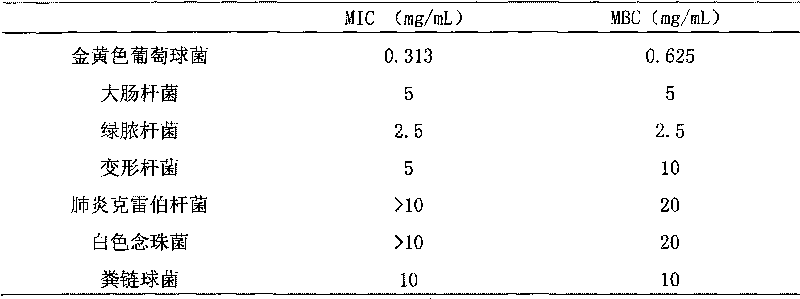
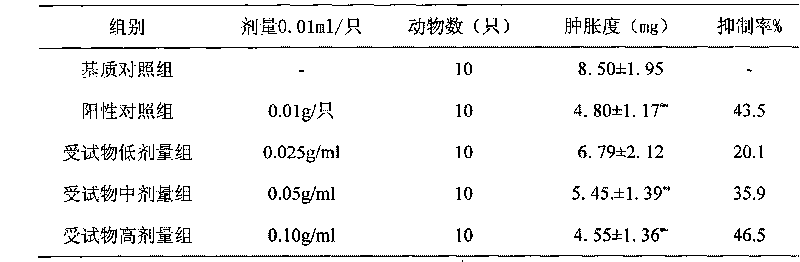
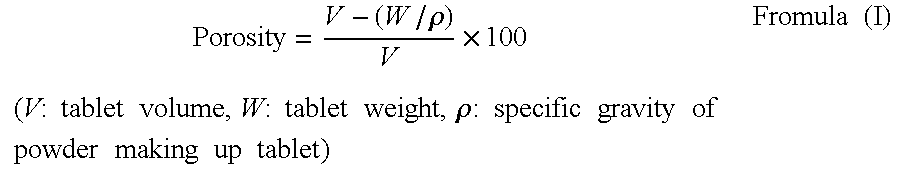Patents
Literature
1410results about How to "Disintegrates quickly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Orally disintegrating tablets and methods of manufacture
ActiveUS20050232988A1Effective taste-maskingOptimal size distributionPowder deliveryGranular deliveryOrally disintegrating tabletAlcohol sugars
A tablet that rapidly disintegrates in the oral cavity comprising a compressed blend of rapidly dispersing microgranules prepared by granulating a sugar alcohol or a saccharide or a mixture thereof having an average particle size less than about 30 microns and a disintegrant, and a taste-masked microcapsule containing at least one drug, the microcapsule being prepared by granulating a pharmaceutically acceptable formulation comprising at least one drug in a therapeutically effective amount and at least one polymeric binder that improves resilience of the microgranules, wet milling the granulated mass, and microencapsulating the milled granules to provide microcapsules.
Owner:ADARE PHARM INC
Oral cavity disintegrating tablet and method of producing the same
ActiveUS20100278930A1Easy to produceDisintegrates quicklyBiocideOrganic active ingredientsSucroseOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing (a) one or more saccharides or sugar alcohols selected from the group consisting of mannitol, lactose, xylitol, sucrose, erythritol and glucose and (b) low substituted hydroxypropylcellulose and substantially free of a starch disintegrant, which tablet is produced by steps of granulating a composition containing the above-mentioned components (a) and (b) by an agitation granulation method, and compression-molding the obtained granulation product. The invention also provides a method of producing an orally disintegrating tablet substantially free of a starch disintegrant, including steps of granulating a composition containing the above-mentioned components by an agitation granulation method, and compression-molding the obtained granulation product.
Owner:SAWAI PHARMA
Controlled release formulations of enzymes, microorganisms, and antibodies with mucoadhesive polymers
InactiveUS20080020036A1Easy to exportDisintegrates quicklyPowder deliveryPeptide/protein ingredientsMicroorganismWater soluble
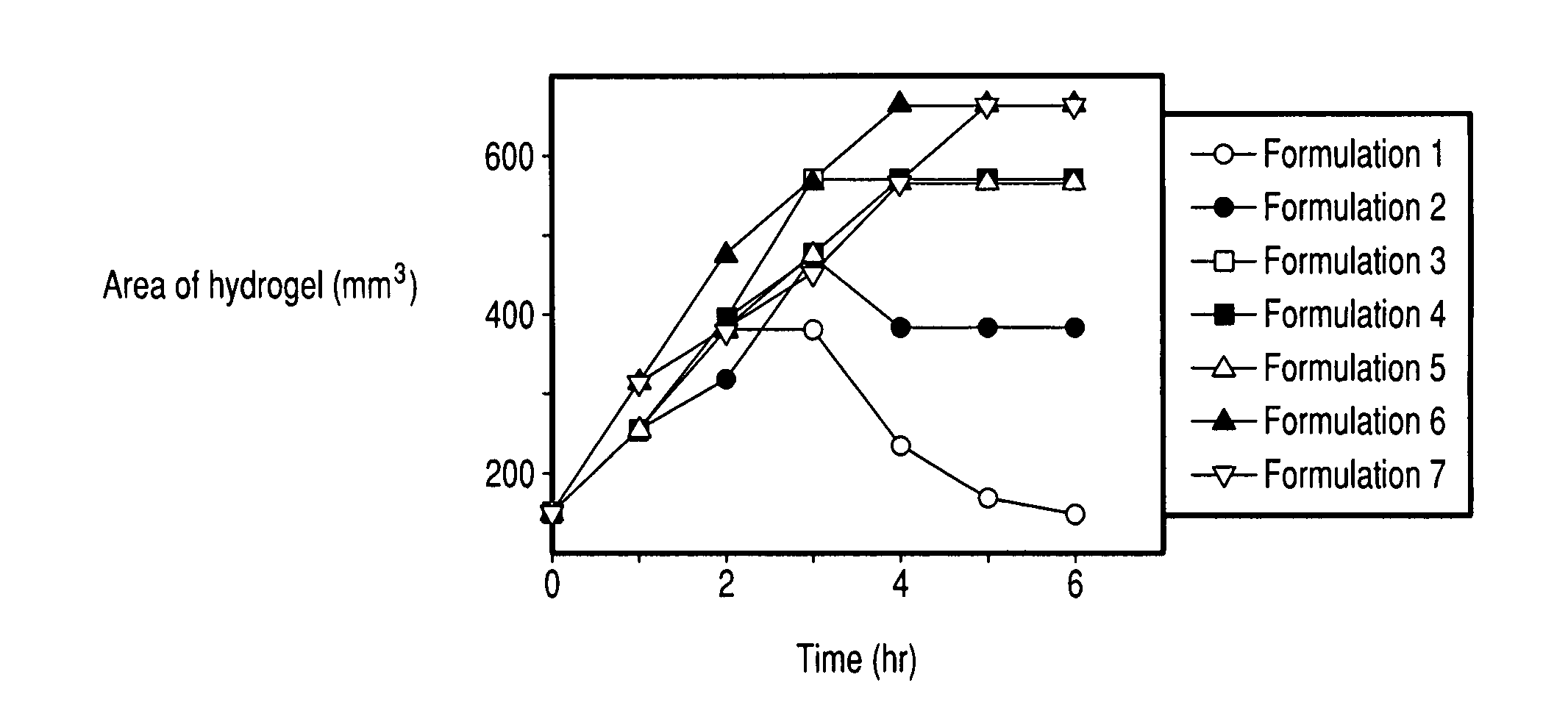
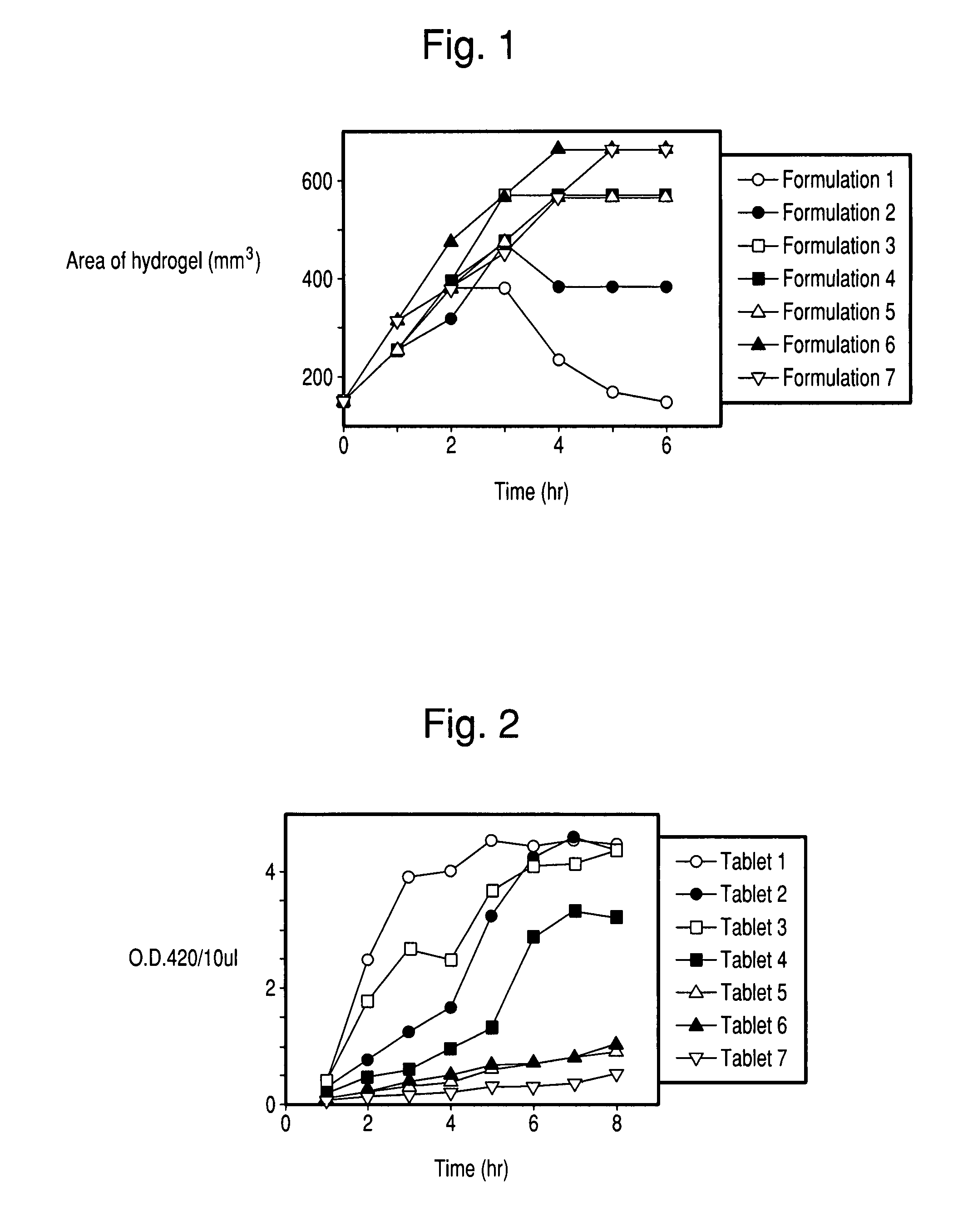
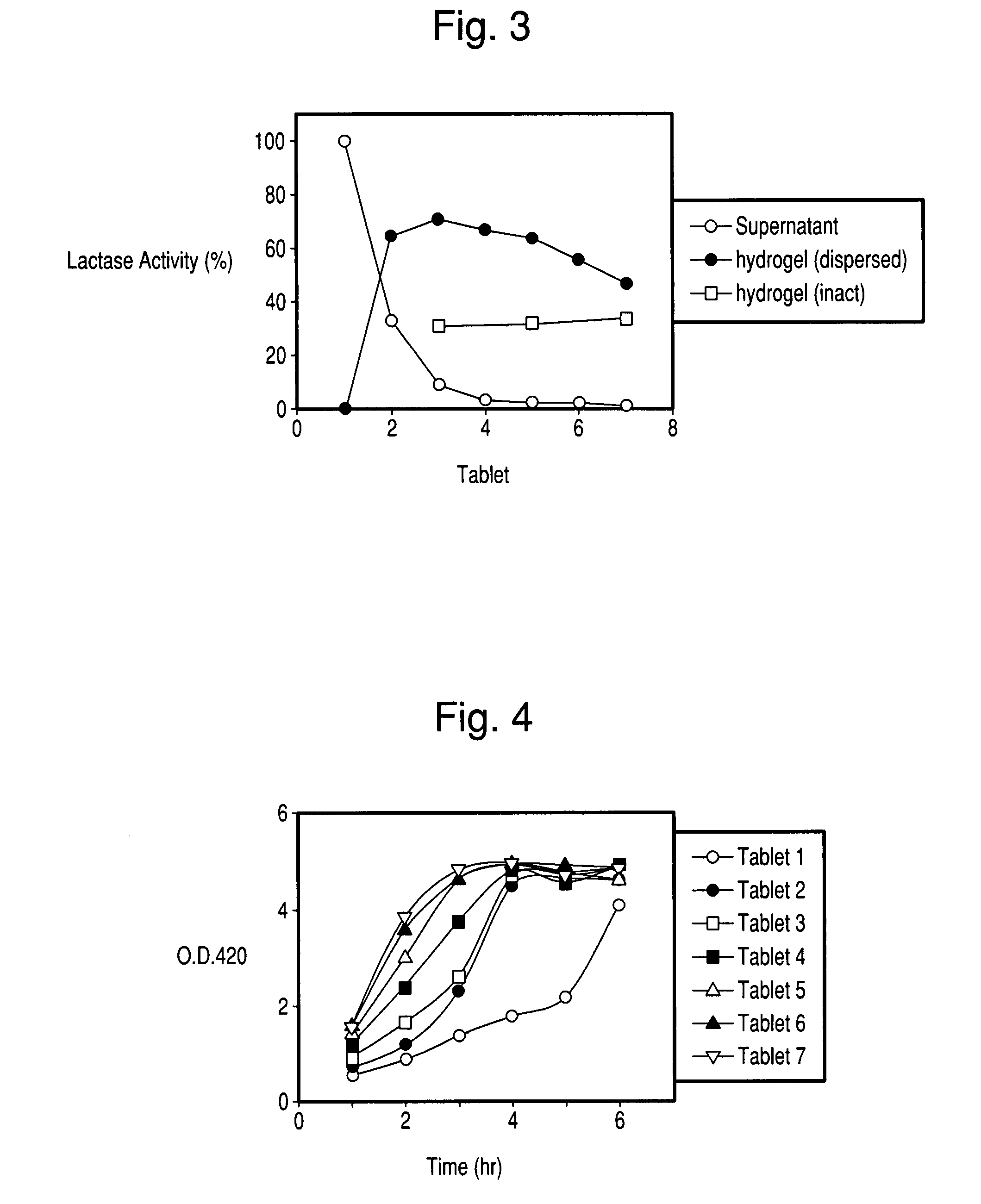
There is provided a composition comprising at least one mucoadhesive polymer that is capable of forming a hydrogel and at one least water soluble polymer, and one or more enzymes, microorganisms, or antibodies. The formulation forms a hydrogel in aqueous solution that has mucoadhesive properties and that is capable of releasing the enzymes, microorganisms, or antibodies over an extended period of time and / or of entrapping enzymes, microorganisms, or antibodies within the hydrogel that is active for an extended time.
Owner:AMANO ENZYME USA CO LTD +1
Quick-disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS6872405B2Improves friabilityHigh strengthPharmaceutical non-active ingredientsDrageesPharmaceutical SubstancesOrganic chemistry
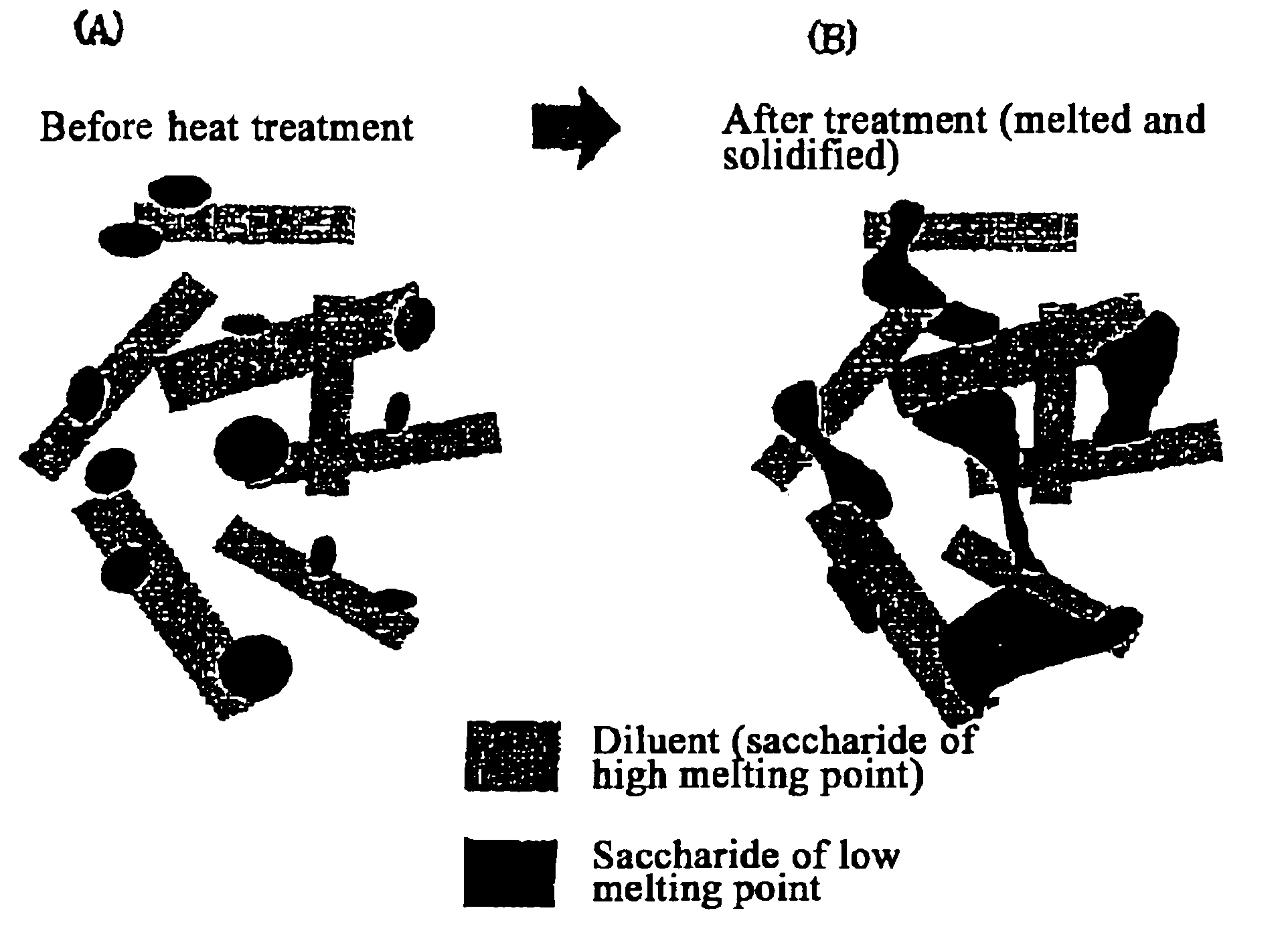
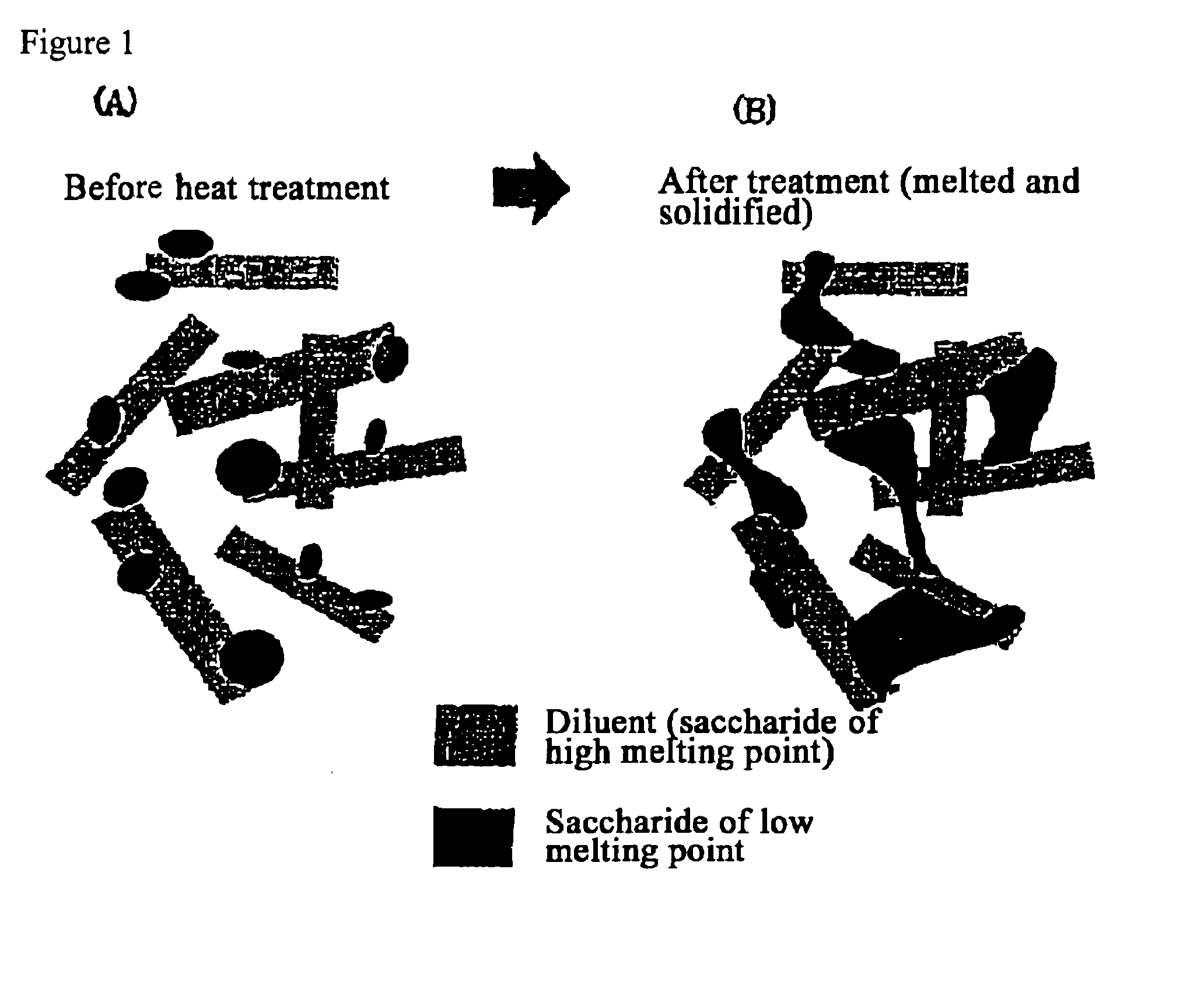
The present invention relates to a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent, which is obtained by uniformly mixing the saccharide with a low melting point in the tablet so that a bridge will be formed between said drug and / or said diluent particles by the product of melting and then solidification of this saccharide with a low melting point. Moreover, the present invention relates to a method of manufacturing a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent and a saccharide with a relatively lower melting point than the drug and the diluent, which comprises (a) the process whereby tablet starting materials including a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent are molded under the low pressure necessary for retaining the shape of a tablet, (b) the process whereby the molded product obtained in process (a) is heated to at least the temperature at which this saccharide with a low melting point will melt, and (c) the process whereby the molded product obtained in process (b) is cooled to at least the temperature at which the molten saccharide with a low melting point solidifies. The present invention presents a quick-disintegrating tablet in the buccal cavity that can be used for practical purposes in that it has almost the same properties as conventional oral pharmaceutical tablets, that is, it has sufficient tablet strength that it can be used with automatic unit dosing machines, and it is produced by conventional tableting machines, and a manufacturing method thereof. Moreover, the present invention presents a quick-disintegrating tablet in the buccal cavity which, in comparison to conventional quick-disintegrating tablets in the buccal cavity, has increased tablet strength and an improved friability without prolonging the disintegration time in the buccal cavity, and a manufacturing method thereof.
Owner:ASTELLAS PHARMA INC
Tablets quickly disintegrating in mouth
InactiveUS20030161879A1Quickly disintegrating in mouthEasy to carryOrganic active ingredientsPill deliveryAdditive ingredientPhospholipid
Tablets quickly disintegrating in the mouth which comprise a bitter drug ingredient and a bitterness-reducing ingredient composed of an essential oil, a high sweetness-sweetener and / or an acidic phospholipid or its lyso-derivative. When taken even without water, these tablets exhibit little bitterness. Thus, a bitter drug ingredient can be formulated without coating into tablets quickly disintegrating in the mouth.
Owner:TAKEDA PHARMA CO LTD
Compressed composition comprising magnesium salt
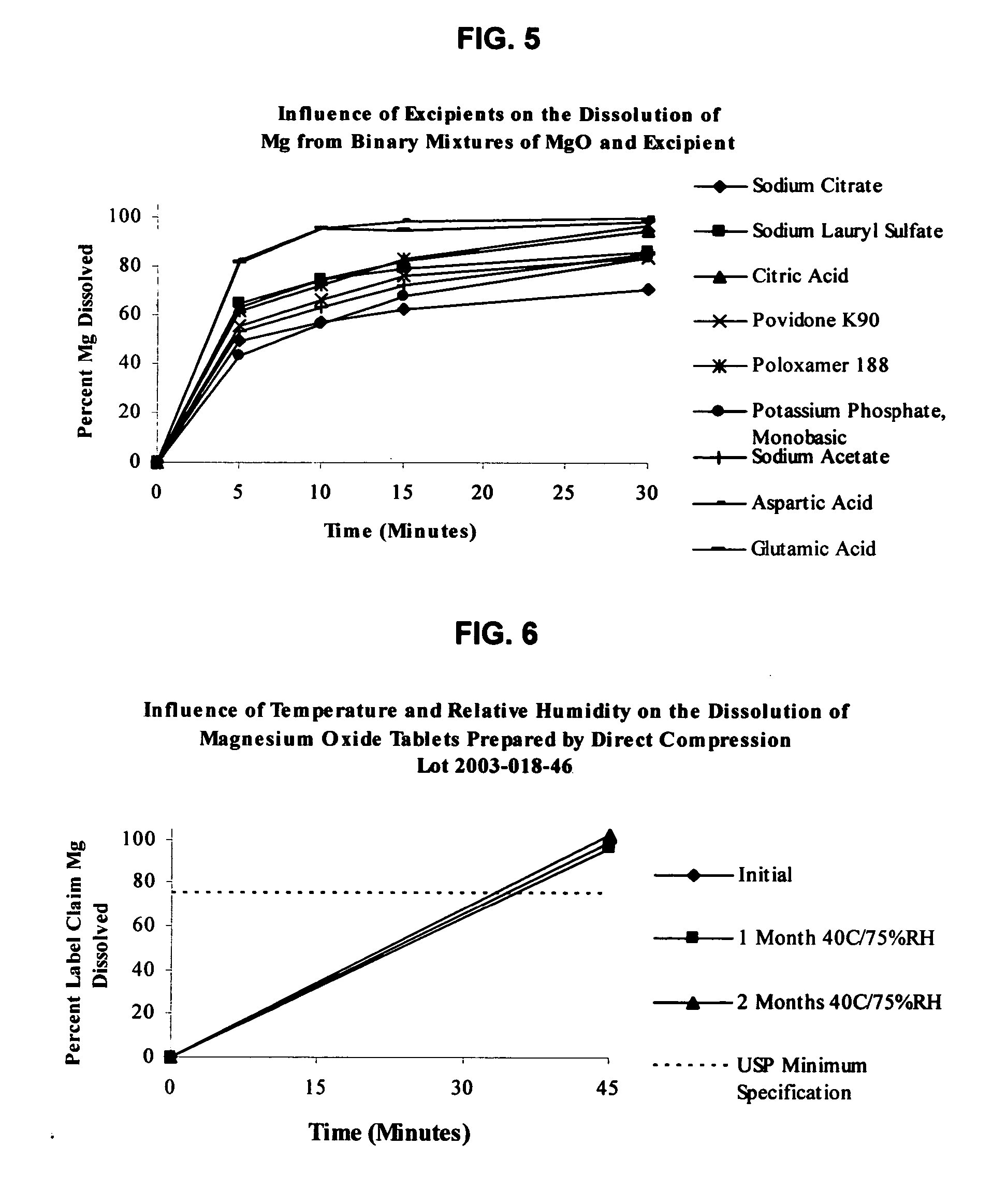
InactiveUS20050220865A1Stable dissolution profileDisintegrates quicklyBiocidePill deliveryInorganic saltsCellulose
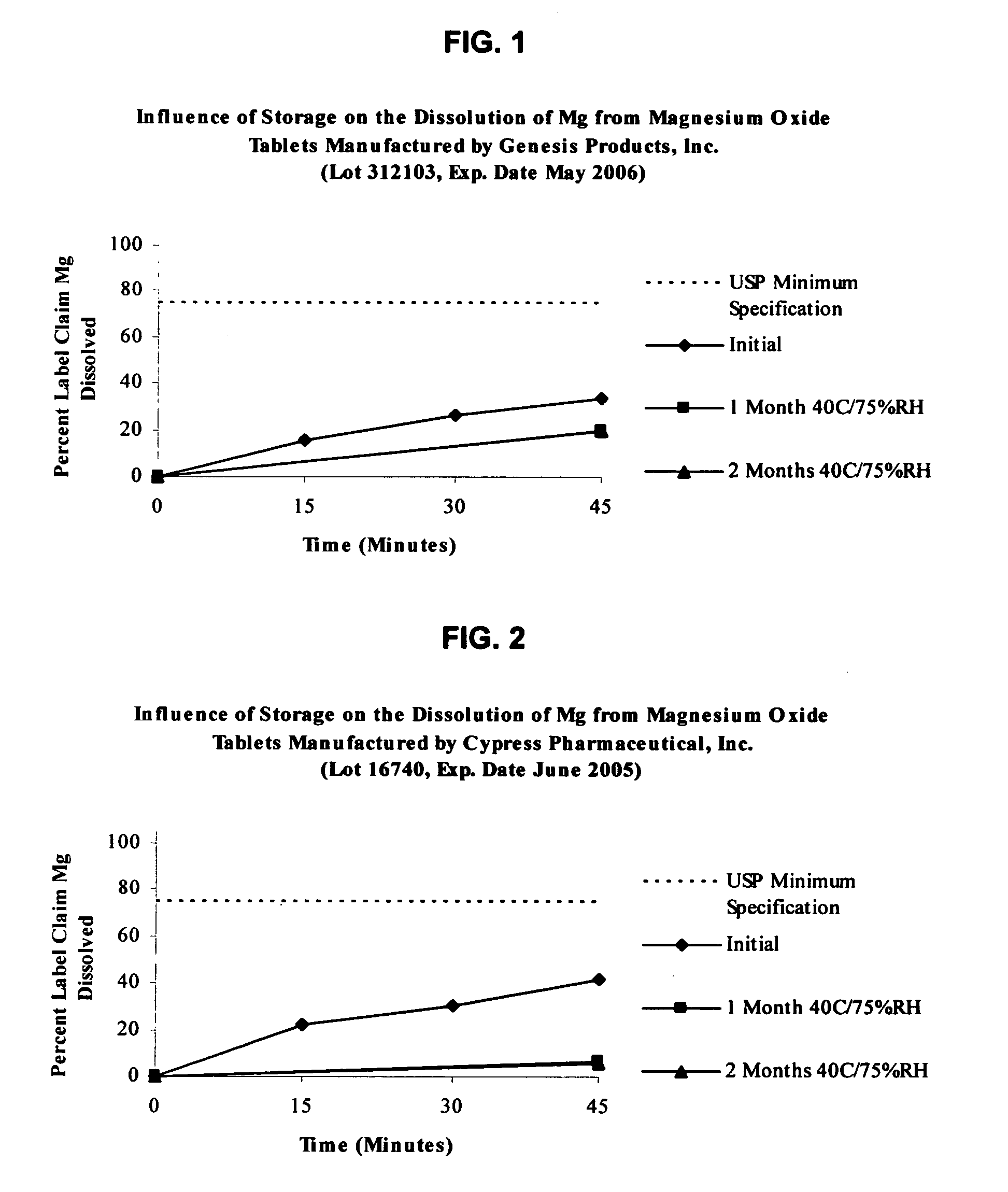
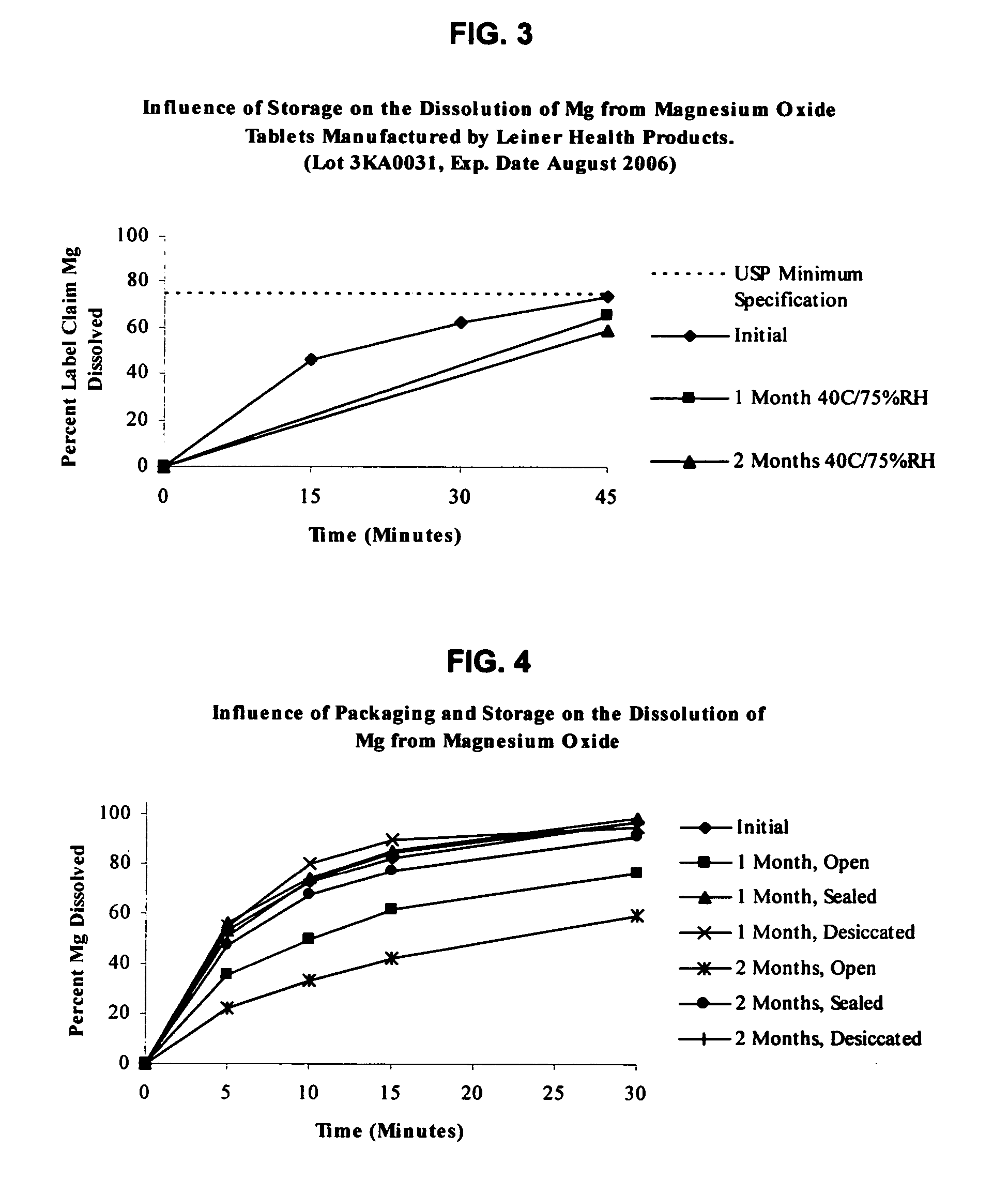
An oral solid compress composition comprising a magnesium salt is provided. The composition provides a rapid dissolution of the magnesium salt, wherein not less than 75% of the magnesium salt dissolves within 45 minutes after placement in hydrochloric acid (0.1 N, 900 mL) as per USP Method <711>. In a particular embodiment, the magnesium salt is an inorganic salt such as MgO, Mg(OH)2, MgCl2, and others. The composition can be prepared by dry granulation, direct compression or another suitable process. The composition provides a substantially stable dissolution profile for the magnesium salt so that the dissolution profile changes only minimally even after an extended period of storage under pharmaceutically acceptable conditions when packaged in a sealed container-enclosure system. The solid composition may also exclude a cellulose-based composition. The compressed composition can be prepared and stored under anhydrous conditions.
Owner:BLAINE PHARMA
Bioadhesive dosage form of steroids
InactiveUS20060013873A1Disintegrates quicklyOrganic active ingredientsPowder deliveryOral mucosaSteroid Compound
Bioadhesive steroid nanoparticles are used to prepare a rapidly disintegrating solid oral dosage form of steroid. The bioadhesive steroid nanoparticles have a bioadhesive polymer surrounding the steroid nanoparticles and with an average diameter of less than 1000 nm. Hence, the solid oral dosage form, containing the bioadhesive steroid nanoparticles, a disintegrant and an effervescent agent, instantly disintegrates in the oral cavity and adheres on the periodontal pocket and oral mucosa to release steroid rapidly.
Owner:MEDICAL & PHARMA IND TECH & DEV CENT
Quick disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS6656492B2Good effectDisintegrates quicklyPowder deliveryLiquid surface applicatorsHigh concentrationLow speed
The present invention pertains to a quick disintegrating tablet in buccal cavity, characterized in that drug-containing particles with a mean particle diameter of approximately 50~approximately 250 mum and an apparent specific gravity of approximately 0.5~approximately 1.2 consisting of a bitter tasting drug and / or drug of inferior fluidity and a pharmaceutical preparation carrier and obtained by spray drying are added to a quick disintegrating tablet in buccal cavity comprising a drug and saccharide. Moreover, the present invention pertains to a method for manufacturing drug-containing particles having a specific mean particle diameter and specific apparent gravity by dissolving and suspending a bitter tasting drug and / or drug of inferior fluidity and a pharmaceutical preparation carrier (preferably containing water-insoluble polymer, particularly at least aqueous ethyl cellulose suspension (preferably containing plasticizer)) to a high concentration in terms of solid concentration in a solvent that is pharmaceutically acceptable and then spray drying this liquid using a rotating disk-type spray dryer, with the disk operating at low speed, and a method for manufacturing a quick disintegrating tablet in buccal cavity comprising said particles.
Owner:ASTELLAS PHARMA INC
Cushioning wax beads for making solid shaped articles
InactiveUS6923984B1High tensile strengthSustained deliveryPowder deliveryPharmaceutical containersWaxCushioning
Biologically inactive cushioning beads comprise at least one compressible cushioning component consisting essentially of a microcrystalline hydrocarbon wax or a natural wax, the said wax being at least 30% by weight of the biologically inactive cushioning beads. Such beads are useful for making solid shaped articles containing biologically active ingredients by compression.
Owner:UNIV GENT
Method for preparing rapidly disintegrating formulation for oral administration and apparatus for preparing and packing the same
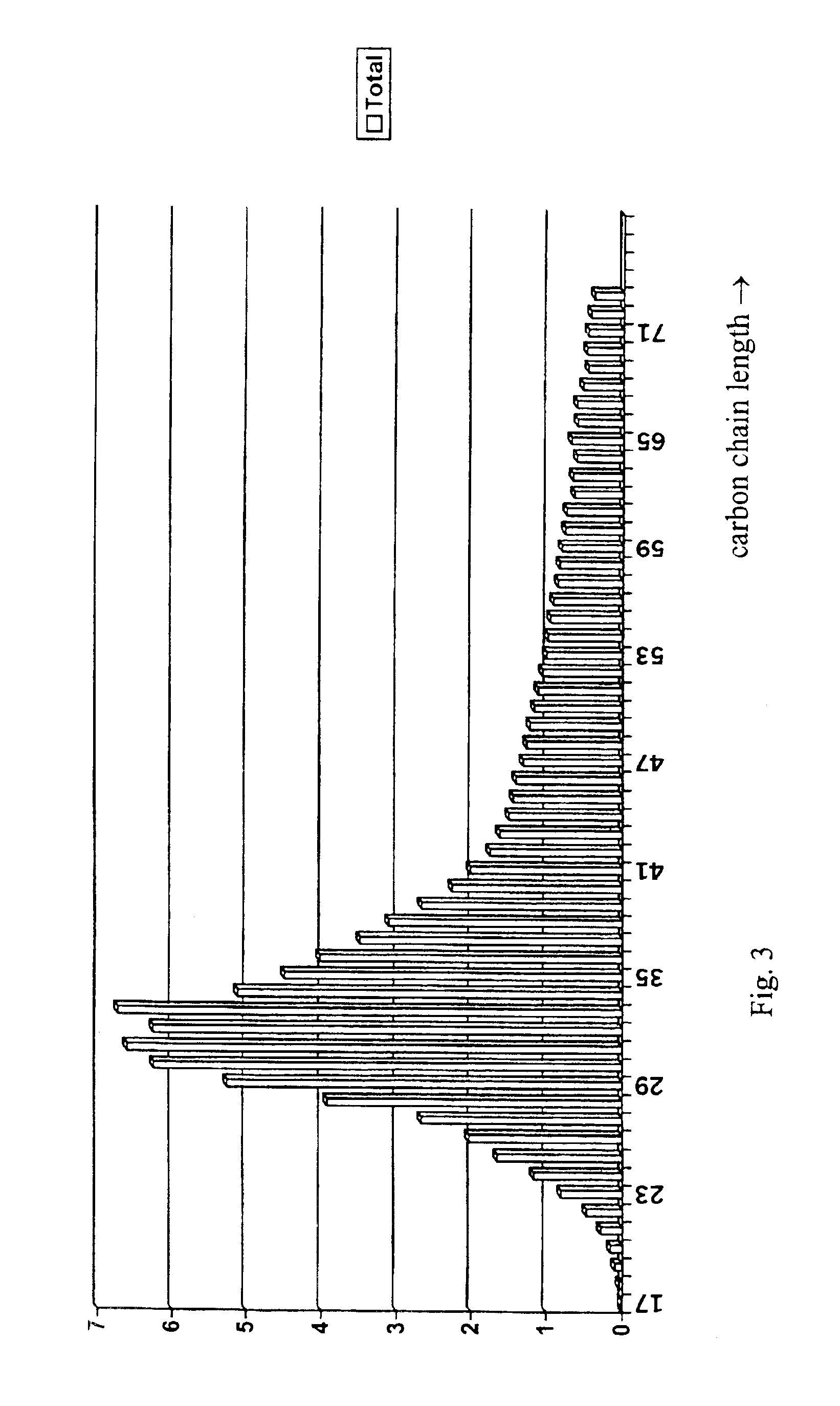
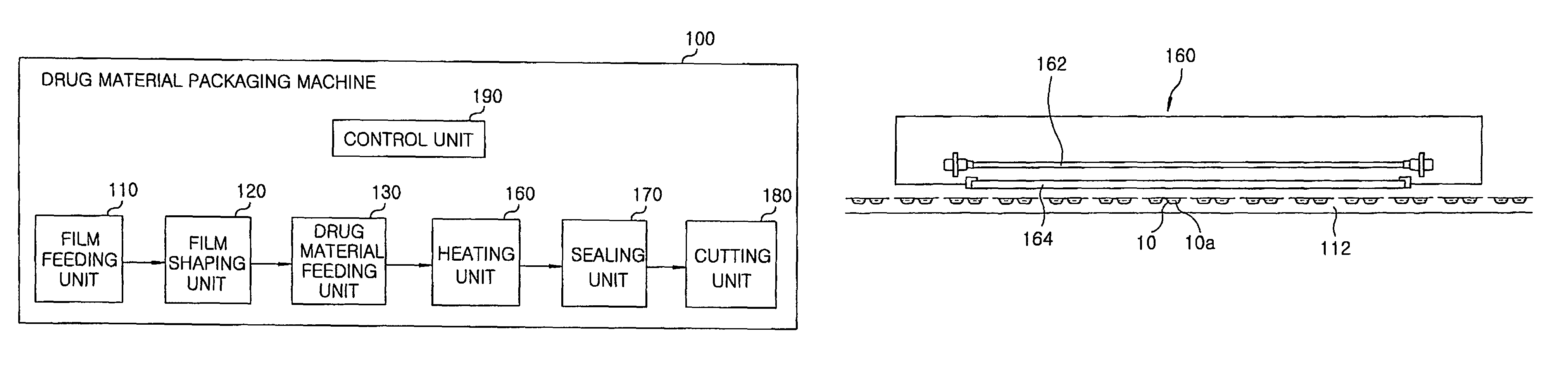
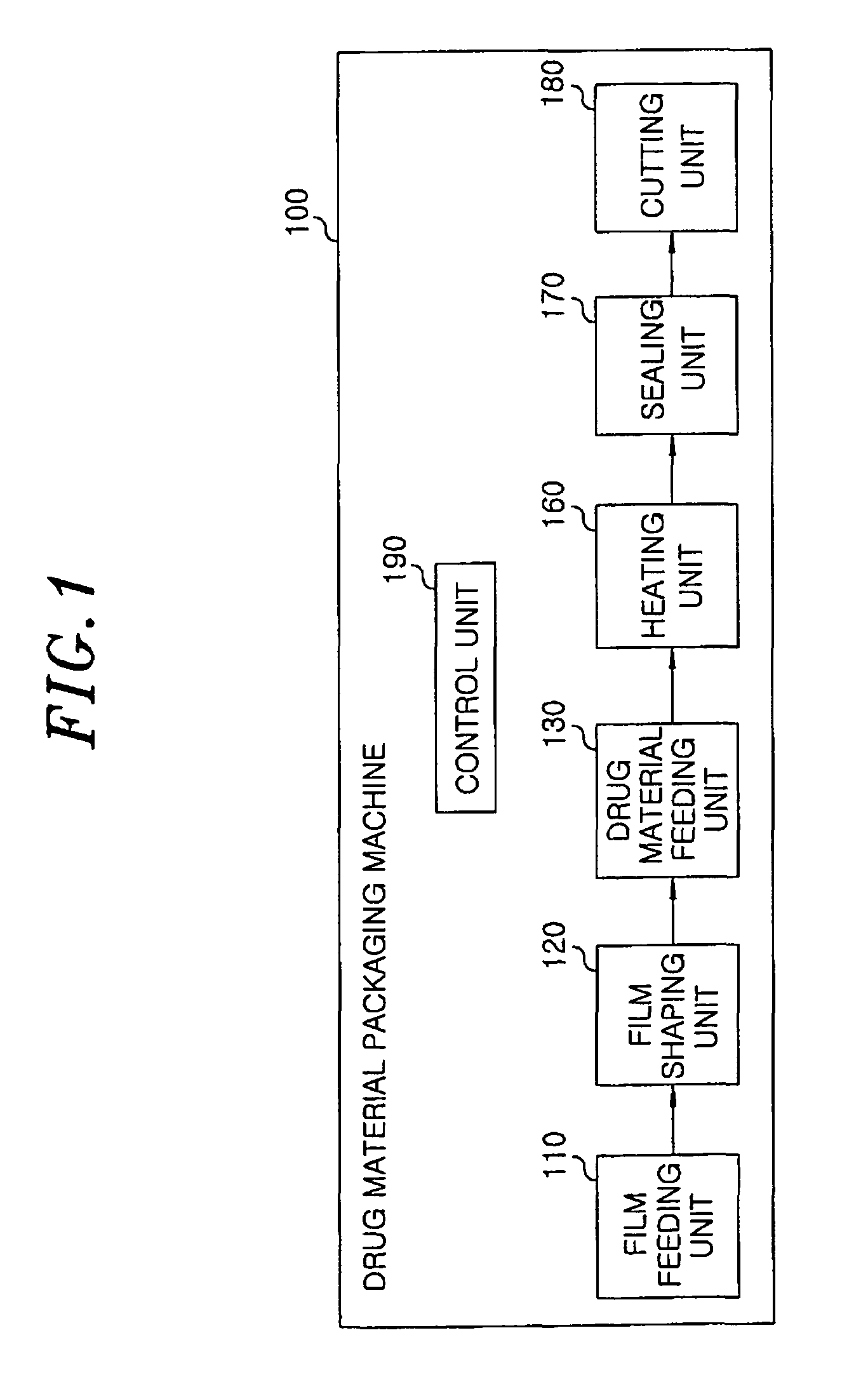
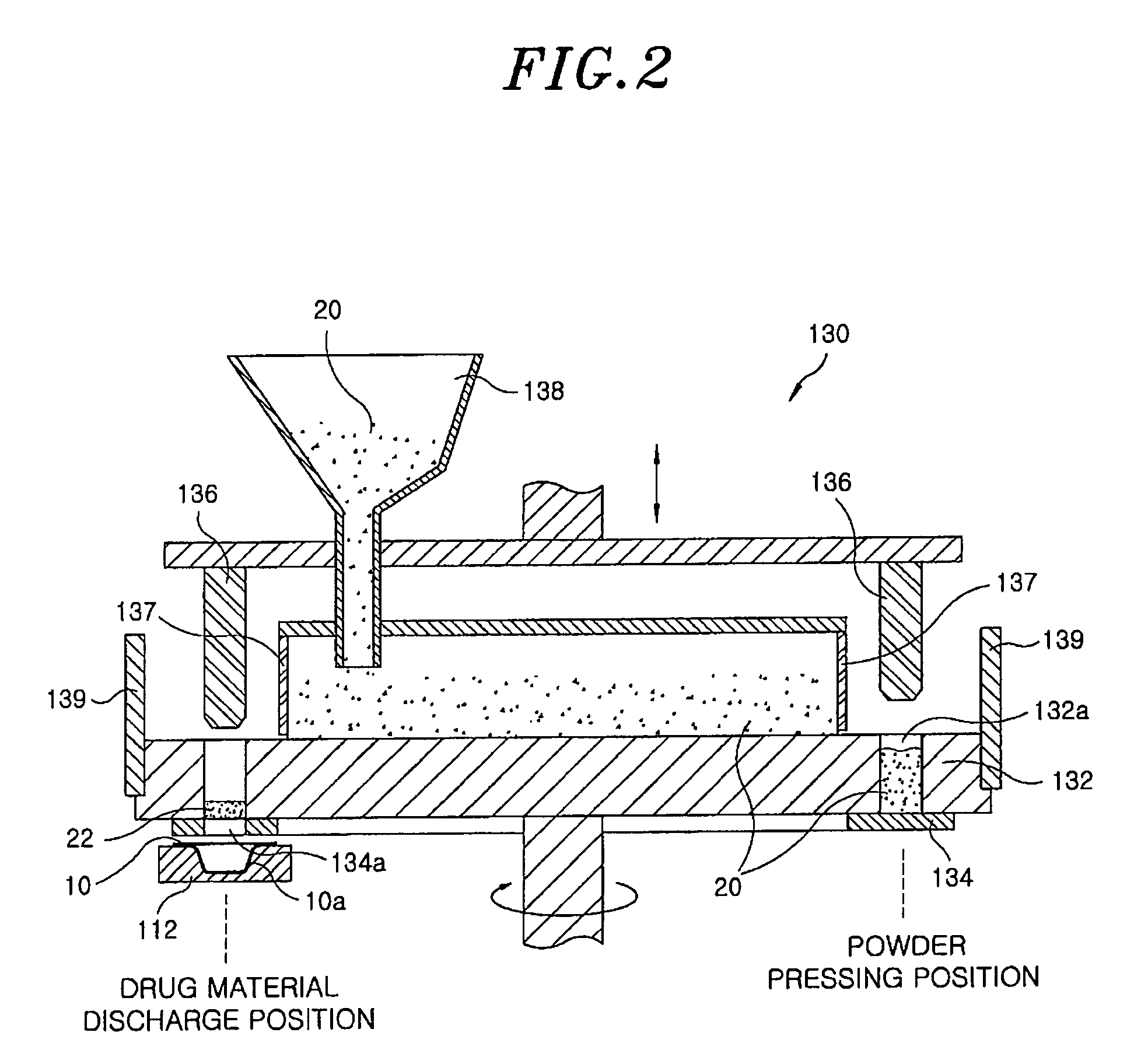
InactiveUS8127516B2Disintegrates quicklyEnhanced patient comfortAntibacterial agentsNervous disorderPowder mixtureOral medication
A method and packaging machine for preparing rapidly disintegrating formulations for oral administration are disclosed. The present invention is characterized in that a powdery mixture including a pharmaceutically active ingredient and a sugar or a sugar alcohol powder is filled into a packaging material and, thereafter, the mixture, filled in the packaging material, is heated. The present invention can simply and economically prepare an oral formulation which undergoes rapid disintegration in the oral cavity and provides for high-quality administration to patients.
Owner:HANMI PHARMA
Mannose-based fast dissolving tablets
Owner:PURDUE RES FOUND INC
Novel Dispersible Tablet Composition
ActiveUS20080312168A1Reduce sedimentation rateDisintegrates quicklyBiocideCarbohydrate active ingredientsBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to a novel dispersible tablet composition, which comprises of a pharmacologically active ingredient and at least one excipient, which reduces the sedimentation rate of active ingredient. This invention further relates to a process for the preparation of a dispersible tablet of a pharmacologically active ingredient.
Washing tablet
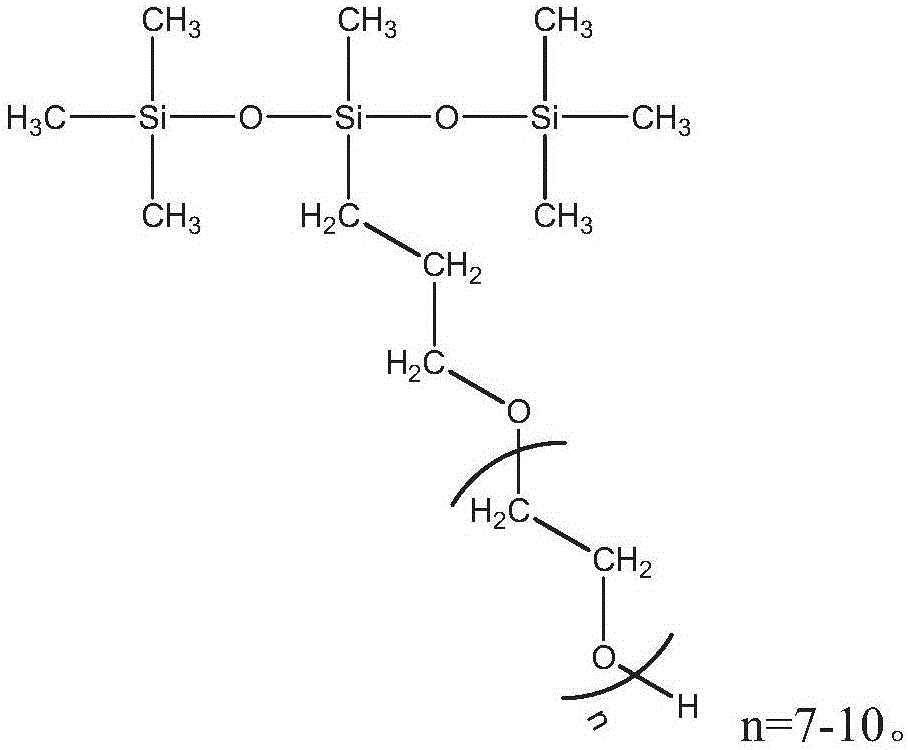
InactiveCN105969550ADisintegrates quicklyImprove washabilityInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsChemistryNonionic surfactant
The invention discloses a washing tablet. The washing tablet comprises, by weight, 20-40 parts of anionic surfactant, 10-20 parts of nonionic surfactant, 0-5 parts of ampholytic surfactant, 0-20 parts of complexing agent, 5-30 parts of disintegrating agent, 0-10 parts of wetting agent, 5-25 parts of film-forming substance, 0-20 parts of washing auxiliaries, 0-1 part of essence, 0-0.1 part of pigment, 0.1-1 part of anticorrosion and bactericidal agent and 40-60 parts of water. The washing tablet has the advantages that the novel organic silicone disintegrating agent is compounded with the common disintegrating agent, the washing tablet is fast in dissolving during use, good in washing effect, easy to rinse, free of residues and pollutions, convenient to use, environmentally friendly, economical, convenient to transport and easy to store.
Owner:苏州禾川化学技术服务有限公司
Method and device for measuring the temperature of a molten metal bath
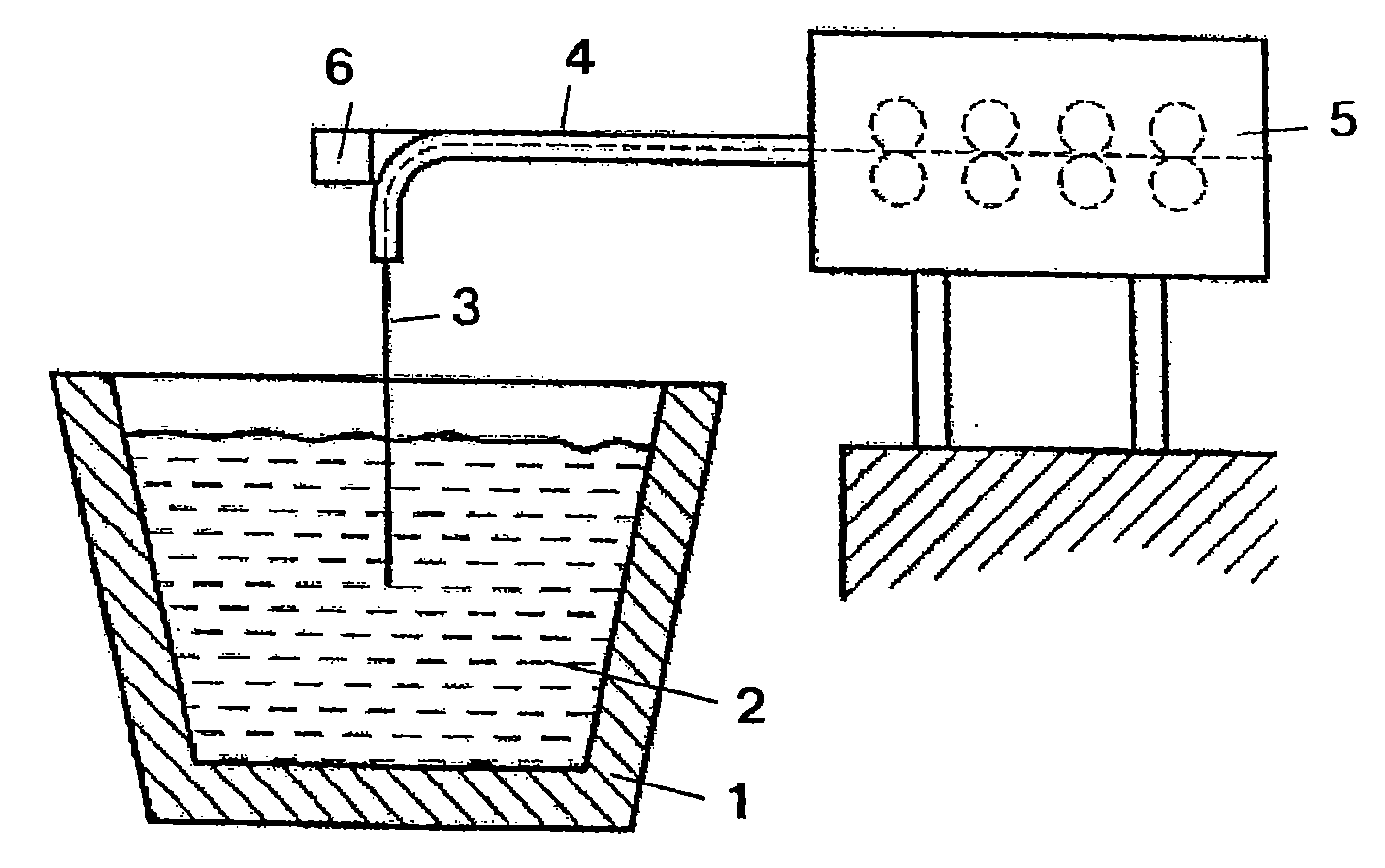
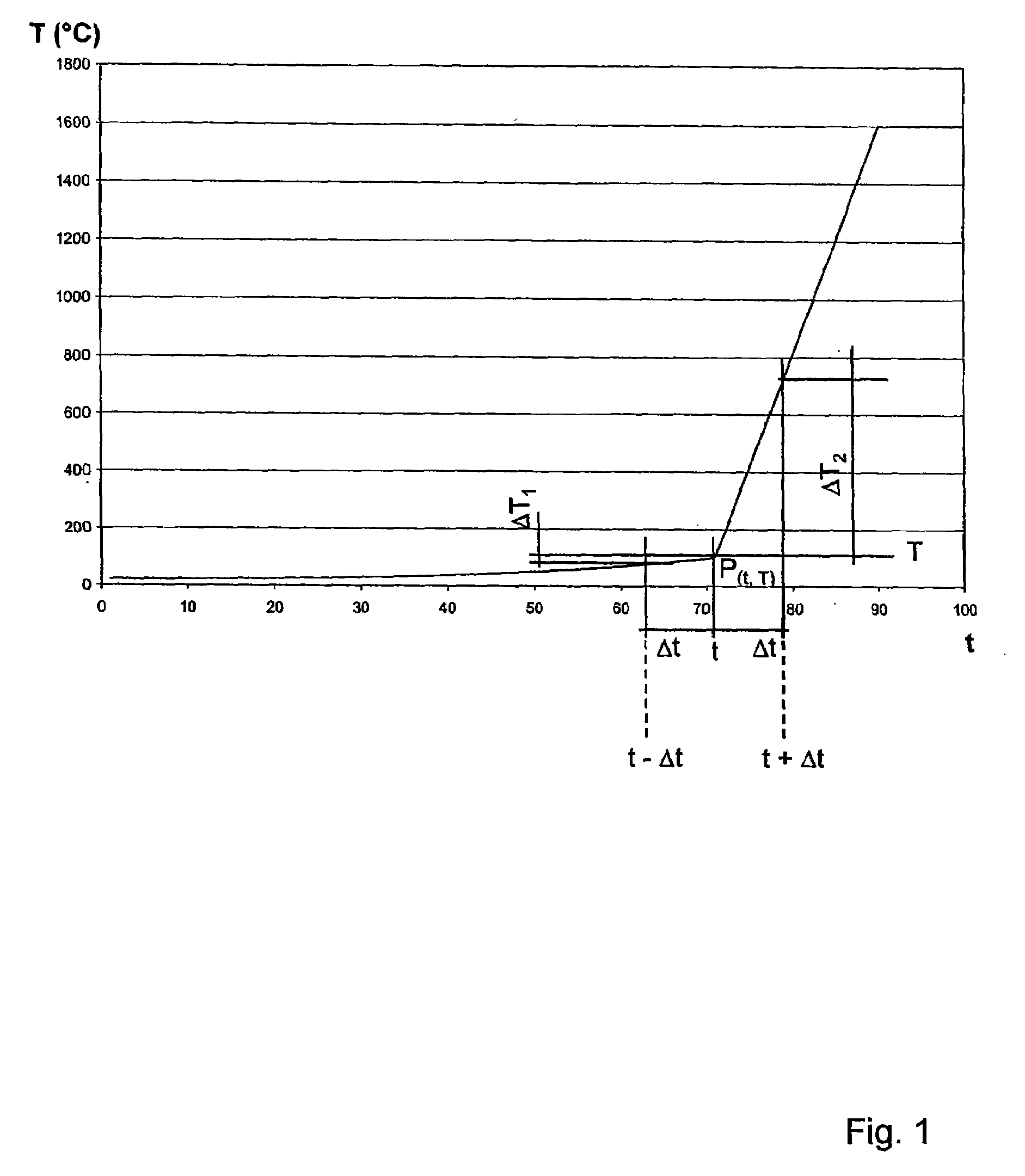
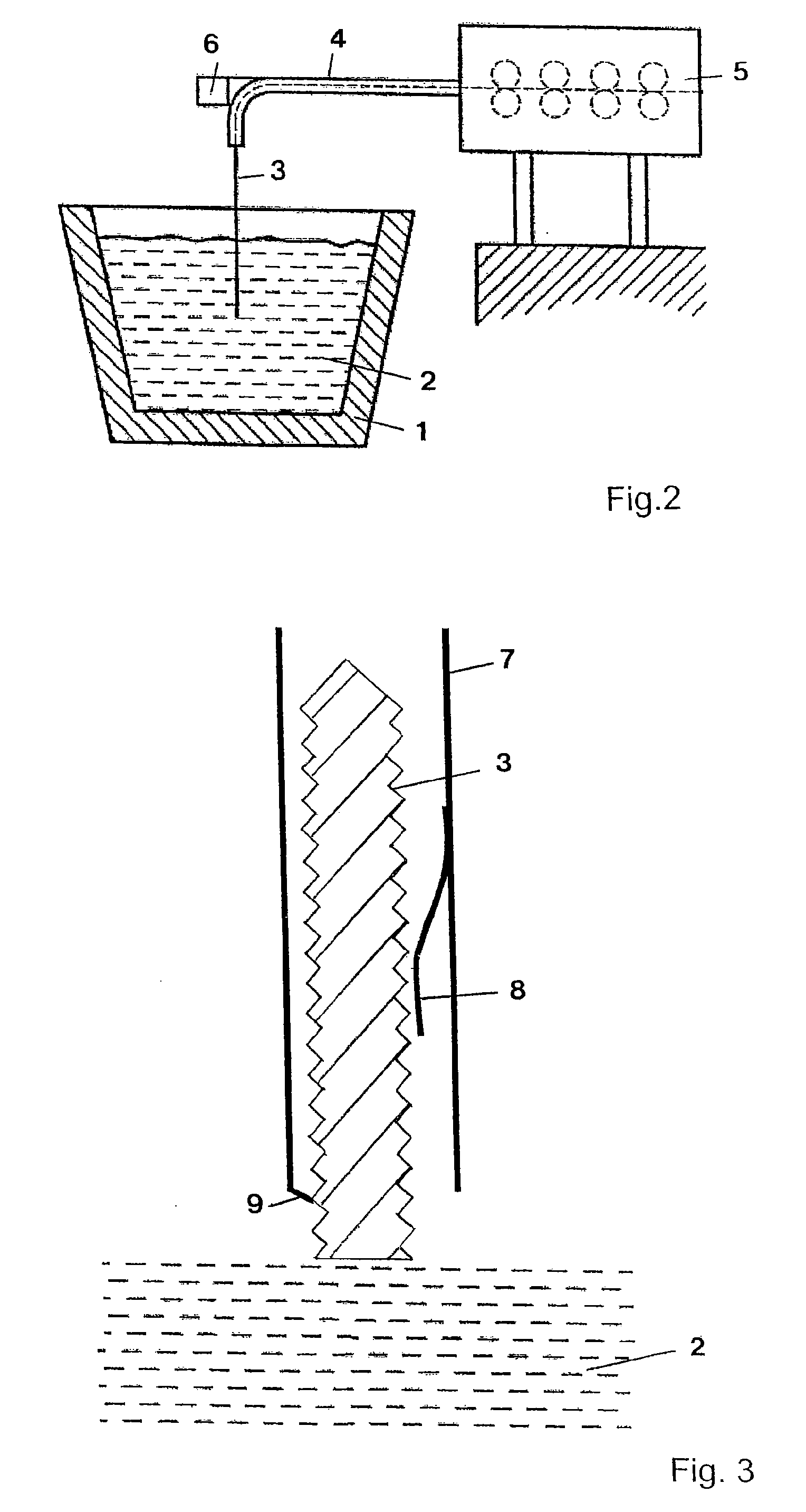
ActiveUS20070268477A1Strong and effective changeIncrease heating speedThermometer detailsThermometers using physical/chemical changesMolten bathMolten metal
A method is provided for measuring a temperature of a molten metal bath by an optical fiber surrounded by a cover. The optical fiber is immersed in the molten bath, and the radiation absorbed by the optical fiber in the molten bath is fed to a detector, wherein the optical fiber is heated when immersed in the molten bath. The heating curve of the optical fiber has at least one point P(t0, T0), wherein the increase ΔT1 in the temperature T of the optical fiber over the time Δt in a first time interval t0−Δt up to the temperature T0 is smaller than the increase ΔT2 in the temperature of the optical fiber over the time Δt in an immediately following second time interval t0+Δt.
Owner:HERALUS ELECTRO NITE INT NV
Rapidly dissolving tablets comprising low surface area calcium phosphates
InactiveUS20070196477A1Effective quick dissolving resultReduce brittlenessCosmetic preparationsToilet preparationsCalcium biphosphateMedicine
This invention pertains to the ability to provide rapidly disintegrating tablets through the inclusion of a calcium phosphate material in combination with other common tablet components. Such a calcium phosphate material must exhibit a sufficiently low surface area in order to boost the ability of the table to separate quickly when introduced into a user's mouth cavity. Such a tablet is dimensionally stable prior to use (low friability) and, when immersed in water the tablet disintegrates therein in less than about 60 seconds.
Owner:WITHIAM MICHAEL C +2
Wet granulation process
InactiveUS20060057073A1Improve solubilityImprove efficiencyOrganic active ingredientsAerosol deliveryBULK ACTIVE INGREDIENTActive ingredient
The invention is in the field of pharmaceutical dosage forms, and more particularly in the field of pharmaceutical granules and processes for making granules. The invention provides a process for preparing pharmaceutical granules which contain an active ingredient in the form of a salt, said process comprising the steps of (a) providing a powder containing the active ingredient as a free base or acid, and (b) agglomerating the powder by adding a granulation liquid to form granules; wherein step (b) is conducted in the presence of a neutralization agent capable of neutralizing the active ingredient, and for a sufficient amount of time to allow the active ingredient to become at least partially converted into a salt. The invention also provides pharmaceutical granules obtainable by said process and pharmaceutical compositions comprising said granules. The invention further provides the use of pharmaceutical granules for the pulmonary delivery of an active ingredient.
Owner:PARI PHARMA GMBH
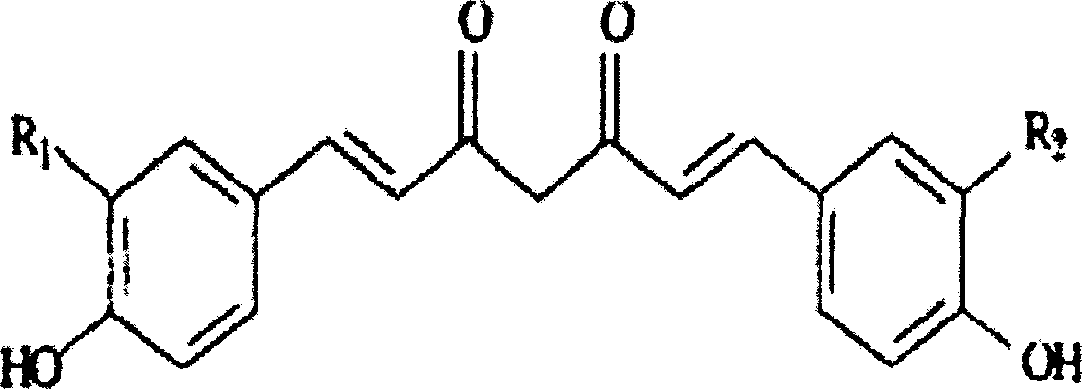
Curcumin preparation and its making method
InactiveCN1895239AImprove solubilityHigh drug loadingOrganic active ingredientsAntipyreticDiseaseEmulsion
A curcumin medicine for preventing and treating cancer and cardiovascular and cerebrovascular disease includes a pre-concentrated curcumin liquid in the form of softgel and prepared from the precursor of curcumin and emulsifier and a fatty curcumin emulsion prepared from the precursor of curcumin, emulsifier, antioxidant, isotonic regulator, stabilizer, pH regulator, and the water for injection. It can also provide energy to the patient.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Traditional Chinese medicine effervescence tablets for feet-washing, and its prepn. method
InactiveCN1947684AConfidenceIntuitive feelingCosmetic preparationsToilet preparationsSodium oxideEffervescent Tablet Dosage Form
A Chinese-medicinal effervescent tablet for preventing and treating beriberi, eczema and itching by foot bath is prepared from zedoary oil, flavescent sophora root and sodium oxide. Its preparing process is also disclosed.
Owner:WENZHOU MEDICAL UNIV +1
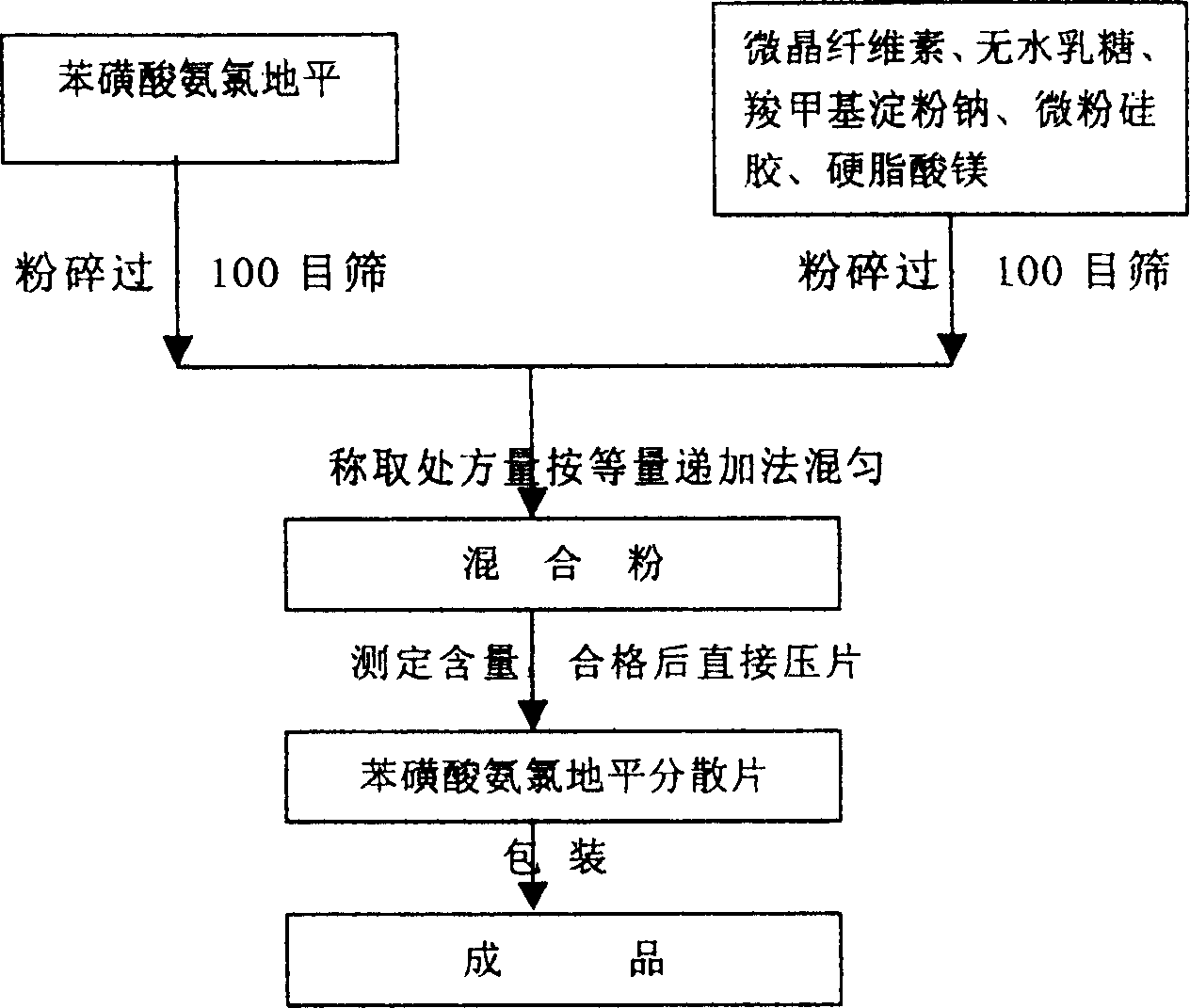
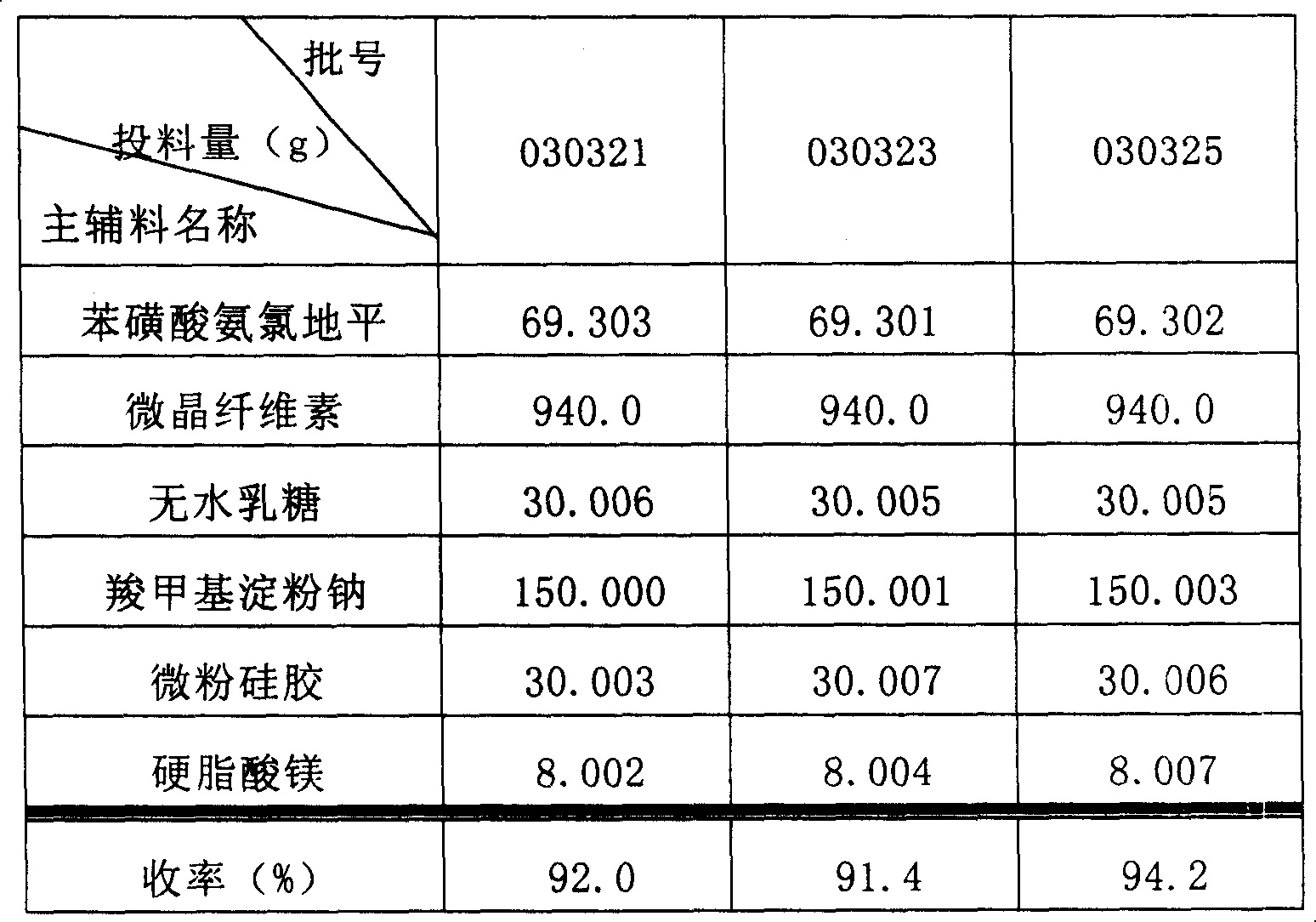
Phenylsulfonic acid amido chloro diping dispersion tablet and its preparation method
InactiveCN1686121AEasy to takeTake fastOrganic active ingredientsPill deliveryCarboxymethyl starchAmlodipine besilate
A dispersing table of amlodipine benzosulfonate for treating hypertension is proportionally prepared from amlodipine benzosulfonate, microcrystalline cellulose, anhydrous lactose, carboxymethyl starch sodium, micropowdered silica gel, and magnesium stearate.
Owner:YUNNAN BAIYAO GRP HEALTH PROD CO LTD
Effervescent and effervescent-dispersion compositions for medicaments and methods of use thereof
InactiveUS20050074489A1Need can be addressedEnhance sensory effectPill deliveryPharmacyPharmaceutical drug
Owner:UNION SPRINGS PHARMA
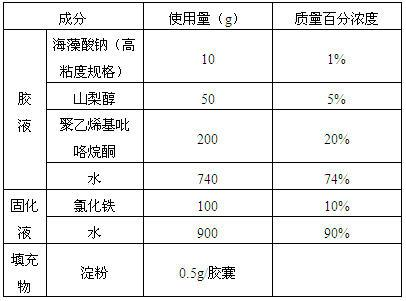
Alginate hard capsule disintegrable at different positions in gastrointestinal tract
ActiveCN102657869AGood heat and humidity stabilityEasy to preparePharmaceutical non-active ingredientsCapsule deliveryHard CapsuleMedicine
Disclosed is an alginate hard capsule disintegrable at different positions in gastrointestinal tract. Processing steps include preparing glue solution, glue solution dipping, solidifying, drying, drawing out, cutting, fastening, optical testing and sterilizing of glue solution and capsule filling. The glue solution is prepared by dissolving univalent alginate, plasticizer and pore former in water, wherein the pore former is a polymer film-forming material, glycerin and sorbitol serve as the plasticizer; the solidifying is that dipped gel solution is made into alginate gel capsule by means of ionic cross-linking or acidulating, wherein solidifying solution is multivalent metal ion water solution or acid solution; and finally active ingredients and disintegrating accelerator are filled in hard capsule shells to obtain finished products, wherein the disintegrating accelerator is a substance which can be in precipitation reaction or complexation with multivalent metal ions. The alginate hard capsule is widely applied to different disintegrating environments in the gastrointestinal tract and disintegrable in vivo at different speeds to release the active ingredients and has the advantages of high hydrothermal stability, simple preparation method and low cost.
Owner:南京健辉生物科技有限公司
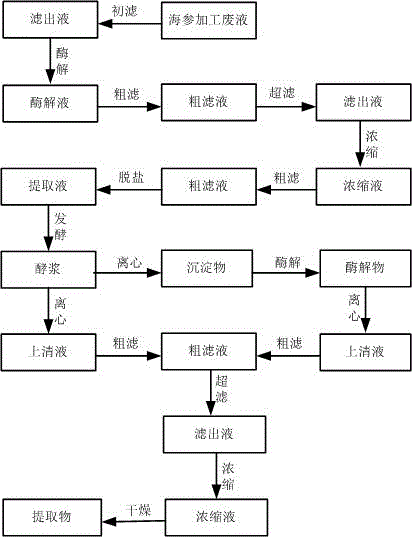
Method for extracting multiple active nutrient contents from sea cucumber processing liquid and application of method
The invention relates to a method for extracting multiple active nutrient contents from sea cucumber processing liquid. The method comprises the following steps: raw material collection, primary filtration, enzymolysis, rough filtration, ultrafiltration, concentration, secondary rough filtration, desalination, fermentation, centrifugation, secondary enzymolysis, secondary centrifugation, third rough filtration, secondary ultrafiltration, secondary concentration and spray drying. The invention further relates to application of the method in orally disintegrating tablets. Compared with the prior art, the method has the advantages that a prepared sea cucumber extract contains multiple nutrient contents, and the total content of sea cucumber polypeptide, small proteins, sea cucumber mucopolysaccharide and sea cucumber glycosides reaches 80%-90%; in the whole technological process, reaction conditions are mild, no organic solvent is used, nutrient contents are hardly damaged, and molecules of the extracted sea cucumber extract are relatively small; after the sea cucumber extract is fermented by using beneficial bacteria, the nutrient contents in the sea cucumber extract are easily absorbed by human bodies, and almost no peculiar smell exists; furthermore, the technological process is very suitable for the industrial expanded production.
Owner:YINGSHANG TIANHAO FOOD
Tablet quickly melting in oral cavity
ActiveUS20060134199A1Easy to produceDisintegrates quicklyDispersion deliveryDigestive systemWater solubleHardness
The object of the present invention is to provide, as a solid preparation for making it easy to take, thus improving patient's compliance etc., an intraorally rapidly disintegrating tablet which can be produced easily without any particular problem by a usual method of producing tablets with a usual tabletting machine, has practically unproblematic hardness, and disintegrate rapidly in the oral cavity. This tablet is produced by tabletting cores coated with a pharmaceutical disintegrating agent, wherein the core is a granule containing a water-soluble medicament or containing a medicament and a sugar.
Owner:NIPPON SHINYAKU CO LTD
Water-Soluble, Quick-Dissolve Flavor Tablets
InactiveUS20080187628A1Easily consumeReadily dissolveMilk preparationButtermilkChemistryWater soluble
The invention is directed to quick-dissolve flavor tablets for use by consumers in flavoring beverages, such as water, and methods for flavoring such beverages. The tablet comprises a flavor component and a quick-dissolve carrier component which disintegrates rapidly upon placement into the beverage container with minimal residue.
Owner:CHAMPION MELINDA L
Pomegranate peel polyphenol antibiosis and antiphlogosis effervescent tablet as well as preparation method and application thereof
ActiveCN101700257AOvercome stabilityOvercoming patient carryAntibacterial agentsAntimycoticsSodium bicarbonateDisease
The invention relates to a pomegranate peel polyphenol antibiosis and antiphlogosis effervescent tablet as well as a preparation method and application thereof. The effervescent tablet is mainly prepared by raw material of pomegranate peel polyphenol, and auxiliary materials of sodium hydrogen carbonate, citric acid or tartaric acid, lactose, polyvinylpyrrolidone, magnesium stearate and superfine silica powder, has the functions of clearing heat, eliminating dampness, killing bugs and stopping itching, has strong suppression effect to women vagina pathogenic bacteria, features high safety, good stability, no obvious toxic or side effect, or drug tolerance, and provides convenience in using, carrying and storing for patients, is used for curing such diseases as vaginitis, vulvitis and the like caused by various trichomonad, epiphyte and bacteria, and can be used as preventability medicine for long-term use.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Tablets quickly disintegrating in mouth
InactiveUS20050147672A1Disintegrates quicklyEasy to carryOrganic active ingredientsBiocideAdditive ingredientPhospholipid
Tablets quickly disintegrating in the mouth which comprise a bitter drug ingredient and a bitterness-reducing ingredient composed of an essential oil, a high sweetness-sweetener and / or an acidic phospholipid or its lyso-derivative. When taken even without water, these tablets exhibit little bitterness. Thus, a bitter drug ingredient can be formulated without coating into tablets quickly disintegrating in the mouth.
Owner:OHMORI SHINJI +2
Orally disintegrating tablets and methods of manufacture
ActiveUS8545881B2Effective taste-maskingLess-friablePowder deliveryGranular deliveryOrally disintegrating tabletAlcohol sugars
A tablet that rapidly disintegrates in the oral cavity comprising a compressed blend of rapidly dispersing microgranules prepared by granulating a sugar alcohol or a saccharide or a mixture thereof having an average particle size less than about 30 microns and a disintegrant, and a taste-masked microcapsule containing at least one drug, the microcapsule being prepared by granulating a pharmaceutically acceptable formulation comprising at least one drug in a therapeutically effective amount and at least one polymeric binder that improves resilience of the microgranules, wet milling the granulated mass, and microencapsulating the milled granules to provide microcapsules.
Owner:ADARE PHARM INC
Orally disintegrating tablet
ActiveUS8377995B2Solve the lack of hardnessDisintegrates quicklyOrganic active ingredientsBiocideCelluloseHydrogen phosphate
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Orally disintegrating tablets
InactiveUS20110053942A1Solve the lack of hardnessDisintegrates quicklyBiocideDigestive systemMANNITOL/SORBITOLMedicine
The present invention relates to an orally disintegrating tablet containing (1) an active ingredient, (2) mannitol, (3) crystalline cellulose and (4) at least two kinds of particular ingredients selected from the group consisting of low-substituted hydroxypropylcellulose, cornstarch and carmellose, wherein the blending ratio of each ingredient relative to 100 wt % of the disintegrating tablet is (1) 0.01 to 50 wt %, (2) 20 to 86 wt %, (3) 10 to 30 wt %, and (4) 1 to 20 wt % for each particular ingredient and 3 to 60 wt % as the total of the particular ingredients to be blended, and an assembly of (3) crystalline cellulose to be blended has a bulk density of not more than 0.18 g / cm3, and can provide an orally disintegrating tablet having both suitable hardness and rapid disintegrability in oral cavity, which maintains orally disintegrability even under moist conditions, and hardness of not less than a predetermined level necessary for using in an automatic packaging machine.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
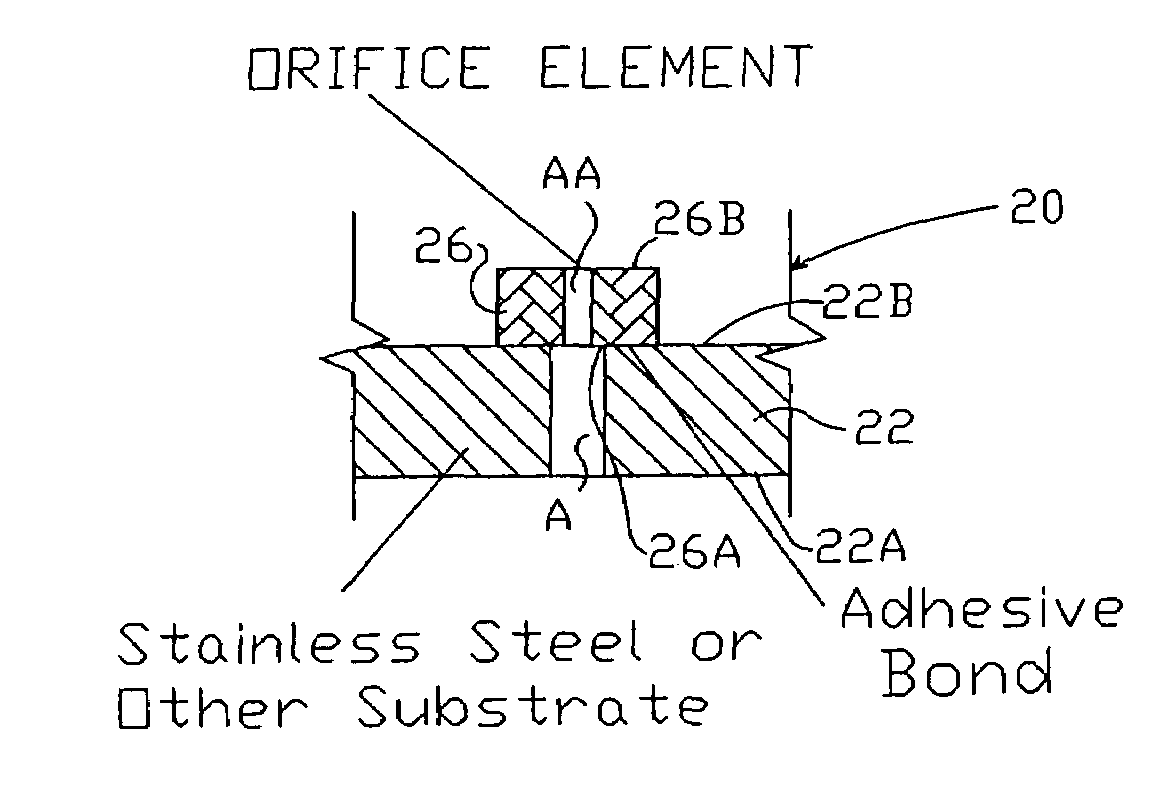
Composite hydroentangling nozzle strip and method for producing nonwoven fabrics therewith
InactiveUS7237308B2Disintegrates quicklyStrong erosionGlass making apparatusPattern makingHardnessNonwoven fabric
A composite nozzle strip for hydroentangling of a fibrous mass is provided to lower nozzle erosion potential and increase operational efficiency. The composite nozzle strip comprises a substrate comprising a material of a first hardness having at least one aperture and at least one orifice element comprising a material of a second hardness greater than the first hardness and further defining an aperture of a second diameter less than the first diameter. The at least one orifice element is affixed to the substrate so that the aperture in the orifice element is aligned with the at least one aperture in the substrate for creation of a constricted water jet when subjected to pressurized water.
Owner:ADVANCED FLUID TECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com