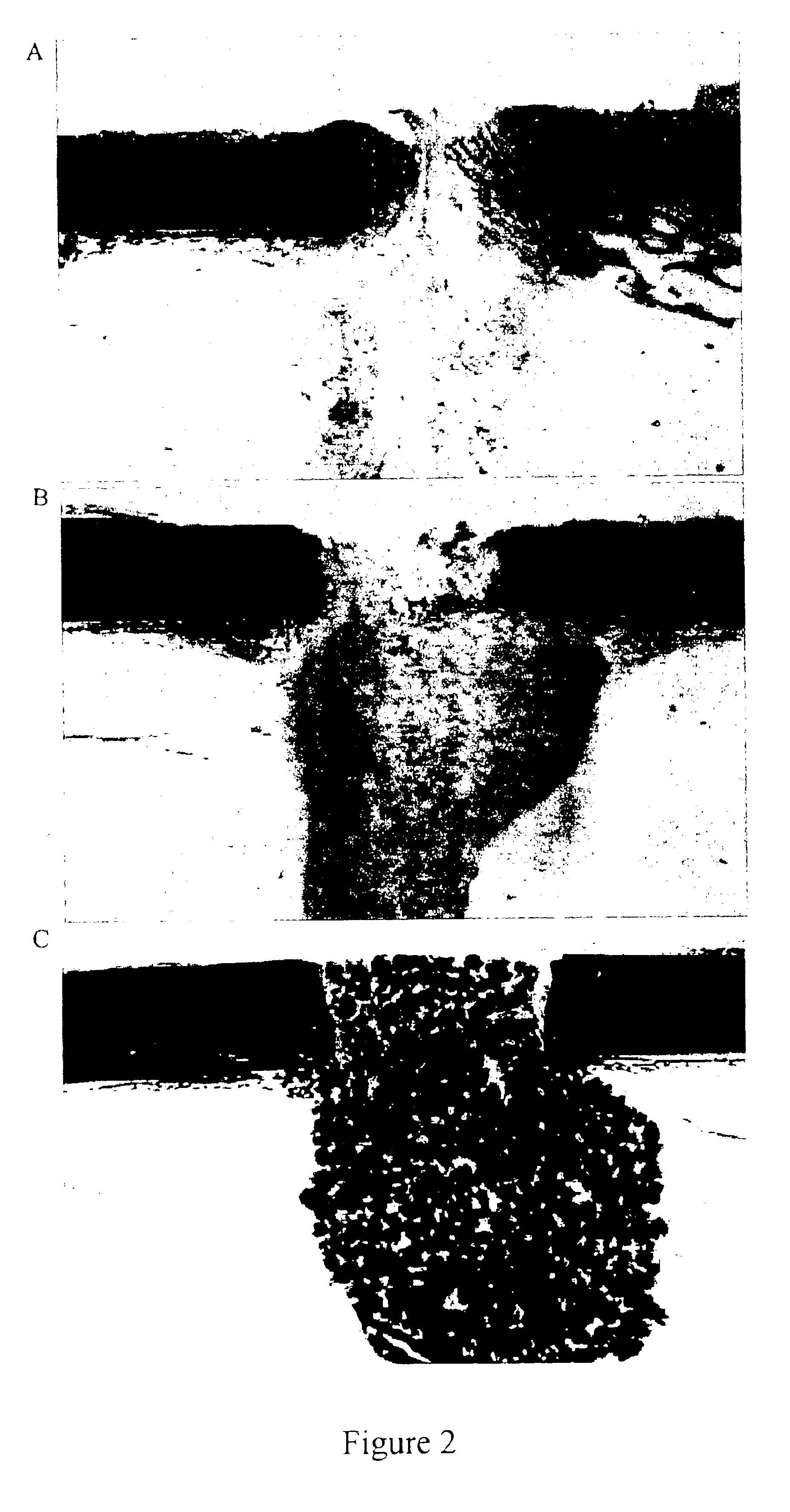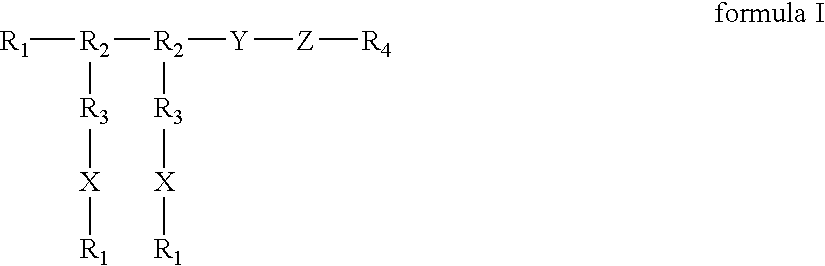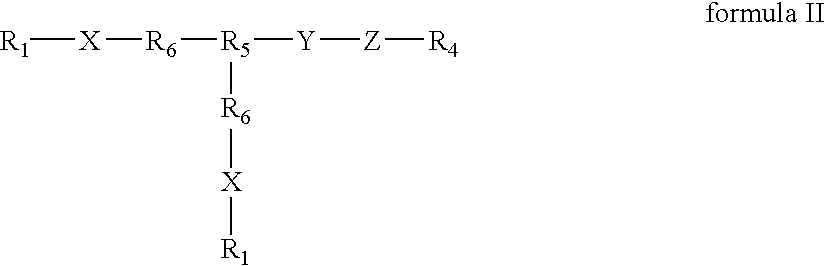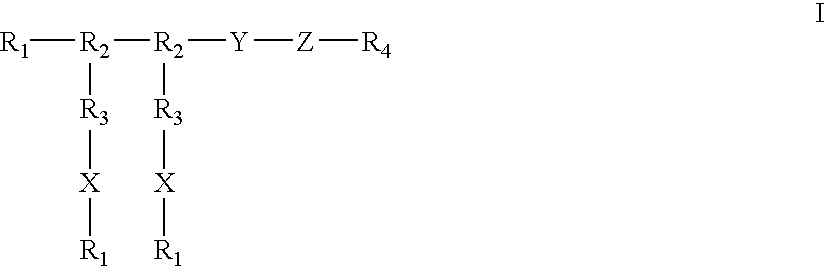Patents
Literature
582 results about "Bone formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
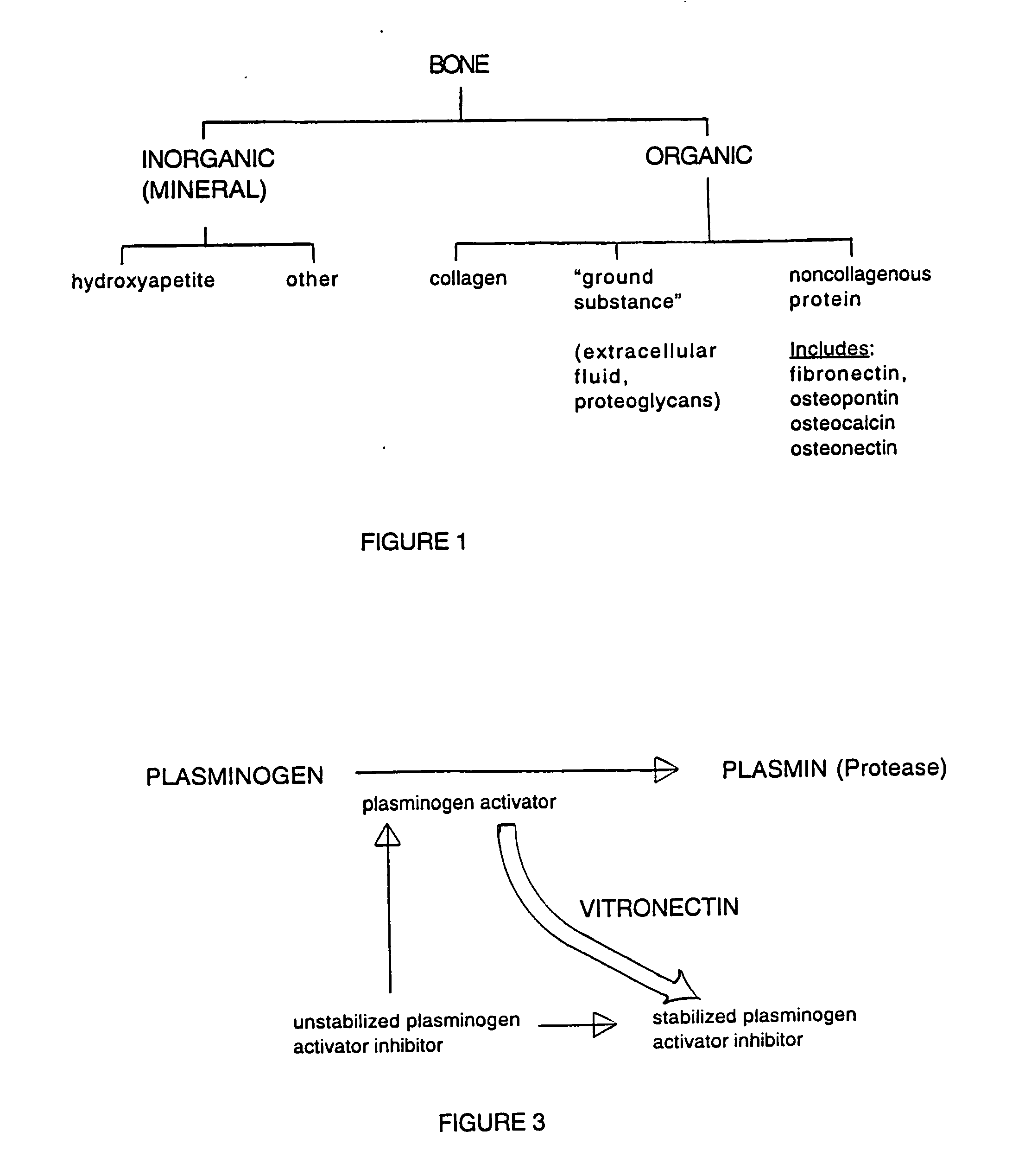
Bone is formed in the embryo in two general ways. For most bones the general shape is first laid down as a cartilage model, which is then progressively replaced by bone (endochondral bone formation). Bone, rigid body tissue consisting of cells embedded in an abundant, hard intercellular material.
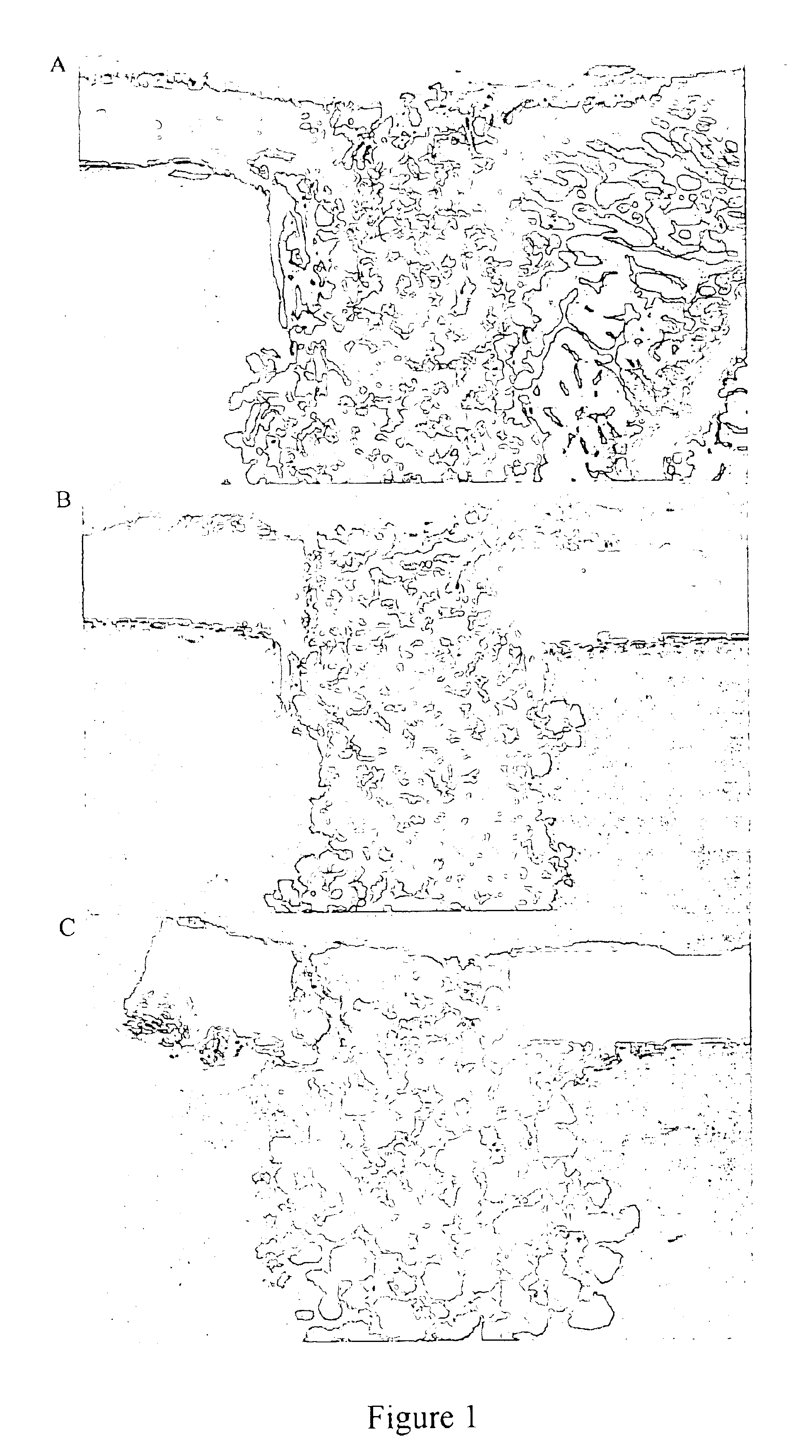
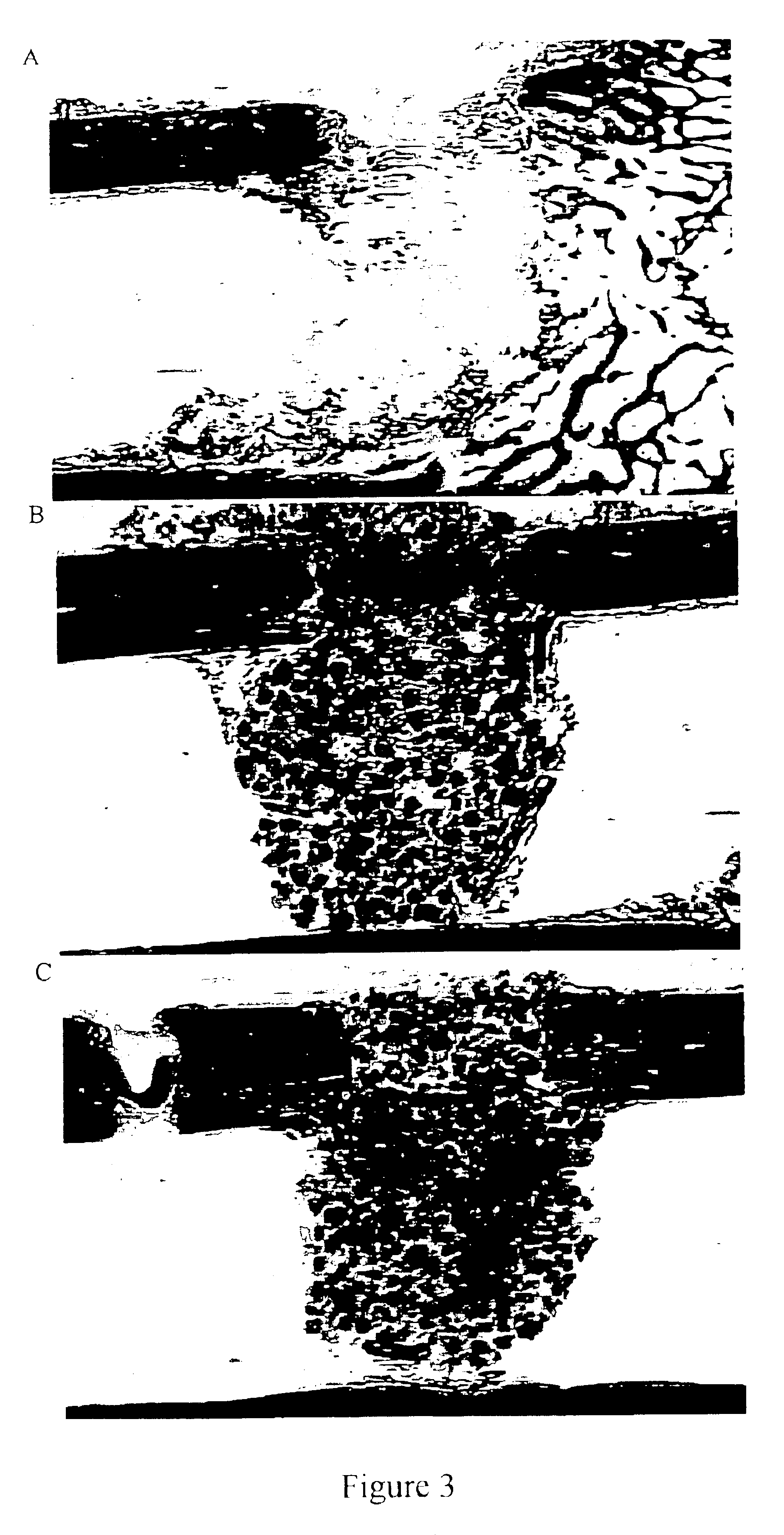
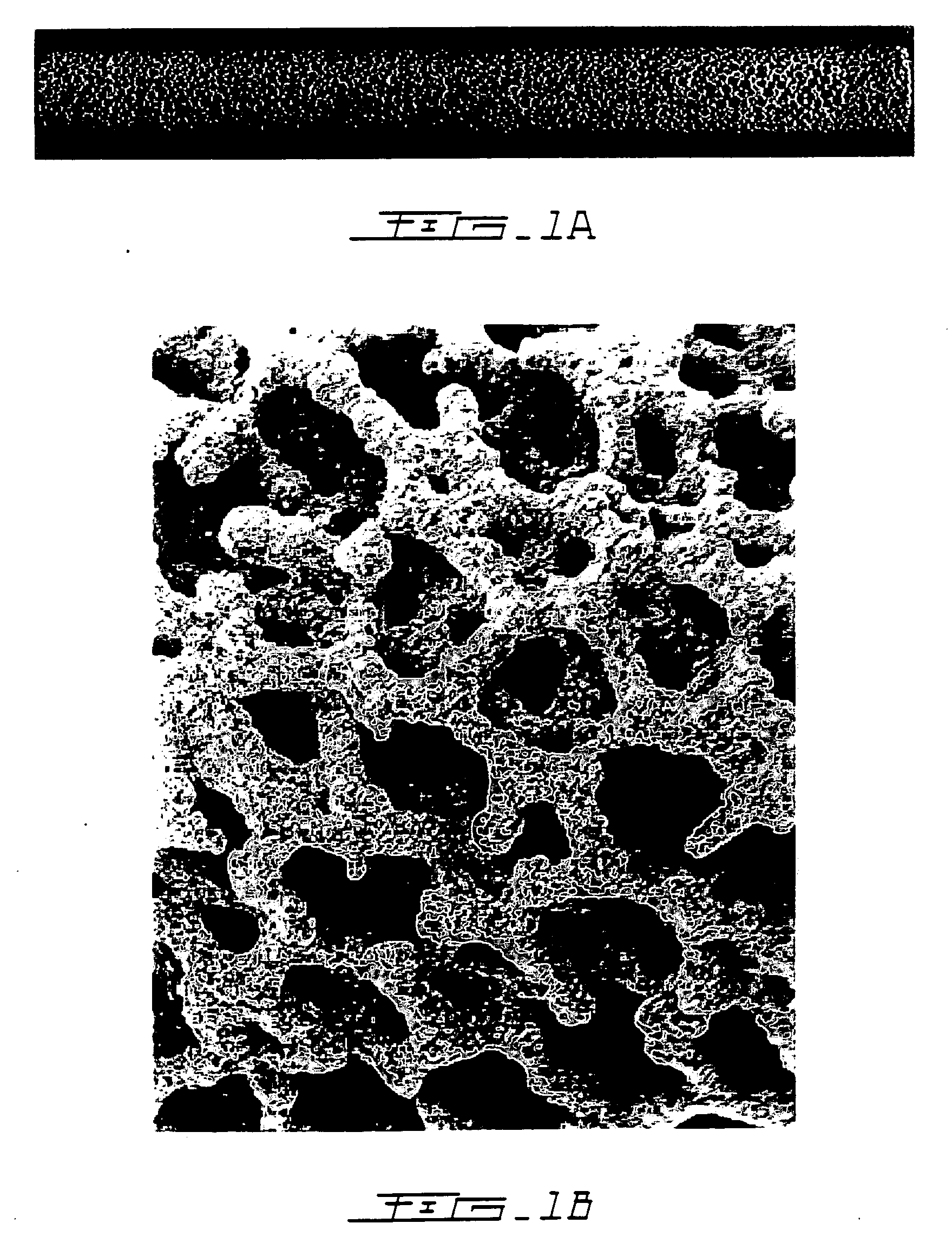
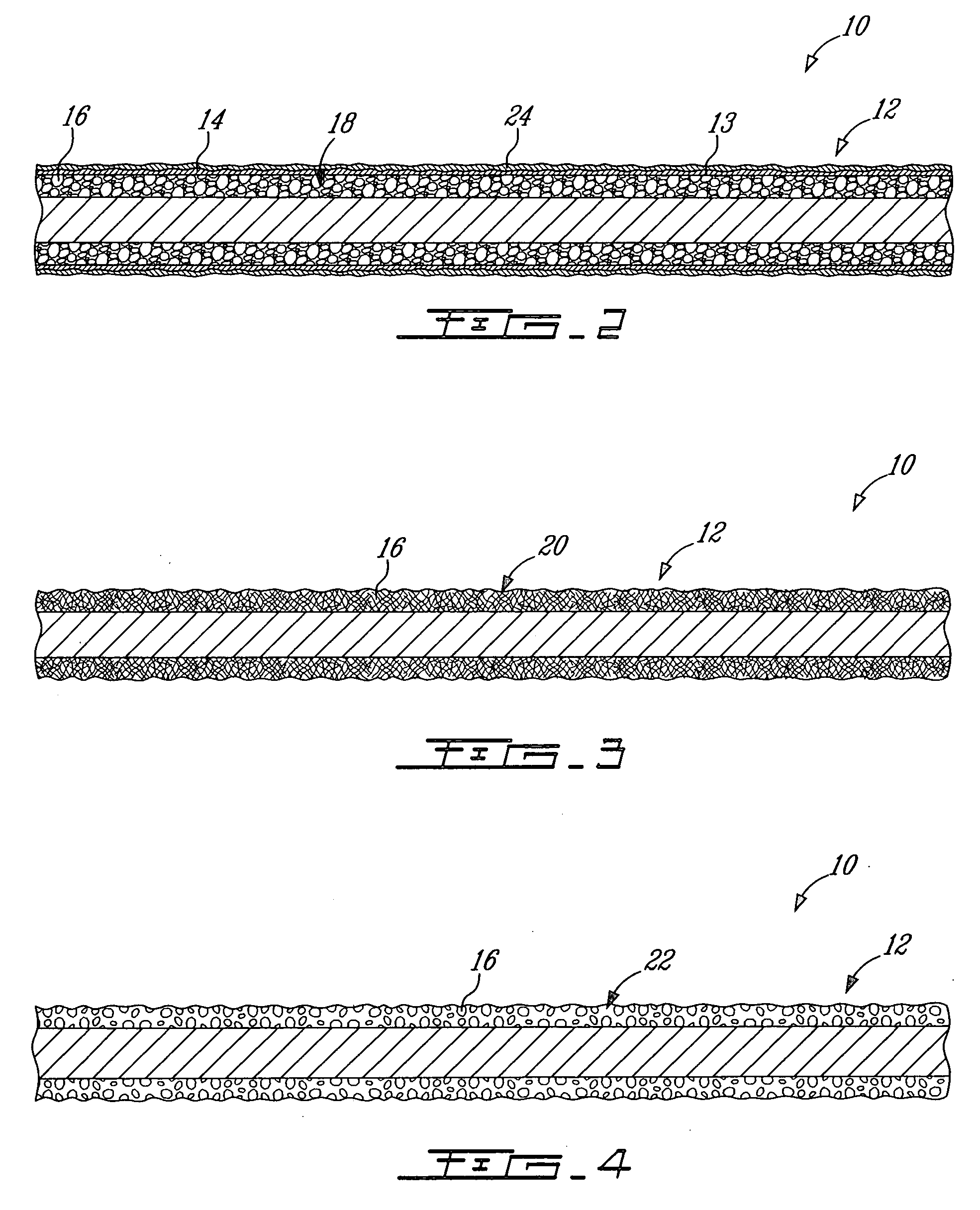
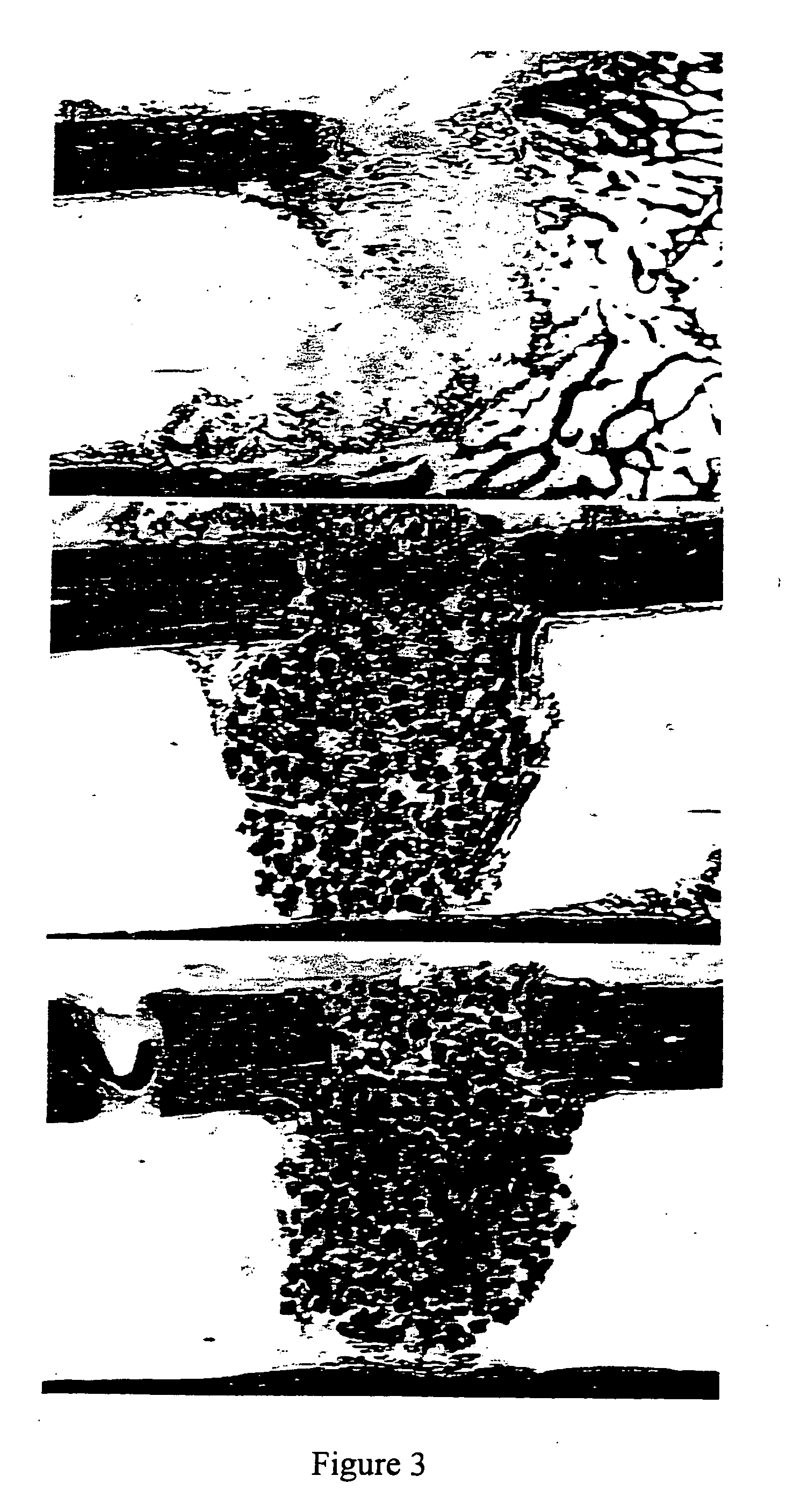
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Regeneration and augmentation of bone using mesenchymal stem cells
InactiveUS6863900B2Reduce functionReduce the numberBiocideBioreactor/fermenter combinationsMedicineBiopolymer
Disclosed are compositions and methods for augmenting bone formation by administering isolated human mesenchymal stem cells (hMSCs) with a ceramic material or matrix or by administering hMSCs; fresh, whole marrow; or combinations thereof in a resorbable biopolymer which supports their differentiation into the osteogenic lineage. Contemplated is the delivery of (i) isolated, culture-expanded, human mesenchymal stem cells; (ii) freshly aspirated bone marrow; or (iii) their combination in a carrier material or matrix.
Owner:MESOBLAST INT
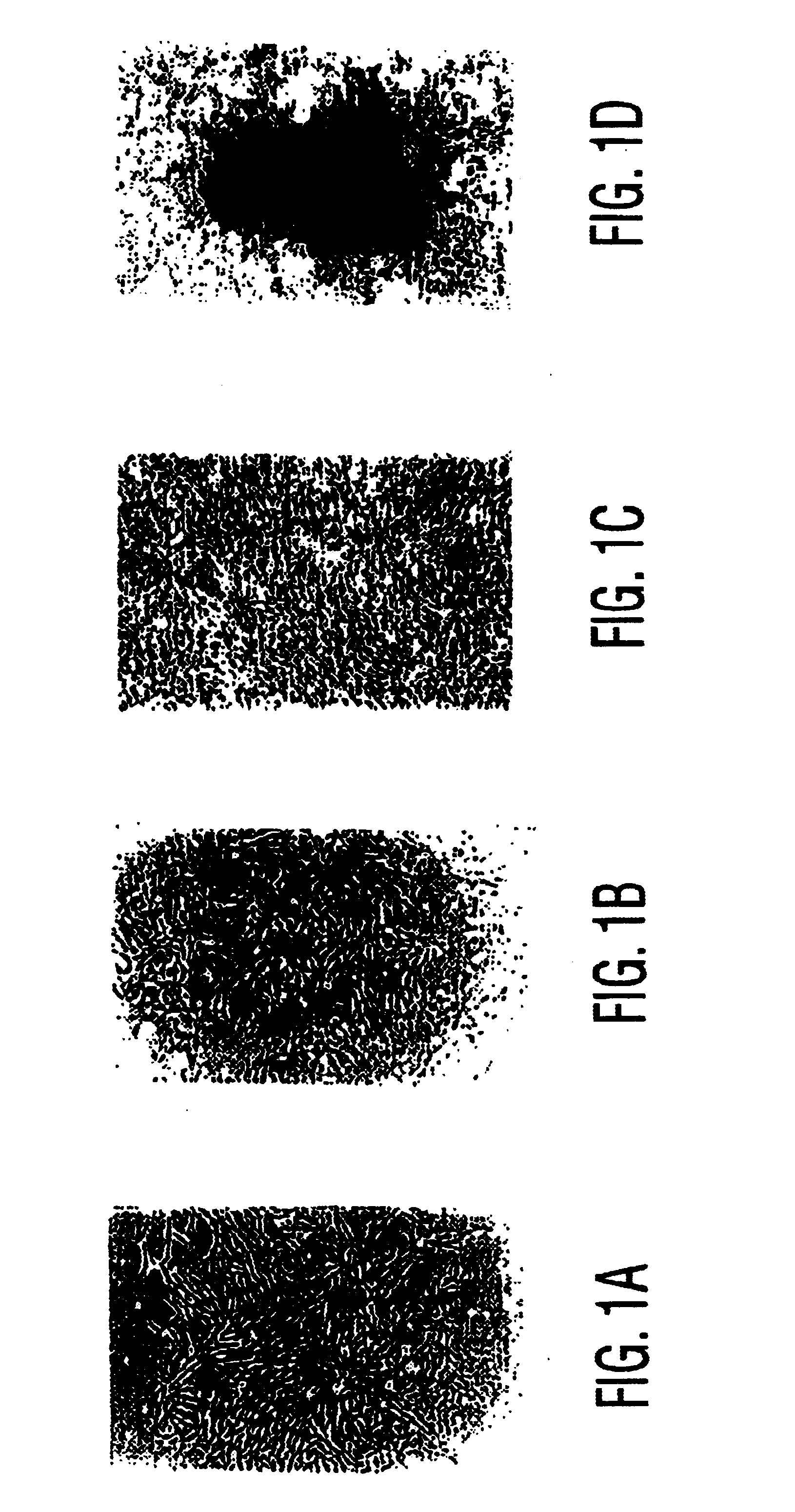
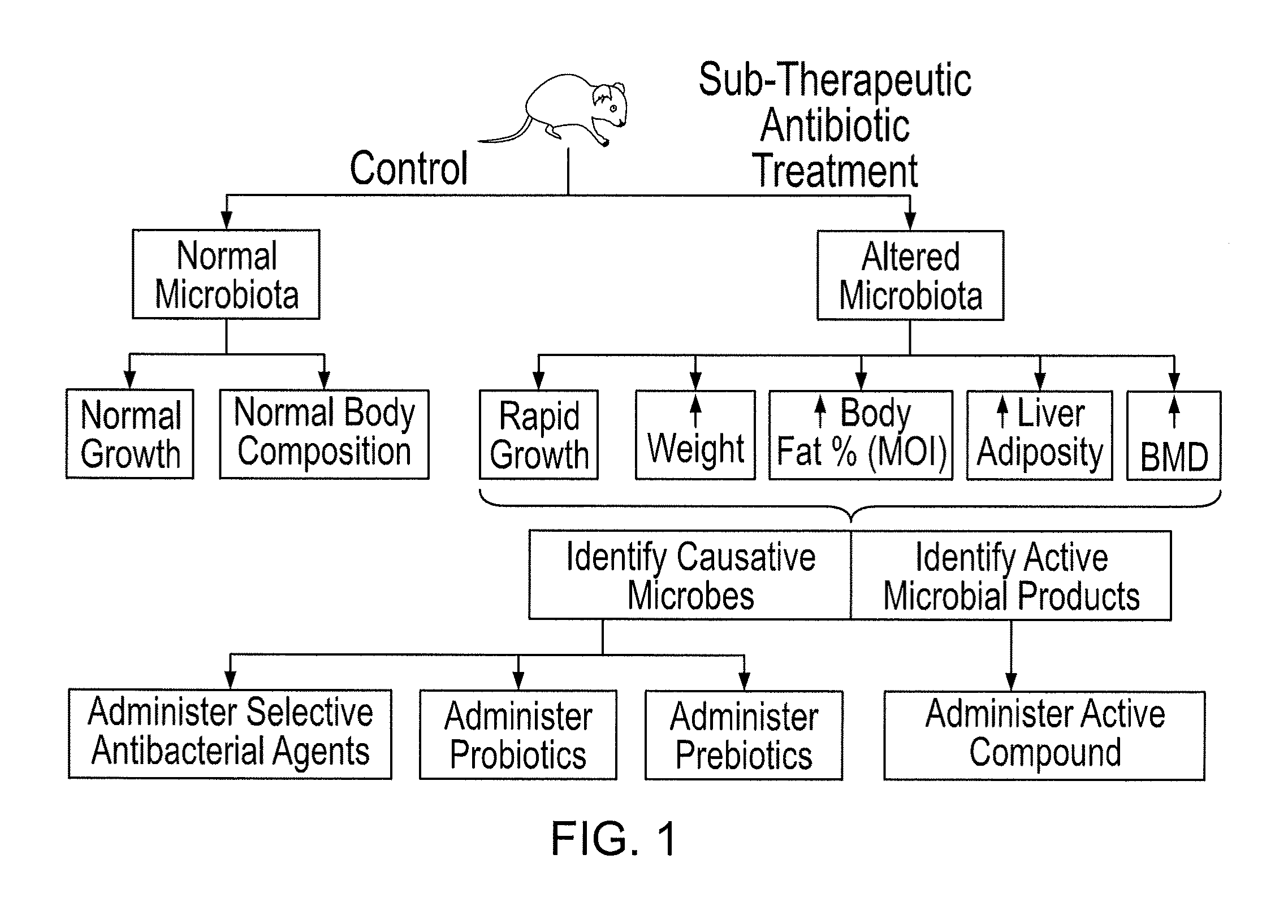
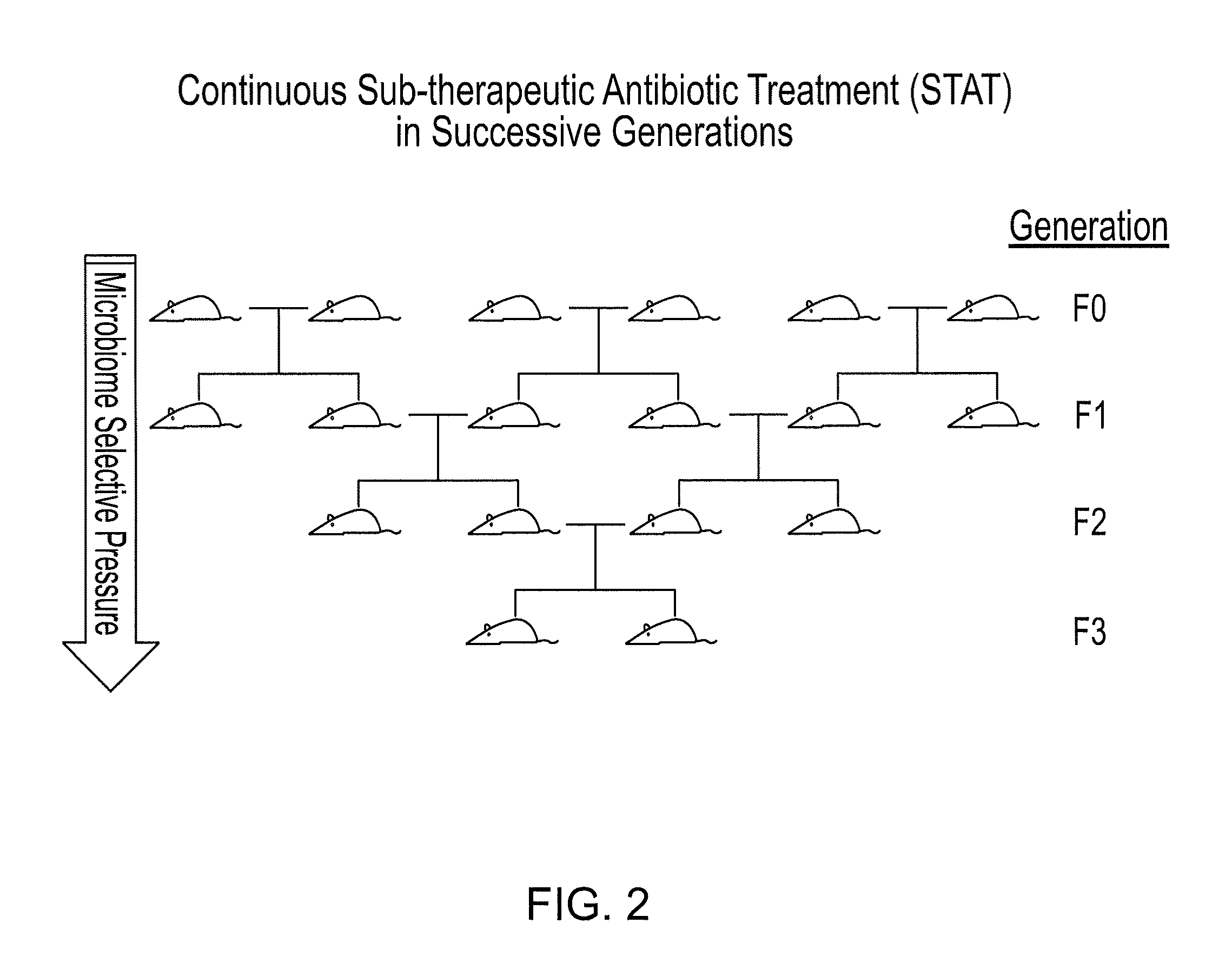
Compositions and methods for treating obesity and related disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS20110280840A1Increased use of antibioticIncreasing adult height and muscle massBiocideMetabolism disorderIntestinal microorganismsBone formation
The present invention relates to characterizing changes in mammalian gastrointestinal microbiota associated with antibiotic treatment and various disease conditions (such as obesity, metabolic syndrome, insulin-deficiency or insulin-resistance related disorders, glucose intolerance, diabetes, non-alcoholic fatty liver, abnormal lipid metabolism, short stature, osteoporosis, and other disorders of bone formation and mineralization, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of probiotics, prebiotics, or narrow spectrum antibiotics / anti-bacterial agents that are capable of restoring healthy mammalian bacterial gastrointestinal microbiota.
Owner:NEW YORK UNIV
Apparatus and methods for bone surgery
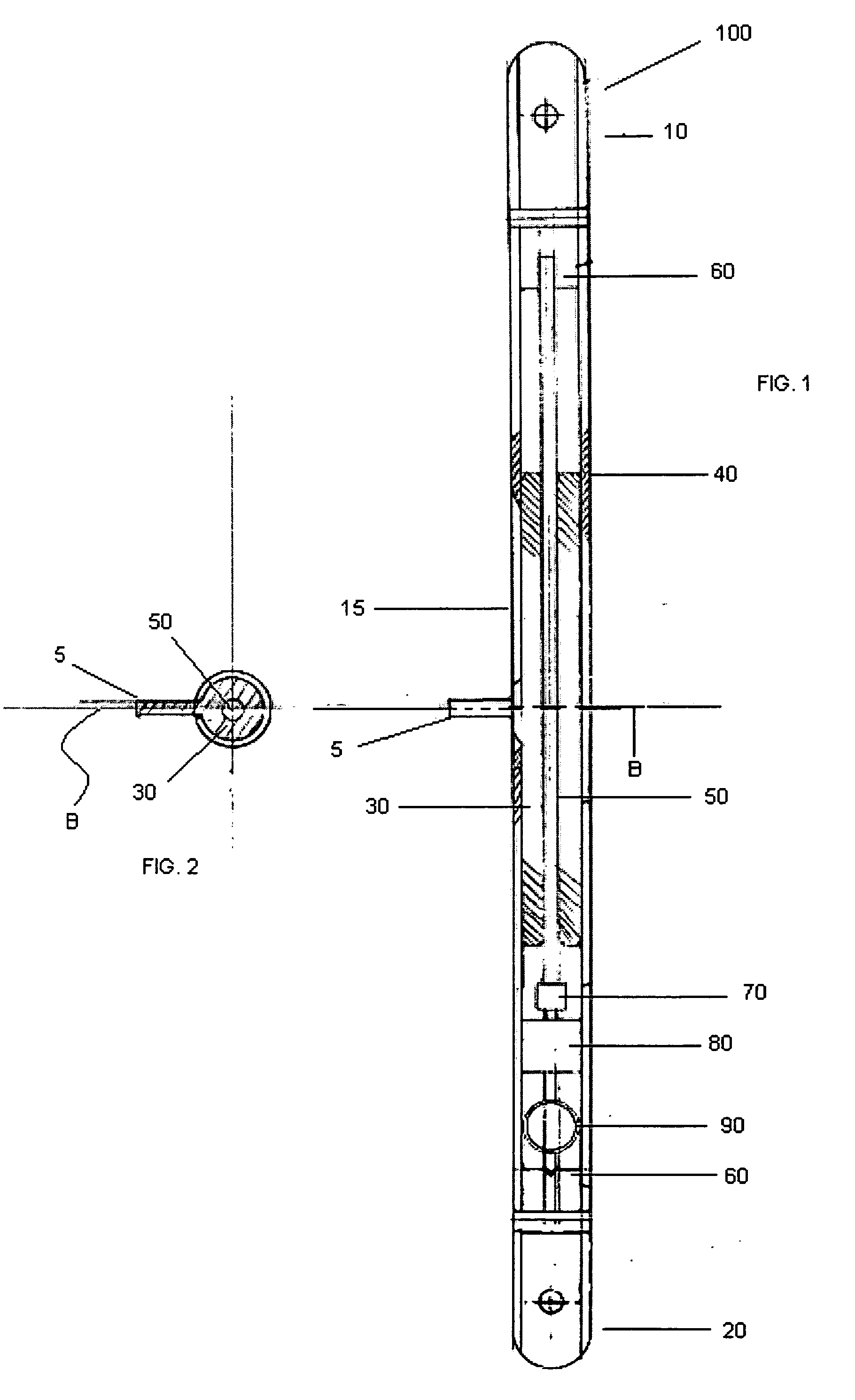
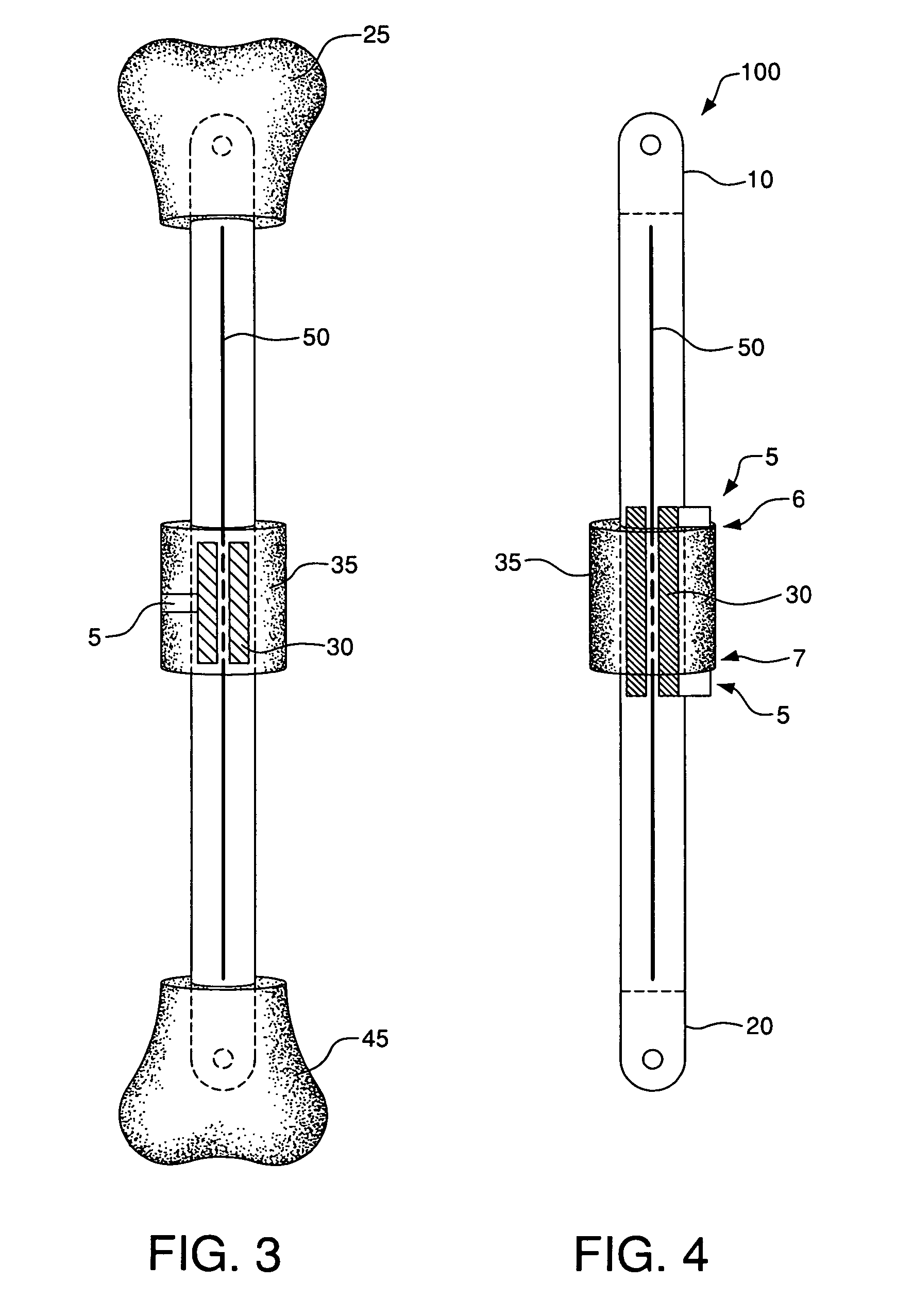
InactiveUS20050059978A1Joint implantsNon-surgical orthopedic devicesRight femoral headLeft femoral head
The surgeon grasps the jig by its handle and manipulates the head of the jig through the patient wound and onto the femoral neck. The head of the jig includes jig location means in the form of an elongate rod which acts as a spacer. The spacer has an end which abuts the trochanteric fossa so as to position the slot of the jig at the required position, which is between 5 mm and 25 mm, and most preferably 15 mm, from the trochantic fossa. Additional jig location means are provided by a surface adapted to receive a bone formation. This surface is provided by contours on the base of the head which are adapted to mate with contours of the femur. The slot is oriented generally perpendicularly to the elongate dimension of the rod. The slot functions as a surgical tool guide means which is positioned by the jig at the correct position for osteotomisation of the neck. Advantageously, osteotomisation takes place while the femoral head is still disposed within the acetabulum.
Owner:INT PATENT OWNERS CAYMAN
Internal bone transport
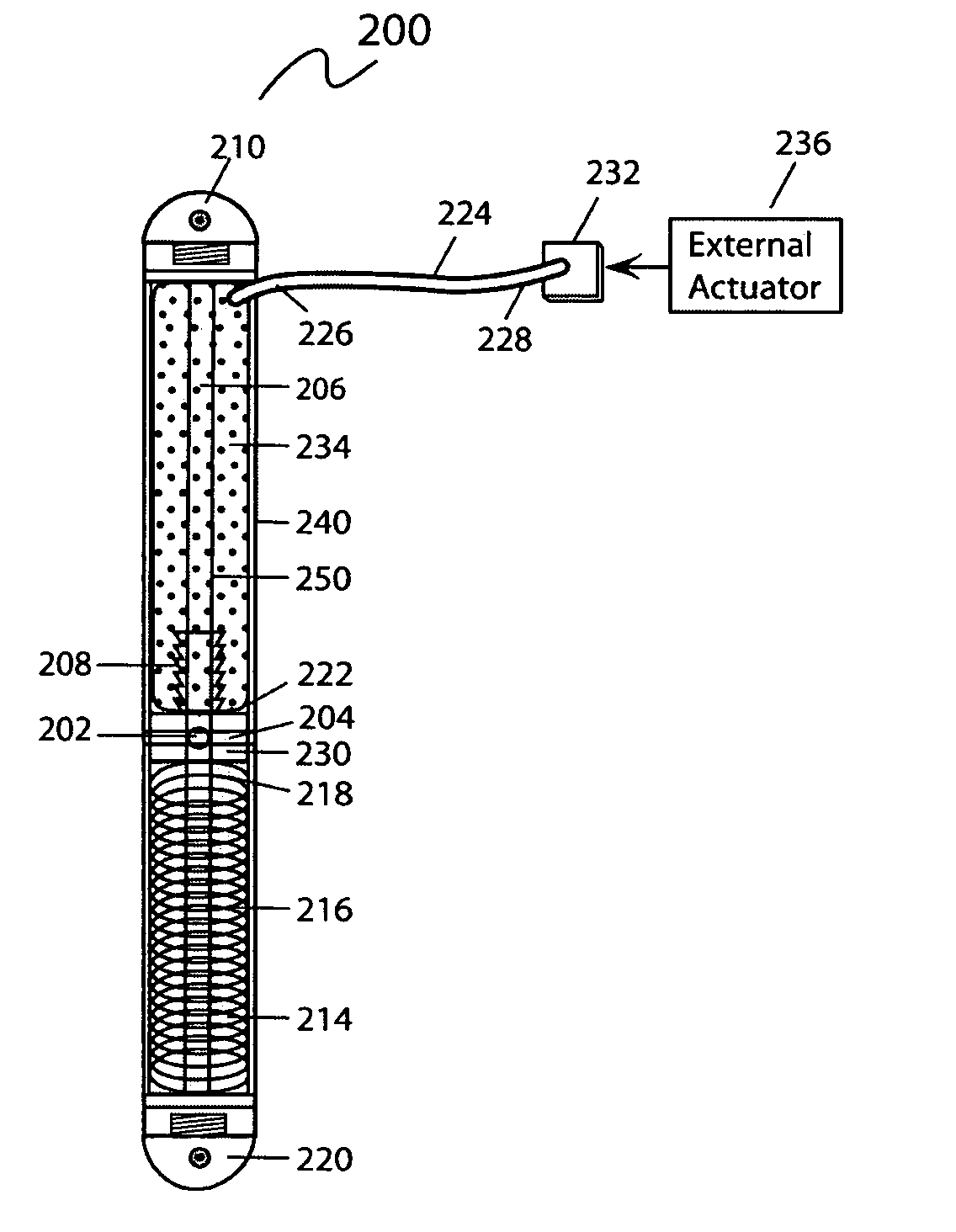
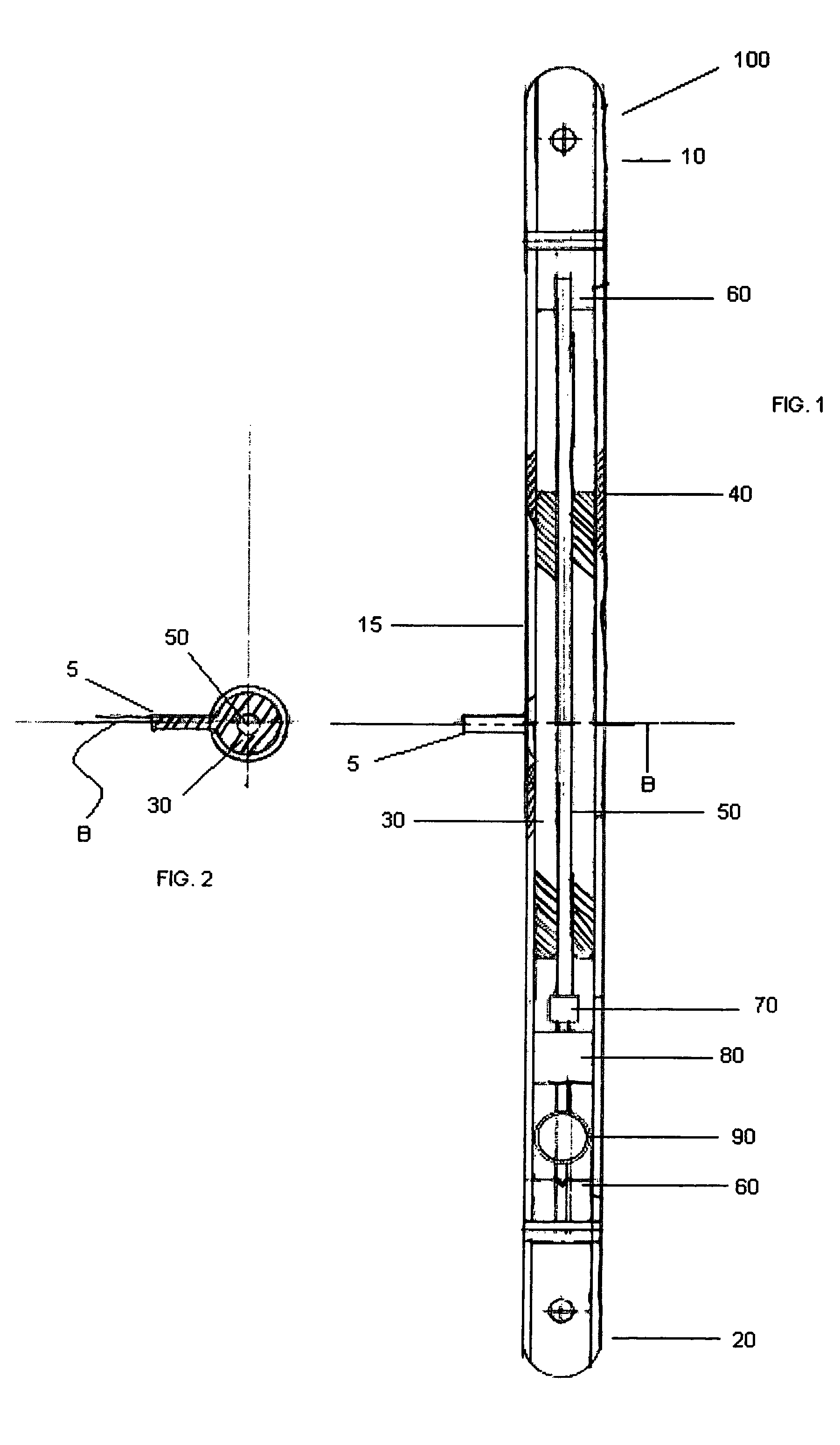
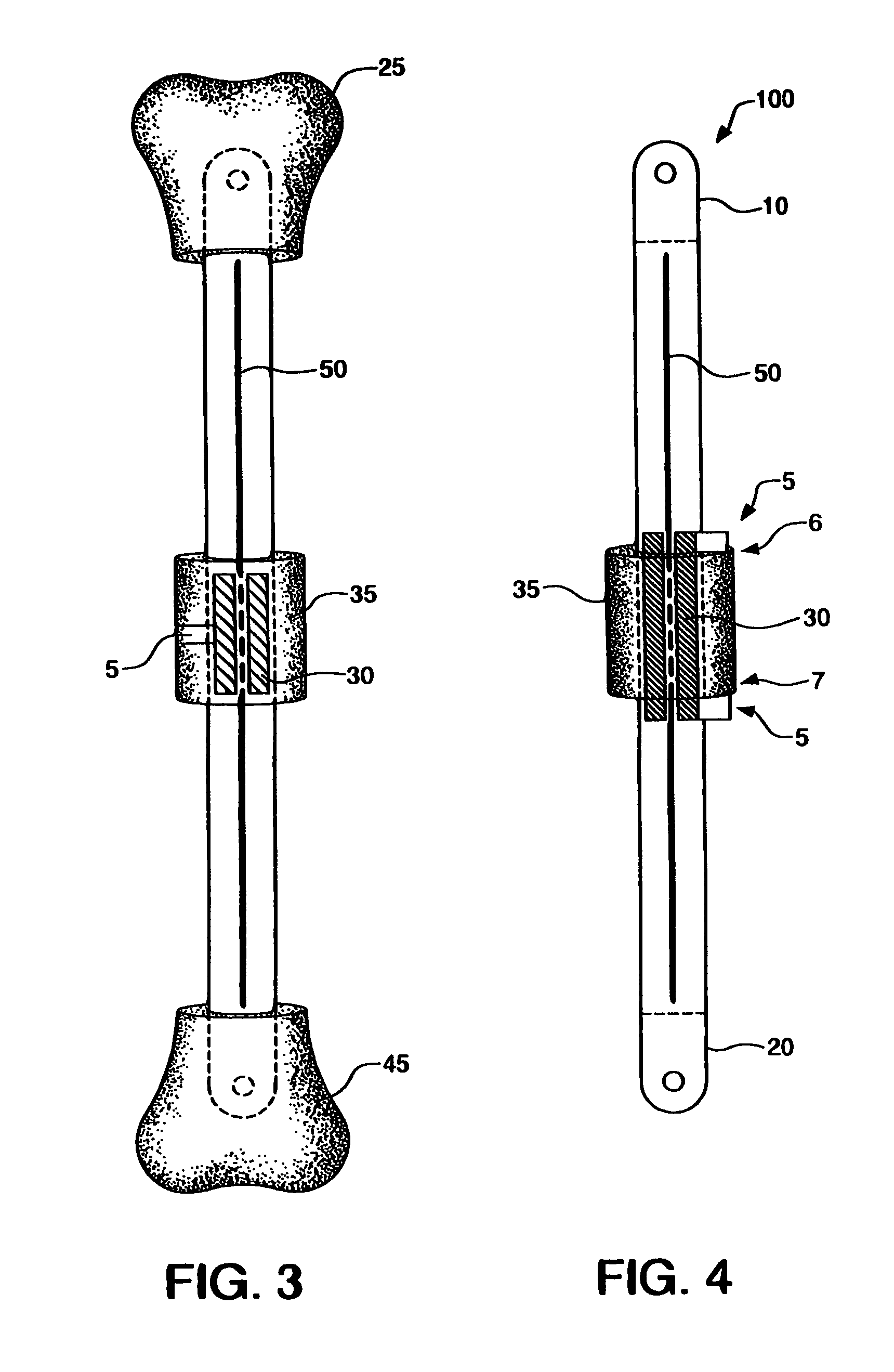
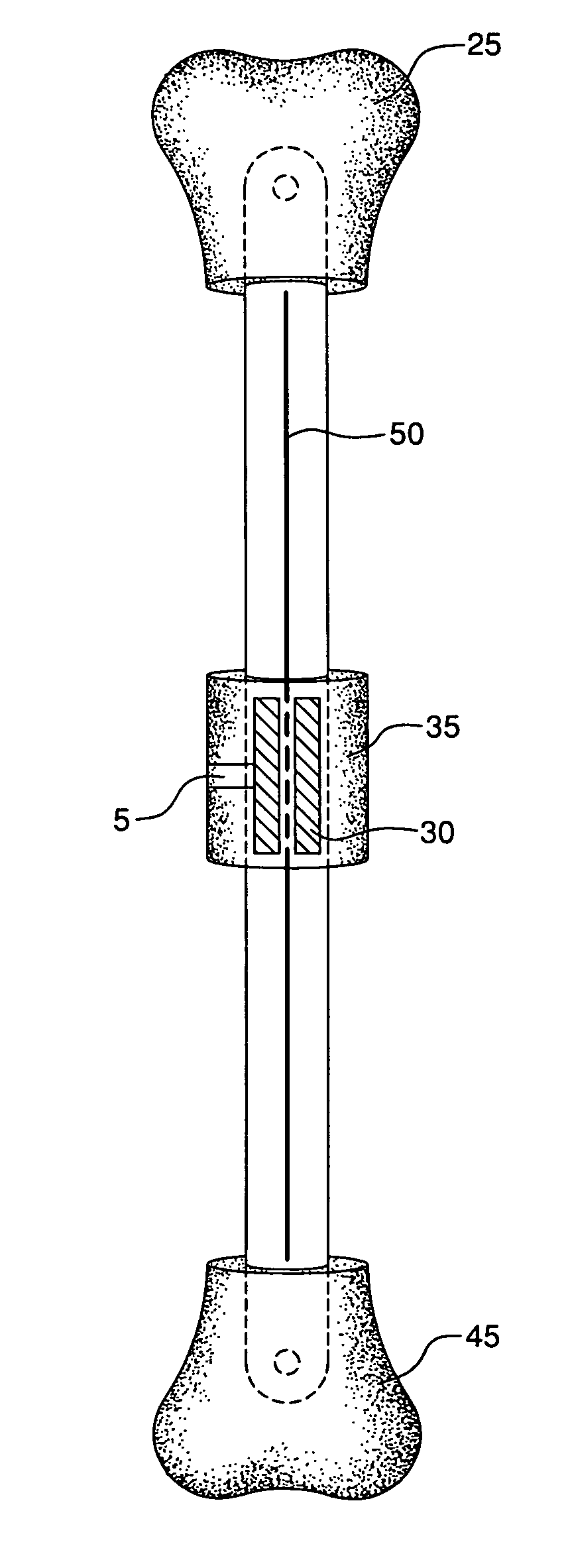
ActiveUS8043299B2Reduce liquid volumeInternal osteosythesisJoint implantsBone transportBone formation
An internal bone transport device and method for lengthen bone that, once surgically implanted will allow for a segment of bone to be transported along the length of the rod without changing the overall length of the rod. The segment from one bone end is transported to the other in a controlled fashion allowing for complete control of the rate of bone transport and adjusting this rate to the quality of the bone formation. The segment is moved either by the application of an externally applied magnetic force or by a fluid actuator in combination with a compression spring. It applies to patients in whom a segment of bone has been removed. This fully internal bone transport allows the bone transport to occur without any external fixation, thus eliminating the problems associated with pin tract infections and pain from the pins cutting through the soft tissue.
Owner:CONWAY JANET
Internal bone transport
An internal bone transport device and method for lengthen bone that, once surgically implanted will allow for a segment of bone to be transported along the length of the rod without changing the overall length of the rod. An externally applied magnetic force is used to drive the segment from one bone end to the other in a controlled fashion allowing for complete control of the rate of bone transport and adjusting this rate to the quality of the bone formation. It applies to patients in whom a segment of bone has been removed. This fully internal bone transport allows the bone transport to occur without any external fixation, thus eliminating the problems associated with pin tract infections and pain from the pins cutting through the soft tissue.
Owner:CONWAY JANET +2
Method of dephosphorylating an endotoxin in vivo with alkaline phosphatase
The invention relates to pharmaceutical compositions suitable for treating or curing clinical complications mediated by endotoxin, including sepsis. The compositions contain components suitable for detoxifying endotoxin rendering it less deleterious to mammals such as humans, in particular to patients with reduced host-defence resistance. The invention also relates to pharmaceutical compositions suitable for stimulating bone formation, e.g. for mending broken bone or for prophylaxis or therapy of metabolic bone diseases such as osteoporosis and osteomalacia and pharmaceutical compositions for decreasing or inhibiting undesired bone formation. The pharmaceutical compositions according to the invention are directed at modulating phosphatase activity in vivo.
Owner:UNIVERSITY OF GRONINGEN
Hydroxyapatite coated nanostructured titanium surfaces
InactiveUS20090035722A1Improve adhesionPromote accumulationDental implantsImpression capsOsteoblast adhesionApatite
Nanotubular structured titanium (Ti) substrates have been coated with nanoparticulate hydroxyapatite (nano-HA). The nano-HA surface is highly adherent to the nanotubular Ti surface and is free of microparticles. The nano-HA coated nanotubular Ti surface promotes osteoblast cell adhesion and is particularly suitable for orthopedic and dental implants where deposition of osteoblasts and other proteins is important in bone formation.
Owner:METASCAPE
Implant improving local bone formation
InactiveUS20060188542A1Improve effectivenessBiocideDental implantsChemical LinkageCalcium biphosphate
A bone implant comprises an active agent on at least a portion thereof. The active agent is locally deliverable to bone proximate the implant in at least a two-phased release scheme. A first phase rapidly releases a first quantity of the active agent, and at least a second phase gradually releases a second quantity of the active agent, whereby bone formation stimulated by the active agent is modulated. In one embodiment, a porous implant comprises a porous portion coated with a calcium phosphate compound and which is contacted with a bisphosphonate compound to form a bisphosphonate layer chemically bound to the calcium phosphate at the surface of the porous portion and to form bisphosphonate molecules being non-chemically attached inside the pores of the porous portion. The non-chemically attached bisphosphonate molecules are released in the subject at a rate greater than that of the chemically bound bisphosphonate layer.
Owner:BOBYN JOHN DENNIS +1
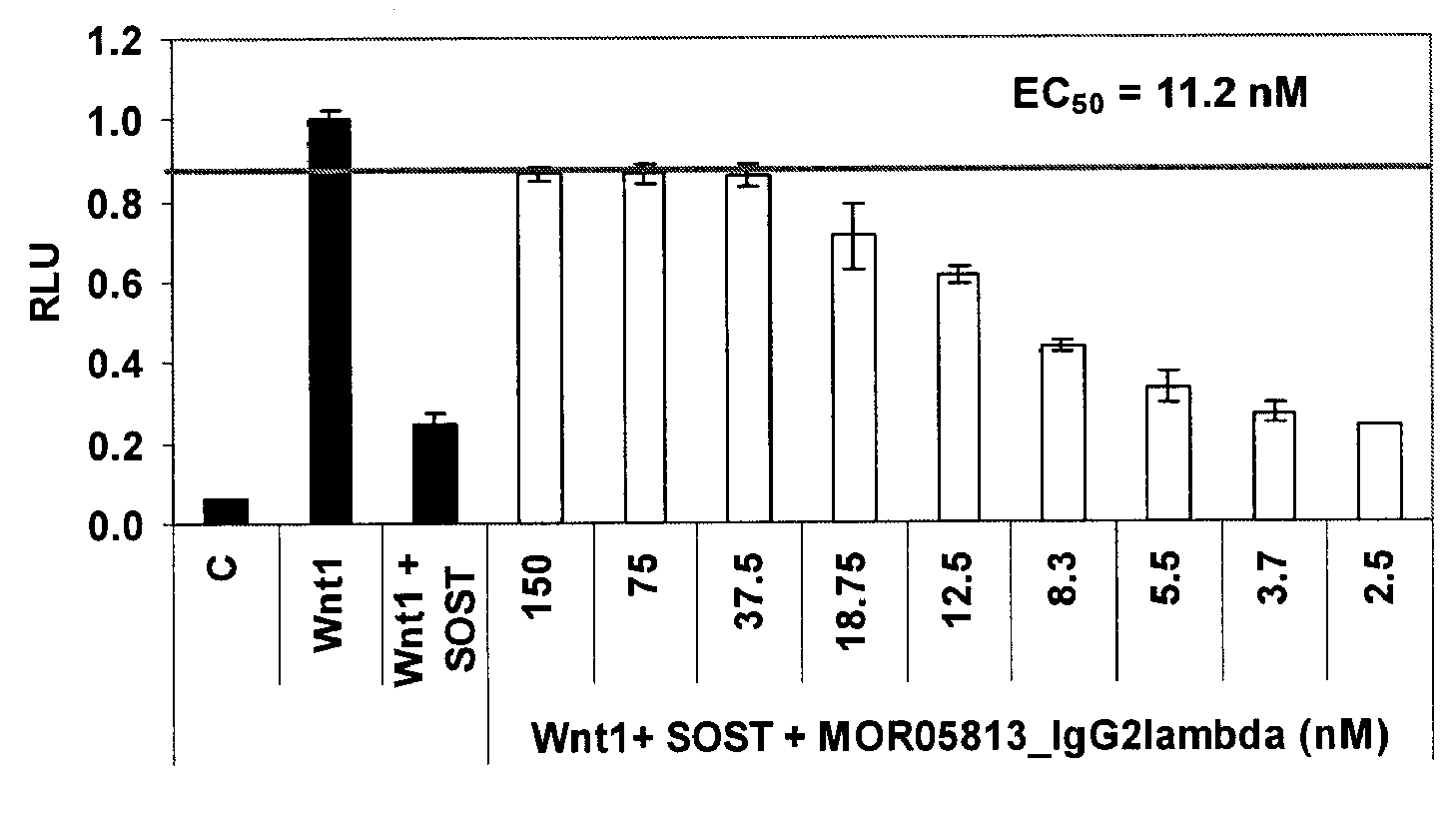
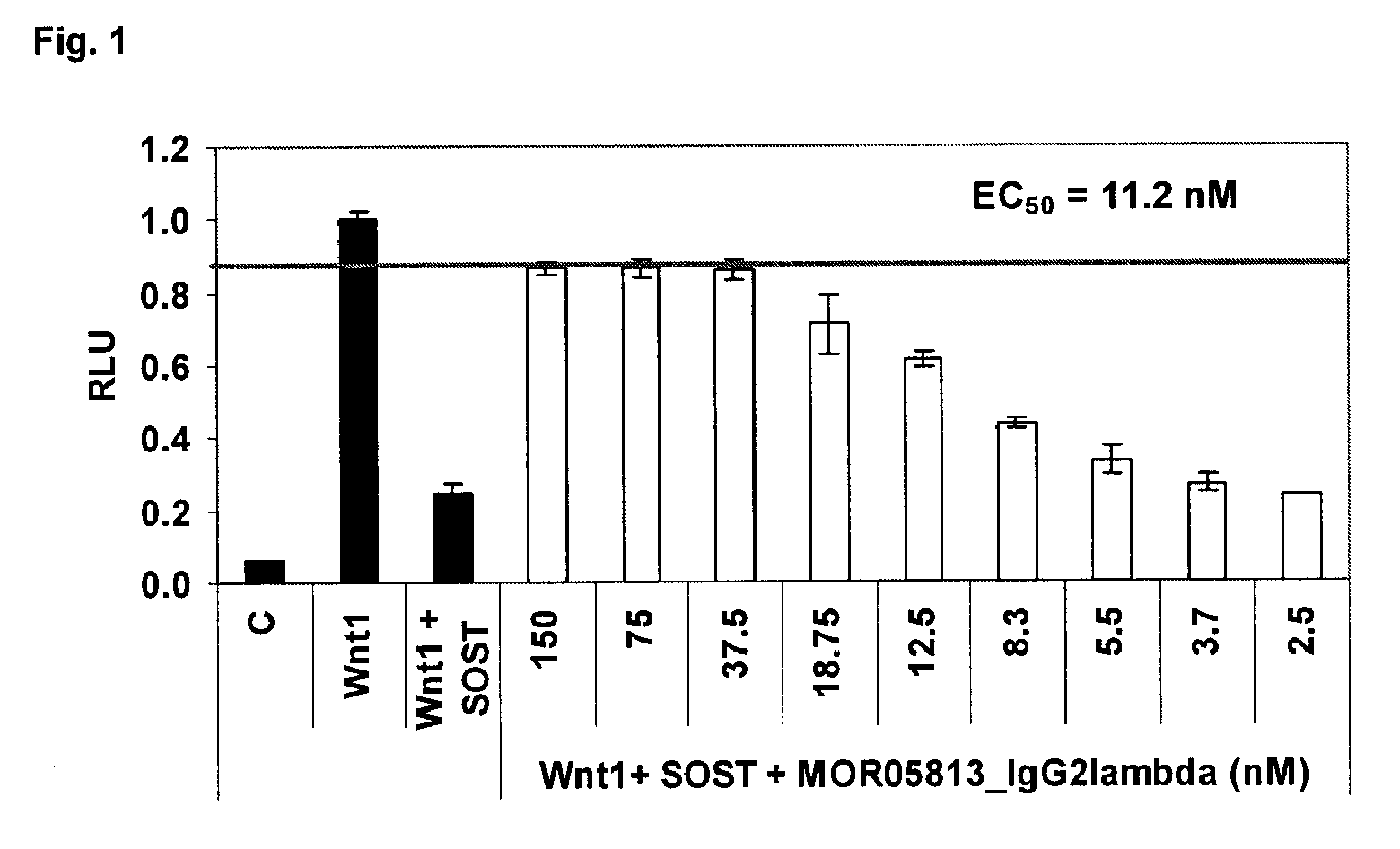
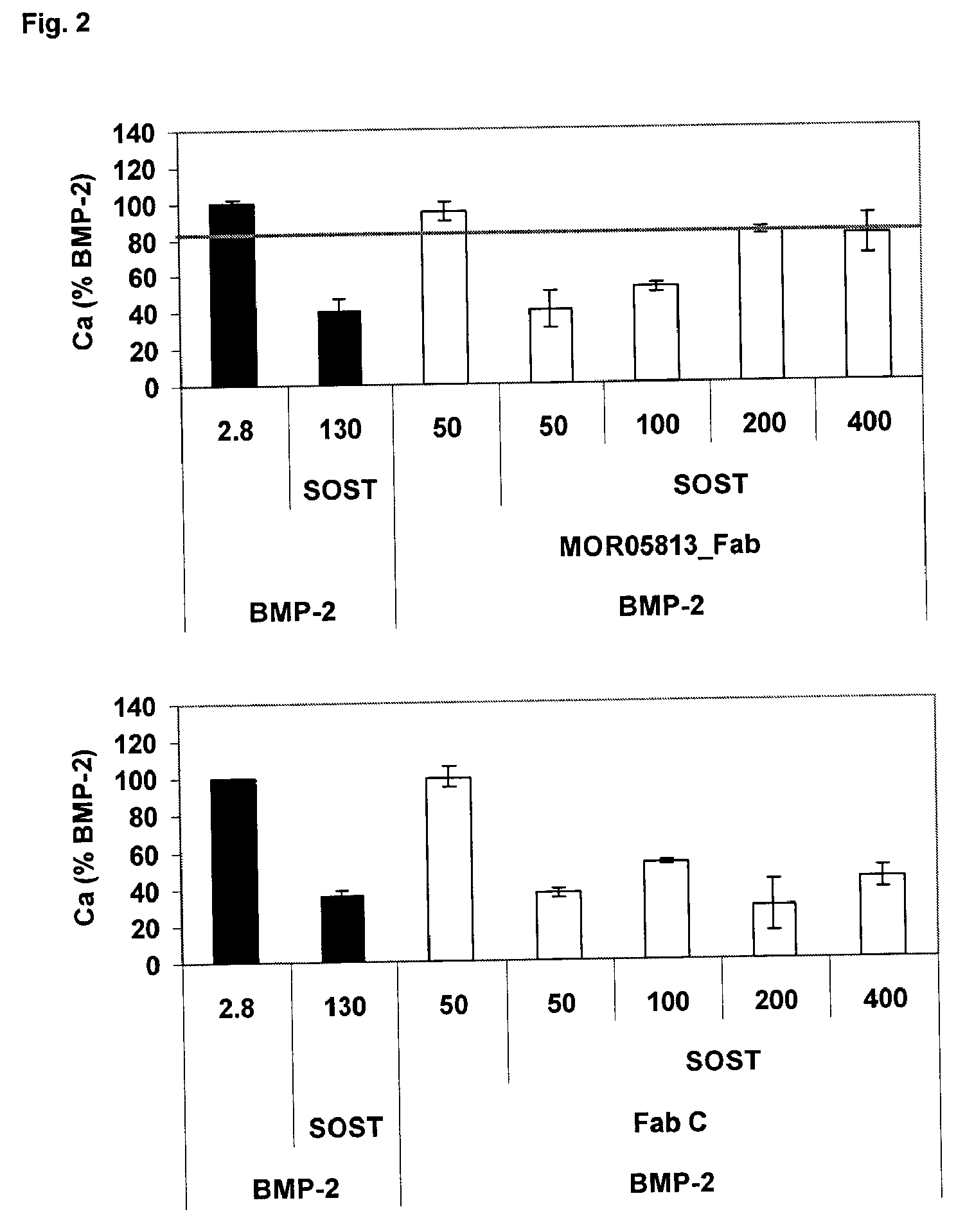
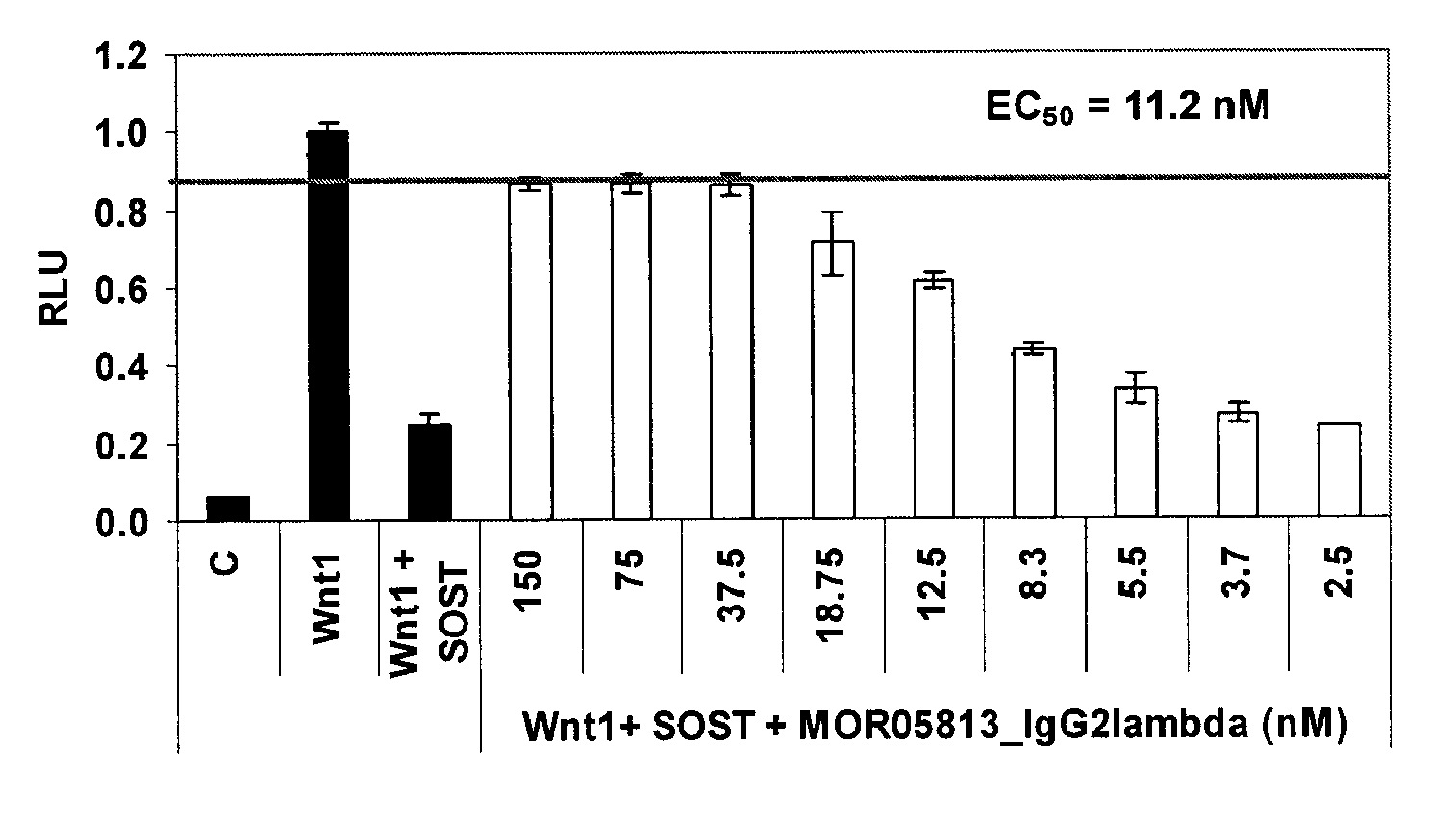
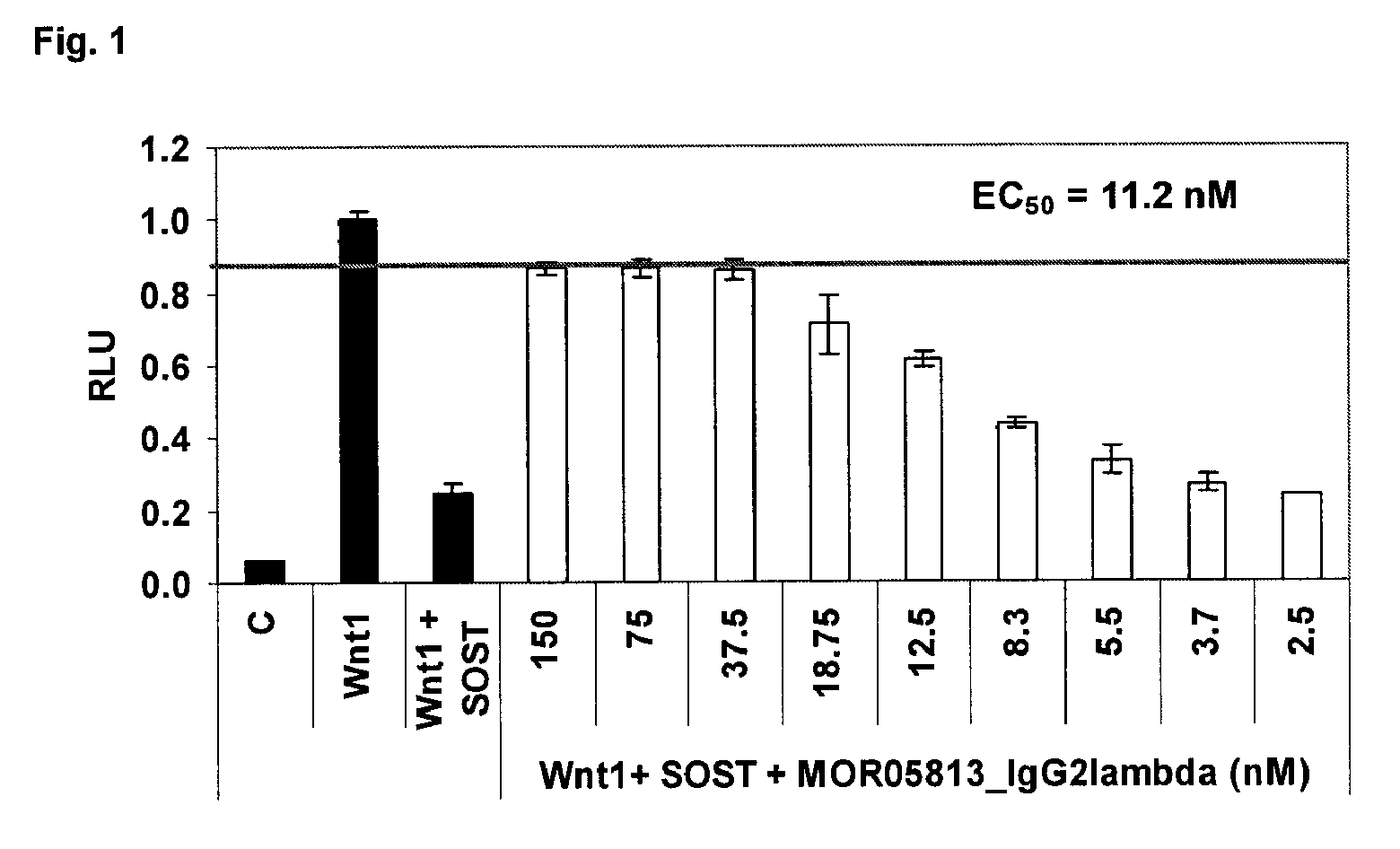
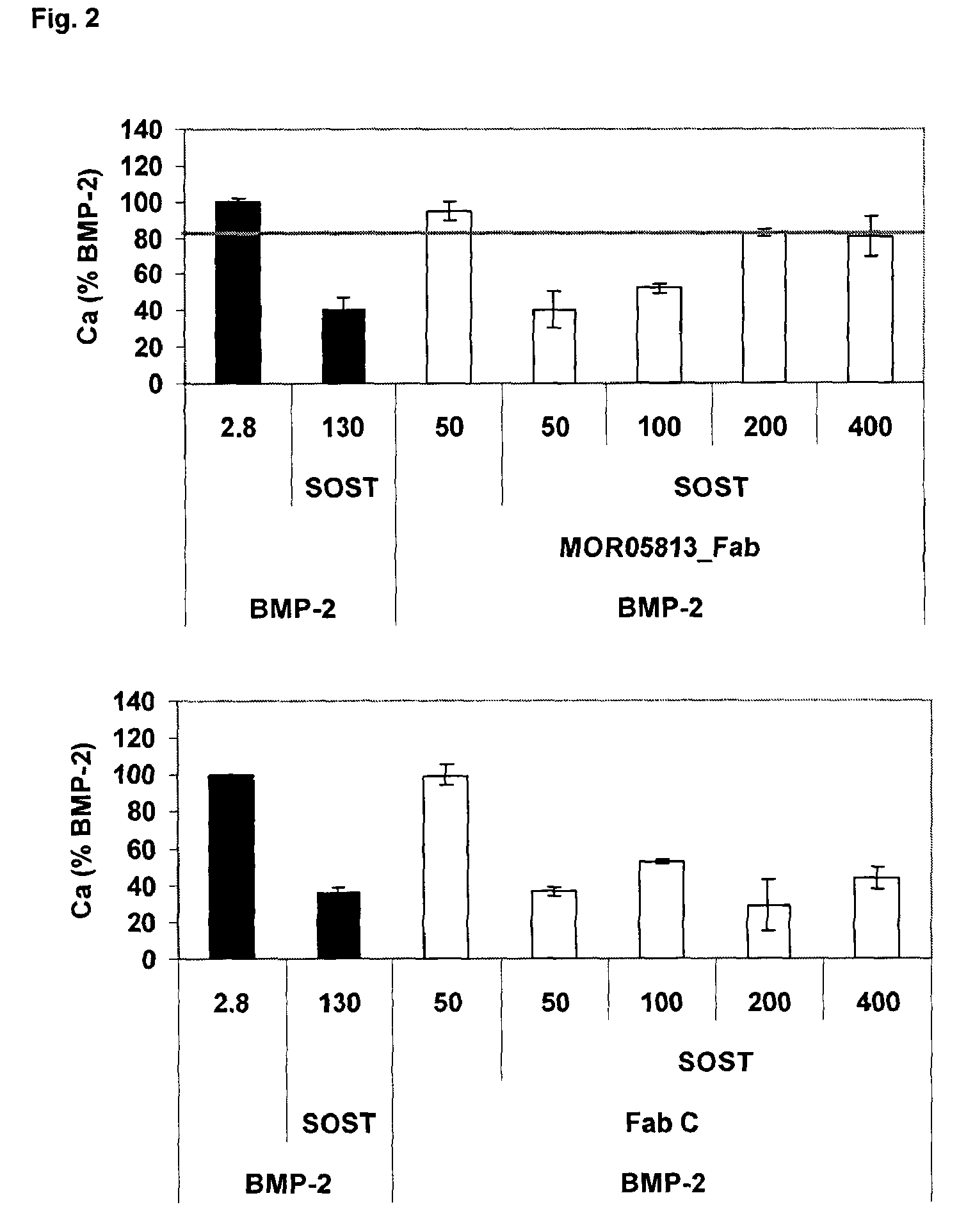
Compositions and methods for use for antibodies against sclerostin
The present invention relates to antibodies against sclerostin and compositions and methods of use for said antibodies to treat disease related to bone abnormalities such as osteoporosis. An embodiment of the invention herein provides an antibody or a functional protein comprising an antigen-binding portion of said antibody for a target in sclerostin polypeptide, characterized in that the antibody or functional protein specifically binds to sclerostin polypeptide and can increase at least one of bone formation, bone mineral density, bone mineral content, bone mass, bone quality and bone strength in a mammal.
Owner:MEREO BIOPHARMA 3 LTD
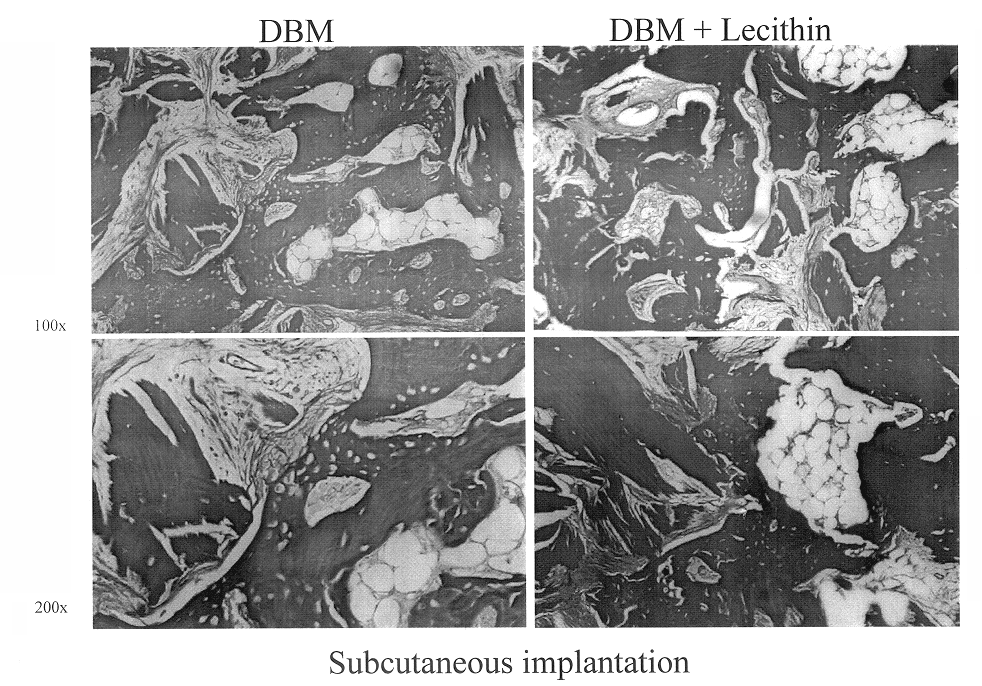
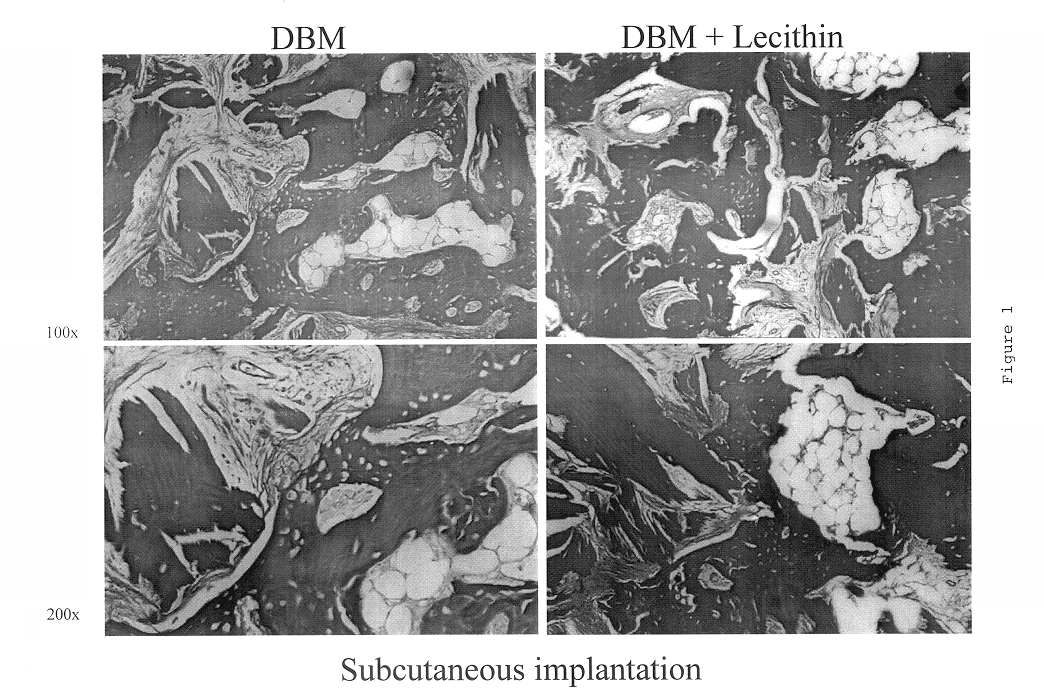
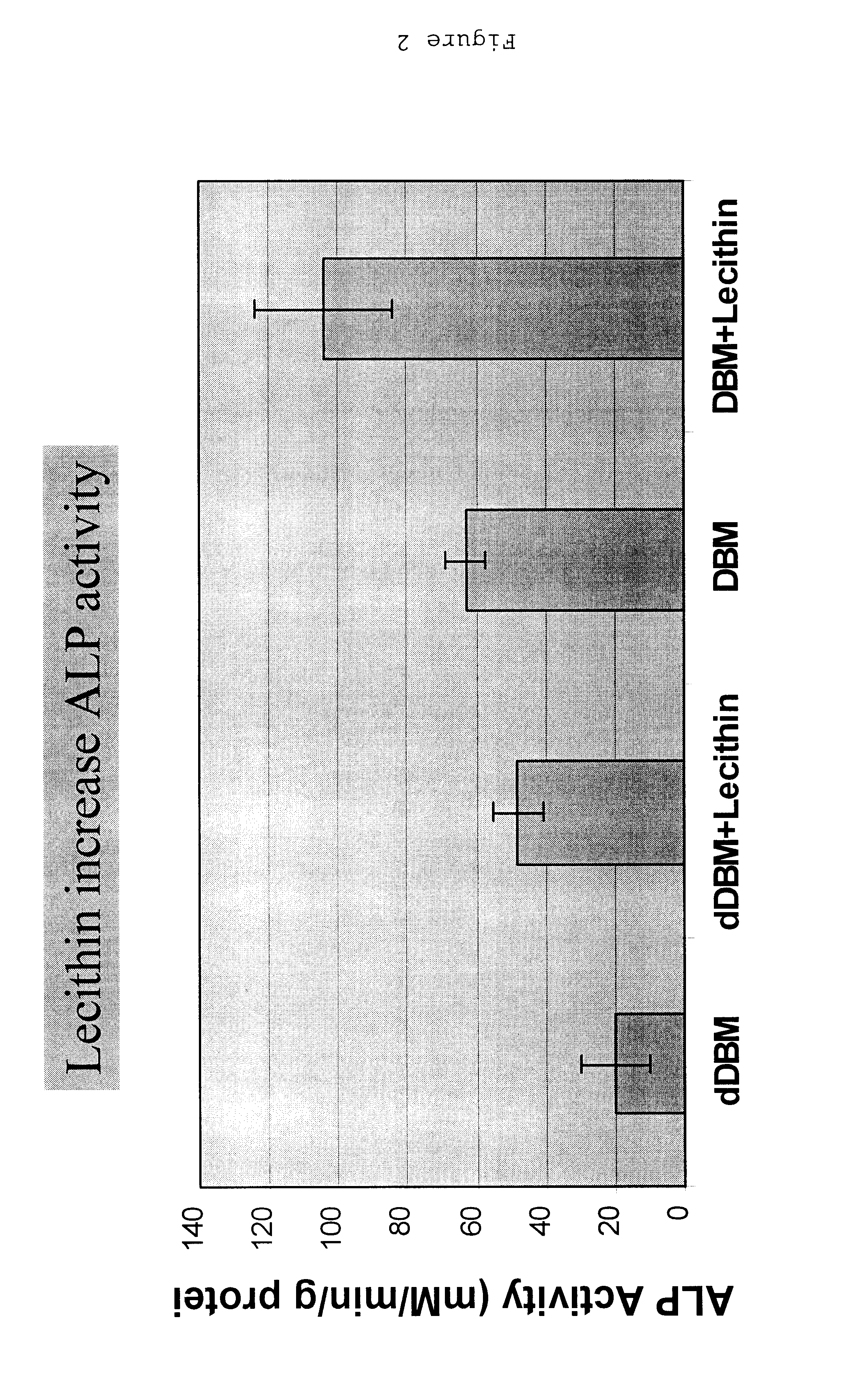
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
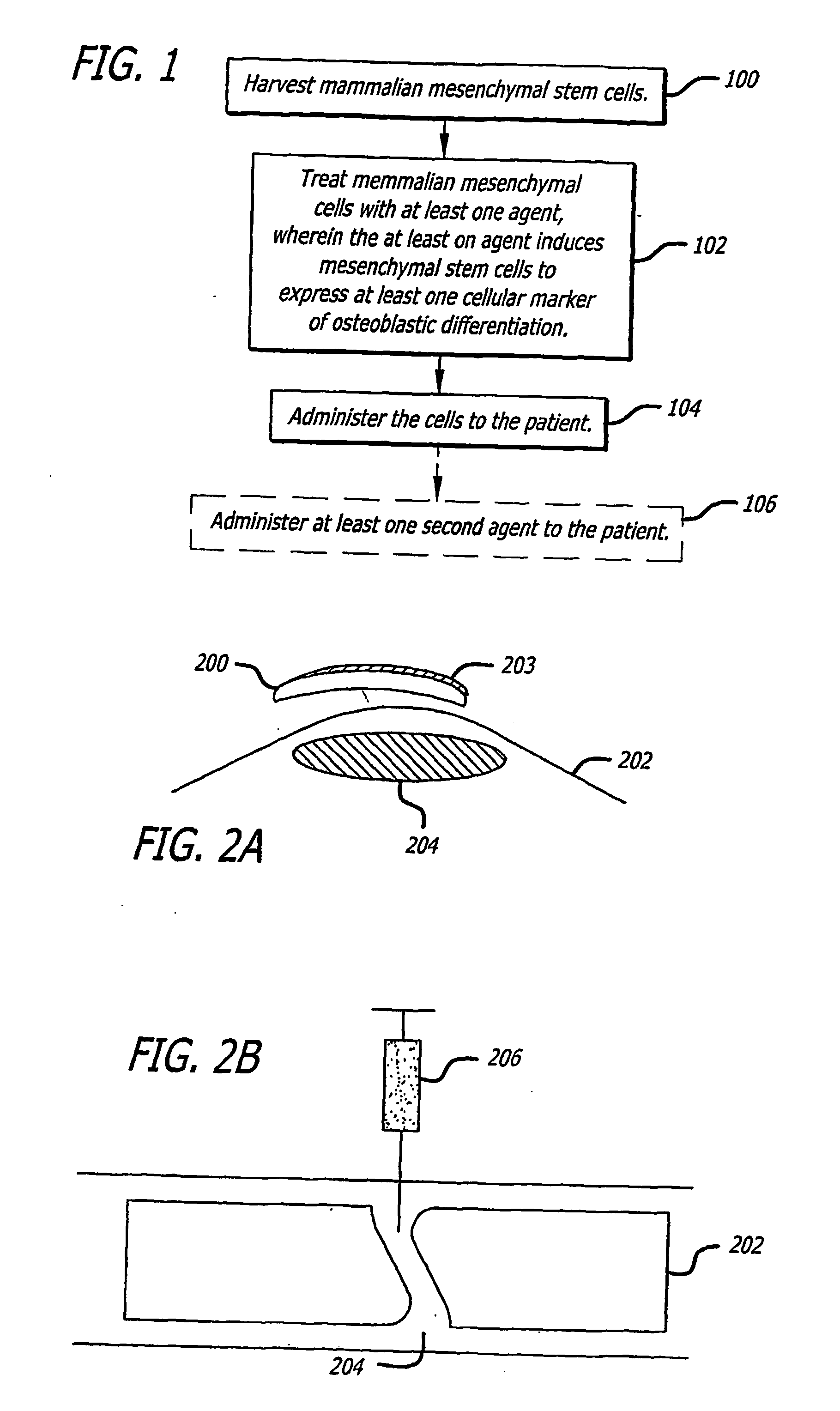
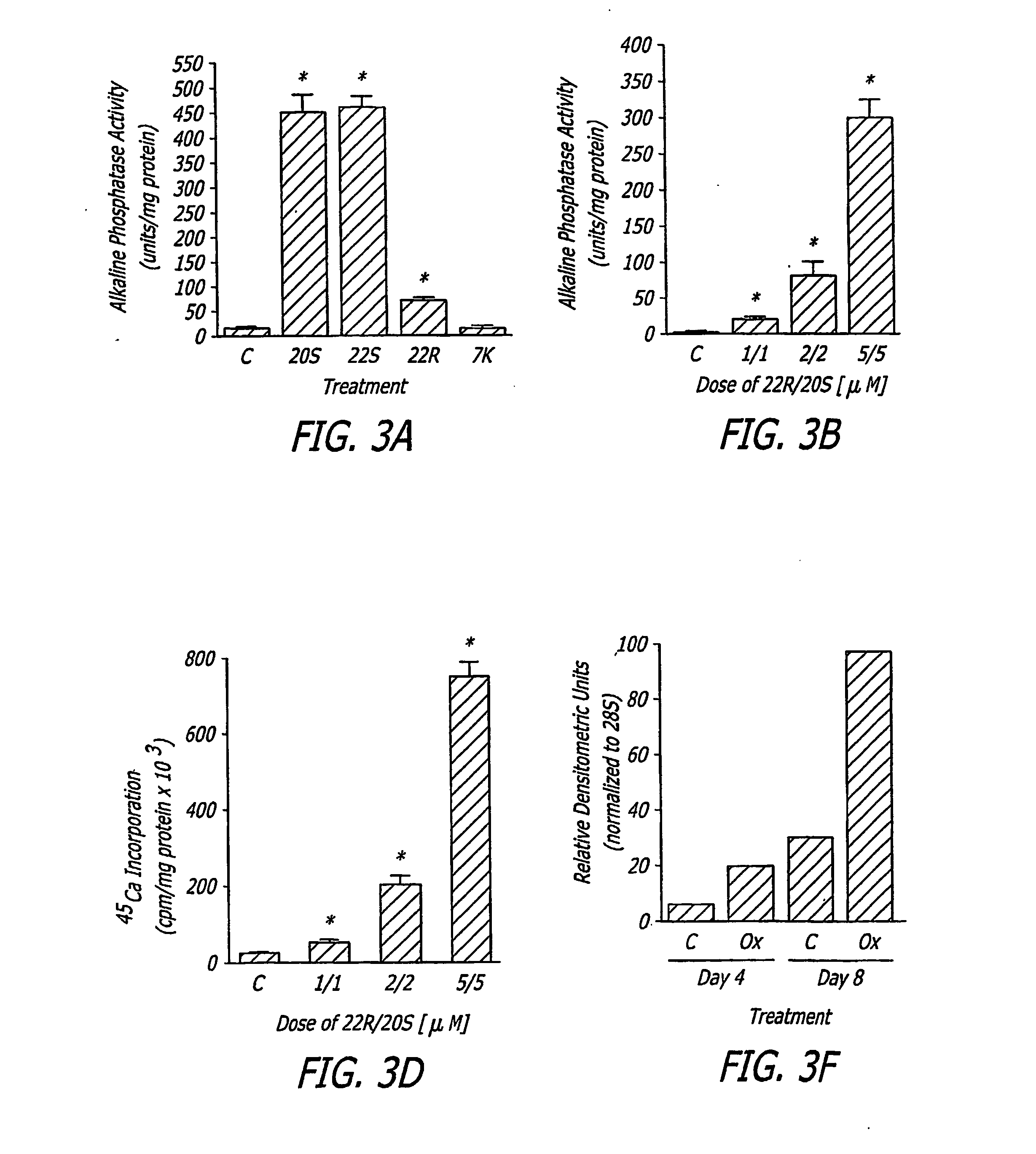
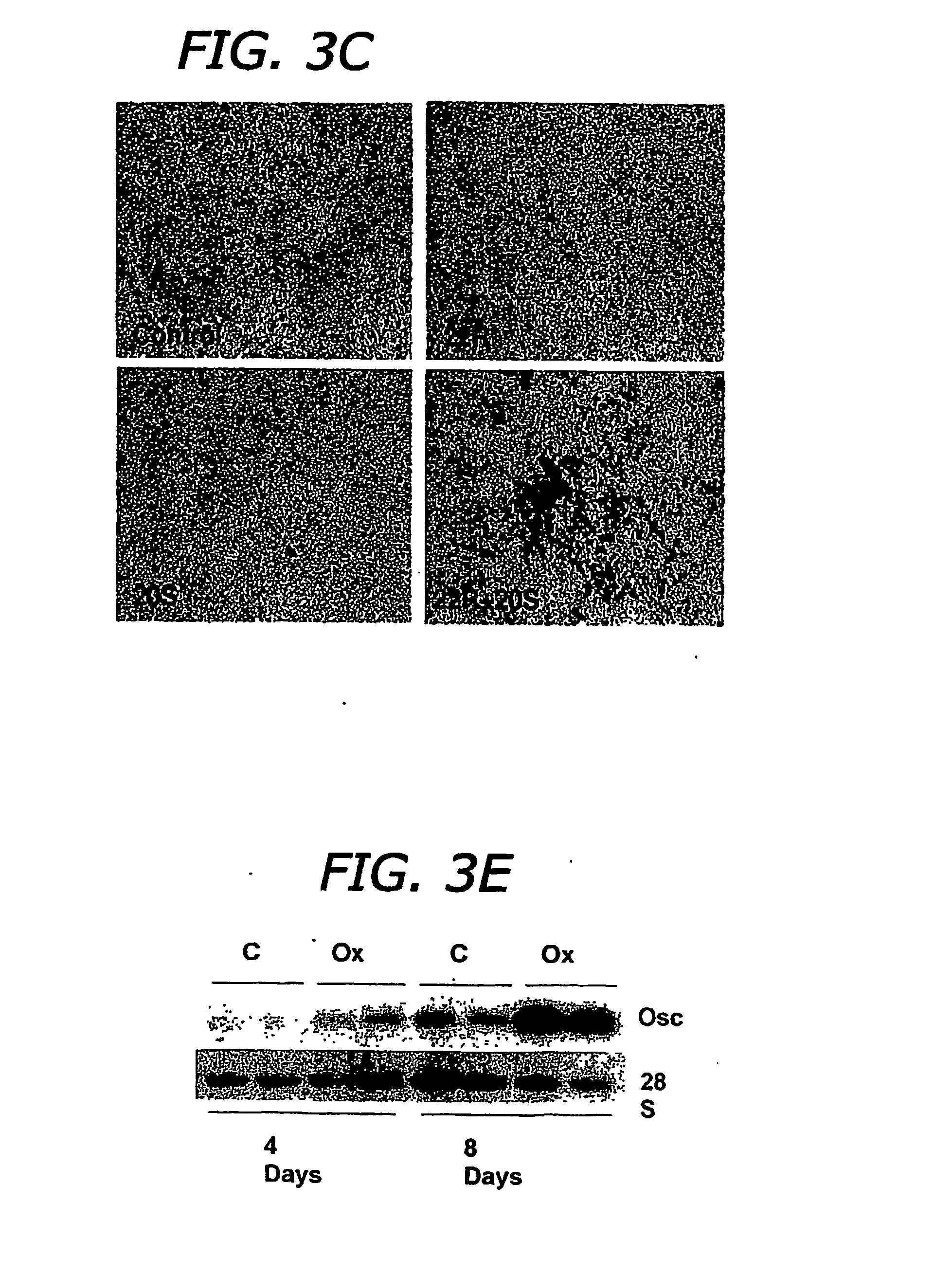
Agents and methods for enhancing bone formation
ActiveUS20060270645A1Improve impactPromotes bone formationBiocidePeptide/protein ingredientsMedicineOxysterol
The present invention discloses agents and methods for inducing osteoblastic cellular differentiation, as well as the use of such agents and method to treat patients to maintain bone mass, enhance bone formation and / or bone repair. Exemplary agents include oxysterols, alone or in combination with particular oxysterols, or other agents known to assist in bone formation. The invention further includes medicaments including oxysterols for the treatment of bone disorders, local injections of oxysterols or cells (206) and implants (202) having agents or cells (203) to facilitate bone repair.
Owner:RGT UNIV OF CALIFORNIA
Cement products and methods of making and using the same
ActiveUS20070032568A1Appropriate flowabilityAppropriate viscosity propertyImpression capsSurgical adhesivesCompound (substance)Bone formation
Disclosed are cement products, methods of forming cement using the cement product, and methods of using the cement product in orthopedic and dental applications. Generally, the disclosed cement product includes a first component comprising a polymerizable resin comprising ethylenic unsaturated double bond, a second component comprising a compound comprising more than one type of amine selected from the group consisting of primary amines, secondary amines, tertiary amines and quaternary amines, and, optionally, the cement product includes a bioactive component to promote bone formation.
Owner:PIONEER SURGICAL TECH INC
Compositions and methods for the stimulation or enhancement of bone formation and the self-renewal of cells
ActiveUS20050261181A1Improve biological activityPromote mineralizationBiocidePeptide/protein ingredientsOsteoblastWnt inhibitor
Compositions and methods for the treatment of bone diseases, bone fractures, bone injuries and other bone abnormalities involving the use of Dkk protein, a Wnt antagonist, a Wnt inhibitor, or any other related protein for the stimulation or enhancement of mineralization and for stimulating the renewal of cells. One Dkk protein, Dickkopf-2 (Dkk-2), acts to stimulate bone formation independently of Wnt proteins which may be inhibited and / or antagonized by Dkk-2. Dkk-2 displayed enhanced specific targeting ability and enhanced biological activity in stimulating or enhancing mineralization. Dkk-2 also played a role in the differentiation and self-renewal of hematopoietic stem cells and mesenchymal stem cells, particularly in osteoblastogenesis and osteoclastogenesis.
Owner:ENZO BIOCHEM
Compositions and methods for use for antibodies against sclerostin
The present invention relates to antibodies against sclerostin and compositions and methods of use for said antibodies to treat disease related to bone abnormalities such as osteoporosis. An embodiment of the invention herein provides an antibody or a functional protein comprising an antigen-binding portion of said antibody for a target in sclerostin polypeptide, characterized in that the antibody or functional protein specifically binds to sclerostin polypeptide and can increase at least one of bone formation, bone mineral density, bone mineral content, bone mass, bone quality and bone strength in a mammal.
Owner:MEREO BIOPHARMA 3 LTD
Inhibitors of proteasomal activity for stimulating hair growth
Compounds that inhibit the activity of NF-κB or inhibit the activity of the proteasome or both promote bone formation and hair growth and are thus useful in treating osteoporosis, bone fracture or deficiency, primary or secondary hyperparathyrdidism, periodontal disease or defect, metastatic bone disease, osteolytic bone disease, post-plastic surgery, post-prosthetic joint surgery, and post-dental implantation; they also stimulate the production of hair follicles and are thus useful in stimulating hair growth, including hair density, in subject where this is desirable.
Owner:OSTEOSCREEN IP +1
Porous beta-tricalcium phosphate granules for regeneration of bone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Cement products and methods of making and using the same
InactiveUS20090270527A1Reduce the amount requiredReduce eliminateImpression capsSurgical adhesivesHindered amine light stabilizersBone formation
Disclosed are cement products, methods of forming cement using the cement product, and methods of using the cement product in orthopedic and dental applications. Generally, the disclosed cement product includes a first component and a second component. The first component comprises a polymerizable resin comprising ethylenic unsaturated double bond, a suitable glycidyl group and / or a suitable isocyanate group. The second component includes a compound comprising more than one type of amine selected from the group consisting of primary amine, secondary amines, tertiary amines and quaternary amines. Alternatively, the second component includes a compound comprising a suitable mercapto (SH—) group, a hindered amine or a dimethylthiotoluenediamine (DMTDA). Optionally, the cement product includes a filler and / or a bioactive component to promote bone formation.
Owner:PIONEER SURGICAL TECH INC
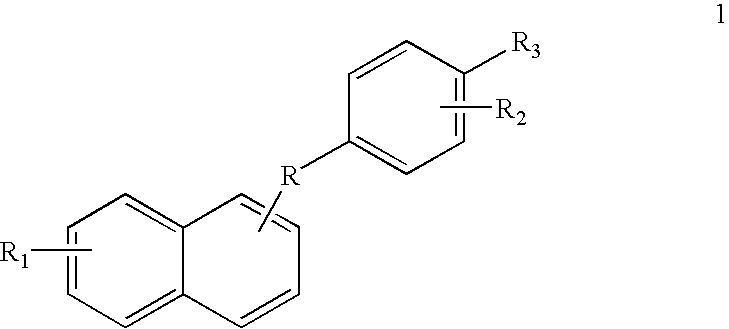
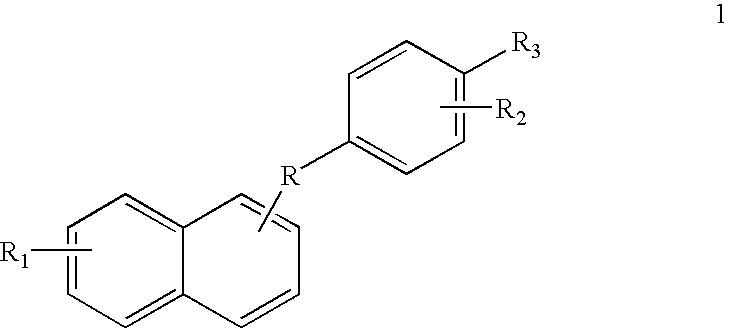
Mercaptophenyl naphthyl methane compounds and synthesis thereof
Novel mercaptophenyl naphthyl methane compounds, their pharmaceutically acceptable salts and compositions comprised thereof are useful for the prevention or treatment of various medical indications associated with estrogen dependent diseases or syndromes related to osteoporosis, bone loss, bone formation, cardiovascular disorders, neurodegenerative disorders, menopausal disorders, physiological disorders, diabetes disorders, prostatic carcinoma, cancer of breast, cancer of uterus, cancer of the cervix and cancer of the colon, threatened or habitual abortion, obesity, ovarian development or function, post-partum lactation and depression.
Owner:COUNCIL OF SCI & IND RES
Positive modulator of bone morphogenic protein-2
InactiveUS20050196425A1Improve biological activityMaximize bioactivityOrganic active ingredientsPeptide/protein ingredientsDiseaseBone formation
Compounds of the present invention of formula I and formula II are disclosed in the specification and wherein the compounds are modulators of Bone Morphogenic Protein activity. Compounds are synthetic peptides having a non-growth factor heparin binding region, a linker, and sequences that bind specifically to a receptor for Bone Morphogenic Protein. Uses of compounds of the present invention in the treatment of bone lesions, degenerative joint disease and to enhance bone formation are disclosed.
Owner:BROOKHAVEN SCI ASSOCS +1
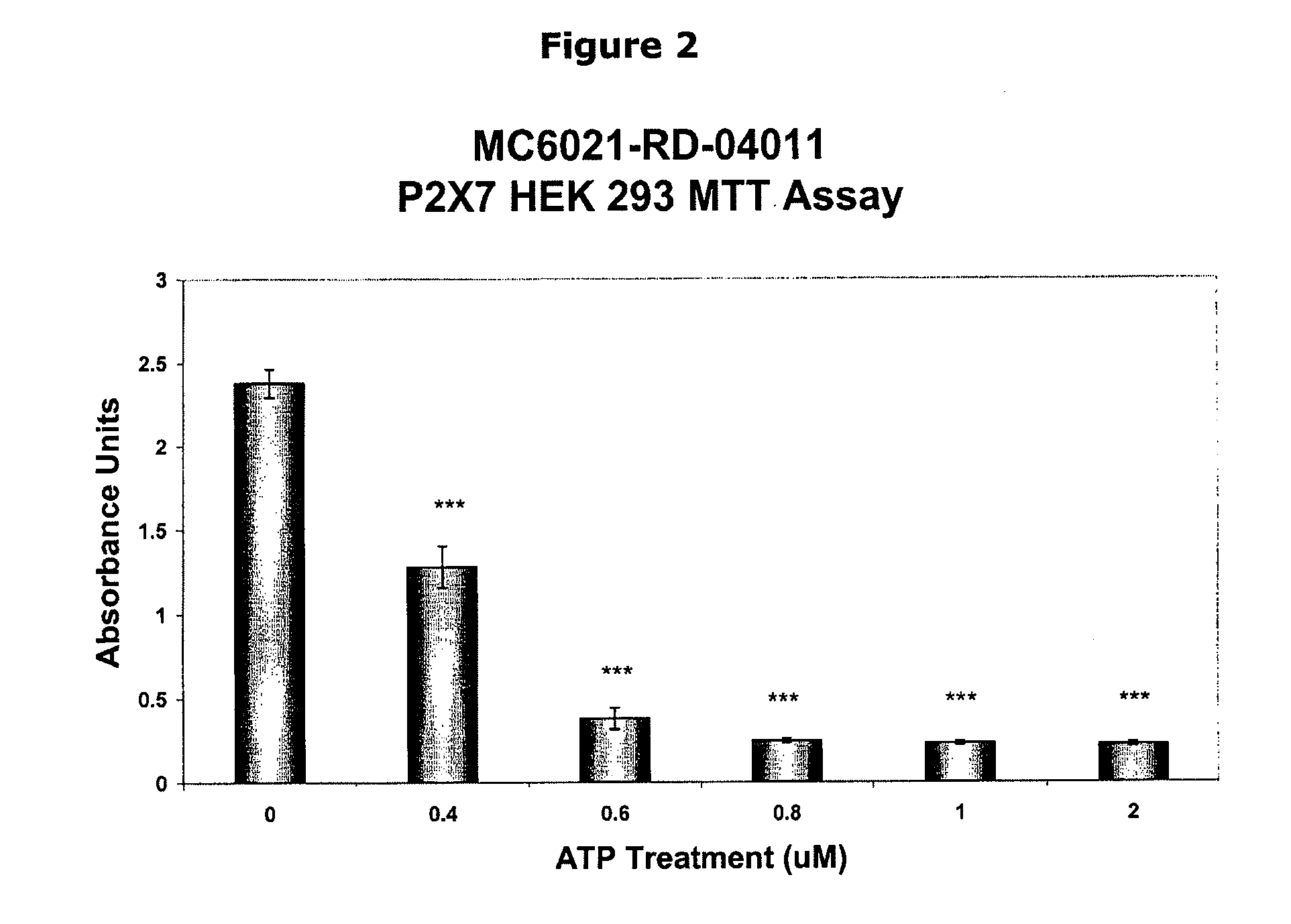
Inhibition of atp-mediated, p2x7 dependent pathways by pyridoxal-5-phosphate and vitamin b6 related compounds
InactiveUS20090215727A1Improve the level ofOrganic active ingredientsBiocideBone formationProstate cancer
P5P can be used as effective treatments for the modulation of P2X7, IL-1β, and inflammation response, and for diseases in which prevention of P2X7-dependent pathways or prevention of release of IL-1β is desirable, such as epithelial cancer, leukemia, brain tumors, spinal cord injury, tuberculosis, Alzheimer's Disease, neurodegenerative diseases, autosomal recessive polycystic kidney disease, diabetes, including type I diabetes, prostate cancer, and osteoporosis, bone formation and resorption.
Owner:MEDICURE INT INC
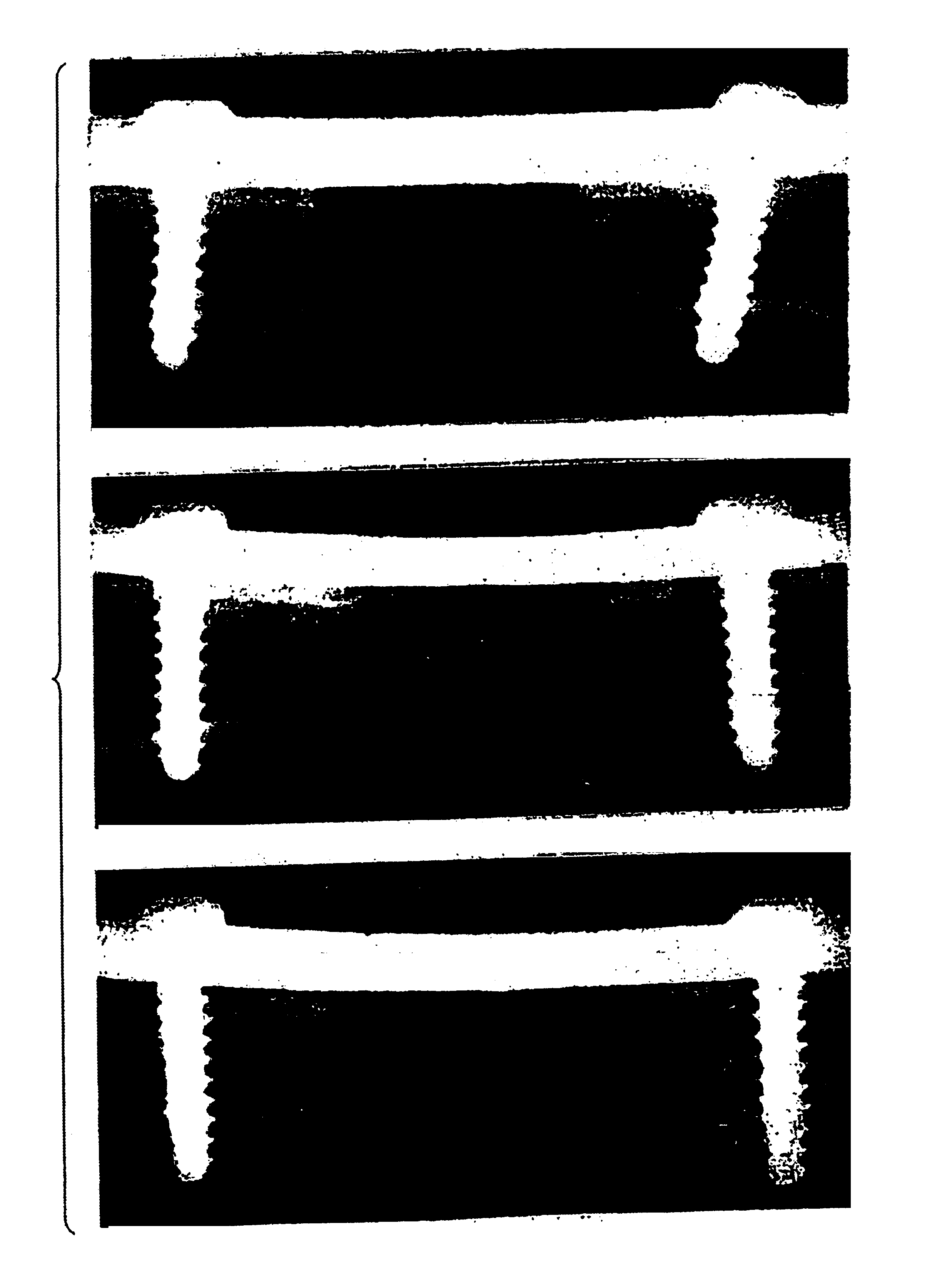
Bone implants and method of manufacture
ActiveUS8414654B1Improve bone formationAdditive manufacturing apparatusInternal osteosythesisMedicineBone formation
An implant device for humans or mammals has a body structure having an exposed surface and one or more selected portions of the exposed surface having a bone formation enhancing 3-dimensional pattern. The exposed surface can be on exterior portions of the body structure or internal portions of the body structure or both. The one or more selected portions of the exposed portions having the bone formation enhancing 3-dimensional patterns are in the external exposed surfaces or in the internal exposed surfaces or both internal and external exposed surfaces.
Owner:BONE PHARM LLC
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous β-tricalcium phosphate (β-TCP).
Owner:CURASAN
Porous beta-tricalcium phosphate granules for regeneration of bone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
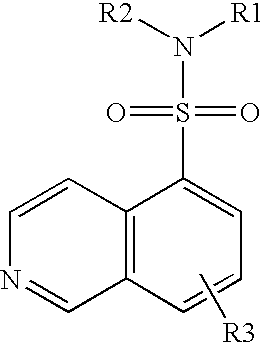
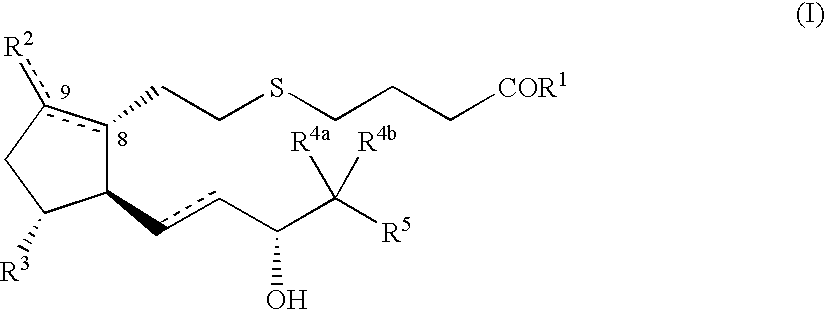
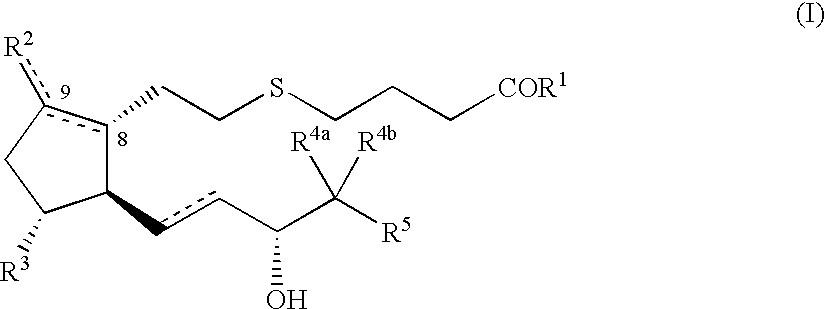
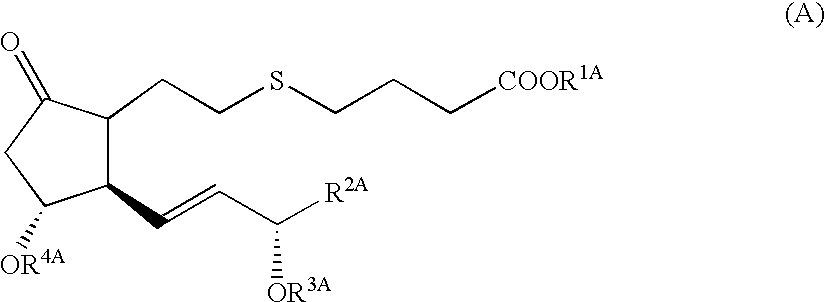
5-thia-omega-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient
The present invention relates to 5-thia-omega-substituted phenylprostaglandin E derivatives of the formula (I)(wherein, all the symbols are as defined in the specification), process for producing them and pharmaceutical compositions comprising them as active ingredient.The compounds of the formula (I) can bind to PGE2 receptors (especially, subtype EP4) strongly, so they are expected to be useful for prevention and / or treatment of immunological diseases (autoimmune diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Sjoegren's syndrome, chronic rheumarthrosis and systemic lupus erythematosus etc., and rejection after organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, lung failure, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardiac ischemia, systemic inflammatory response syndrome, ambustion pain, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, and shock etc. Further, it is thought that EP4 subtype receptor relates to sleeping disorder and blood platelet aggregation, so the compounds of the present invention are expected to be useful for the prevention and / or treatment of such diseases.
Owner:ONO PHARMA CO LTD
Nell peptide expression systems and bone formation activity of nell peptide
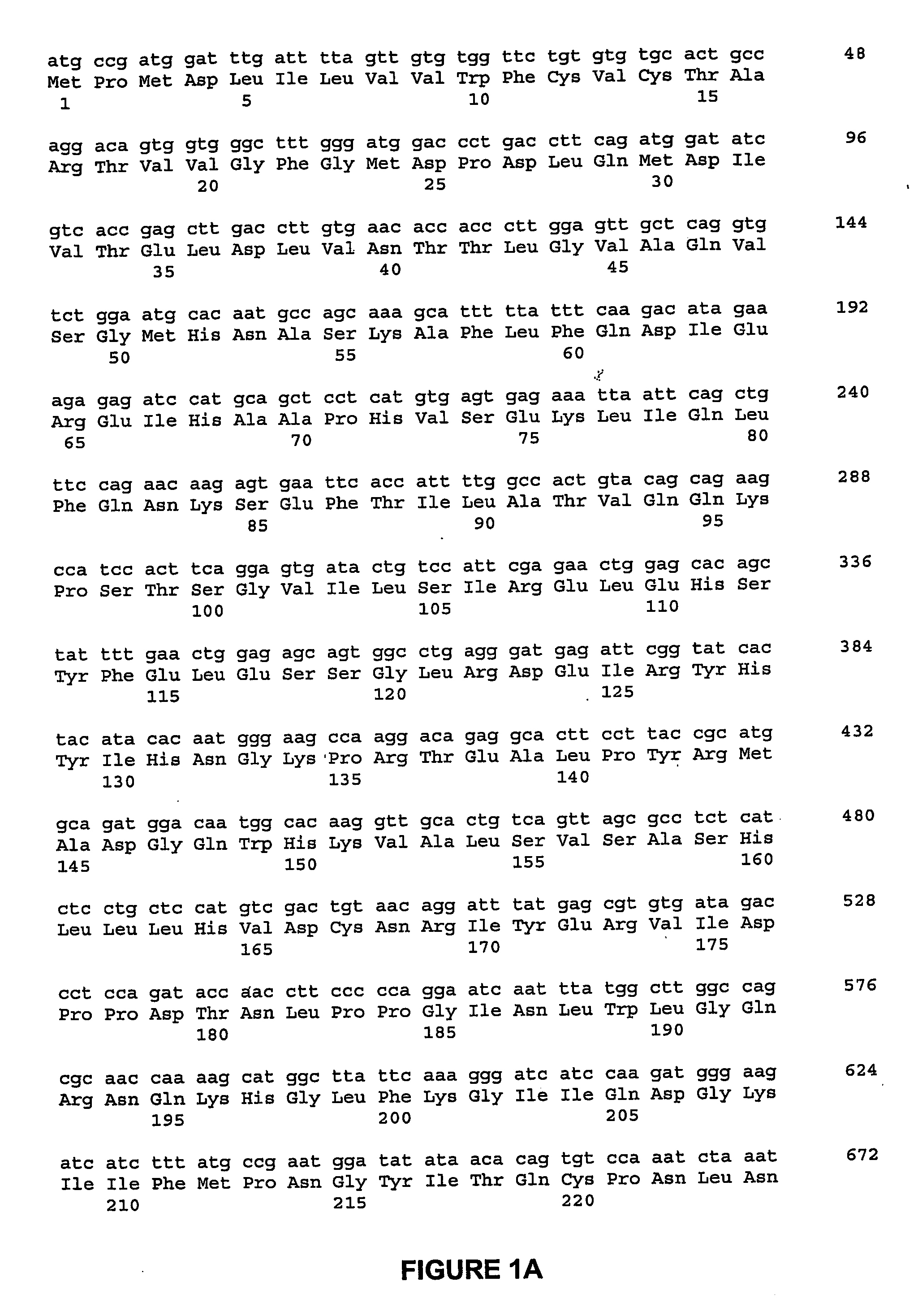
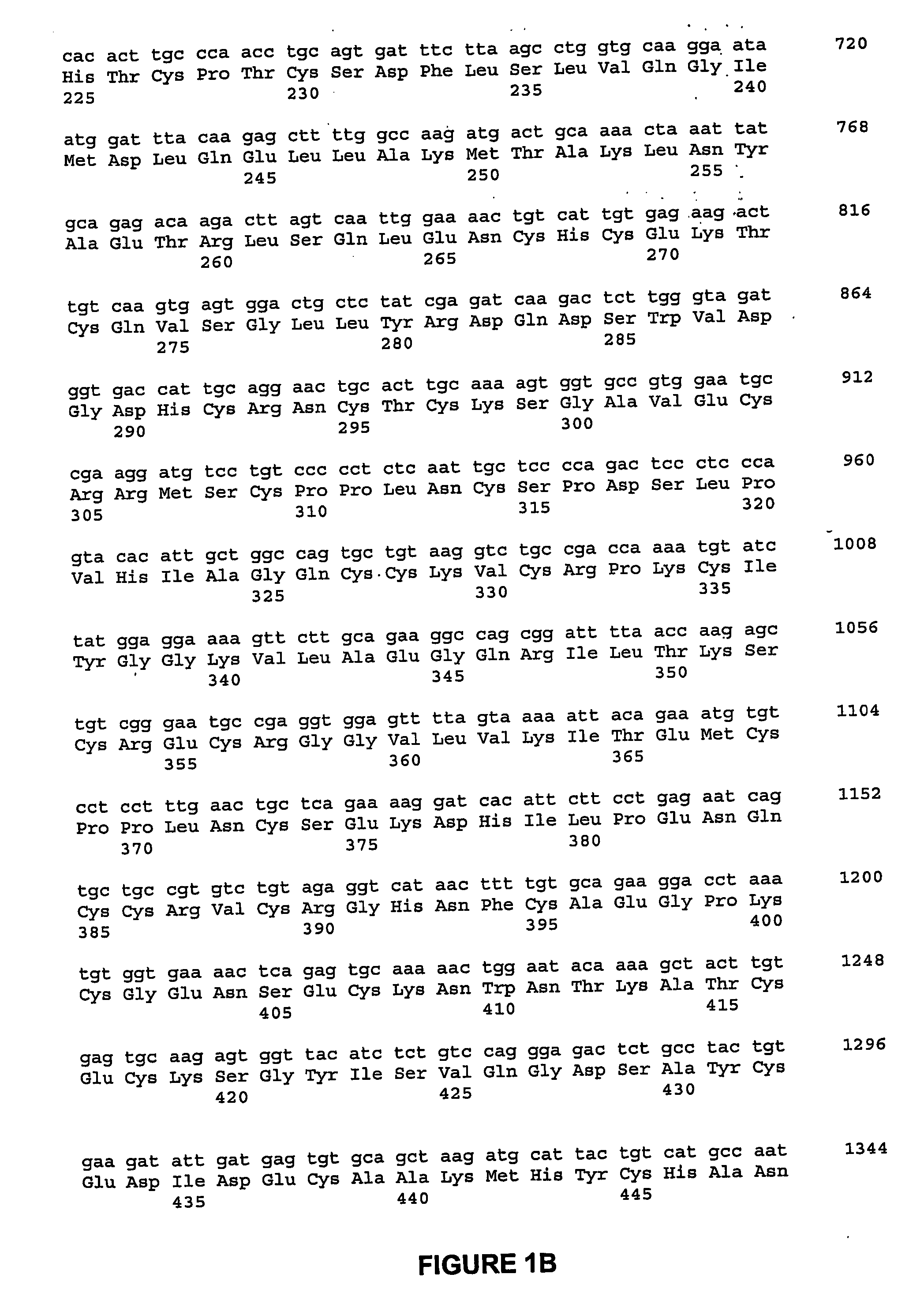
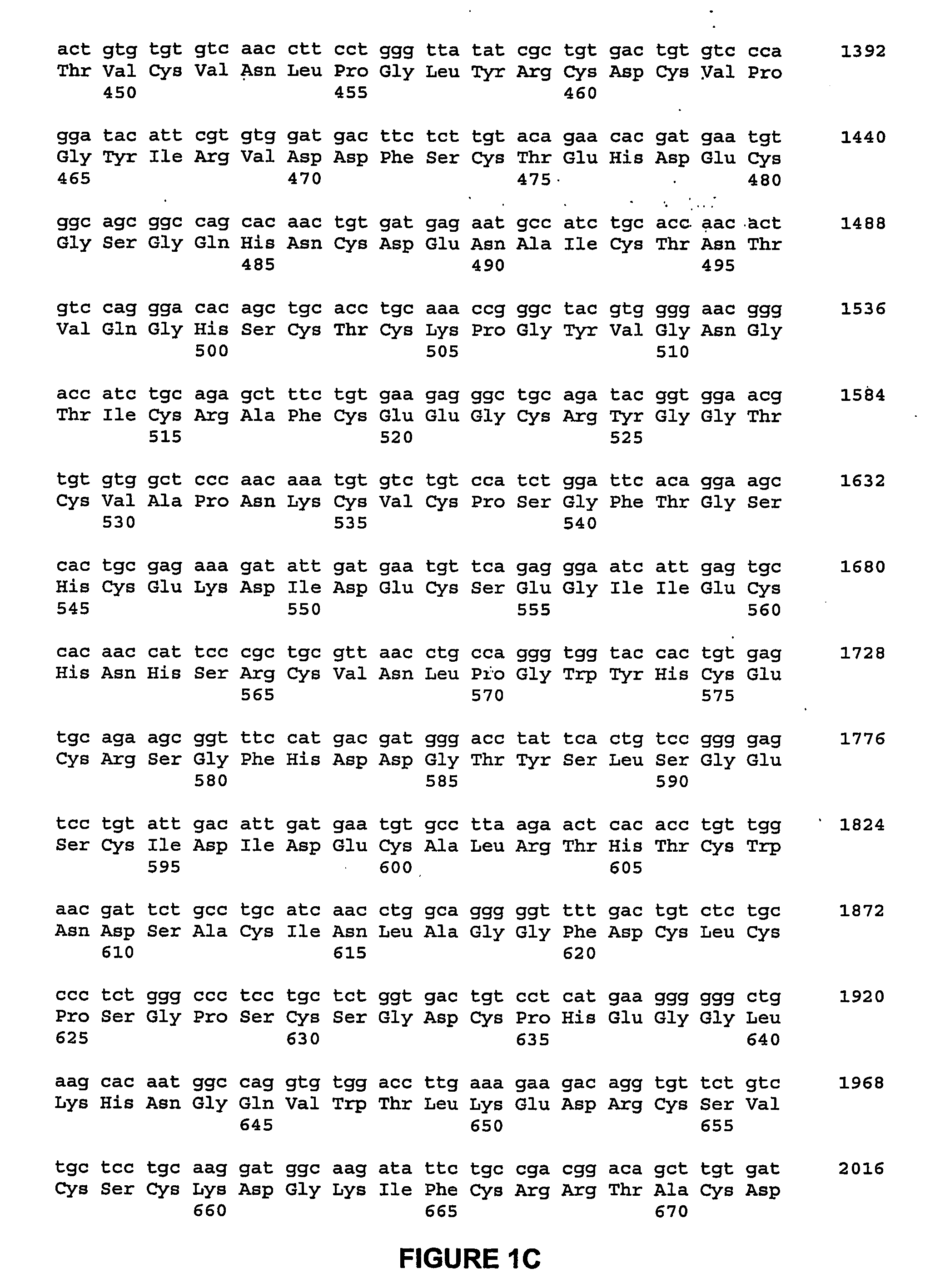
ActiveUS20060292670A1Facilitate protein traffickingFacilitate post production modificationOrganic active ingredientsBacteriaBone formationBone growth factor
The invention generally relates to a bone growth factor, and more particularly to compositions including NELL1, articles of manufacture including NELL1 and methods of using NELL1 to induce bone formation. This invention also provides methods for the expression and purification of NELL1 and NELL2 peptides.
Owner:RGT UNIV OF CALIFORNIA
Resorbable bone replacement and bone formation material
The invention relates to a resorbable bone replacement and bone formation material (augmentation material) based on porous beta-tricalcium phosphate (beta-TCP).
Owner:CURASAN
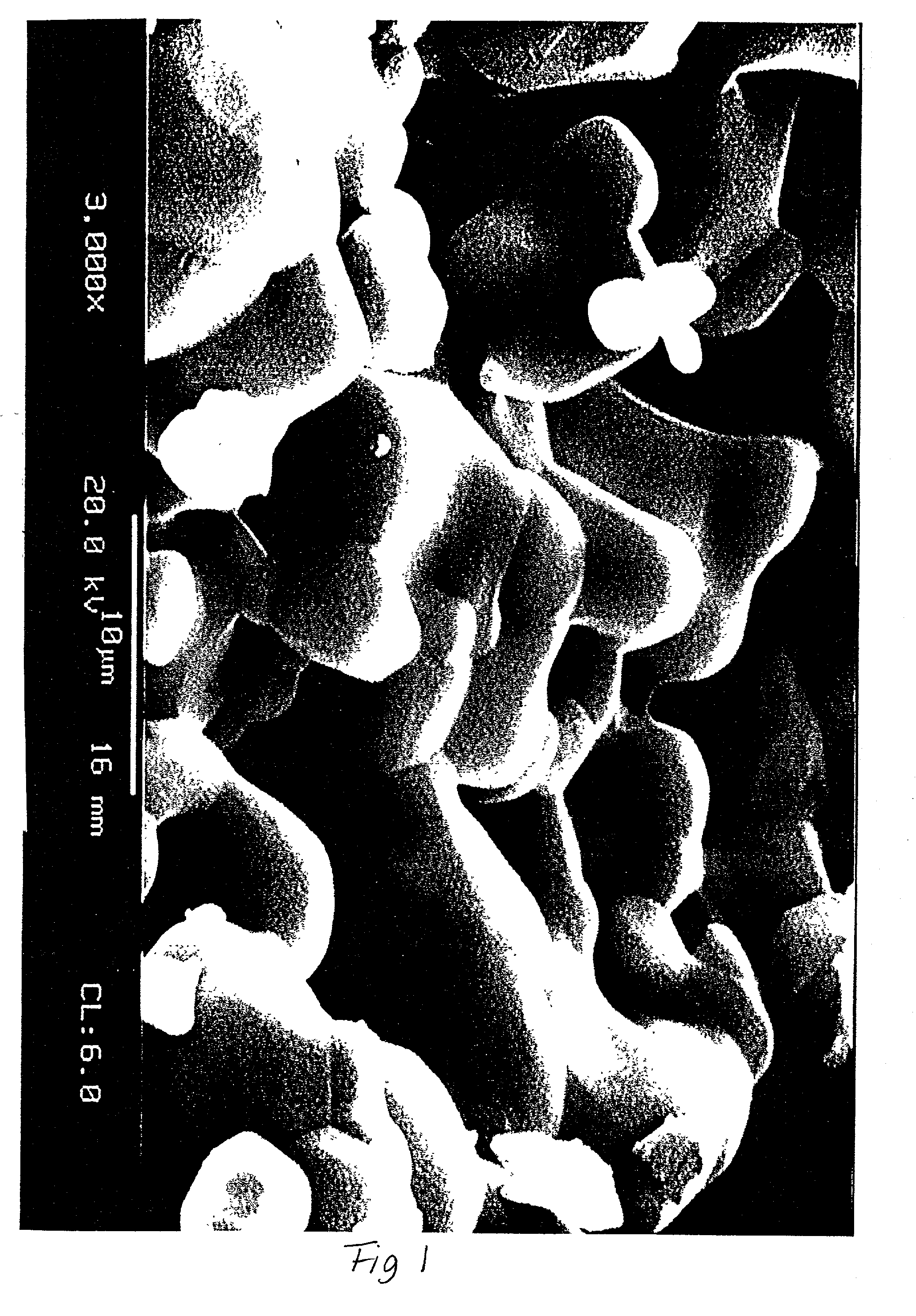
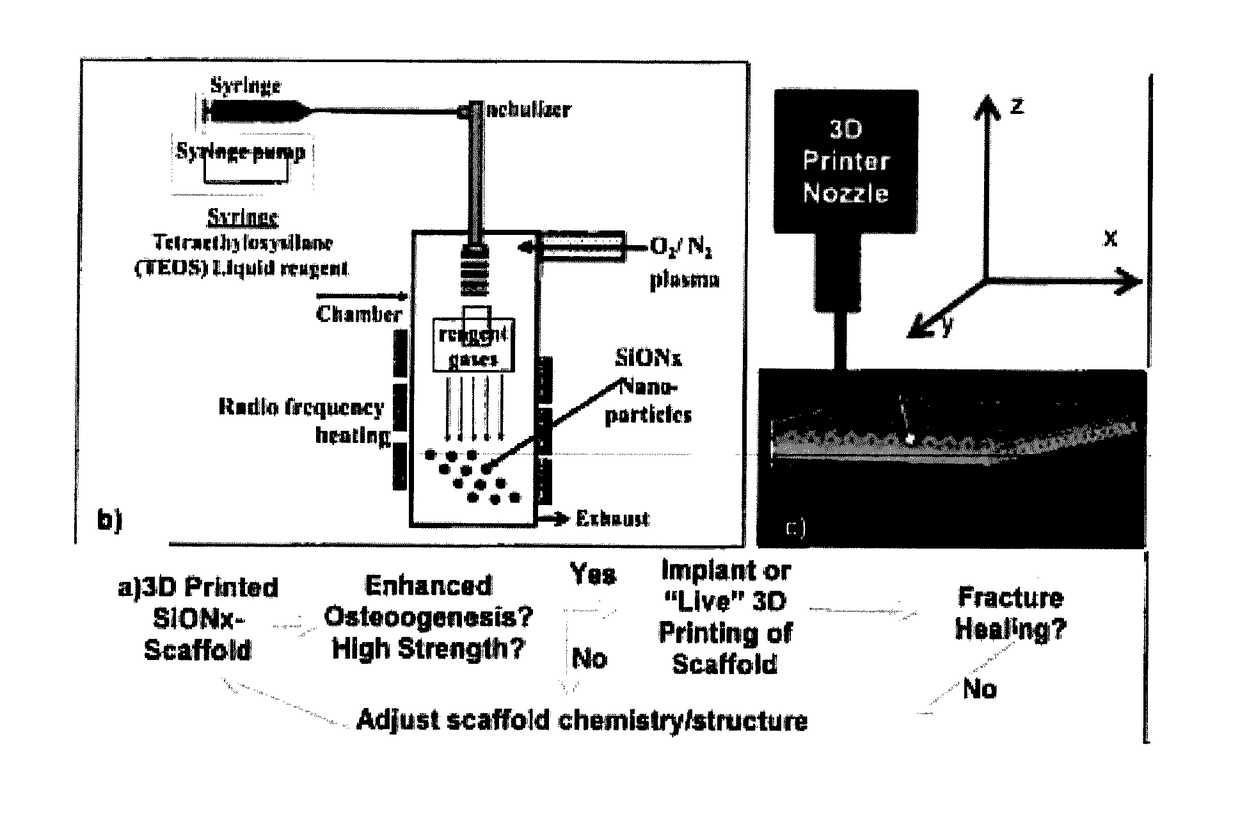
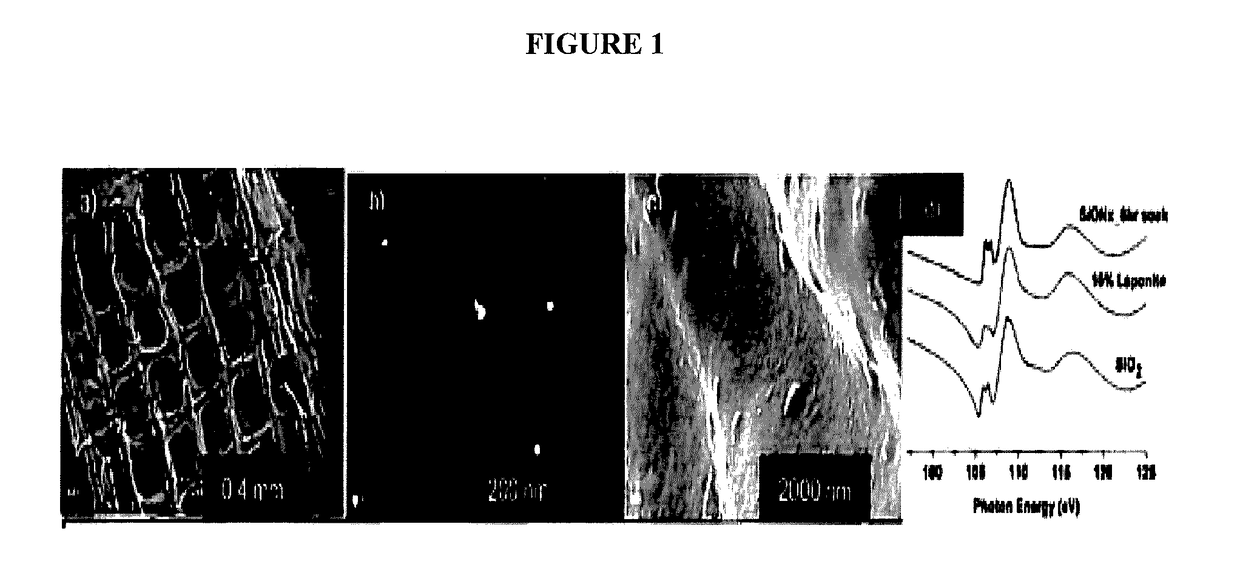
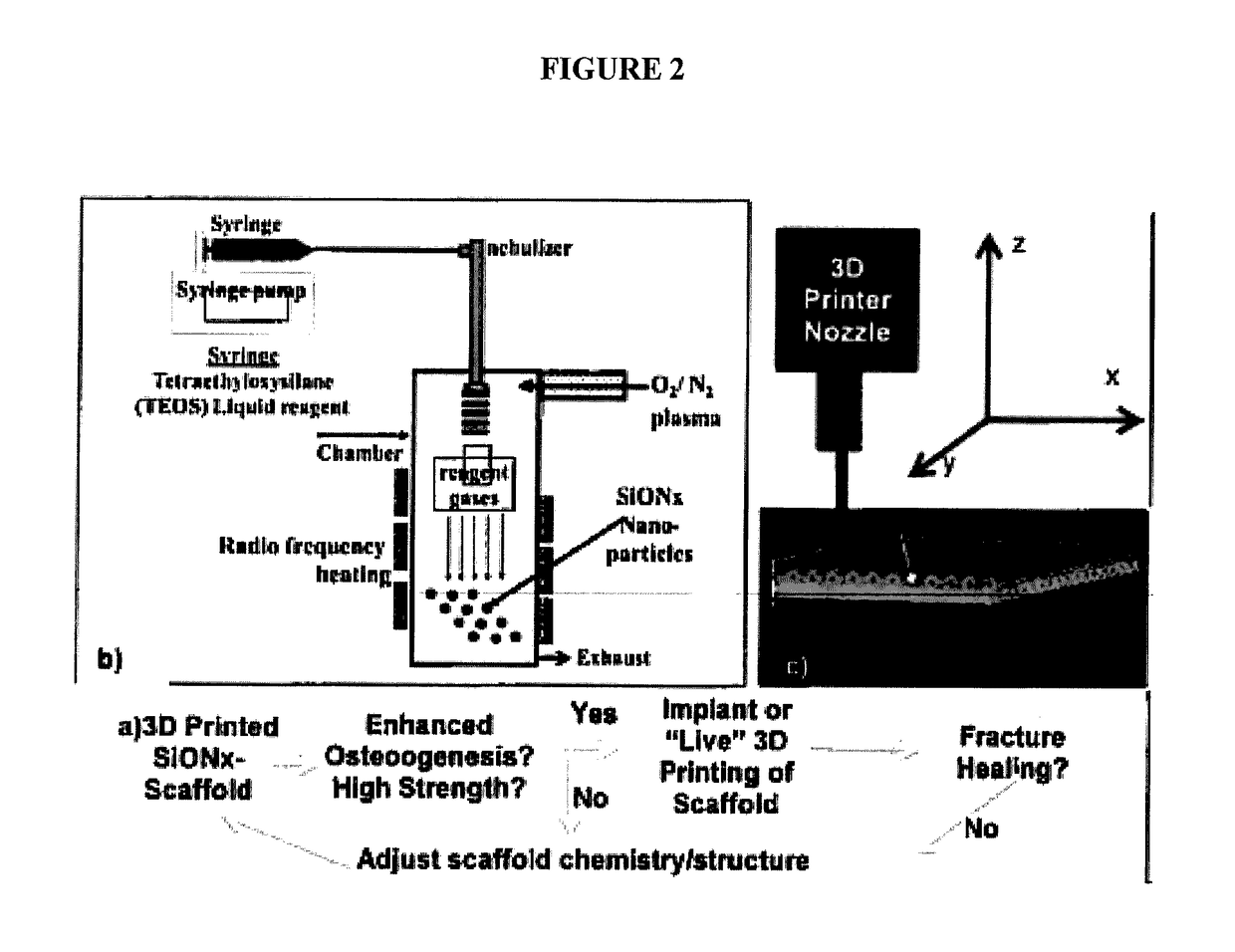
In vivo live 3D printing of regenerative bone healing scaffolds for rapid fracture healing
ActiveUS20170143831A1Promote degradationPowder deliveryOrganic active ingredientsCross-linkPoint of care
Bio-Inks and methods of using compositions comprising the bio-Inks are disclosed. 3-D tissue repair and regeneration through precise and specific formation of biodegradable tissue scaffolds in a tissue site using the bio-inks are also provided. Specific methylacrylated gelatin hydrogels (MAC) and methacrylated chitosan (MACh) preparations formulated with sucrose, a silicate-containing component (such as laponite), and / or a cross-linking agent (such as a photo-initiator or chemical initiator), as well as powdered preparations of these, are also disclosed. Kits containing these preparations are provided for point-of-care tissue repair in vivo. Superior, more complete (up to 99.85% tissue regeneration within 4 weeks applied in situ), and rapid in situ tissue repair and bone formation are also demonstrated.
Owner:TEXAS A&M UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com