Patents
Literature
82 results about "Phenylephrine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylephrine is a medication primarily used as a decongestant, to dilate the pupil, to increase blood pressure, and to relieve hemorrhoids. While marketed as a decongestant, taken by mouth at recommended doses it is of unclear benefit for hay fever. It can be taken by mouth, given by injection into a vein or muscle, or applied to the skin.
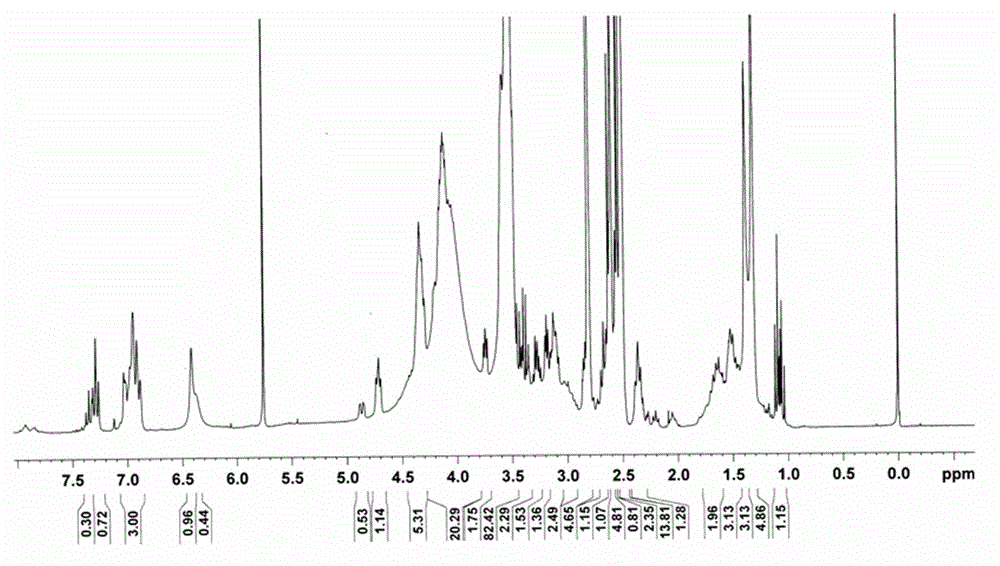
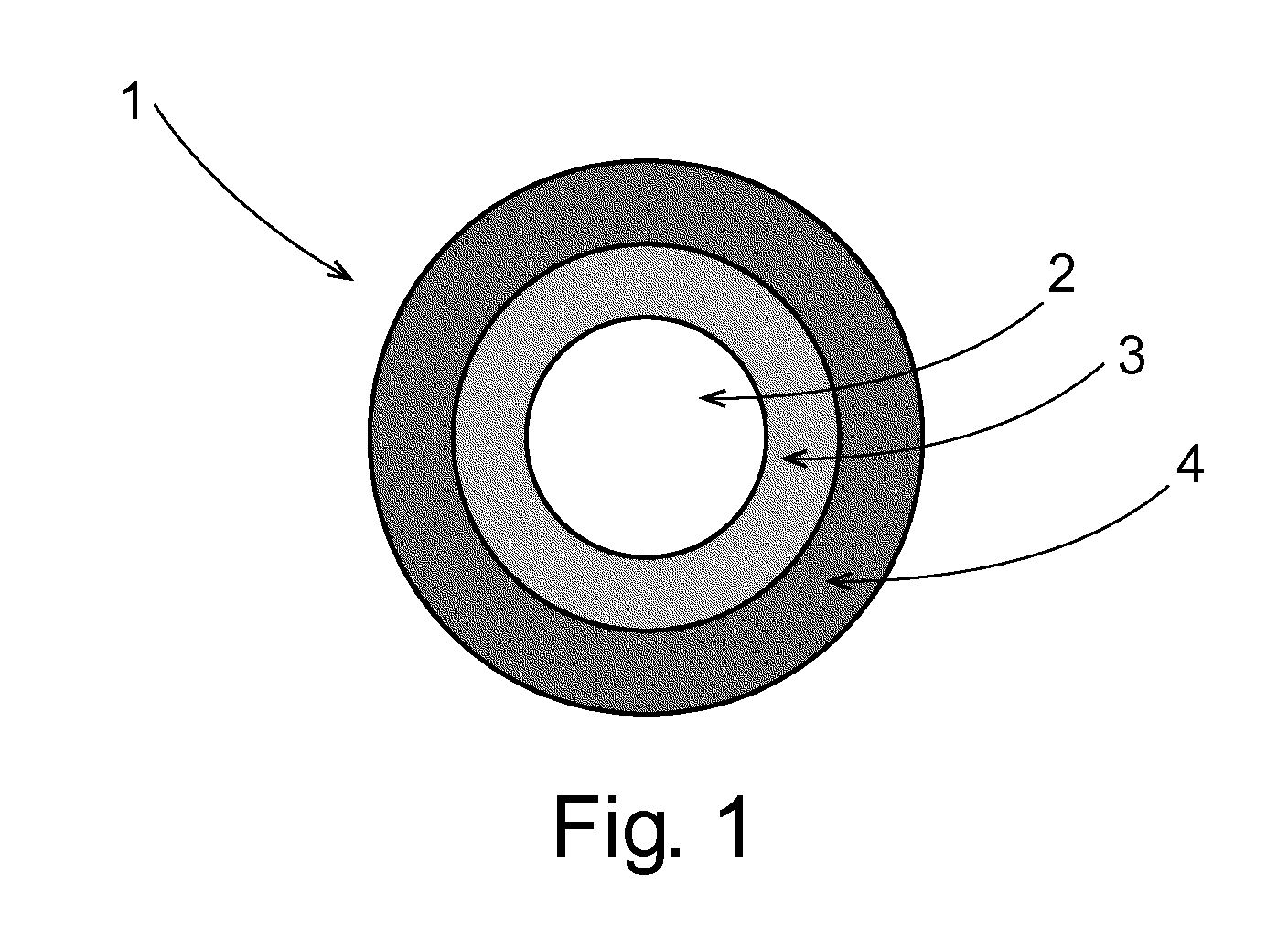
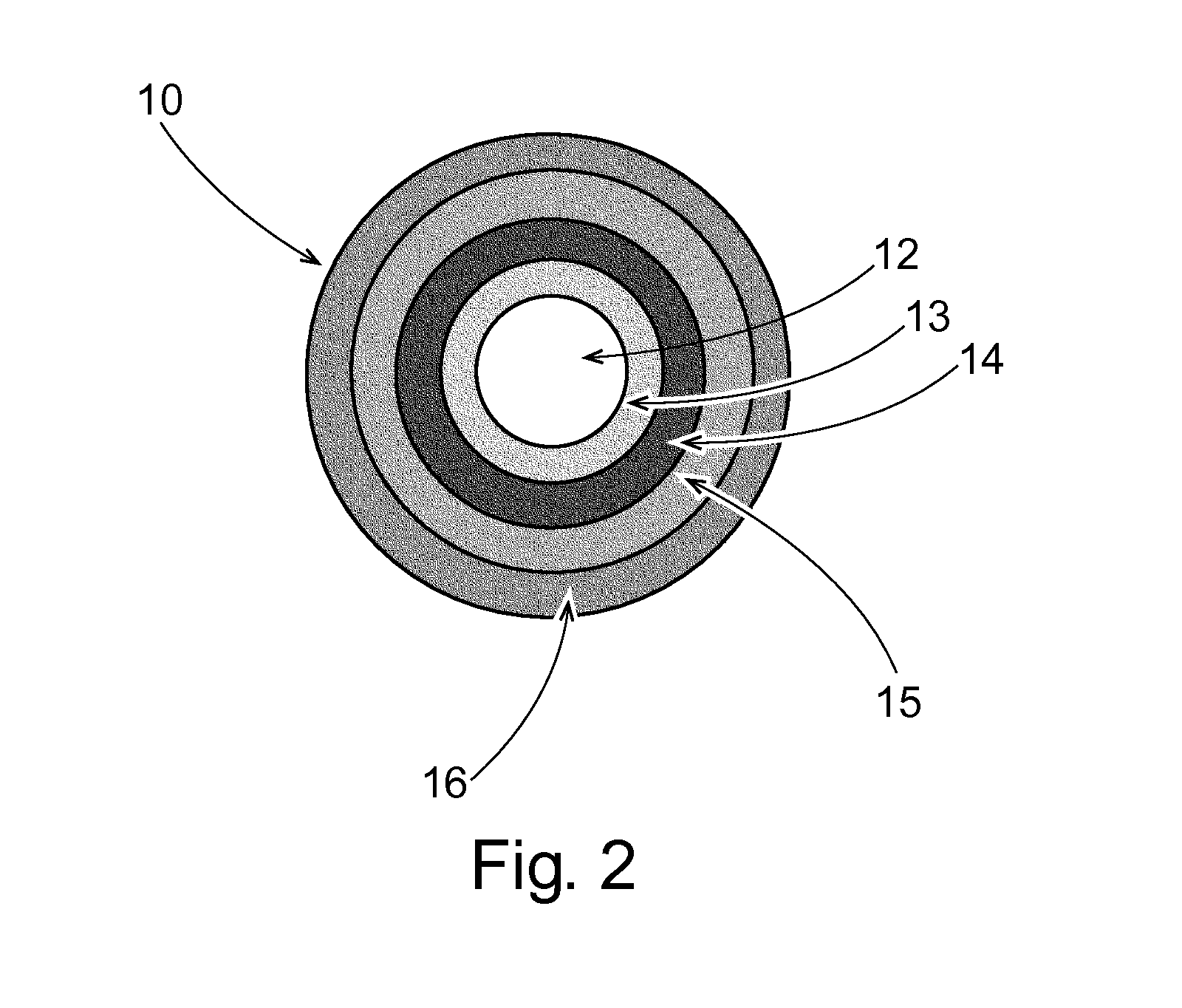
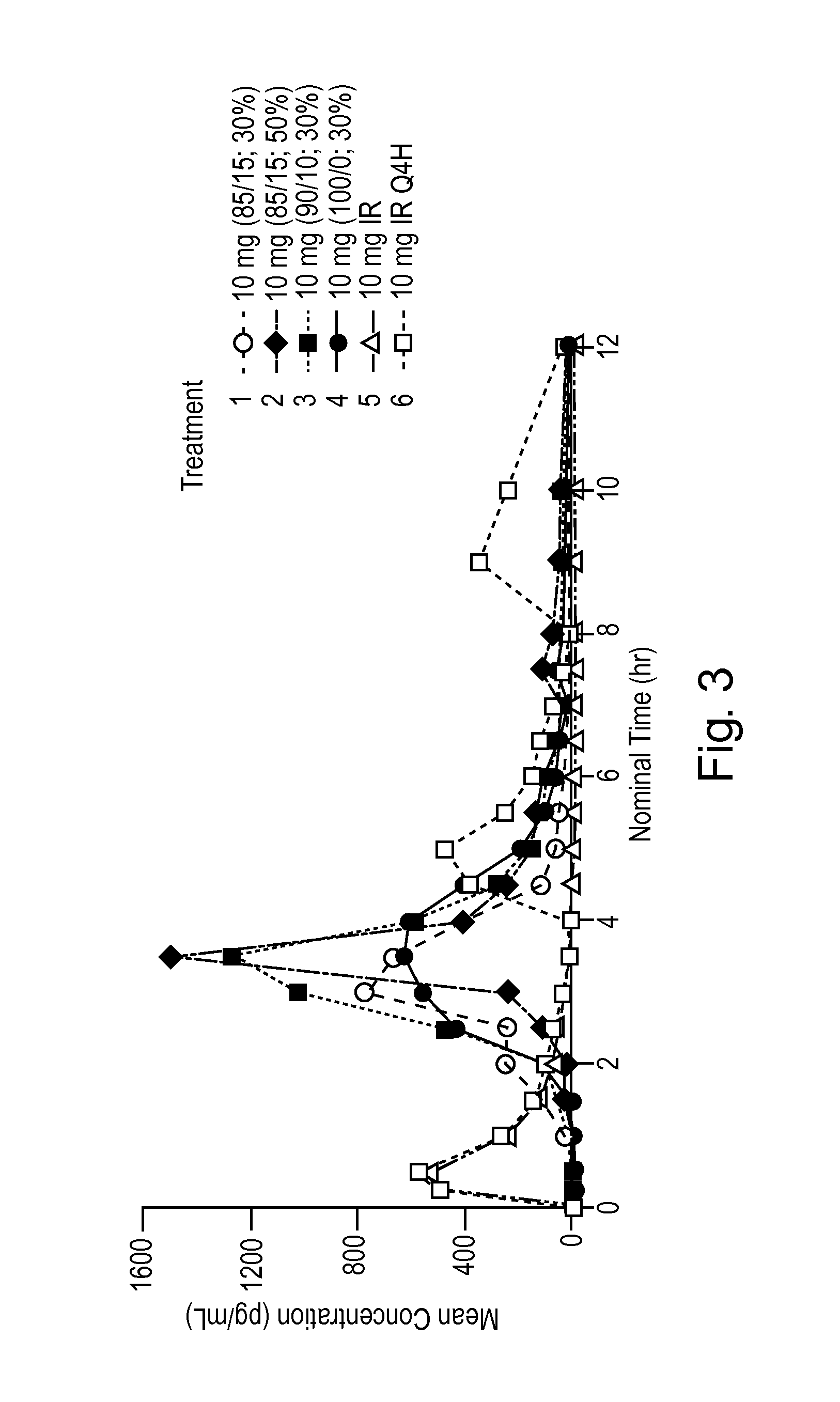
Phenylepherine containing dosage form
ActiveUS20060057205A1Good treatment effectRelieve symptomsBiocidePill deliveryNorphenylephrineBlood plasma
A pharmaceutical dosage form which comprises phenylepherine or a pharmaceutically acceptable salt thereof and a second drug. The dosage form provides a plasma concentration within the therapeutic range of the second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of phenylepherine. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:CAPELLON PHARMA LLC
Enhanced stability phenylephrine liquid compositions
An oral, liquid pharmaceutical composition is provided. The composition comprises phenylephrine and substantially aldehyde-free polyethylene glycol. The composition has phenylephrine stability compatible with the stability required for commercial preparations. Optionally, the composition may comprise one or more additional active agents.
Owner:WYETH LLC
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its free and addition salt forms, and mixtures thereof, alone, or in combination with other pharmaceutical actives. The compositions have a pH of about 2 to about 5 and are substantially free of aldehydes. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its free and addition salt forms, and mixtures thereof alone, or in combination with other pharmaceutical actives, wherein the composition has a pH of from about 2 to about 5 and is substantially free of aldehydes.
Owner:THE PROCTER & GAMBLE COMPANY
Chewable tablet containing phenylephrine
A chewable pharmaceutical composition comprising phenylephrine, artificial sweetener, and a substantially aldehyde-free matrix is provided. The composition has phenylephrine stability suitable for a typical commercial product with a two year shelf life. A method of manufacture of the composition and a method of use are also provided.
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE HLDG US
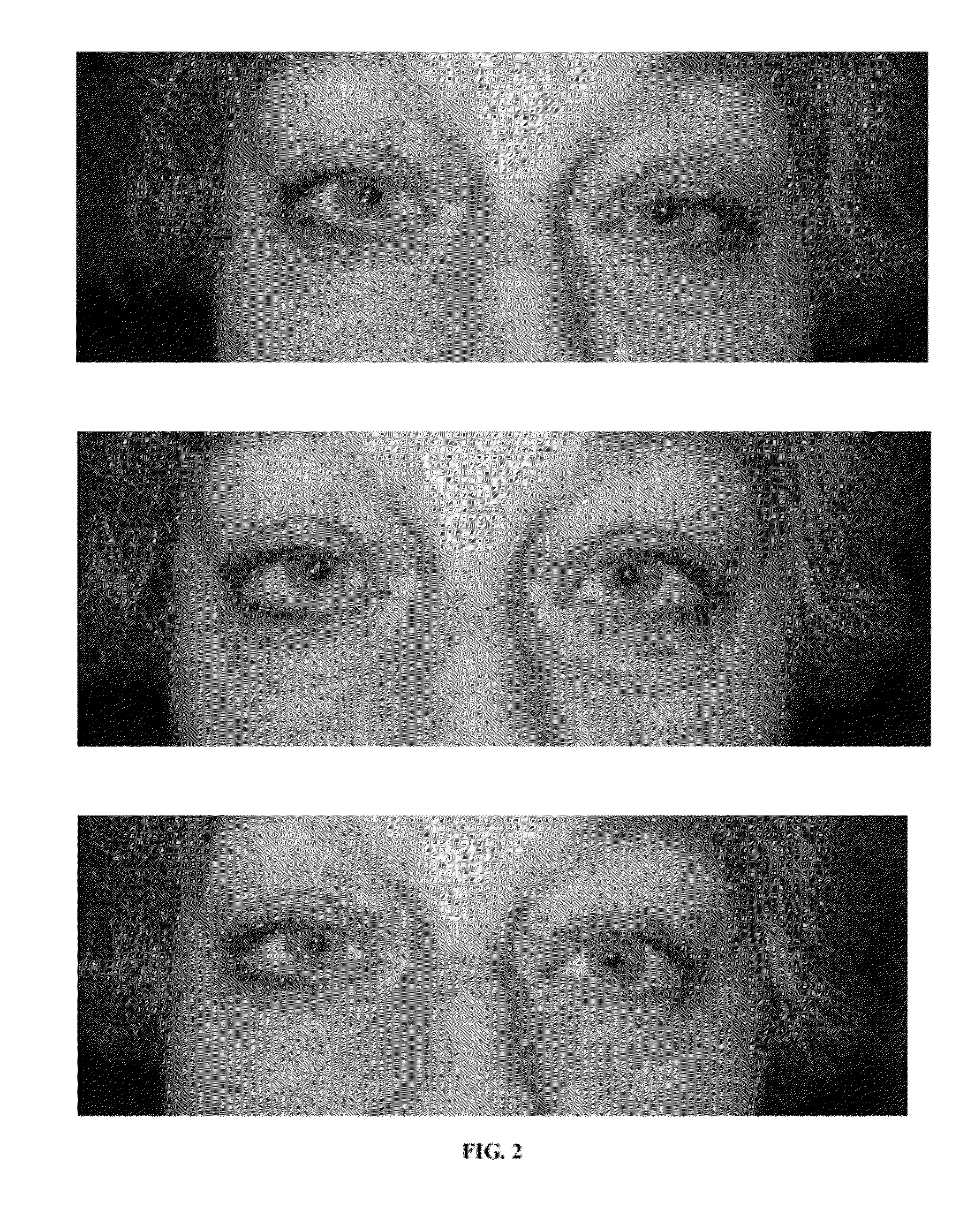
Compositions and methods for non-surgical treatment of ptosis
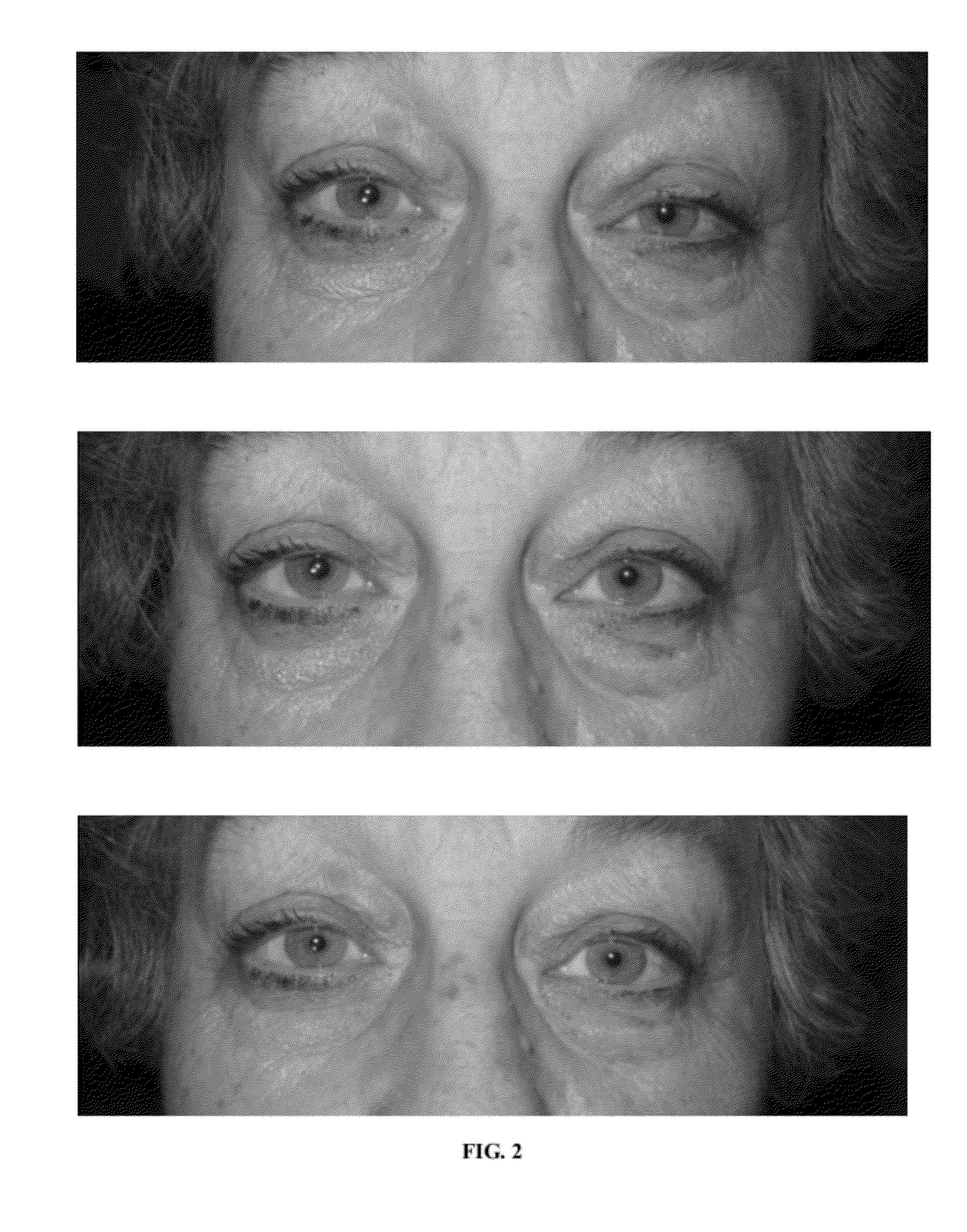
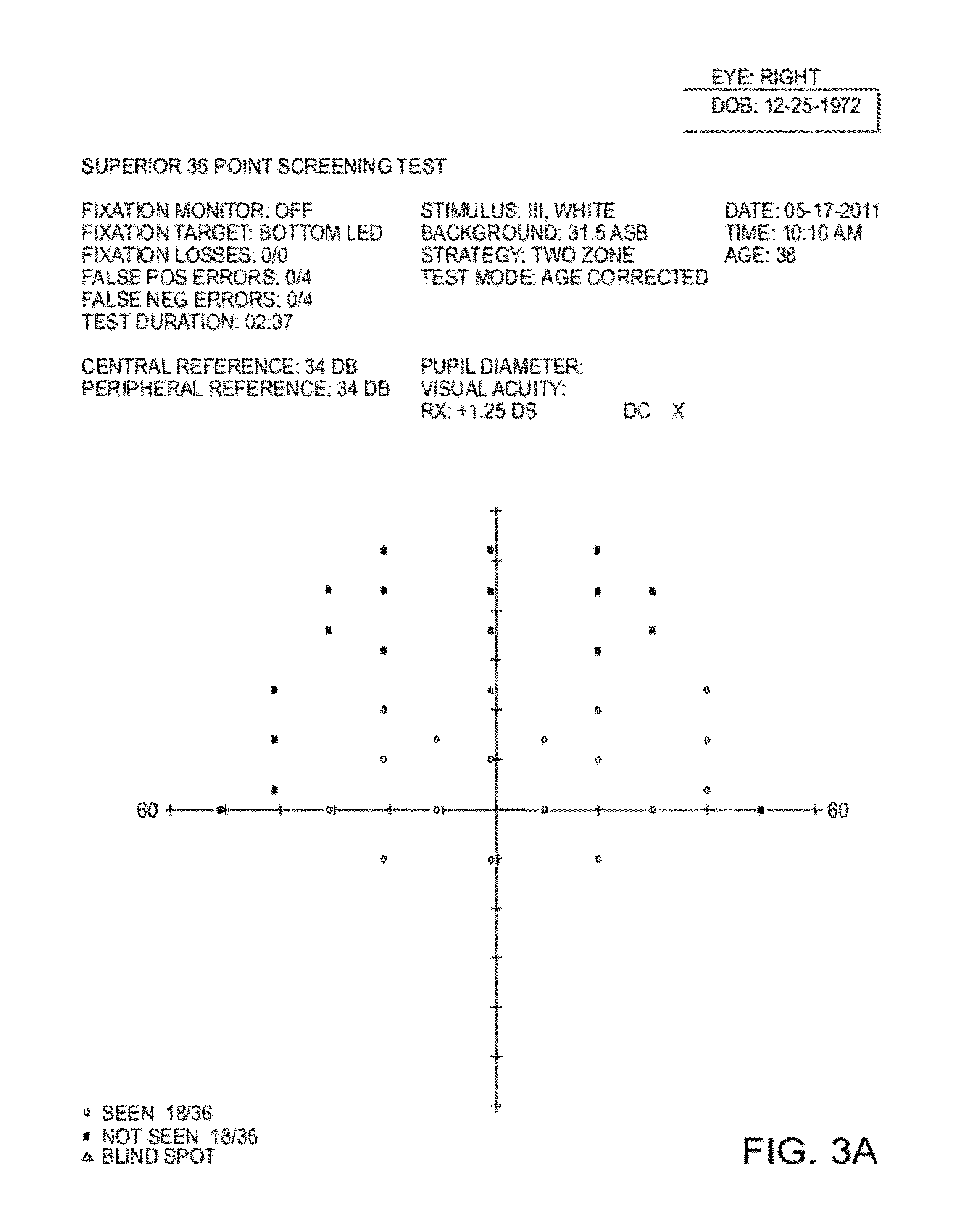
Provided are pharmaceutical compositions, and methods of use of the compositions, for the non-surgical treatment of ptosis (eyelid droop). In one embodiment the composition includes oxymetazoline 0.1% formulated for topical administration to an eye. In one embodiment the composition includes a synergistic combination of oxymetazoline and phenylephrine, formulated for topical administration to an eye. Oxymetazoline alone causes no pupillary dilation (mydriasis), and a synergistic combination of oxymetazoline and phenylephrine induces no clinically significant mydriasis. In addition to providing desirable cosmetic effects, the compositions and methods of the invention can improve visual fields otherwise compromised by ptosis.
Owner:VOOM
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
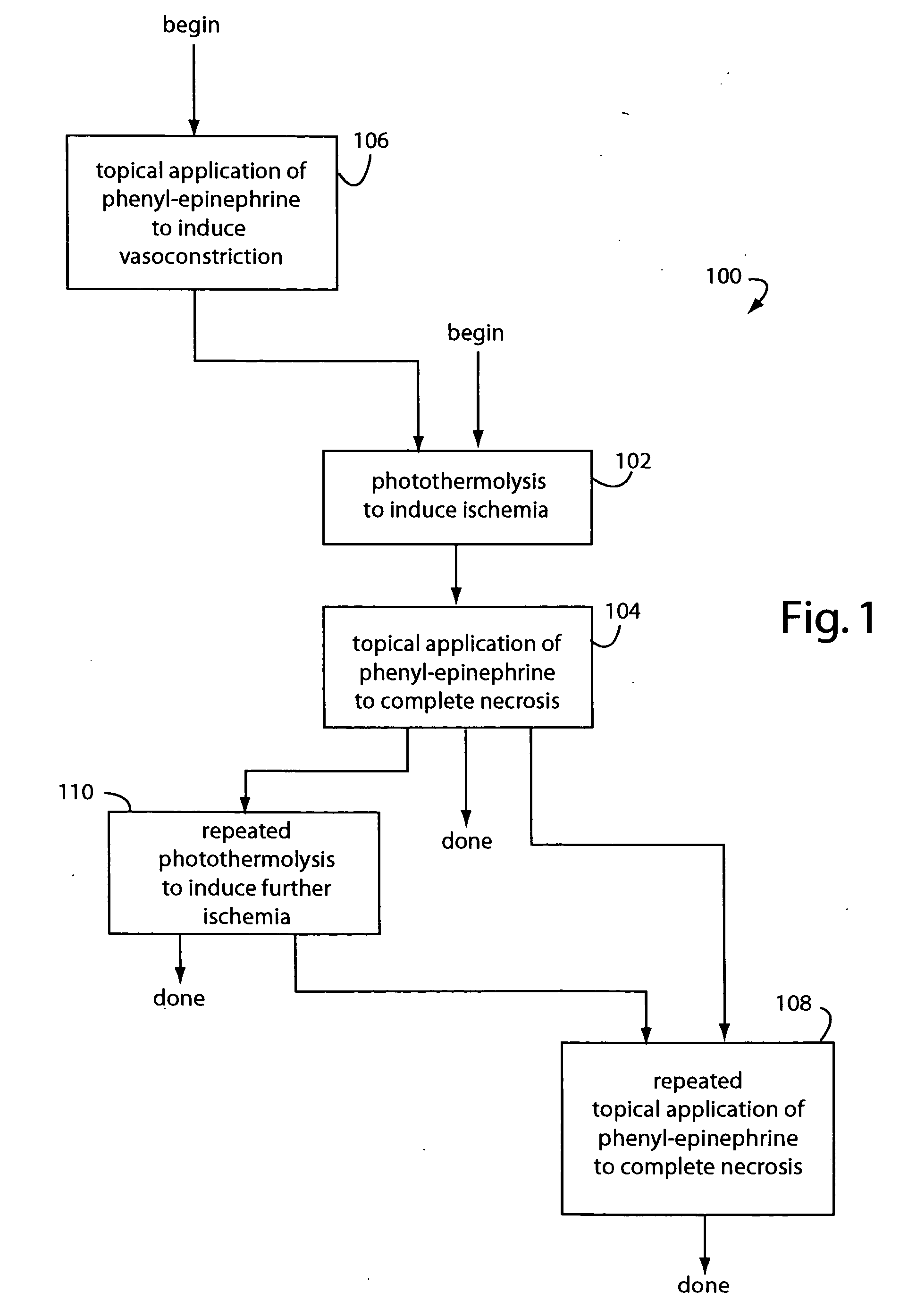
Topical phenyl-epinephrine Rosacea treatment
InactiveUS20050256204A1Safe and effectiveEffective temporary reliefBiocideOrganic active ingredientsArteriolar VasoconstrictionDisease
A near-permanent skin treatment includes photothermolysis of reddened facial skin to induce ischemia. Reperfusion of the photothermolysis treated skin is inhibited by following with regular applications of phenyl-epinephrine carried in a lotion until vascular necrosis is complete. Alternatively, a temporary treatment for reddened facial skin includes only cosmetic as-needed applications of phenyl-epinephrine carried in lotion to induce vasoconstriction in Rosacea and other similarly embarrassing skin disorders.
Owner:BITTER PATRICK H SR
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
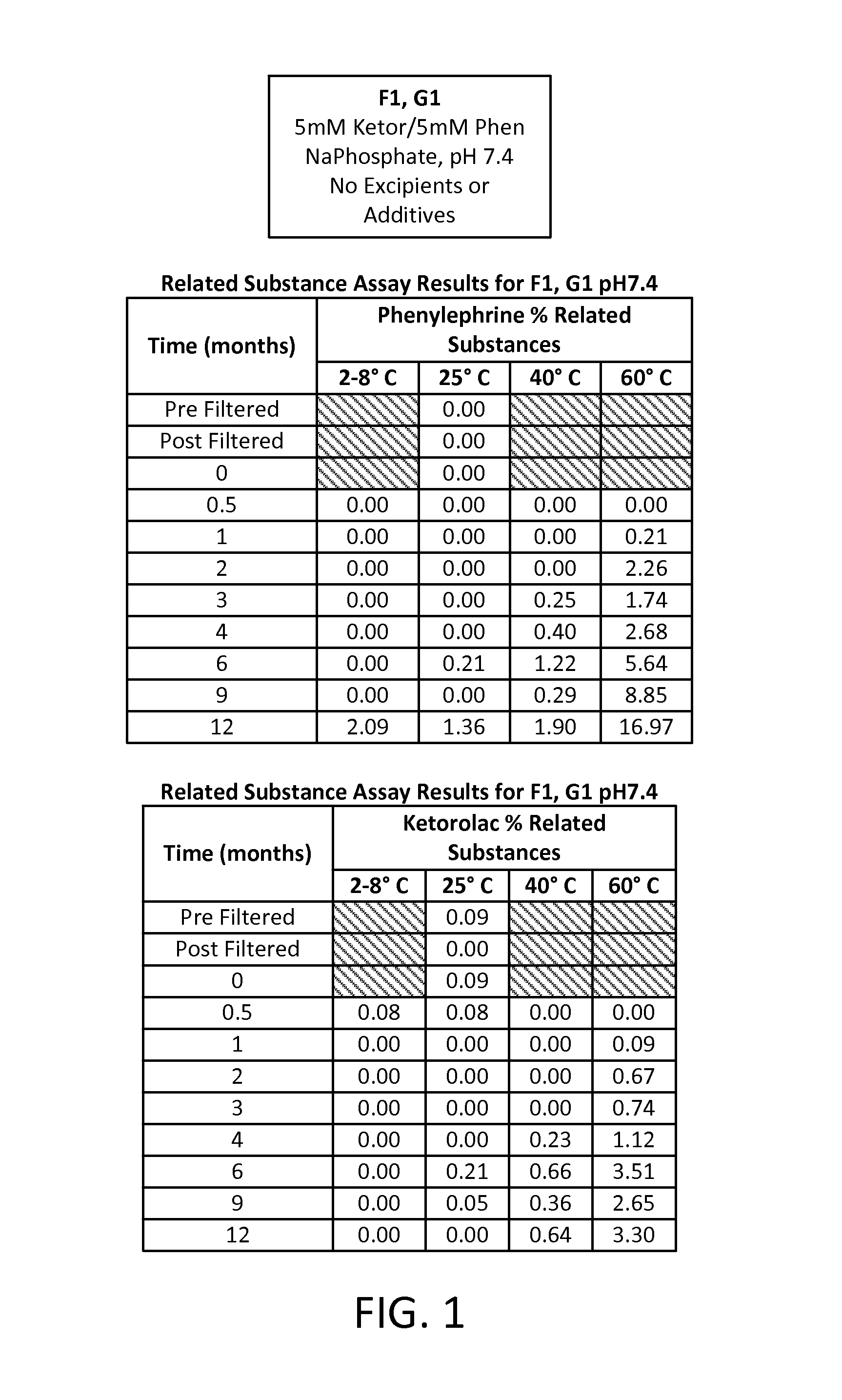
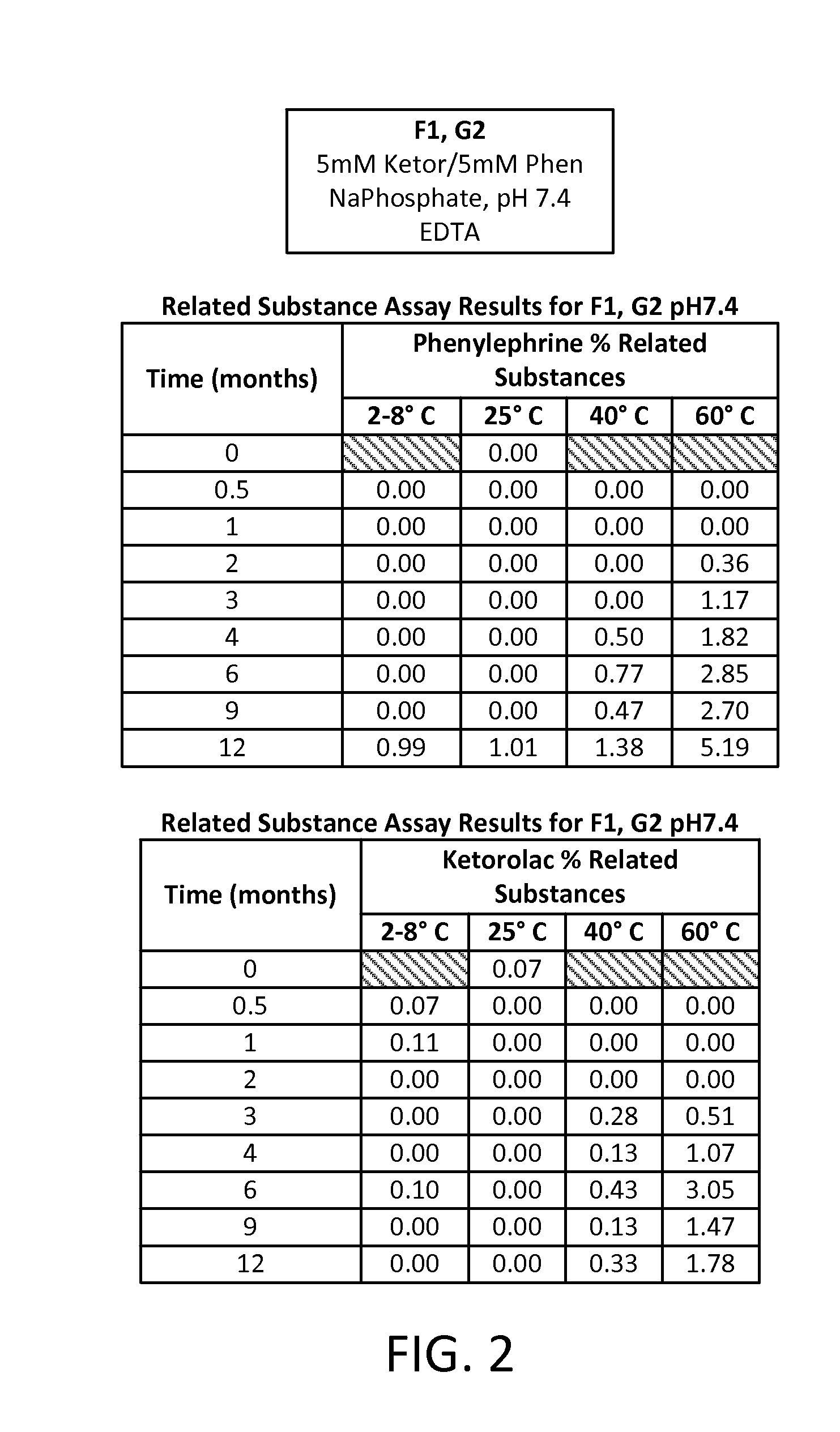
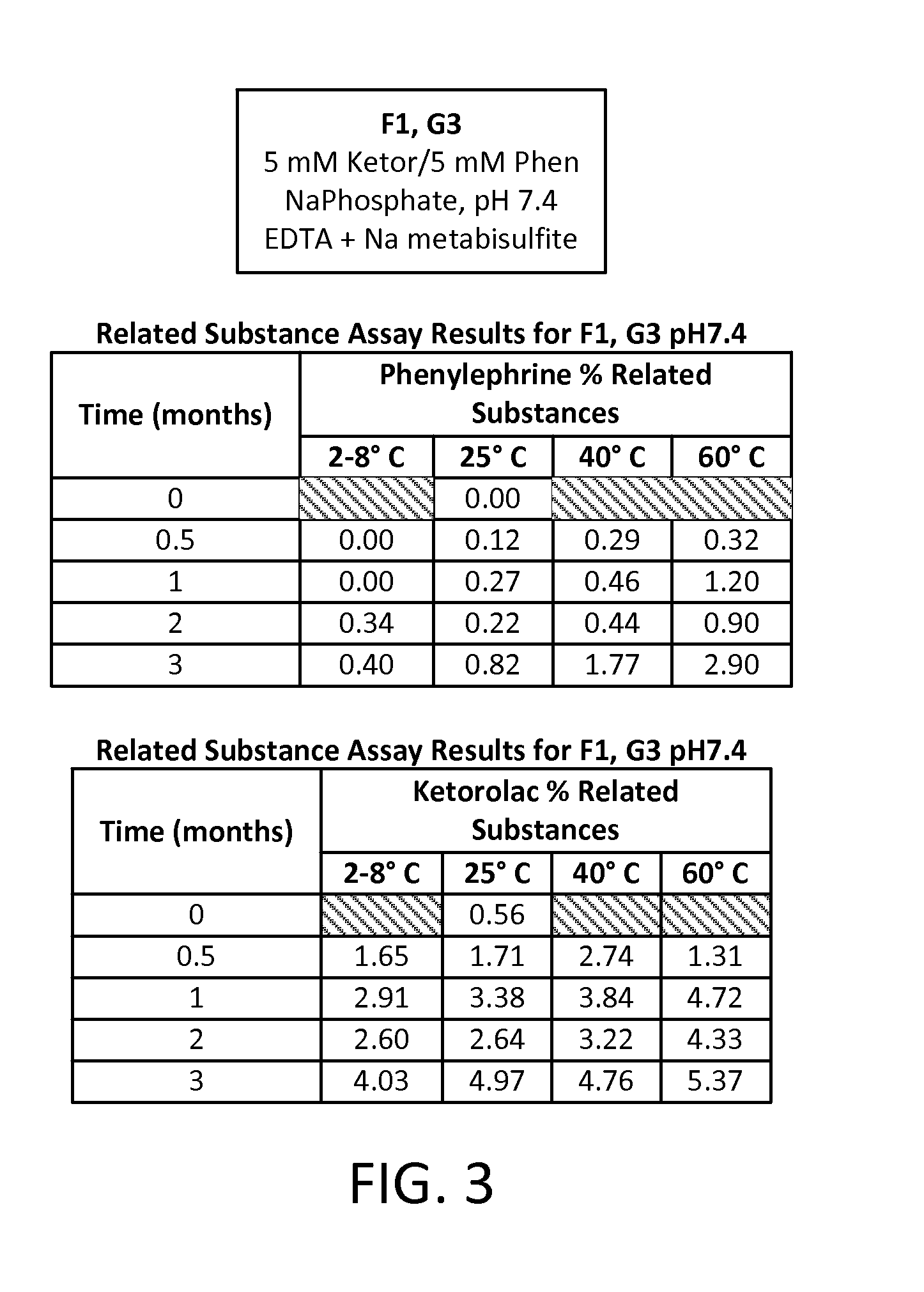
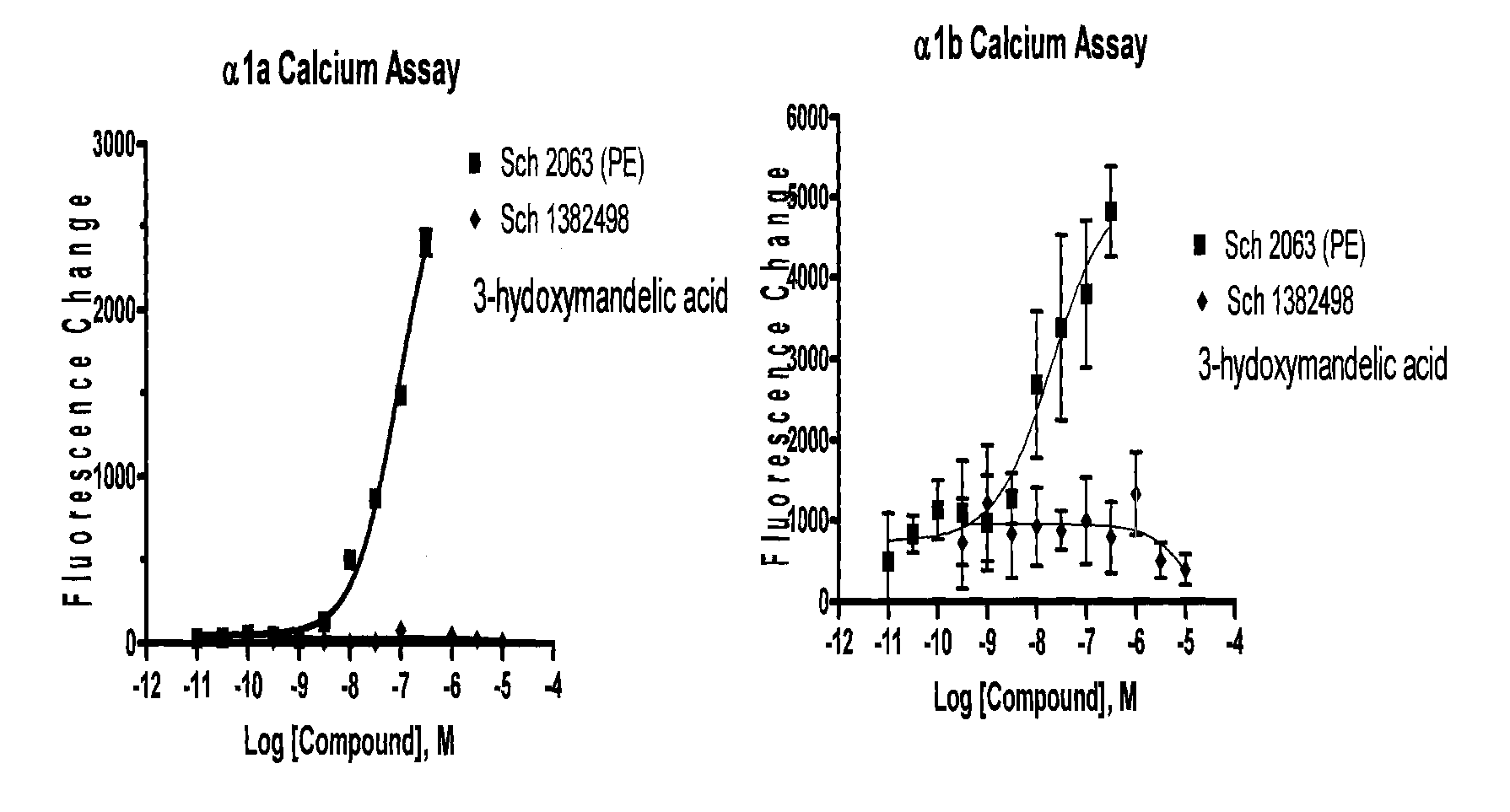
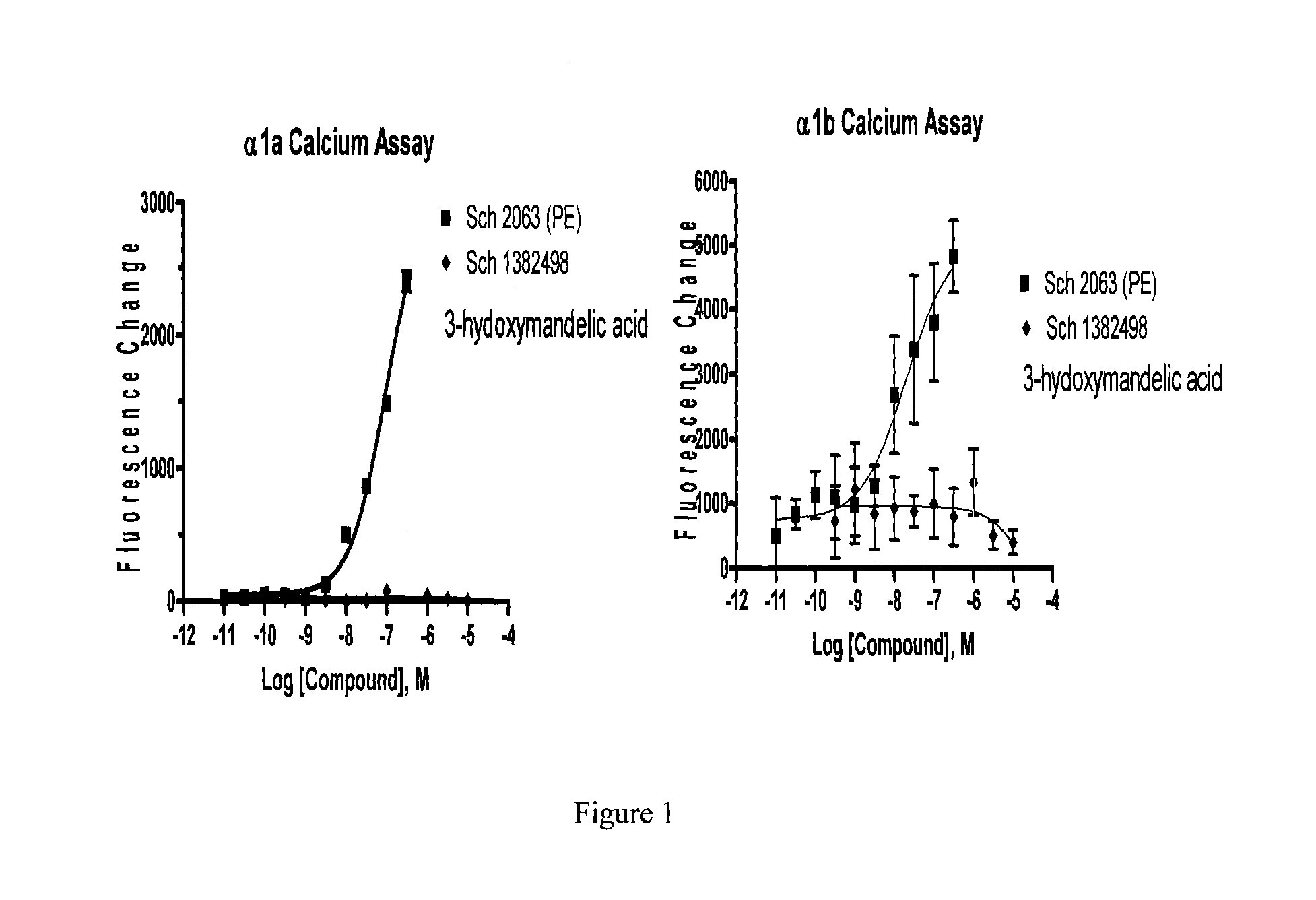
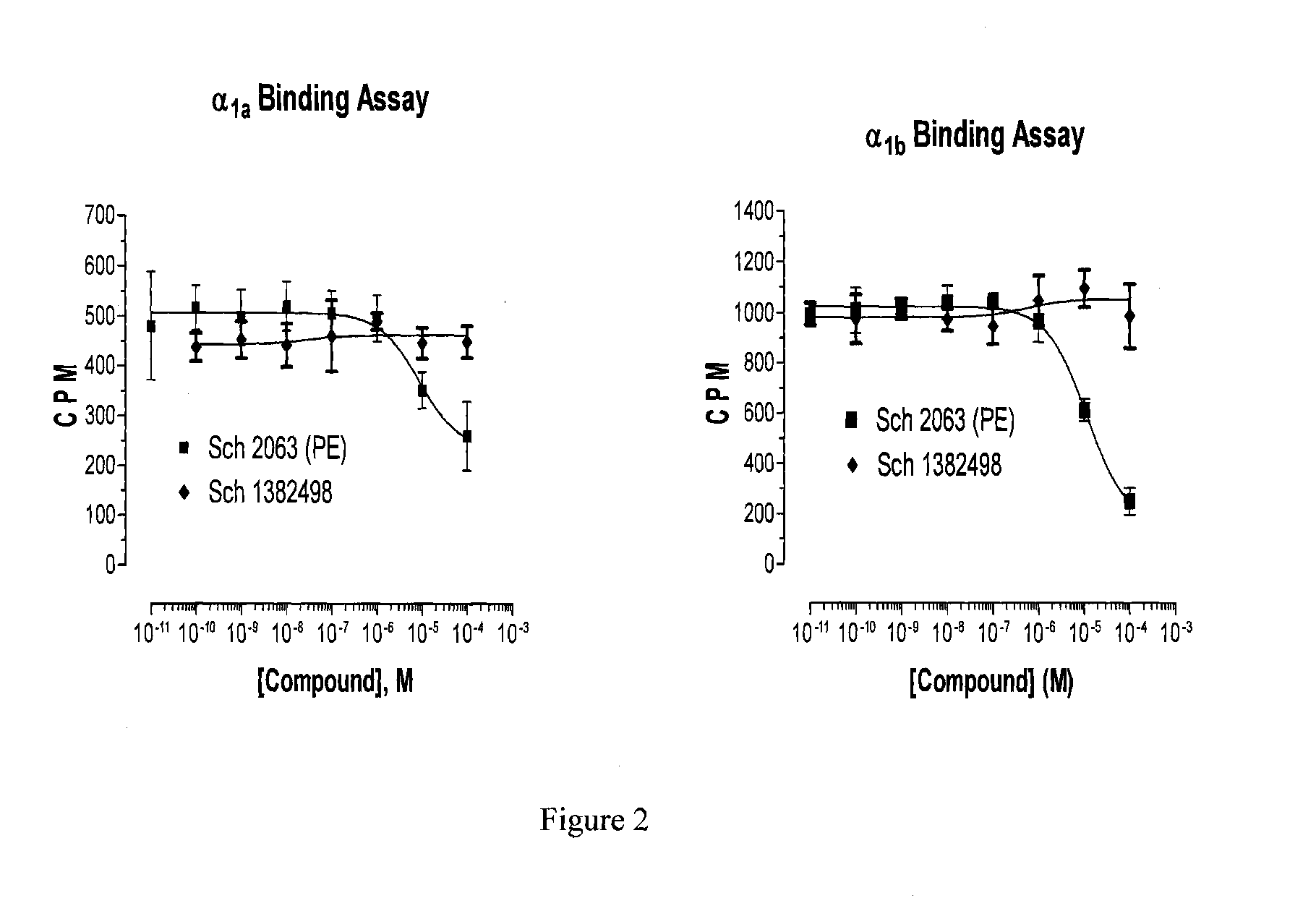
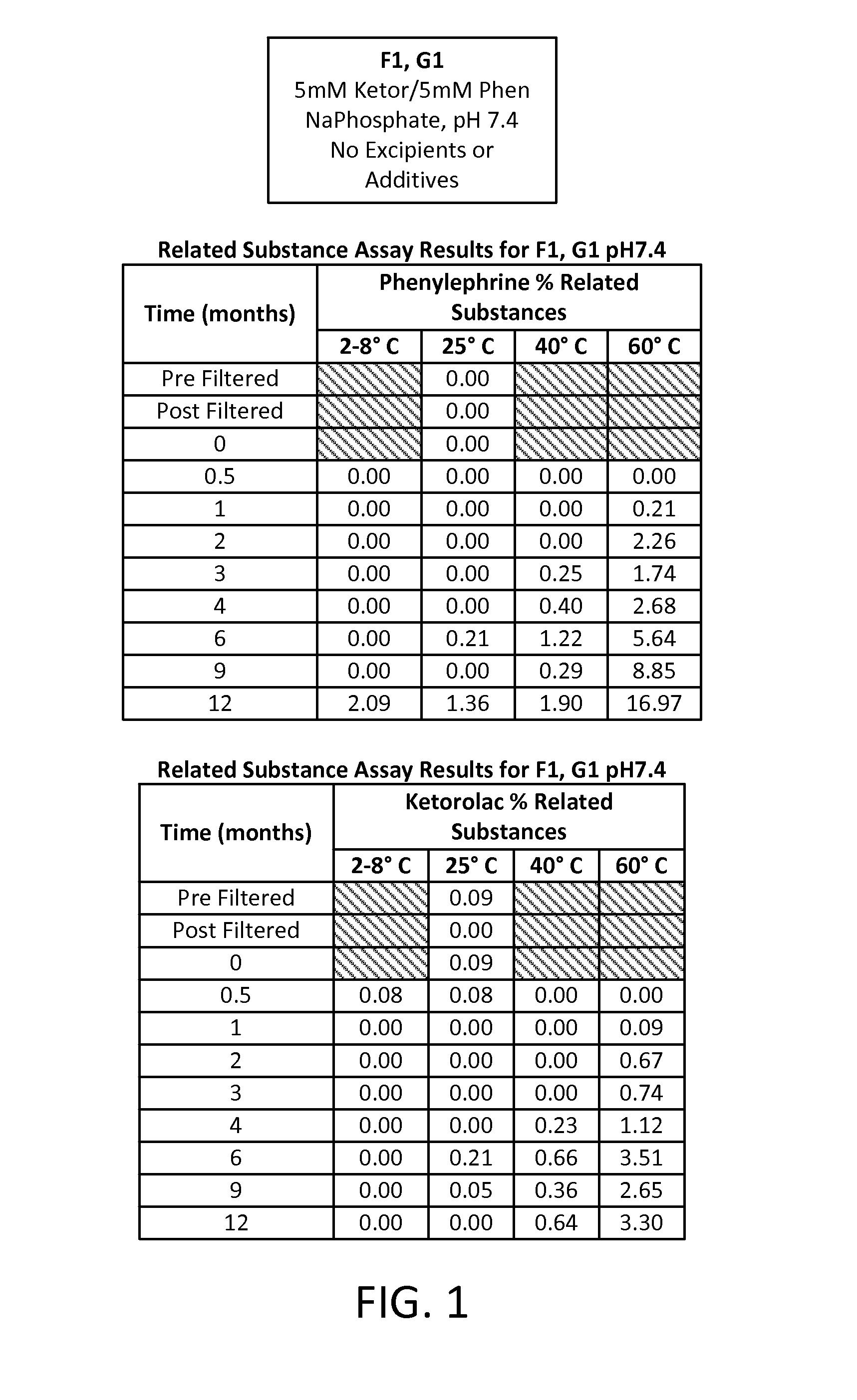
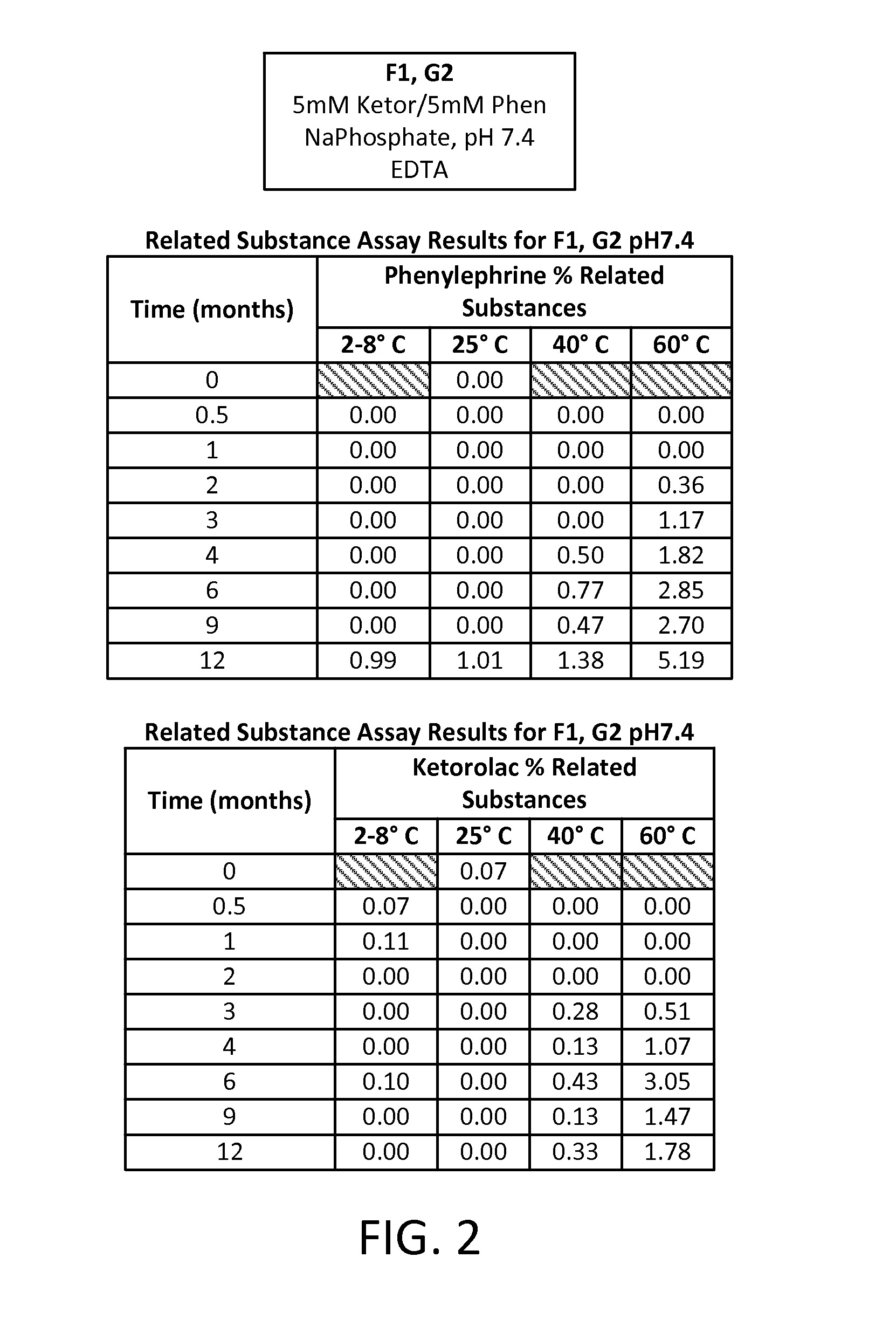
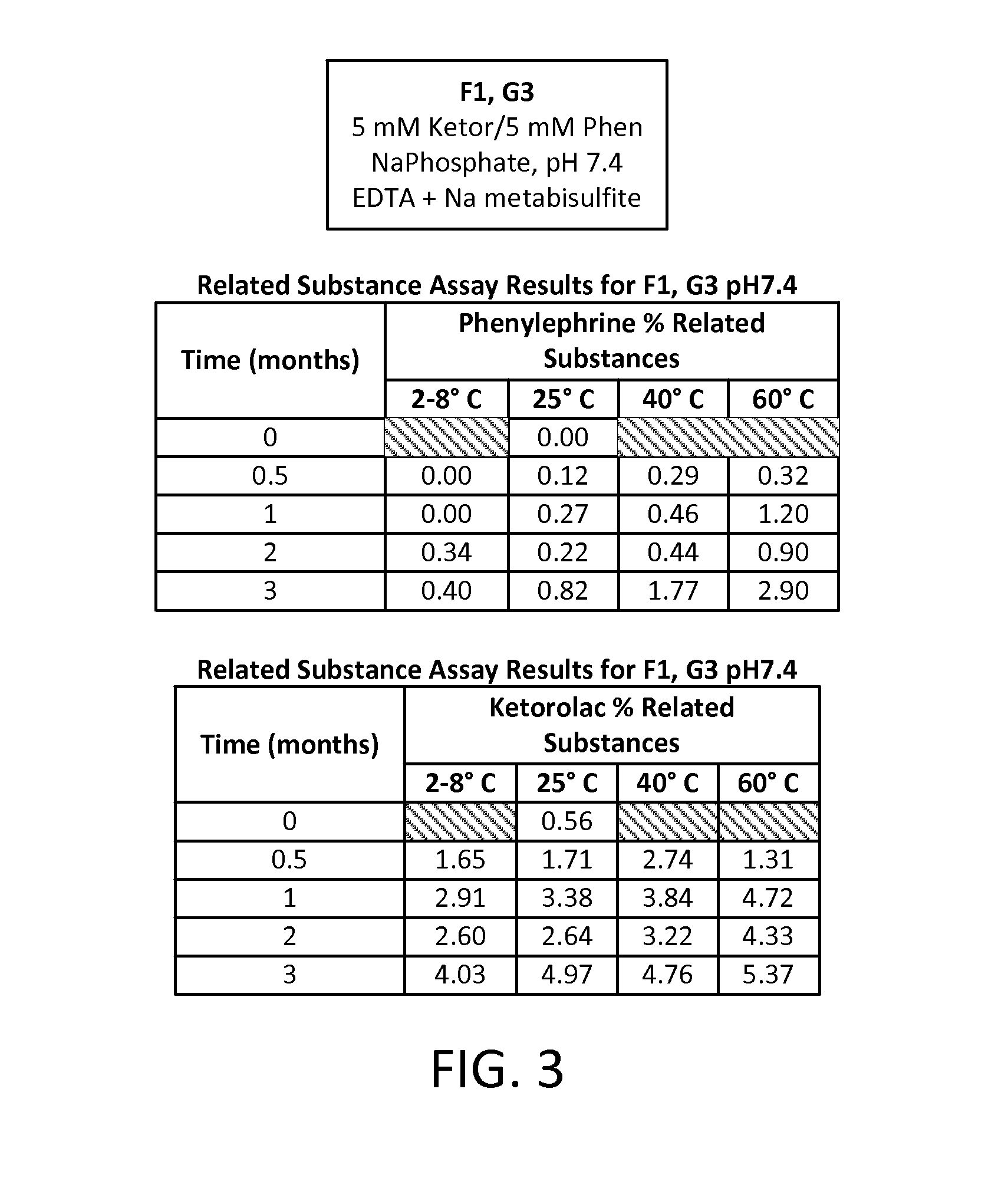
Stable Preservative-free Mydriatic and Anti-inflammatory Solutions for Injection
ActiveUS20140235691A1Avoid Potential ToxicityImprove stabilityBiocideSenses disorderPreservative freeKetorolac
The present invention relates to stable, preservative- and antioxidant-free liquid formulations of phenylephrine and ketorolac for injection.
Owner:RAYNER SURGICAL (IRELAND) LTD
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its salts, and mixtures thereof, in combination with acetaminophen; and optionally in combination with additional pharmaceutical actives. The compositions have a pH of about 6.5 to about 7.5 and may be substantially free of aldehydes. The invention also provides a method of stabilizing phenylephrine. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its salts, and mixtures thereof, in combination with acteaminophen; and optionally in combination with additional pharmaceutical actives, wherein the composition has a pH of from about 6.5 to about 7.5 and may be substantially free of aldehydes.
Owner:THE PROCTER & GAMBLE COMPANY
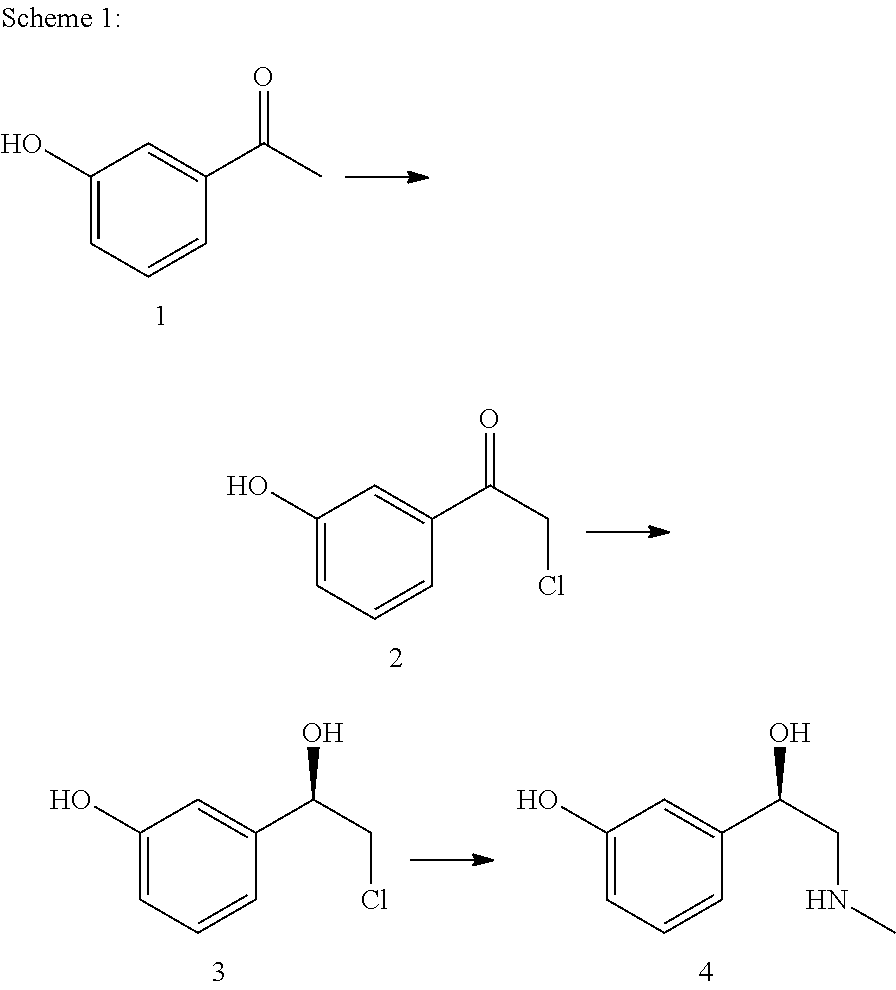
Method for producing l-phenylephrine using an alcohol dehydrogenase of aromatoleum aromaticum ebn1 (azoarcus sp. ebn1)
ActiveUS20110171700A1High stereoselectivityMoreOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
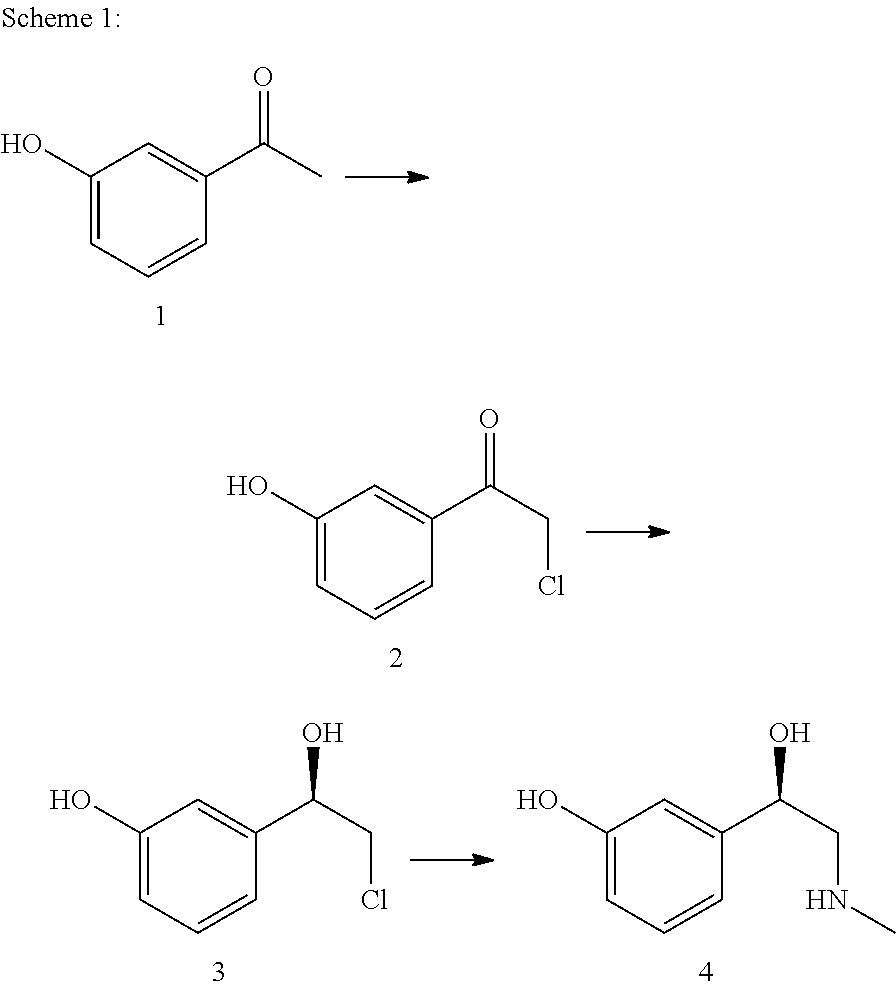
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
Compositions and methods for non-surgical treatment of ptosis
Provided are pharmaceutical compositions, and methods of use of the compositions, for the non-surgical treatment of ptosis (eyelid droop). In one embodiment the composition includes oxymetazoline 0.1% formulated for topical administration to an eye. In one embodiment the composition includes a synergistic combination of oxymetazoline and phenylephrine, formulated for topical administration to an eye. Oxymetazoline alone causes no pupillary dilation (mydriasis), and a synergistic combination of oxymetazoline and phenylephrine induces no clinically significant mydriasis. In addition to providing desirable cosmetic effects, the compositions and methods of the invention can improve visual fields otherwise compromised by ptosis.
Owner:VOOM
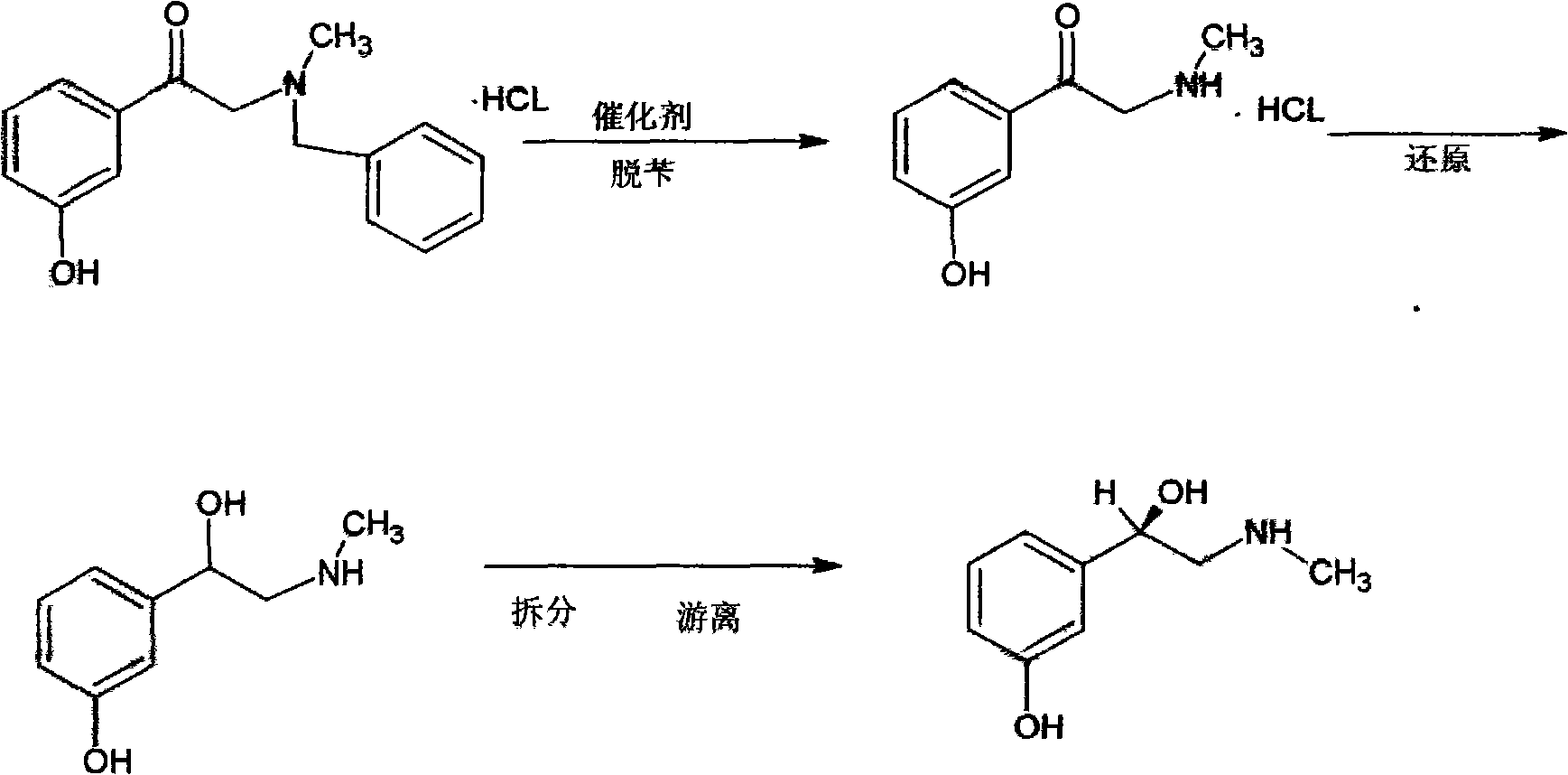
Preparation method of phenylephrine
The invention provides a biological and chemical combined method for preparing phenylephrine. According to the method, on one hand, a biological catalyst is adopted for preparing the phenylephrine and the reaction is mild; and on the other hand, 1-(3-hydroxyphenyl)-2-(methyl (phenylmethyl) amino) ethanone with lower price is taken as raw material.
Owner:SYNCOZYMES SHANGHAI
Compositions and Methods for Non-Surgical Treatment of Ptosis
ActiveUS20120225920A1Good effectBiocideOrganic active ingredientsVisual field lossSurgical treatment
Provided are pharmaceutical compositions, and methods of use of the compositions, for the non-surgical treatment of ptosis (eyelid droop). In one embodiment the composition includes oxymetazoline 0.1% formulated for topical administration to an eye. In one embodiment the composition includes a synergistic combination of oxymetazoline and phenylephrine, formulated for topical administration to an eye. Oxymetazoline alone causes no pupillary dilation (mydriasis), and a synergistic combination of oxymetazoline and phenylephrine induces no clinically significant mydriasis. In addition to providing desirable cosmetic effects, the compositions and methods of the invention can improve visual fields otherwise compromised by ptosis.
Owner:VOOM
Phenylephrine pharmaceutical formulations and compositions for transmucosal absorption
InactiveUS20090280160A1Sustained deliveryBiocideOrganic active ingredientsWhole bodySystemic absorption
Pharmaceutical compositions comprising phenylephrine or a pharmaceutically acceptable salt thereof and methods for administering the pharmaceutical compositions wherein the composition is formulated for systemic absorption of phenylephrine that avoids first pass metabolism. The compositions of the invention are formulated to be applied to oral mucosa of an animal to allow for enhanced systemic delivery of therapeutically active form of phenylephrine.
Owner:MSD CONSUMER CARE INC
Stable preservative-free mydriatic and anti-inflammatory solutions for injection
ActiveUS9066856B2Avoid Potential ToxicityImprove stabilityBiocideSenses disorderPreservative freeKetorolac
The present invention relates to stable, preservative- and antioxidant-free liquid formulations of phenylephrine and ketorolac for injection.
Owner:RAYNER SURGICAL (IRELAND) LTD
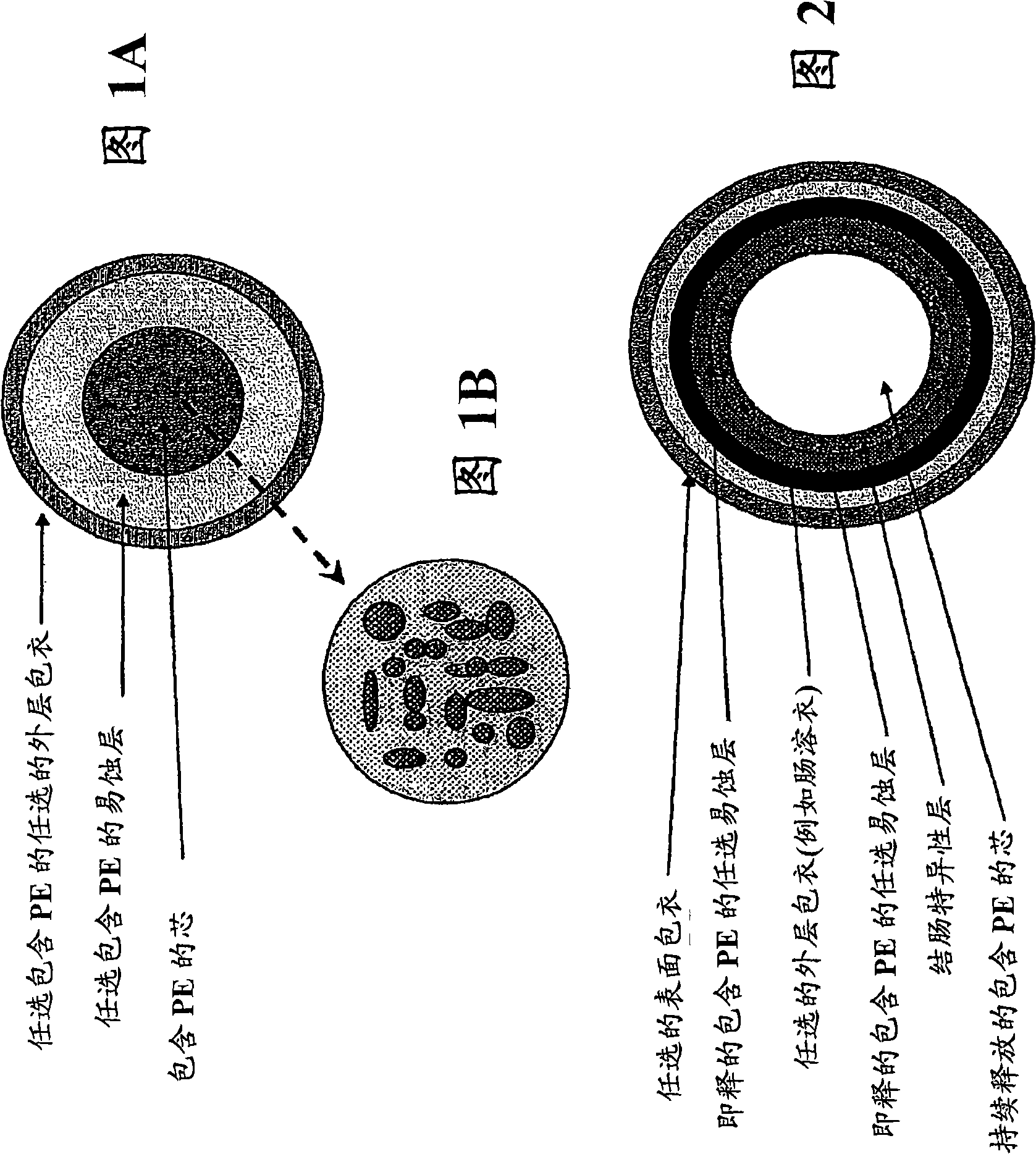
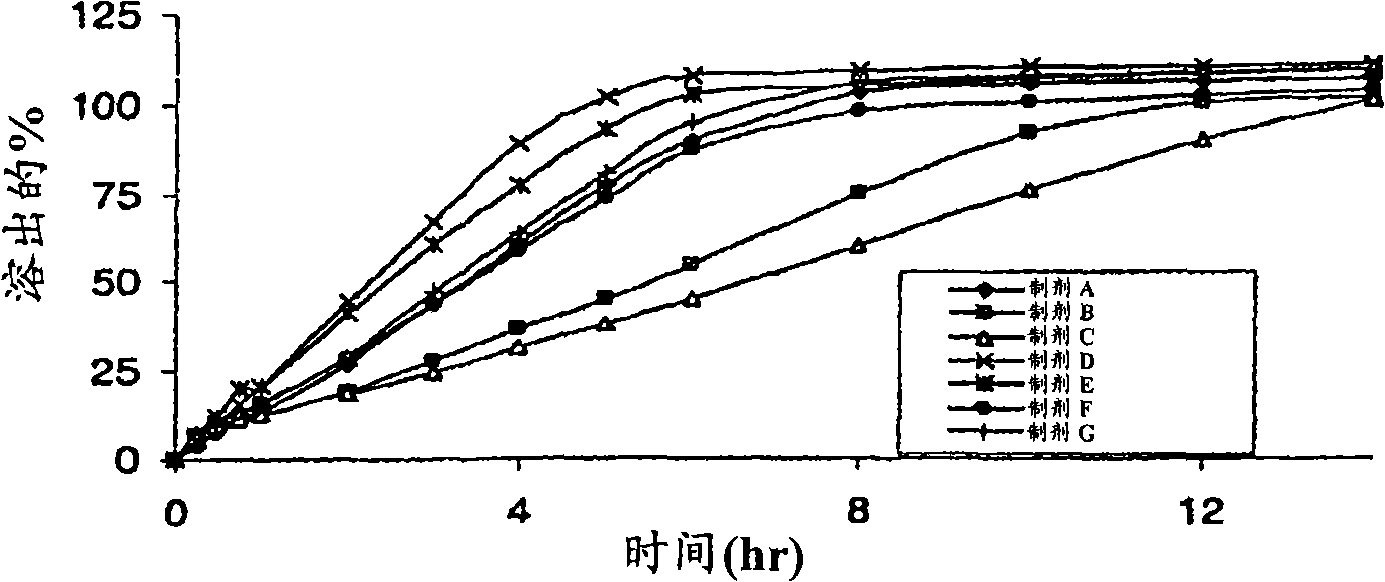
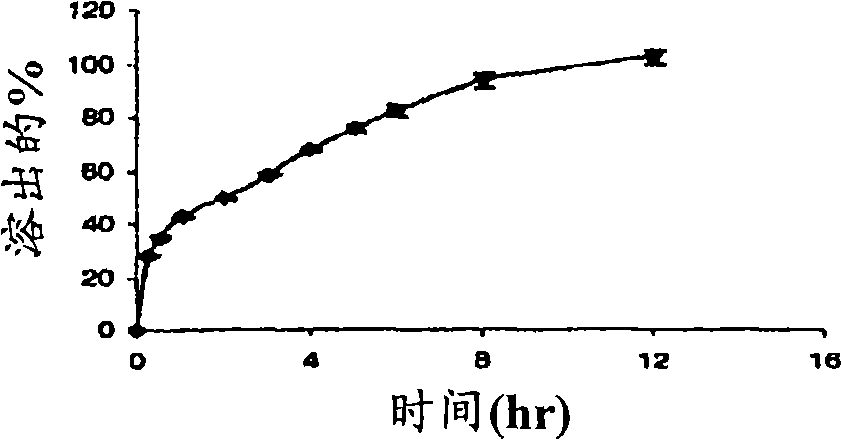
Sustained release pharmaceutical formulation comprising phenylephrine
The invention discloses a pharmaceutical composition comprising phenylephrine or a pharmaceutically acceptable salt thereof and an erodible layer which is for oral administration wherein the composition delivers phenylephrine or a pharmaceutically acceptable salt thereof via absorption in the colon. The pharmaceutical composition comprises a core comprising phenylephrine or a pharmaceutically acceptable salt thereof and an erodible layer which is in a time-dependent, pH-dependent, or colon-specific enzyme-dependent erodible layer that degrades to expose the core to release phenylephrine in the colon. In one preferred embodiment, the erodible layer encases the core. The composition optionally further comprises phenylephrine in the erodible layer or other additional layer(s). The pharmaceutical composition can further comprise one or more additional therapeutically active agents selected from one or more of the group consisting of antihistamines, analgesics, anti-pyretics, and non-steroidal anti-inflammatory agents. The invention also discloses methods of administering phenylephrine via the colon, thereby increasing the bioavailable amount of therapeutically active unconjugated phenylephrine relative to the total phenylephrine in the plasma.
Owner:BAYER CONSUMER CARE
Therapeutic formulations for the treatment of cold and flu-like symptoms
A pharmaceutical formulation of therapeutically effective amounts of acetaminophen, ibuprofen, and a sympathomimetic drug, such as pseudoephedrine (or its prodrug), or phenylephrine used in the treatment of cold and flu-like symptoms. Such symptoms may include fever, pain, nasal congestion, sinus congestion, runny nose, sore throat, myalgia, ear pressure and fullness, and headache. The formulation further includes various excipients used in the formulation process.
Owner:KINGSWAY PHARMA
Compositions and Methods for Non-Surgical Treatment of Ptosis
ActiveUS20120225919A1Good effectOrganic active ingredientsBiocideVisual field lossSurgical treatment
Provided are pharmaceutical compositions, and methods of use of the compositions, for the non-surgical treatment of ptosis (eyelid droop). In one embodiment the composition includes oxymetazoline 0.1% formulated for topical administration to an eye. In one embodiment the composition includes a synergistic combination of oxymetazoline and phenylephrine, formulated for topical administration to an eye. Oxymetazoline alone causes no pupillary dilation (mydriasis), and a synergistic combination of oxymetazoline and phenylephrine induces no clinically significant mydriasis. In addition to providing desirable cosmetic effects, the compositions and methods of the invention can improve visual fields otherwise compromised by ptosis.
Owner:VOOM
Preparation method of phenylephrine
InactiveCN101921197AEmission reductionReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationDissolutionNitrogen gas
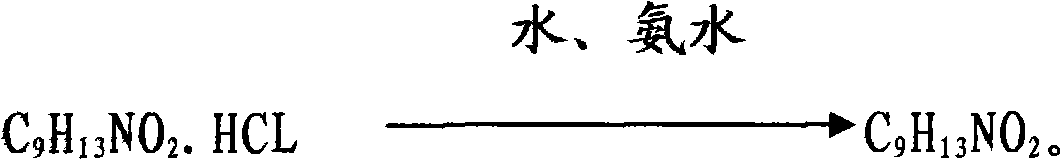
The invention discloses a preparation method of phenylephrine, which is characterized by comprising the following steps of: firstly adding alpha-benzyl methylamino m-hydroxy acetophenone hydrochloride to a solvent, and heating for dissolution; then adding a chiral catalyst at the temperature of 30-60 DEG C; introducing hydrogen after replacement of nitrogen; keeping relative pressure at 0.05+ / -0.01 MPa; carrying out catalytic hydrogenation for 10-20 hours, and then adding 18-25 percent by weight of ammonia water for reaction at the temperature of 15-25 DEG C, wherein the addition proportion of the ammonia water and the alpha-benzyl methylamino m-hydroxy acetophenone hydrochloride is 1.5:1-1:1; and separating and washing reaction products to obtain the phenylephrine. The invention has the advantages of simple production process, easy control of reaction conditions, few reaction byproducts, reduced environmental pollution, enhanced product yield coefficient, reduced production cost and product quality reaching the quality standard of the European pharmacopeia EP5.
Owner:山东潍坊幸福药业有限公司
Phenylephrine pulsed release formulations and pharmaceutical compositions
The invention discloses a pulsed-release formulation or a pharmaceutical composition comprising phenylephrine. The pharmaceutical composition comprises an immediate-release component and an enteric-coated component formulated together either in solid form or in a suspension. The enteric-coated component comprises microcrystals seeded with phenylephrine as an active ingredient and coated with a pH sensitive coating to delay release of the phenylephrine. The pharmaceutical composition can further comprise at least one active selected from the group consisting of an antihistamine, an analgesic, anti-pyretic, non-steroidal anti-inflammatory and mixtures of two or more said actives.
Owner:SCHERING CORP
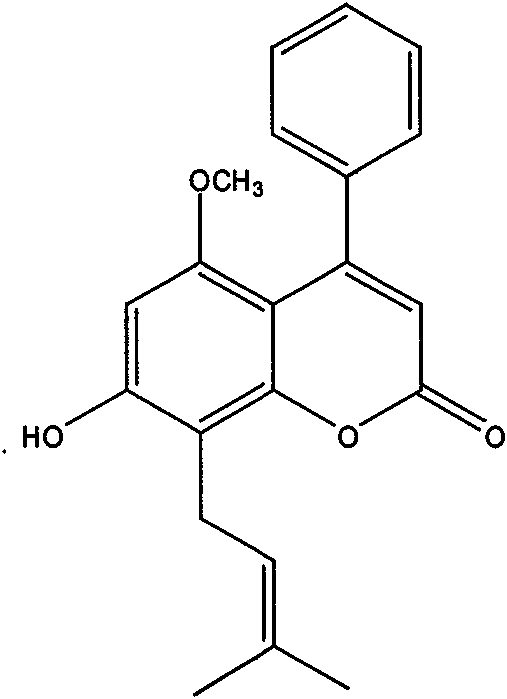
New coumarin active content, preparation method and application thereof
ActiveCN101619054AStrong free radical scavenging activityHigh activityNervous disorderOrganic chemistrySolventPlatelet inhibition
The invention relates to a new coumarin active content, i.e. pigeon pea lactone extracted and separated from cajan leaves, an extraction and separation method and an application thereof. The structure of the compound is shown as a figure 1. The invention adopts the technical scheme that agricultural waste of cajan leaves is used as raw material which is extracted by a solvent; the extracting solution is concentrated and dispersed in water; an ultrasonic oscillation flocculation technology, a bubble column extraction technology and a column chromatography separation technology in positive-phase silica gel are adopted and combined with a low-temperature crystallization and recrystallization technology to obtain the content with the purity higher than 95 percent; and the anti-oxidation activity and the action of the chemical content on cardiovascular diseases are detected by a DPPH model, a beta-renieratene-linoleic acid model, an in-texture SOD enzyme model, a platelet aggregation model induced by ADP in corpora and an aorta systole model induced by phenylephrine. Results show that the active content has favorable free radical scavenging activity, anti-oxidation activity in corpora and in vitro, platelet aggregation restraining action in corpora and a certain hemangiectasis action. Meanwhile, the preparation method of the pigeon pea lactone in the invention is easy and practical and can obtain a product with high purity high yield and high added value in a short period which is suitable for industrialized production and application, can be further developed and researched into a new antioxidant as an additive for medicines, foods and cosmetics.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Method for stabilizing phenylephrine
InactiveUS20090047343A1Easy to manageHigh strengthBiocidePowder deliveryCombinatorial chemistryPhenylephrine
The present invention relates to a process for stabilizing phenylephrine including drying an acidic solution of phenylephrine and pharmaceutical compositions including stabilized phenylephrine.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Pharmaceutical Suspensions and Related Methods
A pharmaceutical suspension having a therapeutically effective amount of phenylephrine and a therapeutically effective amount of a first active agent consisting essentially of a first substantially water insoluble active agent having an average particle size of between about 10 and about 100 microns, an effective amount of non-reducing sweetener; an effective amount of water; and an effective amount of a suspending system; wherein the pharmaceutical suspension has a pH of from about 4 to about 6 and is substantially free of a reducing sugar and related methods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Phenylephrine pharmaceutical formulations and compositions for transmucosal absorption
InactiveCN101938991AOrganic active ingredientsAntipyreticSystemic absorptionPharmaceutical formulation
Pharmaceutical compositions comprising phenylephrine or a pharmaceutically acceptable salt thereof and methods for administering the pharmaceutical compositions wherein the composition is formulated for systemic absorption of phenylephrine that avoids first pass metabolism. The compositions of the invention are formulated to be applied to oral mucosa of an animal to allow for enhanced systemic delivery of therapeutically active form of phenylephrine.
Owner:SCHERING PLOUGH HEALTHCARE PRODUCTS INC
Method for producing L-phenylephrine using an alcohol dehydrogenase of Aromatoleum aromaticum EBN1 (Azoarcus sp. EBN1)
ActiveUS8617854B2MoreHigh stereoselectivityOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
Compositions and methods useful for treatment of respiratory illness
Disclosed are compositions including phenylephrine, its free and addition salt forms, and mixtures thereof, alone, or in combination with other pharmaceutical actives. The compositions have a pH of about 2 to about 5 and are substantially free of aldehydes. Also disclosed are methods of treating respiratory illness through administration of a composition comprising phenylephrine, its free and addition salt forms, and mixtures thereof alone, or in combination with other pharmaceutical actives, wherein the composition has a pH of from about 2 to about 5 and is substantially free of aldehydes.
Owner:PROCTER & GAMBLE CO
Hirsutine analogue and application thereof in preparation of anti-hypertensive drugs
InactiveCN104974154AOvercome the defects of large toxic and side effectsOrganic chemistryBulk chemical productionHypertension medicationsVascular ring
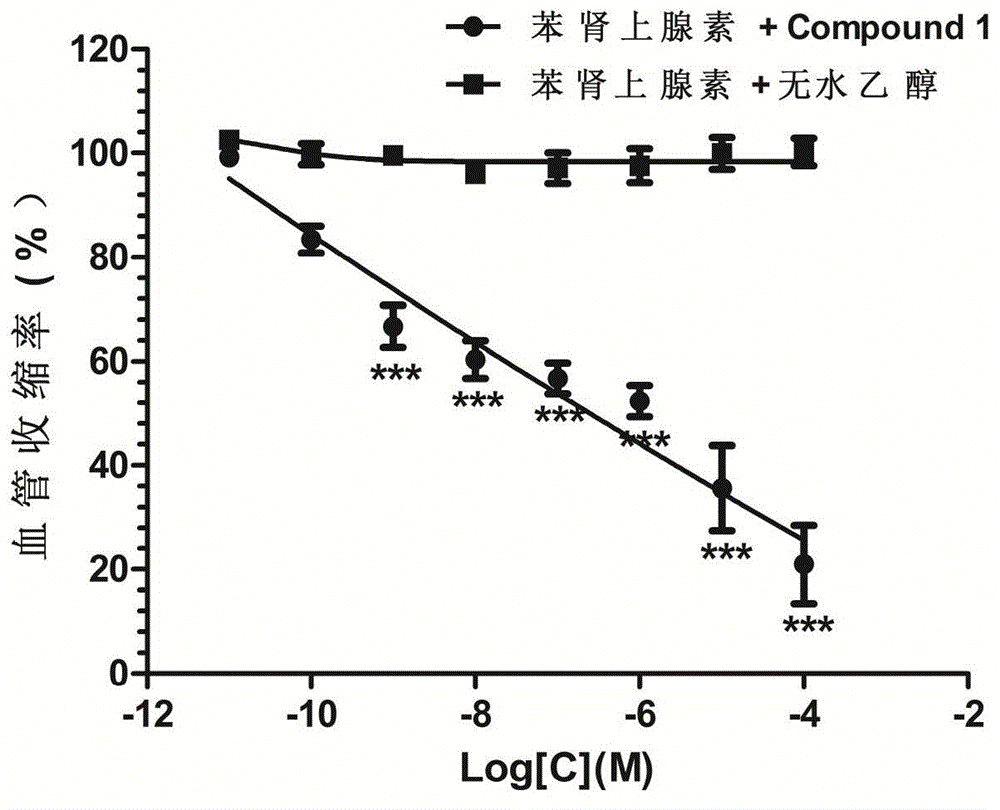
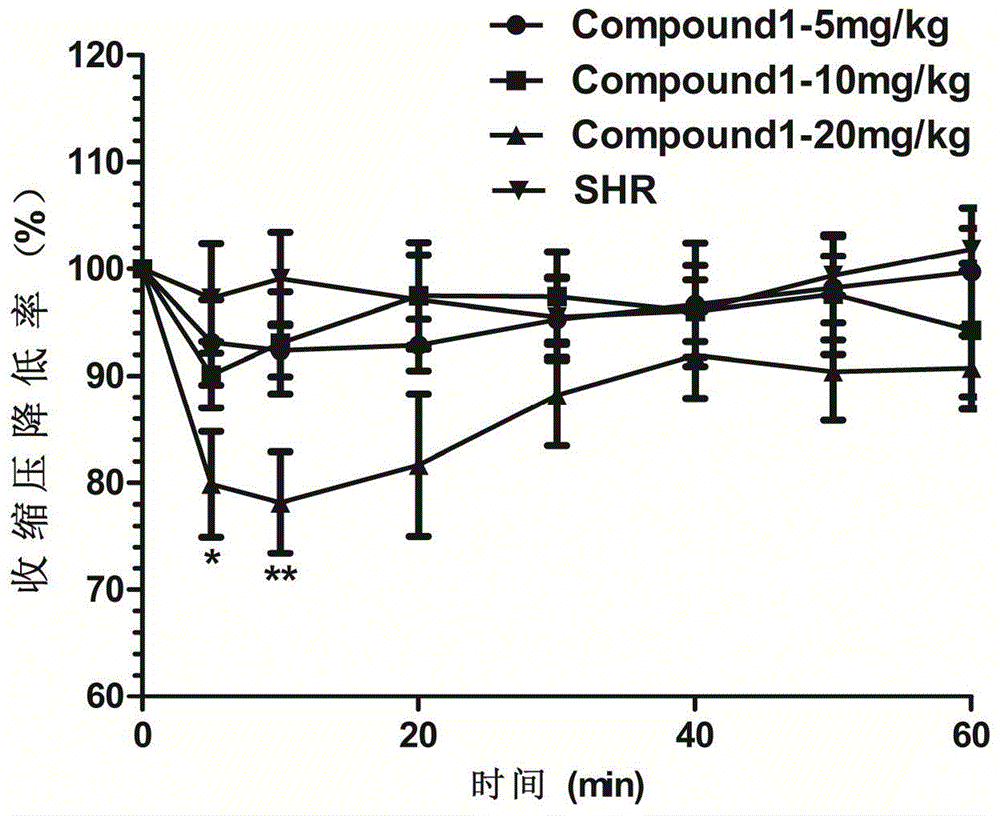
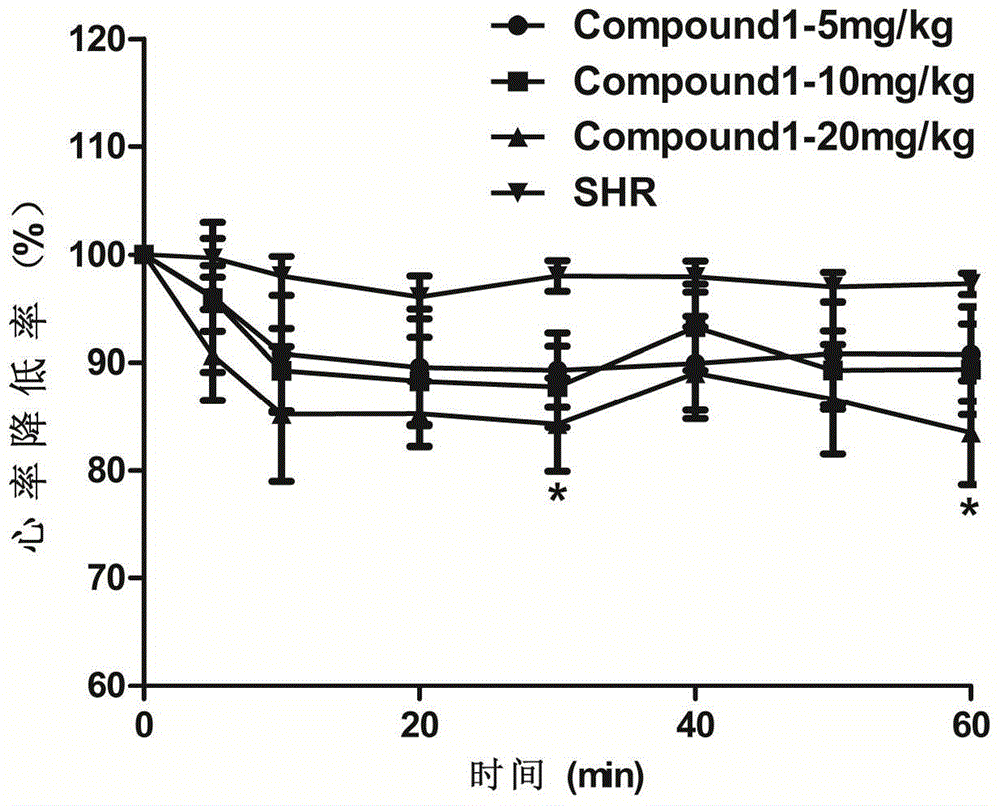
The invention belongs to the field of traditional Chinese medicine production and relates to a traditional Chinese medicine uncaria extract, namely hirsutine analogue compound with the structure represented by formula (1) and application of the hirsutine analogue compound in the preparation of anti-hypertensive drugs. An experiment proves that the hirsutine analogue compound has remarkable pharmacological actions of resisting hypertension and vascular dilation. A spontaneously hypertensive rat (SHR) experiment proves that the blood pressure and the heart rate of an SHR are obviously decreased after 30 minutes of administration, and the blood pressure of the spontaneously hypertensive rat can be rapidly and effectively decreased; an in-vitro vascular ring vasomotion experiment proves that by virtue of an extremely low dose of the hirsutine analogue compound, the vascular ring contraction caused by phenylephrine can be remarkably dilated. The hirsutine analogue compound can be used as a raw material of a pharmaceutical preparation, is further used for preparing drugs for treating hypertension and is particularly used for preparing vascular dilation drugs for treating hypertensive emergencies.
Owner:FUDAN UNIV
Biotin derivative of phenylephrine and preparation method and application thereof
InactiveCN103059288ASmall steric hindranceReduce non-specific adsorptionLuminescent compositionsFluorescencePolyethylene glycol
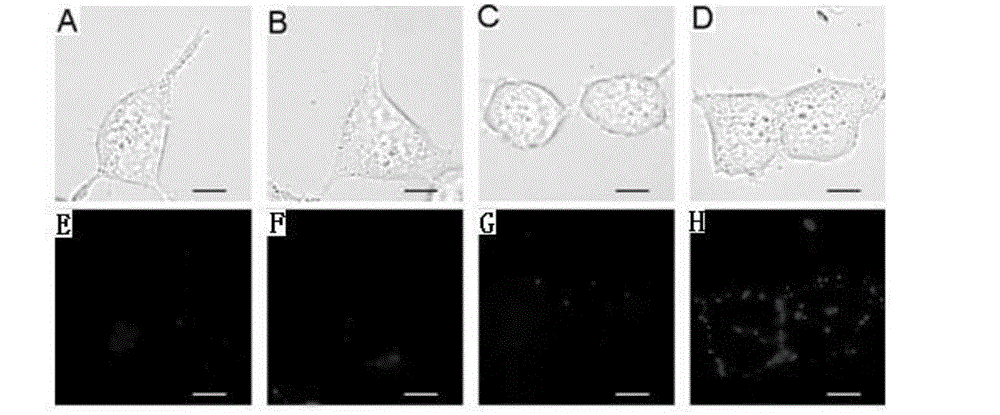
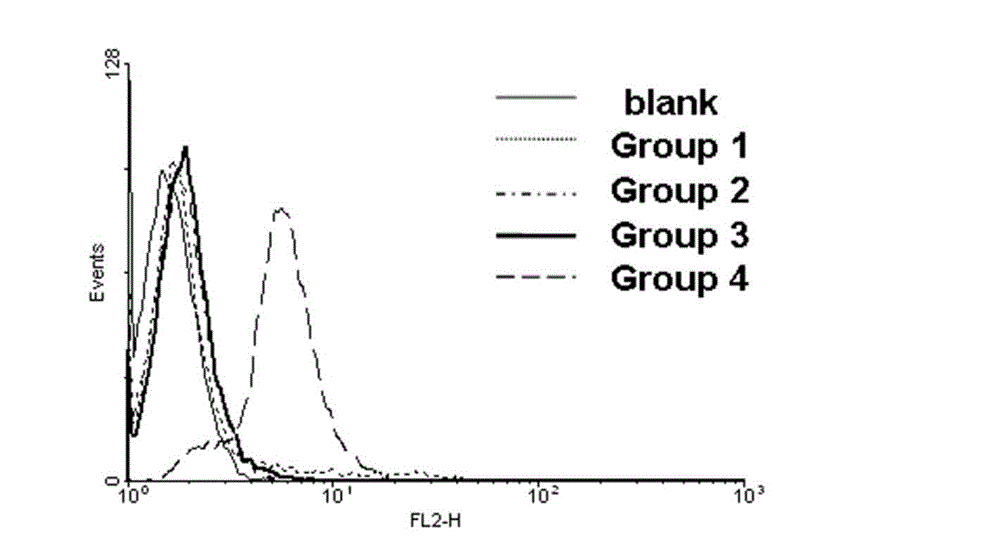
The invention discloses a biotin derivative of phenylephrine and a preparation method and application thereof. The method includes: firstly, the synthetization of double-different-end polyethylene glycol; and secondly, the synthetization of piperazine derivative of phenylephrine; and thirdly, the synthetization of biotinylation of piperazine derivative of phenylephrine. The biotin derivative of phenylephrine and preparation method and application thereof discloses the application of the biotin derivative of phenylephrine as a fluorescence probe. The method firstly synthetizes the biotin derivative capable of being used to be connected with the biotin derivative of phenylephrine of alpha I adrenergic receptor agonist of streptavidin quantum dots, reserves the pharmaceutical activity part of the biotin derivative, and provides a tool for the future application of quantum dots in the research field of alpha I adrenergic receptor. Using PEG to connect alpha I adrenergic receptor agonist with biotin, the nonspecific adsorption of quantum dots and the steric hindrance generated after connected with the agonist are reduced. Because of continuous charging, the method is simple and effective, simple in operation, and good in purification effect. The structural formula is shown in the description, and n=25-60.
Owner:SHANDONG UNIV
Pulsed Release Phenylephrine Dosage Forms
A multi-particle dosage form that can deliver phenylephrine in controlled pulsed doses. The dosage form can contain an immediate release form that can contain phenylephrine or a salt thereof and a plurality of delayed release particles with a coating that can contain phenylephrine or salt thereof and a pH sensitive coating.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com