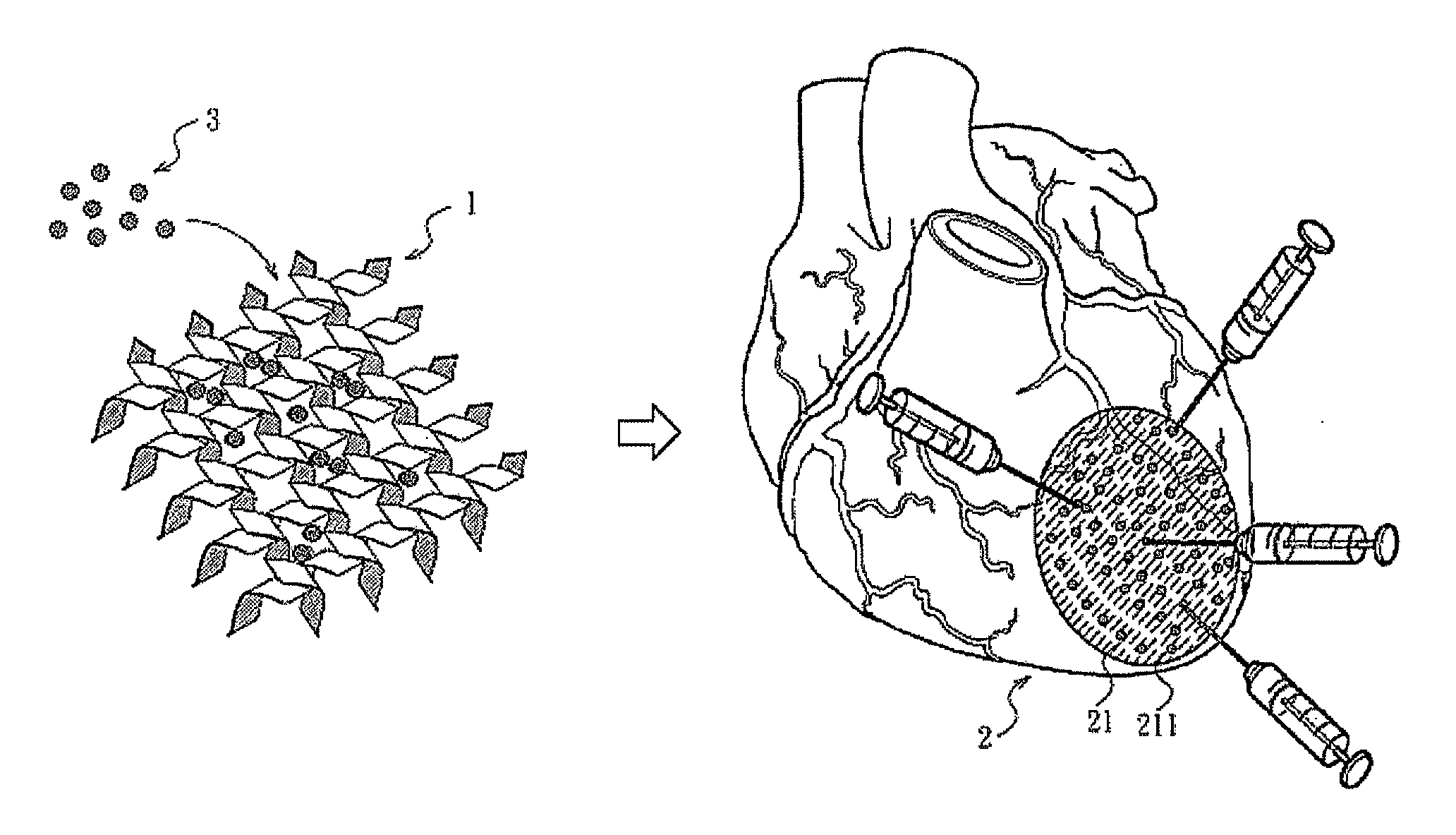
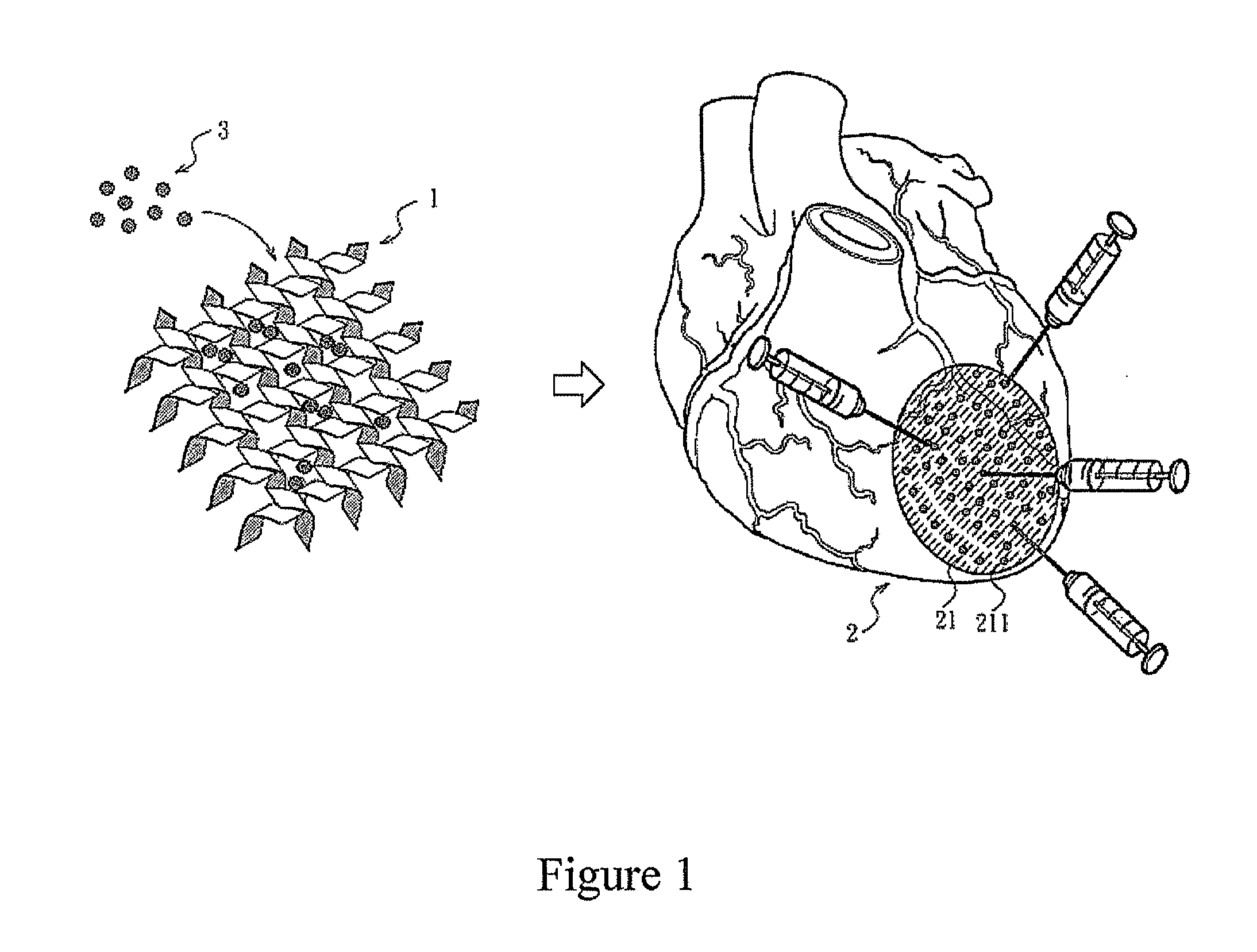
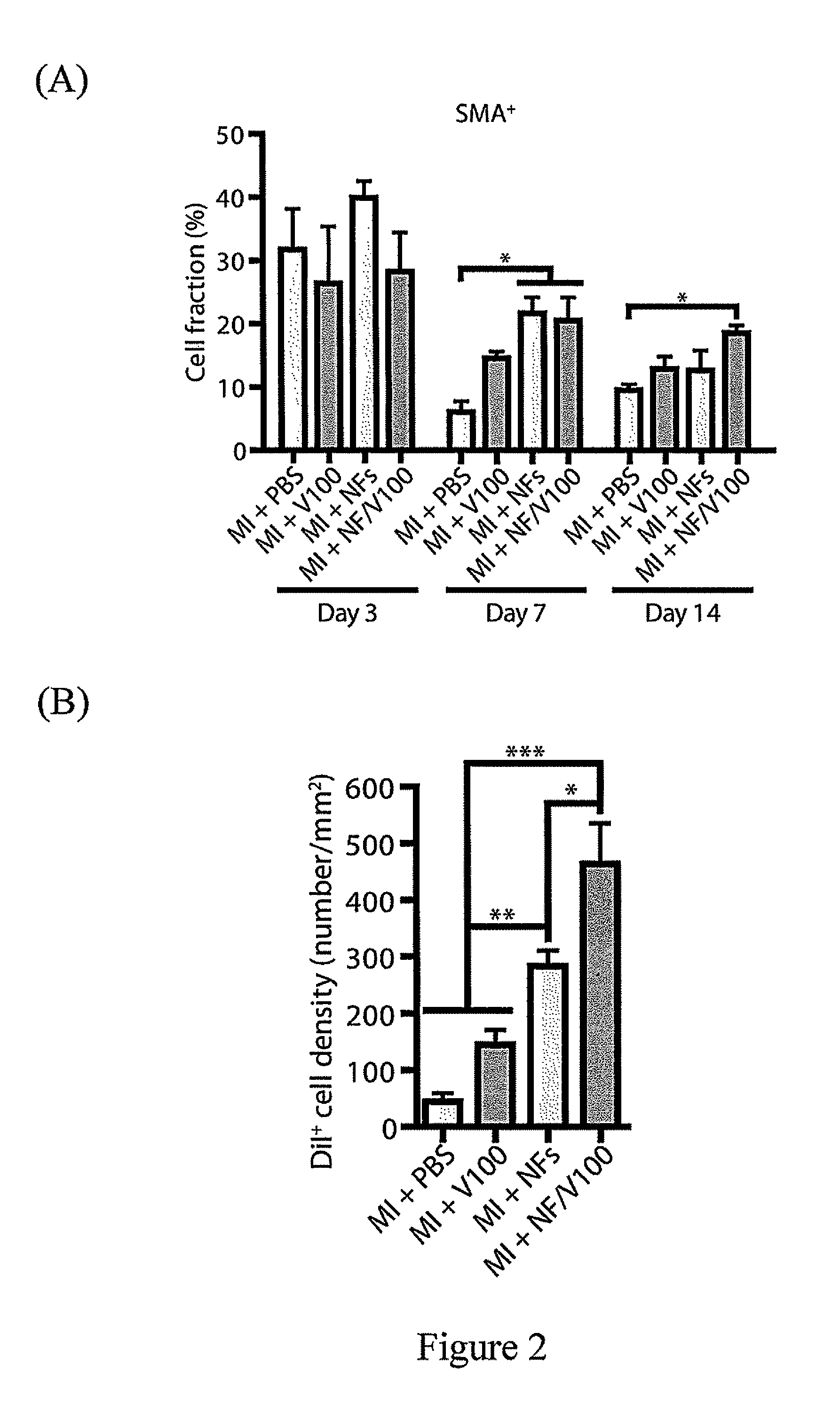
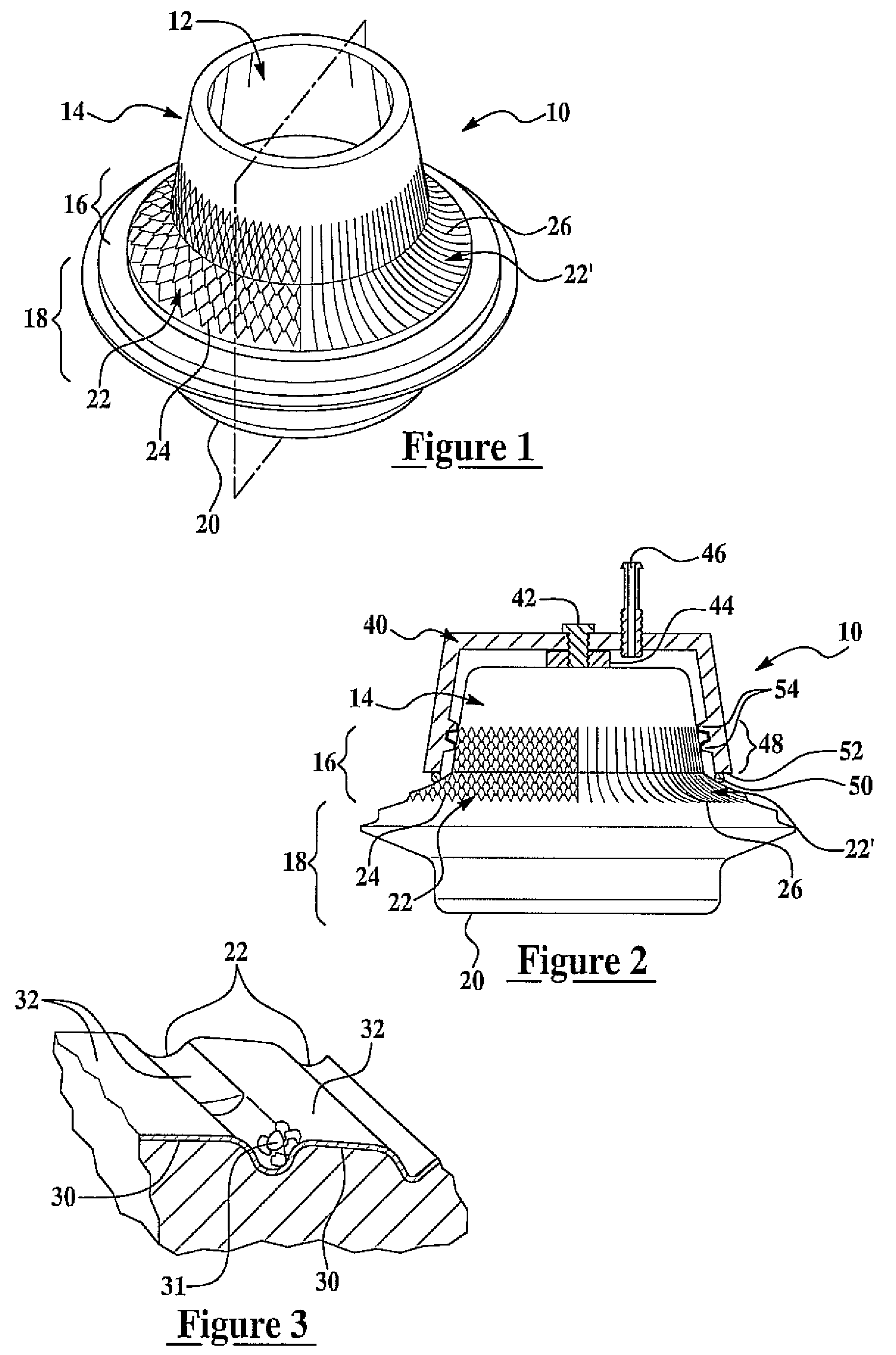
Patents
Literature
203 results about "Autologous cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions for organ and tissue functionality
Materials and methods for treating tissue defects in human or animal tissues using implantable cells are described. Further, culture techniques and factors for enhancing these procedures, and cell survival and adaptation are described. Many of the tissue defects may be treated with autologous cells, while applications involving non-autologous cells or stem cells are also described.
Owner:DASK TECH
Compositions and methods for the augmentation and repair of defects in tissue
InactiveUS20070065415A1Improve successful adaptationBiocidePeptide/protein ingredientsRepair tissueCultured cell
Compositions and methods of treating a defect in a patient are disclosed, including expanding a culture of autologous cells in vitro to form cultured cells, collecting the cultured cells for introduction into the patient, and depositing the cultured cells with ancillary proteins.
Owner:DASK TECH
Regulation of gene expression
InactiveUS6022863APrevention of fetal rejectionSugar derivativesGenetic material ingredientsMHC class IDisease
The present invention relates to utrons, RNA molecules which contain promoter regulatory motif(s) and DNA analogs thereof and DNA molecules that can be transcribed to produce the foregoing. In particular, the invention provides gene promoter suppressing nucleic acids which suppress transcription from a promoter of interest. In a preferred embodiment, the invention provides the TSU gene, nucleotide sequences of the TSU gene and RNA, as well as fragments, homologs and derivatives thereof. Methods of isolating TSU genes are also provided. Therapeutic and diagnostic methods and pharmaceutical compositions are also provided. In particular, the invention relates to methods for cell replacement therapy, gene therapy or organ transplantation wherein TSU nucleic acids suppress MHC class I and II gene expression, thus preventing immuno-rejection of non-autologous cells or organs. The invention also provides methods for treatment of diseases or disorders by suppression of MHC class I, MHC class II, ICAM-1, B7-1, B7-2, and / or Fc gamma R expression by provision of TSU function.
Owner:YALE UNIV
Reprogramming compositions and methods of using the same
InactiveUS20120207744A1Improve versatilityIncrease pluripotencyBiocideOrganic active ingredientsRegenerative medicineCell biology
The present invention provides compositions and methods of using the compositions to alter the developmental potency of a cell. The present invention provides in vivo and ex vivo cell reprogramming and programming methods suitable for autologous cell therapy and regenerative medicine.
Owner:FATE THERAPEUTICS
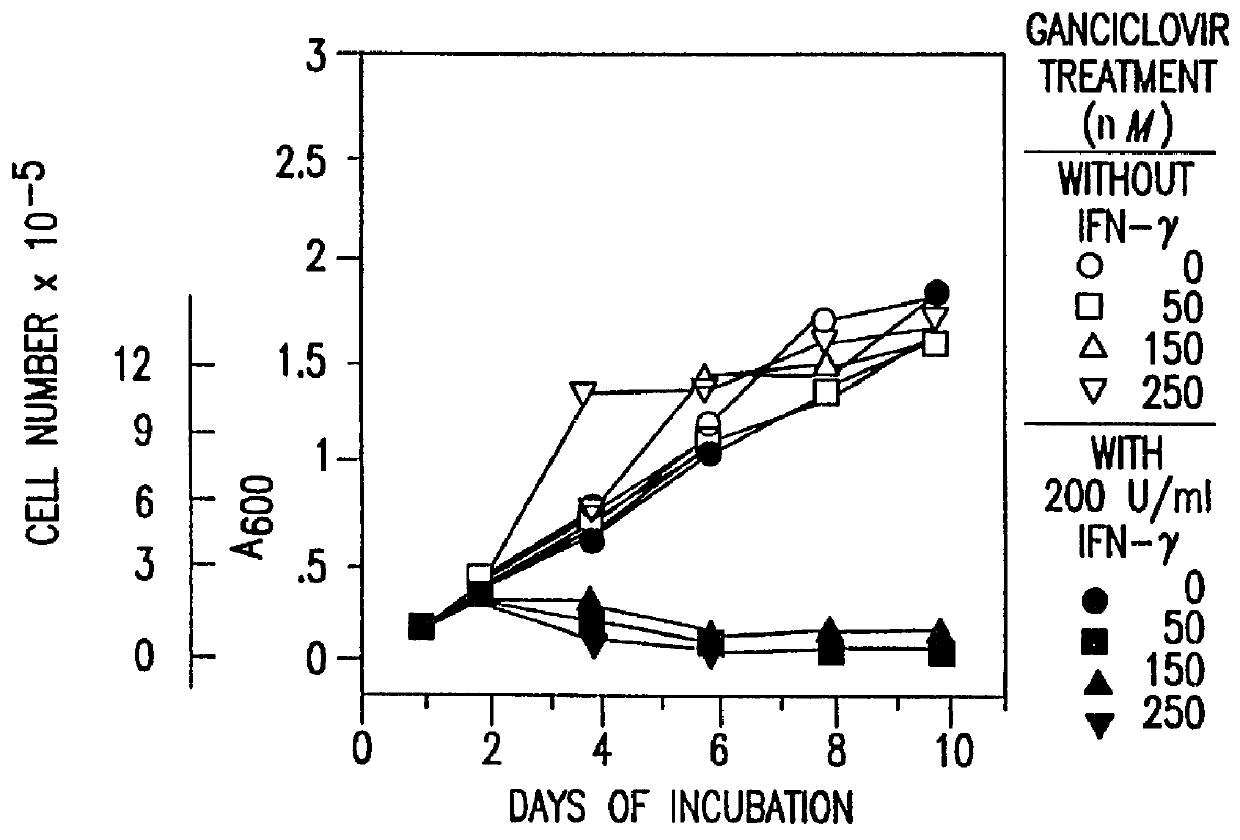
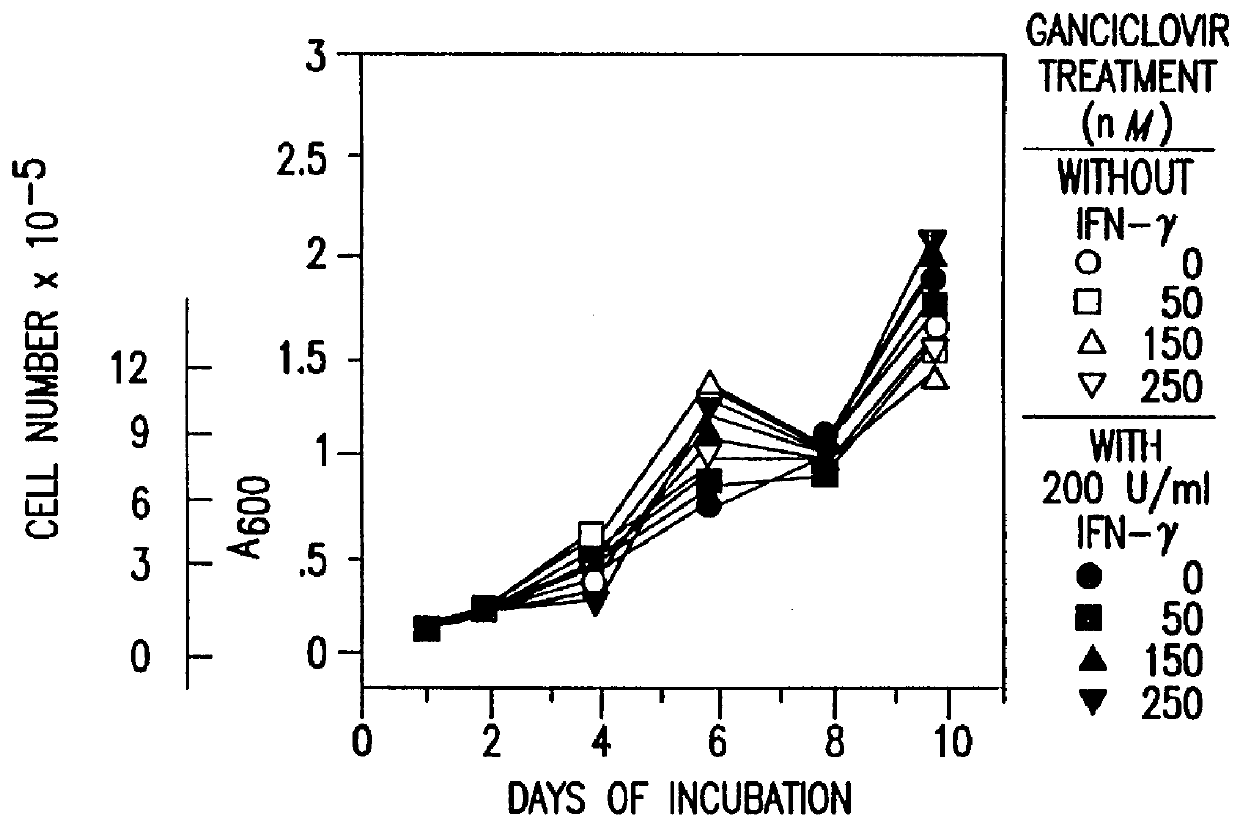
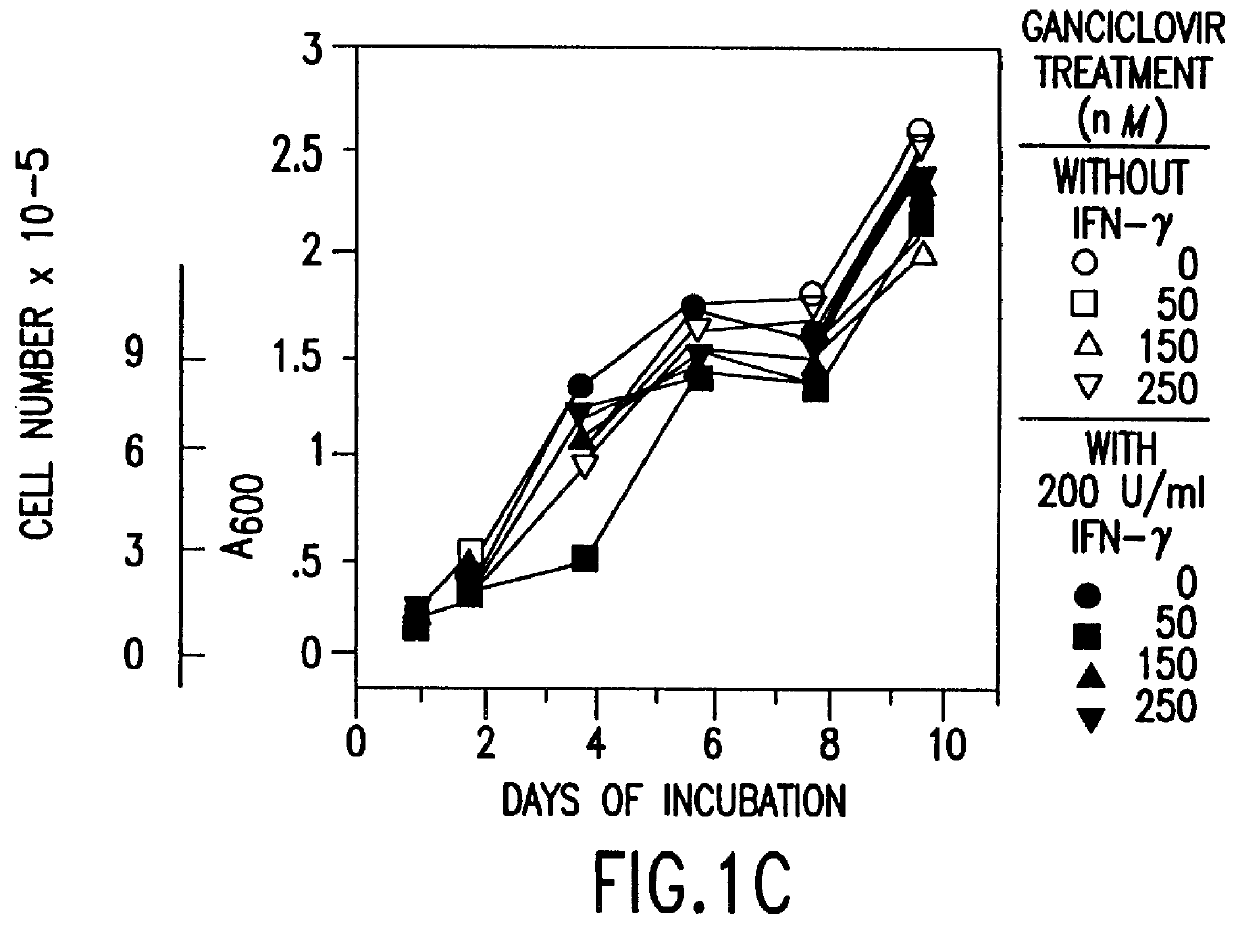
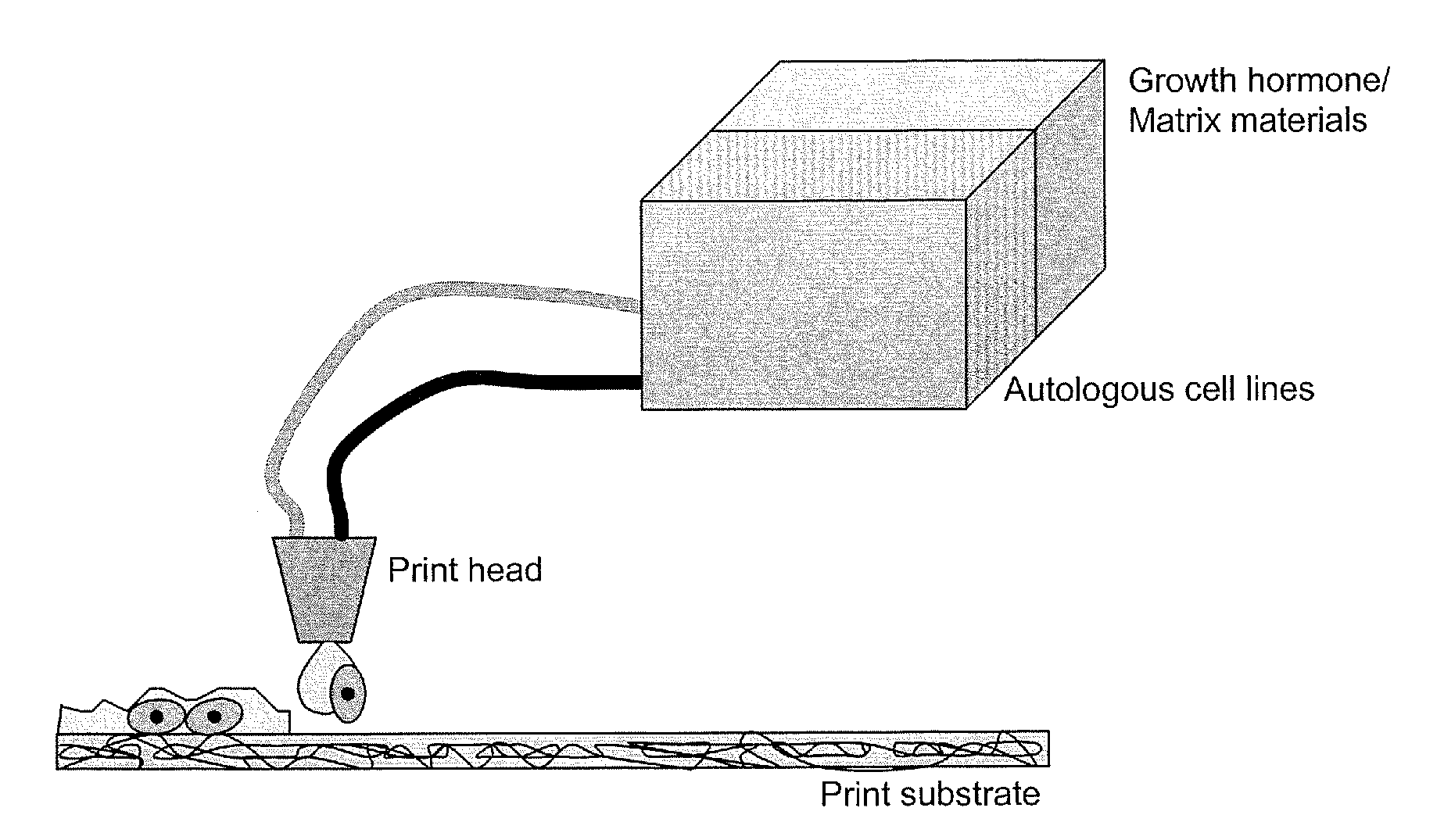
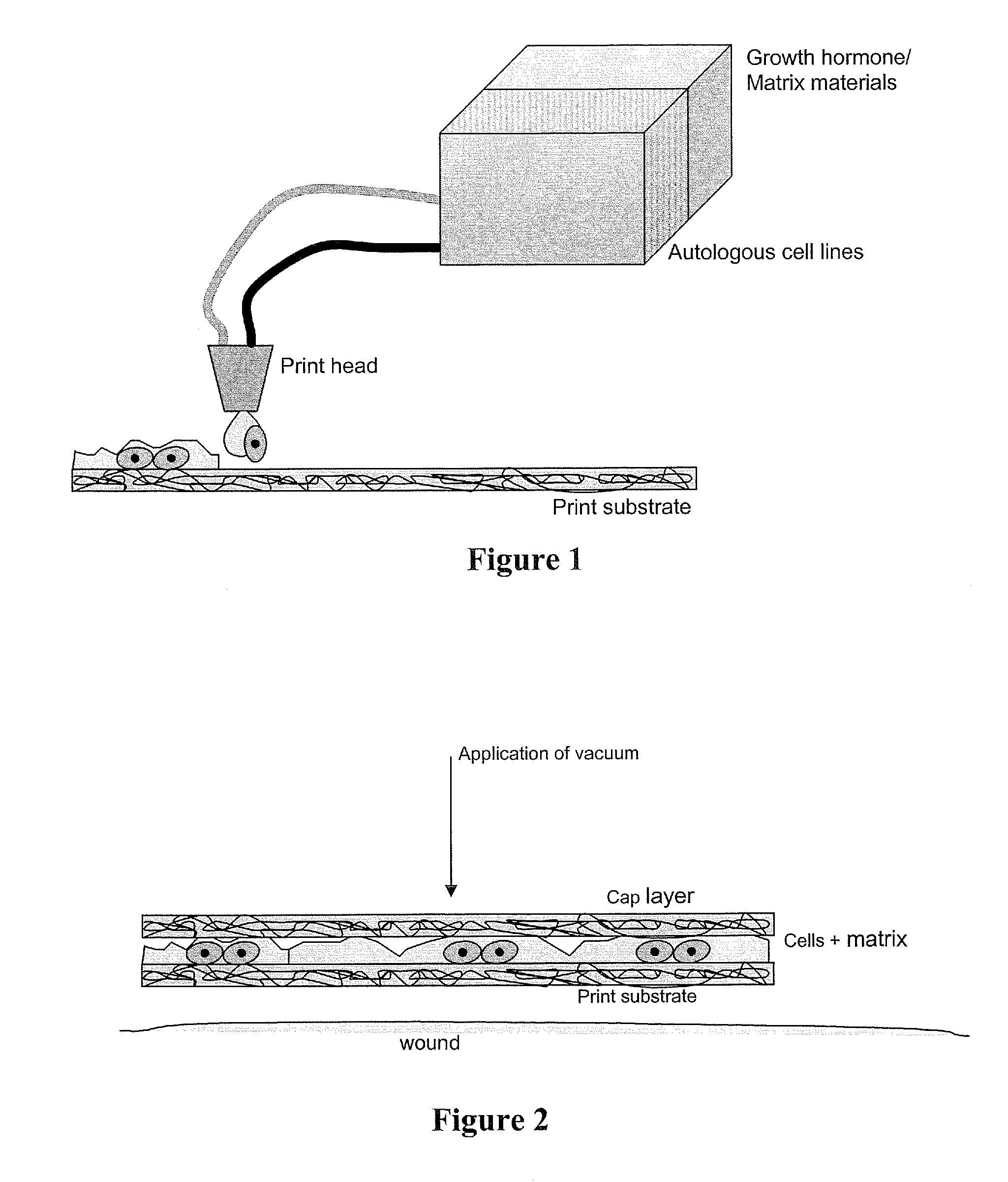
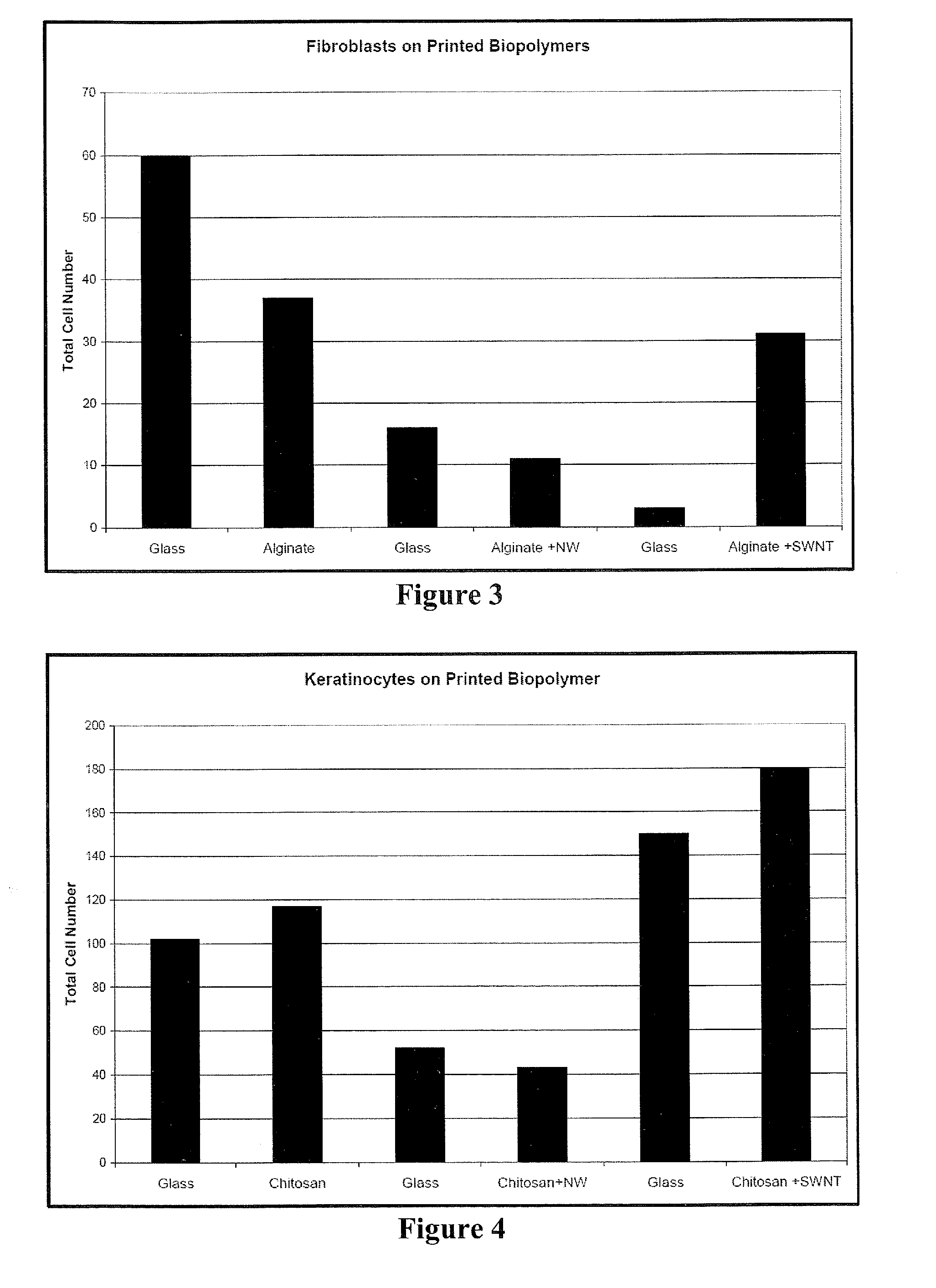
Methods and compositions for printing biologically compatible nanotube composites of autologous tissue
A method of carrying out an autologous tissue implant in a subject in need thereof is carried out by: (a) forming an autologous tissue implant from autologous cells collected from a subject (e.g., by ink-jet printing, the autologous cells and the scaffold, separately or together), and then (b) implanting the autologous tissue implant in said subject.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Cell-Scaffold Constructs
InactiveUS20100131075A1Increase capacityBladder volume capacityUrinary bladderWound drainsAutologous cellOrgan of Corti
Owner:ORGAGEN INC
Brain-Localizing Bone Marrow Progenitor cells
InactiveUS20080075698A1Reduce the number of cellsControlledOrganic active ingredientsBiocideSomaBone marrow cell
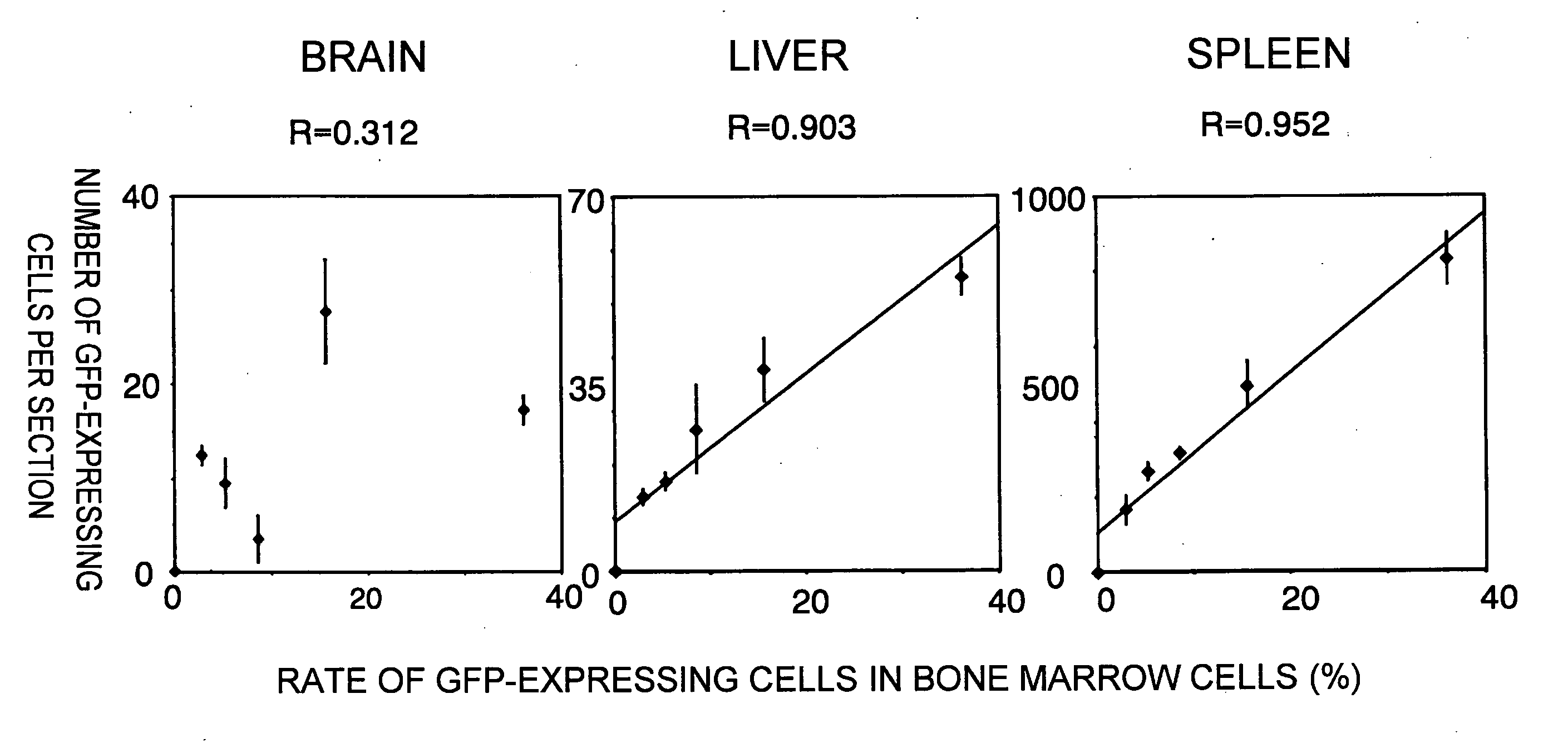
The present inventors discovered that brain-localizing cells exist in the progenitor cell fraction of bone marrow cells. They also elucidated various characteristics of the brain-localizing cells. When these cells are infused into the bloodstream, they circulate in the blood and directly translocate into the cerebral parenchyma from the bloodstream. Furthermore, the present inventors succeeded in developing methods for efficiently preparing brain-localizing cells from bone marrow or bone marrow cells. These methods can be applied to less-invasive regenerative medicines targeting the brain and using autologous cells.
Owner:ACTGEN
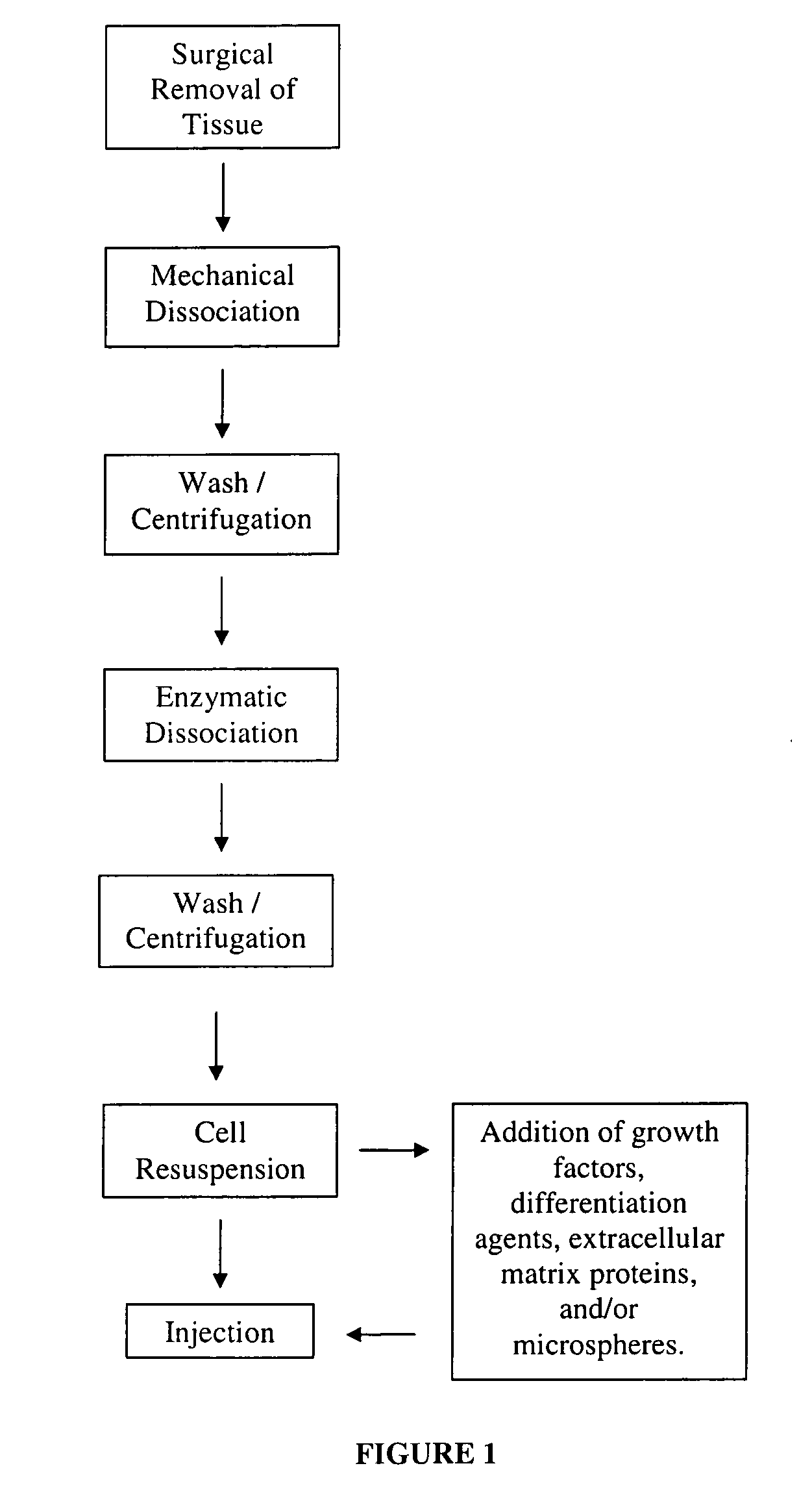
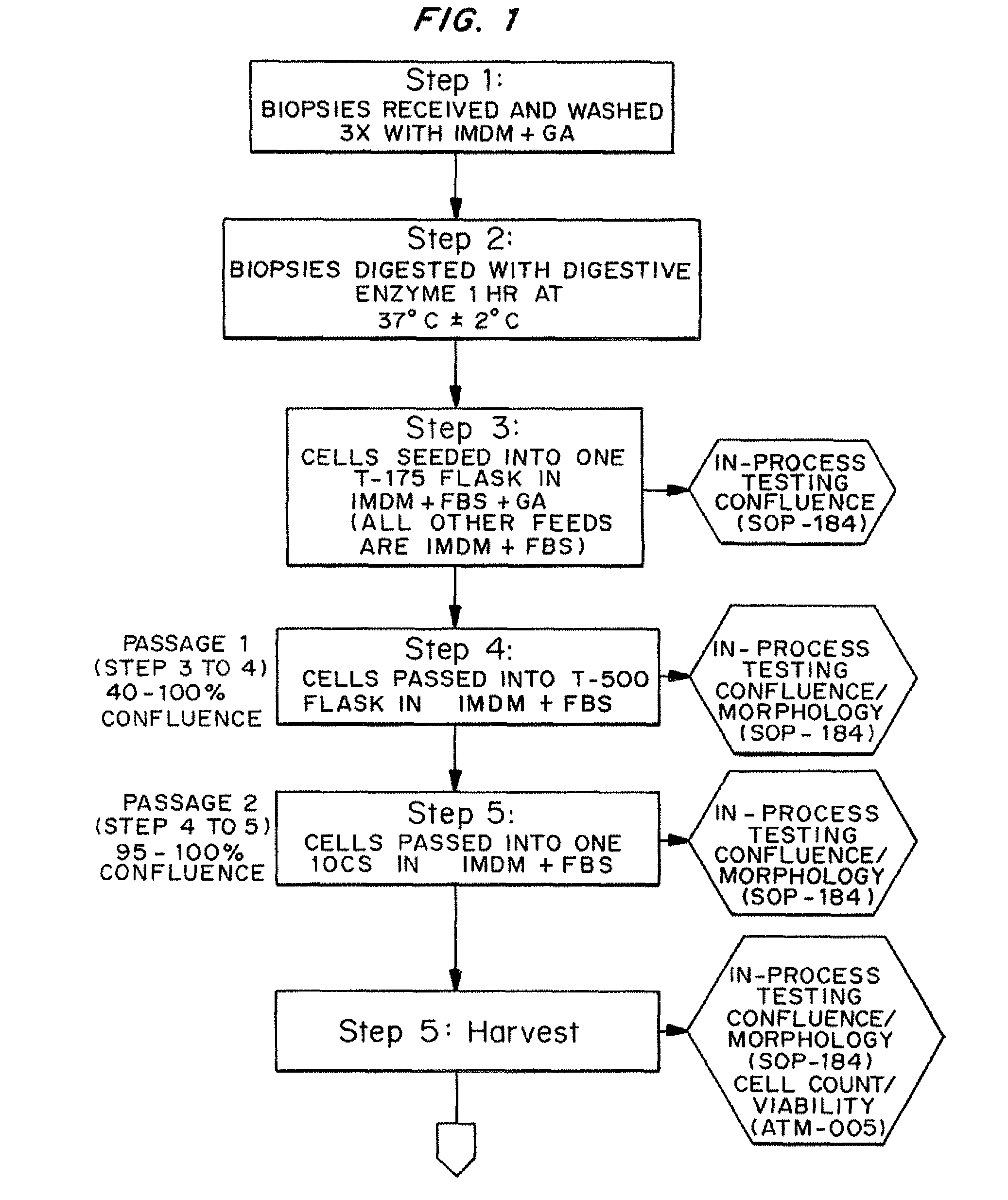
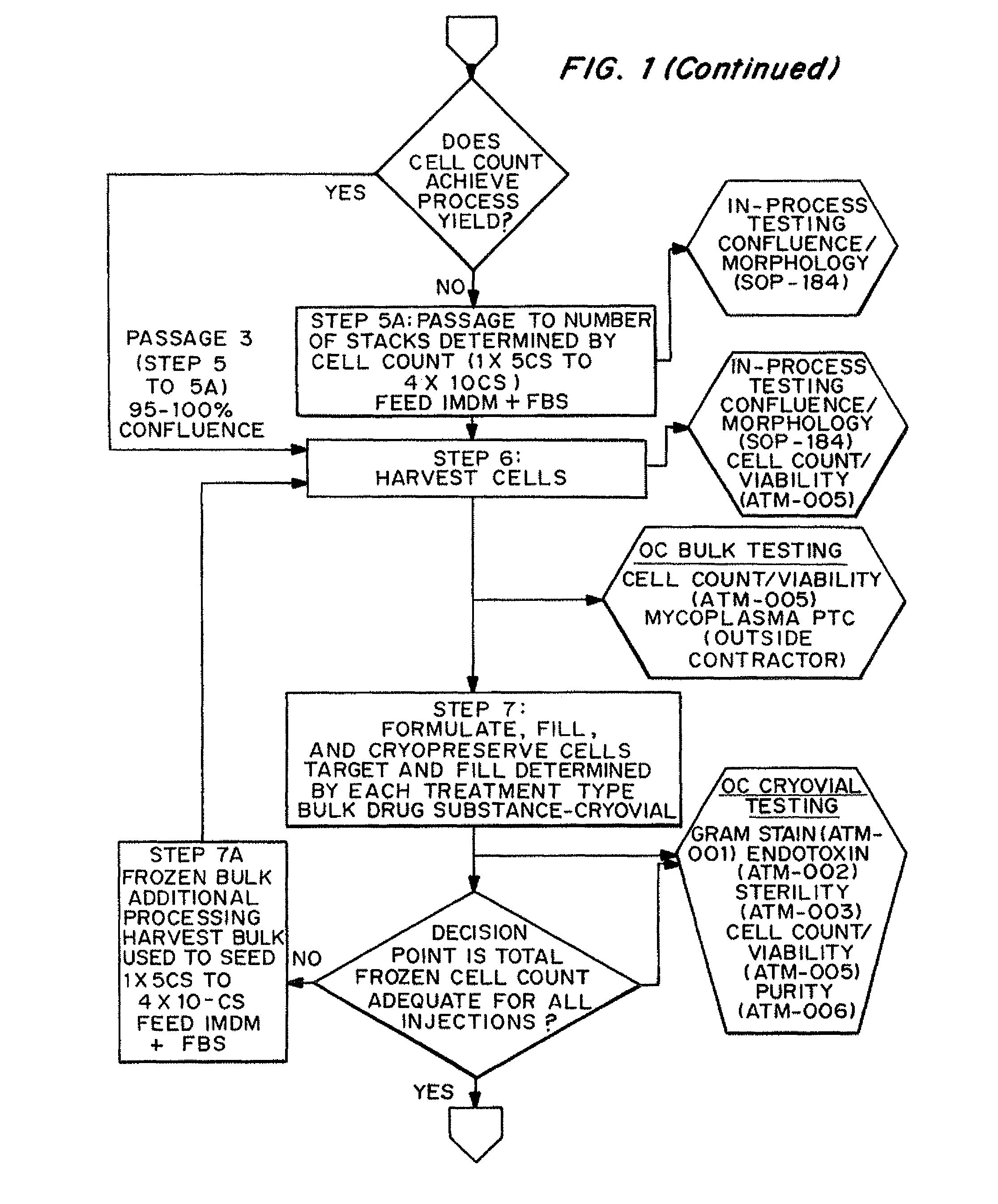
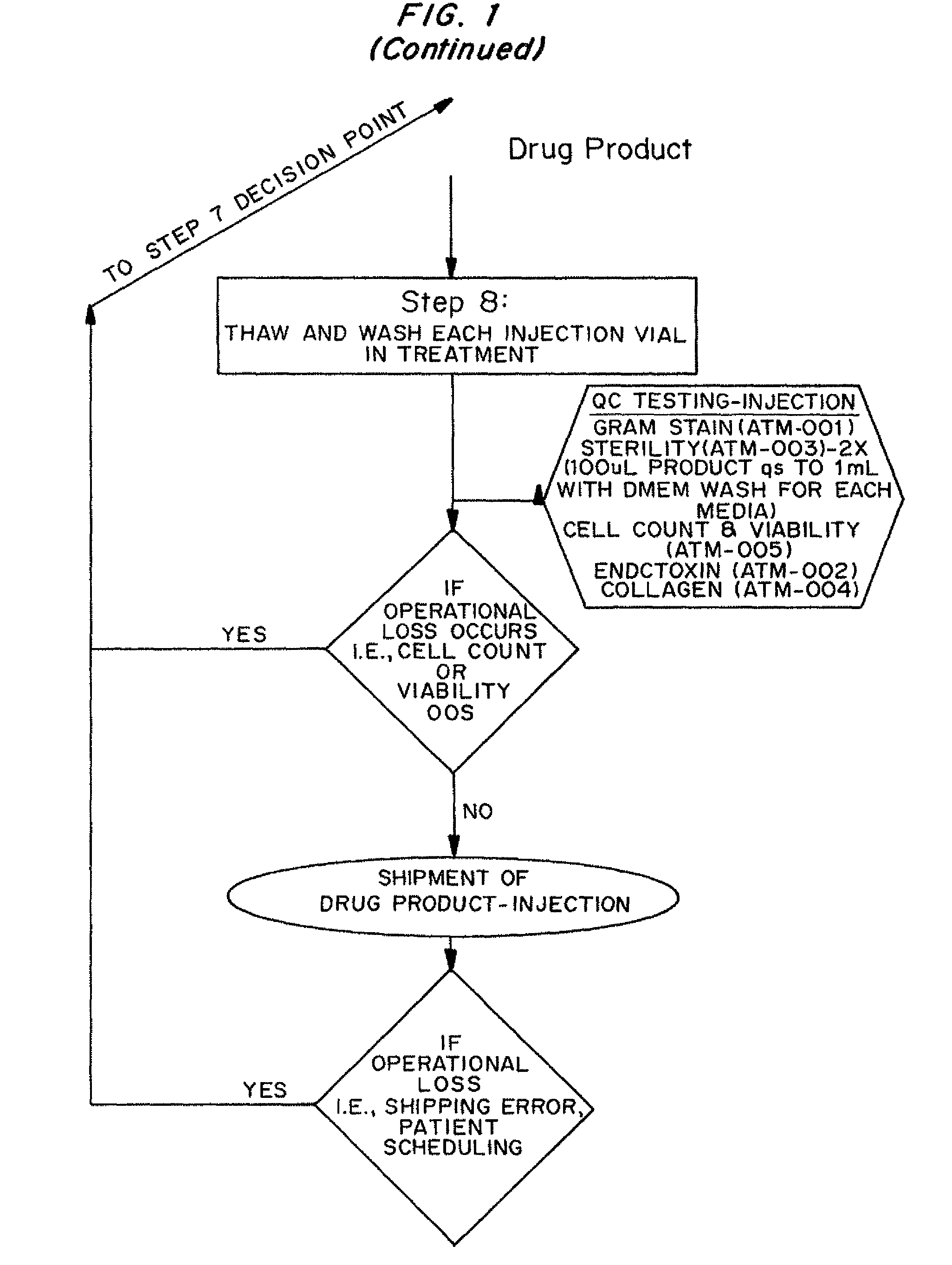
Nonexpansion Protocol for Autologous Cell-Based Therapies
InactiveUS20070224173A1Increased risk of contaminationIncrease the number of cellsBiocideOrganic active ingredientsHeterologousCell-Extracellular Matrix
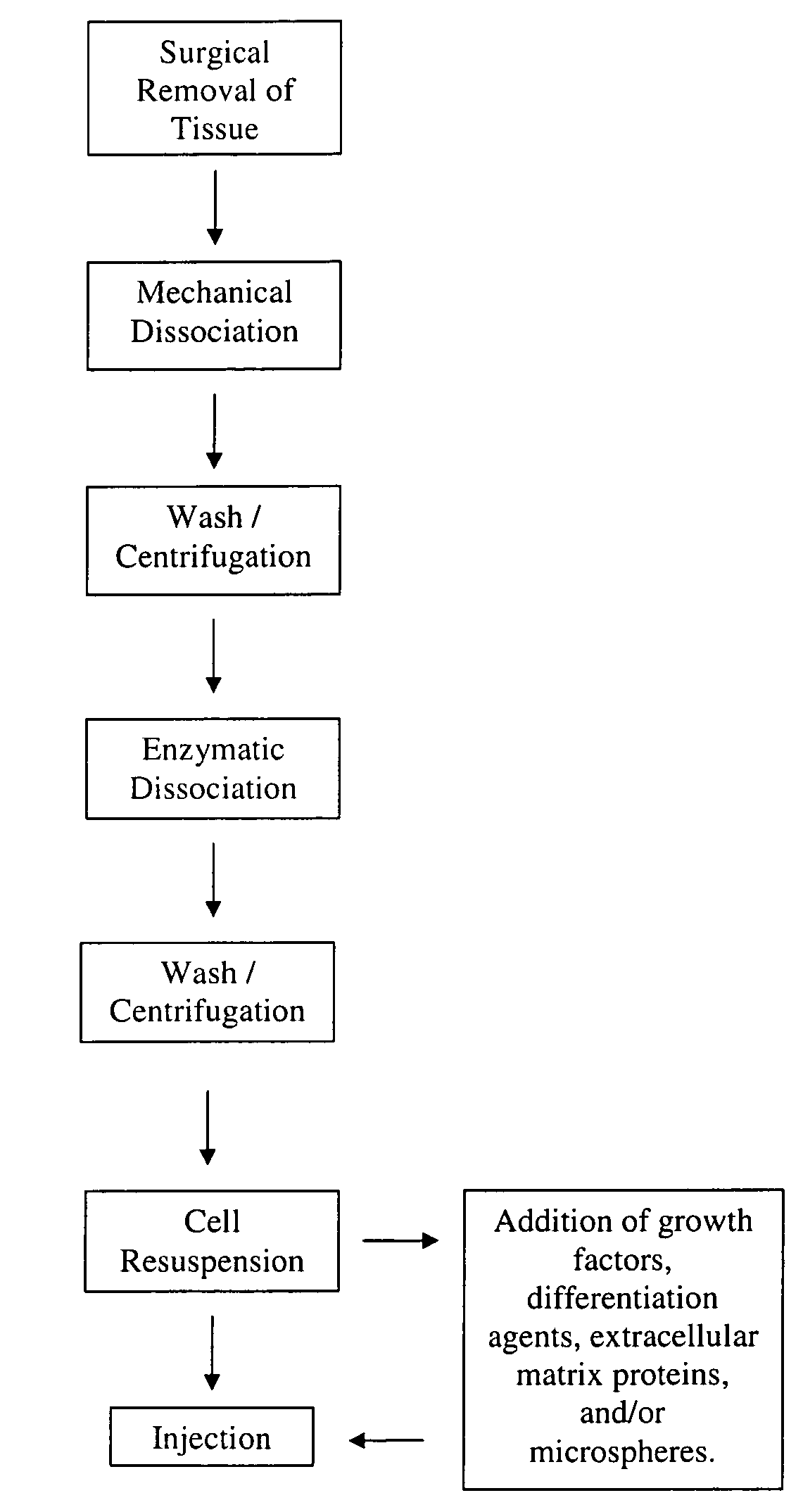
The present application describes various applications of the non-expansion protocol for the preparation of an injectable autologous cell mixture of the present invention that can be used to prevent symptoms in a number of indications. Cells are isolated from surgically derived tissue and are at least partially disaggregated from each other. The heterologous cell mixture is mixed with growth factors, differentiation agents, extracellular matrix proteins and / or microspheres and injected into the patient without cell expansion. The harvesting of tissue, cell isolation, and injection are performed within a single surgical procedure lasting only minutes to hours.
Owner:BOSTON SCI SCIMED INC
Treatment of vocal cords with autologous dermal fibroblast formulation
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated for augmentation or regeneration of vocal cords. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own buccal mucosa or skin using current good manufacturing practices (CGMP) and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL administered three times approximately three to six weeks apart, of cells at a concentration of from 1.0-2.0×107 cells / mL.
Owner:CASTLE CREEK BIOSCIENCES LLC
Artificial articular cartilage based on autologous cells and preparation method thereof
ActiveCN101574543AImprove mechanical propertiesAvoid inconvenienceJoint implantsElectrospinningHYDROSOL
The invention provides artificial articular cartilage based on autologous cells and a preparation method thereof. The artificial articular cartilage comprises a nano bionic support and hydrosol adhered to the nano bionic support, wherein one or more cytokines are coated into the hydrosol. The invention also provides the method for preparing the artificial articular cartilage, which comprises the following steps: preparing an electrostatic spinning solution, a cytokine-containing hydrosol solution and a crosslinking agent solution; using the crosslinking agent solution to receive electrostatic spinning so as to prepare the nano bionic support; and using an ink jet printer to print the cytokine-containing hydrosol solution on the nano bionic support, and curing the hydrosol to obtain the artificial articular cartilage. The three-dimensional support adopted by the invention has ideal degradation speed and is degraded after the regeneration of an articular cartilage layer, can meet the requirement of actual clinical application, can be completely degraded, and has good abrasion resistance and lubricity so as to be capable of replacing the cartilage before the regeneration of the articular cartilage layer.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Methods and compositions for organ and tissue functionality
Materials and methods for treating tissue defects in human or animal tissues using implantable cells are described. Further, culture techniques and factors for enhancing these procedures, and cell survival and adaptation are described. Many of the tissue defects may be treated with autologous cells, while applications involving non-autologous cells or stem cells are also described.
Owner:DASK TECH
Treatment of degenerated disc with autologous cells
InactiveUS20090068270A1Increase the number of cellsAvoid procedurePowder deliveryBiocideIntervertebral diskAutologous cell
The present invention relates to administering autologous uncultured cells into a diseased intervertebral disc.
Owner:DEPUY SYNTHES PROD INC
Dosage unit formulations of autologous dermal fibroblasts
ActiveUS8529883B2Reduce severityAdditional componentBiocideArtificial cell constructsWrinkle skinNasolabial fold
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own skin using current good manufacturing practices (CGMP), and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL, of cells at a concentration of from 1.0-2.0×107 cells / mL. When injected into the nasolabial fold wrinkles (creases on the sides of the nose that extend to the corners of the mouth), the autologous fibroblasts are thought to increase the synthesis of extracellular matrix components, including collagen, reducing the severity of these wrinkles. Dosage and timing of administration have been demonstrated to be critical to achieving clinically significant outcomes.
Owner:CASTLE CREEK BIOSCIENCES LLC
Pharmaceutical composition for promoting arteriogenesis, and preparation method and applications for the same
InactiveUS20130095060A1Improves post-infarction angiogenesisImproves arteriogenesisNervous disorderPeptide/protein ingredientsPeptideAutologous cell
The present invention relates to a pharmaceutical composition for promoting arteriogenesis, and preparation method and applications of the same, wherein said pharmaceutical composition comprises an effective amount of a drug, and a peptide hydrogel, and it forms a microenvironment for autologous cell recruitment and tissue regeneration.
Owner:NAT CHENG KUNG UNIV
Percutaneous access device system facilitating cell growth thereon
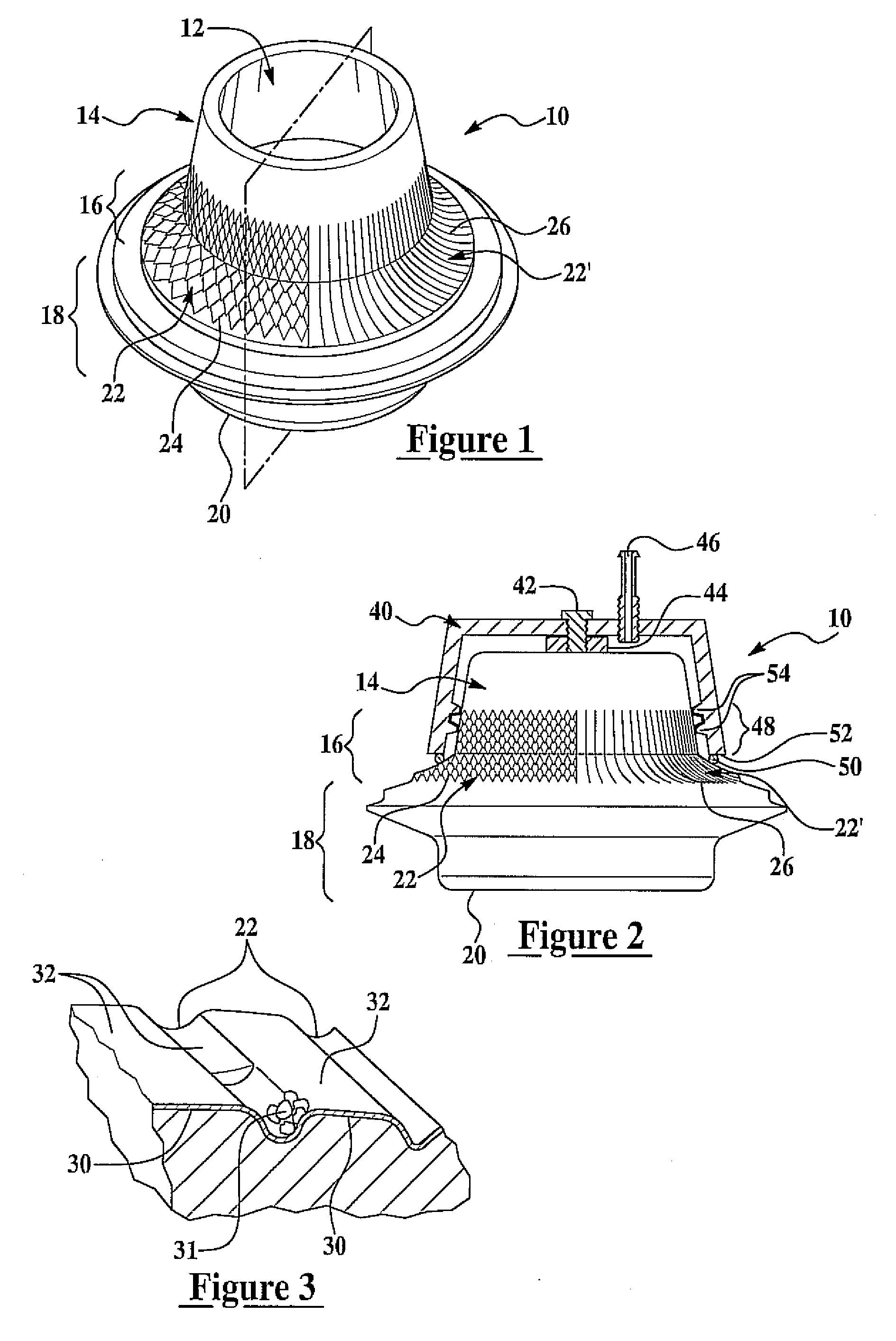
ActiveUS7704225B2Promote autologous cell growthPromote growthBiocideElectrotherapyEngineeringBacterial growth
A biocompatible implantable portal is provided that has a wall defining a communicative passage through an interior bore. The exterior of the portal has a neck region adapted to promote autologous cell growth on the neck region. A series of channels are provided on the exterior neck region to facilitate autologous cell growth while disfavoring fluid pooling and bacterial growth. Typical channel widths are from 20 to 300 microns, with adjacent channels being separated by plateaus having a width of between 0 and 600 microns. Providing the portal exterior neck region with a texture varying on a nanometer length scale facilitates autologous cell growth. Applying a coating such as a tissue scaffolding matrix to the neck region prior to implantation also facilitates cell growth. A coupling or a manifold encompassing the neck region facilitates the draw of vacuum and / or mechanical protection for the growing cells.
Owner:VIADERM LLC
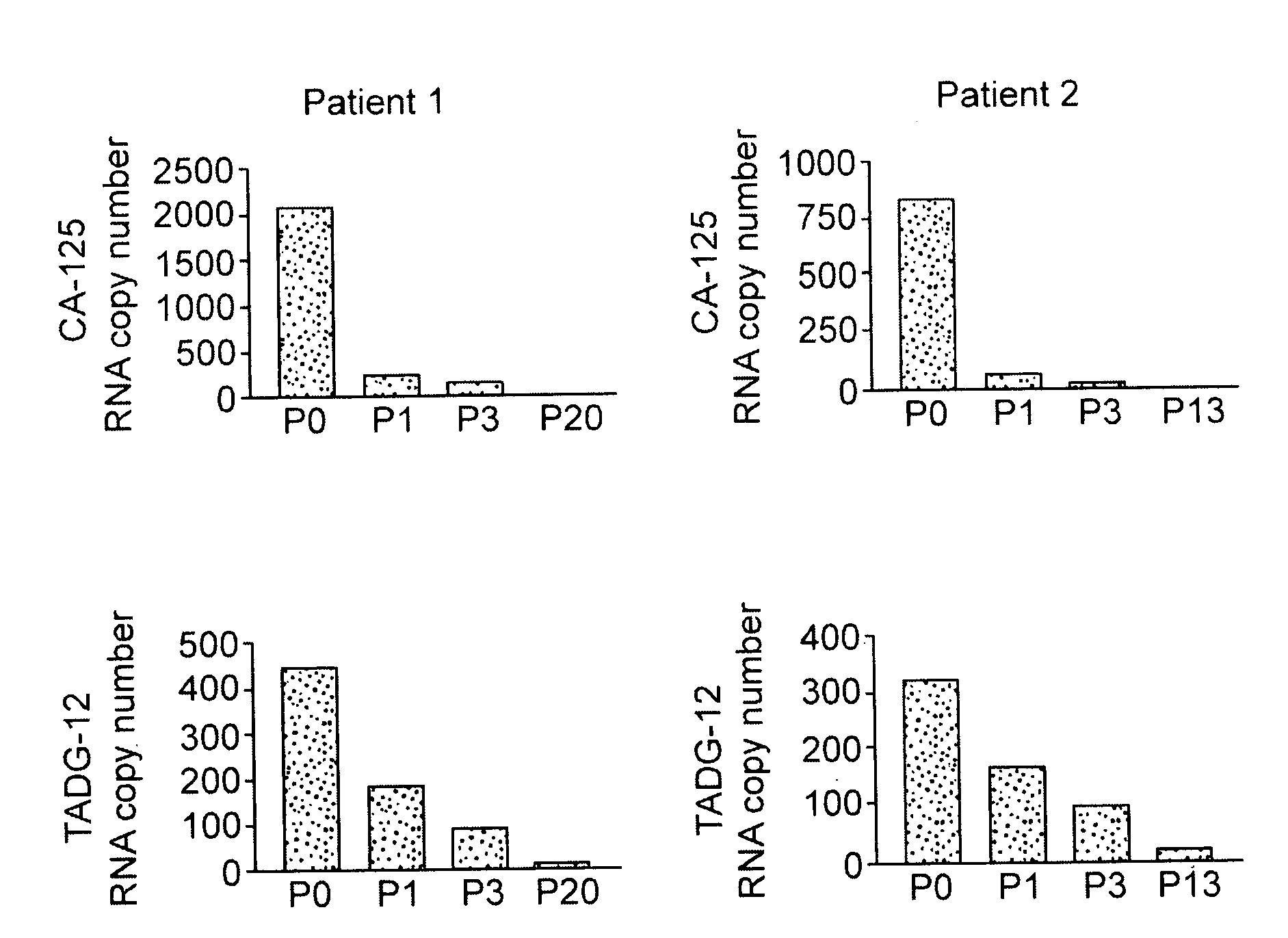
Target peptides for ovarian cancer immunotherapy
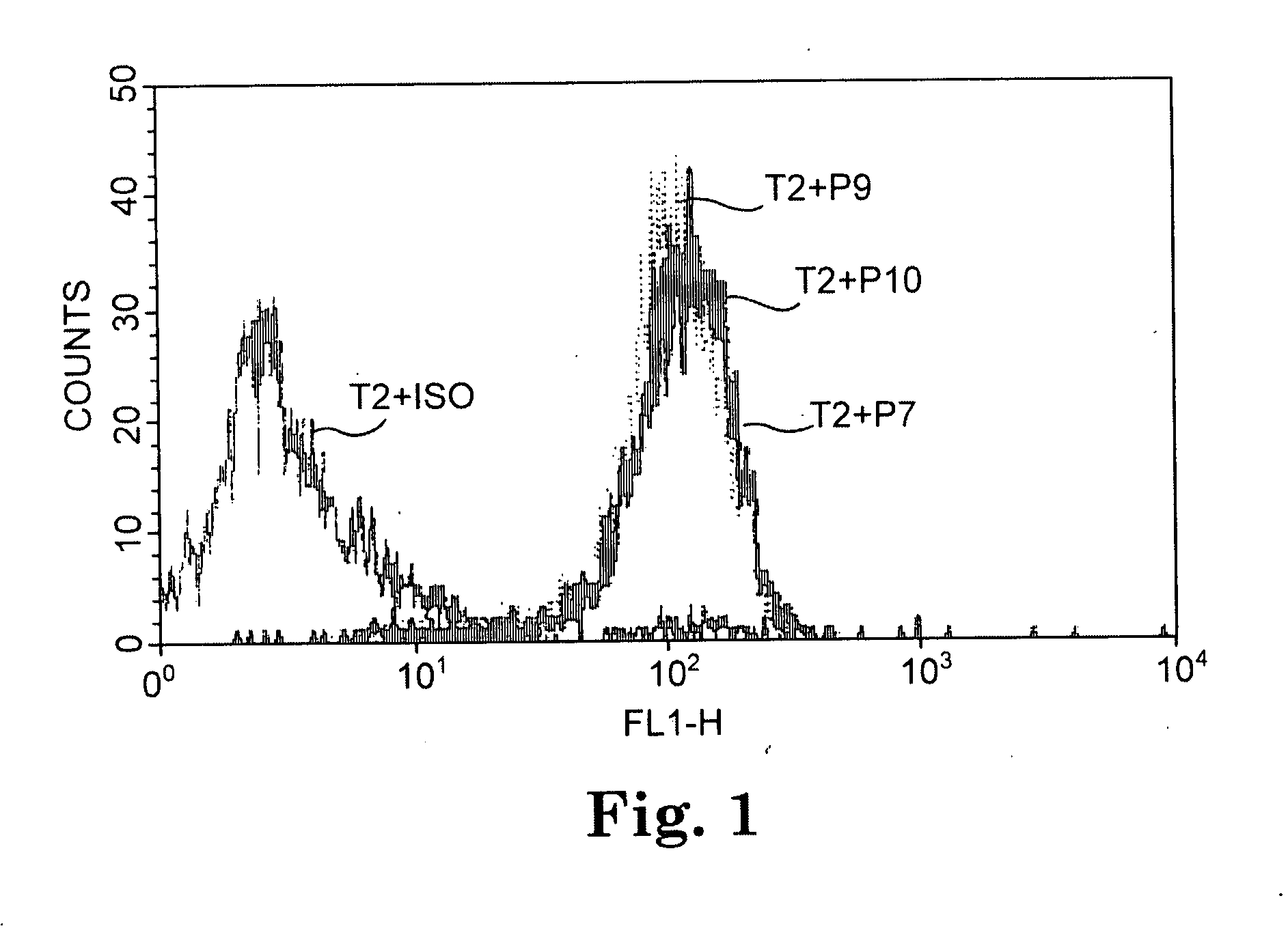
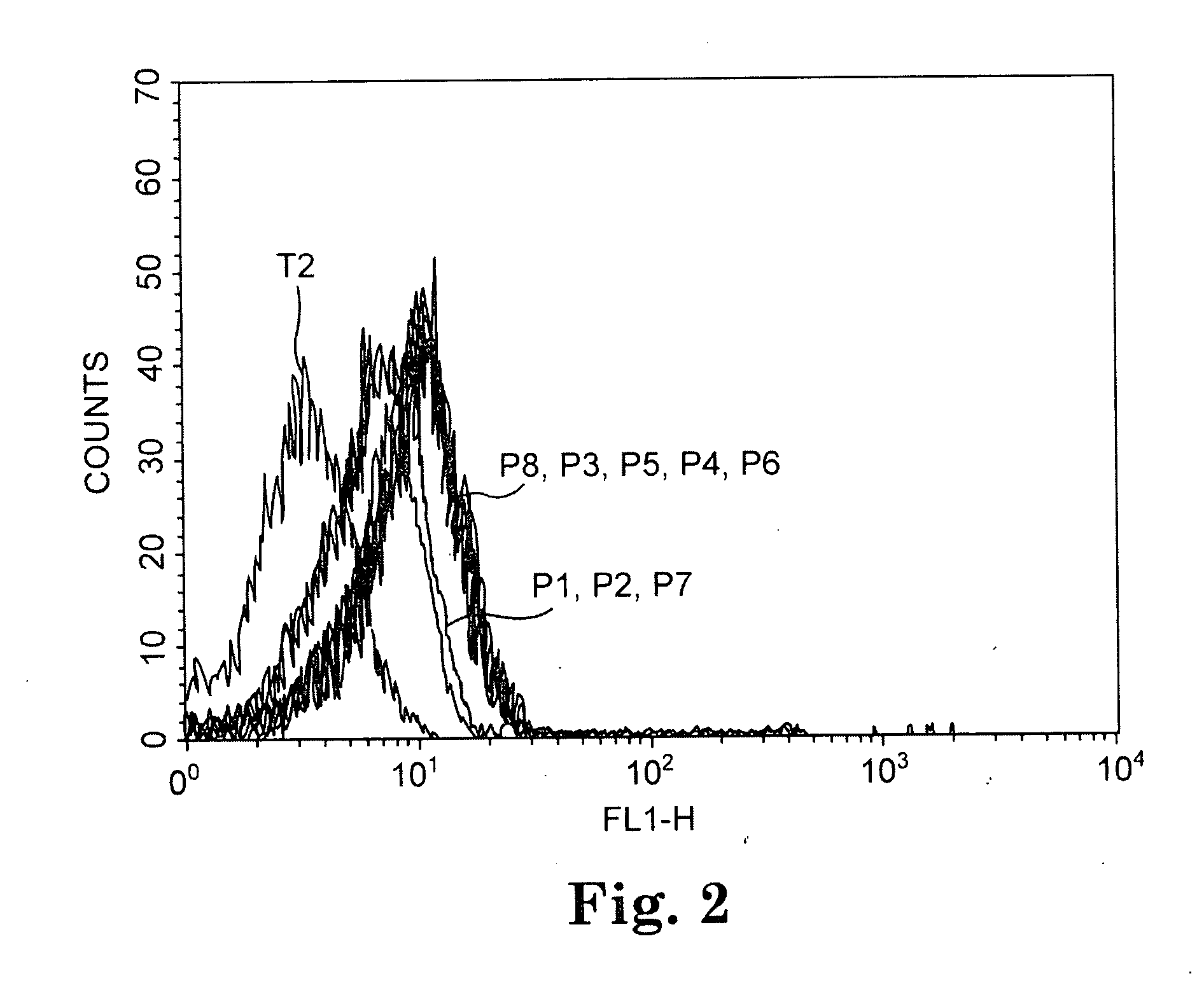
TADG-12 and CA125 are two proteins expressed with high specificity in ovarian cancer tumors. They thus would be potential antigens for immunotherapy in ovarian cancer. The invention is based on the discovery of peptides in TADG-12 and CA125 that can be used to induce an autologous T cell response that lyses ovarian cancer cells expressing TADG-12 or CA125. The peptides are contacted with dendritic cells in vitro to generate peptide-loaded dendritic cells. The peptide-loaded dendritic cells are contacted with T cells in vitro to amplify CD8+ T cells that recognize the peptide. At least one CA125 peptide and at least one TADG-12 peptide were found that amplified CD8+ T cells, even from cancer patients, that lysed autologous CA125-expressing or TADG-12-expressing tumor cells. The peptide-loaded dendritic cells can be administered to a cancer patient to amplify CD8+ T cells in vivo that attack the cancer cells. Alternatively, autologous CD8+ T cells can be amplified ex vivo and then infused into the cancer patient.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Culture medium efficiently amplifying autologous NK cells and cultural method
The invention relates to a culture medium efficiently amplifying autologous NK cells and a culture method. The culture medium internally contains interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNFalpha) and CD3-antibody, can efficiently amplify and activate NK cells so as to obtain a large quantity of highly active immune cells with majority of NK cells to be transferred back to human bodies, and has remarkable curative effect in anti-tumor, anti-virus infection and immune adjustment related diseases. The culture medium efficiently amplifying autologous NK cells comprises the culture medium for cultivating cells and added factors added into the culture medium for cultivating cells, is used for cultivating and amplifying a large quantity of autologous activated NK cells, and is characterized in that the added factor comprises interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNF alpha) and CD3-antibody.
Owner:康思葆(北京)生物技术有限公司
Augmentation and repair of spincter defects with cells including adipocytic cells
InactiveUS20080299213A2Promote resultsPeptide/protein ingredientsMammal material medical ingredientsLigament structureSurgical department
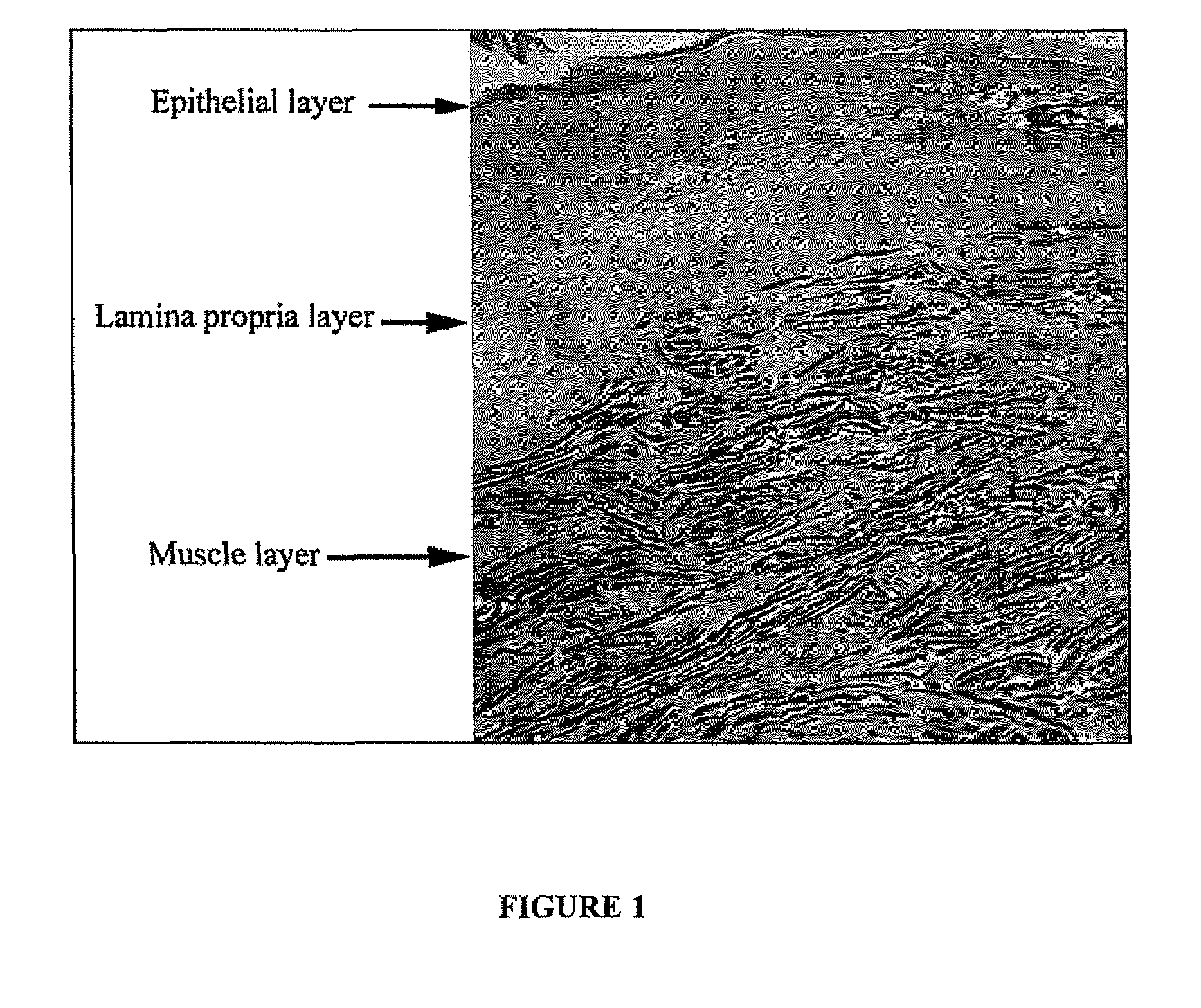
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
Cell-scaffold constructs
InactiveUS8337485B2Increase capacityBladder volume capacityUrinary bladderWound drainsAutologous cellOrgan of Corti
Owner:ORGAGEN INC
Method for cell implantation
An endoscopic method for treating cartilage or bone defects in an animal, said method comprising the steps of: I) identifying the position of the defect, ii) applying cells selected from the group consisting of chondrocytes, chondroblasts, osteocytes and osteoblasts and combinations thereof into the cartilage or bone defect. In particular, the invention relates to a method for arthroscopic or endoscopic implantation of homologous or autologous cells into a defect of an animal body, the method comprising a step of I) arthroscopic or endoscopic application of a fluid to a cavity or surface containing the defect, and the steps of ii) application of the cells to the defect substantially simultaneously with a support material, the application being performed at the defect covered by the fluid, iii) mixing of the cells and the supporting material, iv) solidification of the supporting material so that the defect is covered by a mixture of cells and support material without any significant amount of fluid, and v) optionally, removal of the fluid from the cavity or surface by drainage or suction.
Owner:INTERFACE BIOTECH
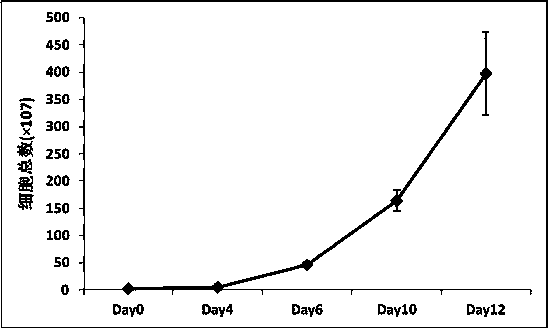
Method for efficiently separating and expanding mesenchymal stem cells in human umbilical cord blood
ActiveCN102876630AStable growthHigh puritySkeletal/connective tissue cellsBlood/immune system cellsMedicineMesenchymal stem cell
The invention relates to an optimal method for efficiently separating and expanding mesenchymal stem cells in umbilical cord blood in cell therapy. The fact that the mesenchymal stem cells with higher differentiative capacity and smaller immunological rejection are contained in the umbilical cord blood has been proved. The establishment of an umbilical cord blood bank provides a basis for achieving transplantation of autologous cells. However, since the content of the mesenchymal stem cells in the umbilical cord blood is very low (only 0.5 to 30 mesenchymal stem cells are contained in 1*108 mononuclear cells), how to efficiently separate and expand the mesenchymal stem cells becomes a problem hindering the transformation of the mesenchymal stem cells in the umbilical cord blood to the clinical application. At present, in most situations, the mesenchymal stem cells in the umbilical cord blood are separated in virtue of adsorption capacity of the mesenchymal stem cells in a culture vessel, but the success ratio is low, and the characteristics of stem cells are difficult to maintain in an expanding process. The method comprises the steps of coating the culture vessel with protein components, adding multiple growth factors in the culture vessel in a coordination manner, and building the expansion environment of the mesenchymal stem cells in the umbilical cord blood, so as to achieve the efficient separation and expansion of the mesenchymal stem cells.
Owner:夏亮
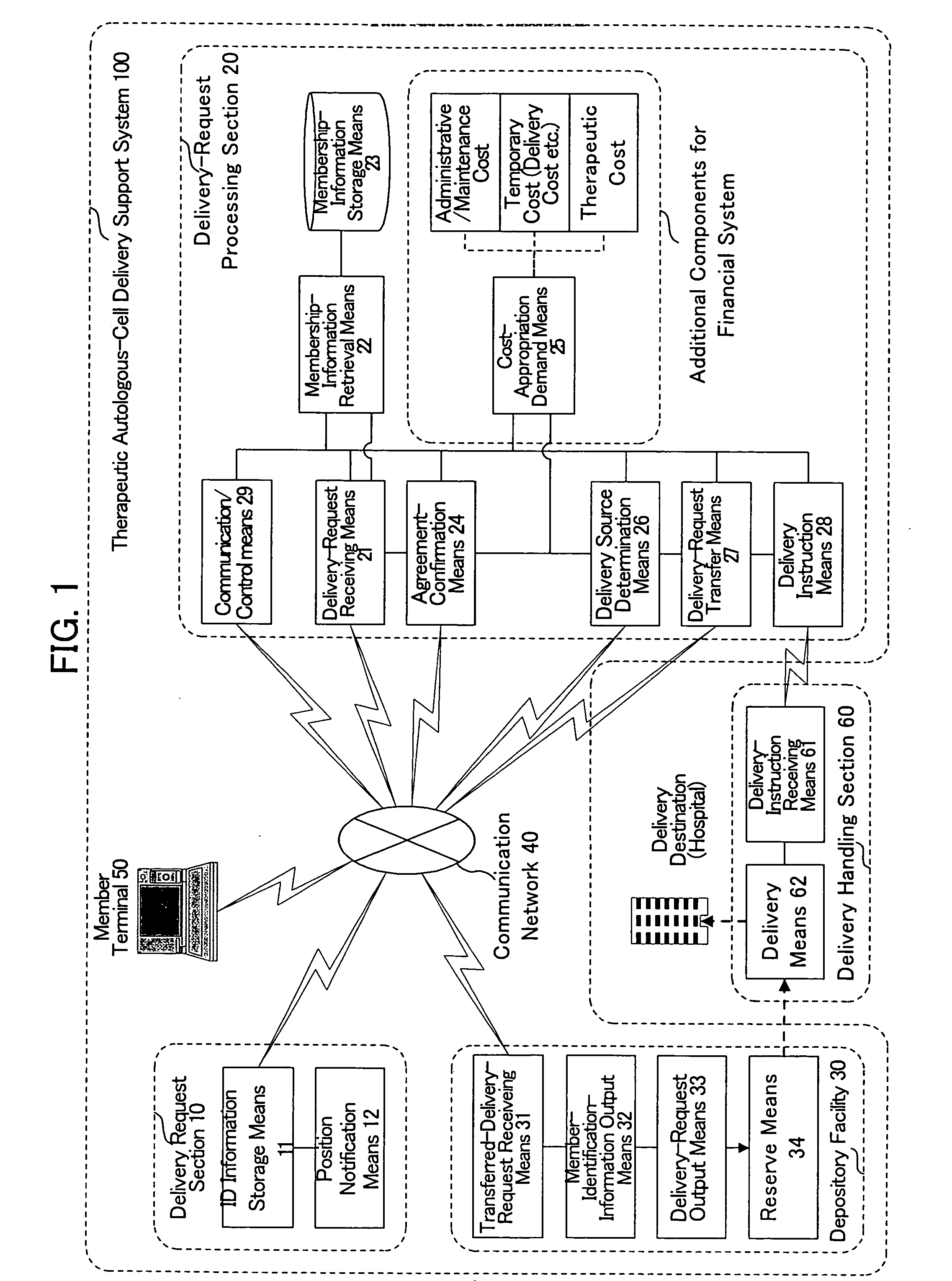
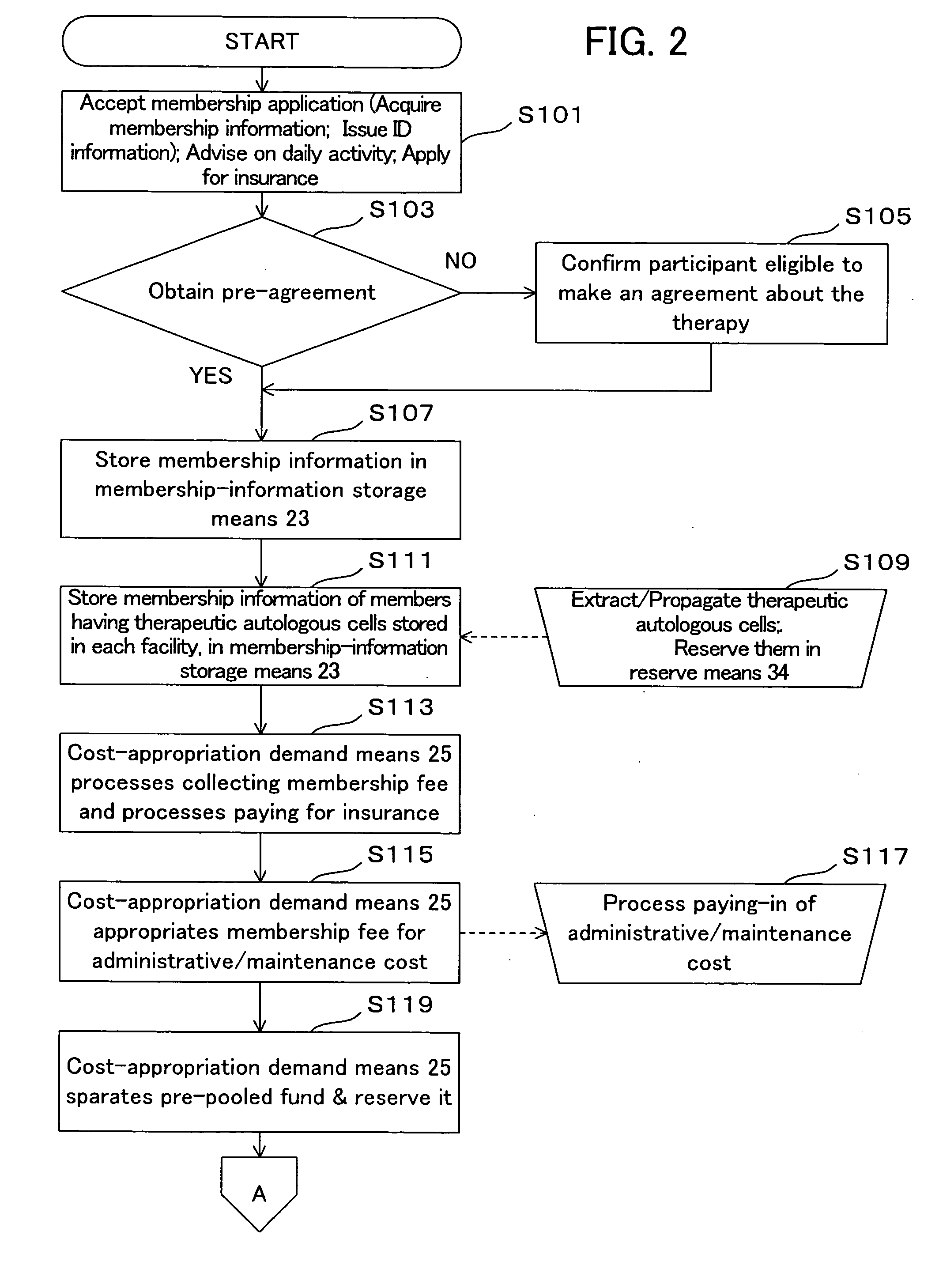
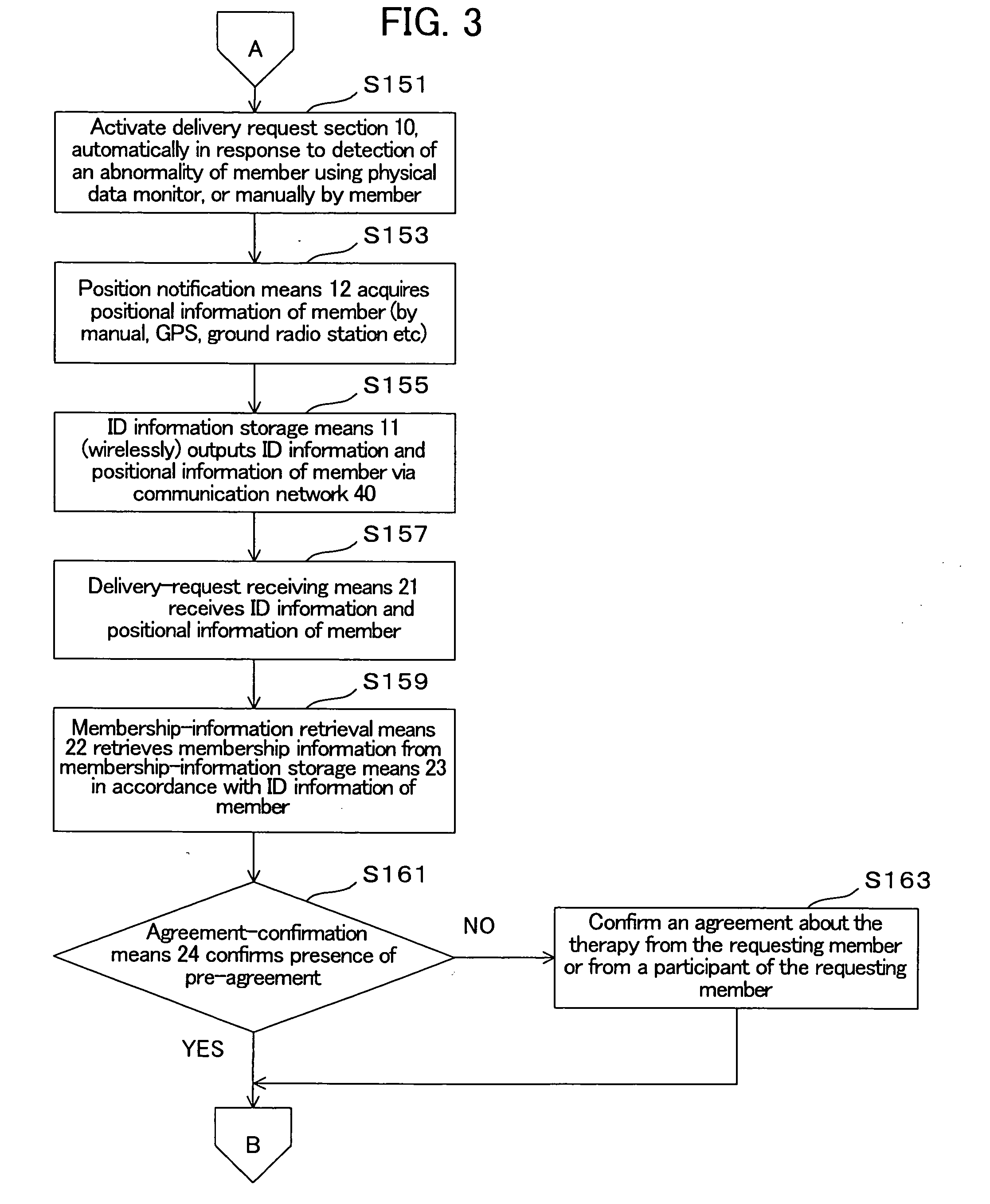
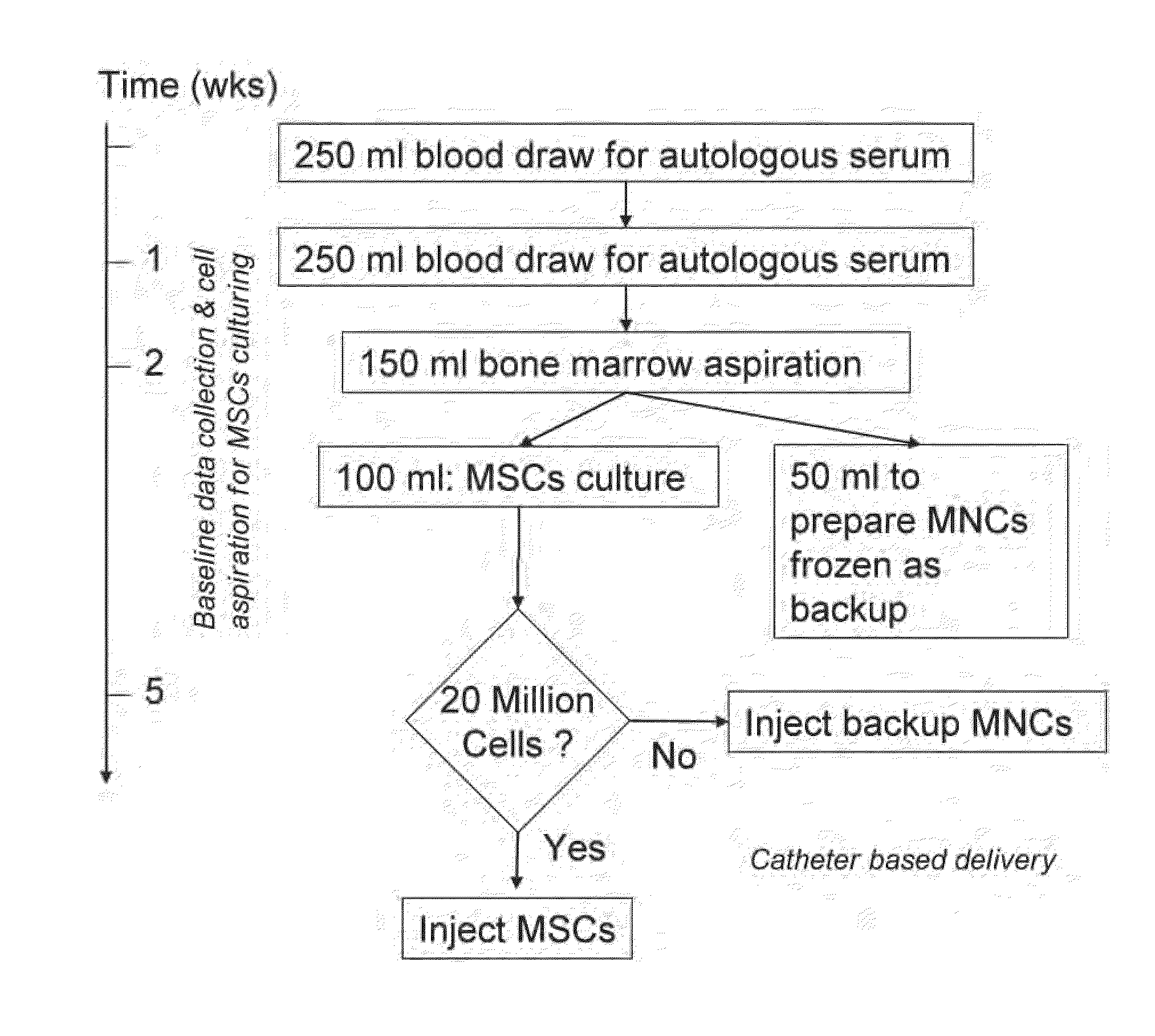
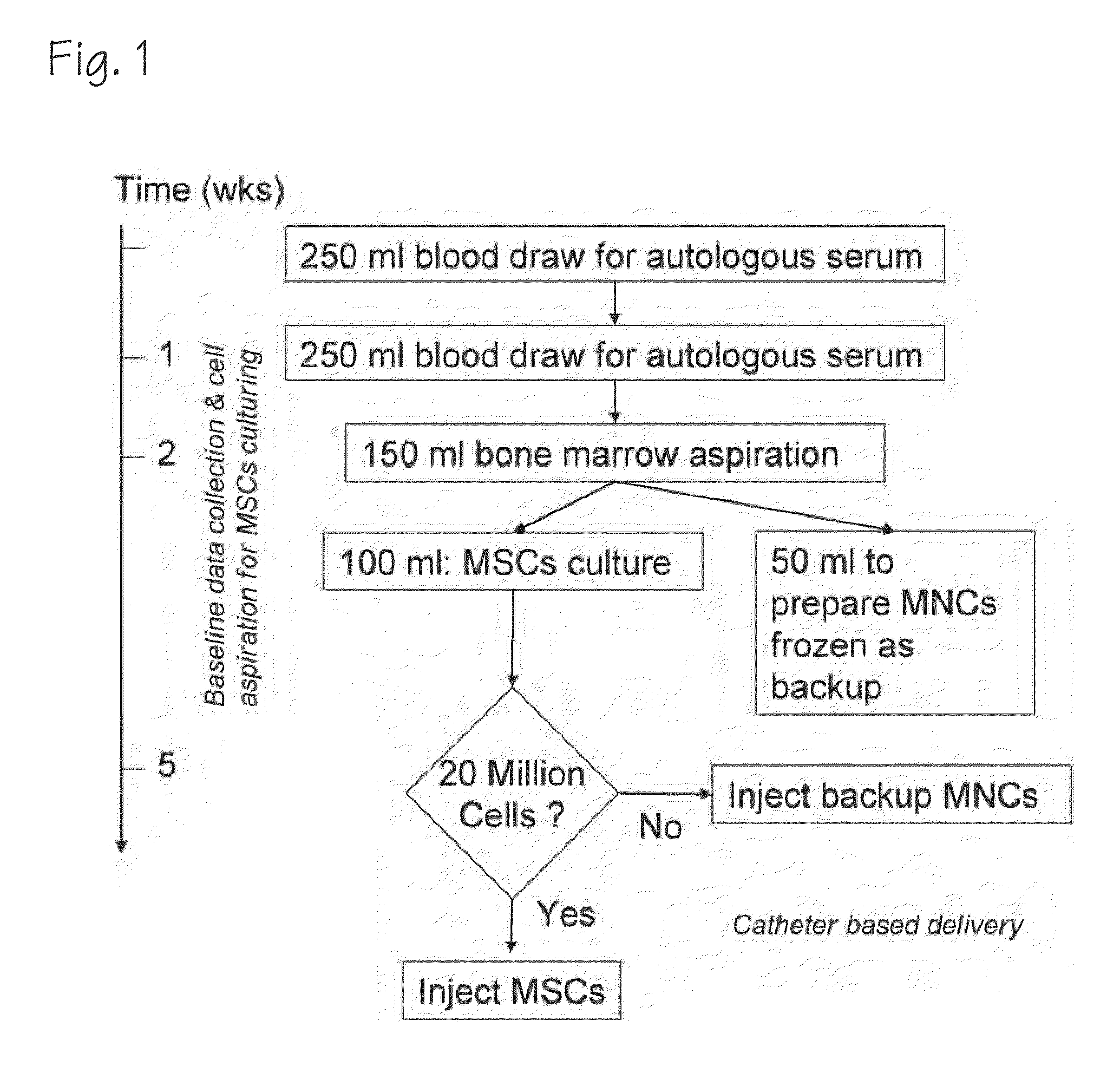
Therapeutic autologous-cell delivery support system, financial system for use therewith, and method therefor
InactiveUS20060062771A1Minimizing delivery timeMinimize timeAntibacterial agentsPhysical therapies and activitiesSupporting systemComputer science
Disclosed is a therapeutic autologous-cell delivery support system 100, in which a therapeutic autologous cell extracted from a member is reserved in reserve means 34, and an autologous-cell delivery request from the member which includes ID information and positional information is received. Membership information is retrieved in accordance with the received ID information, and the membership information and positional information of the member is output. When the reserve means is provided in plural number at geographically different locations, one reserve means having a shortest delivery time is selected in accordance with the positional information included in the delivery request. The delivery request can be issued using ID information storage means 11 for transmitting the ID information, in a simple operation, or automatically issued using a monitor of physical data.
Owner:NC MEDICAL RES +3
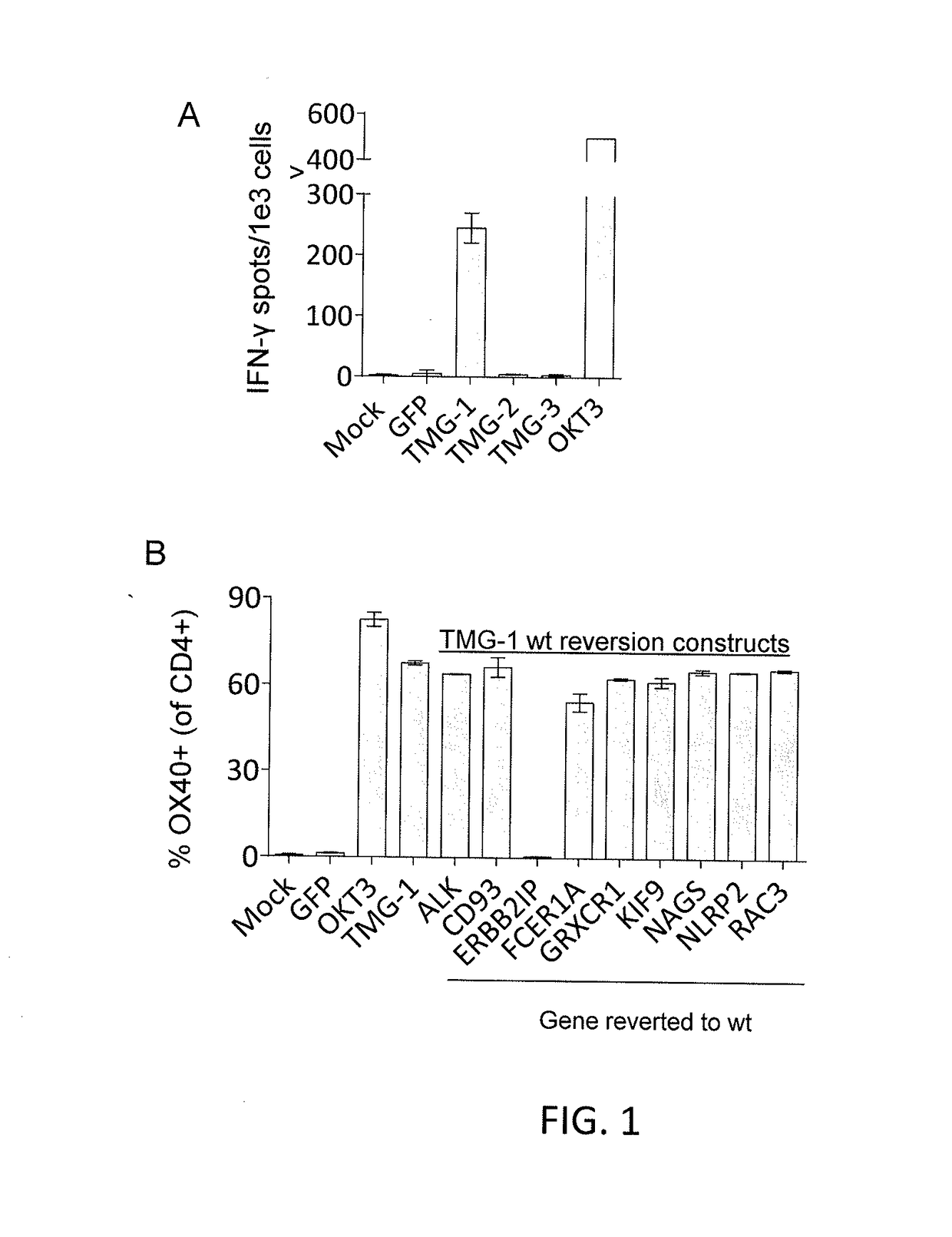
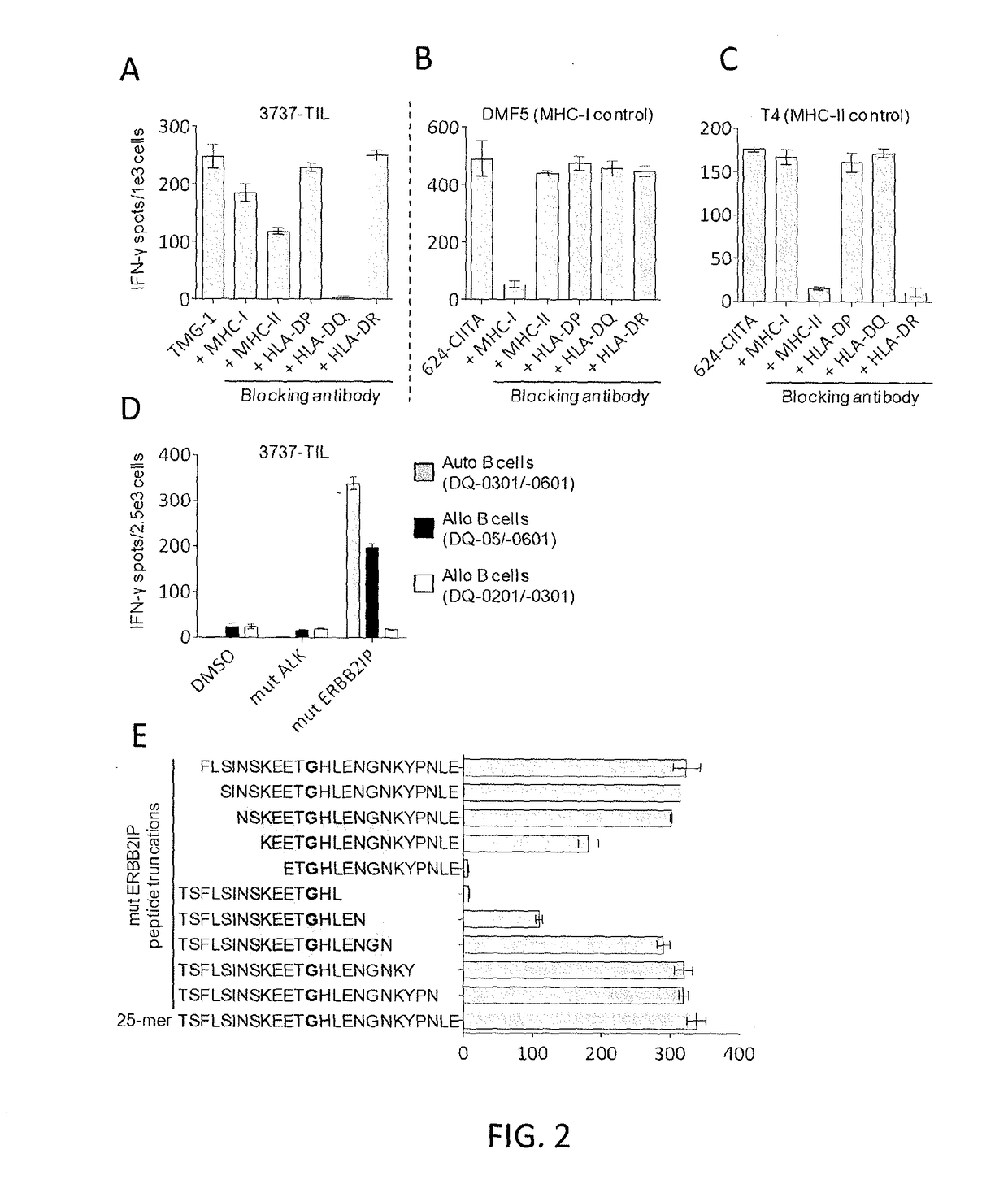
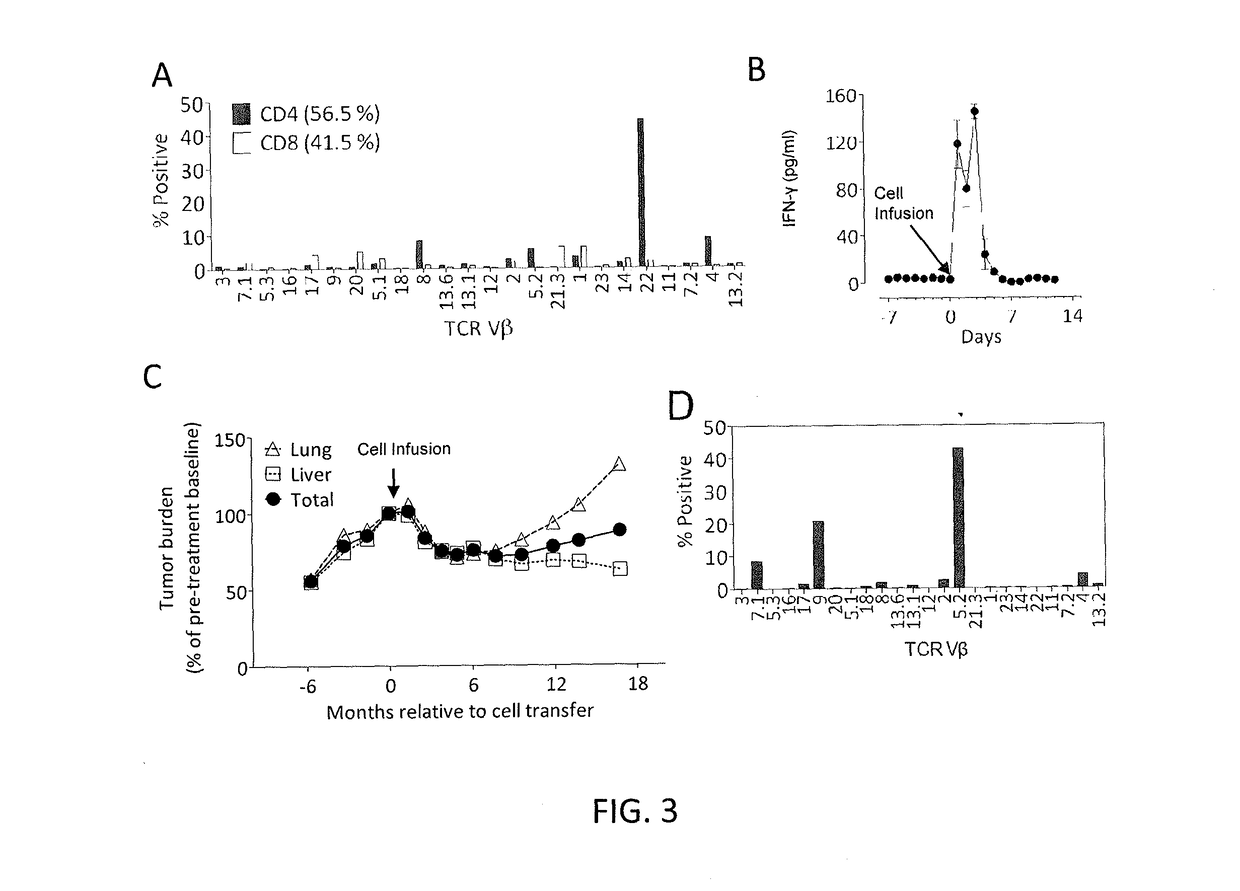
Methods of isolating t cell receptors having antigenic specificity for a cancer-specific mutation
Disclosed are methods of isolating a TCR having antigenic specificity for a mutated amino acid sequence encoded by a cancer-specific mutation, the method comprising: identifying one or more genes in the nucleic acid of a cancer cell of a patient, each gene containing a cancer-specific mutation that encodes a mutated amino acid sequence; inducing autologous APCs of the patient to present the mutated amino acid sequence; co-culturing autologous T cells of the patient with the autologous APCs that present the mutated amino acid sequence; selecting the autologous T cells; and isolating a nucleotide sequence that encodes the TCR from the selected autologous T cells, wherein the TCR has antigenic specificity for the mutated amino acid sequence encoded by the cancer-specific mutation. Also disclosed are related methods of preparing a population of cells, populations of cells, TCRs, pharmaceutical compositions, and methods of treating or preventing cancer.
Owner:UNITED STATES OF AMERICA
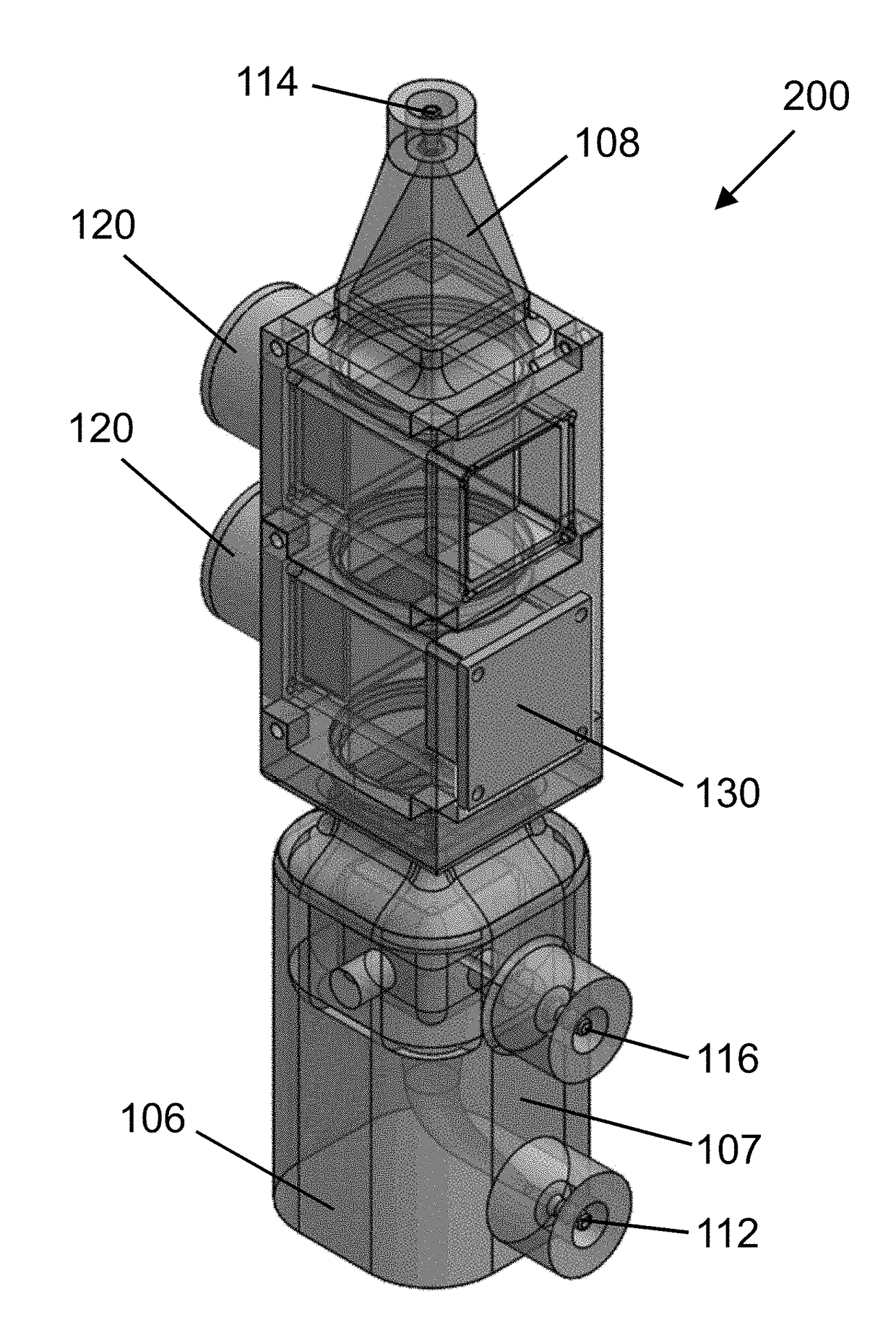
System for generating high concentration factors for low cell density suspensions
ActiveUS20180016570A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiological cellHigh concentration
Acoustophoretic devices and methods for concentrating targeted biological cells in a reduced volume using multi-dimensional acoustic standing waves are disclosed. The methods include flowing a mixture of a host fluid and the biological cells through an acoustophoretic device. The acoustophoretic devices include an inlet, an outlet, and a flow chamber having an ultrasonic transducer-reflector pair. Biological cells, such as T cells, are separated from a host fluid for utilization in allergenic or autologous cell therapies. The disclosed devices and methods are capable of concentrating biological cells to at least 100 times their original cell concentration. The disclosed methods and devices are further capable of decreasing an original feed volume to a final concentrated volume that is less than one percent of the original feed volume.
Owner:FLODESIGN SONICS
Smooth muscle cell constructs
The present invention relates to the regeneration, reconstruction, augmentation or replacement of luminal organs or tissue structures in a subject in need using scaffolds seeded with autologous or non-autologous cell populations that are or are not derived from the corresponding organ or tissue structure that is the subject of the regeneration, reconstruction, augmentation or replacement.
Owner:ORGAGEN INC
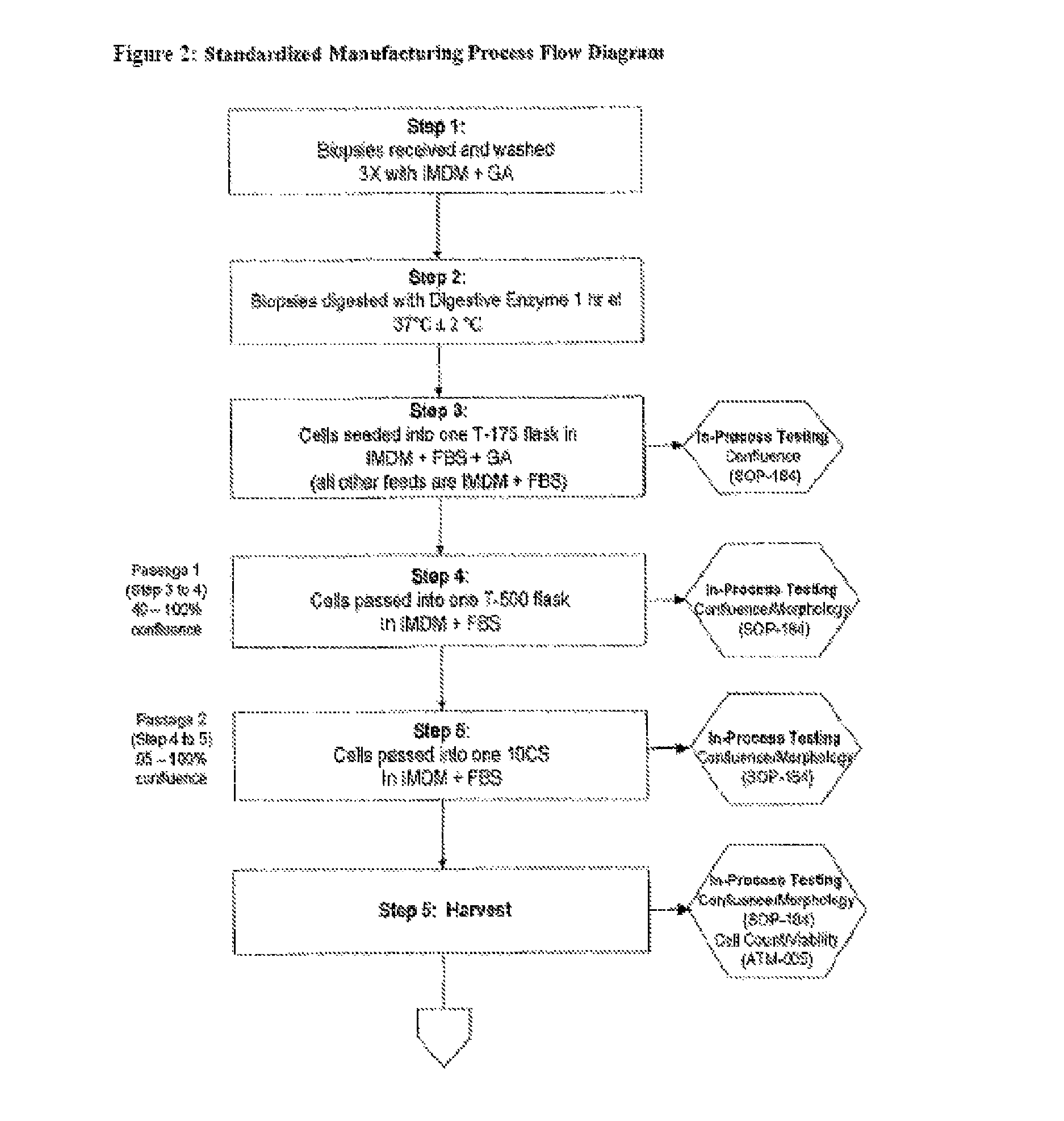
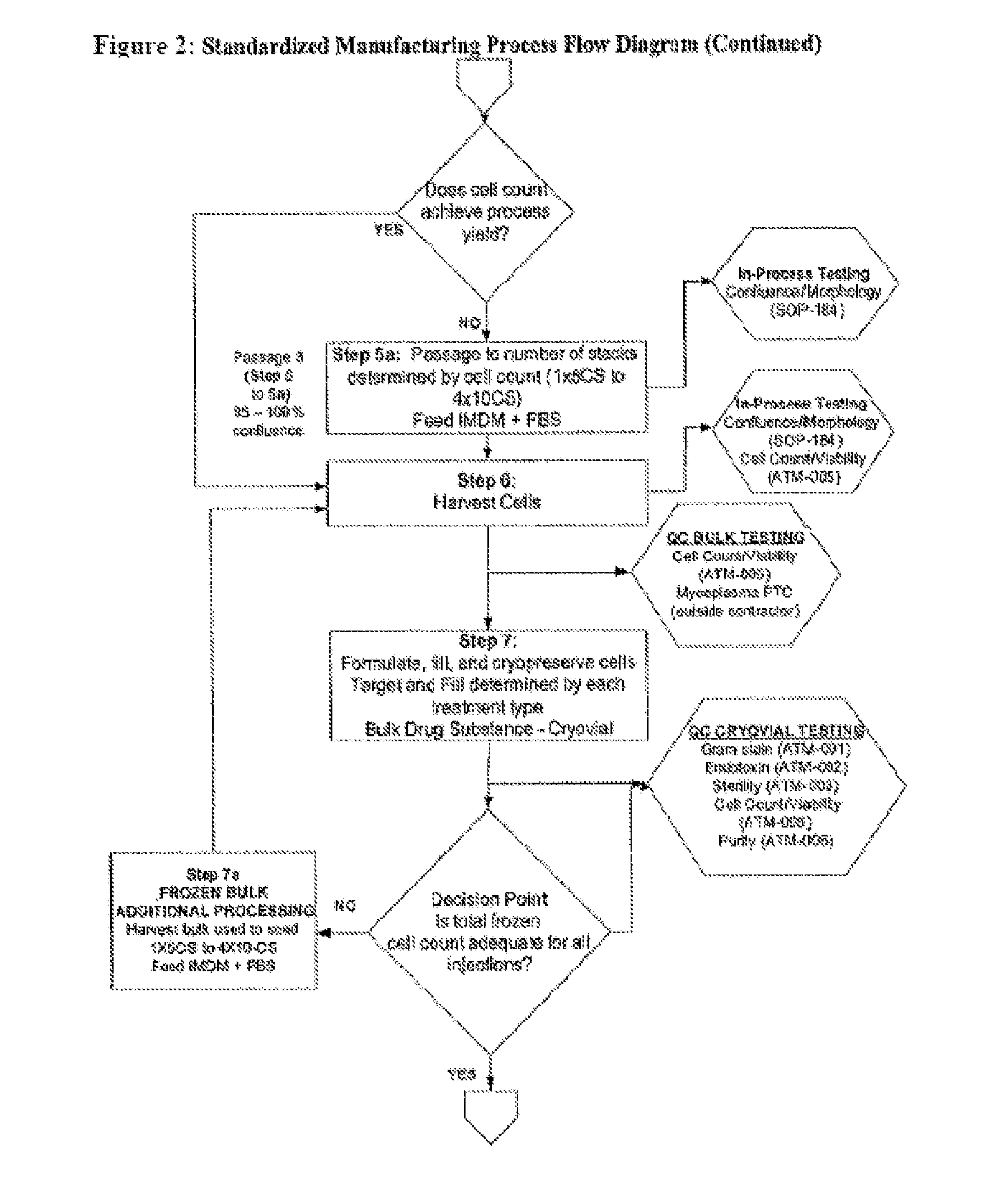
Method of Preparing Autologous Cells and Method of Use for Therapy
A method for expanding mesenchymal cells derived from autologous bone marrow in autologous culture medium which can be used in a clinical setting, and a business method for performing such expansions in the future as a service for patients. A method for expanding mesenchymal cells derived from autologous bone marrow in autologous culture medium including a diagnostic kit for the autologous cell therapy to determine whether a patient will respond to the autologous cell therapy for treatment of a disease, in which said kit comprising a system for detecting gene and protein expression comprising at least two isolated DNA molecules wherein each isolated DNA molecule detects expression of a gene that is differentially expressed in the tissue of the patient that is intended to be the source of the autologous cell therapy.
Owner:BIOCARDIA
Adoptive cell therapy with young t cells
ActiveUS20140030806A1Organic active ingredientsPeptide/protein ingredientsAdoptive cellular therapyTumor response
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Pluripotent mammalian cells
InactiveUS20050210537A1Enhance chromatin remodellingStably integratedNew breed animal cellsGenetic material ingredientsReprogrammingCell type specific
The invention relates to a method of making pluripotent stem cells that does not involve the formation of early preimplantation embryos or fetal tissue. The method has general utility in the production of pluripotent stem cells from many mammalian species but has particular application in man where pluripotent stem cell production can be customized to particular human individual. The method involves the fusion of donor somatic or stem cells (or their karyoplasts) with cytoplasmic, membrane-delimited fragments of mammalian oocytes or zygotes. After the initial genomic reprogramming occurs, the cells can proliferate and thus multiply in vitro yielding a large number of autologous cells for cell therapy application. The result of this process is a cell population genomically identical to the somatic, differentiated cells derived from an individual patient. However, these cells are pluripotent in that upon application of specific growth factors, the cells are capable of differentiating into specific cell types as required by the sought clinical indication.
Owner:DOMINKO TANJA +4
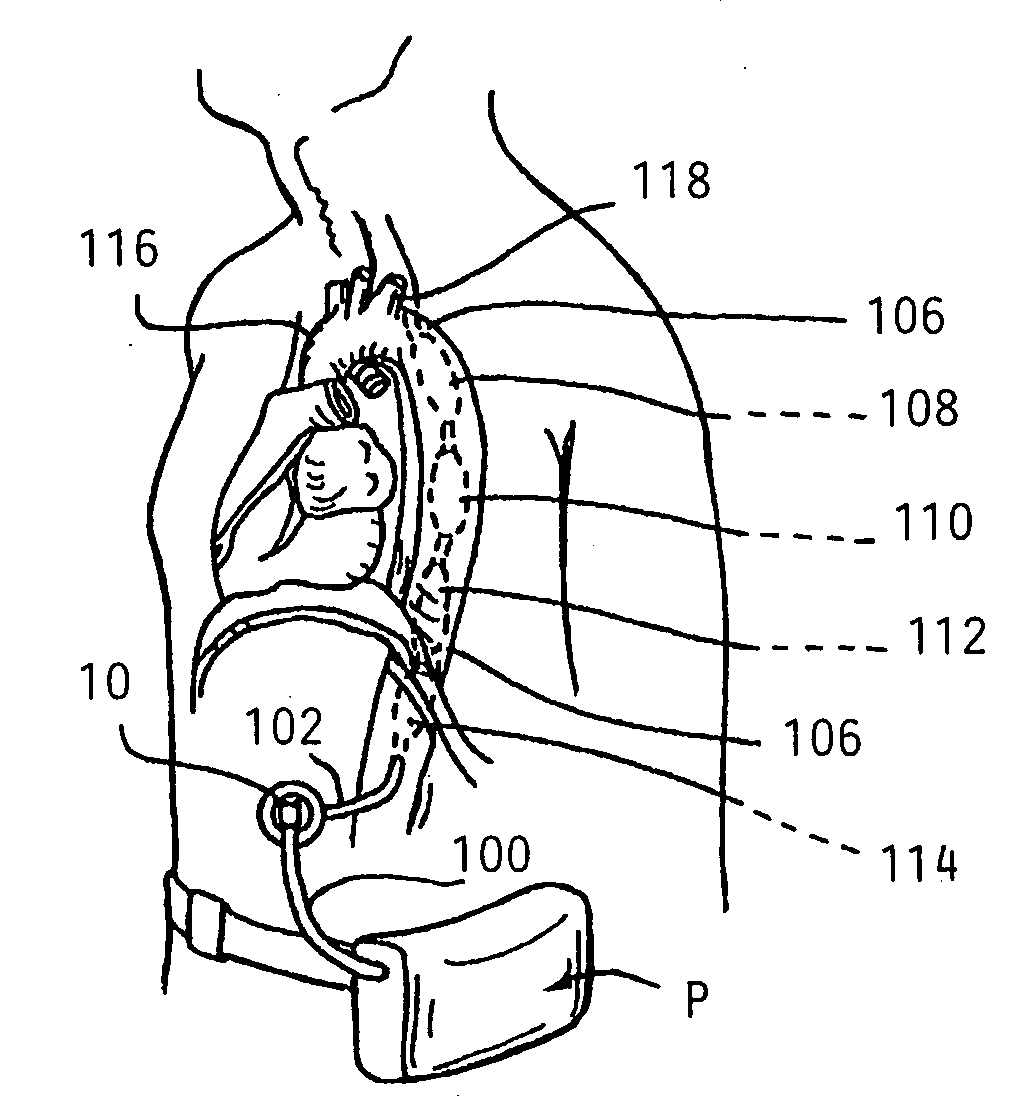
Long term ambulatory intro-aortic balloon pump with percutaneous access device
ActiveUS20080281147A1Assist cardiac functionReduce the overall diameterElectrocardiographyTransvascular endocardial electrodesAmbulatoryAortic balloon pump
Disclosed herein are embodiments of long-term ambulatory intra-aortic balloon pump systems for providing left ventricular cardiac assistance to a patient. The systems can comprise an external drive system for supplying a compressed fluid, the drive system operating in accordance with a control program stored in memory, an intra-luminal balloon pump having an elongate inflatable chamber positionable to be lying completely within a descending aorta of the patient, and a percutaneous access device. The percutaneous access device can be a biocompatible implantable portal comprising a communicative passage through an interior bore and an exterior having a neck region adapted to promote autologous cell growth thereon in an implantable region, the neck region having a plurality of channels extending about the neck region.
Owner:L VAD TECH
Percutaneous access device system facilitating cell growth thereon
ActiveUS20070026032A1Promote autologous cell growthPromote growthBiocideElectrotherapyCouplingEngineering
A biocompatible implantable portal is provided that has a wall defining a communicative passage through an interior bore. The exterior of the portal has a neck region adapted to promote autologous cell growth on the neck region. A series of channels are provided on the exterior neck region to facilitate autologous cell growth while disfavoring fluid pooling and bacterial growth. Typical channel widths are from 20 to 300 microns, with adjacent channels being separated by plateaus having a width of between 0 and 600 microns. Providing the portal exterior neck region with a texture varying on a nanometer length scale facilitates autologous cell growth. Applying a coating such as a tissue scaffolding matrix to the neck region prior to implantation also facilitates cell growth. A coupling or a manifold encompassing the neck region facilitates the draw of vacuum and / or mechanical protection for the growing cells.
Owner:VIADERM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com