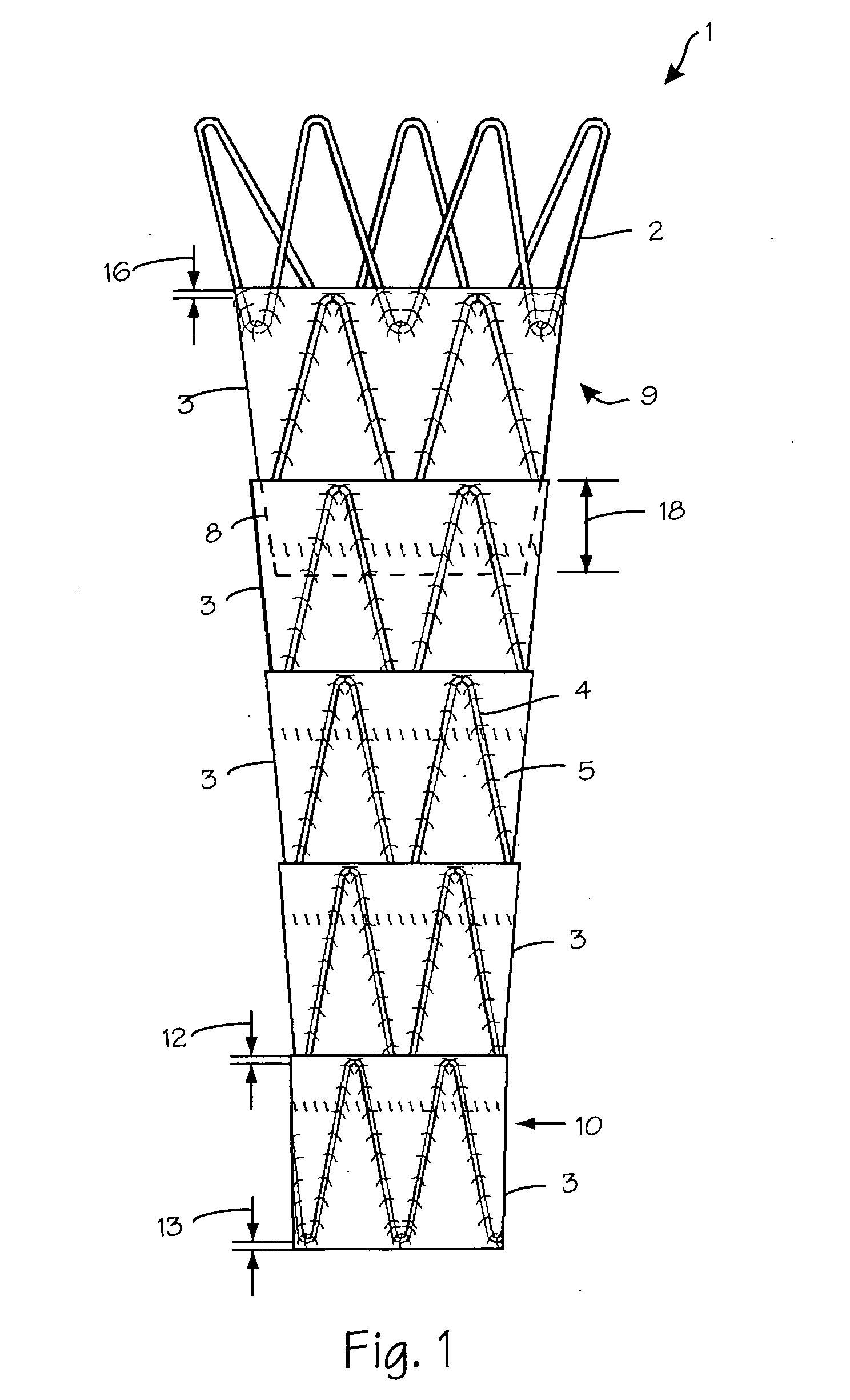
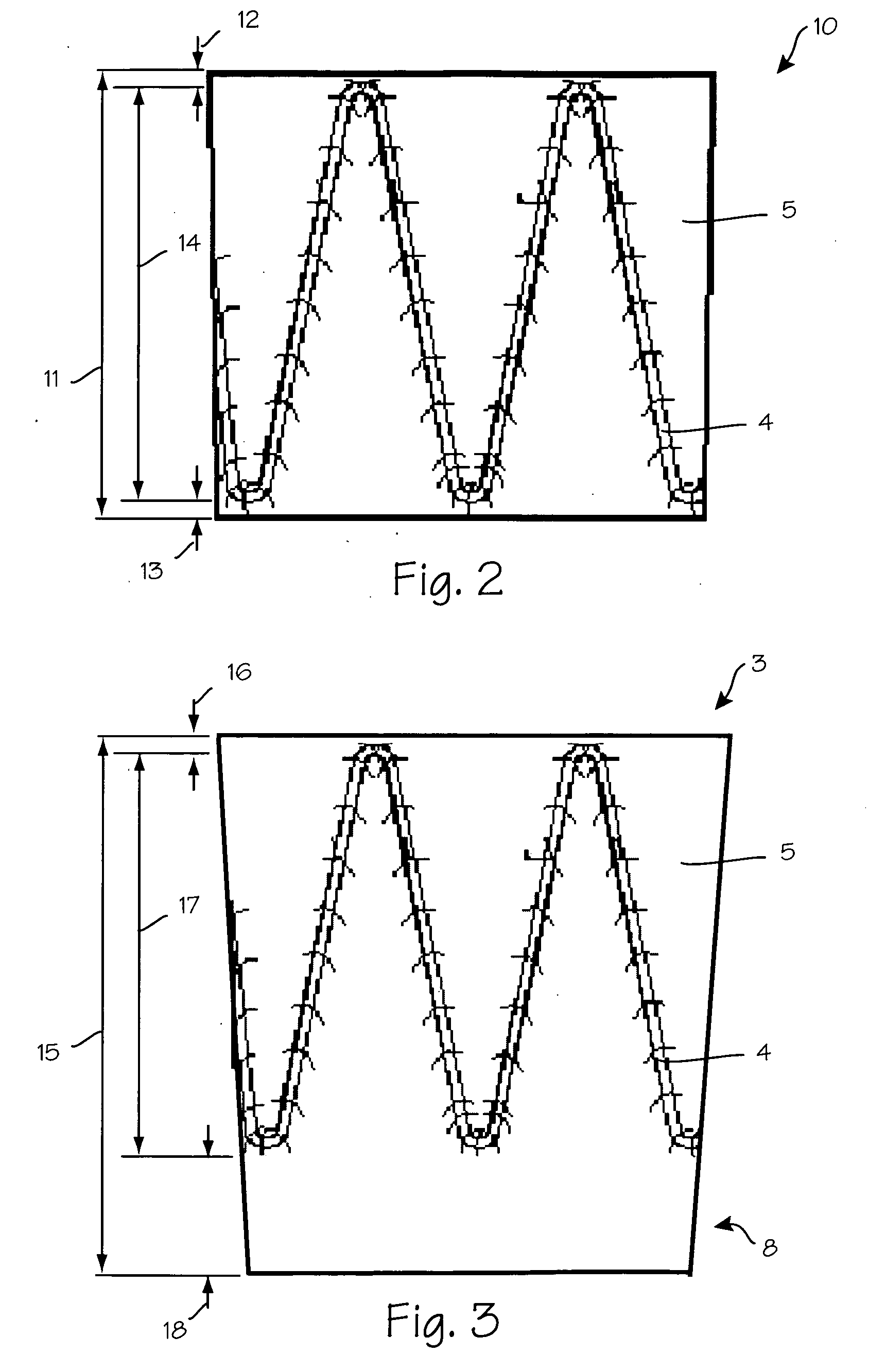
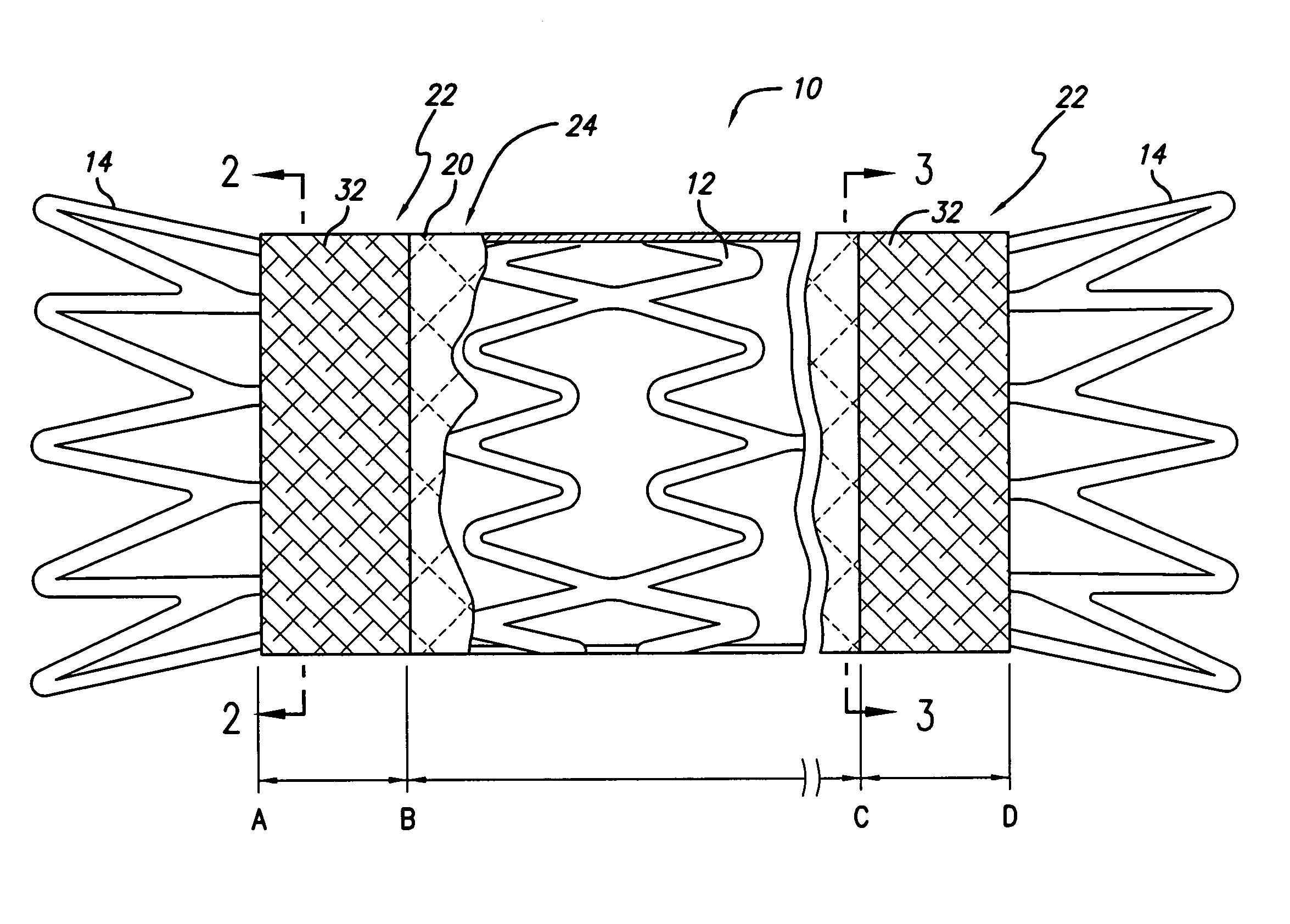
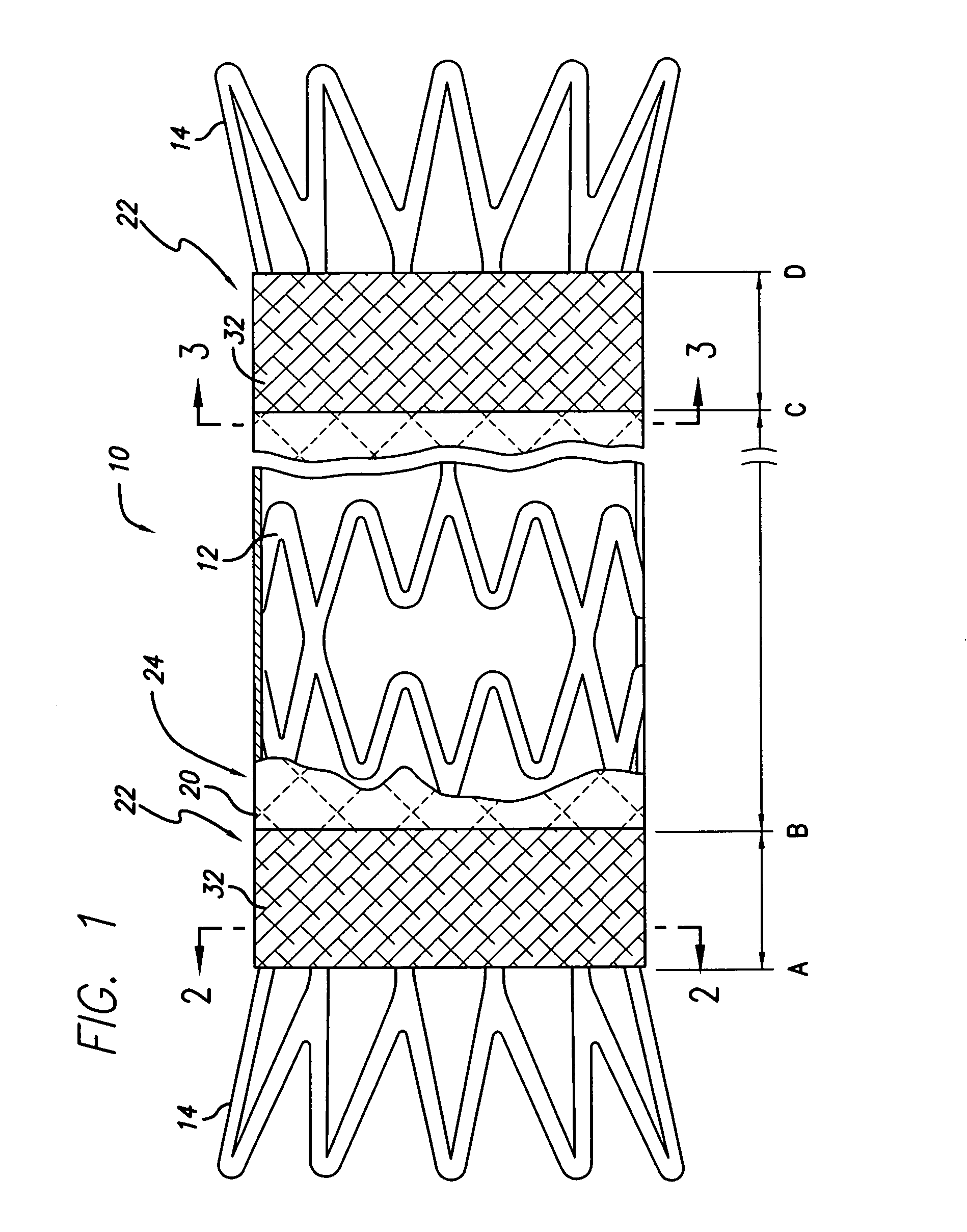
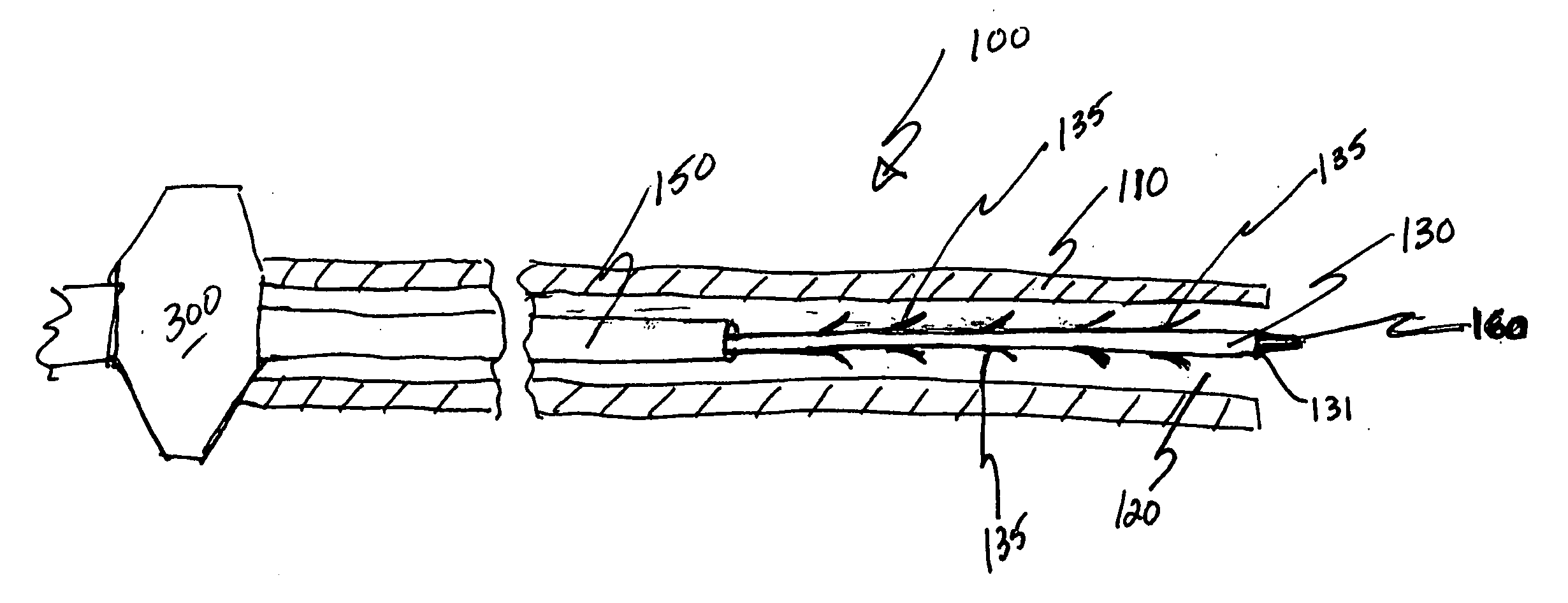
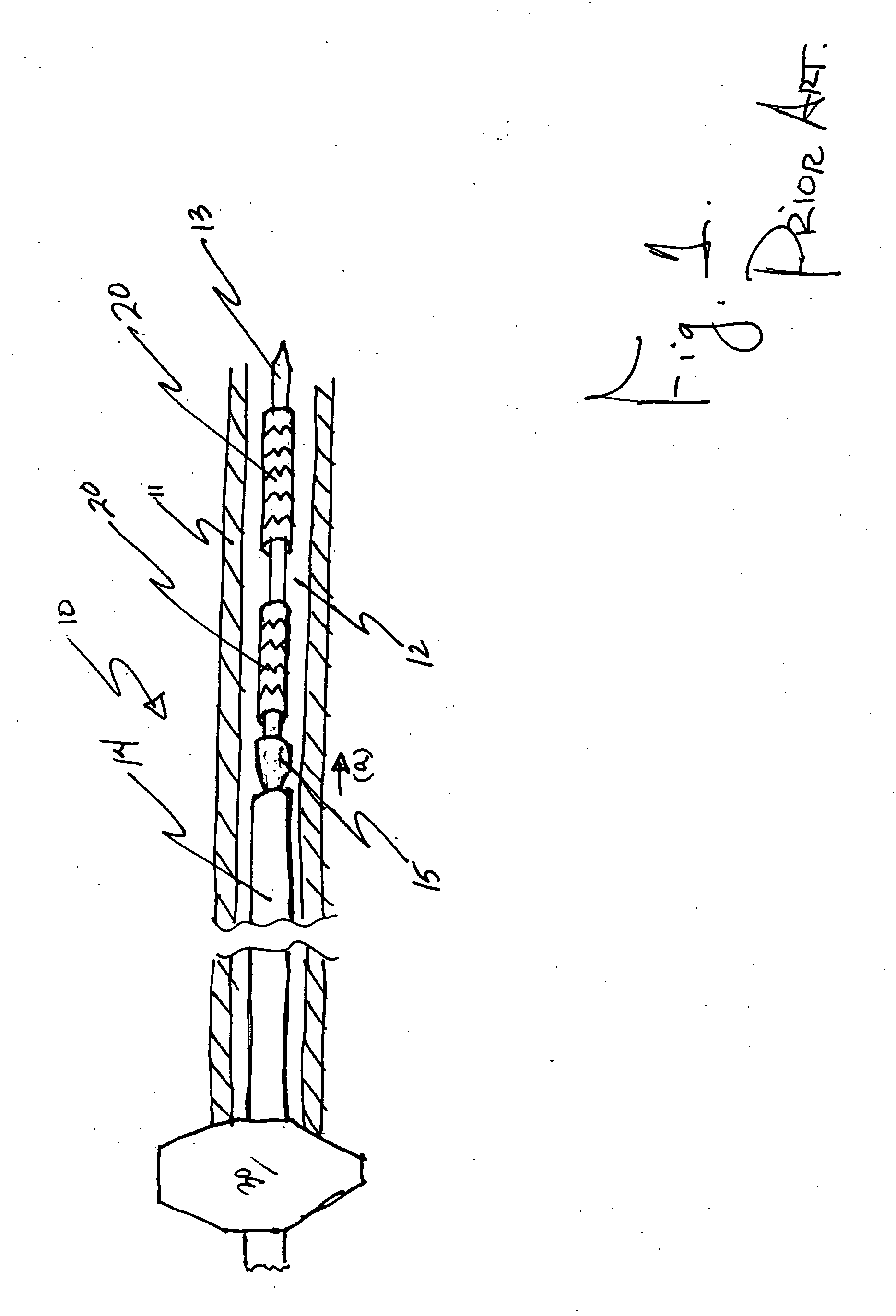
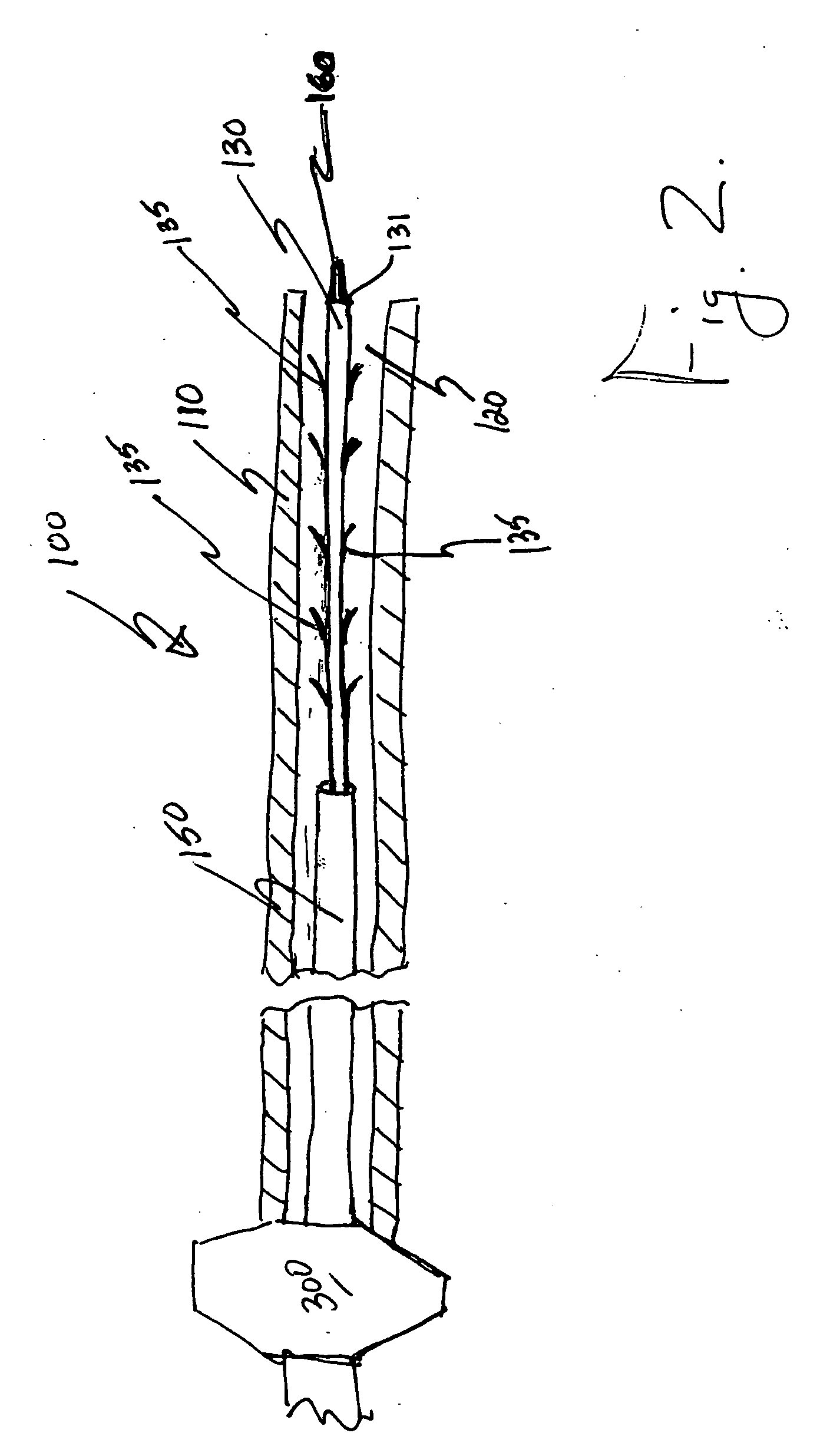
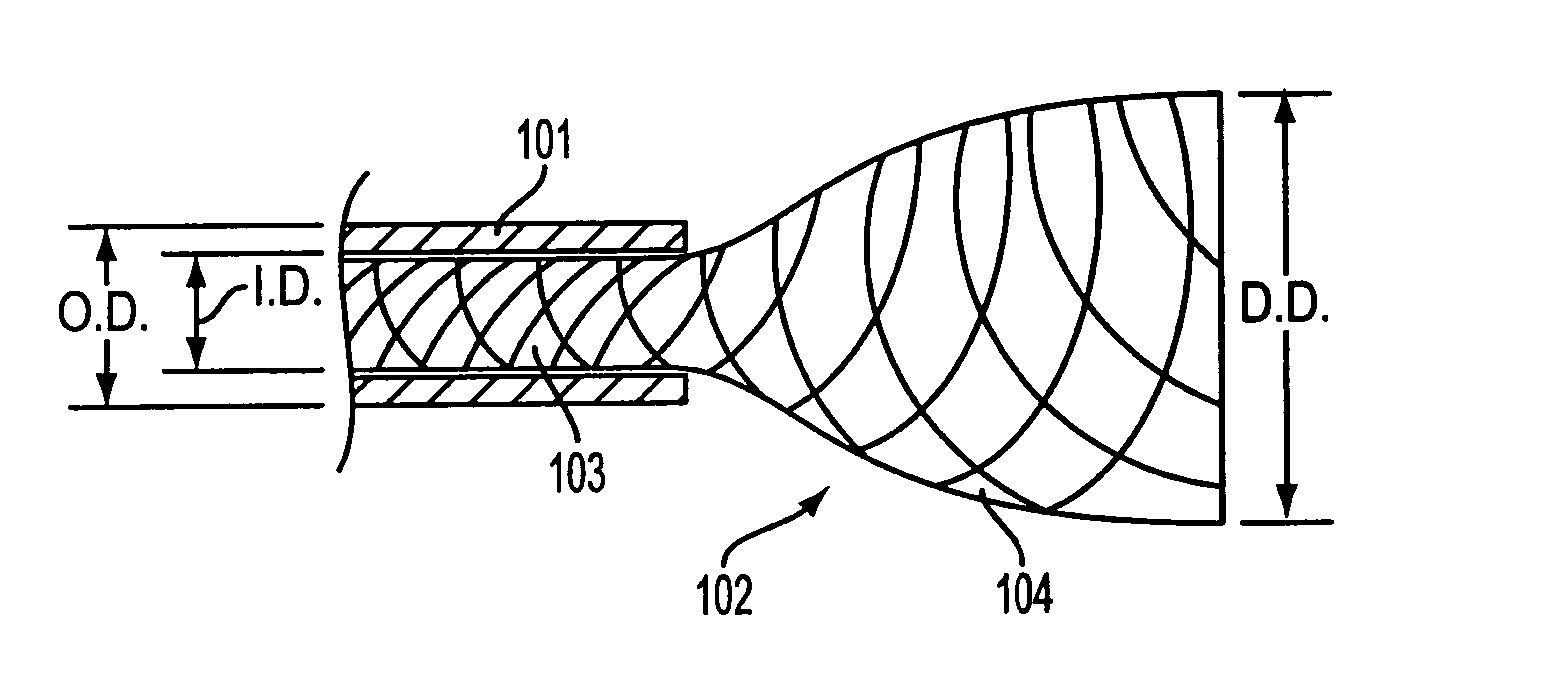
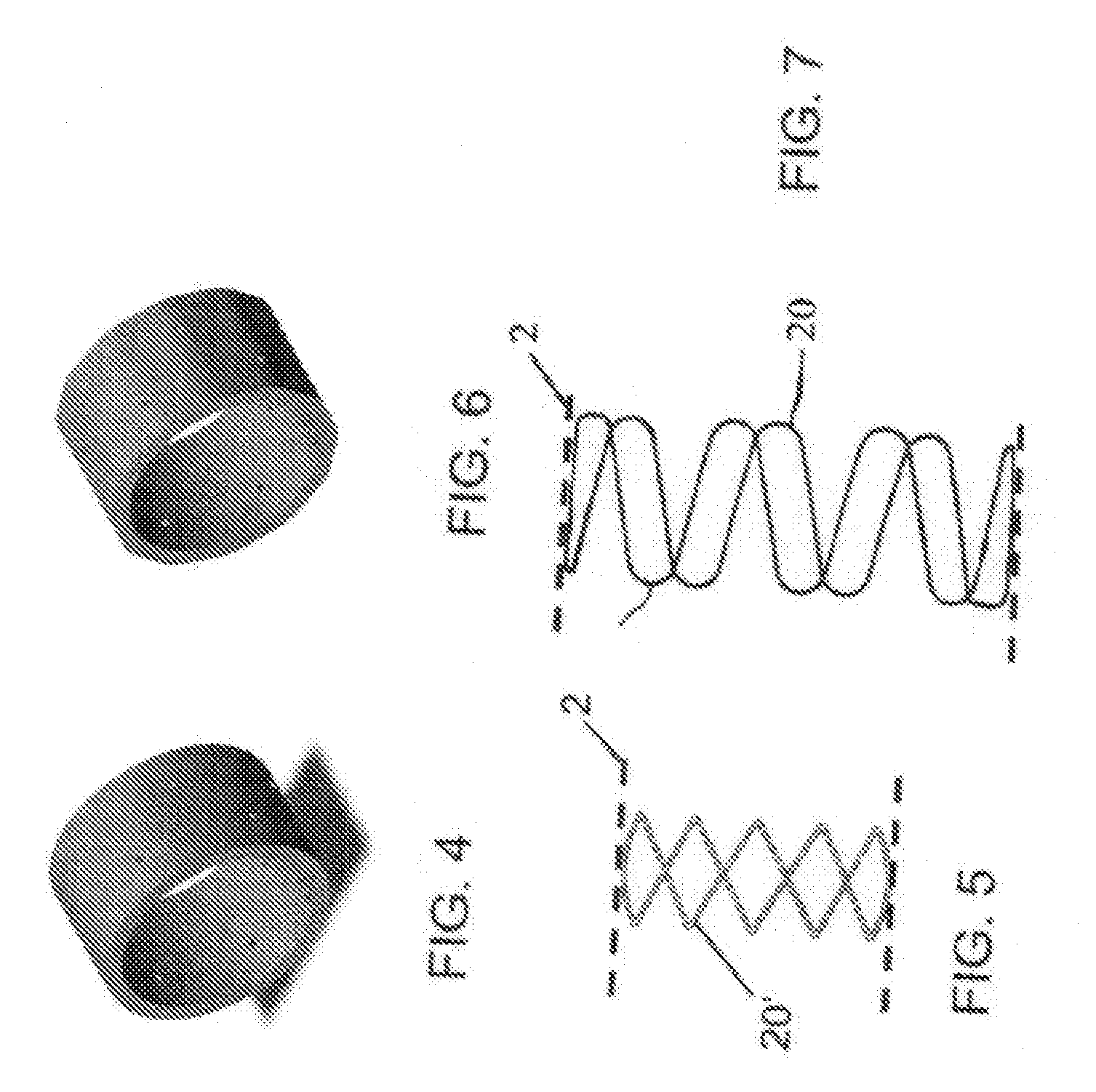Patents
Literature
742 results about "Covered stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A covered stent is a flexible tube used to repair or support a damaged section of a blood vessel. These stents are made of a metallic frame and are covered by an extremely durable fabric. Placing a covered stent into a damaged blood vessel is a relatively simple surgery even when the blood vessels are around the heart.
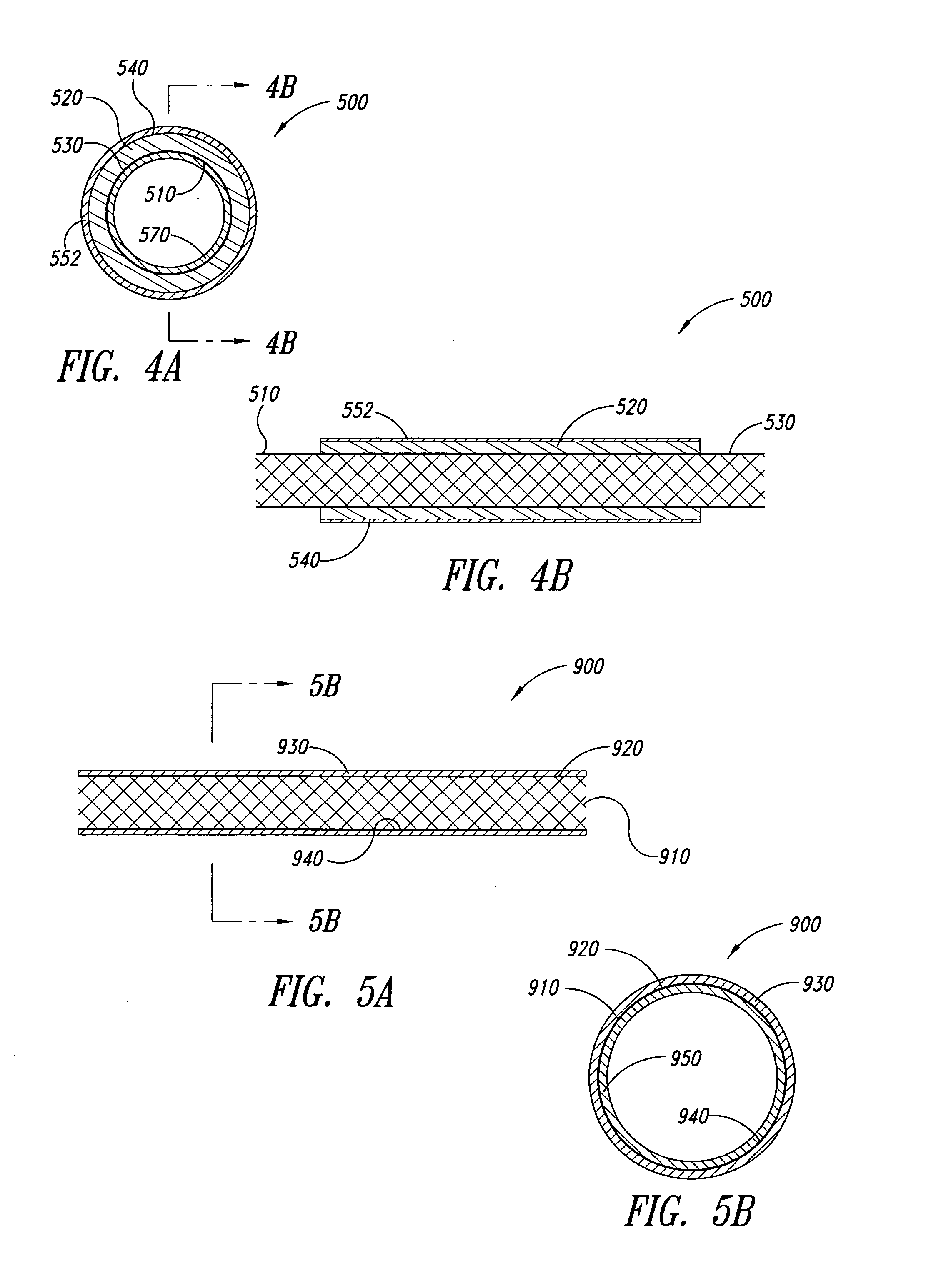
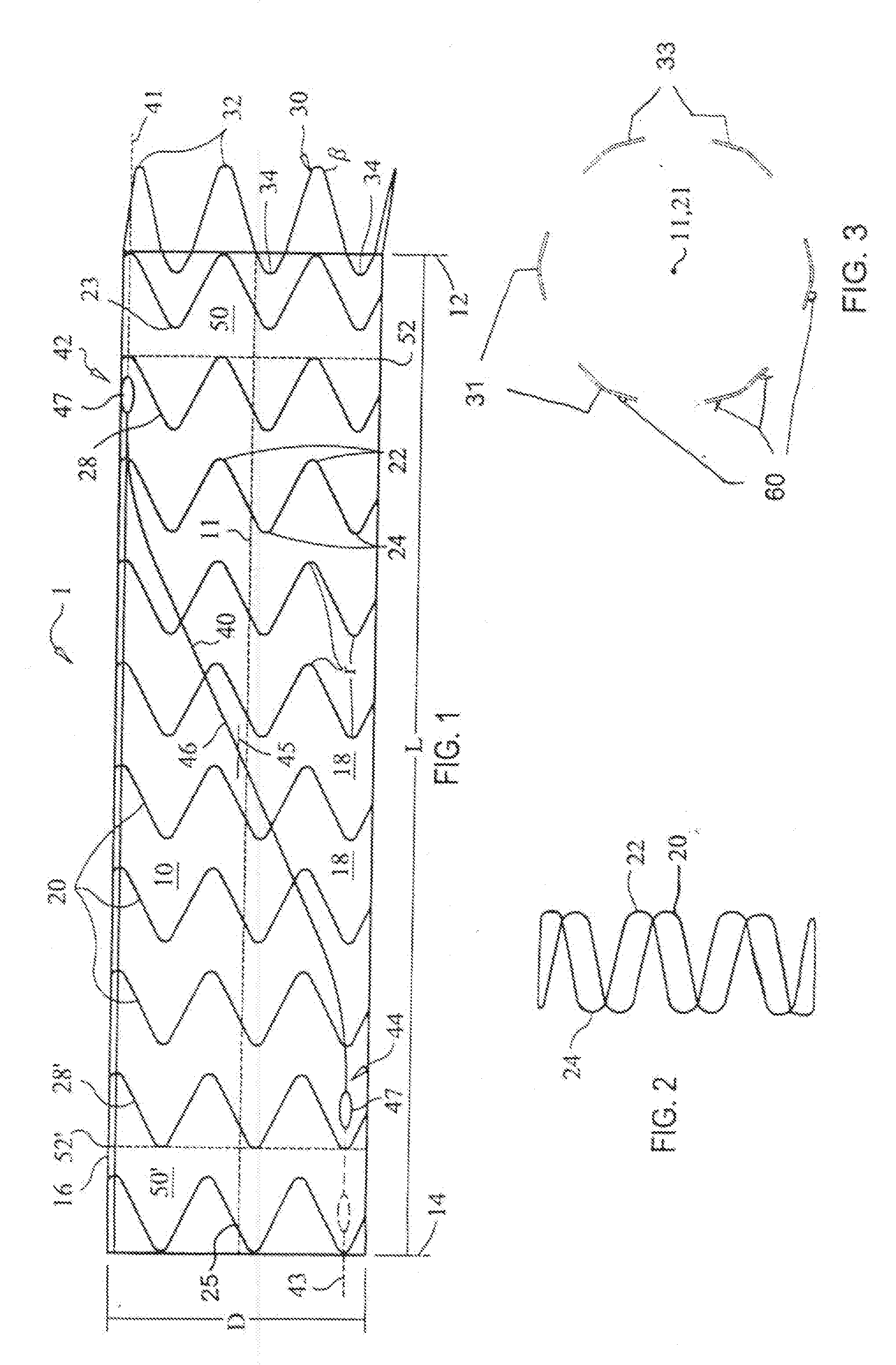
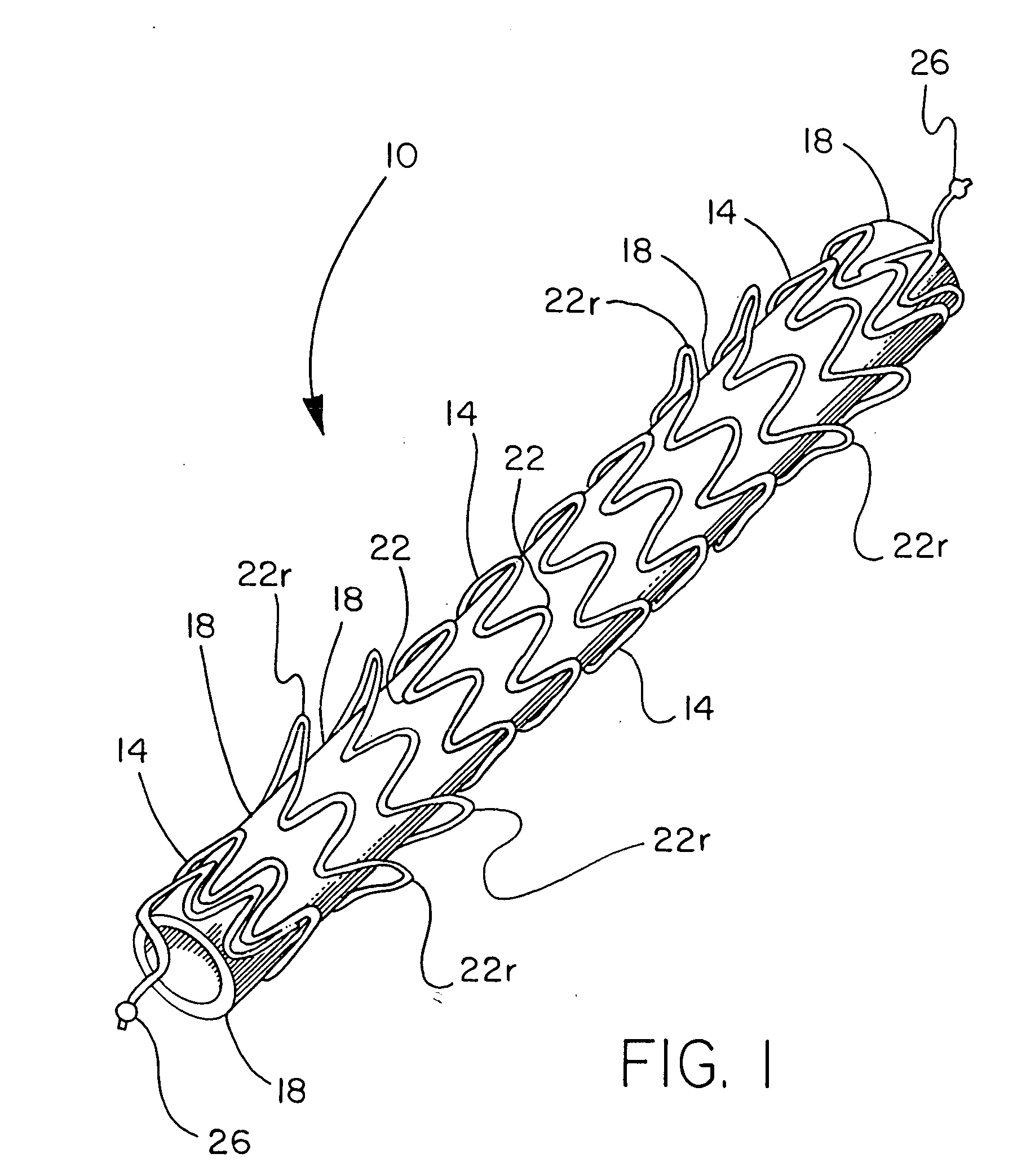
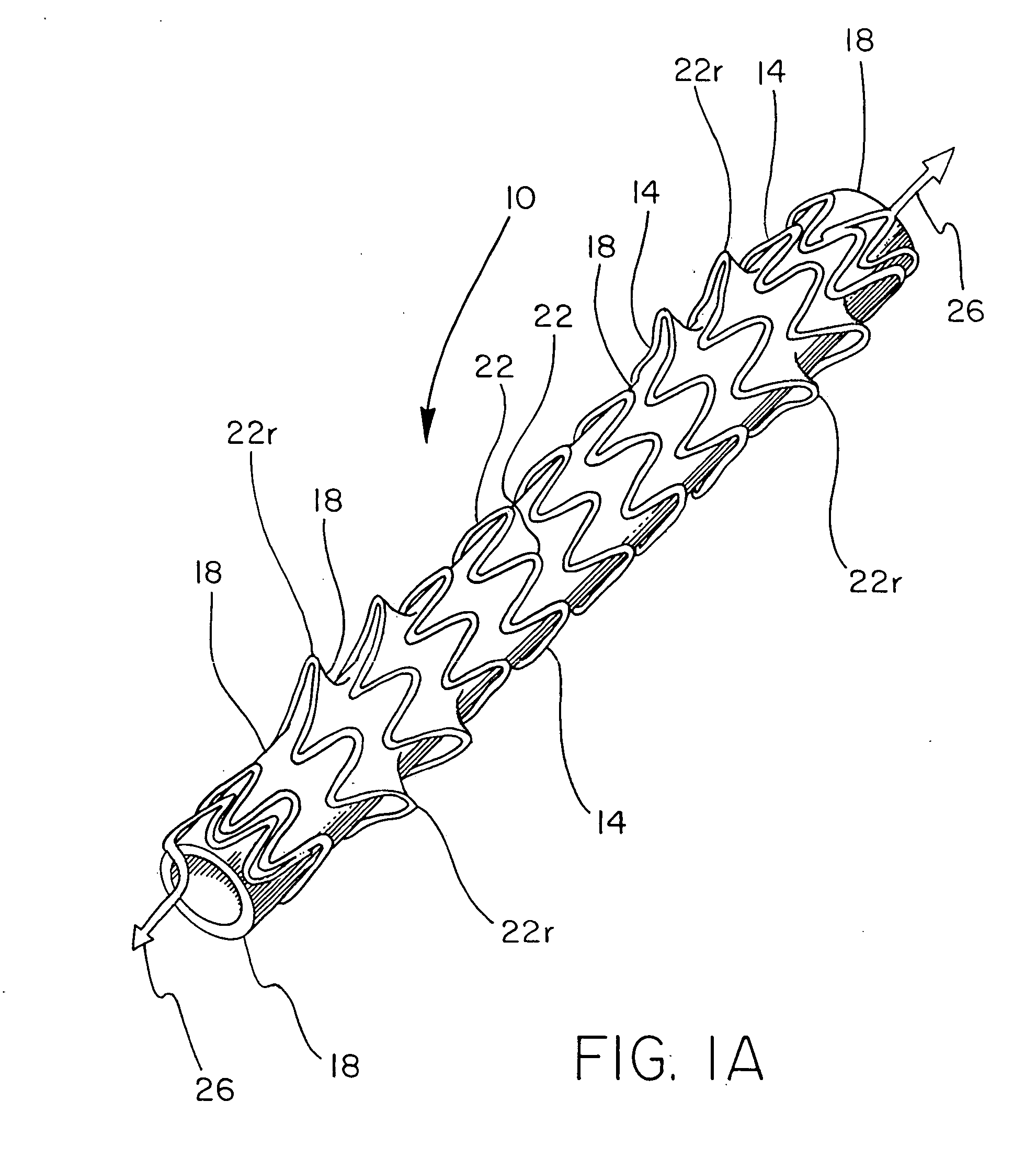
Hybrid intravascular stent
InactiveUS6866805B2Increase flexibilityImprove radial strengthStentsSurgeryCoronary arteriesIntravascular stent
A hybrid stent is formed which exhibits both high flexibility and high radial strength. The expandable hybrid stent for implantation in a body lumen, such as a coronary artery, consists of radially expandable cylindrical rings generally aligned on a common longitudinal axis and interconnected by one or more links. In one embodiment, a dip-coated covered stent is formed by encapsulating cylindrical rings within a polymer material. In other embodiments, at least some of the rings and links are formed of a polymer material which provides longitudinal and flexural flexibility to the stent. These polymer rings and links are alternated with metallic rings and links in various configurations to attain sufficient column strength along with the requisite flexibility in holding open the target site within the body lumen. Alternatively, a laminated, linkless hybrid stent is formed by encapsulating cylindrical rings within a polymer tube.
Owner:ABBOTT CARDIOVASCULAR
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149173A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Covered stent with biologically active material
A composite device provides for the delivery of bioactive agents associated therewith. The device includes a first polymeric liner and a second polymeric liner bonded to the first liner. The device further includes an intermediate structural member interposed between the liner, which is defined by solid segments and openings between the solid segments. The liners are joined through the openings to form at least one pocket about the solid segments. A bioactive agent is located within the pockets about the solid segments of the intermediate structural member.
Owner:BOSTON SCI SCIMED INC
Methods of implanting covered stents with side branch
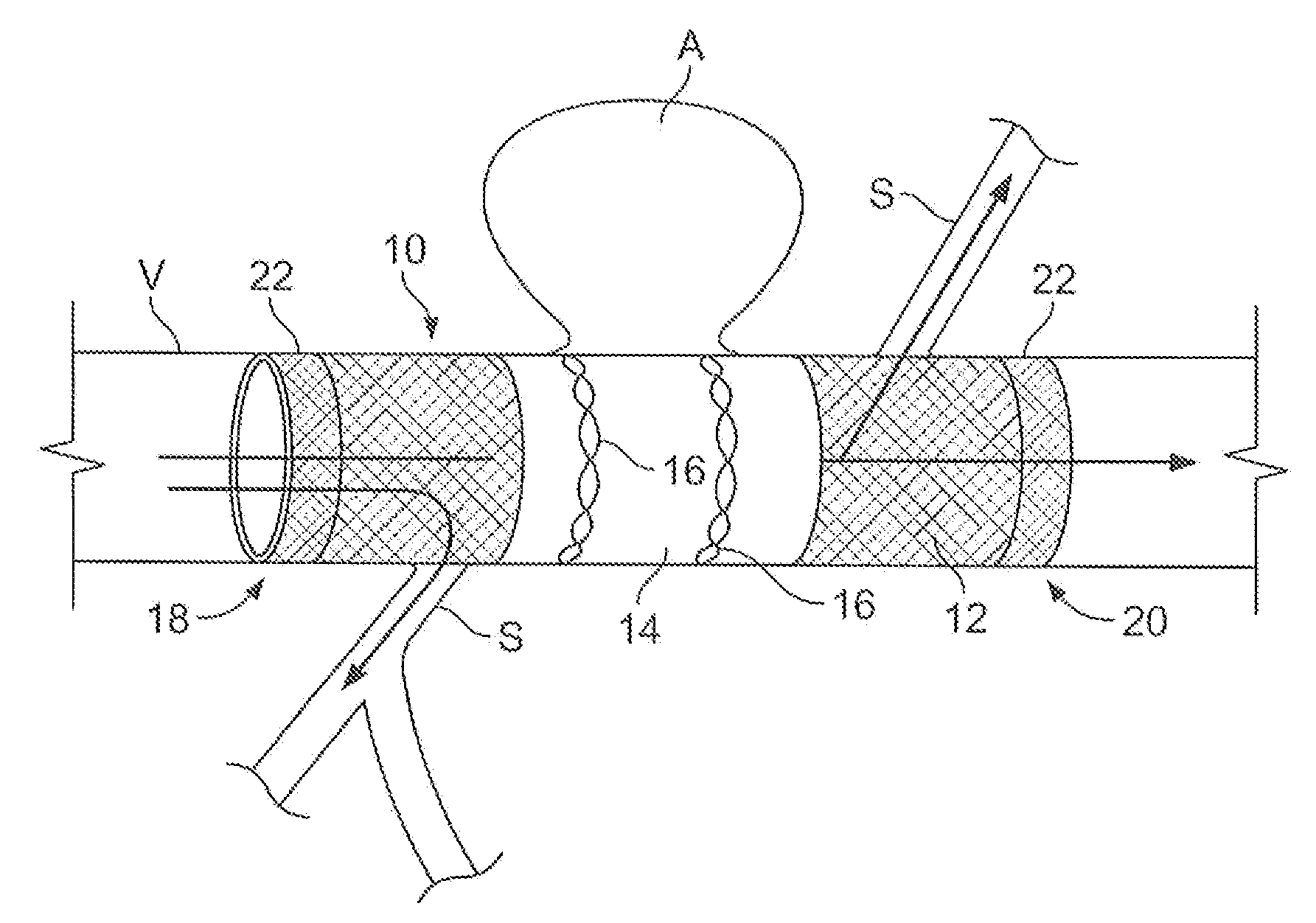
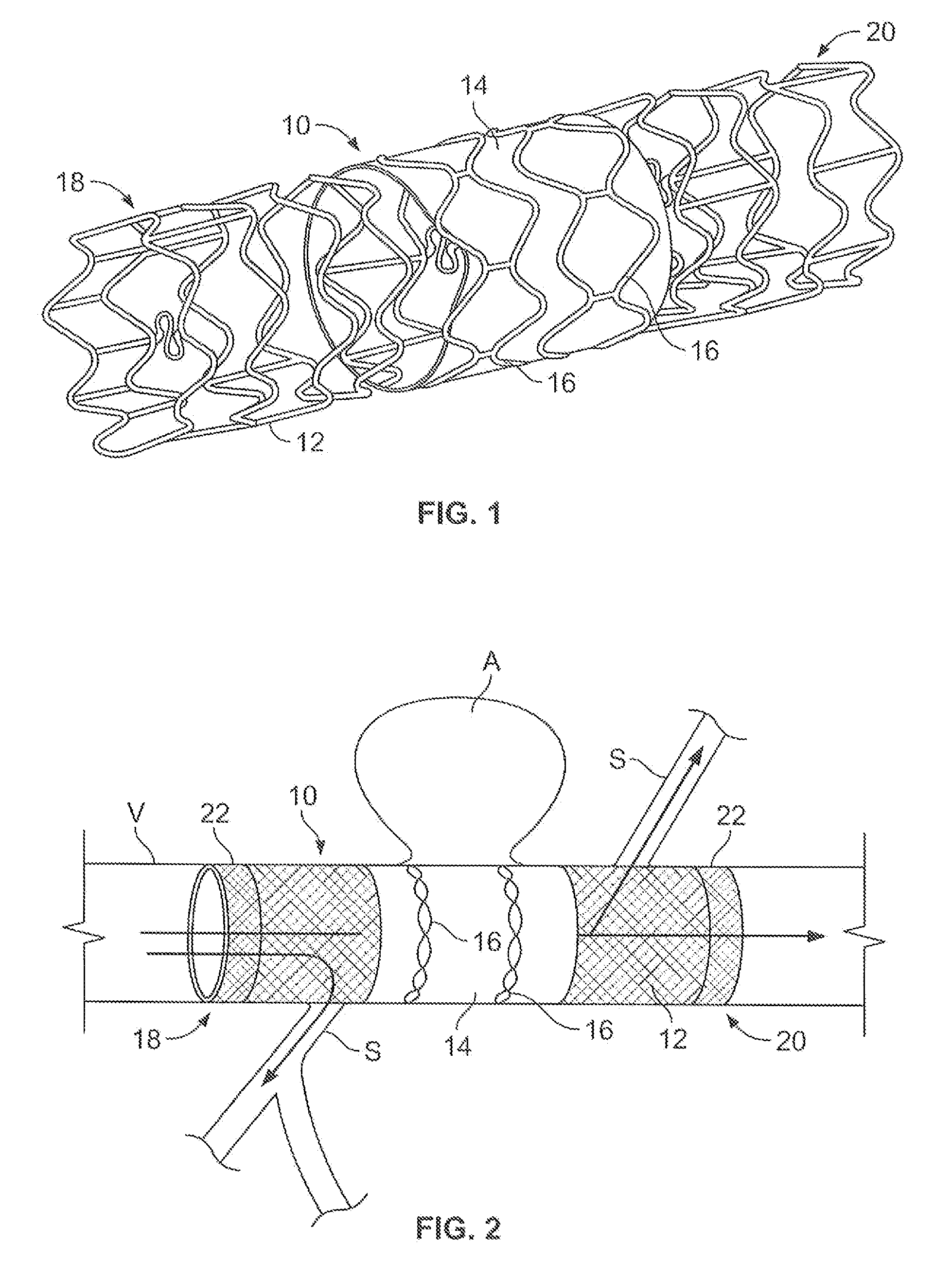
A method of implanting first and second stents with associated grafts within first and second vessel regions extending at an angle with respect to each other comprising inserting a first guidewire to guide a first stent with an associated first graft to the first vessel region, inserting a second guidewire to guide a second stent with an associated second graft to the second vessel region, inserting first and second delivery sheaths containing the first and second stent with the associated grafts over the first and second guidewires and removing the first and second delivery sheaths to enable the first and second stents with the associated grafts to expand against the wall of the first and second vessel regions, respectively. A delivery system is also disclosed.
Owner:REX MEDICAL LP
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149175A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Stent with Anti-migration feature
ActiveUS20090187240A1Reduce migration of stentPrevent and minimize ingrowthStentsSurgeryProsthesisStent removal
An intraluminal prosthesis includes an outer three-dimensional (3D) anti-migration structure that is attached to the outer wall of a fully covered or partially covered stent to prevent migration and still allow stent removal at a later period of time. A method of manufacturing the intraluminal prosthesis includes attaching the anti-migration structure by usage of a polymer such as polyurethane.
Owner:BOSTON SCI SCIMED INC
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
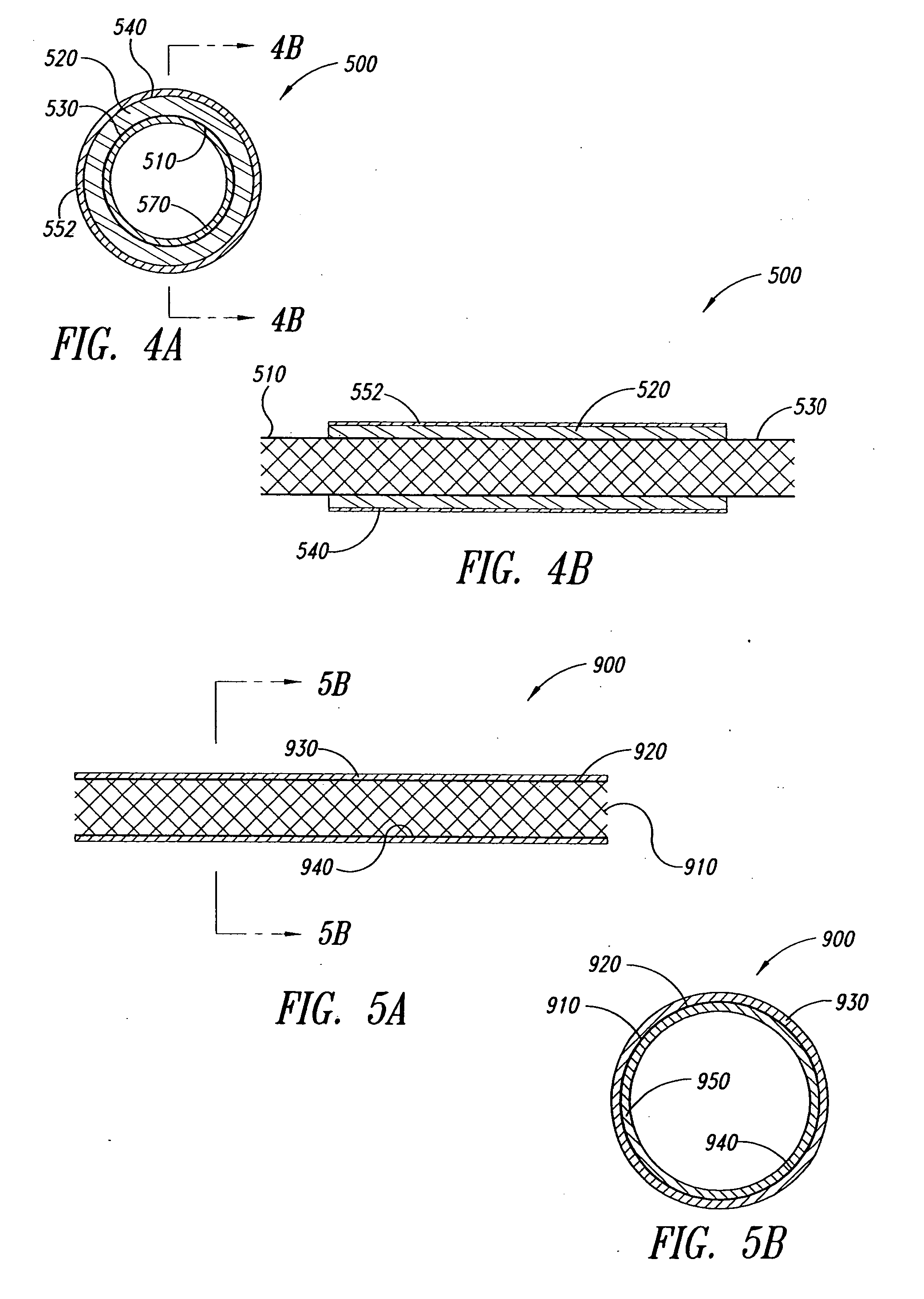
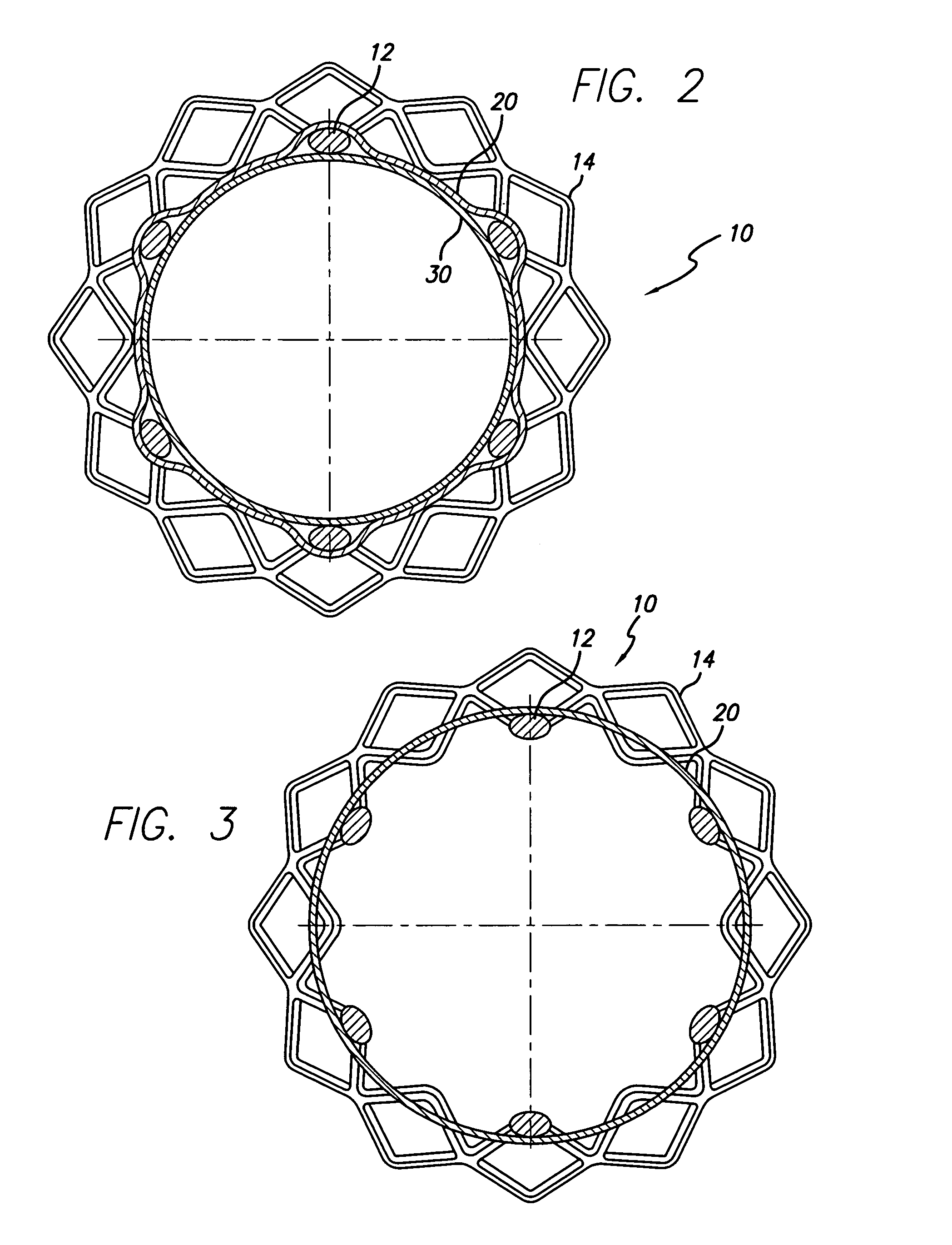
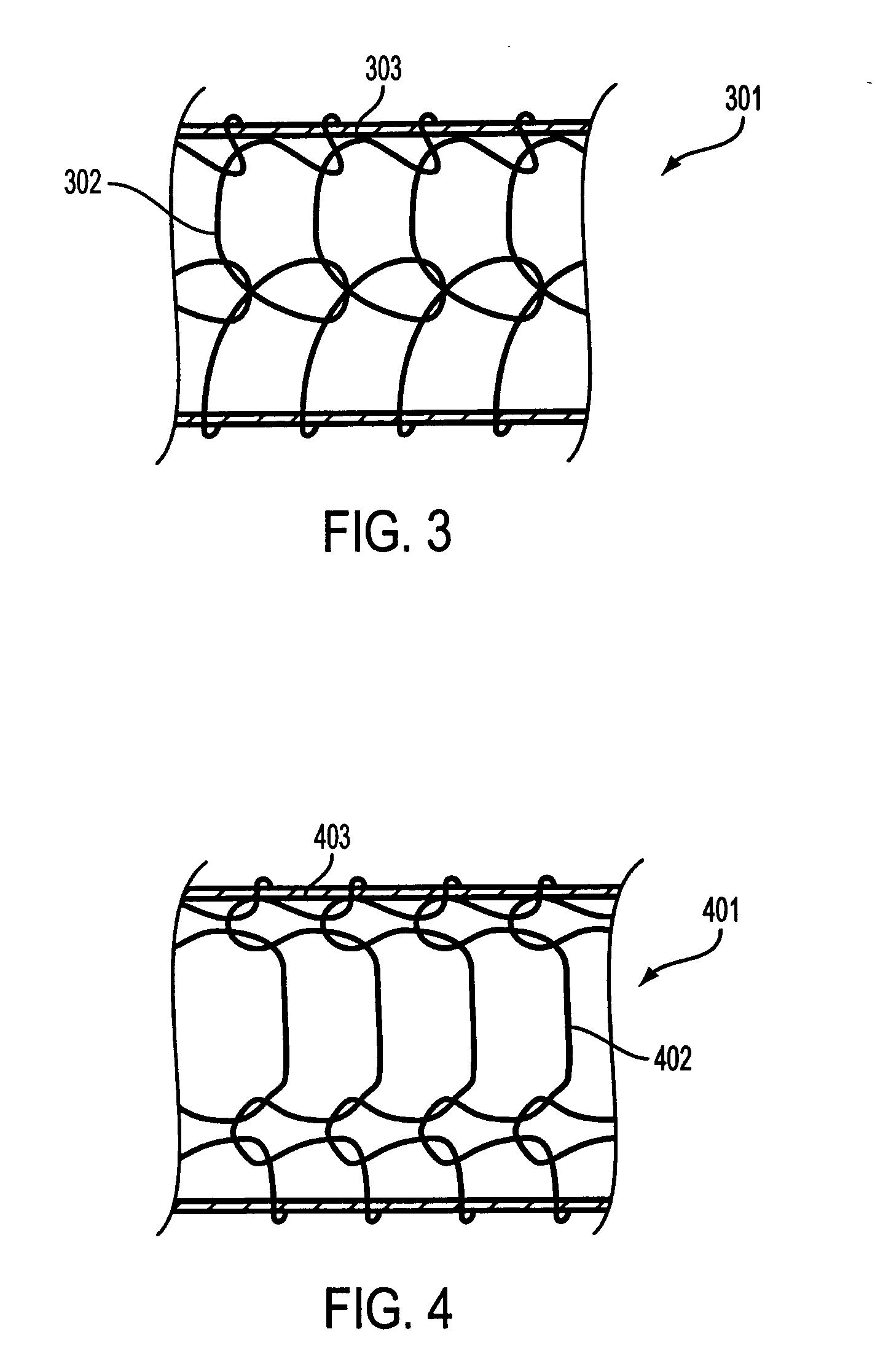
Covered segmented stent
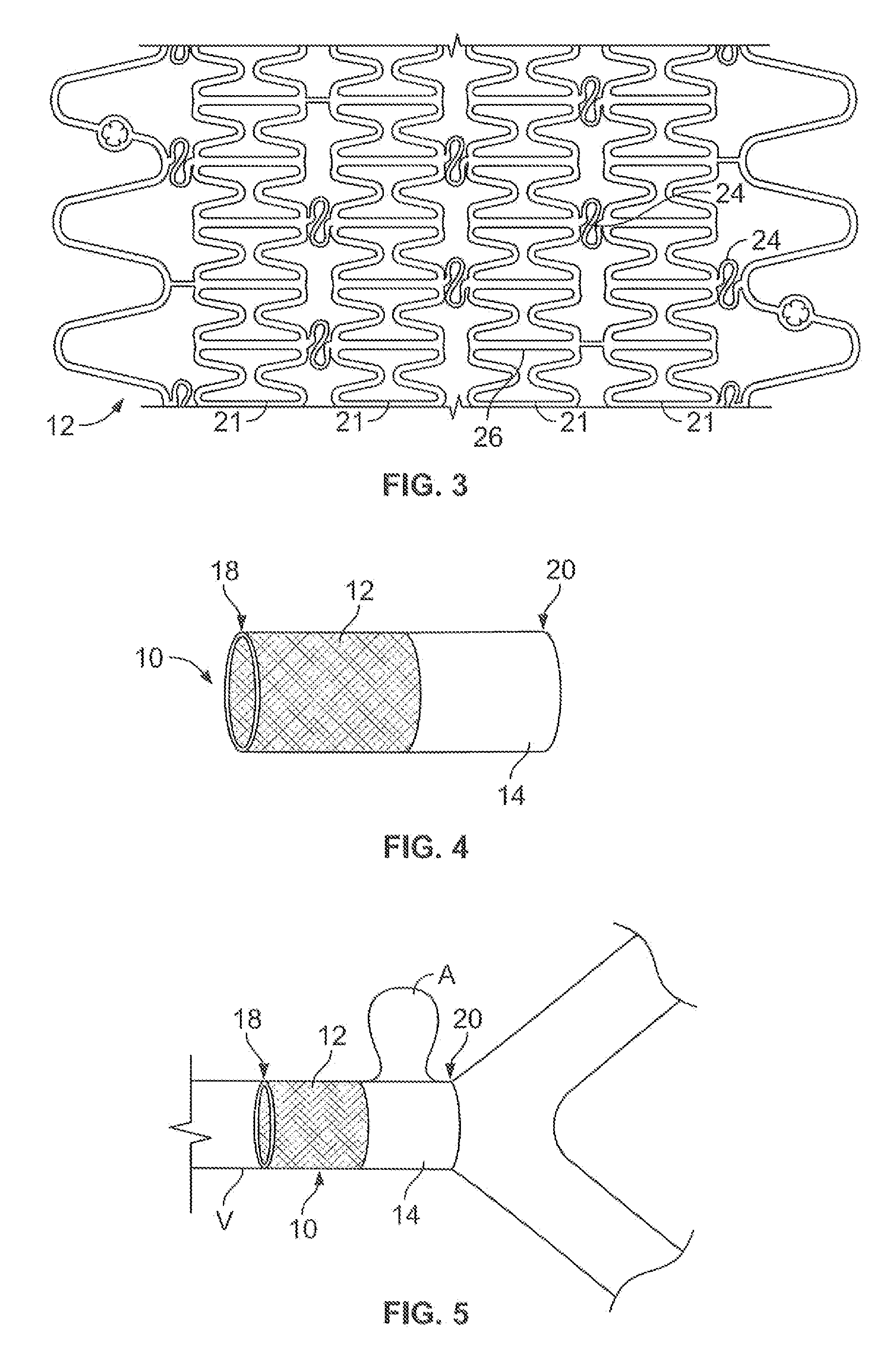
A covered stent comprising individual stent rings alternately loaded inside and outside of the covering material, wherein the rings are not connected to adjacent rings along the longitudinal axis.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
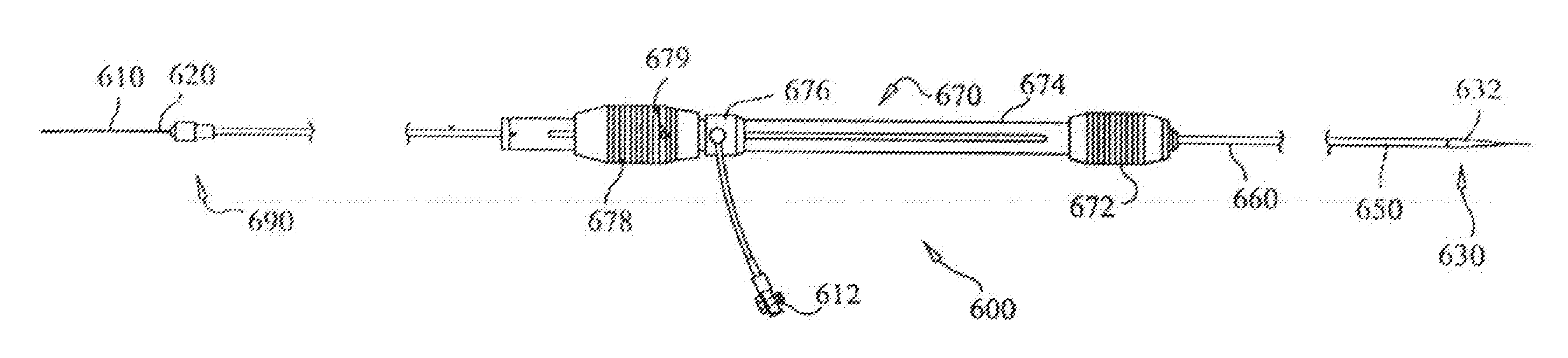
Double sheath deployment system
A stent-graft deployment system (10) includes a stent-graft (15), a flexible catheter tip (12) attached to a catheter shaft (25), a retractable primary sheath (20) containing the stent-graft in a first constrained small diameter configuration around the catheter shaft near the flexible tip. The stent-graft deployment system further includes a flexible secondary sheath (14) disposed within the retractable primary sheath and also containing the stent-graft, wherein when the primary sheath is removed from around the stent-graft, the flexible secondary sheath contains the stent-graft in a second constrained small diameter configuration around the catheter shaft near the flexible tip. The removal of the secondary sheath releases the stent-graft from a radial constraint so that stent-graft deployment may proceed.
Owner:MEDTRONIC VASCULAR INC
Stent for Endovascular Procedures
A stent graft for deploying a stent graft at a lesion site is provided. The stent graft comprises a tubular graft and a support stent, at least a portion of which lies within the tubular graft. The graft has a proximal end and a distal end. The support stent has a proximal end and a distal end, and comprises at least one wire forming a first helical wire portion having a first translational direction about an axis of the support stent and a second helical wire portion having a second translational direction about the axis opposite the first translational direction. When the support stent is in a compressed delivery configuration, the support stent has a first length, and when the support stent is in an expanded deployed configuration, the support stent has a second length which is less than the first length. When in the delivery configuration, the support stent is attached to the graft only at or near the proximal end of the graft.
Owner:VASCULAR INNOVATIONS
Stent Graft Repair Device
ActiveUS20070219620A1Avoid possibilityHigh tensile strengthStentsBlood vesselsStent graftingInsertion stent
A repair device (10) for affixing a migrating stent graft (30) to the interior surface of a vessel wall (31). The repair device includes tubular graft (11) with a bare or uncovered stent (16) affixed to the proximal end (12). The bare stent includes a plurality of distally pointed barbs (17) for securing the repair device to a vessel wall. A second stent (15) is positioned in the passage (14) of the tubular graft to expand the graft against the interior surface of the migrating stent graft (30). Proximally pointing barbs (20) are affixed to the struts of the second stent and extend through the graft material for securing the repair device to the migrating stent graft. Biological glue (22) and other sealing material (23) can be applied to the tubular graft and / or stents for sealing the repair device against the vessel wall and / or the interior of the migrating stent graft.
Owner:COOK MEDICAL TECH LLC
Method for Operating a Stent Graft Delivery System Handle
ActiveUS20080077227A1Reduce the possibilityIncreases blood-tightStentsBlood vesselsStent graftingImplantation Site
A method for delivering a stent graft includes the steps of moving lumens retaining a stent graft within a multi-lumen stent-graft-delivery catheter towards an implantation site to place the stent graft at a first position prior to reaching the implantation site. The delivery catheter has at least one interior lumen holding the stent graft. A handle assembly of the delivery catheter is activated to extend distally the at least one interior lumen towards the implantation site to thereby place the stent graft at a second position substantially within the implantation site while a lumen surrounding the at least one interior lumen of the delivery catheter remains substantially at the first position. The handle assembly is activated to proximally retract a sheath holding the stent graft to at least partially deploy the stent graft at the implantation site.
Owner:BOLTON MEDICAL INC
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154454A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154445A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Intravascular devices and fibrosis-inducing agents
InactiveUS20050154453A1Facilitate “anchoring”Good curative effectStentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Partially covered stent devices and methods of use
InactiveUS20070219619A1Avoid flowMaintain blood flowStentsBlood vesselsFilling materialsCovered stent
Devices, systems and methods are provided for treating aneurysms, particularly cerebral aneurysms. Such treatment is achieved minimally invasively without the need for conventional filling materials and methods. Such treatments may be used for aneurysms located near blood vessel side-branches and bifurcations.
Owner:NFOCUS NEUROMEDICAL
Multi-unit stent-graft
InactiveUS20060195172A1Lower performance requirementsRaise the potentialStentsBlood vesselsMulti unitStent grafting
A new stent-graft is provided with an improved structure design which comprises multiple stent-graft units with / without one or two open stents sutured to combine the uniform prosthesis without a connector bar in the place where flexing required. Each stent-graft unit comprises a stent sutured to a tubular graft. The new stent-graft can bend, provides a smooth inner surface and is stable after placement within an inner lumen of the human body.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD +1
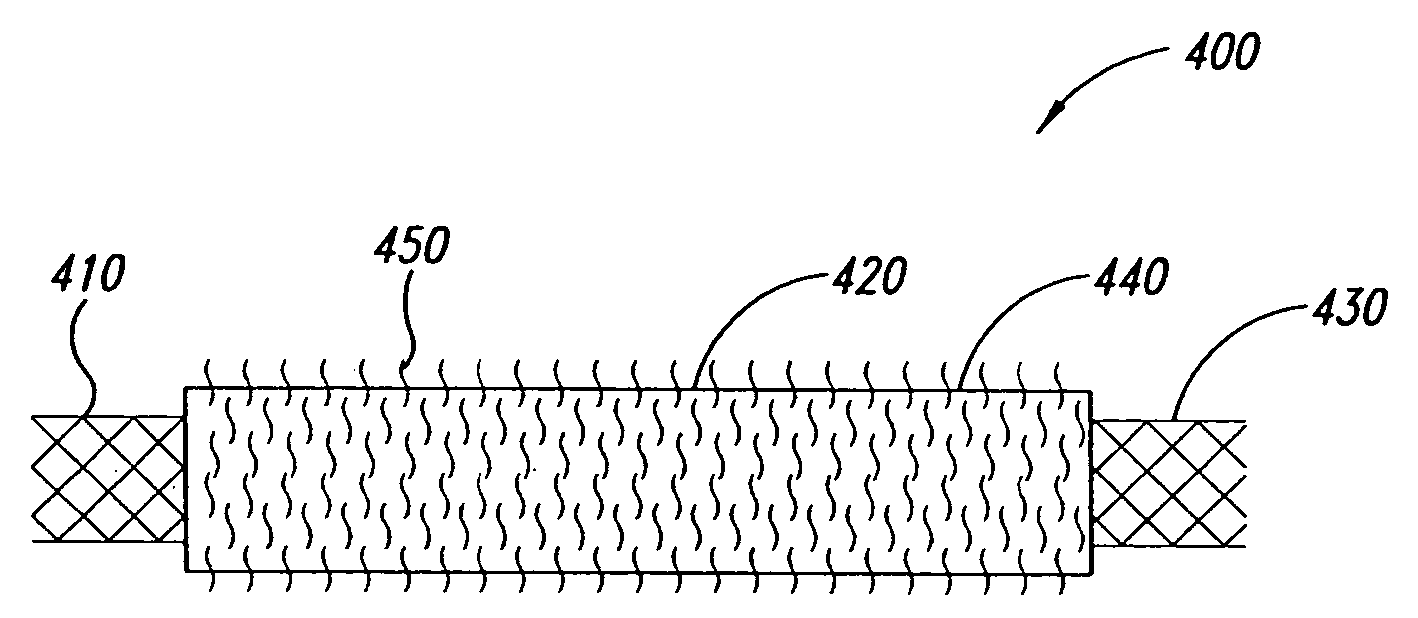
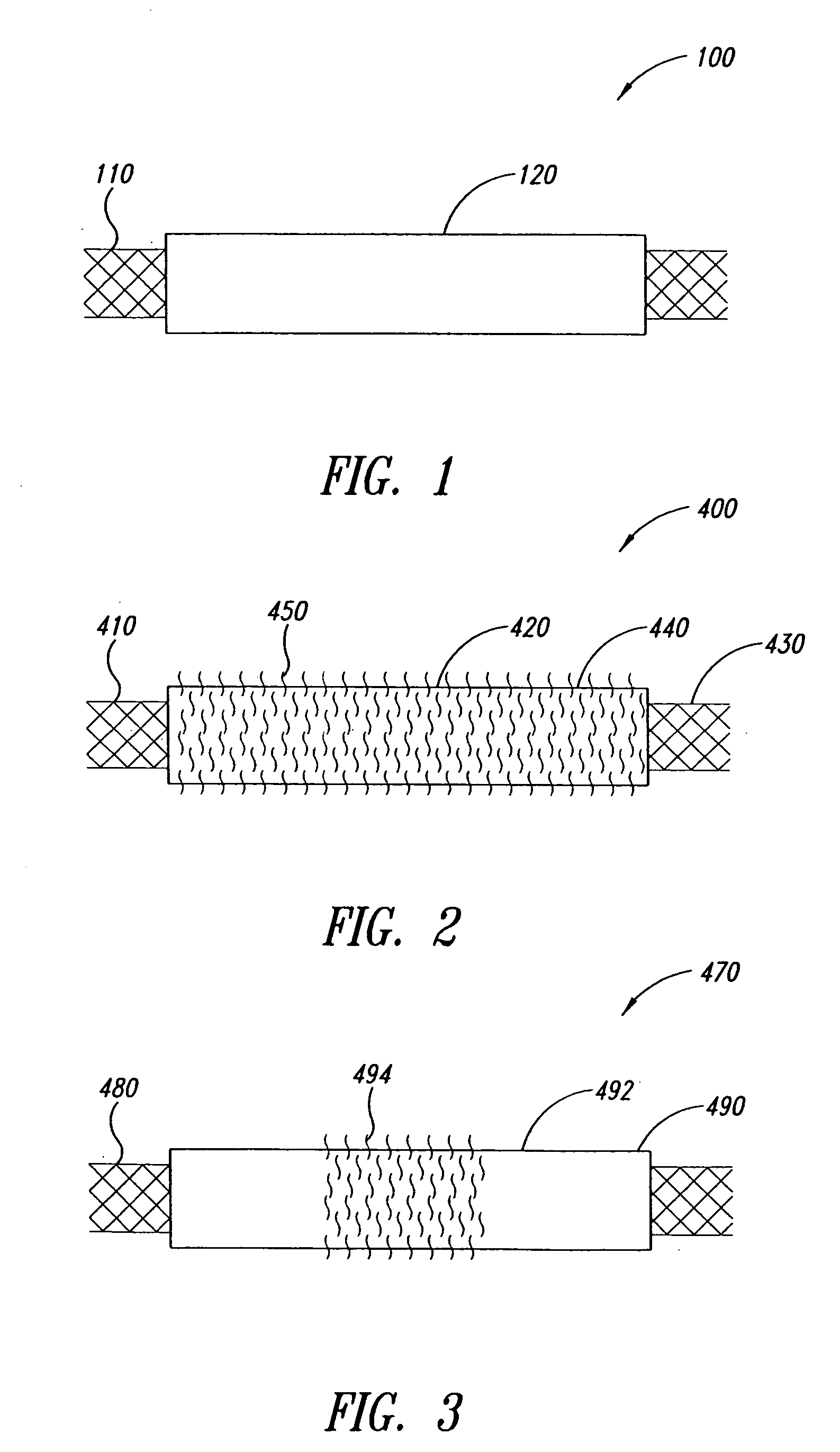
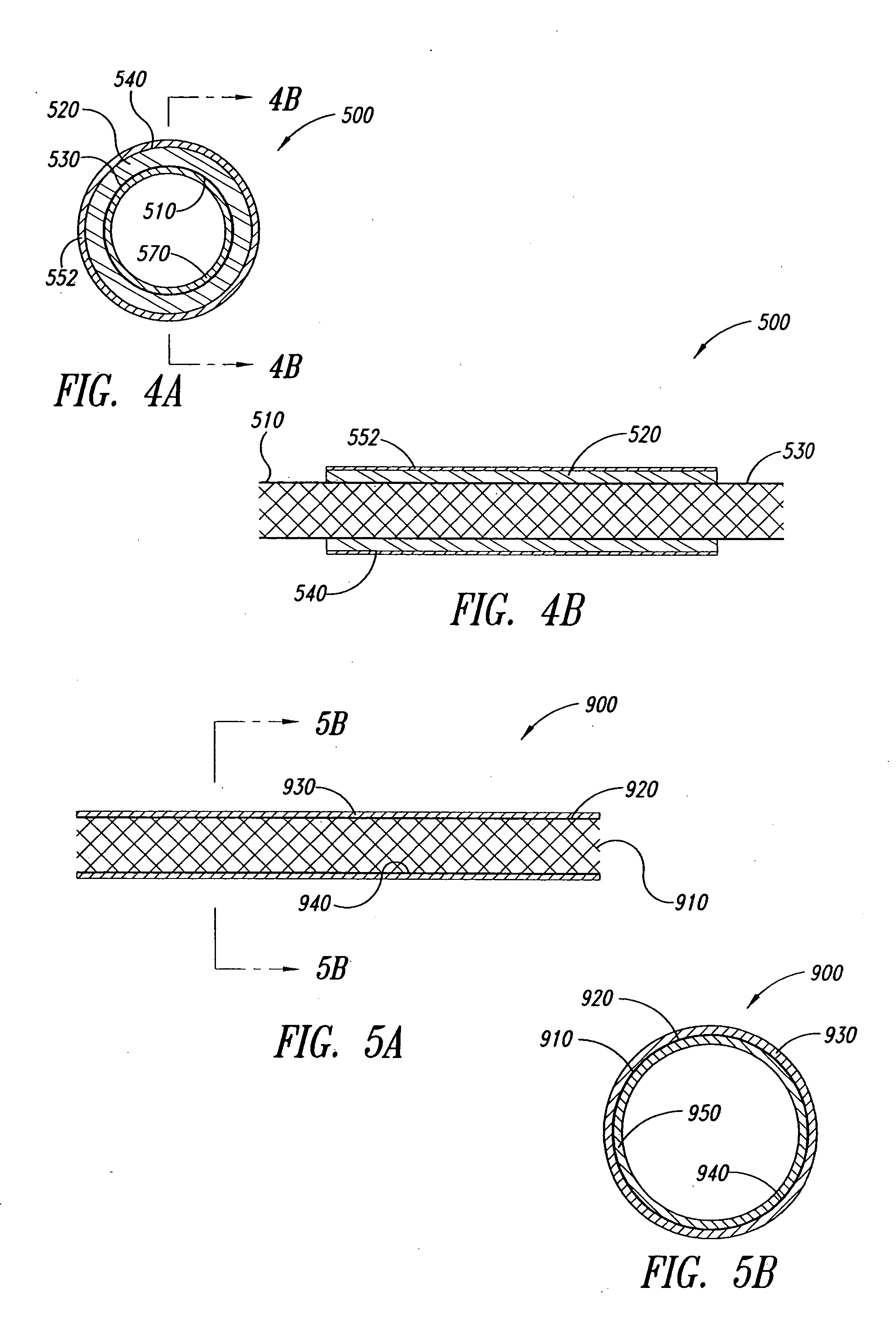
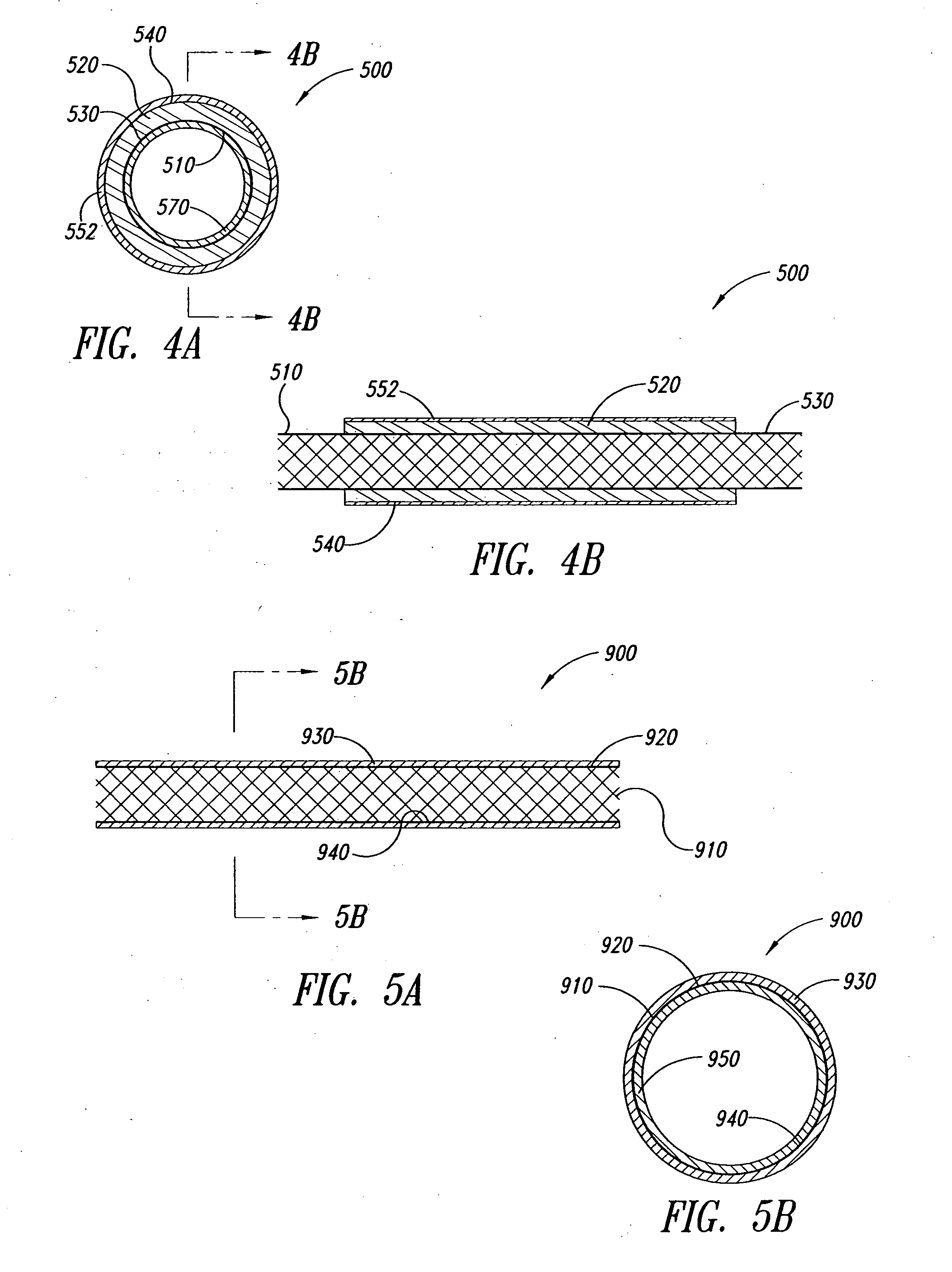
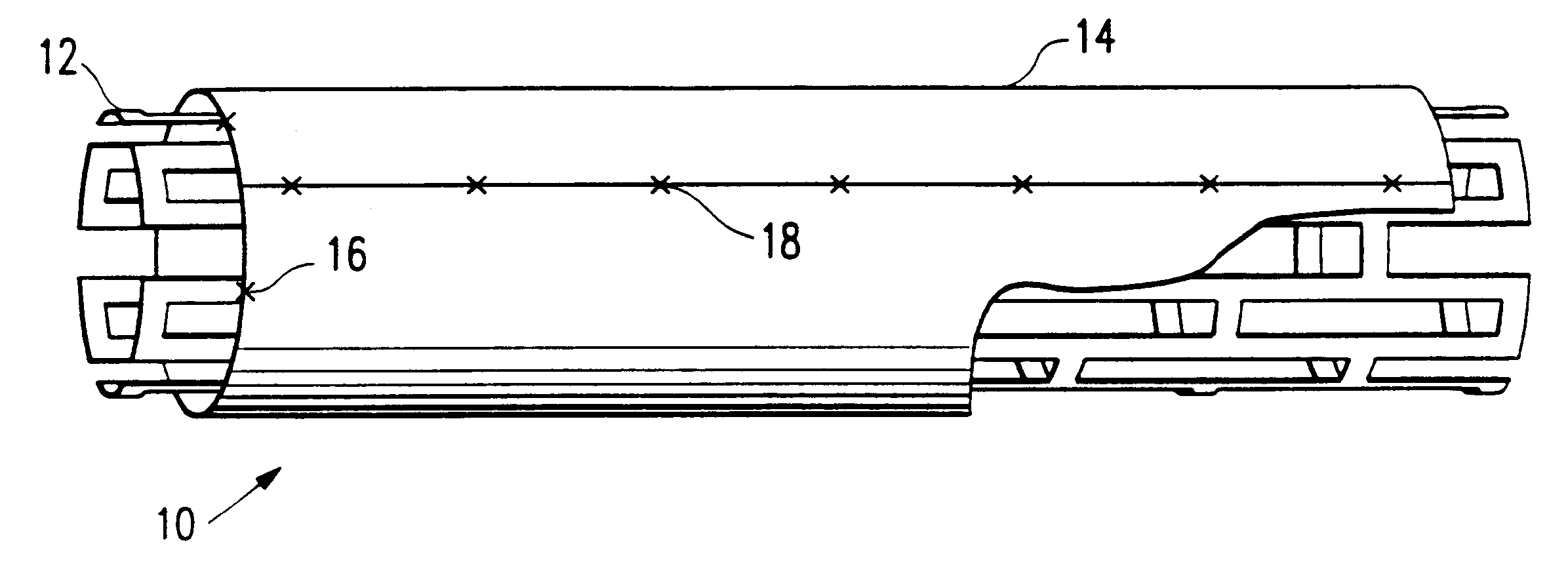
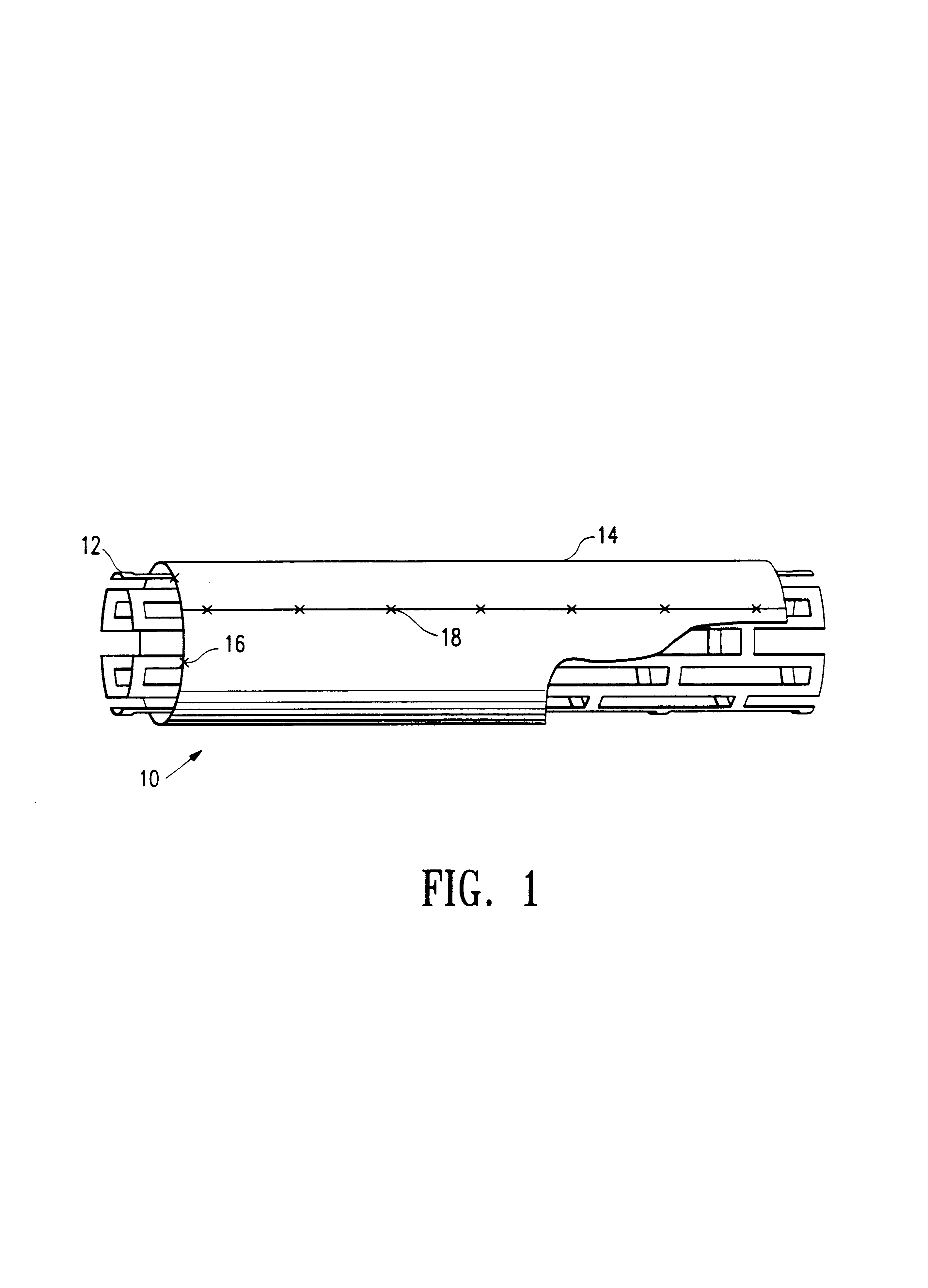
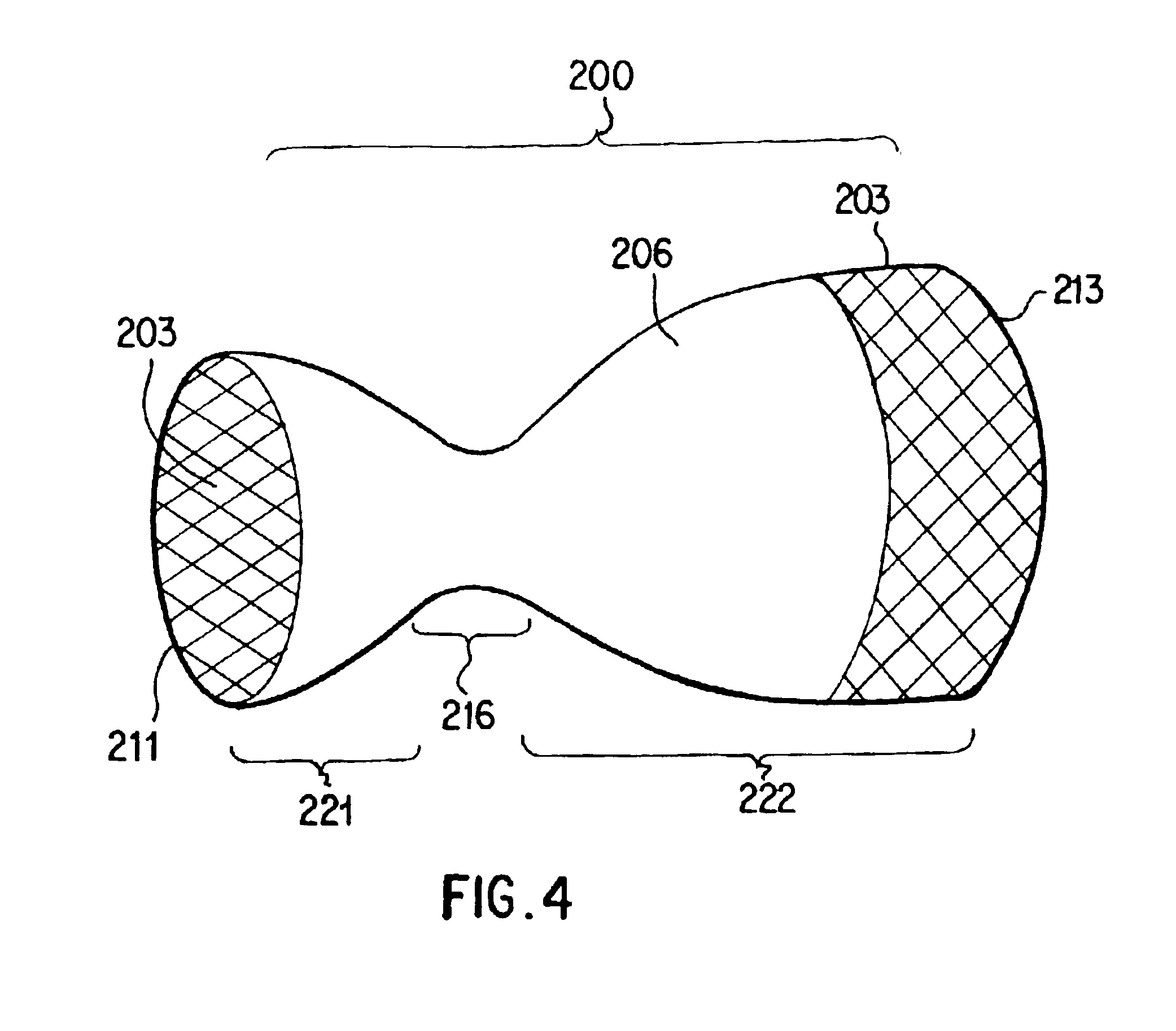
Covered stent with encapsulated ends
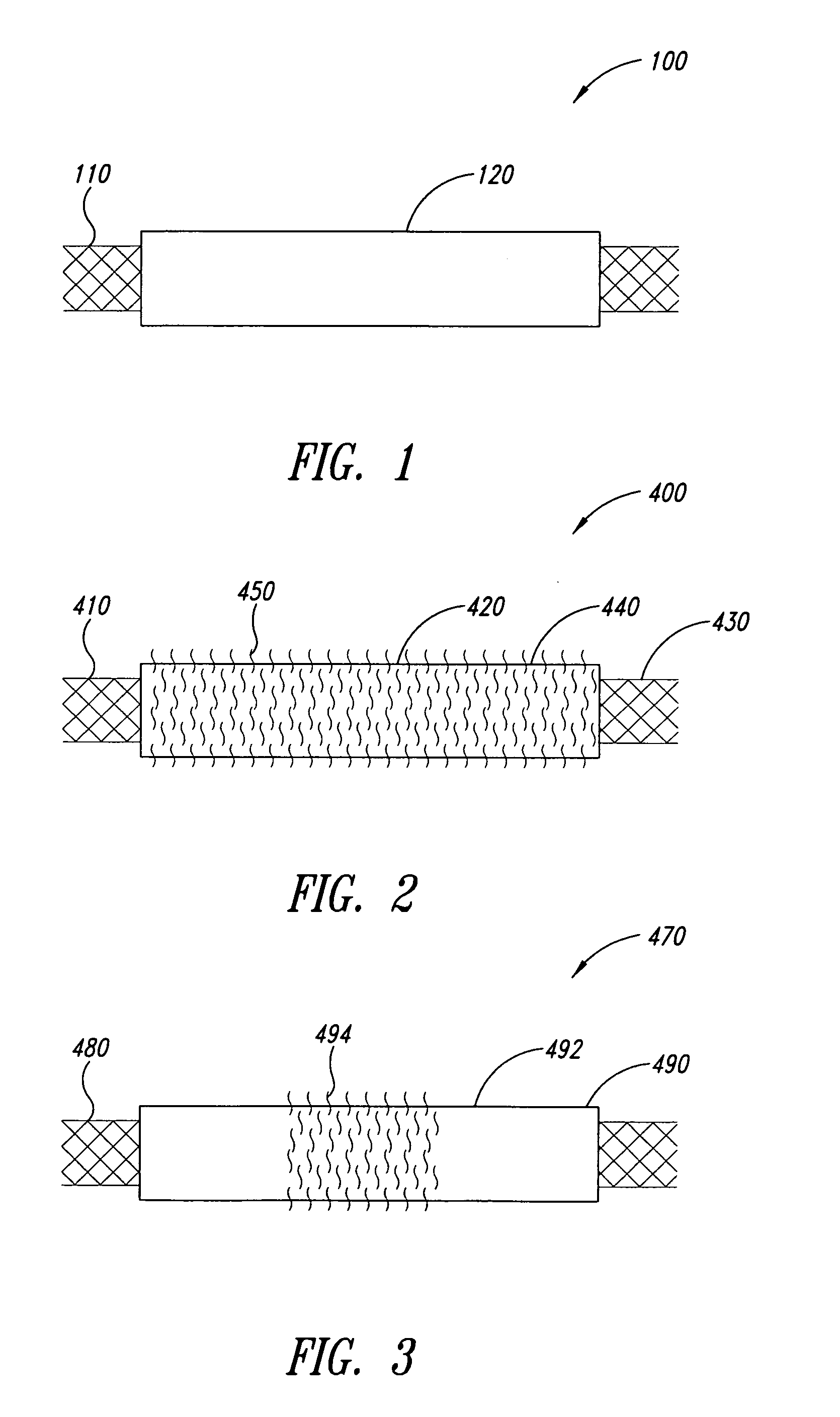
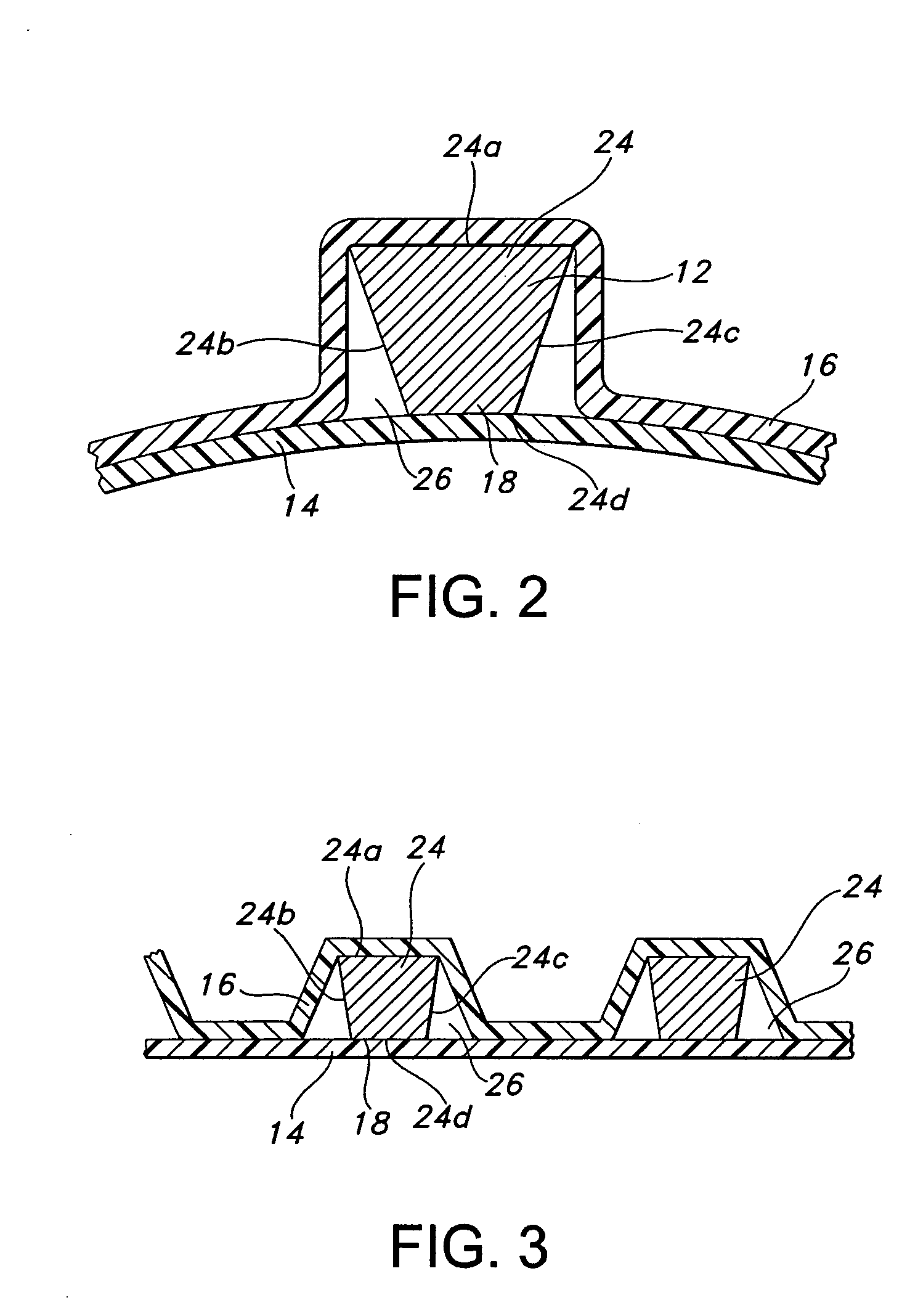
InactiveUS7083640B2Increase flexibilityFlexibility of stent is retainedStentsSurgeryInsertion stentCovered stent
A flexible covered stent having a stent covered on a first surface by a first layer of biocompatible material and on a second surface by both a second and third layer of biocompatible material, the first and second layers and the first and third layers of biocompatible material being bonded to one another through openings in a wall in the stent. The first layer of biocompatible material is longer than both the second and third layers of biocompatible material such that at least a portion of the second surface of the stent is not covered by either second or third layer, imparting flexibility to the stent.
Owner:CR BARD INC
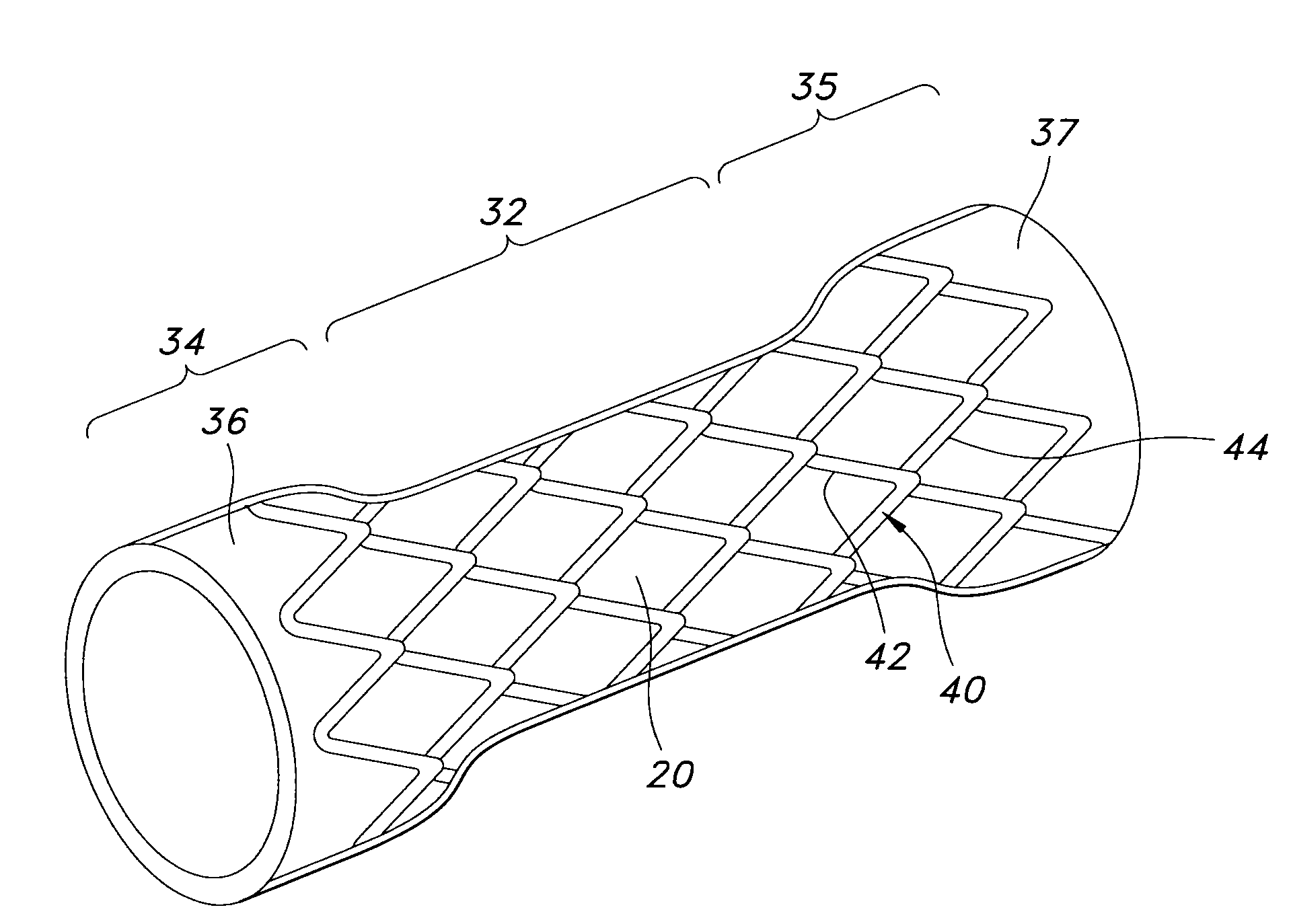
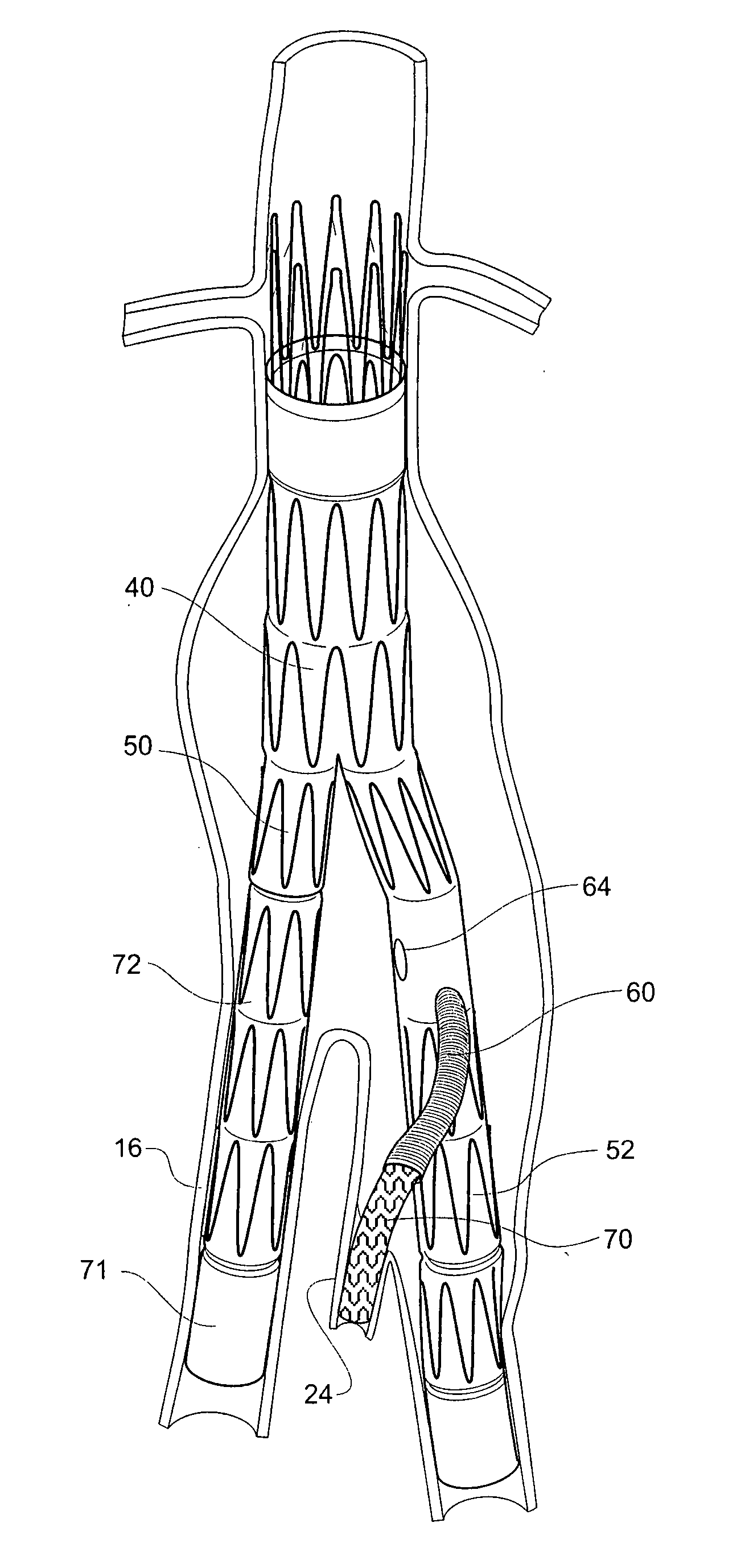
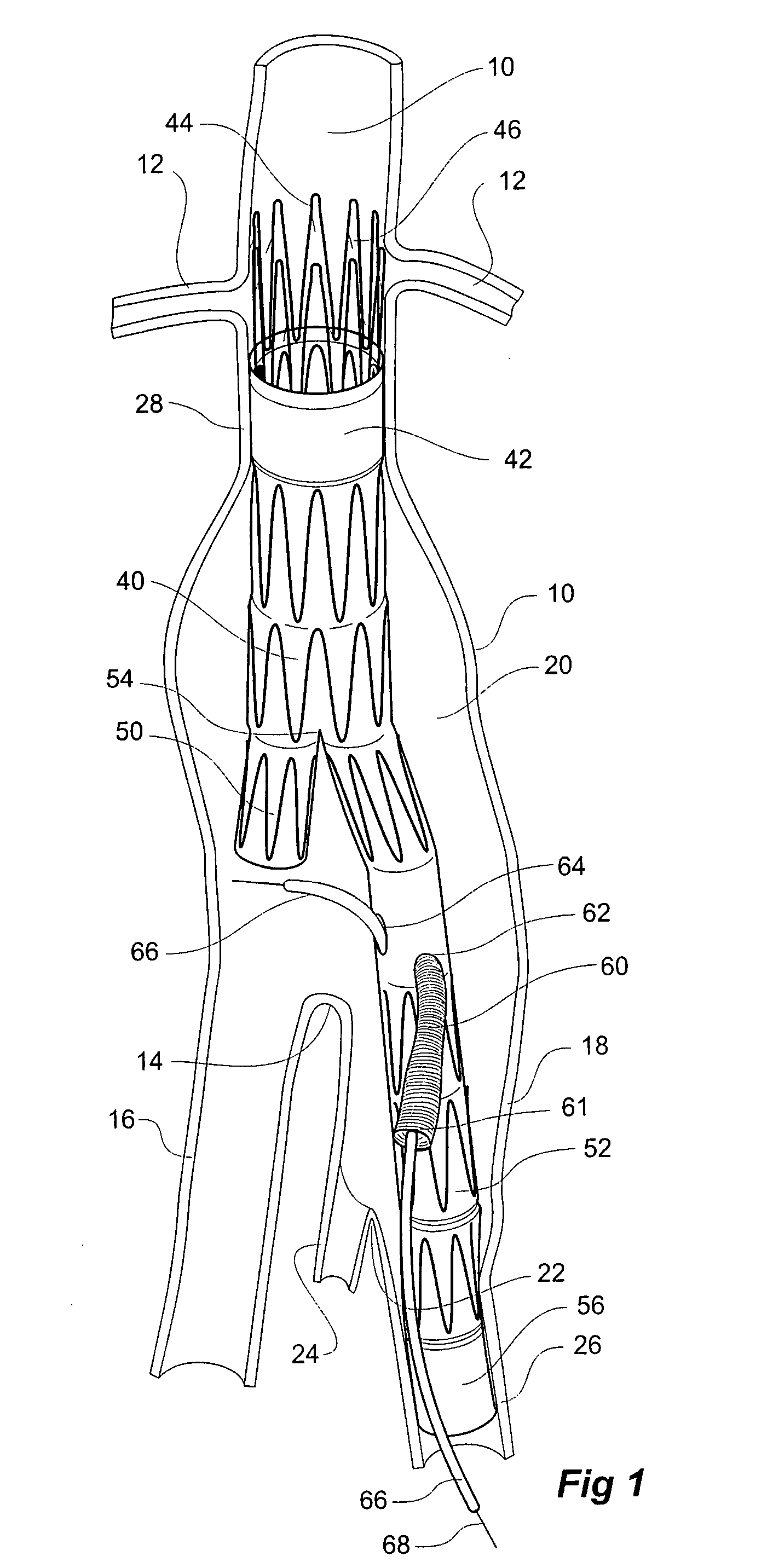
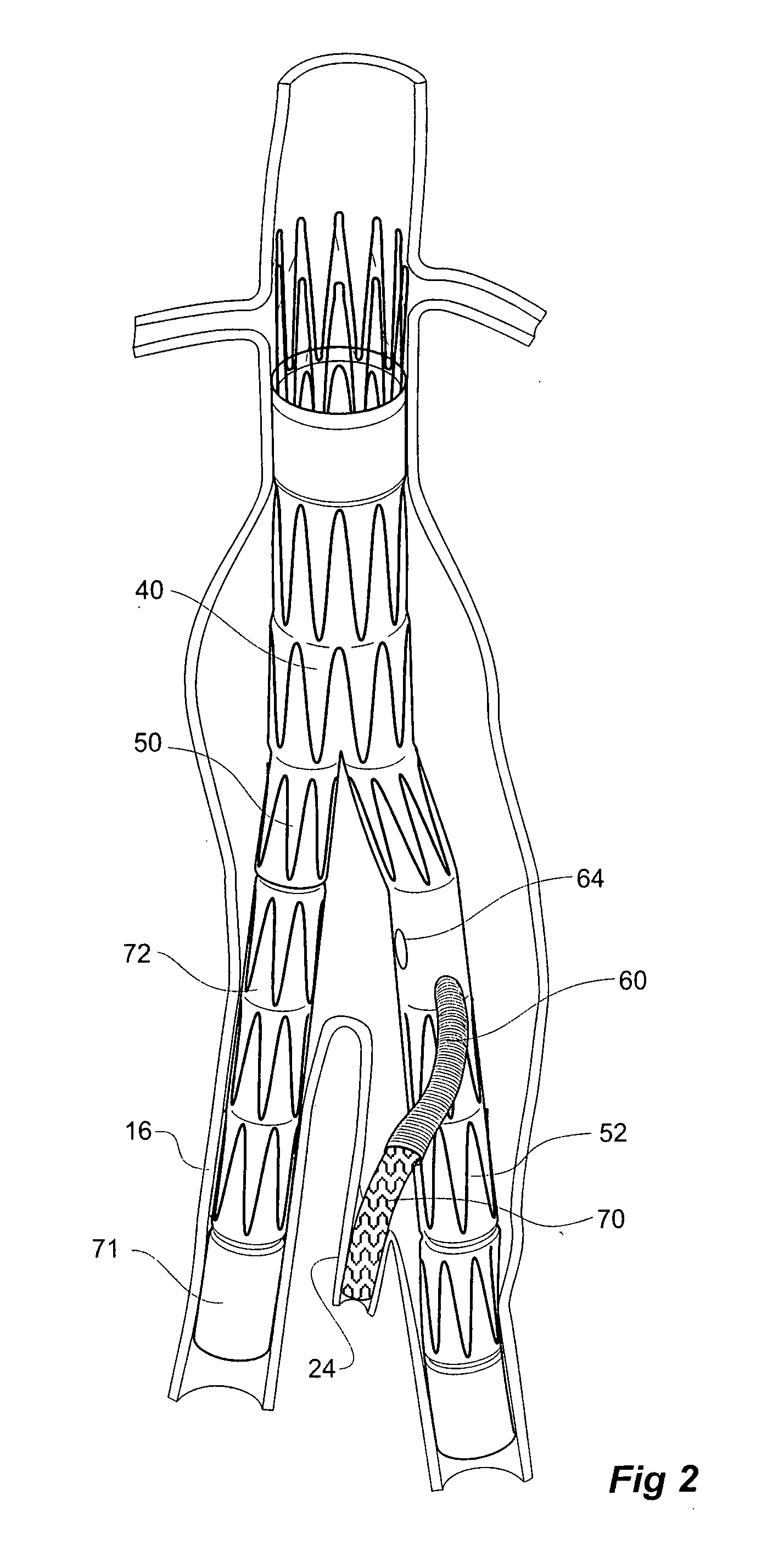
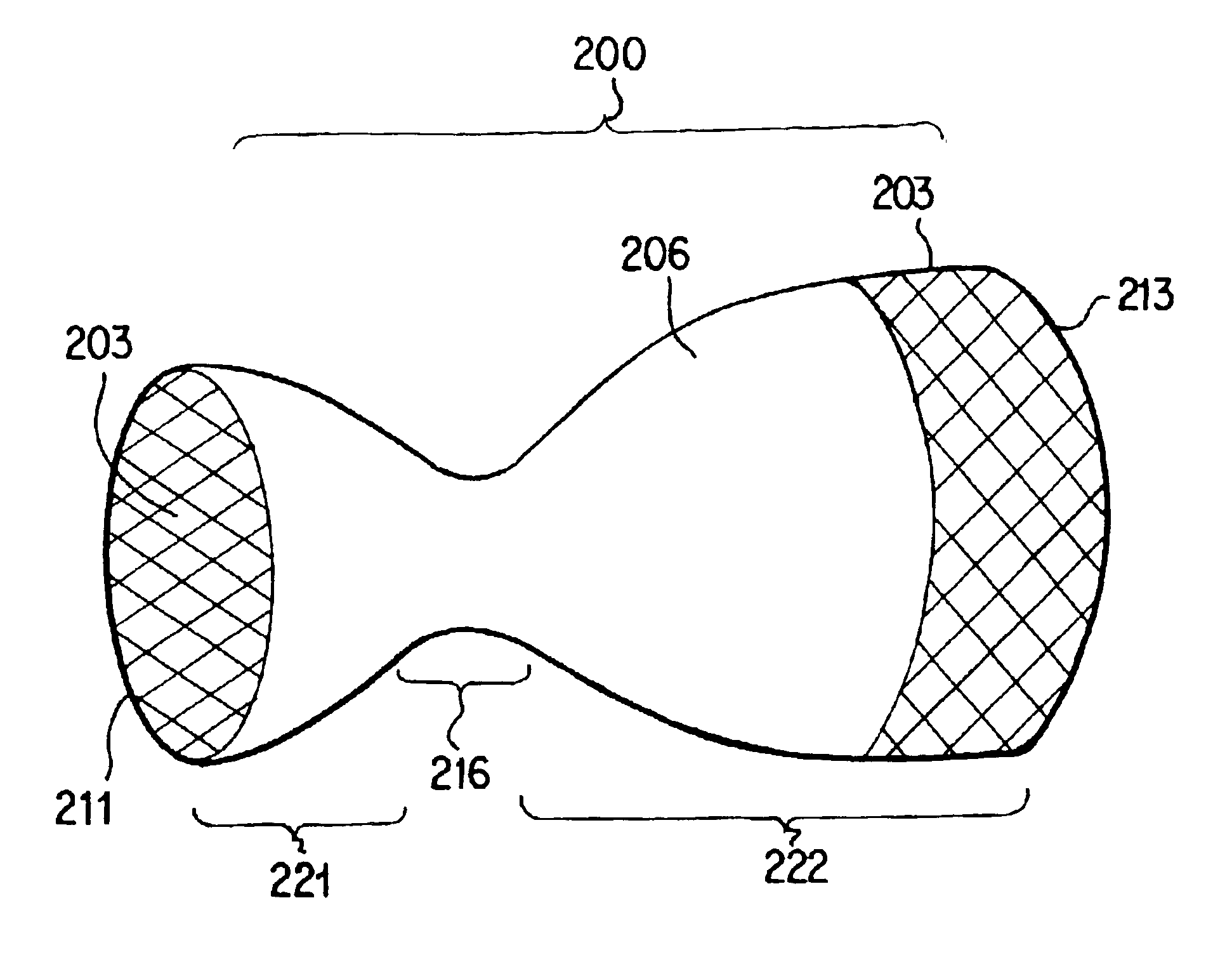
Twin bifurcated stent graft
A stent graft (40) has a tubular body with a first bifurcation (54) with first and second legs (50, 52) extending from the bifurcation. One of the legs (52) has a further bifurcation (62) to define a side arm. The stent graft can be deployed into the vasculature of a patient with the tubular body being in an aorta of the patient, a first leg extending down an iliac artery, a second leg being directed towards a contralateral iliac artery and the side arm directed to an internal artery of one of the iliac arteries. One of the legs can include a valved aperture to enable the placement of an indwelling catheter therethrough
Owner:COOK MEDICAL TECH LLC +1
Medical devices having MEMs functionality and methods of making same
ActiveUS20050177223A1High data densitySacrificing rangeInertial sensorsMeasuring/recording heart/pulse rateElectromagnetic potentialEngineering
Implantable medical devices, including stents, grafts, covered stents, catheters, patches or the like having regions of the device which are functionalized employing microelectromechanical systems that are capable of acting as electromechanical sensors or biosensors in response to either an endogenous event, such as tissue growth, biochemical binding events, pressure changes, or respond to an externally applied stimulus, such as RF energy, to cause a change in the state of the device, such as to induce an oscillation signal which may be interrogated and interpreted external the body or may generate an induced electrical or electromagnetic potential in the device to activate micromotors to effect a geometric change in the device.
Owner:VACTRONIX SCI LLC
Removable stent-graft
A removable device such as a stent-graft, intended for applications where it may be desirable to remove the device at some time following implantation. The stent-graft of the present invention includes a helically-wound stent component provided with a covering of graft material. It is removable by gripping an end of the helically-wound stent component with a retrieval device and applying tension to the stent component in the direction in which it is intended to be withdrawn from the site of implantation. The use of such a retrieval device allows the stent-graft to be removed remotely, such as via a catheter inserted into the body at a different location from the implantation site. The design of the stent-graft is such that the stent component is extended axially while the adjacent portion of the graft separates between windings of the stent component. The axial extension of the stent component, with portions of the graft still joined to the stent component, allows the device to be “unraveled” (or “unwound”) and removed through a catheter of diameter adequately small to be inserted into the body cavity that contained the stent-graft. It is removed atraumatically, without incurring significant trauma to the body conduit in which it had been deployed.
Owner:WL GORE & ASSOC INC
Stent covered with heterologous tissue
A covered stent assembly comprising a tubular, expandable stent having a metallic framework covered with a cylinder of biocompatible, non-thrombogenic expandable material, such as heterologous tissue. In a preferred embodiment, the metallic framework is positioned coaxially within a cylinder of bovine pericardial tissue. A catheter may be used to deliver the stent assembly to a desired region in the lumen of a patient. The metallic framework is then expanded to seat the assembly within the lumen.
Owner:AMNIS THERAPEUTICS
System and method for deploying an endoluminal prosthesis at a surgical site
A system for implanting a prosthesis includes a control lumen and a nose cone affixed at a distal end of the control lumen. At least one supporting wire is affixed at one end, is substantially parallel to a major axis of the control lumen and is free at an opposite end, wherein the free end of at least one of the supporting wires is arcuate. Alternatively, a system for implanting a prosthesis includes at least one suture extending from a nose cone affixed to a distal end of a control lumen. The suture extends from the nose cone to a proximal end to a stent graft extending about the control lumen and from the stent graft to a fixed location on the control lumen. The suture is releasable from the stent graft by remote activation, whereby the suture separates from the nose cone to thereby deploy the stent graft.
Owner:BOLTON MEDICAL INC
Methods, materials and apparatus for deterring or preventing endoleaks following endovascular graft implantation
Methods and apparatus for treating or preventing endoleaks after an endovascular graft (e.g., a stent, tubular graft, stent-graft, coated stent, covered stent, intravascular flow modifier or other endovascular implant that affects, limits or prevents blood flow into a vascular defect such as an aneurysm, arterio-venous fistula, arterio-venous malformation, vessel wall perforation, etc.) has been implanted in the vasculature of a human or veterinary patient. An expansile polymeric material, such as a swellable polymer (e.g., a hydrogel), a flexible or elastomeric polymer foam (e.g. silicone, polyurethane, etc.) or a carrier member (e.g, a coil, filament, wire, etc) that carries a quantity of such expansile polymer is delivered into a perigraft space (i.e., space between the endovascular graft and the surrounding blood vessel wall) such that the polymeric material expands in situ to substantially fill the perigraft space or a portion thereof. The expansile polymeric material is delivered into he perigraft space through a catheter and / or cannula that is placed prior to, during or after the implantation of the endovascular graft. The invention includes an injector apparatus that is useable to deliver the expansile polymeric material through the wall of a previously implanted graft. After delivery into the perigraft space, the expanded polymeric material expands so as to fill all or an intended portion of the perigraft space in a manner that substantially prevents additional blood from leaking or flowing into such perigraft space. One type of blood-absorbing, porous, expansile polymeric material useable in this invention is a super-expansile hydrogel.
Owner:MICROVENTION INC
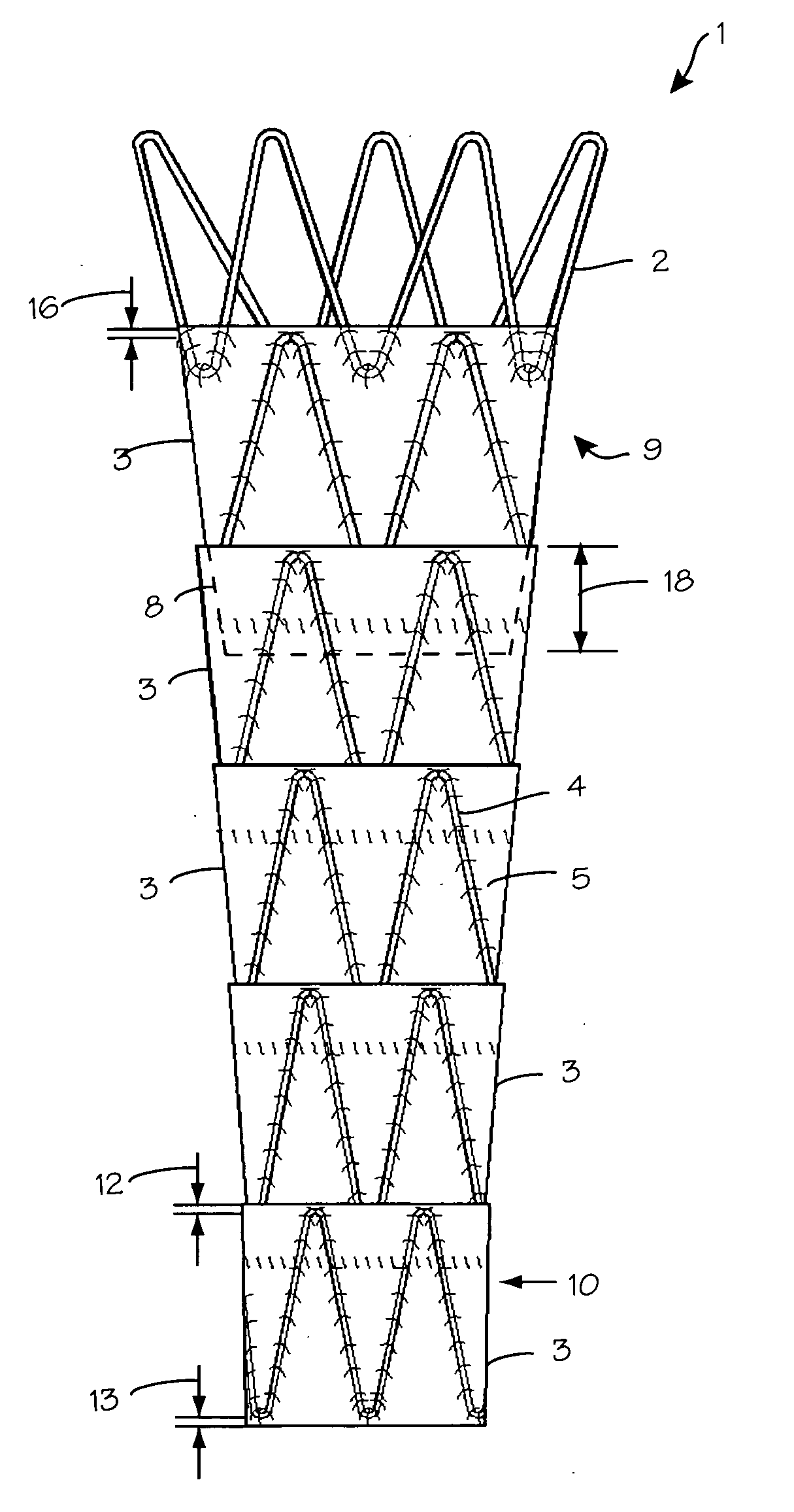
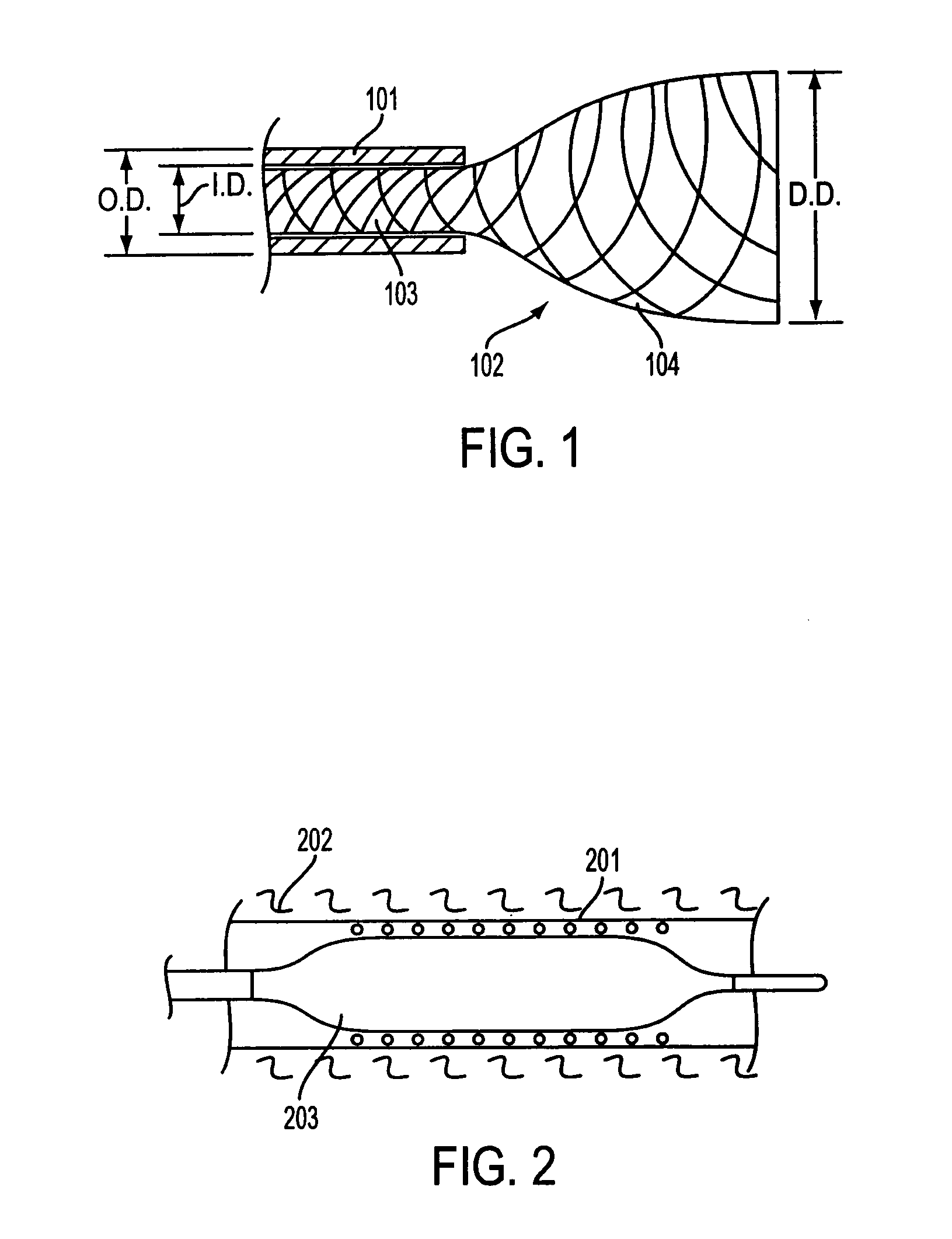
Three-dimensional braided covered stent
InactiveUS7052513B2Reduce manufacturing costSolve the lack of tensionStentsBlood vesselsYarnProsthesis
A prosthesis for transluminal implantation consists of a flexible tubular three-dimensionally braided structure of metal or polymeric monofilaments, and polymeric multifilament yarns. The prosthesis can be elastically deformed to reduce its diameter through axial elongation. The monofilaments and multifilament yarns are arranged in axially spaced apart helices, concentric on a common central axis of the prosthesis. The monofilaments are selectively shaped before their interbraiding with the multifilament yarns, either by an age-hardening or other heat-setting stage, or a cold-working stage that controllably plastically deforms the strands. The shaped structural strands cooperate to impart to the prosthesis its nominal shape and resilience. The textile strands cooperate to provide one or more layers of sheeting that reduce permeability and thereby enhance the utility of the prosthesis as a vascular graft. An alternative embodiment prosthesis includes elastically and plastically deformable structural strands, selectively plastically deformed by cold working, then three-dimensionally braided to form the prosthesis.
Owner:LIFESHIELD SCI
Medical devices having MEMs functionality and methods of making same
ActiveUS7235098B2Accelerate endothelializationPromote formationInertial sensorsMeasuring/recording heart/pulse rateElectricityElectromagnetic potential
Implantable medical devices, including stents, grafts, covered stents, catheters, patches or the like having regions of the device which are functionalized employing microelectromechanical systems that are capable of acting as electromechanical sensors or biosensors in response to either an endogenous event, such as tissue growth, biochemical binding events, pressure changes, or respond to an externally applied stimulus, such as RF energy, to cause a change in the state of the device, such as to induce an oscillation signal which may be interrogated and interpreted external the body or may generate an induced electrical or electromagnetic potential in the device to activate micromotors to effect a geometric change in the device.
Owner:VACTRONIX SCI LLC
Stent delivery system with improved delivery force distribution
InactiveUS20070156223A1Equally distributedMaximize distributionStentsBlood vesselsStent graftingInsertion stent
A stent delivery system that helps equalize delivery forces throughout a vascular prosthesis during delivery thereof to an intended treatment site. The delivery system comprises a catheter having a lumen therethrough, an inner core over which the one or more stents or stent grafts is mounted for delivery from the catheter, and an outer sheath overlying the one or more stent or stent graft and the inner core. The inner core of the further comprises a series of outwardly flared members that engage segments or fill voids in or between the stent, stents or stent grafts, respectively. The outwardly flared members of the inner core thus help maintain the segments of the stent or the one or more stents or stent grafts in spaced relation with respect to one another as delivery thereof occurs and as removal of the outer sheath occurs to position the one or more stents or stent grafts at the intended treatment site. The outwardly flared members thus help to distribute delivery forces more evenly throughout the one or more stents or stent grafts being delivered from the catheter. The outwardly flared members further aid accurate placement of the one or more stents or stent grafts by maintaining longitudinal spacing thereof during delivery.
Owner:CORDIS CORP
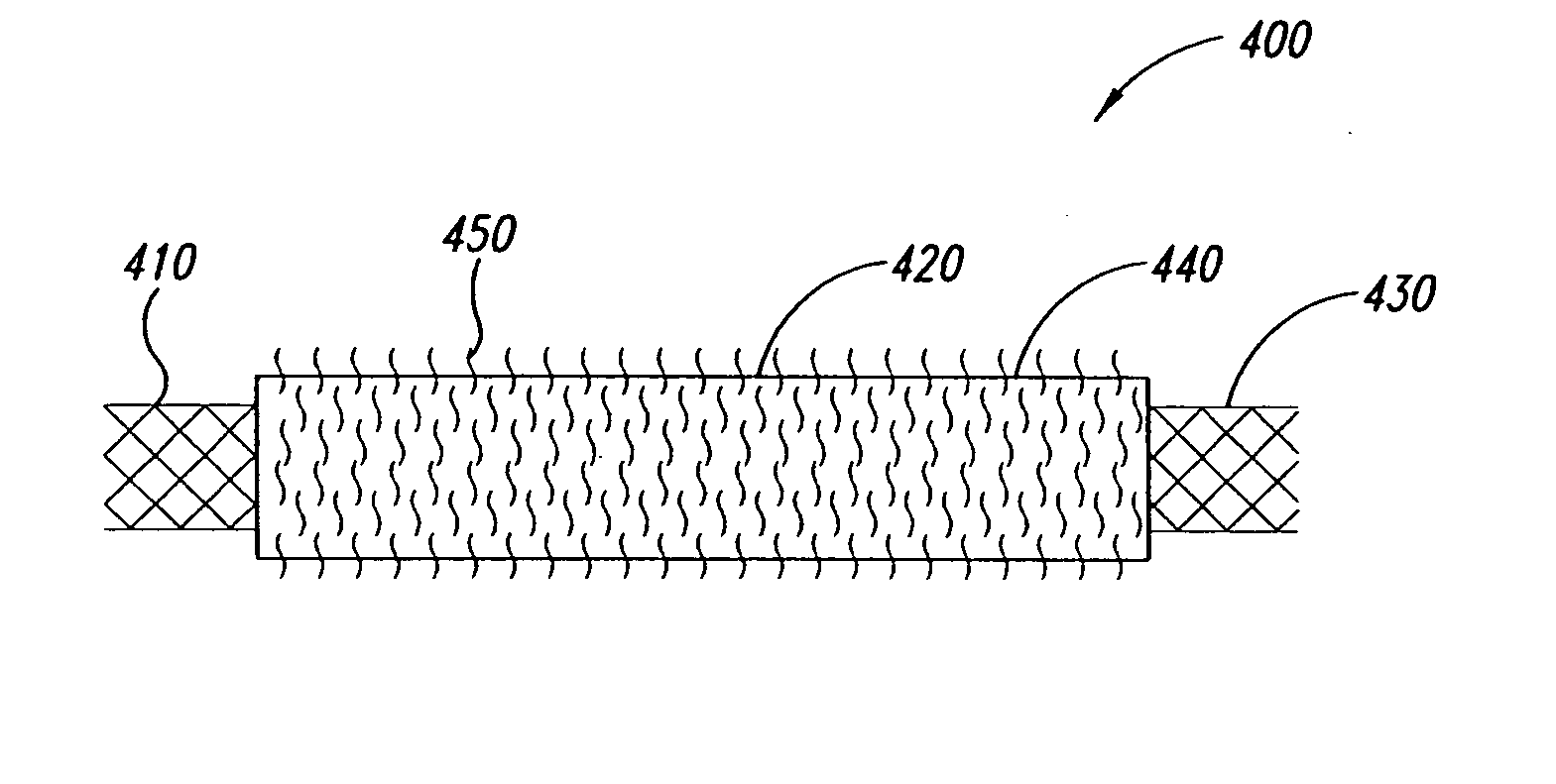
Composite stent with inner and outer stent elements and method of using the same
An endoprosthesis comprising a stent, a cover fully covering the stent wherein the cover has variable porosity in the radial direction; and an adhesion layer connecting the stent to the cover. Another aspect of the invention is a method of implanting an endoprosthesis which includes a stent, providing a cover with variable porosity in the radial direction, connecting the stent to the cover with an adhesion layer to form a covered stent, and implanting the covered stent within a body lumen of a patient.
Owner:BOSTON SCI SCIMED INC
Two-piece stent combination for percutaneous arterialization of the coronary sinus and retrograde perfusion of the myocardium
The present invention contemplates a method for percutaneously providing oxygenated blood retrogradely from the left ventricle via coronary sinus to the heart tissue without a significant left-to-right shunt. The present invention contemplates a two-piece stent combination generally having an arterializing stent and a restricting covered stent.
Owner:MARTIN ERIC C
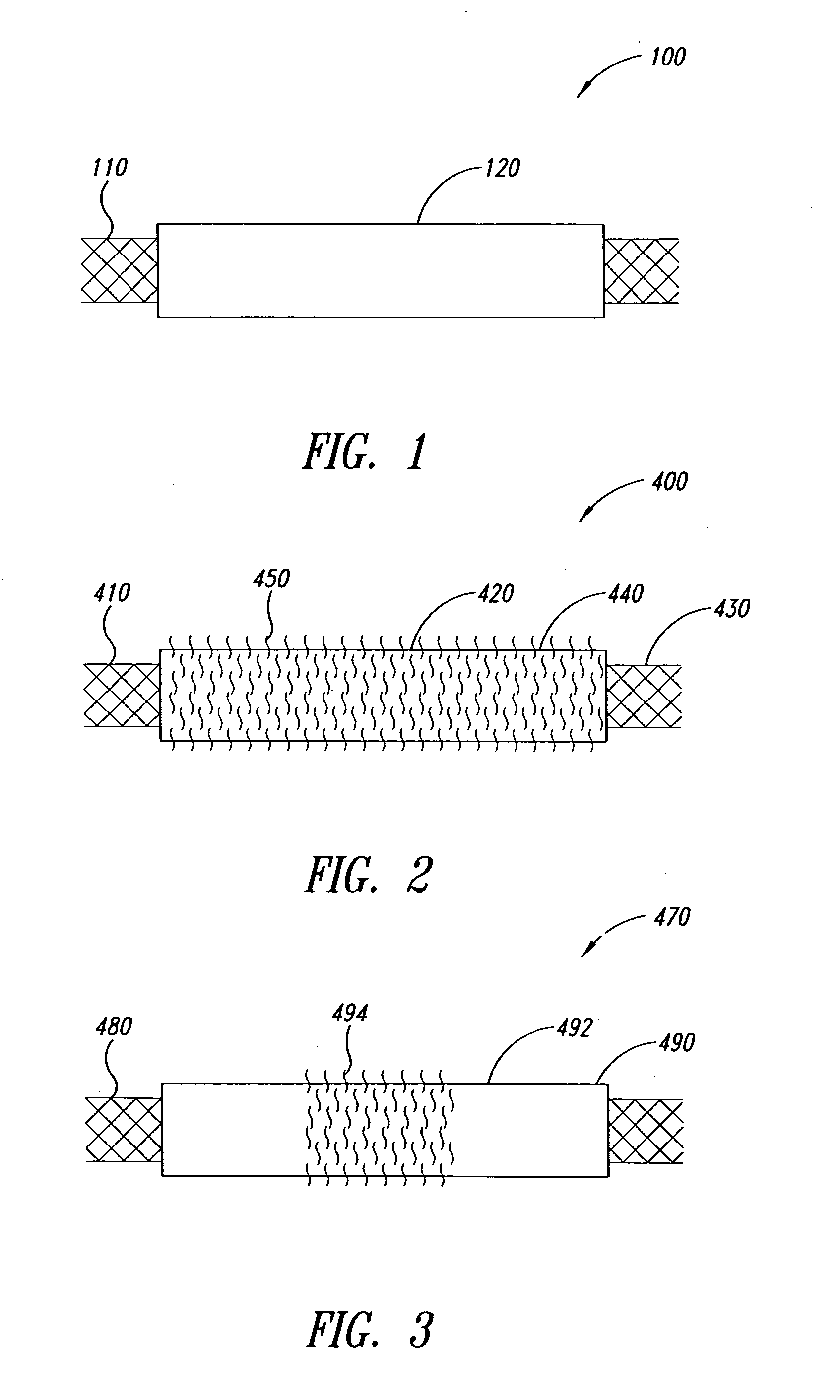
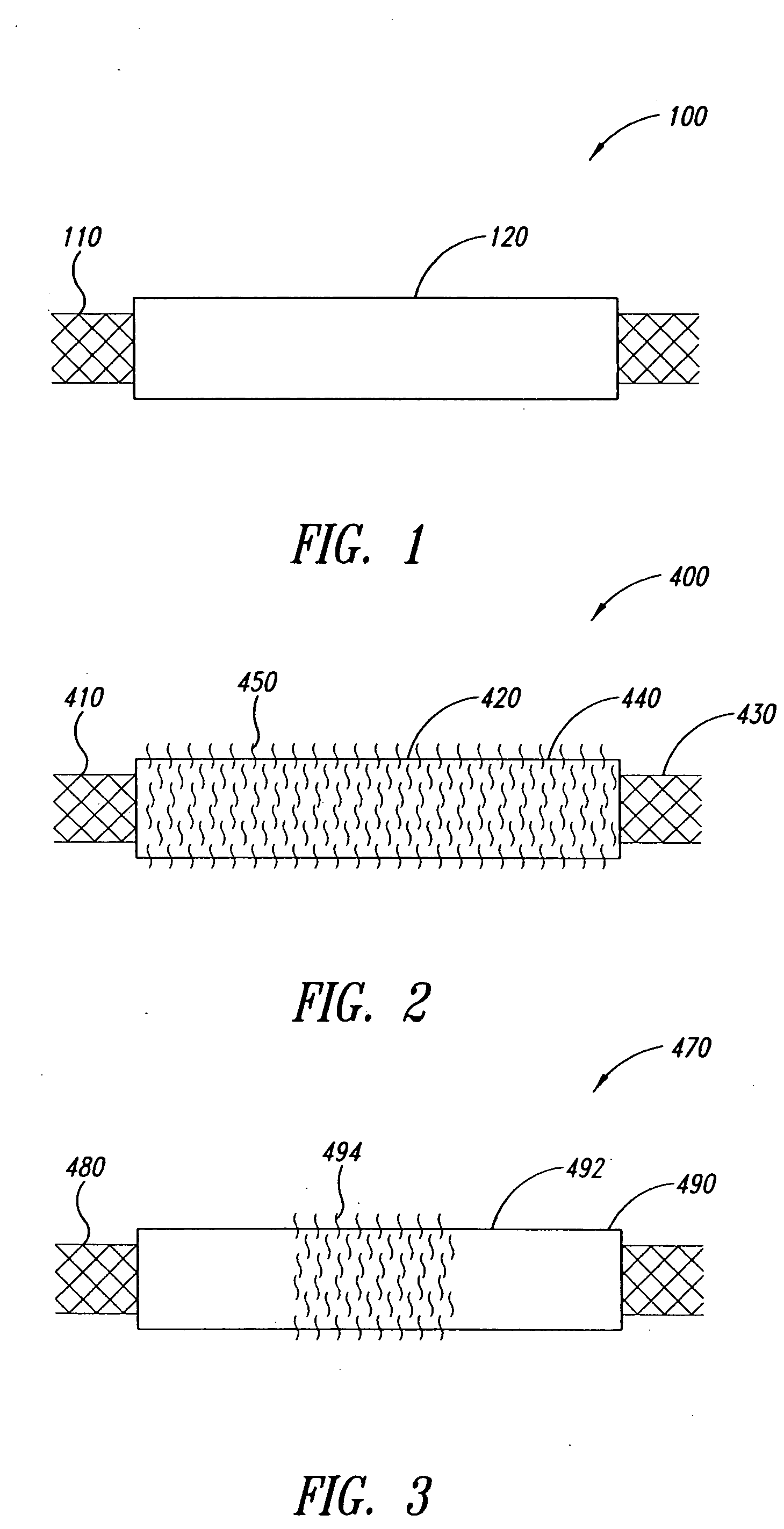
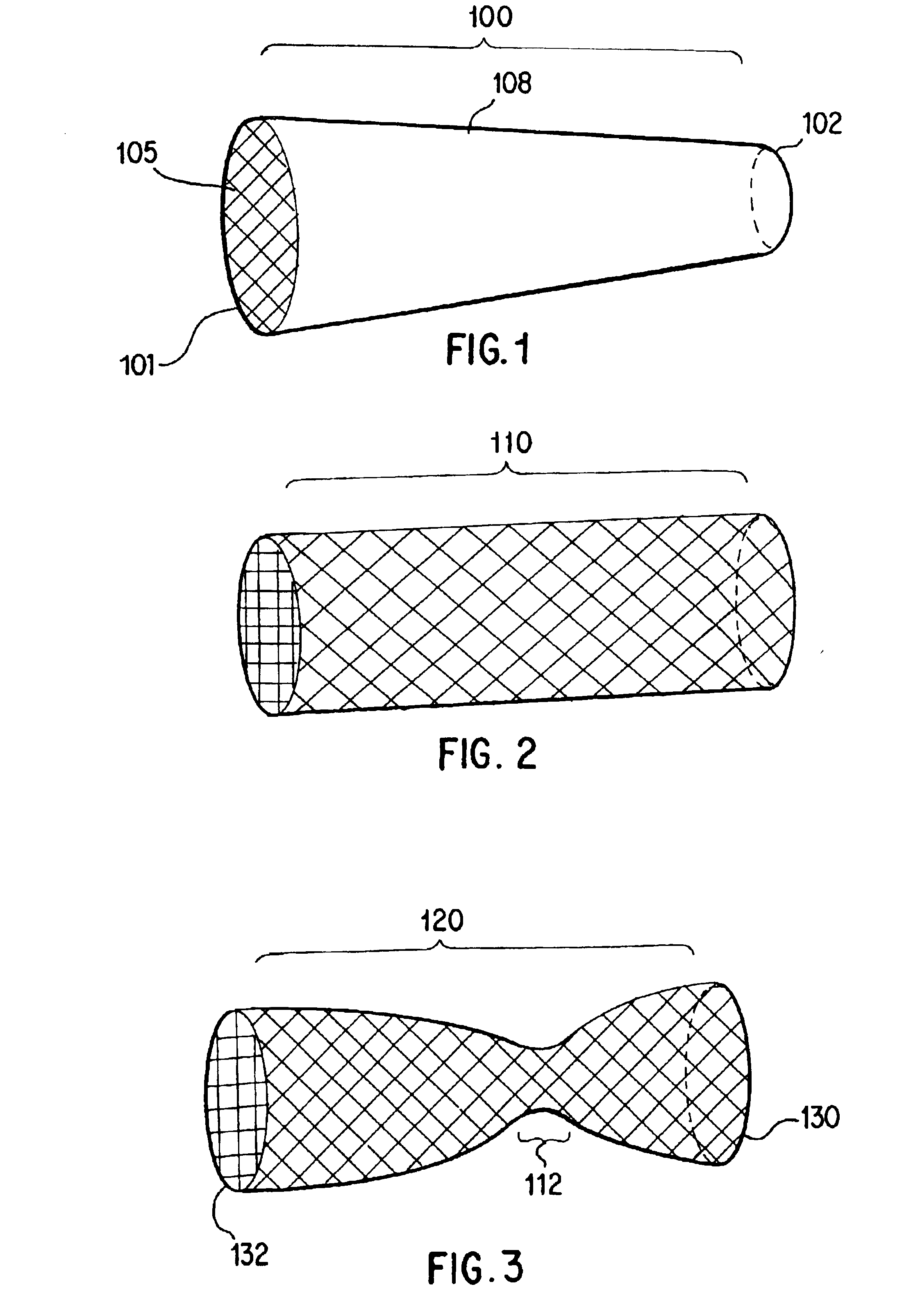
Tubular Polymer Stent Coverings
The present invention concerns thin, flexible tubings useful as stent coverings; covered stents; methods for applying such coverings to stents; and methods for reinforcing biological lumens, such as the intracranial vasculature, by introducing the covered stent into the biological lumen and positioning the covered stent at a target site, such as the site of a vascular defect.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com