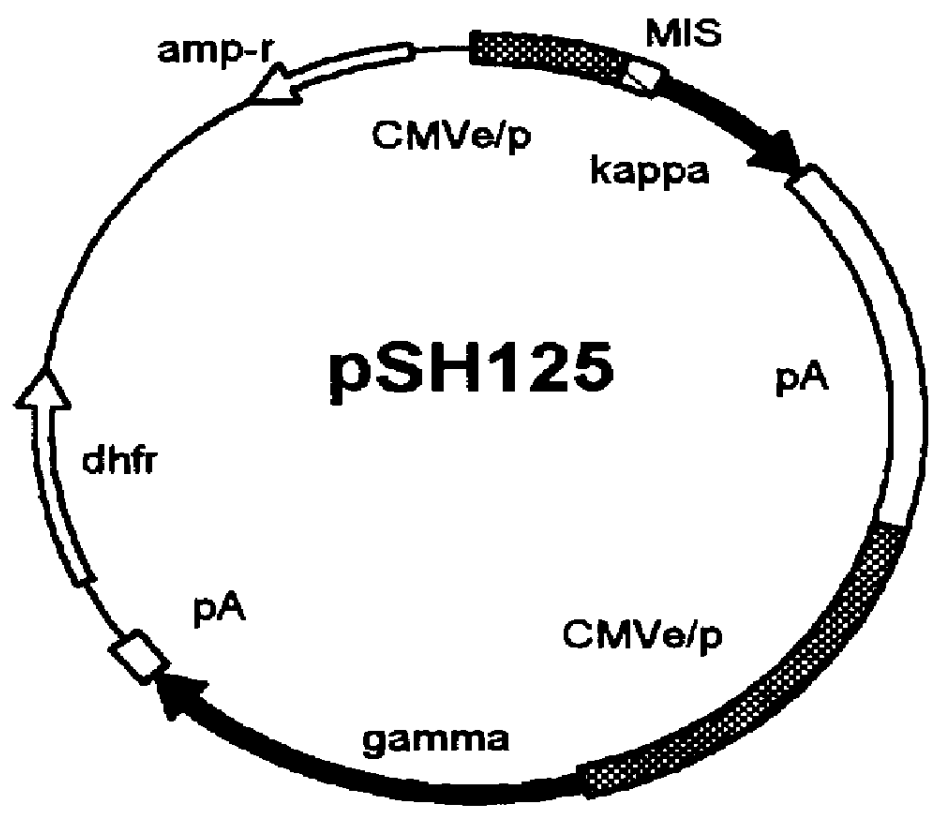
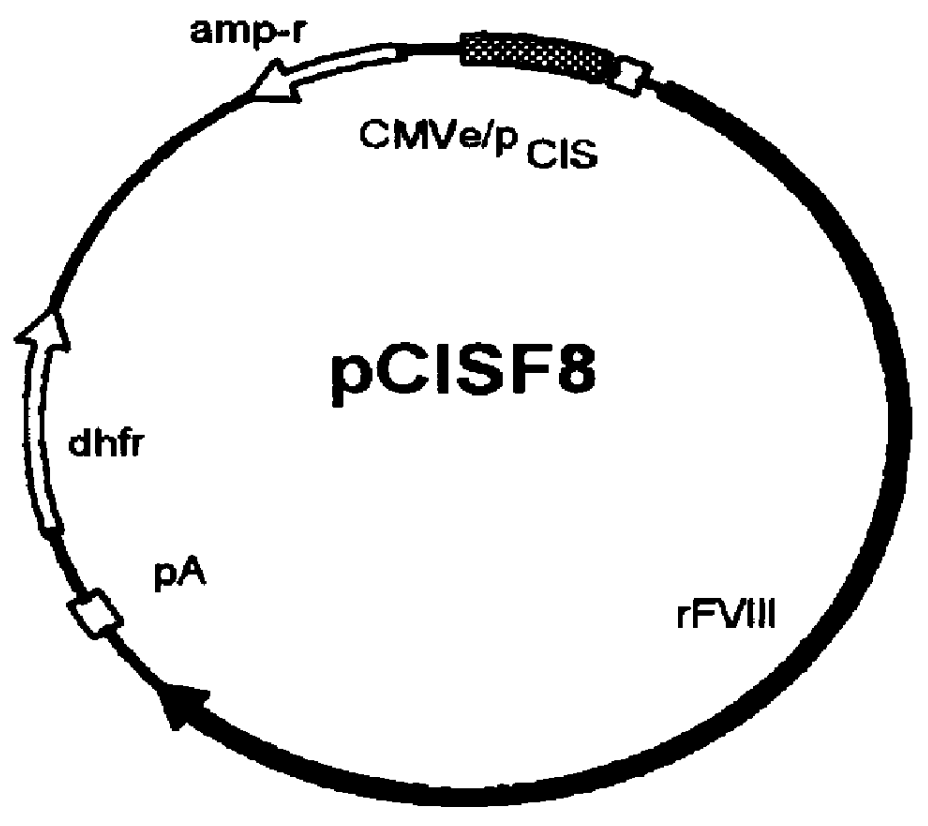
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
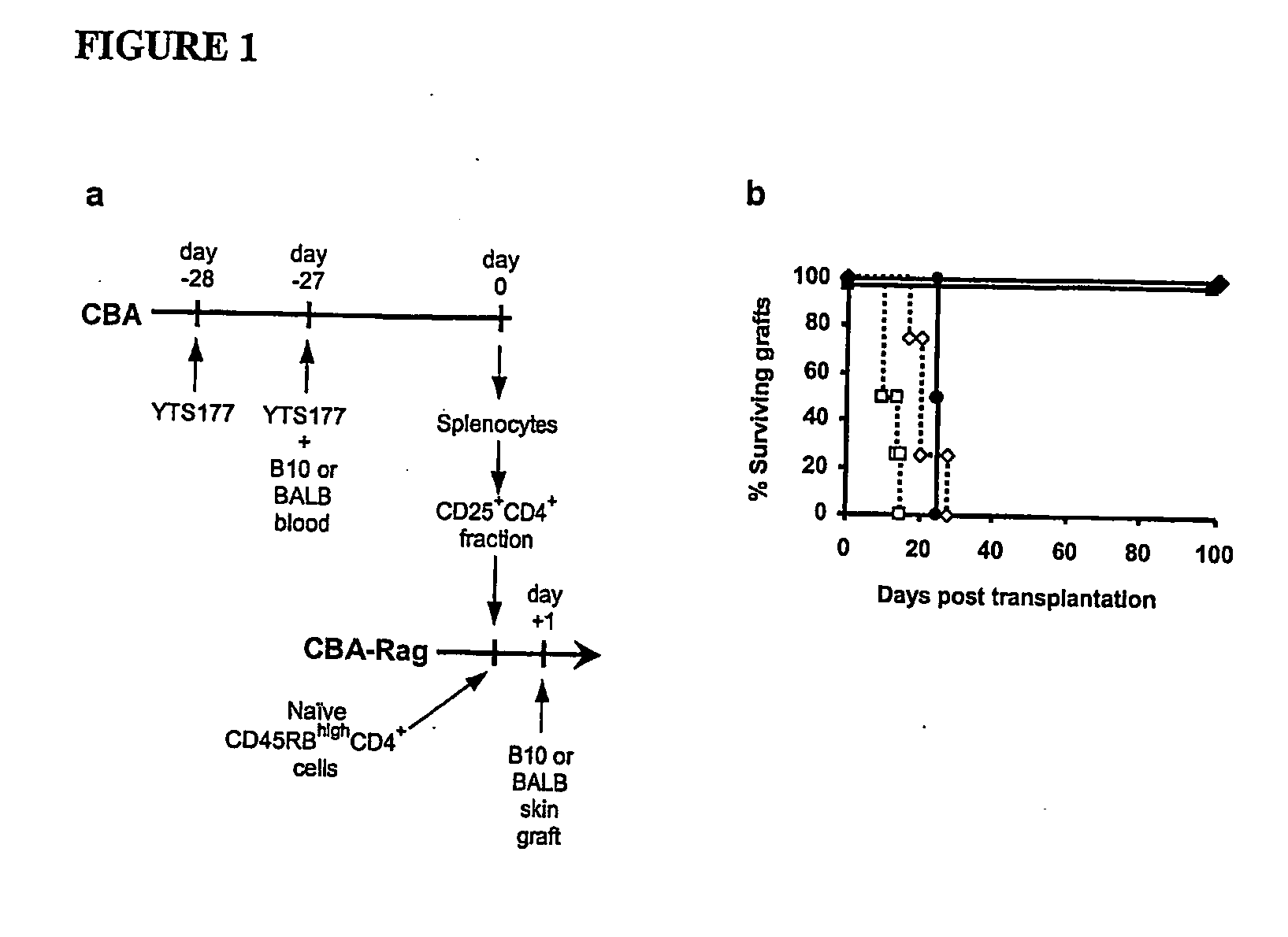
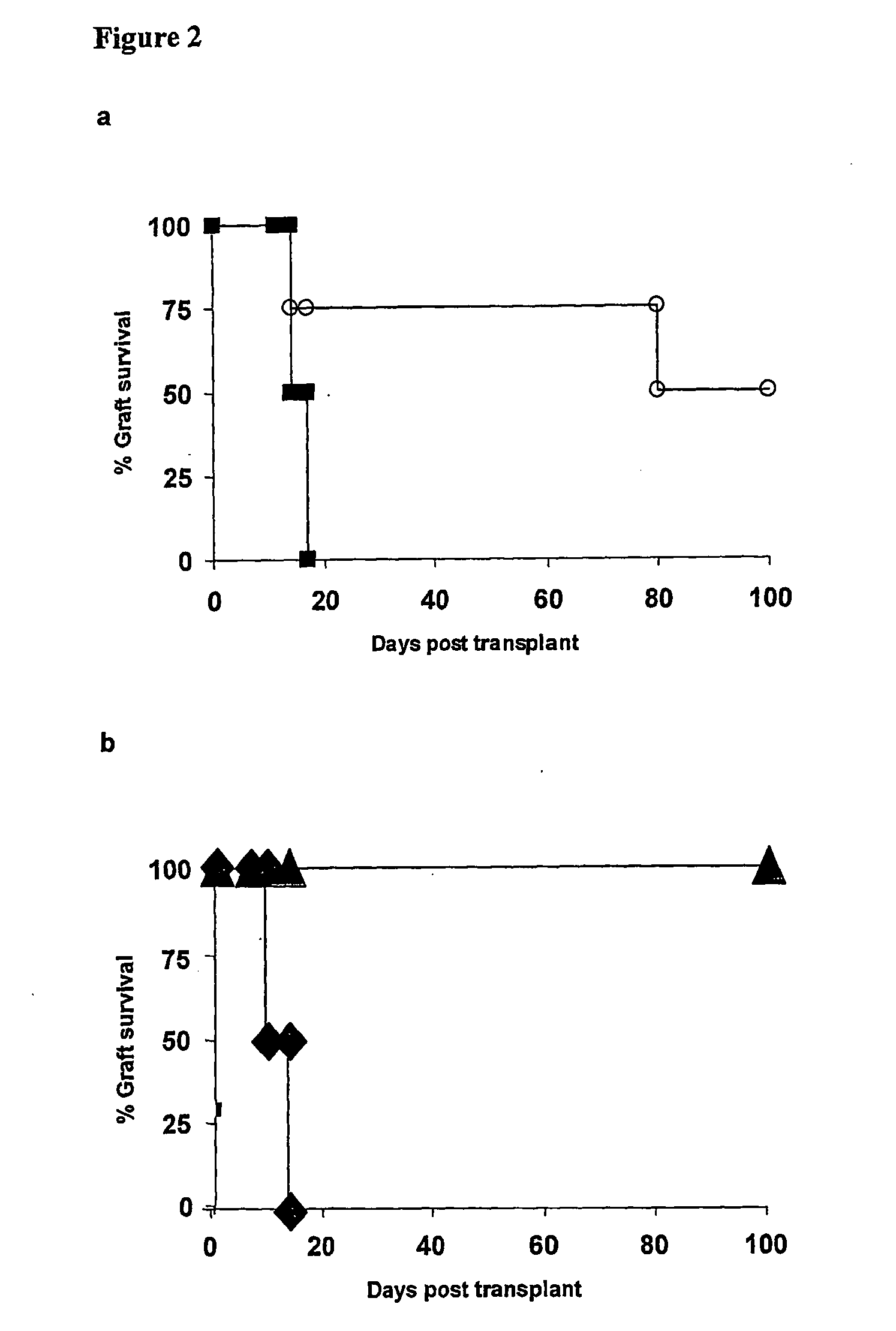
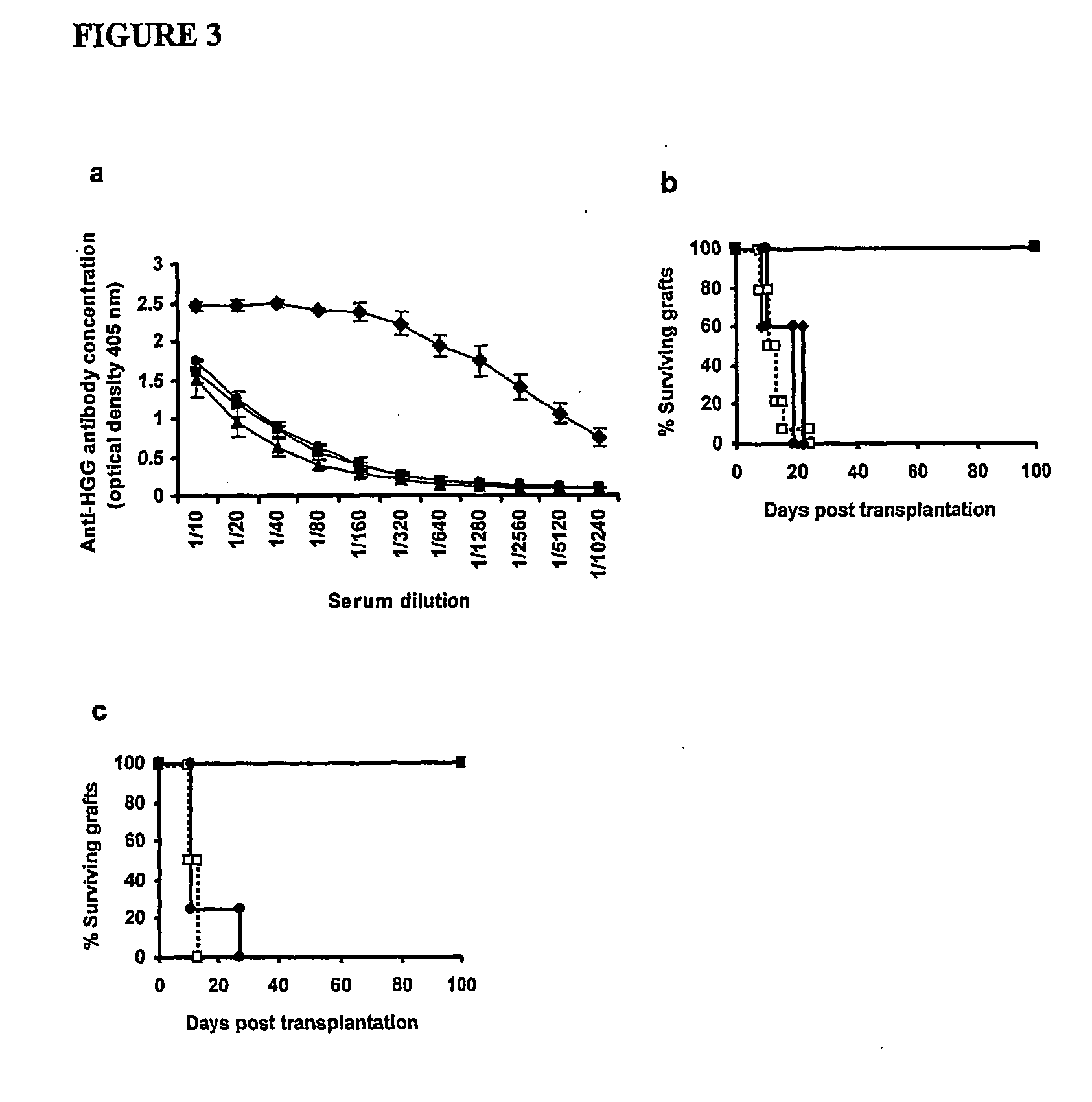
Suppression of transplant rejection
InactiveUS20070166307A1Vertebrate cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionRegulatory T cell
The present invention relates to a transplant rejection in an animal suppressed by administration of an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, preferably an anti-CD4 antibody, together with a non-cellular protein antigen to generate in the animal a population of regulatory T-lymphocytes; reactivating said population of regulatory T-lymphocytes by further administration to the animal of the non-cellular protein antigen; and transplanting said organ or tissue whilst said population of regulatory T-lymphocytes is activated. Regulatory T cells can be generated ex vivo by culturing T cells with an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, in the presence of cells that present either alloantigen or a non-cellular protein antigen. Ex vivo generated T-lymphocytes can be used as an alternative method of overcoming transplant rejection or in combination with the in vivo method. A similar approach can be adopted for the treatment of autoimmune conditions.
Owner:ISIS INNOVATION LTD
Methods to identify polynucleotide and polypeptide sequences which may be associated with physiological and medical conditions
InactiveUS20090304653A1Extend your lifePositive evolutionarily significant changeBiocideGenetic material ingredientsNucleotideTherapy related
Disclosed are methods to identify an agent which may modulate resistance to HIV-1-mediated disease, comprising contacting at least one agent to be tested with a cell comprising human ICAM-1, and detecting the cell's resistance to HIV-1 viral replication, propagation, or function, wherein an agent is identified by its ability to increase the cell's resistance to HIV-1 viral replication, propagation, or function. Also disclosed are human mutant ICAM-1 polypeptides and methods to treat HIV-1 viral replication, propagation, or function in a human subject by ICAM-1 gene therapy relating to one or more of the following 10 mutations to human ICAM-1: L18Q, K29D, P45G, R49W, E171Q, wherein the mutant ICAM-1 is otherwise identical to human ICAM-1.
Owner:EVOLUTIONARY GENOMICS LLC
Methods and kits for the diagnosis of acute coronary syndrome
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
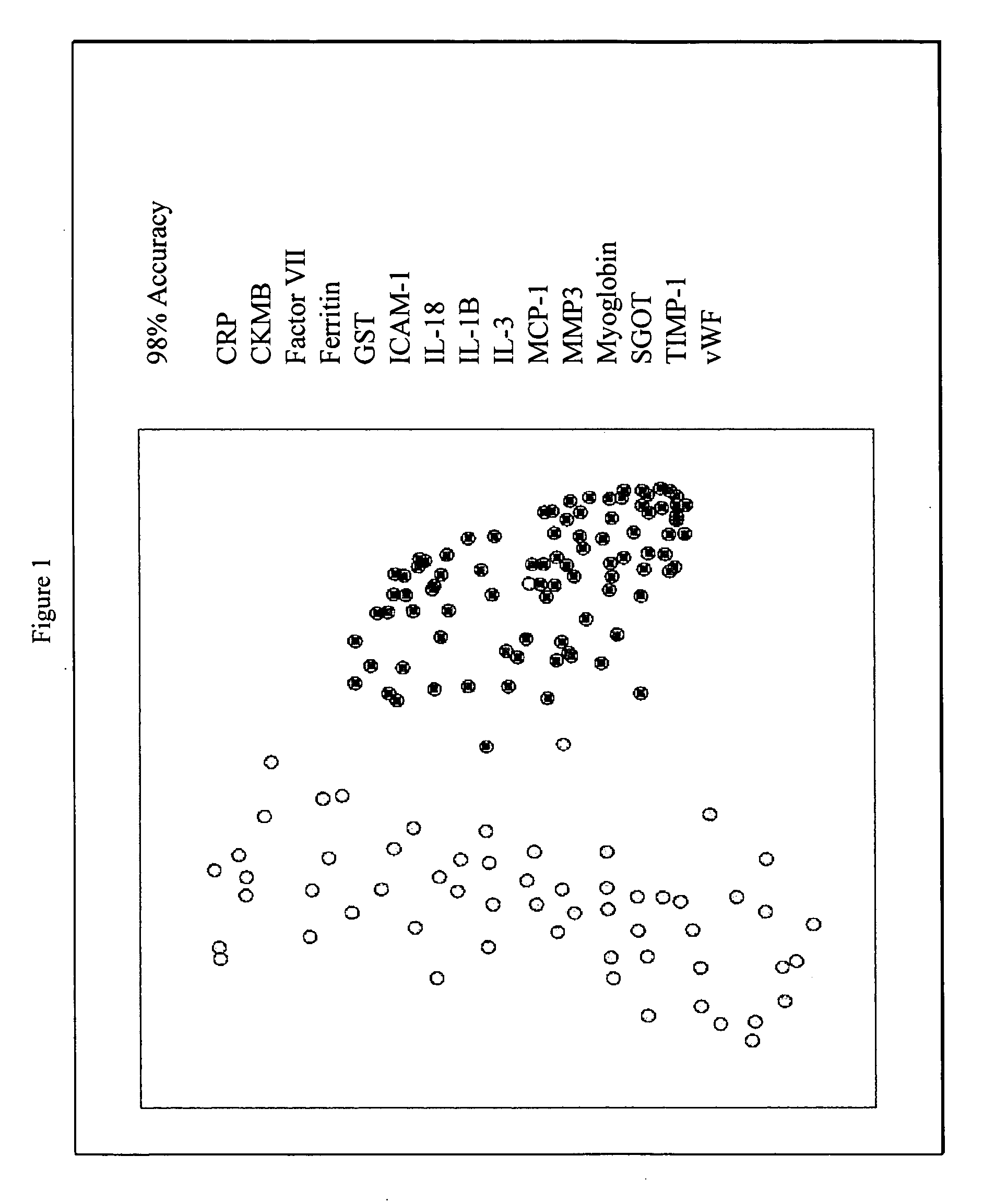
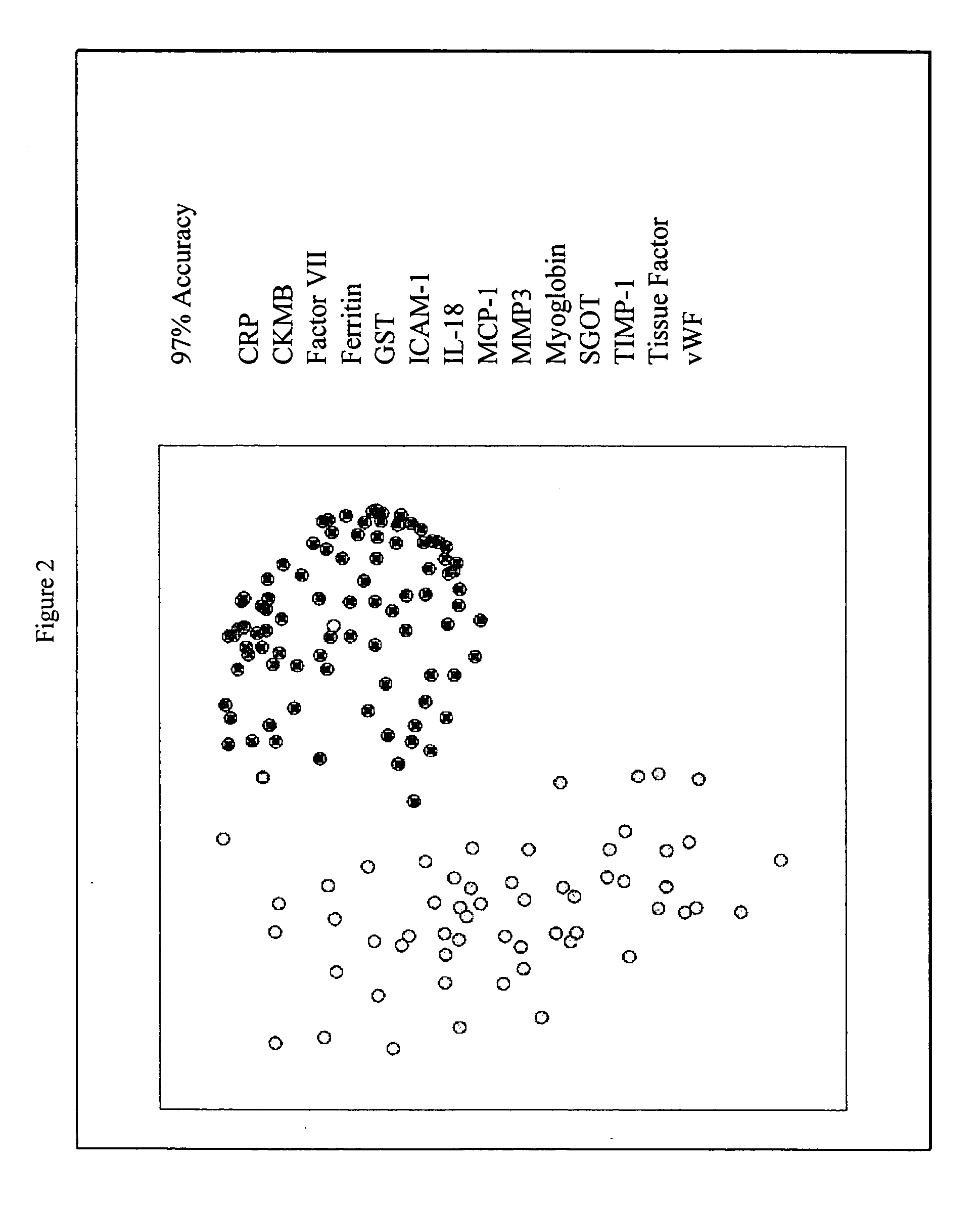
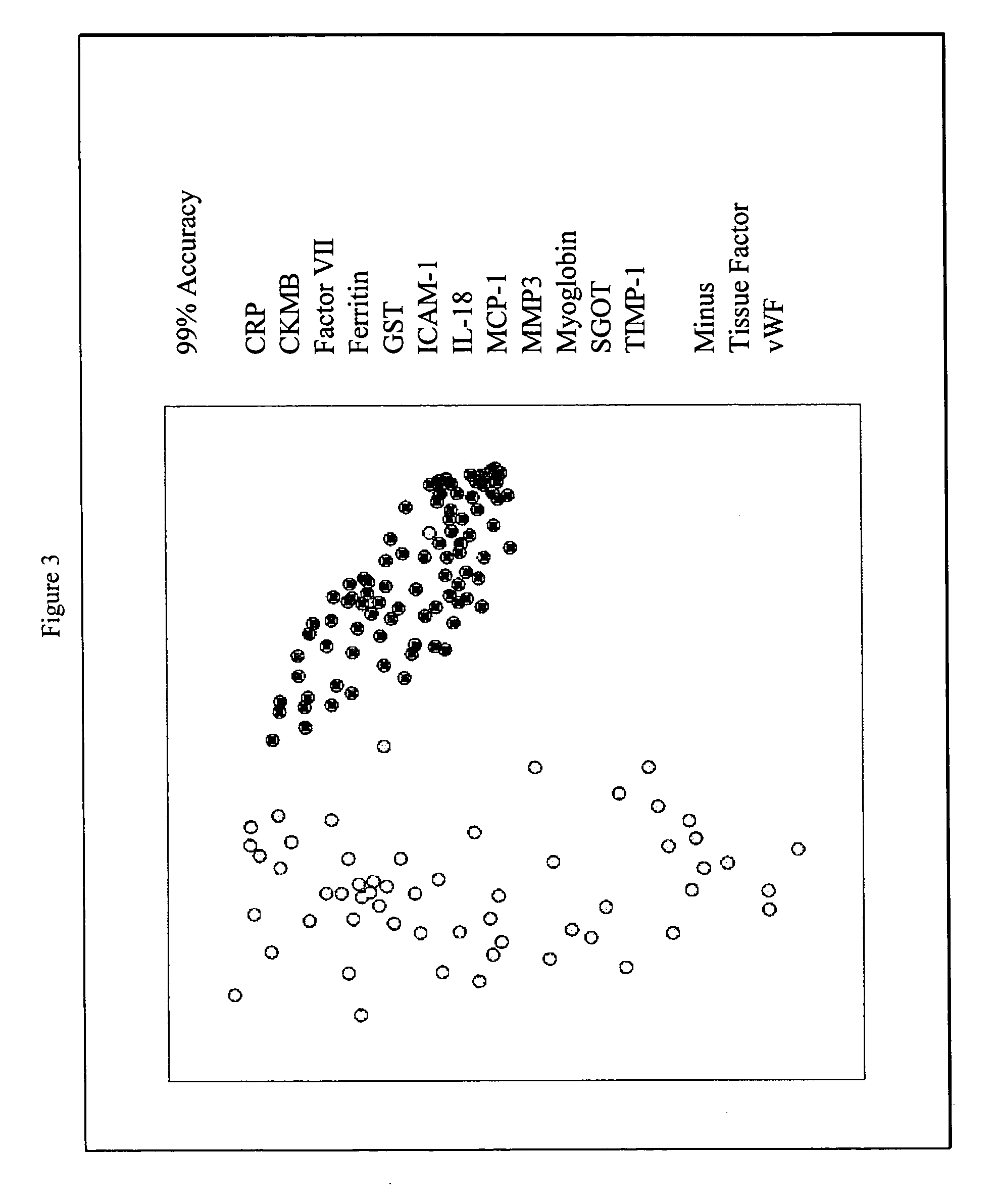
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
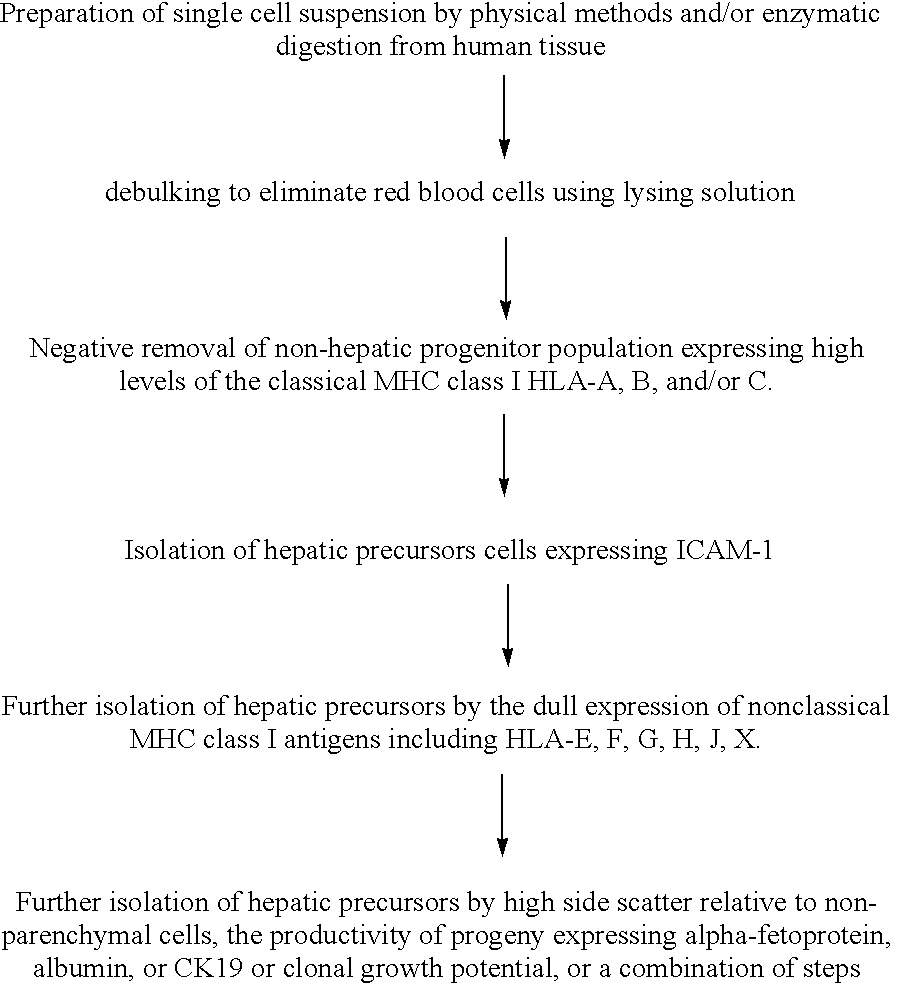
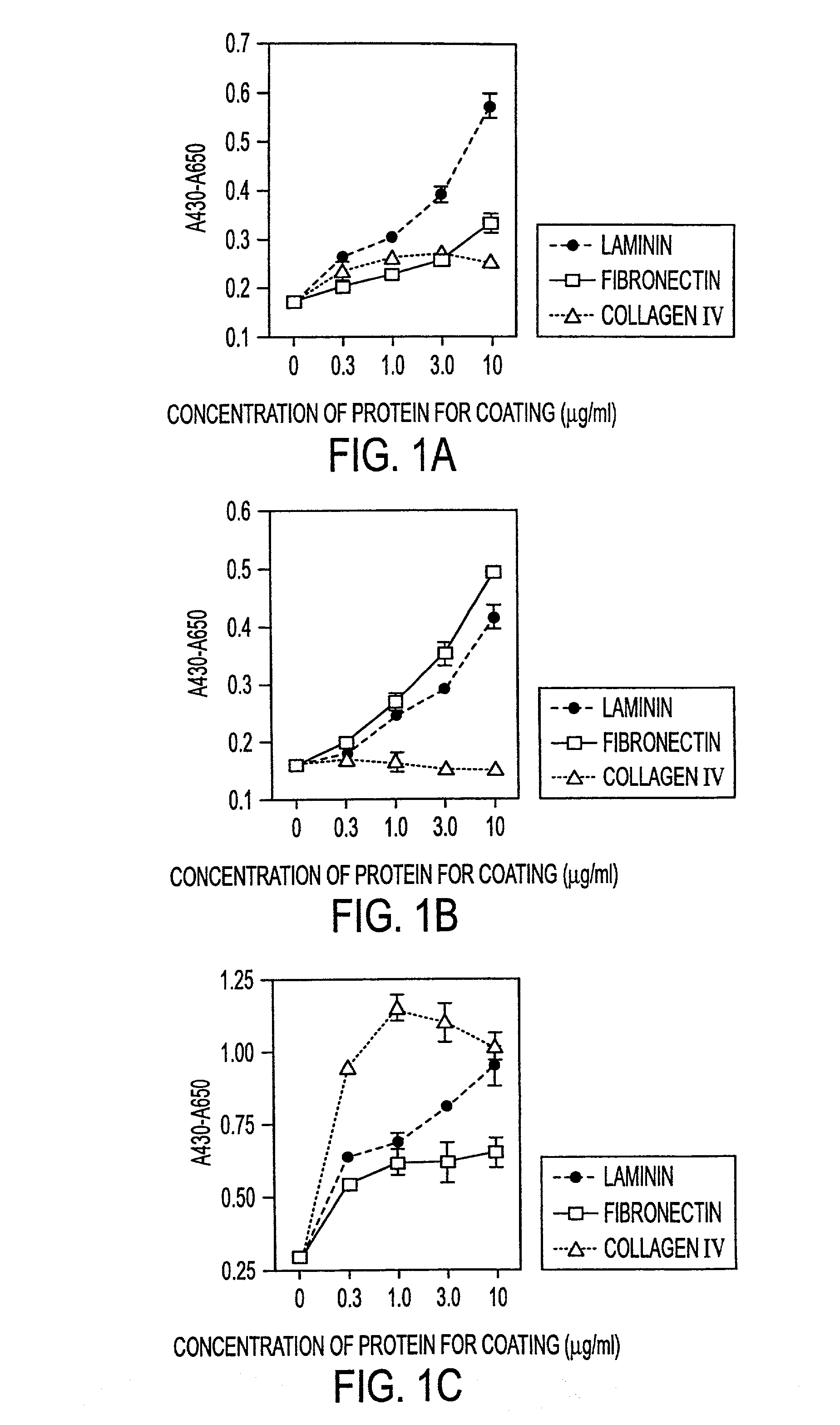
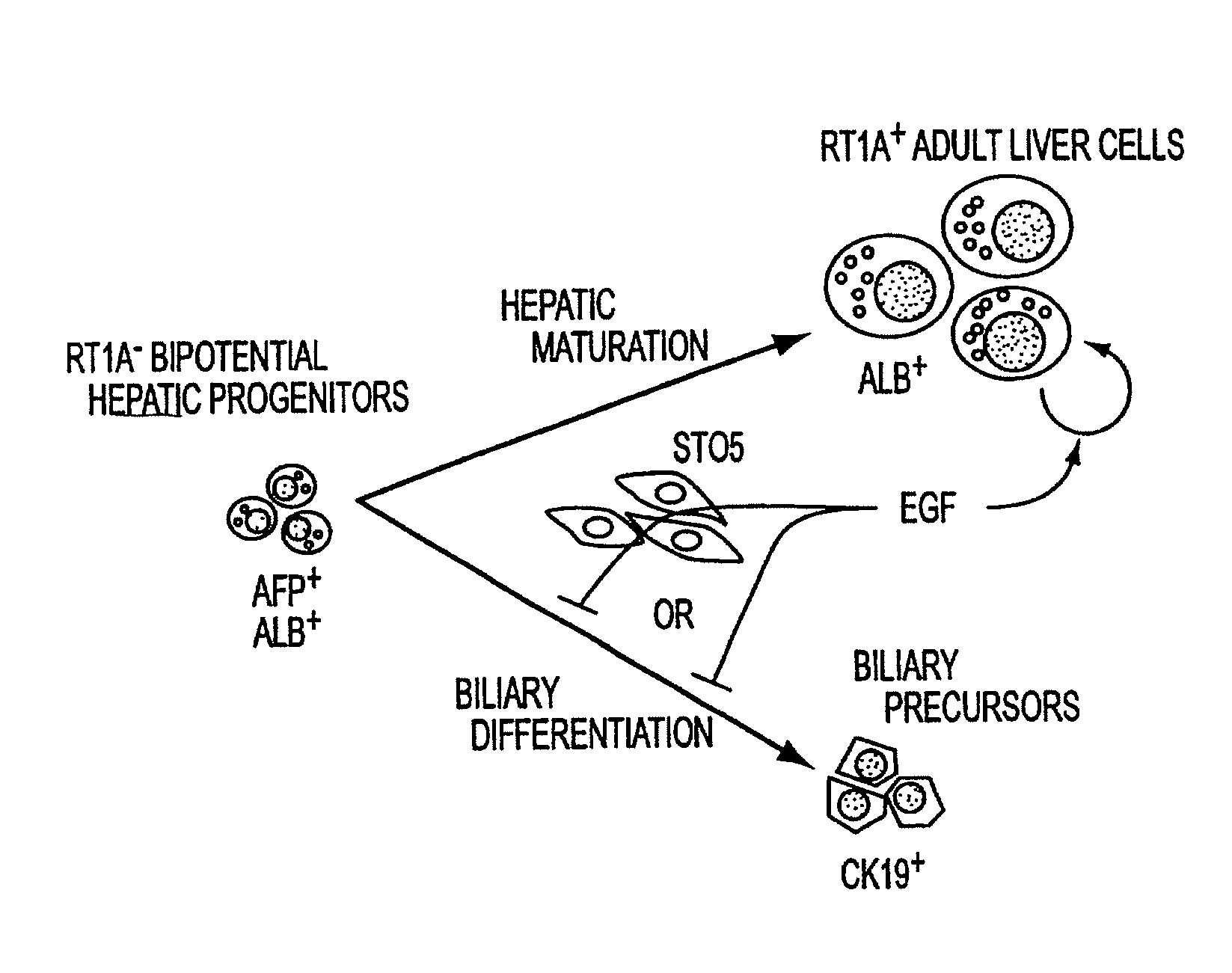
Methods of isolating bipotent hepatic progenitor cells
A method of obtaining a mixture of cells enriched in hepatic progenitors is developed which comprises methods yielding suspensions of a mixture of cell types, and selecting those cells that are classical MHC class I antigen(s) negative and ICAM-1 antigen positive. The weak or dull expression of nonclassical MHC class I antigen(s) can be used for further enrichment of hepatic progenitors. Furthermore, the progenitors can be selected to have a level of side scatter, a measure of granularity or cytoplasmic droplets, that is higher than that in non-parenchymal cells, such as hemopoietic cells, and lower than that in mature parenchymal cells, such as hepatocytes. Furthermore, the progeny of the isolated progenitors can express alpha-fetoprotein and / or albumin and / or CK19. The hepatic progenitors, so isolated, can grow clonally, that is an entire population of progeny can be derived from one cell. The clones of progenitors have a growth pattern in culture of piled-up aggregates or clusters. These methods of isolating the hepatic progenitors are applicable to any vertebrates including human. The hepatic progenitor cell population is expected to be useful for cell therapies, for bioartificial livers, for gene therapies, for vaccine development, and for myriad toxicological, pharmacological, and pharmaceutical programs and investigations.
Owner:KUBOTA HIROSHI +1
Herbal composition for treating various disorders including psoriasis, a process for preparation thereof and method for treatment of such disorders
InactiveUS20030194456A1Safe and well-toleratedMinimal effectBiocideAntipyreticPhosphodiesteraseEnzyme inhibition
The invention provides a novel herbal composition containing the extracts of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant, optionally containing the extracts of the fruits of <italic>Cuminum cyminum< / highlight>, which exhibits useful in vitro, in vivo and interesting immunological and pharmacological activities; a process for preparation thereof; and a method of treatment of psoriasis and related immunological and biological disorders by administration of the said novel herbal composition. The useful in vitro, in vivo and interesting immunological and pharmacological activities exhibited by the extracts and fractions of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant include immunosuppression, lymphoproliferation inhibition, cytokine modulation such as IL-2 inhibition, IFNgamma inhibition, IL-10 induction, keratinocyte proliferation inhibition, keratolytic activity, endothelial cell proliferation inhibition, inhibition of cell adhesion molecule expression such as ICAM-1, MEST inhibition, and enzymes inhibition such as p60src Tyrosine kinase, which are known to be involved in anti-psoriatic activity. The novel herbal composition(s) is useful in the treatment of various disorders, such as psoriasis including plaque psoriasis, gutatte psoriasis, pustular psoriasis and psoriasis of the nails; dermatitis and scleroderma; eczema; inflammatory disorders and other autoimmune diseases like psoriatic arthritis, rheumatoid arthritis, Crohn's disease, multiple sclerosis, irritable bowel disease, ankylosing spondilitis, systemic lupus erythremetosus and Sjogren's syndrome; allergies like asthma and chronic obstructive pulmonary disease and is safe, well-tolerated, non-toxic, with minimal and reversible adverse reactions or side effects, and most importantly, with minimal relapse or recurrence of the disease following completion of a treatment regimen. The invention also describes the presence of phosphodiesterase (III, IV and V) inhibition and 5-Lipoxygenase inhibition in the aqueous, ethanolic or aqueous-ethanolic extracts of fruits of <italic>Cuminum cyminum < / highlight>plant.
Owner:LUPIN LTD
Premature rupture of membrane fast examination tool using ICAM-1 as examination index and examination kit
The invention discloses a premature rupture of membrane (PROM) fast examination tool using an intercellular adhesion molecular (ICAM)-1 as an examination index. The tool comprises a gasket, an absorbent pad, a nitrocellulose membrane, a conjugate pad and a sample pad, wherein the absorbent pad, the nitrocellulose membrane, the conjugate pad and the sample pad are connected from top to bottom and adhered to the gasket; the conjugate pad is covered by the sample pad part; the nitrocellulose membrane is provided with a goat-anti-human ICAM-1 polyclonal antibody coated examination line and a goat-anti-mouse immunoglobulin G polyclonal antibody coated control line; the examination line is below and away from the control line at intervals; and the conjugate pad is a water absorbing fibber coated with conjugated mouse-anti-human ICAM-1 monoclonal antibody conjugate. A PROM fast examination kit using the ICAM-1 as the examination index consists of a kit body and the examination tool arranged in the kit body.
Owner:ORIGISSAY BIOLOGICS TECH
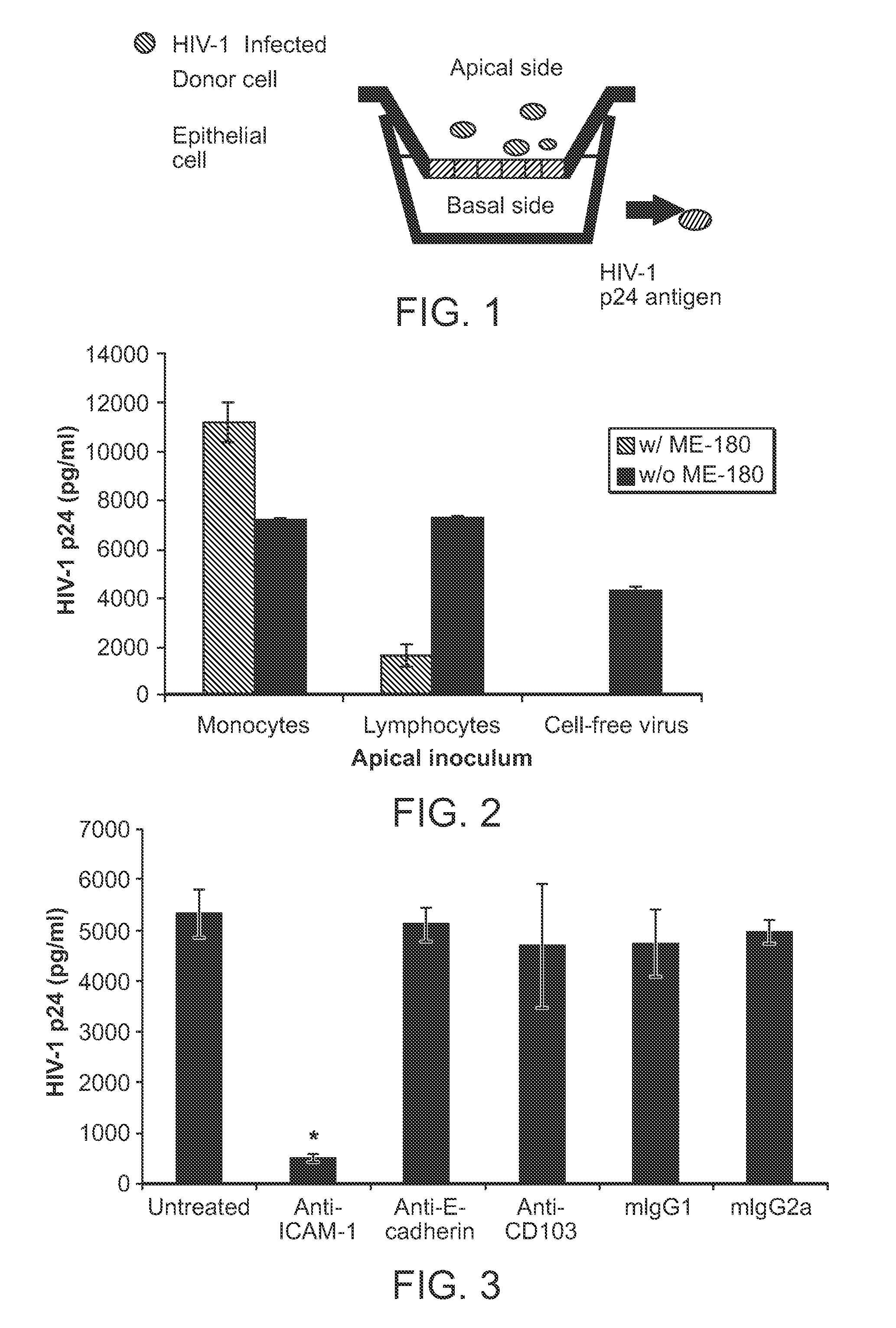
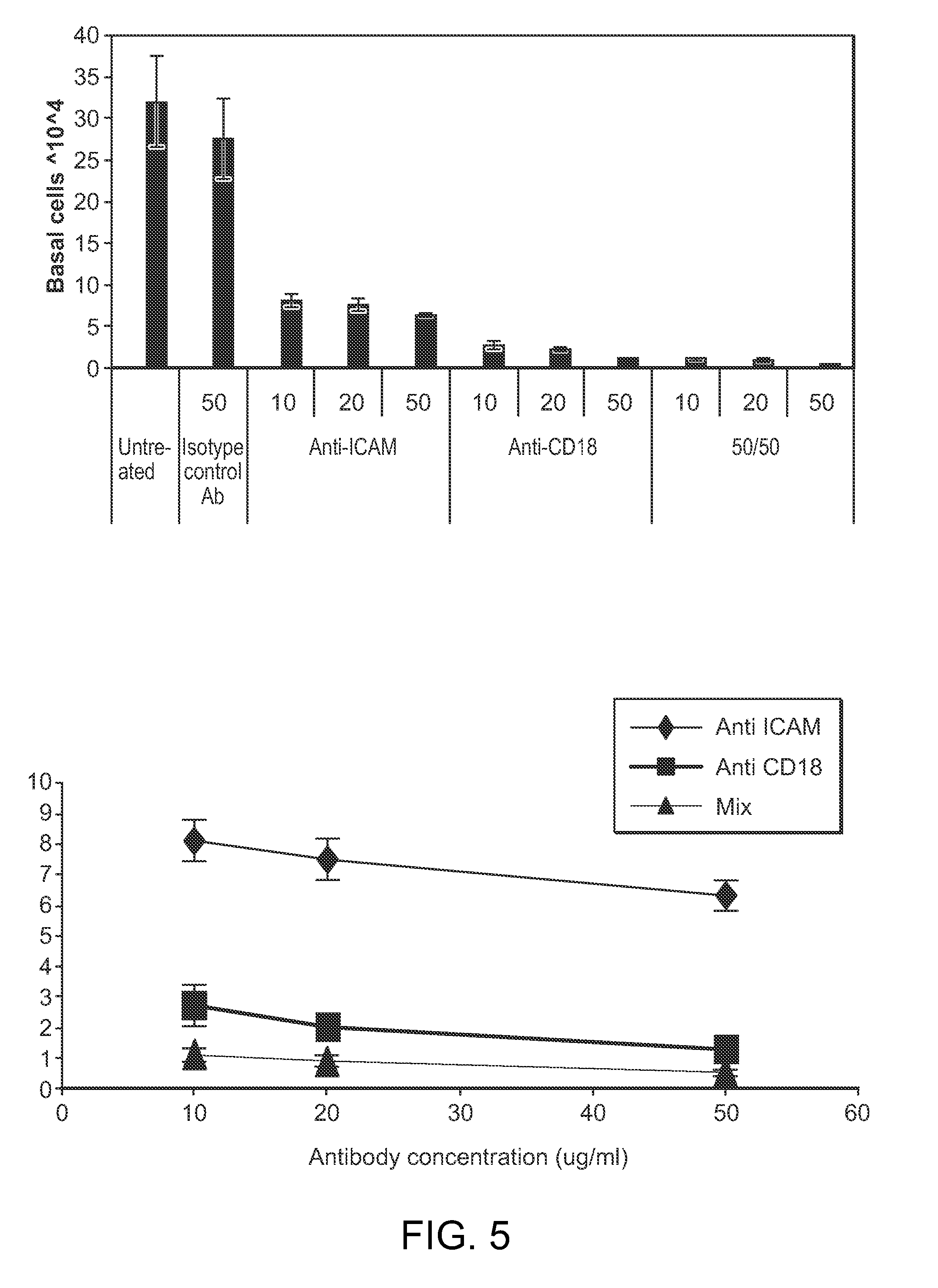
Use of camelid-derived variable heavy chain variable regions (VHH) targeting human cd18 and icam-1 as a microbicide to prevent hiv-1 transmission
InactiveUS20130164307A1Reduce and prevents transmissionReduces and prevents sexual transmissionBiocideBacteriaHeavy chainViral infection
The invention provides methods, compositions, and kits featuring a camelid-derived antibody for use in preventing or inhibiting a viral infection.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
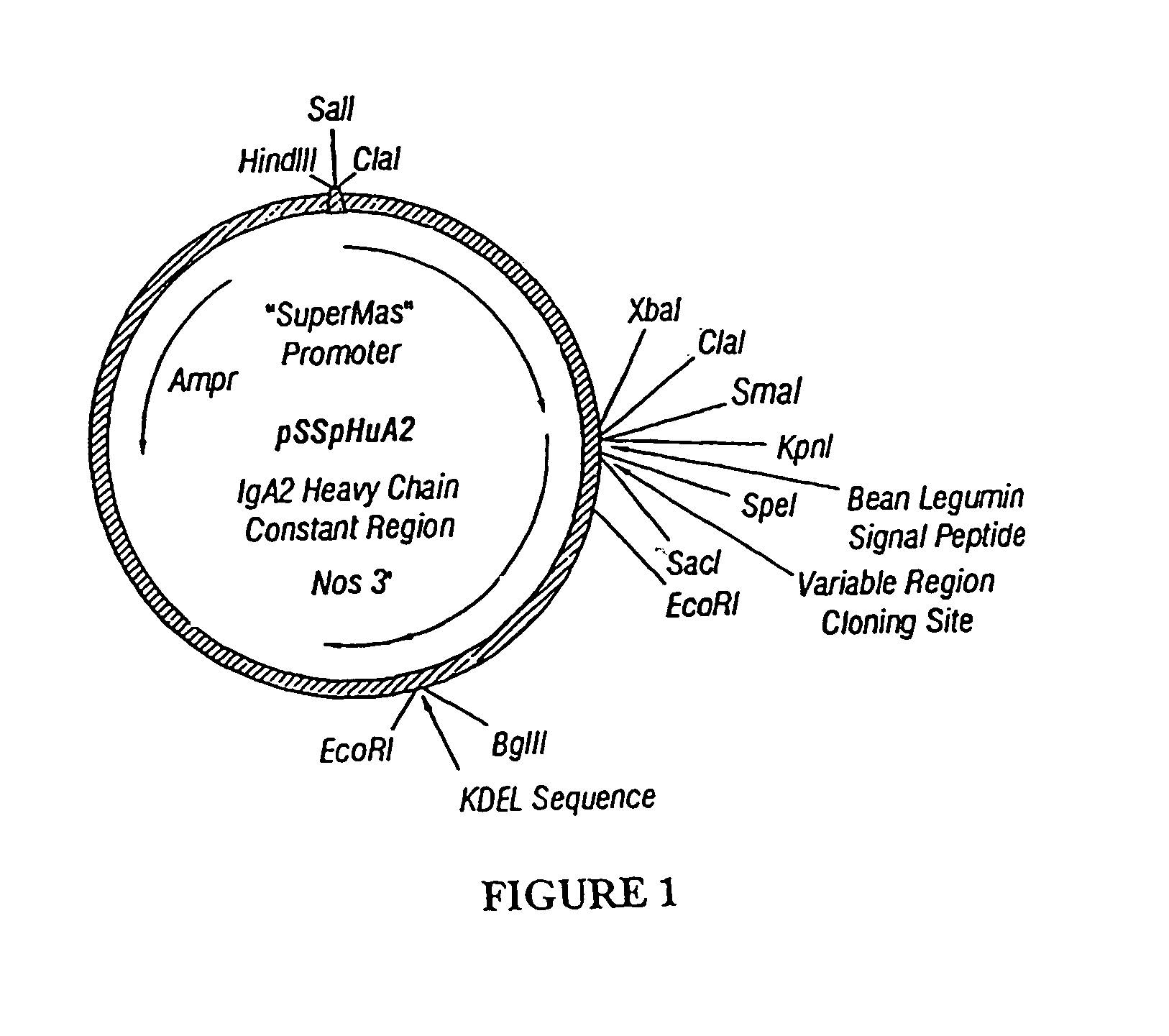
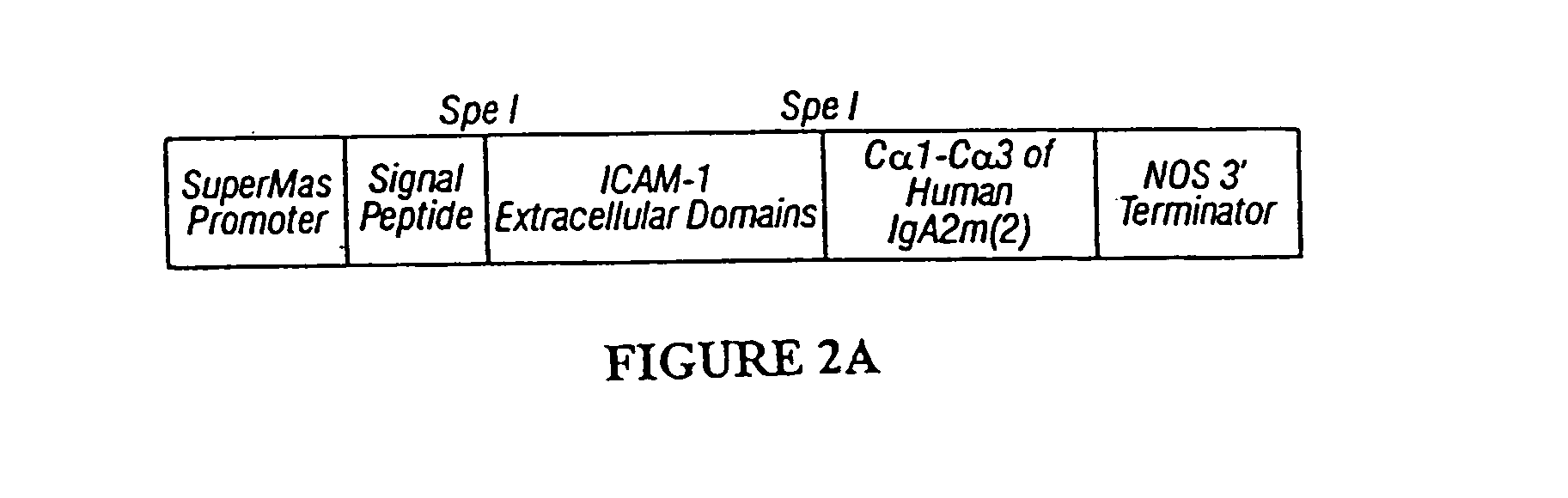
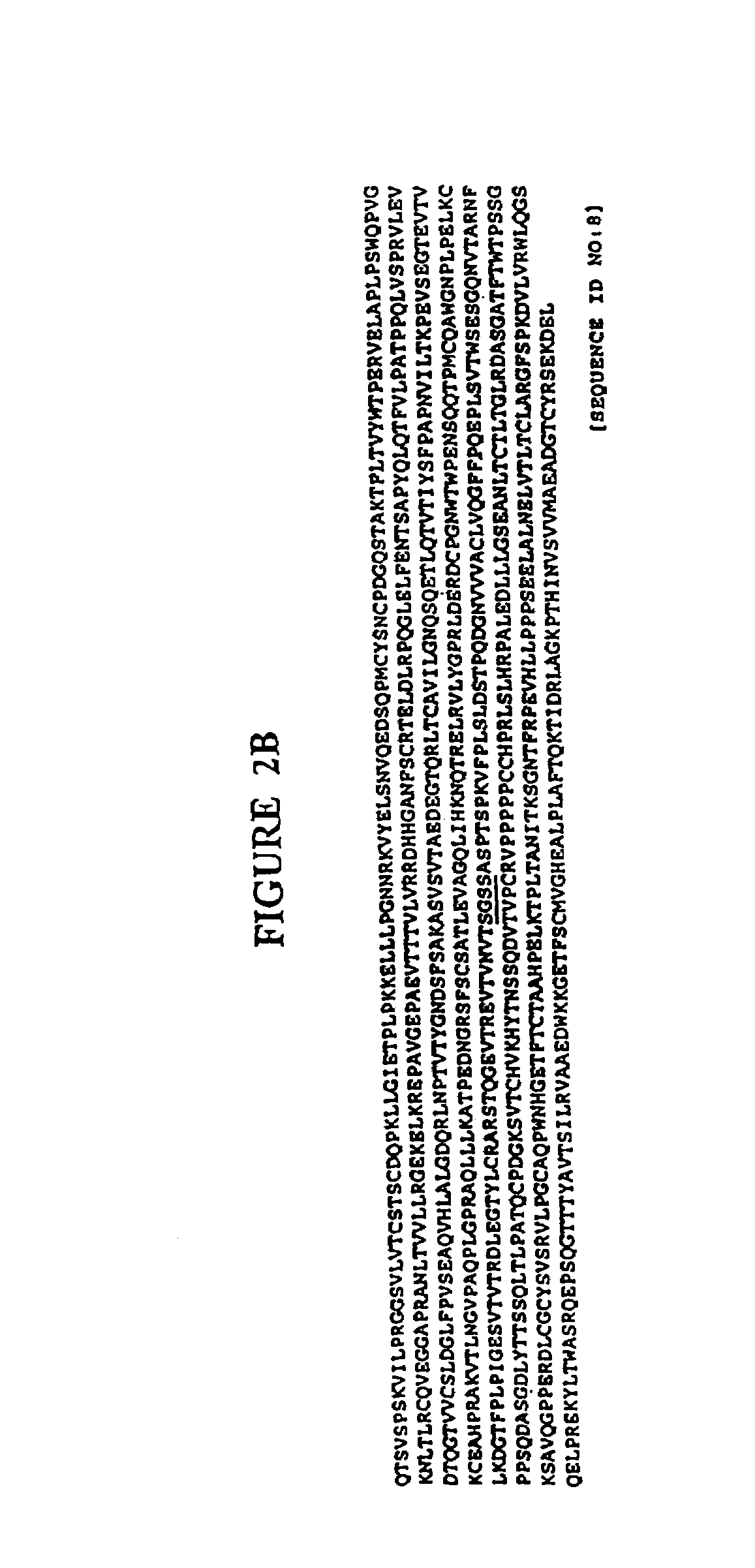
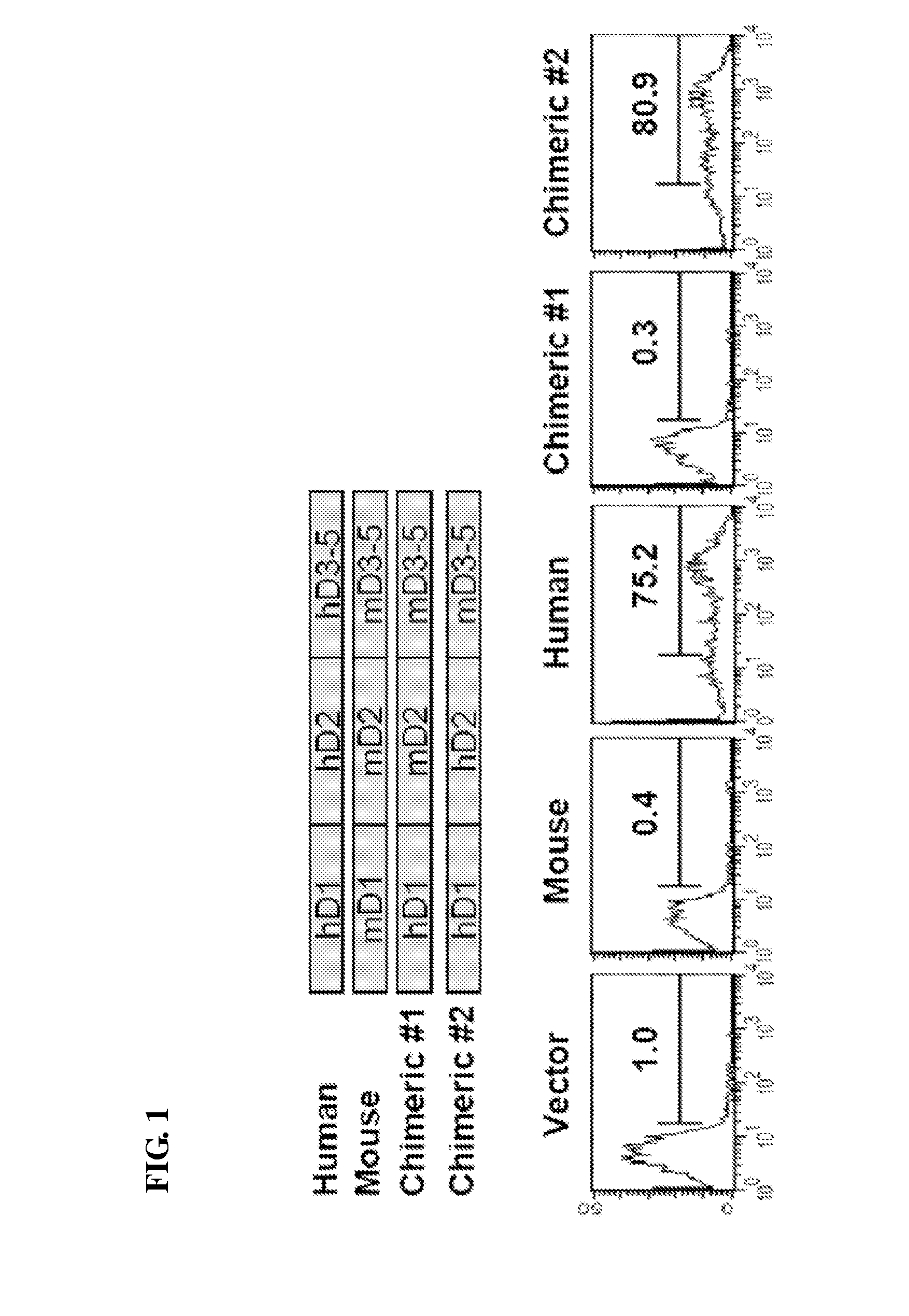
Immunoadhesin comprising a chimeric ICAM-1 molecule produced in a plant
InactiveUS7951378B2Improve efficiencyIncrease in valencySsRNA viruses positive-sensePeptide/protein ingredientsMammalADAMTS Proteins
The immunoadhesions of the present invention are useful in treating rhinovirus infections. The immunoadhesions contain a chimeric ICAM molecule and may optionally also contain J chain and secretory compounds. The chimeric ICAM molecule is a fusion protein that has a rhinovirus receptor protein linked to an immunoglobulin protein. This invention also includes the greatly increased and improved method of producing immunoadhesions in plants. Each of the components of an immunoadhesin is produced in a plant cell and thereby assembles within the plant cell. This method of producing the immunoadhesions of the present invention results in the efficient and economic production of these molecules. The present invention also contemplates the production of immunoadhesions in a variety of eukaryotic cells including plants and mammalian cells. The immunoadhesions of the present invention are useful as a therapeutic against the common cold in humans which is caused by rhinoviruses.
Owner:PLANET BIOTECH
Premature rupture of membrane (PROM) detection kit using ICAM-1 as examination index and preparation method
ActiveCN101871942AQuantitative detection of accurate contentImprove accuracyMaterial analysisChromogenic SubstratesMonoclonal antibody
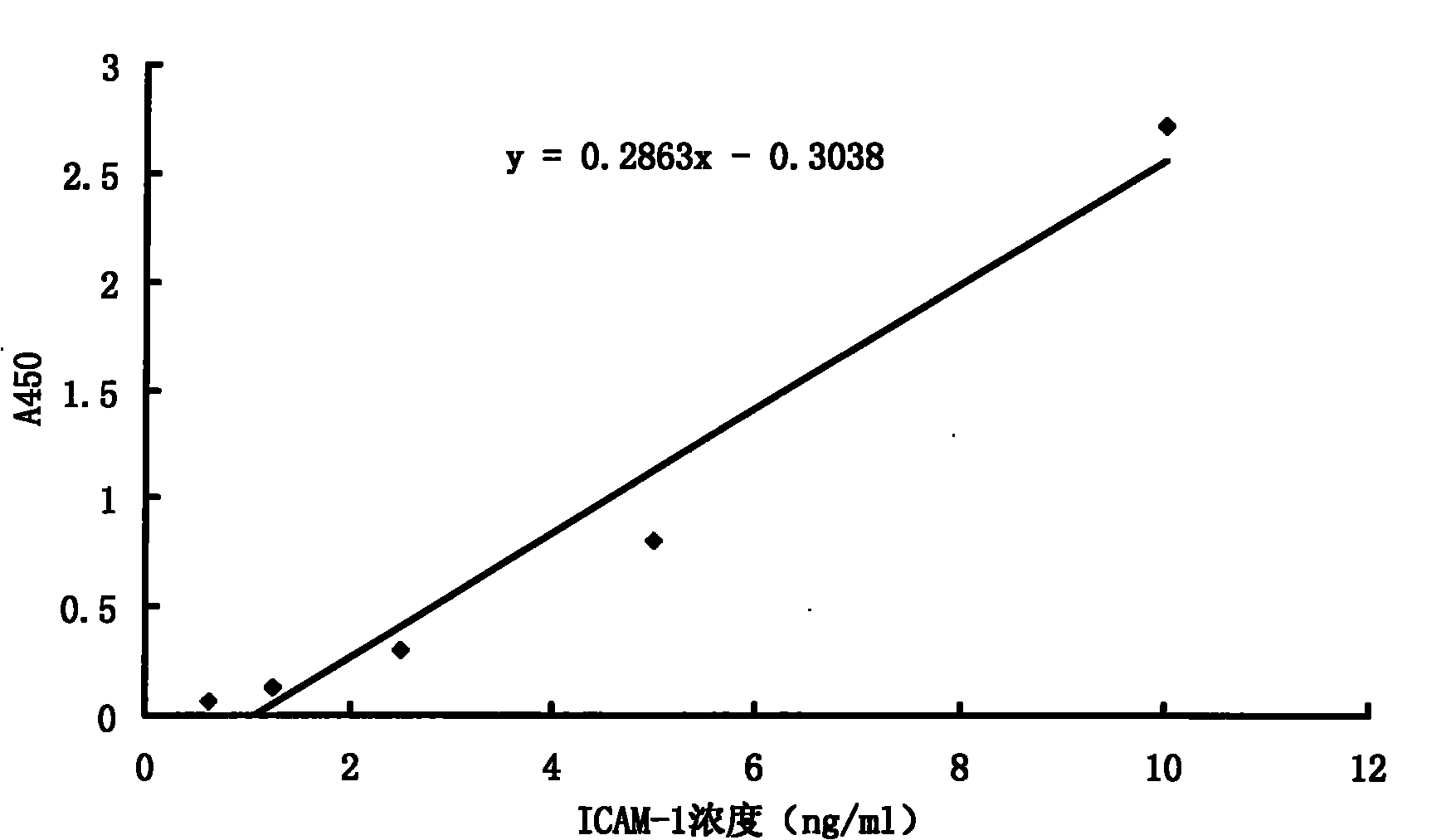
The invention discloses a premature rupture of membrane (PROM) detection kit using an intercellular adhesion molecular (ICAM)-1 as an examination index. The kit comprises a perforated plate coated with an ICAM-1 monoclonal antibody, biotin-labeled ICAM-1 monoclonal antibody (biotin-ICAM-1Ab) examination fluid, aidin-horseradish peroxidase combined with the biotin-labeled ICAM-1 monoclonal antibody, chromogenic substrate 3',3',5,5'-tetramethylbenzidine and ICAM-1 protein standard. A preparation method of the kit comprises the following steps of: (1) preparing the perforated plate coated with the ICAM-1 monoclonal antibody; (2) preparing the biotin-labeled ICAM-1 monoclonal antibody examination fluid; and (3) preparing the aidin-horseradish peroxidase, the chromogenic substrate 3',3',5,5'-tetramethylbenzidine and the ICAM-1 protein standard.
Owner:ORIGISSAY BIOLOGICS TECH
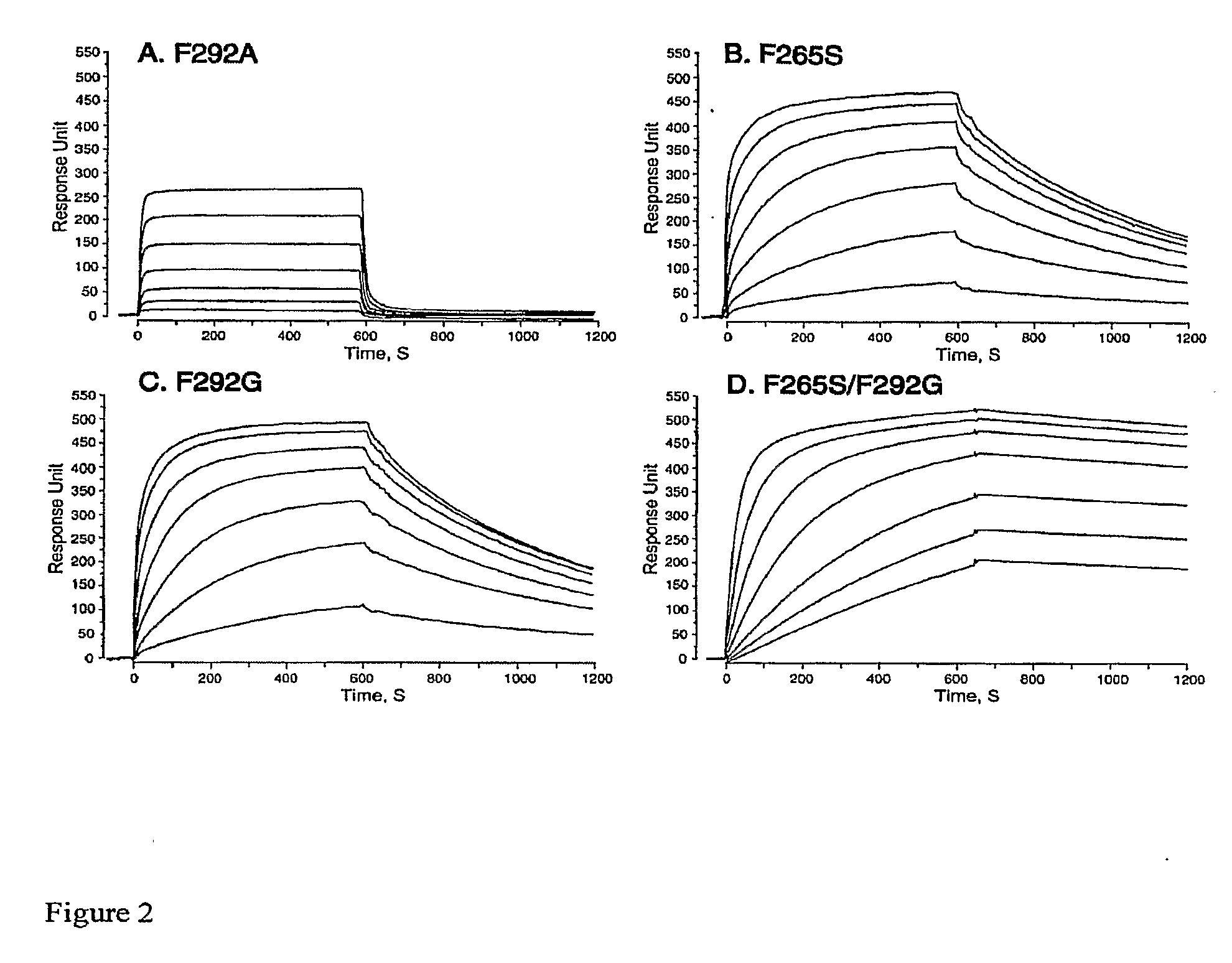
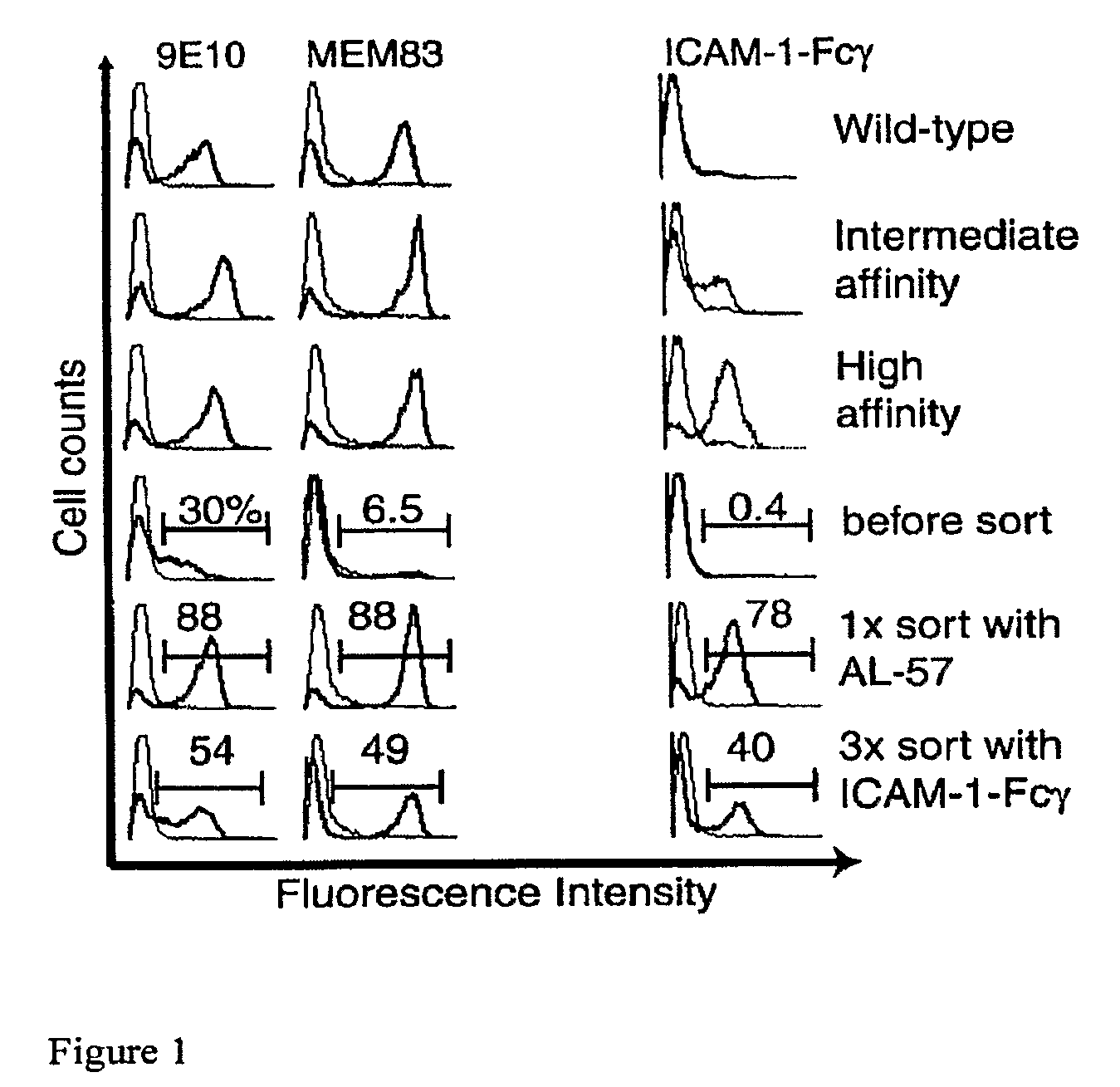
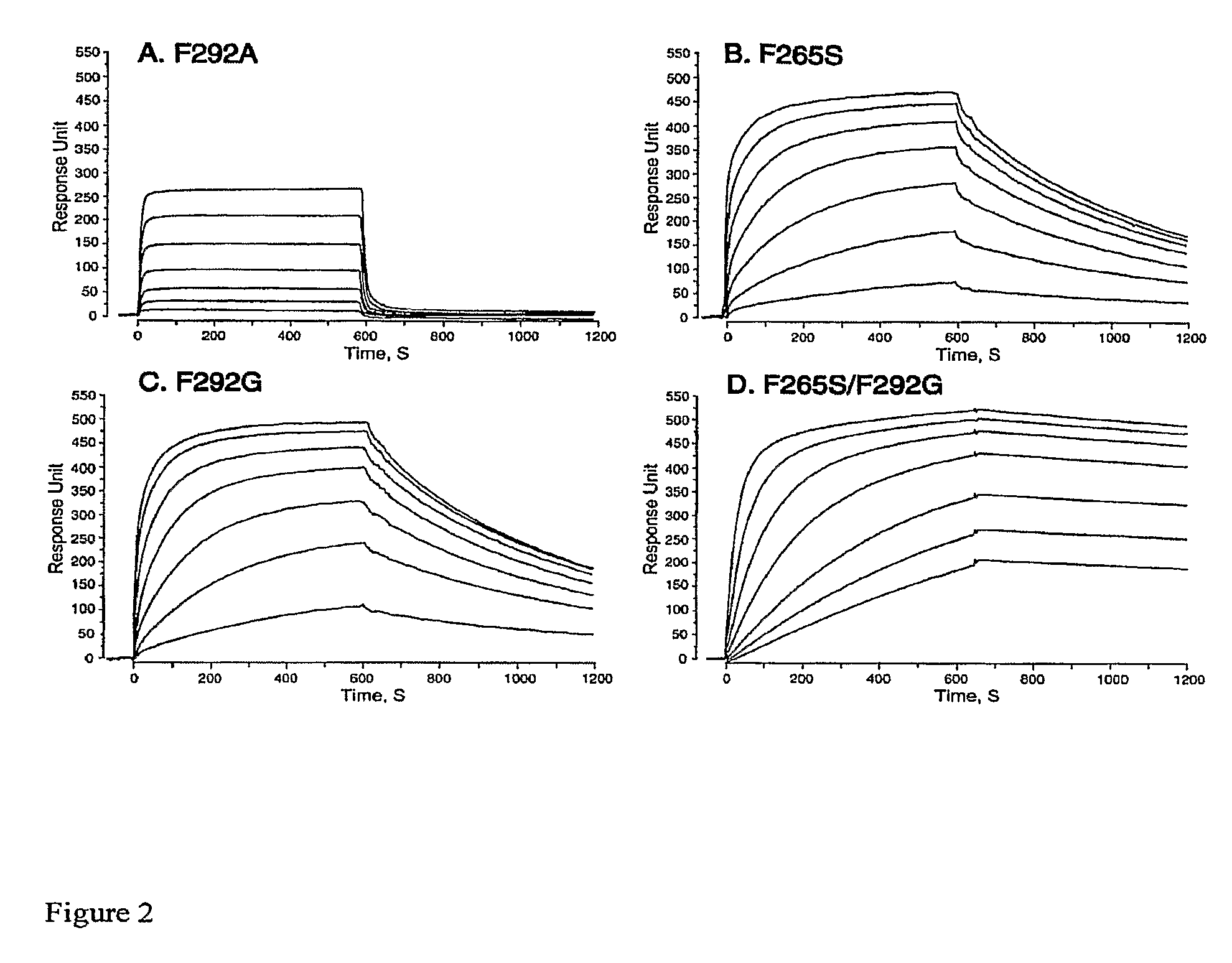
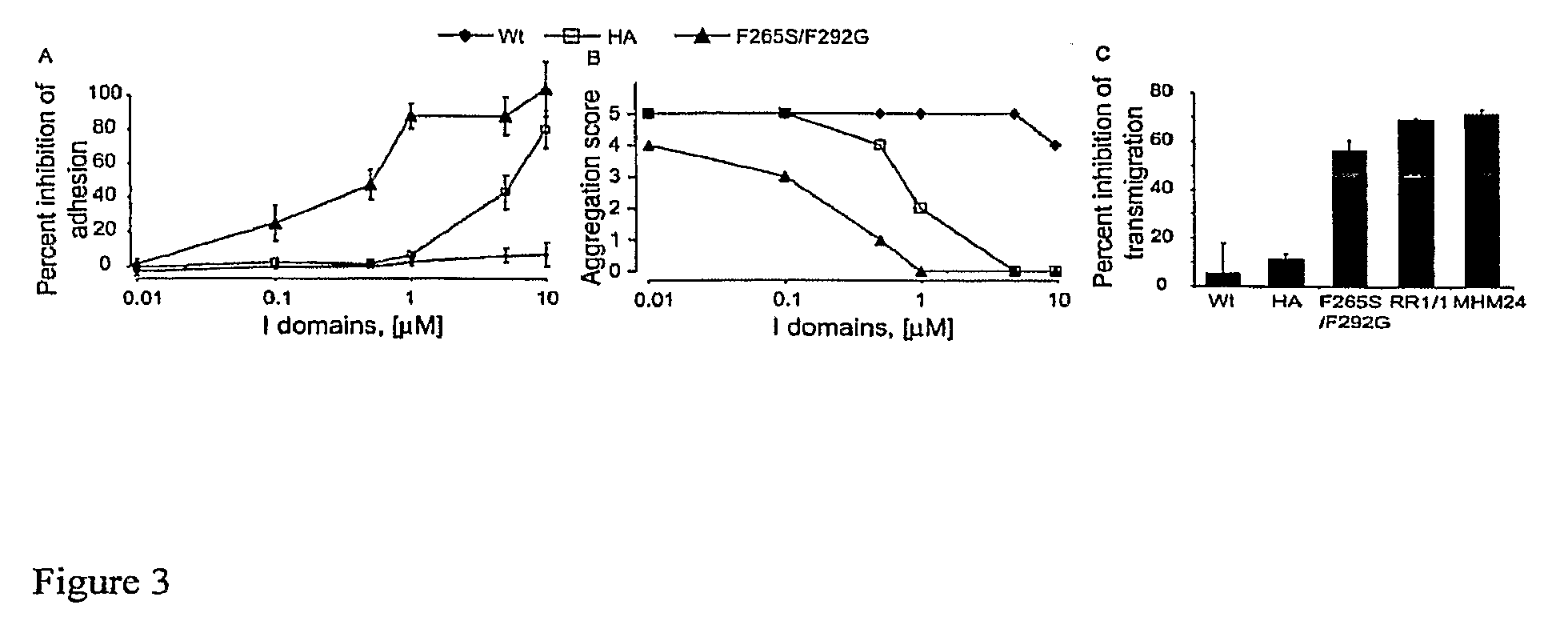
Integrin Alpha L I Domain Mutants with Increased Binding Affinity
The present invention provides an isolated polypeptide capable of binding to aCAM-1, comprising the integrin (XL I domain or biologically active portion thereof, wherein one or more residues is substituted, wherein the substituted polypeptide binds ICAM-I at a higher affinity than wild type integrin CCL protein. The invention provides a method for inhibiting ICAM-I and a pharmaceutical composition comprising an integrin (XL I domain polypeptide or biologically active portion of the polypeptides. The invention also provides a method of treating or preventing an LFA-I mediated ICAM-1 associated disease such as inflammation, artherosclerosis, allograft rejection, diabetes, T-cell mediated sensitization reaction, psoriasis, HIV infection, or rheumatoid arthritis.
Owner:IMMUNE DISEASE INST INC
Integrin alpha L I domain mutants with increased binding affinity
The present invention provides an isolated polypeptide capable of binding to aCAM-1, comprising the integrin (XL I domain or biologically active portion thereof, wherein one or more residues is substituted, wherein the substituted polypeptide binds ICAM-I at a higher affinity than wild type integrin CCL protein. The invention provides a method for inhibiting ICAM-I and a pharmaceutical composition comprising an integrin (XL I domain polypeptide or biologically active portion of the polypeptides. The invention also provides a method of treating or preventing an LFA-I mediated ICAM-1 associated disease such as inflammation, artherosclerosis, allograft rejection, diabetes, T-cell mediated sensitization reaction, psoriasis, HIV infection, or rheumatoid arthritis.
Owner:IMMUNE DISEASE INST INC
Methods to identify polynucleotide and polypeptide sequences which may be associated with physiological and medical conditions
InactiveUS7247425B2Reduce resistanceImprove developmentCompound screeningApoptosis detectionPolynucleotideICAM-1
The present invention provides methods for identifying evolutionarily significant polynucleotide and polypeptide sequences in human and / or non-human primates which may be associated with a physiological condition, such as enhanced resistance to AIDS infection. The invention also provides methods for identifying evolutionarily significant polynucleotides with mutations that are correlated with susceptibility to diseases, such as ICAM 1. The methods employ comparison of human and non-human primate sequences using statistical methods. Sequences thus identified may be useful as host therapeutic targets and / or in screening assays.
Owner:EVOLUTIONARY GENOMICS LLC
Anti-ICAM-1 single domain antibody and uses thereof
ActiveUS8623369B2Easy and inexpensive to produceShorten the timeUltrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsLama glamaSingle-domain antibody
Owner:NAT RES COUNCIL OF CANADA
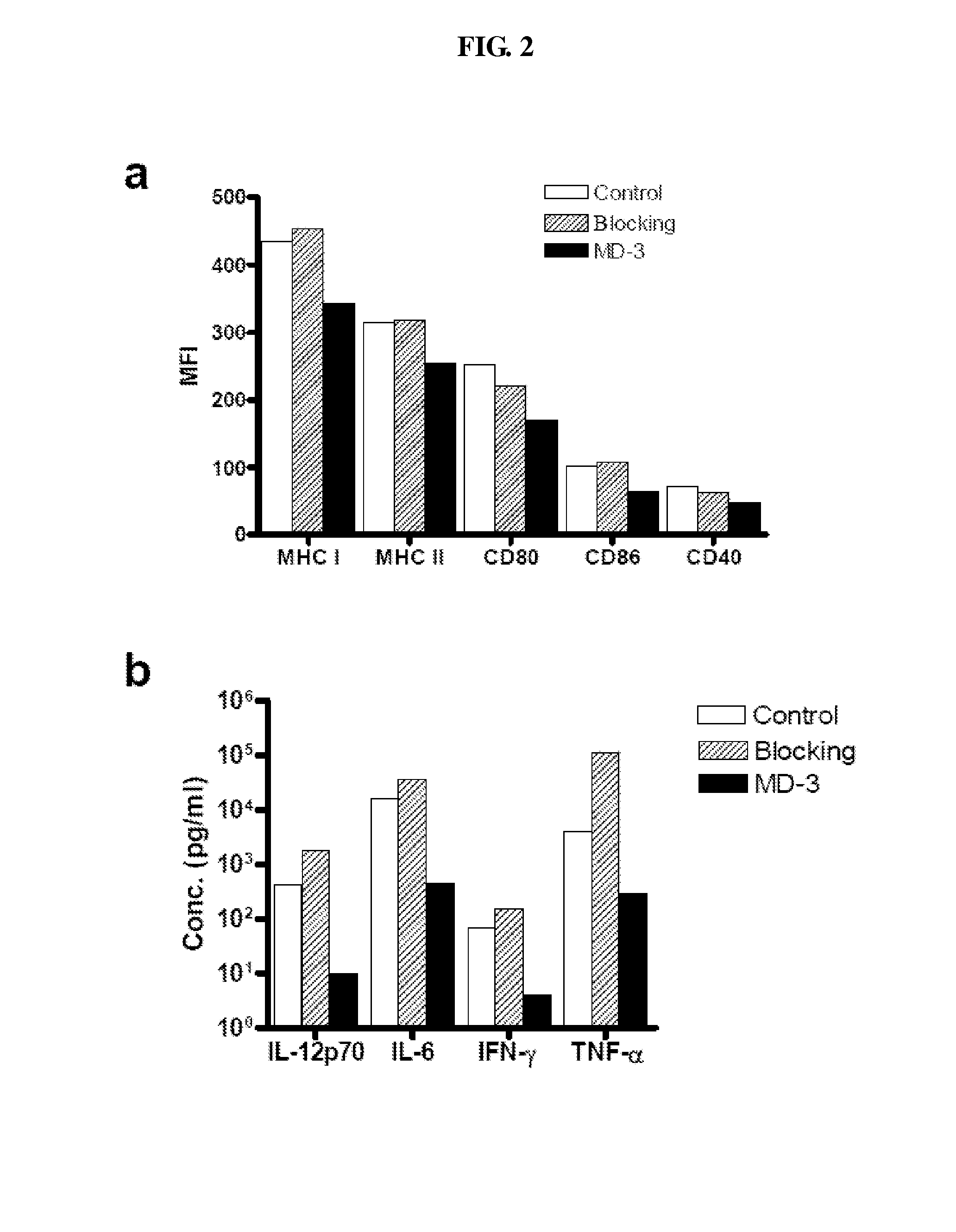
Antibody modulating the differentiation and function of dendritic cells via binding intercellular adhesion molecule-1 and use thereof
InactiveUS20100168394A1Modulate differentiationModulate functionAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsEpidermal Dendritic CellsGraft survival
The present invention relates to an antibody binding to human intercellular adhesion molecule-1 (ICAM-1) where the antibody is able to modulate the differentiation status of dendritic cells and prolong the graft survival. In addition, the present invention provides a pharmaceutical composition comprising the antibody, and method of using them for the treatment of disease.
Owner:DINONA INC
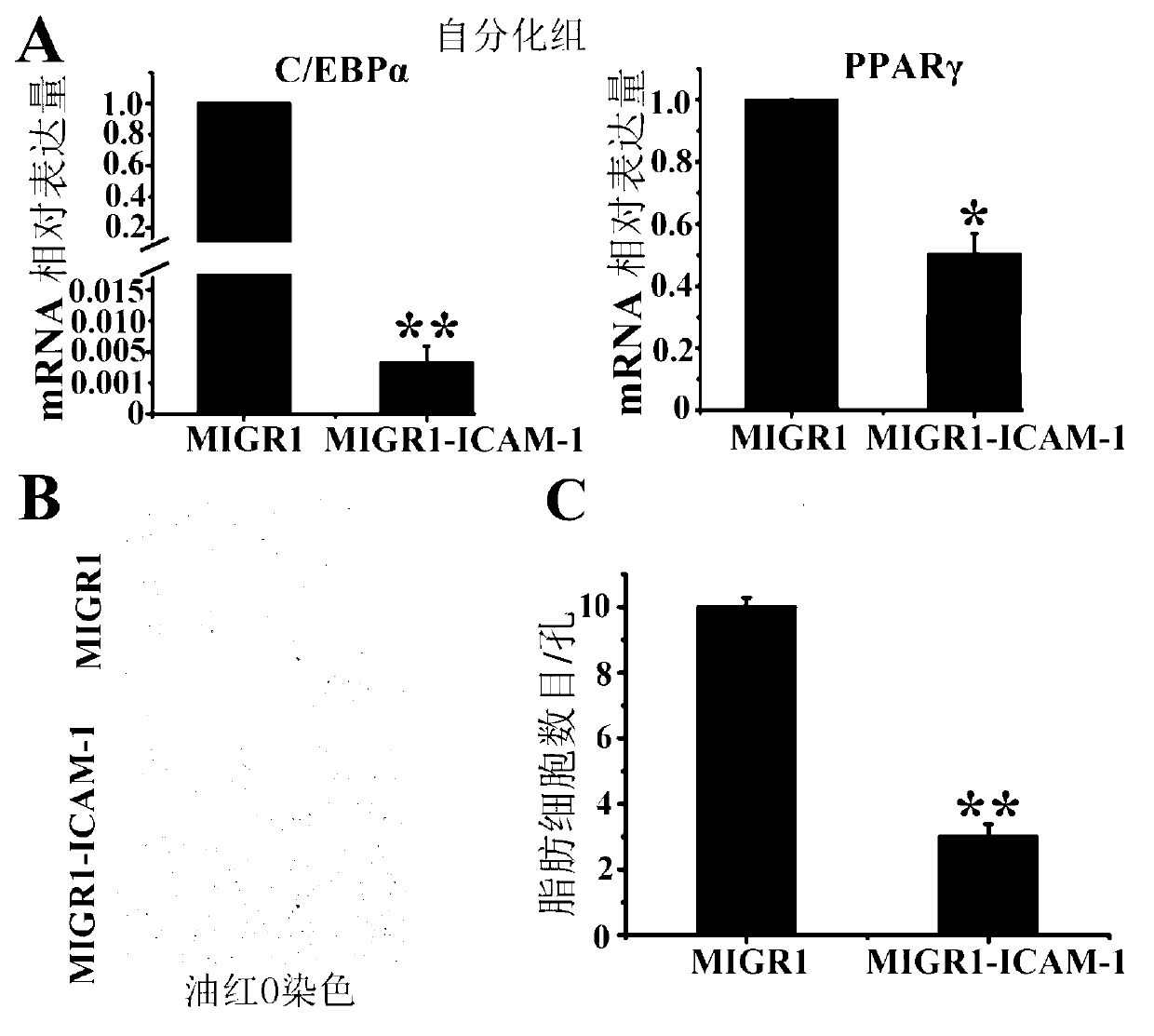
Method for restraining mesenchymal stem cells from differentiating into fat cells
InactiveCN103103213AEasy to operateVigorous proliferation in vitroGenetic engineeringFermentationIntercellular cell adhesion moleculeVirus type
The invention belongs to the technical field of gene engineering and particularly relates to a method for restraining mesenchymal stem cells from differentiating into fat cells. A purpose of restraining the mesenchymal stem cells from differentiating into the fat cells is realized by the following steps of: constructing recombinant retrovirus plasmids containing ICAM-1 (intercellular cell adhesion molecule) genes, carrying out co-transfection on the packaging cells by the recombinant retrovirus plasmids together with packaging plasmids, collecting relevant viral supernatants, and infecting the mesenchymal stem cells. According to the method for restraining the mesenchymal stem cells from differentiating into the fat cells, the separation method is simple in operation, convenient and practical, the efficiency (more than 90% of ICAM-1 can be highly expressed) for transfecting the mesenchymal stem cells is high, and the obtained mesenchymal stem cells are exuberant in in-vitro multiplication and can be passed down for multiple times (more than 50 generations). Thus, as the method and a culture technique system for restraining mesenchymal stem cells from differentiating into fat cells are established, a foundation for research and application of the mesenchymal stem cells is laid.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Methods of isolating bipotent hepatic progenitor cells
A method of obtaining a mixture of cells enriched in hepatic progenitors is developed which comprises methods yielding suspensions of a mixture of cell types, and selecting those cells that are classical MHC class I antigen(s) negative and ICAM-1 antigen positive. The weak or dull expression of nonclassical MHC class I antigen(s) can be used for further enrichment of hepatic progenitors. Furthermore, the progenitors can be selected to have a level of side scatter, a measure of granularity or cytoplasmic droplets, that is higher than that in non-parenchymal cells, such as hemopoietic cells, and lower than that in mature parenchymal cells, such as hepatocytes. Furthermore, the progeny of the isolated progenitors can express alpha-fetoprotein and / or albumin and / or CK19. The hepatic progenitors, so isolated, can grow clonally, that is an entire population of progeny can be derived from one cell. The clones of progenitors have a growth pattern in culture of piled-up aggregates or clusters. These methods of isolating the hepatic progenitors are applicable to any vertebrates including human. The hepatic progenitor cell population is expected to be useful for cell therapies, for bioartificial livers, for gene therapies, for vaccine development, and for myriad toxicological, pharmacological, and pharmaceutical programs and investigations.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods for treating malignancies expressing ICAM-1 using coxsackie a group viruses
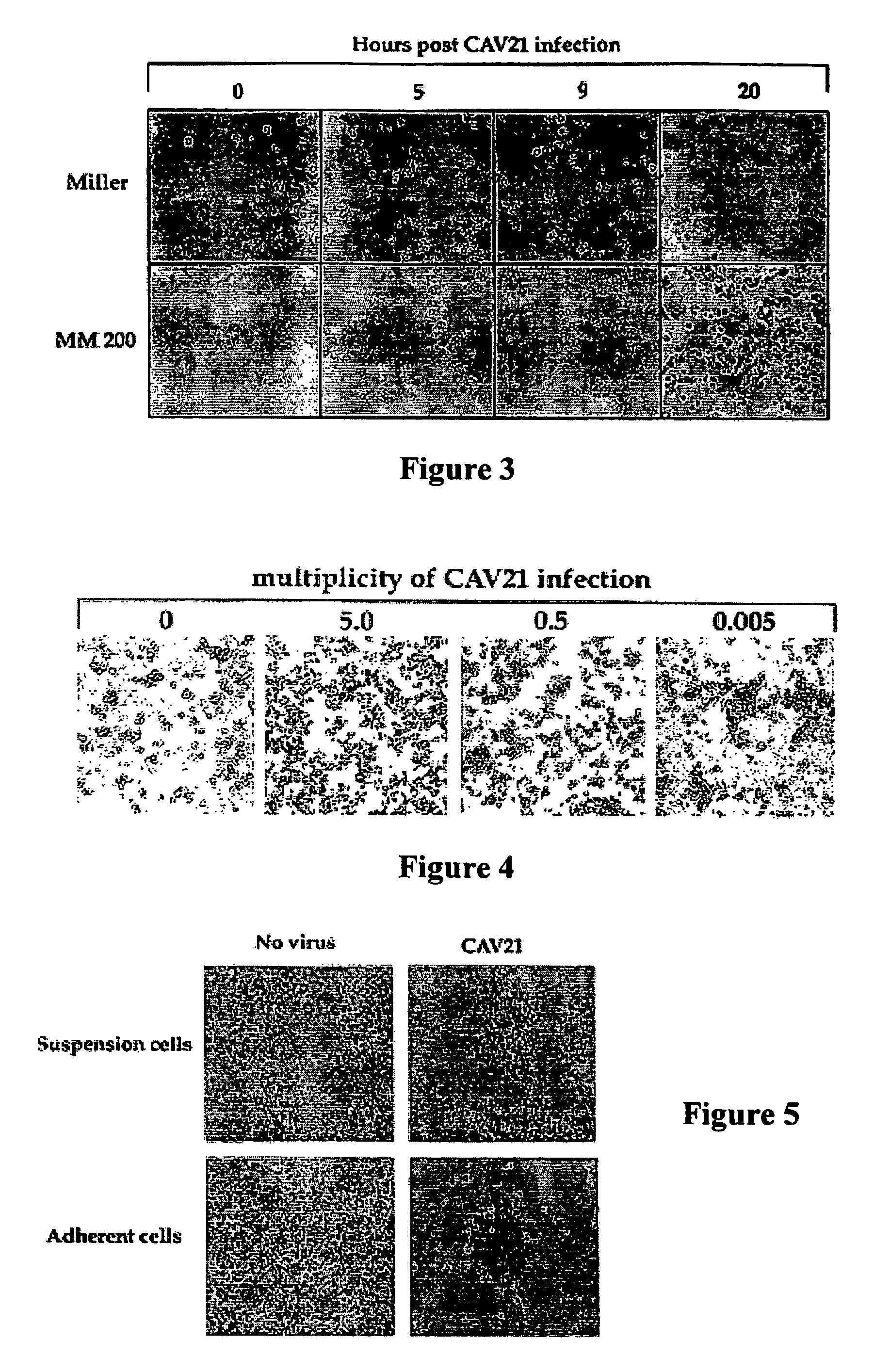
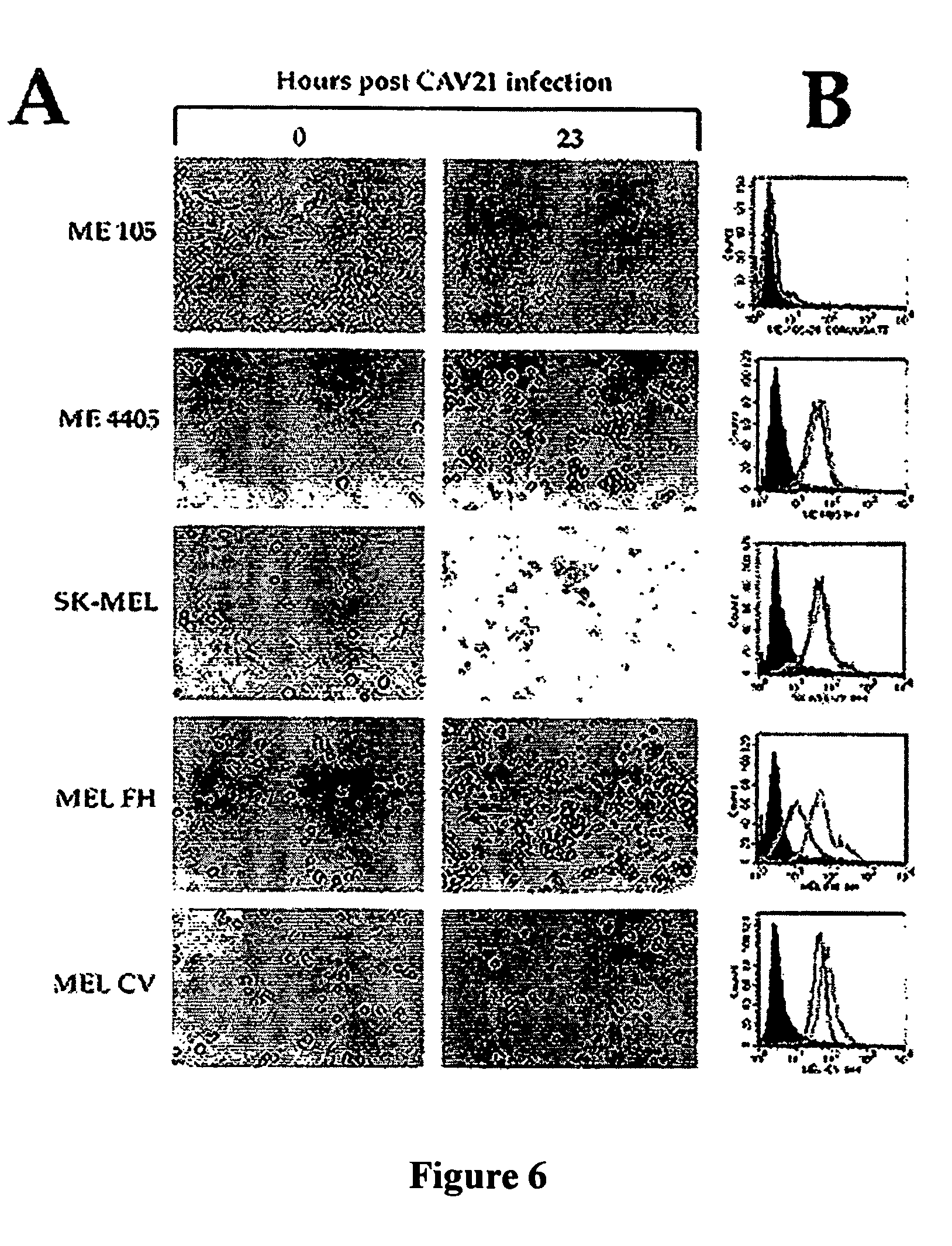
InactiveUS7361354B1Effective targetingImprove immunityBiocideSsRNA viruses positive-senseMelanomaMalignancy
There is disclosed a method of killing abnormal cells such as malignant cells including melanoma cells, using a virus recognizing at least one of a cell adhesion molecule and a complement regulatory protein. The virus may be a member of the Picornaviridae family. Coxsackie A-group viruses have been found to be particularly suitable. The cell adhesion molecule is desirably a member of the immunoglobulin (Ig) superfamily. Typically, the complement regulatory protein will be DAF.
Owner:MERCK SHARP & DOHME CORP
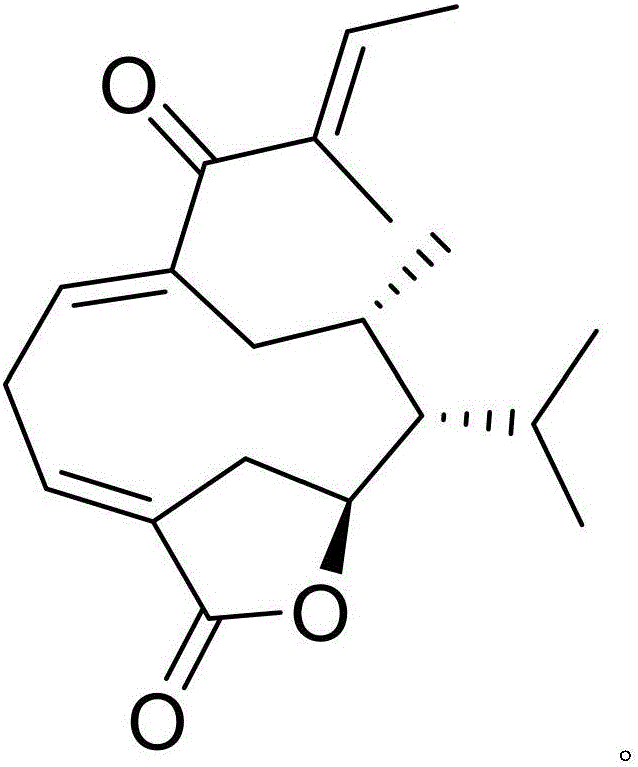
Pharmaceutical composition of cefdinir and medical application thereof
InactiveCN105820144AImprove protectionProtectiveOrganic active ingredientsOrganic chemistryReperfusion injuryNatural product
The invention discloses a pharmaceutical composition of cefdinir and its medical application. The pharmaceutical composition of cefdinir provided by the invention contains cefdinir and a novel natural product compound (I), cefdinir Compound (Ⅰ) can significantly improve the neurological function scores of rats with cerebral ischemia-reperfusion and significantly reduce the expression levels of inflammatory cytokines TNF-α, IL-1β and adhesion molecules VCAM-1 and ICAM-1. The combined effect is better than single use, and can be developed into a drug for protecting cerebral ischemia-reperfusion injury.
Owner:吴珺
Method for detecting or treating triple negative breast cancer
PendingUS20170173005A1Increased riskHigh riskPowder deliveryOrganic active ingredientsNanoparticleImaging agent
A method of detecting triple negative breast cancer (TNBC) is provided. Overexpression of ICAM-1 is linked to an increased risk of TNBC. A composition of matter is also provided that binds an anti-ICAM˜1 antibody to a nanoparticle. The composition may be used as an imaging agent and / or a therapeutic targeting agent. A therapeutically active molecule may be bound to the composition to provide targeted therapy.
Owner:CHILDRENS MEDICAL CENT CORP
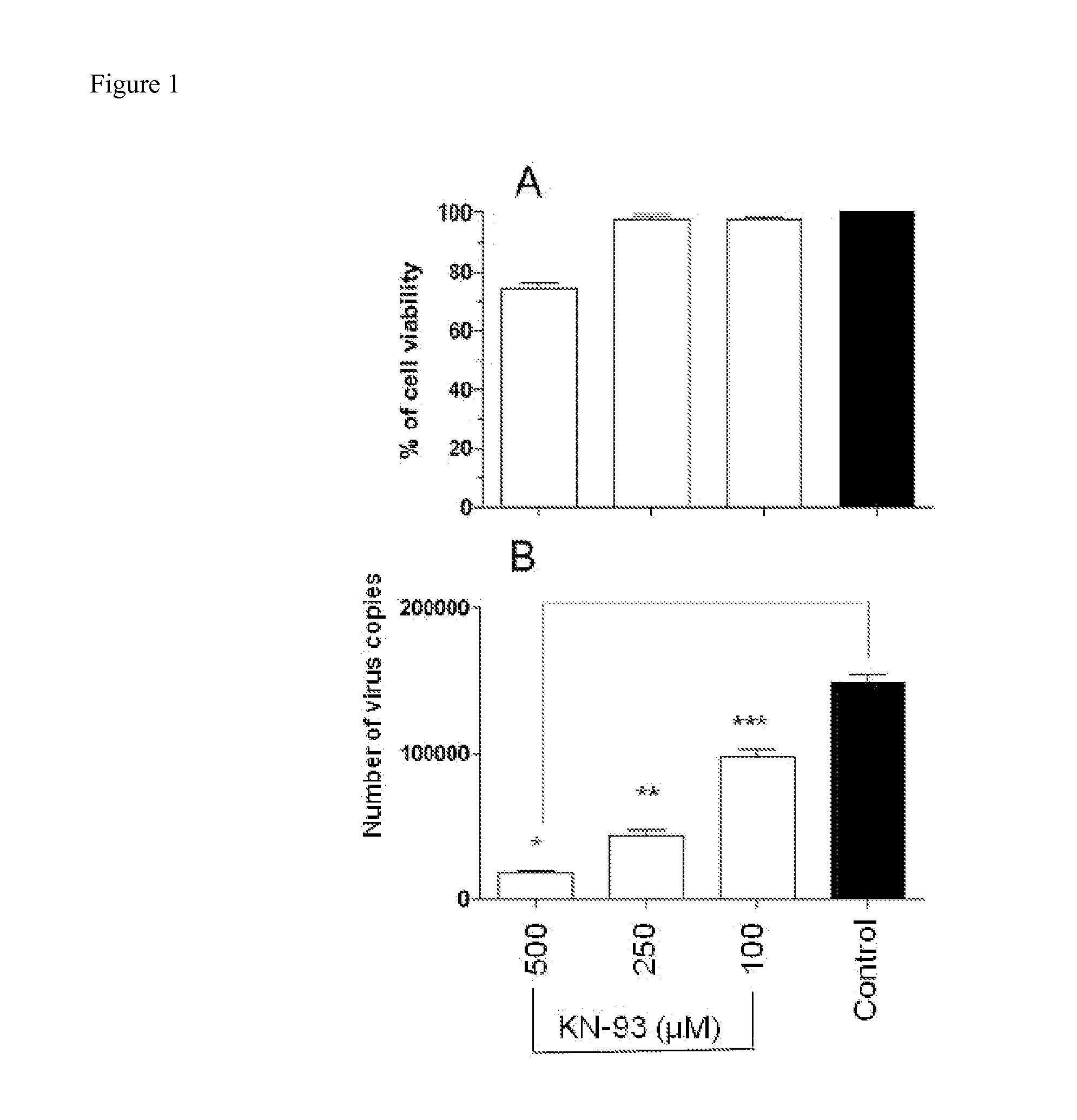
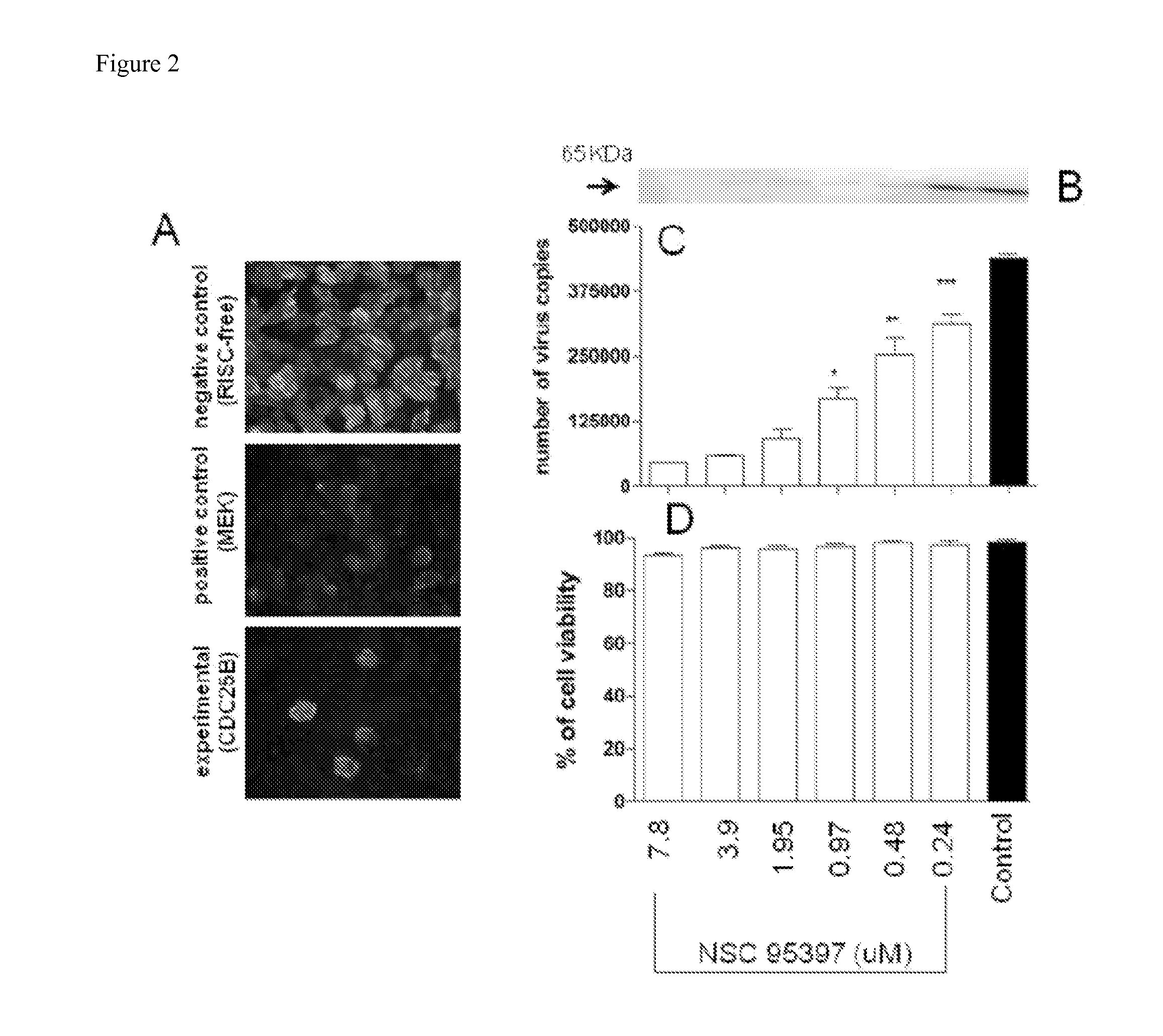
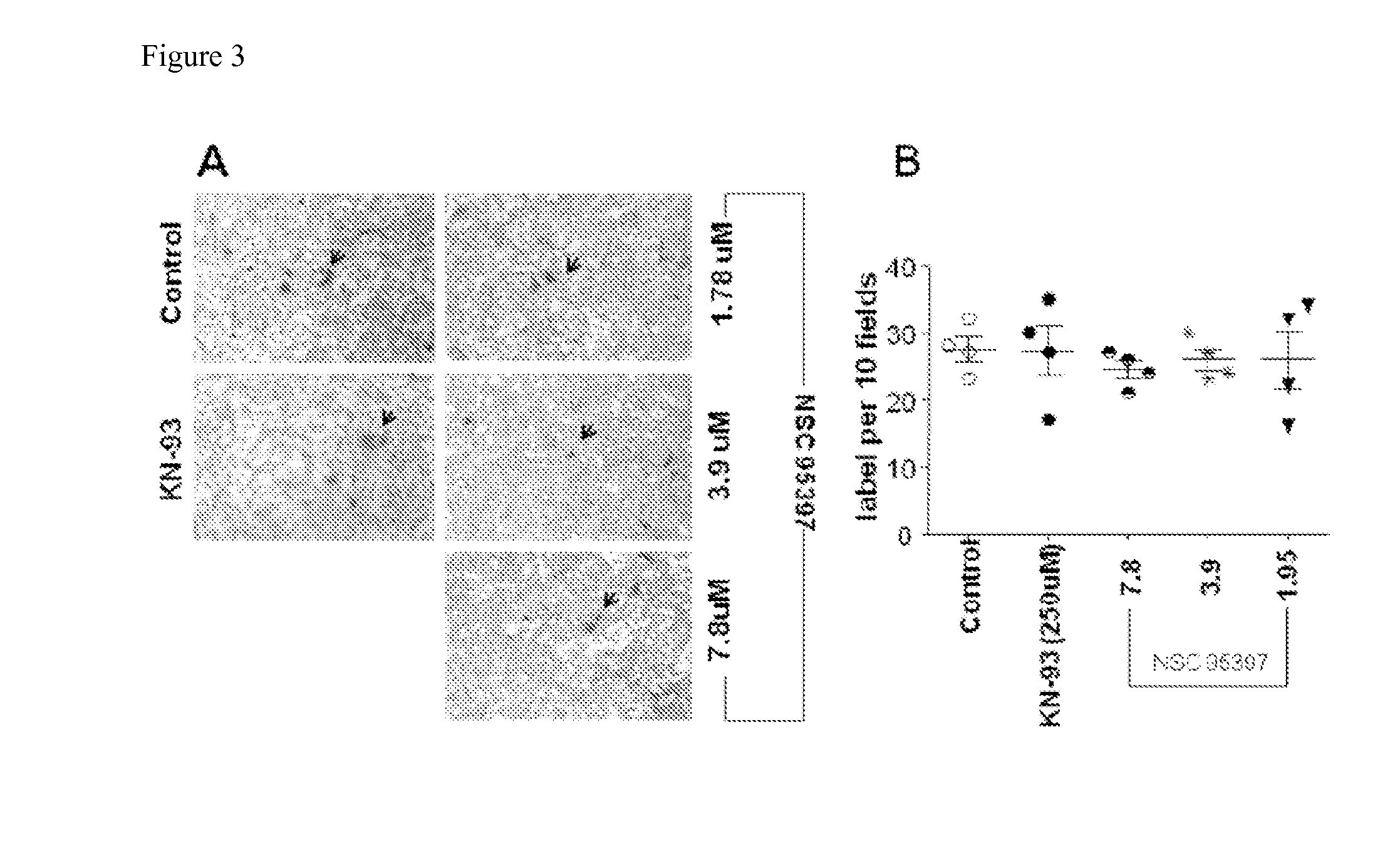
Methods for Inhibiting Virus Replication
The present invention provides methods for treating a subject having, or at risk of having, a viral infection. The methods include, but are not limited to, the use of 4-(dipropylsulfamoyl)benzoic acid, an inhibitor of CDC25B phosphatase, an inhibitor of ICAM-1, an inhibitor of CamK2B, or a combination thereof.
Owner:UNIV OF GEORGIA RES FOUND INC
Clinic detection kit of multiple biomarkers in ischemic cerebral apoplexy
The invention relates to SHIP-1, IGF-1, SDPR, MBL, ICAM-1, Hs-CRP, LP(a) and MMP-9 chip clinic diagnosis reagents. Any other combined or single methodological clinic diagnosis reagents except an Hs-CRP biochemical reagent and methodologies are included in claims 1-6. The invention also relates to identification and selection of 8 serum protein markers, identification and selection of any combinations of biomarkers of the 8 serum protein markers, and preparation methods and quality standards of antigens of the 8 serum protein markers, and corresponding antibodies thereof. The 8 serum protein markers are differentially expressed in blood, and can be used for early warning, diagnosis, monitoring, health state evaluation and predication of ischemic cerebral apoplexy diseases, and clinic detection of judgment prognosis and treatment effect evaluation.
Owner:陈惠 +1
Targeting and prolonging association of drugs to the luminal surface of the pulmonary vascular endothelial cells using antibodies that bind to ICAM-1
Methods for targeting and prolonging association of a selected drug to the luminal surface of pulmonary vascular endothelium of an animal are provided wherein a selected drug is administered to an animal in combination with a non-internalizable ICAM-1 antibody which binds to an antigen on the luminal surface of the pulmonary vasculature. This method is particularly useful in dissolution of fibrin clots or prevention of the intravascular coagulation in the pulmonary vasculature.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for treatment of pouchitis
ActiveUS20120270920A1Complication and expenseOrganic active ingredientsAntipyreticMedicineSuppository
The present invention relates methods of treating pouchitis by administering a pharmaceutical formulation suitable for rectal use, such as an enema or suppository, comprising an antisense oligonucleotide targeted to ICAM-1 to an individual
Owner:IONIS PHARMA INC
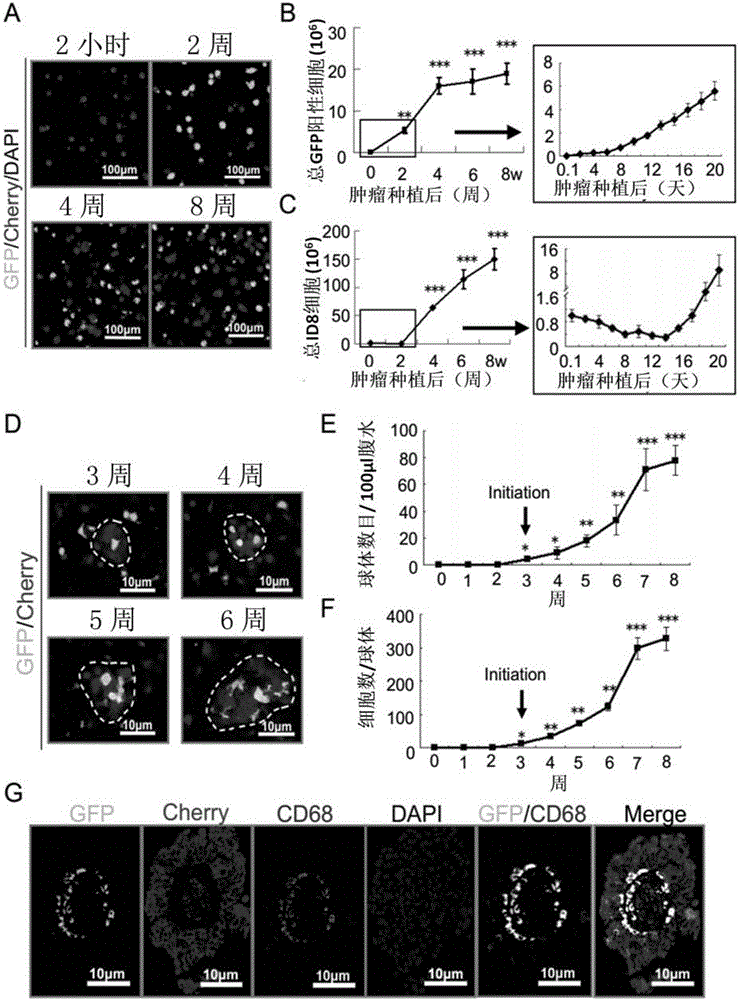
Application of ICAM-1 or alphaMbeta2 integral protein in screening of medicine for diagnosing or treating implantation metastatic cancer
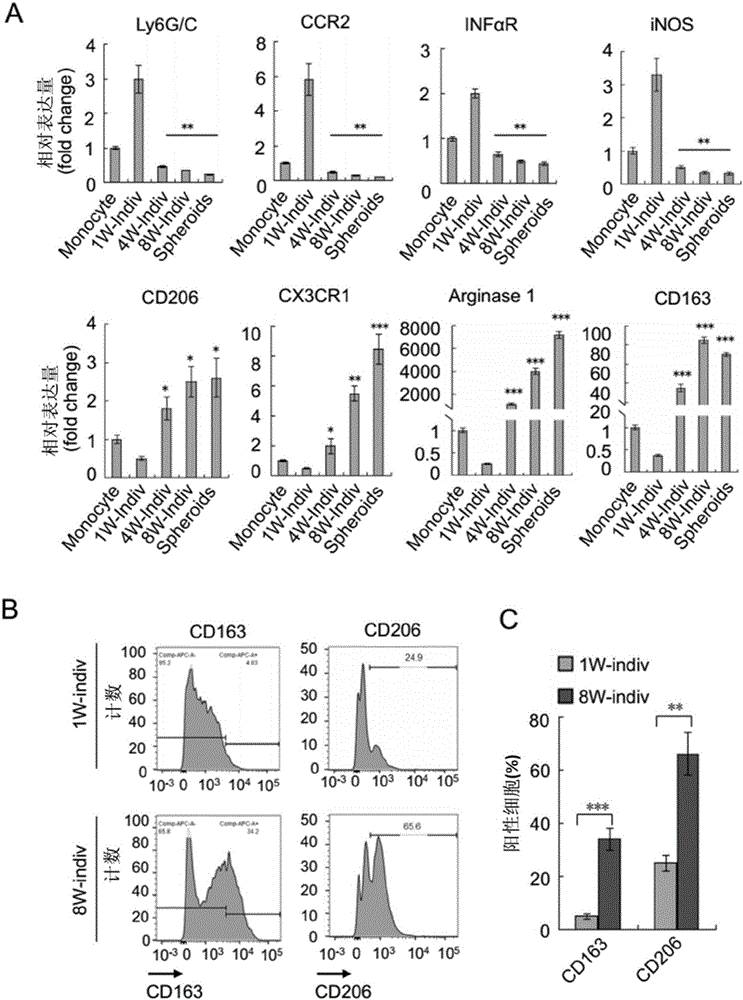
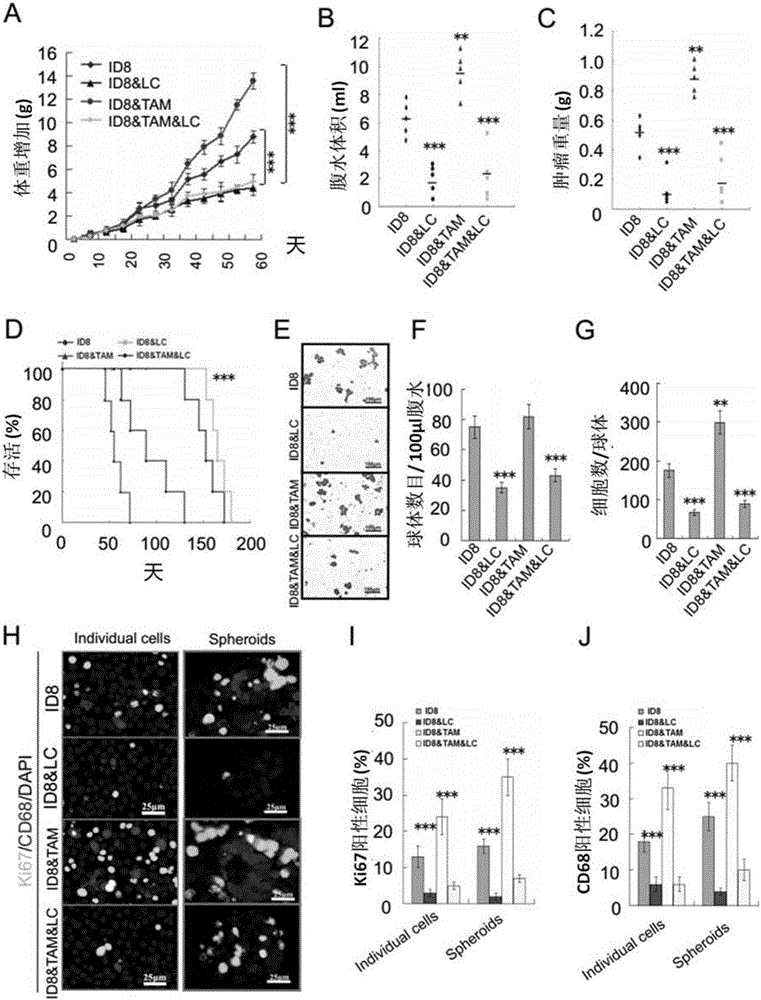
The invention discloses an application of ICAM-1 or alphaMbeta2 integral protein in screening of medicine for diagnosing or treating implantation metastatic cancer. The application firstly verifies that the ICAM-1 or alphaMbeta2 integral protein plays an important regulating effect in the ovarian cancer implantation metastasis process on a mice in-situ ovarian cancer model and discloses the formation basis mechanism of the ovarian cancer sphere, and can provide theoretical foundation for the screening of the medicine for diagnosing or treating implantation metastatic cancer.
Owner:广州道瑞医药科技有限公司
Antibody that binds domain 2 of ICAM-1 and methods of treatment
ActiveUS8900586B2Effective prevention and treatmentMinimize adverse effectsAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsDendritic cellT cell mediated immunity
The present invention relates to an antibody binding to the domain 2 of human intercellular adhesion molecules-1 (ICAM-1) where the antibody is able to modulate the differentiation status of dendritic cells and induce antigen-specific T cell tolerance, thereby be effective in the prevention and / or treatment of T cell-mediated immune disorders such as transplantation rejection, graft-versus-host disease, and autoimmune disease. In addition, the present invention provides a pharmaceutical composition comprising the antibody, and method of using them for the treatment of disease.
Owner:KUMHO HT +1
Human ICAM-1 extramembranous I-III domain gene expression product ,its prep. and application in radioimmunological technology
InactiveCN1448509AEasy to purifySimple and fast operationCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseImmune profiling
The present invention relates to gene engineering technology. The I-III functional encoding gene outside intercellular adhesion molecule-1 (ICAM-1) membrane is recombined to expression plasmid pET42a, transferred into prokaryotic expression bacteria, and purified to obtain expression product GST-ICAM-1. The expression product is used as antigen in immunizing rabbit to obtain rabbit anti-human polyclonal antibody. Thus prepared expression product of human I-III functional encoding gene outside ICAM-1 membrane in prokaryotic system is easy to purify, low in cost and high in yield. The human serum sICAM-1 radioimmunoassay technology is developed and used in clinical test and has important significance in diagnosing the treating effect of Graves' disease.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Diagnostic markers of wound infection III
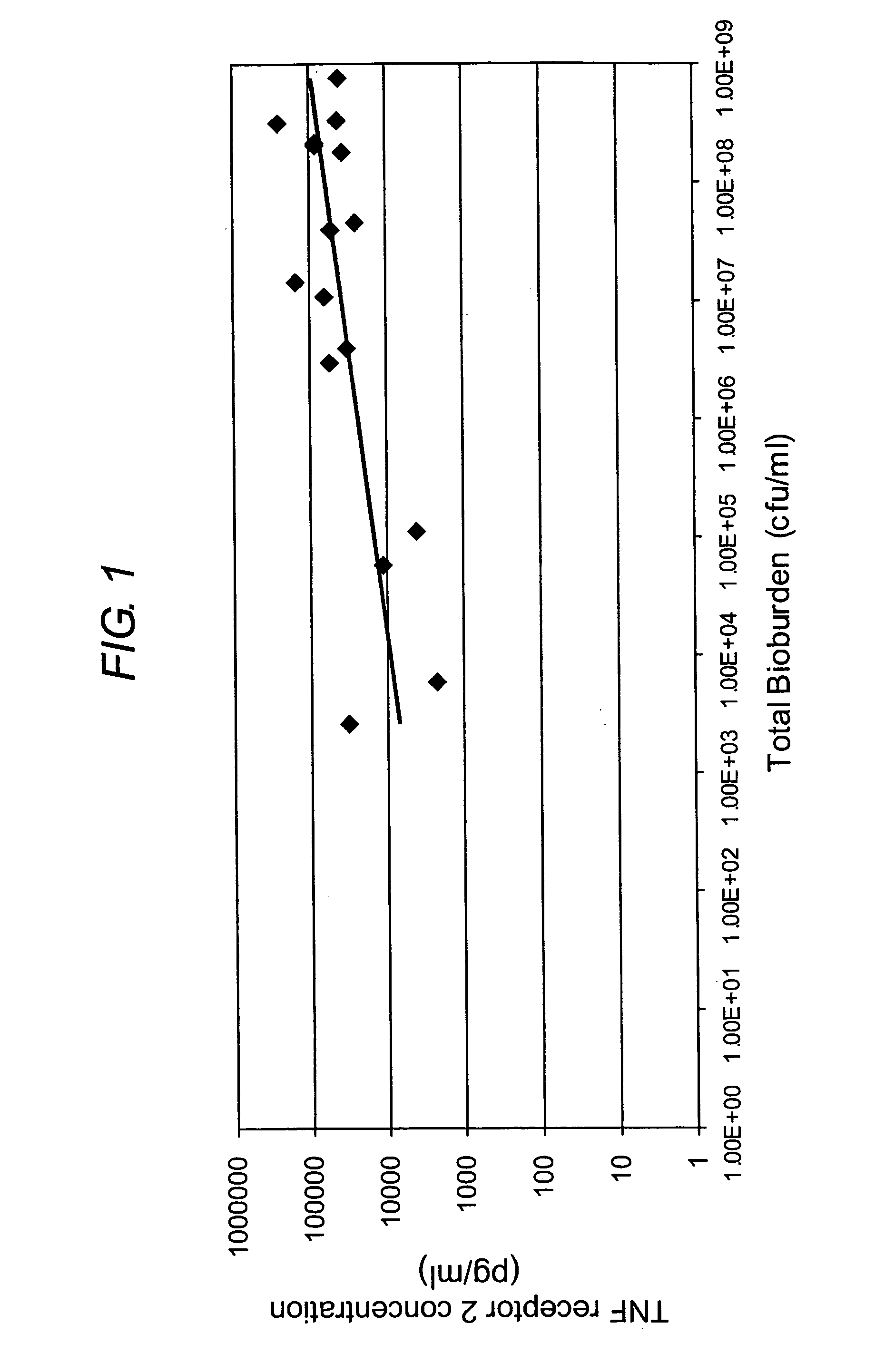
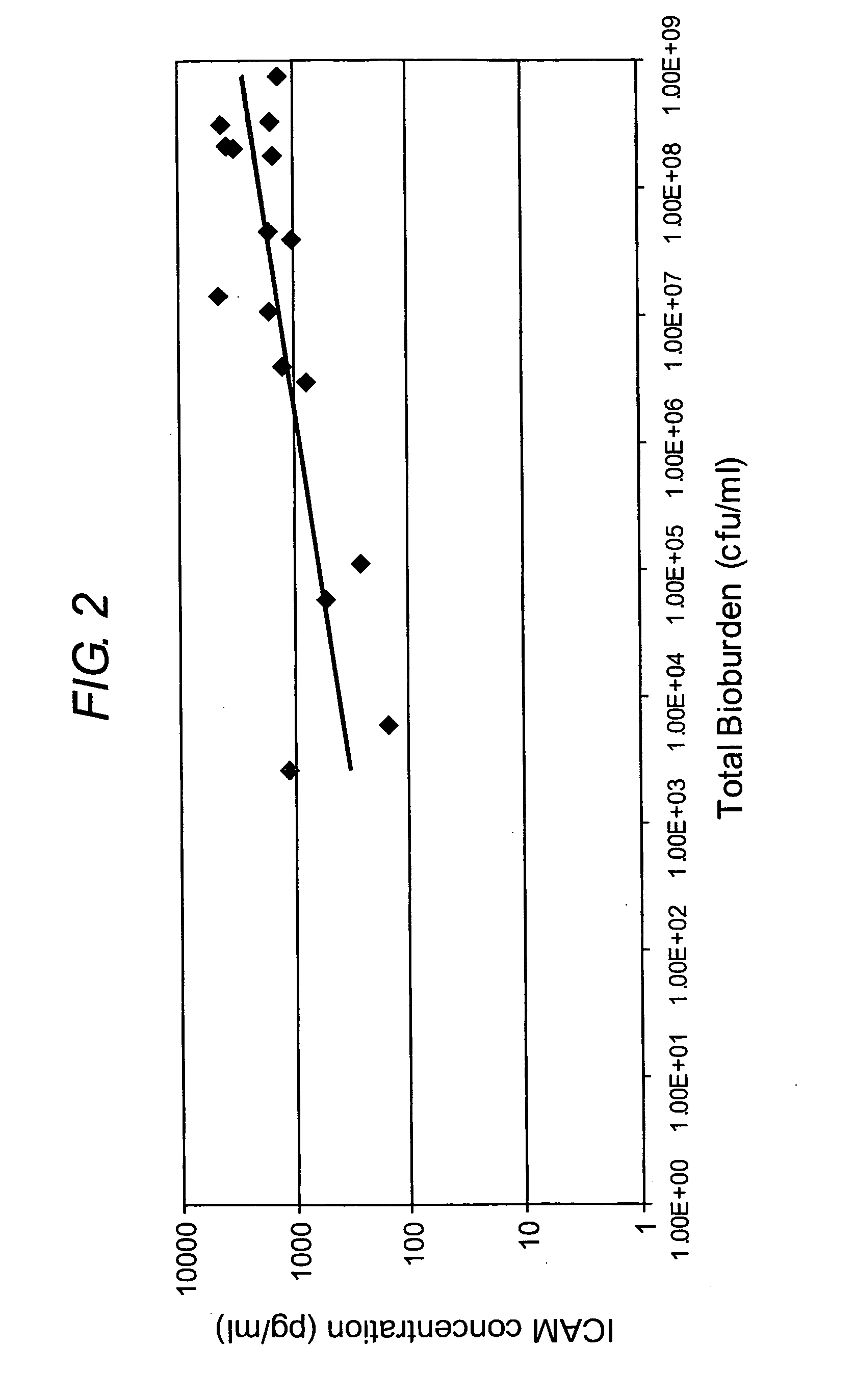
The present invention relates to a method of diagnosis or prognosis of a mammalian wound infection, said method comprising the step of measuring the level of at least one cell surface receptor in a sample of wound fluid. The preferred cell surface receptors are Intercellular adhesion molecule-1 (ICAM 1) and Tumor Necrosis Factor Receptor-2 (TNF-RII). The present invention also provides devices (e.g. biosensors) for use in such methods, and methods and products for diagnosing and treating infected wounds.
Owner:WOUNDCHECK LAB US
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com