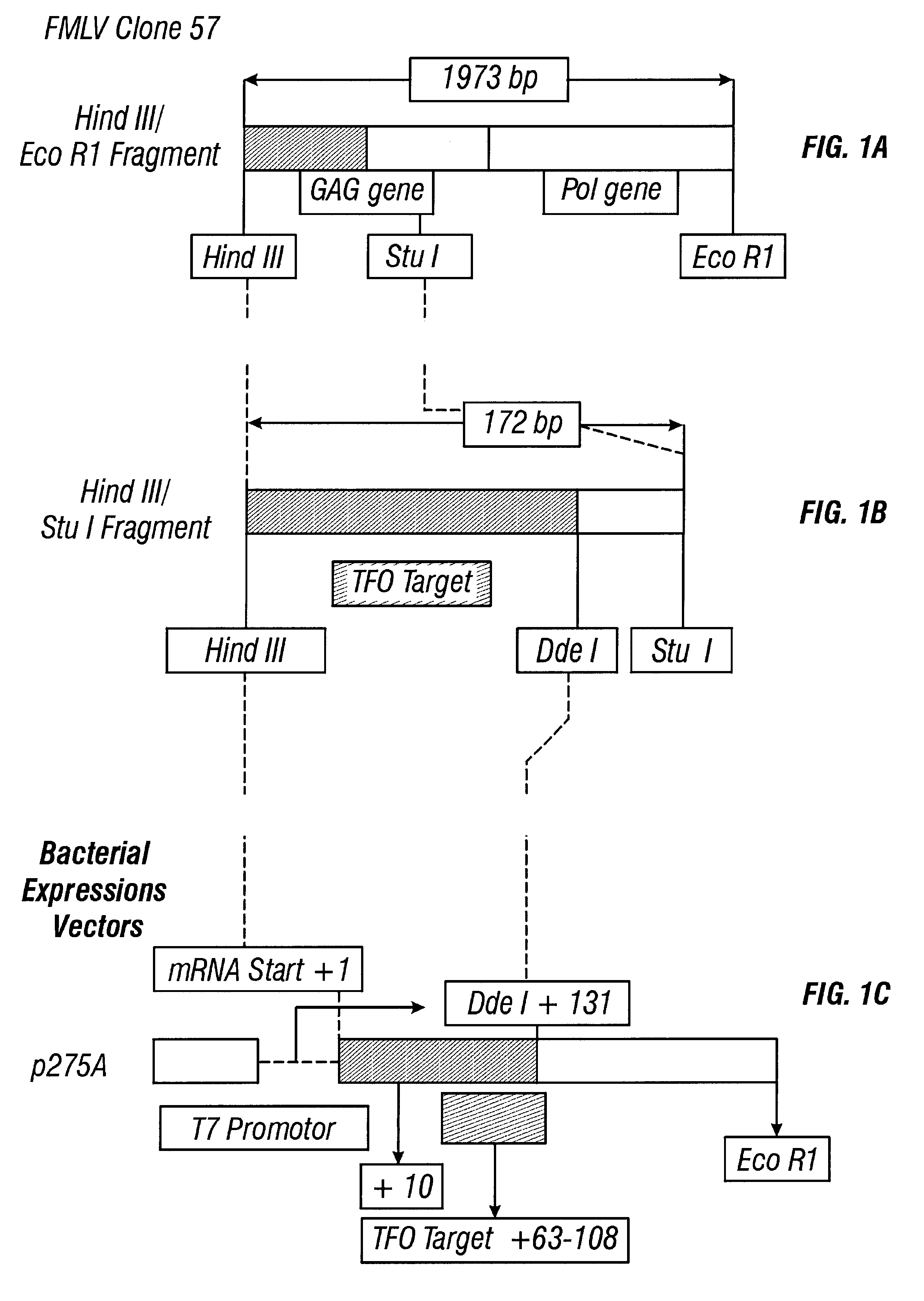
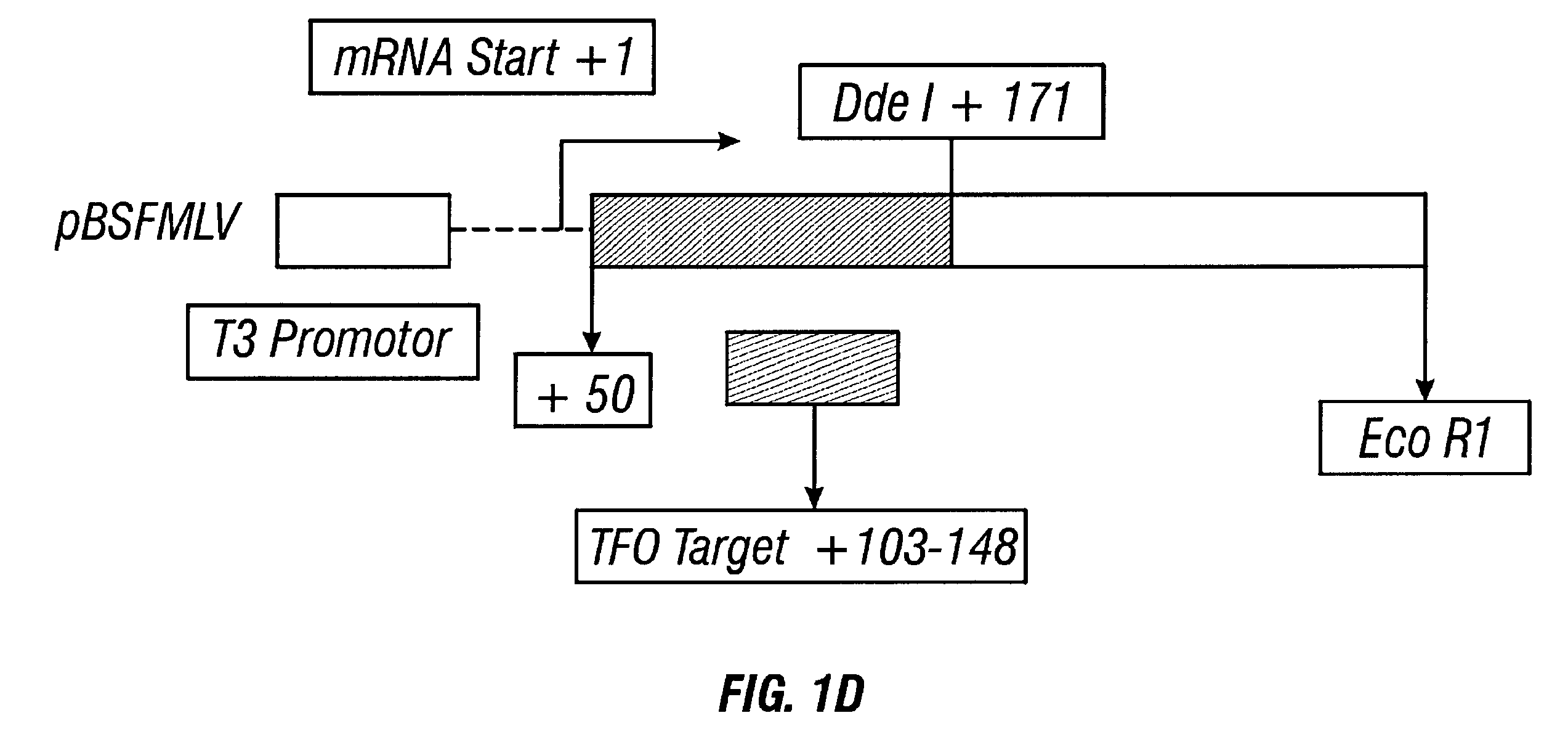
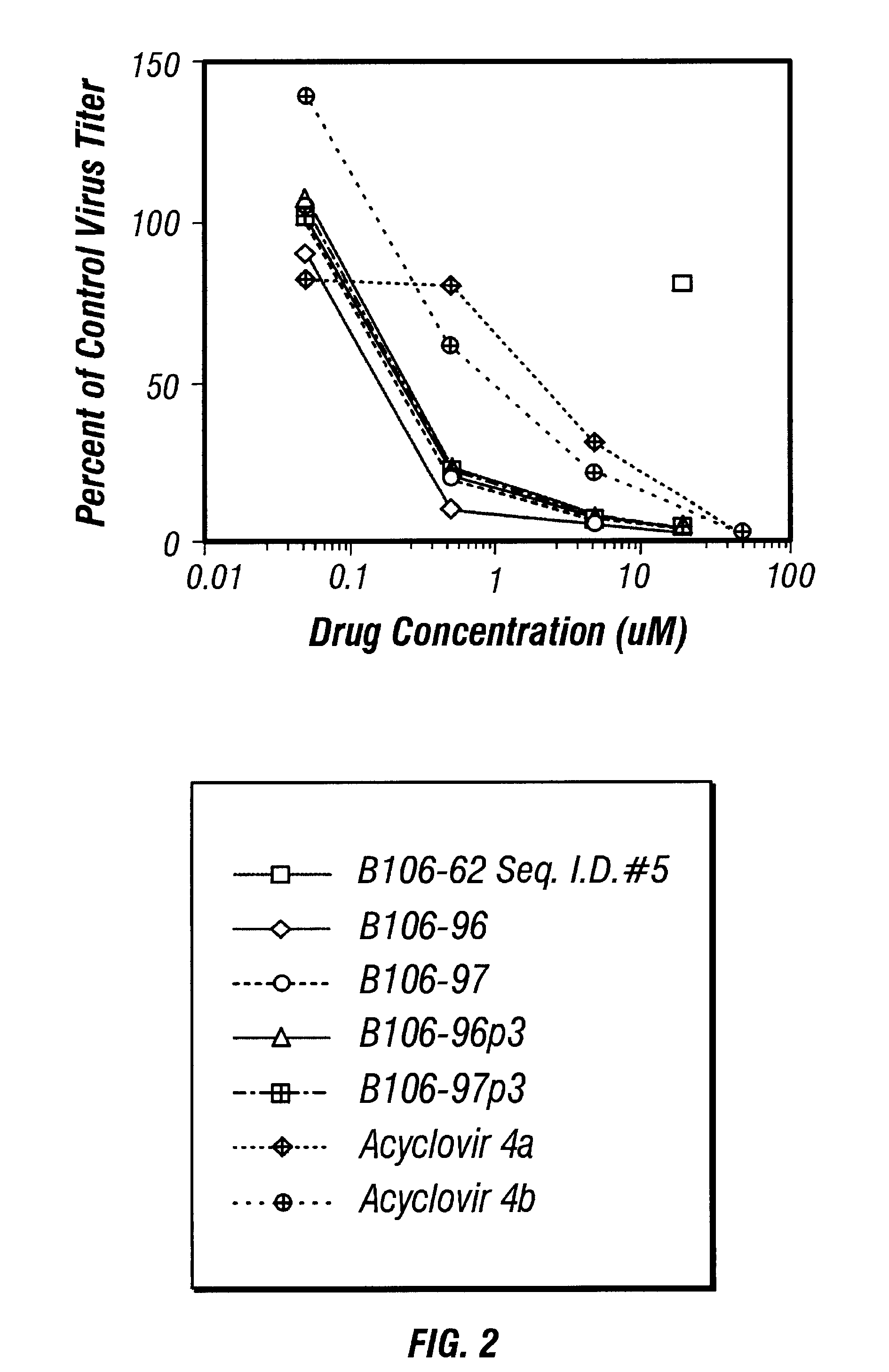
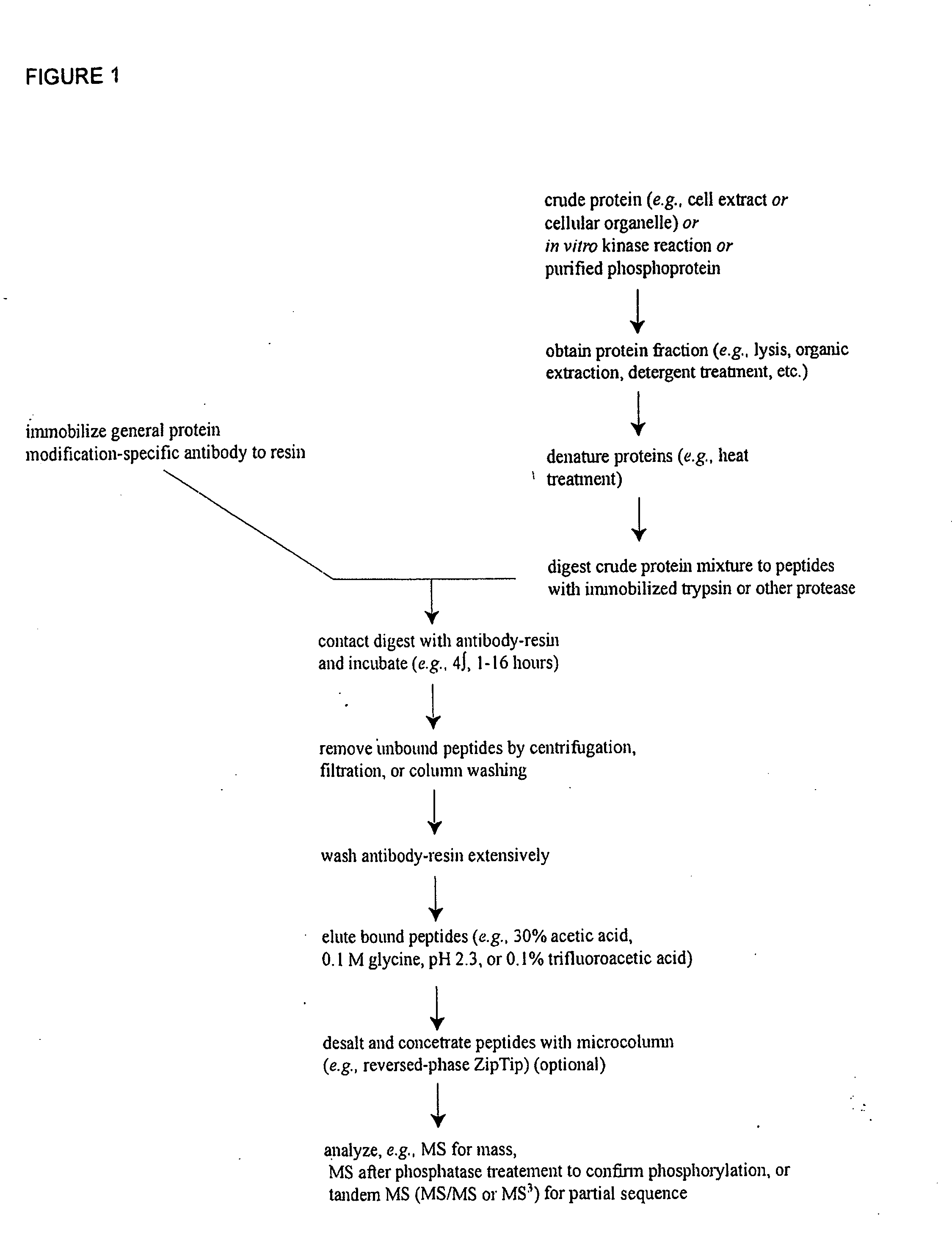
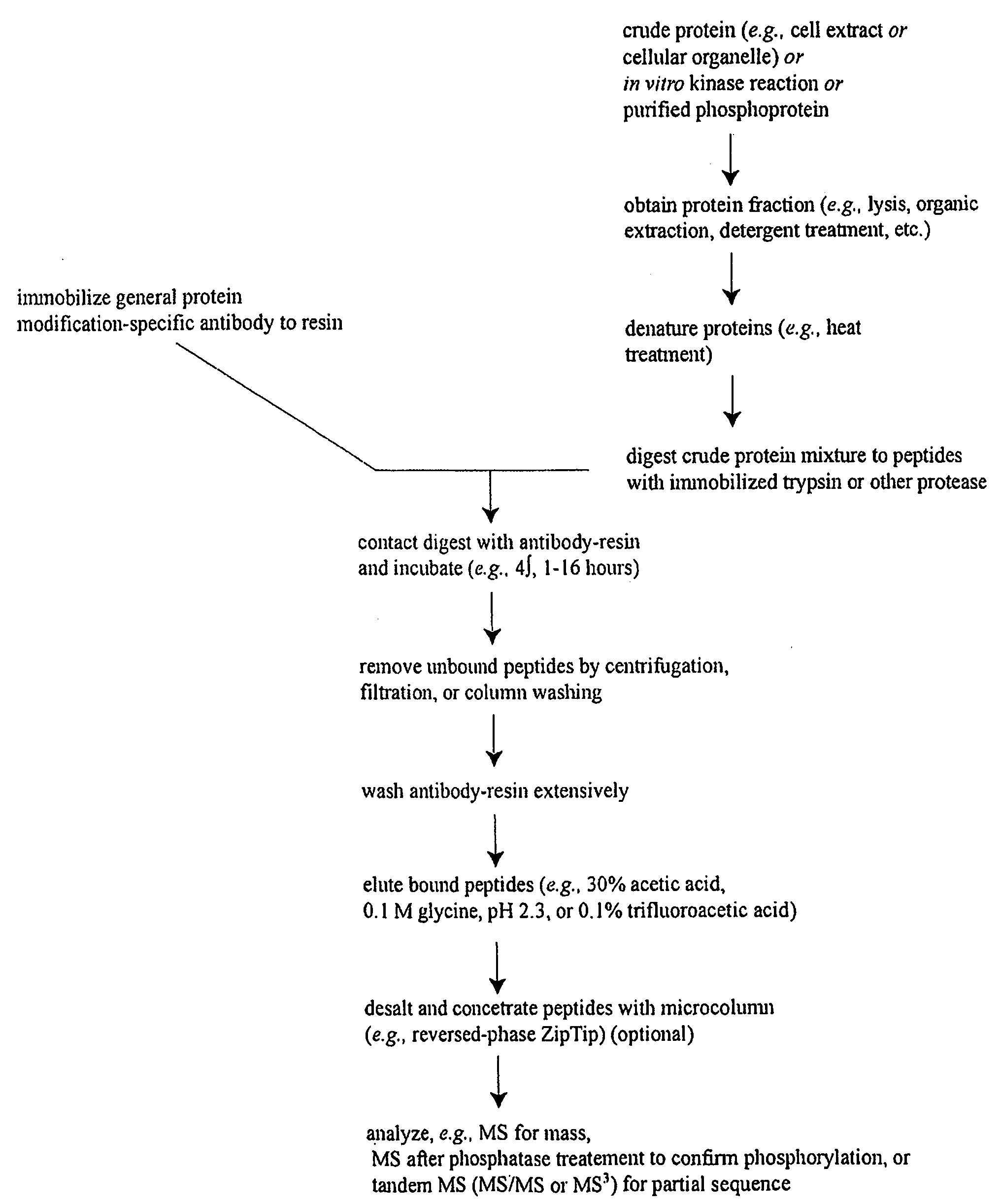
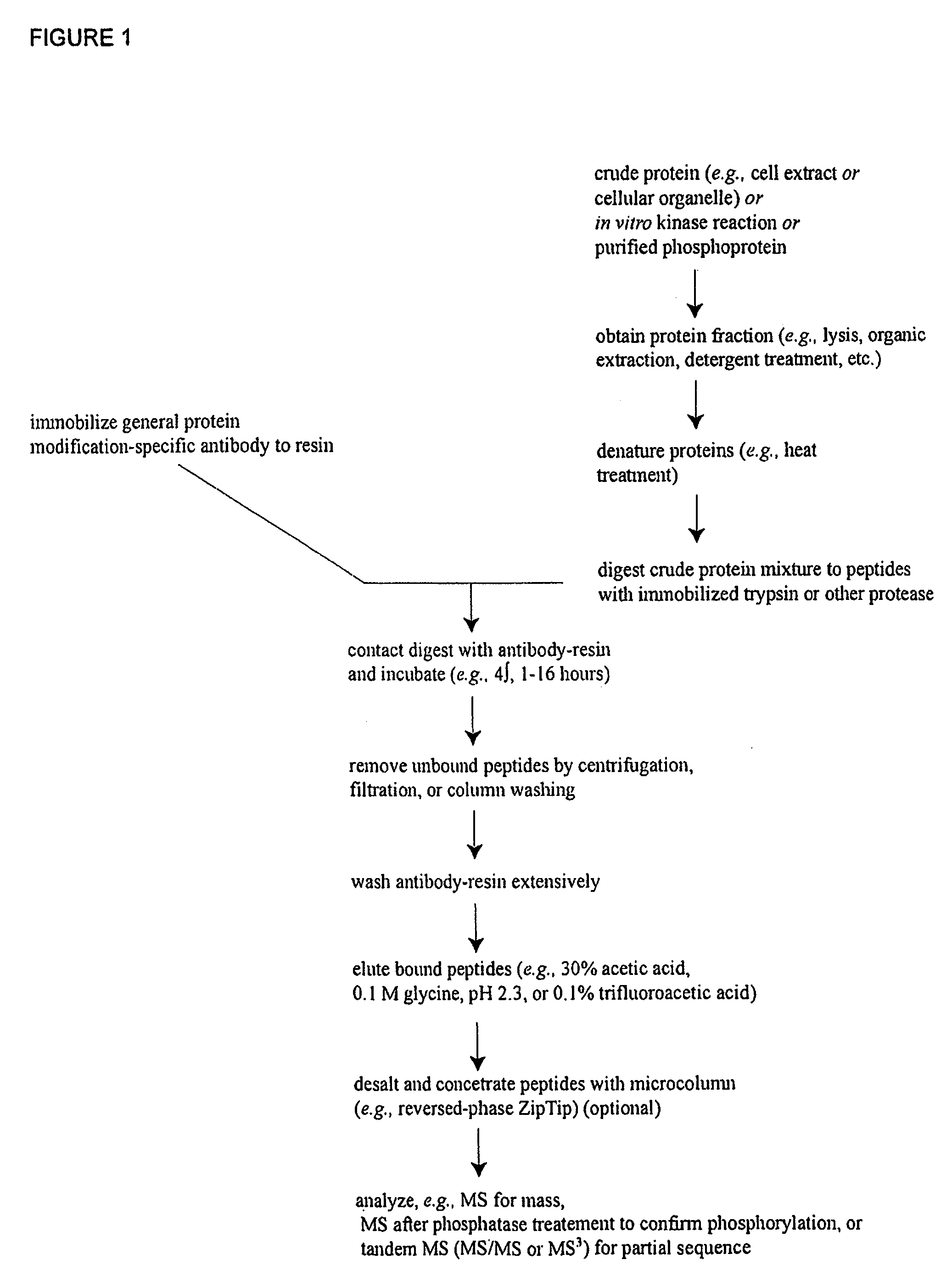
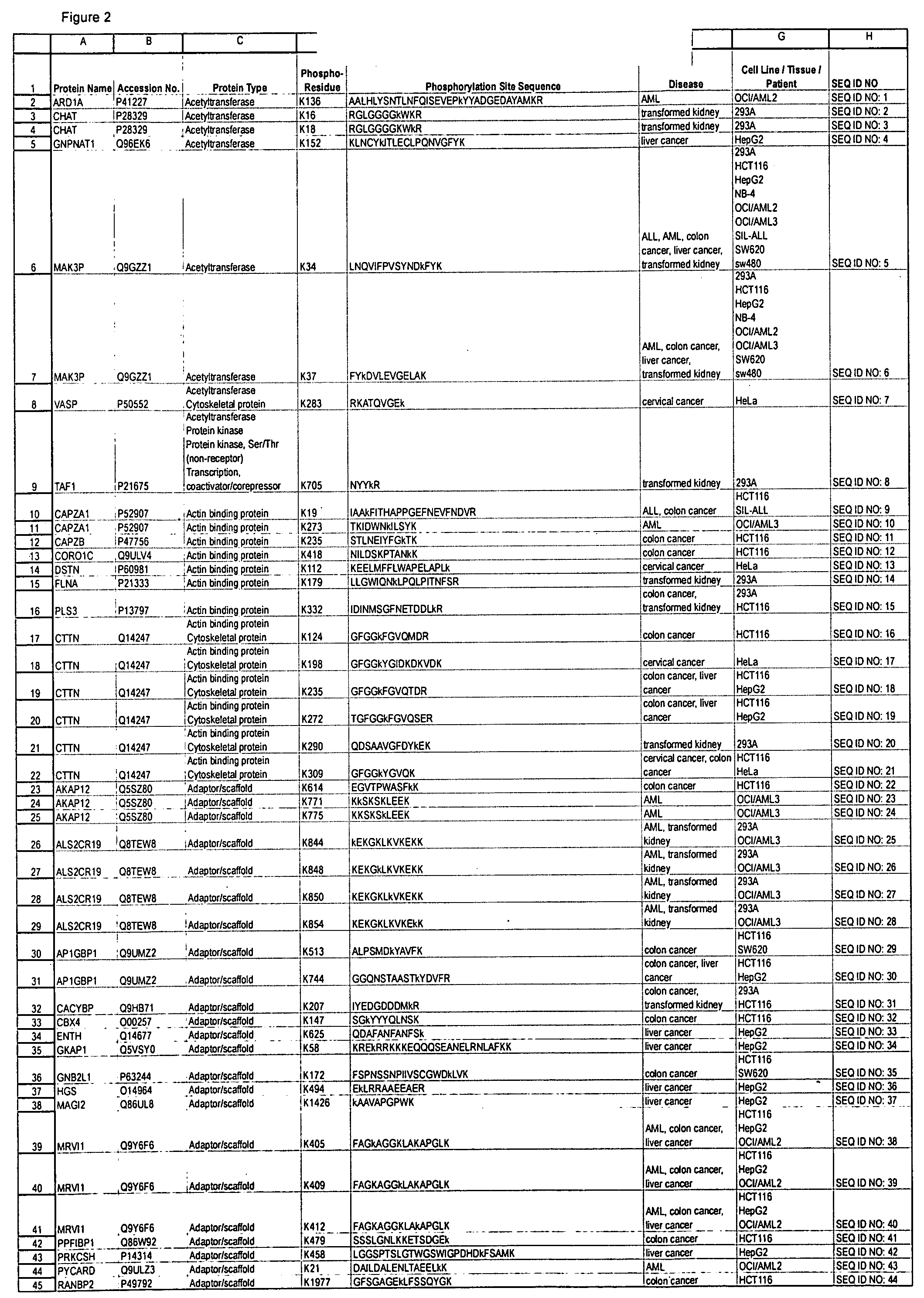
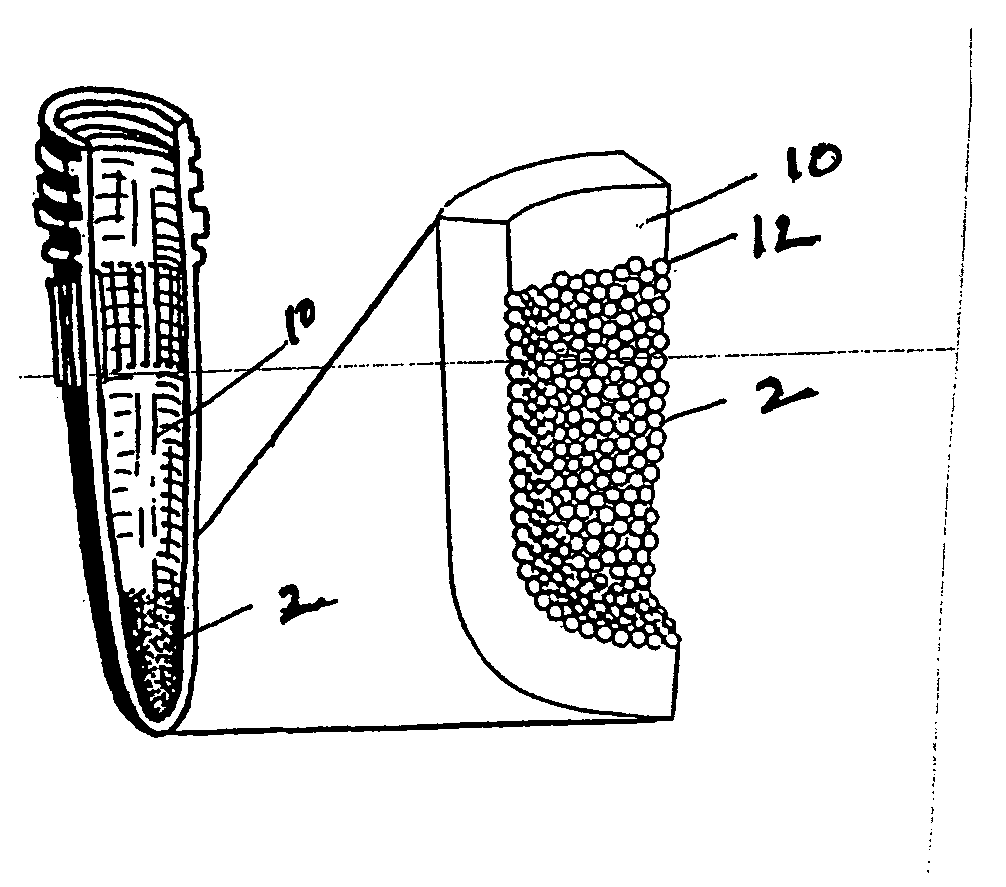
Patents
Literature
41 results about "Guanine Nucleotides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
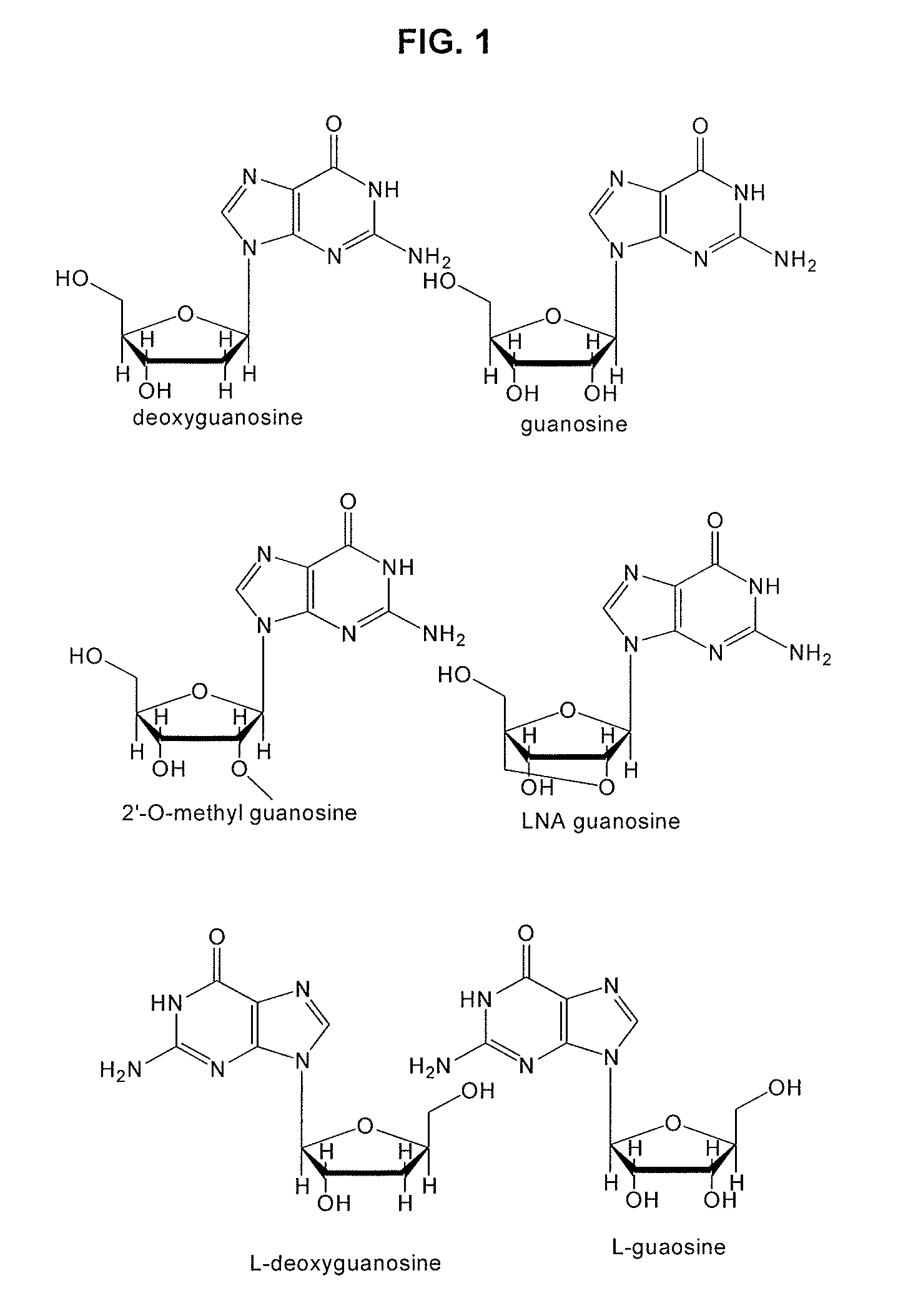
Guanine (/ˈɡwɑːnɪn/; or G, Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.
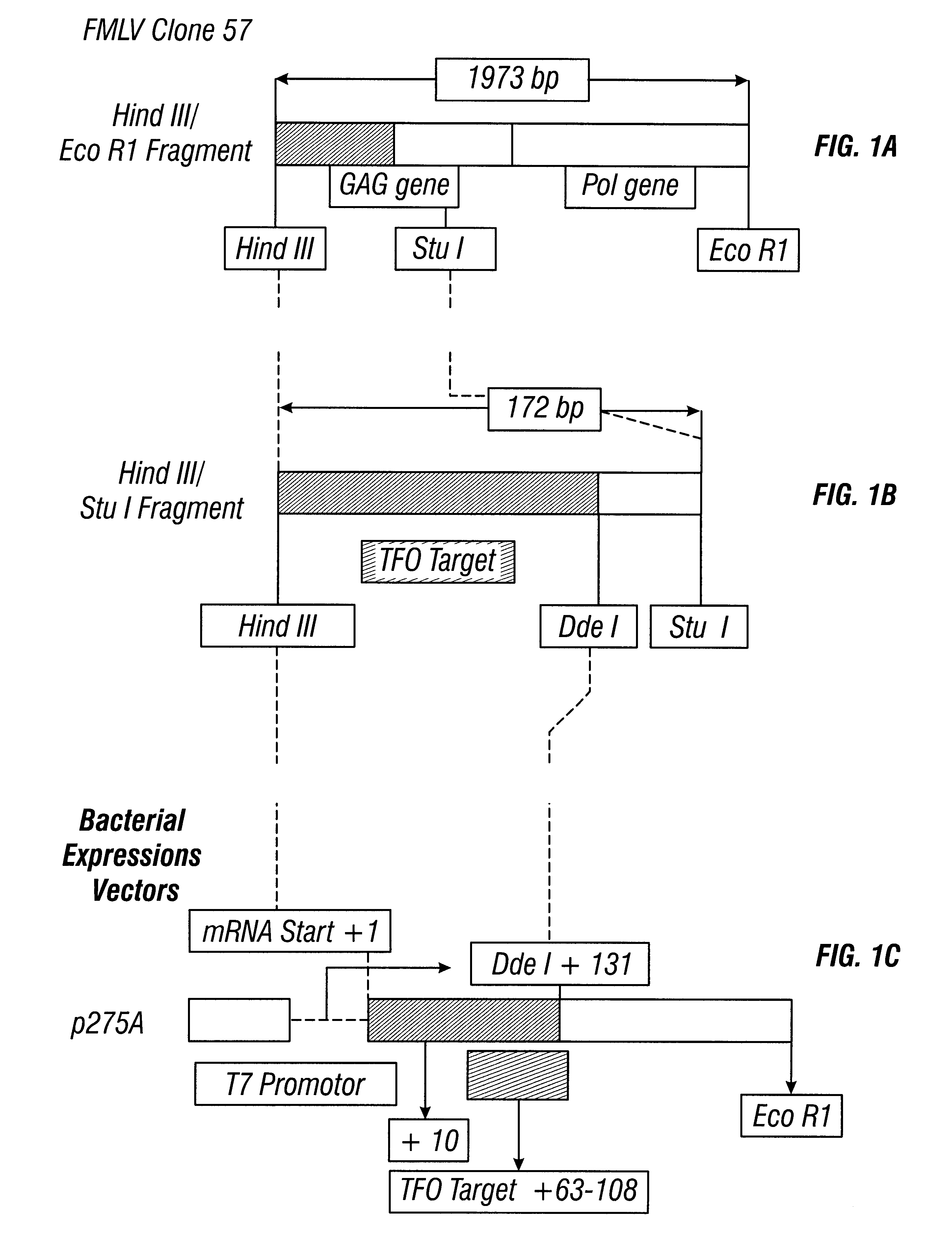
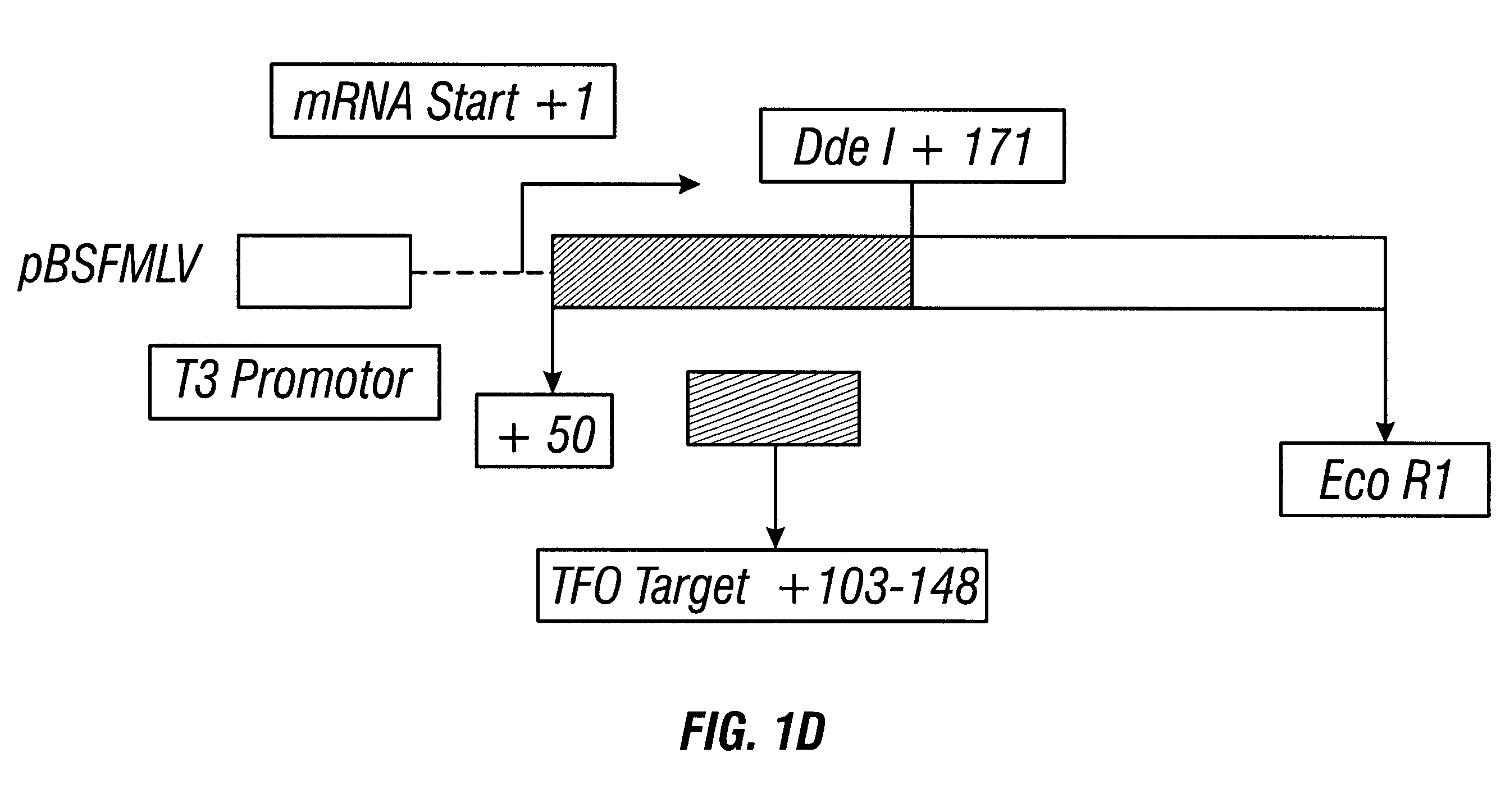
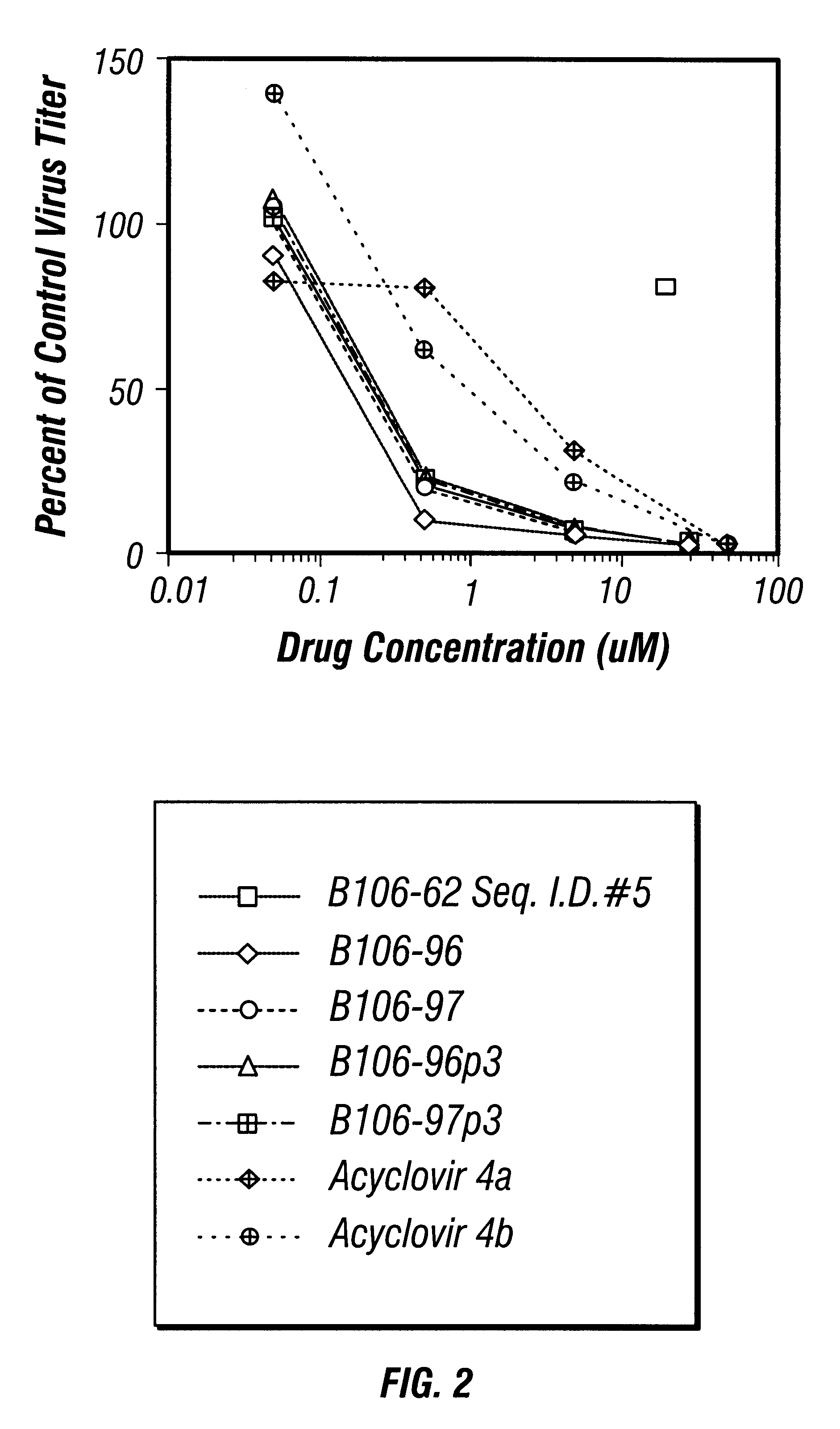
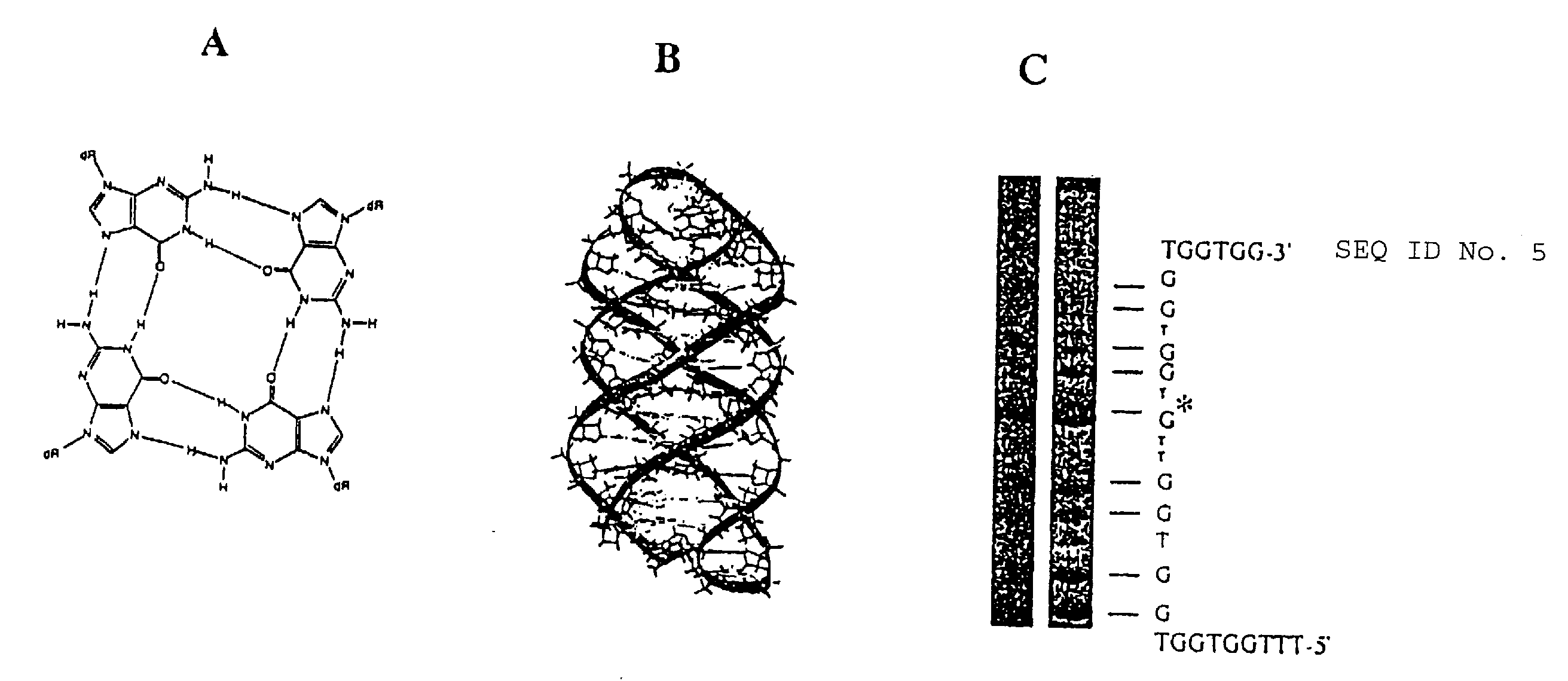
Anti-viral guanosine-rich oligonucleotides and method of treating HIV
A method and compositions for treating viral infection in vitro and in vivo using a guanosine-rich oligonucleotide. The oligonucleotides have sufficient guanosine to form a guanosine tetrad. Also provided are oligonucleotides of at least two runs of at least two guanosines. Also provided are guanosine-rich oligonucleotides and methods for treating viral infections in humans, and a method for designing guanosine-rich oligonucleotides having anti-viral activity and integrase inhibition activity.
Owner:ARONEX PHARMA +2
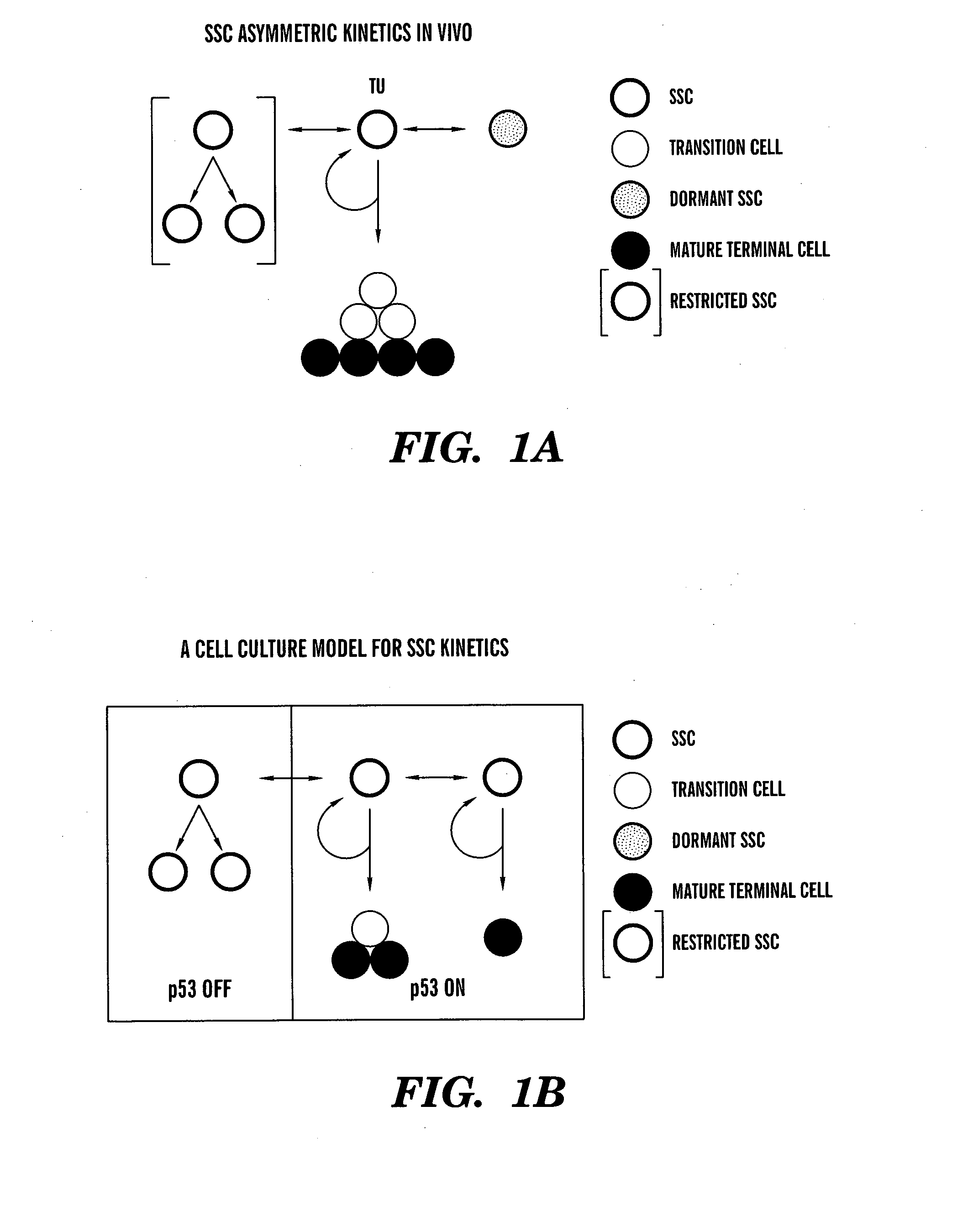
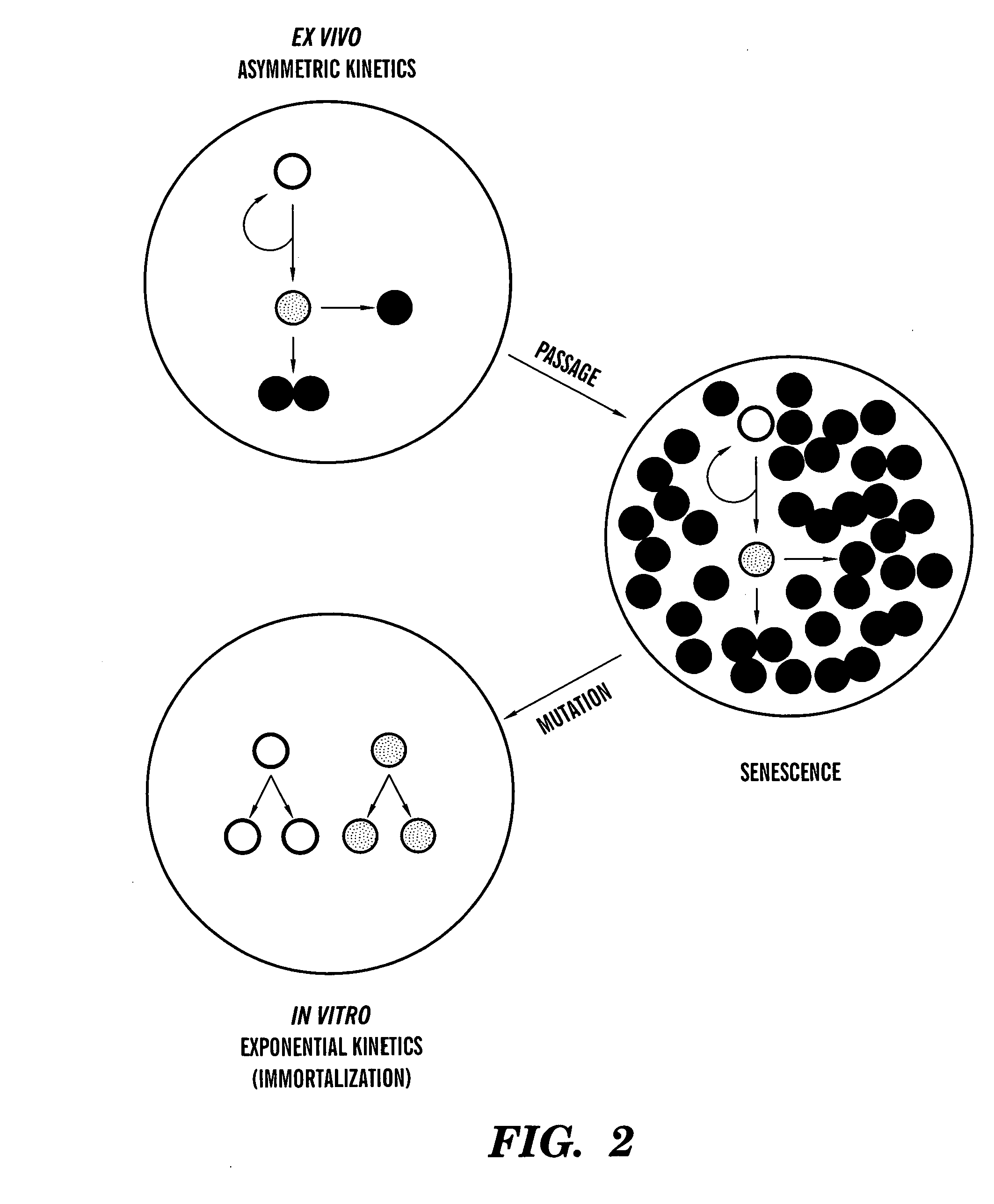
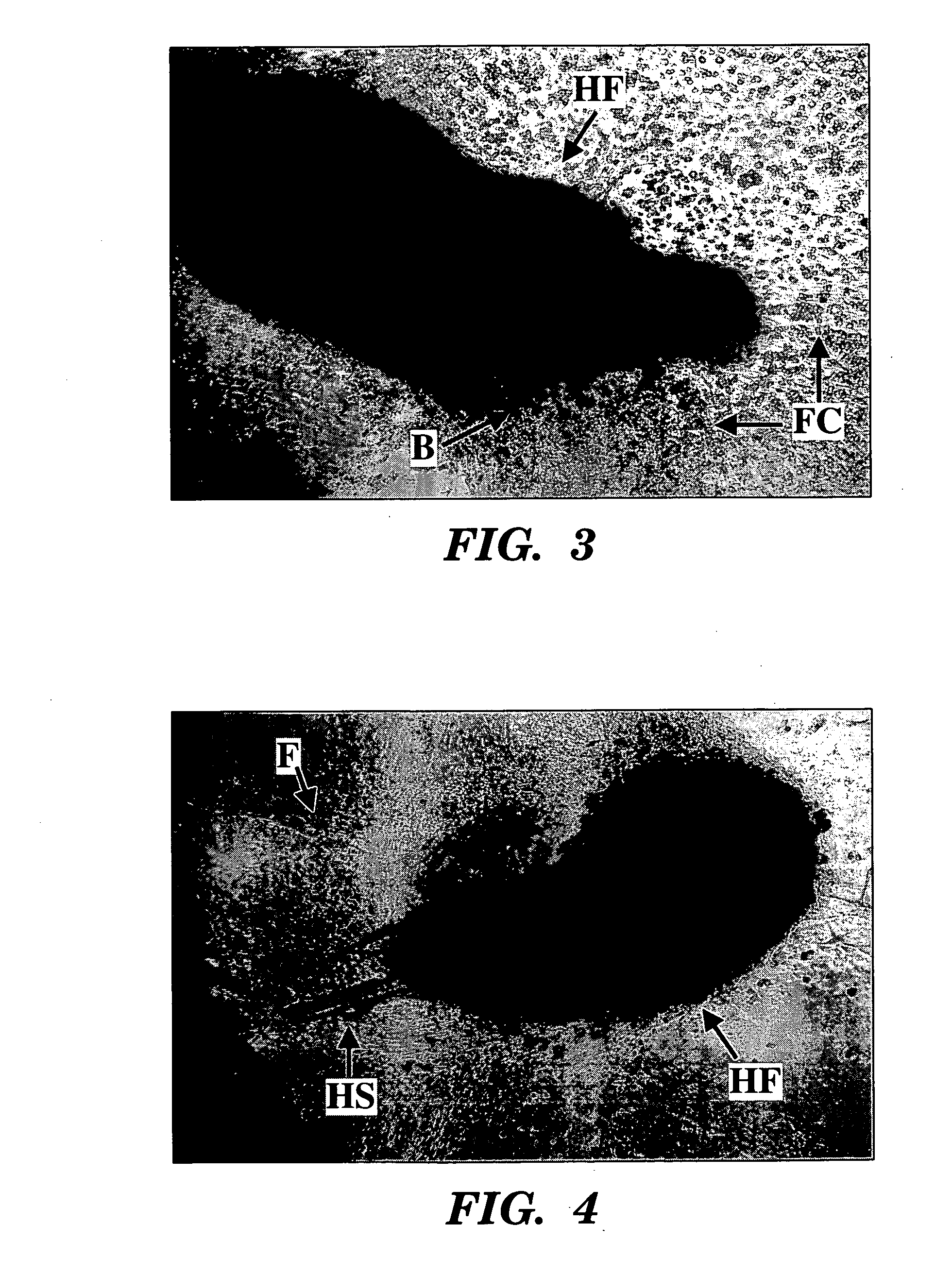
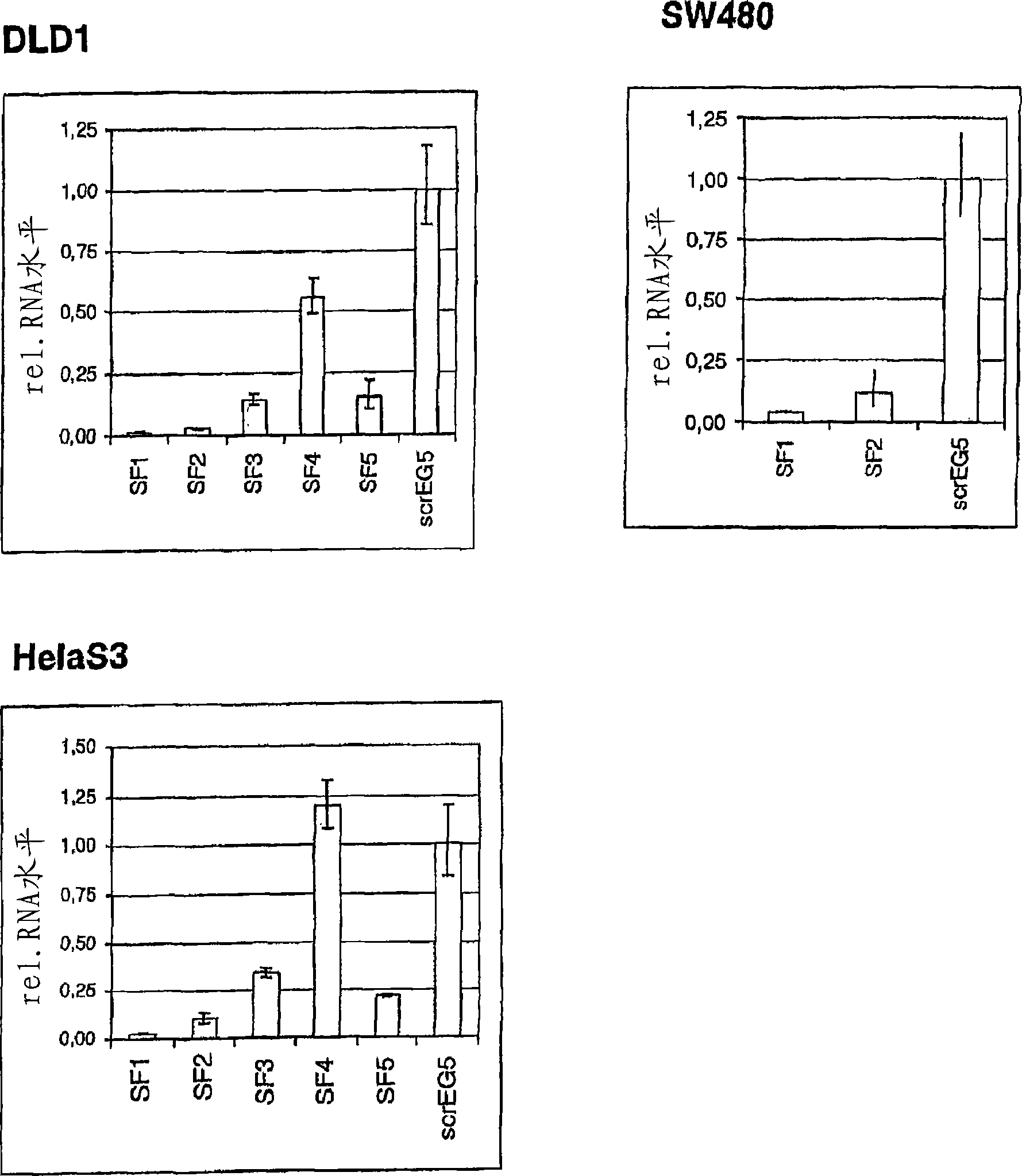
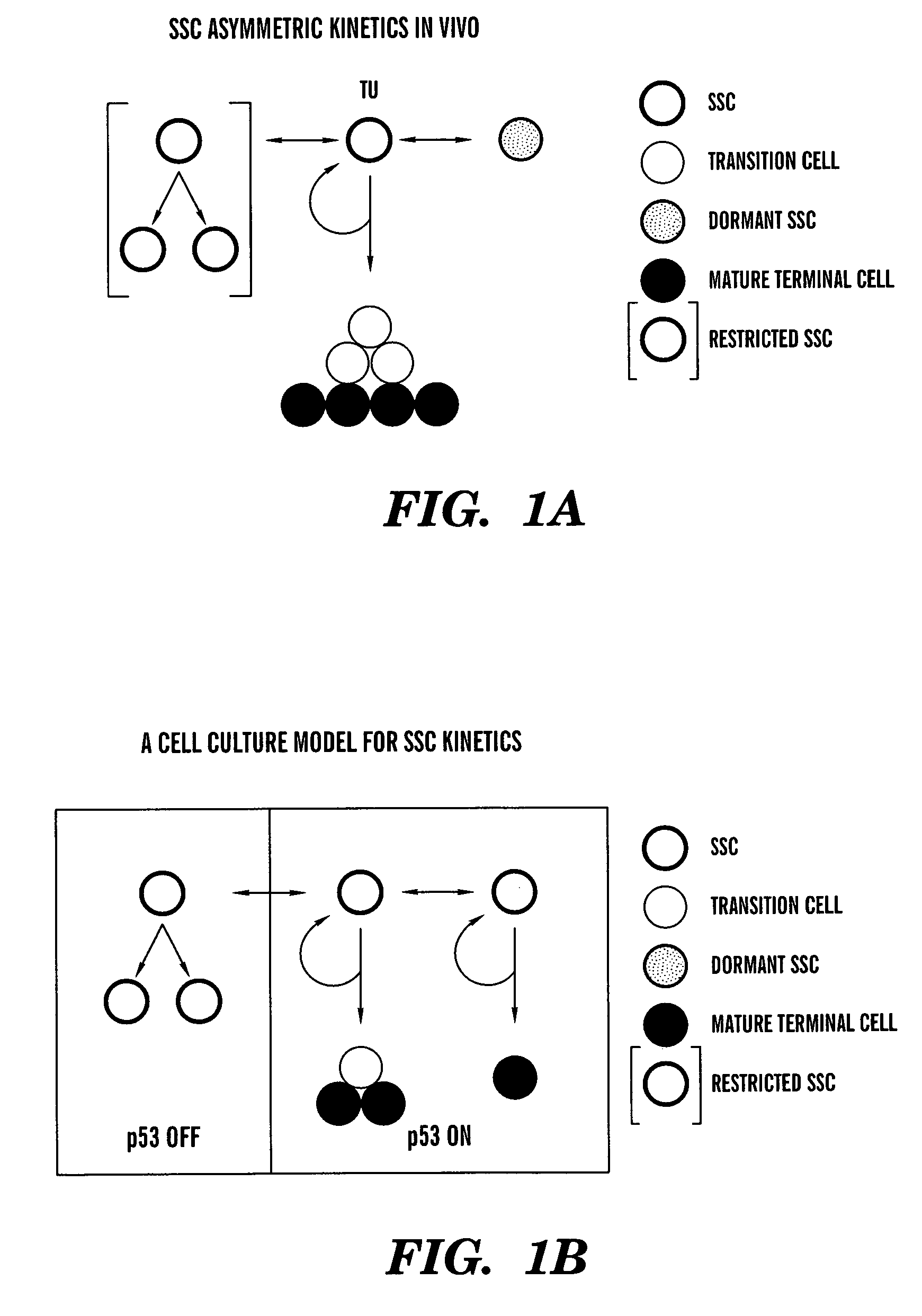
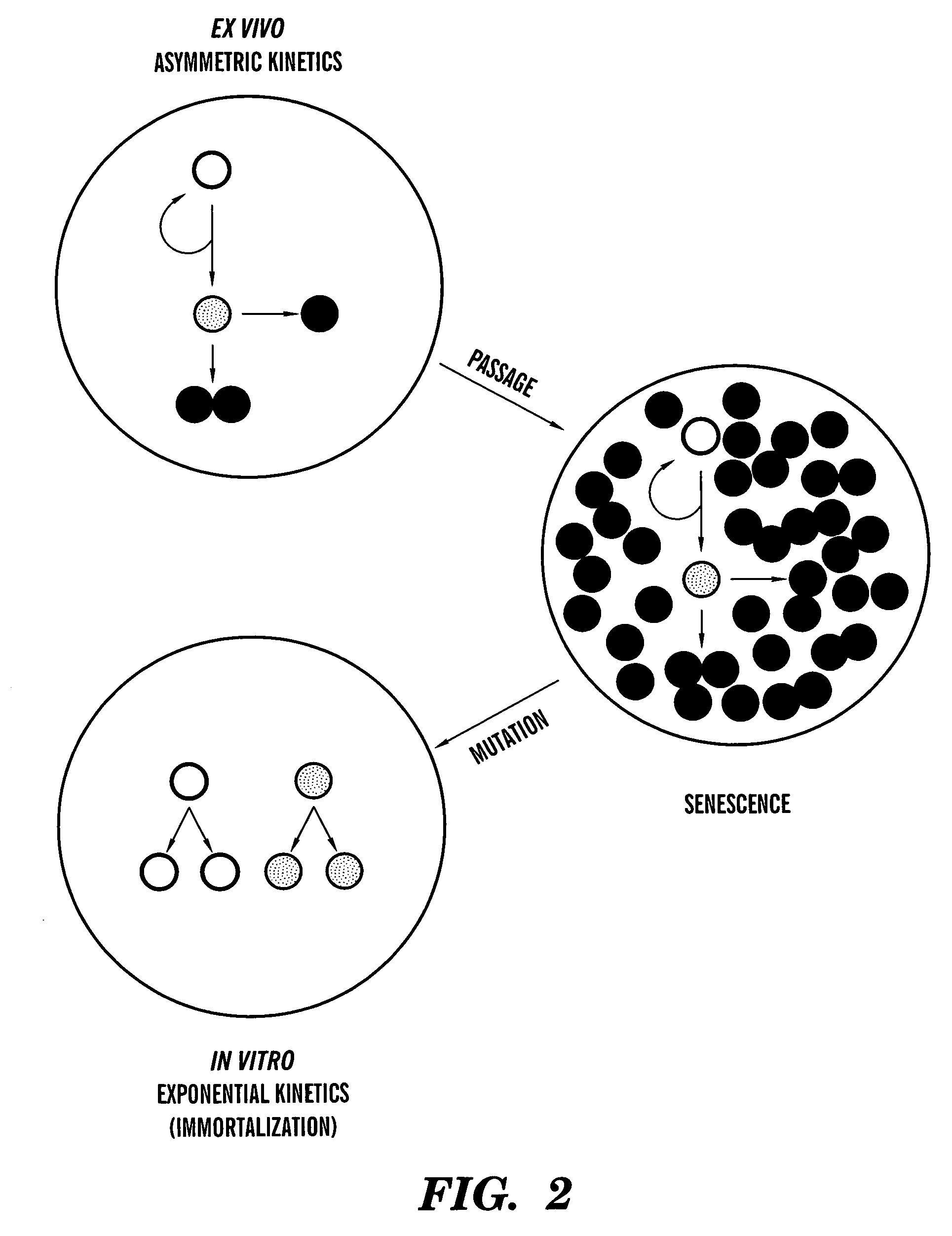
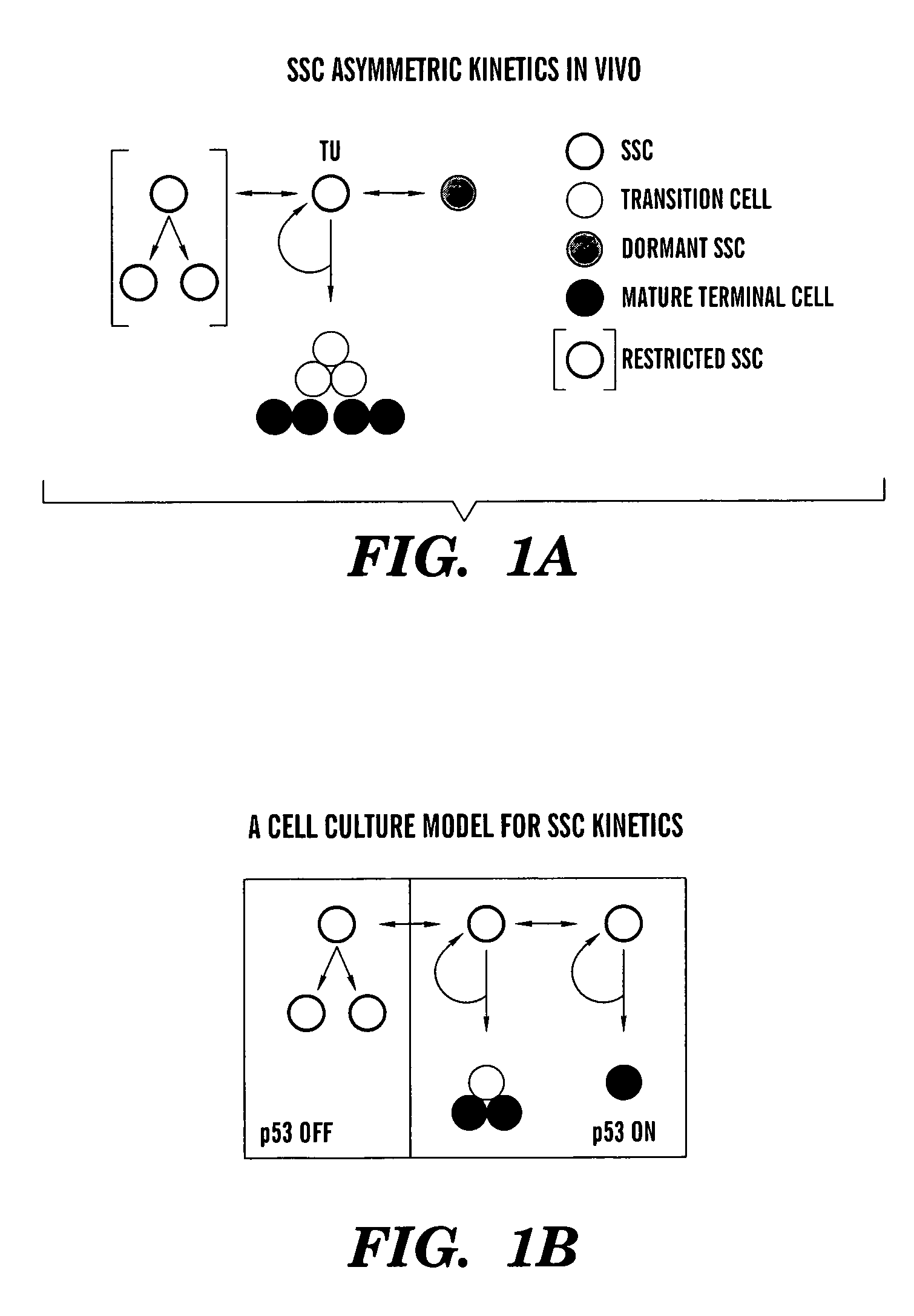
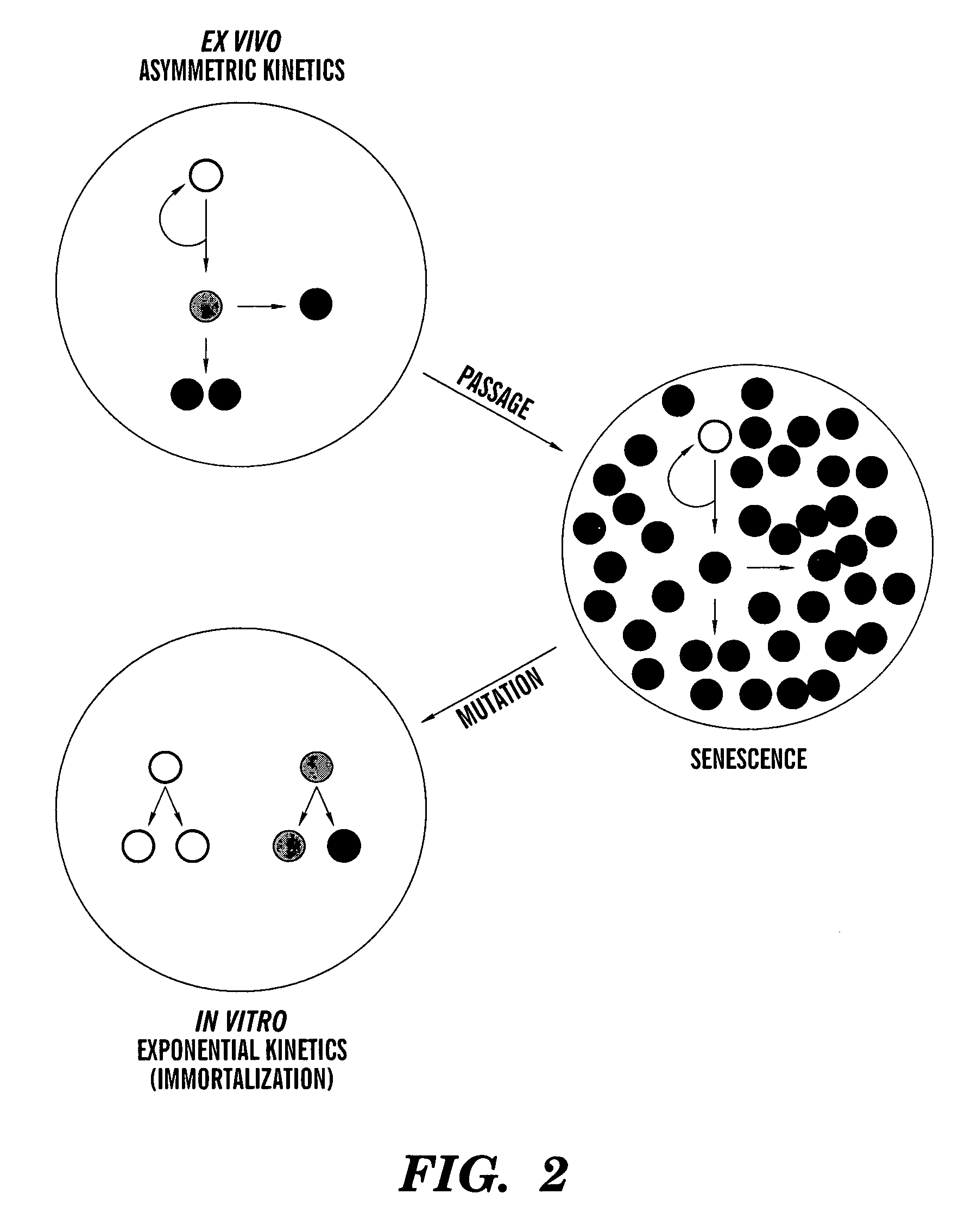
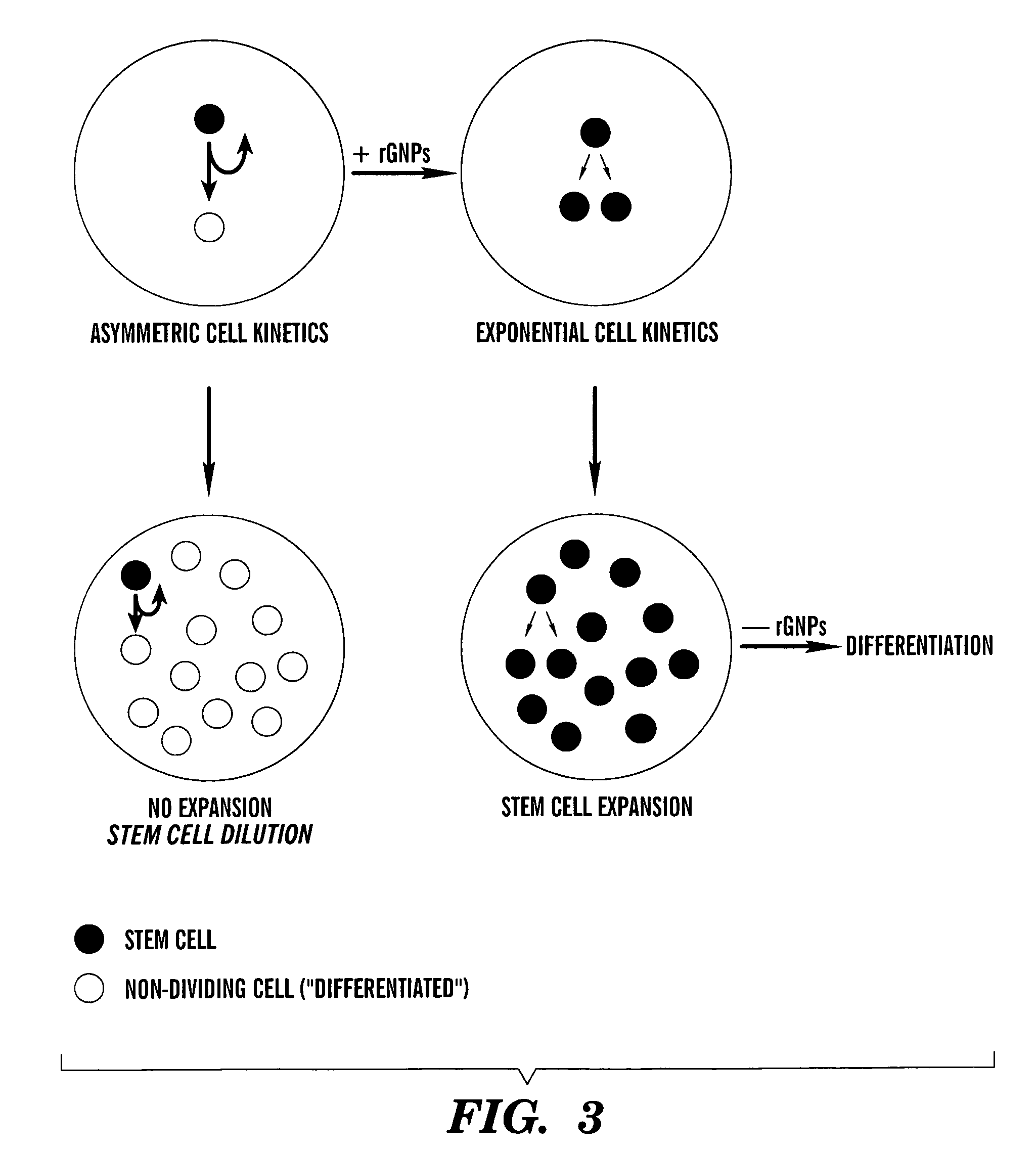
Methods for ex vivo propagation of somatic hair follicle stem cells
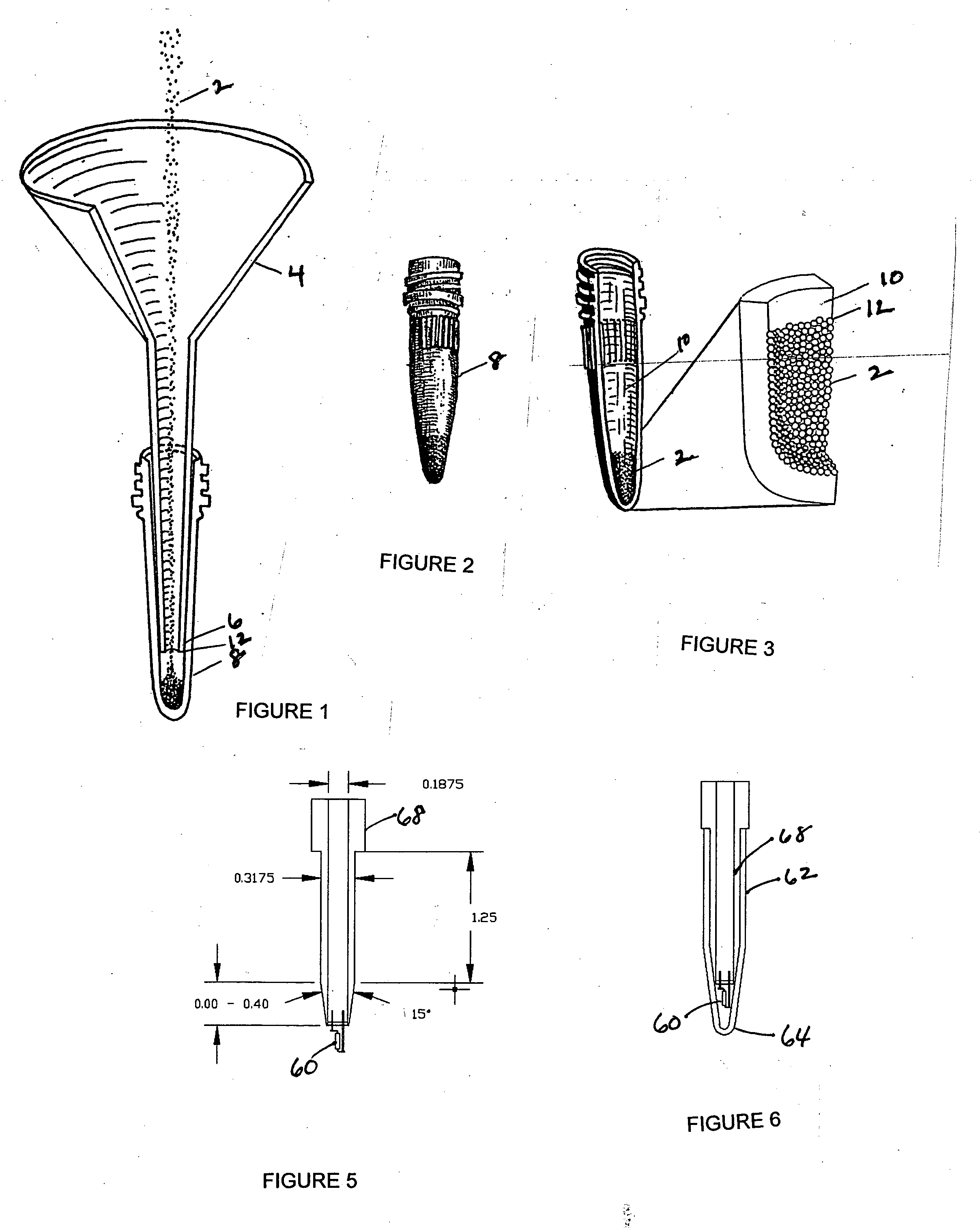
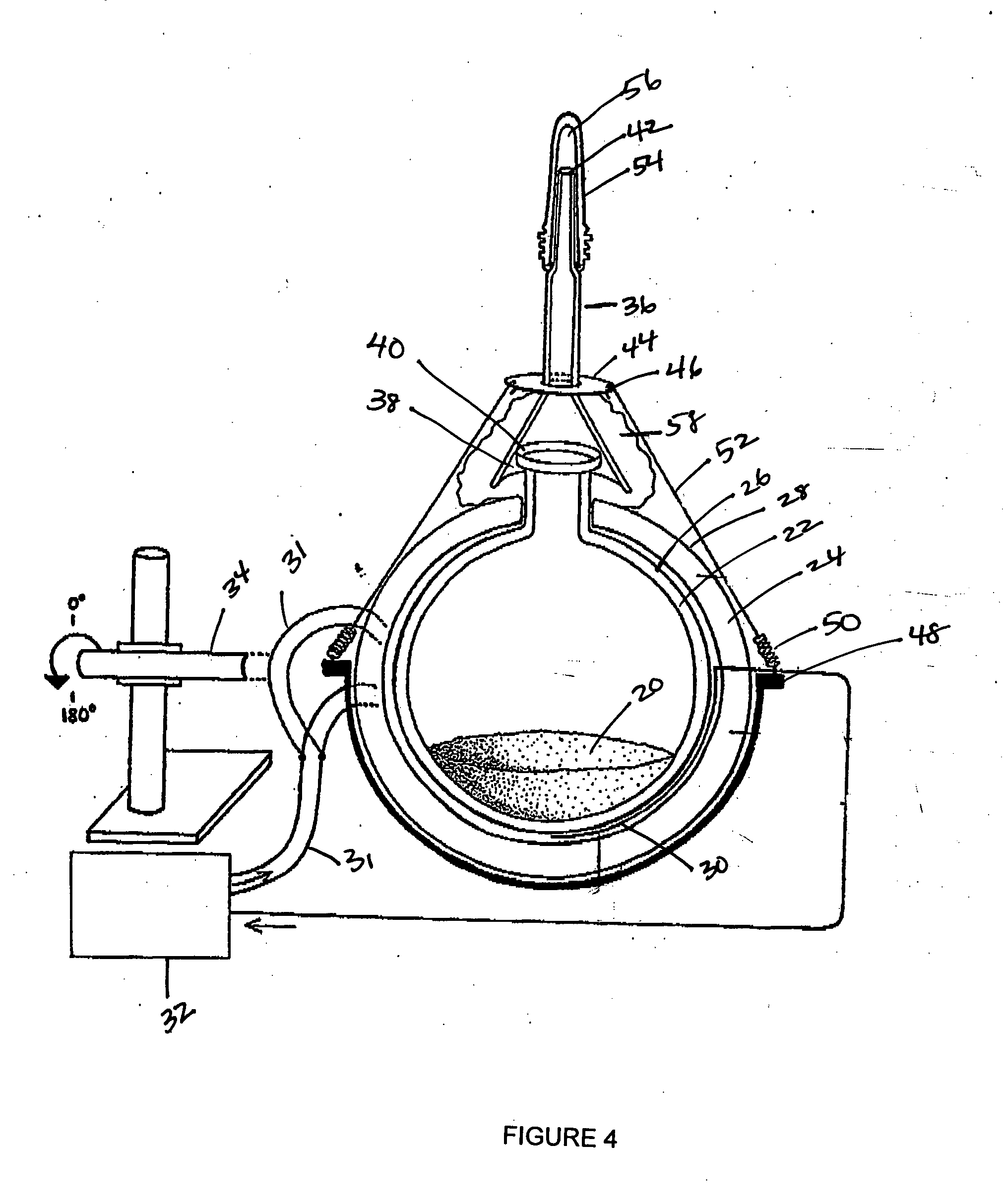
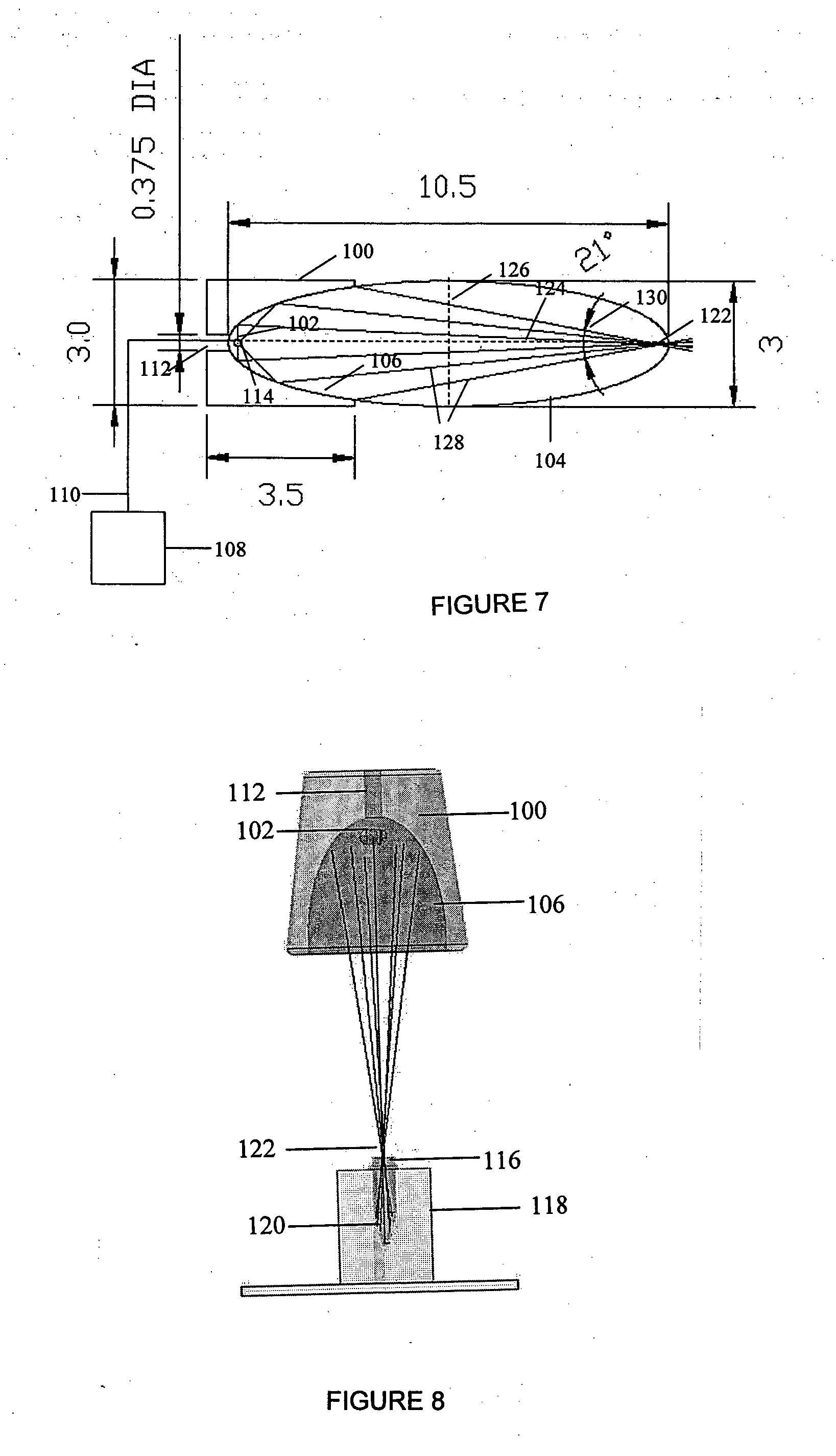
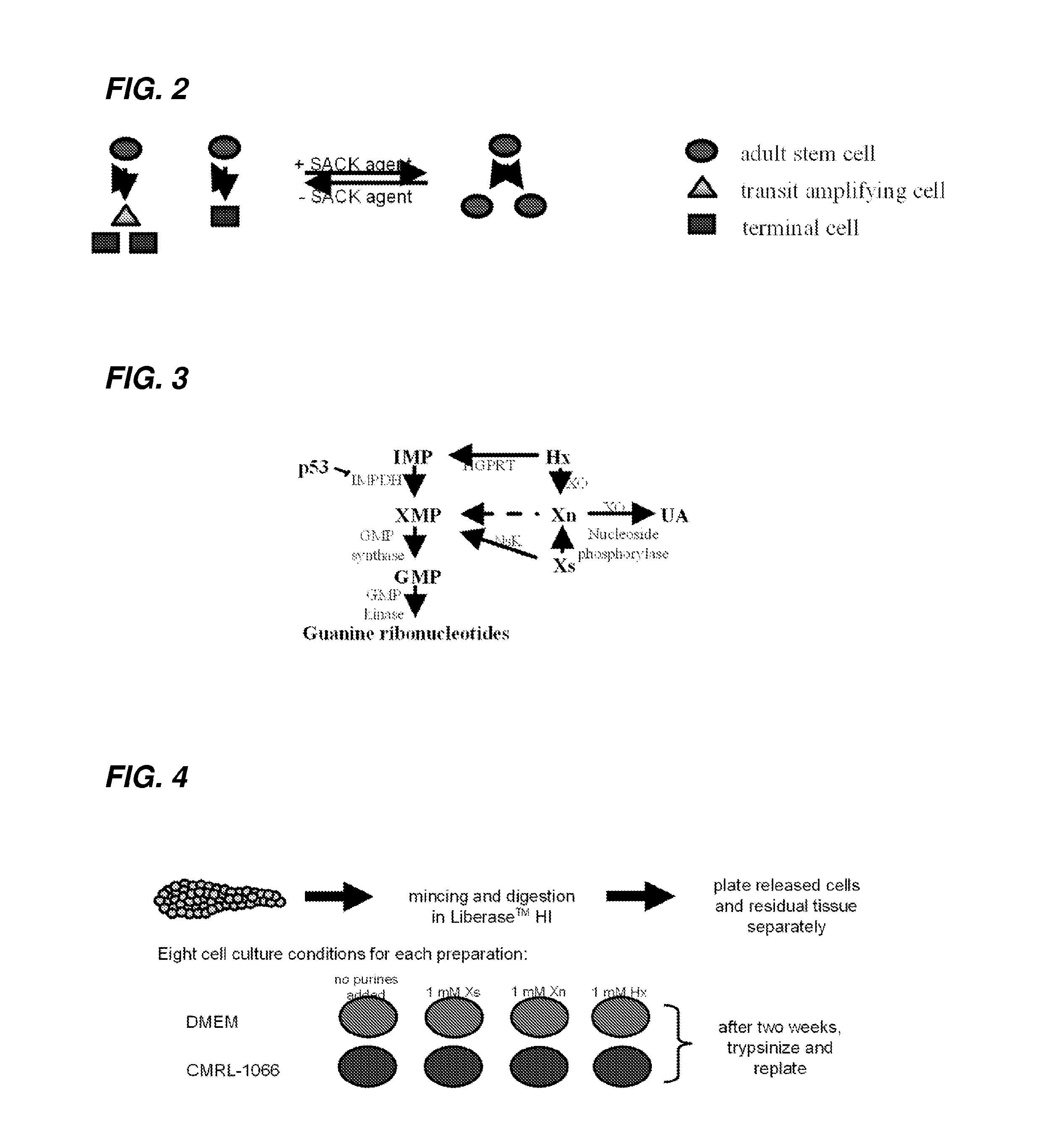
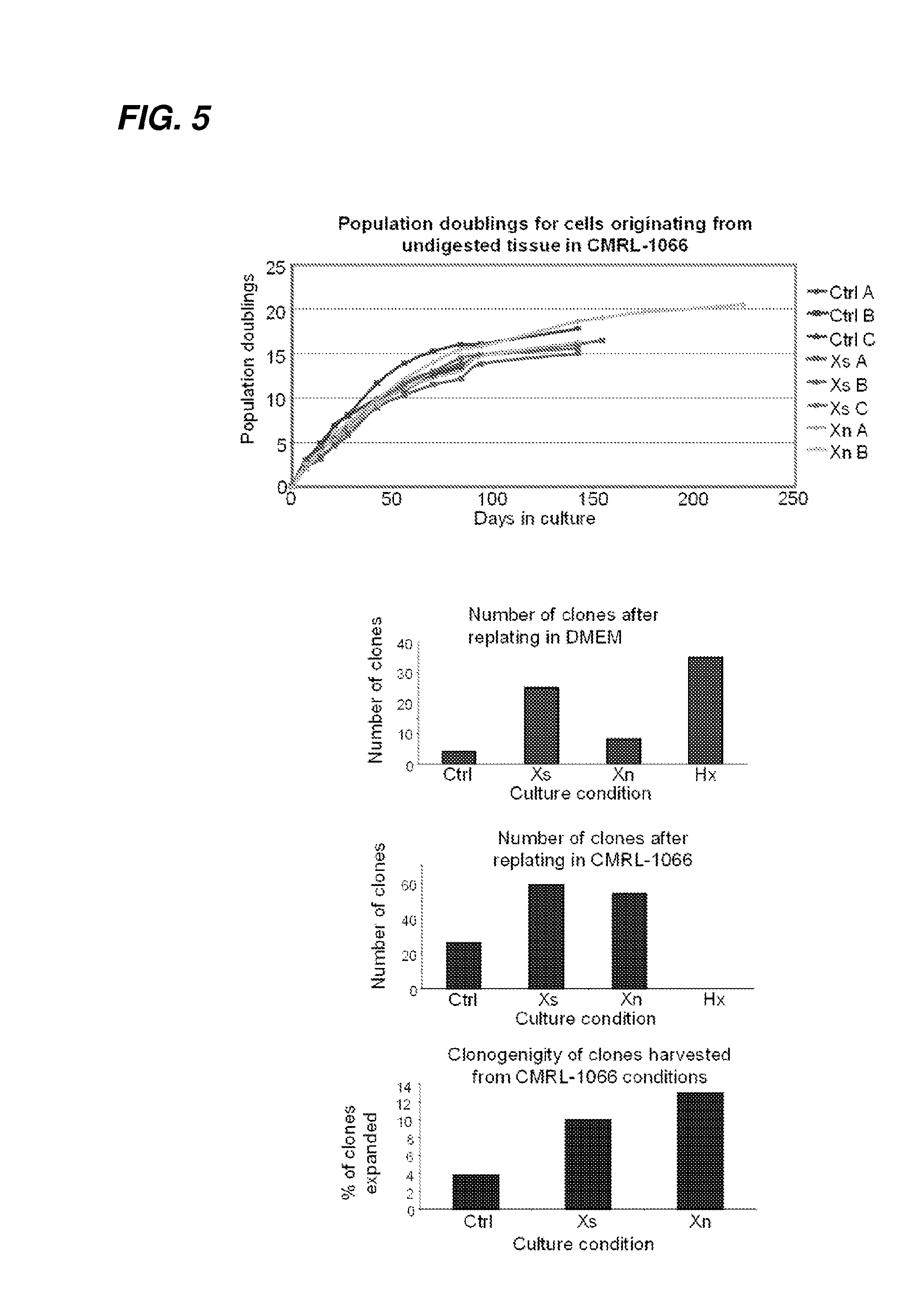
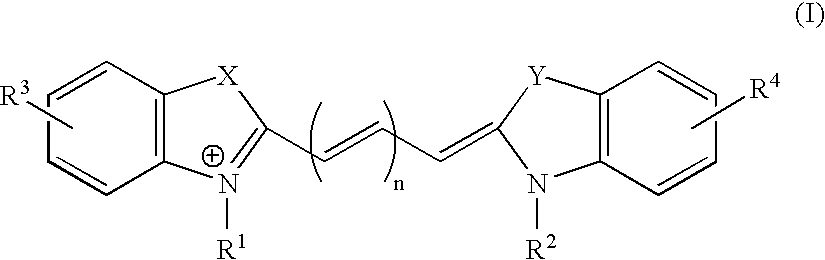
ActiveUS20050272147A1Enhance guanine nucleotide biosynthesisSuppressing asymmetric cell kineticsBiocideEpidermal cells/skin cellsCell kineticsSomatic cell
The present invention is directed to methods for readily propagating somatic hair follicle stem cells or melanocyte stem cells. The methods comprise enhancing guanine nucleotide (GNP) biosynthesis, thereby expanding guanine nucleotide pools. This in turn conditionally suppresses asymmetric cell kinetics in the explanted cells. The methods of the invention include pharmacological methods and genetic methods. For example, the resulting cultured somatic hair follicle stem cells can be used for a variety of applications including cell replacement therapies such as hair transplants, gene therapies, and tissue engineering applications, such as the generation of artificial skin and skin regeneration strategies including skin grafts.
Owner:MASSACHUSETTS INST OF TECH
Idiopathic pulmonary fibrosis urine protein marker, and application of same to diagnosis and prognosis
The invention relates to an idiopathic pulmonary fibrosis urine protein marker, and application of the same to diagnosis and prognosis. Specifically, the invention relates to application of a urine protein marker obtained by using an idiopathic pulmonary fibrosis model and mass spectrometry to early-stage diagnosis, pathogenesis monitoring and curative effect assessment of human pulmonary fibrosis. The urine protein marker comprises collagen alpha-1(I) chain, vimentin, protein RUFY3, keratin type-II skeleton 8, a sodium-hydrogen exchange regulation factor NHE-RF2, a threonine synthase sample 2, zinc-actin binding repeat protein 2, low-density lipoprotein receptor-associated protein 4, alpha-11 of a guanine nucleotide binding protein subunit, an alpha-1 chain of tropomyosin, mitochondrial stress-70 protein, NSFL1 cofactor P47, ubiquitin carboxyl-terminal hydrolase isozyme L1, etc.
Owner:BEIJING NORMAL UNIVERSITY
Enzyme synthesized by Chinese caterpillar fungus hirsutella sinensis to metabolize guanine nucleotide and genes and application of enzyme
InactiveCN102690796AIncrease productionEnhance expressive abilityFungiBacteriaBiotechnologyBiosynthetic genes
The invention relates to IMP (inosine monophosphate) dehydrogenase synthesized by 'Bailing' producing fungus Chinese caterpillar fungus hirsutella sinensis to metabolize guanine nucleotide based on xanthosine monophosphate and coding genes and application of the IMP dehydrogenase. The IMP dehydrogenase comprises proteins shown by SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3, and coding genes of the IMP dehydrogenase correspond to nucleotide sequences shown by SEQ ID No.4, SEQ ID No.5 and SEQ ID No.6. Detailed studies on metabolic pathways of the xanthosine monophosphate synthesizing the guanine nucleotide in principle are carried out and include that cloned DNAs (deoxyribonucleic acids) in the provided nucleotide sequences can be transferred into engineering bacteria by means of transduction, transformation and transconjunction, and high expressivity is given to the host guanine nucleotide by adjusting expressions of guanine nucleotide biosynthetic genes, so that an effective way for increasing output of the guanine nucleotide is provided, and the IMP dehydrogenase and the coding genes have significant application prospects.
Owner:ZHEJIANG UNIV OF TECH +1
Guanosine-rich oligonucleotide integrase inhibitors
A method and compositions for treating viral infection (IV) vitro and in vivo using a guanosine-rich oligonucleotide. The oligonucleotides have sufficient guanosine to form a guanosine tetrad. Also provided are oligonucleotides of at least two runs of at least two guanosines. Also provided are guanosine-rich oligonucleotides and methods for treating viral infections in humans, and a method for designing guanosine-rich oligonucleotides having anti-viral activity and integrase inhibition activity.
Owner:ARONEX PHARMA
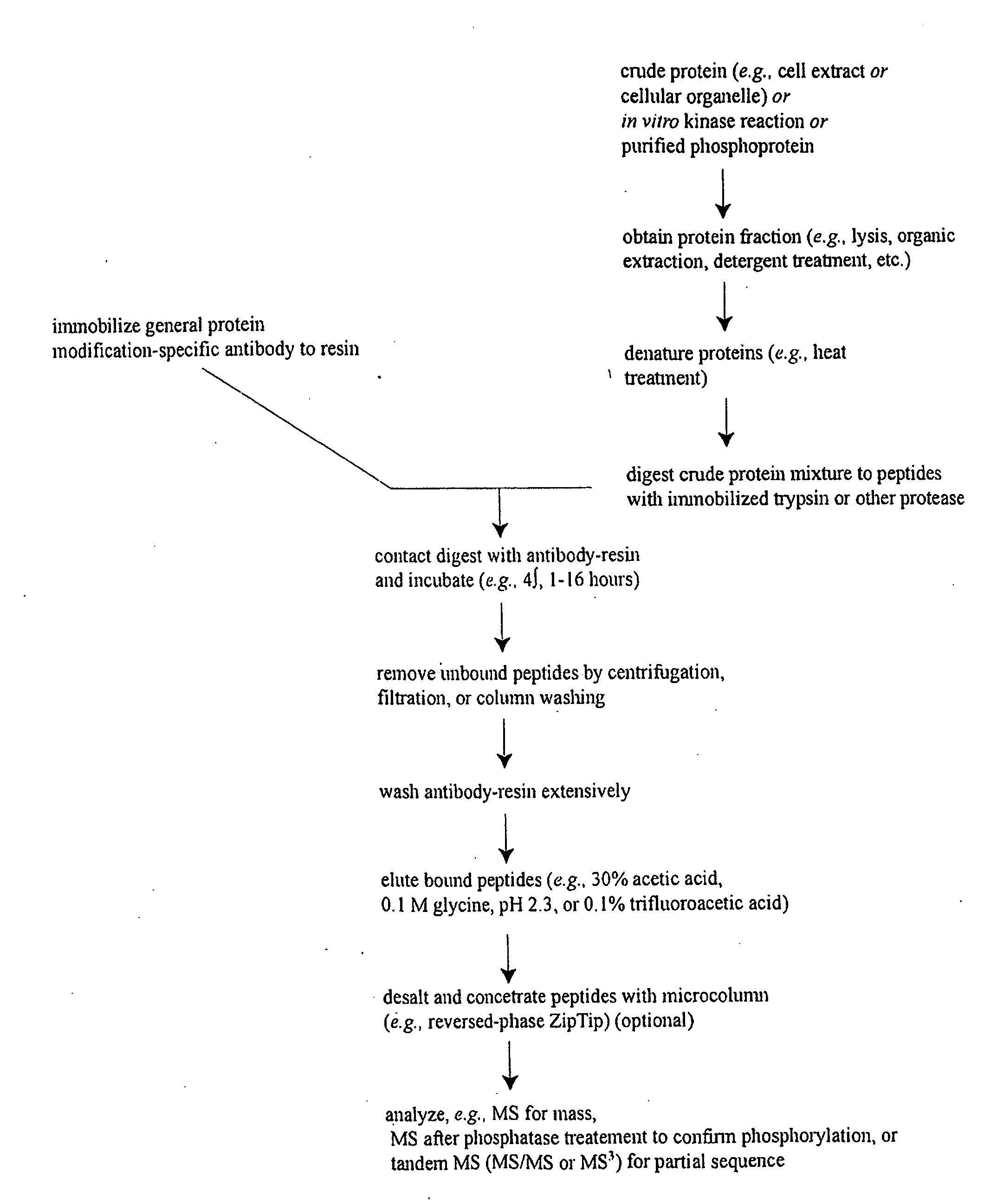
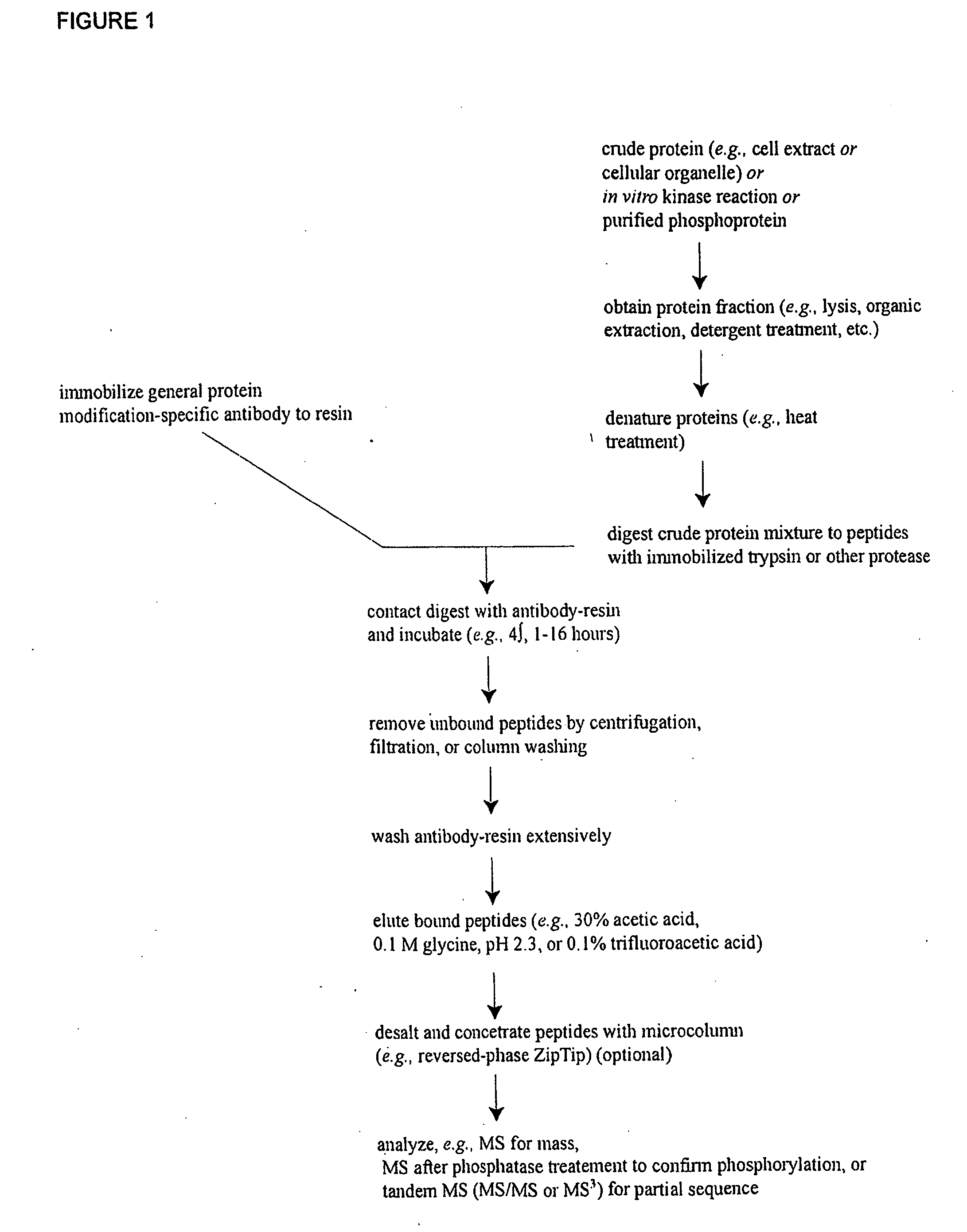
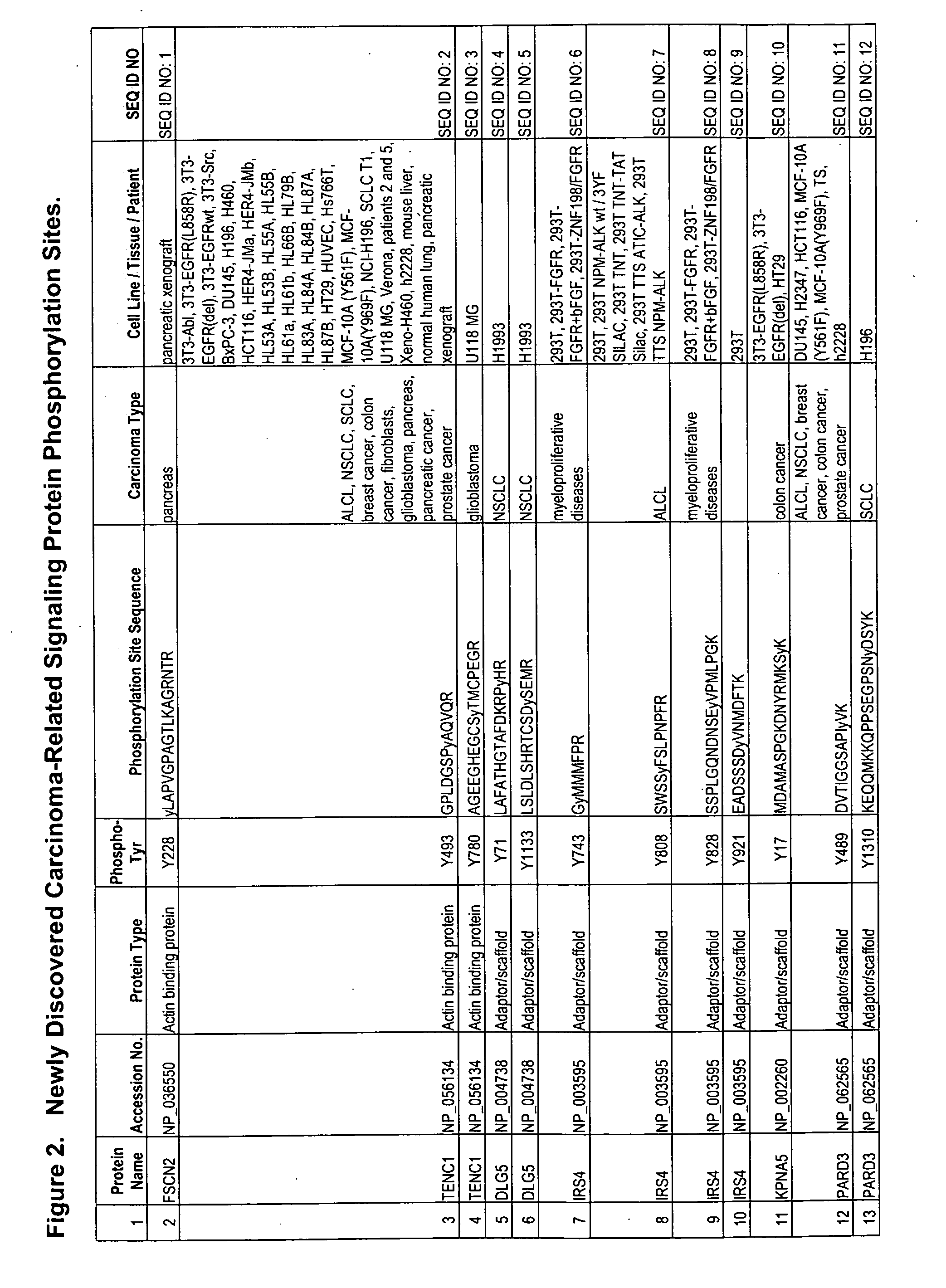
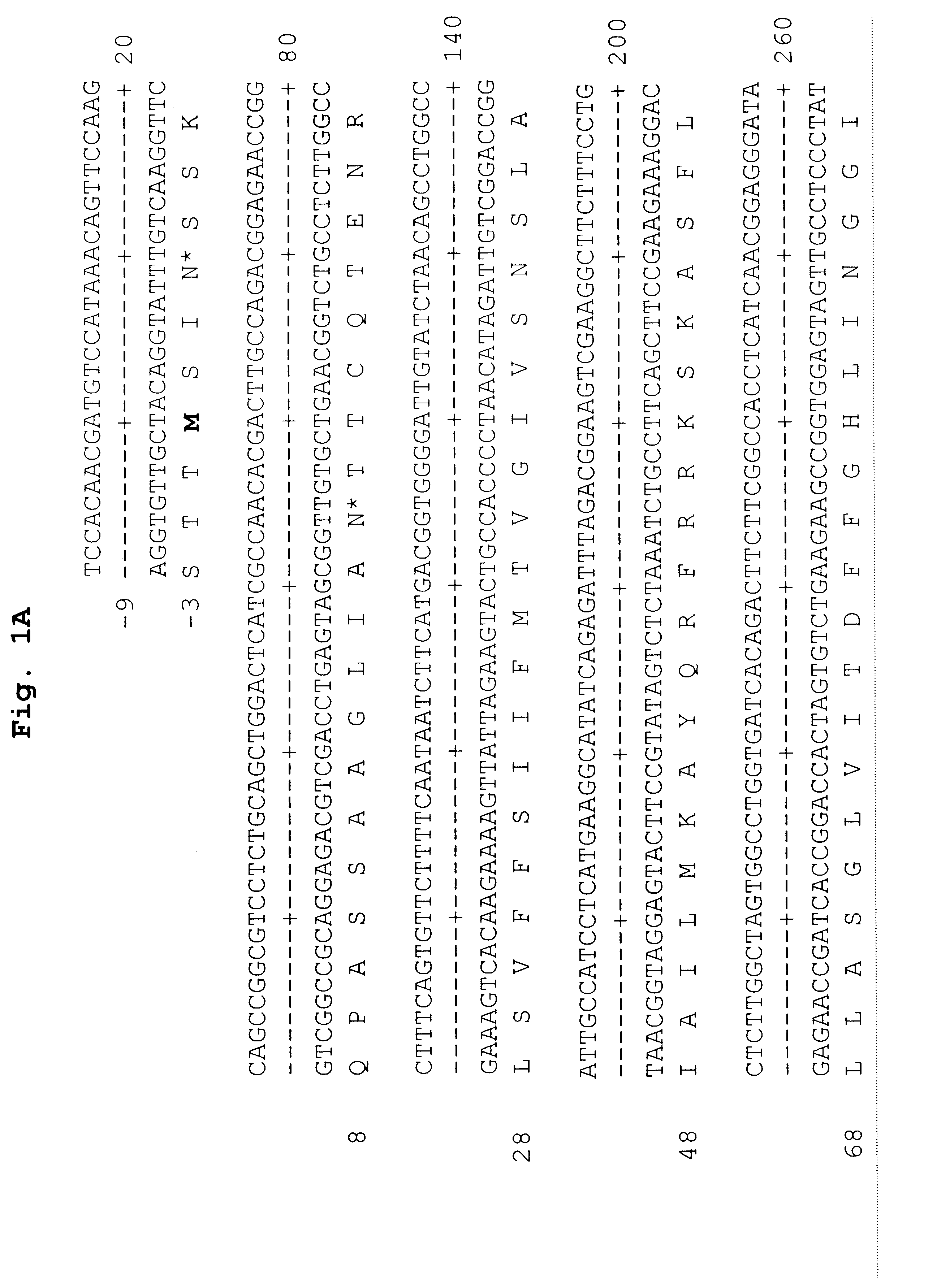
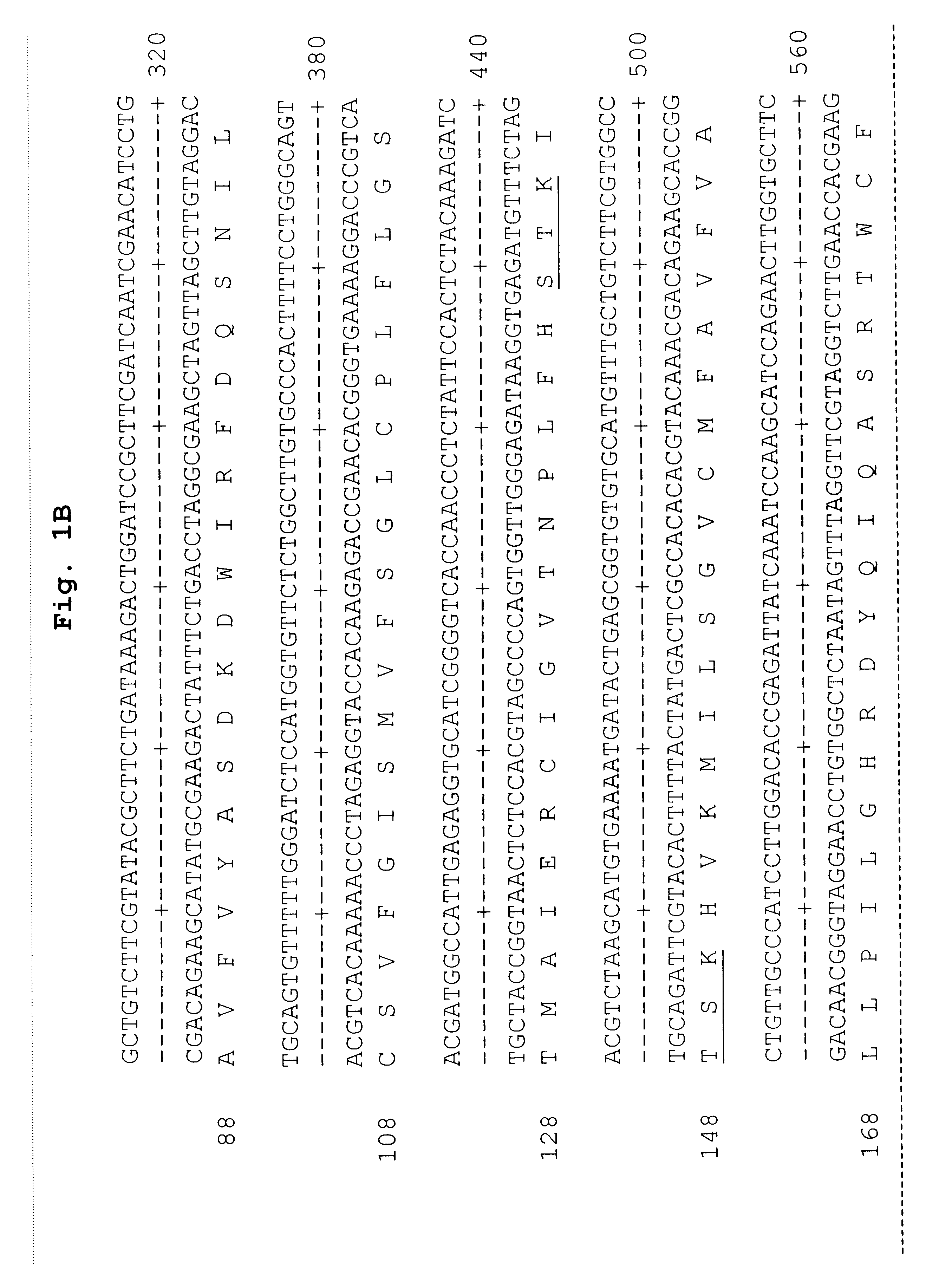
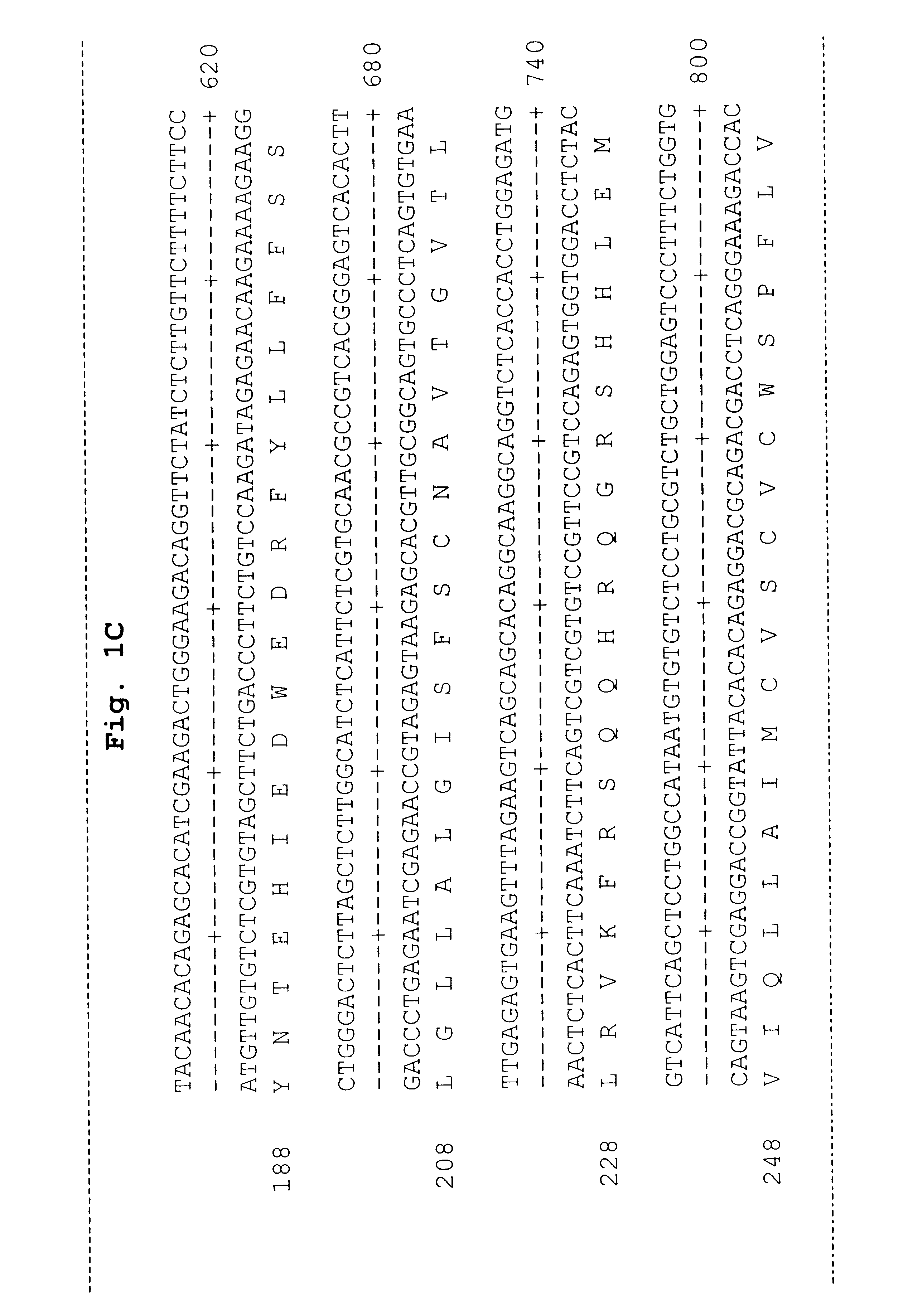
Reagents for the detection of protein phosphorylation in carcinoma signaling pathways
InactiveUS20090258442A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationPhospholipaseADAMTS Proteins
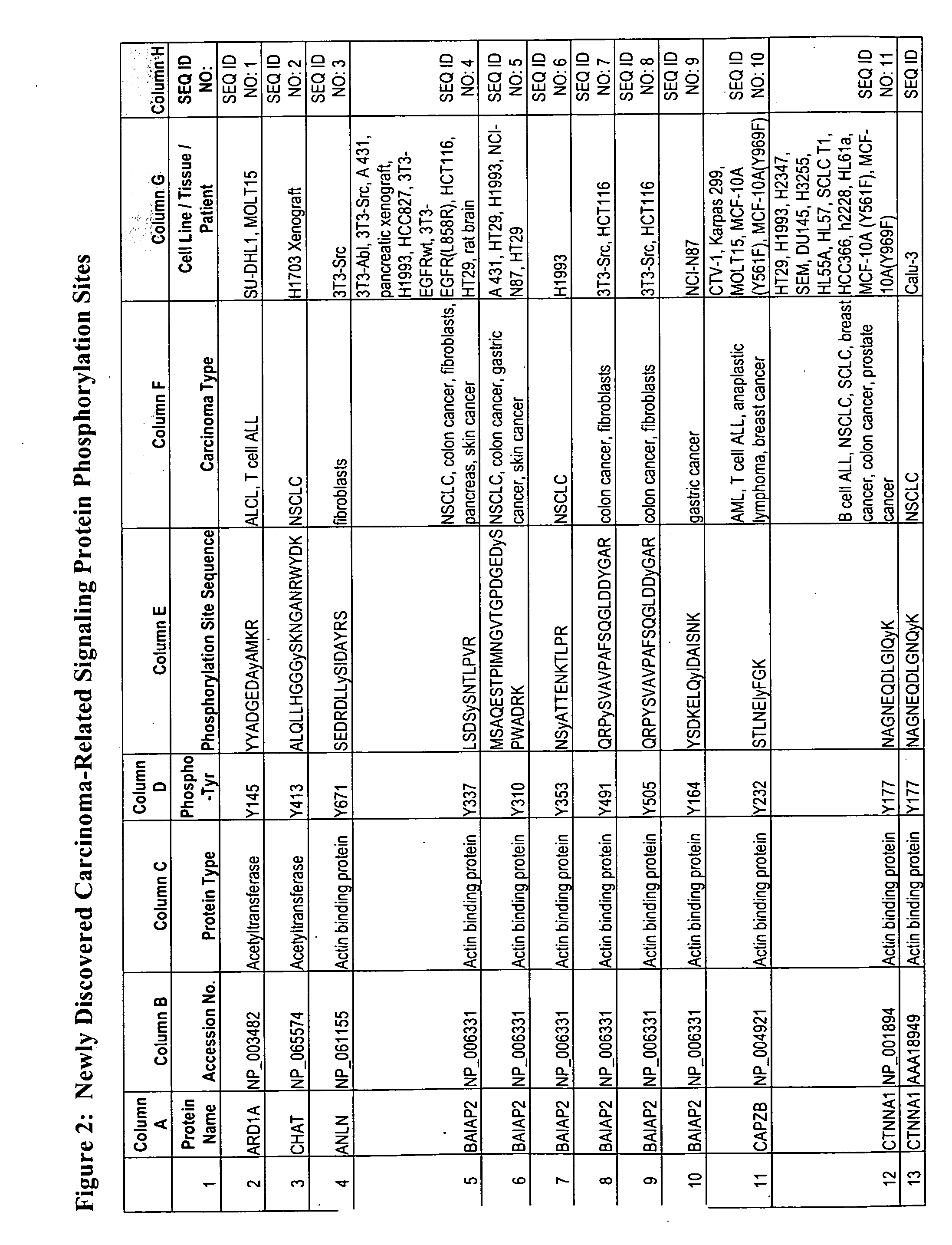
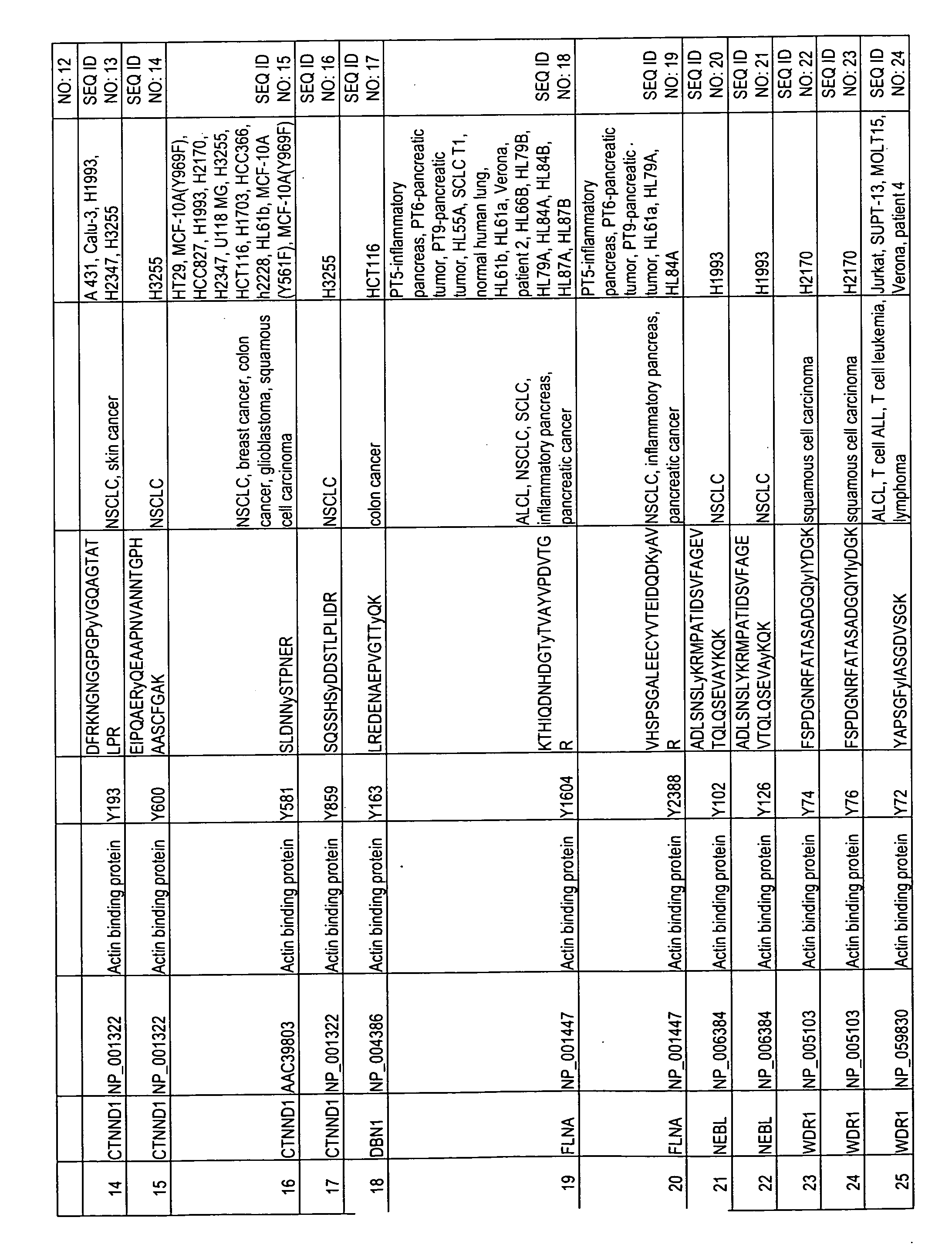
The invention discloses nearly 474 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human carcinoma, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Kinase, Adaptor / Scaffold proteins, Phosphatase, G protein Regulator / Guanine Nucleotide Exchange Factors / GTPase Activating Proteins, Cytoskeleton Proteins, DNA Binding Proteins, Phospholipase, Receptor Proteins, Enzymes, DNA Repair / Replication Proteins, Adhesion Proteins, and Proteases, as well as other protein types.
Owner:CELL SIGNALING TECHNOLOGY
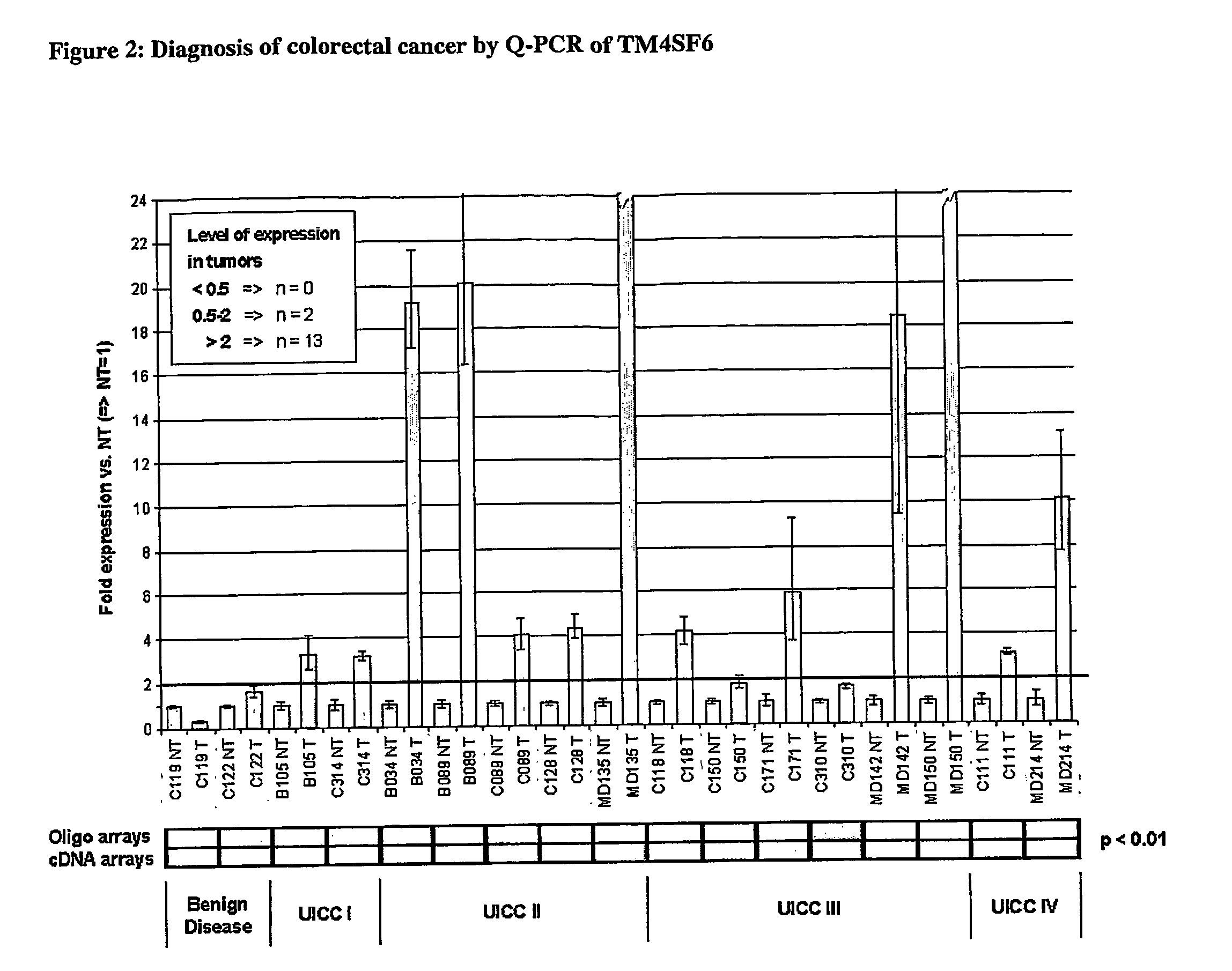
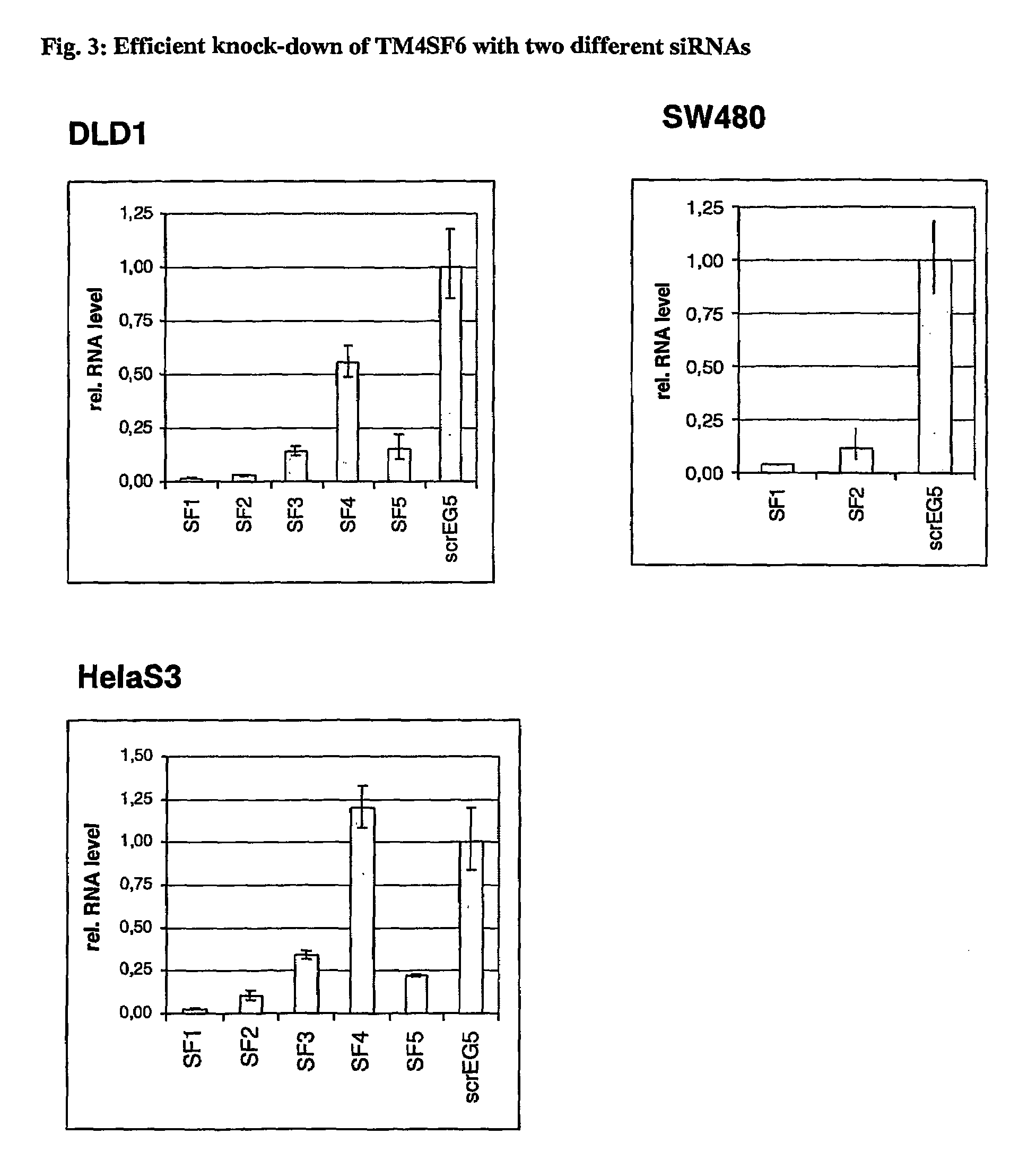
Differentially expressed tumour-specific polypeptides for use in the diagnosis and treatment of cancer
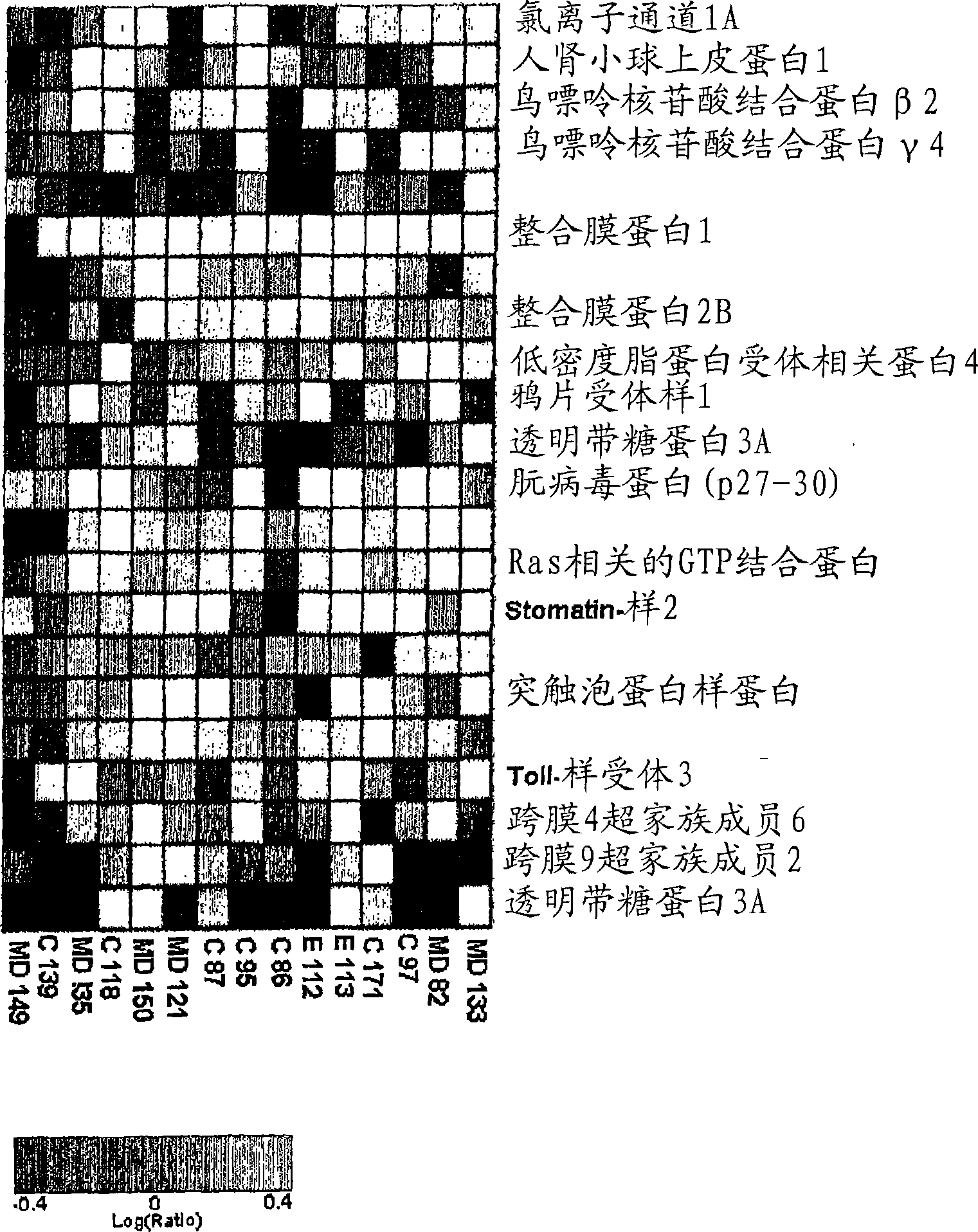
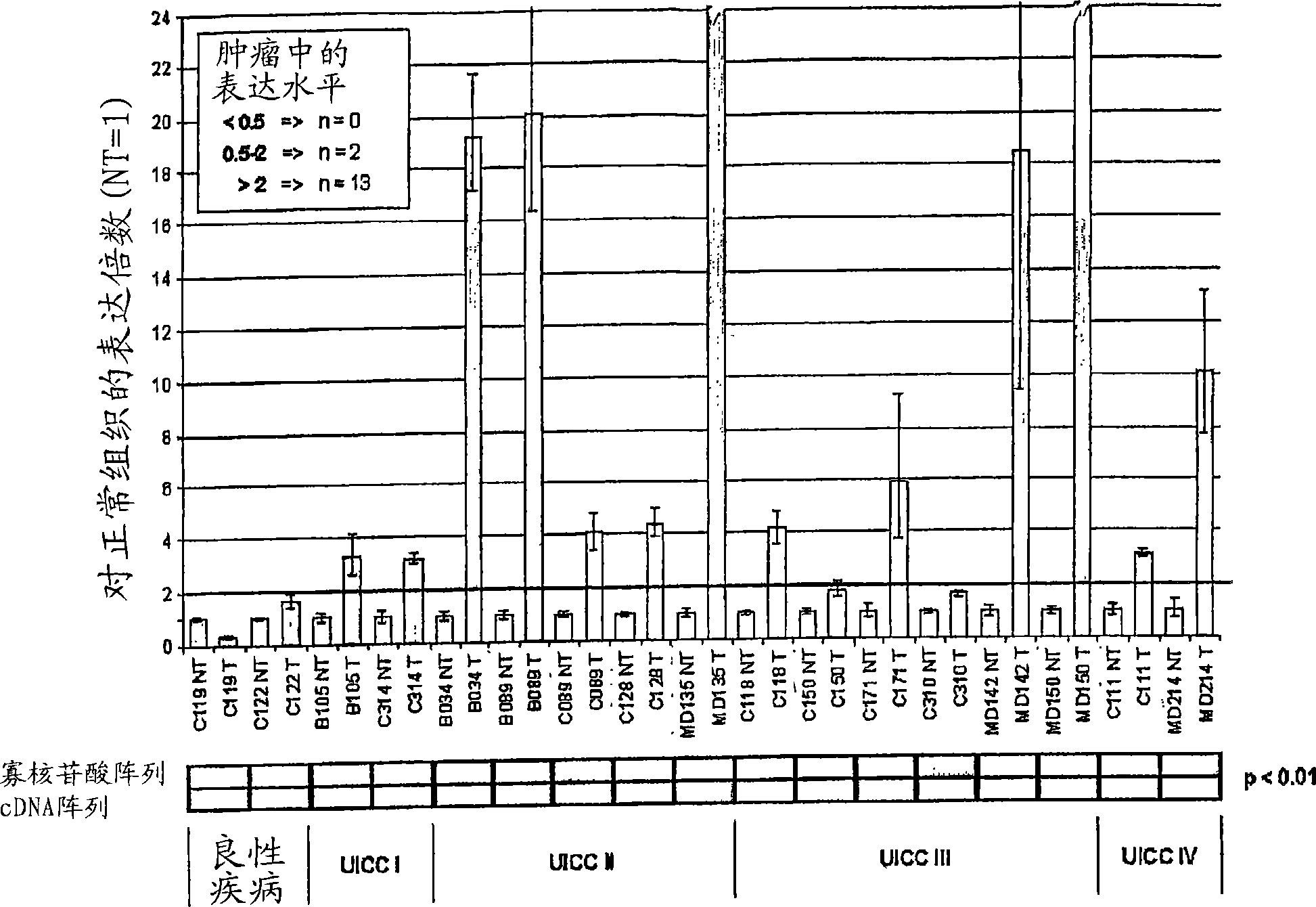
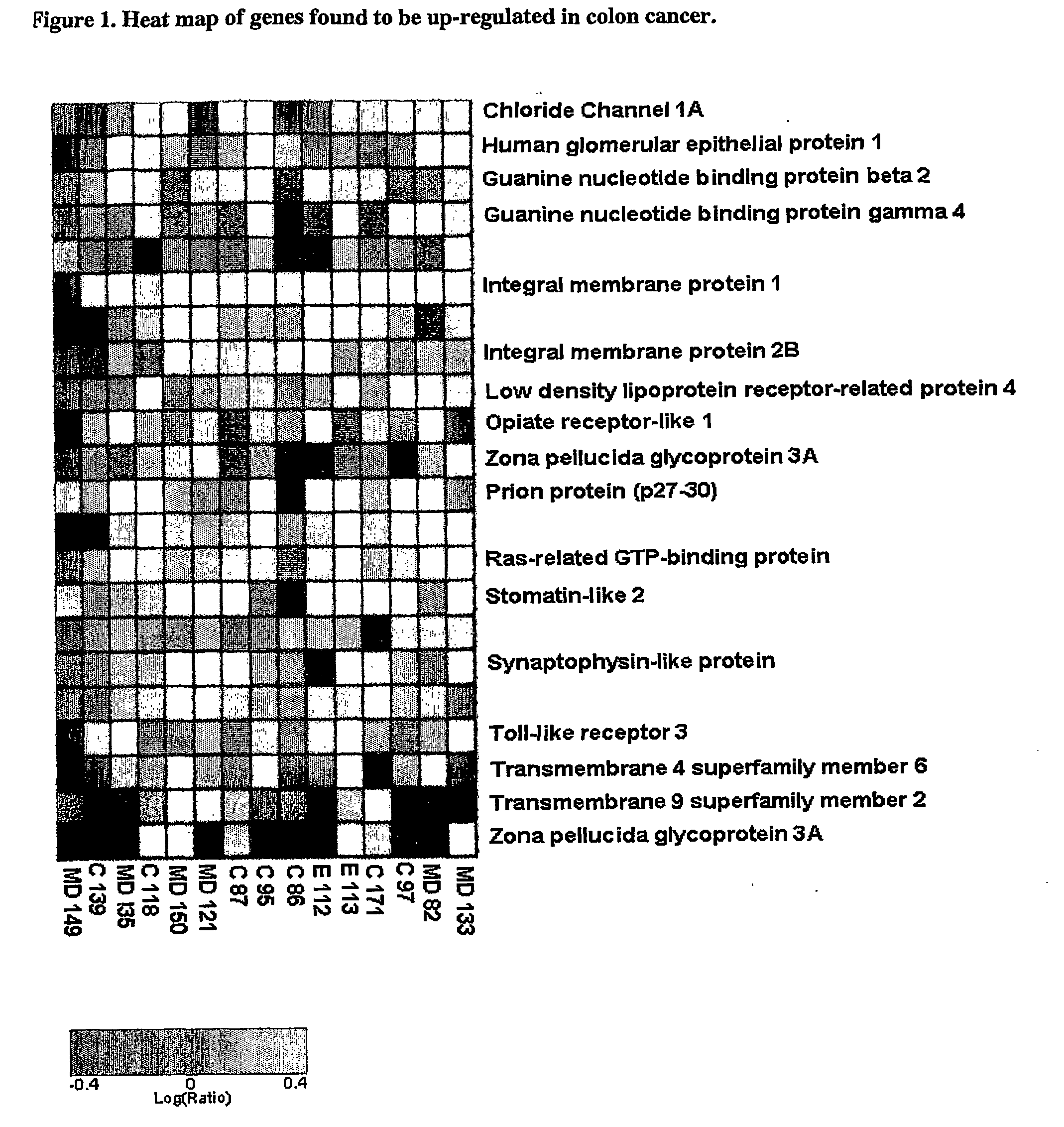
The present invention relates to reagents and methods for the diagnosis, prognosis and treatment of cancer. Specifically, the present invention relates to the use of proteins encoding transmembrane superfamily member 6 (TM4SF6), synaptophysin-like protein (SYPL), stomatin-like 2 (STOML2), Ras-associated GTP-binding protein (RAGA), nucleotide-sensitive Chloride channel 1A (CLNS1A), prion protein (p27-30) (PRNP), guanine nucleotide-binding protein β2-like 1 (GNB2L1), guanine nucleotide-binding protein 4 (GNG4), integral membrane protein 2B (ITM2B), integral membrane protein 1 (ITM1), transmembrane 9 superfamily member 2 (TM9SF2), opiate receptor-like 1 protein (OPRL1), low-density lipoprotein receptor-related protein 4 (LRP4), human kidney Nucleic acid and amino acid sequences of glomerular epithelin 1 (GLEPP1), toll-like receptor 3 (TLR3), and / or zona pellucida glycoprotein 3A (ZP3) for early and advanced non-steroid-specific cancer diagnosis, cancer prognosis, and For screening therapeutic agents that modulate the gene expression and / or biological activity of the protein. The invention further relates to biotechniques designed to inhibit gene expression and / or biological activity of said proteins, including the use of agents identified in the screening assays described herein, vector delivery of antisense polynucleotide sequences, and the Antibody targeting the protein. In specific embodiments, the protein is of human origin.
Owner:GENMAB AS
Methods for ex vivo propagation of somatic hair follicle stem cells
ActiveUS7655465B2Enhance guanine nucleotide biosynthesisSuppressing asymmetric cell kineticsBiocideCulture processCell kineticsSomatic cell
The present invention is directed to methods for readily propagating somatic hair follicle stem cells or melanocyte stem cells. The methods comprise enhancing guanine nucleotide (GNP) biosynthesis, thereby expanding guanine nucleotide pools. This in turn conditionally suppresses asymmetric cell kinetics in the explanted cells. The methods of the invention include pharmacological methods and genetic methods. For example, the resulting cultured somatic hair follicle stem cells can be used for a variety of applications including cell replacement therapies such as hair transplants, gene therapies, and tissue engineering applications, such as the generation of artificial skin and skin regeneration strategies including skin grafts.
Owner:MASSACHUSETTS INST OF TECH
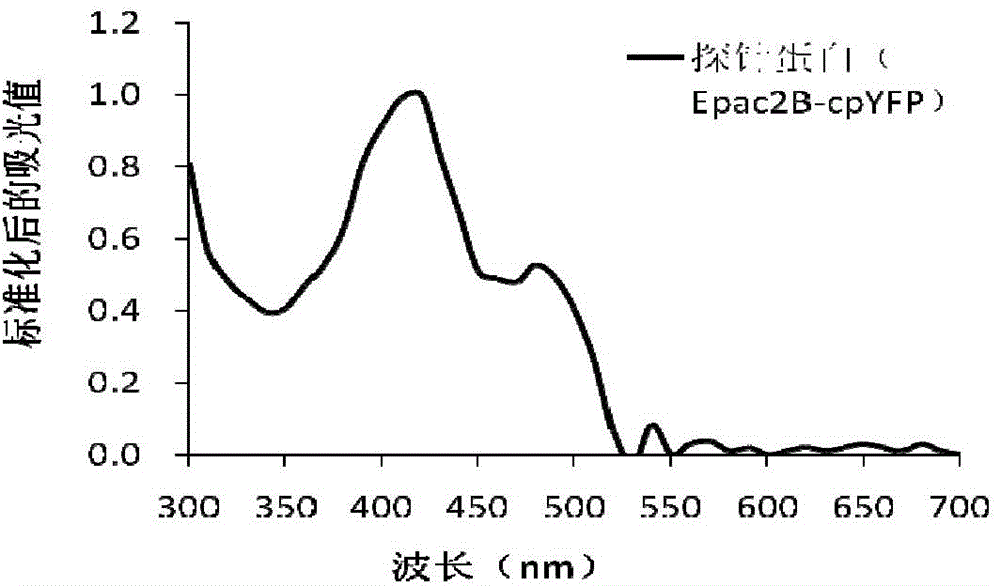
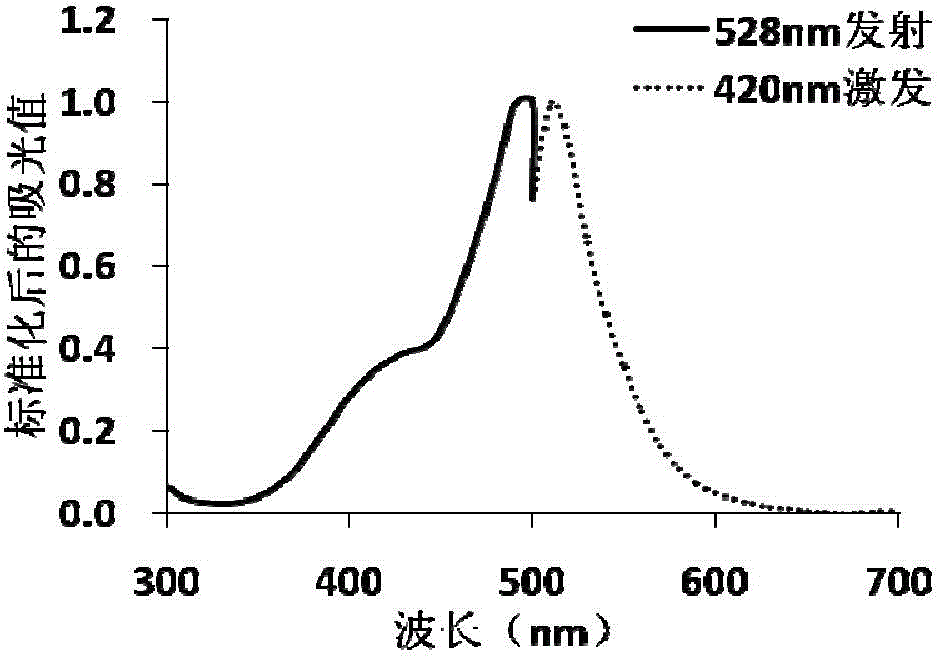
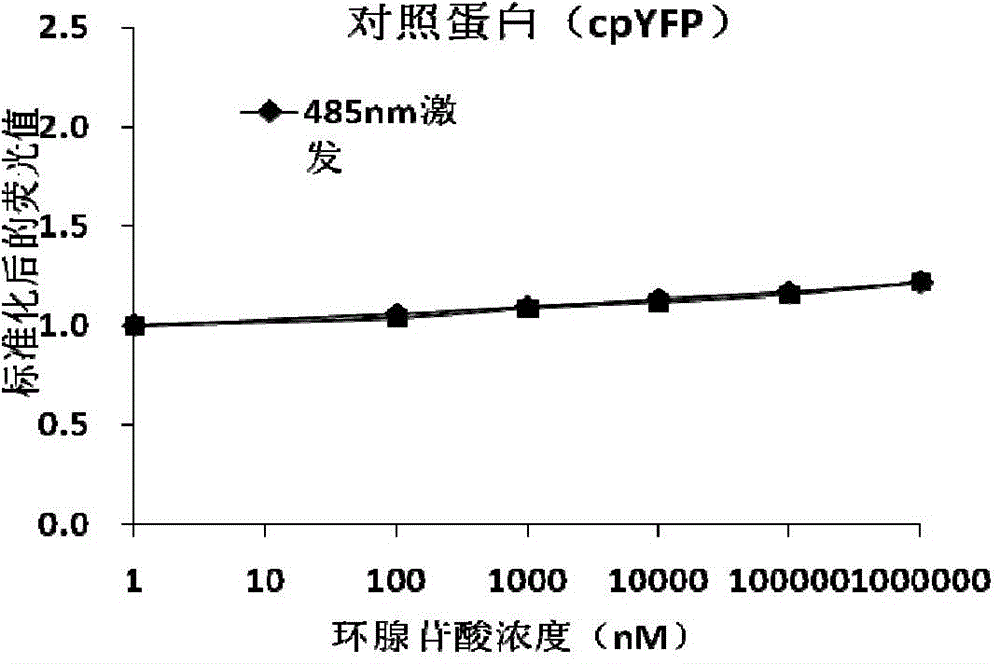
A gene-encoded cyclic adenylic acid fluorescence probe, and a preparing method and applications thereof
InactiveCN105646716AMicrobiological testing/measurementFluorescence/phosphorescenceVisibilityFluorescence
The invention provides a gene-encoded cAMP fluorescence probe containing a fluorescence protein sequence or derivatives thereof and a polypeptide sensitive to cAMP. The polypeptide sensitive to the cAMP comprises (1) a CNBD structural domain with cAMP binding characteristics and / or is derived from family proteins of a guanine nucleotide exchange factor Epac sensitive to the cAMP and directly activated with the cAMP. The invention also discloses a preparing method of the gene-encoded cAMP fluorescence probe. The fluorescence probe is applied in cAMP detection, can detect the cAMP in the physiological level and the subcellsular level, and is excellent in specificity, visibility and sensitivity.
Owner:EAST CHINA UNIV OF SCI & TECH
Methods for ex vivo propagation of adult hepatic stem cells
InactiveUS7824912B2Enhance guanine nucleotide biosynthesisSuppressing asymmetric cell kineticsCompound screeningApoptosis detectionArtificial liverNucleotide
The present invention is directed to methods for readily propagating somatic liver stem cells. The methods comprise enhancing guanine nucleotide (GNP) biosynthesis, thereby expanding guanine nucleotide pools. This in turn conditionally suppresses asymmetric cell kinetics in the explanted cells. The methods of the invention include pharmacological methods and genetic methods. For example, the resulting cultured somatic liver stem cells can be used for a variety of applications including cell replacement therapies, gene therapies, drug discovery applications, and tissue engineering applications, such as the generation of artificial liver.
Owner:MASSACHUSETTS INST OF TECH
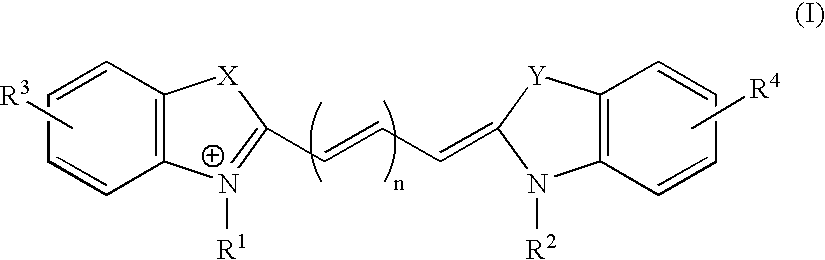
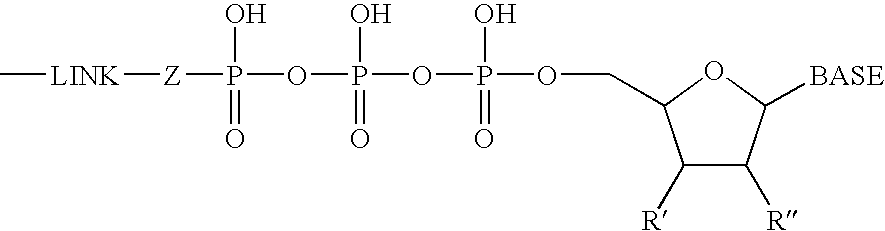
Fluorescent Nucleotide Analogues
The present invention provides fluorescent cyanine dye-based nucleotide analogues, in which the cyanine dye is coupled to the γ-phosphate group of a nucleoside triphosphate. Preferred embodiments of the invention are directed to fluorescent cyanine dye-based GTP analogues which may be employed in an homogeneous FRET-based assay to measure the binding of guanine nucleotides to GPCR polypeptides, or alternatively, to measure the effect of an exogenous ligand on GPCR protein binding.
Owner:GE HEALTHCARE LTD
Compounds for treating bacterial infections
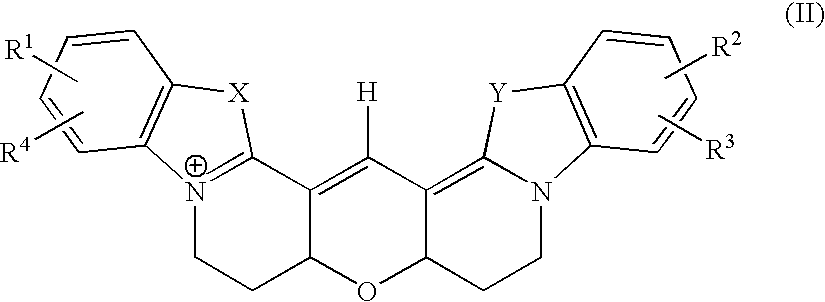
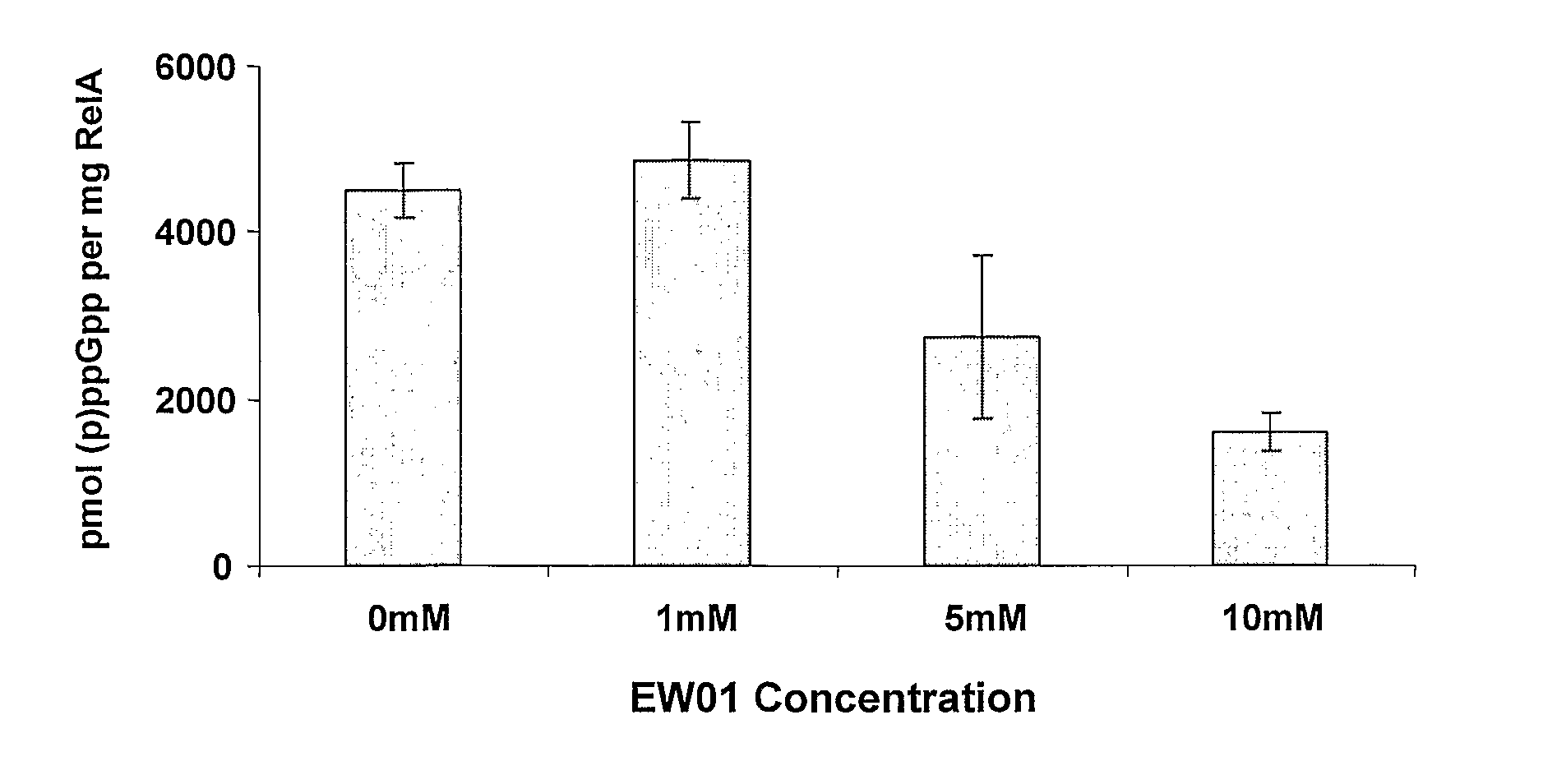
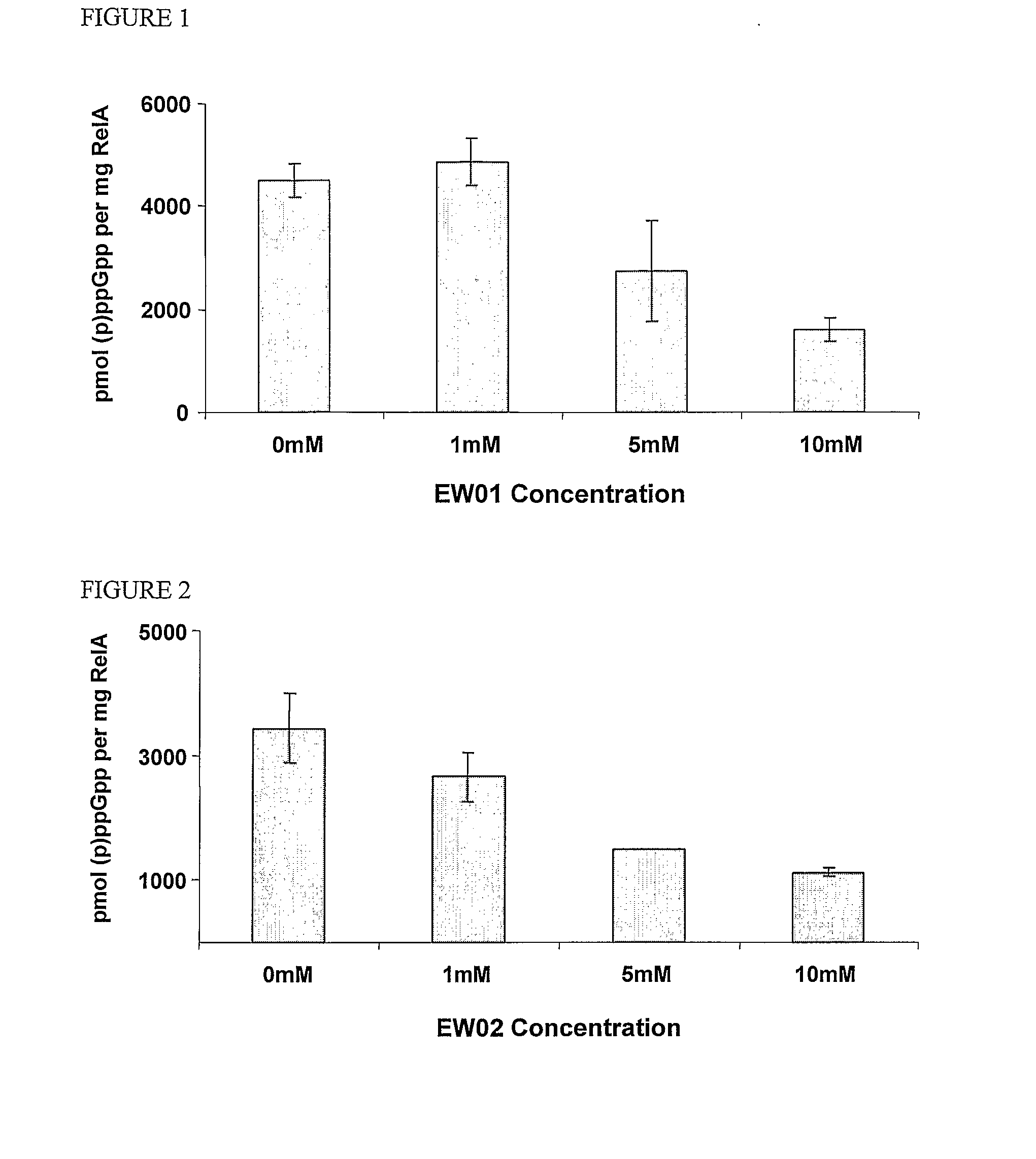
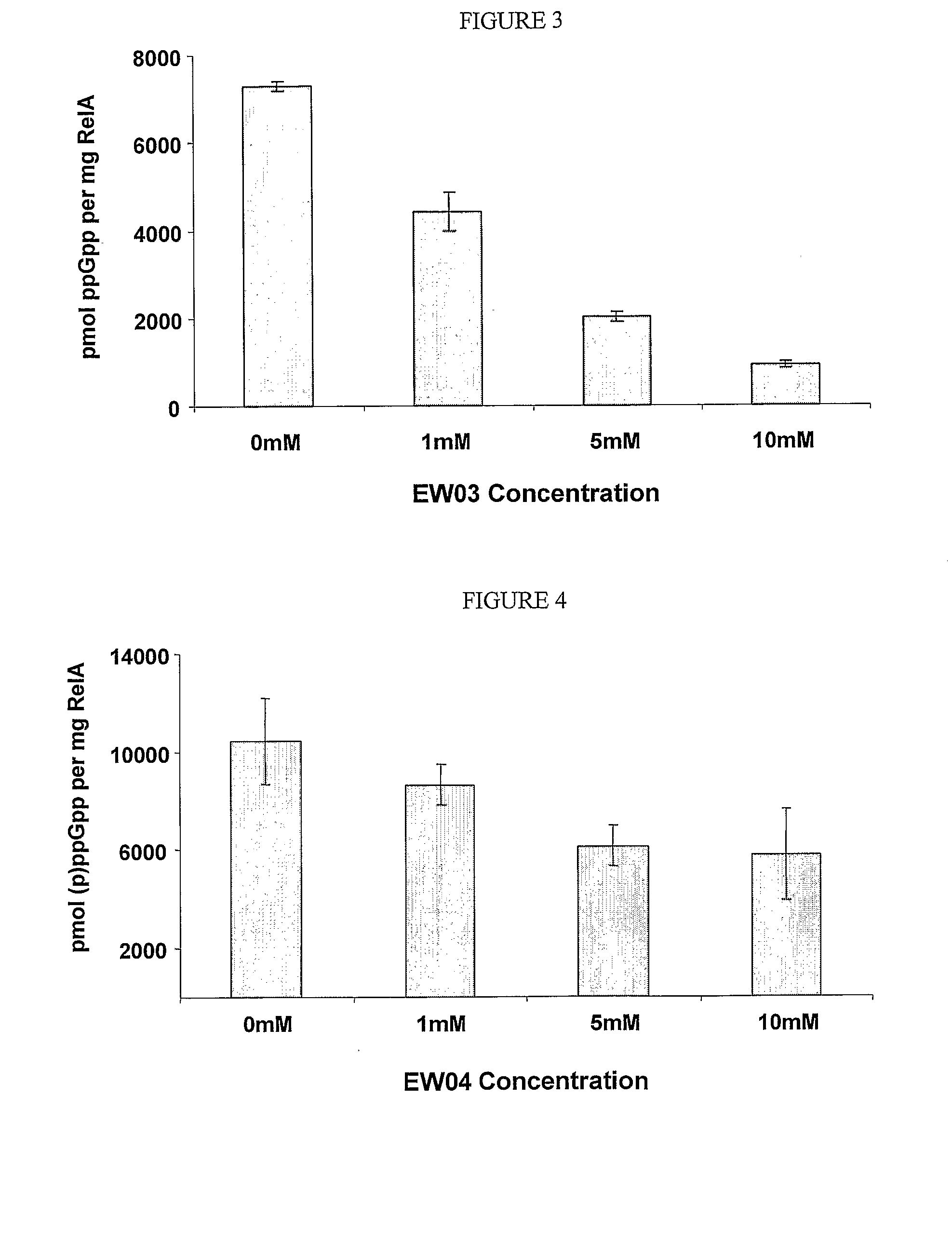
InactiveUS20110086813A1Prevented being sensedAntibacterial agentsBiocideGuanine NucleotidesAntibacterial activity
The present invention relates to a novel class of guanine nucleotide analogs which inhibit RelA and Relseq synthetic activity and which possess anti-bacterial activity. The present invention also relates to pharmaceutical compositions that include such compounds, and to methods of use of such compounds or compositions for combating bacteria and treating bacterial infections.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
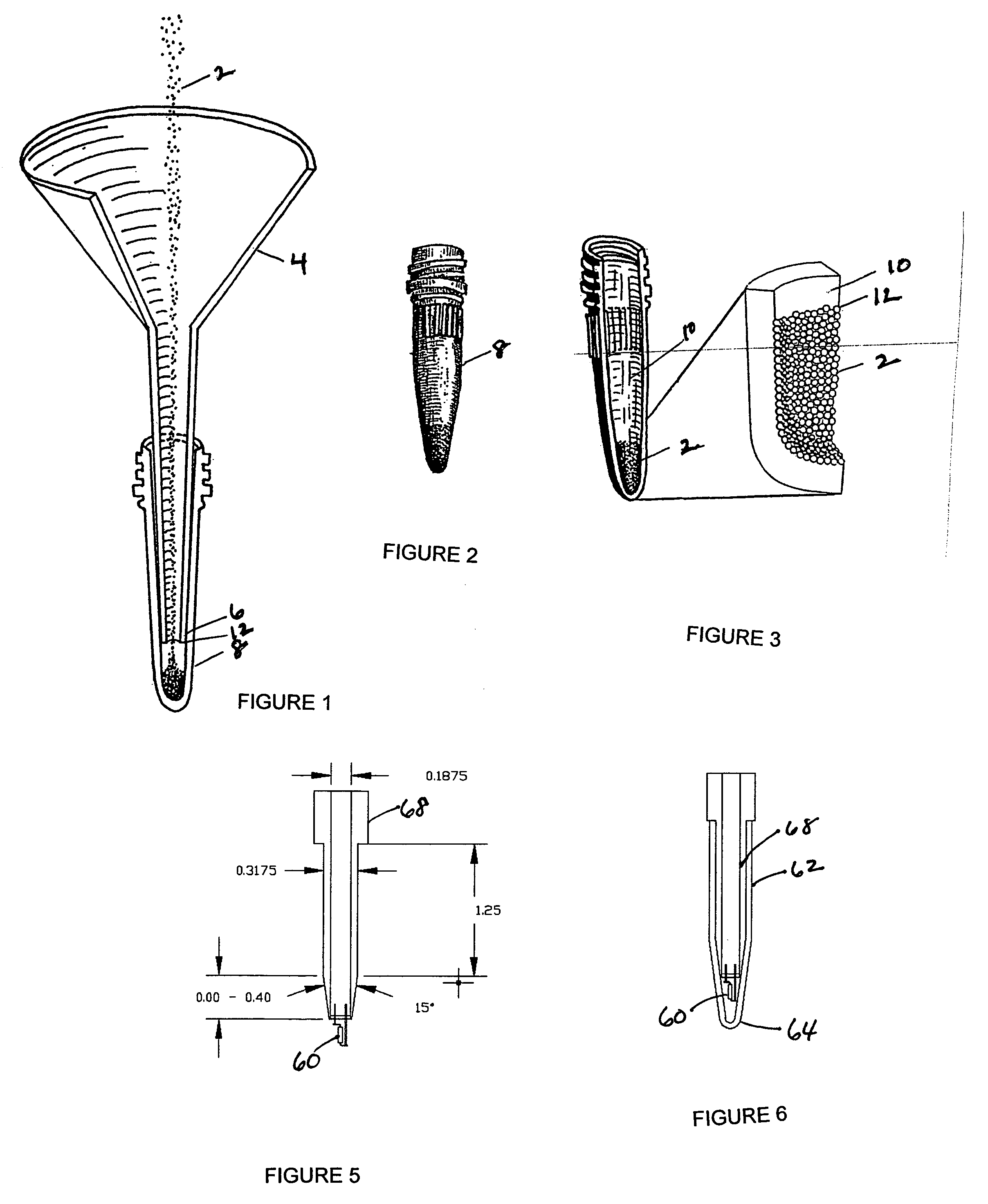
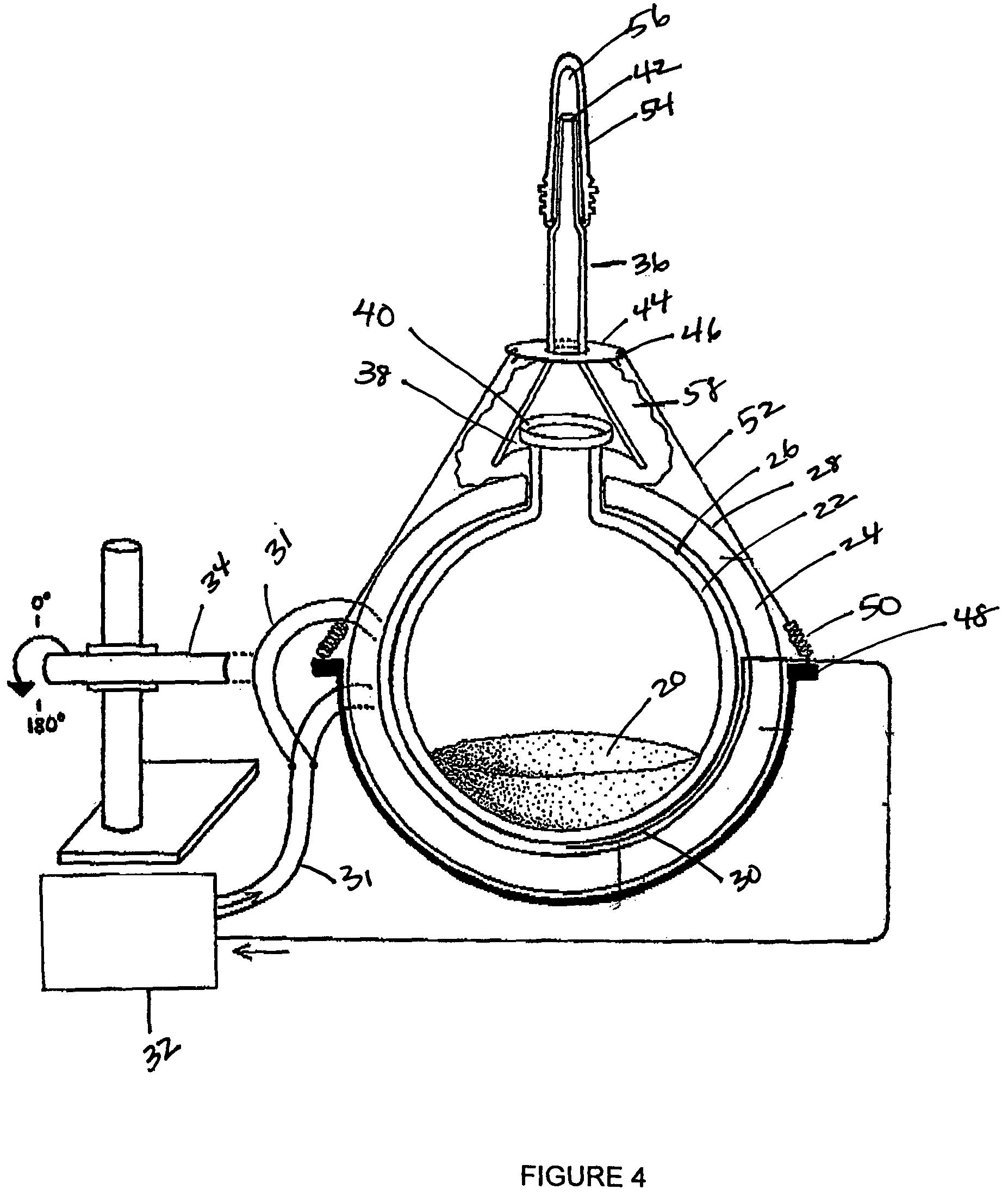
Solid phase isolation of proteins, nucleic acids and other macromolecules
InactiveUS20050164408A1Rapid fashionPeptide preparation methodsG-proteinsCrystallographyGuanine Nucleotide Binding Protein
The invention is a method for the isolation of molecules of interest using tubes in which at least a portion of the inner walls of the tube are coated with microbeads that are coated with a capture reagent to bind the molecule of interest. The microbeads may be glass or polymer beads. The invention is a method and apparatus for preparation of the tubes for use in the method of the invention. The invention is a method for determining ratios of guanine nucleotides bound to guanine-nucleotide binding proteins.
Owner:CA RGT UNIV OF
Differentially expressed tumour-specific polypeptides for use in the diagnosis and treatment of cancer
The invention relates to agents and methods for the diagnosis, prognosis and treatment of cancer. Specifically, the invention relates to the use of nucleic and amino acid sequences encoding transmembrane superfamily member 6 (TM4SF6), synaptophysin like protein (SYPL), stomatin like 2 (STOML2), Ras related GTP binding protein RAGA), nucleotide sensitive chloride channel 1A (CLNS1A), prion protein (p27-30) (PRNP), guanine nucleotide binding protein beta 2-like 1 (GNB2L1), guanine nucleotide binding protein 4 (GNG4), integral membrane protein 2B (ITM2B), integral membrane protein 1 (ITM1), transmembrane 9 superfamily member 2 (TM9SF2), opiate receptor-like 1 protein (OPRL1), low density lipoprotein receptor-related protein 4 (LRP4), human glomerular epithelial protein 1 (GLEPP1), toll-like receptor 3 (TLR3), and / or zona pellucida glycoprotein 3A (ZP3) for the diagnosis of both early and late stage non-steroid specific cancers, cancer prognosis, as well as screening for therapeutic agents that regulate the gene expression and / or biological activity of said proteins. This invention further relates to the biological technologies designed to inhibit the gene expression and / or biological activity of said proteins including using agents identified in screening assays described herein, vector delivery of antisense polynucleotide sequences, and antibody targeting of said proteins. In specific embodiments, the proteins are of human origin.
Owner:GENMAB AS
Antiproliferative activity of G-rich oligonucleotides and method of using same to bind to nucleolin
InactiveUS20090326047A1Prevent proliferationHeavy metal active ingredientsBiocideADAMTS ProteinsGuanine Nucleotides
The present invention provides a method for inhibiting the proliferation of malignant and / or hyperplastic cells in a subject by administering to the subject a therapeutically effective amount of a guanosine rich oligonucleotide. The present invention also provides oligonucleotides which are capable of being specifically bound to a specific cellular protein which is nucleolin and / or nucleolin in nature, which is implicated in the proliferation of cells, specifically malignant and / or hyperplastic cells, and a method for their selection.
Owner:ADVANCED CANCER THERAPEUTICS
Quality control method for ribonucleic acid II for injection
ActiveCN102818867AHigh technology contentImprove securityComponent separationInternational marketHydrolysis
The invention discloses a quality control method for ribonucleic acid II for injection. The method comprises the following steps that the nucleic acid enzyme hydrolysis solution of a substance to be measured is subjected to high-performance liquid chromatogram analysis, an adopted chromatographic column is an Agilent ZORBAX SB-AQC18 chromatographic column, and a flowing phase is a mixture of a formic acid solution and an acetonitrile solution; after the analysis, if the substance to be measured is determined to contain five substances as follows: cytidylate, uridine monophosphate, guanine nucleotide, guanosine and adenosine, the substance to be measured is the ribonucleic acid II for injection or is the ribonucleic acid II for injection as a candidate; and if not, the substance to be measured is not the ribonucleic acid II for injection or is not the ribonucleic acid II for injection as the candidate. A high-performance liquid chromatographic technique is utilized, and the strong-specificity quality control method for the ribonucleic acid II for injection is established. The method has important meanings on increasing the technological content of the medicine, increasing the safety and effectiveness, reducing the cost, enlarging the production scale, increasing the market occupancy, and going forward to the international market.
Owner:JILIN AODONG PHARMACEUTICAL INDUSTRY GROUP YANJI CO LTD
Reagens for the Detection of Protein Acetylation Signaling Pathways
InactiveUS20090124023A1Immunoglobulins against animals/humansBiological testingCell Surface ProteinsMetabolic enzymes
The invention discloses 432 novel acetylation sites identified in signal transduction proteins and pathways underlying human protein acetylation signaling pathways, and provides acetylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these acetylated sites / proteins, as well as methods of using the reagents for such purpose. Among the acetylation sites identified are sites occurring in the following protein types: Acetyltransferases, Adaptor / Scaffold proteins, Actin binding proteins, Adhesion proteins, Apoptosis proteins, Calcium-binding proteins, Cell Cycle Regulation proteins, Cell Surface proteins, DNA binding proteins, DNA replication proteins, Channel proteins, Chaperone proteins, Cellular Metabolism enzymes, Cytoskeletal proteins, DNA repair proteins, Endoplasmic reticulum proteins, Enzyme proteins, G protein and GTPase Activating proteins, Guanine Nucleotide Exchange Factors, Helicase proteins, Isomerase proteins, Extracelluar matrix proteins, Hydrolases, Ligase proteins, Lipid kinases, Inhibtor proteins, Lipid Binding proteins and Lyases.
Owner:CELL SIGNALING TECHNOLOGY
Solid phase isolation of proteins, nucleic acids and other macromolecules
InactiveUS7981694B2Rapid fashionPeptide preparation methodsG-proteinsCrystallographyGuanine Nucleotide Binding Protein
Owner:CA RGT UNIV OF
Reagents for the detection of protein phosphorylation in carcinoma signaling pathways
The invention discloses nearly 443 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human carcinoma, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Protein kinases (including Serine / Threonine dual specificity, and Tyrosine kinases), Adaptor / Scaffold proteins, Transcription factors, Phospoatases, Tumor supressors, Ubiquitin conjugating system proteins, Translation initiation complex proteins, RNA binding proteins, Apoptosis proteins, Adhesion proteins, G protein regulators / GTPase activating protein / Guanine nucleotide exchange factor proteins, and DNA binding / replication / repair proteins, as well as other protein types.
Owner:CELL SIGNALING TECHNOLOGY
DNA encoding a prostaglandin F2alpha receptor, a host cell transformed therewith and an expression product thereof
Molecular cloning and expression of a prostaglandin F2alpha receptor which is linked to the signal transduction pathways via guanine nucleotide binding regulatory (G) proteins and measured by, for example, cAMP, IP3 or intracellular calcium. By constructing cell lines that express a prostaglandin F2alpha receptor, the affinities and efficacies of agonist and antagonist drugs with the receptor can be assessed. A recombinant DNA construct includes a vector and a DNA fragment encoding a prostaglandin F2alpha receptor. A host cell is transformed with a recombinant DNA construct, so that the DNA fragment is expressed and a prostaglandin F2alpha receptor is produced. Suitable host systems include eukaryotic and prokaryotic cells, especially mamalian cells such as rat or human. Additionally, for diagnostic purposes, antibodies to a prostaglandin F2alpha receptor can be prepared by producing all or a portion of the receptor protein and injecting these into various types of mammals. Using the resulting antibodies, expression of an F2alpha receptor cDNA, i.e. receptor protein in tissue and cells can be measured.
Owner:PHARMACIA & UPJOHN AB
Methods for identifying chemical modulators of Ras superfamily GTPase activity
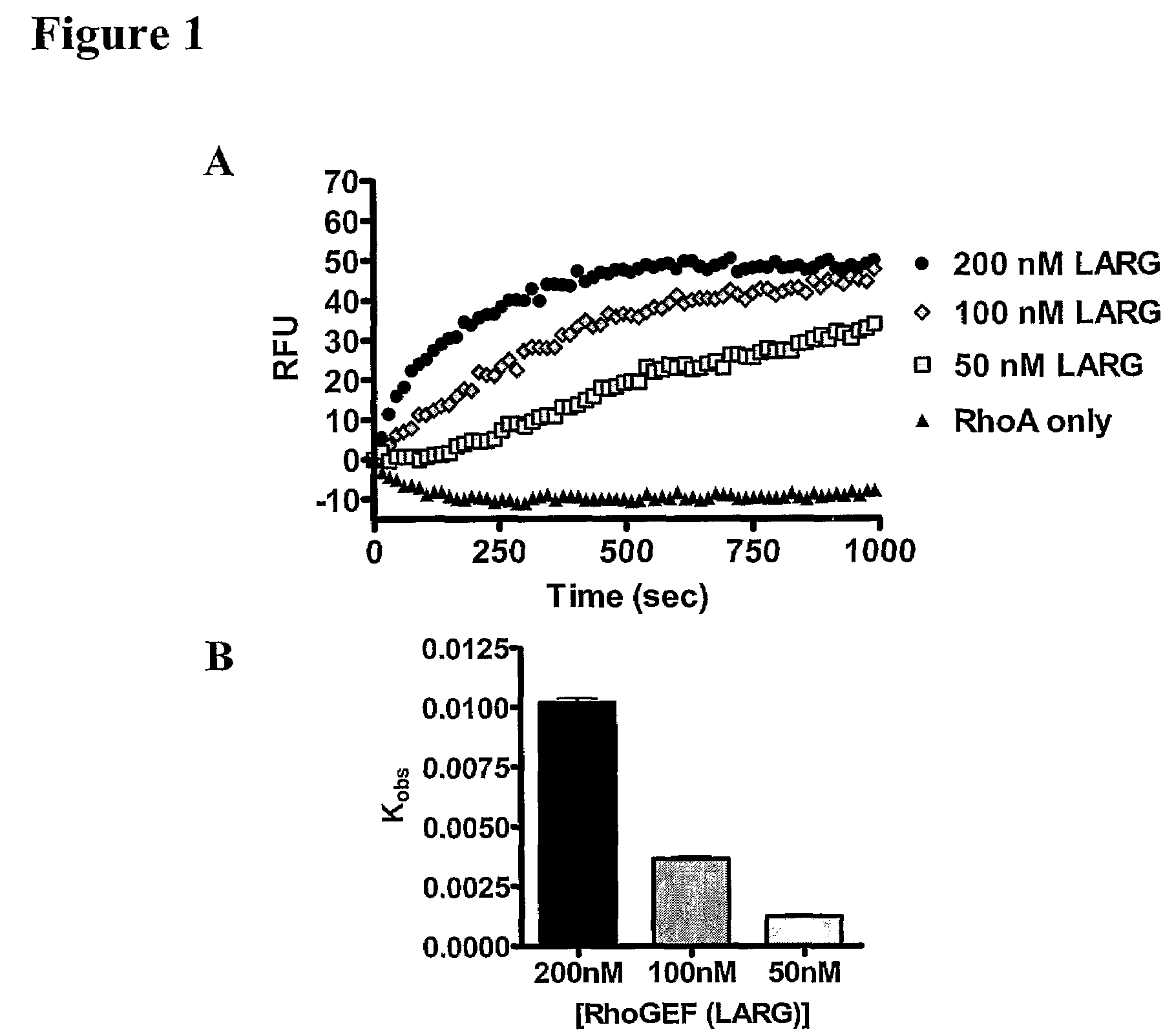
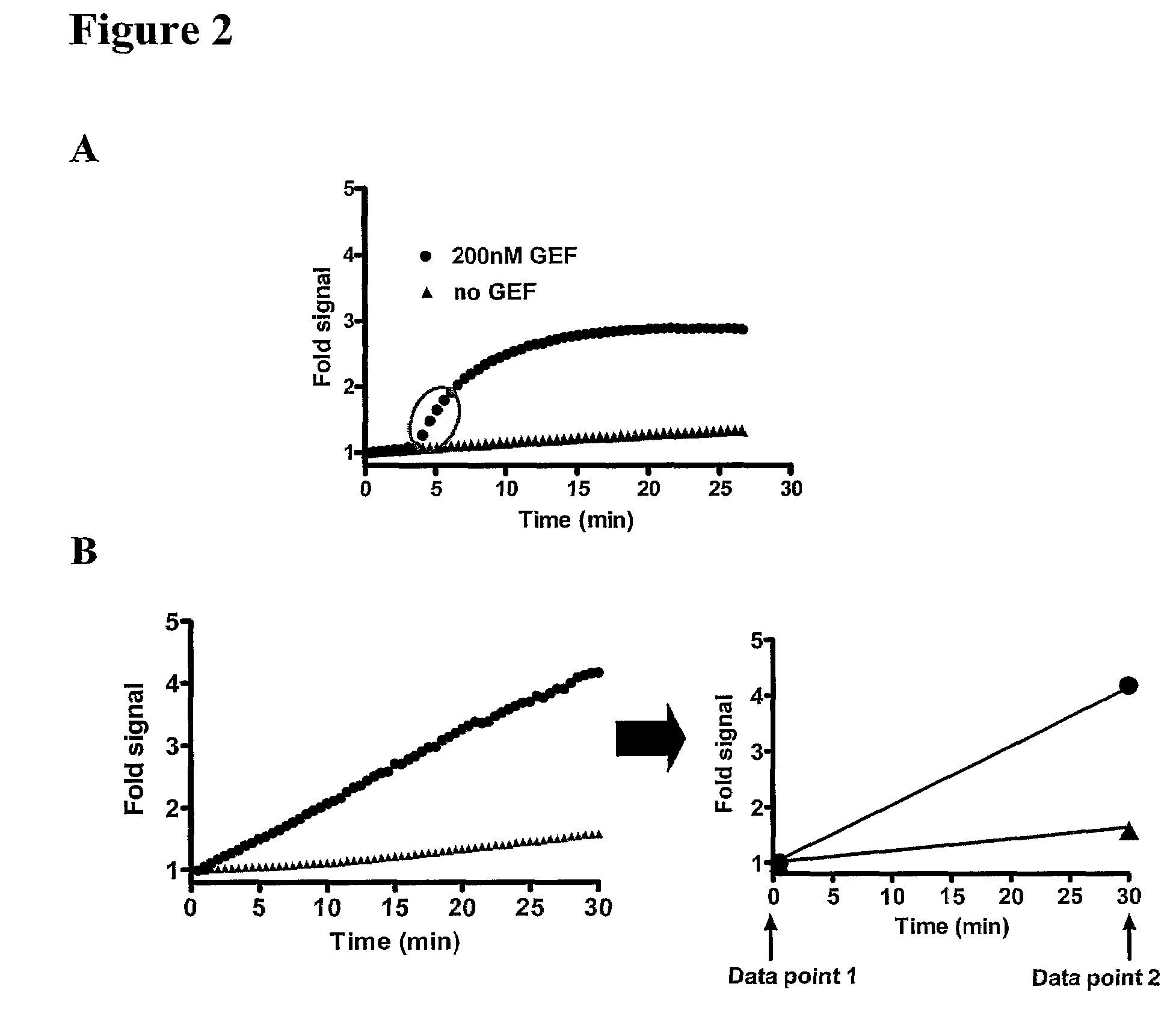
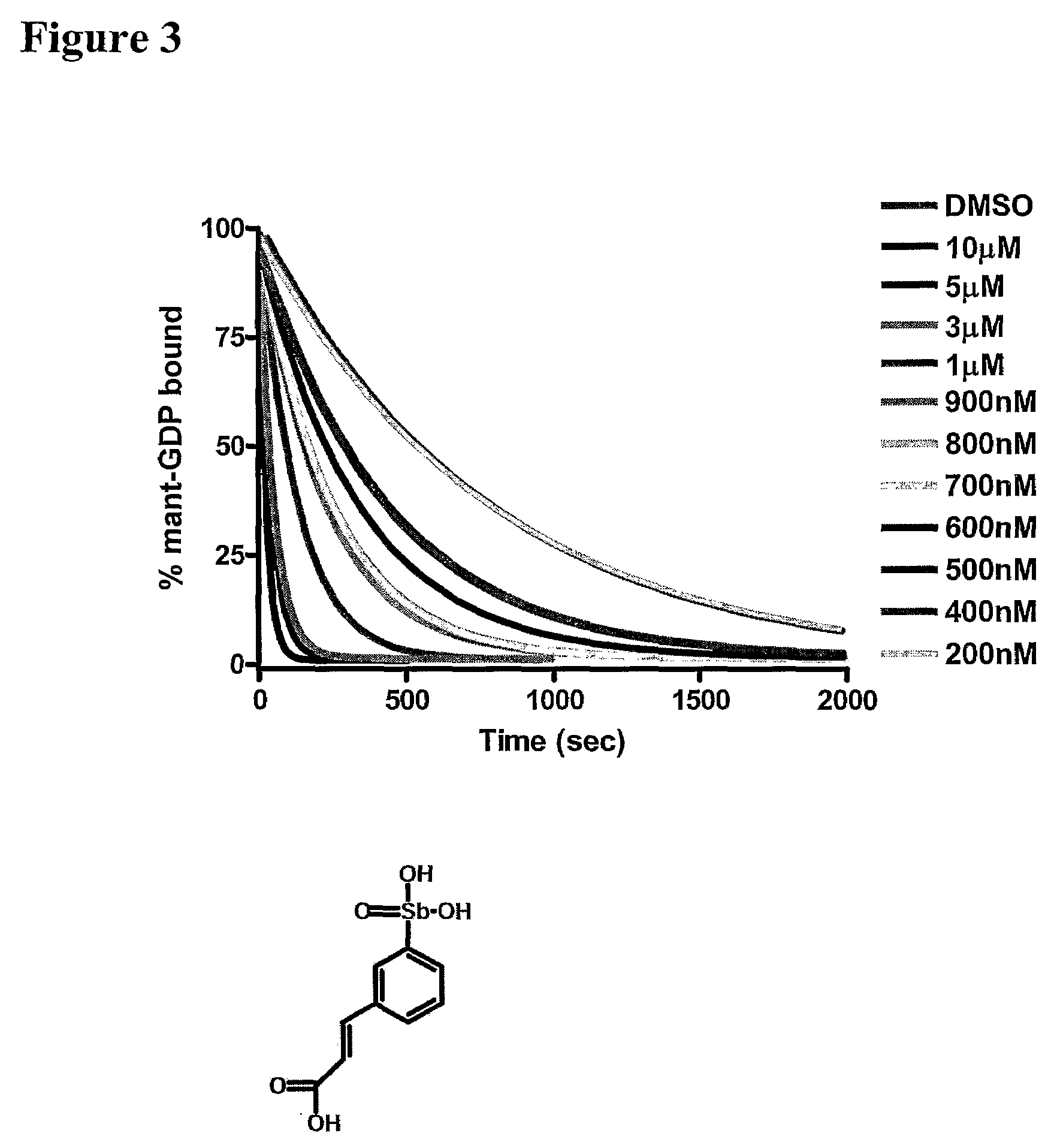
The present invention provides a method of identifying a compound having the ability to modulate the guanine nucleotide exchange cycle of a Ras superfamily GTPase, comprising: a) contacting the compound with a guanine nucleotide exchange factor and a GTPase and obtaining a baseline fluorescence measurement; b) contacting the guanine nucleotide exchange factor and the GTPase without the compound and obtaining a baseline fluorescence measurement; c) adding a fluorophore-conjugated GTP to the components of (a) and (b), respectively; d) obtaining fluorescence measurements of the respective components of (c) over time; e) subtracting the respective baseline fluorescence measurements of (a) and (b) from each fluorescence measurement of (d); and f) comparing the resulting fluorescence values of (e), wherein a decrease or increase in the rate of fluorescence change with the compound as compared with the rate of fluorescence change without the compound identifies a compound having the ability to modulate the guanine nucleotide exchange cycle of a Ras superfamily GTPase. Further provided are compounds of the invention and pharmaceutical compositions comprising compounds of the invention useful for the treatment of cancer and neurological disorders.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Feed for preventing and treating wattle-necked softshell turtle red spot disease and a preparation method thereof
The present invention discloses feed for preventing and treating wattle-necked softshell turtle red spot disease and a preparation method thereof, and belongs to the wattle-necked softshell turtle feed processing technology field. The feed consists of the following raw materials: bone meal, locust powder, pumpkin powder, sweet potato powder, table salt, dimethyl-beta-propiothetin, betaine, phenylalanine, guanine, sodium hydrogen di, disodium 5'-guanylate and Chinese herbal medicine preparation. The feed is prepared by drying, crushing, sieving, mixing, granulating, drying, cooling, packaging and other steps. The feed can play roles in resisting bacteria and diminishing inflammation, clearing away heat and detoxifying the body, cooling the blood and eliminating edema, promoting digestion, improving body immunity, effectively preventing and treating the wattle-necked softshell turtle red spot disease, and reducing mortality of the wattle-necked softshell turtle with the comprehensive utilization of various Chinese herbal medicine efficacies. The feed is reasonable in preparation and high in palatability, and can increase feed intake and improve growth rate, shorten growth cycle for the wattle-necked softshell turtle.
Owner:广西高企科技有限公司
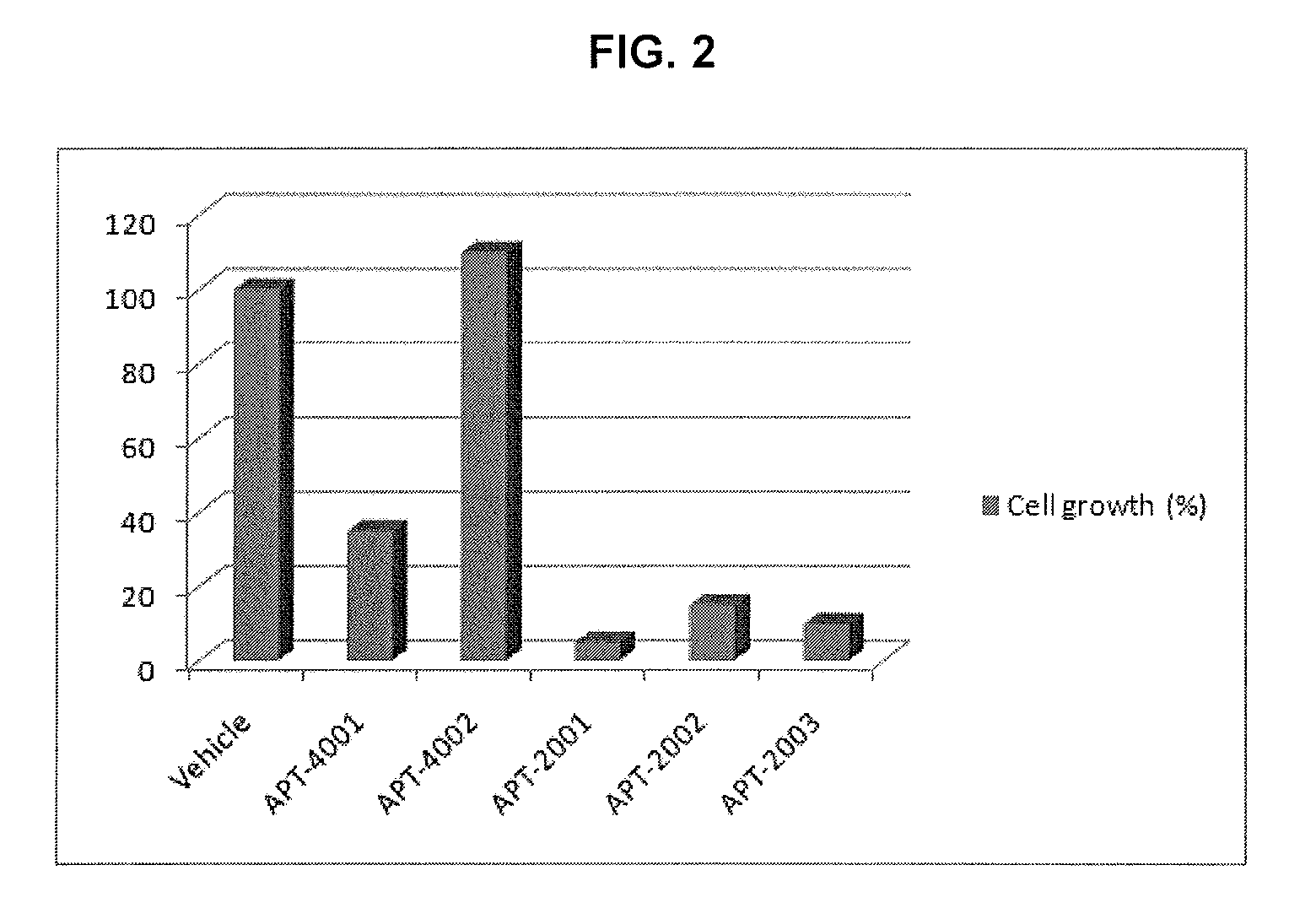
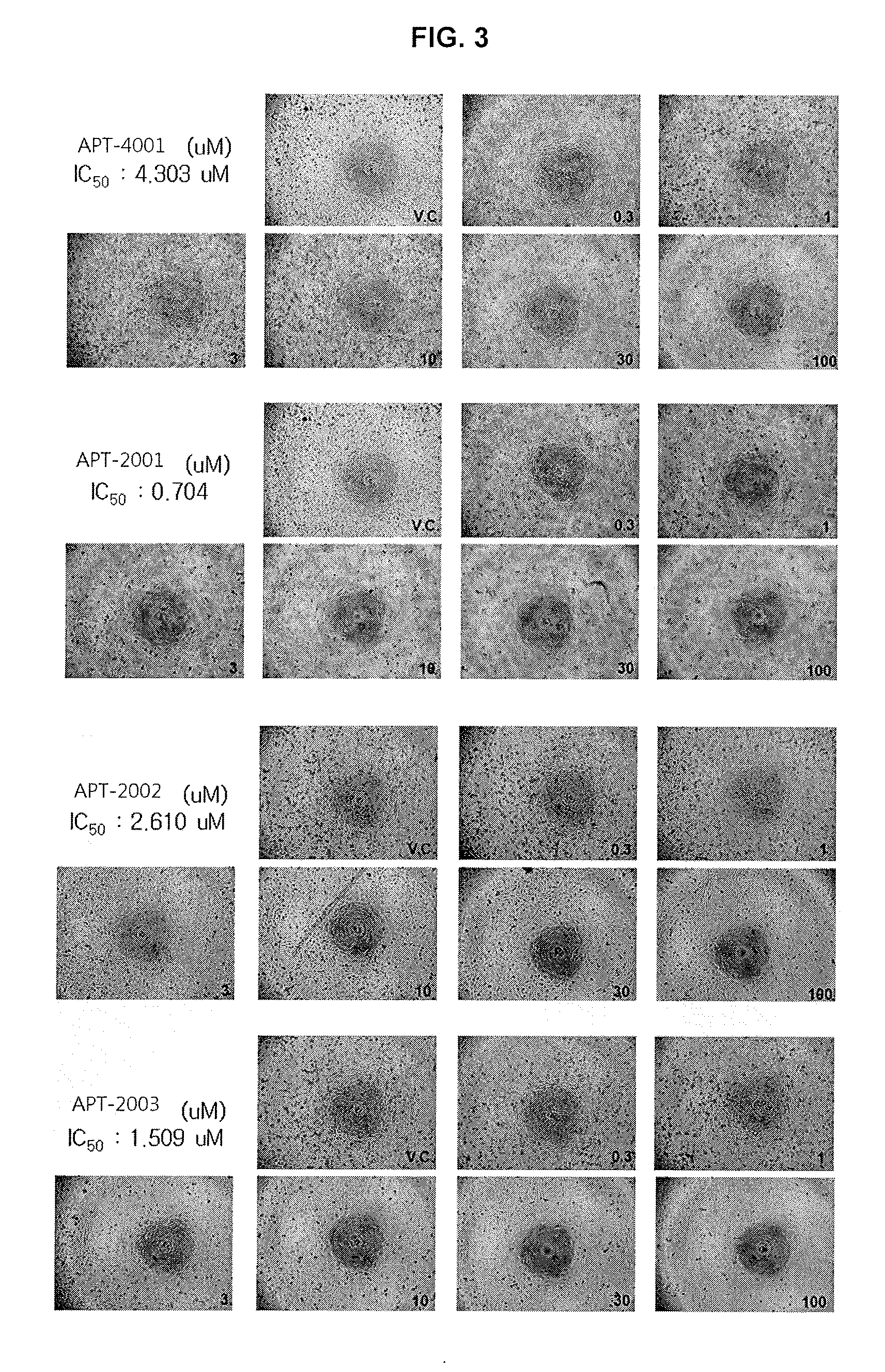
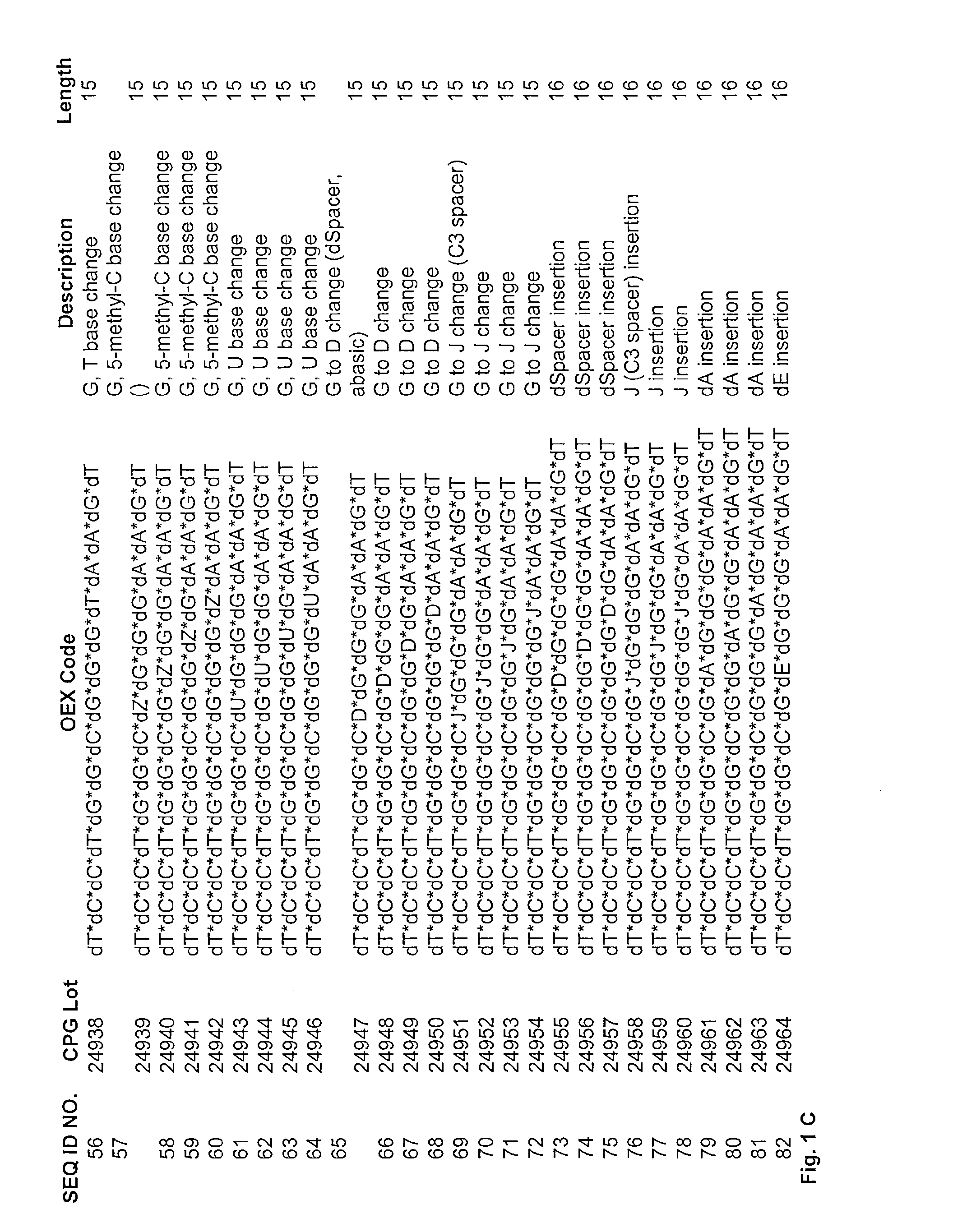
Guanosine-rich modified oligonucleotides and antiproliferative activity thereof
ActiveUS9056886B2High activityOrganic active ingredientsSugar derivativesCancer cellGuanine Nucleotides
The present invention relates to a novel modified oligonucleotide comprising at least one guanosine molecule and a modified nucleic acid with therapeutic efficacies. The present invention also relates to a pharmaceutical composition having cell apoptotic activity against cancer cells for preventing or treating cancer comprising a guanosine-rich modified oligonucleotide with at least one therapeutically effective modified nucleic acid (N), or its pharmaceutically acceptable salt as active ingredient.
Owner:APTABIO THERAPEUTICS
Inhibitory oligonucleotides and their use in therapy
Inhibitory oligonucleotide having the general formula:X1 C C N1 N2 N3 X2 N4 N5 GGG N6 X3 N7 (I)are disclosed which can be used in pharmaceutical compositions, whereby in formula (I)C is cytidine or a derivative thereof, whereby the cytidine derivative is selected from the group consisting of 5-methylcytidine, a cytidine-like nucleotide having a chemical modification involving the cytosine base, cytidine nucleoside sugar, or both the cytosine base and the cytidine nucleoside sugar, 2′-O-methylcytidine, 5-bromocytidine, 5-hydroxycytidine, ribocytidine and cytosine-β-D-arabinofuranoside,G is guanosine or a derivative thereof, whereby the guanosine derivative is selected from the group consisting of 7-deazaguanosine, a guanosine-like nucleotide having a chemical modification involving the guanine base, the guanosine nucleoside sugar or both the guanine base and the guanosine nucleoside sugar,X1 and X3 is any nucleotide sequence with 0 to 12 bases and each nucleotide is independent of any other, X2 is any nucleotide sequence having 0 to 3 nucleotides,N1, N2 and N3are each independently any nucleotide,N4 and N7 is a pyrimidine or a modified pyrimidine,N5 is a purin or a modified purin,N6 is a modified pyrimidine, A or a modified purin,wherein at least two of the nucleotides N4, N5, N6 or N7 are modified purins or modified pyrimidines.
Owner:SAREPTA THERAPEUTICS INC
Systems and methods for the assessment of g-protein activation
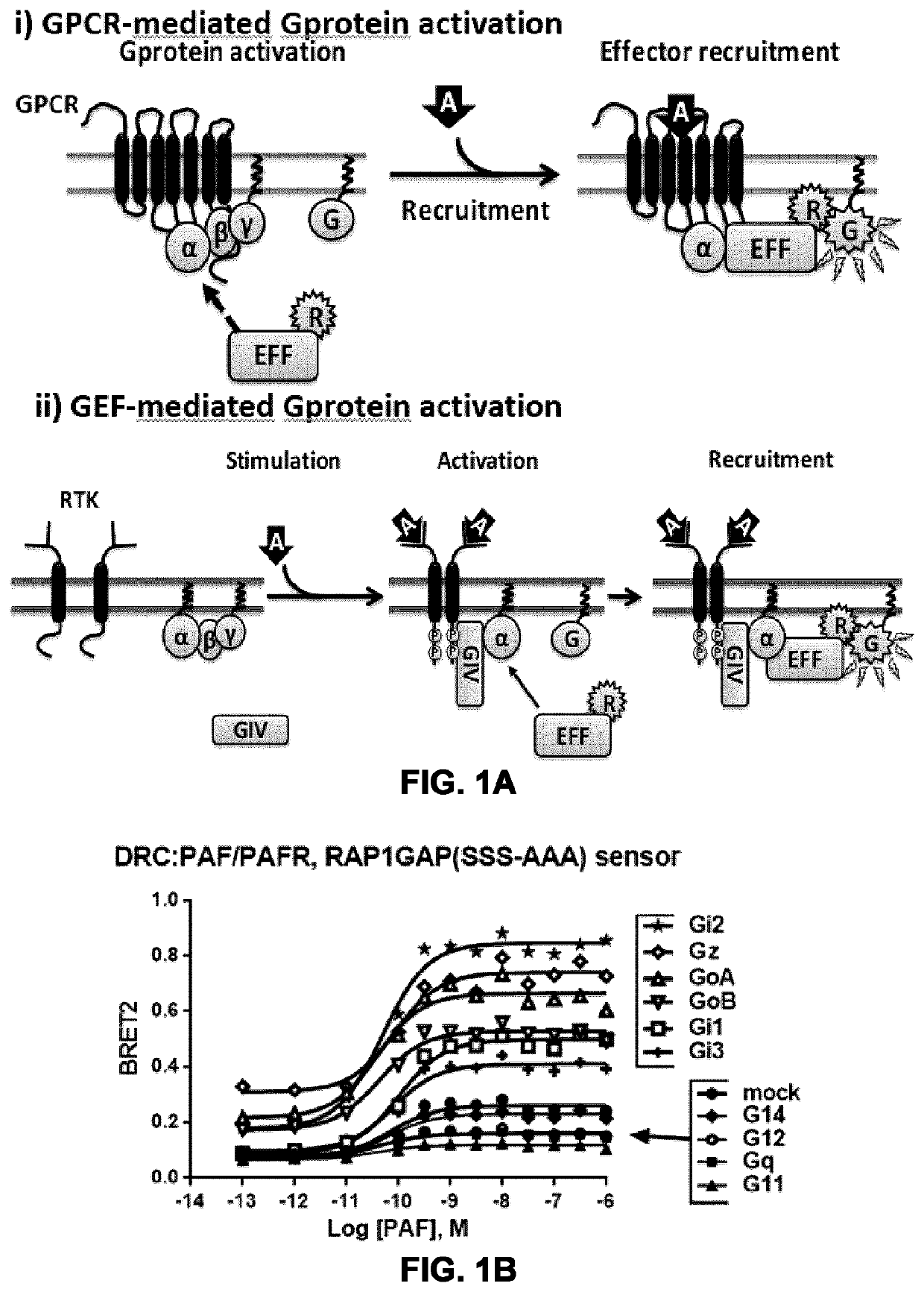
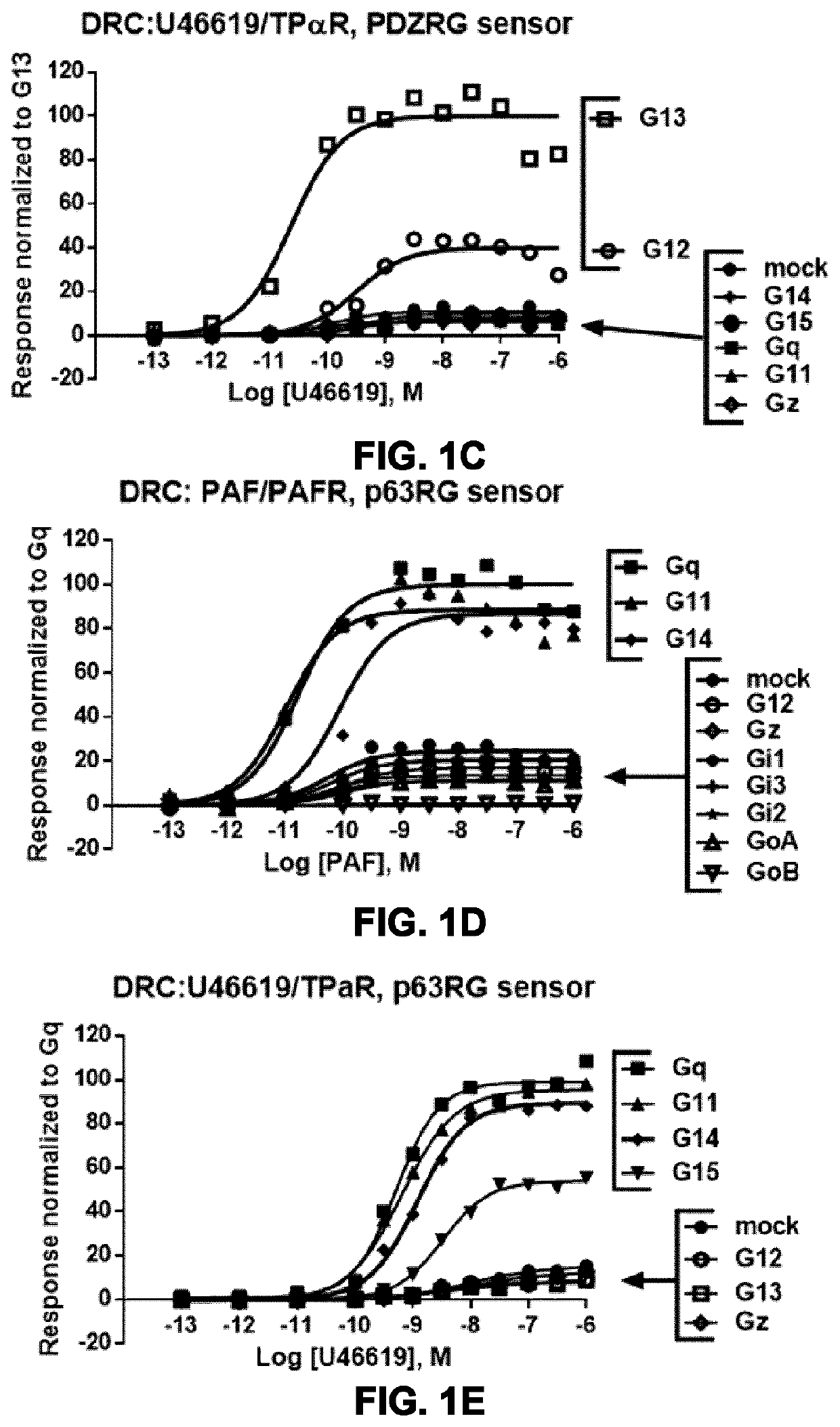
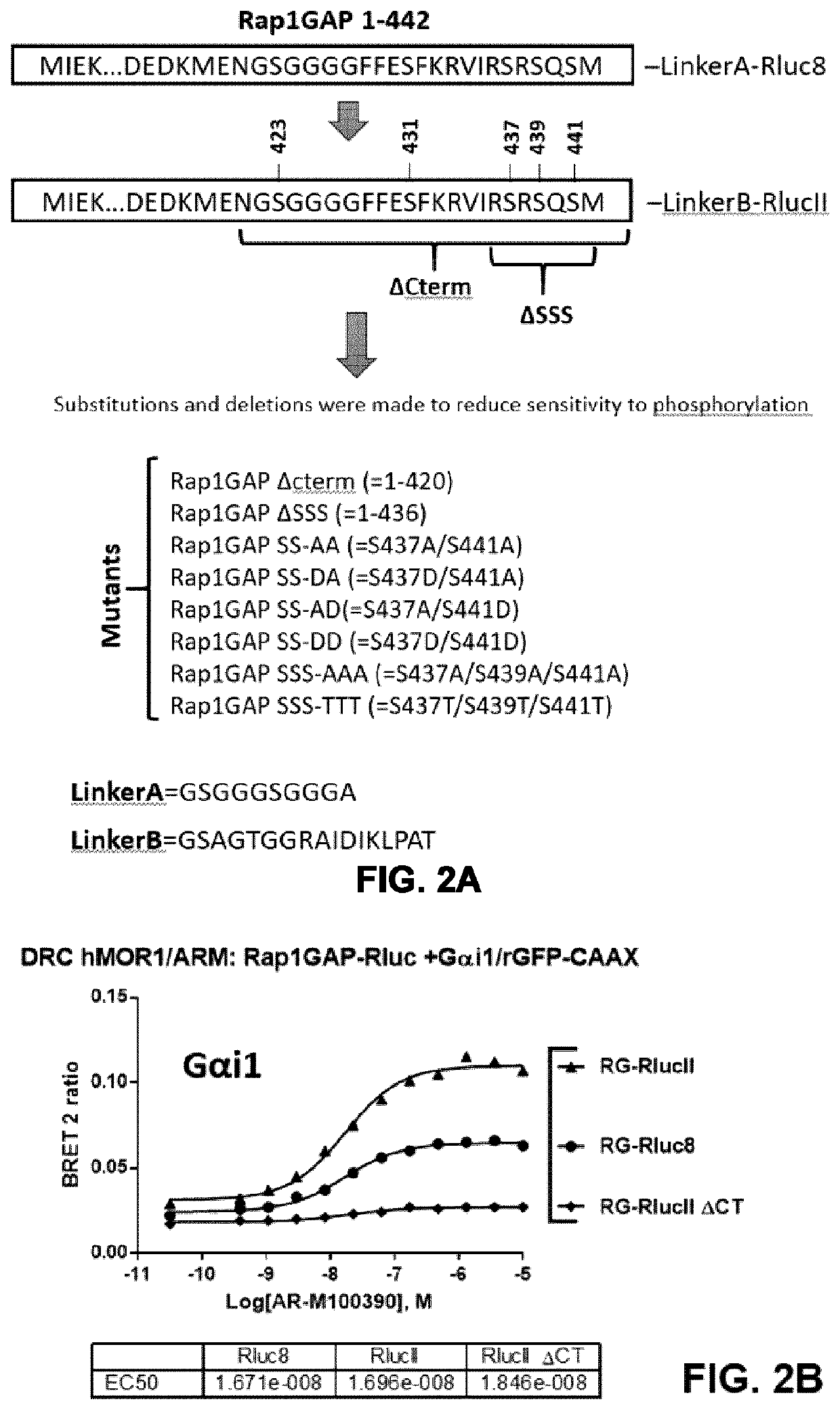
Systems and methods for the monitoring of G protein activation at various cell compartments, such as the plasma membrane and the endosomes, and in a Gα protein subunit family-selective manner are described. These systems and methods also allows the monitoring of G protein-coupled receptor (GPCR)-mediated as well as non-receptor guanine nucleotide exchange factor (GEF)-mediated G protein activation, and are based on the use of the G protein-binding domains of specific effectors of G proteins and cellular compartment markers, tagged with suitable energy (BRET) donors and acceptors.
Owner:UNIV DE MONTREAL +1
Methods for producing pancreatic precursor cells and uses thereof
InactiveUS20140193910A1Easily propagatedSolve the shortageCell dissociation methodsMetabolism disorderGuanine NucleotidesPrecursor cell
The present invention is directed to methods for readily propagating somatic pancreatic precursor cells. The methods comprise isolating cells from intact pancreatic samples and enhancing guanine nucleotide (GNP) biosynthesis in cultures comprising these cells, thereby expanding guanine nucleotide pools. This in turn conditionally suppresses asymmetric cell kinetics in the cells, thereby generating pancreatic precursor cells.
Owner:SHERLEY JAMES
Fluorescent nucleotide analogues
The present invention provides fluorescent cyanine dye-based nucleotide analogues, in which the cyanine dye is coupled to the γ-phosphate group of a nucleoside triphosphate. Preferred embodiments of the invention are directed to fluorescent cyanine dye-based GTP analogues which may be employed in an homogeneous FRET-based assay to measure the binding of guanine nucleotides to GPCR polypeptides, or alternatively, to measure the effect of an exogenous ligand on GPCR protein binding.
Owner:GE HEALTHCARE LTD
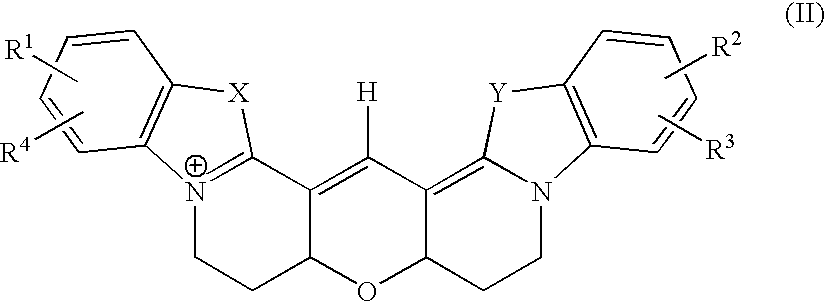
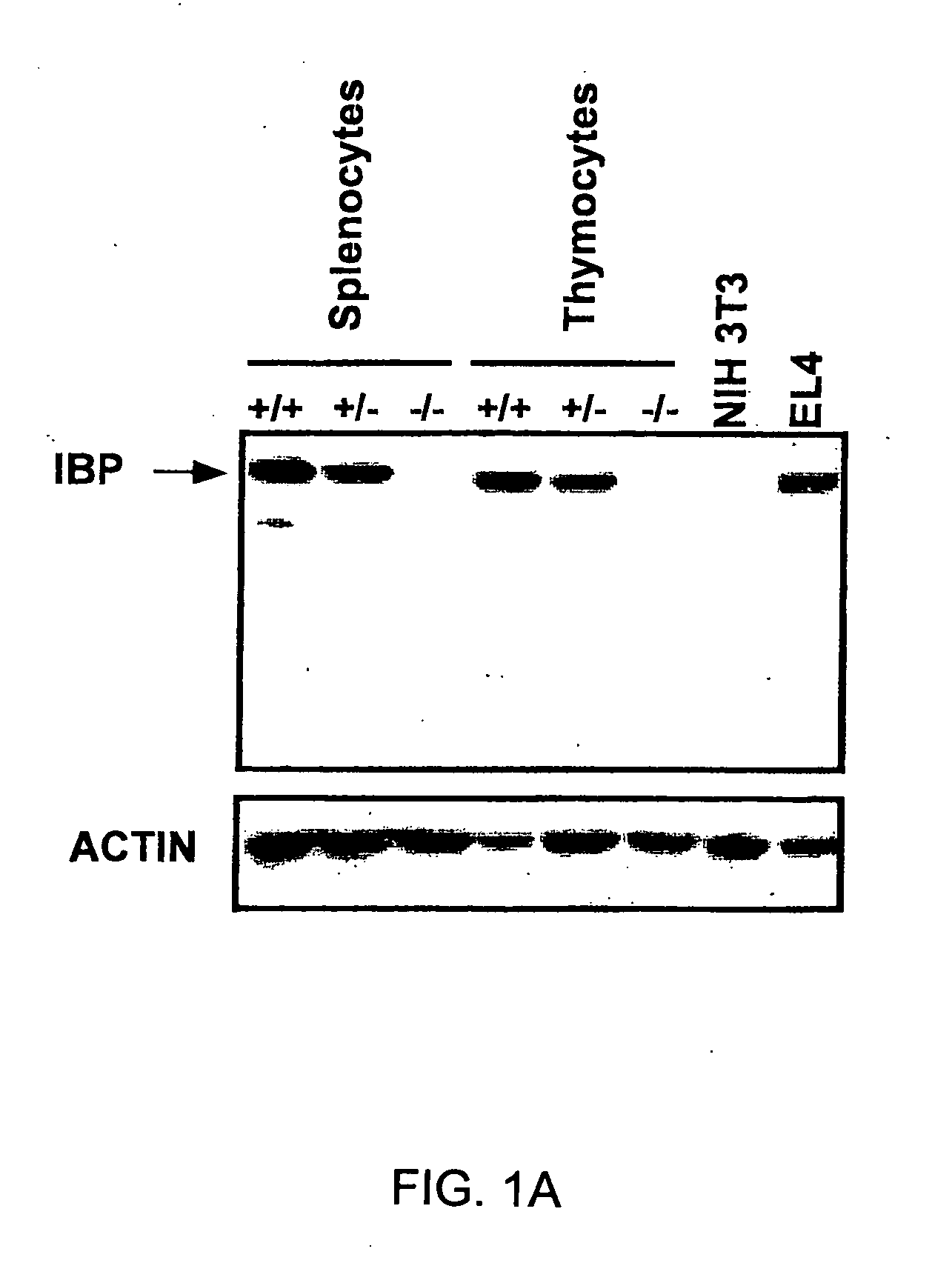
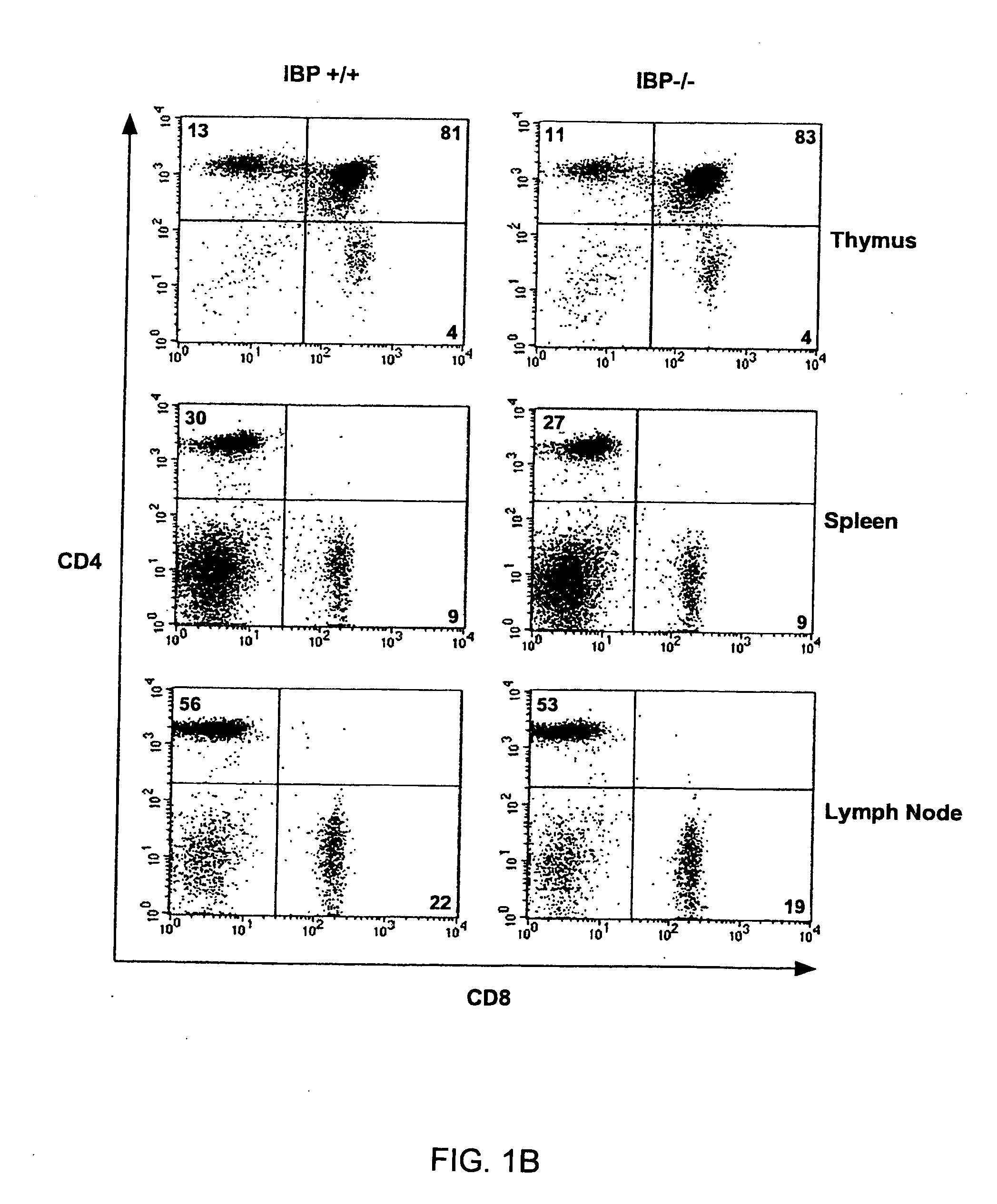
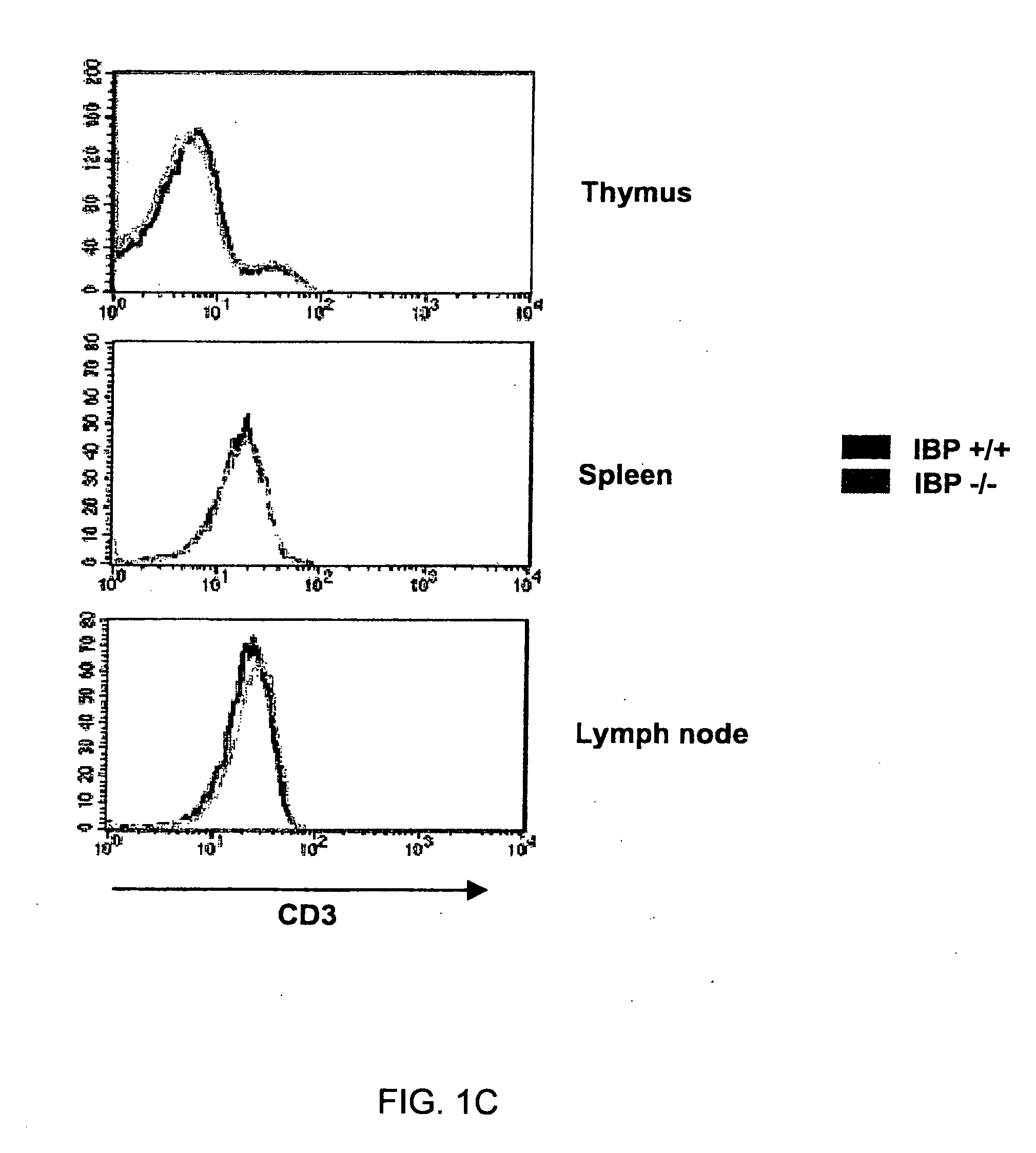
Method to modulate the immune system with a novel guanine nucleotide exchange factor
InactiveUS20060019915A1Compound screeningApoptosis detectionGuanine NucleotidesGuanine nucleotide exchange factor
The present invention provides a method of modulating T cell receptor (“TCR”) dependant regulation of a signaling factor in a T cell, and a method of modulating the proliferation and / or differentiation of a T cell, which includes administering to the T cell an IBP modulator in an amount effective to modulate the function of IBP. The present invention further provides a method and a kit for identifying a modulator of IBP-Lck interaction, a modulator of IBP-PI(3,4,5)P3 interaction, a modulator of a signaling factor in a T cell. Also provided are compositions containing an IBP modulator.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compound nucleotide oral liquor for improving immunity and its preparing method
InactiveCN100467028CImprove efficacyImprove immunityOrganic active ingredientsSolution deliveryUracil nucleosideAdenine nucleotide
The invention discloses a new oral liquid of compound nucleotide and preparation thereof. Each ml. of said compound nucleotide oral liquid comprising the following nucleotide in weight: adenylic acid 0.68-0.76mg, guanylic acid 0.43-0.52mg, uridylate 0.5-0.67mg, cytidylic acid 0.42-0.55mg and thymidylic acid 0.22-0.25mg.
Owner:庄田 +1
Immobilized enzyme for oriented catalysis of esterification reaction and preparation method and application of immobilized enzyme
PendingCN111394345AEasy to prepareIncrease enzyme activityHydrolasesOn/in organic carrierFreeze-dryingCandida antarctica
The invention belongs to the technical field of biochemical engineering and in particular discloses an immobilized enzyme for oriented catalysis of an esterification reaction and a preparation methodand application of the immobilized enzyme. The method comprises the following steps: mixing candida antarctica lipase with an HEPES (hydroxyethyl piperazin ethanesulfonic) buffer so as to obtain an HEPES-lipase liquid; and mixing the obtained HEPES-lipase liquid with GMP (guanine nucleotide) or AMP (adenine nucleotide) mother liquor so as to obtain a mixed solution, further uniformly mixing the mixed solution with metal ions, performing filtering, and performing freeze drying, so as to obtain the immobilized enzyme. The immobilized enzyme preparation method disclosed by the invention is simple, and the obtained immobilized enzyme is high in enzyme activity, is capable of effectively catalyzing the esterification reaction, in addition, is good in repeated utilization, but cannot catalyze alcoholysis reactions, has an oriented catalysis property, that is, is capable of catalyzing the esterification reaction but not alcoholysis reactions, and thus has very good application prospects.
Owner:GUANGDONG PHARMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com