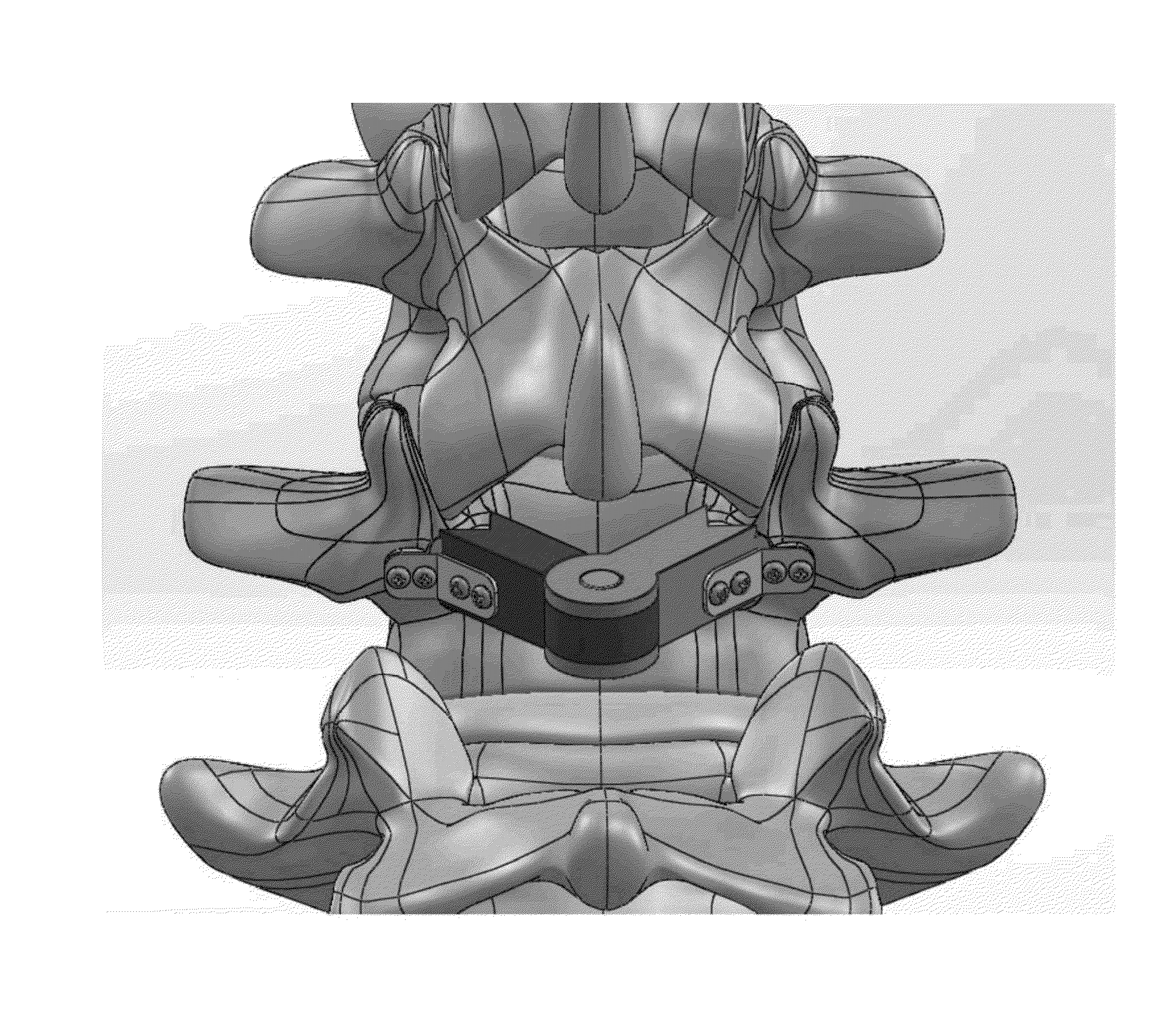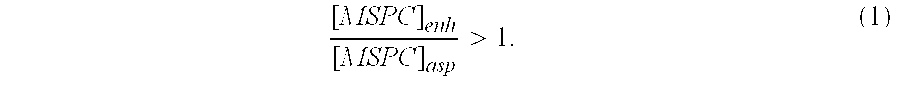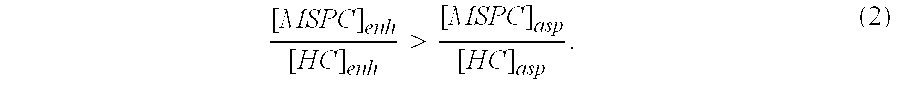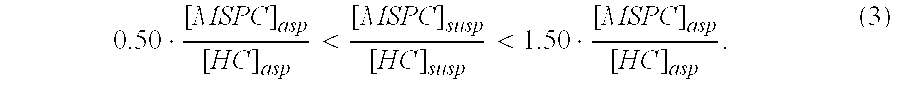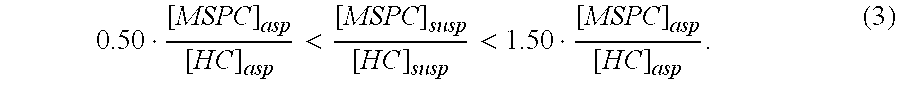Patents
Literature
178 results about "Autologous bone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cartilage repair methods
The present application discloses methods for repairing hyaline cartilage defects. The methods comprise a combination of introducing autologous bone mesenchymal stem cells to a joint, and applying to the joint a membrane comprising a polyester entangled with a polysaccharide. In some aspects, the bone mesenchymal stem cells are mesenchymal stem cells originating in bone underlying the joint. In these aspects, contact between the joint and the mesenchymal stem cells can be effected by introducing apertures through the bone using standard surgical techniques such as microfracture, abrasion, or drilling. Cartilage which forms in response to application of these methods is hyaline cartilage rather than fibrocartilage.
Owner:ISTO TECH II LLC
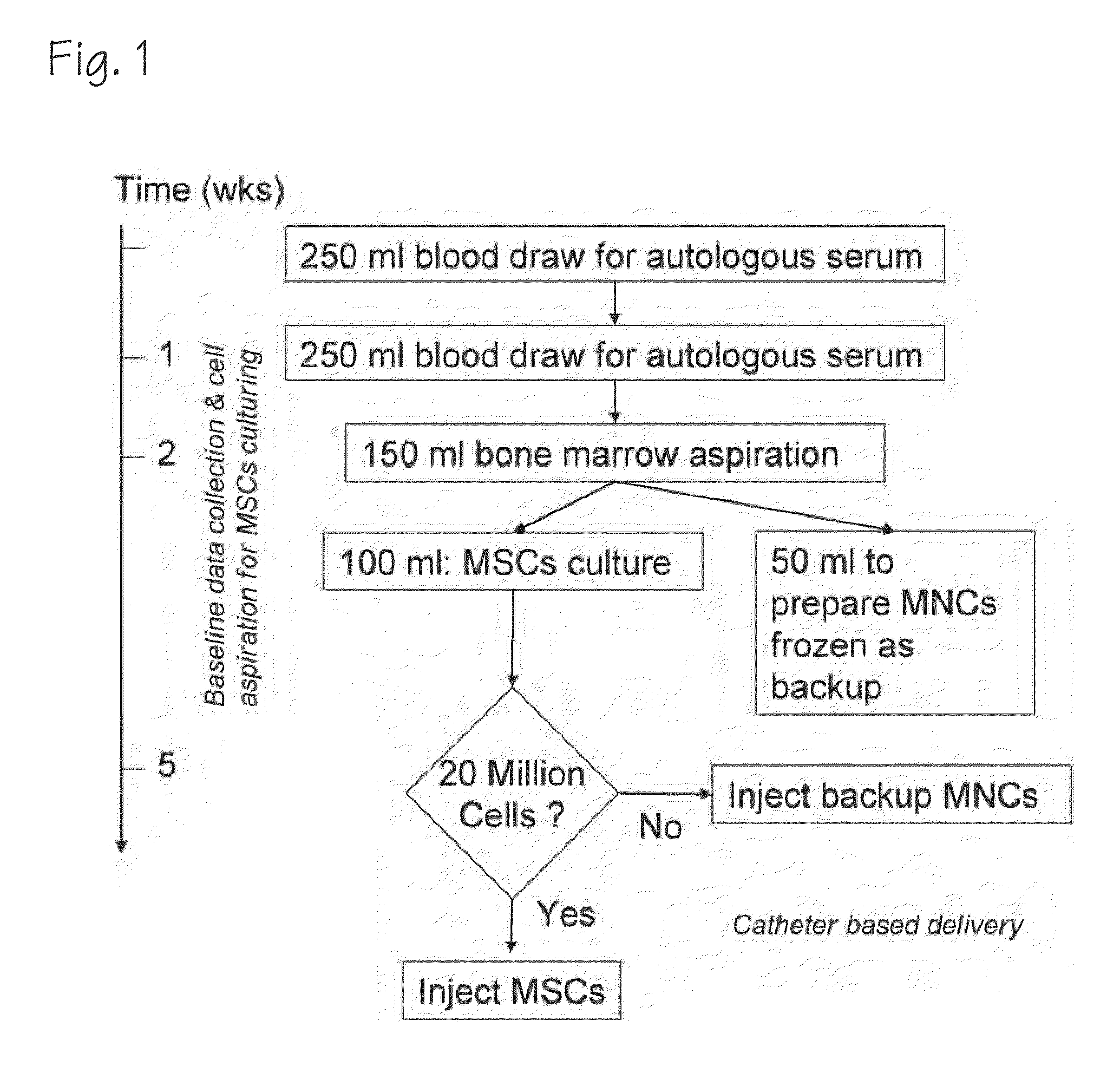
Intramyocardial injection of autologous bone marrow
InactiveUS7097832B1Enhance formationIncrease perfusionBiocidePeptide/protein ingredientsAngiogenesis growth factorPharmaceutical drug
Owner:MYOCARDIAL THERAPEUTICS INC +3
Biosynthetic composite for osteochondral defect repair
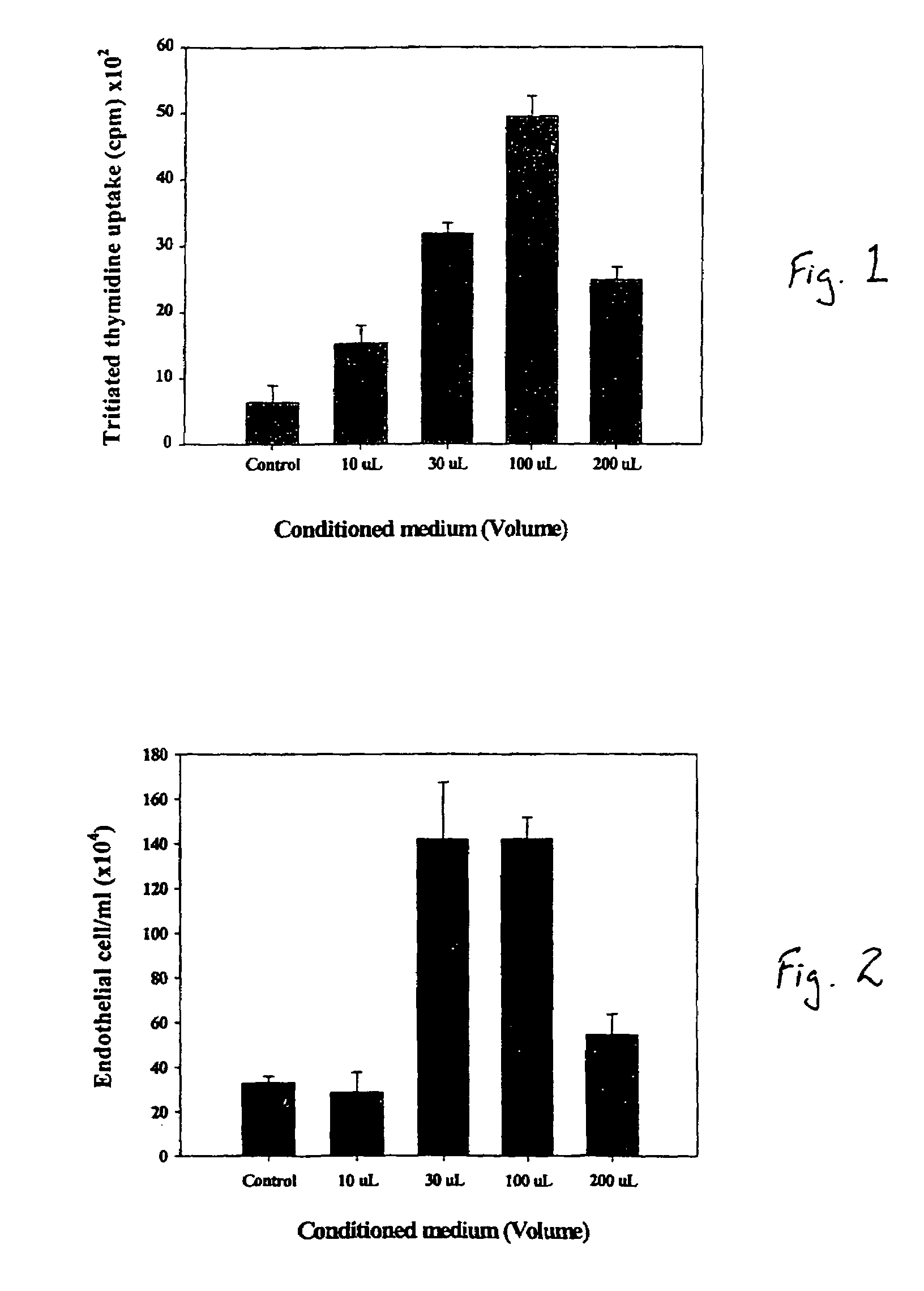
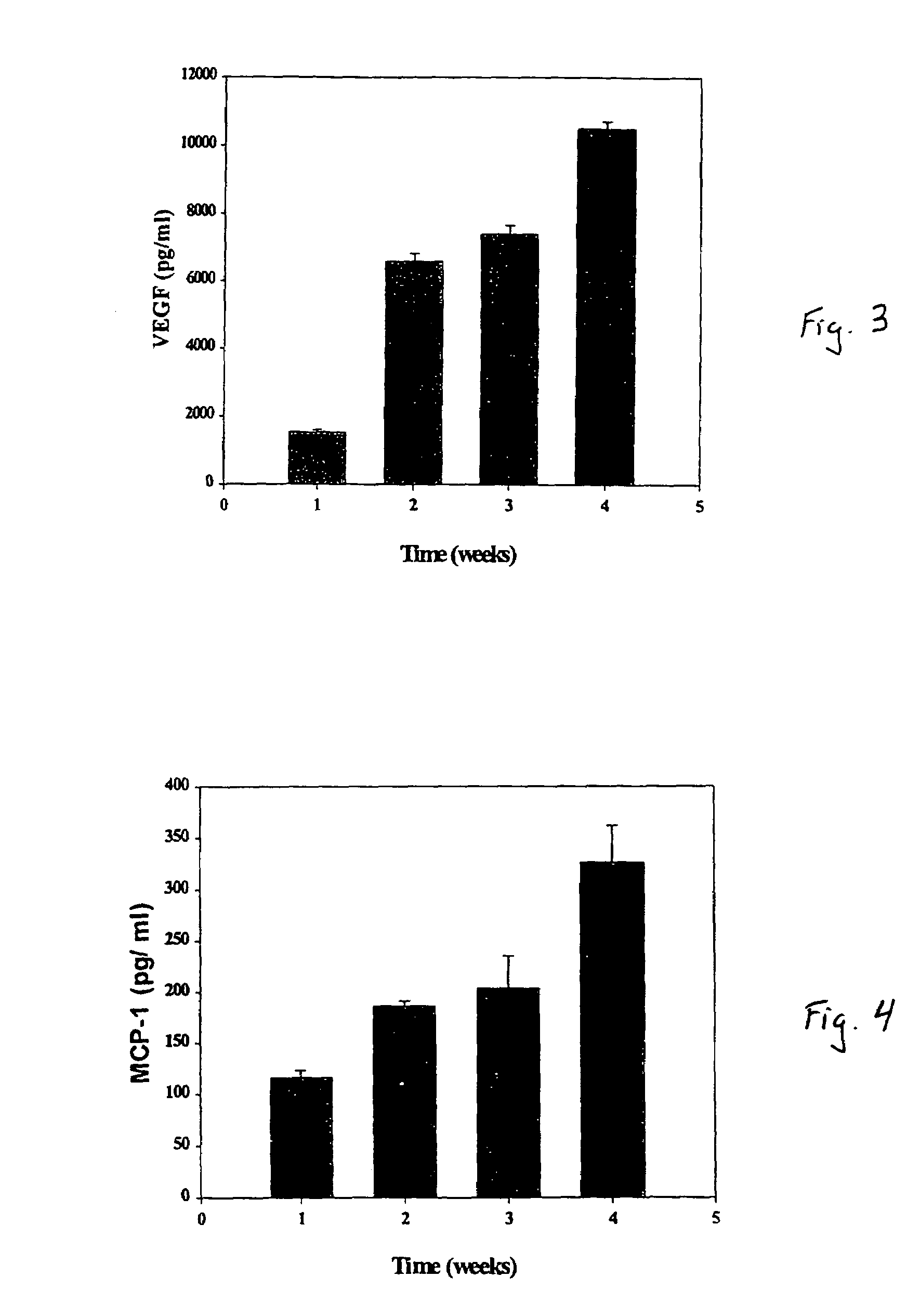
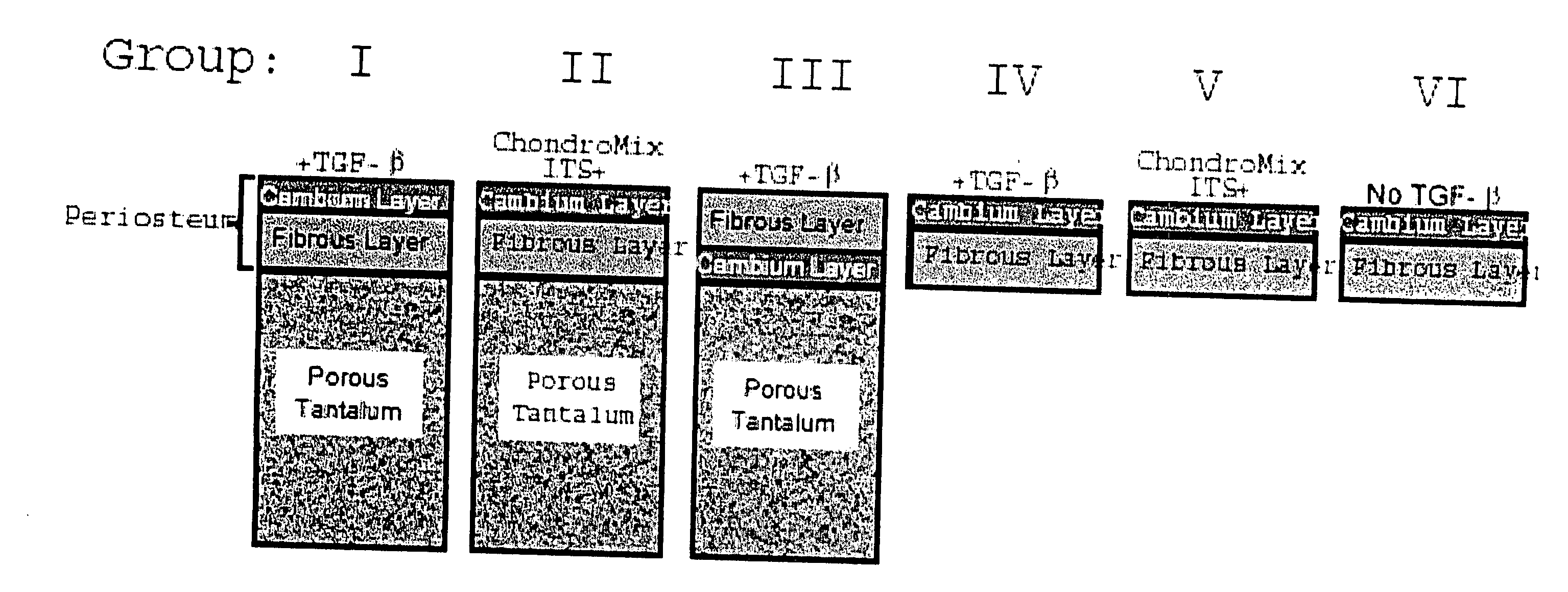
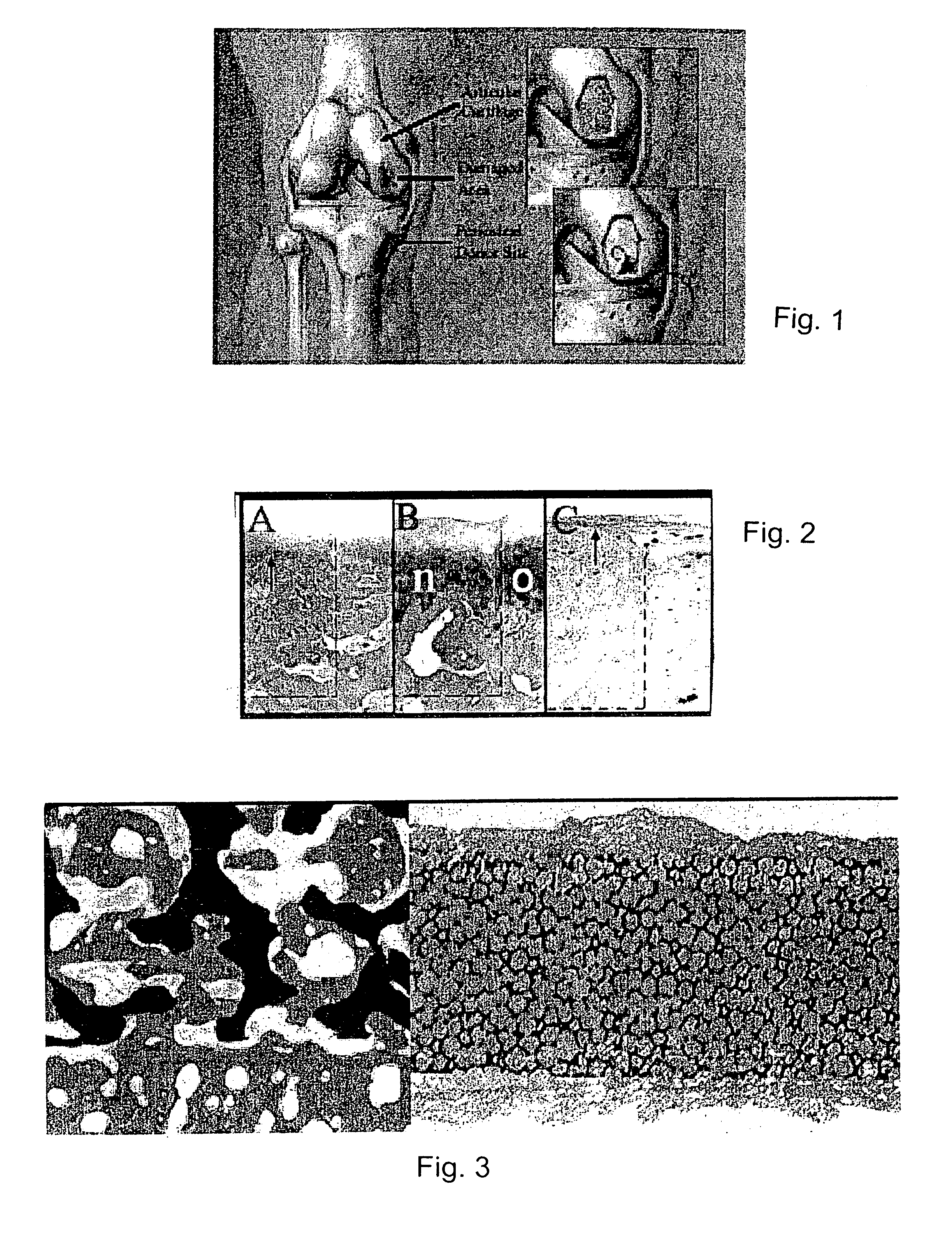
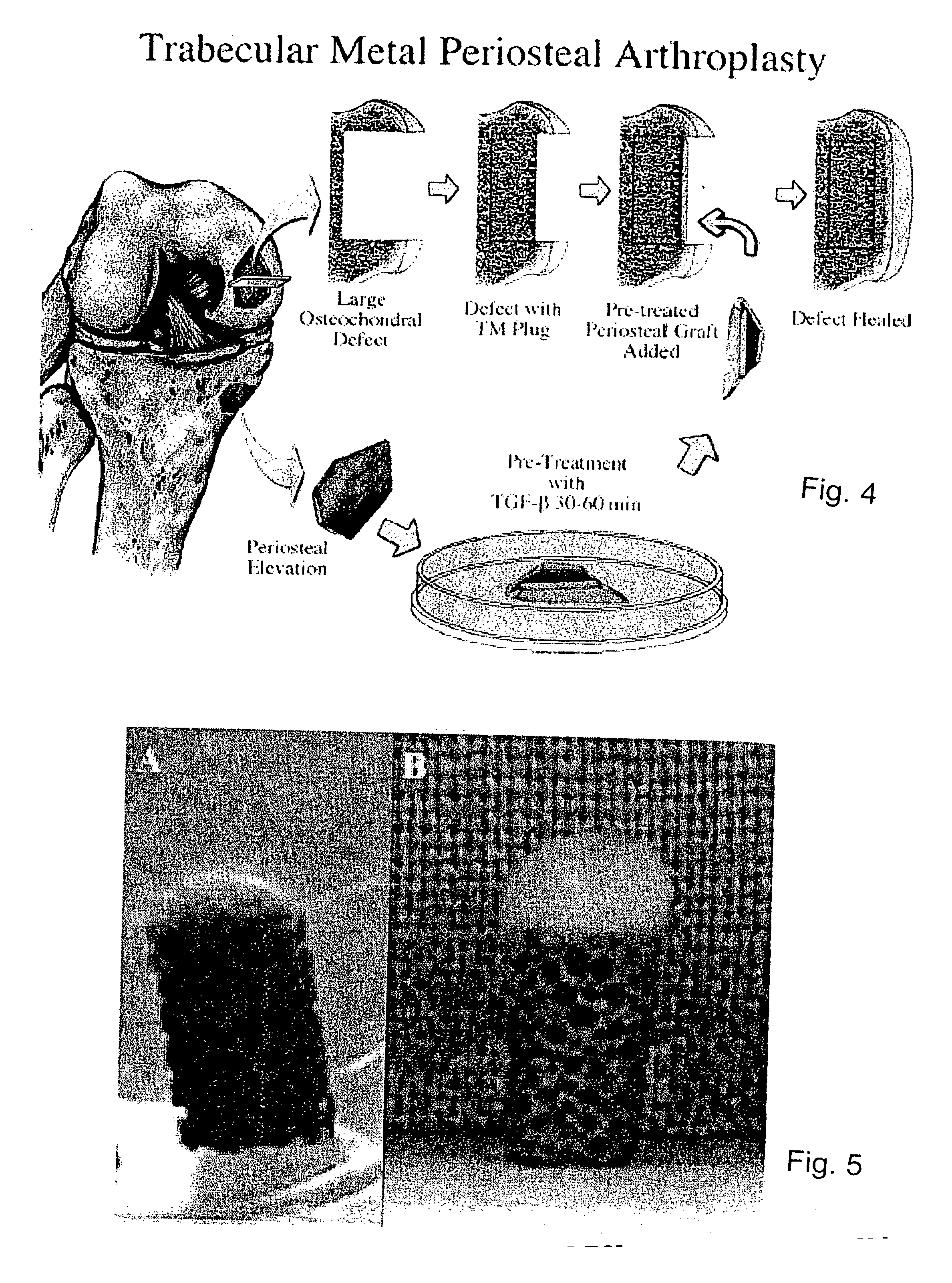
A composite for osteochondral defect repair includes a porous scaffold and a periosteal graft secured to a surface of the scaffold. The composite provides cartilage growth from autologous periosteum chondrogenesis. Biological resurfacing of large osteochondral defects, or a complete joint is feasible using the porous scaffold / autologous periosteal composite. The use of this composite eliminates the necessity of using normal cartilage surface as a donor site and its respective associated morbidity. In one form, the strong bone integration capacity of a porous metal (e.g., tantalum) scaffold and the high grade of integration observed from periosteal chondrogenesis into the normal cartilage eliminates the lack of chondral-chondral integration observed in the autologous osteochondral graft technique.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Structural/biological implant system
InactiveUS20050033427A1Increasing bone heightIncrease volumeDental implantsInternal osteosythesisMedicineProviding material
A structural / biological implant and method of use. The implant being utilized as a single or multiple staged system that is designed to encourage new alveolar bone growth with or without the need to obtain autologous bone. The implant has an apical portion that is fastened into existing bone, with the remainder of the implant left outside of existing bone. The exposed portion of the implant may have an external shape or configuration with a variety of attached and / or integrally formed mechanical retention and stability elements. Osteotropic / angiotropic material may be associated with the implant to induce and or conduct new bone growth and possible vascularization, thus, rather than fitting the implant into the bone, the bone is grown integratively with the implant. The osteotropic / angiotropic materials may be simultaneously placed with the staged implant to provide consistent stabilization for the materials and to provide an immediately available surface for bone cell adhesion and growth. The invention thus allows implants to be used in location where the volume and / or shape of the bone would not be adequate for existing implant systems.
Owner:UNIV OF CONNECTICUT
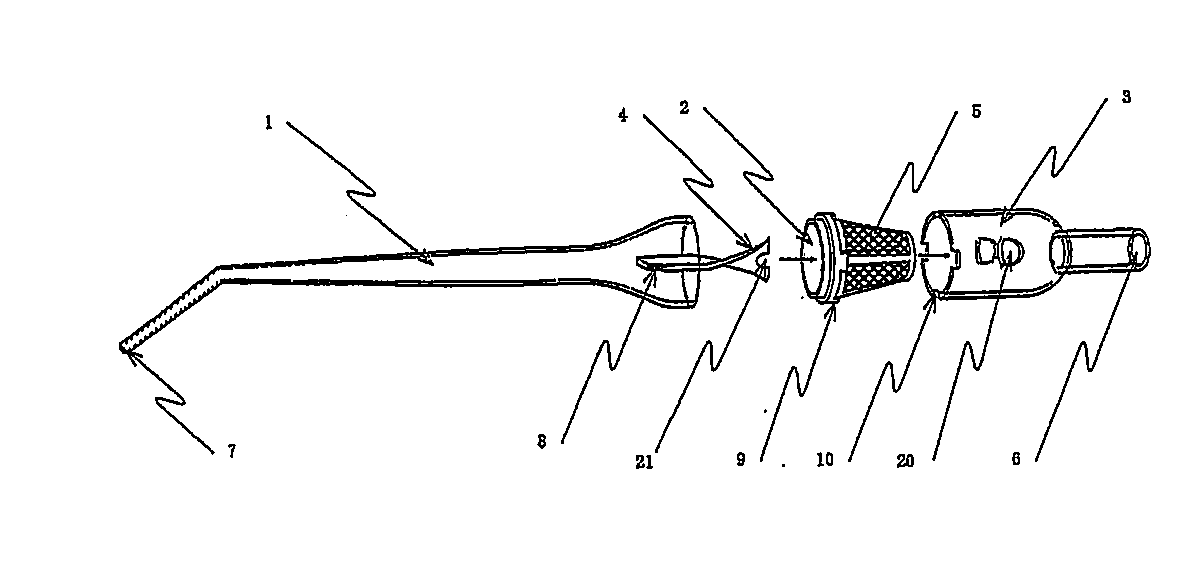
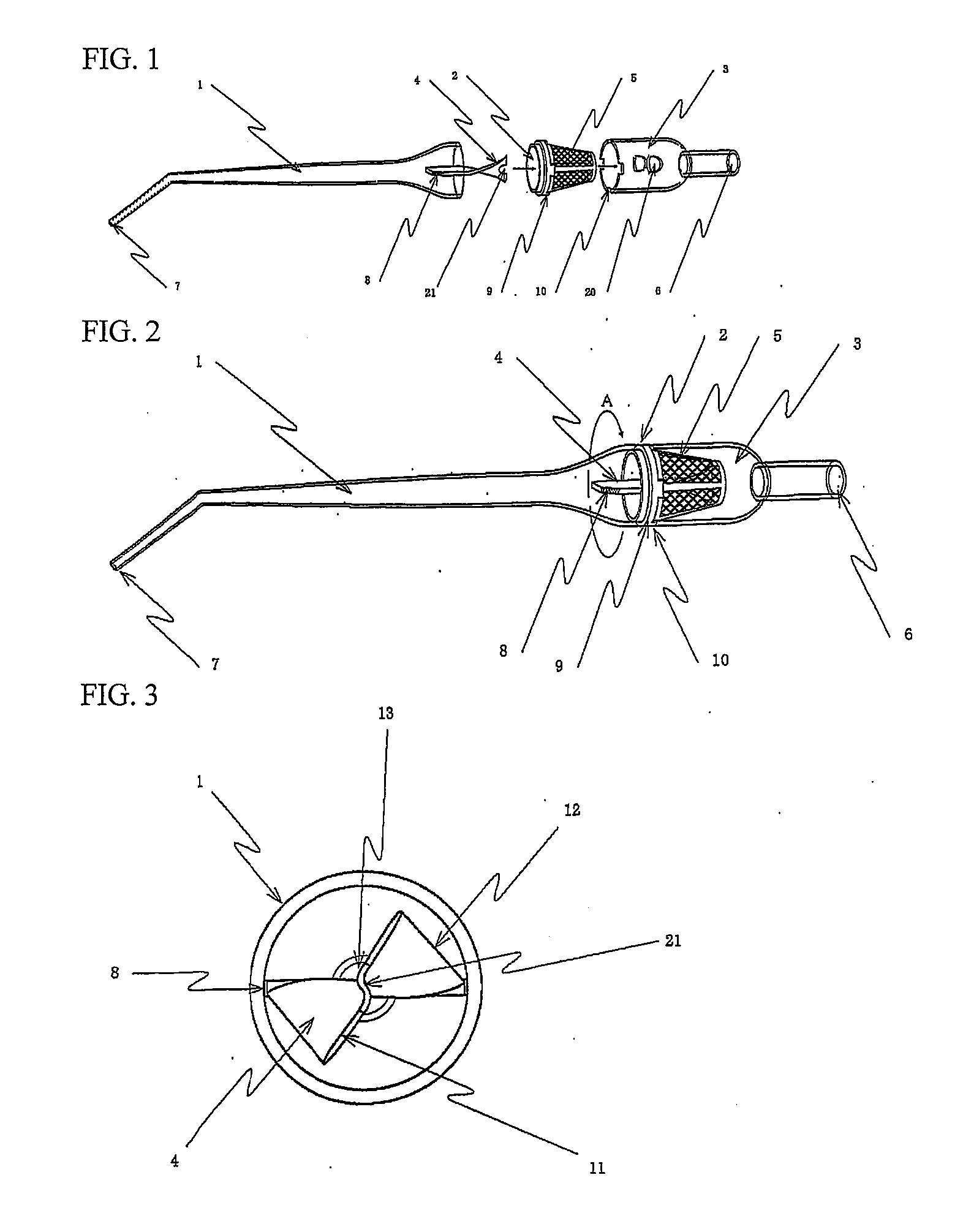
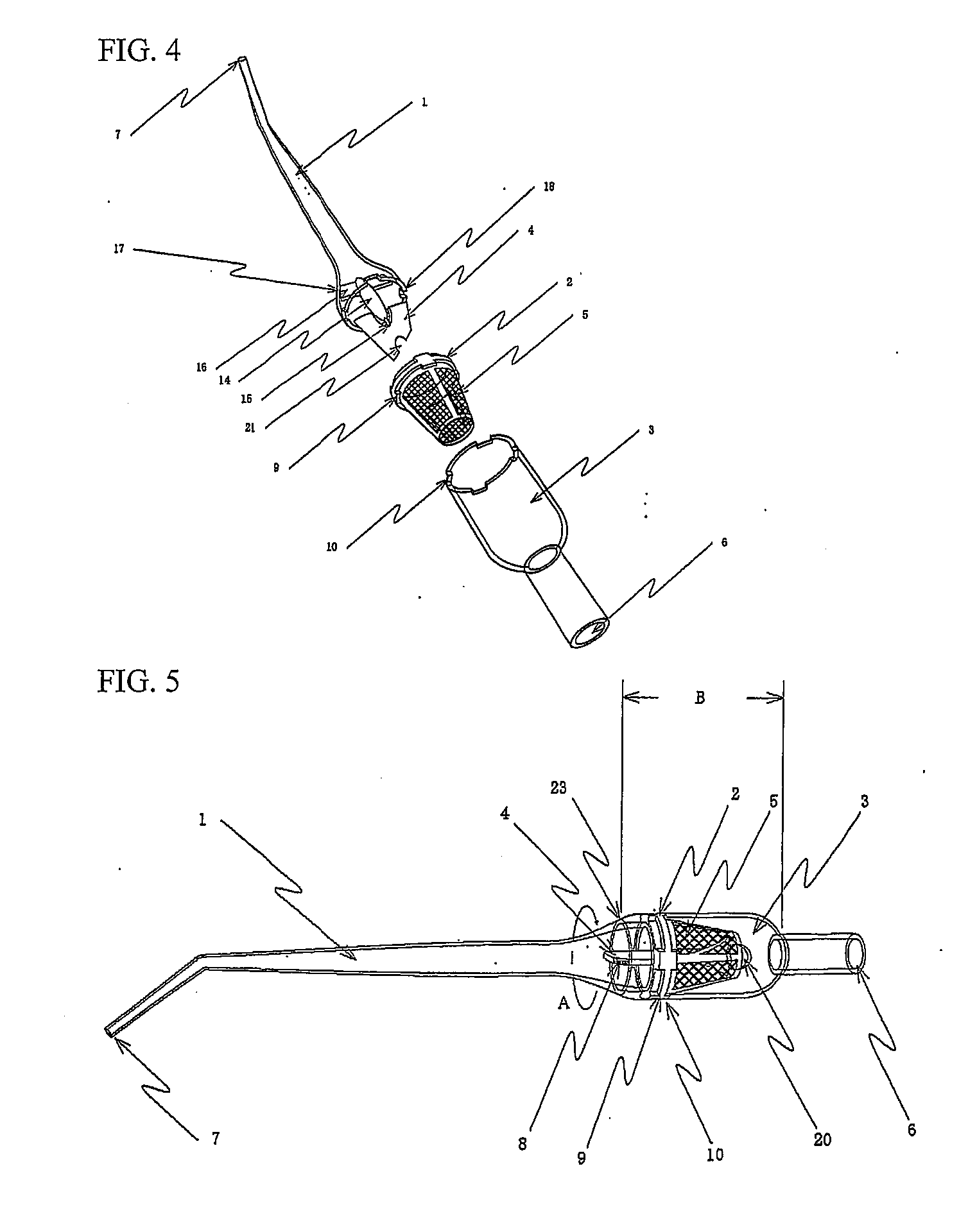
Autologous bone collection device having enhanced suction efficiency
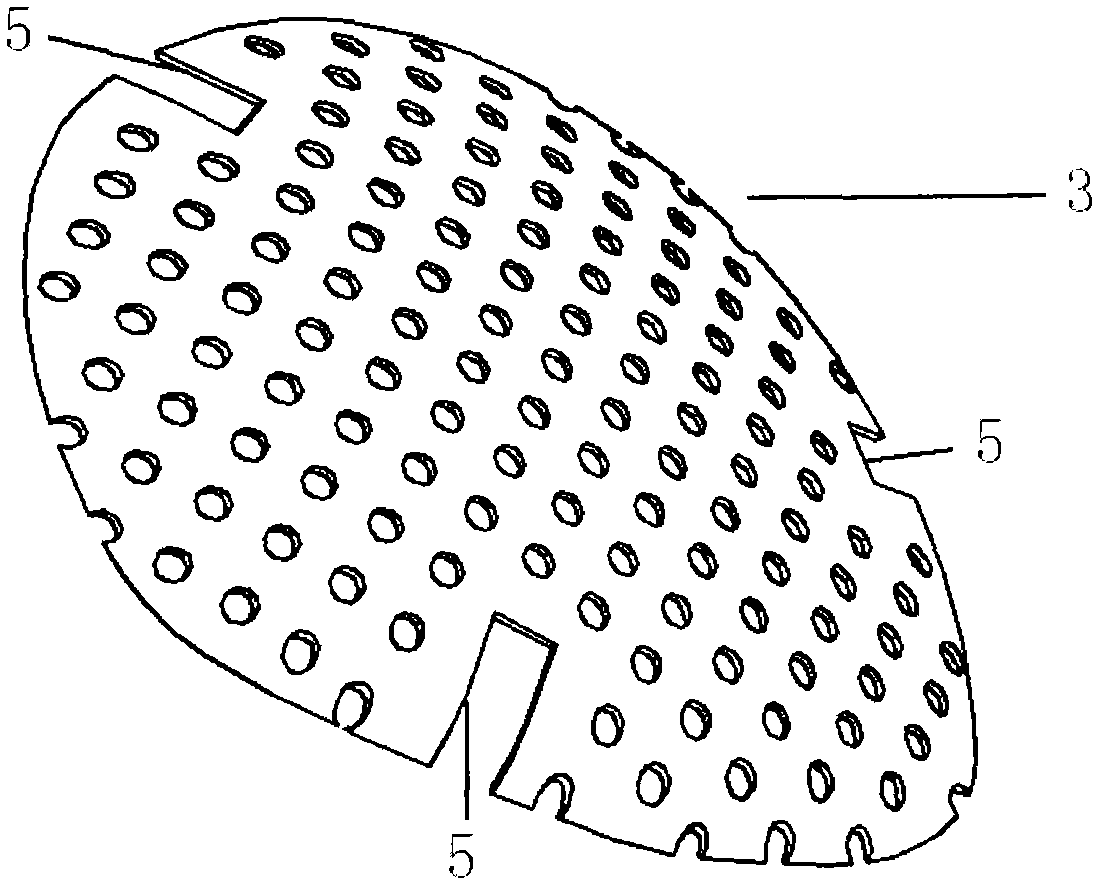
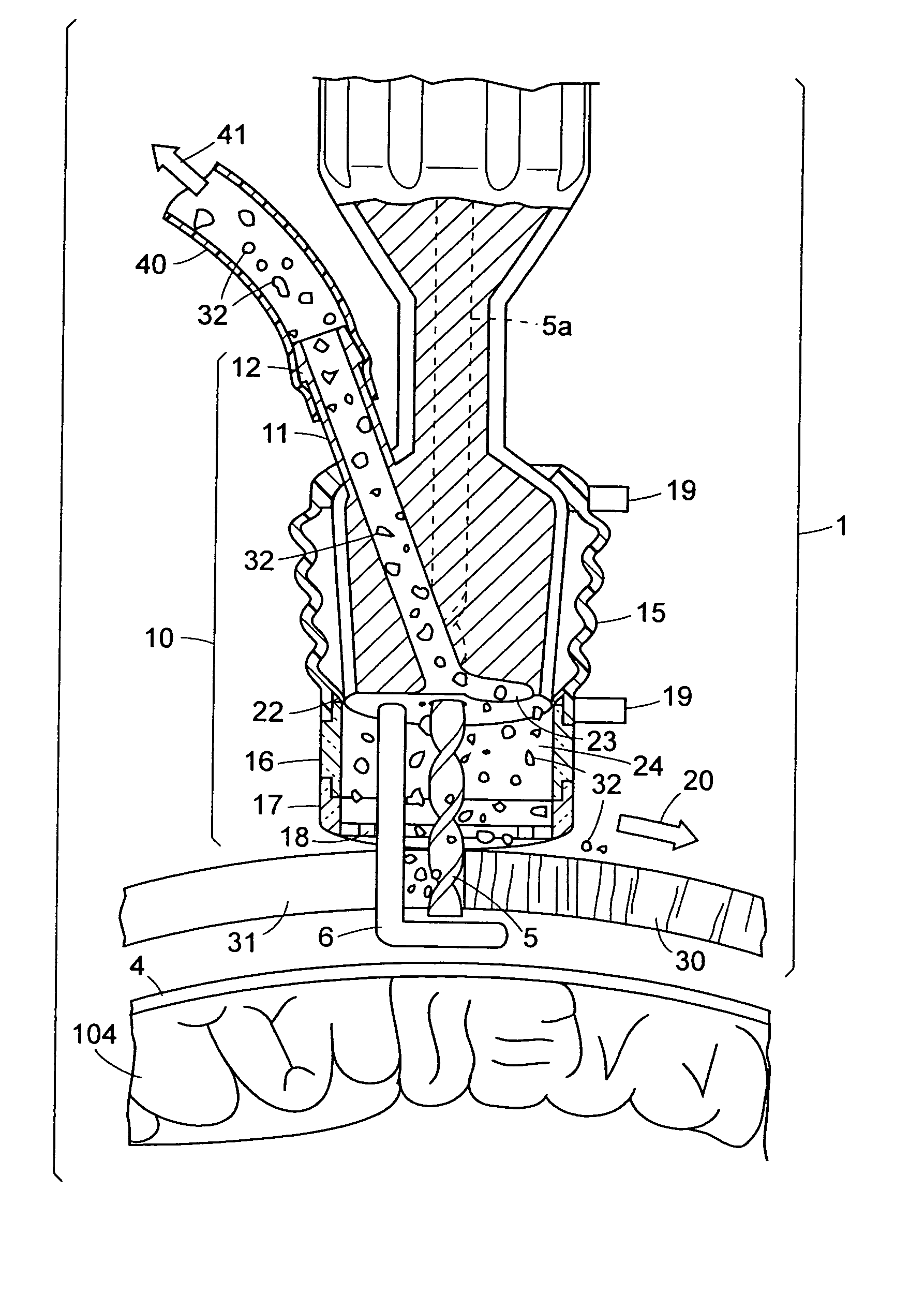
InactiveUS20090306669A1Constant suction efficiencySuction efficiency is maximizedSurgeryVaccination/ovulation diagnosticsMotor driveBone chip
A bone piece collector wherein a blade (4) coming into tight contact with the surface of a filter (2) under an appropriate pressure is provided in order to prevent lowering in the efficiency of suction work due to sampled bone chips adhering to the surface of the filter (2) in the bone piece collector provided in a suction line in order to sample a bone, i.e. a transplantation material in autologous bone transplantation, the blade (4) is rotated while being pressed against the surface of the filter (2) with a finger pressure or a motor drive force, and the sampled bone chips are moved in a certain direction so that clogging of the filter (2) is eliminated and the suction efficiency can be recovered at any time.
Owner:TAKAHASHI ATSUSHI
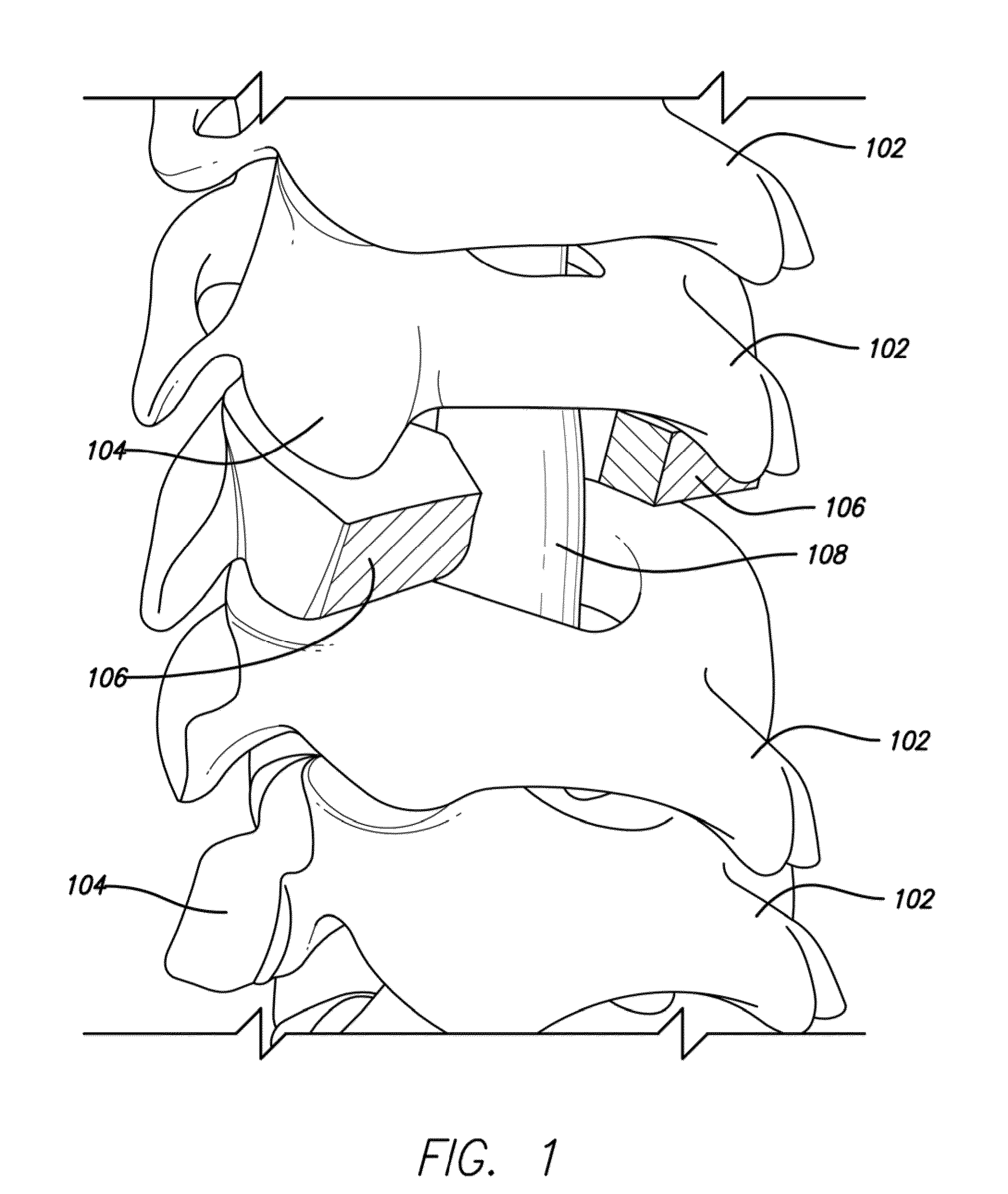
Lamina implant and method
A prosthetic implant for restoring lamina after a laminectomy. The implant is generally a lamina-sized construct having a hollow interior. The lamina removed during the laminectomy may be converted to autologous bone that may then be placed inside the hollow interior of the implant. The implant may then be secured to the spine at the site of the laminectomy so that lamina restoration can occur as the hollow interior of the implant solidifies with bone growth.
Owner:GLOBUS MEDICAL INC
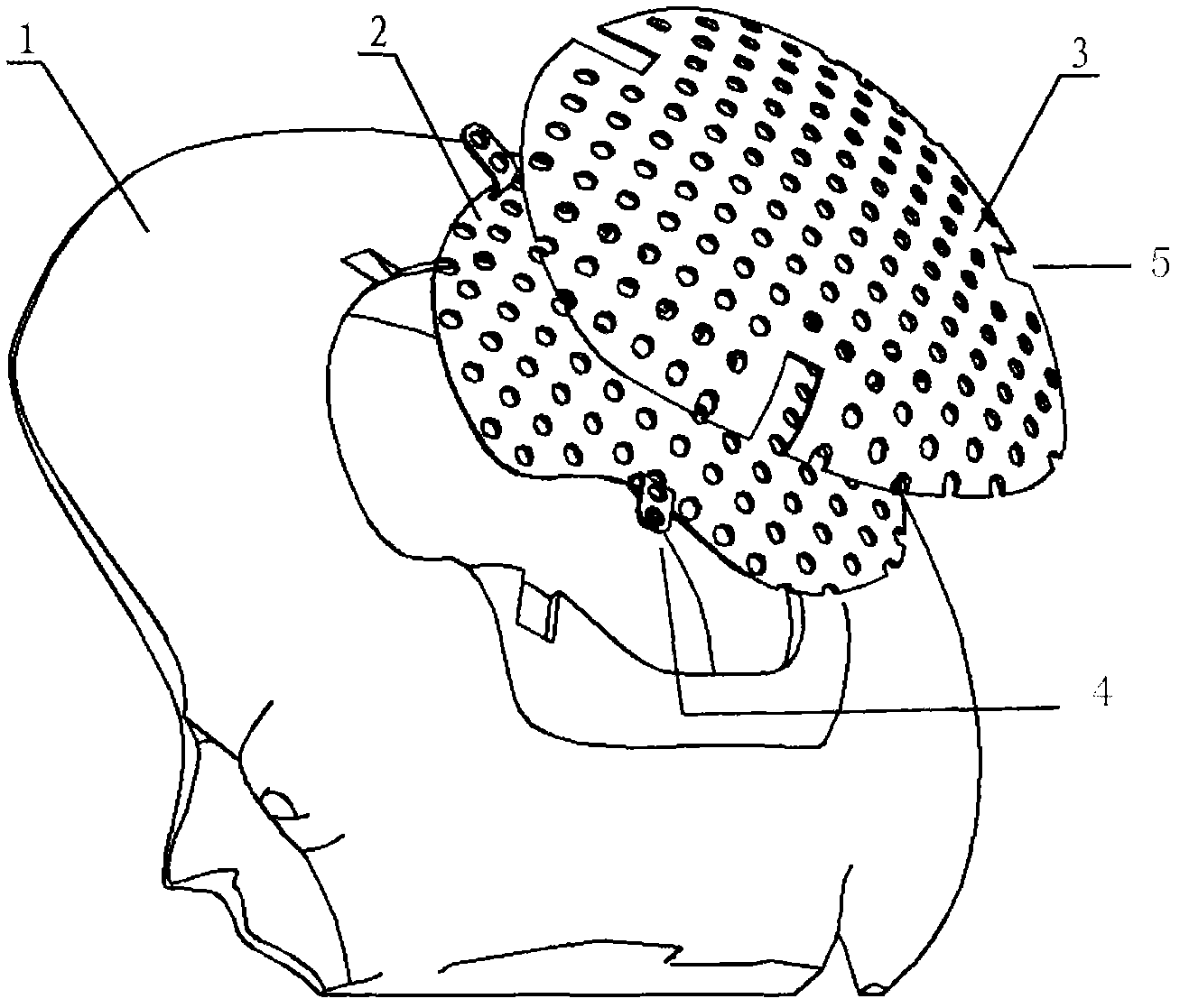
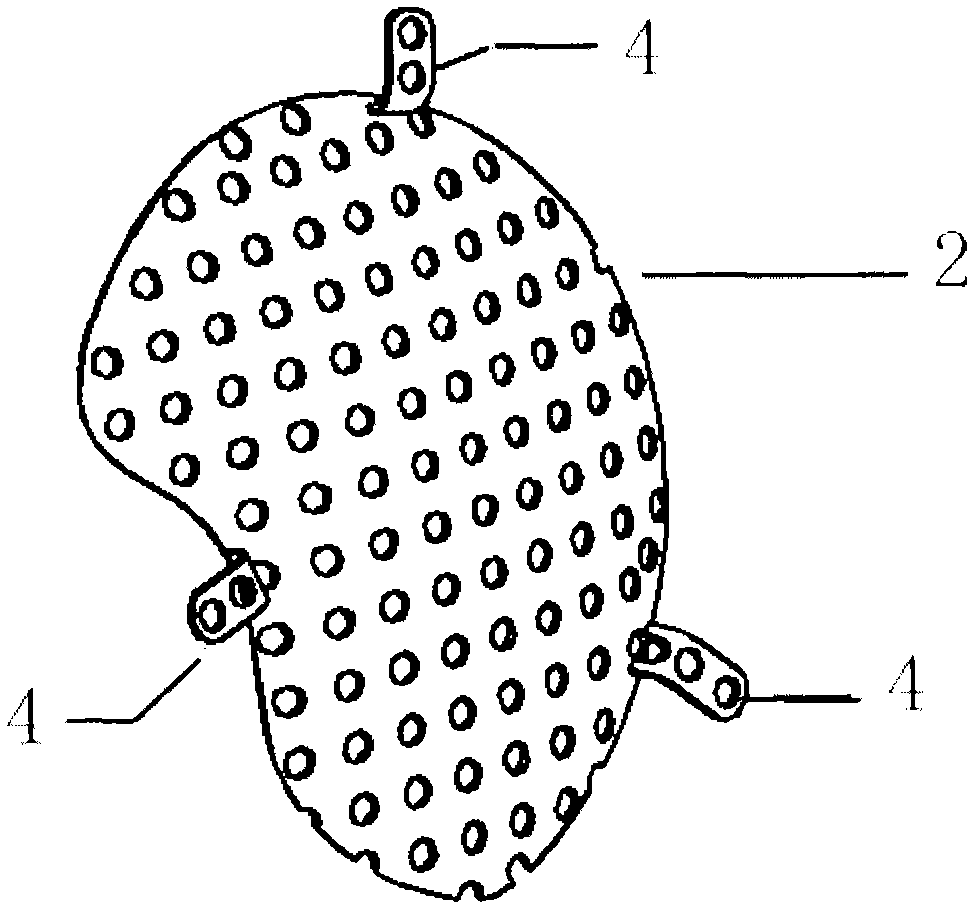
Double-layer titanium mesh for cranioplasty
The invention relates to a double-layer titanium mesh for cranioplasty, which is characterized by comprising an inner-layer titanium mesh and an outer-layer titanium mesh, wherein the outer-layer titanium mesh coincides with the defective edge on the outer surface of the cranial bone; the curvature of the outer-layer titanium mesh conforms to the appearance of the head; the gap between the inner-layer titanium mesh and the outer-layer titanium mesh is filled with autologous bone granules or artificial bone granules; the outer-layer titanium mesh comprises a scalp contact surface and fixed-end grooves; the curvature of the outer-layer titanium mesh conforms to the outer surface of the skull; the edge of the outer-layer titanium mesh coincides with the defective edge on the outer surface of the cranial bone; the outer-layer titanium mesh is fixed on the defective position on the outer surface by screws, so that the outer surface of the cranial bone can be closed; and the outer-layer titanium mesh is provided with holes. The titanium mesh can simultaneously repair the inner surface and the outer surface of the cranial bone, thereby realizing full anatomical and functional recovery and avoiding the brain tissue damage caused by inner surface defects.
Owner:中国人民解放军第四军医大学唐都医院
Tissue engineering nerve graft and application thereof
The invention discloses a chitosan artificial nerve graft containing a neurotrophic factor and a preparation method thereof. In the chitosan artificial nerve graft, chitosan is processed into a nerve conduit with a porous structure and high tensile strength by performing processes such as weak acid dissolving, injection molding, molding, neutralization fixing, cleaning, freeze drying and the like under the condition of not adding any foaming agent or crosslinking agent. The nerve conduit contains cells or tissues which have treating effects and are included or distributed inside or on the surfaces of tubular body pores, including autologous bone marrow stem cells, autologous bone mesenchymal stem cells, schwann cells or dorsal root ganglion tissues or combinations thereof. A tissue engineering nerve can be used for repairing peripheral nerve defect and can be applied to repairing of spinal cord injury simultaneously.
Owner:NANTONG UNIVERSITY
Methods for collecting and processing autografts, processed autografts, kits for collecting and transporting autografts, and tools for preparing autografts
ActiveUS20110160857A1Easy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsAnatomyAutogenous graft
The present invention is directed to methods for collecting and processing autografts, processed autografts, kits for collecting and transporting autografts, and tools for preparing autografts. It is also directed to autologous bone grafts, and methods of preparing them.
Owner:LIFENET HEALTH
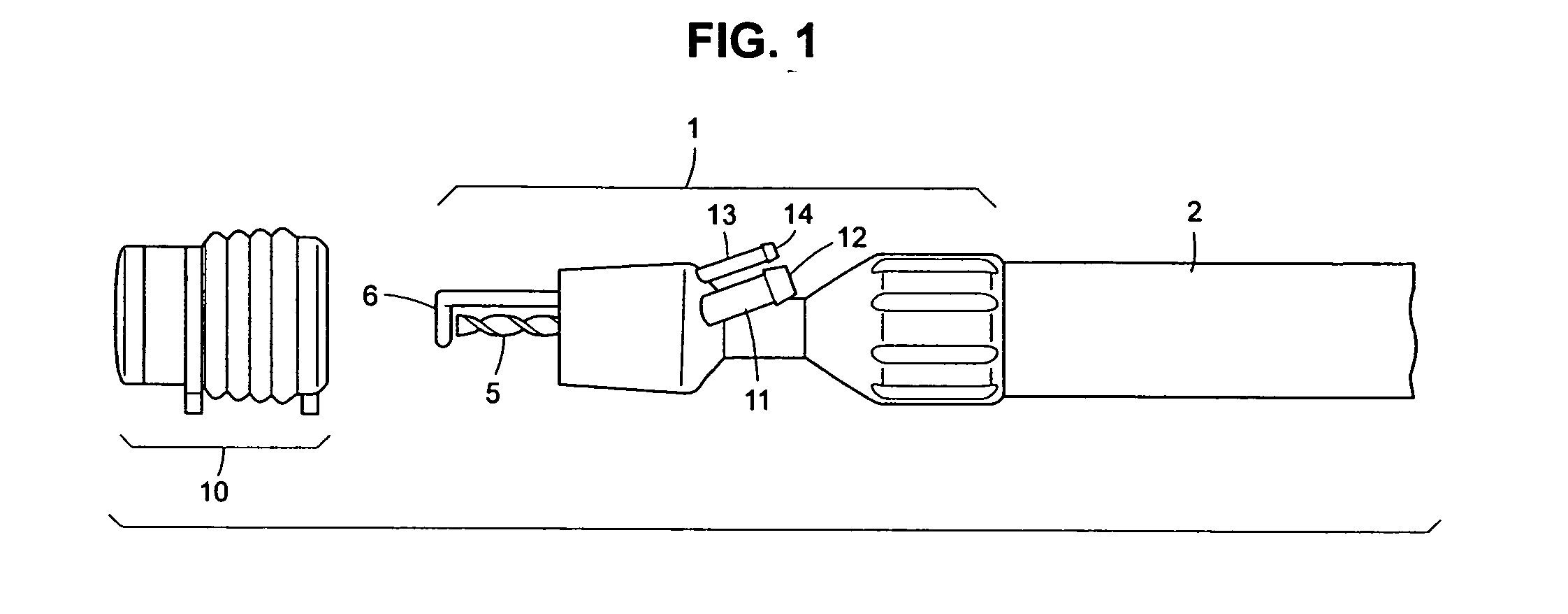
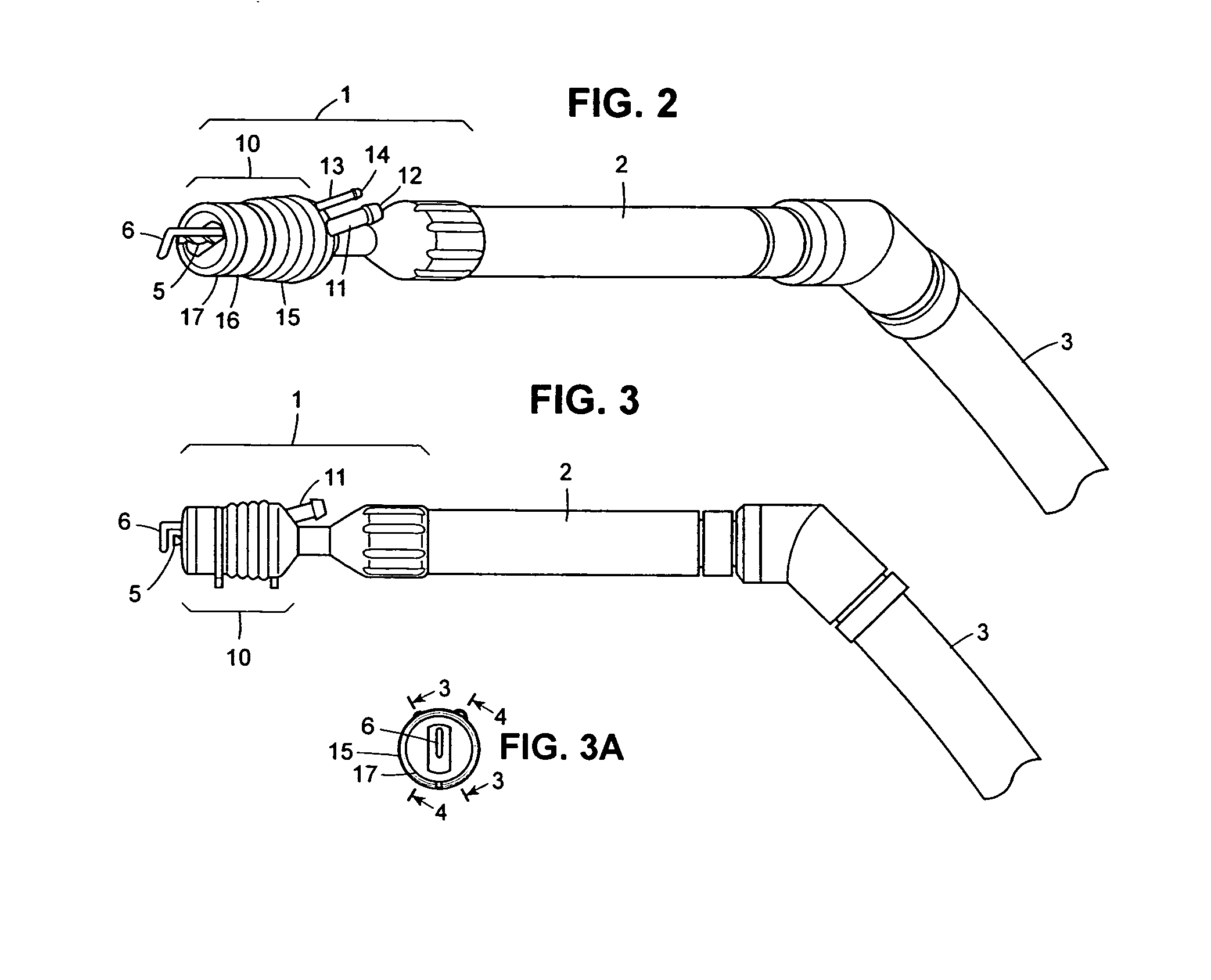
Autologous bone harvest during osteotomy and bone drilling procedures
InactiveUS20070270771A1Reduce pollutionReliable sourceSuction devicesProsthesisBones fusionSurgical site
An apparatus and method for collecting particulate bone from the operating site during an osteotomy or bone drilling procedure so that it can be used subsequently to augment the bone fusion process. A bone cutting or drilling tool is provided with a module for collecting particulate bone simultaneously with cutting or drilling the bone. The collected particulate bone is transferred continuously to a sterile containment module and maintained under sterile conditions until it is prepared for re-use in the patient.
Owner:FIRST COMMERCE BANK
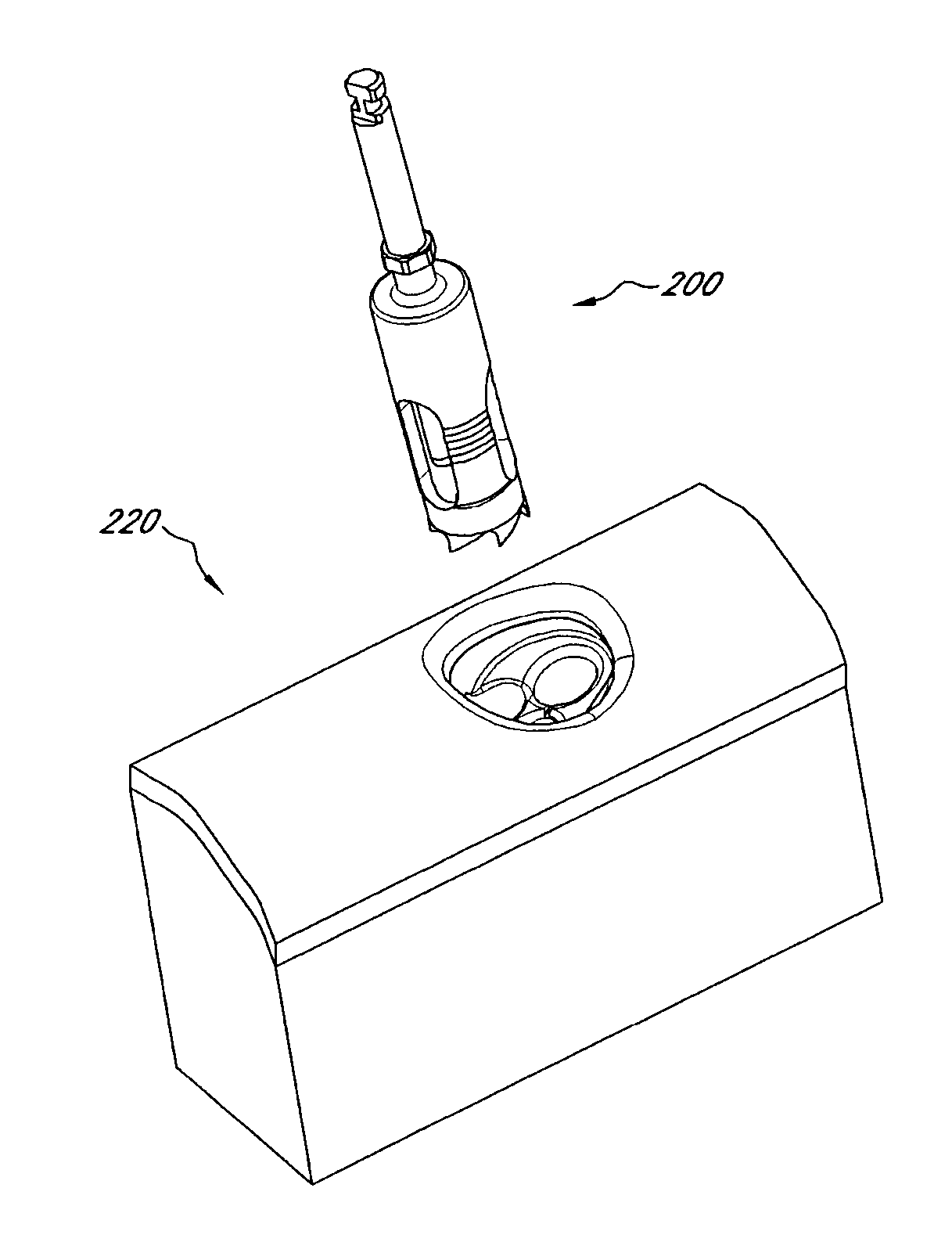
Device and procedure for implanting a dental implant
InactiveUS20120129126A1Easy to controlImprove cutting accuracyDental implantsDental toolsOsteotomyCoring
Various tools and procedures are provided for implantation of an implant at a target site. The procedure can comprise performing an osteotomy at the target site; placing a guide sleeve into the osteotomy; inserting a coring tool into the guide sleeve; coring the target site up to a depth less than the depth of the osteotomy, the coring tool being configured to collect autogenous bone material from the target site during the coring of the target site; placing the implant at the implant site; and grafting the autogenous bone material into selected portions of the target site.
Owner:NOBEL BIOCARE SERVICES AG
Autologous surgical bone collection and filtration
Apparatus and methods are disclosed for use during surgical procedures for collecting autologous bone. A preferred bone collection assembly collects bone by compressing blood products away from trapped bone using a press. Such bone collecting assembly is easy and uncomplicated to use, and can be easily integrated into an operation where the need for collection and utilization of autologous bone exists.
Owner:HENSLER SURGICAL PROD
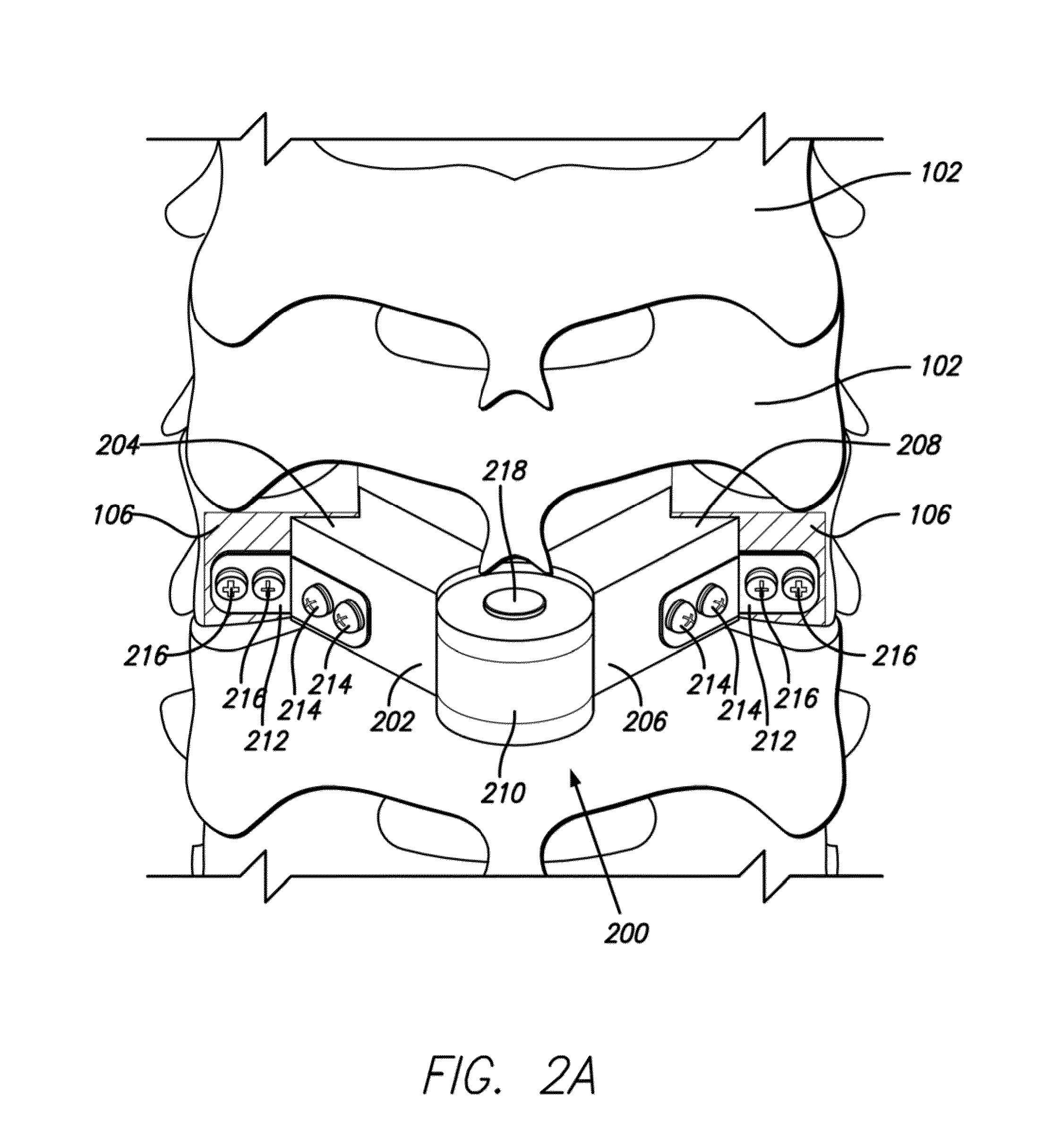
Multi-component cortical bone assembled implant
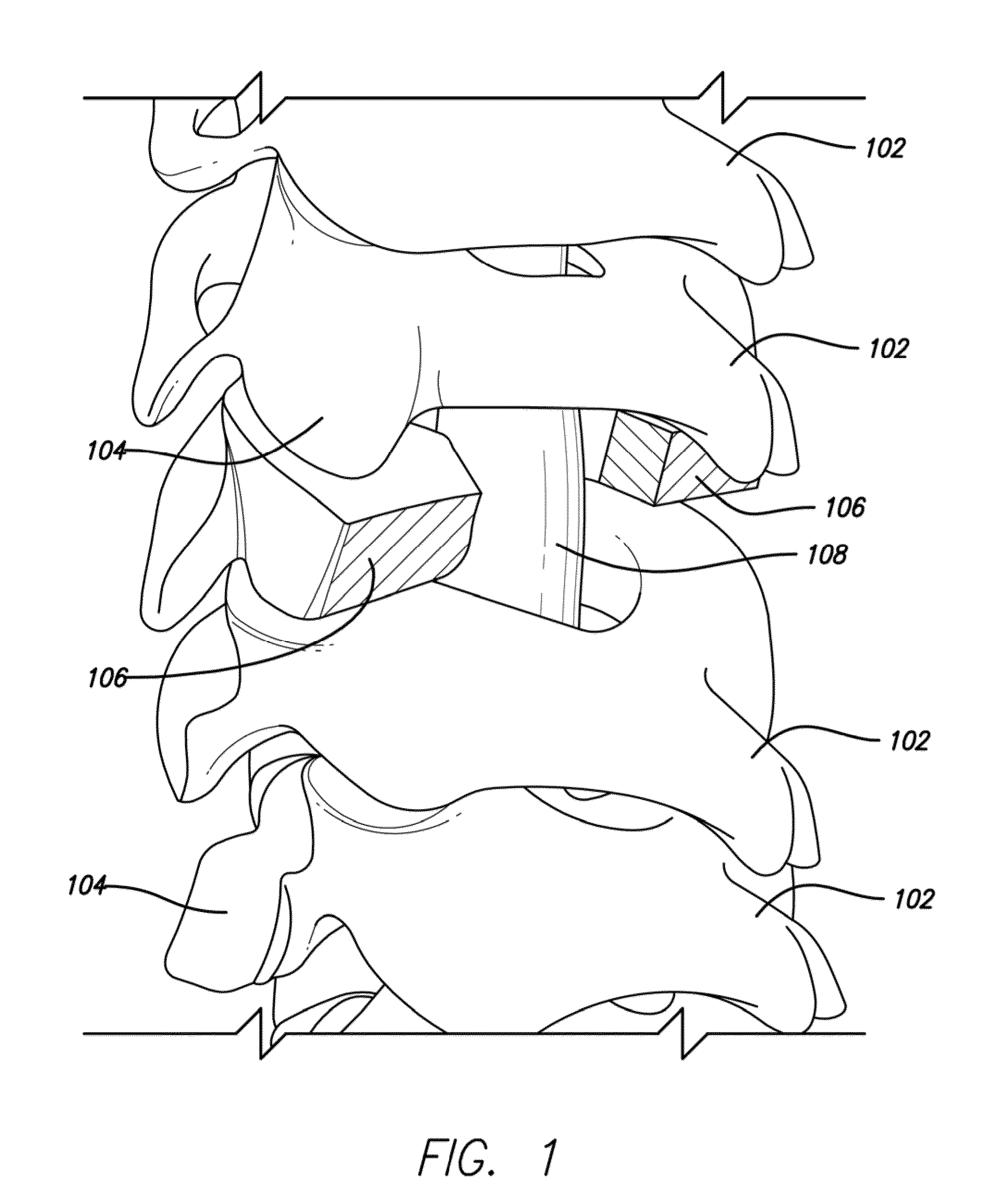
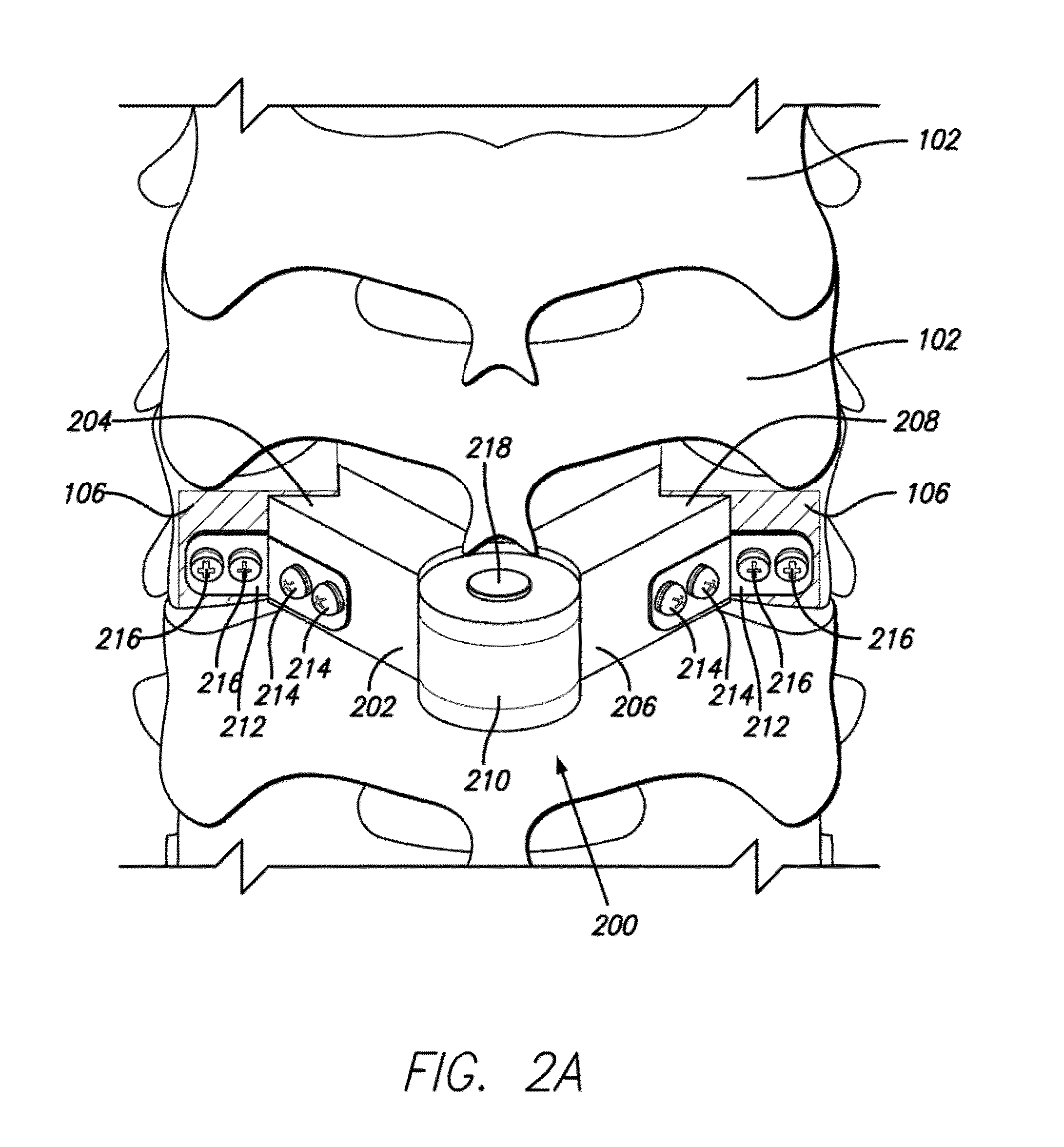
An implant composed substantially of cortical bone is provided for use in cervical Smith-Robinson vertebral fusion procedures. The implant is derived from allograft or autograft cortical bone sources, is machined to form a symmetrically or asymmetrically shaped (e.g. a substantially “D”-shaped) implant having a canal running therethrough according to methods of this invention, and inserted into the space between adjacent cervical vertebrae to provide support and induce fusion of the adjacent vertebrae. Osteogenic, osteoinductive or osteoconductive materials may be packed into the canal of the implant to expedite vertebral fusion and to allow autologous bony ingrowth.
Owner:RTI BIOLOGICS INC
Lamina implant and method
A prosthetic implant for restoring lamina after a laminectomy. The implant is generally a lamina-sized construct having a hollow interior. The lamina removed during the laminectomy may be converted to autologous bone that may then be placed inside the hollow interior of the implant. The implant may then be secured to the spine at the site of the laminectomy so that lamina restoration can occur as the hollow interior of the implant solidifies with bone growth.
Owner:GLOBUS MEDICAL INC
Autologous Bone Graft Material
This invention relates to a musculoskeletogenic MSG graft composite made from whole bone marrow aspirate BMA having native levels of musculoskeletal progenitor cells MSPCs, comprising: a) a suspension of fractionated BMA comprising: i) MSPCs present at a level greater than their native level in whole BMA, and ii) red blood cells RBCs present at a level less than their native level in whole BMA, and b) a porous sterile matrix having an average pore size of at least 20 μm.
Owner:DEPUY SPINE INC (US)
Autologous Bone Harvest During Osteotomy and Bone Drilling Procedures
ActiveUS20100298835A1Reduce pollutionReliable sourceProsthesisBone drill guidesSurgical siteBones fusion
An apparatus and method for collecting particulate bone from the operating site during an osteotomy or bone drilling procedure so that it can be used subsequently to augment the bone fusion process. A bone cutting or drilling tool is provided with a module for collecting particulate bone simultaneously with cutting or drilling the bone. The collected particulate bone is transferred continuously to a sterile containment module and maintained under sterile conditions until it is prepared for re-use in the patient.
Owner:NEW AMSTERDAM LLC
Moldable bone repairing material for bone repairing and preparation method thereof
ActiveCN103800945AAppropriate degradation rateSuitable compressive strengthProsthesisBiocompatibility TestingRepair material
The invention provides a moldable bone repairing material for bone repairing. The material is prepared from the following raw materials in parts by weight: 20-90 parts of alpha-calcium sulfate hemihydrate and hydroxyapatite, 20-40 parts of bioactive mineral powder, 10-80 parts of autologous bone powder particles or DBM (demineralized bone matrix) particles, and hydrogel, wherein the ratio of hydrogel to the total usage of the raw materials is 1:0.5-1:15, the hydrogel is optimally PEO-PPO-PEO (polyoxyethylene-polyoxypropylene-polyoxyethylene) segmented copolymer solution. The prepared moldable bone repairing material for bone repairing is applicable to in-situ curing after wound injection and in-vitro quick curing for bone repairing, wherein all components are proved to be degradable materials with excellent biocompatibility.
Owner:北京科健生物技术有限公司
Treatment for Chronic Myocardial Infarct
ActiveUS20090148415A1BiocidePharmaceutical delivery mechanismChronic myocardial infarctionMCA Infarction
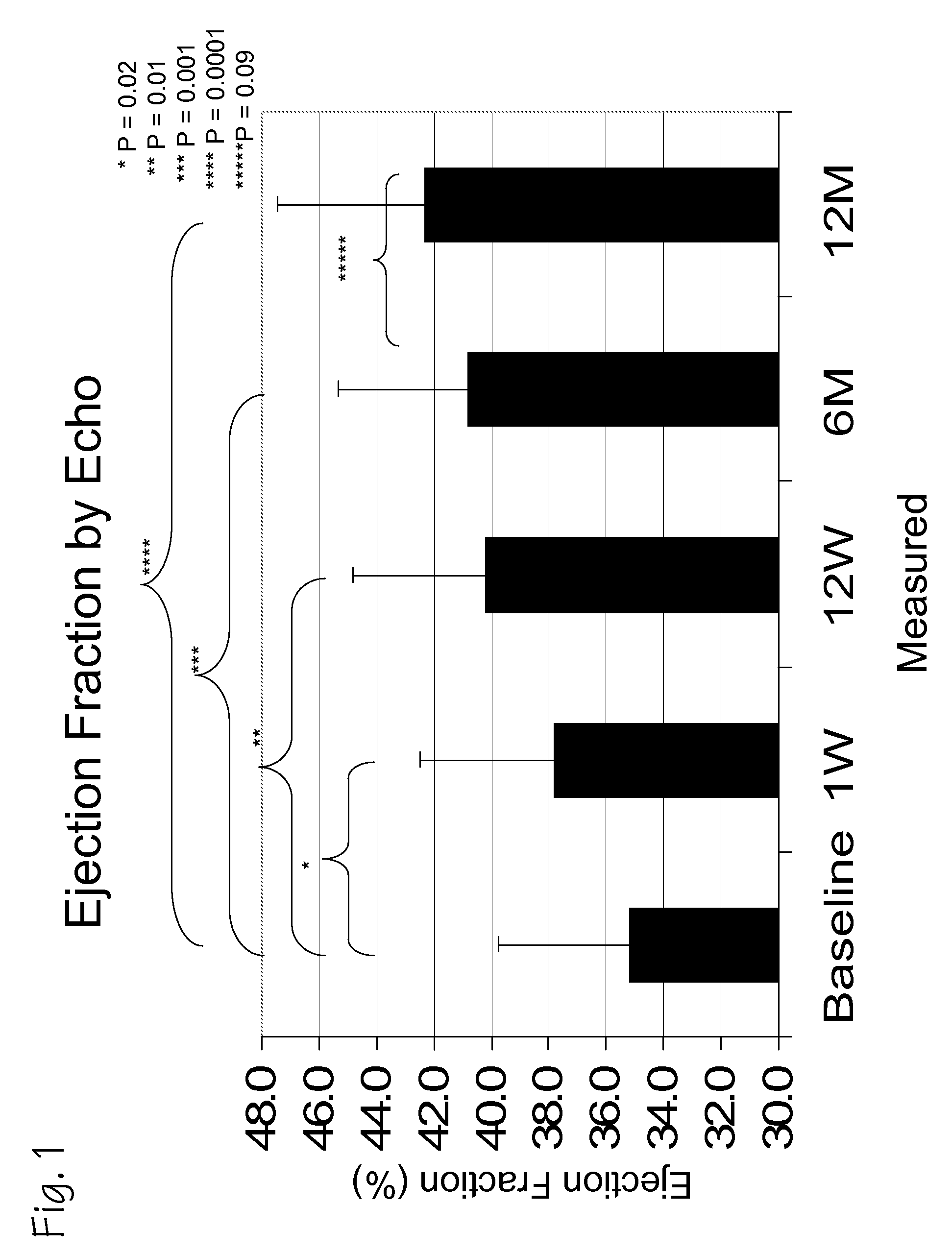
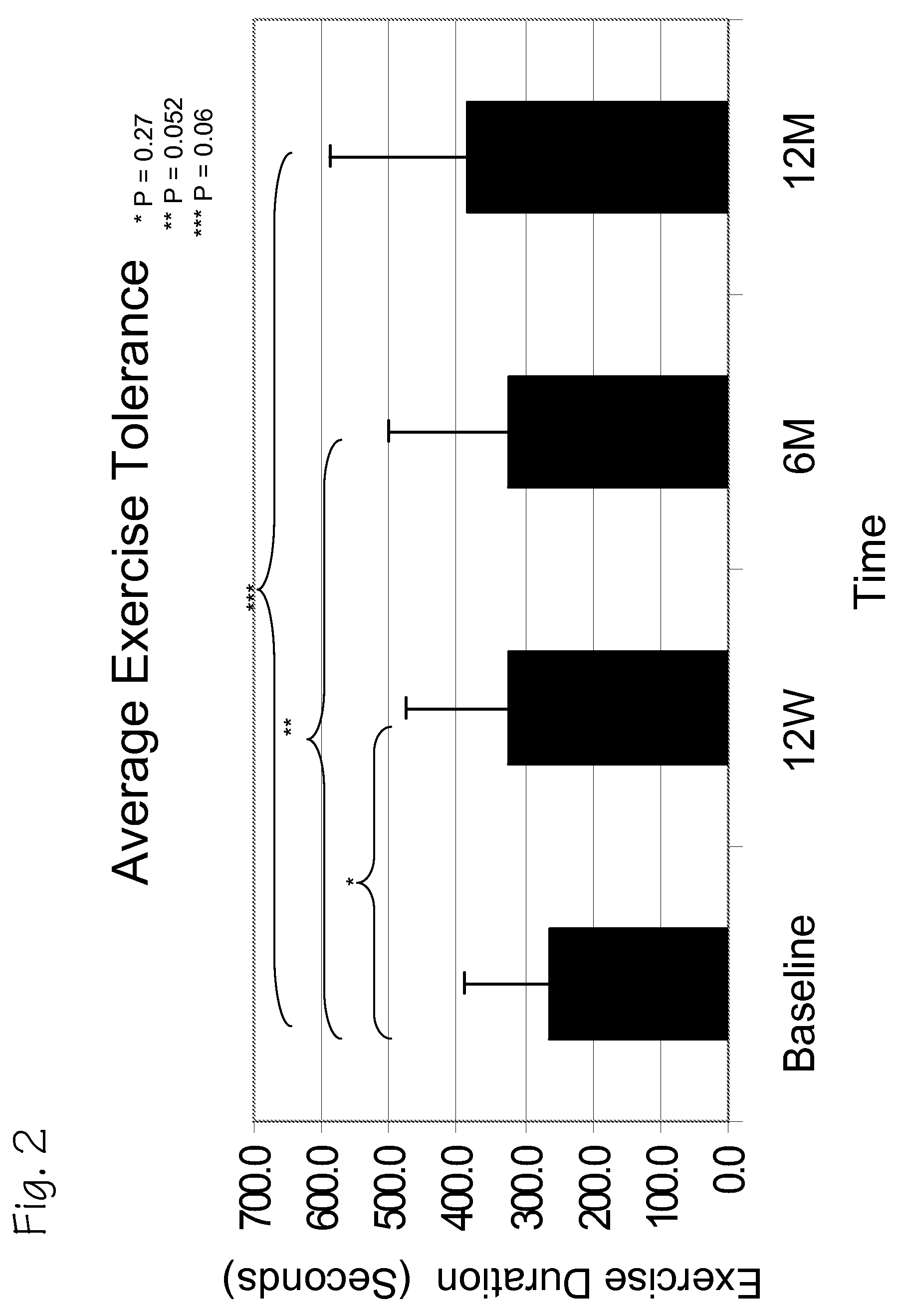
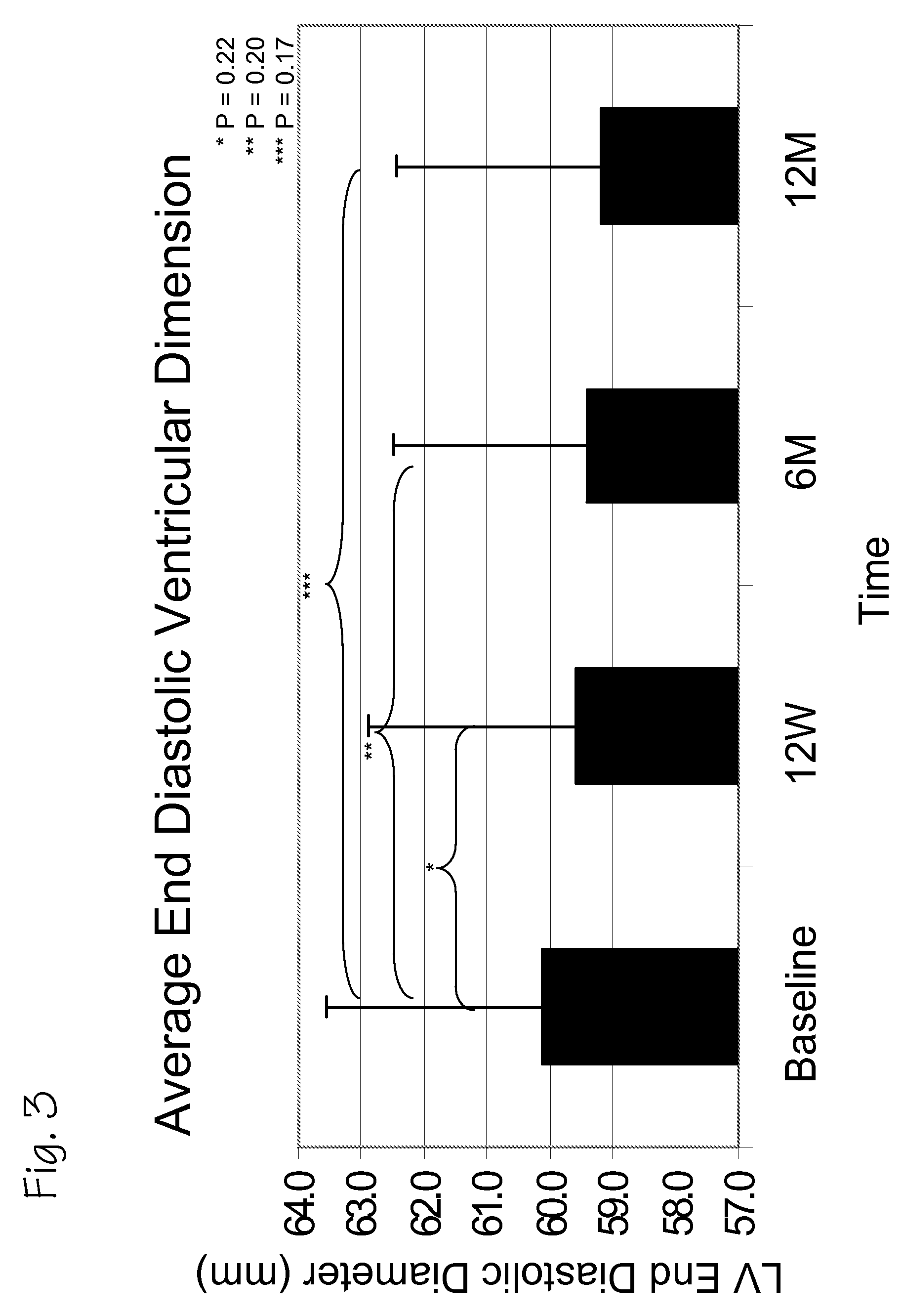
A method of treating chronic post-myocardial infarction including helical needle transendocardial delivery of autologous bone marrow (ABM) mononuclear cells around regions of hypo or akinesia in chronic post-myocardial infarction (MI) patients. The treatment is safe and improves ejection fraction (EF).
Owner:BIOCARDIA
Manufacturing method of individualized stent for repairing defect of more than four tooth positions on one side of low jawbone
InactiveCN103315829AGood biocompatibilityImprove biological activityBone implantBone formationCombined method
The invention discloses a manufacturing method of an individual stent for repairing defect of more than four tooth positions on one side of a low jawbone. The manufacturing method includes designing a repairing model of personalized appearance and an internal microstructure of individual defect through a CT (computed tomography) scan and image combined method and a reverse engineering method, obtaining a to-be-repaired body of a defect area through a cutting algorithm and manufacturing the to-be-repaired body through an EBSM (electron beam selective melting) method after being designed by a BOOL algorithm; implanting a three-dimensional metal stent into the low jawbone defect area; adopting titanium bolts to fix the to-be-repaired body and broken ends of two fractured bones; adding osteogenesis materials and autologous bone marrow strmal cells to the broken ends of the two fractured bones; suturing soft tissues and implanting an implant after bone formation is performed; finally completing repairing of false teeth. By the manufacturing method, bone growth can be better promoted and guided, effect of bone combination of the implant and surrounding bones is good, and repairing is facilitated in the long run.
Owner:GENERAL HOSPITAL OF PLA
Stem cells obtained from pulp of deciduous or permanent teeth and of dental germ, able to produce human bone tissue
ActiveUS20090035376A1Cell dissociation methodsPowder deliveryCell-Extracellular MatrixSub populations
In this invention is described a method that foresees the isolation of a new subpopulation of stem cells derived form dental pulp, whose differentiation is osteoblasts lead to the subsequent production and employment of a bone tissue, called LAB (Living Autologous Bone). Specifically, the invention describes: 1) the isolation of stem cells from the pulp of deciduous and permanent teeth and of dental germs, obtained from human subjects; 2) the growth of these cells in vitro, under specific conditions that allow the isolation of a cellular sub-population, which, after differentiation in osteoblasts, is able to produce in vitro an extracellular matrix, identical to that detectable in bone tissue; 3) the use of this selected and differentiated cell population in order to produce autologous bone tissue in vitro, containing vital osteoblasts; 4) the preservation of the LAB under conditions which guarantee cellular vitality; 5) the use of the LAB in donor patients to reconstruct bone tissue, as required in the daily practice in dentistry, maxillo-facial surgery and orthopedics.
Owner:CARINCI FRANCESCO +5
Cell injection for treating bone injury and preparation method thereof
InactiveCN102232970AReduce the impactSimple and fast extraction procedureSkeletal disorderUnknown materialsCell injectionTemperature sensitive
The invention relates to a cell injection for treating bone injury and a preparation method thereof, belonging to the field of tissue engineering. The injection is a compound formed by mesenchymal stem cells from autologous bone marrow, temperature sensitive gel and effective trophic factors separated and extracted from autoblood. The preparation method comprises the steps of: after extracting the bone marrow of a patient, separating and extracting the mesenchymal stem cells from the bone marrow, adding no animal serum in the amplification culture process, and mixing 1*(105-108) cells with 1-5ml of biological scaffold material to form the injectable compound. The cell injection for treating bone injury is mainly used for treating bone injuries including osteoarthritis, femoral head necrosis and delayed union and disunion of bone fracture, is convenient to use, has notable effect without immunological rejection, and can alleviate the great pain of patients caused by bone injury.
Owner:董运海
Autologous bone graft material
This invention relates to a musculoskeletogenic MSG graft composite made from whole bone marrow aspirate BMA having native levels of musculoskeletal progenitor cells MSPCs, comprising:a) a suspension of fractionated BMA comprising:i) MSPCs present at a level greater than their native level in whole BMA, andii) red blood cells RBCs present at a level less than their native level in whole BMA, andb) a porous sterile matrix having an average pore size of at least 20 μm.
Owner:DEPUY SPINE INC (US)
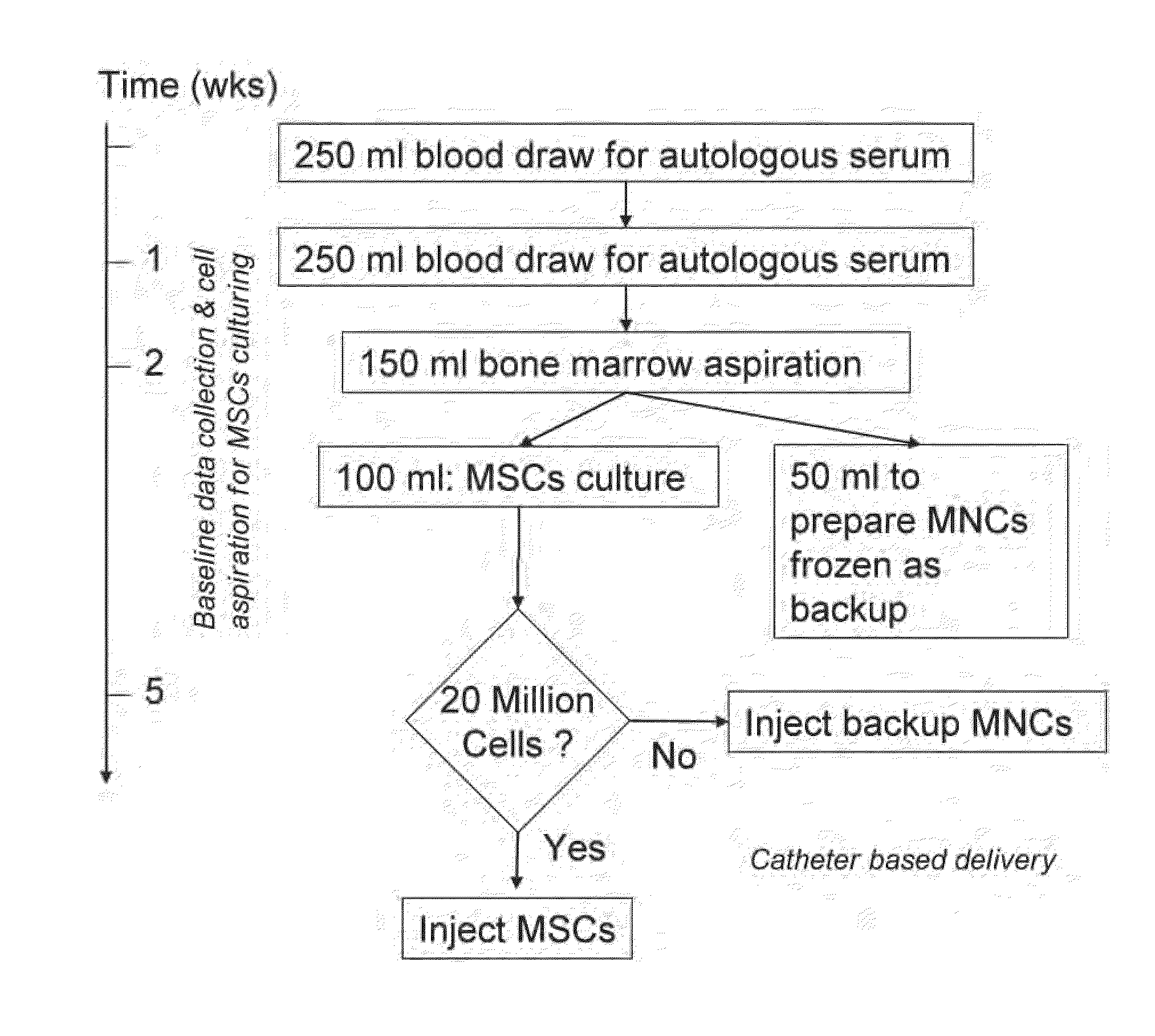
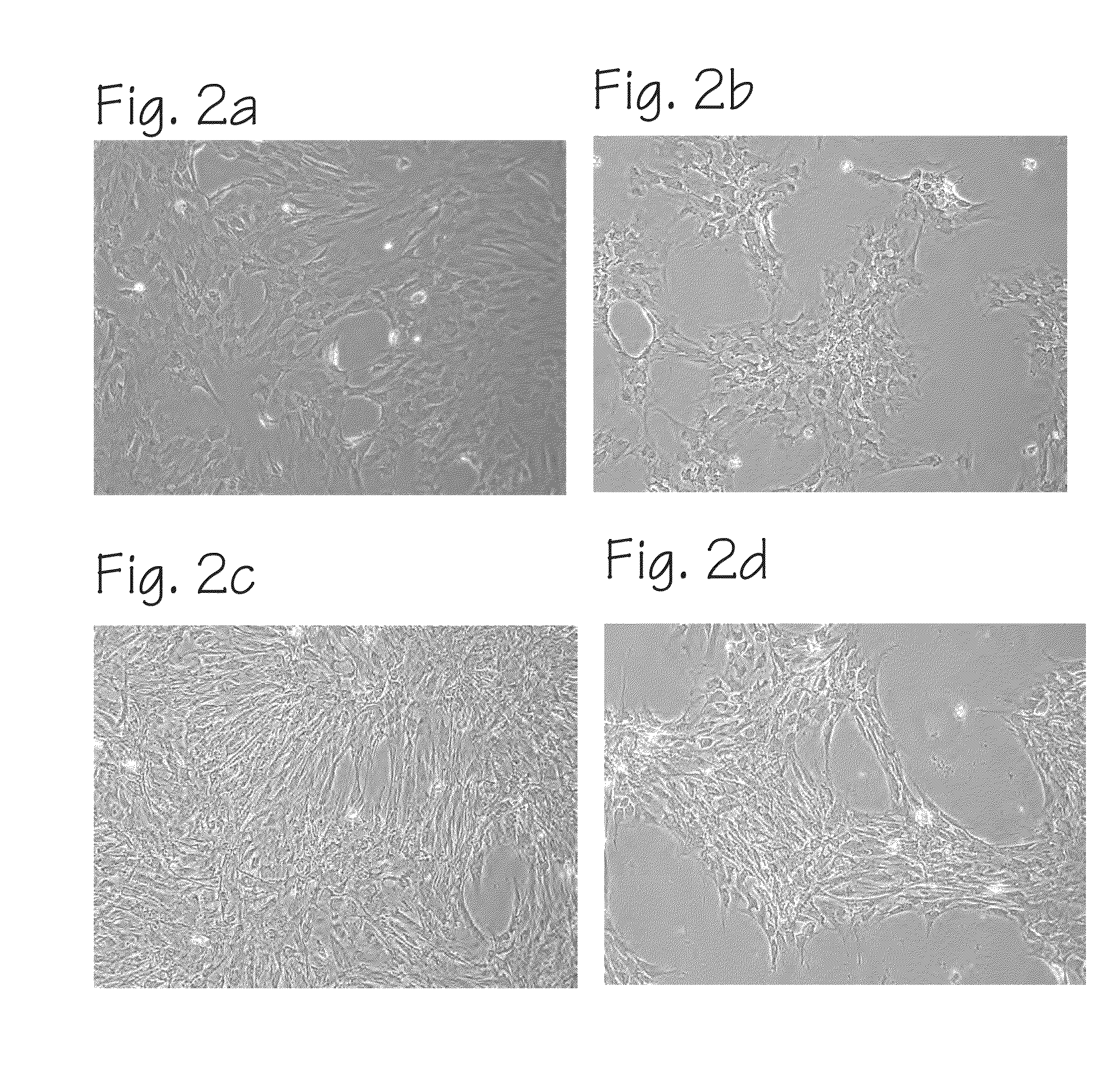
Method of Preparing Autologous Cells and Method of Use for Therapy
A method for expanding mesenchymal cells derived from autologous bone marrow in autologous culture medium which can be used in a clinical setting, and a business method for performing such expansions in the future as a service for patients. A method for expanding mesenchymal cells derived from autologous bone marrow in autologous culture medium including a diagnostic kit for the autologous cell therapy to determine whether a patient will respond to the autologous cell therapy for treatment of a disease, in which said kit comprising a system for detecting gene and protein expression comprising at least two isolated DNA molecules wherein each isolated DNA molecule detects expression of a gene that is differentially expressed in the tissue of the patient that is intended to be the source of the autologous cell therapy.
Owner:BIOCARDIA
Bone defect regeneration and repair tissue-engineered bone, as well as construction method and application thereof
ActiveCN105013016AGood mechanical propertiesGood biological propertiesProsthesisCell-Extracellular MatrixTreatment effect
The invention discloses a bone-defect regeneration and repair tissue-engineered bone, as well as a construction method and an application thereof. The tissue-engineered bone is a composite laminated structural body formed by three layers of mesenchymal stem cell polymers and two layers of bone matrix particles, wherein the composite laminated structural body is a complex formed by mesenchymal stem cell polymers and bone matrix particles which are alternately arranged; the mesenchymal stem cell polymer comprises mesenchymal stem cells and extracellular matrix which is secreted by the mesenchymal stem cells and is distributed around the mesenchymal stem cells. The tissue-engineered bone formed by adopting the construction method can be used for simultaneously compounding living cells and active factors, the repairing and reconstructing process is quick, the joint of new bone and surrounding autogenous bone is complete and uniform in thickness. The tissue-engineered bone is suitable for bone-defect regeneration and repair, physiological regeneration of bones is realized, and the repairing treatment effect of bone defect is improved.
Owner:广东芙金干细胞再生医学有限公司
Cartilage repair methods
The present application discloses methods for repairing hyaline cartilage defects. The methods comprise a combination of introducing autologous bone mesenchymal stem cells to a joint, and applying to the joint a membrane comprising a polyester entangled with a polysaccharide. In some aspects, the bone mesenchymal stem cells are mesenchymal stem cells originating in bone underlying the joint. In these aspects, contact between the joint and the mesenchymal stem cells can be effected by introducing apertures through the bone using standard surgical techniques such as microfracture, abrasion, or drilling. Cartilage which forms in response to application of these methods is hyaline cartilage rather than fibrocartilage.
Owner:ISTO TECH II LLC
Intramyocardial injection of autologous bone marrow
InactiveUS20040161421A1Enhance therapeutic myocardial angiogenesisGood effectBiocidePeptide/protein ingredientsAngiogenesis growth factorCardiac muscle
A method of treating cardiac or myocardial conditions comprises the administration of an effective amount of autologous bone marrow. The bone marrow may optionally be stimulated and / or administered in combination with a pharmaceutical drug, protein, gene or other factor or therapy that may enhance bone marrow production of angiogenic growth factors and / or promote endothelial cell proliferation or migration or blood vessel formation.
Owner:KOMOWSKI RAN +3
Tissue engineered bone constructed from allogeneic bone marrow mesenchymal stem cells and application thereof
InactiveCN103768656AIncrease success rateQuality improvementProsthesisComposite Tissue AllograftBone defect
The invention discloses an allogeneic tissue engineered bone implant, which comprises a biodegradable material and bone marrow mesenchymal stem cells, wherein the bone marrow mesenchymal stem cells are in vitro cultured allogeneic bone marrow mesenchymal stem cells. The tissue engineered bone constructed from allogeneic bone marrow mesenchymal stem cells can repair bone defects, and the effect has no significant difference compared with conventional tissue engineered bone constructed from autologous bone marrow mesenchymal stem cells. The invention also provides a preparation method and application of the tissue engineered bone.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Preparation and Applications of 3D Bioprinting Bioinks for Repair of Bone Defects, Based on Cellulose Nanofibrils Hydrogels with Natural or Synthetic Calcium Phosphate Particles
ActiveUS20190307923A1Induce bone formationRepair large defectPharmaceutical delivery mechanismSkeletal/connective tissue cellsPorosityBiopolymer
The present invention relates to preparation of bioink composed of cellulose nanofibril hydrogel with native or synthetic Calcium containing particles. The concentration of the calcium containing particles can be between 1% and 40% w / v. Such bioink can be 3D Bioprinted with or without human or animal cells. Coaxial needle can be used where cellulose nanofibril hydrogel filled with Calcium particles can be used as shell and another hydrogel based bioink mixed with cells can be used as core or opposite. Such 3D Bioprinted constructs exhibit high porosity due to shear thinning properties of cellulose nanofibrils which provides excellent printing fidelity. They also have excellent mechanical properties and are easily handled as large constructs for patient-specific bone cavities which need to be repaired. The porosity promotes vascularization which is crucial for oxygen and nutrient supply. The porosity also makes it possible for further recruitment of cells which accelerate bone healing process. Calcium containing particles can be isolated from autologous bone, allogenic bone or xenogeneic bone but can be also isolated from minerals or be prepared by synthesis. Preferable Calcium containing particles consist of β-tricalcium phosphate which is resorbable or natural bone powder, preferably of human or porcine origin. The particles described in the present invention have particle size smaller than 400 microns, or more preferably smaller than 200 microns, to make it possible to handle in printing nozzle without clogging and to obtain a good resolution. Cellulose nanofibrils can be produced by bacteria orbe isolated from plants. They can be neutral, charged or oxidized to be biodegradable. The bioink can be additionally supplemented by other biopolymers which provide crosslinking. Such biopolymers can be alginates, chitosans, modified hyaluronic acid or modified collagen derived biopolymers.
Owner:CELLINK AB
Stem cells obtained from pulp of deciduous or permanent teeth and of dental germ, able to produce human bone tissue
In this invention is described a method that foresees the isolation of a new subpopulation of stem cells derived form dental pulp, whose differentiation is osteoblasts lead to the subsequent production and employment of a bone tissue, called LAB (Living Autologous Bone). Specifically, the invention describes: 1) the isolation of stem cells from the pulp of deciduous and permanent teeth and of dental germs, obtained from human subjects; 2) the growth of these cells in vitro, under specific conditions that allow the isolation of a cellular sub-population, which, after differentiation in osteoblasts, is able to produce in vitro an extracellular matrix, identical to that detectable in bone tissue; 3) the use of this selected and differentiated cell population in order to produce autologous bone tissue in vitro, containing vital osteoblasts; 4) the preservation of the LAB under conditions which guarantee cellular vitality; 5) the use of the LAB in donor patients to reconstruct bone tissue, as required in the daily practice in dentistry, maxillo-facial surgery and orthopedics.
Owner:POLO TECH PIEMONTESE SRL
Method for preparing biological scaffold by 3D printing
InactiveCN105903078AGood biocompatibilityNo toxicityAdditive manufacturing apparatusProsthesisBiologic scaffoldNormal bone
The invention provides a method for preparing a biological scaffold by 3D printing. The method comprises the steps of grinding allogeneic bones or heterogeneous bones at a low temperature in a freezing grinder to obtain a printing material, modeling 3D printing with the aid of a computer, and then performing post treatment in a genipin solution. The prepared biological scaffold has good biological compatibility, and the bone graft material does not have toxicity, rejection, mutagenicity or antigenicity in vivo, and does not disturb bone and tissue regeneration; the prepared biological scaffold can be gradually degraded and absorbed and replaced by autologous bone tissues, can bear the pressure close to 20MPa with normal bone cortex, and has good initial mechanical properties; and the elastic modulus is gradually reduced, stress shielding is avoided, fracture, collapse and loosening of implants in the long-term healing process are avoided, bone fusion can be accelerated, and the implants can be finally completely converted into autologous bone tissues.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com