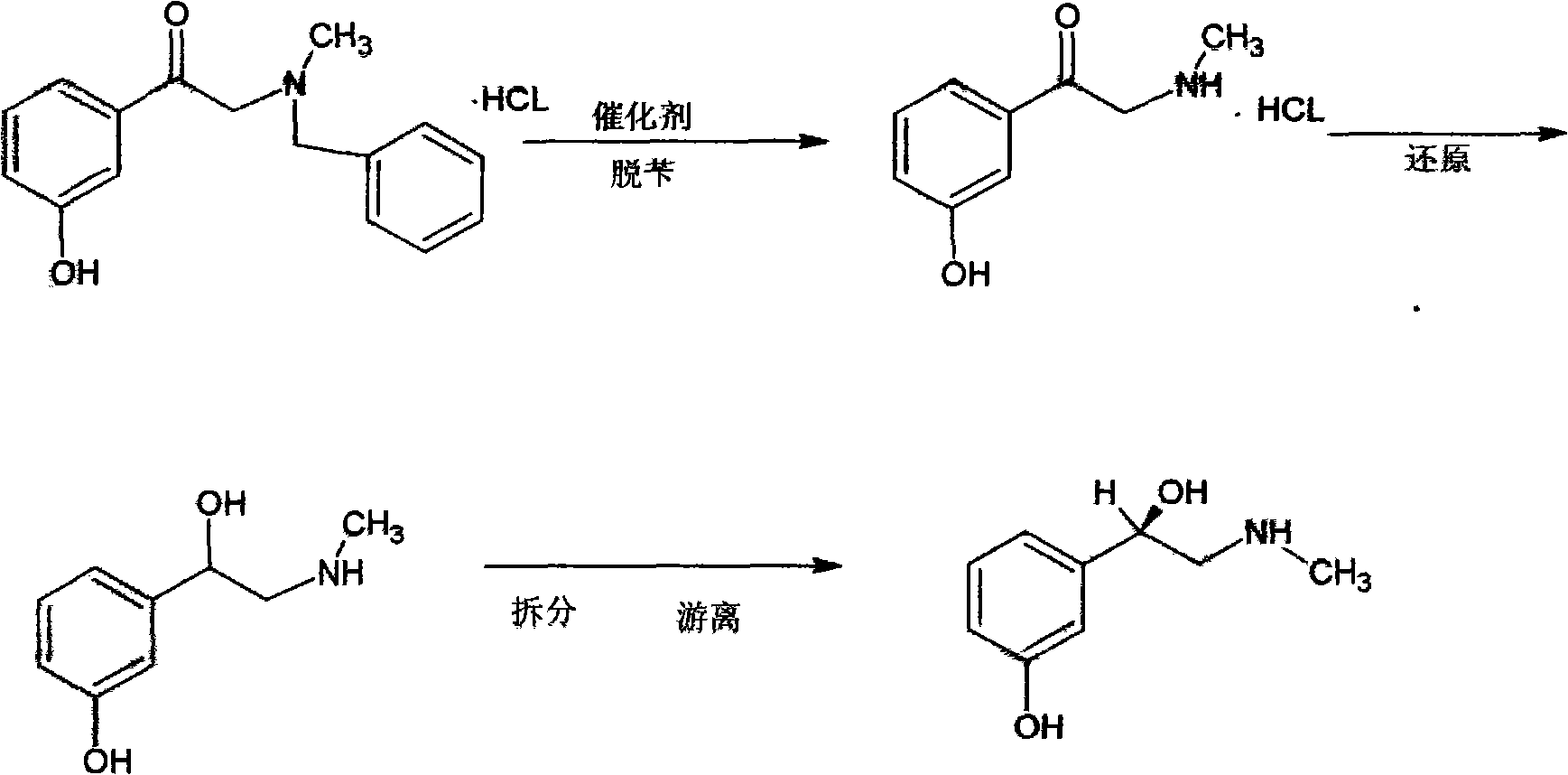
Preparation method of phenylephrine
A technology of phenylephrine and benzylmethylamino-m-hydroxyacetophenone hydrochloride, which is applied in the field of medicinal chemistry, can solve the problems of difficult mother liquor handling, complicated steps, and inadequate reaction, and achieve good environmental and economic benefits , the reaction conditions are easy to control, and the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
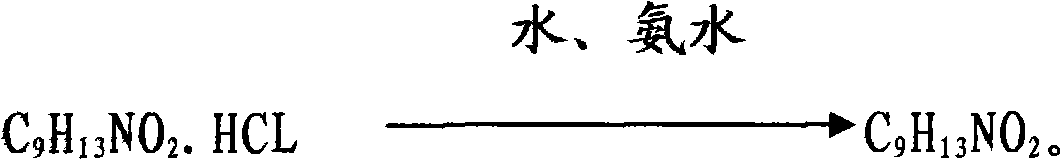
[0028] α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is dropped into 99.5wt% methanol solution, and the feeding weight ratio of the α-benzylmethylamino-m-hydroxyacetophenone hydrochloride to solvent is 1:10 , heated to dissolve, add 2.0% chiral catalyst palladium complex of α-benzylmethylamino-m-hydroxyacetophenone hydrochloride when the temperature is 35 ° C, pass through hydrogen after nitrogen replacement, at a relative pressure of Catalytic hydrogenation reaction is carried out at 0.05MPa, the toluene produced by the reaction is purified to more than 99wt% after split distillation, and then recycled. After 18 hours of reaction, the reaction solution is concentrated to viscous, and 18wt% ammonia water is added when the temperature is 18°C , the addition ratio of ammonia water and α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is 1.4:1, and the reaction product is centrifuged and washed with deionized water to obtain phenylephrine.
Embodiment 2
[0030] α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is dropped into 99.5wt% methanol solution, and the feeding weight ratio of α-benzylmethylamino-m-hydroxyacetophenone hydrochloride to solvent is 1:12 , heated to dissolve, add 1.5% chiral catalyst palladium complex of α-benzylmethylamino-m-hydroxyacetophenone hydrochloride when the temperature is 40 ° C, pass through hydrogen after nitrogen replacement, at a relative pressure of Catalytic hydrogenation reaction is carried out at 0.05MPa, the toluene produced by the reaction is purified to more than 99wt% after split distillation, and then recycled. After 15 hours of reaction, the reaction solution is concentrated to viscous, and 20wt% ammonia water is added when the temperature is 20°C , the addition ratio of ammonia water and α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is 1.1:1, and the reaction product is centrifuged and washed with deionized water to obtain phenylephrine.
Embodiment 3
[0032] α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is dropped into 99.5wt% methanol solution, and the feeding weight ratio of α-benzylmethylamino-m-hydroxyacetophenone hydrochloride to solvent is 1:15 , heated to dissolve, add 1.8% chiral catalyst palladium complex of α-benzylmethylamino-m-hydroxyacetophenone hydrochloride when the temperature is 50°C, pass through hydrogen after nitrogen replacement, at a relative pressure of Carry out catalytic hydrogenation reaction when 0.05MPa, the toluene produced by the reaction is purified to more than 99wt% after split distillation, and then recycled. After reacting for 16 hours, the reaction solution is concentrated to viscous, and the temperature is 22 ℃ to add 19wt% ammonia water , the addition ratio of ammonia water and α-benzylmethylamino-m-hydroxyacetophenone hydrochloride is 1.3:1, and the reaction product is centrifuged and washed with deionized water to obtain phenylephrine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com