Patents
Literature
43results about How to "Sustained delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
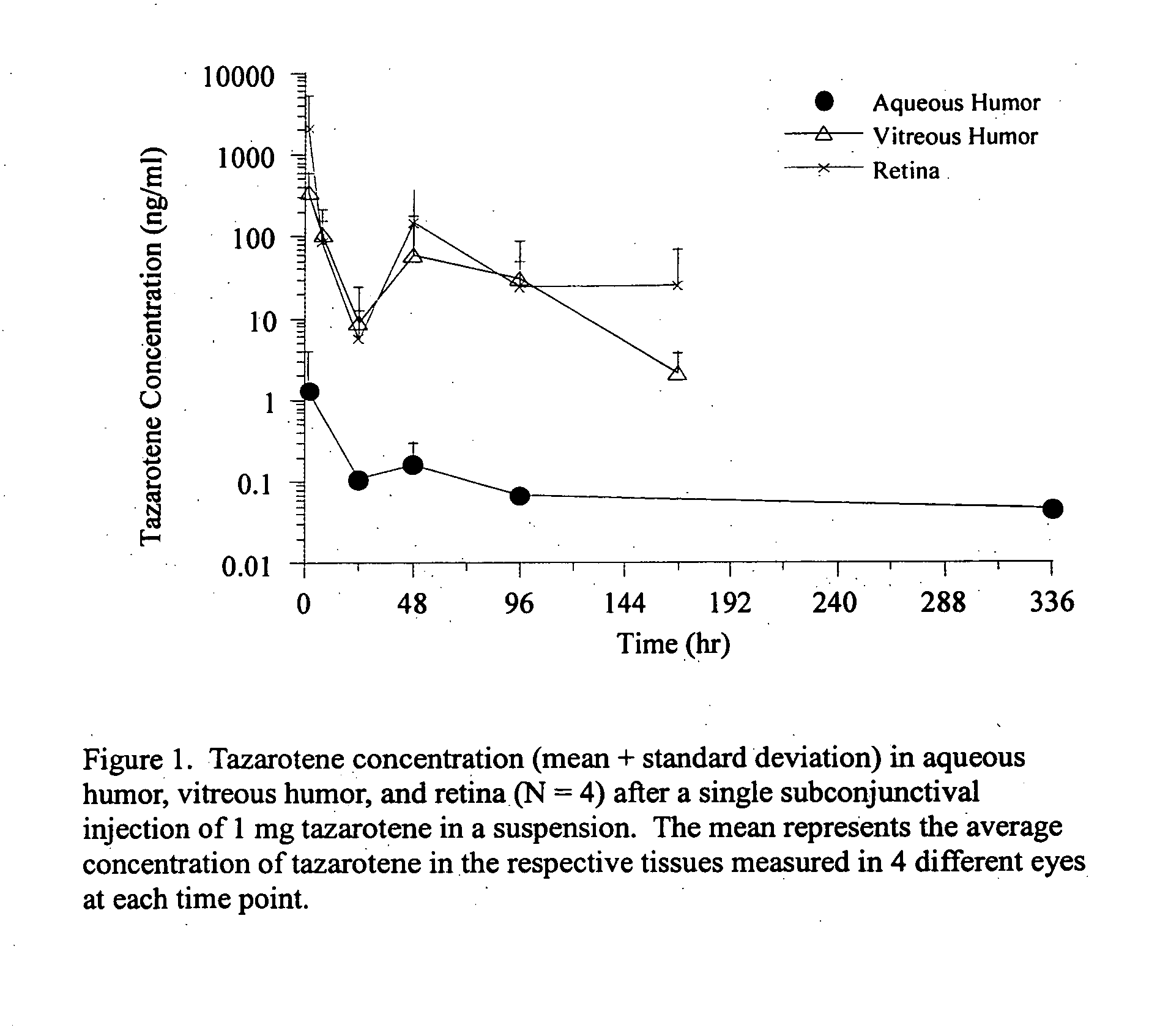
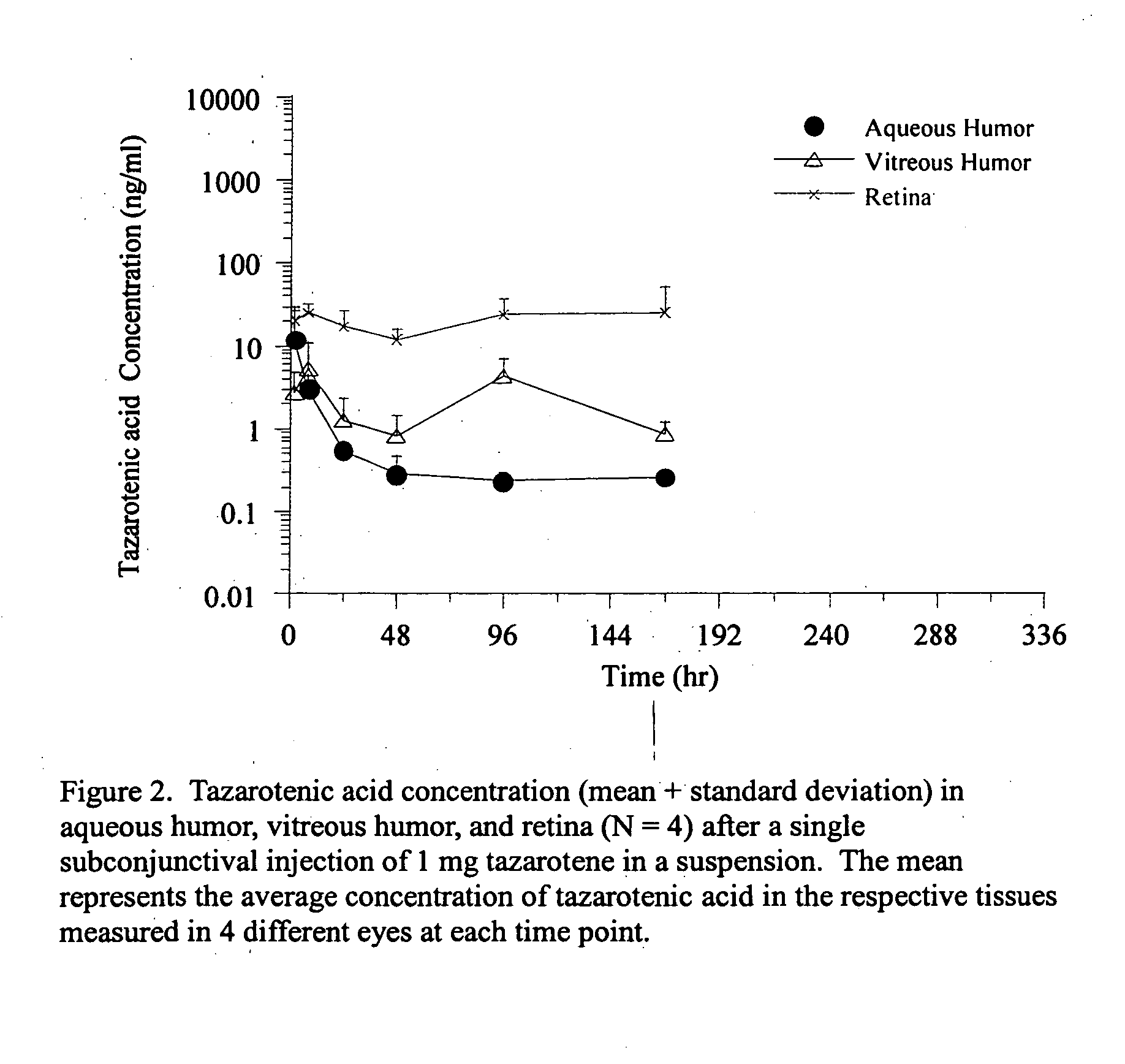
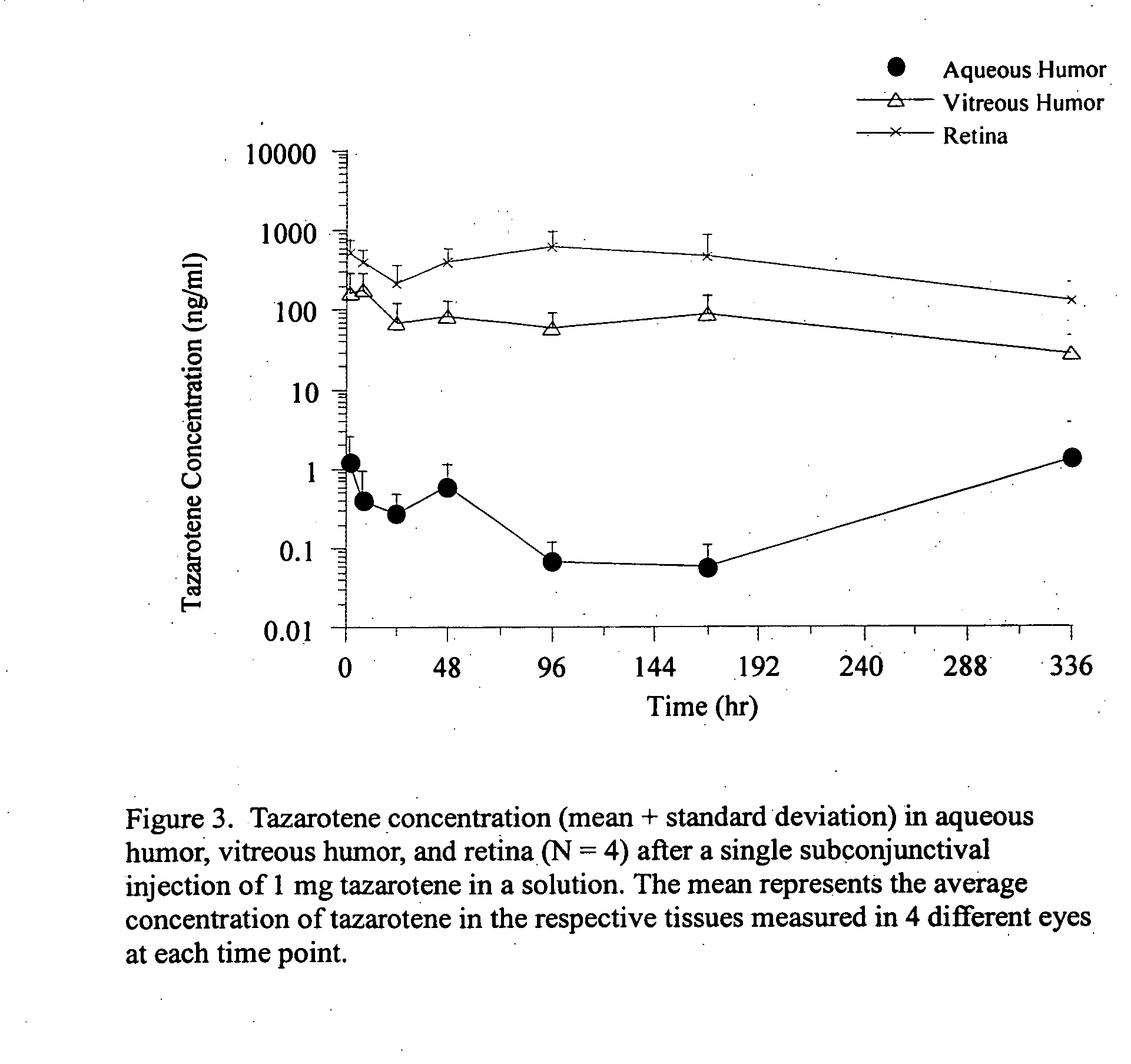
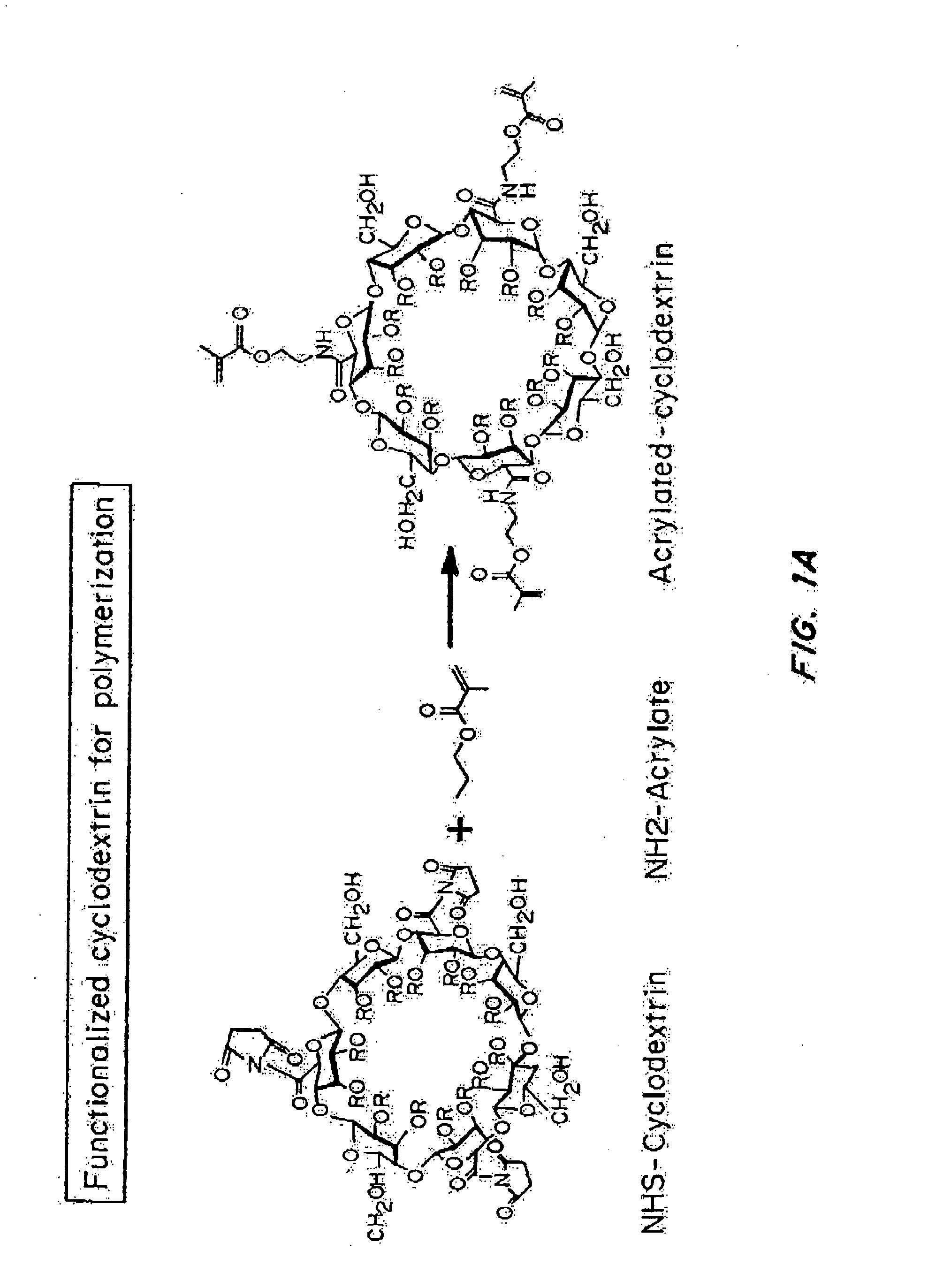
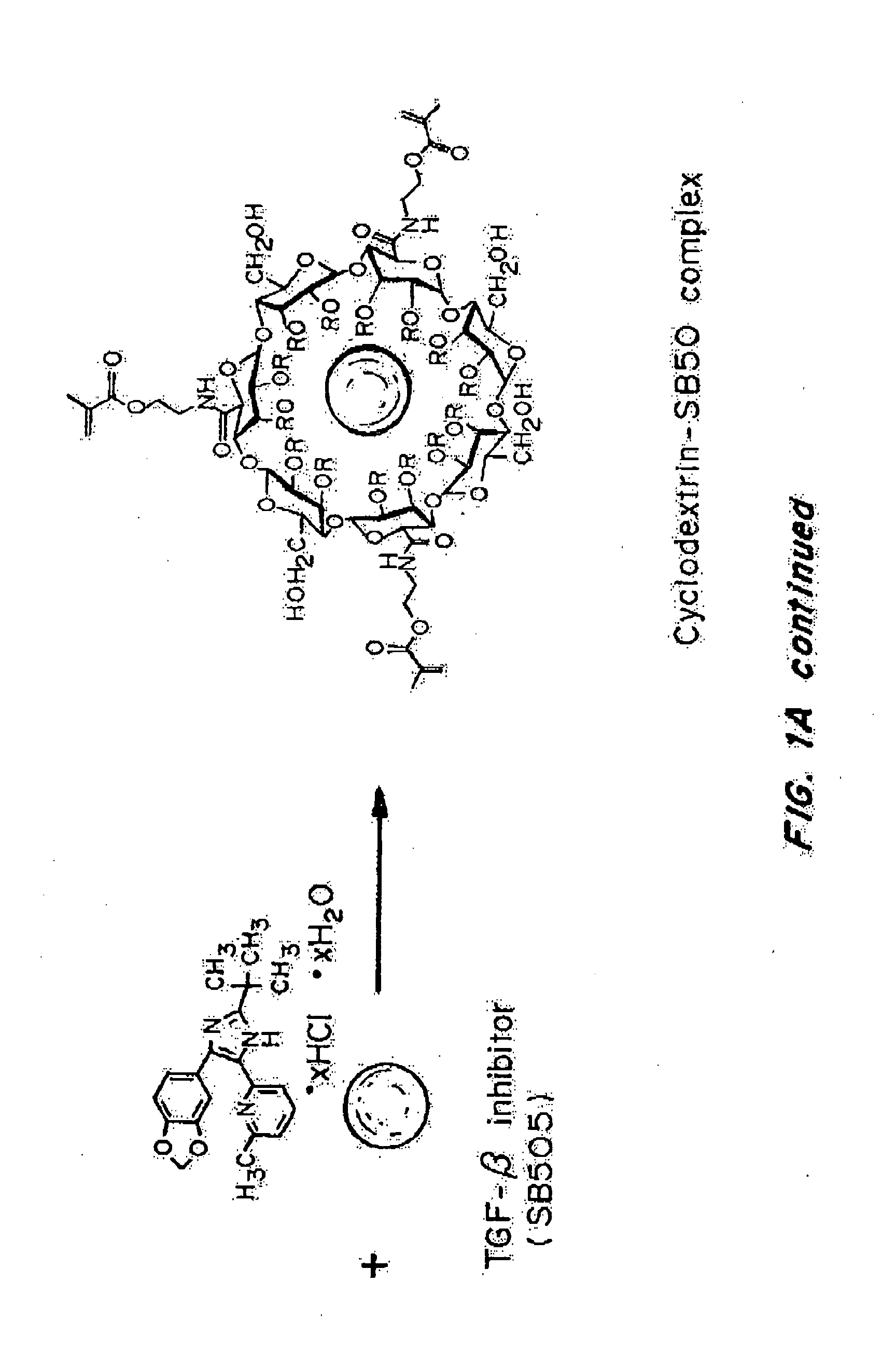
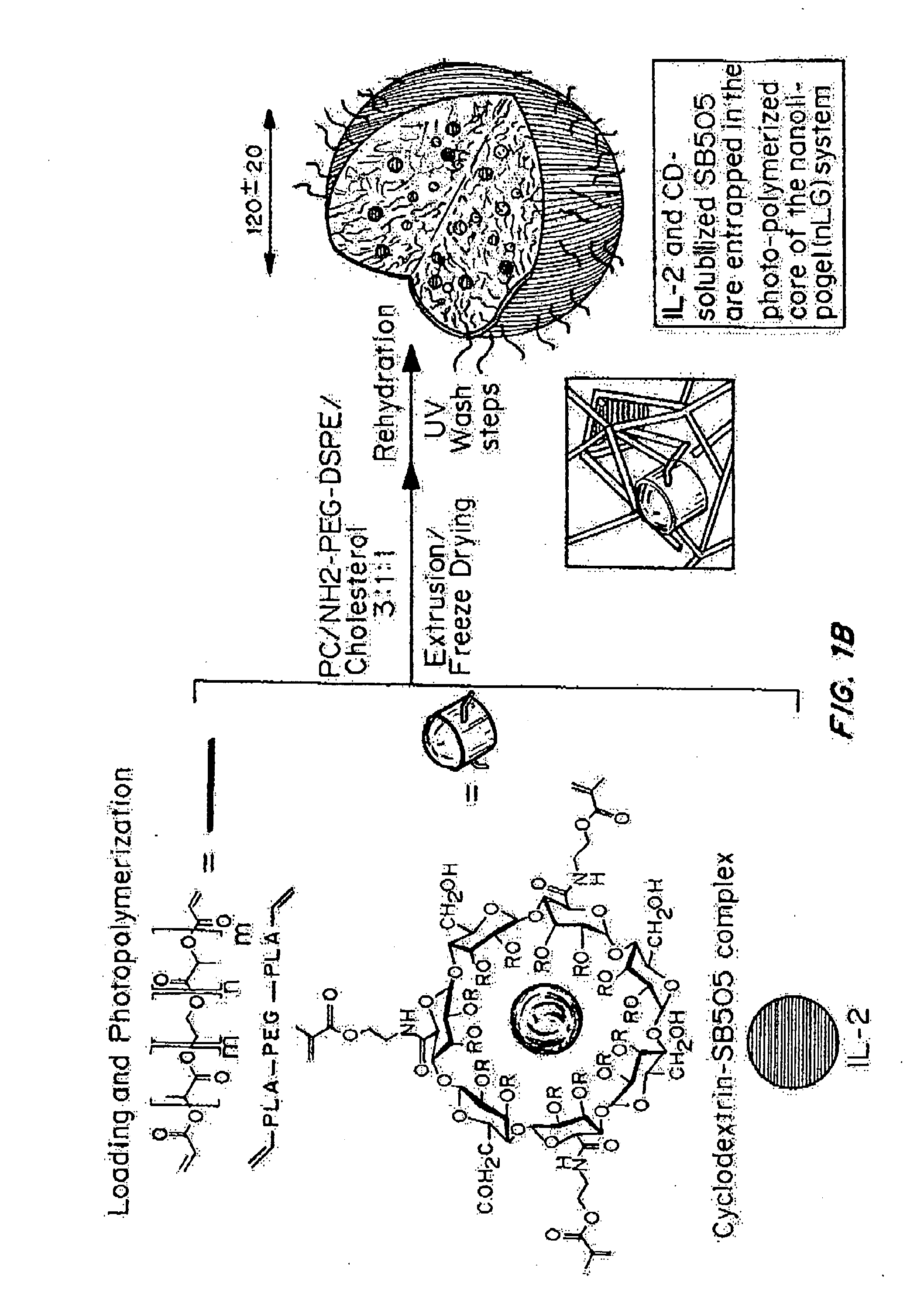
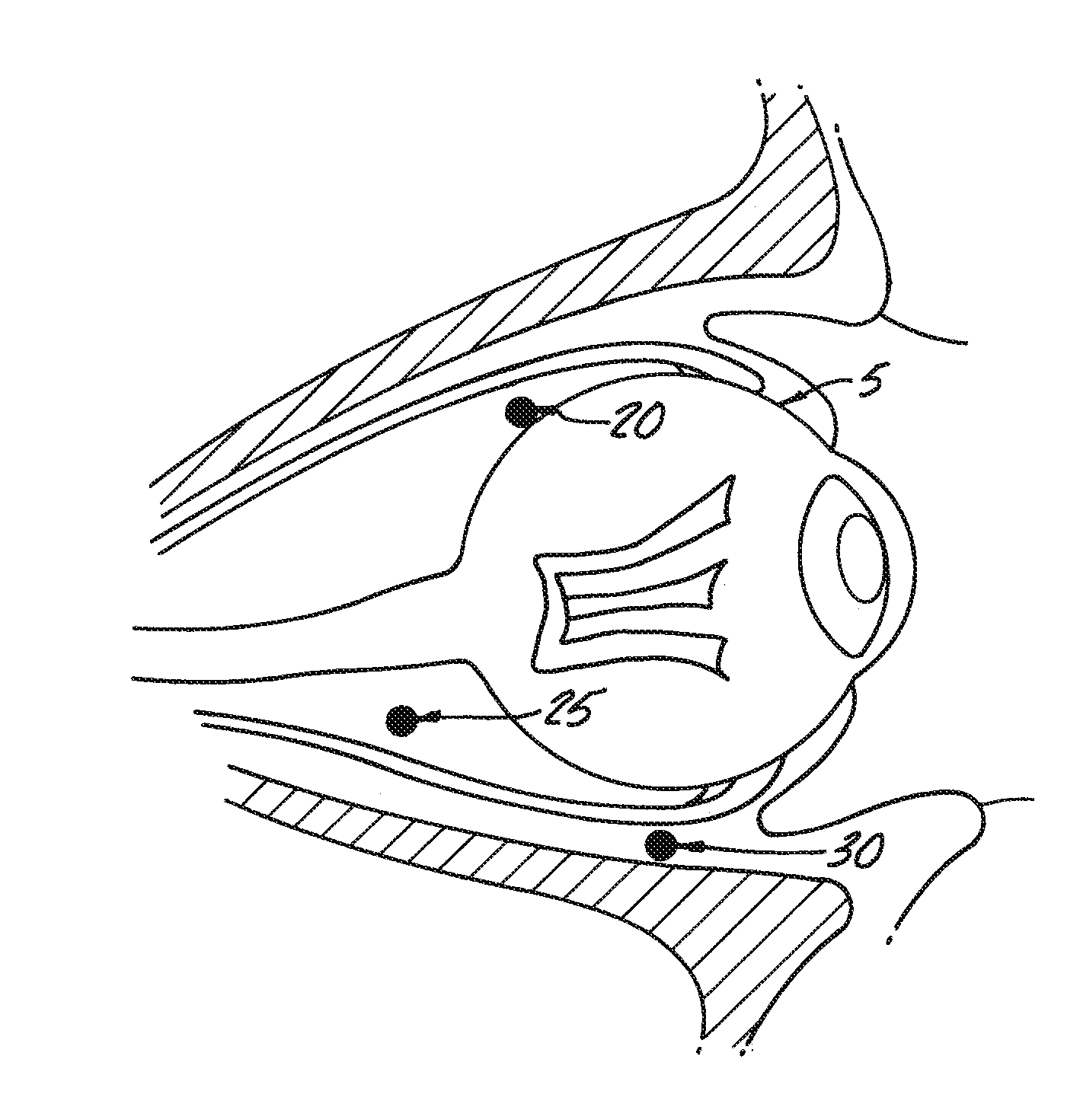
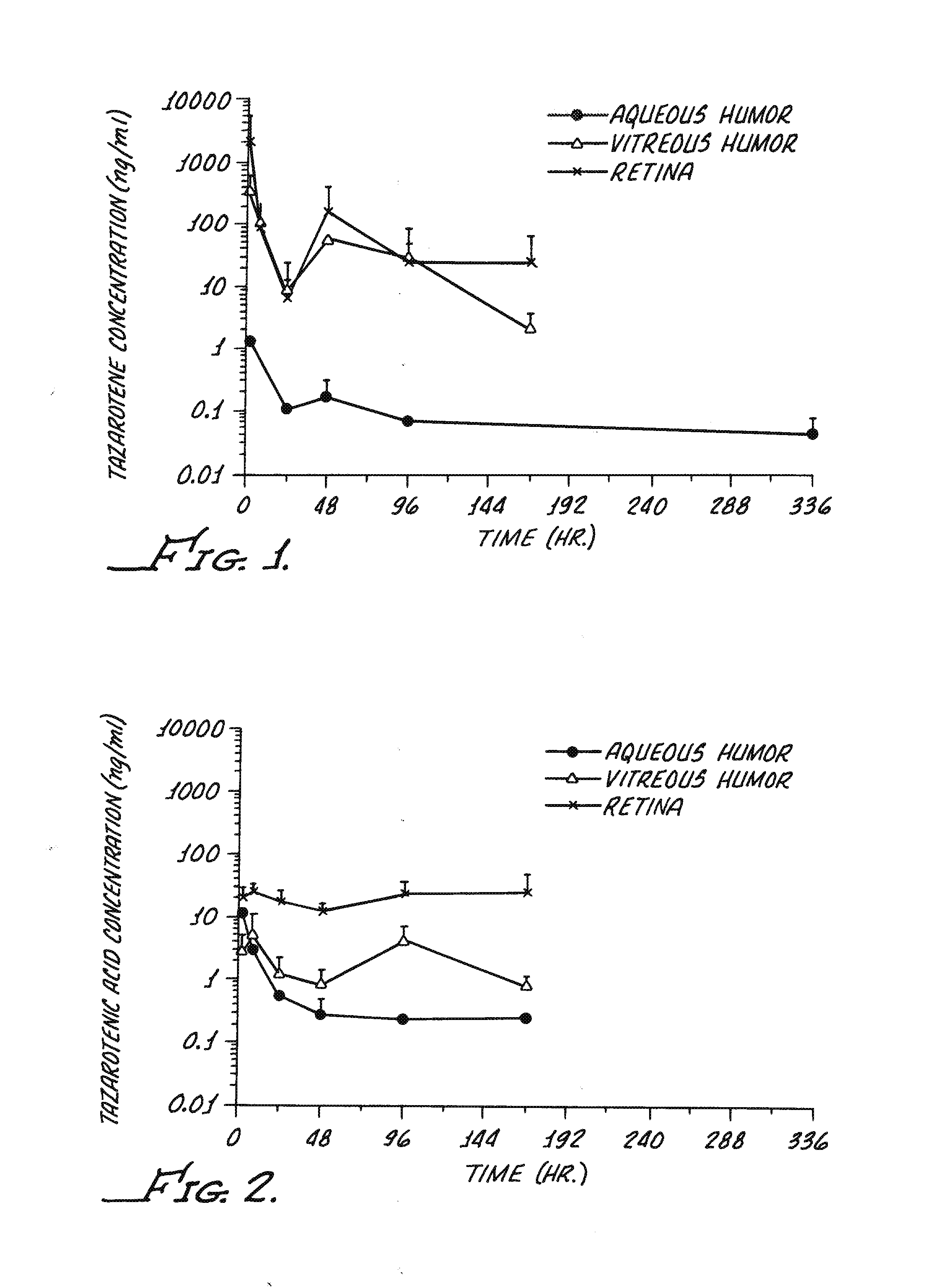
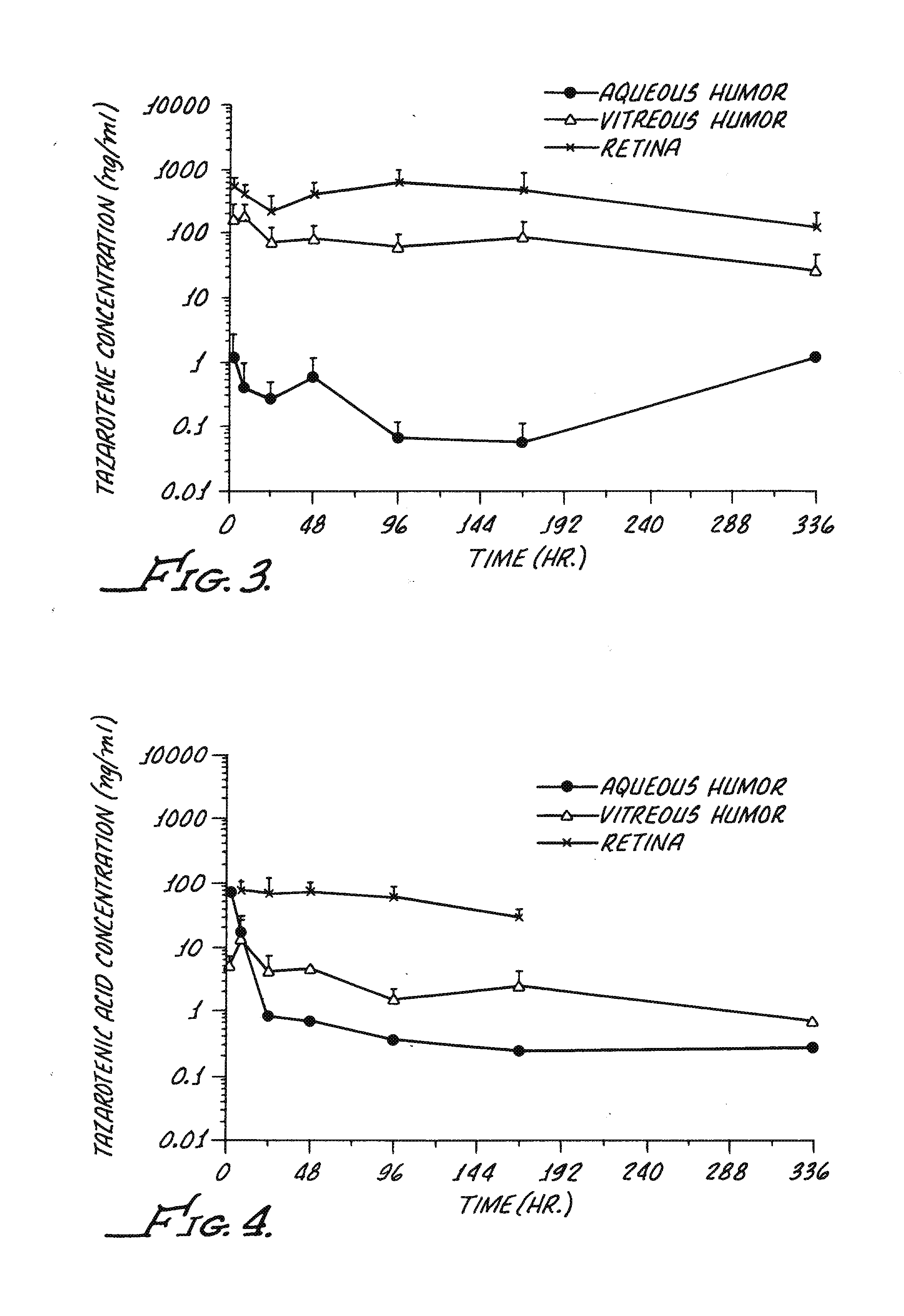
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20050009910A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaEster prodrug
The present invention relates to method of sustained-delivery of an active drug to a posterior part of an eye of a mammal to treat or prevent a disease or condition affecting said mammal, wherein said disease or condition can be treated or prevented by the action of said active drug upon said posterior part of the eye, comprising administering an effective amount of an ester prodrug of the active drug subconjunctivally or periocularly. Preferably, the active drug is more than about 10 times as active as the prodrug. Other aspects of this invention deal with the treatment of certain diseases by the periocular or subconjunctival delivery of an ester prodrug, and certain pharmaceutical products containing ester prodrugs for periocular or subconjunctival administration.
Owner:ALLERGAN INC
Immune enhancing compositions and methods of use thereof
InactiveUS20050271726A1Easy to synthesizeEffective absorptionPowder deliveryOrganic active ingredientsGlycineBlood level
A method of administering parenterally, particularly intramuscularly, glutamine and cystine and glycine plus selenium; or lactalbumin plus selenium; or lactalbumin and glutamine and cystine and glycine plus selenium, through a long-acting pharmaceutically acceptable carrier to a patient. The method comprises injecting a mixture of glutamine, cystine, glycine, lactalbumin and selenium in order to maintain the mixture systemically or locally for a sufficient time period so as to maintain blood levels of glutathione within an improved therapeutic range.
Owner:CRUM ALBERT
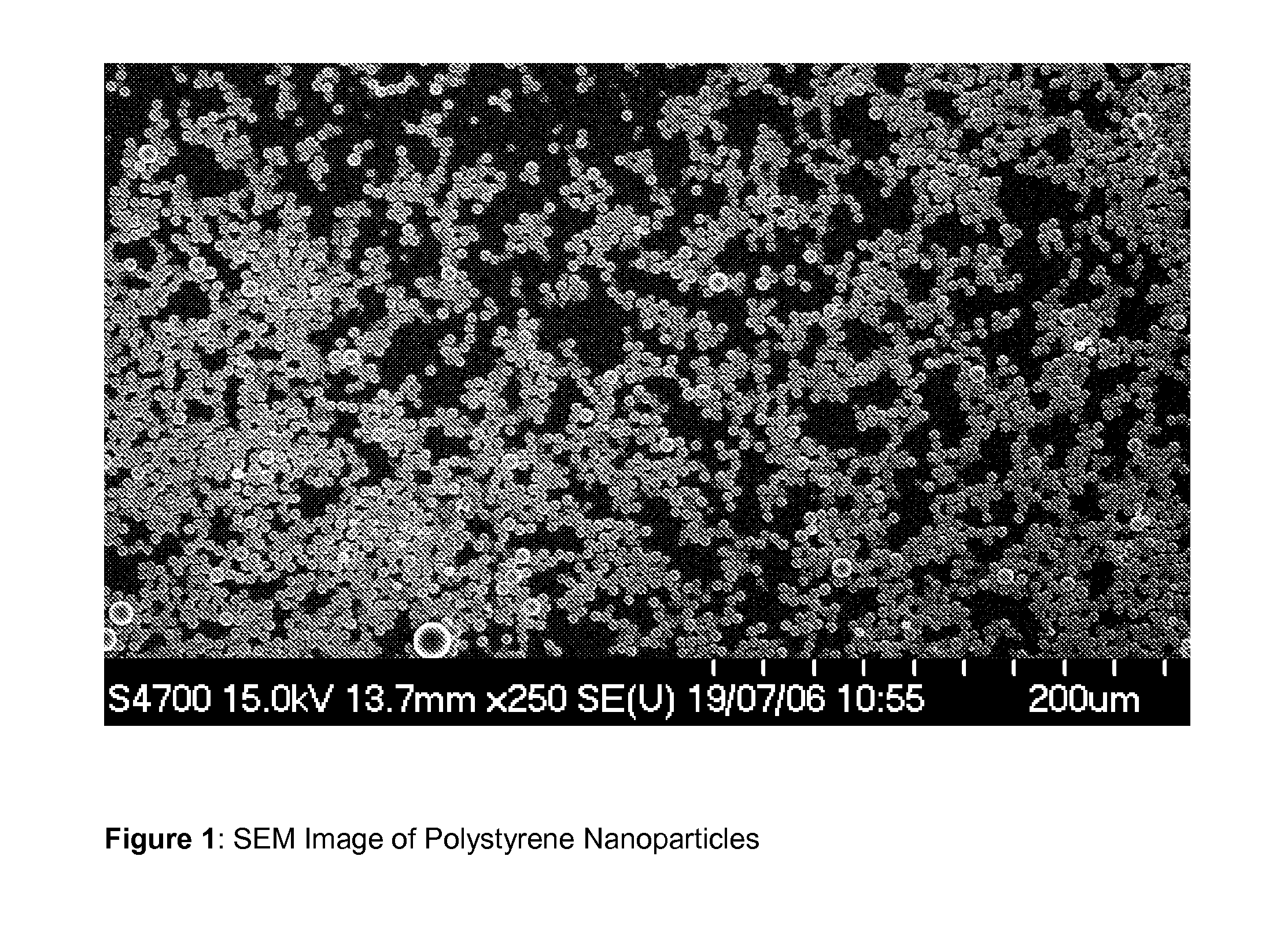
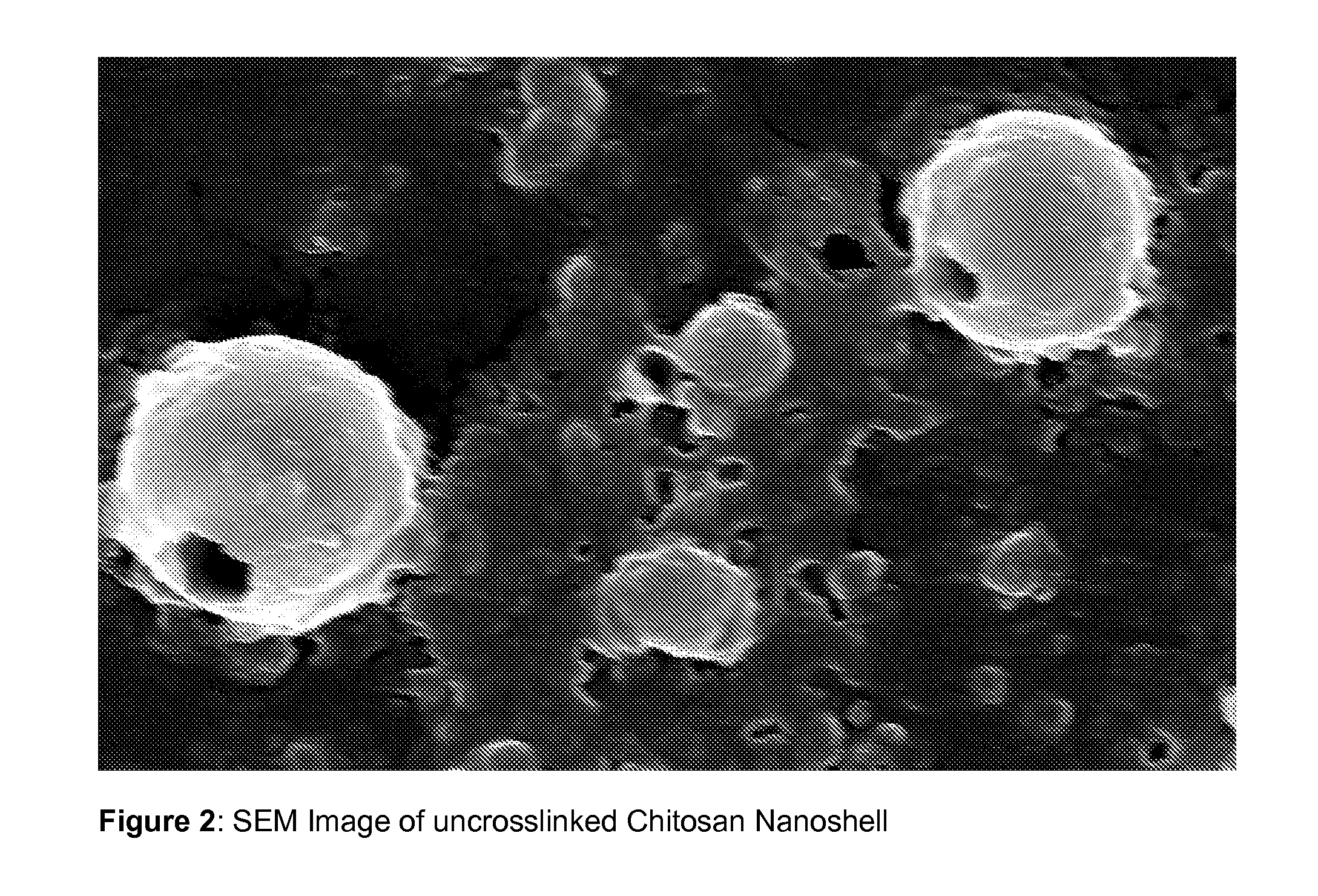
Hollow biodegradable nanospheres and nanoshells for delivery of therapeutic and/or imaging molecules
InactiveUS20110123456A1Increase payload capacityIncrease load capacityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsBiomedical engineering
A polymeric hollow nanoshell or nanosphere for release of an agent is described, wherein the hollow nanosphere comprises at least one biodegradable polymer, characterised in that the polymer is cross-linked. The biodegradable mono-disperse nanospheres described are suitable for use as carriers of biomolecules, therapeutic agents and / or imaging agents.
Owner:THE NAT UNIV OF IRELAND GALWAY
Cushioning wax beads for making solid shaped articles
InactiveUS6923984B1High tensile strengthSustained deliveryPowder deliveryPharmaceutical containersWaxCushioning
Biologically inactive cushioning beads comprise at least one compressible cushioning component consisting essentially of a microcrystalline hydrocarbon wax or a natural wax, the said wax being at least 30% by weight of the biologically inactive cushioning beads. Such beads are useful for making solid shaped articles containing biologically active ingredients by compression.
Owner:UNIV GENT
Acylated uridine and cytidine and uses thereof
ActiveUS6258795B1No untoward pharmaceutical effectEfficient managementBiocideSugar derivativesDiseaseDiabetes mellitus
The invention relates to compositions comprising acyl derivatives of cytidine and uridine. The invention also relates to methods of treating hepatopathies, diabetes, heart disease, cerebrovascular disorders, Parkinson's disease, infant respiratory distress syndrome and for enhancement of phospholipid biosynthesis comprising administering the acyl derivatives of the invention to an animal.
Owner:WELLSTAT THERAPEUTICS
Pharmaceutical formulations for sustained release
InactiveUS20060293217A1Sustained deliveryOrganic active ingredientsBiocideHigh concentrationActive component
Sustained delivery pharmaceutical compositions comprising a solid ionic complex of a pharmaceutically active compound and an ionic macromolecule are provided by the present invention. The pharmaceutical compositions of the invention allow for loading of high concentrations of pharmaceutically active compounds and for delivery of a pharmaceutically active compound for prolonged periods of time, e.g., one month, after administration. Methods for preparing these pharmaceutical compositions, as well as methods of using them to treat a subject are also provided.
Owner:PRAECIS PHARM INC
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20120157499A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaDisease
Owner:ALLERGAN INC
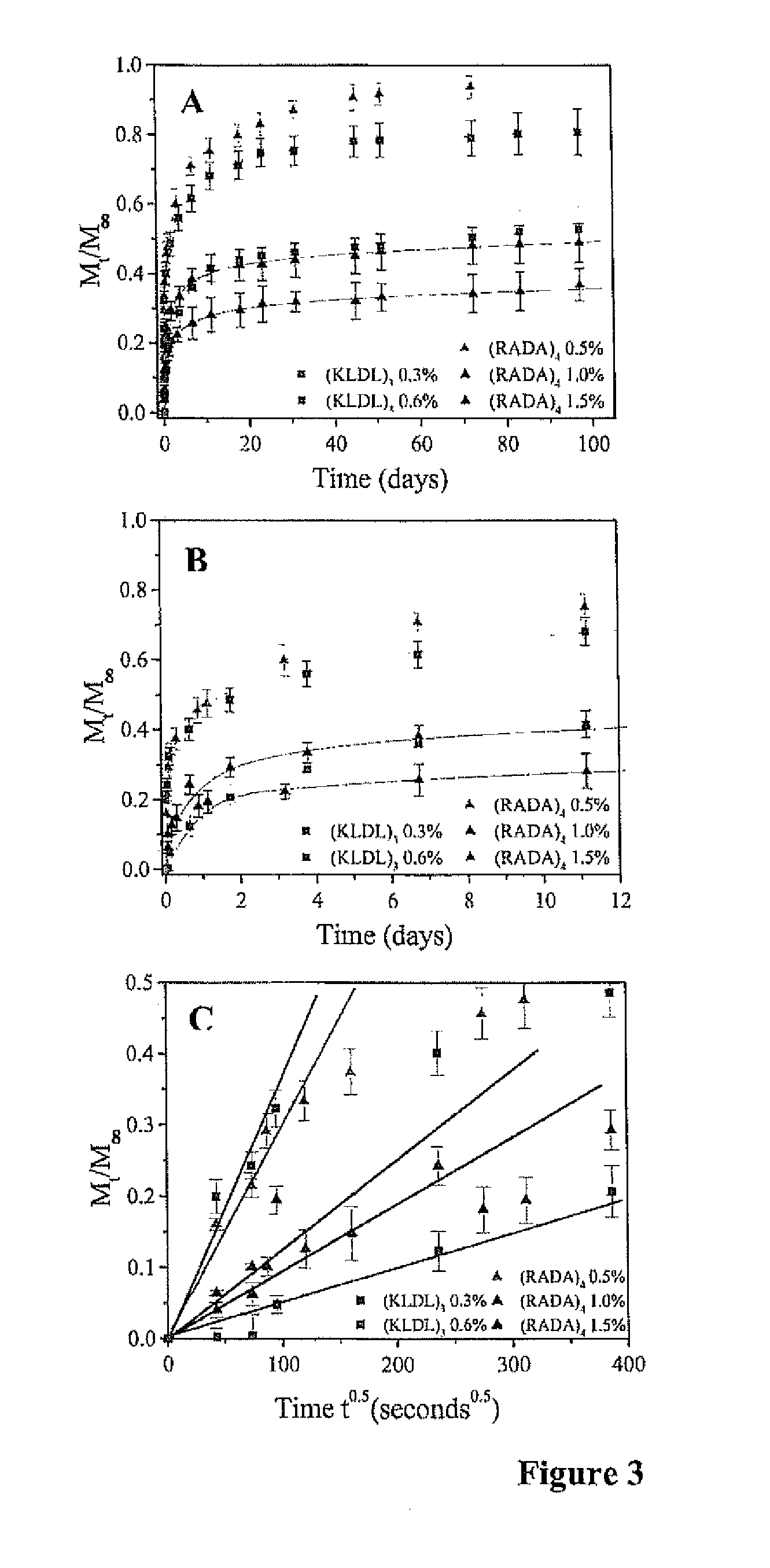
Multi-layered injectable self-assembling peptide scaffold hydrogels for long-term sustained release of human antibodies
ActiveUS20140302144A1Antibody diffusivities can be decreasedHigh densityPowder deliveryPeptide/protein ingredientsAntibody fragmentsPharmaceutical formulation
The invention relates to a pharmaceutical formulation for sustained delivery of a therapeutic agent, preferably a protein, polypeptide, an antibody or an antibody fragment, comprising one or more gel forming peptides wherein the formulation exhibits sustained delivery for at least two weeks, three weeks, four weeks, five weeks, six weeks, seven weeks, eight weeks, nine weeks, ten weeks, eleven weeks, twelve weeks or more. In one embodiment, the invention relates to a formulation comprising self-assembling peptides that undergo sol-gel transition in the presence of an electrolyte solution such as biological fluids and salts. The formulation can provide sustained release of antibody and antibody fragments, in particular, IgG. Antibody diffusivities can be decreased with increasing hydrogel nanofiber density, providing a means to control the release kinetics.
Owner:MASSACHUSETTS INST OF TECH
Acylated uridine and cytidine and uses thereof
InactiveUS6274563B1No untoward pharmaceutical effectEfficient managementBiocideSugar derivativesDiseaseDiabetes mellitus
The invention relates to compositions comprising acyl derivatives of cytidine and uridine. The invention also relates to methods of treating hepatopathies, diabetes, heart disease, cerebrovascular disorders, Parkinson's disease, infant respiratory distress syndrome and for enhancement of phospholipid biosynthesis comprising administering the acyl derivatives of the invention to an animal.
Owner:WELLSTAT THERAPEUTICS
Pharmaceutical formulations for sustained release
InactiveUS20060198815A1Sustained deliveryBiocideNervous disorderHigh concentrationPharmaceutical formulation
Sustained delivery pharmaceutical compositions comprising a solid ionic complex of a pharmaceutically active compound and an ionic macromolecule are provided by the present invention. The pharmaceutical compositions of the invention allow for loading of high concentrations of pharmaceutically active compounds and for delivery of a pharmaceutically active compound for prolonged periods of time, e.g., one month, after administration. Methods for preparing these pharmaceutical compositions, as well as methods of using them to treat a subject are also provided.
Owner:GLAXO SMITHKLINE LLC
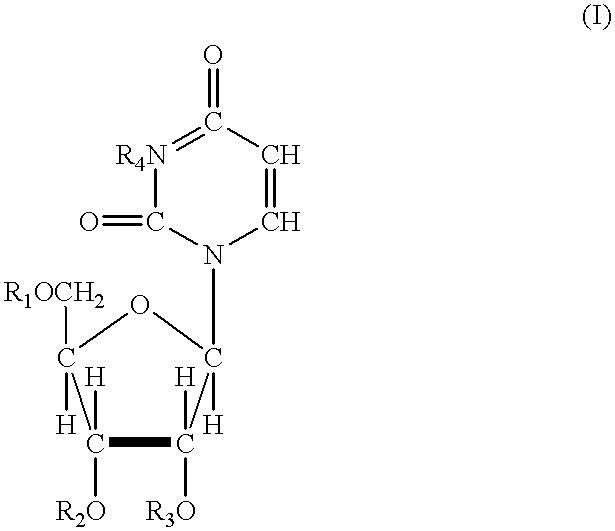
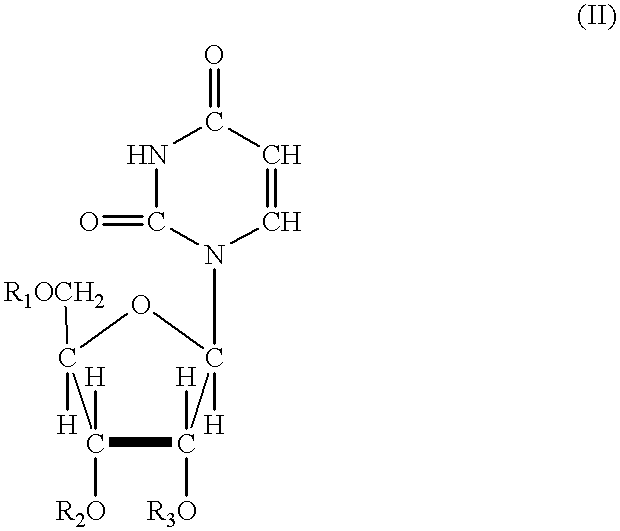
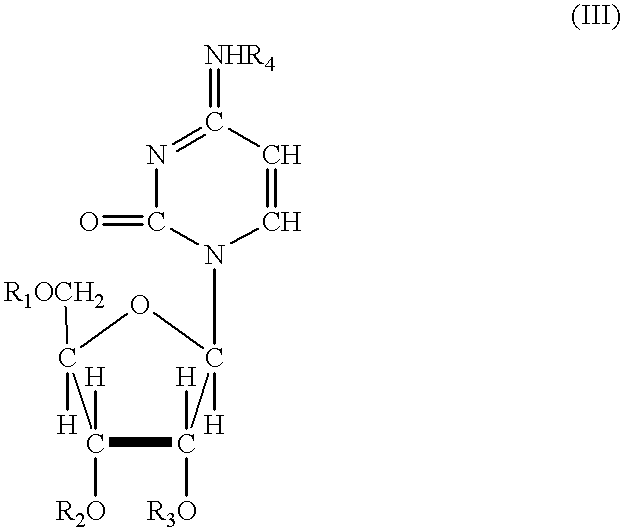
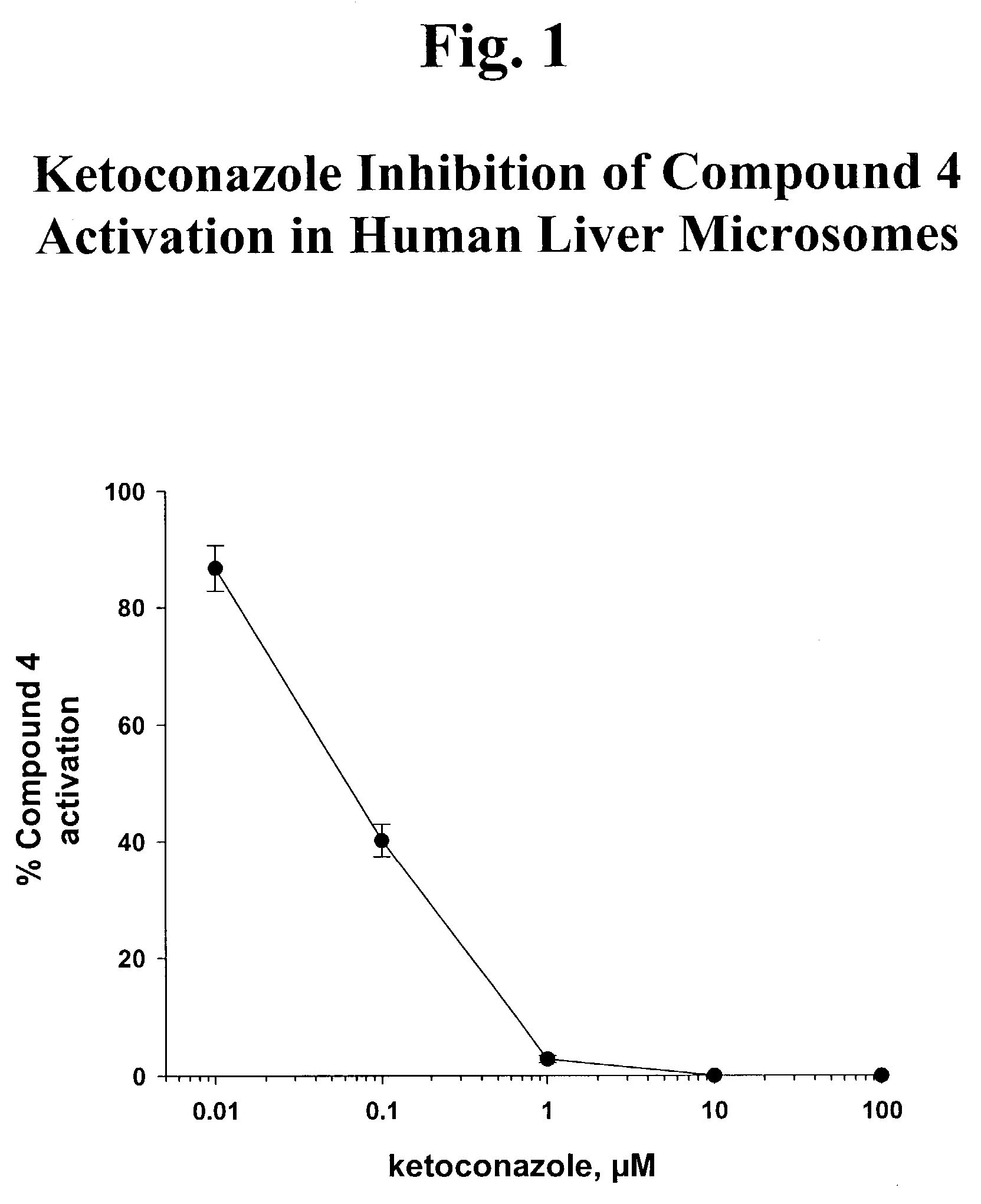
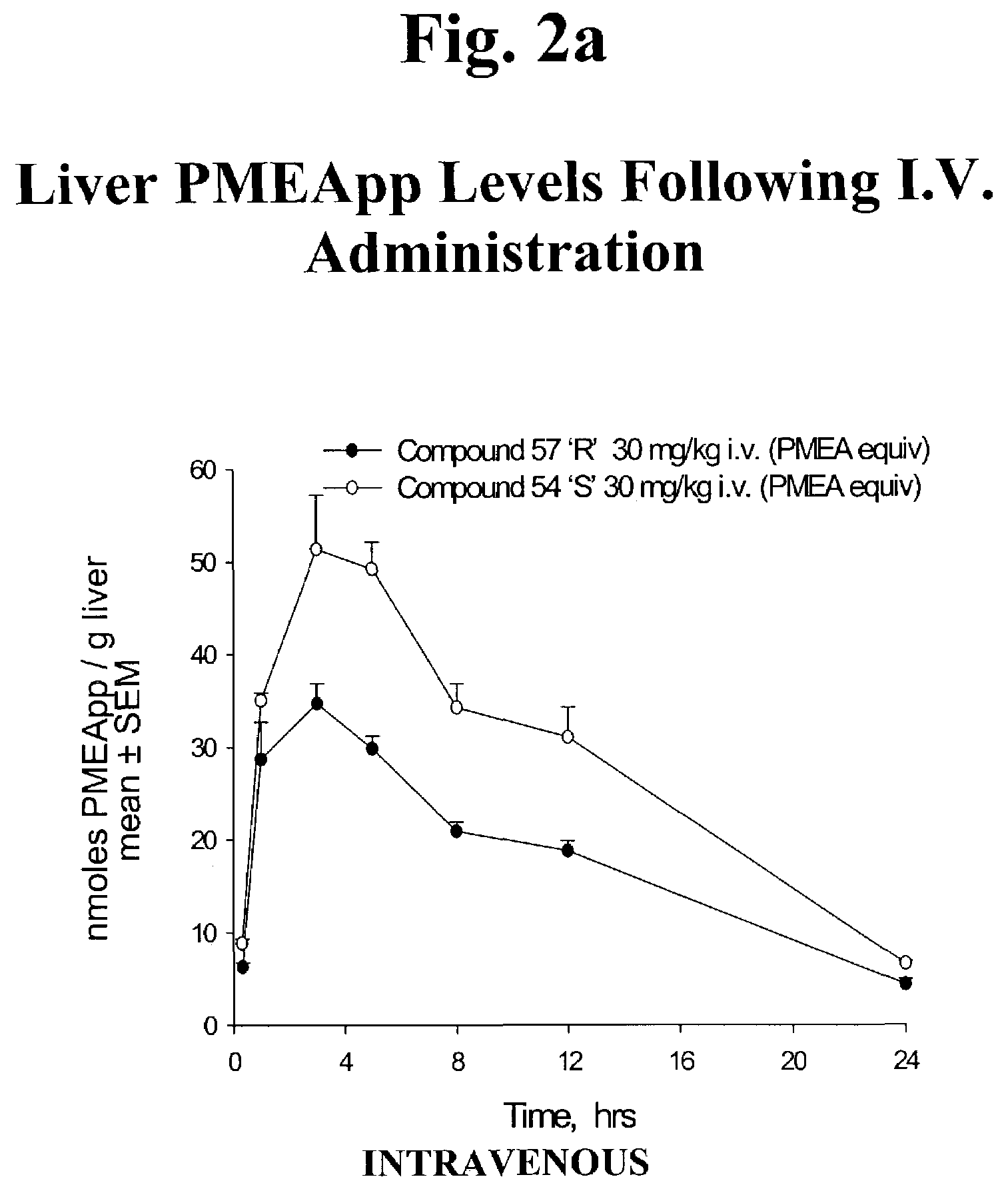
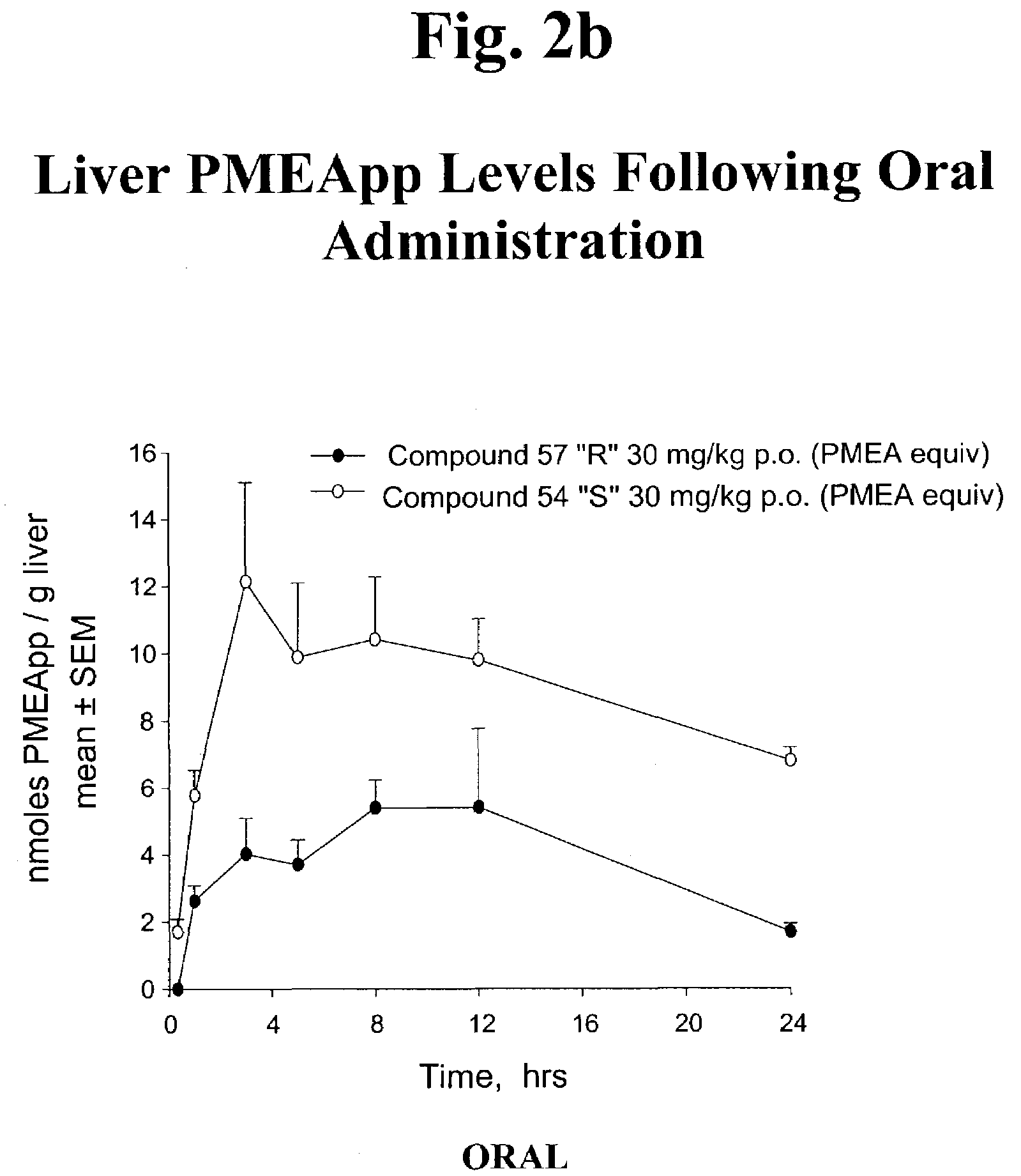
Phosphonic acid based prodrugs of PMEA and its analogues
InactiveUS7214668B2Enhanced drug distributionExtended half-lifeOrganic active ingredientsBiocide2,6-DiaminopurineAlkyl
Prodrugs of Formula I, their uses, their intermediates, and their method of manufacture are described:wherein:M and V are cis to one another and MPO3H2 is a phosphonic acid selected from the group consisting of 9-(2-phosphonylmethoxyethyl)adenine, (R)-9-(2-phosphonylmethoxy propyl)adenine, 9-(2-phosphonylmethoxyethyl)guanine, 9-(2-phosphonylmethoxy ethyloxy)adenine, 9-(2-phosphonylmethoxyethyl)-2,6-diaminopurine, (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine, 9-(3-hydroxy-2-phosphonylmethoxypropyl)guanine, and (S)-9-(3-fluoro-2-phosphonyl methoxypropyl)adenine;V is selected from a group consisting of phenyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-furanyl, 3-furanyl, 2-thienyl, and 3-thienyl, all optionally substituted with 1-3 substituents selected from a group consisting of F, Cl, Br, C1-C3 alkyl, CF3 and OR6;R6 is selected from the group consisting of C1-C3 alkyl, and CF3;and pharmaceutically acceptable salts thereof.
Owner:METABASIS THERAPEUTICS INC
Method of treating neurodegenerative brain disease with a composite comprising superparamagnetic nanoparticles and a therapeutic compound
InactiveUS20080014285A1High retention rateIncrease volumeHeavy metal active ingredientsOrganic active ingredientsCompound aInjury brain
A method of treating brain injury involving intrathecally administering a composite powder comprising superparamagentic nanoparticle and a therapeutic agent compound, and then magnetically transporting the composite into an injured brain.
Owner:DEPUY SYNTHES PROD INC
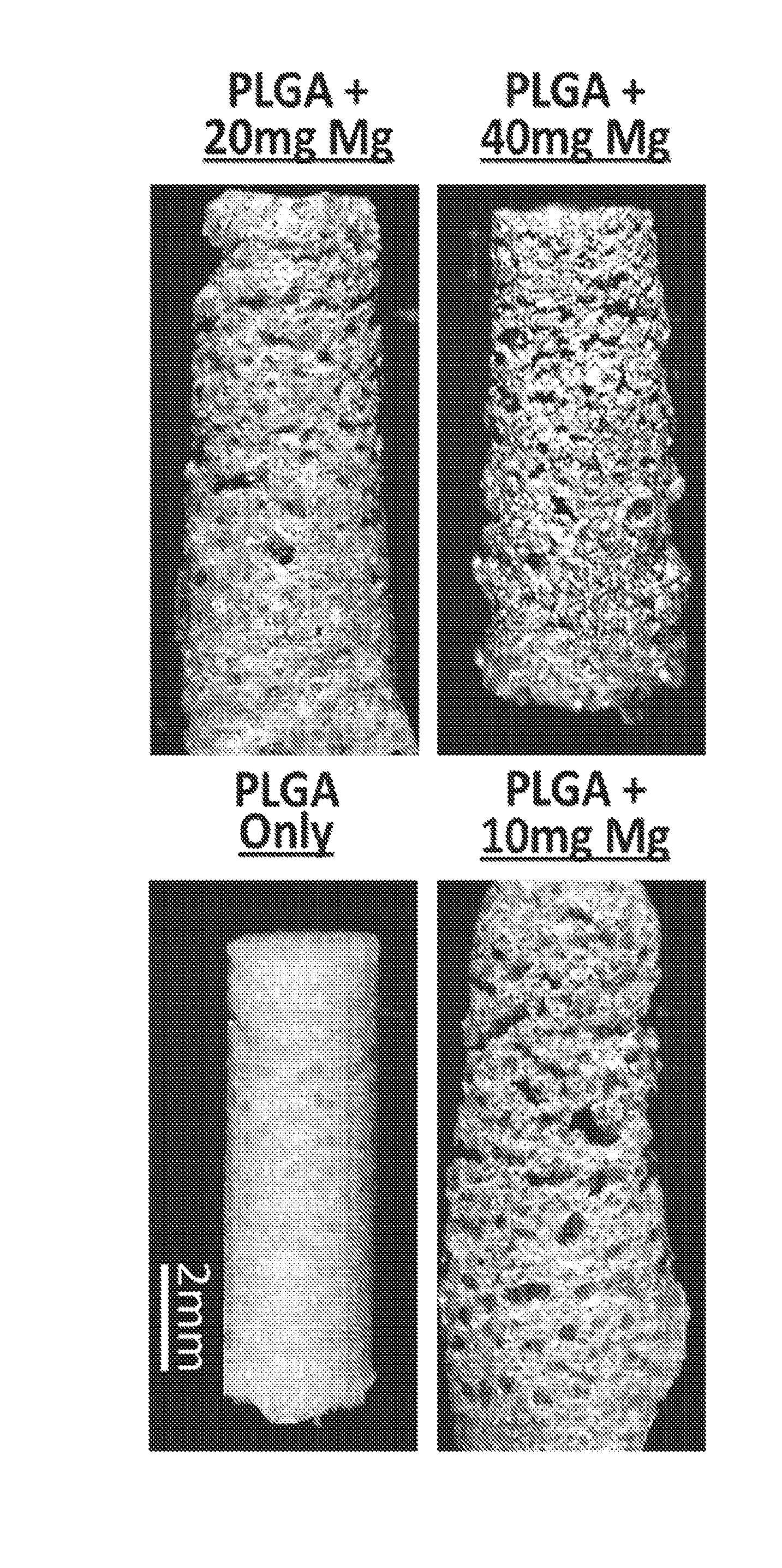
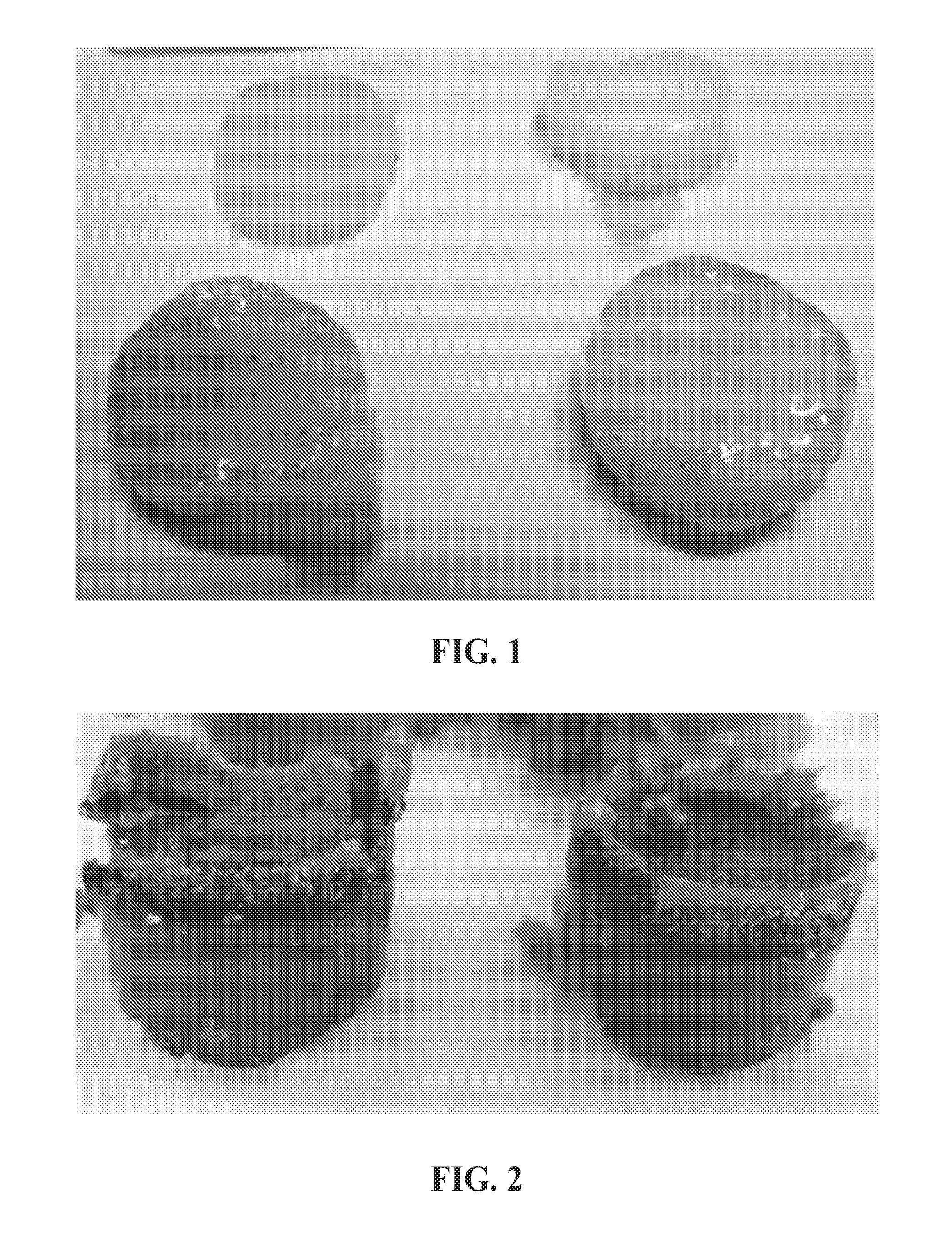
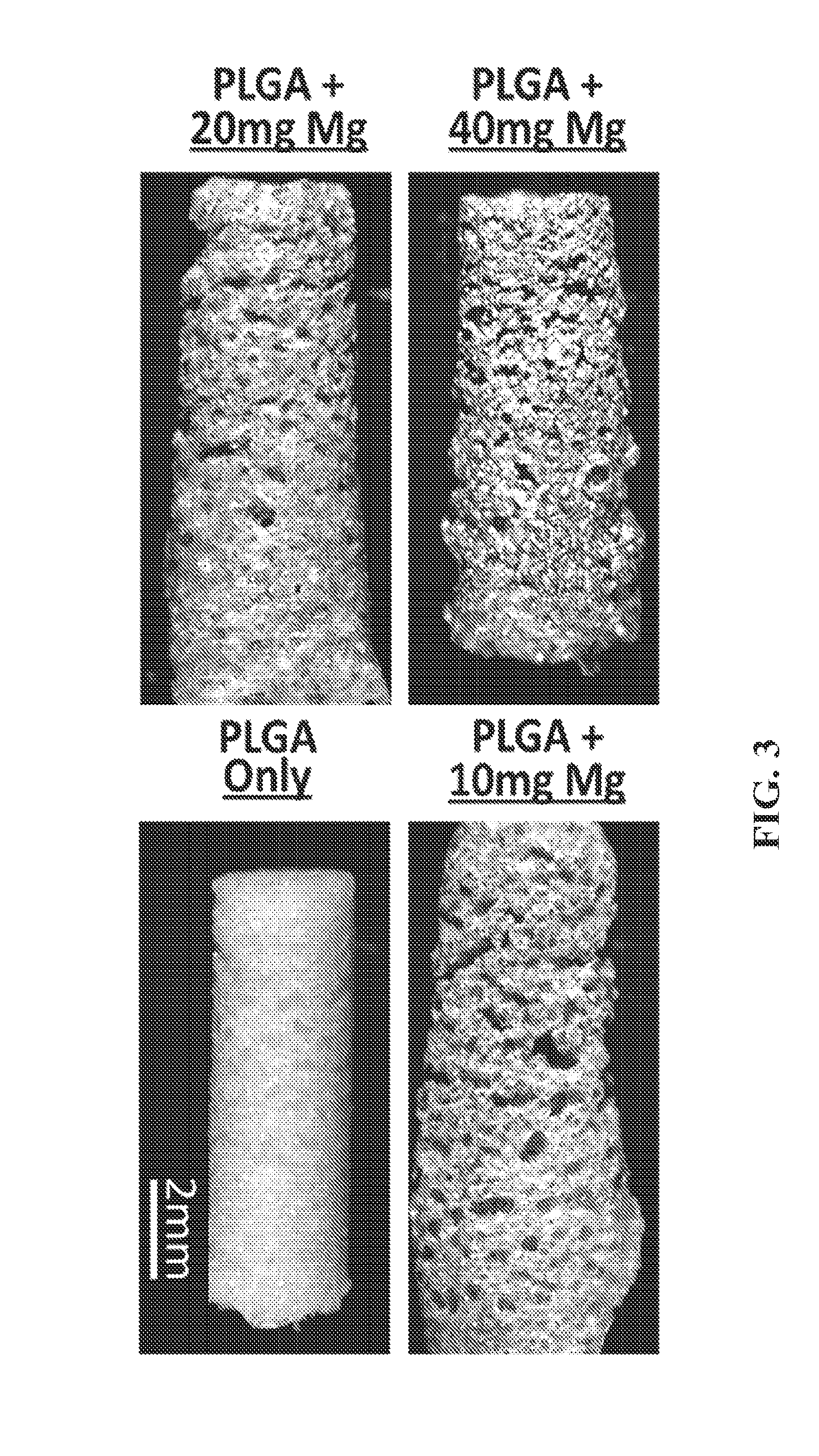
Magnesium/polymer composite-containing scaffolds to enhance tissue regeneration
The invention relates to magnesium-polymer composites, methods for their preparation and applications for their use. The composites include a combination of magnesium particles and polymer matrix. The polymer can include, but is not limited to, poly(lactic co-glycolic) acid. In certain embodiments, the composites of the invention are particularly useful for forming medical devices for implantation into a body of a patient. In certain other embodiments, the magnesium-polymer composites are useful for wound healing compositions for administration to an exterior surface of a body of a patient.
Owner:UNIVERSITY OF PITTSBURGH
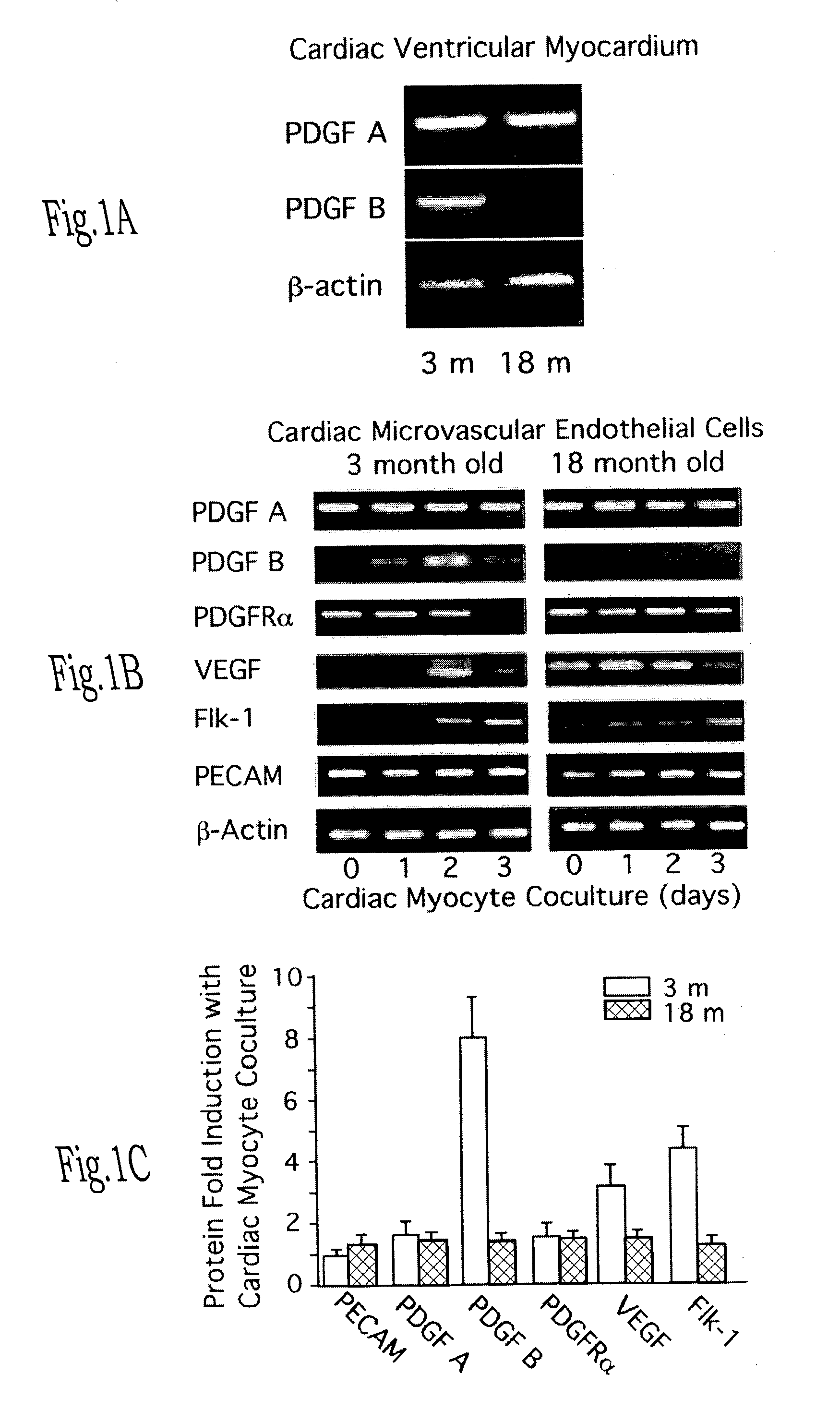
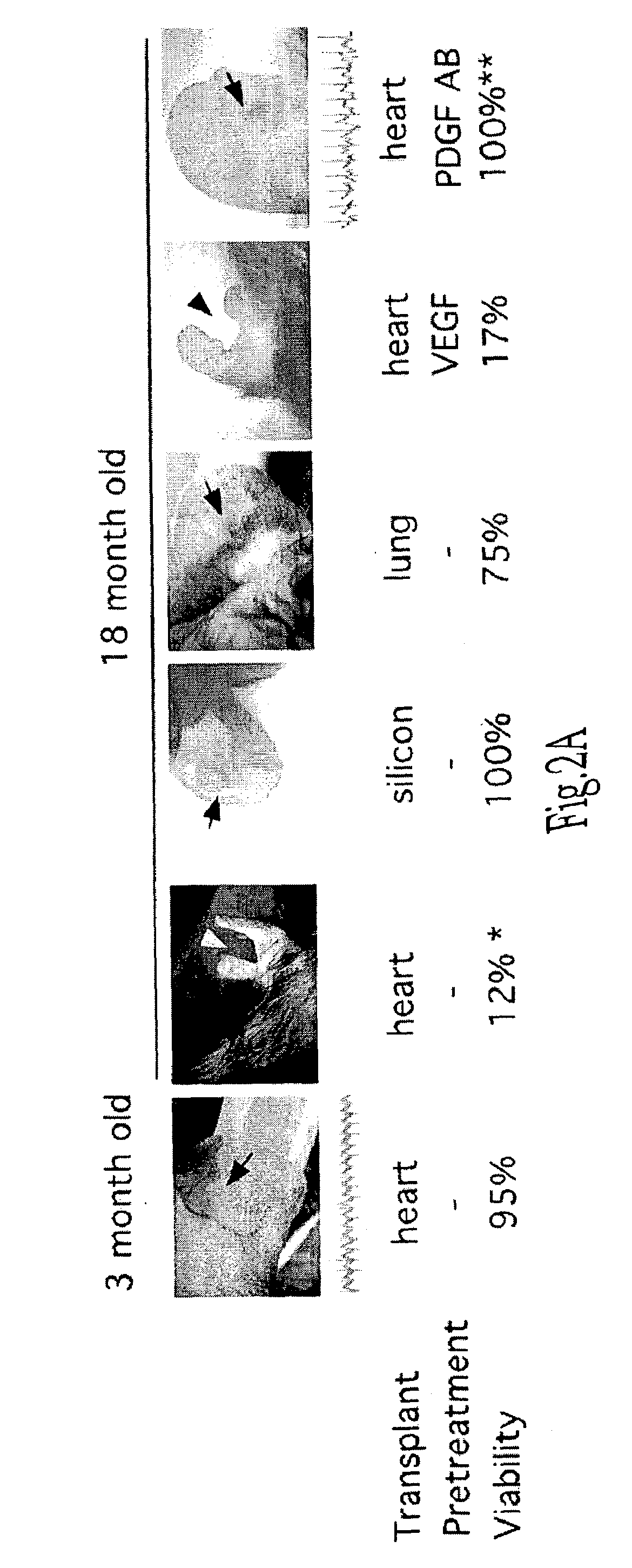
Endothelial precursor cells for enhancing and restoring vascular function
InactiveUS7135171B2Reverses effectRestore angiogenesisBiocidePeptide/protein ingredientsBlood vesselVascular function
Owner:CORNELL RES FOUNDATION INC
Method for treating insulin sensitivity by long-acting GLP-1 receptor mimetibody agonists
ActiveUS7833531B2Reduce food intake and body weightImproved profileAntibacterial agentsSenses disorderAgonistObesity
Owner:CENTOCOR
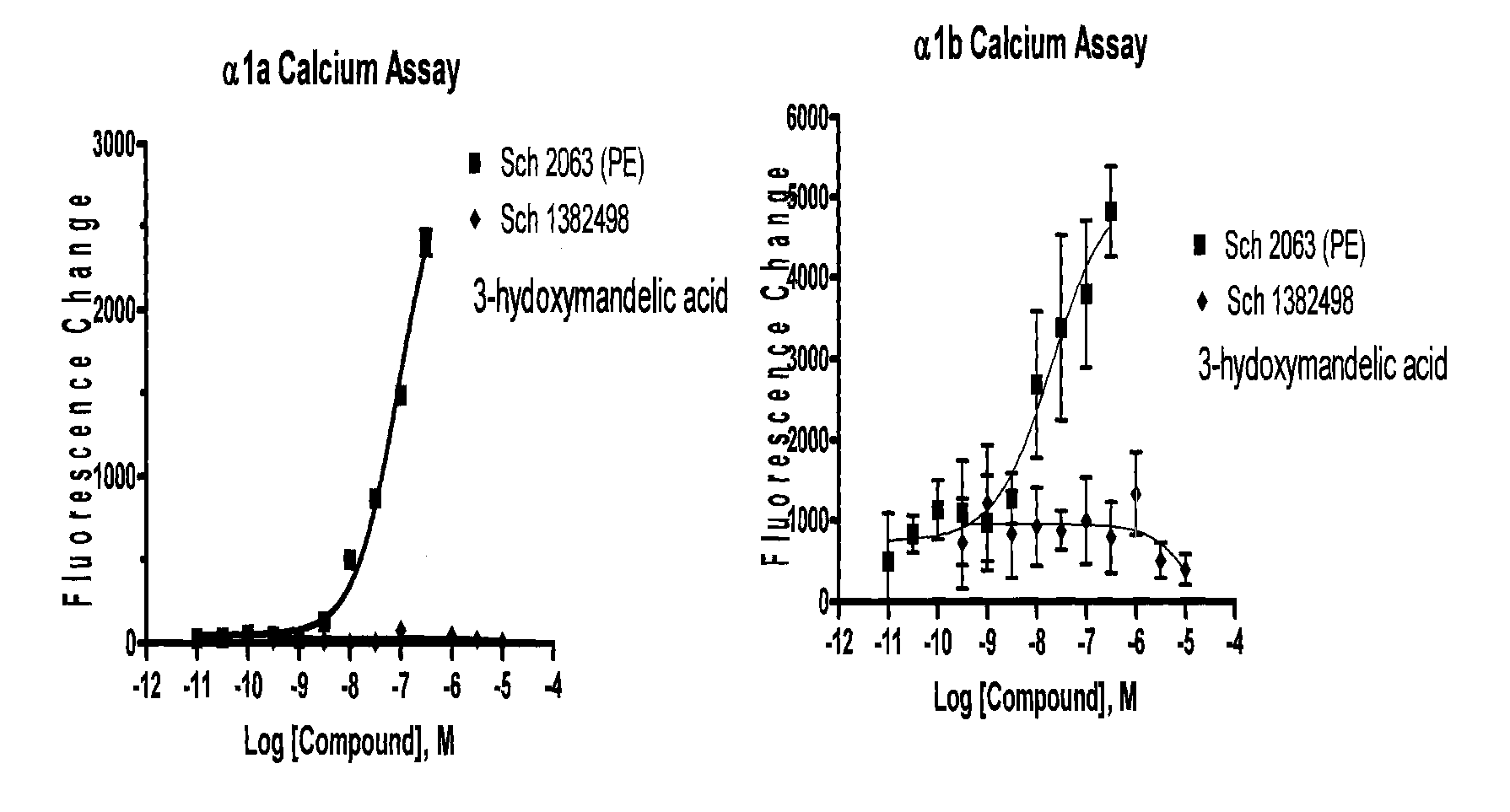
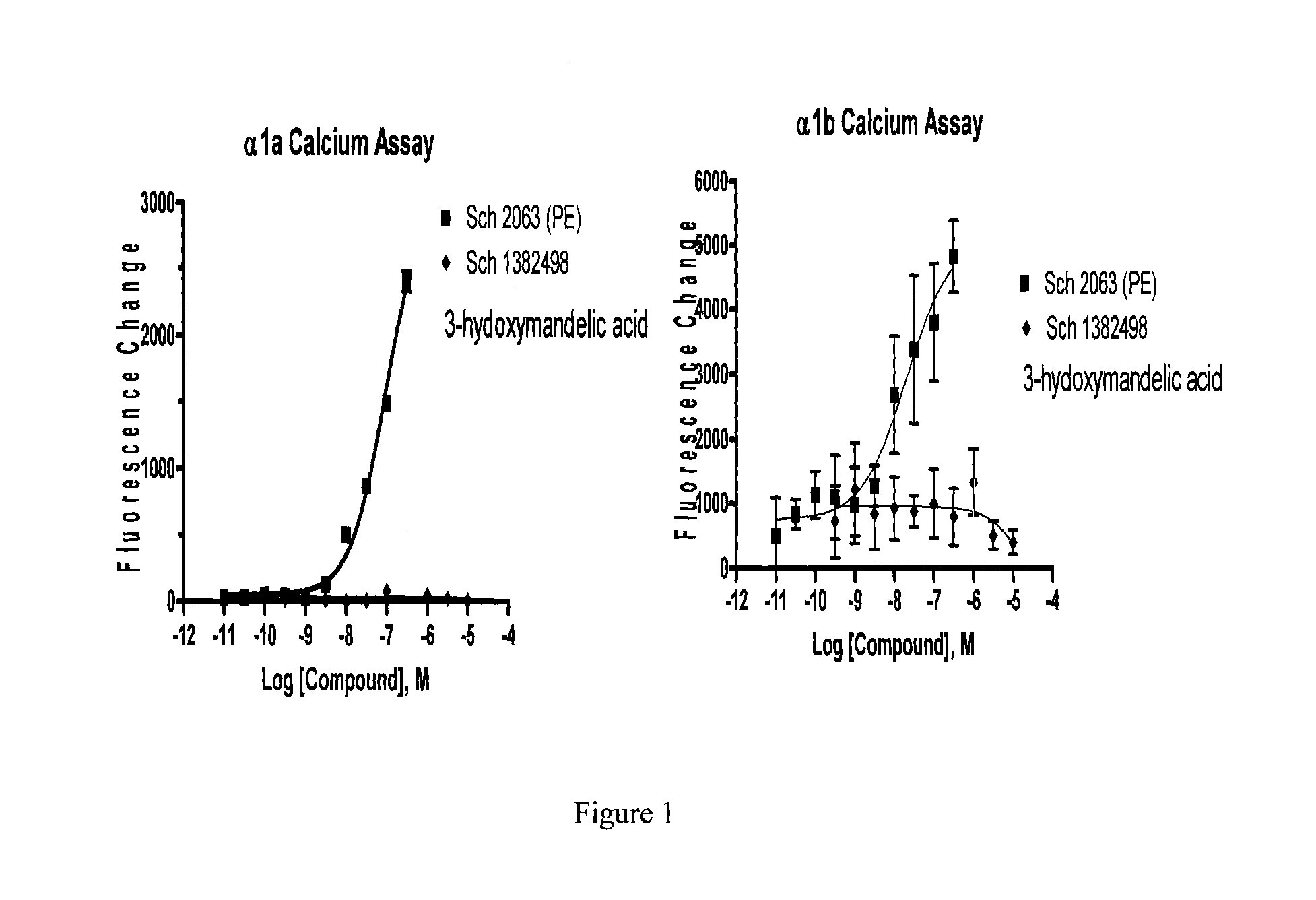
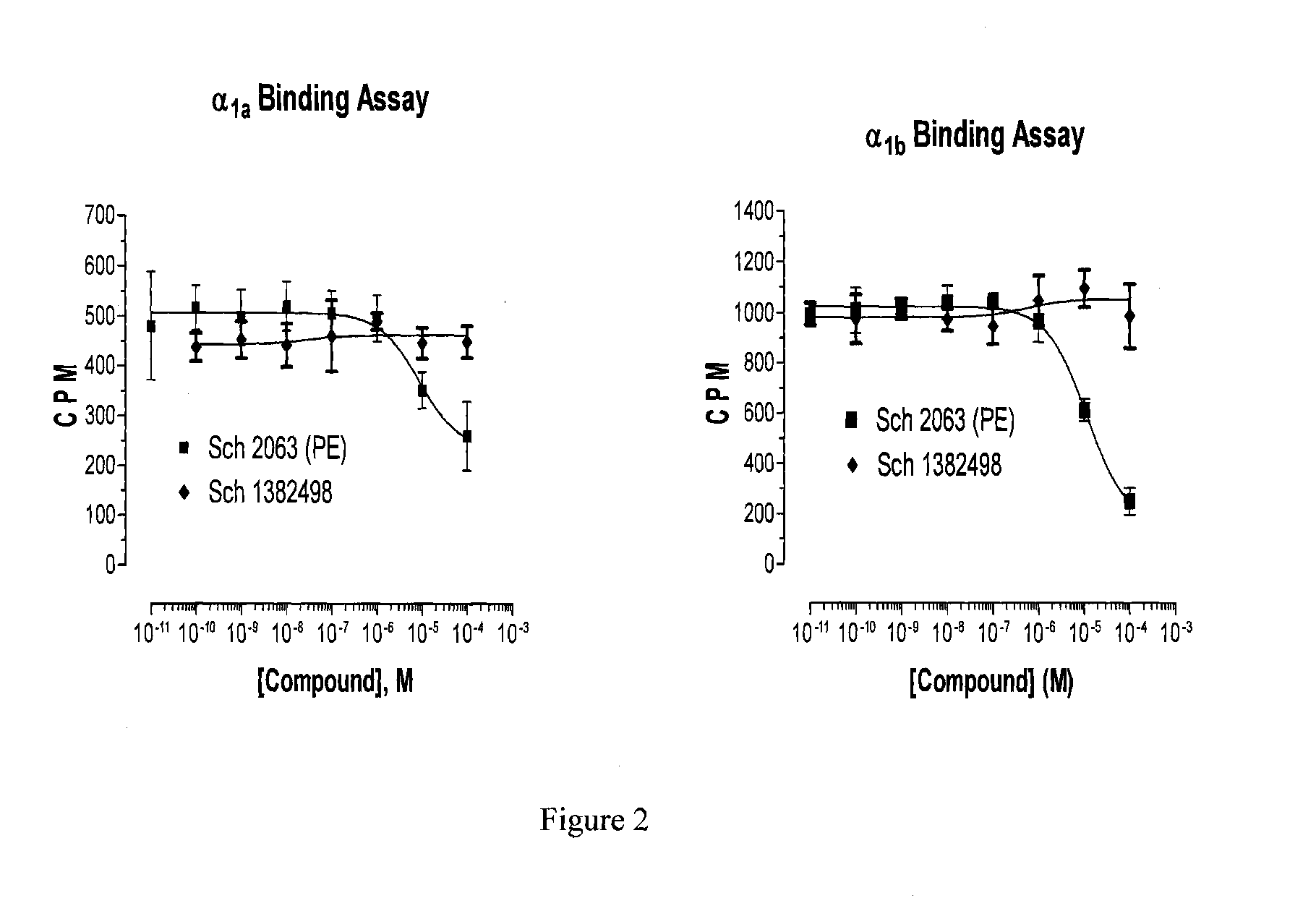
Phenylephrine pharmaceutical formulations and compositions for transmucosal absorption
InactiveUS20090280160A1Sustained deliveryBiocideOrganic active ingredientsWhole bodySystemic absorption
Pharmaceutical compositions comprising phenylephrine or a pharmaceutically acceptable salt thereof and methods for administering the pharmaceutical compositions wherein the composition is formulated for systemic absorption of phenylephrine that avoids first pass metabolism. The compositions of the invention are formulated to be applied to oral mucosa of an animal to allow for enhanced systemic delivery of therapeutically active form of phenylephrine.
Owner:MSD CONSUMER CARE INC
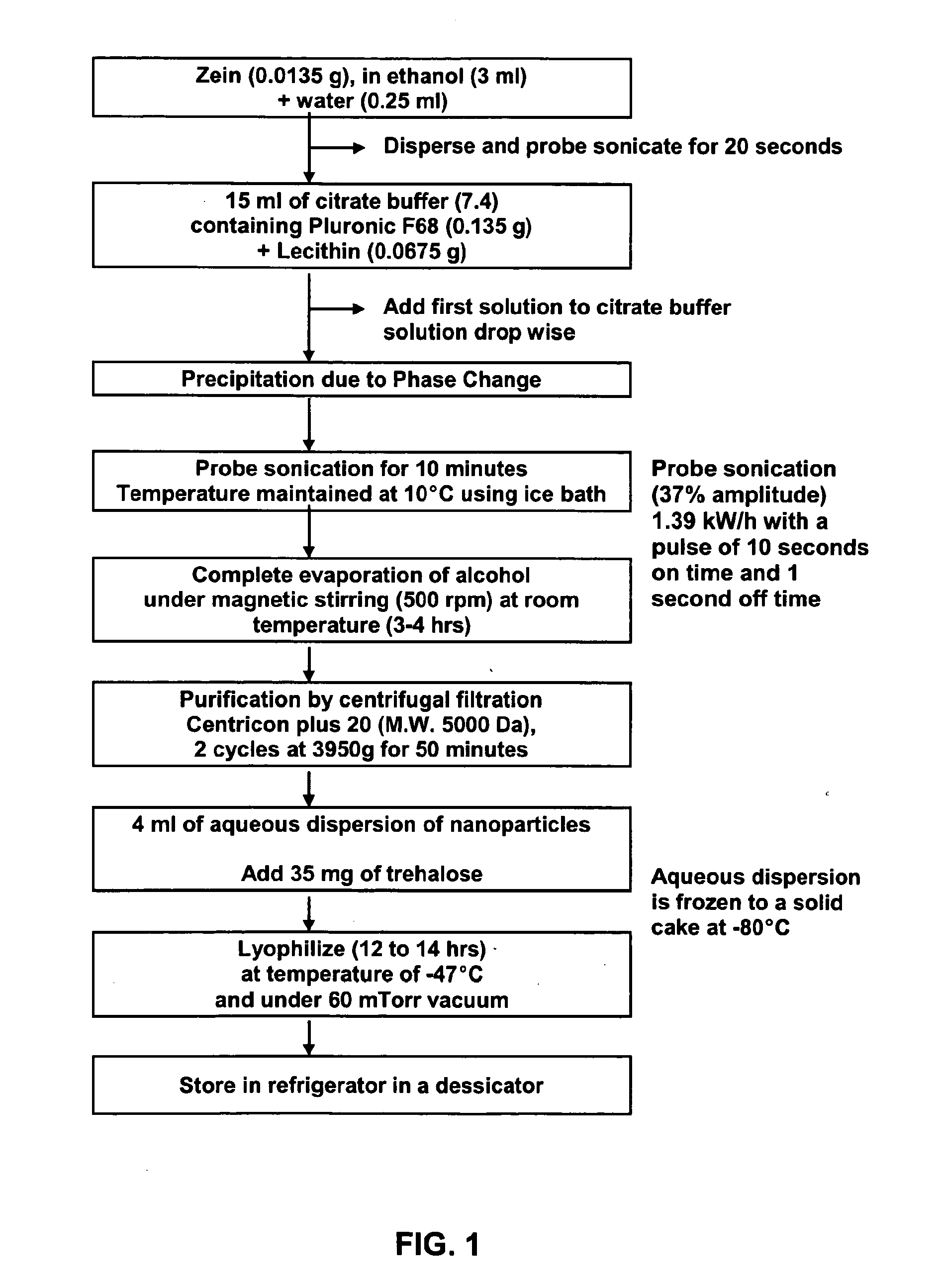
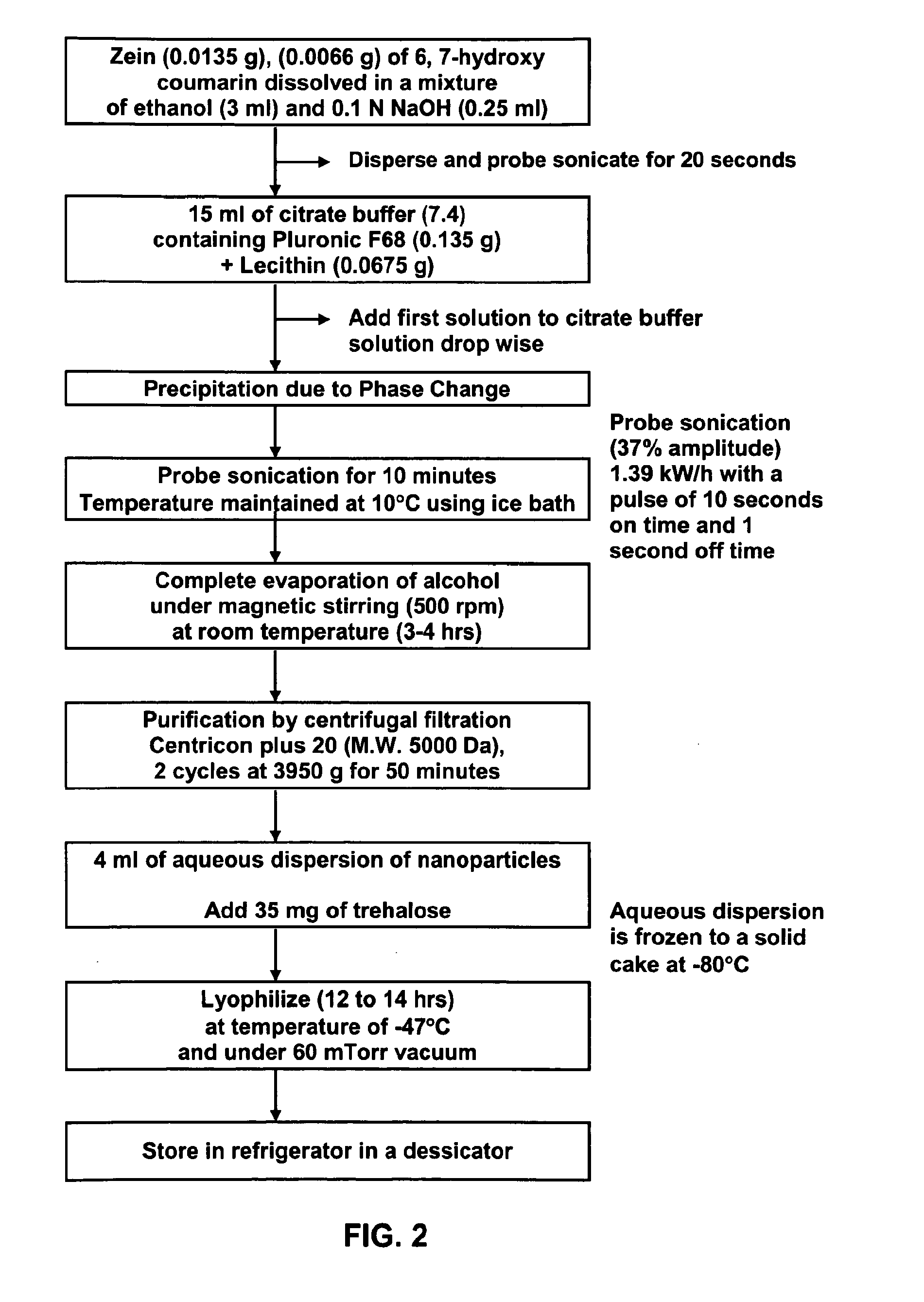
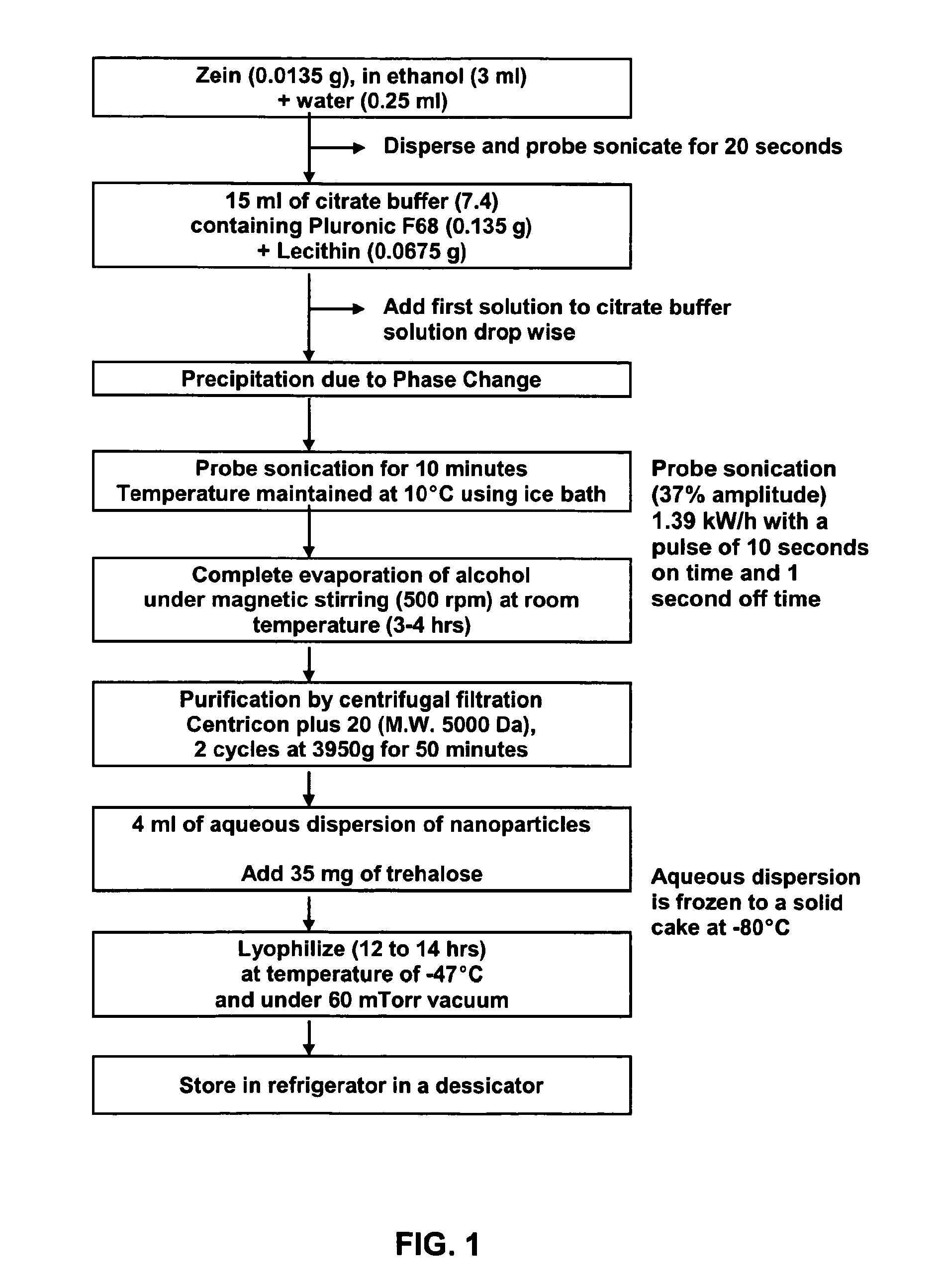
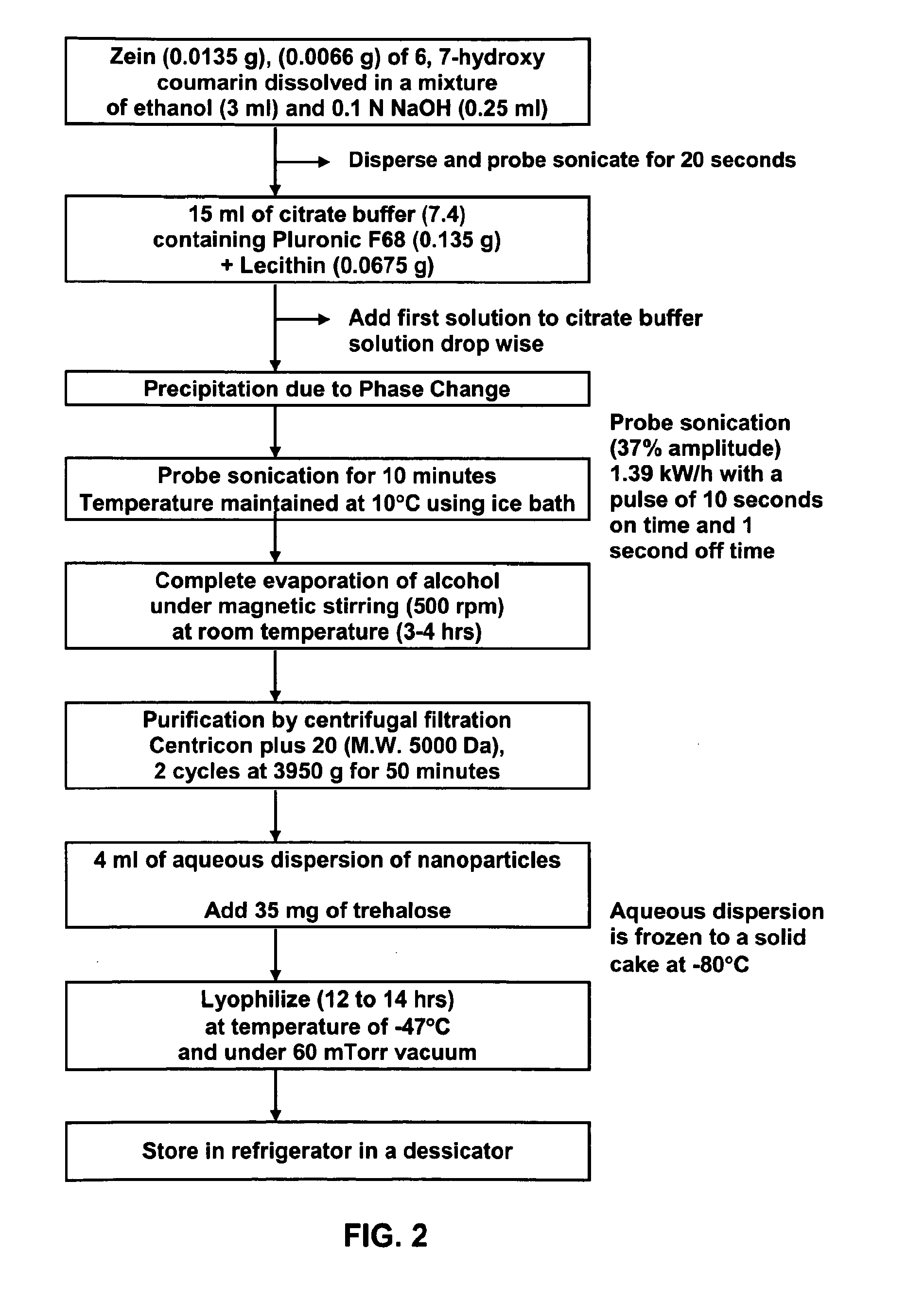
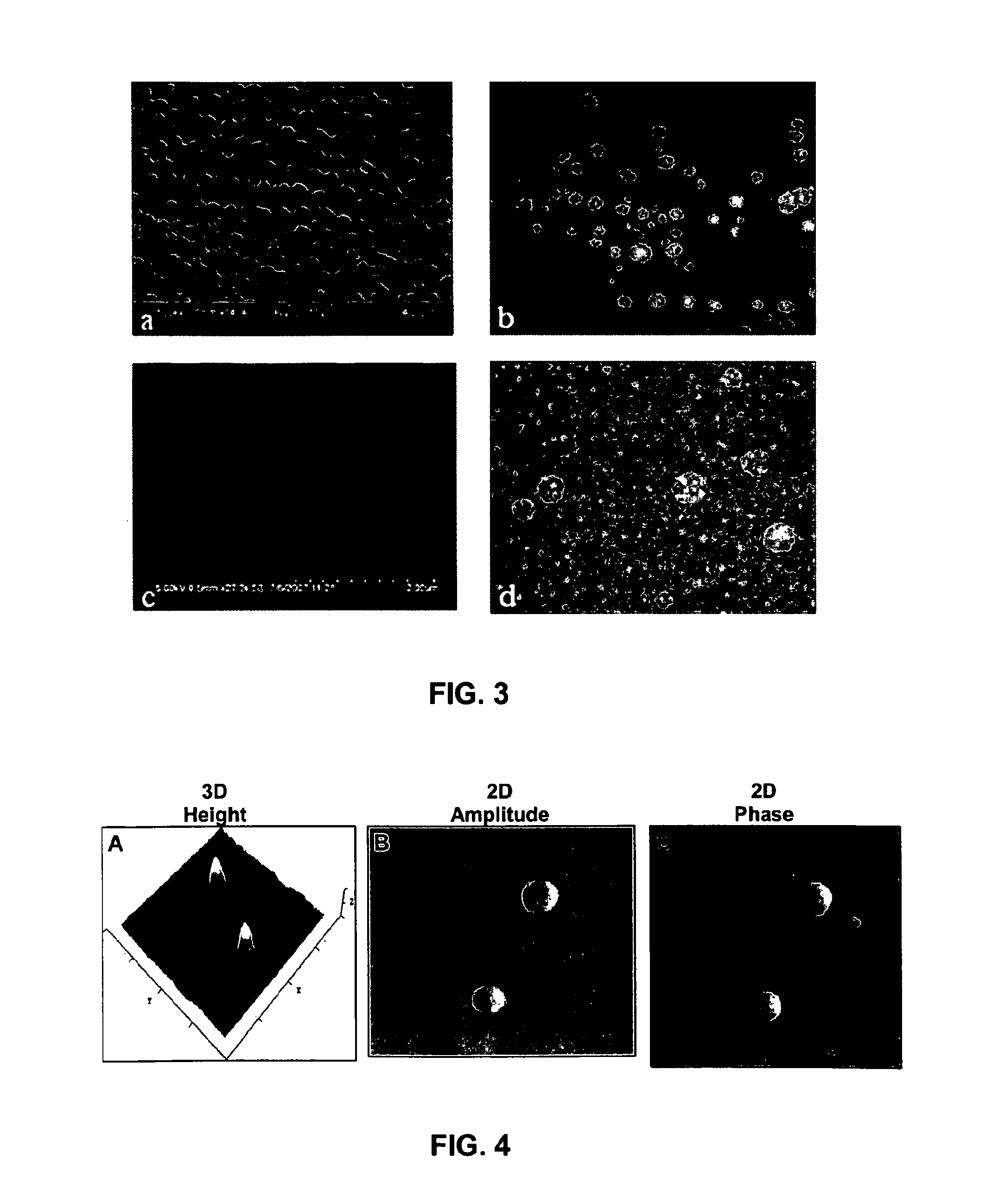
Method of forming non-immunogenic hydrophobic protein nanoparticles and uses therefor
Methods are described for producing non-immunogenic nanoparticles from protein sources by controlling the pH in a nanoprecipitation process. The nanoparticles that are produced by the disclosed methods range in diameter size from about 100 ran to about 400 nm, with a preferred diameter size of from approximately 100 nm to approximately 300 nm, thereby rendering them non-immunogenic. The invention further discloses methods for producing nanoconjugates that are suitable for a variety of therapeutic, diagnostic and other uses.
Owner:SOUTH DAKOTA STATE UNIVERSITY
Hollow biodegradable nanospheres and nanoshells for delivery of therapeutic and/or imaging molecules
InactiveUS20140004251A1Increase payload capacityIncrease load capacityPowder deliveryMicroencapsulation basedCross-linkImaging agent
A polymeric hollow nanoshell or nanosphere for release of an agent is described, wherein the hollow nanosphere comprises at least one biodegradable polymer, characterised in that the polymer is cross-linked. The biodegradable mono-disperse nanospheres described are suitable for use as carriers of biomolecules, therapeutic agents and / or imaging agents.
Owner:THE NAT UNIV OF IRELAND GALWAY
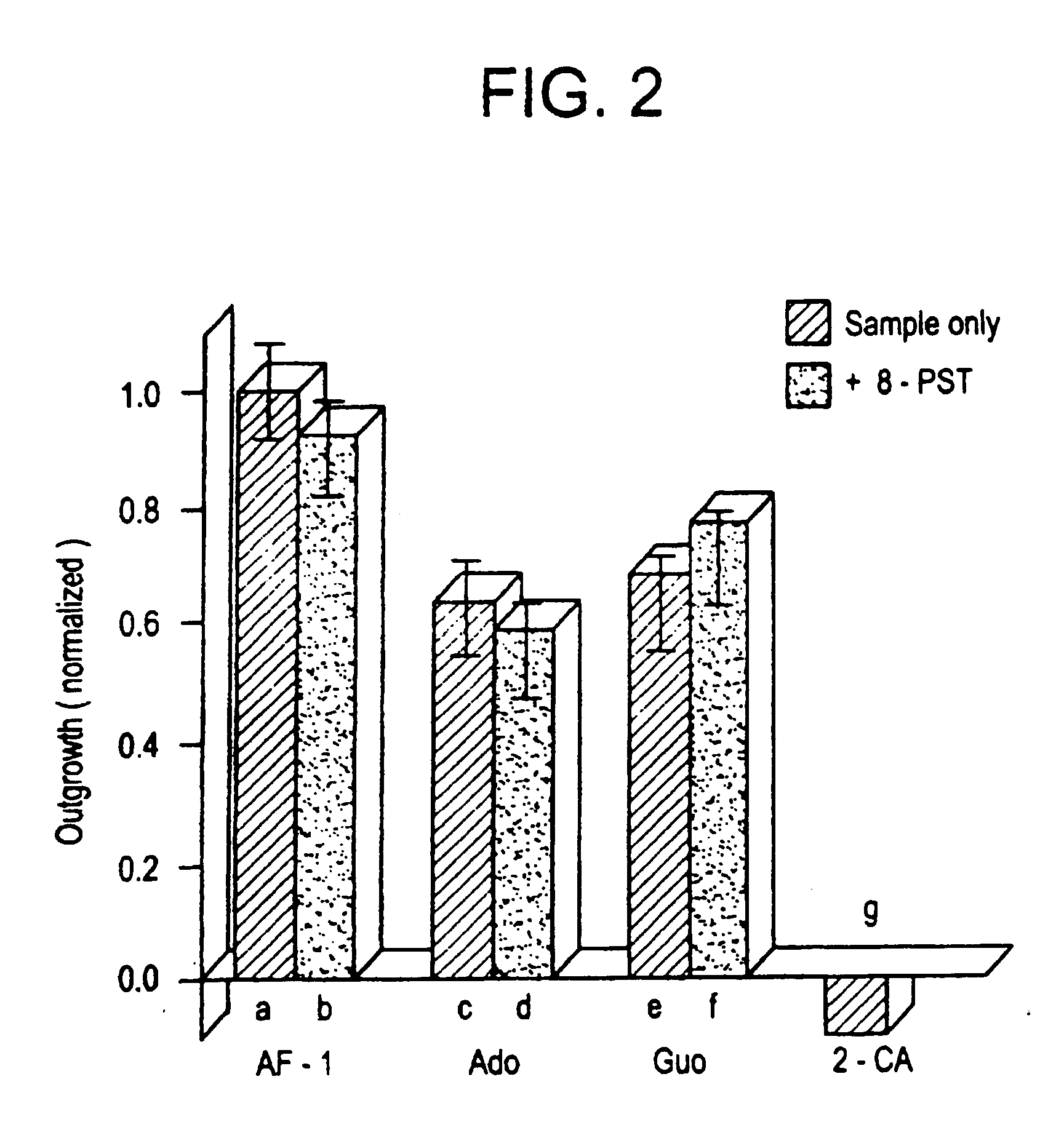
Methods for modulating the axonal outgrowth of central nervous system neurons
Methods for modulating the axonal outgrowth of central nervous system neurons are provided. Methods for stimulating the axonal outgrowth of central nervous system neurons following an injury (e.g., stroke, Traumatic Brain Injury, cerebral aneurism, spinal cord injury and the like) and methods for inhibiting the axonal outgrowth of central nervous system neurons are also provided. Finally, a packed formulation comprising a pharmaceutical composition comprising an inosine nucleoside and a pharmaceutically acceptable carrier packed with instructions for use of the pharmaceutical composition for treatment of a central nervous system disorder is provided.
Owner:CHILDRENS MEDICAL CENT CORP
Method of forming non-immunogenic hydrophobic protein nanoparticles and uses therefor
Owner:SOUTH DAKOTA STATE UNIVERSITY
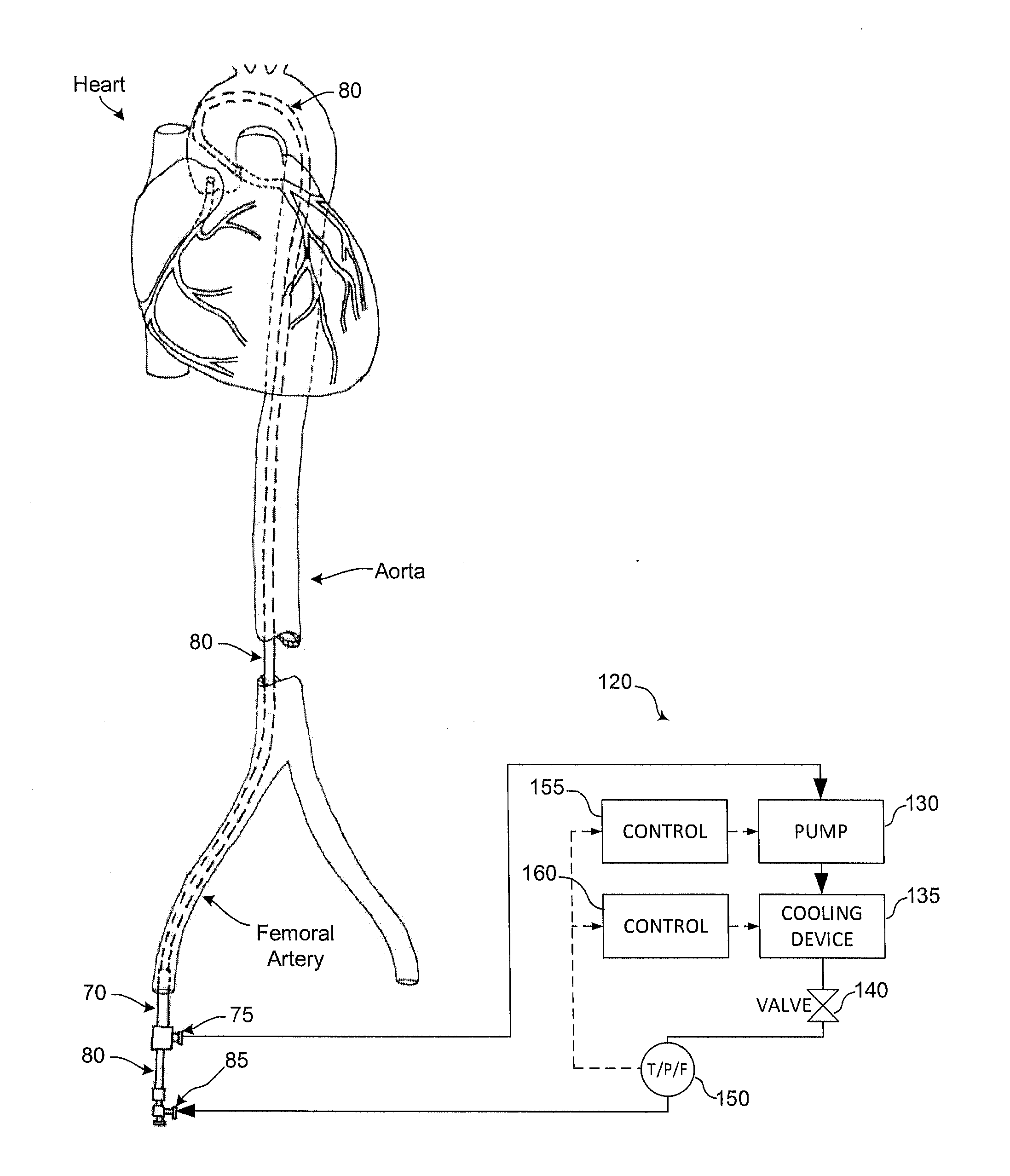
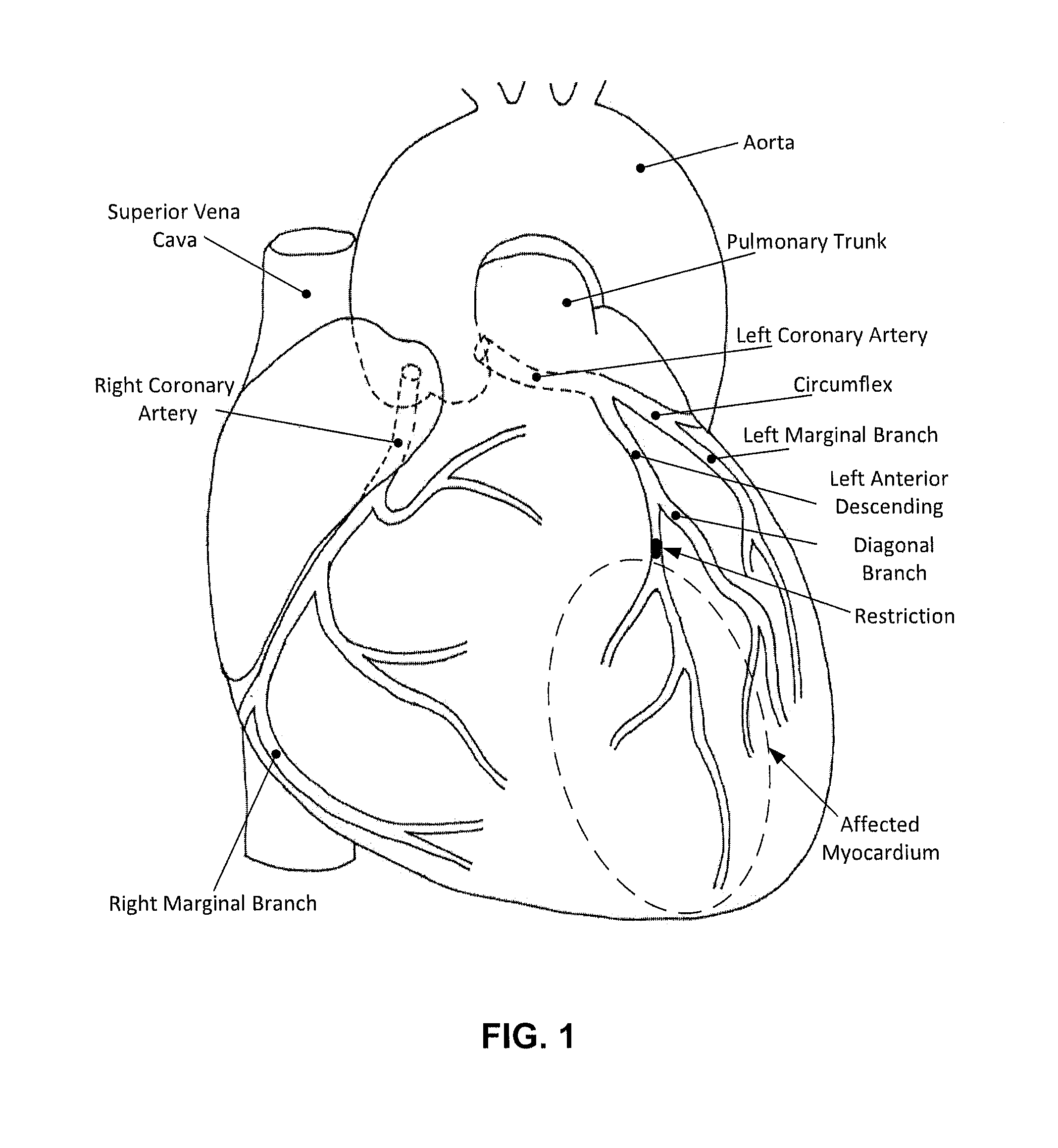
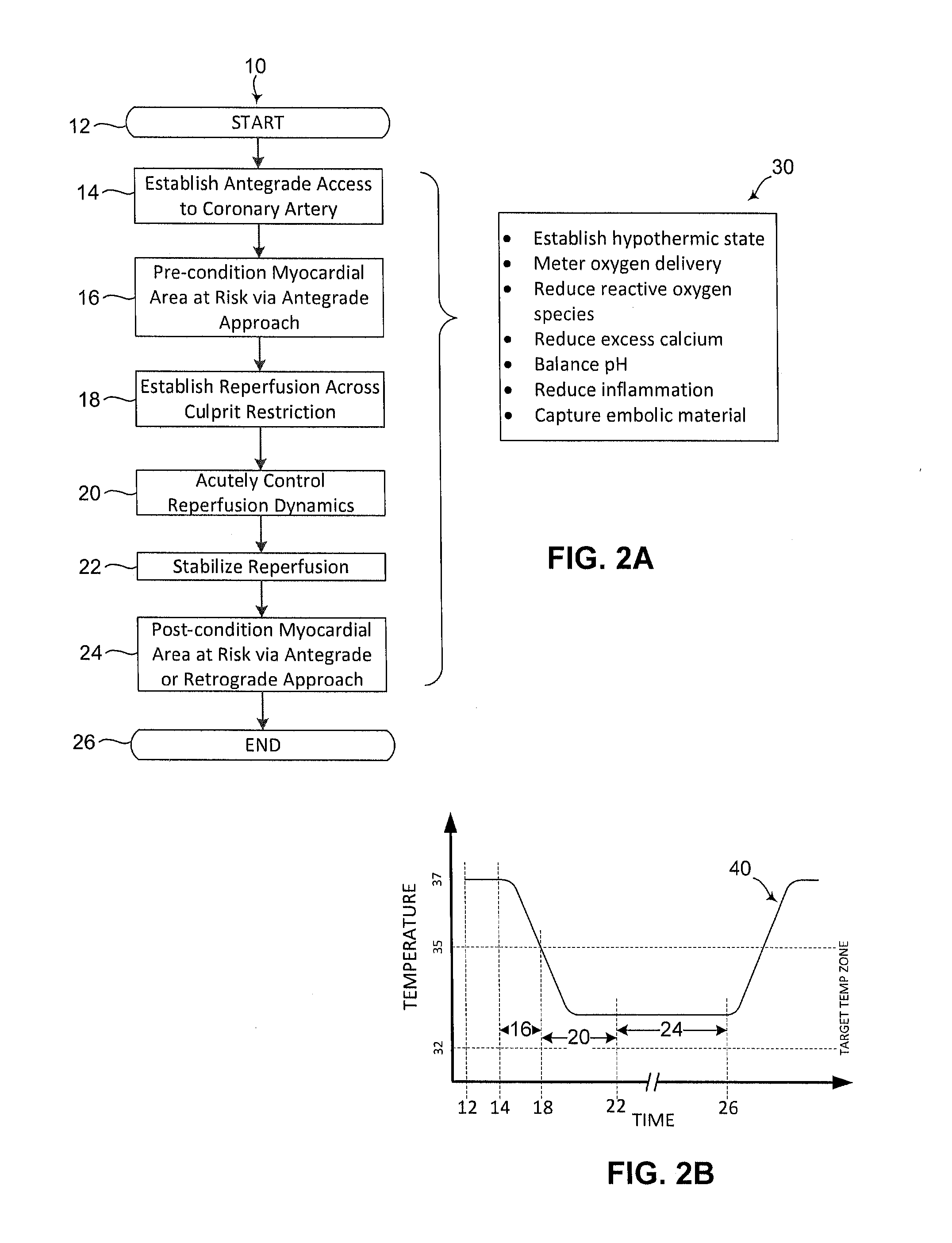
Devices and methods to reduce myocardial reperfusion injury
InactiveUS20140025143A1Reduce probabilityReduce presenceMedical devicesTherapeutic coolingCardiac muscleCvd risk
Devices and methods that mitigate reperfusion injury (RI) in a clinically practical manner so as to avoid significantly increasing time to reperfusion. In general, these systems and methods involve an antegrade approach to deliver a fluid to the myocardium at risk of RI before, during and after reperfusion is established by a percutaneous coronary intervention such as aspiration and stenting.
Owner:PROSPEX MEDICAL III
Bioadhesive composition and patch
InactiveUS8173113B1Sustained deliveryReduce lossesAbsorbent padsSynthetic polymeric active ingredientsBioadhesiveTraditional medicine
A bioadhesive composition that adheres suitably to a mucosal surface and is capable of delivering drugs in sustained fashion, and a patch comprising the bioadhesive composition. Methods of using and processes for preparing the bioadhesive composition are also described.
Owner:3M INNOVATIVE PROPERTIES CO
Composition for the delivery of live cells and methods of use thereof
InactiveUS20090041851A1Enhances primary therapeutic effectSustained deliveryBiocidePowder deliveryActive agentTherapeutic effect
The invention relates to an improved method for administering live cells to a patient and compositions useful in the method. The composition comprises live cells and biocompatible, biodegradable polymer microparticles. The cells and microparticles of the cell / microparticle composition can be contacted immediately prior to administration, or can be contacted in culture for a specified period of time prior to administration. In the method of the invention, an effective amount of the cell / microparticle composition is administered to a patient in need thereof by injection to a treatment site of the patient to provide a therapeutic effect in the patient. The therapeutic effect can be, for example, the formation of new tissue at the treatment site, or the production and secretion of a biologically active secretory molecule at the treatment site. The therapeutic effect resulting from injection of the cell / microparticle composition into a treatment site, is determined by the type of cell present in the composition. The composition comprising lives cells and biocompatible, biodegradable polymer microparticles can further comprise a biologically active agent. In a preferred embodiment, the biologically active agent is incorporated into the microparticle. The biologically active agent can be, for example, factors which modulate cell growth.
Owner:COSTANTINO HENRY R +2
Use of long-acting glp-1 receptor agonists to improve insulin sensitivity and lipid profiles
ActiveUS20090098108A1Lose weightDecrease food intakeAntibacterial agentsOrganic active ingredientsLipid formationAgonist
The present invention provides to at least one novel human GLP-1 receptor agonist, or specified portion or variant, including isolated nucleic acids that encode at least one GLP-1 receptor agonist, or specified portion or variant, GLP-1 receptor agonist, or specified portion or variants, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including the use of long acting GLP-1 receptor agonists to improve insulin sensitivity or lipid profiles in obesity and related therapeutic and / or diagnostic compositions, methods and devices.
Owner:CENTOCOR
Cosmetic and Therapeutic Stick Products
InactiveUS20100008959A1Improve stabilitySustained deliveryBiocideCosmetic preparationsIncompatible ComponentDelivery system
A composition wherein phase or chemically incompatible components are introduced into a stick composition through the use of a microparticle delivery system.
Owner:AMCOL INTERNATIONAL CORPORATION
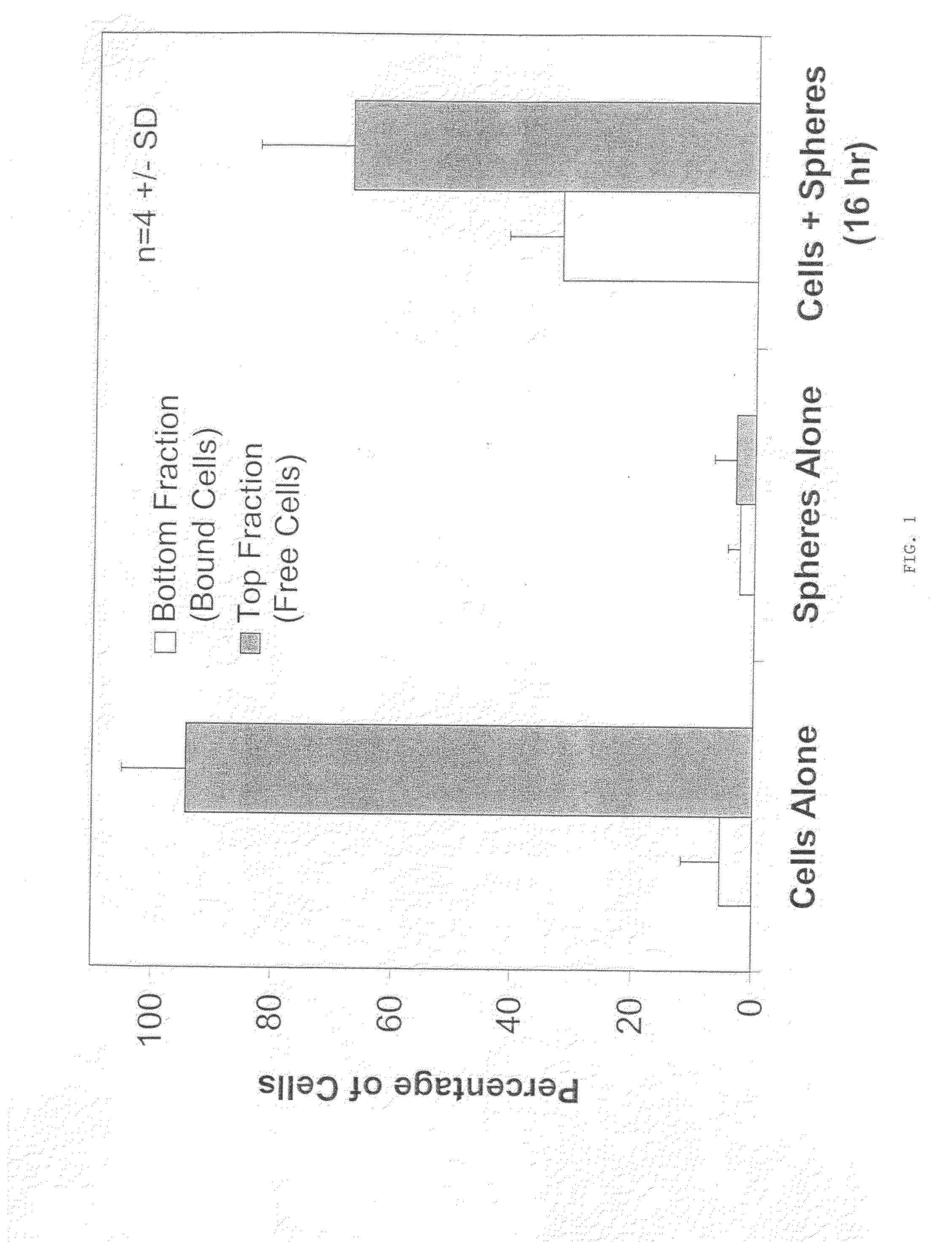
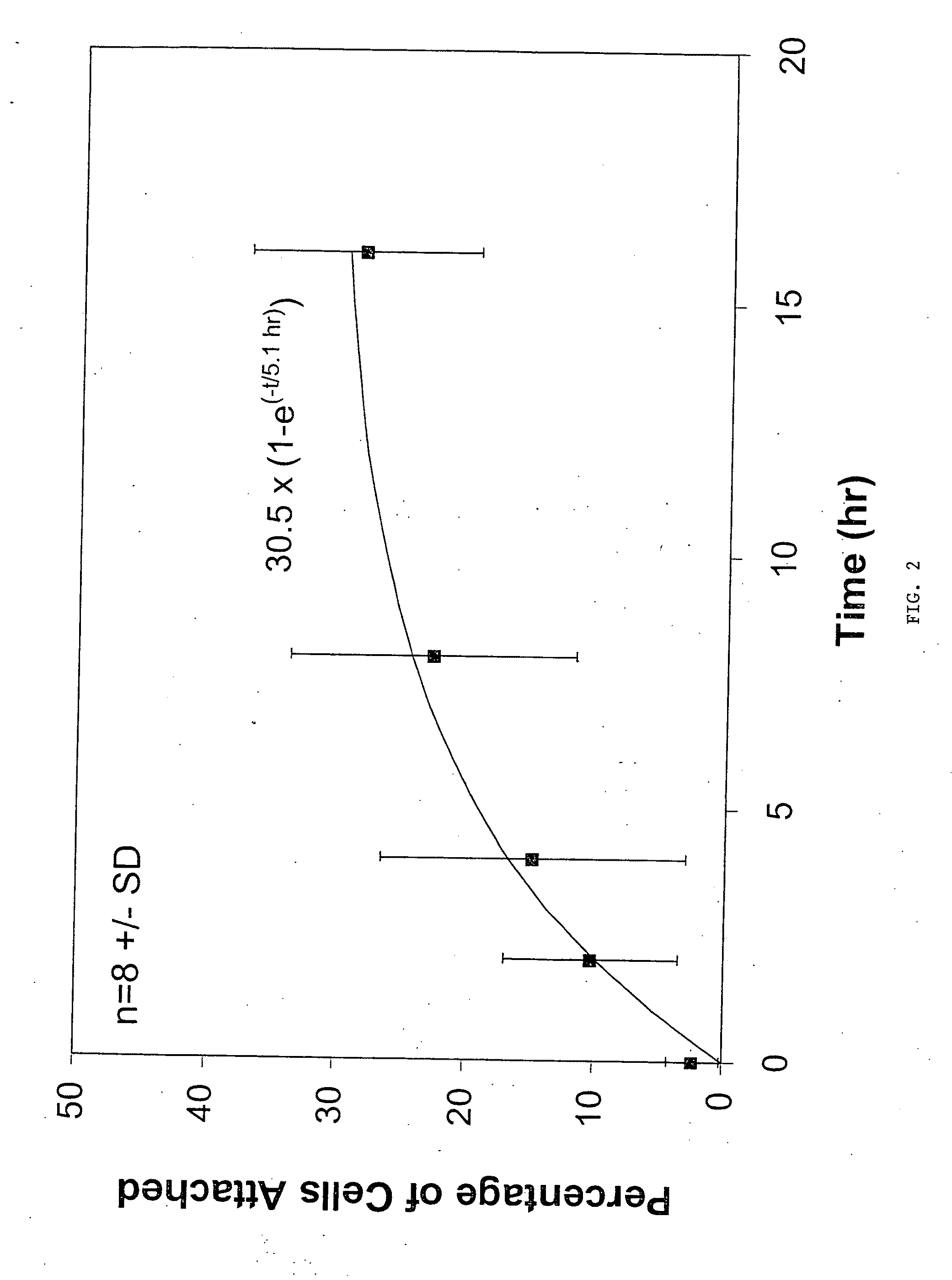
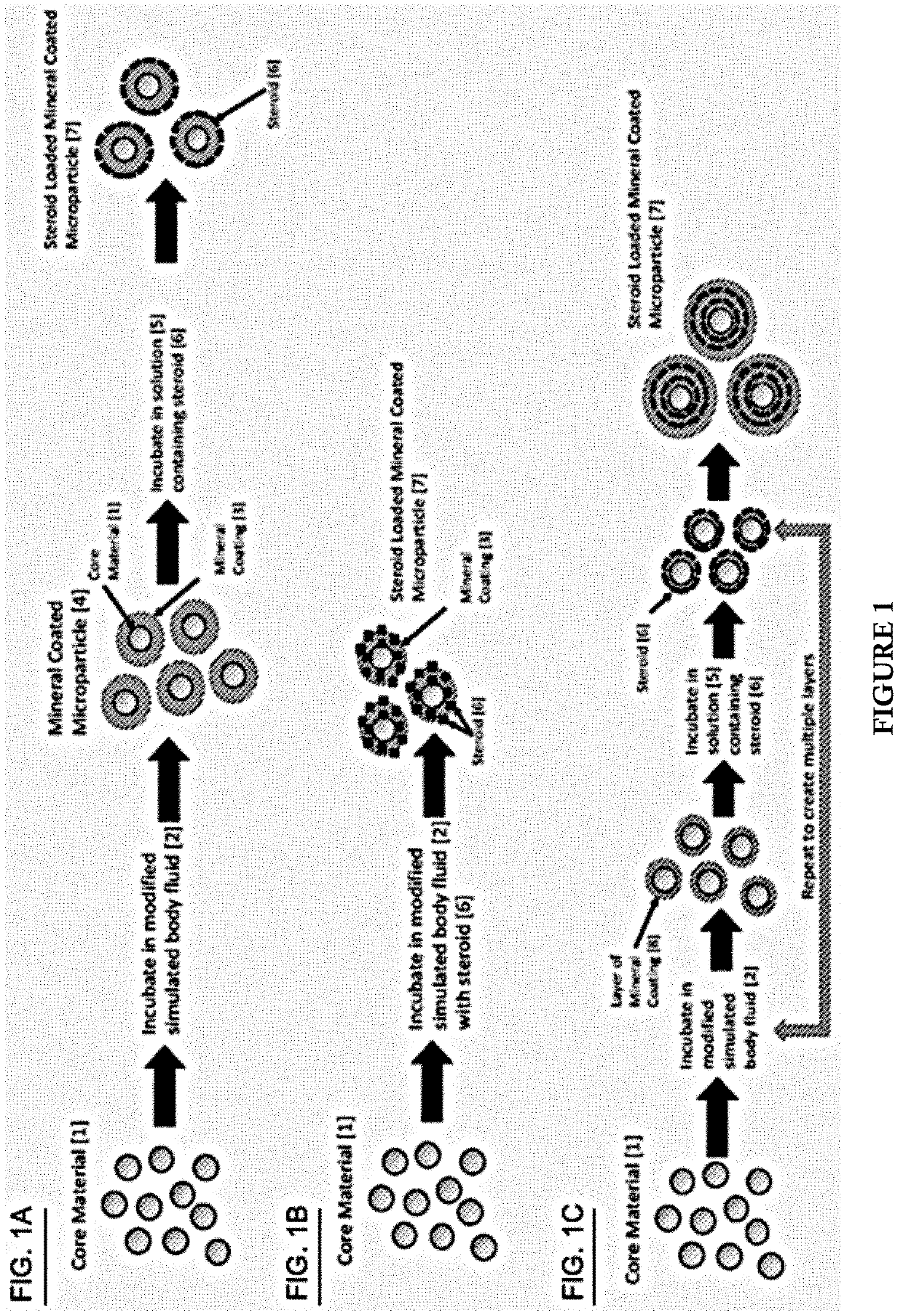
Mineral coated microparticles for sustained delivery of steroids
InactiveUS20190358344A1Improve efficiencyLimit achievable doseOrganic active ingredientsPowder deliveryMedicineMicroparticle
Disclosed are formulations for providing a steroid. Formulations include a mineral coated microparticles wherein a steroid is adsorbed to the mineral coating or incorporated within the mineral coating. Also disclosed are methods for sustained delivery of a steroid and methods for treating inflammation or pain using a formulation for providing sustained delivery of a steroid.
Owner:DIANOMI THERAPEUTICS INC
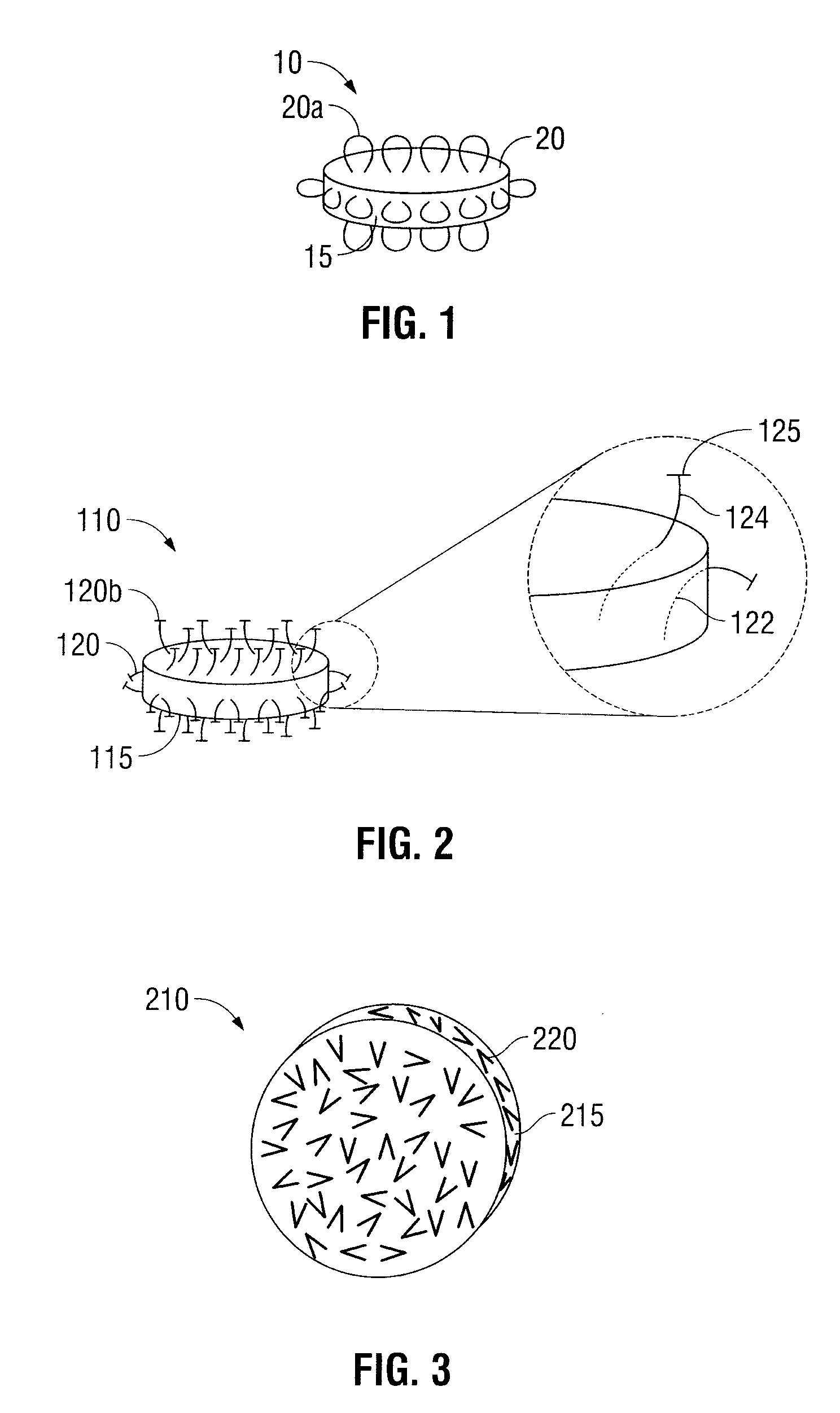
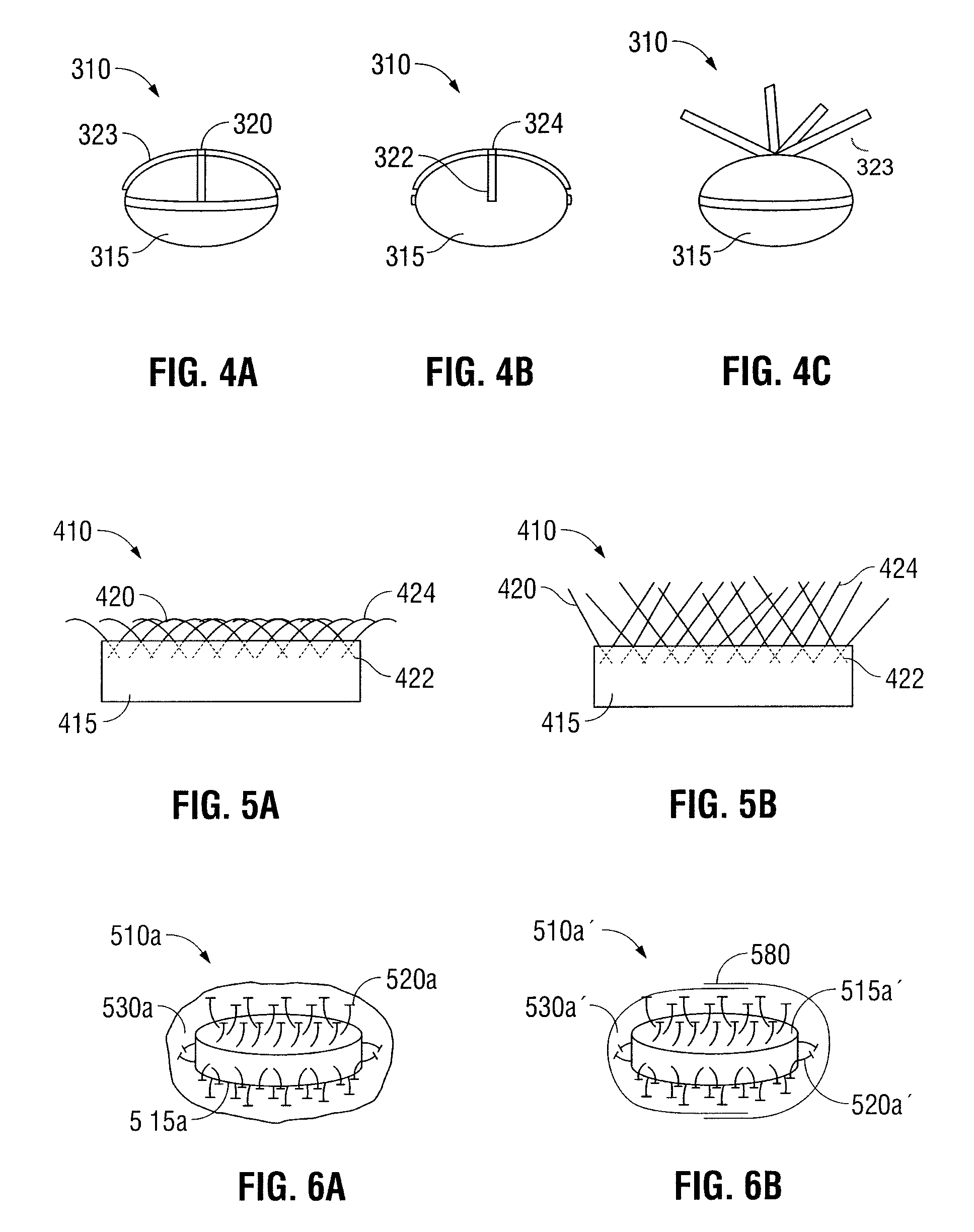
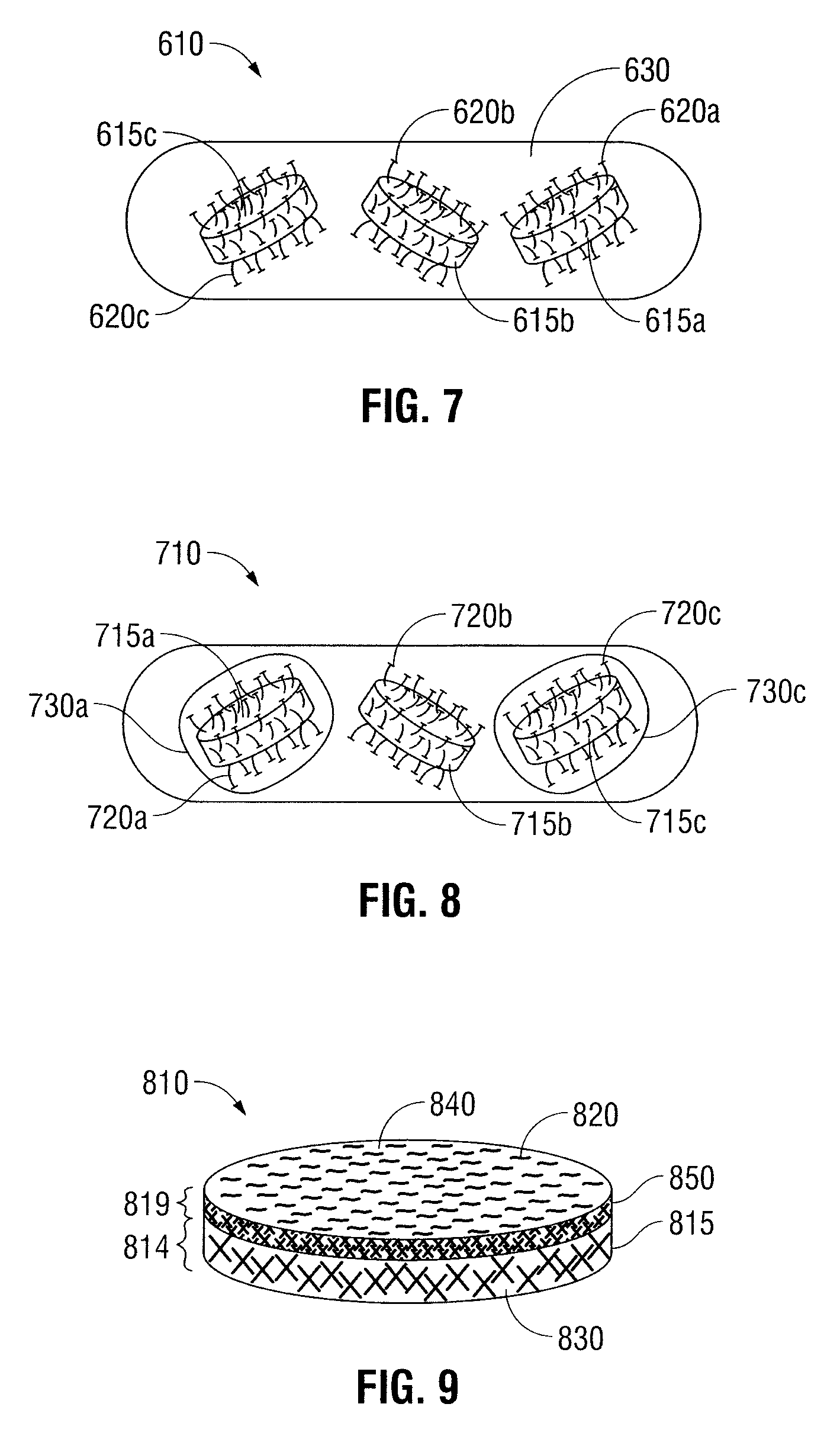
Oral Dosage Forms for Delivery of Therapeutic Agents
ActiveUS20110123614A1High activitySustained deliveryPowder deliveryPharmaceutical product form changeBiomedical engineeringGastrointestinal tract
Oral dosage forms for the delivery of therapeutic agents include mechanical fasteners for engaging tissue of the gastrointestinal tract.
Owner:TYCO HEALTHCARE GRP LP
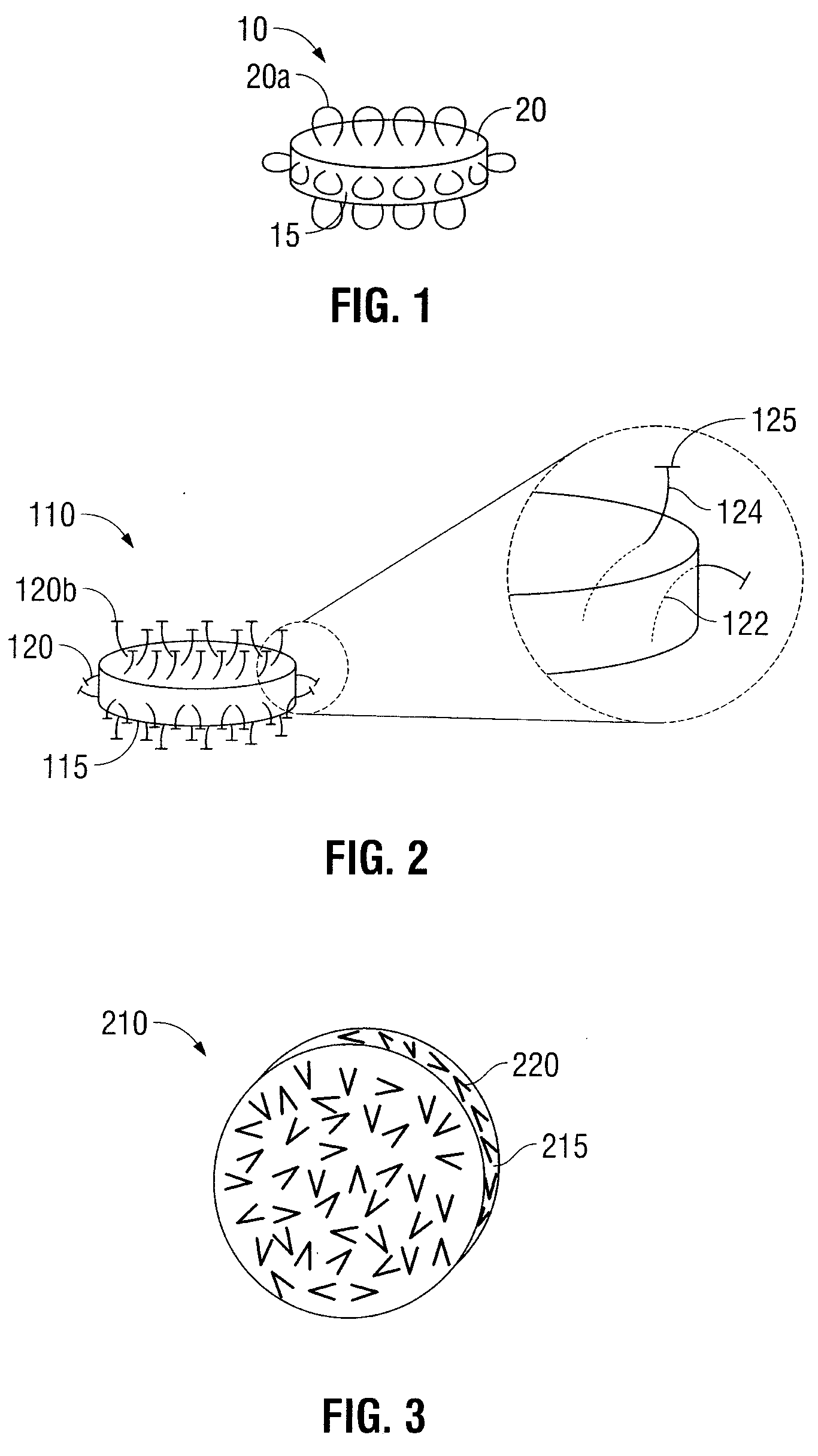
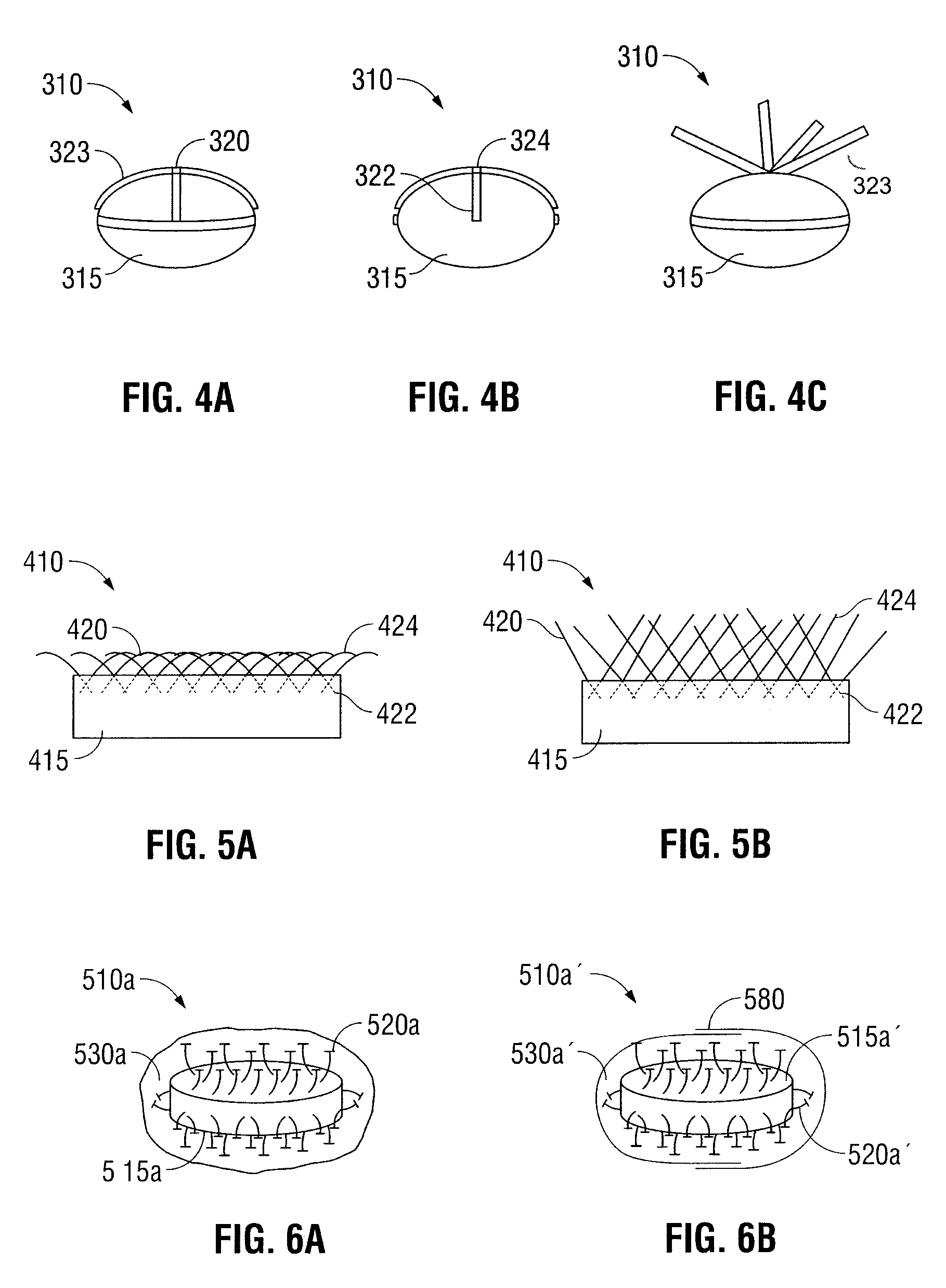
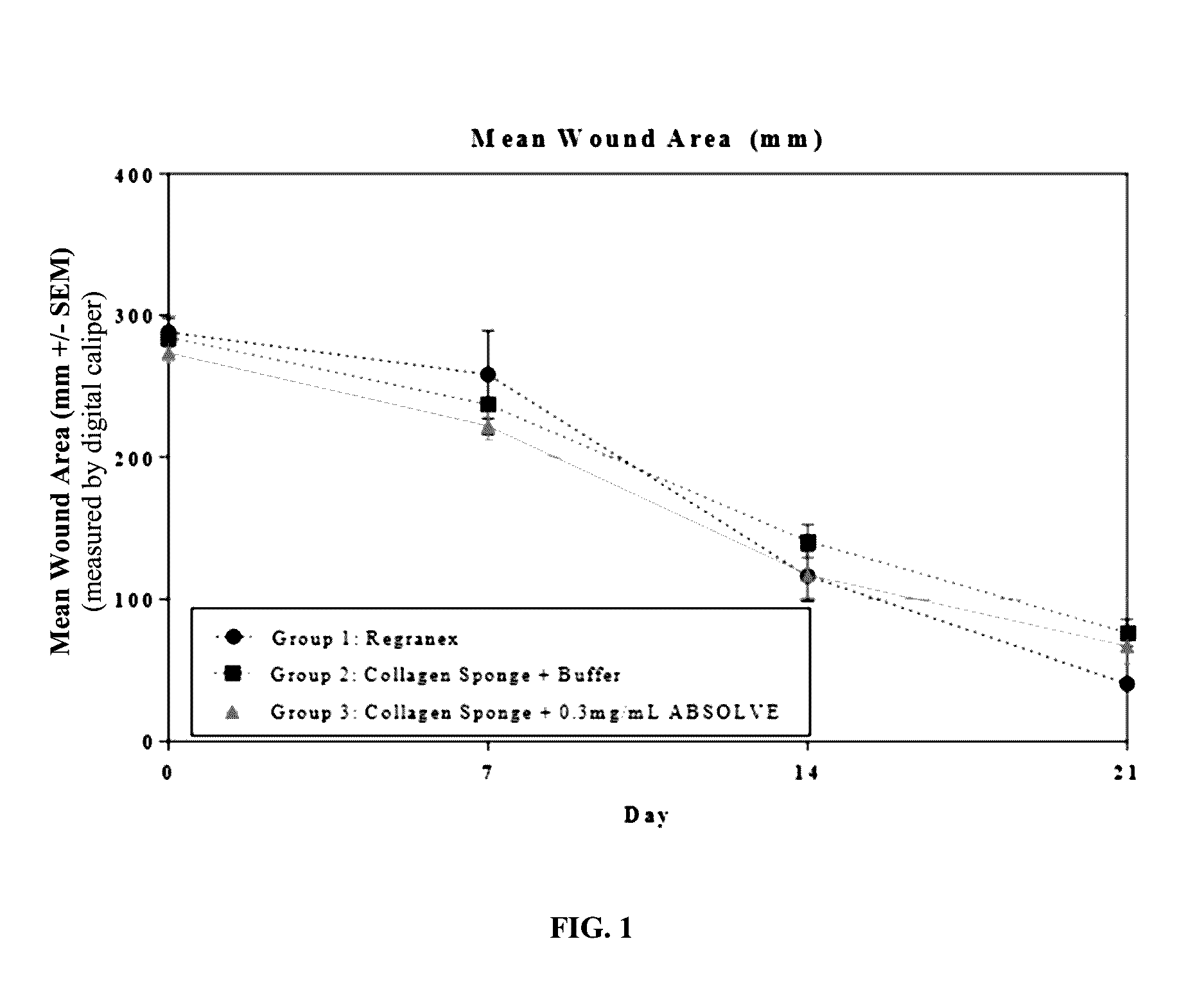
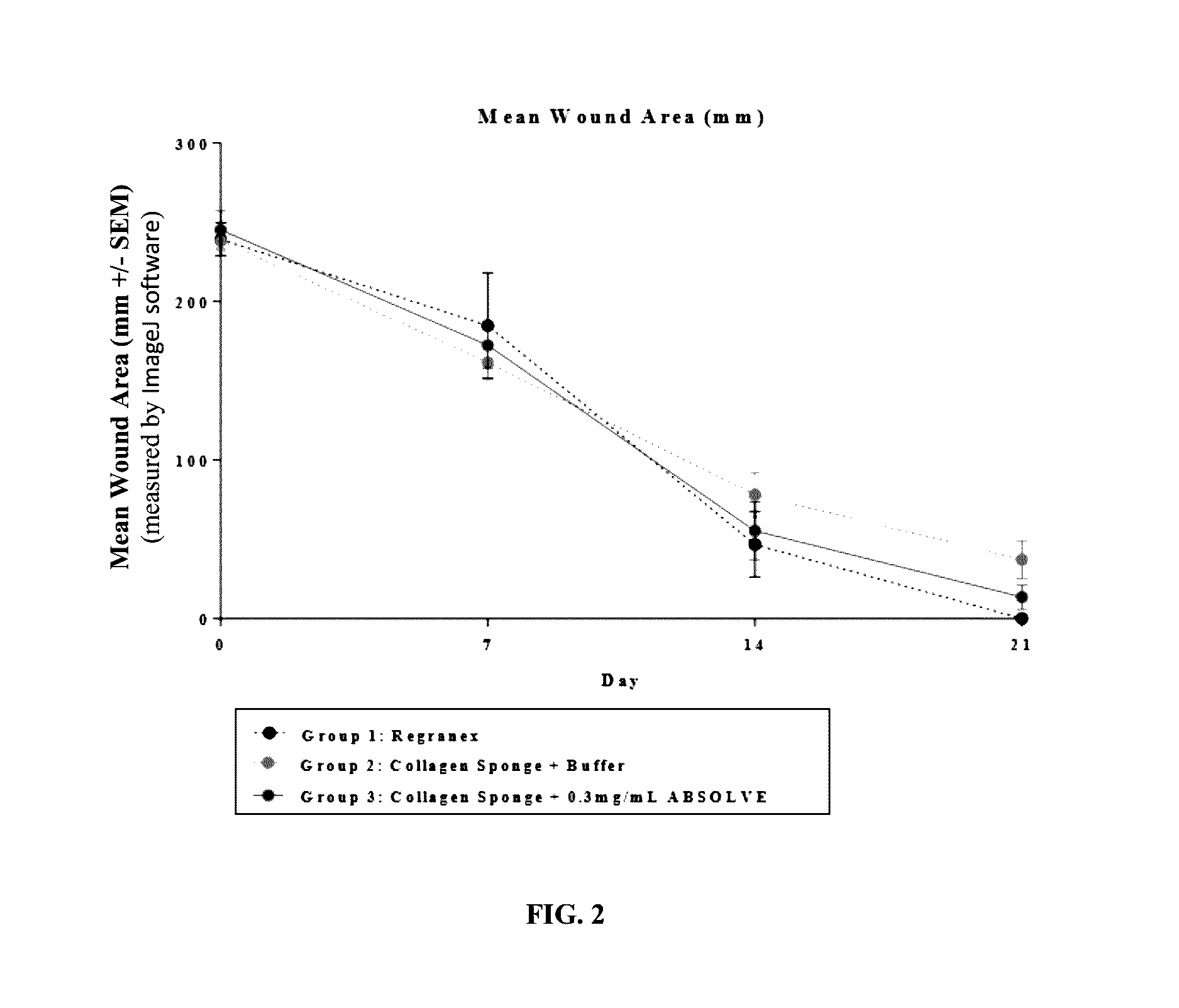
Compositions for treating wounds
InactiveUS20170021054A1Easy maintenanceEasy to handlePeptide/protein ingredientsPharmaceutical delivery mechanismPlateletPharmacology
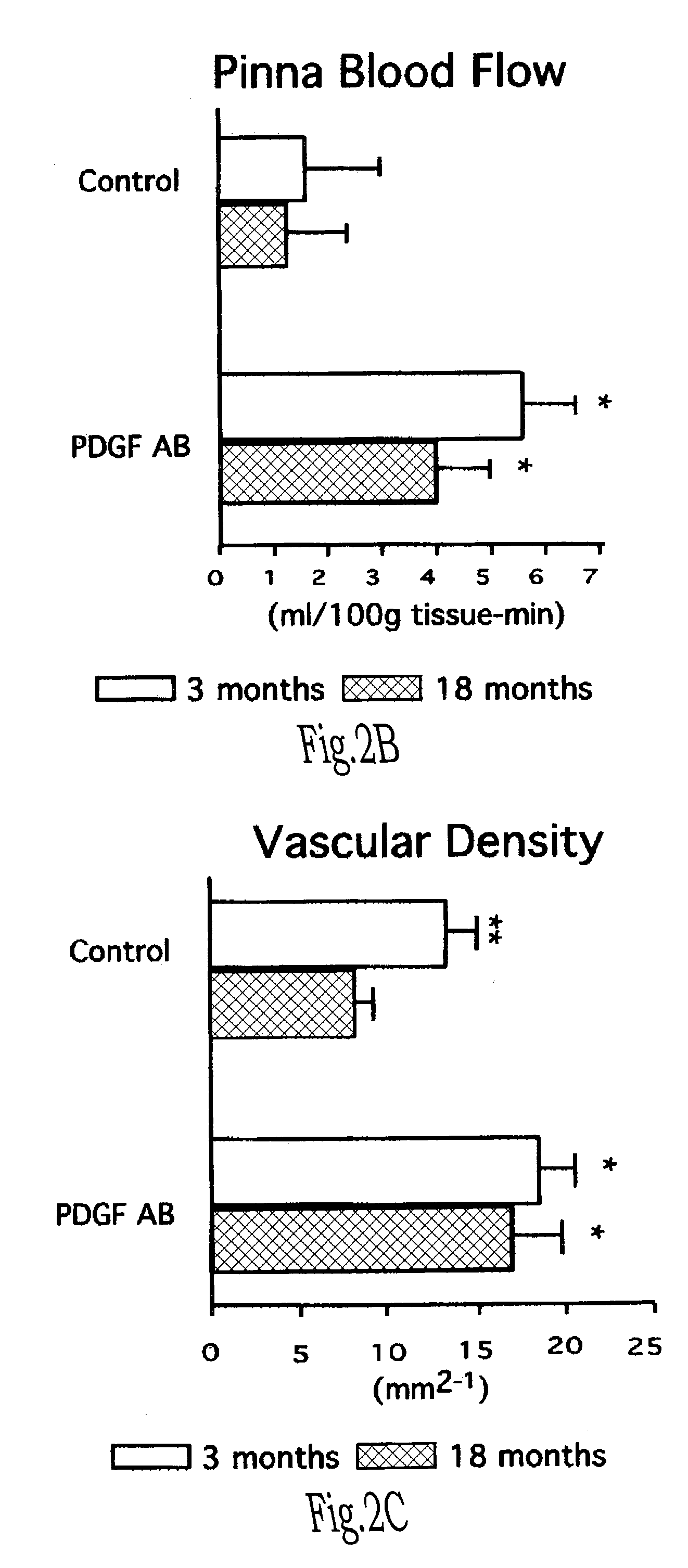
Novel compositions for treating wounds and promoting the healing thereof are described, including composition containing novel combinations of a carrier and recombinant platelet derived growth factor having fewer isoforms and enhanced biostability. Methods of treating wounds with novel therapeutic composition using dosing procedures leading to effective results with a minimal number of treatment applications are also described.
Owner:LYNCH SAMUEL E +1
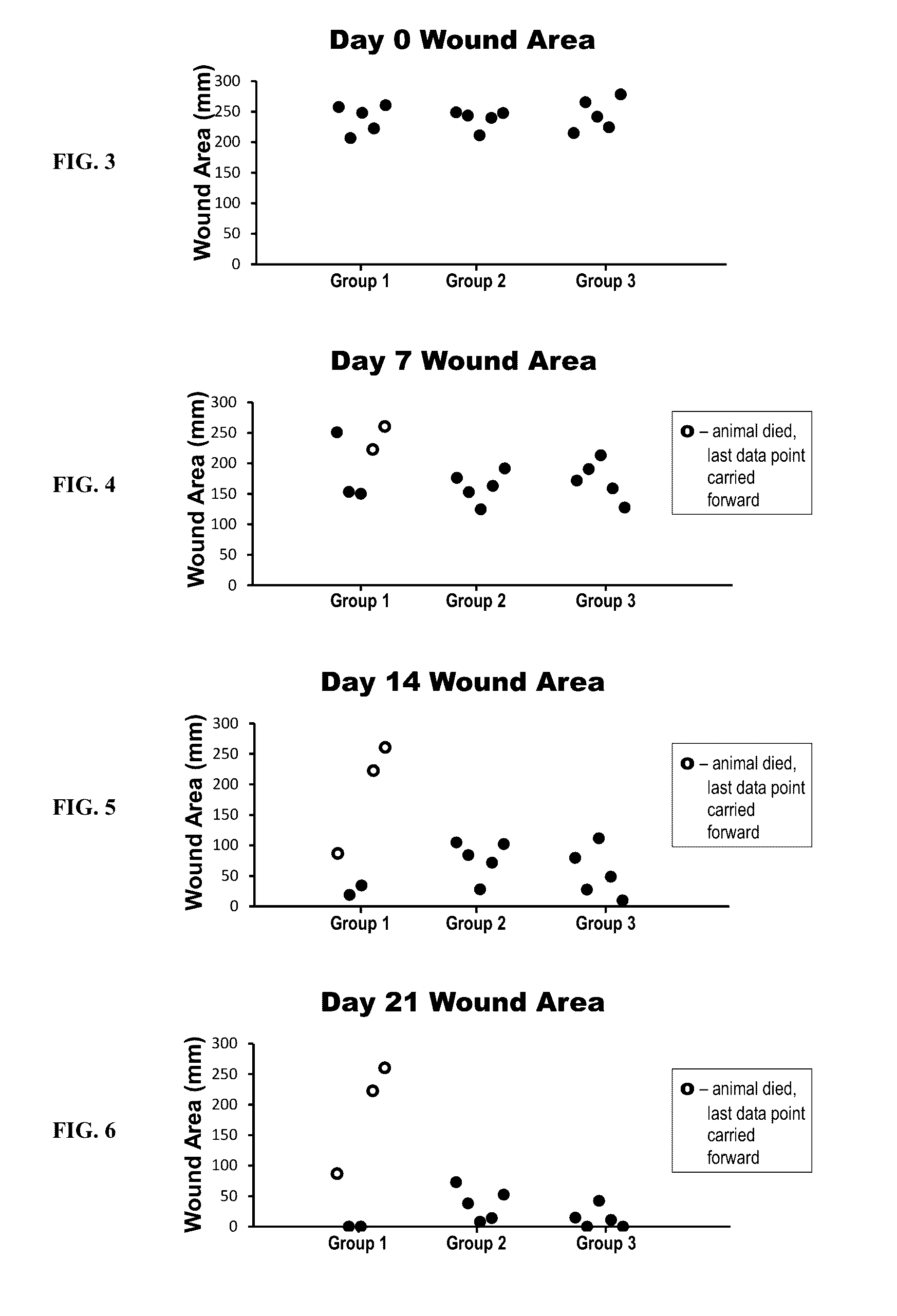
Oral dosage forms for delivery of therapeutic agents
ActiveUS9421169B2High activitySustained deliveryPowder deliveryPharmaceutical product form changeMedicineBiomedical engineering
Oral dosage forms for the delivery of therapeutic agents include mechanical fasteners for engaging tissue of the gastrointestinal tract.
Owner:COVIDIEN LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com