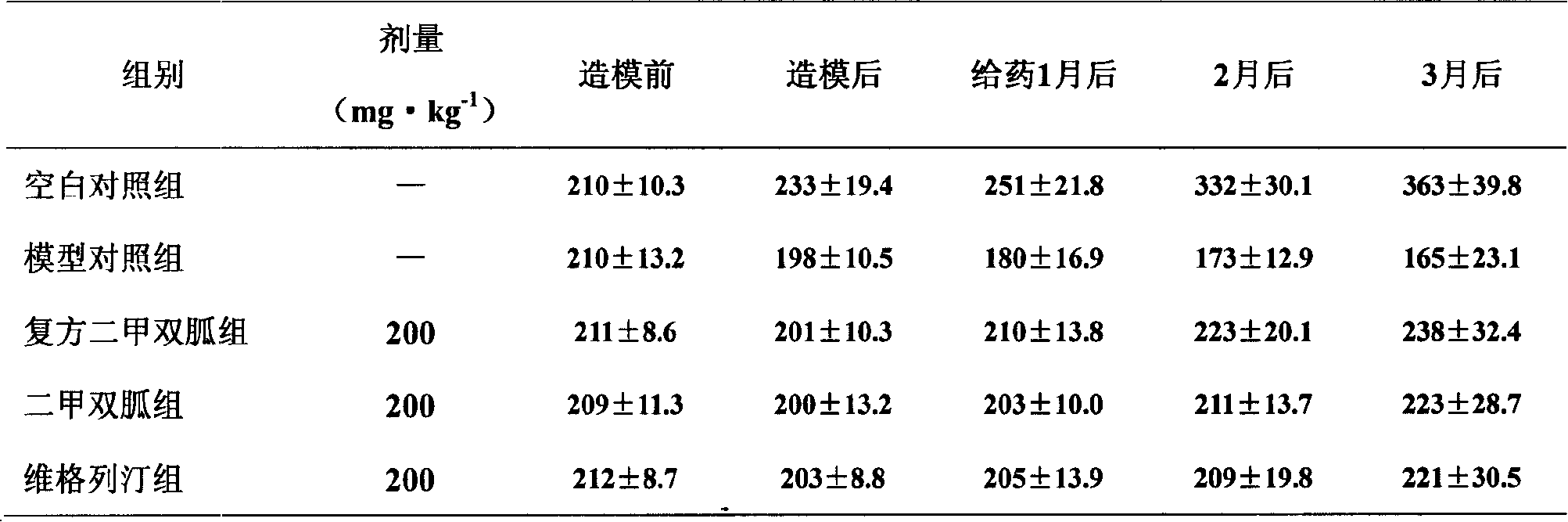
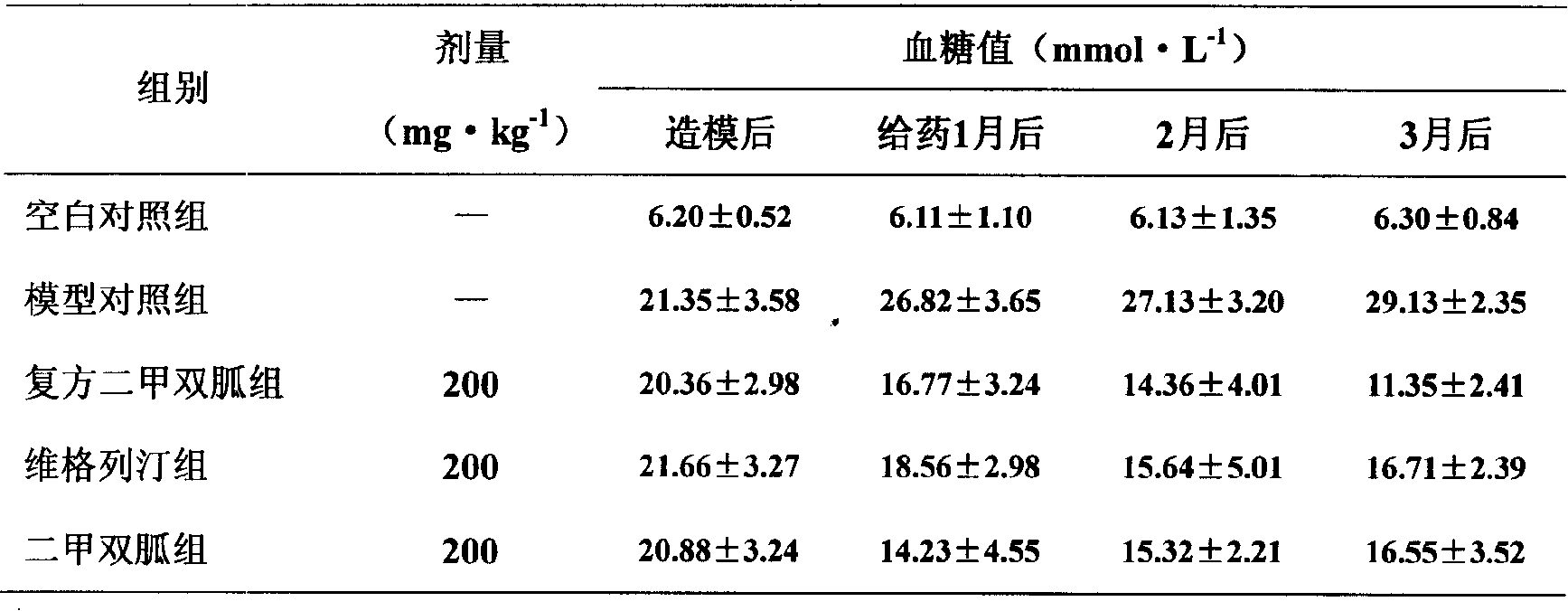
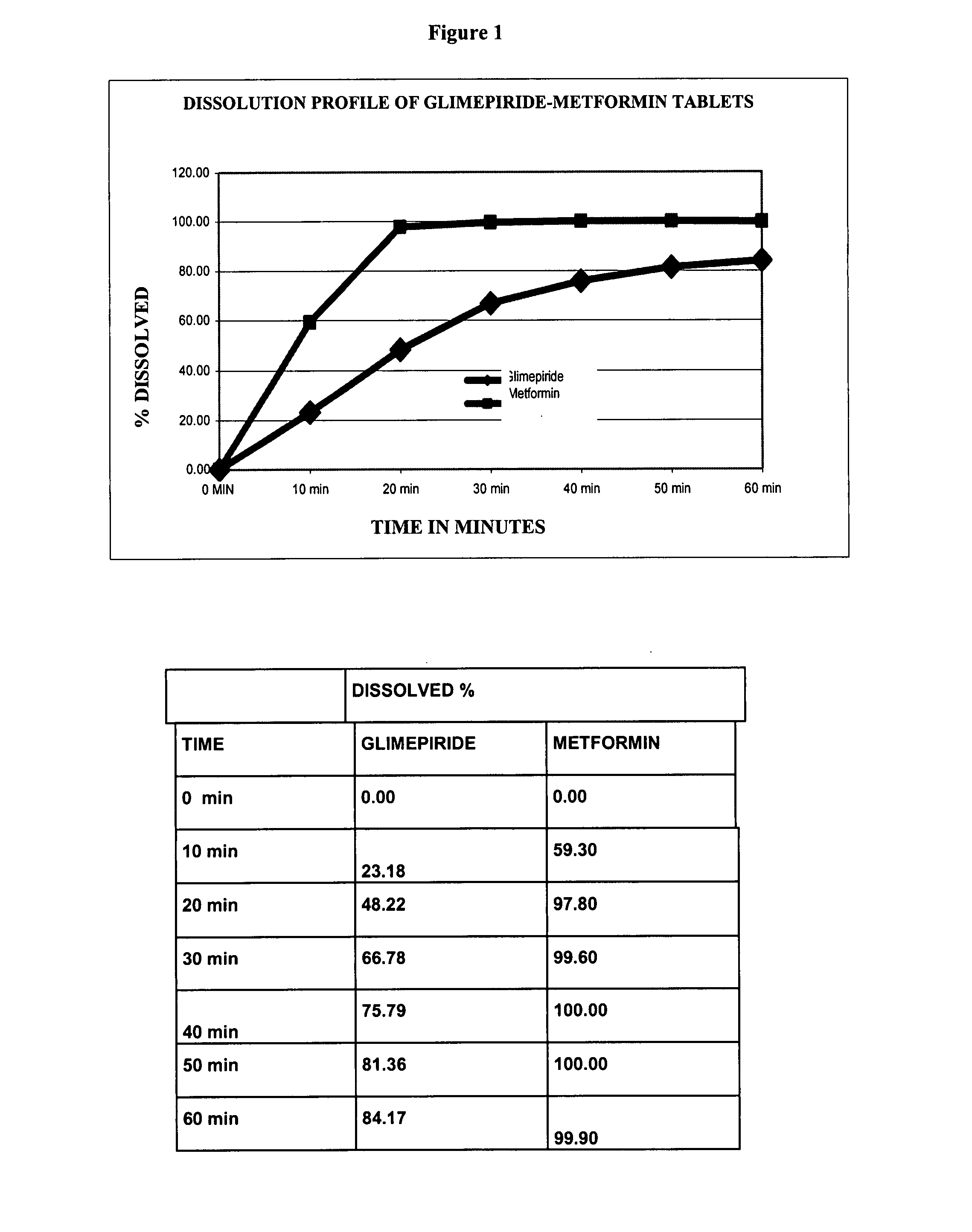
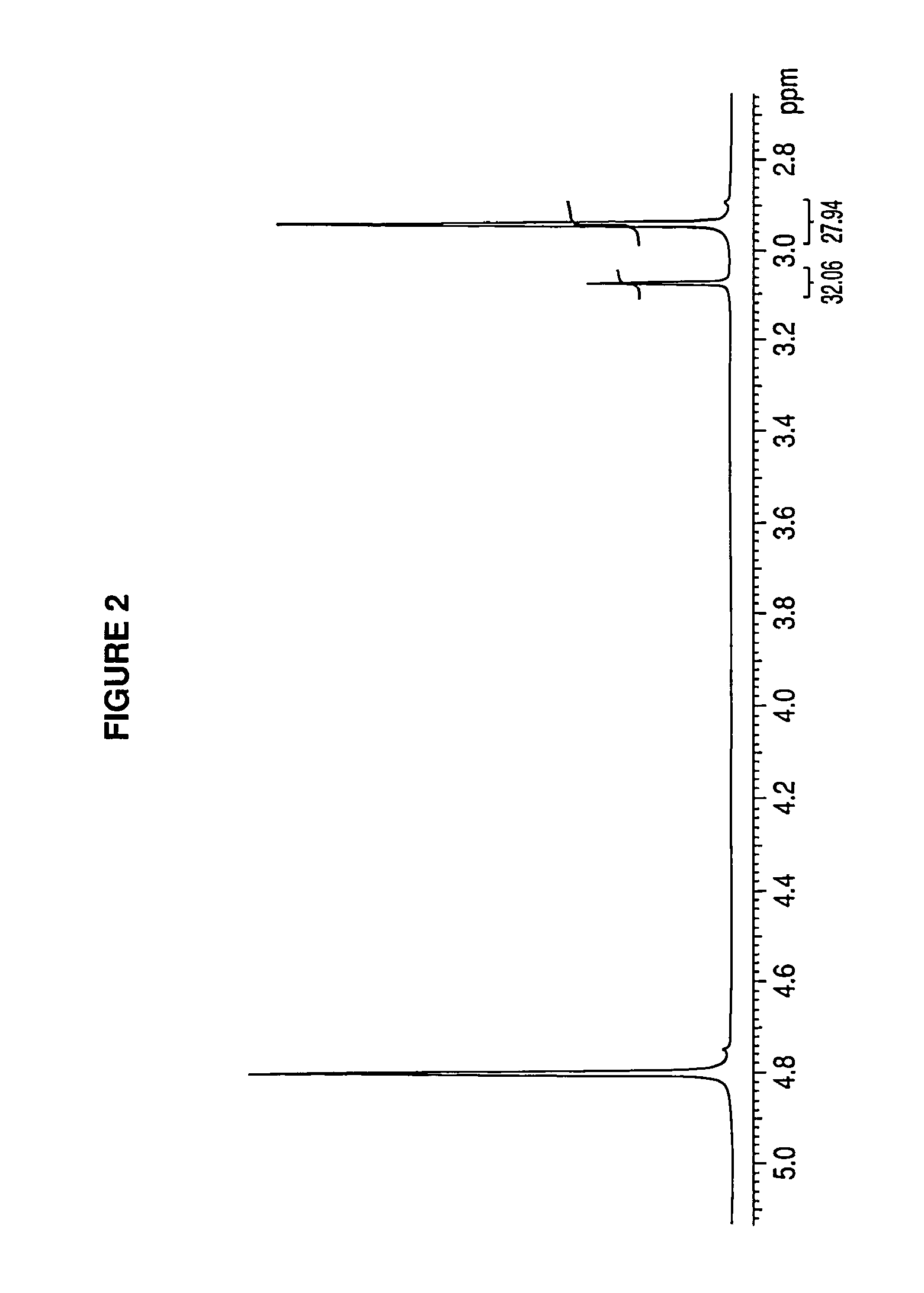
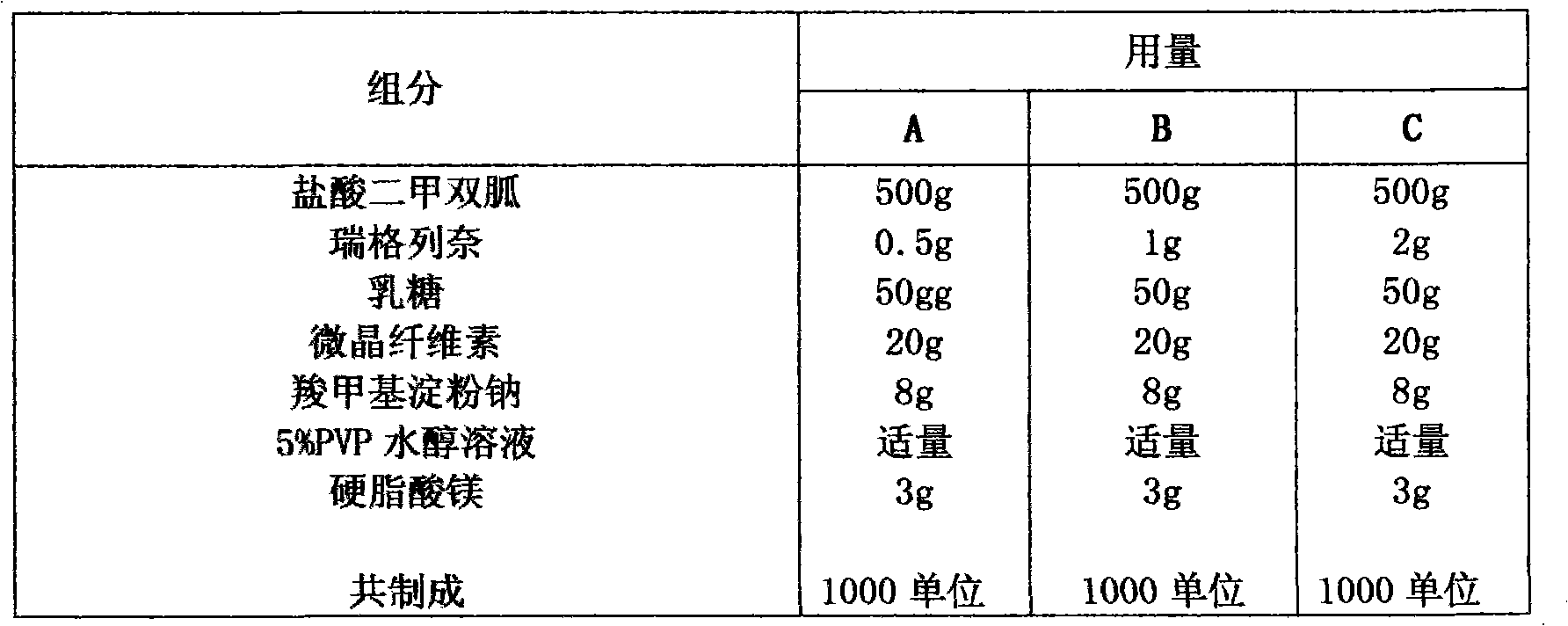
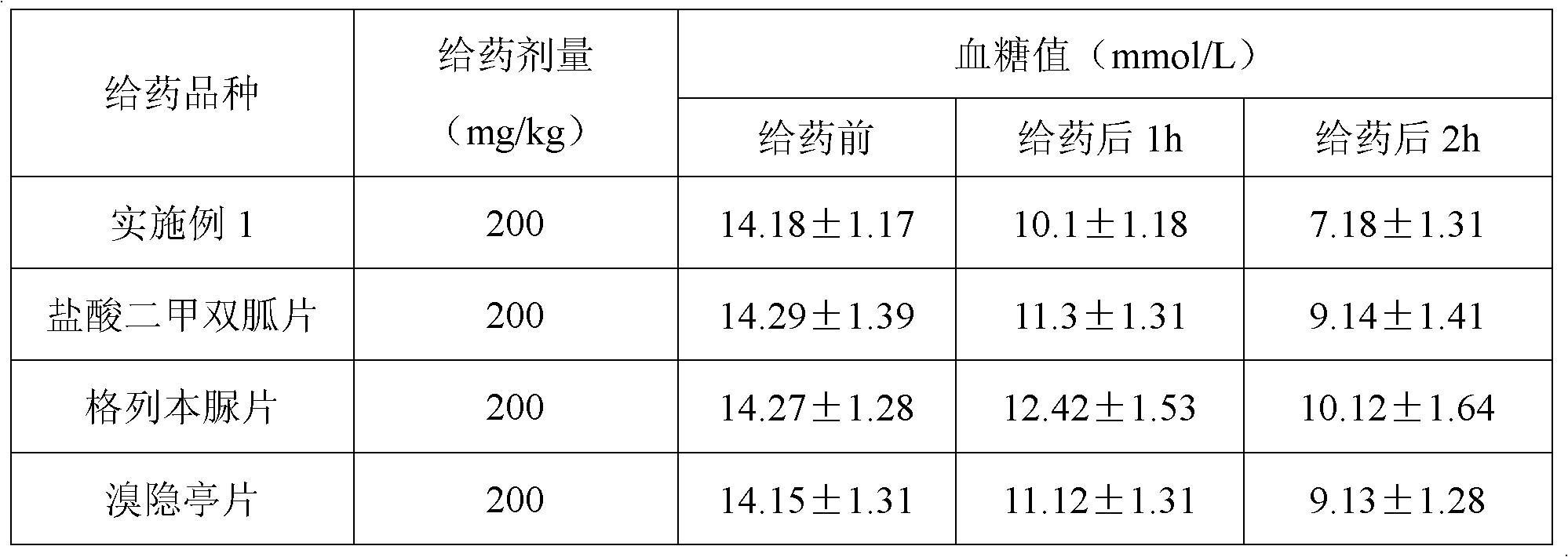
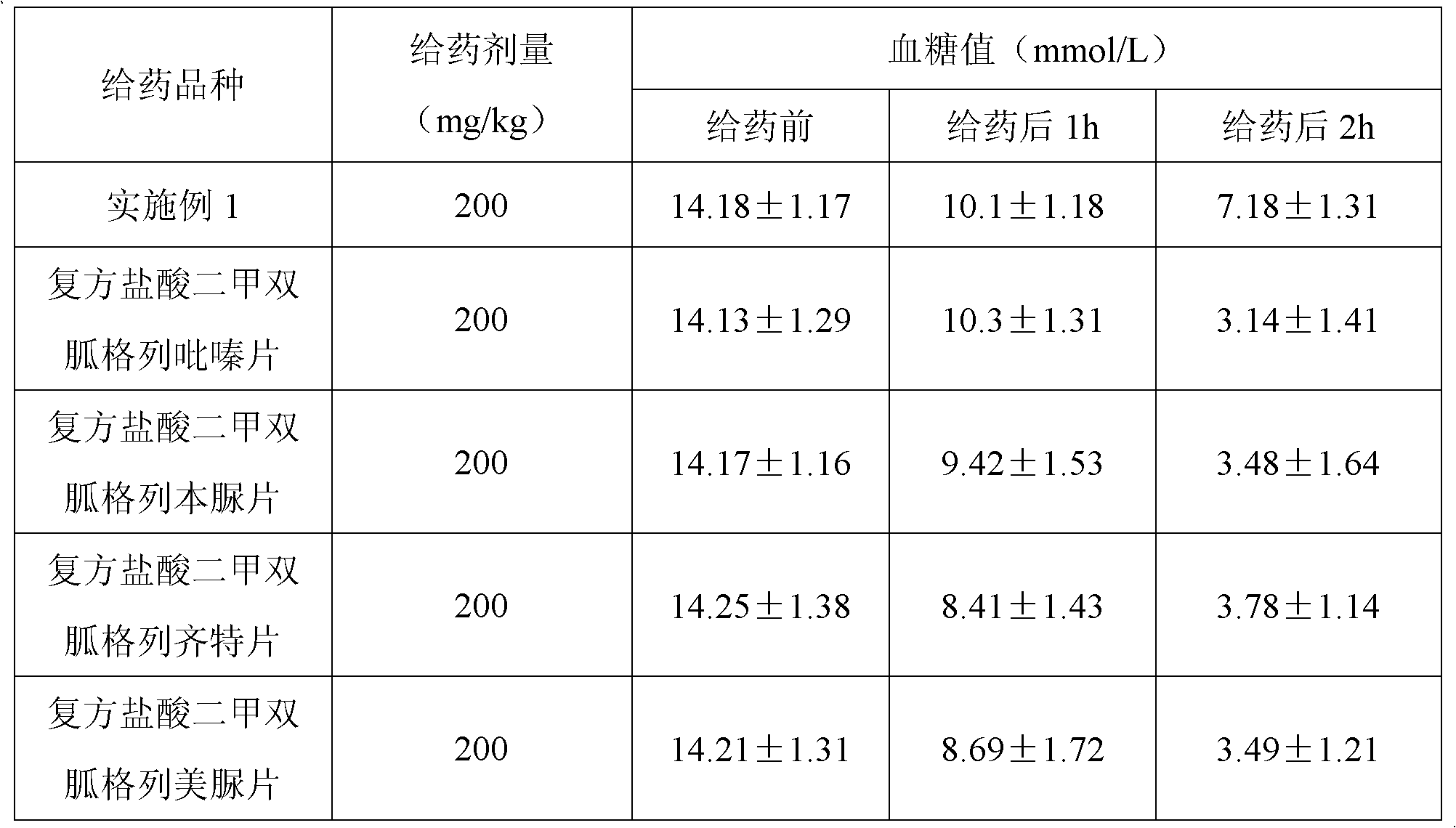
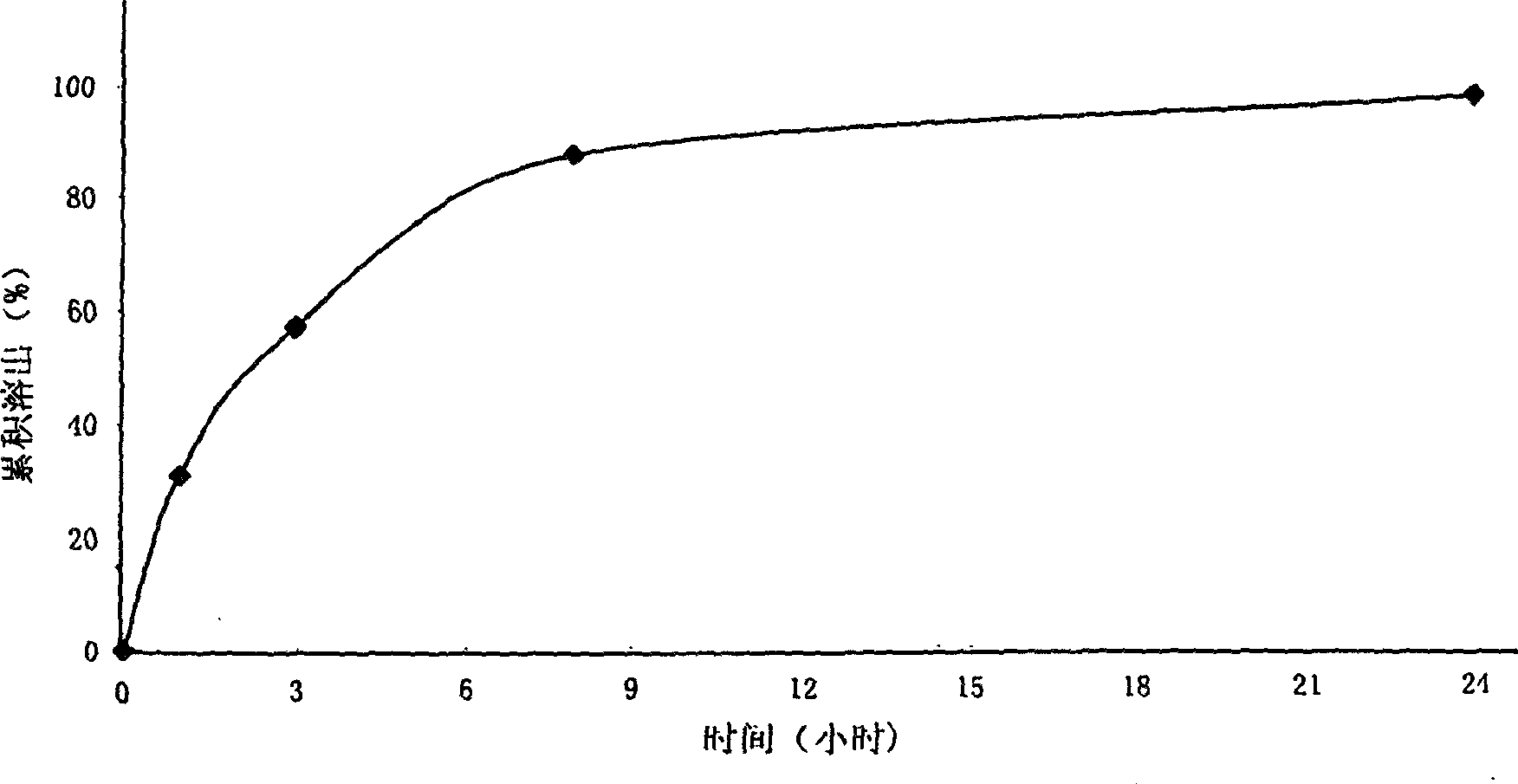
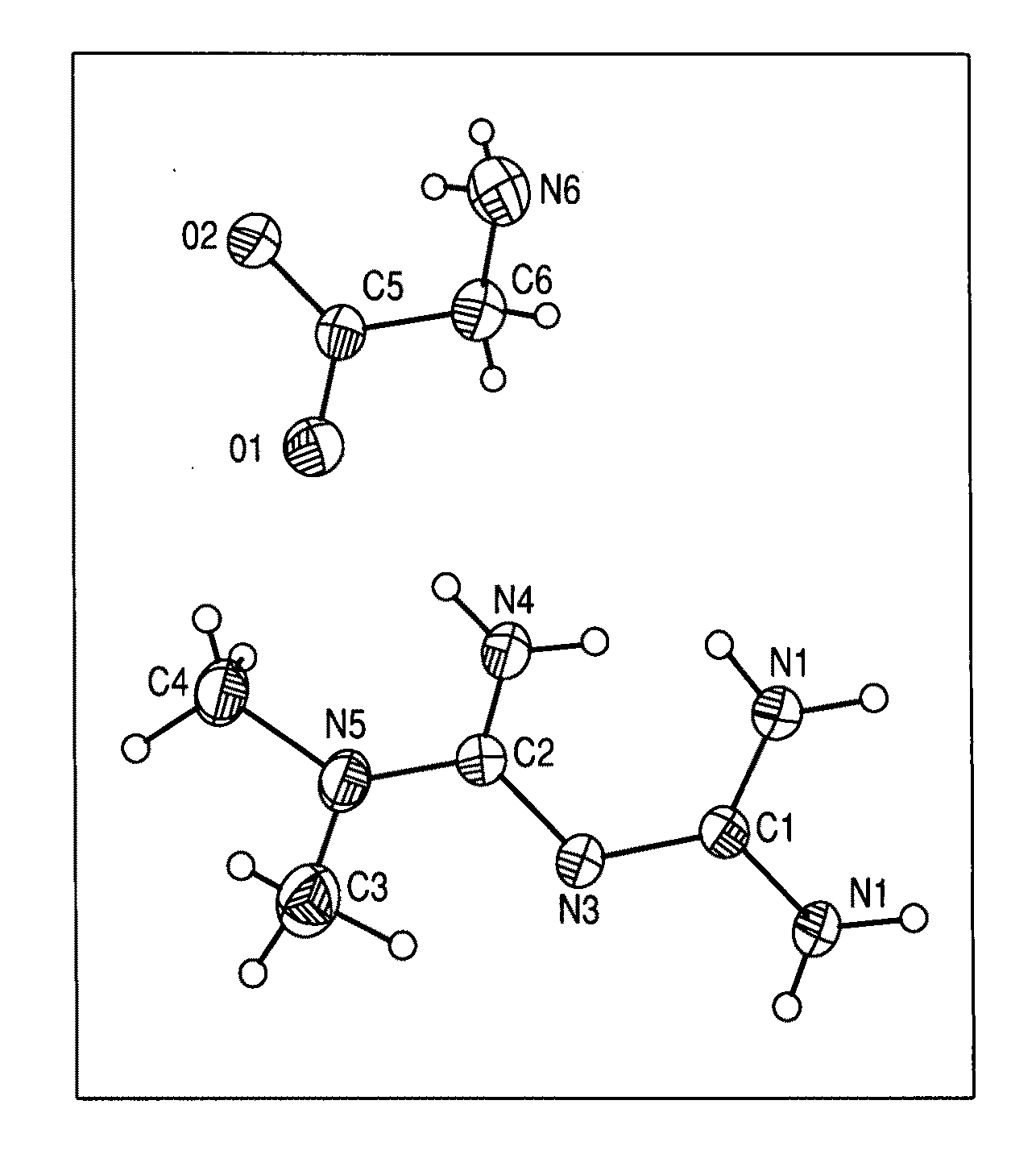
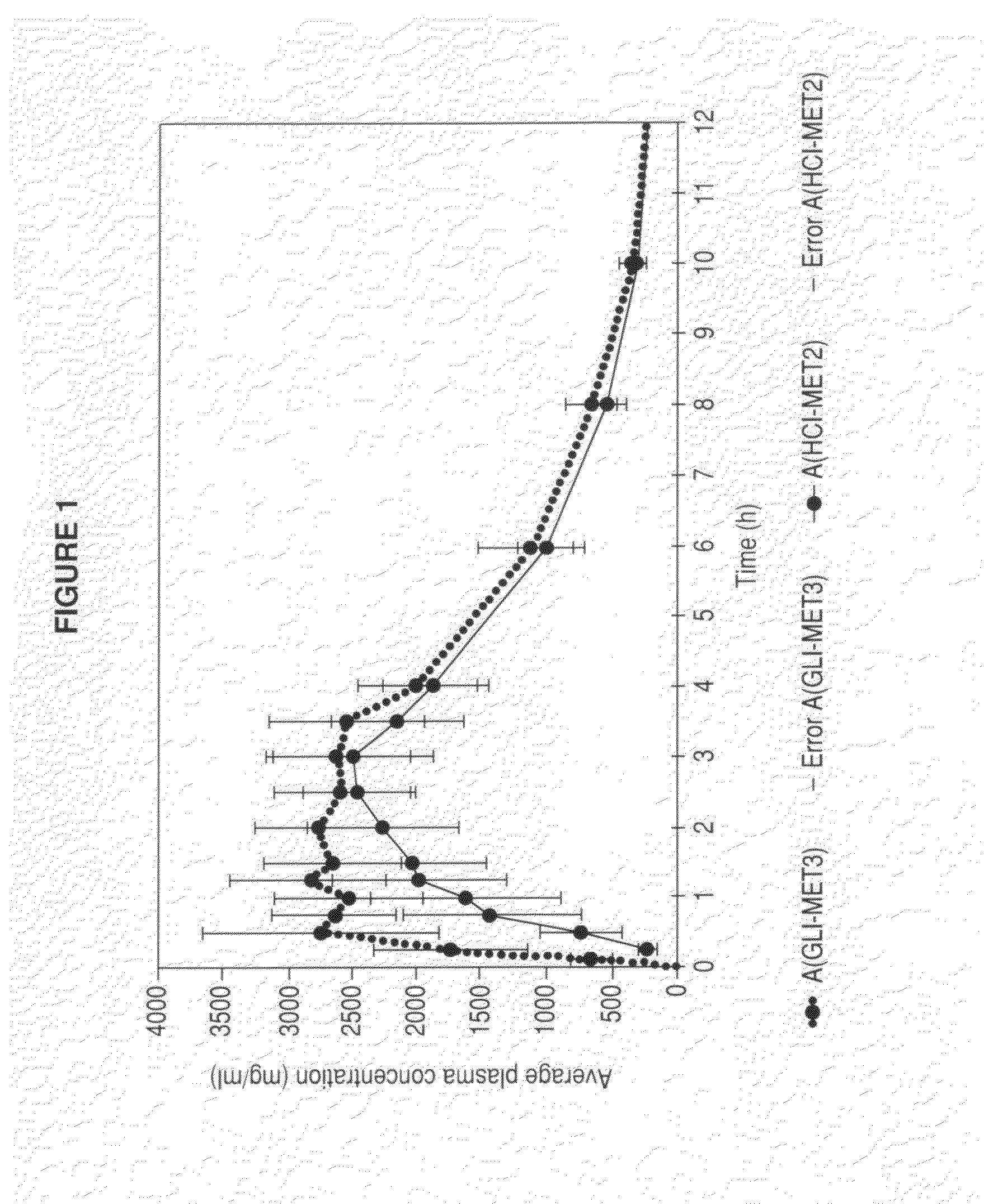
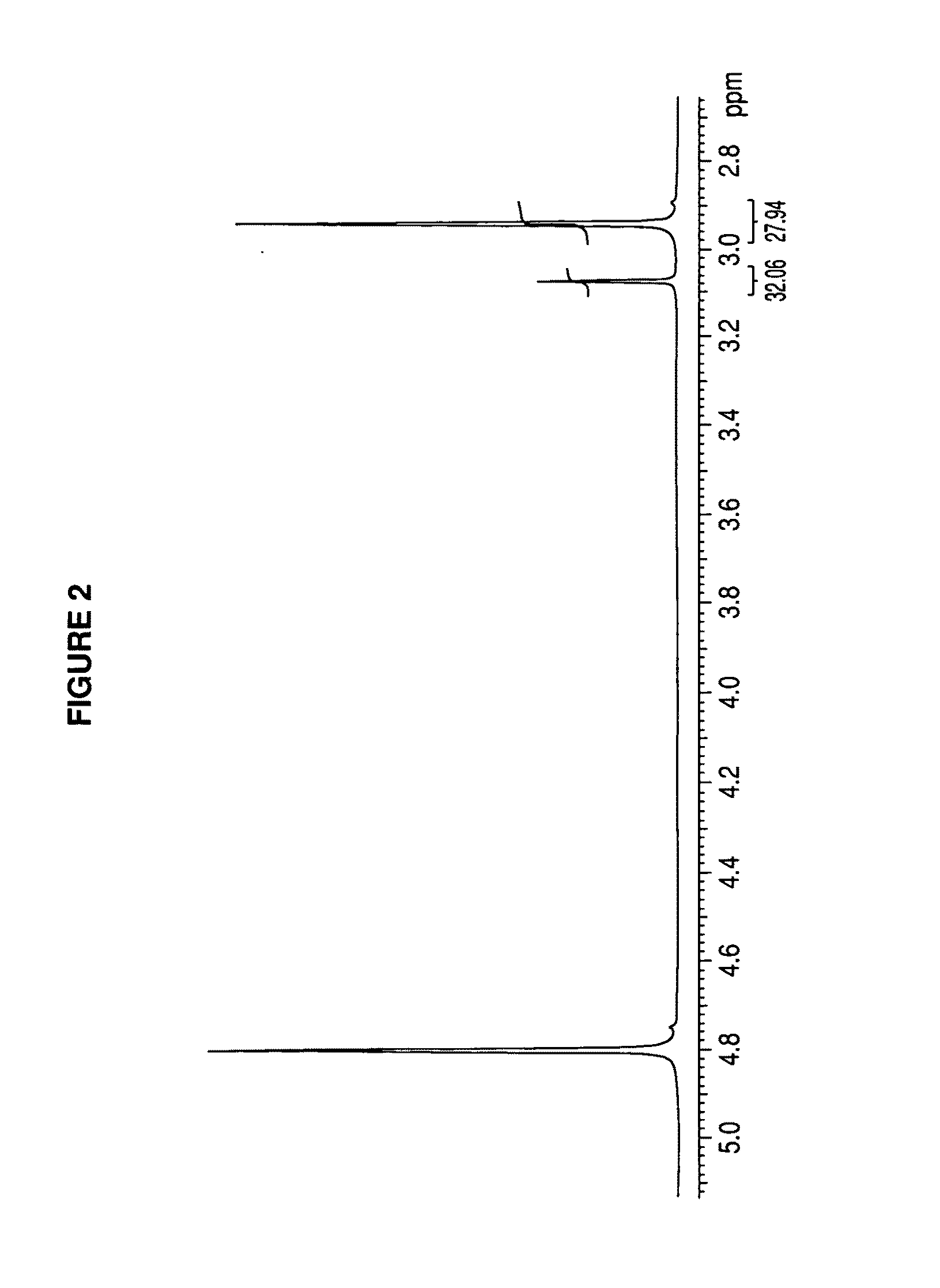
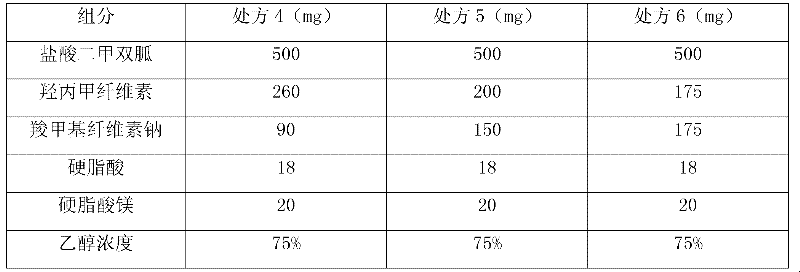
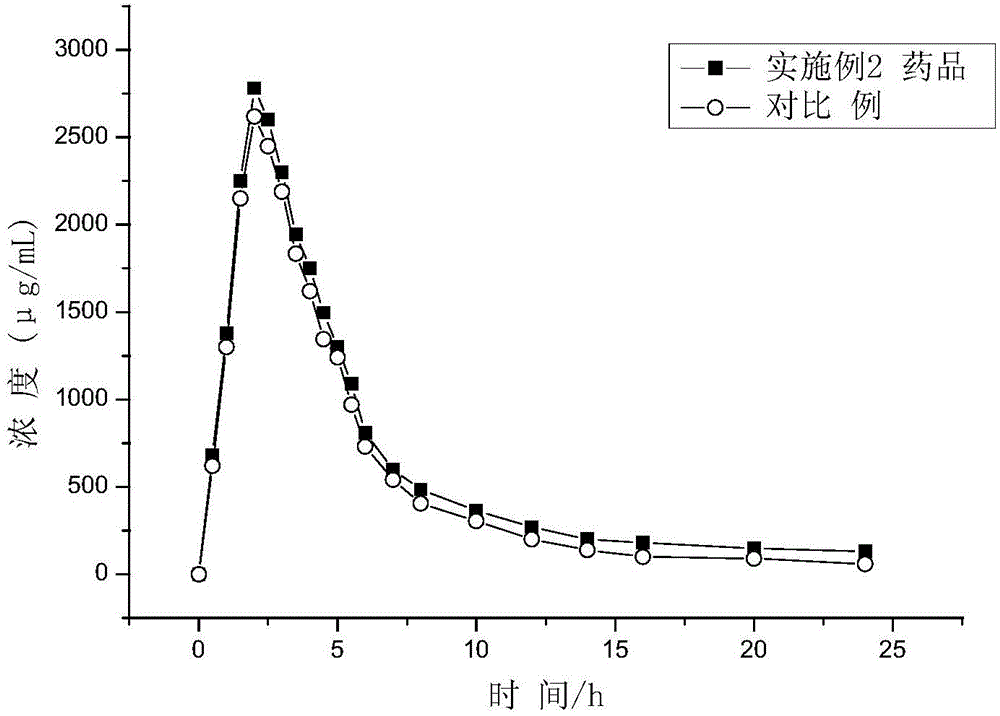
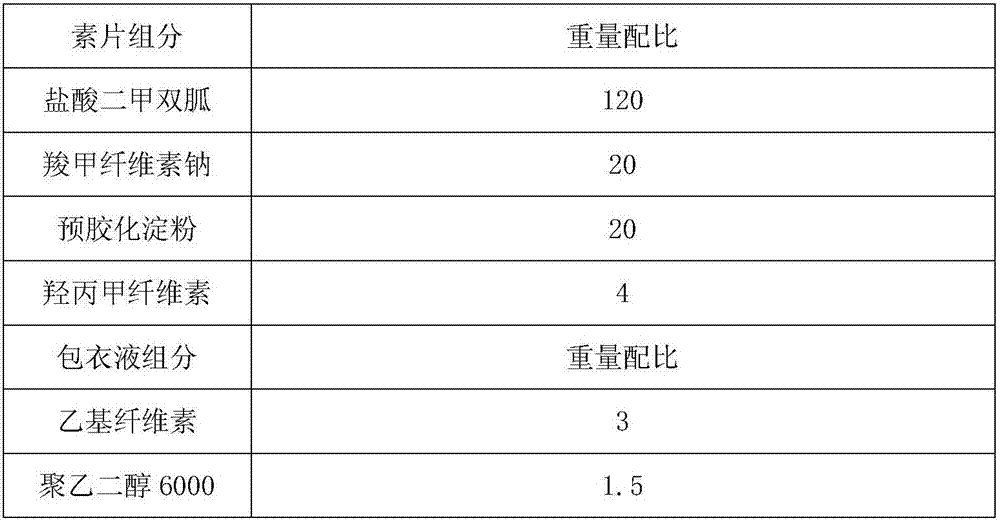
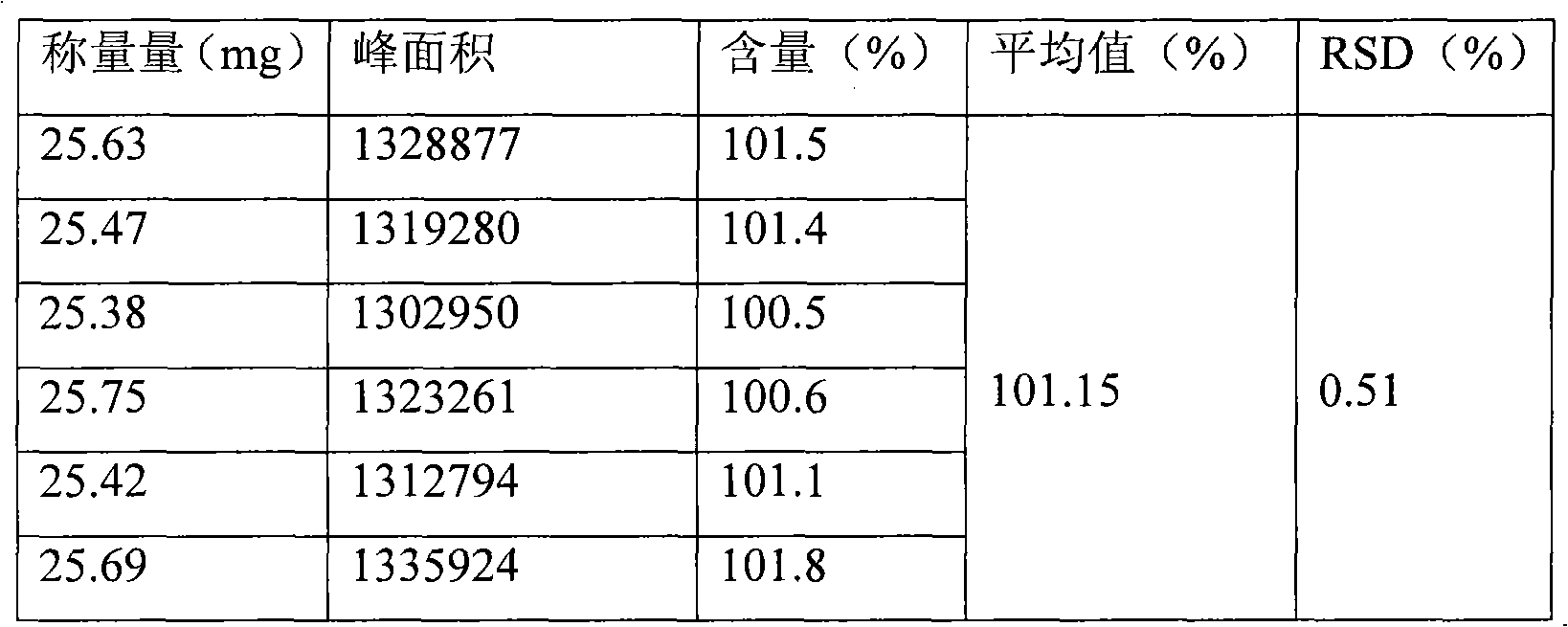
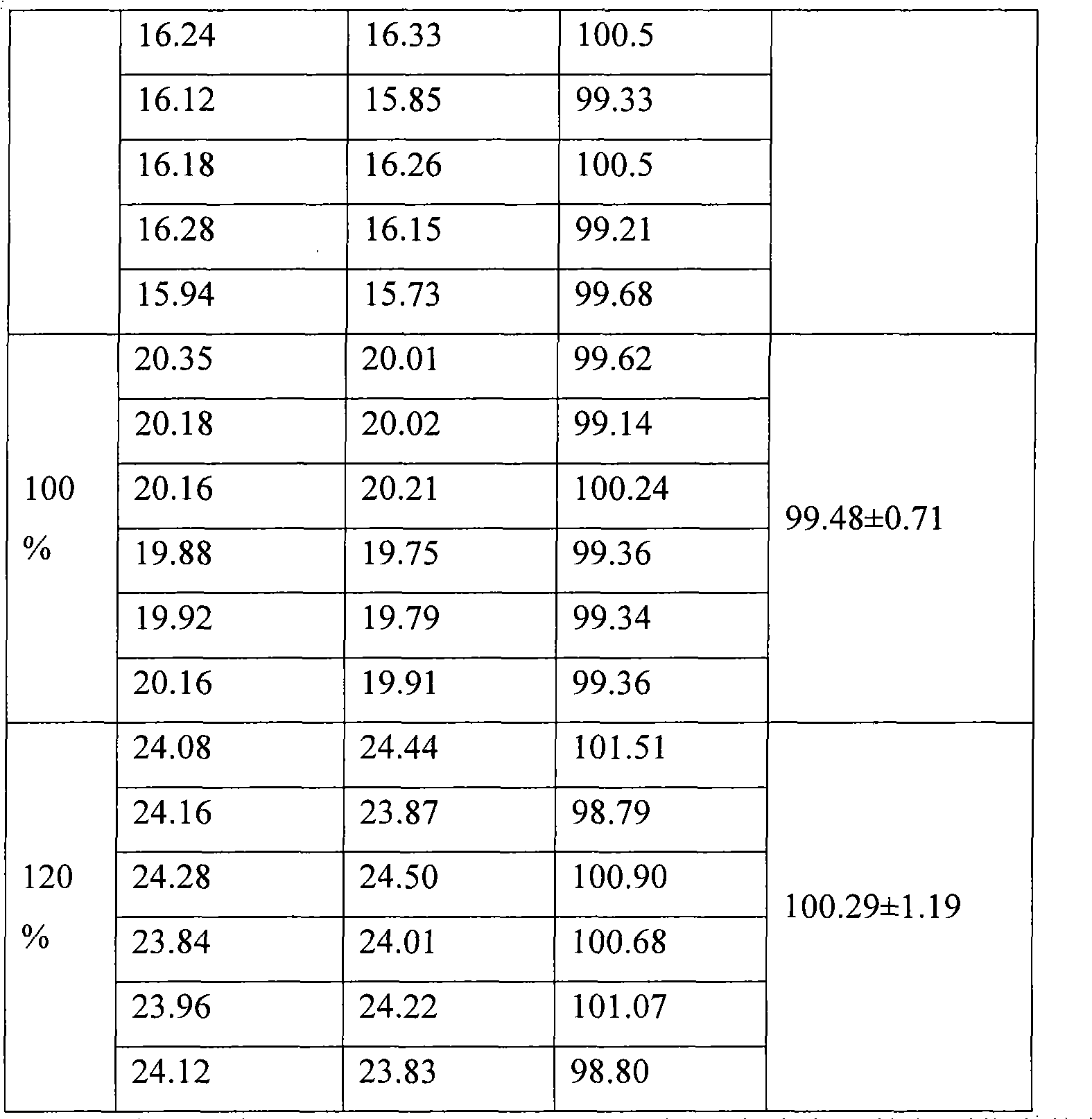
Patents
Literature
294 results about "Metformin Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
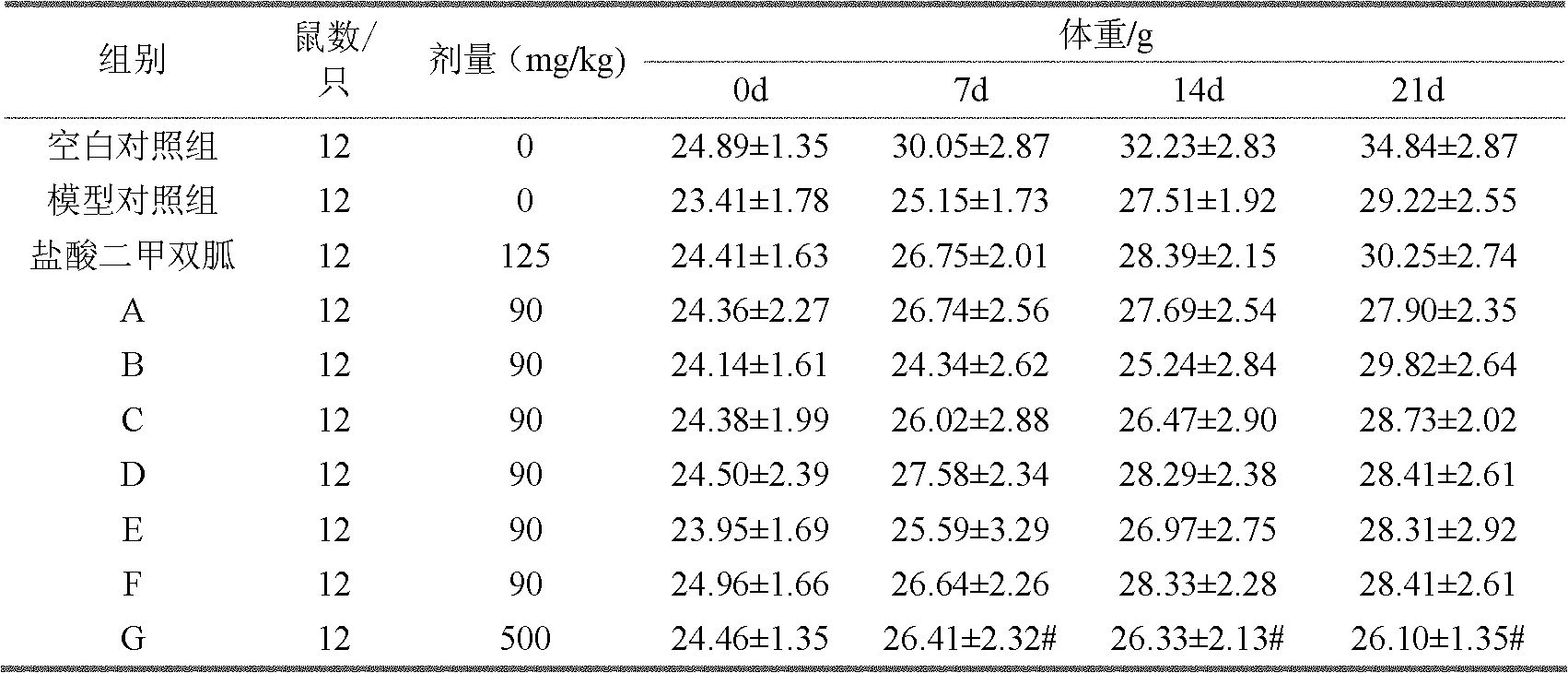
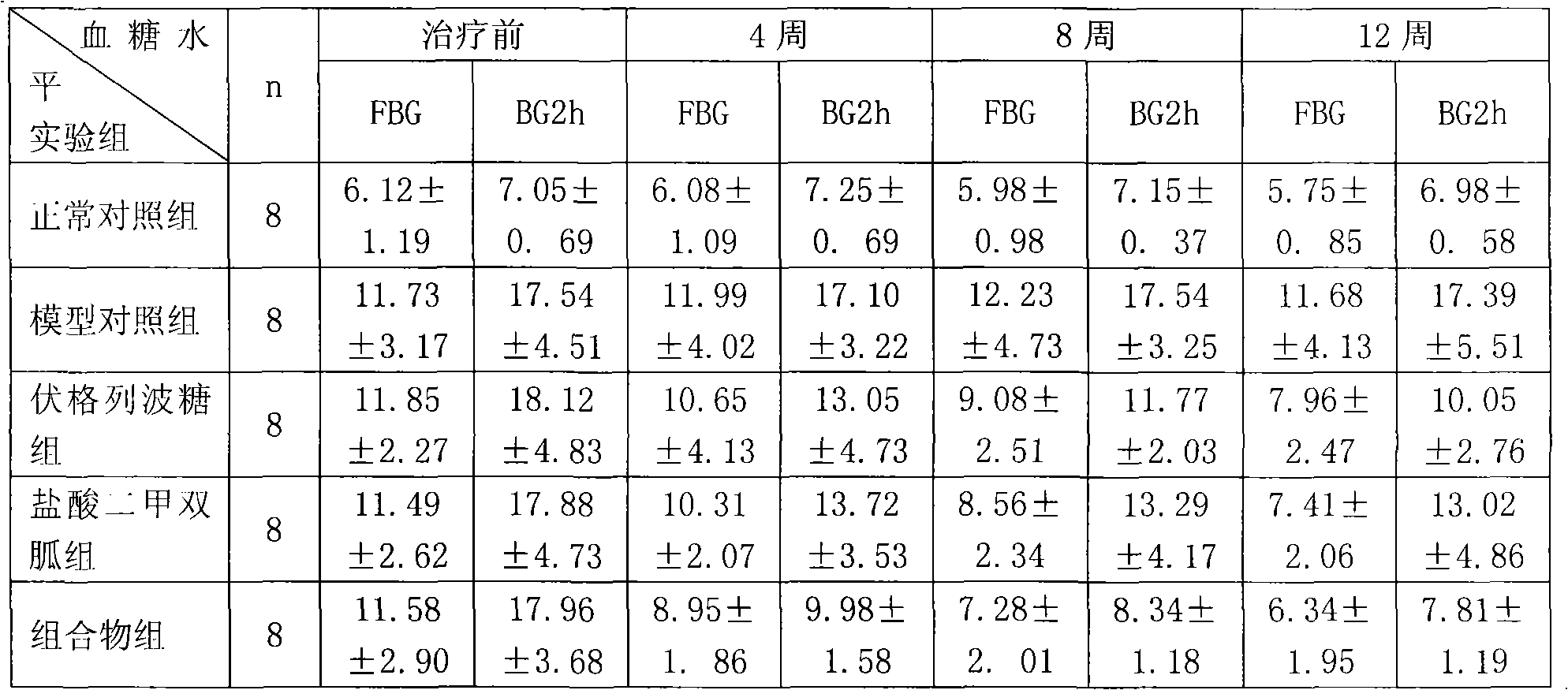
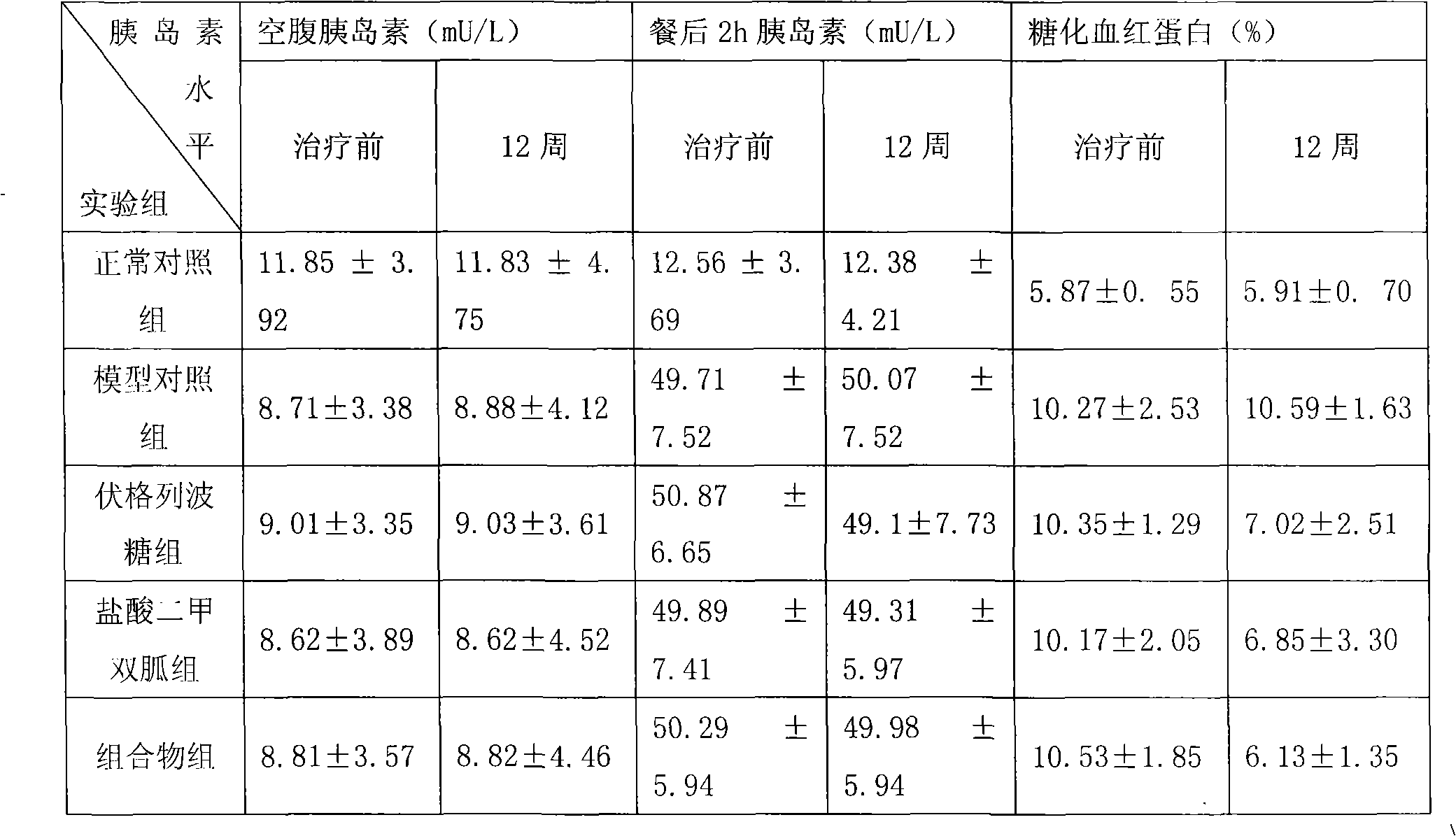
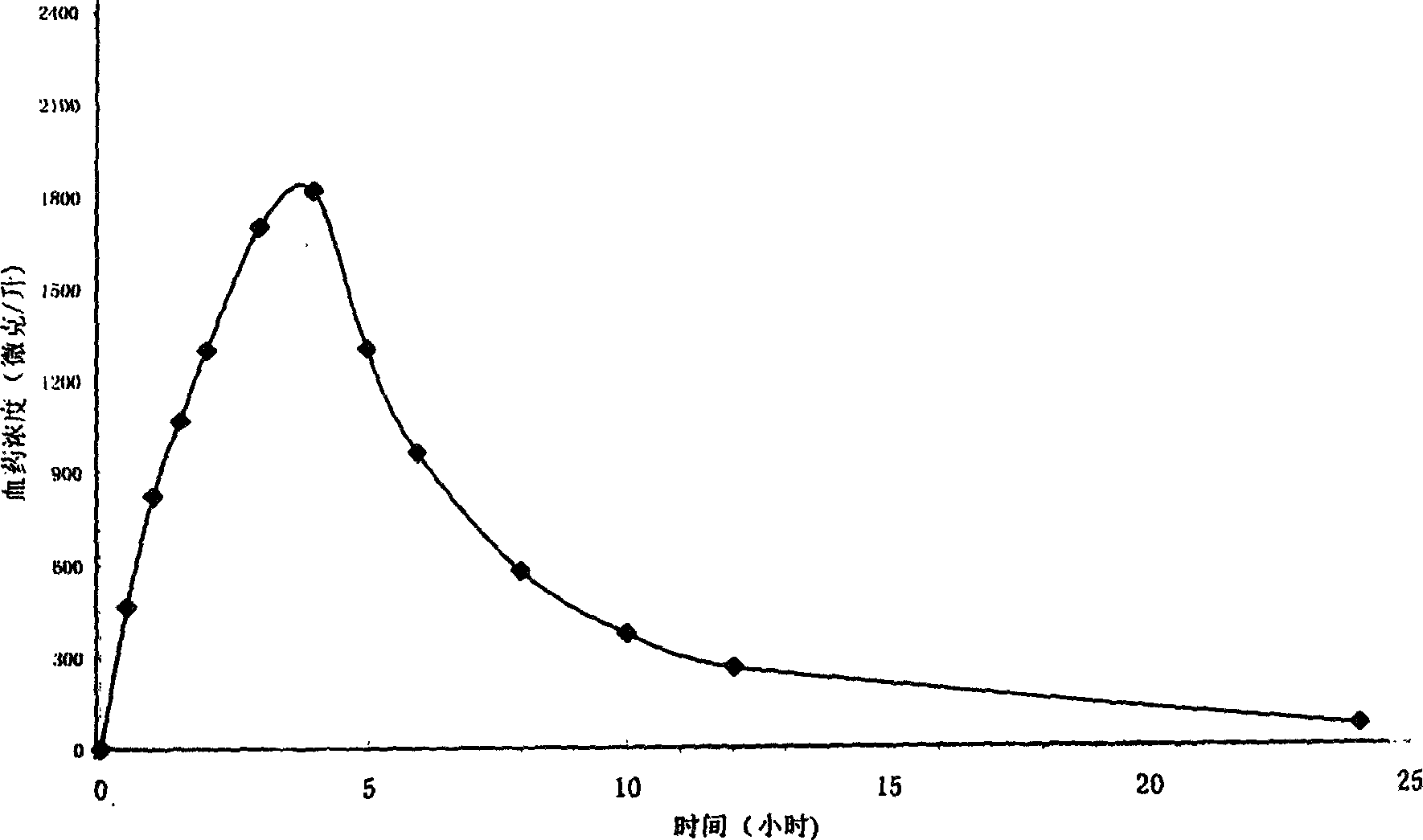
For patients switching from combined therapy with separate metformin and rosiglitazone preparations, the usual initial dosage of the fixed combination is the same as the patient's existing dosage of the individual drugs. 247 If additional glycemic control is needed following transfer, increase daily dosage in increments of 500 mg of metformin hydrochloride and/or 4 mg of rosiglitazone until ...
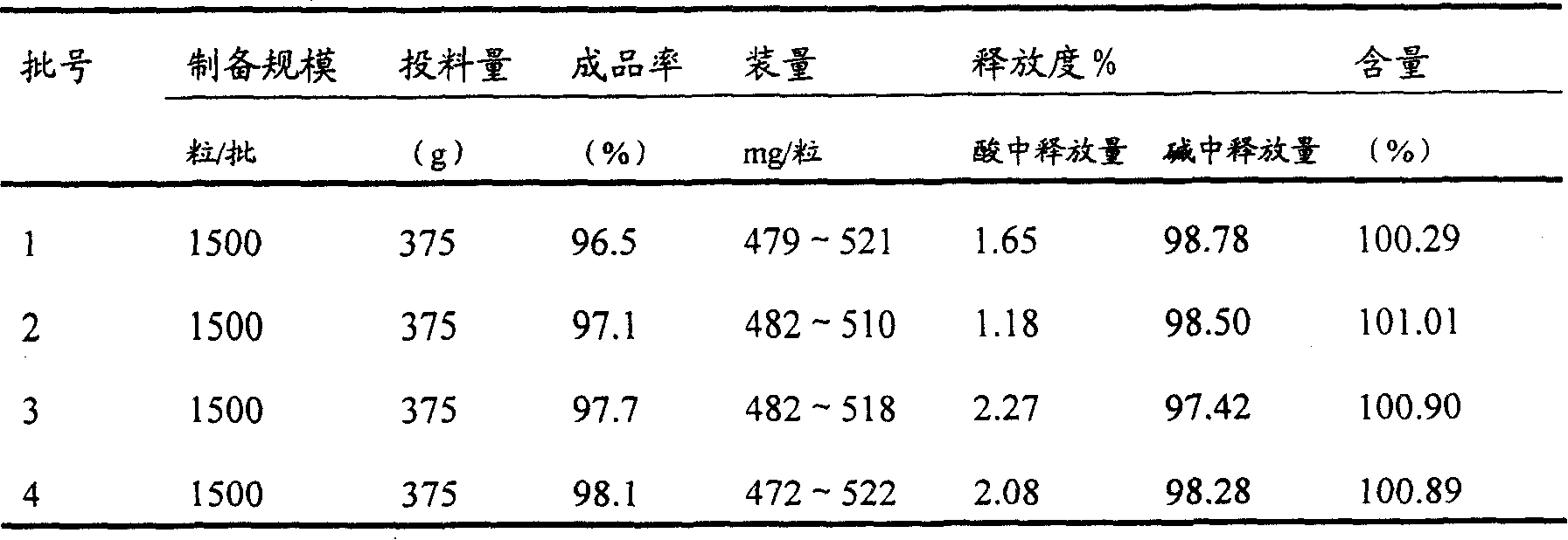
Direct compression metformin hydrochloride tablets
InactiveUS6117451AGood compressibilityImproved flowabilityPowder deliveryBiocideMetformin HydrochlorideHigh doses
Metformin Hydrochloride (herein referred to as metformin HCl) that may be 98.5%-100% pure is a high dose drug capable of being directly compressed with specific excipients into tablets having desired, hardness, disintegrating ability, and acceptable dissolution characteristics. Metformin HCl is not inherently compressible and thus presents formulation problems. Excipients used in the formulation enhance the flow and compaction properties of the drug and tableting mix. Optimal flow contributes to uniform die fill and weight control. The binder used ensures sufficient cohesive properties that allow metformin HCl to be compressed using the direct compression method. The tablets produced provide an acceptable in-vitro dissolution profile.
Owner:PHARMALOGIX
Pharmaceutical composition containing diabetosan and vildagliptin and preparation thereof
InactiveCN101234105AInhibits non-enzymatic glycation reactionsControl fluctuationsMetabolism disorderPill deliveryEffervescent tabletAdditive ingredient
The invention relates to medical composition containing metformin and Vildagliptin and a preparation method thereof. The medical composition is formed by taking metformin chloride and Vildagliptin as medical active components and blending with pharmaceutically acceptable auxiliary materials; the invention adopts the metformin chloride and Vildagliptin as materials and adds auxiliary materials with some special kinds and proportions to prepare and develop diversified oral preparations such as tablets, capsules, granules, dispersible tablets, chewable tablets, buccal tablets, effervescent tablets, effervescent granules according to the technical method of the invention. The medical composition of the invention can be used for treating diabetes type II that can not be properly cured by alimentary control and sports and also for diabetes type II that can not be controlled by simply using metformin chloride or Vildagliptin.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
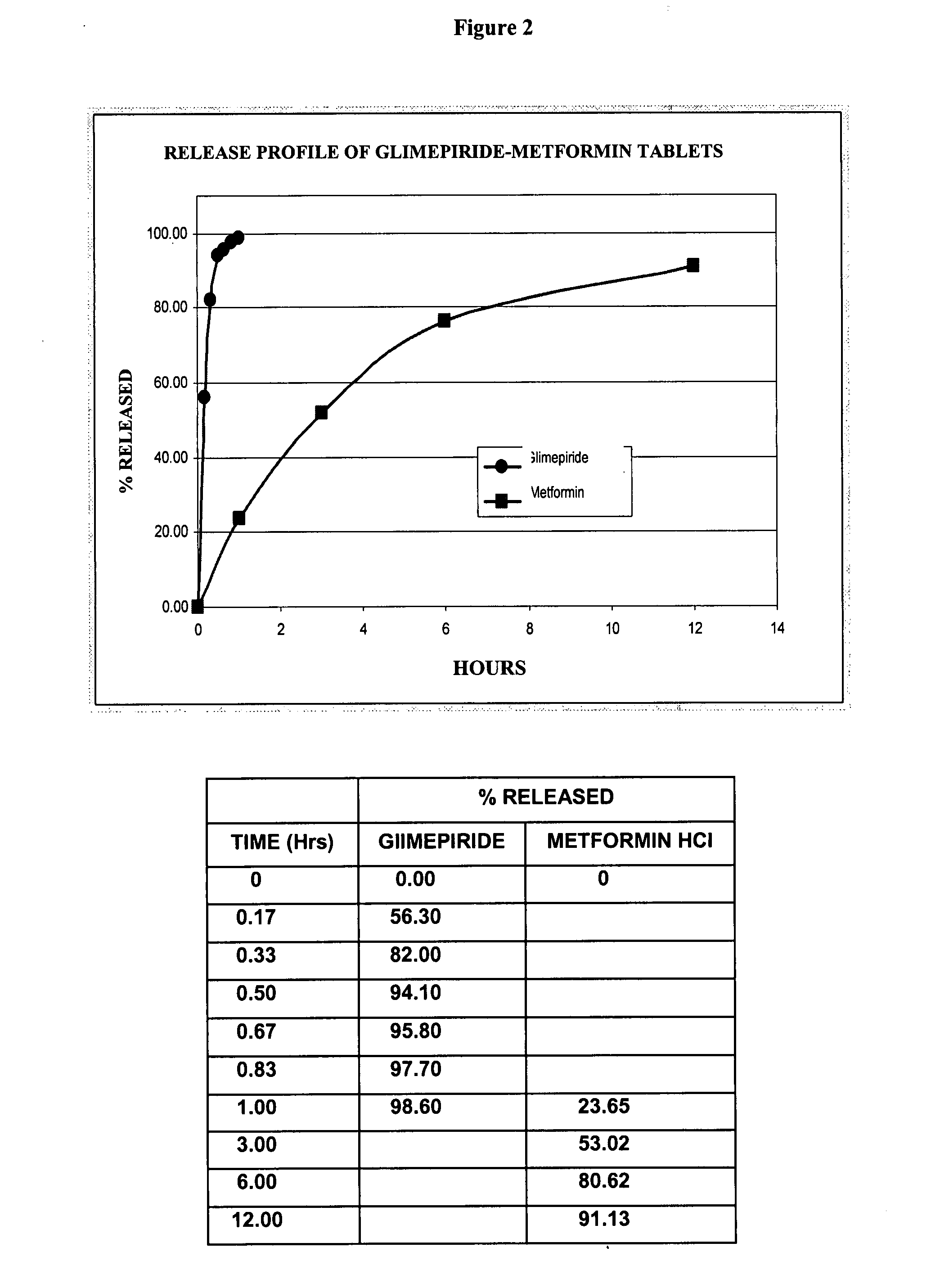
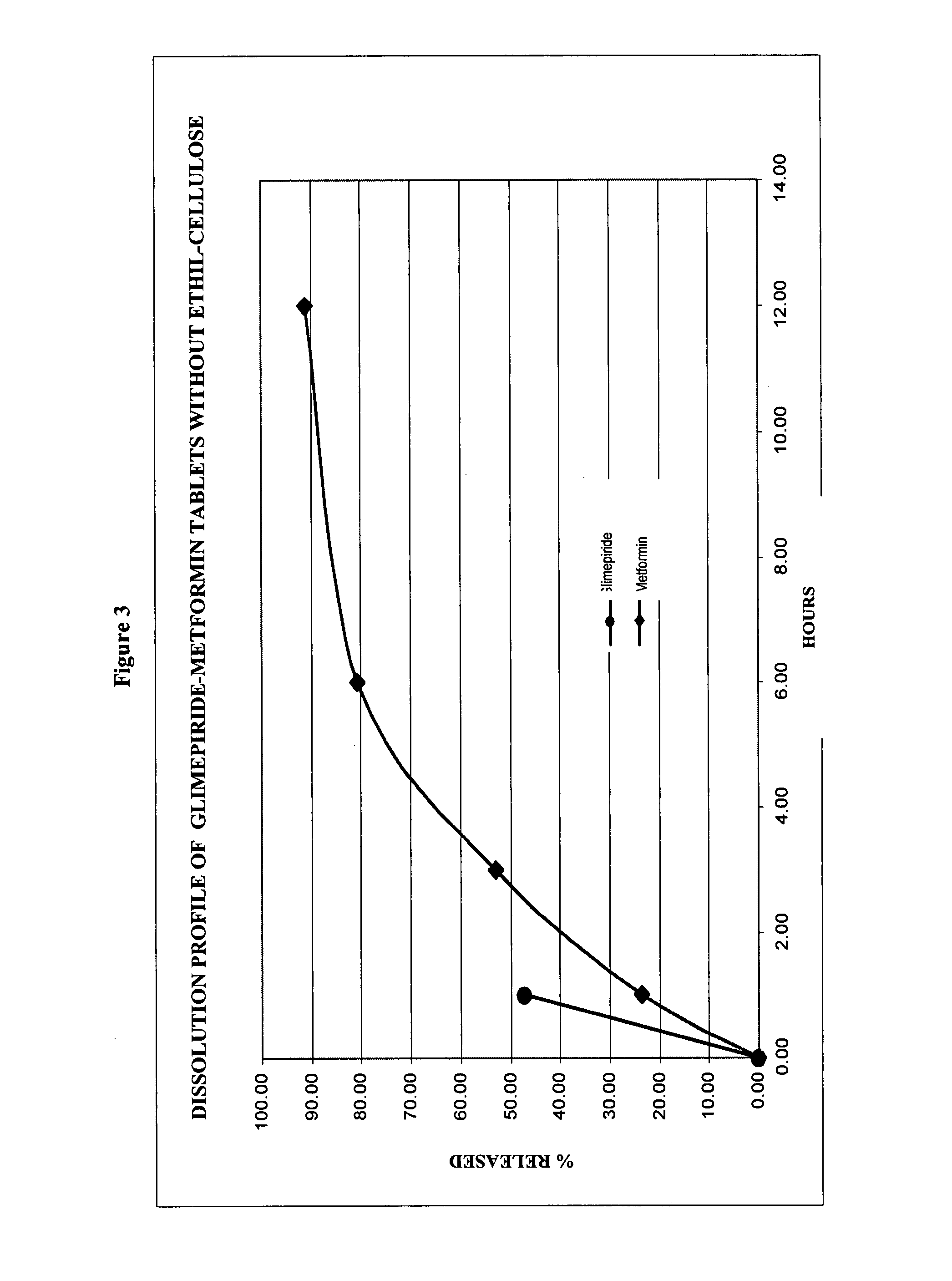
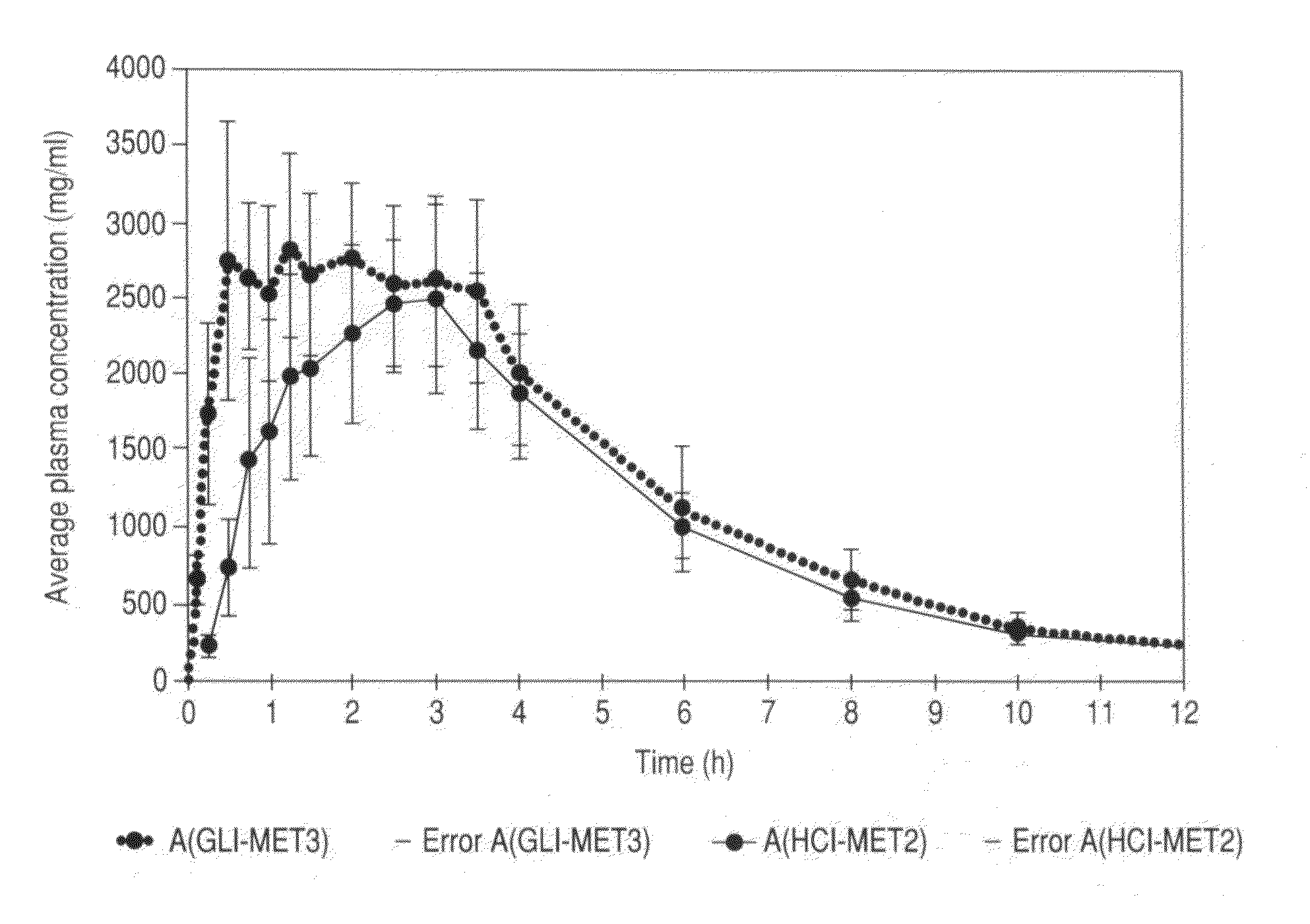
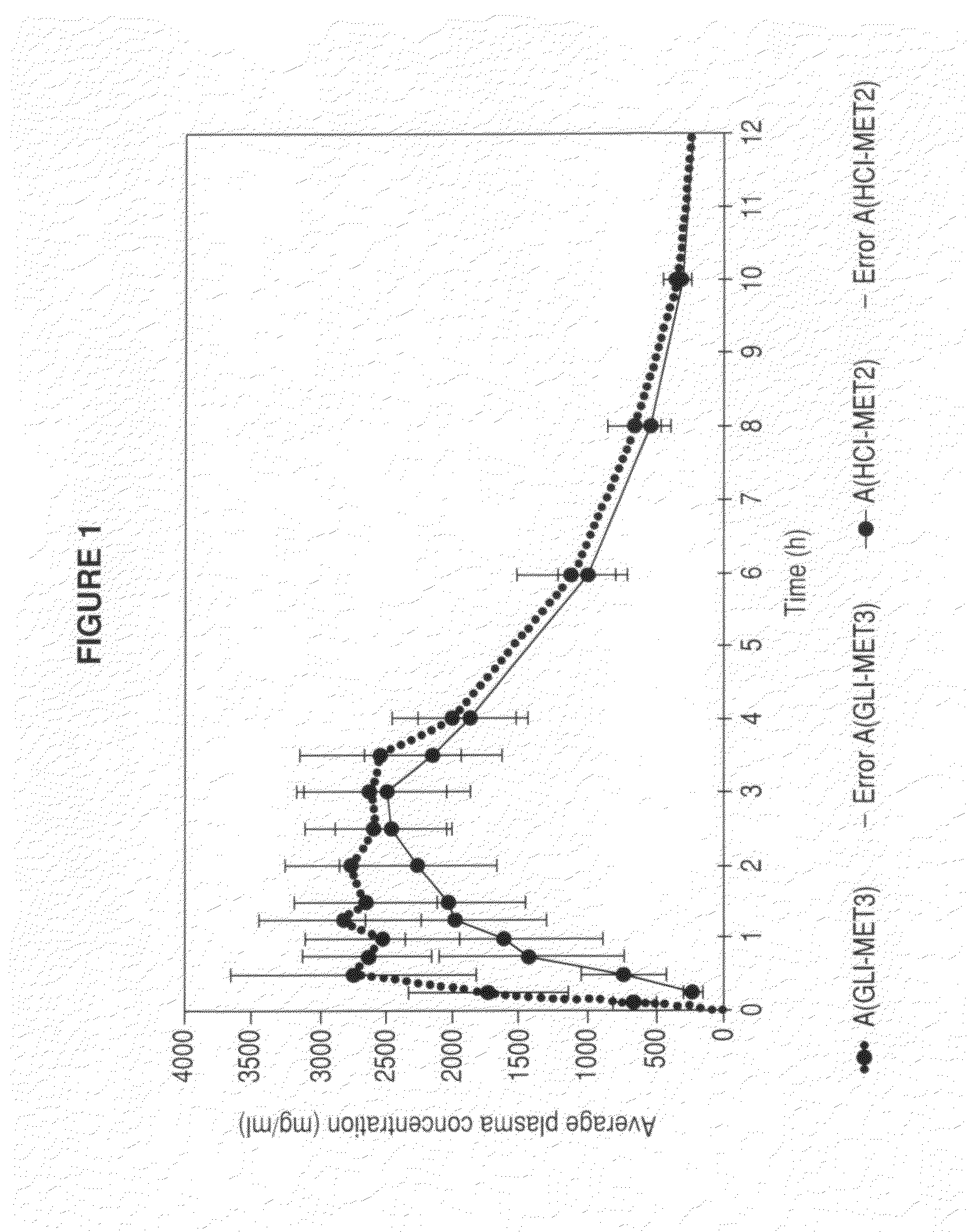
Stable pharmaceutical composition of immediate-release glimepiride and extended-release metformin
InactiveUS20070264331A1Avoid mixingBiocideMetabolism disorderImmediate releaseMetformin Hydrochloride
This invention is directed to a pharmaceutical composition in the form of a tablet with improved stability, as well as the process for obtaining said composition. The tablet of the present invention comprises two active ingredients comprising two oral hypoglycemic agents: one phase with a sulphonylurea, such as immediate release Glimepiride, and a second phase with a biguanide, such as extended-release Metformin hydrochloride (Metformin HCl). The biphasic tablet, which can include over 500 mg of Metformin HCl (i.e. up to 1,000 or 1,500 mg, depending on the daily requirements of each patient), is to be orally administered once or twice a day. The combination of these hypoglycemic agents has a synergic effect and therefore a greater effectiveness in controlling the blood glucose level in patients with diabetes mellitus, type 2.
Owner:LAB SILANES
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Directly compressible extended-release matrix formulation for metformin hydrochloride
InactiveUS6524618B1Acceptable degreeAcceptable of friabilityPowder deliveryBiocideMetformin HydrochlorideDissolution
An extended-release matrx formulation capable of being directly compressed into tablets comprising metformin hydrochloride blended with specific excipients. The excipients used in the formulation enhance the flow and compaction properties of the drug and insure that the formulation is directly compressible into a tablet containing about 100 mg to about 800 mg, preferably about 250 mg to about 750 mg, of metformin hydrochloride in unit dosage form. Each tablet produced by direct compression of the formulaton has the desired hardness and dissolution characteristics such that the drug is released in the body of the subject over an extended period of time.
Owner:PHARMALOGIX
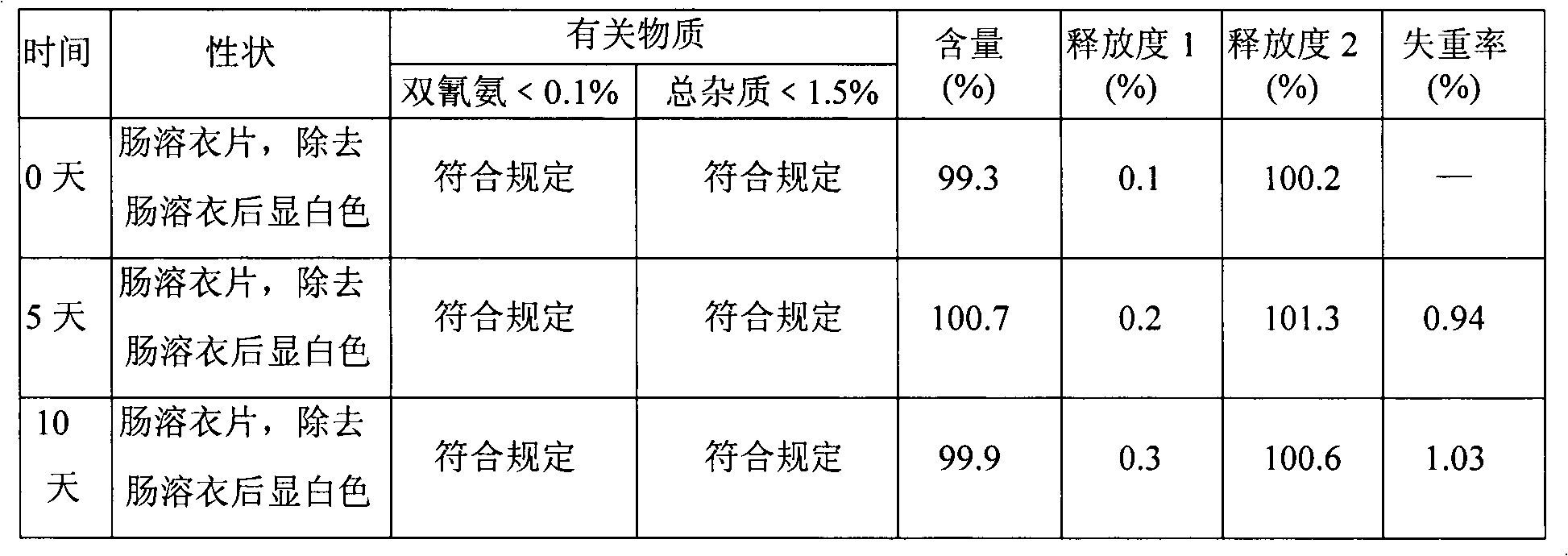
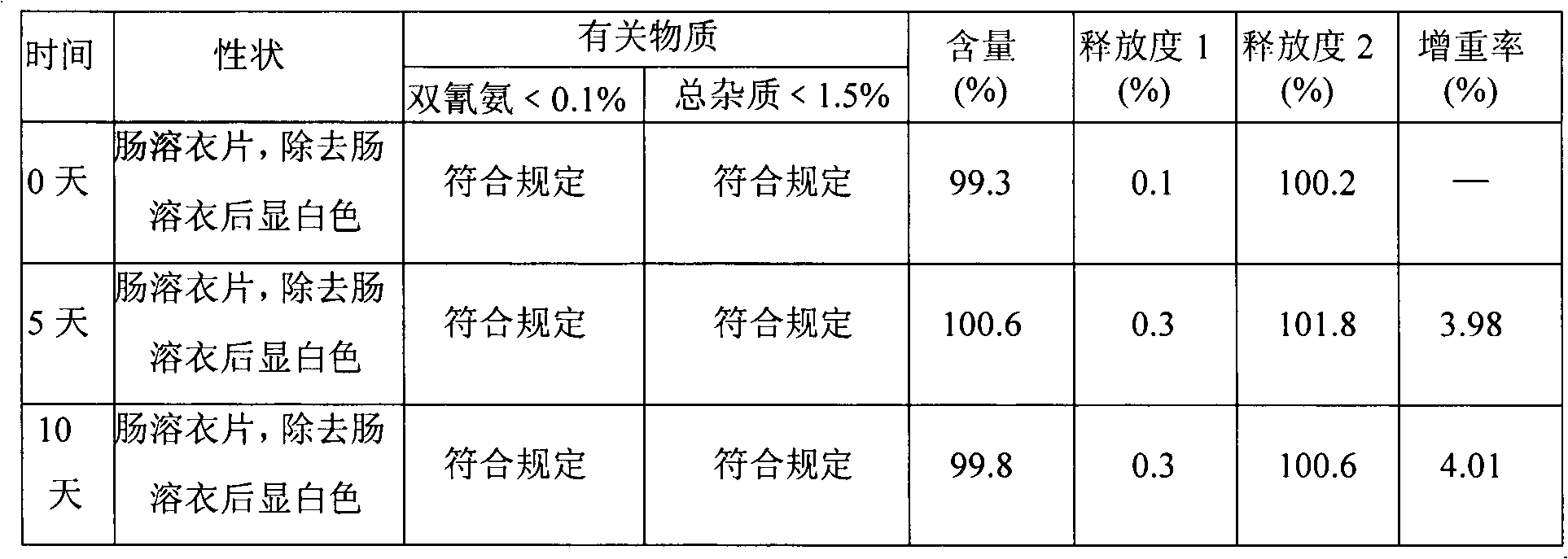
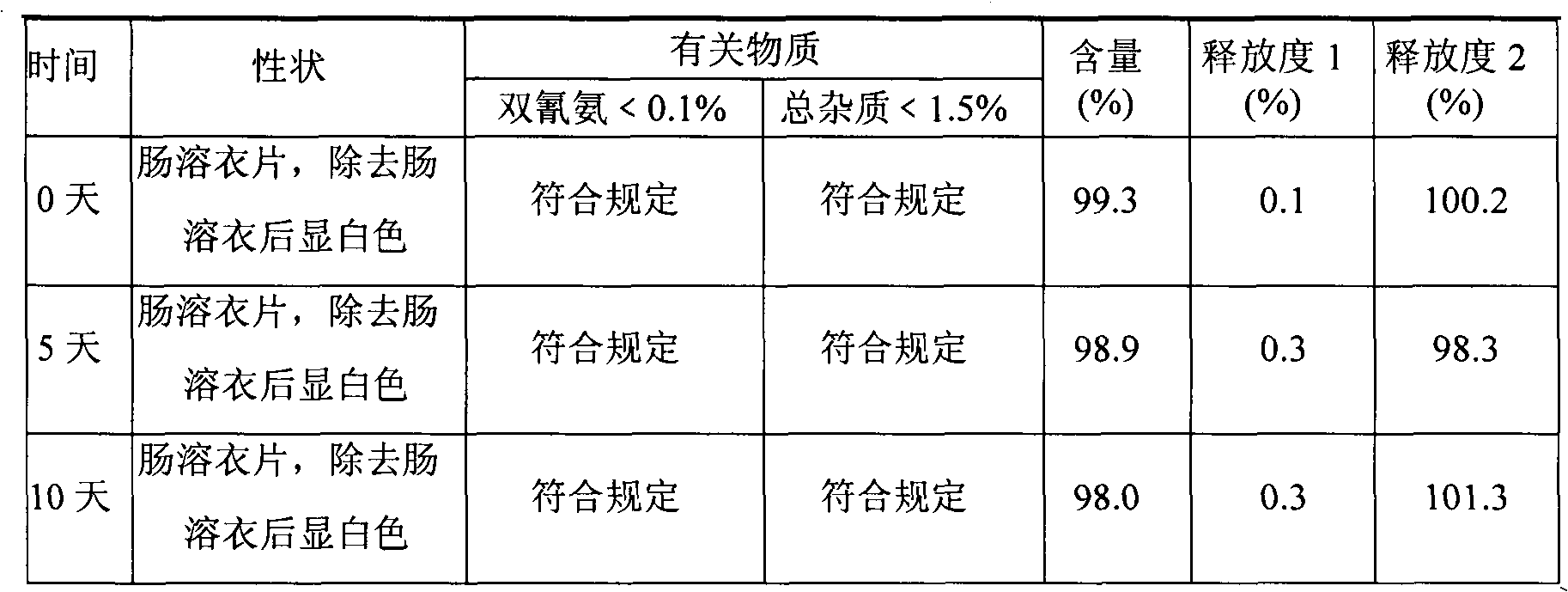
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Fuscoporia obliqua active ingredients capable of lowering blood sugar and preparation method and application of fuscoporia obliqua active ingredients
InactiveCN102038720AReduce adverse side effectsStrong market competitive advantageMetabolism disorderFungi medical ingredientsCelluloseReduction Activity
The invention discloses fuscoporia obliqua active ingredients capable of lowering blood sugar and a preparation method and application of the fuscoporia obliqua active ingredients. The preparation method takes fuscoporia obliqua fruit body as raw material and comprises the following steps: respectively extracting, filtering and concentrating the fuscoporia obliqua fruit body with normal temperature water and high temperature water; adding alcohol into concentrate and depositing to obtain crude polysaccharide; respectively pouring the polysaccharide extracted with normal temperature water and the crude polysaccharide extracted with high temperature water to flow through a (diethylaminoethanol) DEAE-52 cellulose column; carrying out subsection elution by using distilled water and NaCl solutions with different concentrations; and collecting stepwise elution peak sugar solution. Internal blood sugar reduction activity experiment shows that 0.2mol / L NaCl-section eluted sugar of the crude polysaccharide extracted with normal temperature water and 0.2mol / L NaCl-section eluted sugar of the crude polysaccharide extracted with high temperature water both have obvious blood sugar reduction activity, same blood sugar reduction activity with the blood sugar reduction medicine of metformin hydrochloride, and no obvious toxic or side effect.
Owner:CHINA AGRI UNIV
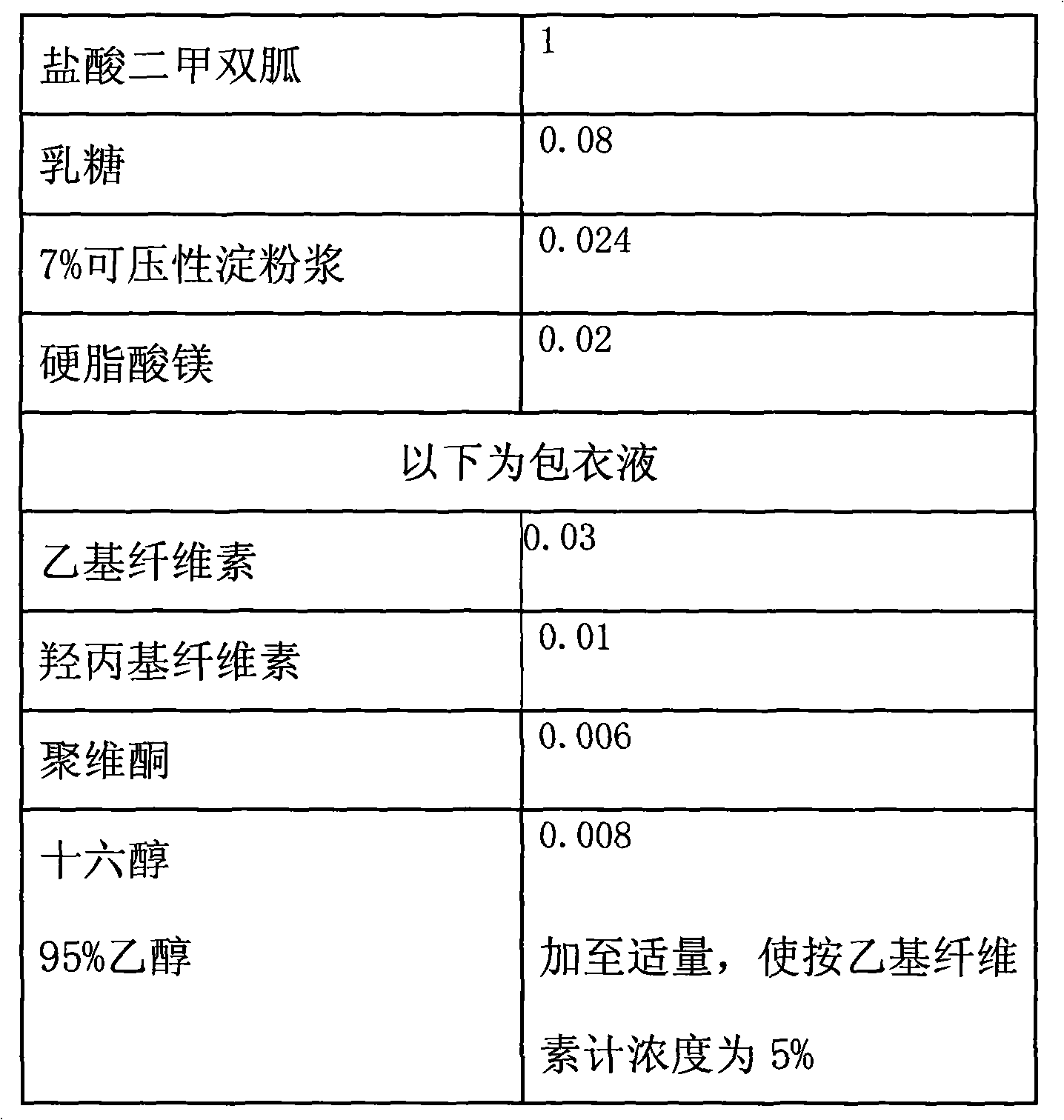
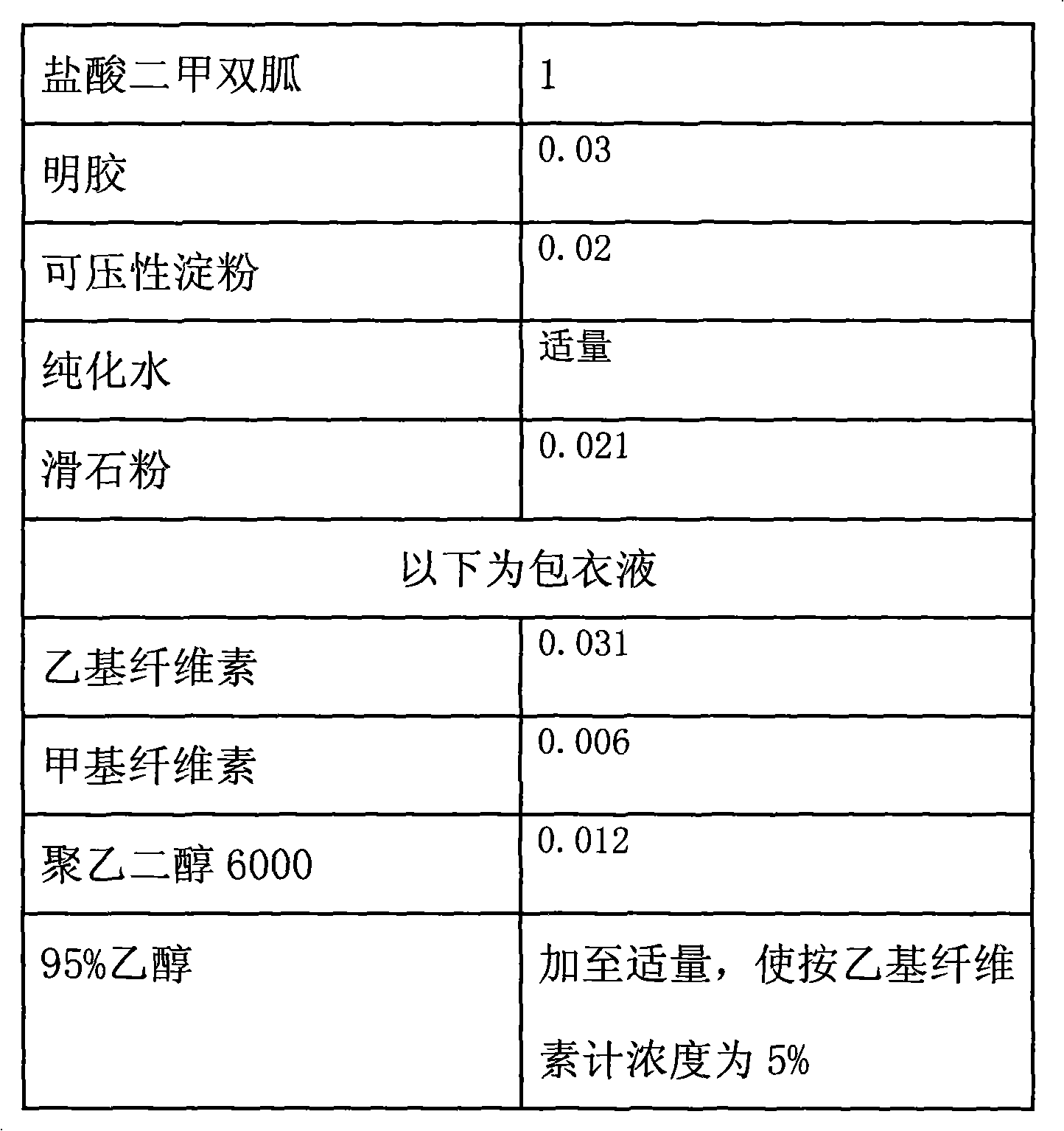
Metformin hydrochloride enteric-coated tablets and preparation method thereof
The invention belongs to the technical field of medicinal preparations, in particular relates to metformin hydrochloride enteric-coated tablets and a preparation method thereof, and provides stable metformin hydrochloride enteric-coated tablets. Each metformin hydrochloride enteric-coated tablet comprises a tablet core, an insulation coating and an enteric coating, wherein the tablet core is prepared from metformin hydrochloride, dextrin, hyprolose, magnesium stearate and talcpowder by adopting the ethanol aqueous solution of hypromellose as an adhesive; the insulation coating is prepared from a gastric soluble film coating premixed suspension agent and purified water; and the enteric coating is prepared from an enteric film coating premixed suspension agent and the purified water.
Owner:BEIJING JINGFENG PHARMA GRP
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
Enteric medicinal composition for treating diabetes and preparation method thereof
ActiveCN101190179AUniform drug releaseStrong quality controllabilityOrganic active ingredientsMetabolism disorderActive componentMetformin Hydrochloride
The invention discloses a biguanide anti-diabetes medicine, in particular to a metformin hydrochloride enteric-coated medicine compound. The medicine compound is provided with a. a hollow pill core; b. a biguanide anti-diabetes medicine active component layer which is covered outside the hollow pill core; c. an enteric-coated layer which is covered outside the biguanide anti-diabetes medicine active component layer. The invention also discloses a method of preparing the medicine compound.
Owner:北京利龄恒泰药业有限公司
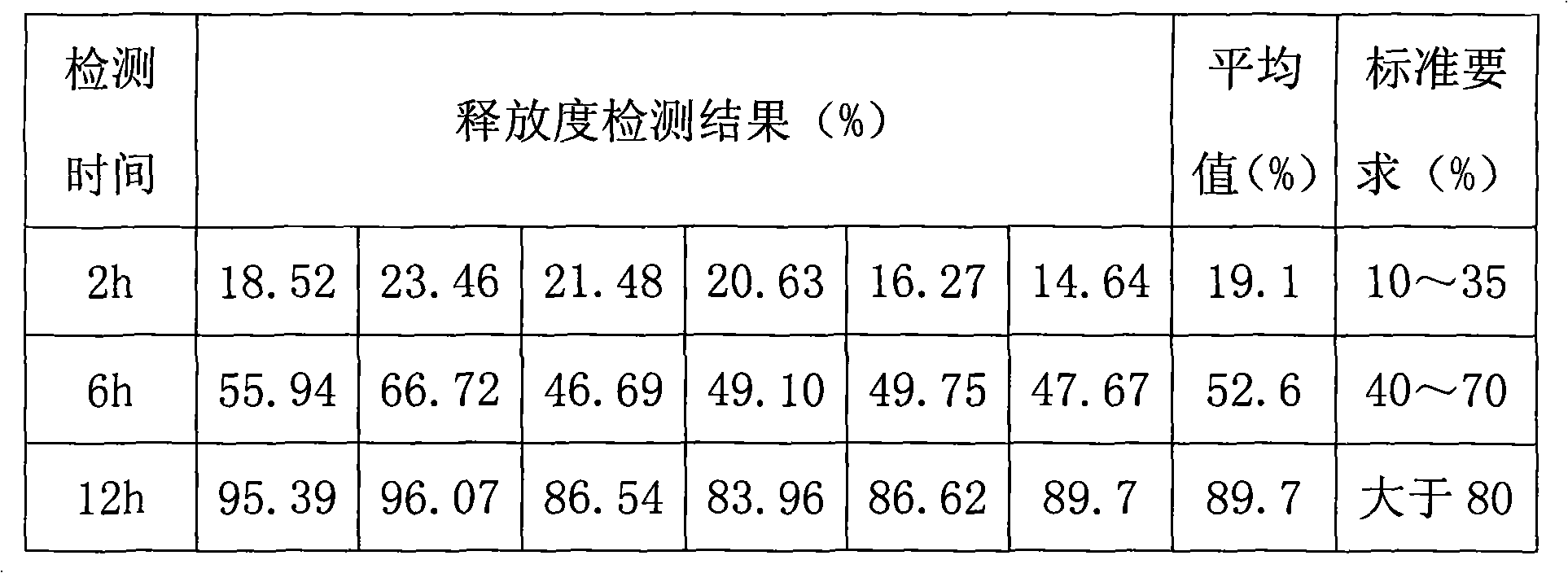
Metformin hydrochloride controlled-release tablet and preparation method thereof
ActiveCN101579325ALess weight gainIncrease production capacityOrganic active ingredientsMetabolism disorderPharmaceutical industryMetformin Hydrochloride
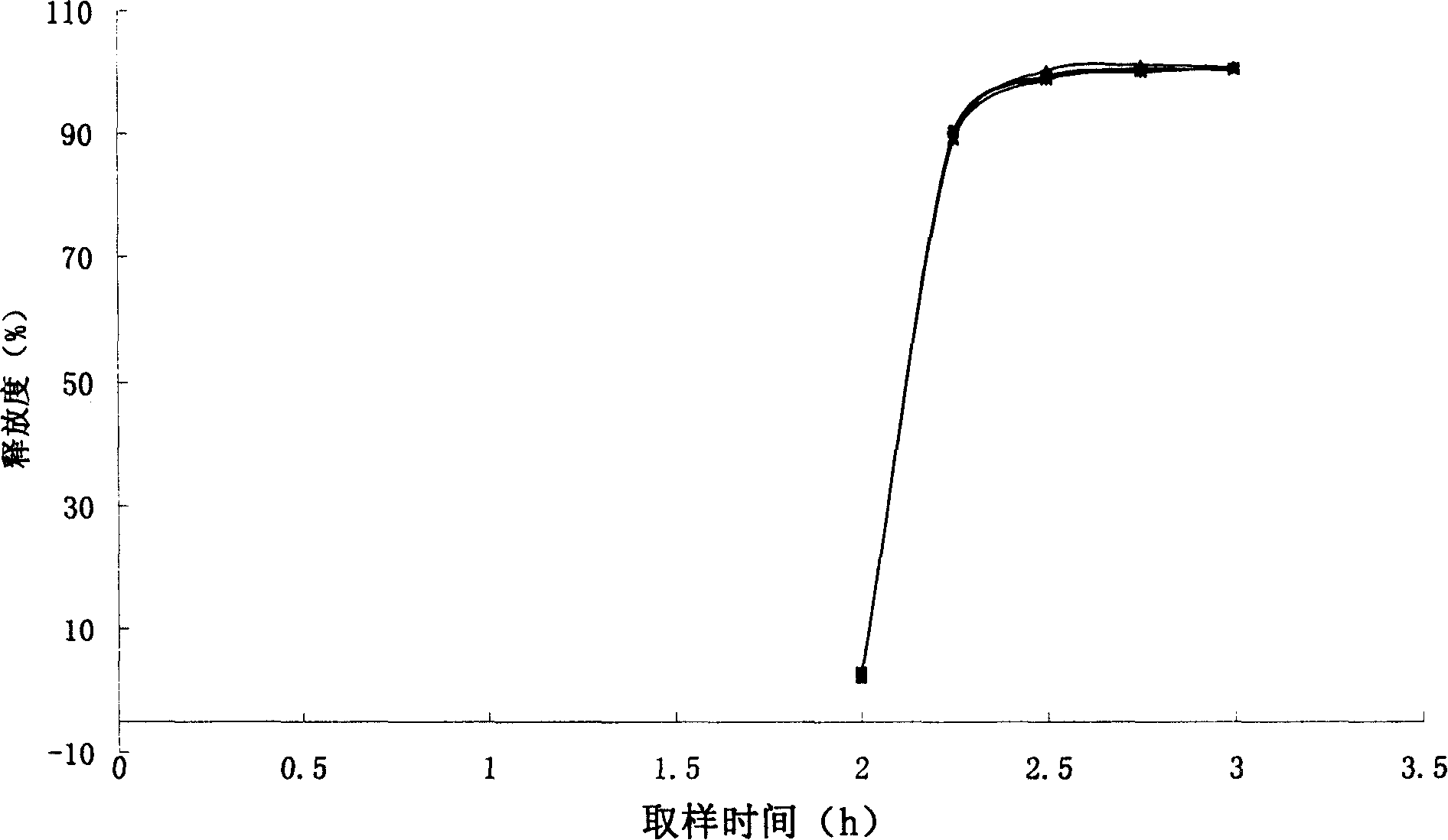
The invention provides a metformin hydrochloride controlled-release tablet, which comprises a metformin hydrochloride controlled-release tablet with effective dose and pharmaceutical accessories and is characterized in that the metformin hydrochloride controlled-release tablet uses the common tabletting, and the controlled-release effect is controlled by totally depending on the film coating technique. The coating adopted by the invention is a releasing system comprising the prescription which can cause the medicine to reach release degree standard in vitro. The preparation method in the invention is simple and convenient; the process conditions are easy to control and suitable for batch production, can use conventional production equipment in pharmaceutical industries for economically and conveniently producing the metformin hydrochloride controlled-release tablet on a large scale, can effectively and stably cause the release degree of the metformin hydrochloride controlled-release tablet in the second hour to be 10% to 35%, the release degree to be 40% to 70% in the sixth hour and the release degree to be more than 80% in the twelfth hour.
Owner:CHONGQING CONQUER PHARML
Process for producing diabecron sustained release tablet
InactiveCN101428007AImprove bioavailabilityOrganic active ingredientsMetabolism disorderCelluloseMetformin Hydrochloride
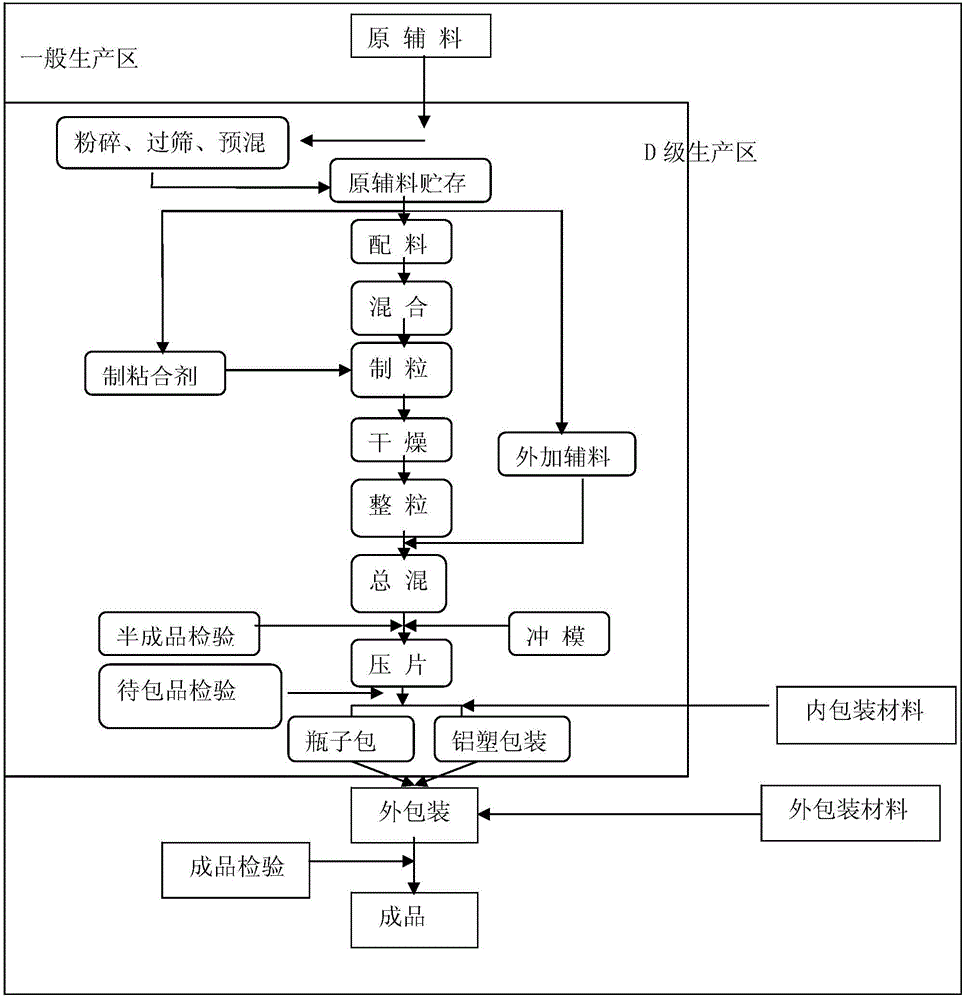
The invention relates to a preparation method for metformin hydrochloride sustained-release tablets. The preparation method comprises the following steps: firstly, dissolving ethyl cellulose into ethanol solution, blending the ethyl cellulose with metformin hydrochloride to produce granules, and drying and sieving the granules at 45 to 55 DEG C; secondly, dissolving hydroxypropyl methylcellulose into ethanol solution to produce bonding agent, blending the granules prepared in step one, hydroxypropyl methylcellulose, ethyl cellulose and pore forming agent, adding the bonding agent, performing sieving and granulation, adding hydroxypropyl methylcellulose and lubricant, evenly blending, detecting the content, determining the tablet weight, and pressing into plain tablets; thirdly, dissolving film forming agent, plasticizer, masking agent and glidant for hydroxypropyl methylcellulose and ethyl cellulose into ethanol solution, completely stirring for more than 1 hour to produce sustained-release preparation coating solution, and finally obtaining the metformin hydrochloride sustained-release tablets by performing film coating to the plain tablets. The sustained-release preparation not only embodies the main characteristics of the sustained-release preparation for continuous sustained release of drug, but also presents high bioavailability.
Owner:上海天赐福生物工程有限公司
Metformin hydrochloride purification method
ActiveCN101450918AHighlight substantive featuresSignificant progressOrganic chemistryOrganic compound preparationChemical synthesisPurification methods
The invention discloses a method for purifying metformin hydrochloride in the technical field of chemical synthesis. The invention adopts a technical proposal that: a crude product of metformin hydrochloride obtained through synthesis of dimethylammonium chloride and dicyandiamide is put into a purified water of which the weight is three times of that of the crude product of metformin hydrochloride, heated to 65 to 75 DEG C for circumfluence to be dissolved completely, and then is cooled to separate a mother liquid; and thus refined metformin hydrochloride is obtained. The obtained product avoids blocking the filter press pipes easily, and does not need heat preservation in the filter press pipes, filter press of 200kg of metformin hydrochloride takes about 0.5 hour, and the method has easy operation and decreased cost.
Owner:JIANGSU DEYUAN PHARMA
Metformin hydrochloride enteric-coated tablet
InactiveCN102357088AAvoid situations of rapid aggregate denaturationReduce use costOrganic active ingredientsMetabolism disorderManufacturing cost reductionClinical efficacy
The invention discloses a metformin hydrochloride enteric-coated tablet, which comprises a tablet core and an enteric-coated layer. The tablet core is prepared by the following raw materials by weight: 100 parts of metformin hydrochloride, 3-5 parts of povidone K30, 8-12 parts of 75% ethanol water containing 10% povidone K30 and 0.5-1.0 part of magnesium stearate. The enteric-coated layer is prepared by the following raw materials by weight: 100 parts of eudragit L30D-55, 10-30 parts of talcum powder, 2-4 parts of polyethylene glycol 4000, 4-6 parts of 4% sodium hydroxide solution and 80-120 parts of purified water. The preparation process includes the steps of weighing all raw materials according to proportion, preparing the tablet core, preparing enteric-coated solution and then obtaining the metformin hydrochloride enteric-coated tablet. The metformin hydrochloride enteric-coated tablet simplifies preparation technology, effectively reducing manufacture cost, can simultaneously control releasing speed, and effectively guarantees clinical effects of products.
Owner:HEBEI JAMESHILL PHARMA
Compound with metformin and repaglinide, preparation method thereof and application thereof
InactiveCN101822672ALower glucose toleranceLower natural responsesOrganic active ingredientsMetabolism disorderInsulin dependent diabetesOrally disintegrating tablet
The invention relates to a composite composition with metformin hydrochloride and repaglinide as active ingredients, a preparation method thereof and application thereof and belongs to the technical field of medicaments. The composite composition is a medicinal composition which is mixed by using the metformin hydrochloride and repaglinide as the active ingredients and by using a carrier and can be prepared into sustained-release tablets, sustained-release granules, sustained-release capsules, common troches and capsules, granules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, liquid capsules, soft capsules, drop pills and other oral preparations. The composite composition is used for treating patients with I-type diabetes or II-type diabetes (non-insulin-dependent diabetes) and has synergistic effect on controlling blood sugar.
Owner:深圳南方盈信制药有限公司 +1
Medicinal composition for treating diabetes
InactiveCN102188429AGood curative effectReduce high blood sugarMetabolism disorderHeterocyclic compound active ingredientsDiabetes mellitusSide effect
The invention discloses a medicinal composition for treating diabetes, which comprises bromocriptine and metformin hydrochloride. In the medicinal composition for treating diabetes, the dose of a main medicine is small; and compared with other compound preparations of metformin hydrochloride, the side effect is reduced greatly, patients can taken the medicinal composition for a long time, and the obedience in the patients is high. In addition, the medicinal composition also has the advantage that the medicine price is lower than other metformin hydrochloride compound preparation treatment medicines.
Owner:吴四清 +2
Metformin hydrochloride sustained-release tablet and method for preparing the same
ActiveCN1543937AConvenient amountReduce dosageOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
The invention provides a Metformin Hydrochloride slow release tablet and process for preparation, wherein the slow release tablet comprises metformin hydrochloride 46.5-70%, hydroxypropylcellulose 13.5-33.0%, micronization ethyl cellulose 10.0-14.0%, filling agent 1.3%-9.4%, and lubricating agent 1.2-1,5%. The preparation process comprises processing the prescribed raw material of metformin hydrochloride, cellulose glycollic ether, and the filling agent through the conventional tablet production process of granulation, drying, granulating, charging micronization ethyl cellulose, mixing homogenously with lubricant and tabletting.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Oral solid drug composition of metformin hydrochloride repaglinide
The invention discloses an oral solid drug composition containing metformin hydrochloride repaglinide, which contains pharmaceutically acceptable carriers. The preparation process is simple, the problem of chipping is effectively solved, and simultaneously, the repaglinide has good content uniformity and dissolution; and the oral solid drug composition is used for treating type II diabetes.
Owner:万全万特制药(厦门)有限公司
Pharmaceutical composition comprising an ampk activator and a serotonergic agent and methods of use thereof
InactiveUS20140350064A1Easy to transportImprove toleranceBiocideOrganic active ingredientsDiseaseMetformin Hydrochloride
The present invention is based on the unexpected discovery that a combination of certain known drugs exhibits synergistic effects in treating metabolic syndrome and various other diseases. In particular, the invention comprises a pharmaceutical composition comprising: (1) a therapeutically effective quantity of a first agent that is an AMPK activator; and (2) a therapeutically effective quantity of a second agent that possesses or maintains serotonin activity. A preferred composition comprises metformin hydrochloride and melatonin. The invention further comprises methods for the use of these compositions for the treatment of metabolic syndrome, hyperproliferative diseases including cancer, and other diseases and conditions.
Owner:ALS MOUNTAIN
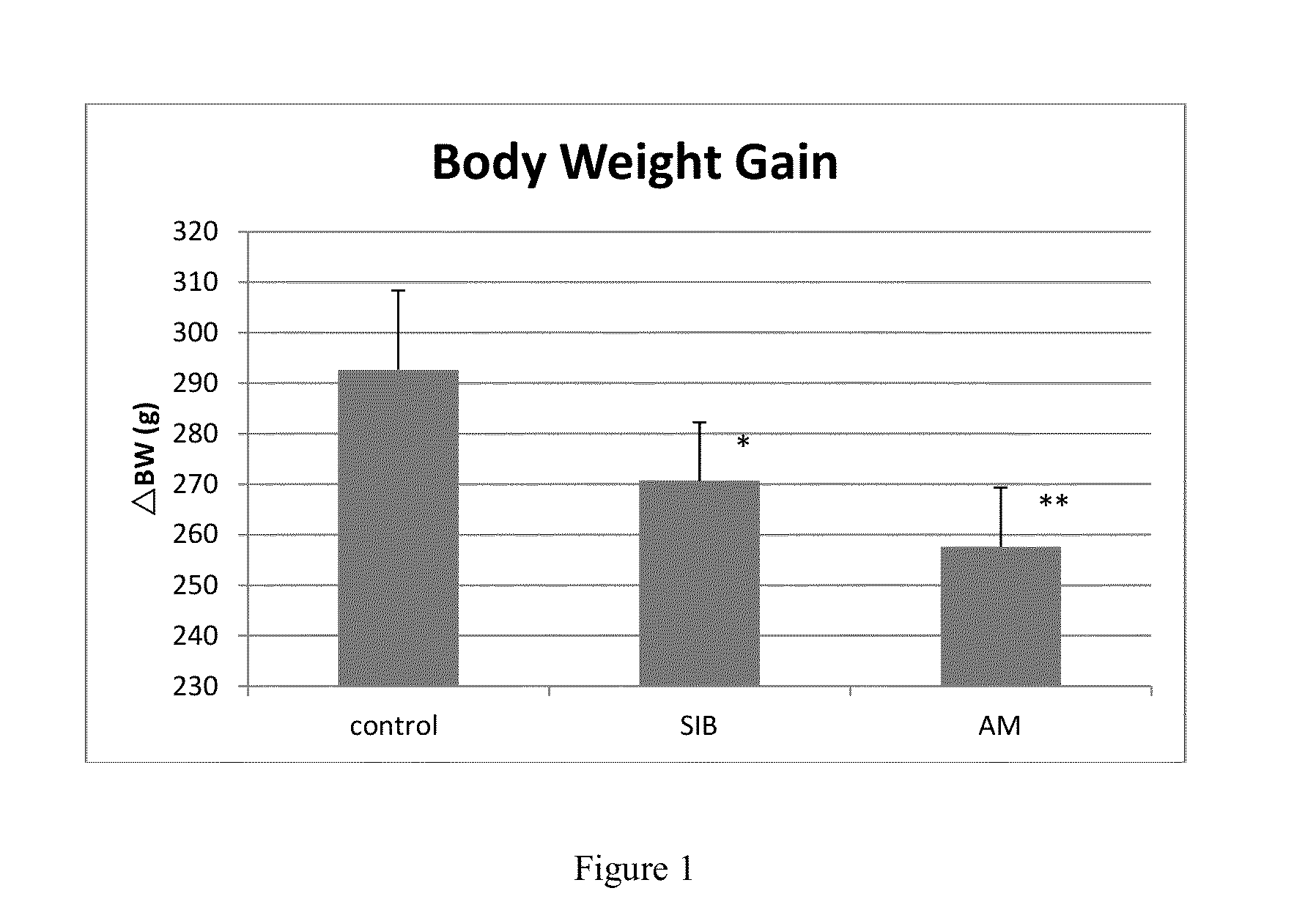
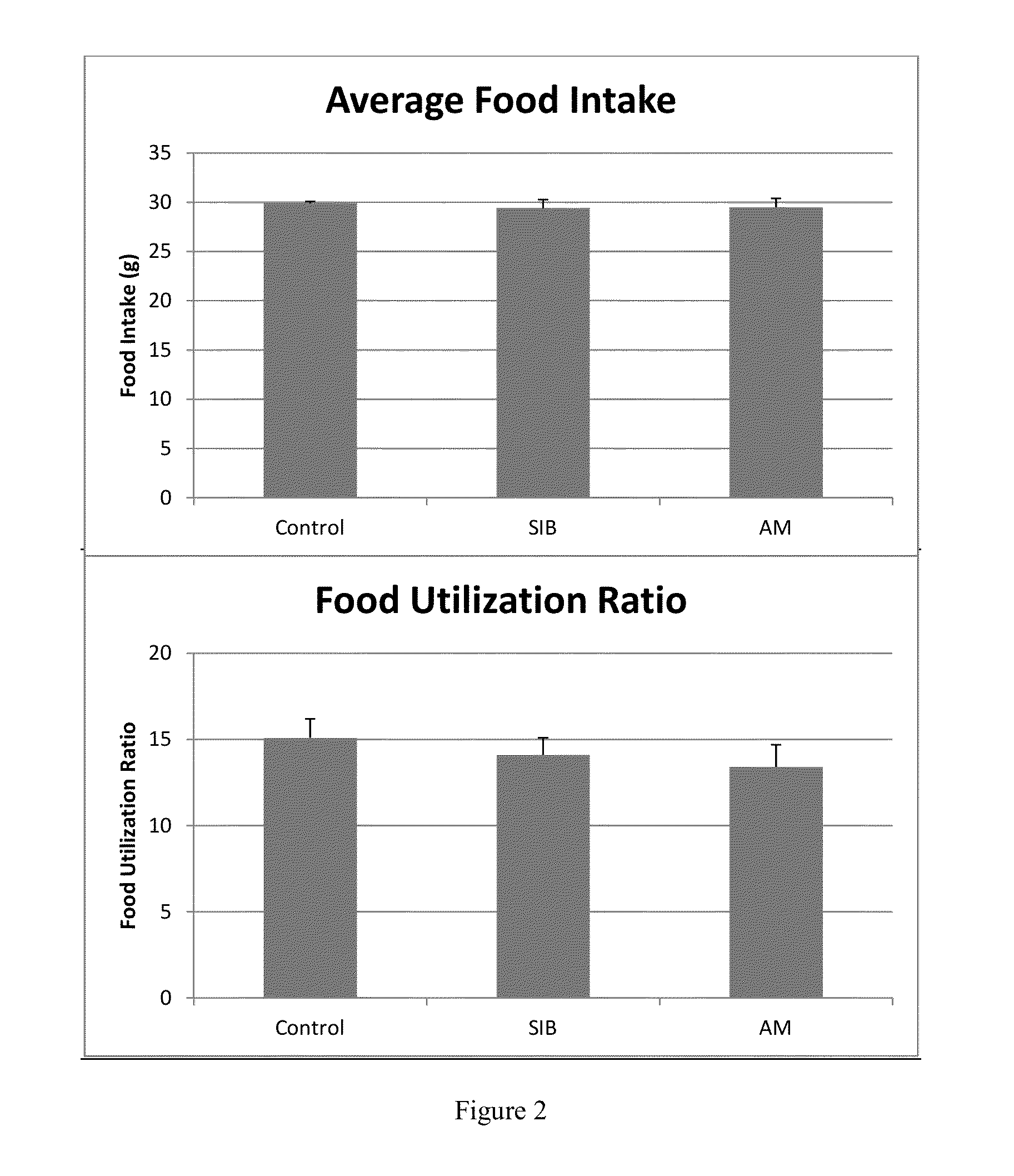
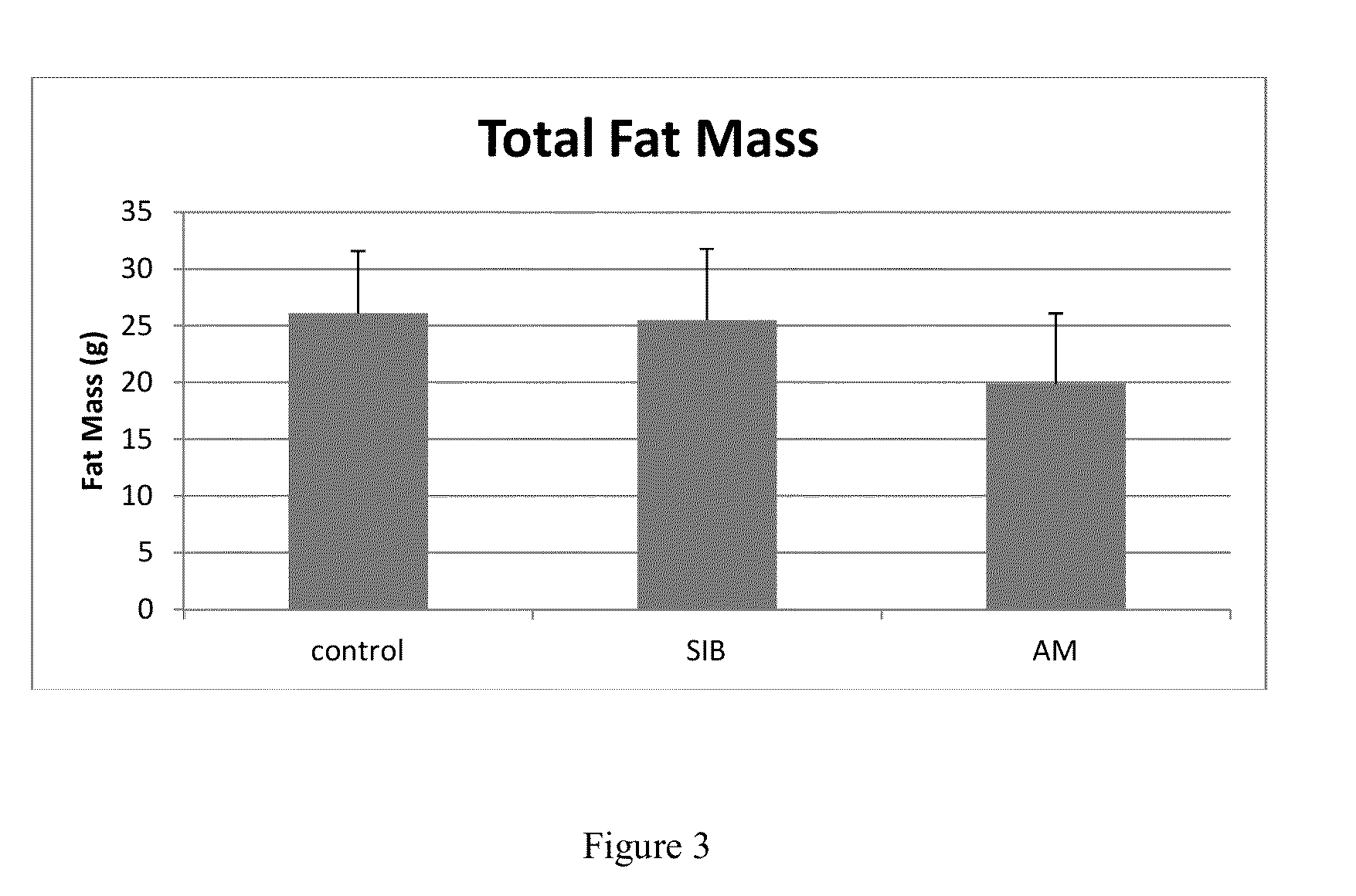
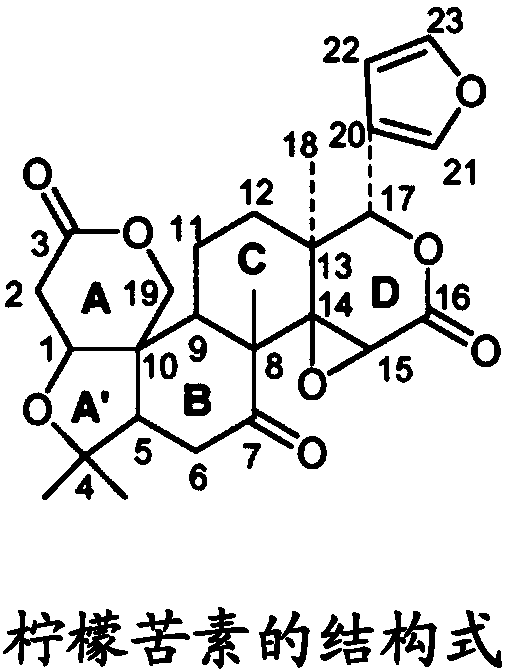
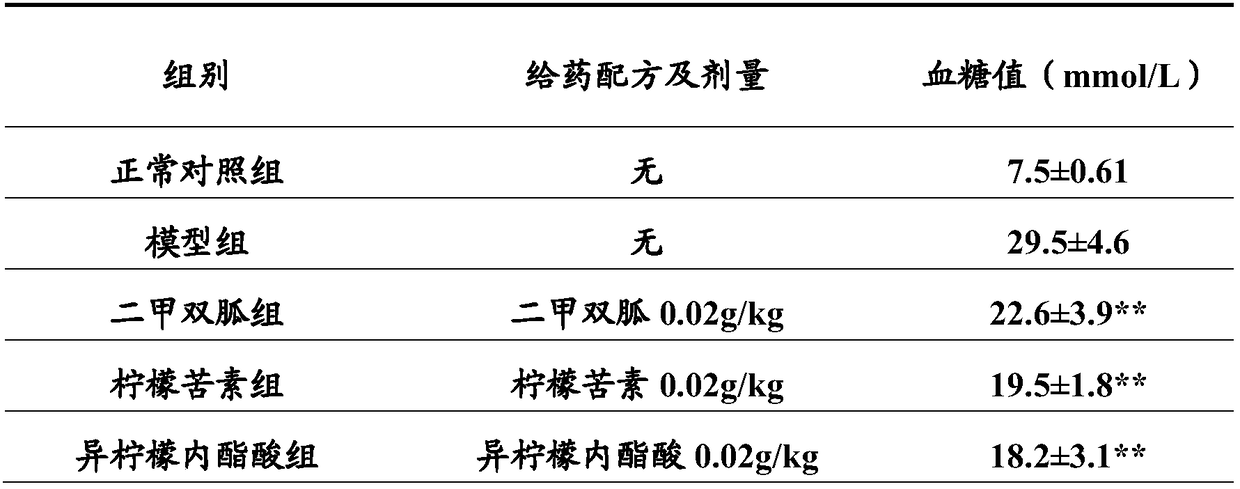
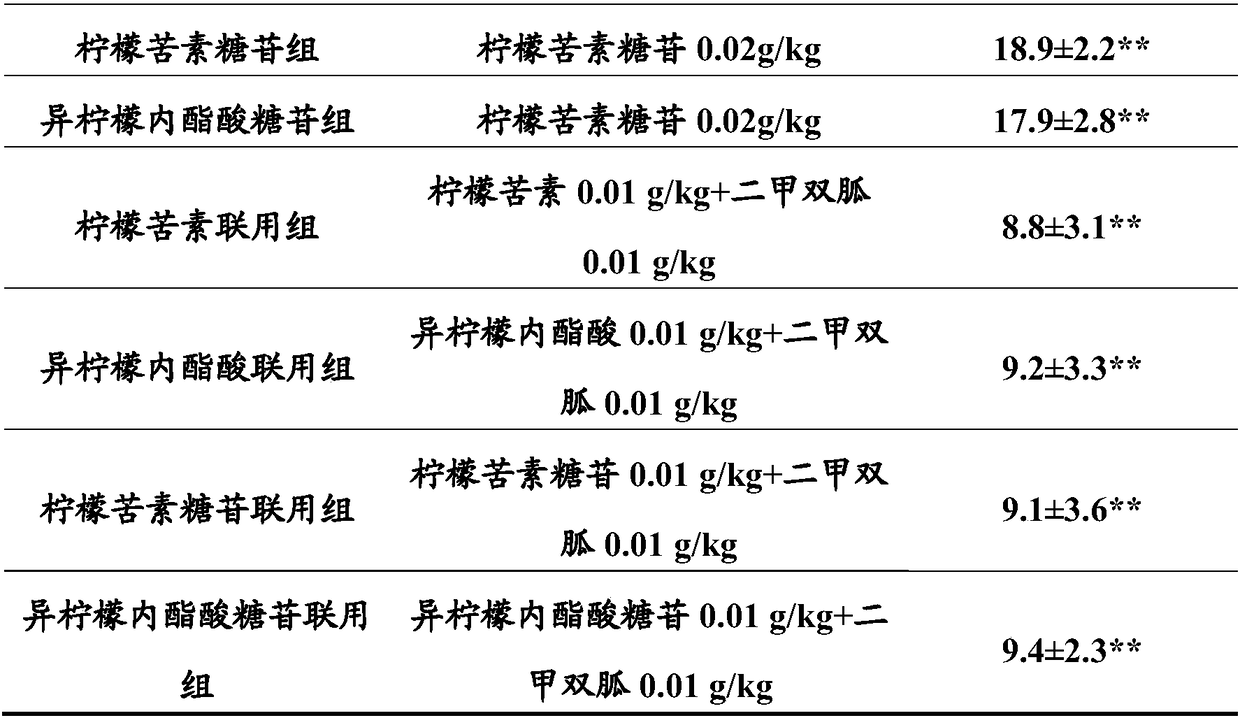
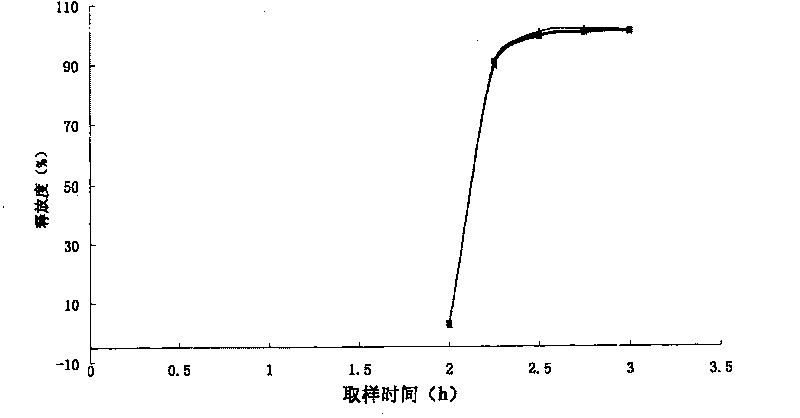
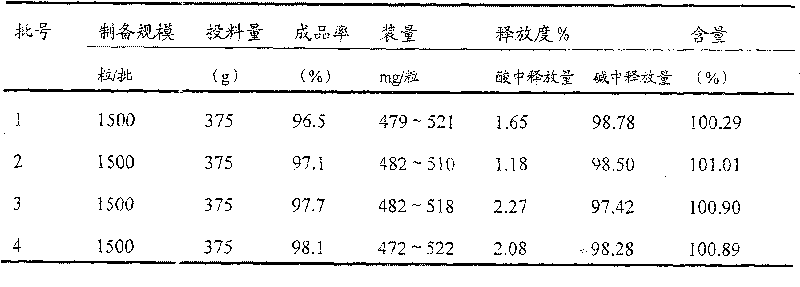
Combined product containing limonin compounds and biguanide compounds
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Process for preparing metformin hydrochloride
InactiveCN1844093ALow costHigh yieldOrganic chemistryOrganic compound preparationAcetic acidEthylene diamine
The invention relates to a method of diabecron, using dimethylamine aqueous solution of 40% concentration one value, dropping hydrochloric acid in dimethylamine aqueous solution until the PH value is 2, stopping drop acid, vacuum concentrating solution, cooling to 50deg C after there is crystal in reactor, adding ethanol 0.3 shares, dicyandiamide 0.62-0.67 shares, heating to 80deg C,doing vacuum distillation to distill ethanol, reacting to produce diabecron when heating to 130deg C, adding ethanol 2-3 shares when cooling to 50deg C, discharging when cooling to 5deg C, centrifugal rinsing and drying, drying, recovering ethanol, getting diabecron crude product, drying crude product, adding deionized water 2.5-3 shares, activated char 0.02 shares, ethylene diamine tetraacetic acid disodium 0.003 shares, heating-up to 75-80deg C, cooling to 50deg C again after agitating, adsorbing, filtering, concentrating, adding ethanol 2 shares and agitating, cooling to 5deg C, discharging and centrifugal rinsing and drying, drying to recover the ethanol, getting diabecron completed product, on-spec product's yield of this invention is 75% -80%,the cost is decreased 15%-25% compared with primal method, hasing advantages of high product yield and low cost.
Owner:翟树军
Enteric medicinal composition for treating diabetes and preparation method thereof
ActiveCN101190179BUniform drug releaseImprove quality controllabilityOrganic active ingredientsMetabolism disorderMedicineActive component
The invention discloses a biguanide anti-diabetes medicine, in particular to a metformin hydrochloride enteric-coated medicine compound. The medicine compound is provided with a. a hollow pill core; b. a biguanide anti-diabetes medicine active component layer which is covered outside the hollow pill core; c. an enteric-coated layer which is covered outside the biguanide anti-diabetes medicine active component layer. The invention also discloses a method of preparing the medicine compound.
Owner:北京利龄恒泰药业有限公司
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Metformin hydrochloride sustained-release tablets and preparation method thereof
ActiveCN102440975AStable blood concentrationExtended half-lifeOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseHalf-life
The invention discloses metformin hydrochloride sustained-release tablets which are characterized in that: every 10000 tablets are prepared from medicine materials and auxiliary materials of, by weight: 5000g of metformin hydrochloride, 1750g of hypromellose, 1750g of sodium carboxymethyl cellulose, 180g of stearic acid, 200g of magnesium stearate, and an appropriate amount of 75% ethanol. The metformin hydrochloride sustained-release tablets can be slowly released in vivo. With the tablets, stability of blood drug level can be maintained, and a half-life period is prolonged. The tablets are safe, can be used for treating type II diabetes, and are advantaged in high efficiency, low toxicity, and convenient administration. The invention also provides a preparation method of the metformin hydrochloride sustained-release tablets.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Metformin hydrochloride sustained-release tablet
ActiveCN103816130ALow costGood slow releaseOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
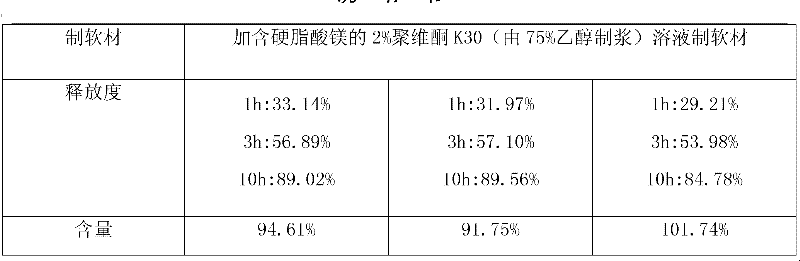
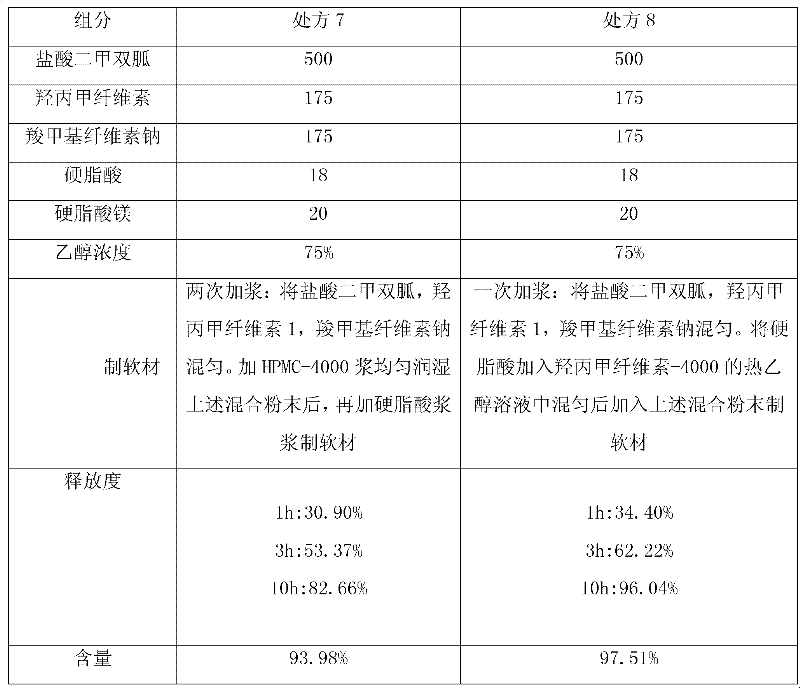
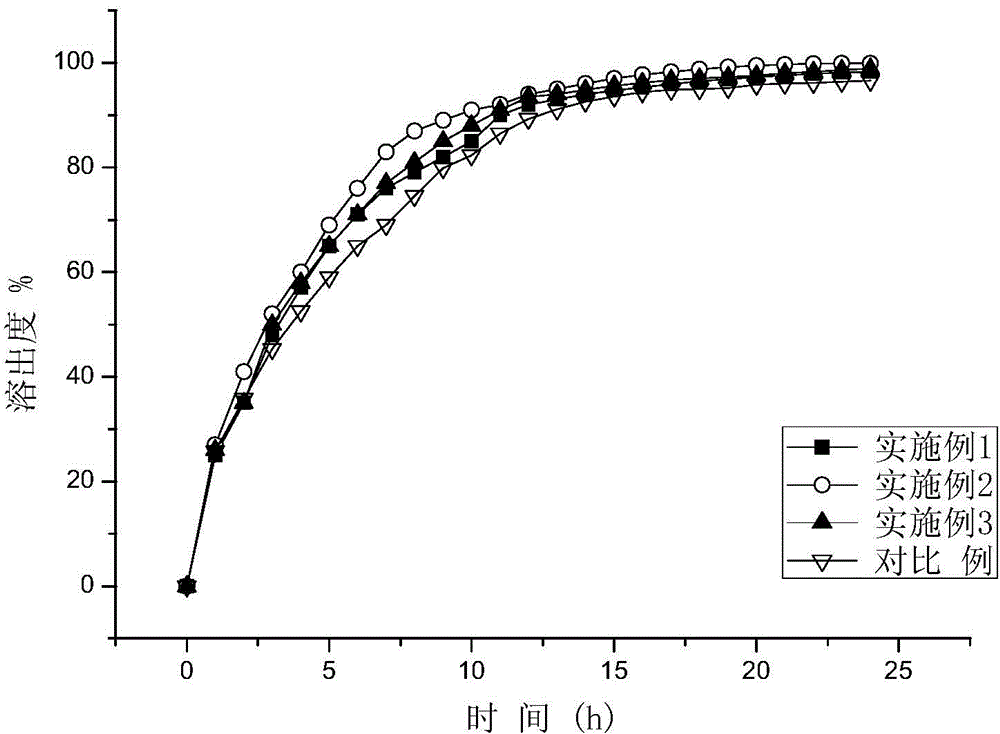
The invention provides a metformin hydrochloride sustained-release tablet. The metformin hydrochloride sustained-release tablet is prepared from the components by weight: 400-600 parts of metformin hydrochloride, 30-60 parts of sodium carboxymethylcellulose, 200-250 parts of hydroxypropyl methylcellulose, 180-220 parts of ethyl acrylate-methyl methacrylate copolymer aqueous dispersion and 5-10 parts of magnesium stearate. Suitable auxiliary materials and a suitable preparation method are adopted to prepare the metformin hydrochloride sustained-release tablet with lower raw material cost and simpler process, and the sustained-release performance of the obtained product is good, the release amounts at 1 hour, 3 hours and 10 hours are respectively 20-45 percent, 45-75 percent and above 80 percent, and the drug has good stability and can be preserved for 24 months at the room temperature.
Owner:YOUCARE PHARMA GROUP
Metformin hydrochloride sustained-release tablets and preparation method thereof
InactiveCN107049980ASimple preparation processSolve the sudden releaseOrganic active ingredientsMetabolism disorderAdhesiveMetformin Hydrochloride
The invention discloses metformin hydrochloride sustained-release tablets and a preparation method thereof. The sustained-release tablets achieve a slow release effect by means of a slow release agent and a coating, wherein the slow release agent is a composition prepared from sodium carboxymethyl cellulose and pregelatinized starch; a coating solution is prepared by dissolving ethyl cellulose and other components in ethanol with mass fraction of 95%, wherein other components comprise polyethylene glycol 6000 and hexadecanol; hydroxypropyl methylcellulose is dissolved in purified water so as to be prepared into adhesive. The metformin hydrochloride sustained-release tablets can enable medicines to be slowly released in a human body and maintain an effective blood concentration, thus improving the compliance and safety of patients who take the medicines. The preparation method of the product is safe and reliable, and can realize large-scale production.
Owner:CHONGQING CONQUER PHARML
Preparation method of metformin hydrochloride
InactiveCN105481726AInhibitionShort reaction timeOrganic chemistryOrganic compound preparationAlcoholMetformin Hydrochloride
The invention discloses a preparation method of metformin hydrochloride. The method is characterized by including the following steps of firstly, adding dicyandiamide and dimethylamine hydrochloride in a reaction still, and adding cyclohexanol, wherein the mole ratio of dicyandiamide to dimethylamine hydrochloride is 1:1.2, and the mole ratio of cyclohexanol to dicyandiamide is 3:1; secondly, starting a stirring device, controlling the rotating speed at 45-50 r / min, regulating the vacuum degree in the reaction still to 0.08-0.09 MPa, heating the mixture to 135-145 DEG C, and preparing crude metformin hydrochloride after the reaction is conducted for 2.5 hours; thirdly, conducting recrystallization on the crude product through ethyl alcohol, and conducting filtering and drying to obtain the metformin hydrochloride. By means of the method, the reaction time is greatly shortened, the production cost is reduced, side reactions can be effectively restrained, the impurity content is reduced, and therefore the yield of the product is greatly improved.
Owner:SHIJIAZHUANG POLEE PHARMA CO LTD
Content measuring method of metformin hydrochloride enteric coated tablet
InactiveCN101776660AElimination of Assay EffectsImprove accuracyComponent separationMedicineMetformin Hydrochloride
The invention provides a content measuring method of a metformin hydrochloride enteric coated tablet, which comprises the following steps: using metformin hydrochloride as a reference substance and measuring the content of the metformin hydrochloride in the enteric coated tablet by using a high performance liquid chromatography. Compared with the prior art, the invention uses the high performance liquid chromatography to measure the content of the metformin hydrochloride enteric coated tablet, improves the accuracy compared with the prior ultraviolet spectrophotometric method, and provides better standards for the quality control of the metformin hydrochloride enteric coated tablet.
Owner:贵州天安药业股份有限公司
Compound pioglitazone hydrochloride/metformin hydrochloride bilayer osmotic pump controlled release preparation and preparation method thereof
InactiveCN102008472AImprove controllabilityStable storageOrganic active ingredientsMetabolism disorderCoated tabletsControl release
The invention provides a compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The compound preparation structurally comprises the following parts from inside to outside in sequence: a tablet core comprising a drug layer and a digestive layer, an insulation coating layer, a controlled release coating film with drug release holes, a rapid pioglitazone hydrochloride release layer and an unnecessary attractive coating. The invention also provides a preparation method of the compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The preparation method comprises the following steps of: (1) preparing the drug layer; (2) preparing the digestive layer; (3) tabletting the tablet core; (4) coating insulation coating layer; (5) coating the controlled release coating; (6) punching the coated tablet: (7) coating the rapid pioglitazone hydrochloride release layer; and (8) coating the attractive coating.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com