Patents
Literature
380 results about "Guanosine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Guanosine is a purine nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a β-N₉-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These forms play important roles in various biochemical processes such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction (cGMP). When guanine is attached by its N9 nitrogen to the C1 carbon of a deoxyribose ring it is known as deoxyguanosine.
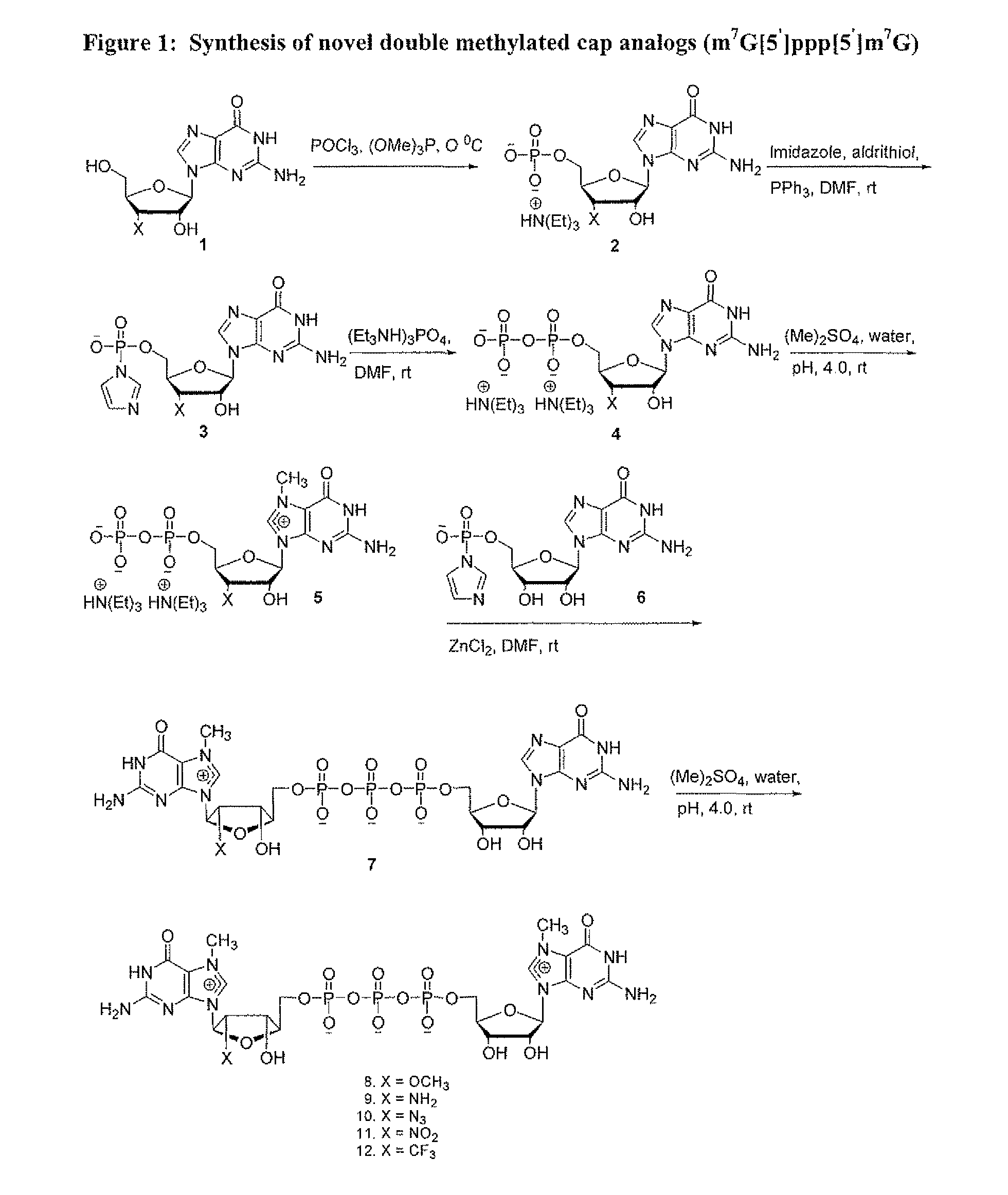
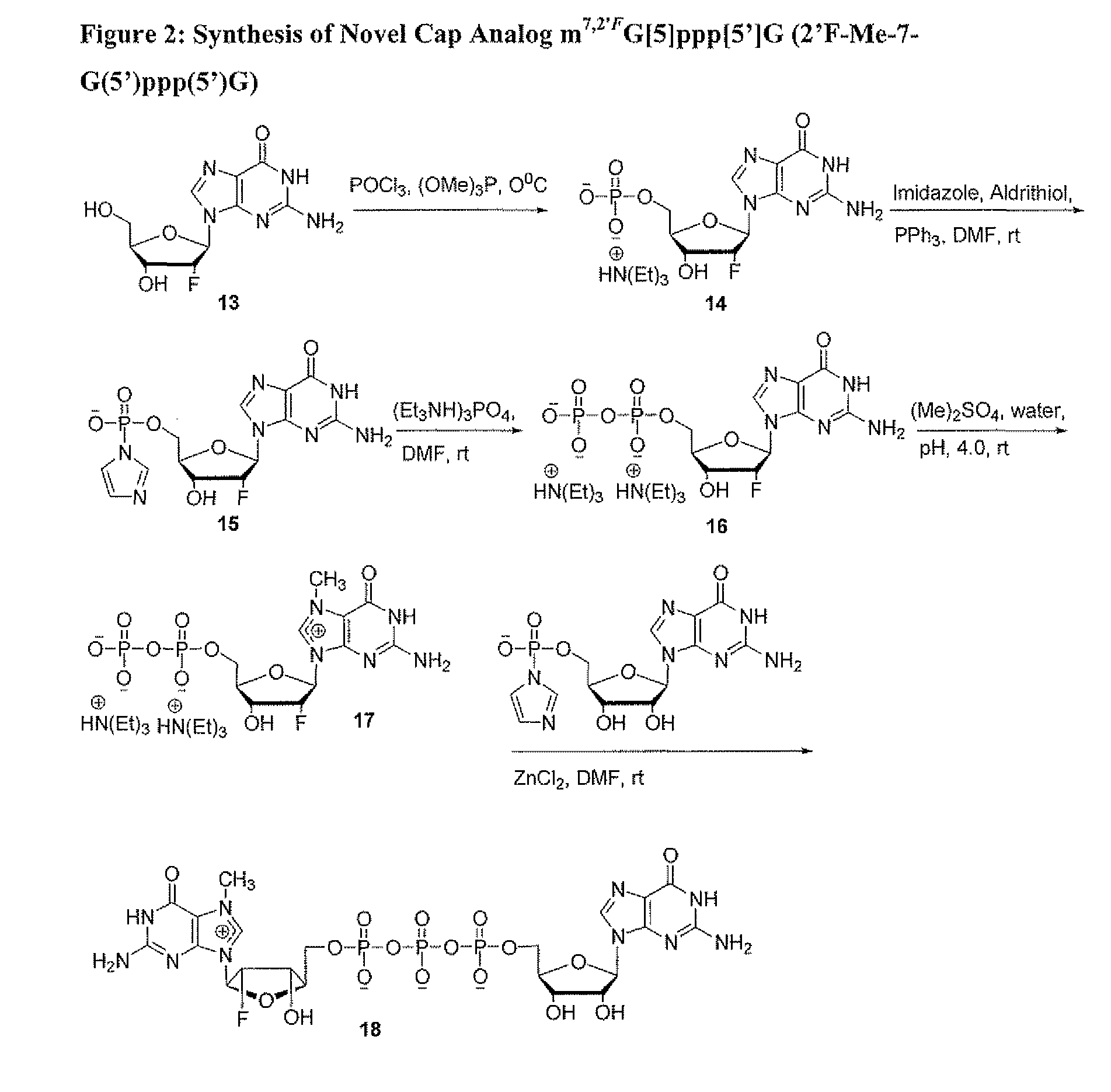
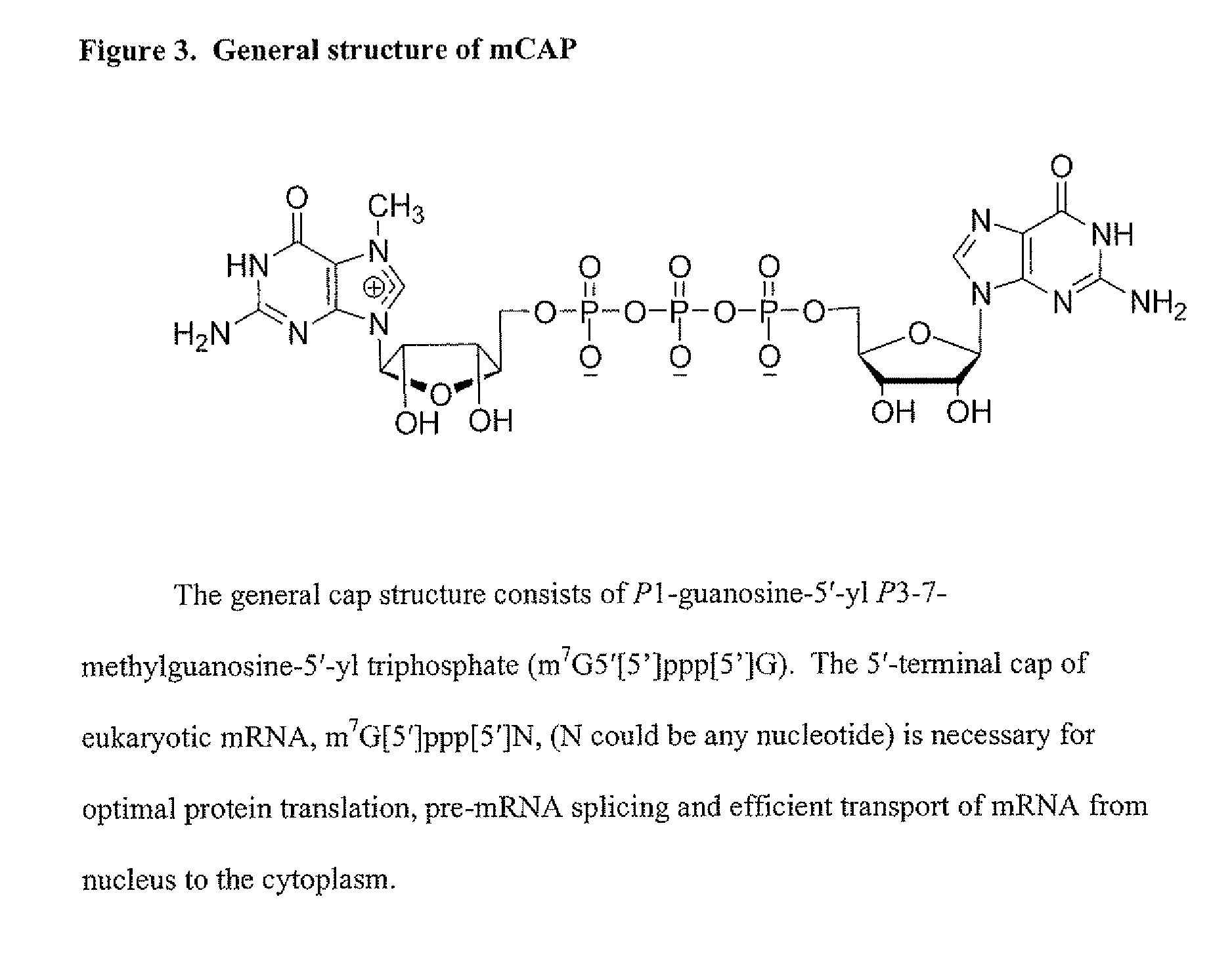
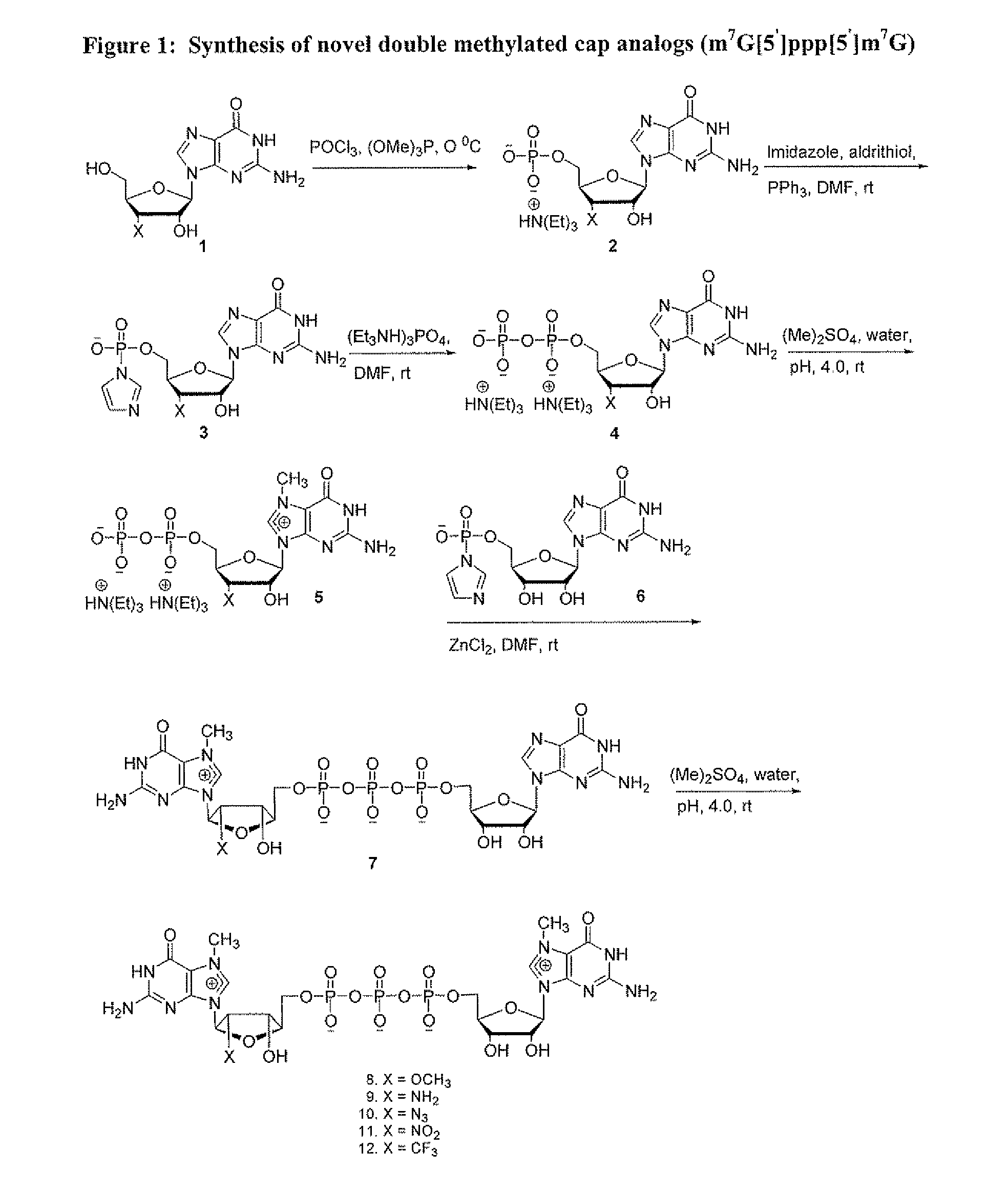
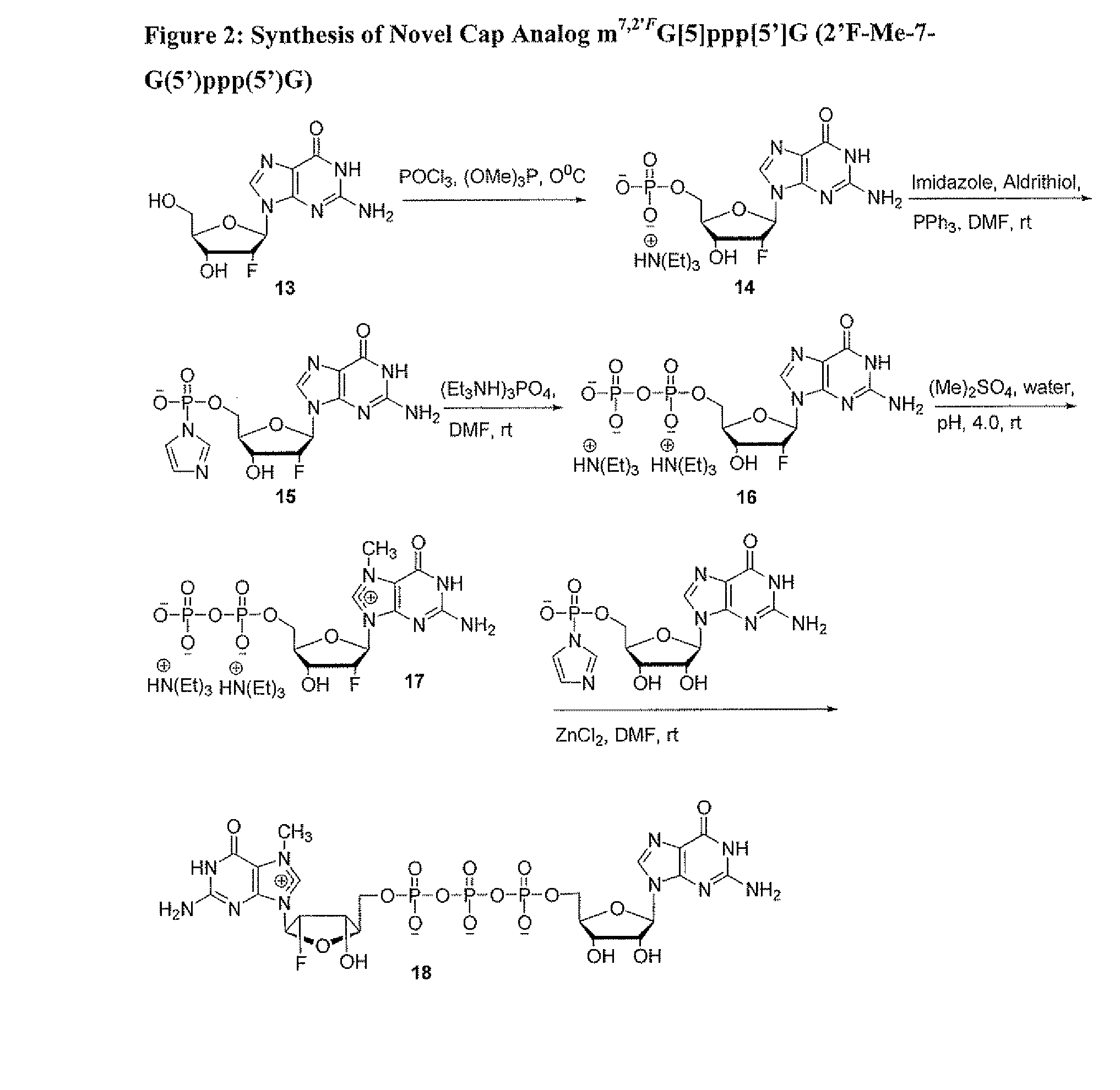
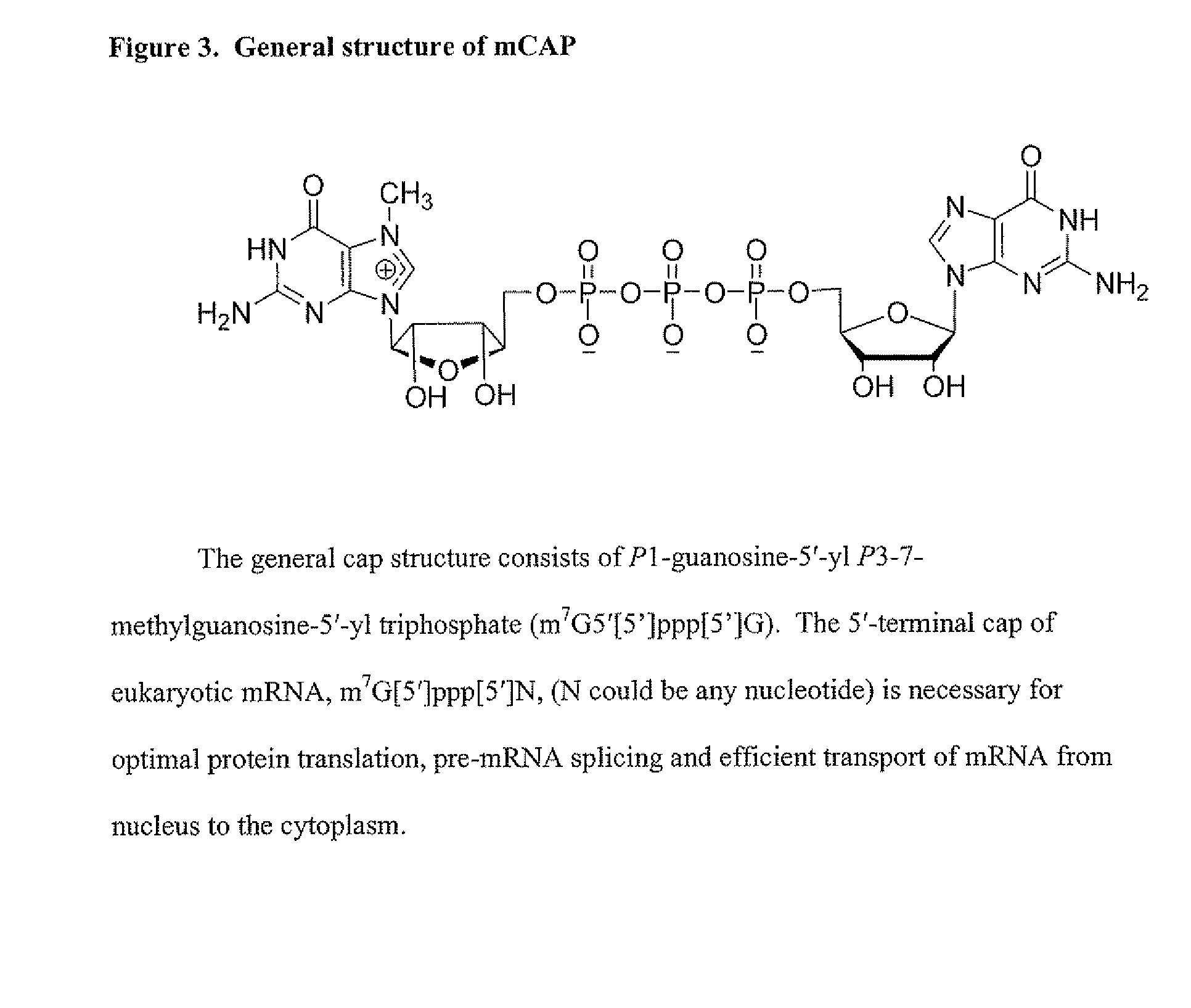
Dinucleotide MRNA cap analogs
ActiveUS8304529B2Increase opportunitiesImprove translationSugar derivativesActivity regulationPyrimidine NucleotidesRibose
Novel cap analogs which are easily synthesized, resulting in high levels of capping efficiency and transcription and improved translation efficiencies are provided. Such caps are methylated at the N7 position of one or both guanosines of the dinucleotide cap as well as at the 3′ position on the ribose ring. Substituent groups on the ribose ring also result in the cap being incorporated in the forward orientation. Also provided are methods useful for preparing capped analogs and using mRNA species containing such analogs are also contemplated herein, as well as kits containing the novel cap analogs.
Owner:APPL BIOSYSTEMS INC
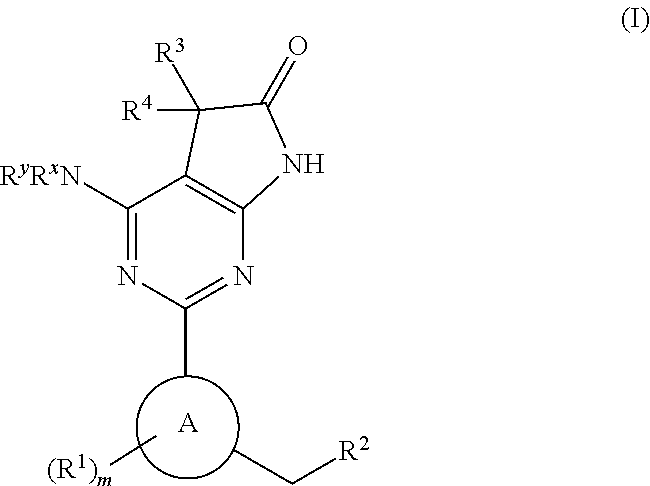
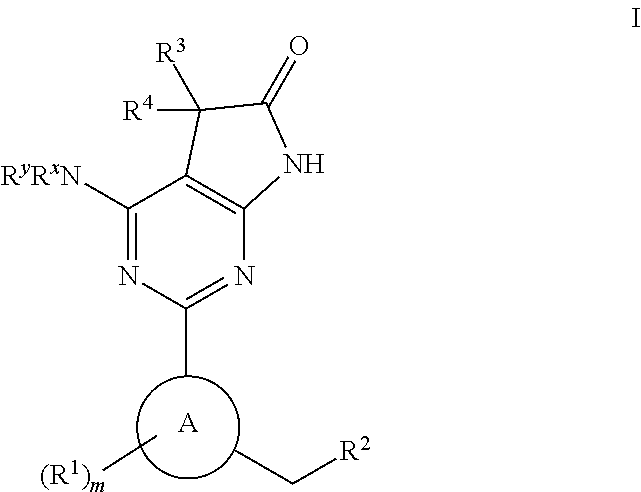
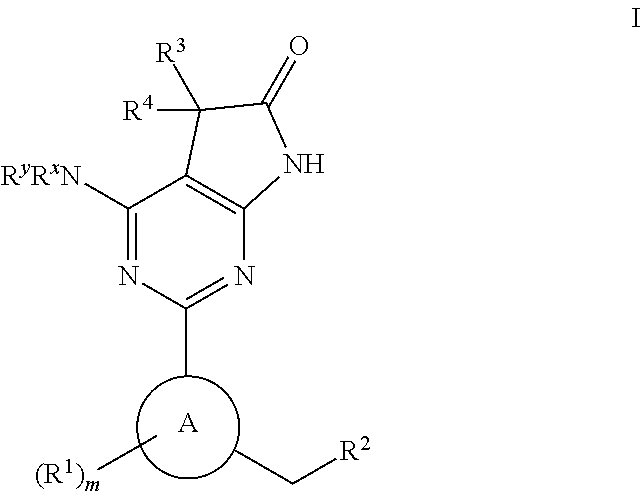
Tetracyclic cyclic GMP-specific phosphodiesterase inhibitors, process of preparation and use
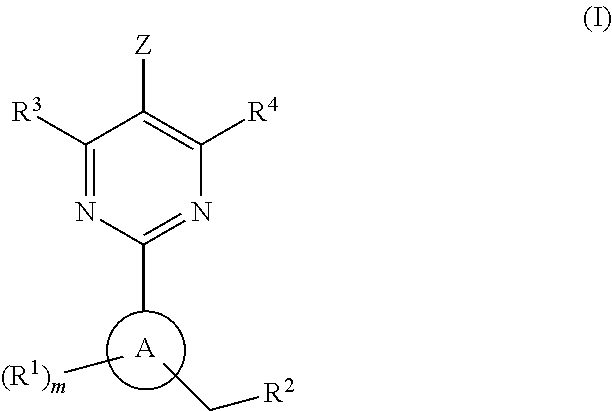
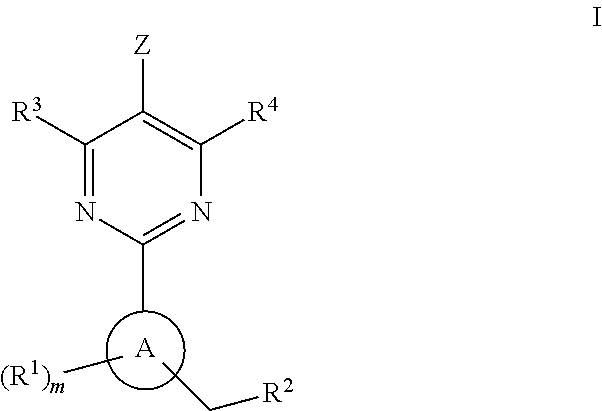
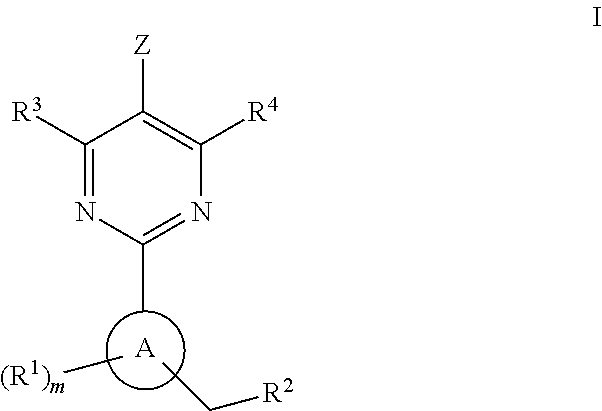
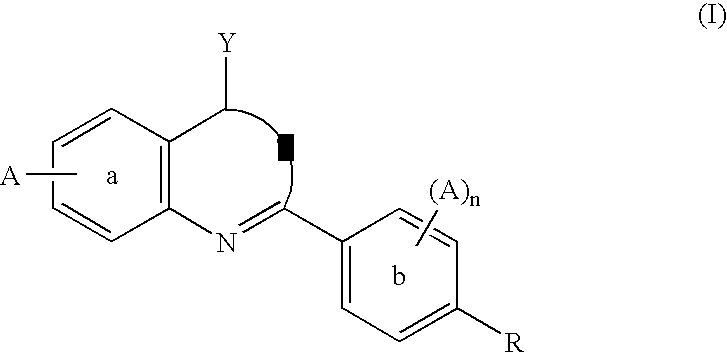
A compound of formula (I) and salts and solvates thereof, in which: R0 represents hydrogen, halogen, or C1-6alkyl; R1 represents hydrogen, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, haloC1-6alkyl, C3-8cycloalkyl, C3-8cycloalkylC1-3alkyl, arylC1-3alkyl, or heteroarylC1-3alkyl; R2 represents an optionally substituted monocyclic aromatic ring selected from benzene, thiophene, furan, and pyridine, or an optionally substituted bicyclic ring (a) attached to the rest of the molecule via one of the benzene ring carbon atoms, and wherein the fused ring (A) is a 5- or 6-membered ring which may be saturated or partially or fully unsaturated, and comprises carbon atoms and optionally one or two heteroatoms selected from oxygen, sulphur, and nitrogen; and R3 represents hydrogen or C1-3alkyl, or R1 and R3 together represent a 3- or 4-membered alkyl or alkenyl chain. A compound of formula (I) is a potent and selective inhibitor of cyclic guanosine 3',5'-monophosphate specific phosphodiesterase (cGMP specific PDE) having a utility in a variety of therapeutic areas where such inhibition is beneficial, including the treatment of cardiovascular disorders and erectile dysfunction.
Owner:ICOS CORP
Carboline derivatives
Carboline derivatives of formula (I) are potent and selective inhibitors of cyclic guanosine 3',5'-monophosphate specific phosphodiesterase (cGMP-specific PDE) and have utility in a variety of therapeutic areas where such inhibition is thought to be beneficial, including the treatment of cardiovascular disorders and erectile dysfunction.
Owner:ICOS CORP
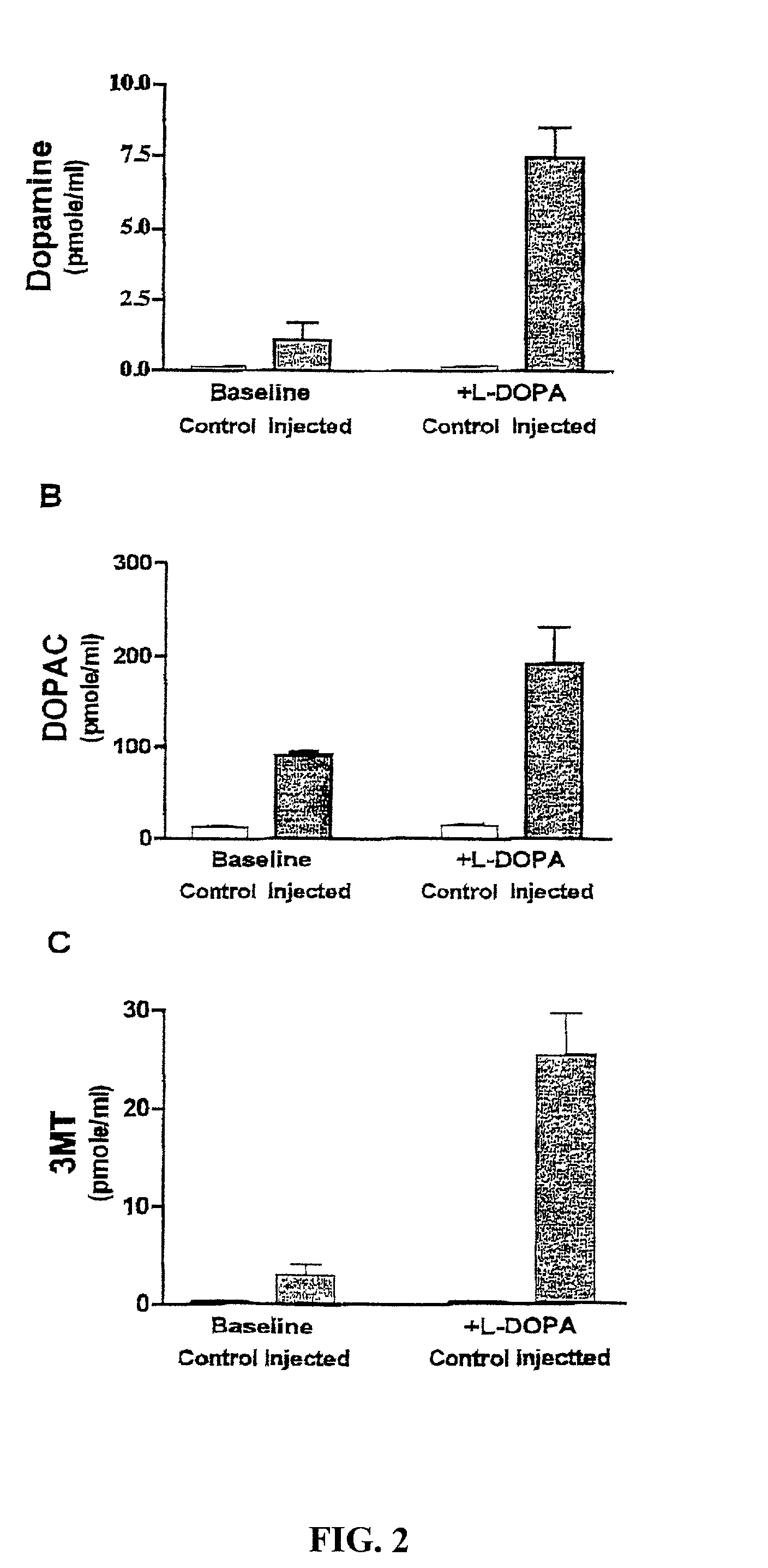
Methods of treating Parkinson's disease using recombinant adeno-associated virus virions
ActiveUS7588757B2Reduce deliveryIncrease in fine motor taskingBiocidePeptide/protein ingredientsGene deliveryDisease
Methods for treating Parkinson's disease (PD) are provided. Recombinant adeno-associated virus (rAAV) virions are used to deliver genes encoding dopamine-synthesizing enzymes to the central nervous system of a primate. Once delivered, the genes are expressed, which then results in dopamine synthesis and amelioration in the clinical signs and symptoms of PD. The methods of the present invention can be used to deliver the three central dopamine synthesizing enzymes: tyrosine hydroxylase, aromatic L-amino acid decarboxylase, and guanosine triphosphate cyclohydrolase I thereby enhancing dopamine biosynthesis and providing for enhanced therapeutic efficacy.
Owner:GENZYME CORP
Soluble guanylate cyclase activators
A compound of Formula (I): or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Dinucleotide MRNA CAP Analogs
ActiveUS20100261231A1Increase opportunitiesImprove translationSugar derivativesActivity regulationRibosePyrimidine Nucleotides
Novel cap analogs which are easily synthesized, resulting in high levels of capping efficiency and transcription and improved translation efficiencies are provided. Such caps are methylated at the N7 position of one or both guanosines of the dinucleotide cap as well as at the 3′ position on the ribose ring. Substituent groups on the ribose ring also result in the cap being incorporated in the forward orientation. Also provided are methods useful for preparing capped analogs and using mRNA species containing such analogs are also contemplated herein, as well as kits containing the novel cap analogs.
Owner:APPL BIOSYSTEMS INC
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
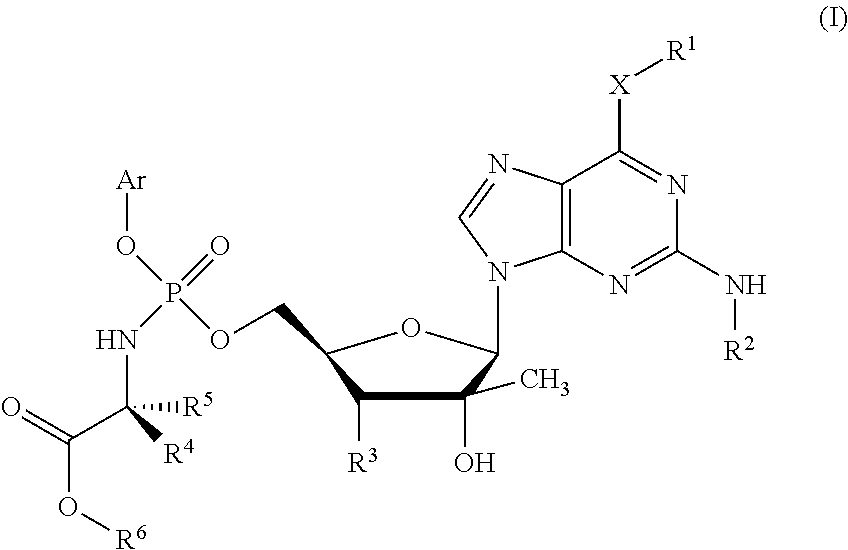
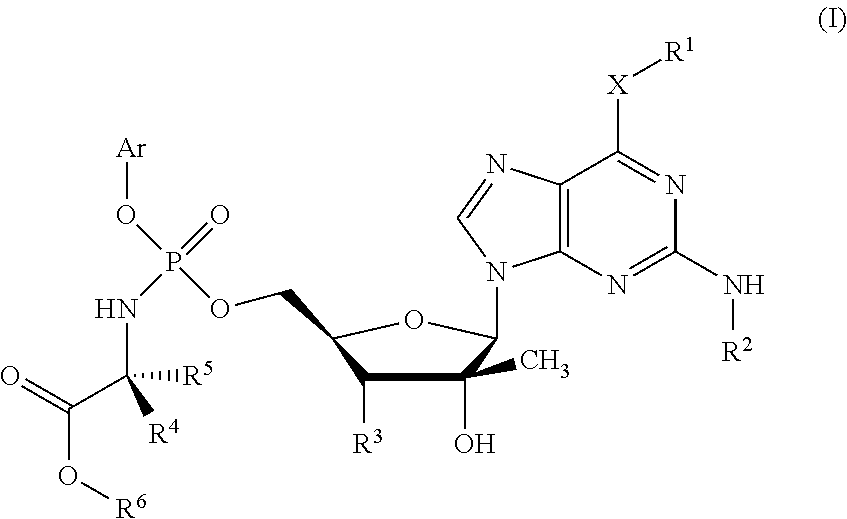
Phosphoramidate derivatives of guanosine nucleoside compounds for treatment of viral infections
Phosphoramidate compounds derived from guanine bases having enhanced therapeutic potency are provided, and these compounds in particular have enhanced potency with respect to treatment of viral infections, such as hepatitis C virus. Pharmaceutical compositions, methods of preparing the compounds, and methods of using the compounds and compositions to treat viral infections are also provided.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Compositions comprising phosphodiesterase inhabitors for the treatment of sexual disfunction
The present invention relates to highly selective phosphodieterase (PDE) enzyme inhibitors and to their use in pharmaceutical articles of manufacture. In particular, the present invention relates to potent inhibitors of cyclic guanosine 3′,5′-monophosphate specific phosphodiesterase type 5 (PDE5) that when incorporated into a pharmaceutical product at about 1 to about 20 mg unit dosage are useful for the treatment of sexual dysfunction.
Owner:ICOS CORP
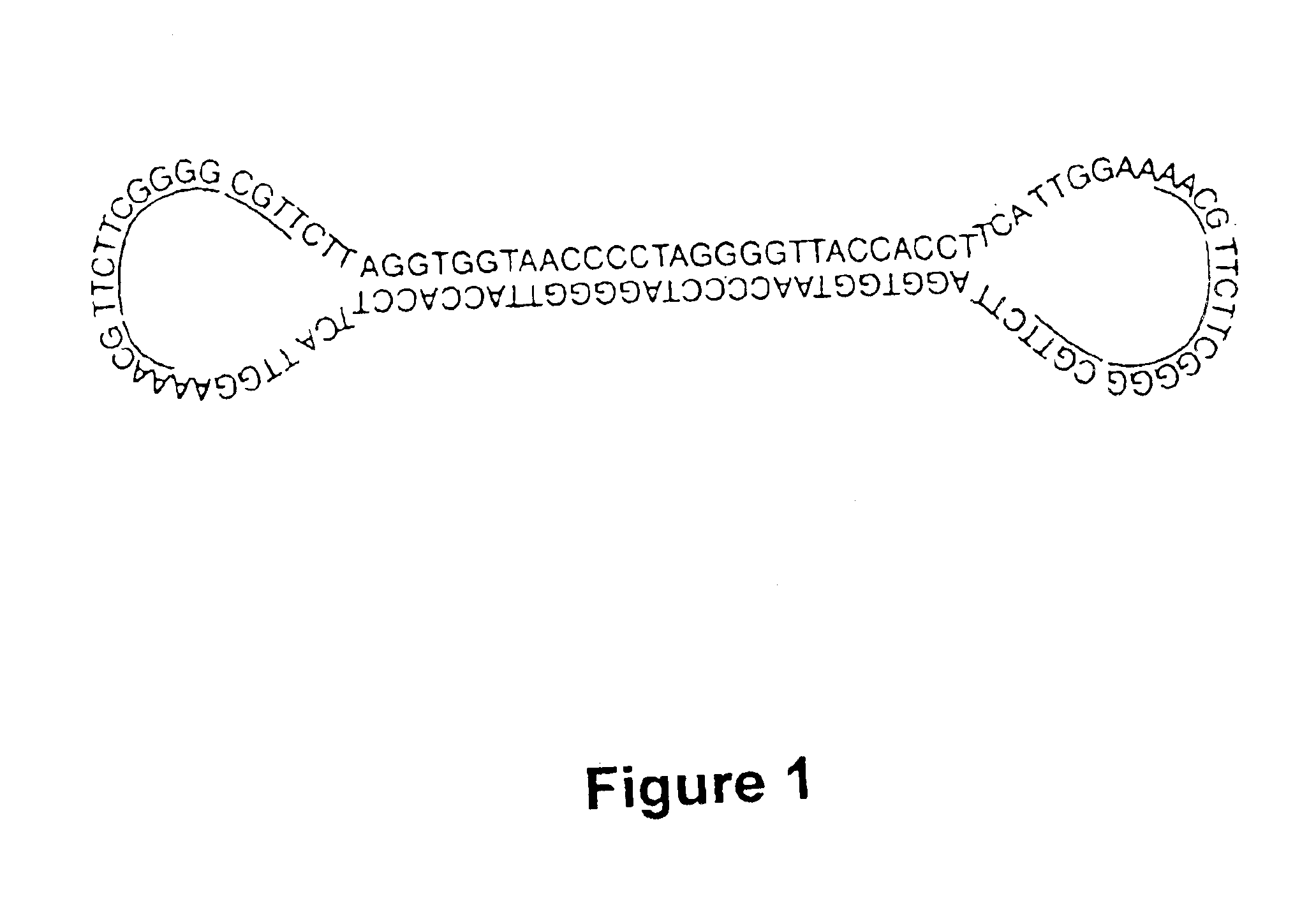
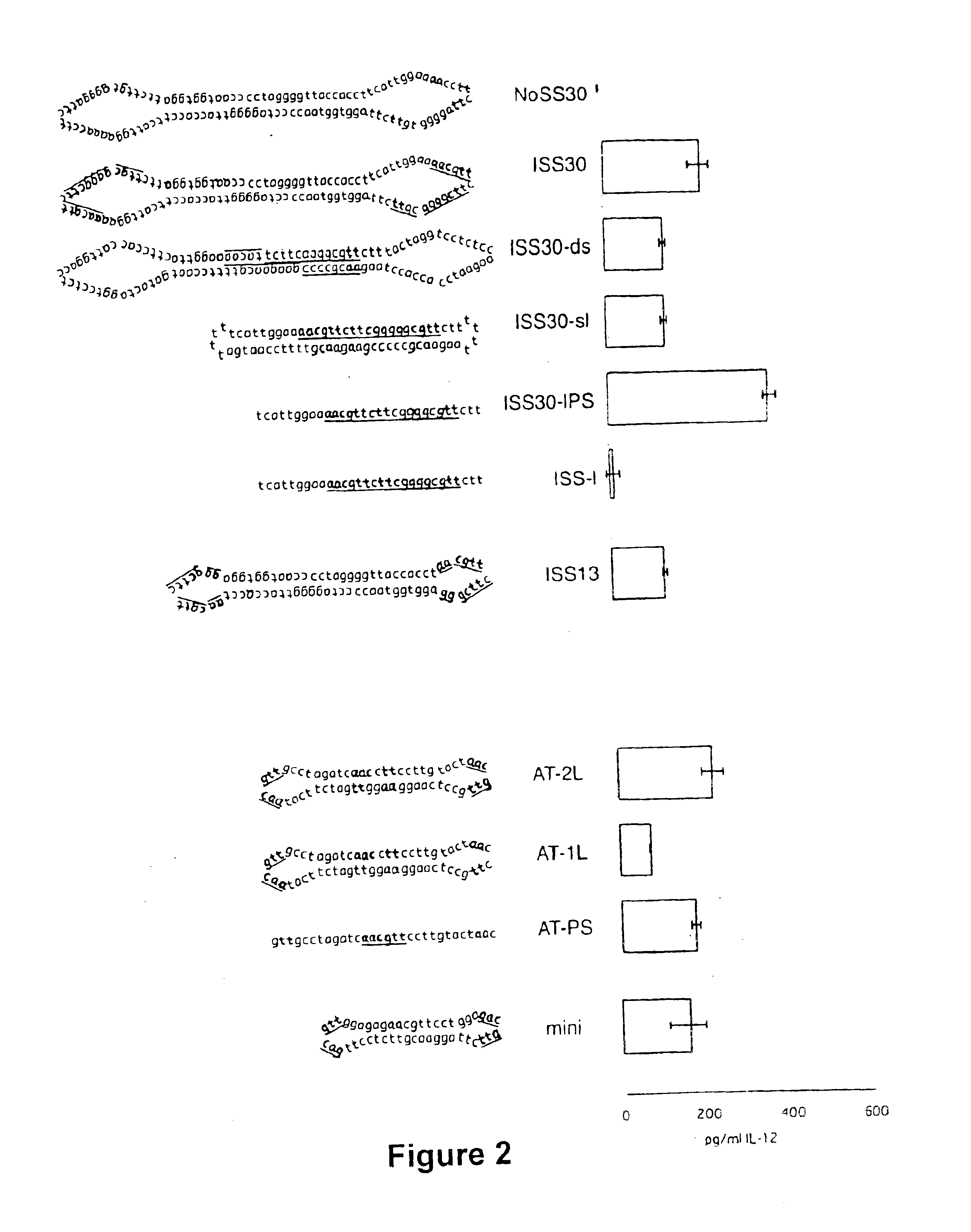
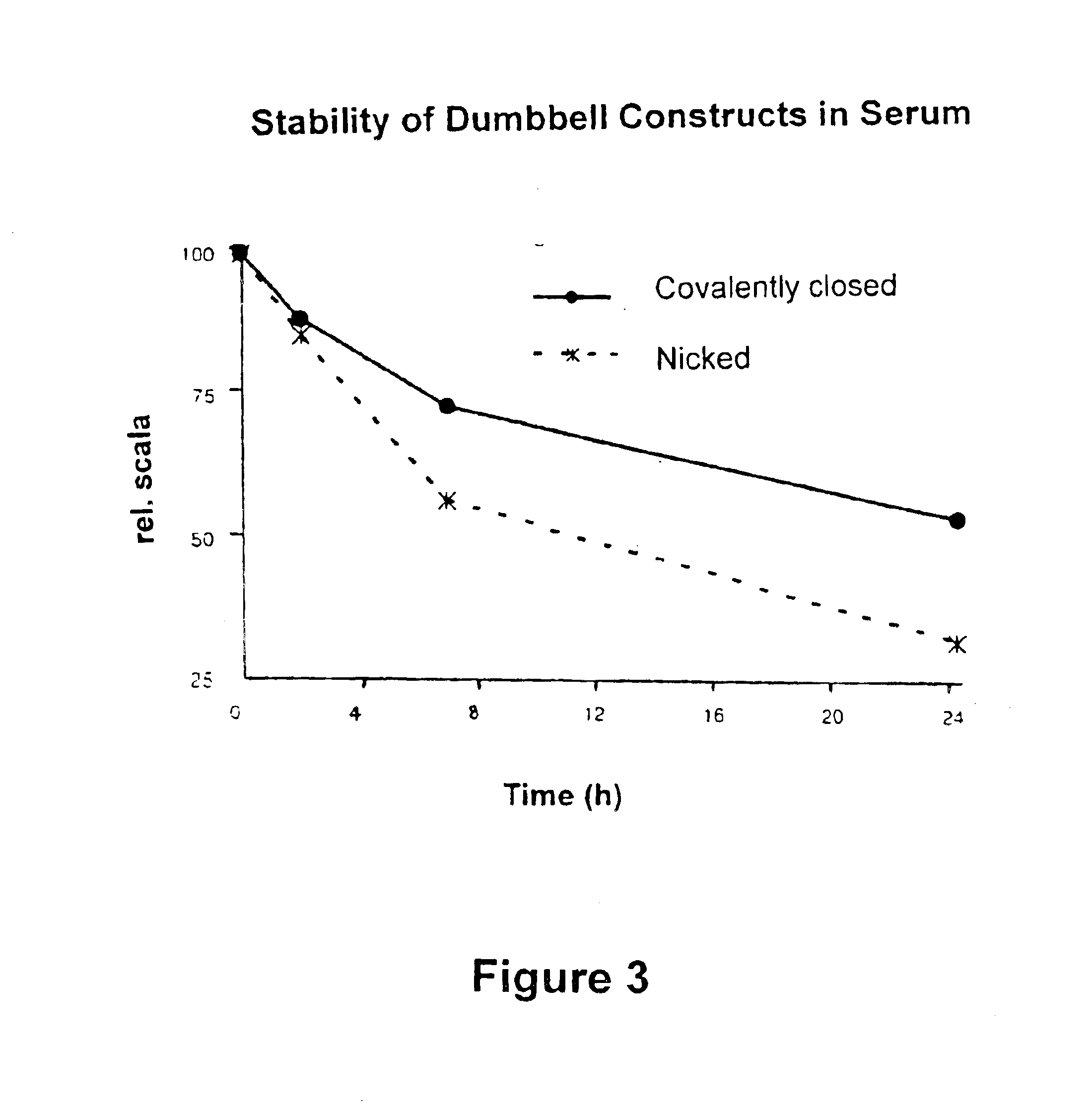
Covalently closed nucleic acid molecules for immunostimulation
InactiveUS6849725B2Improve the level ofIncrease irritationOrganic active ingredientsSugar derivativesCytosineSingle strand
Short deoxyribonucleic acid molecules that are partially single-stranded, dumbbell-shaped, and covalently closed, which contain one or more unmethylated cytosine guanosine motif (CpG motif) and exhibit immunomodifying effects. Such molecules can be used for immunostimulation applications in humans or vertebrates.
Owner:GILEAD SCI INC
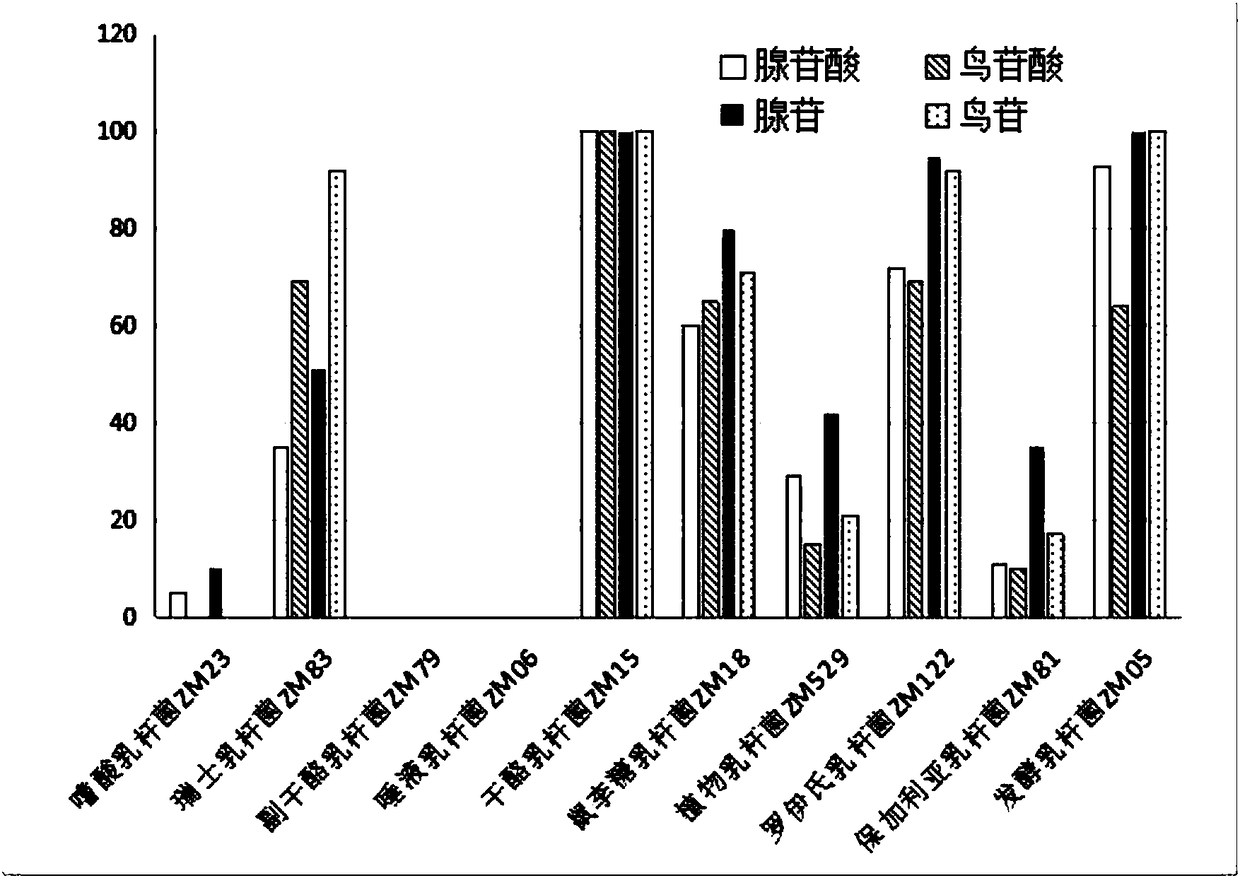
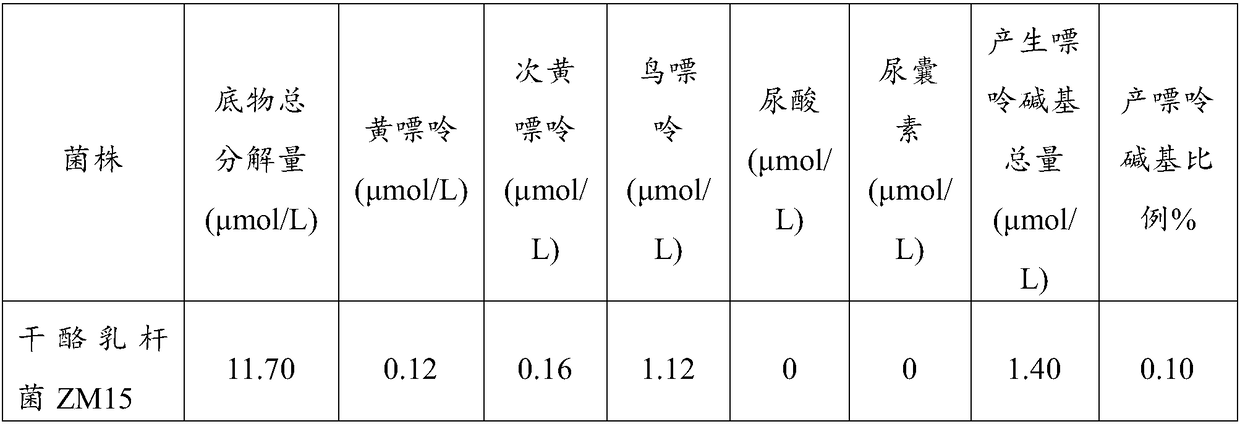
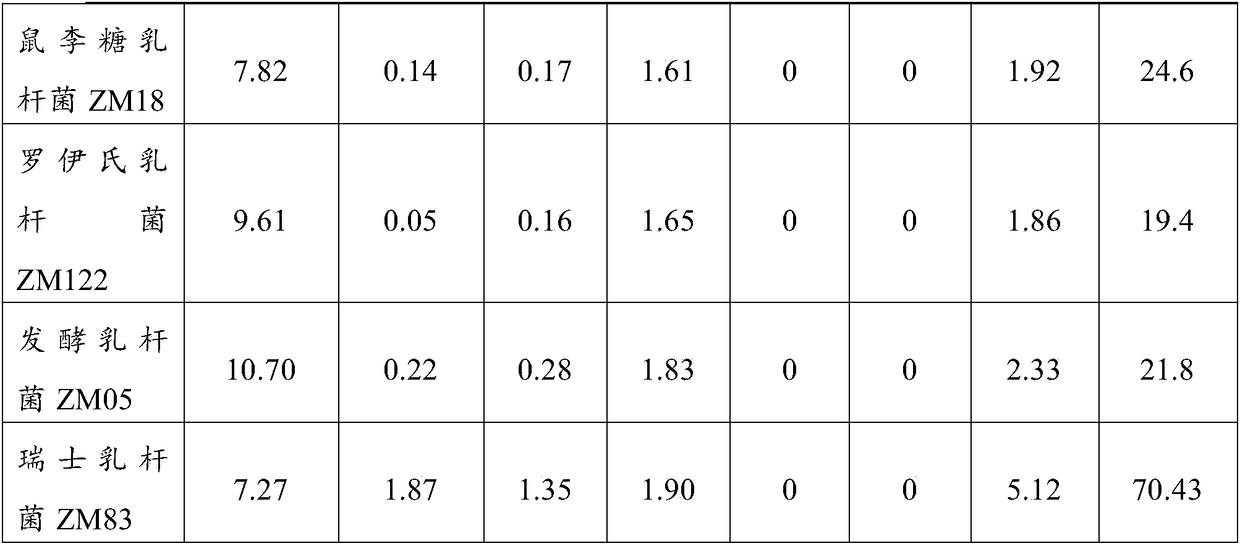
Lactobacillus casei strain and probiotic composition for lowering blood uric acid and application thereof
The invention provides a lactobacillus casei strain and a probiotic composition for lowering blood uric acid and application thereof, and belongs to the technical field of food medicines. The lactobacillus casei strain ZM15 for lowering the blood uric acid has the collection number of CGMCC No.13980. The lactobacillus casei strain ZM15 has the rates of degrading adenylic acid, guanylic acid, adenosine and guanosine as high as 100%; a degraded product does not contain uric acid and allantoin; in addition, the degraded product has a low purine base proportion of only 0.10%, so that the product can be prevented from being further decomposed into the uric acid, and the human blood uric acid content can be effectively reduced. The probiotic composition provided by the invention is obtained by compounding four strains of ZM15, ZM18, ZM122 and ZM05; through mutual coordination of the four strains, the blood uric acid lowering effect of the probiotic composition is significantly better than that during independent application of ZM15, ZM18, ZM122 and ZM05 strains, so that the blood uric acid lowering effect can be further improved.
Owner:JIAXING INNOCUL PROBIOTICS CO LTD
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Phosphoramidate Derivatives of Guanosine Nucleoside Compunds for Treatment of Viral Infections
Phosphoramidate compounds derived from guanine bases having enhanced therapeutic potency are provided, and these compounds in particular have enhanced potency with respect to treatment of viral infections, such as hepatitis C virus. Pharmaceutical compositions, methods of preparing the compounds, and methods of using the compounds and compositions to treat viral infections are also provided.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Method for synthesizing nelarabine
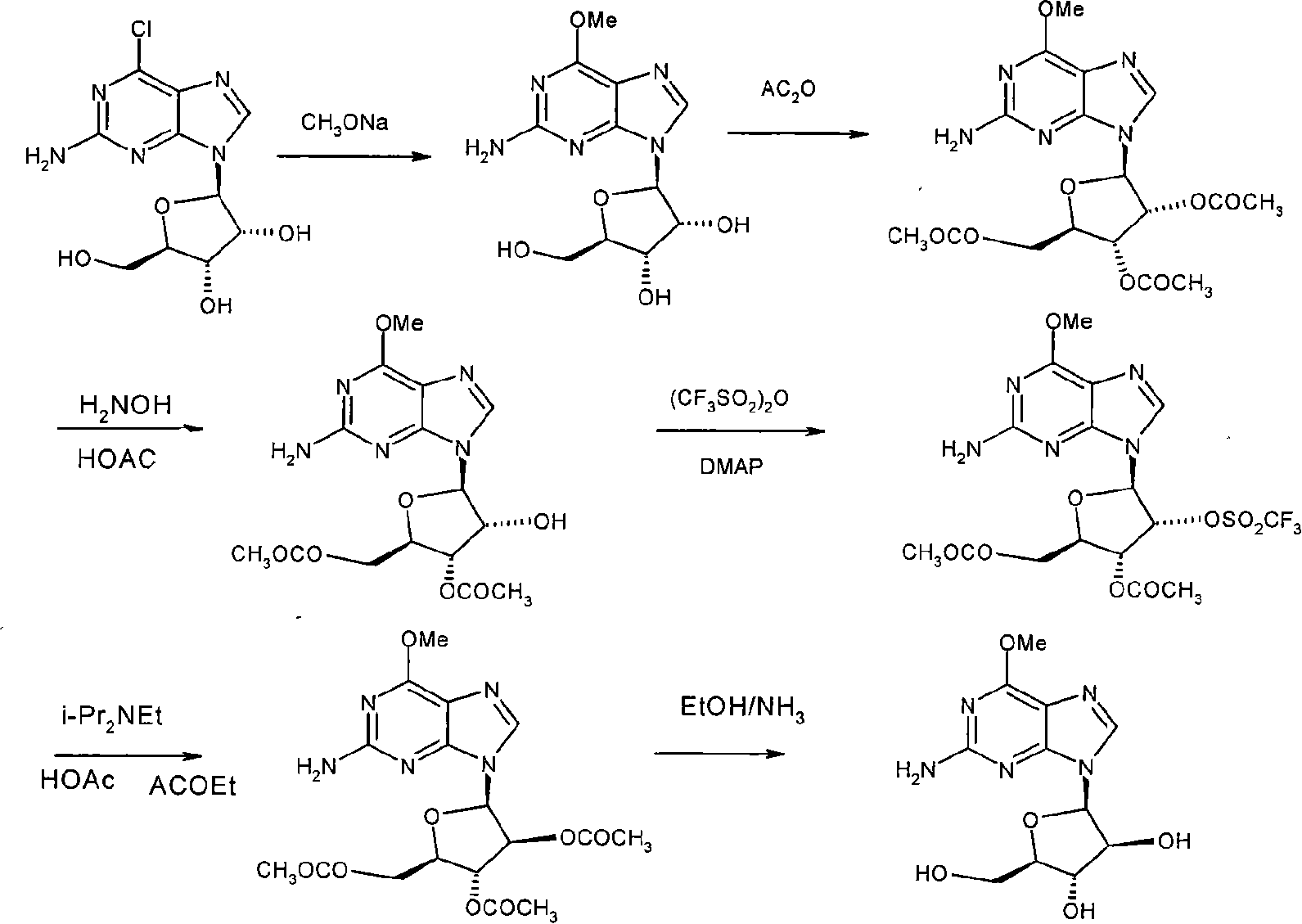
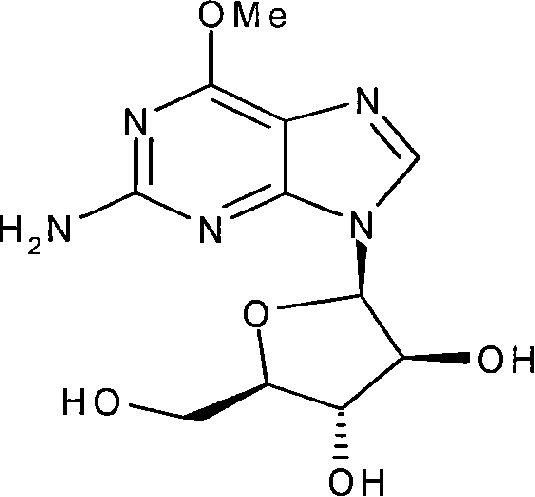
This invention discloses a method for synthesizing nelarabine. The method comprises: utilizing 6-chloroguanosine as the raw material, and performing chemical synthesis to convert ribofuranose configuration into arabinofuranosyl configuration and obtain nelarabine. The method has such advantages as abundant raw materials, simple process, easy operation and high yield, and is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Anti-viral guanosine-rich oligonucleotides and method of treating HIV
A method and compositions for treating viral infection in vitro and in vivo using a guanosine-rich oligonucleotide. The oligonucleotides have sufficient guanosine to form a guanosine tetrad. Also provided are oligonucleotides of at least two runs of at least two guanosines. Also provided are guanosine-rich oligonucleotides and methods for treating viral infections in humans, and a method for designing guanosine-rich oligonucleotides having anti-viral activity and integrase inhibition activity.
Owner:ARONEX PHARMA +2
Soluble guanylate cyclase activators
A compound of Formula (I): or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Self-healing injectable supramolecular hydrogel and preparation method and application thereof
The invention relates to self-healing injectable supramolecular hydrogel and a preparation method and application thereof. The hydrogel is prepared by heating commercial guanosine and arylboronic acidcompound in brine or aqueous alkali and cooling. The preparation process is simple and green, is convenient to operate, requires no tedious synthesis or purification steps, has low production cost and is suitable for large-scale production. The prepared supramolecular hydrogel has good self-healing performance, viscoelasticity, injectability, ionic conductivity, mechanical property regulation anduse safety, and has a good application prospect in the field of ion skin and gel electrolytes.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
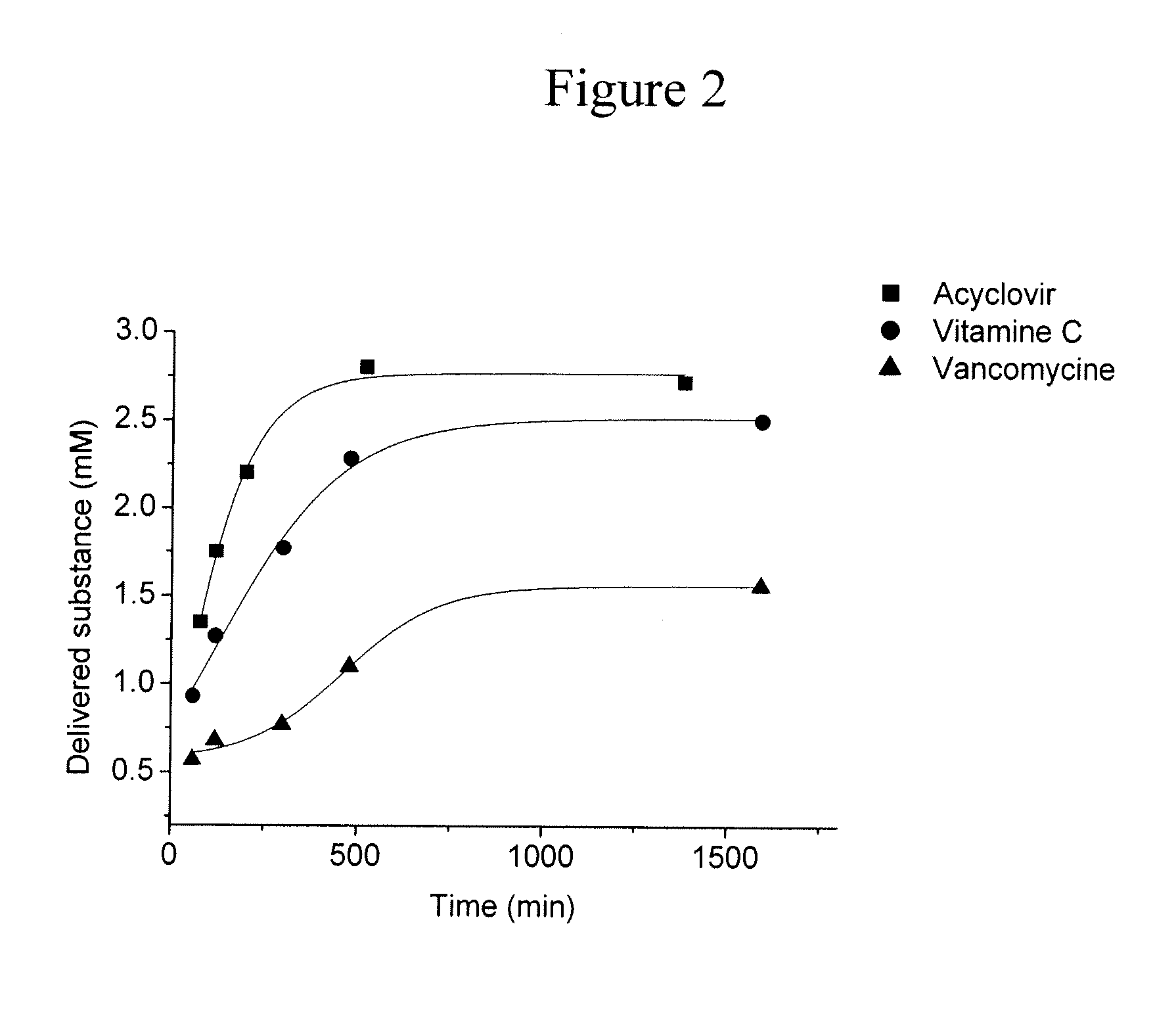
Hydrogels for the controlled release of bioactive materials
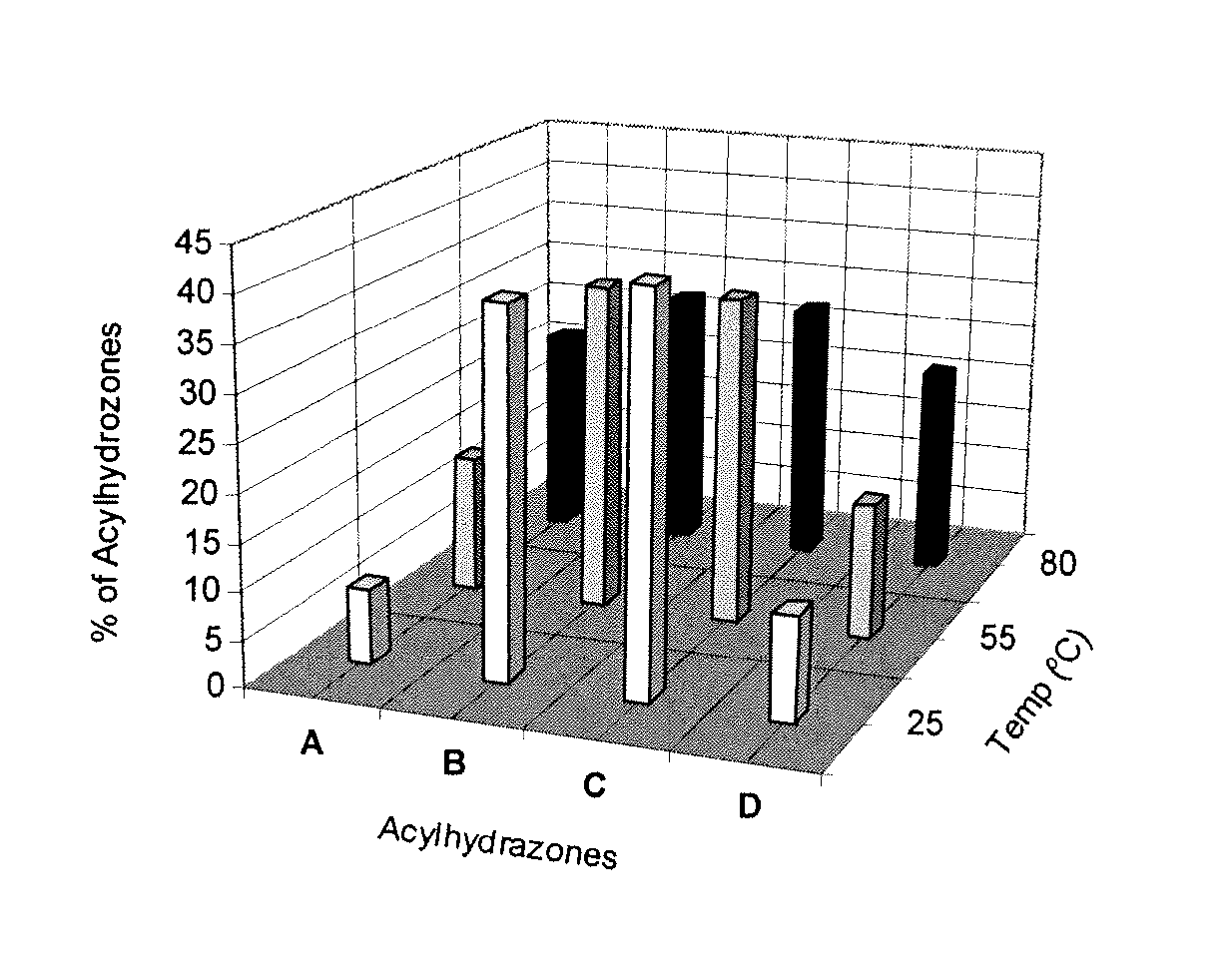
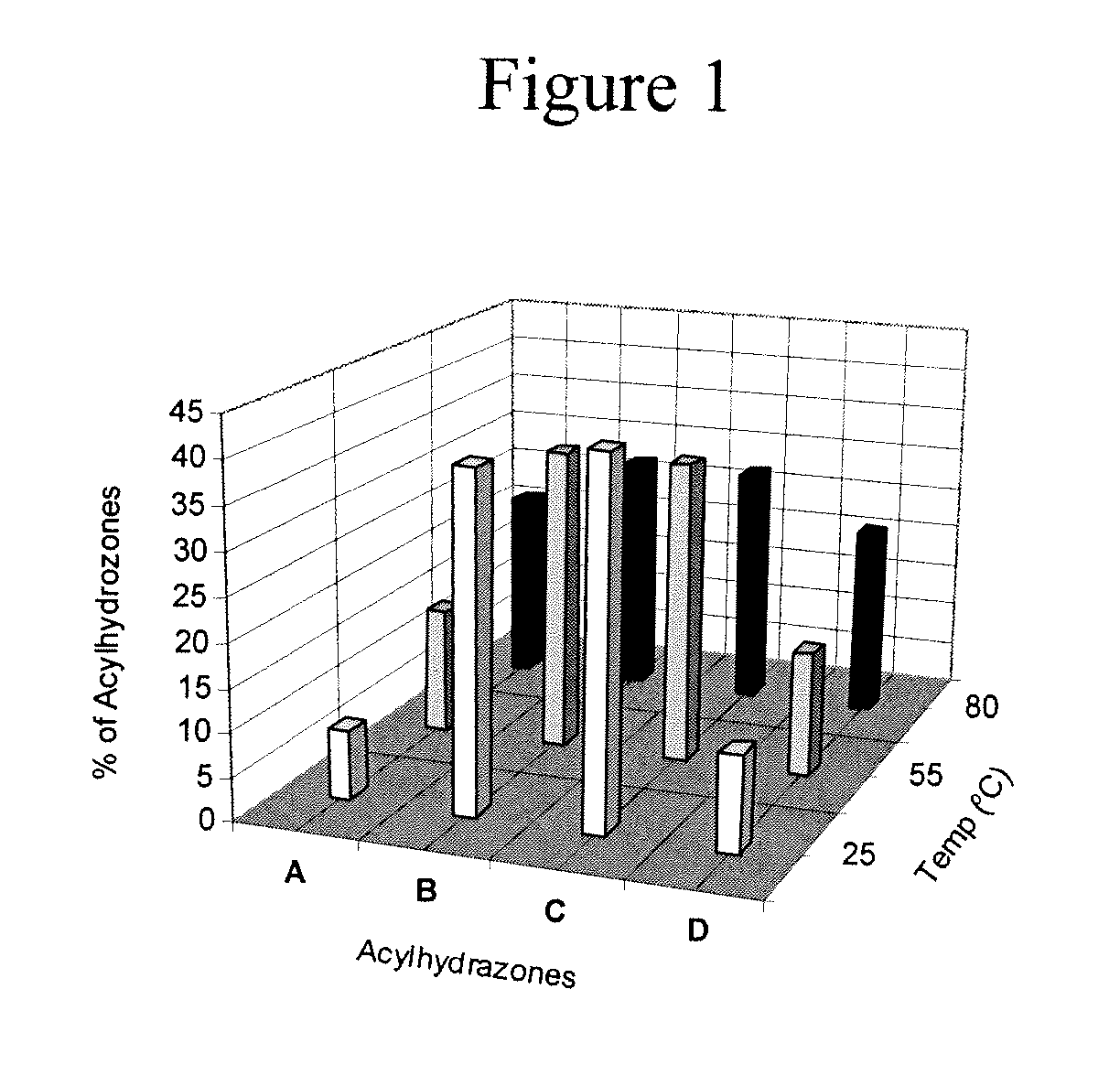
The present invention relates to the formation of hydrogels based on guanosine hydrazide derivatives in the presence of cations. The hydrogels can be used as a carrier / delivery system for biologically active substances such as flavors, fragrances, insect attractants or repellents, bactericides, fungicides, pharmaceuticals or agrochemicals.
Owner:UNIV LOUIS PASTEUR ULP +2
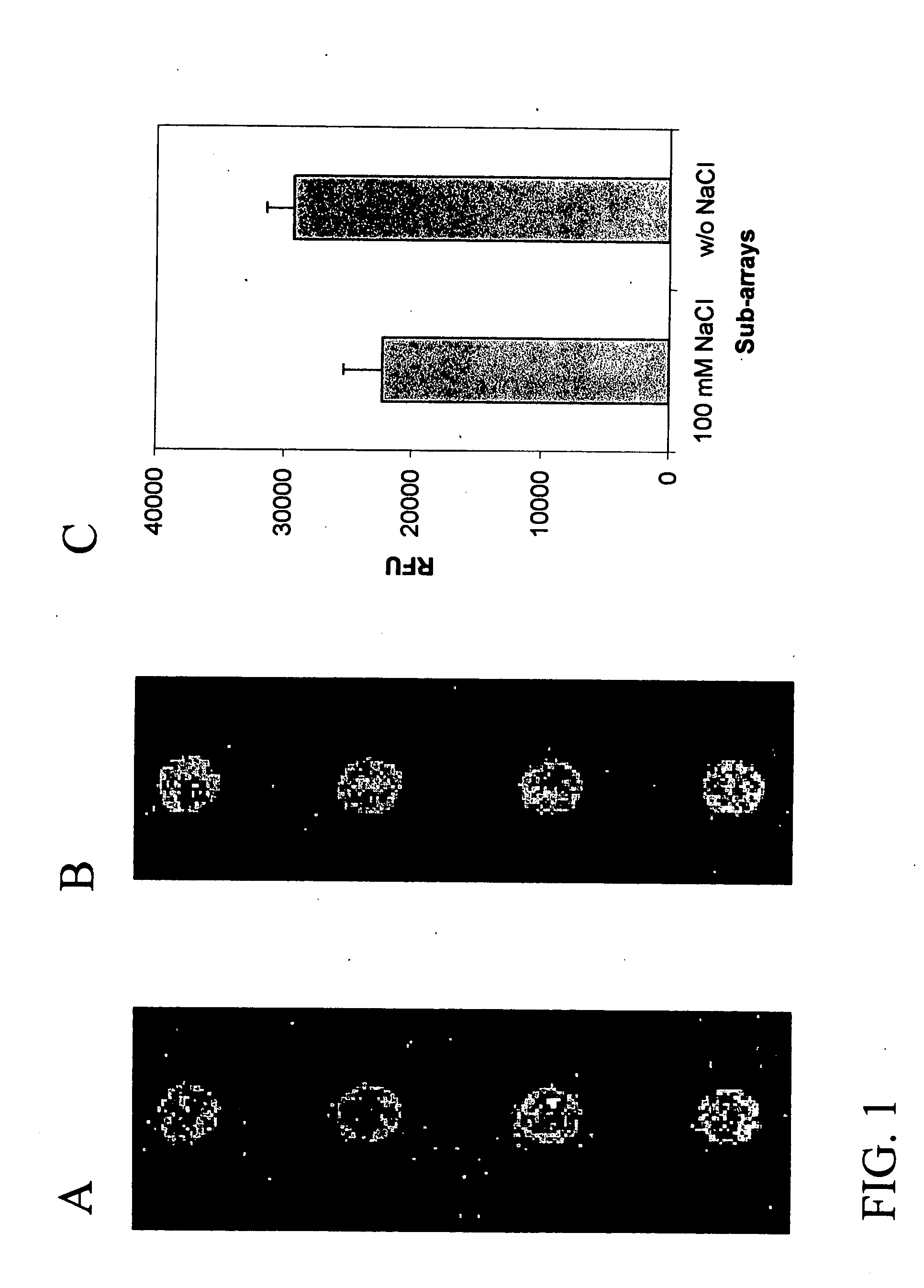
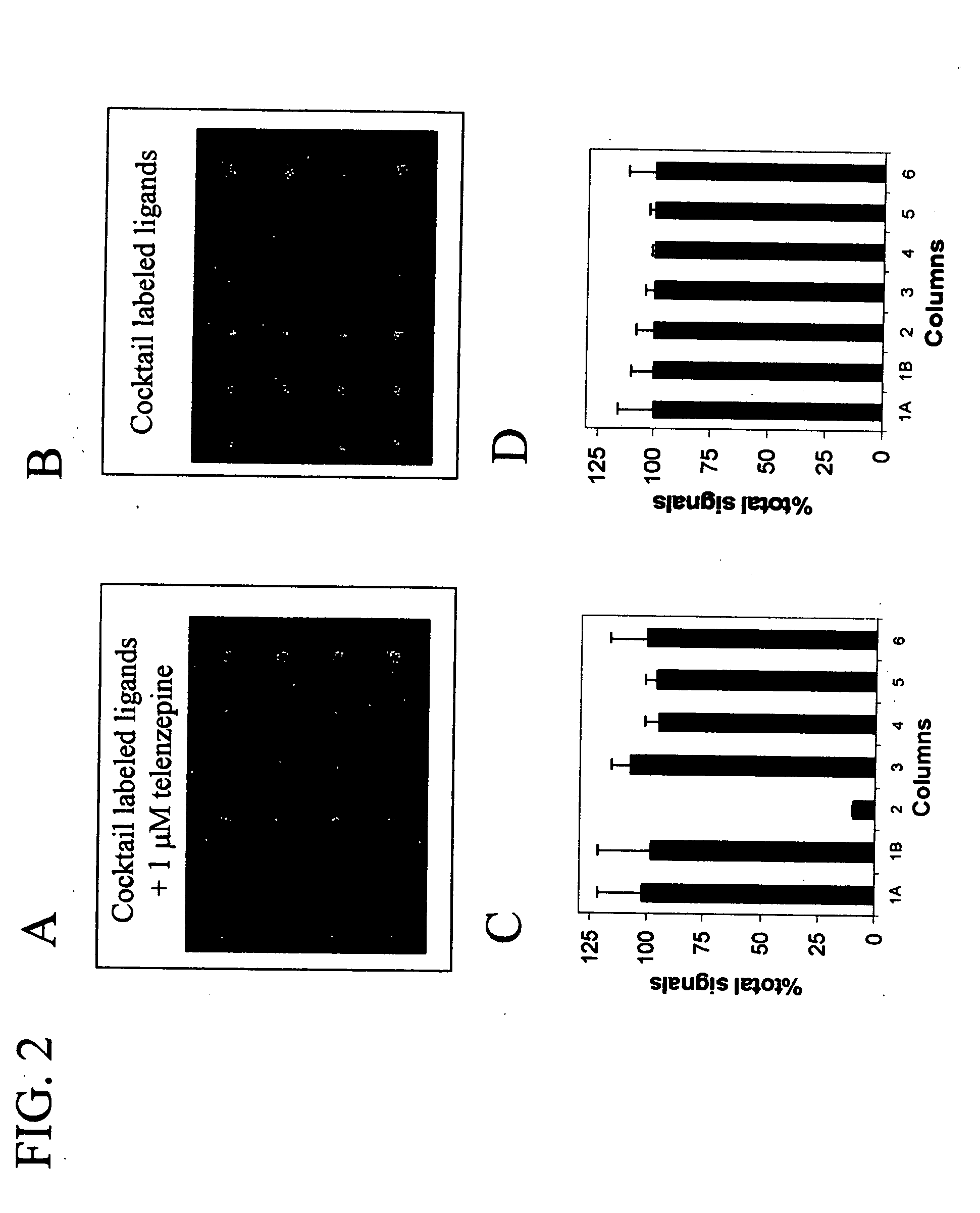
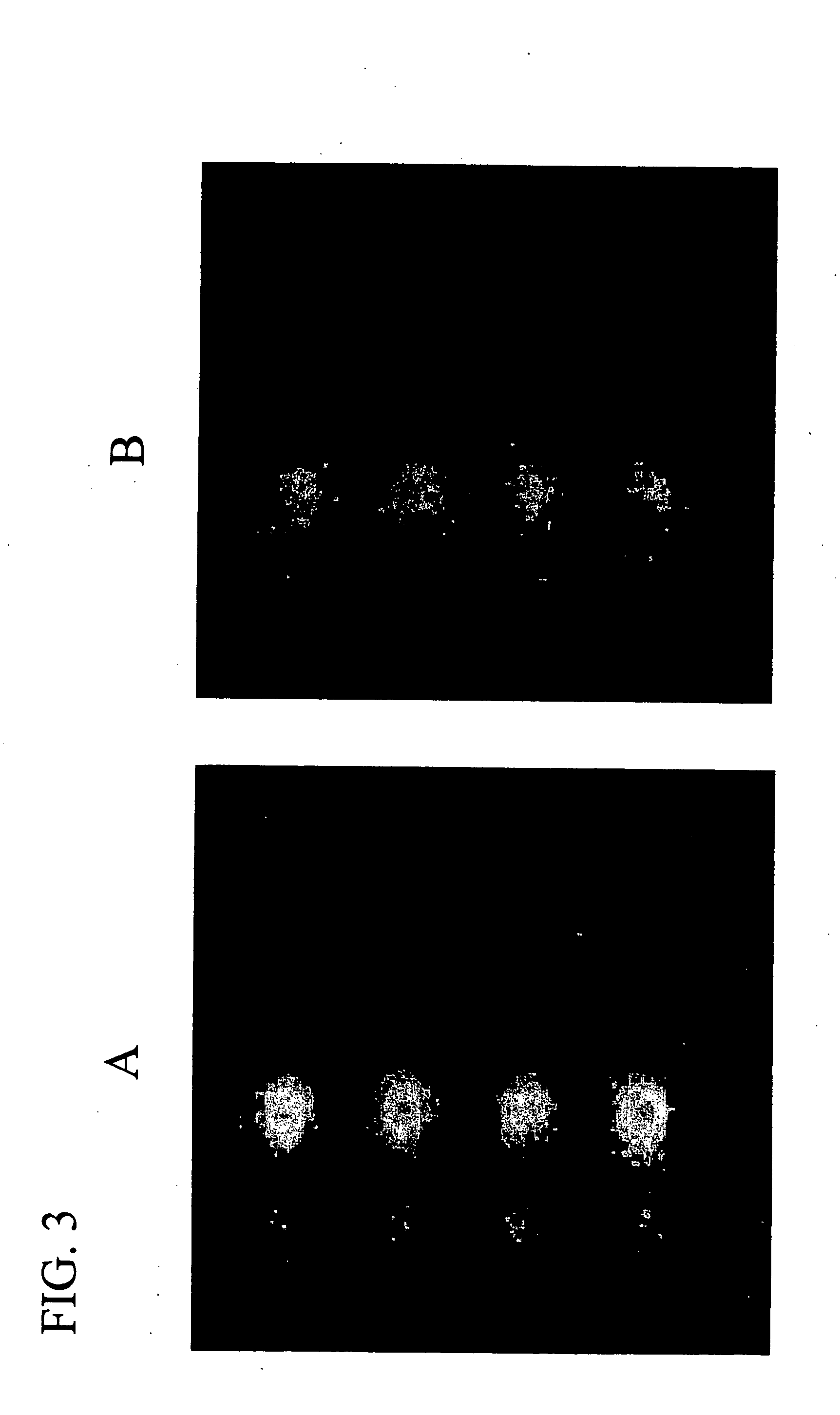
Assay solution compositions and methods for GPCR arrays
InactiveUS20050069953A1Reduce background signalBiological material analysisBiological testingInorganic saltsFunctional assay
Buffered assay solutions for performing 1) binding or 2) functional assays on GPCR arrays, along with methods for their use are described. The buffered assay solution has an underlying composition having: a buffer reagent with a pH in the range of about 6.5 to about 7.9; an inorganic salt of either a monovalent or divalent species, at a concentration from about 1 mM to about 500 mM; and optionally a combination of: c) a blocker reagent at a concentration of about 0.01 wt. % to about 2 wt. % of the composition, or d) protease-inhibitor at a concentration of about 0.001 mM to about 100 mM. In an embodiment for functional assay uses, the composition is modified to also include a GTP-analogue, a guanosine 5′-diphosphate (GDP) salt, and / or an anti-oxidant reagent.
Owner:CORNING INC
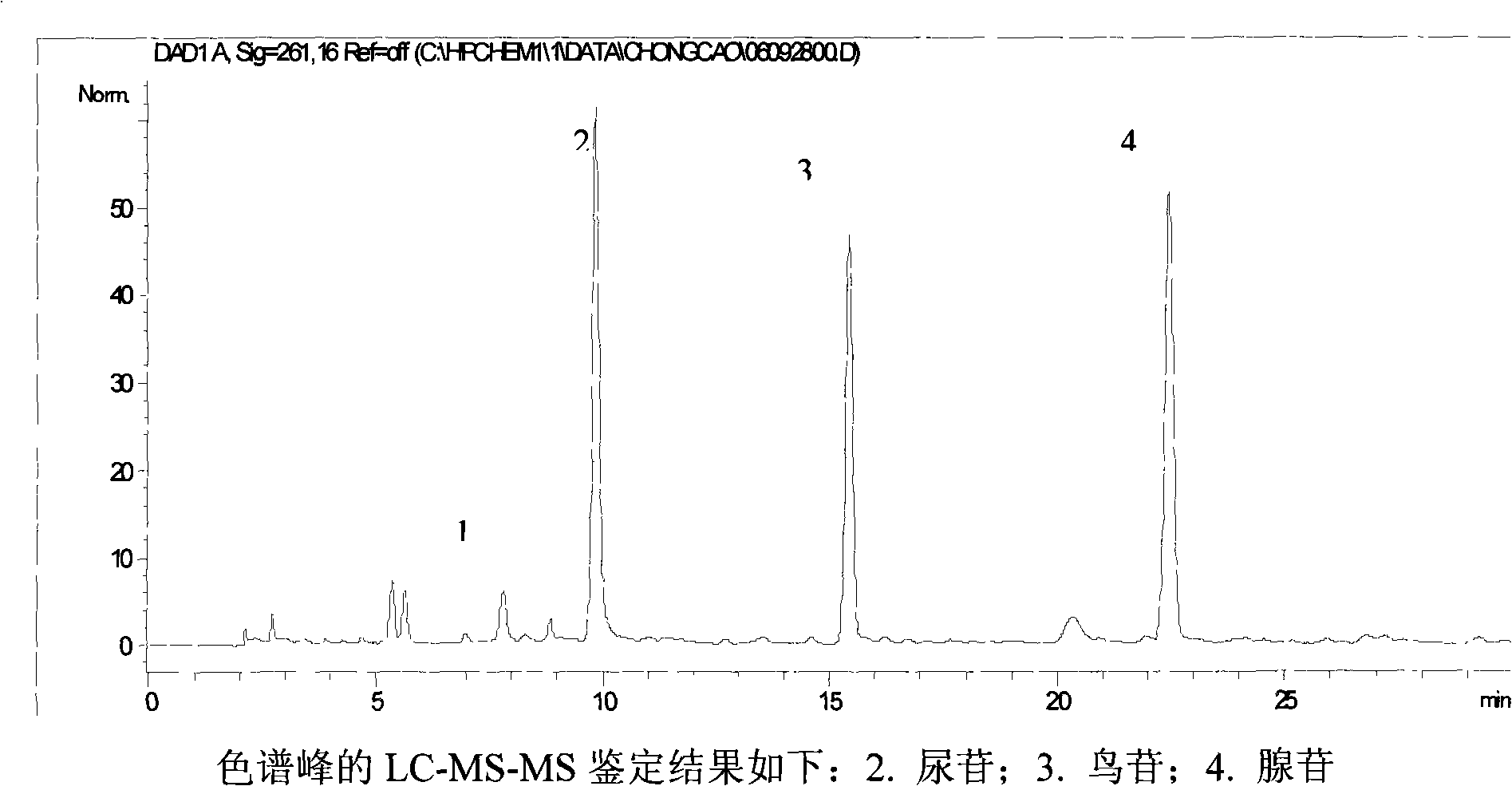
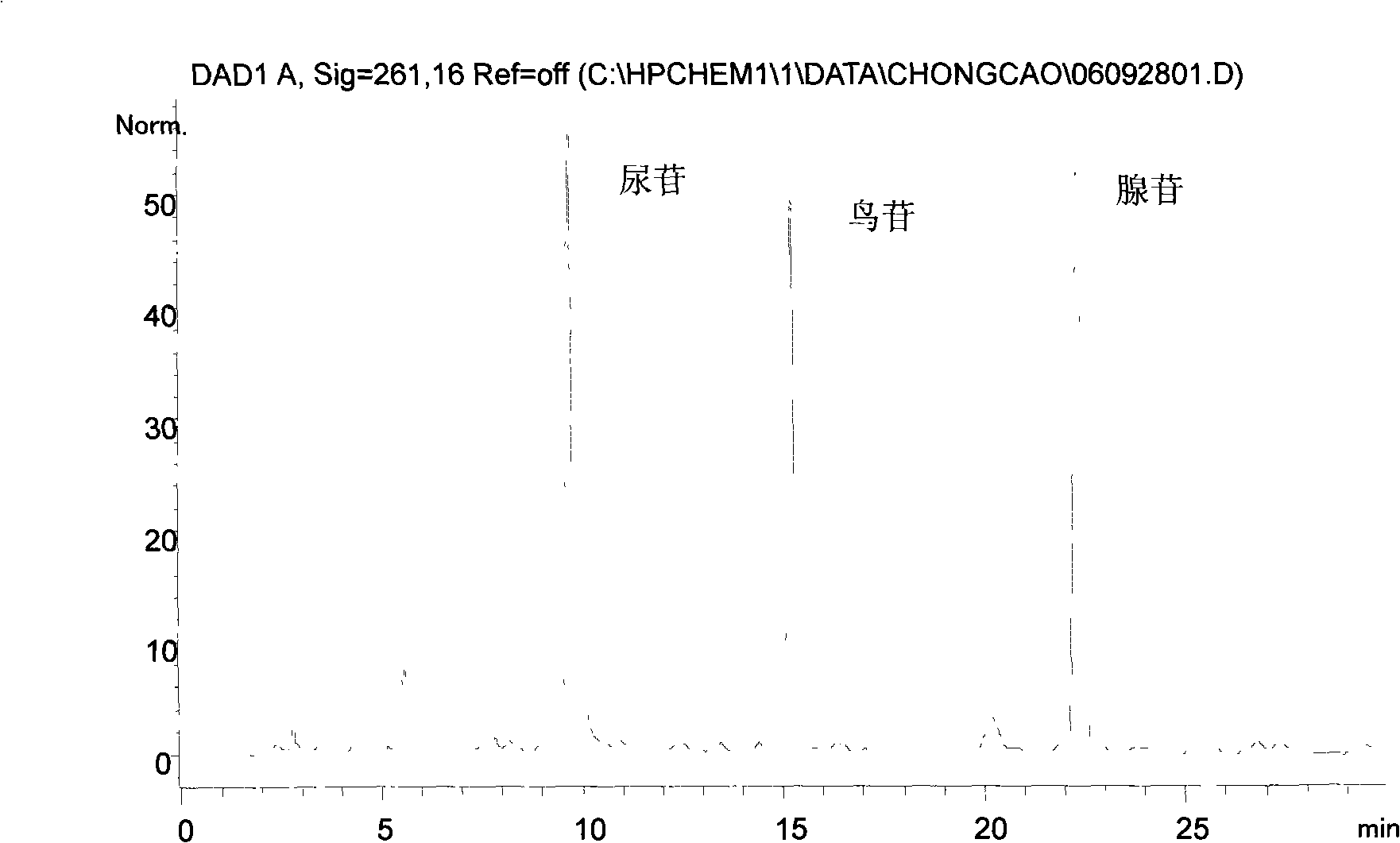
Fingerprint pattern quality control method for cordyceps sinensis bacterium powder raw material in herbs medicaments for strengthening the body resistance and activating blood and dissolving stasis
ActiveCN101293002AGuarantee normal implementationHigh sensitivityFungiComponent separationHplc fingerprintRetention time
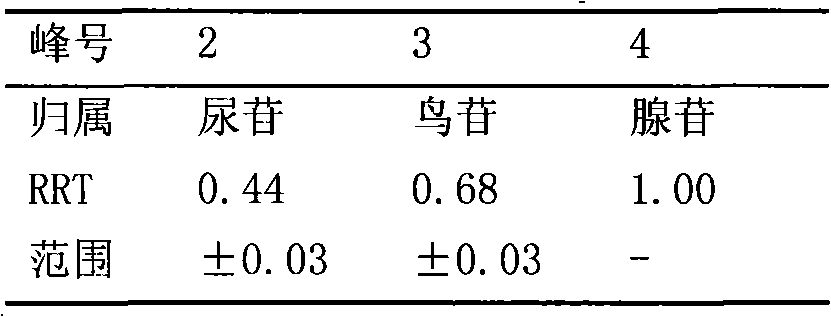
The invention relates to a control method of the fingerprint spectrum quality of cordyceps sinensis powder raw material in botanical drug for strengthening vital qi and removing blood stasis, comprising the steps that: (1) cordyceps sinensis powder is extracted: 0.100g of cordyceps sinensis powder is taken, purified water is added, the ultrasonic extraction, the filtration and the sample injection are carried out; (2) the gradient elution with mobile phase is carried out: octadecyl silane bonded silica gel is taken as a filler, water and acetonitrile are taken as mobile phase to carry out the gradient elution for 0 to 30min and 0 to 7 percent B; (3) a standard fingerprint spectrum is established: the HPLC standard fingerprint spectrum of the cordyceps sinensis powder is determined, and 3 characteristic peaks are selected; (4) the quality control of the fingerprint spectrum is carried out: the relative retention time of No.2 peak uridine, No.3 peak guanosine and No.4 peak adenosine are 0.44 plus or minus 0.03, 0.68 plus or minus 0.03 and 1.00 respectively; the HPLC fingerprint spectrum of the sample is compared with the contrast fingerprint spectrum. The similarity calculated by the 5 common peaks is not less than 0.9, (5) the preparation of the cordyceps sinensis powder raw material is carried out; the control method has good repetitivity and can fully reflect the basic characteristics of nucleoside ingredients of the cordyceps sinensis powder.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Substituted, non-coding nucleic acid molecule for therapeutic and prophylactic stimulation of the immune system in humans and higher animals
ActiveUS8017591B2High expressionGood effectOrganic active ingredientsSugar derivativesCytosineSingle strand
Short deoxyribonucleic acid molecules that are partially single-stranded, dumbbell shaped, and covalently closed, which contain one or more unmethylated cytosine guanosine motif (CpG motif) and exhibit immunomodifying effects. Such molecules can be used for immunostimulation applications in humans and vertebrates.
Owner:GILEAD SCI INC
Phosphodiesterase inhibitors and nitric oxide donors, compositions and methods of use
The invention provides novel compositions containing at least one phosphodiesterase inhibitor, and at least one compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and / or one or more vasoactive agents. The invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing diseases induced by the increased metabolism of cyclic guanosine 3′,5′-monophosphate (cGMP).
Owner:NITROMED
Composition and preparation method of targeting liposome-cyclic dinucleotide and application of targeting liposome-cyclic dinucleotide to anti-tumor
ActiveCN106667914AProlong metabolic cycleEnhance the effect of anti-tumor therapyOrganic active ingredientsNervous disorderTumor targetCholesterol
The invention belongs to the technical field of medicines, and particularly discloses a composition and a preparation method of cyclic dinucleotide cGAMP (cyclic Guanosine Adenosine Monophosphate) encapsulated by a targeting liposome and application of the cyclic dinucleotide cGAMP to anti-tumor. The targeting liposome consists of lecithin, cholesterol, polyethylene glycol and the like as well as a link targeting molecule of the targeting liposome; a cGAMP slow release drug encapsulated by the targeting liposome can be used for enhancing the intensifying cell osmosis action, enhancing an immune reaction, effectively targeting drug delivery and intensifying the inhibition on the growth of multiple tumor cells. Therefore, the cGAMP encapsulated by the targeting liposome can be used for preparing an anti-tumor targeting slow release drug, and has important potential application in the field of targeting immune anti-tumor.
Owner:HANGZHOU XINGAO BIOTECH CO LTD
Method for separating and purifying four nucleoside chemical ingredients from trichosanthes bark
ActiveCN103304613ASimple compositionNot pollutedSugar derivativesChemical recyclingPharmacologyChinese herbology
The invention relates to a method for separating and purifying four nucleoside chemical ingredients (namely adenine, guanosine, 6-iso inosine and adenosine) from traditional Chinese medicine trichosanthes bark. According to the method, four high-purity monomeric compounds, namely adenine, guanosine, 6-iso inosine and adenosine, are obtained from the trichosanthes bark through the following steps of: (1) preparing a crude trichosanthes bark extract; (2) carrying out crude separation by using a macroporous adsorption resin column; and (3) carrying out separation and purification by using semi-preparative high-performance liquid chromatography: carrying out separation and purification on obtained samples by using the semi-preparative high-performance liquid chromatography, wherein the mobile phase is methanol-water. The method disclosed by the invention has the advantages that the process flow is environment-friendly, the damage to the environment is not serious, and the comprehensive cost is low.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE +2
Assay solution compositions and methods for GPCR arrays
InactiveUS20060148006A1Peptide/protein ingredientsSnake antigen ingredientsInorganic saltsFunctional assay
Buffered assay solutions for performing 1) binding or 2) functional assays on GPCR arrays, along with methods for their use are described. The buffered assay solution has an underlying composition having: a buffer reagent with a pH in the range of about 6.5 to about 7.9; an inorganic salt of either a monovalent or divalent species, at a concentration from about 1 mM to about 500 mM; and optionally a combination of: c) a blocker reagent at a concentration of about 0.01 wt. % to about 2 wt. % of the composition, or d) protease-inhibitor at a concentration of about 0.001 mM to about 100 mM. In an embodiment for functional assay uses, the composition is modified to also include a GTP-analogue, a guanosine 5′-diphosphate (GDP) salt, and / or an anti-oxidant reagent.
Owner:CORNING INC
Synthesis of new fucose-containing carbohydrate derivatives
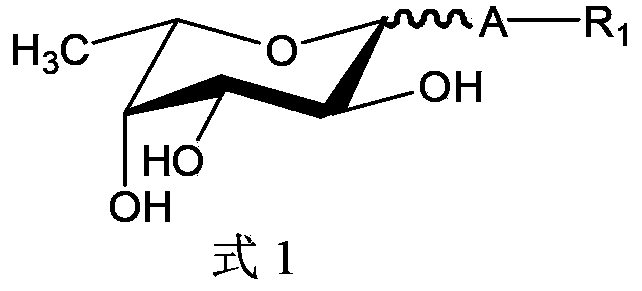
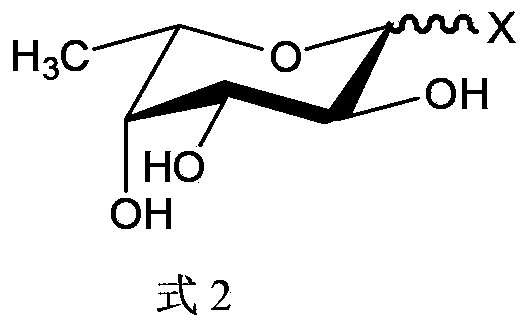
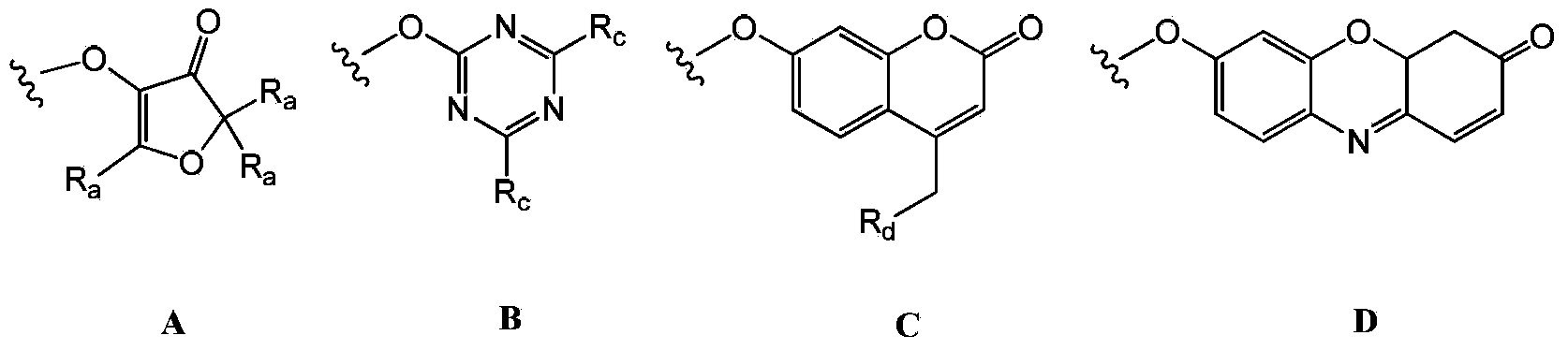
A method for the synthesis of a compound of formula (1) or a salt thereof, wherein A is a carbohydrate linker which is a lactosyl moiety or which consists of a lactosyl moiety and at least one monosaccharide unit selected from the group consisting of: glucose, galactose, N-acetylglucosamine, fucose and N-acetyl neuraminic acid; and wherein R1 is one of the following anomeric protecting groups: a) -OR2, wherein R2 is a protecting group removable by catalytic hydrogenolysis; b) -SR3, wherein R3 is an optionally substituted alkyl, an optionally substituted aryl or an optionally substituted benzyl; c) -NH- C(R")=C(R')2, wherein each R' independently is one of the following electron withdrawing groups: -CN, -COOH, -COO-alkyl, -CO-alkyl, -CONH2, -CONH- alkyl or -CON(alkyl)2, or wherein the two R'-groups are linked together and form -CO-(CH2)2-4-CO- and thus form, together with the carbon atom to which they are attached, a 5-7 membered cycloalkan-1,3-dione, in which dione any of the methylene groups is optionally substituted with 1 or 2 alkyl groups, and R" is H or alkyl, in which a fucosyl donor of formula (2) wherein X is selected from the group consisting of: a guanosine diphosphatyl moiety, a lactose moiety, azide, fluoride, optionally substituted phenoxy-, optionally substituted pyridinyloxy-, optionally substituted 3-oxo-furanyloxy- of formula (A), optionally substituted 1,3,5-triazinyloxy- of formula (B), 4-methylumbelliferyloxy-group of formula (C), and a group of formula (D) wherein Ra is independently H or alkyl, or two vicinal Ra groups represent a=C(Rb)2 group, wherein Rb is independently H or alkyl, Rc is independently selected from the group consisting of alkoxy, amino, alkylamino and dialkylamino, Rd is selected from the group consisting of H, alkyl and -C(=O)Re, wherein Re is OH, alkoxy, amino, alkylamino, dialkylamino, hydrazino, alkylhydrazino, dialkylhydrazino or trialkylhydrazino, is reacted with an acceptor of formula H-A-R1 or a salt thereof, wherein A and R1 are as defined above, under the catalysis of an enzyme capable of transferring fucose. A compound of formula 1', its use in manufacture of human milk oligosaccharides, a method of manufacture of human milk oligosaccharides, and a fucosyl donor are also provided.
Owner:GLYCOM AS (DK)
Synthetic circulation production process for guanine nucleoside
InactiveCN101492484AEfficient use ofIn line with the new concept of green environmental protectionSugar derivativesAnimal feeding stuffFiltration membraneUltrafiltration
The invention provides a comprehensive recycling production process of guanosine. The bacterial suspension collected from the process in which guanosine is extracted and refined from guanosine fermented liquid is dried as feed additive or made into feed protein; the primary mother liquid acquired from the first four times during micro-filtrate membrane filtration, crude crystallization and pressure filtration and separation is sent into a reverse osmosis membrane for condensation and then mixed with the guanosine fermented liquid for internal recycling; the primary mother liquid acquired from the fifth time undergoes evaporation concentration, crystallization, drying and granulation to produce ammonium sulfate and other bio-compound fertilizers; the secondary mother liquid acquired from ultrafiltration membrane treatment is reused as micro-filtration membrane dialysis water; the third mother liquid acquired from extract crystallization and pressure filtration and separation is respectively reused as anti-crystallinic water of anti-crystallinic jar and dialysis water of micro-filtration membrane and ultrafiltration membrane. The invention overcomes the defects of the traditional process such as incomprehensive utilization, resources waste and environmental pollution; the comprehensive cycling process is reasonable and feasible and conforms to the green environmental protection concept, thus cleaning the environment, promoting the ecological balance, reducing the production cost and strengthening the social and economic benefits.
Owner:湖南汉晶瑞氨基酸有限公司
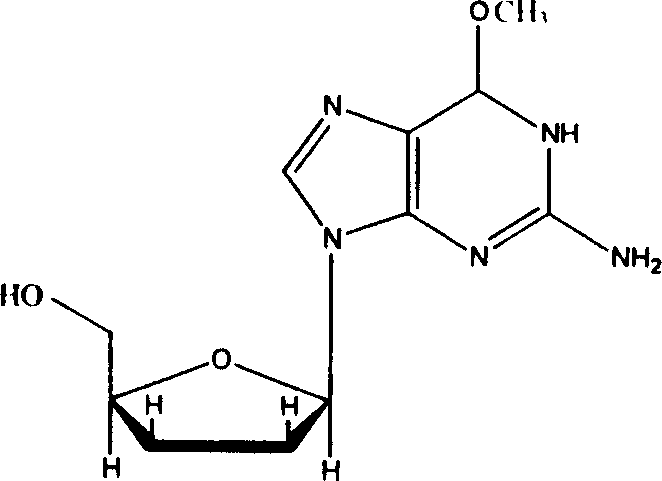
Application of 6-methocy bideoxy bideoxy guanosine in preparation of antihepatitis B medicine
InactiveCN1493301AImprove bioavailabilityOrganic active ingredientsDigestive systemHigh resistanceHepatitis B virus
Owner:GUANGZHOU YIPINHONG PHARMA
Method for detecting quality of traditional Chinese medicine indigowoad root granules
ActiveCN101912428AGuaranteed to be scientificGuaranteed growthComponent separationAntiviralsAdenosineElectro spray
The invention provides a method for detecting the quality of traditional Chinese medicine indigowoad root granules. The method comprises the following steps of: detecting the traditional Chinese medicine indigowoad root granules by using a soft-ionization mass spectrometry technique; performing primary evaluation on the number and strength of characteristic fingerprint peaks in an electro-spray fingerprint; measuring by high performance liquid chromatography to obtain the peak area of a chromatographic peak corresponding to each of cytidine, adenine, uridine, guanosine and adenosine serving as standard reference substances; and measuring according to a standard curve to obtain the cytidine content, adenine content, uridine content, guanosine content and adenosine content of one gram of the traditional Chinese medicine indigowoad root granules: 0.234 to 0.238 milligrams of cytidine, 0.116 to 0.118 milligrams of adenine, 0.472 to 0.474 milligrams of uridine, 0.331 to 0.334 milligrams of guanosine and 0.447 to 0.450 milligrams of adenosine. The method has the advantages of ensuring the scientificity and progressiveness of the method for detecting the quality of the traditional Chinese medicine indigowoad root granules, along with qualitative and quantitative performance.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Compositions for reducing the deleterious effects of stress and aging
InactiveUS20120045426A1Reduce the amount requiredIncreasing tissue perfusionOrganic active ingredientsDipeptide ingredientsAdjuvantMedicine
The invention provides a formulation for treating stress and lessening fatigue. The formulation can be combined with water or another suitable liquid to provide a beverage for ease of administration. The formulation can include one or more of an energy compound, a vasodilator, a vasodilator adjuvant, and an antioxidant enhancer. In a typical formulation the energy compound is D-ribose or guanosine. The formulation can improve energy and alertness, and reduce the effects of stress and fatigue.
Owner:HYDROPEP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com