Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
A metformin hydrochloride enteric and metformin hydrochloride technology, which is applied in the field of metformin hydrochloride enteric-coated sustained-release tablets and the preparation thereof, and can solve the problems such as failure to conduct a metformin hydrochloride release test and unsatisfactory effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
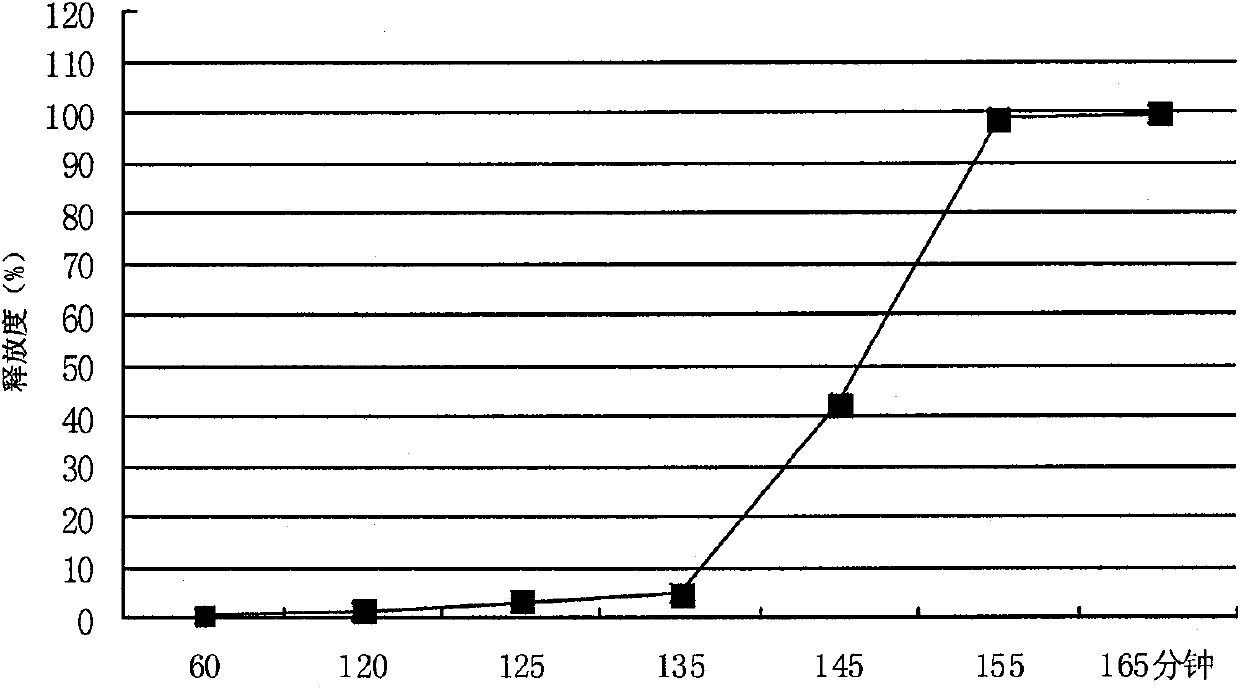
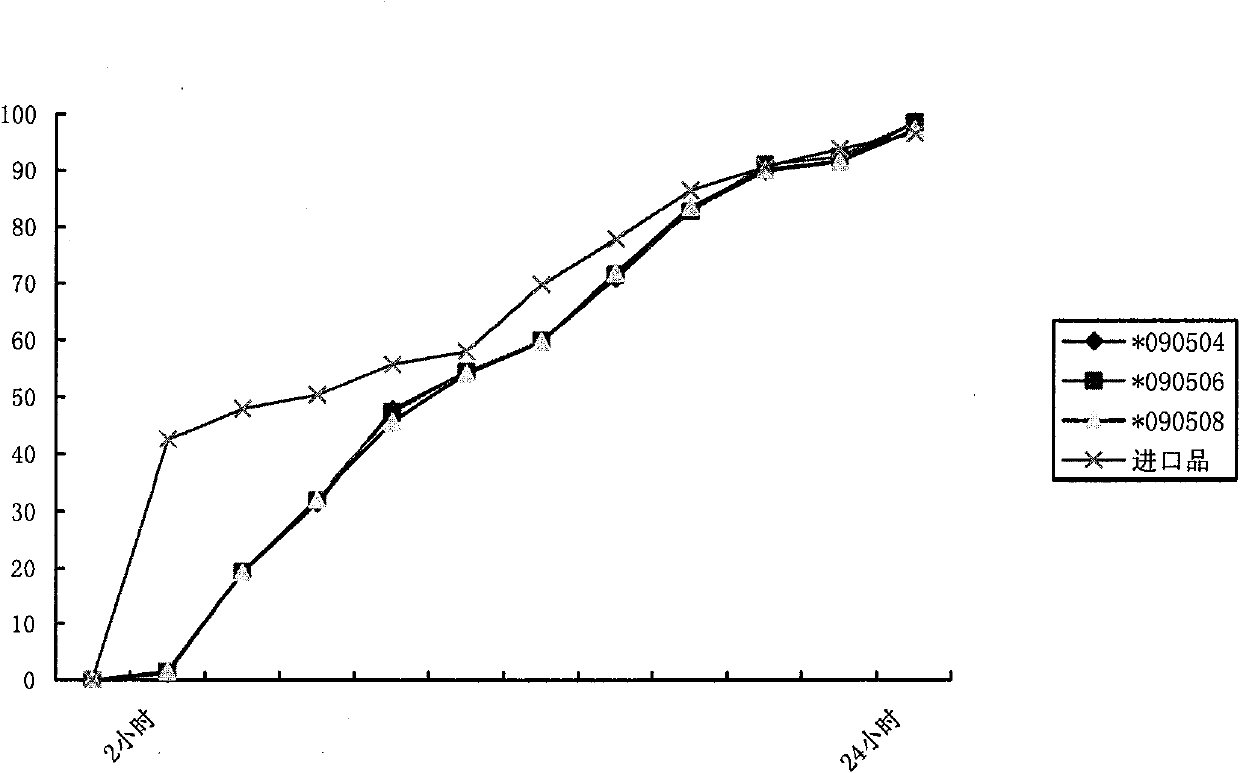
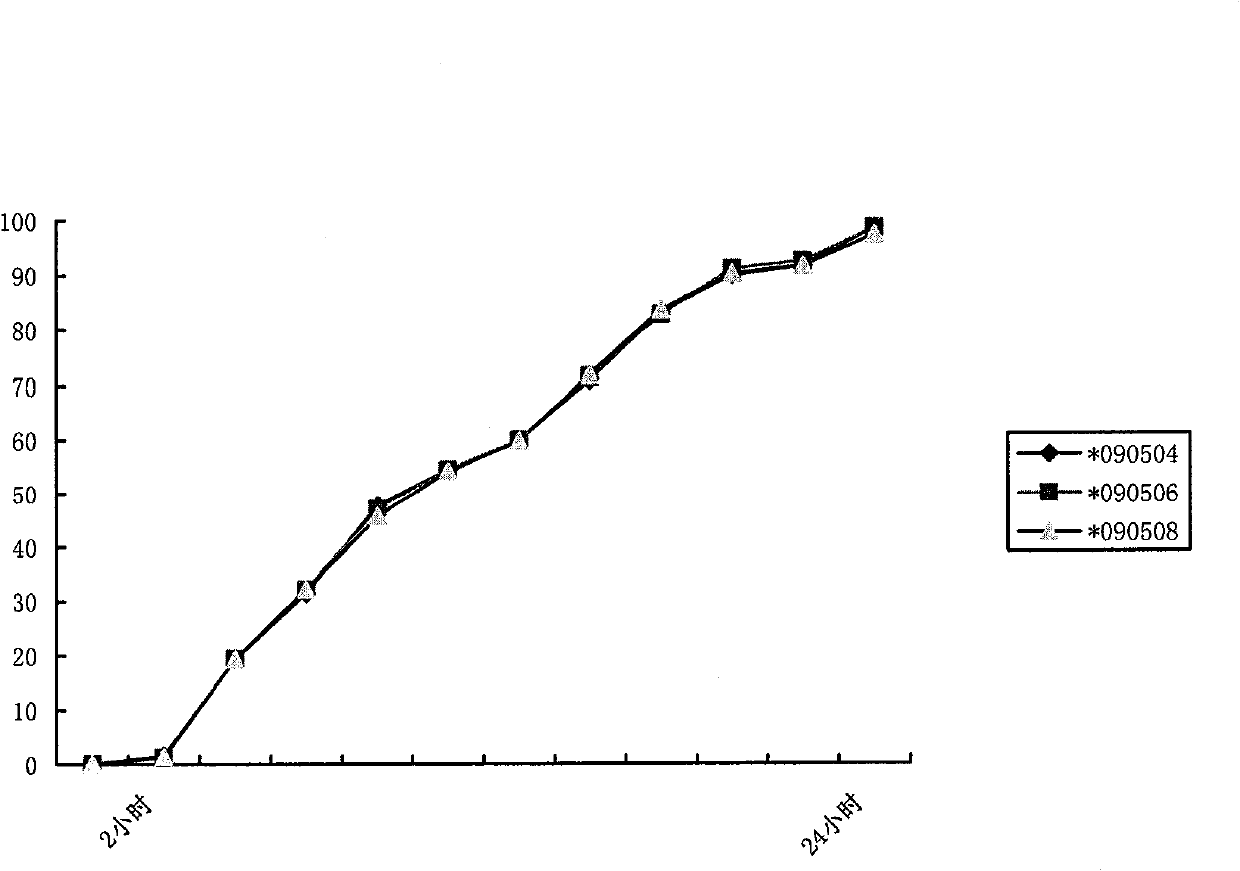
[0055] 1. Preparation prescription: Metformin hydrochloride 500g, sodium carboxymethylcellulose 40g, hypromellose 200g, polyvinylpyrrolidone K30 80g, 30% polyacrylic acid resin (No. IV ethanol solution 60-120ml, magnesium stearate 1% , enteric-coated powder (off-white, the enteric-coated agent of Huzhou Prospect Pharmaceutical Co., Ltd.) and an amount of ethanol, the enteric-coated sustained-release tablet of 500mg metformin hydrochloride / tablet is made.
[0056] 2. Preparation process
[0057] (1) Preparation of adhesive
[0058] Accurately weigh an appropriate amount of polyacrylic acid resin No. IV, and prepare a solution with a concentration of 30% with 95% ethanol for subsequent use.
[0059] (2) Preparation of tablet core
[0060] Take the main drug metformin hydrochloride and the auxiliary materials sodium carboxymethylcellulose, hypromellose, and polyvinylpyrrolidone K30, mix well and pass through a 60-mesh sieve, use 60-120ml of 30% polyacrylic acid resin IV ethanol s...
Embodiment 2
[0064] Preparation prescription: metformin hydrochloride 500g, sodium carboxymethylcellulose 30g, hypromellose 300g, polyvinylpyrrolidone K30 116g, 20% polyacrylic resin No. IV 60-120ml, magnesium stearate 1.0%, enteric coating powder (off-white, the enteric-coated coating agent of Huzhou Prospect Pharmaceutical Co., Ltd.) and ethanol are appropriate, make the enteric-coated slow-release tablet of 500mg metformin hydrochloride / tablet according to the method for embodiment 1
Embodiment 3
[0066] Preparation prescription: metformin hydrochloride 500g, sodium carboxymethylcellulose 30g, hypromellose 300g, polyvinylpyrrolidone K30 116g, 20% polyacrylic resin No. II 60-120ml, magnesium stearate 1.0%, enteric coating powder (off-white, the enteric-coated coating agent of Huzhou Prospect Pharmaceutical Co., Ltd.) and ethanol are appropriate, make the enteric-coated slow-release tablet of 500mg metformin hydrochloride / tablet according to the method for embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com