Patents
Literature
92 results about "FOOD EFFECT" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical compositions comprising fenofibrate and atorvastatin
InactiveUS20070014846A1Improve bioavailabilityReducing inter-individual variationBiocidePill deliveryParticulatesHMG-CoA reductase
Pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor atorvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCatorvastatin) of between about 250 and about 10,000. The solid compositions are manufactured without any need of addition of water or aqueous medium. Atorvastatin is optionally provided as a controlled release or a delayed release formulation resulting in a maintained LDL-lowering effect at a reduced dosage, and fenofibrate is provided in a formulation having increasing bioavailability and reduced food effect.
Owner:VELOXIS PHARMA
Novel fenofibrate formulations and related methods of treatment
InactiveUS20090149533A1Readily bioavailableProperty is unexpectedBiocideOrganic active ingredientsFOOD EFFECTGram
The invention provides novel omega-3 ester-based oil solutions of fenofibrate. These solutions are substantially free of any food effect, effective in small volumes, and readily bioavailable. Notably, because the solutions of the invention contain an omega-3 ester-based oil as the major ingredient, they not only provide an antihyperlipidemic effect due to the fenofibrate active ingredient, they also provide recommended daily dosages of omega-3 oils (i.e., approximately 1 gram of omega-3 oil per day), or a portion thereof.
Owner:ALMBURG
Pharmaceutical Compositions Comprising Sirolimus and/or an Analogue Thereof
InactiveUS20080275076A1Improve safety/efficacy ratioReduce impactAntibacterial agentsBiocideParticulatesSide effect
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising sirolimus (rapamycin) and / or derivatives and / or analogues thereof. Compositions of the invention exhibit an acceptable bioavailability of sirolimus and / or a derivative and / or an analogue thereof. The pharmaceutical compositions of the invention are designed to release sirolimus in a controlled manner so that the plasma levels stays within the narrow therapeutic window that exist for this class of substances. An extended release profile, where the peak concentration has been reduced without loosing significant bioavailability, together with less variable absorption, is expected to improve the safety / efficacy ratio of the drug. Furthermore, compositions according to the invention provide for a significant reduced food effect and a delayed release of sirolimus is expected to reduce the number of gastro-intestinal related side effects.
Owner:LIFECYCLE PHARMA AS
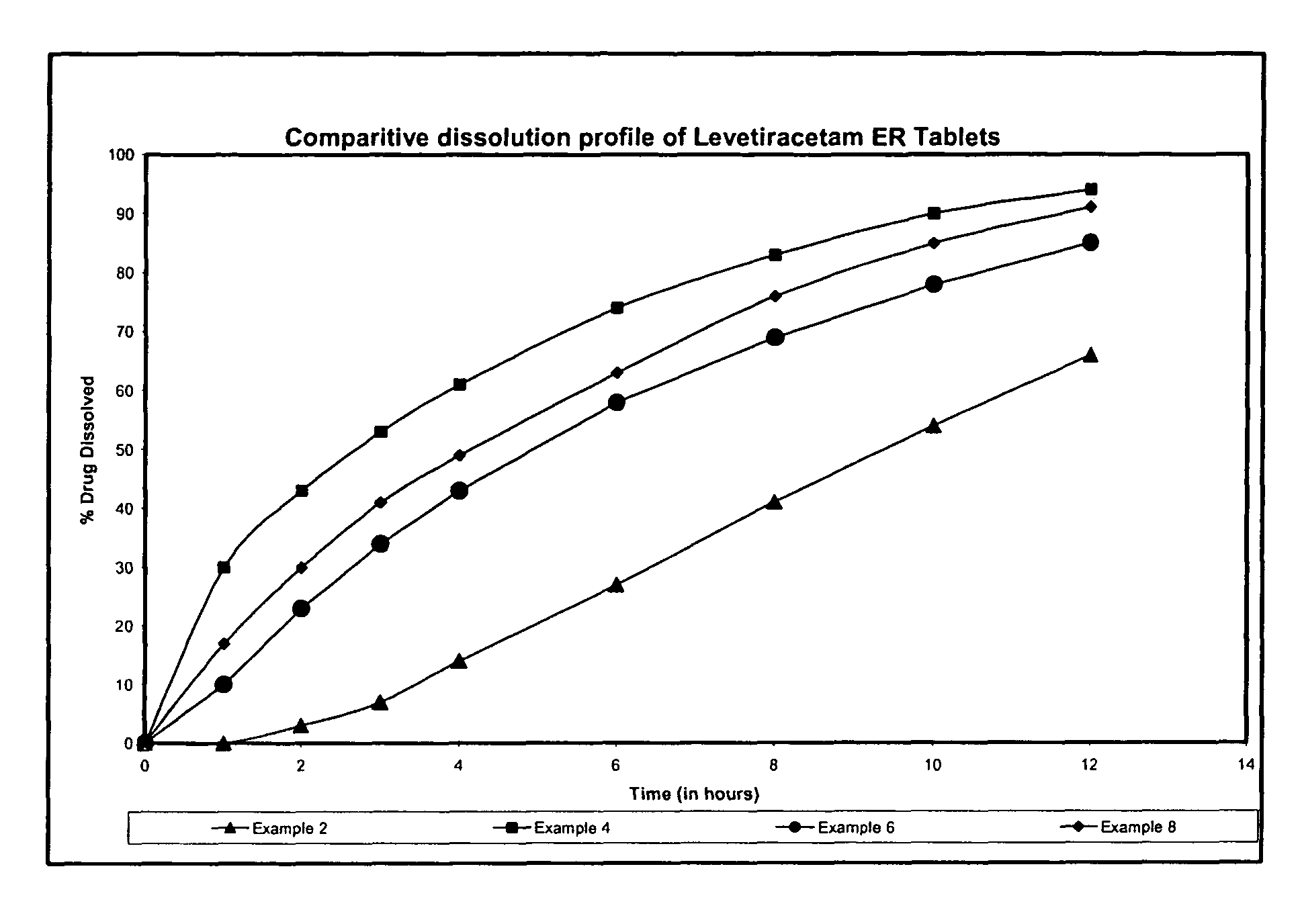
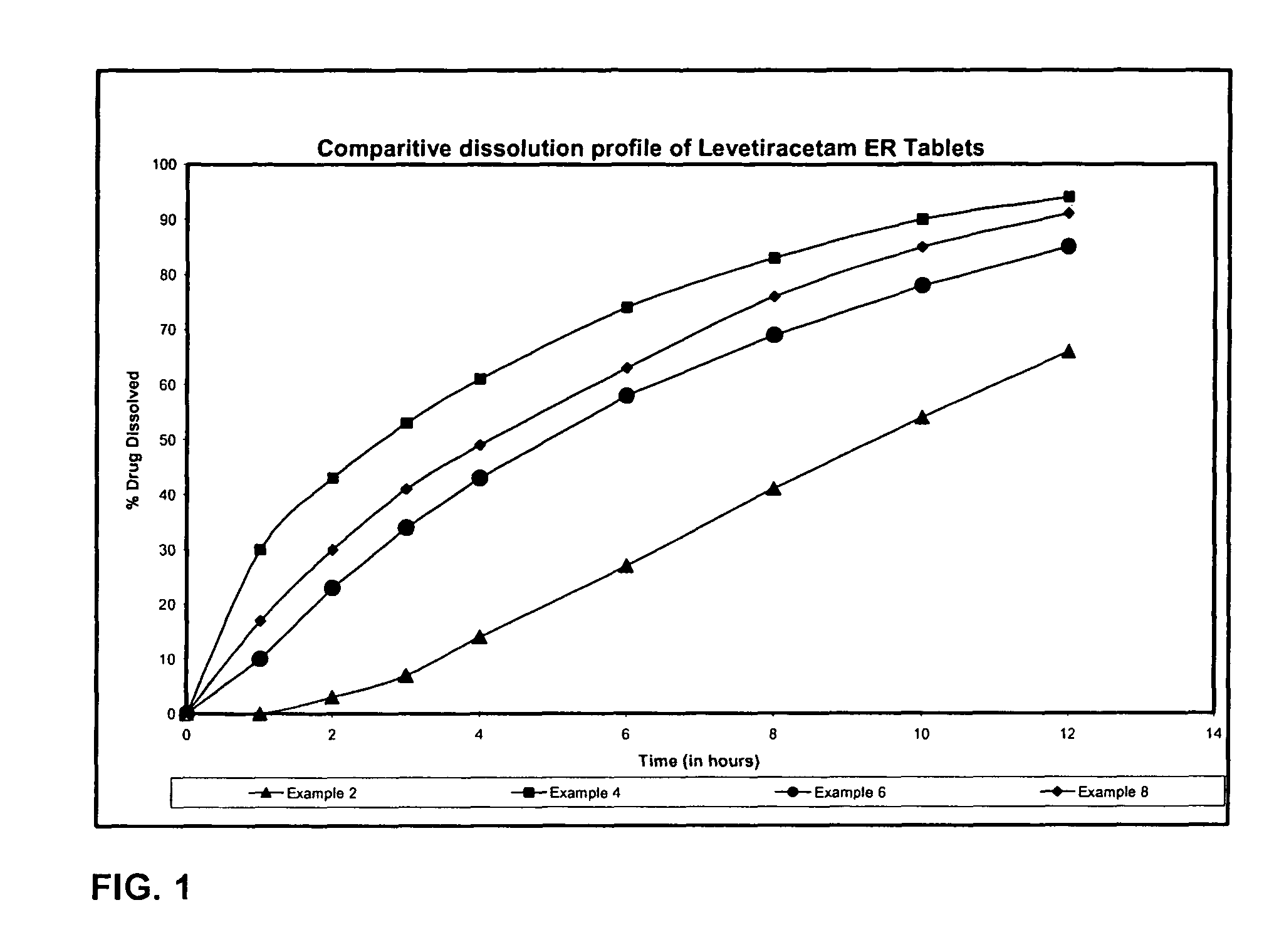
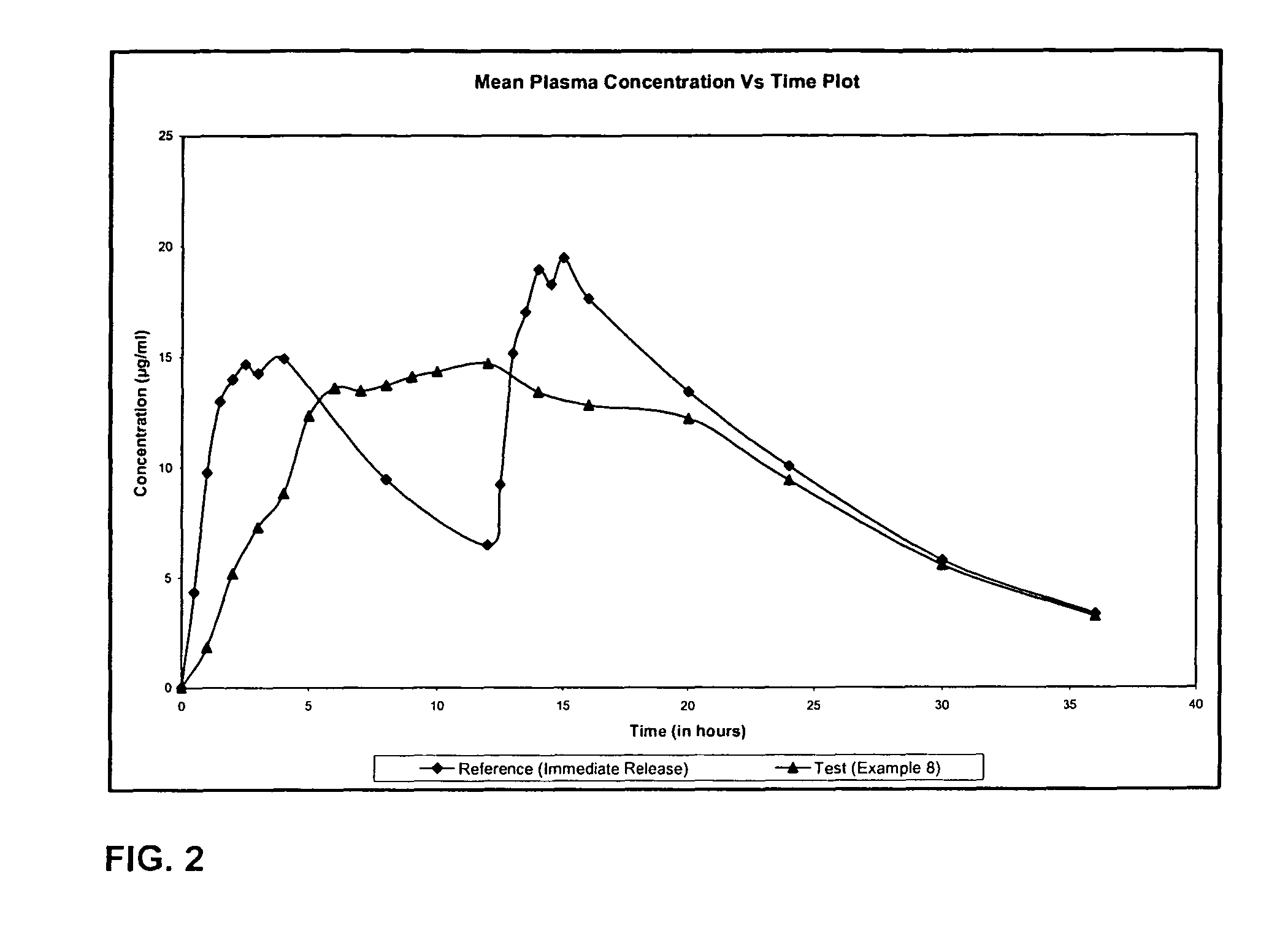
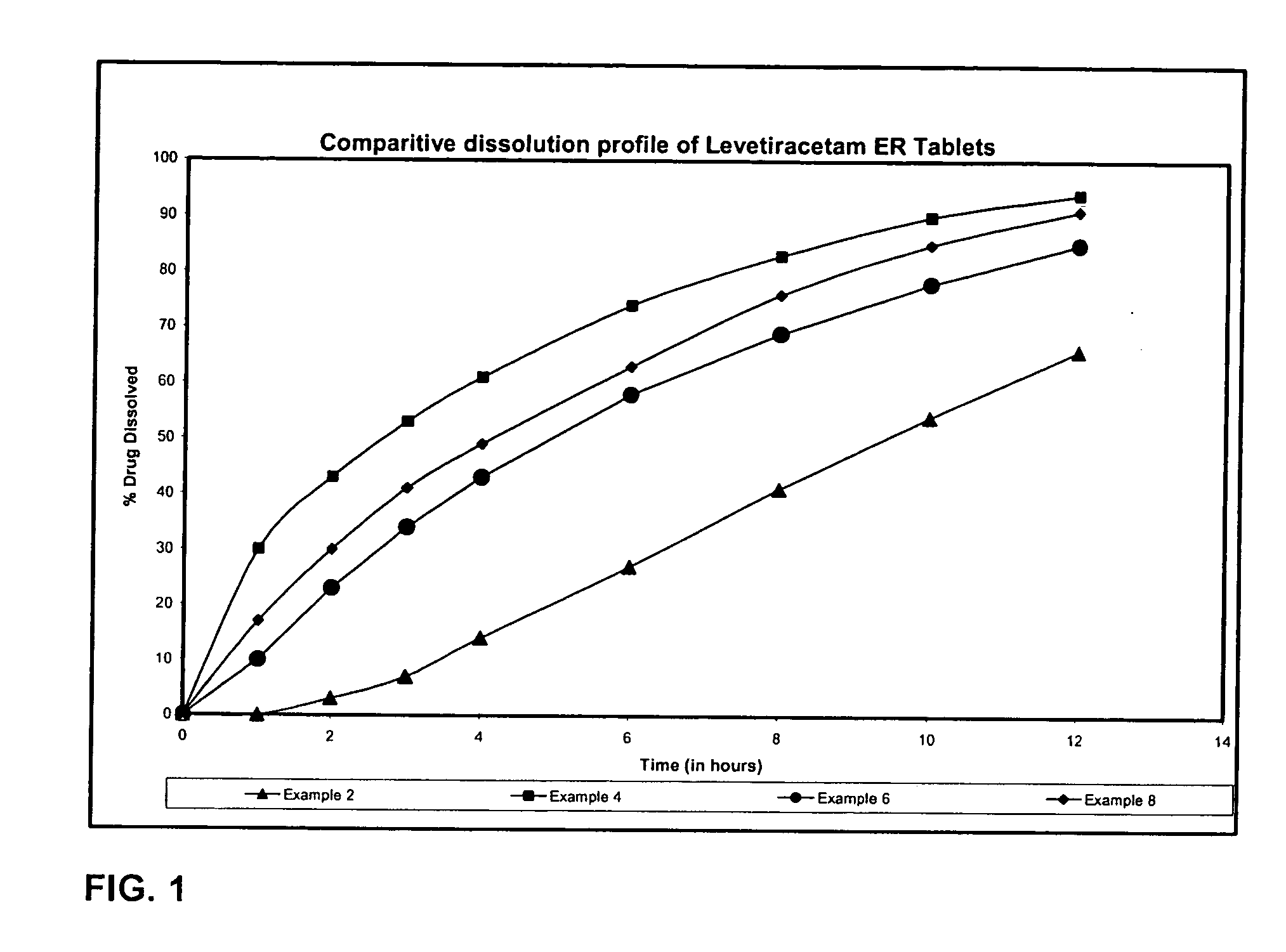
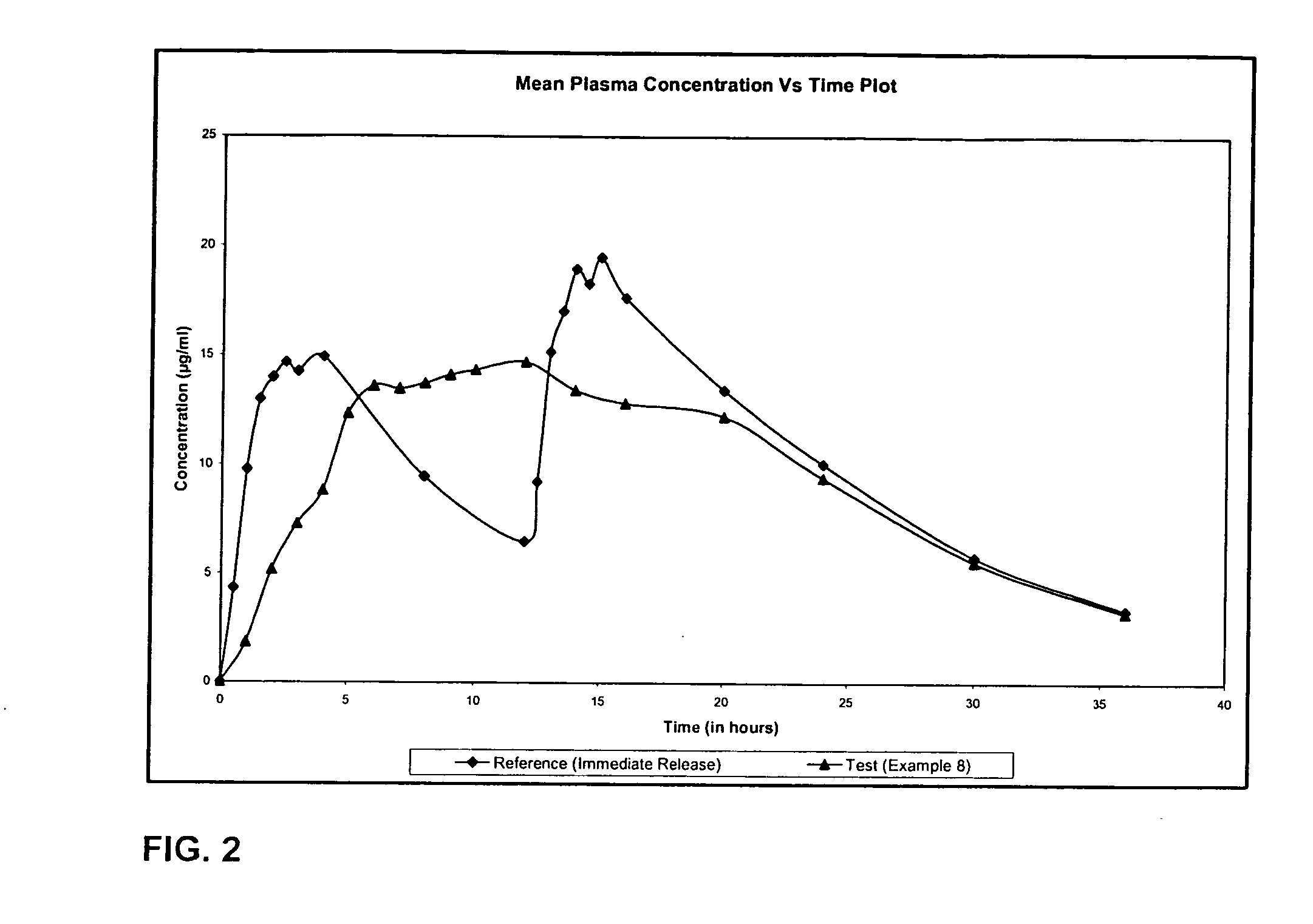
Extended release formulation of Levetiracetam
ActiveUS7863316B2Reduced inter subject variabilityBiocideNervous disorderWater dispersibleFOOD EFFECT
Owner:UCB PHARMA SA
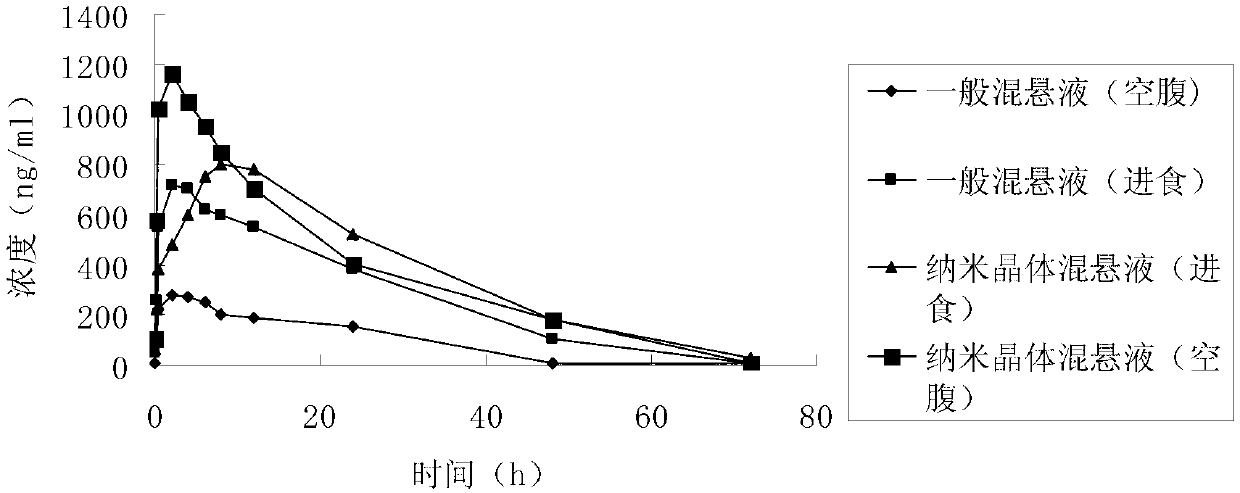
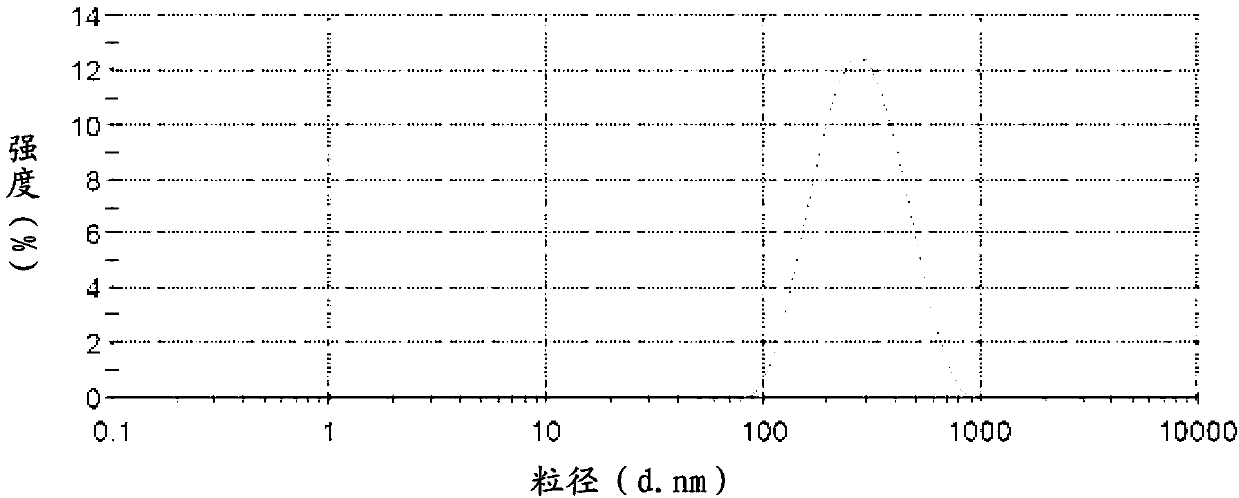
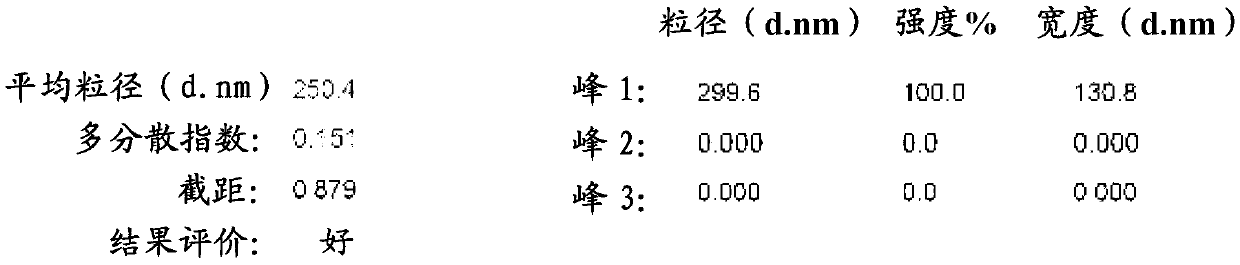
Puerarin nanocrystalline medical composition and preparation method thereof
InactiveCN103211759AImprove stabilityImprove solubilityOrganic active ingredientsMetabolism disorderSolubilityFOOD EFFECT
The invention belongs to the technical field of medicines and relates to a puerarin nanocrystalline medical composition and a preparation method and application thereof. Specifically, the puerarin composition is a puerarin nanocrystalline composition. The puerarin composition provided by the invention remarkably improves the solubility and bioavailabilityof puerarin and reduces or eliminates the food effect of the medicine, and is good in stability and can be prepared into various common preparation forms easily, the stability and effect of the medicine are improved, so that the composition has good prospect.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
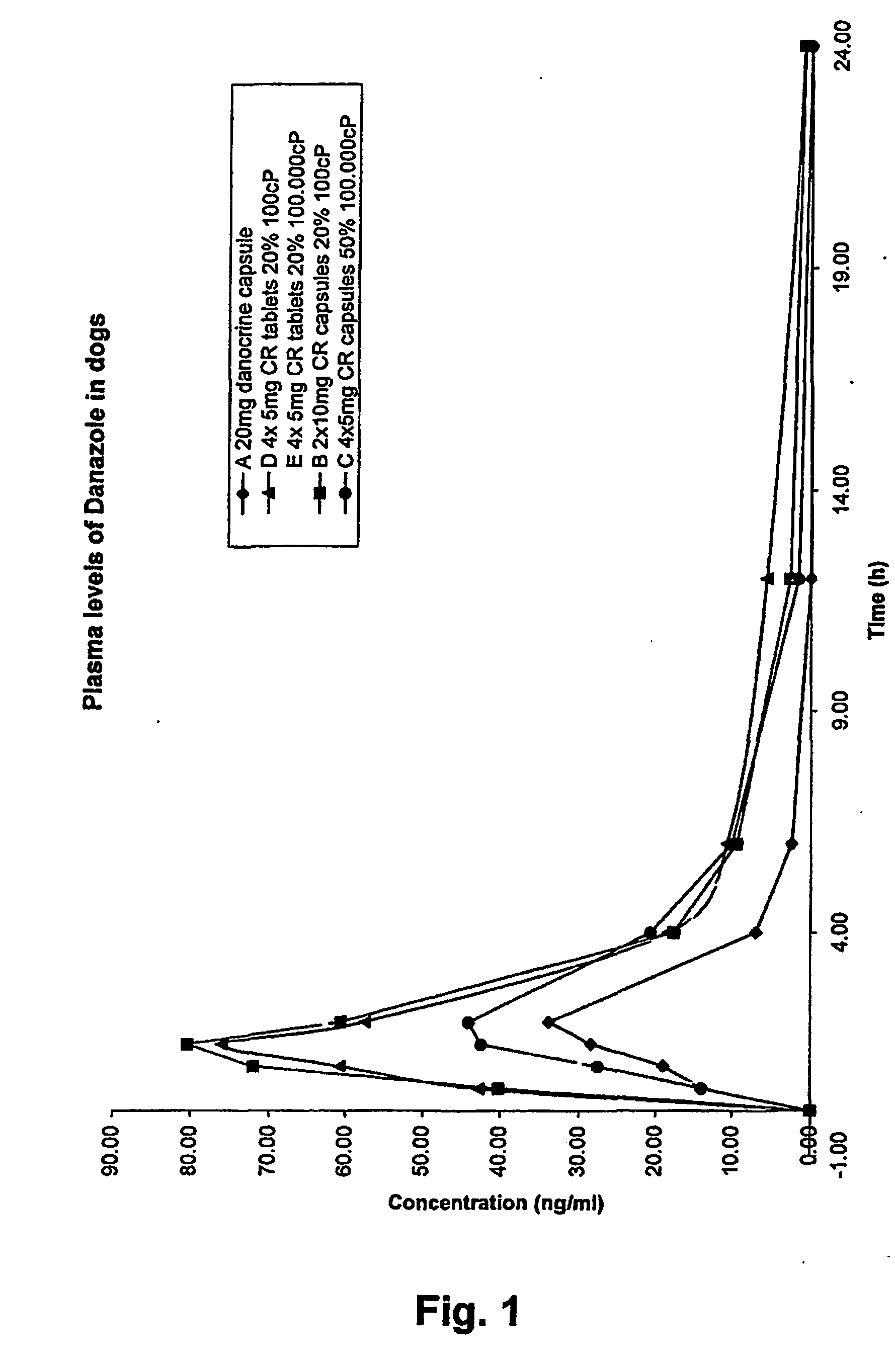
Pharmaceutical Compositions Comprising Danazol
A controlled release pharmaceutical comprising danazol has the property of slow release of danazol over an extended period of time and markedly increased bioavailability compared to commercially available danazol-containing products. The pharmaceutical composition comprises danazol dissolved in a solid vehicle or carrier and is especially suitable for oral solid dosage forms. The composition significantly reduces food effect and may reduce side effects.
Owner:LIFECYCLE PHARMA AS
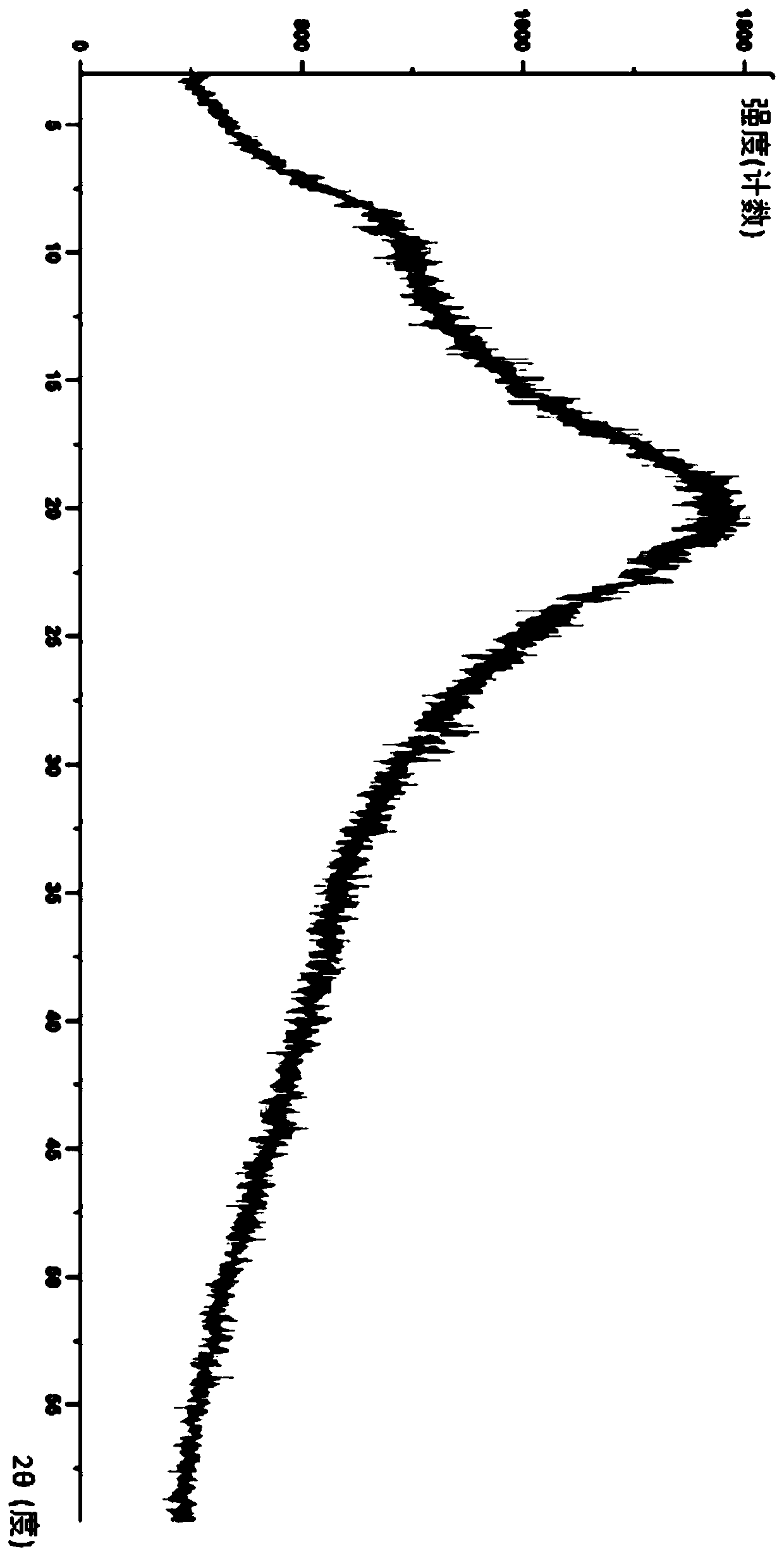
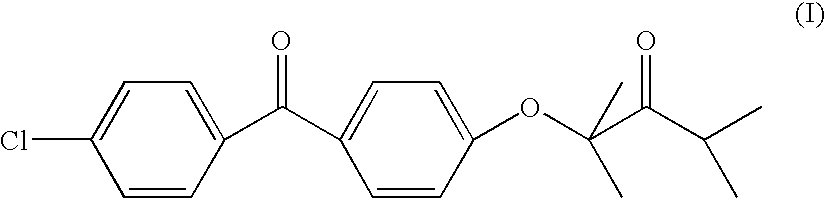
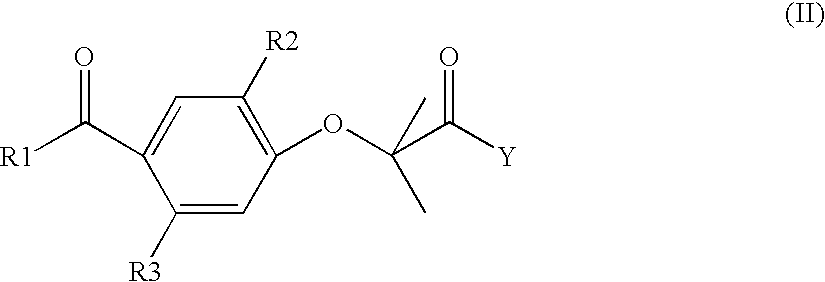
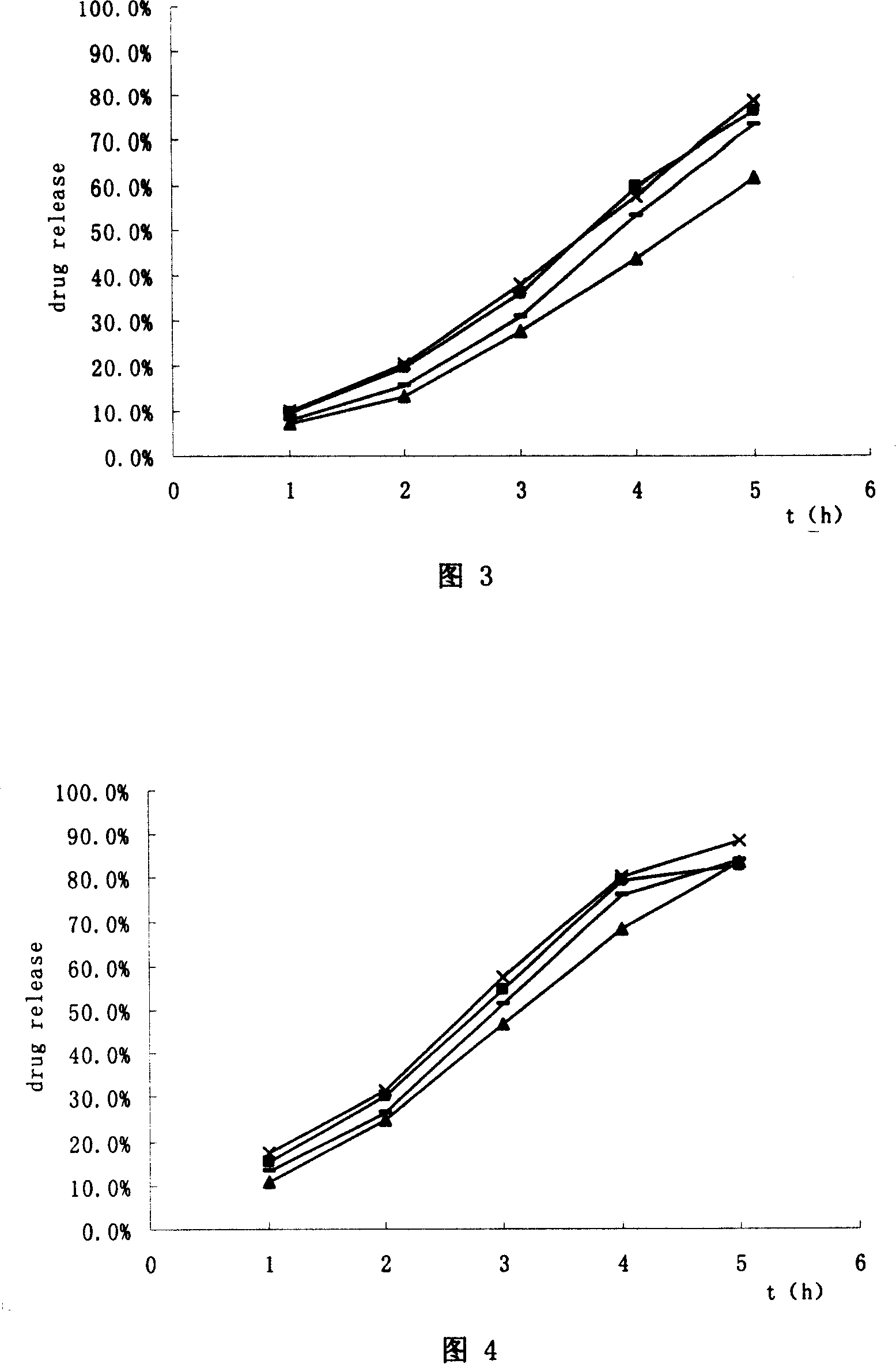
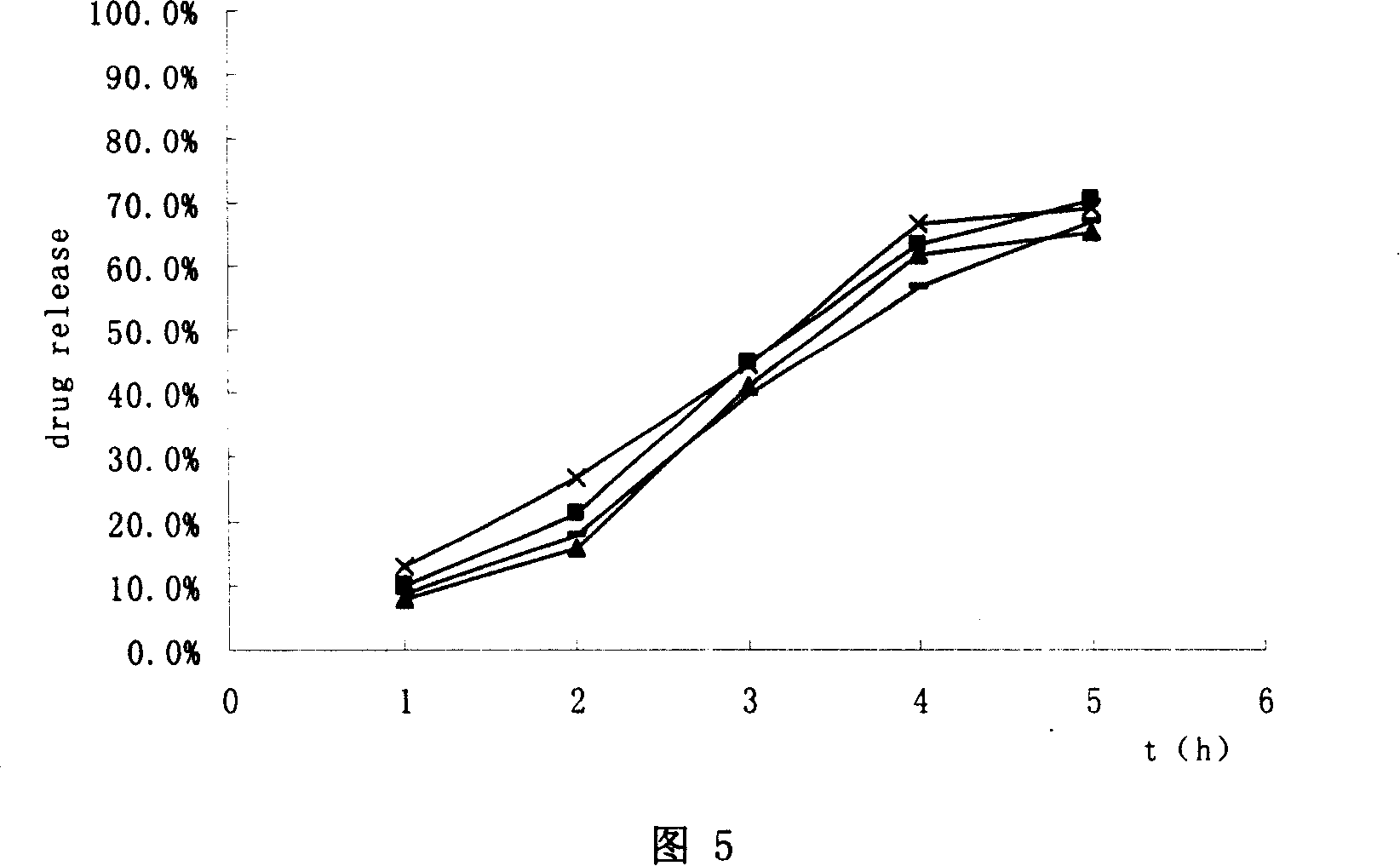
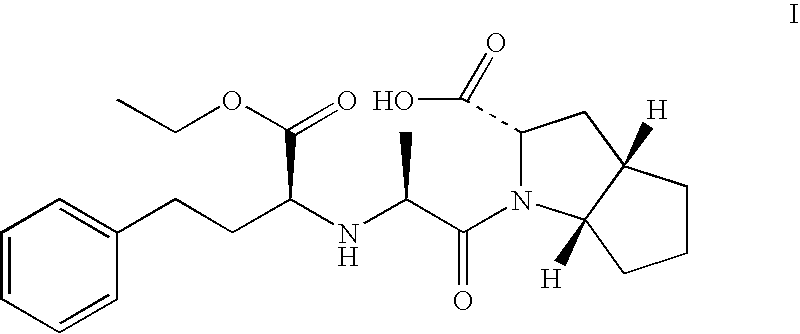
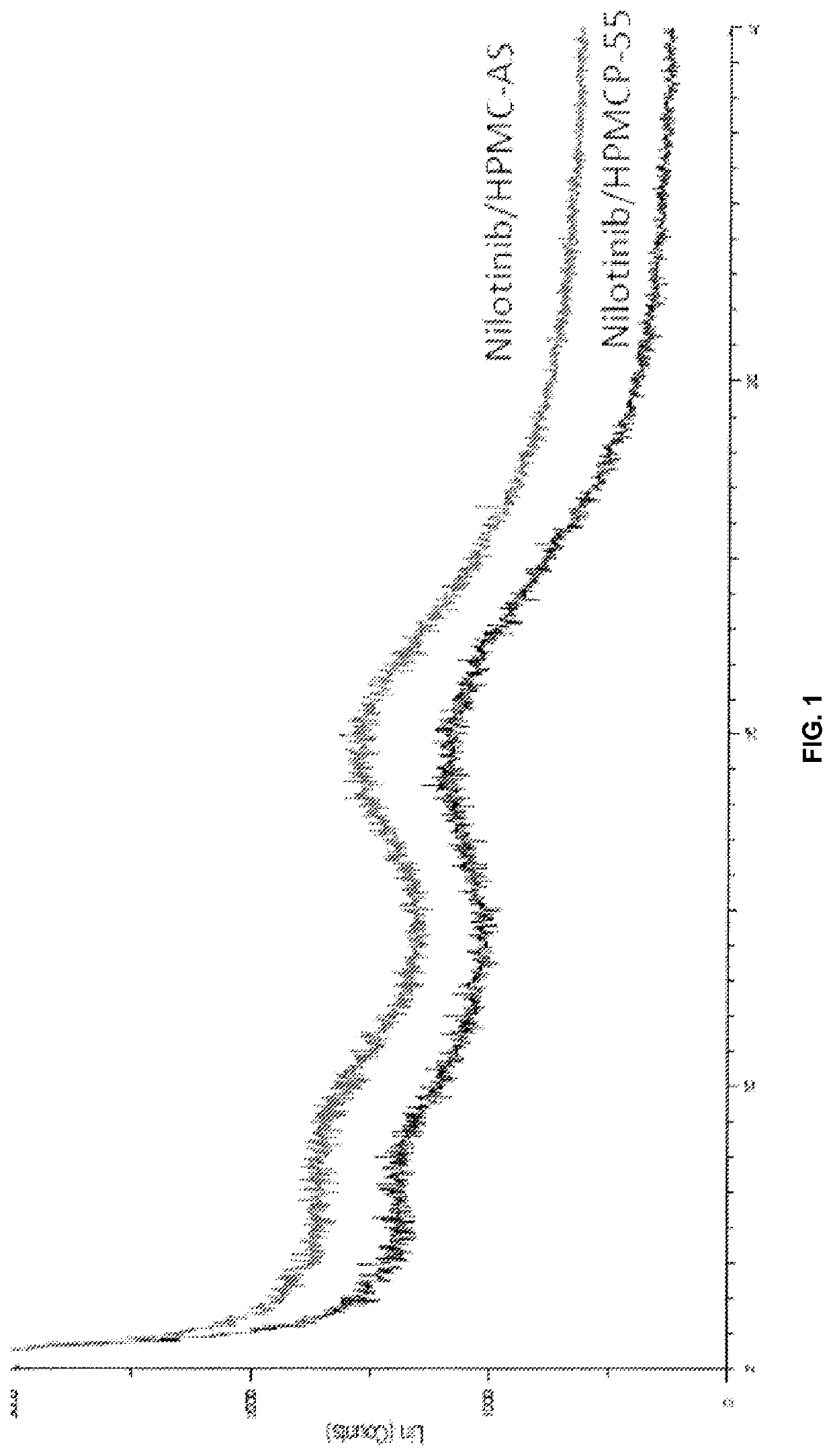
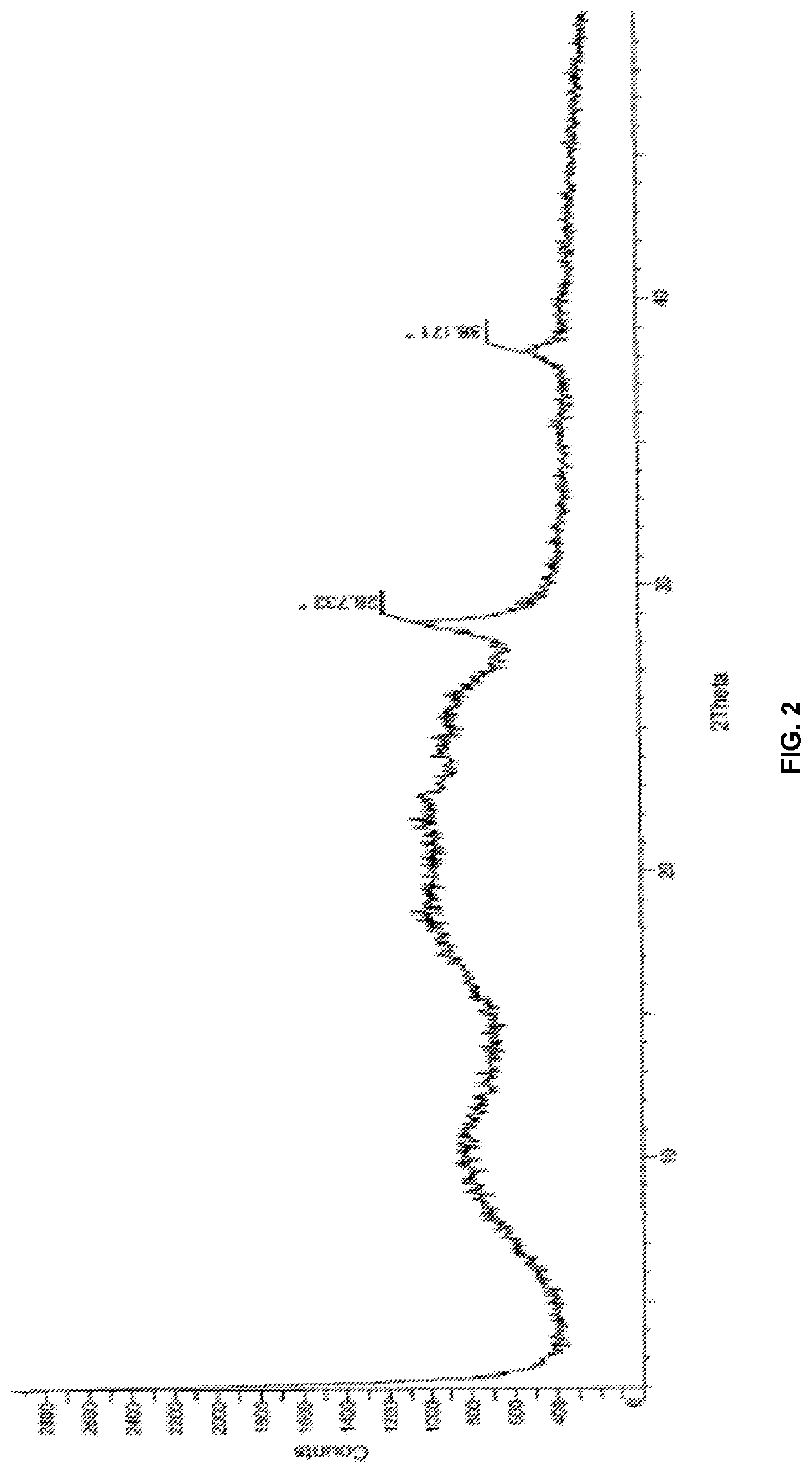
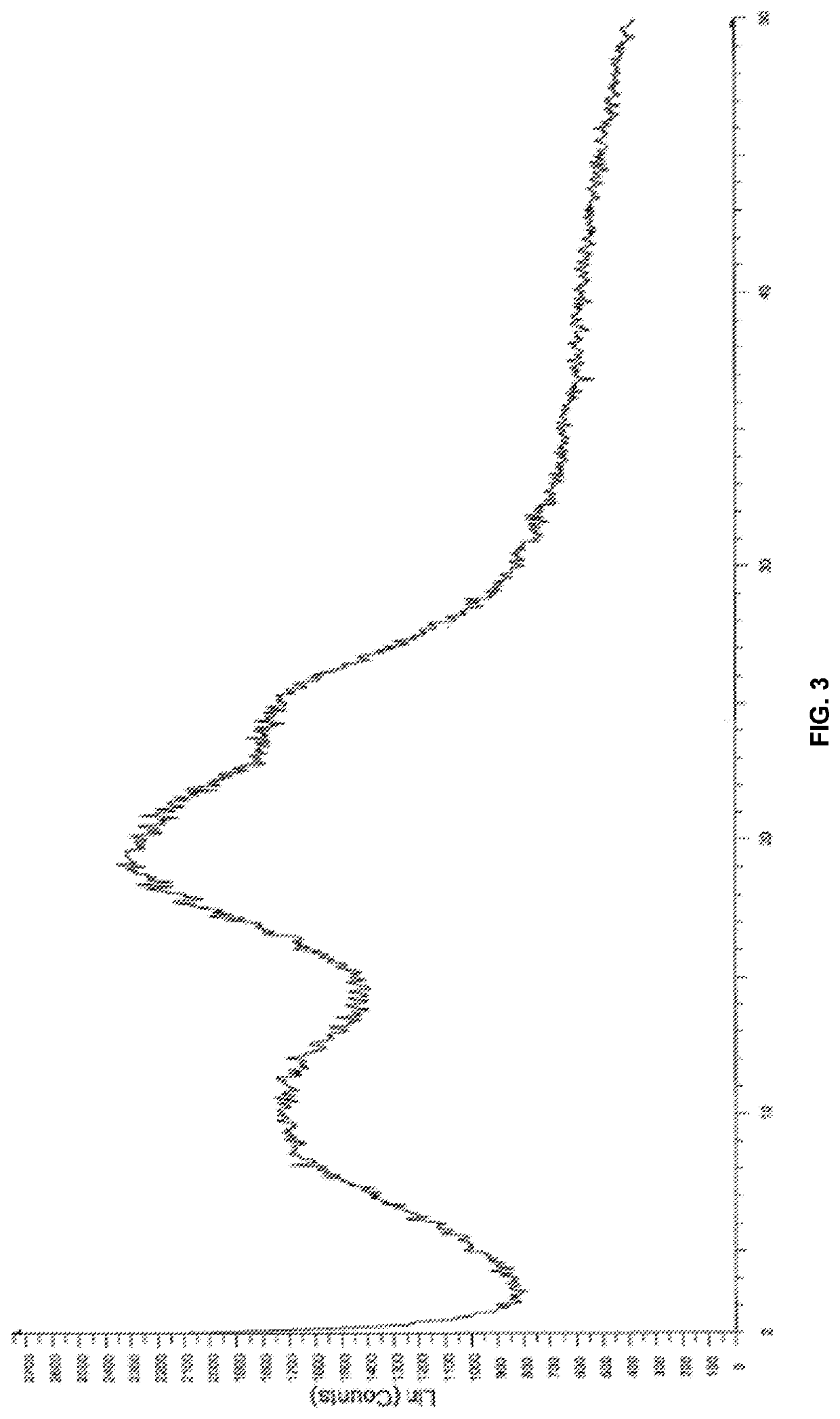
Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids
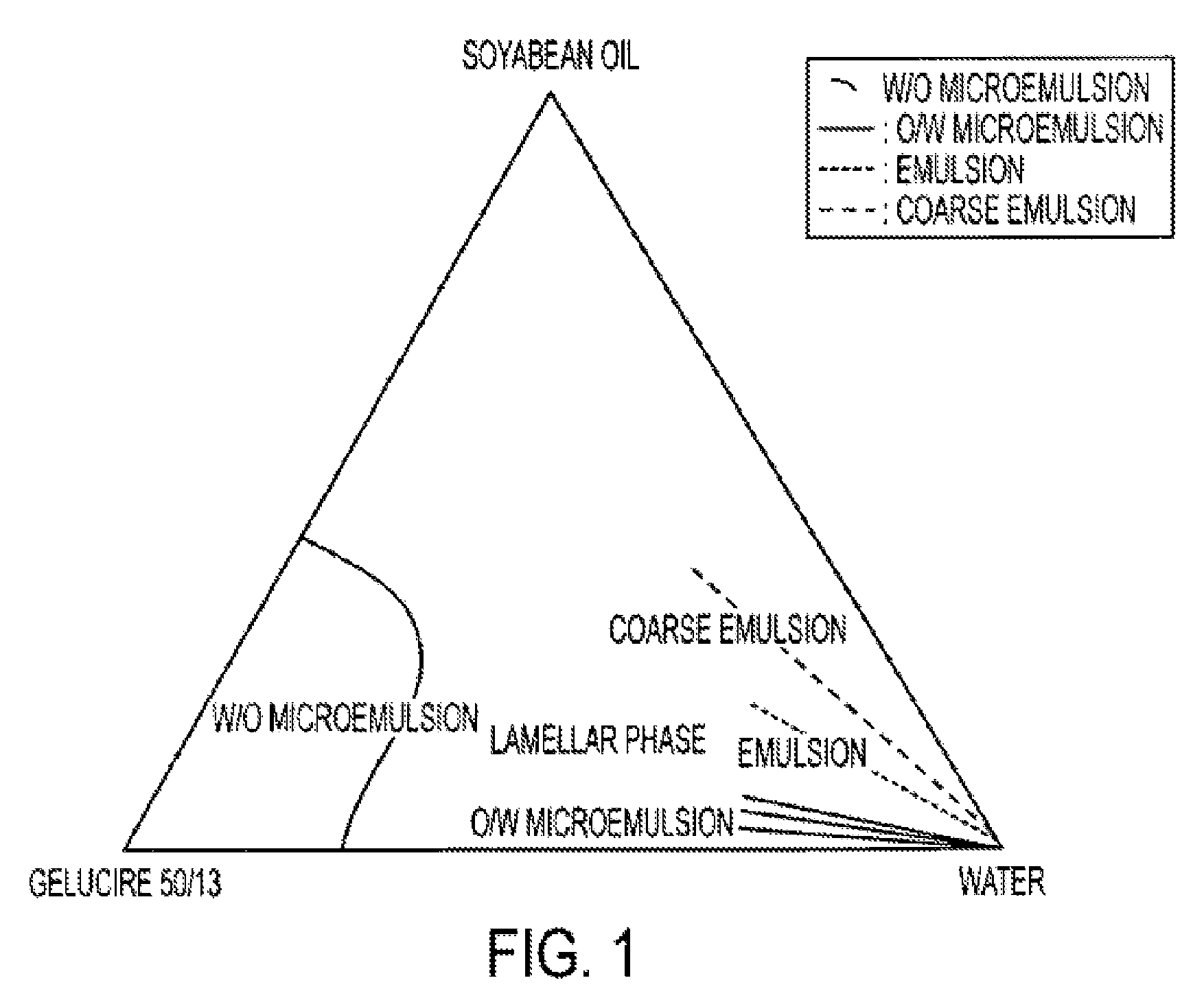
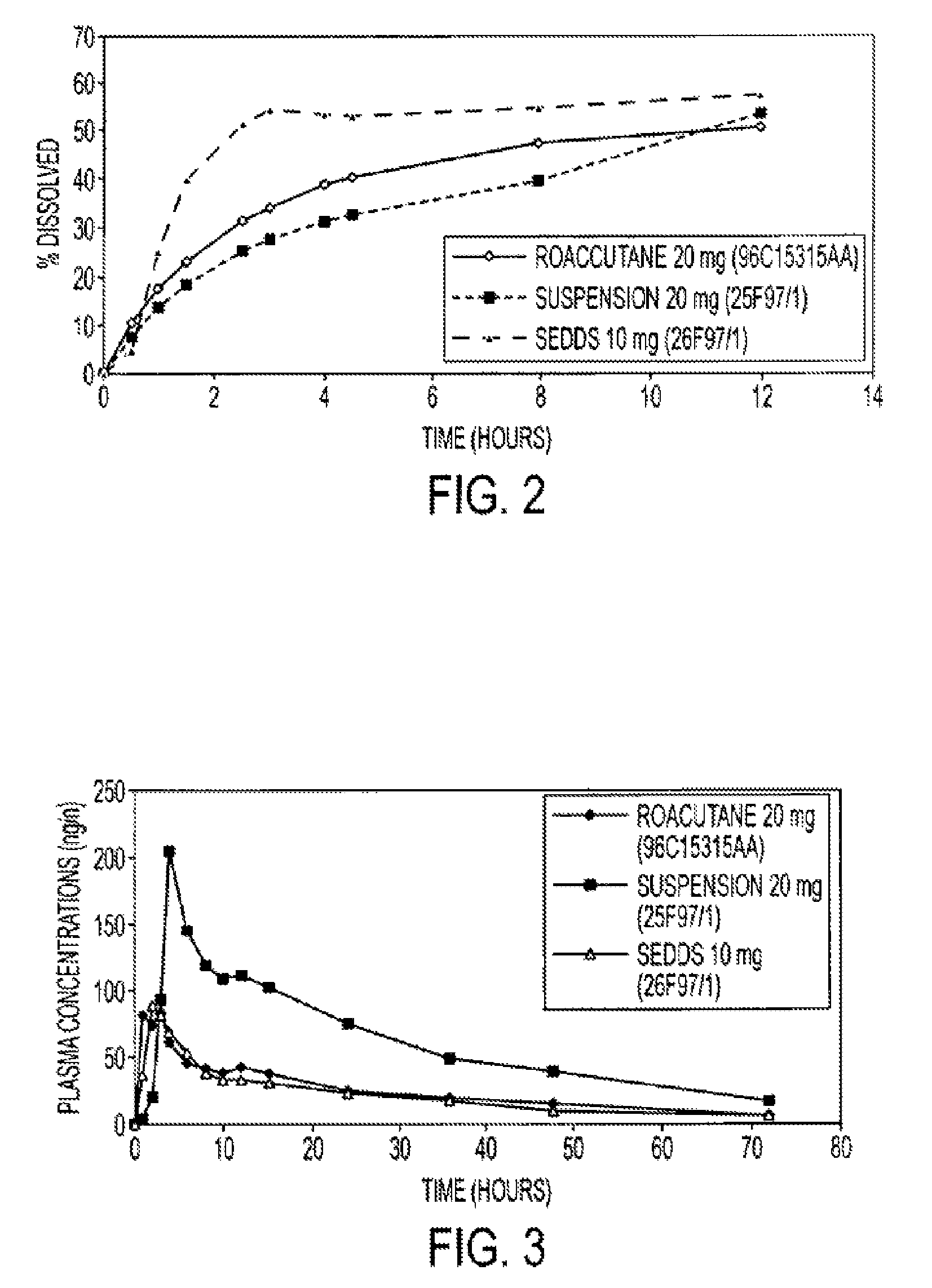
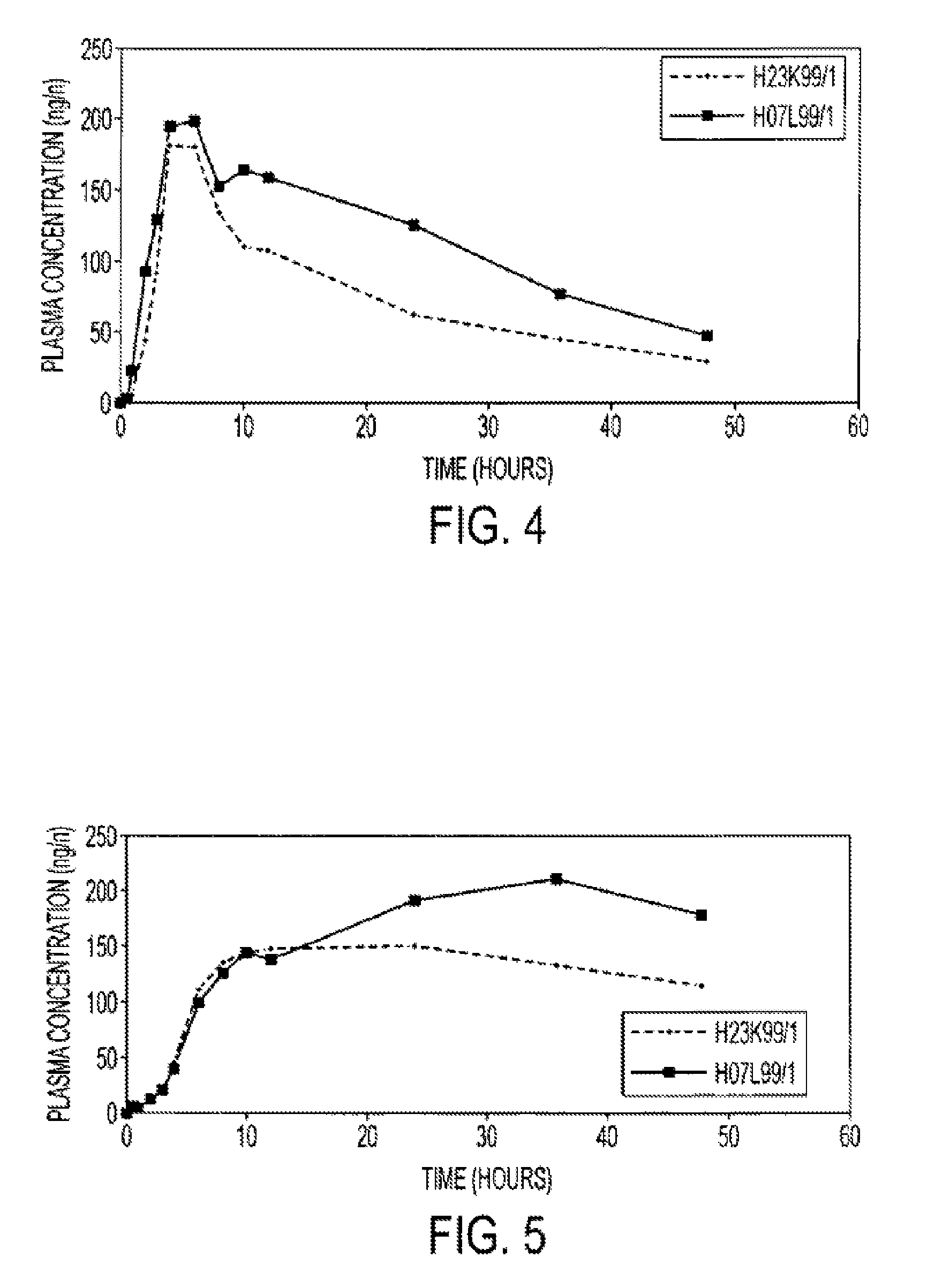
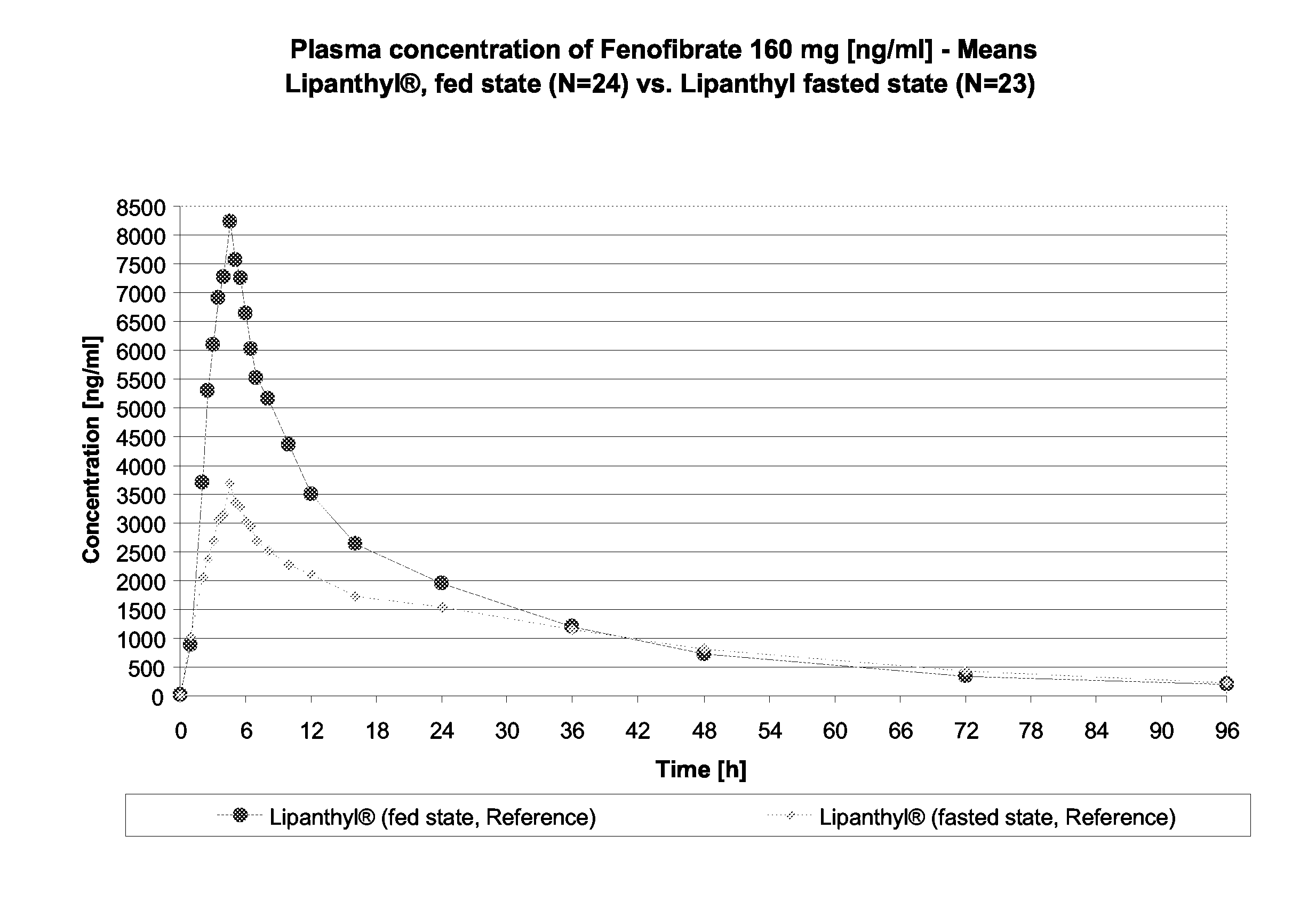
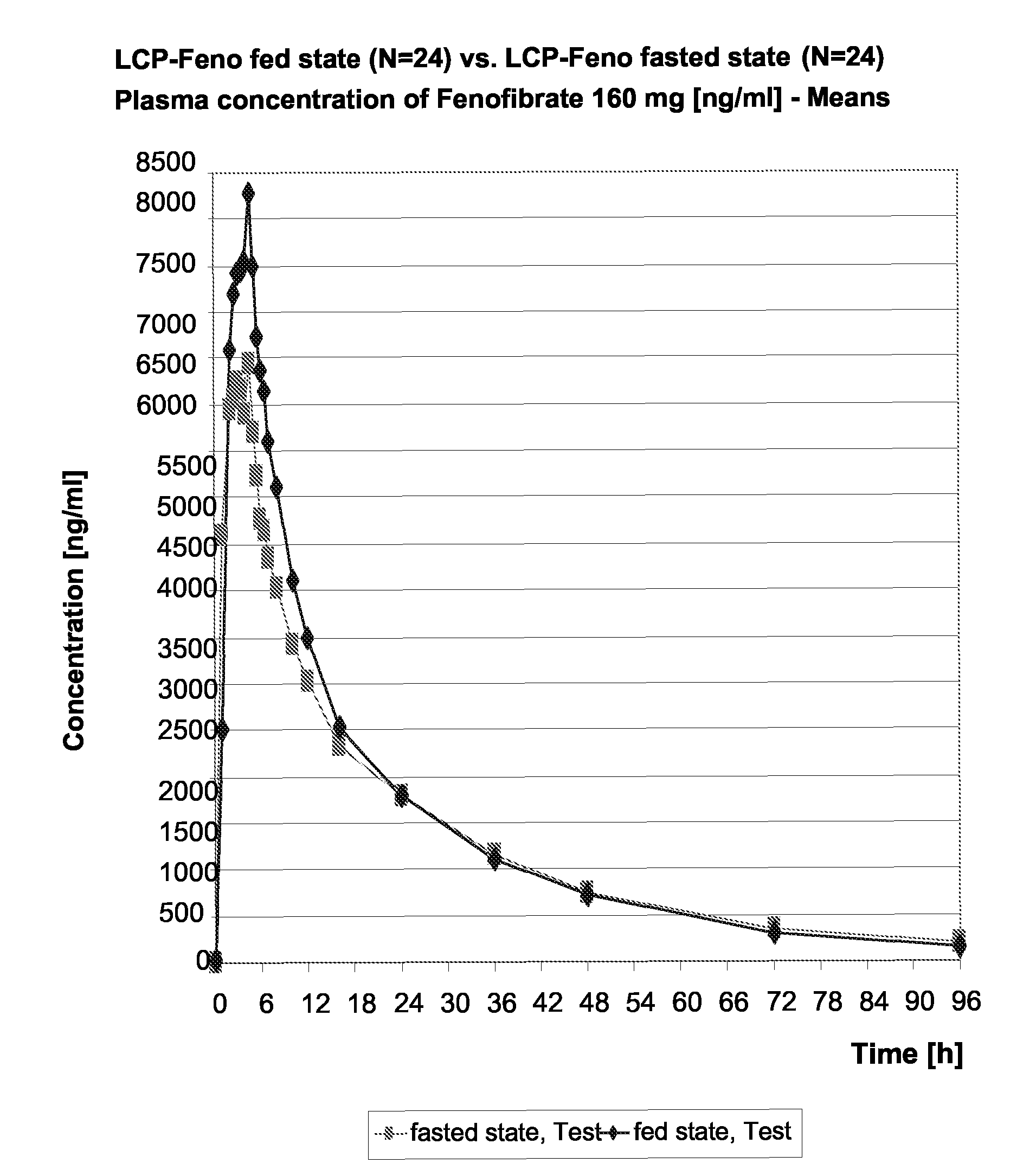
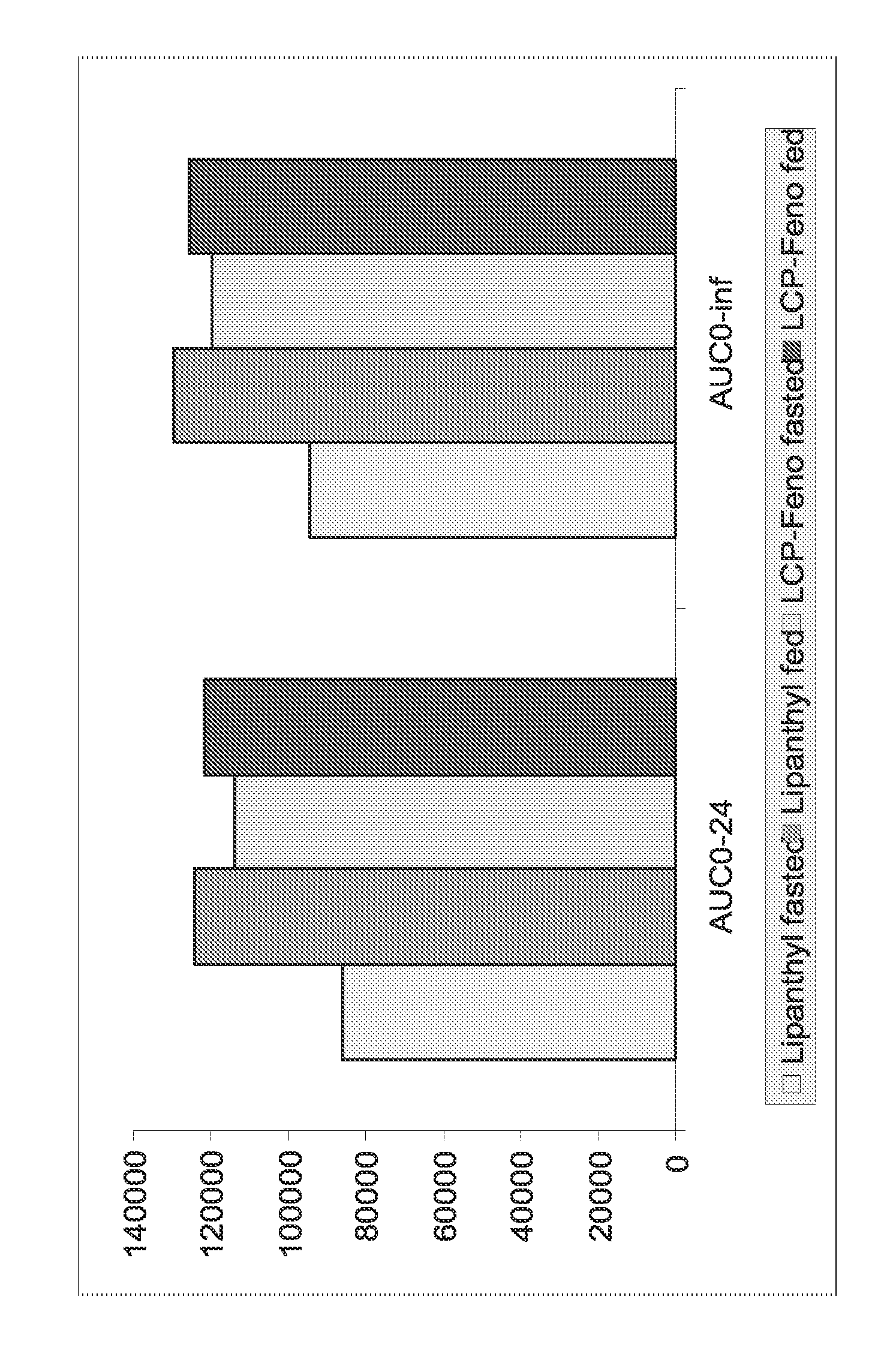
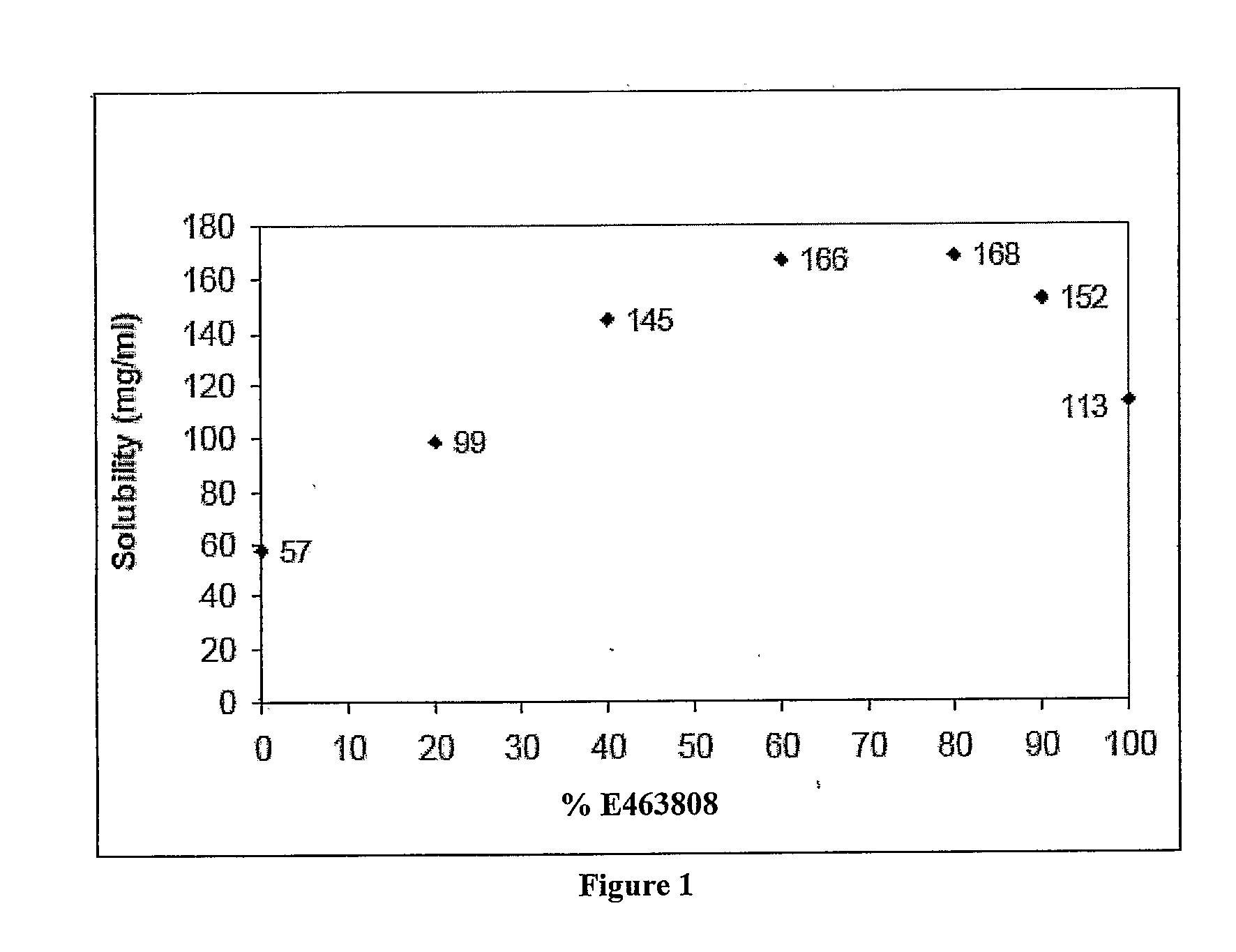
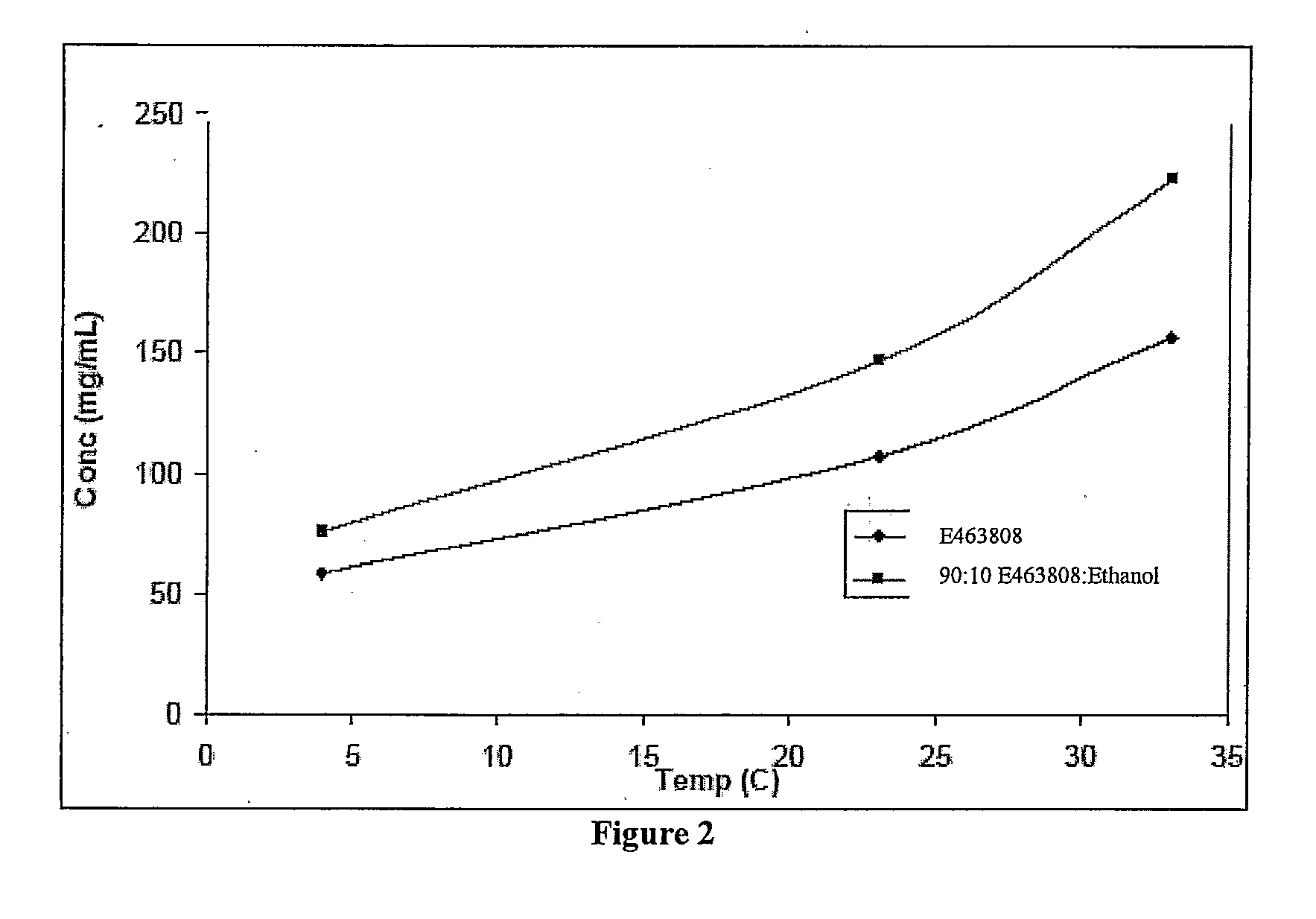
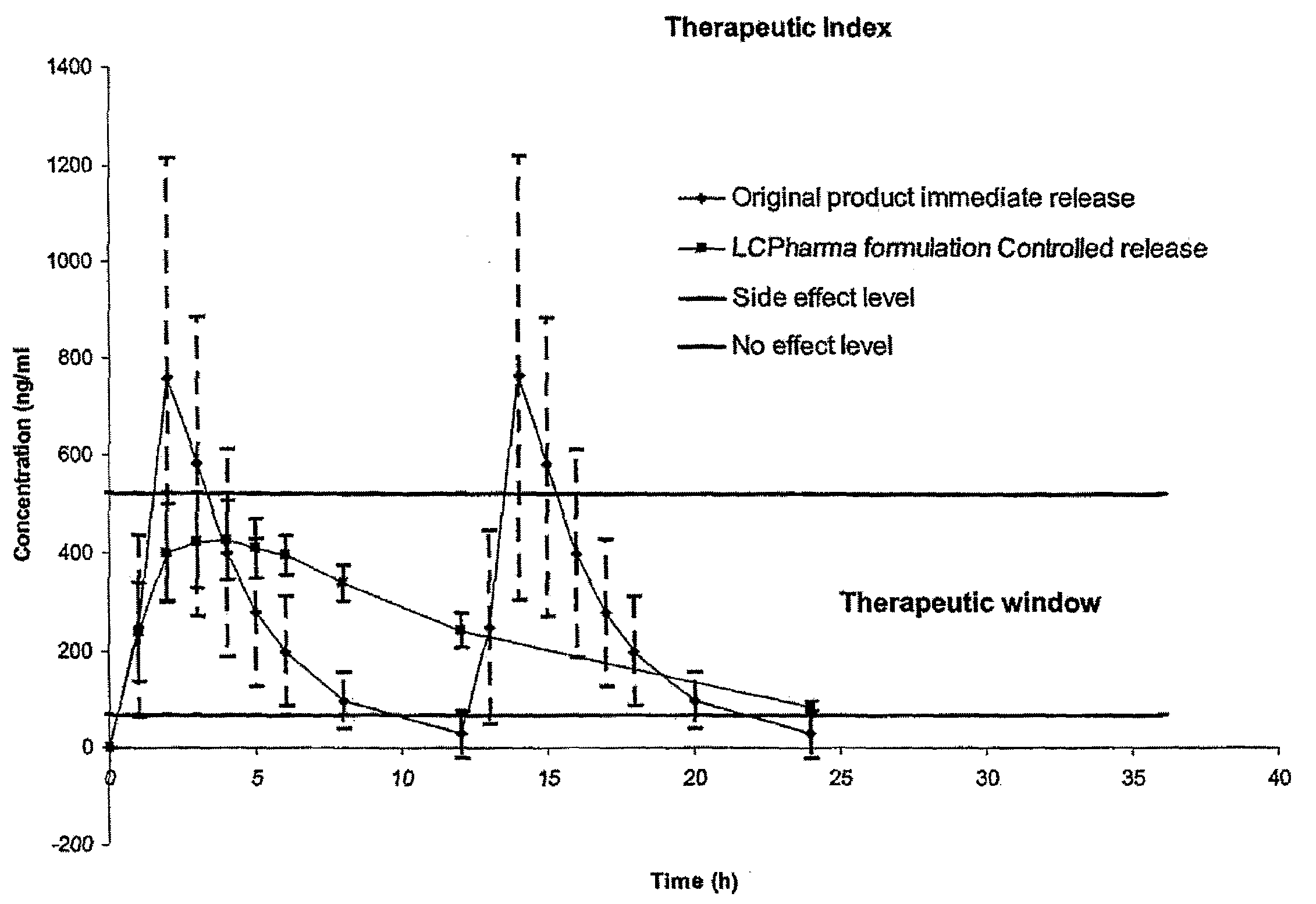
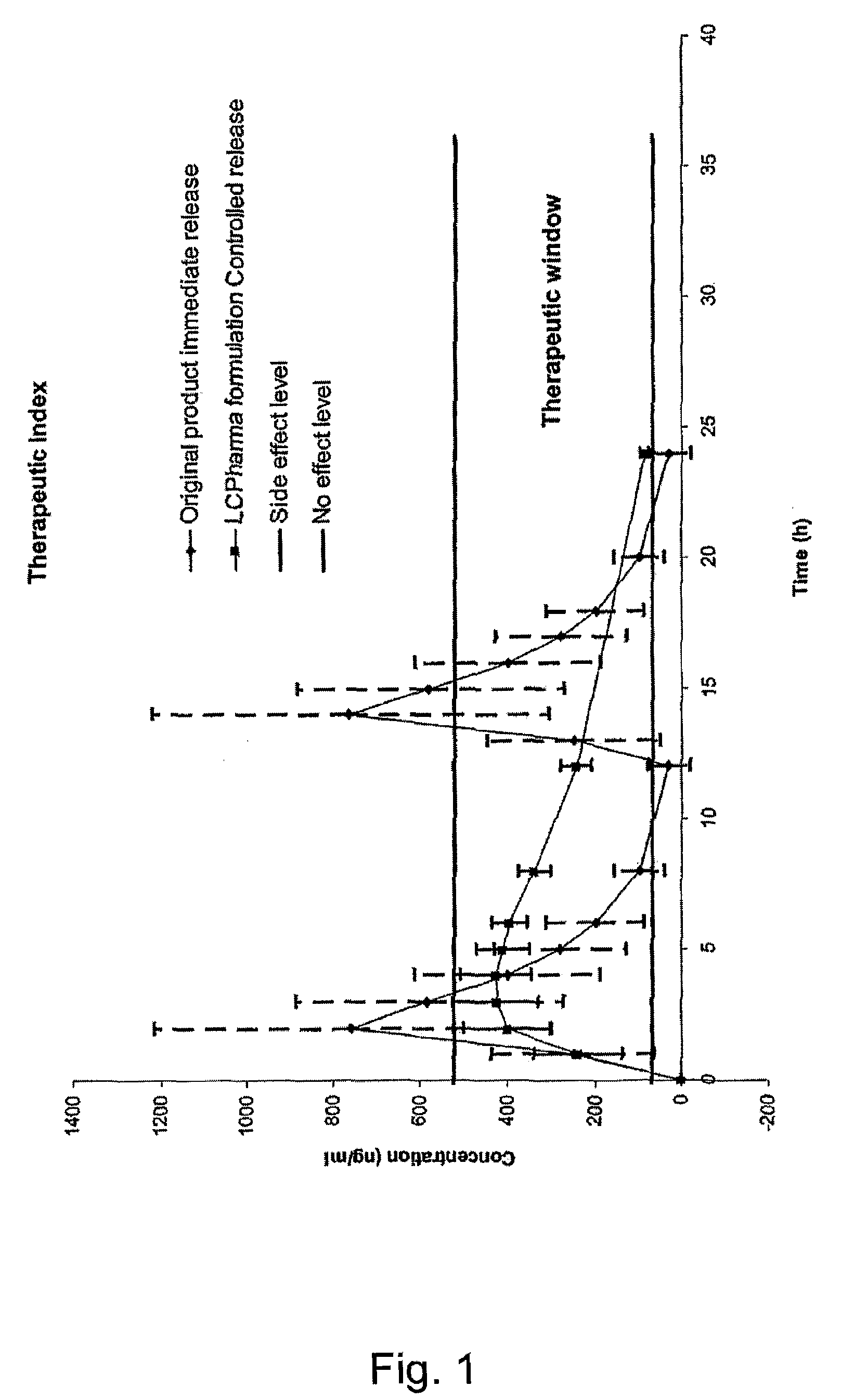
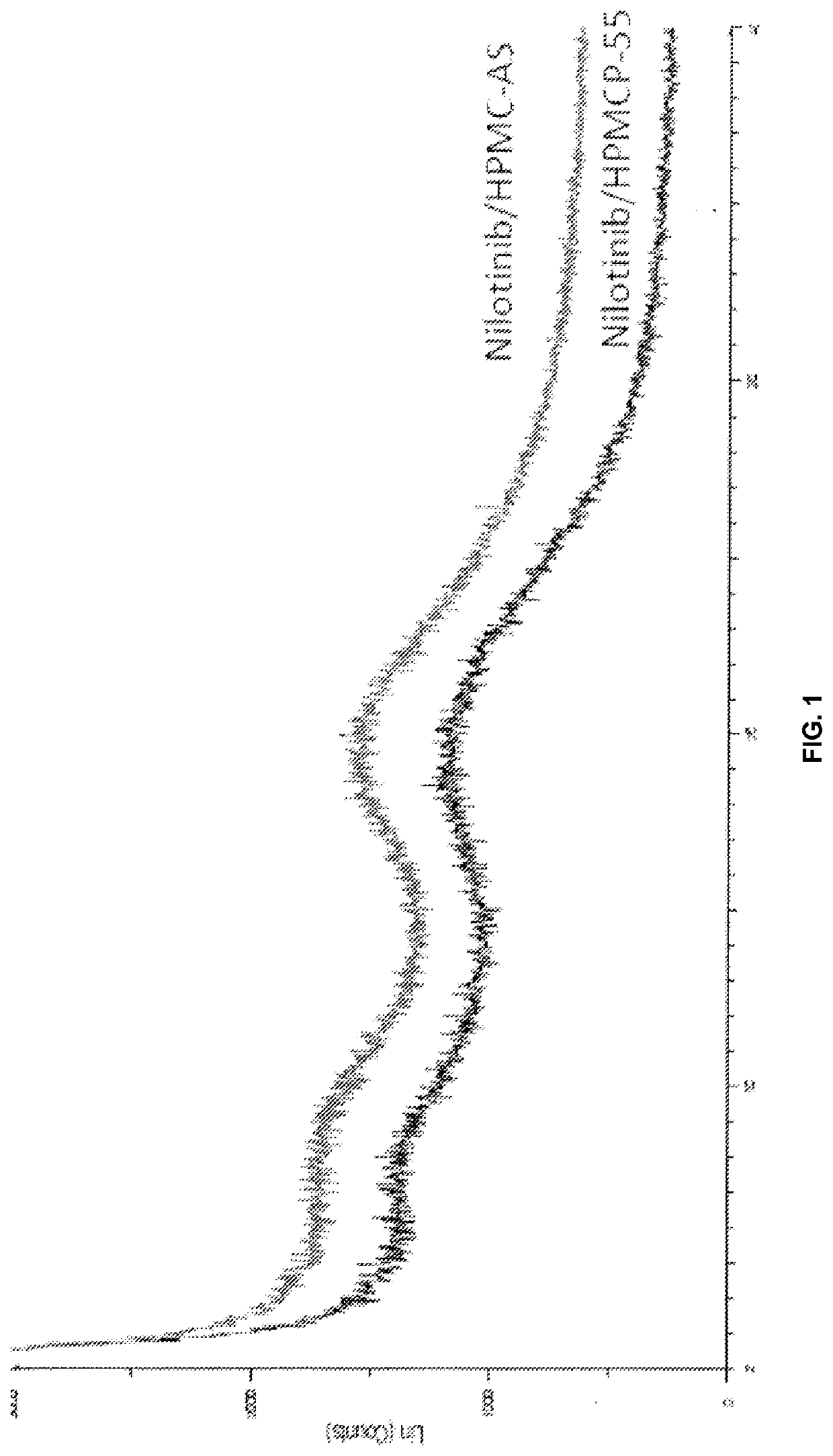
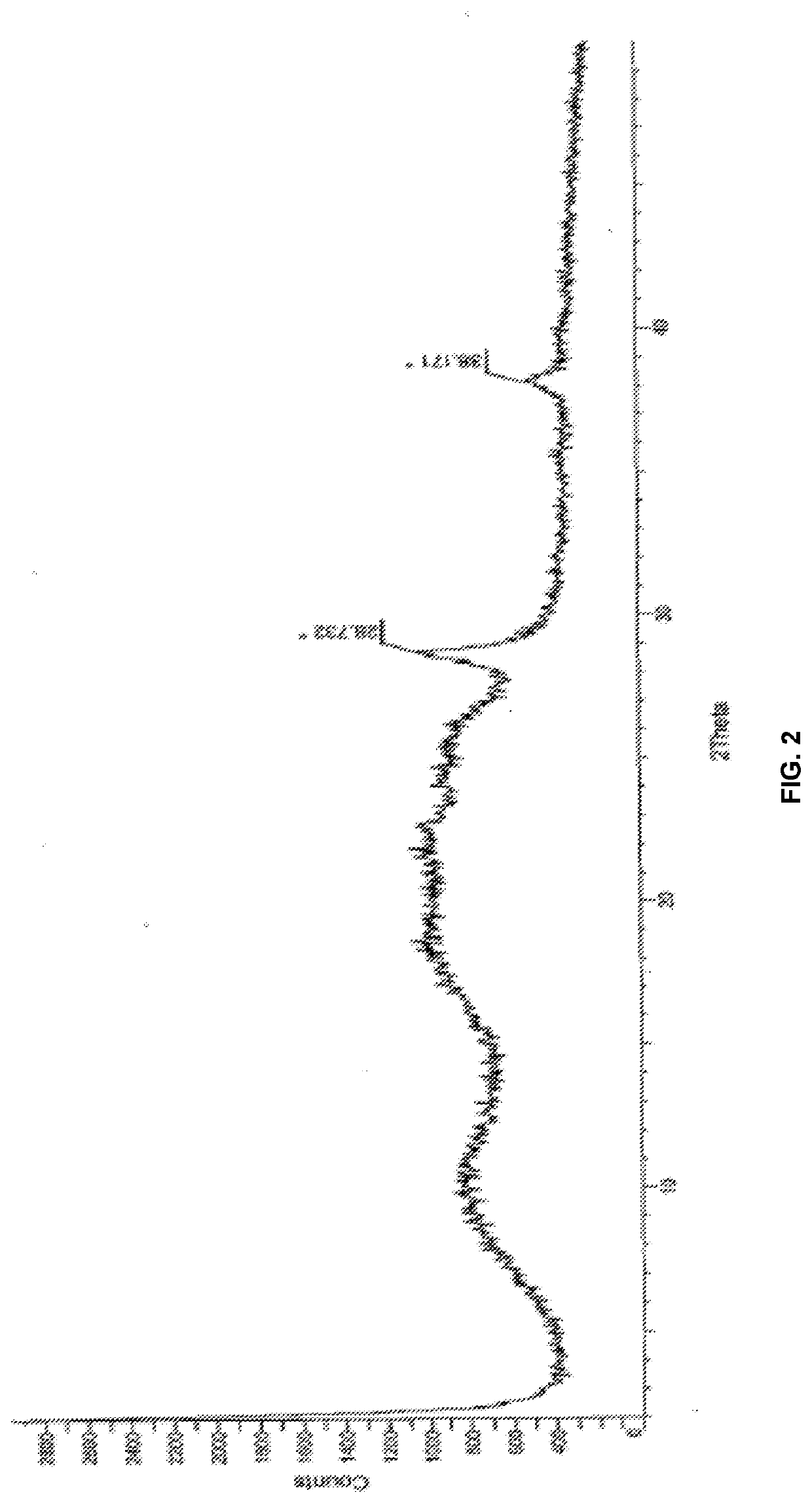
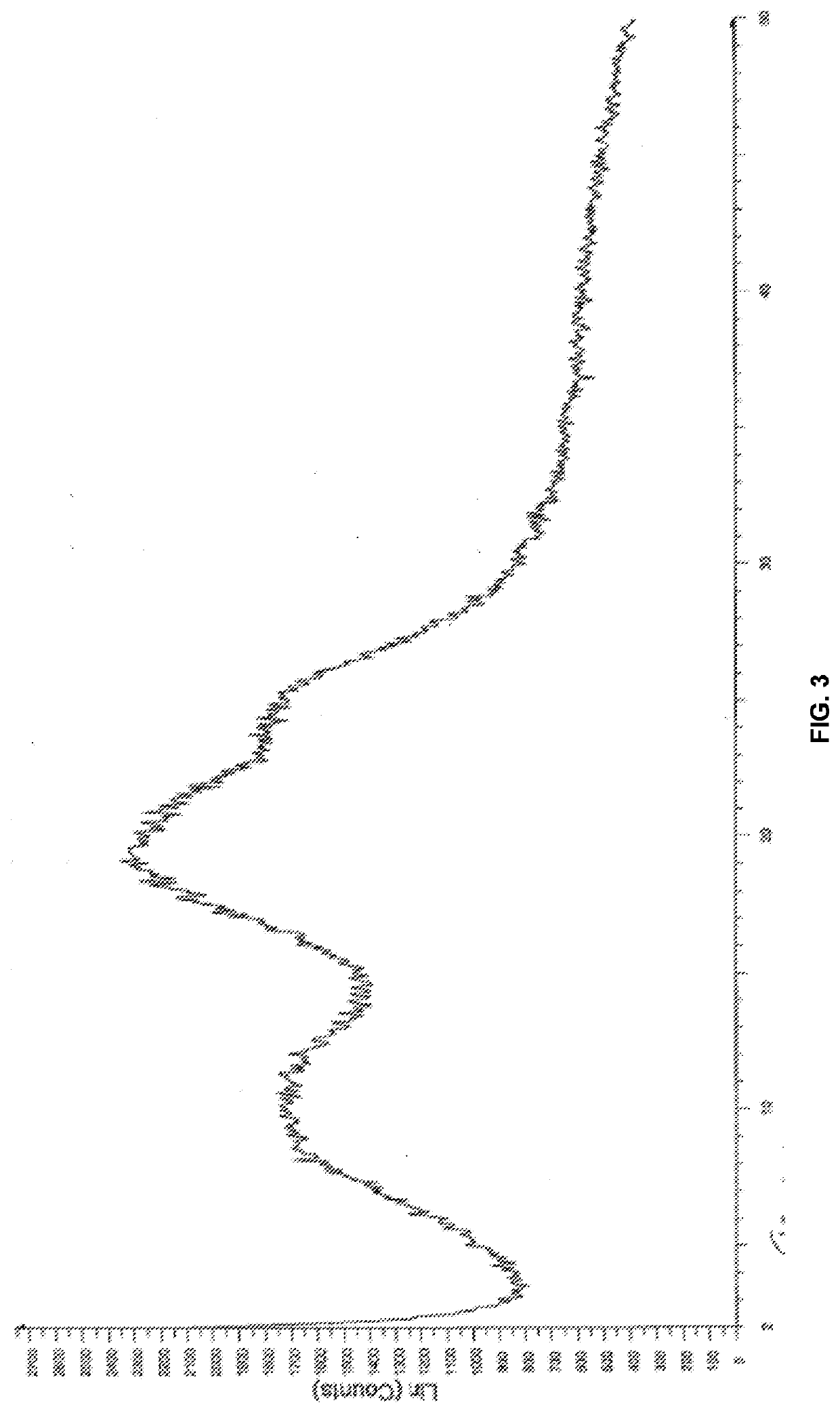
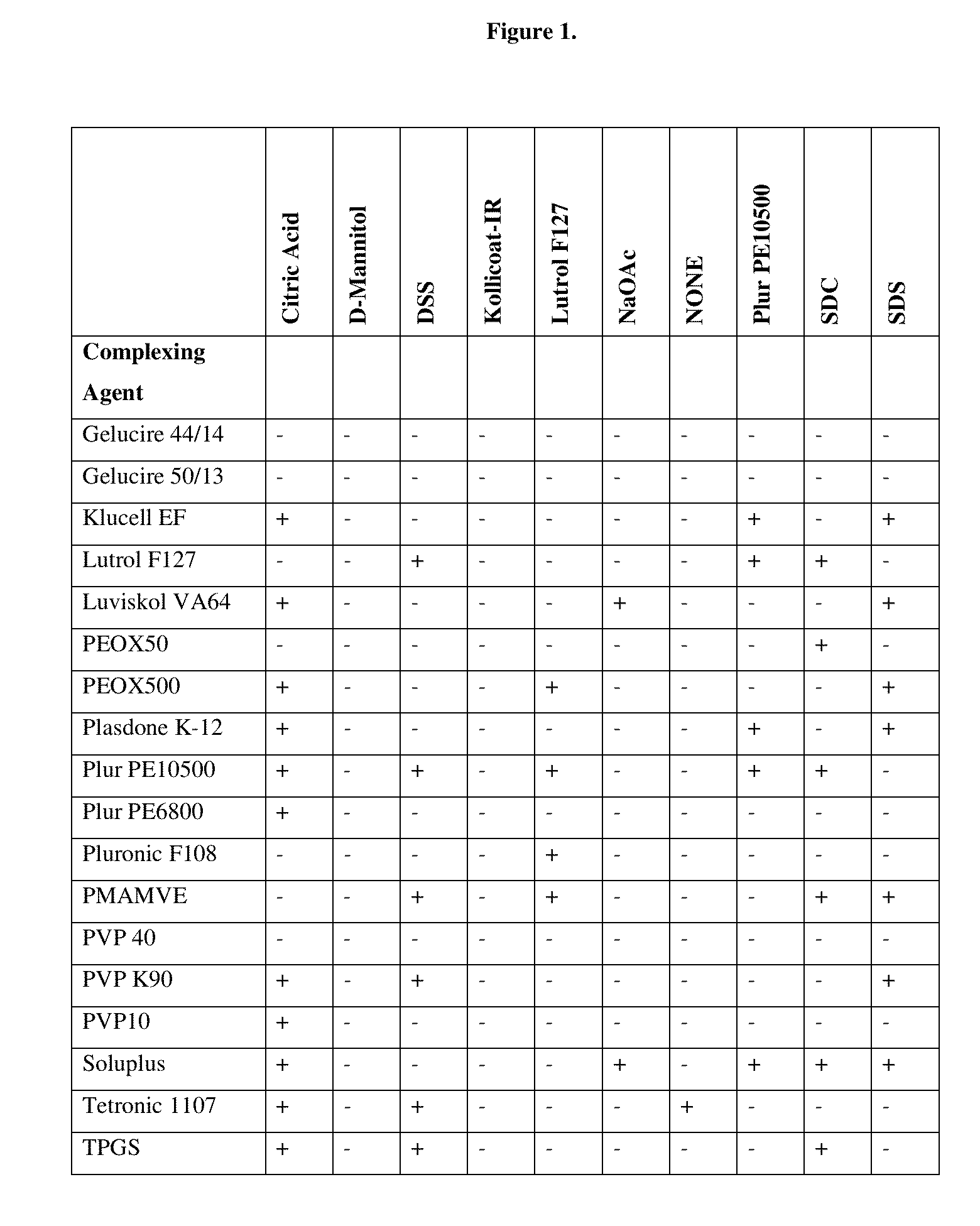
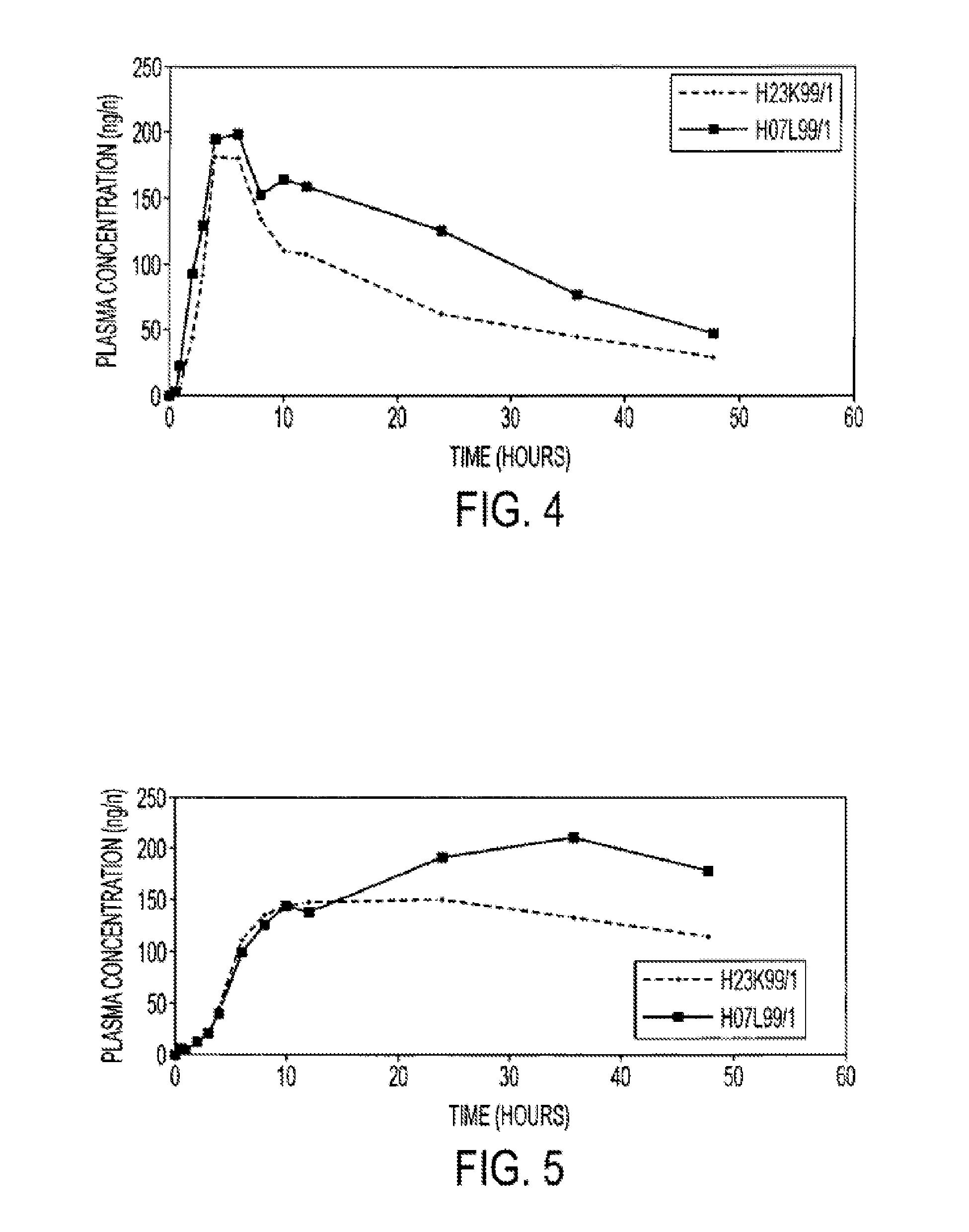
InactiveUS20150273070A1Improve bioavailabilitySuppressing food effectBiocideOrganic active ingredientsOrganic acidFOOD EFFECT
Soluble pharmaceutical compositions of nilotinib or a pharmaceutically acceptable salt thereof were invented using one or more organic acids that function as a solubilizing agent, increasing the bioavailability of nilotinib and supressing the food effect associated with certain compositions of nilotinib. The pharmaceutical compositions are in the form of solid oral dosage forms, including capsules and tablets.
Owner:NOVARTIS AG
Method of treatment for improved bioavailability
InactiveUS20050147663A1Minimizes inter-patient differenceReducing bioavailability food effectBiocideAnimal repellantsFOOD EFFECTActive agent
A method of treatment to avoid bioavailability food effects and improve bioavailability variability, by administering a pharmaceutical dosage form containing a pharmaceutical active agent and a disintegrant in a core, a swellable coating surrounding the core, and an optional enteric coating surrounding the swellable coating.
Owner:DR REDDYS LAB LTD +1
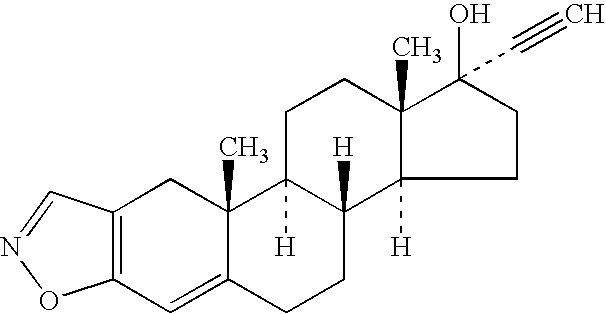
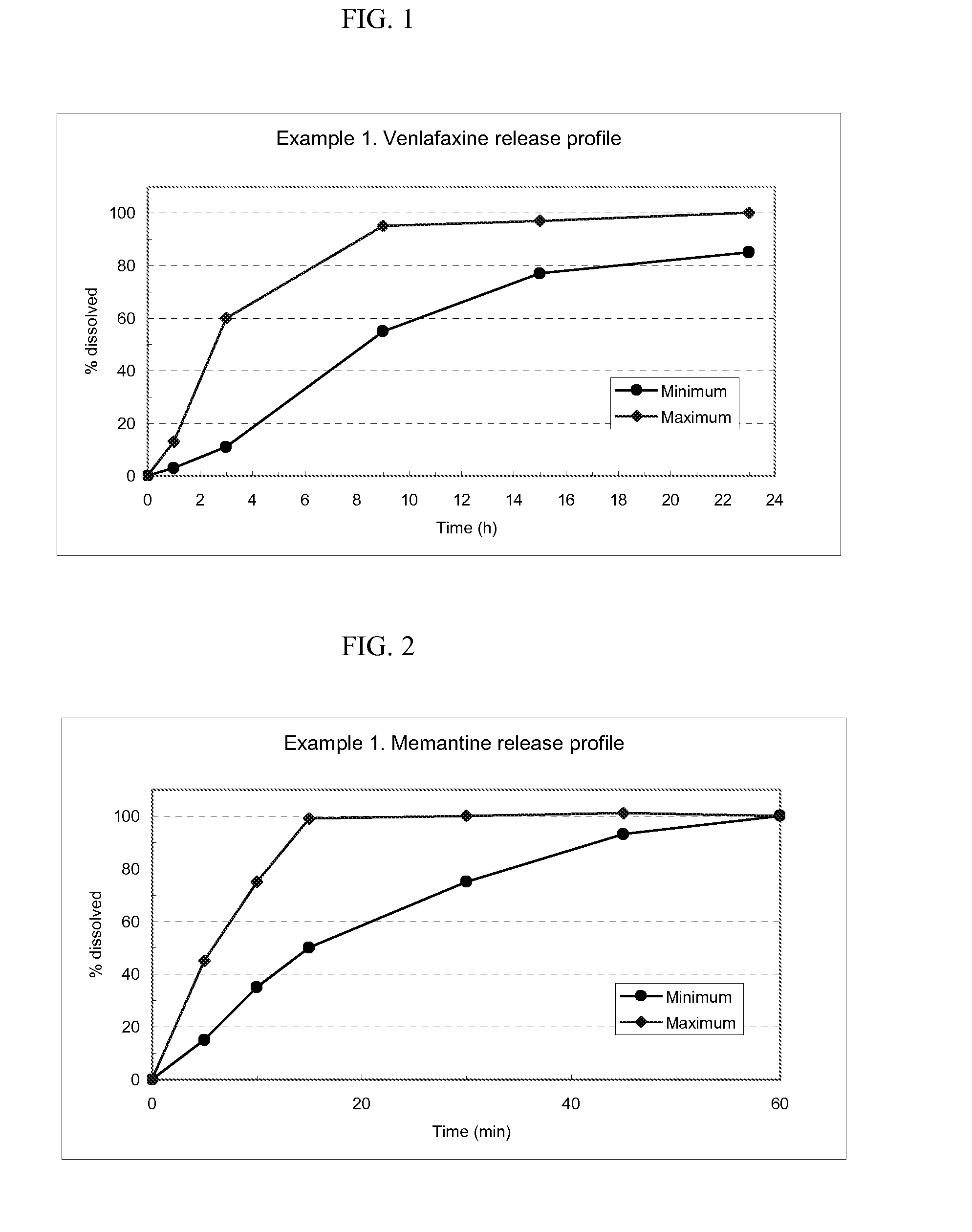
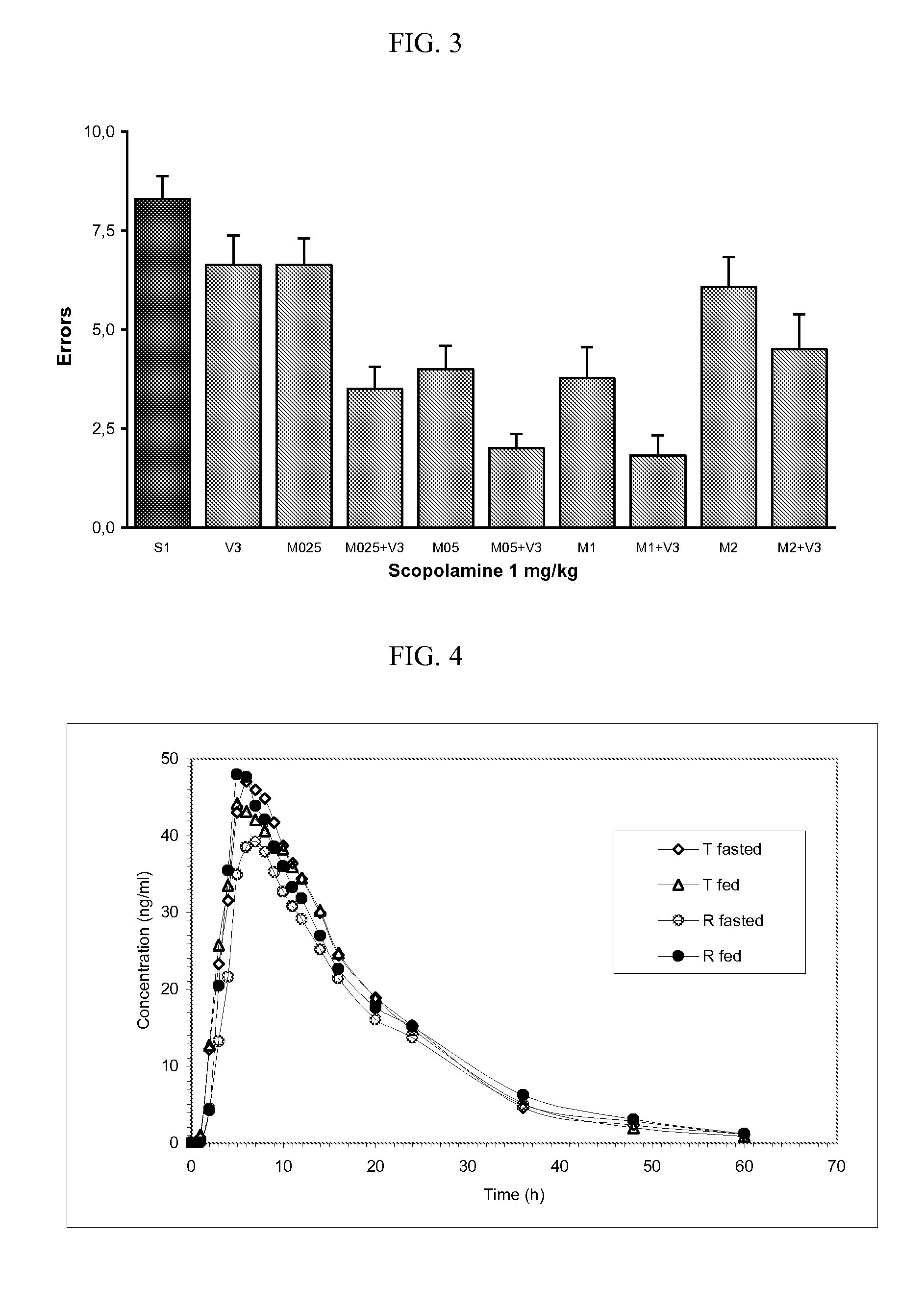
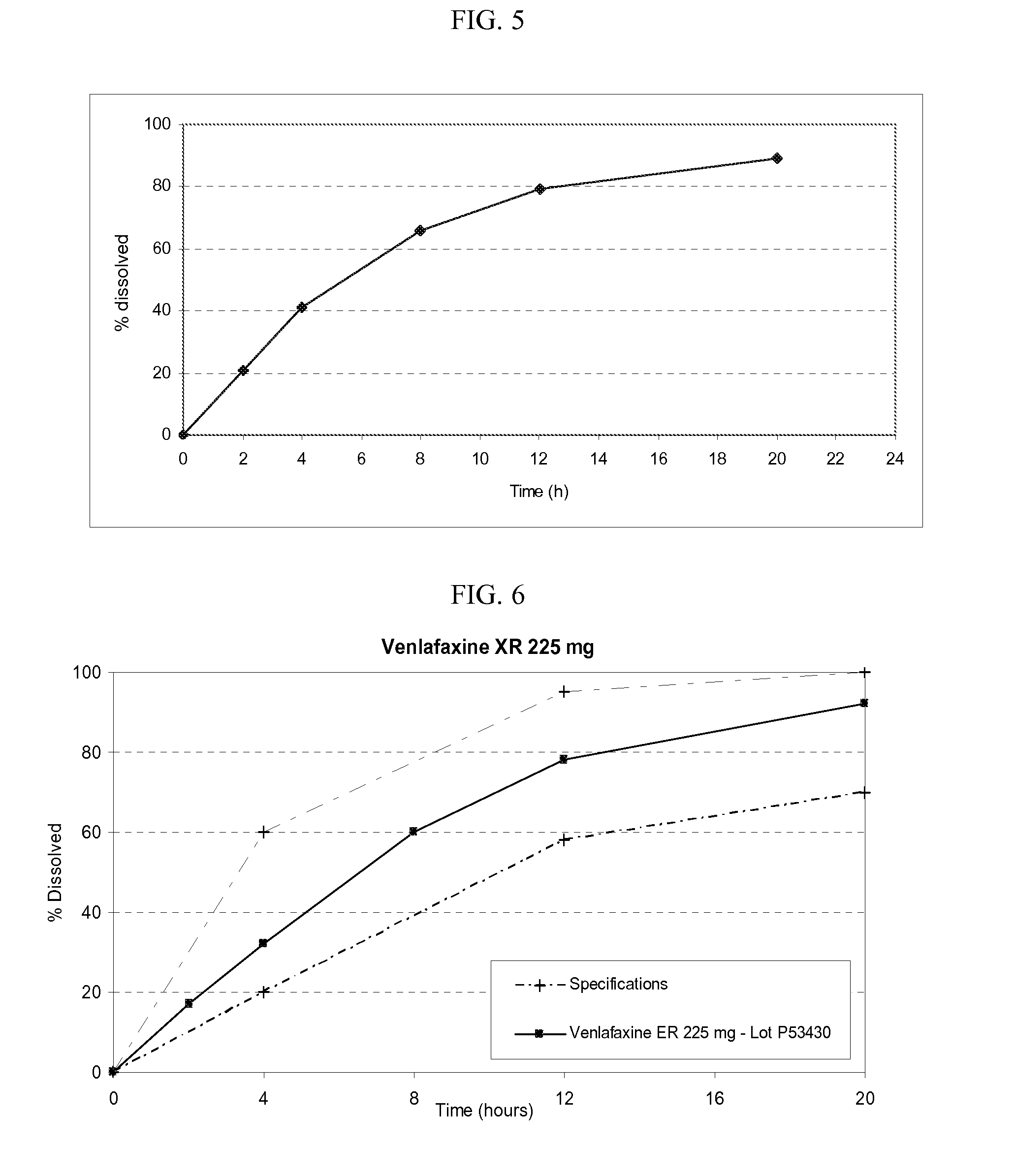
Venlafaxine osmotic device formulation
InactiveUS20070077301A1Reduced food effectReducing food effectBiocideOrganic active ingredientsImmediate releaseNeurological disorder
The present invention provides an osmotic device containing controlled release venlafaxine in the core, wherein the osmotic device exhibits a reduced food effect as compared to a reference controlled release capsule formulation. Some embodiments include venlafaxine in controlled release form in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's and / or Parkinson's patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Self-emulsifying formulations of fenofibrate and/or fenofibrate derivatives with improved oral bioavailability and/or reduced food effect
InactiveUS7022337B2Improve bioavailabilityBiocideCapsule deliveryTG - TriglyceridePolyethylene glycol
A fibrate self-emulsifying oral formulation with improved bioavailability when compared to commercially available formulations containing a therapeutically effective dose of fenofibrate, derivative of fenofibrate or mixtures thereof dissolved in a fibrate solubilizer selected from N-alkyl derivative of 2-pyrrolidone, mono- or di- or polyethylene glycol monoethers, C8-12 fatty acid mono- or di-esters of propylene glycol, or combinations thereof, one or more surfactants and optionally one or more stabilizers useful in the treatment of hypercholesterolaemia or hypertriglyceridaemia in mammals in the fed or fasted state.
Owner:SUPERNUS PHARM INC
Extended release formulation of levetiracetam
ActiveUS20070092569A1Reduced inter subject variabilityBiocideNervous disorderWater dispersibleExtended release tablets
The present invention relates to extended release pharmaceutical compositions of Levetiracetam and processes for preparing the same. The extended release tablet of Levetiracetam is with a core comprising of Levetiracetam and water dispersible rate controlling polymer, and the tablet core is optionally functional coated comprising a combination of water non-dispersible and / or water dispersible polymer. It provides extended therapeutically effective plasma levels over a twenty four hour period with diminished incidences of neuropsychiatric adverse events by eliminating the troughs and peaks of drug concentration in a patient's blood plasma. The composition also exhibits no food effect.
Owner:UCB PHARMA SA
Pharmaceutical compositions of nilotinib
ActiveUS20200261449A1Improve bioavailabilityReduced strength/dosePowder deliveryOrganic active ingredientsFOOD EFFECTOral medication
Amorphous solid dispersions of nilotinib fumarate or nilotinib tartrate are provided, as well as pharmaceutical compositions thereof, wherein the compositions exhibit enhanced bioavailability in the fasted state. Preferably, the compositions may be orally administered to a patient in either the fed or fasted state, with a decrease or elimination of the food effect. Preferably, following oral administration of the pharmaceutical compositions, there is no substantial difference in the pharmacokinetic parameters (e.g., Cmax, AUC0-t and / or AUC0-infinity) of nilotinib, regardless of whether the pharmaceutical compositions are administered to a subject in the fed or fasted state.
Owner:SLAYBACK PHARMA LLC
Purple corn paste and production method thereof
The invention relates to a purple corn paste and a production method thereof. The method comprises the following steps: mixing and crushing purple corn grains and frumentum accessories; spraying water and humidifying the crushed raw materials; then, carrying out puffing processing and cutting the major ingredient into short strips; crushing the puffed short strips into major ingredient powder; stir-frying flavor and nutrition accessories to ripeness and crushing the ripe accessories; evenly mixing the flavor and nutrition accessories and the major ingredient powder; carrying out drying and sterilization on the mixture; finally, adding seasoning and evenly mixing the mixture. The invention has the advantages that the purple corn paste is purely natural anthocyanin food, anthocyanin in the purple corn is the best variety in terms of effect among all plant anthocyanins and is superior to other anthocyanin food in terms of food effect; the purple corn paste contains a large amount of purple corn anthocyanin, thus being nutritious and safe to eat; the paste enjoys typical corn food flavor and achieves a good combination of color, aroma and taste; the paste is in line with the traditional dietary habit and meets the demand of mass consumption; the purple corn features high yield of raw material per unit area, low plantation cost and simple cultivation technique, and can be planted widely, thus enjoying abundant sources of the raw material and product processing features simple technology, easy operation, low cost and high economic benefit.
Owner:SHENYANG AGRI UNIV
Hydrochloric tamsulosin sustained-release capsule and its preparation method
ActiveCN101125134APrecise Controlled ReleaseControl releaseOrganic active ingredientsPharmaceutical delivery mechanismSide effectOral medication
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
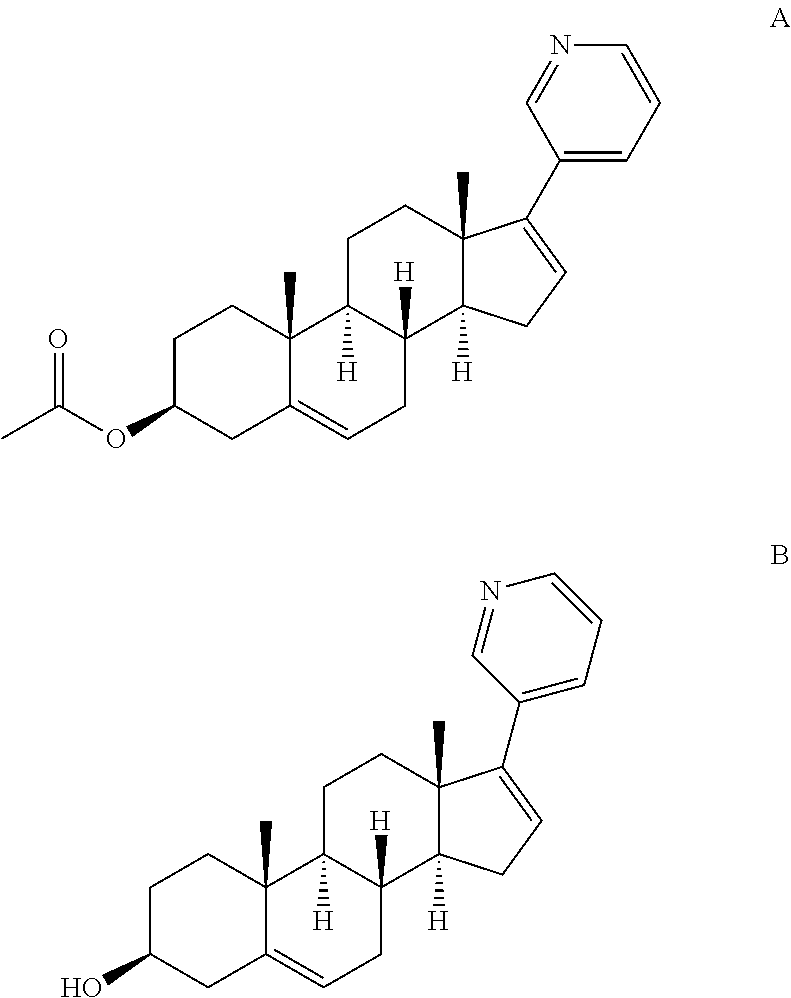
Complexes of abiraterone acetate, process for the preparation thereof and pharmaceutical compositions containing them
ActiveUS20160228455A1Improved physicochemical characteristicImprove biological performanceOrganic active ingredientsPowder deliveryFOOD EFFECTDose reduction
The present disclosure relates to pharmaceutically acceptable complex formulae comprising complexes of Abiraterone acetate and pharmaceutically acceptable excipients, process for the preparation thereof and pharmaceutical compositions containing them. The complex formulae of the present disclosure have improved physicochemical properties which results in reduced food effect which allows significant dose reduction and the abandoning of the requirement of taking the drug on an empty stomach.
Owner:TAVANTA THERAPEUTICS HUNGARY INC
Efficient roller-type traditional Chinese medicine drying equipment
InactiveCN107255399AImprove drying efficiencyImprove shock absorption and cushioning effectDrying gas arrangementsDrying chambers/containersFOOD EFFECTPulp and paper industry
The invention discloses a high-efficiency drum-type drying equipment for Chinese herbal medicines, which comprises a bottom plate, rollers and a drying cylinder. The drying cylinder is provided with a fixed groove, and a driven gear is arranged on the fixed groove. The driven gear The lower end of the drive gear is connected with a driving gear, the right end of the driving gear is connected with a rotating shaft, and the right end of the rotating shaft is connected with a motor. When the equipment is working, the medicinal materials first enter the drying cylinder from the feeding port, and the operation of the equipment is controlled by the controller. Driven by the motor, the drying cylinder rotates under the action of the driving gear and the driven gear, and the rotating fixed wheel starts to rotate. To the role of fixed rotation, and the fan continuously blows air into the drying cylinder, under the heating effect of the steam heater, the air in the drying cylinder becomes hot, forming a heating and drying effect on the grain, and with the rotation of the drying cylinder, The lifting board can continuously stir the grain, which is helpful to the drying effect of the grain and can improve the drying efficiency of the grain.
Owner:惠安县灿鑫新材料科技有限公司
Ramipril formulation
InactiveUS20070053975A1Fast absorptionGreat C max valueBiocideNervous disorderFOOD EFFECTPharmacology
A Ramipril formulation rapidly disintegrates after ingestion and exhibits substantially no food effect.
Owner:SELAMINE
Pharmaceutical compositions comprising lercanidipine
A controlled release pharmaceutical composition comprising lercanidipine dissolved or dispersed in a solid vehicle at ambient temperature, thus forming a solid dispersion, achieves delayed release of lercanidipine over an extended period of time, reduced food effect and increased bioavailability compared to commercially available lercanidipine containing products.
Owner:生命周斯药物公司
Pharmaceutical semi-solid composition of isotretinoin
InactiveUS20140107203A1Improve solubilityPromote absorptionBiocideHydroxy compound active ingredientsCholic acidFOOD EFFECT
The invention relates to an oral pharmaceutical composition of isotretinoin at least two excipients, one of the excipients being a hydrophilic excipient having an HLB value greater than or equal to 10 and the other excipient being an oily vehicle. The oral pharmaceutical composition is substantially devoid of food effect as characterized by a dissolution profile wherein at least 70% of the oral pharmaceutical composition is dissolved after about four hours in a USP2 dissolution apparatus at a paddle speed of 100 rpm, and a dissolution media composed of 900 mL of pH 7.5 buffer containing 0.11% pancreatin, 4.7% cholic acid, 0.14% sodium dihydroxide phosphate and 0.5% sodium hydroxide at 37° C.
Owner:GALEPHAR PHARMA RES
Controlled release compositions with reduced food effect
InactiveCN103037849AOrganic active ingredientsPeptide/protein ingredientsControlled releaseFOOD EFFECT
The present invention provides a controlled release pharmaceutical composition which exhibits reduced food effect.
Owner:TWI PHARMA
Pharmaceutical compositions of nilotinib
ActiveUS10874671B2Improve bioavailabilityReduced strength/dosePowder deliveryOrganic active ingredientsFOOD EFFECTOral medication
Amorphous solid dispersions of nilotinib fumarate or nilotinib tartrate are provided, as well as pharmaceutical compositions thereof, wherein the compositions exhibit enhanced bioavailability in the fasted state. Preferably, the compositions may be orally administered to a patient in either the fed or fasted state, with a decrease or elimination of the food effect. Preferably, following oral administration of the pharmaceutical compositions, there is no substantial difference in the pharmacokinetic parameters (e.g., Cmax, AUC0-t and / or AUC0-infinity) of nilotinib, regardless of whether the pharmaceutical compositions are administered to a subject in the fed or fasted state.
Owner:SLAYBACK PHARMA LLC
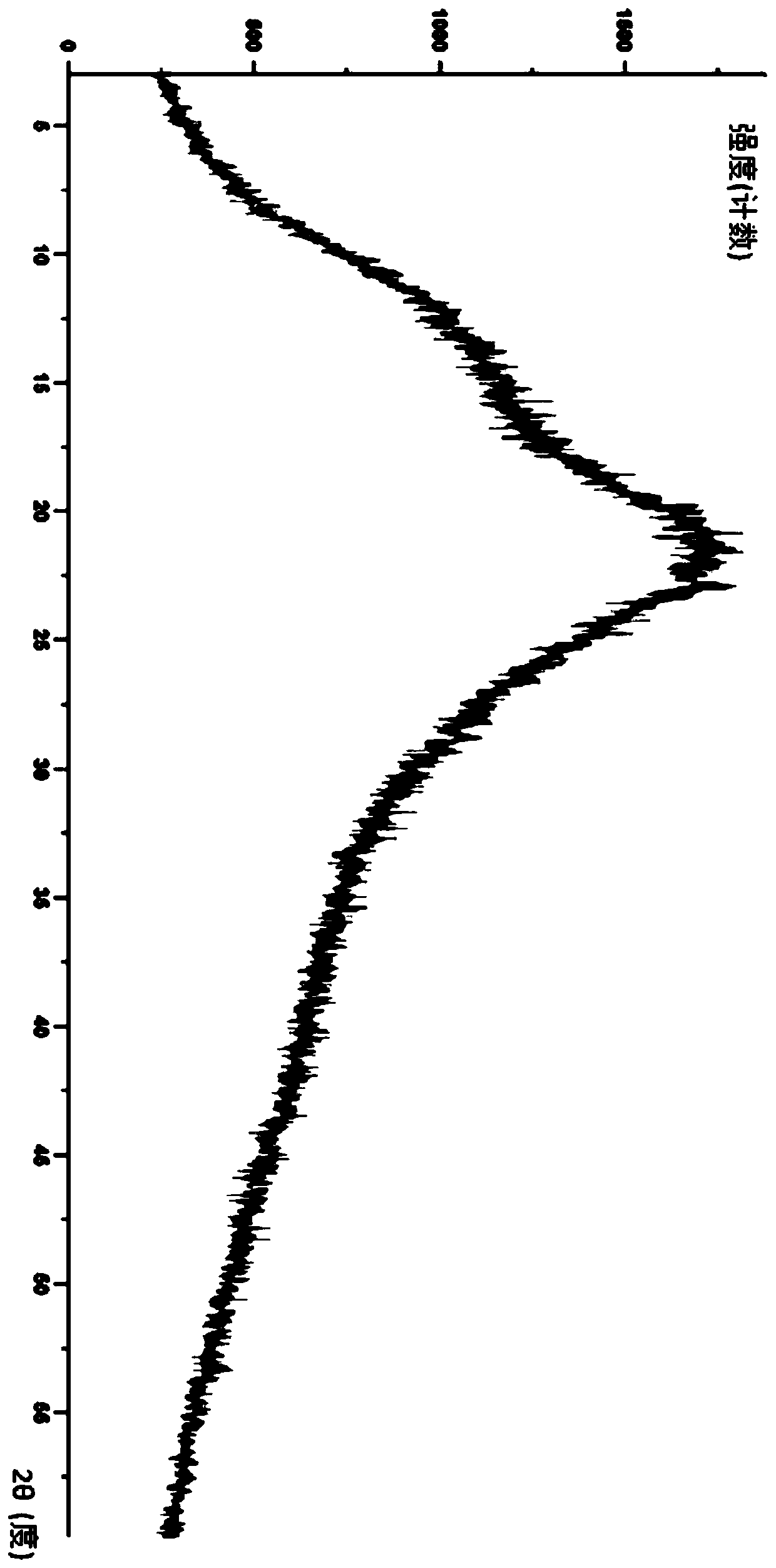
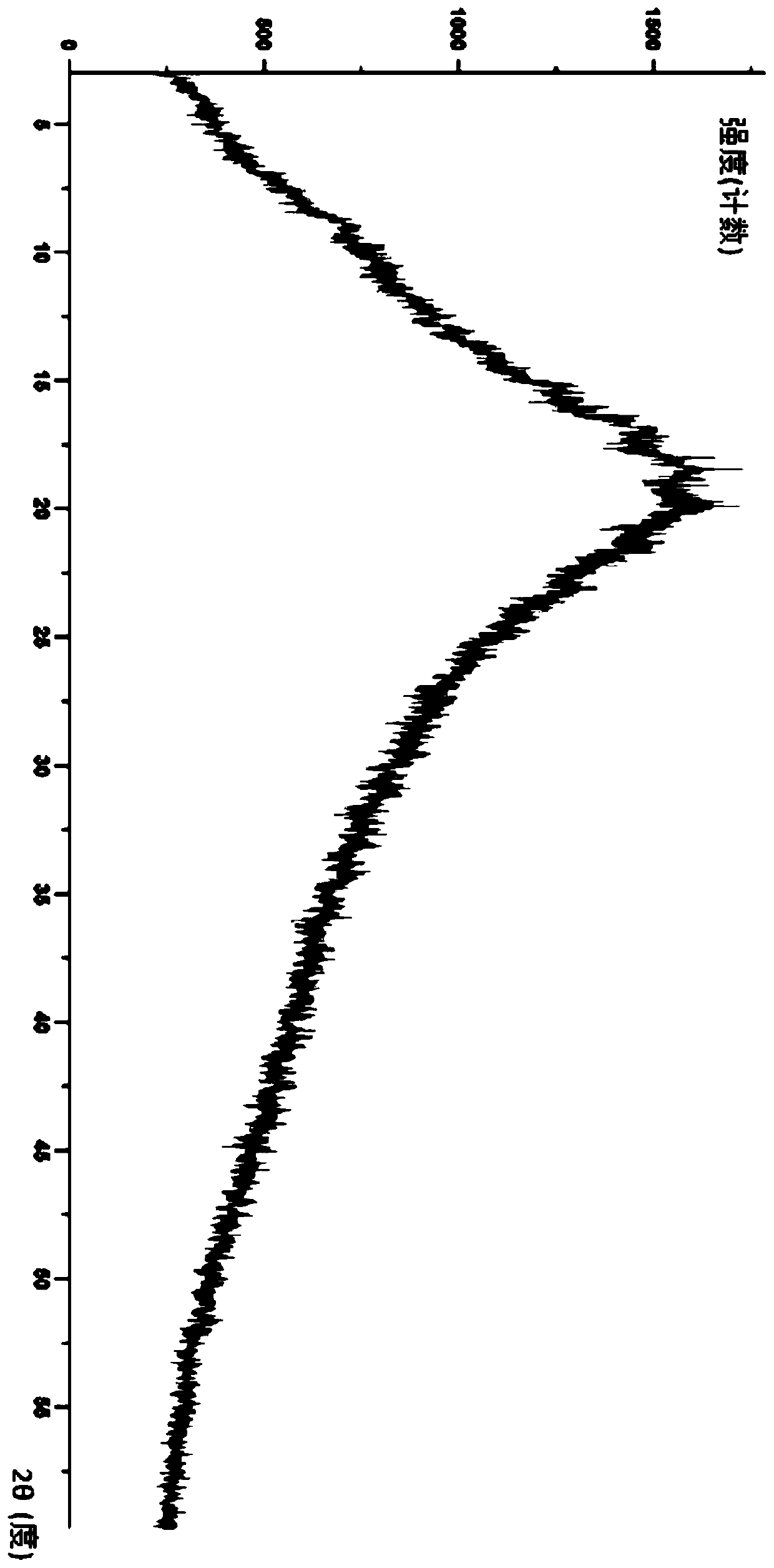
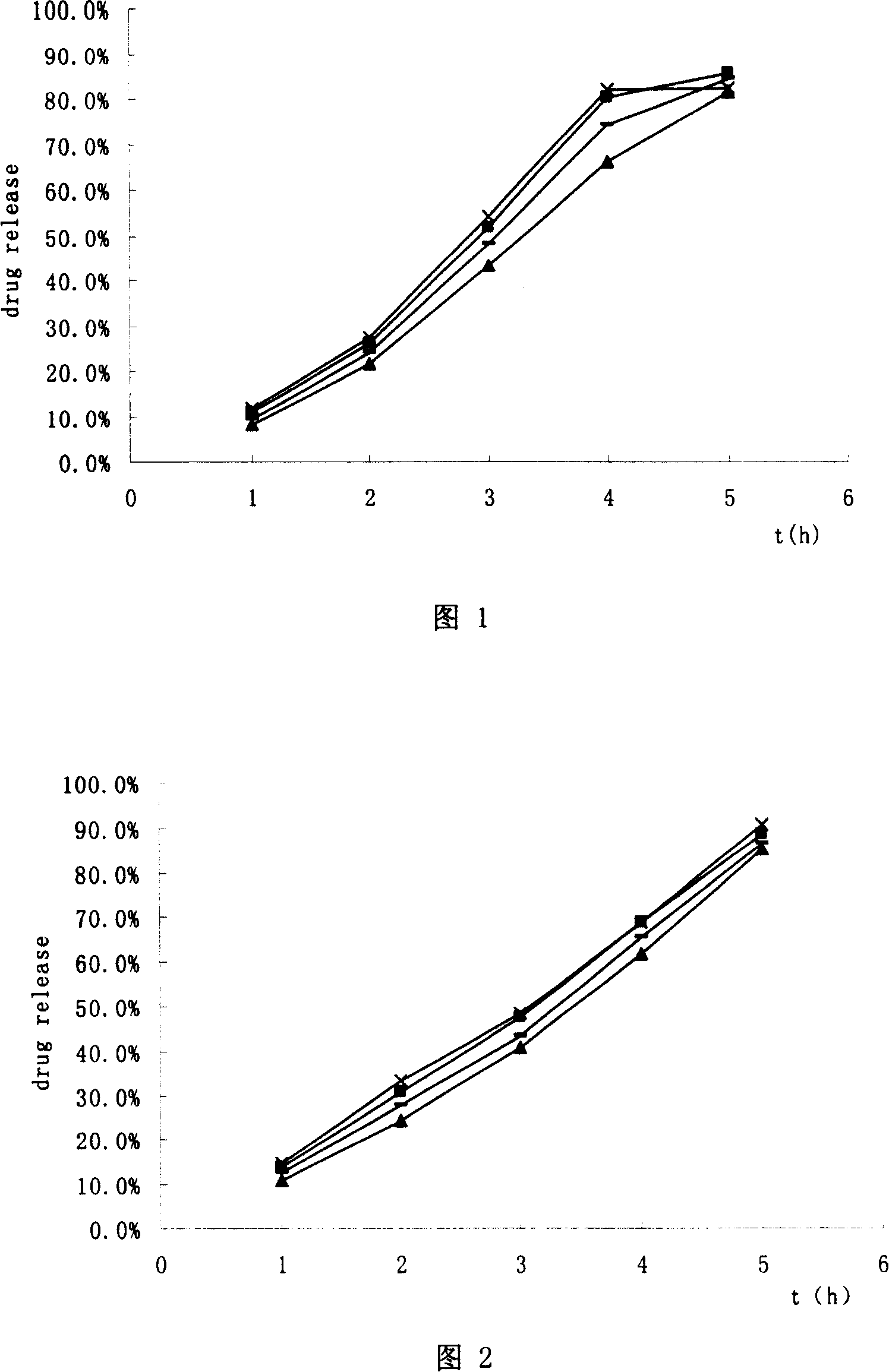
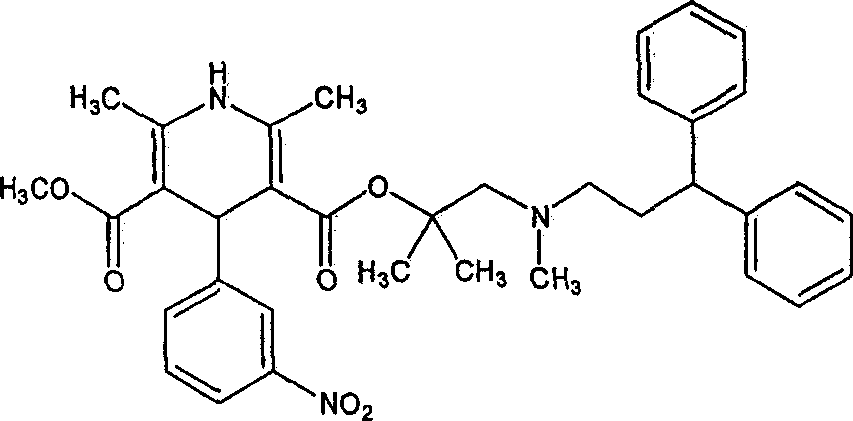
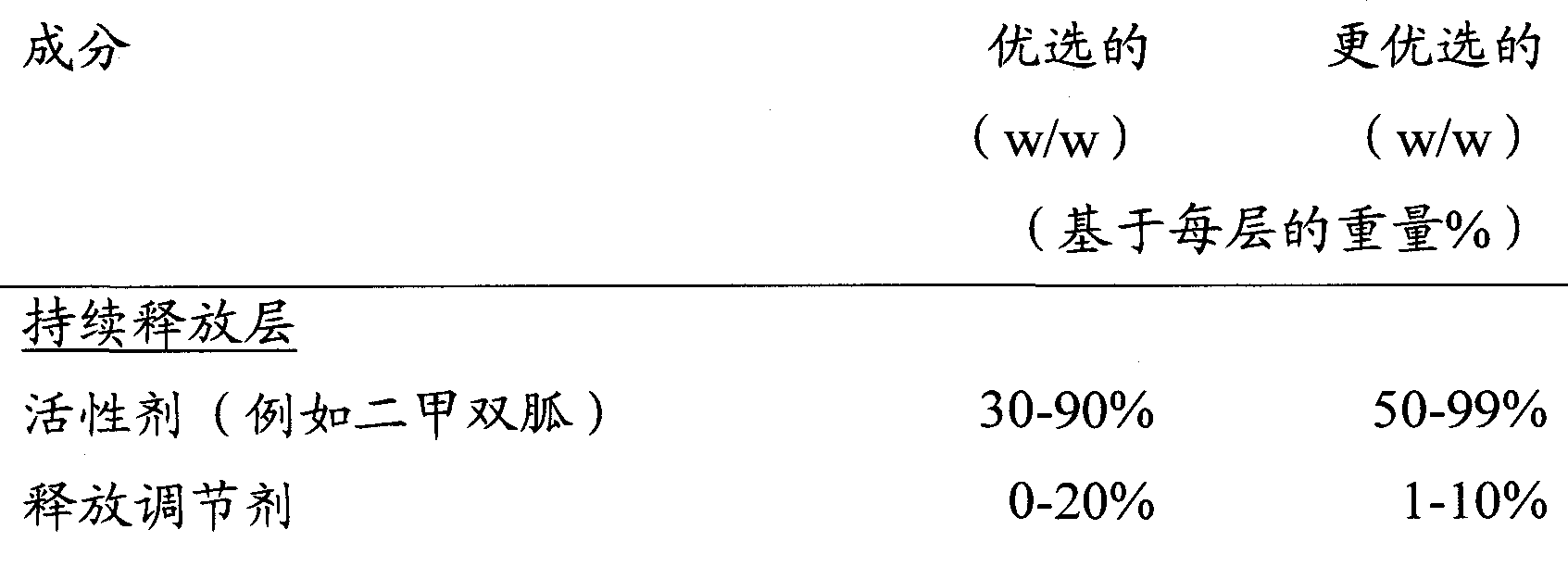
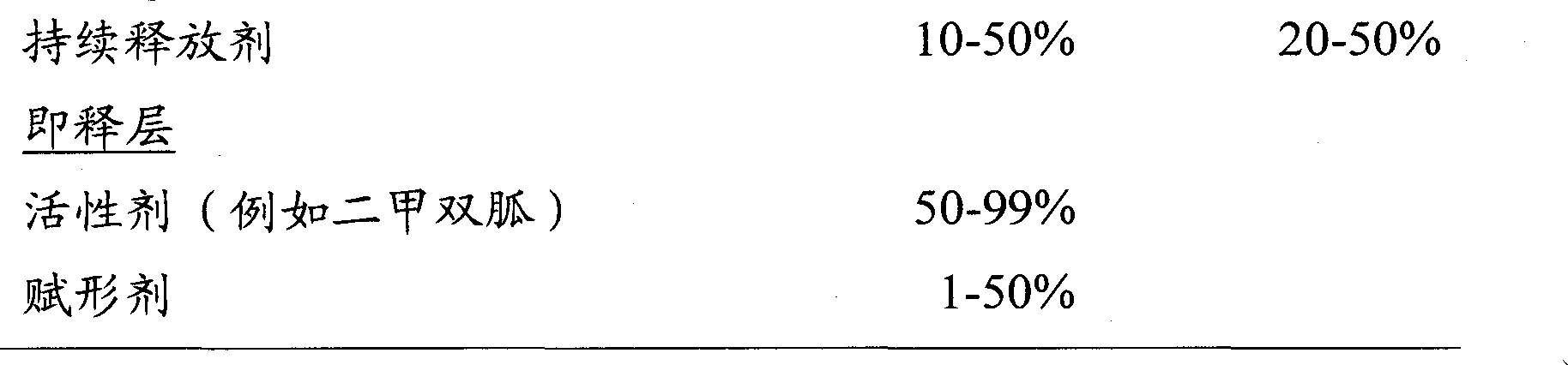
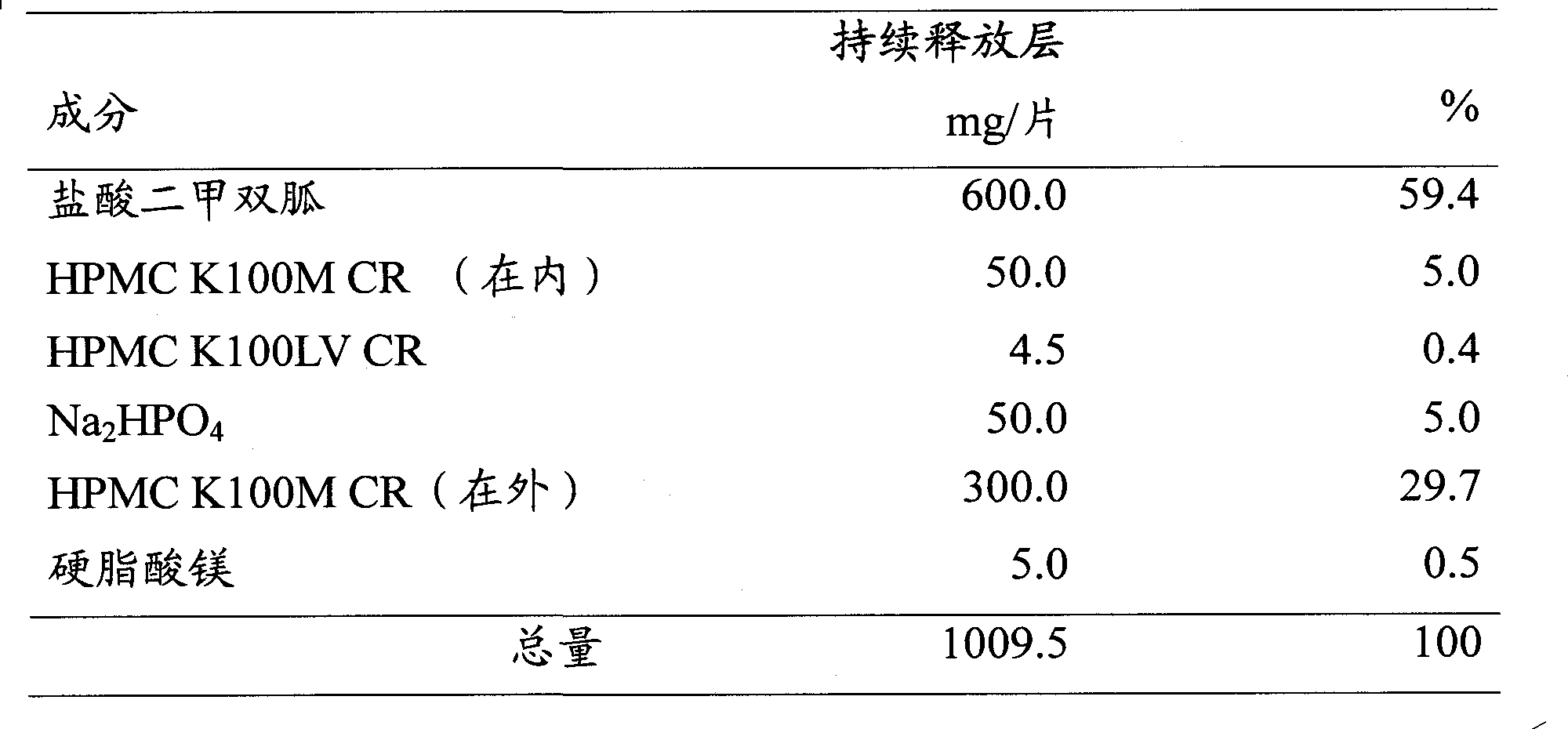
Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids
Soluble pharmaceutical compositions of amorphous nilotinib or a pharmaceutically acceptable salt thereof were invented using one or more organic acids that function as a solubilizing agent, increasing the bioavailability of nilotinib and supressing the food effect associated with certain compositions of nilotinib. The pharmaceutical compositions are in th form of solid oral dosage forms, including capsules and tablets.
Owner:NOVARTIS AG
Plant enzymatic cooking wine and preparation method thereof
InactiveCN104012924ARegulate acid-base balanceImprove immunityAlcoholic beverage preparationFood ingredient functionsMedicinal herbsFOOD EFFECT
The invention relates to the technical field of fermented food processing, and in particular relates to a plant enzymatic cooking wine and a preparation method of the plant enzymatic cooking wine. The cooking wine is obtained by fermenting plant stems, medicinal herbs and edible flavoring plants. The plant enzymatic cooking wine and the preparation method of the plant enzymatic cooking wine are scientific in formula, simple in technology and low in production cost; all the plants are combined and fermented, the plant enzymatic cooking wine does not contain additive and contains medicinal components, and the vitamins, mineral substances, amino acids and various enzymes beneficial to the human body in the plant raw materials are protected to the utmost extent, so that the food effects and the use safety are greatly improved; the plant enzymatic cooking wine has the functions of balancing the nutrition and the five internal organs, is used for seasoning the dishes which are eaten in the daily life, and has the effects of adjusting the acid-base balance of the human body, eliminating fatigue, reducing cholesterol and improving the immunity of the human body.
Owner:ANHUI WEIXIAN FOOD
Pharmaceutical semi-solid composition of isotretinoin
InactiveUS9078925B2Improve oral bioavailabilityLow absolute bioavailabilityHydroxy compound active ingredientsCapsule deliveryCholic acidFOOD EFFECT
The invention relates to an oral pharmaceutical composition of isotretinoin at least two excipients, one of the excipients being a hydrophilic excipient having an HLB value greater than or equal to 10 and the other excipient being an oily vehicle. The oral pharmaceutical composition is substantially devoid of food effect as characterized by a dissolution profile wherein at least 70% of the oral pharmaceutical composition is dissolved after about four hours in a USP2 dissolution apparatus at a paddle speed of 100 rpm, and a dissolution media composed of 900 mL of pH 7.5 buffer containing 0.11% pancreatin, 4.7% cholic acid, 0.14% sodium dihydroxide phosphate and 0.5% sodium hydroxide at 37° C.
Owner:GALEPHAR PHARMA RES
Lurasidone solid dispersion and preparation method thereof
It relates to a lurasidone solid dispersion and a preparation method, wherein the method comprises melting treatment of a mixture containing lurasidone, a medicinal hot melt carrier, optionally an acidic regulator and plasticizer in order to obtain the solid dispersion described herein, and wherein the lurasidone is provided in a form of free base. The lurasidone solid dispersion obtained by the preparation method according to the example of the invention has the characteristics of high dissolution rate (dissolution rate can reach 30%-70%) in a partial neutral medium (e.g. pH 6.0). The bioavailability of lurasidone solid dispersion increased significantly and the food effect of lurasidone solid dispersion prepared from the example decreased remarkably. It overcomes the limitation of too many medication in the prior art and avoids the reduction of curative effect of the improper medication for the patient or even invalid, ensures the normal efficacy, thereby increases the patient's medication flexibility and compliance.
Owner:SUNSHINE LAKE PHARM CO LTD
Chinese yam and buckwheat biscuit having healthcare effect on diabetes
InactiveCN103783119AIncrease appetiteAchieve the effect of integrationBakery productsCooking & bakingDiabetes mellitus
The invention provides a Chinese yam and buckwheat biscuit having the healthcare effect on the diabetes. According to the biscuit, after Chinese yam powder and buckwheat powder are mixed according to the weight ratio of 1:1-4, baking is performed according to a conventional biscuit making method, and then the biscuit can be made and packaged. According to the biscuit, the Chinese yam and the buckwheat which have both the medicine effect and the food effect are adopted as components and mixed reasonably, the obtained Chinese yam and buckwheat biscuit having the healthcare effect on the diabetes is formed by ingeniously combining pure medicine and food, the biscuit has great advantages for reconstructing a glucostasis system of a human body, the appetite of a diabetes patient can be increased through biological and chemical reaction in the human body, meanwhile the glucose index can also be reduced, the biscuit can be taken for a long time, no toxic and side effect exists, the effect of integrating food, healthcare products and medicine is achieved, the medicine making method of traditional Chinese medicine is carried forward continuously, the making method is simple, raw materials are easy to obtain, cost is low, and the curative effect is good.
Owner:牛正华
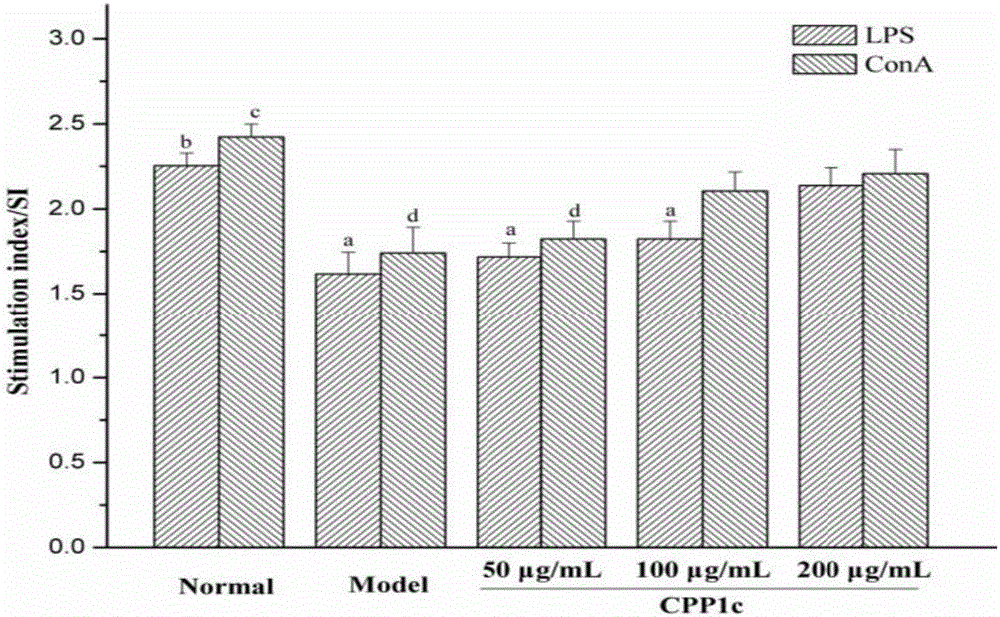
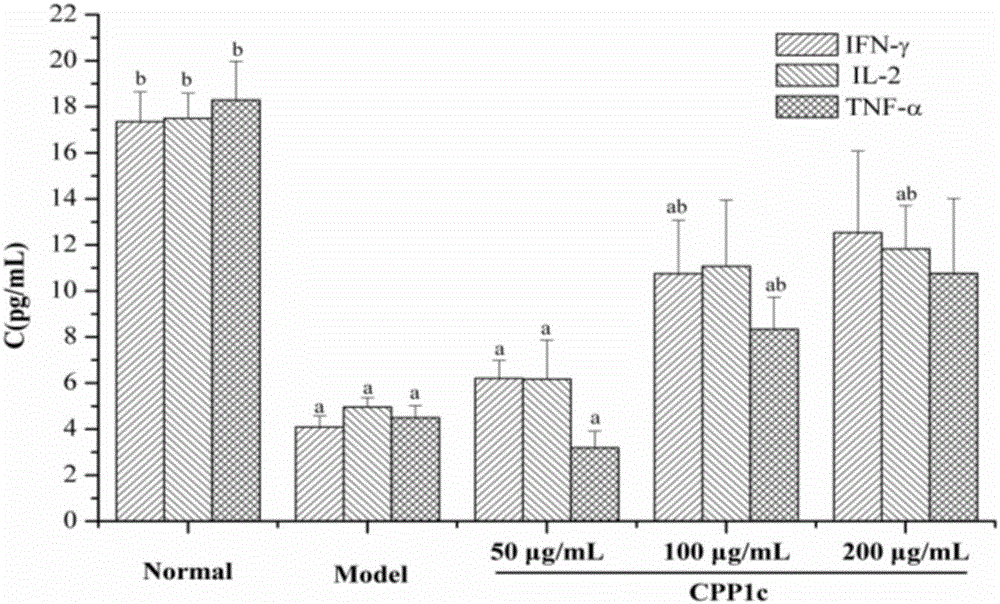
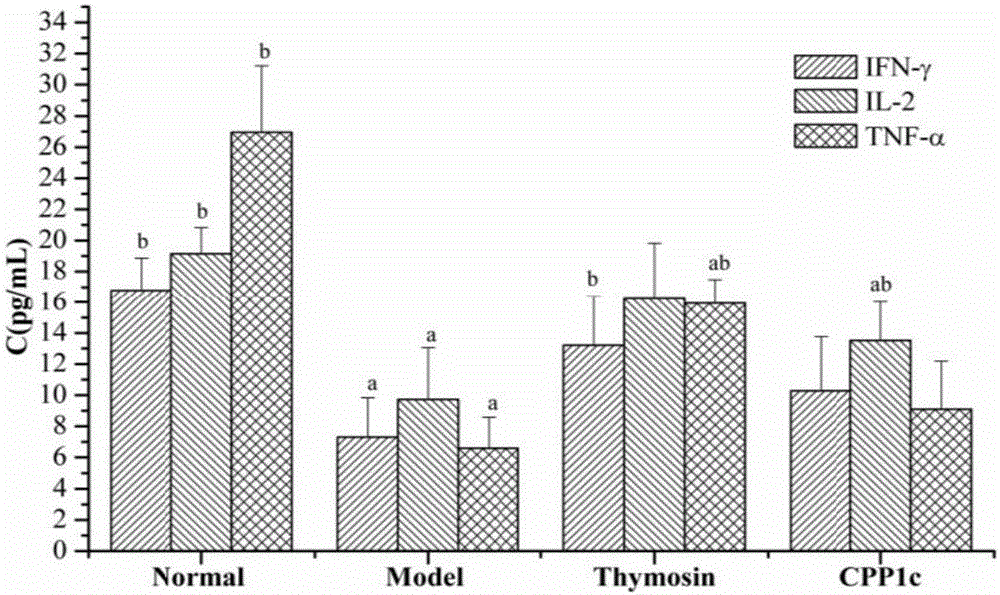
Application of codonopsis pilosula pectic polysaccharides (CPP1c) and drug and health product thereof
ActiveCN106727624APromote proliferationPromote secretionOrganic active ingredientsImmunological disordersLymphocyte proliferationFOOD EFFECT
The invention provides application of codonopsis pilosula pectic polysaccharides (CPP1c) and a drug and a health care product thereof, and belongs to the field of biomedical engineering. The codonopsis pilosula pectic polysaccharides (CPP1c) can promote the proliferation of lymphocyte, improve the activity of lymphocyte, promote the expression of immune related molecules and related gene and promote the homing of lymphocyte. The codonopsis pilosula pectic polysaccharides (CPP1c) have high medicine value and are high in health care function; the drug and the health care product prepared by the codonopsis pilosula pectic polysaccharides (CPP1c) have food effect and are worthy of popularization and application.
Owner:LANZHOU UNIVERSITY
Nitrendipine sustained-release preparation and preparation method thereof
InactiveCN101683336AOrganic active ingredientsPharmaceutical delivery mechanismFOOD EFFECTSustained release drug
The invention discloses a sustained-release drug composite containing nitrendipine, which is composed of nitrendipine with effective dose and accessories accepted physiologically; the nitrendipine sustained-release drug composite can release the nitrendipine in a sustained way, the food effect is reduced and the bioavailability is increased.
Owner:COSCI MED TECH CO LTD
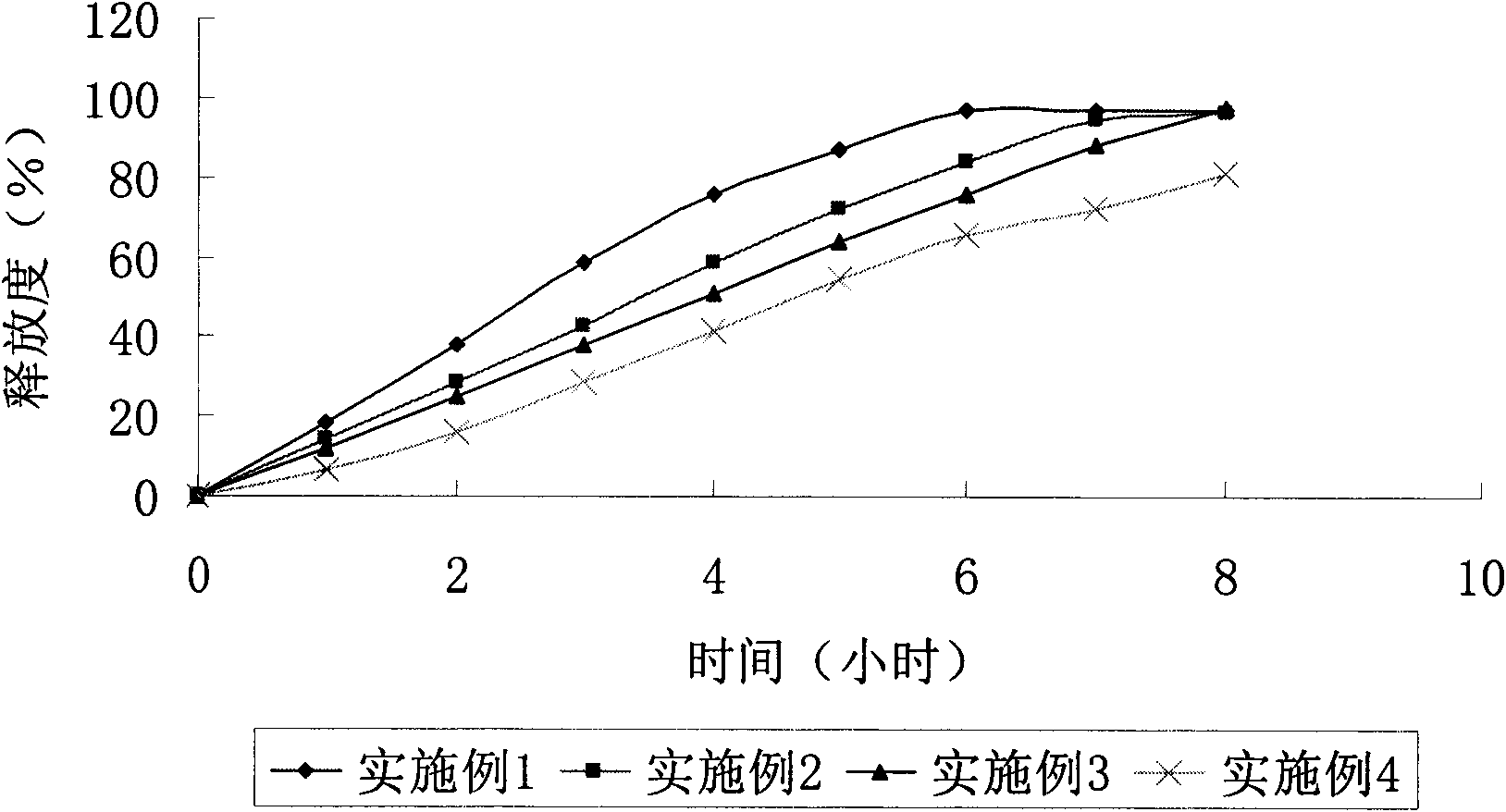
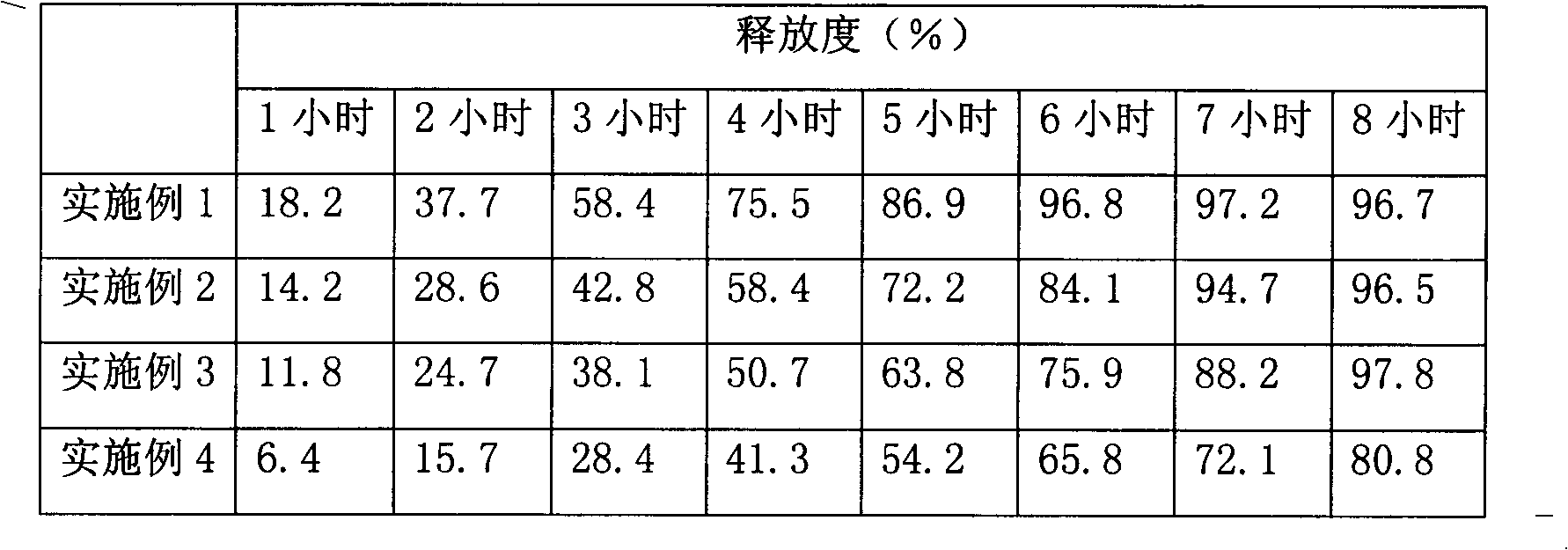
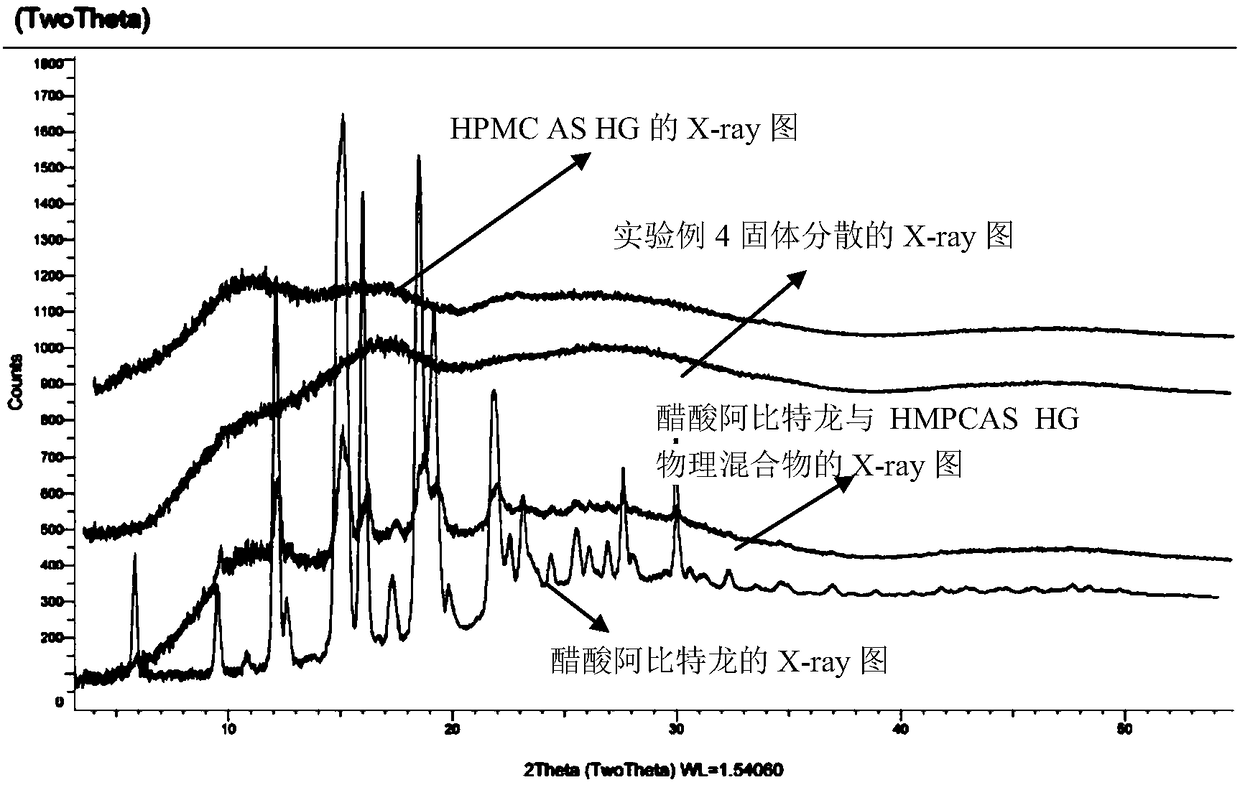
Solid dispersion and preparation method thereof
The invention relates to a solid dispersion and a preparation method of the solid dispersion, in particular to the solid dispersion. The solid dispersion contains abiraterone or a derivative of the abiraterone and a carrier material HPMCAS, wherein DS<Ac> in HPMCAS is not greater than 0.50, and DS<Ac>+DS<s> is not smaller than 0.83, The preparation prepared by the solid dispersion system has gooddissolution and stability, and can eliminate individual differences after patients take medicines and food effects and the like caused by fasting and satiety administration to some extent.
Owner:SUNCADIA PHARM CO LTD
Substitutional tea with effects of loosening bowel to relieve constipation, melting turbidity and reducing lipid
The invention discloses substitutional tea with the effects of loosening the bowel to relieve constipation, melting turbidity and reducing lipid. The substitutional tea is characterized in that the tea is prepared from bunge cherry seeds, semen cassia, almonds, peach seeds, lotus leaves and parched hawthorn fruit, raw materials are cleaned and processed into a powder extract, and the powder extract is matched with black sesame honey powder, and the substitutional tea is prepared through the steps of mixing, pelletizing and packing. According to the substitutional tea with the effects of loosening the bowel to relieve constipation, a Chinese medical theory is exerted, food materials with the homology of medicine and food are chosen for scientific matching, in the formula, the bunge cherry seeds can loosen the bowel to relieve constipation, descend qi and disinhibit water, semen cassia can remove liver-fire for improving eyesight, and loosen the bowel to relieve constipation, the two components have the effects of inhibiting depletion of fluid causing intestinal dryness, food accumulation and qi depression and abdominal distension and constipation, almonds, peach seeds, black sesame and honey can moisten the intestines and relax the bowels, lotus leaves and parched hawthorn fruit have the effects of reducing lipid, the medical and food effects of all the matched components have complementary advantages, and jointly have the effects of moistening the intestines and relaxing the bowels, melting turbidity and reducing lipid, and it is proved by senior herbalist doctors and consumer crowds through long-term application that the substitutional tea with the effects of loosening the bowel to relieve constipation, melting turbidity and reducing lipid is not only good in effect, but also low in price, convenient to drink and good in mouthfeel.
Owner:山东岐伯堂生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00001.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00002.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-[5-(4-methyl-1h-imidazol-1-yl)-3-(triflouoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/875af486-3c8c-4b61-8e3c-c06c6a1a32f3/US20150273070A1-20151001-D00003.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000011.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000021.PNG)
![Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids Modified release of 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-n-5-(4-methyl-1h-imidazol-1-yl)-3-(trifluoromethyl)phenyl] benzamide solubilized using organic acids](https://images-eureka.patsnap.com/patent_img/fc3b0caa-94ba-475c-ad99-cceb5f1e418f/HDA0000438795460000022.PNG)