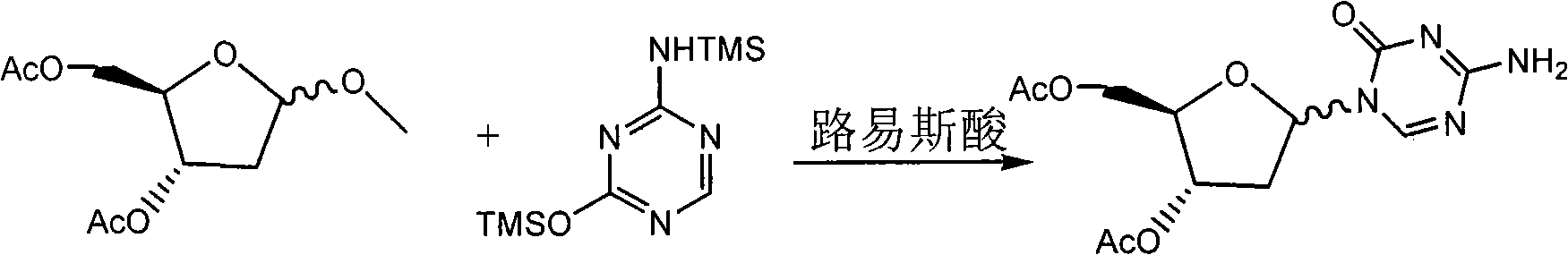
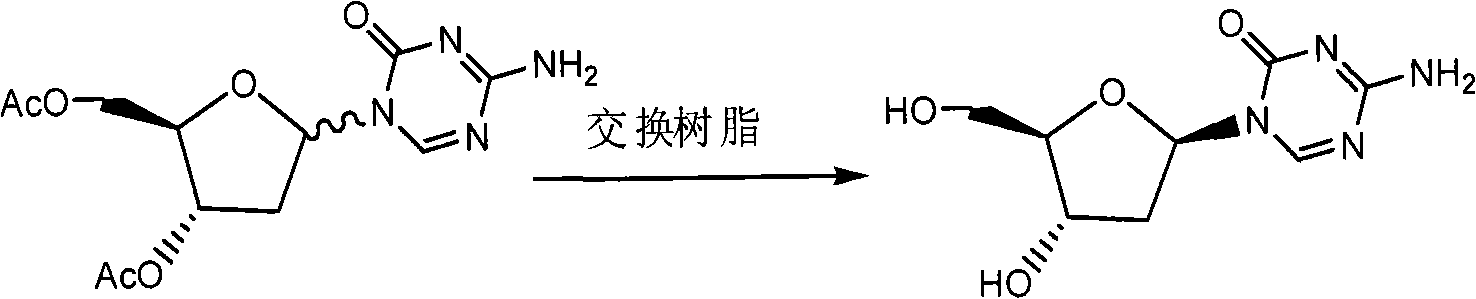
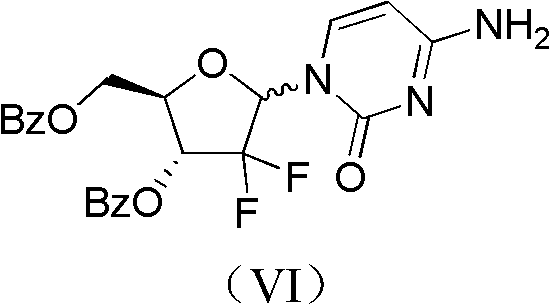
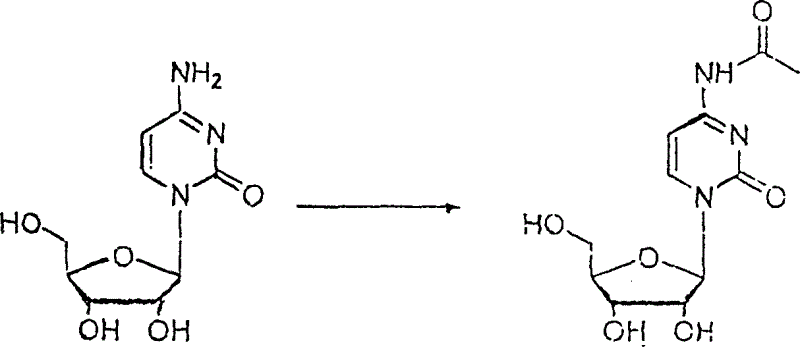
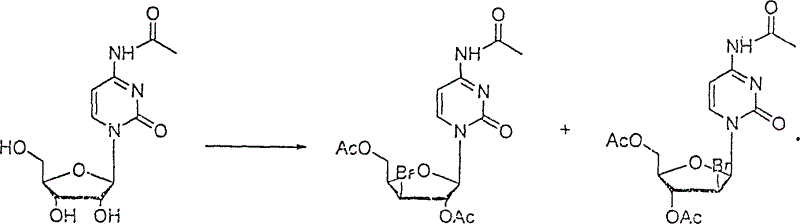
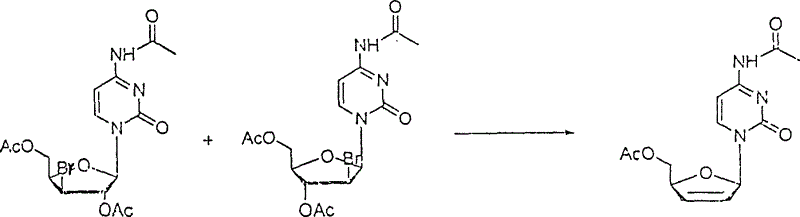
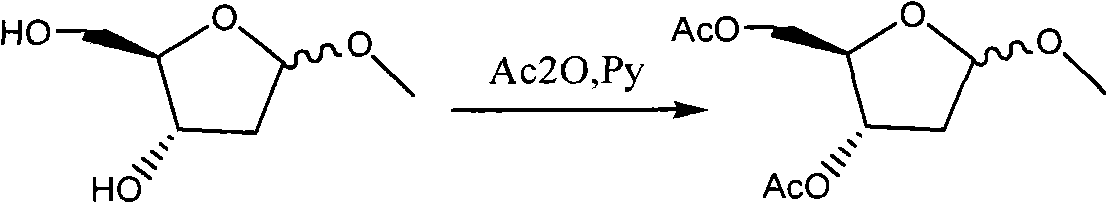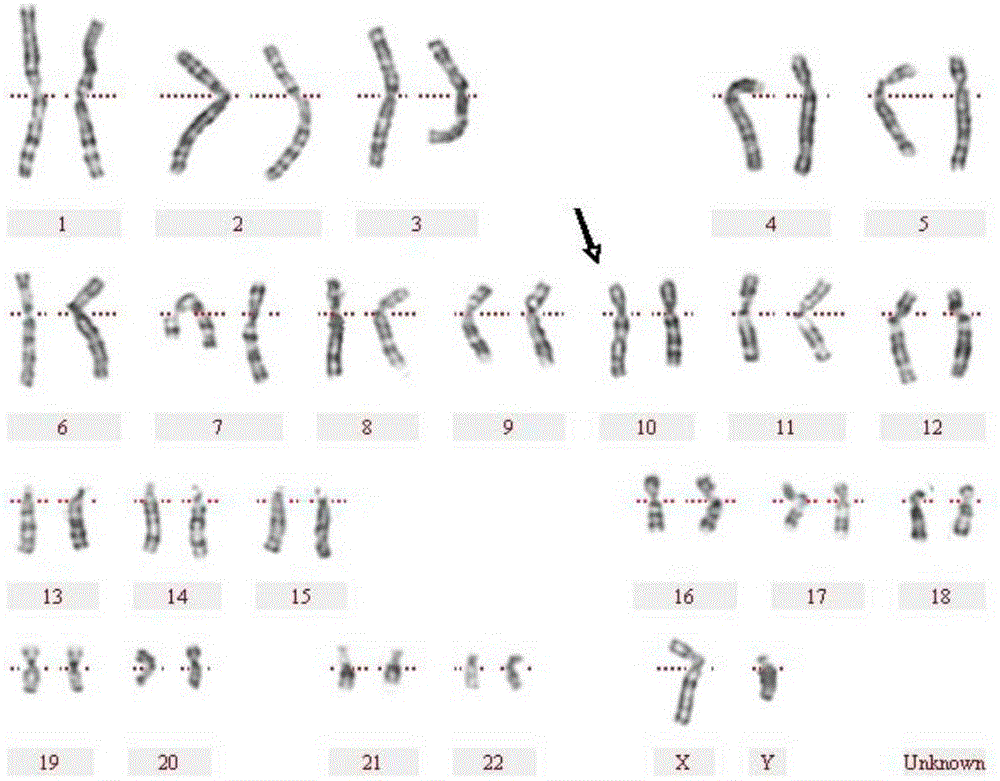Patents
Literature
99 results about "Deoxycytidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
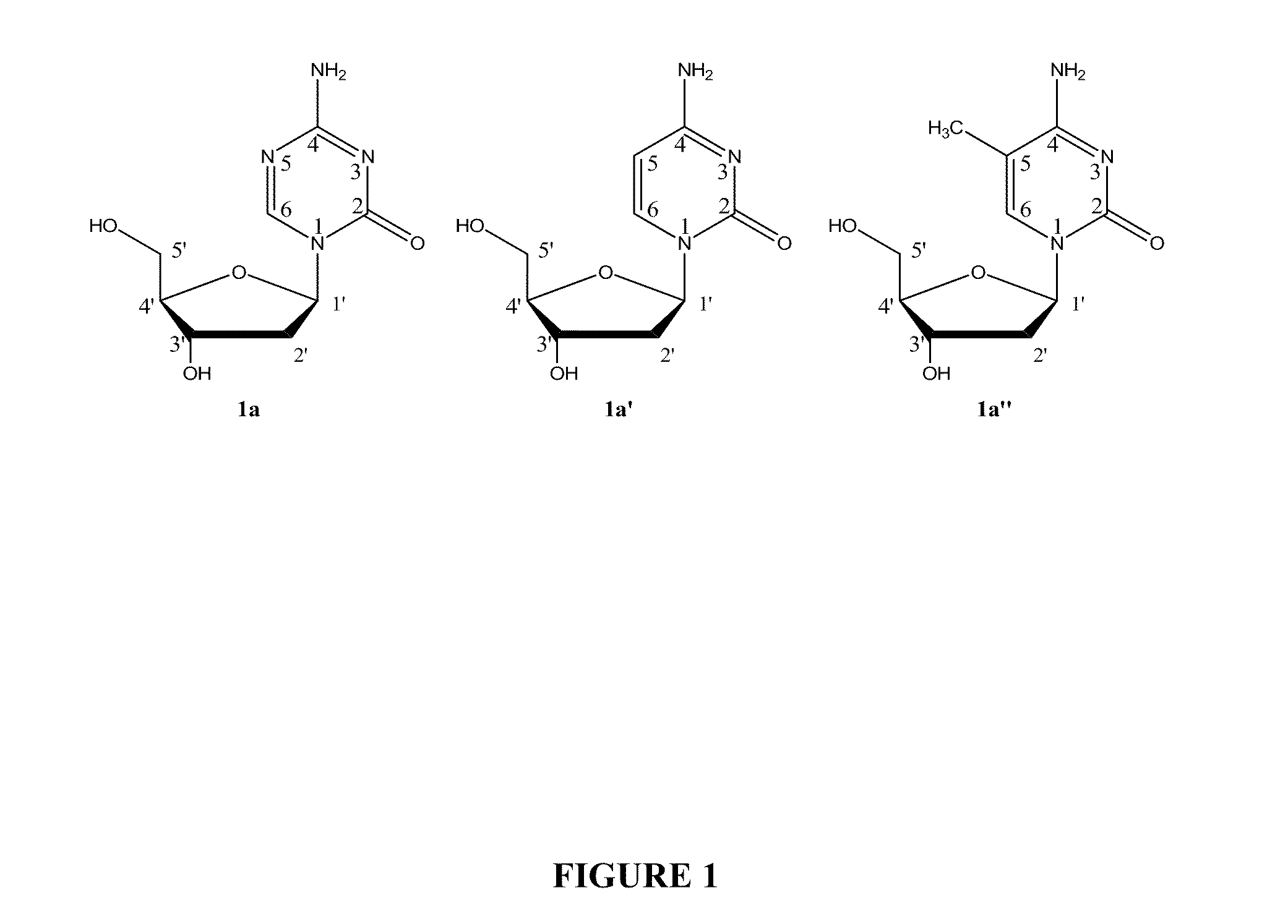
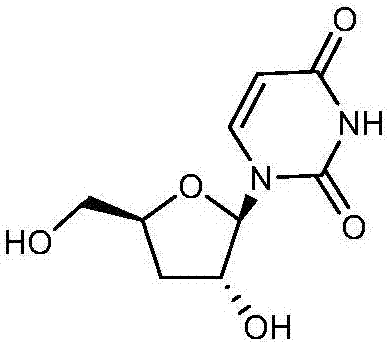
Deoxycytidine is a deoxyribonucleoside, a component of deoxyribonucleic acid. It is similar to the ribonucleoside cytidine, but with one hydroxyl group removed from the 2' position. Deoxycytidine can be phosphorylated by deoxycytidine kinase (DCK).
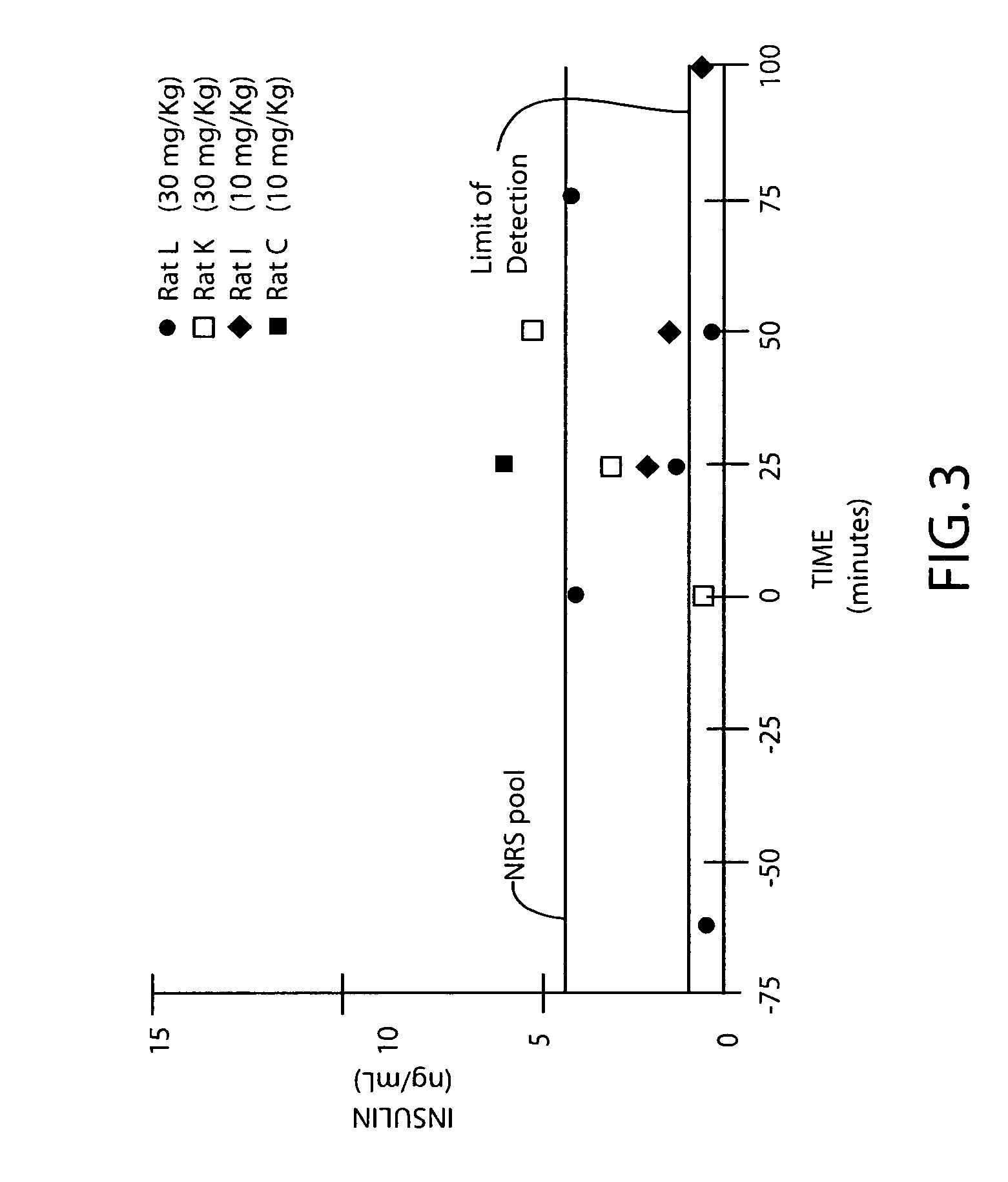
Pharmaceutical formulation comprising dinucleoside polyphosphates and salts thereof
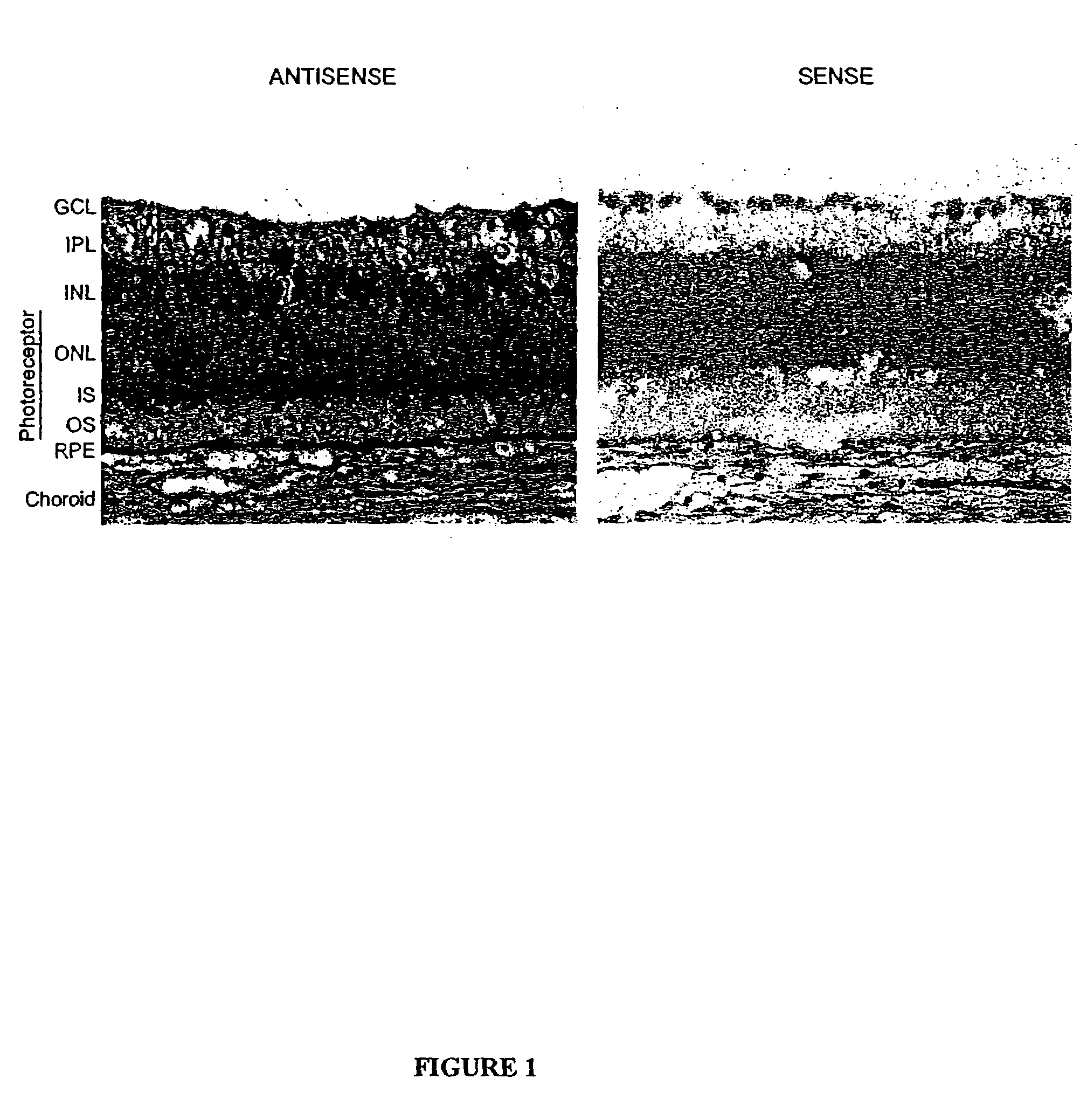
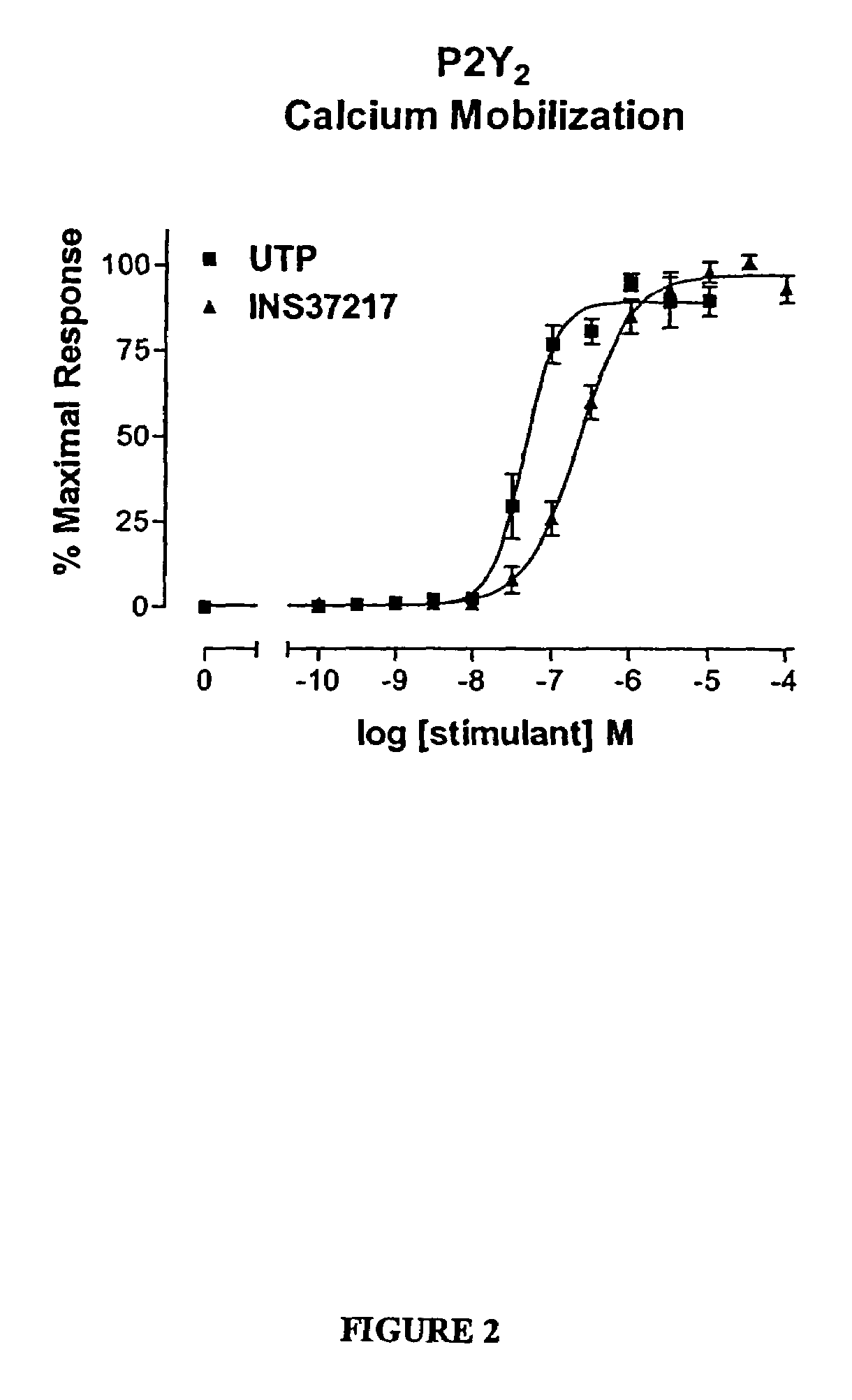
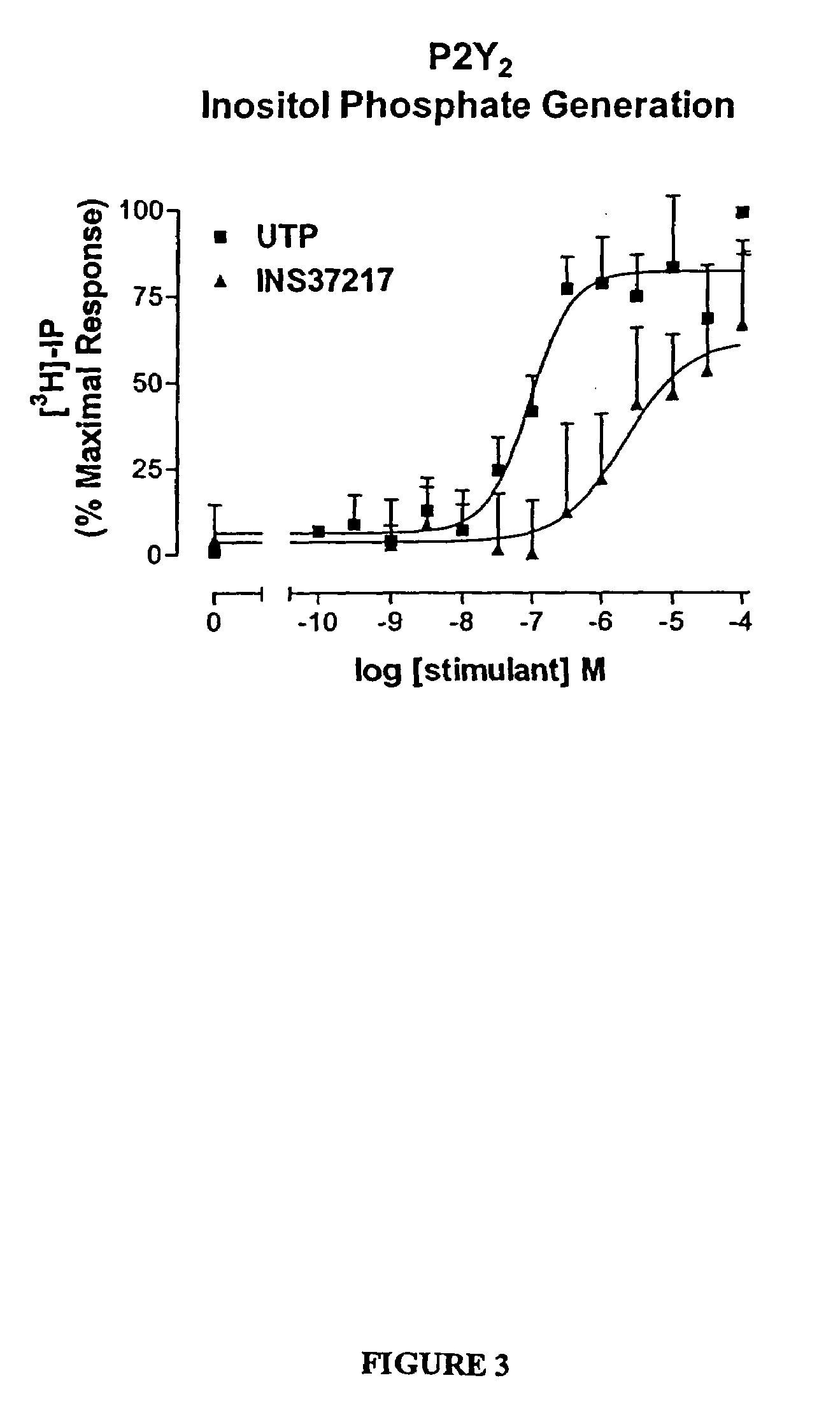
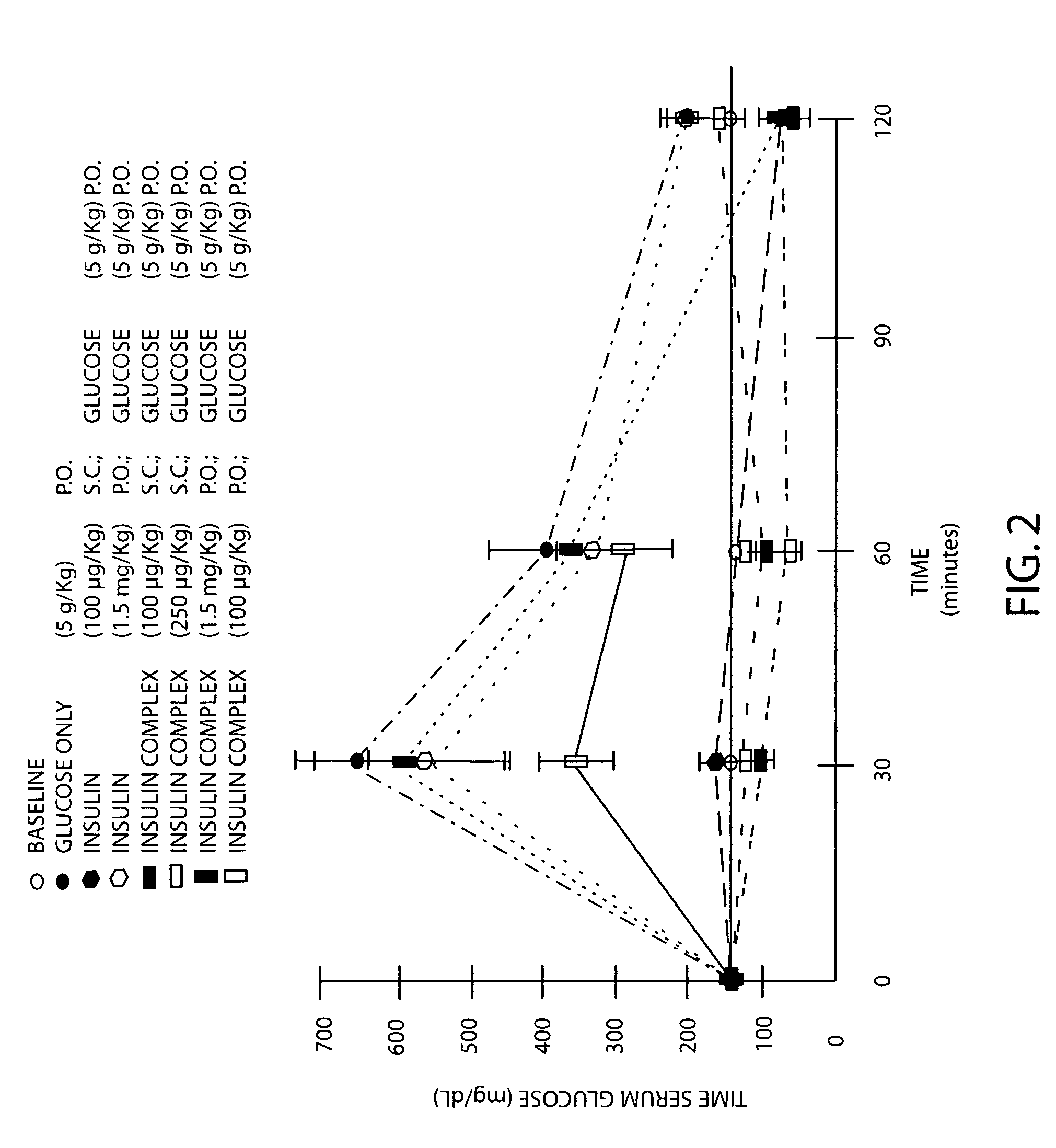
The present invention provides a method of treating edematous retinal disorders. The method comprises administration of a pharmaceutical formulation comprising a hydrolysis-resistant P2Y receptor agonist to stimulate the removal of pathological extraneous fluid from the subretinal and retinal spaces and thereby reduce the accumulation of said fluid associated with retinal detachment and retinal edema. The P2Y receptor agonist can be administered with therapeutic and adjuvant agents commonly used to treat edematous retinal disorders. The pharmaceutical formulation useful in this invention comprises a P2Y receptor agonist with enhanced resistance to extracellular hydrolysis, such as dinucleoside polyphosphate compounds, or hydrolysis-resistant mononucleoside triphosphate salts. The present invention also provides P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salts such as tetrasodium, tetralithium, tetrapotassium, and mixed (tetra-alkali metal) salts. The present further provides a pharmaceutical formulation comprising a P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salt, in a pharmaceutically acceptable carrier.
Owner:MERCK SHARP & DOHME LLC
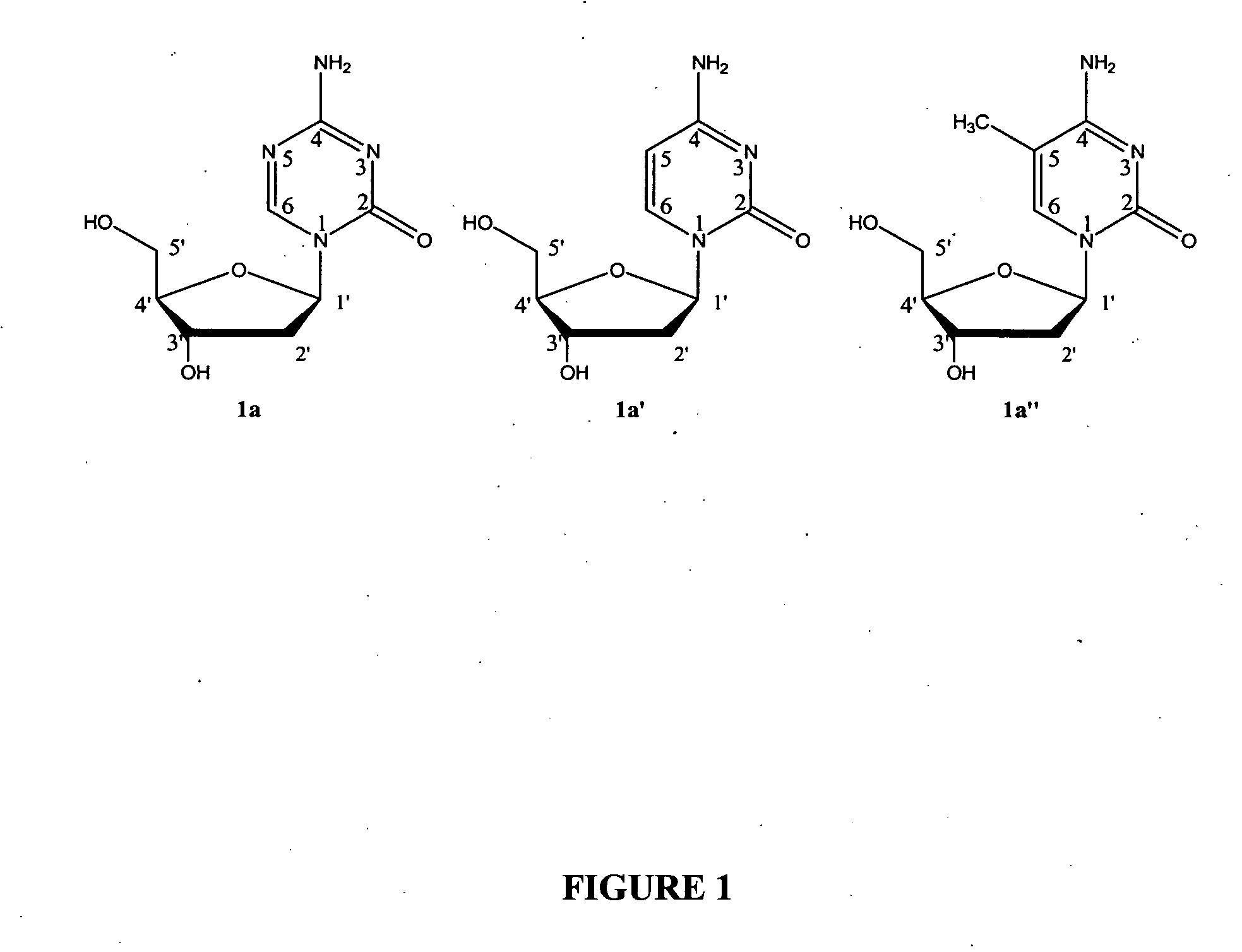
Oligonucleotide analogues incorporating 5-aza-cytosine therein
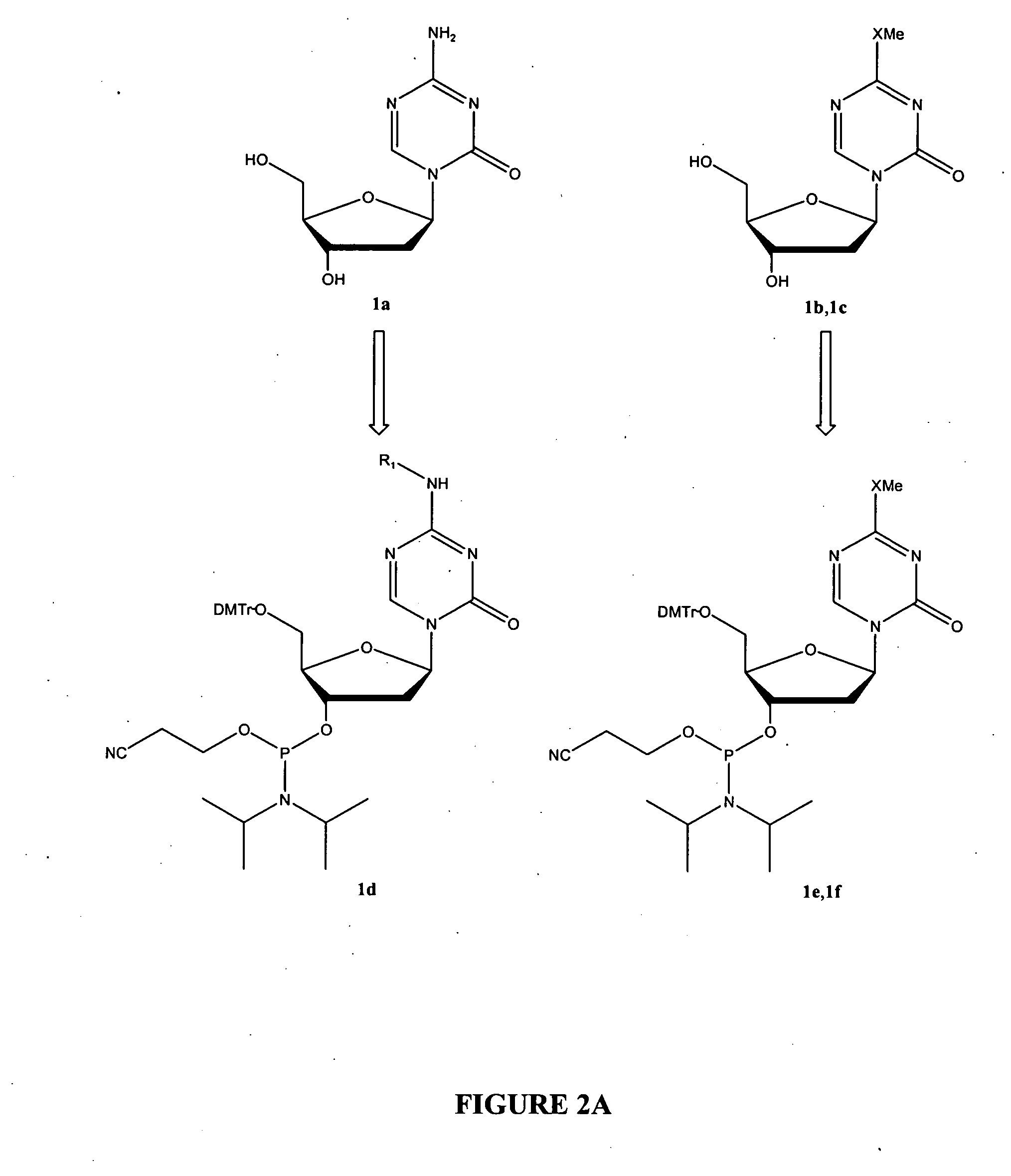
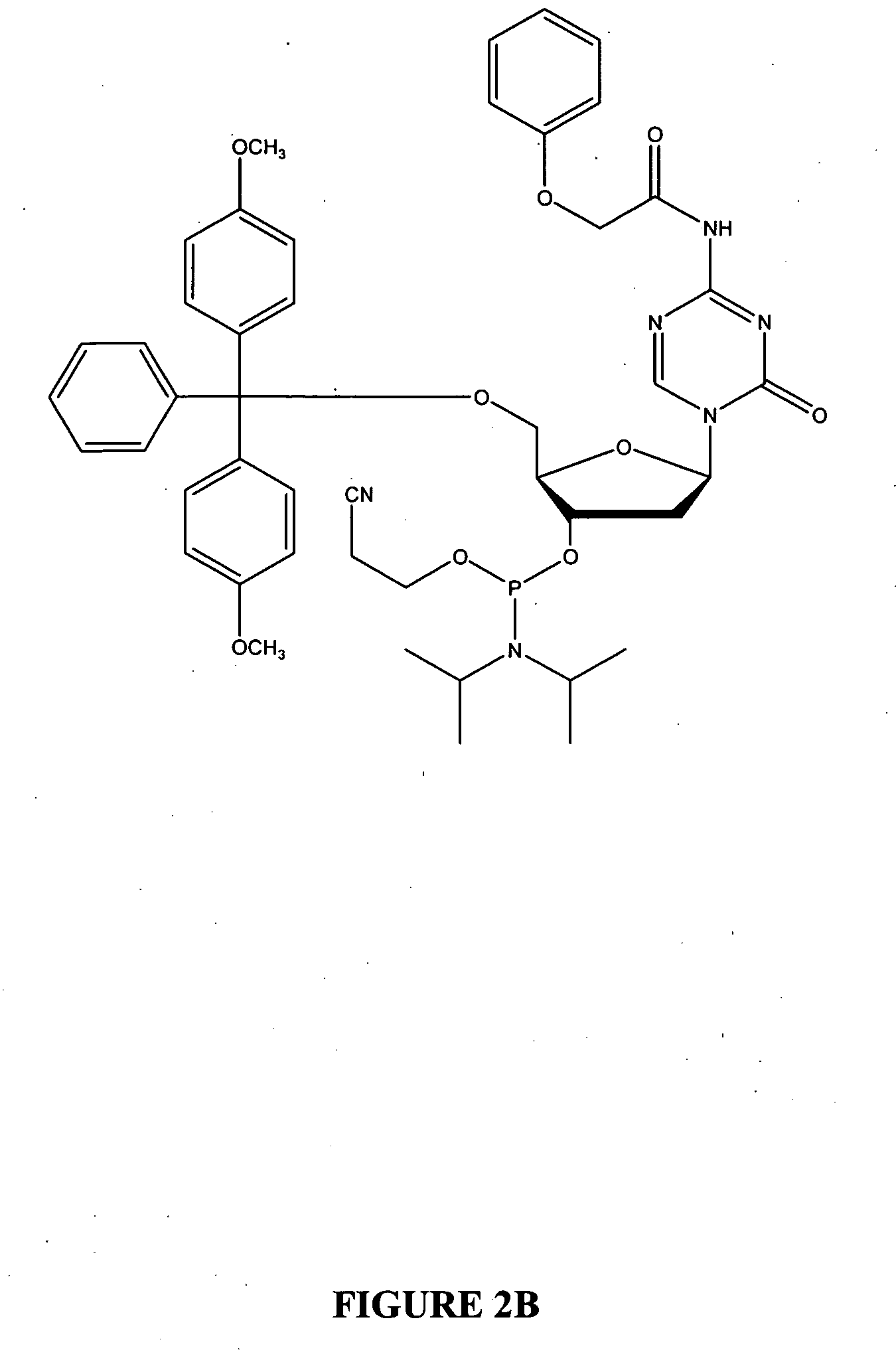
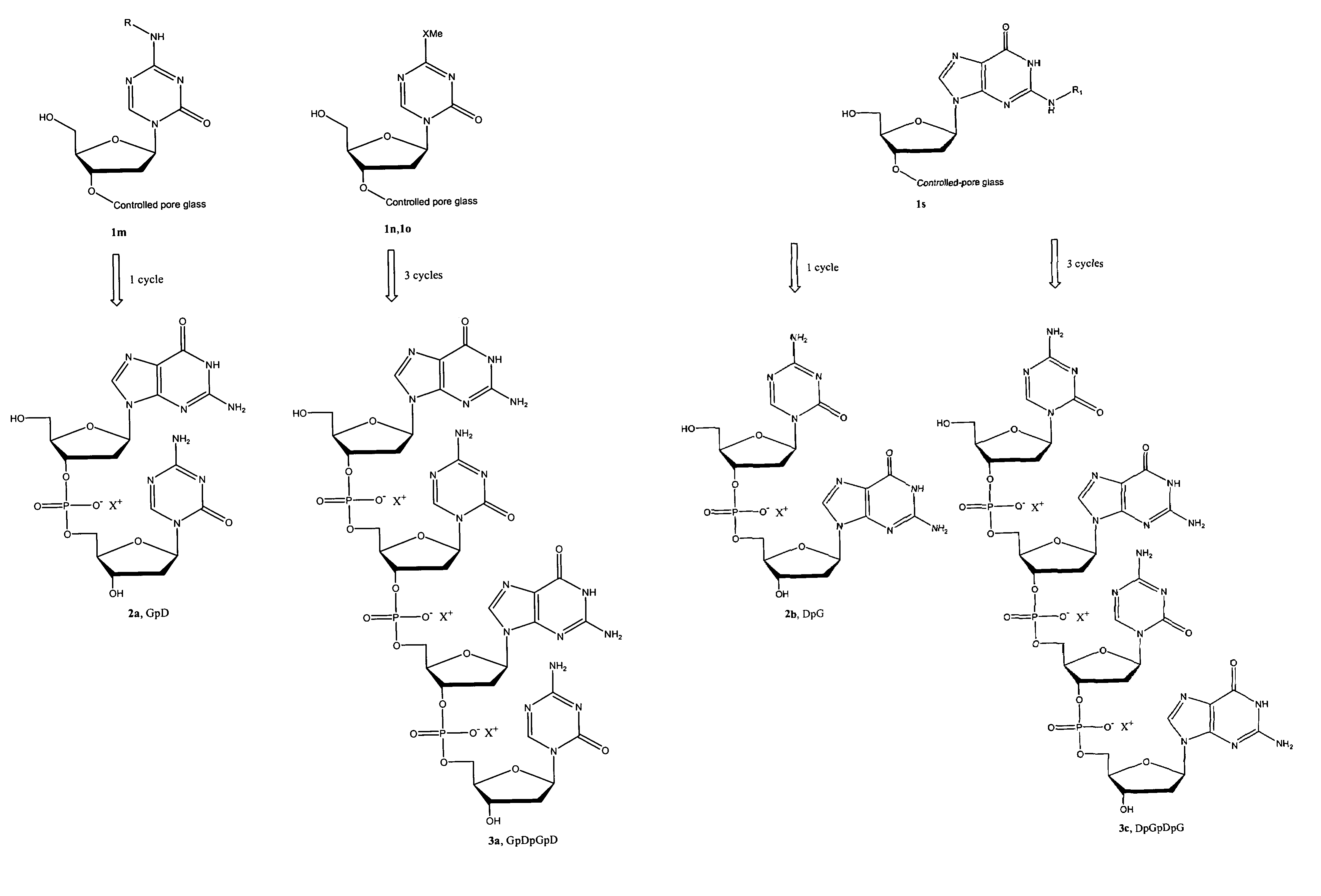
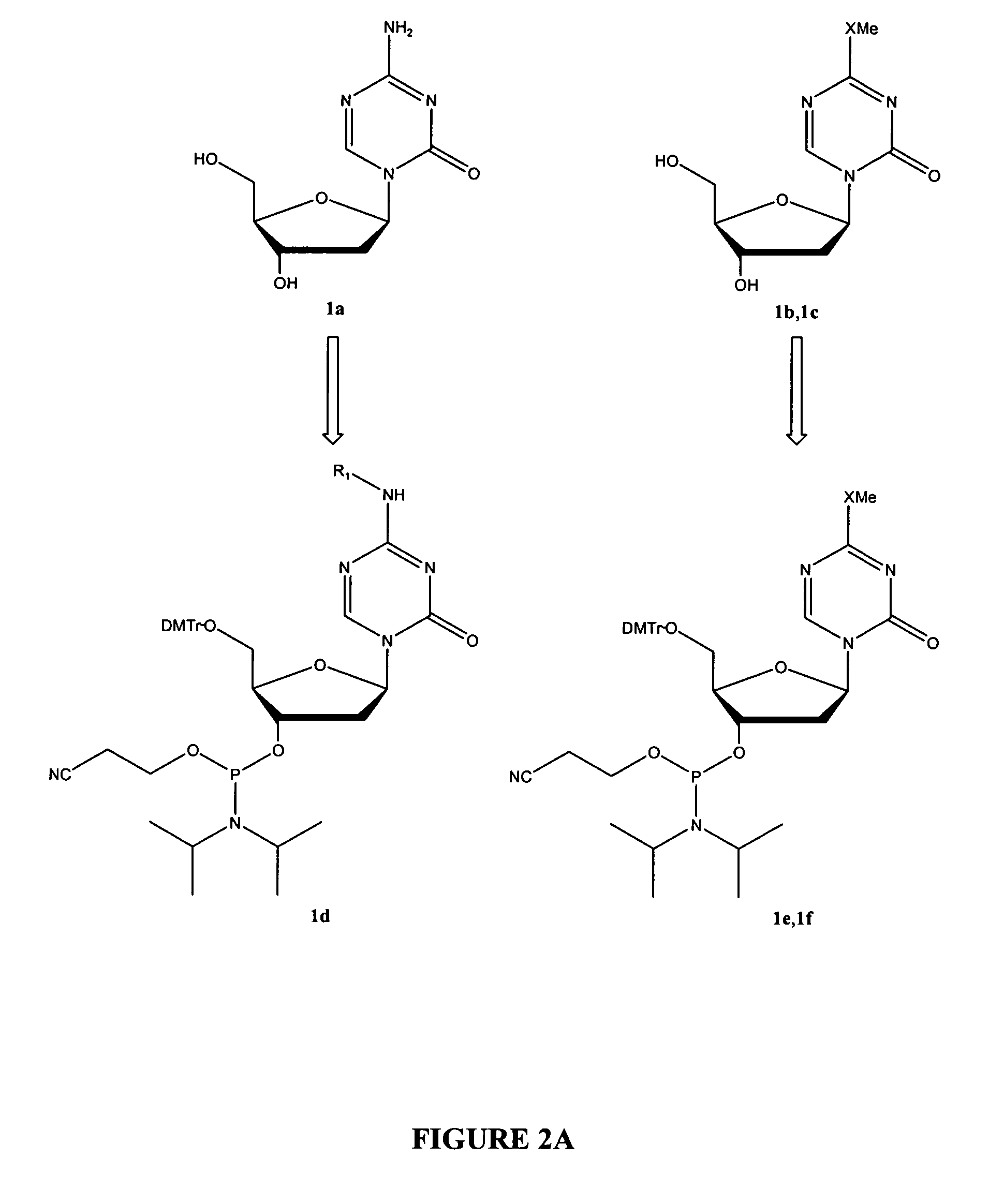
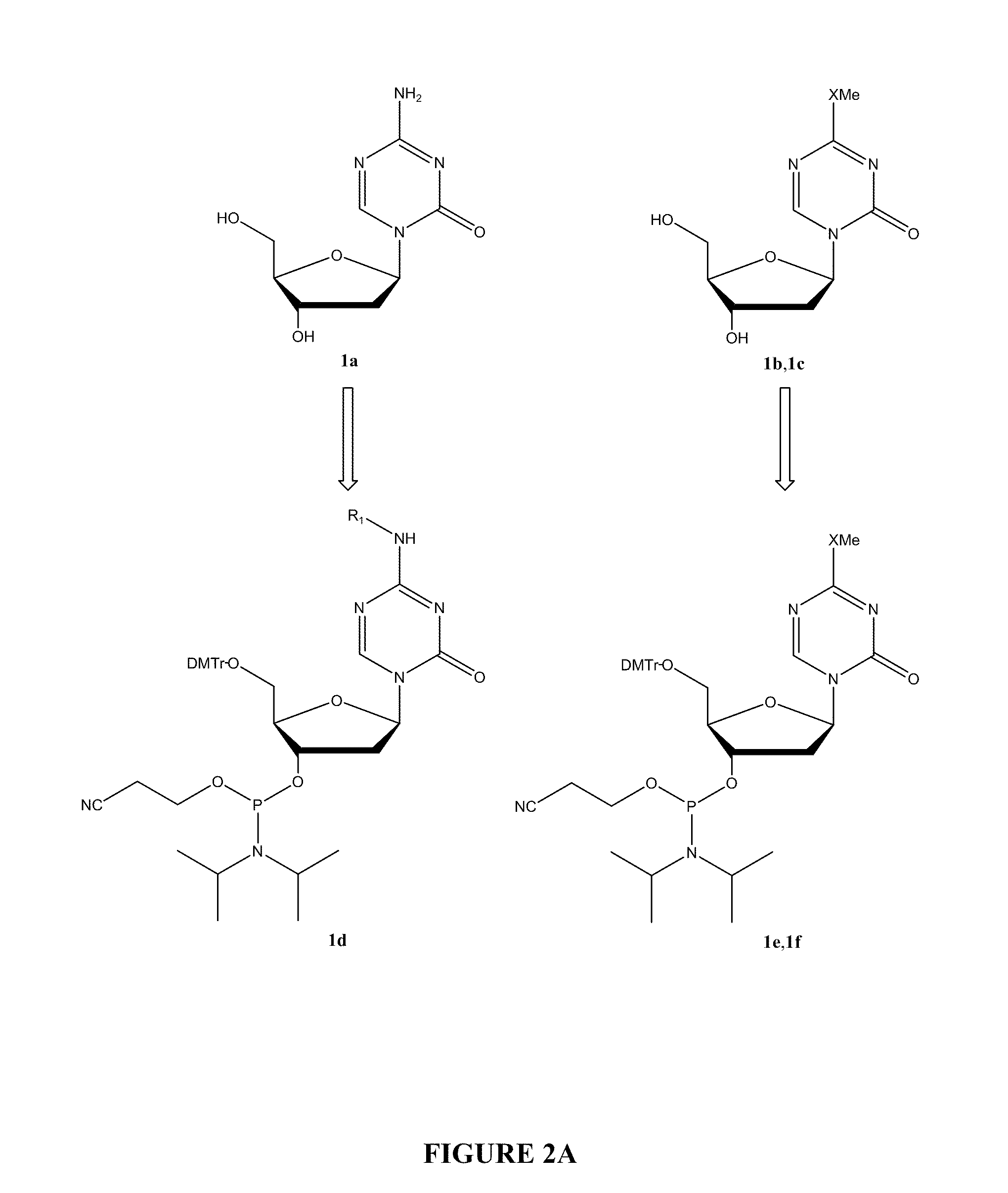
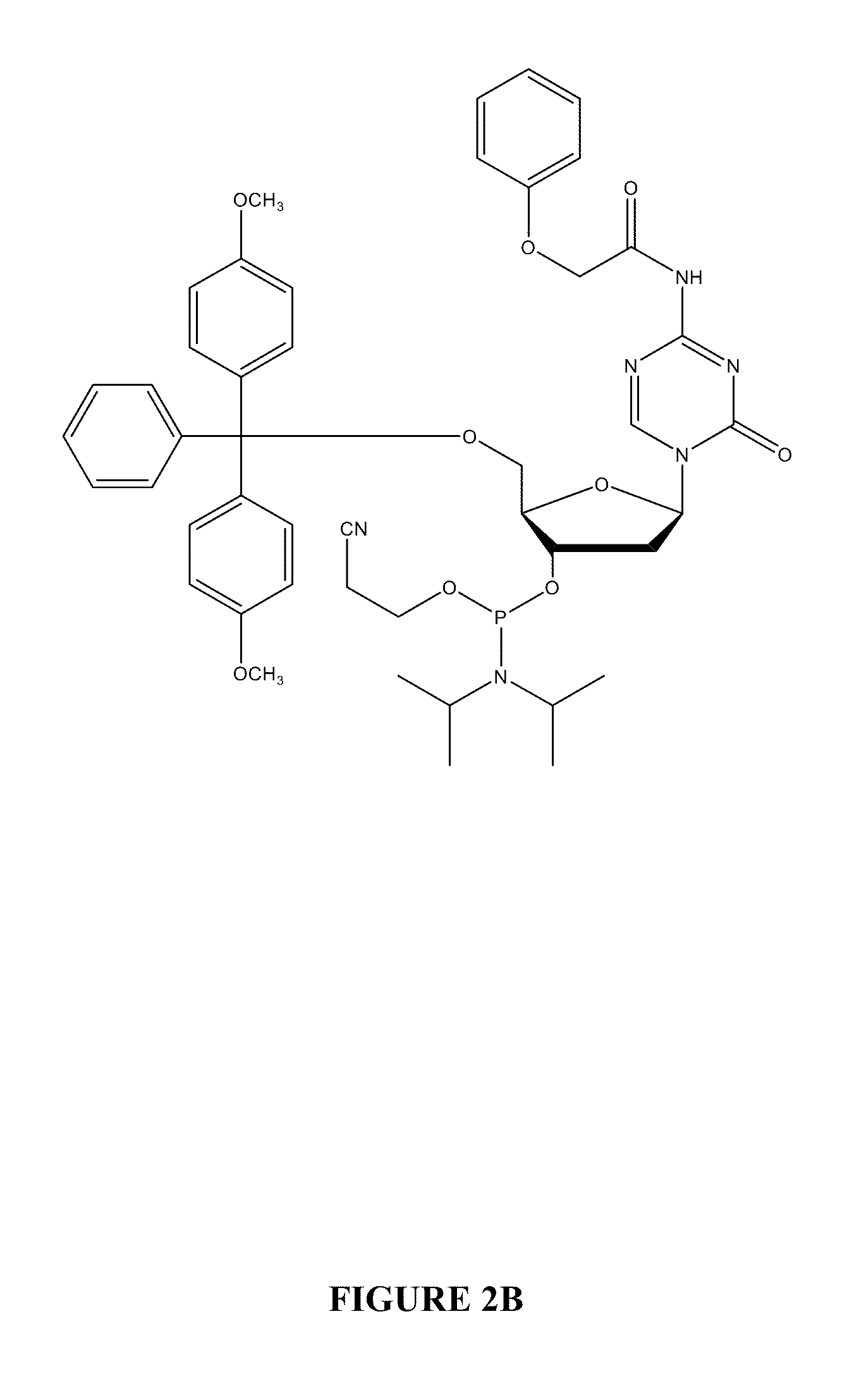
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Pharmaceutical formulation of decitabine
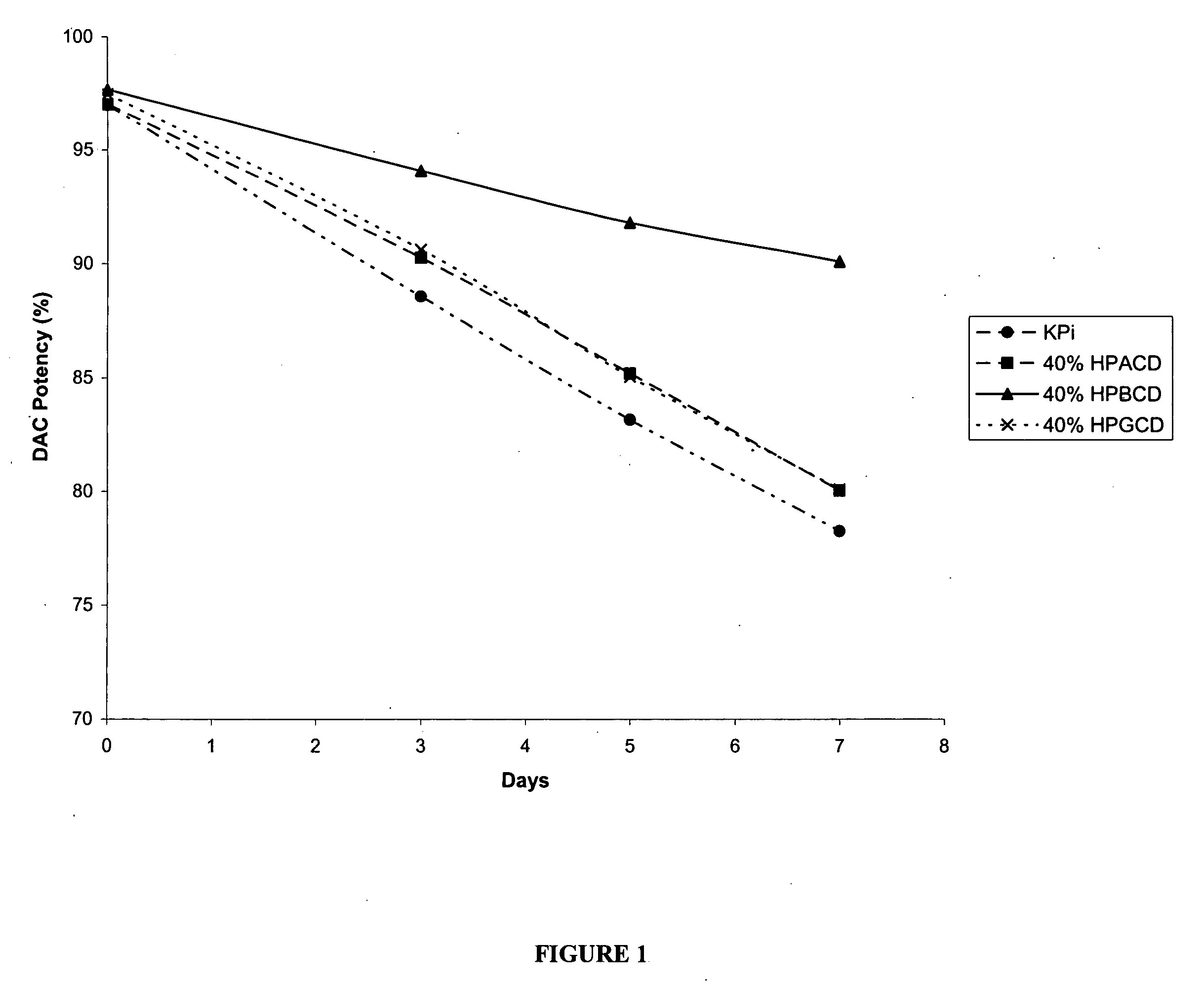
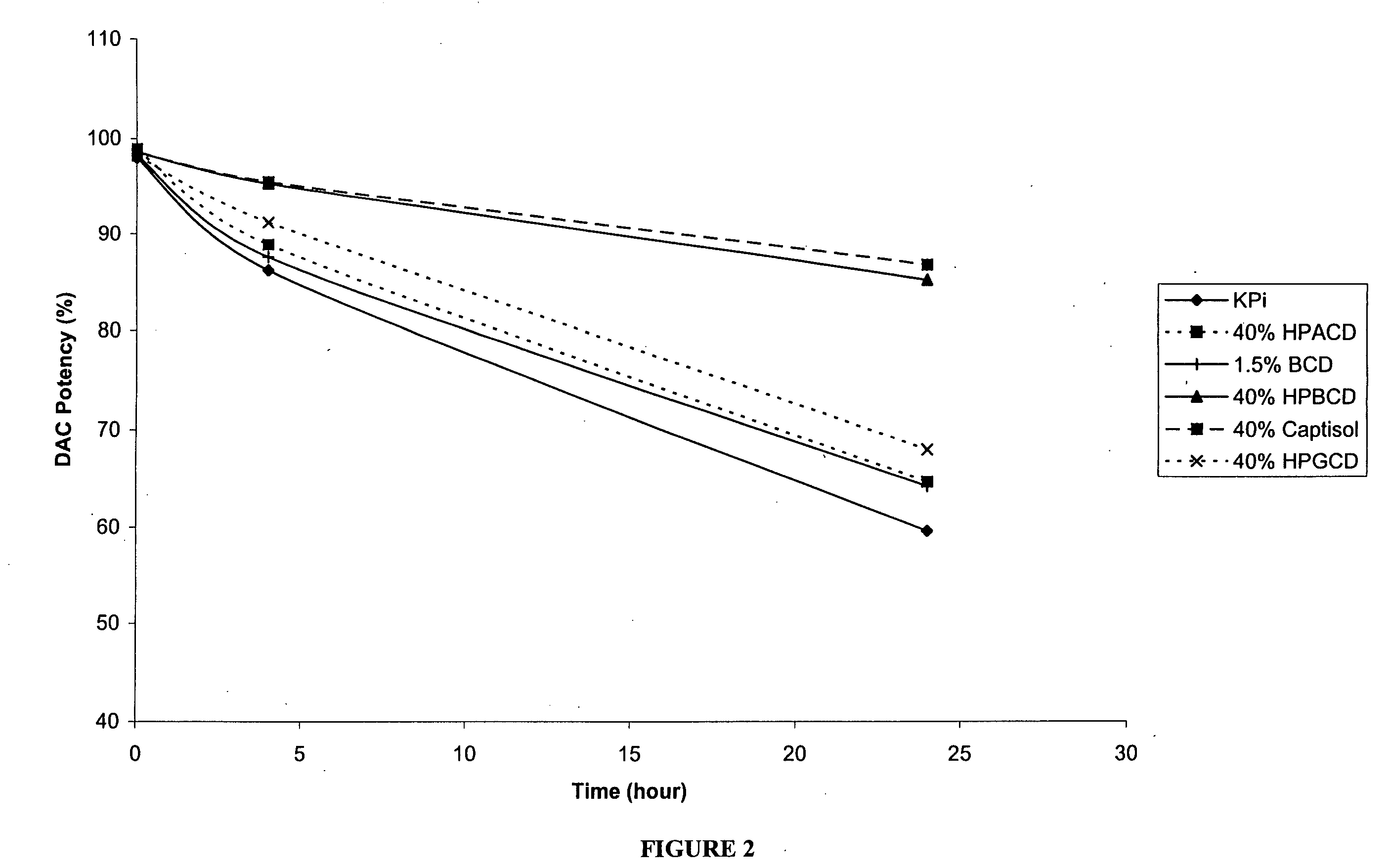
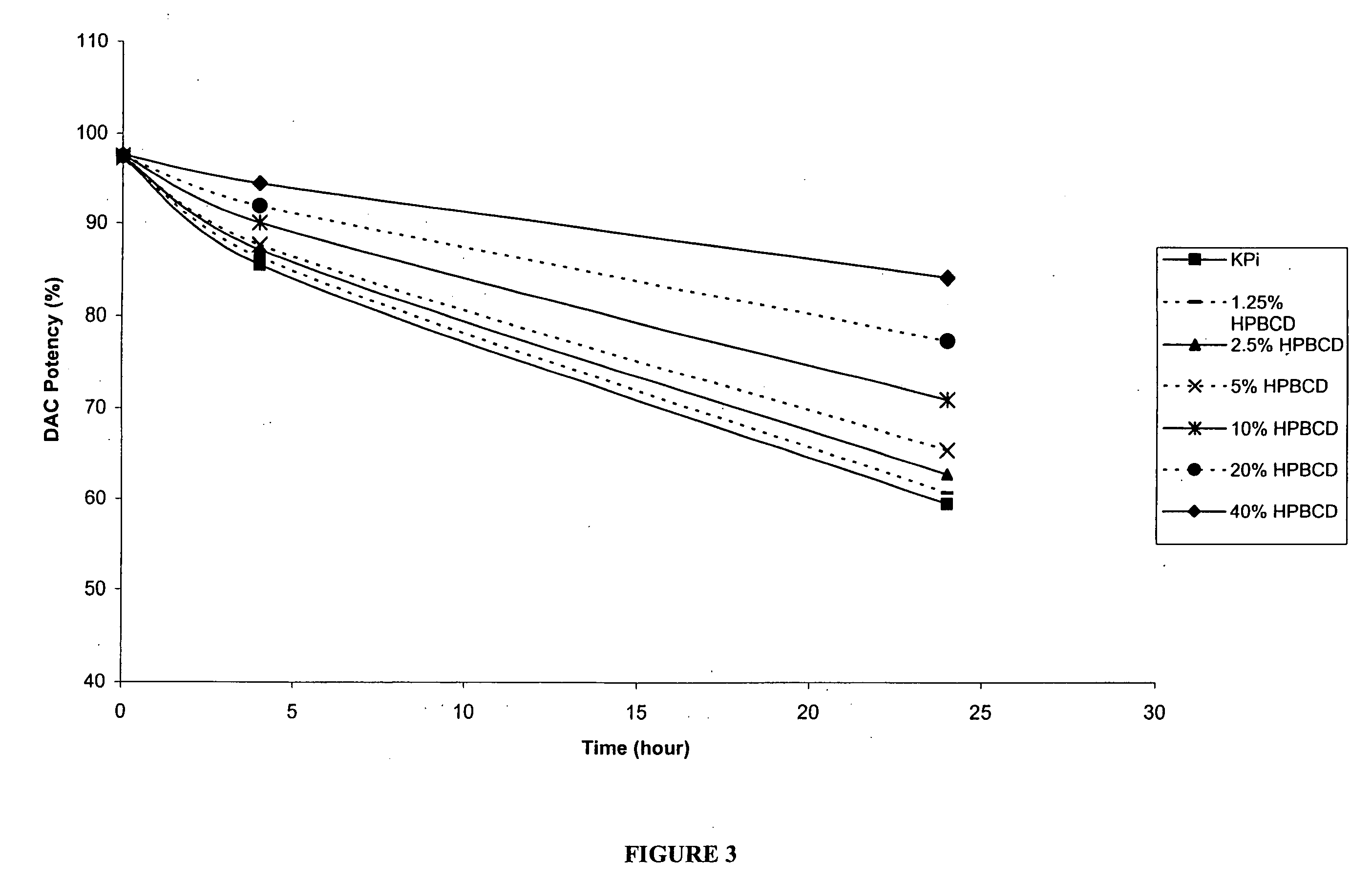
The present invention provides pharmaceutical formulations of decitabine or 5-aza-2′-deoxycytidine as well as methods of manufacturing the formulations. In particular, decitabine is formulated with a cyclodextrin compound to stabilize and / or enhance solubility of the drug. Kits and methods for using the pharmaceutical formulations are also provided, including methods of administering decitabine to treat conditions or diseases, such as cancer and hematological disorders.
Owner:SUPERGEN
Oligonucleotide analogues incorporating 5-aza-cytosine therein
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
N2S2 chelate-targeting ligand conjugates
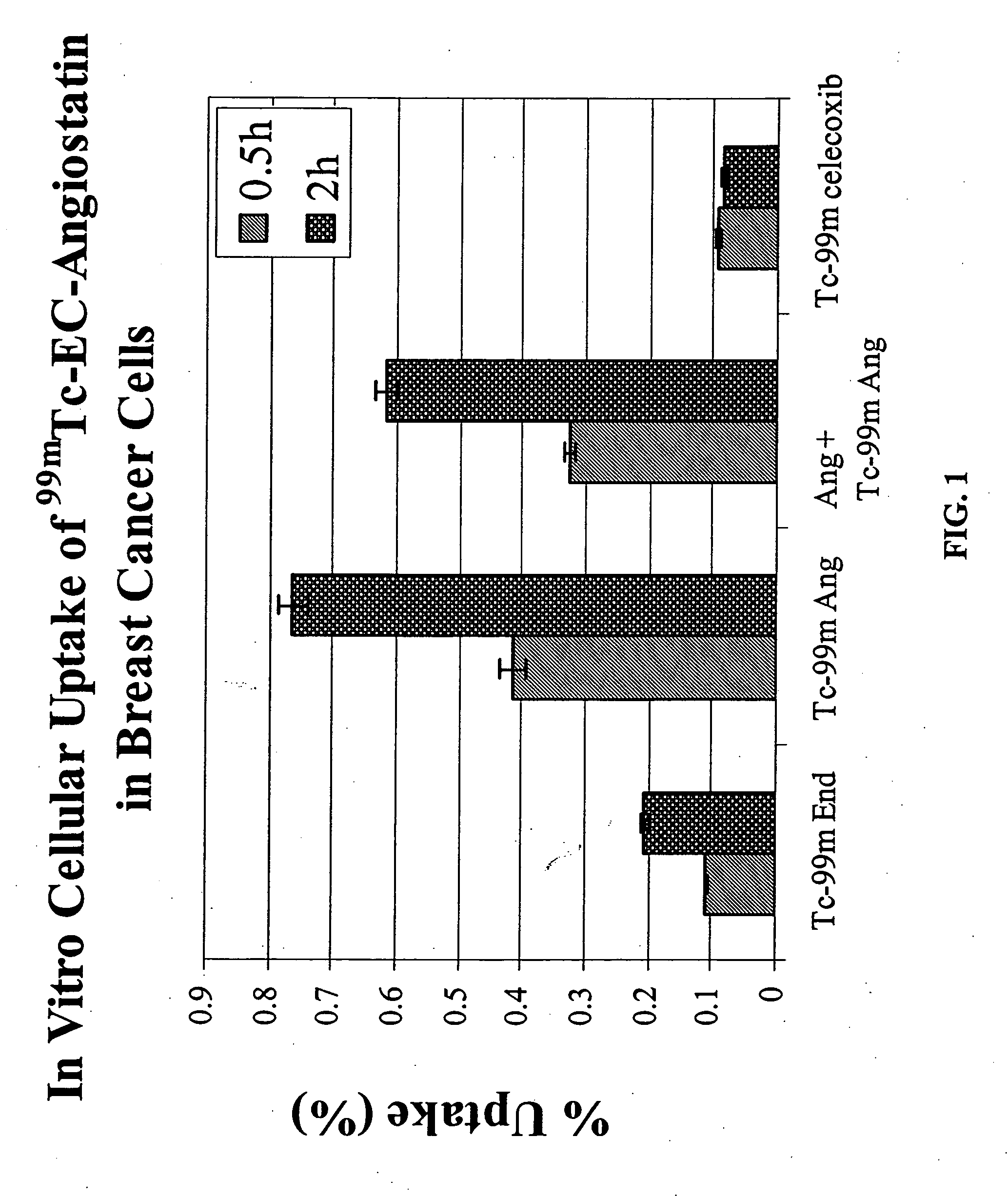
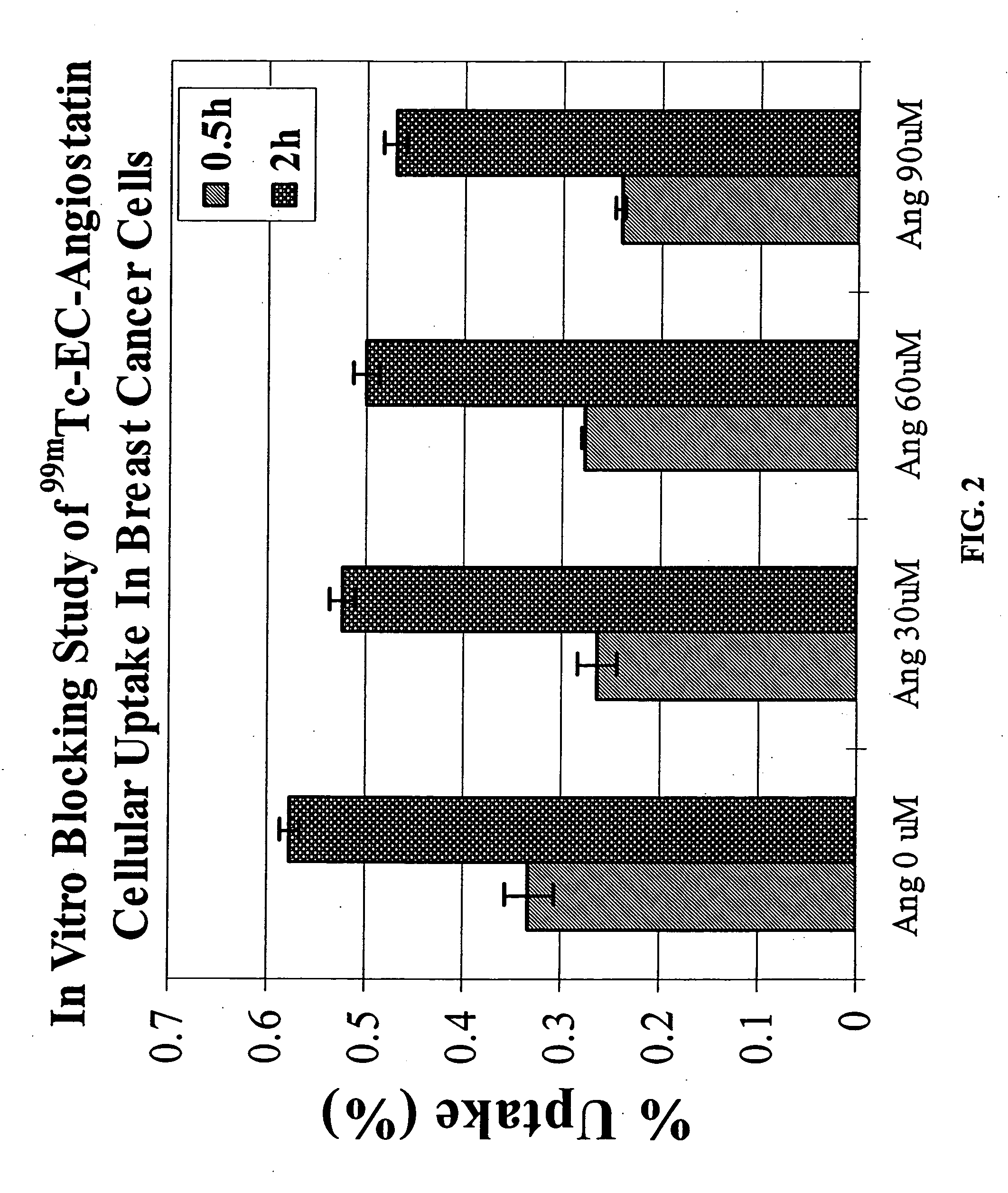
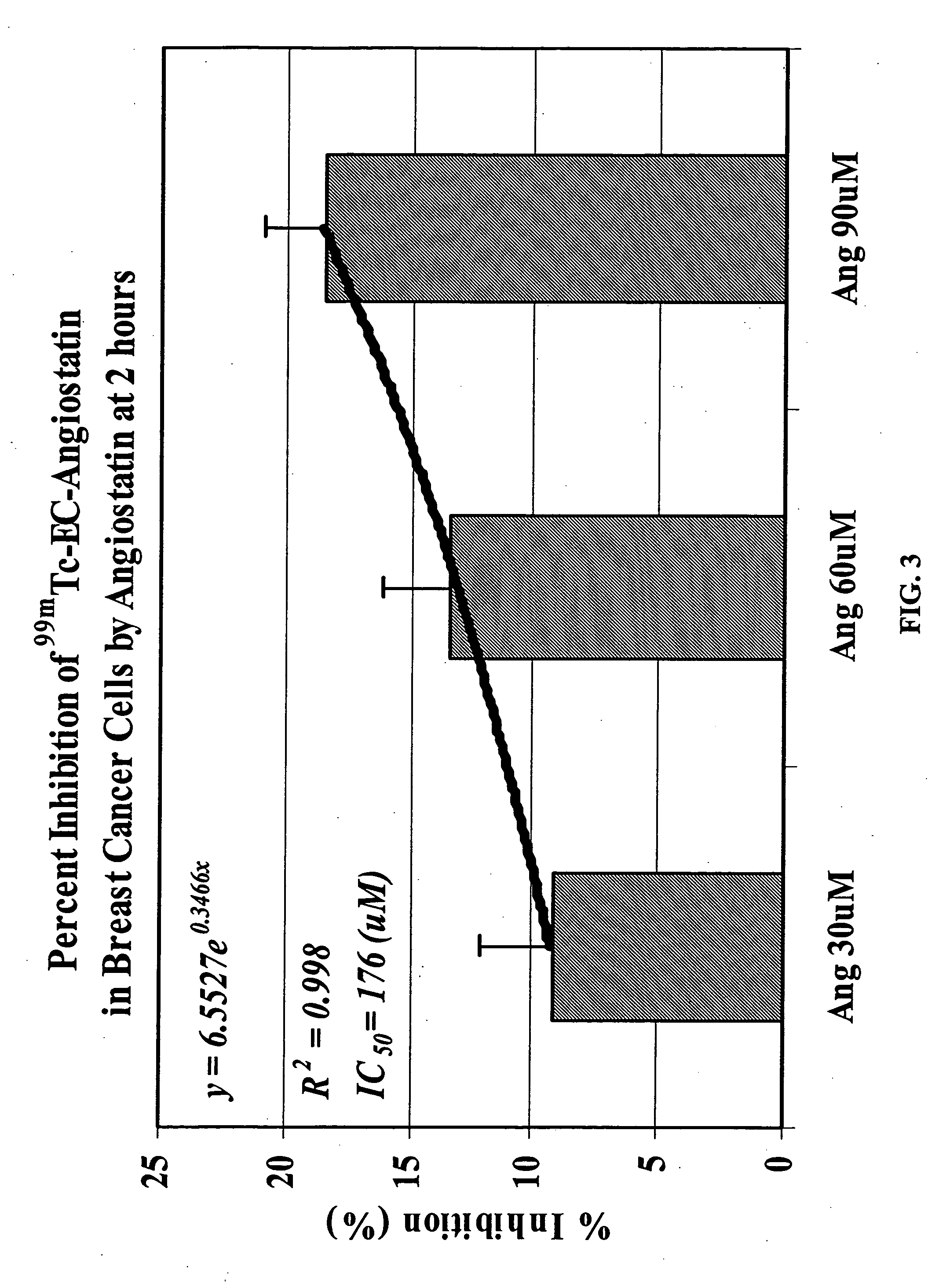
ActiveUS20050129619A1Sufficient amountHybrid immunoglobulinsRadioactive preparation carriersAngiostatinAbnormal tissue growth
The invention provides, in a general sense, a new labeling strategy employing compounds that are are N2S2 chelates conjugated to a targeting ligand, wherein the targeting ligand is a disease cell cycle targeting compound, a tumor angiogenesis targeting ligand, a tumor apoptosis targeting ligand, a disease receptor targeting ligand, amifostine, angiostatin, monoclonal antibody C225, monoclonal antibody CD31, monoclonal antibody CD40, capecitabine, a COX-2 inhibitor, deoxycytidine, fullerene, herceptin, human serum albumin, lactose, leuteinizing hormone, pyridoxal, quinazoline, thalidomide, transferrin, or trimethyl lysine. The present invention also pertains to kits employing the compounds of interest, and methods of assessing the pharmacology of an agent of interest using the present compounds.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for testing sequence of nucleic acid single molecule
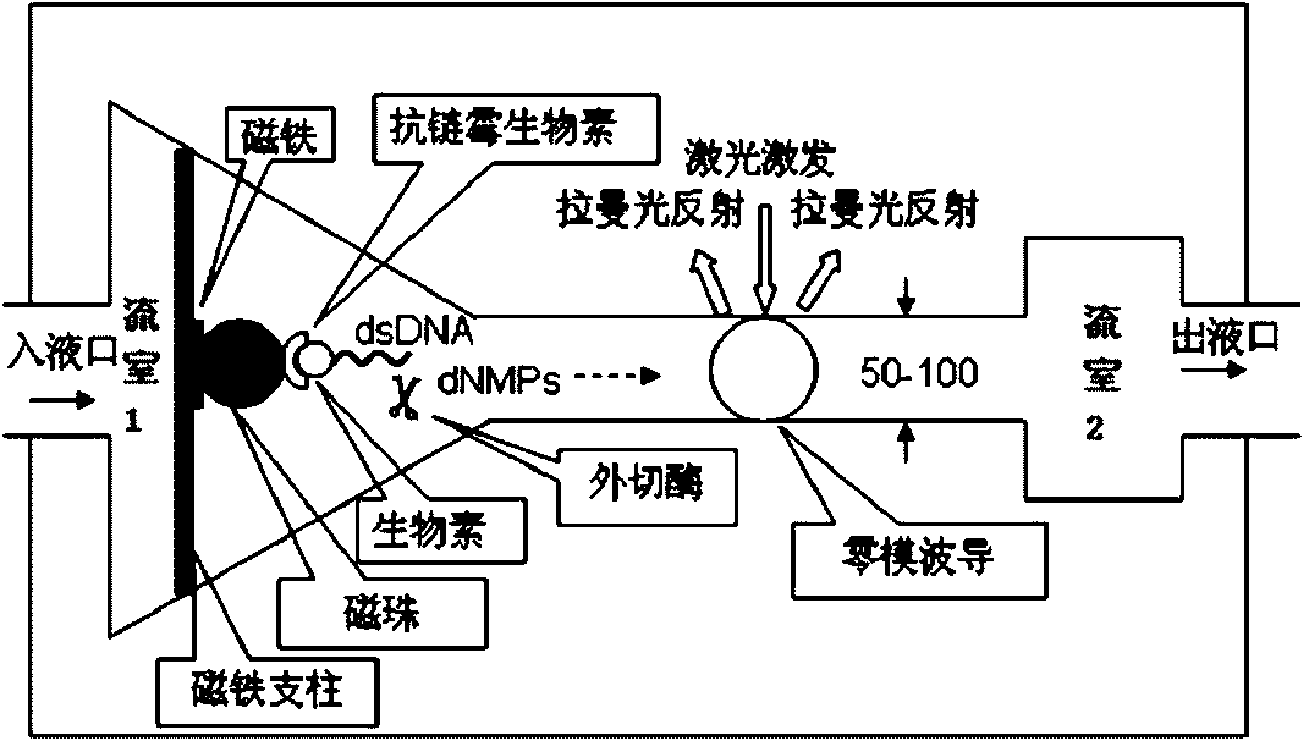
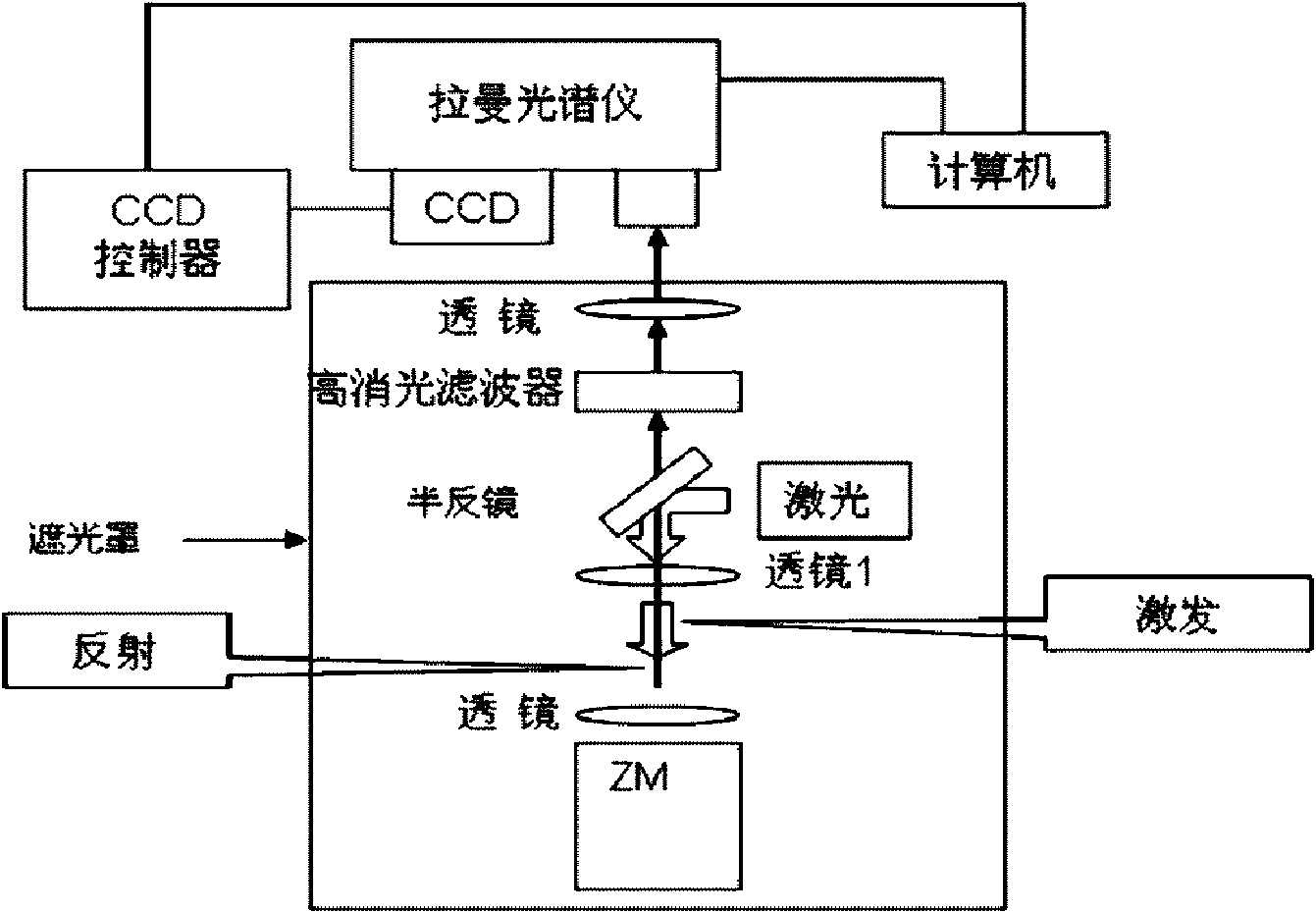
InactiveCN101654712AFast measurementHigh assay qualityMicrobiological testing/measurementRaman scatteringPhosphoric acidWaveguide
The invention relates to a method for testing a sequence of a nucleic acid single molecule in the technical field of biology, which comprises the following steps: respectively detecting Raman spectrumsignals of deoxyadenosine 5'-phosphoric acid, deoxyguanosine 5'-phosphoric acid, deoxycytidine 5'-phosphoric acid, deoxythymidine 5'-phosphoric acid, methyldeoxycytidine 5'-phosphoric acid, adenosine5'-phosphoric acid, vernine 5'-phosphoric acid, cytidine 5'-phosphoric acid and uridine 5'-phosphoric acid and establishing a standard curve; cutting a nucleic acid molecule to be detected by exonclease and detecting the Raman spectrum signal transmitted by a cut product when passing through a zero mode waveguide by the trigger of a laser; converting the Raman spectrum signal obtained in the step2 into concrete ribotide according to the standard curve obtained in the step 1 and obtaining the concrete sequence of the nucleic acid molecule to be detected by combining the cutting direction of the exonclease. The method can directly detect a natural nucleic acid sequence without a mark and has high detecting speed and detecting quality and low detecting cost.
Owner:SHANGHAI JIAO TONG UNIV
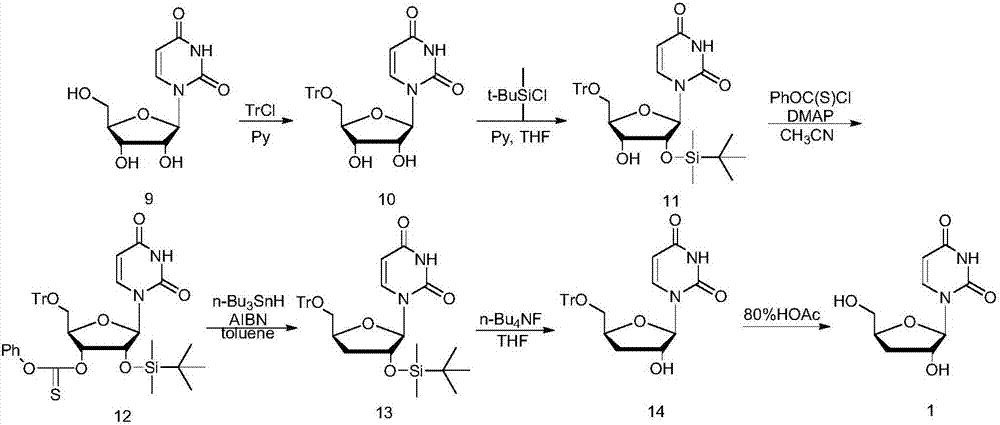
Methods for preparing 5-Aza-2'-deoxycytidine and intermediate product thereof
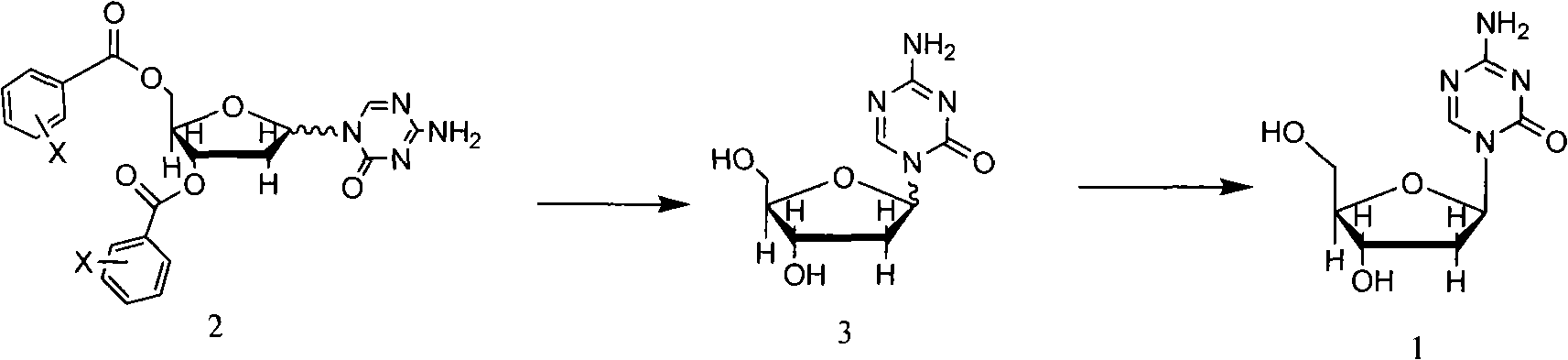
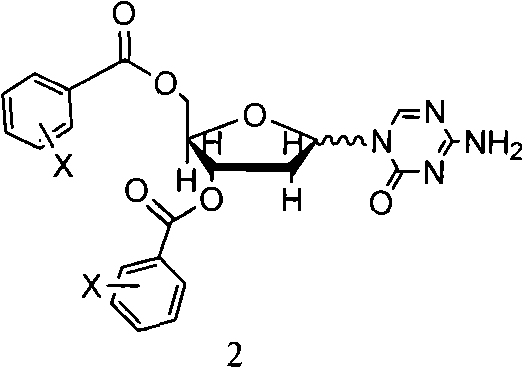
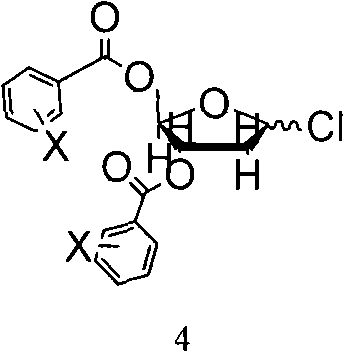
InactiveCN101570559AHigh yieldHigh puritySugar derivativesDrug compositionsSeparation technologyDisplacement reactions
The invention relates to methods for preparing 5-Aza-2'-deoxycytidine and an intermediate product thereof. The method for preparing the preparing 5-Aza-2'-deoxycytidine comprises the following steps: a compound (shown in formula 2) is hydrolyzed under alkaline matter to remove acyl-oxygen protecting group, a diastereomeric compound (shown in formula 3) is obtained, and then the diastereomeric compound is separated by a general separation technology to obtain an isomer. The invention also relates to a compound (shown in formula 2) used as the intermediate product and a preparation method thereof, wherein the preparation method comprises the following steps: 5-Aza cytimidine is activated by a silanization method, and then makes displacement reaction with a compound (shown in formula 4), and further removes the protecting group to obtain the compound (shown in formula 2). The preparation method has simpler process, good reproduction quality and little toxicity and is suitable to the industrialized production; and meanwhile, used raw materials and reagent are easy to obtain.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Oligonucleotide analogues incorporating 5-aza-cytosine therein
ActiveUS20100215729A1Heavy metal active ingredientsGenetic material ingredientsDiseaseHuman DNA sequencing
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Novel HIV integrase inhibitors and HIV therapy based on drug combinations including integrase inhibitors
InactiveUS20050049242A1Strong synergyBiocideAnimal repellantsCombination drug therapyResistant virus
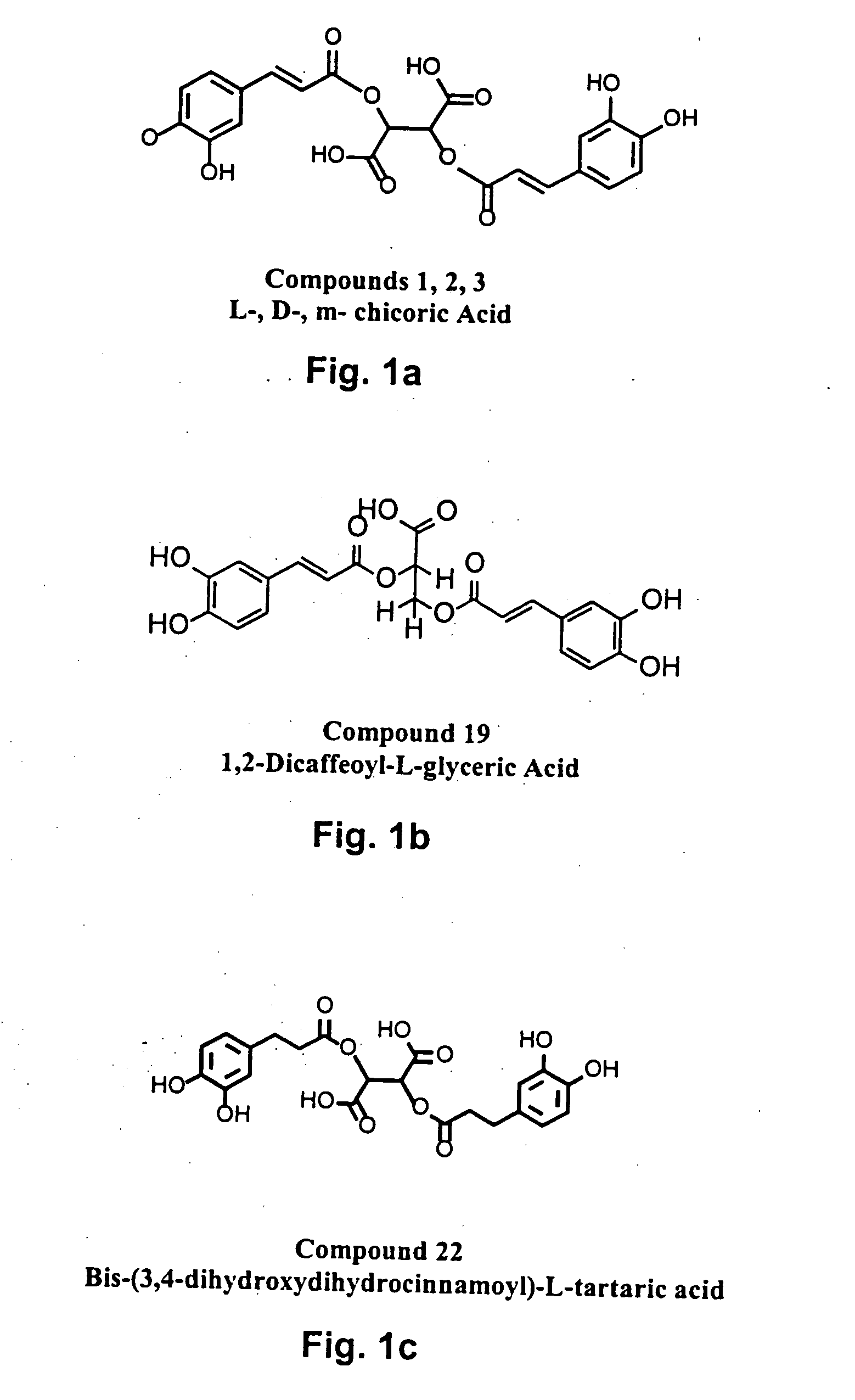
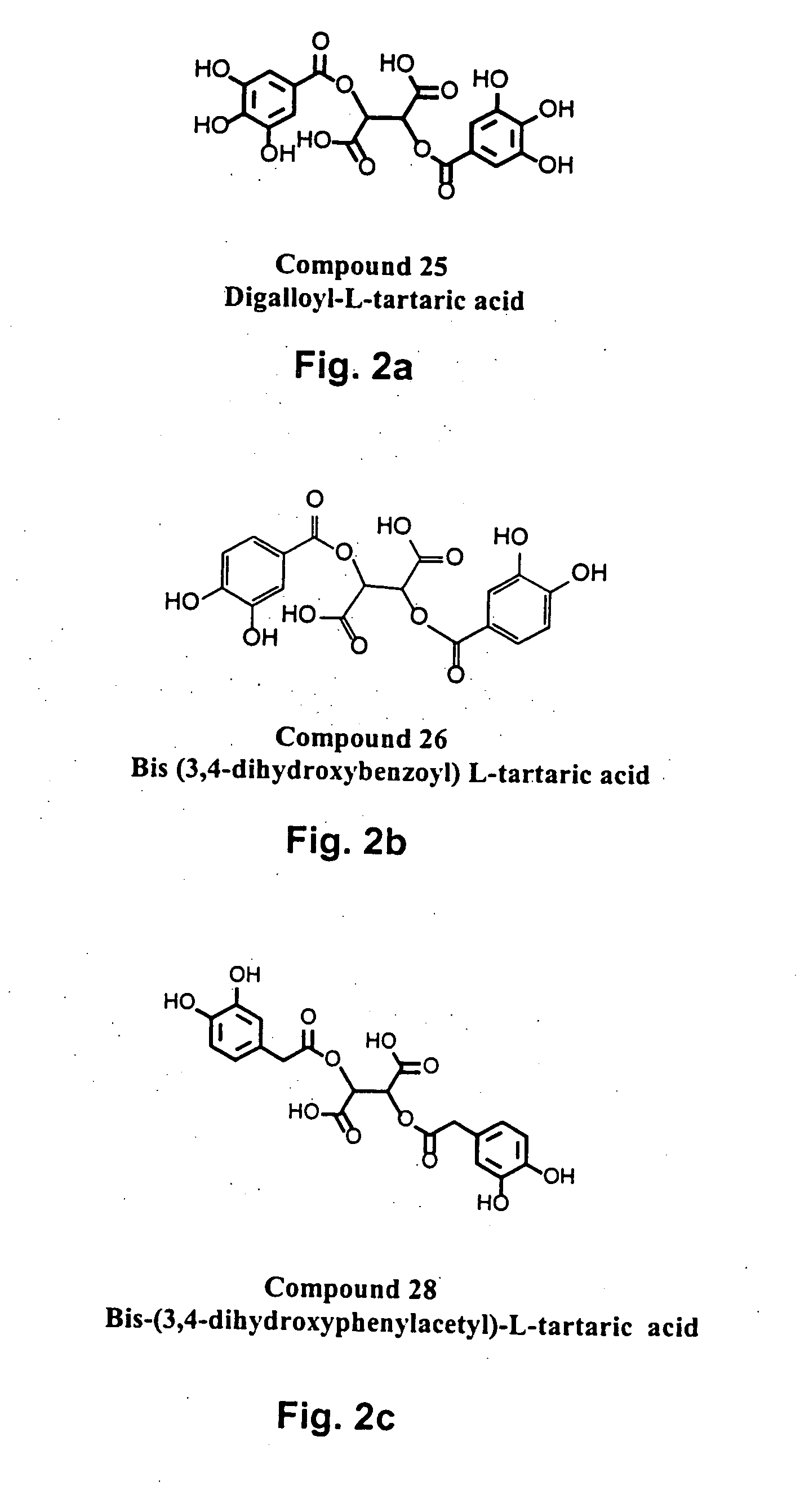
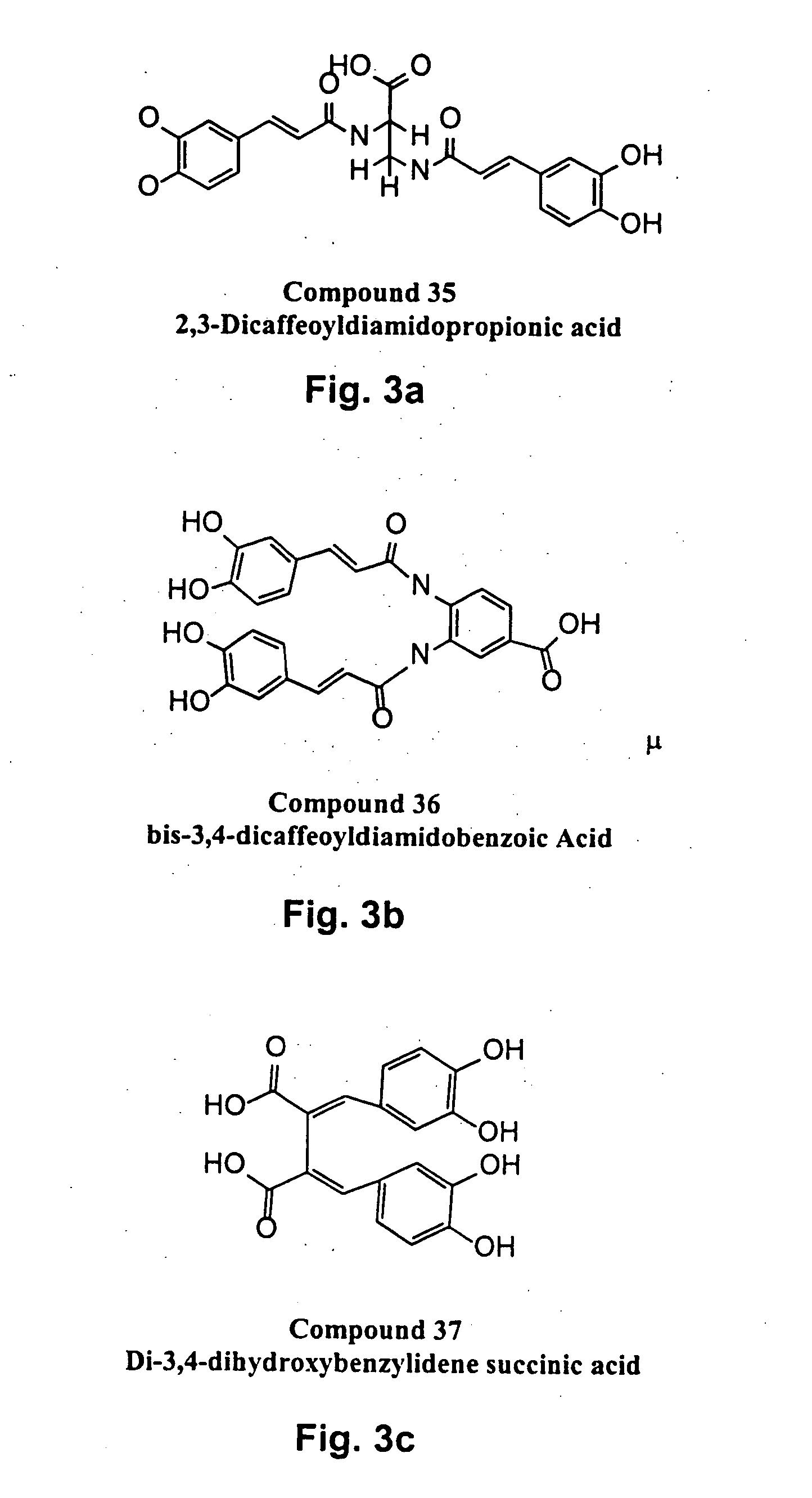
The present invention includes a group of novel compounds that are demonstrated to potently and selectively inhibit HIV integrase (IN) activity in vitro and to potently inhibit HIV replication in live, cultured cells at non-toxic concentrations. The novel compounds disclosed include 2,3-di(3,4-dihydroxy-dihydroxydihydrocinnamoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxybenzoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxyphenylacetyl)-L-tartaric acid, 2,3-di-(3,4,5-trihydroxybenzoyl-L-tartaric acid, 2,3-dicaffeoyldiamidopropionic acid, 1,2,-dicaffeoyl-L-glyceric acid, bis,-3,4-dicaffeoyldiamidobenzoic acid, di-3,4-dihydroxybenzylidene succinic acid, di-3,4-dihydrodihydroxybenzylidine succinic acid, 2,3-dicaffeoyl-L-serine, bis-dicaffeoyl-L-isoserine and 1,4-dicaffeoyl-L-lysine. Tests of integrase inhibitors with 2′,3′-dideoxycytidine, zidovudine and nelfinavir (protease inhibitor) indicated a potent synergy against reverse transcriptase inhibitor resistant virus. The potential benefit from the addition of integrase inhibitors to combination drug therapies is significant.
Owner:ROBINSON W EDWARD JR +2
Periplaneta americana preparation and applications thereof in preparing medicines for treating gastric cancer
ActiveCN105878289APrevent proliferationInhibit expressionOrganic active ingredientsAnthropod material medical ingredientsPharmaceutical drugGastric carcinoma
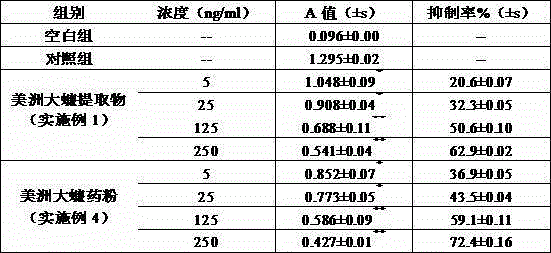
The invention provides a periplaneta americana preparation and applications of the periplaneta americana preparation in preparing medicines for treating gastric cancer. Periplaneta americana is treated by adopting an extraction process or an ultralow-temperature grinding process. The research shows that periplaneta americana extract and periplaneta americana medicinal powder can effectively inhibit the expression of VEGF in gastric cancer cells SGC-7901, and have an obvious inhibiting effect for the proliferation of the gastric cancer cells or gastric cancer tumors. The research further shows that after the periplaneta americana extract and the periplaneta americana medicinal powder are respectively mixed with 5-aza-2'-deoxycytidine for use, the inhibition ratios for the proliferation of the gastric cancer cells SGC-7901 cultured in vitro are high, and are respectively 75.1% and 83.9%; after the periplaneta americana extract and the periplaneta americana medicinal powder are mixed with TNP-470 for use, the tumor inhibition rates for the transplantation tumor of tumor-bearing mice are respectively 75.3% and 86.5%.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
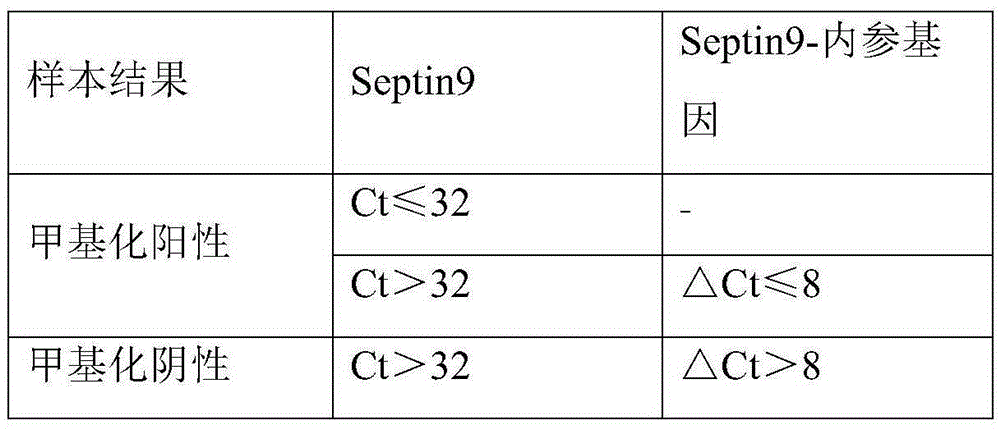
Detection reagent kit for methylation of septin 9 genes in human peripheral blood circulation tumor DNA
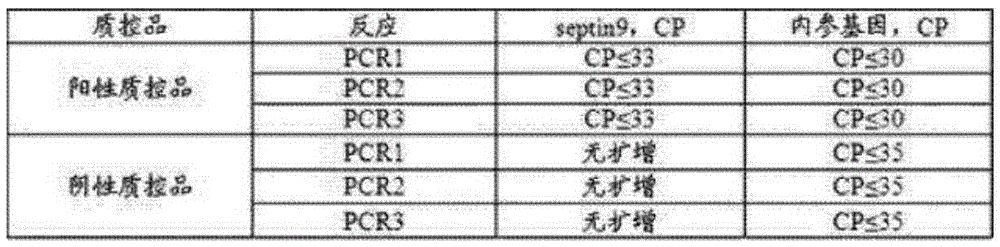
ActiveCN105331727AEasy to detectEasy to operateMicrobiological testing/measurementCpG siteOperability
Owner:湖南宏雅基因技术有限公司
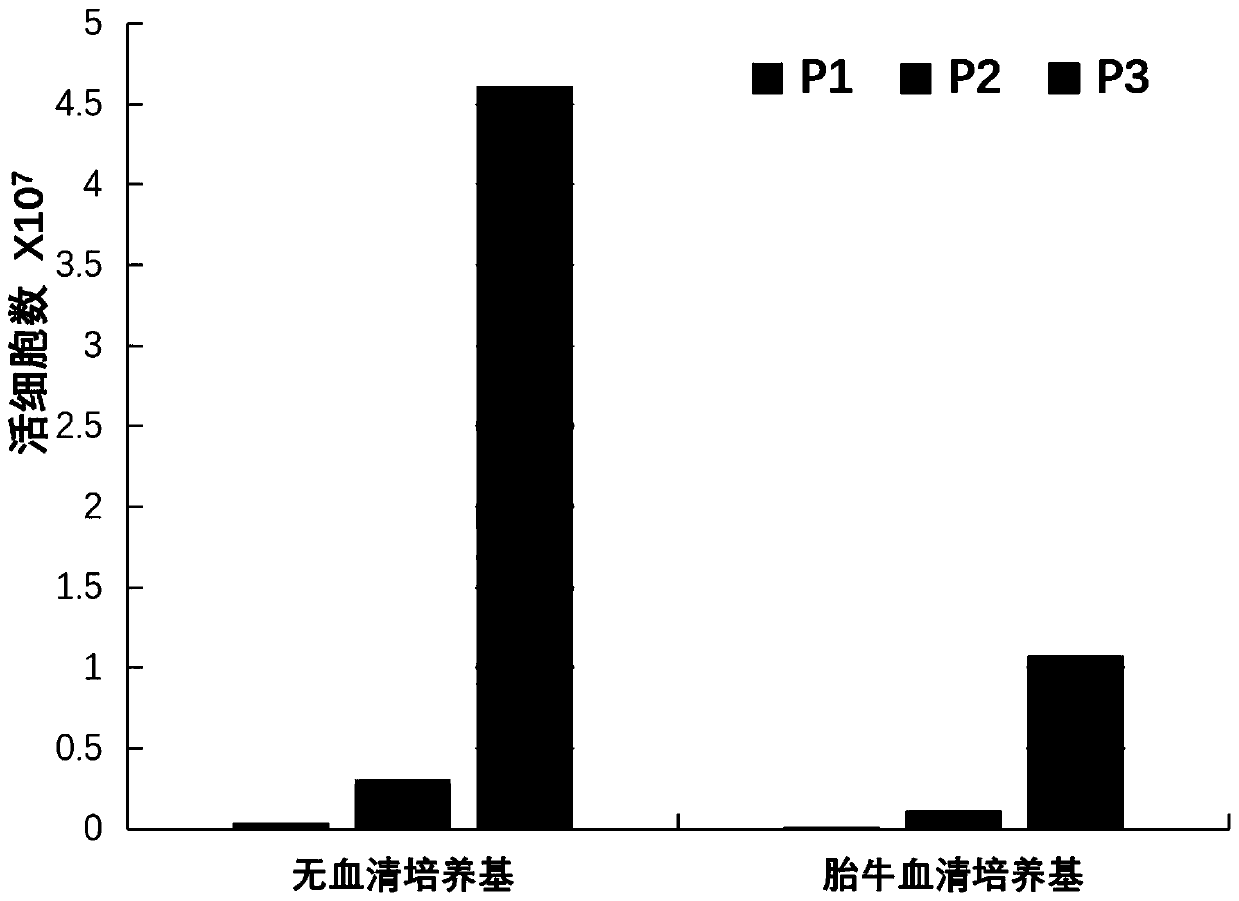
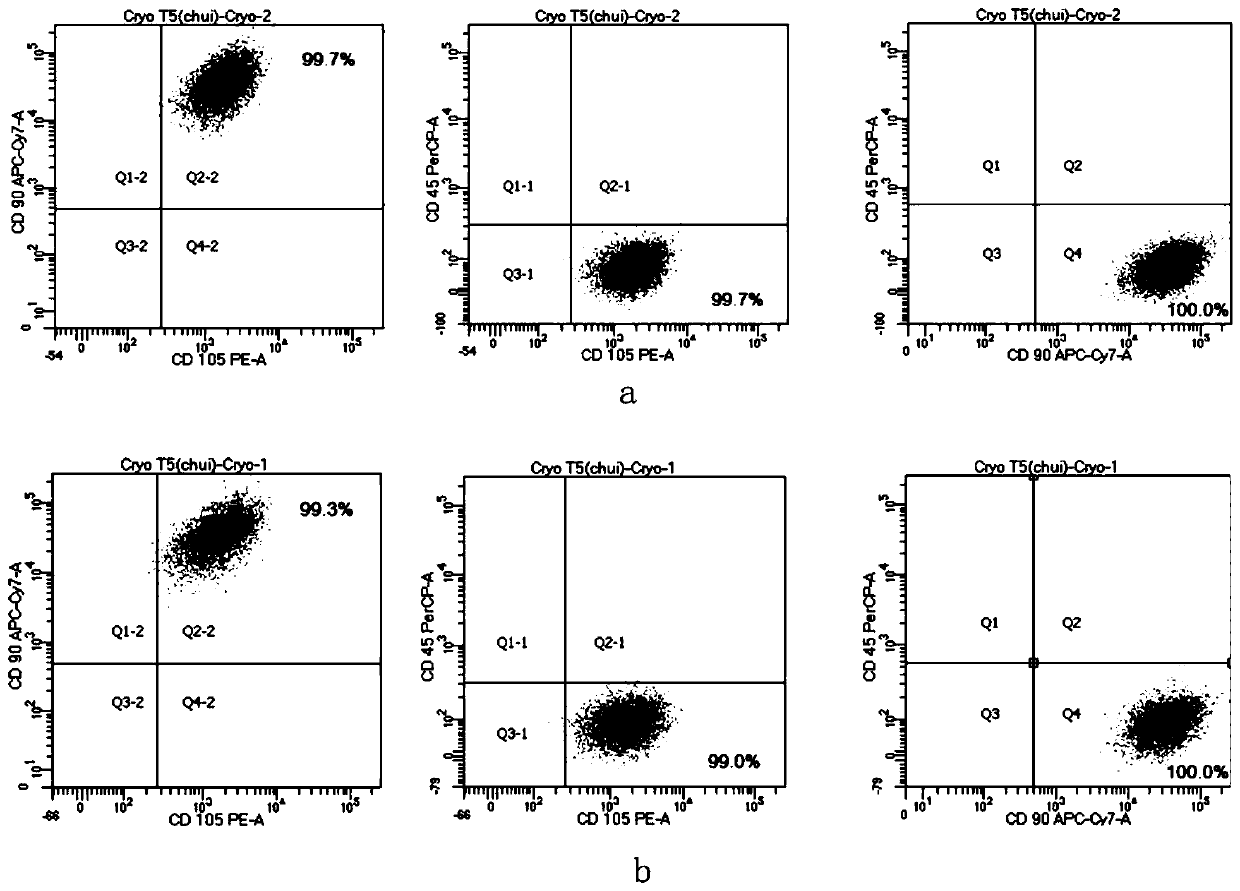
Serum-free culture medium for mesenchymal stem cells and application thereof
ActiveCN110331130AShort cycleImprove consistencyCulture processSkeletal/connective tissue cellsHeterologousCell cycle
The invention relates to a serum-free culture medium for mesenchymal stem cells and application thereof. The serum-free culture medium comprises a basal culture medium and additive components. The additive components comprise the following components: 2'-Deoxyadenosine, 2'-Deoxycytidine-HCl, 2'-Deoxyguanosine, L-glutamine, human serum albumin, recombinant human transferrin, recombinant human insulin, rhPDGF-BB, rhFGF-b, rhTGF-beta1, rhEGF, sodium selenate and hydrocortisone. The serum-free culture medium disclosed by the invention does not contain animal-derived heterologous component such asbovine serum, and can effectively replace serum to culture bone marrow mesenchymal stem cells; and due to the synergistic effect of the additive components, the culture medium has the advantages of shortening cell cycle, rapid proliferation and good cell consistency while culturing bone marrow mesenchymal stem cells, and can effectively maintain the molecular characteristics and differentiation potential of the bone marrow mesenchymal stem cells. The bone marrow mesenchymal stem cells obtained by the culture medium are suitable for further scientific research and clinical application research.
Owner:苏州依科赛生物科技股份有限公司
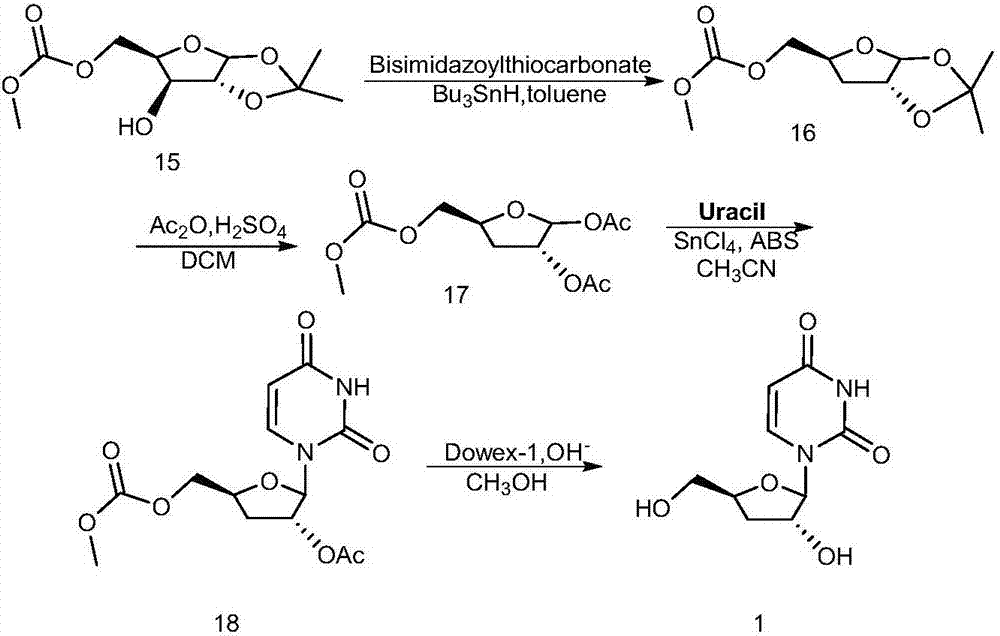
Preparation method of decitabine
ActiveCN101560233AShort reaction stepsLow costSugar derivativesSugar derivatives preparationAcetic anhydrideIon-exchange resin
Owner:SHANGHAI QINGSONG PHARMA
Novel application of 5-bit modified 2'deoxycytidine derivative or phosphate thereof in the preparation of medicaments
ActiveCN102125579AInhibition retentionInhibit synthesisOrganic active ingredientsAntineoplastic agentsPhosphateMedicine
The invention discloses application of a 5-bit modified 2'deoxycytidine analogue or phosphate thereof in the preparation of medicaments for treating cancers. The 5-bit modified 2' deoxycytidine analogue or the phosphate thereof can also be used in combination with deoxythymidine or phosphate thereof to form a medicinal composition for treating tumors. A 5-bit modified 2' deoxycytidine derivative is added with an appropriate amount of thymidine or 5' monophosphate when administrated, so that the toxicity to normal cells is greatly reduced to keep the effect of inhibiting tumor cell proliferation.
Owner:GUANGZHOU RIBOBIO
Method for preparing 2-deoxidized-2, 2-hydrochloric acid difluoro deoxycytidine
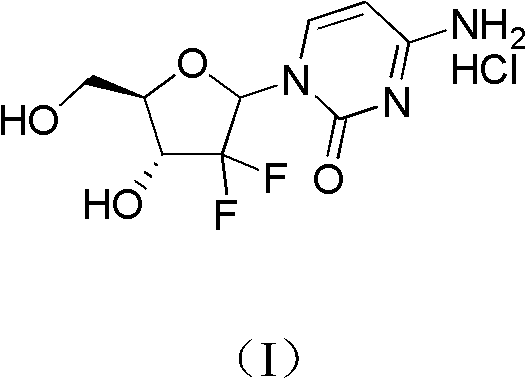
InactiveCN102617677ASimple and fast operationHigh yieldSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideChemistry
The invention relates to a 2-deoxidized-2, 2-hydrochloric acid difluoro deoxycytidine compound as shown in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method is simple in process, high in yield and quite suitable for industrial production, the purity of a product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing 2', 3'2-dideoxycytidine
In this invention, cytidine reacts with acetic oxide to produce 4-N-acetylcytidine, then reacting with hydrogen bromide in acetic acid saturated solution, in the presence of acitic oxide as catalyst to produce bromo-mixture; then reacting in the presence of Zn-Cu couple as catalyst to produce 4-N-acetyl-2,3-dihydro-2,3-didioxy cytidine-5-acetate; then, reacting, to proceed hydrogenation reaction, in the presence of Pd / C, to produce 4-N-acetyl-2,3-dideoxy guanosine-5-acetate; then, reacting in the presence of methanol and triethylamine to produce 2,3-dideoxy cytidine. This invention has advantages of: simple process, easy to operate, available raw material, high yield over 90%.
Owner:陆锦康
Method for ultra-sensitively simultaneously detecting multiple DNA glycosylases by using intrinsic fluorescent nucleotide
ActiveCN107083437AAchieving Simultaneous DetectionEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationA-DNAFluorophore
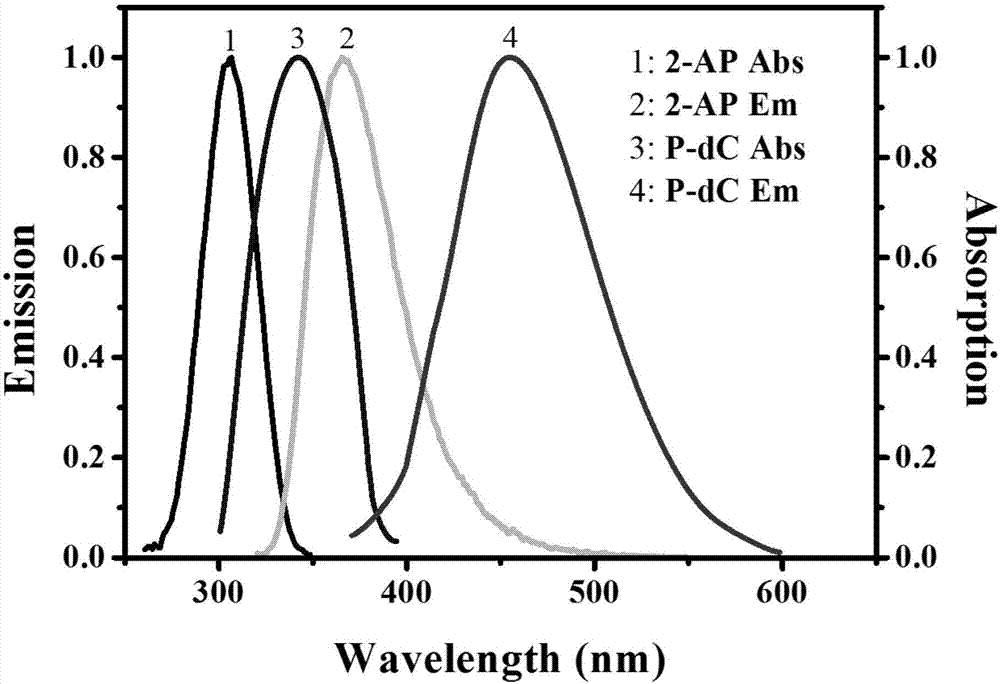
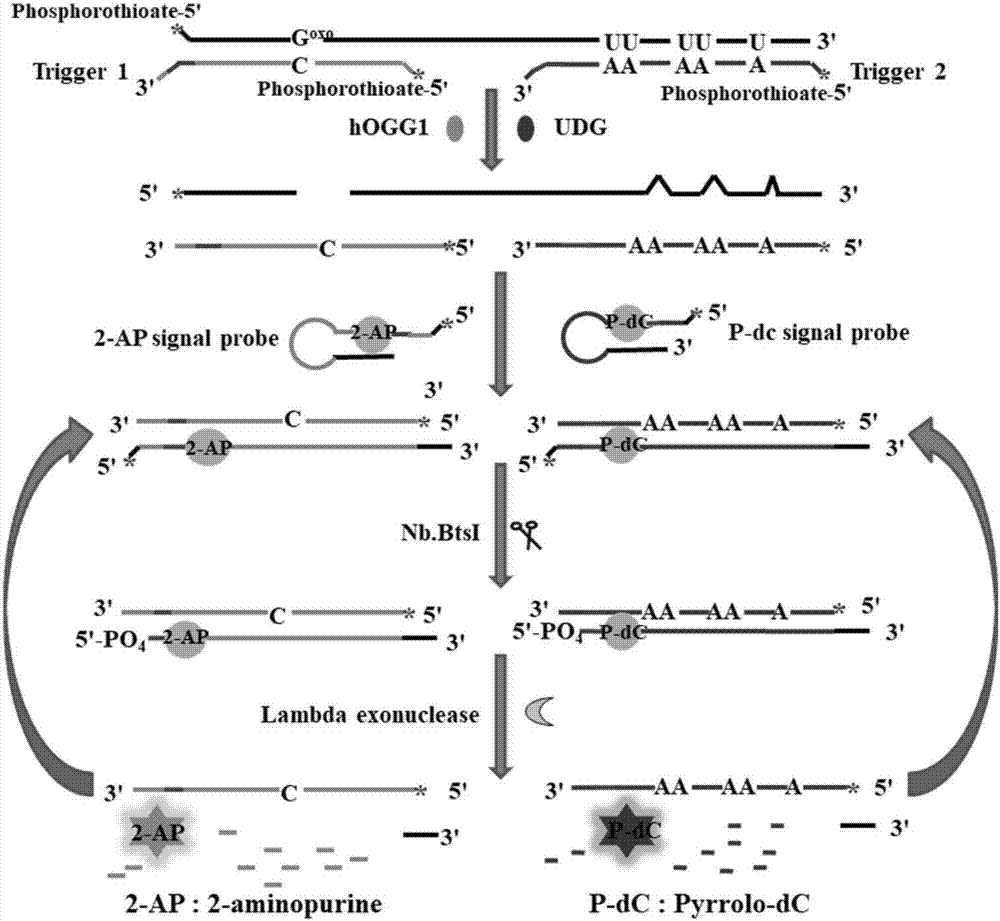
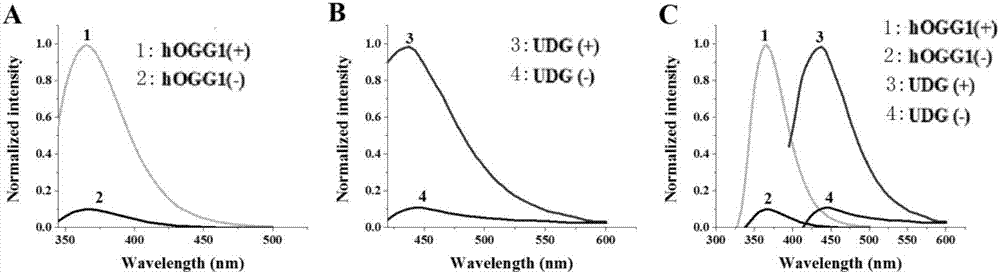
The invention discloses a method for ultra-sensitively simultaneously detecting multiple DNA glycosylases by using intrinsic fluorescent nucleotide. The method is a fast and sensitive fluorescence method for simultaneously detecting multiple DNA glycosylases by adopting 2-aminopurine and pyrrole-deoxycytidine as fluorophores and a DNA molecule as an intrinsic quenching agent and combining excision enzyme-assisted circulating signal amplification; and extra fluorophore and quenching group are not needed for marking, so that the target of simple, intuitive and high-sensitivity detection of an actual sample is achieved, and above all, simultaneous detection of multiple DNA glycosylases is achieved.
Owner:SHANDONG NORMAL UNIV
Dinucleotide crystals
The present invention is directed to crystals of P<1>-(2'-deoxycytidine 5'-)P<4>-(uridine 5'-)tetraphosphate (dCP4U) or a salt thereof and to a process for producing the crystals. The present invention also provides a process for producing dCP4U involving reacting uridine 5'-monophosphate (UMP), 2'-deoxycytidine 5'-monophosphate (dCMP), diphenyl phosphochloridate (DPC), and pyrophosphate (PPi). The crystals of dCP4U obtained through the process according to the present invention have high purity and high stability and no hygroscopicity as compared with a freeze-dried product, and thereby serve as a useful raw material for preparing a pharmaceutical. The process for producing dCP4U according to the present invention permits use of inexpensive UMP as a raw material and realizes high yield. Thus, the process is suitable for large-scale synthesis of dCP4U.
Owner:MERCK & CO INC
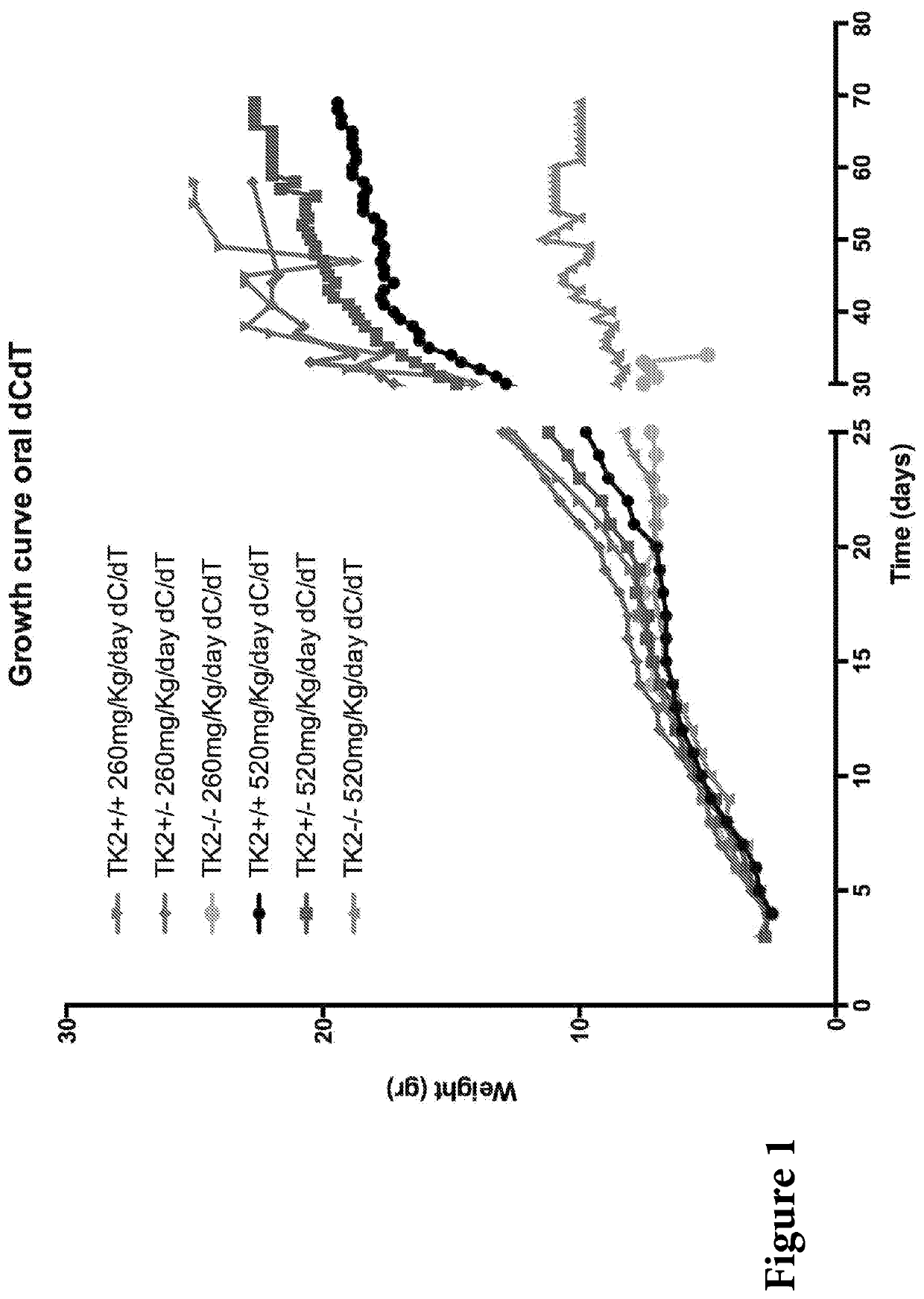
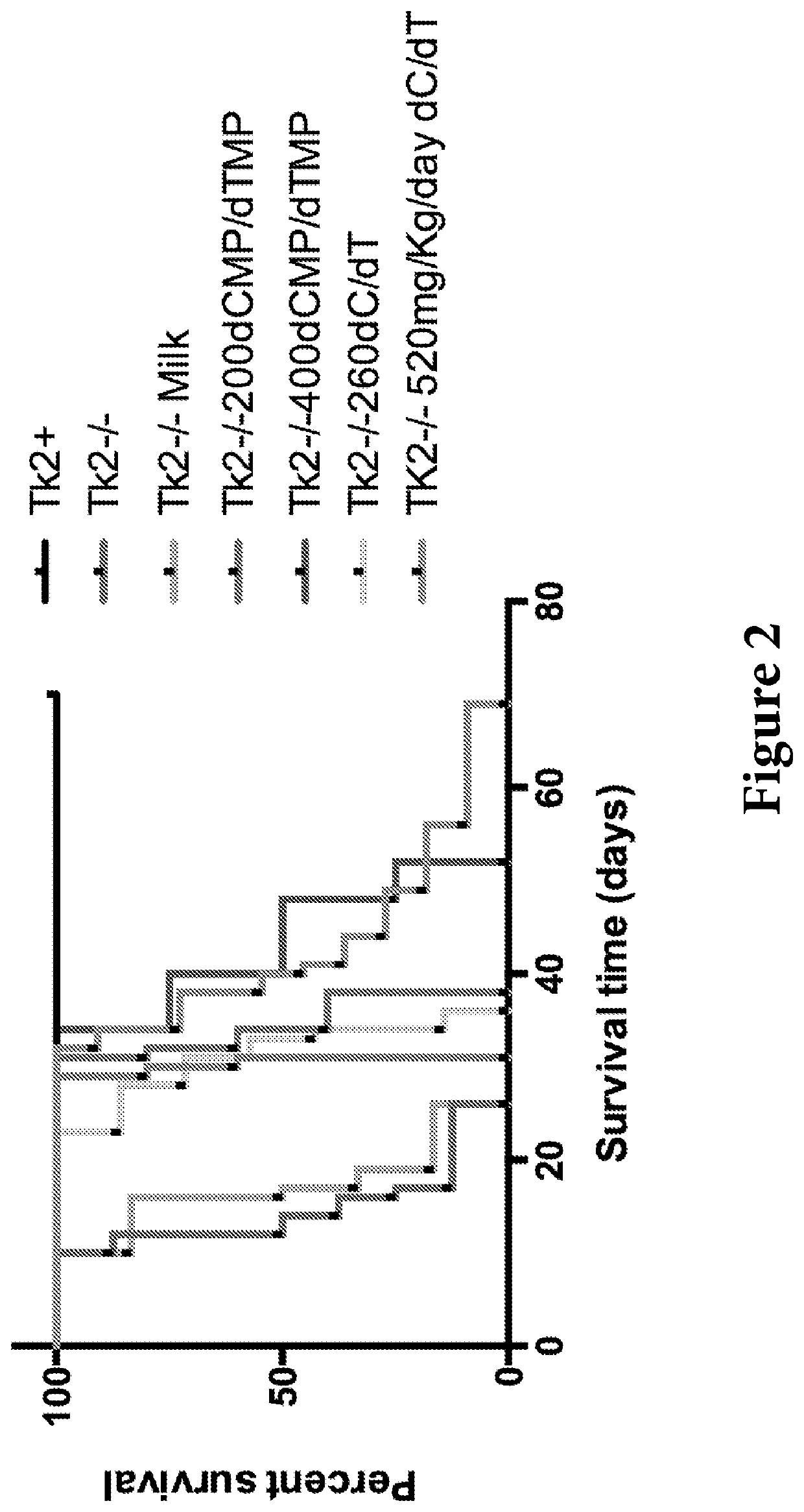
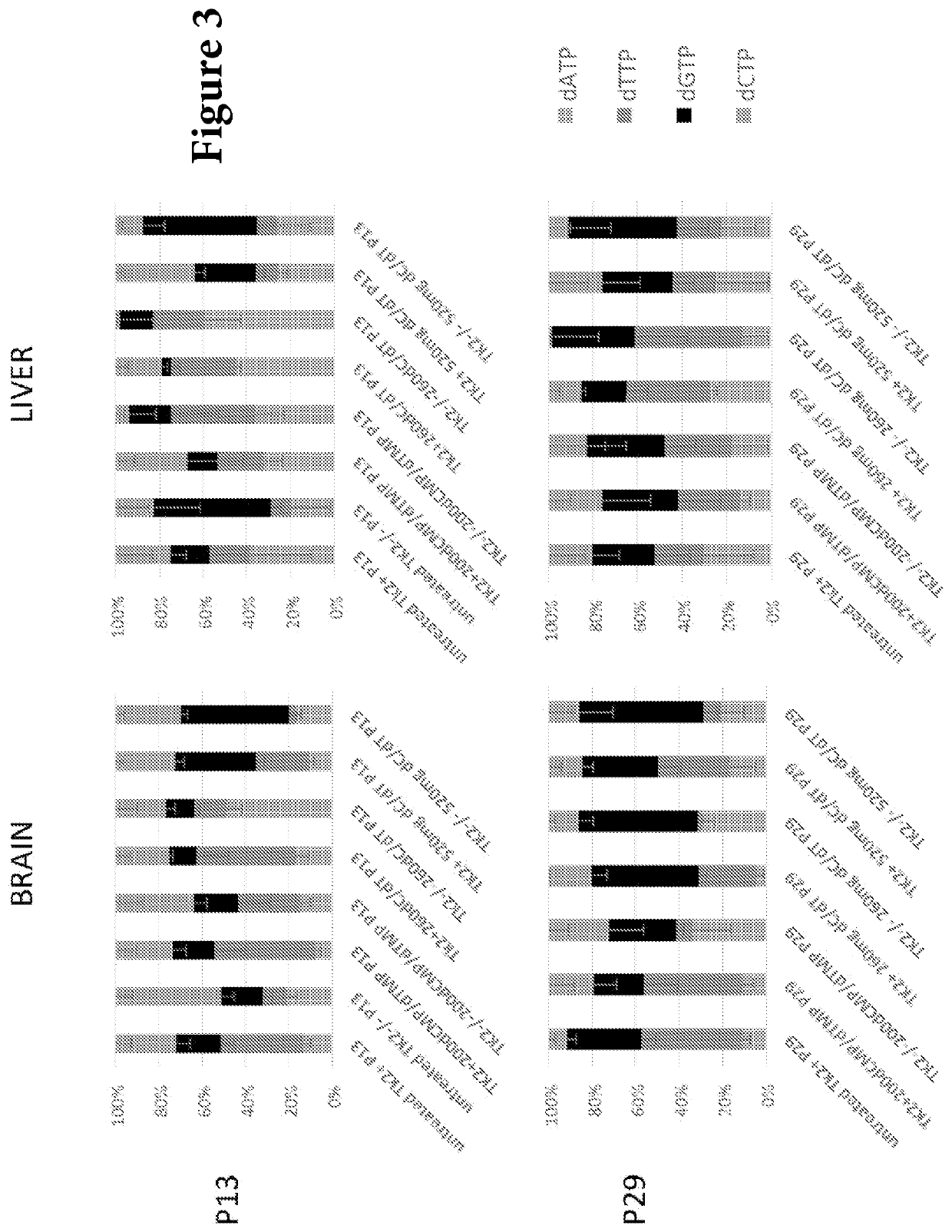
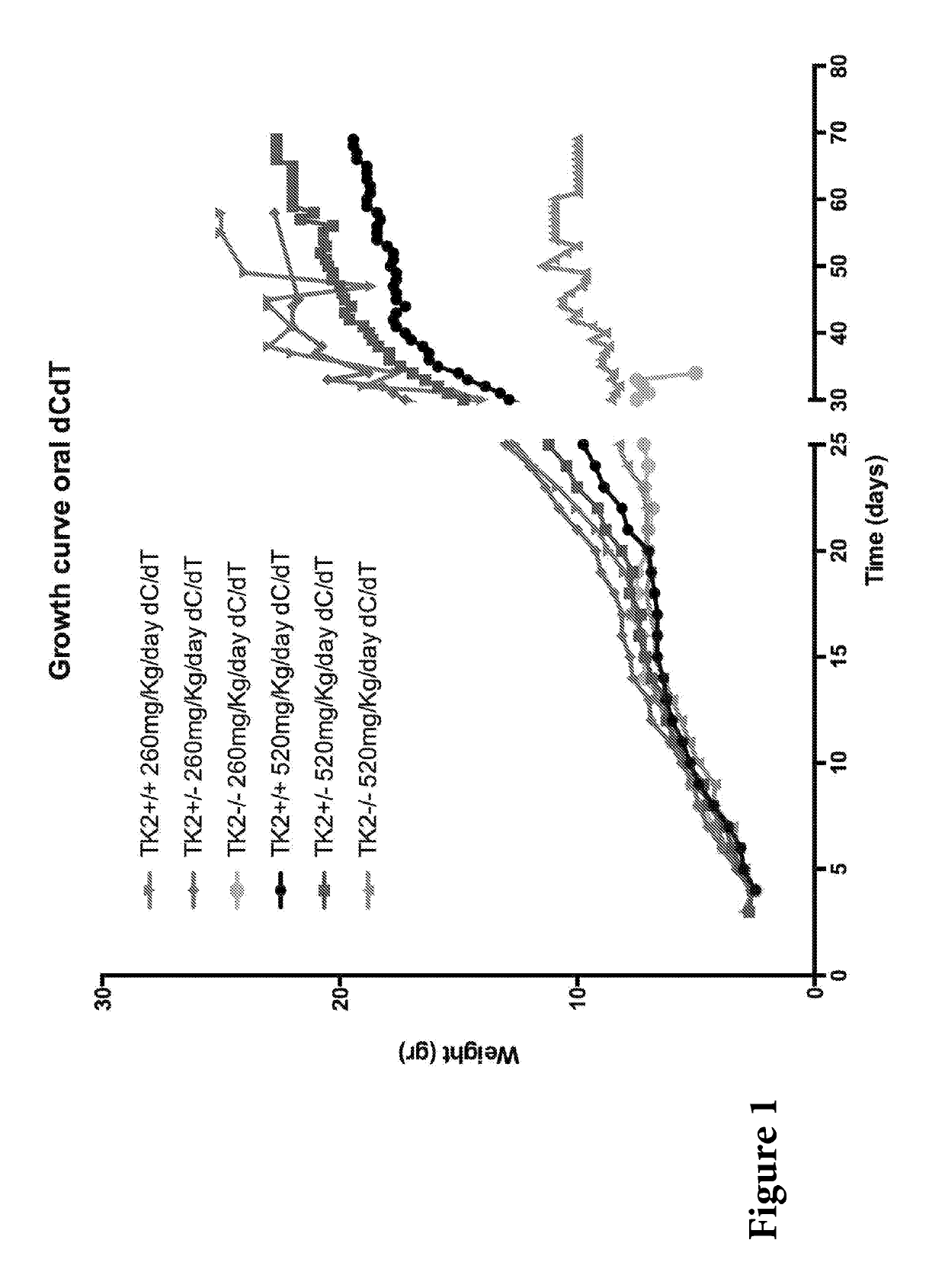
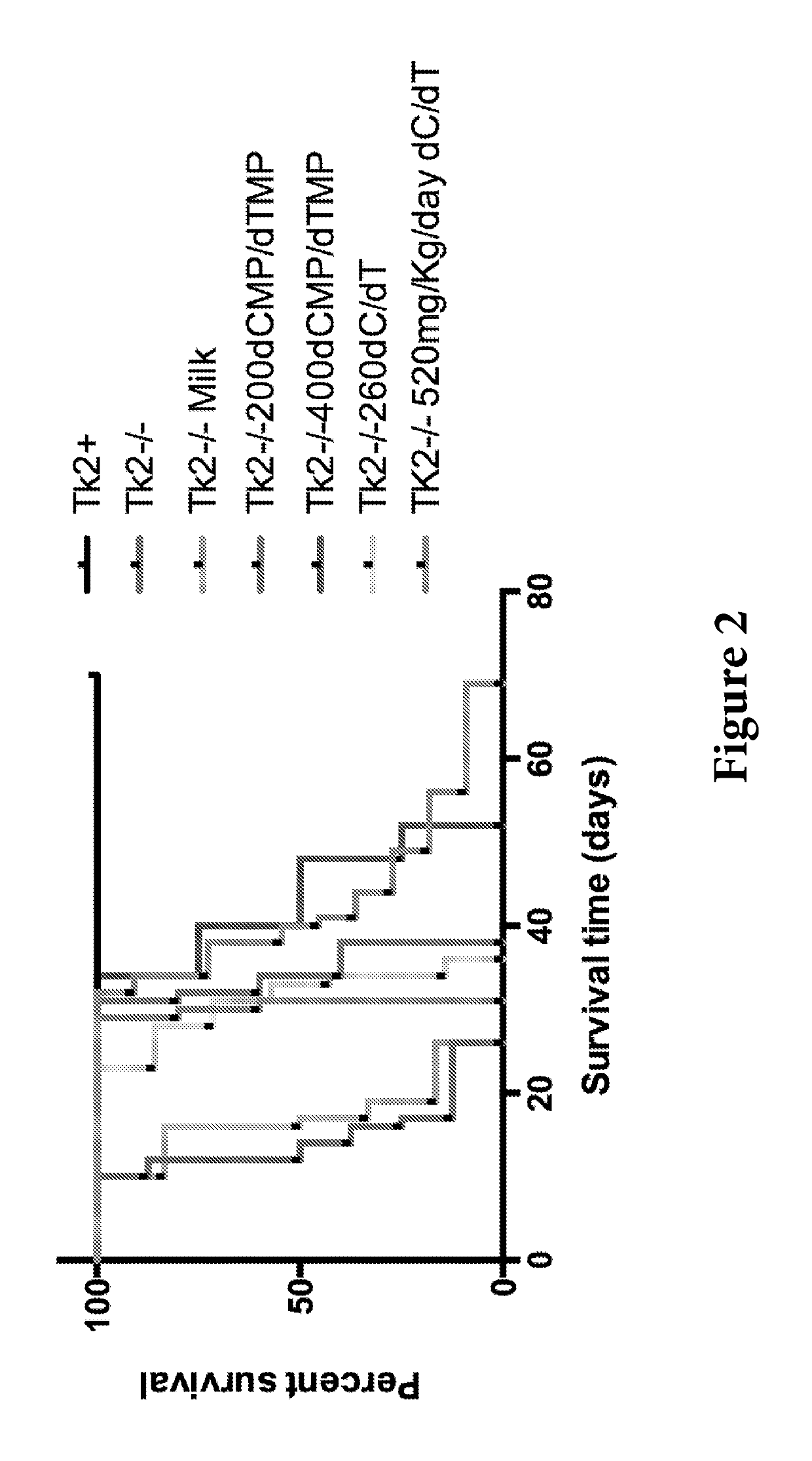
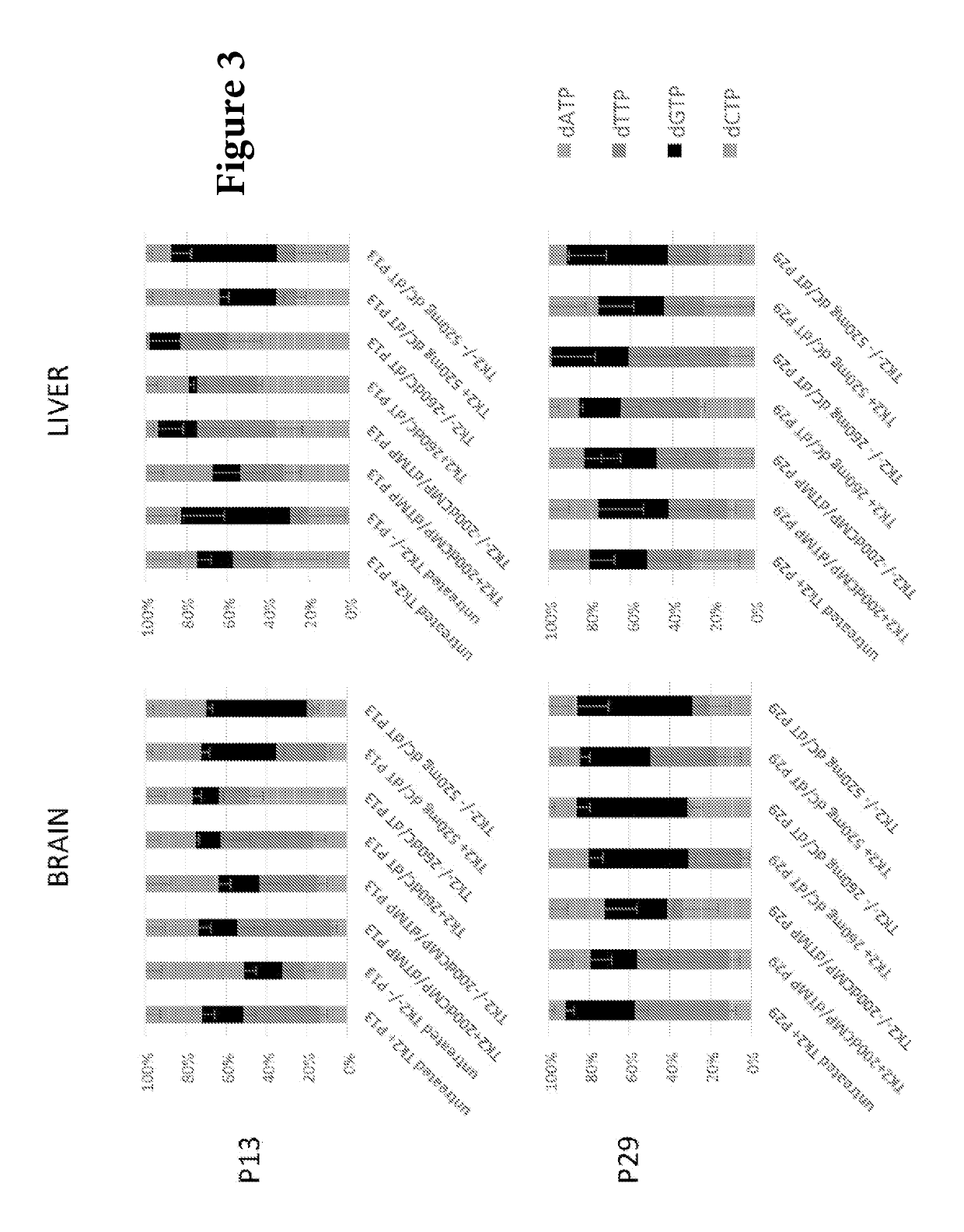
Nucleotide pools including mitochondrial DNA depletion syndromes
The invention relates generally to a pharmacological therapy for human genetic diseases, specifically those characterized by unbalance nucleotide pools, more specifically mitochondrial DNA depletion syndromes, and more specifically, thymidine kinase 2 (TK2) deficiency. The pharmacological therapy involves the administration of at least one deoxynucleoside, or mixtures thereof. For the treatment of TK2 deficiency, the pharmacological therapy involves the administration of either deoxythymidine (dT) or deoxycytidine (dC), or mixtures thereof. This administration of deoxynucleosides is applicable to other disorders of unbalanced nucleotide pools, especially those found in mitochondrial DNA depletion syndrome.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Deoxynucleoside therapy for diseases caused by unbalanced nucleotide pools including mitochondrial DNA depletion syndromes
The invention relates generally to a pharmacological therapy for human genetic diseases, specifically those characterized by unbalance nucleotide pools, more specifically mitochondrial DNA depletion syndromes, and more specifically, thymidine kinase 2 (TK2) deficiency. The pharmacological therapy involves the administration of at least one deoxynucleoside, or mixtures thereof. For the treatment of TK2 deficiency, the pharmacological therapy involves the administration of either deoxythymidine (dT) or deoxycytidine (dC), or mixtures thereof. This administration of deoxynucleosides is applicable to other disorders of unbalanced nucleotide pools, especially those found in mitochondrial DNA depletion syndrome.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
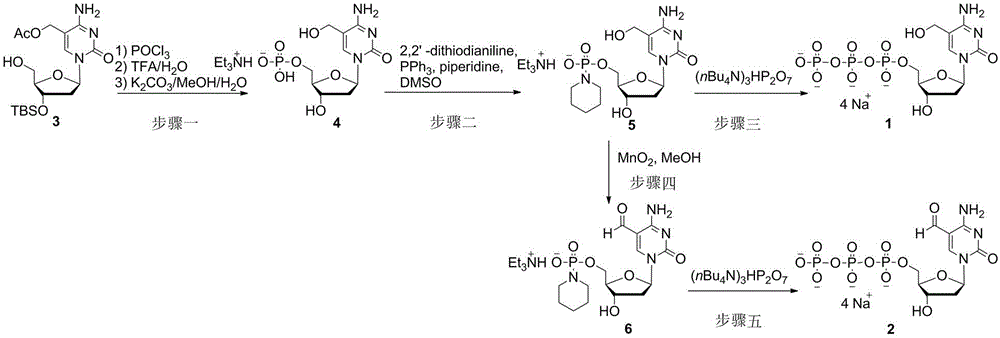
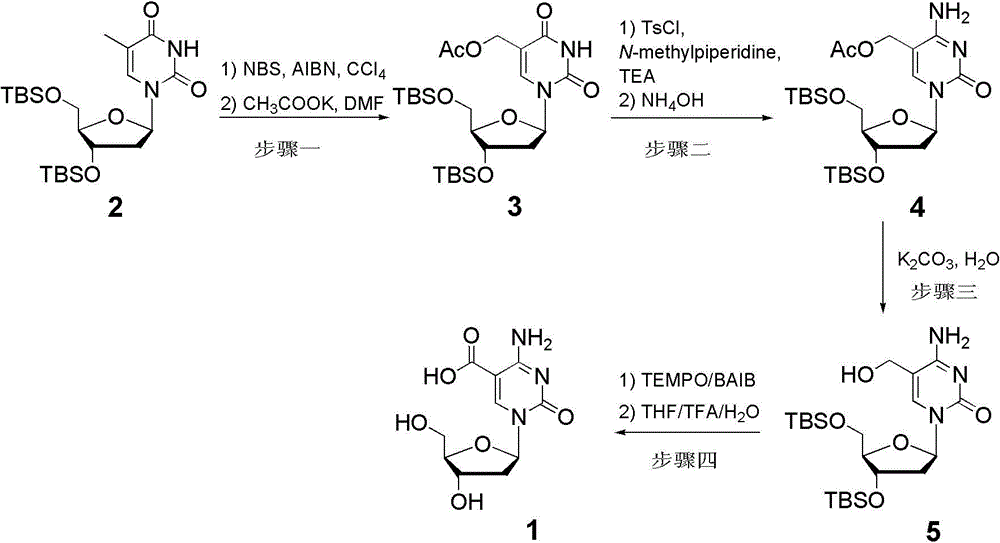
Method for synthesizing tetrasodium 5-hydroxymethyl and 5-aldehyde-2'-deoxycytidine triphosphate
InactiveCN104592334AHigh yieldEasy to operateSugar derivativesSugar derivatives preparationDeoxycytidine monophosphateChlorethoxyfos
The invention relates to a method for synthesizing tetrasodium 5-hydroxymethyl and 5-aldehyde-2'-deoxycytidine triphosphate. The method comprises the following steps of reacting 3'-tert-butyl-dimethylsilyl-5-acetoxymethyl-2'-deoxycytidine (3) and phosphorus oxychloride, removing TBS protecting groups with trifluoroacetic acid / water and removing acetyl groups with potassium carbonate / methanol / water to obtain triethylamine 5-hydroxymethyl-2'-deoxycytidine monophosphate (4); condensing the triethylamine 5-hydroxymethyl-2'-deoxycytidine monophosphate (4) and piperidine in the presence of 2,2'-dimercapto-diphenylamine / triphenylphosphine to obtain 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5); activating the 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5) and tris(tetrabutyl)ammonium pyrophosphate in the presence of 4,5-dicyanoimidazole to obtain tetrasodium 5-hydroxymethyl deoxycytidine triphosphate; and oxidizing the 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5) with activated manganese dioxide to obtain 5-aldehyde-2'-deoxycytidine phosphoryl piperidine triethylamine salt (6) and activating 5-aldehyde-2'-deoxycytidine phosphoryl piperidine triethylamine salt (6) and tris(tetrabutyl)ammonium pyrophosphate in the presence of 4,5-dicyanoimidazole to obtain tetrasodium 5-aldehyde-2'-deoxycytidine triphosphate.
Owner:JIANGXI SCI & TECH NORMAL UNIV
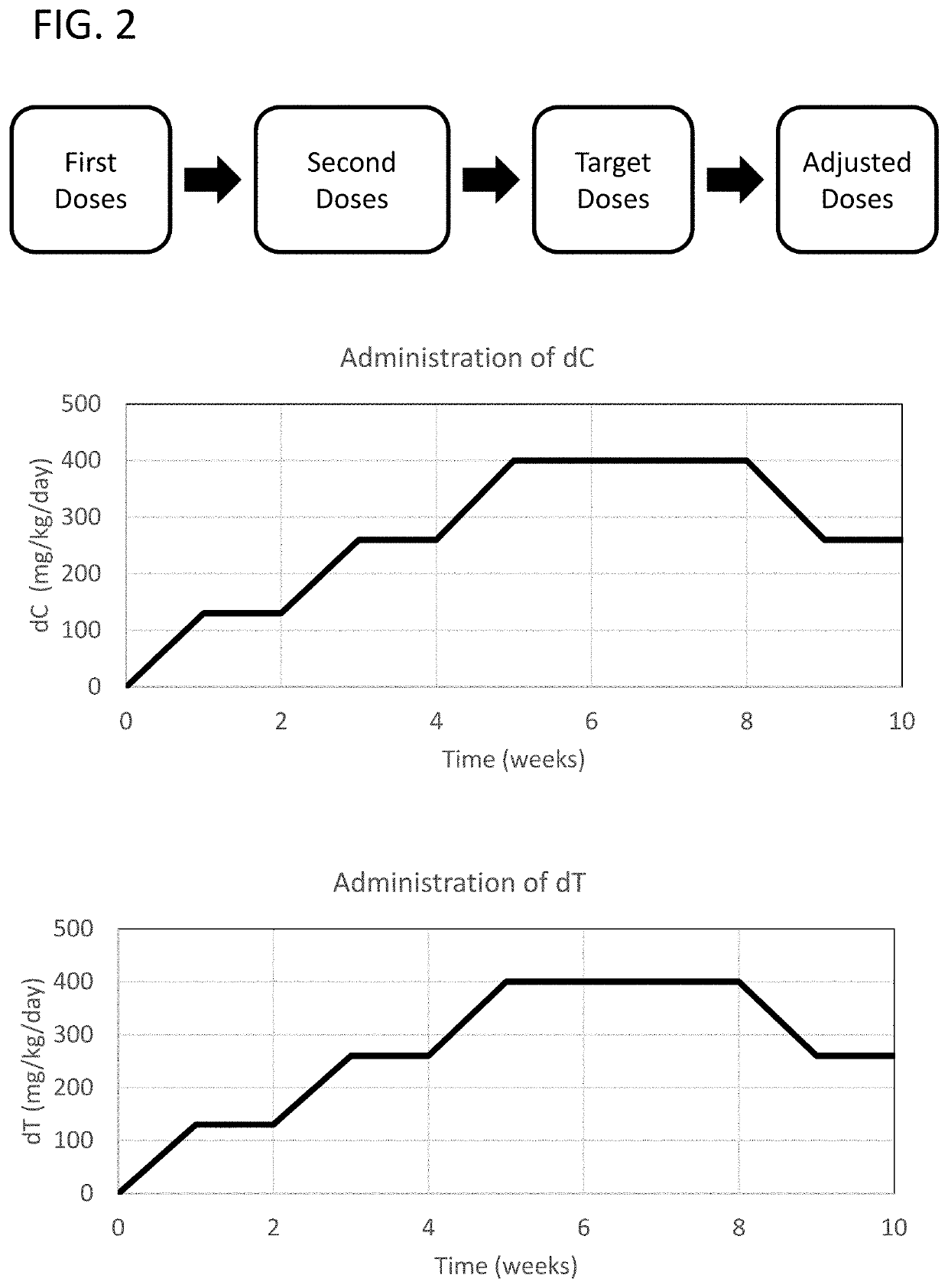
Methods of treating thymidine kinase 2 deficiency by administering deoxycytidine and deoxythymidine
Provided is a method of treating thymidine kinase 2 (TK2) deficiency in a subject by administering deoxycytidine (dC) and deoxythymidine (dT). The method can include administering dC and dT to a subject in doses between 200 mg / kg / day and 600 mg / kg / day for each of dC and dT. In some cases, the method includes performing test administrations with lower dosages of dC and dT, administering adjusted doses after administration of the target doses, or a combination thereof.
Owner:ZOGENIX INT
Industrially scalable nucleoside synthesis
InactiveUS20050004357A1Good yieldEfficient and cost-effective procedureSugar derivativesMedical preparationsTwo stepOrganic chemistry
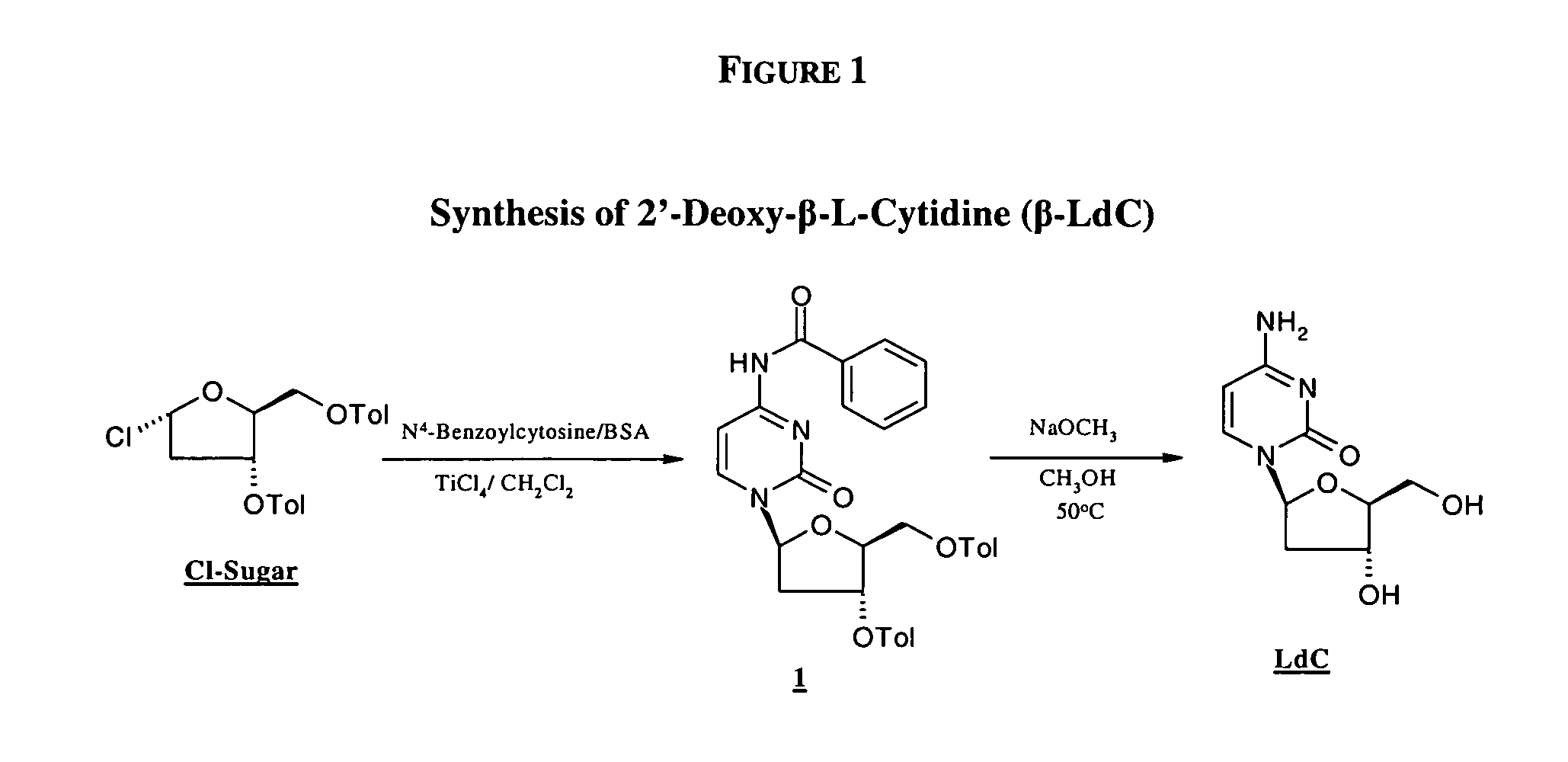
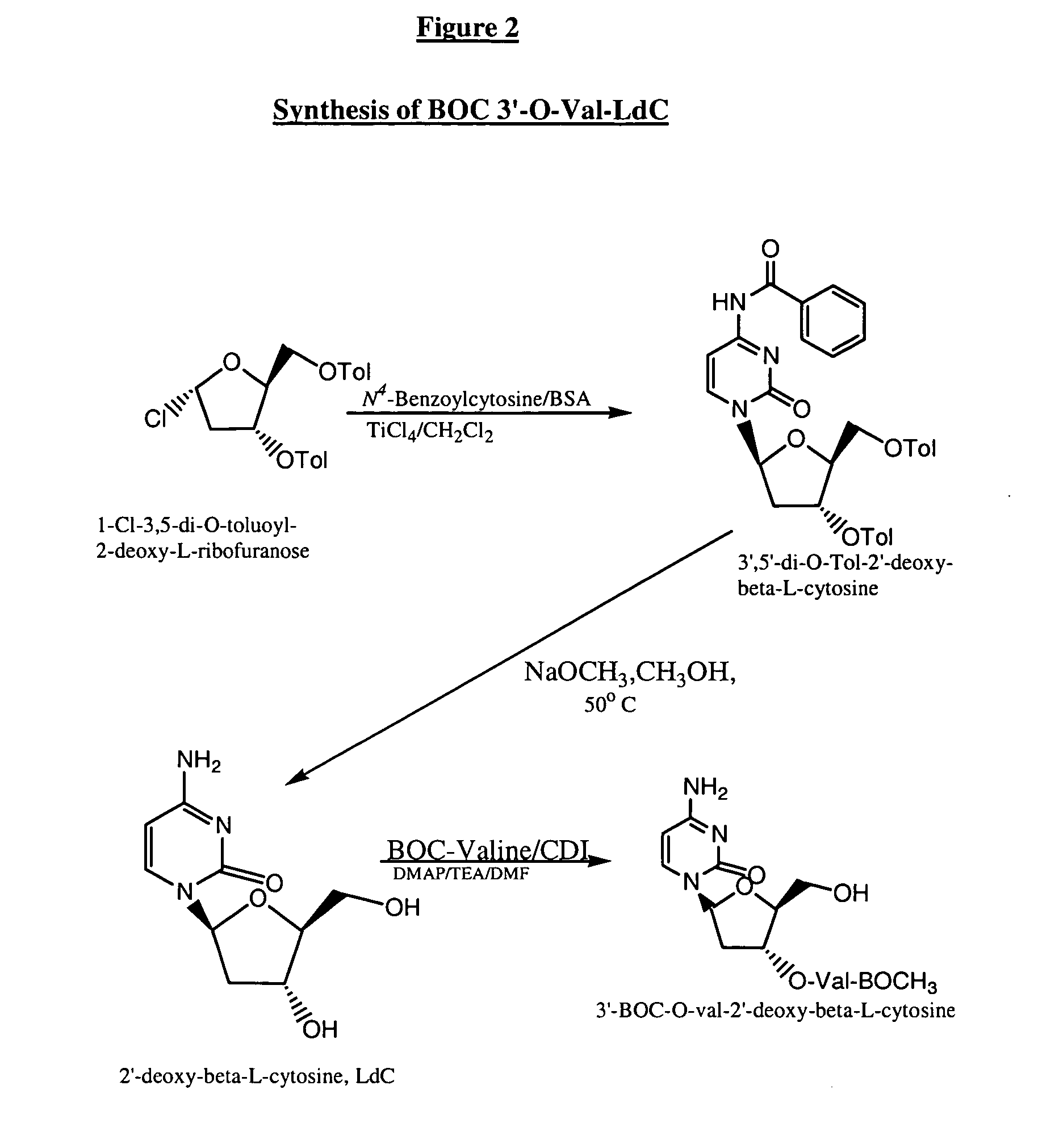
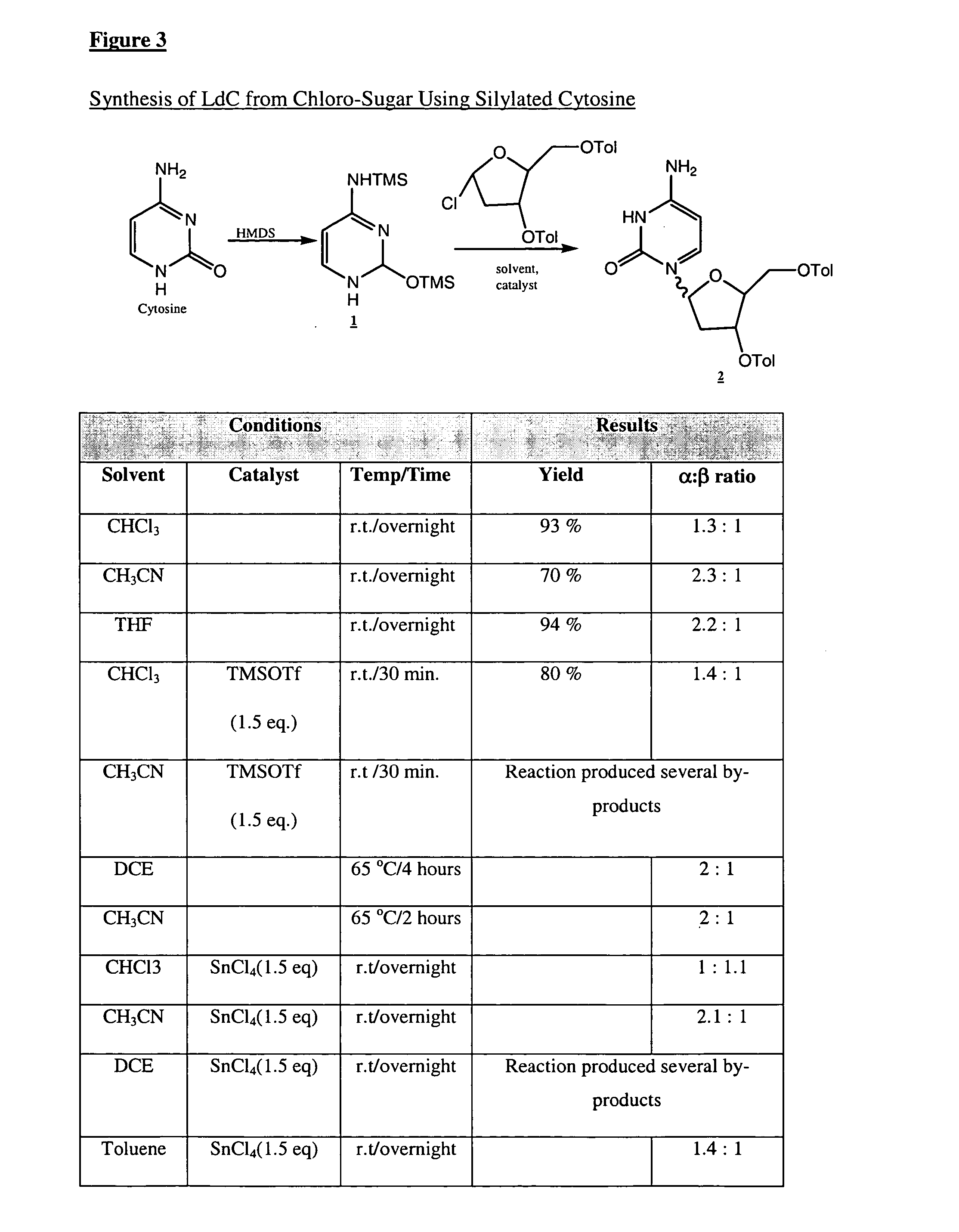
An industrially scalable two-step process for preparing a β-L-2′-deoxy-nucleoside that results in a predominance of the β- over the γ-anomeric form of the compound is described. An optional third step may be used to prepare 3′-prodrugs of desirable β-L-2′-deoxy-nucleosides for the delivery of these pharmaceuticals effective for treating viral diseases. The synthetic process is applicable in particular to the formation of β-L-2′-deoxy-cytidine, a pharmaceutically acceptable salt or prodrug thereof. The process can provide a relatively uncontaminated product that may require no further isolation or purification, thereby making the synthesis easily scalable for industrial manufacture.
Owner:NOVARTIS AG
Preparation method of 3'-deoxyuridine
ActiveCN107033205AReduce usageAvoid generatingSugar derivativesSugar derivatives preparationAcetic anhydrideHigh pressure water
The invention relates to the field of pharmaceutical synthesis and particularly relates to a preparation method of 3'-deoxyuridine. The method comprises the steps: by adopting a compound 3 as a raw material, firstly protecting amino through acetic anhydride to obtain a compound 4, obtaining a compound 5 under the action of acetyl bromide, reducing through a hypophosphite system to obtain a compound 6; removing deacetylated amino under the action of high-pressure water vapor and an organic solvent to obtain a compound 8 or removing N-acetyl to obtain a compound 7; and finally removing all acetyl to obtain a mixture of 3'-deoxyuridine and 3'-deoxycytidine; separating and purifying to obtain 3'-deoxyuridine crystal and 3'-deoxycytidine crystal separately, or directly removing all acetyl through the compound 6 to obtain the 3'-deoxycytidine. Available natural products are taken as initial raw materials, so that the method is simple in operation and convenient to purify, and industrial large-scale production is extremely easy to implement.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
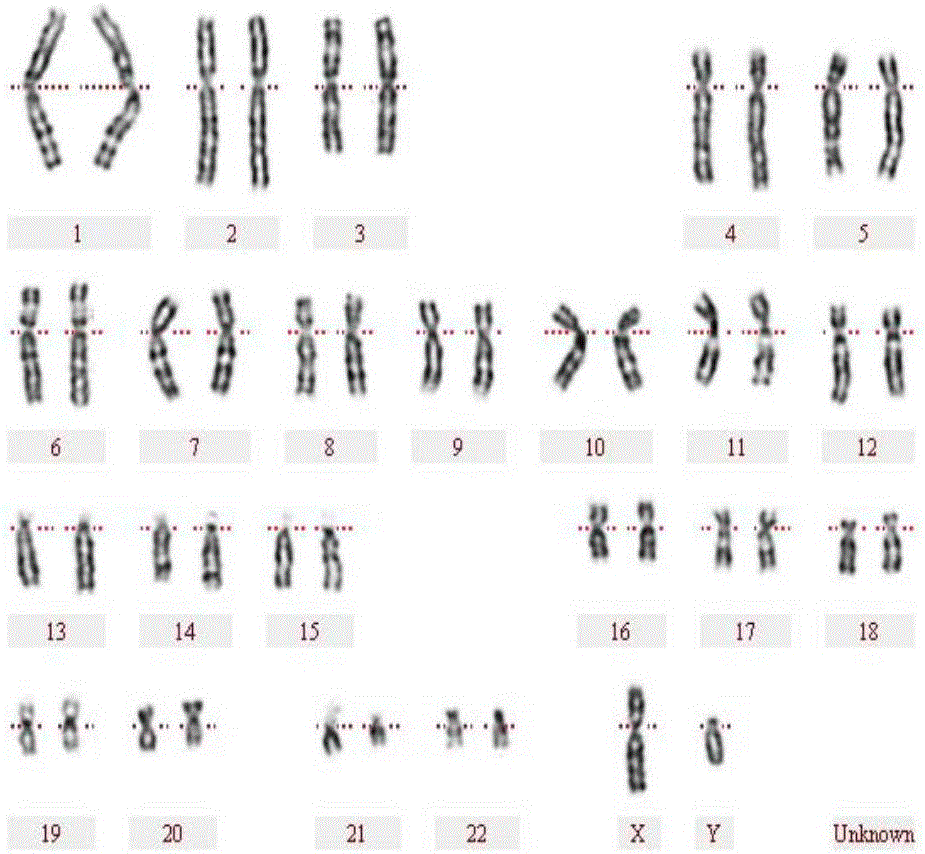
Human peripheral blood chromosome synchronization preparation kit
InactiveCN106501040AAvoid toxic effectsHighly toxicPreparing sample for investigationColchicineCulture mediums
The invention discloses a human peripheral blood chromosome synchronization preparation kit which comprises thymidine, 2'-deoxycytidine, colchicine, a 1640 culture medium and a fixing solution. According to the kit, growth cycles of peripheral blood lymphocytes are synchronized, enough 500-550 chromosome karyotypes with stripes can be obtained, so that chromosomal aberration and small abnormal structures are accurately identified. The synchronization kit adopting excessive thymidine single occlusion, deoxycytidine releasing and ethanol acetic acid harvesting is capable of increasing resolution ratio, simple in operation steps, capable of reducing the contact frequency of toxic reagents, and suitable for wide and conventional application.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV +1
Compositions Comprising a GPR109 Ligand For Treating Disorders of the Digestive Tract and/or Cancer
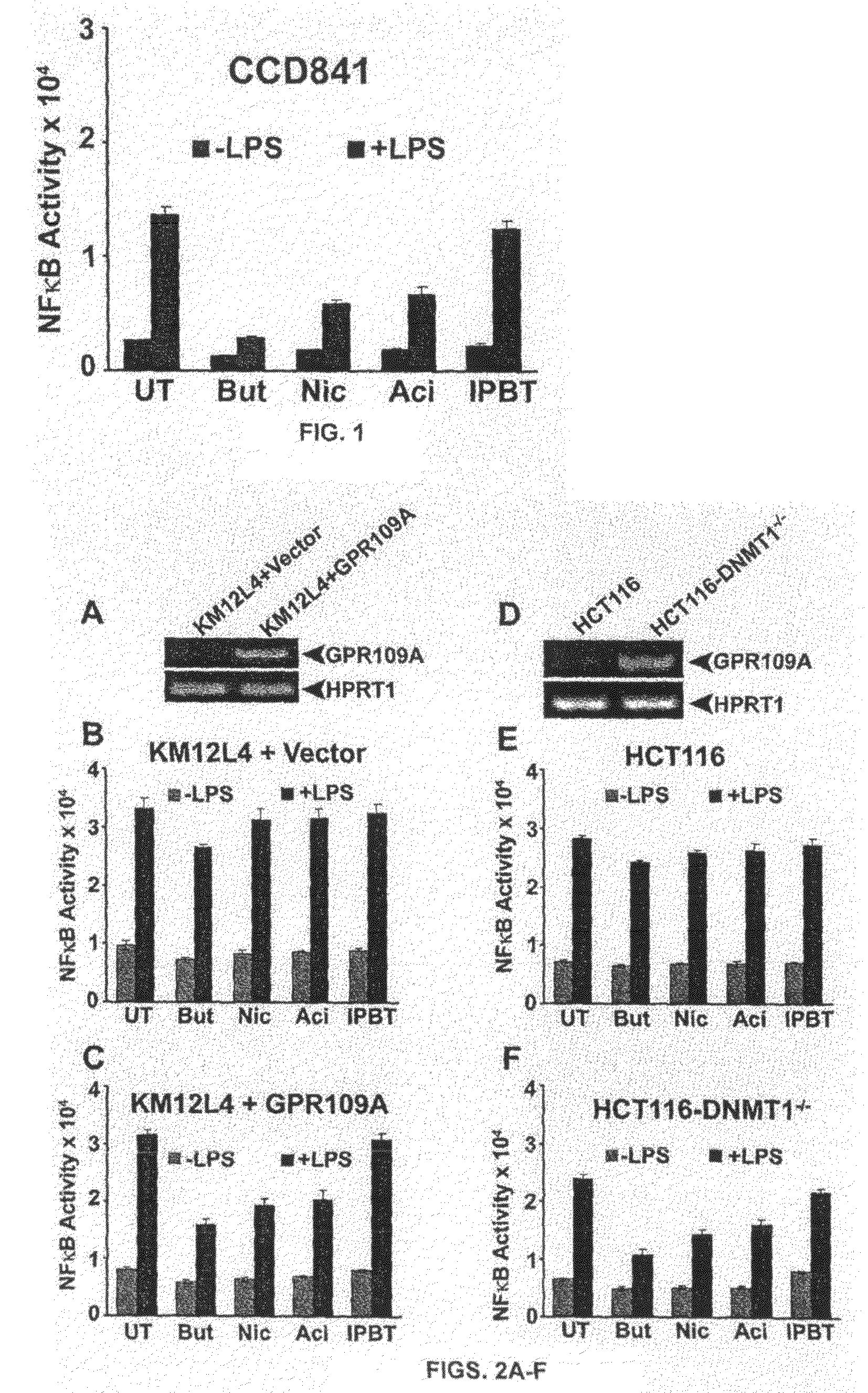
Pharmaceutical compositions containing an effective amount of a ligand for GPR109 to decrease intracellular cAMP levels of a subject in combination with an effective amount of a DNA methyl transferase inhibito to reduce or inhibit downregulation of GPR109 in the intestinal epithelial cells of the subject relative to a control are provided. It has been discovered that ligands for GPR109 can be used to treat one or more symptoms of cancer, inflammatory disorders, and diarrhea. Representative CPR 109 ligands include, but are not limited to butyrate, β-hydroxybutyrate, nicotinic acid, acifran, and octanoate. Suitable DNA methyl transferase inhibitors include 5-azacytidine, 5-aza-2′-deoxytidine, 1-β-D-arabinfαmosyl-5-azacytosine and dihydro-5-azacytidine. Typically, the compositions are formulated to achieve a GPR 109 ligand serum blood level of about 1 to about 1000 μM. The compositions are useful for the treatment of one or more symptoms of cancer. Preferred cancers that can be treated using the disclosed compositions include, but are not limited to colon cancer, breast cancer and leukemia. Methods for treating cancer, inflammatory disorders, and diarrhea are also provided.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Amphiphilic oligomers
InactiveUS20050181976A1Promote absorptionGood componentPeptide/protein ingredientsHydrolasesAmpicillinDaunorubicin
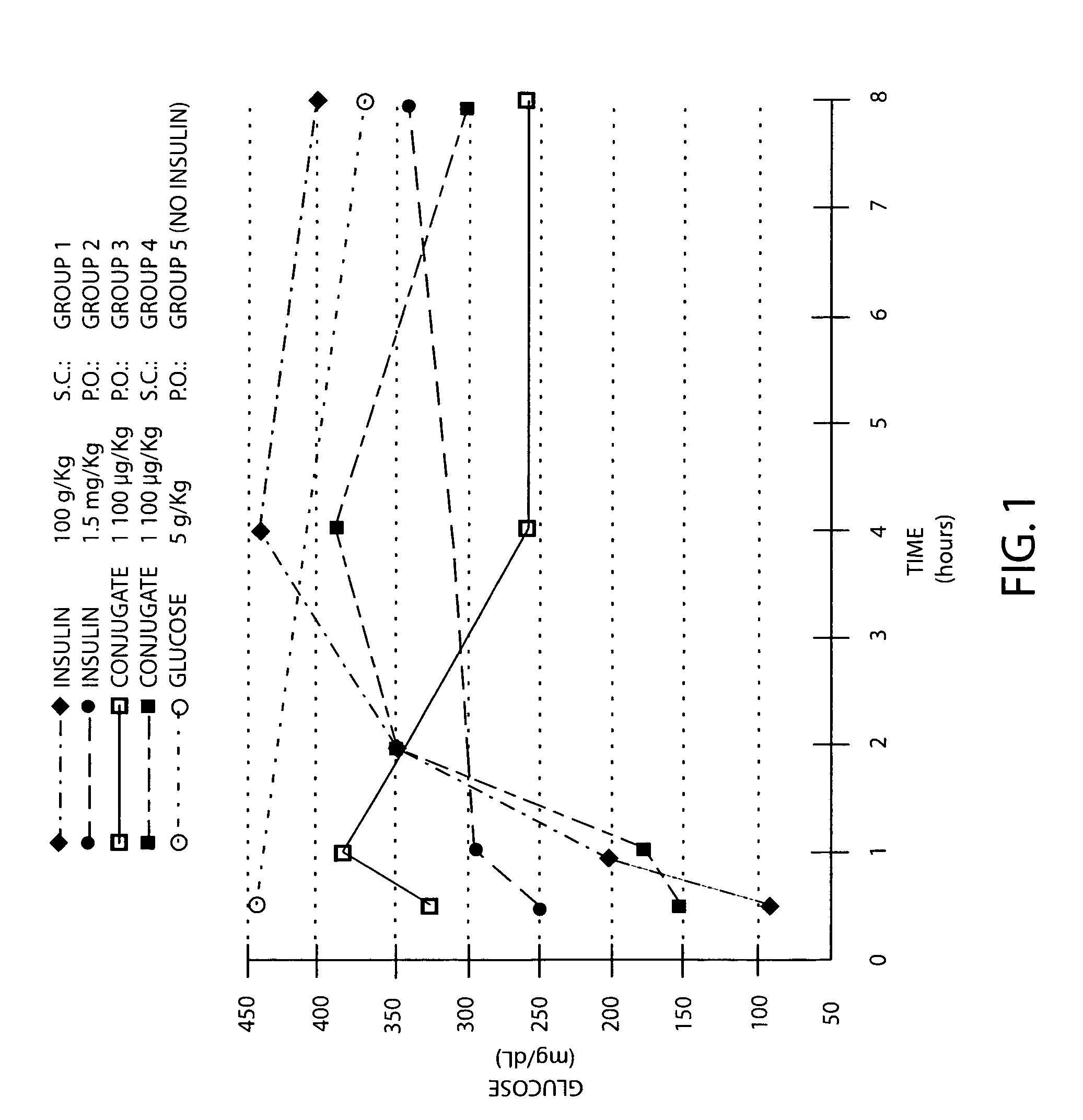
A therapeutic formulation comprising a microemulsion of a therapeutic agent in free and / or conjugatively coupled form, wherein the microemulsion comprises a water-in-oil (w / o) microemulsion including a lipophilic phase and a hydrophilic phase, and has a hydrophilic and lipophilic balance (HLB) value between 3 and 7, wherein the therapeutic agent may for example be selected from the group consisting of insulin, calcitonin, ACTH, glucagon, somatostatin, somatotropin, somatomedin, parathyroid honnone, erythropoietin, hypothalamic releasing factors, prolactin, thyroid stimulating hormones, endorphins, enkephalins, vasopressin, non-naturally occurring opioids, superoxide dismutase, interferon, asparaginase, arginase, arginine deaminease, adenosine deaminase, ribonuclease, trypsin, chymotrypsin, papain, Ara-A (Arabinofuranosyladenine), Acylguanosine, Nordeoxyguanosine, Azidothym id ine, Didesoxyadenosine, Dideoxycytidine, Dideoxyinosine Floxuridine, 6-Mercaptopurine, Doxorubicin, Daunorubicin, or I-darubicin, Erythromycin, Vancomycin, oleandomycin, Ampicillin; Quinidine and Heparin. In a particular aspect, the invention comprises an insulin composition suitable for parenteral as well as non-parenteral administration, preferably oral or parenteral administration, comprising insulin covalently coupled with a polymer including (i) a linear polyalkylene glycol moiety and (ii) a lipophilic moiety, wherein the insulin, the linear polyalkylene glycol moiety and the lipophilic moiety are conformationally arranged in relation to one another such that the insulin in the composition has an enhanced in vivo resistance to enzymatic degradation, relative to insulin alone. The microemulsion compositions of the invention are usefully employed in therapeutic as well as non-therapeutic, e.g., diagnostic, applications.
Owner:BIOCON LTD
Novel method for synthesizing 5-carboxyl-2'-deoxycytidine
InactiveCN104592333AHigh yieldThe synthesis method is simpleSugar derivativesSugar derivatives preparationCarboxyl radicalAceric acid
Owner:JIANGXI SCI & TECH NORMAL UNIV
Self-assembled nano material as well as preparation method and application thereof
ActiveCN111686815ALow priceEasy to scale applicationMaterial nanotechnologyOrganic-compounds/hydrides/coordination-complexes catalystsFluorescenceThymidine monophosphate
The invention discloses a self-assembled nano material. formed by nucleic acid or nucleotide, an amino acid derivative or polypeptide and copper ions through self-assembly. The type of the amino acidderivative is lysine, histidine, methionine or cysteine; the chiral configuration is L-shaped or D-shaped; the polypeptide contains histidine, methionine, lysine or cysteine; the length of the peptideis 2 peptide-40 peptide; the nucleic acid is DNA; the molar ratio of guanine deoxynucleotide in DNA is 10%-100%, the total number of the nucleotides is 4-59, and the nucleotide is guanosine-5'-monophosphate, adenosine-5'-monophosphate, cytidine-5'-monophosphate, uridine-5'-monophosphate, deoxyguanosine-5'-monophosphate, deoxyadenosine-5'-monophosphate, deoxycytidine-5'-monophosphate or thymidine-5'-monophosphate. According to the invention, the simulated enzyme catalytic reaction can be monitored through a light absorption or fluorescence photometer.
Owner:BEIJING UNIV OF CHEM TECH
Means for the treatment of HIV
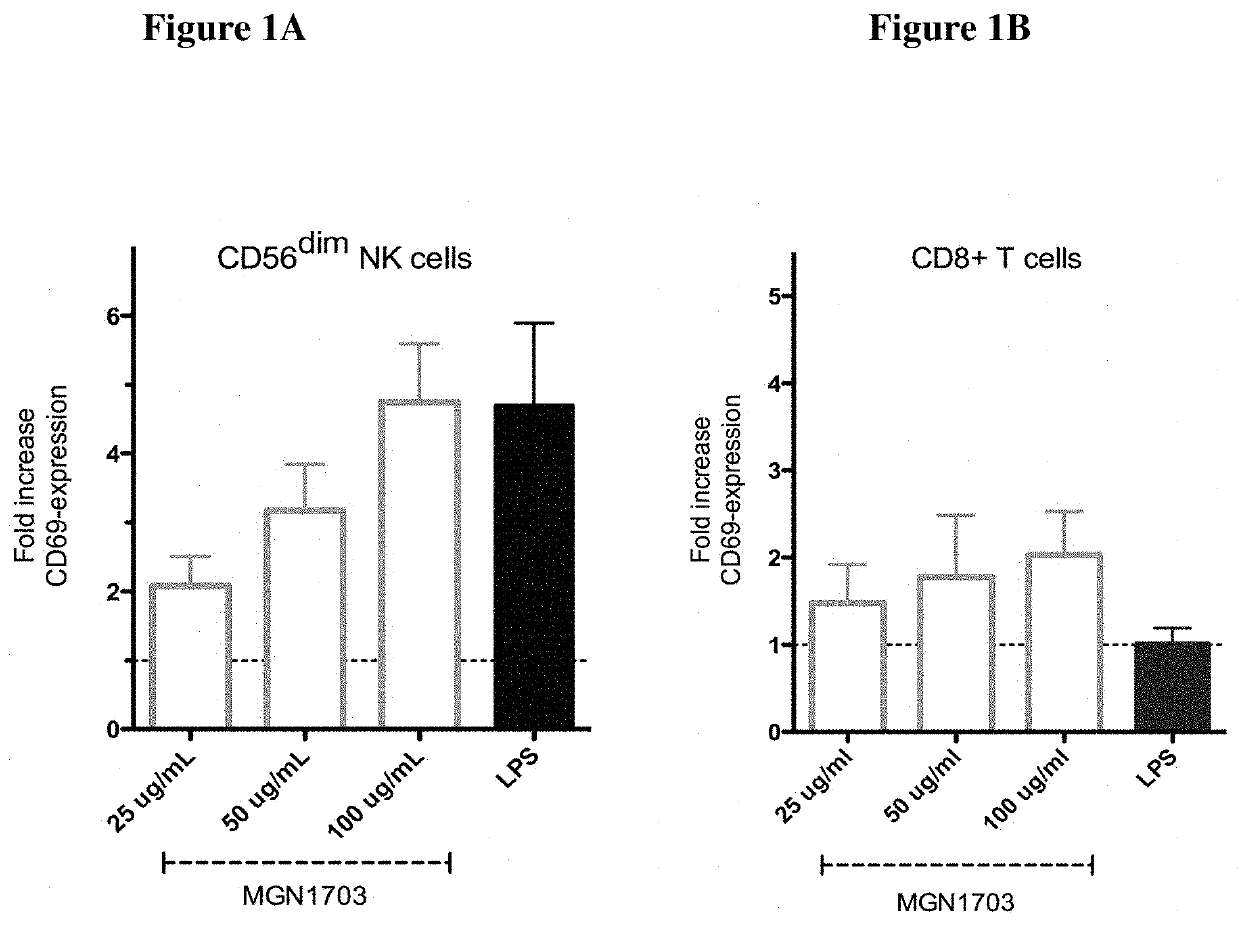
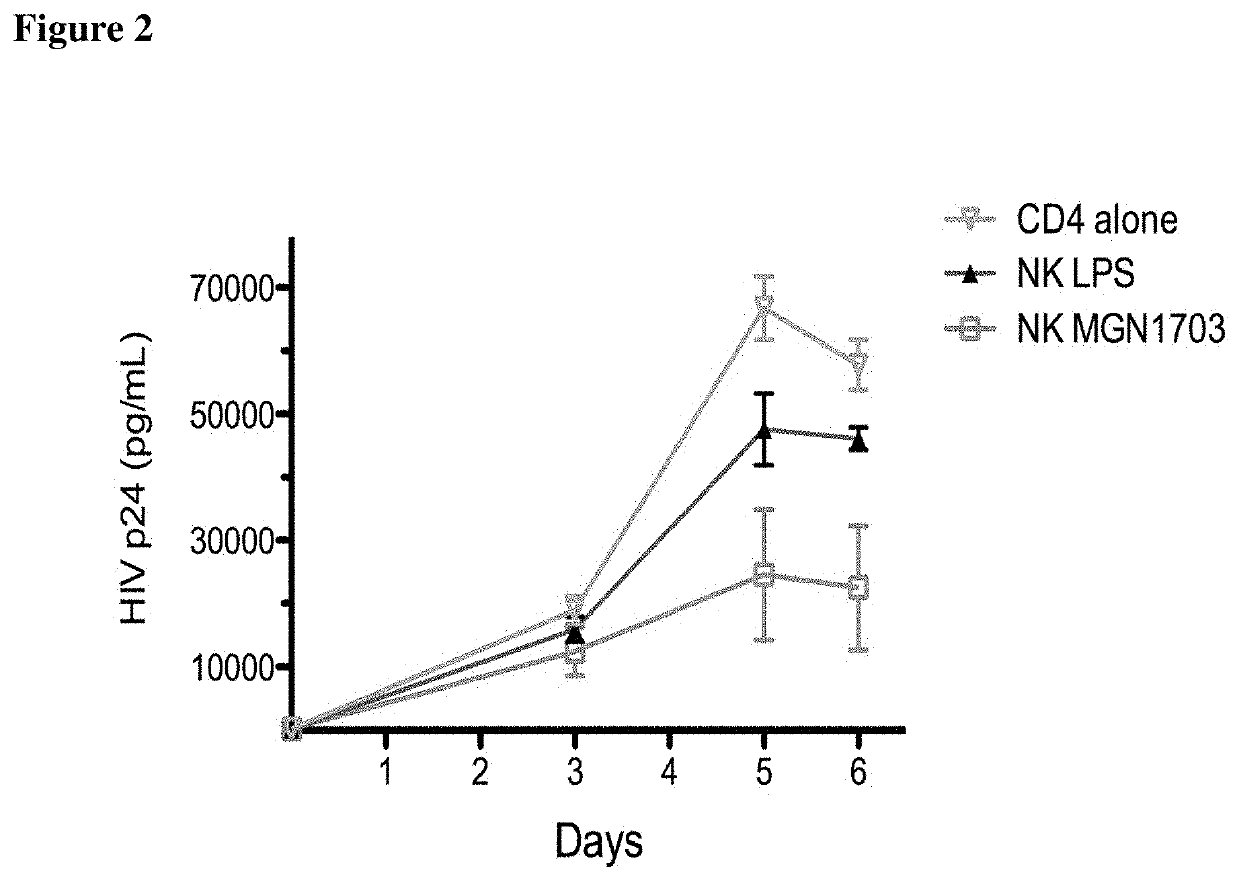
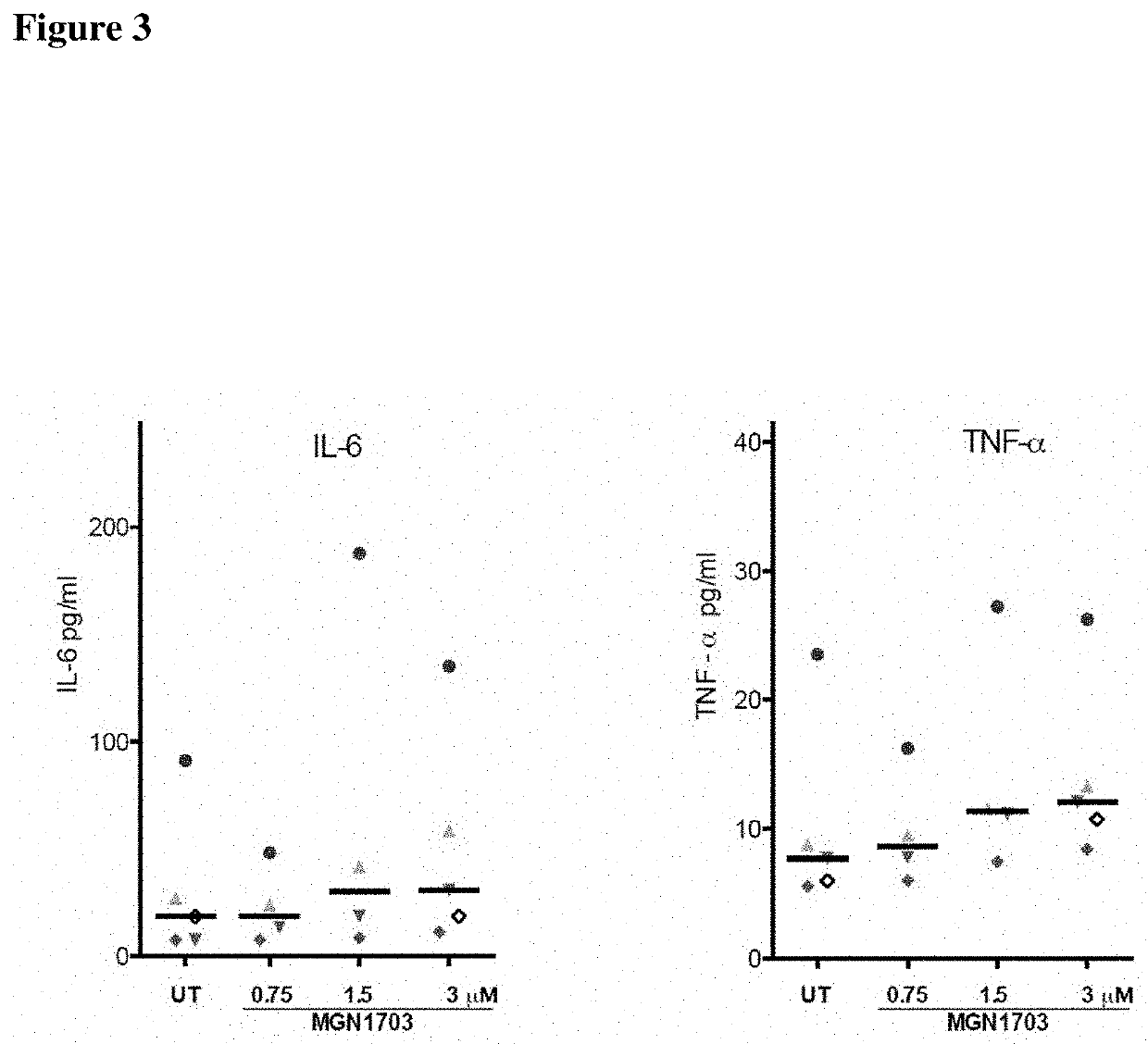
The invention relates to a non-coding sequence of deoxyribonucleic acids comprising at least one sequence motif N1N2CGN3N4, wherein N is a nucleotide comprising A, C, T, or G, and C is deoxycytidine, G is deoxyguanosine, A is deoxyadenosine and T is deoxy-thymidine for the treatment of viral infections. In particular, the non-coding sequence of deoxyribonucleic acids is used in combination with antiretroviral therapy and / or histone de-acetylase inhibitors.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com