Structural/biological implant system
a bio-injection and implant technology, applied in the field of bio-injection systems, can solve the problems of inadequate bone height and/or width for the placement of standard endosseous dental implants, bone resorption after tooth loss, and insufficient bone volume, etc., to achieve the effect of increasing bone height and volume in the patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
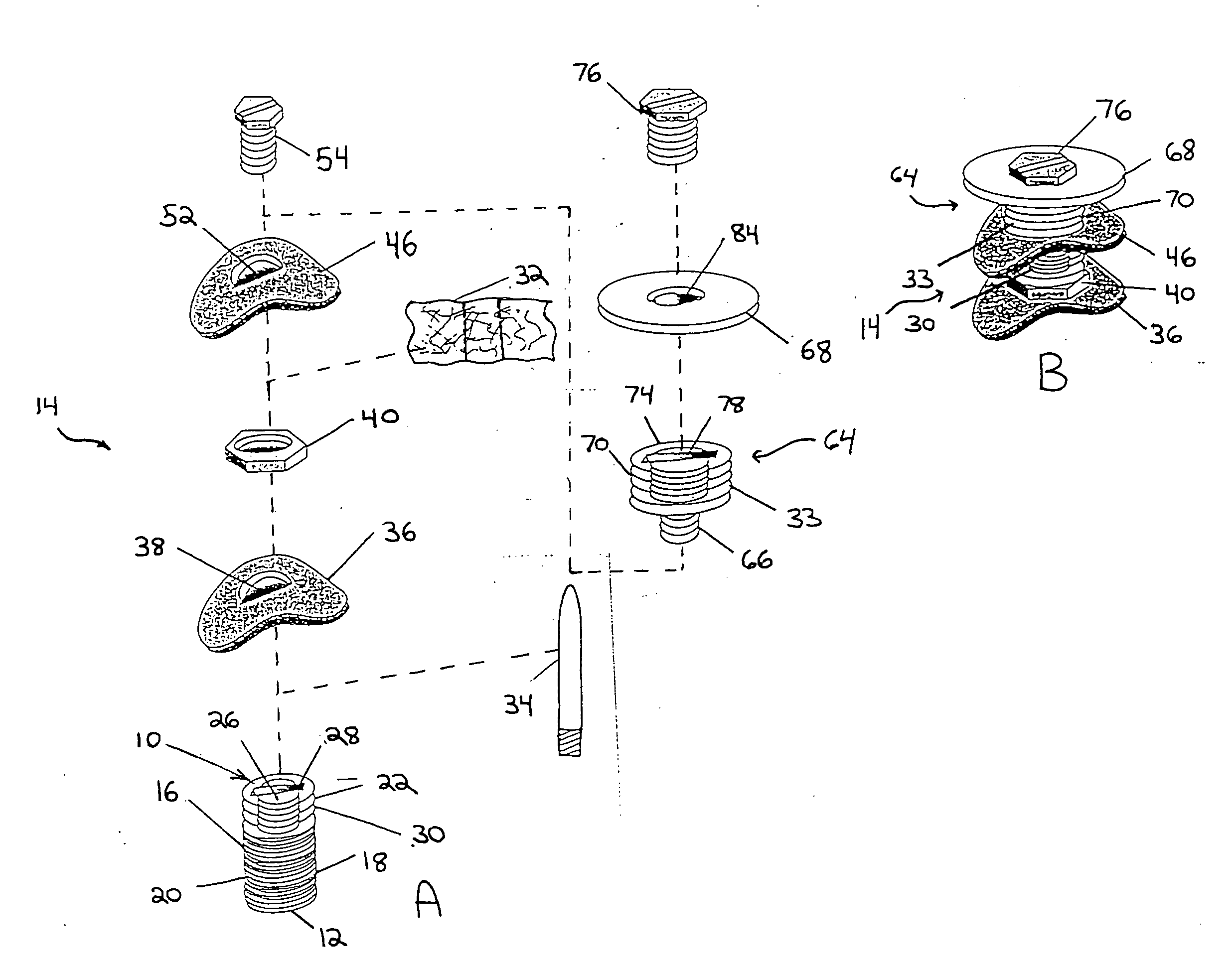
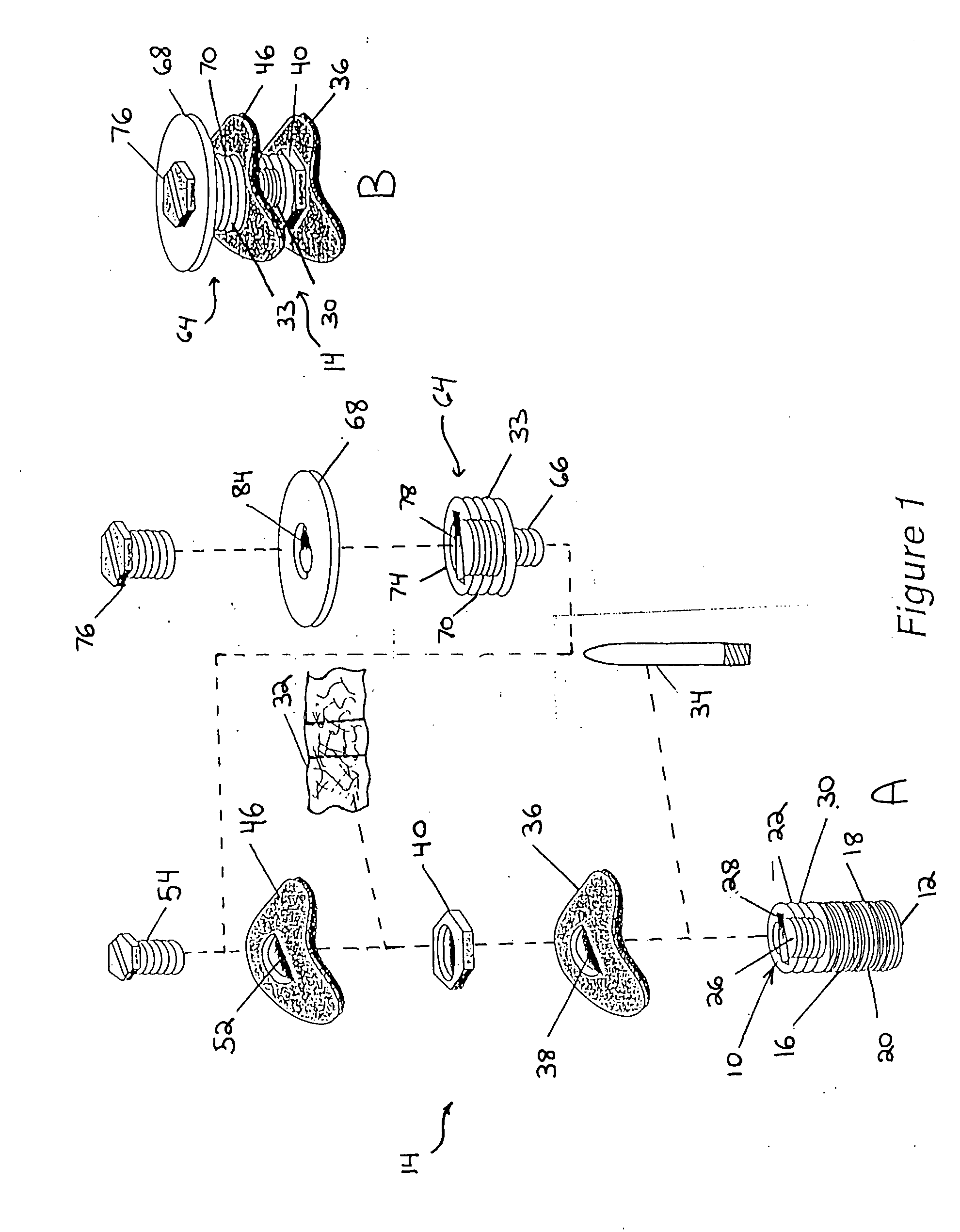
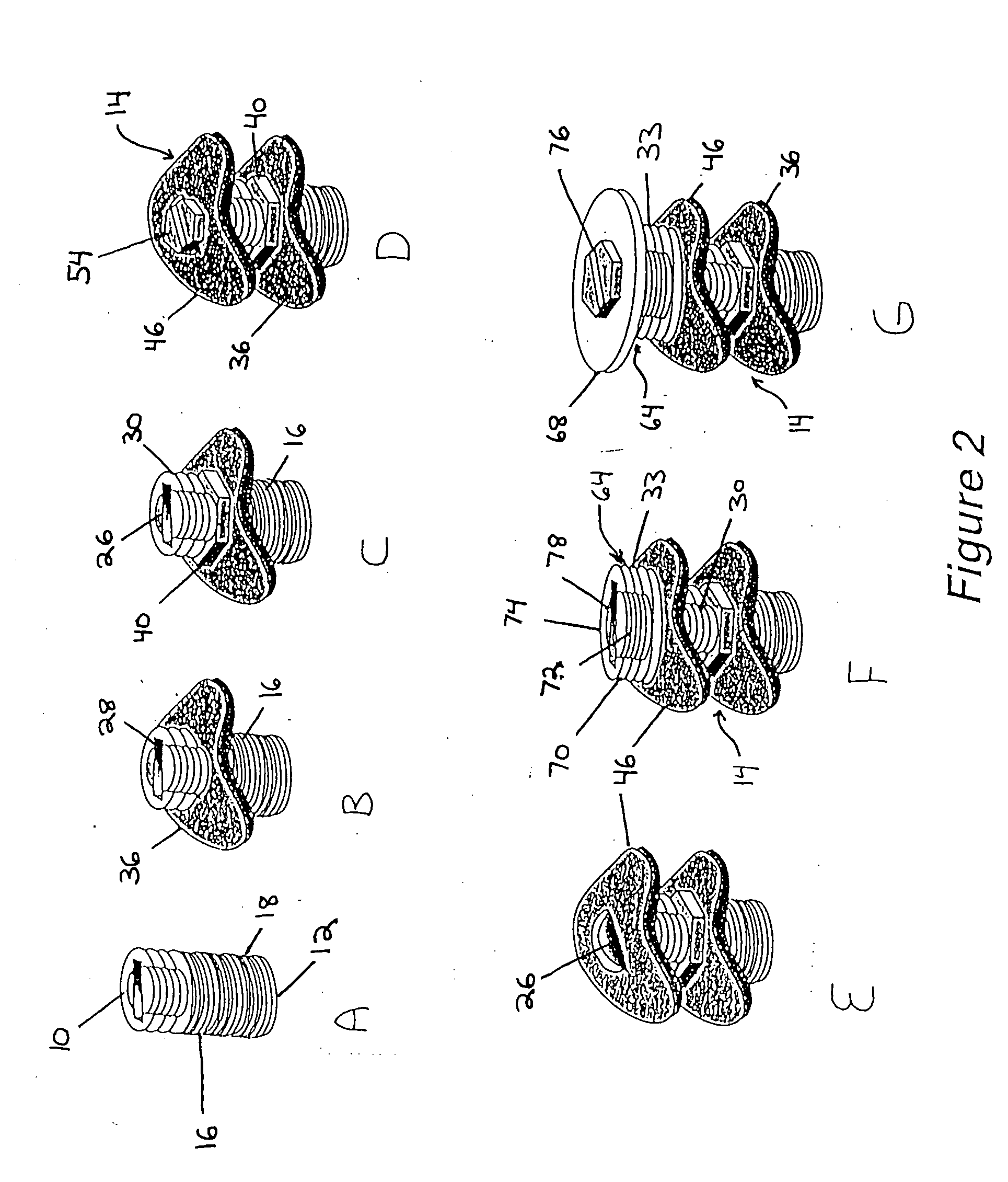
[0035] The implant in one embodiment of the invention as illustratively shown in FIG. 1 has a central axis (not shown) extending between a coronal end 10 and an apical end 12. The implant includes a level I assembly 10 having an initial implant portion 16 with an anchor portion 18. The anchor portion 18 may be substantially axially symmetric to the central axis. The anchor portion 18 has an exterior surface. In one embodiment the anchor portion 18 exterior surface has fastening means such as screw threads 20 helically disposed thereon. The threads advantageously have a minimal spacing, for example, less than 1 mm, and are self-tapping for optimal engagement and adaptation to existing alveolar bone. Other fastening means may be disposed on the anchor portion 18 exterior surface to help secure the anchor portion 18 to existing bone. The anchor portion 18 may for example be between about 3 mm and about 6 mm in length. The initial implant portion 16 may also have a transmucosal portion ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Biological properties | aaaaa | aaaaa |
| Bioavailability | aaaaa | aaaaa |
| Bioabsorbable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com