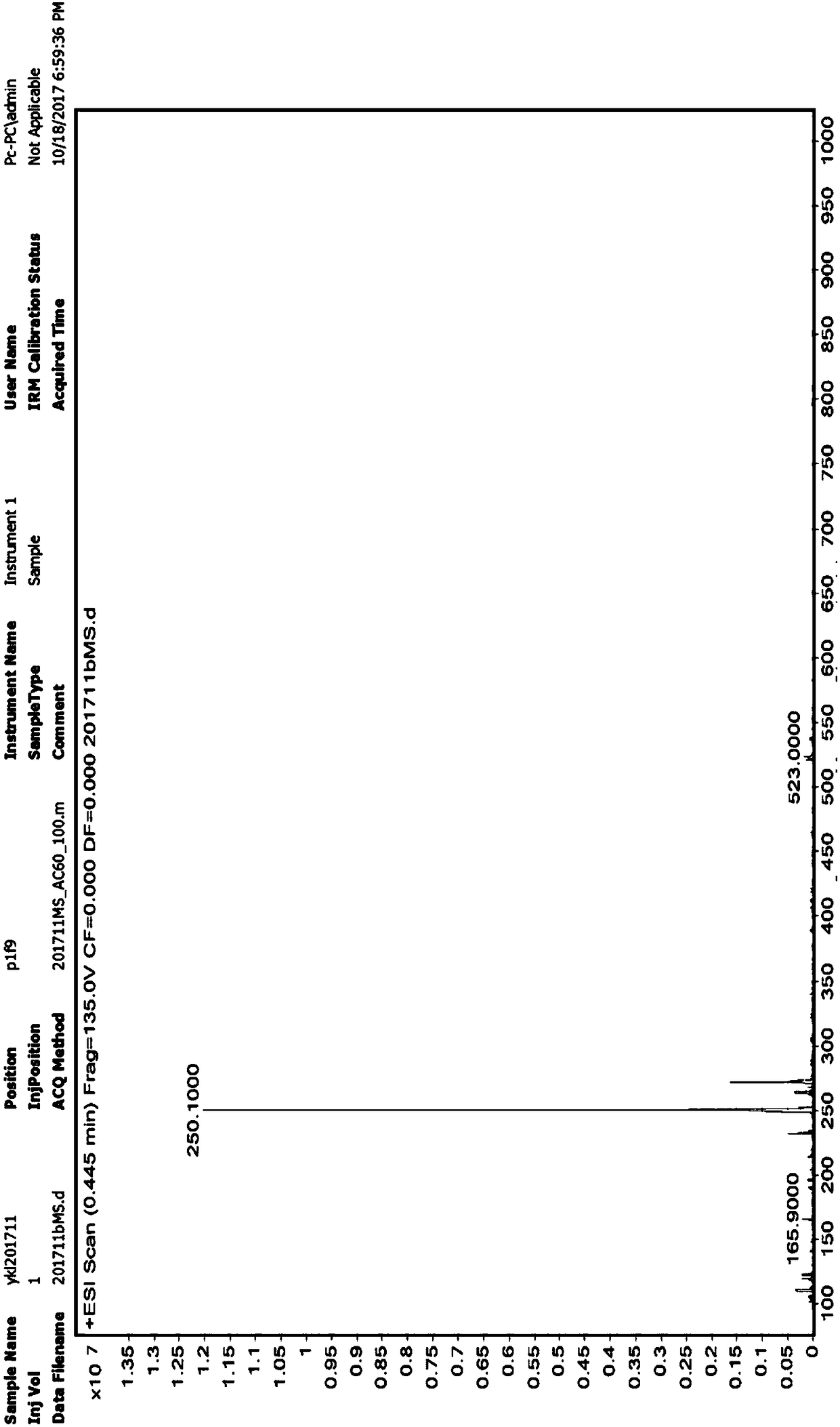
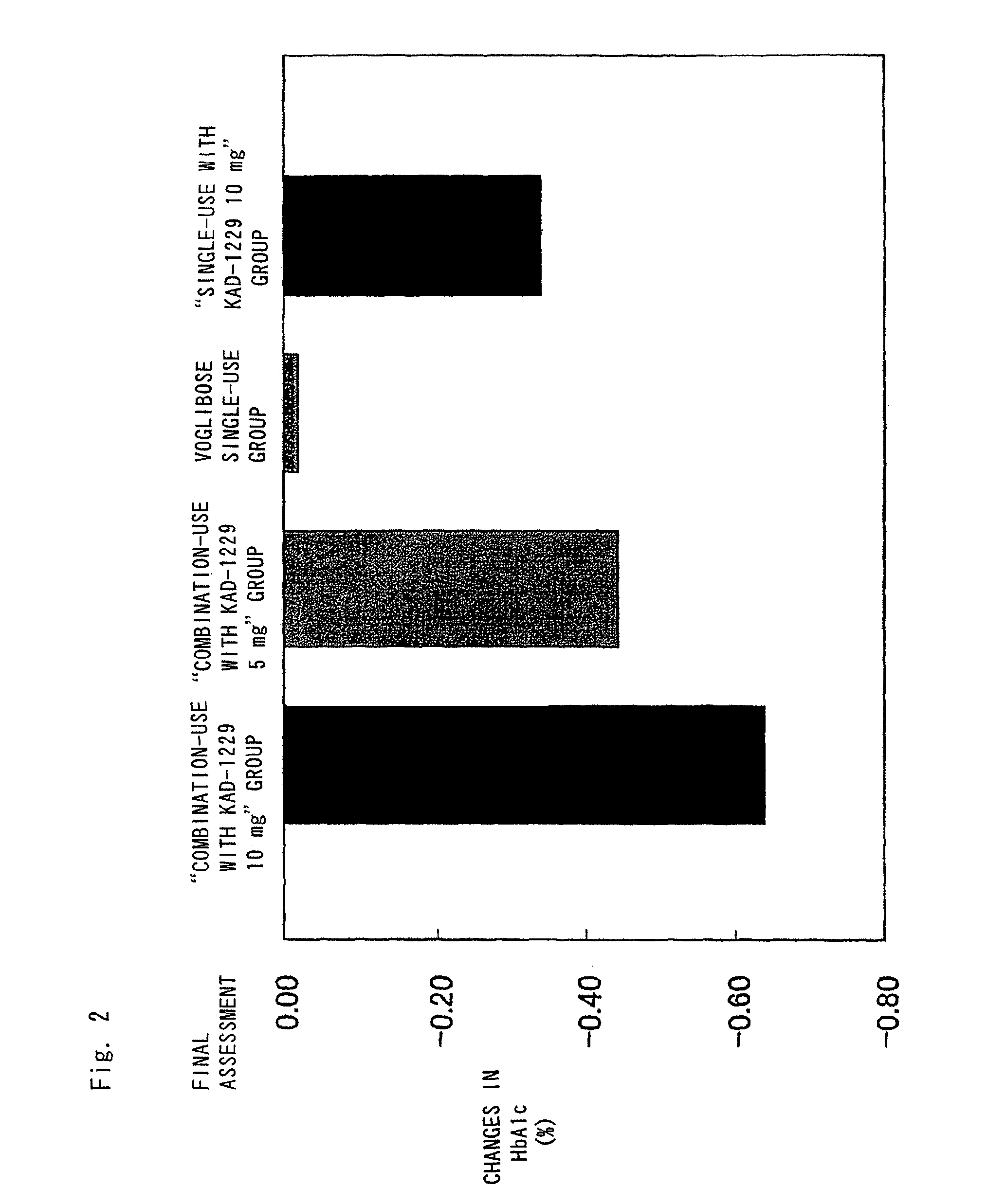
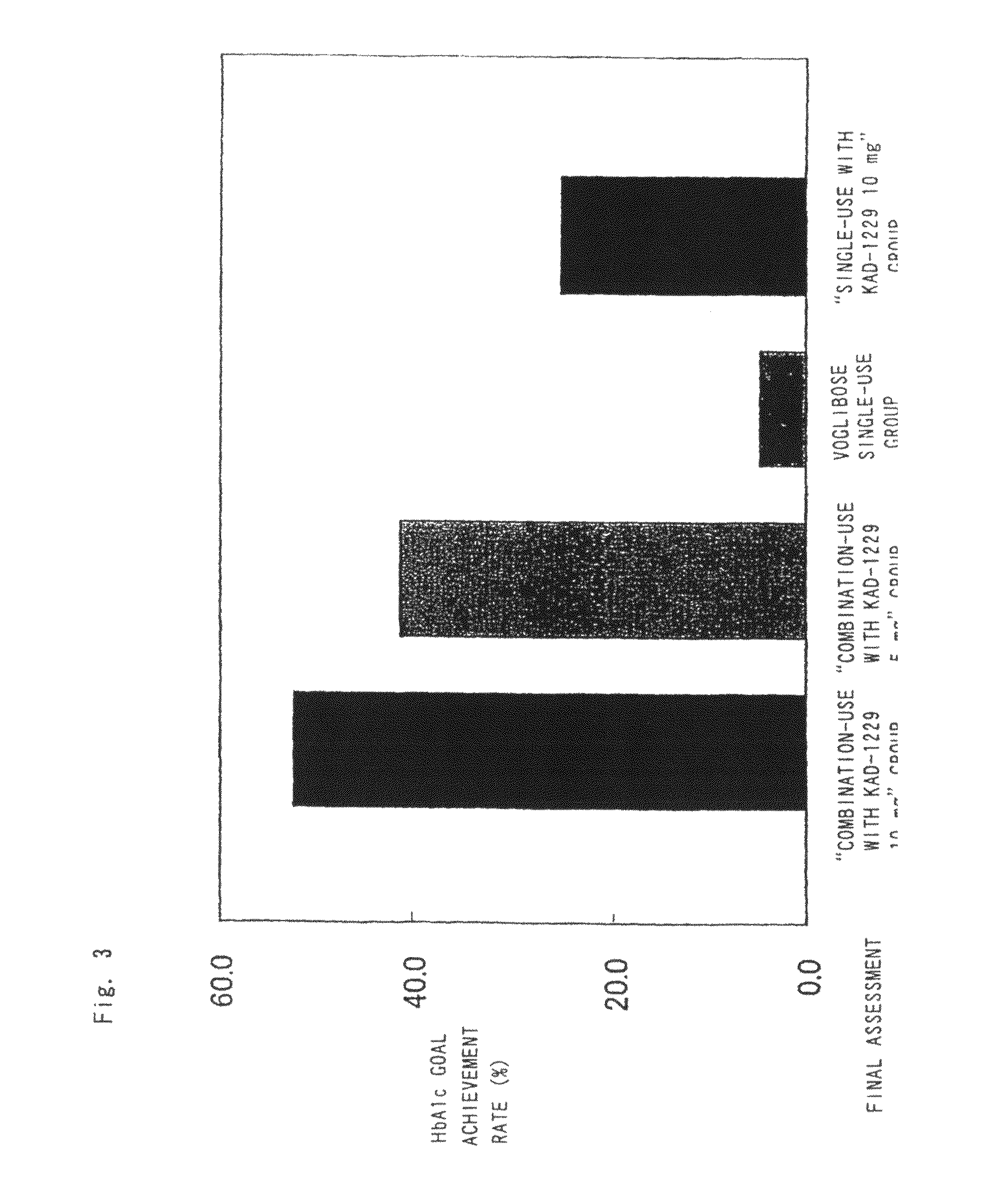
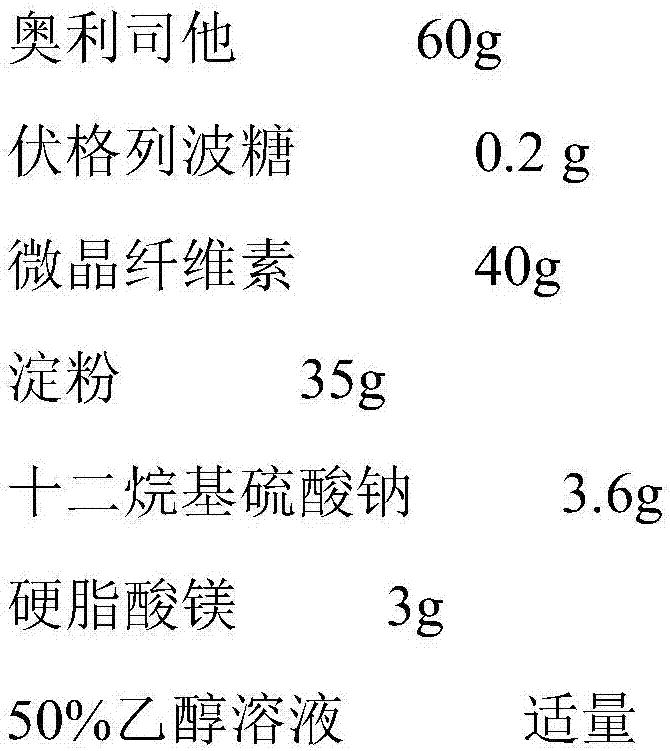
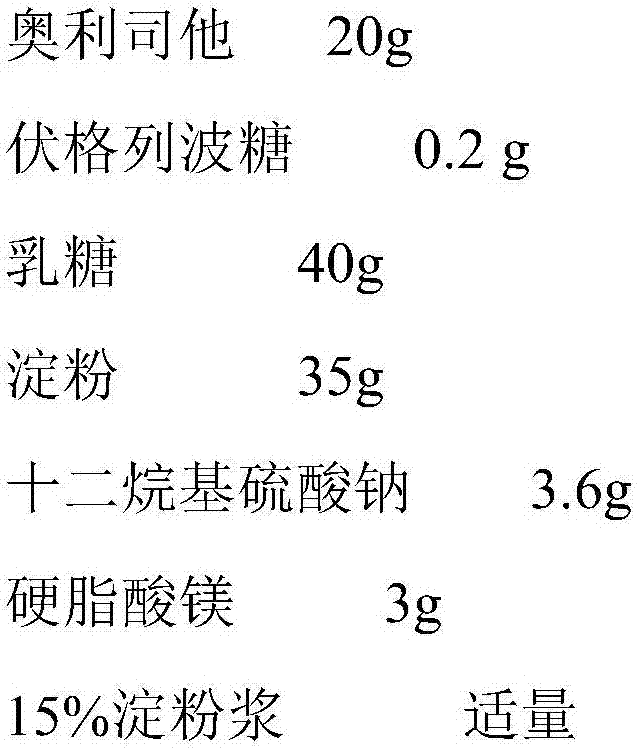
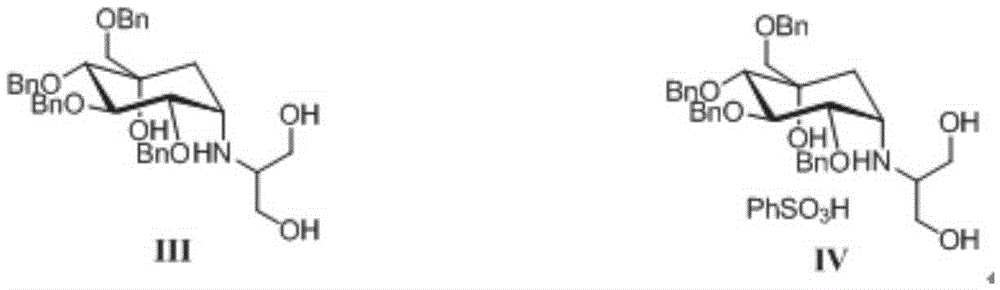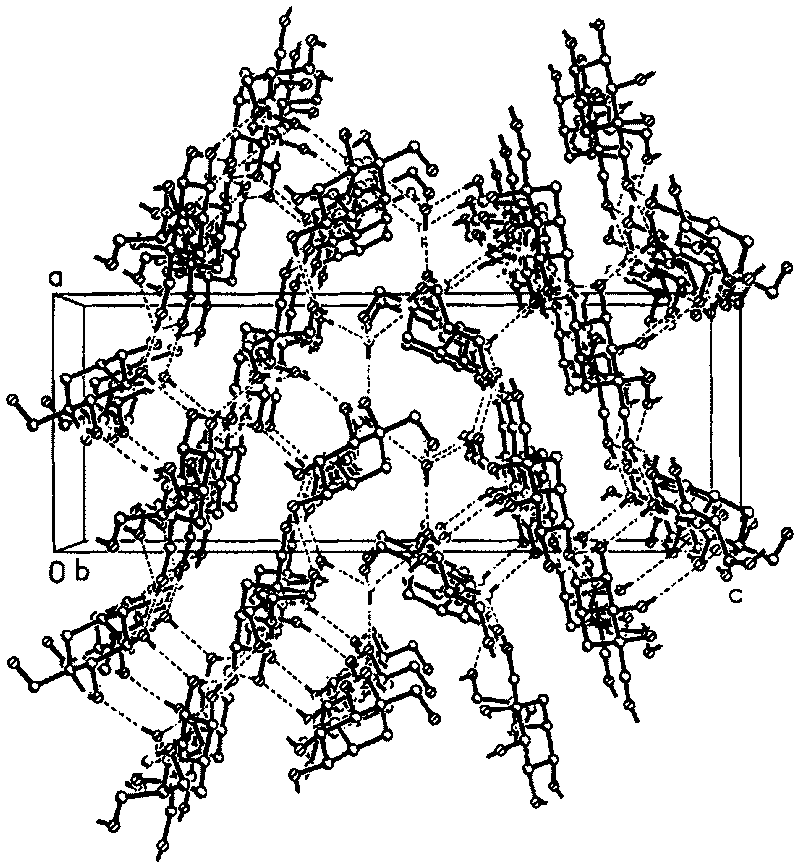Patents
Literature
36 results about "Voglibose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
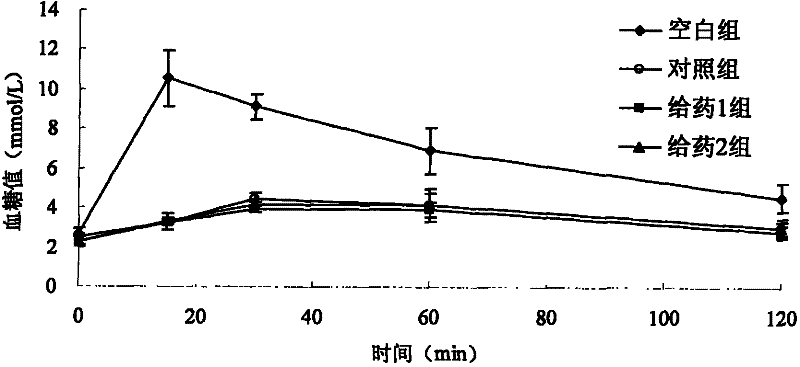
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Voglibose was first launched in 1994, under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.
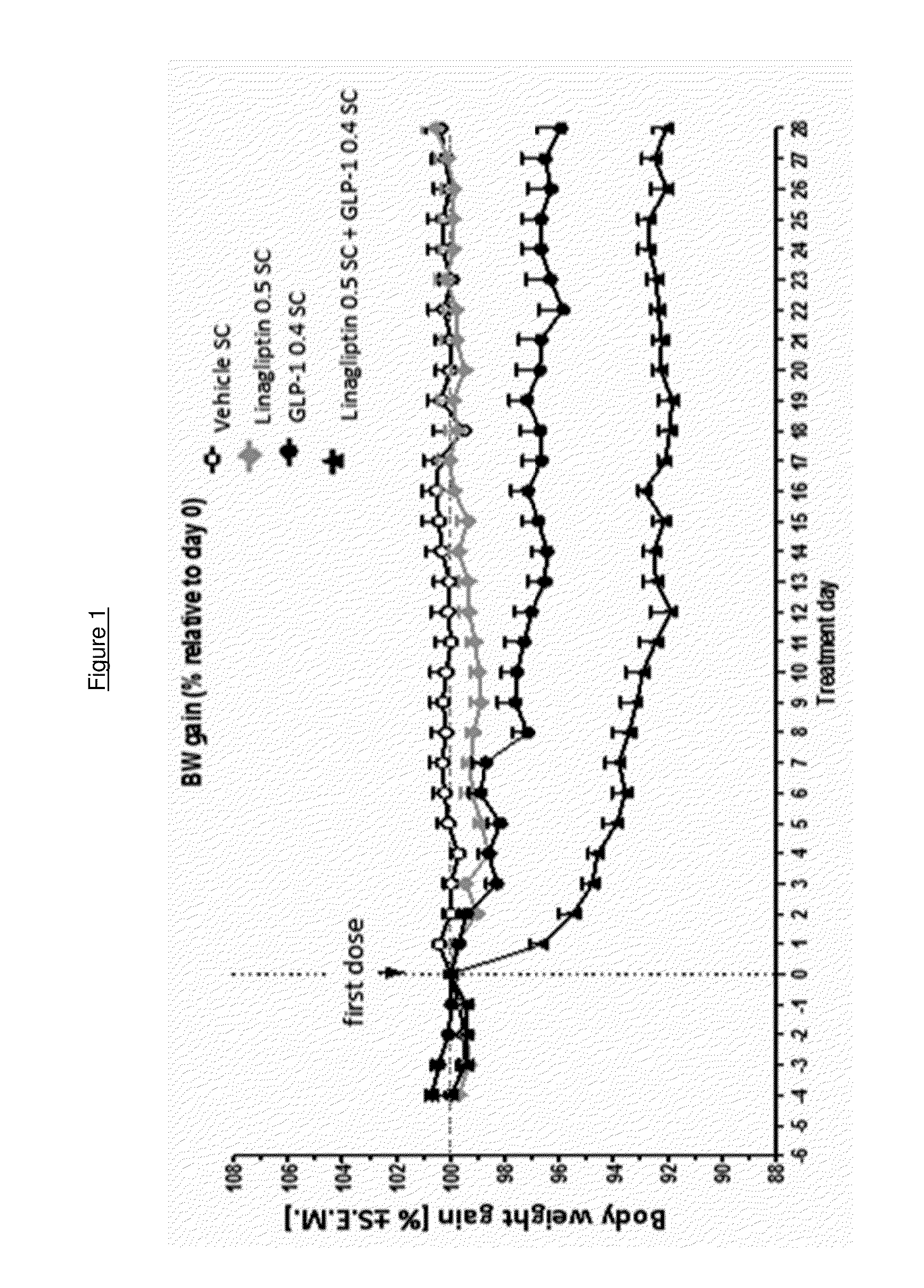
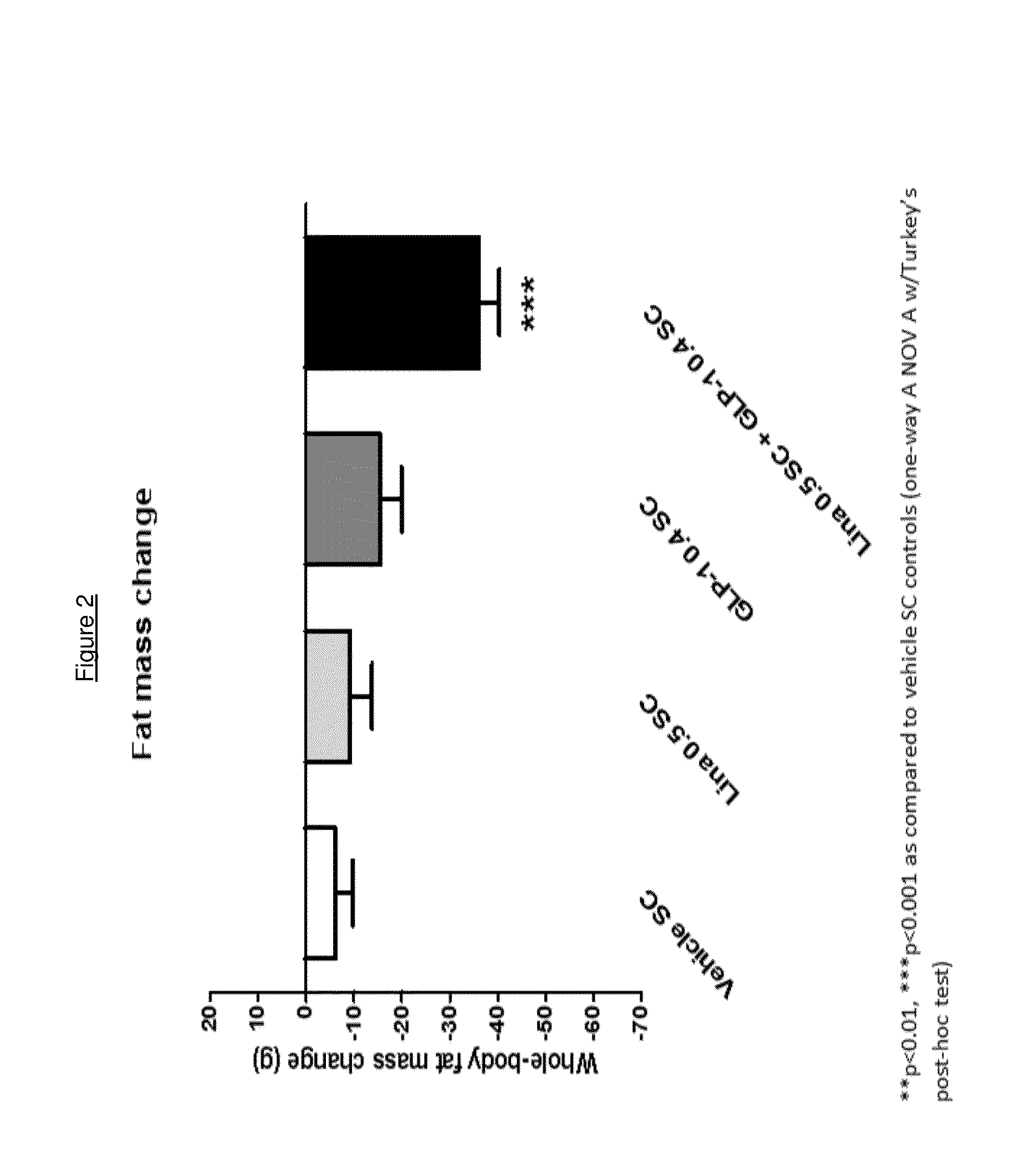
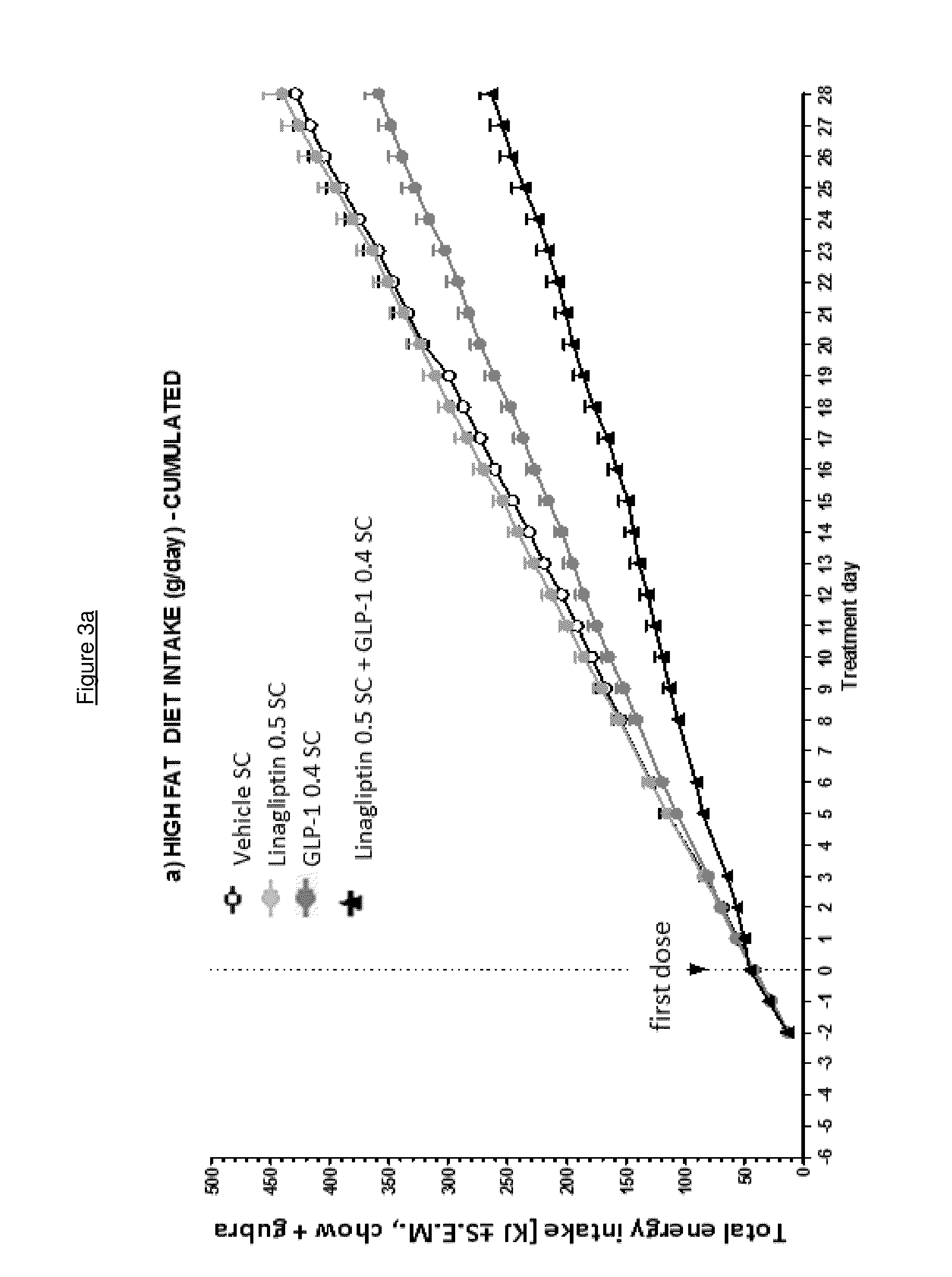
Combination of a certain dpp-4 inhibitor and voglibose
InactiveUS20140343014A1Improving glycemicImproving body weight controlBiocideMetabolism disorderDiseaseGlycosidase inhibitor
The present invention relates to the use of a combination of a certain DPP-4 inhibitor and an alpha-glucosidase inhibitor for use in therapy, e.g. for use in treating and / or preventing a metabolic disease such as type 2 diabetes mellitus and / or conditions related thereto. The invention also relates to the use of such combination for improving body weight control, reducing body weight, inducing satiety, inhibiting gastric emptying and / or reducing food intake, in a patient in need thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
Voglibose dispersible tablet, capsule and method for preparing the same
InactiveCN101219127APromote dissolutionPromote absorptionOrganic active ingredientsMetabolism disorderSucroseLow-substituted hydroxypropylcellulose
The invention relates to a voglibose dispersible tablet and capsule and a preparation method thereof. The voglibose dispersible tablet comprises: 0.01-5 percent of voglibose, 1-99 percent of disintegrating agent, 0-98 percent of diluting agent; 0.5-20 percent of lubricant and fluidizer, 0.1-20 percent of bonding agent; wherein, the disintegrating agent is one or more selected from starch, modified starch, cellulose, microcrystalline cellulose, cross-linked polyvinyl pyrrolidone, sodium carboxymethyl starch, cross-linked sodium carboxymethyl cellulose, low-substituted hydroxypropyl cellulose, alginic acid and colloid magnesium aluminum silicate; the diluting agent is one or more selected from lactose, mannitol, sorbitol, sucrose, calcium sulfate, kaolin, dextrine and sodium chloride. The capsule preparation does not contain the bonding agent. The invention has the advantages that the voglibose dispersible tablet disintegrates swiftly and disperses evenly, the voglibose dispersible tablet can be disintegrated swiftly into fine particles and scattered evenly after being taken orally, which is beneficial for the dissolution and the absorption of the voglibose dispersible tablet with the short onset time. Disintegrated swiftly in three minutes; after being taken orally, the capsule shell quickly swells and splits, which conceals the discomfort caused by the capsule taken in mouth.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Pharmaceutical composition having alpha-glucosidase inhibition activity, and applications thereof
ActiveCN104984346AGood hypoglycemic effectHypoglycemic effect achievedOrganic active ingredientsMetabolism disorderSide effectHypoglycemia
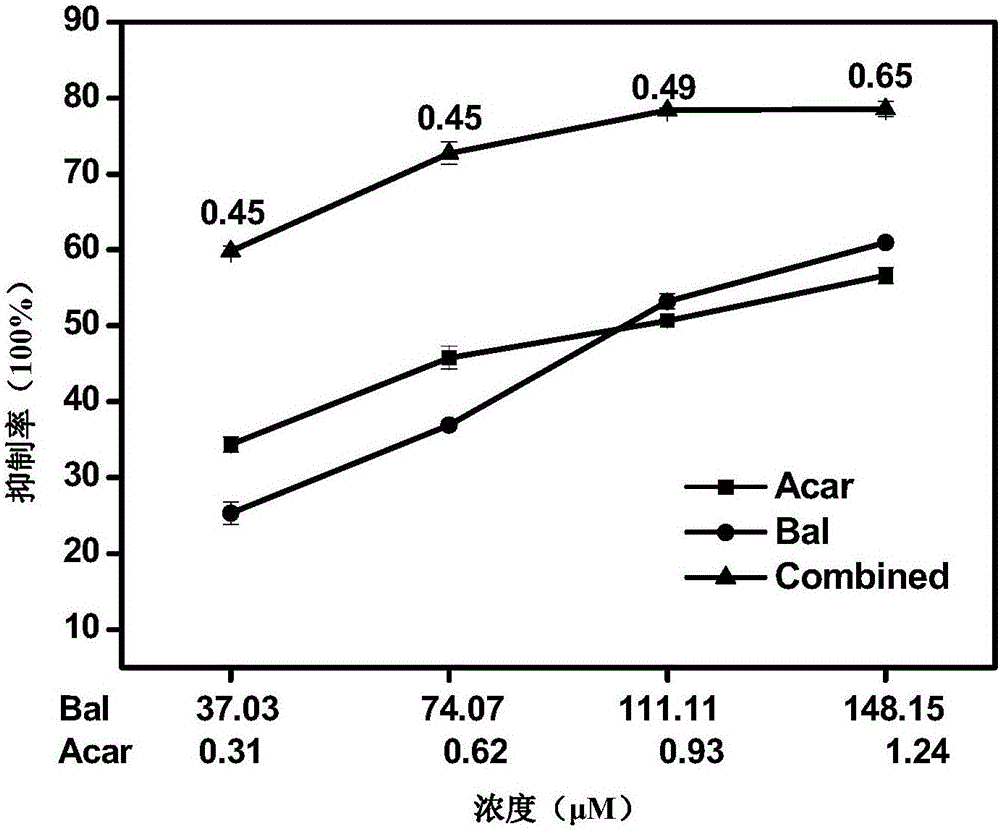
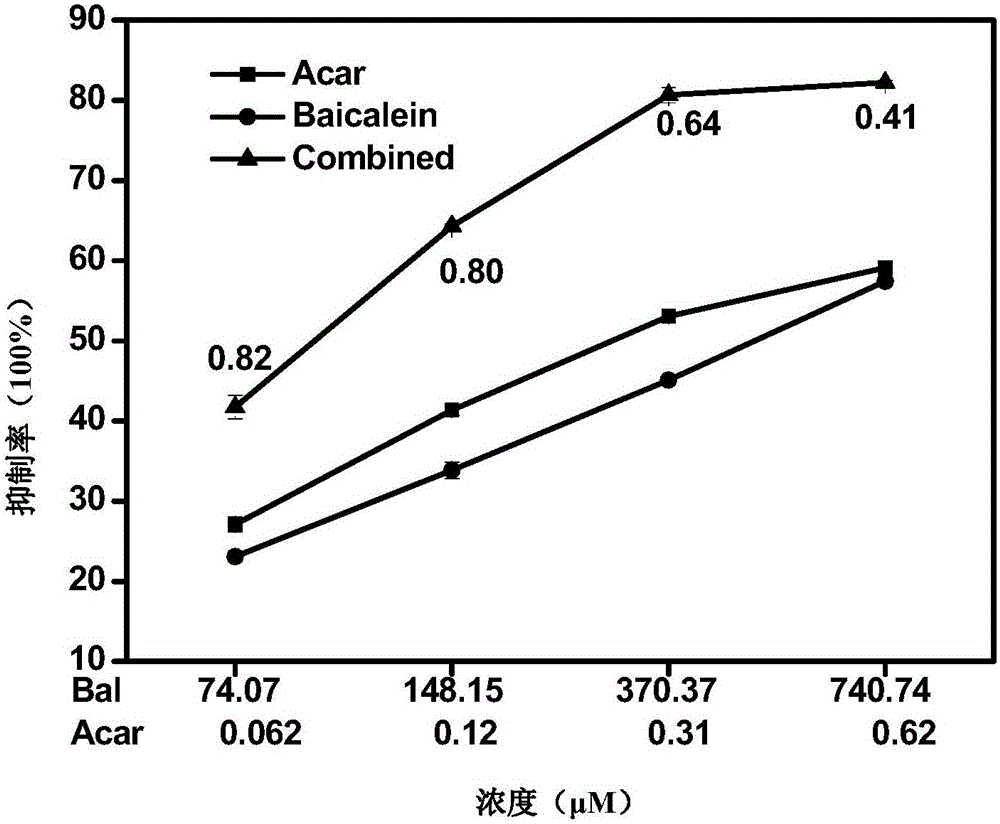
The invention relates to a pharmaceutical composition having alpha-glucosidase inhibition activity, wherein the pharmaceutical composition comprises a flavone compound and an alpha-glucosidase inhibitor, the flavone compound is at least one selected from a monomer such as baicalein, quercetin, luteolin, baicalein-7-O-glucoside and catechin, an organic salt of the monomer, and an inorganic salt of the monomer, and the alpha-glucosidase inhibitor is at least one selected from a monomer such as acarbose, voglibose and miglitol, an organic salt of the monomer, and an inorganic salt of the monomer. According to the present invention, the pharmaceutical composition can effectively reduce postprandial blood glucose, can inhibit the activity of alpha-glucosidase adopting starch, maltose and sucrose as substrates, and less uses the alpha-glucosidase inhibitor, such that the efficacy can be improved, the side effect of the alpha-glucosidase inhibitor can be effectively reduced, and hypoglycemia and other problems easily caused by drug combination are effectively solved.
Owner:上海皋鱼医药科技有限公司
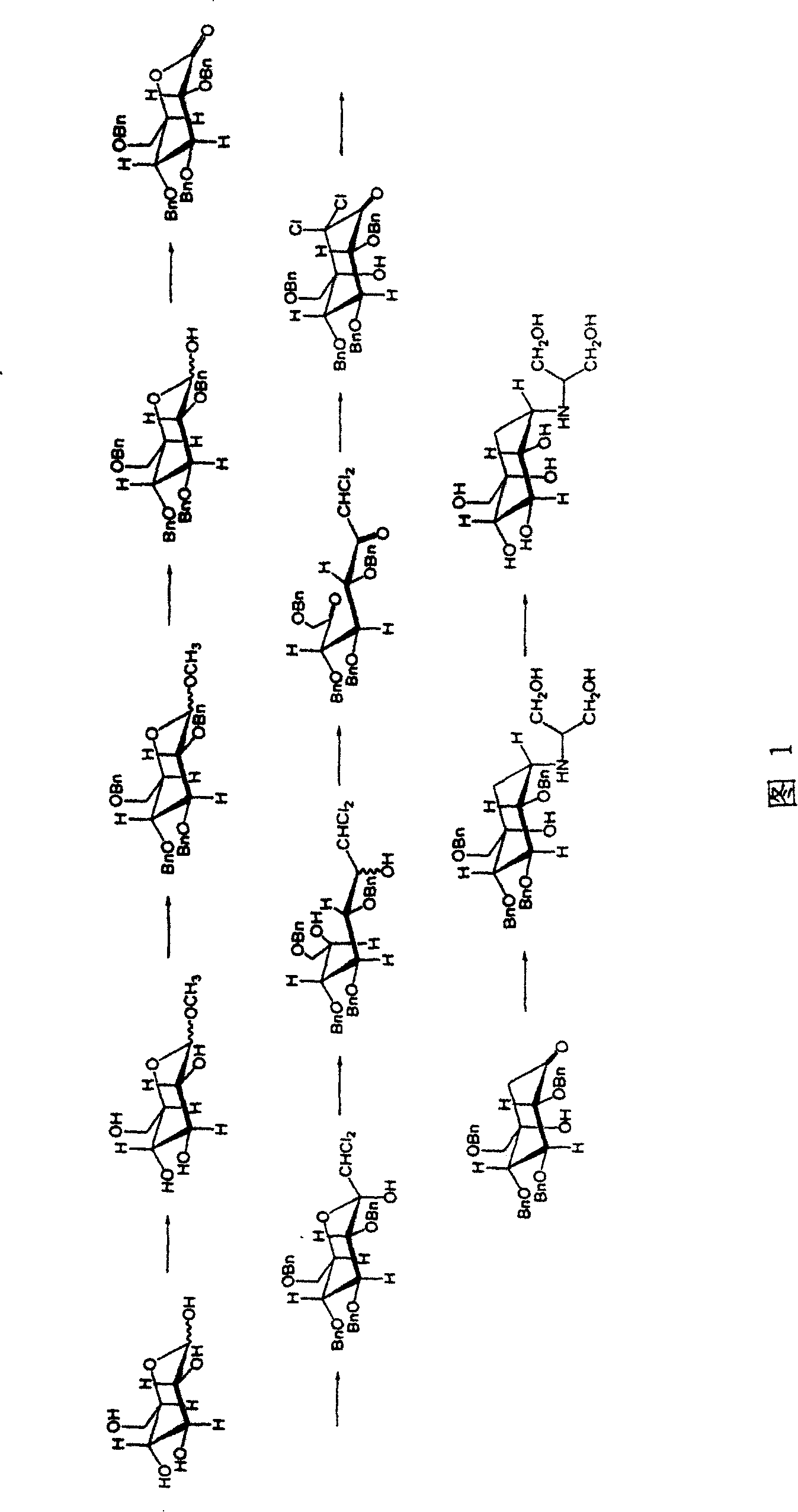
Tetrabenzyl voglibose crystallizing and preparing process
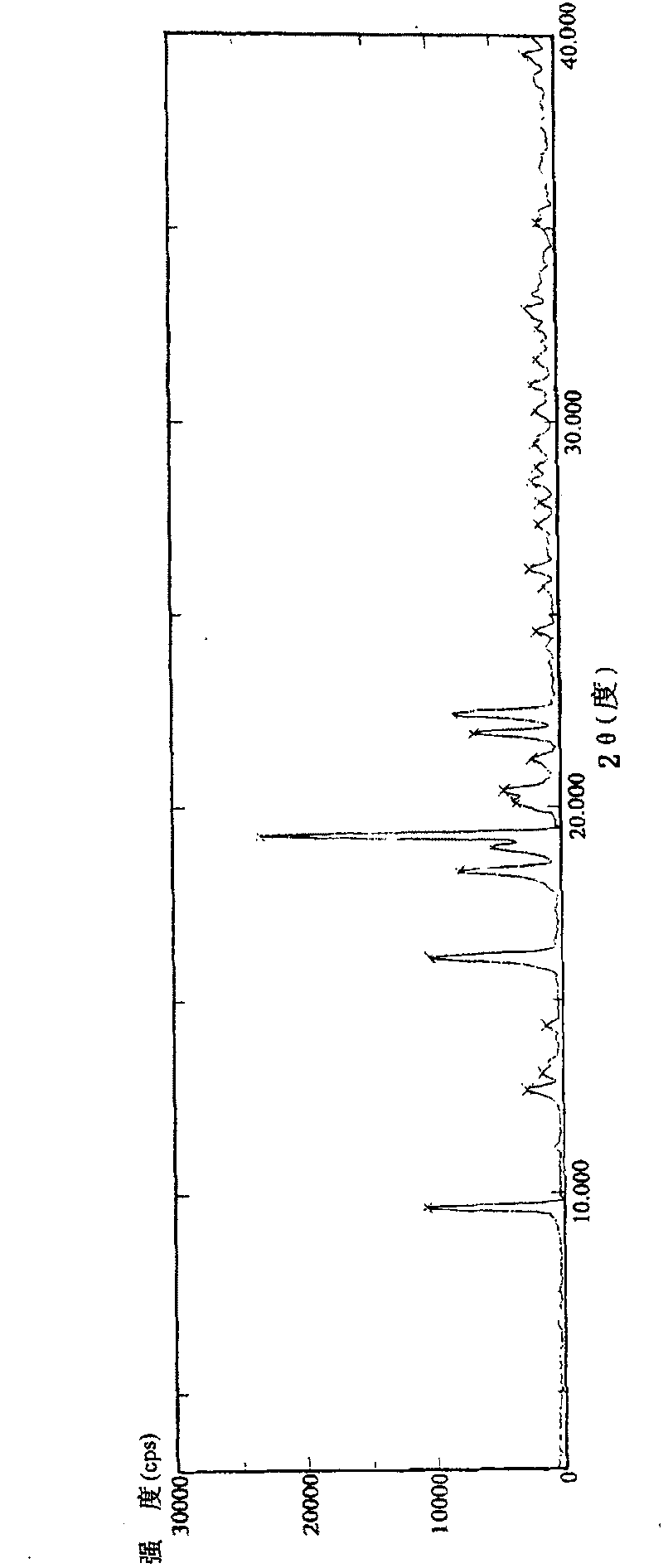
InactiveCN1931826AHigh purityIncrease contentOrganic compound preparationMetabolism disorderX-raySolvent
The present invention relates to crystallized (1S)-(1(hydroxy), 2, 4, 5 / 1, 3)-2, 3, 4-tri-oxo-benzyl-5-[(2-hydroxy-1-(hydroxymethyl) ethyl) amino]-1-carbo-methylbenzylmethyl-1, 2, 3, 4-cyclohexane tetrol, which has smelting point of 89.7 deg.c; monocrystalline X ray diffraction data of orthorhombic system, P2(1)2(1)2(1) space groupp, unit cell parameters a=7.8487, b=20.746, c=20.988 and R 0.0748; and X ray diffraction peak of crystal powder of 16.76, 18.90 and 24.00+ / -0.15 deg. It is prepared through dissolving oily tetrabenzyl voglibose in one kind of polar non-protonic solvent, adding one other kind of non-polar solvent via stirring at room temperature to crystallize, stilling, filtering and vacuum drying to obtain the crystal. Compared with oily tetrabenzyl voglibose, the crystallized tetrabenzyl voglibose has higher purity, convenient transportation and maintenance and easy application.
Owner:PHARMAXYN LAB
Method for preparing voglibose particles
InactiveCN108635332ASimple production processShorten production timeOrganic active ingredientsMetabolism disorderAdhesiveDiluent
The invention provides a method for preparing voglibose particles. The particles are prepared through a one-step prilling process,the friability is low,and the roundness is good. Voglibose can be dissolved out fast,the content uniformity is good,and the voglibose particles are suitable for being further prepared into capsules,tables (including dispersible tablets and orally disintegrating tablets)and granules. According to the preparation idea,the voglibose is added into a polar solvent containing a water-soluble adhesive,and the mixture is sprayed into a one-step pelletizer containing a diluent and a disintegrating agent in a top spraying prilling mode. The preparation method is easy to operate,the labor intensity is reduced,and the preparation method is suitable for large-scale production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Process for the preparation of voglibose
The invention relates to processes for the preparation of pure voglibose. The invention also relates to the preparation of acid addition salts of voglibose. More particularly, it relates to the preparation of crystalline hydrochloride salt of voglibose. The invention also relates to pharmaceutical compositions that include the pure voglibose or voglibose hydrochloride and use of said compositions for treatment or prevention of hyperglycemic symptoms and various disorders caused by hyperglycemia such as diabetes, obesity, and hyperlipemia.
Owner:RANBAXY LAB LTD
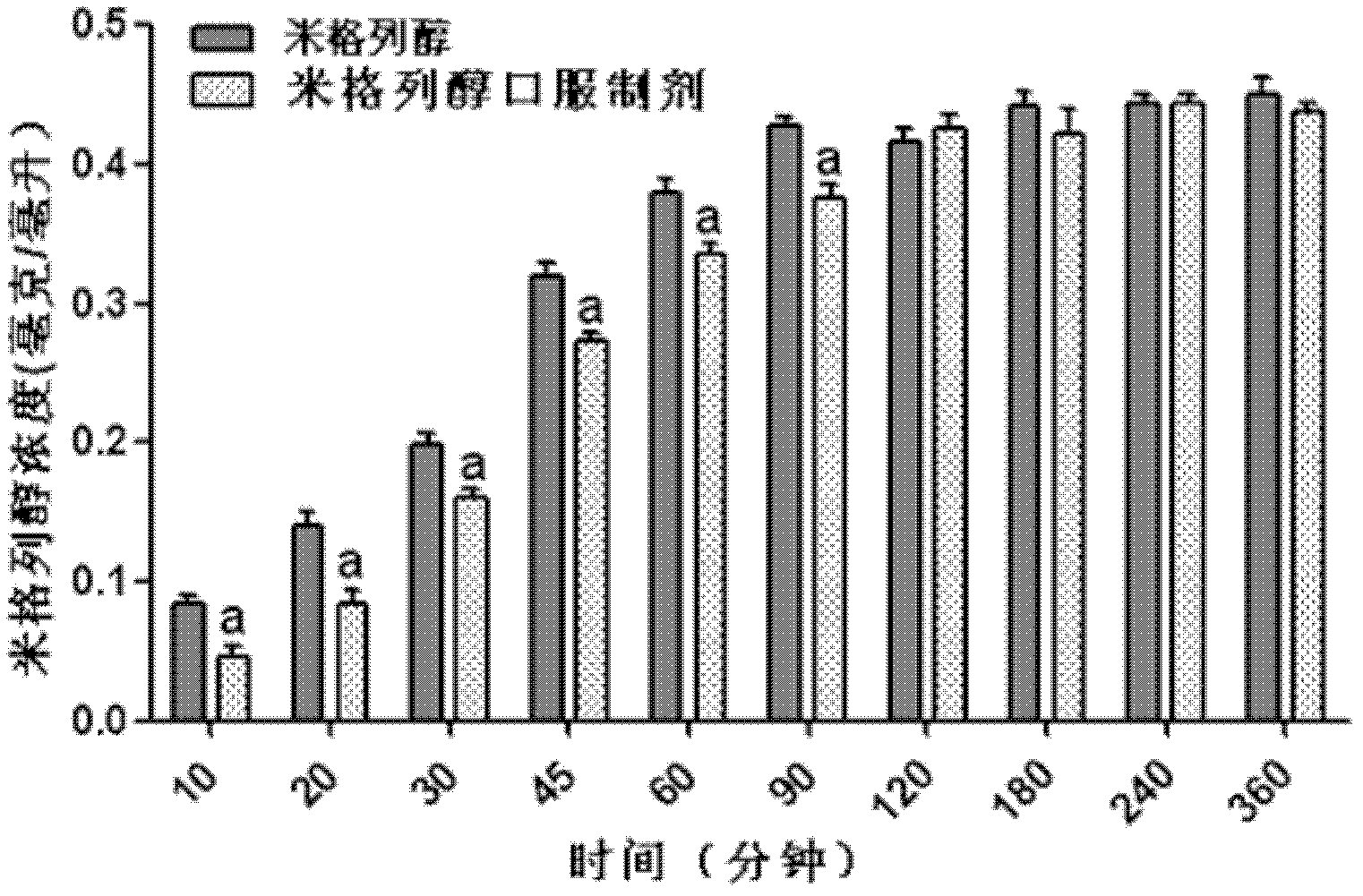
Voglibose film and preparation method thereof
ActiveCN101732286ANice appearanceEasy to carryOrganic active ingredientsMetabolism disorderFlavouring agentPlasticizer
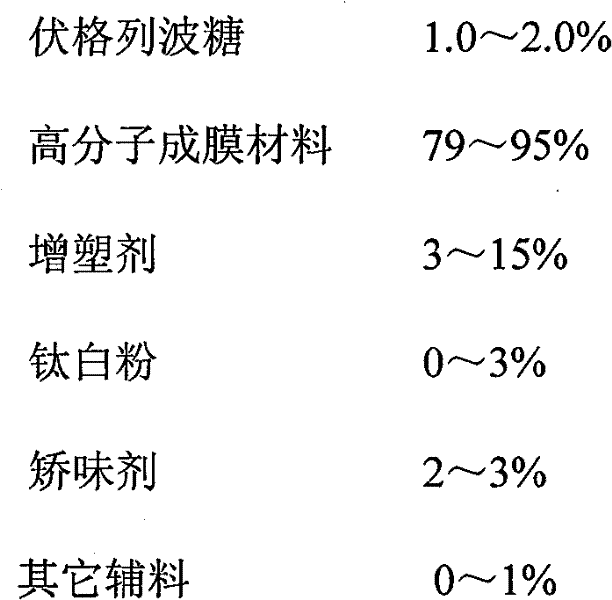
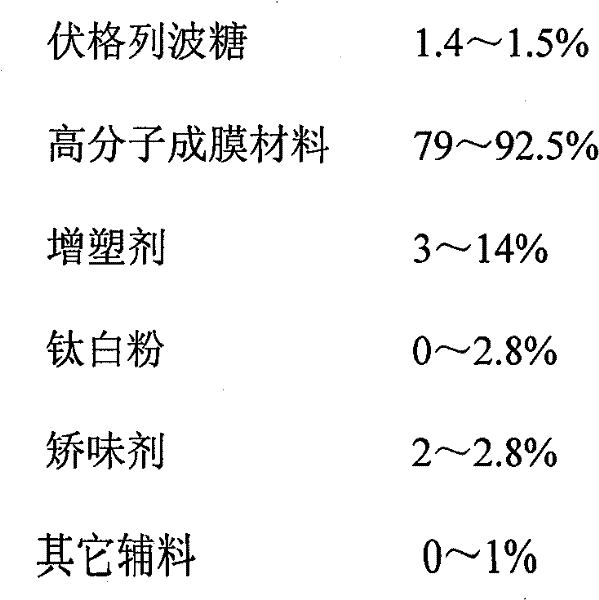
The invention discloses a voglibose film and a preparation method thereof. The voglibose film comprises the following components by weight percent: 0.2-15% of voglibose, 40-99.8% of high molecular filming material, 0-20% of plasticizer, 0-5% of titanium pigment and 0-20% of flavouring agent, wherein the molecular weight of the high molecular filming material is 20000-300000. The invention is the voglibose film prepared by a solid dispersion technology, the medicine is highly dispersed in a carrier material and is encircled by the sufficient molecules of the carrier material, the high dispersivity of the medicine is ensured, the dissolving out of the medicine is quickened, the medicine enters the digestive tract along with saliva quickly, and the medicine effect is exerted.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Process for preparation of voglibose
InactiveUS20050165257A1Low costOrganic compound preparationBulk chemical productionCyclohexanoneVoglibose
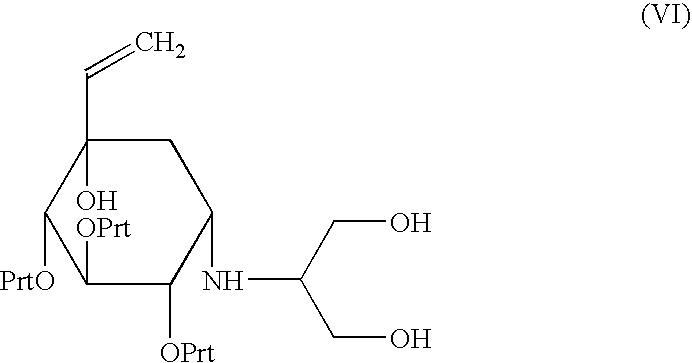
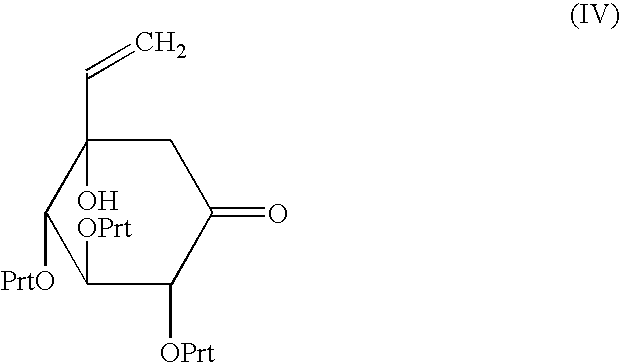
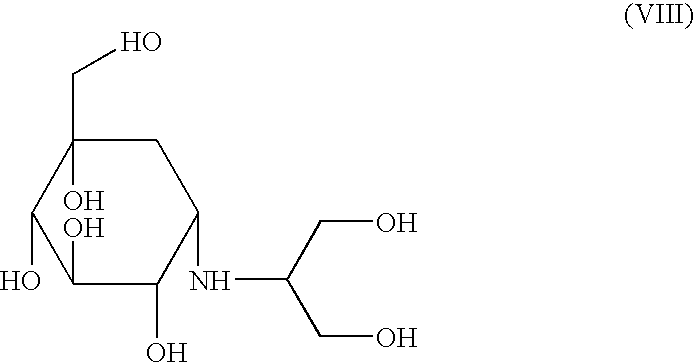
A process capable of conveniently preparing voglibose at a low cost in a safe process, and an intermediate which can be suitably used in the process and a process for preparing the intermediate are provided. An inositol derivative represented by the formula (VI): wherein Prt is a protecting group of hydroxyl group; a process for preparing the inositol derivative, wherein a cyclohexanone compound represented by the formula (IV): wherein Prt is as defined above, is dihydroxyaminated using a dihydroxyaminating agent and a reducing agent; and a process for preparing voglibose represented by the formula (VIII): wherein the inositol derivative is oxidized to give an inositol compound, and the protecting group, Prt of the inositol compound is deprotected.
Owner:SAWAI PHARMA
Pharmaceutical composition for improving complications of high-fat and high-sugar diet and application thereof
ActiveCN104840962AOvercoming hypoglycemic effect is not obviousOvercoming large doseMetabolism disorderDigestive systemMiglitolSide effect
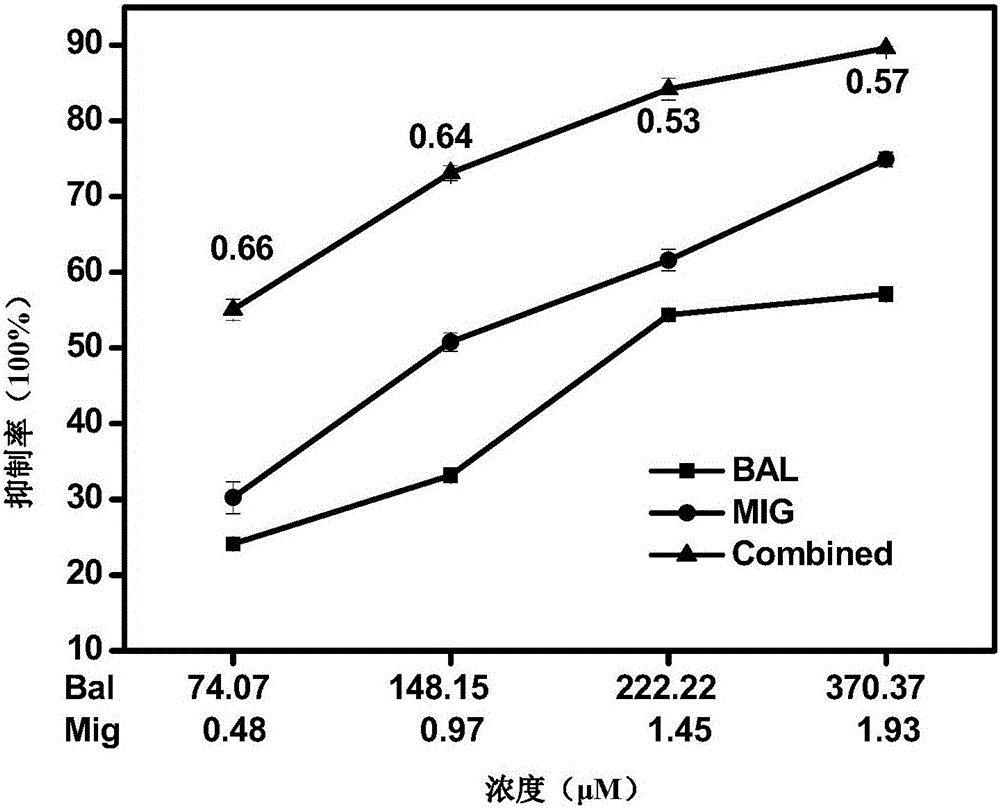
The invention provides a pharmaceutical composition for improving complications of high-fat and high-sugar diet. The pharmaceutical composition comprises flavonoid and an alpha-glucosidase inhibitor, wherein the flavonoid is at least one selected from the group consisting of monomer baicalein, monomer chrysin, organic salts of the monomers and inorganic salts of the monomers, and the alpha-glucosidase inhibitor is one selected from the group consisting of monomer acarbose, monomer voglibose, monomer migltol, organic salts of the monomers and inorganic salts of the monomers. The composition provided by the invention can effectively reduce the dosage of the alpha-glucosidase inhibitor; desired effects can be obtained by mixing the flavonoid with the alpha-glucosidase inhibitor with a dosage 0.01 to 0.75 time of a normal dosage; the disadvantages of a great dosage and great side-effects in individual usage of the alpha-glucosidase inhibitor are overcome; the disadvantages of unobvious hypoglycemic effect, a great dosage, a long administration period and the like in individual usage of the flavonoid are overcome; and the disadvantages of complex components and difficult quality control of a traditional Chinese medicine compound drug are overcome.
Owner:上海皋鱼医药科技有限公司
Oral preparation for slowing down absorption of alpha-glycosidase inhibitor and enhancing hypoglycemic drug effect
ActiveCN102210866AReduce absorptionProlong the action timeMetabolism disorderMacromolecular non-active ingredientsDiseaseCellulose
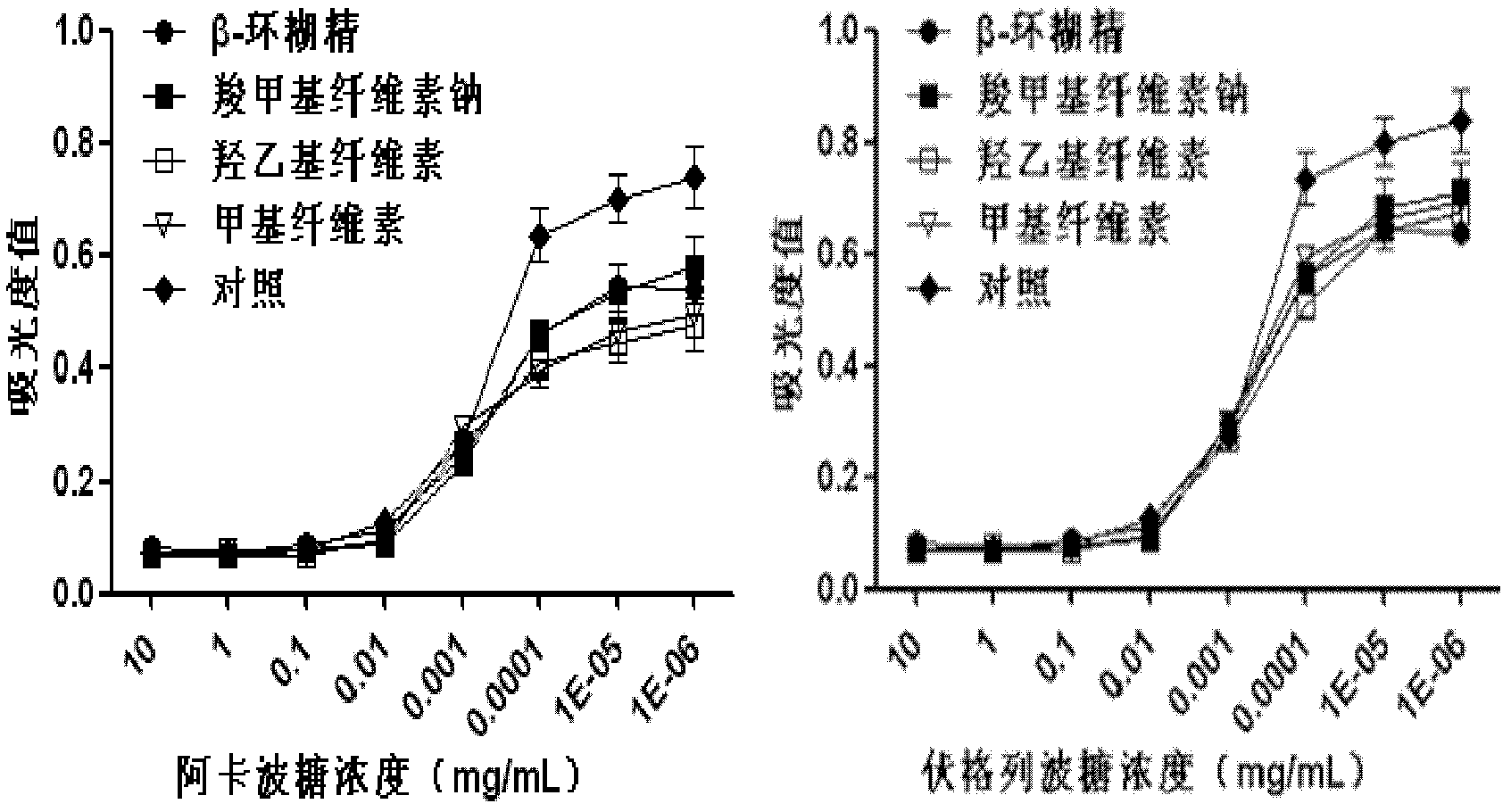
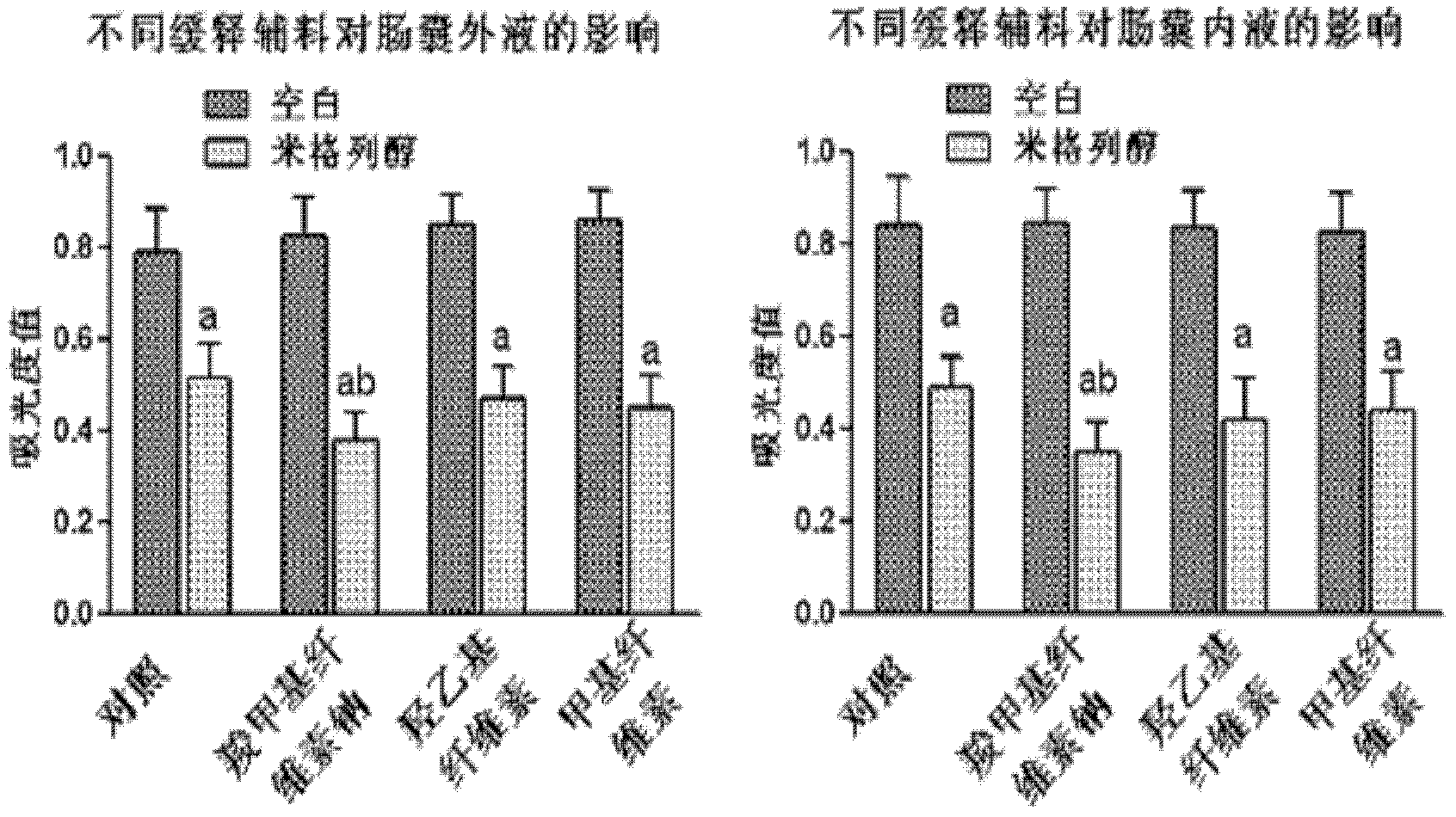
The invention discloses an oral preparation for slowing down the absorption of an alpha-glycosidase inhibitor and enhancing a hypoglycemic drug effect. The preparation is an oral preparation prepared by adding the following adhesive auxiliary materials based on the certain proportion: carboxymethyl cellulose, hydroxyethyl cellulose, methyl cellulose or beta-cyclodextrin and the like, to each alpha-glycosidase inhibitor (acarbose, voglibose, miglitol, 1-deoxynojirimycin and extract of the 1-deoxynojirimycin). Compared with singly taken alpha-glycosidase inhibitors, the adhesives can be used for prolonging the action time of the alpha-glycosidase inhibitor in the intestine, slowing down the absorption of a medicament and glucose in the intestine and further reducing a plasma concentration, thus the hypoglycemic drug effect is improved. The oral preparation disclosed by the invention can be applied to the prevention and therapy of diseases, such as diabetes, obesity and the like.
Owner:NANKAI UNIV +1
Tetrabenzyl voglibose crystallizing and preparing process
InactiveCN100393694CHigh purityIncrease contentOrganic compound preparationMetabolism disorderX-raySolvent
The present invention relates to crystallized (1S)-(1(hydroxy), 2, 4, 5 / 1, 3)-2, 3, 4-tri-oxo-benzyl-5-[(2-hydroxy-1-(hydroxymethyl) ethyl) amino]-1-carbo-methylbenzylmethyl-1, 2, 3, 4-cyclohexane tetrol, which has smelting point of 89.7 deg.c; monocrystalline X ray diffraction data of orthorhombic system, P2(1)2(1)2(1) space groupp, unit cell parameters a=7.8487, b=20.746, c=20.988 and R 0.0748; and X ray diffraction peak of crystal powder of 16.76, 18.90 and 24.00+ / -0.15 deg. It is prepared through dissolving oily tetrabenzyl voglibose in one kind of polar non-protonic solvent, adding one other kind of non-polar solvent via stirring at room temperature to crystallize, stilling, filtering and vacuum drying to obtain the crystal. Compared with oily tetrabenzyl voglibose, the crystallized tetrabenzyl voglibose has higher purity, convenient transportation and maintenance and easy application.
Owner:PHARMAXYN LAB
Synthesis method of voglibose
PendingCN113214094AMild reaction conditionsEasy to operateEsterified saccharide compoundsSugar derivativesSodium acetateBiochemical engineering
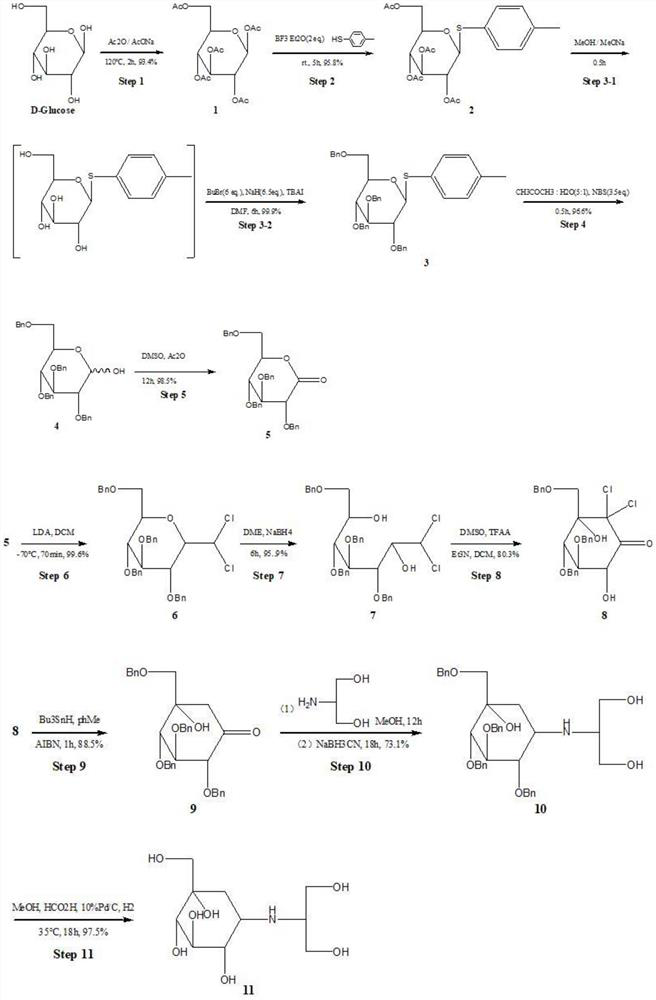
The invention provides a synthesis method of voglibose, and solves the technical problems that in an existing synthesis method of voglibose, raw materials are difficult to obtain, high in price, large in investment, low in yield and not suitable for industrial production. The synthesis method comprises the steps: synthesizing a compound V by taking glucose monohydrate and sodium acetate as raw materials through eleven reaction steps; and preparing a compound VIII from the compound V through an addition reaction, a ring-opening reaction and an aldol condensation reaction, and thus obtaining voglibose through amination reduction of the compound VIII. The synthesis method of voglibose can be widely applied to the technical field of voglibose synthesis methods.
Owner:WEIFANG TIANFU CHEM TECH
Glipizide/voglibose hypoglycemic oral preparation composition and preparation method thereof
InactiveCN105687213AMulti-targetImprove complianceMetabolism disorderSulfonylurea active ingredientsPatient complianceGlimepiride
The present invention discloses a glipizide / voglibose hypoglycemic oral preparation composition and a preparation method thereof, and the glipizide / voglibose hypoglycemic oral preparation composition comprises the following raw materials: 2-6% by weight of main components, 50-90% by weight of a filler, 5-15% by weight of a disintegrant, 0.1-3% by weight of a binder, 0.01-5% by weight of a lubricant. The main components comprise 2.5mg-5mg of glipizide and 0.1mg-0.3mg of voglibose, and auxiliary materials comprises the filler, the disintegrant, the binder and the lubricant. The glipizide / voglibose hypoglycemic oral preparation composition has the advantages of complementary main material action mechanism, multi targets, good patient compliance, and the like, and is more applicable to type 2 diabetes patients of which blood-glucose rises after meals and fasting blood-glucose rises insignificantly.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Preparation method of voglibose impurity vinyl voglibose
InactiveCN108276298ASimple processEasy to operateOrganic compound preparationAmino-hyroxy compound preparationVogliboseValienamine
The invention relates to a preparation method of voglibose impurity vinyl voglibose. According to the preparation method, valienamine and 1,3-dihydroxy acetone are adopted, one kettle method is adopted to prepare vinyl voglibose through reduction using a reducing agent. The preparation method is simple; operation is convenient; subsequent process is simple; technology advantages are obvious; and purity and yield are high.
Owner:SHANDONG XINHUA PHARMA CO LTD
A kind of voglibose tablet and preparation method thereof
ActiveCN108309946BHigh dissolution rateImprove efficacyOrganic active ingredientsMetabolism disorderTablet dissolutionMagnesium stearate
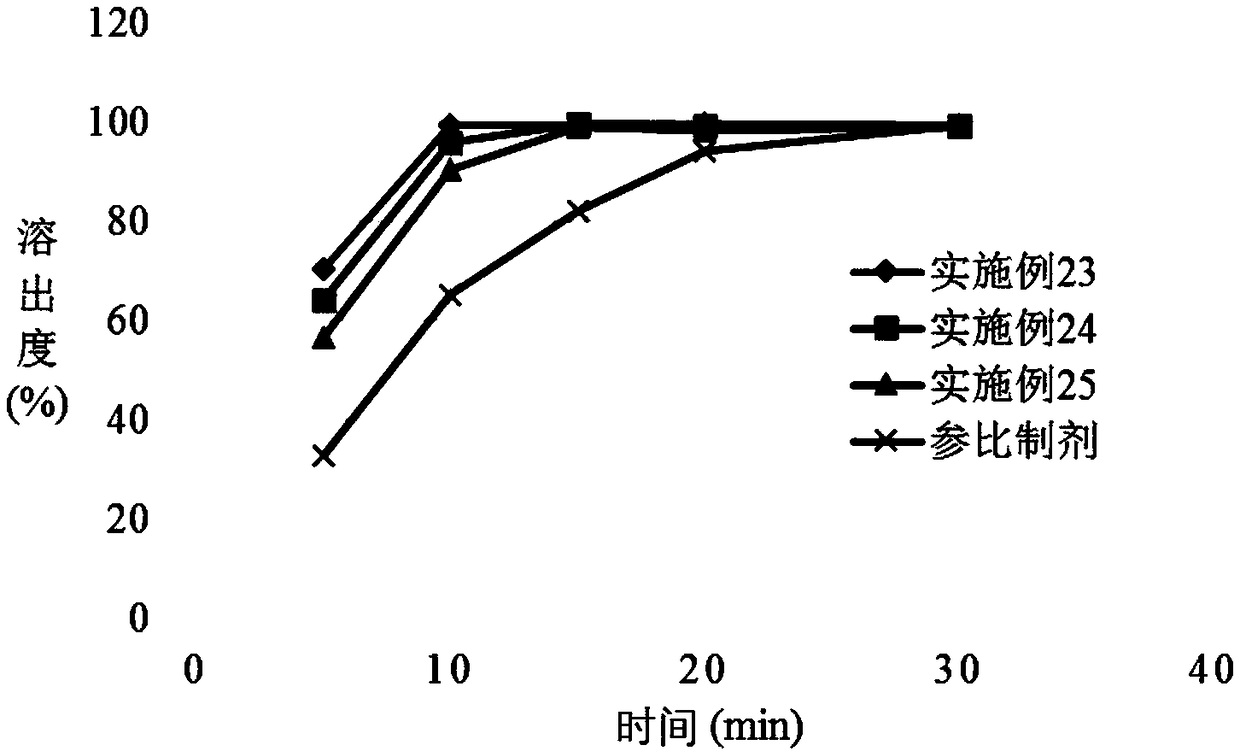
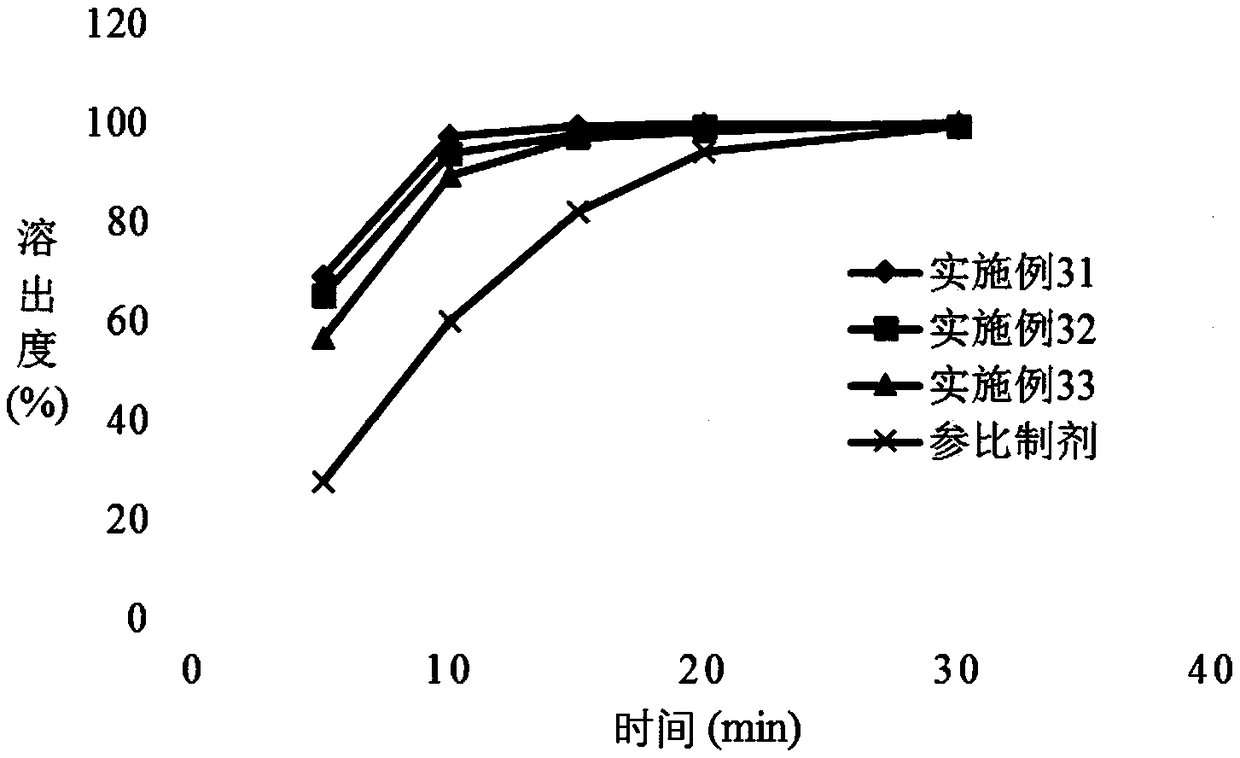
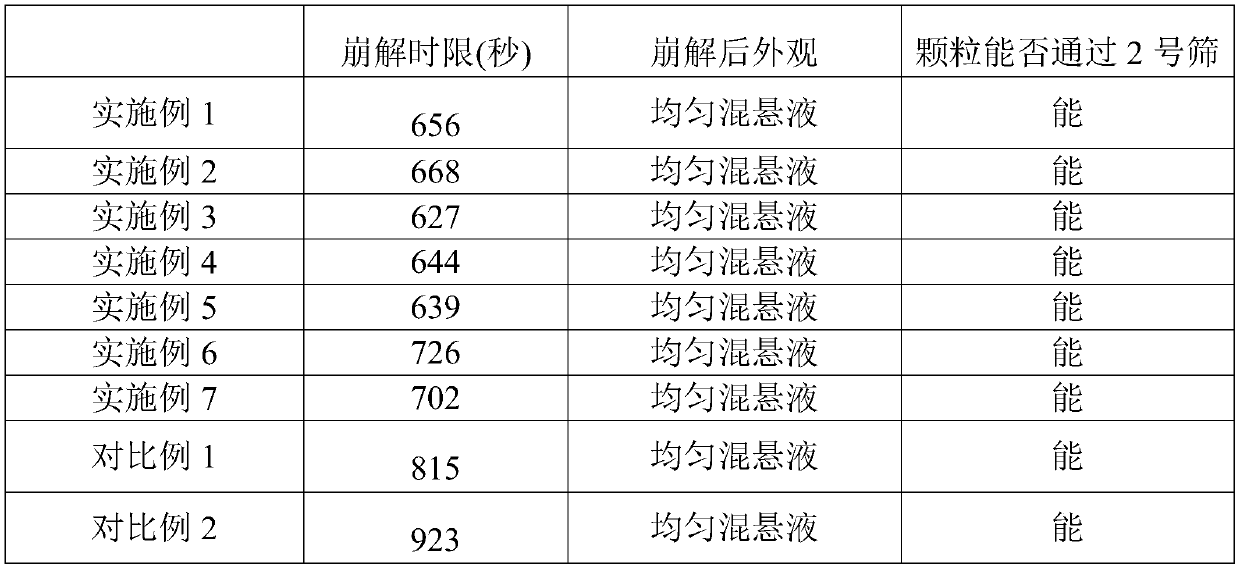
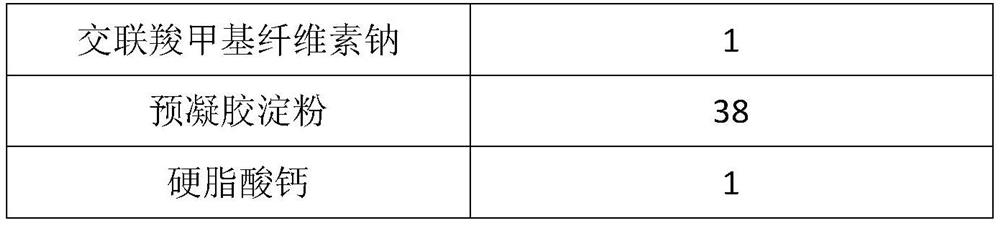
The invention provides a voglibose tablet and a preparation method thereof. The voglibose tablet is prepared from 1 part by weight of voglibose, 450-550 parts by weight of lactose, 130-170 parts by weight of starch, 20-30 parts by weight of high substituted hydroxypropyl fibers, 3-4 parts by weight of magnesium stearate, 0.1-0.5 parts by weight of propolis, 5-10 parts by weight of sodium lauryl sulfate, 4-6 parts by weight of polysorbate 80, 30-40 parts by weight of a co-solvent and 40-50 parts by weight of a disintegrant. The voglibose is used as the main drug, and high-substituted hydroxypropyl fiber, propolis, polysorbate 80 synergistic voglibose, lactose, starch, magnesium stearate and sodium lauryl sulfate are used in a good ratio so that the tablet dissolution degree is greatly improved, the disintegration time limit is optimized and the drug efficacy is improved. The voglibose tablet has a good dissolution degree and good disintegration effects, can well exert the efficacy of the voglibose tablet and can improve the drug efficiency. Through combination of the initial mixing process and low / high-speed agitation, the tablet dissolution degree is further improved and the quality of the tablet is improved.
Owner:HAINAN HUALON PHARM
Preparing method for tetra-benzyl-voglibose
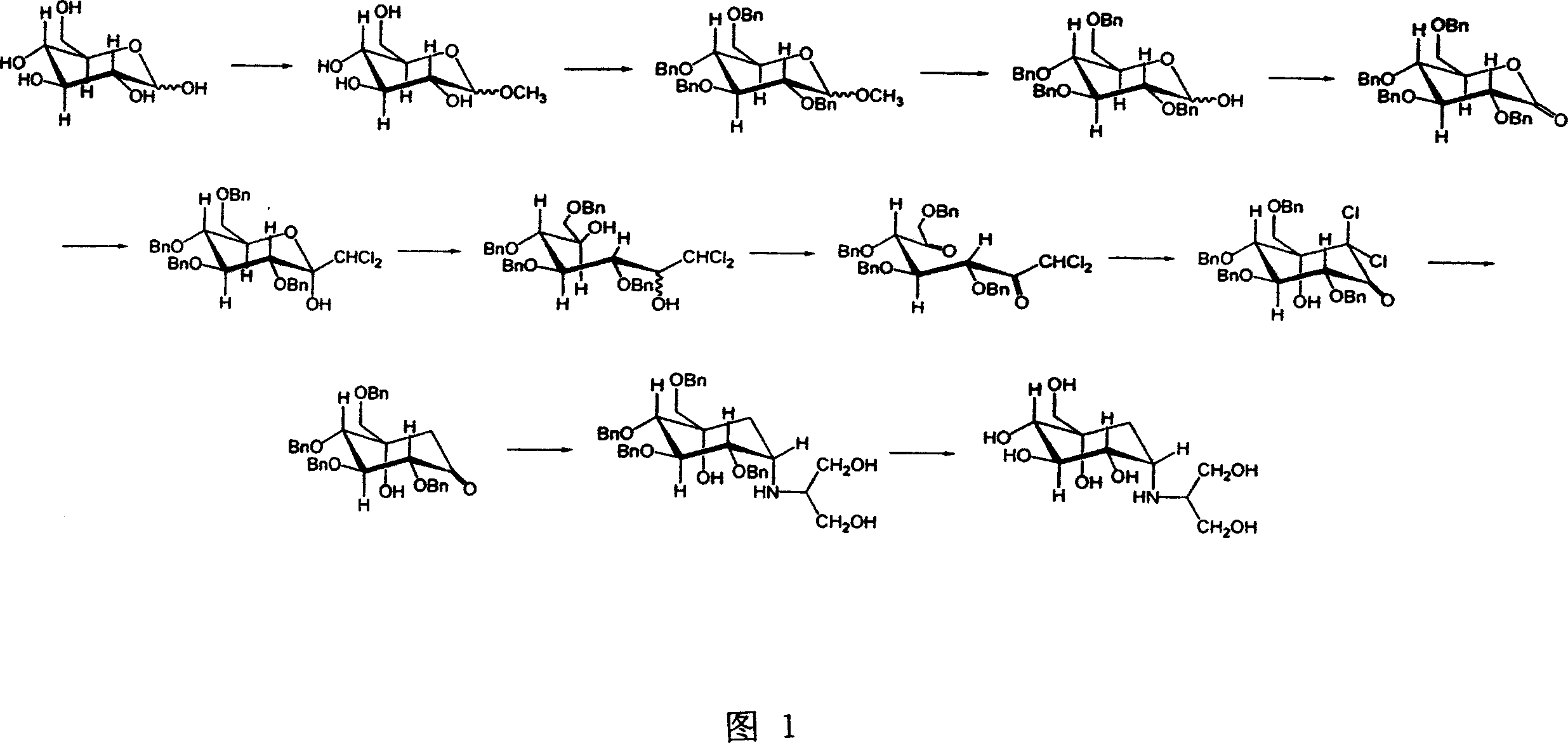
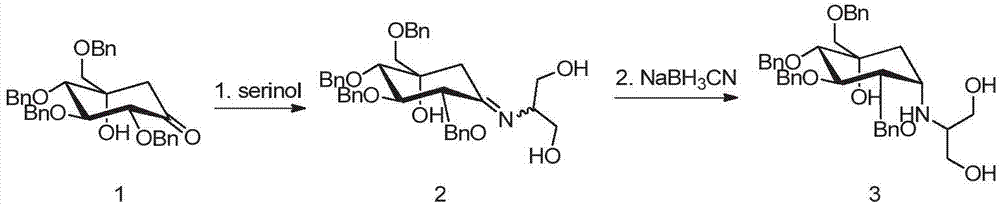
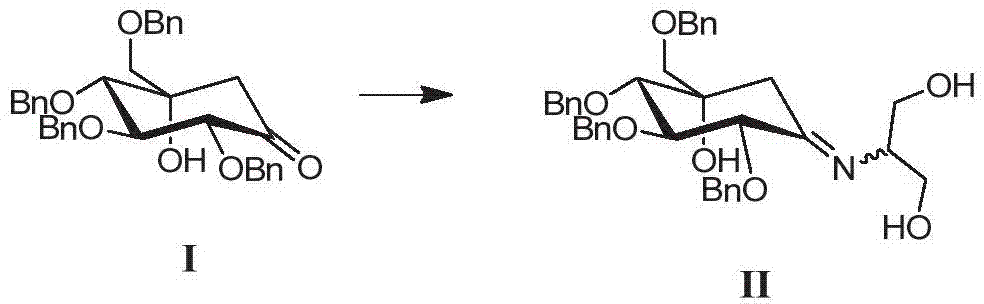
ActiveCN105254514ALow costImprove production safetyOrganic compound preparationSulfonic acids salts preparationCyclohexanoneSolvent
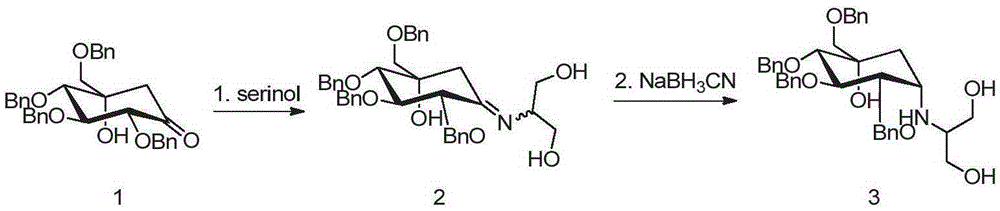
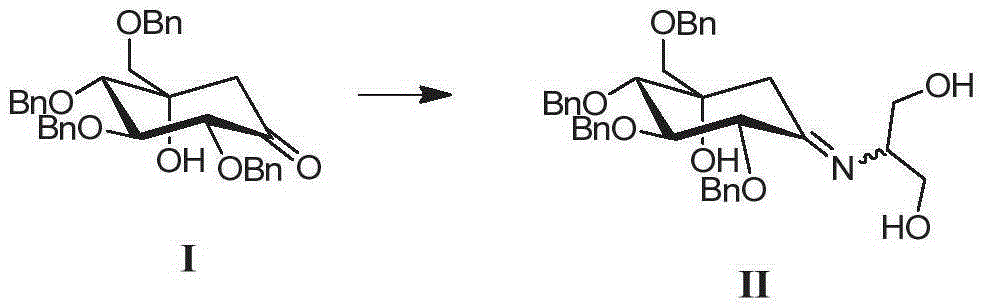
The invention discloses a preparing method for tetra-benzyl-voglibose, namely, (1S)-(1(hydroxyl),2,45 / 1,3)-2,3,4-trioxyl-benzyl-5-[(2-hydroxyl-1-(hydroxymethyl)ethyl)amino]-1-carbon-benzyloxy methyl-1,2,3,4-cyclohexane tetraol or benzene sulfonate thereof. The method comprises the steps that (2R, 3S, 4S, 5S)-5-hydroxyl-2,3,4-tri(benzyloxy)-5-[(benzyloxy)methyl]-cyclohexanone reacts with serinol in protonic solvent under organic acid catalysis to be prepared into an intermediate amine compound, and the intermediate amine compound is reduced to be tetra-benzyl-voglibose. The method has the advantages that reaction reagent is low in price, safety is good, solvent toxicity is low, reaction time is short, aftertreatment is simple, the product yield is large, purity is high, and the method is suitable for industrial production.
Owner:CHONGQING ZEN PHARMACEUTICAL CO LTD
Voglibose tablet and preparation method thereof
InactiveCN112618501ADisintegrates quicklyPromote dissolutionOrganic active ingredientsMetabolism disorderVogliboseHot melt
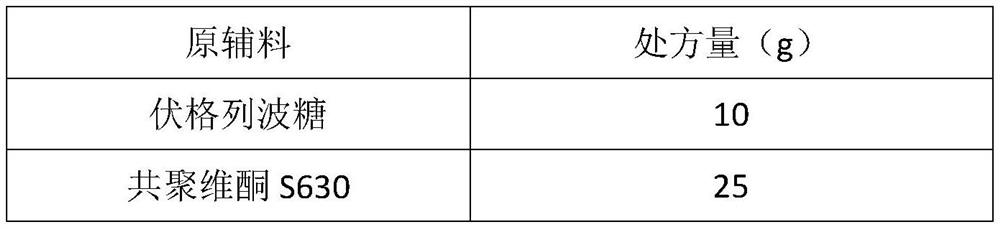
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a voglibose-containing tablet and a preparation method thereof. The tablet is composed of 20-50% of a voglibose solid dispersion, 0.5-5% of a disintegrating agent, 30-60% of a filling agent and 0.5-2% of a lubricating agent, wherein the voglibose solid dispersion is prepared by uniformly mixing voglibose and copovidone, and performing heating melt extrusion through a hot melt extruder. The tablet provided by the invention has the advantages of rapid disintegration, rapid dissolution, high bioavailability, simple preparation process and few steps, and is beneficial to industrial large-scale production.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
A kind of voglibose tablet and preparation method thereof
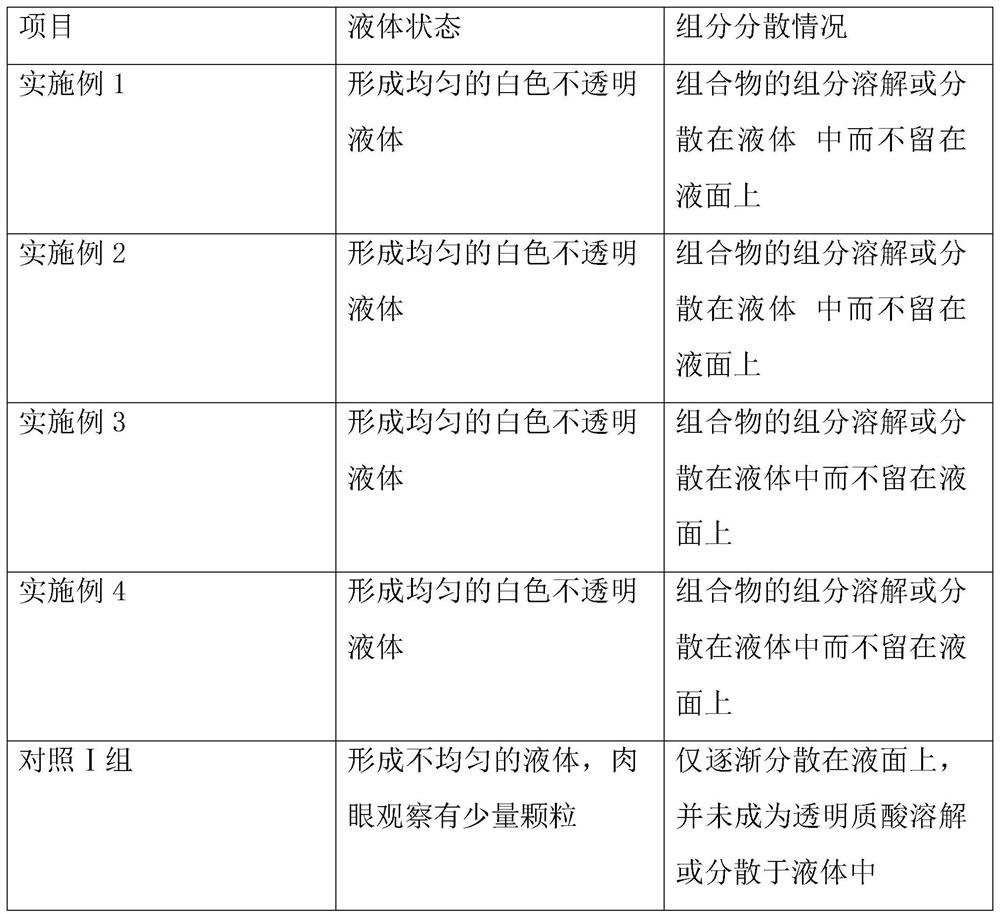
ActiveCN110101671BHigh dissolution rateGood content uniformityOrganic active ingredientsMetabolism disorderFormularyPotassium hydroxide
The invention discloses a voglibose tablet and a preparation method thereof, comprising the following raw materials: voglibose, lactose, microcrystalline cellulose, hydroxypropyl cellulose, crospovidone and magnesium stearate , polysorbate 80, micropowder silica gel, macromolecular polysaccharide, magnesium carbonate, isopropylene glycol, carmellose calcium, sodium alginate, hydrogenated phospholipids, vitamin E ester, sodium hydroxide, potassium hydroxide, povidone K30, Using a new raw material formula, setting a high-quality ratio, and setting process parameters, etc., the obtained voglibose tablets have good dissolution rate and content uniformity, and while ensuring high dissolution rate and uniformity, it will not Causing gastric mucosal damage can better exert the efficacy of voglibose tablets and improve its drug efficacy.
Owner:JIANGSU CHENPAI PHARM GRP CO LTD
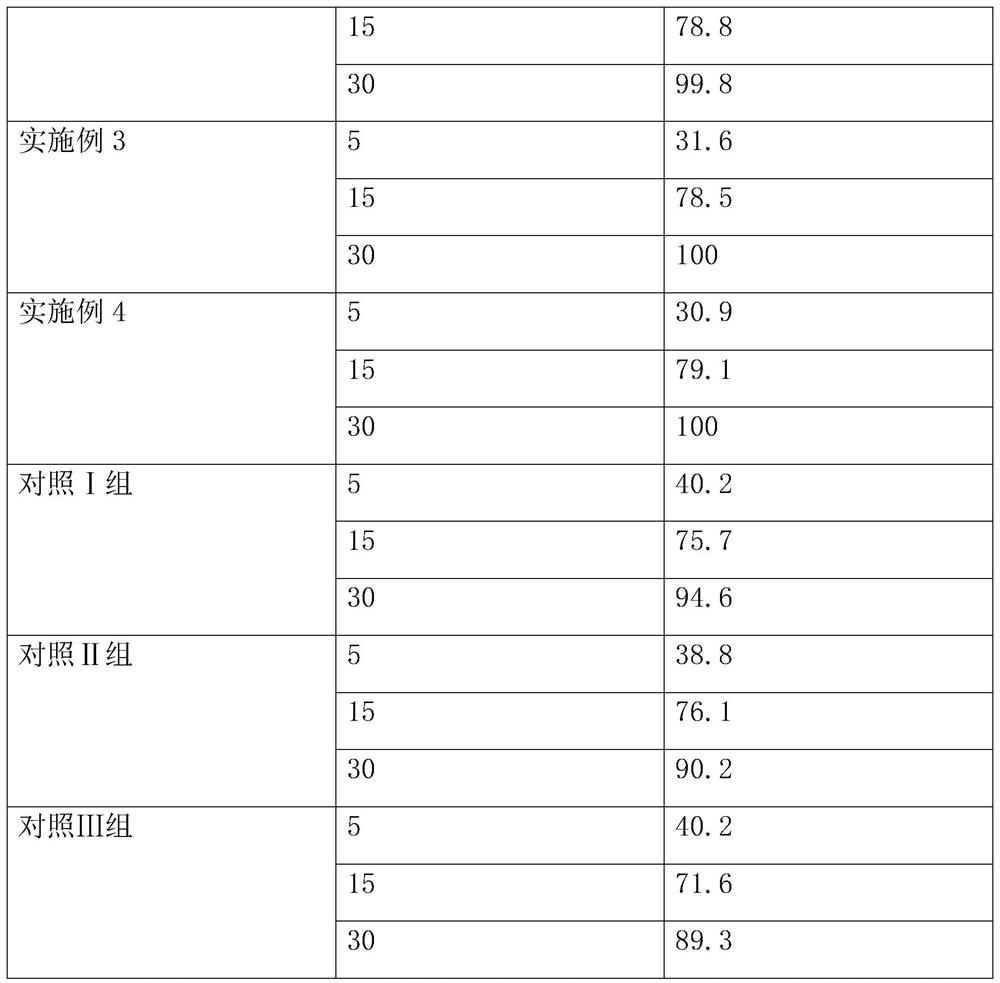
A kind of preparation method of voglibose capsule
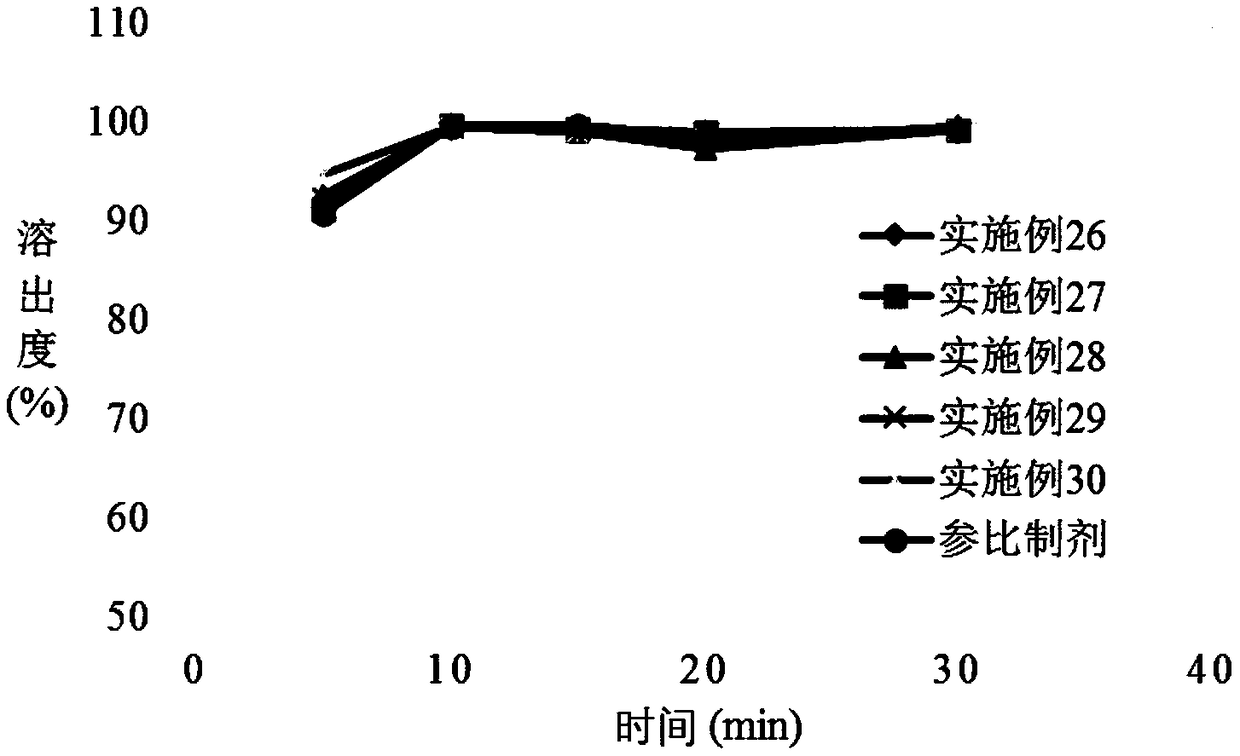
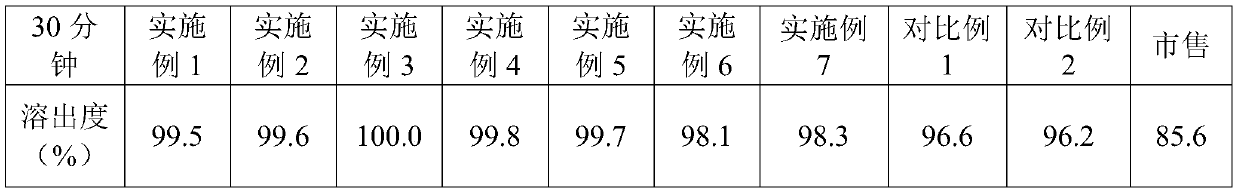
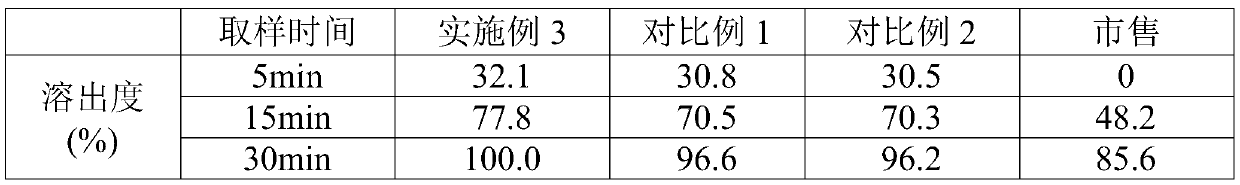
ActiveCN114699383BHigh dissolution rateGood uniformity of voglibose contentOrganic active ingredientsMetabolism disorderFreeze-dryingMedicine
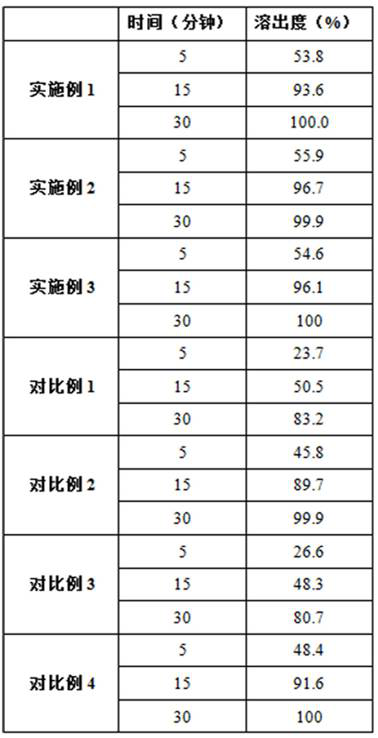
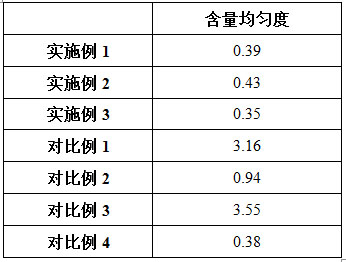
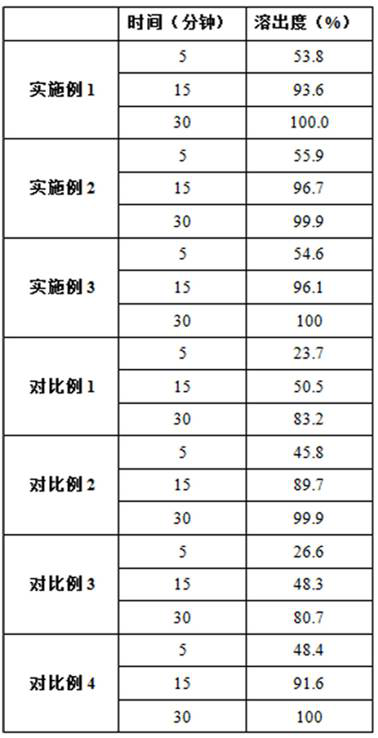
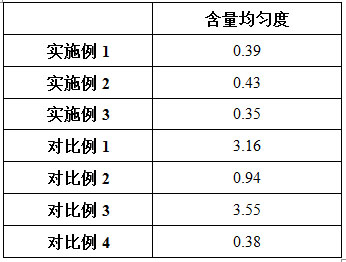
The invention discloses a preparation method of voglibose capsules, belonging to the field of pharmaceutical preparations, comprising five steps of preparation of original medicinal liquid, film drying, micronization, freeze drying and capsule filling. The invention prepares voglibose capsules with high dissolution rate and good uniformity of voglibose content, and the dissolution rates in 5 minutes, 15 minutes and 30 minutes are respectively 53.8-55.9%, 93.6-96.7% and 99.9%. ~100%, the voglibose content uniformity is 0.35~0.43; the present invention inhibits voglibose by adding polyvinylpyrrolidone and micropowder silica gel to the voglibose aqueous solution and cooperating with the rapid evaporation operation of coating film drying. The crystallization phenomenon during the precipitation process of boose yields voglibose in an amorphous powder state, so that the final capsule preparation has excellent dissolution and content uniformity.
Owner:SHANDONG WEIFANG PHARMA FACTORY
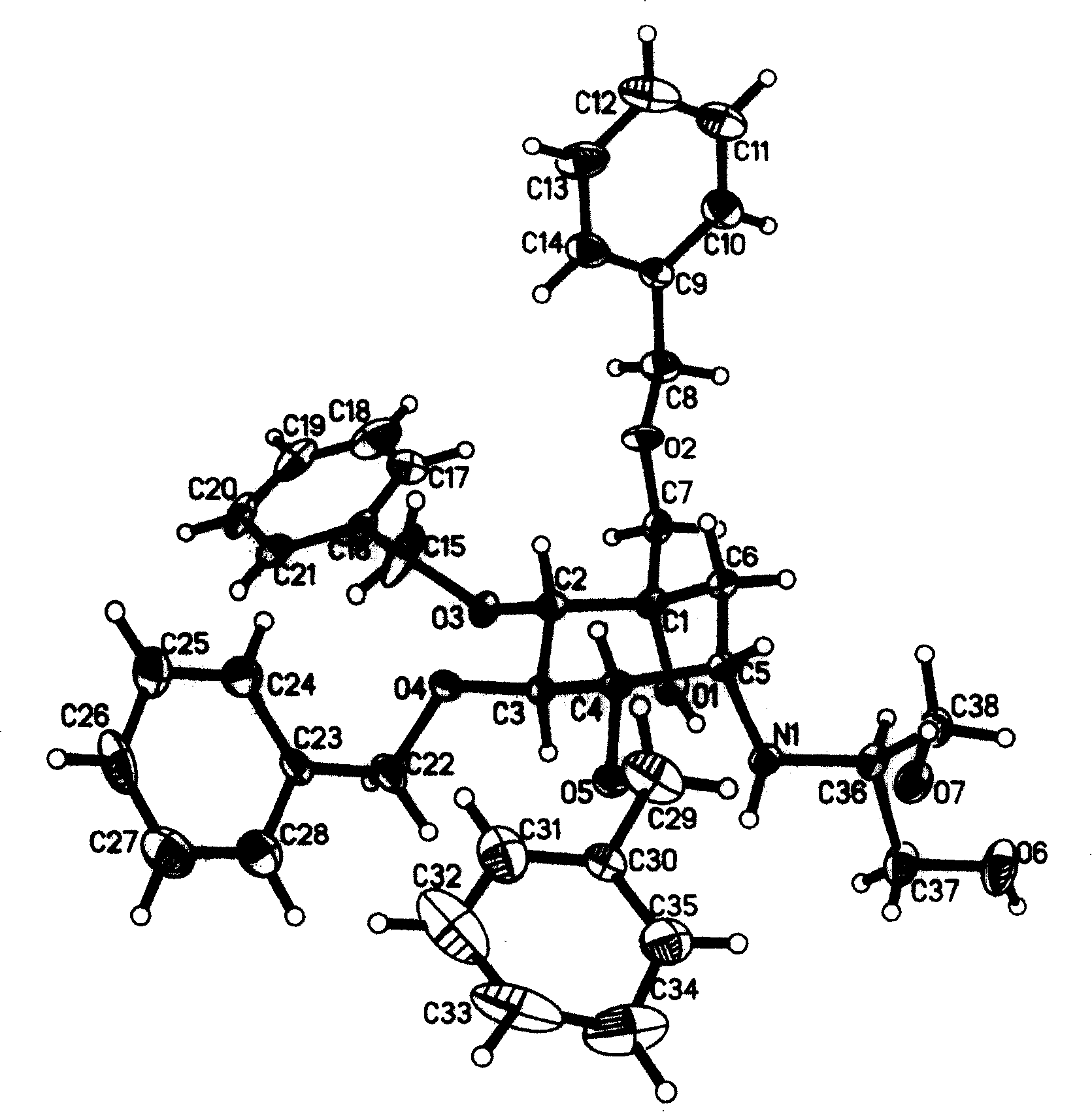
Voglibose semi-hydrated crystal, its preparation method and its uses in medicament formulation
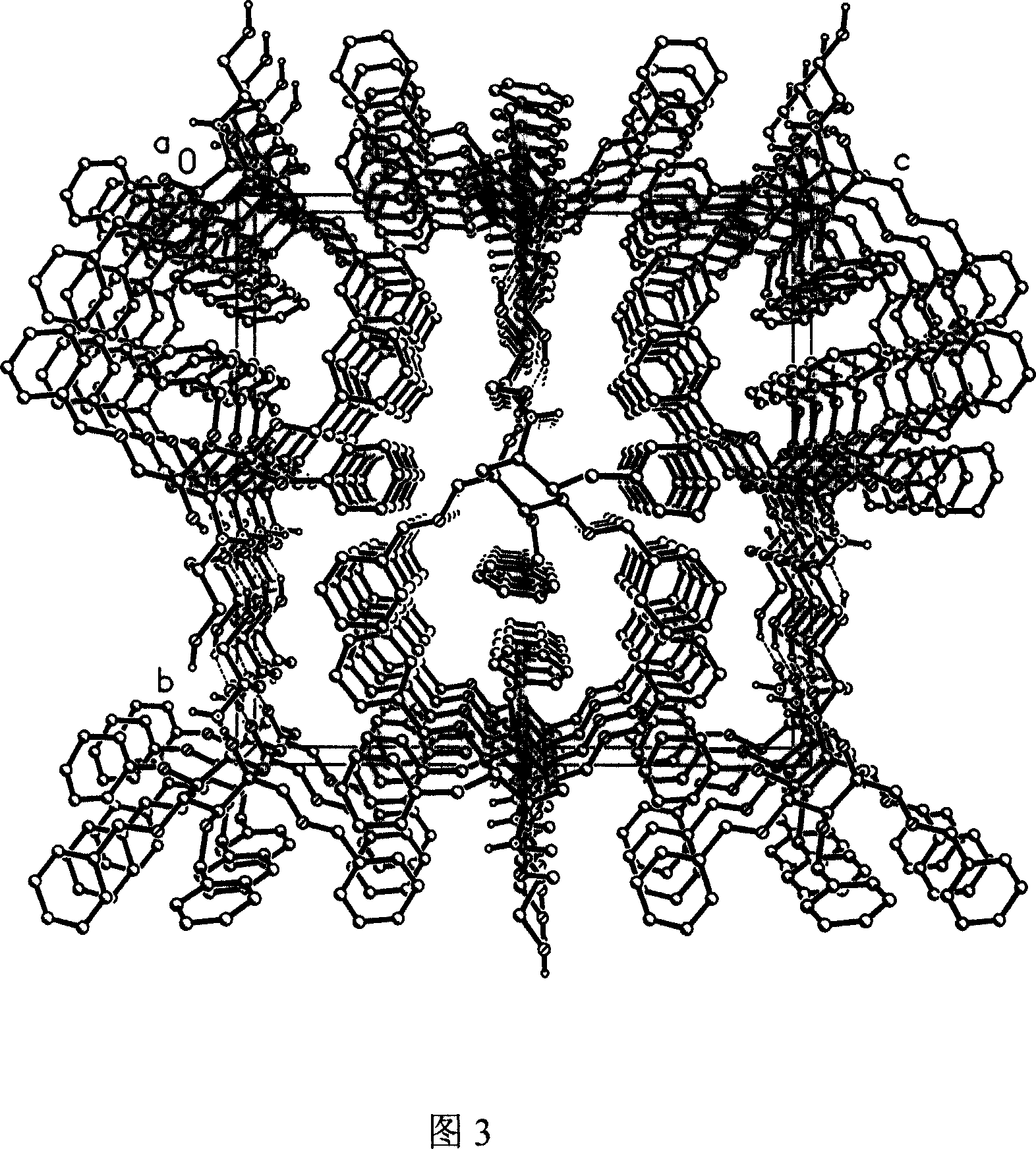
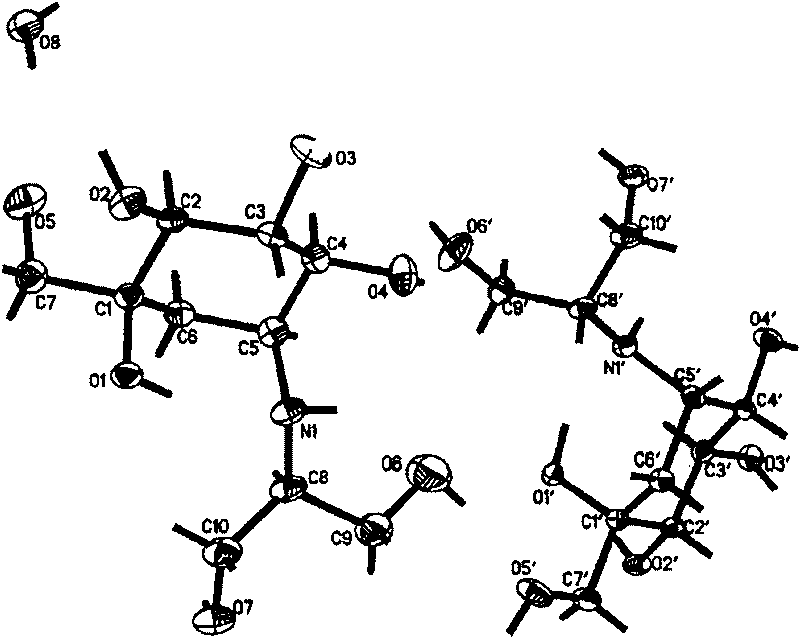
InactiveCN101007771BHigh purityHigh yieldOrganic active ingredientsMetabolism disorderCrystal systemInfrared
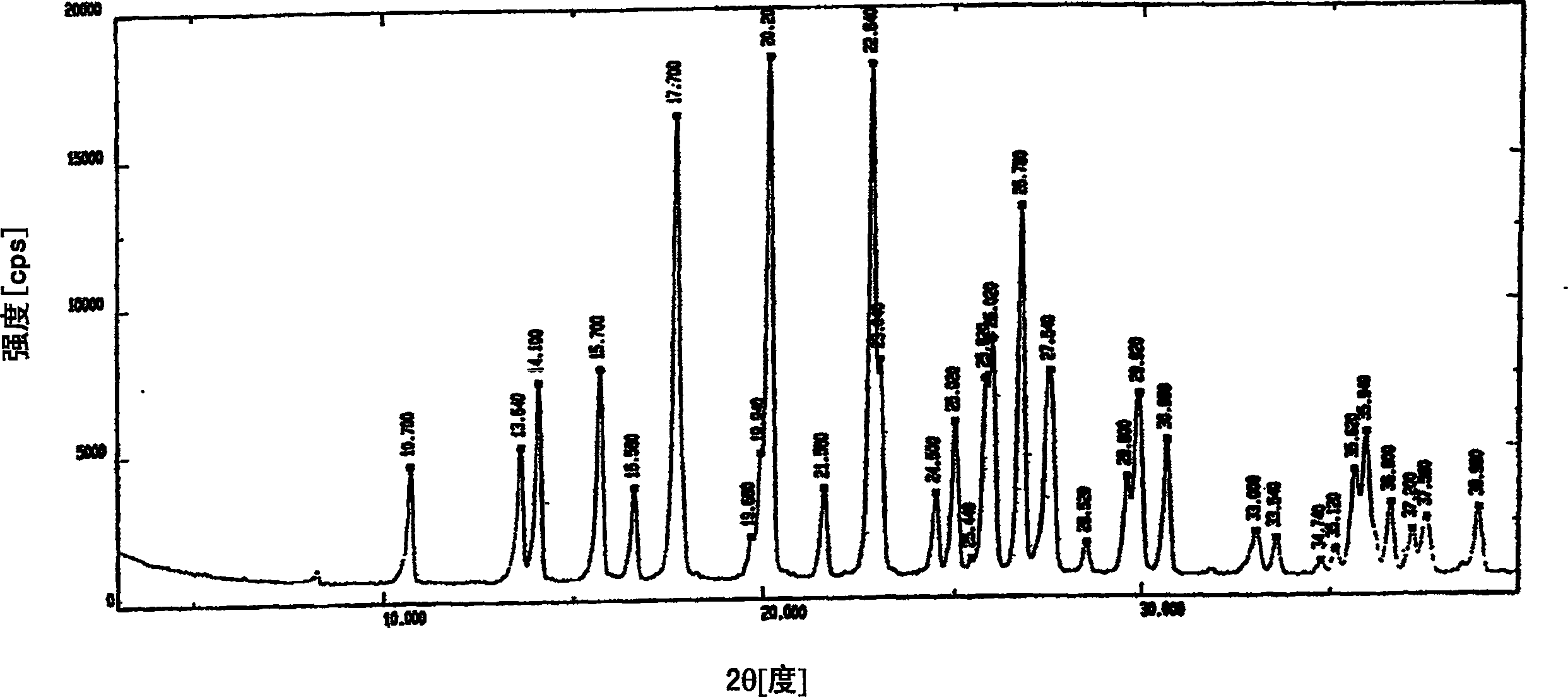
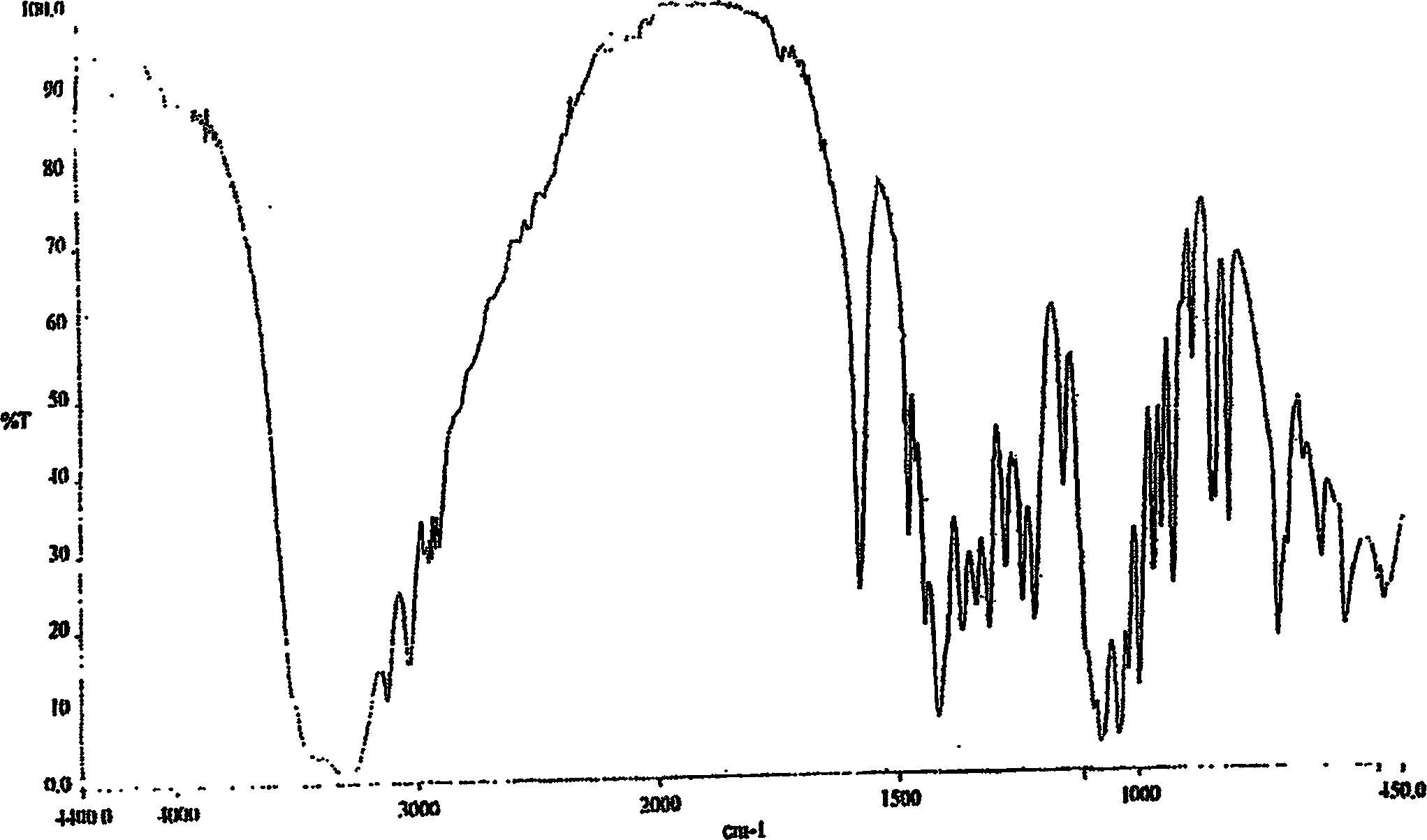
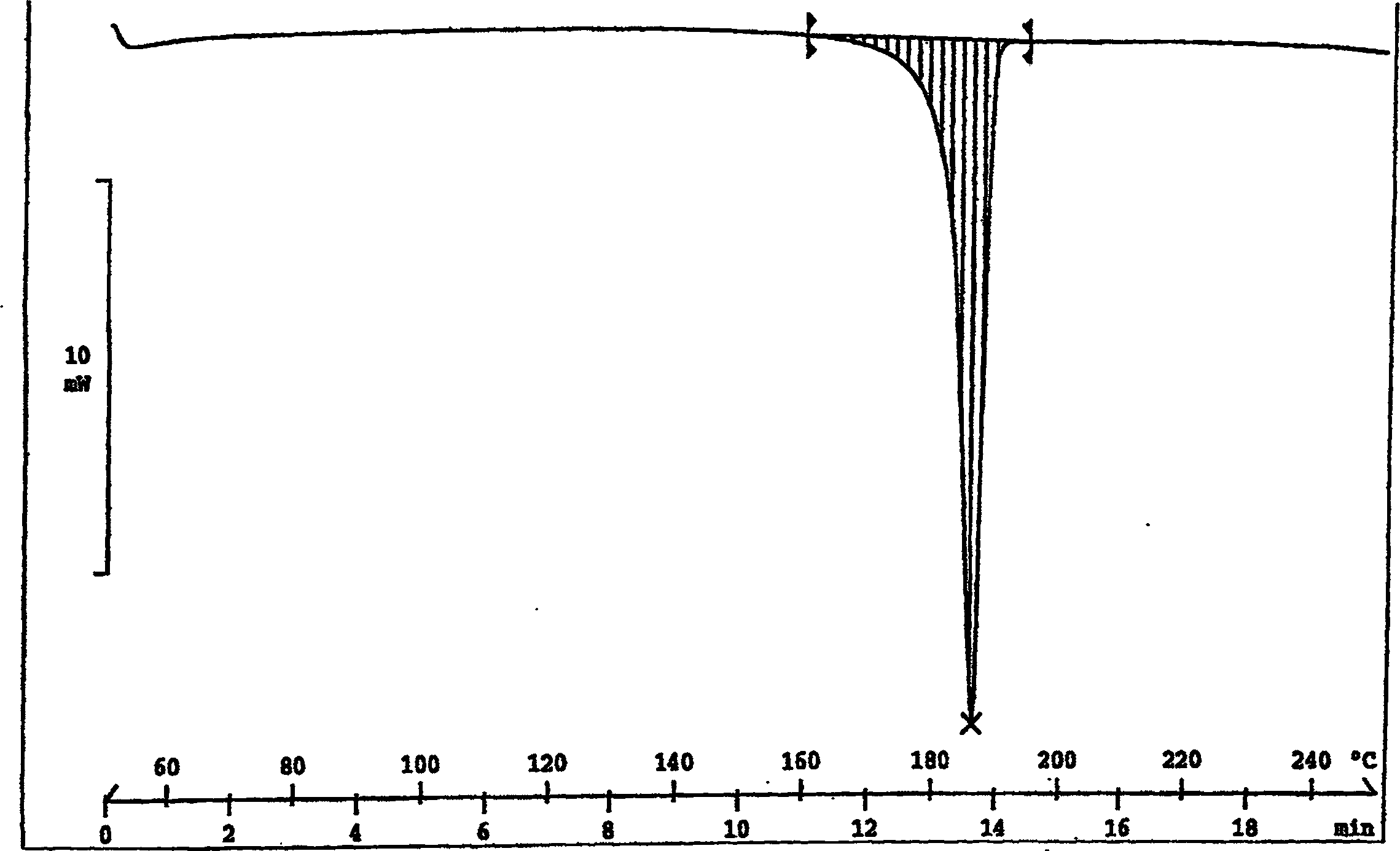
The invention discloses a Fuge train wave hemi-hydrated crystalline with 0.5 molecular crystallinic water, whose structure and character is simulated by single-crystal X-ray diffraction, powder X-raydiffraction, thermogravimetric analysis (TG), differential scanning calorimetry (DSC), infrared spectrum (IR), element analysis and specific rotation, wherein the molecular structure is C10H21NO7 .0.5H2O, which belongs to orthogonal crystal system in the P2(1)2(1)2(1) spatial group; the cell parameter a is 9.877(2); b is 9.905(2); c is 26.760(5); alpha is 90 deg; beta is 90deg; gamma is 90 deg; Ris 0.0324.
Owner:PHARMAXYN LAB
Voglibose film and preparation method thereof
ActiveCN101732286BNice appearanceEasy to carryOrganic active ingredientsMetabolism disorderFlavouring agentPlasticizer
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Preparation method of valienamine from acarbose and/or acarbose derivatives using trifluoroacetic acid
InactiveCN1282638CEasy to separateEasy to getOrganic active ingredientsOrganic compound preparationAlgluceraseOrganic acid
The present invention relates to a method for the preparation of vistilide from acarbose and / or acarbose derivatives using the organic acid TFA (trifluoroacetic acid). Using the method of the present invention, the selective hydrolysis of acarbose and / or acarbose derivatives using TFA produces a large amount of voglibose core which is a strong inhibitor of α-glucosidase and can be used for the treatment of diabetes Precursor, that is, villetamide.
Owner:B T GIN · CO LTD
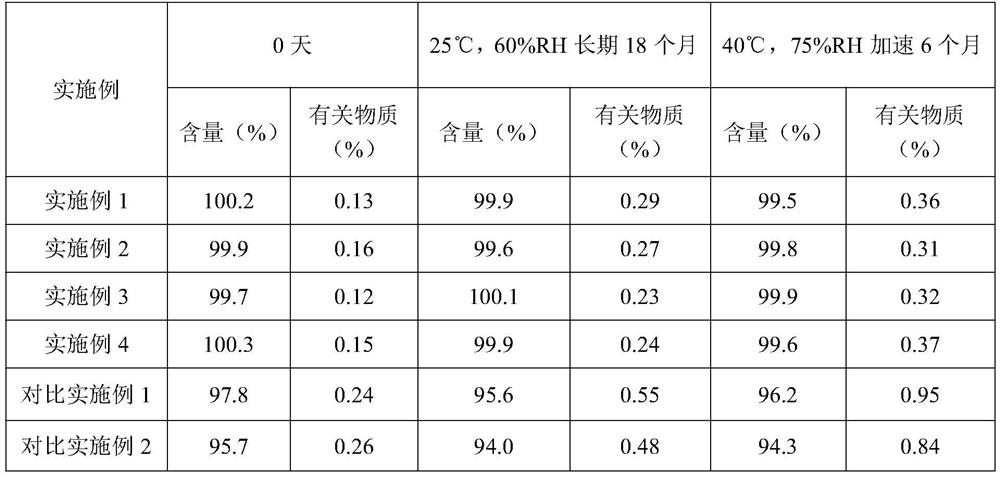
A method for determining related substances in voglibose raw materials and preparations
ActiveCN111855841BGuaranteed validityEnsure safetyComponent separationOther chemical processesFluid phaseBiochemical engineering
Owner:CISEN PHARMA
Voglibose tablet with high dissolution rate and preparation method thereof
PendingCN114796136AAdvantages and Significant AdvancementsGood dissolution effectOrganic active ingredientsMetabolism disorderVogliboseEngineering
The invention discloses a voglibose tablet capable of being quickly dissolved out and a preparation method of the voglibose tablet, and belongs to the technical field of pharmaceutical preparations. According to the voglibose tablet, firstly, a solid dispersion of voglibose and a carrier material is prepared by adopting a solvent method and a spray drying technology, and then the solid dispersion is uniformly mixed with a filling agent, a disintegrating agent and a lubricating agent and is directly tableted. According to the voglibose tablet prepared by the method, the dissolution rate and the content uniformity are improved, meanwhile, the quality is stable and controllable, and the voglibose tablet is suitable for industrial large-scale production.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Combined pharmaceutical preparation for treatment of type 2 diabetes
InactiveUS7888382B2Improving a course of postprandial blood glucoseDecreasing a morning fastingBiocideOrganic chemistryPharmacometricsVoglibose
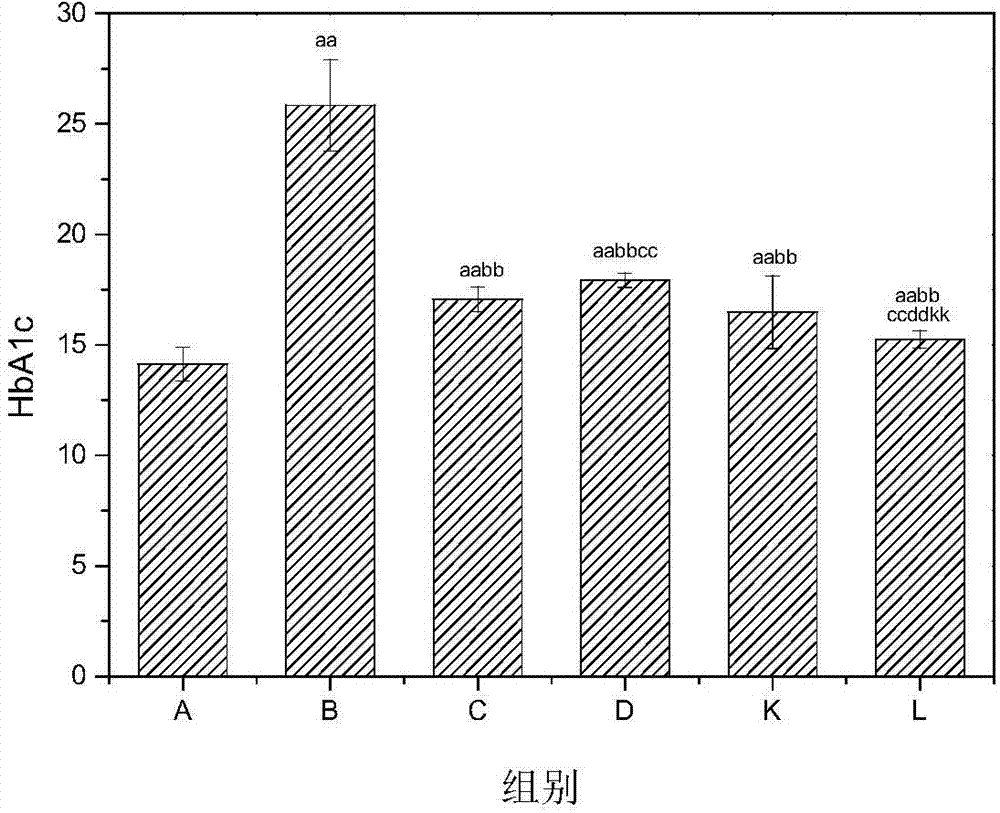
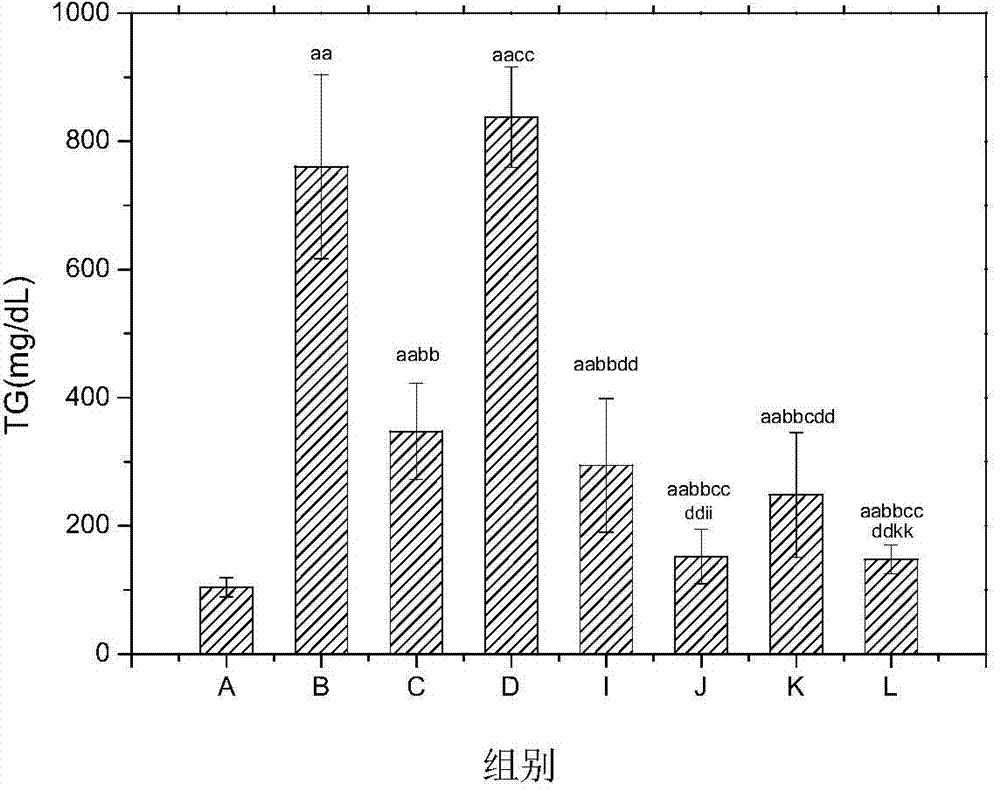
For controlling the condition of type 2 diabetes, a pharmaceutical including a combination of mitiglinide, a pharmacologically acceptable salt thereof or a hydrate thereof and an α-glucosidase inhibitor such as voglibose or acarbose, and a therapeutic method using the pharmaceutical are provided. The pharmaceutical according to the present invention has an extremely strong effect of decreasing a morning fasting blood glucose level, a postprandial blood glucose level and HbA1C of a patient with type 2 diabetes, and can improve glucose spike, insulin resistance and lipid metabolism.
Owner:KISSEI PHARMA
Medicine composition for treating or preventing adiposis and metabolic syndrome and application thereof
InactiveCN107308154AAdvantageous therapeuticAdvantage preventionOrganic active ingredientsMetabolism disorderAntiobesity drugIslet cells
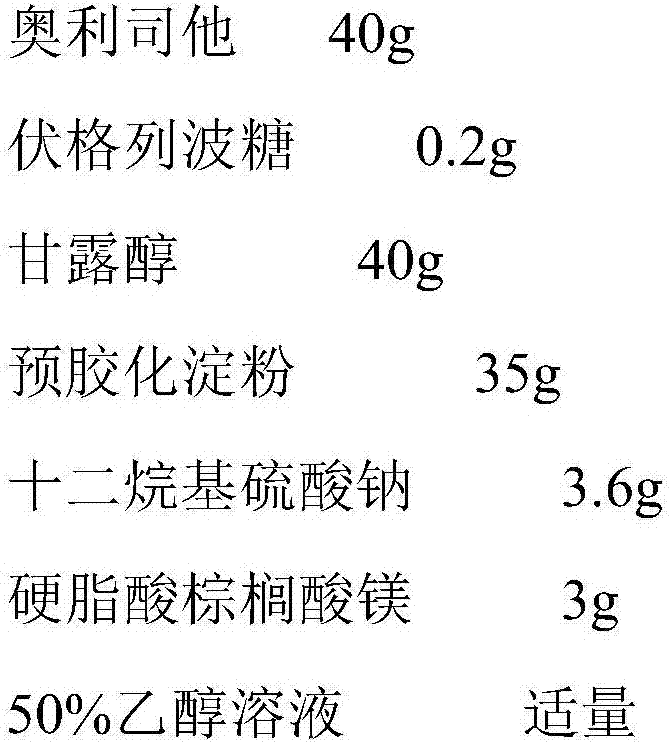
The invention belongs to the field of medicines, and particularly relates to a medicine composition for treating or preventing adiposis and metabolic syndrome and application thereof. Active ingredients of the medicine composition are orlistat and voglibose, wherein the mass ratio of the orlistat to the voglibose is (10 to 30) to 0.1. According to the medicine composition, the metabolism and endocrine system drug voglibose is creatively combined with the conventional antiobesity drug orlistat for use, and the purposes of remarkably reducing postprandial blood sugar and enabling the postprandial blood sugar to be stable can be realized by greatly reducing the dosage of the single drugs, so that the pressure of islet cells is lowered, and the probability of diabetic complication of an adiposis patient is retarded or prevented. When the composition is used for treating or preventing the adiposis and the metabolic syndrome, not only obvious advantage is shown on the treating and preventing of the adiposis, but also effective control is performed on the indexes of blood fat, blood glucose and the like, and the positive treating and preventing effects are realized on the metabolic syndrome.
Owner:CHONGQING ZEN PHARMACEUTICAL CO LTD
Biological preparation method of Jinggang mycylamine
ActiveCN106566850BSolve pollutionReduce cumbersome operationsTransferasesMicroorganism based processesChemical synthesisEnamine
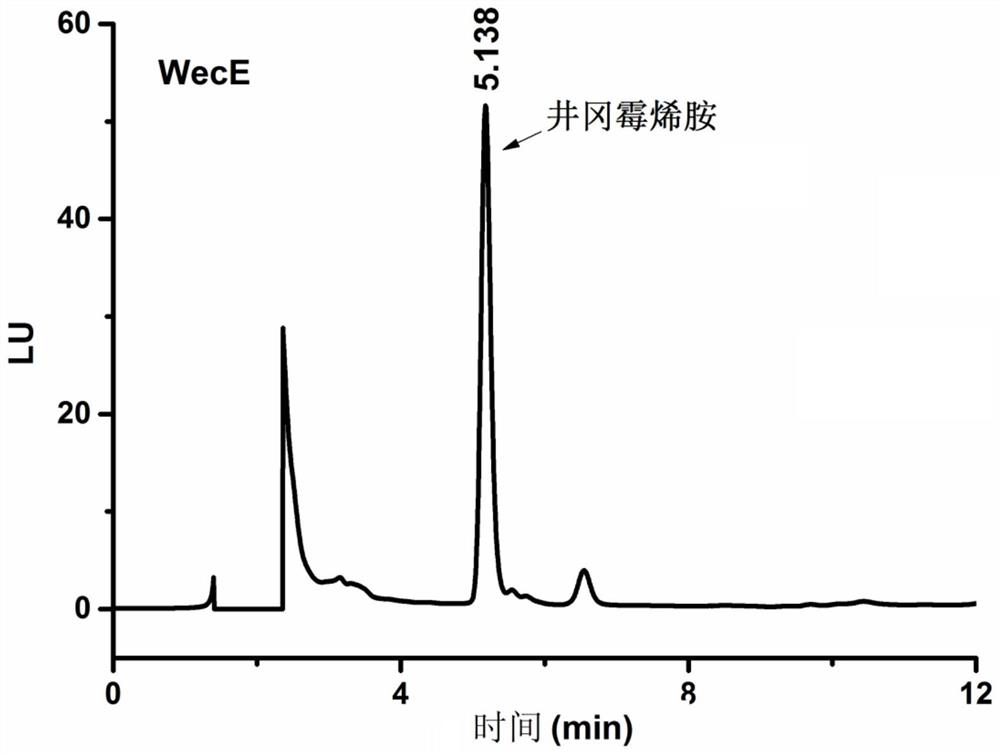
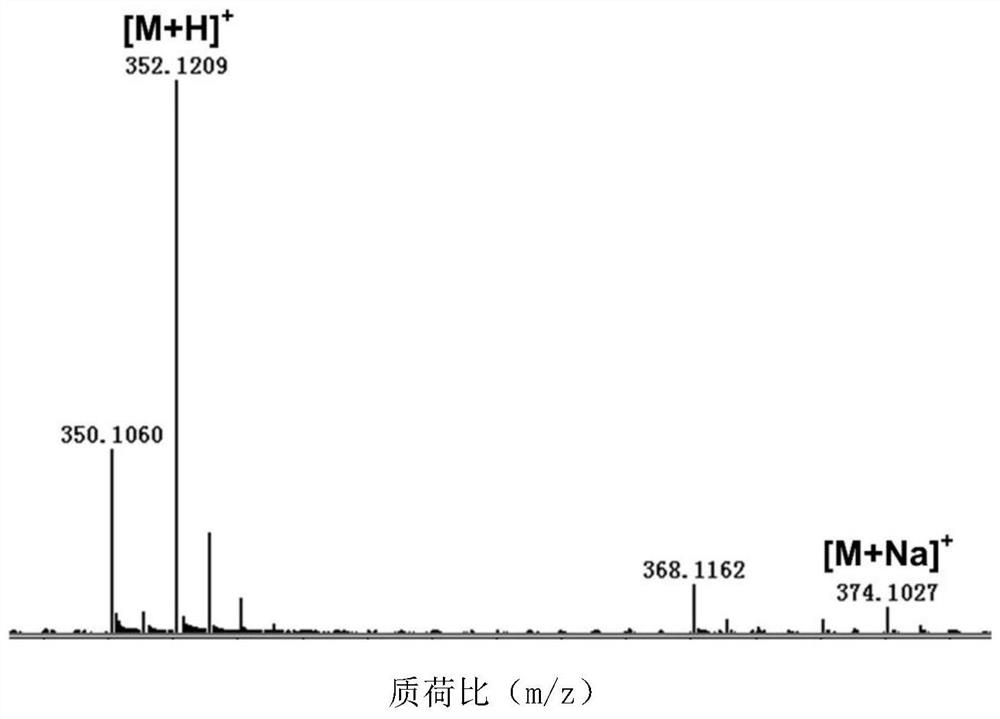
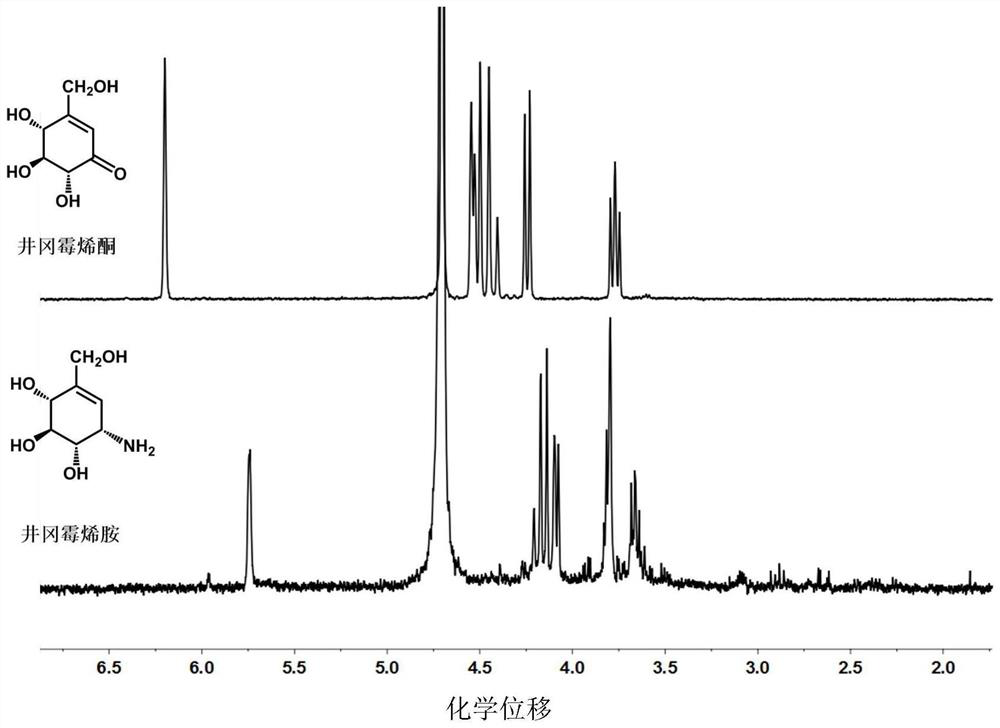
The invention discloses a biological preparation method of valienamine. The preparation method comprises a step of biologically converting valienone into valienamine by using amino transferase (WecE), or integrating WecE gene into bacterial strains that can generate the precursor compounds of valienone so that the bacterial strains integrated with WecE gene can directly produce valienamine through fermentation. The conventional chemical synthesis method has the disadvantages such as many reaction steps, low yield, strict reaction conditions, organic reagent pollution, and the like, and the provided biological preparation method overcomes the abovementioned problems. The biological preparation method is based on amino transferase catalyzed amino transferring reactions, the direct biological synthesis is convenient and high efficient, the stereo-selectivity is high, and the valienamine can be used as a synthetic intermediate for synthesis of voglibose for treating II type diabetes, synthesis of acarbose, and development of glycosidase inhibitors.
Owner:SHANGHAI JIAOTONG UNIV
A kind of preparation method of tetrabenzyl voglibose
ActiveCN105254514BLow costImprove production safetyOrganic compound preparationSulfonic acids salts preparationCyclohexanoneOrganic acid
The invention discloses a preparing method for tetra-benzyl-voglibose, namely, (1S)-(1(hydroxyl),2,45 / 1,3)-2,3,4-trioxyl-benzyl-5-[(2-hydroxyl-1-(hydroxymethyl)ethyl)amino]-1-carbon-benzyloxy methyl-1,2,3,4-cyclohexane tetraol or benzene sulfonate thereof. The method comprises the steps that (2R, 3S, 4S, 5S)-5-hydroxyl-2,3,4-tri(benzyloxy)-5-[(benzyloxy)methyl]-cyclohexanone reacts with serinol in protonic solvent under organic acid catalysis to be prepared into an intermediate amine compound, and the intermediate amine compound is reduced to be tetra-benzyl-voglibose. The method has the advantages that reaction reagent is low in price, safety is good, solvent toxicity is low, reaction time is short, aftertreatment is simple, the product yield is large, purity is high, and the method is suitable for industrial production.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Preparation method of voglibose capsule
ActiveCN114699383AHigh dissolution rateGood uniformity of voglibose contentOrganic active ingredientsMetabolism disorderFreeze-dryingPyrrolidinones
A preparation method of voglibose capsules belongs to the field of pharmaceutical preparations and comprises five steps of preparation of original liquid medicine, coating drying, micronization, freeze drying and capsule filling. The voglibose capsule with high dissolution rate and good voglibose content uniformity is prepared, the dissolution rates in 5 minutes, 15 minutes and 30 minutes are respectively 53.8 to 55.9 percent, 93.6 to 96.7 percent and 99.9 to 100 percent, and the voglibose content uniformity is 0.35 to 0.43; according to the voglibose capsule preparation and the preparation method thereof, the polyvinylpyrrolidone and the superfine silica powder are added into the voglibose aqueous solution, and the rapid evaporation operation of coating drying is matched, so that the crystallization phenomenon in the voglibose precipitation process is inhibited, the voglibose in an amorphous powder state is obtained, and the final capsule preparation has excellent dissolution rate and content uniformity.
Owner:SHANDONG WEIFANG PHARMA FACTORY
A kind of preparation method of voglibose impurity I hydrochloride
ActiveCN110511152BRaise quality standardsImprove medication safetyCarbamic acid derivatives preparationOrganic compound preparationCyclohexanoneMethyl palmoxirate
The present invention provides a kind of preparation method of voglibose impurity I hydrochloride, comprising the steps of: (S)-(oxirane methyl) carbamate tert-butyl ester and tetrabenzyl Jinggang mycosamine Amination of epoxide to obtain the compound of formula III; the hydroxyl group of the compound of formula III is protected by benzyl group to obtain the compound of formula IV; the amino protecting group of the compound of formula IV is deprotected to obtain the compound of formula V; 5S)-5-hydroxyl-2,3,4-tris(benzyloxy)-5-[(benzyloxy)methyl]-cyclohexanone undergoes a condensation reduction reaction to obtain a compound of formula VI. The preparation method of voglibose impurity I hydrochloride provided by the present invention has a simple process flow and fills up the gap in the current technology for preparing voglibose impurity I. The prepared voglibose impurity I hydrochloride The salt can be used for qualitative and quantitative research on impurities in the production of voglibose, which can improve the quality standard of voglibose, thereby improving the drug safety of voglibose.
Owner:无锡富泽药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com