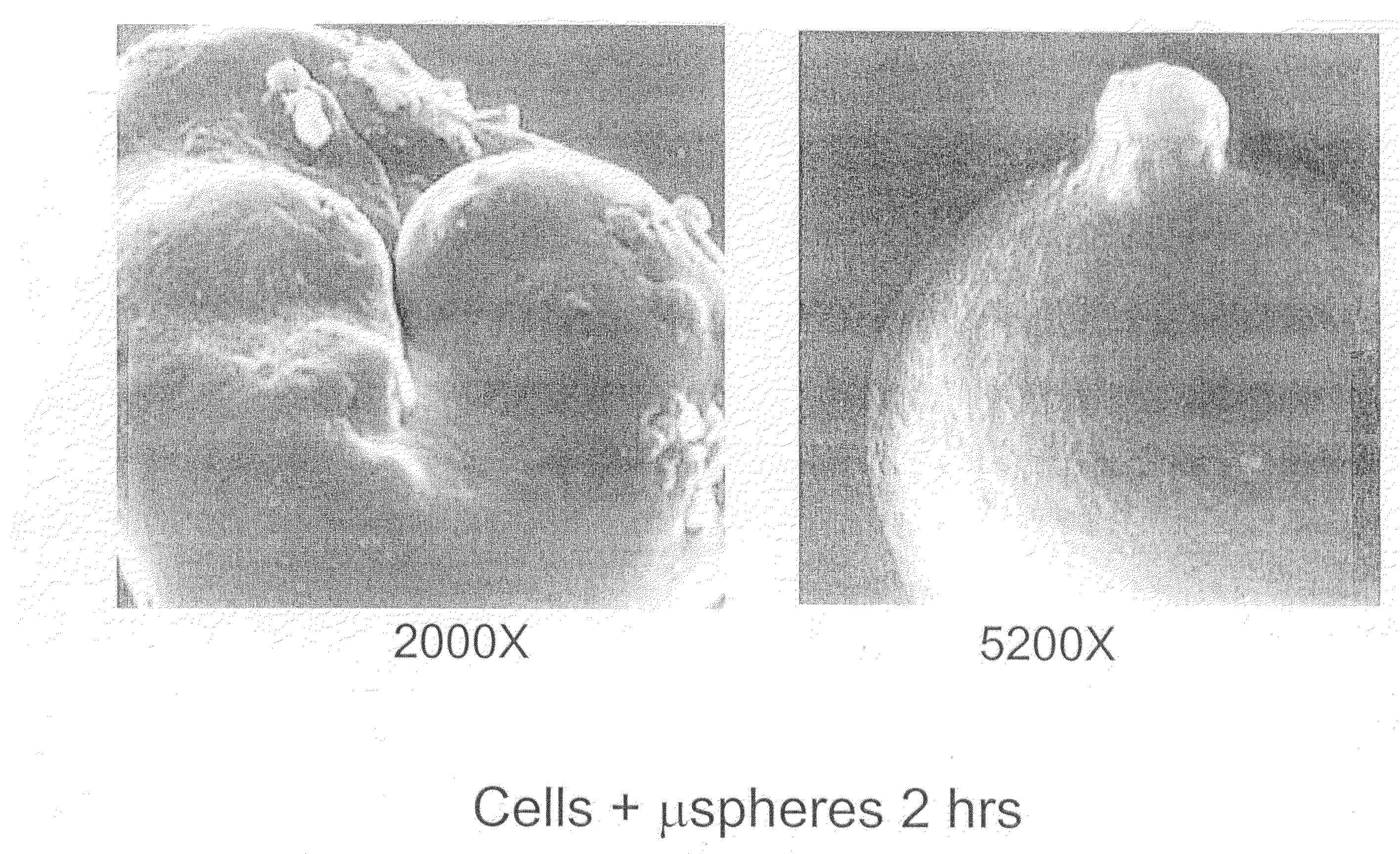Composition for the delivery of live cells and methods of use thereof
a technology for live cells and liquid systems, applied in drug compositions, skeletal/connective tissue cells, metabolic disorders, etc., can solve the problems of difficult control of liquid systems within a particular area, risk of infection, invasive and painful procedures, etc., and achieve the effect of improving the primary therapeutic effect and shortening the time period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Microparticles
[0096]Microparticles were fabricated from PLG (50:50 lactide:glycolide, uncapped (—COOH), Mw˜10 kDa) using the General Process outlined above.
example 2
Cell Isolation-Chondrocytes
[0097]Bovine articular cartilage was harvested from the gelnohumeral and humeroulnar joints of neonatal calves and digested in 0.3% Type II collagenase at 37° C. for 12-16 hours overnight with shaking. Chondrocytes were passed through a 180 ÿm filter to remove large particulate material. Cells were washed 3 times with PBS and resuspended in complete medium (Ham's F12, 10% FBS, 0.3% carboxymethylcellulose, pen / strep / amphotericin B, and ascorbic acid).
example 3
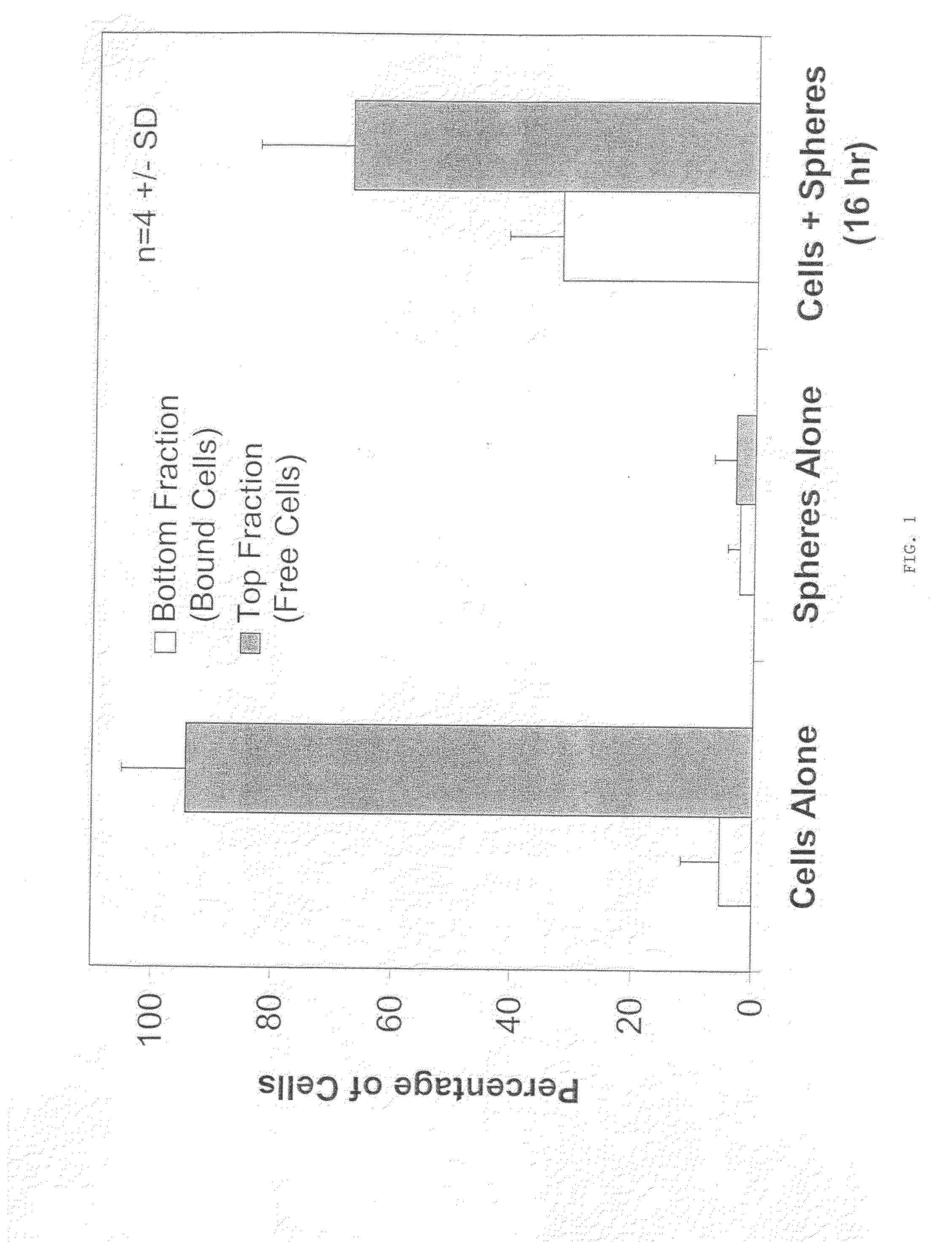
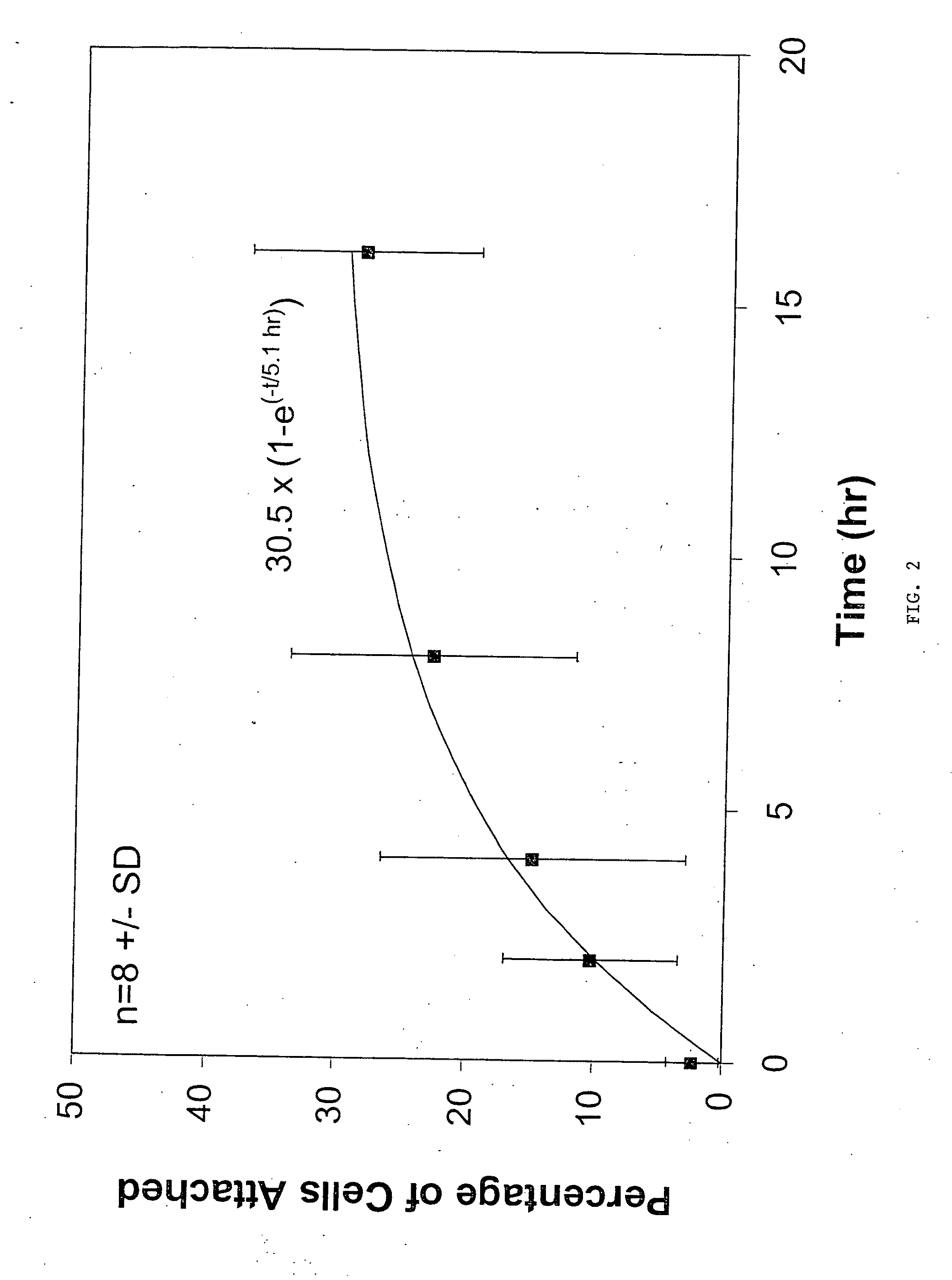
Chondrocyte / Microparticle Adhesion Assay
[0098]Chondrocytes were mixed with 3.57 mg / mL of PLG (50:50 L:G) microparticles, prepared as described above, to a final concentration of 1×106 cells / mL. Control groups included cells alone and microparticles alone at the same concentrations as described above. The mixture of cells and microparticles contained phosphate buffered saline and cell culture medium.
[0099]The combined cell / microparticle suspension and controls were incubated on a shaker plate at 37° C. At 0, 2, 4, 16 hours, 1 mL samples were removed and assayed to determine the amount of cells which had adhered to the microparticles. In order to determine the amount of cells which had adhered to the microparticles, each sample was loaded onto a 3 mL histopaque density gradient and centrifuged for 5 min at 5000 rpm. The unattached cells were removed by decanting the less dense media fraction on top of the histopaque (approximately 1 mL). The remaining portion (approximately 3 mL) con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com