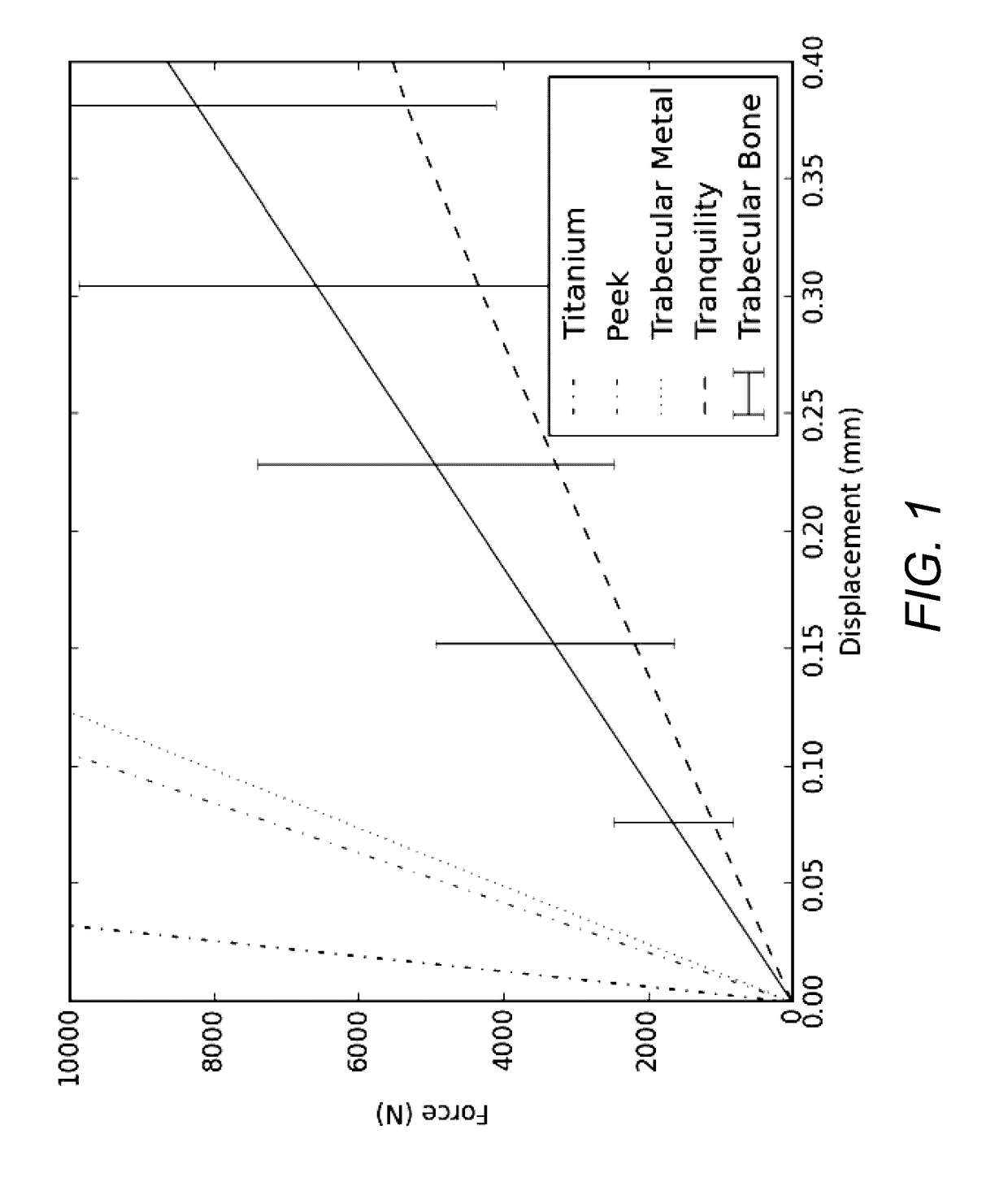
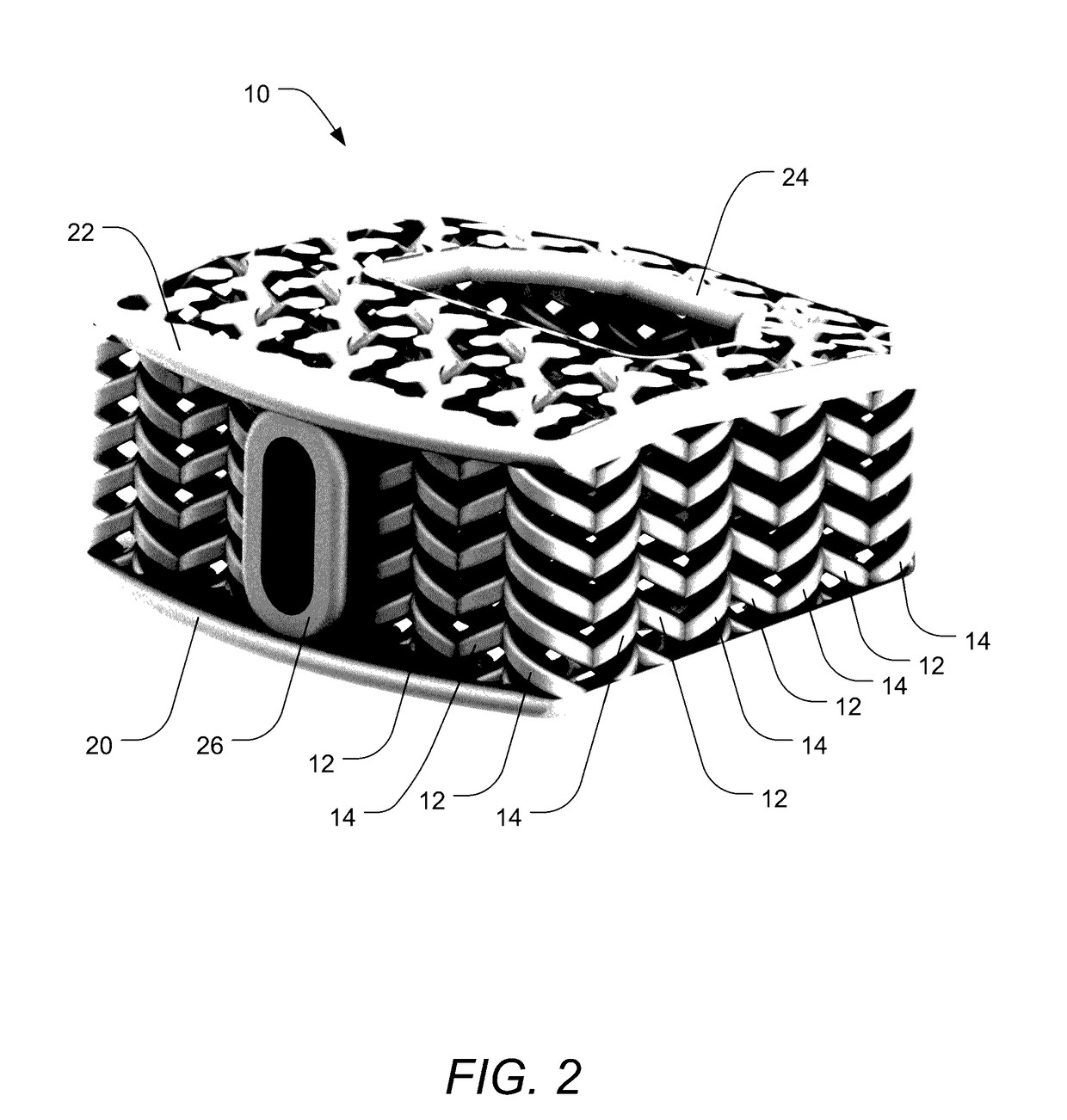
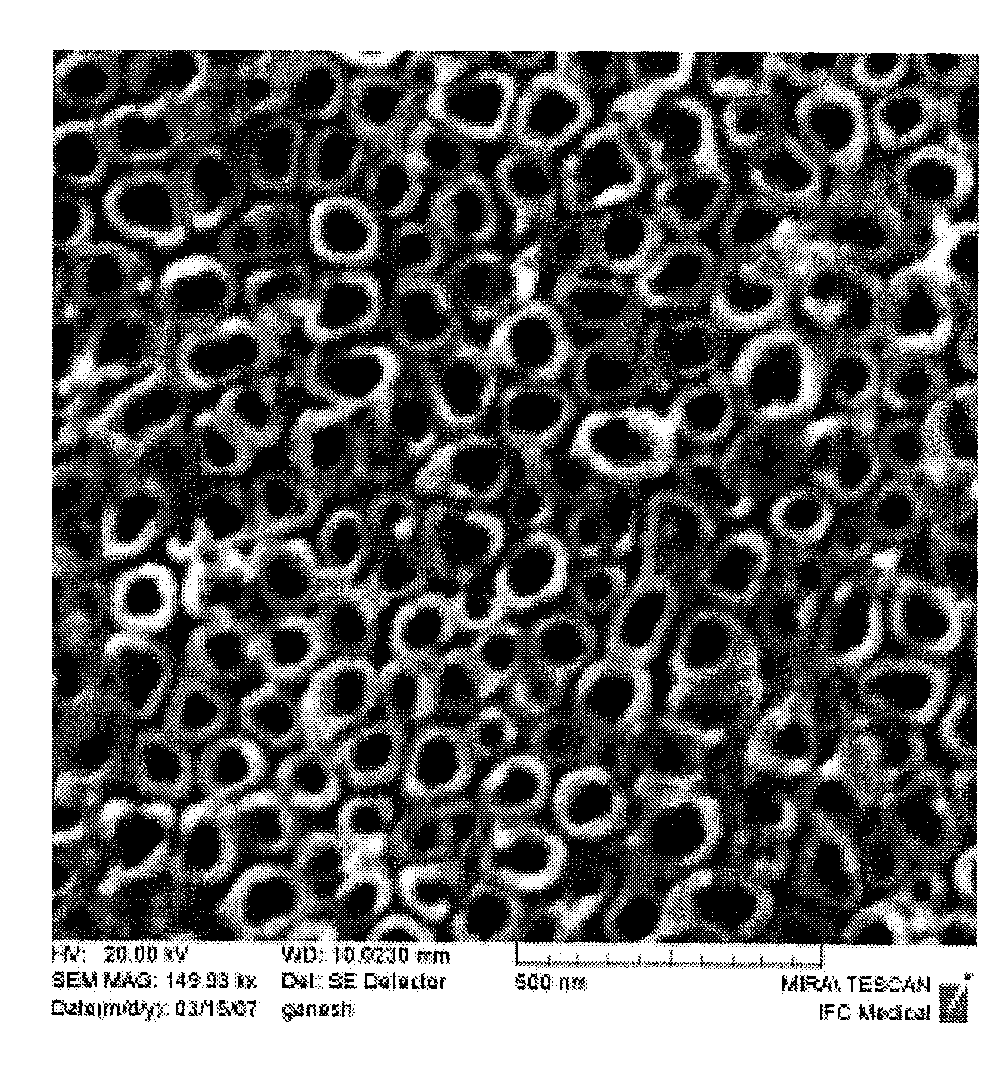
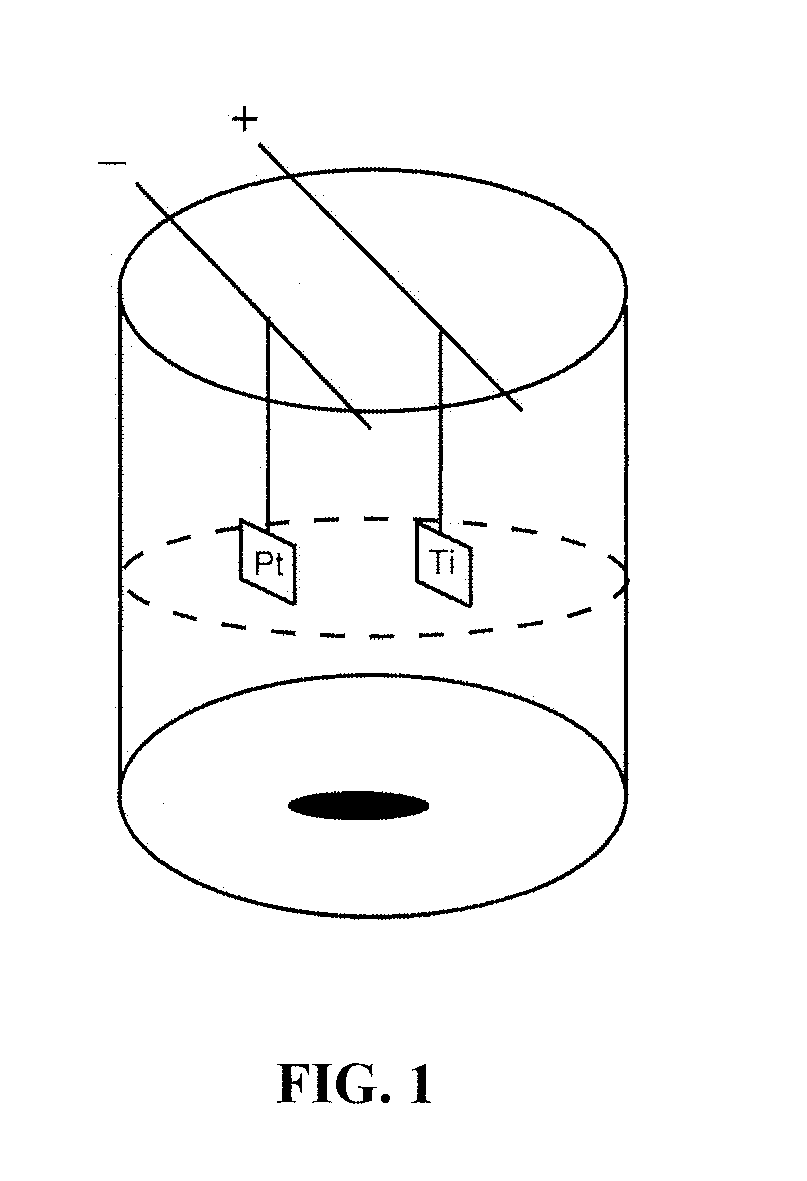
Patents
Literature
833 results about "Bone cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bone cell' Bone cells', which are found within the bone tissue, are responsible for the make-up of the skeleton of vertebrates. They are made up of different bone cells: there is the compact tissue which is the hard outer part of the bone, the cancellous tissue which is all the spongy tissue inside of the compact tissue, and the subchondral tissue which is the smooth tissue at the end of each bone."All About Bone." All About Bone. Ohio State University, n.d. Web. 19 Mar. 2013. Bone cells all work together inside of the bones to help keep up the skeletal system. The bone cells do many things for the skeletal system such as the development of new bones, the maintenance of bones, and the regulation of minerals in the body. Types There are four main categories of bone cells which include: lining cells, which protect the boneOursler, Merry J., and Teresita Bellido Bellido. "B>one Cells." ASBMR Educational Materials. ASBMR Educational Materials, 12 Sept. 2003. Web. 19 March 2013. Osteoclasts Osteoclasts are very large multinucleate cells that are responsible for the breakdown of bones. The breakdown of bone is very important in bone health because it allows for bone remodeling.
Biocompatible compositions and methods of using same
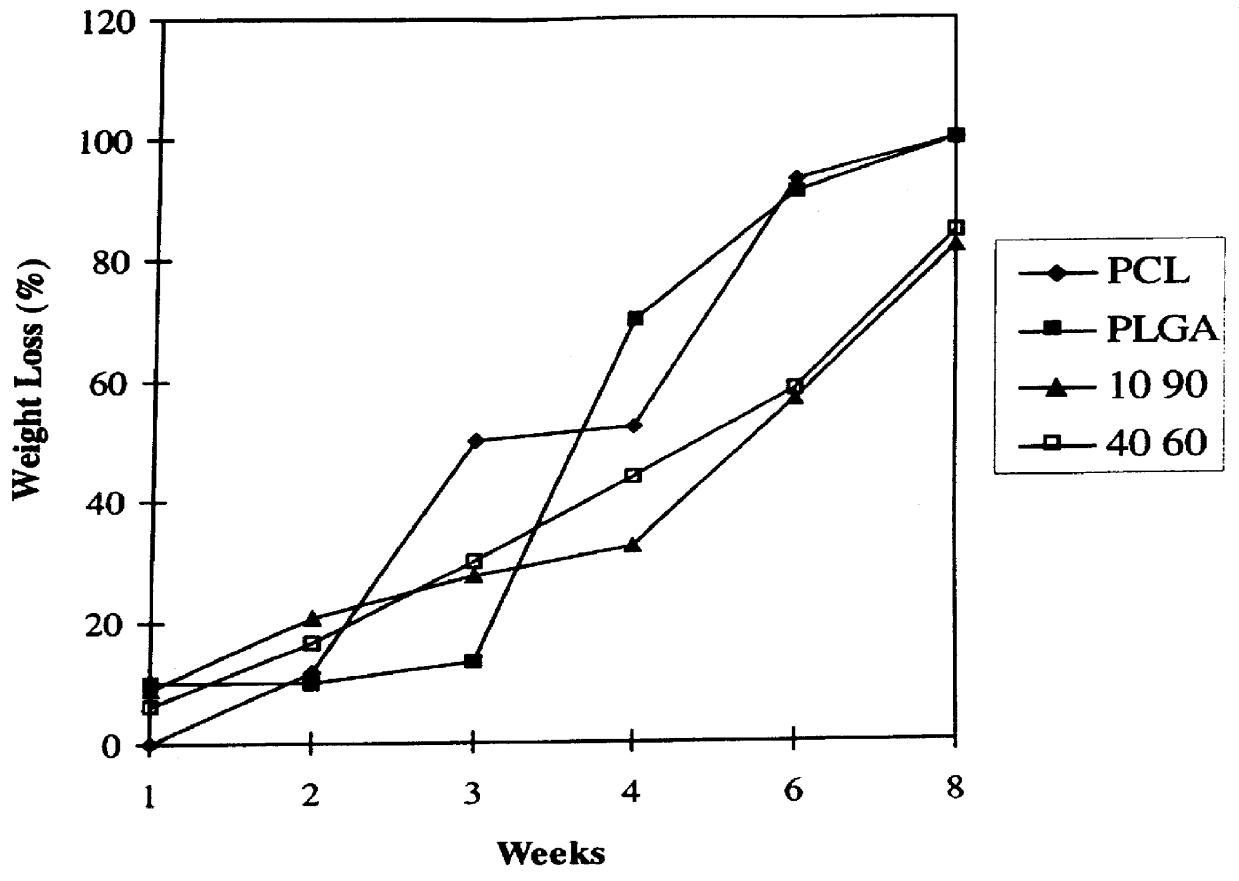
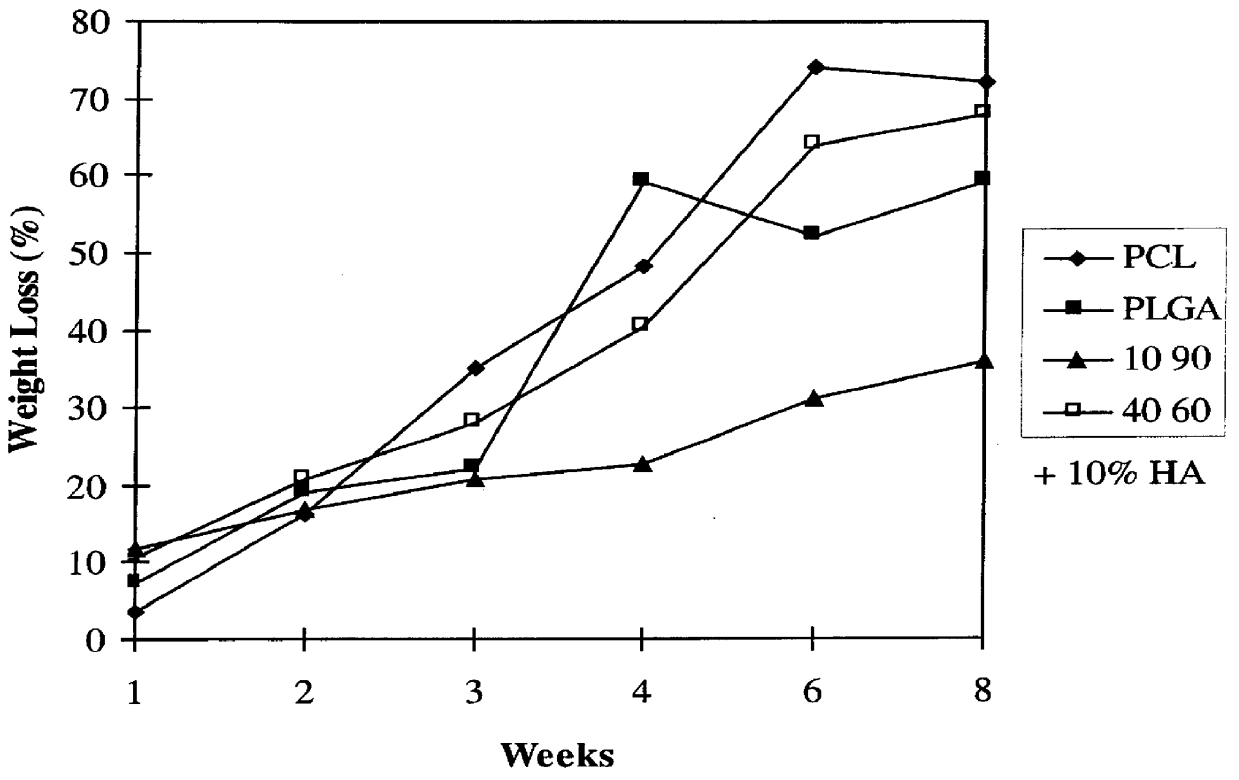
Blends of biodegradable polymers, preferably poly(caprolactone) and poly(D,L-lactic-co-glycolic) acid are discussed as well as their applications in the medical field, particularly with regard to bone tissue engineering. Preferably, hydroxyapatite ("HA") granules are incorporated into the blends and the resulting blends have desirable mechanical, physical, and biological characteristics. Even more preferably the compositions of the present invention are utilized to form osteoconductive composites that supported bone cell growth on the surface as well as throughout the scaffold.
Owner:CARNEGIE MELLON UNIV +1
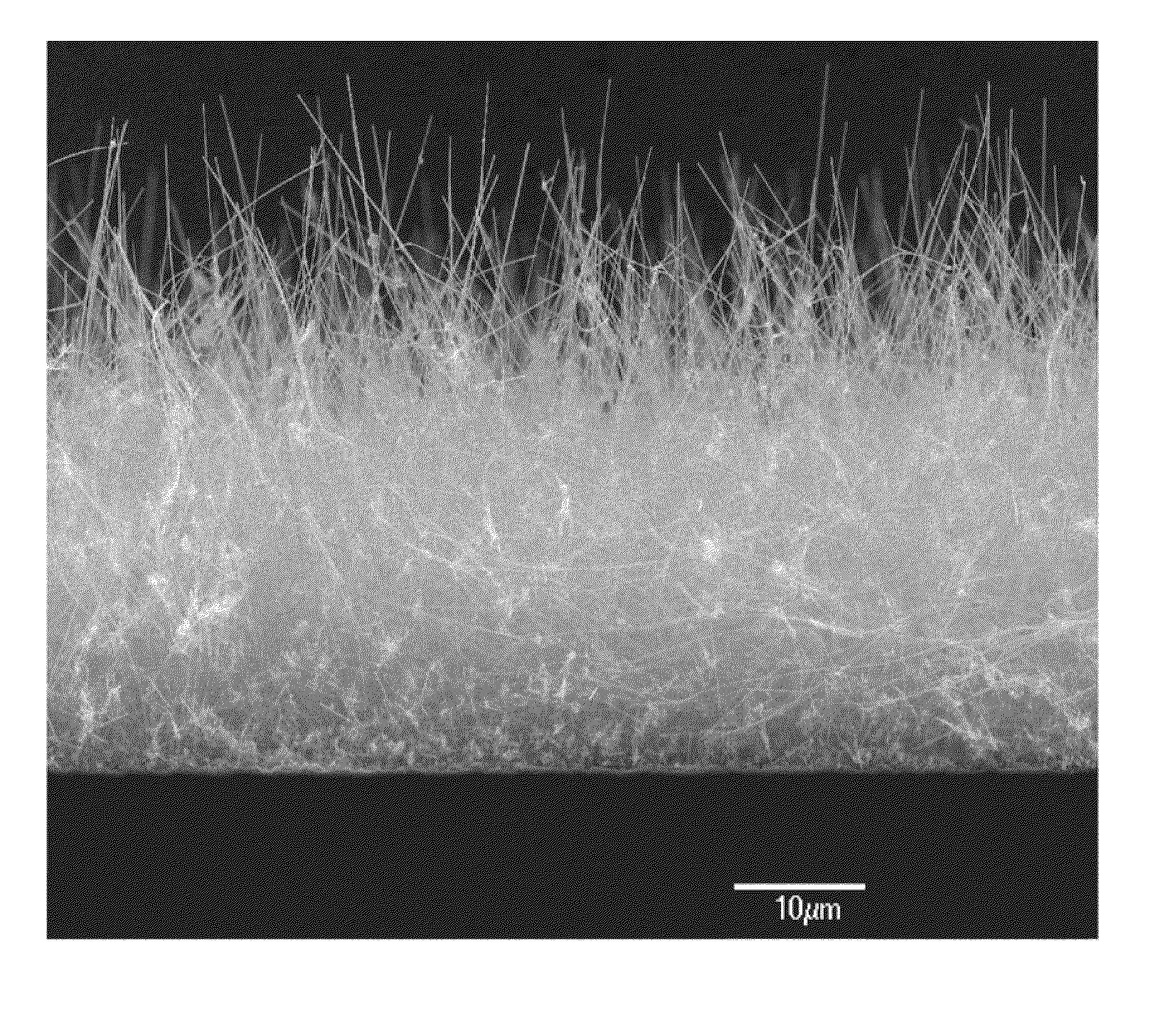
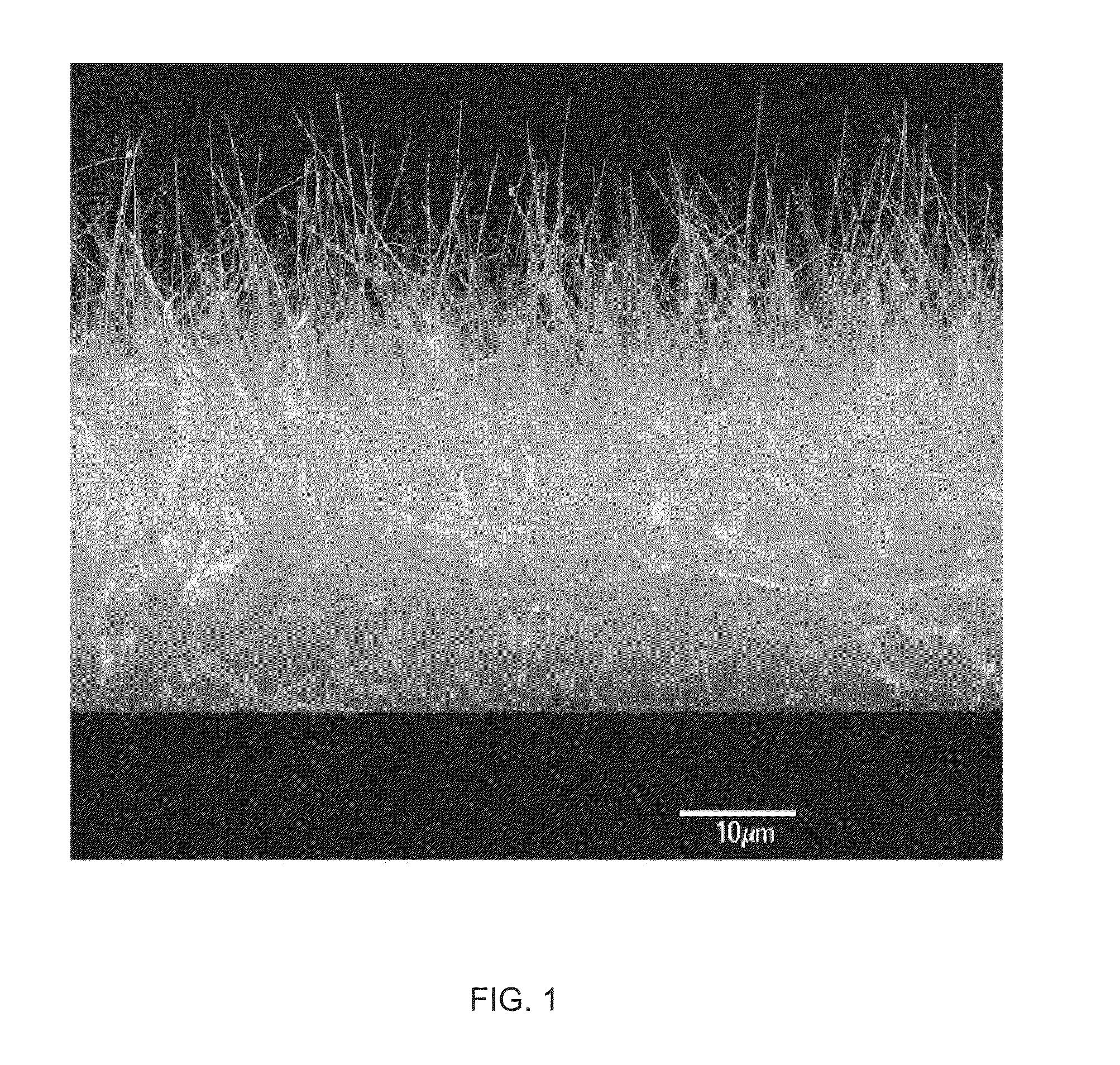
Medical device applications of nanostructured surfaces
InactiveUS20050221072A1Fine surfacePrevent/reduce bio-foulingNanomedicinePharmaceutical delivery mechanismMedicineOsteoblast
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:NANOSYS INC
Resorbable polymeric device for localized drug delivery
InactiveUS20050177118A1Good adhesionMinimizes procedural discomfortMedical devicesMammal material medical ingredientsOsteoblastHigh doses
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. Another embodiment is arranged for the local delivery of therapeutic agent. A preferred embodiment is a porous resorbable implant, wherein the therapy delivery may be localized in nature, rather than systemic, such that higher doses at the target site may be allowed than would be tolerable by the body systemically.
Owner:KENSEY NASH CORP
Device for regeneration of articular cartilage and other tissue
InactiveUS20050074481A1Good adhesionMinimizes procedural discomfortBone implantMammal material medical ingredientsSubchondral boneOsteoblast
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. A hydrophobic barrier exists between the regions, of this embodiment. In one embodiment, the polyelectrolytic complex transforms to hydrogel, following the implant procedure.
Owner:KENSEY NASH CORP
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
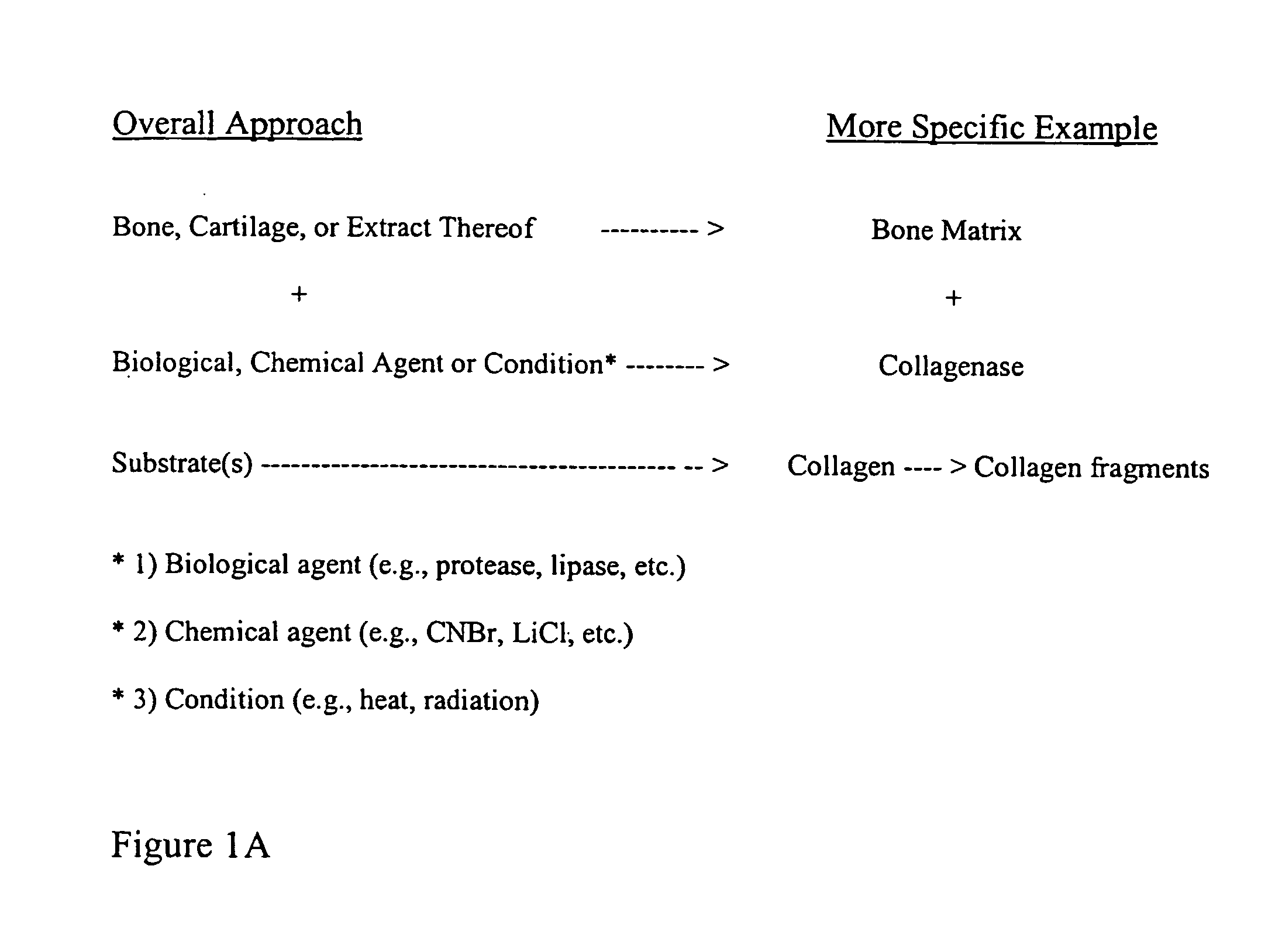
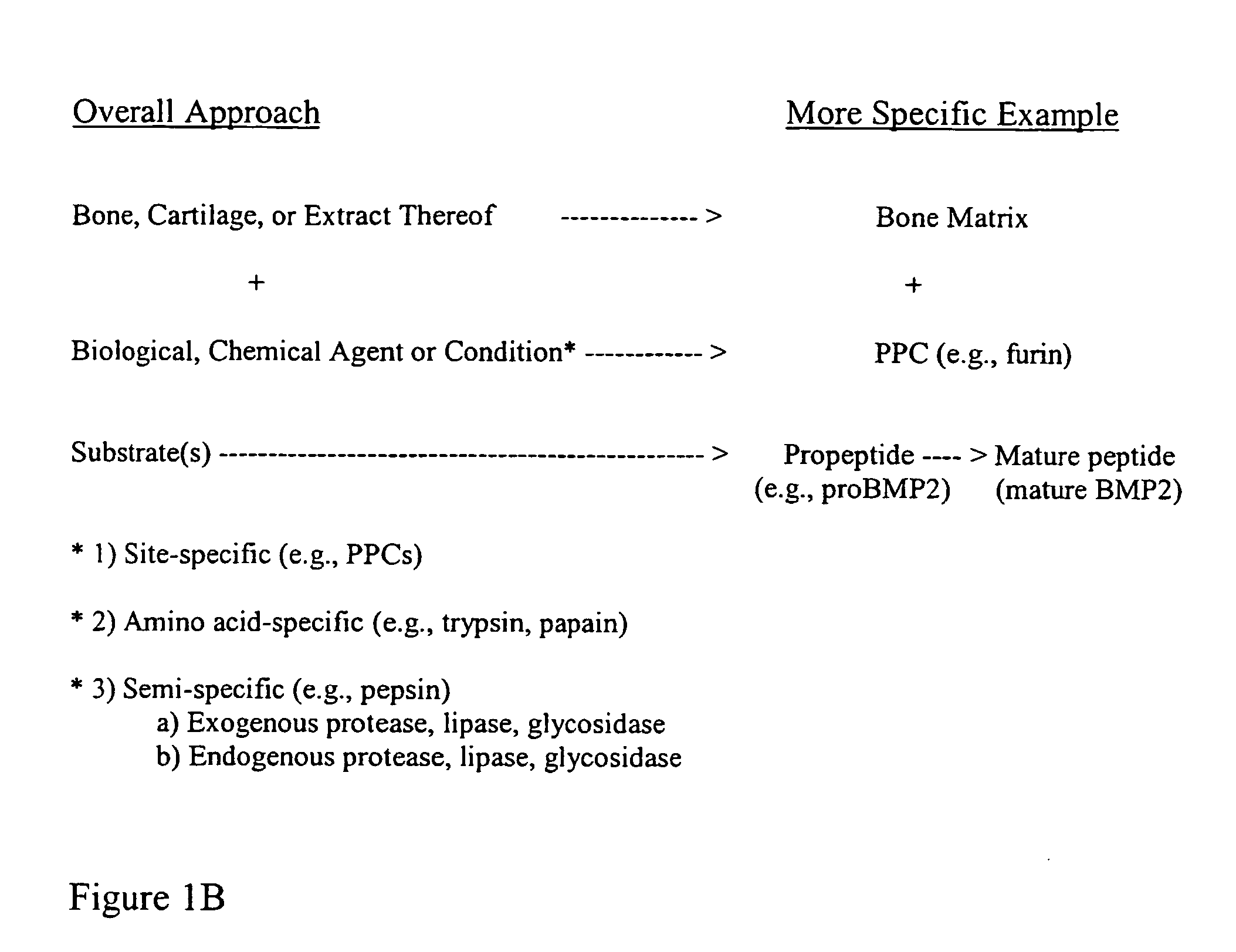
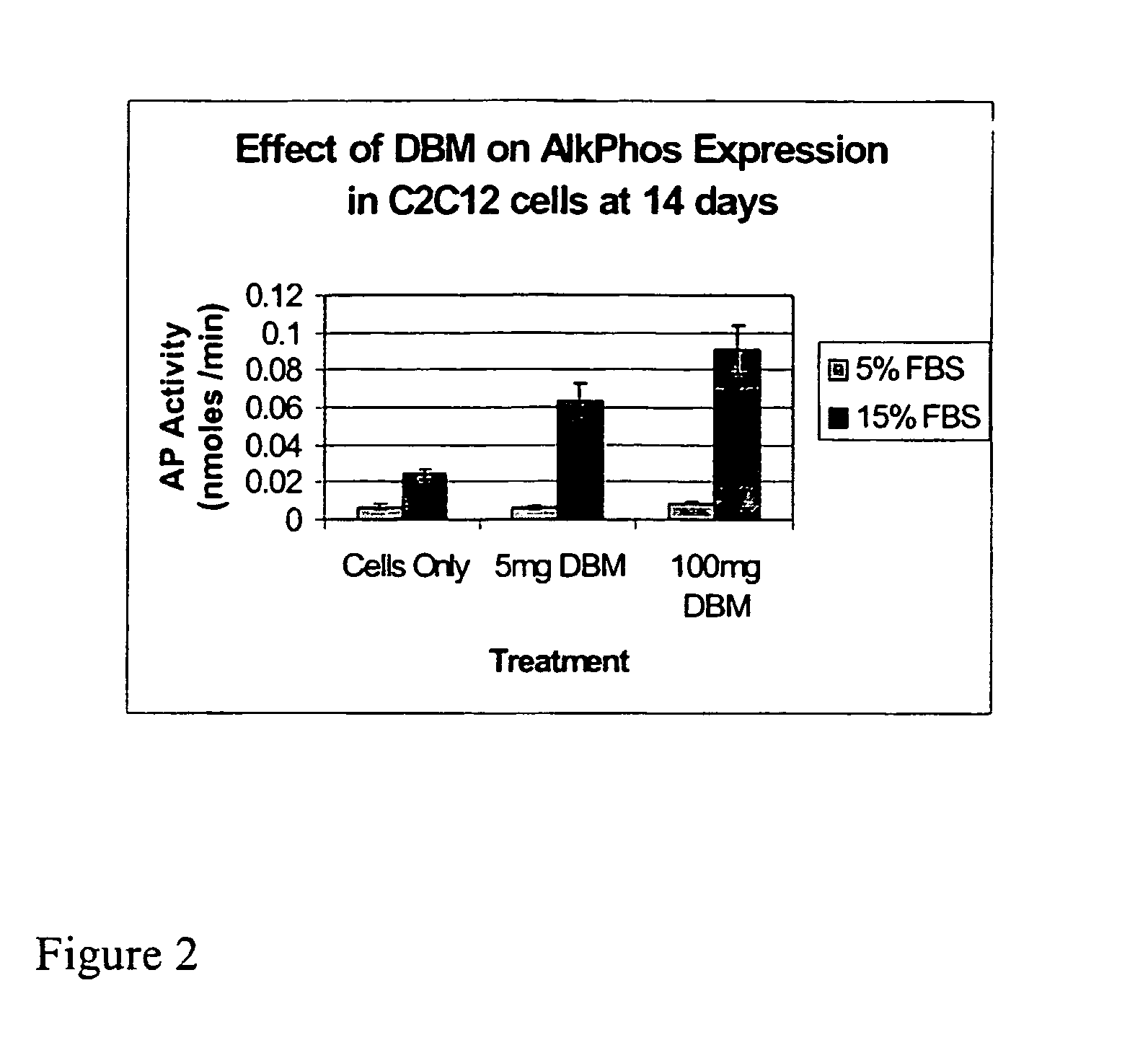
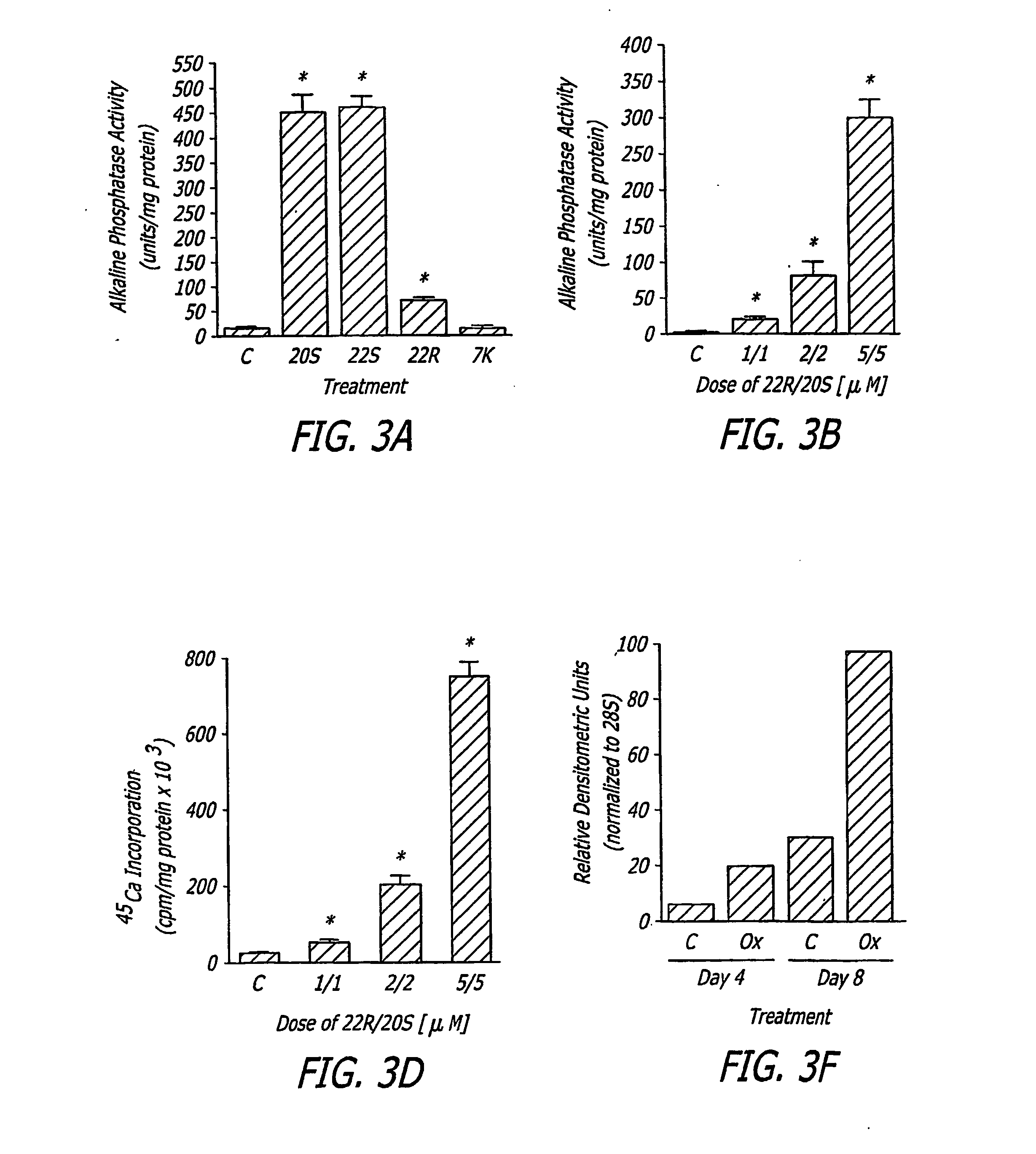
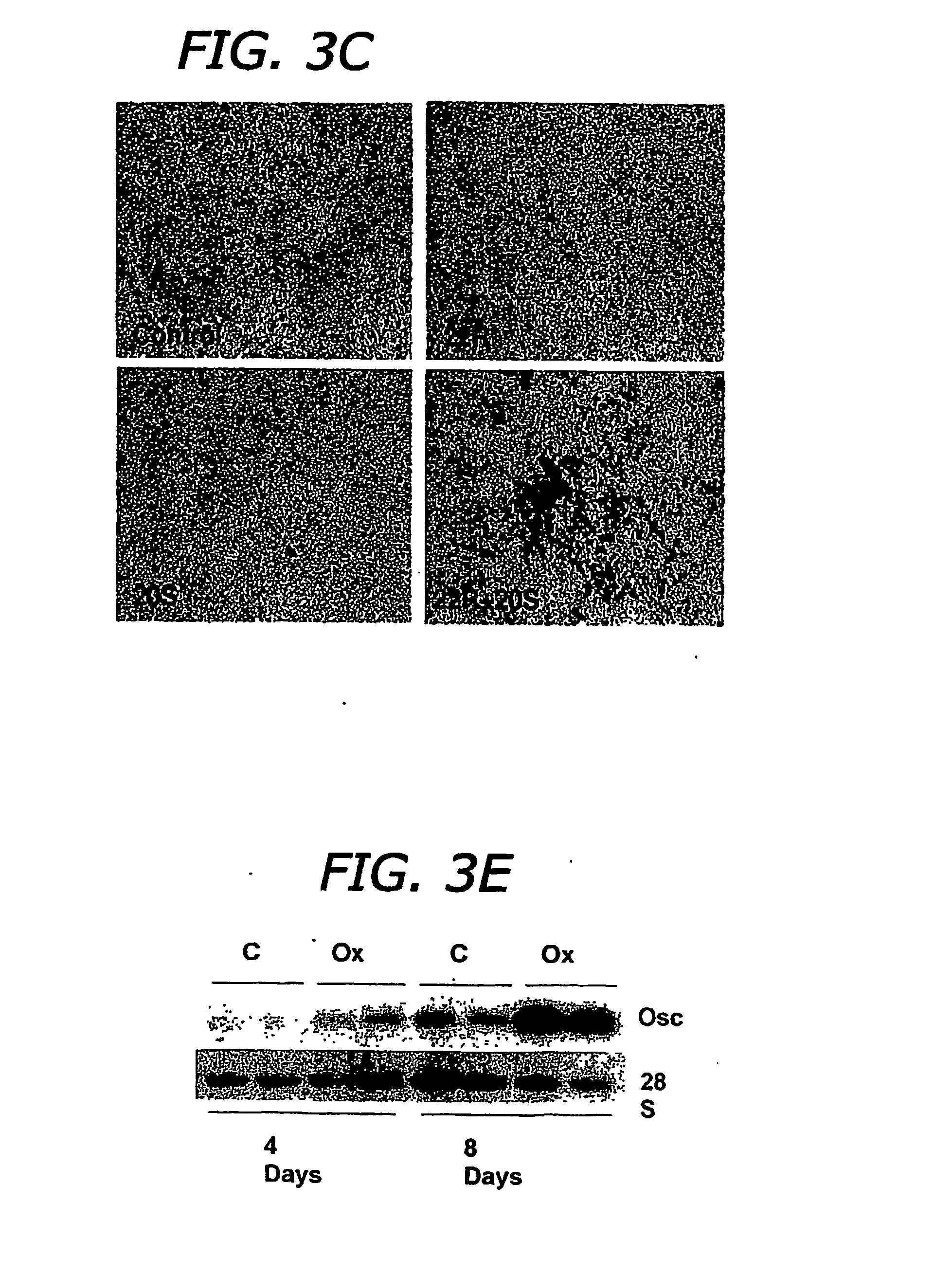
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Medical Device Applications of Nanostructured Surfaces
InactiveUS20110201984A1Improve adhesionIncrease frictionMaterial nanotechnologyInternal electrodesFiberOsteoblast
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:NANOSYS INC
Porous Interbody Spacer
Orthopedic implants, particularly interbody spacers, have a combination of correct pore size and stiffness / flexibility. When the implants have the proper pore size and stiffness, osteocytes are able to properly bridge the pores of the implant and then experience a proper compressive load to stimulate the bone cells to form bone within the pores. An implant includes a body formed of an osteoconductive material and having a stiffness of between 400 megapascals (MPa) and 1,200 MPa. Additionally, the body includes a plurality of pores having an average size of between 150 microns and 600 microns. The pores permit the growth of bone therein. The body is formed of packs of coils which may be formed using an additive manufacturing process and using traditional orthopedic implant materials such as titanium and titanium alloys while still achieving desired stiffness and pore sizes of the implants.
Owner:NEXUS SPINE L L C
Biocompatible Coated Nanostructured Titanium Surfaces
InactiveUS20100028387A1Promote bone growthStrong cell adhesionBiocideTetrapeptide ingredientsNano sizeCell adhesion
Bioactive molecules have been coated on nanotubular structured titanium substrates by molecular plasma deposition. The coatings promote cell adhesion and are particularly suited for orthopedic implants that provide improved bone cell adhesion and new tissue growth. Nanodimensional features on titanium substrates are engineered using electrochemical anodization techniques. The nanostructured surfaces provide superior support for a wide selection of polypeptide coatings.
Owner:METASCAPE
Hydroxyapatite coated nanostructured titanium surfaces
InactiveUS20090035722A1Improve adhesionPromote accumulationDental implantsImpression capsOsteoblast adhesionApatite
Nanotubular structured titanium (Ti) substrates have been coated with nanoparticulate hydroxyapatite (nano-HA). The nano-HA surface is highly adherent to the nanotubular Ti surface and is free of microparticles. The nano-HA coated nanotubular Ti surface promotes osteoblast cell adhesion and is particularly suitable for orthopedic and dental implants where deposition of osteoblasts and other proteins is important in bone formation.
Owner:METASCAPE
Use of bioactive glass compositions to stimulate osteoblast production
InactiveUS20040009598A1Expand the populationRapid apoptosisSkeletal/connective tissue cellsCell culture supports/coatingOsteoblastMammal
Compositions comprising bioactive glass compositions or extracts thereof which include ions in an appropriate concentration and ratio that they enhance osteoblast production, and methods of preparation and use thereof, are disclosed. The compositions can be included in implantable devices that are capable of inducing tissue formation in autogeneic, allogeneic and xenogeneic implants, for example as coatings and / or matrix materials. Examples of such devices include prosthetic implants, sutures, stents, screws, plates, tubes, and the like. Aqueous extracts of the bioactive glass compositions, which extracts are capable of stimulating osteoblast production, are also disclosed. The compositions can be used, for example, to induce local tissue formation from a progenitor cell in a mammal, for accelerating allograft repair in a mammal, for promoting in vivo integration of an implantable prosthetic device to enhance the bond strength between the prosthesis and the existing target tissue at the joining site, and for treating tissue degenerative conditions.
Owner:NOVATHERA
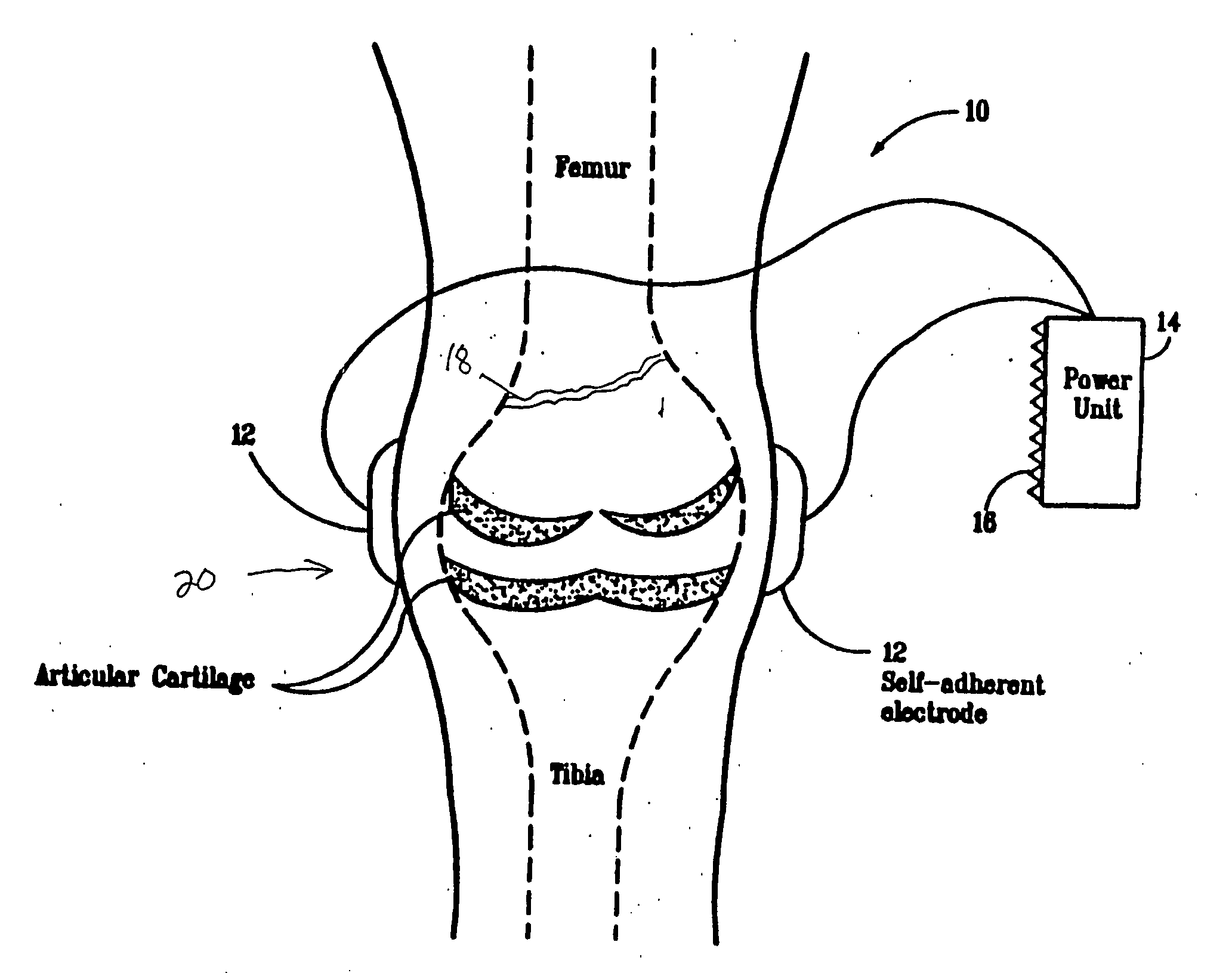
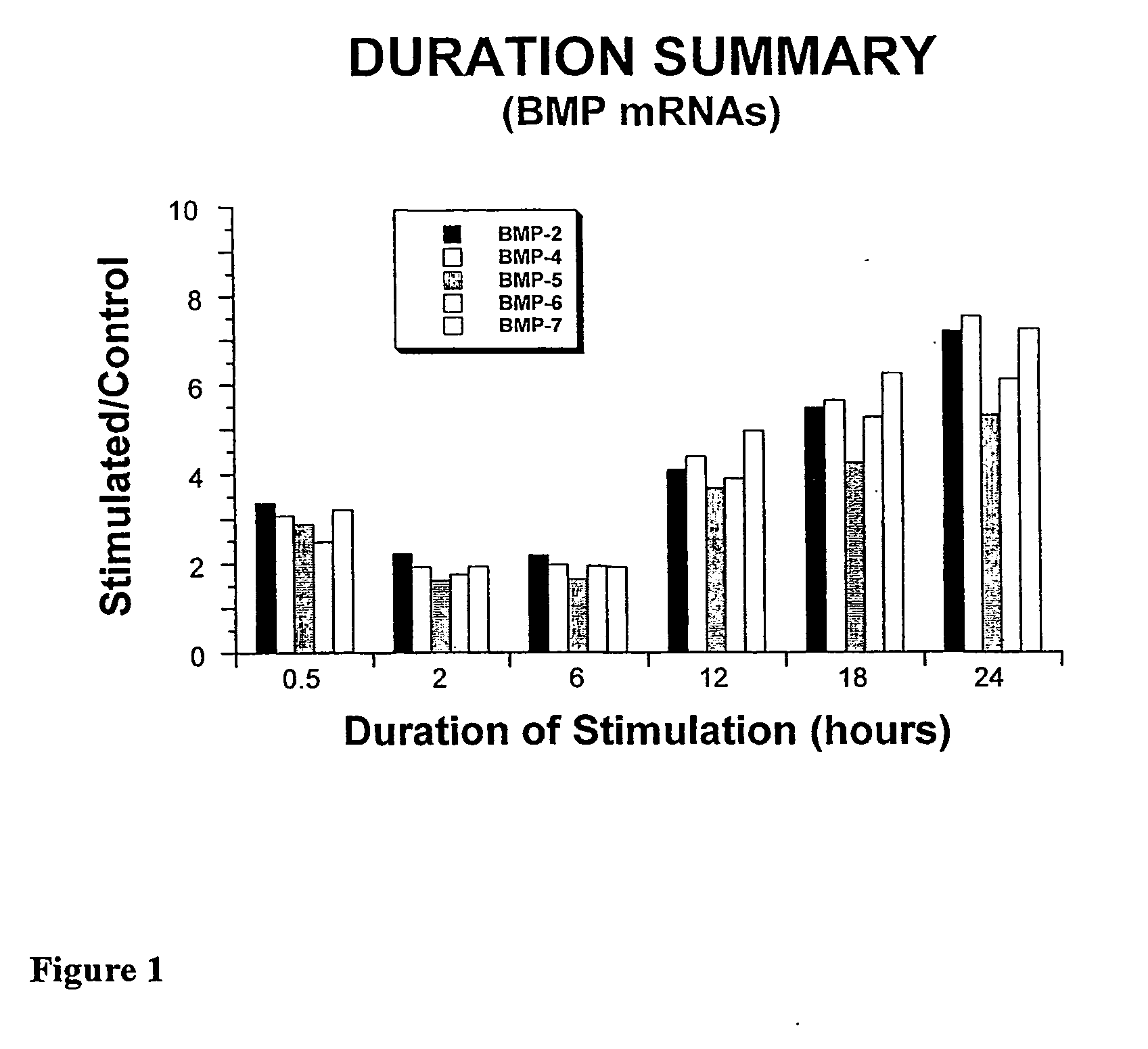
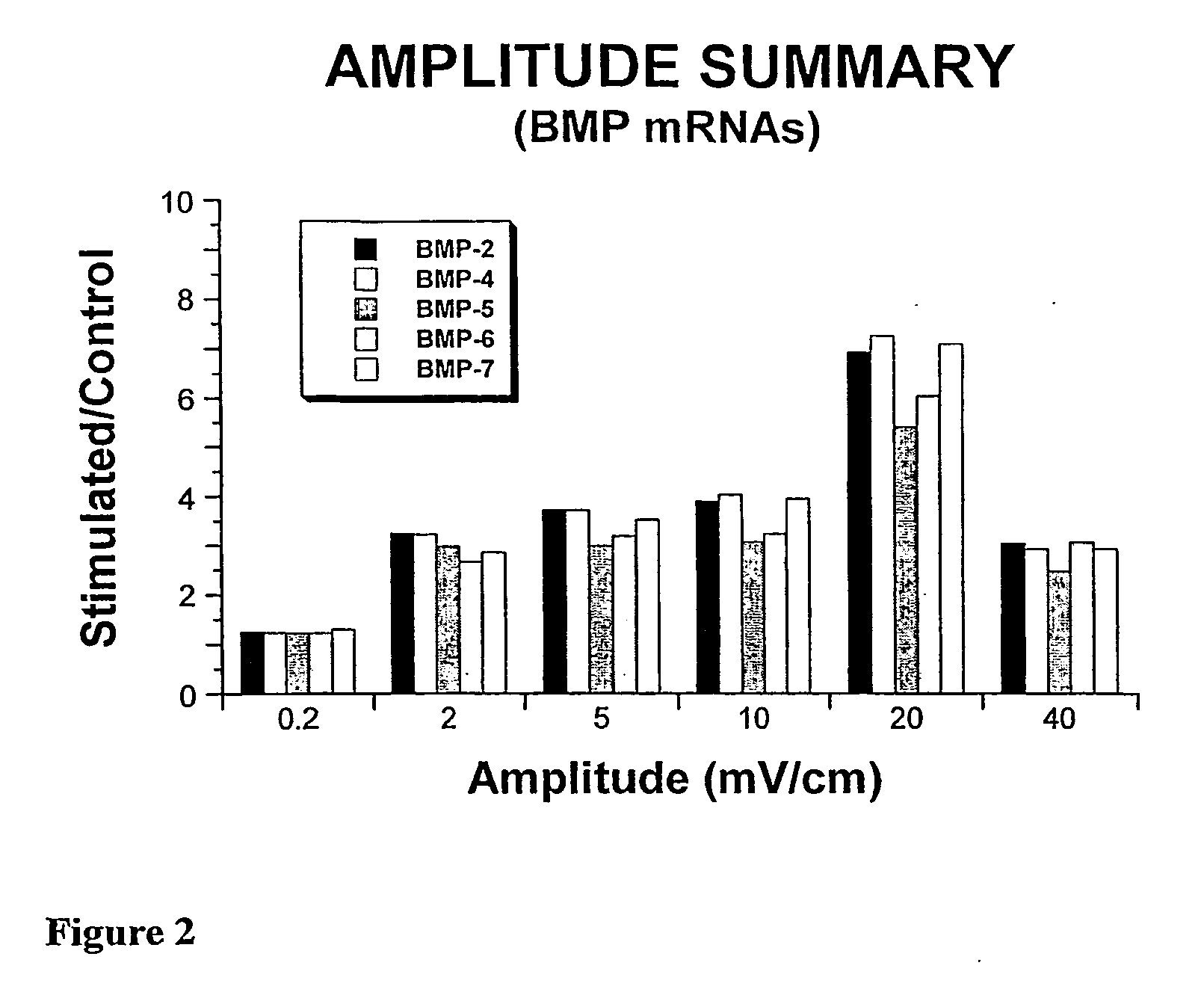
System and Method of Up-Regulating Bone Morphogenetic Proteins (Bmp) Gene Expression in Bone Cells Via the Application of Fields Generated by Specific and Selective Electric and Electromagnetic Signals
InactiveUS20070299472A1High expressionBiocideElectrotherapyHuman DNA sequencingBone Morphogenetic Protein Gene
Methods and devices are described for the regulation of bone morphogenetic protein gene expression in bone cells via the application of fields generated by specific and selective electric and electromagnetic signals in the treatment of diseased or injured bone. By gene expression is meant the up-regulation or down-regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased bone tissue that include generating specific and selective electric and electromagnetic signals that generate fields optimized for increase of bone morphogenetic protein gene expression and exposing bone to the fields generated by specific and selective signals so as to regulate bone morphogenetic protein gene expression in such bone tissue. The resulting methods and devices are useful for the targeted treatment of bone fractures, fractures at risk, delayed unions, nonunion of fractures, bone defects, spine fusions, osteonecrosis or avascular necrosis, as an adjunct to other therapies in the treatment of one or all of the above, and in the treatment of osteoporosis.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
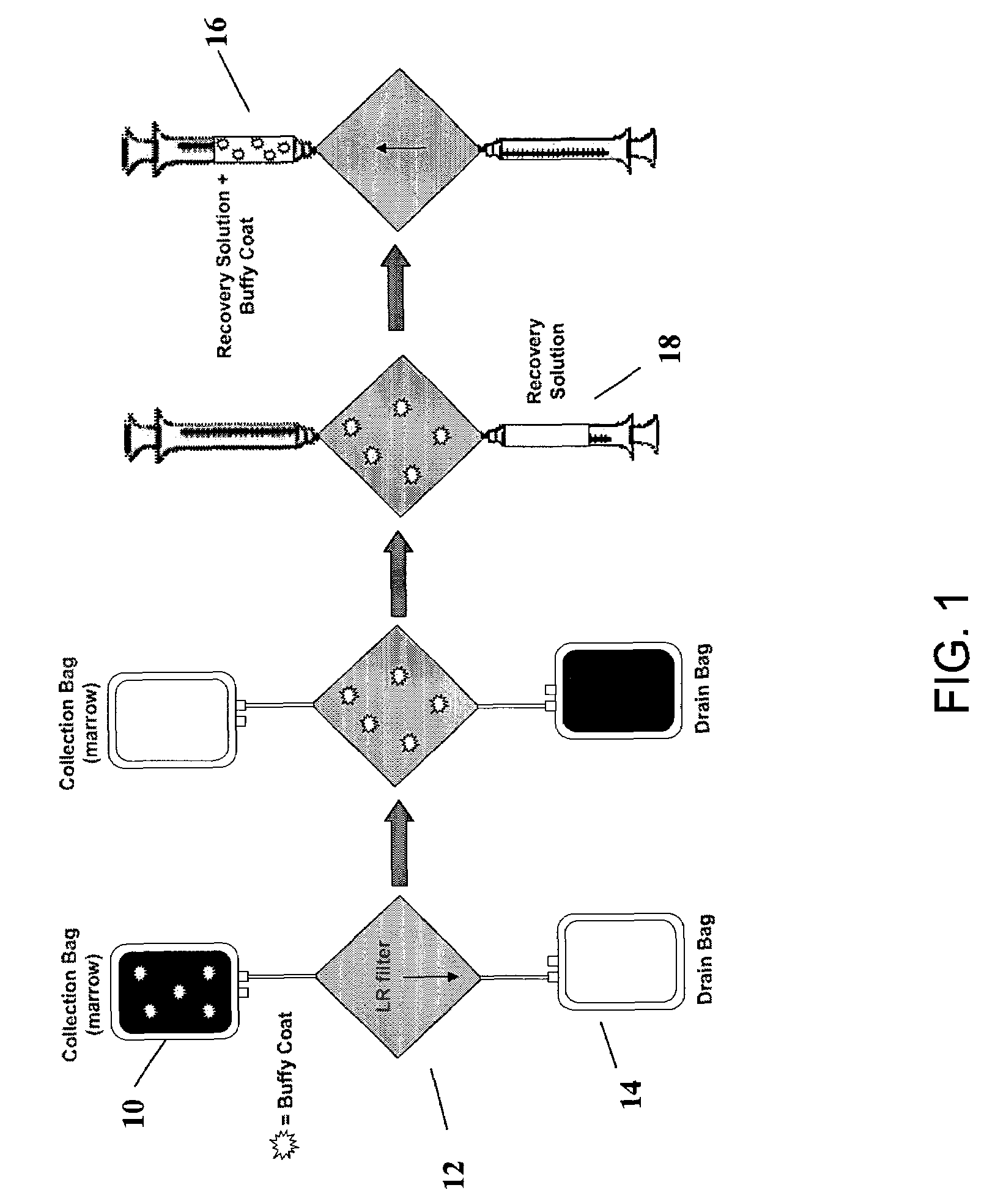
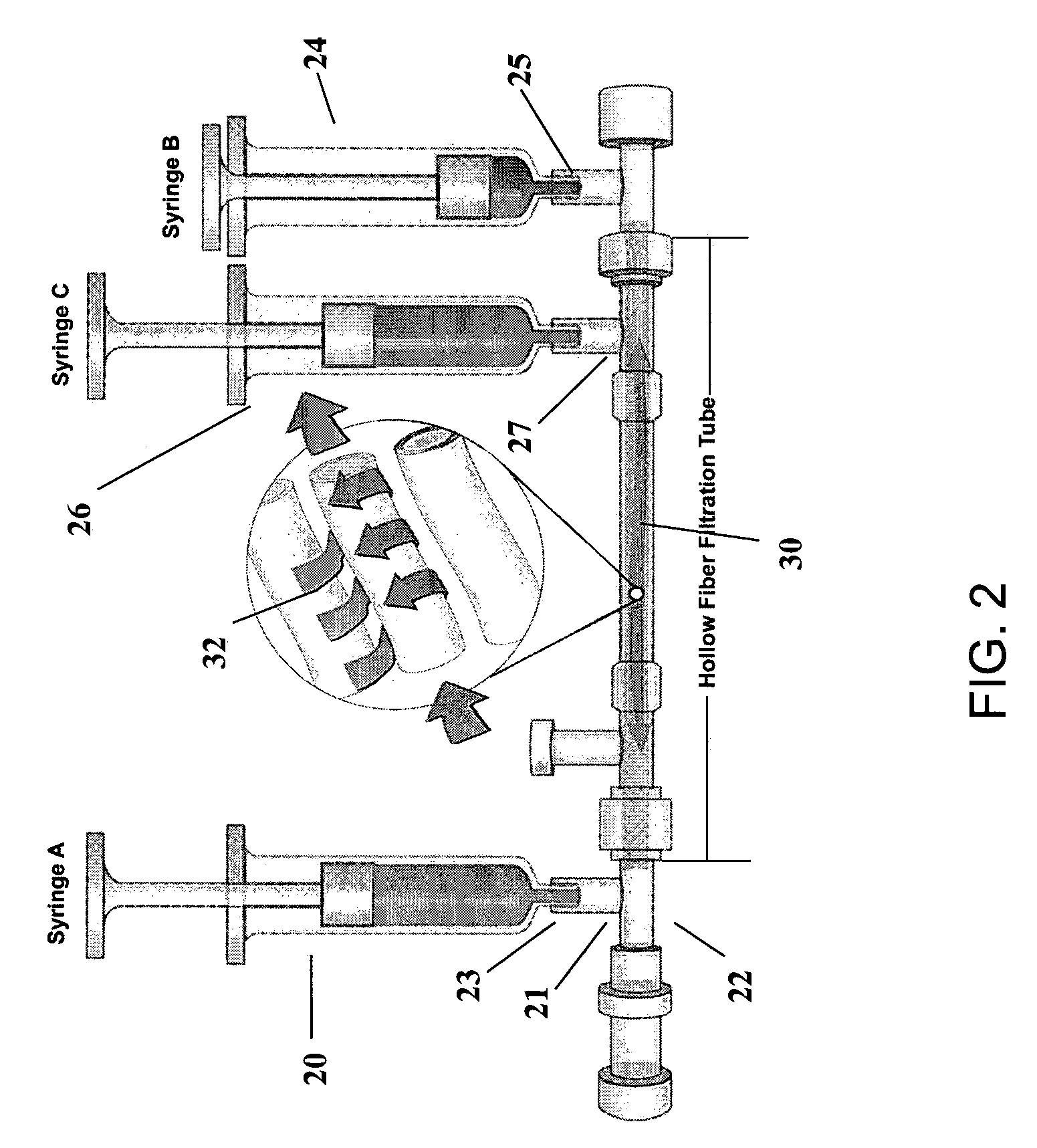
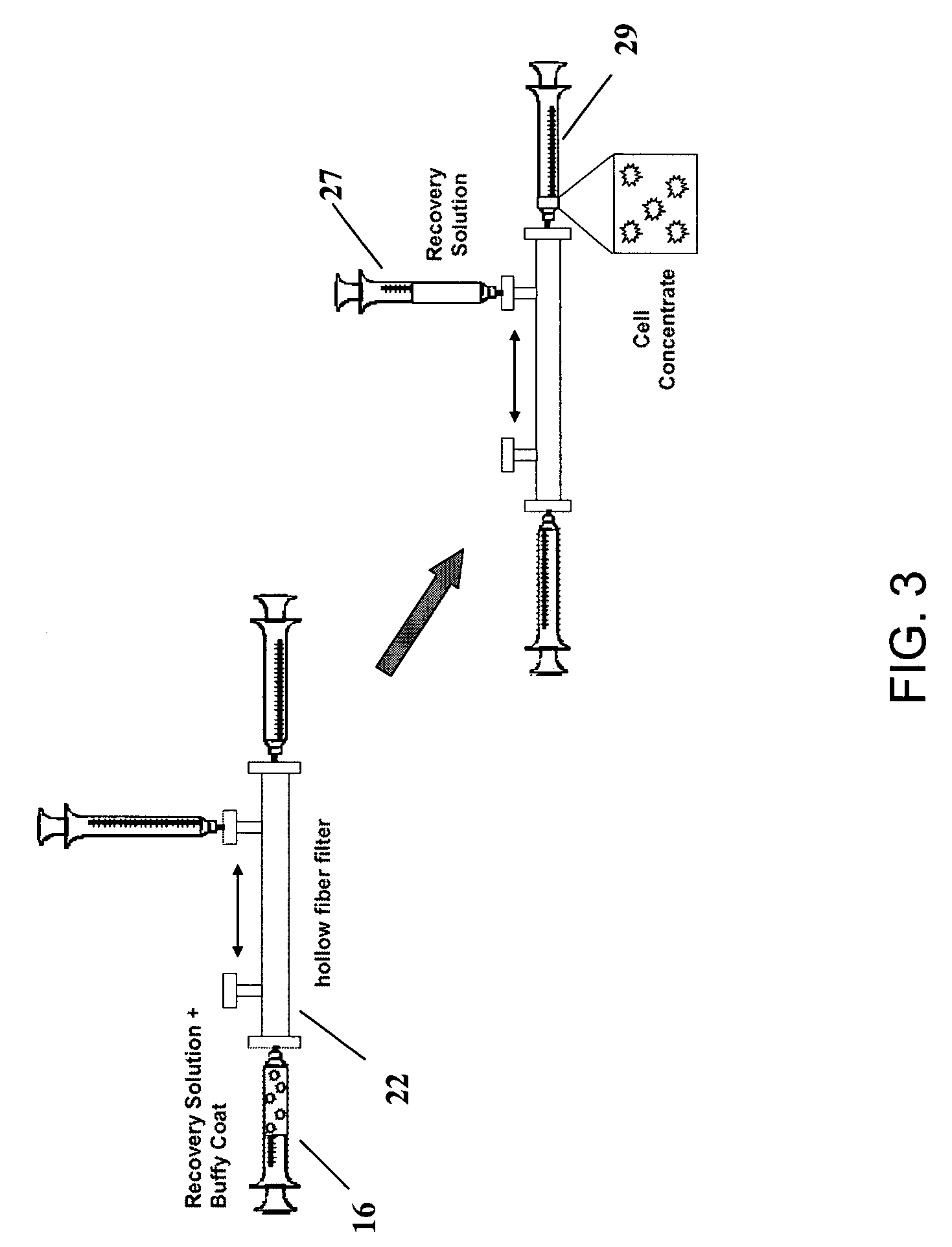
Preparation of a cell concentrate from a physiological solution
The present invention is directed to methods and compositions regarding the preparation of an cell concentrate, such as, for example, an osteogenic cell concentrate, from a physiological solution, such as bone marrow aspirate, blood, or a mixture thereof. In specific embodiments, the invention provides methods and compositions utilizing two physiological solution-processing techniques, particularly in a point of care environment, wherein centrifugation is not employed.
Owner:SMITH & NEPHEW INC +1
Implant for recreating verterbrae and tubular bones
InactiveUS6989033B1High mechanical strengthExtend range of possible usBone implantSpinal implantsExtracellular materialBiomedical engineering
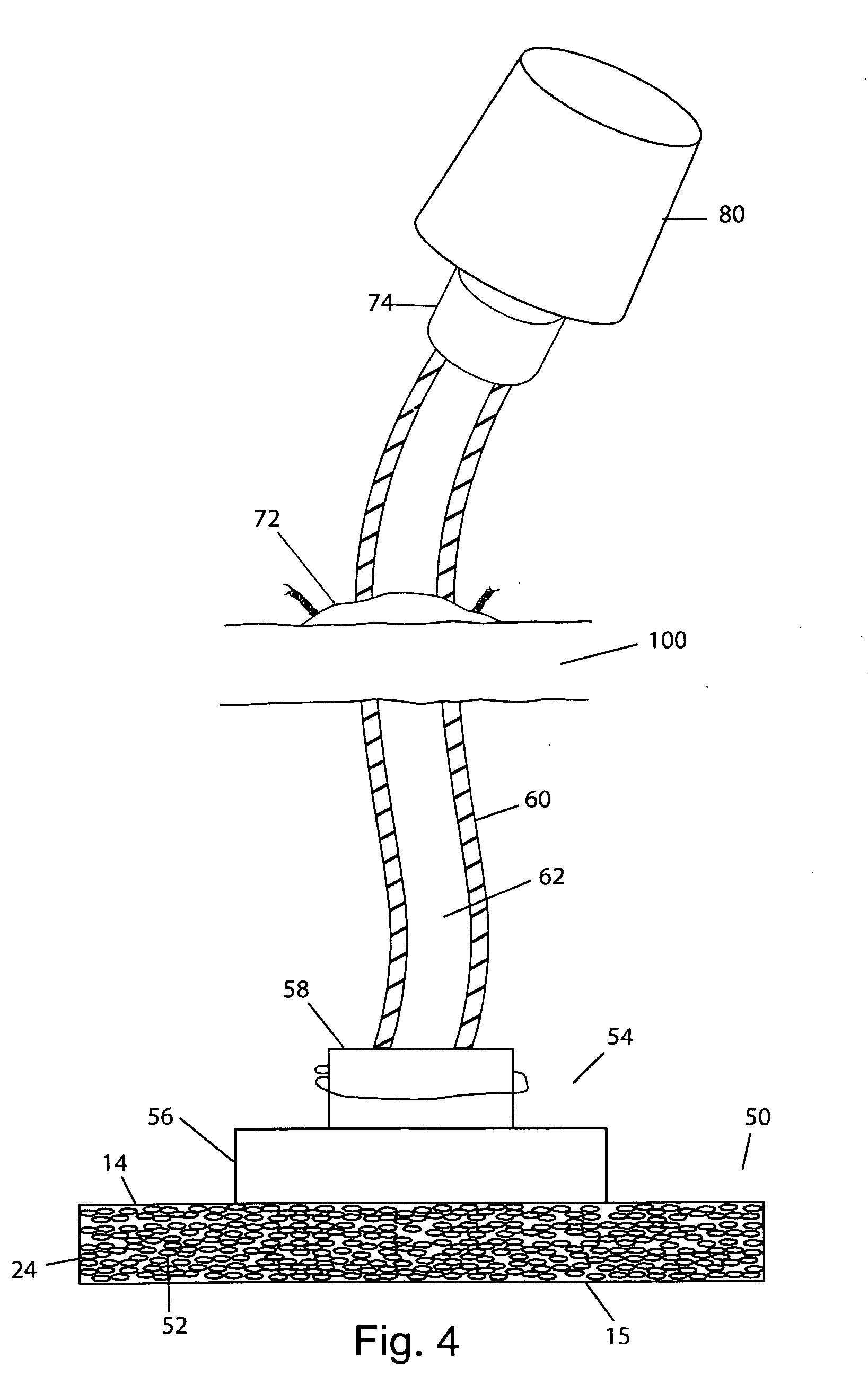
The invention relates to an implant for at least partially creating, recreating or stabilizing vertebral bodies or tubular bones. In said implant, a metallic, nonmetallic or ceramic hollow body is coated with an active substance complex or comprises said active substance complex. This active substance complex comprises the following components which differ from one another and which are specifically adapted for creating bone: at least one structural component based on extracellular material which is specifically adapted to the cells of the bone which is to be created, at least one recruiting component, at least one adhesion component, and at least one growth and / or maturation component.
Owner:OSSACUR +1
Remotely Activated Piezoelectric Pump for Delivery of Biological Agents to the Intervertebral Disc and Spine
ActiveUS20110092948A1Without significant loss of power efficiencyHighly miniaturized devicesBone implantMedical devicesMathematical modelBone fusion
The present disclosure describes a remotely activated piezoelectric pump for delivery of biological agents to the intervertebral disc and spine in order to achieve spinal fusion. A spinal pump is implanted on the vertebrae of a patient and a spinal cage is inserted in between two adjacent vertebrae after removal of the vertebrae disc. A piezoelectric motor drives the pump and pushes osteogenetic agent through the spinal cage and into a sponge disposed within the cage. The pump is charged by an external removable induction belt worn by the patient. Delivery duration and delivery frequency may be changed before implantation of the spine pump according to the specific needs of the patient. The current device employs a mathematical model that enables the regulation as well as attenuation of the bone fusion process by extending and generalizing the model to enhance and optimize the delivery of osteogenetic agent in a regulated manner.
Owner:COGNOS THERAPEUTICS INC
Agents and methods for enhancing bone formation
ActiveUS20060270645A1Improve impactPromotes bone formationBiocidePeptide/protein ingredientsMedicineOxysterol
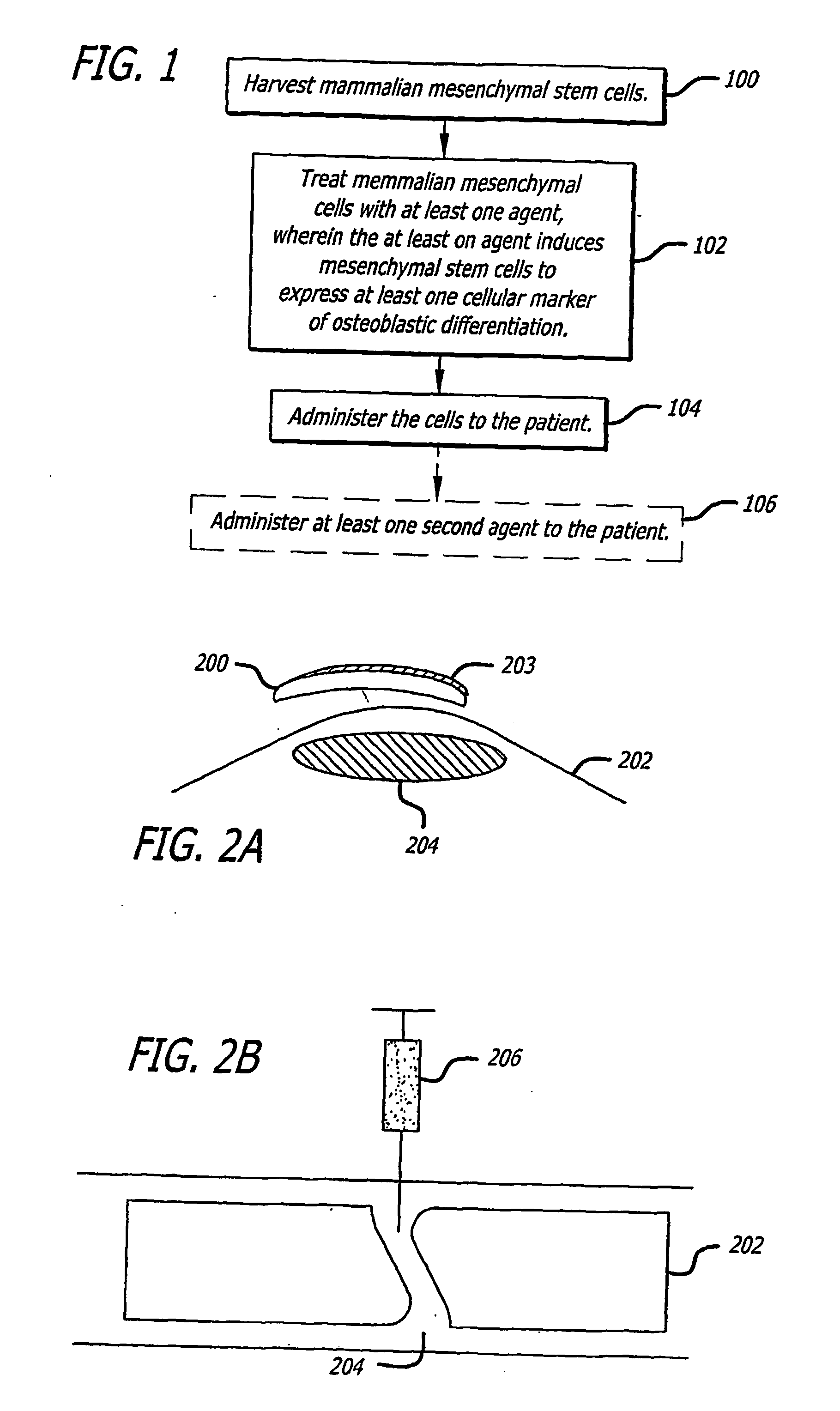
The present invention discloses agents and methods for inducing osteoblastic cellular differentiation, as well as the use of such agents and method to treat patients to maintain bone mass, enhance bone formation and / or bone repair. Exemplary agents include oxysterols, alone or in combination with particular oxysterols, or other agents known to assist in bone formation. The invention further includes medicaments including oxysterols for the treatment of bone disorders, local injections of oxysterols or cells (206) and implants (202) having agents or cells (203) to facilitate bone repair.
Owner:RGT UNIV OF CALIFORNIA
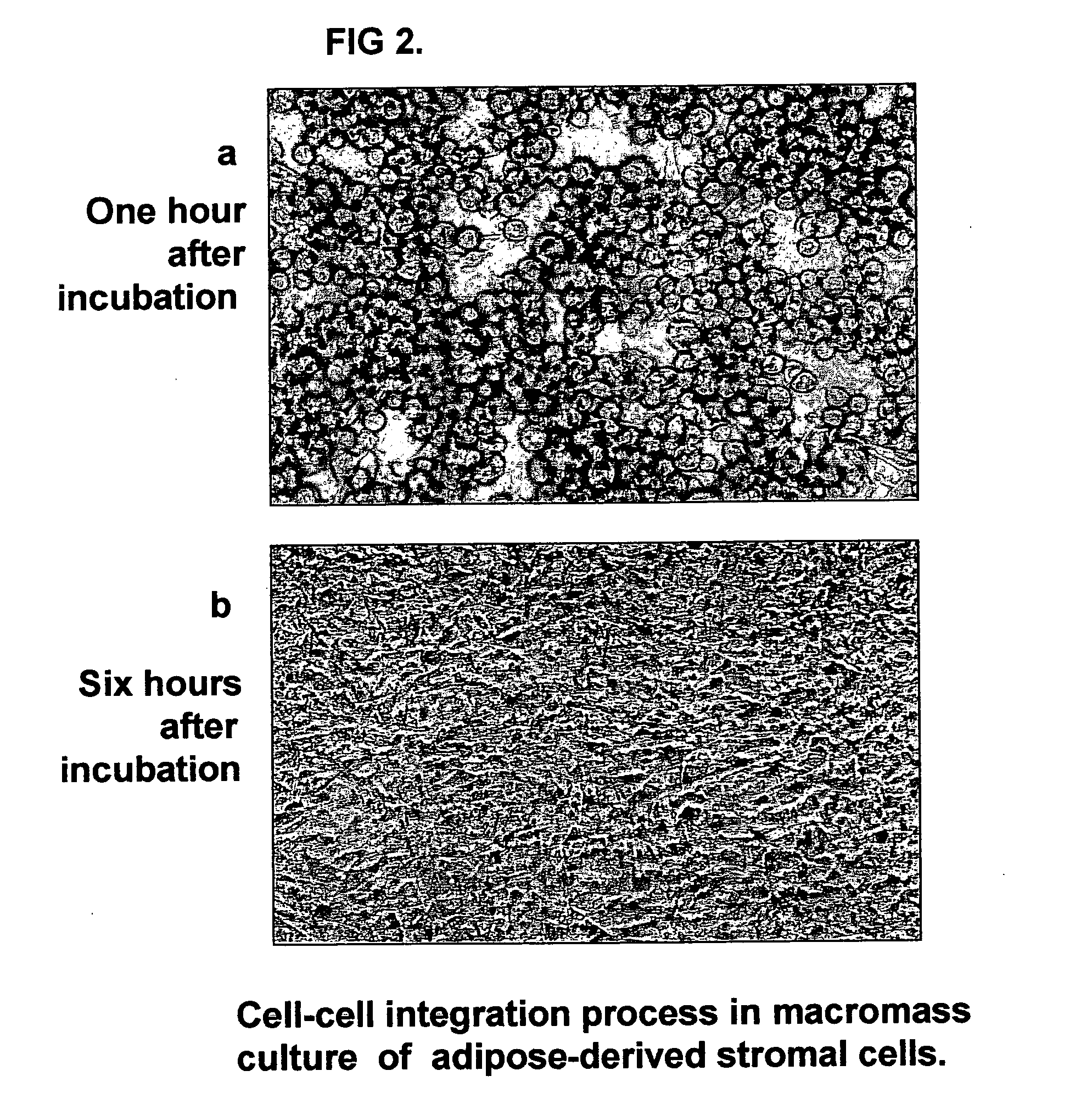
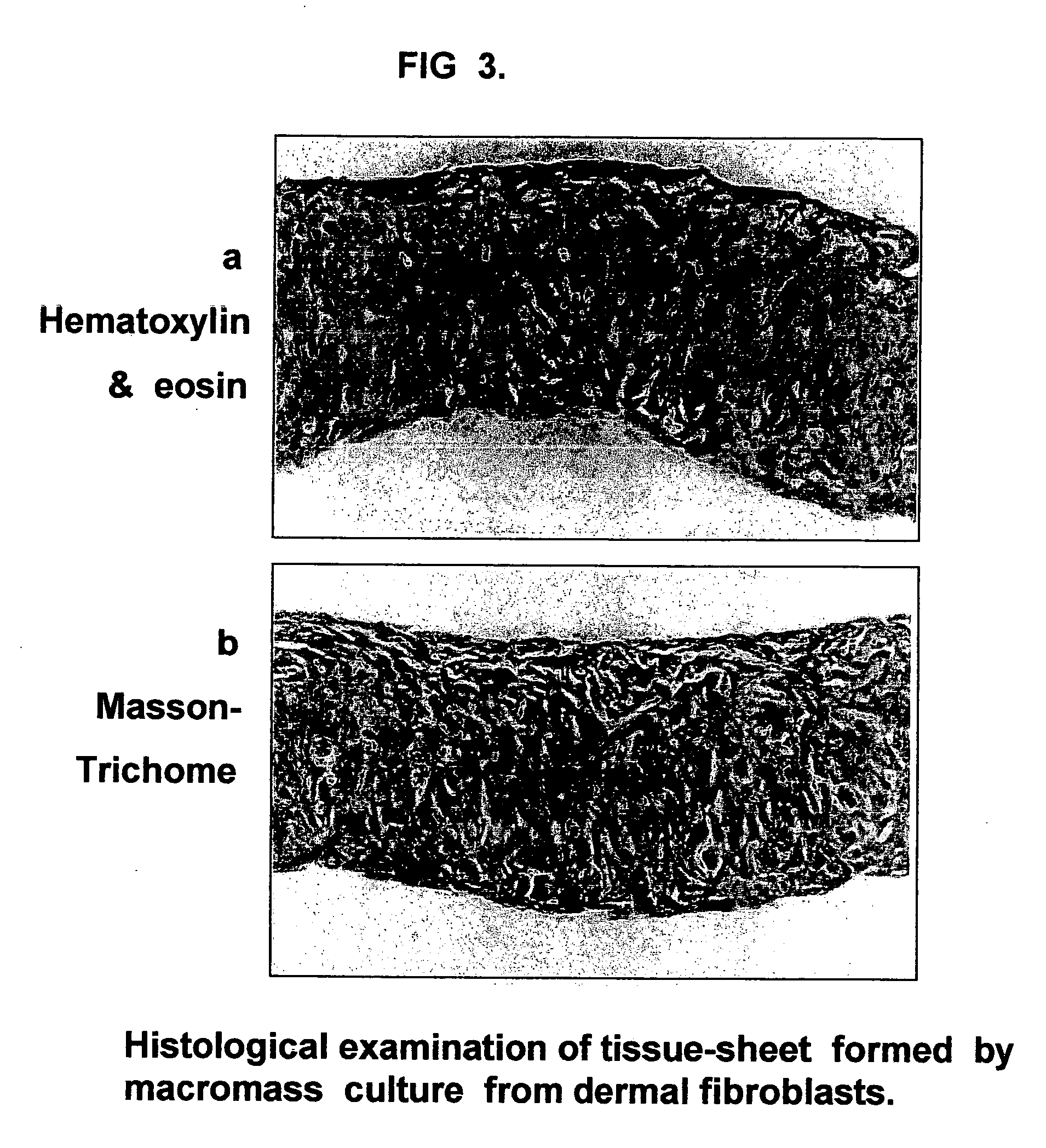
Tissue-like organization of cells and macroscopic tissue-like constructs, generated by macromass culture of cells, and the method of macromass culture
InactiveUS20040082063A1Sectioned easilySmall sizeEpidermal cells/skin cellsMammal material medical ingredientsHigh cellFiber
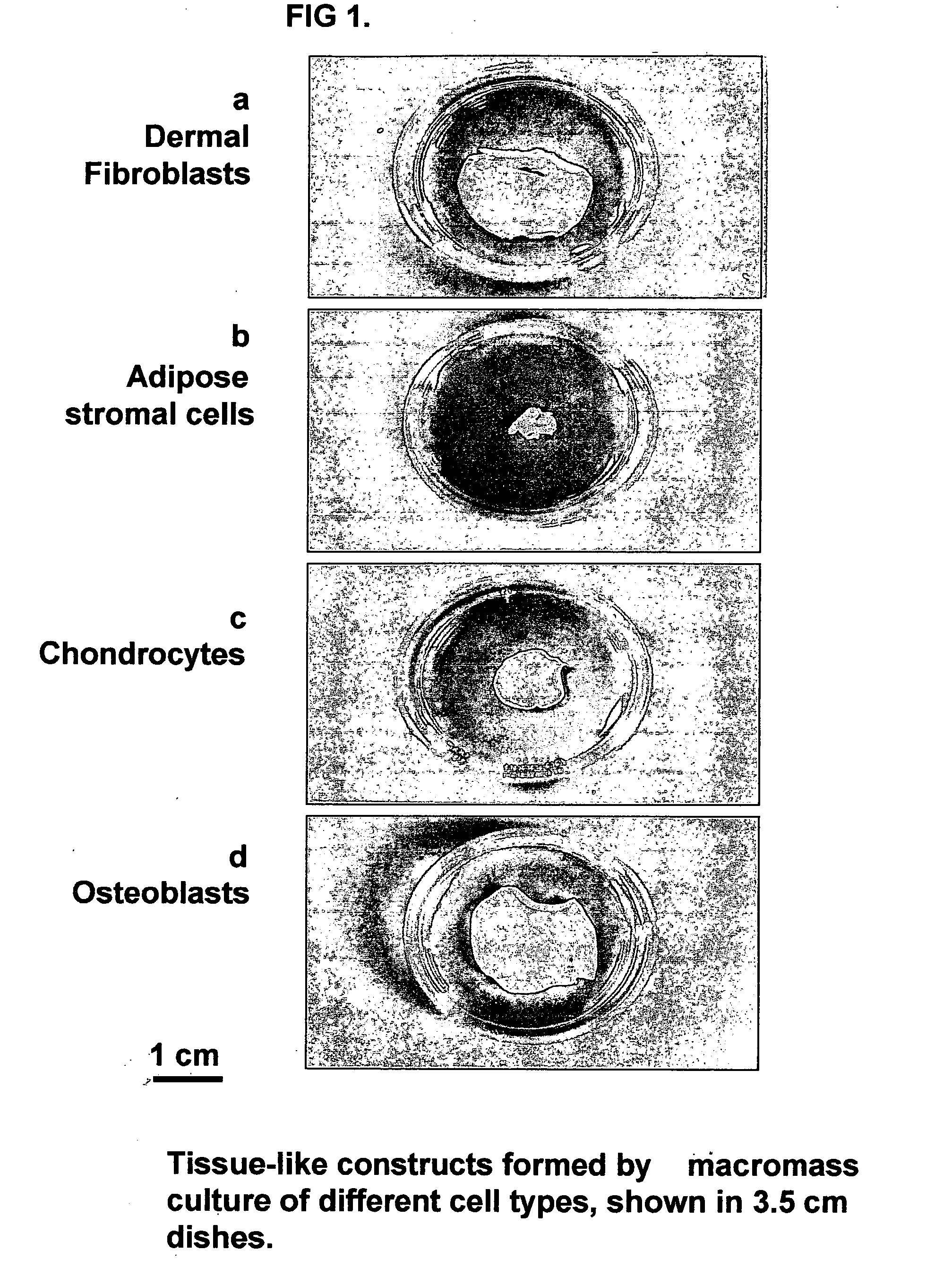
Three-dimensional tissue-like organization of cells by high cell-seeding-density culture termed as macromass culture is described. By macromass culture, cells can be made to organize themselves into a tissue-like form without the aid of a scaffold and three-dimensional macroscopic tissue-like constructs can be made wholly from cells. Tissue-like organization and macroscopic tissue-like constructs can be generated from fibroblastic cells of mesenchymal origin (at least), which can be either differentiated cells or multipotent adult stem cells. In this work, tissue-like organization and macroscopic tissue-like constructs have been generated from dermal fibroblasts, adipose stromal cells-derived osteogenic cells, chondrocytes, and from osteoblasts. The factor causing macroscopic tissue formation is large scale culture at high cell seeding density per unit area or three-dimensional space, that is, macromass culture done on a large scale. No scaffold or extraneous matrix is used for tissue generation, the tissues are of completely cellular origin. No other agents (except high cell-seeding-density) that aid in tissue formation such as tissue-inducing chemicals, tissue-inducing growth factors, substratum with special properties, rotational culture, etc, are employed for tissue formation. These tissue-like masses have the potential for use as tissue replacements in the human body. Tissue-like organization by high cell-seeding-density macromass culture can also be generated at the microscopic level.
Owner:RELIANCE LIFE SCI PVT
Compositions and methods for the stimulation or enhancement of bone formation and the self-renewal of cells
ActiveUS20050261181A1Improve biological activityPromote mineralizationBiocidePeptide/protein ingredientsOsteoblastWnt inhibitor
Compositions and methods for the treatment of bone diseases, bone fractures, bone injuries and other bone abnormalities involving the use of Dkk protein, a Wnt antagonist, a Wnt inhibitor, or any other related protein for the stimulation or enhancement of mineralization and for stimulating the renewal of cells. One Dkk protein, Dickkopf-2 (Dkk-2), acts to stimulate bone formation independently of Wnt proteins which may be inhibited and / or antagonized by Dkk-2. Dkk-2 displayed enhanced specific targeting ability and enhanced biological activity in stimulating or enhancing mineralization. Dkk-2 also played a role in the differentiation and self-renewal of hematopoietic stem cells and mesenchymal stem cells, particularly in osteoblastogenesis and osteoclastogenesis.
Owner:ENZO BIOCHEM
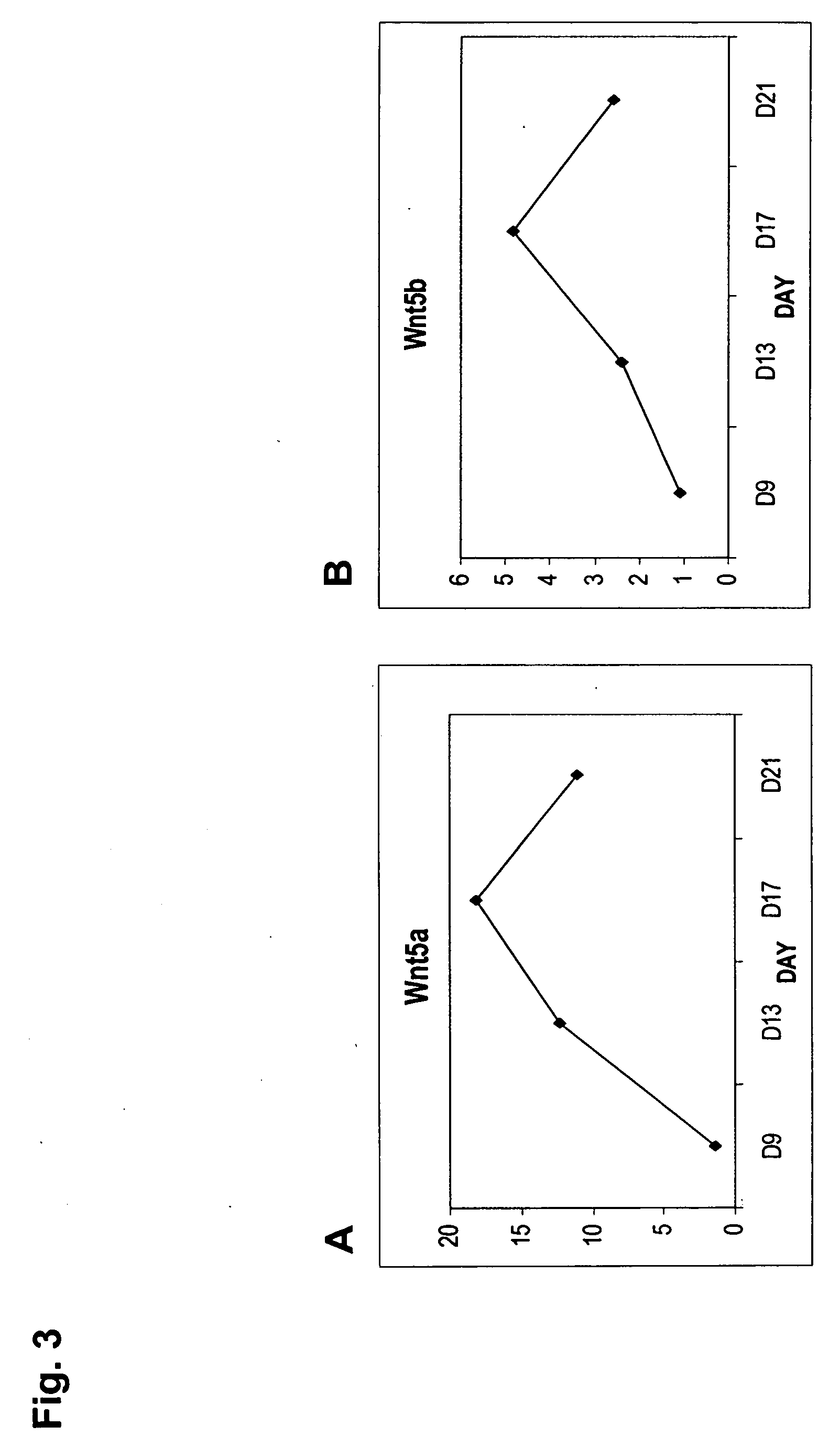
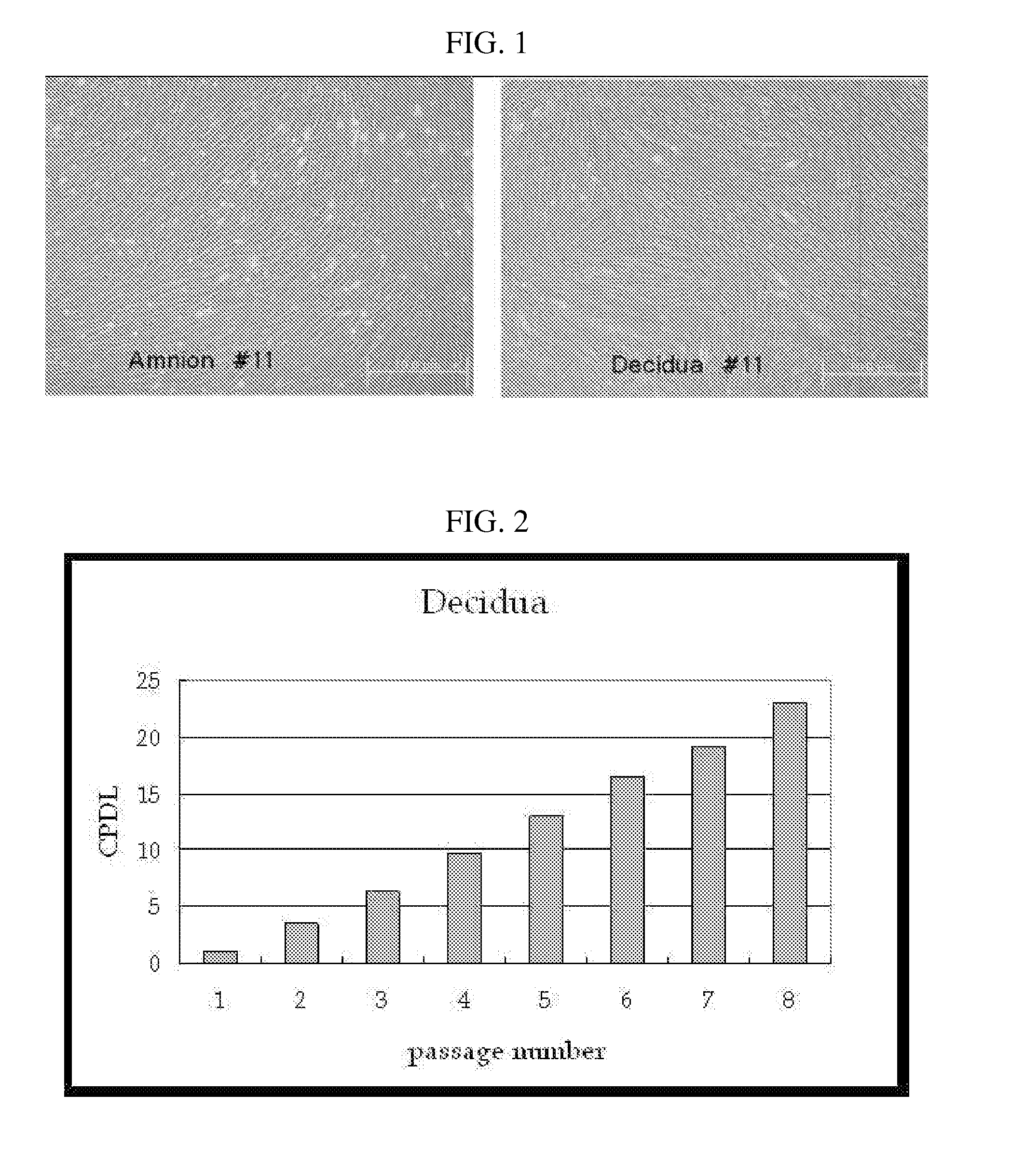
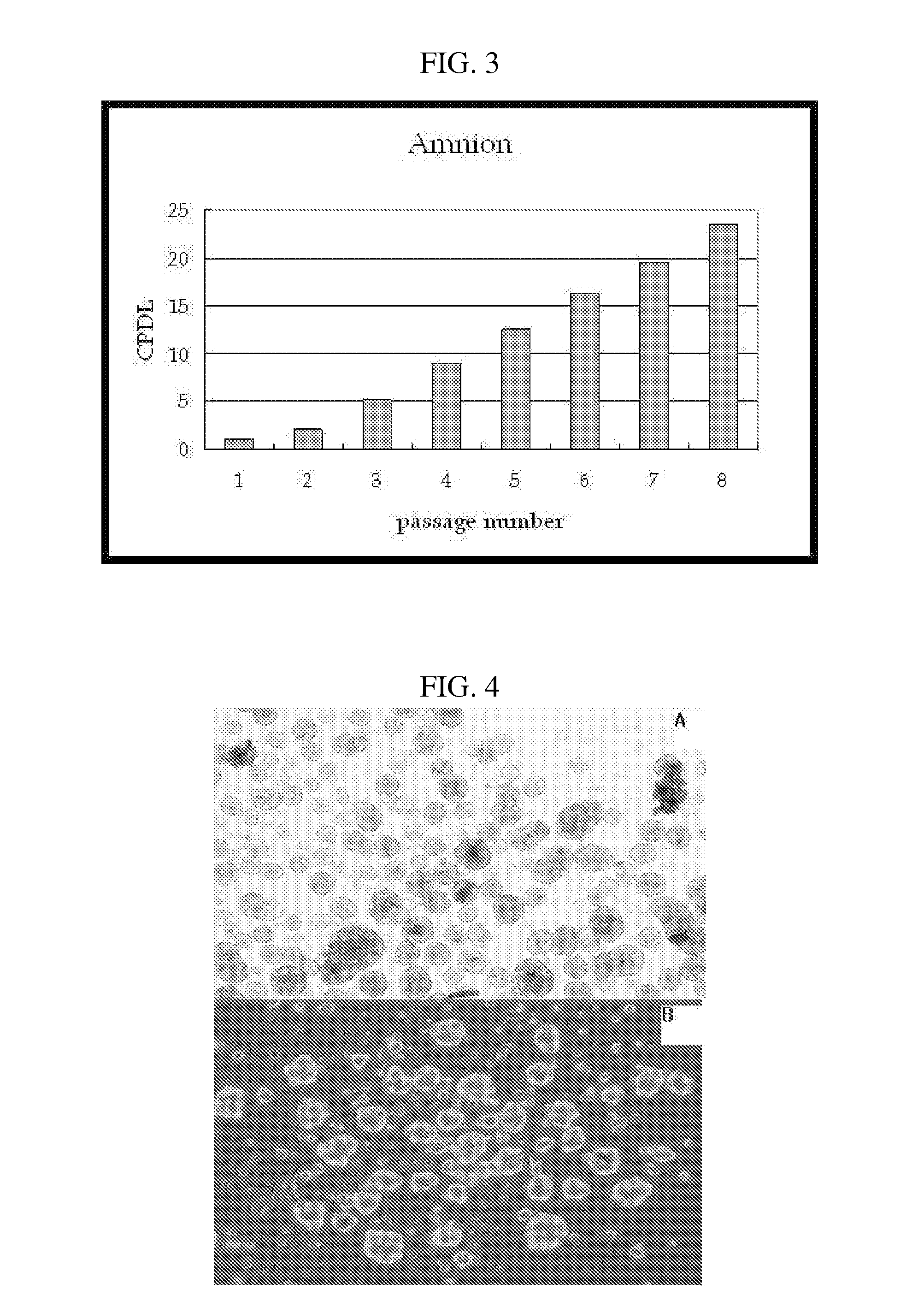
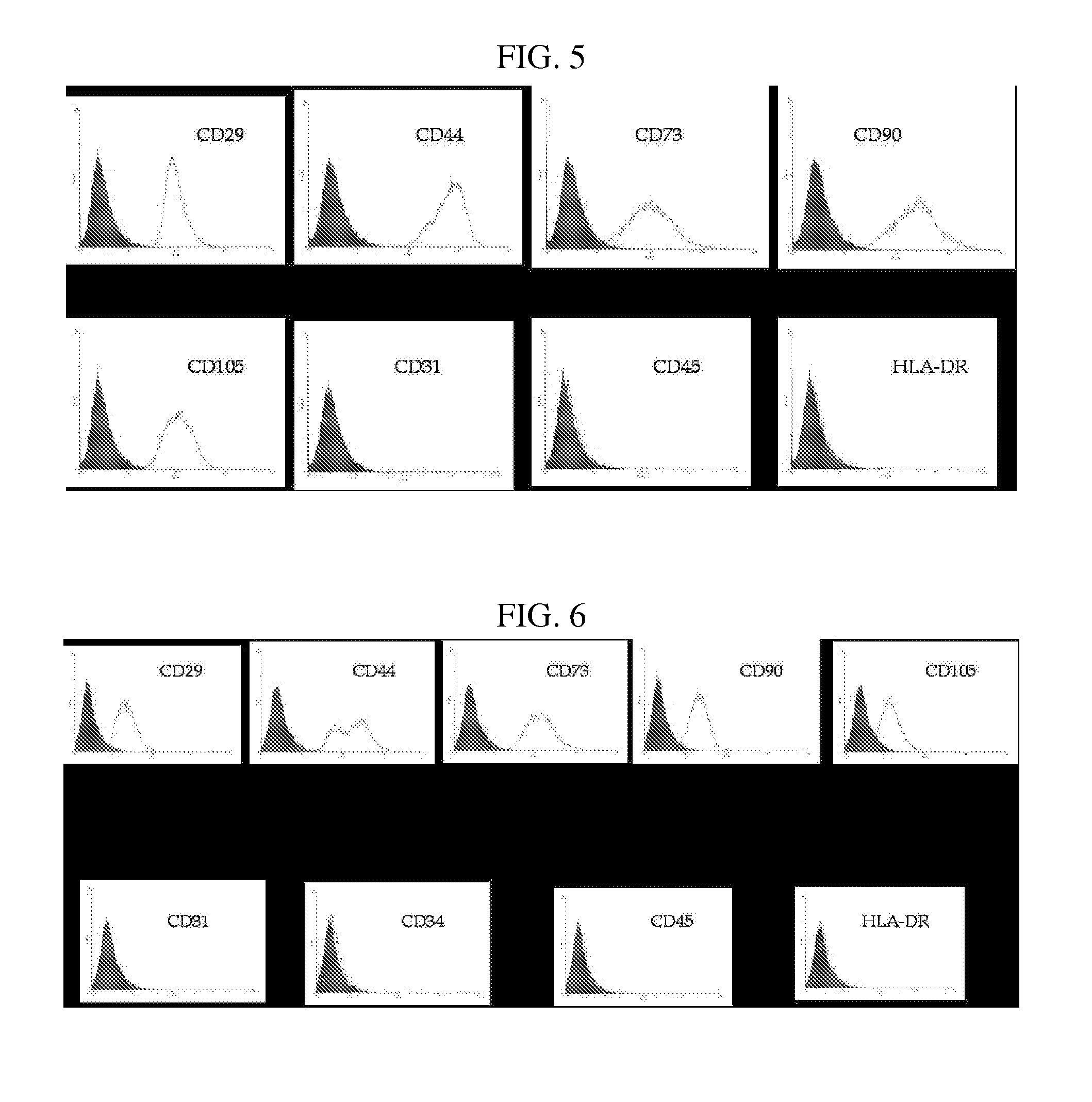
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Bone cell covered arthroplasty devices
InactiveUS20040024471A1Reduce shear stressPromote bone growthInternal osteosythesisDiagnosticsShear stressSacroiliac joint
Owner:FERREE BRET A
Novel peptide with osteogenic activity
The present invention provides a composition including an isolated or recombinant peptide component that has osteogenic cell proliferative activity. The peptide, which promotes proliferation of osteoblasts, is useful for treatment of fractures, as a filler in deficient sites of bone, for inhibition of decrease in bone substance related to osteoporosis and periodontic diseases, and for prevention of fractures associated with osteoporosis and rheumatoid arthritis. The peptide, or cells that have been genetically engineered to produce the peptide, can be combined with a bone-compatible matrix to facilitate slow release of the peptide to a treatment site and / or provide a structure for developing bone.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
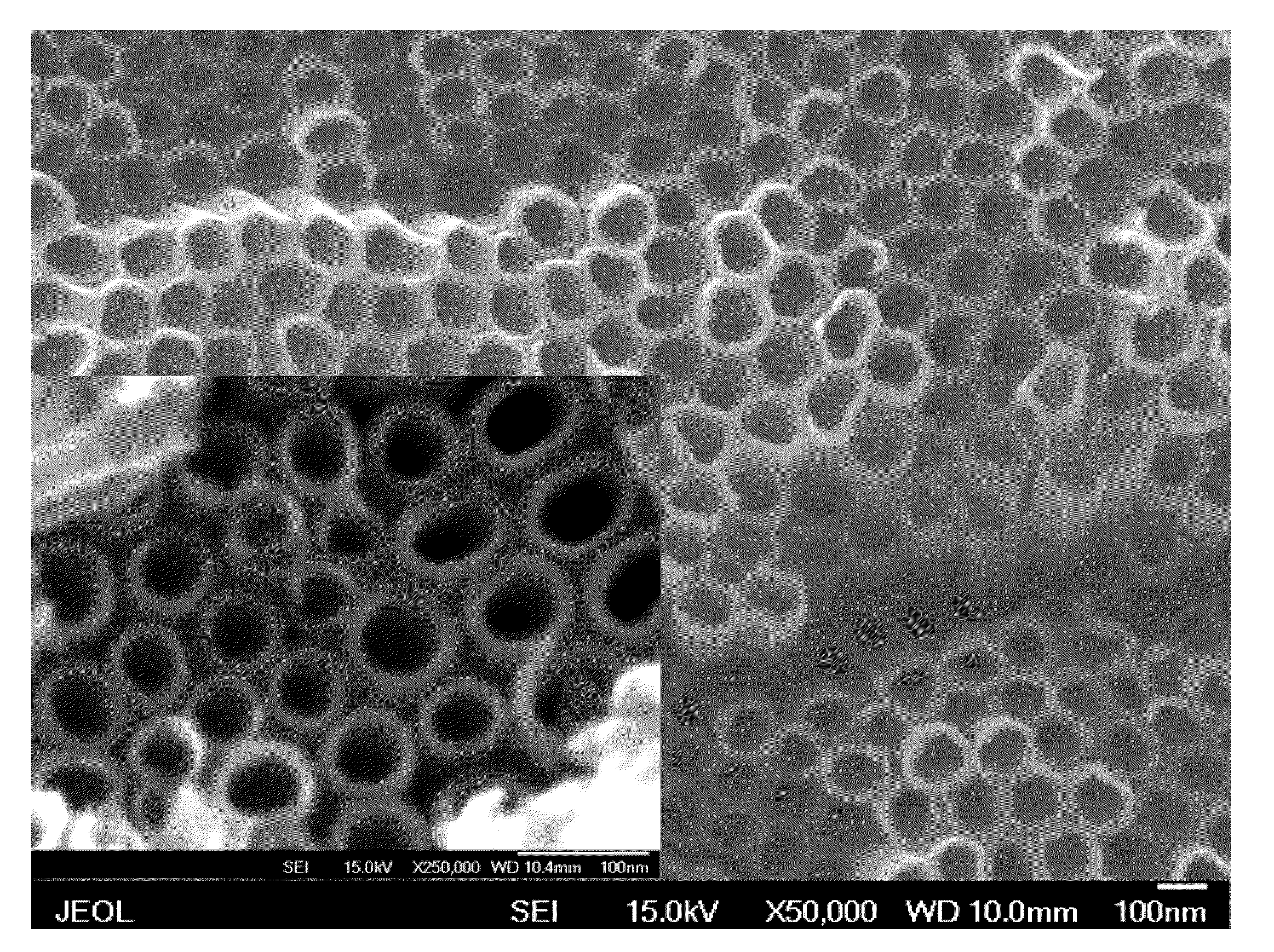
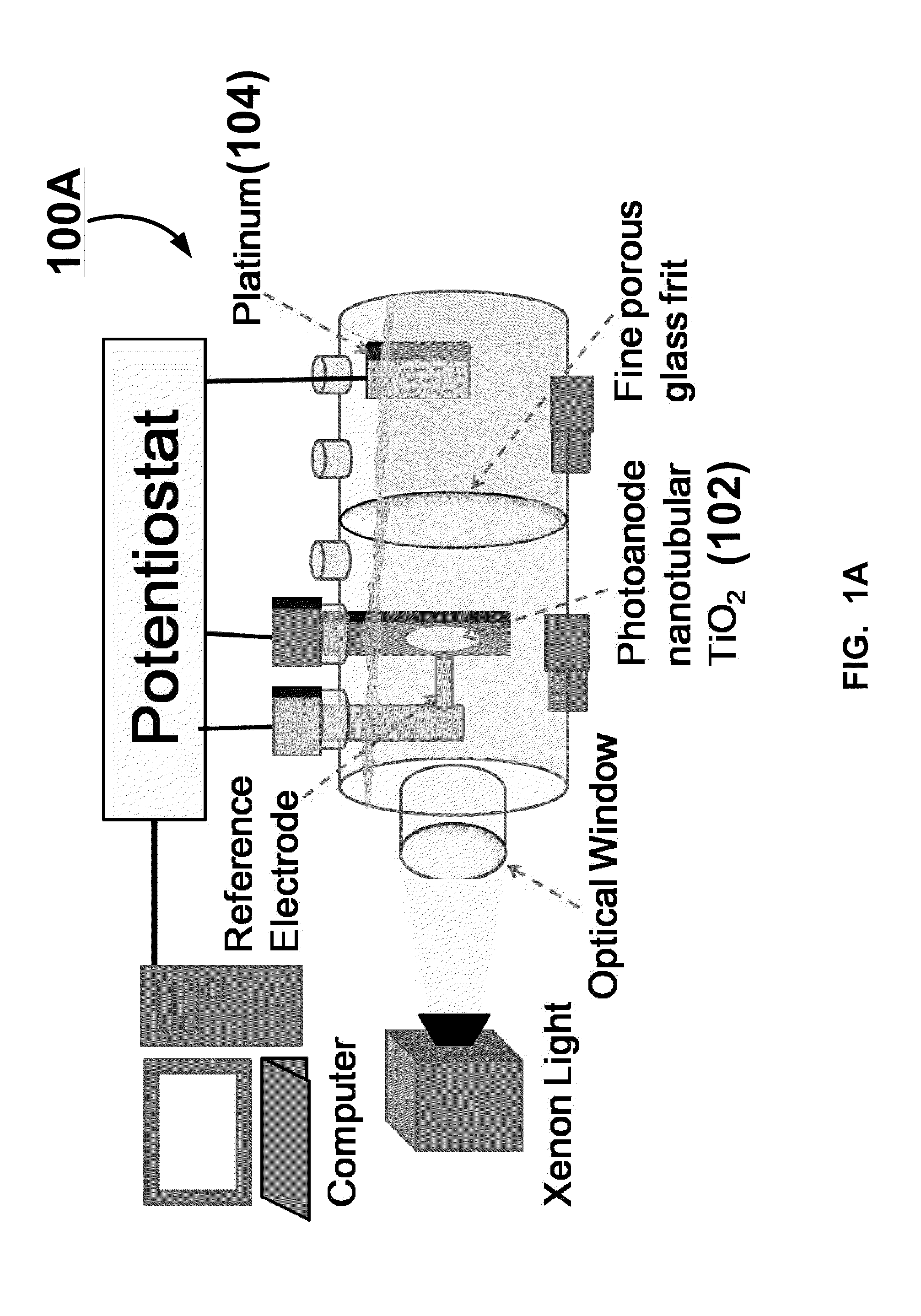
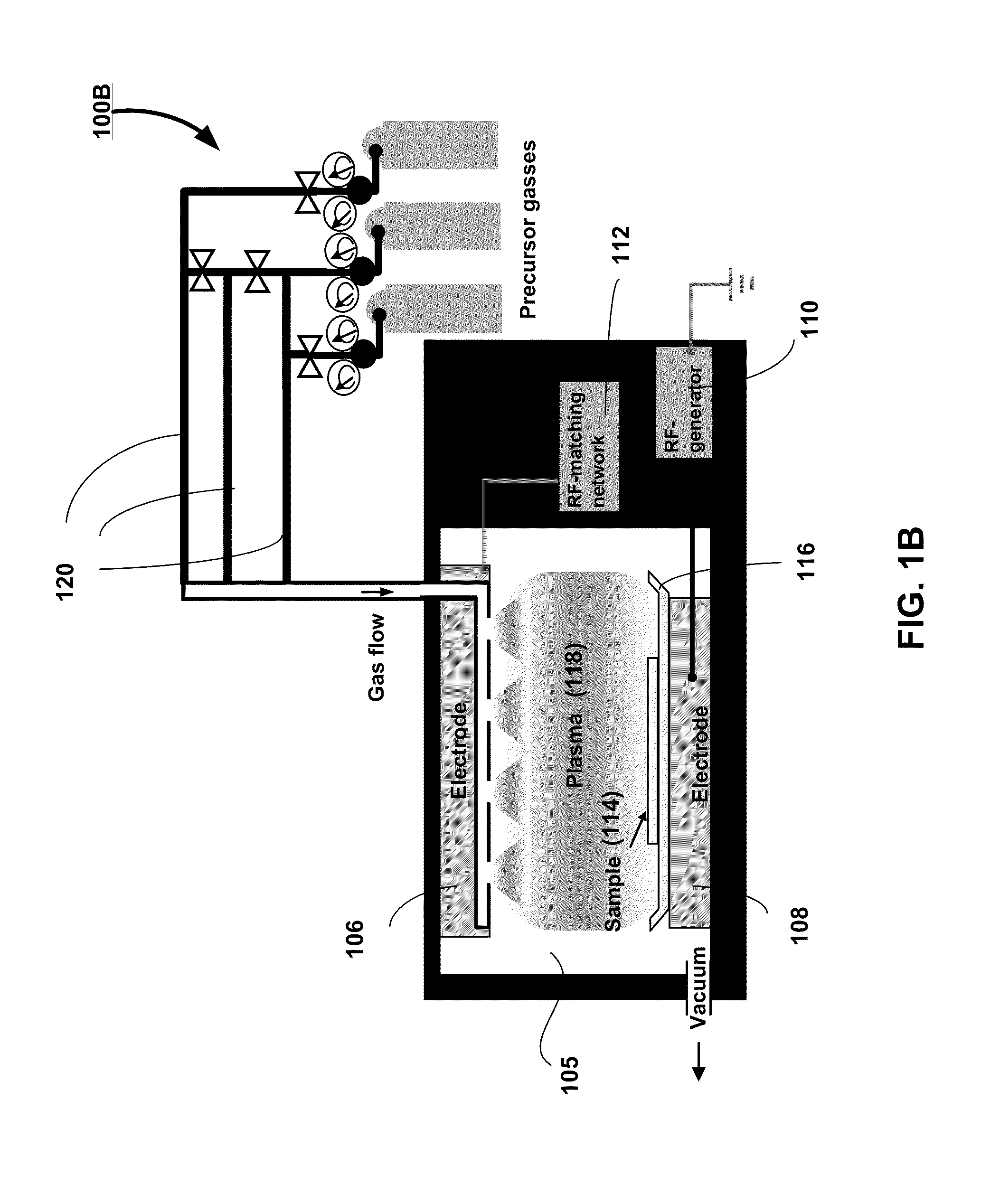
ENHANCED BONE CELLS GROWTH AND PROLIFERATION ON TiO2 NANOTUBULAR SUBSTRATES TREATED BY RADIO-FREQUENCY PLASMA DISCHARGE
A method for growing bone cells. In one aspect, the present invention provides a method for growing bone cells, comprising the steps of (a) anodizing a titanium substrate to form an array of titanium dioxide nanotubes on a surface of the titanium substrate, (b) subjecting the anodized titanium substrate to a radio frequency plasma discharge to chemically modify the array of titanium dioxide nanotubes formed on the surface of the titanium substrate, (c) seeding bone cells onto the surface of the titanium substrate that has an array of titanium dioxide nanotubes thereon after the subjecting step, and (d) incubating the seeded bone cells for a period of time effective for the cells to grow and proliferate.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Transplant material and method for fabricating the same
InactiveUS6989030B1Improved bone tissue repair speedGood biocompatibilityBone implantTissue cultureCell adhesionOsteoblast
A transplant material which is capable of imparting desired mechanical properties, elevating bone tissue repair speed and improving biocompatibility. This transplant material comprises an artificial and biologically inactive material, which is to be implanted in vivo as a substitute for bone tissue, and at least one type of cells selected from among osteoblasts and precursory osteoblasts which are adhered to the surface of the artificial material so that the artificial material is coated with the bone matrix produced by the cells. The artificial material involves not only a biologically inactive material but also a biologically inactive material coated with a biologically active substrate. This transplant material is produced by culturing mesenchymal stem cells collected from a living body to differentiate into at least one type of cells selected from among osteoblasts and precursory osteoblasts and then culturing the cells together with the artificial material to thereby adhere the differentiated cells on the surface of the artificial material and coat the surface of the artificial material with the bone matrix produced by the differentiated cells.
Owner:JAPAN TISSUE ENG
Methods for modulating osteochondral development using bioelectrical stimulation
ActiveUS20060293724A1Maximize utilizationMaximize applicationElectrotherapyStress based microorganism growth stimulationCo administrationOsteoblast
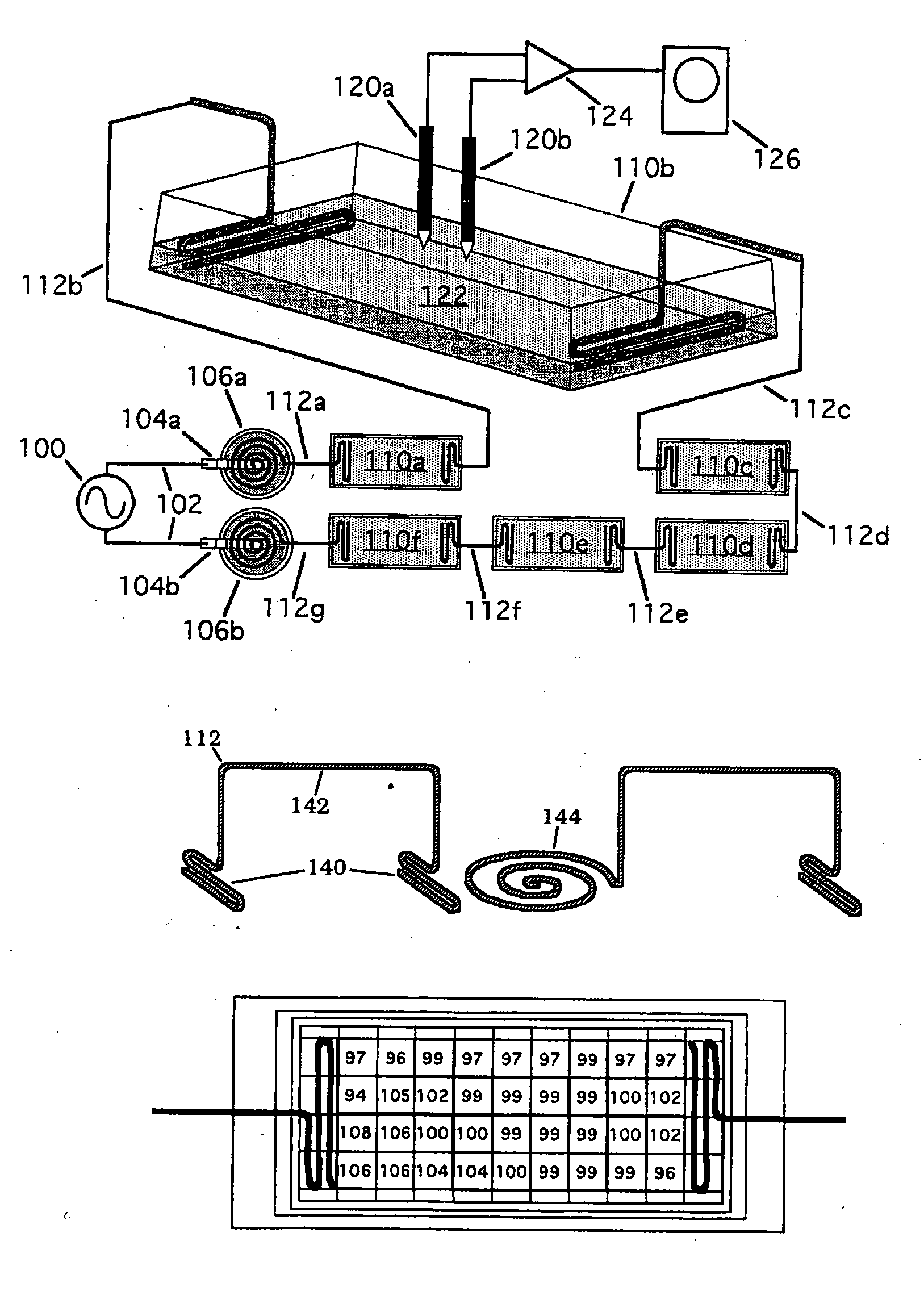
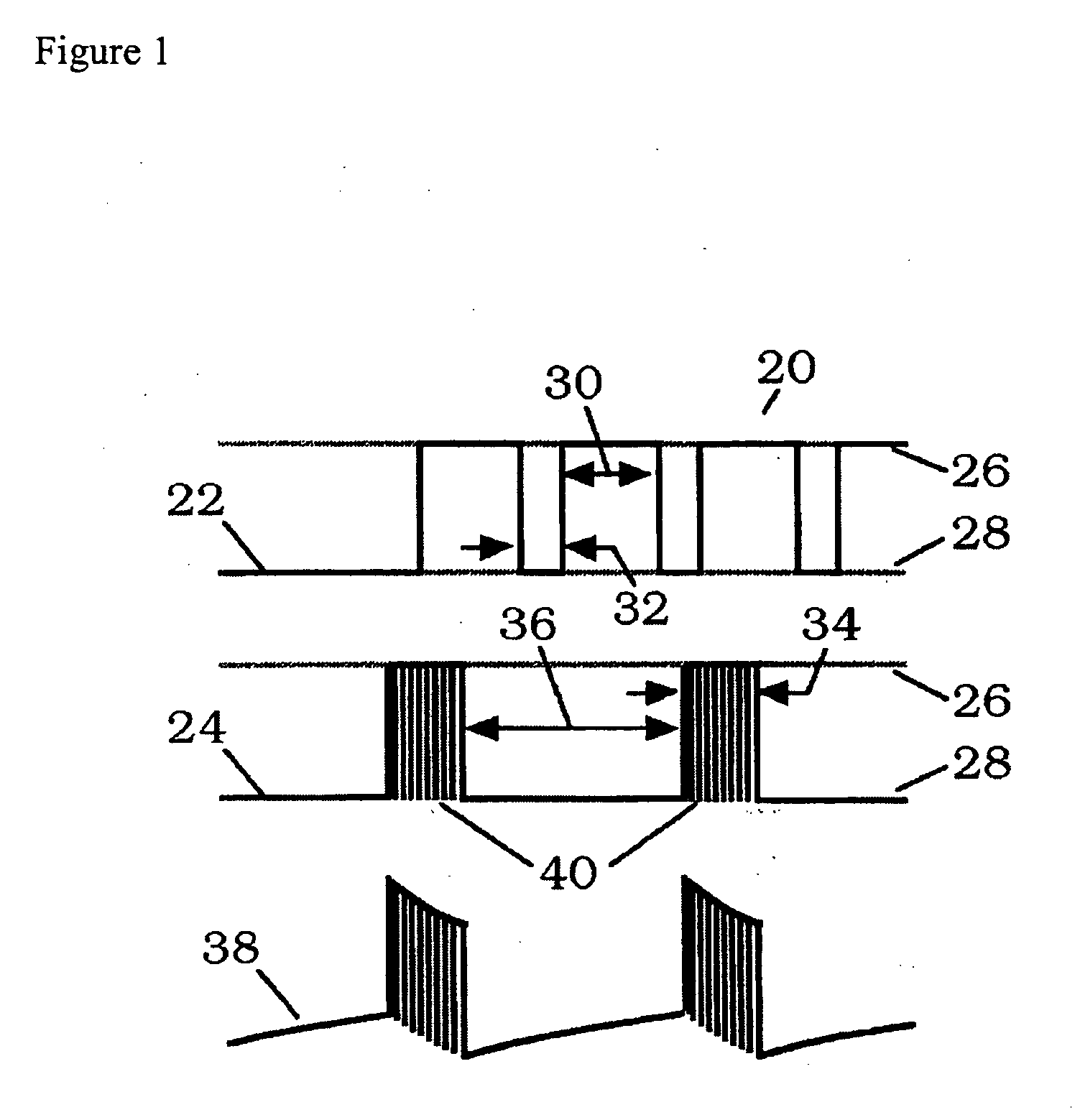
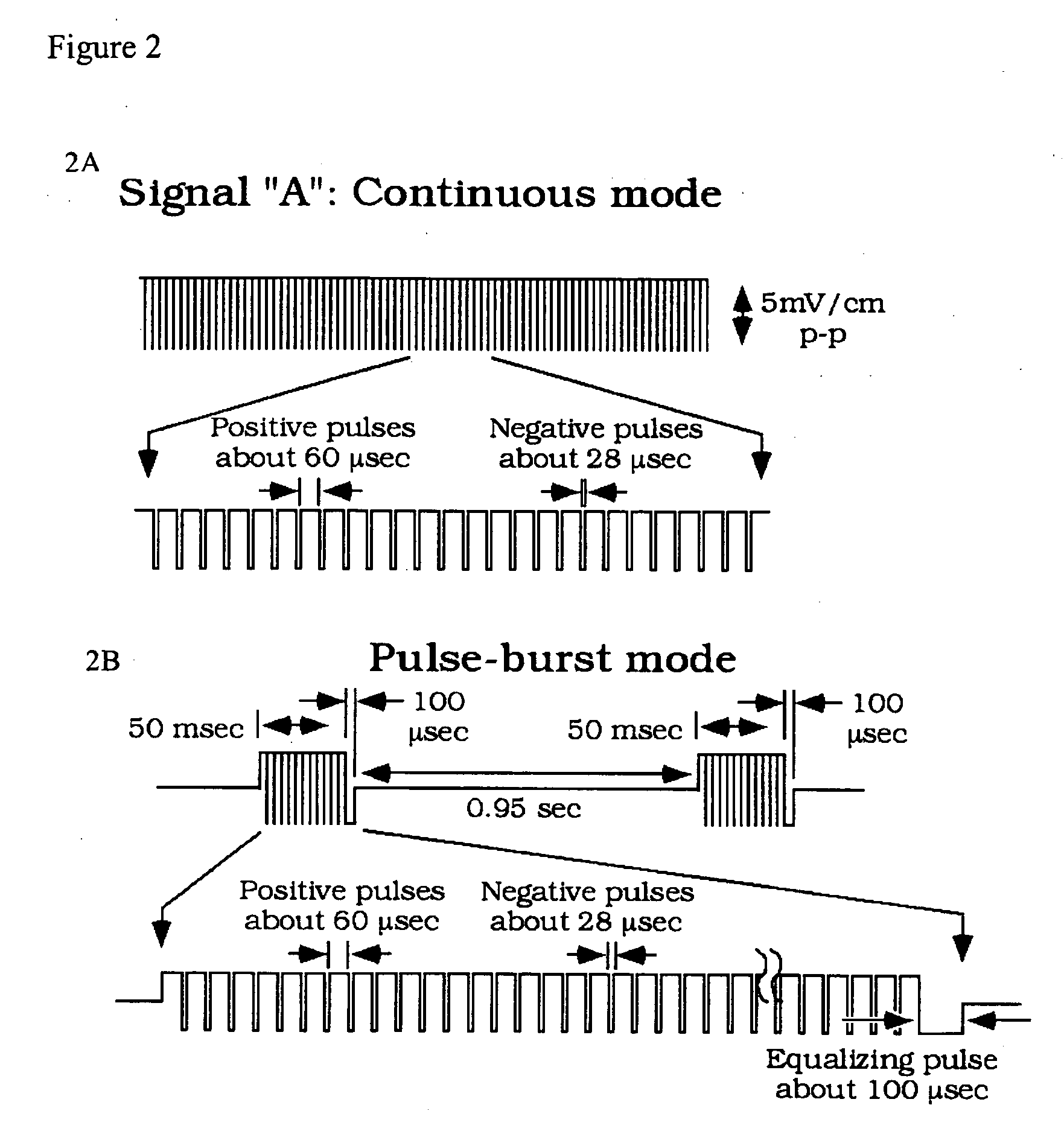
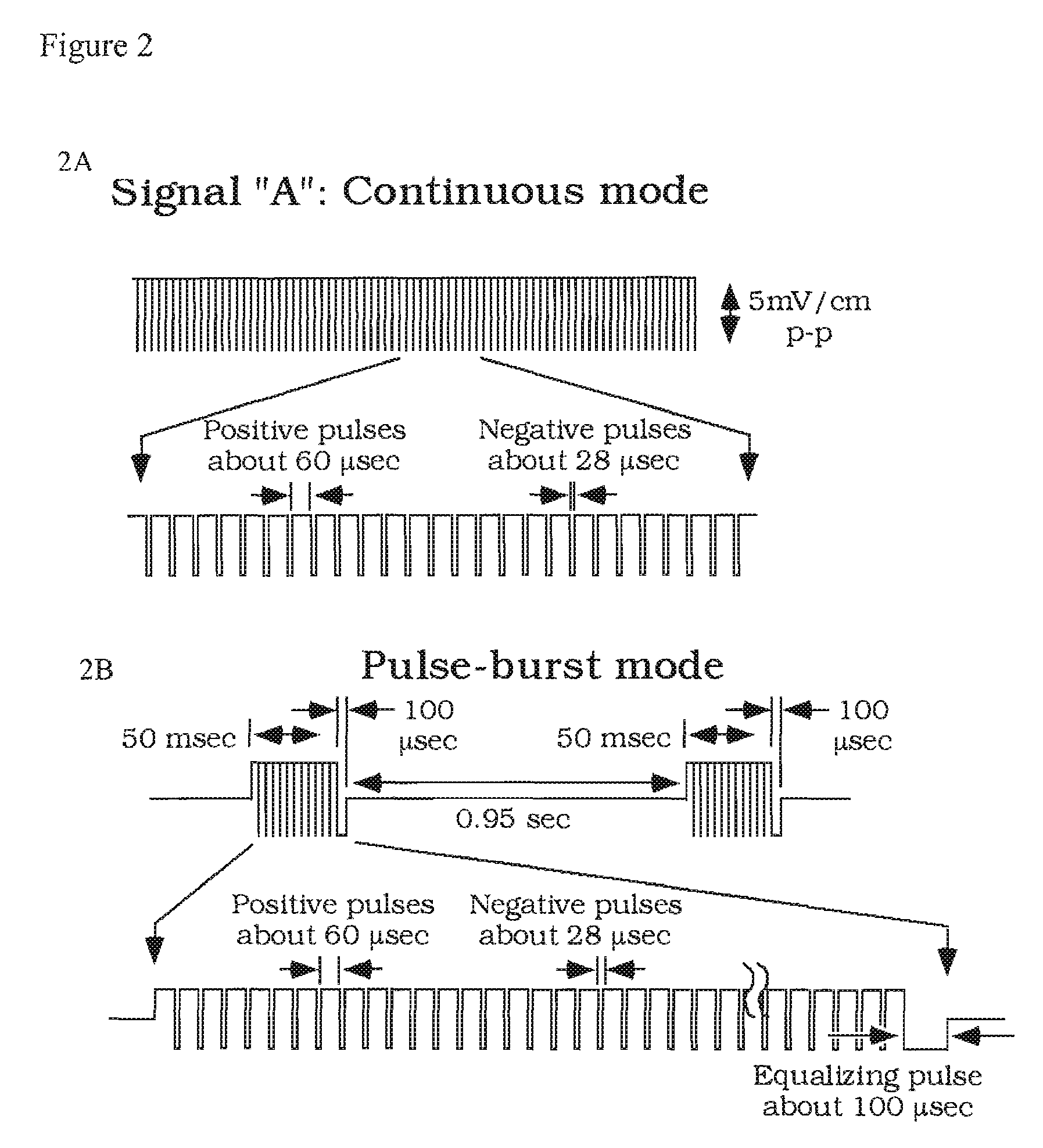
Compositions and methods are provided for modulating the growth, development and repair of bone, cartilage or other connective tissue. Devices and stimulus waveforms are provided to differentially modulate the behavior of osteoblasts, chondrocytes and other connective tissue cells to promote proliferation, differentiation, matrix formation or mineralization for in vitro or in vivo applications. Continuous-mode and pulse-burst-mode stimulation of cells with charge-balanced signals may be used. Bone, cartilage and other connective tissue growth is stimulated in part by nitric oxide release through electrical stimulation and may be modulated through co-administration of NO donors and NO synthase inhibitors. Bone, cartilage and other connective tissue growth is stimulated in part by release of BMP-2 and BMP-7 in response to electrical stimulation to promote differentiation of cells. The methods and devices described are useful in promoting repair of bone fractures, cartilage and connective tissue repair as well as for engineering tissue for transplantation.
Owner:MEDRELIEF
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
Methods for modulating chondrocyte proliferation using pulsing electric fields
Compositions and methods are provided for modulating the growth, development and repair of cartilage, bone or other connective tissue. Devices and stimulus waveforms are provided to differentially modulate the behavior of chondrocytes, osteoblasts and other connective tissue cells to promote proliferation, differentiation, matrix formation or mineralization for in vitro or in vivo applications. Continuous-mode and pulse-burst-mode stimulation of cells with charge-balanced signals may be used. Cartilage, bone and other connective tissue growth is stimulated in part by nitric oxide release through electrical stimulation and may be modulated through co-administration of NO donors and NO synthase inhibitors. The methods and devices described are useful in promoting repair of bone fractures, cartilage and connective tissue repair as well as for engineering tissue for transplantation.
Owner:HEALTHONICS INC
Multiplex composite bone tissue engineering bracket material capable of degrading gradiently and preparation method thereof
The invention discloses a multiplex composite engineering scaffold material capable of gradually decomposing bone tissue and a preparation method thereof. The composite scaffold material consists of calcium phosphate bone cement, biological compatible degradable synthetic high polymer and biological compatible degradable natural high polymer, has better mechanical property and gradient degradation characteristic, and can achieve the aim of regenerating and repairing bone tissue defect by implanting a bone growth factor to induce in-vivo stem cells to be differentiated into bone cells, thereby obviously improving initial strength and toughness of the scaffold material, and ensuring enough strength and toughness of the scaffold material during operating and implanting. After compounded with the high polymer material, the scaffold has excellent flexibility, so that the scaffold can be subjected to certain machining, such as cutting and the like.
Owner:SOUTH CHINA UNIV OF TECH
Biomimetic artificial hip joint with internal growth function
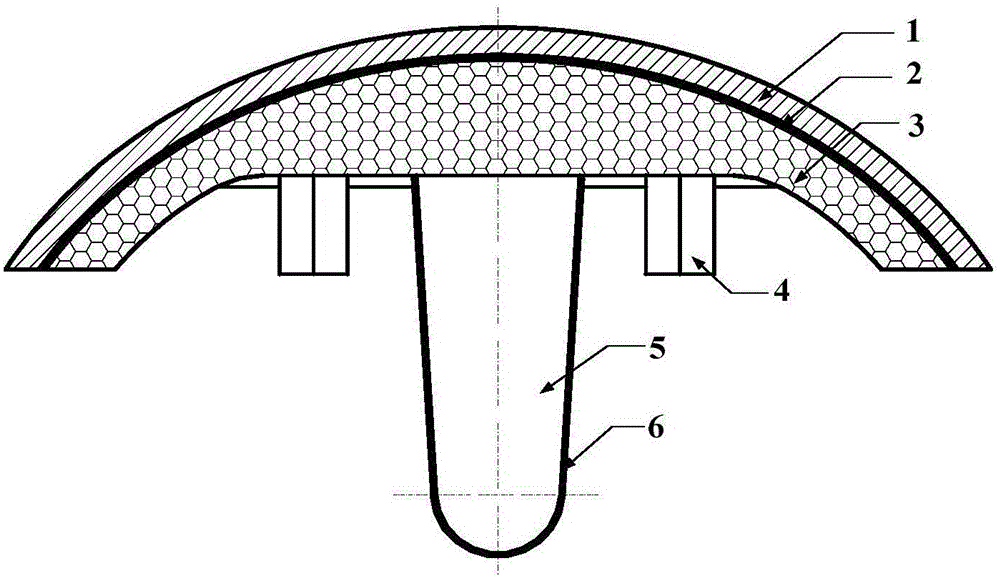
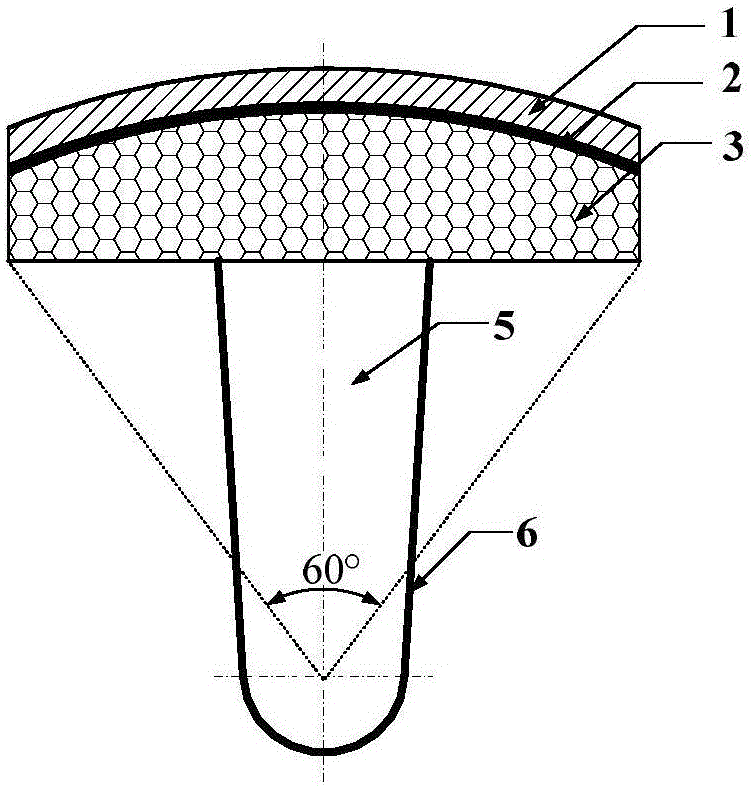
ActiveCN105105875AImprove tribological propertiesImprove fault toleranceJoint implantsHip jointsArticular surfacesBiomechanics
The invention discloses a biomimetic artificial hip joint with an internal growth function. The joint is composed of an artificial cartilage layer, an interface bonding layer and a porous bracket, wherein the artificial cartilage layer and the porous bracket have elliptic surfaces; the surface wrapping angle of the joint is 60-120 degrees; when the wrapping angle is 80-120 degrees, 3-6 convex columnar bodies, which are uniformly distributed, are designed on the inner surface of a prosthesis along the peripheral direction; a porous coating with biological activity is prepared on the outer surface of a femoral component and materials of the coating have gradient changes from inside to outside; the porous bracket is designed into a porous structure with gradient according to a finite element optimization result, and the pore diameter is 300-800 microns; the porosity is 20%-85%. According to the biomimetic artificial hip joint disclosed by the invention, bone mass and biomechanical characteristics of thigh bones can be kept to the greatest extent; the biomimetic artificial hip joint has good mechanical properties and tribological properties; the growth of bone cells is induced or promoted so as to guarantee effective interface bonding intensity between a prosthesis implantation material and a natural bone, the stability of the planted prosthesis is improved and the service life is prolonged.
Owner:XI AN JIAOTONG UNIV
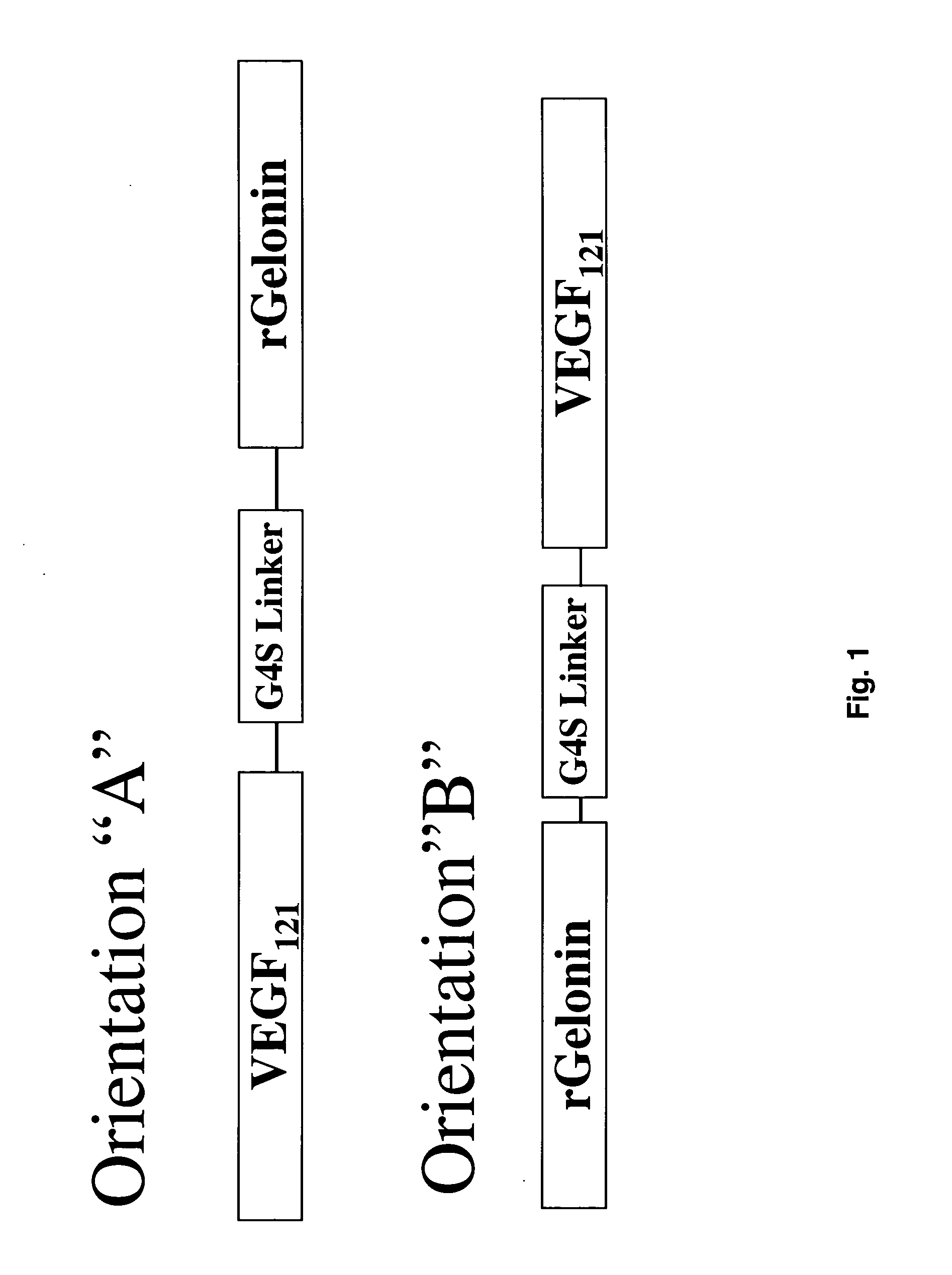
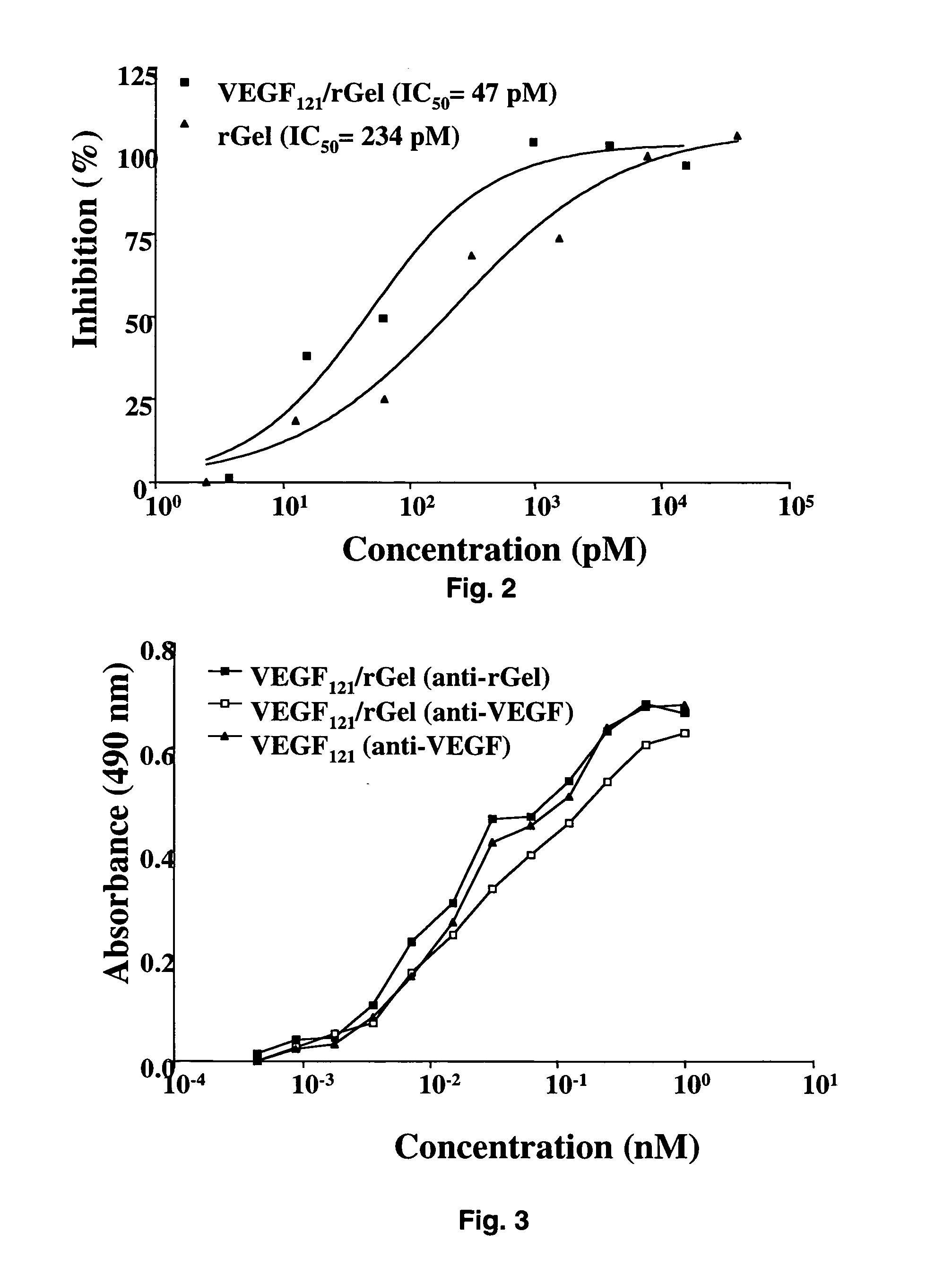
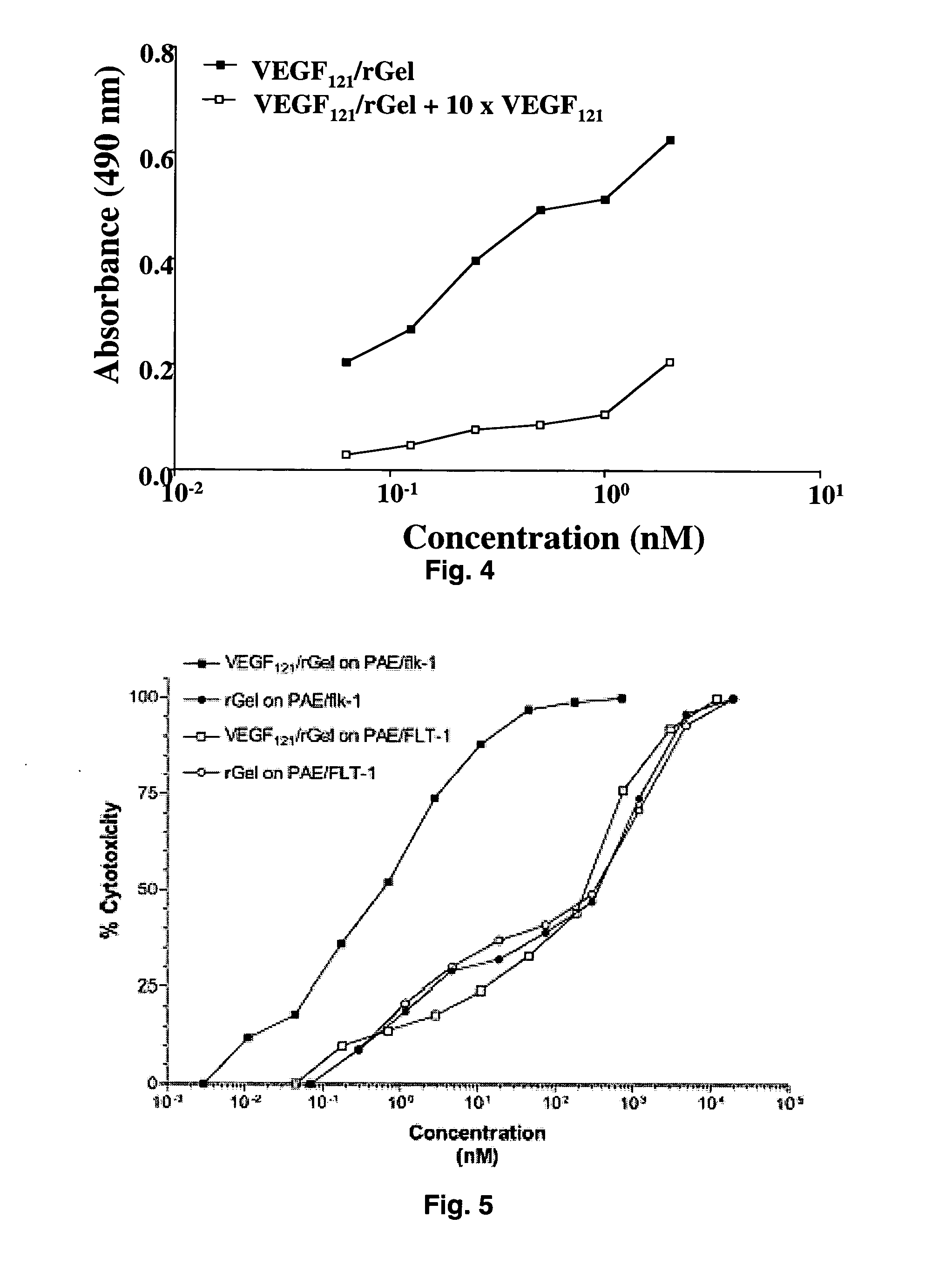
Vascular endothelial growth factor fusion constructs and uses thereof
InactiveUS20050037967A1Inhibiting metastatic spreadInhibiting vascularizationPeptide/protein ingredientsAntibody mimetics/scaffoldsGeloninAbnormal tissue growth
The 121-amino acid isoform of vascular endothelial growth factor (VEGF121) is linked by a flexible G4S tether to a cytotoxic molecule such as toxin gelonin or granzyme B and expressed as a soluble fusion protein. The VEGF12, fusion protein exhibits significant anti-tumor vascular-ablative effects that inhibit the growth of primary tumors and inhibit metastatic spread and vascularization of metastases. The VEGF121 fusion protein also target osteoclast precursor cells in vivo and inhibits osteoclastogenesis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
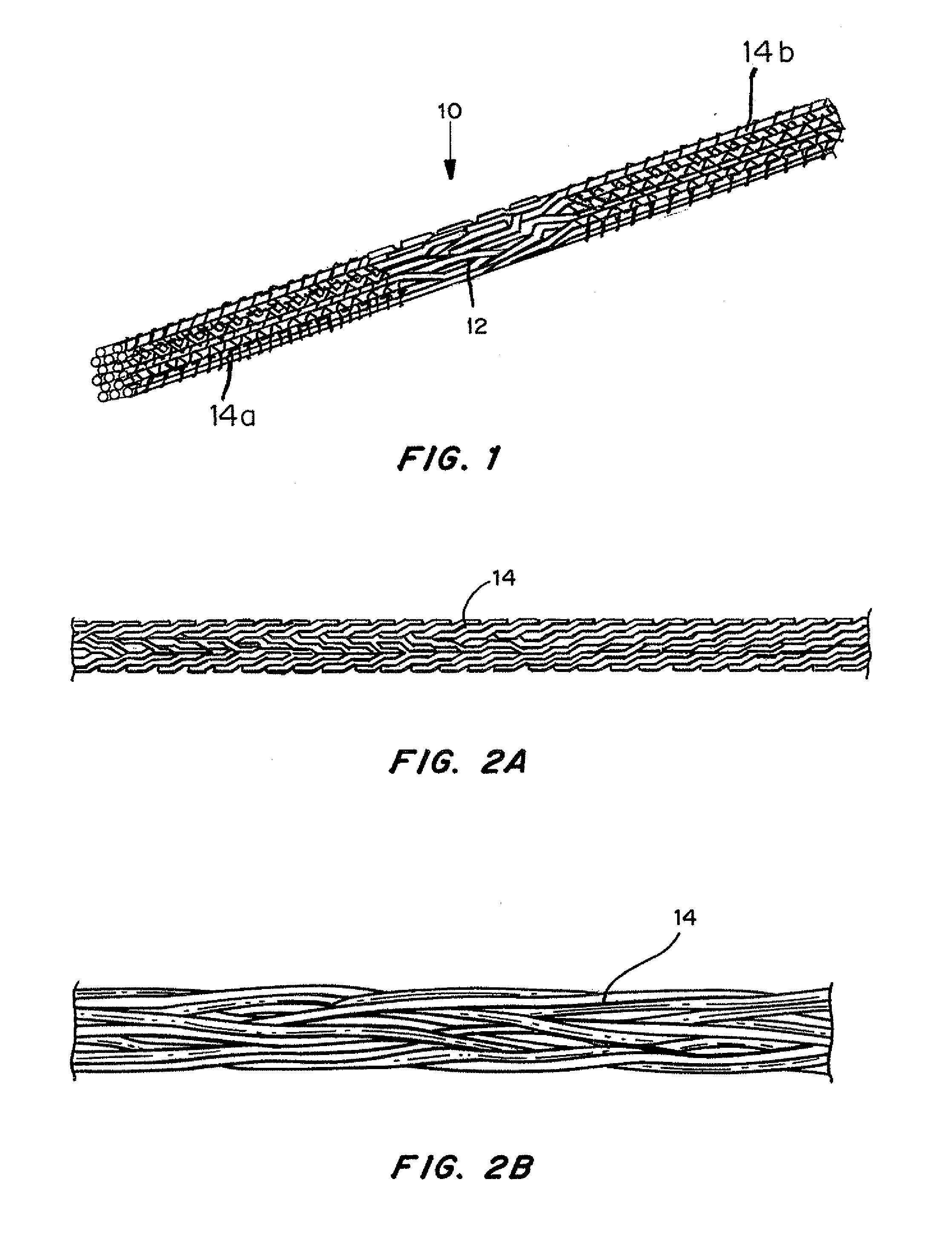
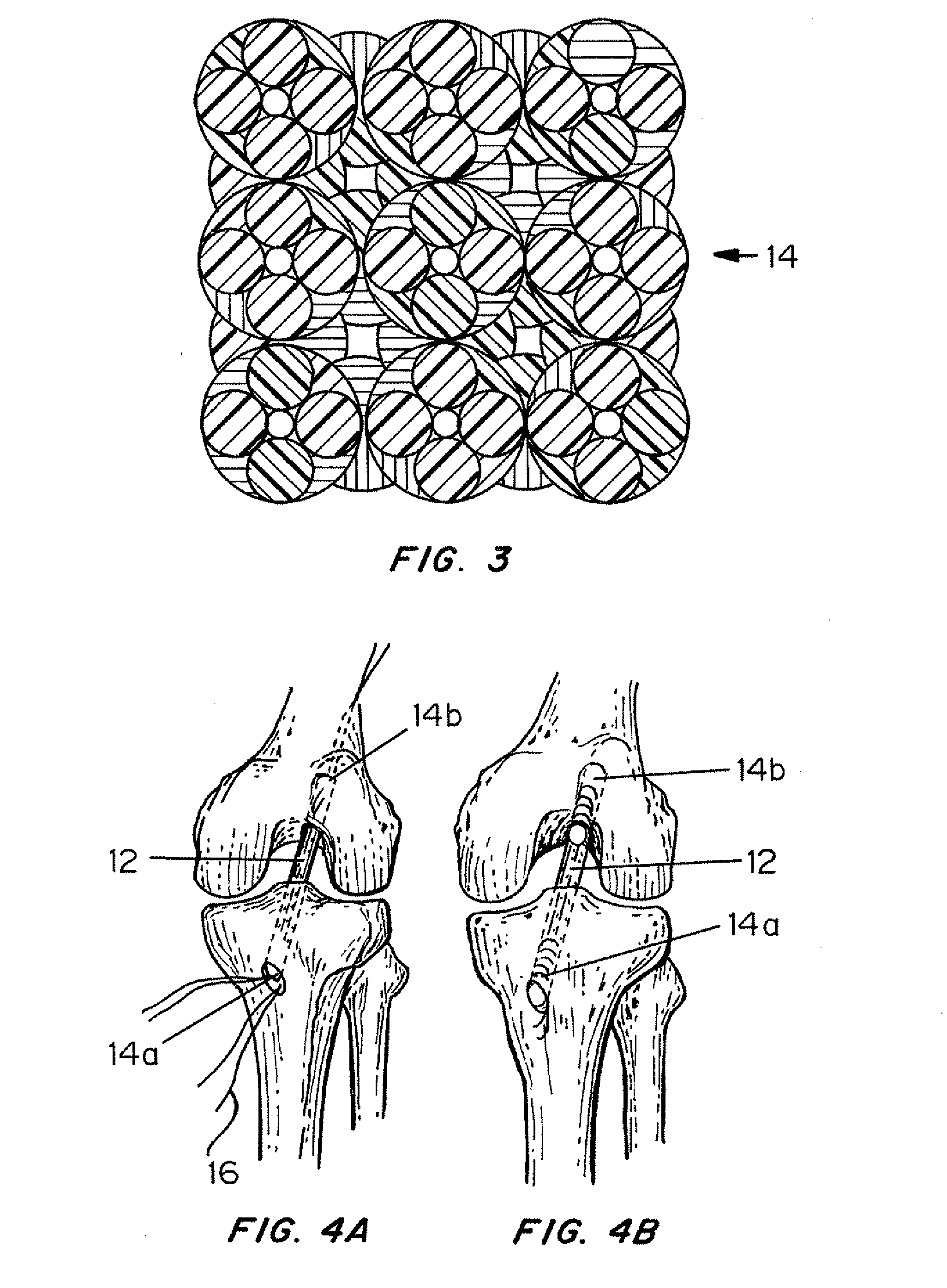
Mechanically competent scaffold for ligament and tendon regeneration
A multi-region device for repair, regeneration or reconstruction in articular tissue injury such as torn ligaments and tendons is provided. The device comprises at least one degradable material and biocompatible non-degradable polymeric fiber-based material, in a three-dimensional braided scaffold. The two end sections are designed for attachment of the device at the site of implantation and are designed to allow bone cell ingrowth, and one or more middle regions are designed to allow ligament or tendon cell ingrowth.
Owner:SOFT TISSUE REGENERATION
Injectable composite material capable of promoting bone regeneration and repair and preparation method thereof
The invention discloses an injectable composite material capable of promoting bone regeneration and repair. The injectable composite material is prepared by mixing sodium alginate, chitosan, multiple trace element, calcium phosphate porous microsphere and bioactive glass nanometer granules, preparing the mixture by using deionized water and cell culture fluid, and compounding the prepared mixture. The injectable composite material comprises the following components in percentage by mass: 0.10 to 0.50 percent of sodium alginate, 0.01 to 0.20 percent of chitosan, 5 to 30 percent of multiple trace element codoped calcium phosphate porous microsphere, 0.05 to 0.50 percent of bioactive glass, 25 to 55 percent of cell culture fluid and 30 to 45 percent of deionized water. The preparation process is simple; the prepared injectable composite material has the characteristics of excellent injectability and quick degradation; and a hydrogel network can concentrate calcium, phosphorous ion and trace elements degraded and released by inorganic particles and can promote the migration, growth, multiplication and differentiation of bone cell, thereby having effects of quickly inducing bone regeneration and promoting bone repair on endosteal microdamage, fracture or bone defect.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com