Patents
Literature
1742 results about "Prosthetic graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prosthetic grafts. Abstract. translated from. An improved prosthetic graft for the bypass, replacement or repair of vessels and organs that are in contact with blood flow is disclosed. The prosthetic graft includes a porous prosthetic implant and adherent cells adhered to the outer surface of the implant.
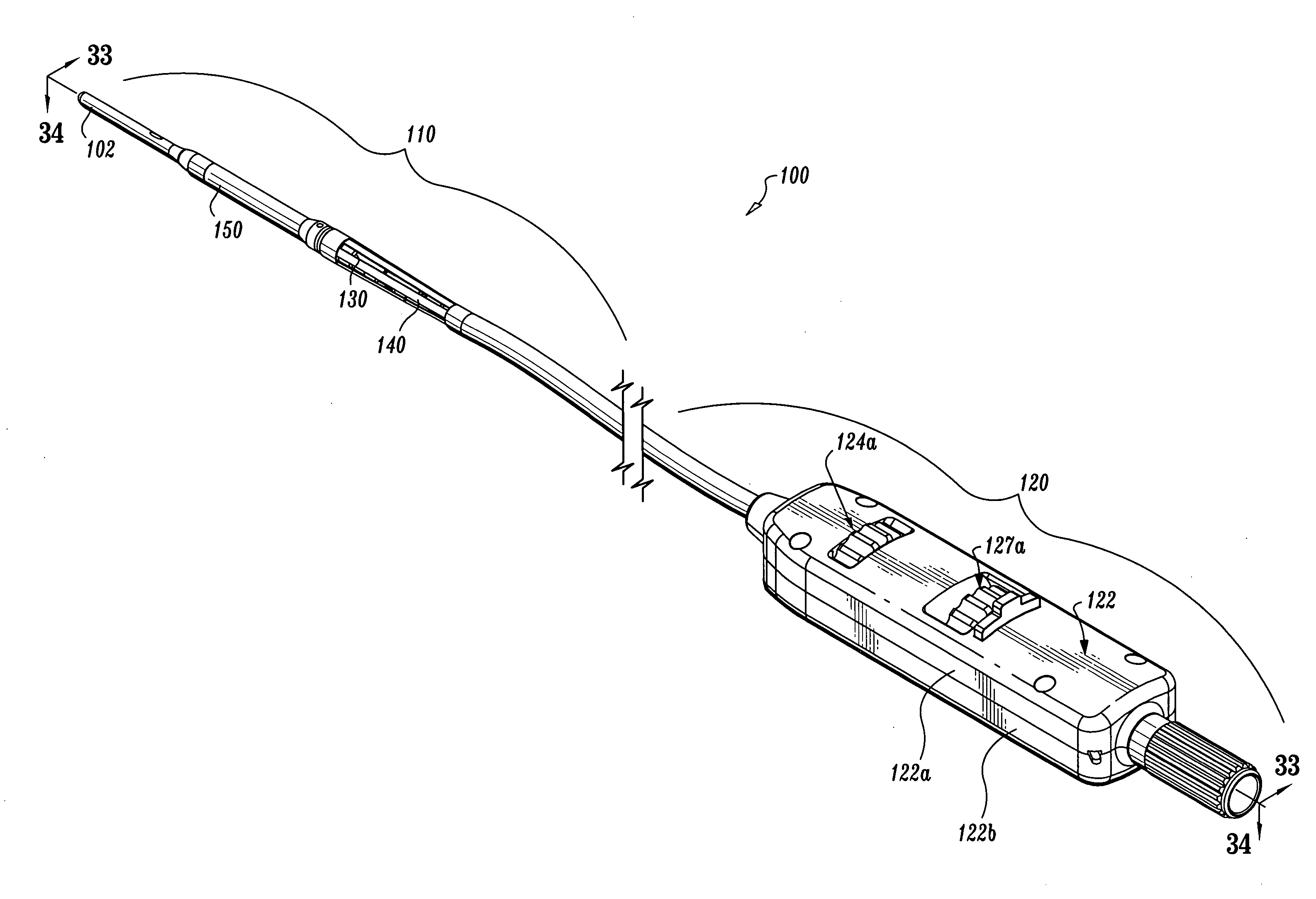
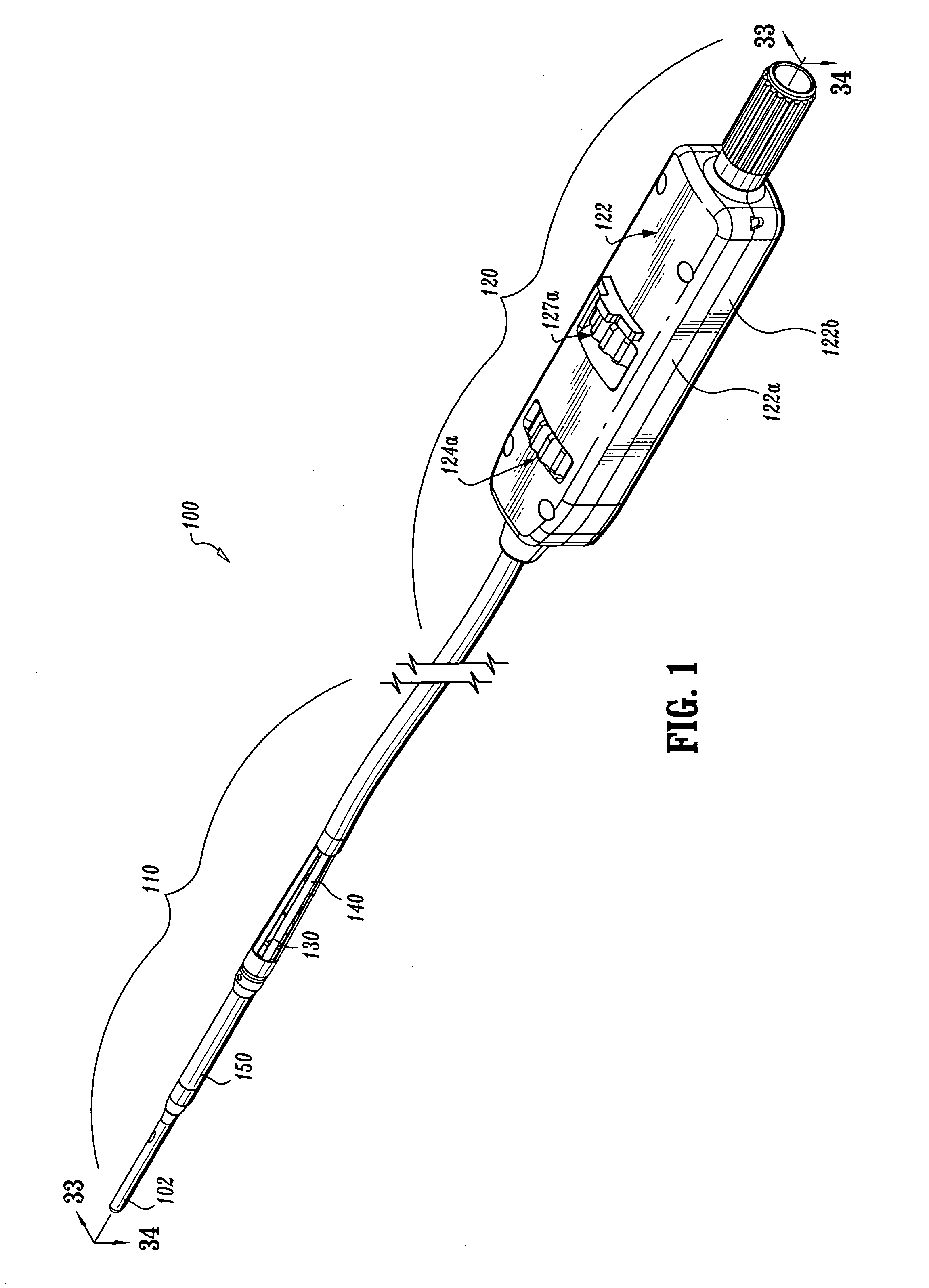
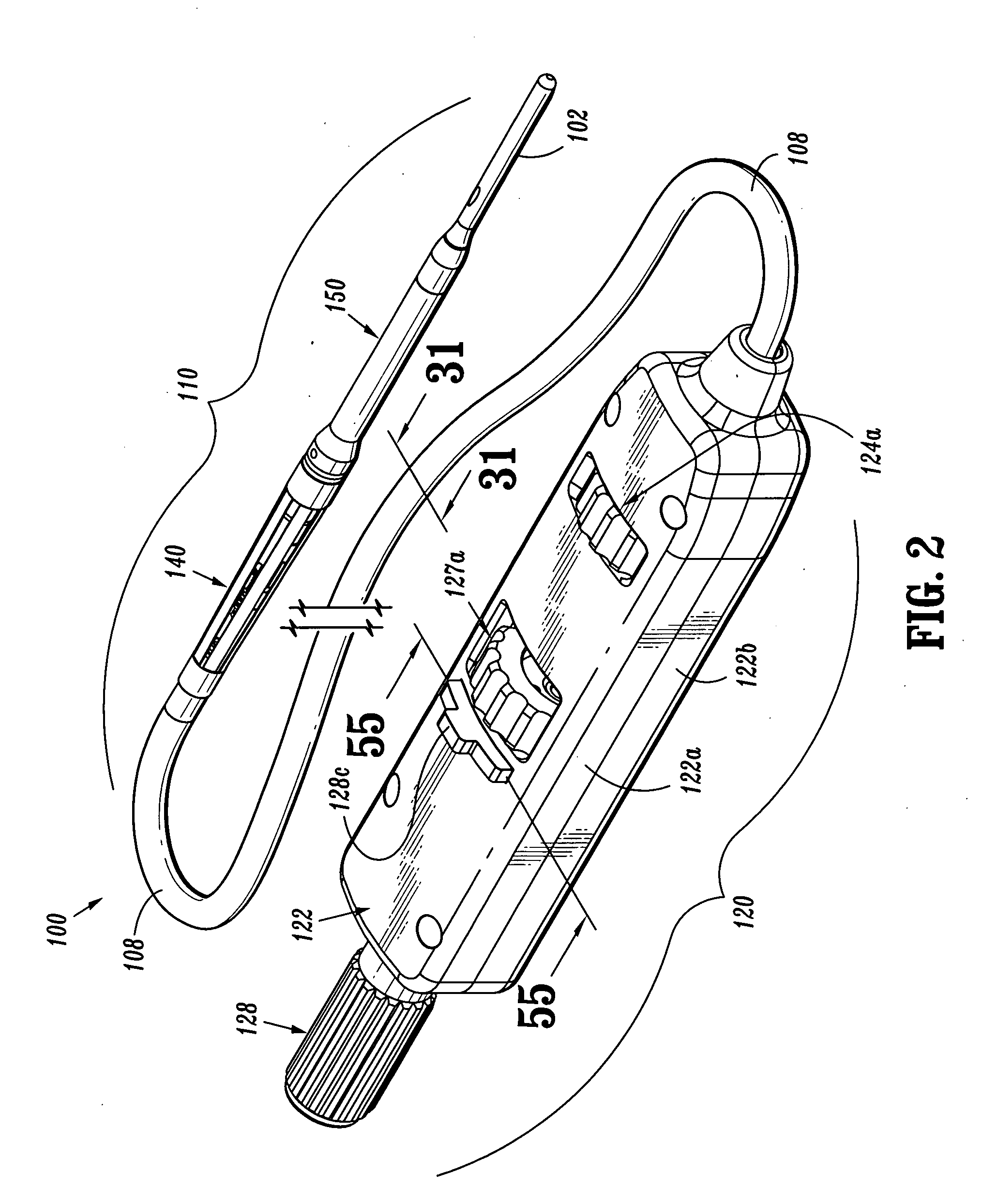
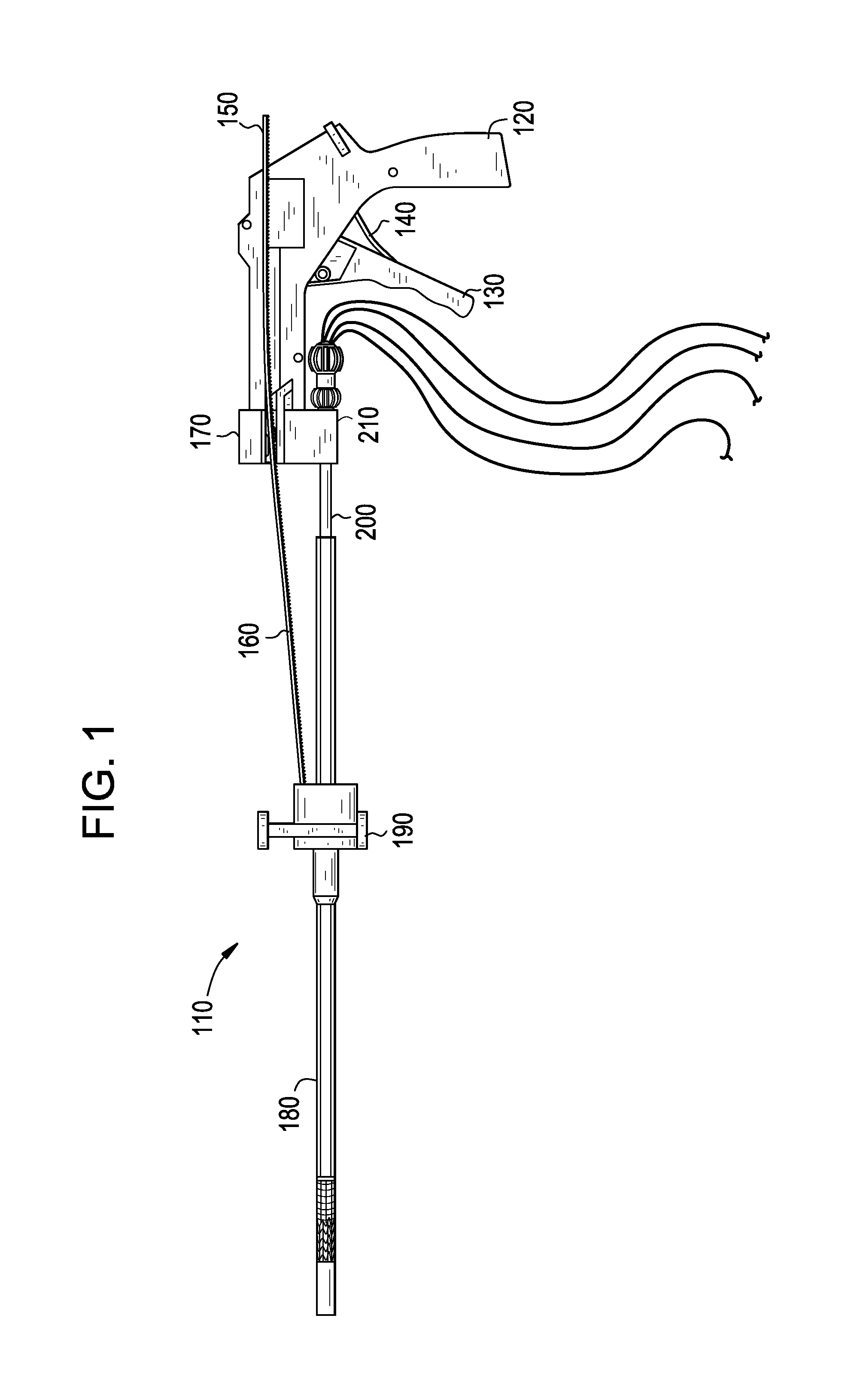
Endovascular fastener applicator
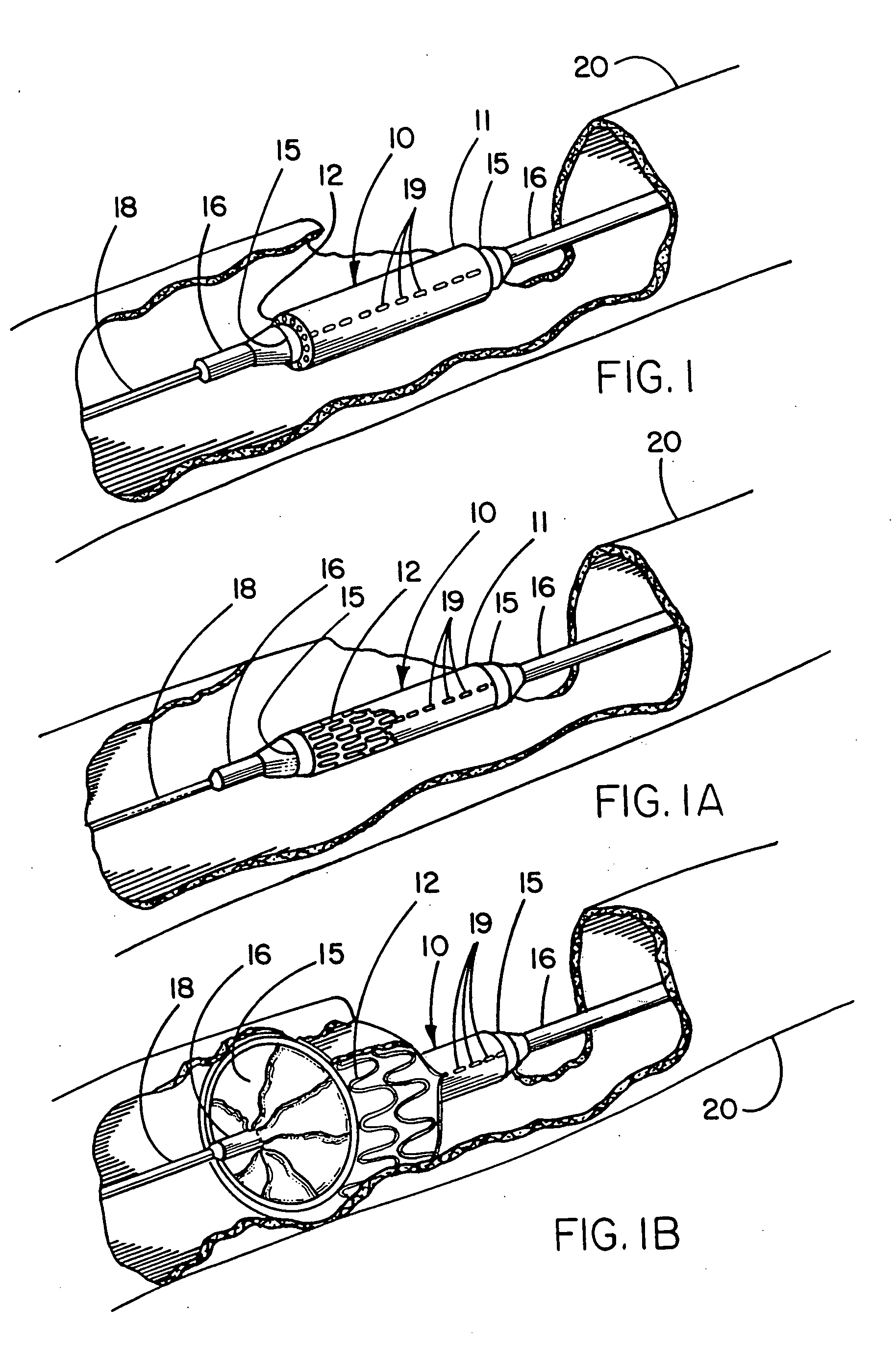
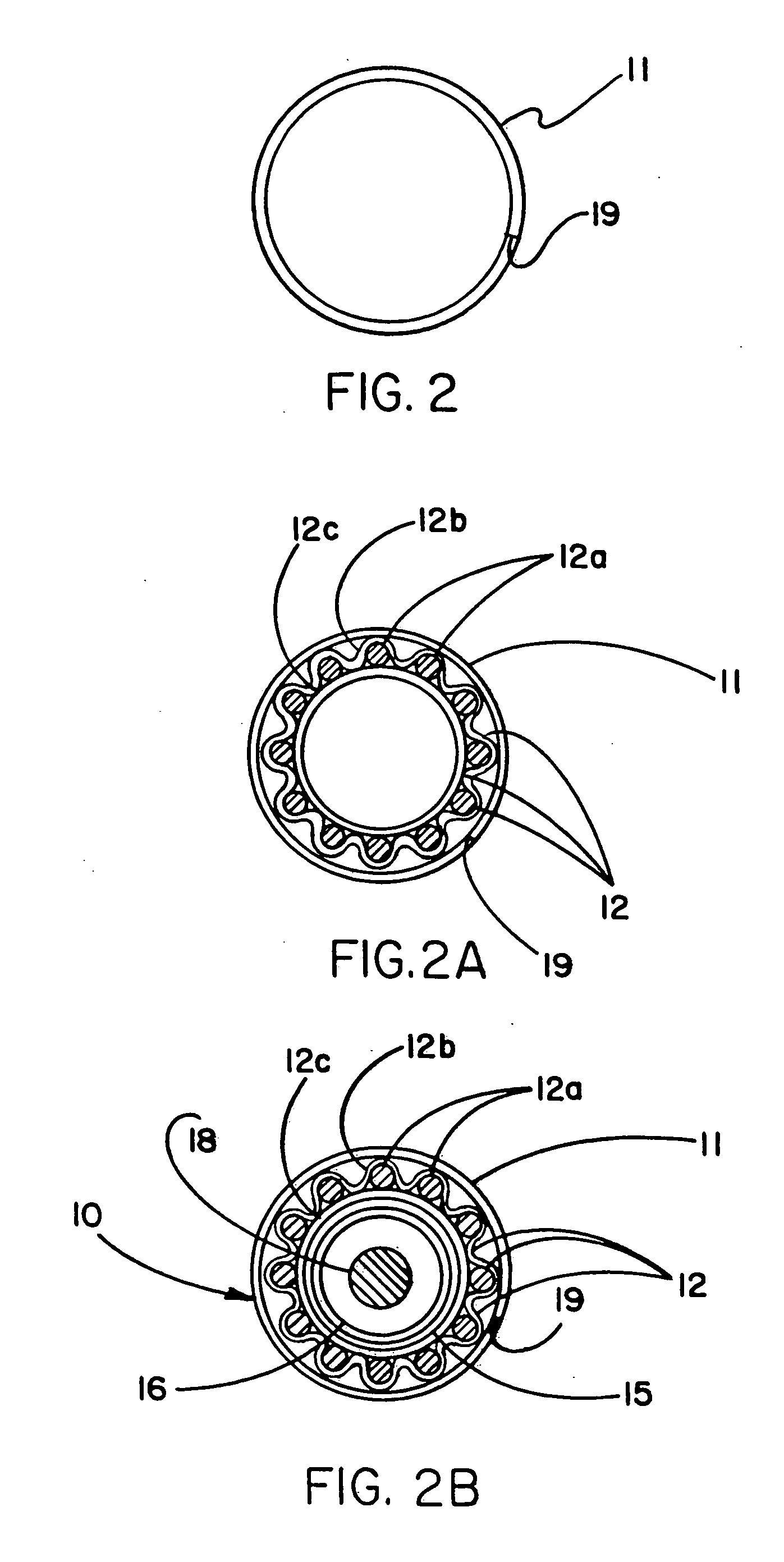
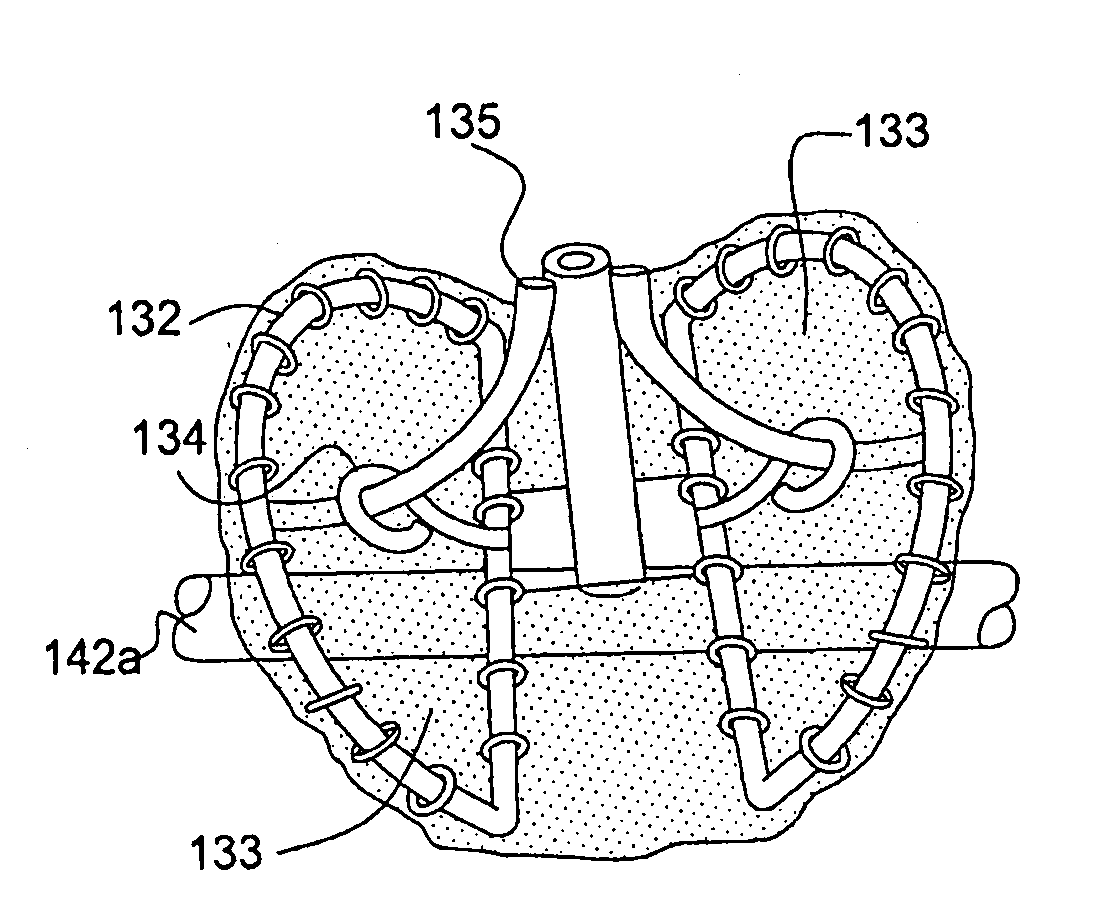
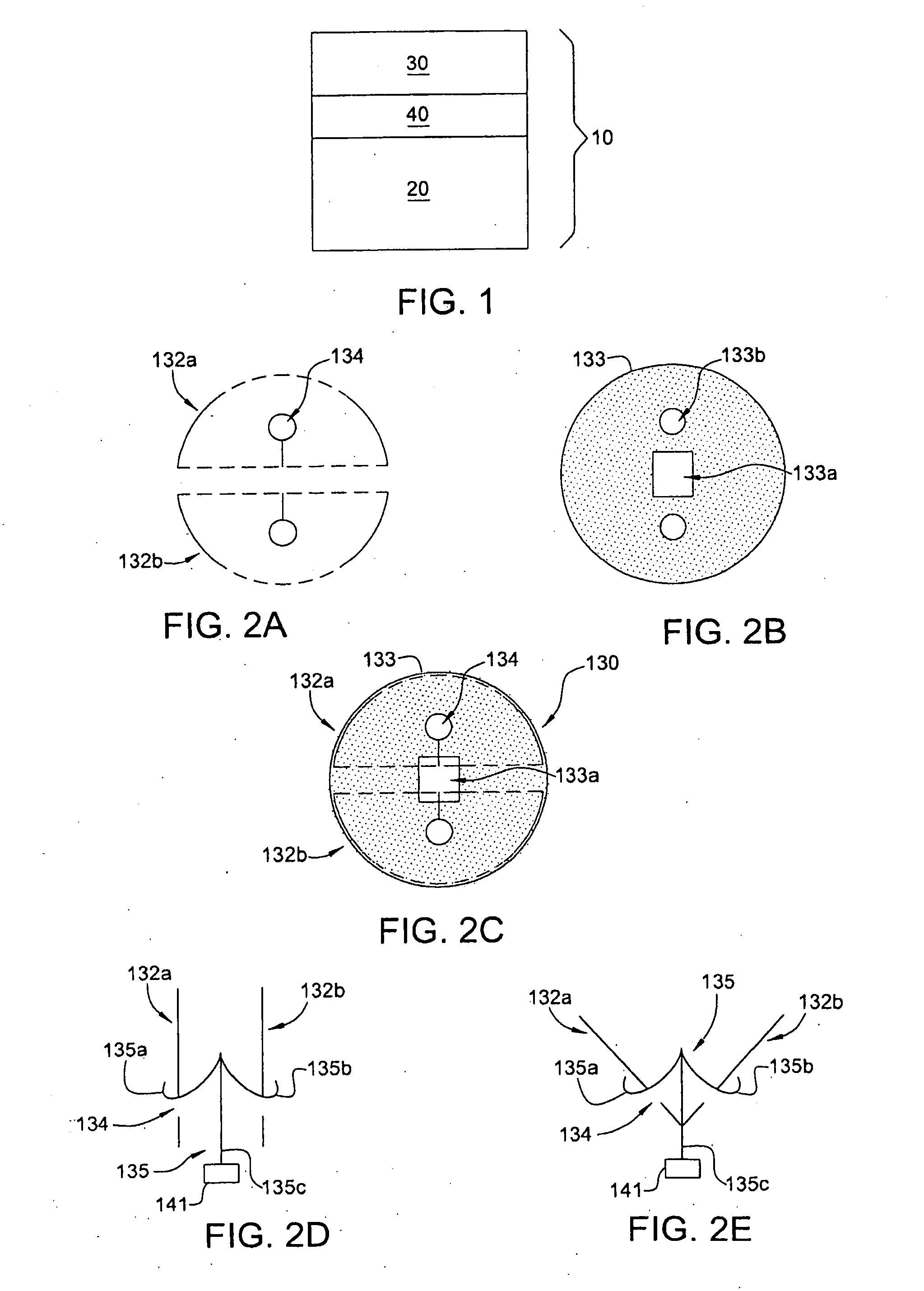
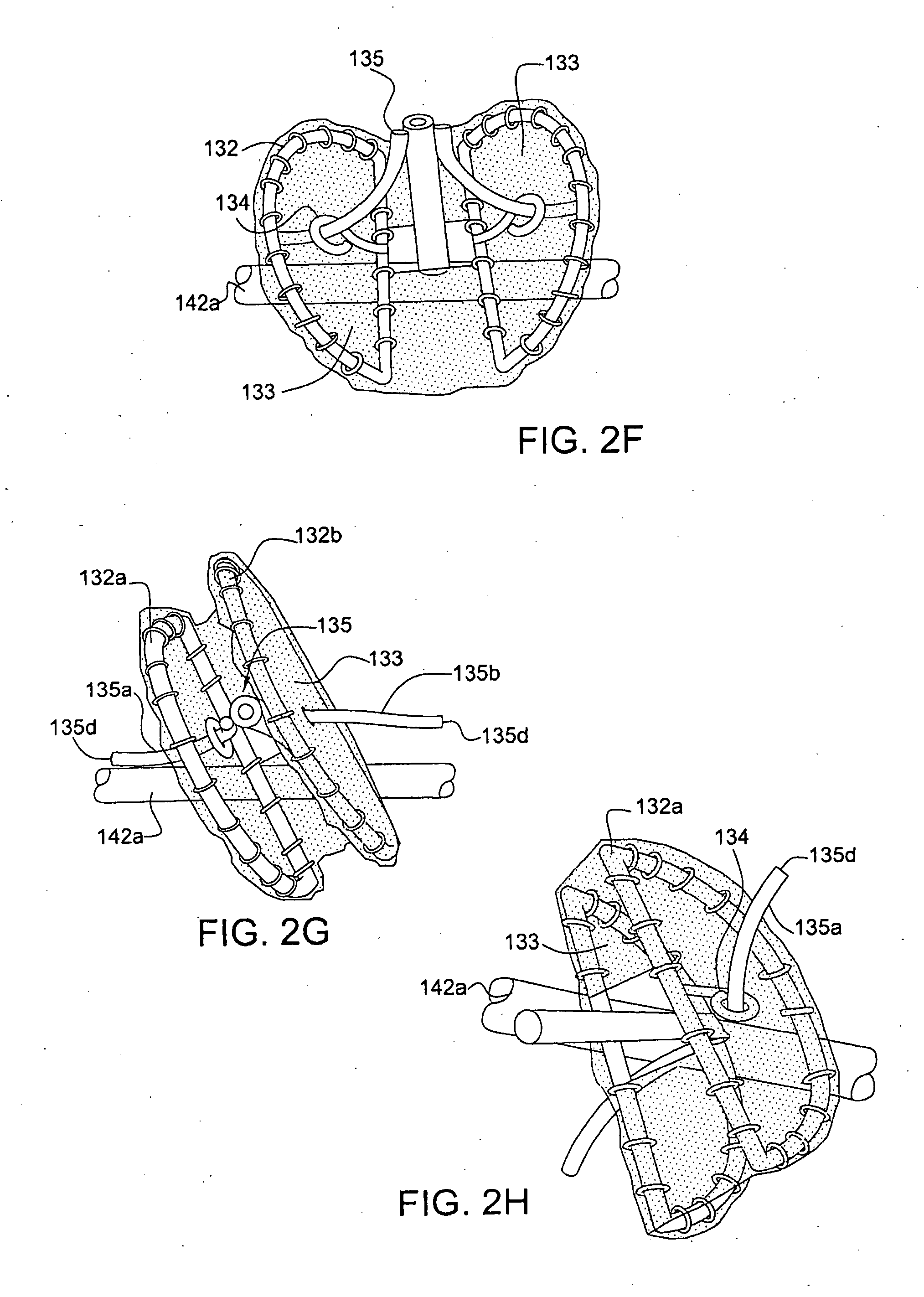
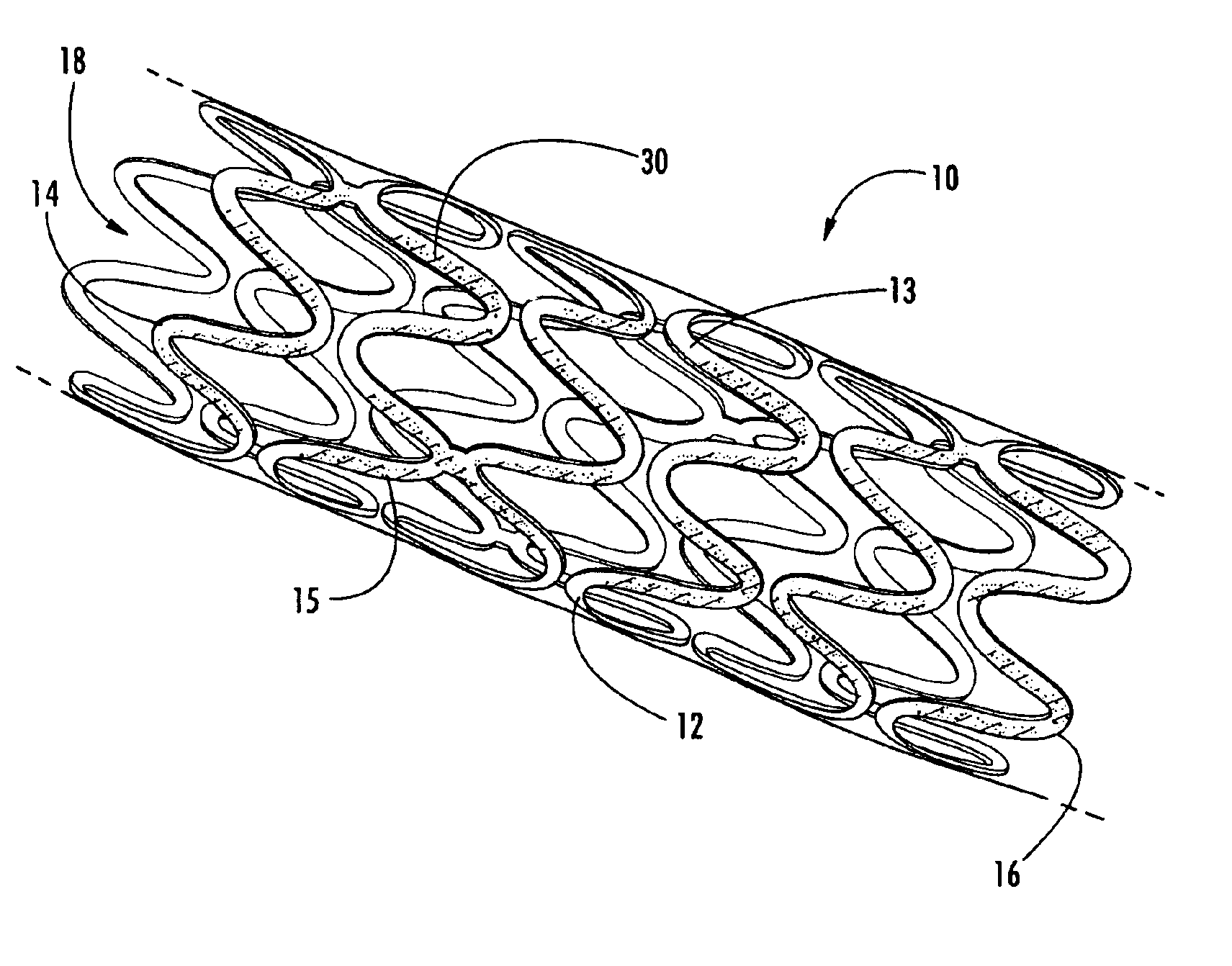
Endovascular fastener applicator for endoluminally fastening prosthetic grafts to vessels, are provided. The endovascular fastener applicator includes a delivery assembly configured for positioning within a vessel, and a control assembly mounted to a proximal end of the outer sheath for extracorporeal control of the delivery assembly. The delivery assembly includes an expandable portion disposed adjacent a distal end of an outer sheath and being expandable to support a prosthetic in contact with an inner surface of a vessel; a yoke assembly disposed within the expandable portion; an applicator head assembly pivotably mounted to the yoke assembly and movable between a loading position longitudinally aligned with the yoke assembly, and a firing position oriented substantially perpendicular to the yoke assembly; and a fastener assembly connectable to a distal end of the expandable portion, the fastener assembly retaining at least one fastener therein.
Owner:TYCO HEALTHCARE GRP LP
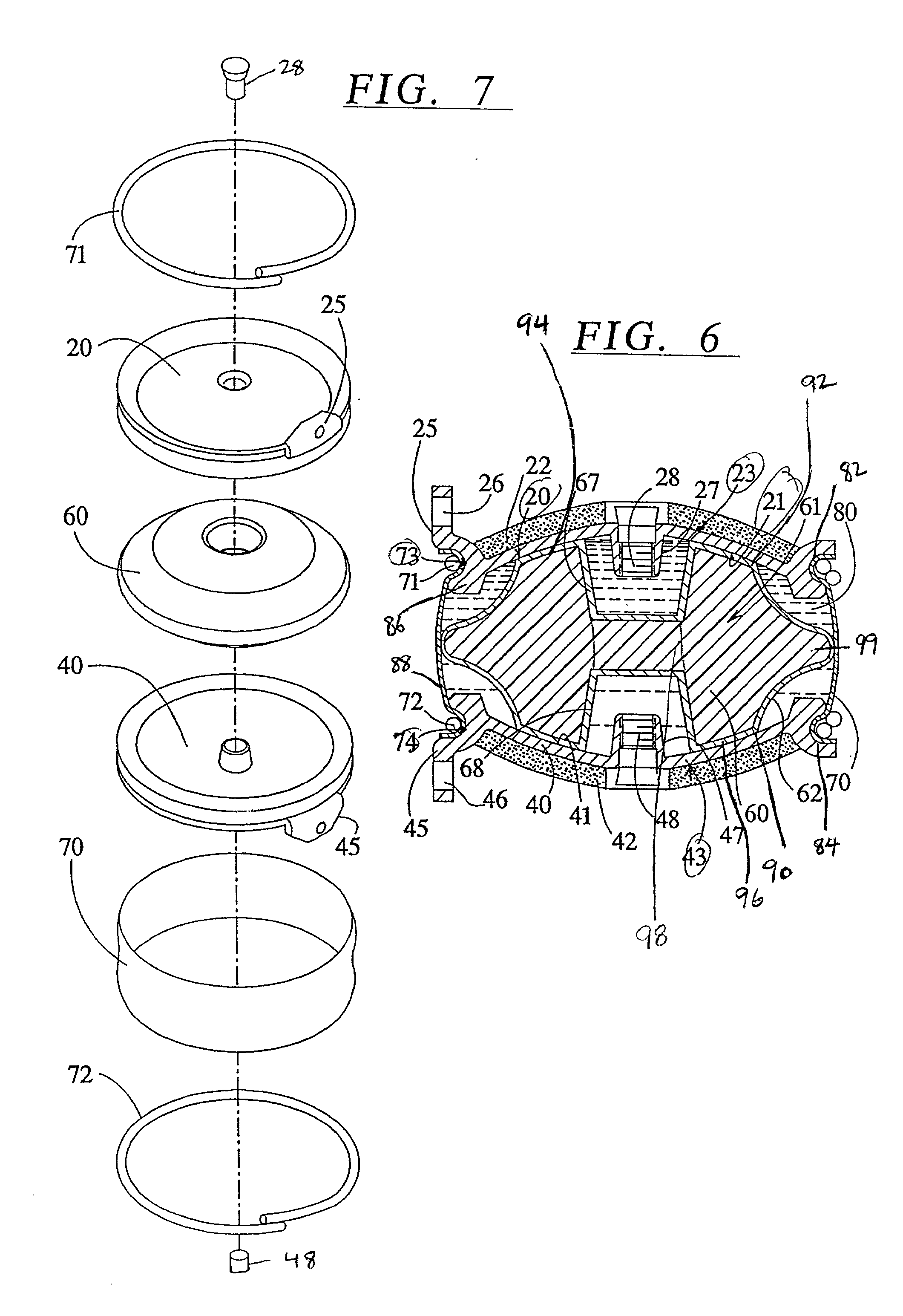
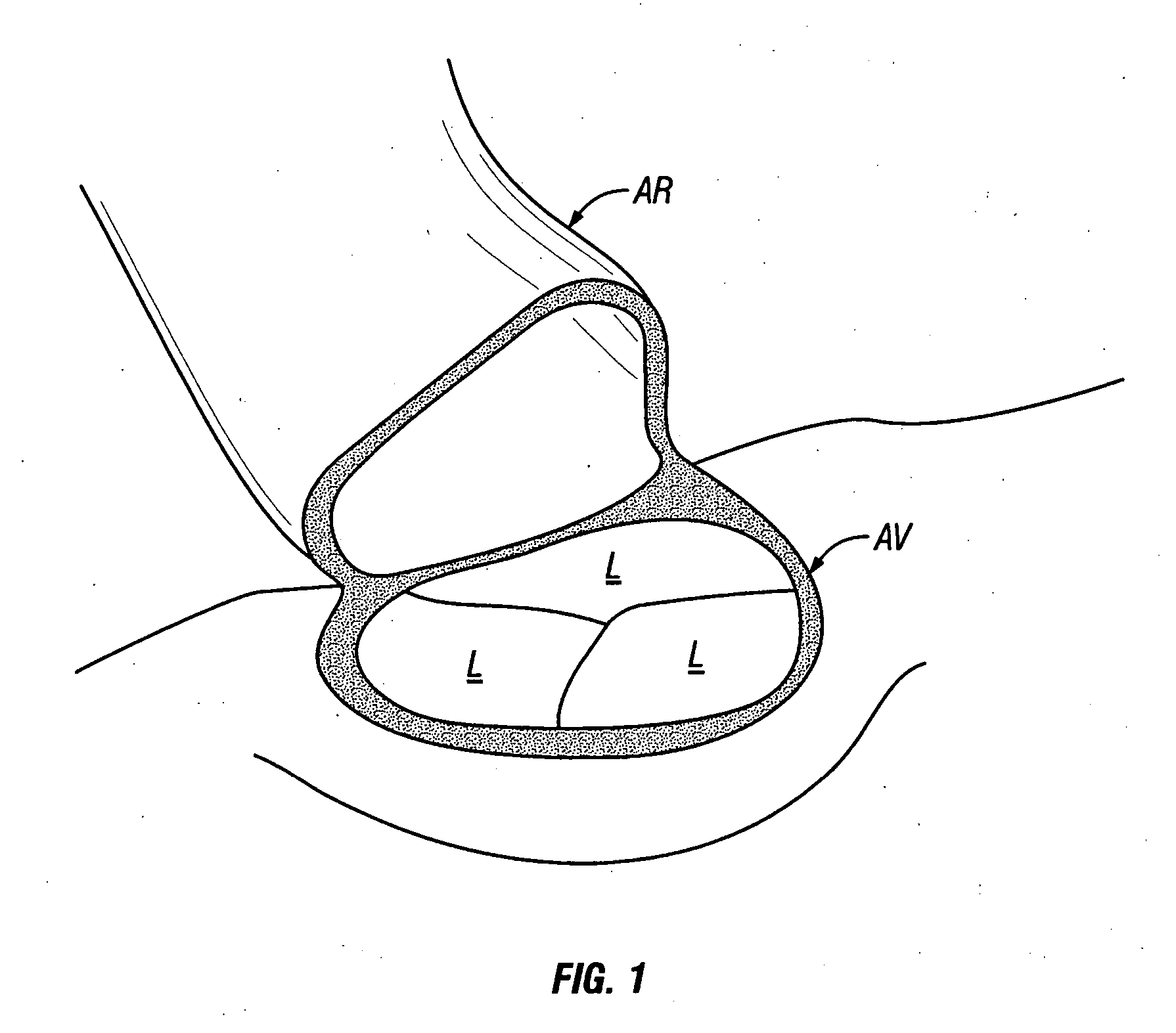
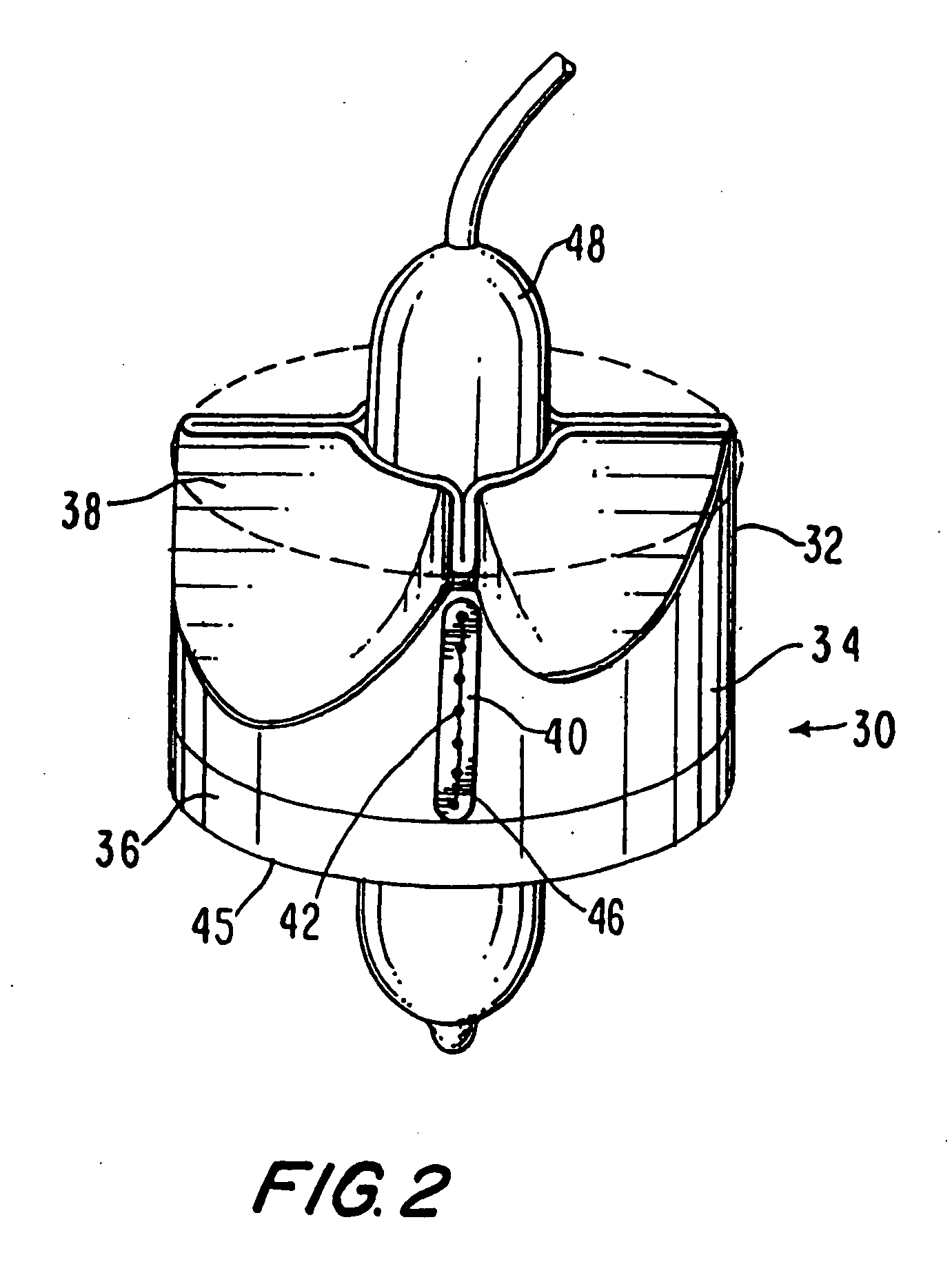
Percutaneous Heart Valve Prosthesis
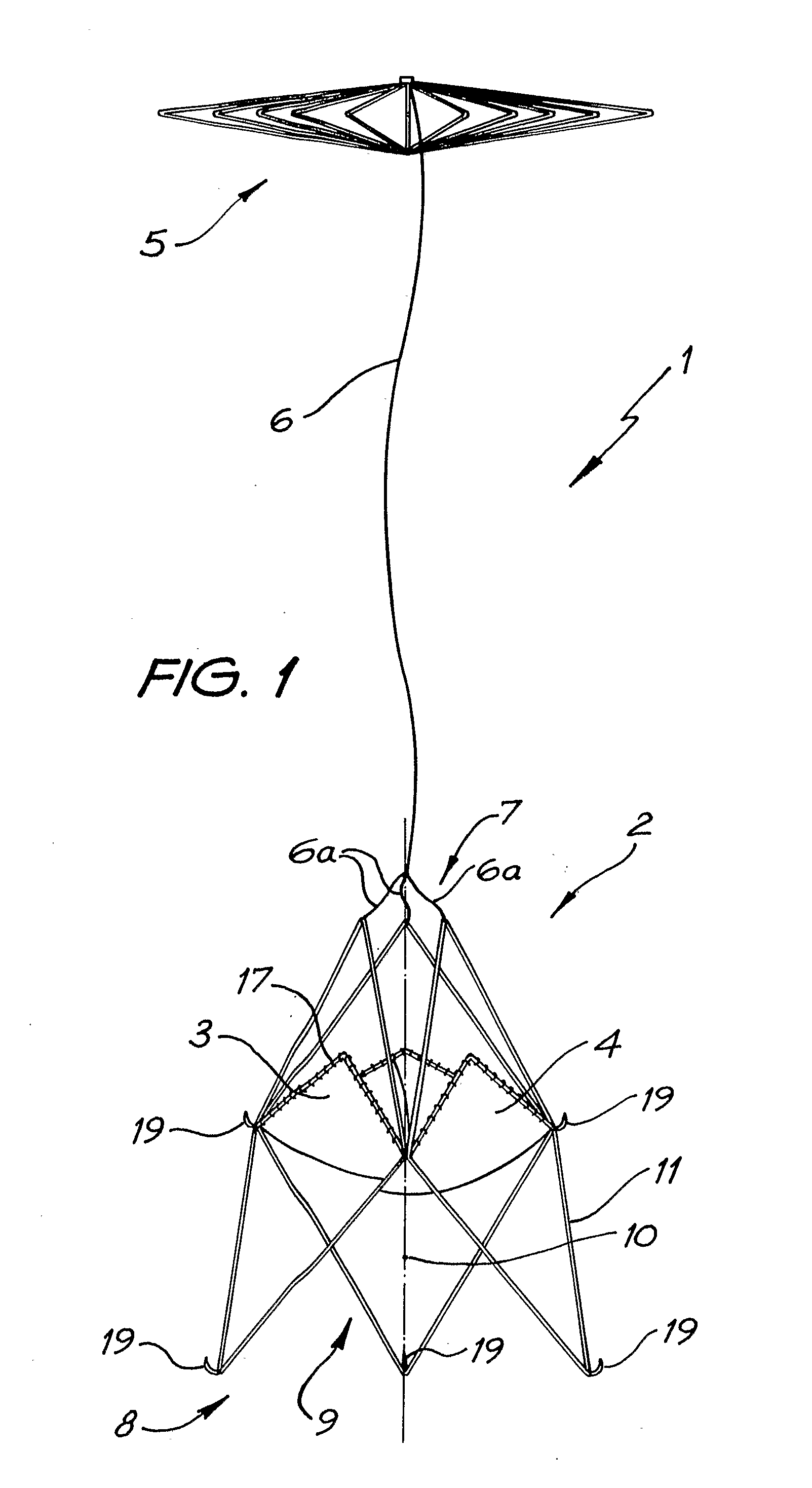
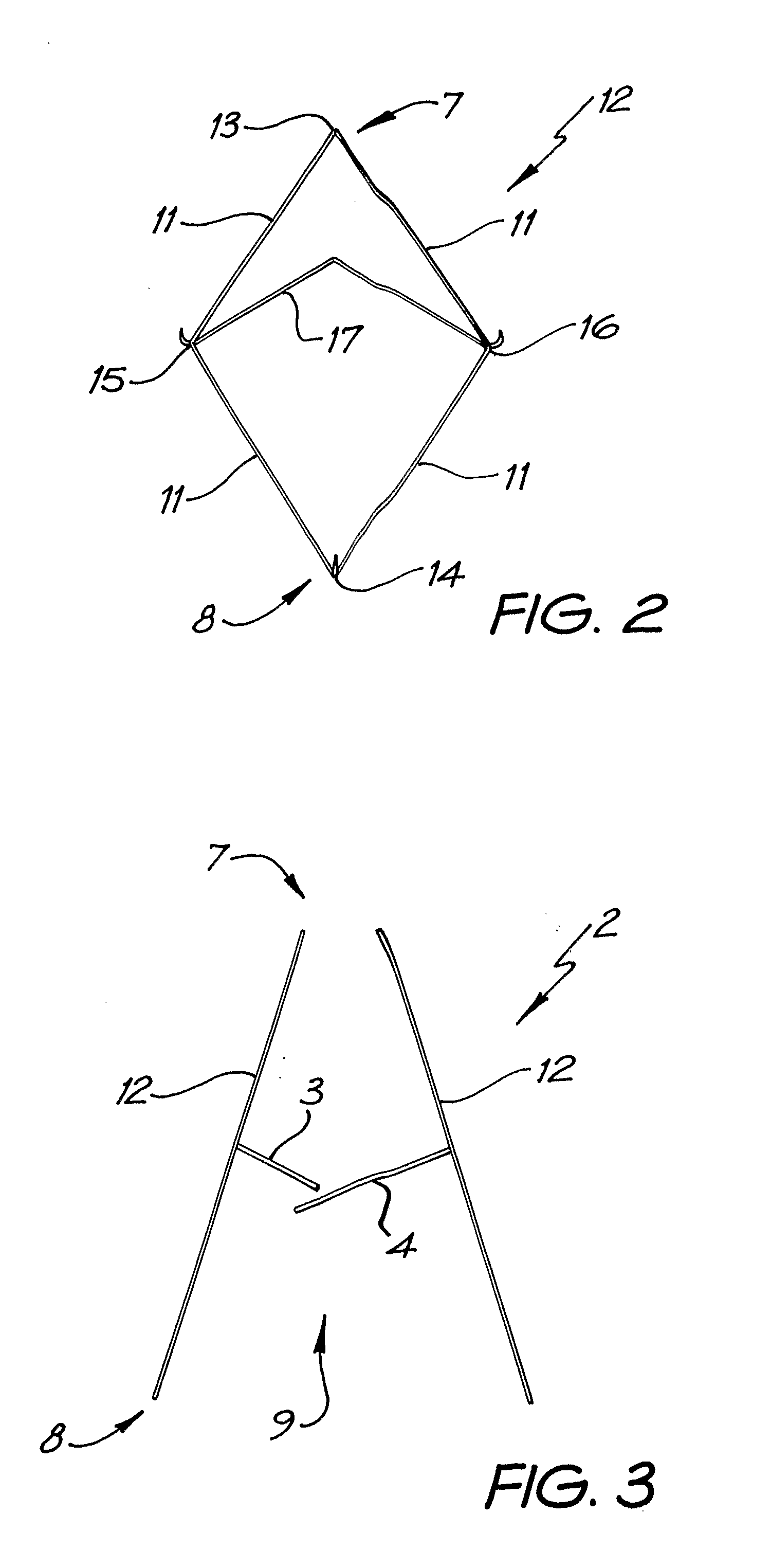
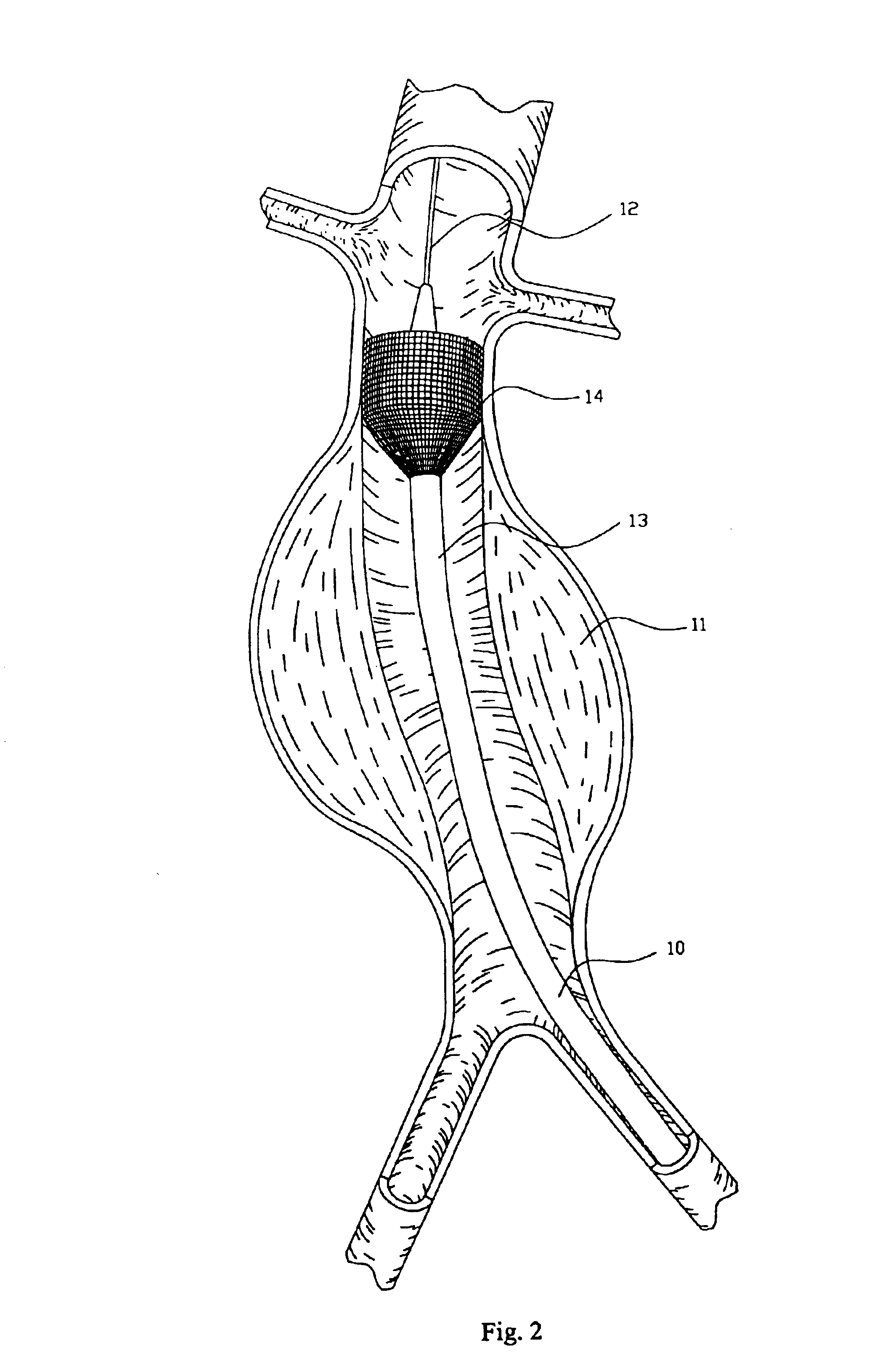
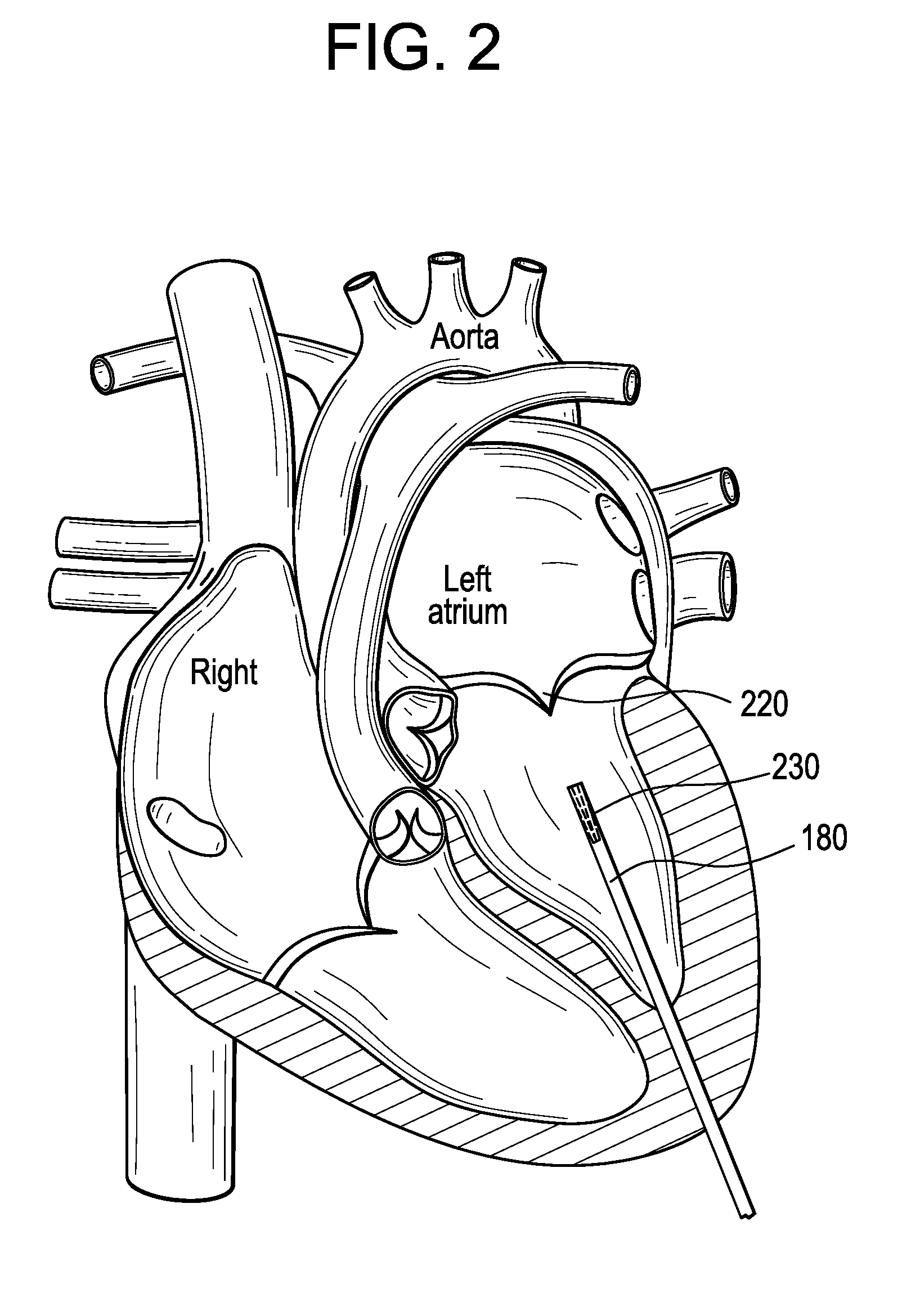
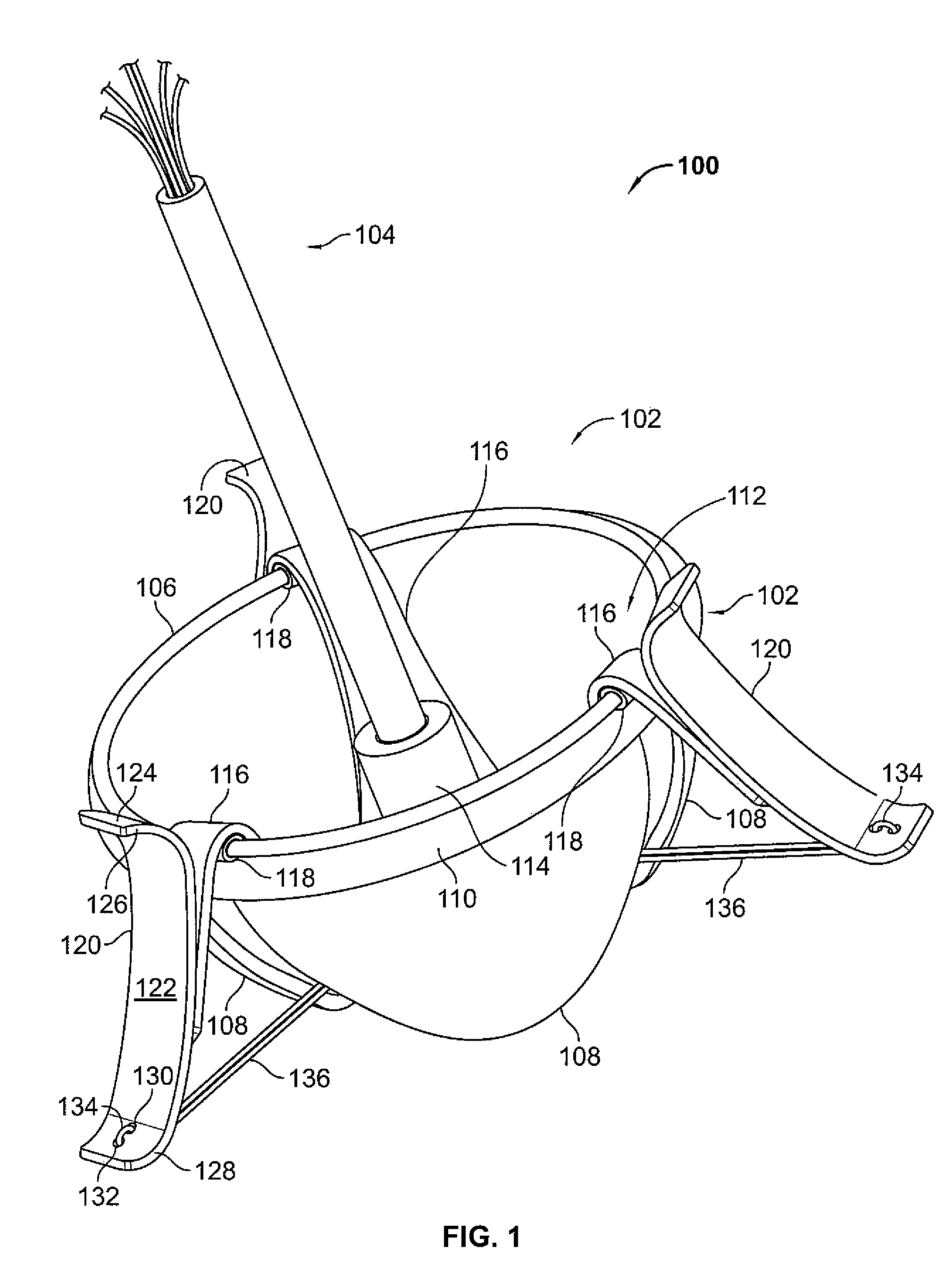
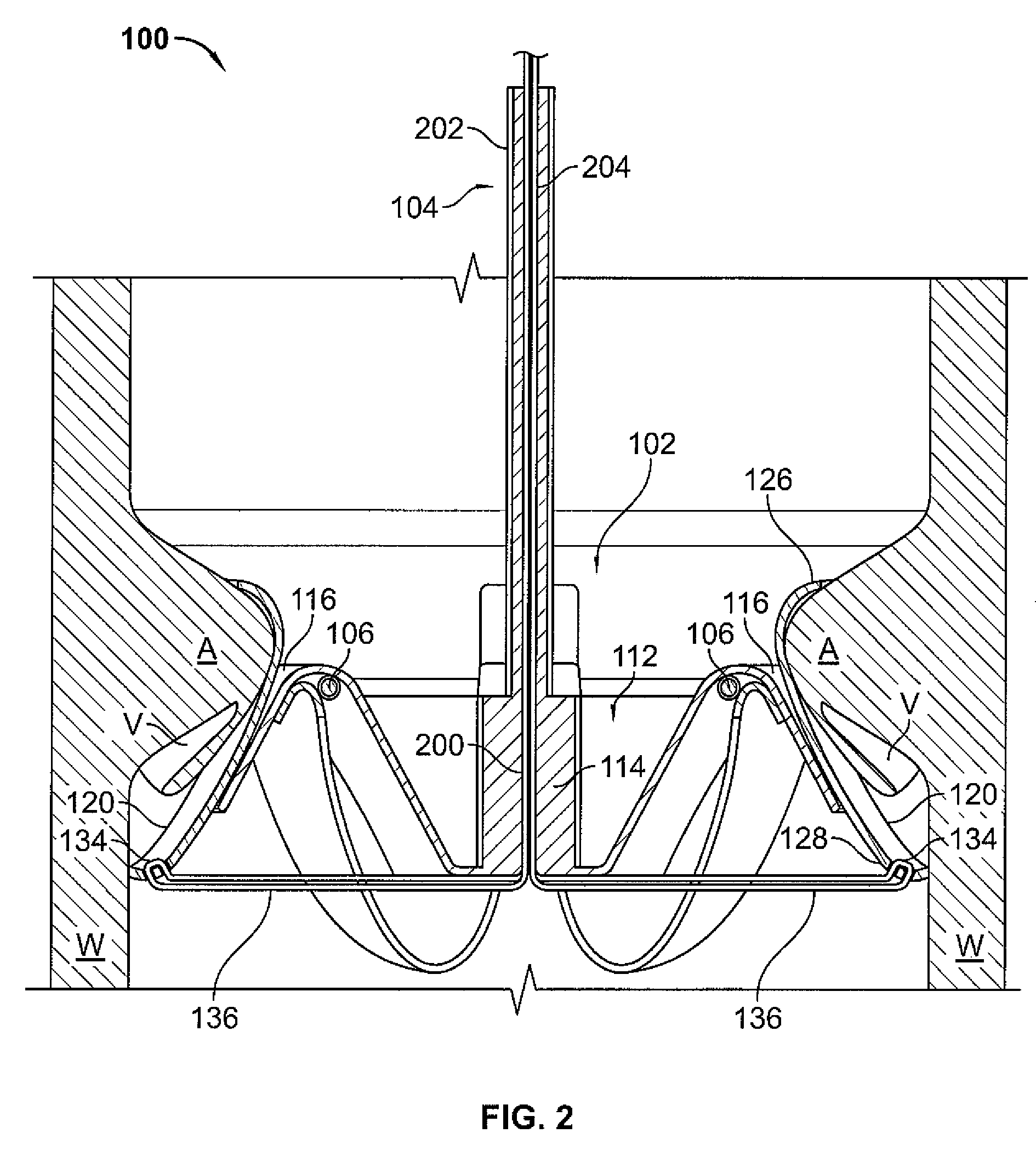
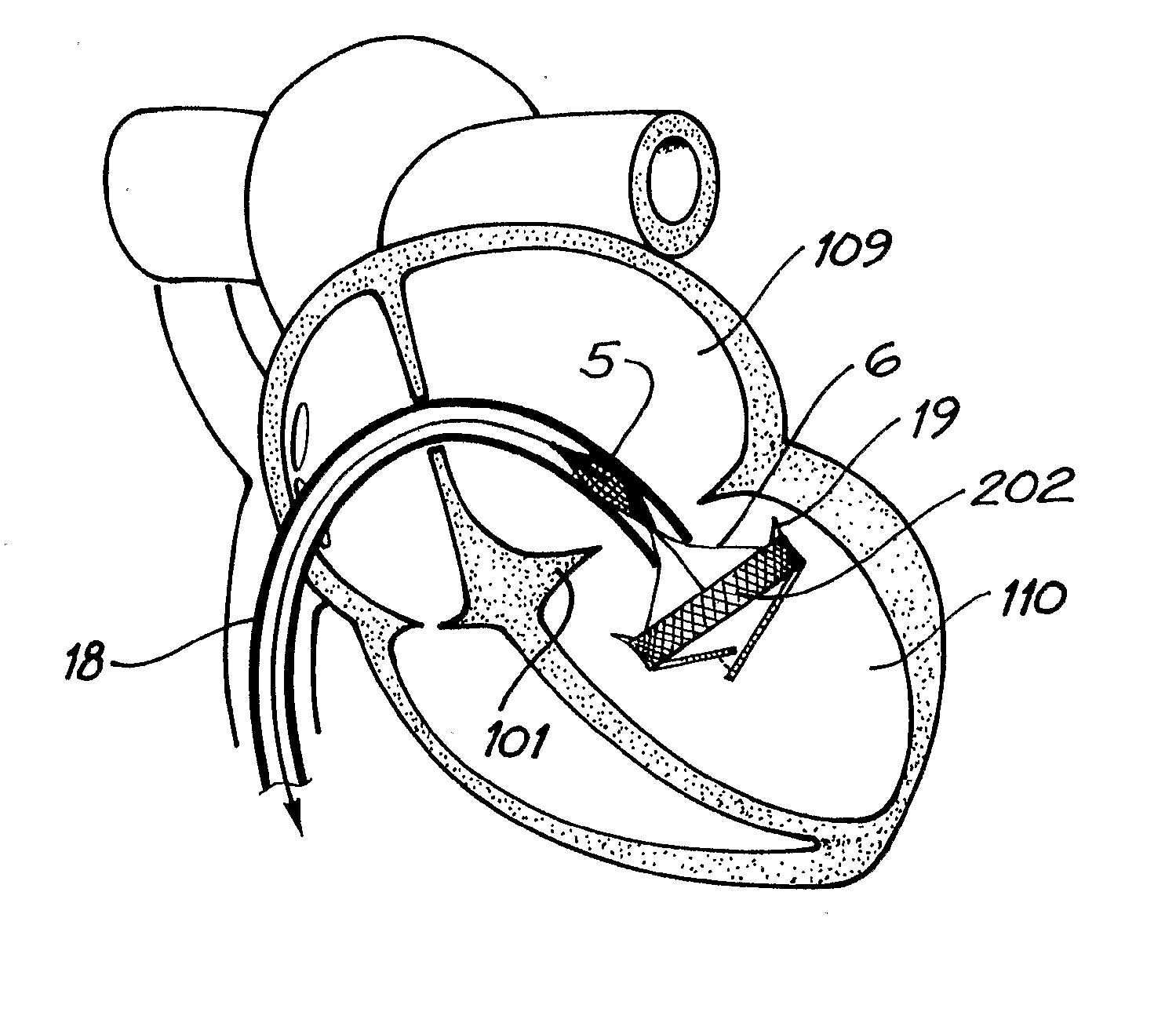
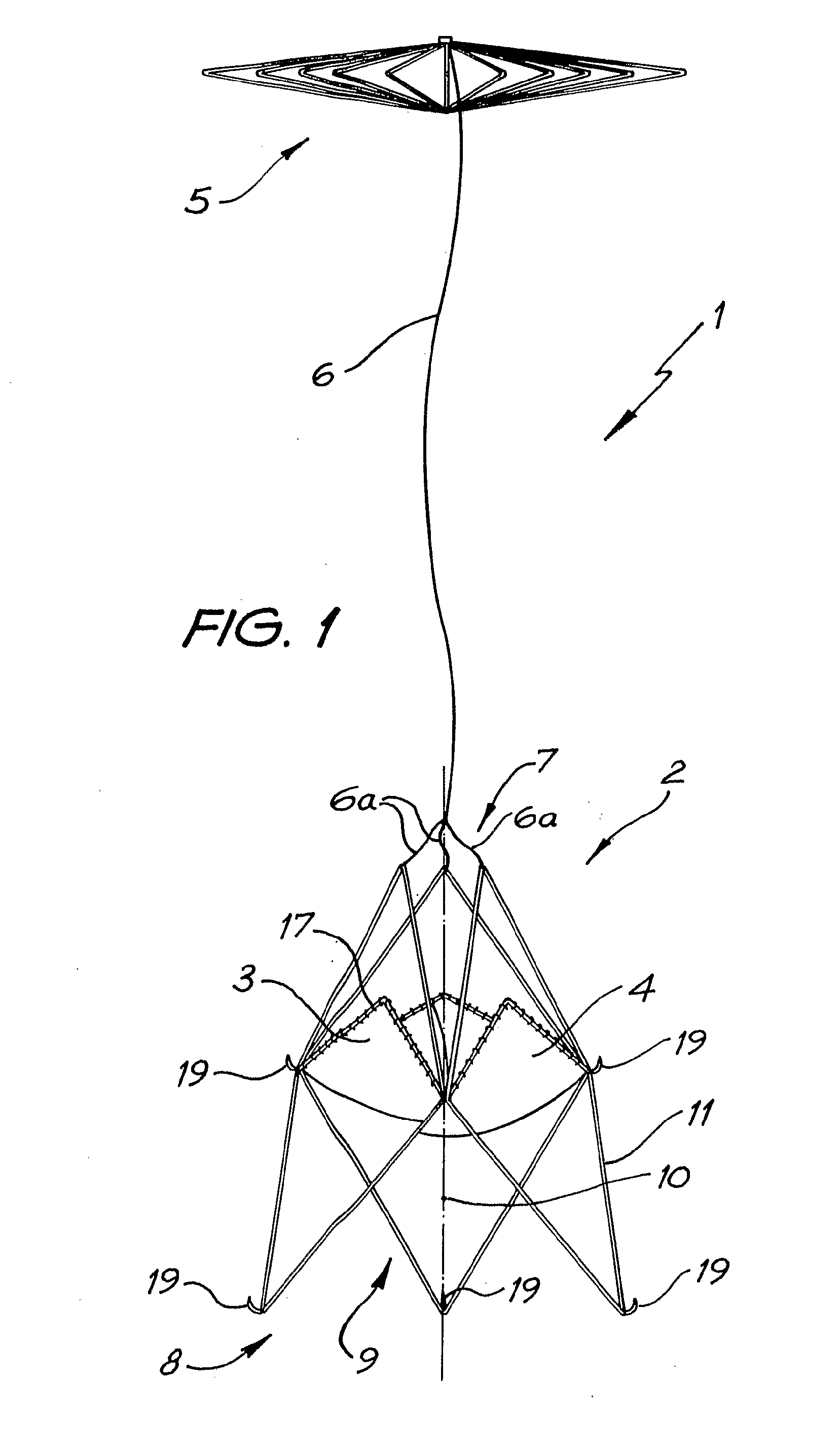
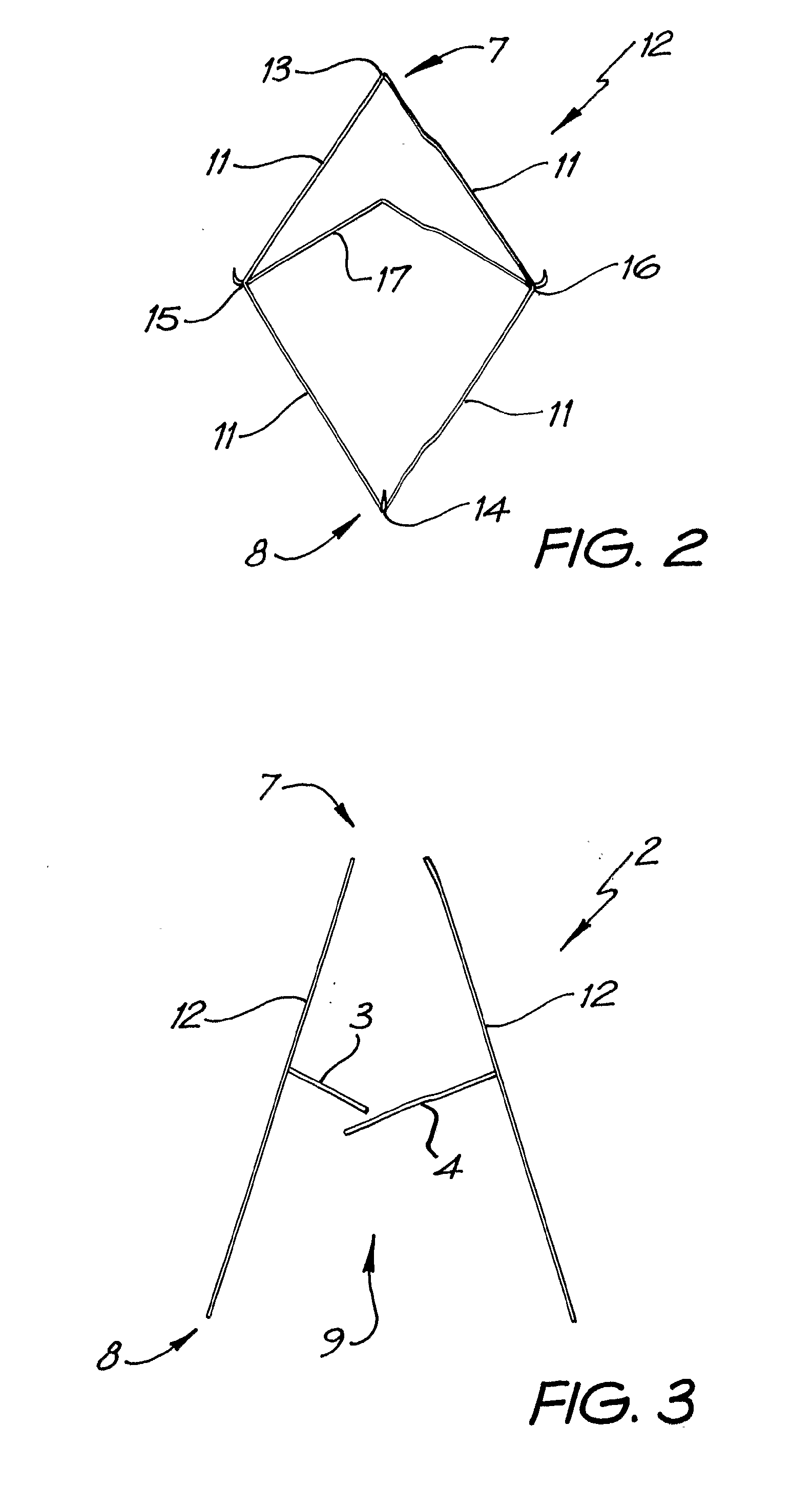
A percutaneous heart valve prosthesis (1) has a valve body (2) with a passage (9) extending between the first and second ends (7, 8) of the valve body (2). The valve body (2) is collapsible about a longitudinal axis (10) of the passage (9) for delivery of the valve body (2) via a catheter (18). One or more flexible valve leaflets (3, 4) are secured to the valve body (2) and extend across the passage (9) for blocking bloodflow in one direction through the passage (9). An anchor device (5), which is also collapsible for delivery via catheter (18), is secured to the valve body (2) by way of an anchor line (6). A failed or failing mitral heart valve (101) is treated by percutaneously locating the valve body (2) in the mitral valve orifice (102) with the anchor device (5) located in the right atrium (107) and engaging the inter-atrial septum (103), such that the taught anchor line (6) acts to secure the valve body (2) within the mitral valve orifice (102).
Owner:PERCUTANEOUS CARDIOVASCULAR SOLUTIONS
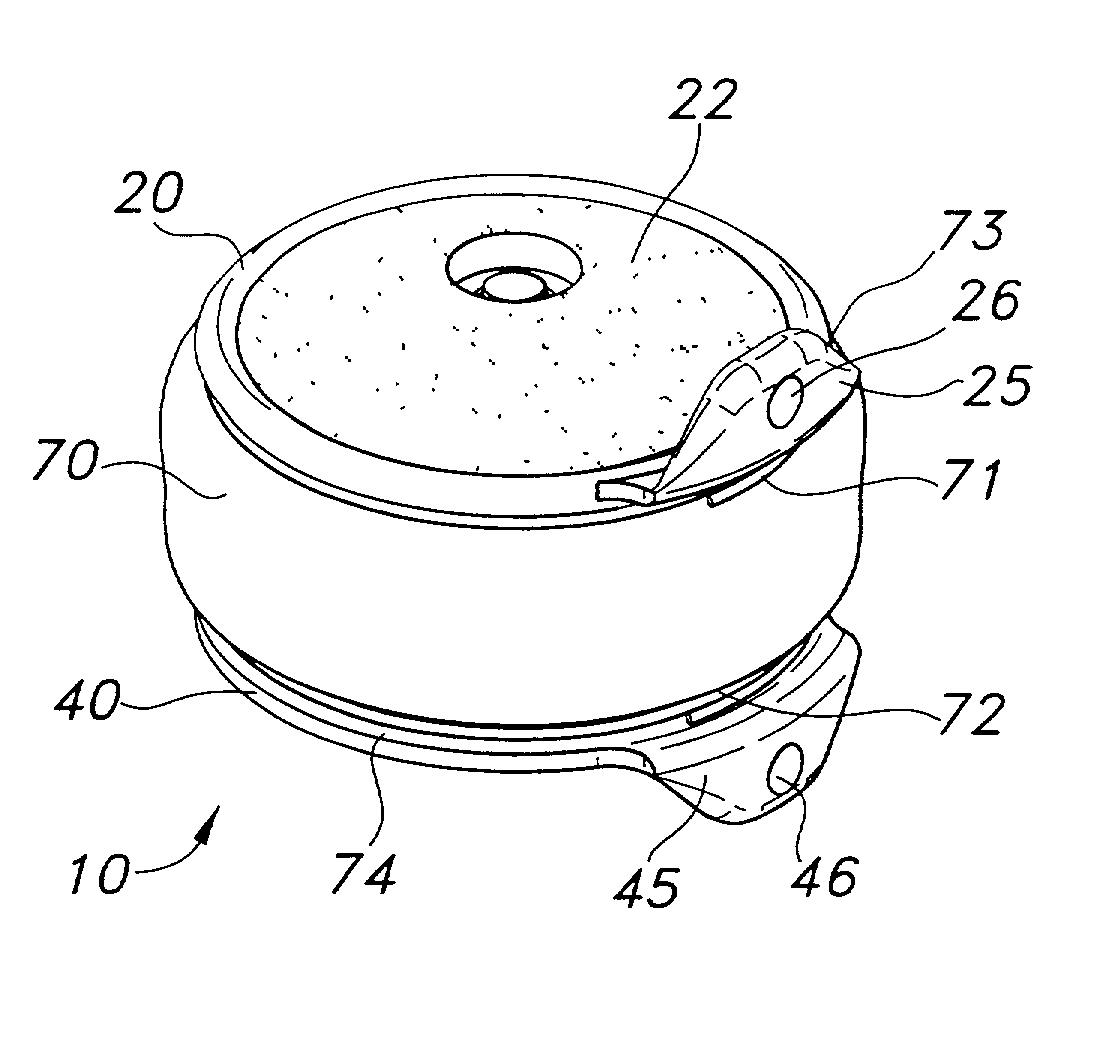
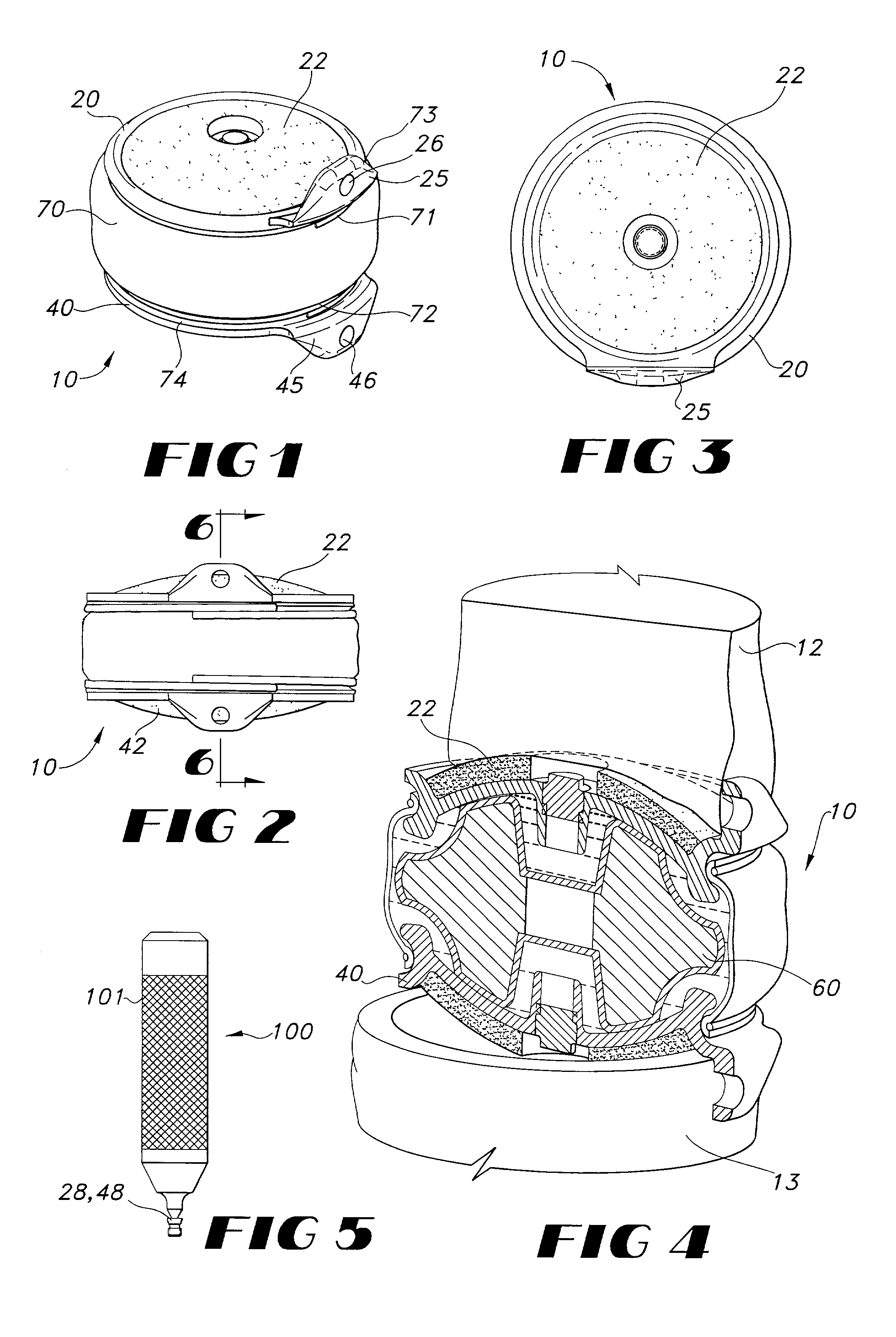
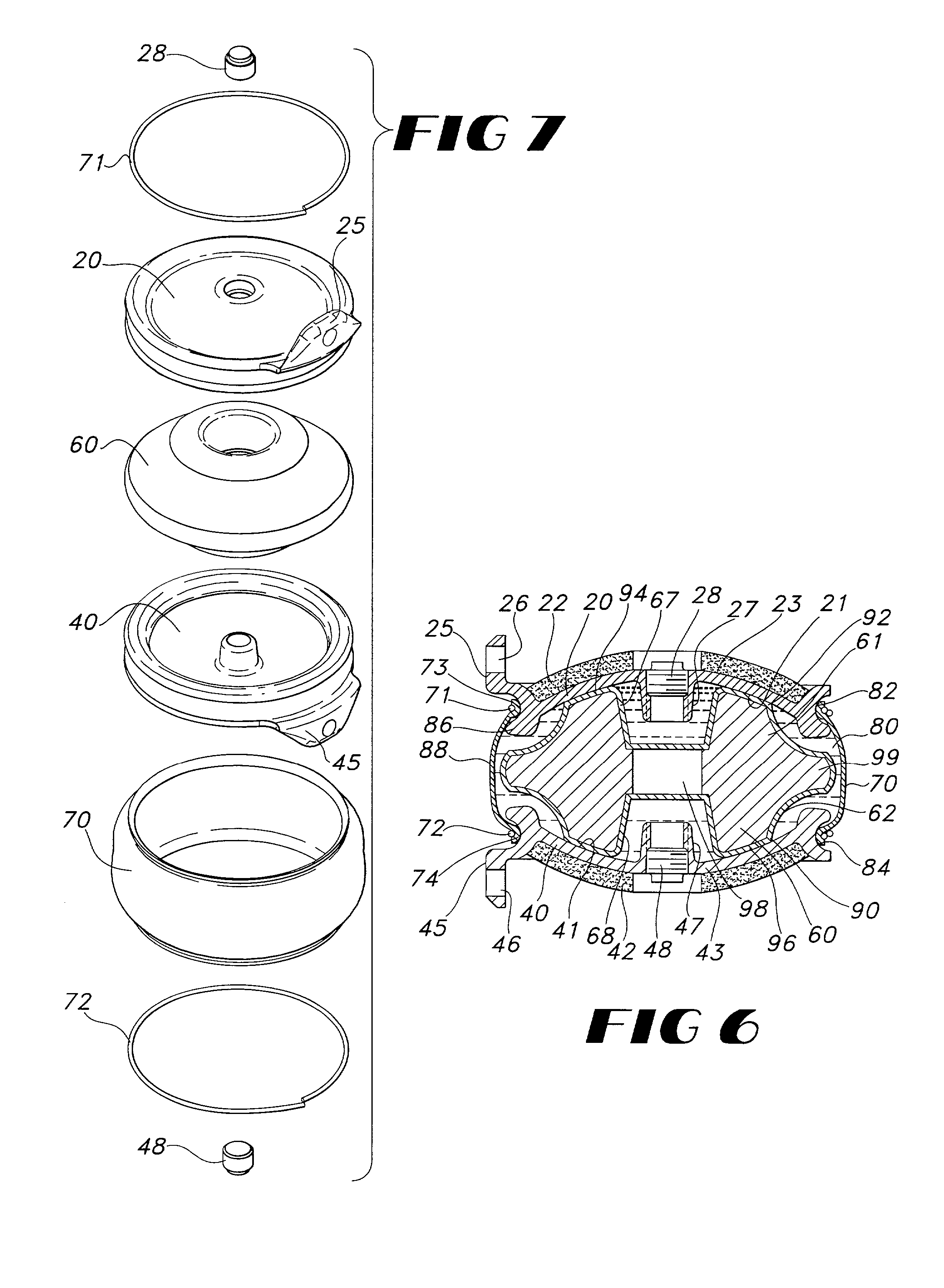
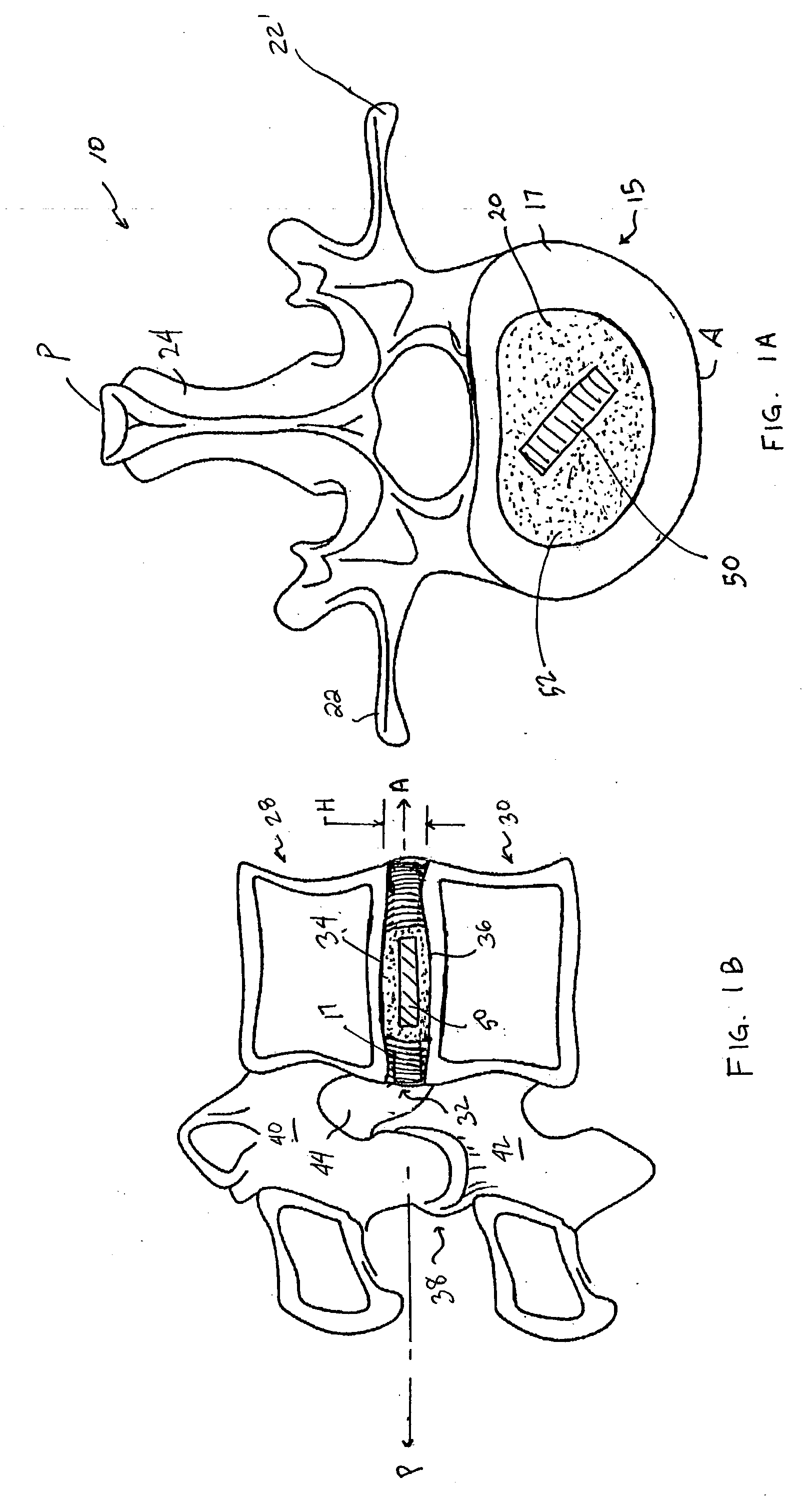
Intervertebral disc implant
InactiveUS6187048B1Speed up the flowReduce and eliminate any adverse effectJoint implantsSpinal implantsIntervertebral discProsthesis
An implant for forming an intervertebral disc nucleus pulposus prosthesis includes a conformable material adapted to fill cavities within the disc and to at least partially polymerize in-situ to form a shaped, resiliently deformable prosthesis.
Owner:HOWMEDICA OSTEONICS CORP
Implantable joint prosthesis
InactiveUS20020035400A1Improve wear resistanceImprove tribological propertiesDiagnosticsJoint implantsRange of motionIntervertebral disc
The invention relates to a surgical implant that provides an artificial diarthroidal-like joint, suitable for use in replacing any joint, but particularly suitable for use as an intervertebral disc endoprosthesis. The invention contains two rigid opposing shells, each having an outer surface adapted to engage the surfaces of the bones of a joint in such a way that the shells are immobilized by friction between their outer surfaces and the surfaces of the bone. These outer surfaces are sufficiently rough that large frictional forces strongly resist any slippage between the outer surface and the bone surfaces in the joint. They may be convex, and when inserted into a milled concavity, are immediately mechanically stable. Desirably, the outer surfaces of the shells are adapted to allow for bony ingrowth, which further stabilizes the shells in place. The inner surfaces of the shells are relatively smooth, and adapted to slide easily across a portion of the outer surface of a central body disposed between the shells. The central body has a shape that cooperates with the shape of the inner surface of the shell so as to provide a range of motion similar to that provided by a healthy joint. A flexible sheath extends between edges of the opposing shells. The inner surface of this sheath, together with the inner surfaces of the rigid shells, defines a cavity encasing the central body. At least a portion of this cavity is filled with a fluid lubricant, further decreasing the frictional force between inner surfaces of the shell and the surface of the central body.
Owner:SPINAL DYNAMICS CORP
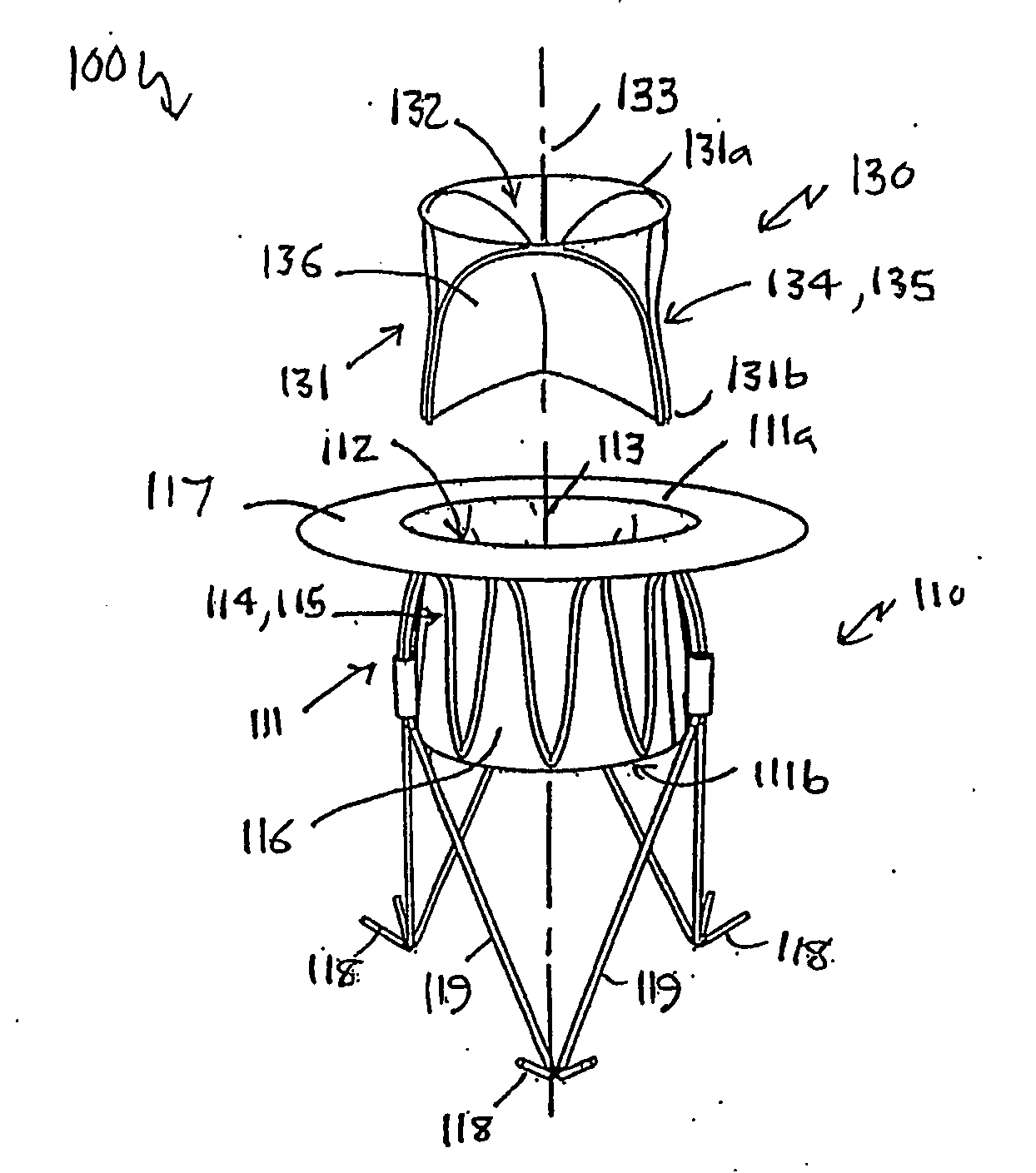
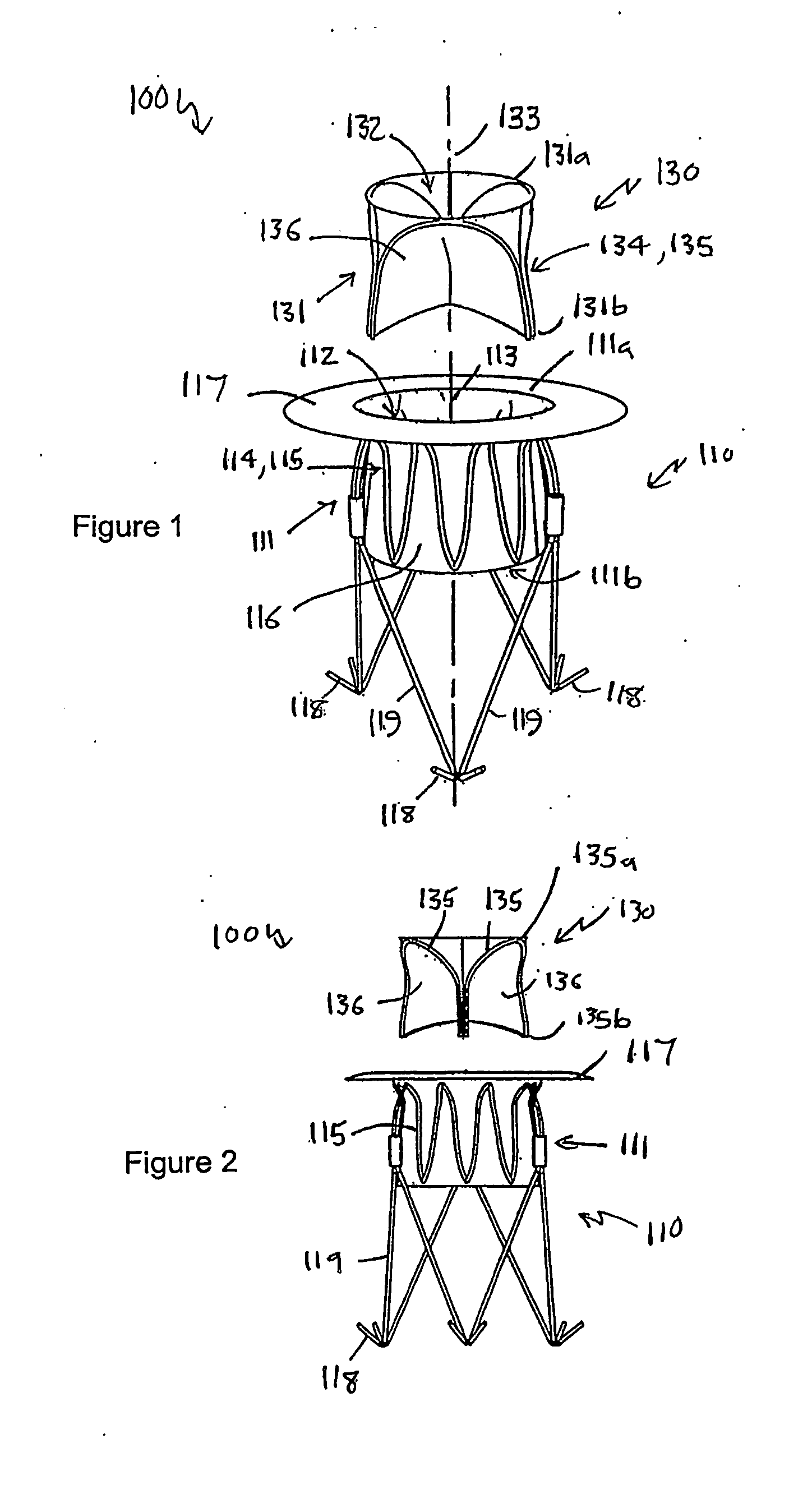
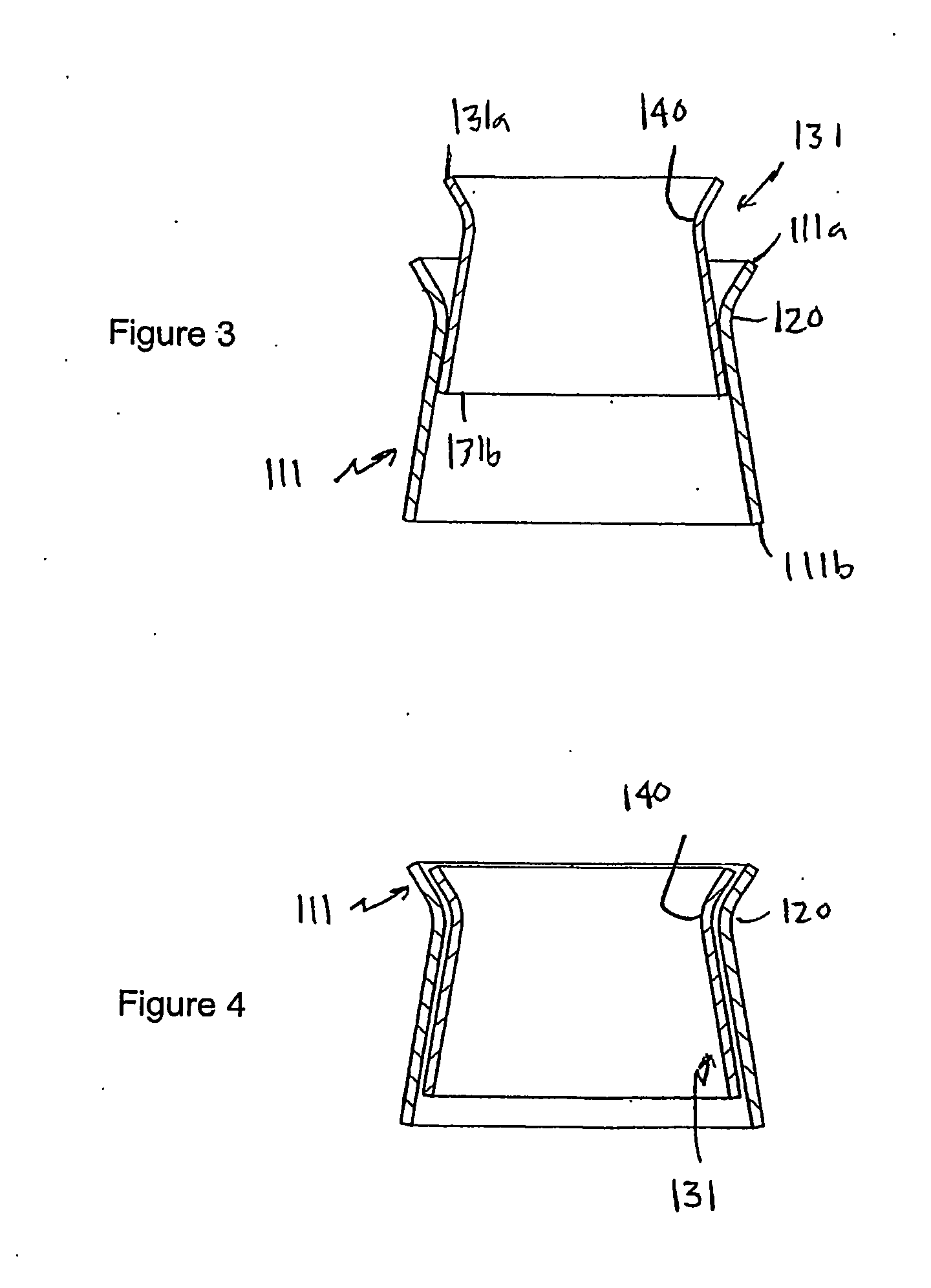
Heart valve prosthesis and method
A heart valve prosthesis (100) comprises a housing component (110) and a valve component (130). The housing component (110) comprises a housing body (111) having a housing passage (112) extending therethrough. The housing body (111) is configured to be located in, or adjacent to and communicating with, a native valve orifice (16) of a heart (10) and to engage structure of the heart (10) to fix the housing body (111) in relation to the valve orifice (161). The housing component (111) is collapsible for delivery via catheter (2). The valve component (130) comprises a valve body (131) having a valve passage (132) extending therethrough. The valve body (131) is configured to be fixed within the housing passage (112) with the valve passage (132) extending along the housing passage (112). One or more flexible valve elements (131) is / are secured to the valve body and extend across the valve passage (132) for blocking blood flow in a first direction through the valve passage (132) whilst allowing blood flow in the opposing direction. The valve component (130) is collapsible for delivery via catheter (2) separate to the housing component (110). An associated method of replacing a failed or failing heart valve utilising the heart valve prosthesis (100) is also disclosed.
Owner:PERCUTANEOUS CARDIOVASCULAR SOLUTIONS
Implantable joint prosthesis
ActiveUS20020128715A1Increased durabilityImprove stabilityDiagnosticsJoint implantsIntervertebral discSurgical implant
The invention relates to a surgical implant that provides an artificial diarthroidal-like joint, suitable for use in replacing any joint, but particularly suitable for use as an intervertebral disc endoprosthesis. The invention contains two rigid opposing shells, each having an outer surface adapted to engage the surfaces of the bones of a joint in such a way that the shells are immobilized by friction between their outer surfaces and the surfaces of the bone. These outer surfaces are sufficiently rough that large frictional forces strongly resist any slippage between the outer surface and the bone surfaces in the joint. They may be convex, and when inserted into a milled concavity, are immediately mechanically stable. Desirably, the outer surfaces of the shells are adapted to allow for bony ingrowth, which further stabilizes the shells in place. The inner surfaces of the shells are relatively smooth, and adapted to slide easily across a portion of the outer surface of a central body disposed between the shells. The central body has a shape that cooperates with the shape of the inner surface of the shell so as to provide a range of motion similar to that provided by a healthy joint. A flexible sheath extends between edges of the opposing shells. The inner surface of this sheath, together with the inner surfaces of the rigid shells, defines a cavity encasing the central body. At least a portion of this cavity is filled with a fluid lubricant, further decreasing the frictional force between inner surfaces of the shell and the surface of the central body.
Owner:COMPANION SPINE LLC
Prostheses, systems and methods for replacement of natural facet joints with artificial facet joint surfaces
InactiveUS6974478B2Desired range of mobilityLessen and alleviate spinal painSuture equipmentsInternal osteosythesisArticular surfacesSpinal column
Cephalad and caudal vertebral facet joint prostheses and methods of use are provided. The prostheses provide an artificial facet joint structure including an artificial articular configuration unlike the preexisting articular configuration. The radii and material stress values of the prostheses are configured to sustain contact stress. The cephalad prosthesis provides for posterior-anterior adjustment. Both prostheses permit lateral adjustment and adjustment to accomodate interpedicle distance. Further, the prostheses may be customized to provide a pre-defined lordotic angle and a pre-defined pedicle entry angle.
Owner:GLOBUS MEDICAL INC
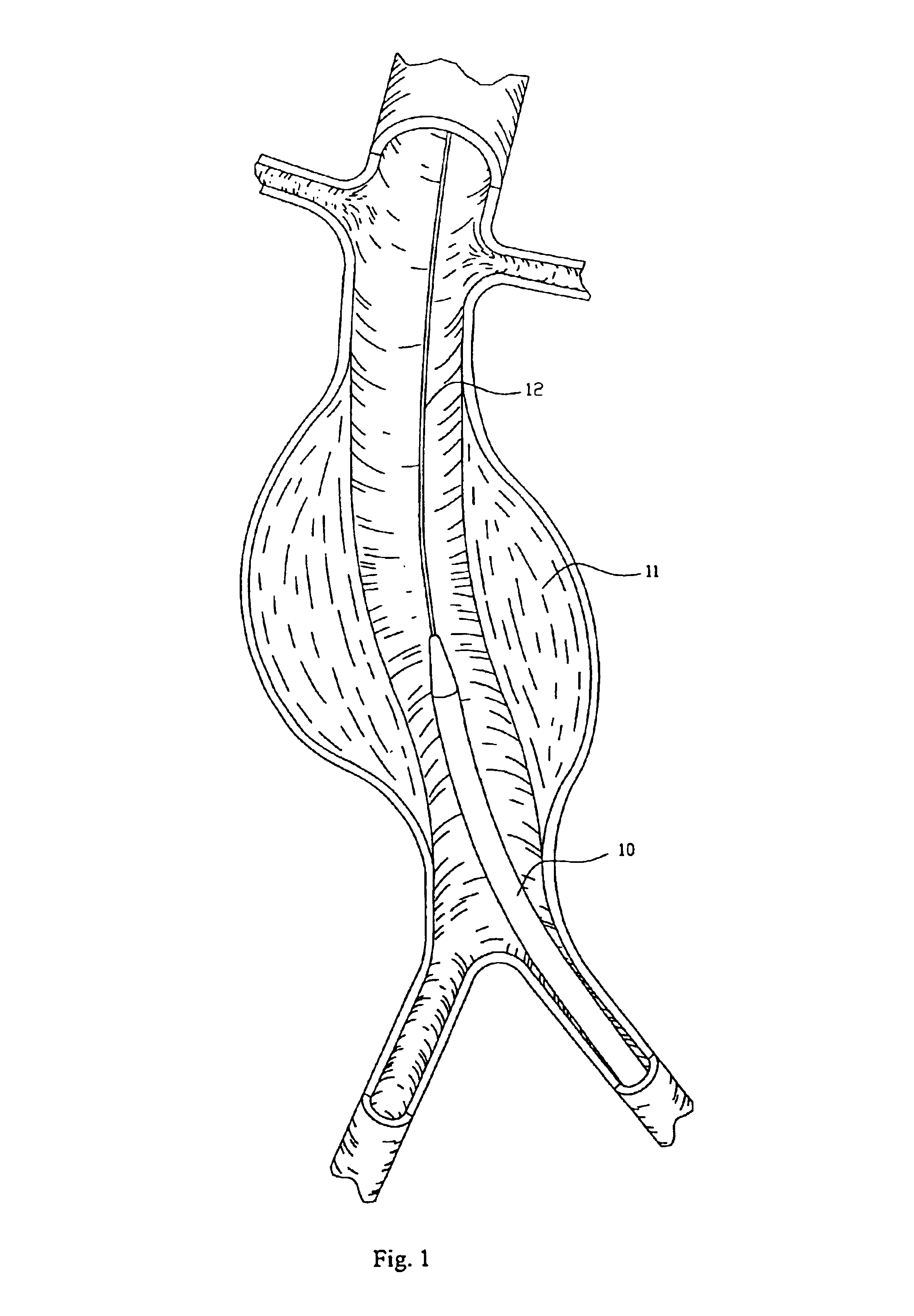
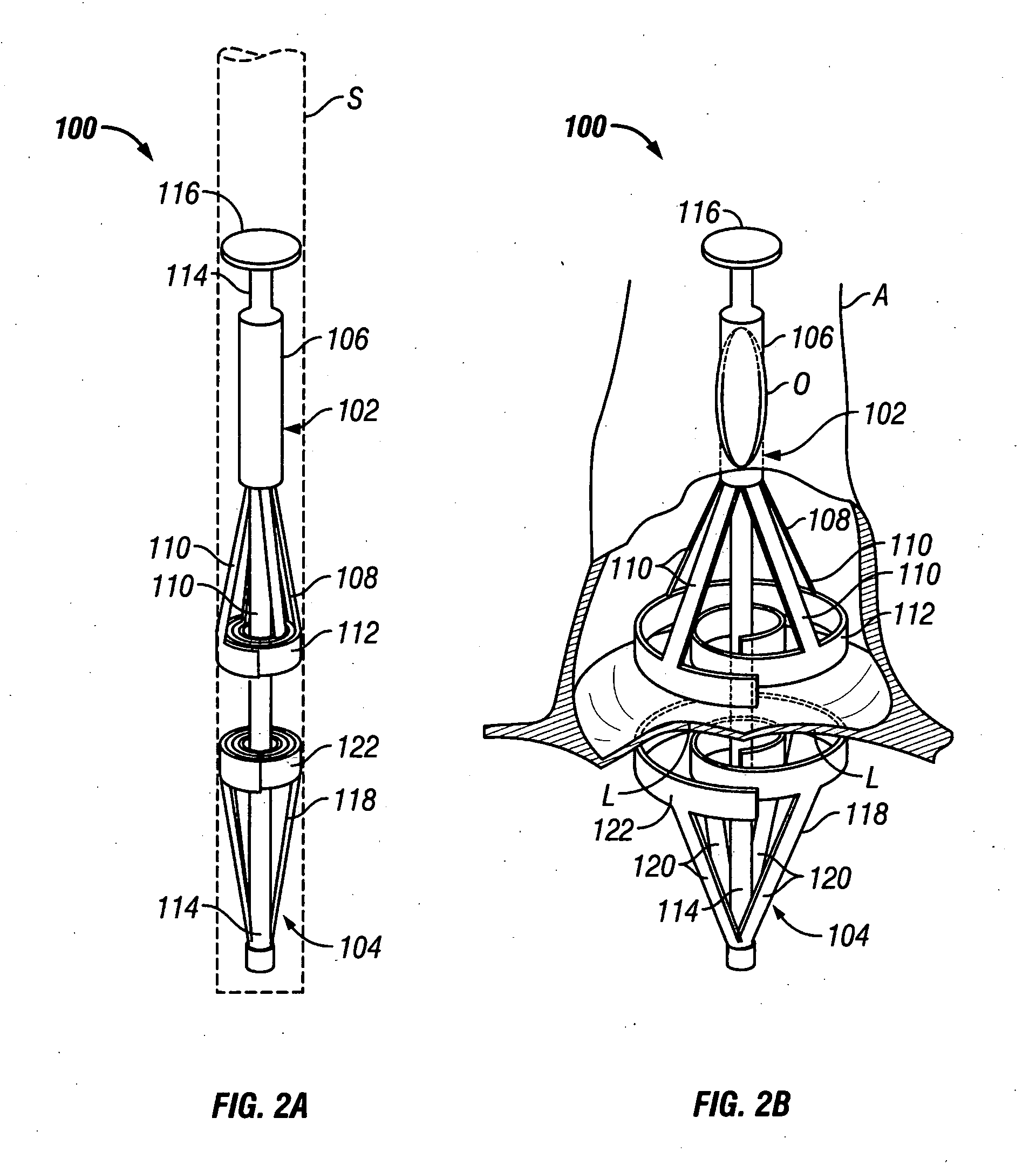
Endovascular aneurysm repair system
InactiveUS6960217B2Low profileEffective and less traumaticStentsEar treatmentEndovascular aneurysm repairBiomedical engineering
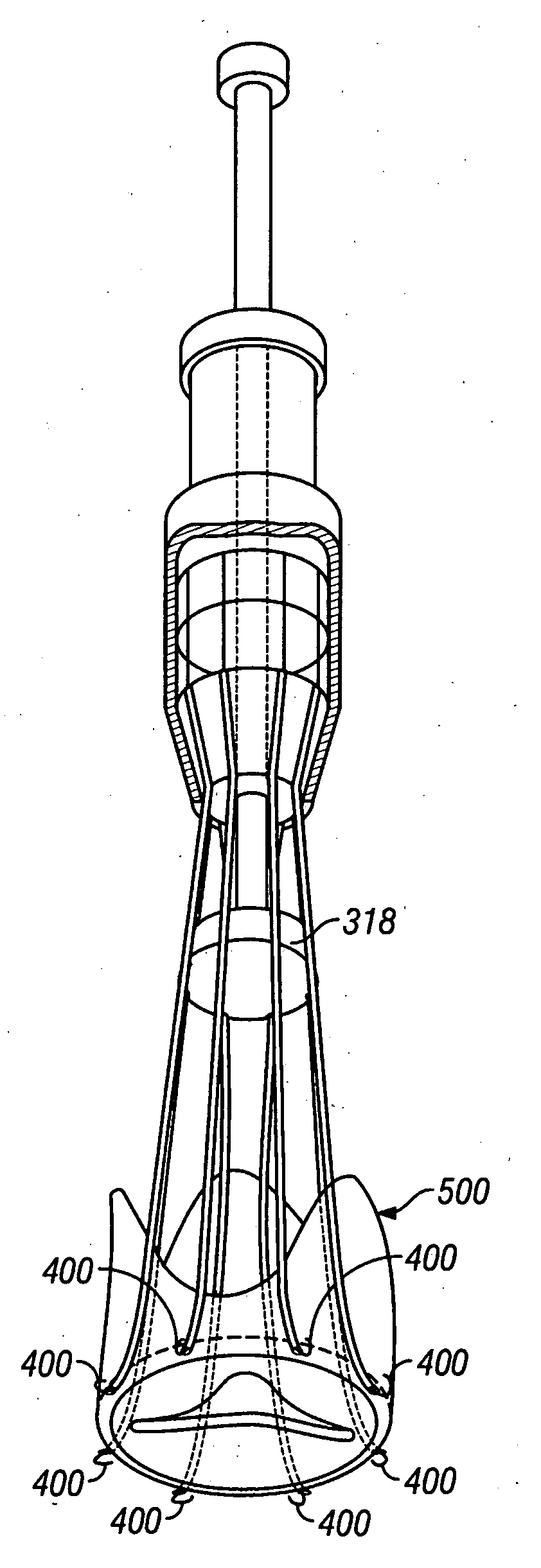
Method and apparatus for implanting radially expandable prostheses in the body lumens rely on tacking or anchoring of the prostheses with separately introduced fasteners. The prostheses may be self-expanding or balloon expandable. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners at at least one prosthesis end, usually as each end of the prosthesis. The fasteners are usually helical fasteners which are delivered from a helical track in the fastener applier by rotation with a rotator wire. The fasteners will be applied singly, typically in circumferentially spaced-apart patterns about the interior of each end of the prosthesis.
Owner:MEDTRONIC VASCULAR INC
Multi-component designs for heart valve retrieval device, sealing structures and stent assembly
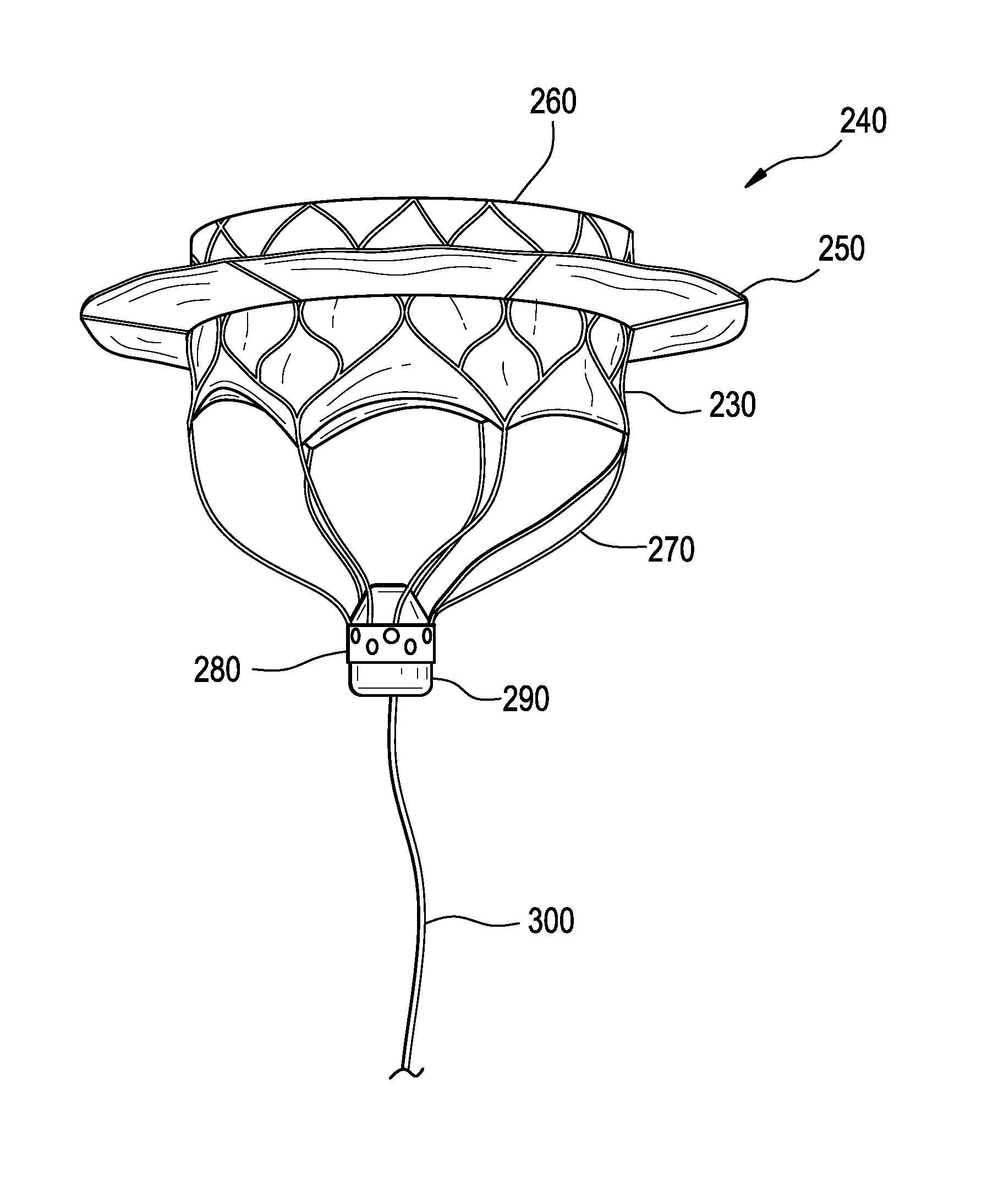
ActiveUS20150142103A1Prevent perivalvular leakEasy to take backStentsHeart valvesValve prosthesisVALVE PORT
This invention relates to the design and function of a device which allows for retrieval of a previously implanted valve prosthesis from a beating heart without extracorporeal circulation using a transcatheter retrieval system, including a guide component to facilitate the compression of the valve and retraction into a retrieval catheter, as well as an improved prosthetic transcatheter heart valve having one or more of: a series of radially extending tines having a loop terminus to improve sealing a deployed prosthetic mitral valve against hemodynamic leaking, a pre-compressible stent-in-stent design, or an articulating cuff attached to a covered stent-valve and a commissural sealing skirt structure attached to the underside of the articulating cuff.
Owner:TENDYNE HLDG
Cutting guide apparatus and surgical method for use in knee arthroplasty
InactiveUS7104997B2Avoiding minimizing errorPrecise alignmentSurgical sawsProsthesisSurgical approachSurgical incision
Novel cutting guides and surgical methods for use in knee arthroplasty are described. Embodiments of the inventive cutting guide apparatus include fixed and adjustable cutting guide blocks having a series of slots designed to accommodate a cutting saw. The cutting guides and surgical method are designed to allow for the provision of all desired surgical cuts upon the distal end of the femur, for subsequent implantation of a prosthesis thereto, without having to remove the cutting guide block.
Owner:LIONBERGER DAVID +1
Deployment system for intraluminal devices
ActiveUS20060015171A1Short working lengthRisk minimizationStentsDiagnosticsProsthesisBiomedical engineering
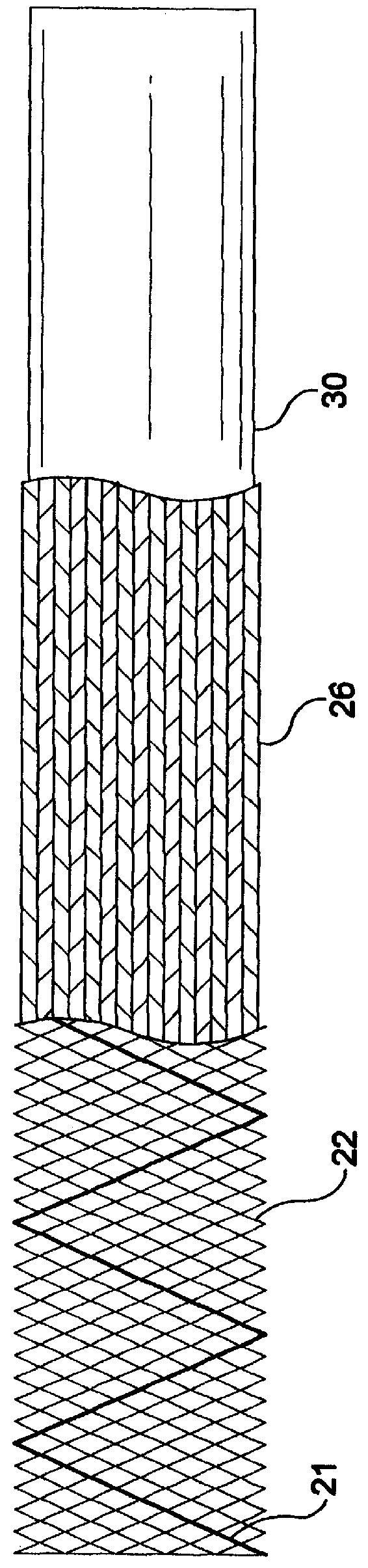
A constraining sheath for use around an endoprosthesis (e.g., a stent device, with or without a graft covering), which may be a balloon expandable endoprosthesis but more preferably is a self-expanding prosthesis. The endoprosthesis is coaxially enclosed within and substantially covered by the constraining sheath, which is an outer, removable tubular sheath, preferably made of ePTFE. The sheath is preferably corrugated circumferentially along at least a portion of the length of the endoprosthesis. The constraining sheath and endoprosthesis are preferably mounted together as an assembly at the distal end of a delivery means such as a catheter shaft, for delivery of the endoprosthesis to a desired location within a body conduit such as an artery. The constraining sheath is removed by the application of tension to a tensile member such as a tether to cause sequential pulling out of the corrugations followed by release and deployment of the endoprosthesis. The use of a corrugated constraining sheath in comparison to a non-corrugated sheath results in a more smoothly applied tensile force to effect the endoprosthesis release as well as requiring less maximum force.
Owner:WL GORE & ASSOC INC
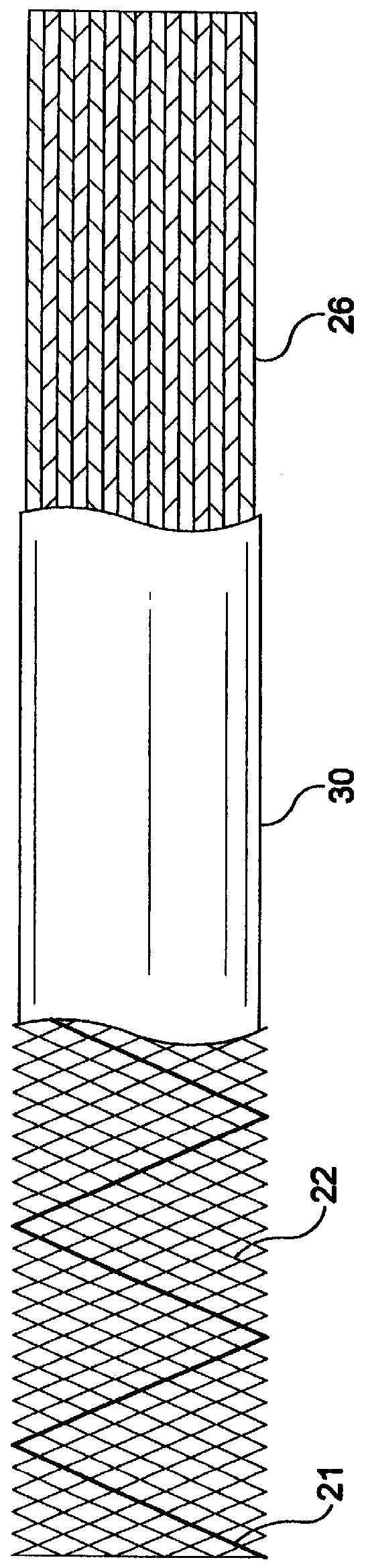
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
Techniques for percutaneous mitral valve replacement and sealing
Apparatus is described for use with a native heart valve of a subject, the apparatus including (1) a prosthetic valve support, comprising an upstream support portion, the upstream support portion having (a) a compressed configuration and an uncompressed configuration in which the upstream support portion has an inner perimeter that defines an opening; and (2) a prosthetic valve, advanceable into the opening defined by the upstream support portion, and intracorporeally couplable to the upstream support portion by being expanded within the opening defined by the upstream support portion, the apparatus being configured such that, when the prosthetic valve is expanded within the opening defined by the upstream support portion, the expansion of the prosthetic valve is restricted by the inner perimeter of the upstream support portion, without causing the prosthetic valve support to apply a radially-expansive force to the native annulus. Other embodiments are also described.
Owner:CARDIOVALVE LTD
Percutaneous valve prosthesis and system and method for implanting same
InactiveUS20090306768A1Minimizes gradientIncrease the areaHeart valvesBlood vesselsInsertion stentBalloon dilatation
A heart valve prosthesis includes a cylindrical valve cage stent constructed to be implanted percutaneously in the planar axis of a native valve annulus, an elastic and compressible, multi-leaflet valve insertable percutaneously into the body, and an attachment mechanism for attaching the valve to the superior rim of the valve cage stent. The valve can be of a bi-leaflet or a tri-leaflet type and includes a valve frame made from a memory metal and a tissue cover attached to the valve frame. The valve cage stent is self-expanding or balloon expandable, made respectively from memory metal or stainless steel but otherwise structurally the same.
Owner:EDWARDS LIFESCI CARDIAQ
Methods and apparatus for valve repair
A valve delivery device is provided. The device comprises a heart valve prosthesis support having a proximal portion and a distal portion; a plurality of fasteners ejectably mounted on the support; a heart valve prosthesis being releasably coupled to said distal portion of said heart valve prosthesis support; and where the heart valve prosthesis and support are configured for delivery to the heart through an aortotomy formed in the patient's aorta. The device may include an anvil movable along a longitudinal axis of the device to engage tissue disposed between the anvil and the valve prosthesis.
Owner:REALYVASQUEZ FIDEL
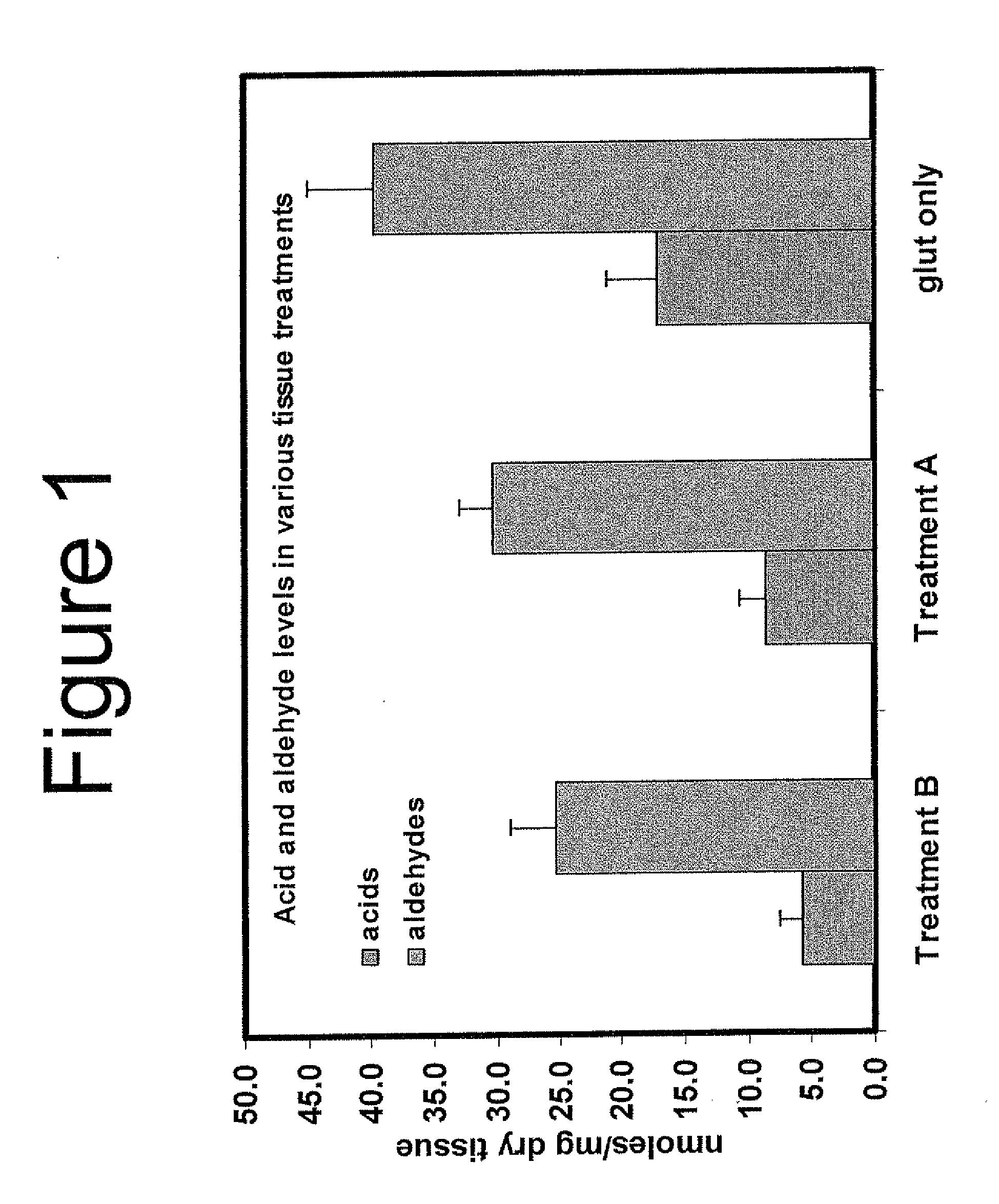
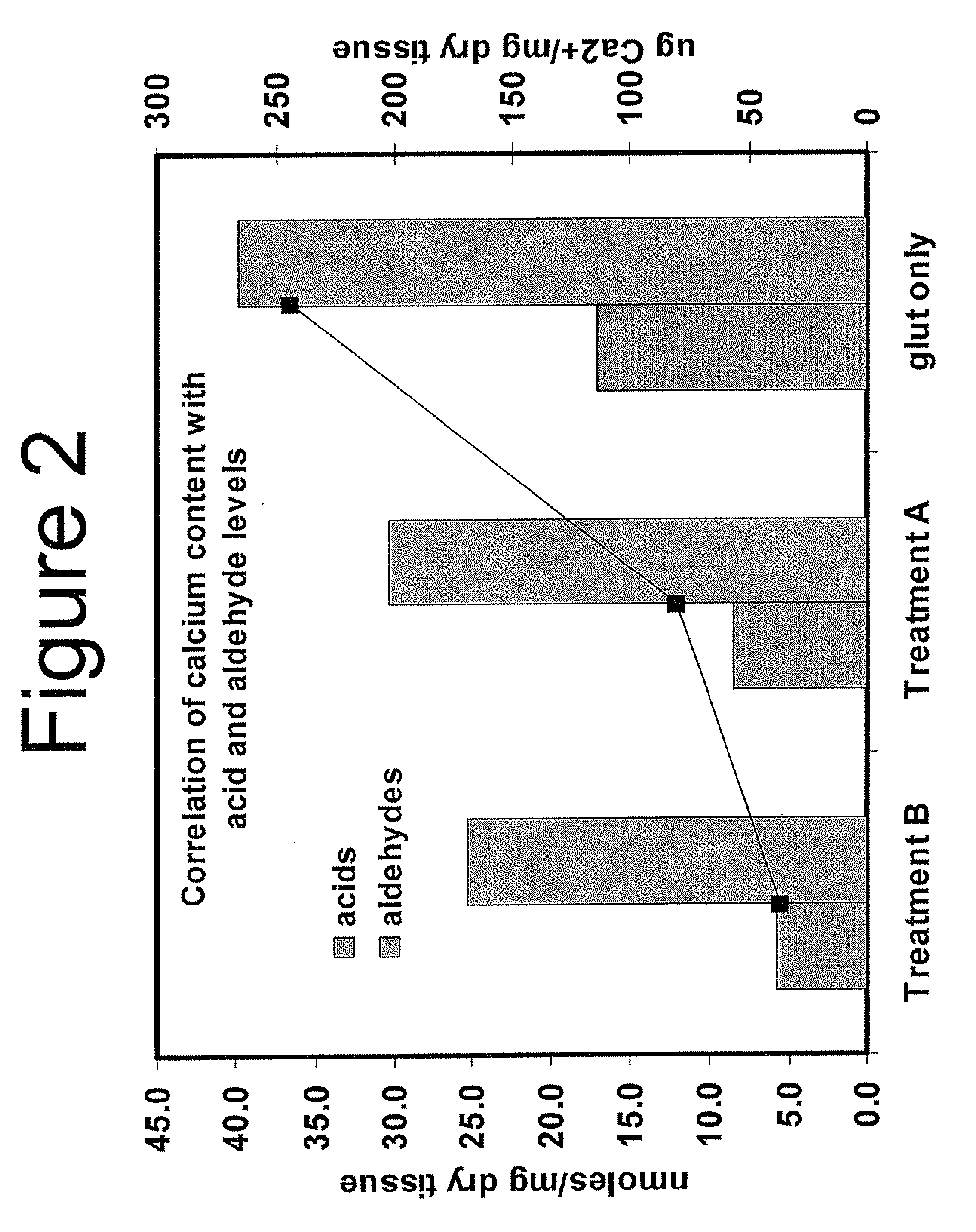
Capping Bioprosthetic Tissue to Reduce Calcification
A treatment for bioprosthetic tissue used in implants or for assembled bioprosthetic heart valves to reduce in vivo calcification. The method includes applying a calcification mitigant such as a capping agent or an antioxidant to the tissue to specifically inhibit oxidation in tissue. Also, the method can be used to inhibit oxidation in dehydrated tissue. The capping agent suppresses the formation of binding sites in the tissue that are exposed or generated by the oxidation and otherwise would, upon implant, attract calcium, phosphate, immunogenic factors, or other precursors to calcification. In one method, tissue leaflets in assembled bioprosthetic heart valves are pretreated with an aldehyde capping agent prior to dehydration and sterilization.
Owner:EDWARDS LIFESCIENCES CORP
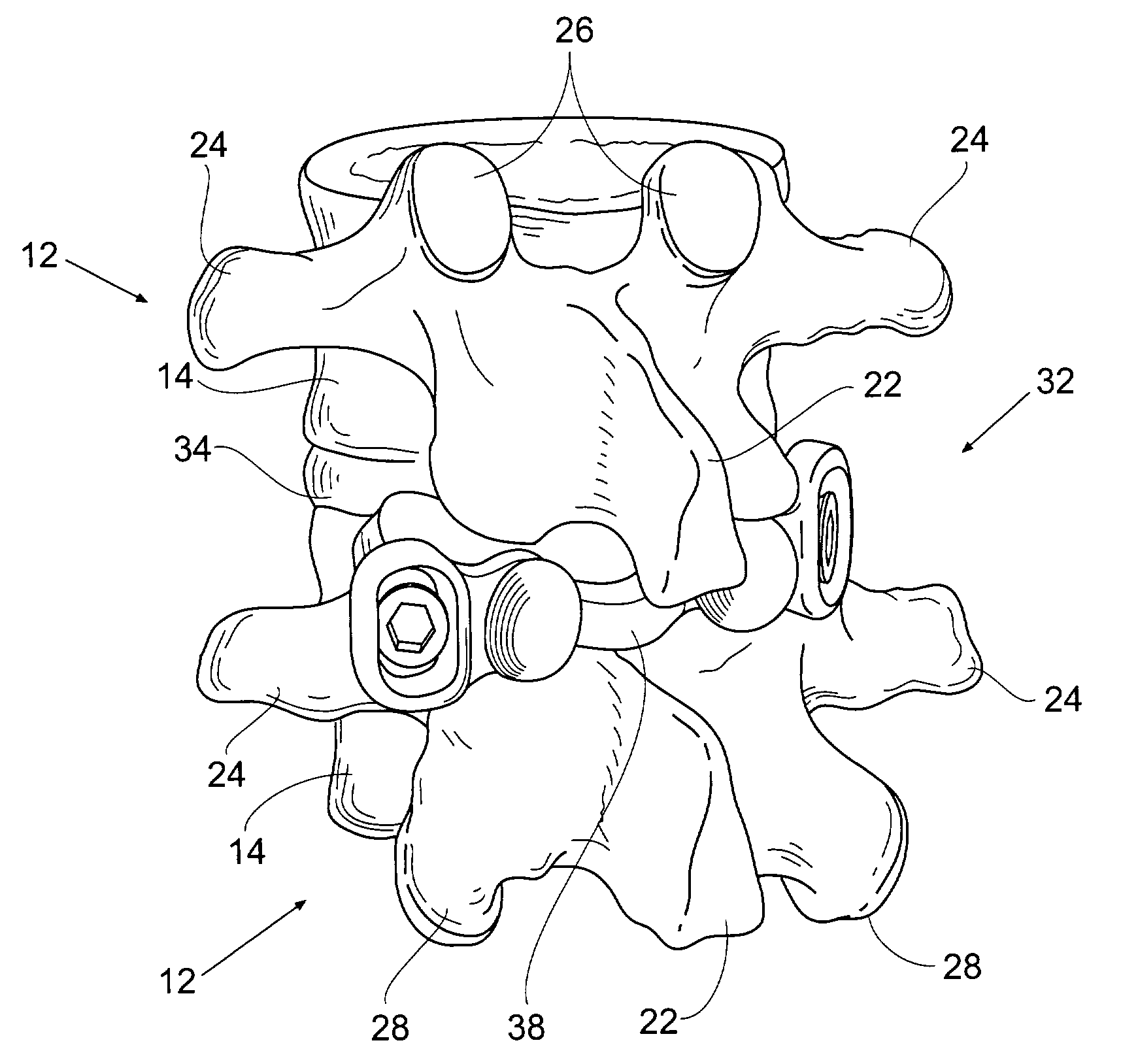
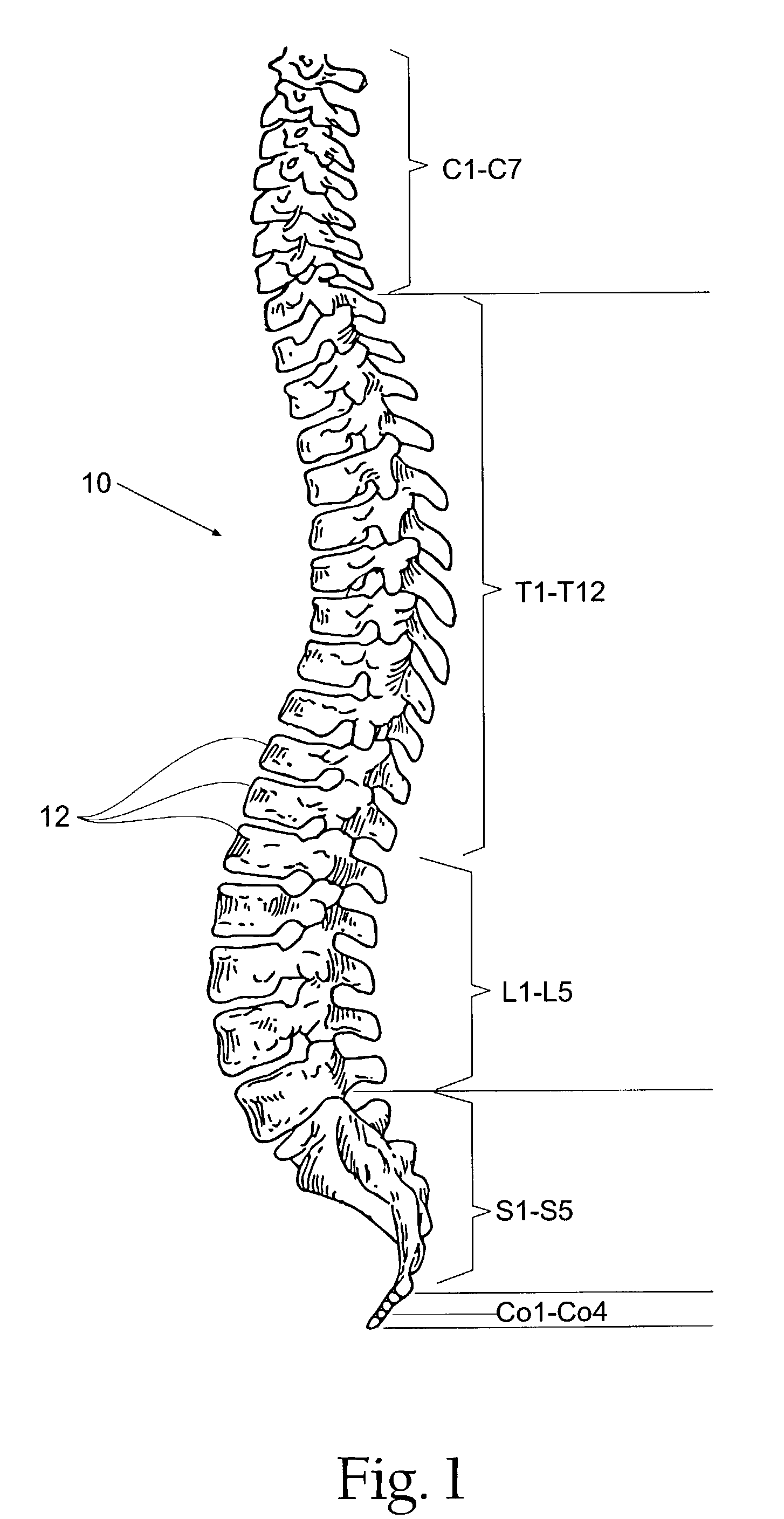
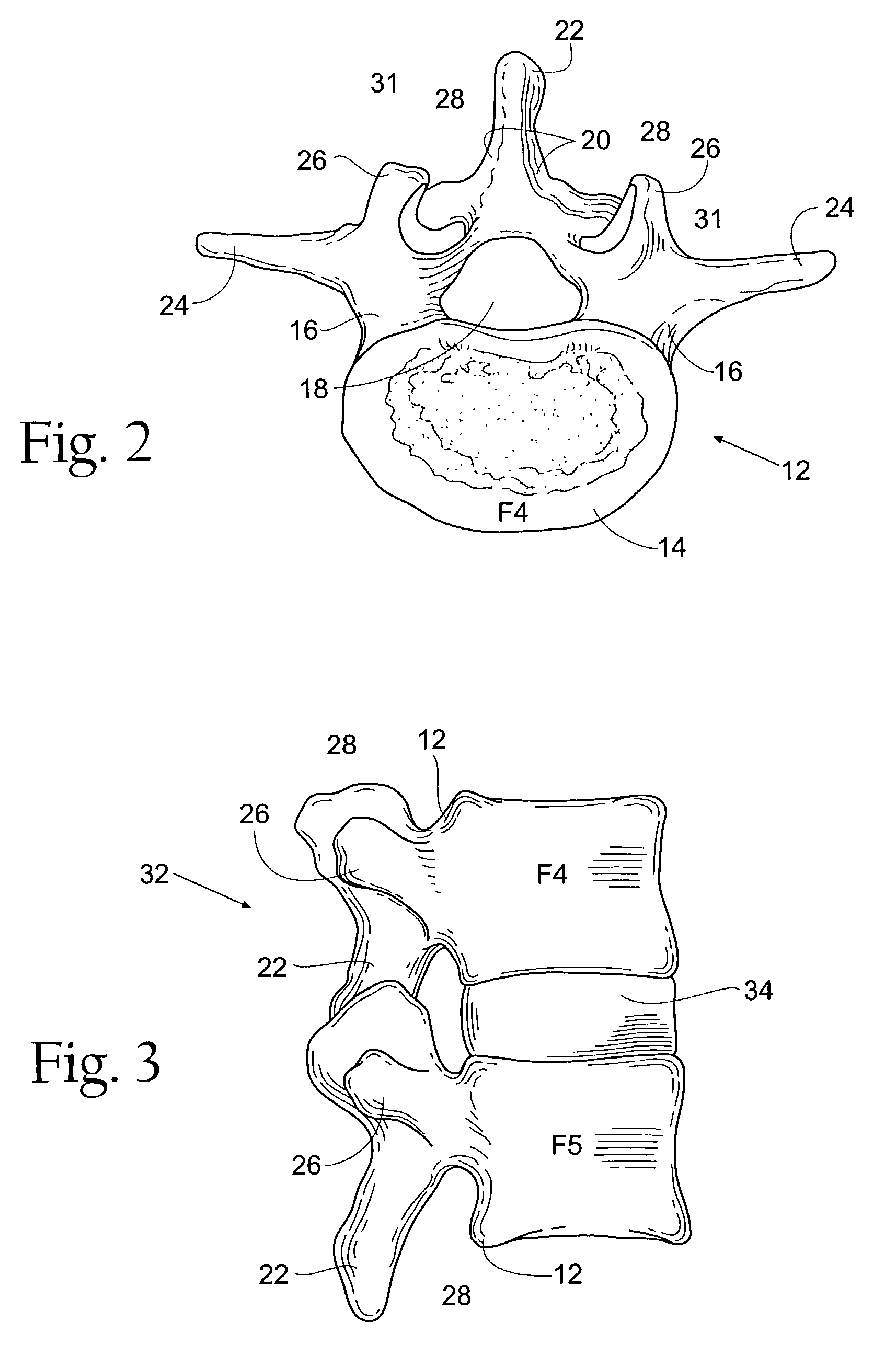
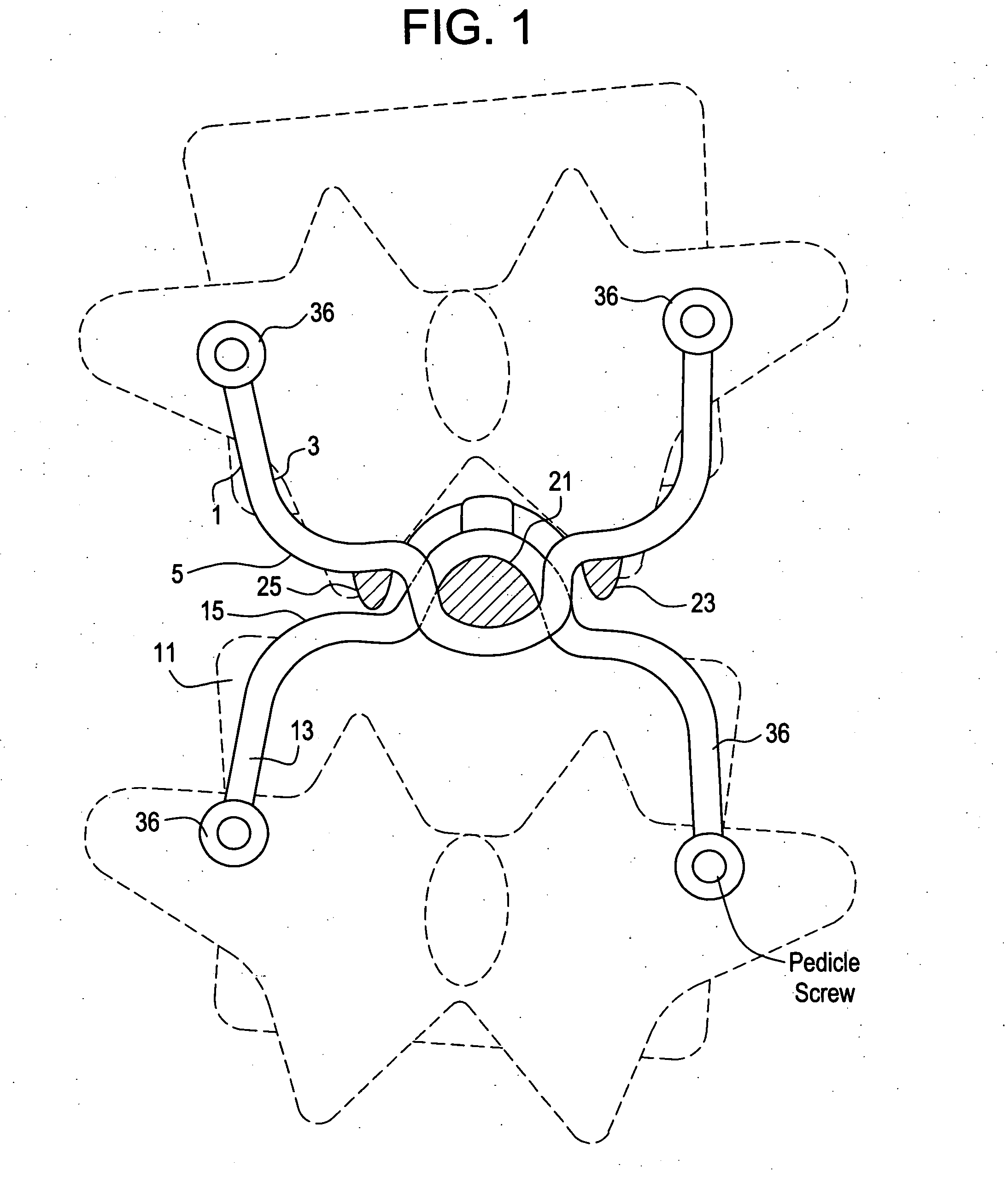
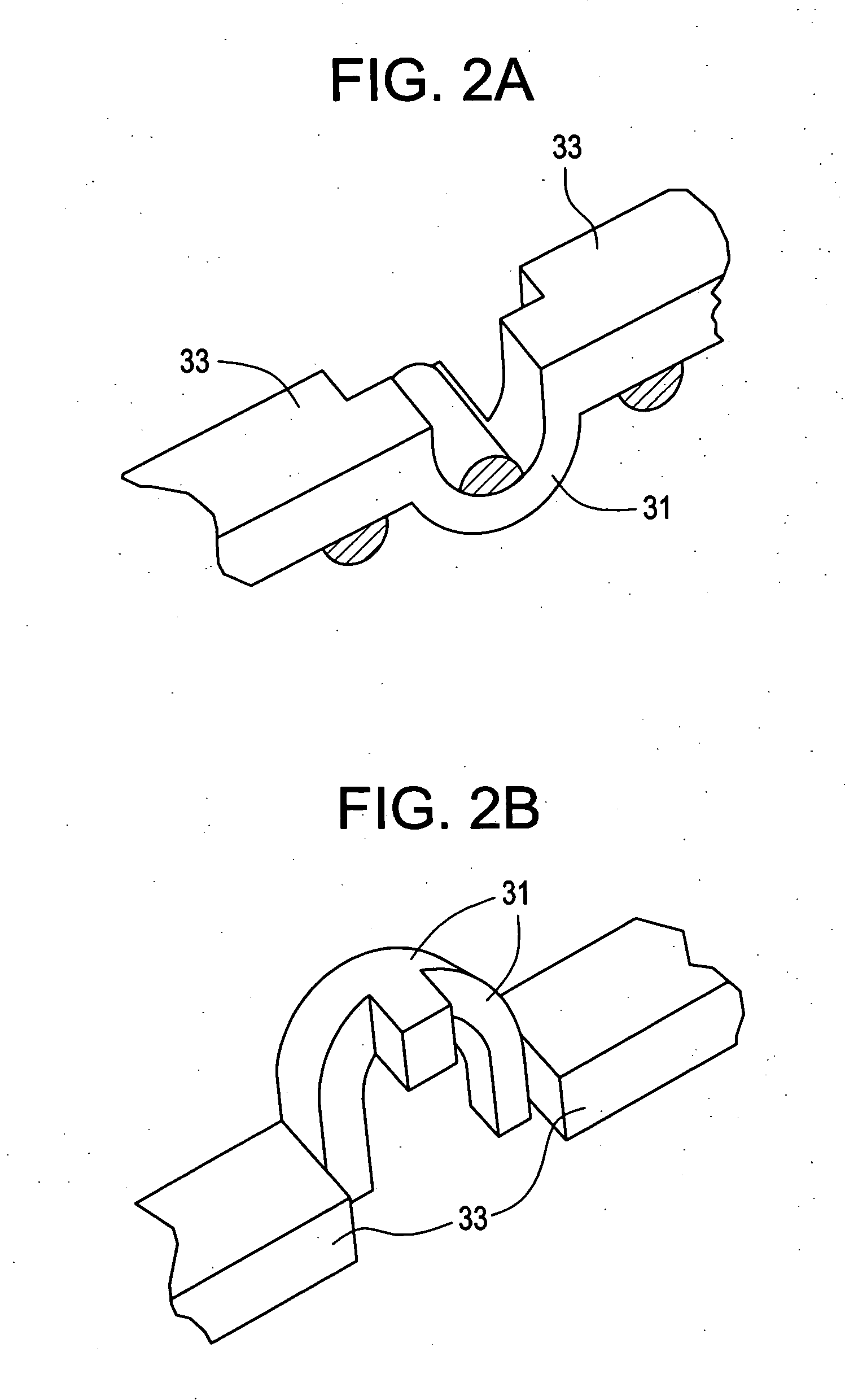
Prosthesis for the replacement of a posterior element of a vertebra
A prosthetic replacement for a posterior element of a vertebra comprising portions that replace the natural lamina and the four natural facets. The prosthetic replacement may also include portions that replace one or more of the natural spinous process and the two natural transverse processes. If desired, the prosthesis replacement may also replace the natural pedicles. A method for replacing a posterior element of a vertebra is also provided.
Owner:GLOBUS MEDICAL INC
Mitral valve system
Valve prostheses are disclosed that are adapted for secure and aligned placement relative to a heart annulus. The valve prostheses may be placed in a non-invasive manner, e.g., via transcatheter techniques. The valve prosthesis may include a resilient ring, a plurality of leaflet membranes mounted with respect to the resilient ring, and a plurality of positioning elements movably mounted with respect to the flexible ring. Each of the positioning elements defines respective proximal, intermediate, and distal tissue engaging regions cooperatively configured and dimensioned to simultaneously engage separate corresponding areas of the tissue of an anatomical structure, including respective first, second, and third elongate tissue-piercing elements. The proximal, distal, and intermediate tissue-engaging regions are cooperatively configured and dimensioned to simultaneously engage separate corresponding areas of the tissue of an anatomical structure so as to stabilize a position of the valve prosthesis with respect to the anatomical structure, including wherein for purposes of so simultaneously engaging the separate corresponding areas of tissue, at least one of the first, second, and third elongate tissue-piercing elements is pointed at least partially opposite the direction of blood flow, and at least another thereof is pointed at least partially along the direction of blood flow. The valve prosthesis may also include a skirt mounted with respect to the resilient ring for sealing a periphery of the valve prosthesis against a reverse flow of blood around the valve prosthesis.
Owner:ENDOVALVE +1
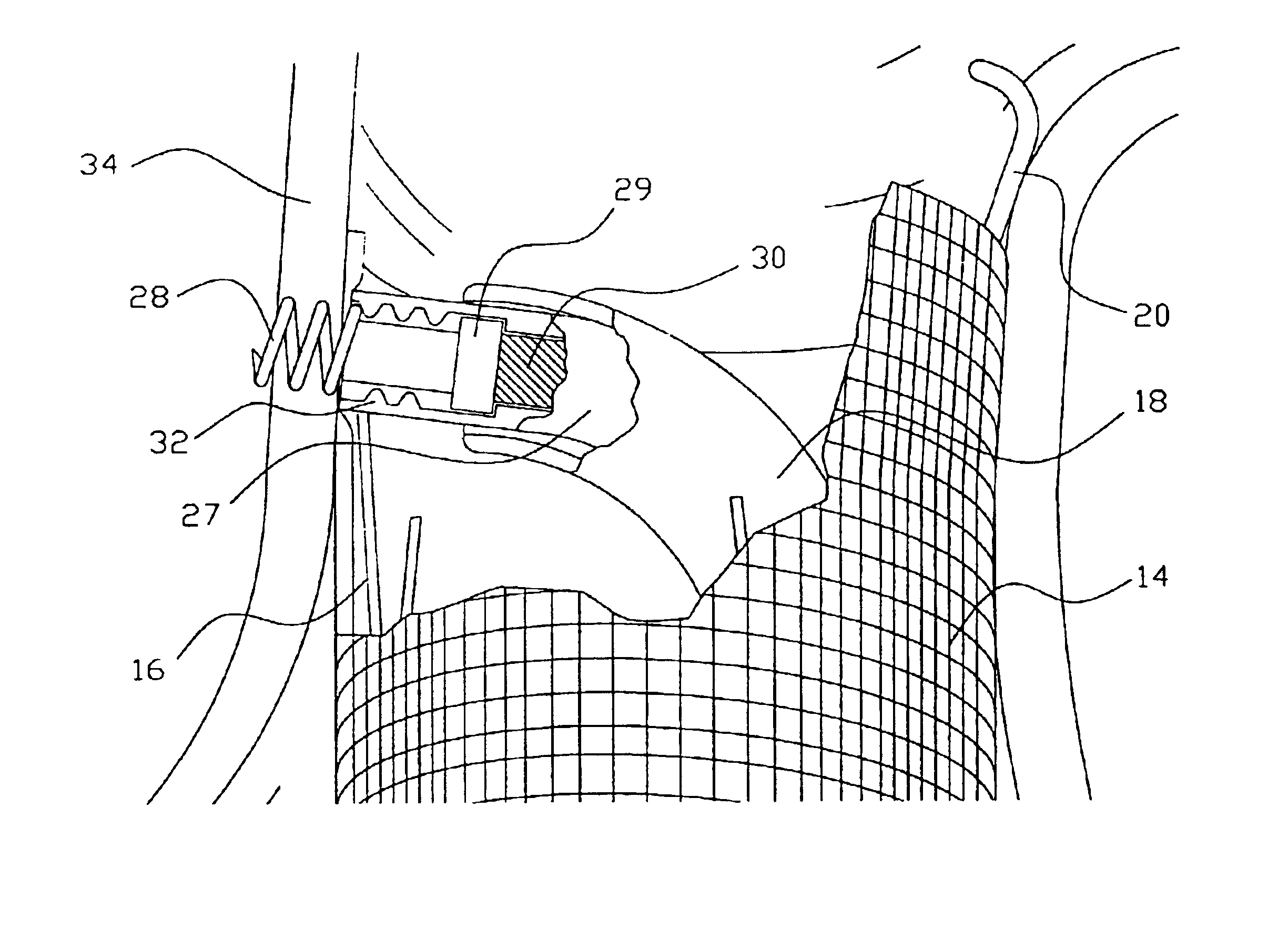
Artificial facet joint device having a compression spring
A prosthetic facet joint having compression springs interposed between articulating surfaces for providing gradual resistance to extreme movements.
Owner:DEPUY SPINE INC (US)
Implantable prosthetic valve
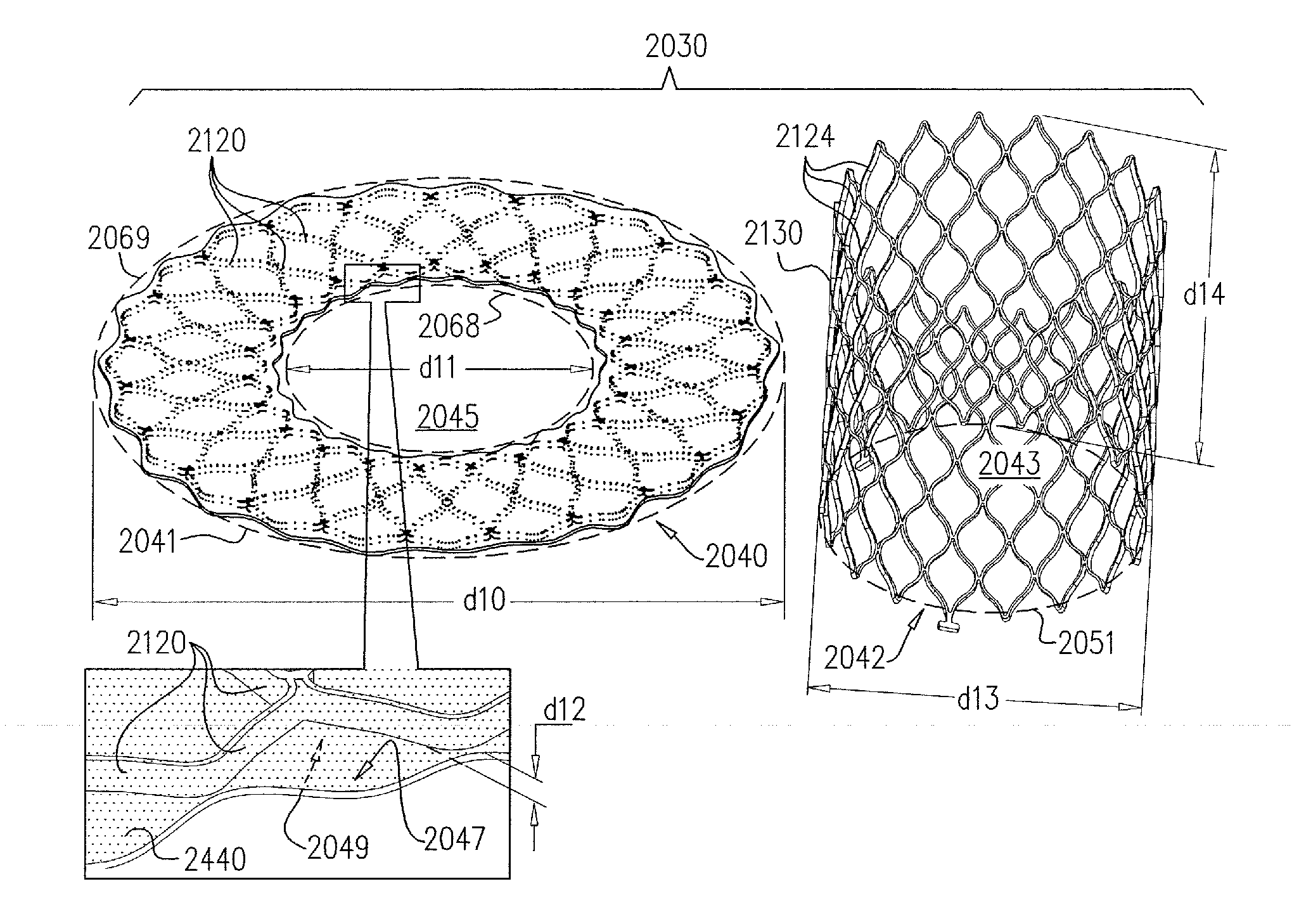
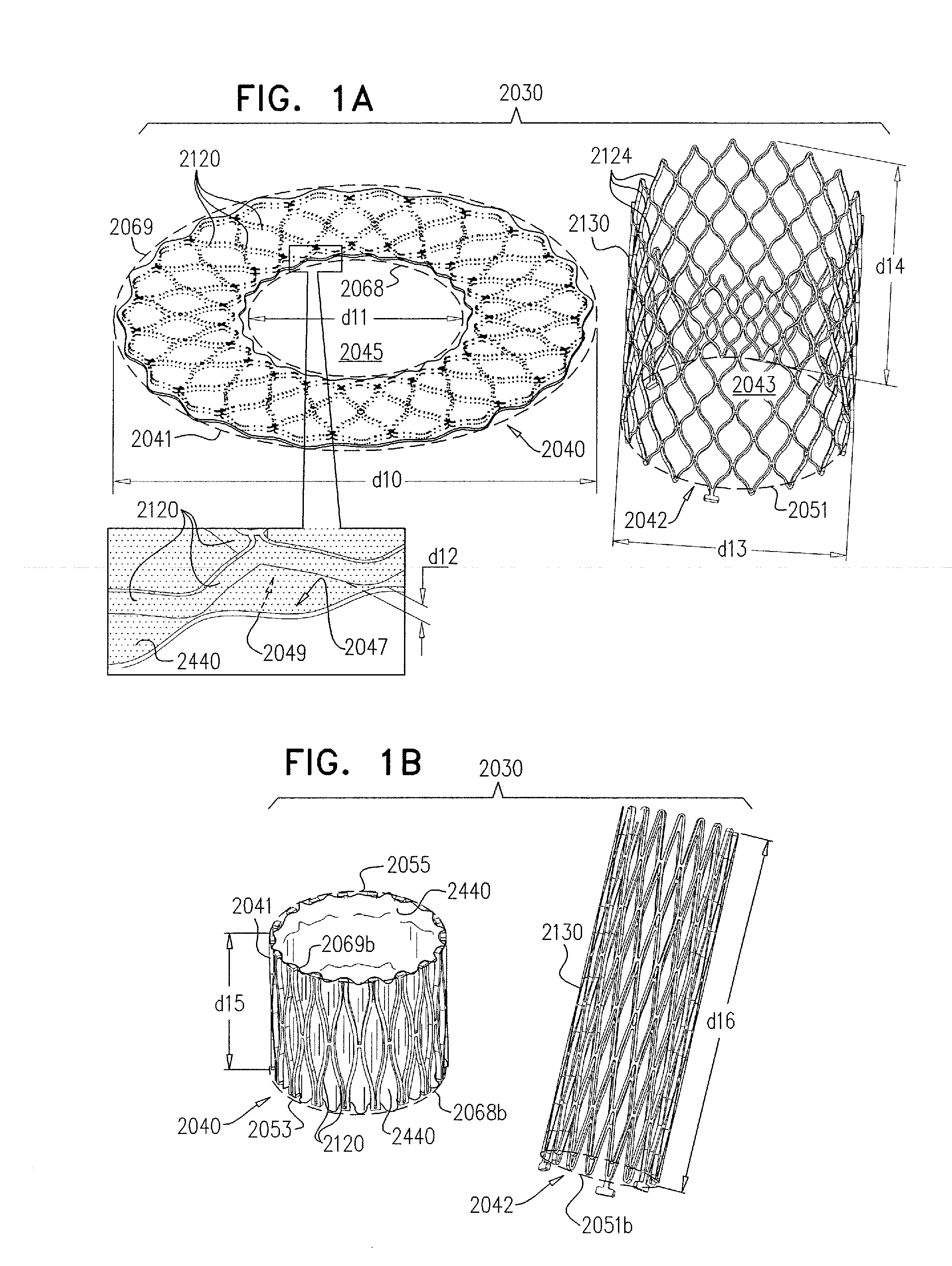
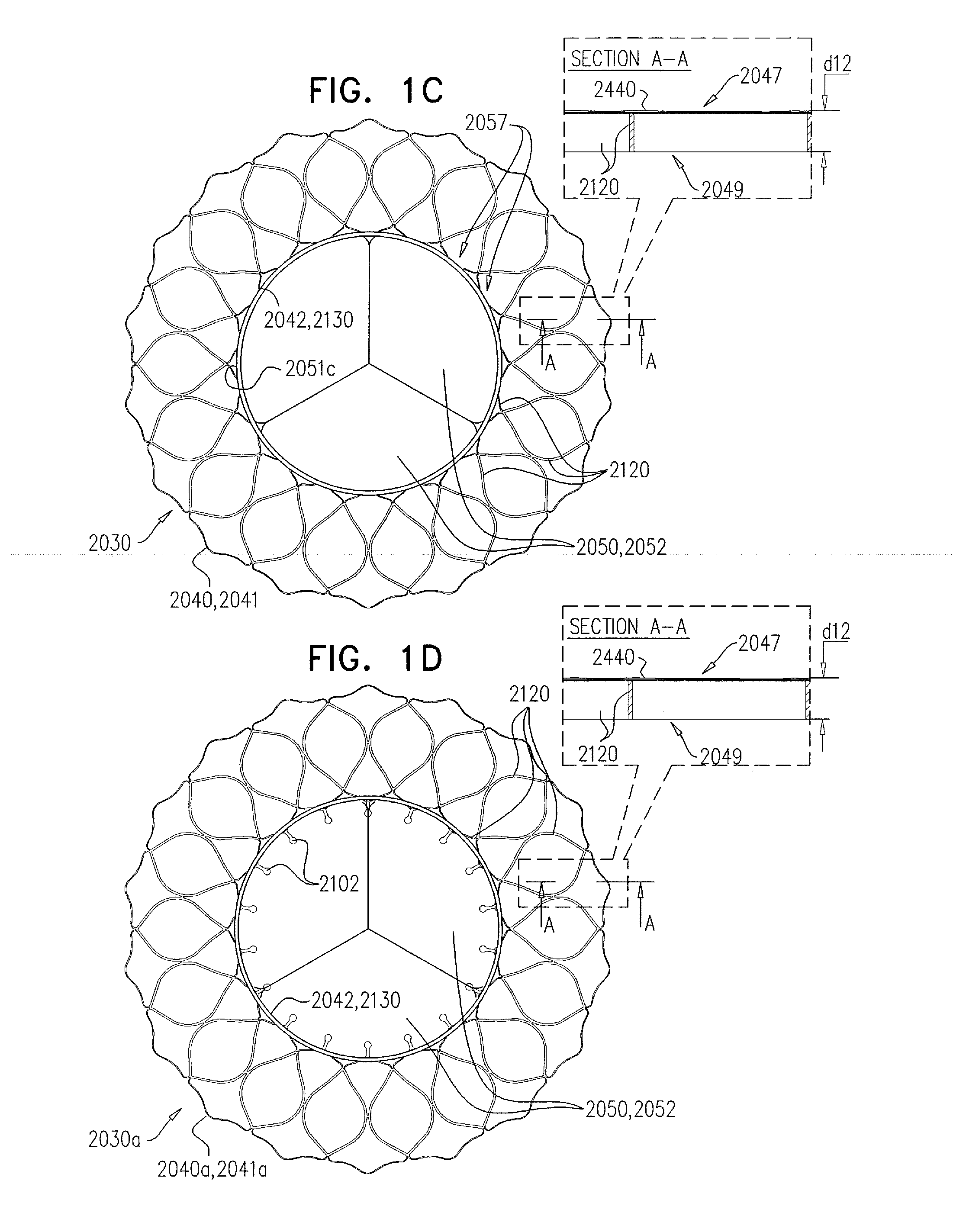
A valve prosthesis device is disclosed suitable for implantation in body ducts. The device comprises support stent, comprised of a deployable construction adapted to be initially crimped in a narrow configuration suitable for catheterization through the body duct to a target location and adapted to be deployed by exerting substantially radial forces from within by means of a deployment device to a deployed state in the target location, the support stent provided with a plurality of longitudinally rigid support beams of fixed length; valve assembly comprising a flexible conduit having an inlet end and an outlet, made of pliant material attached to the support beams providing collapsible slack portions of the conduit at the outlet. When flow is allowed to pass through the valve prosthesis device from the inlet to the outlet the valve assembly is kept in an open position, whereas a reverse flow is prevented as the collapsible slack portions of the valve assembly collapse inwardly providing blockage to the reverse flow.
Owner:EDWARDS LIFESCI PVT
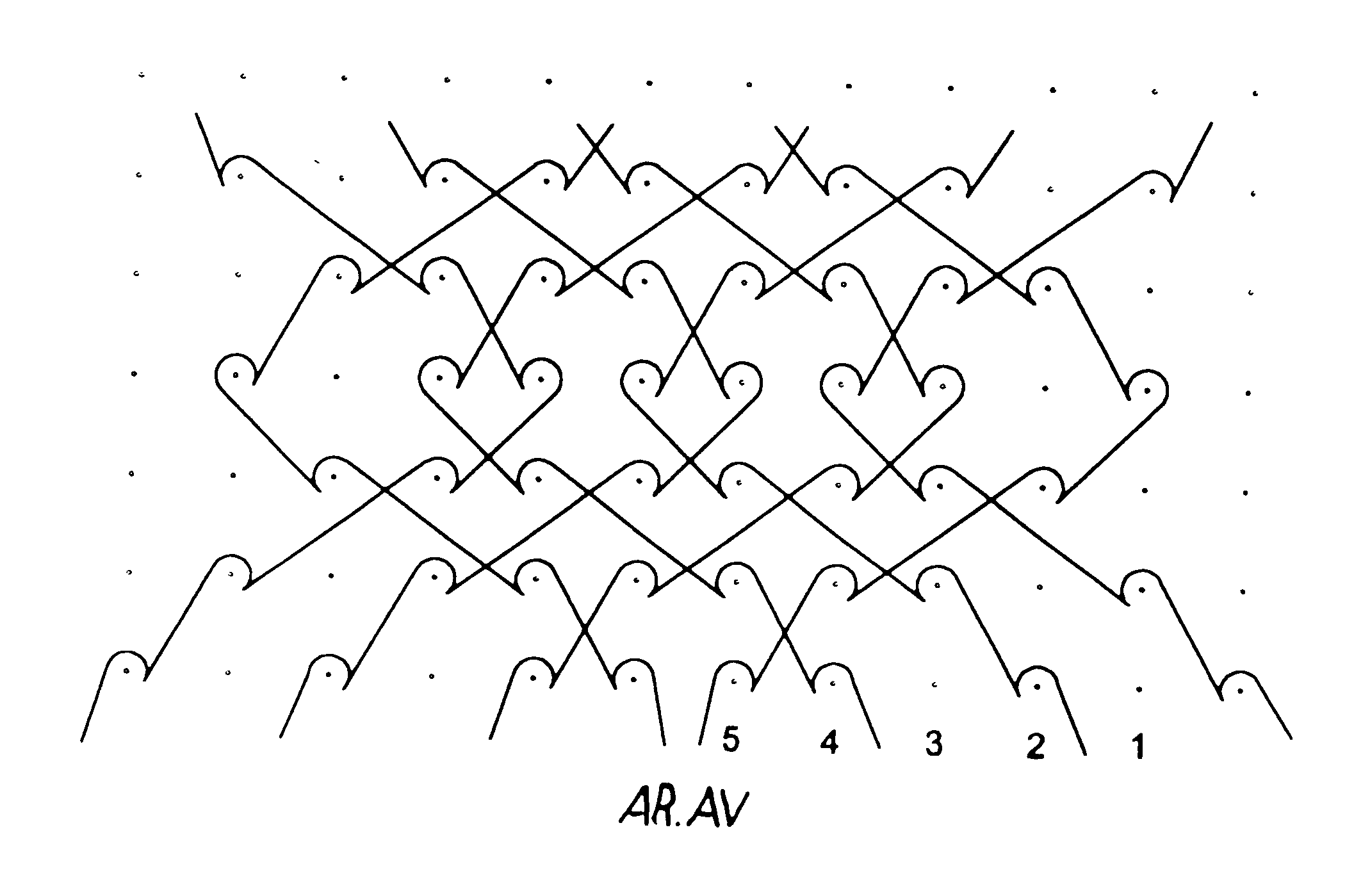
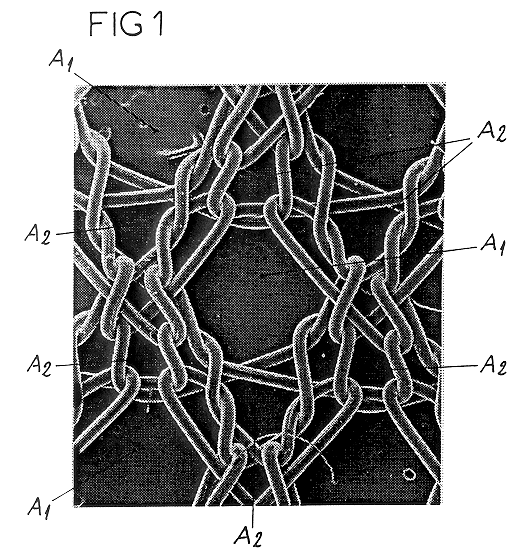
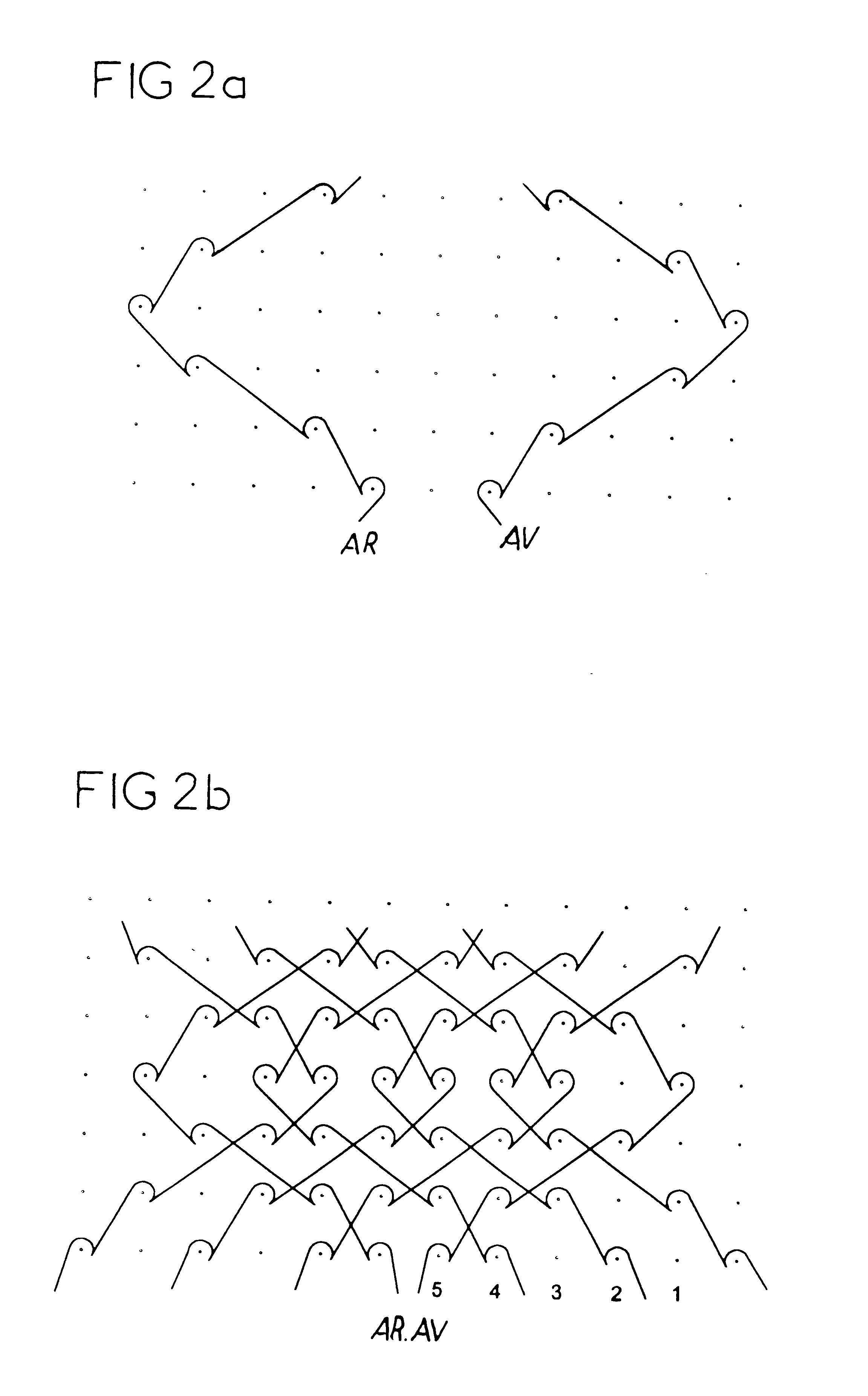
Isoelastic prosthetic filet stitch fabric
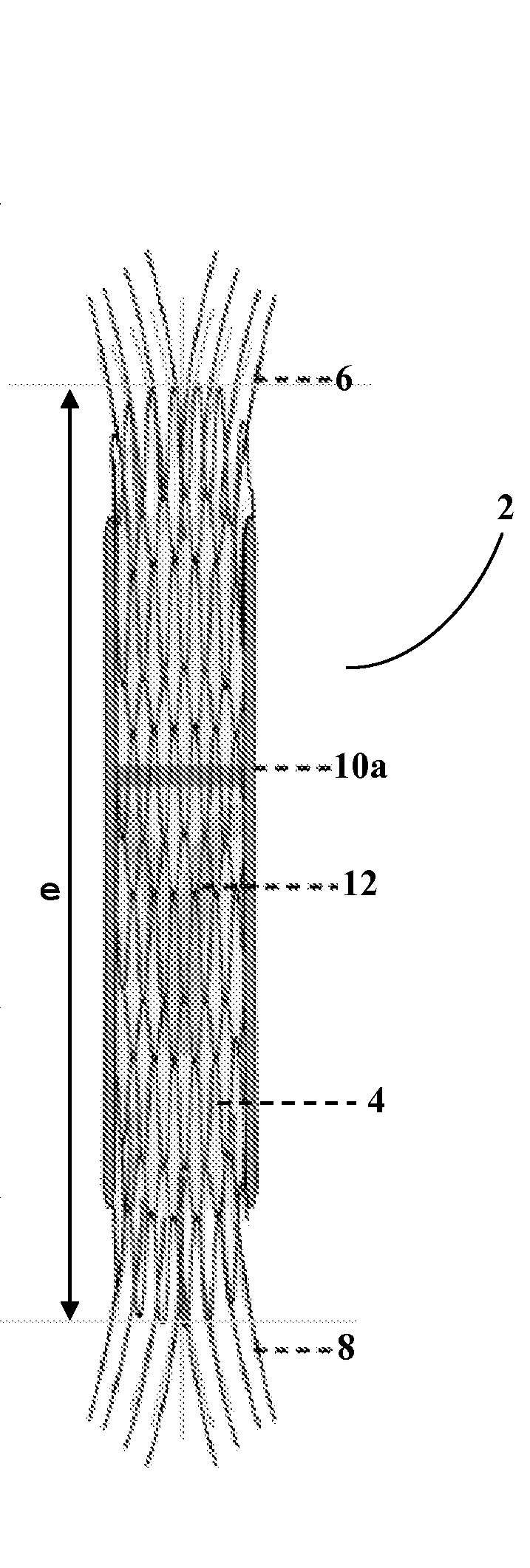
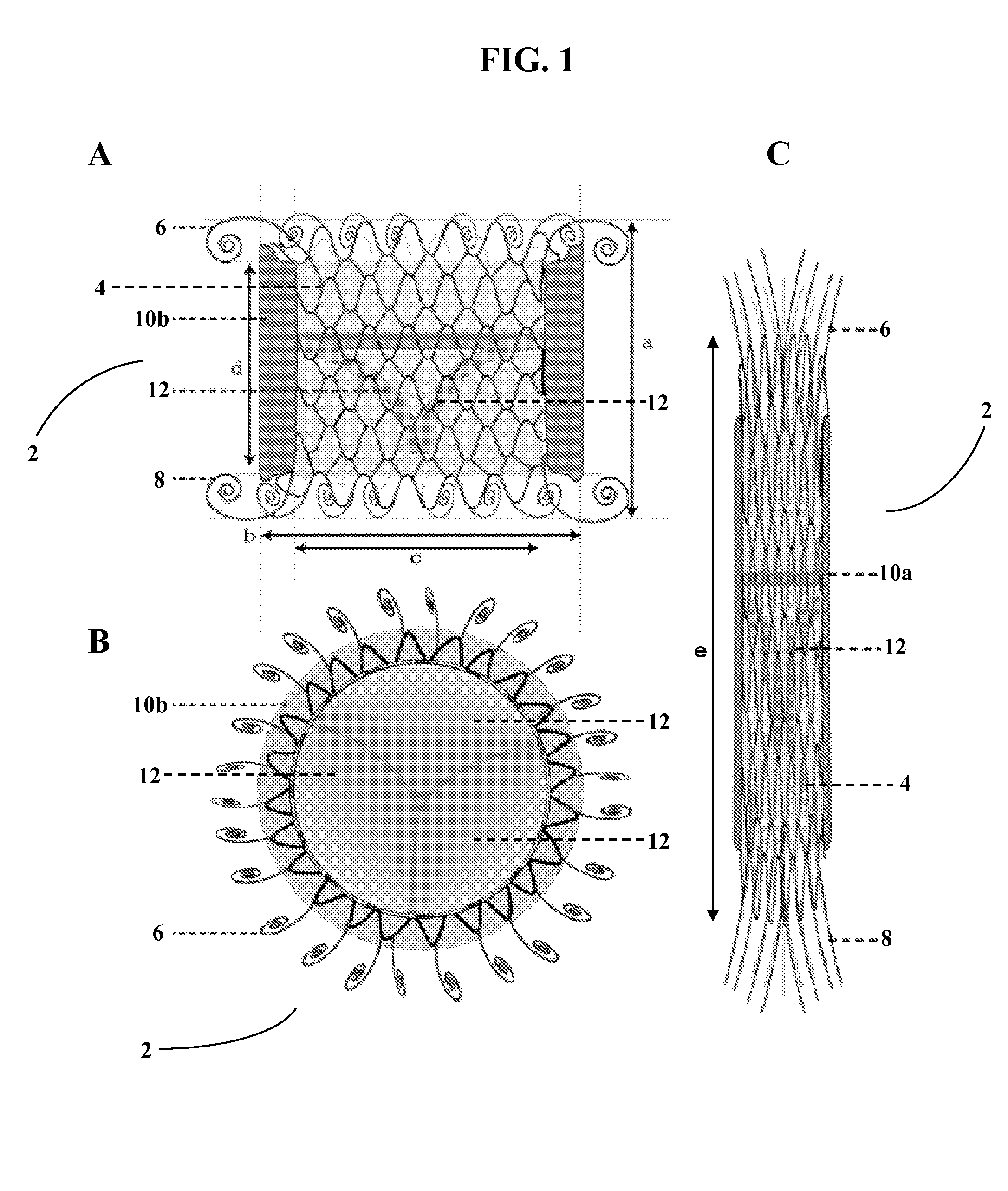
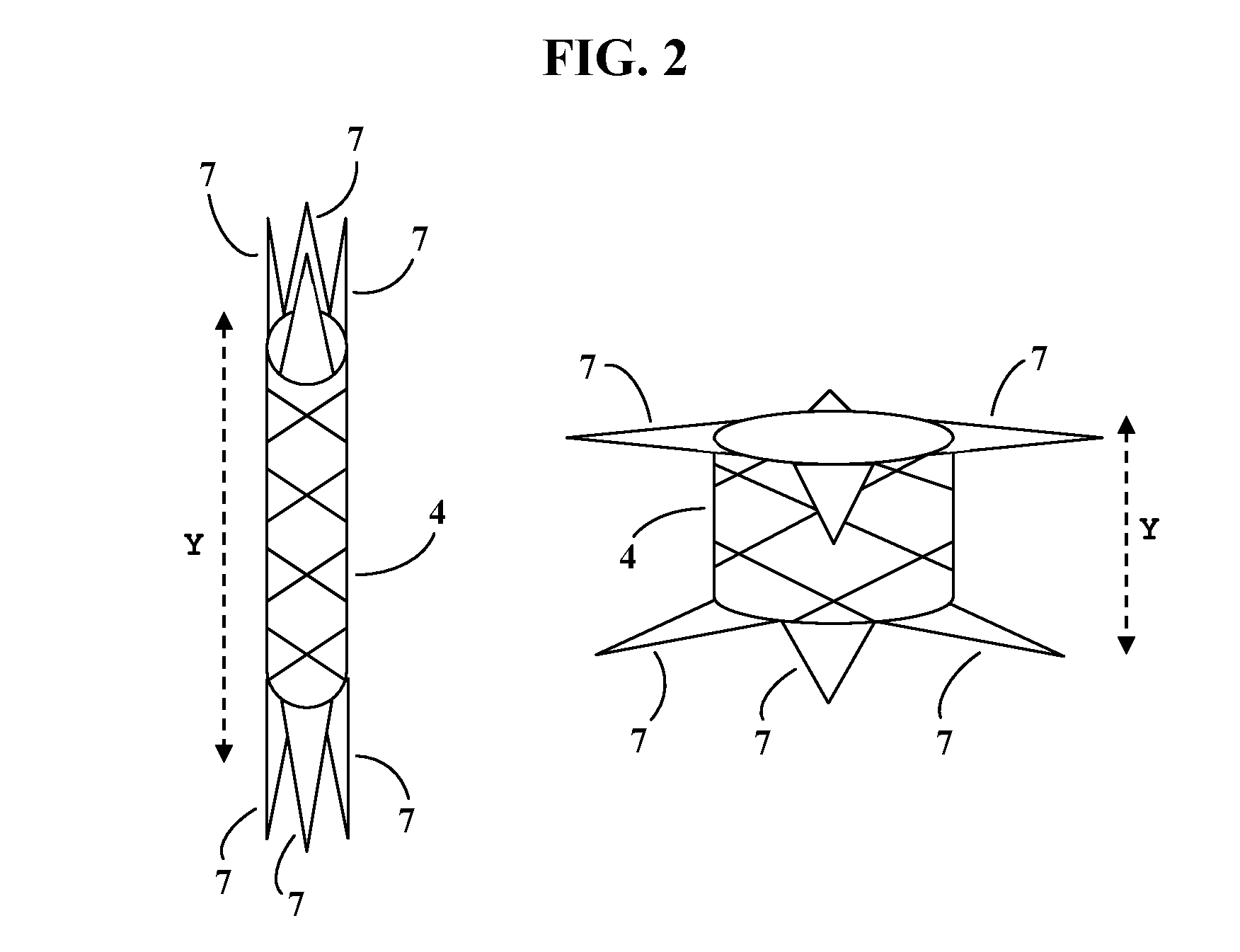
This knit is produced on the basis of a biocompatible polymer material monofilament, whose pattern is defined by a front lap and a rear lap of yarns knitted together and determines a plurality of cells each having a substantially polygonal shape. The pattern gives the knit a multidirectional tensile behavior such as obtained by a front lap capable of being obtained by knitting according to a scheme 5-4 / 4-3 / 2-1 / 0-1 / 1-2 / 3-4 and by a rear lap capable of being obtained by knitting according to a scheme 0-1 / 1-2 / 3-4 / 5-4 / 4-3 / 2-1.
Owner:SOFRADIM PROD SAS
Valve prosthesis
The present disclosure relates to valve replacement devices that are foldable for catheter-based deployment to the site of implantation, as well as systems for the delivery of valve prostheses, including prostheses having the special characteristics of the disclosed valve replacement devices. The devices include highly effective adhering mechanisms for secure and enduring precision implantation. The adhering mechanisms may employ a unique sealing mechanism that includes a cuff that expands slowly whereby the device is not secured in place until the completion of the implantation procedure. The implanted device, optionally together with the cuff, prevents perivalvular leaks and incorporate an appropriate leaflet system for reliable functioning in situ.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
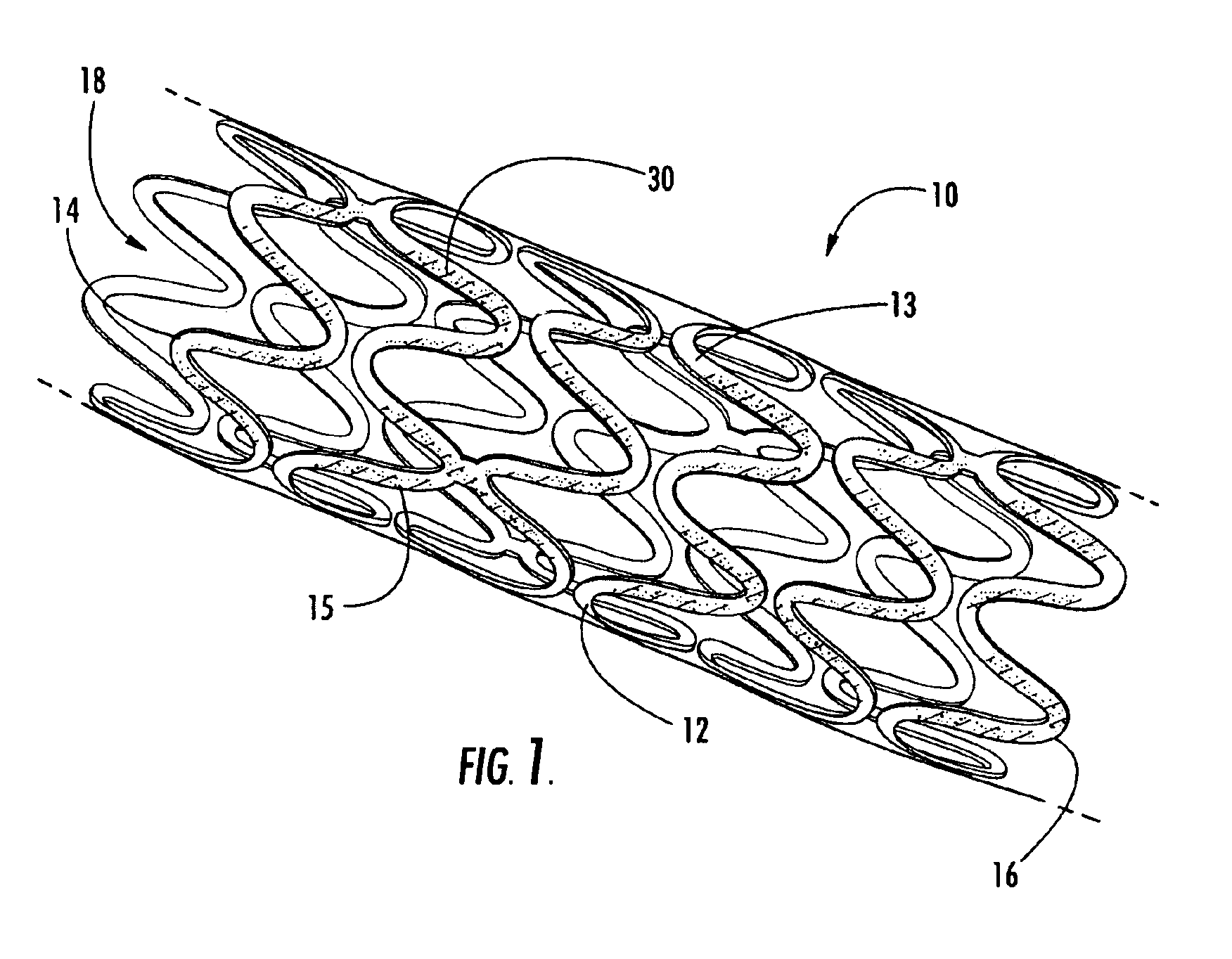
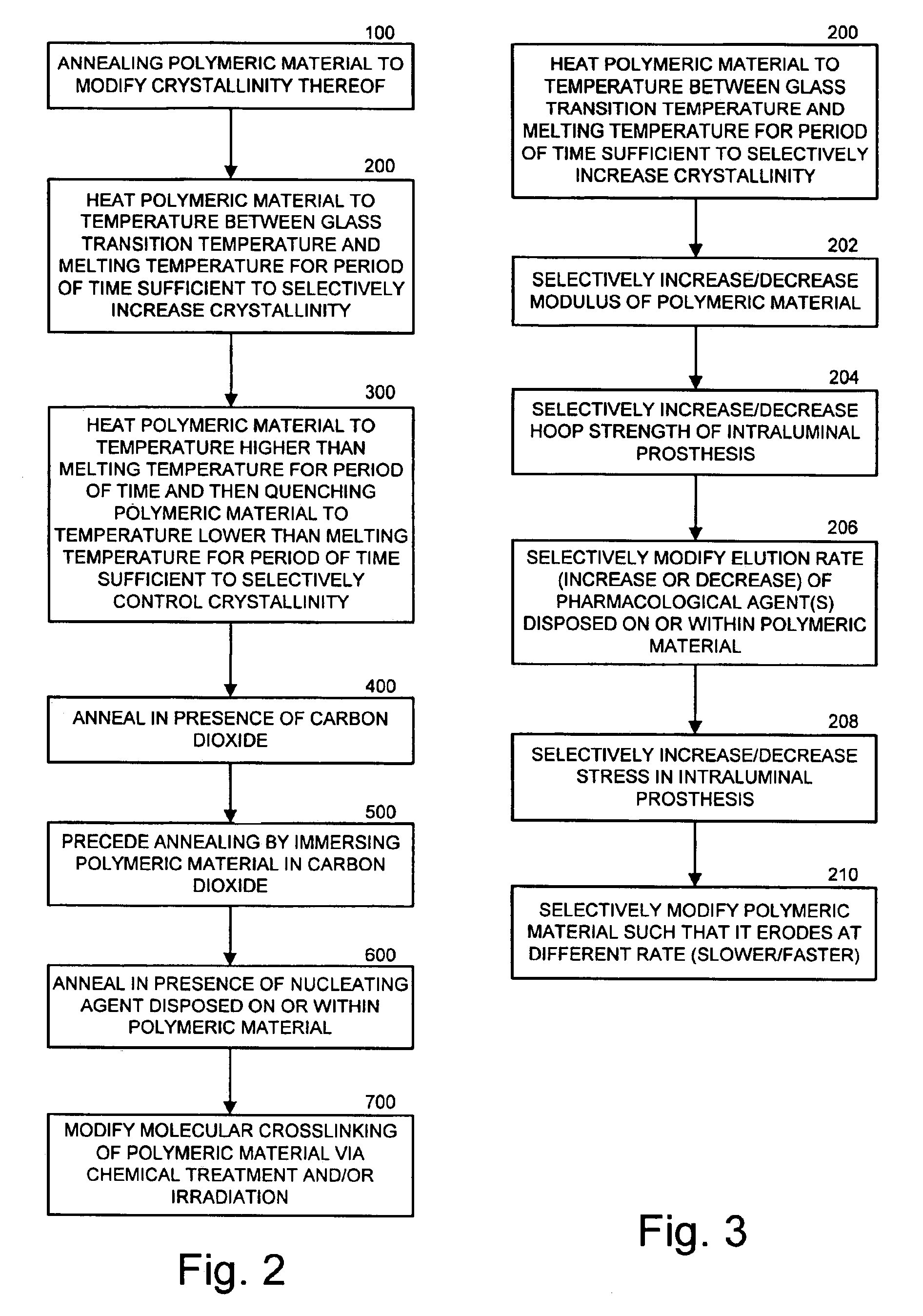
Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same
Methods of manufacturing polymeric intraluminal prostheses include annealing the polymeric material to selectively modify the crystallinity thereof. Annealing may be utilized to selectively modify various properties of the polymeric material of an intraluminal prosthesis, including: selectively increasing the modulus of the polymeric material; selectively increasing the hoop strength of the intraluminal prosthesis; selectively modifying the elution rate (increase or decrease) of a pharmacological agent subsequently disposed on or within the annealed polymeric material; selectively increasing / decreasing stress in the intraluminal prosthesis; and selectively modifying the polymeric material such that it erodes at a different rate.
Owner:SYNECOR LLC
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS20030060782A1Safely and permanently excludingPreventing initial or recurrent aneurysmal subarachnoid hemorrhageStentsCatheterIn situ polymerizationProsthesis
Devices for excluding aneurysms and treating atherosclerotic disease, for intra-aneurysmal occlusion; and devices for preventing distal emboli. The devices are generally pliable and collapsible thin film devices which can be delivered via a microcatheter into the desired location where they are deployed and undergo either a shape memory phase transformation or in situ polymerization to assume the stable configuration of a permanent endoluminal prosthesis. Prior to being caused to assume their final shape, the devices remain soft, collapsible and pliable to ensure atraumatic delivery through the vascular system. Upon reaching the endoluminal defect in the vessel, the device is extruded from the microcatheter. Devices are also provided for retrieving clots.
Owner:NEW YORK UNIV
Percutaneous heart valve prosthesis
A percutaneous heart valve prosthesis (1) has a valve body (2) with a passage (9) extending between the first and second ends (7, 8) of the valve body (2). The valve body (2) is collapsible about a longitudinal axis (10) of the passage (9) for delivery of the valve body (2) via a catheter (18). One or more flexible valve leaflets (3, 4) are secured to the valve body (2) and extend across the passage (9) for blocking bloodflow in one direction through the passage (9). An anchor device (5), which is also collapsible for delivery via catheter (18), is secured to the valve body (2) by way of an anchor line (6). A failed or failing mitral heart valve (101) is treated by percutaneously locating the valve body (2) in the mitral valve orifice (102) with the anchor device (5) located in the right atrium (107) and engaging the inter-atrial septum (103), such that the taught anchor line (6) acts to secure the valve body (2) within the mitral valve orifice (102).
Owner:PERCUTANEOUS CARDIOVASCULAR SOLUTIONS PTY LTD
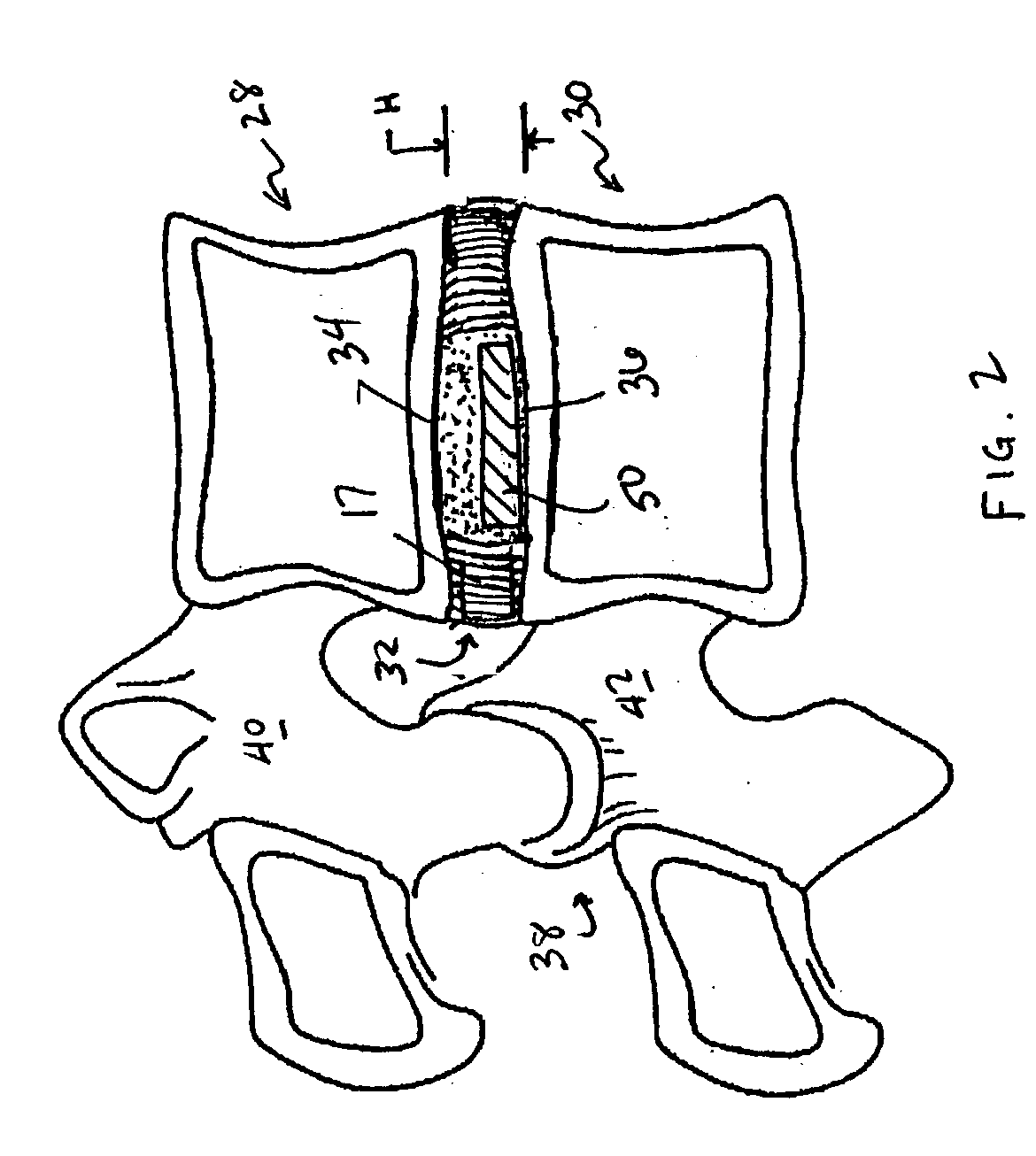
Devices and method for augmenting a vertebral disc
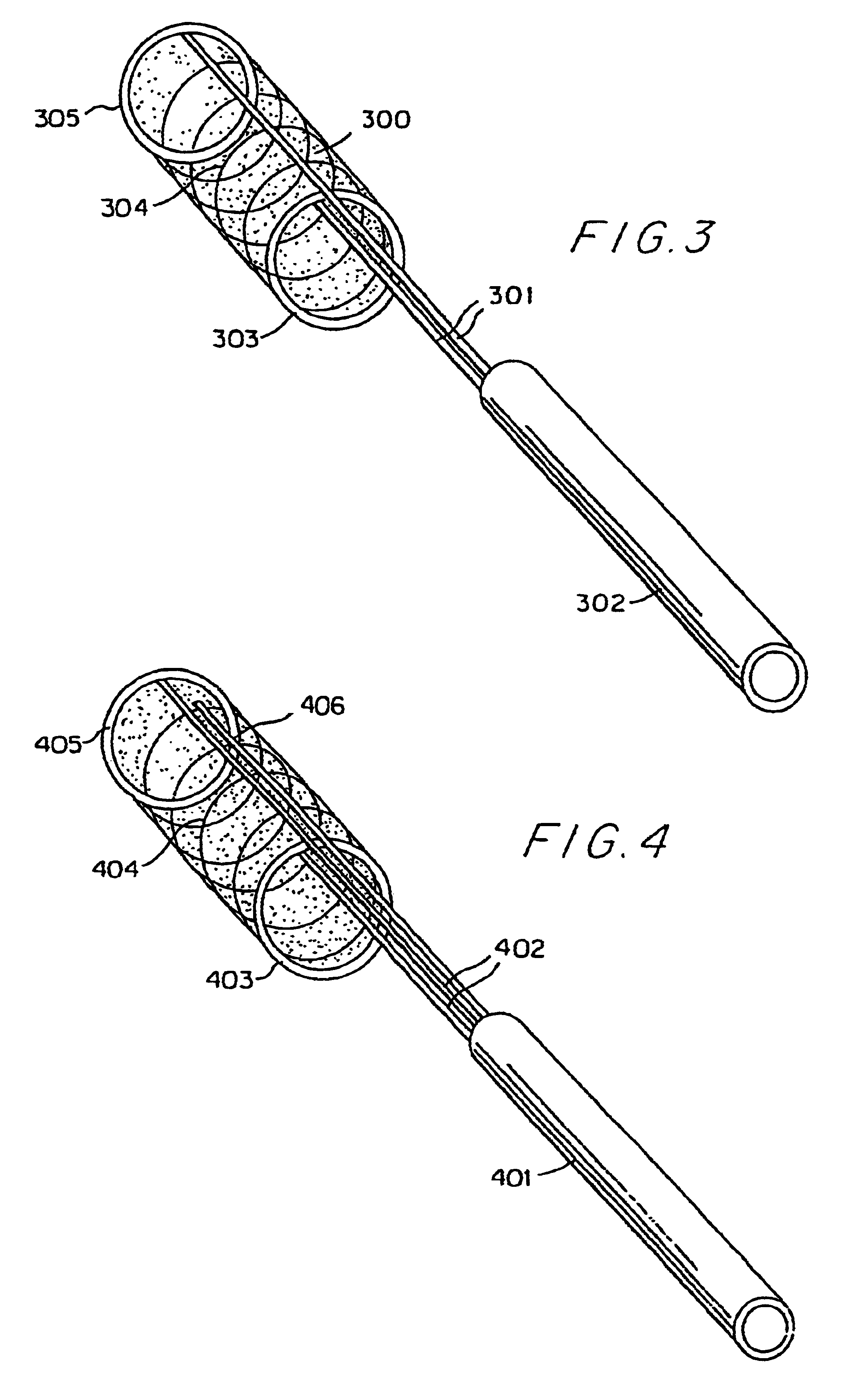
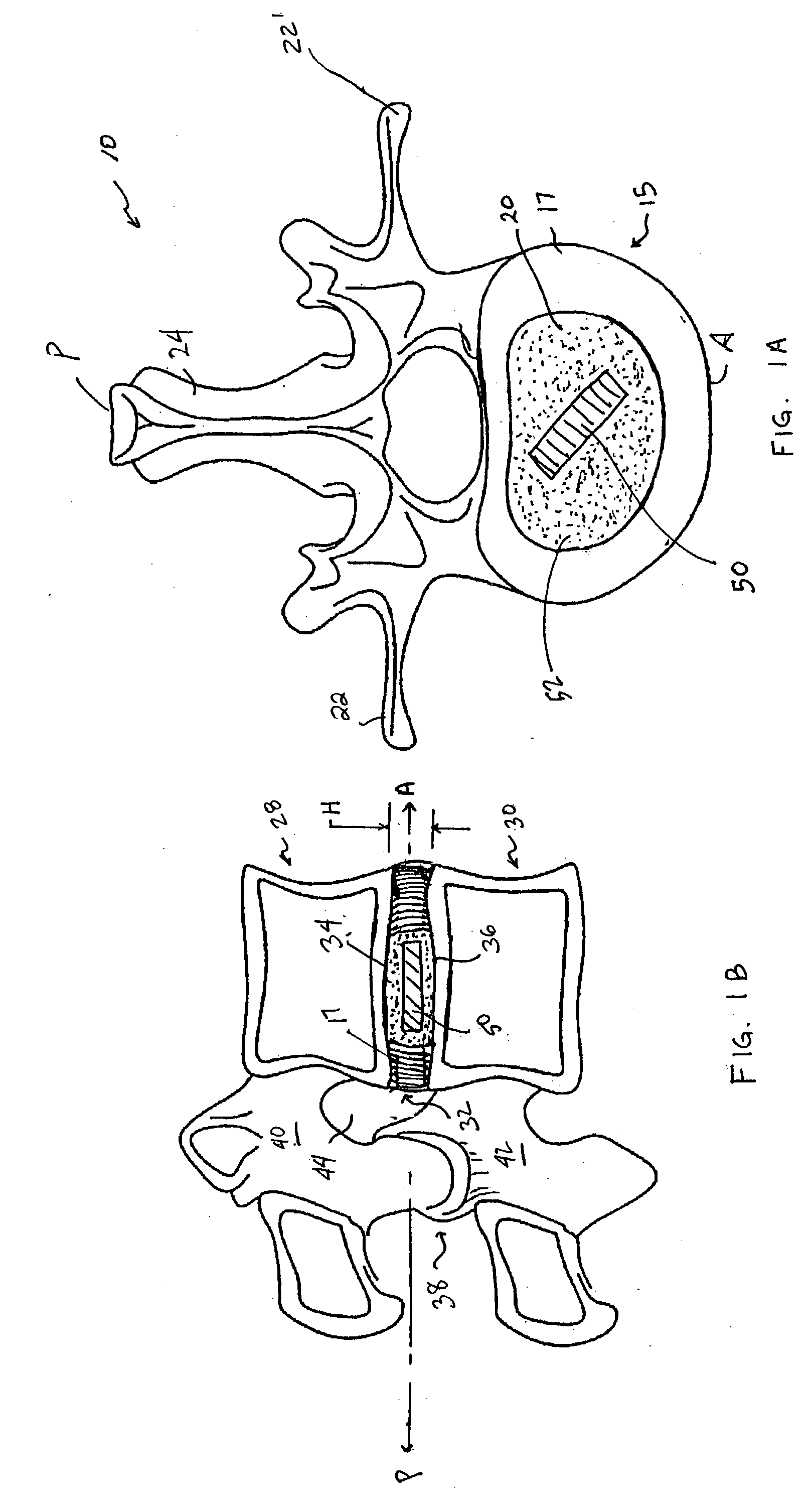
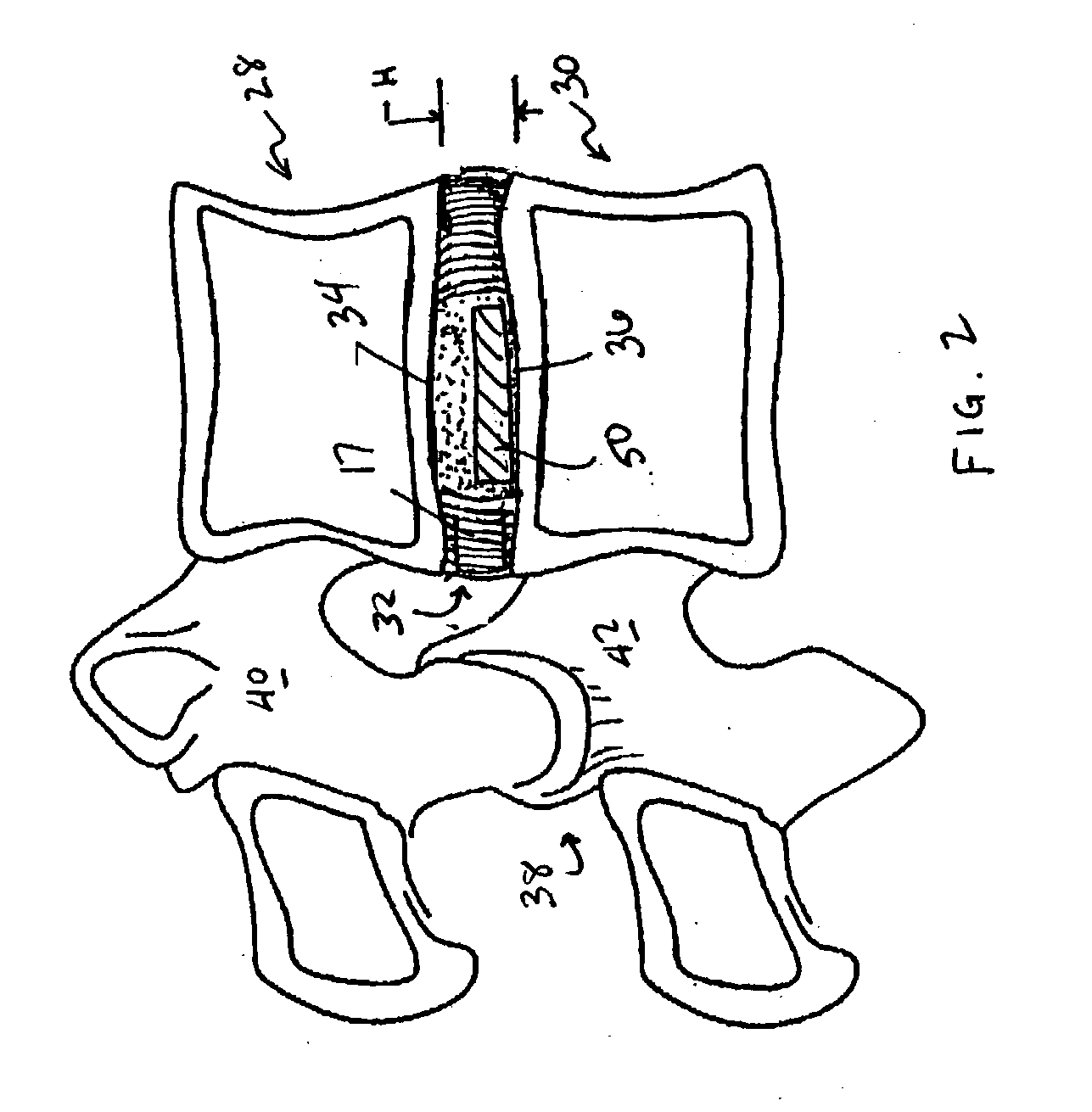
A vertebral disc prosthesis, a method of implanting a prosthesis and a deployment device is provided. The prosthesis may be implanted into the interior region of the vertebral disc so as to displace existing vertebral tissue, such as NP. The size or amount of the prosthesis inserted into the interior region of the vertebral disc may be a characteristic of the disc or the prosthesis. For example, the amount or size of prosthesis inserted into the disc may be dependent upon restoring the functionality of the disc (e.g., the ability of the disc to transfer nutrients or otherwise survive, the ability of the disc to carry the required loads and absorb stress or the reduction of pain). Restoring disc function may be determined by the resulting disc height desired, the resulting disc pressure desired or the resulting disc volume desired. The prosthesis may be sized or positioned within the interior of the vertebral disc such that it is spaced from at least one of the end plates of the vertebral disc. The prosthesis may be formed of a material having a compression strength that is less than 4 mn / m<2>. A deployment device may be used to facilitate placement of the prosthesis within the vertebral disc. The prosthesis may include a grouping of multiple components that can be deployed as group.
Owner:INTRINSIC THERAPEUTICS
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS6666882B1Treating and preventing ischemic and hemorrhagic strokeInhibit migrationStentsOcculdersIn situ polymerizationDistal embolization
Devices for excluding aneurysms and treating atherosclerotic disease, for intra-aneurysmal occlusion, and devices for preventing distal emboli. The devices are generally pliable and collapsible thin film devices which can be delivered via a microcatheter into the desired location where they are deployed and undergo either a shape memory phase transformation or in situ polymerization to assume the stable configuration of a permanent endoluminal prosthesis. Prior to being caused to assume their final shape, the devices remain soft, collapsible and pliable to ensure atraumatic delivery through the vascular system. Upon reaching the endoluminal defect in the vessel, the device is extruded from the microcatheter. Devices are also provided for retrieving clots.
Owner:NEW YORK UNIV
Devices and method for augmenting a vertebral disc
A vertebral disc prosthesis, a method of implanting a prosthesis and a deployment device is provided. The prosthesis may be implanted into the interior region of the vertebral disc so as to displace existing vertebral tissue, such as NP. The size or amount of the prosthesis inserted into the interior region of the vertebral disc may be a characteristic of the disc or the prosthesis. For example, the amount or size of prosthesis inserted into the disc may be dependent upon restoring the functionality of the disc (e.g., the ability of the disc to transfer nutrients or otherwise survive, the ability of the disc to carry the required loads and absorb stress or the reduction of pain). Restoring disc function may be determined by the resulting disc height desired, the resulting disc pressure desired or the resulting disc volume desired. The prosthesis may be sized or positioned within the interior of the vertebral disc such that it is spaced from at least one of the end plates of the vertebral disc. The prosthesis may be formed of a material having a compression strength that is less than 4 mn / m<2>. A deployment device may be used to facilitate placement of the prosthesis within the vertebral disc. The prosthesis may include a grouping of multiple components that can be deployed as group.
Owner:LAMBRECHT GREGORY +3
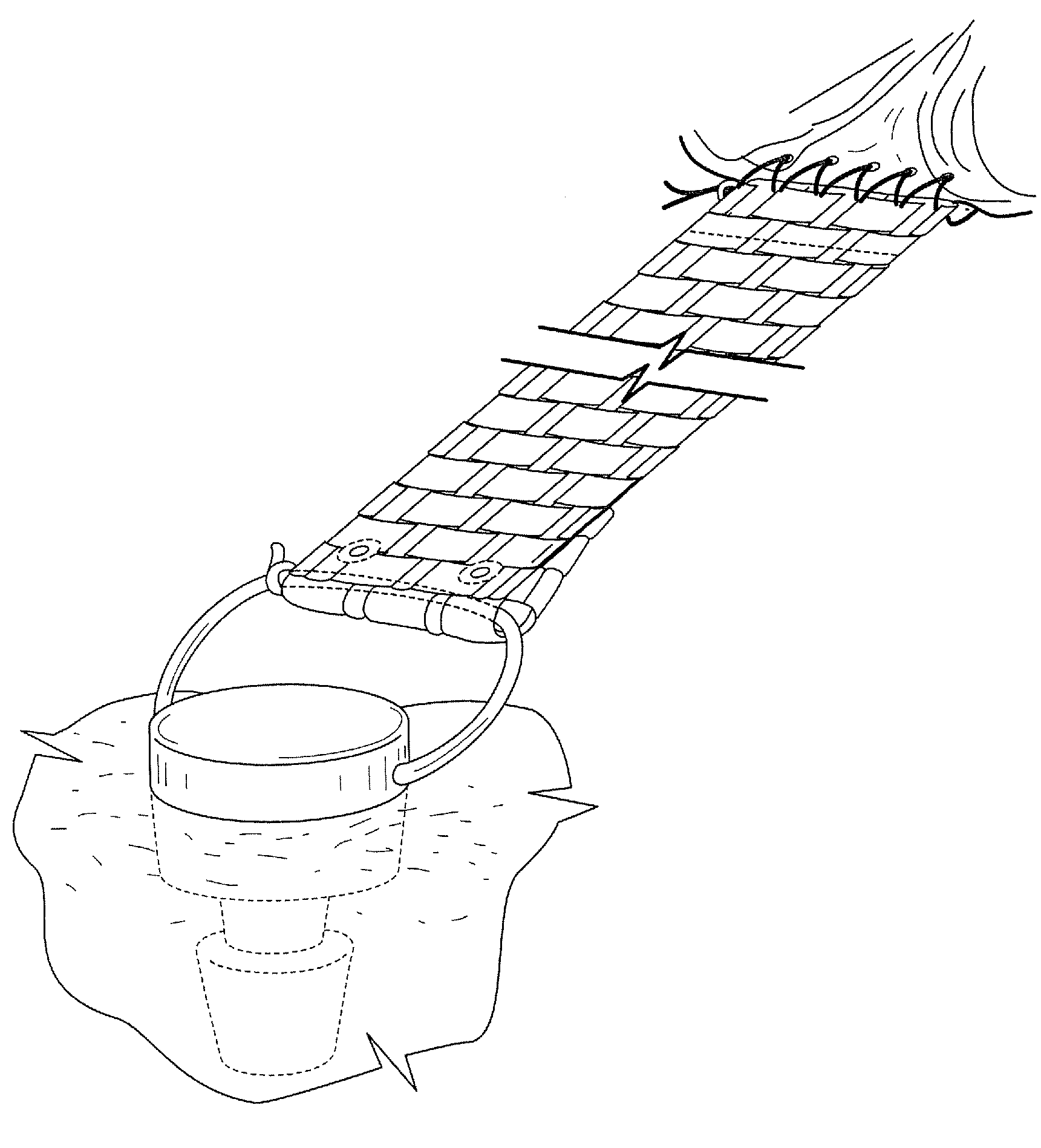
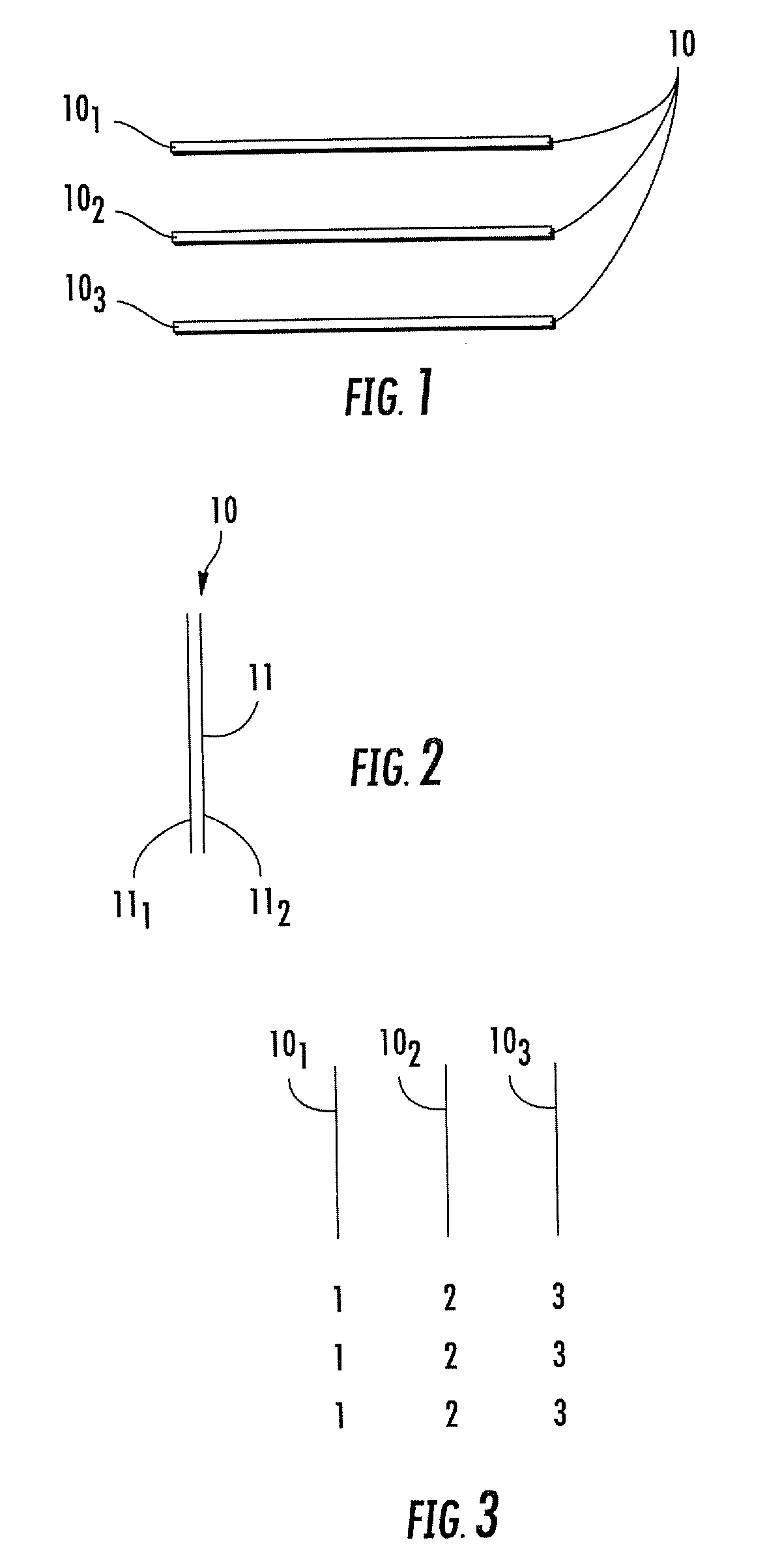
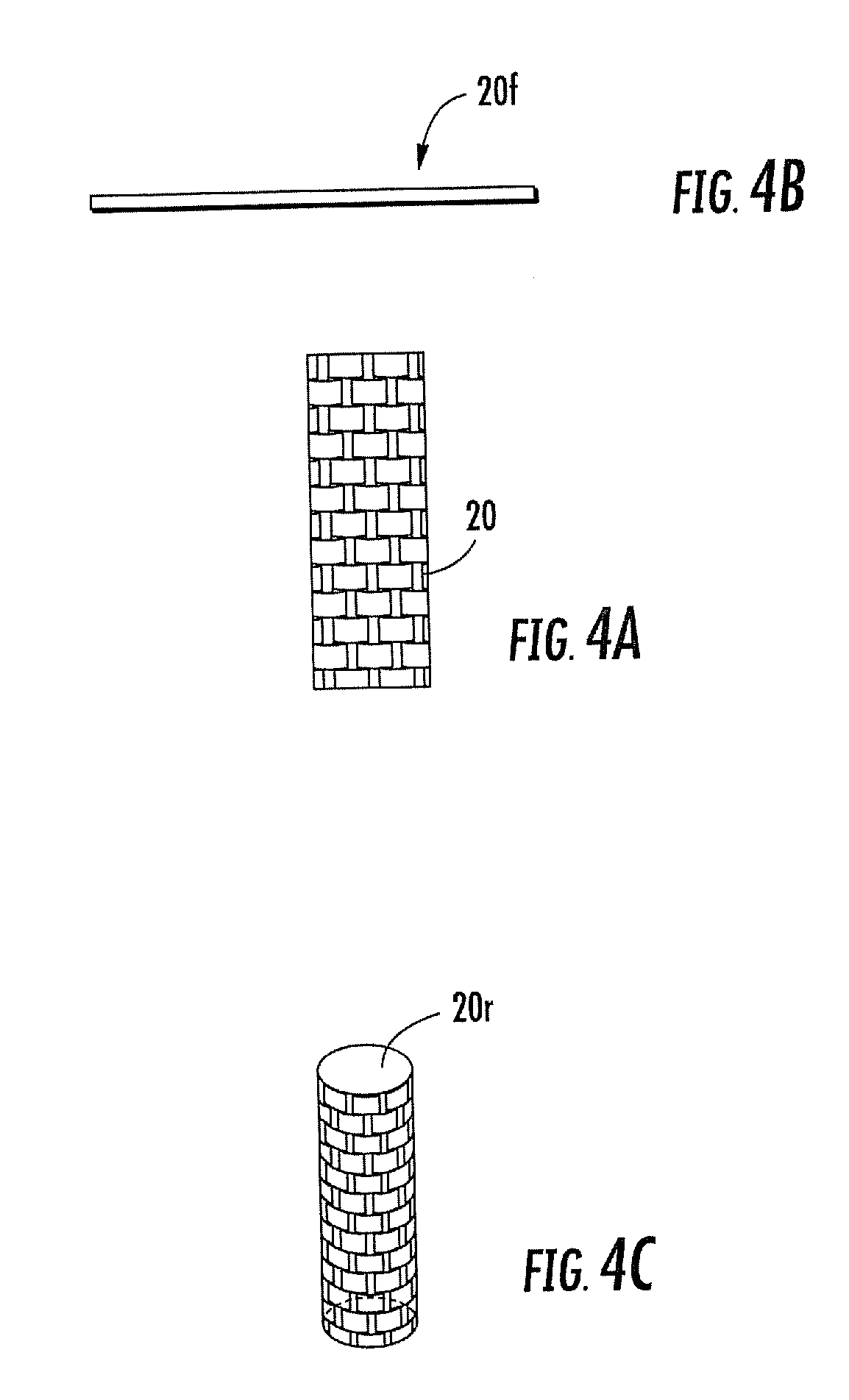
Woven and/or braided fiber implants and methods of making same
Owner:SHRINERS HOSPITALS FOR CRIPPLED CHILDREN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com