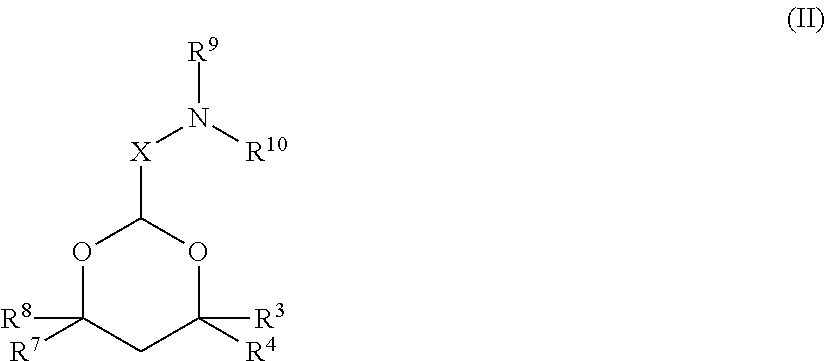Patents
Literature
2662 results about "Low dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
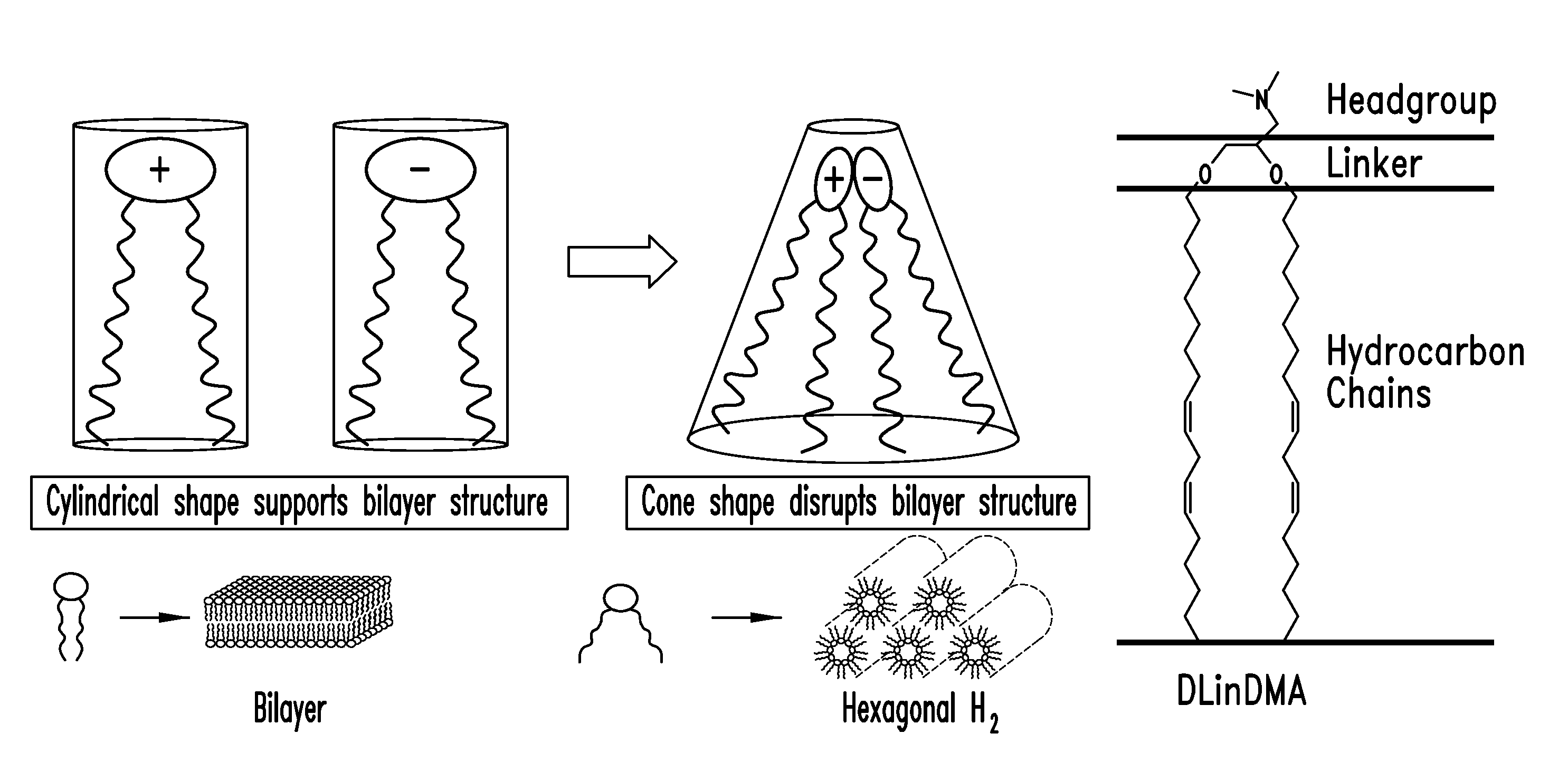
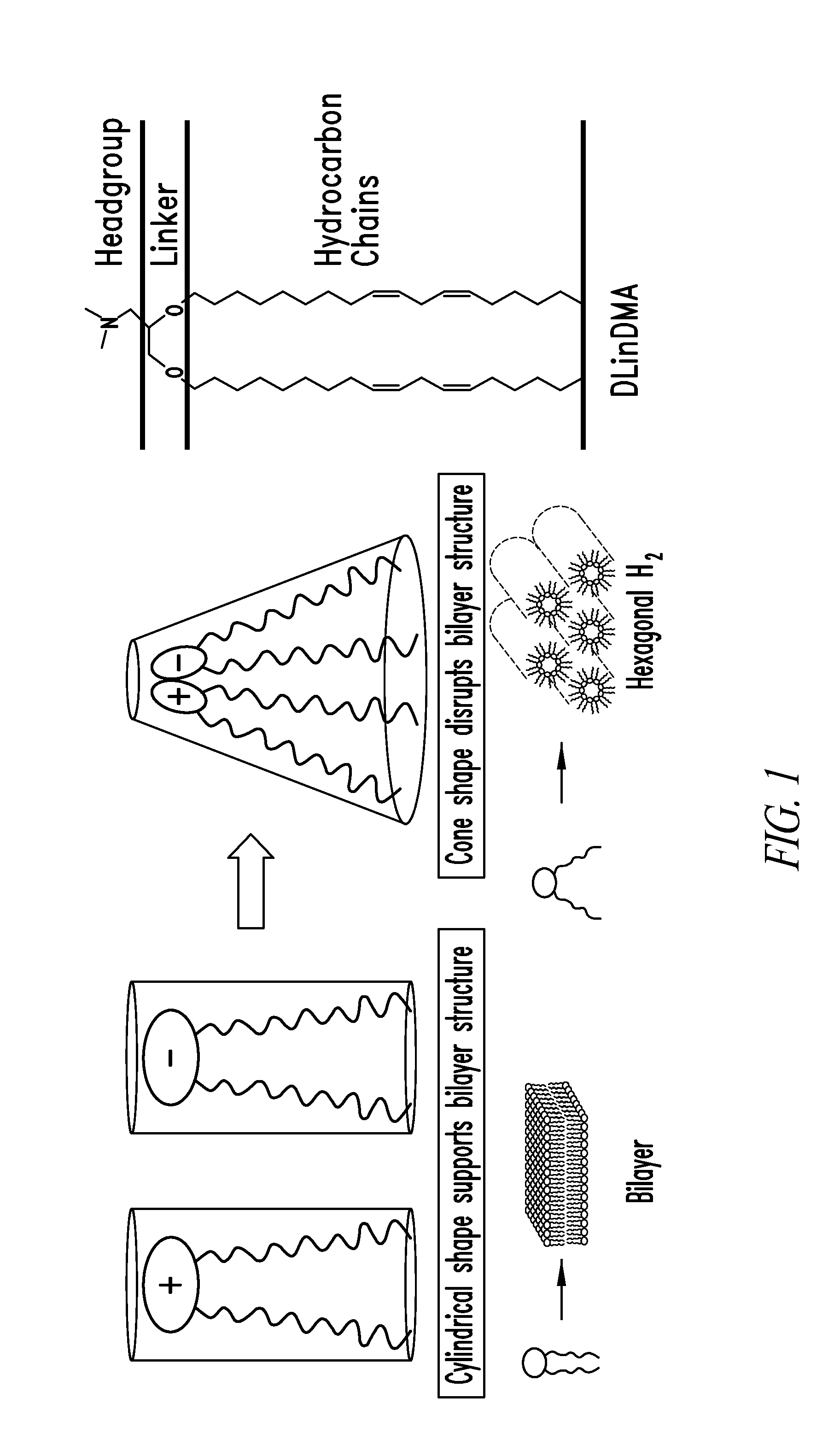
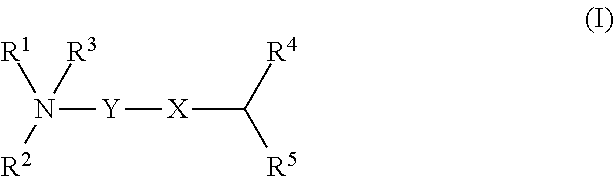
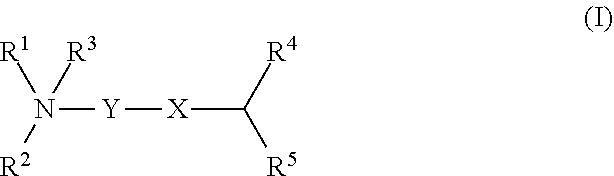
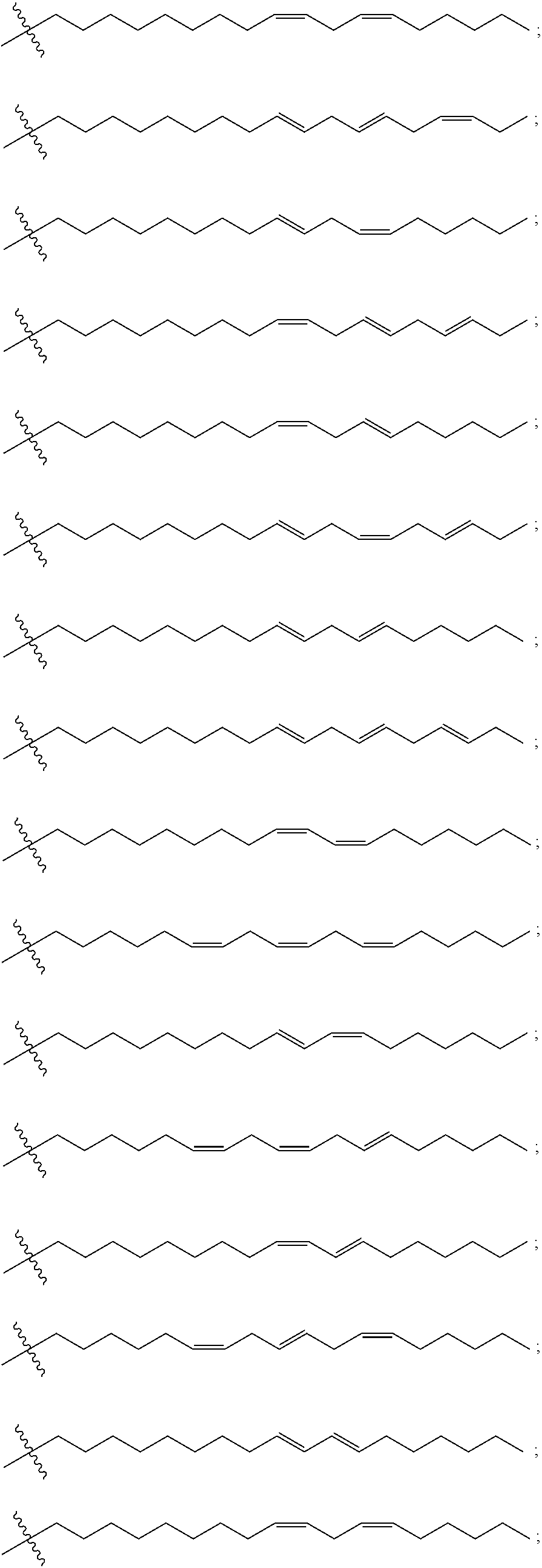
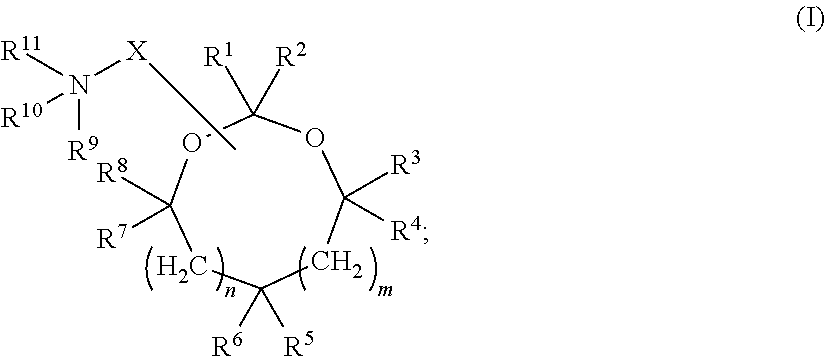
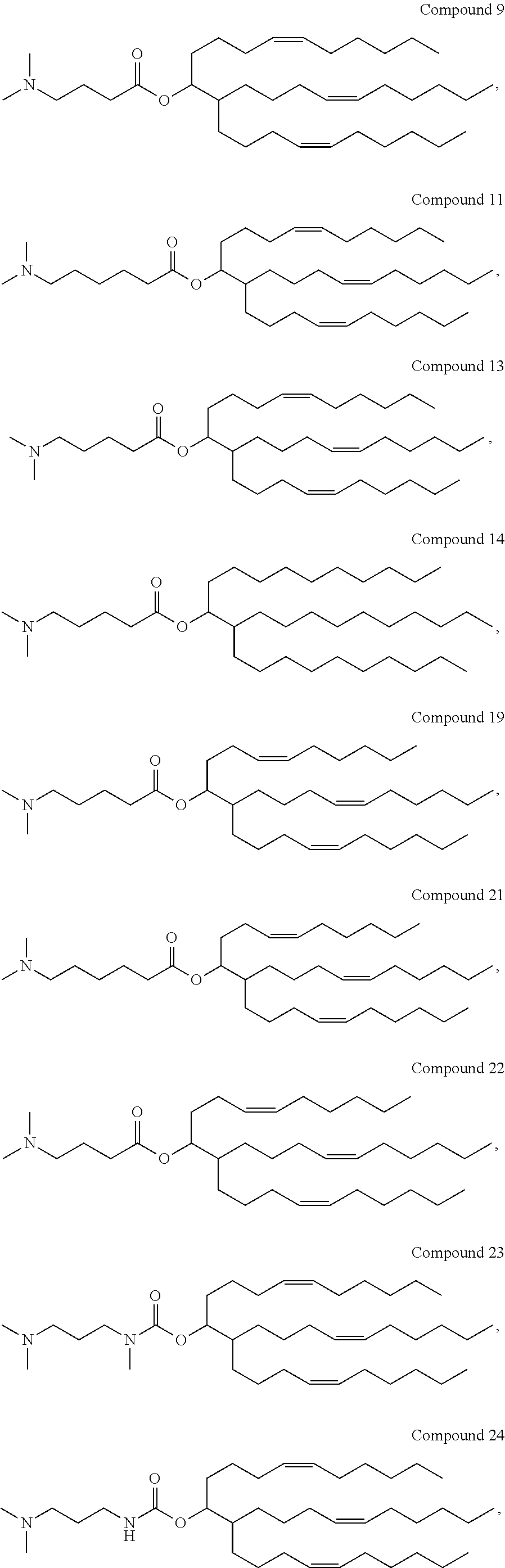
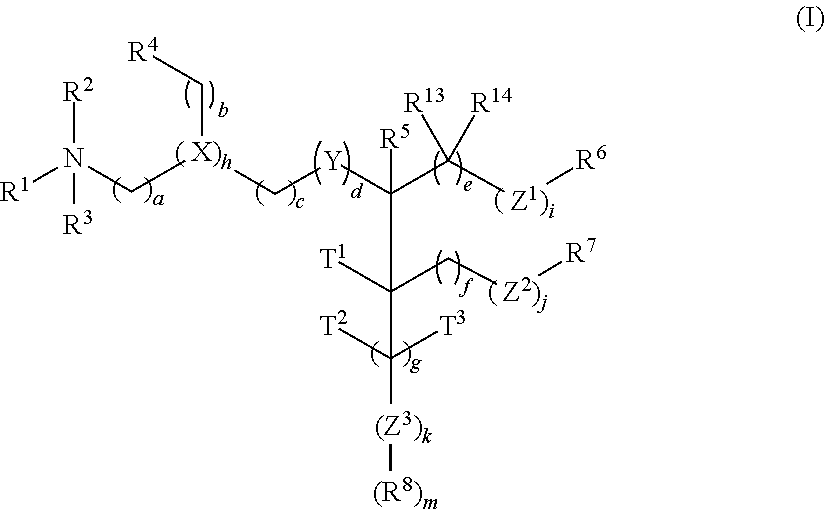
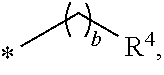
Cationic lipids and methods for the delivery of therapeutic agents
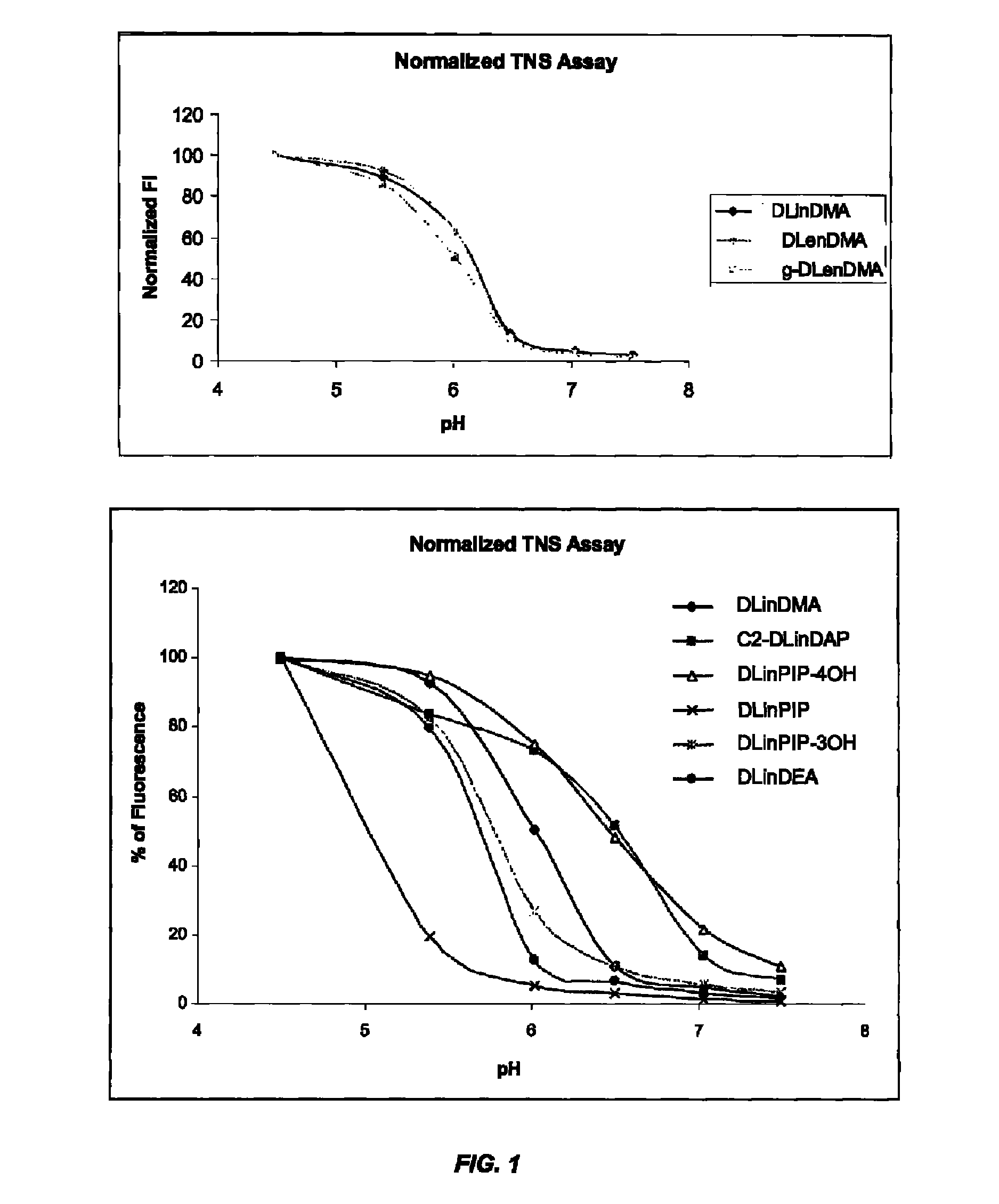
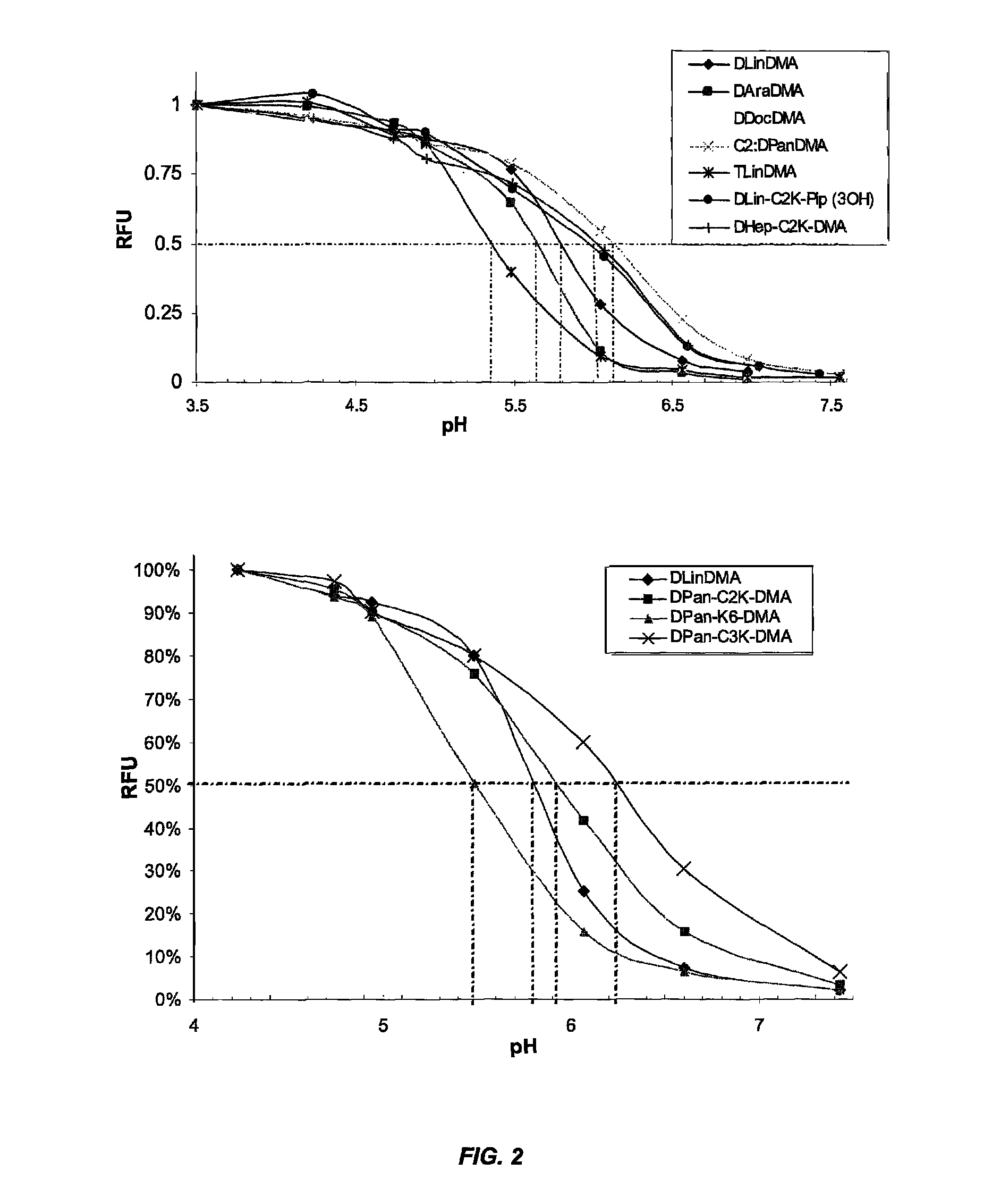
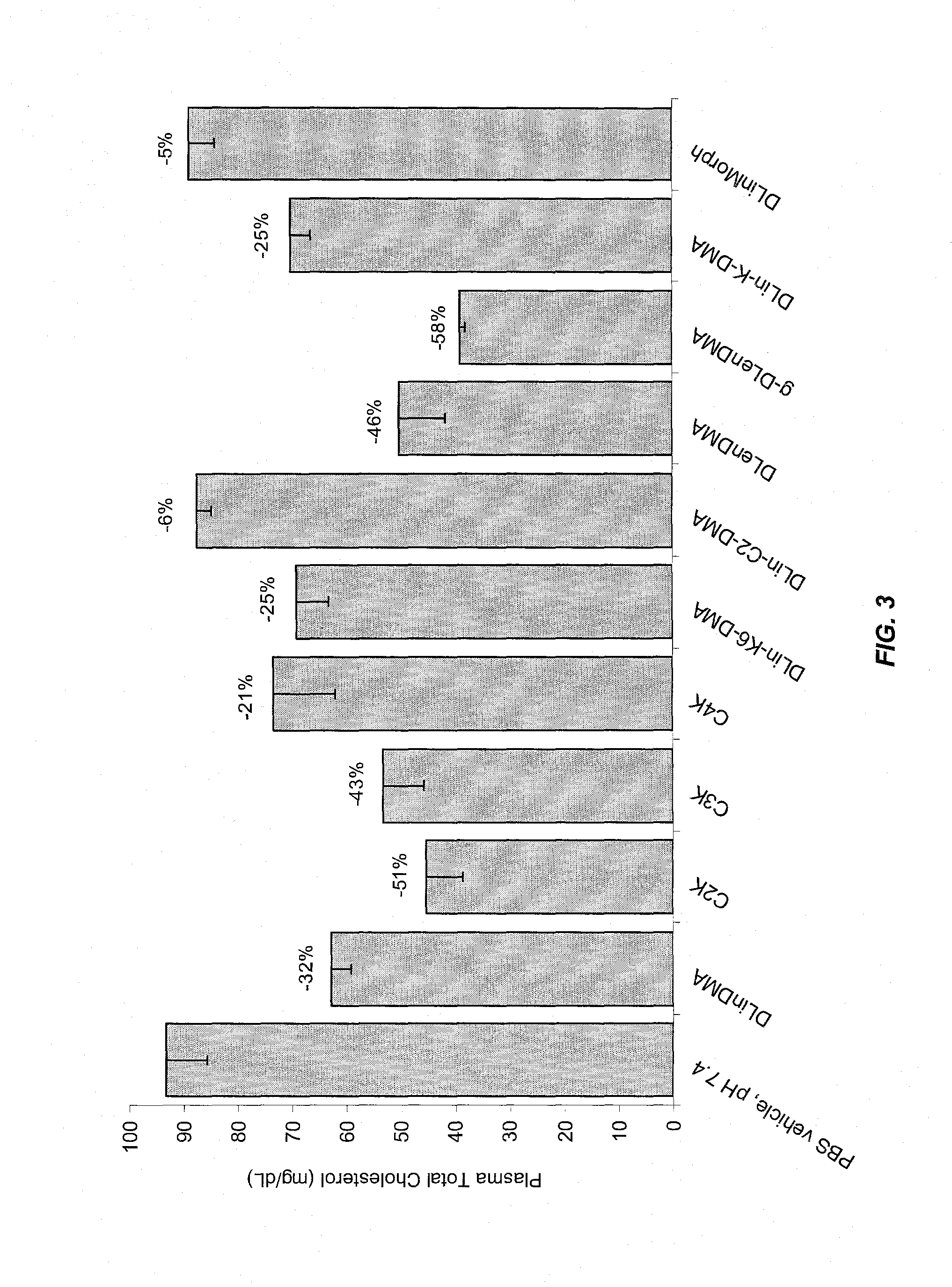
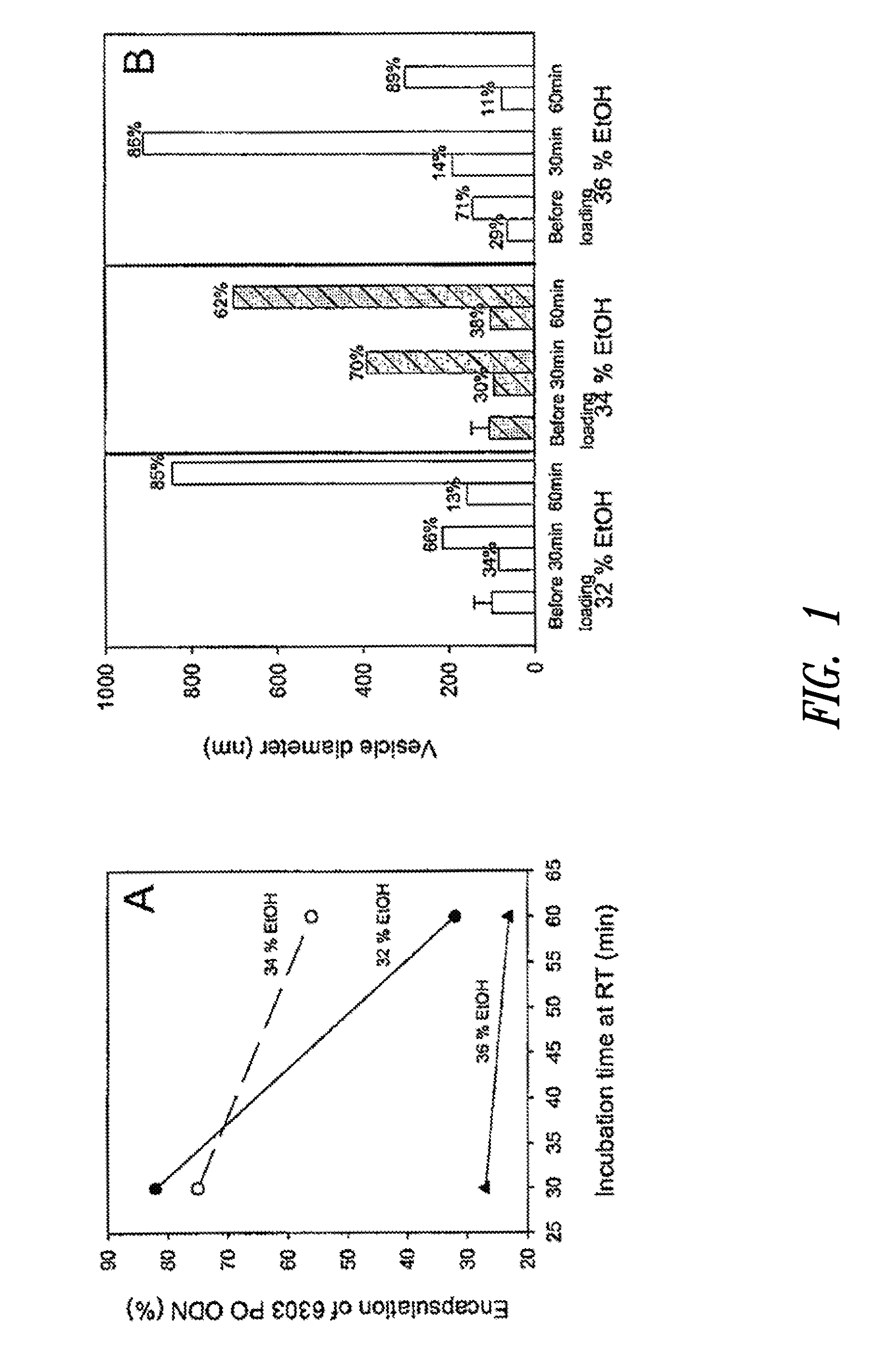
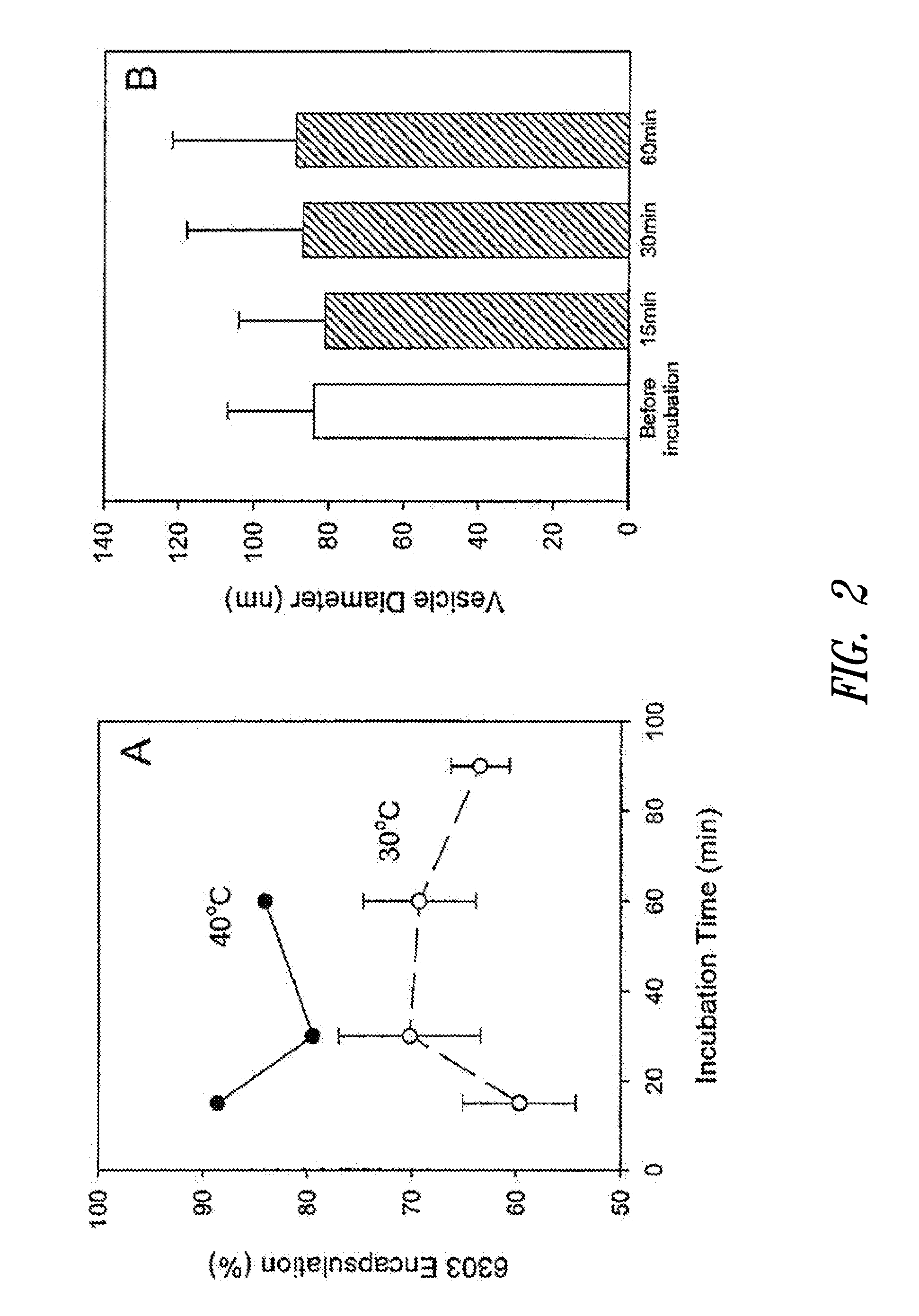
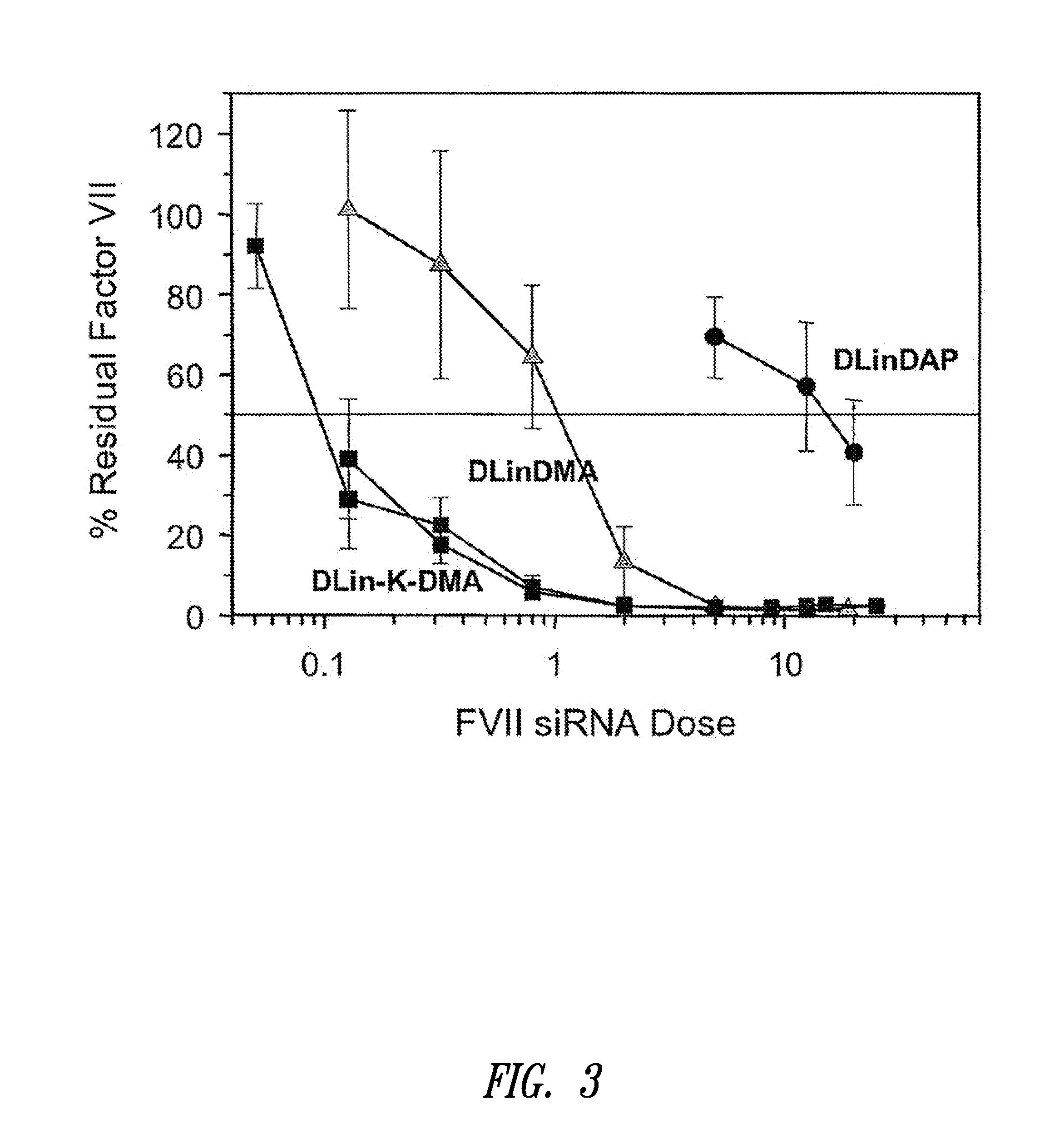
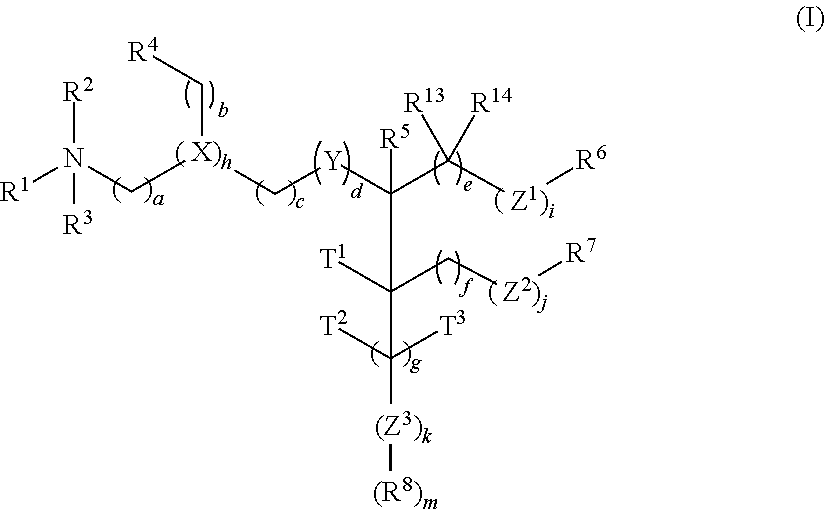
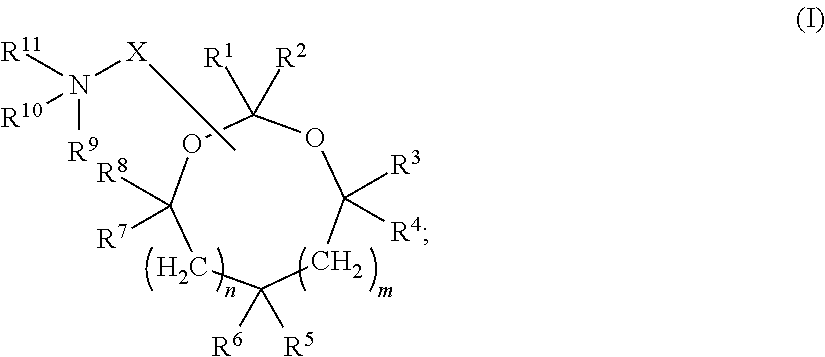
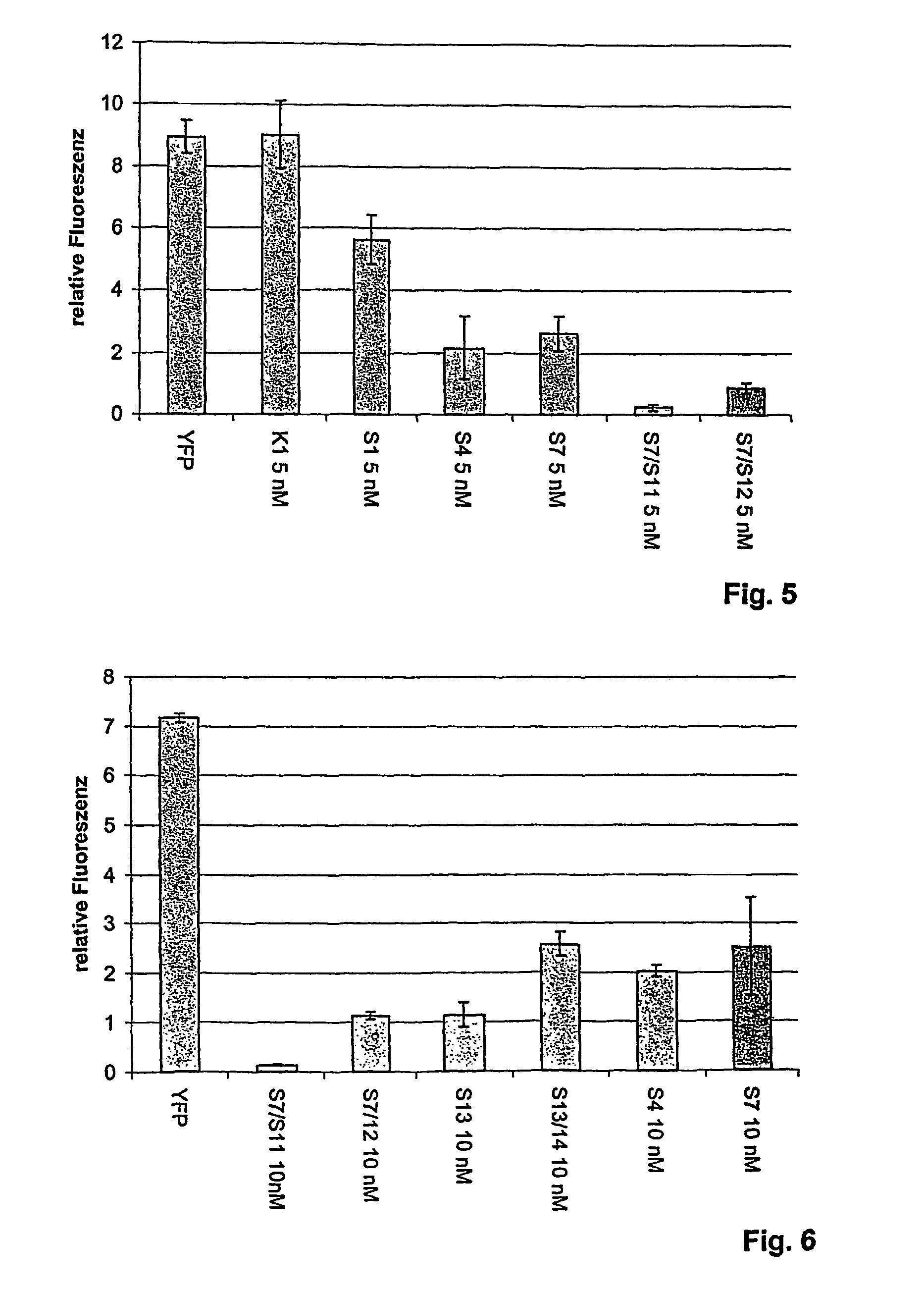
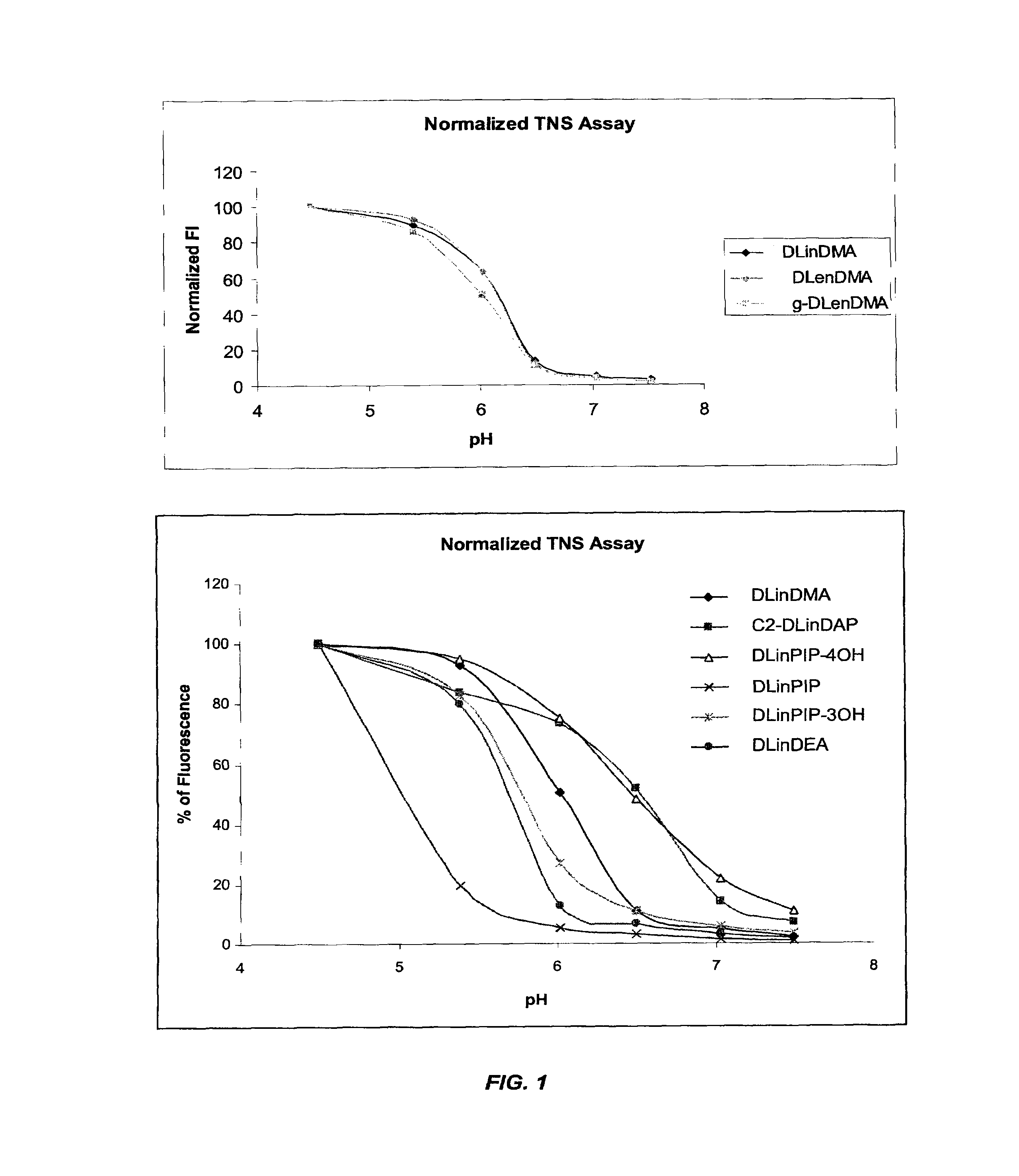
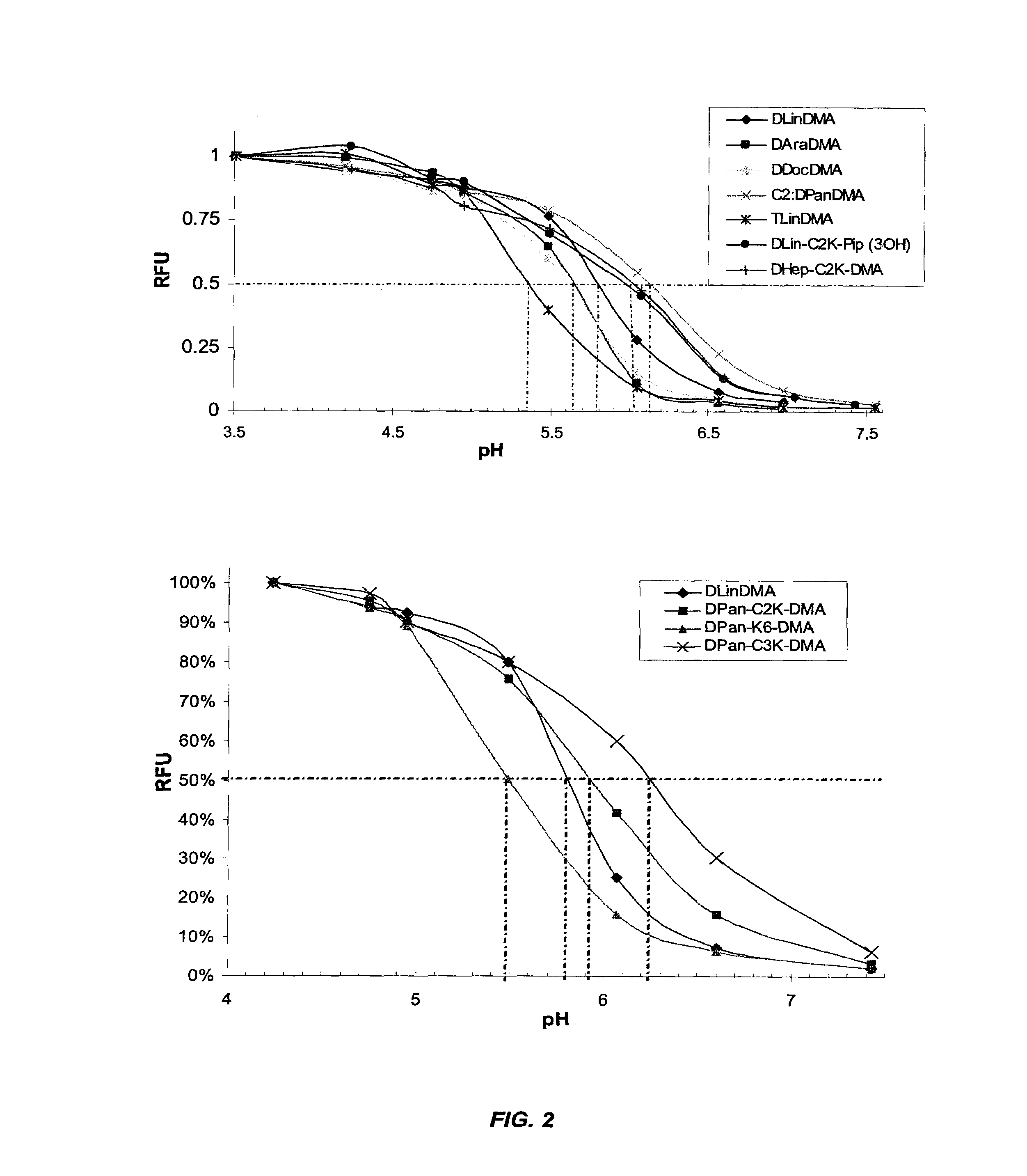
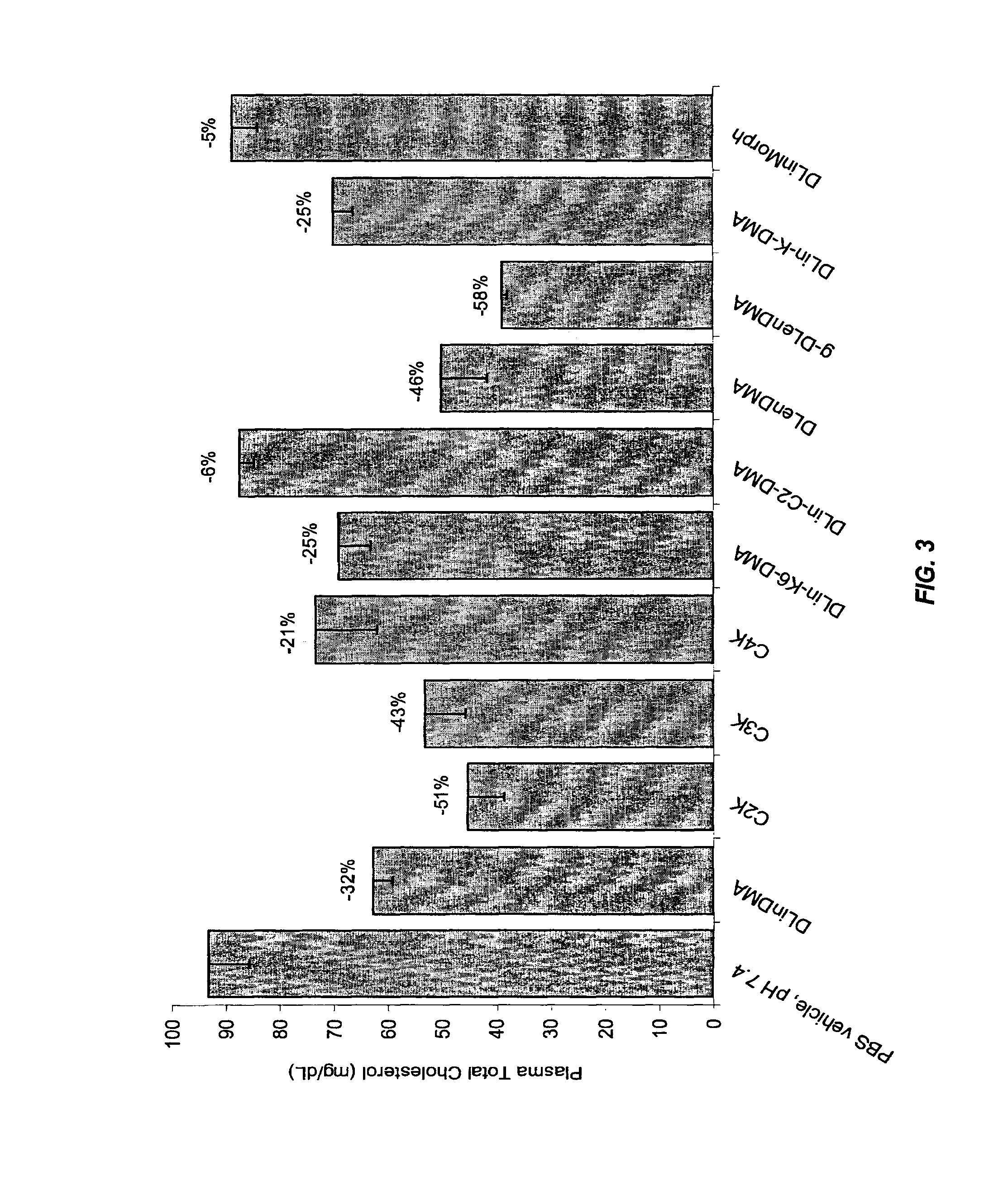
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
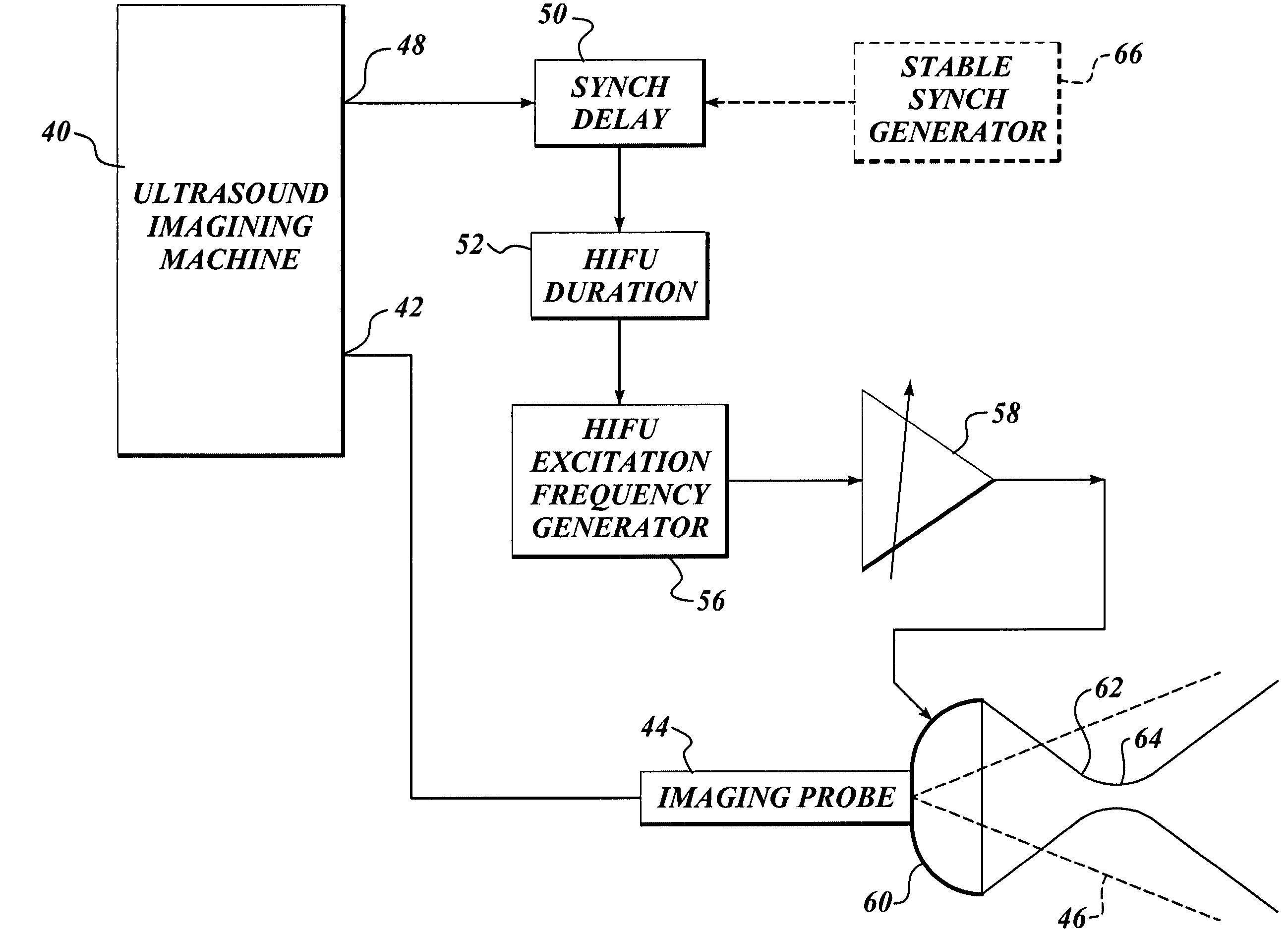
Ultrasound guided high intensity focused ultrasound treatment of nerves
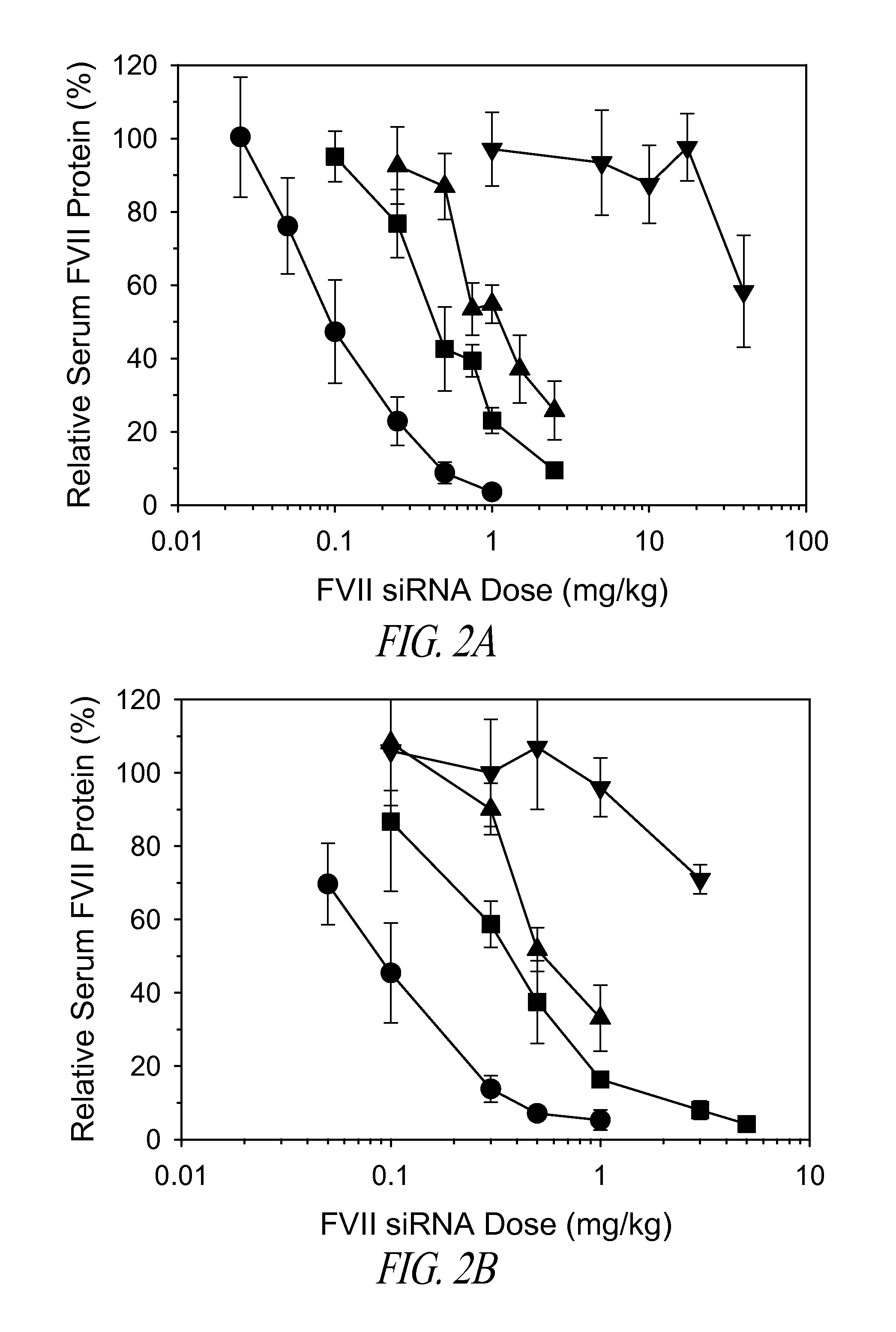
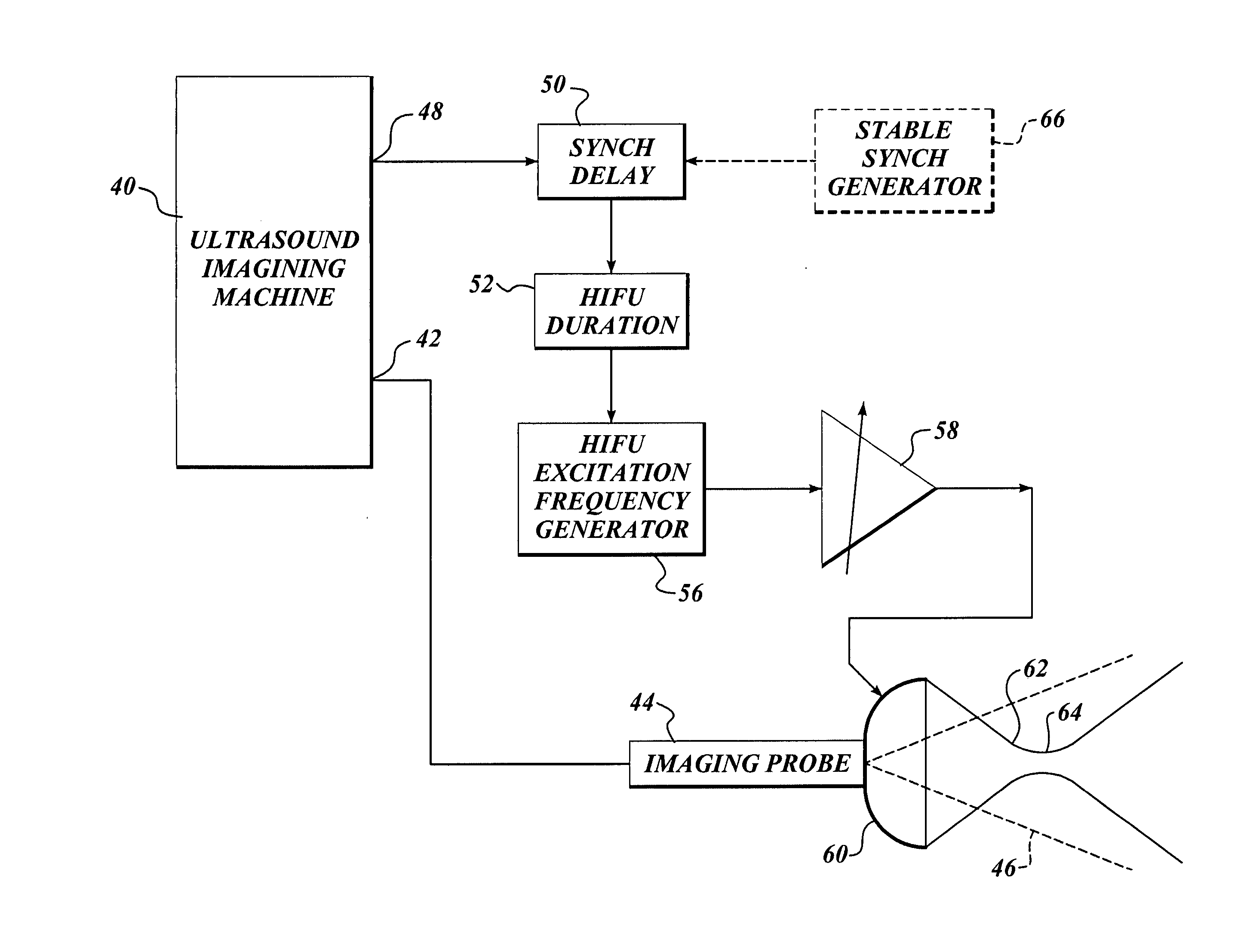
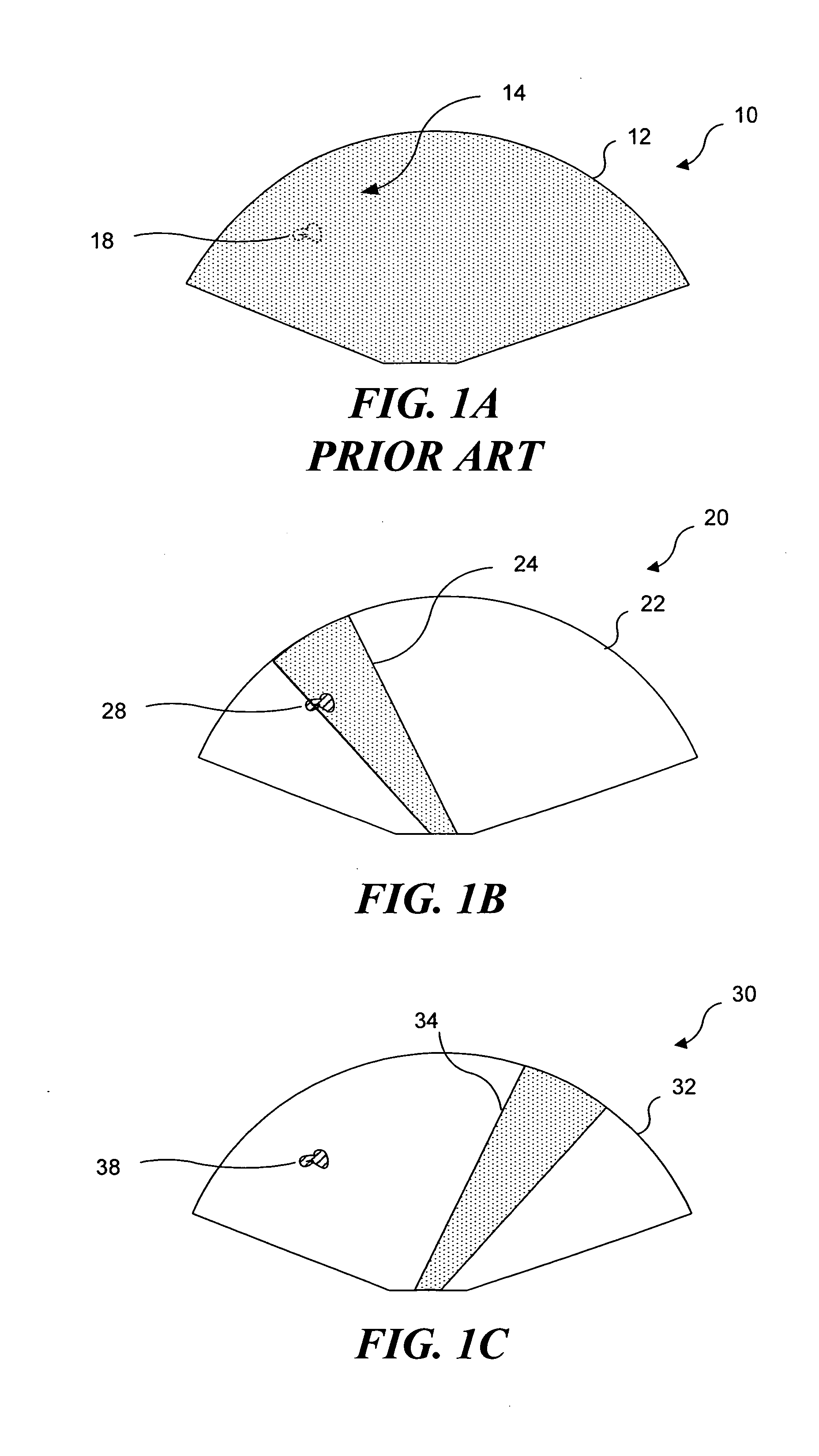
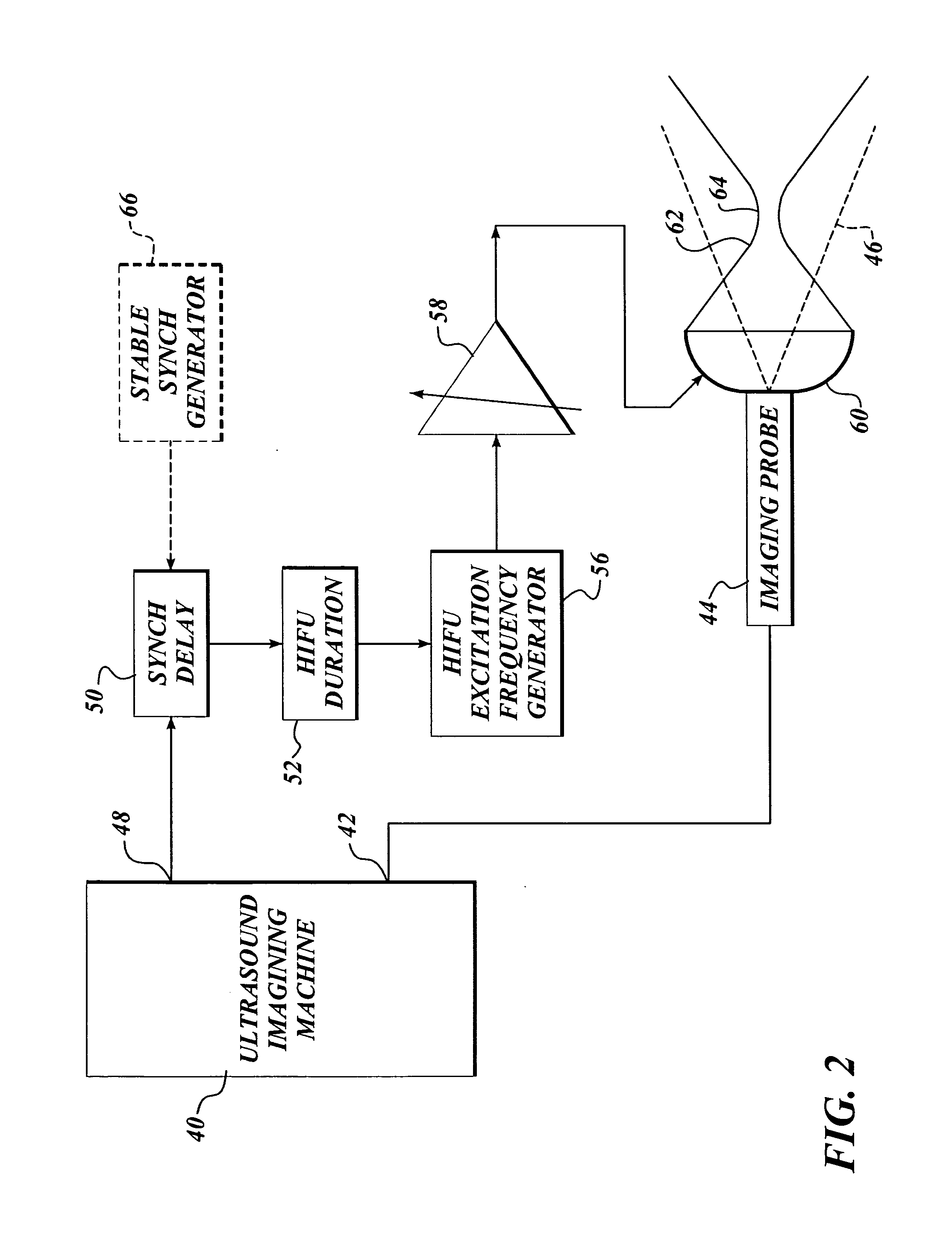
InactiveUS7510536B2Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesSonificationHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS20050240126A1Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesAbnormal tissue growthHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
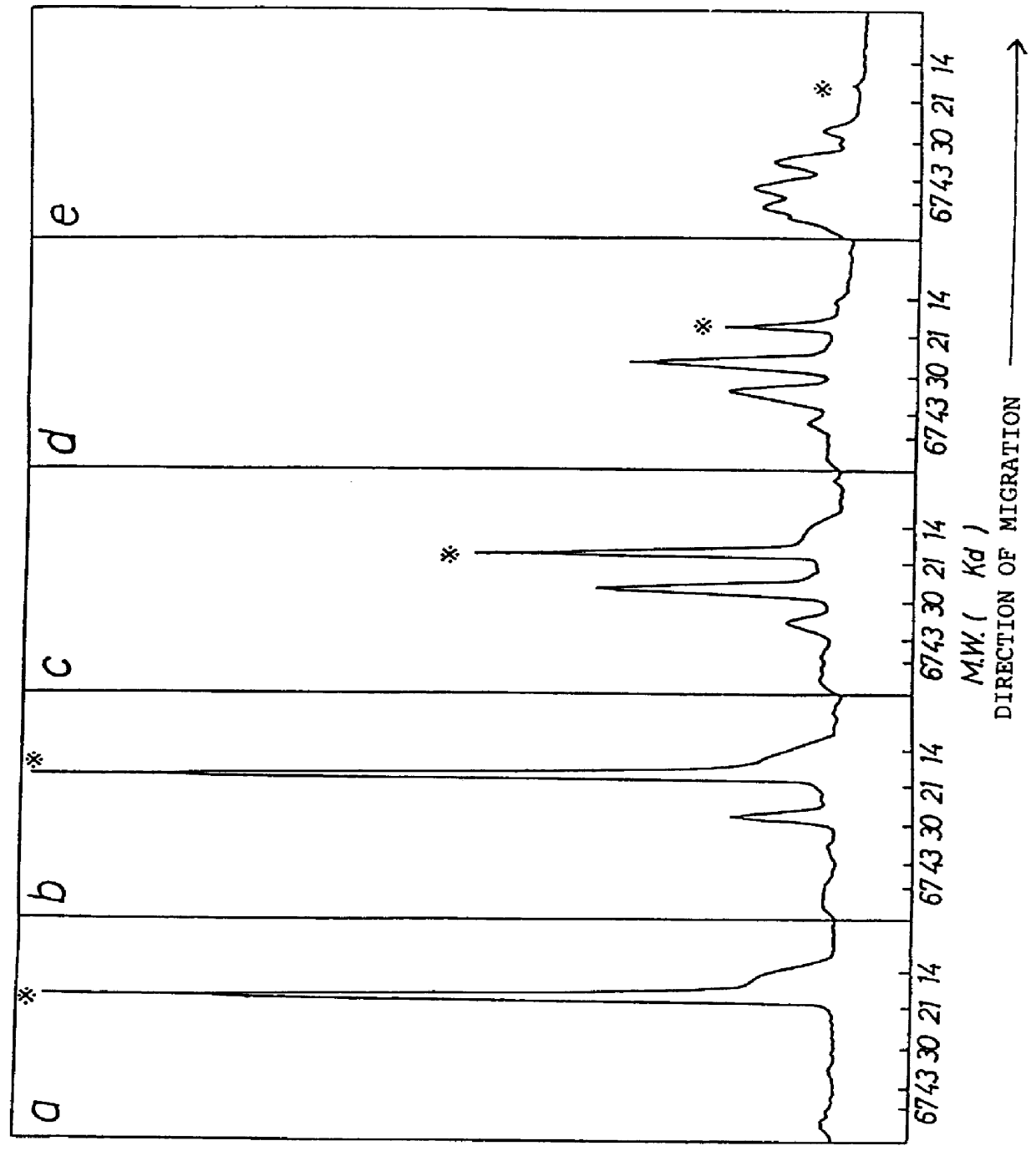
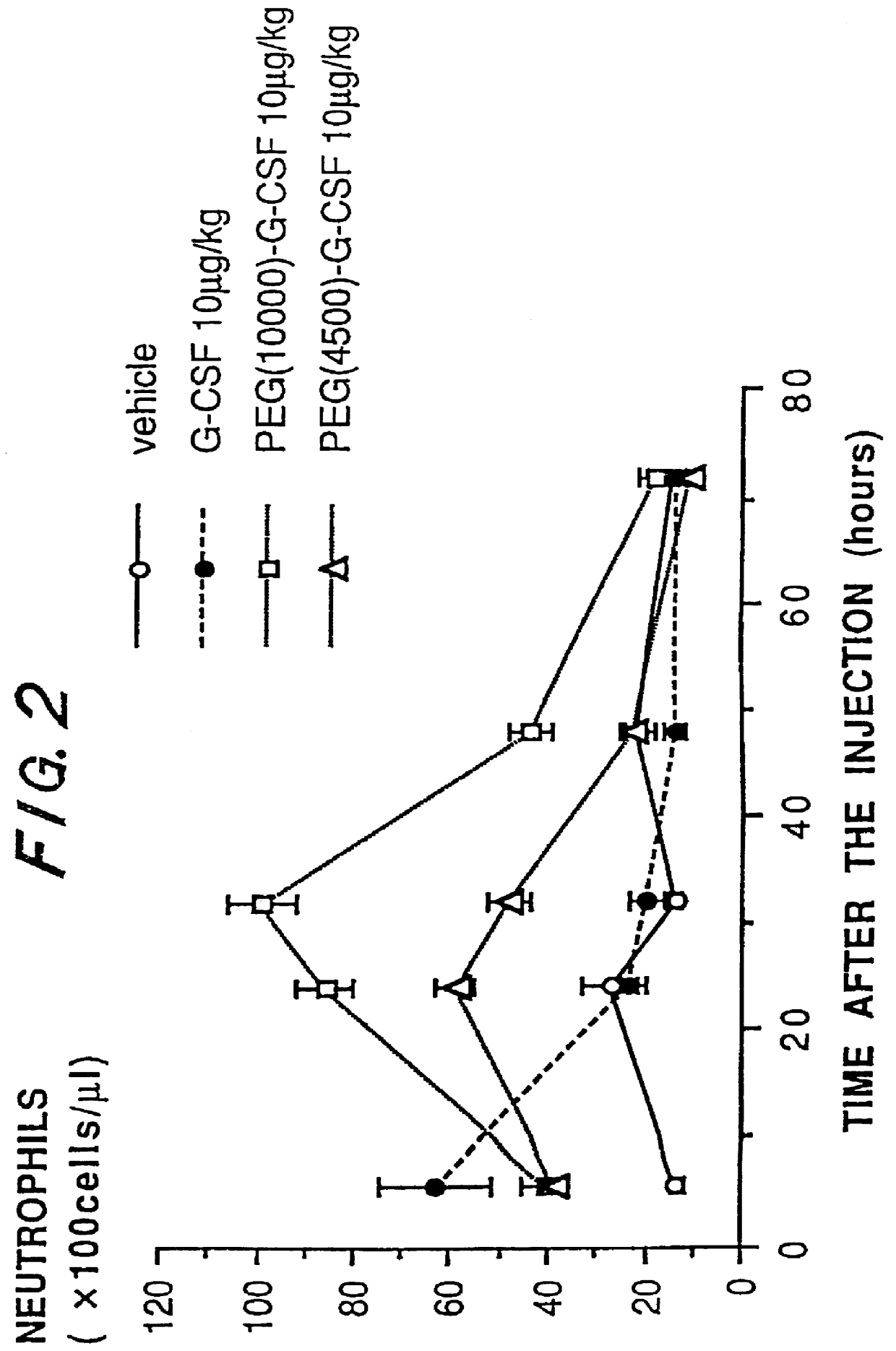
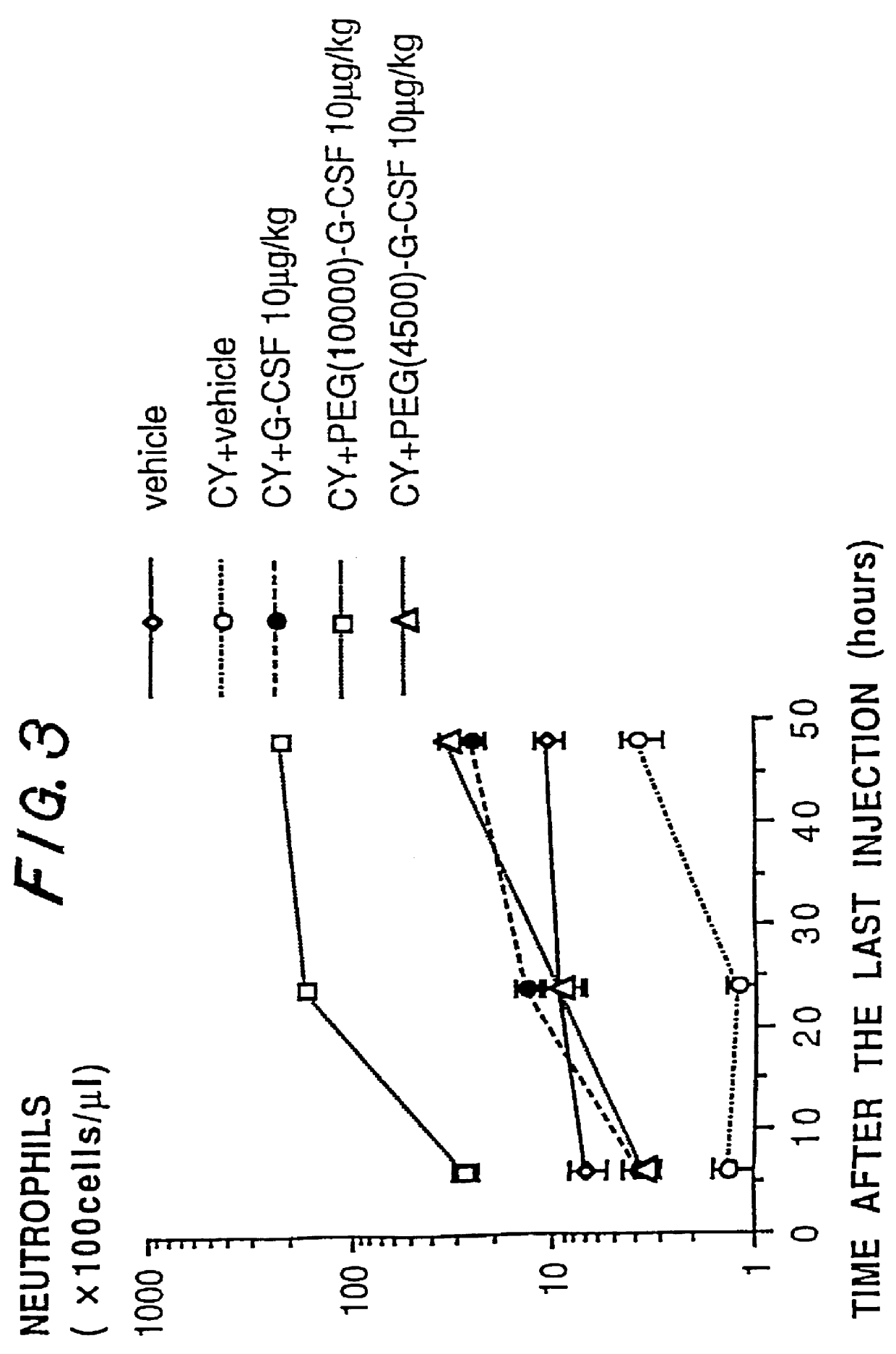
Chemically-modified G-CSF
InactiveUS6166183APharmacological effectPromote recoveryDepsipeptidesPeptide preparation methodsExogenous DNAPolyethylene glycol
The present invention provides a chemically-modified protein prepared by binding polyethylene glycol to a polypeptide characterized by being the product of expression by a host cell of an exogenous DNA sequence and substantially having the following amino acid sequence: - - (Het)n - - Thr Pro Leu Gly Pro Ala Ser Ser Leu Pro Gln - - Ser Phe Leu Leu Lys Cys Leu Glu Gln Val Arg - - Lys Ile Gln Gly Asp Gly Ala Ala Leu Gln Glu - - Lys Leu Cys Ala Thr Tyr Lys Leu Cys His Pro - - Glu Glu Leu Val Leu Leu Gly His Ser Leu Gly - - Ile Pro Trp Ala Pro Leu Ser Ser Cys Pro Ser - - Gln Ala Leu Gln Leu Ala Cly Cys Leu Ser Gln - - Leu His Ser Gly Leu Phe Leu Tyr Gln GIY Leu - - Leu Gln Ala Leu Glu Gly Ile Ser Pro Glu Leu - - Gly Pro Thr Leu Asp Thr Leu Gln Leu Asp Val - - Ala Asp Phe Ala Thr Tbr Ile Trp Gln Gln Het - - Glu Glu Leu Gly Het Ala Pro Ala Leu Gln Pro - - Thr Gln Gly Ala Het Pro Ala Phe Ala Ser Ala - - Phe Gln Arg Arg Ala Gly Gly Val Leu Val Ala - - Ser His Leu Gln Ser Phe Leu Glu Val Scr Tyr - - Arg Val Leu Arg His Leu Ala Gln Pro - (n = 0 or 1) - The chemically-modified protein according to the present invention has a neutrophils-increasing activity much more lasted than that of the intact human G-CSF, enabling fewer numbers of administration with a lower dose.
Owner:KIRIN AMGEN
Cationic lipids and methods for the delivery of therapeutic agents
InactiveUS20120202871A1Cyclic stabilitySize requirementBiocideOrganic active ingredientsLipid formationLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
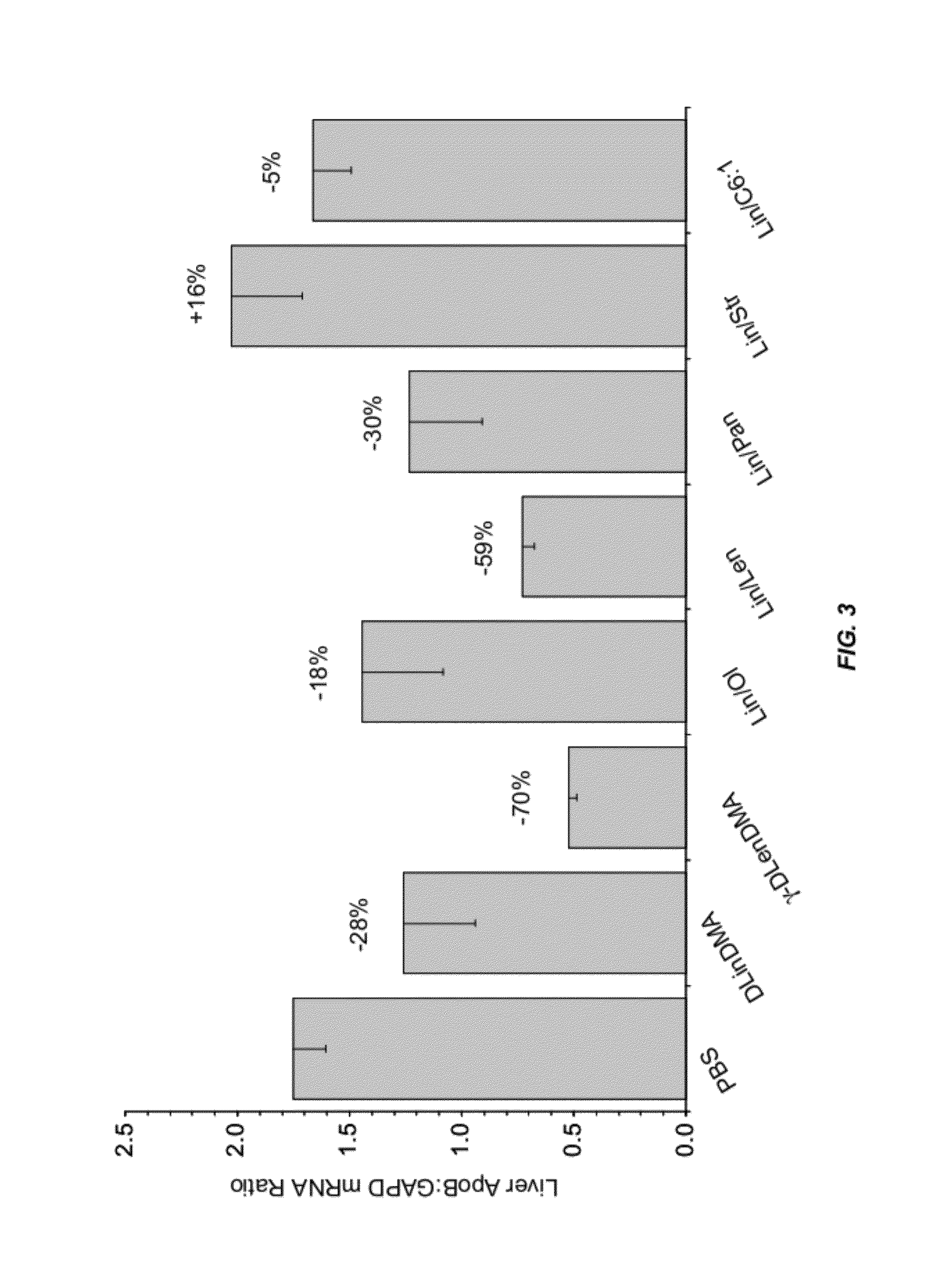
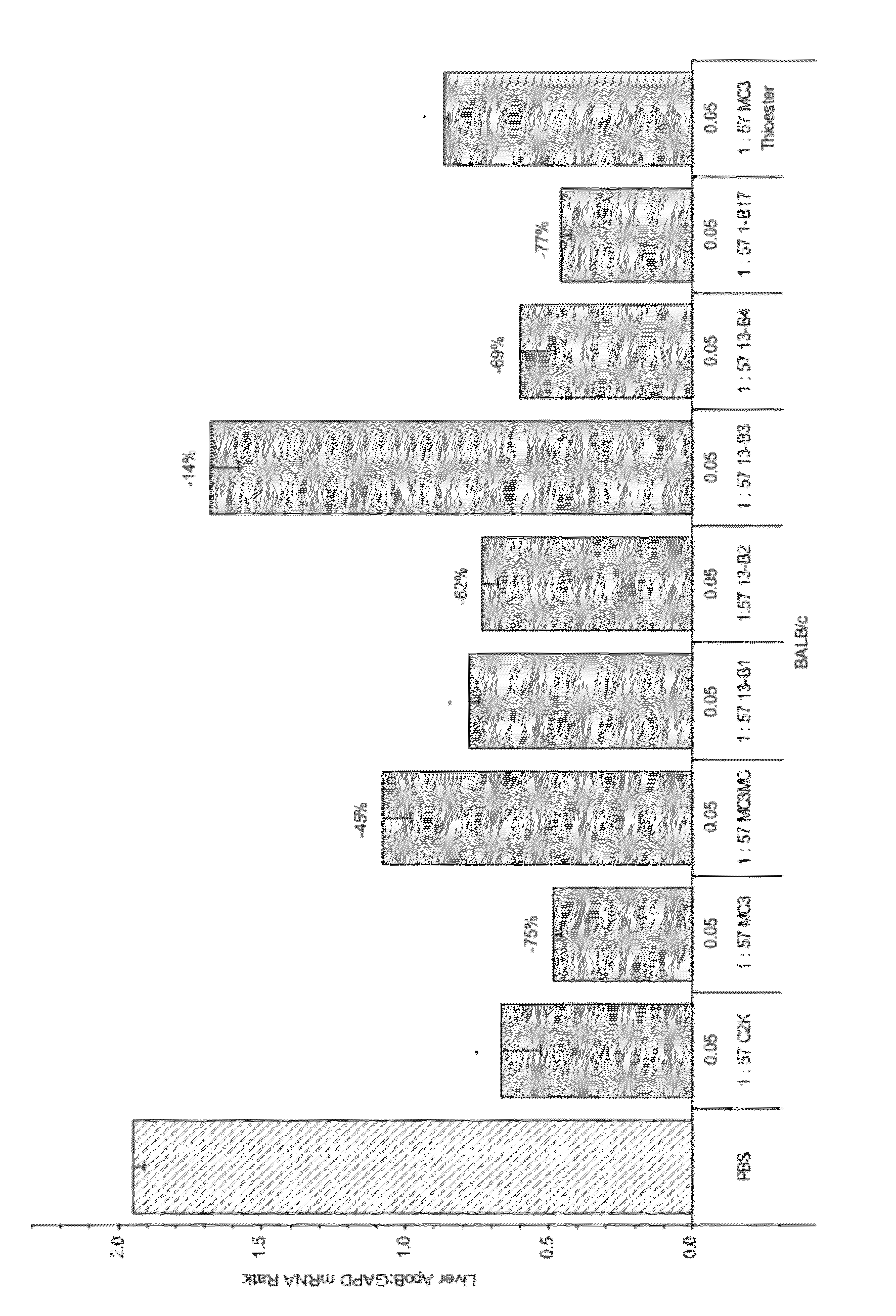
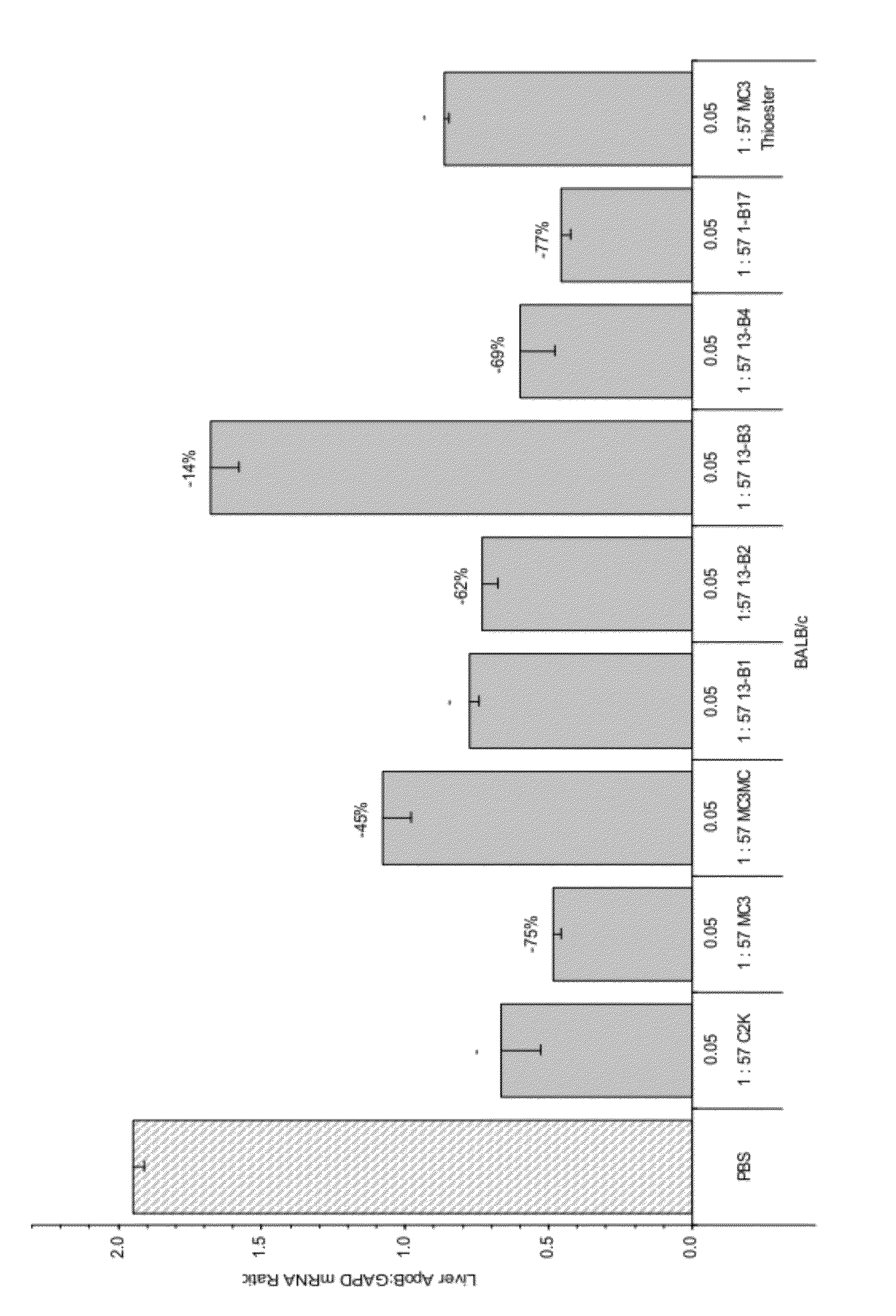
Compositions and methods for silencing apolipoprotein B
InactiveUS8236943B2Improve effectivenessHigh activityOrganic active ingredientsNanotechLipid particleApolipoproteins b
The present invention provides compositions and methods for the delivery of interfering RNAs that silence APOB expression to liver cells. In particular, the nucleic acid-lipid particles provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of APOB at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Novel cationic lipids and methods of use thereof
InactiveUS20130123338A1Cyclic stabilitySize requirementOrganic active ingredientsBiocideProtein targetLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
Trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Novel cationic lipids and methods of use thereof
ActiveUS20130064894A1Cyclic stabilitySize requirementPowder deliveryBiocideLipid formationLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel, trialkyl, cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
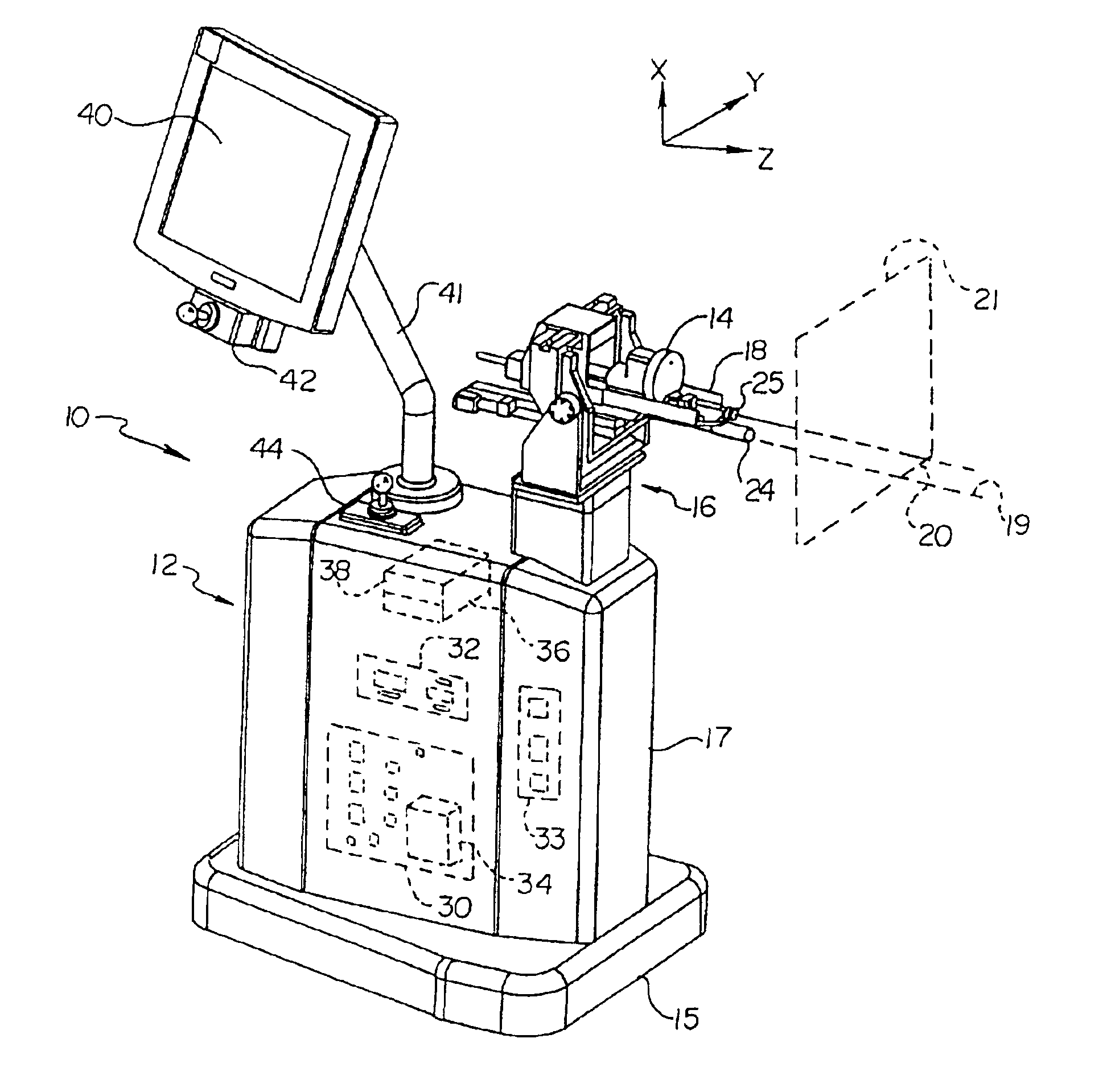
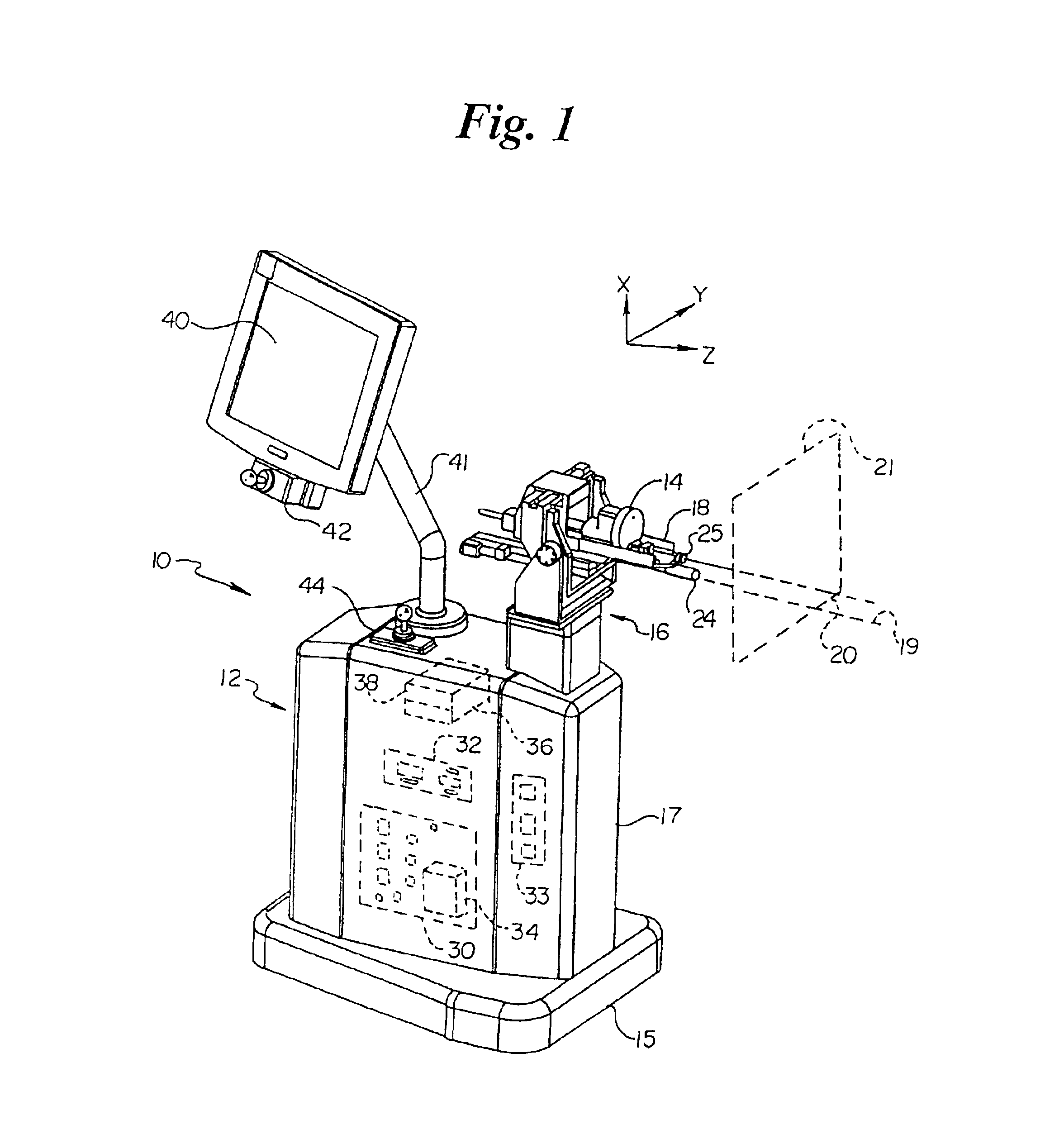
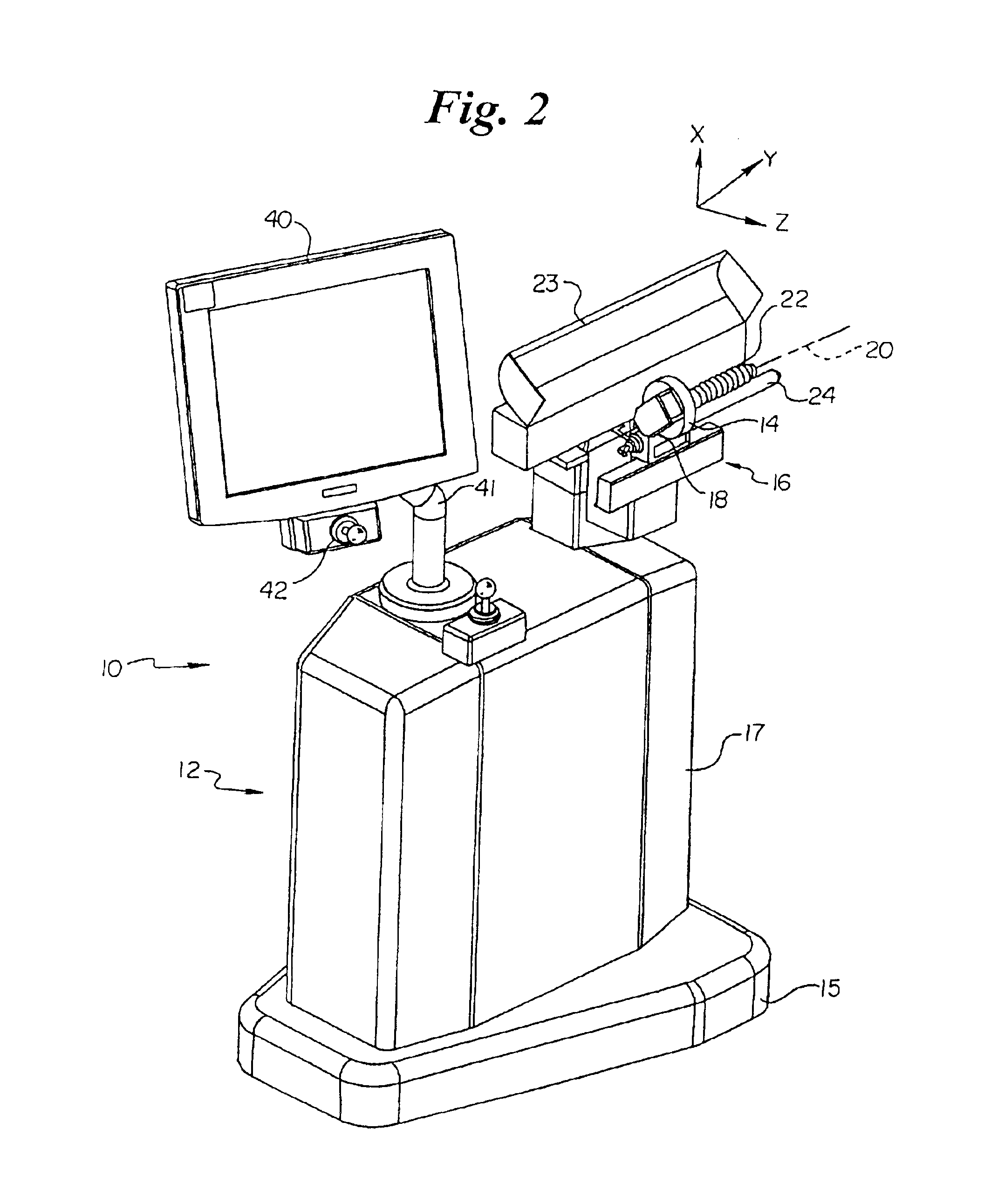
Automated implantation system for radioisotope seeds
InactiveUS6869390B2Medical devicesX-ray/gamma-ray/particle-irradiation therapyAdemetionineMovement control
An automated implantation system assists the implantation of low dose radioisotope seeds in a patient as part of a brachytherapy procedure. A Z-axis automated motion control system and an X-Y axis automated motion control system control a needle assembly. The X-Y axis automated motion control system positions an insertion axis of the needle assembly relative to the patient. The Z-axis automated motion control system selectively moves the needle assembly along the insertion axis to implant at least one radioisotope seed. This process is repeated for a plurality of locations on a base plane perpendicular to the insertion axis. Preferably, the radioisotope seeds are contained in a replaceable cartridge and the needle assembly is also replaceable.
Owner:THERAGENICS CORP
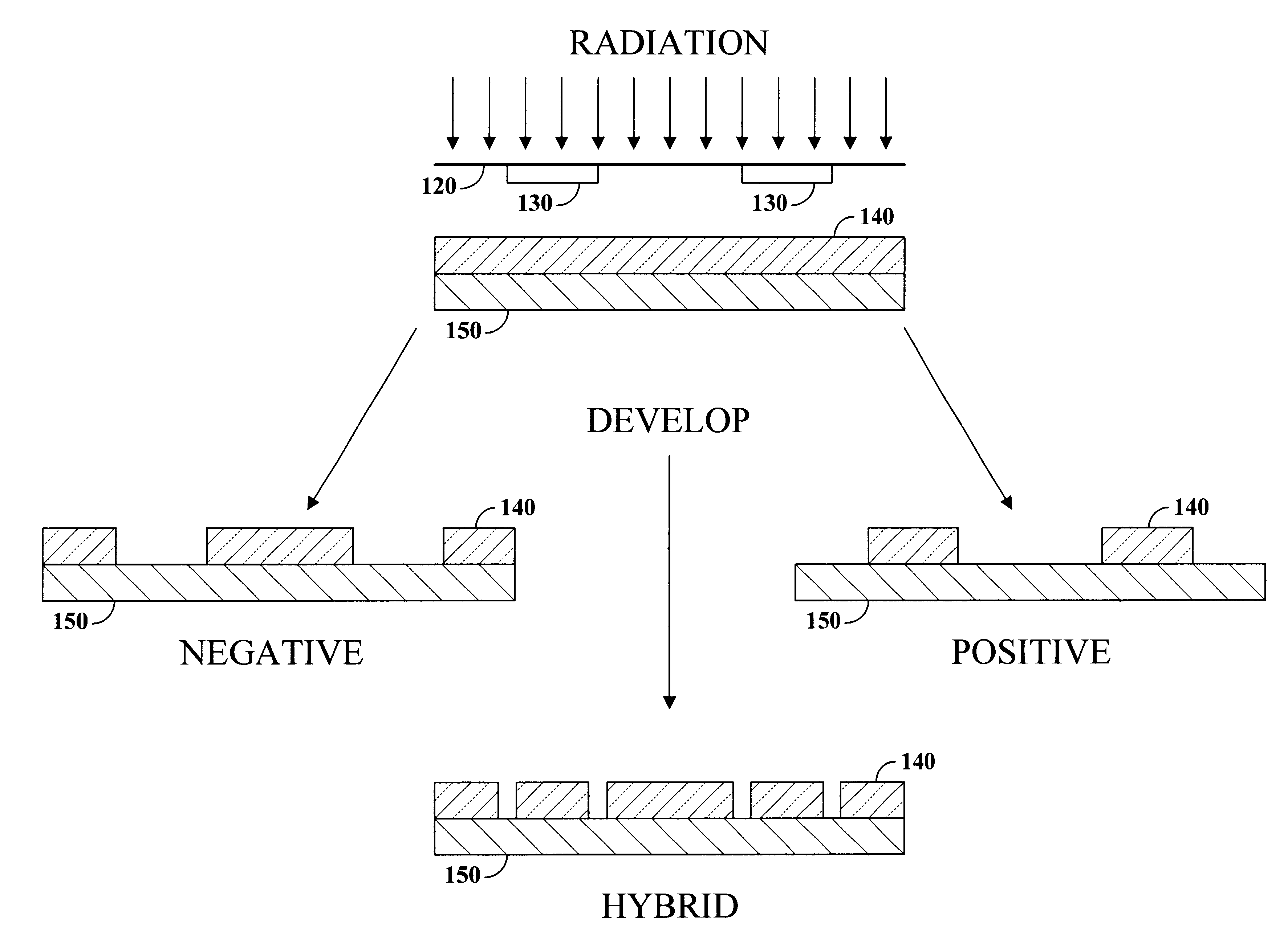
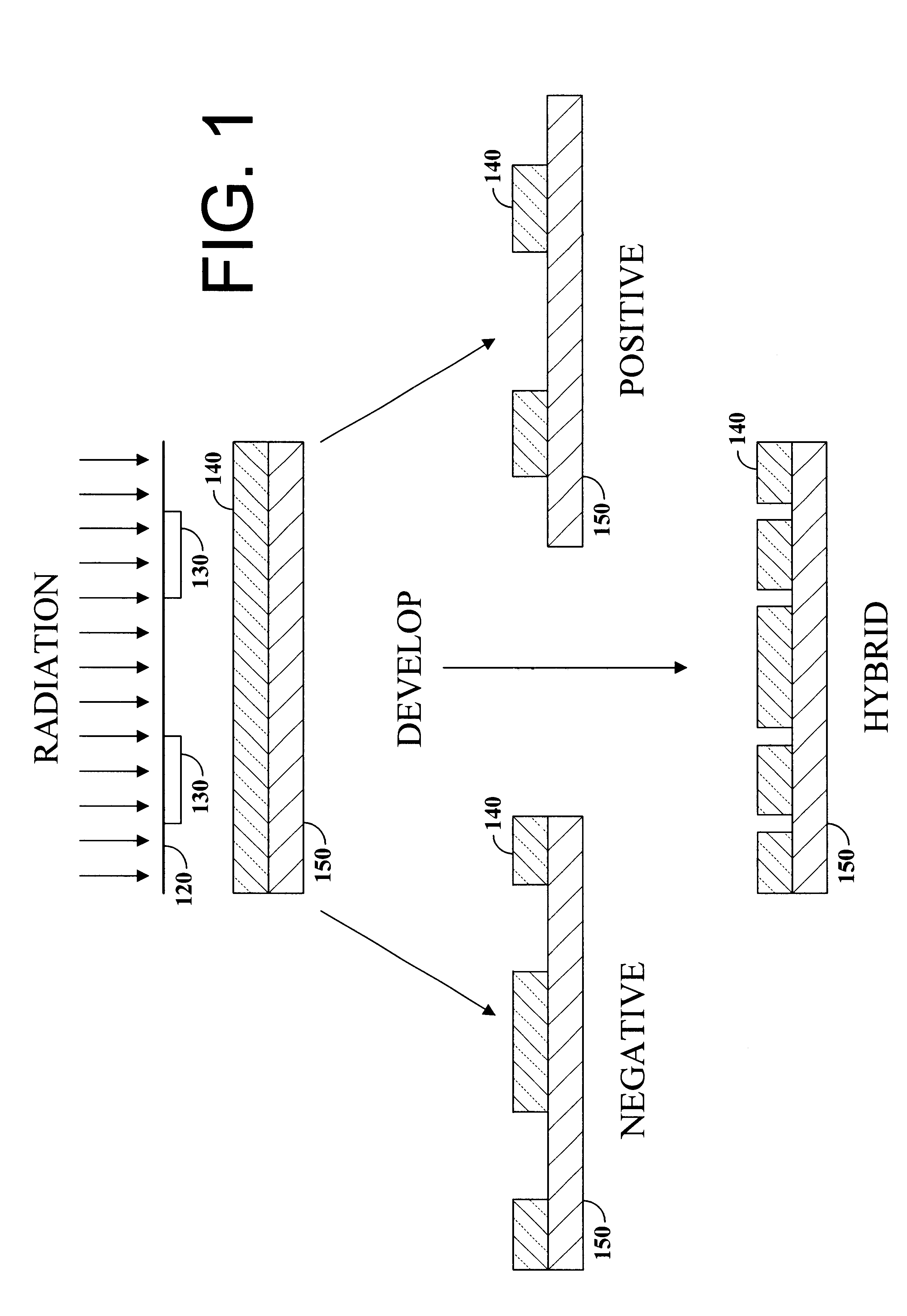
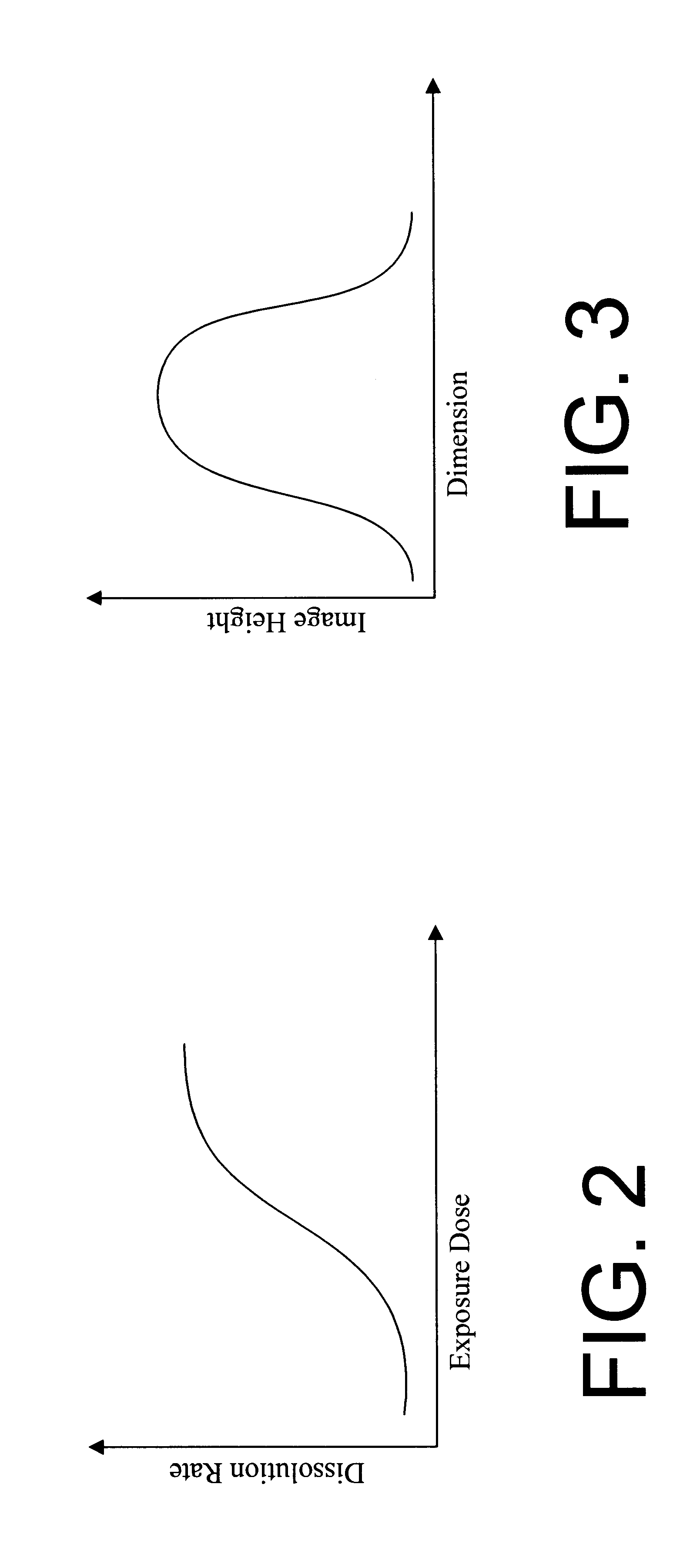
Hybrid resist based on photo acid/photo base blending
A photo resist composition contains a polymer resin, a first photo acid generator (PAG) requiring a first dose of actinic energy to generate a first photo acid, and a photo base generator (PBG) requiring a second dose of actinic energy, different from the first dose, to generate a photo base. The amounts and types of components in the photo resist are selected to produce a hybrid resist image. Either the first photo acid or photo base acts as a catalyst for a chemical transformation in the resist to induce a solubility change. The other compound is formulated in material type and loading in the resist such that it acts as a quenching agent. The catalyst is formed at low doses to induce the solubility change and the quenching agent is formed at higher doses to counterbalance the presence of the catalyst. Accordingly, the same frequency doubling effect of conventional hybrid resist compositions may be obtained, however, either a line or a space may be formed at the edge of an aerial image. Feature size may also be influenced by incorporating a quenching agent into the resist composition that does not require photo generation.
Owner:IBM CORP
Radiation curable and jettable ink compositions
The present invention is directed to radiation curable and jettable ink compositions and particularly to such compositions which exhibit enhanced elongation, when cured at low doses, and are advantageously used, for example, in digital inkjet printing. The compositions include a polyfunctional component and a monofunctional monomer, and may optionally include an additional monofuctional component and / or chain transfer agent. The compositions have a viscosity at 25° C. of not greater than about 70 cP and are radiation curable to form a cured ink having an elongation of between about 40 and about 150%. In addition, the compositions, when cured at low dose, exhibit a tack free surface and a low coefficient of friction.
Owner:COLLINS INK CORP
Compositions and methods for inhibiting expression of a target gene
ActiveUS7829693B2Inhibit expression of target geneOrganic active ingredientsNervous disorderDiseaseNucleotide
The present invention relates to a double-stranded ribonucleic acid (dsRNA) having a nucleotide sequence which is substantially identical to at least a part of a target gene and which is no more than 49, preferably less than 25, nucleotides in length, and which comprises a complementary (antisense) RNA strand having a 1 to 4 nucleotide overhang at the 3′-end and a blunt 5′-end. The invention further relates to a pharmaceutical composition comprising the dsRNA and a pharmaceutically acceptable carrier. The pharmaceutical compositions are useful for inhibiting the expression of a target gene, as well as for treating diseases caused by expression of the target gene, at low dosages (i.e., less than 5 milligrams, preferably less than 25 micrograms, per kg body weight per day). The invention also relates to methods for inhibiting the expression of a target gene, as well as methods for treating diseases caused by the expression of the gene.
Owner:ALNYLAM PHARM INC
Selective cellular targeting: multifunctional delivery vehicles, multifunctional prodrugs, use as antineoplastic drugs
InactiveUS20030138432A1Improve targeting selectivityHigh affinityNanomedicineAntibody ingredientsDelivery vehicleTherapeutic intent
The present invention relates to the compositions, methods, and applications of a novel approach to selective cellular targeting. The purpose of this invention is to enable the selective delivery and / or selective activation of effector molecules to target cells for diagnostic or therapeutic purposes. The present invention relates to multi-functional prodrugs or targeting vehicles wherein each functionality is capable of enhancing targeting selectivity, affinity, intracellular transport, activation or detoxification. The present invention also relates to ultra-low dose, multiple target, multiple drug chemotherapy and targeted immunotherapy for cancer treatment.
Owner:DRUG INNOVATION & DESIGN
Immunisation of large mammals with low doses of RNA
ActiveUS20130149375A1Conveniently preparedImprove stabilityAntibacterial agentsSsRNA viruses negative-senseMammalImmunity response
RNA encoding an immunogen is delivered to a large mammal at a dose of between 2 μg and 100 μg. Thus the invention provides a method of raising an immune response in a large mammal, comprising administering to the mammal a dose of between 2 μg and 100 μg of immunogen-encoding RNA. Similarly, RNA encoding an immunogen can be delivered to a large mammal at a dose of 3 ng / kg to 150 ng / kg. The delivered RNA can elicit an immune response in the large mammal
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Cleaving process to fabricate multilayered substrates using low implantation doses
InactiveUS7056808B2Improve efficiencyQuality improvementSolid-state devicesSemiconductor/solid-state device manufacturingHigh concentrationControl manner
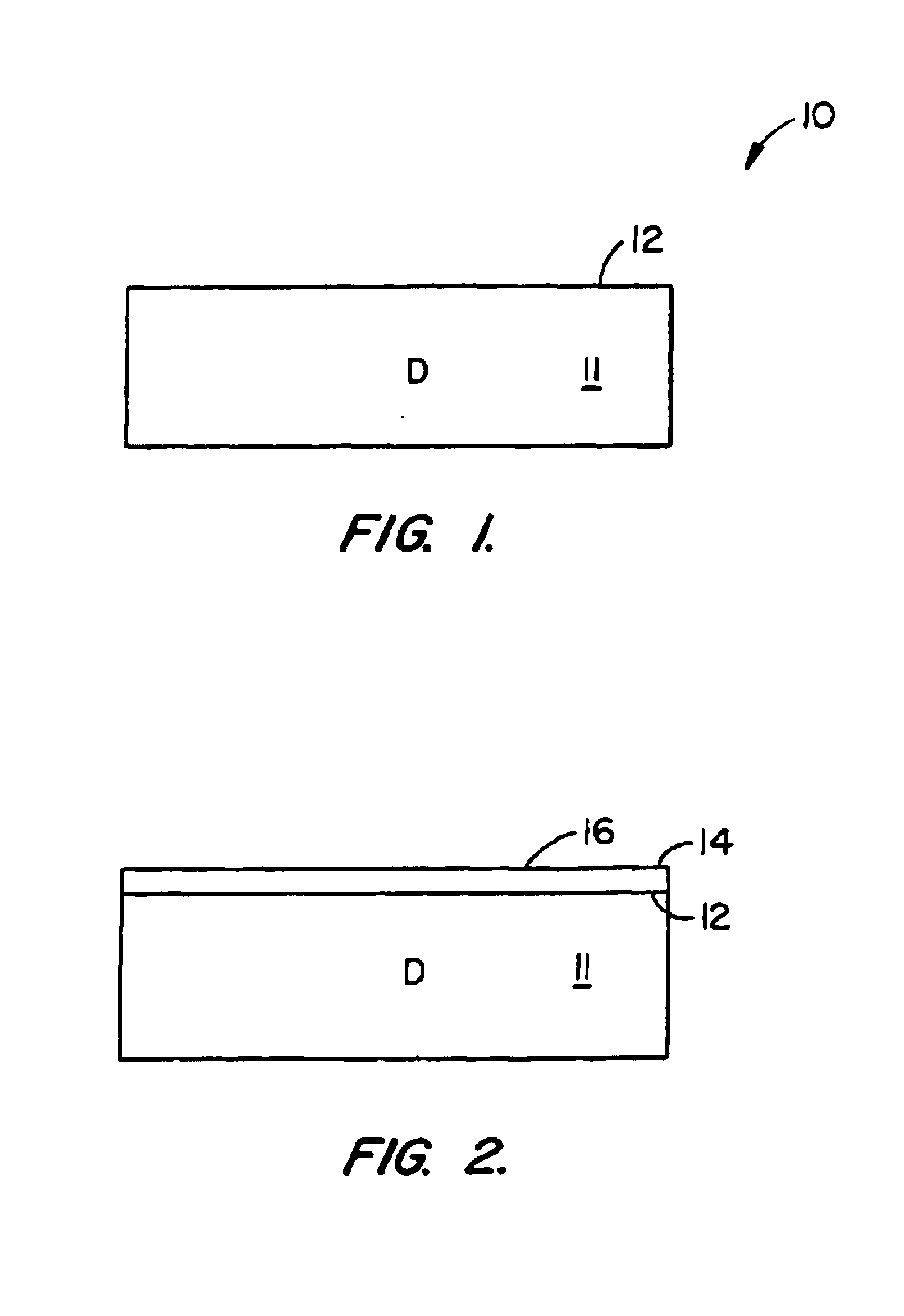
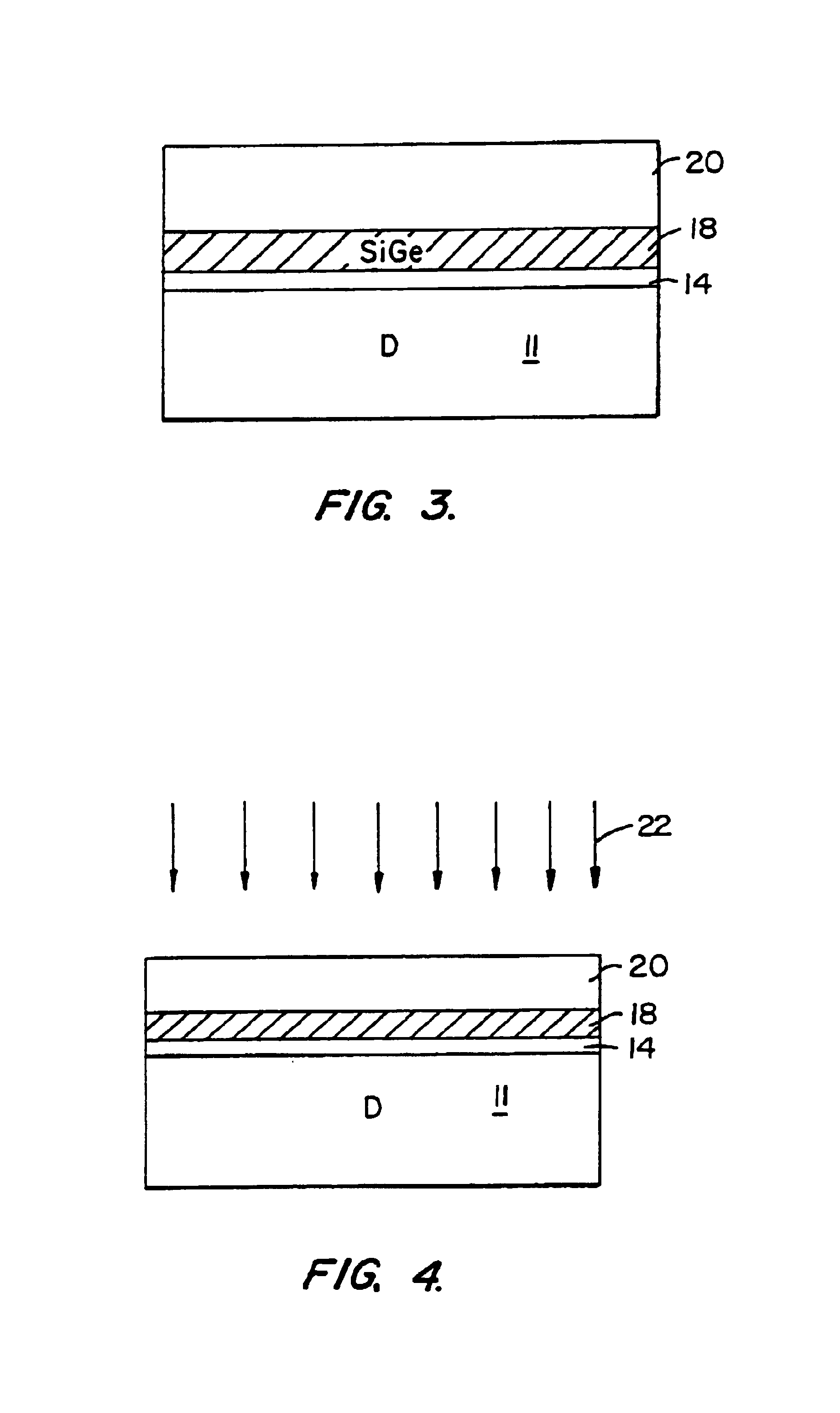
A method of forming substrates, e.g., silicon on insulator, silicon on silicon. The method includes providing a donor substrate, e.g., silicon wafer. The method also includes forming a cleave layer on the donor substrate that contains the cleave plane, the plane of eventual separation. In a specific embodiment, the cleave layer comprising silicon germanium. The method also includes forming a device layer (e.g., epitaxial silicon) on the cleave layer. The method also includes introducing particles into the cleave layer to add stress in the cleave layer. The particles within the cleave layer are then redistributed to form a high concentration region of the particles in the vicinity of the cleave plane, where the redistribution of the particles is carried out in a manner substantially free from microbubble or microcavity formation of the particles in the cleave plane. That is, the particles are generally at a low dose, which is defined herein as a lack of microbubble or microcavity formation in the cleave plane. The method also includes providing selected energy to the donor substrate to cleave the device layer from the cleave layer at the cleave plane, whereupon the selected energy is applied to create a controlled cleaving action to remove the device layer from a portion of the cleave layer in a controlled manner.
Owner:SILICON GENERAL CORPORATION
Method And System For Dynamic Low Dose X-ray Imaging
InactiveUS20080118023A1Reduce decreaseReduce detectionTomosynthesisHandling using diaphragms/collimetersX-rayX ray dose
A method and system for performing fluoroscopic imaging of a subject has high temporal and spatial resolution in a center portion of the captured dynamic images. The system provides for reduced X-ray dose to the patient associated with that part of the X-ray beam associated with a peripheral portion of the captured images although temporal, and in some embodiments spatial, resolution is reduced in the peripheral portion of the image. The system uses a rotating collimator to produce an X-ray beam having narrow wing portions associated with the peripheral portions of the image, and a broader central region associated with the high resolution center portion of the images.
Owner:FOREVISION IMAGING TECH LLC
Novel cyclic cationic lipids and methods of use
ActiveUS20130116307A1Cyclic stabilitySize requirementBiocideOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Ct system for use in multi-modality imaging system
InactiveUS20120256092A1Reduced footprintMaterial analysis using wave/particle radiationRadiation/particle handlingImaging qualityX-ray
A computed tomography (CT) imaging system is disclosed. The CT imaging system may be used in a multi-modality imaging context or other context. In one embodiment, the CT imaging system provides for both fast rotation of the rotating X-ray source and detection components and low dose of X-rays generated by the source providing several clinical and economic benefits such as low dose and sufficient image quality and no or insignificant investment in room shielding associated with diagnostic CT dose.
Owner:GENERAL ELECTRIC CO
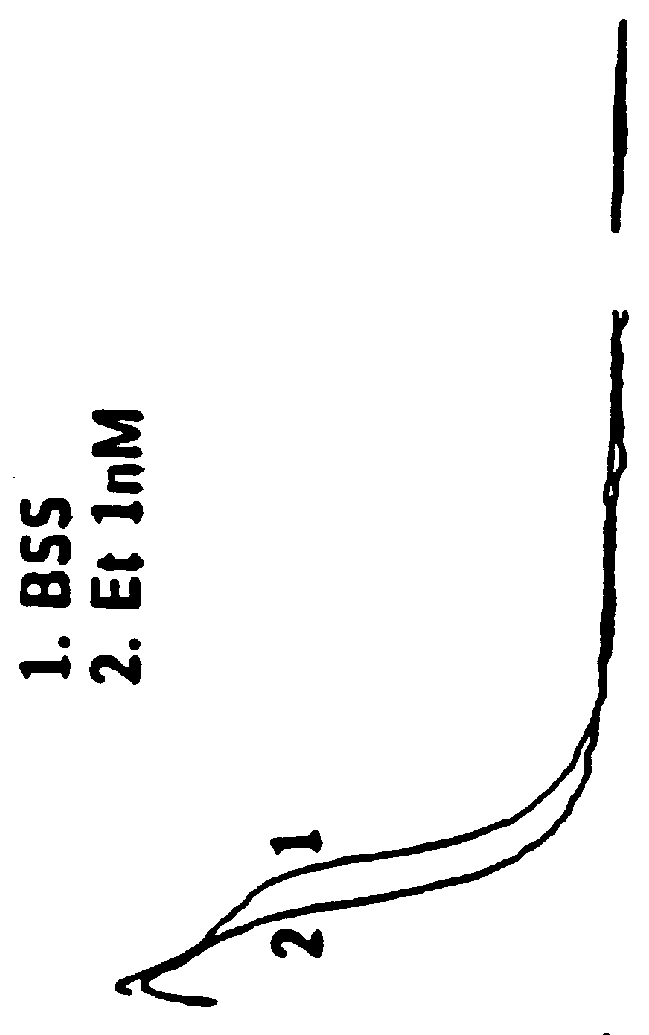
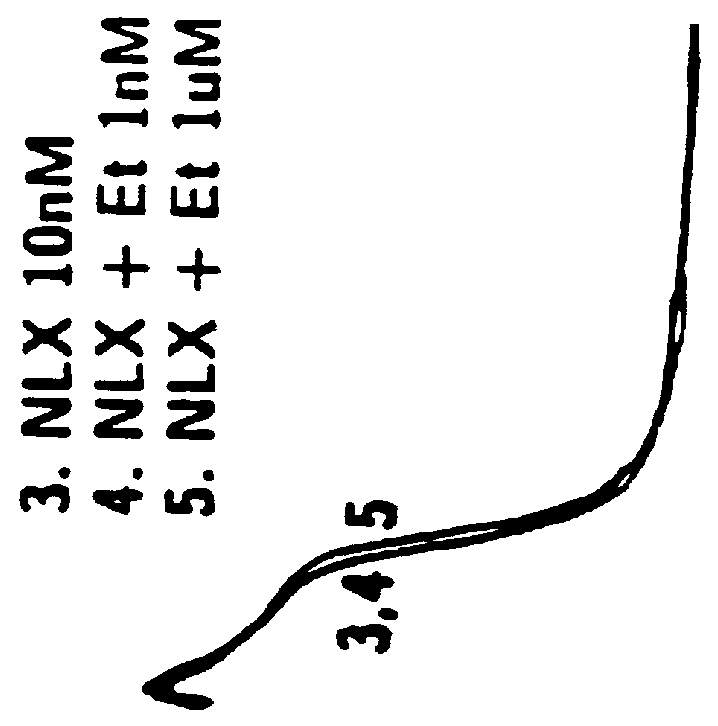
Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
InactiveUSRE36547E1Enhance analgesic potencyDecrease dependence liabilityCompound screeningBiocideEndogenous OpiatesNervous system
This invention relates to a method of selectively enhancing the analgesic potency of morphine and other clinically used bimodally-acting opioid agonists and simultaneously attenuating development of physical dependence, tolerance and other undesirable side effects caused by the chronic administration of said bimodally-acting opioid agonists comprising the co-administration of a bimodally-acting opioid agonist which activates both inhibitory and excitatory opioid receptor-mediated functions of neurons in the nociceptive (pain) pathways of the nervous system and an opioid receptor antagonist which selectively inactivates excitatory opioid receptor-mediated side effects. This invention also relates to a method of using excitatory opioid receptor antagonists alone to block the undesirable excitatory side effects of endogenous bimodally-acting opioid agonists which may be markedly elevated during chronic pain. This invention further relates to a method of long-term treatment of previously detoxified opiate, cocaine and alcohol addicts utilizing said excitatory opioid receptor antagonists, either alone or in combination with low-dose methadone, to prevent protracted physical dependence, and to compositions comprising an excitatory opioid receptor antagonist of the invention and a bimodally-acting opioid agonist.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Topical aminolevulinic acid-photodynamic therapy for the treatment of acne vulgaris
InactiveUS6897238B2Reduce sebum productionSmall sizeBiocideOrganic active ingredientsBacteroidesDisease
Light treatments of sebaceous gland disorders with 5-aminolevulinic acid and photodynamic therapy are disclosed. A preferred treatment includes topical application of 5-aminolevulinic acid to the skin followed by light exposures with repeated treatment at various intervals. At low doses of ALA and photodynamic therapy (PDT) in single or multiple treatments, improvement in the sebaceous gland disorder, e.g., acne, provides the discovery that diminishment in sebum secretion and the eradication of bacteria occurs. At high doses of ALA and a single high energy PDT treatment, permanent changes to the sebaceous gland and sebum secretion have been discovered.
Owner:THE GENERAL HOSPITAL CORP
Methods and low dose regimens for treating red blood cell disorders
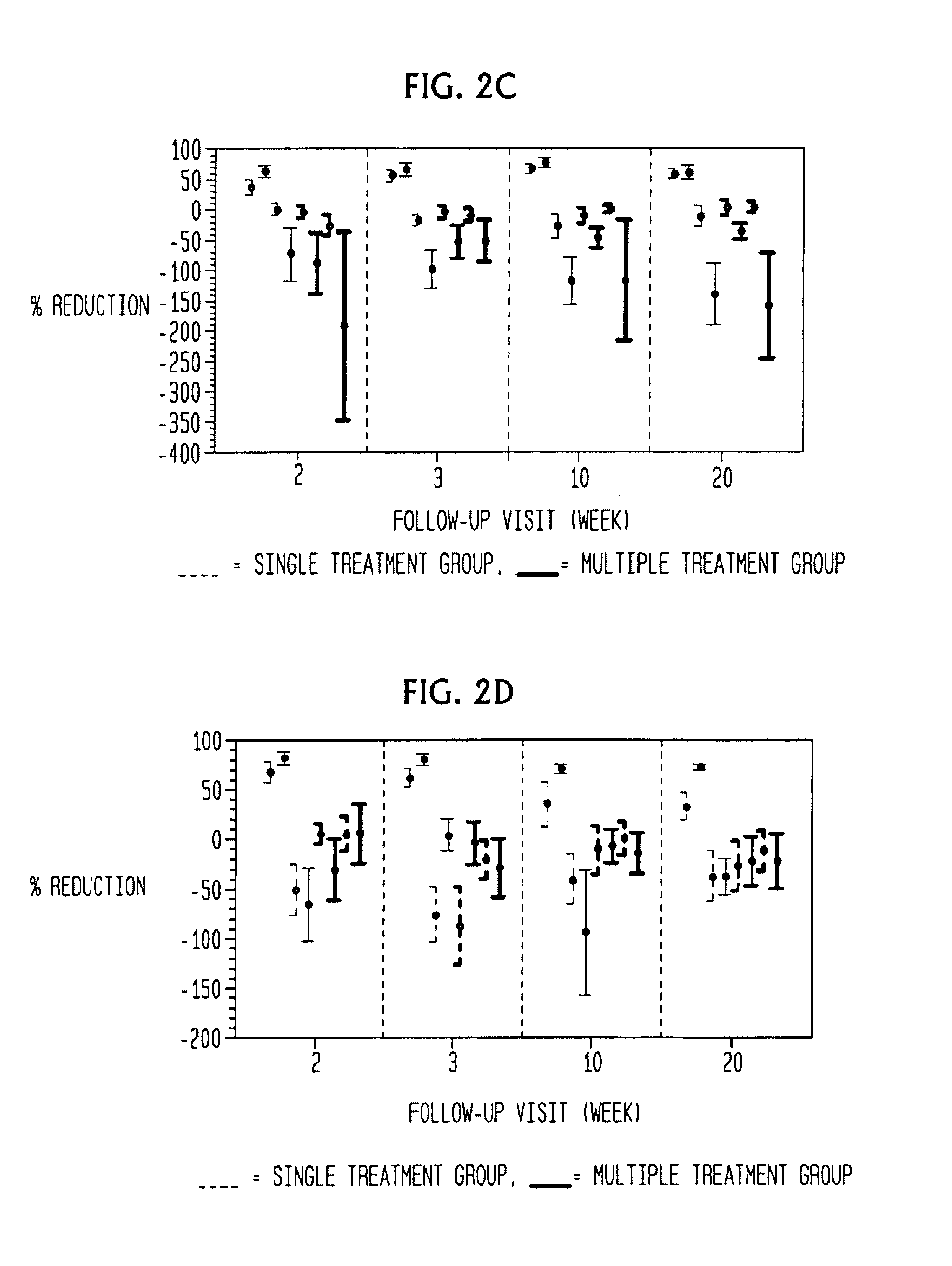
ActiveUS20110251149A1Increase volumeIncrease the number ofBiocideCarbohydrate active ingredientsBeta thalassemiaRegimen
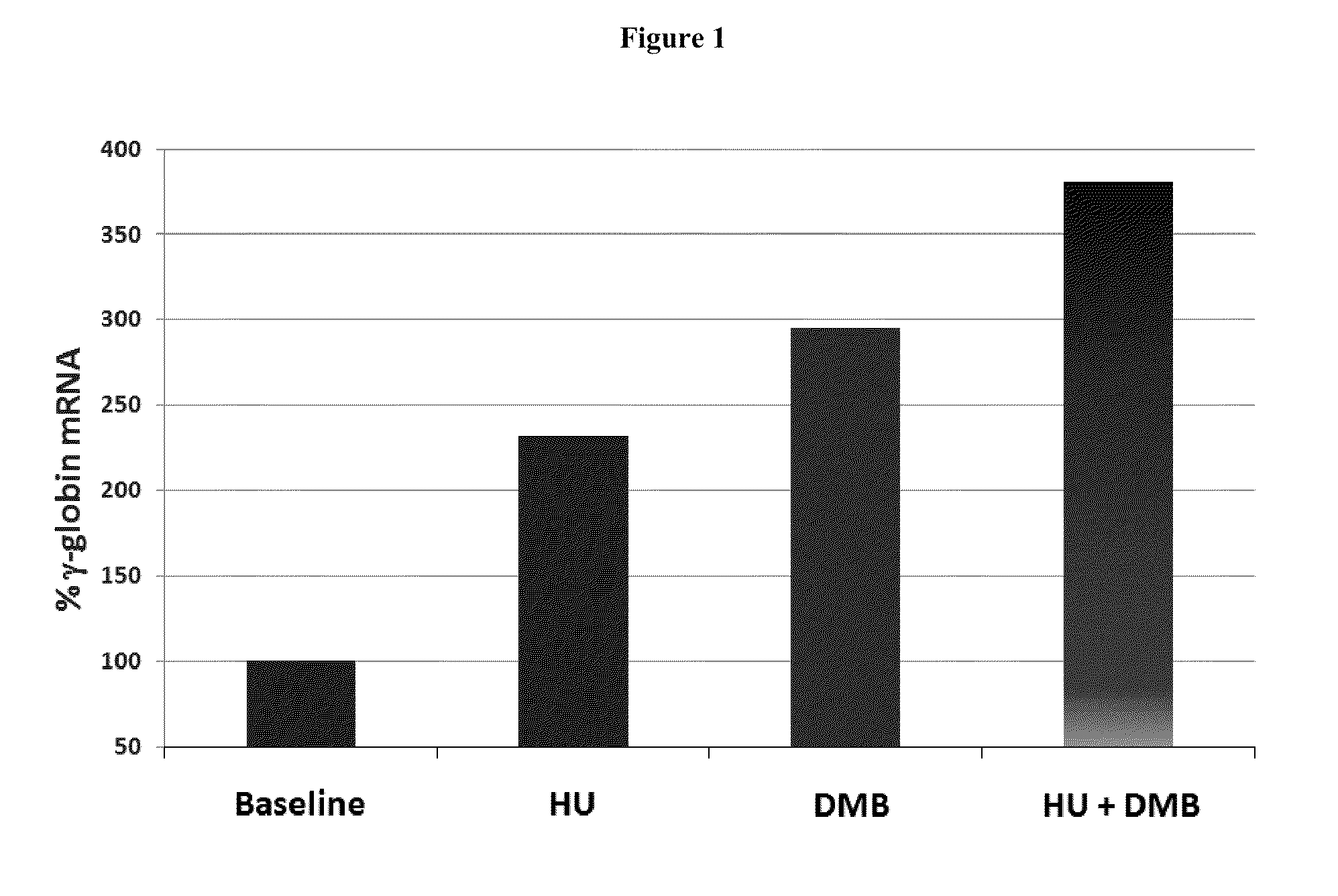
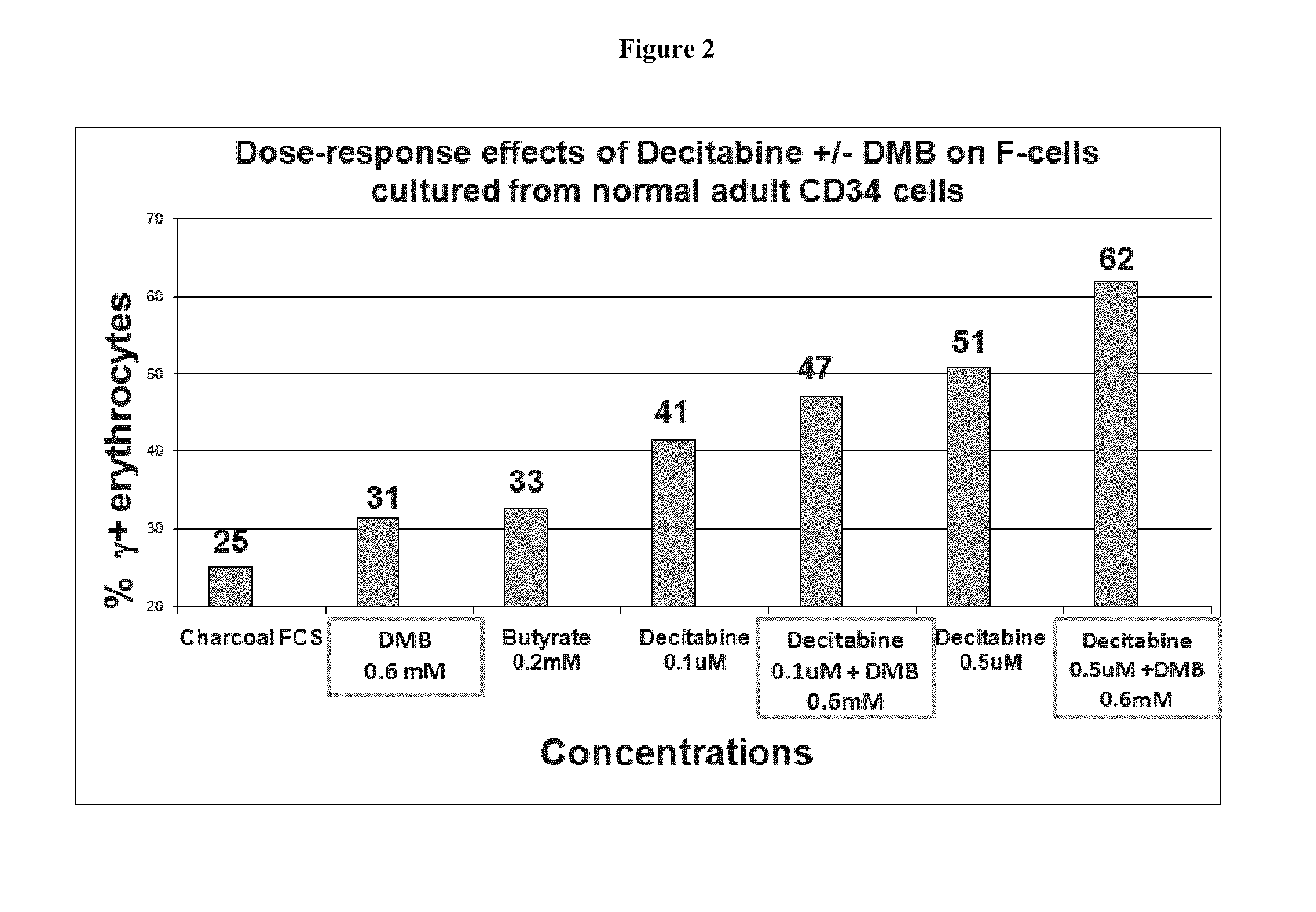
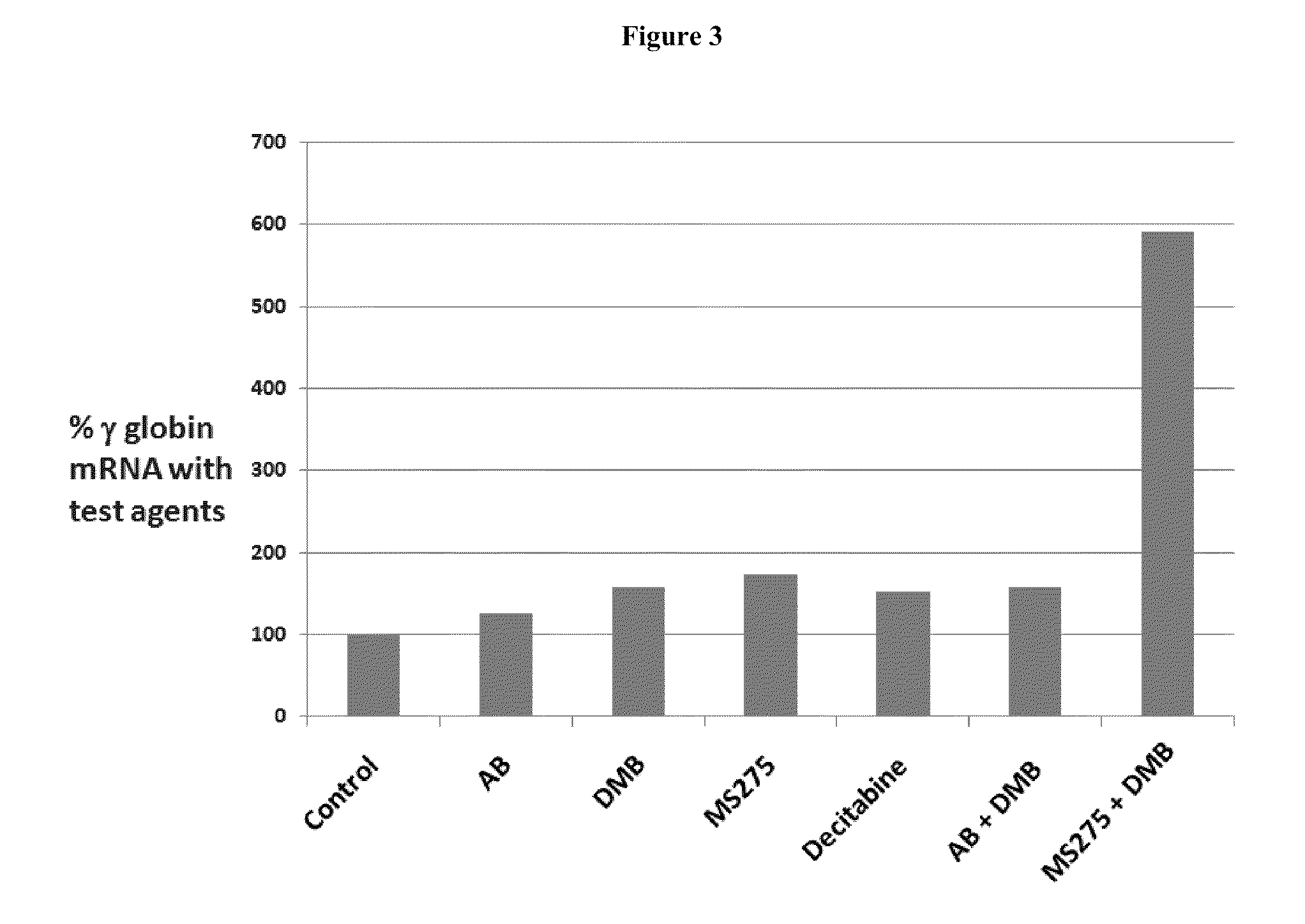
Disclosed herein are methods and low dose regimens for increasing fetal hemoglobin levels in patients with red blood cell disorders, such as beta thalassemia, sickle cell disease, other anemias, or blood loss. Fetal and total hemoglobin levels and red blood cell counts are increased by administering 2,2-dimethylbutyrate (DMB) alone or in combination with hydroxyurea, decitabine or an HDAC inhibitor. Treatment can be continued for at least two weeks.
Owner:HEMAQUEST PHARMA INC +1
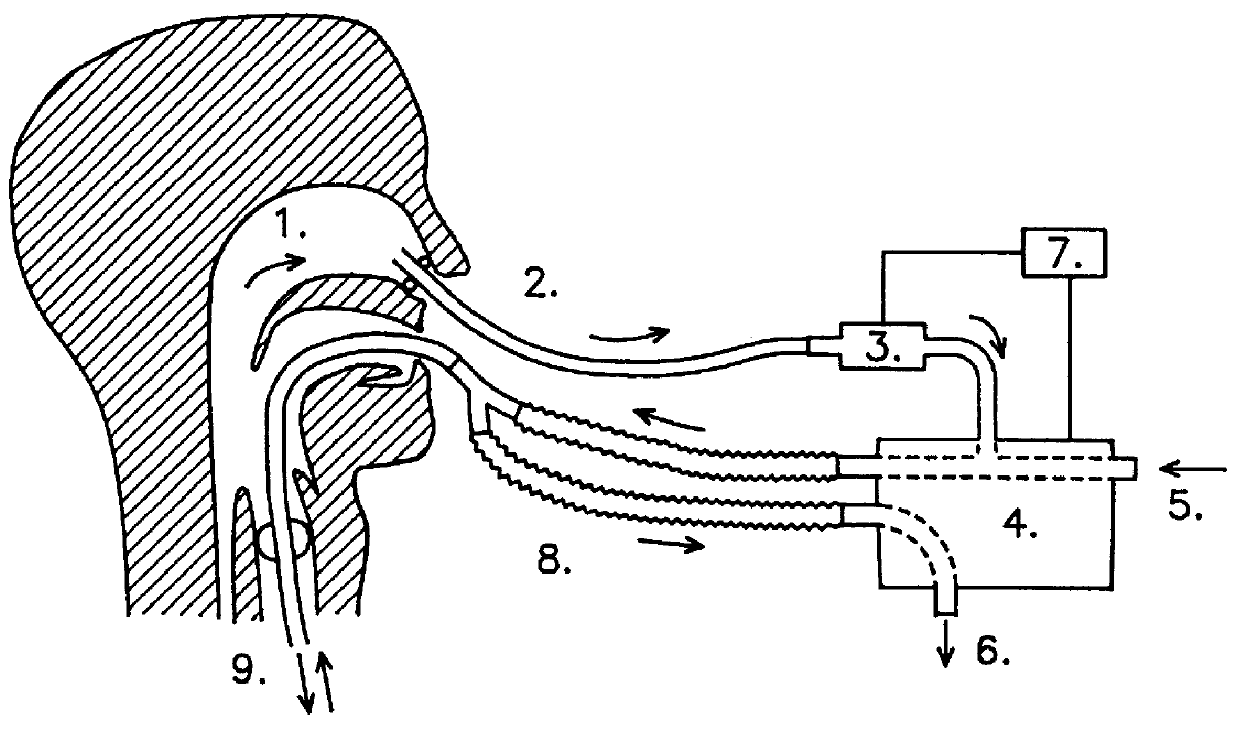
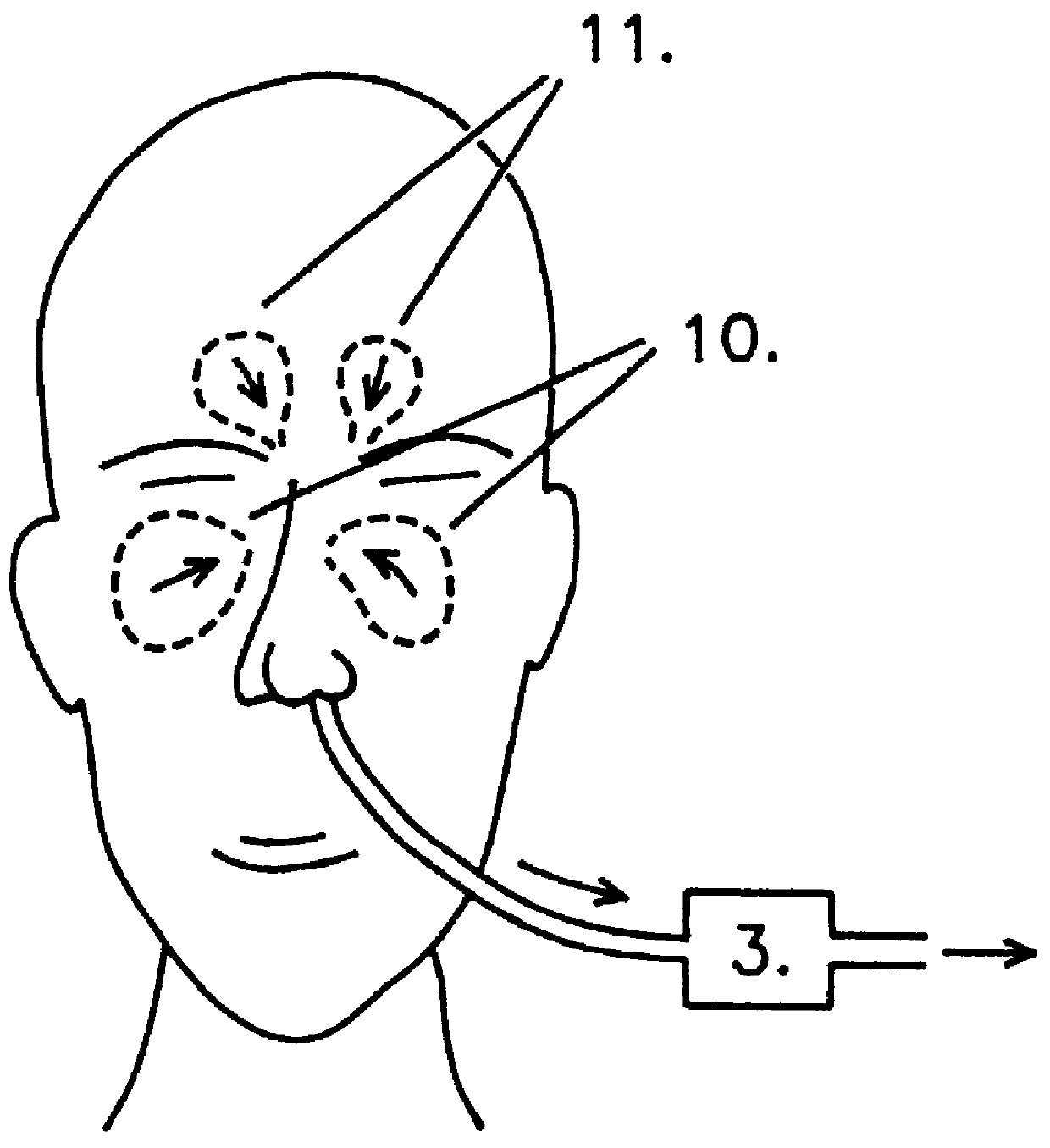
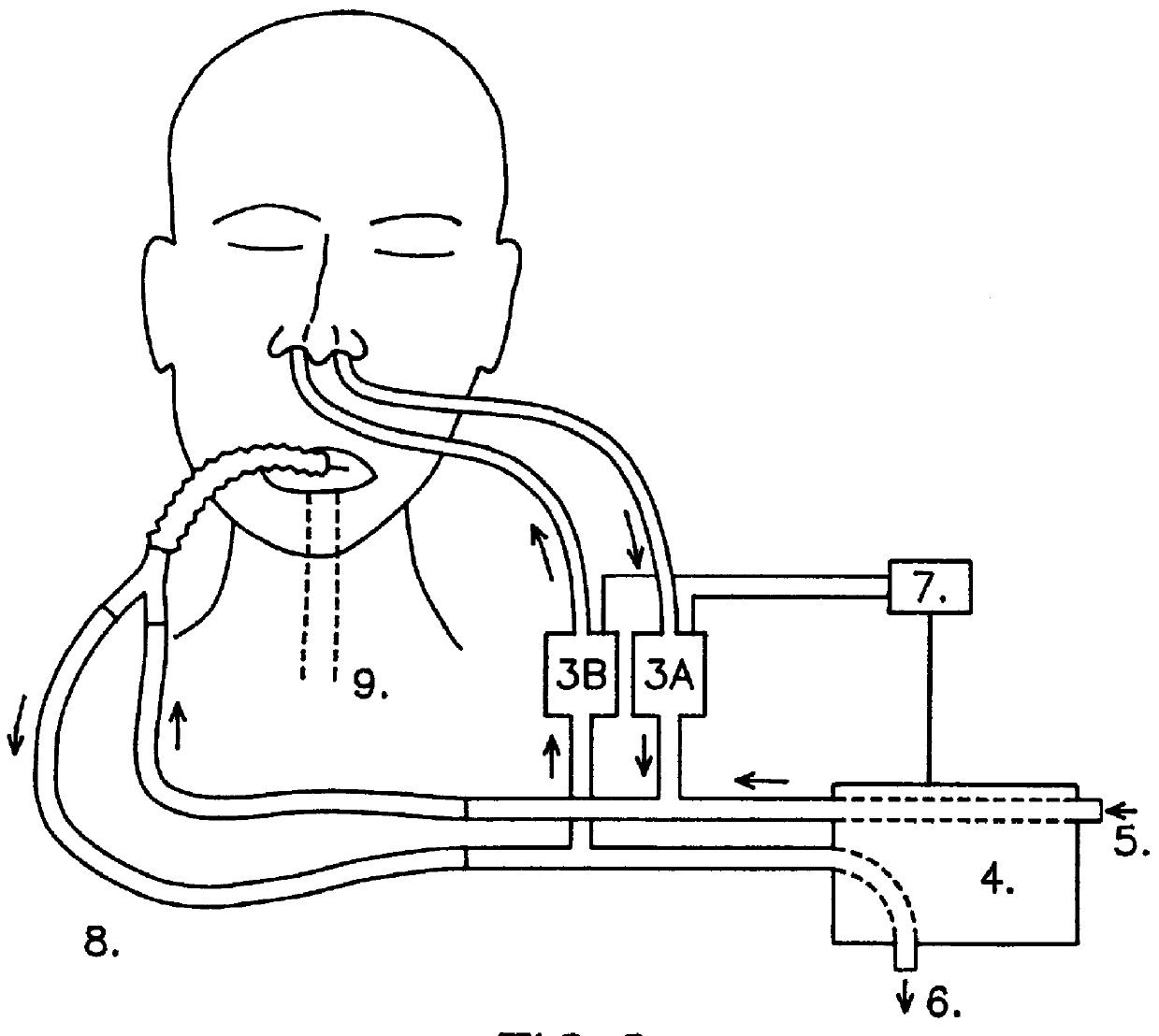
Ventilator device
InactiveUS6019100AIncrease ciliary beat frequencyRestore natural nasal breathing patternTracheal tubesOperating means/releasing devices for valvesActive agentNitric oxide
The present invention restores the normal low-dose flushing of the lower airways with air from the upper airways, containing nitric oxide (NO) and possible other biologically active agents by aspiration of air from the upper airways and introducing said air in the inspiratory airflow of a ventilator. The inventive apparatus and method is free from the risks associated with traditional administration of exogenous NO.
Owner:ALVING KJELL +3
Novel trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
High performance CMOS devices and methods for making same
ActiveUS8067280B2Improve performanceImprove short channel effectTransistorSemiconductor/solid-state device manufacturingCapacitanceCMOS
An integrated circuit having high performance CMOS devices with good short channel effects may be made by forming a gate structure over a substrate; forming pocket implant regions and source / drain extensions in the substrate; forming spacers along sides of the gate structure; and thermal annealing the substrate when forming the spacers, the thermal annealing performed at an ultra-low temperature. An integrated circuit having high performance CMOS devices with low parasitic junction capacitance may be made by forming a gate structure over a substrate; forming pocket implant regions and source / drain extensions in the substrate; forming spacers along sides of the gate structure; performing a low dosage source / drain implant; and performing a high dosage source / drain implant.
Owner:TAIWAN SEMICON MFG CO LTD
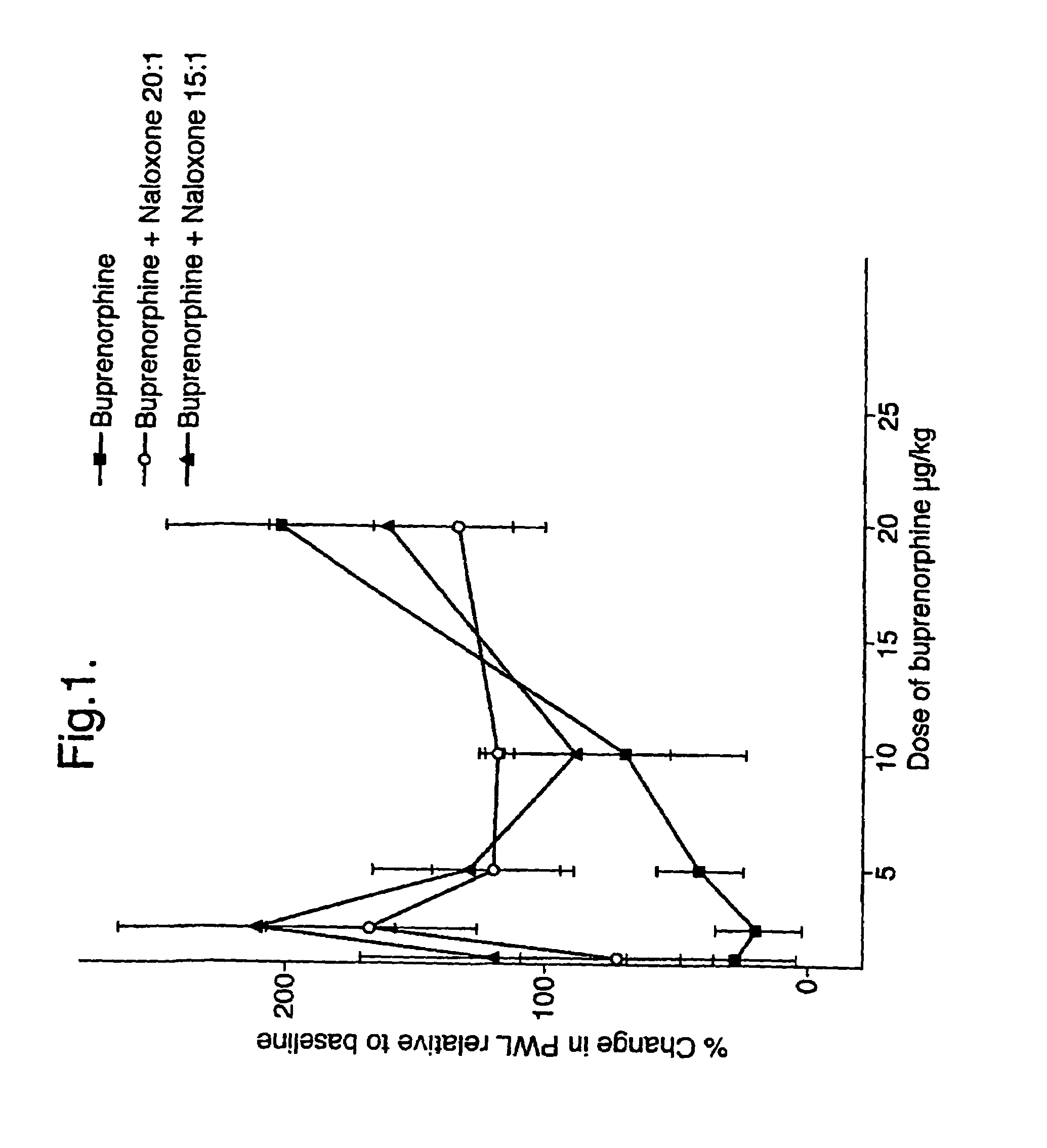
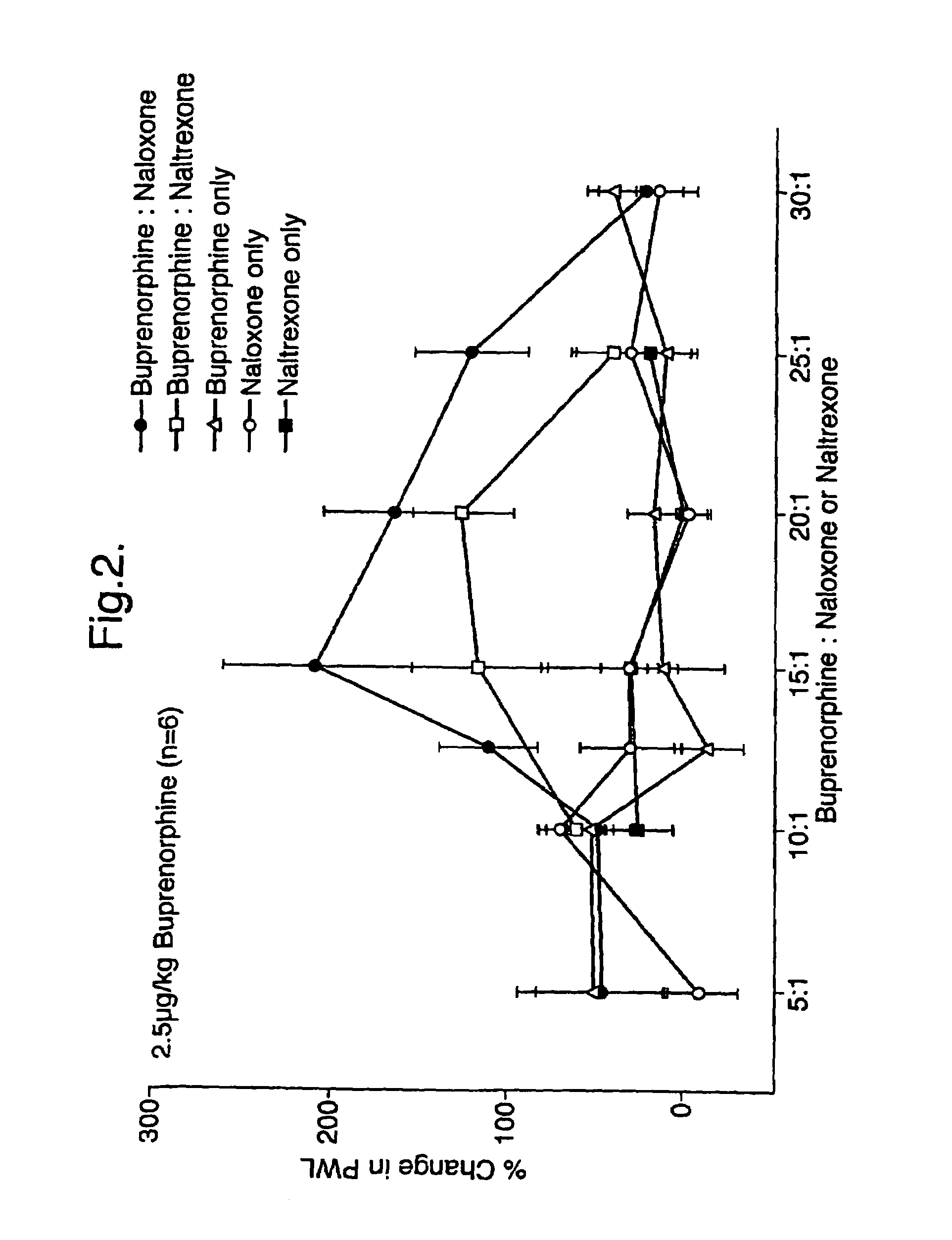
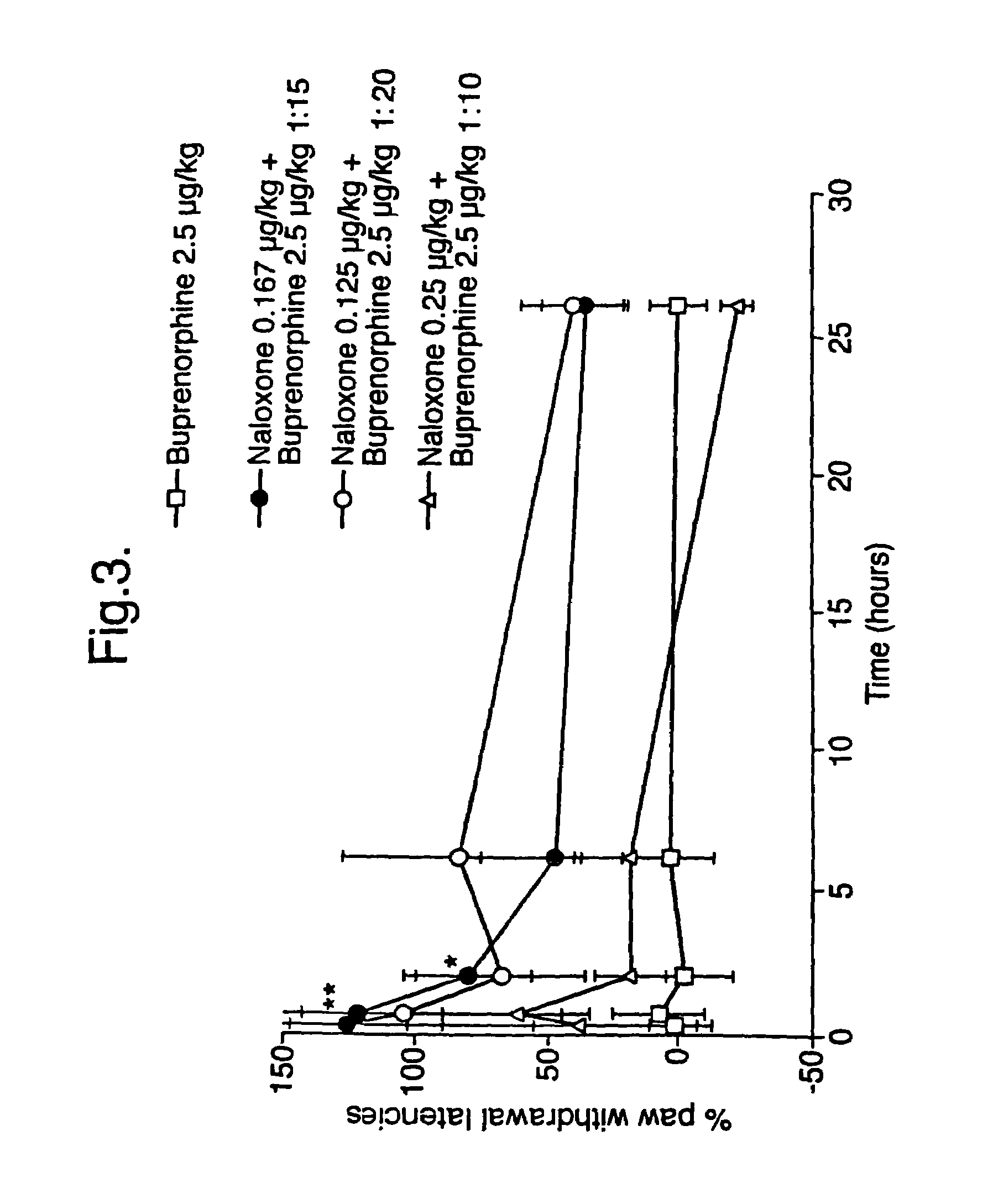
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
Cationic lipids and methods for the delivery of therapeutic agents
Owner:PROTIVA BIOTHERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com