Removable stent-graft
a stent and graft technology, applied in the field of stent grafts, can solve the problems of living tissue having a number of limitations and/or reactions, and no commercially available devices are designed to be removable,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
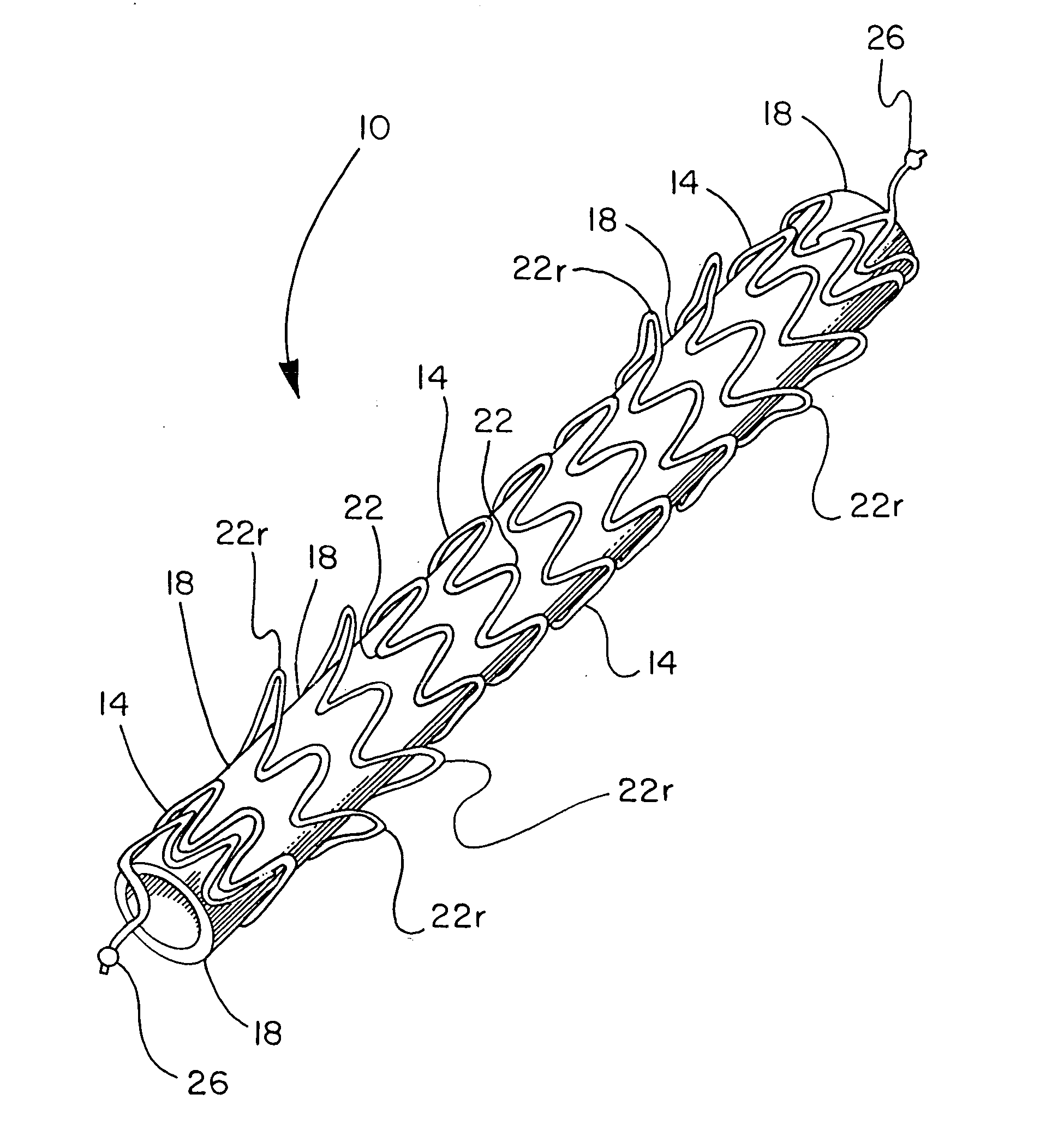
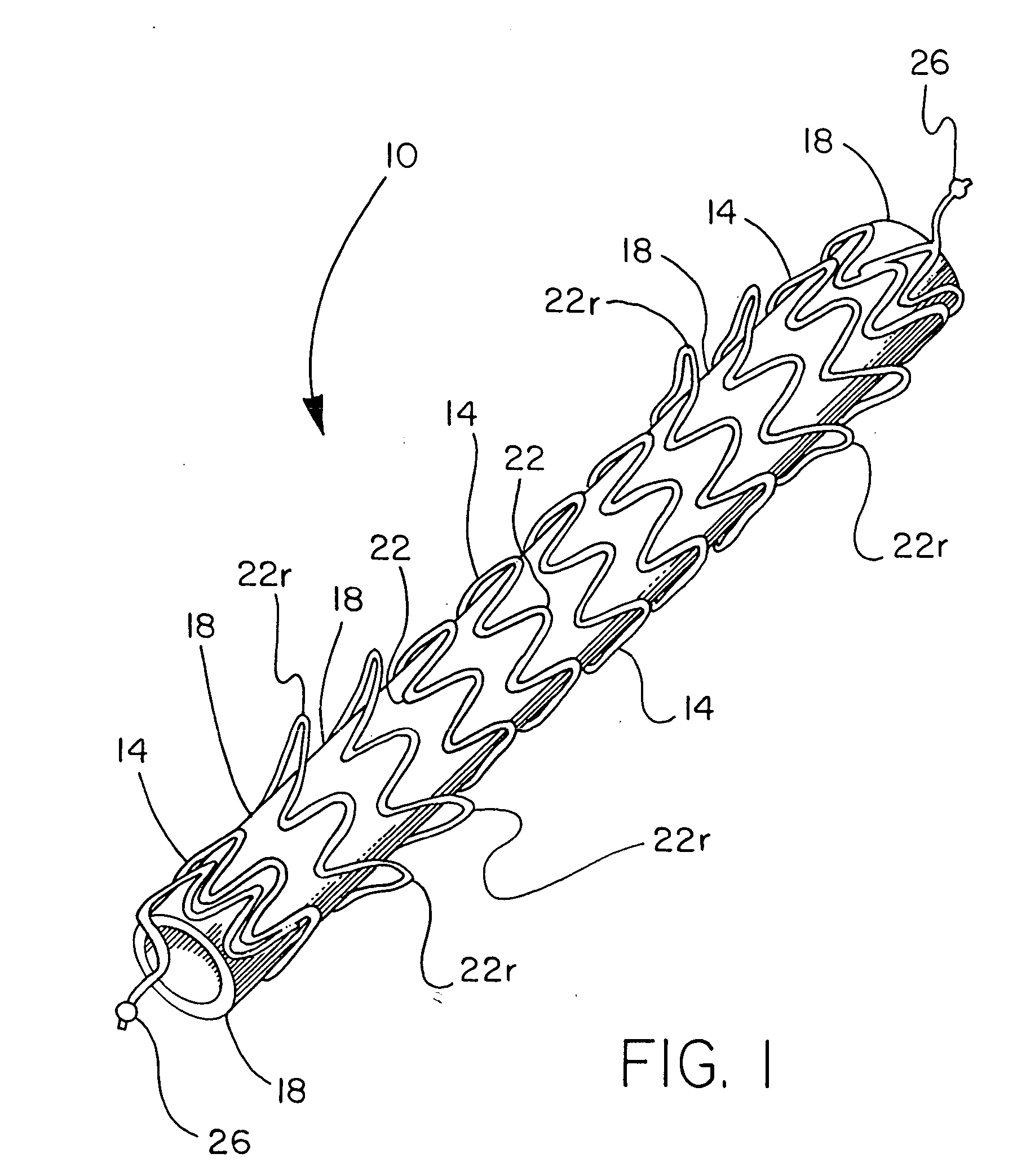
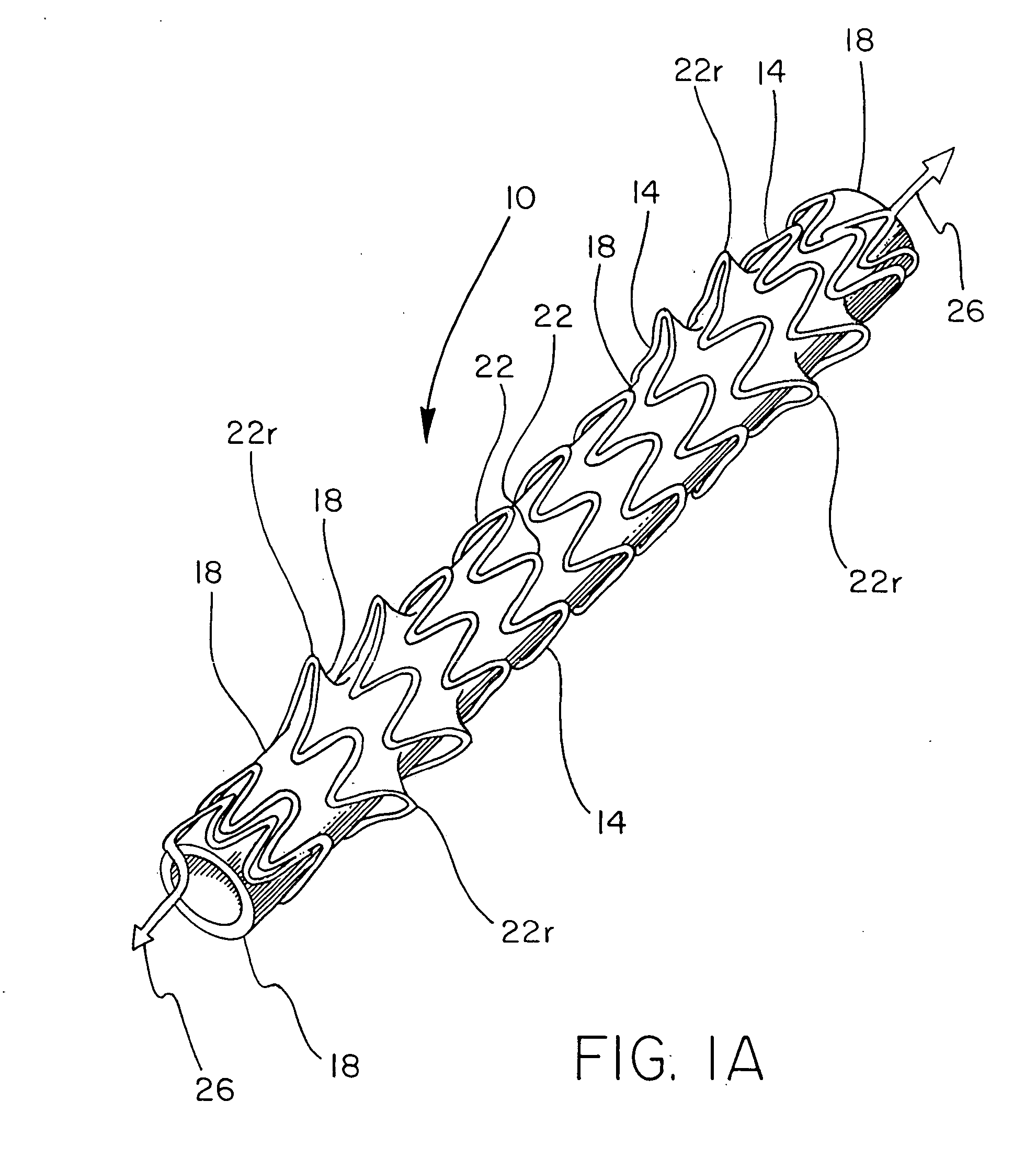
[0057] A stent component was produced by winding a 0.25 mm diameter nitinol wire (SMA Inc, Santa Clara Calif.) onto an 8 mm diameter wire forming fixture, creating a stent component as shown in FIG. 1. The wire-wound fixture was then subjected to heat treatment and quench cycles sufficient to set the wire into the desired form. FEP powder (Daikin America, Orangeburg N.Y.) was applied to the stent component by first stirring the powder into an airborne “cloud” in a standard kitchen-type blender and suspending the frame in the cloud until a uniform layer of powder was attached to the wire. The stent component was then subjected a thermal treatment of 320° C. for approximately one minute to cause the powder to melt and adhere as a coating over the stent component.
[0058] A sacrificial 7 mm inside diameter, 0.1 mm thick ePTFE tube that had been previously heated above 380° C., was pulled onto an 8 mm diameter mandrel, which involved slight stretching of the ePTFE tube. This tube was int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com