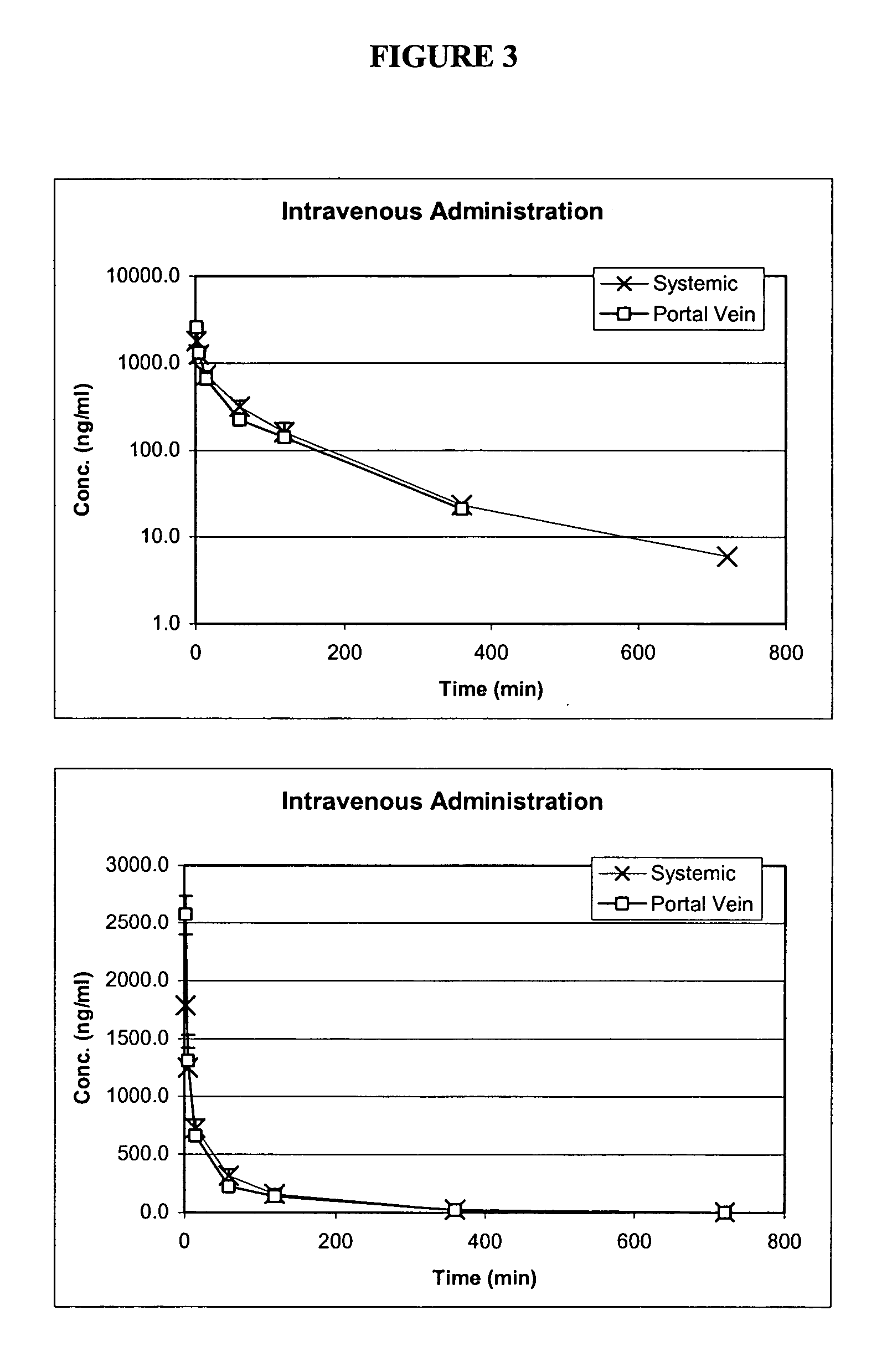
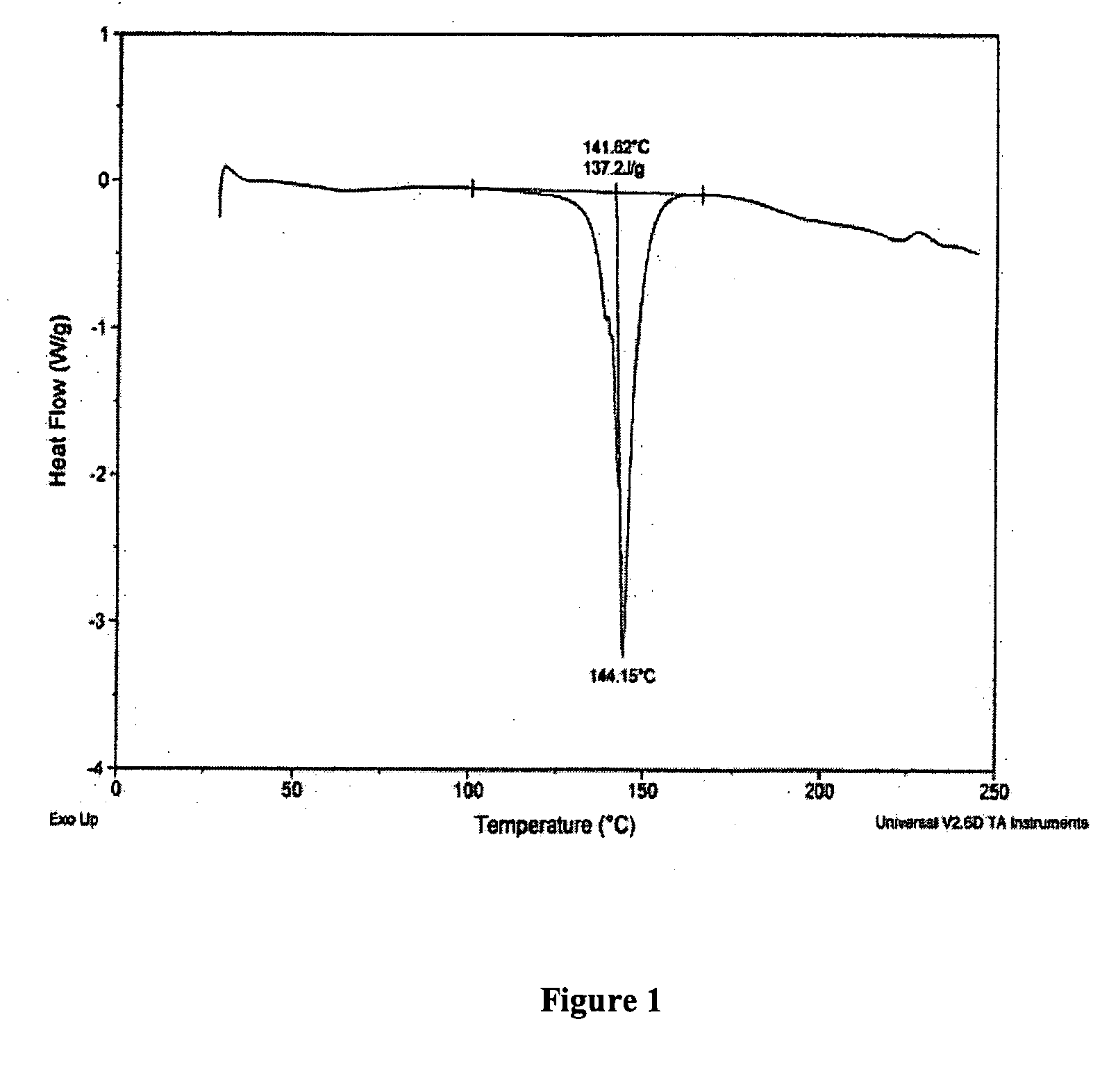
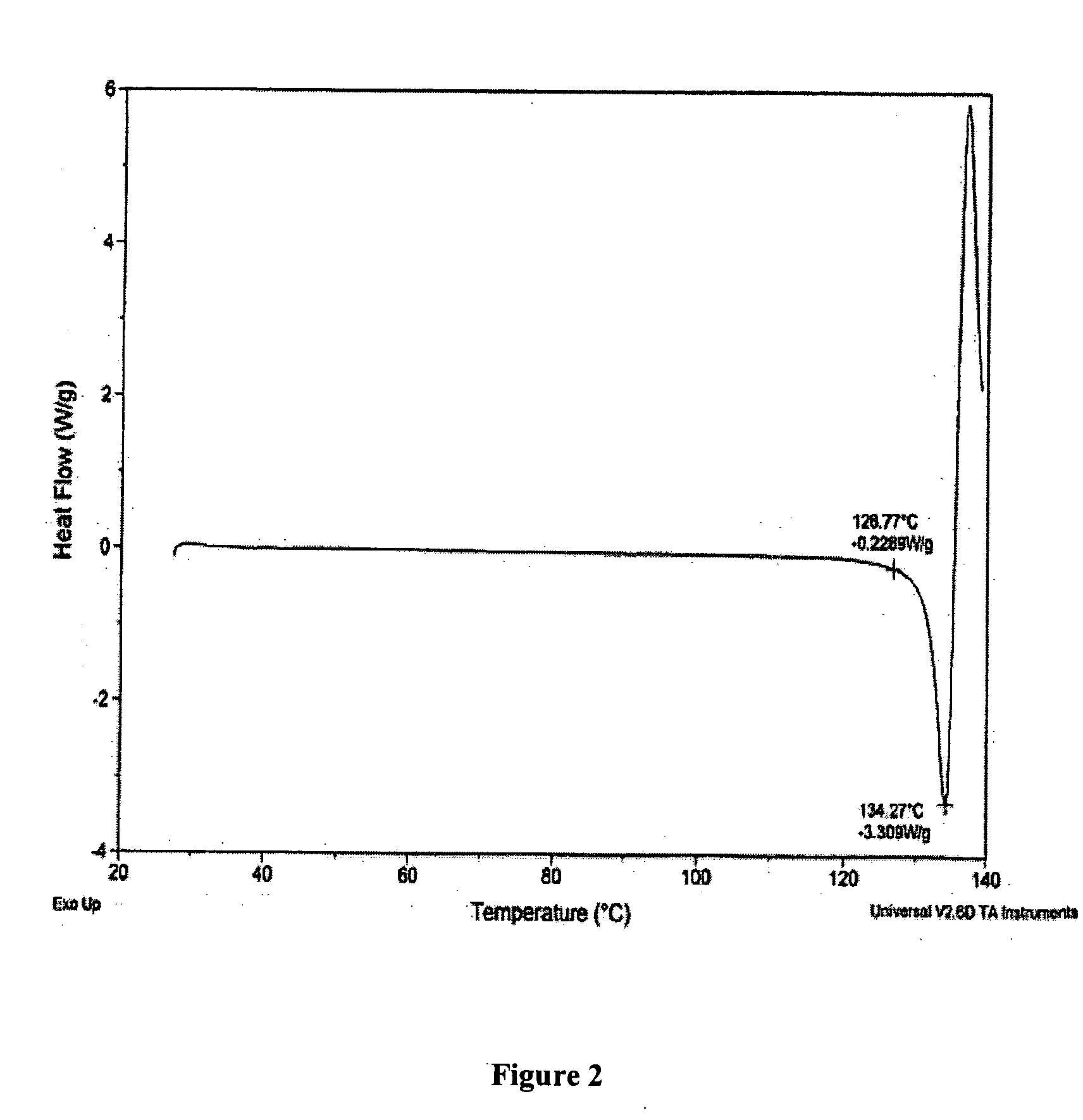
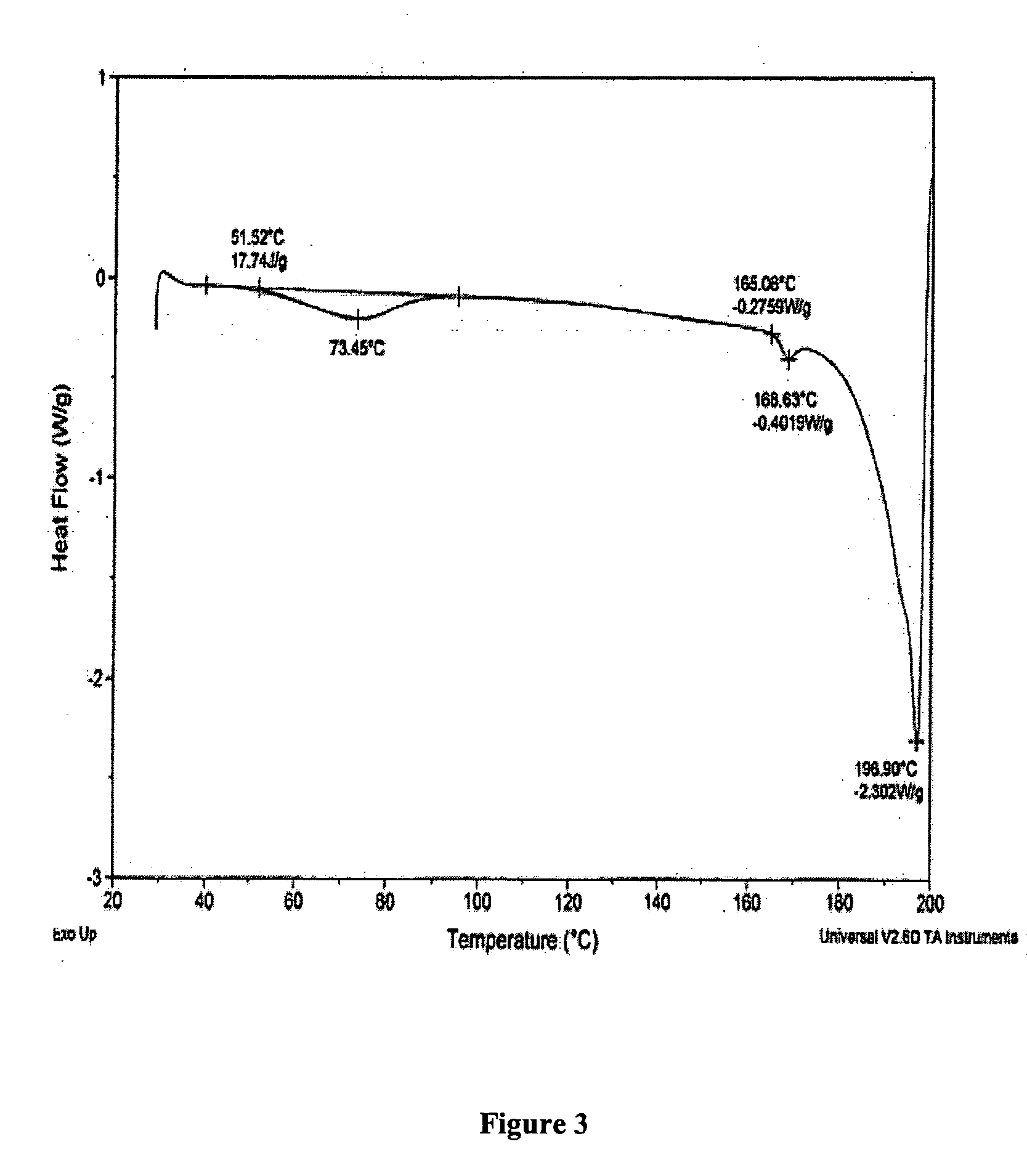
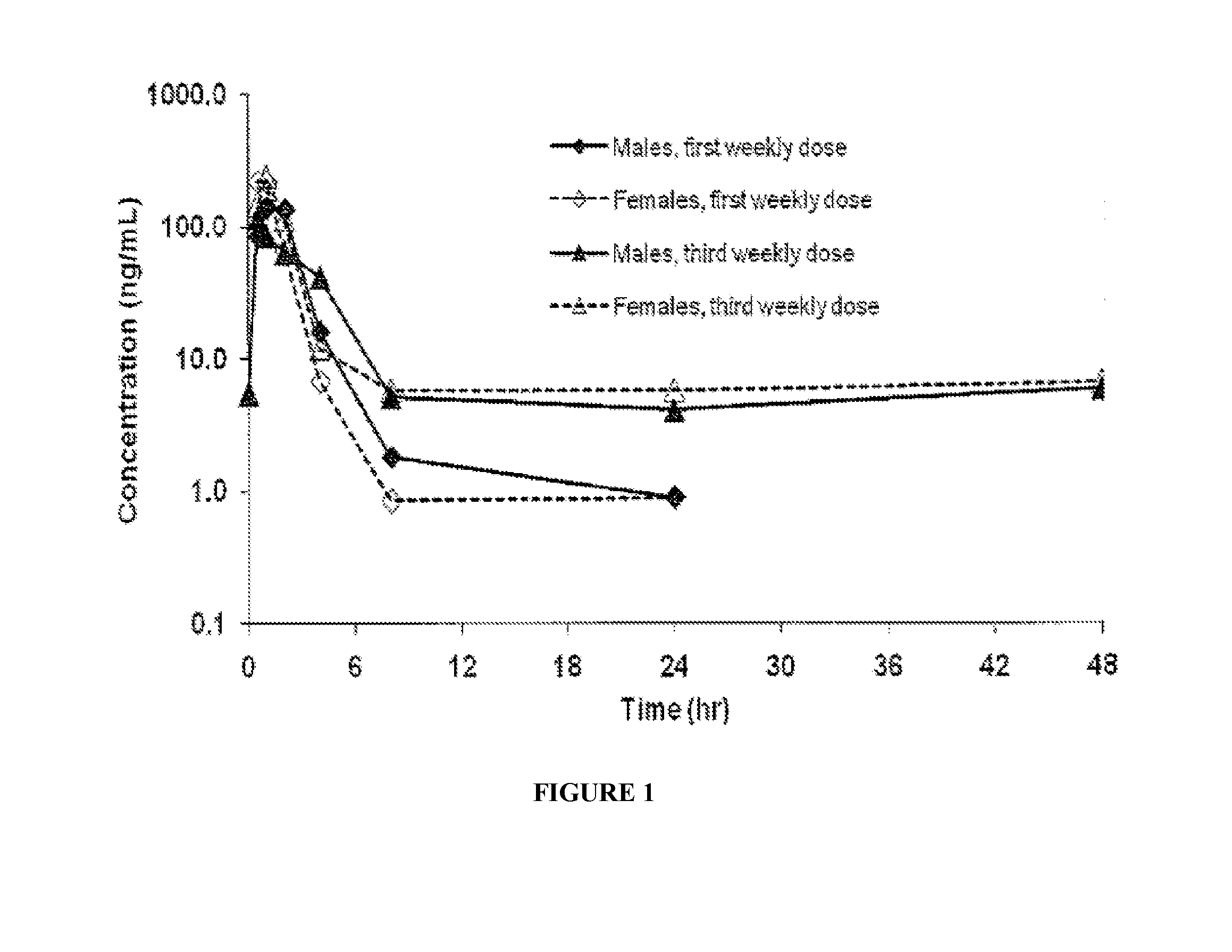
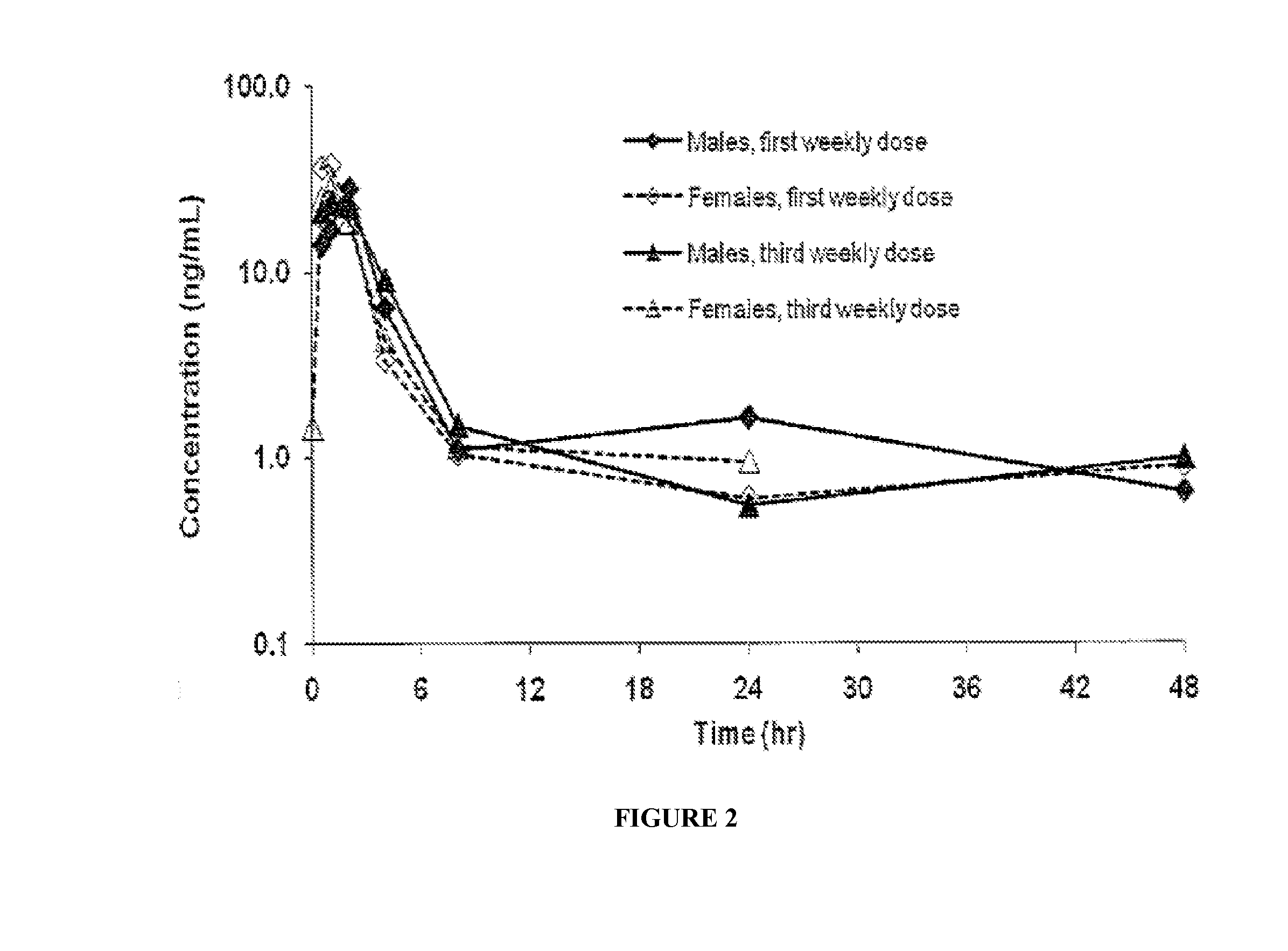
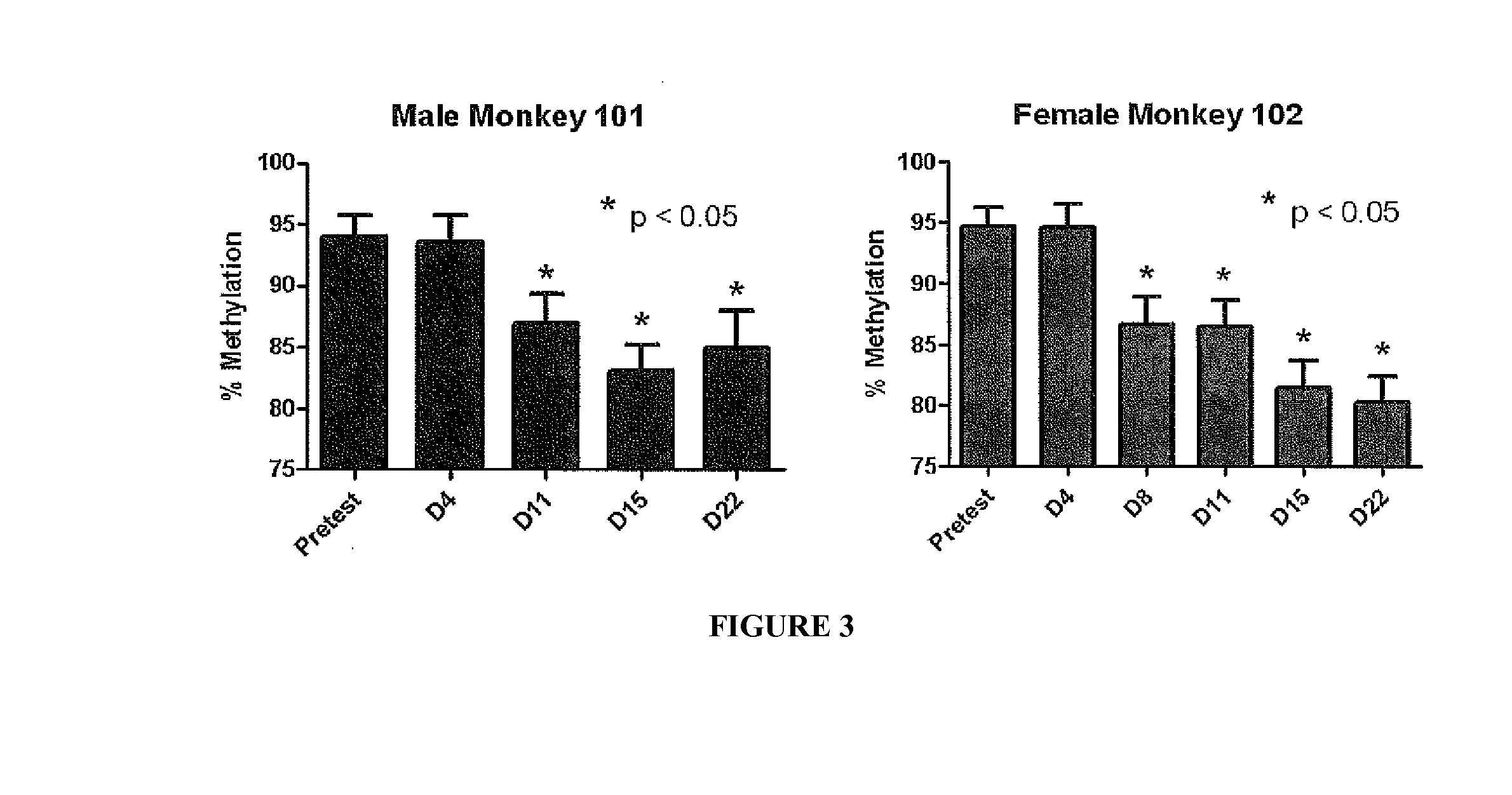
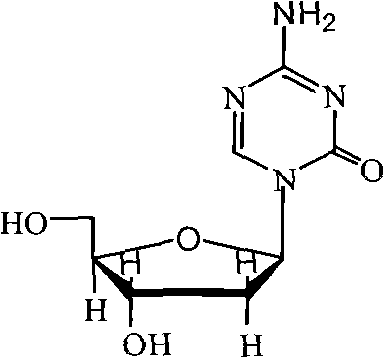
Patents
Literature
141 results about "Decitabine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
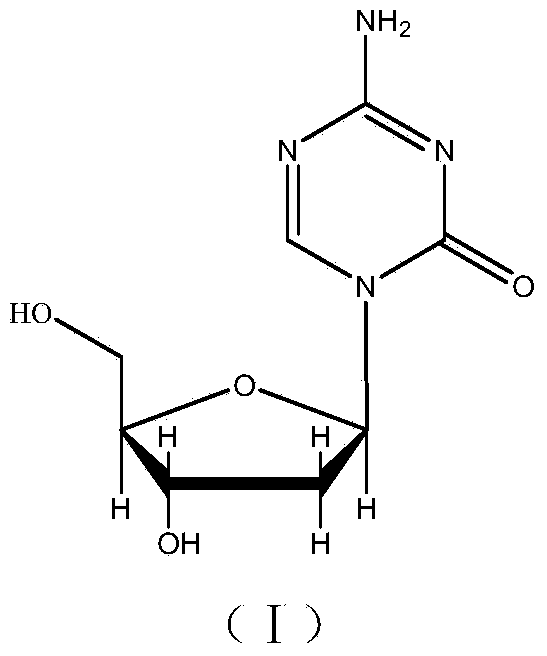
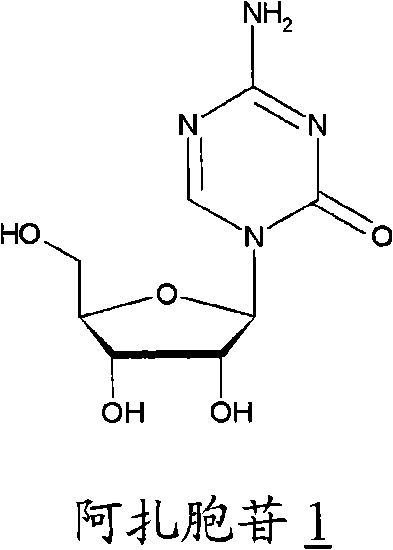
This medication is used to treat a group of blood/bone marrow disorders (myelodysplastic syndromes-MDS) in which the bone marrow does not produce enough healthy blood cells.
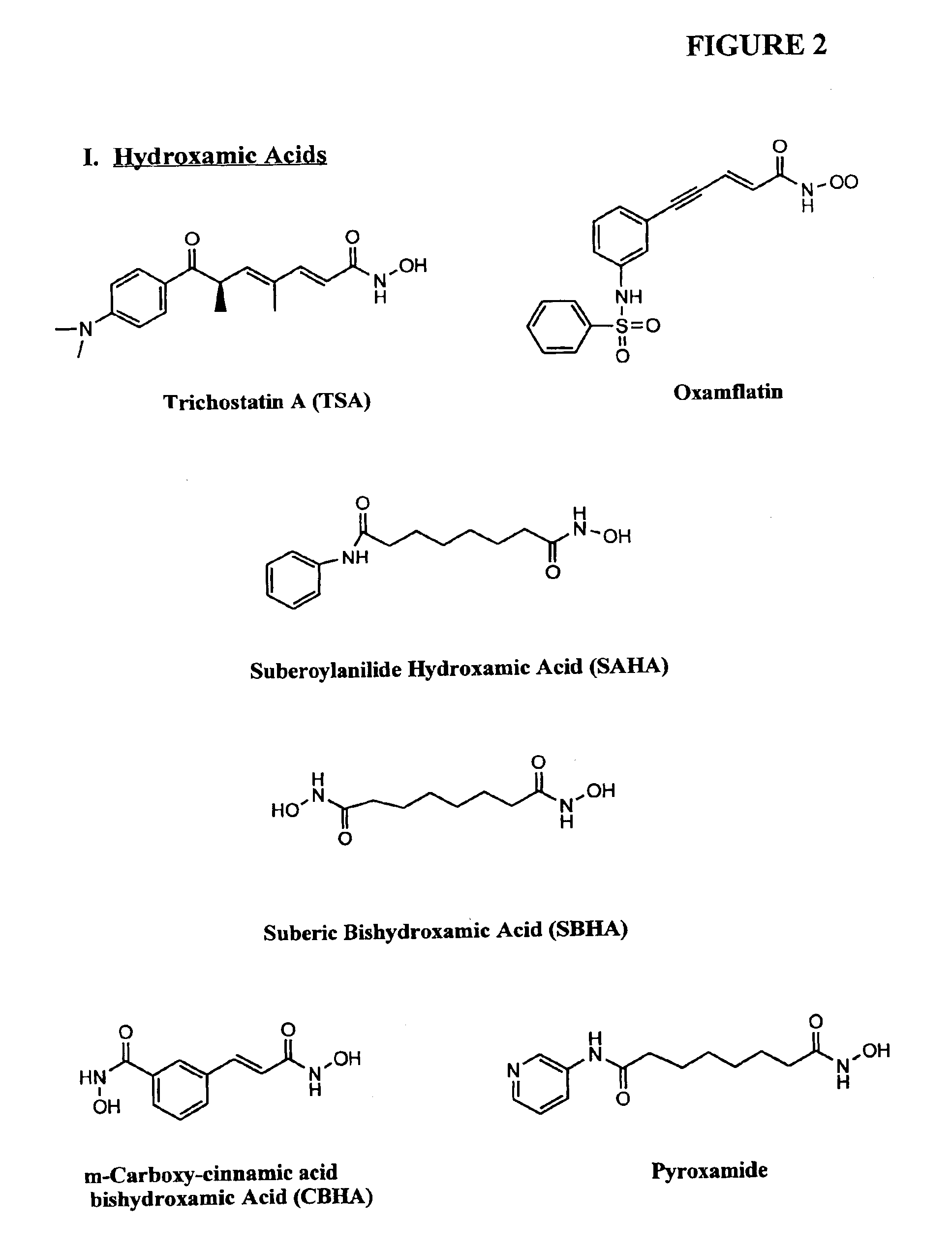
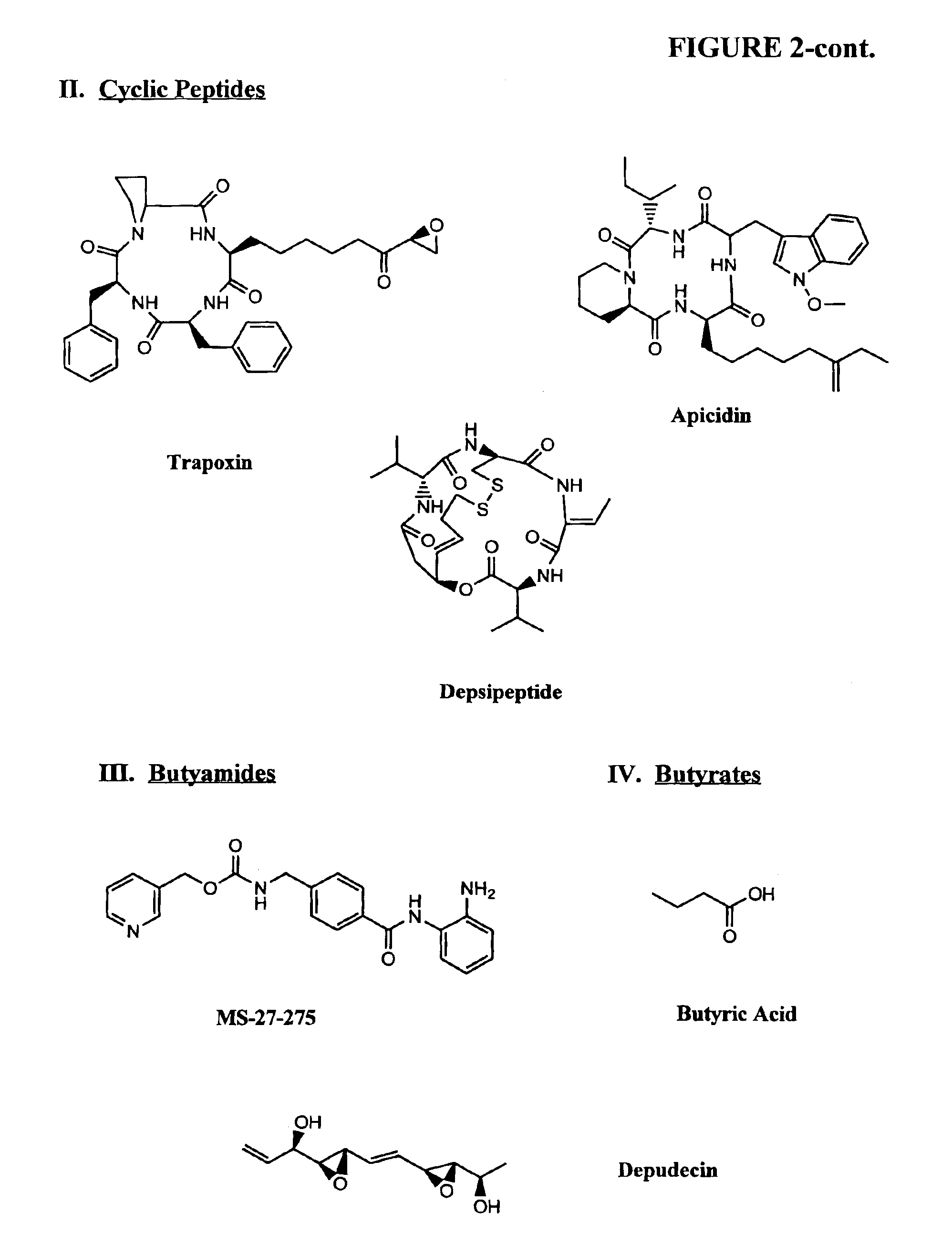
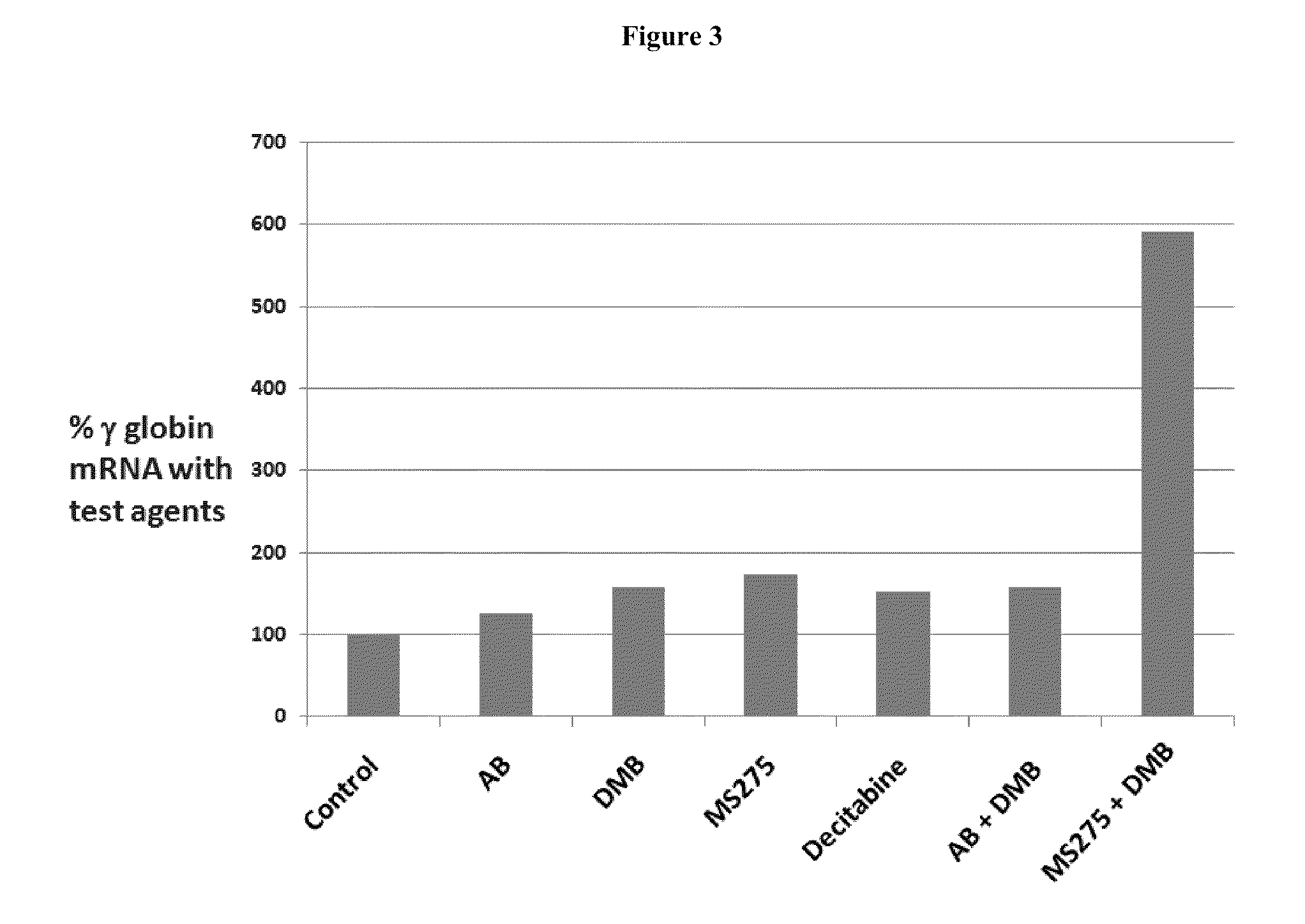
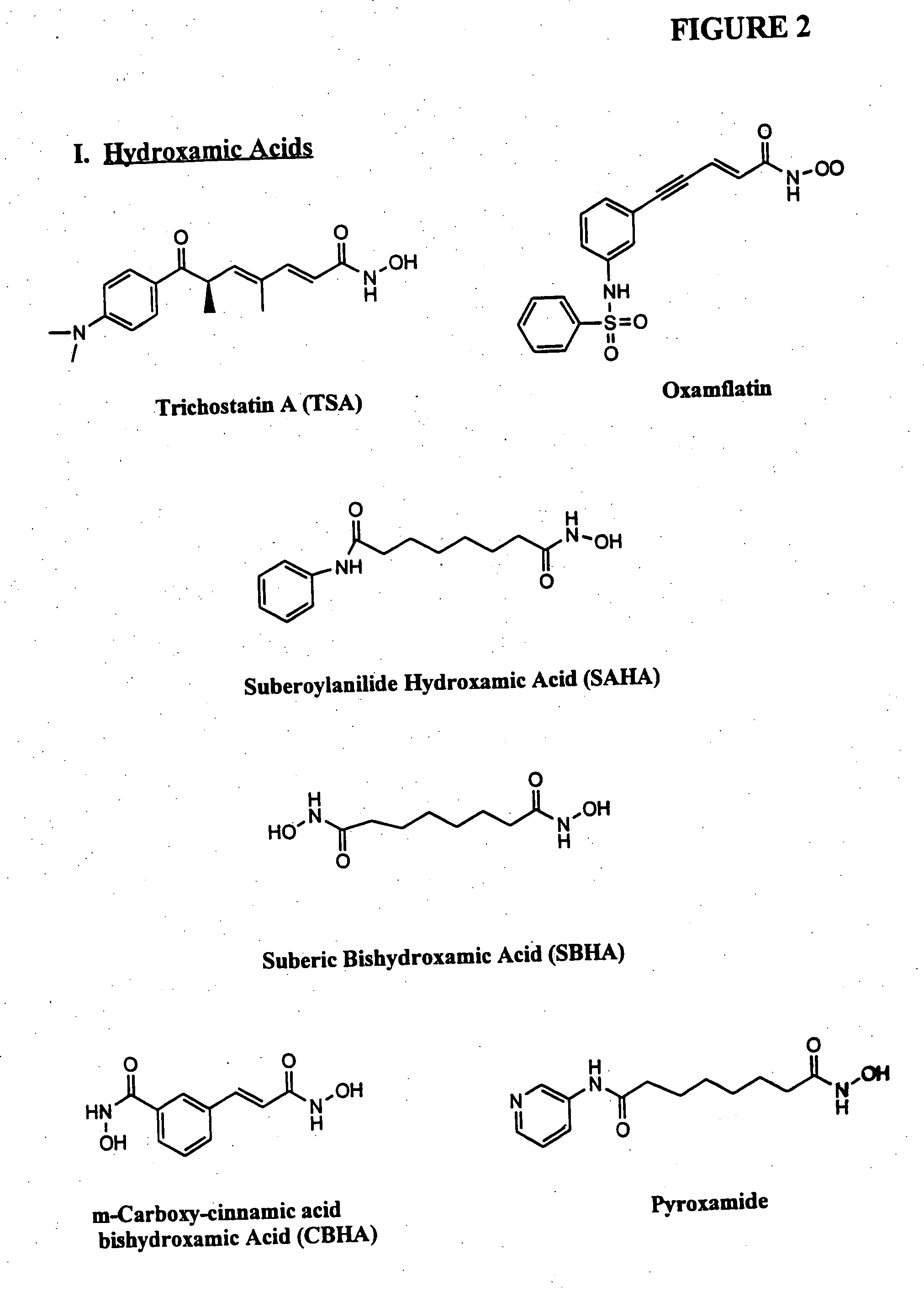
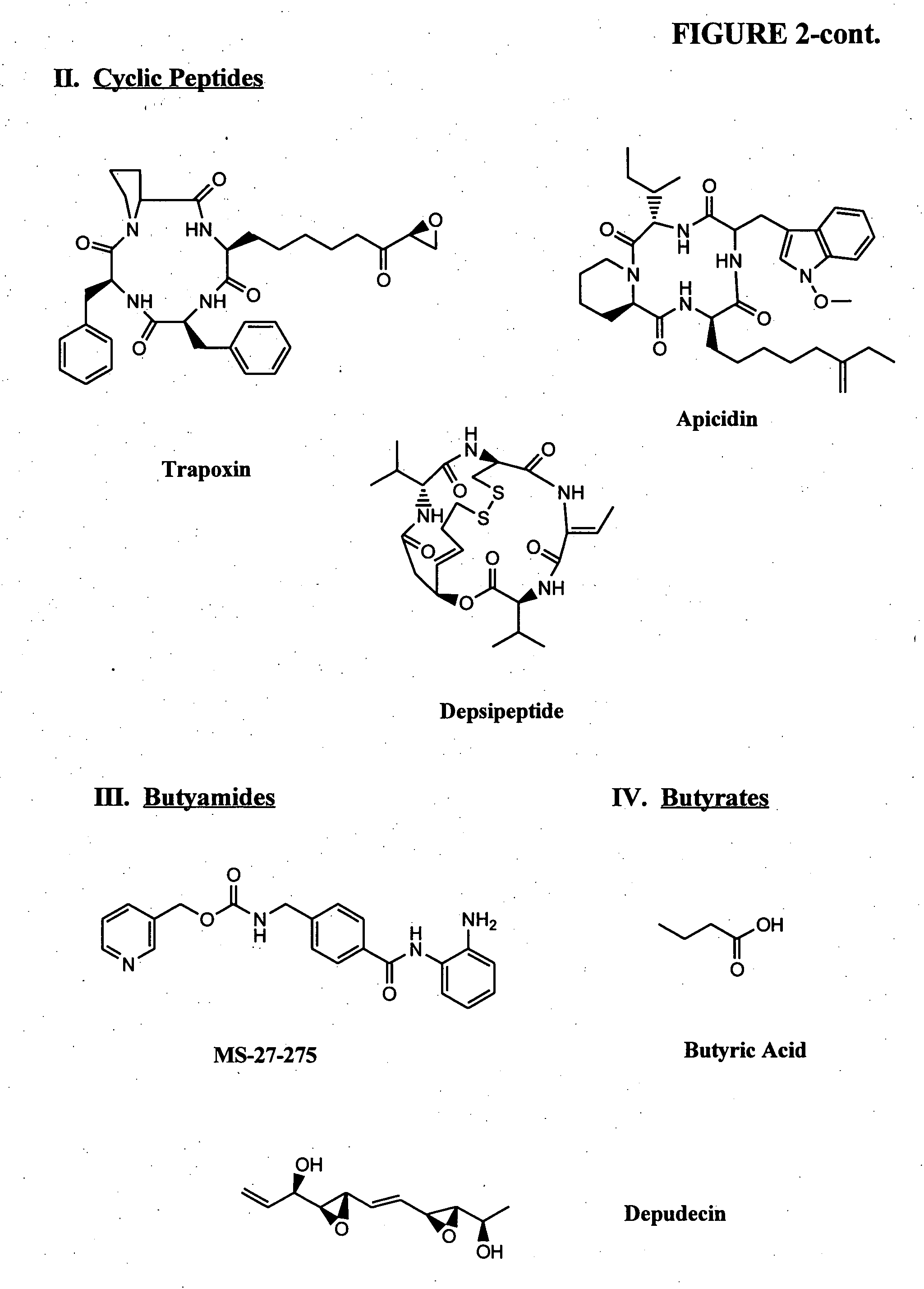
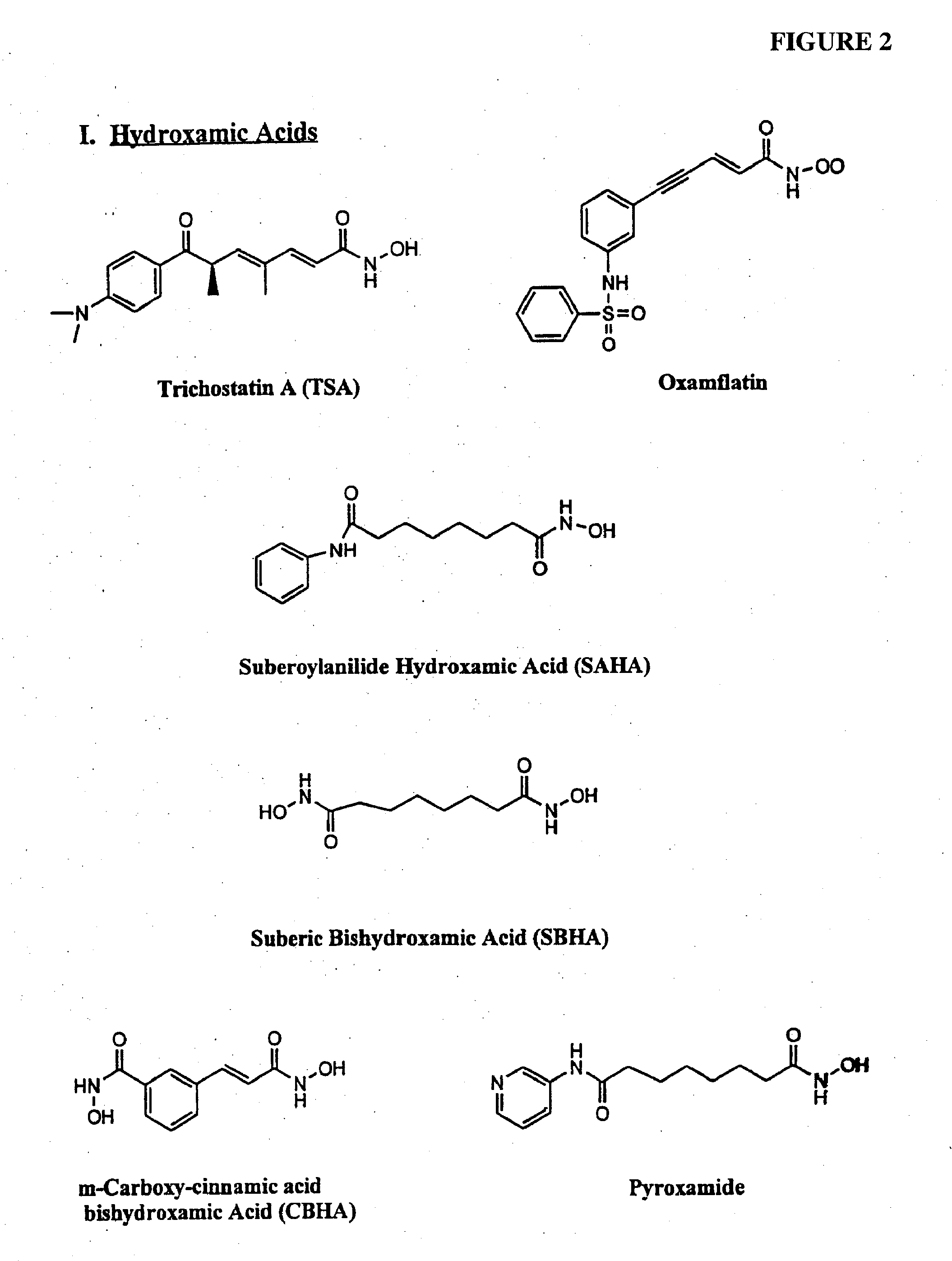
Compositions and methods for reestablishing gene transcription through inhibition of DNA methylation and histone deacetylase
InactiveUS6905669B2Better clinical outcomeReduce dosageBiocideCell receptors/surface-antigens/surface-determinantsCyclic peptideDisease
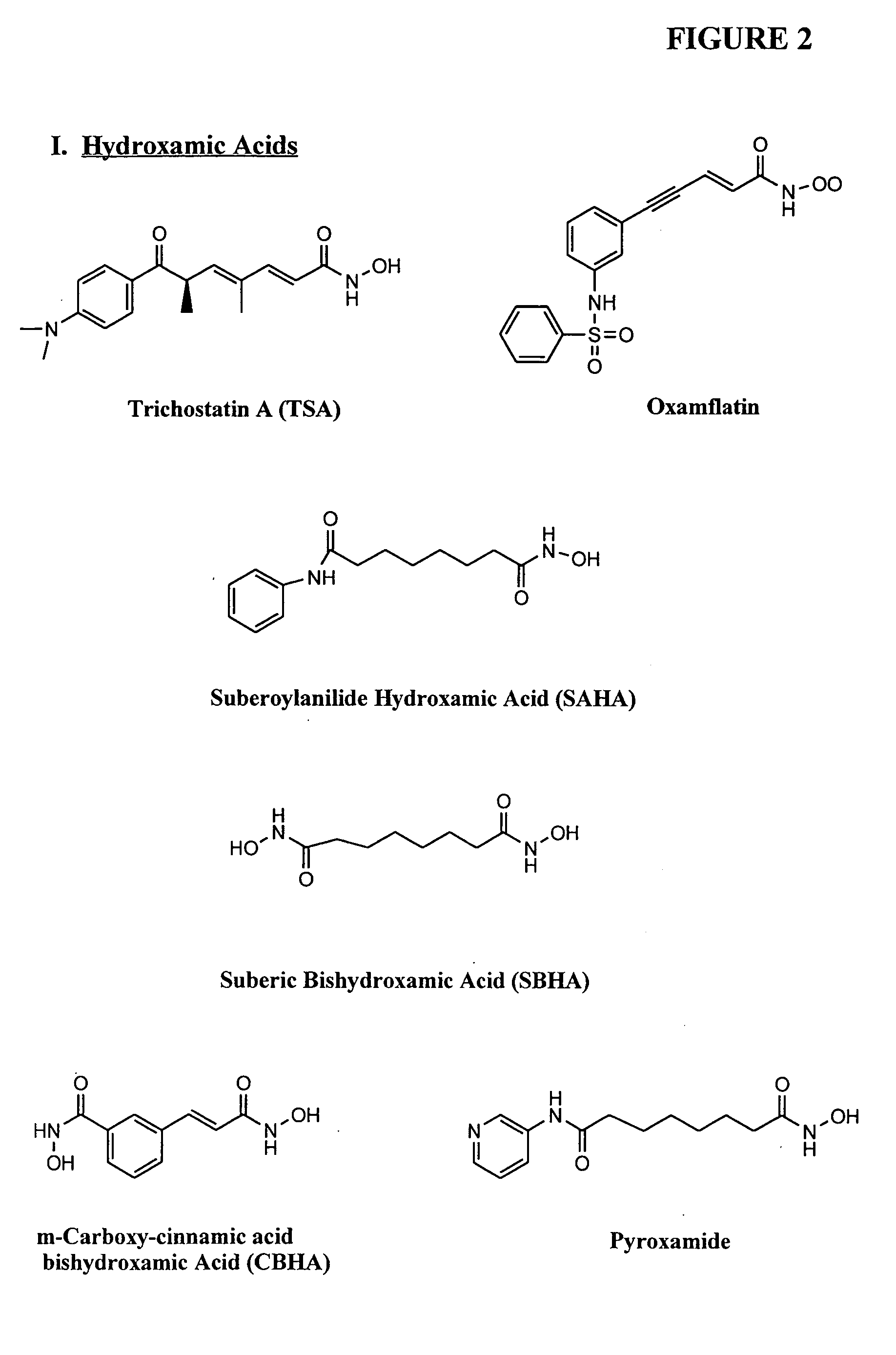
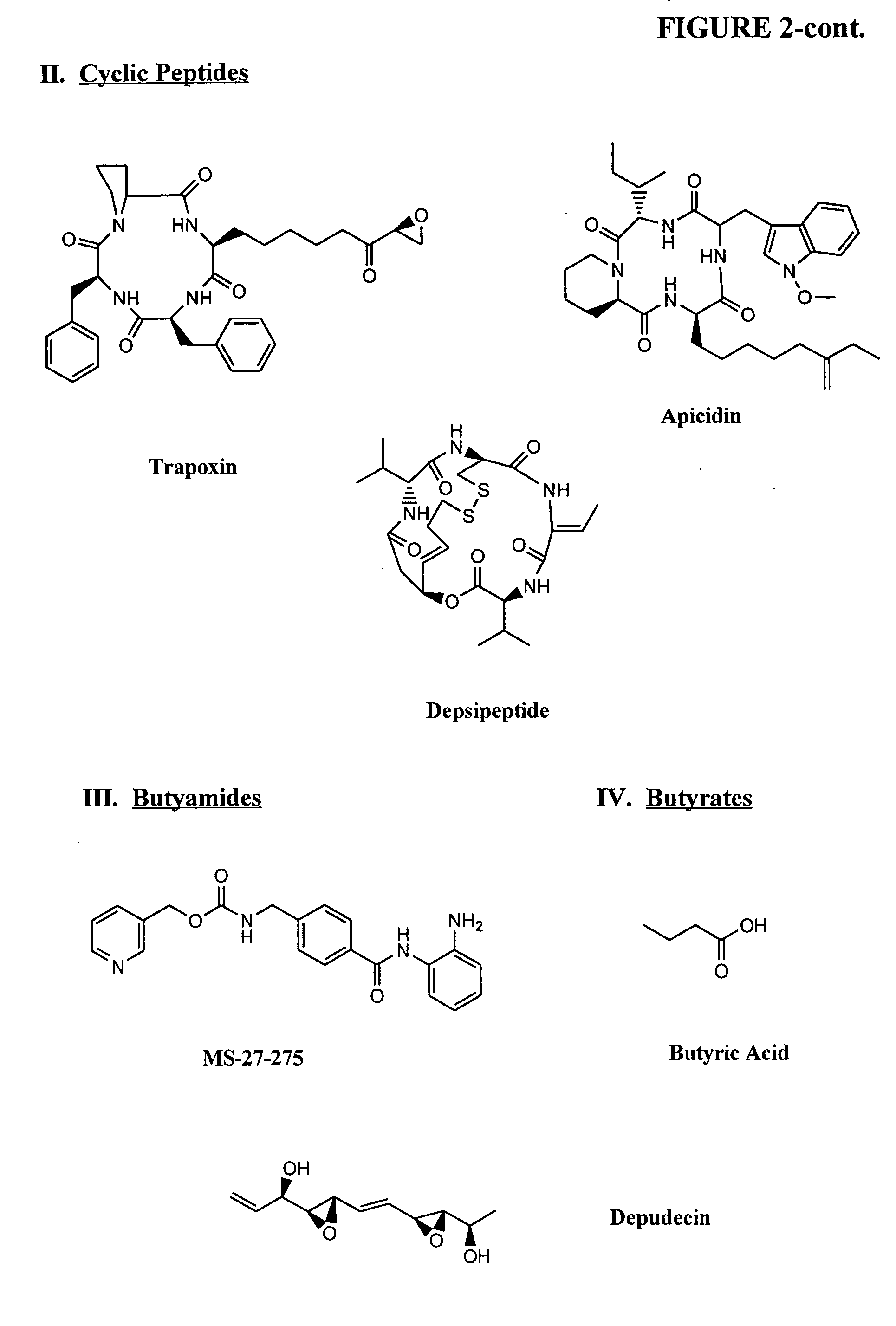
Compositions and methods are provided for treating diseases associated with aberrant silencing of gene expression such as cancer by reestablishing the gene expression through inhibition of DNA hypomethylation and histone deacetylase. The method comprises: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
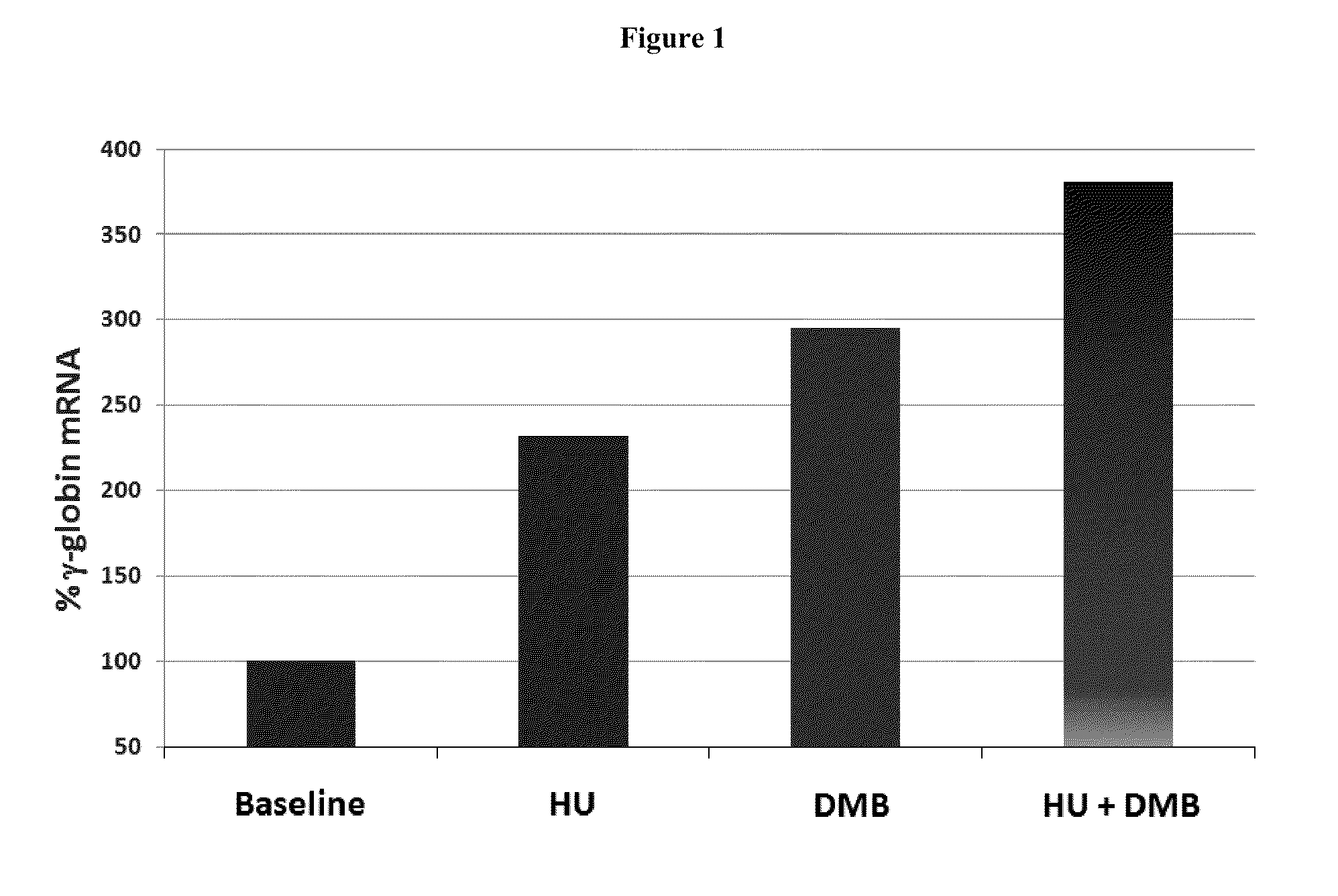
Methods and low dose regimens for treating red blood cell disorders
ActiveUS20110251149A1Increase volumeIncrease the number ofBiocideCarbohydrate active ingredientsBeta thalassemiaRegimen
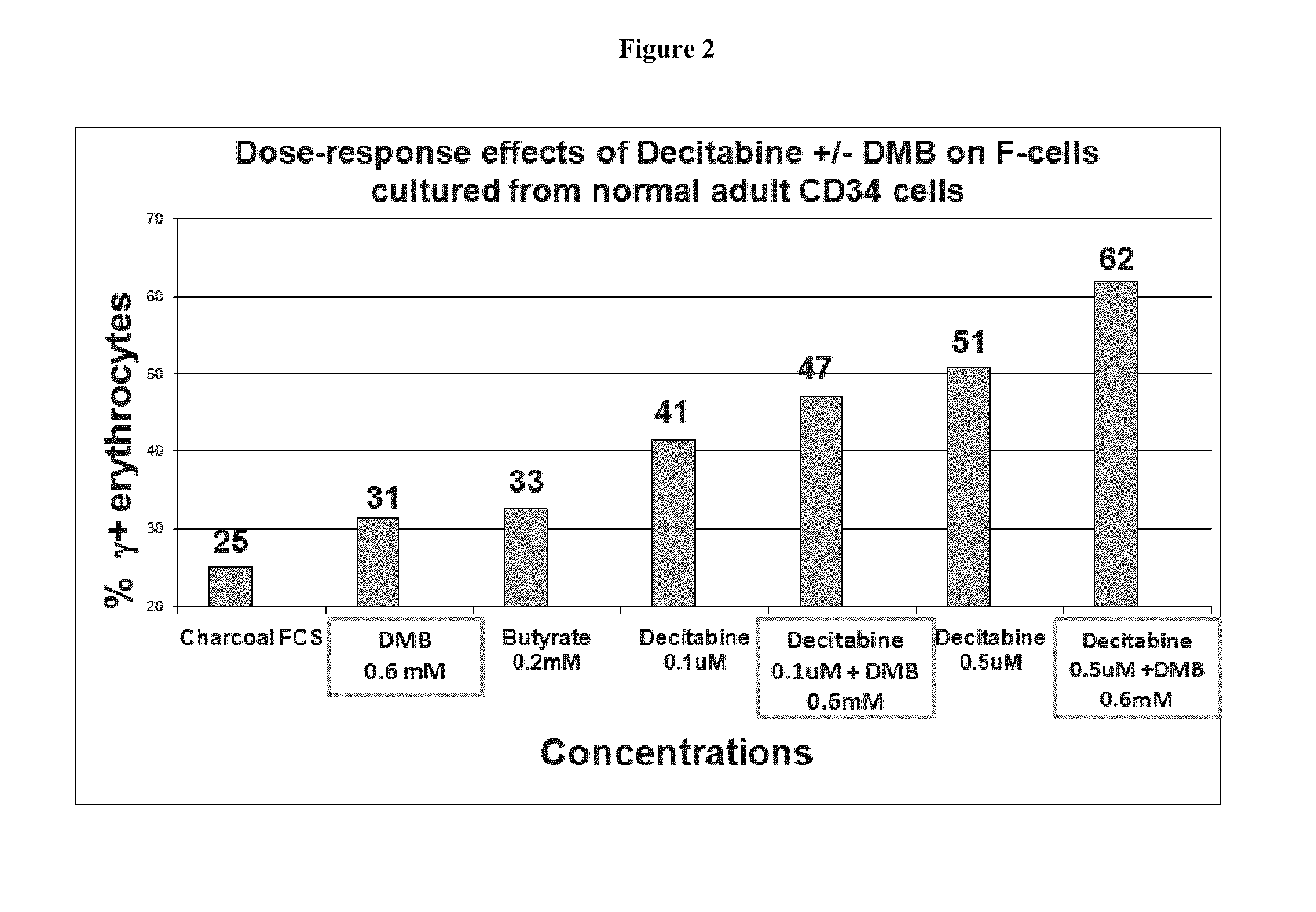
Disclosed herein are methods and low dose regimens for increasing fetal hemoglobin levels in patients with red blood cell disorders, such as beta thalassemia, sickle cell disease, other anemias, or blood loss. Fetal and total hemoglobin levels and red blood cell counts are increased by administering 2,2-dimethylbutyrate (DMB) alone or in combination with hydroxyurea, decitabine or an HDAC inhibitor. Treatment can be continued for at least two weeks.
Owner:HEMAQUEST PHARMA INC +1
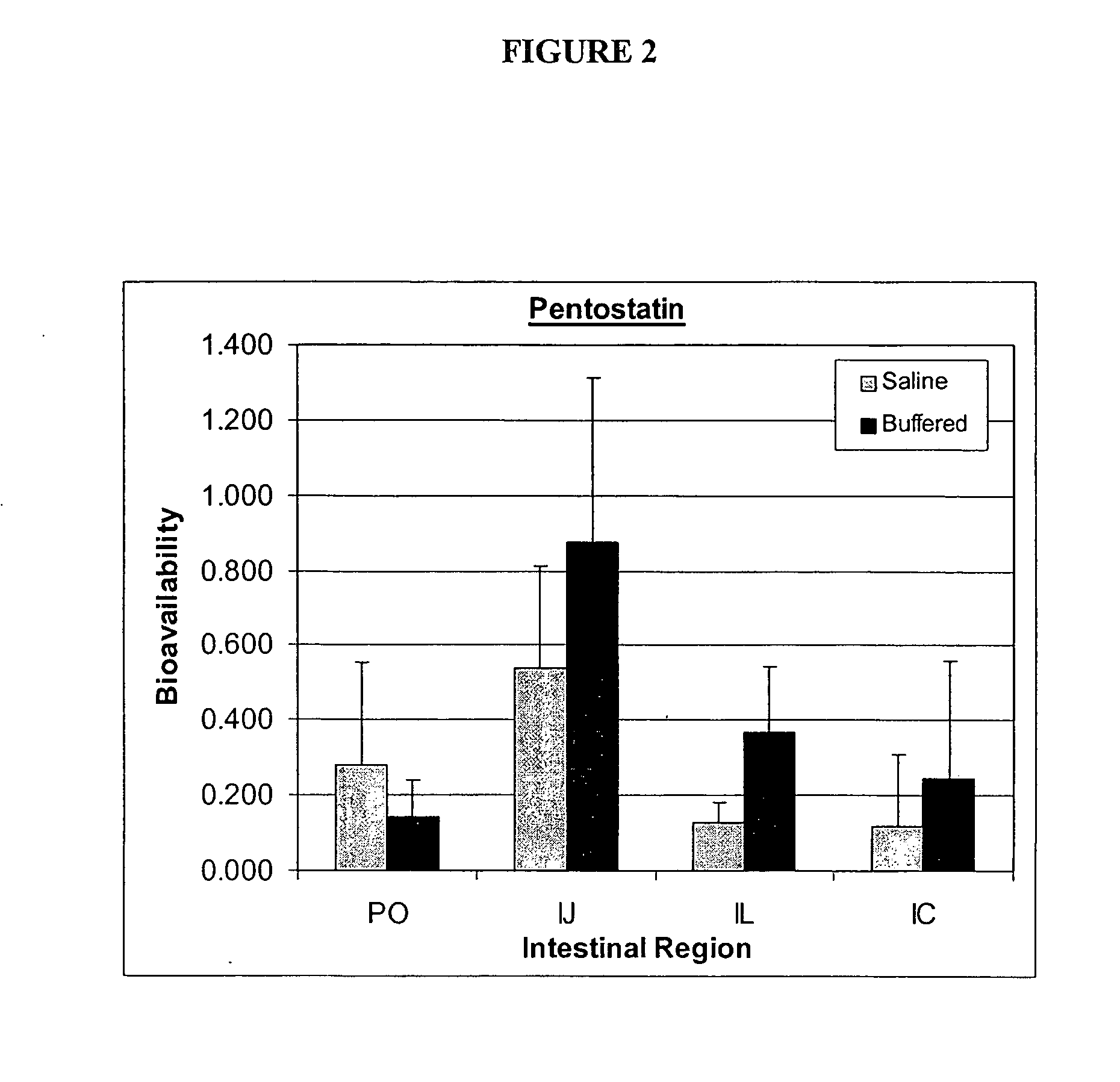
Pharmaceutical formulations targeting specific regions of the gastrointesinal tract
Oral formulations of pharmaceuticals are provided with enhanced bioavailability by targeting specific regions of the gastrointestinal tract. Particularly, water soluble and acid-labile drugs such as cytidine analogs (e.g., decitabine) and 2'-deoxyadenosine analogs (e.g., pentostatin) are formulated with pH-sensitive polymers so that these drugs are preferably absorbed in the upper regions of the small intestine, such as the jejunum. In addition, drugs with poor oral bioavailability such as camptothecin compounds (e.g., 9-nitro-camptothecin) can also be formulated using similar strategies in order to significantly improve their oral bioavailability. These formulations can be used to treat a wide variety of diseases or conditions, such hematological disorders, benign tumors, cancer, restenosis, inflammatory diseases, and autoimmune diseases.
Owner:MAYNE PHARMA USA
Liquid formulation of decitabine and use of the same
Pharmaceutical formulations, kits and vessels are provided for delivering decitabine to a patient suffering from a disease in need of treatment with decitabine. The pharmaceutical formulation comprises decitabine solvated in a non-aqueous solvent that comprises glycerin, propylene glycol, polyethylene glycol, or combinations. Such formulations are more chemically stable than conventional liquid formulations of decitabine containing more than 40% water in volume. The pharmaceutical formulations can be used for any disease that is sensitive to the treatment with decitabine, such as hematological disorders and cancer.
Owner:SUPERGEN
Oral administration of decitabine salt
The present invention relates to salts of decitabine as well as methods for synthesizing the salts described herein. Pharmaceutical compositions and methods of using the decitabine salts are also provided, including methods of orally administering the salts or pharmaceutical compositions thereof to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Salts of decitabine
The present invention relates to salts of decitabine as well as methods for synthesizing the salts described herein. Pharmaceutical compositions and methods of using the decitabine salts are also provided, including methods of administering the salts or pharmaceutical compositions thereof to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
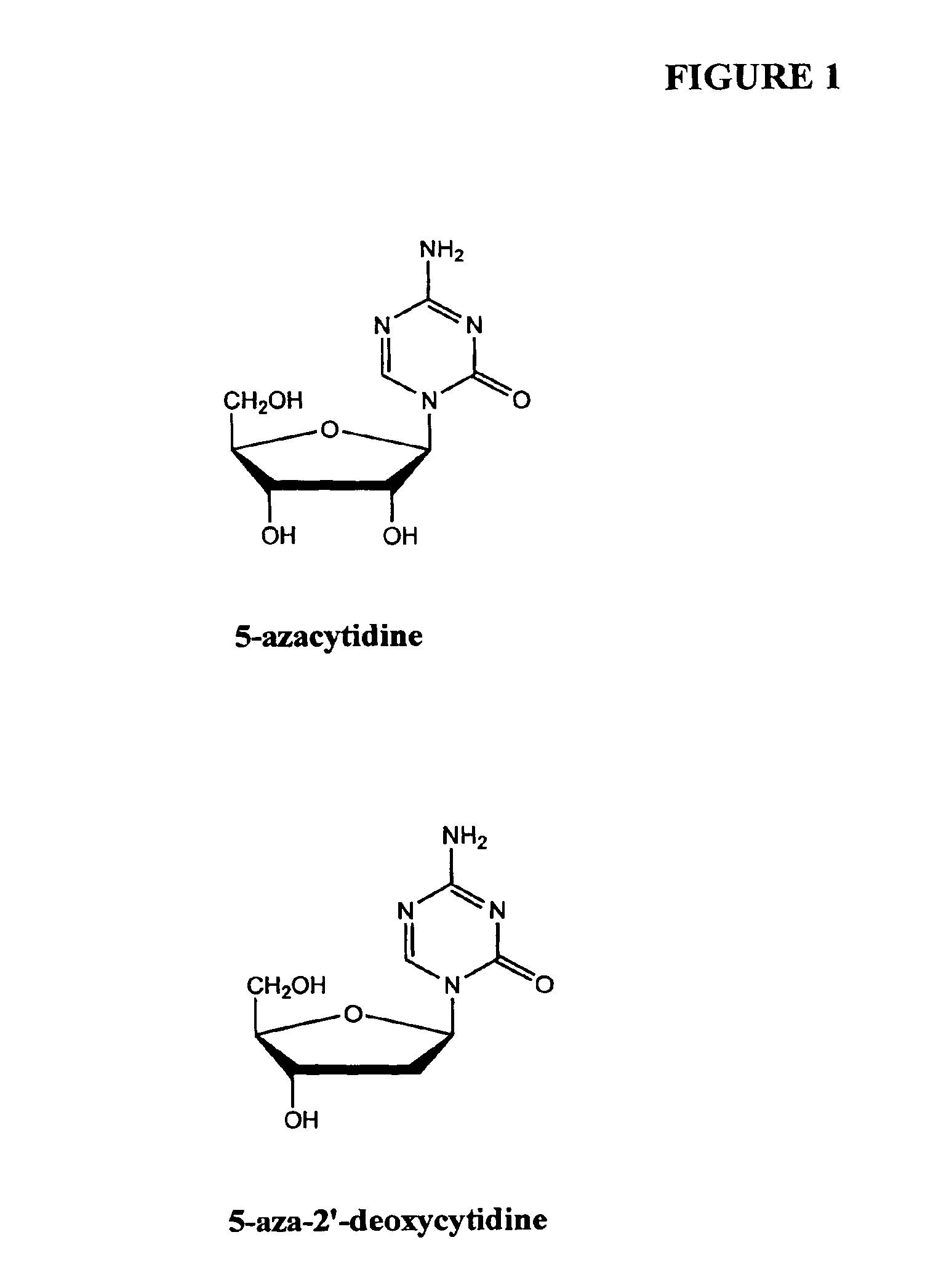
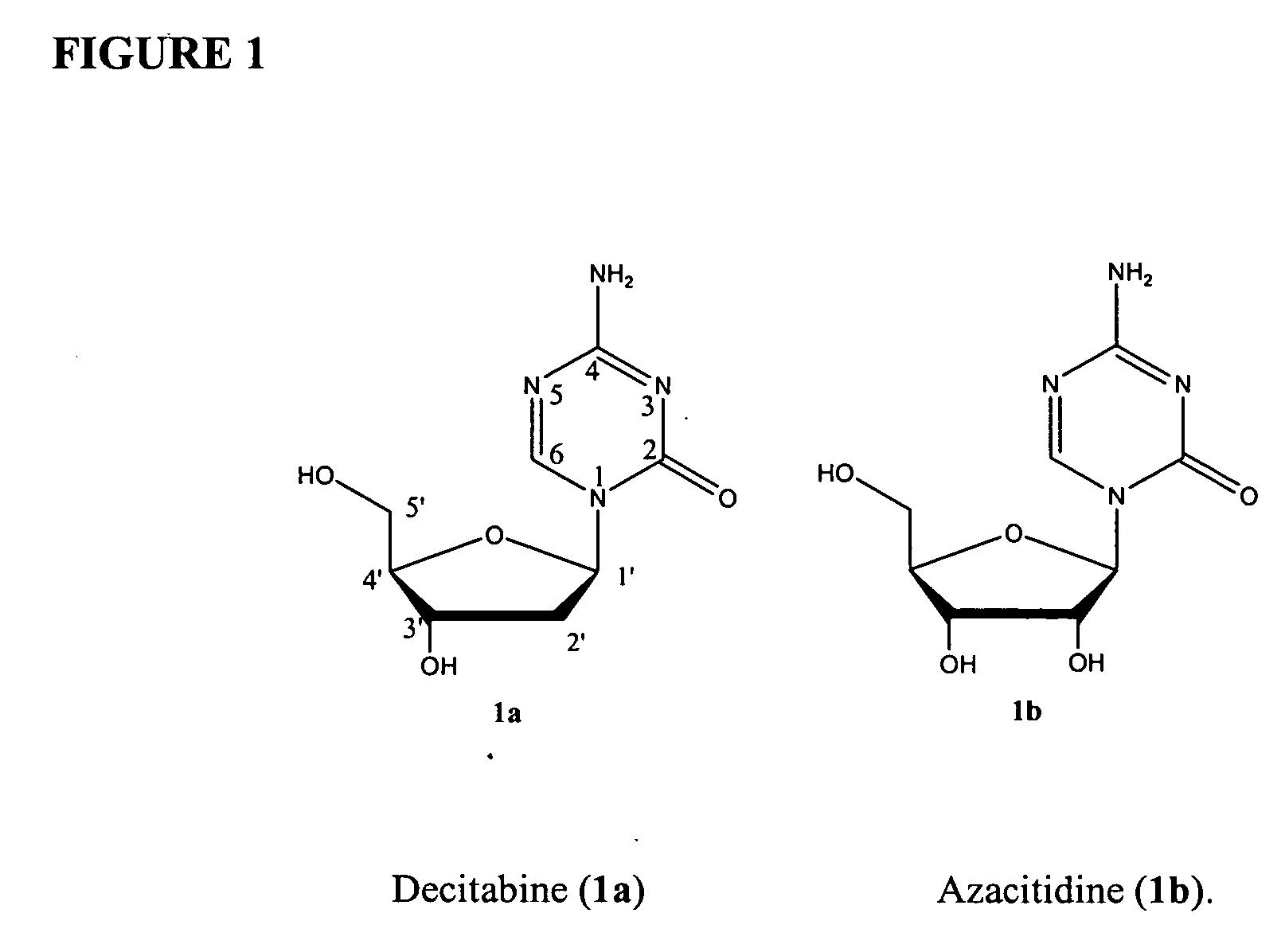
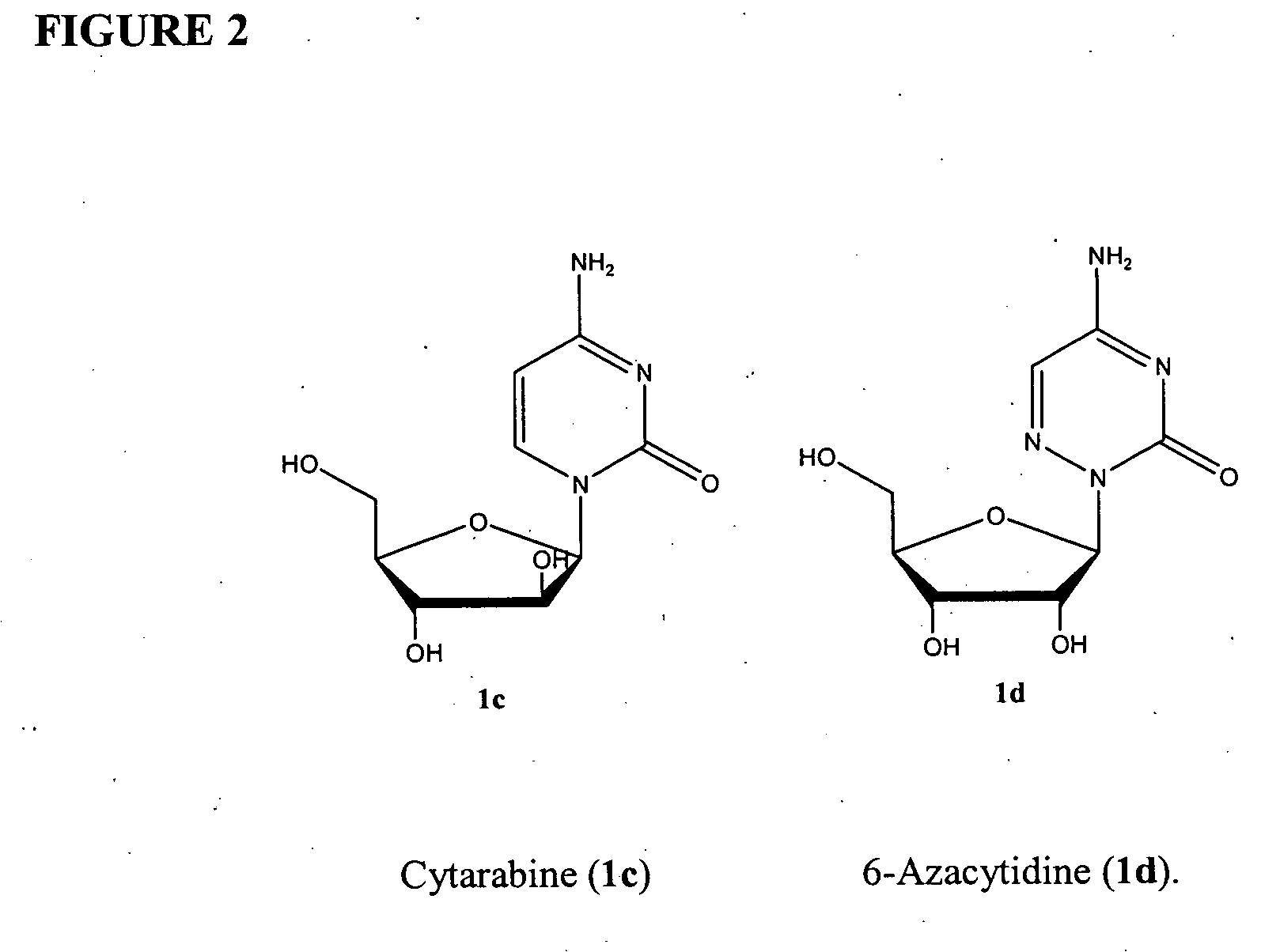
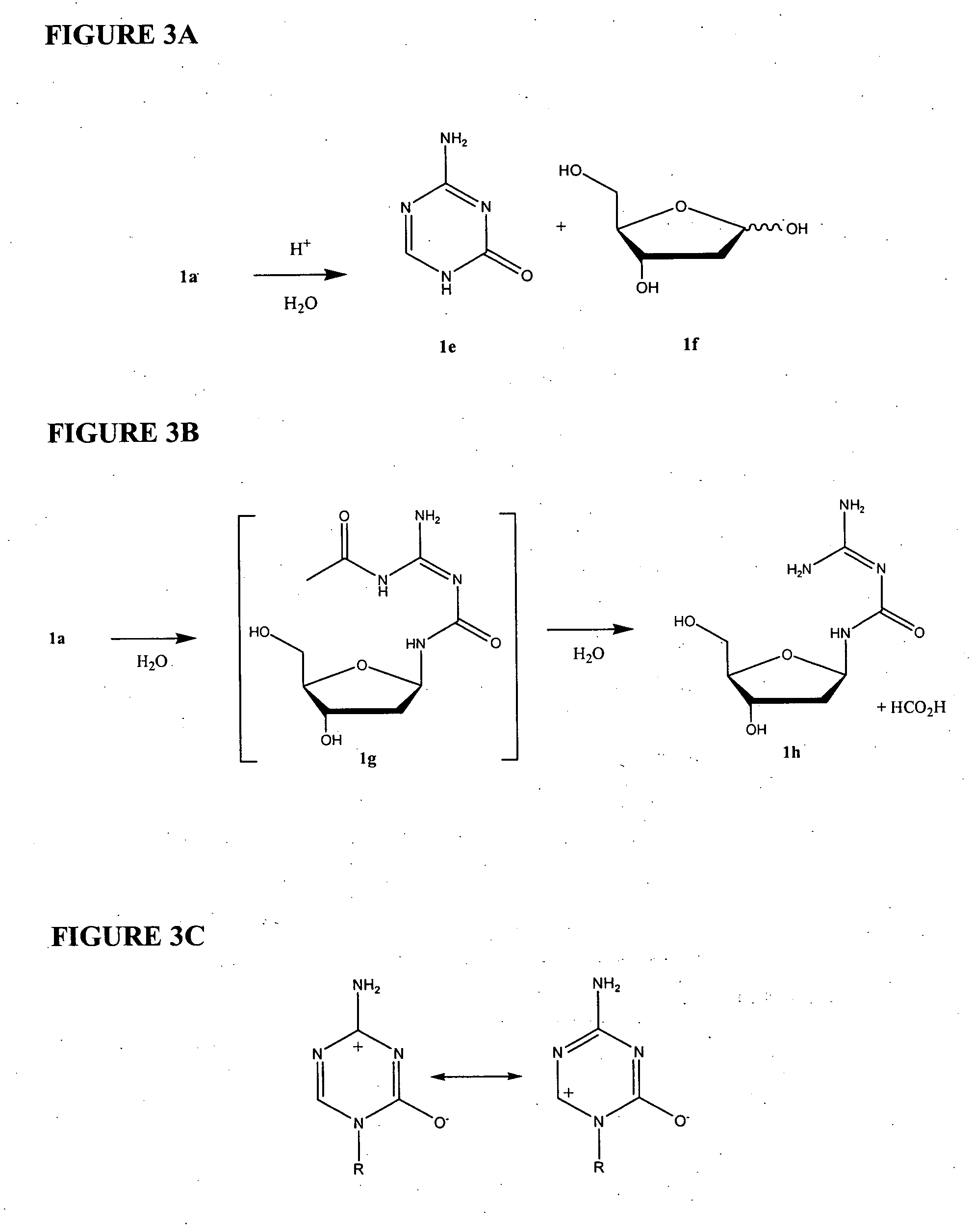
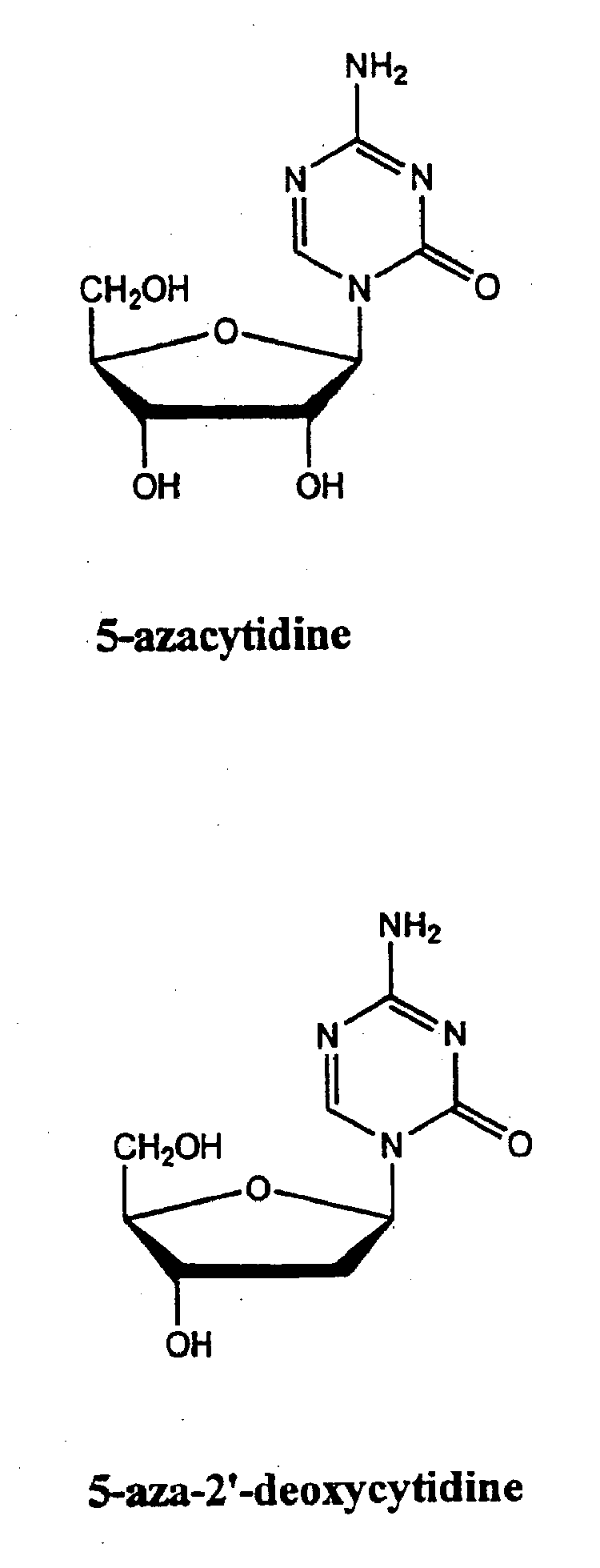
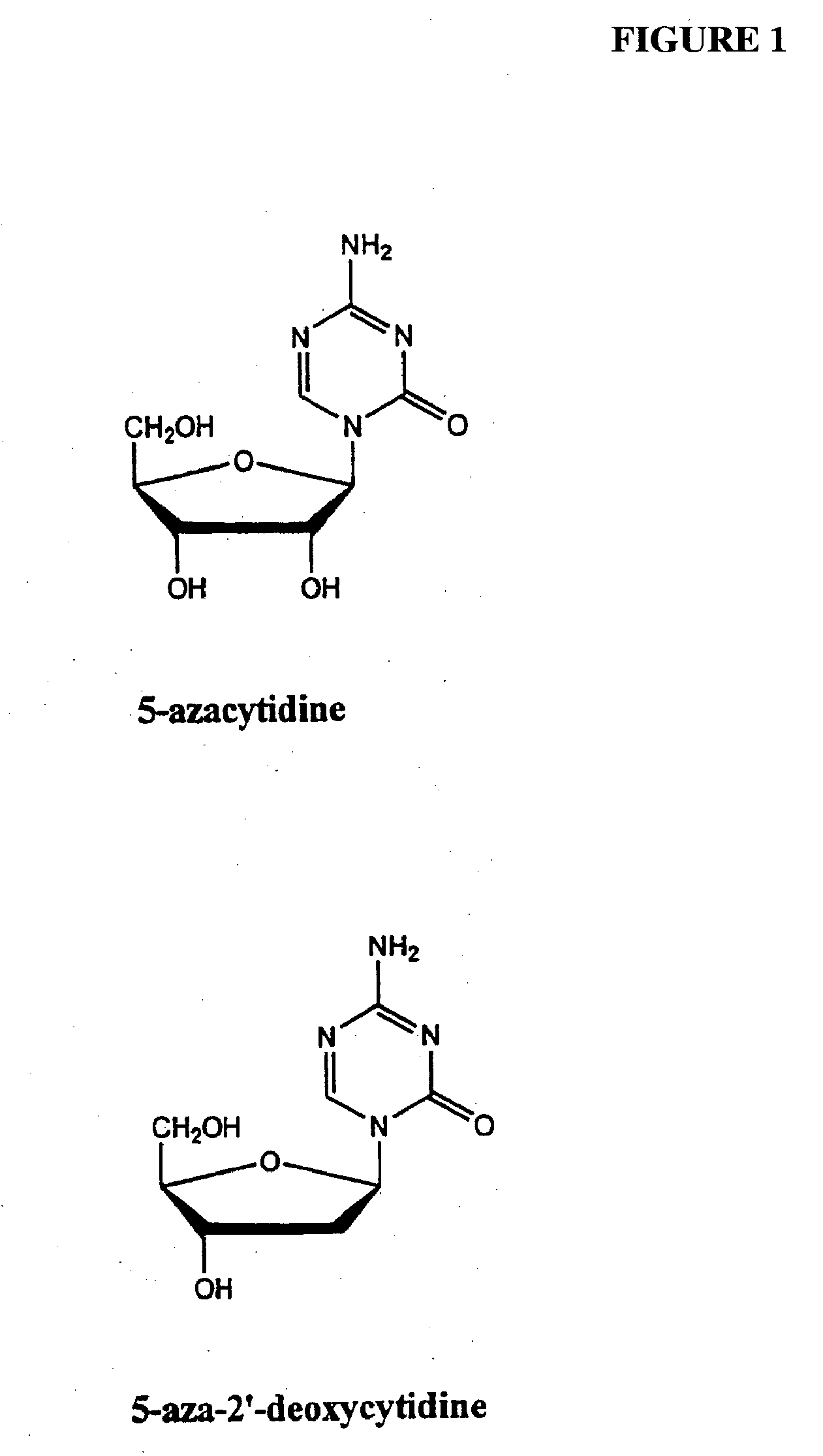
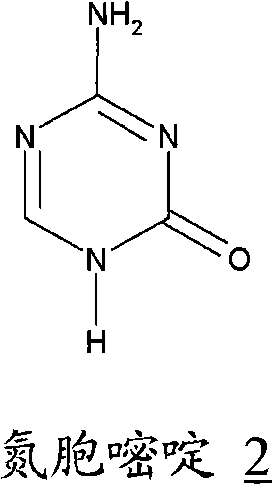
Azacytosine analogs and derivatives
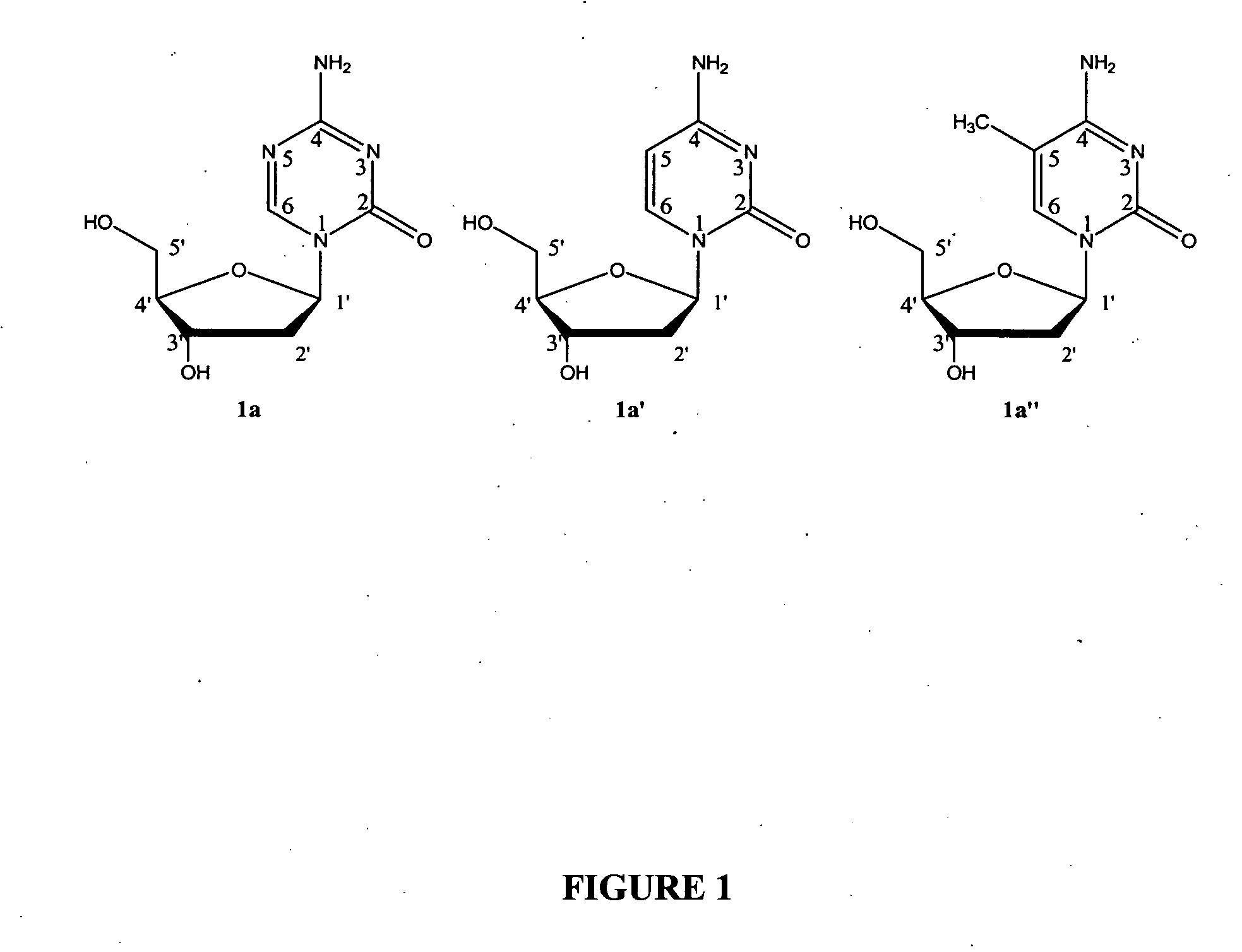
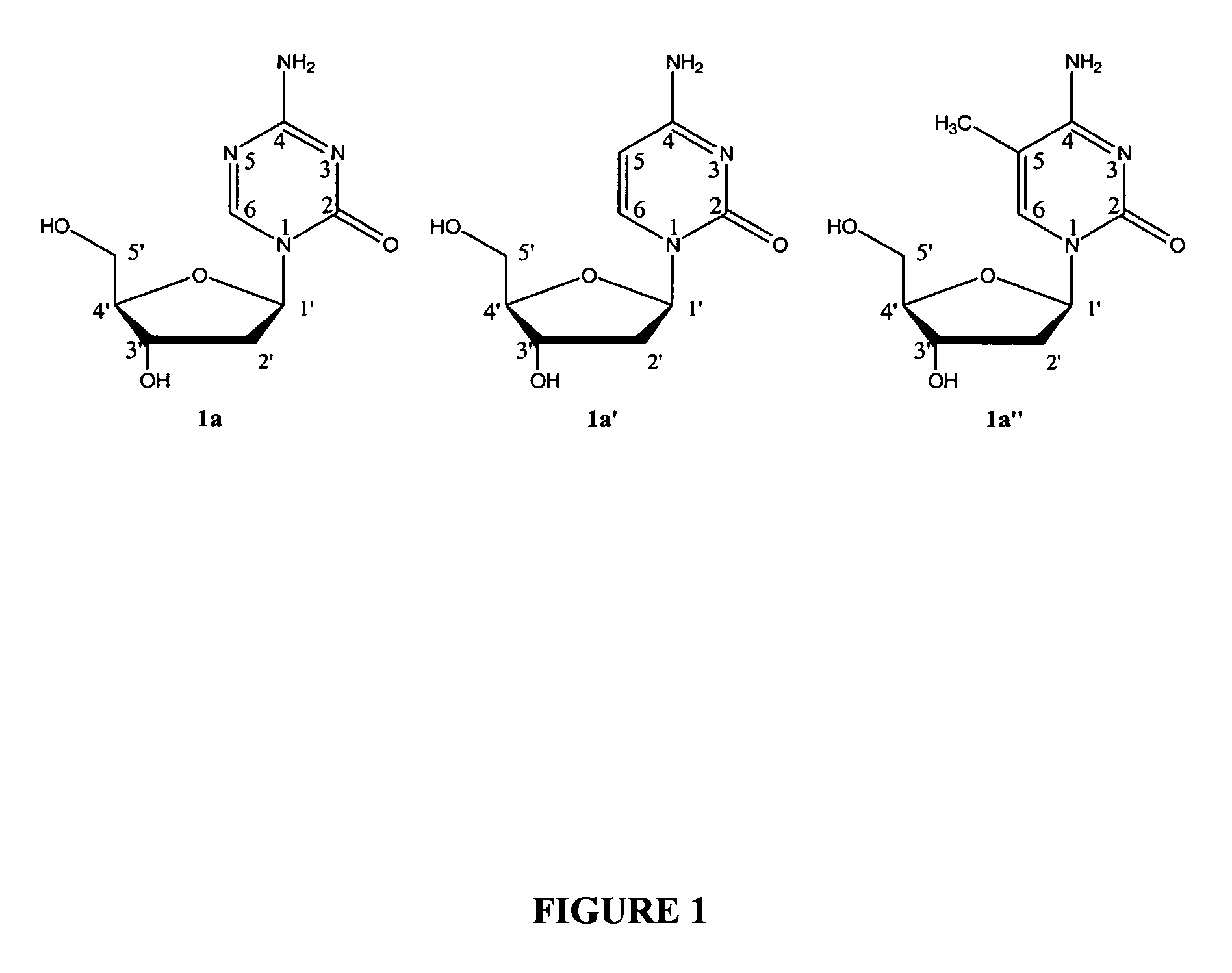
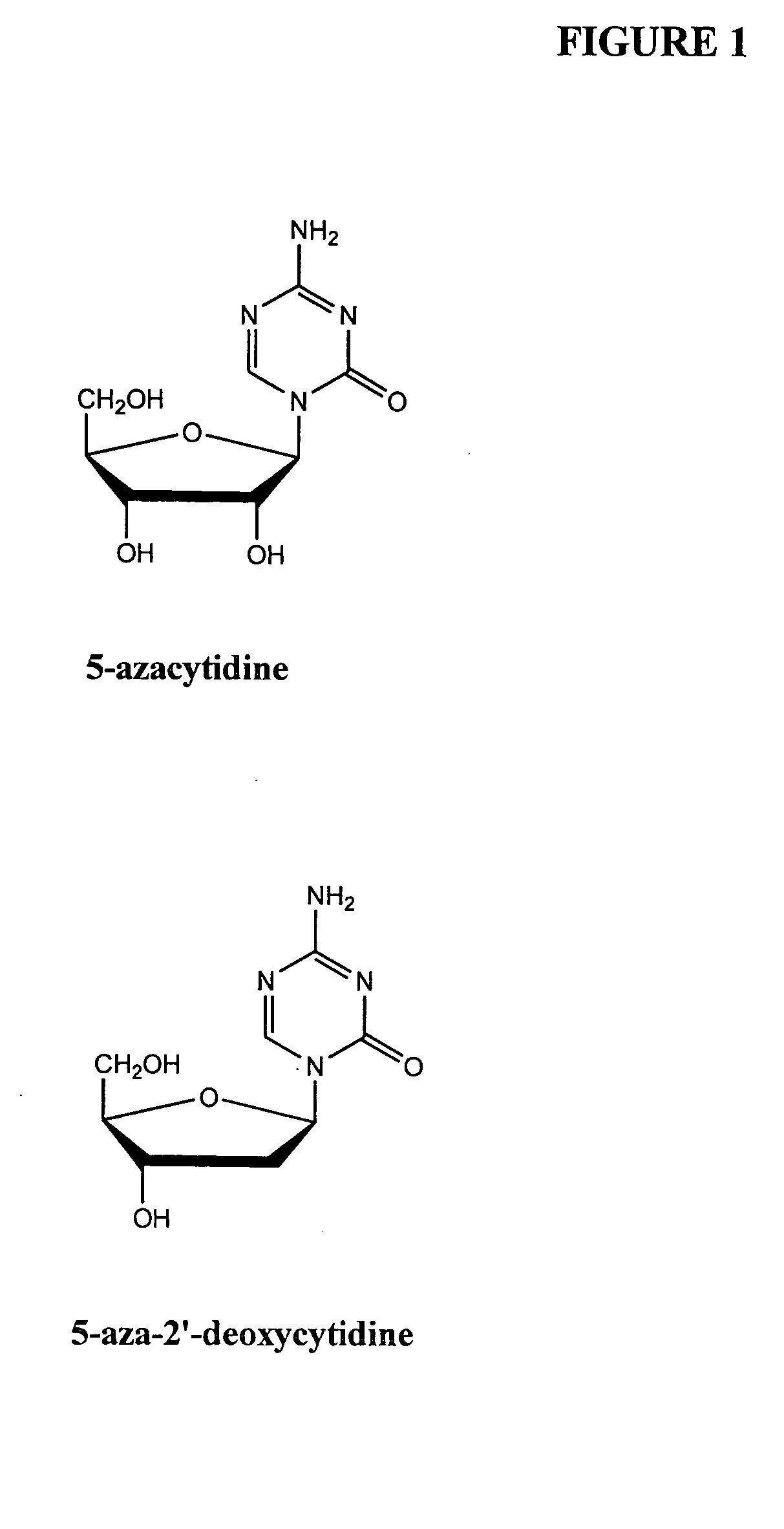
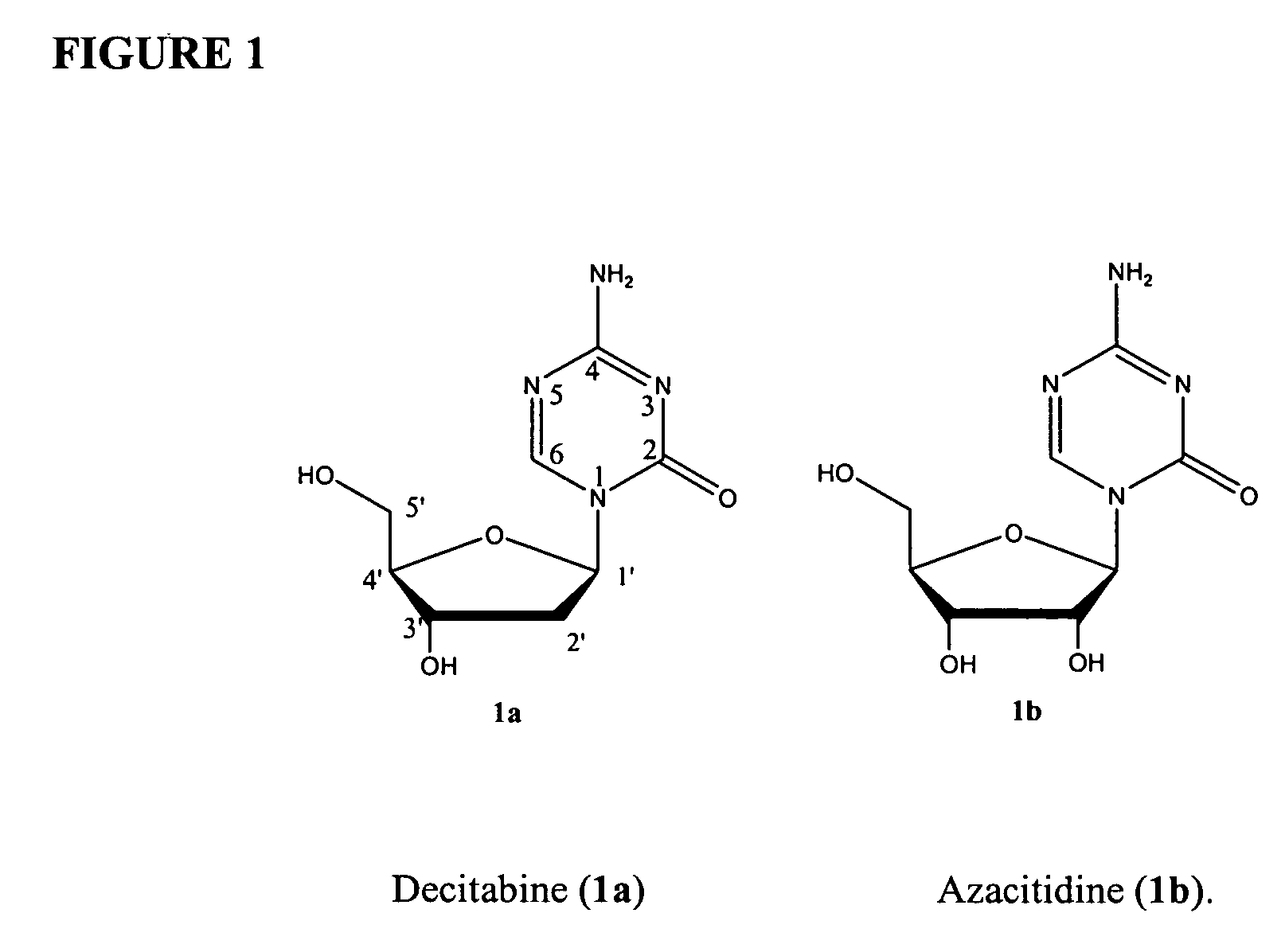
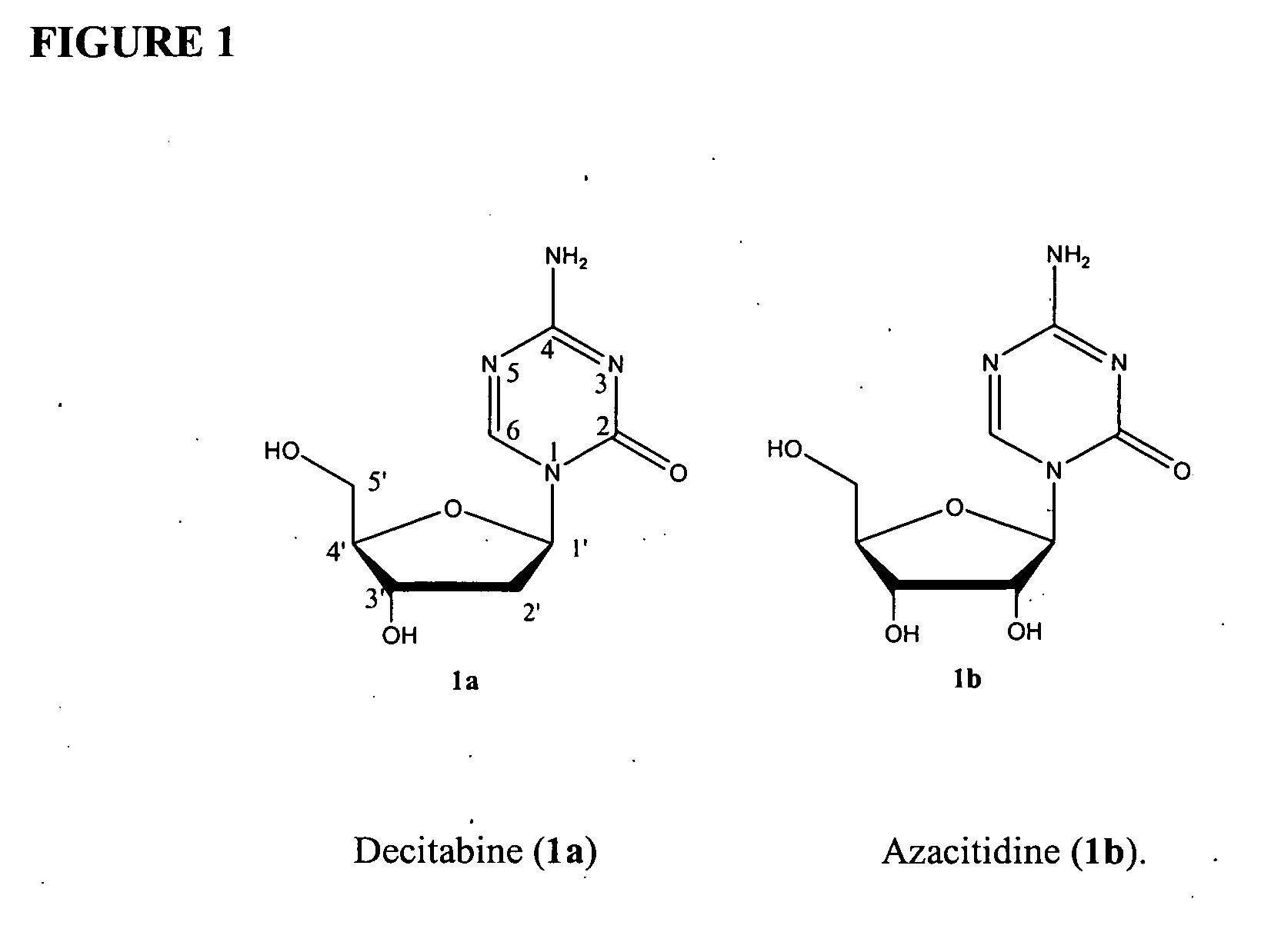
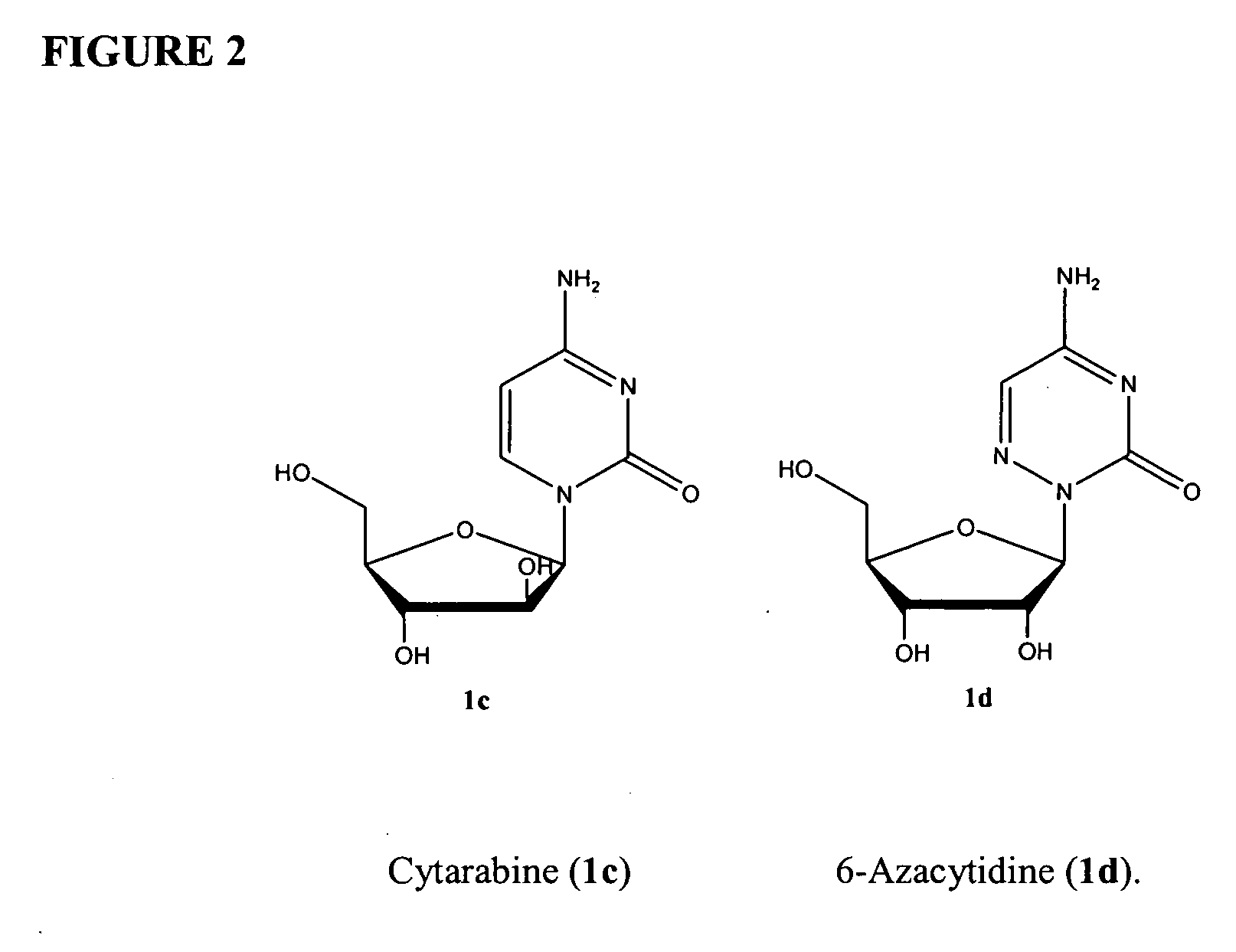
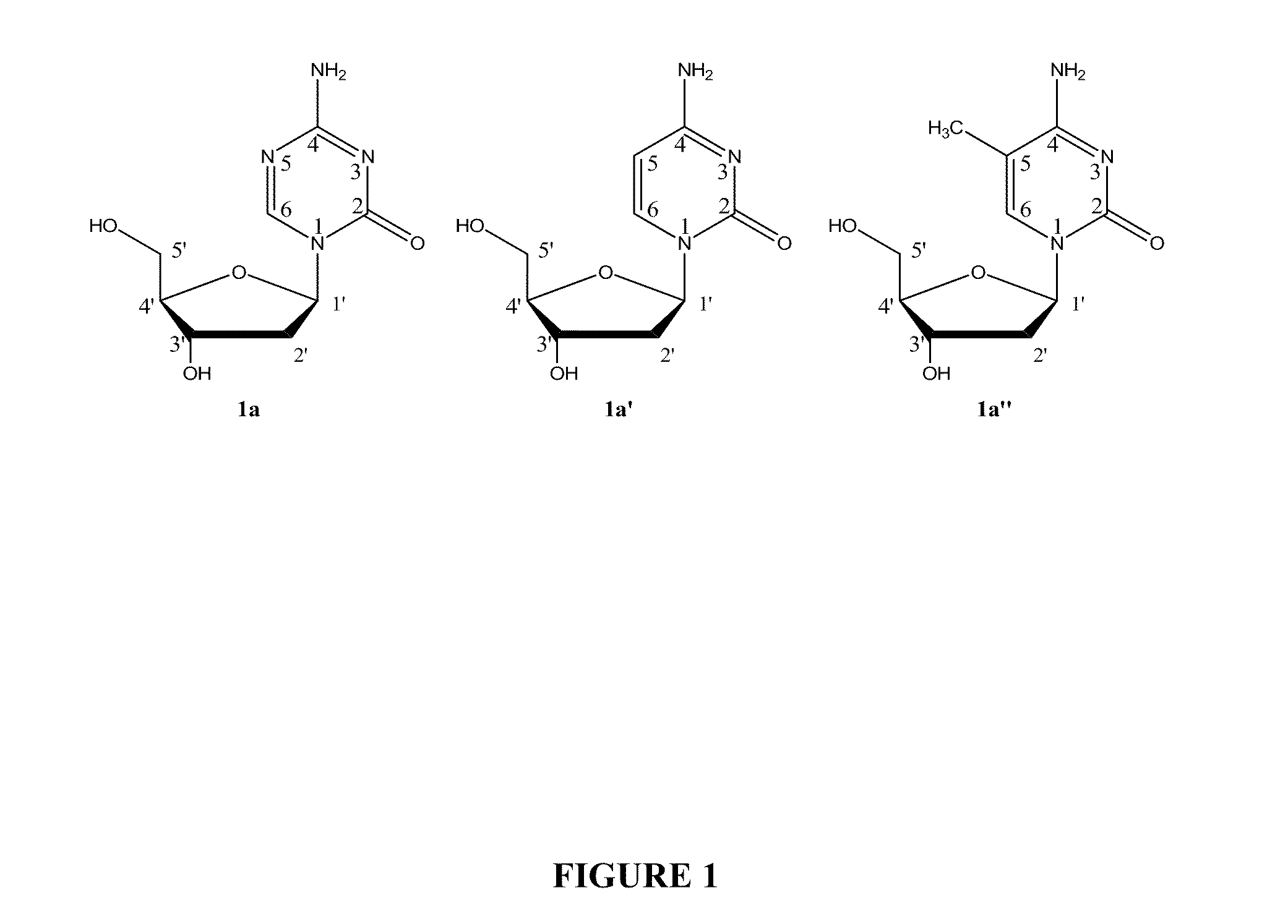
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
Composition and method for treating neurological disorders
InactiveUS20050037992A1Effective treatmentBetter clinical outcomeBiocideNervous disorderNervous systemFragile X chromosome
Compositions, kits and methods are provided for treating or preventing neurological disorders associated with aberrant silencing of gene expression by reestablishing the gene expression through inhibition of DNA methylation and / or histone deacetylase. The compositions and methods include administering to a patient suffering from the neurological disorder a therapeutically effective amount of a DNA methylation inhibitor, such as decitabine, preferably in combination with an effective amount of a histone deacetylase inhibitor. The compositions, kits and methods can be used to treat or present neurological disorders such as Lou Gehrig's disease, fragile X syndrome, Parkinson's disease and Alzheimer's disease.
Owner:SUPERGEN
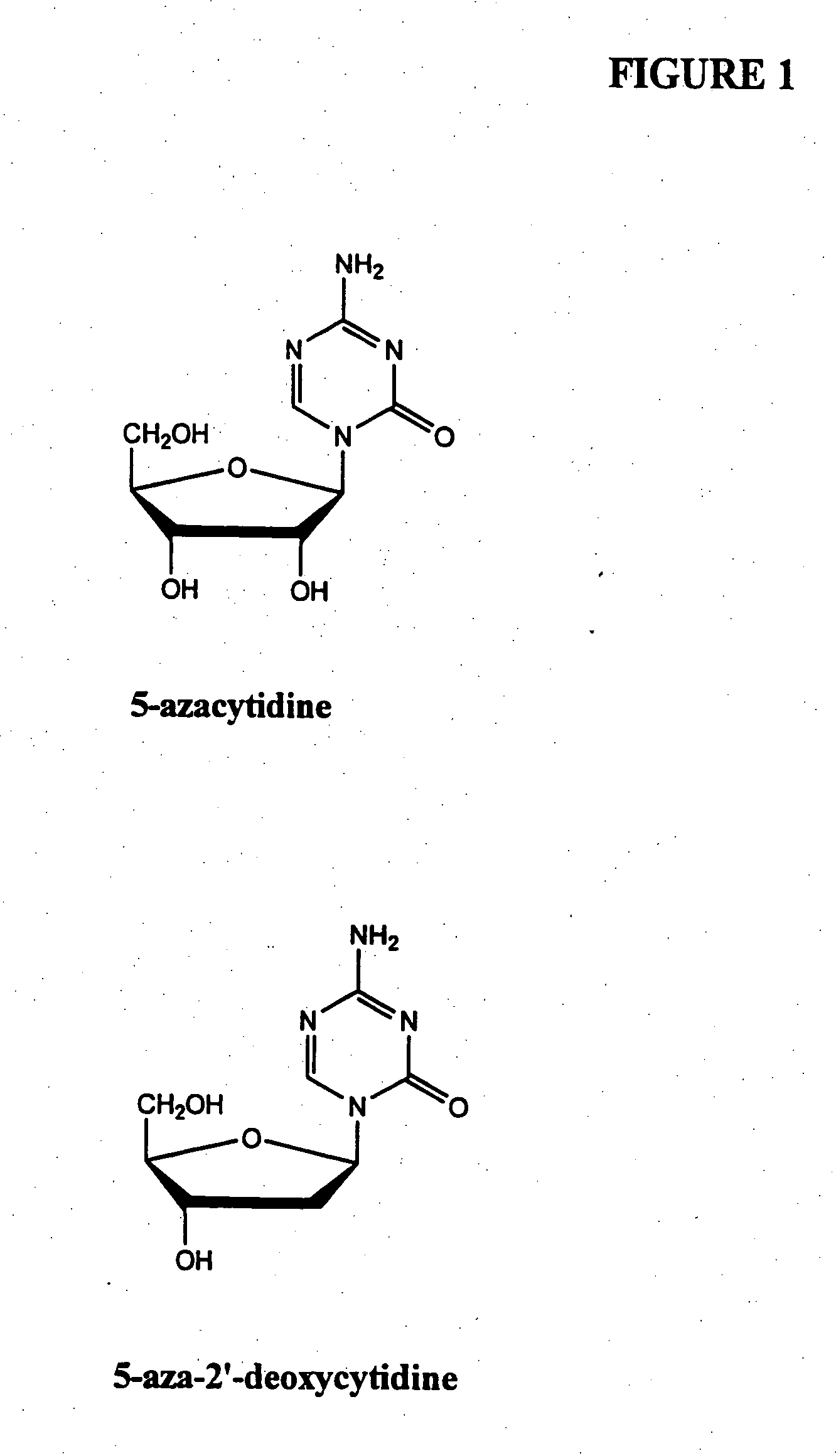
Oligonucleotide analogues incorporating 5-aza-cytosine therein
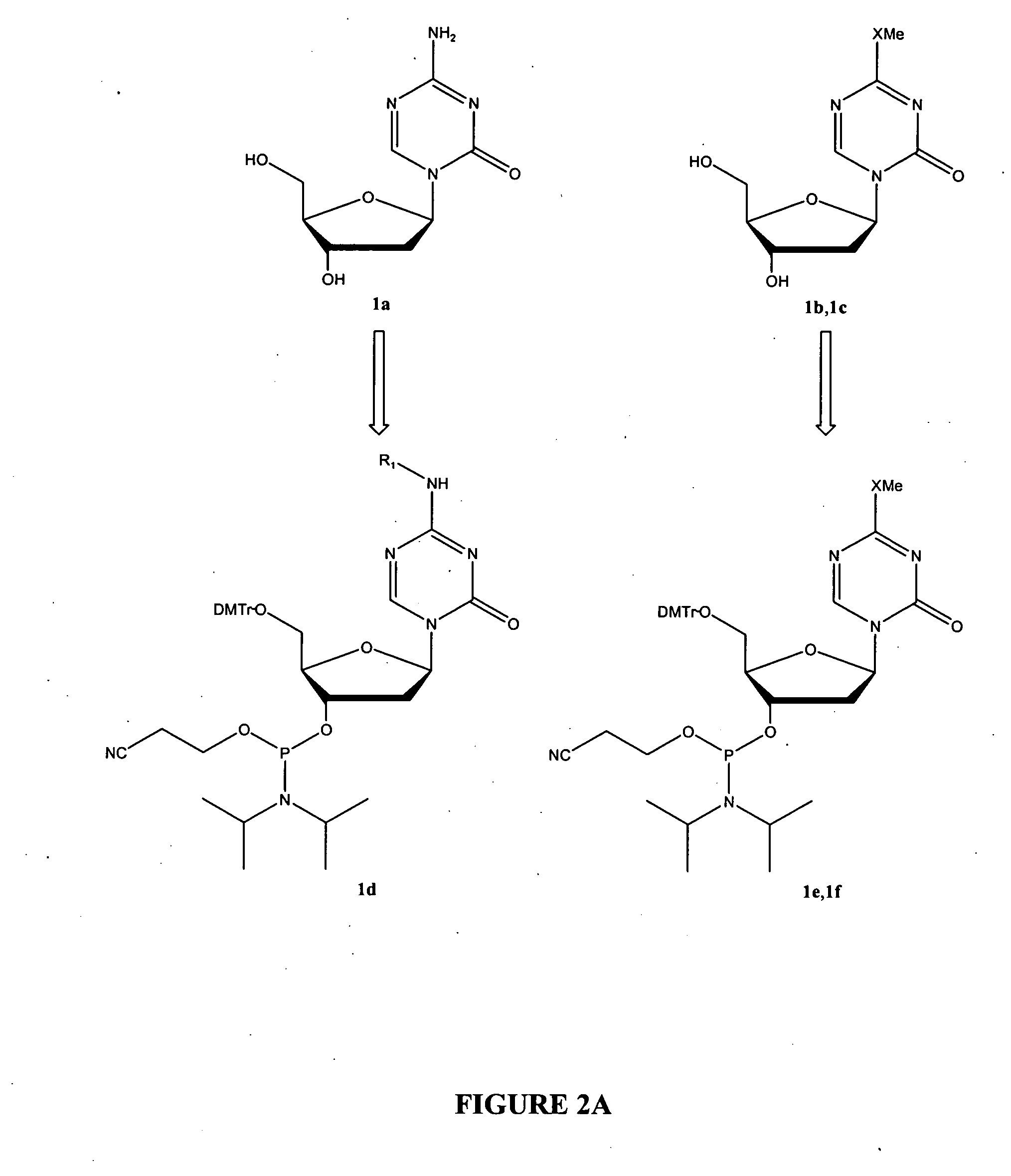
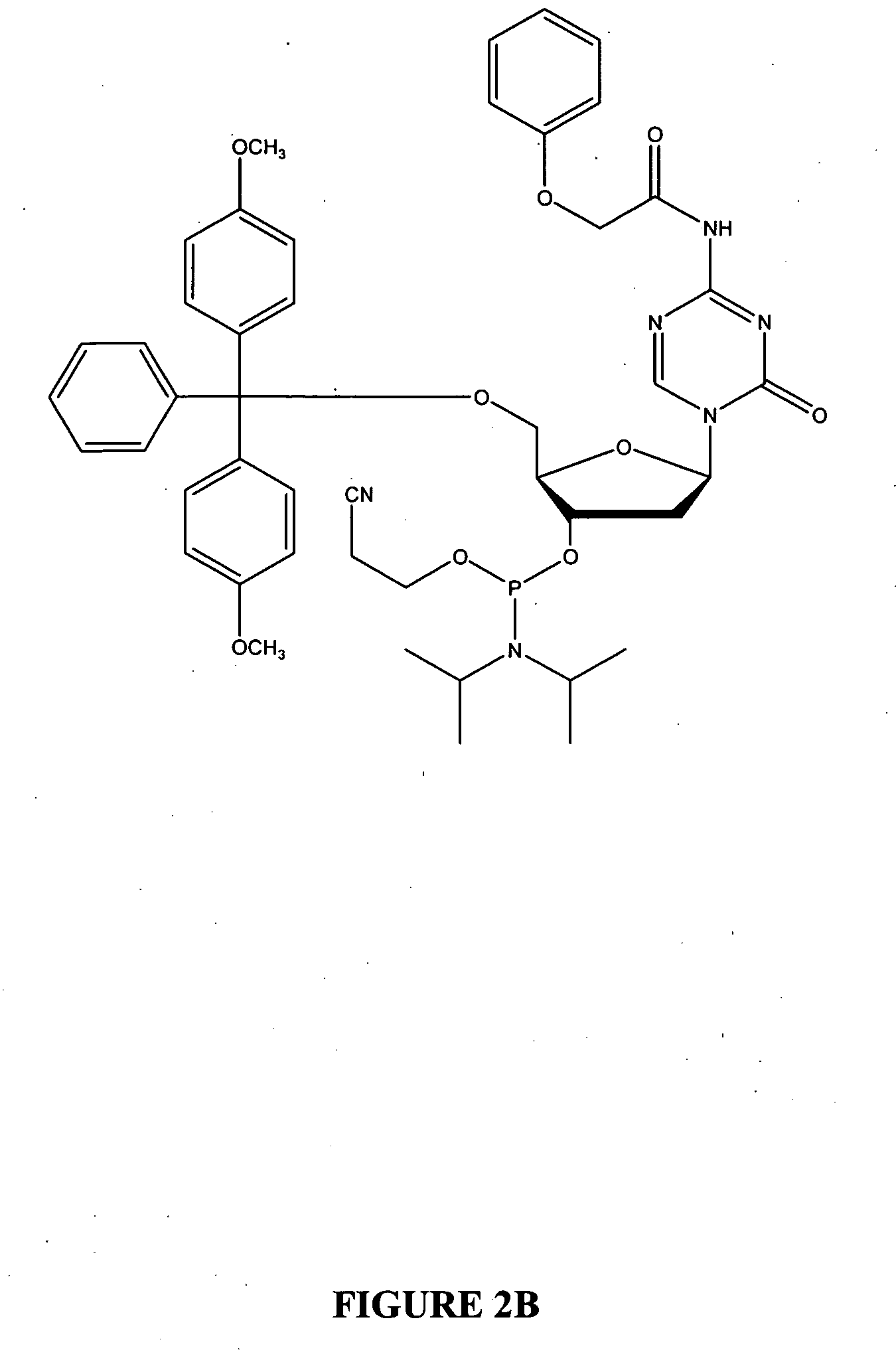
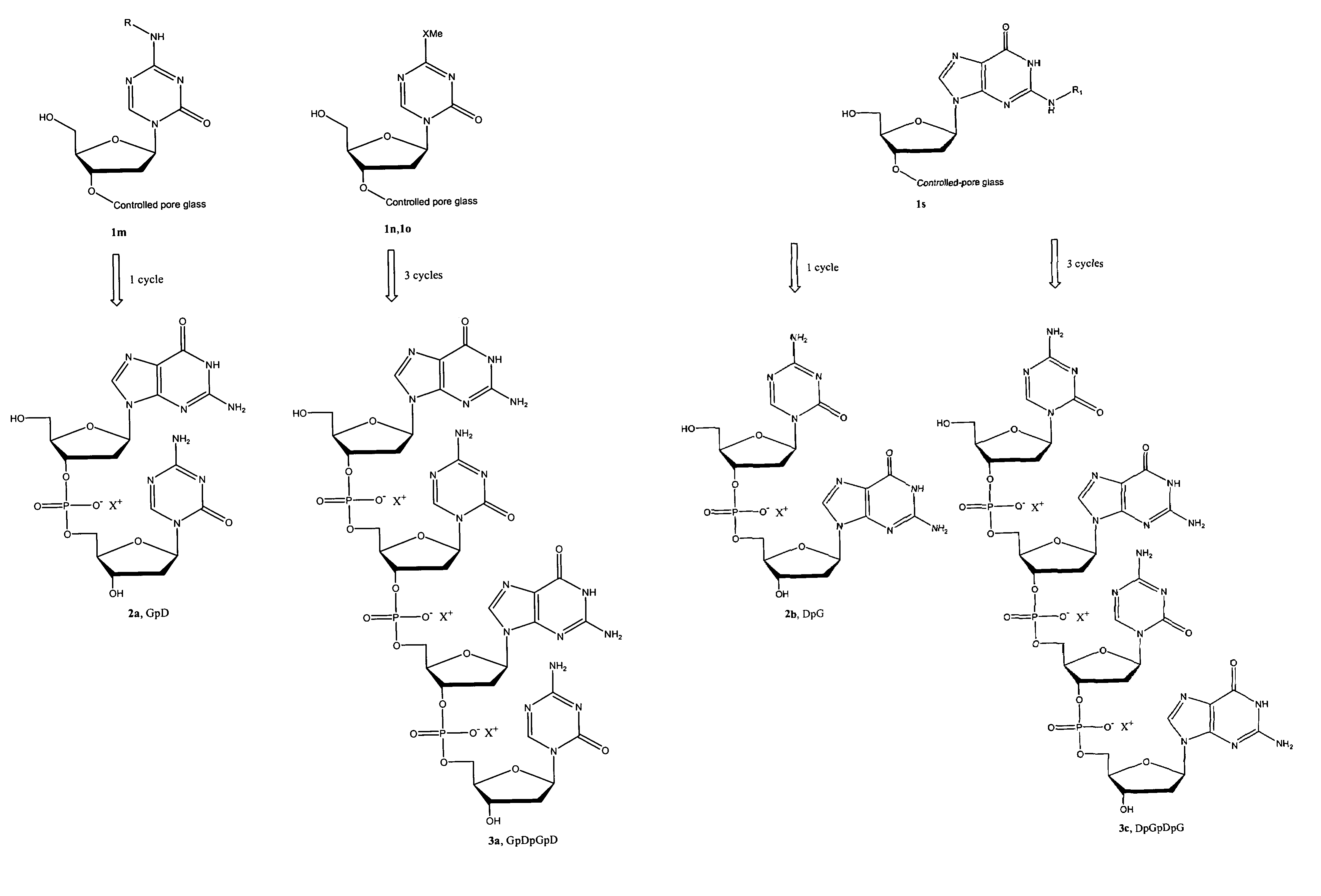
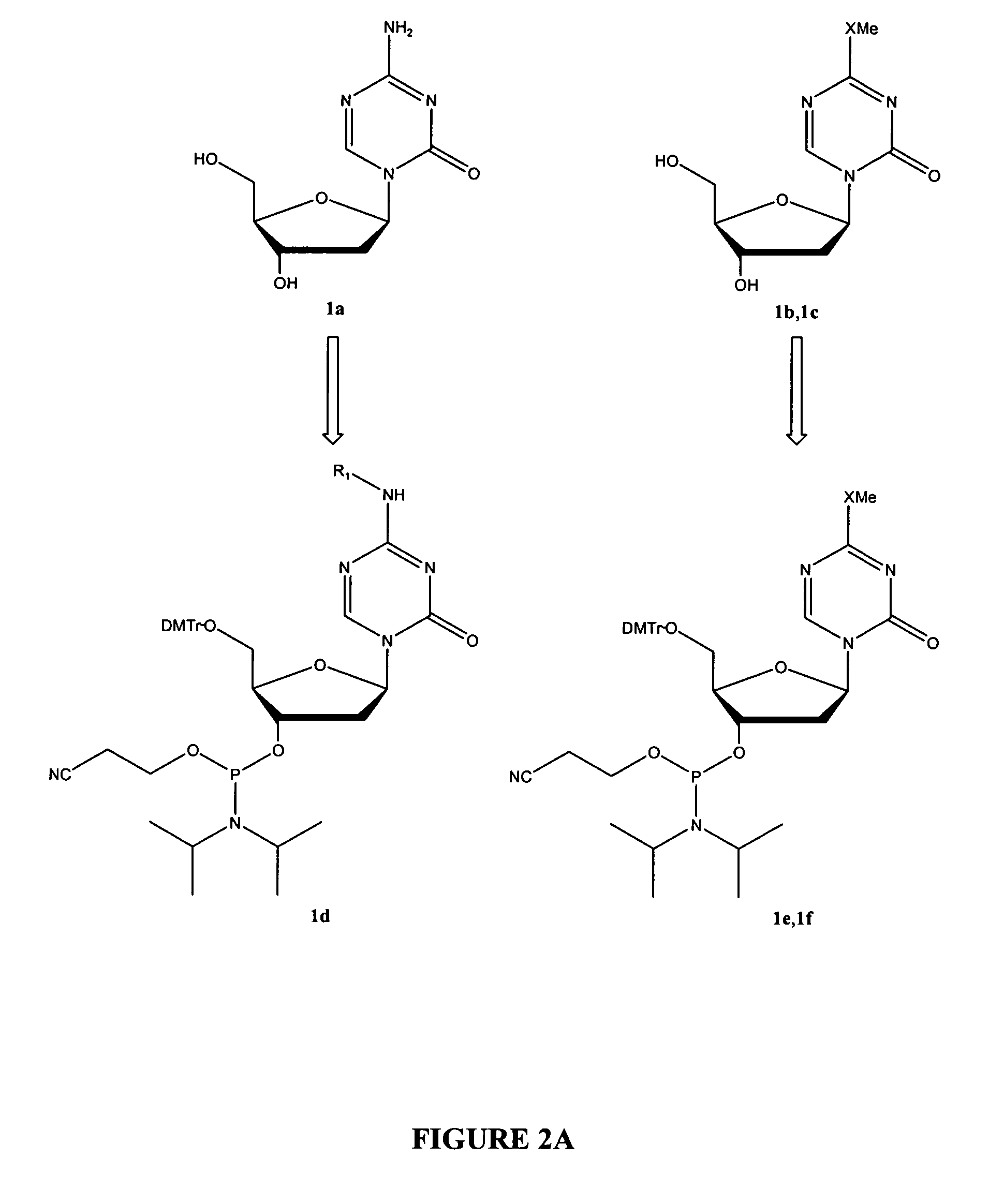
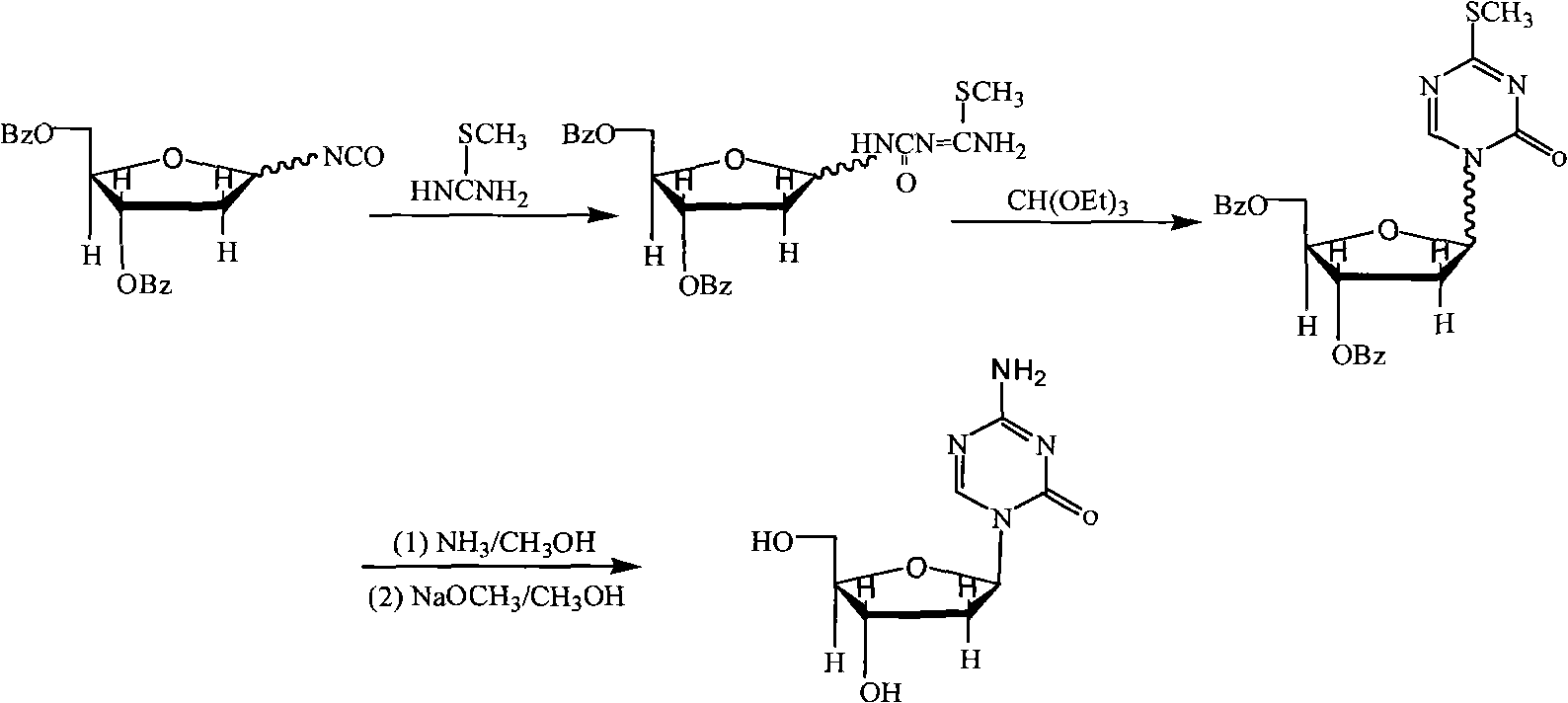
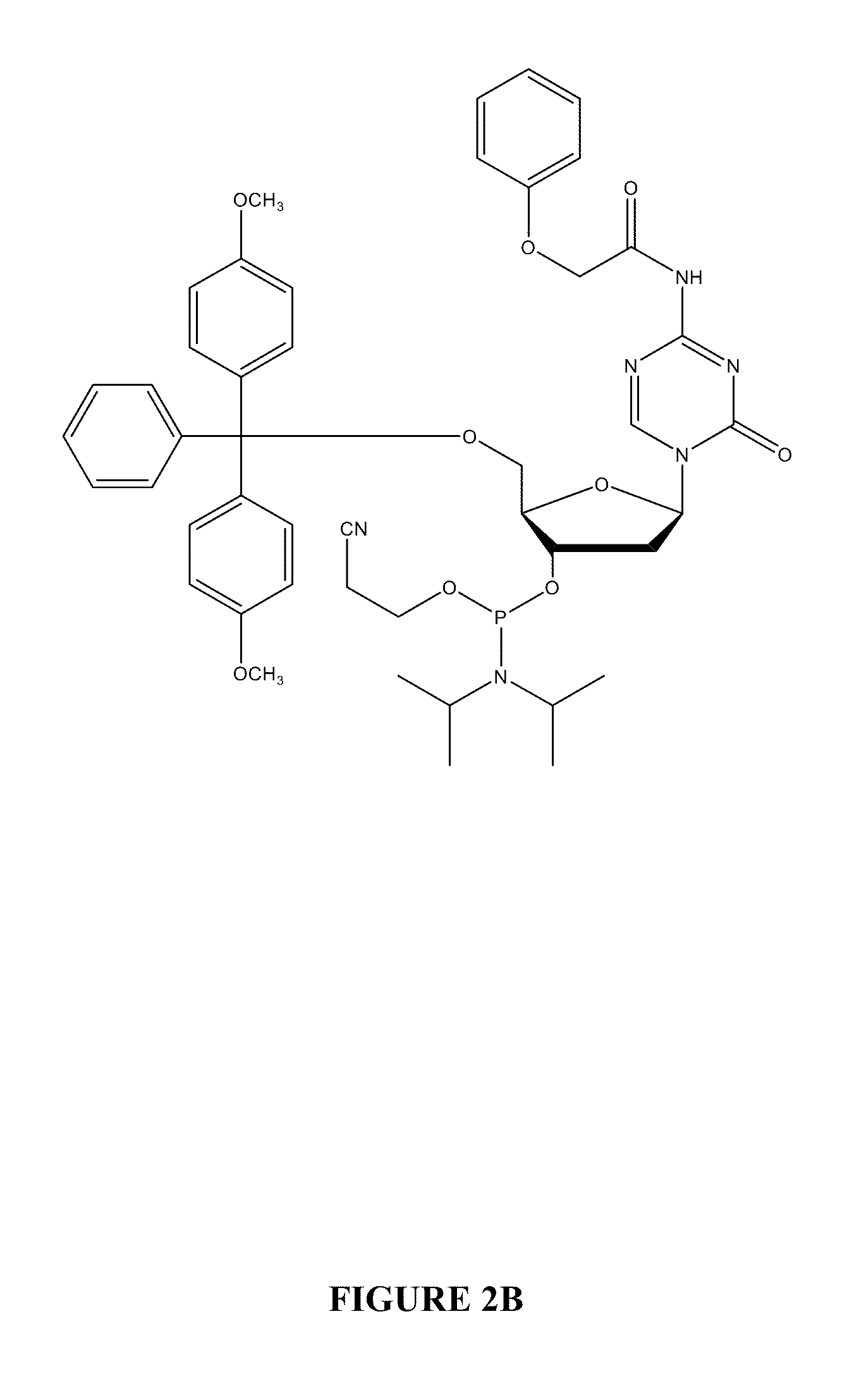
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Pharmaceutical formulation of decitabine
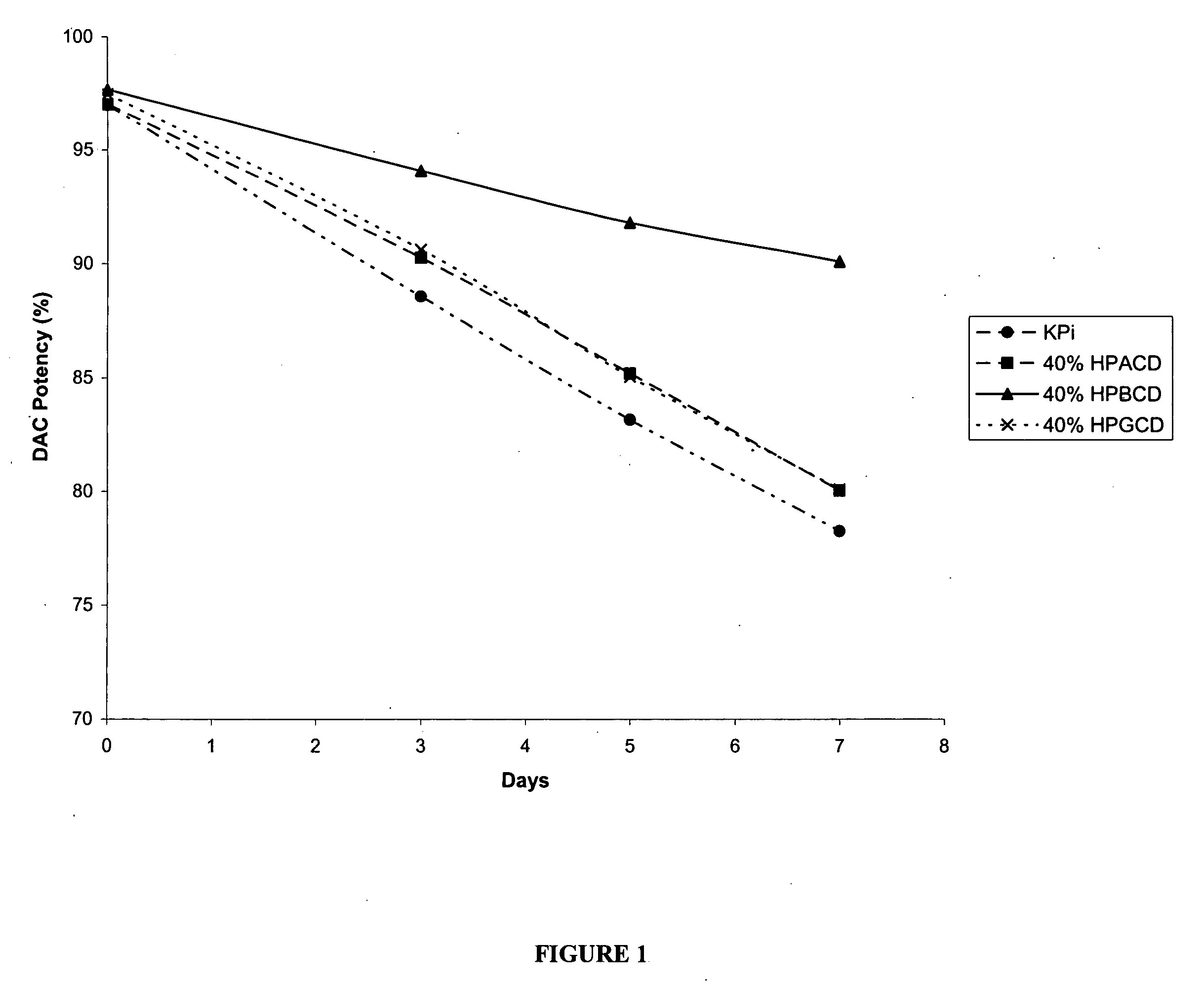
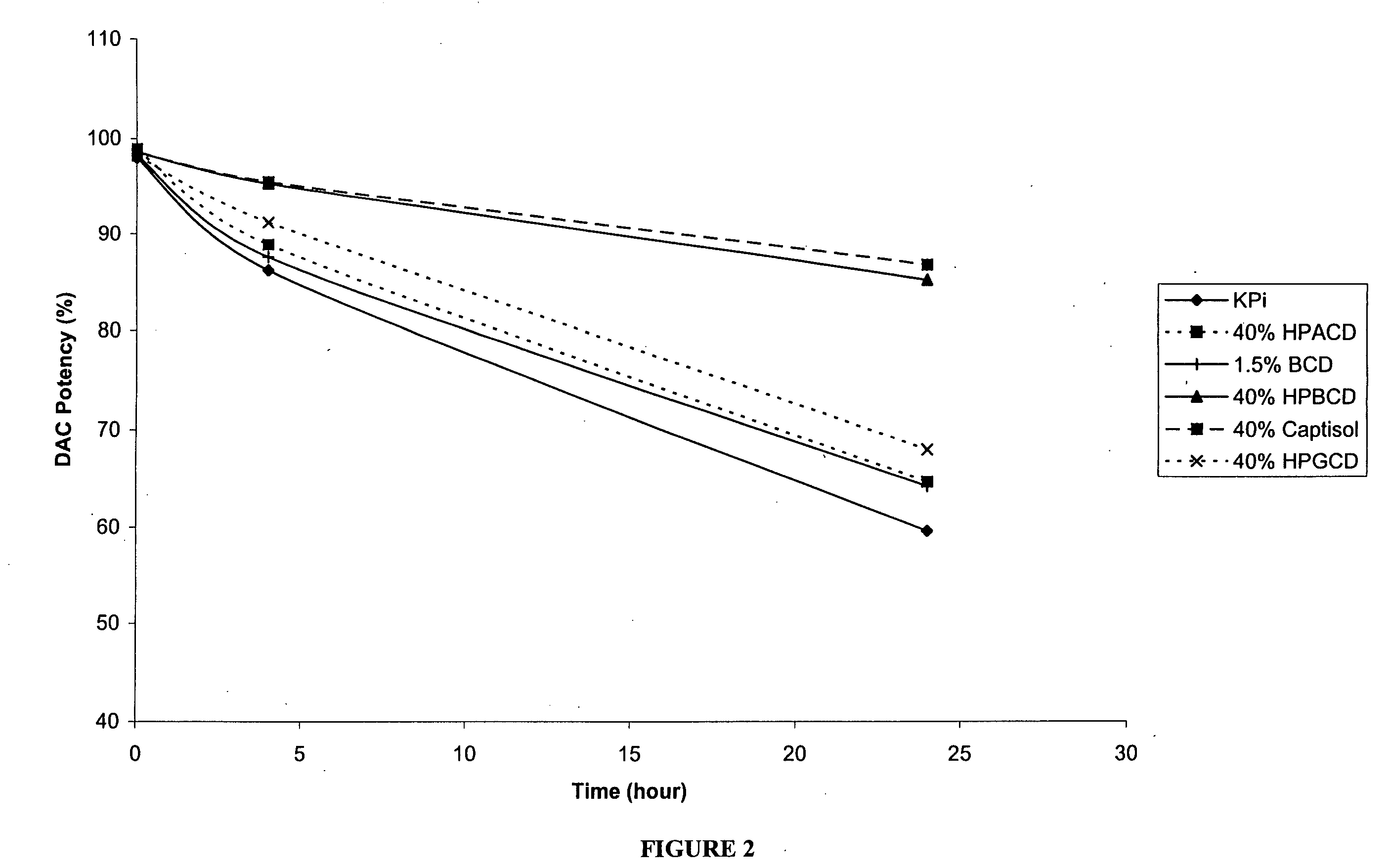
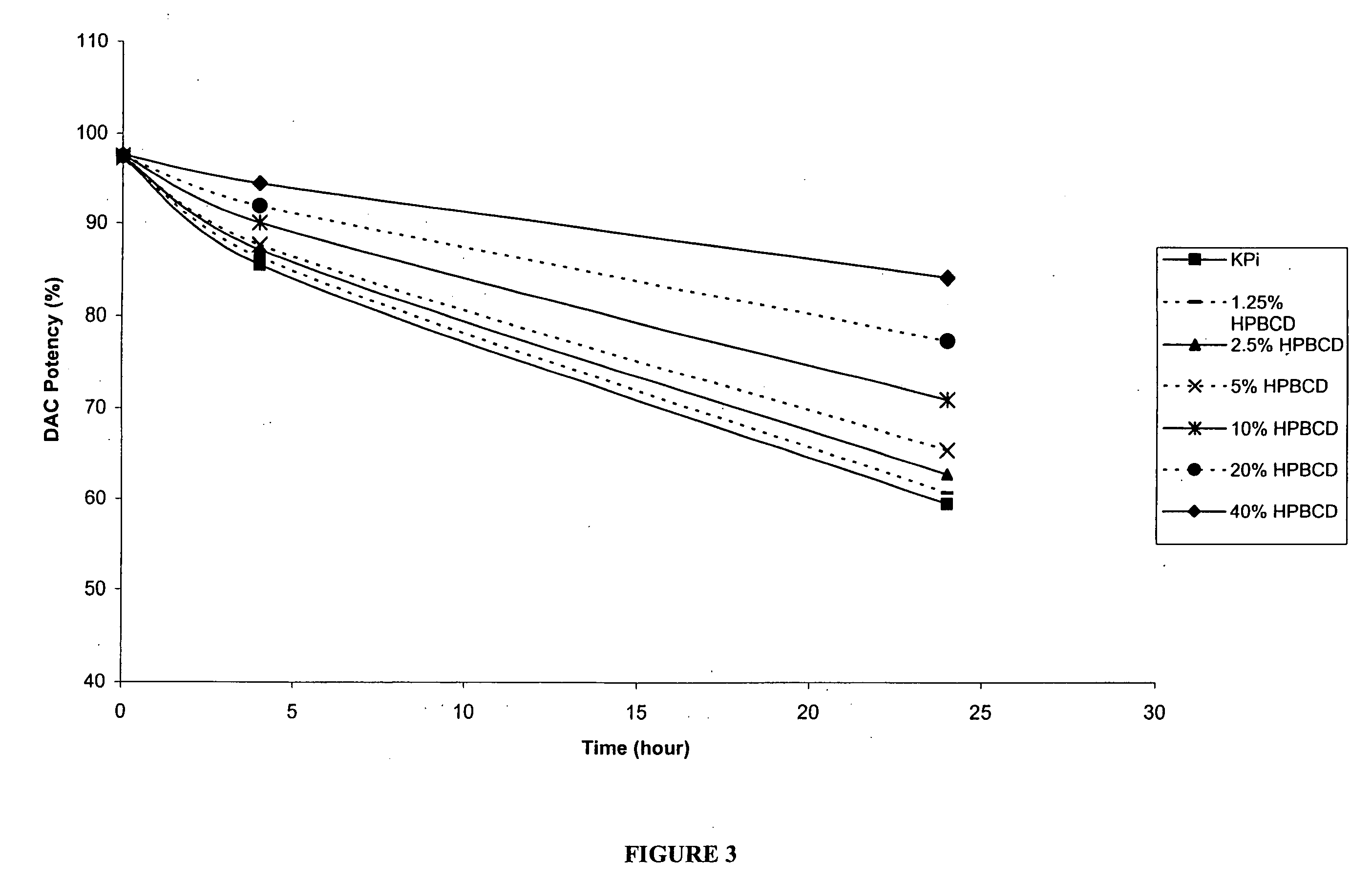
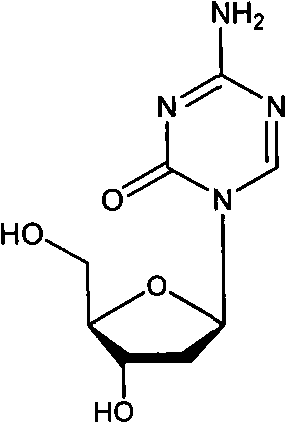
The present invention provides pharmaceutical formulations of decitabine or 5-aza-2′-deoxycytidine as well as methods of manufacturing the formulations. In particular, decitabine is formulated with a cyclodextrin compound to stabilize and / or enhance solubility of the drug. Kits and methods for using the pharmaceutical formulations are also provided, including methods of administering decitabine to treat conditions or diseases, such as cancer and hematological disorders.
Owner:SUPERGEN
Stable preparation method of decitabine freeze-dry preparation
InactiveCN101361718AAvoid degradationReduce the rate of degradationOrganic active ingredientsPowder deliveryOrganic solventFiltration
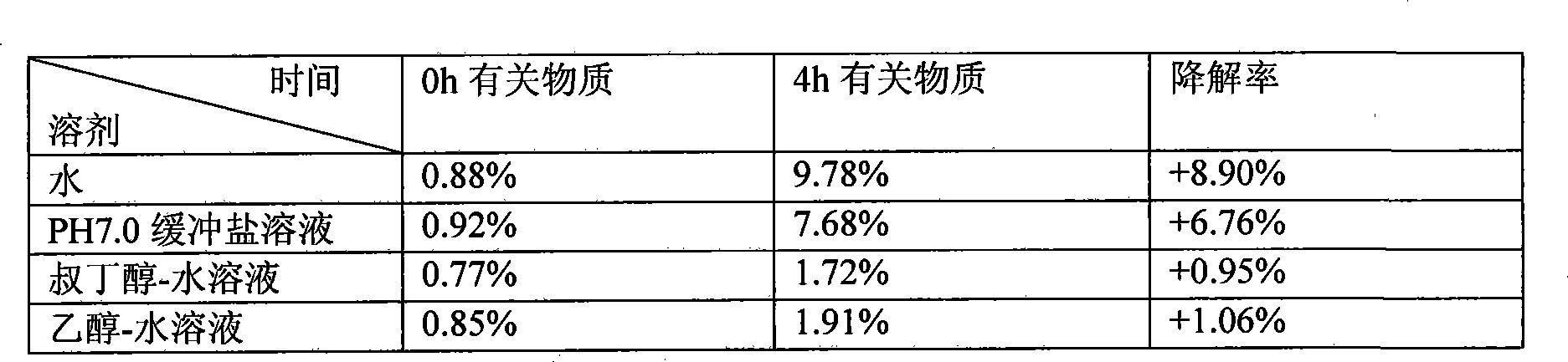
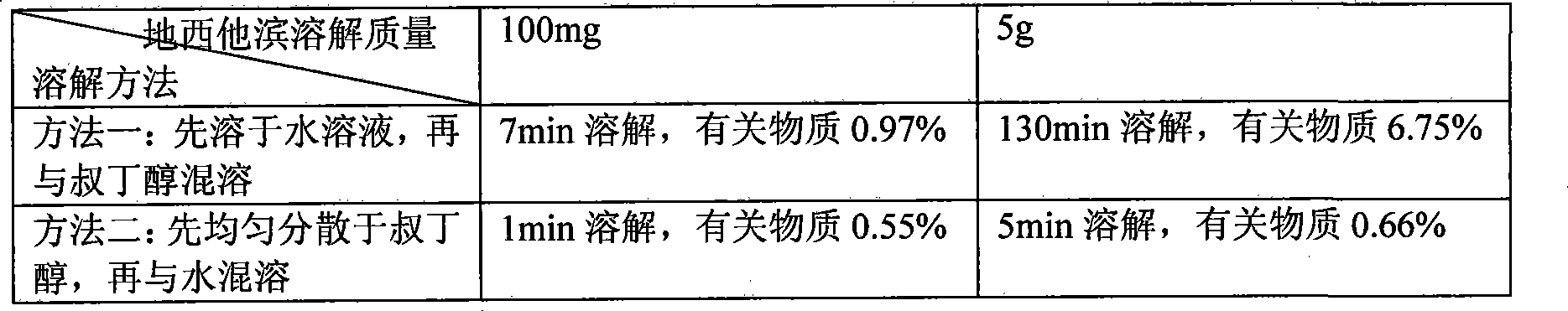
The invention discloses a method for preparing a stable Decitabine freeze-dried preparation, which comprises the following steps: Decitabine is uniformly dispersed in at least one of organic solvents including tertiary butyl alcohol, ethanol and methanol or dissolved in the organic solvent dimethyl sulfoxide; and then the mixed solution is mixed with water for injection or the water for injection with freeze-dried propping agents and / or pH regulators dissolved, thus obtaining a new mixed solution and the organic solvent accounting for 5-80 percent of the mixed solution in volume; and organic solution is removed after filtration and freeze drying. The method can prepare a stable Decitabine freeze-dried preparation, in which the residual quantity of organic solvent is not more than 1 percent.
Owner:深圳万乐药业有限公司
Oligonucleotide analogues incorporating 5-aza-cytosine therein
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Methods for treating hematological disorders through inhibition of DNA methylation and histone deacetylase
InactiveUS20050159347A1Address bad outcomesReduce dosageAntibacterial agentsBiocideCyclic peptideHydroxamic acid
Methods are provided for treating hematological disorders by inhibition of DNA hypomethylation and histone deacetylase. Such disorders include, for example, acute promyelocytic leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, myelodysplastic syndromes, and sickle cell anemia. The methods comprise: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
Azacytosine analogs and derivatives
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′–6′ position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
Azacytosine analogs and derivatives
InactiveUS20060205687A1Decrease electrophilicityBiocideSugar derivativesAbnormal tissue growthPyrimidine analogue
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 2-, 4-, or 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of using, synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease associated with aberrant DNA methylation, or a disease or condition that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders, tumors and cancers.
Owner:SUPERGEN
Decitabine freeze-dried powder injection
ActiveCN101584670AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryPhosphateFreeze-drying
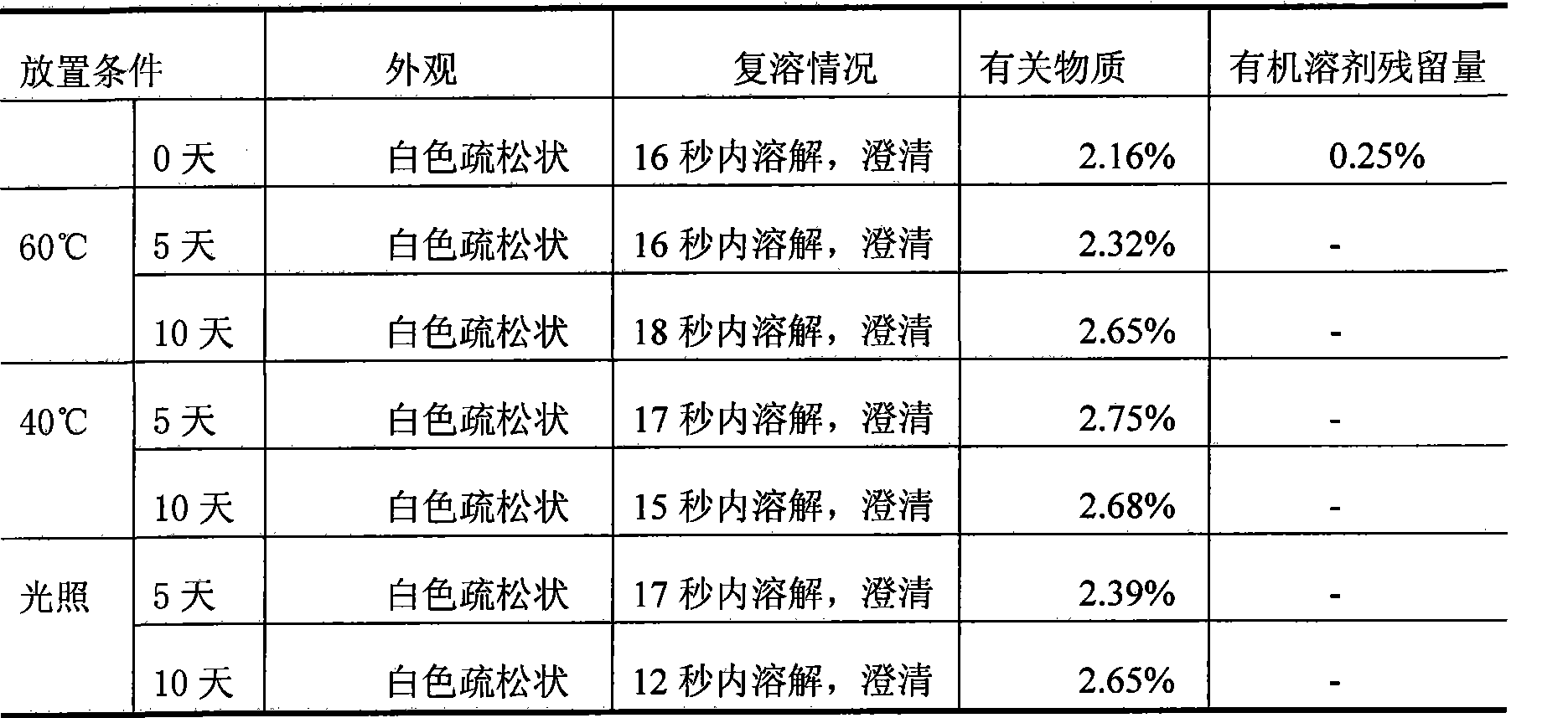
The invention relates to a decitabine freeze-dried powder injection and a preparing method thereof. The prepared decitabine freeze-dried powder injection is used for treating myelodysplastic syndrome (MDS). The decitabine freeze-dried powder injection contains decitabine, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the decitabine in the mixed solvents is 2.5-5 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The potassium dihydrogen phosphate and the sodium hydroxide may be added for the pH regulator. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, potassium dihydrogen phosphate and sodium hydroxide, stirring and mixing evenly, cooling to 2-15 DEG C, heat preserving, adding decitabine, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
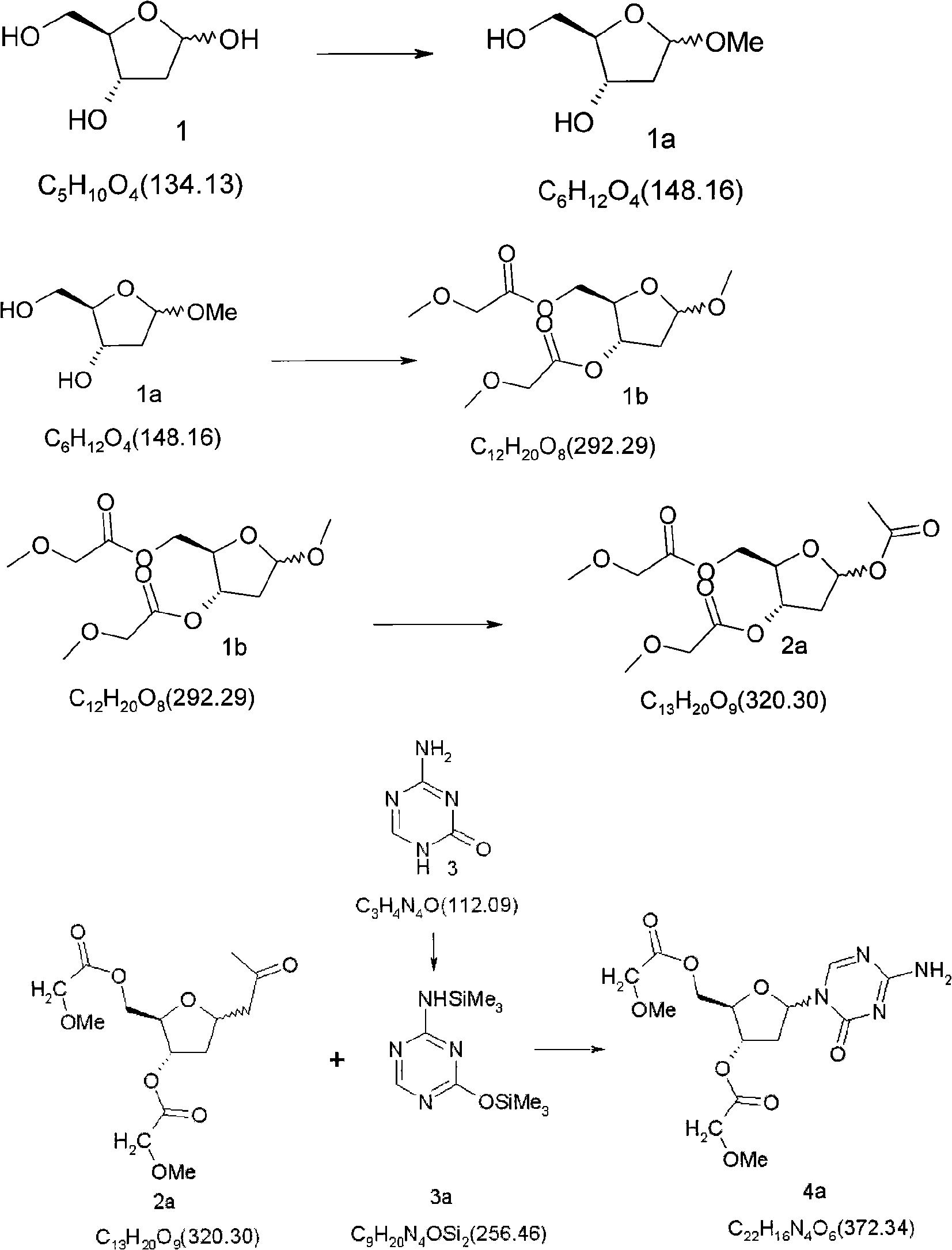
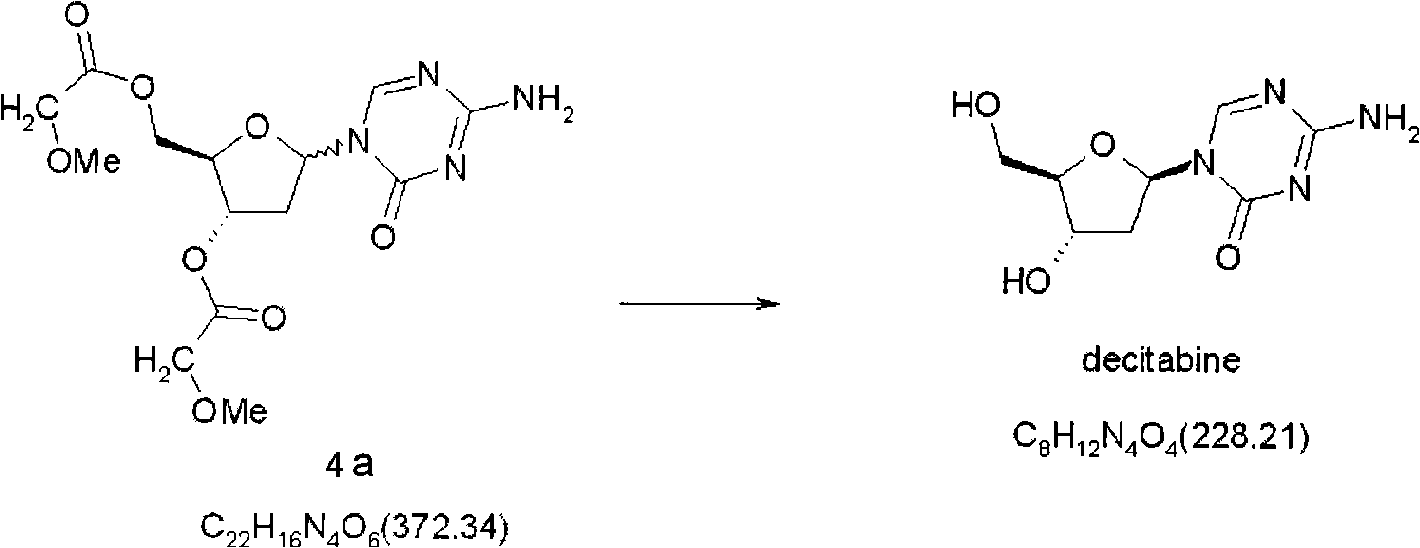
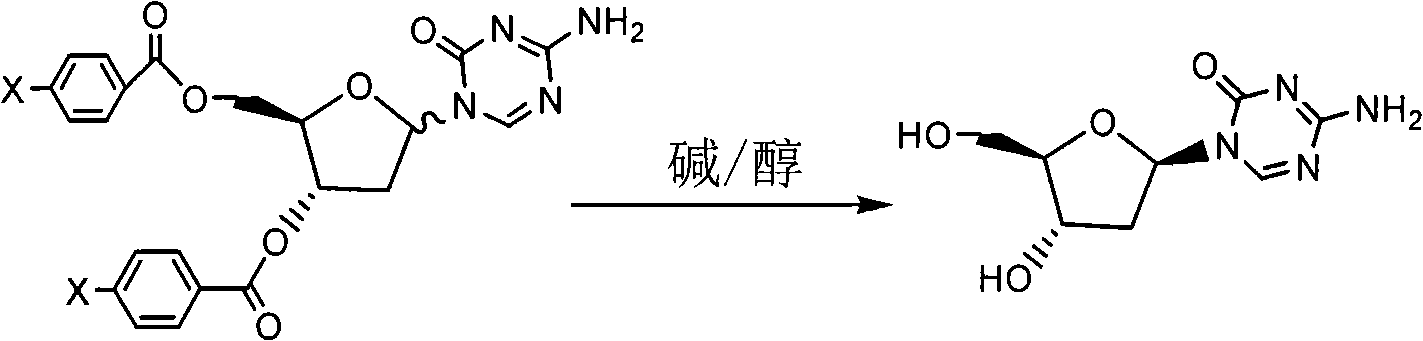
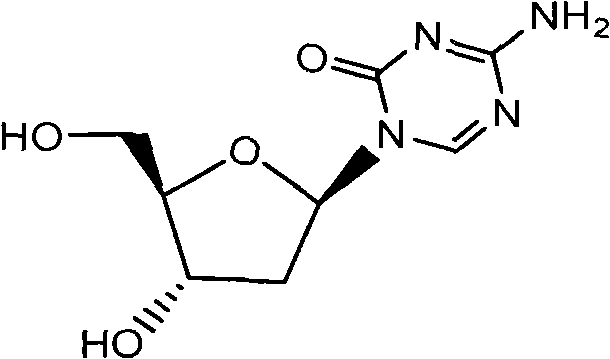
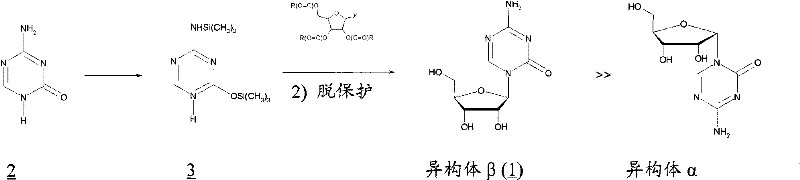
Synthetic process of decitabine
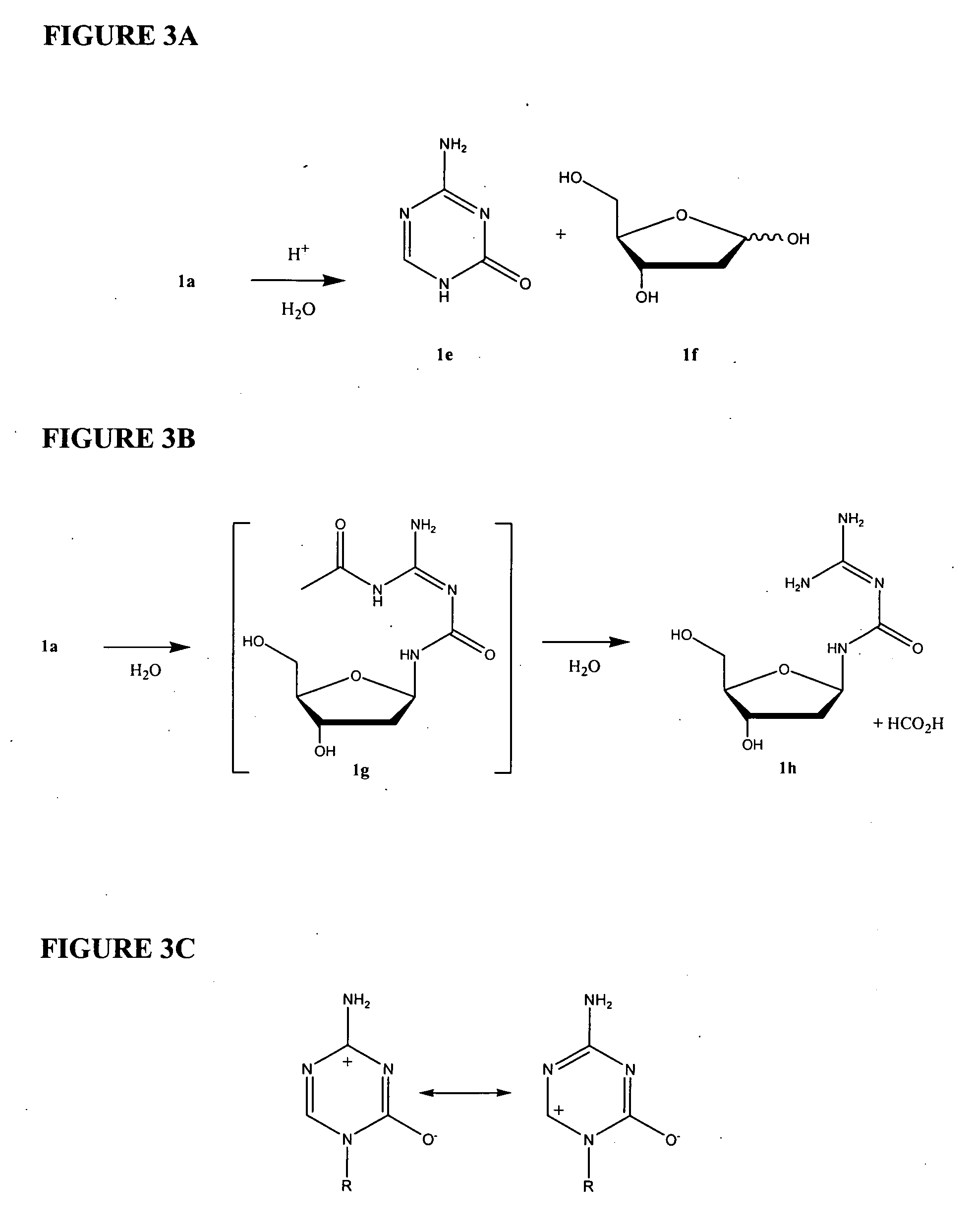
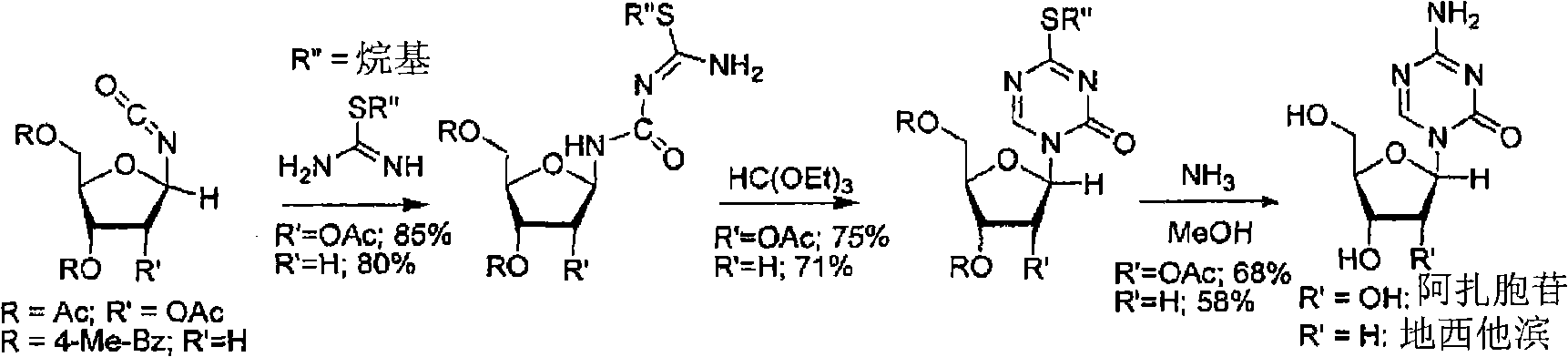
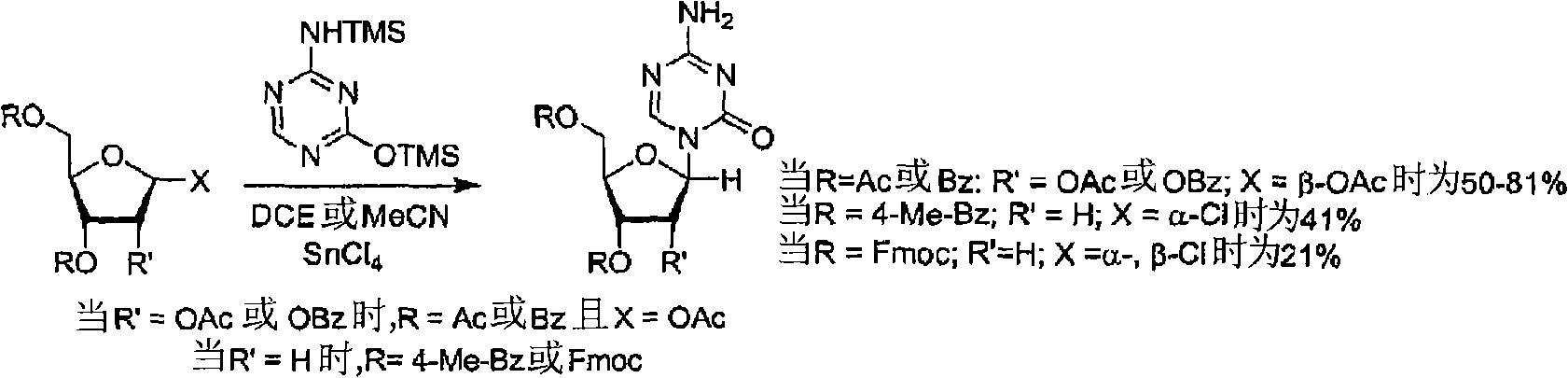
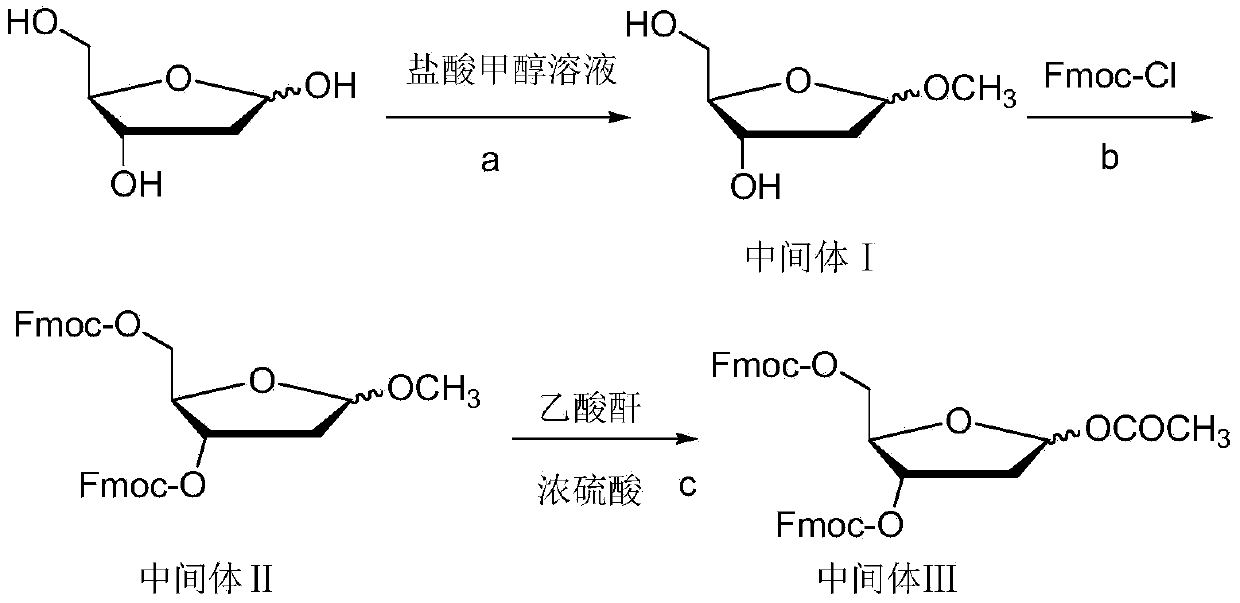
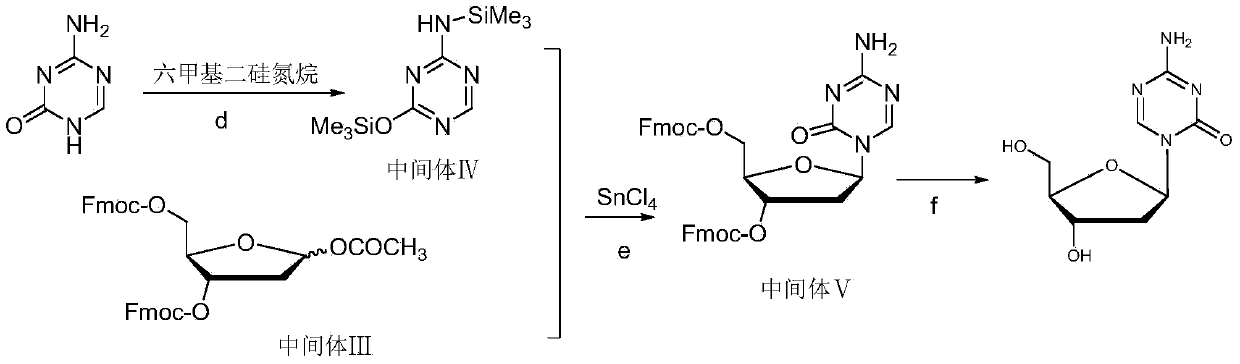
The invention relates to a method for preparing Decitabine. The particular proposal for solving the technical problem is as follows: 2-deoxidtion-D-ribose, 10 percent of HCL methanol solution, methoxyacetic acetic anhydride, HMDS, acetic anhydride, tri-silicyl tri-fluorine methane sulfonic acid ester, acetic acid amine, etc. are adopted as raw materials to synthesize the Decitabine; the target product of the Decitabine is obtained through the five steps of reactions, namely, methylation, acylation, trimethyl silication, ammoniation and deacylation with a total yield of above 18.4 percent and a product purity of above 99.7 percent.
Owner:GUIZHOU UNIV
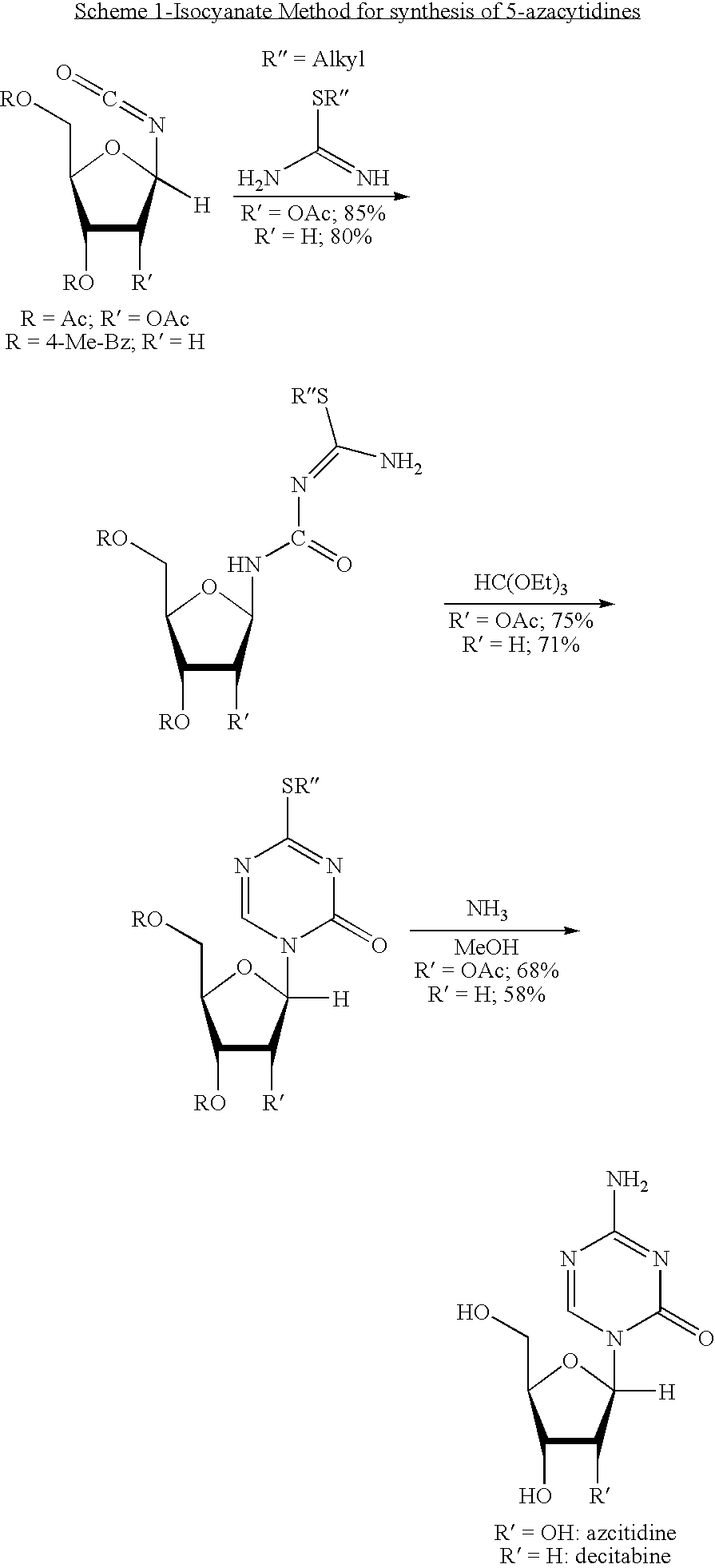
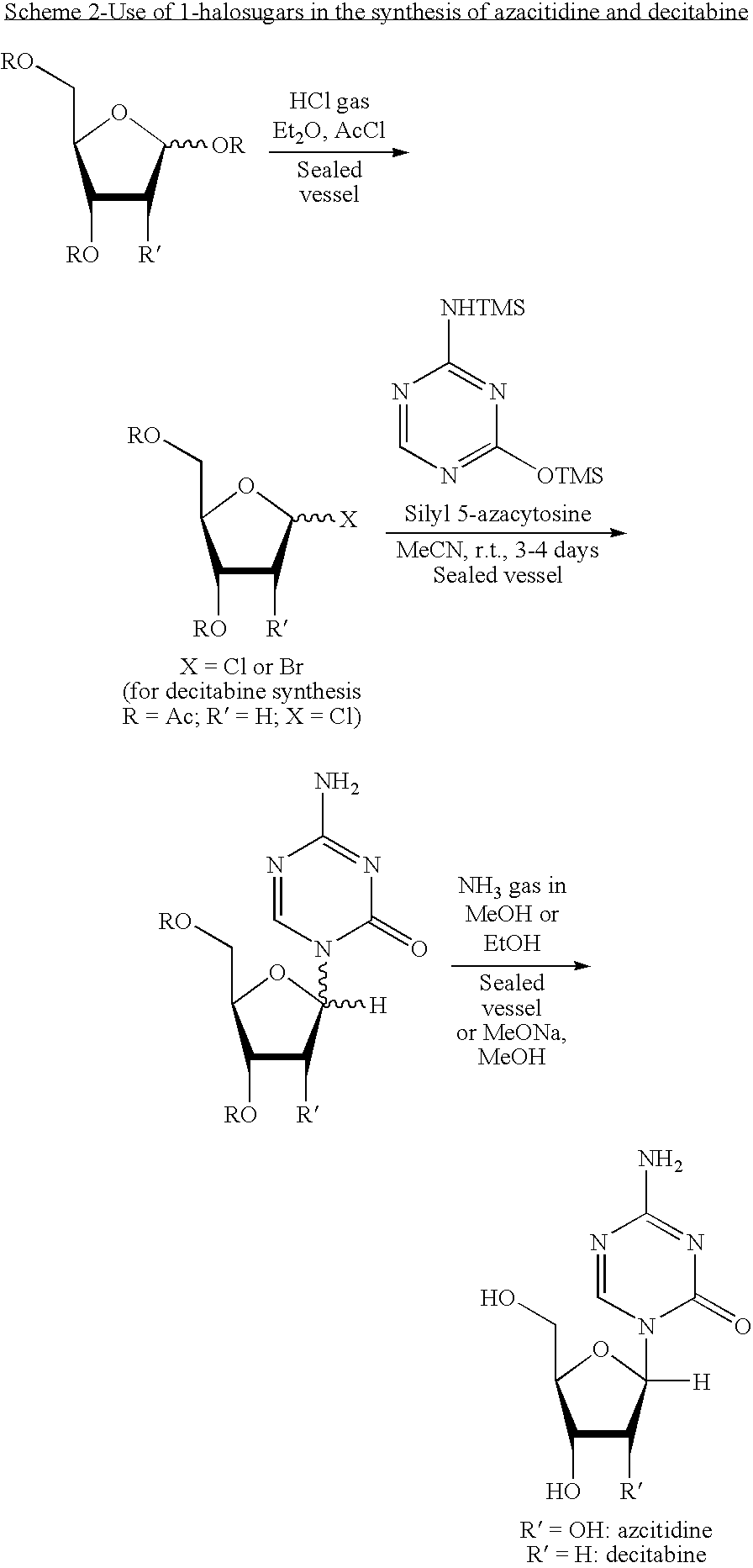
Process for Making 5-Azacytosine Nucleosides and Their Derivatives
ActiveUS20100036112A1High boiling pointIncrease polarityEsterified saccharide compoundsOrganic active ingredientsDecitabineSilylation
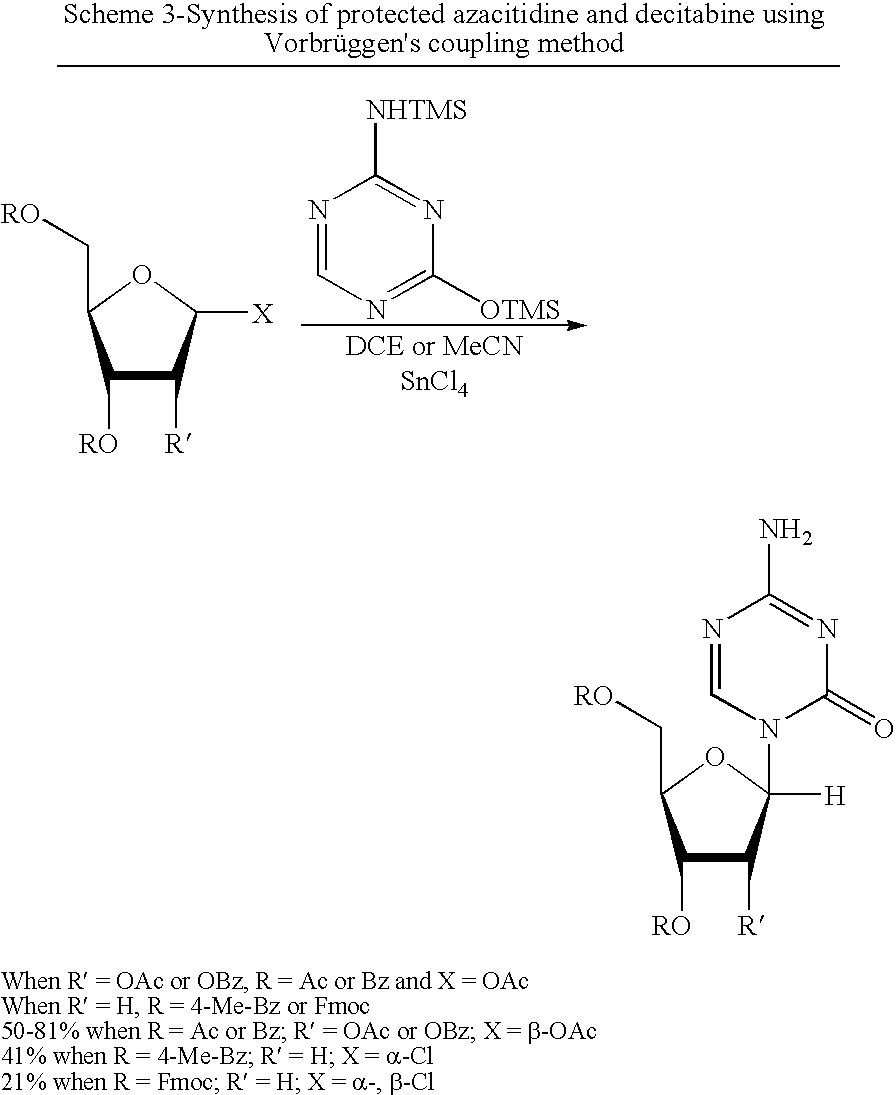
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Process for making 5-azacytosine nucleosides and their derivatives
ActiveCN102216315AShorten the timeSuitable for large-scale synthesisEsterified saccharide compoundsBiocideDecitabine5-azacytosine
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Synthesis of decitabine
The present invention provides a method for producing a protected precursor of Decitabine as well as the Decibatine final product in high yield and purity.
Owner:SCINOPHARM TAIWAN LTD
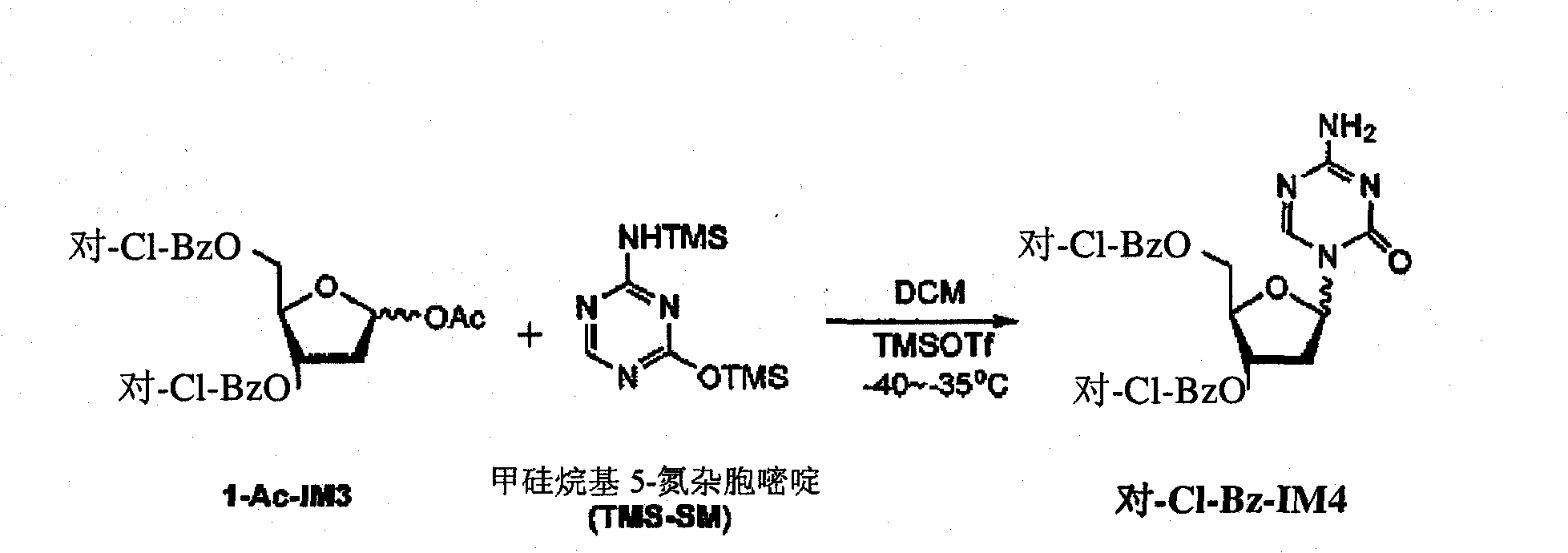
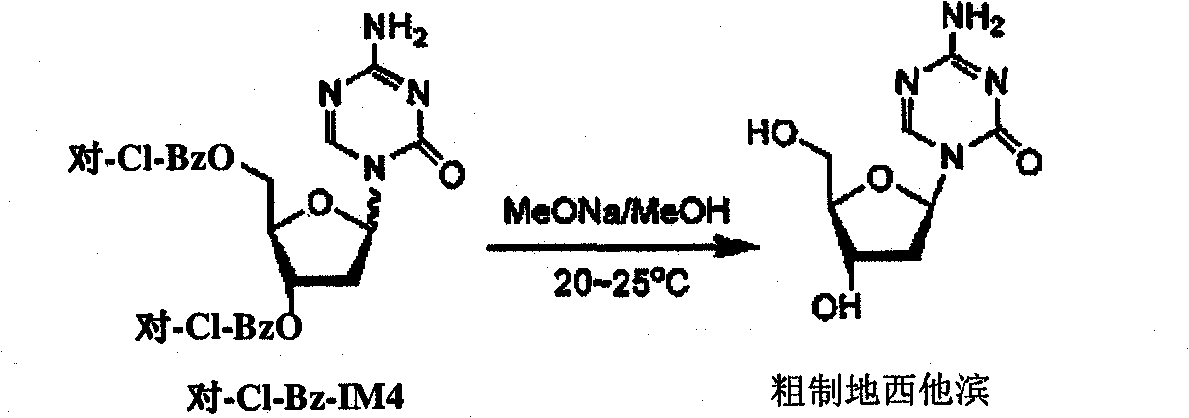
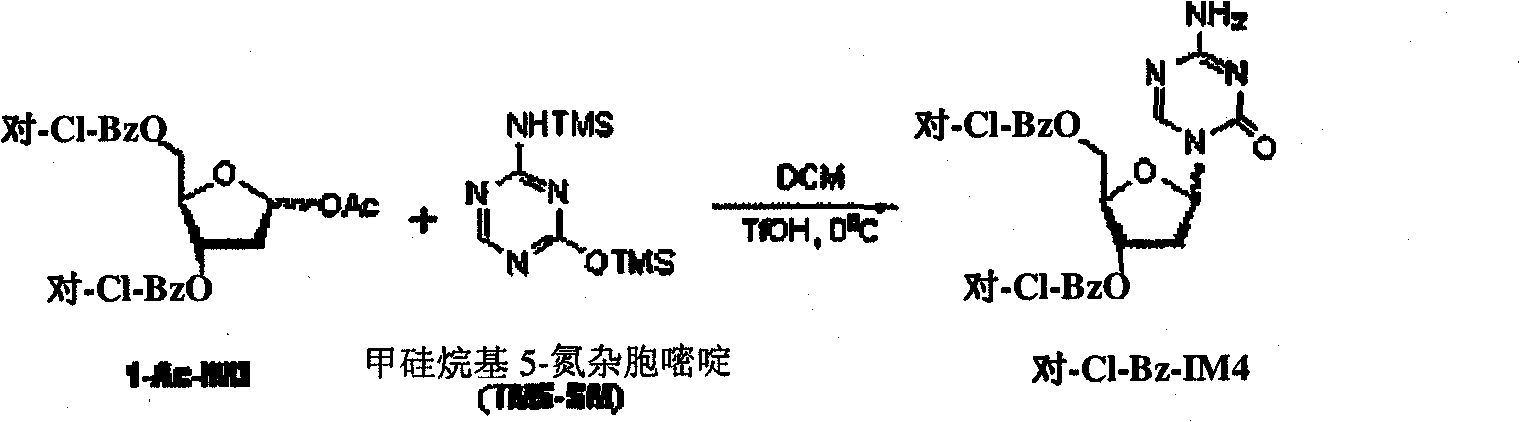
Preparation method of decitabine intermediate
ActiveCN103739636AReduce usageImprove protectionEsterified saccharide compoundsSugar derivativesCombinatorial chemistryDecitabine
The invention discloses a preparation method of a decitabine intermediate compound 1-acetoxyl-2-deoxidized-3,5-bi-O-fluorene methyl cyslohexyl-D-ribofuranose. The structural formula of the compound is shown in img file='DDA0000455164900000011.TIF' wi='648' he='304' / , and the preparation method comprises the following steps: o-methylation of 2'-deoxidized-D-ribose 1-bit hydroxyl; b) protection of 3,5-bit hydroxyl; and c) acylation of 1-bit-O-methyl. The invention also discloses a method for preparing decitabine employing the intermediate as a raw material.
Owner:SHANDONG NEWTIME PHARMA
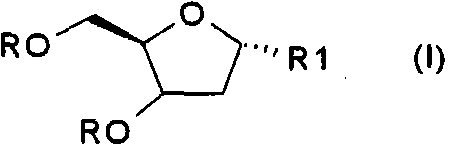
Method for preparing improved decitabine
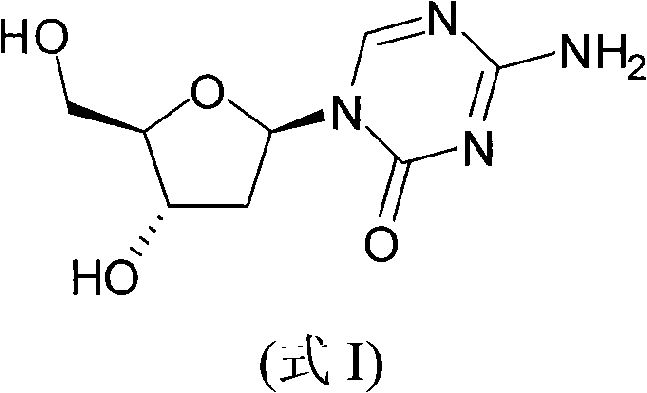
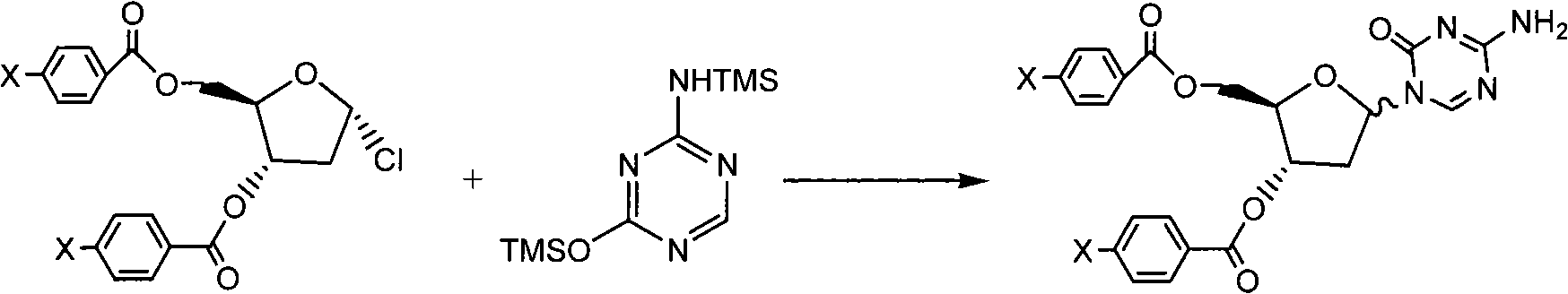
InactiveCN101560232AHigh purityShort stepsOrganic active ingredientsSugar derivativesRiboseDecitabine
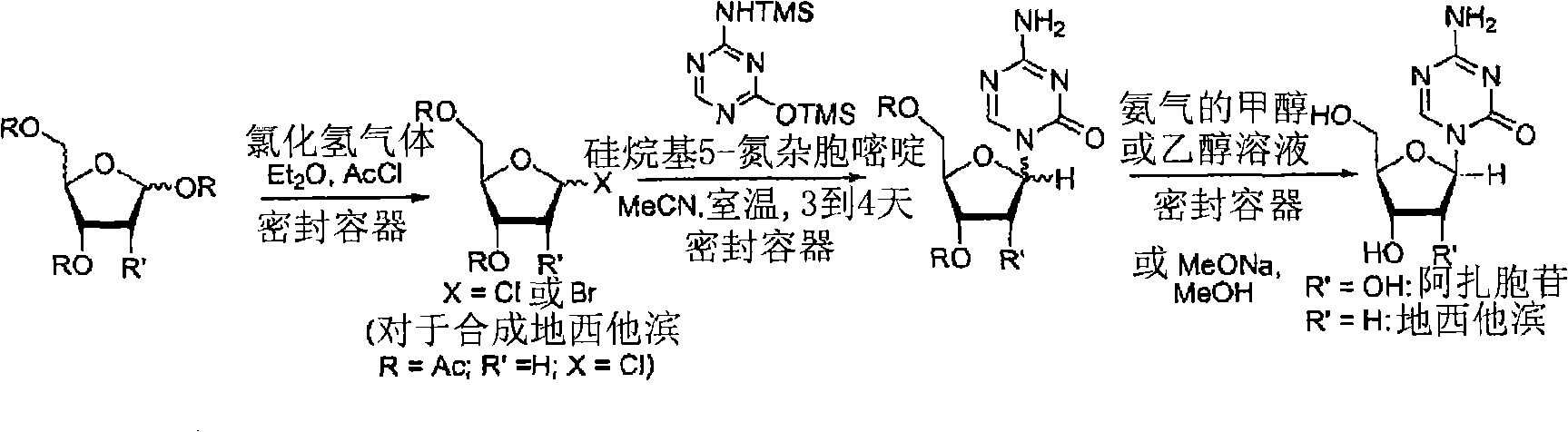
The invention discloses a method for preparing improved decitabine (formula I), comprising: using 1-alpha-chlorine-3,5-two pairs of halo-benzoyl-2-deoxidation-D-ribose (formula II) as materials, concentrating with 2-4-bi-(trimethyl silicane)-5-azacytosine (formula III) to obtain I by de-protecting group. Materials used in the invention are easy to obtain at a low price. The invention also has the advantages of convenient operation and a high reaction yield, and is suitable for industrialized production.
Owner:SHANGHAI QINGSONG PHARMA
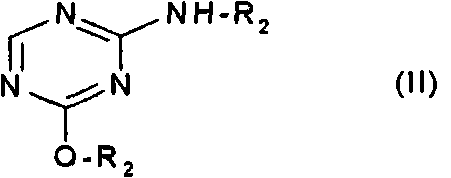
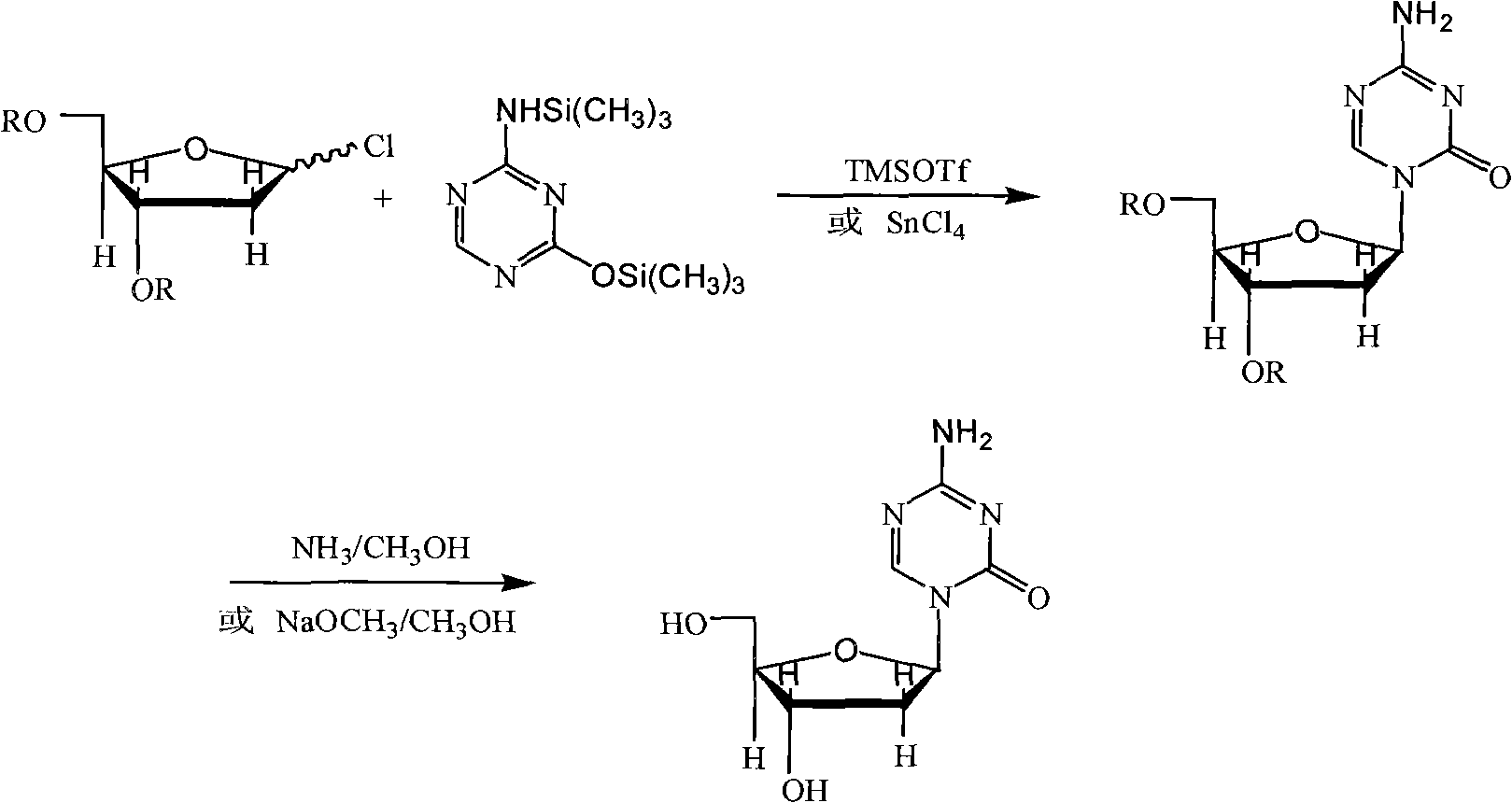
Method of producing 2'-deoxy-5-azacytidine (decitabine)
The invention relates to a method of producing 2'-deoxy-5-azacytidine (Decitabine) by providing a compound of formula (I) shown in the description, wherein R is a removable substituent known per se; and R1 is a removable substituent; further providing a silylated base of formula (II) shown in the description, wherein R2 is a protecting group, preferably a trimethylsilyl TMS )-residue; reacting the compound of formula (I) and the compound of formula (II) together in a suitable anhydrous solvent and in the presence of a suitable catalyst; and removing the substituents R from the compound obtained in order to obtain the compound 2'-deoxy-5-azacytidine (Decitabine), characterized in that said catalyst is selected from the group comprising a salt of an aliphatic sulphonic acid or a salt of a strong inorganic acid.
Owner:CILAG
Method of producing nucleosides
Method of producing a free nucleoside compound, the compound 2′-deoxy-5-azacytidine (Decitabine) being excluded, by reacting a glycoside donor preferably a 1-halogen derivative, or 1-O-acyl, 1-O-alkyl, or an imidate preferably a trichloromethyl derivative, or a thio-alkyl derivative of a blocked monosaccharide or oligosaccharide preferably ribose and 2-desoxyribose derivatives with a protected nucleoside base, in a suitable anhydrous solvent and in the presence of a catalyst, and removing the protecting groups from said blocked nucleoside compound, wherein said catalyst is selected from the group comprising salts of an aliphatic sulphonic acid and / or salts a strong inorganic acid containing a non-nucleophilic anion.
Owner:CILAG
Drug combinations
InactiveUS20160015805A1Good effectLow toxicityBoron compound active ingredientsAntibody ingredientsDiseaseHaematological disorders
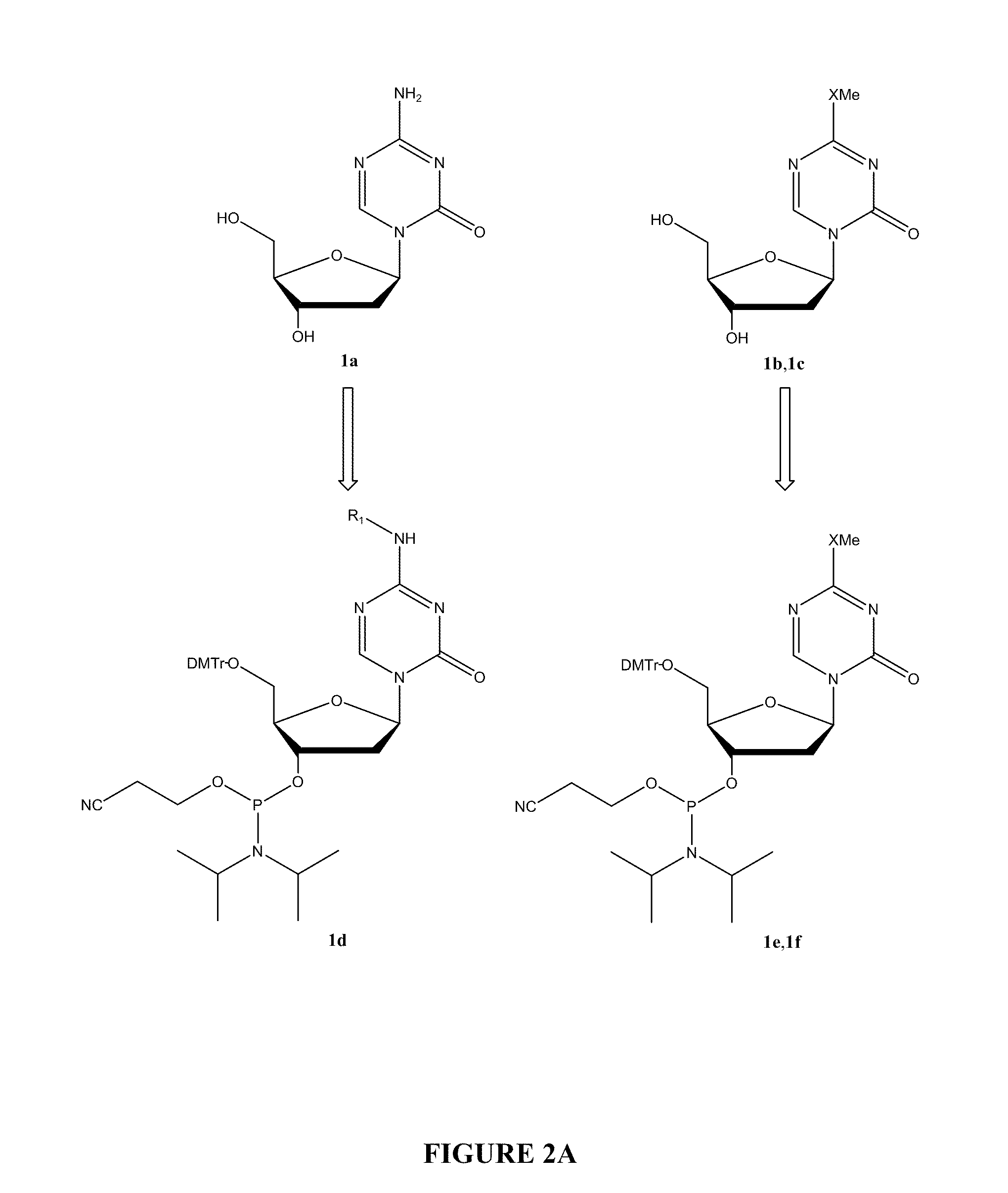
The invention provides combinations of derivatives of decitabine and other active agents, including T-cell activating agents, cancer vaccines, and adjuvants. Some derivatives of decitabine exhibit superior chemical stability and shelf life, with similar physiological activity. Methods of treating one or more myelodysplasia syndromes, cancers, haematological disorders, or diseases associated with abnormal haemoglobin synthesis using the combinations are described.
Owner:SUPERGEN
Industrialized production method for high-purity decitabine
InactiveCN101948493AReduce the impactMeet quality requirementsSugar derivativesSugar derivatives preparationSodium methoxideSolvent
The invention provides an industrialized production method for high-purity decitabine. The method comprises the following steps of: 1, performing silanization reaction of 5-azacytosine, bis(trimethylsilyl)amine and trimethyl chlorosilane, which serve as raw materials, to prepare 2,4-bis(trimethylsilyl)-5-azacytosine; 2, performing reaction of the product obtained by the step 1 and 1-chloro-3,5-bis-(4-chlorobenzoyl)-2-deoxy-D-ribofuranose, which serve as raw materials, to prepare a crude product of 1-(3,5-bis-(4-chlorobenzoyl)-2-deoxy-beta-D-ribofuranose)-5-azacytosine; 3, dissolving the product obtained by the step 2 in a C5 to C7 hydrocarbon, stirring, filtering and drying to obtain a refined product; and 4, producing the high-purity decitabine by using the product obtained by the step 3, methyl alcohol and sodium methoxide as raw materials. The method overcomes the disadvantages of need of column purification, low purity, difficult industrial production in the prior art, and has the advantages of simple and convenient operation, small solvent consumption, small influence on the environment, low labor intensity, short period, high product purity, single impurity and less than 0.1 percent total impurity content.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Oligonucleotide analogues incorporating 5-aza-cytosine therein
ActiveUS20100215729A1Heavy metal active ingredientsGenetic material ingredientsDiseaseHuman DNA sequencing
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Composition and method for treating neurological disorders
InactiveUS20070254835A1Effective treatmentAddress bad outcomesBiocideNervous disorderNervous systemFragile X chromosome
Owner:SUPERGEN
Process for the synthesis of azacitidine and decitabine
ActiveCN102206240AHigh yieldAvoid processing powerGroup 4/14 element organic compoundsSugar derivativesOrganic solventTrimethylsilyl
Described herein is a process for the synthesis of azacitidine or decitabine, comprising the silylation of azacytosine in the presence of N,O-bis-trimethylsilyl)-trifluoroacetamide. Such reaction is performed in an organic solvent, preferably aprotic, even more preferably selected from among dichloromethane, dichloroethane and / or acetonitrile. According to a further aspect of the process, 2 to 3 moles of N,O-bis-trimethylsilyl-trifluoroacetamide are used per mole of azacytosine, preferably from 2.2 to 2.5.
Owner:CHEMI SPA
Preparation method of decitabine freeze-dried powder injection
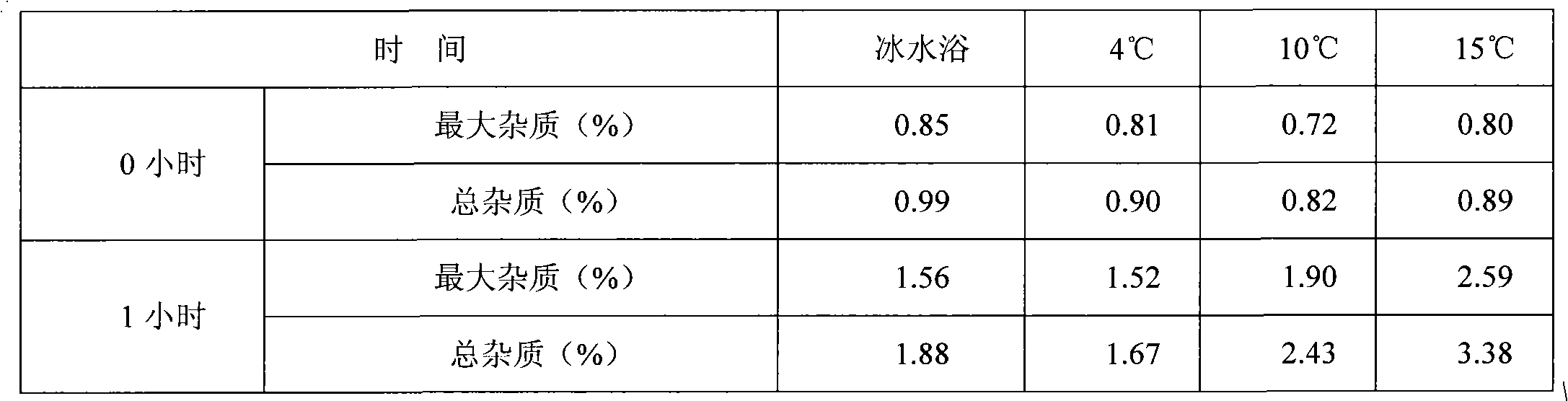
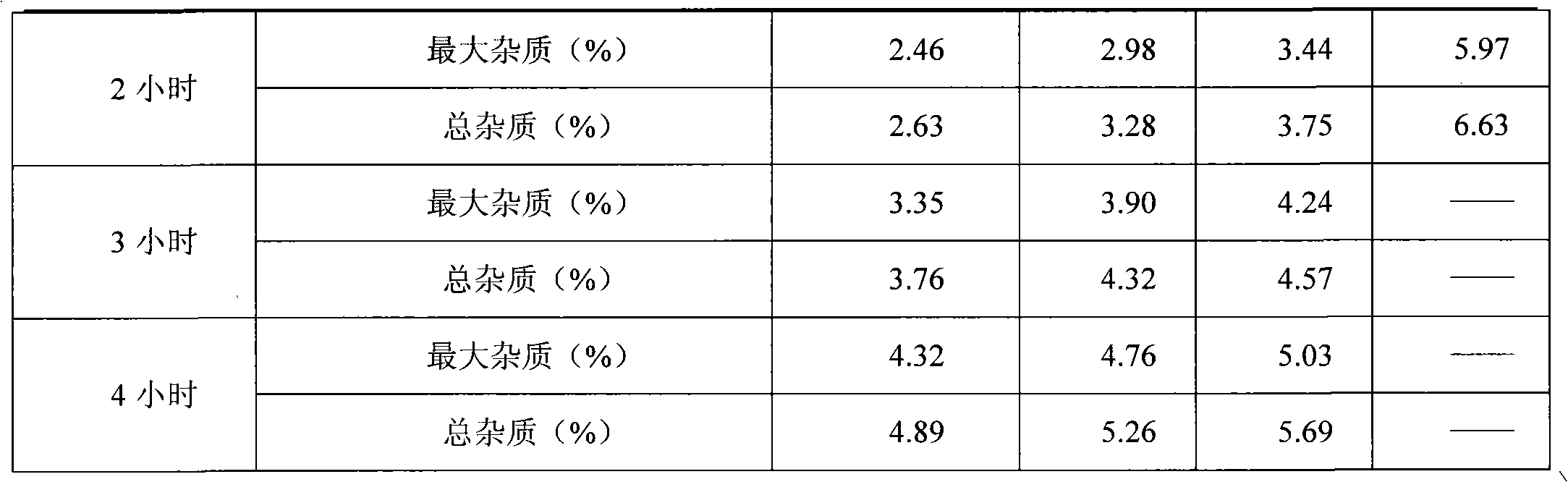
InactiveCN101843592ALow content of related substancesSafe and non-flammableOrganic active ingredientsPowder deliveryAdjuvantOrganic solvent
The invention discloses a preparation method of a decitabine freeze-dried powder injection, which is characterized by comprising the following steps: adding a pharmaceutic adjuvant into water and dissolving; adjusting the pH value to be 6.0-8.0 to obtain adjuvant solution; refrigerating the adjuvant solution to 0-10DEG C for later use; adding decitabine into the adjuvant solution and dissolving; keeping the liquid medicine at 0-10DEG C; adjusting the pH value to be 6.0-8.0; and adding active carbon, filtering, filling and freeze-drying to obtain the decitabine freeze-dried powder injection. In the process method, an organic solvent is not added and is replaced by water; and by controlling the pH value and the temperature, the contents of relative substances of the obtained preparation are low.
Owner:连云港杰瑞药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com