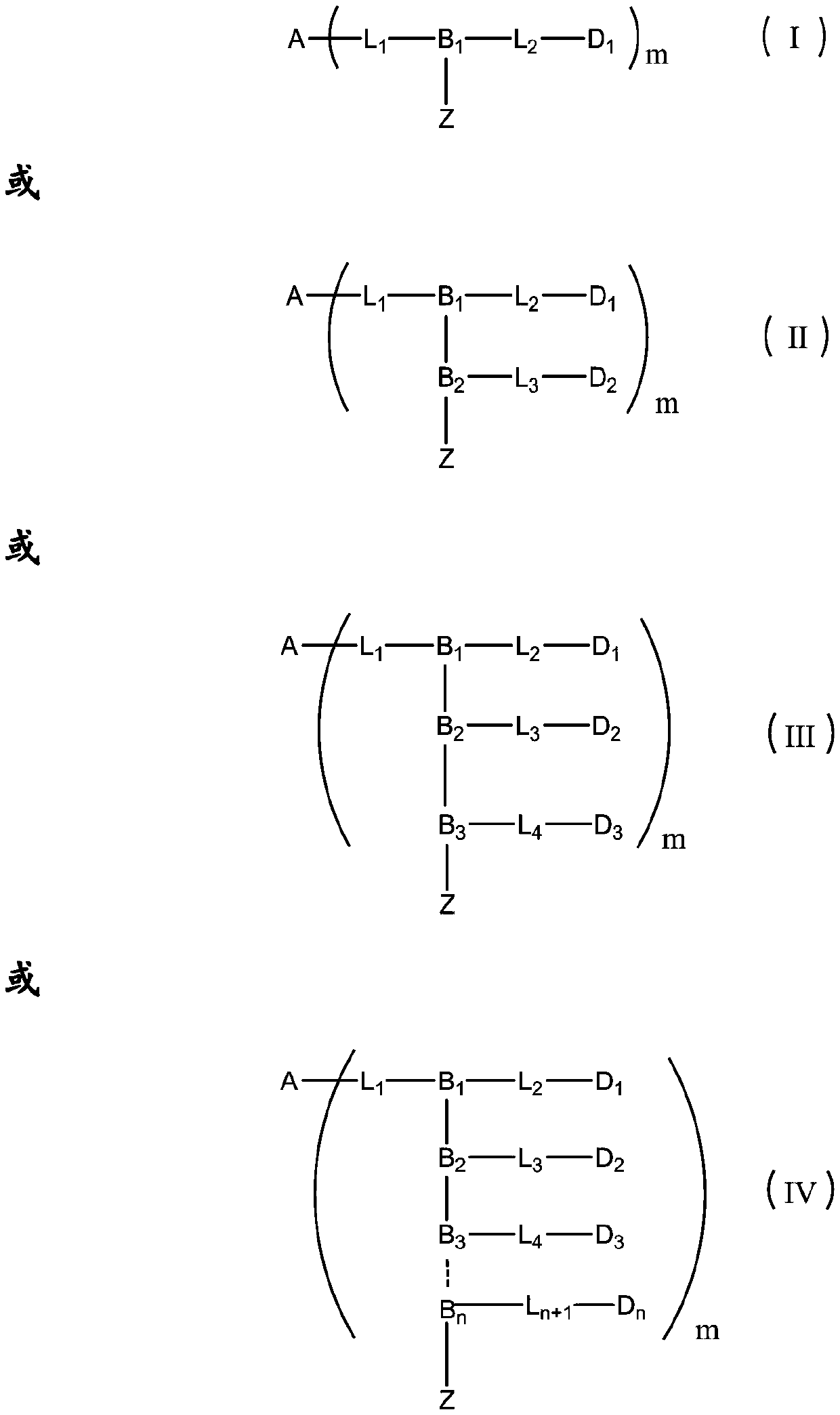
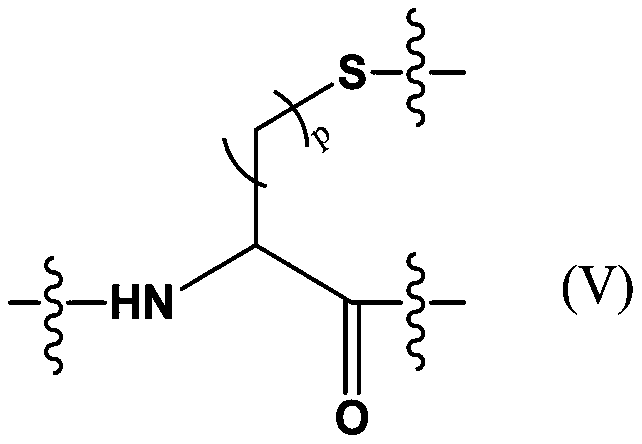
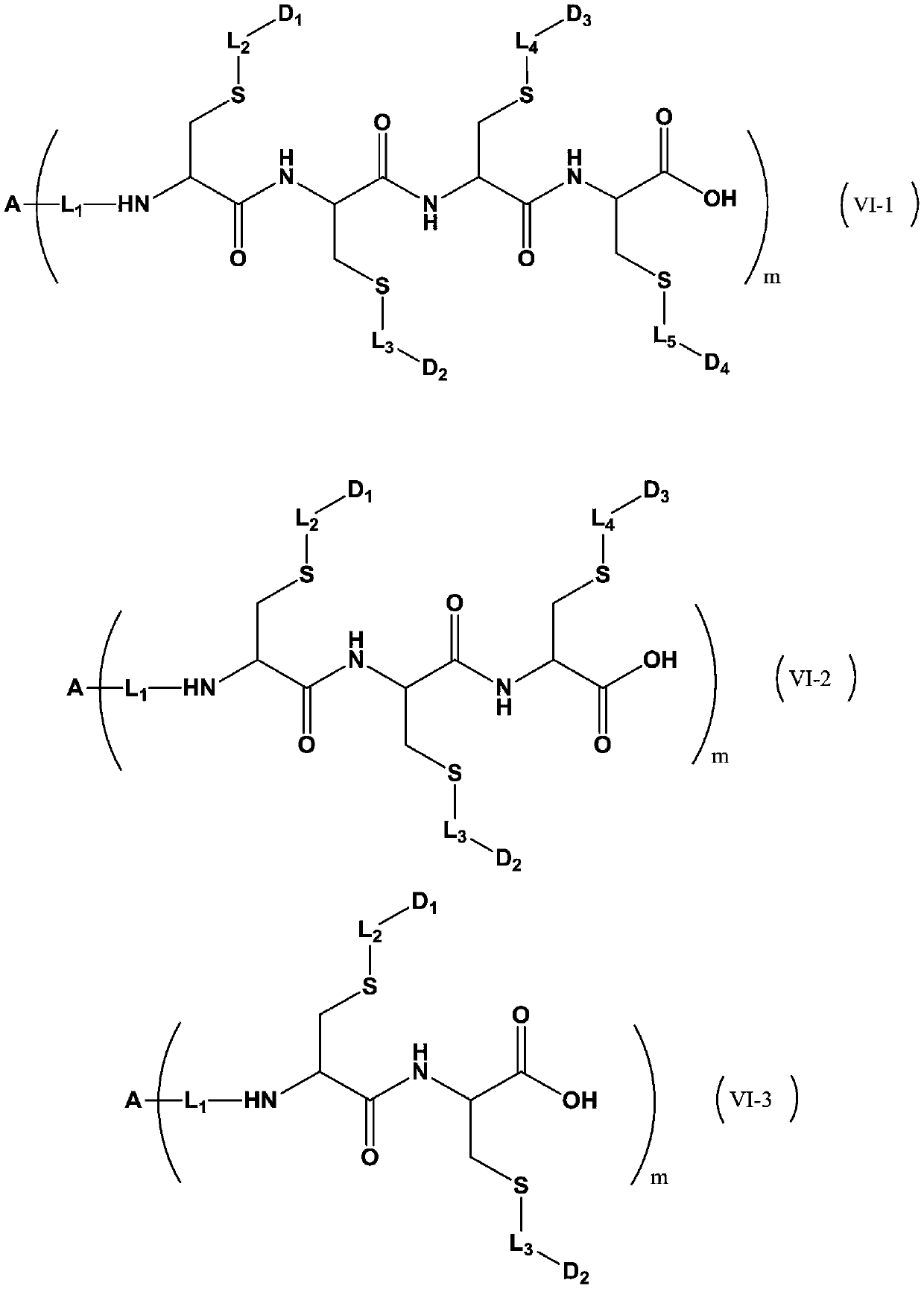
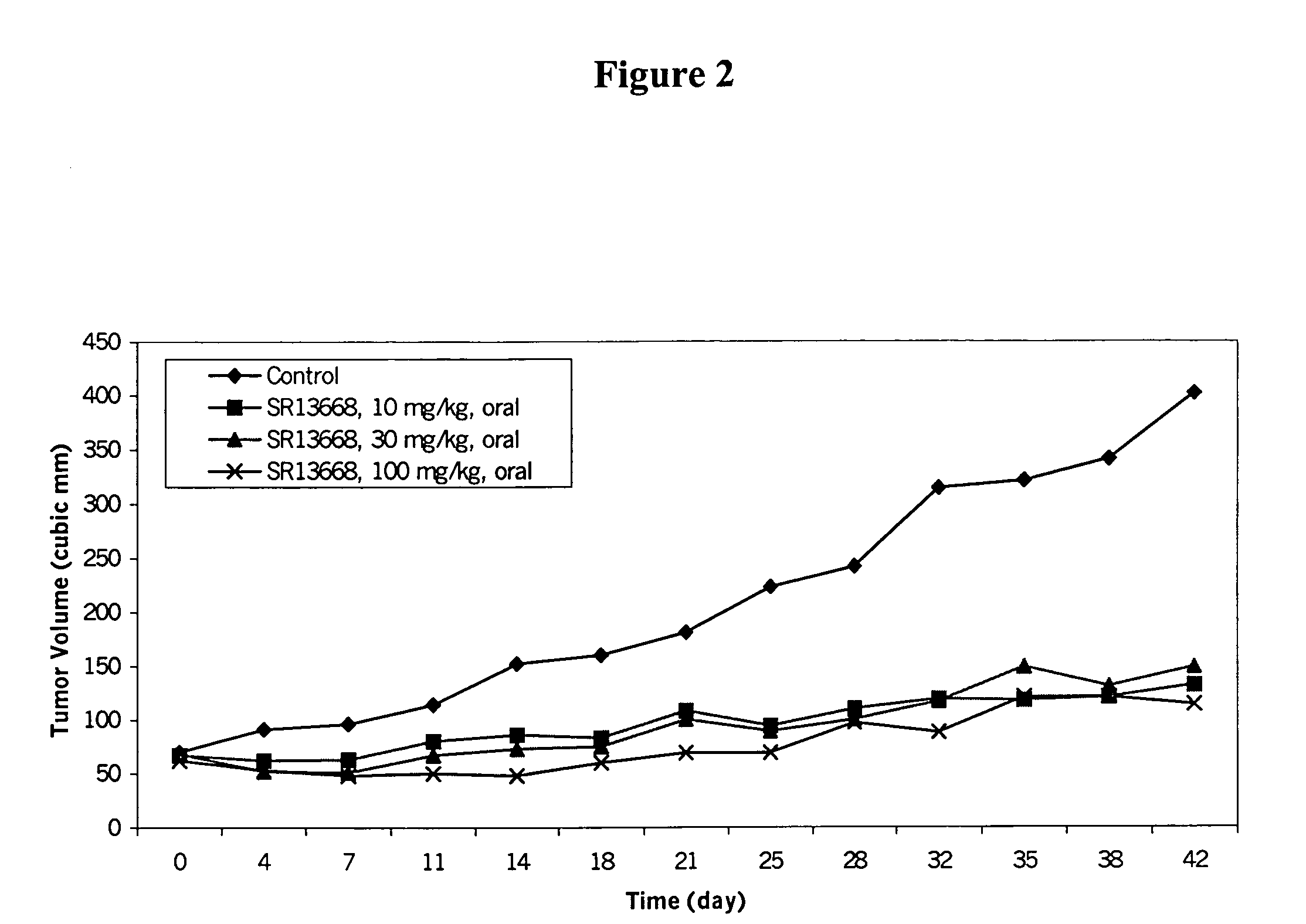
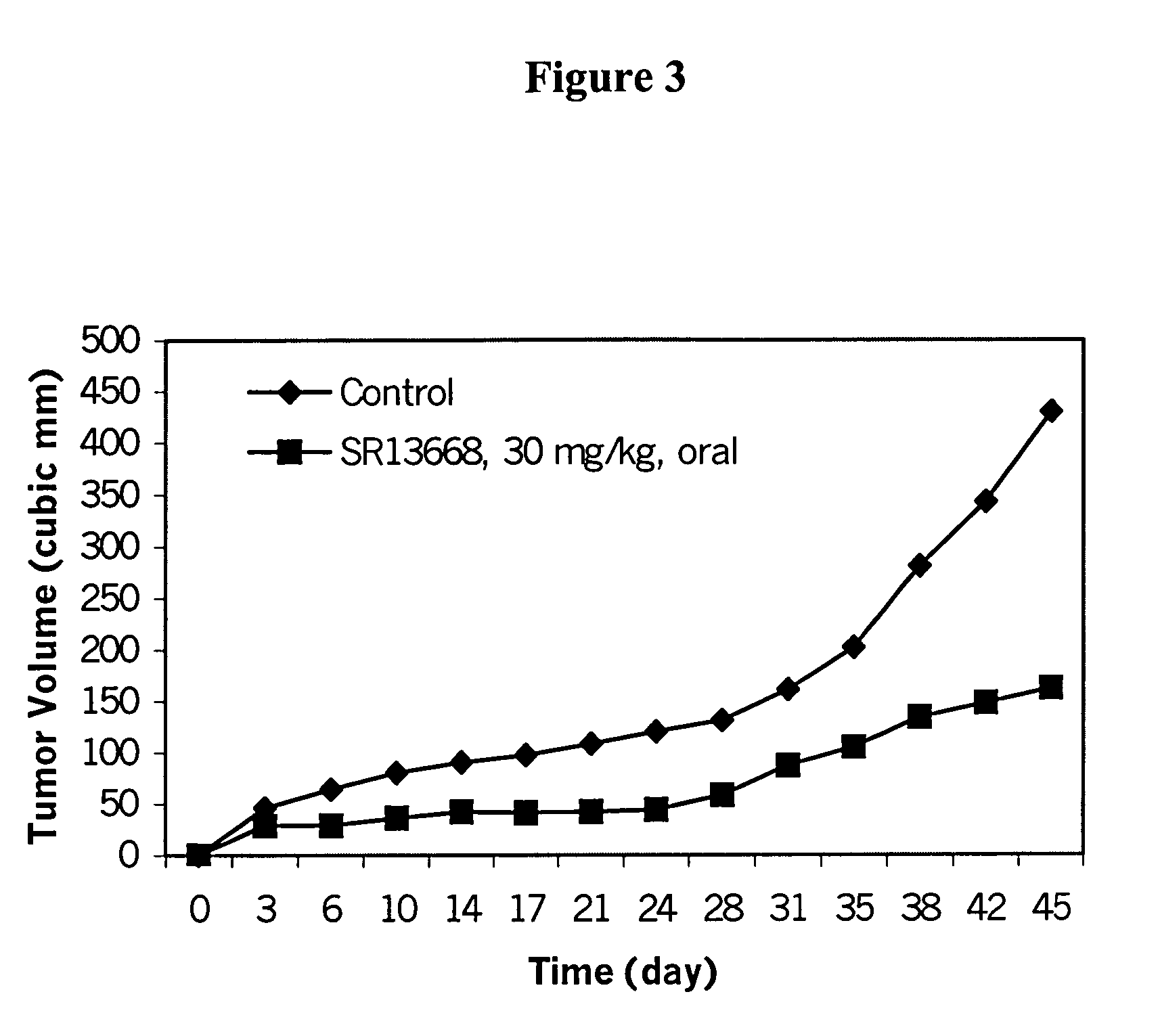
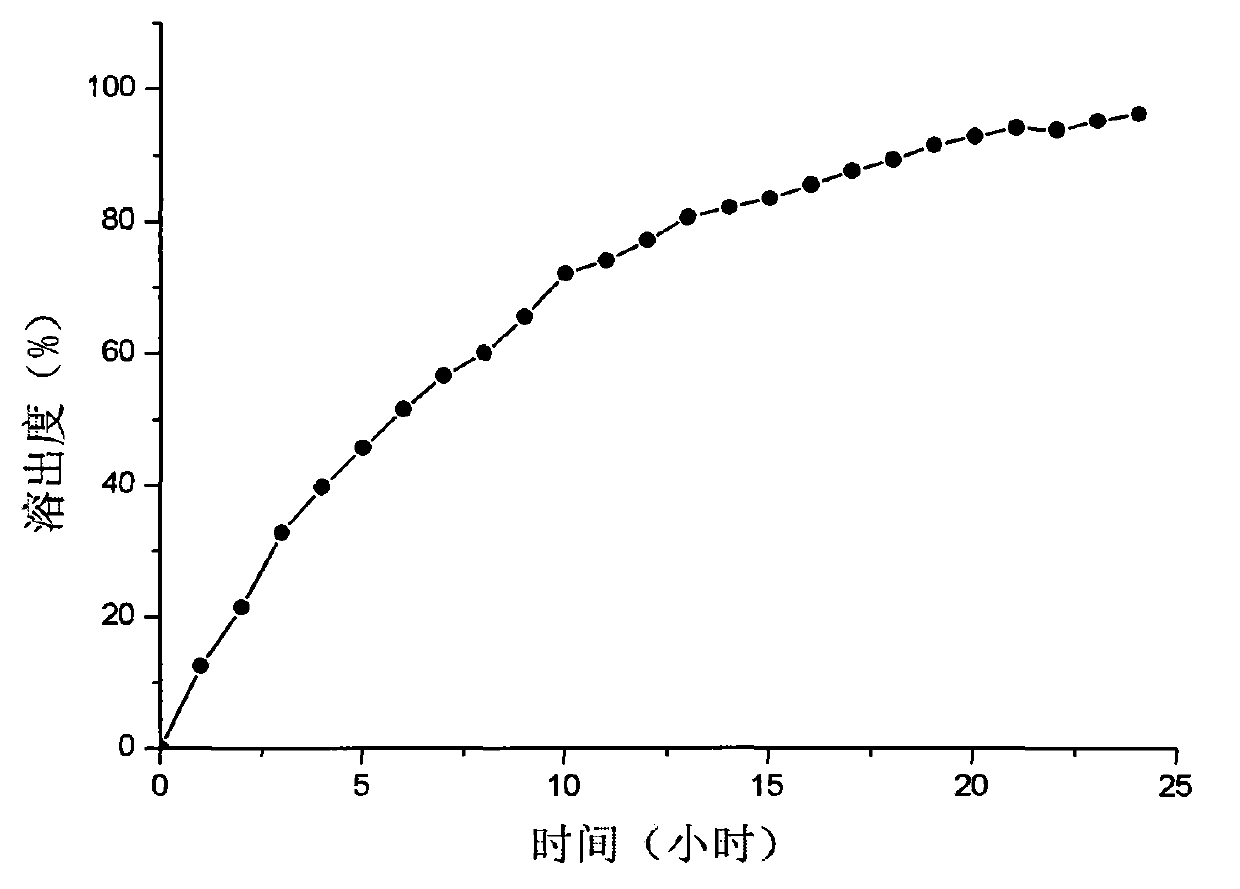
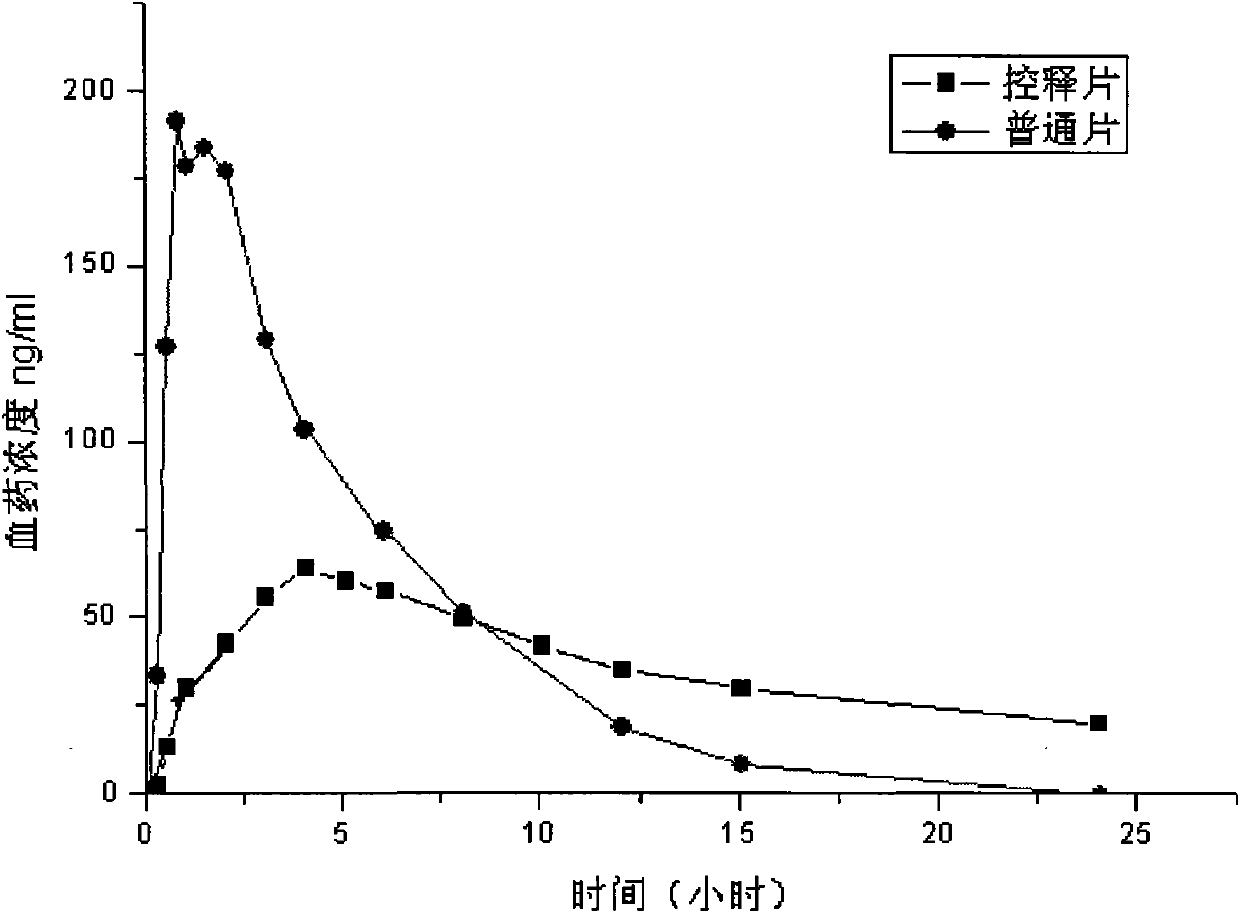
Patents
Literature
137 results about "Therapeutic window" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Therapeutic window of a drug is the range of drug dosages which can treat disease effectively while staying within the safety range. In other words, it is the dosages of a medication between the amount that gives an effect and the amount that gives more adverse effects than desired effects. Medication with a small pharmaceutical window must be administered with care and control, frequently measuring blood concentration of the drug, since it easily gives adverse effects, including irreversible damage.
Threshold optimization for tissue stimulation therapy
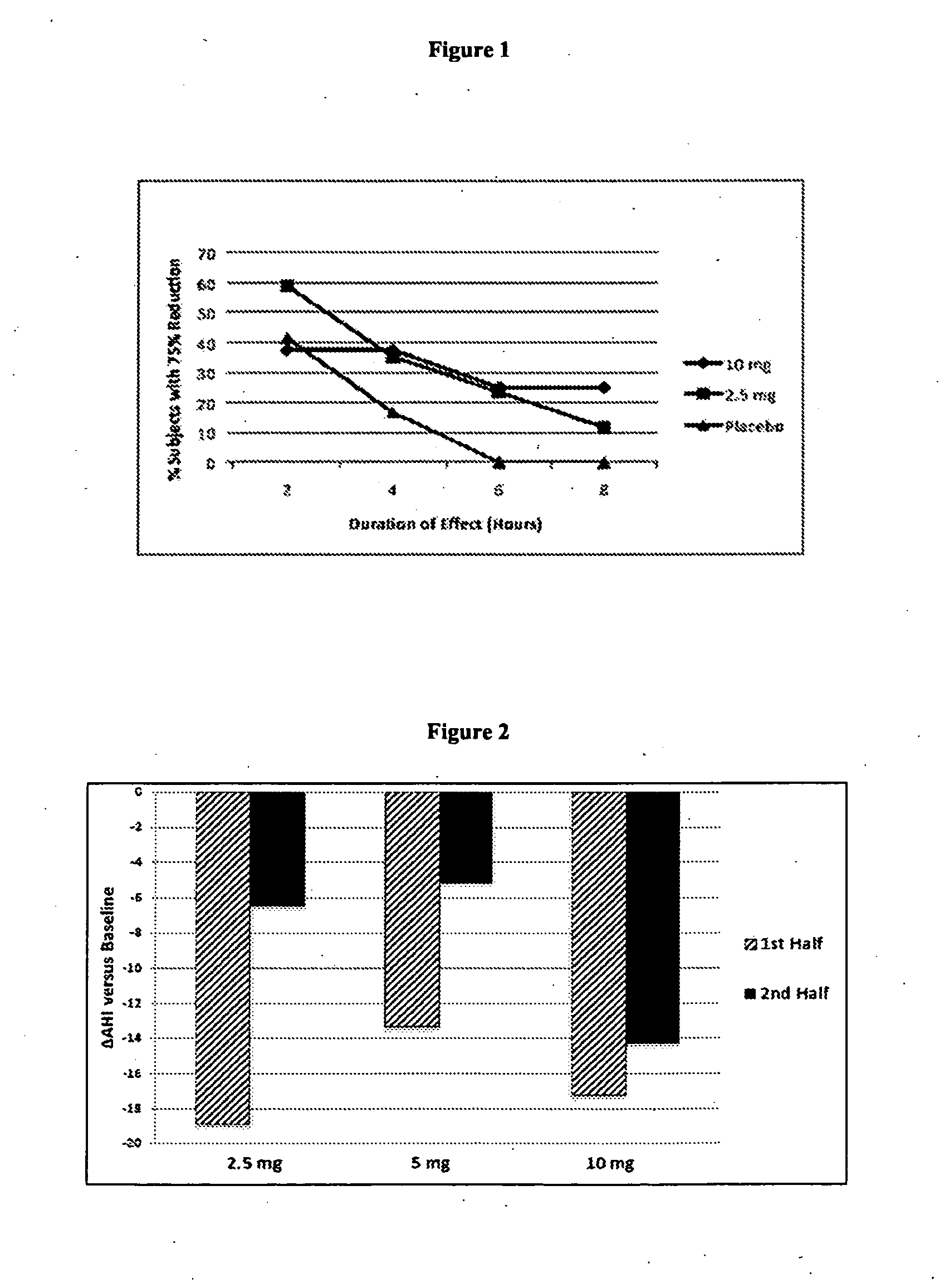
ActiveUS7869885B2Energy efficiencyElectrotherapyArtificial respirationSensory stimulation therapyTherapeutic window
Methods and systems for determining an optimal therapeutic window of parameter settings for nerve stimulation therapy are described herein. The disclosed techniques generally utilize one or more parameter sweeps to determine upper and lower threshold settings. The determination of the optimal therapeutic window may be performed during or after implantation.
Owner:LIVANOVA USA INC
Sustained release cannabinoid medicaments
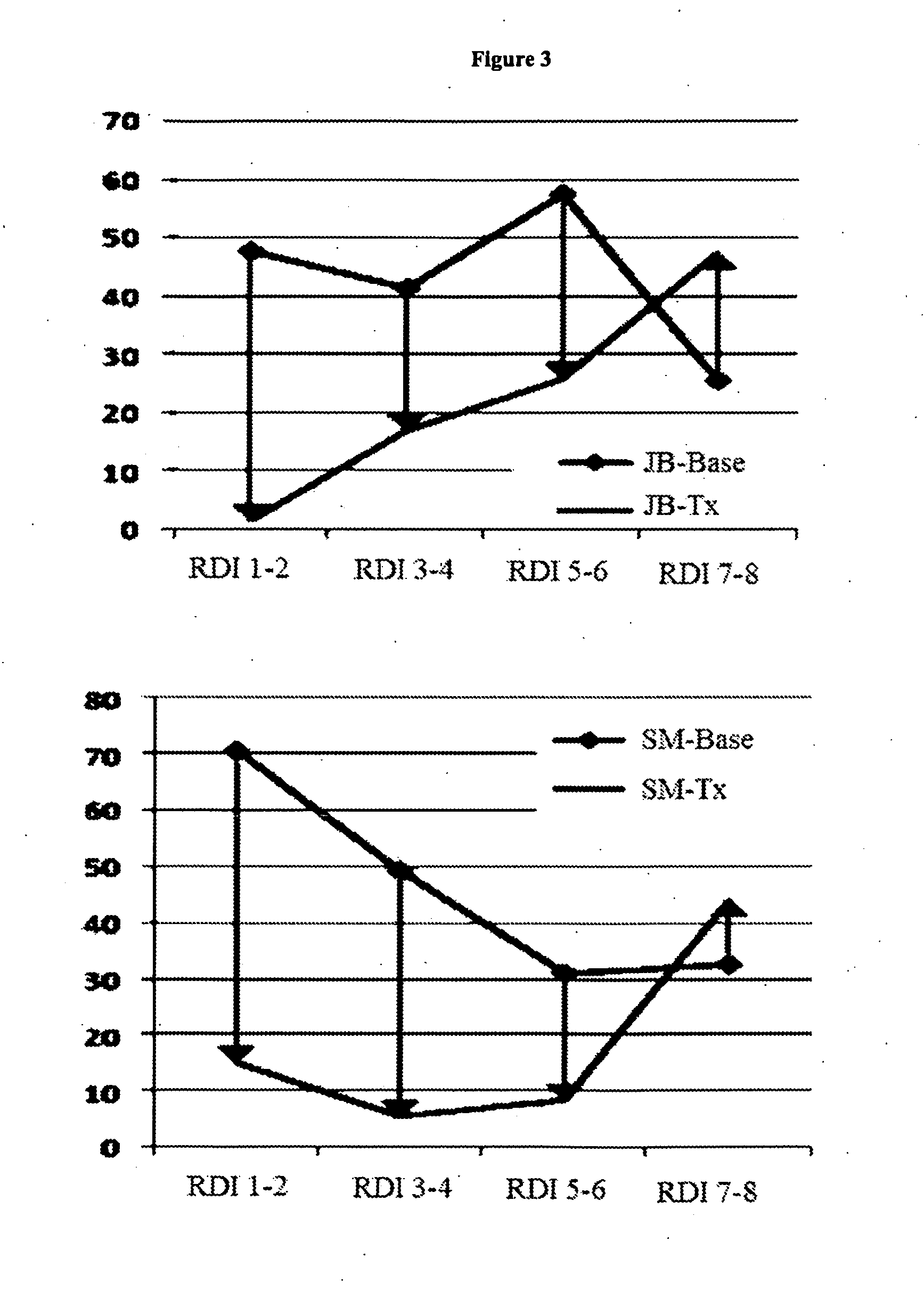
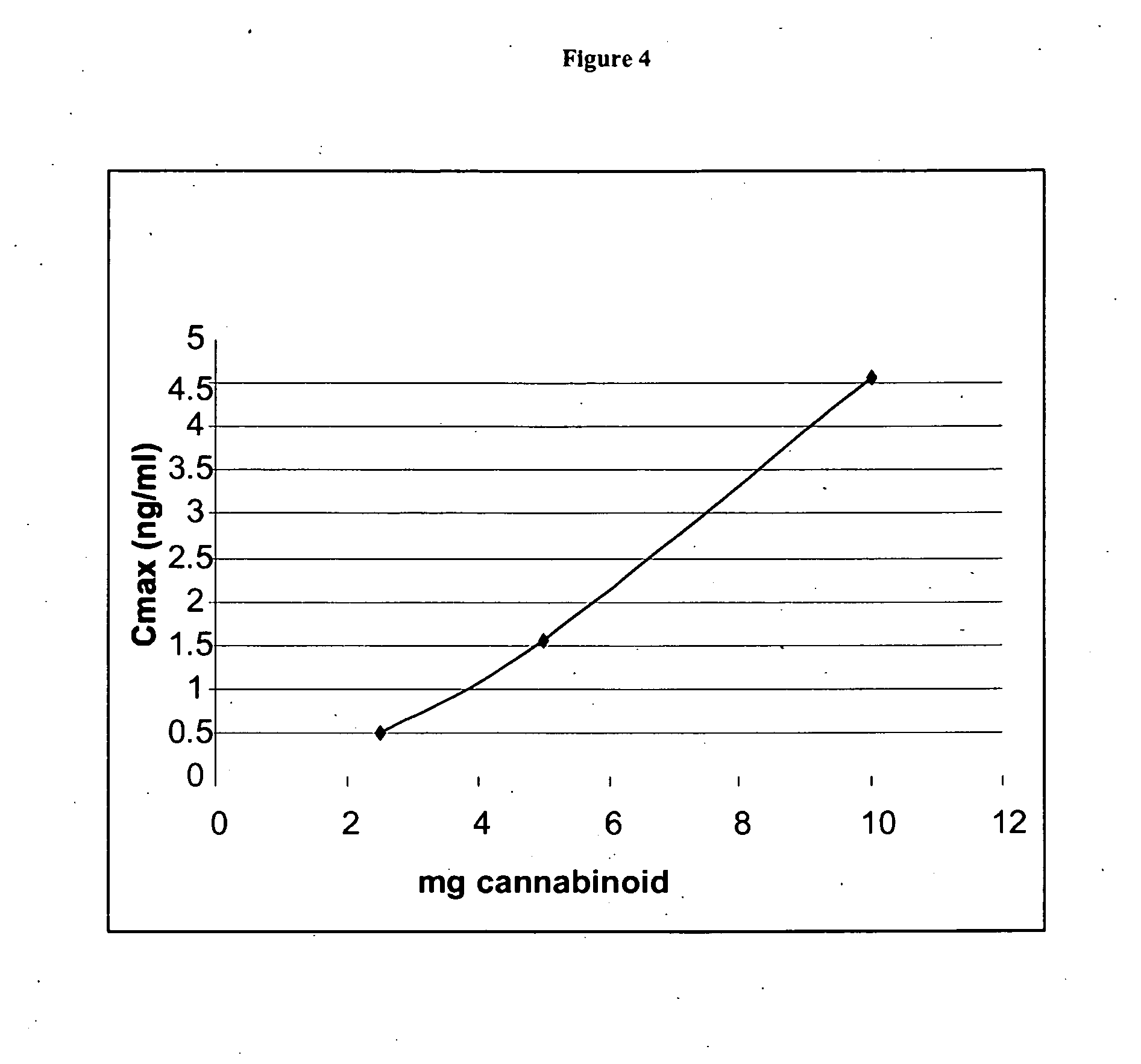
The present invention provides a medicament which results in delivery of a therapeutic level of one or more cannabinoids during a clinically relevant therapeutic window. The therapeutic window is a longer window than provided by an immediate release medicament such as Marinol containing an equivalent amount of the cannabinoid. Oral administration of the present compositions provides therapeutic dosing while maintaining safe, side effect sparing, levels of a cannabinoid. The present invention also provides methods of treating cannabinoid-sensitive disorders.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Melphalan prodrugs
InactiveUS20050214310A1High specificity of actionImprove stabilityNanomedicineAntibody ingredientsSolubilityTherapeutic window
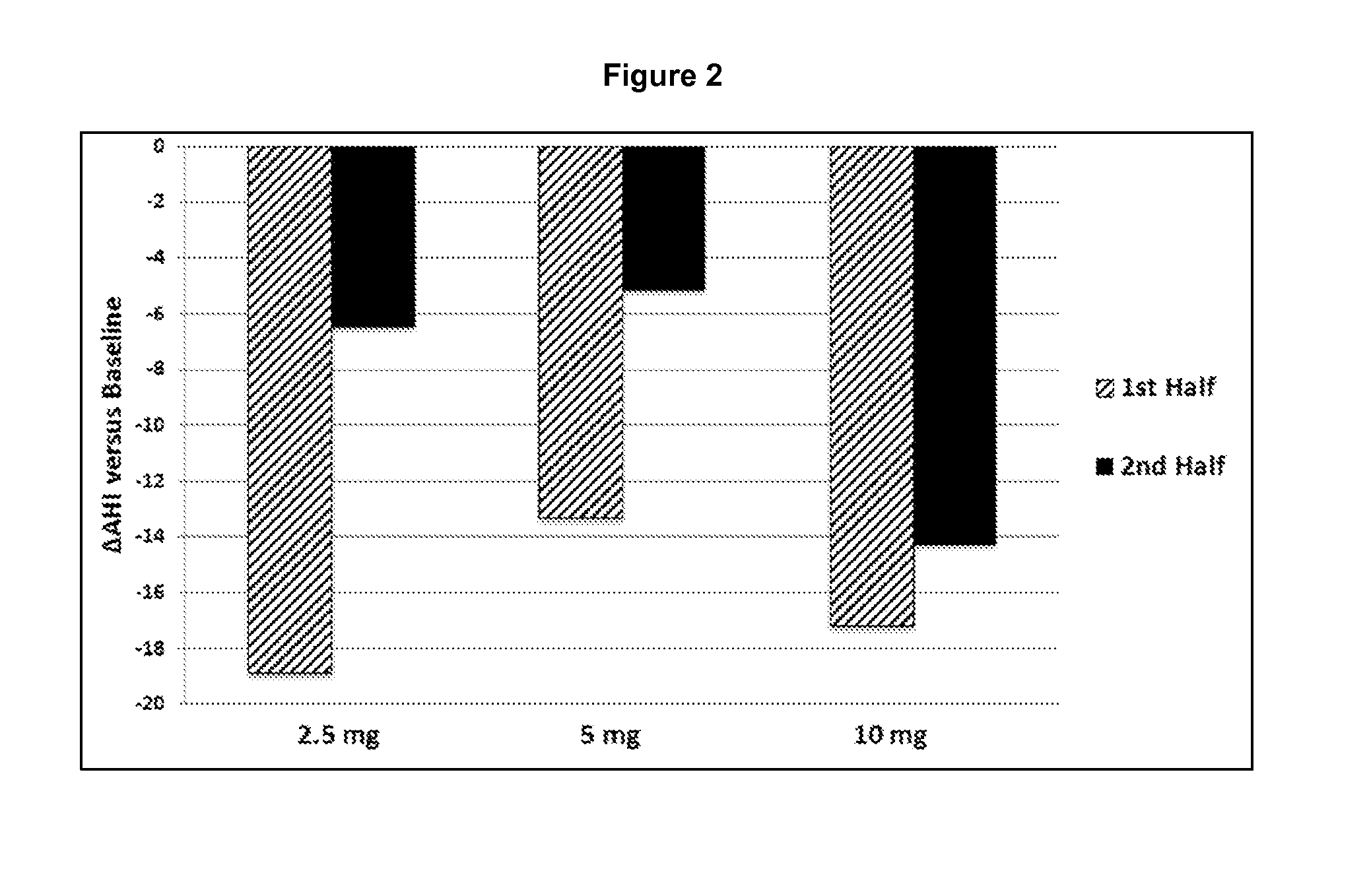
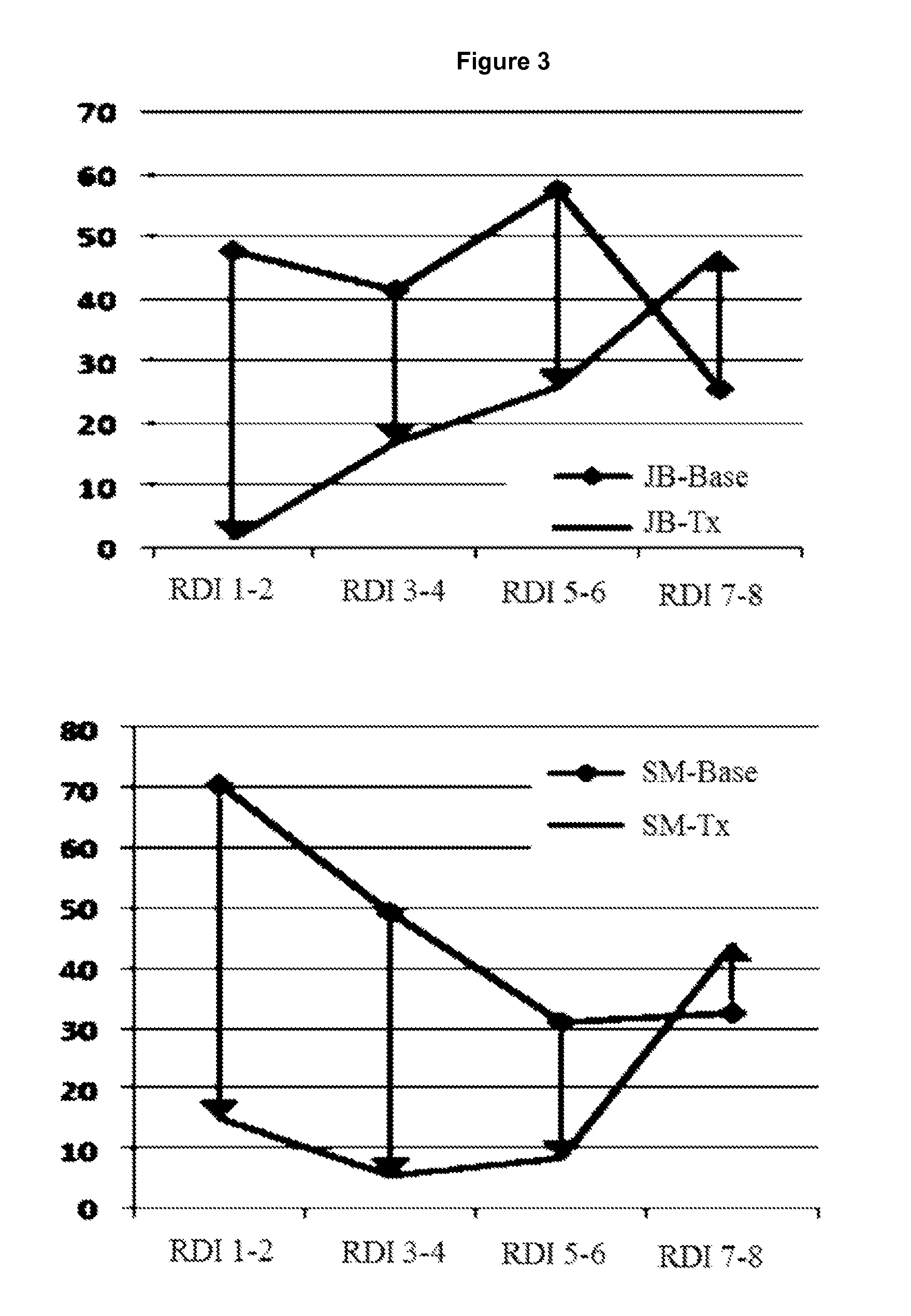
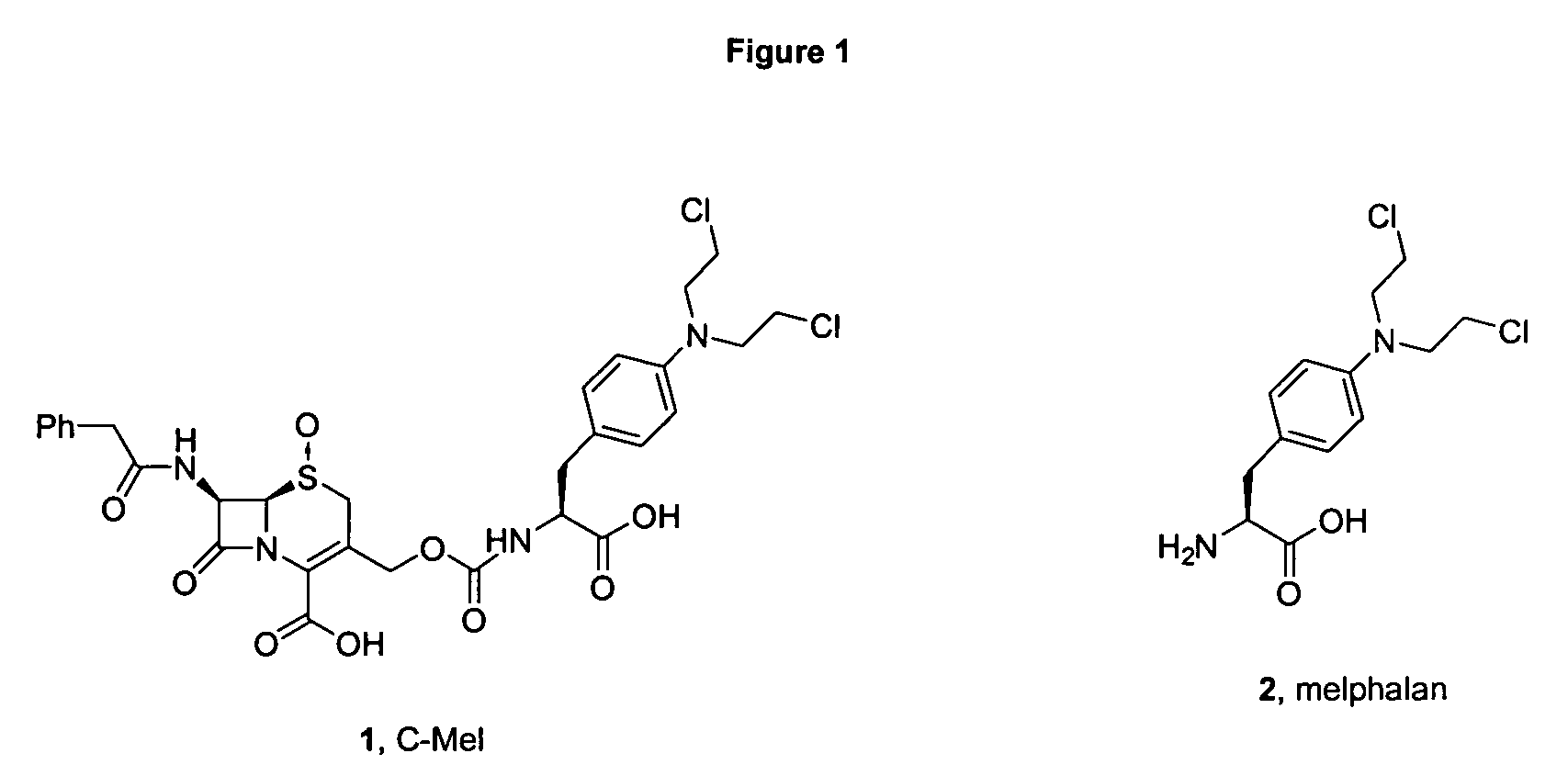
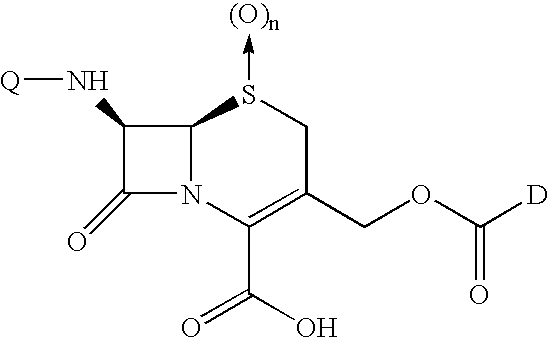
Shown and described are the synthesis of more potent forms of C-Mel, a prodrug used in Antibody-Directed Enzyme Prodrug Therapy, that releases the clinically used anticancer alkylating agent melphalan extracellularly. Shown and described are the synthesis of a variety of melphalan analogues with the intention to promote facile intracellular drug access. Esters, amides, and peptides of melphalan are shown. Cephalosporin prodrugs of the most interesting melphalan derivatives were synthesized and evaluated for potency, toxicity, therapeutic window, plasma stability, and solubility.
Owner:SEATTLE GENETICS INC
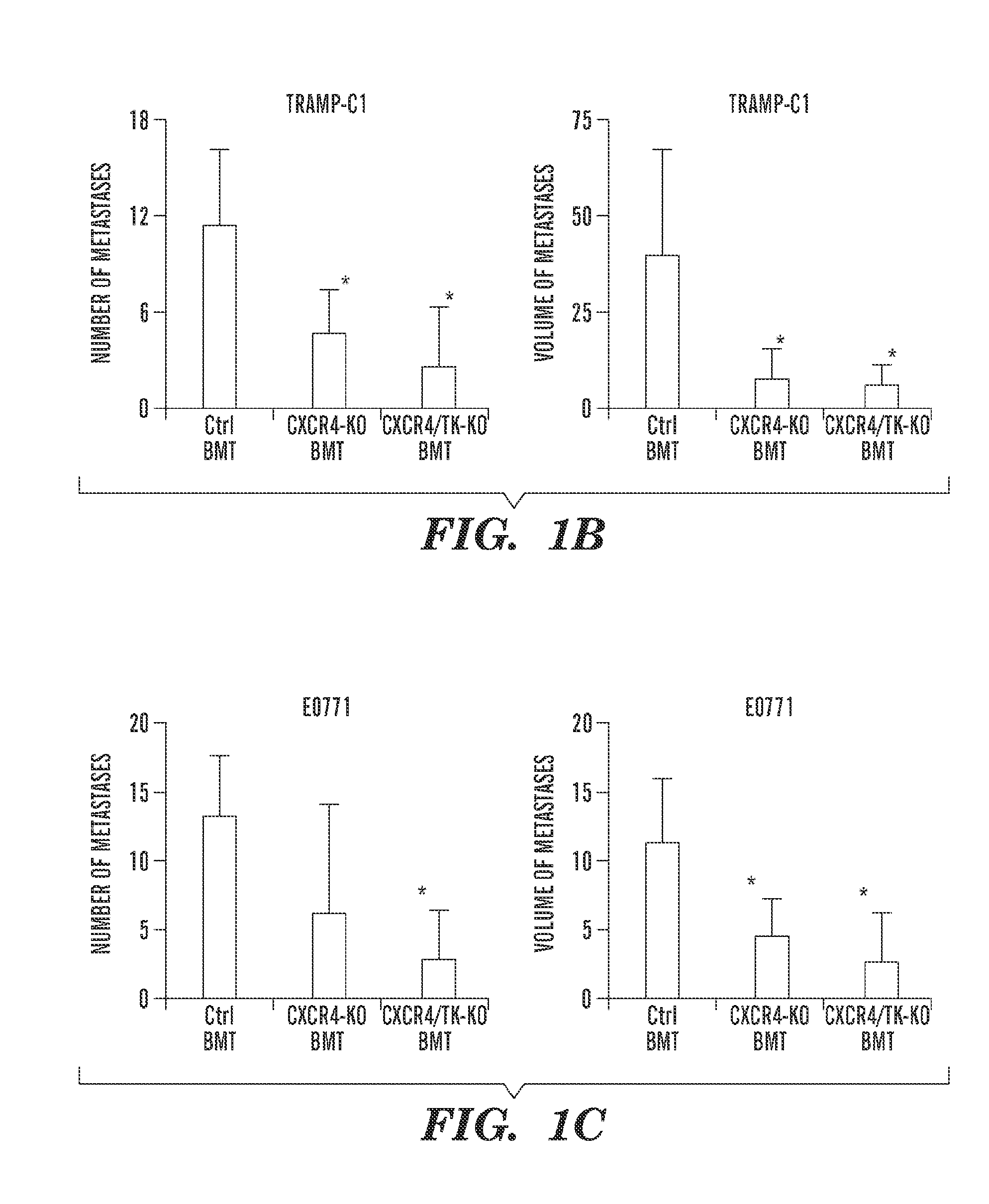
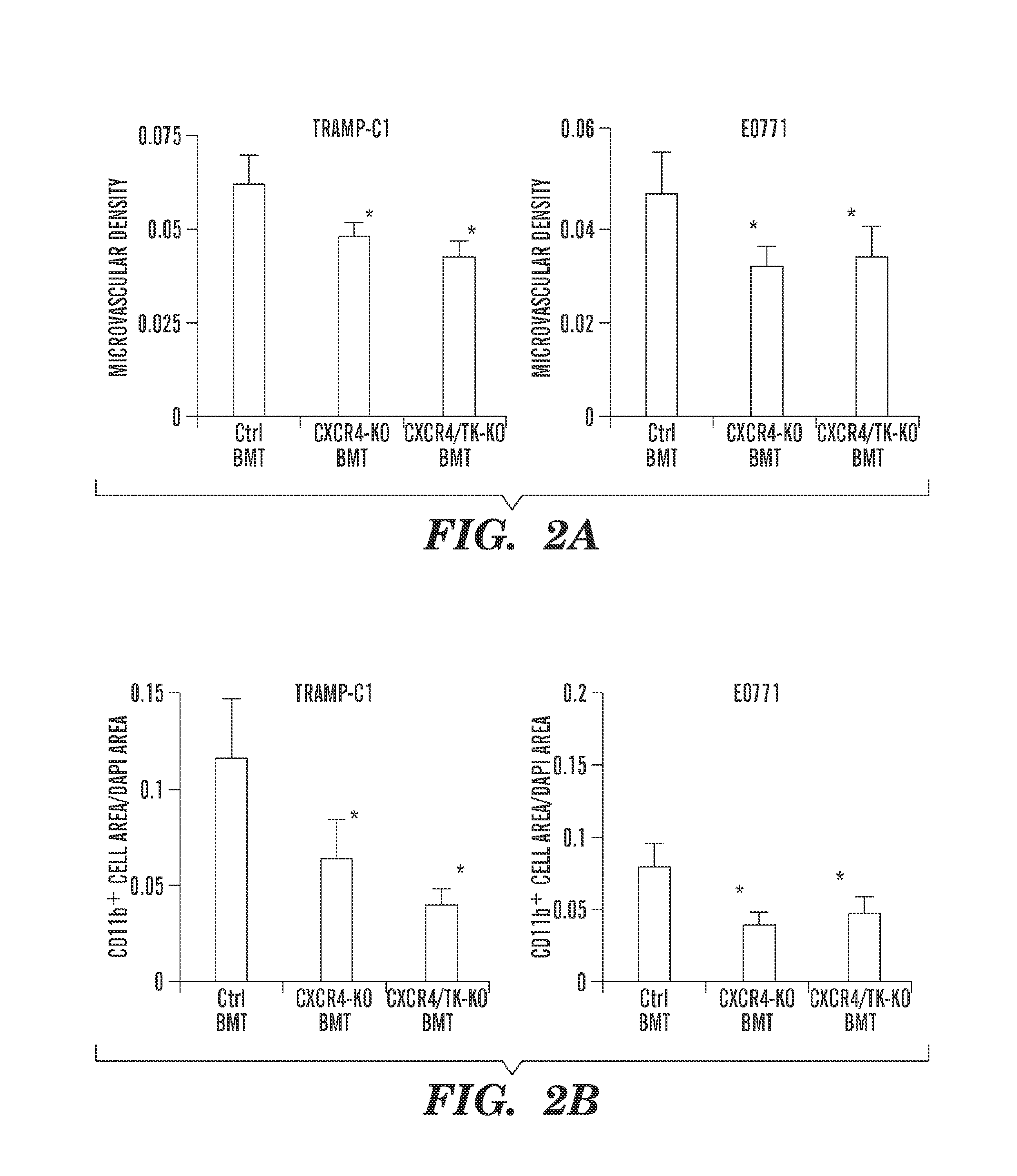
Anti-cxcr4 as a sensitizer to cancer therapeutics
ActiveUS20130216531A1Promote cell survivalPrevent infiltration of tumorBiocidePeptide/protein ingredientsCXCR4CXCR4 Inhibitor
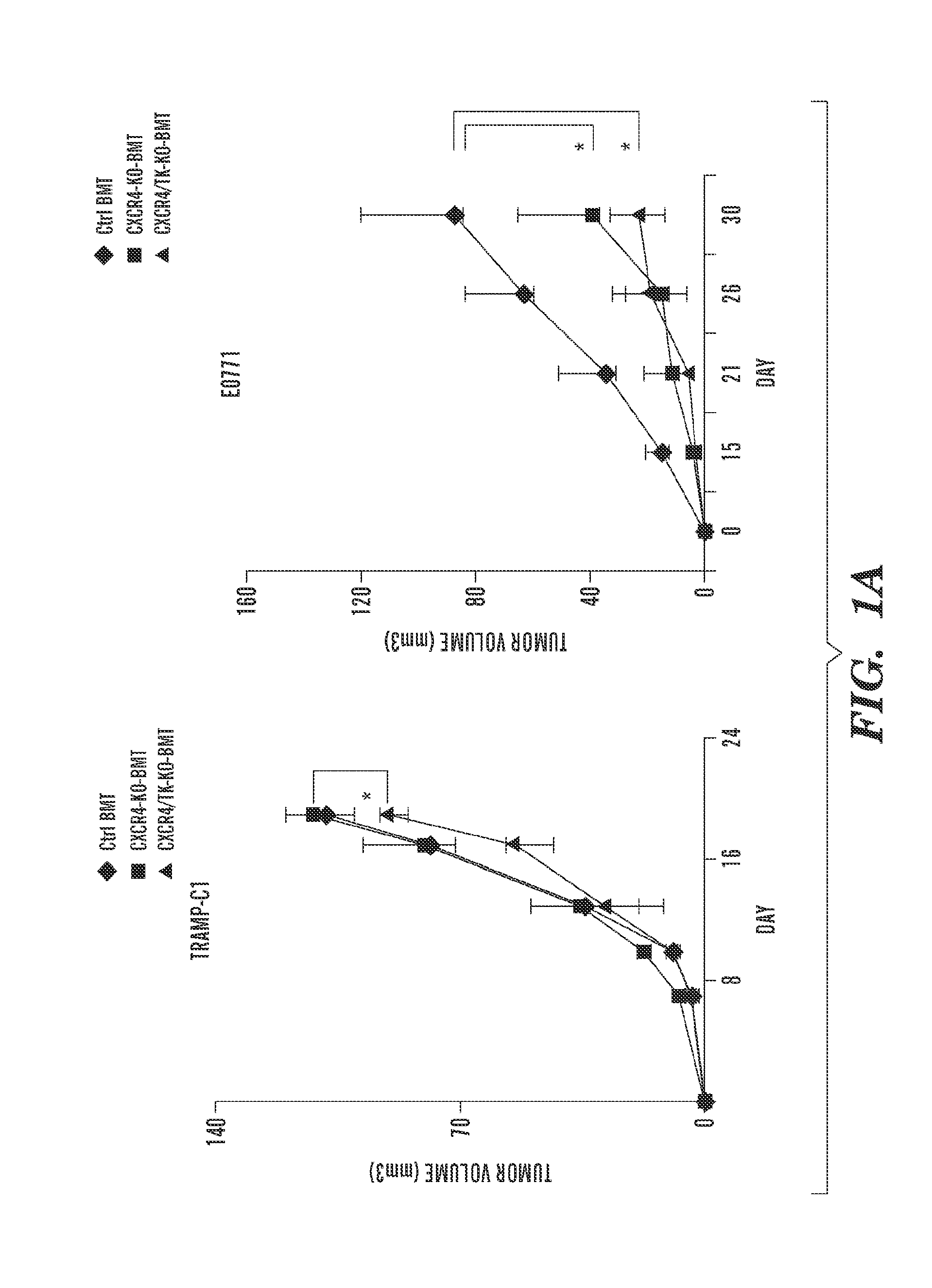
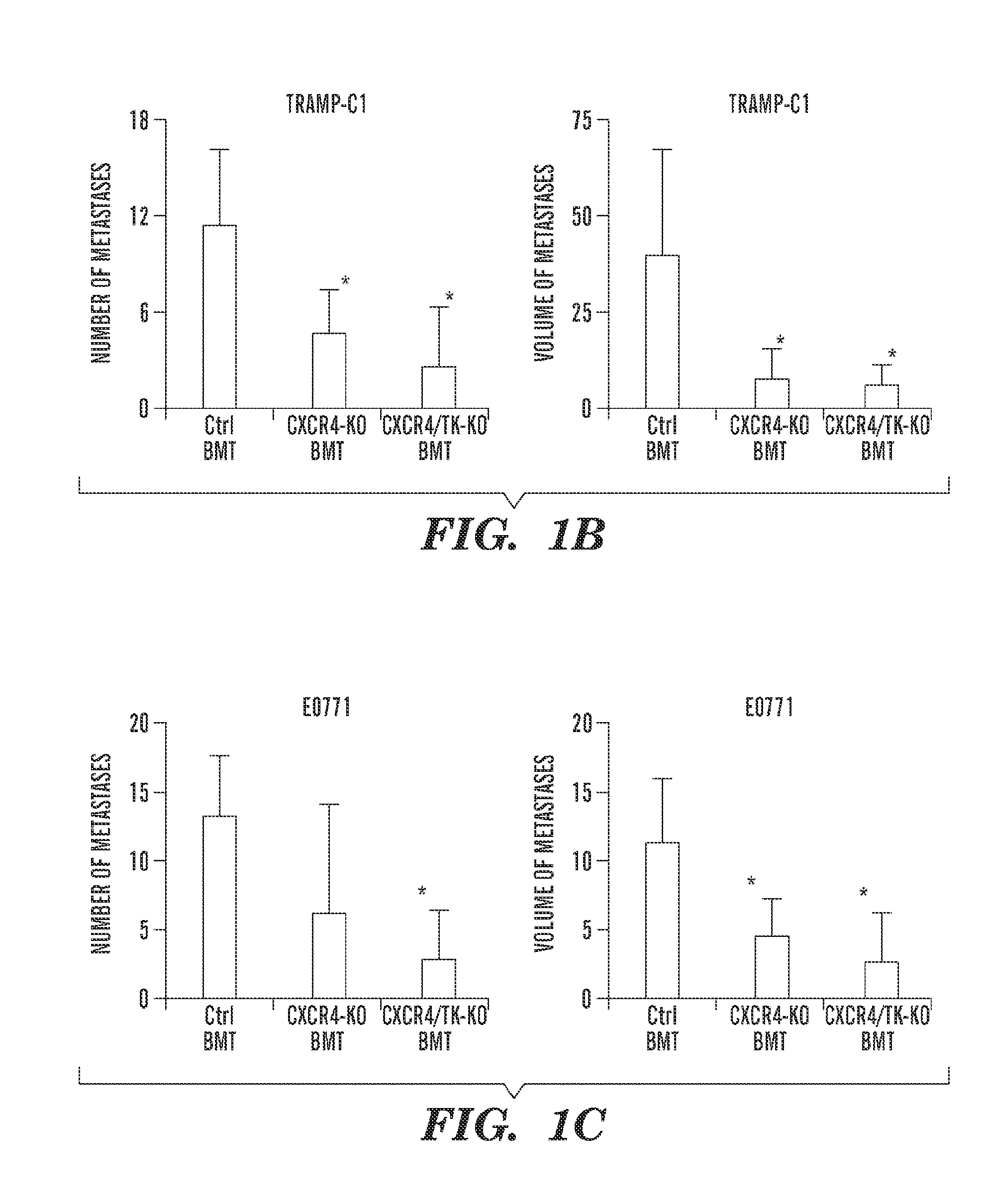
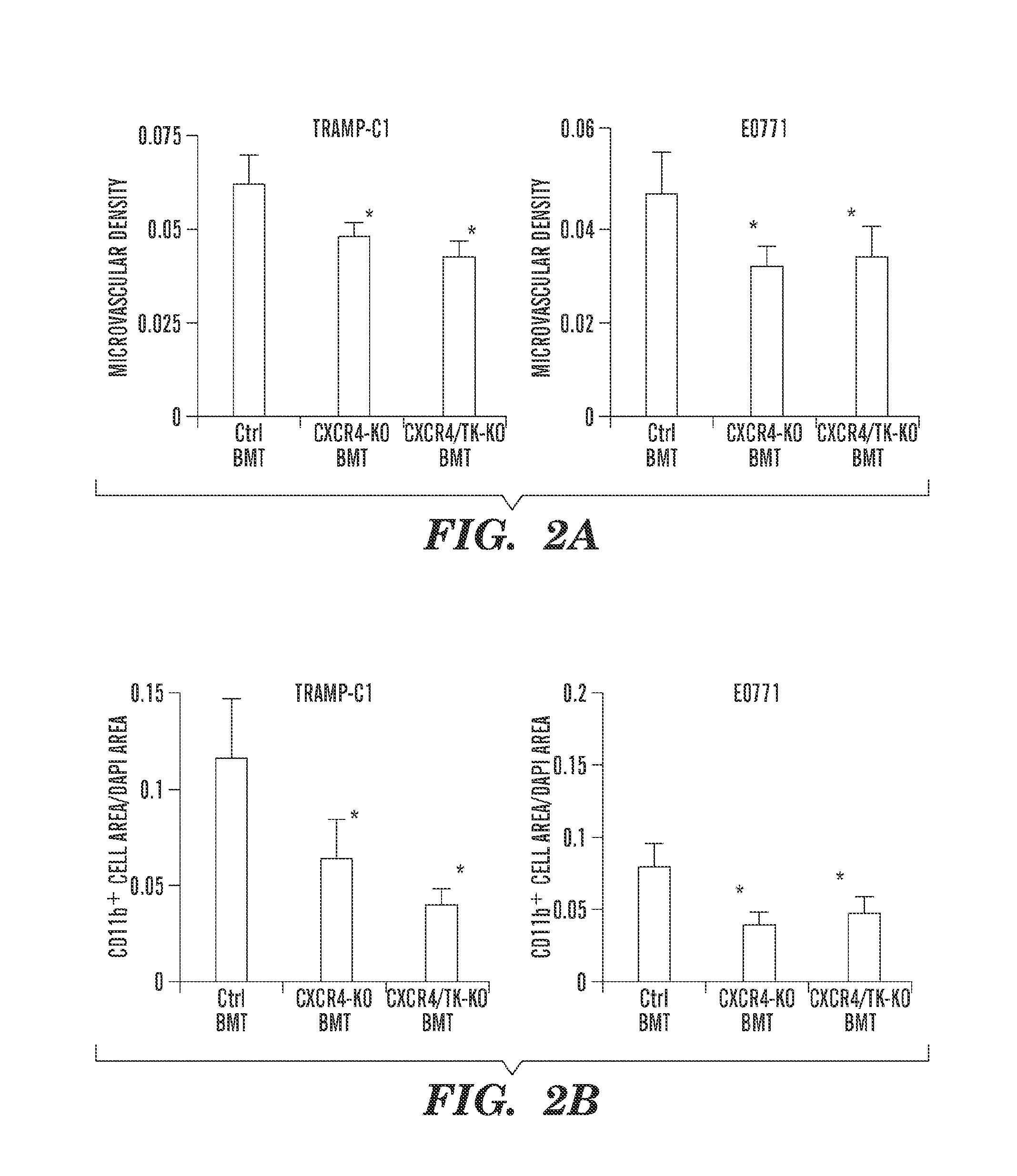
Inhibition of CXCR4 can inhibit tumor growth and metastasis during certain therapeutic windows. Disclosed are novel methods for treating and preventing cancer in a subject related to administration of CXCR4 inhibitors during a therapeutic window following treatment with another anti-tumor therapy.
Owner:THE GENERAL HOSPITAL CORP
Pharmaceutical Compositions Comprising Sirolimus and/or an Analogue Thereof
InactiveUS20080275076A1Improve safety/efficacy ratioReduce impactAntibacterial agentsBiocideParticulatesSide effect
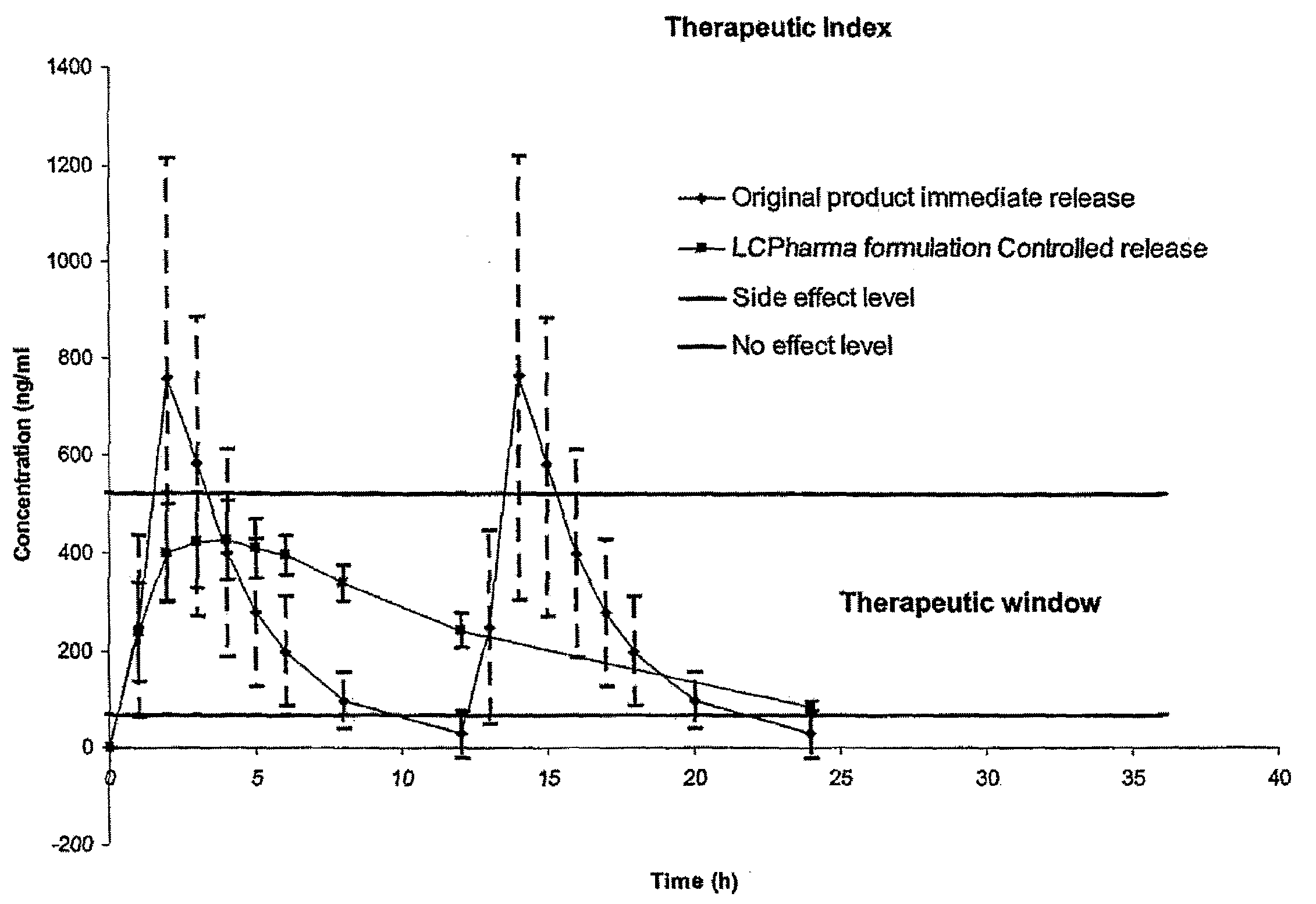
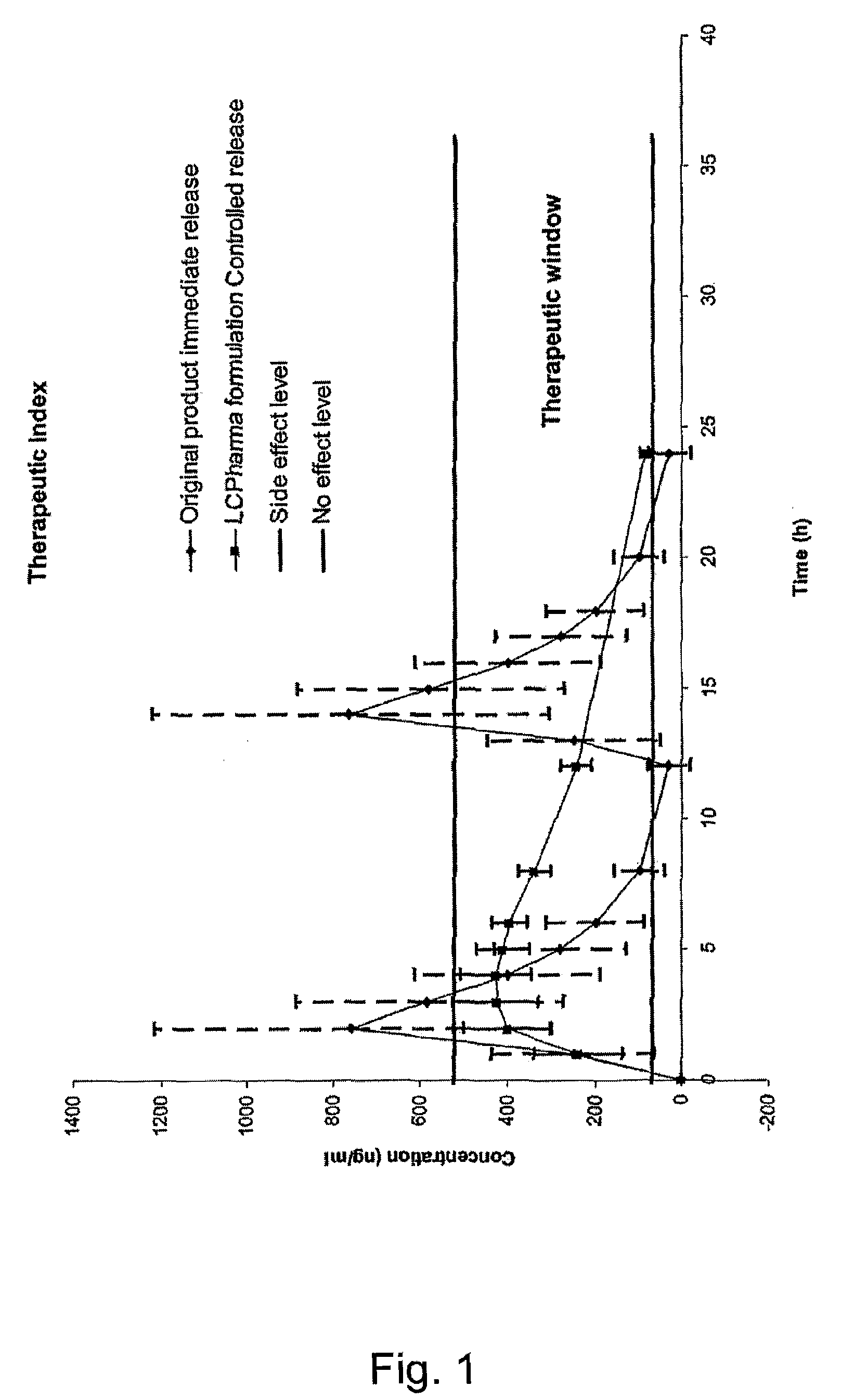
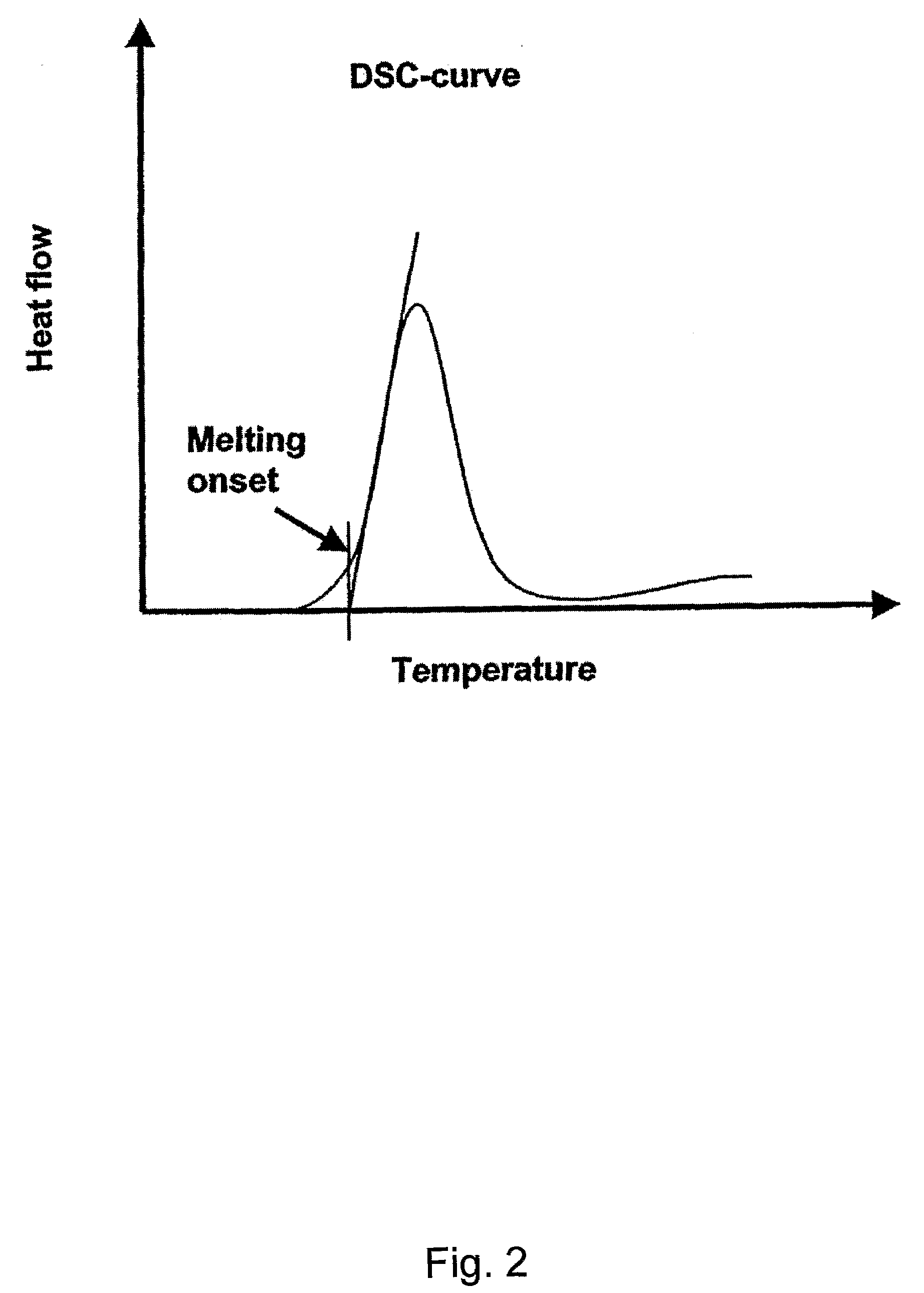
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising sirolimus (rapamycin) and / or derivatives and / or analogues thereof. Compositions of the invention exhibit an acceptable bioavailability of sirolimus and / or a derivative and / or an analogue thereof. The pharmaceutical compositions of the invention are designed to release sirolimus in a controlled manner so that the plasma levels stays within the narrow therapeutic window that exist for this class of substances. An extended release profile, where the peak concentration has been reduced without loosing significant bioavailability, together with less variable absorption, is expected to improve the safety / efficacy ratio of the drug. Furthermore, compositions according to the invention provide for a significant reduced food effect and a delayed release of sirolimus is expected to reduce the number of gastro-intestinal related side effects.
Owner:LIFECYCLE PHARMA AS
Low dose cannabinoid medicaments
The present invention provides methods for treating cannabinoid-sensitive disorders with a lose-dose oral cannabinoid which results in delivery of a therapeutic level during an extended clinically-relevant therapeutic window. These methods provide therapeutic dosing while maintaining safe, side effect sparing, levels of a cannabinoid. The present invention also provides methods of determining optimal dosing in treated patients.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
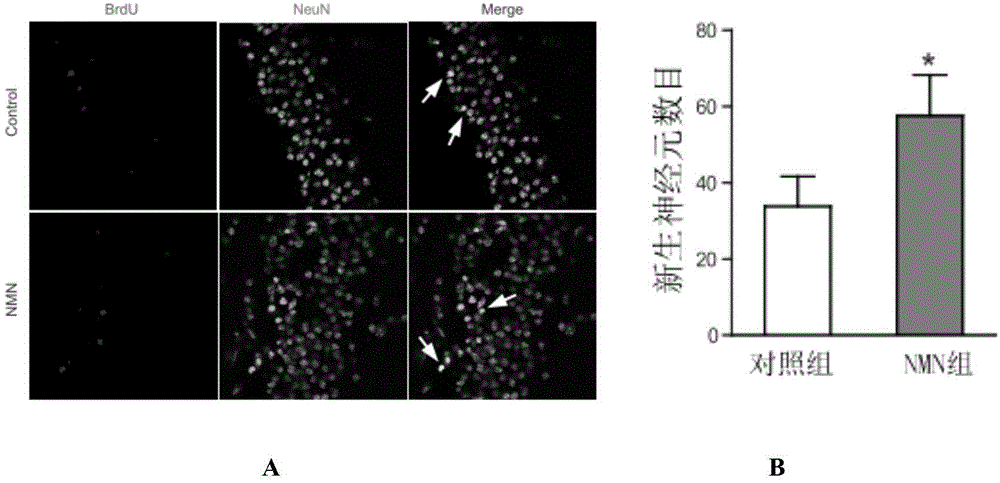
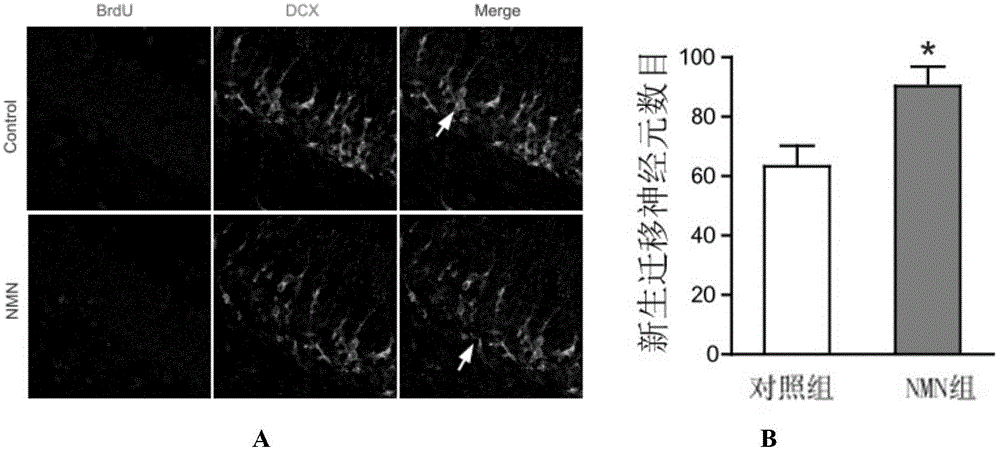
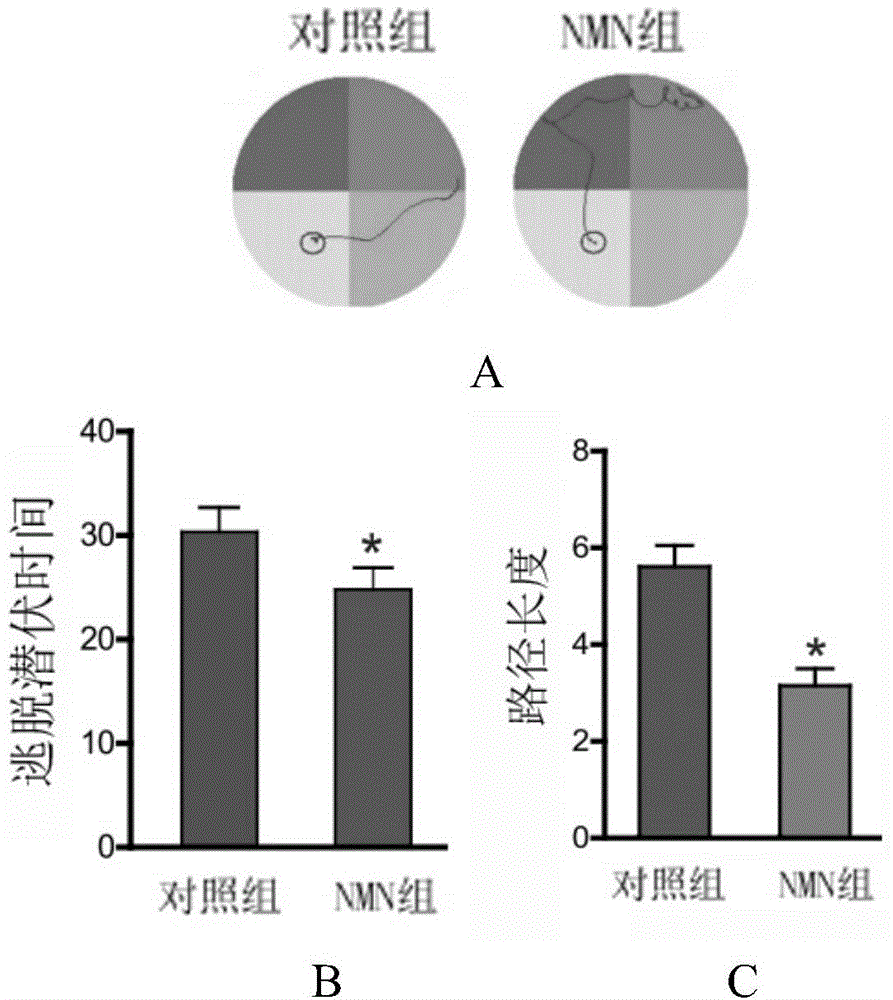
Application of nicotinamide mononucleotide in preparation of medicines for promotion of nerve regeneration after cerebral ischemia
ActiveCN104367587APromote differentiationImprove neurological impairmentOrganic active ingredientsNervous disorderNerves regenerationNicotinamide mononucleotide
The invention relates to the technical field of medicines. The present invention provides application of nicotinamide mononucleotide in preparation of medicines for promotion of nerve regeneration after cerebral ischemia, and the nicotinamide mononucleotide can be used as an active pharmaceutical ingredient and a pharmaceutically acceptable supplementary material for preparation of medicine compositions. The nicotinamide mononucleotide compound itself is an endogenous protective substance, and the available data do not report the adverse reaction, so that the nicotinamide mononucleotide as a medicine or a health product is high in safety and wide in therapeutic window.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
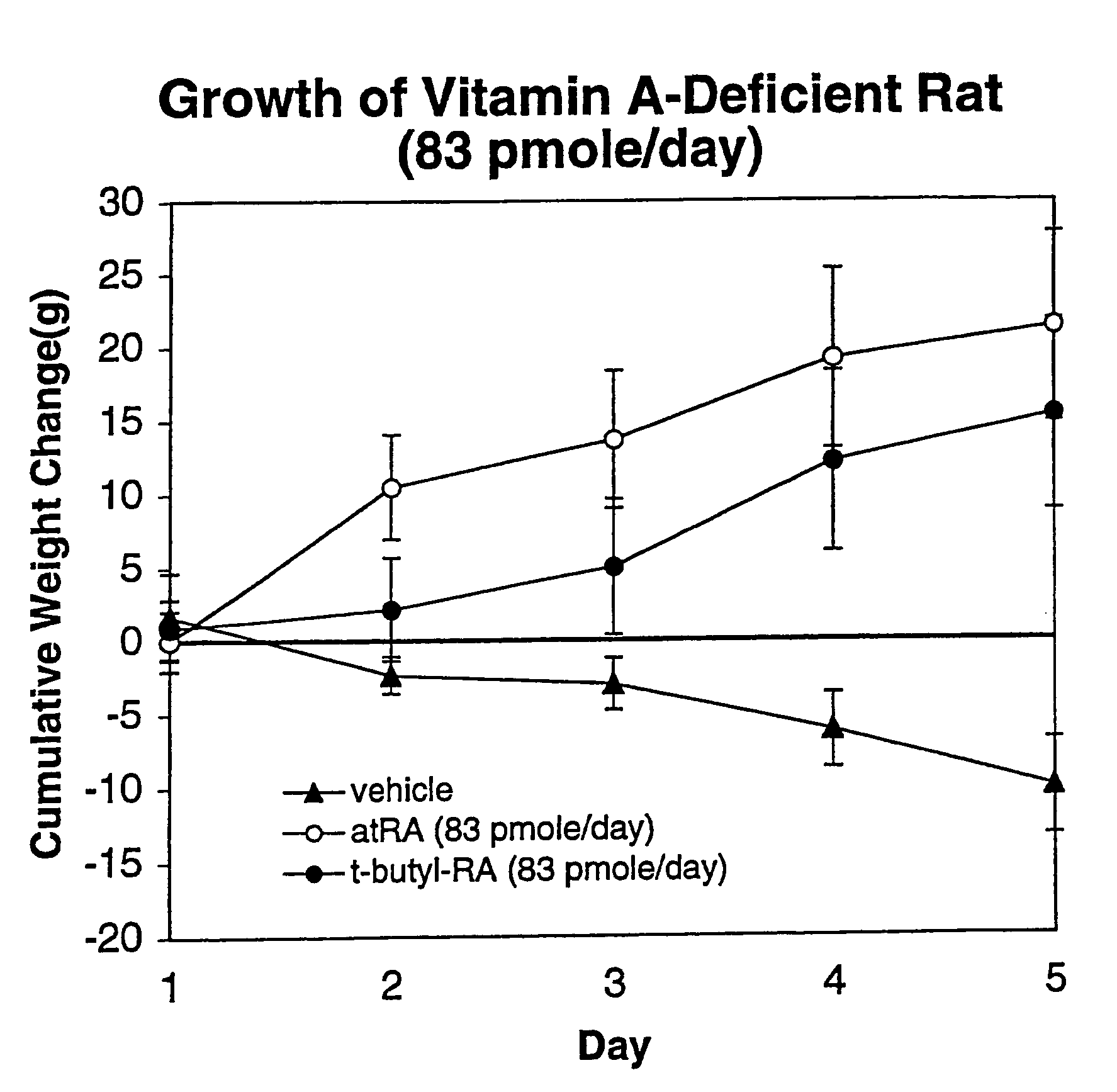
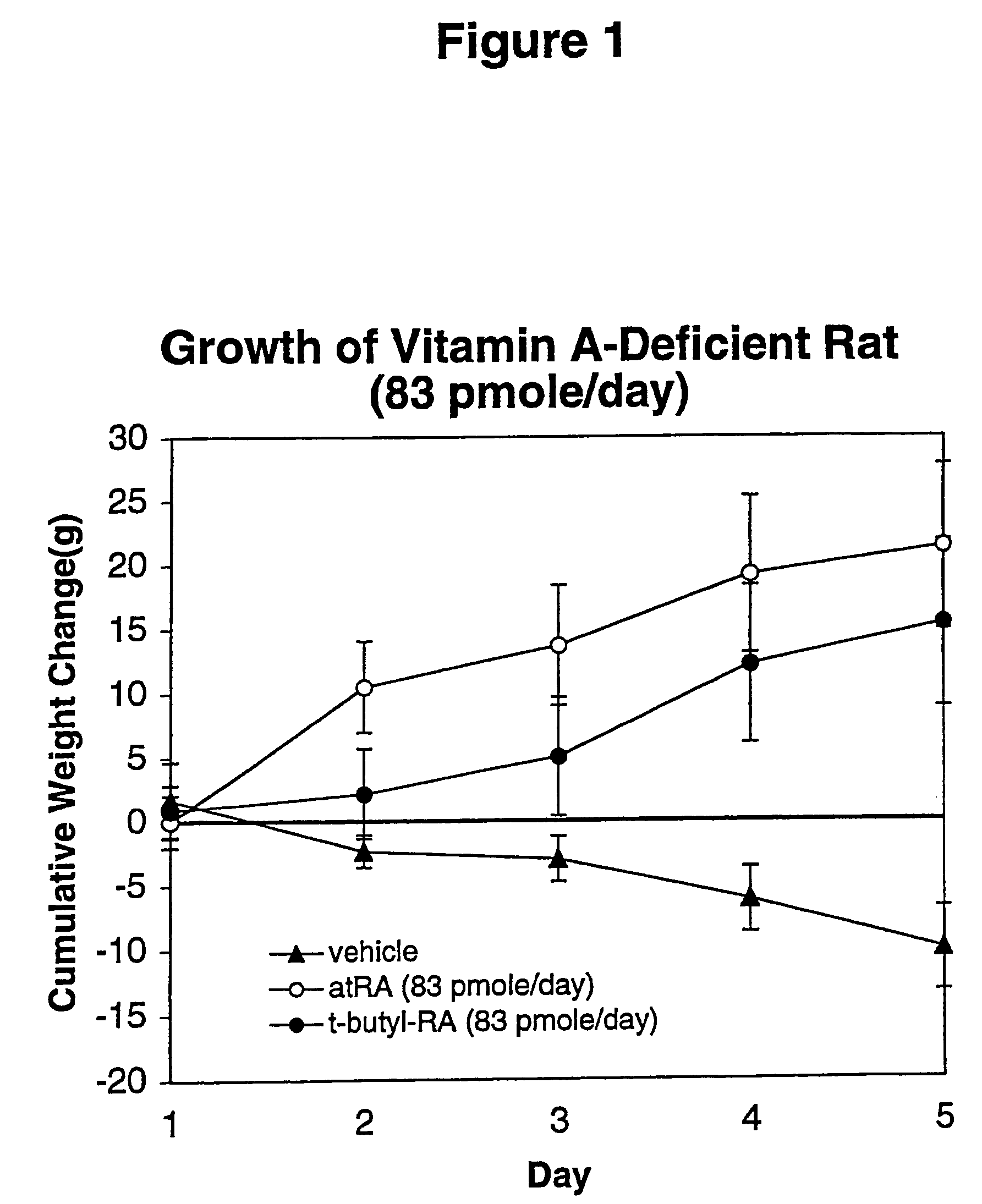
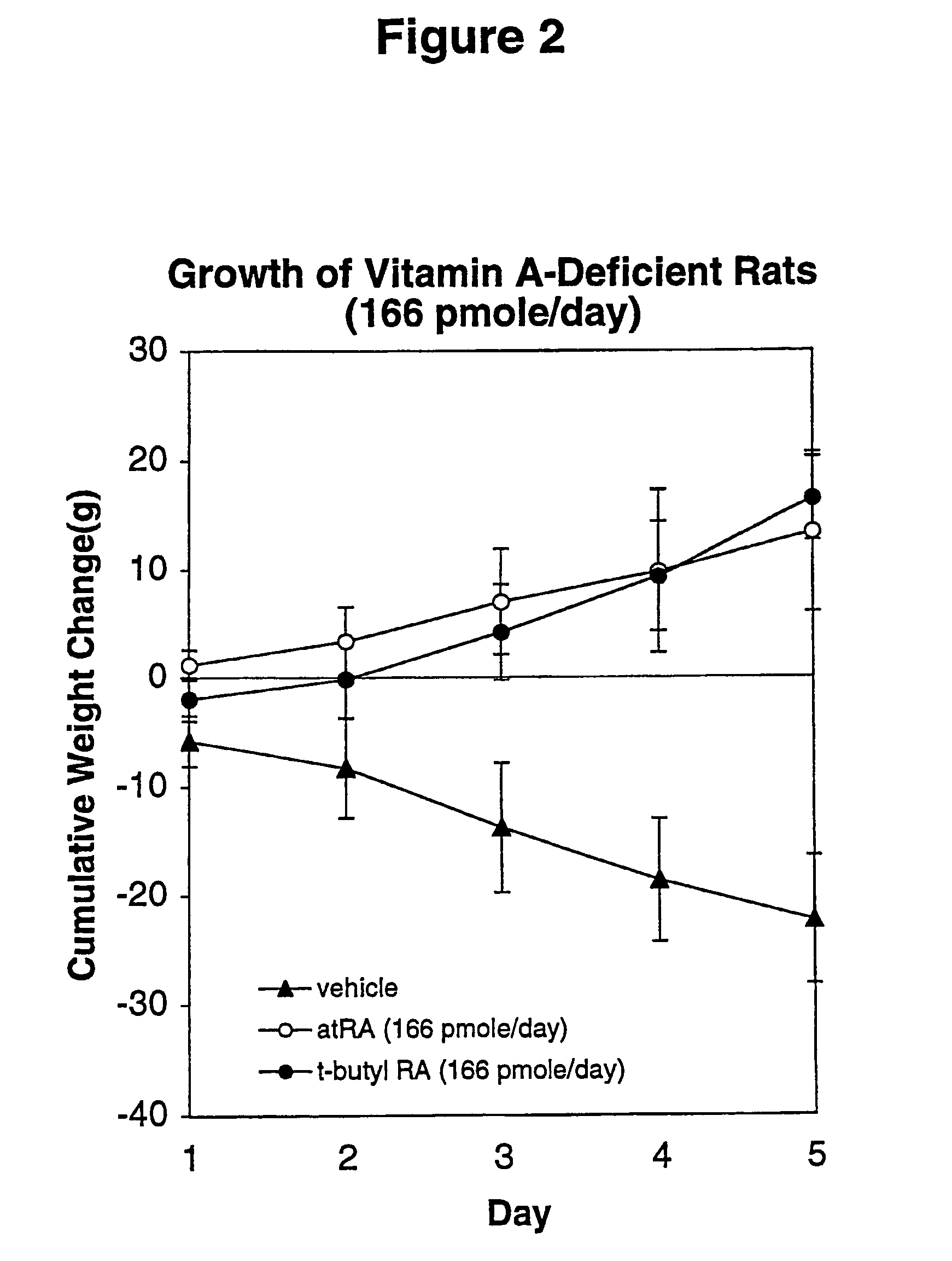
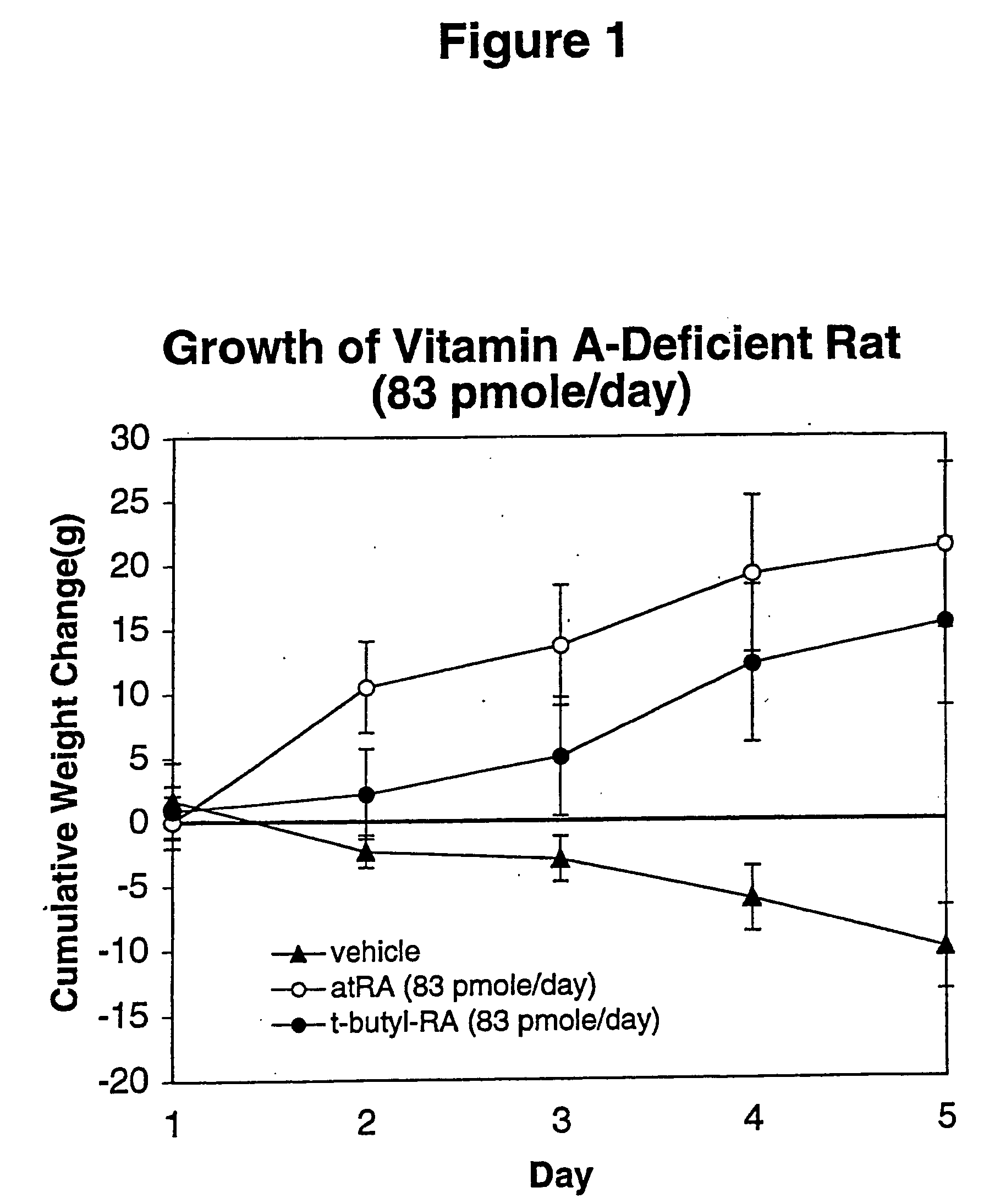
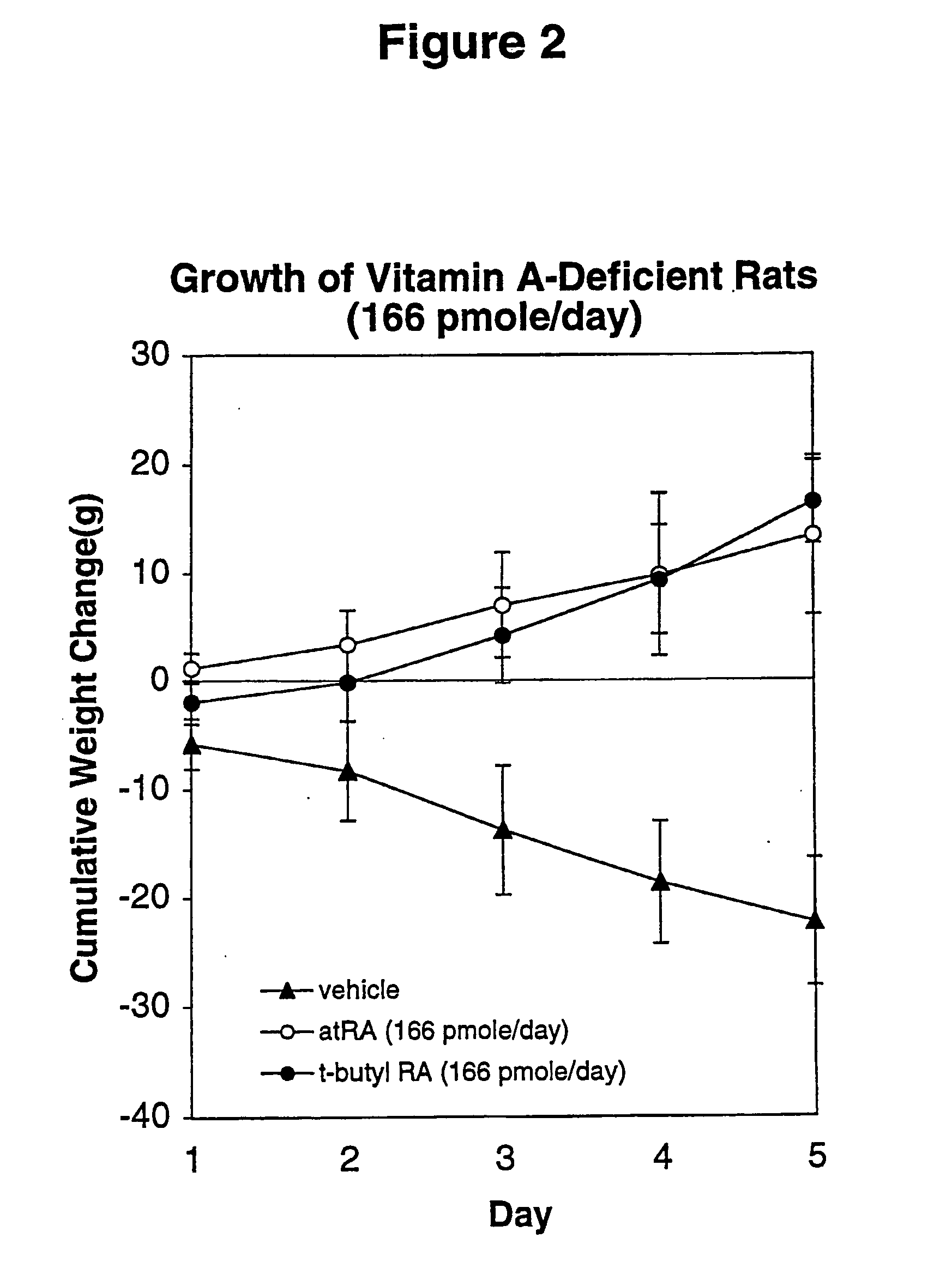
Method of reducing toxicity of retinoids
InactiveUS7126017B2Minimal orLow toxicityFatty oils/acids recovery from wasteOrganic active ingredientsRetinoidAlcohol
A method of minimizing or reducing the toxicity of a retinoid having a free carboxyl group is described. The method comprises the step of esterifying the carboxyl group of the retinoid with a highly sterically hindered compound, which is preferably an alcohol. The resulting retinoid esters are rendered much less toxic than the starting or parent retinoid. This process provides a retinoid ester analog of reduced toxicity so that it may be administered orally with minimal side effects and with a much greater therapeutic window.
Owner:WISCONSIN ALUMNI RES FOUND
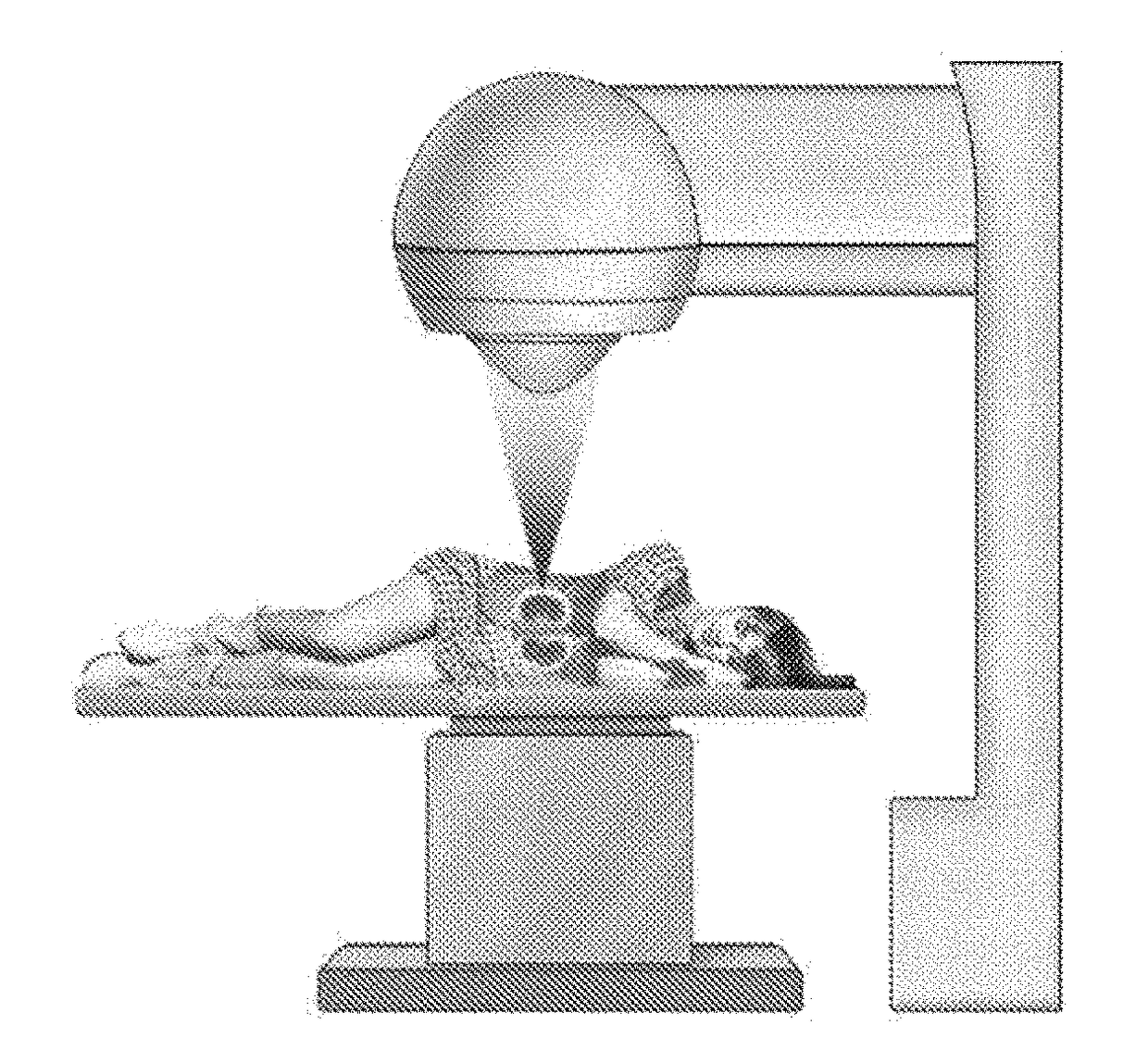
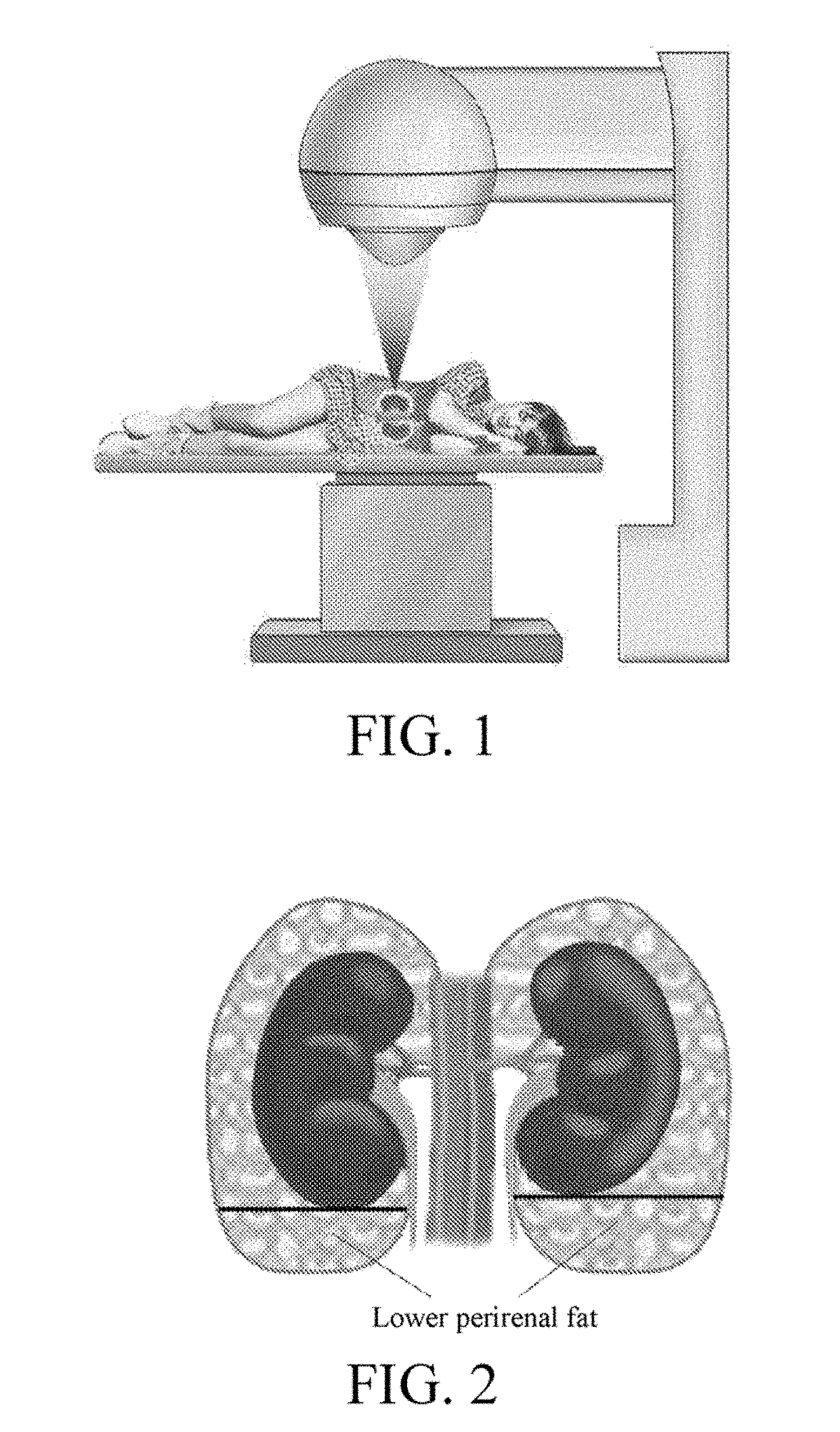
Application of high-intensity focused ultrasound system to treatment of essential hypertension
InactiveUS20180154184A1Blood pressure level is reducedSmall doseUltrasound therapySurgical instrument detailsNervous systemWhole body
An application of a high-intensity focused ultrasound system to treatment of essential hypertension. A treatment process includes: conducting ultrasonic positioning measurement on both sides of perirenal fat tissue of a patient, setting a therapeutic window and parameters of the system, and setting corresponding power parameter of the system, so as to make local temperature of the tissue during treatment reach 40-70° C., wherein treatment portions are both sides, and treatment scope is one third to all of the whole tissue; starting the system to treat one side of the tissue according to the set parameters and then the other. By treating a secondary center for regulating the activity of a whole-body sympathetic system, the activity of the whole-body sympathetic system can be reduced, so that the blood pressure level of the patient can be reduced, and fewer kinds and smaller dosage of antihypertensive drugs can be taken or ceased.
Owner:NANJING GUANGCI MEDICAL TECH
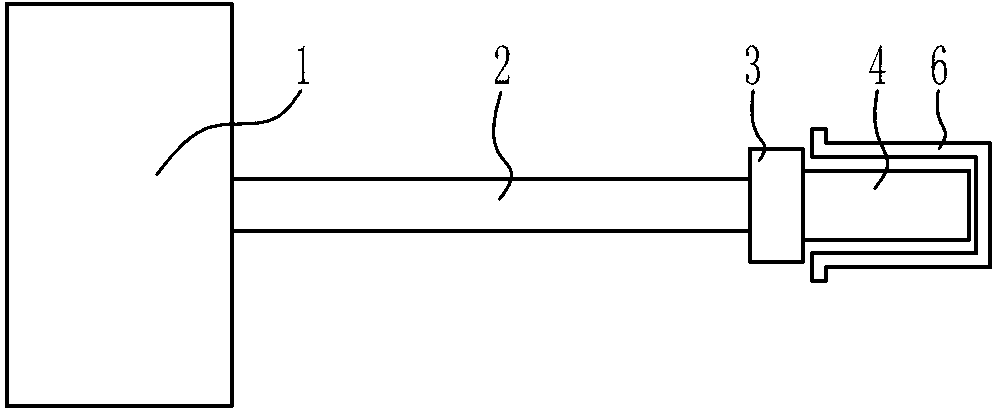
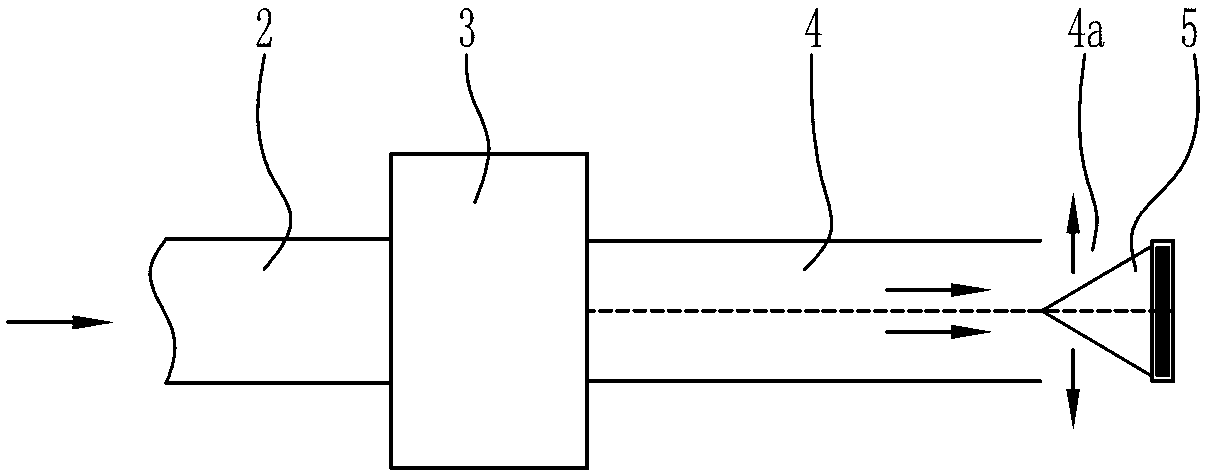
Laser therapeutic instrument for vagina tightening
InactiveCN103211651AGuaranteed minimum occlusionGuaranteed occlusionSurgical instrument detailsLight beamEngineering
The invention discloses a laser therapeutic instrument for vagina tightening. A laser therapeutic main machine (1) is connected with an image emitter (3) through a light beam transmission system (2). The image emitter (3) is fixed with one end of a therapeutic handle (4). A circle of therapeutic window (4a) is arranged on the outer periphery of the other end of the therapeutic handle (4). A pyramid prism (5) is arranged in the circle of therapeutic window. The tip end of the pyramid prism (5) is towards the image emitter (3). An included angle of 45 degrees is formed between the central line of each oblique face of the pyramid prism (5) and the axial line of the therapeutic handle (4). The laser therapeutic instrument can be compatible with various lasers between 1500 nm and 1100 nm, be beneficial to regeneration of skin collagen cells and well tighten skin, the therapeutic handle irradiates target tissue in a 360-degree-all-direction mode, operation is greatly simplified, the shortcoming that in a traditional technology, a therapeutic process is complex is overcome, and therapeutic effect is greatly improved.
Owner:CHONGQING DEMA PHOTOELECTRIC TECH
Antibody drug conjugate and application thereof
PendingCN110997010AEasy to manufactureHigh drug loadingImmunoglobulinsPharmaceutical non-active ingredientsDrug conjugationAntiendomysial antibodies
According to the invention, one or more cysteine residues or cysteine derivative residues are used as a drug connection carrier; one or more drugs are coupled to limited connecting sites of the antibody at the same time, so that drugs with higher drug loading capacity are prepared or drugs with lower toxicity can be selected to prepare ADC products, and ADC products with larger treatment windows are obtained. Furthermore, since a plurality of drug molecules can be coupled at one connection site, the ADC product obtained by the method provided by the invention has better uniformity under the condition of preparing antibody drug conjugates with the same DAR value. The use amount of the antibody required in production can be greatly reduced, so that the production cost is effectively reduced.Compared with the antibody drug conjugate of which the same site can only be connected with one drug molecule, the antibody drug conjugate prepared by adopting the method disclosed by the invention still has the same inhibiting or killing effect on tumor cells under the condition that the total amount of the coupled drug molecules is greatly reduced.
Owner:MABPLEX INT LTD
Anti-CXCR4 as a sensitizer to cancer therapeutics
ActiveUS9155723B2Prevent infiltrationConducive to survivalBiocidePeptide/protein ingredientsCXCR4CXCR4 Inhibitor
Inhibition of CXCR4 can inhibit tumor growth and metastasis during certain therapeutic windows. Disclosed are novel methods for treating and preventing cancer in a subject related to administration of CXCR4 inhibitors during a therapeutic window following treatment with another anti-tumor therapy.
Owner:THE GENERAL HOSPITAL CORP
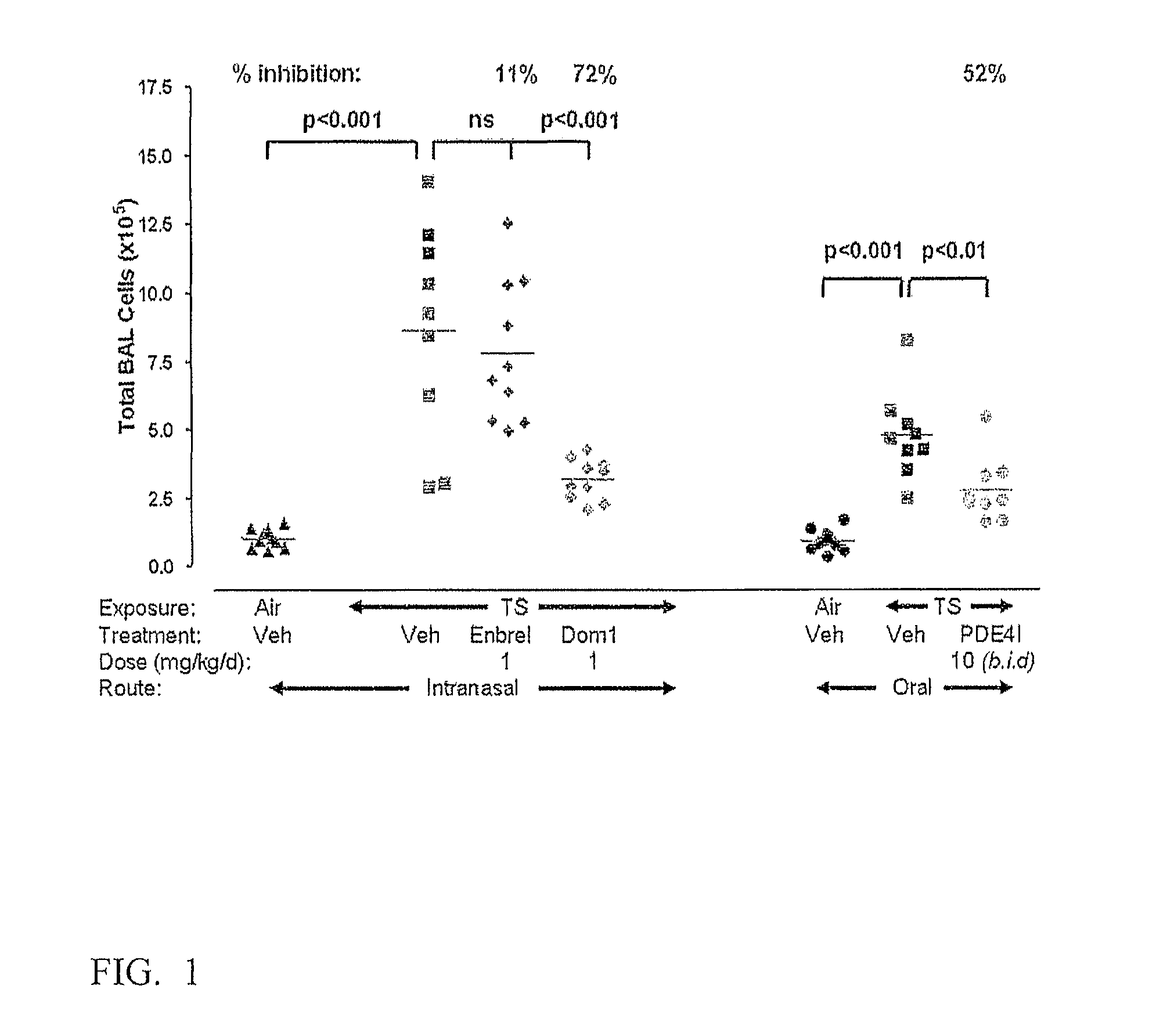
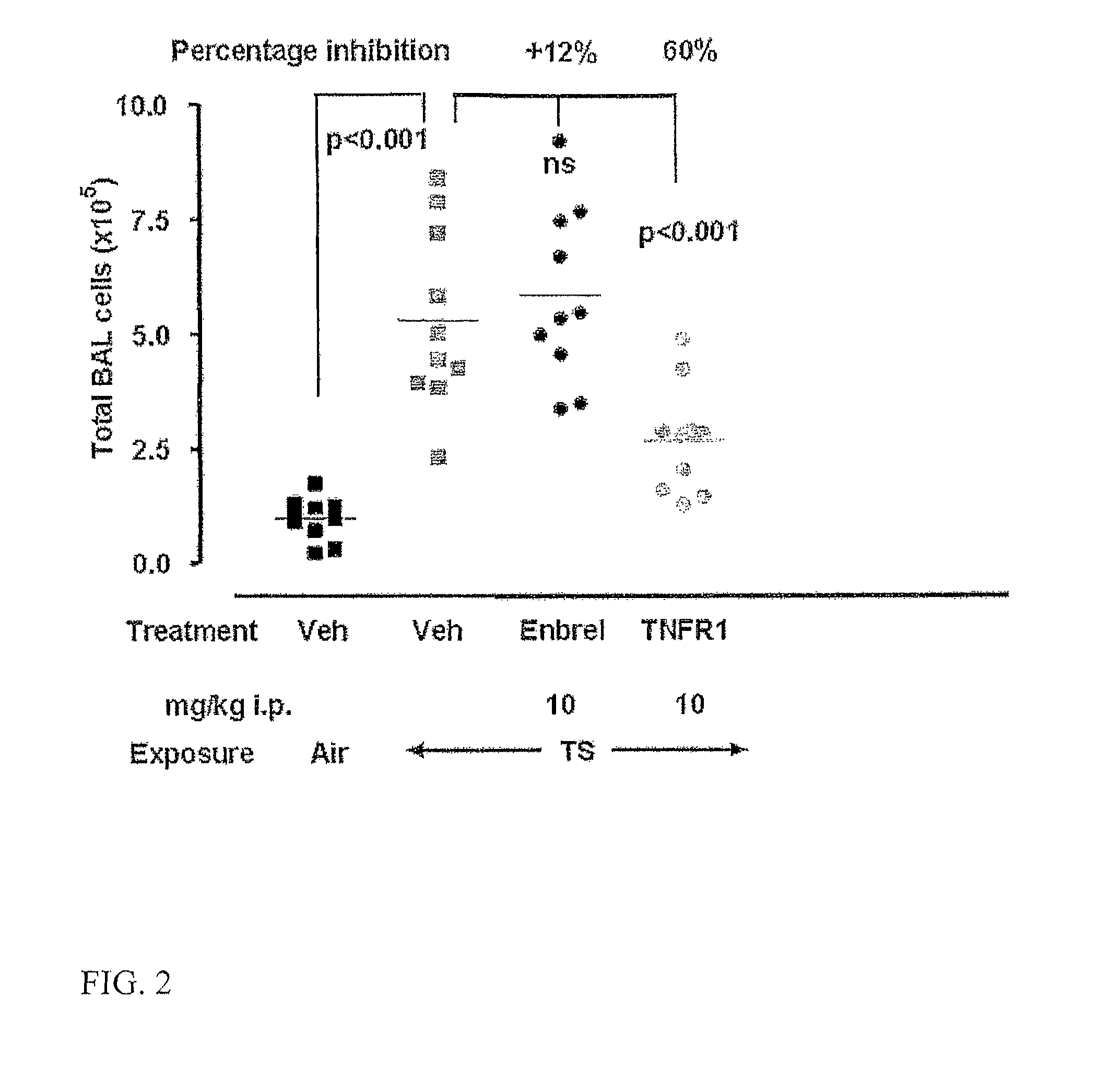
Agents that bind a target in pulmonary tissue for targeting respiratory diseases
Disclosed is the use of an agent (e.g., antibody fragment, antagonist, ligand, dAb monomer) that binds a target in pulmonary tissue for the manufacture of a long action or long therapeutic window formulation for local delivery to pulmonary tissue, and methods for administering an agent that binds a target in pulmonary tissue to a subject to produce a long therapeutic window in pulmonary tissue. The formulation is for, and the method comprises, administering locally to pulmonary tissue. Also disclosed is the use of antagonists of TNFR1 for the manufacture of a formulation or medicament for treating, preventing or suppressing lung inflammation or a respiratory disease, and methods of treating such diseases. Also disclosed are the use of agents a for the manufacture of a delivery device (e.g., inhaler, intranasal delivery device) for the treatment or prevention of lung inflammation or a respiratory disease, and a delivery device for the treatment or prevention of lung inflammation or a respiratory disease that contains an agent as described herein.
Owner:DORMANTIS LTD +1
Modified Retinoid Compounds and Their Uses
InactiveUS20080194534A1Low toxicityGreat therapeutic windowBiocideCosmetic preparationsDiseaseRetinoid
A method of minimizing or reducing the toxicity of a retinoid having a free carboxyl group and the resulting modified retinoids are described. The method comprises the step of esterifying the carboxyl group of the retinoid with a highly sterically hindered compound, which is preferably a secondary or tertiary alcohol. The resulting retinoid esters are rendered much less toxic than the starting or parent retinoid. This process provides a retinoid ester analog of reduced toxicity so that it may be administered orally with minimal side effects and with a much greater therapeutic window. The modified retinoid compounds are useful in the treatment and prophylaxis of all diseases and disorders where retinoid compounds have been shown effective.
Owner:WISCONSIN ALUMNI RES FOUND
Pain relieving bilayer controlled-release tablet and preparation method thereof
ActiveCN103655505AImprove physical stabilityTightly boundAntipyreticAnalgesicsSide effectBlood concentration
The invention provides a pain relieving bilayer controlled-release tablet which comprises a quick release layer and a slow release layer, wherein holes are formed in the slow release layer; the holes are filled with quick release particles; the quick release layer and the quick release particles consist of pain relieving drugs and pharmaceutical adjuvant; the slow release layer consists of pain relieving drugs, slow release materials and pharmaceutical adjuvant. The pain relieving bilayer controlled-release tablet has the following technical effects: 1) the bilayer tablet has better physical stability than a common bilayer tablet, so that the storage and the transportation are convenient; 2) the disintegration time limit of the quick release layer of the bilayer controlled-release tablet is 10-30 seconds through the detection of the dissolving experiment; the slow release layer presents the zero-level release mode; the medicine taking effectiveness and safety of patients are largely improved. In the preparation process of the bilayer tablet, the quick release particles are filled in the holes. The quick disintegration of the quick release layer after the medicine taking is guaranteed through the drug release mode of combining the quick release with the slow release, so that the blood concentration can quickly achieve the range of a therapeutic window; the slow release layer is slowly released in a longer time period to continuously maintain the treatment effect; the toxic or side effects are effectively controlled.
Owner:越洋医药(广州)开发有限公司
Method for enhancing cognition or inhibiting cognitive decline
InactiveUS20100137403A1Increase awarenessCognitive declineBiocideOrganic active ingredientsWhole bodyChannel blocker
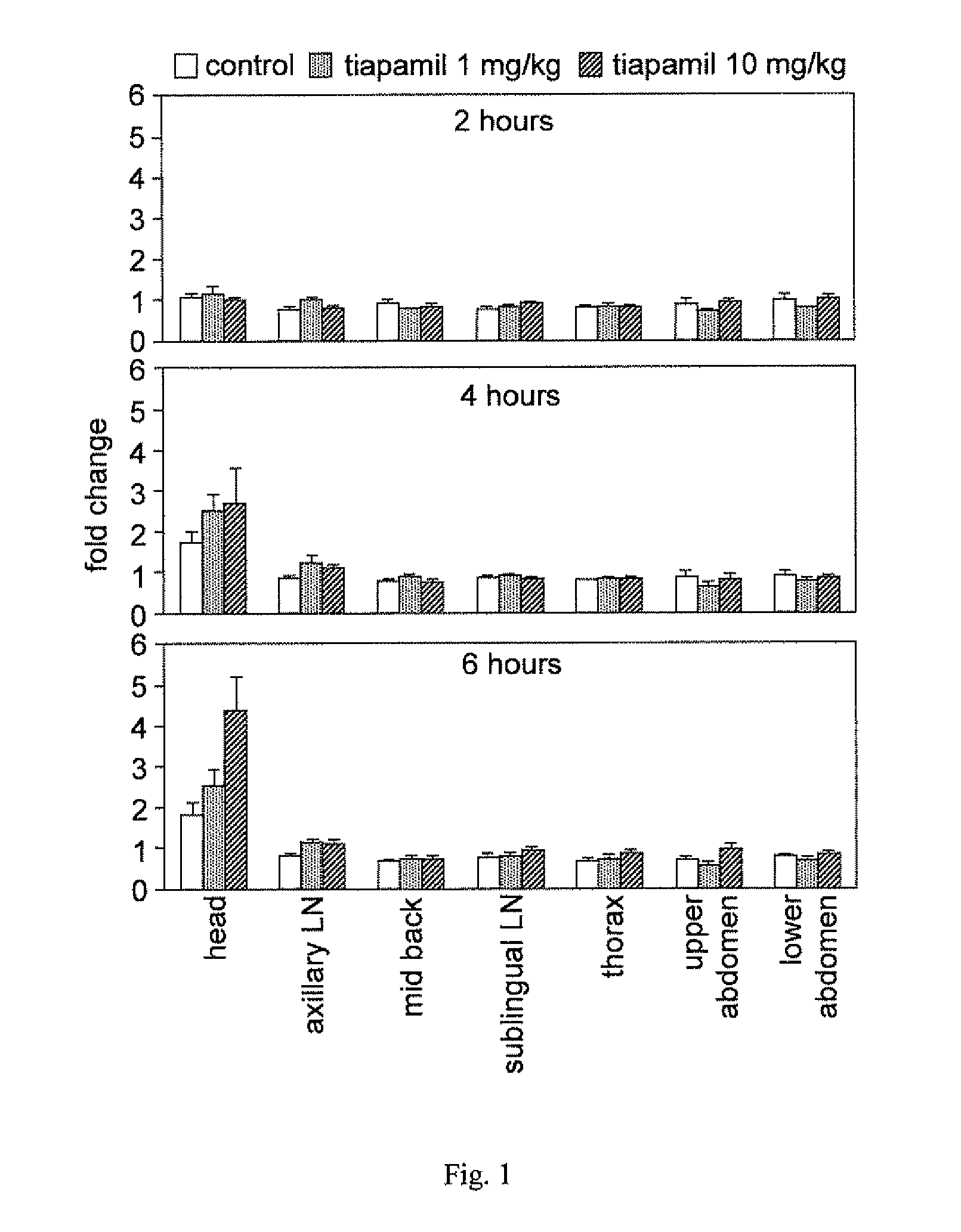
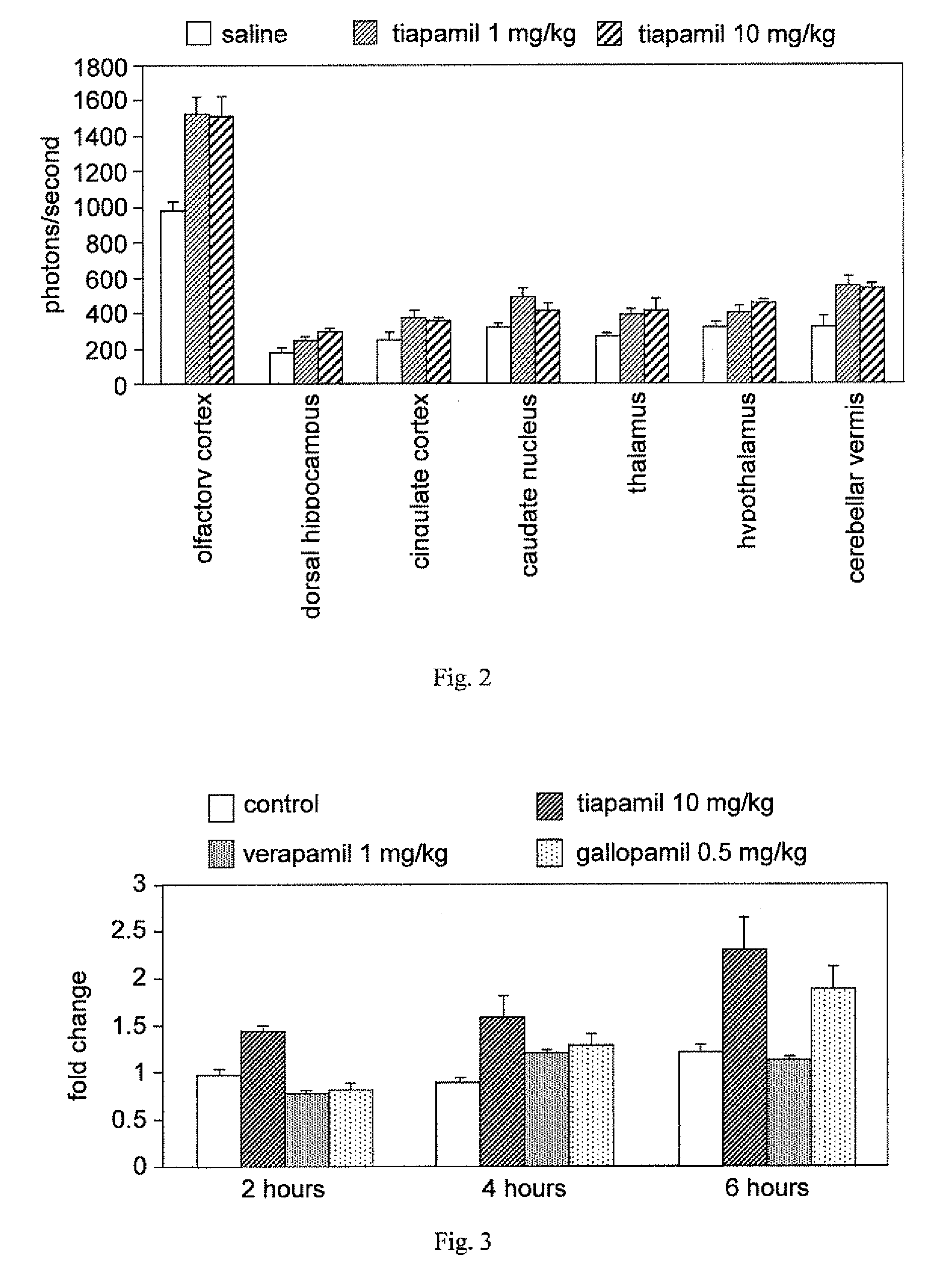
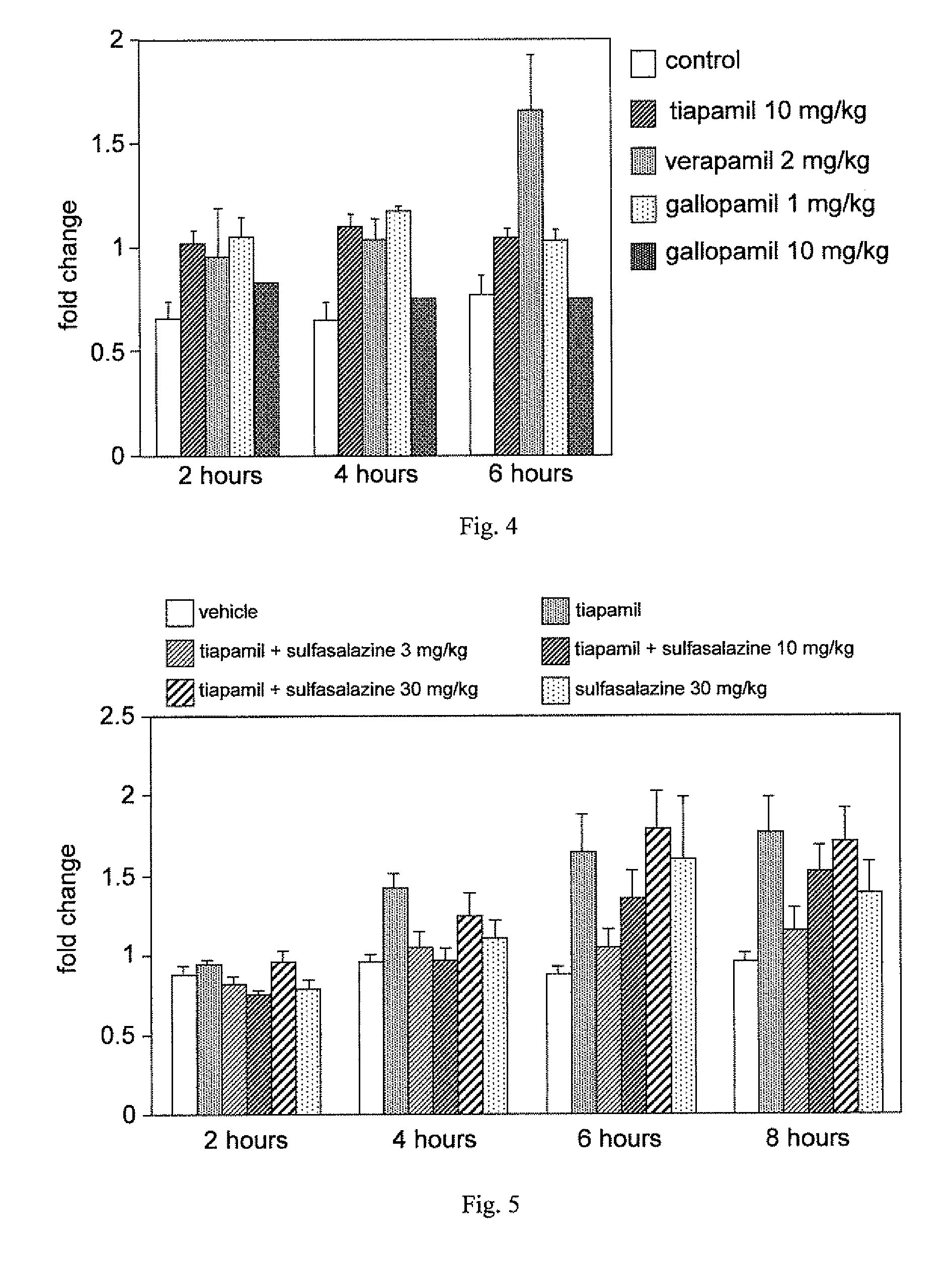
A method for enhancing cognition or inhibiting cognitive decline in a subject comprises selecting a Ca2+ channel blocker that is effective, when administered intravenously to an animal in a nontoxic amount, to increase NF-κB expression in the brain of the animal; and administering the selected Ca2+ channel blocker to the subject, via a systemic route that affords an adequate therapeutic window for cognition-enhancing or cognitive decline-inhibiting effectiveness of the selected Ca2+ channel blocker, in an amount within the therapeutic window. The selected Ca2+ channel blocker can be, for example, tiapamil or a pharmaceutically acceptable salt or prodrug thereof.
Owner:ORE PHARMA
Method of reducing toxicity of retinoids
InactiveUS20050085539A1Increase doseNon toxicFatty oils/acids recovery from wasteOrganic active ingredientsRetinoidAlcohol
A method of minimizing or reducing the toxicity of a retinoid having a free carboxyl group is described. The method comprises the step of esterifying the carboxyl group of the retinoid with a highly sterically hindered compound, which is preferably an alcohol. The resulting retinoid esters are rendered much less toxic than the starting or parent retinoid. This process provides a retinoid ester analog of reduced toxicity so that it may be administered orally with minimal side effects and with a much greater therapeutic window.
Owner:WISCONSIN ALUMNI RES FOUND
Long-acting interleukin-2 capable of realizing targeted regulation of T cell, and application of long-acting interleukin-2 for treating autoimmunity disease
ActiveCN110642934AExpanding Therapeutic WindowEffectivePeptide/protein ingredientsMetabolism disorderIMMUNE SUPPRESSANTSWhite blood cell
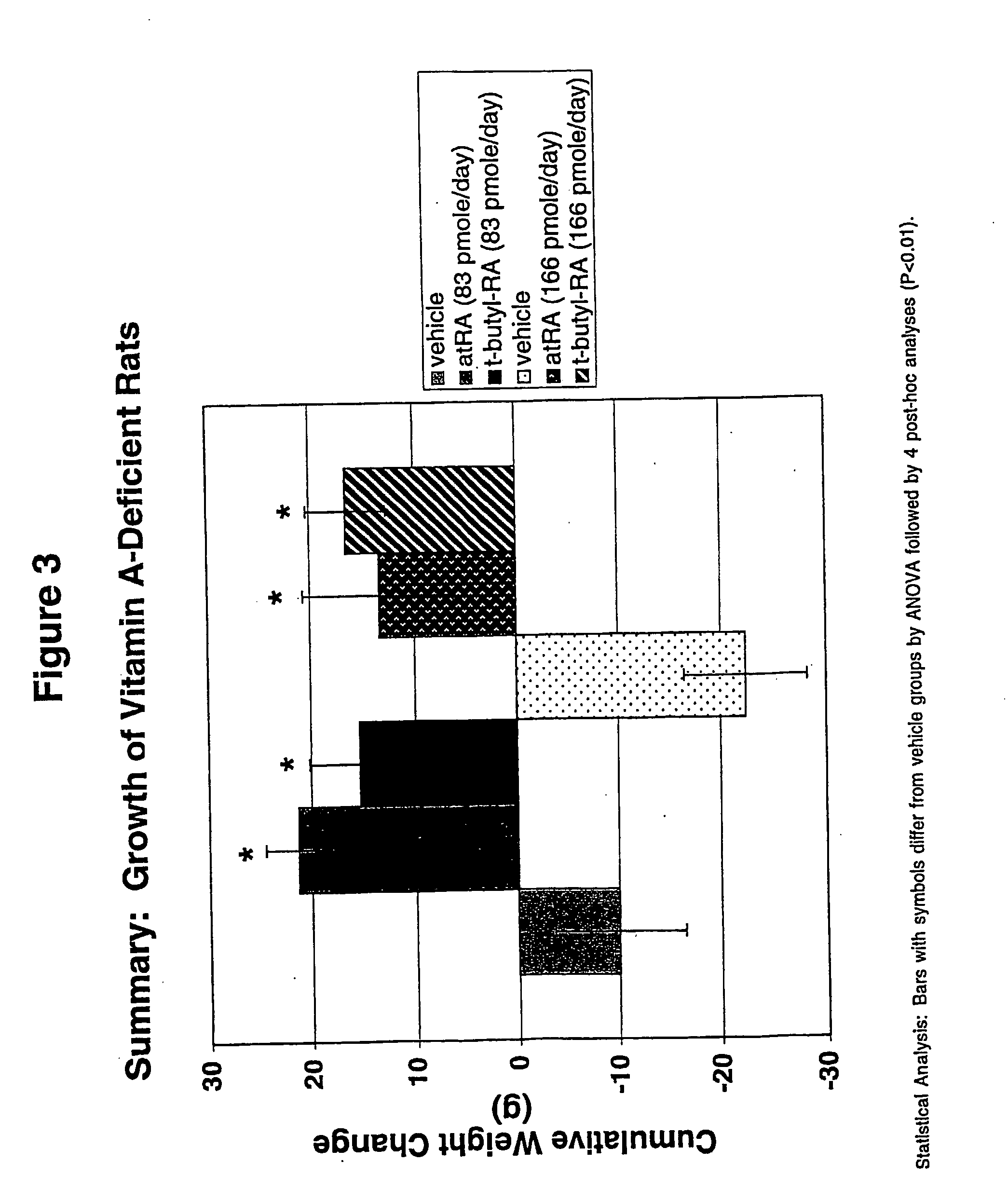
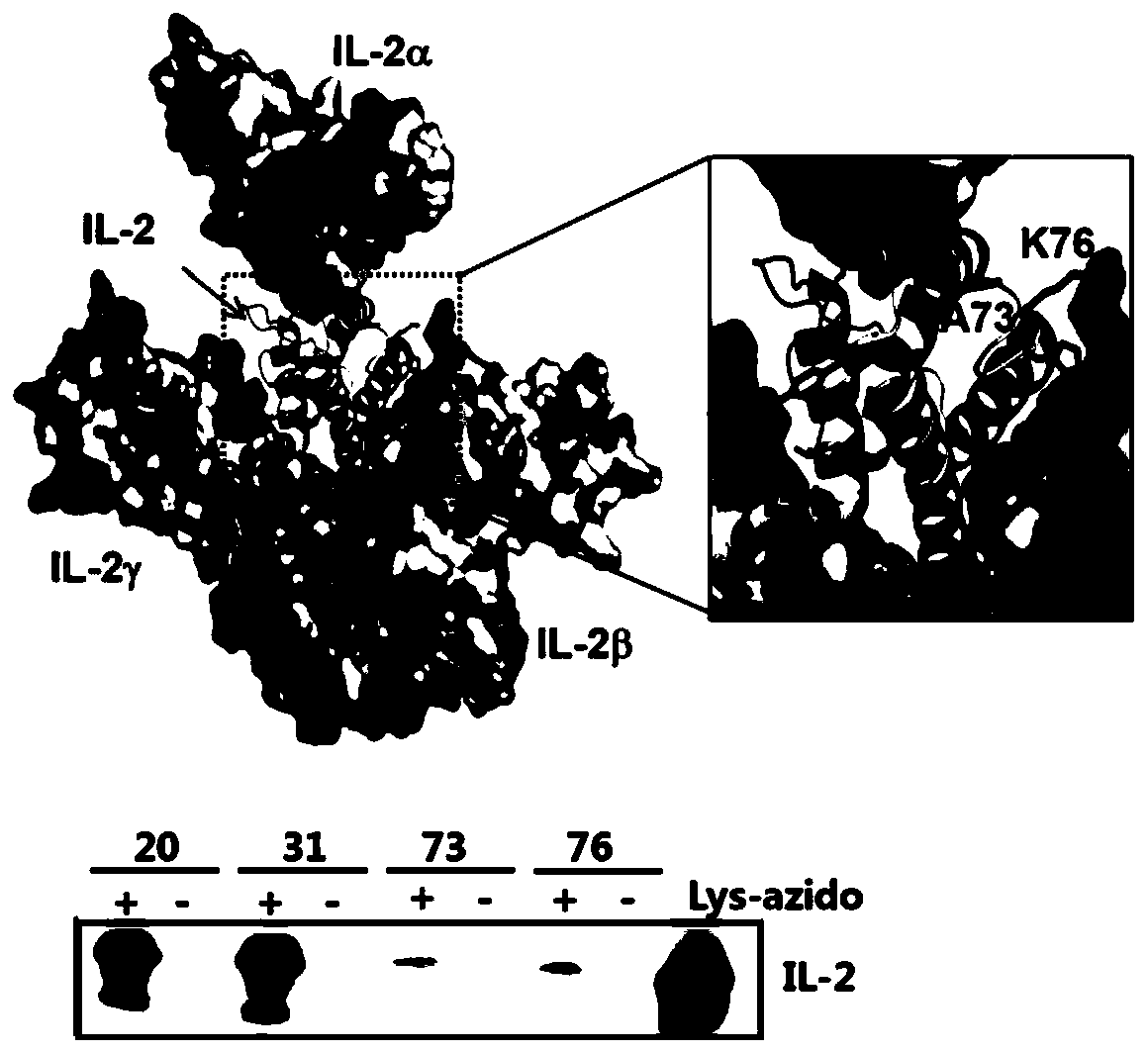
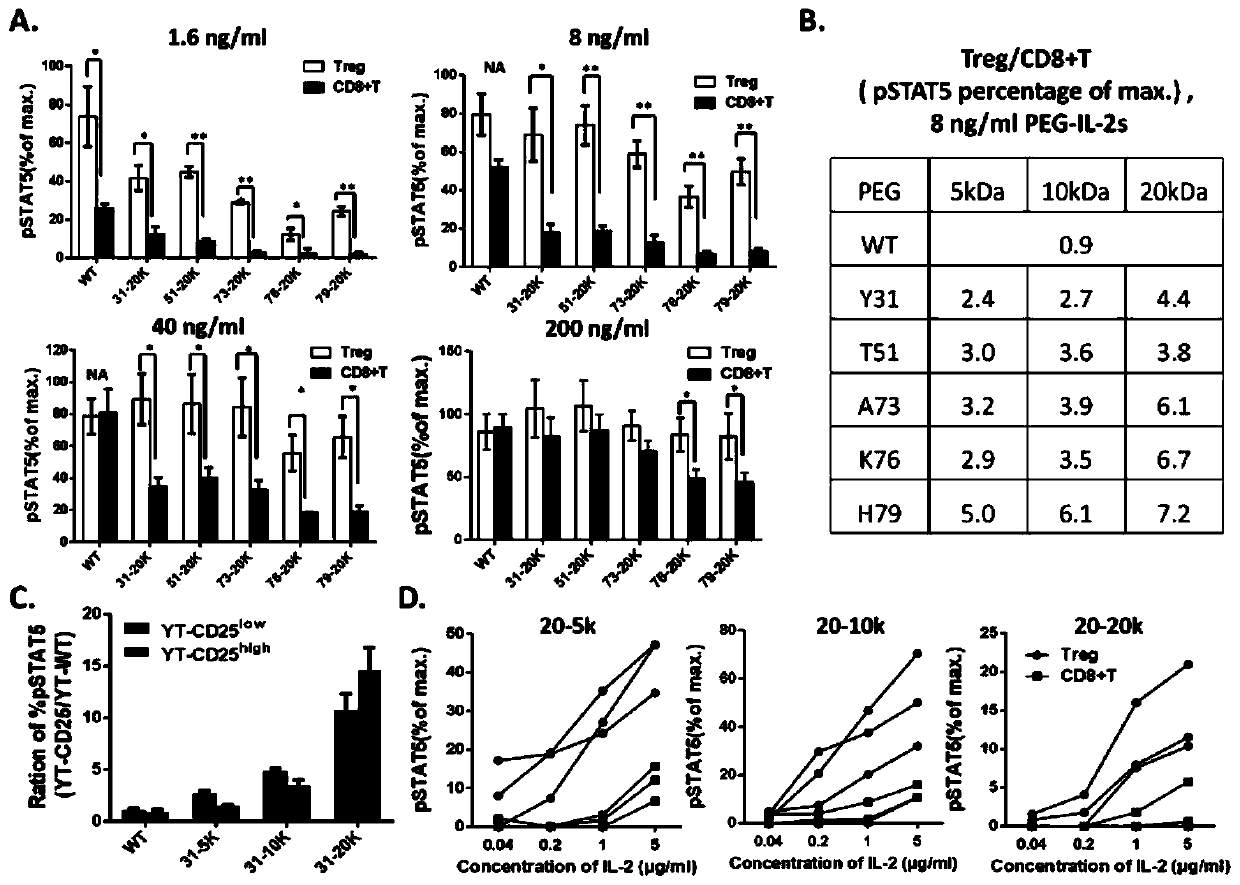
The invention relates to modification sites capable of enabling human interleukin-2 to carry out targeted activation on Treg, human interleukin-2 subjected to site-directed mutation on the modification sites, and human interleukin-2 subjected to site-directed modification on the modification sites. The modified long-acting interleukin-2 can carry out targeted activation regulation on T cells in awide therapeutic window, slightly and evenly does not activate other effector cells, and performs a long-acting systematic immunorepressive effect. The invention further relates to application of thecategory of site-directed mutation or modification interleukin-2, and purposes of the site-directed mutation or modification interleukin-2 for treating various autoimmunity diseases as a stable and long-acting immunosuppressor.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Combination therapy
InactiveUS20100266590A1High dose levelReduced incidence and severityOrganic active ingredientsPeptide/protein ingredientsEverolimusDose level
Disclosed are methods for treating various cancers. Methods encompass the administration of a first drug such as AP23573, temsirolimus or everolimus in combination with a second drug selected from Remicade, Humira, Enbrel, Raptiva, Abatacept, Actermra, Cimzia or anakinra.The methods are aimed at providing a desirable therapeutic window while maintaining prior, if not higher, dose levels of the first drug.
Owner:DEMETRI GEORGE D +2
Liposomal compositions of epoxyketone-based proteasome inhibitors
Liposomal compositions comprising peptide epoxyketone compounds are described, as well as methods of making and using such liposomal compositions. These liposomal compositions enhance the therapeutic window of peptide epoxyketone compounds by improving in vivo half-life relative to non-liposomal compositions comprising peptide epoxyketone compounds, providing desirable pharmacodynamic profiles, and providing anti-tumor activity in a human tumor xenograft model, greater than or equal to non-liposomal compositions comprising peptide epoxyketone compounds. Further, experiments performed in support of the present invention demonstrated improved tolerability of liposomal compositions comprising peptide epoxyketone compounds.
Owner:ONYX THERAPEUTICS
Hypnotic double-layer controlled release tablet and preparation method thereof
ActiveCN103690505AImprove physical stabilityTightly boundOrganic active ingredientsNervous disorderSide effectBlood concentration
The invention provides a hypnotic double-layer controlled release tablet, comprising a rapid-release layer and a slow-release layer, wherein the rapid-release layer is provided with pores which are filled with rapid-release particles; the rapid-release layer and the rapid-release particles are composed of hypnotic drug and pharmaceutical excipients; the slow-release layer is composed of a hypnotic drug, a slow-release material and pharmaceutical excipients. The hypnotic double-layer controlled release tablet has the following technical effects that 1) the physical stability of the double-layer tablet provided by the invention is better than that of the common double-layer table, which is advantageous for storage and transportation, and 2) a dissolution test detects that the disintegration time limit of the rapid-release layer of the double-layer tablet provided by the invention ranges from 20 seconds to 30 seconds; and the slow-release layer is in a zero-order release pattern, and therefore the effectiveness and safety of medication for a patient are greatly improved. In the preparation process of the double-layer tablet, the rapid-release particles fill in the pores. The drug release pattern of combined rapid release and slow release guarantees that the rapid-release layer is disintegrated rapidly after the drug is taken; as a result, the blood concentration is capable of quickly reaching the therapeutic window range; the slow-release layer is slowly released so that the therapeutic action is maintained for a long period of time and toxic and side effects are effectively controlled.
Owner:OVERSEAS PHARMA
Analogs of indole-3-carbinol metabolites as chemotherapeutic and chemopreventive agents
InactiveUS7078427B2Improve oral bioavailabilityBroad therapeutic windowBiocideOrganic chemistryMetaboliteTolerability
Novel compounds useful as chemotherapeutic and chemopreventive agents are provided. The compounds are analogs of indole-3-carbinol metabolites wherein the structures and substituents of the compounds are selected to enhance the compounds' overall efficacy, particularly with respect to therapeutic activity, oral bioavailability, long-term safety, patient tolerability, and therapeutic window. The compounds are useful not only in treatment of cancer but also in prevention of cancer. One preferred class of the novel compounds have the structure of formula (I)wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, and R12 are defined herein. Pharmaceutical compositions are provided as well, as are methods of synthesis and use.
Owner:SRI INTERNATIONAL
Metoprolol controlled release mixed matrix tablet and preparation method thereof
InactiveCN101904828AControl release speedGuaranteed uniformityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseBlood concentration
The invention discloses a metoprolol controlled release mixed matrix tablet, belonging to the pharmaceutical field, comprising the following components: 4-60% of metoprolol or metoprolol salt, 0.05-10% of lauryl sodium sulphate, 15-60% of high viscosity hydrophilic polymer and 2-60% of non-disintegration matrix material; the non-disintegration matrix material forms a matrix system with equal gaps; the mixture of the metoprolol or metoprolol salt and the high viscosity hydrophilic polymer is filled in the matrix system. The metoprolol matrix tablet can slowly release for 24 hours and can be taken one time per day; the blood concentration is in the range of the therapeutic window of the metoprolol, which is suitable for the request of the treatment. Simultaneously, the invention provides a method for preparing the metoprolol controlled release mixed matrix tablet; the other auxiliary materials and pharmaceutical preparations are mixed with the insoluble non-disintegration matrix material; the insoluble non-disintegration matrix material can form rigid matrix and effectively control the release speed of the drugs.
Owner:GUANGZHOU HANFANG PHARMA
Sirolimus preparation and preparation process thereof
ActiveCN101632662AGood dissolution effectLow dissolutionOrganic active ingredientsAntimycoticsOral medicationBioavailability
The invention provides a Sirolimus preparation and a preparation process thereof. The process comprises the following steps: preparing an intermedium by a solid dispersion technology; preparing pellets by an extrusion spheronization process; and finally filling capsules. The preparation and the process of the preparation ensure the high leaching ability of Sirolimus, achieve the aim of slow release, have smaller leaching difference among the capsules and better solve the application limit caused by low bioavailability and narrow therapeutic window of the oral administration of the Sirolimus.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
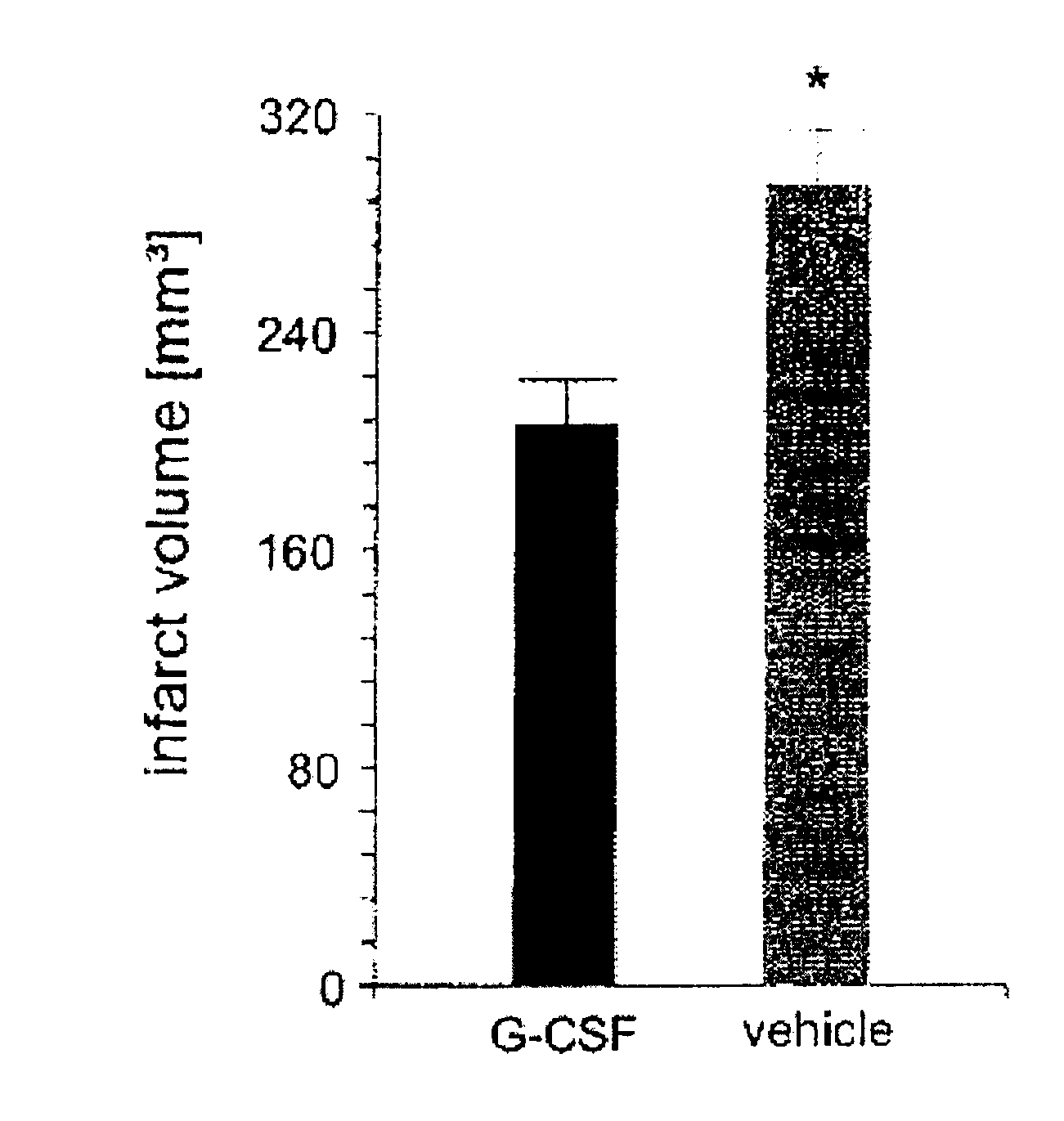
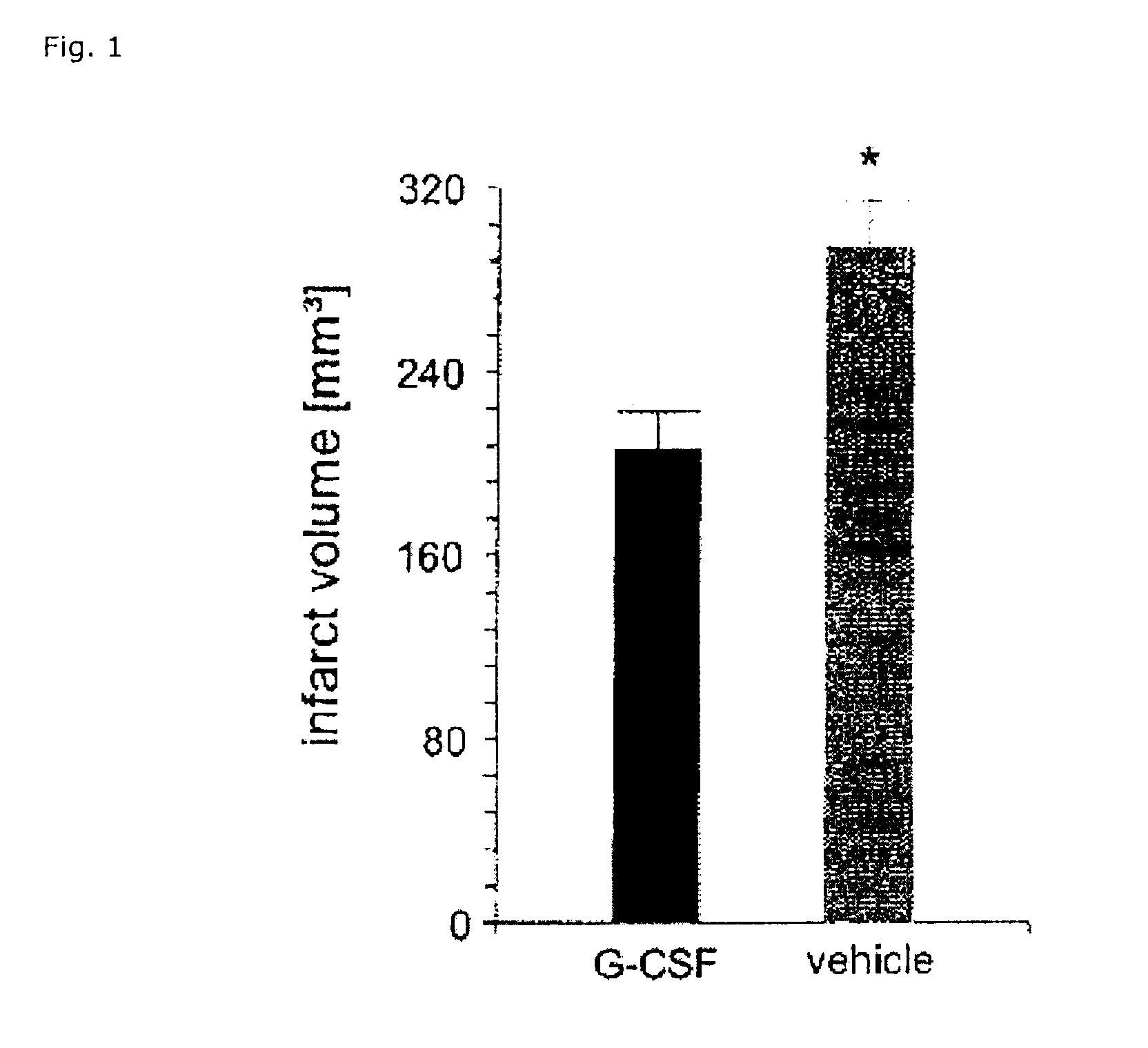
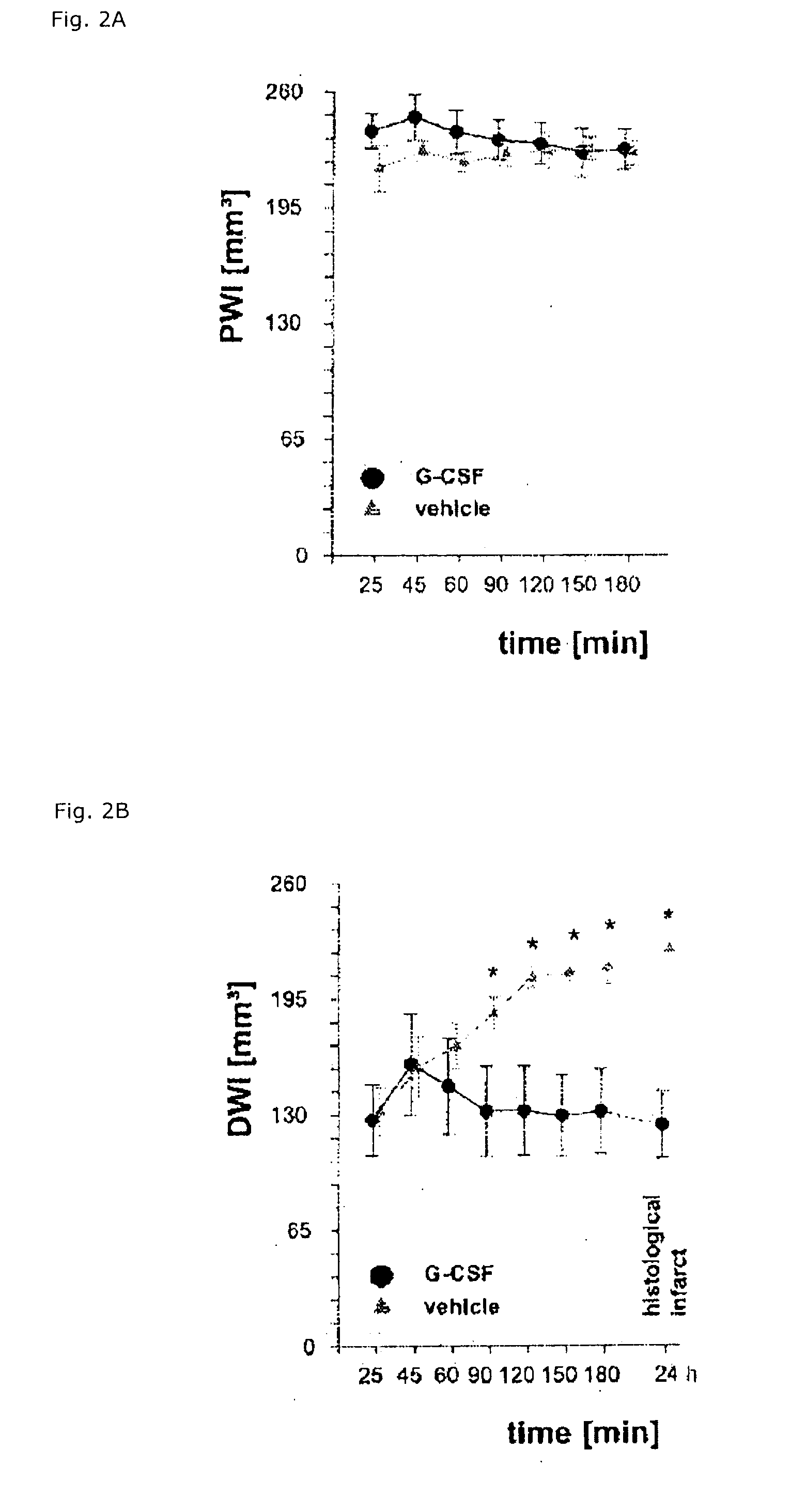
Use of g-csf for the extension of the therapeutic time-window of thrombolytic stroke therapy
InactiveUS20120070403A1Effective treatmentImprove clinical outcomesNervous disorderHydrolasesSide effectMedicine
The present invention relates to the use of G-CSF and derivatives thereof for extending the therapeutic window of subsequent thrombolytic treatment of acute stroke, and thereby, allowing the diagnostic examinations which are necessary prior to the thrombolytic treatment in order to avoid hemorrhagic and other severe adverse side effects of the thrombolysis.
Owner:SYGNIS BIOSCIENCE GMBH & CO KG
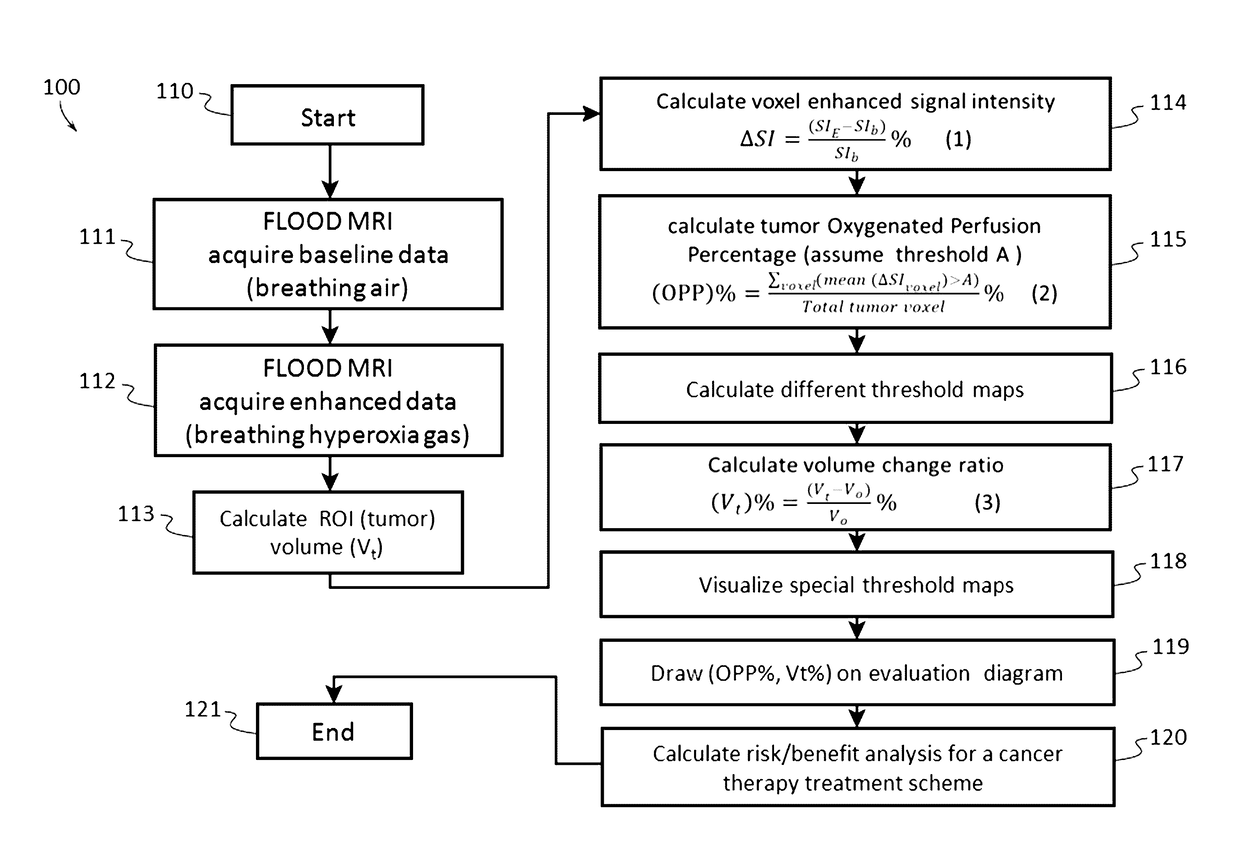
Cancer therapeutic window evaluation method
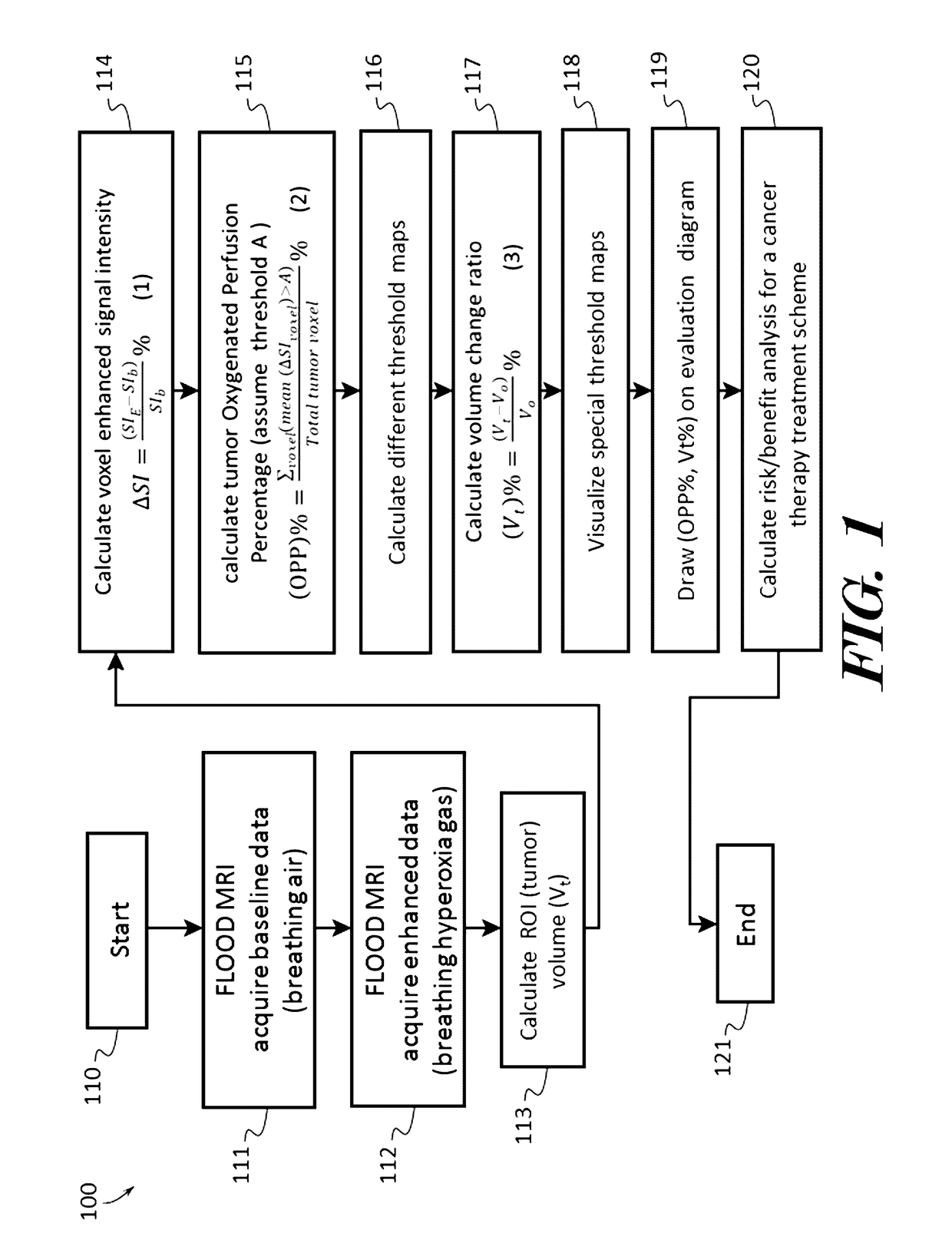
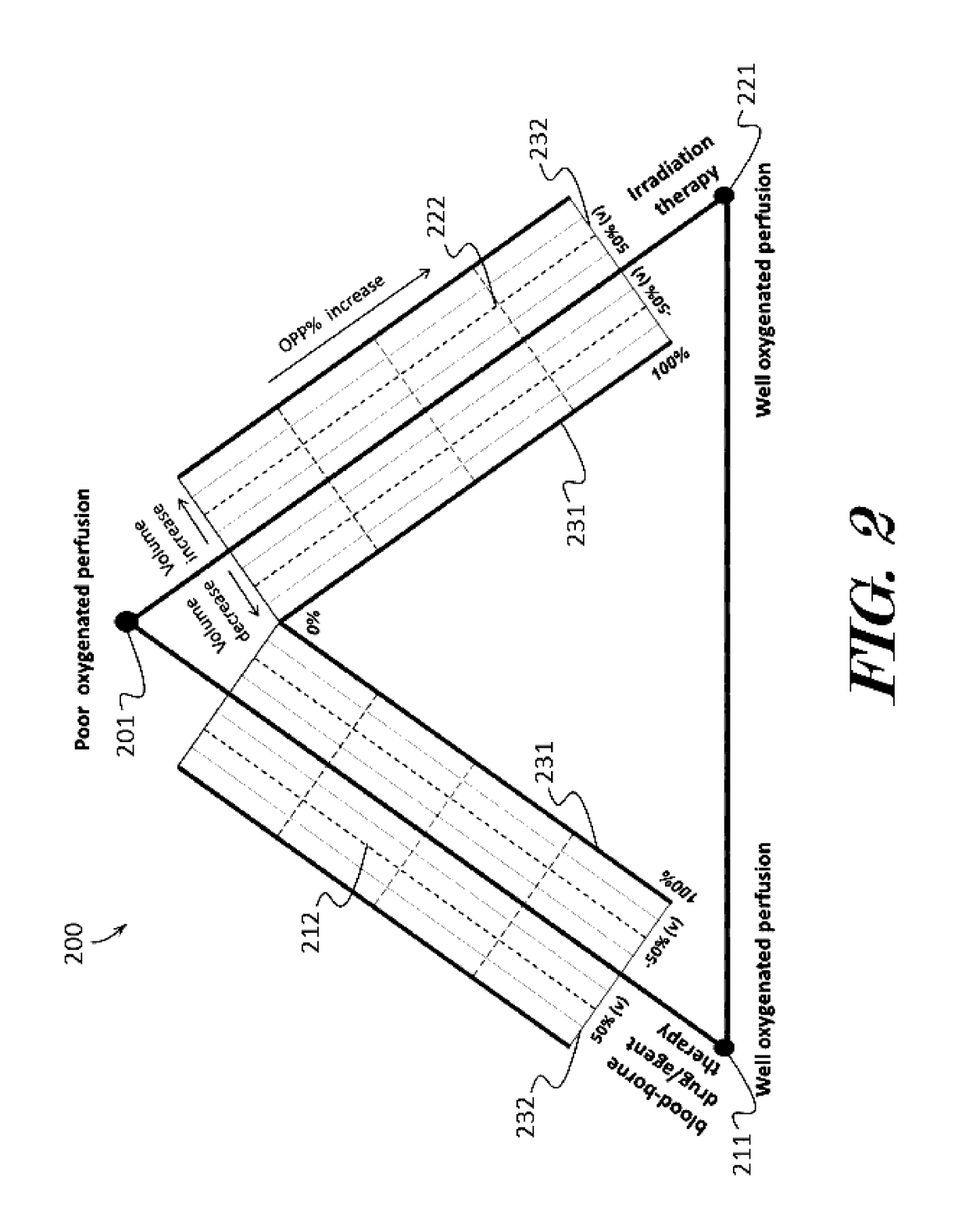
InactiveUS20170091409A1Easy to understandIts social cost may become even heavierTelemedicinePatient personal data managementBaseline dataVoxel
A cancer therapeutic window evaluation method is provided. In some embodiments, the method may comprise: detecting tumor oxygenated perfusion by having a patient breathe air to acquire MRI baseline data; inhalation of hyperoxia gas to generate higher than baseline HbO2 blood circulating in body to acquire MRI enhanced data; the region-of-interest (ROI), which in this case is a tumor volume (V0), and which may be performed by volume contour tracing / region-of-interest (ROI) analysis and 3D tumor volumetry methods; calculating voxel's enhanced signal intensity (ΔSI); calculating tumor oxygenated perfusion percentage (OPP %); selecting different threshold and calculating maps such as a reconstruction OPP % pseudo color map; calculating tumor volume change ratio (Vt %); overlaying reconstruction OPP % pseudo color map to original images for visualizing tumor response data; drawing or plotting the OPP % and Vt % may on a cancer treatment evaluation diagram, and calculating risk / benefit analysis based on pooled collected data.
Owner:JIANG LAN
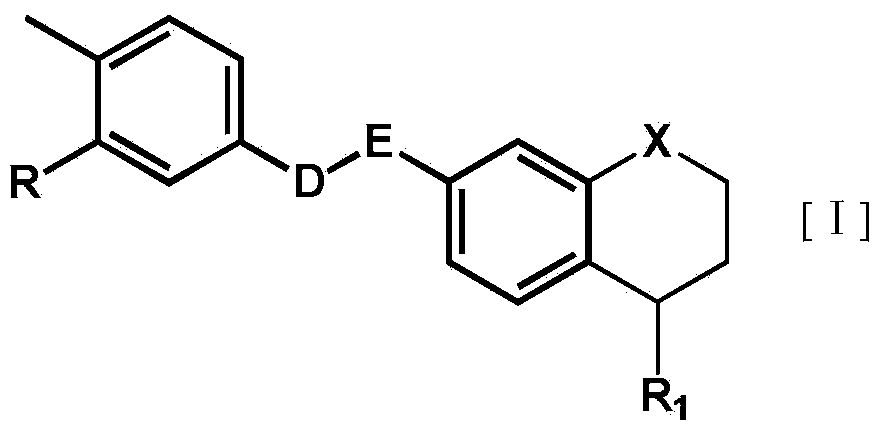
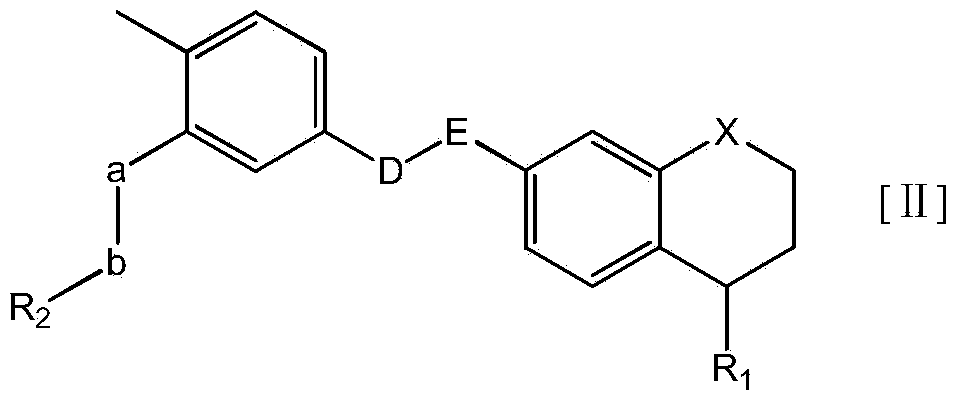
Substituted tetrahydronaphthalene amide compound, pharmaceutically acceptable salt thereof, and preparation method and application
ActiveCN104250253AGood antitumor activityImprove securityOrganic active ingredientsOrganic chemistryTetralinTherapeutic window
The invention relates to substituted tetrahydronaphthalene amide compound as shown in a general formula I (in the Specification), pharmaceutically acceptable salt thereof, and a preparation method and application. The substituted tetrahydronaphthalene amide compound can be used as an antitumor drug, the invention also provides a preparation method of compounds similar to the substituted tetrahydronaphthalene amide compound and pharmaceutical compositions containing the compounds, and in vitro and in vivo anti-tumor effect research results and acute toxicity research results. The substituted benzo-cyclic amide compound as the antitumor drug, has better antitumor activity and safety, particularly resists imatinib mesylate resistance tumors, can be applied to curing tumors, such as leukemia, gastrointestinal stromal tumor, lung cancer, colon cancer, ovarian cancer and kidney cancer, is wide in therapeutic window, and as antitumor agent, the compound has high application value in the field of medicine.
Owner:LIAONING UNIVERSITY
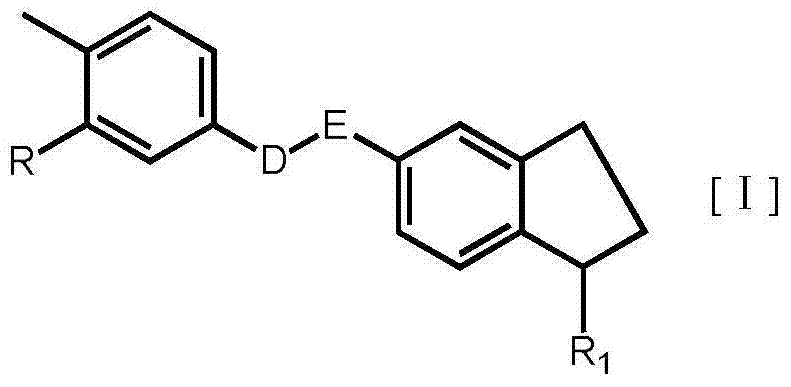
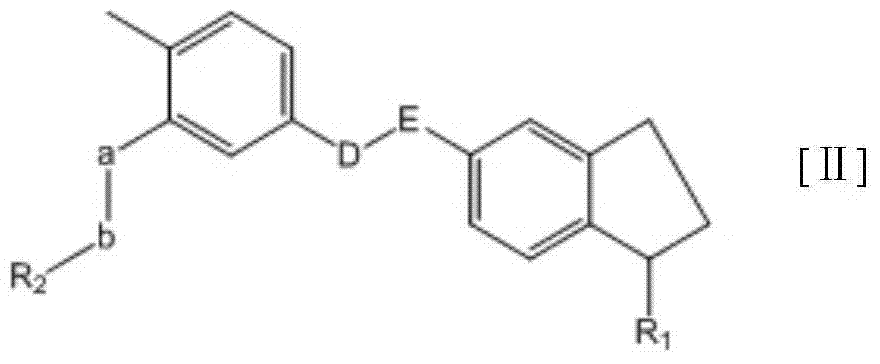
Substituted-indanyl amide-type compounds and pharmaceutically acceptable salt and preparation method and application thereof
InactiveCN104327083AGood antitumor activityImprove securityOrganic active ingredientsOrganic chemistryAcute toxicity testingImatinib
The invention relates to substituted-indanyl amide-type compounds as shown in the general formula I, pharmaceutically acceptable salt of the compounds and a preparation method and an application thereof. The invention also provides a pharmaceutical composition of the compounds. Through in vitro and in vivo anti-tumor effect research results and acute toxicity research results, the anti-tumor drug substituted-indanyl amide-type compounds have more excellent antitumor activity and safety, especially resisting imatinib drug-resistant tumors, and can be applied in curing tumors such as leukemia, gastrointestinal stromal tumor, lung cancer, colon cancer, ovarian cancer, kidney cancer and the like. Thus, the compounds have wide therapeutic window and have application value when used as an anti-tumor agent in the field of medicine.
Owner:LIAONING UNIVERSITY
Reversal core pharmaceutical system and method
InactiveUS20080312166A1Control deteriorationImprove faultBiocideNervous disorderTherapeutic windowDrug release
A medication delivery system is disclosed to mitigate or reverse the pharmacologic effect of a medication beyond the desired therapeutic window. The system provides sequential delivery of medications including a first agent which induces a physiologic change, a second agent for reversing the physiologic change, and a medication release delaying agent for delaying the release of the second agent so that, upon receipt of the medication delivery system into the body the first agent is released before the second agent so that the physiologic effect of the first agent is mitigated by the second agent. The system can be a pill with the first agent comprising an outer medication and the second agent an inner medication with the medication release delaying agent intermediate the outer medication and the inner medication. In an example, the first agent can comprise a sleep aid and the second agent an arousal agent.
Owner:LYNN DARYL JOHN +3
Intranasal delivery of cell permeant therapeutics
The present invention relates to compositions and methods for the inhibition of apoptosis associated with ischemic injury in the central nervous system. In addition, the present invention relates to compositions and methods useful for extending the therapeutic window associated with ischemic injury.
Owner:SANFORD BURNHAM MEDICAL RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com