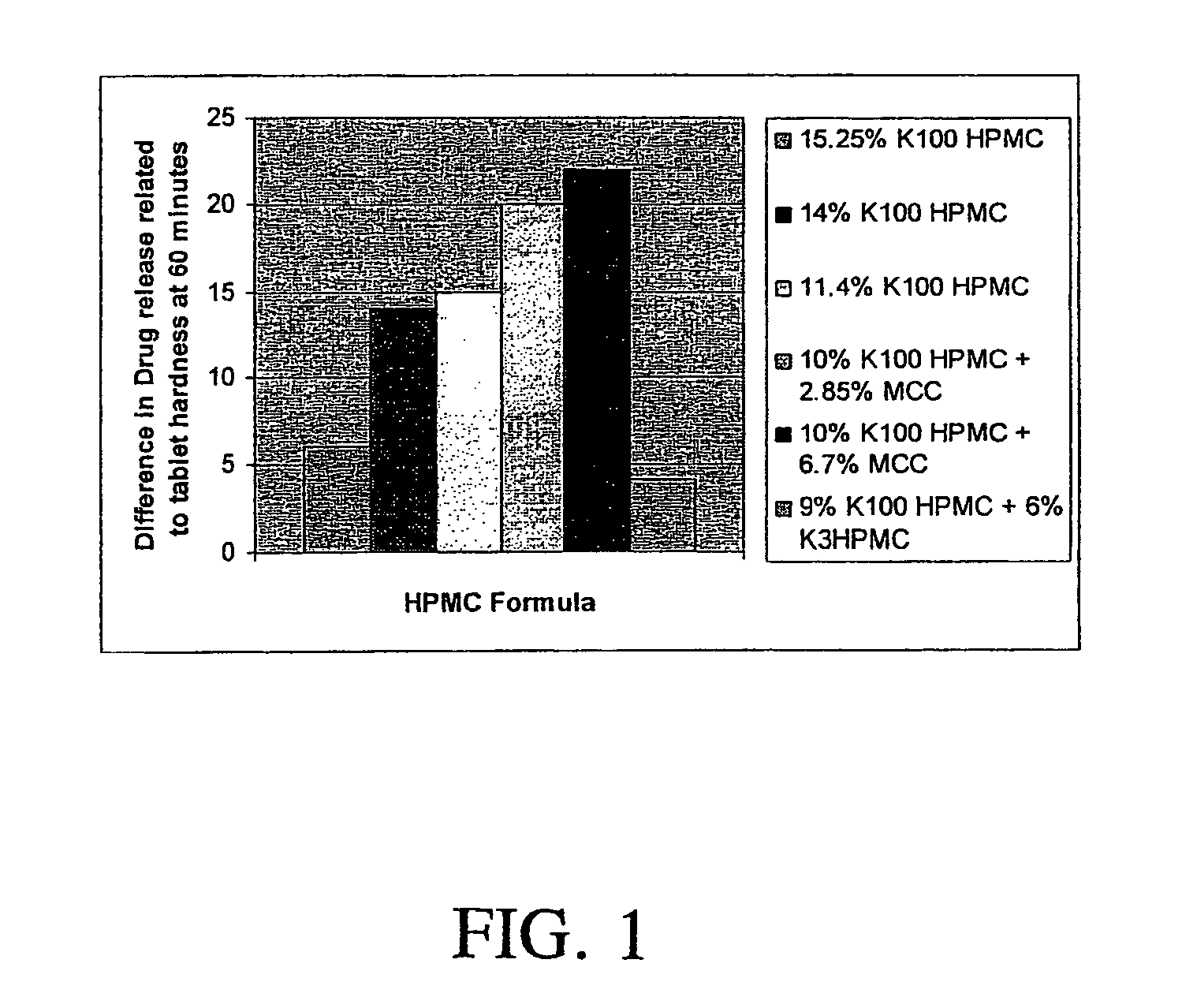
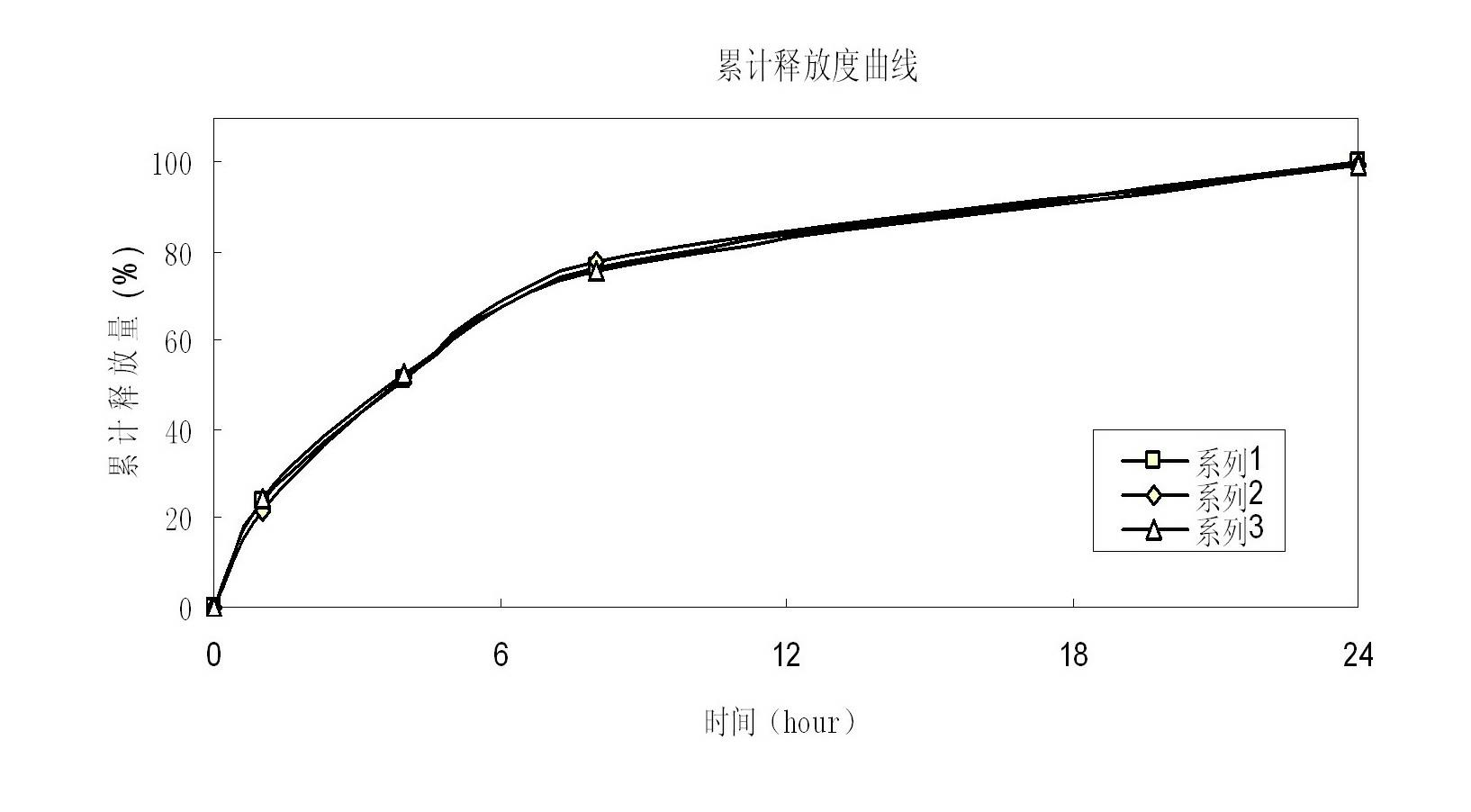
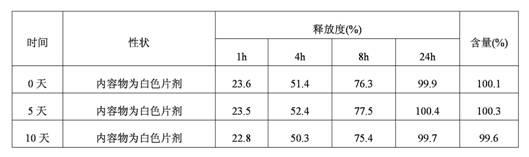
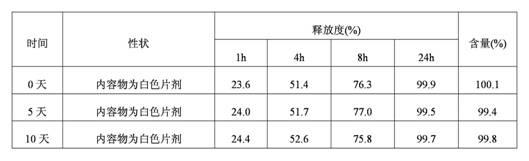
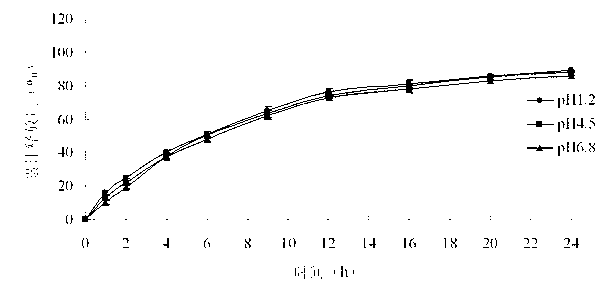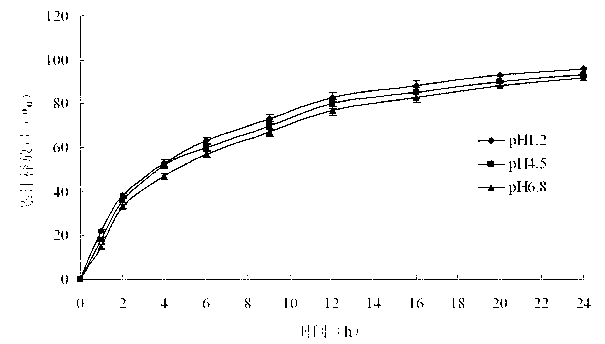Patents
Literature
178 results about "Extended release tablets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
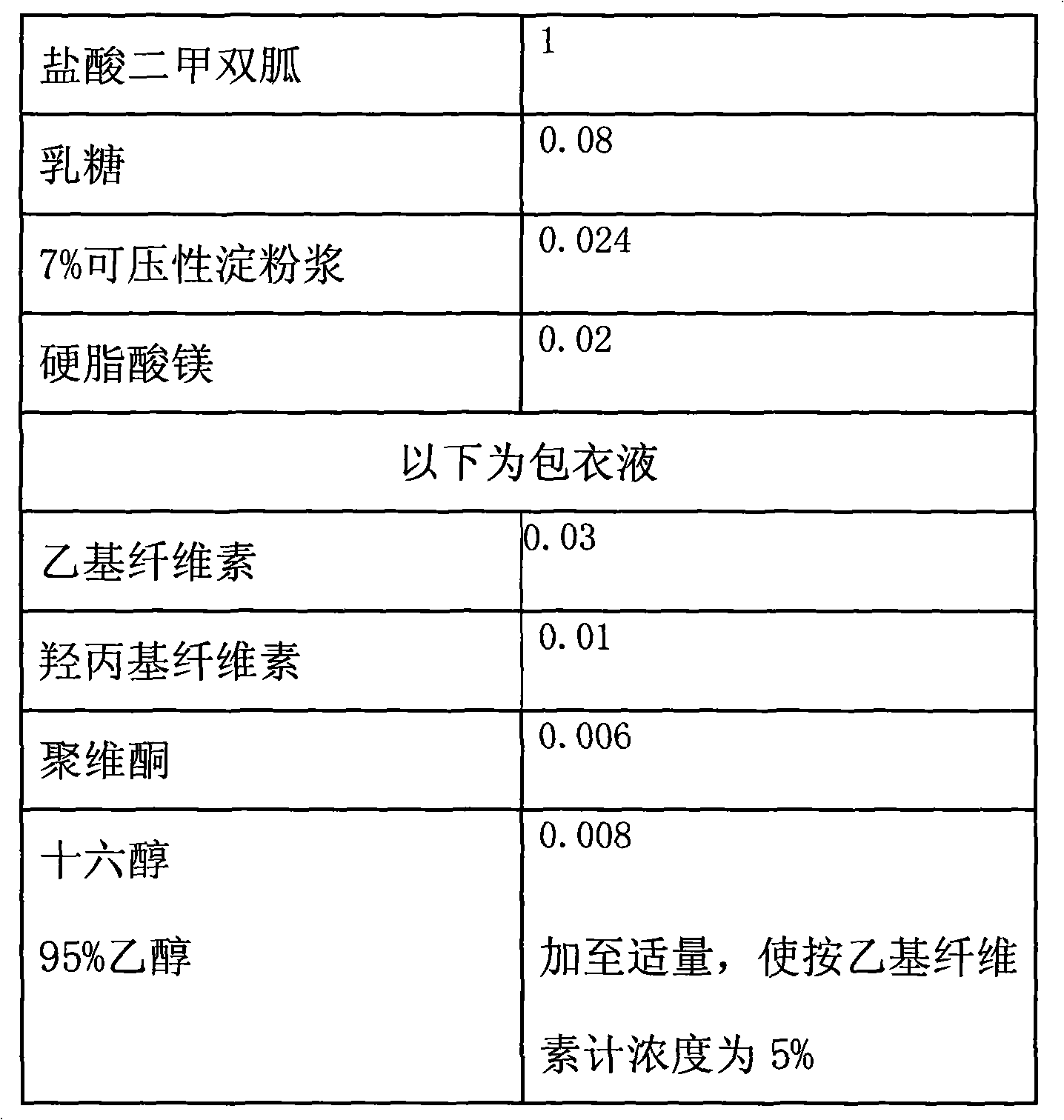
Metformin hydrochloride extended-release tablets, USP is a white to off-white crystalline compound with a molecular formula of C 4 H 11 N 5 • HCl and a molecular weight of 165.63. Metformin hydrochloride extended-release tablets, USP is freely soluble in water and is practically insoluble in acetone, ether, and chloroform.
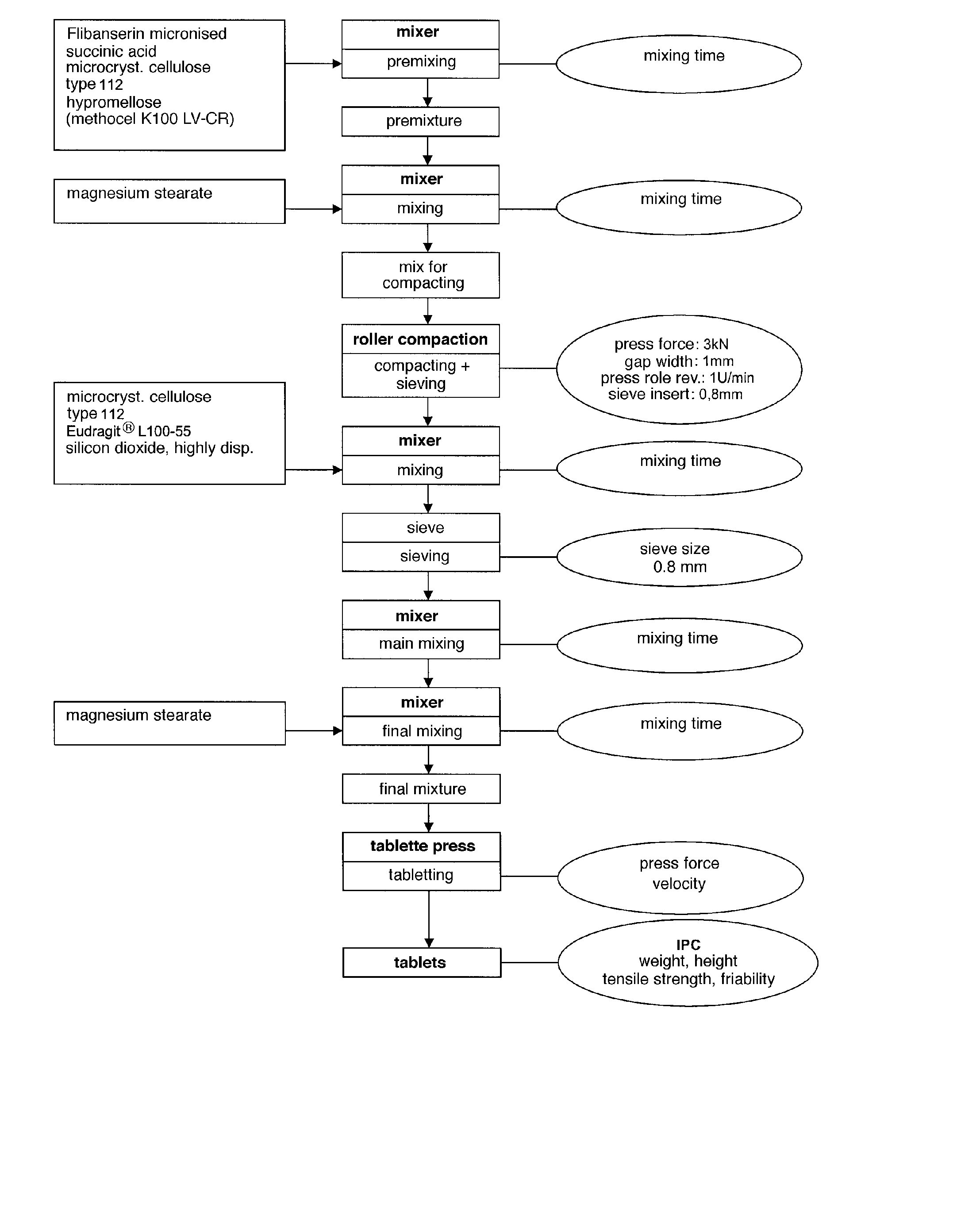
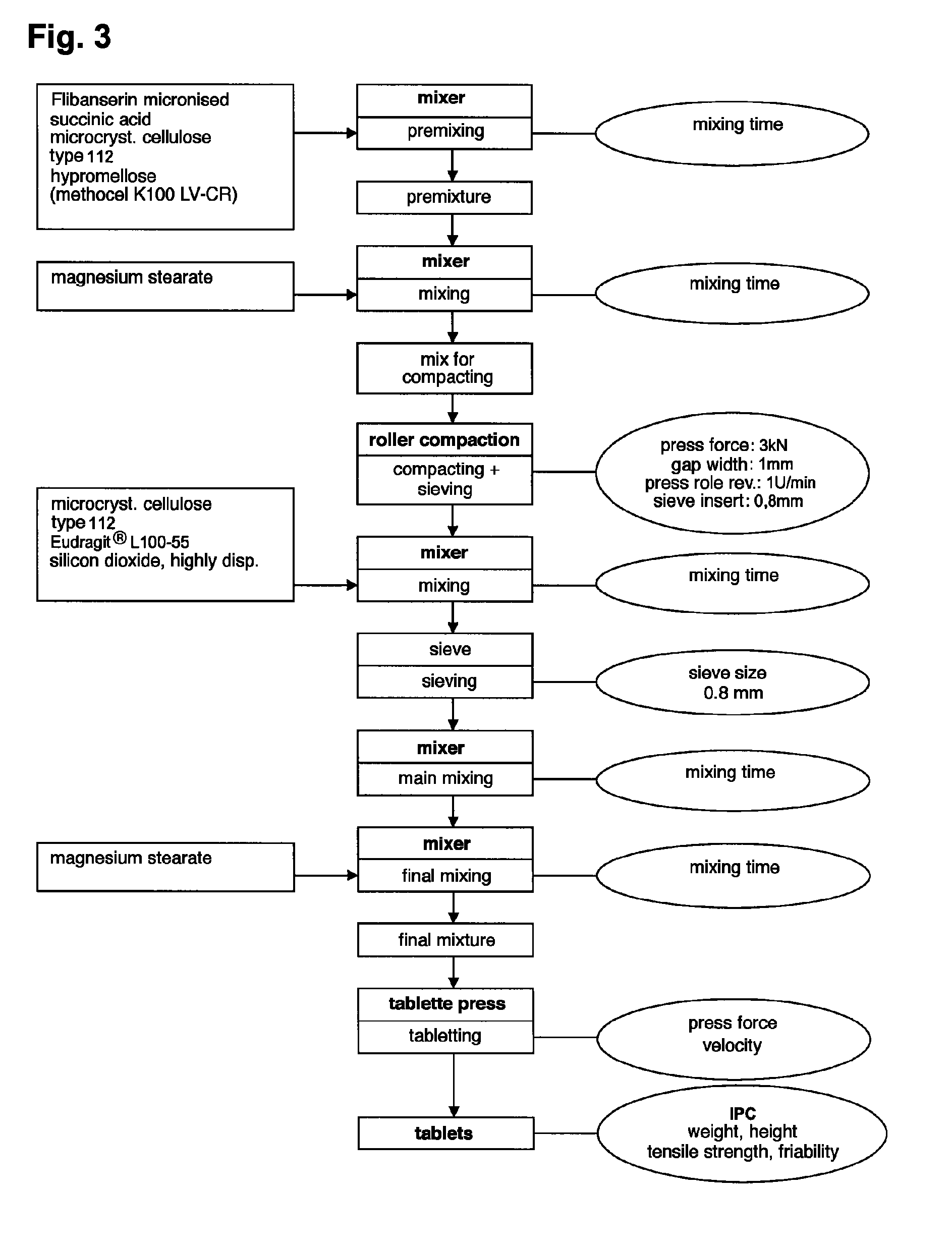
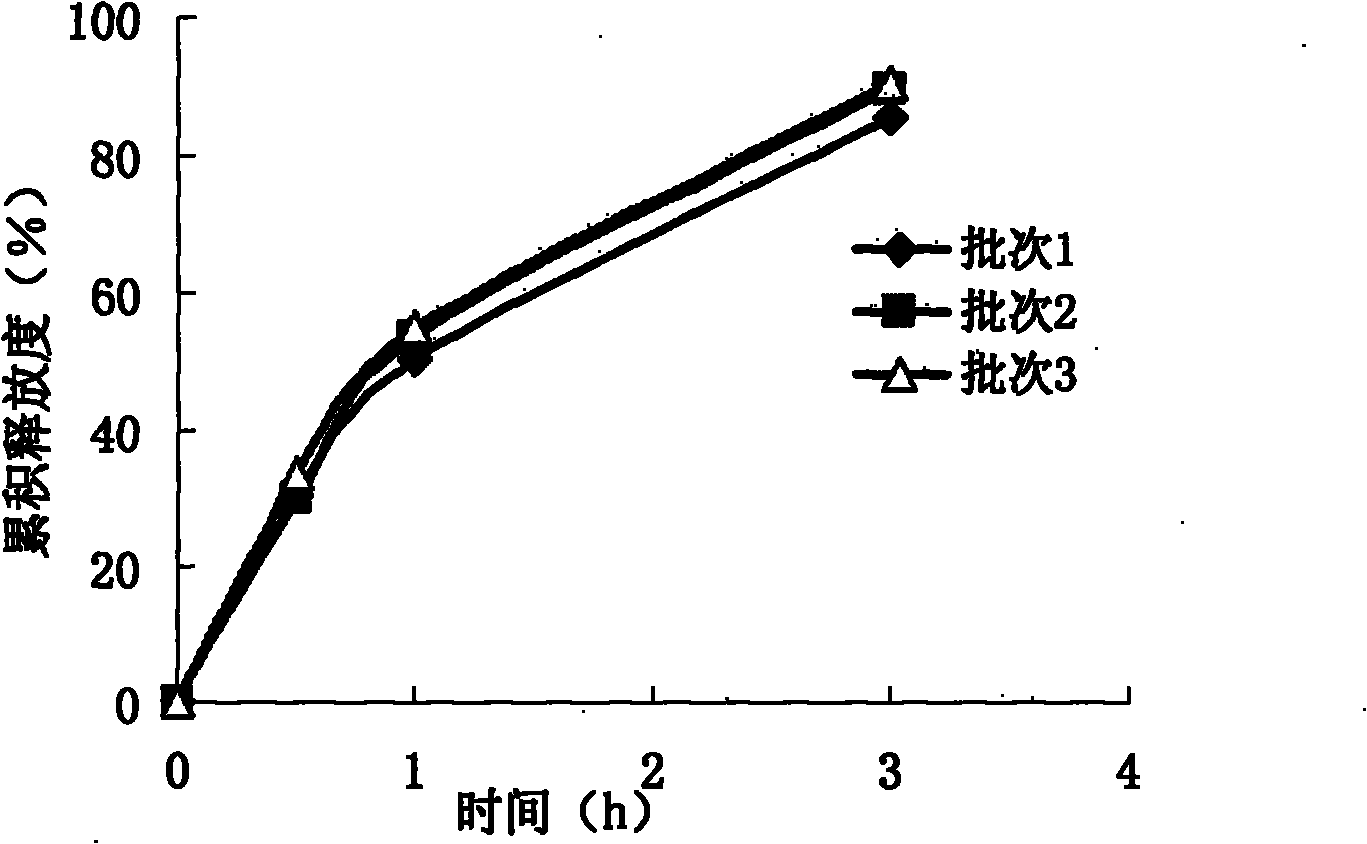
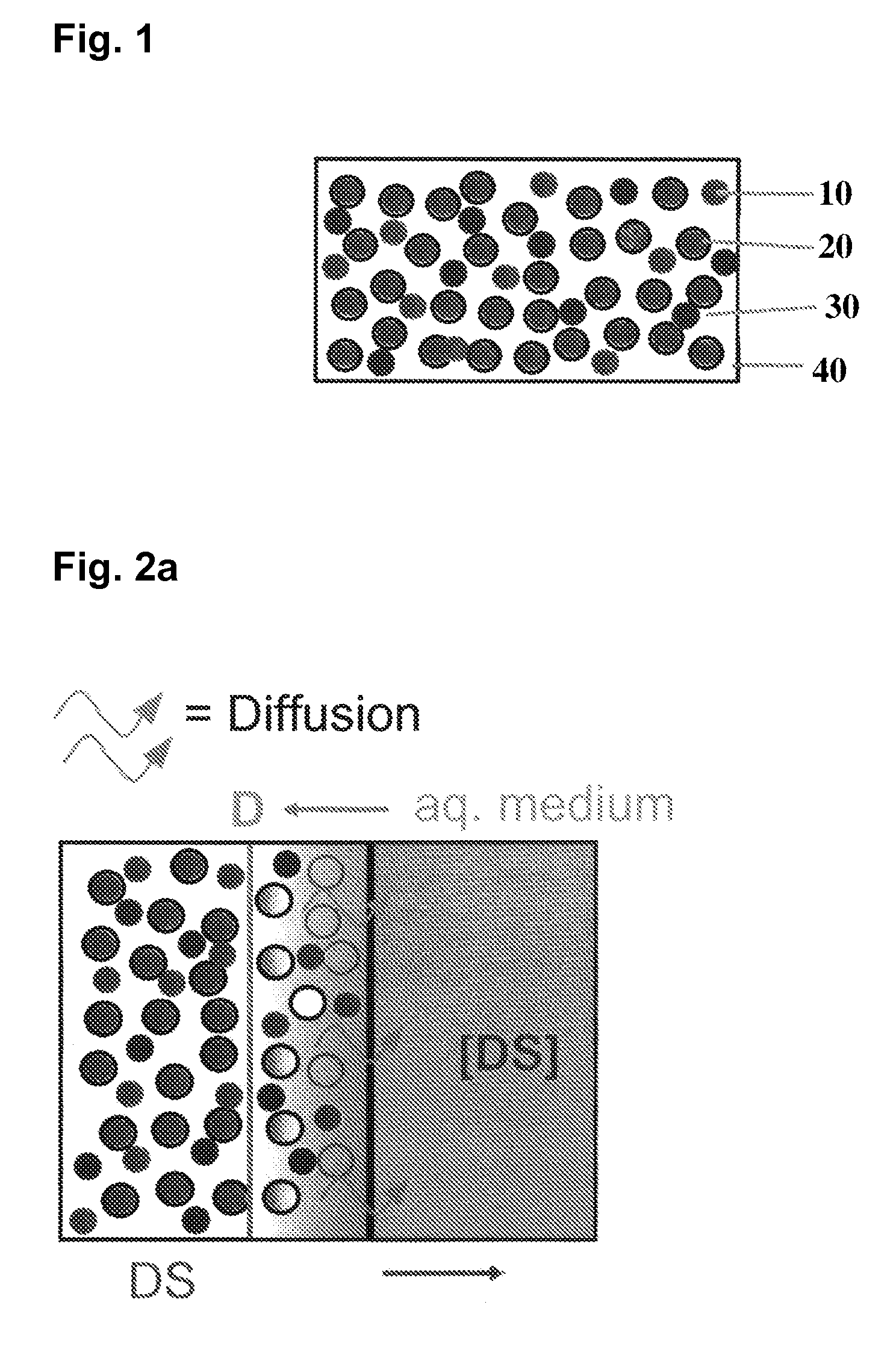
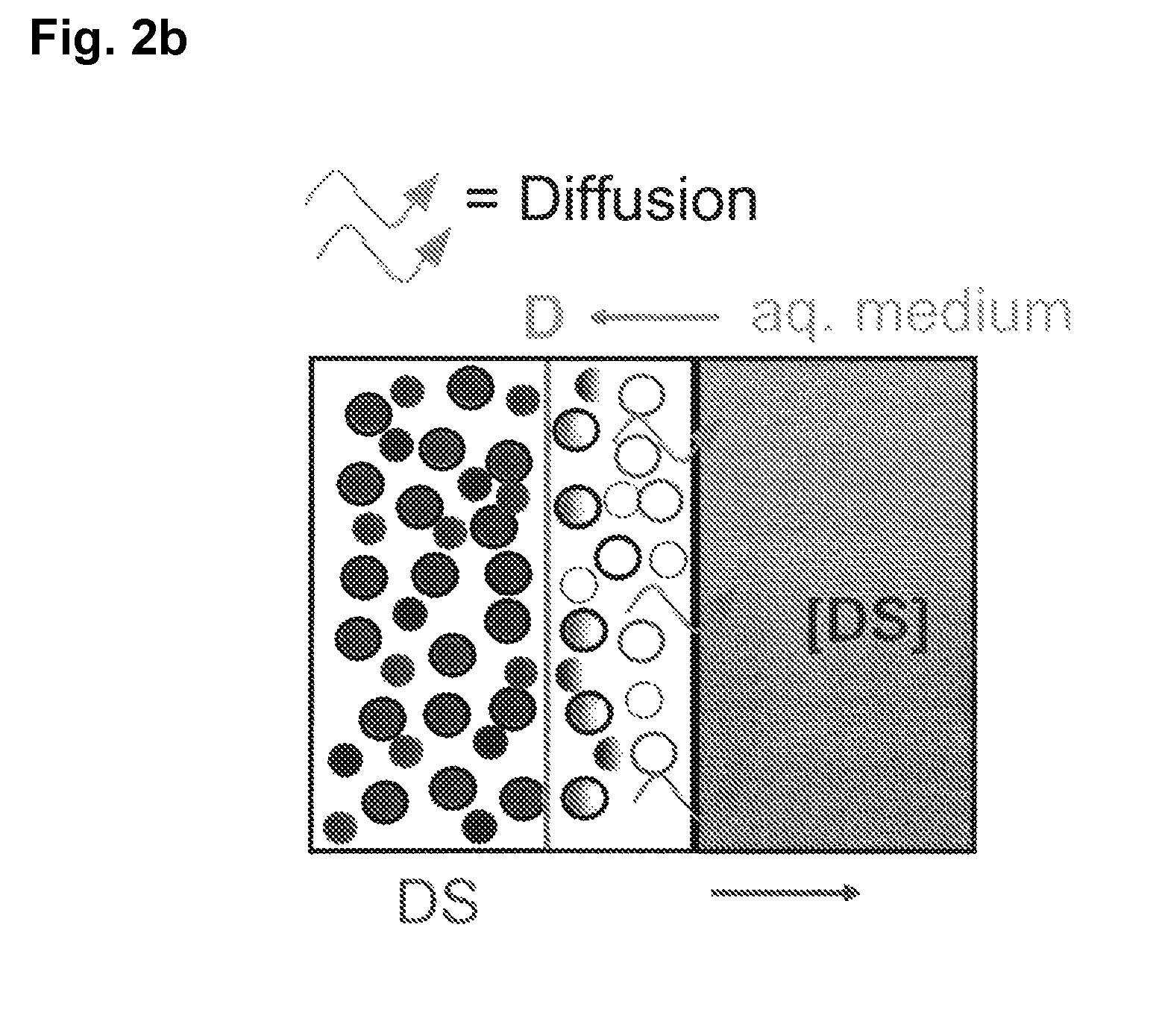
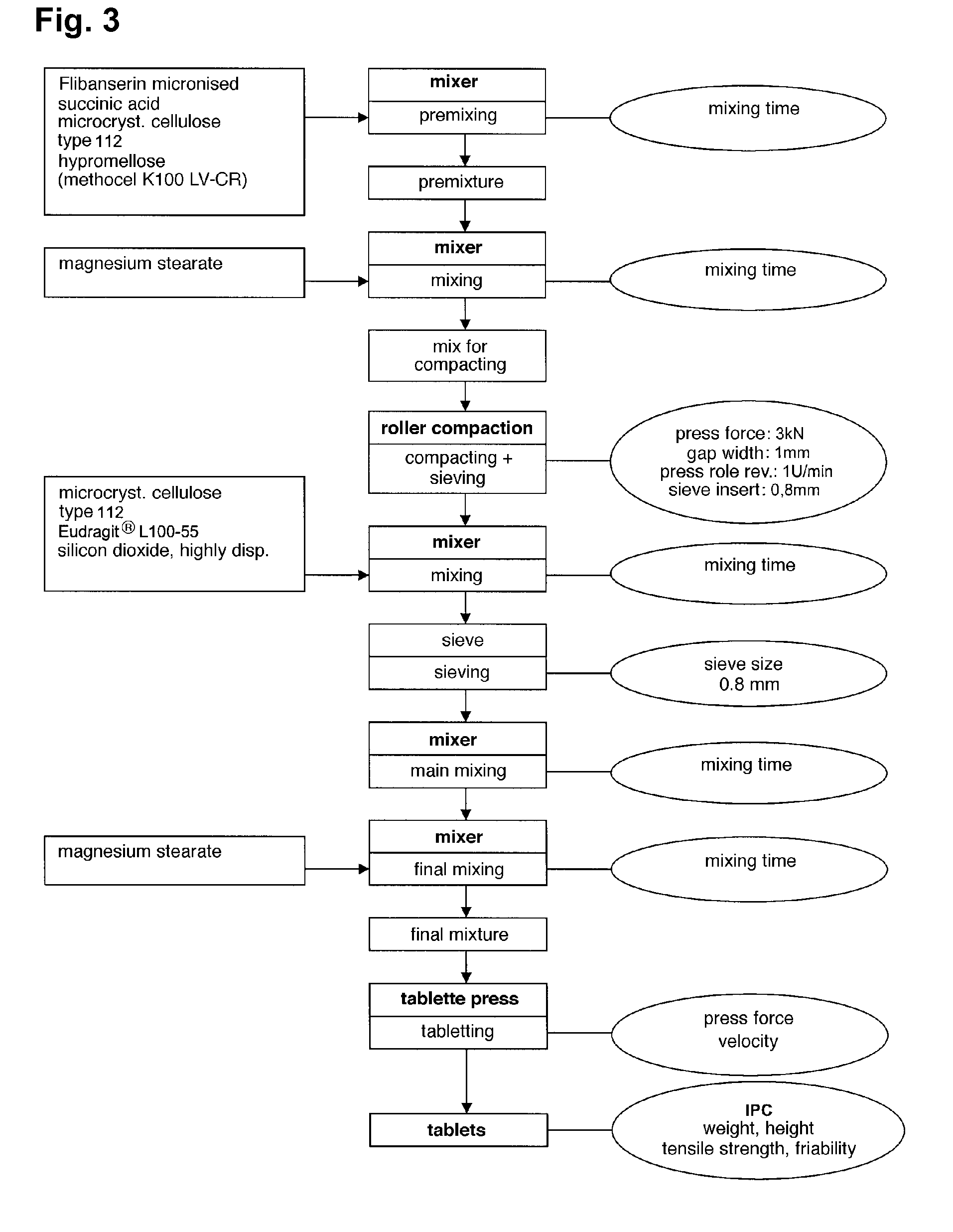
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
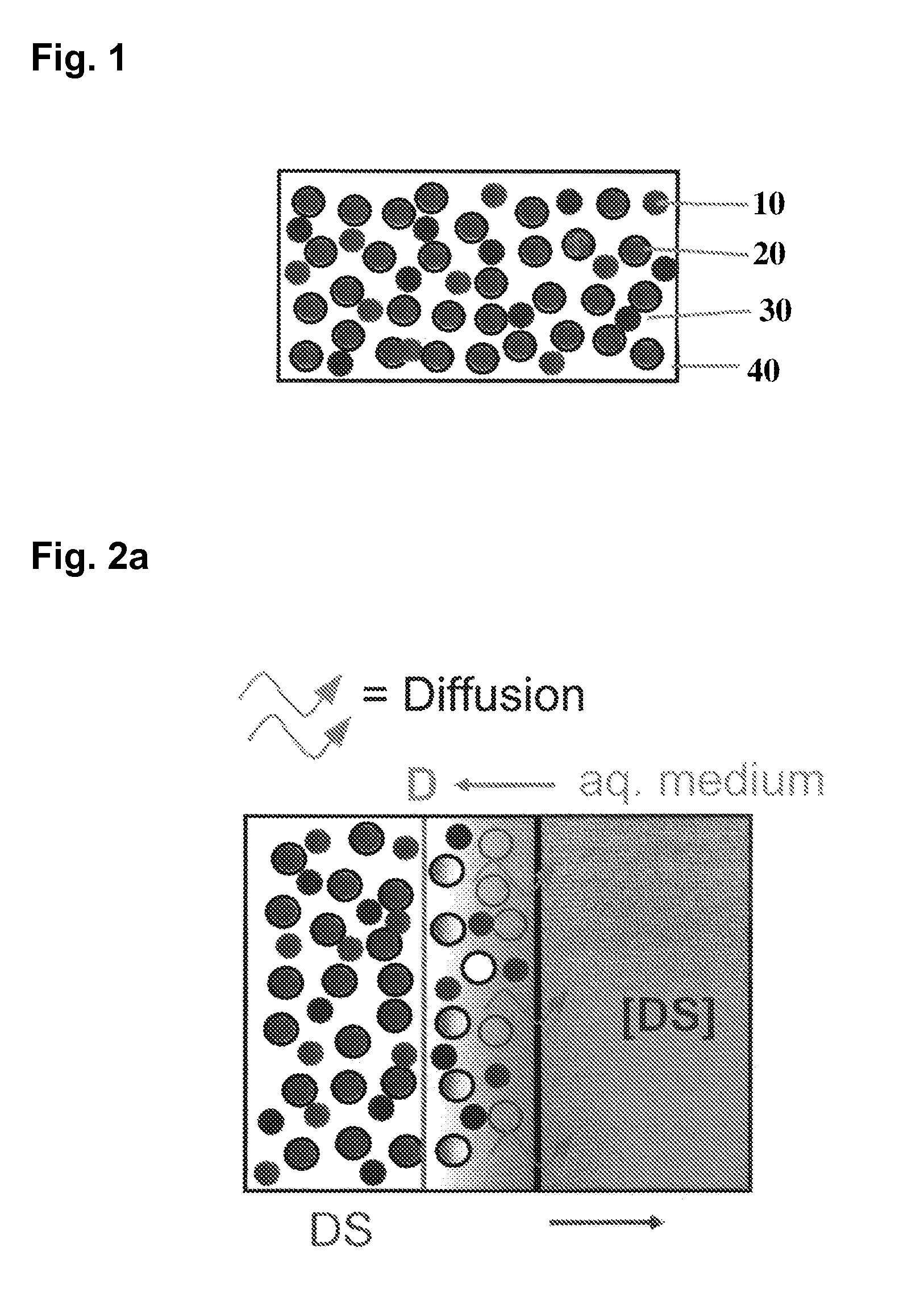
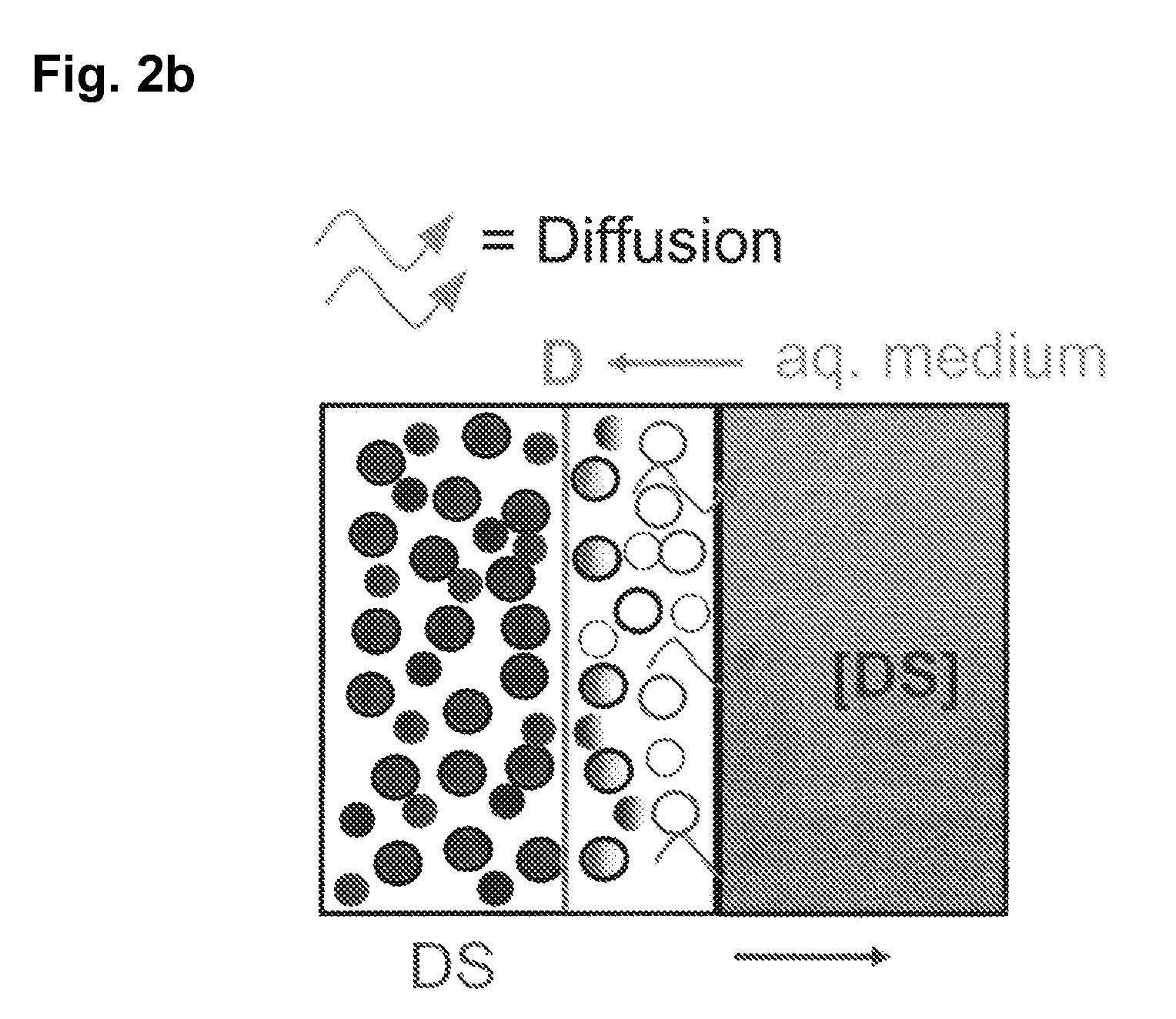
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
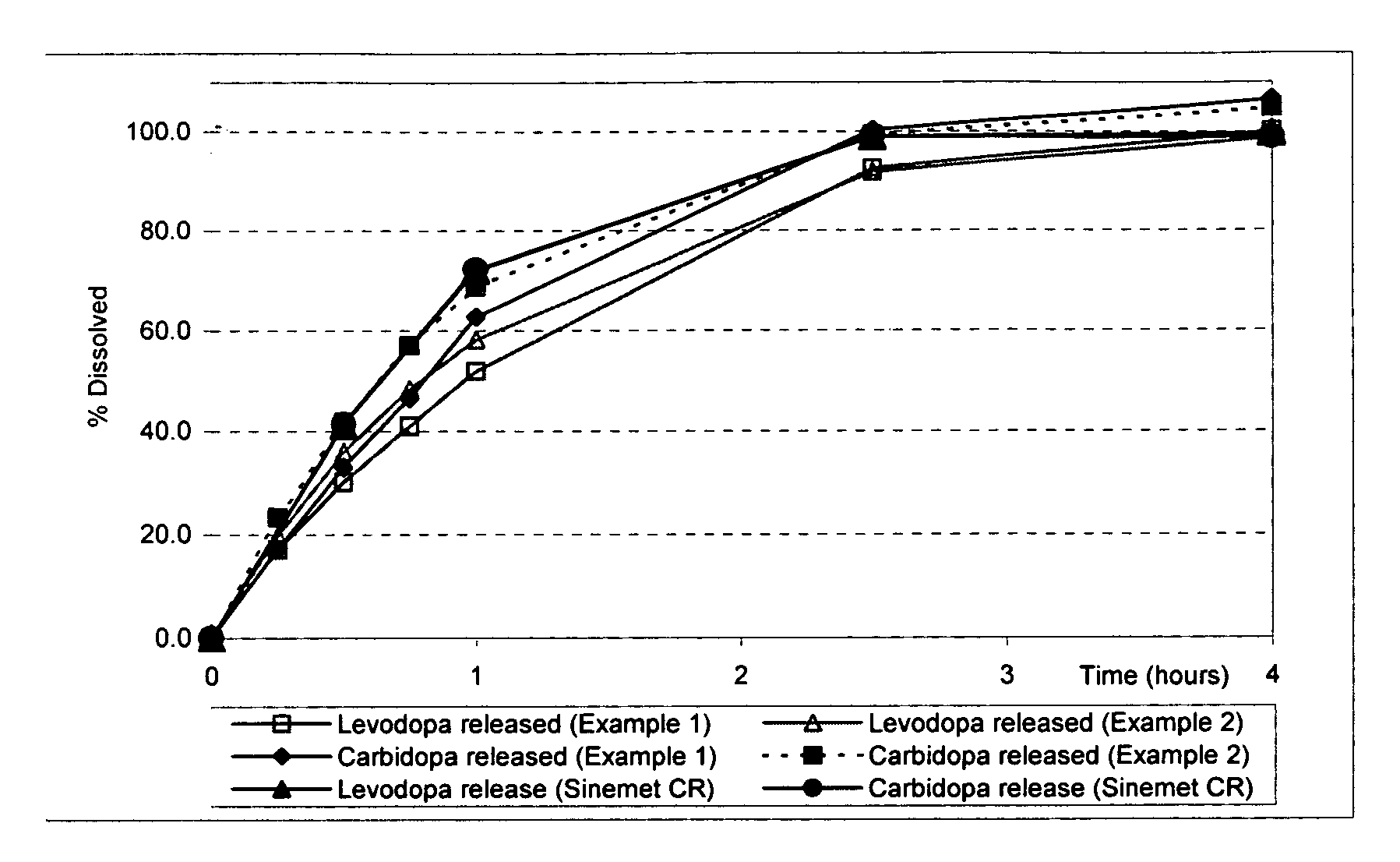
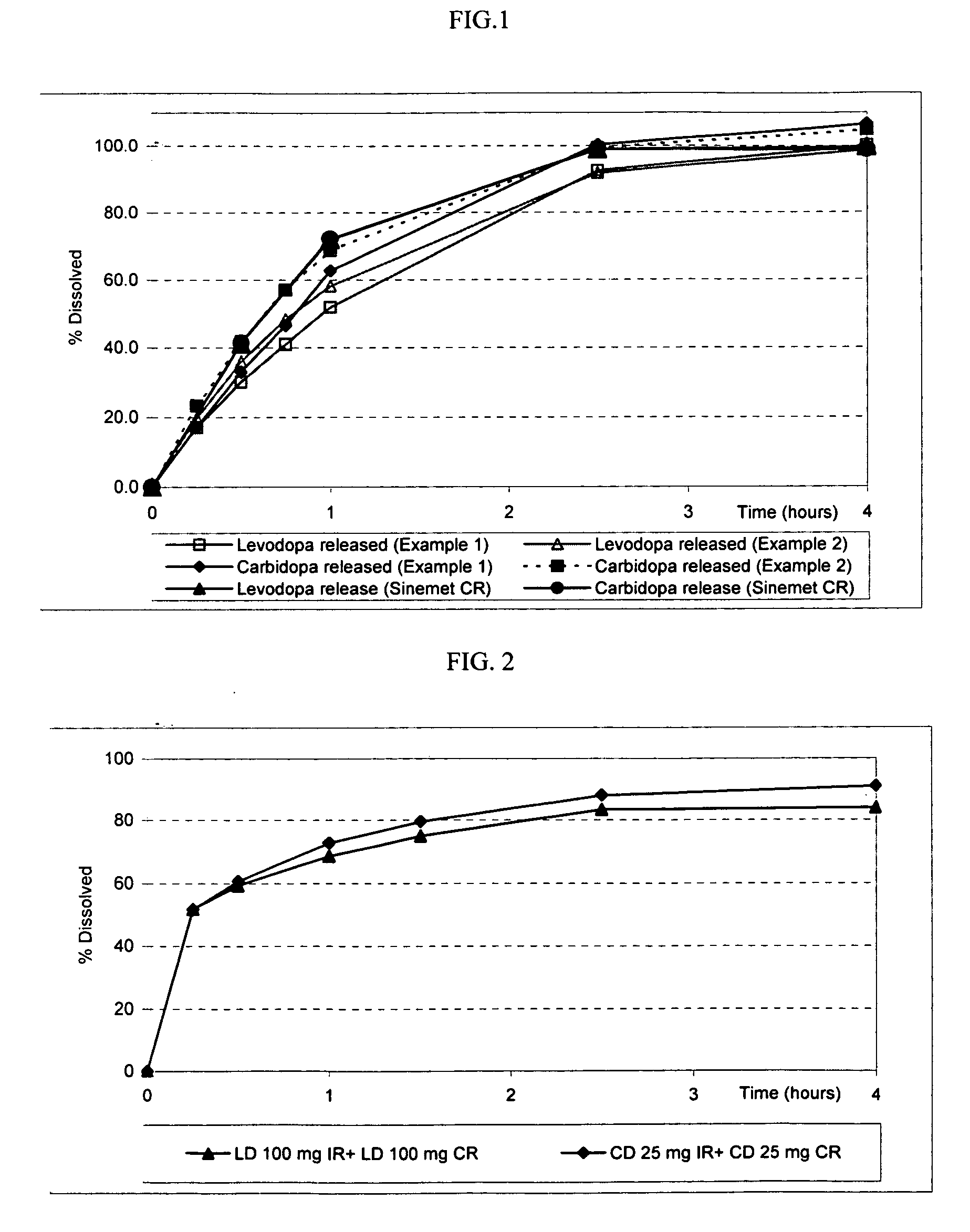
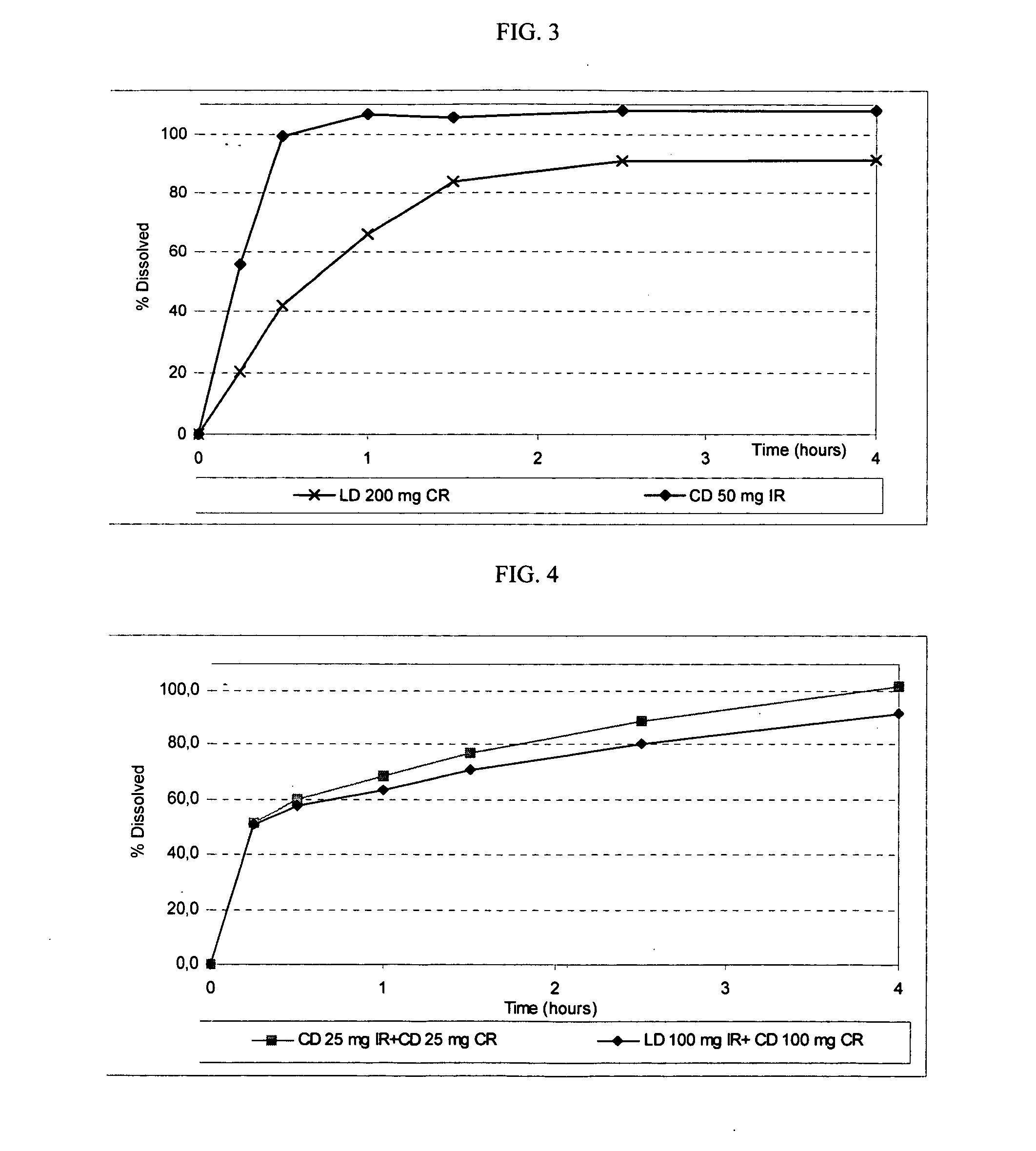
Extended release solid pharmaceutical composition containing carbidopa and levodopa
InactiveUS20070275060A1Improve aestheticsImprove bioavailabilityOrganic active ingredientsBiocideExtended release tabletsImmediate release
The invention provides a compressed tablet that provides a extended release tablet containing a extended release form of carbidopa and a extended release form of levodopa. The tablet optionally further comprises an immediate or rapid release composition of carbidopa and / or levodopa. The extended release composition in the tablet excludes a release rate-controlling polymer, and a release rate-controlling coating; however, the release of the carbidopa and / or levodopa is independently optionally delayed for a lag time. The invention also provides a tablet having a extended release form of levodopa and a rapid or immediate release form of carbidopa. A tablet can contain levodopa present in extended release form and rapid or immediate release form, and carbidopa present in extended release form and rapid or immediate release form. The tablet is used to treat Parkinson's disease and other movement related disorders, diseases or syndromes.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038347A1Reduce wearExtended shelf lifeOrganic active ingredientsNervous disorderExtended release tabletsDrug release
The present invention provides pharmaceutical release systems comprising an therapeutically effective amount of flibanserin and at least one pharmaceutically acceptable excipient, characterized in that said pharmaceutical release systems exhibit a pharmacokinetic profile that is characterized by an average maximum flibanserin plasma concentration Cmax lower than 300 ng / mL, preferably lower than 200 ng / mL after administration of a single dose to healthy volunteers in fasted state or directly after a meal.
Owner:BOEHRINGER INGELHEIM INT GMBH
Extended release tablet
InactiveUS20060257473A1Reduce manufacturing costBiocidePowder deliveryParticulatesExtended release tablets
A single tablet layer having an extended release profile comparable to the release profile of a bi-layer tablet having both an immediate release and an extended release layer is prepared from a pharmaceutical granulation containing a pharmaceutically active compound, a hydrophilic polymer, and a water in-soluble, non-swellable particulate channeling agent.
Owner:L PERRIGO
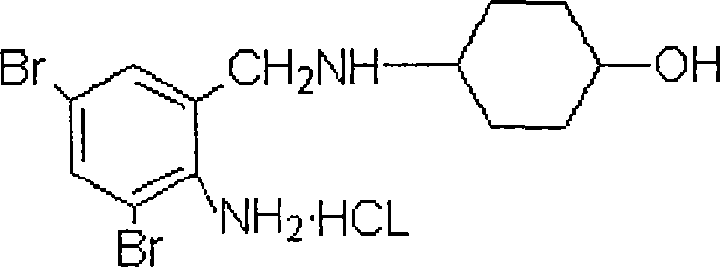
Salt of leonurine and its preparation
InactiveCN1415603ALess irritatingGuaranteed stabilityOrganic active ingredientsOrganic chemistryLeonurineOrganic acid
Owner:李晓祥
Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
ActiveCN101785763AOrganic active ingredientsMetabolism disorderPatient complianceSustained-Release Preparations
The invention discloses a metformin hydrochloride enteric-coated sustained release tablet which is prepared by enteric coating the metformin hydrochloride sustained release tablet. Compared with the prior art, the sustained release tablet integrates with the enteric coating technology to prepare a new form of the metformin hydrochloride enteric-coated sustained release tablet. By using the enteric coating technology, the metformin hydrochloride does not disintegrate in the stomach or stimulate the gastric mucosa, and the adverse reaction of nausea, stomachache and diarrhea caused by medicine taking can be avoided; meanwhile, the metformin hydrochloride is prevented from being damaged by gastric juice, and the bioavailability is improved. The product is a sustained release preparation, the medicine can stably release in vivo, the effective blood concentration can be maintained for a long time, the toxic and side effects caused by over-high blood concentration in a short time are avoided, the medicine taking frequency is decreased, and the patient compliance is improved as well.
Owner:贵州天安药业股份有限公司
Clarithromycin formulations having improved bioavailability
InactiveUS20050163857A1Excellent solubility propertiesPowder deliveryBiocideExtended release tabletsClarithromycin
A pharmaceutical composition includes micronized clarithromycin and exhibits improved dissolution characteristics relative to a pharmaceutical composition that includes unmicronized clarithromycin. The clarithromycin may have a particle size less than approximately 35 microns. One process for preparing an extended release tablet of the clarithromycin includes micronizing the clarithromycin; blending the micronized clarithromycin with one or more rate controlling polymers and pharmaceutically acceptable excipients; granulating the blend; and compressing to form a tablet. To treat a bacterial infection in a mammal in need of treatment, a patient may be administered a pharmaceutical composition that includes micronized clarithromycin.
Owner:RANBAXY LAB LTD
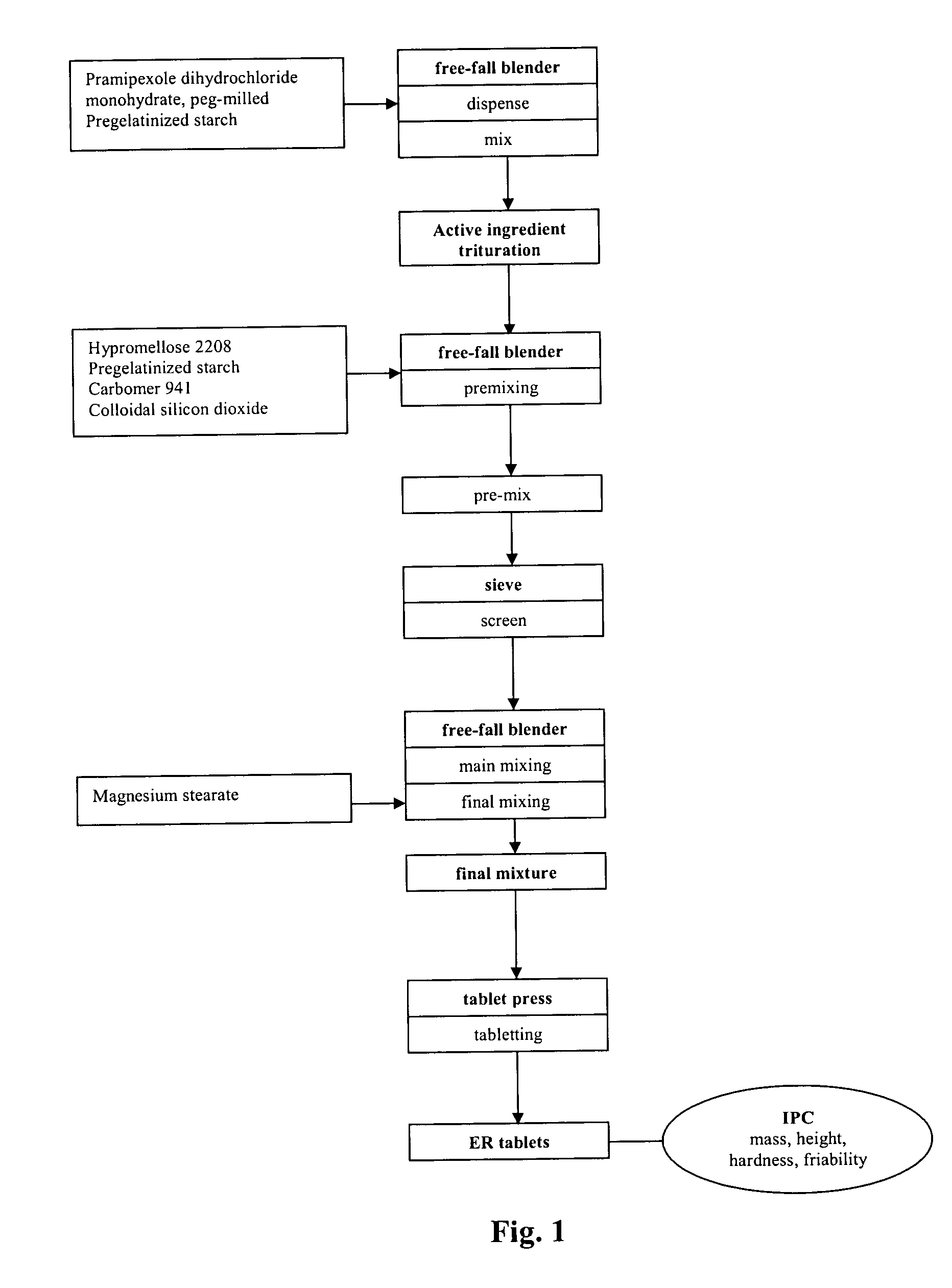
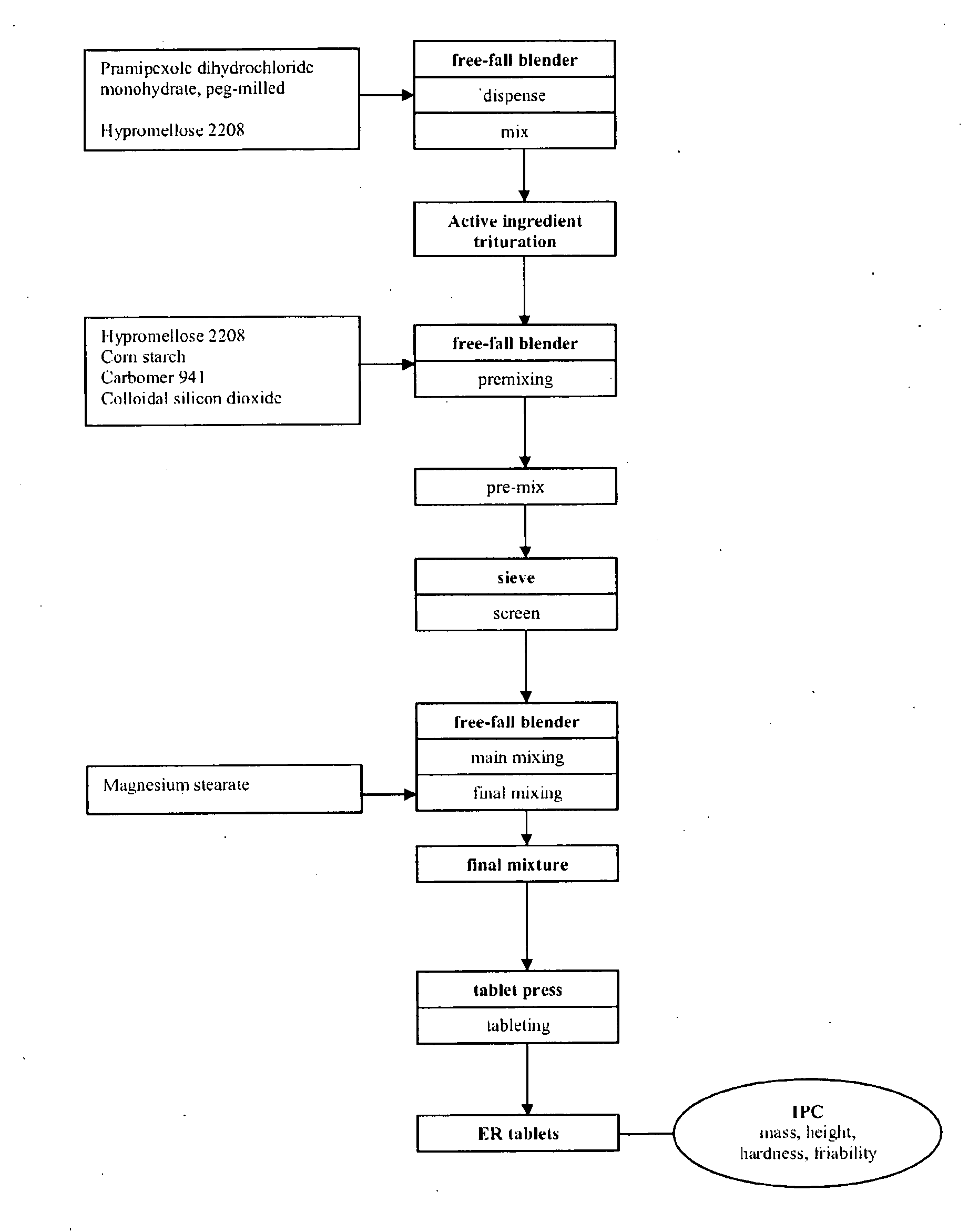
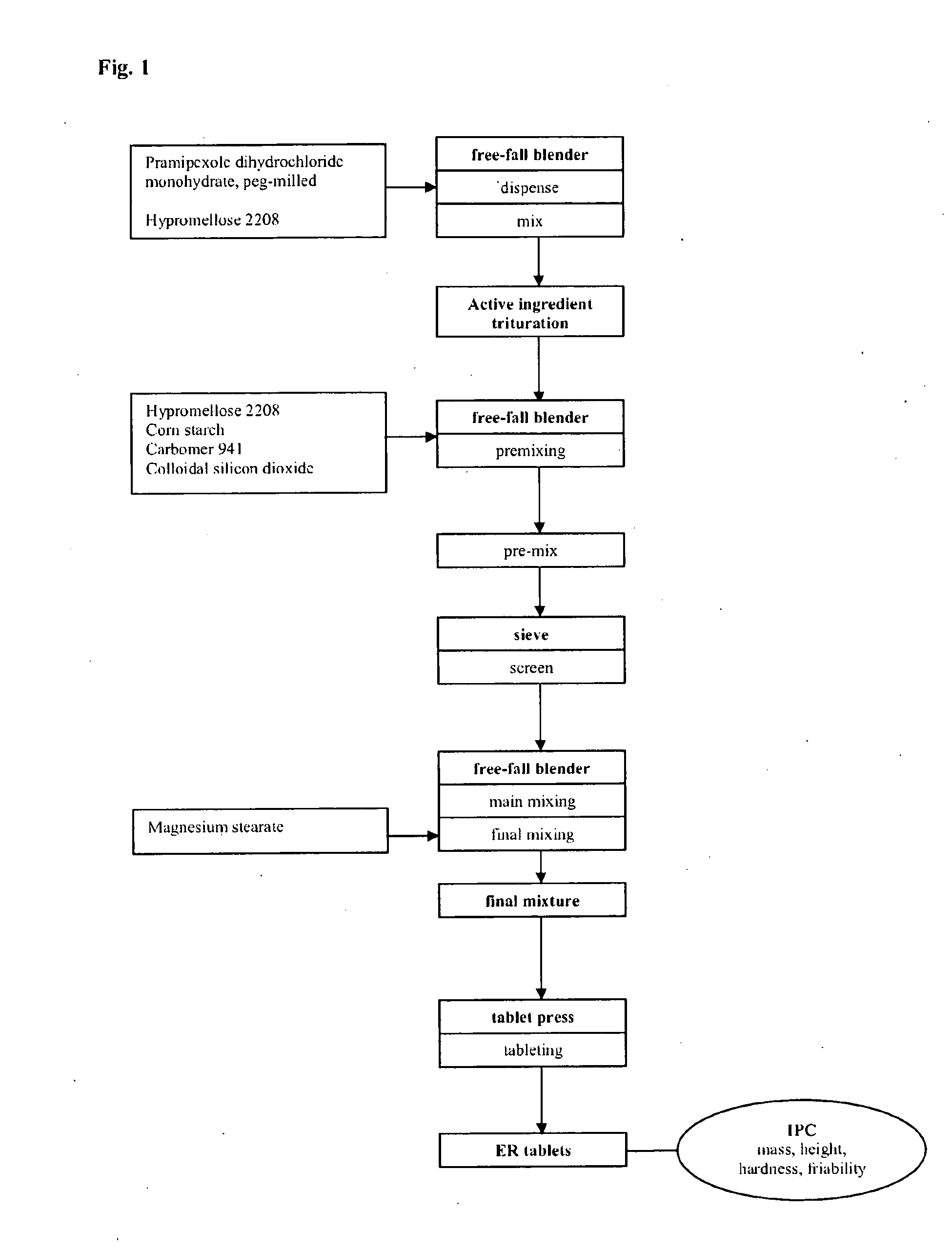
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
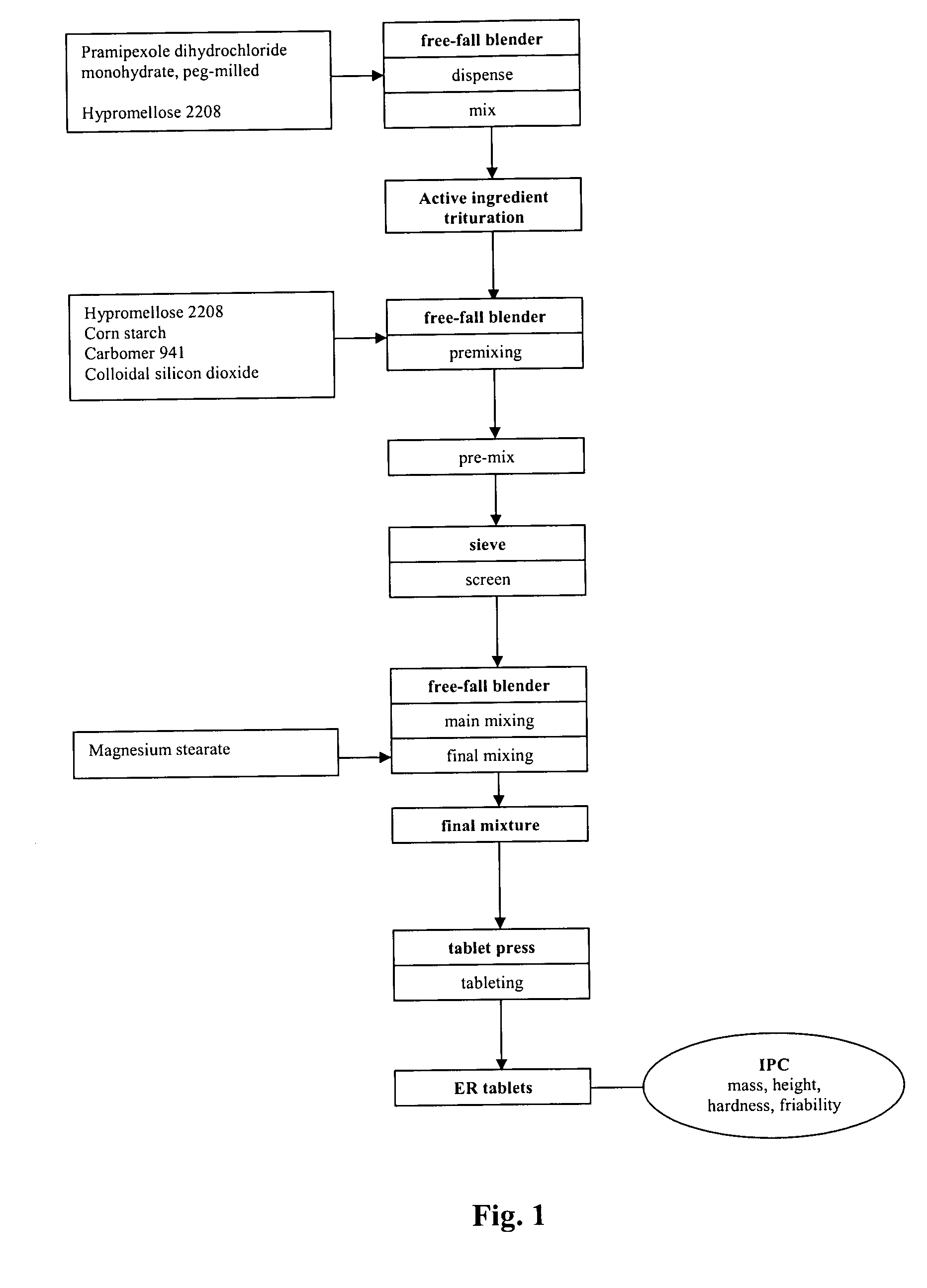
InactiveUS20060051417A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
An extended release tablet formulation comprising pramipexole or a pharmaceutically acceptable salt thereof in a matrix, the matrix comprising at least two water swelling polymers, wherein one of the polymers is pregelatinized starch, and wherein another one of the polymers is an anionic polymer.
Owner:BOEHRINGER INGELHEIM INT GMBH
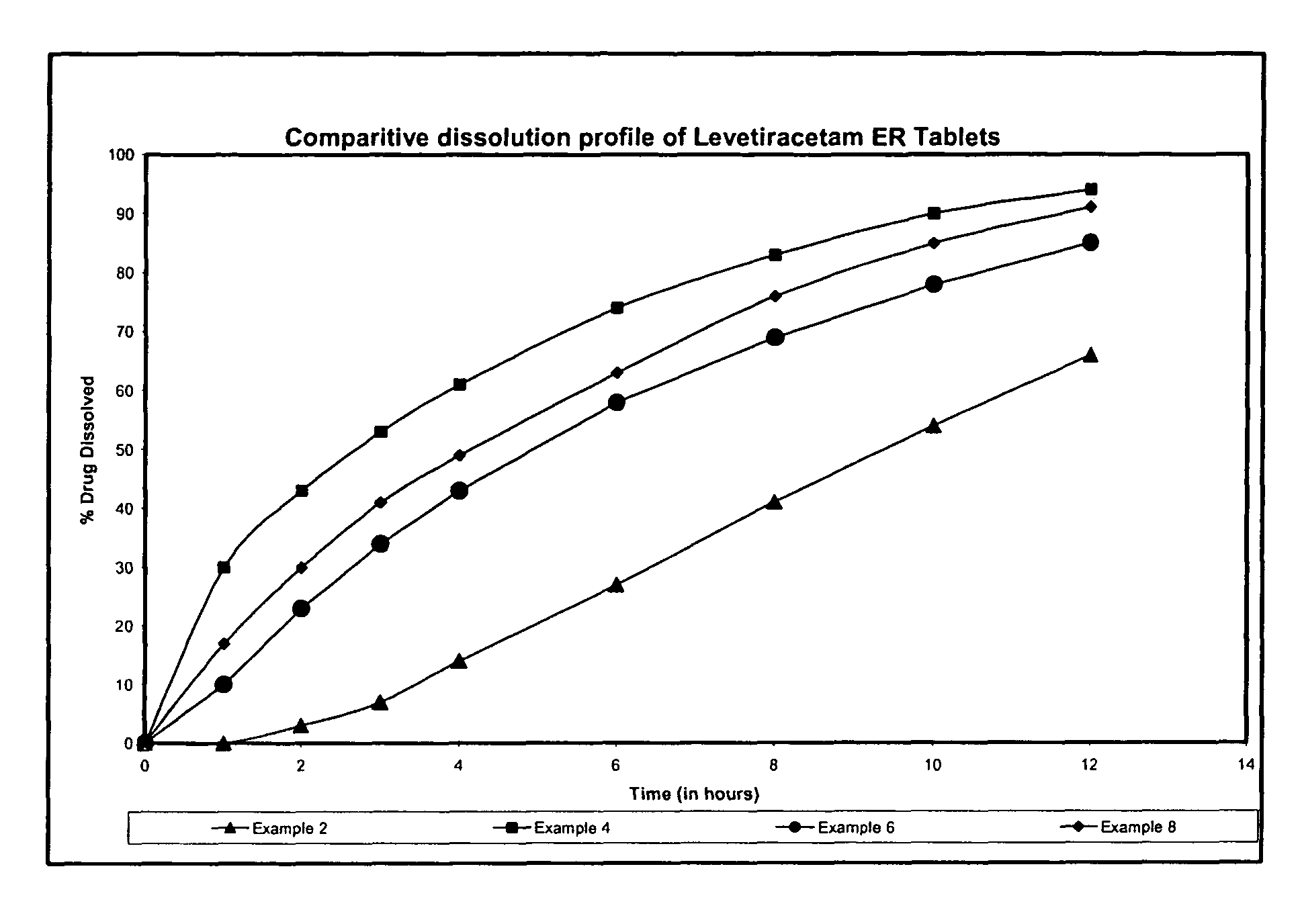
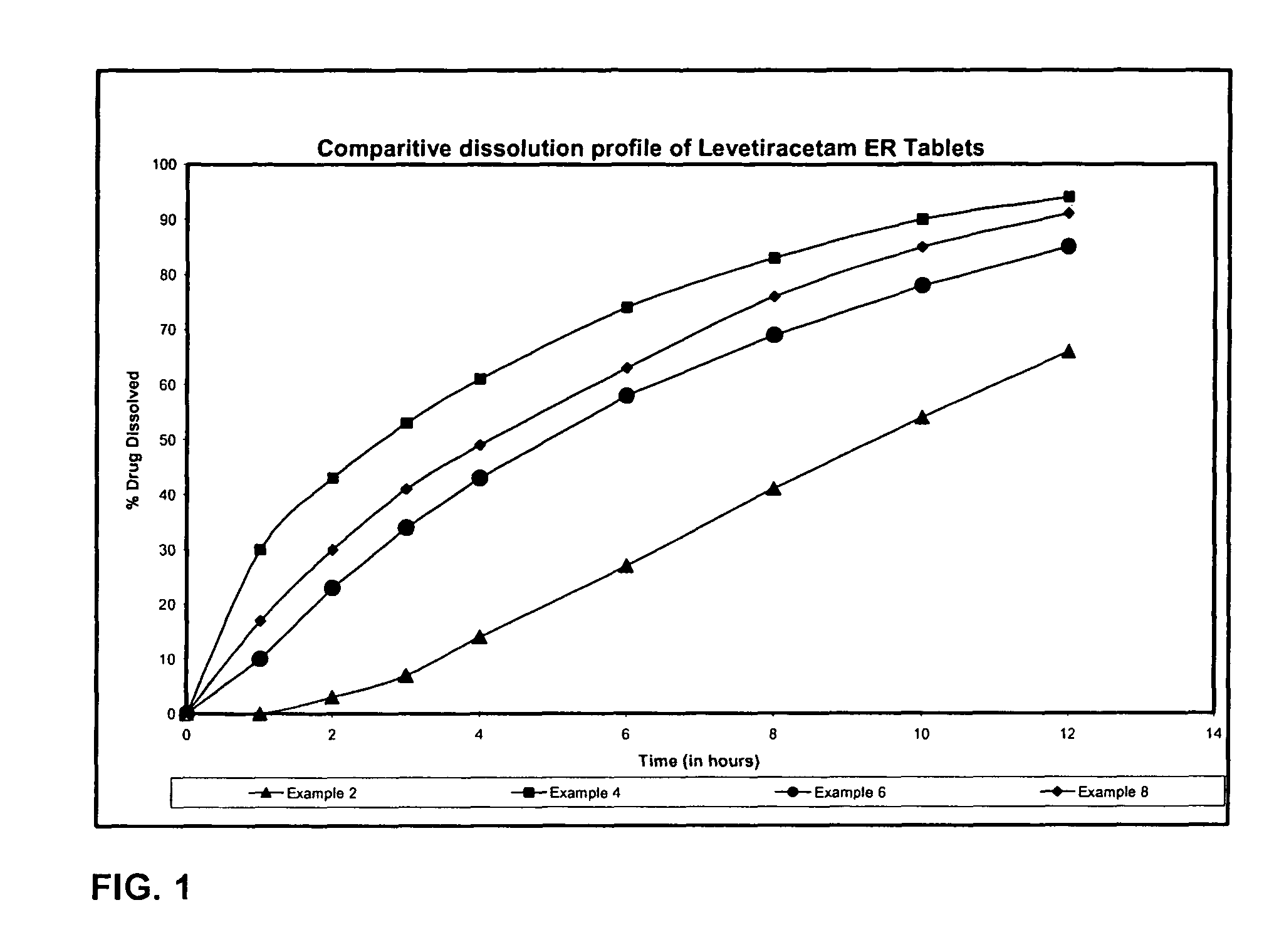
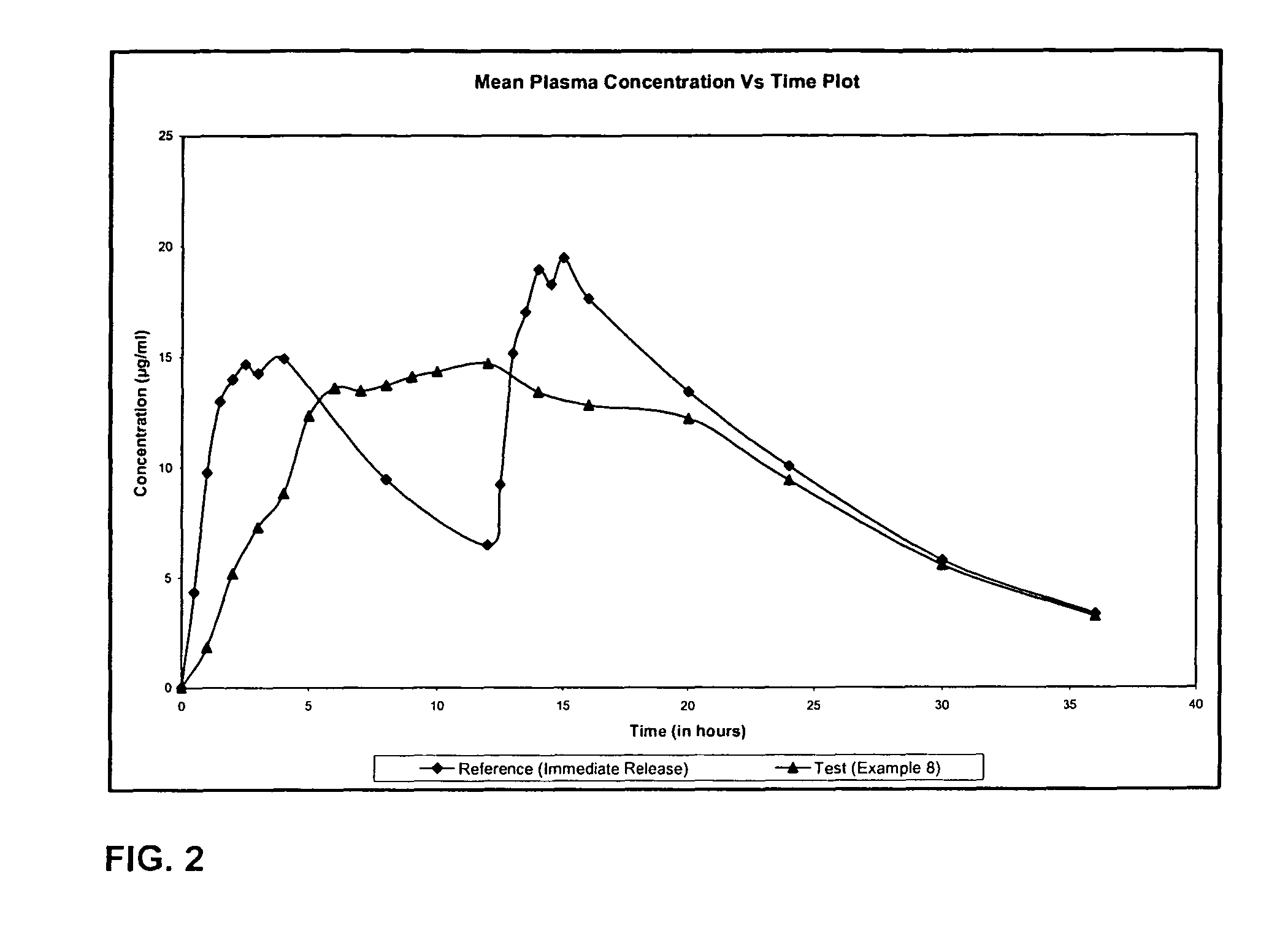
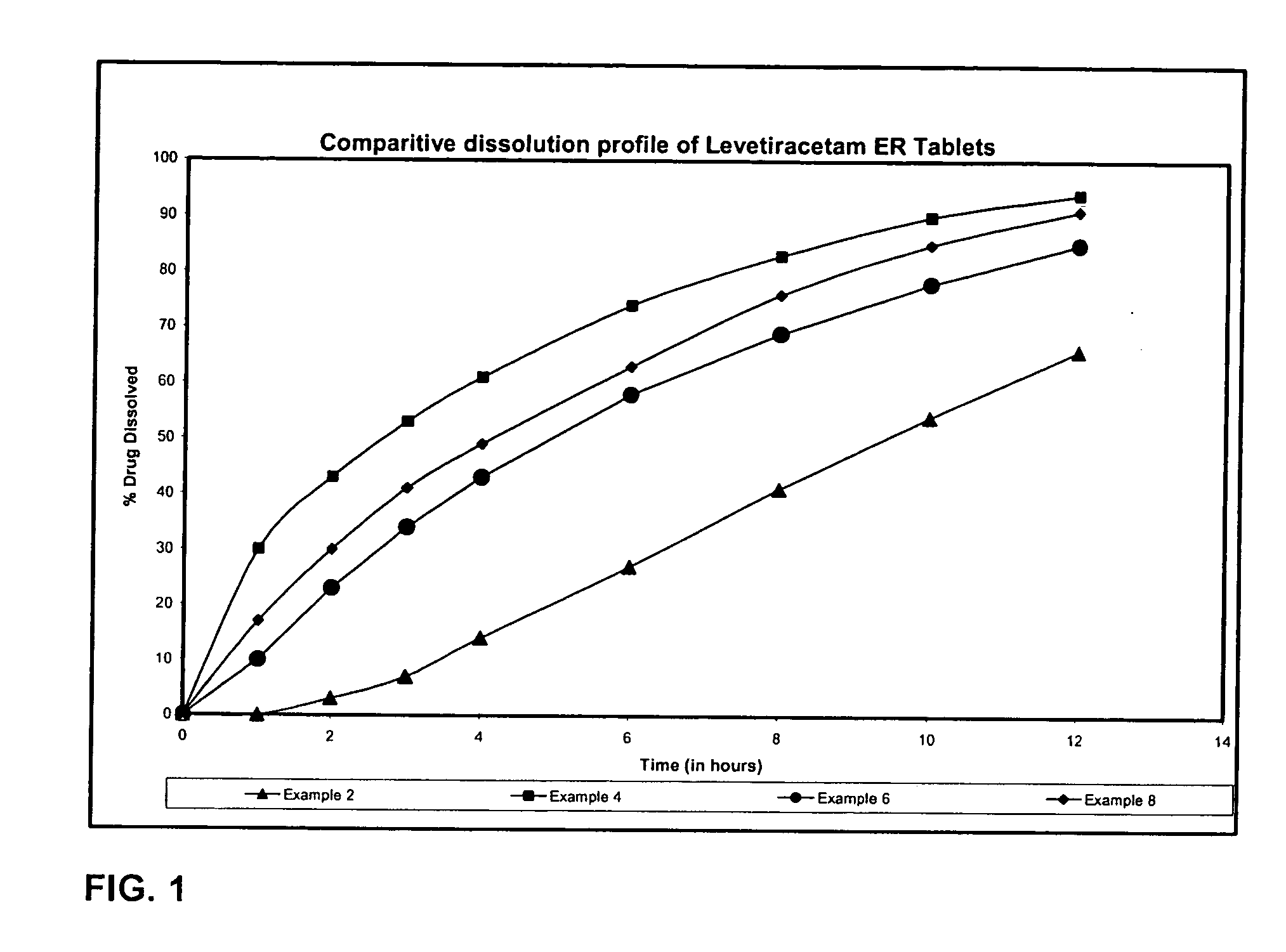
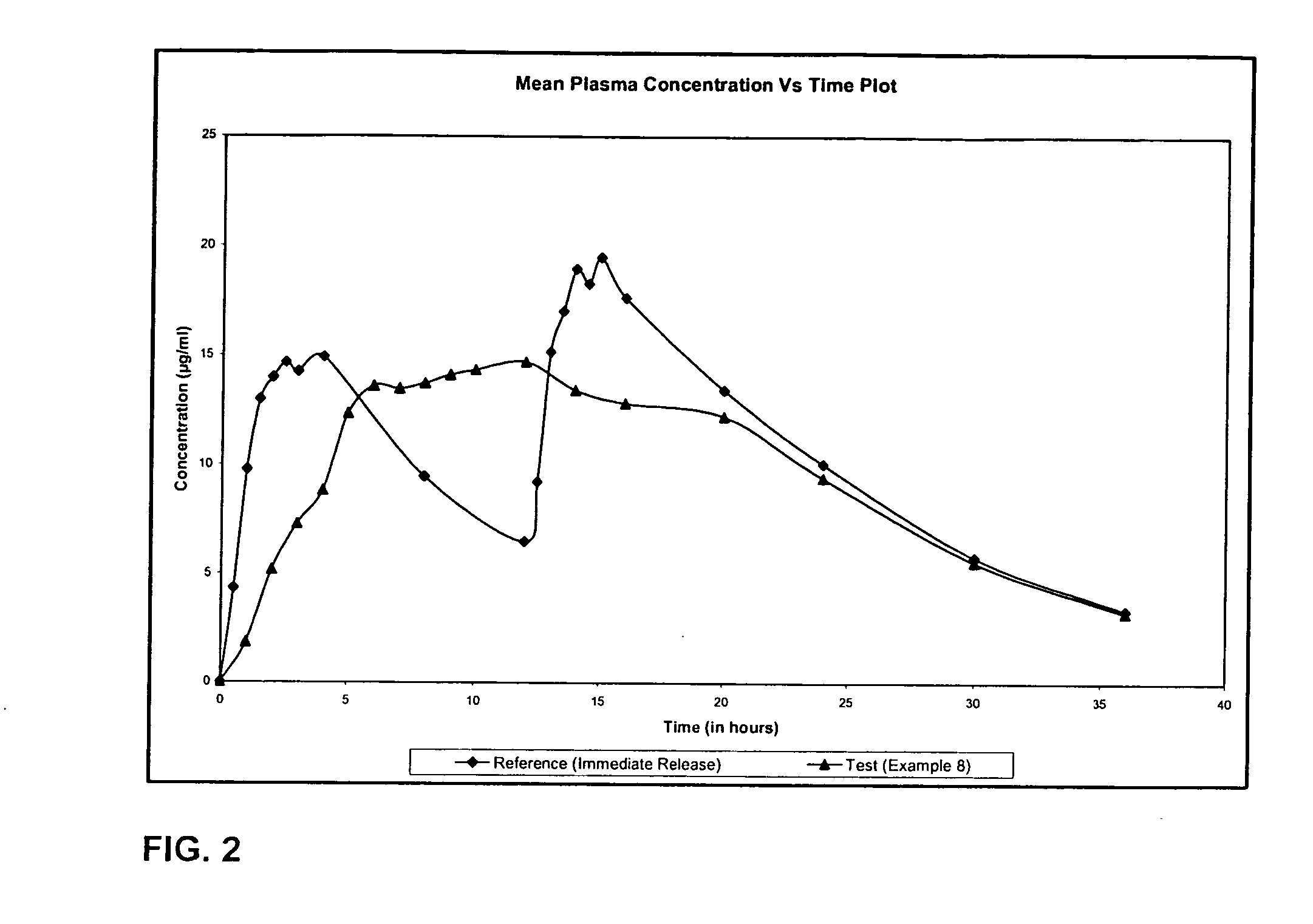
Extended release formulation of Levetiracetam
ActiveUS7863316B2Reduced inter subject variabilityBiocideNervous disorderWater dispersibleFOOD EFFECT
Owner:UCB PHARMA SA
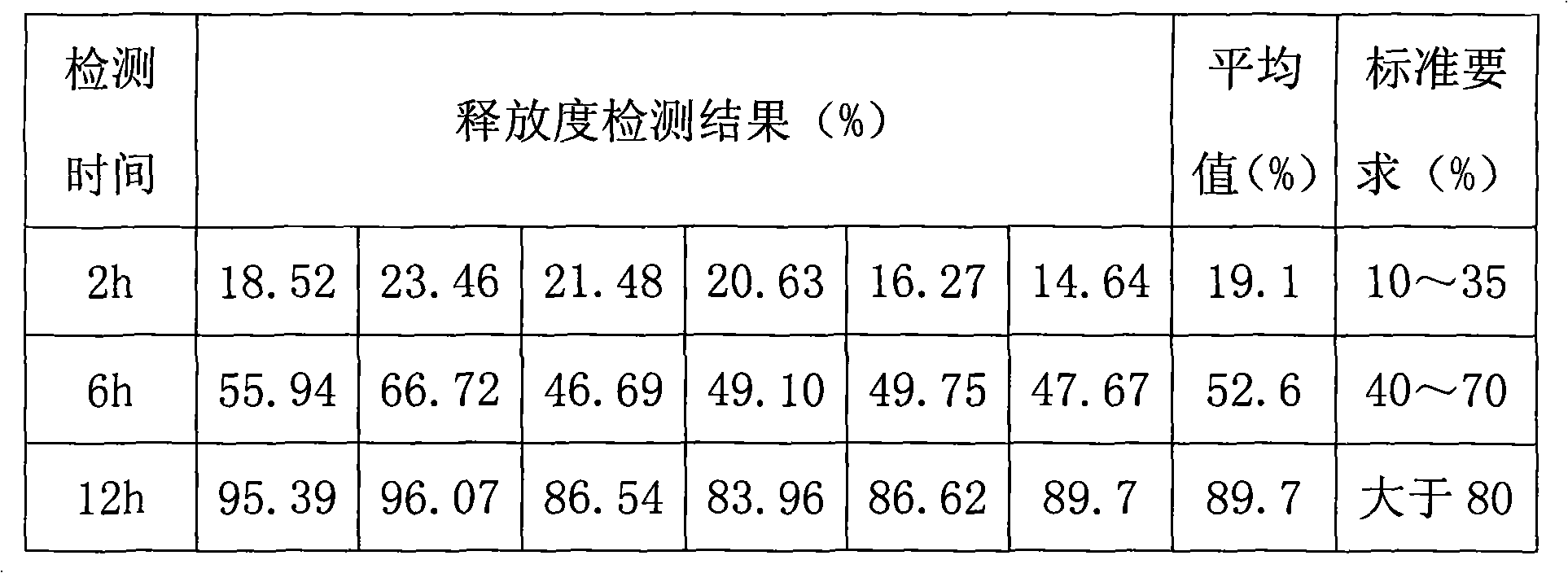
Metformin hydrochloride controlled-release tablet and preparation method thereof
ActiveCN101579325ALess weight gainIncrease production capacityOrganic active ingredientsMetabolism disorderPharmaceutical industryMetformin Hydrochloride
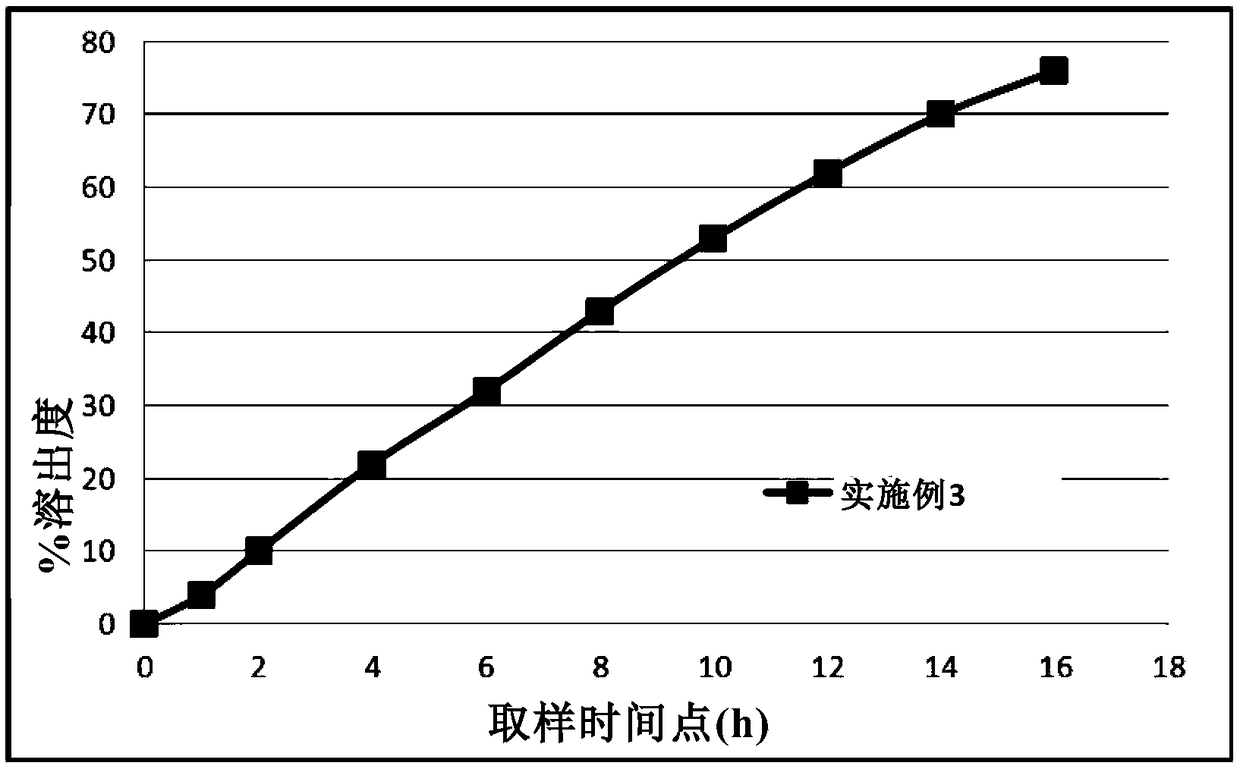
The invention provides a metformin hydrochloride controlled-release tablet, which comprises a metformin hydrochloride controlled-release tablet with effective dose and pharmaceutical accessories and is characterized in that the metformin hydrochloride controlled-release tablet uses the common tabletting, and the controlled-release effect is controlled by totally depending on the film coating technique. The coating adopted by the invention is a releasing system comprising the prescription which can cause the medicine to reach release degree standard in vitro. The preparation method in the invention is simple and convenient; the process conditions are easy to control and suitable for batch production, can use conventional production equipment in pharmaceutical industries for economically and conveniently producing the metformin hydrochloride controlled-release tablet on a large scale, can effectively and stably cause the release degree of the metformin hydrochloride controlled-release tablet in the second hour to be 10% to 35%, the release degree to be 40% to 70% in the sixth hour and the release degree to be more than 80% in the twelfth hour.
Owner:CHONGQING CONQUER PHARML
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
ActiveUS20060198887A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
Modified Release Formulation
InactiveUS20090041844A1Improve complianceImprove convenienceBiocideOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
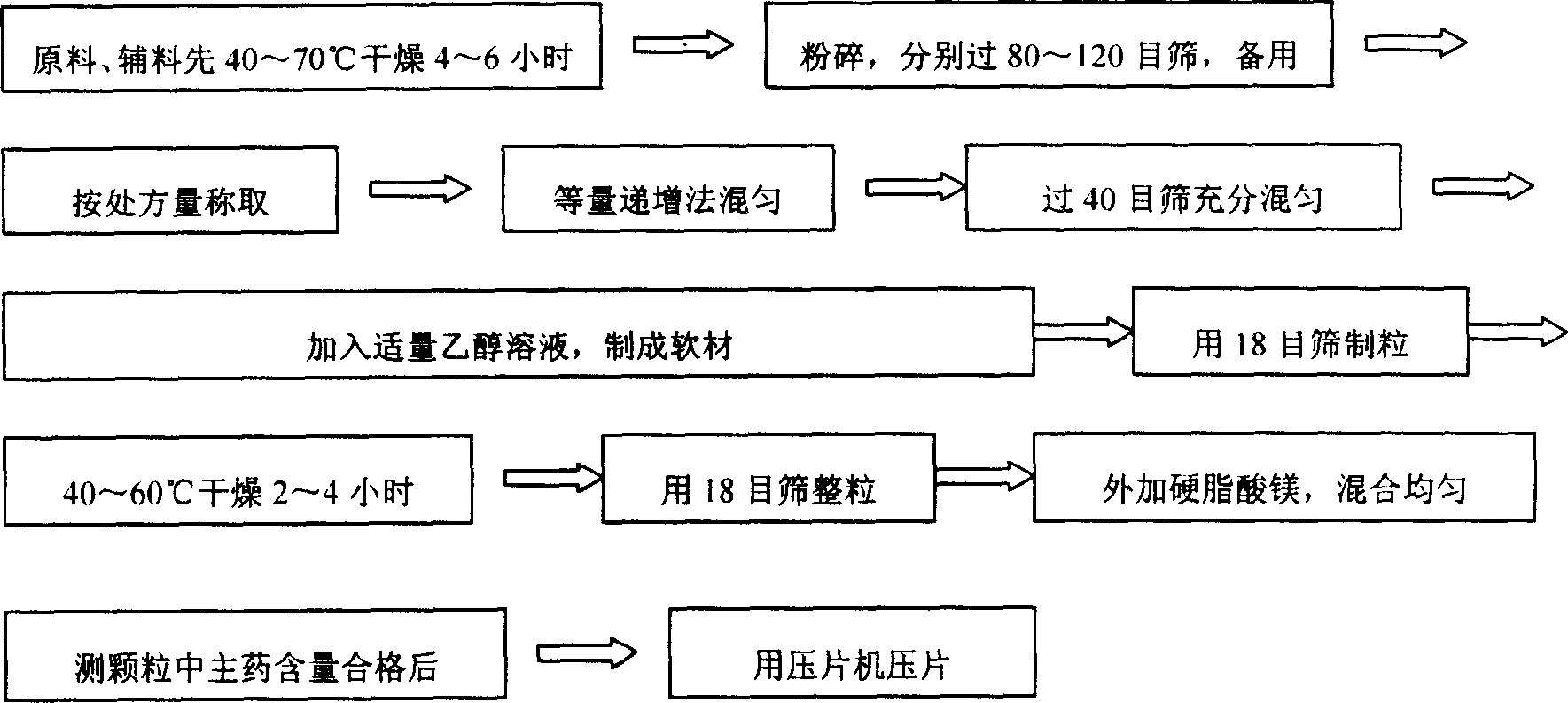
Metformin hydrochloride slowly released tablet and its preparation method
InactiveCN1415288APromote aerobic metabolismIncrease intakeOrganic active ingredientsMetabolism disorderExtended release tabletsAlcohol
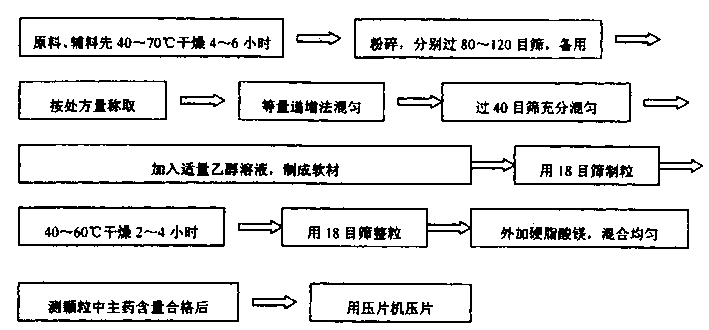
A slowly-releasing mellitin tablet is prepared from fluamine, excipient, adhesive and lubricant through drying at 40-70 deg.C for 4-6 hrs, pulverizing, sieving by 80-120 meshes, proportionally mixing, mixing with alcohol solution, granularating by 18-mesh sieving, drying at 40-60 deg.C for 2-4 hrs, mixing with lubricant, and tabletting. Its advantages are long half-life (0.9-2.6 hr), low dosage and low by-effect.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
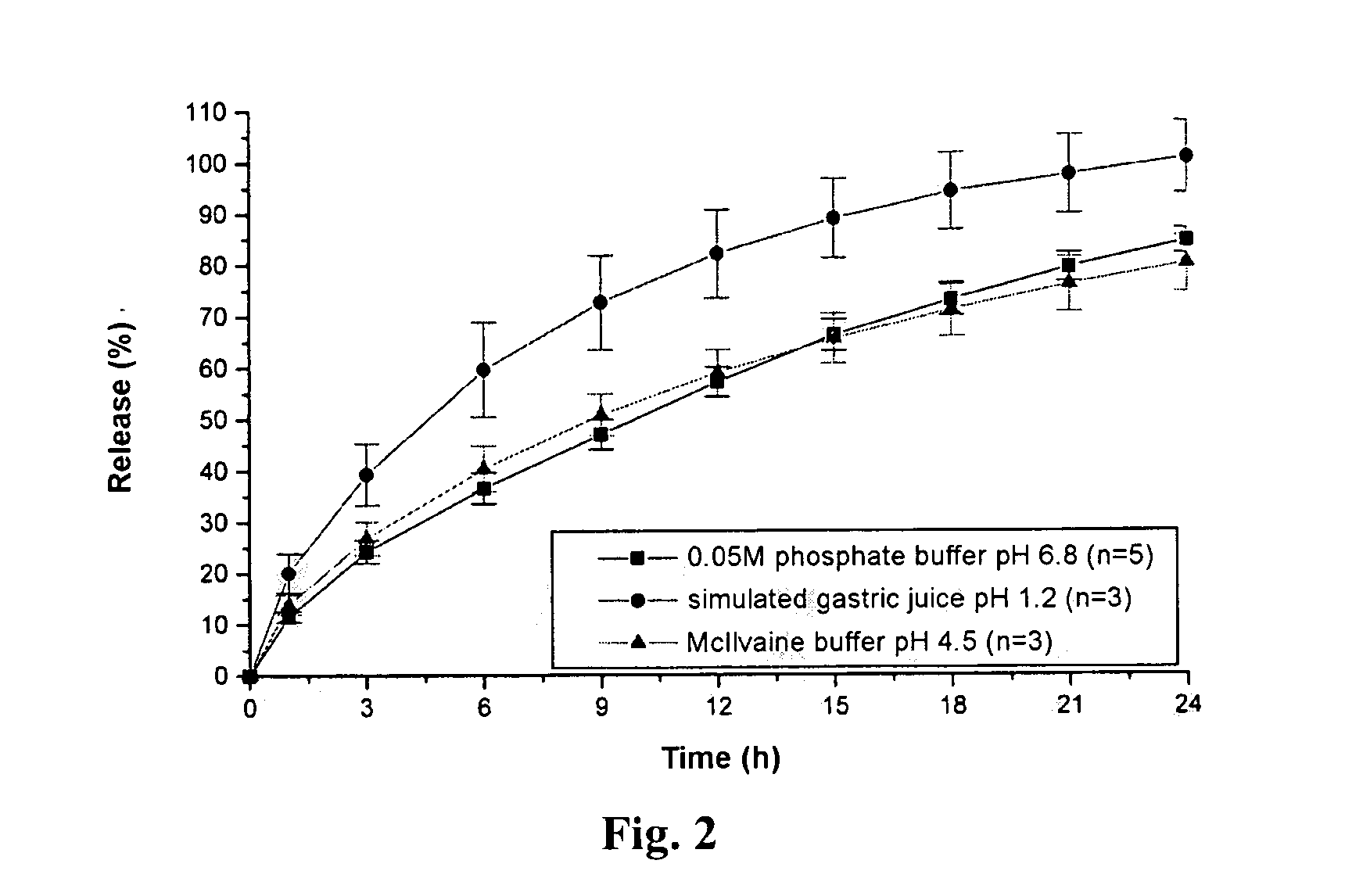
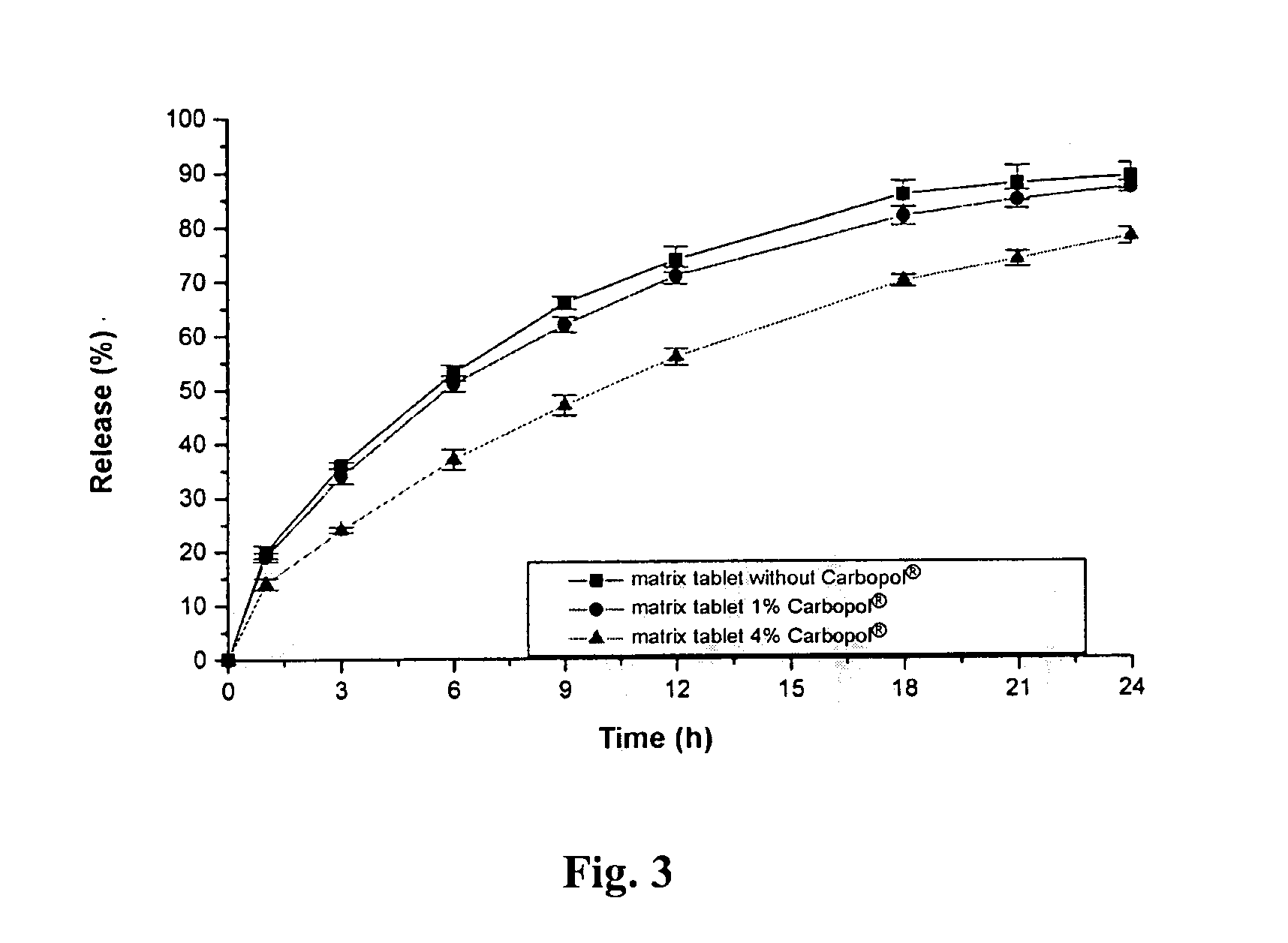
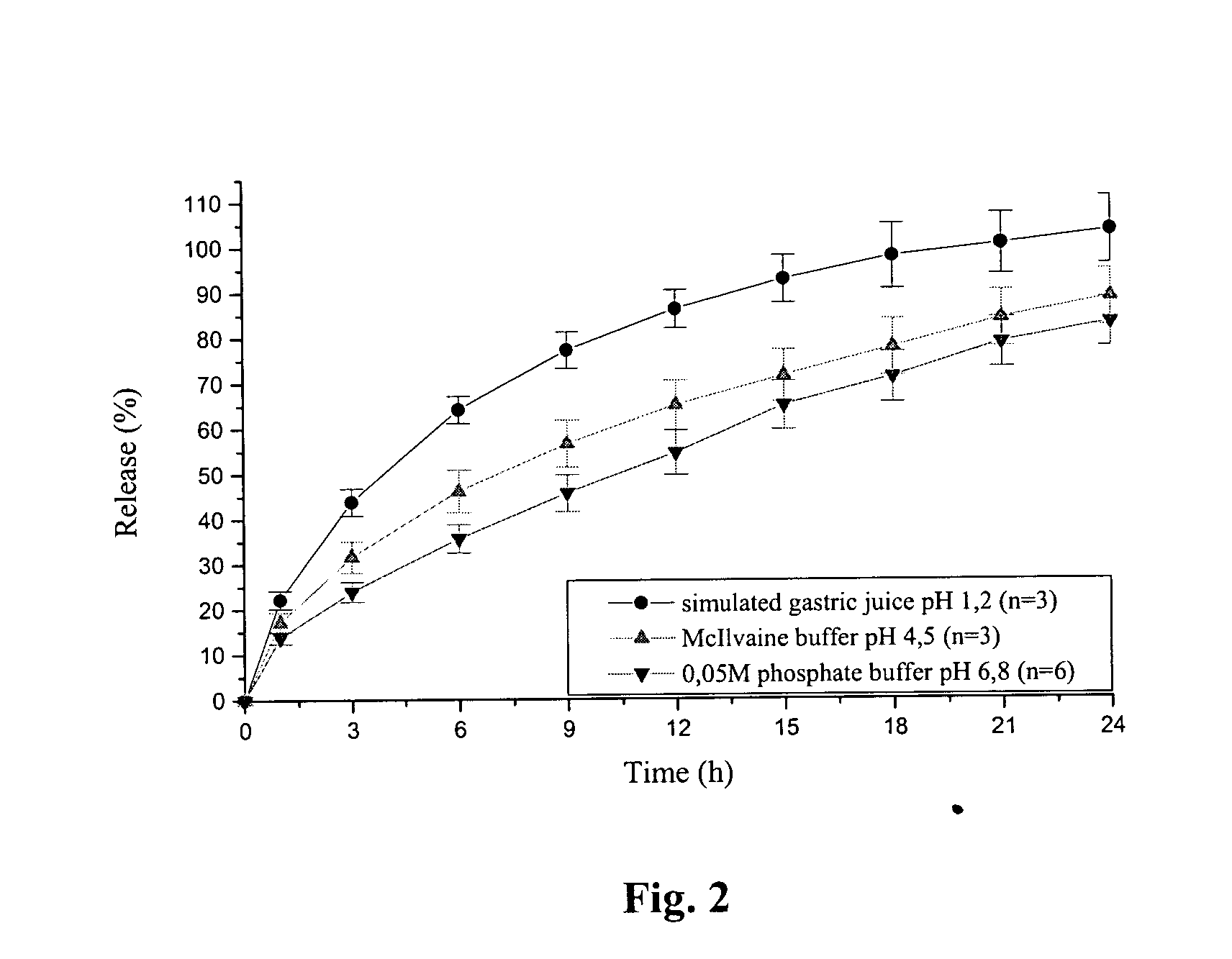
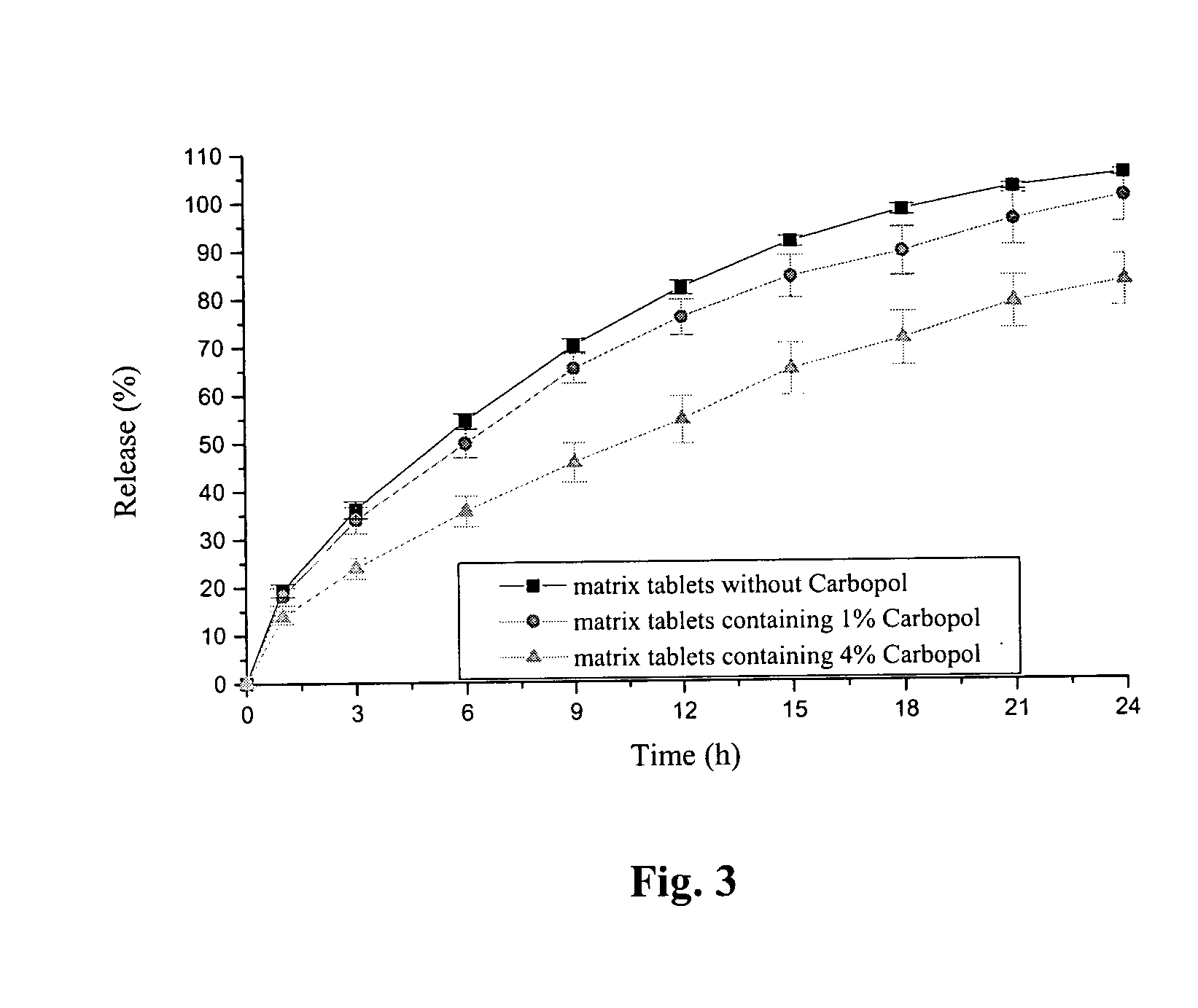
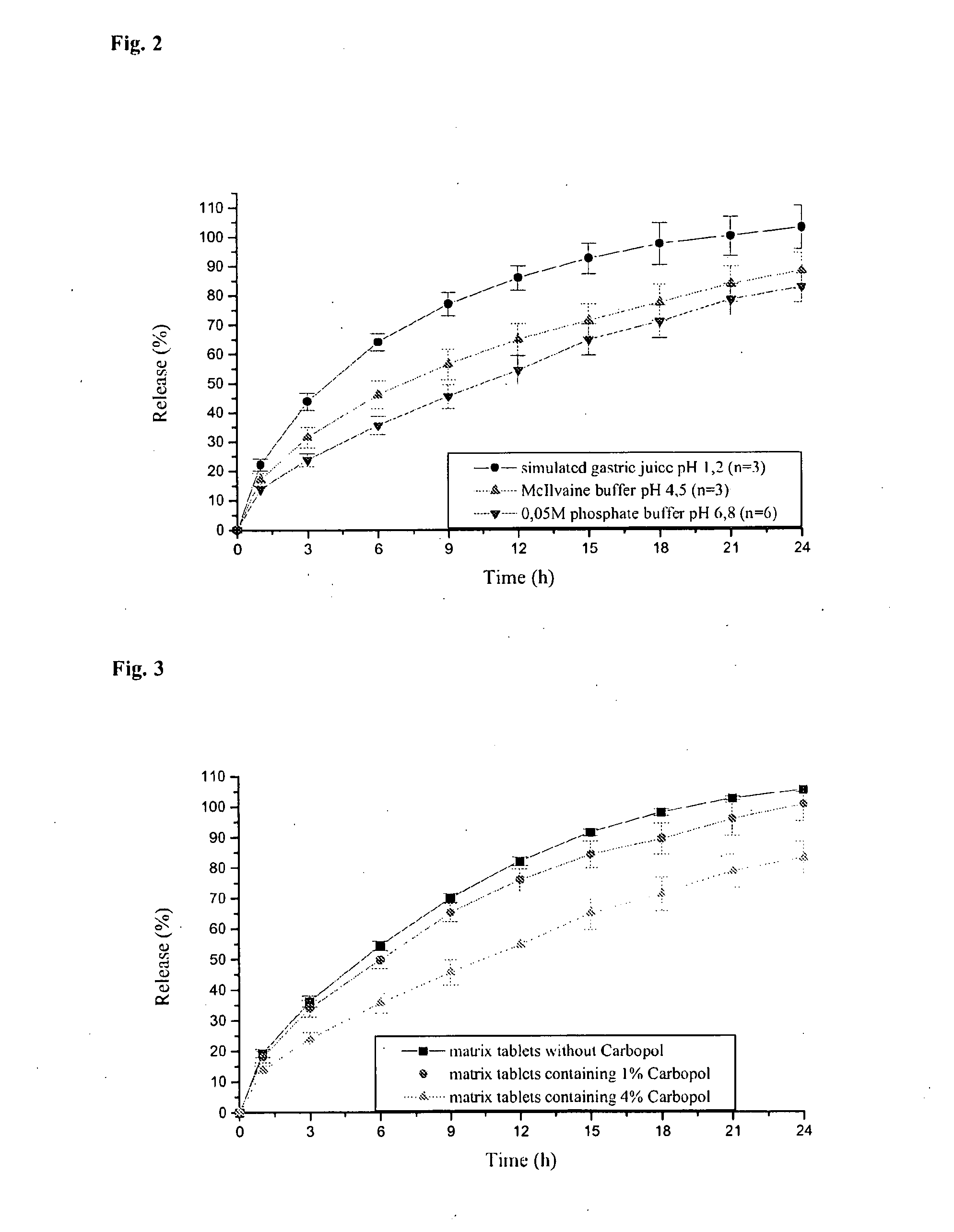
Pramipexole dihydrochloride slow-release tablet with high content uniformity and preparation method thereof
InactiveCN102836137AIncreasing the thicknessEffectively adjust the drug release curveOrganic active ingredientsNervous disorderHydrogenRelative standard deviation
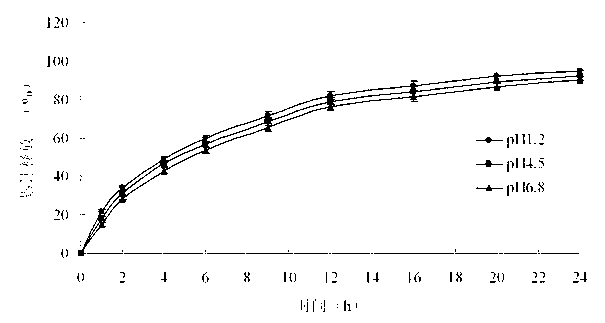
The invention belongs to the field of pharmaceutic preparations, and particularly provides a pramipexole dihydrochloride slow-release tablet with high content uniformity and a preparation method thereof. The invention provides the pramipexole dihydrochloride slow-release tablet which is independent of pH (potential of hydrogen) during drug release, is slowly released in buffer solutions with pHs of 1.2, 4.5 and 6.8 for 24 hours, reaches final release amounts above 85 percent without exception and only needs to be taken once every day. The preparation method provided by the invention is simple and practicable, relative standard deviations (RSDs) of the content uniformity and the release rate of the low-dose medicine, namely the pramipexole dihydrochloride can be guaranteed to be far below the RSD in a limit requirement, the between difference is small and the repeatability is high.
Owner:SHANDONG QIDU PHARMA
Clonidine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352473AImprove stabilityDefinite curative effectOrganic active ingredientsPharmaceutical product form changeExtended release tabletsPlastic packaging
The invention provides clonidine hydrochloride sustained release tablets. The clonidine hydrochloride sustained release tablets are prepared from 0.2 part of clonidine hydrochloride, 70-90 parts of sustained release skeleton material and 10-20 parts of lubricating agent by weight. A preparation method of the clonidine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The clonidine hydrochloride sustained release tablets and the preparation method have the beneficial effects that the clonidine hydrochloride sustained release tablets have good stability and definite curative effects and can be used for effectively treating hypertension; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the clonidine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Tablets exhibiting reduced drug release variability
ActiveUS7897172B2Low variabilityReduce and eliminate effectPowder deliveryBiocideExtended release tabletsHardness
Owner:L PERRIGO
Guanidine hydrochloride sustained release preparation and preparation method thereof
ActiveCN102579381AImprove stabilitySimple manufacturing processOrganic active ingredientsGranular deliveryBlood drug concentrationTableting
The invention discloses guanidine hydrochloride sustained release preparation and a preparation method thereof. The guanidine hydrochloride sustained release preparation is mainly prepared by guanidine hydrochloride, a filling agent, sustained release materials and potential of hydrogen (pH) sensitive materials. The preparation method comprises the steps of firstly preparing all raw materials according to proportion, evenly mixing the guanidine hydrochloride, the filling agent, the sustained release materials and the pH sensitive materials at high speed, granulating and drying the evenly mixed powder, finally adding a flow agent, a lubrication agent and an adhesion agent, tableting according to a general method, and achieving guanidine hydrochloride sustained release tablets. The guanidine hydrochloride sustained release preparation is small in side effect, obviously reduces difference of preparation of different batches, improves stability of samples, is convenient long term curing of patients and improves compliance of medicine. A patient can take the guanidine hydrochloride sustained release tablets once a day, so that effective drug concentration in bodies can be guaranteed for 24 hours, and nervous centralis side reactions caused by the fact that a blood concentration peak value is high due to general preparation is reduced.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8545886B2Reduce solubilitySufficiently slow releasePowder deliveryNervous disorderExtended release tabletsOral medication
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting ofa) flibanserin or a pharmaceutically acceptable derivative thereof as active substance;b) one or more pharmaceutically acceptable pH-dependent polymers;c) one or more pharmaceutically acceptable pH-independent polymers;d) one or more pharmaceutically acceptable acids; ande) optionally one or more additives.The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
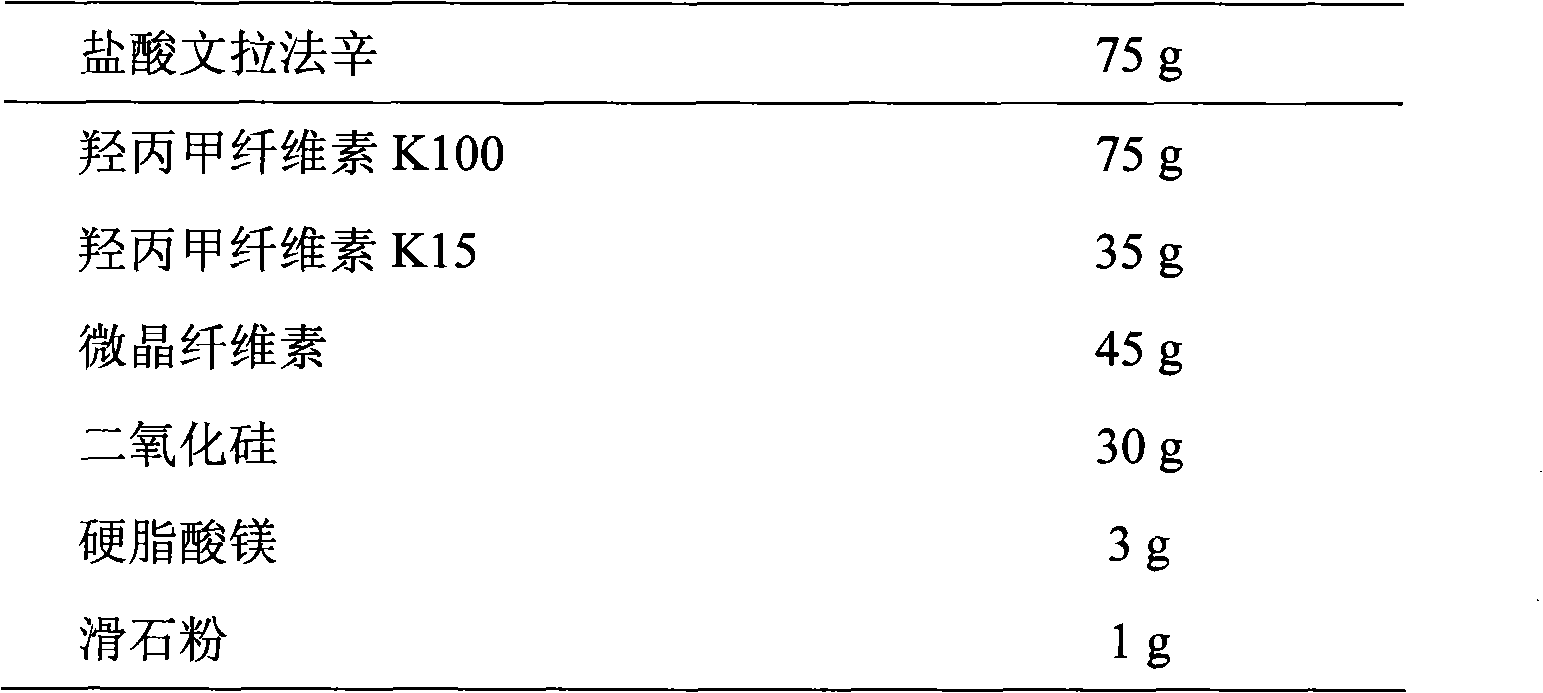
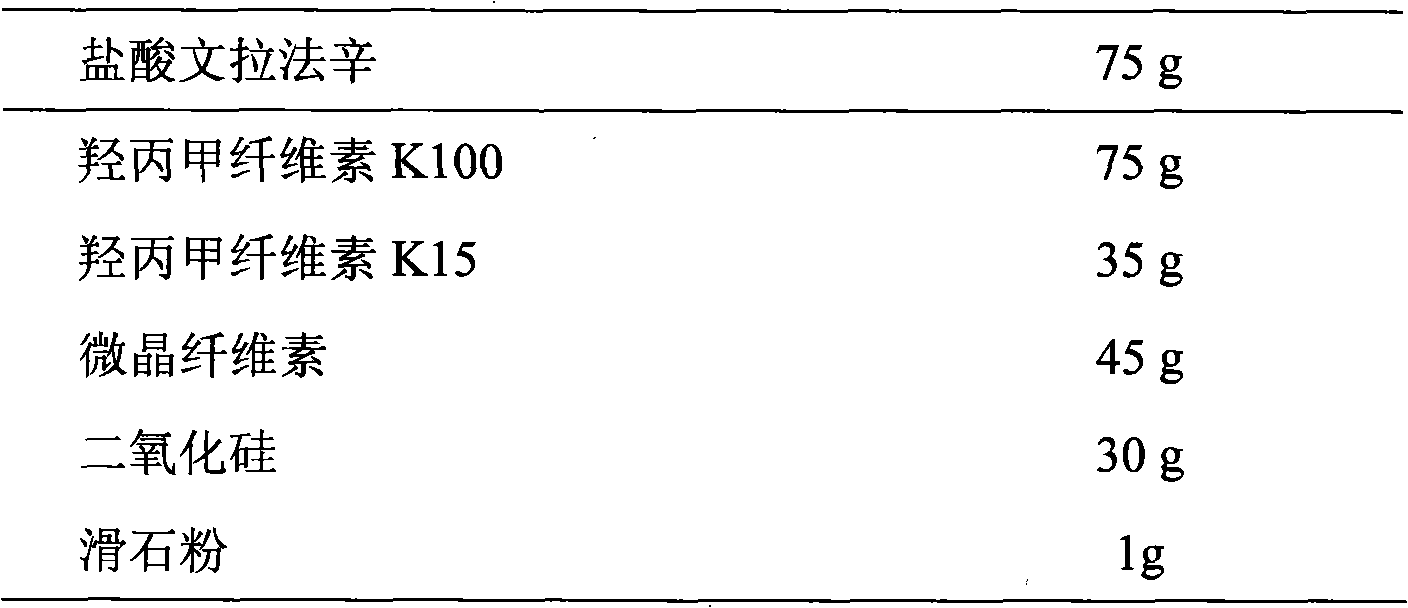
Extended release tablet formulations of venlafaxine
This invention relates to an extended release tableted dosage formulation of the antidepressant venlafaxine hydrochloride or an optical form thereof having improved bioavailability.
Owner:WYETH LLC
Metformin hydrochloride sustained-release tablet
ActiveCN103816130ALow costGood slow releaseOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
The invention provides a metformin hydrochloride sustained-release tablet. The metformin hydrochloride sustained-release tablet is prepared from the components by weight: 400-600 parts of metformin hydrochloride, 30-60 parts of sodium carboxymethylcellulose, 200-250 parts of hydroxypropyl methylcellulose, 180-220 parts of ethyl acrylate-methyl methacrylate copolymer aqueous dispersion and 5-10 parts of magnesium stearate. Suitable auxiliary materials and a suitable preparation method are adopted to prepare the metformin hydrochloride sustained-release tablet with lower raw material cost and simpler process, and the sustained-release performance of the obtained product is good, the release amounts at 1 hour, 3 hours and 10 hours are respectively 20-45 percent, 45-75 percent and above 80 percent, and the drug has good stability and can be preserved for 24 months at the room temperature.
Owner:YOUCARE PHARMA GROUP
Method for preparing potassium citrate sustained-release tablets
InactiveCN101791299ARelease stabilityGood slow-release propertiesOrganic active ingredientsPharmaceutical delivery mechanismALLYL SUCROSEStearic acid
The invention relates to a formula and a process for preparing potassium citrate sustained-release tablets, belonging to the technical field of the medicine. The potassium citrate sustained-release tablets are prepared by mixing pore-forming agent, filler, lubricant and coloring agent which are pharmaceutically acceptable to stearic acid or stearic acid derivative, ethyl cellulose, polyacrylic acid resin and glyceryl monostearate which are used as insoluble framework materials according to a certain proportion. The potassium citrate sustained-release tablets have attractive appearance and can release stably in various release medium and even for 6 months in the acceleration test at 40 DEG C.
Owner:南京泽恒医药技术开发有限公司
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS8658207B2Reduce wearExtended shelf lifePowder deliveryBiocideExtended release tabletsDrug release
The present invention provides pharmaceutical release systems comprising an therapeutically effective amount of flibanserin and at least one pharmaceutically acceptable excipient, characterized in that said pharmaceutical release systems exhibit a pharmacokinetic profile that is characterized by an average maximum flibanserin plasma concentration Cmax lower than 300 ng / mL, preferably lower than 200 ng / mL after administration of a single dose to healthy volunteers in fasted state or directly after a meal.
Owner:BOEHRINGER INGELHEIM INT GMBH
Extended release formulation of levetiracetam
ActiveUS20070092569A1Reduced inter subject variabilityBiocideNervous disorderWater dispersibleExtended release tablets
The present invention relates to extended release pharmaceutical compositions of Levetiracetam and processes for preparing the same. The extended release tablet of Levetiracetam is with a core comprising of Levetiracetam and water dispersible rate controlling polymer, and the tablet core is optionally functional coated comprising a combination of water non-dispersible and / or water dispersible polymer. It provides extended therapeutically effective plasma levels over a twenty four hour period with diminished incidences of neuropsychiatric adverse events by eliminating the troughs and peaks of drug concentration in a patient's blood plasma. The composition also exhibits no food effect.
Owner:UCB PHARMA SA
Venlafaxine hydrochloride sustained-release tablet preparation and preparation method thereof
InactiveCN101584674AGood sustained release effectOrganic active ingredientsNervous disorderSustained Release TabletAdhesive
The invention discloses a venlafaxine hydrochloride sustained-release tablet preparation which comprises venlafaxine hydrochloride, framework material, diluent, lubricant, adhesive and coating material. The experiments demonstrate that the venlafaxine hydrochloride sustained-release tablet achieves better sustained-release effect, can reduce the dosing frequency and is convenient for the patients to use. The invention also provides a preparation method thereof.
Owner:上海医药科技发展有限公司
Compound ambroxol hydrochloride sustained-release tablet and preparation method thereof
InactiveCN101084912AImprove complianceReduce the number of dosesAntibacterial agentsOrganic active ingredientsSustained Release TabletAdjuvant
The invention provides a preparation method of compound ambroxol hydrochloride sustained release matrix tablet. The inventive ambroxol hydrochloride sustained release tablet specifically comprises (g / g) ambroxol hydrochloride 5-20%, roxithromycin 15-60%, skeletal material 5-40%, diluent 5-50%, pH regulator 0-20%, proper amount of binding agent, and proper amount of lubricant. The preparation method comprises mixing the materials and tabletting directly; or granulating part of the materials, mixing with the rest materials, mixing, and preparing into sustained release tablet; or mixing part of the raw materials with adjuvant, preparing into quick-release part, mixing the rest raw material, skeletal material, pH regulator and diluent as slow-released part, and preparing into double layer tablet with quick-release layer and sustained release layer. The method for preparing granulate comprises dry method, wet method, melting or fusion.
Owner:SHANDONG INST OF PHARMA IND
Compound preparation for treating and preventing thrombosis and apoplexia as well as its preparing method
InactiveCN1480144AReduce manufacturing costSmall fluctuations in performanceSalicyclic acid active ingredientsBlood disorderBenzoic acidDipyridamole
A slowly-releasing compound medicine in the form of tablet for treating and preventing thrombosis and apoplexy is prepared from aspirin, 2-(acetoxy) benzoic acid, slow-releasing dipyridamole, 2,2',2'',2''', [(4,8-dipiperidylpyrimido[5,4-d] pyrimidine-2,6-diyl) dihyponitido]-tetraethanol through granulating, tabletting and coating. Its advantages are high curative effect and equickly taking its effect.
Owner:CHENGDU LIST PHARMA
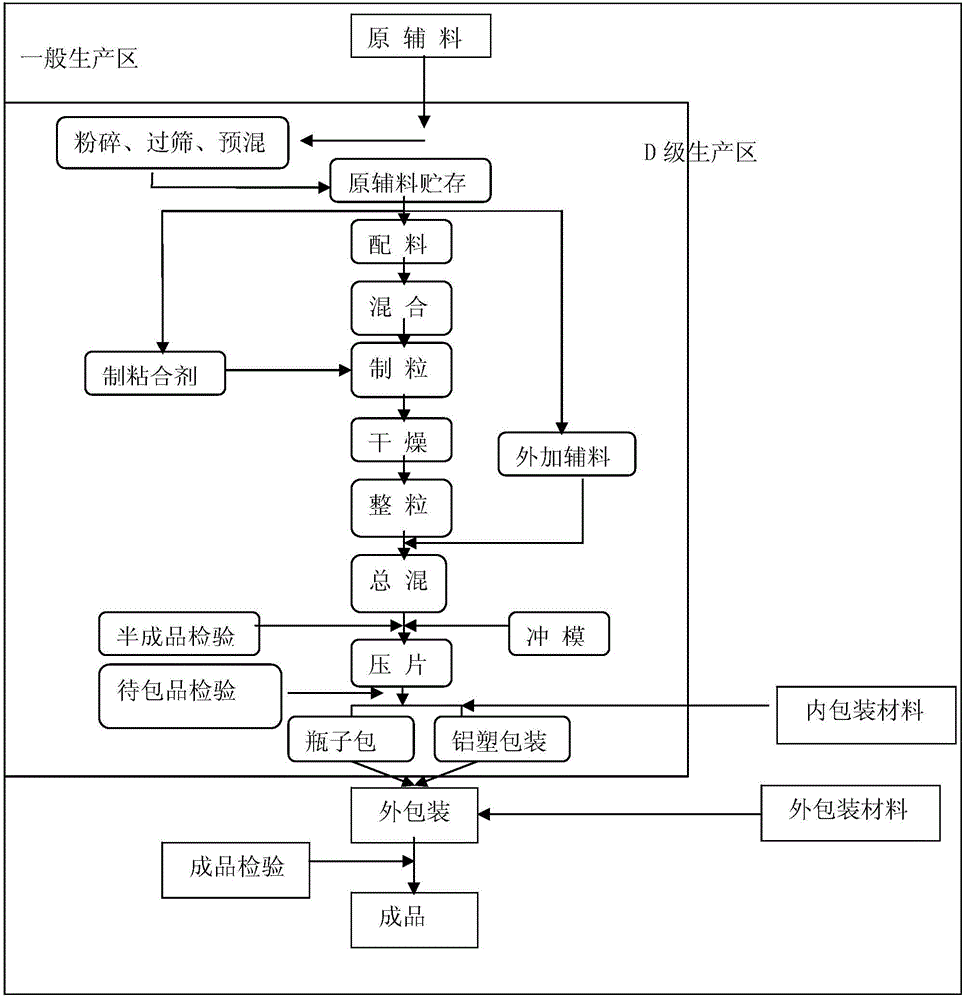
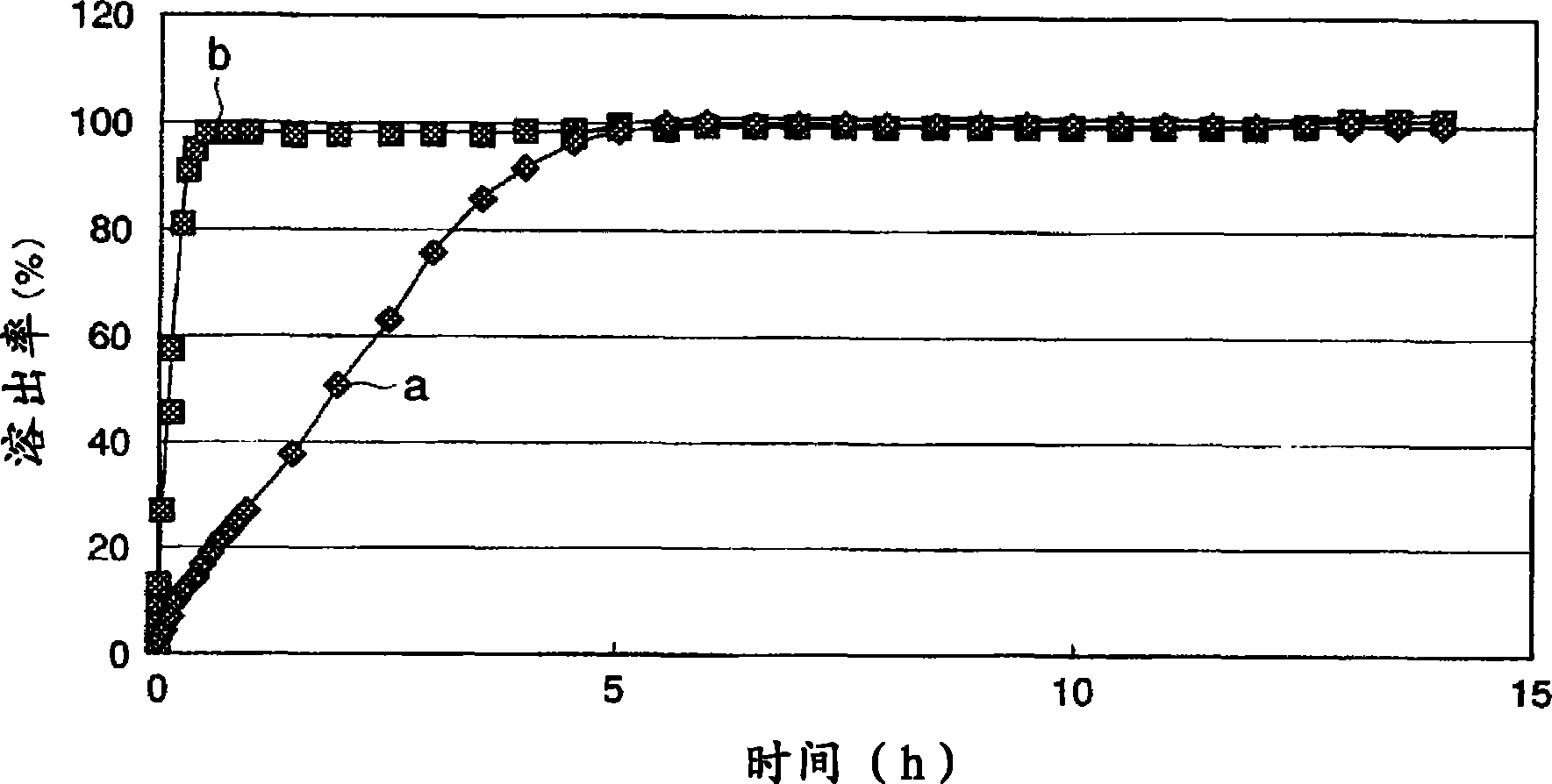
Method for producing extended release tablet
ActiveCN101420937AEfficient preparationDissolution inhibitionAntipyreticAnalgesicsCelluloseExtended release tablets
Disclosed is a method for producing an extended release tablet, which enables to efficiently produce such an extended release tablet having excellent slow release property that is suppressed in initial release of a drug but capable of completing release of the drug after a predetermined time. Specifically disclosed is a method for producing an extended release tablet wherein a mixture, which is composed of a hydroxyalkyl cellulose (A) whose 2% by mass aqueous solution has a viscosity at 20 DEG C of 1-50 mPa s, a hydroxyalkyl cellulose (B) whose 2% by mass aqueous solution has a viscosity at 20 DEG C of not less than 100 mPa s, an active ingredient and an additive, is granulated by dry granulation and then the thus-obtained granules are formed into tablets, or alternatively the mixture is directly formed into tablets.
Owner:NIPPON SODA CO LTD
Metformin hydrochloride floating sustained-release tablet and preparation method thereof
InactiveCN108969501AGood floating performanceGood sustained release effectOrganic active ingredientsMetabolism disorderDrugMetformin Hydrochloride
The invention provides a metformin hydrochloride floating sustained-release tablet and a preparation method thereof. The metformin hydrochloride floating sustained-release tablet comprises a tablet core and a non-gastric-soluble coating film wrapped outside the tablet core, wherein the tablet core comprises metformin hydrochloride, a floating material, an adhesive and other excipients, and non-gastric-soluble coating film comprises a film forming material and other excipients. The tablet core is prepared by matching the above materials, and is matched with the non-gastric-soluble coating filmoutside the tablet core, and the density of the tablet core is less than 1g / cm<3>, so that the metformin hydrochloride floating sustained-release tablet can immediately float in water or gastric juice, thereby reducing the possibility of the tablet being discharged from the stomach into the duodenum, and prolonging the time in which the metformin hydrochloride sustained-release tablet floats in the stomach; the metformin hydrochloride floating sustained release tablet can float until the contents of the stomach are emptied, thereby ensuring that the medicine is released in the stomach; the metformin hydrochloride floating sustained-release tablet provided by the invention does not swell substantially in water or gastric juice, thereby reducing the possibility of abdominal distension feeling of a patient.
Owner:奕利制药有限公司
Modified release venlafaxine hydrochloride tablets
InactiveUS20050048118A1Organic active ingredientsNervous disorderVenlafaxine tabletExtended release tablets
A modified release tablet of venlafaxine hydrochloride is formed by a core containing a lipophilic matrix and venlafaxine hydrochloride and a water-insoluble, permeable coating thereover.
Owner:SYNTHON IP
Pharmaceutical compositions and formulations of metformin extended release tablets
InactiveUS20070275061A1High viscositySmall average pore sizeBiocideOrganic active ingredientsDrug release rateBlood level
The present invention relates to a metformin extended release tablet, particularly to a metformin extended release tablet comprising metformin, which is effective in treating non-insulin dependent diabetes mellitus, as active ingredient, and a matrix in which hydrophilic polymers and hydrophobic materials are mixed at a specific proportion. The hydrophilic polymers enable controlling of the pore size of the gel layer formed by water swelling, thereby enabling primary control of drug release rate. And, the hydrophobic materials block the pores of the gel layer, thereby enabling secondary control of drug release rate. Therefore, the metformin extended release tablet of the present invention has better dissolution properties than conventional extended release tablets and, thus, enables extended drug release at constant rate even with less matrix. A constant blood level can be maintained with one administration a day and the tablet can be made smaller, which makes the administration easier and the production simpler.
Owner:HANALL PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com