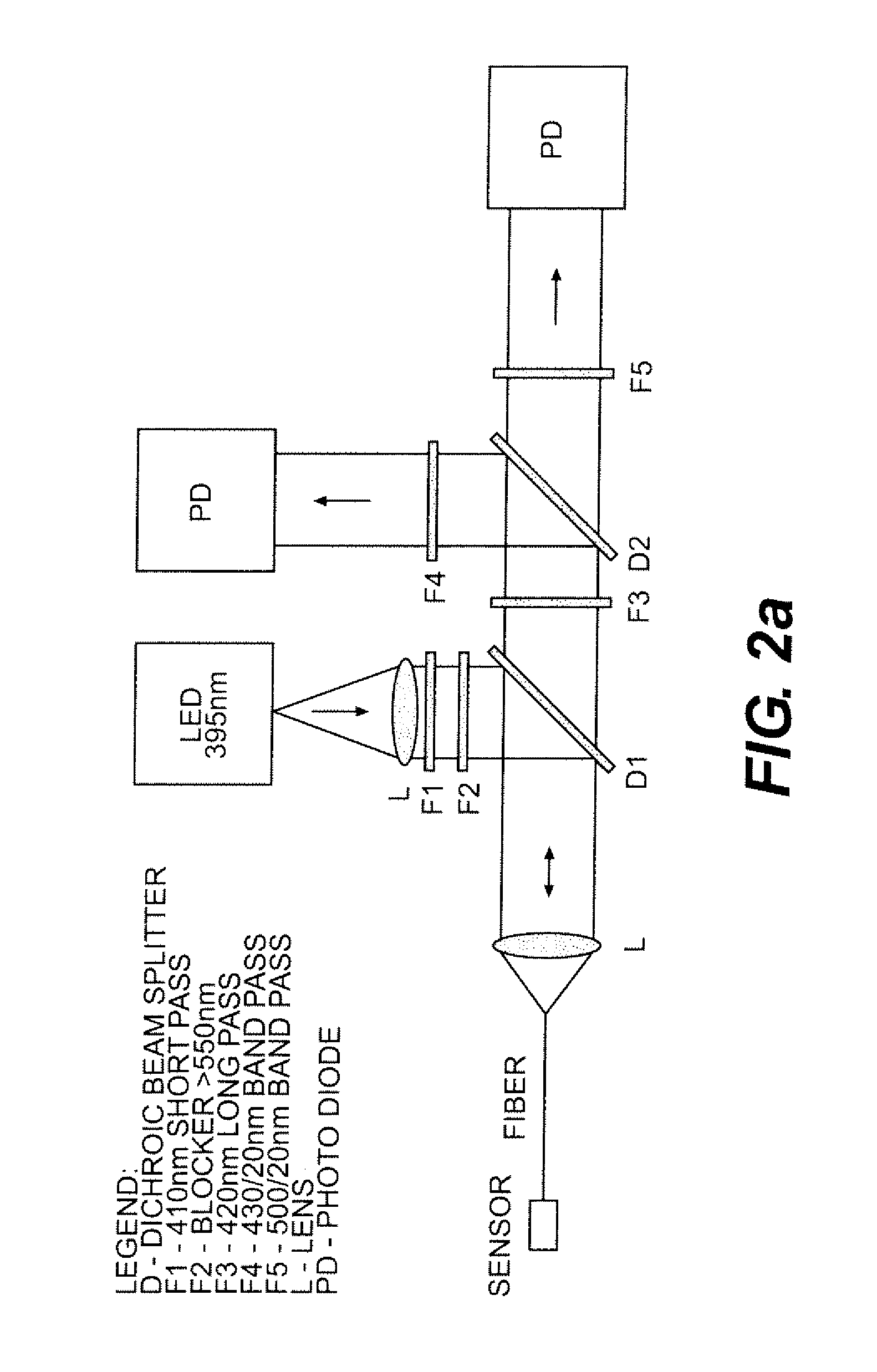
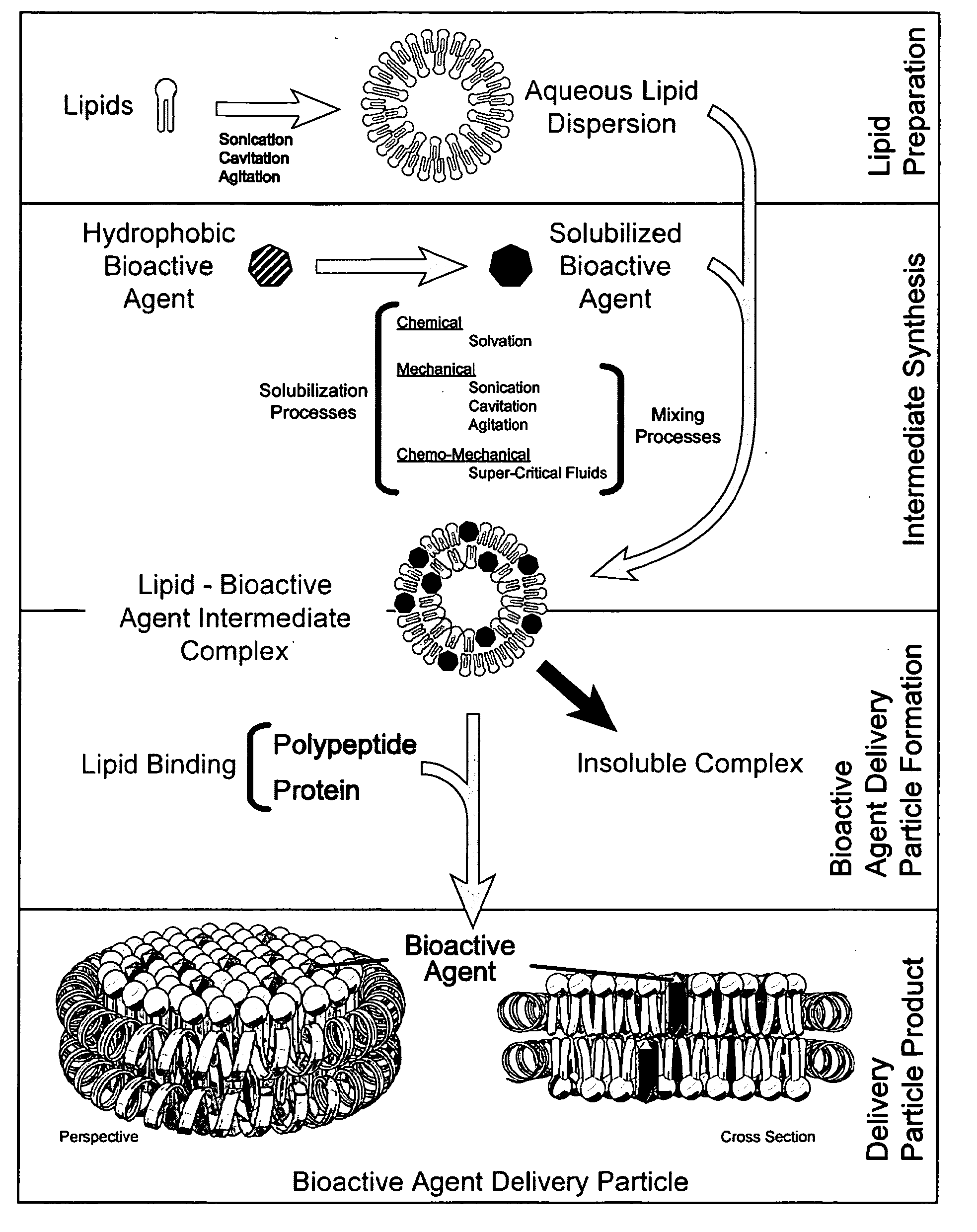
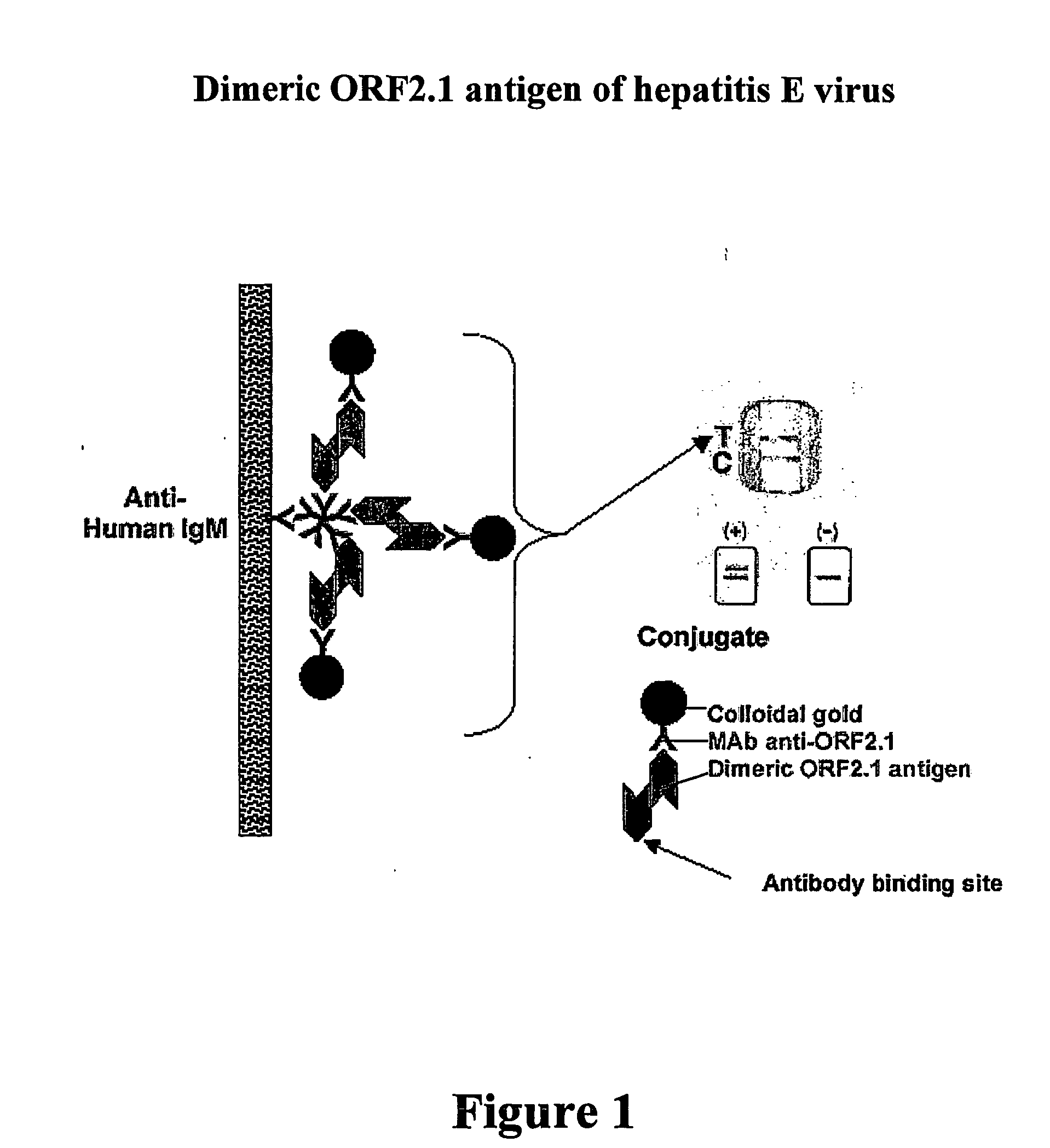
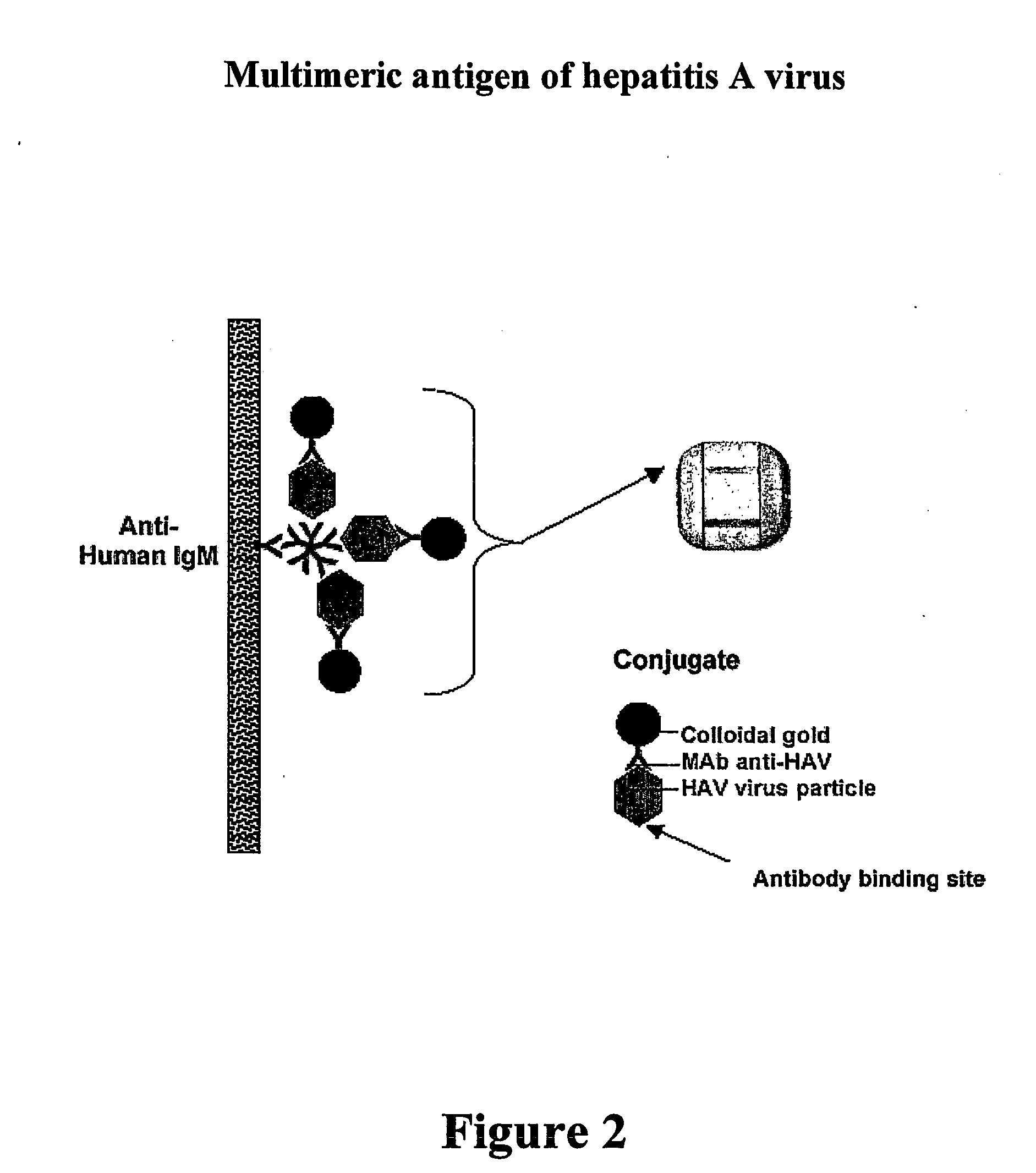
Patents
Literature
52 results about "Lipid binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infant formulas for early brain development
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis, at least about 5 mg / L of gangliosides, at least about 150 mg / L of phospholipids, at least about 70 mg / L of total sialic acid with at least about 2.5% as lipid-bound sialic acid, at least about 0.13% docosahexaenoic acid by weight of total fatty acids, and at least about 0.25% arachidonic acid by weight of total fatty acids. Also disclosed are methods of accelerating brain development, neural migration, and cognitive development in an infant by administering the infant formulas during the first 2-4 months of life, preferably as a sole source of nutrition.
Owner:ABBOTT LAB INC
Hybrid phosphoinositide phospholipids: compositions and uses
InactiveUS20050148042A1Facilitate recruitment of PtdlnsPPeptide/protein ingredientsMicrobiological testing/measurementLipid formationChemical Moiety
The methods and compositions disclosed herein concern the synthesis of a novel class of “two-headed” phospholipid-phosphoinositide hybrids possessing a carbon backbone, such as 2,3-diacylthreitol, erythritol or a synthetic module. The second phospholipid head group allows introduction of a biochemical or chemical moiety in a position orthogonal in space to those occupied by the phosphoinositide head group and the two acyl chains. The diacyl moieties allow for the incorporation of Pea-PIP2 into a lipid bilayer, while the Ptdlns(4,5)P2 moiety in the aqueous layer is specifically recognized by lipid binding proteins. In alternative embodiments of the invention, reporters, for example biotin, fluorophores and / or spin labels, are attached to the free amino group of the head groups of such molecules to specifically target the reporters to the lipid-water interface.
Owner:PRESTWICH GLENN +5
Artificial tear formulation
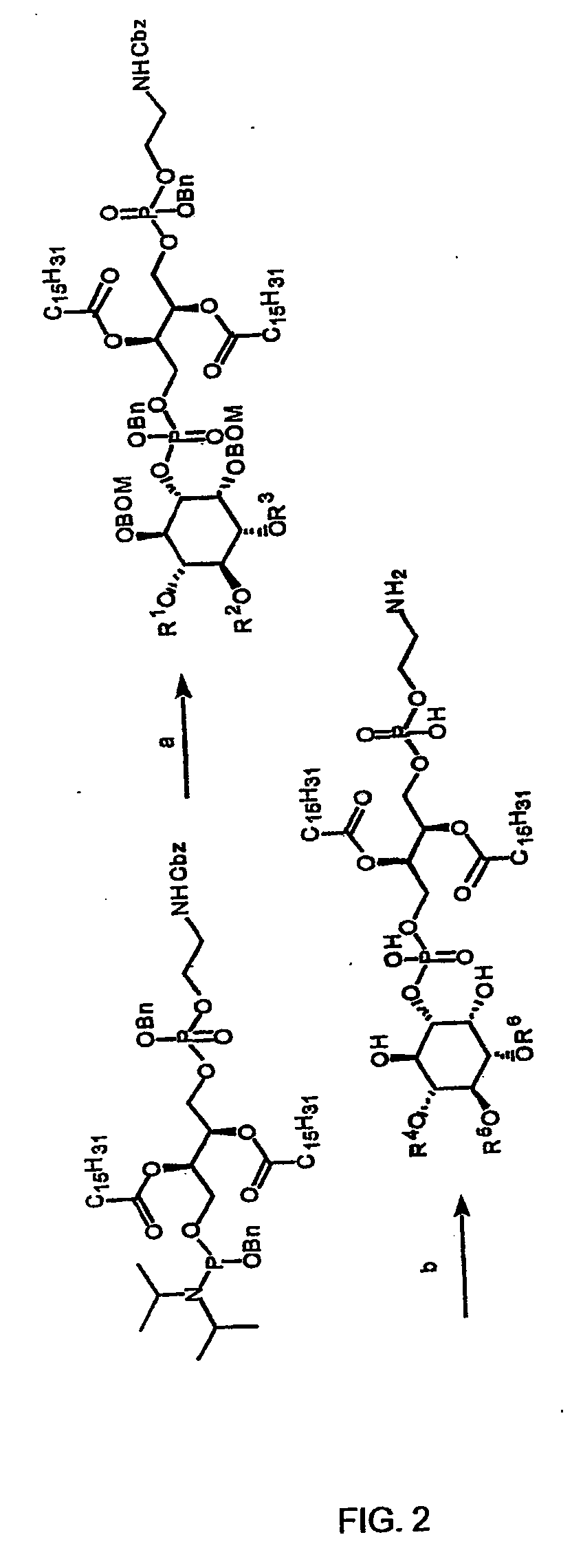
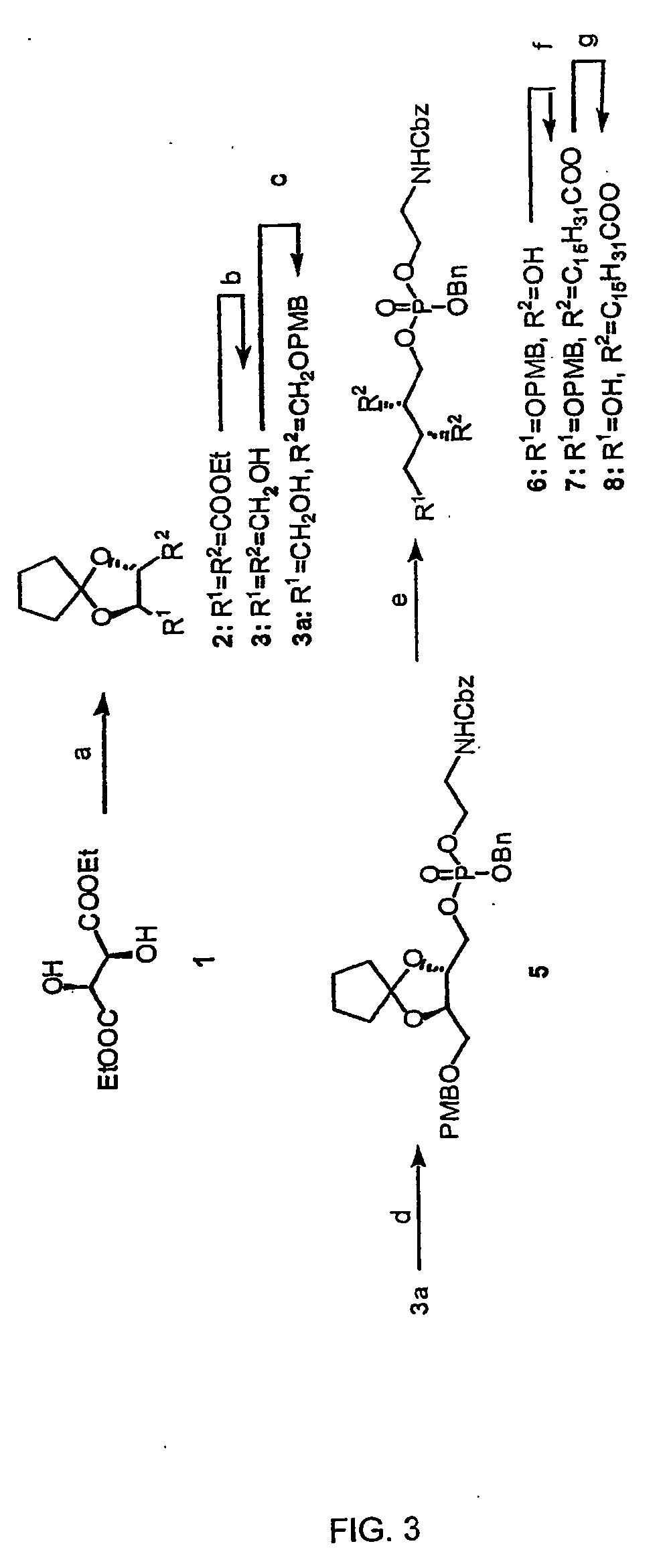
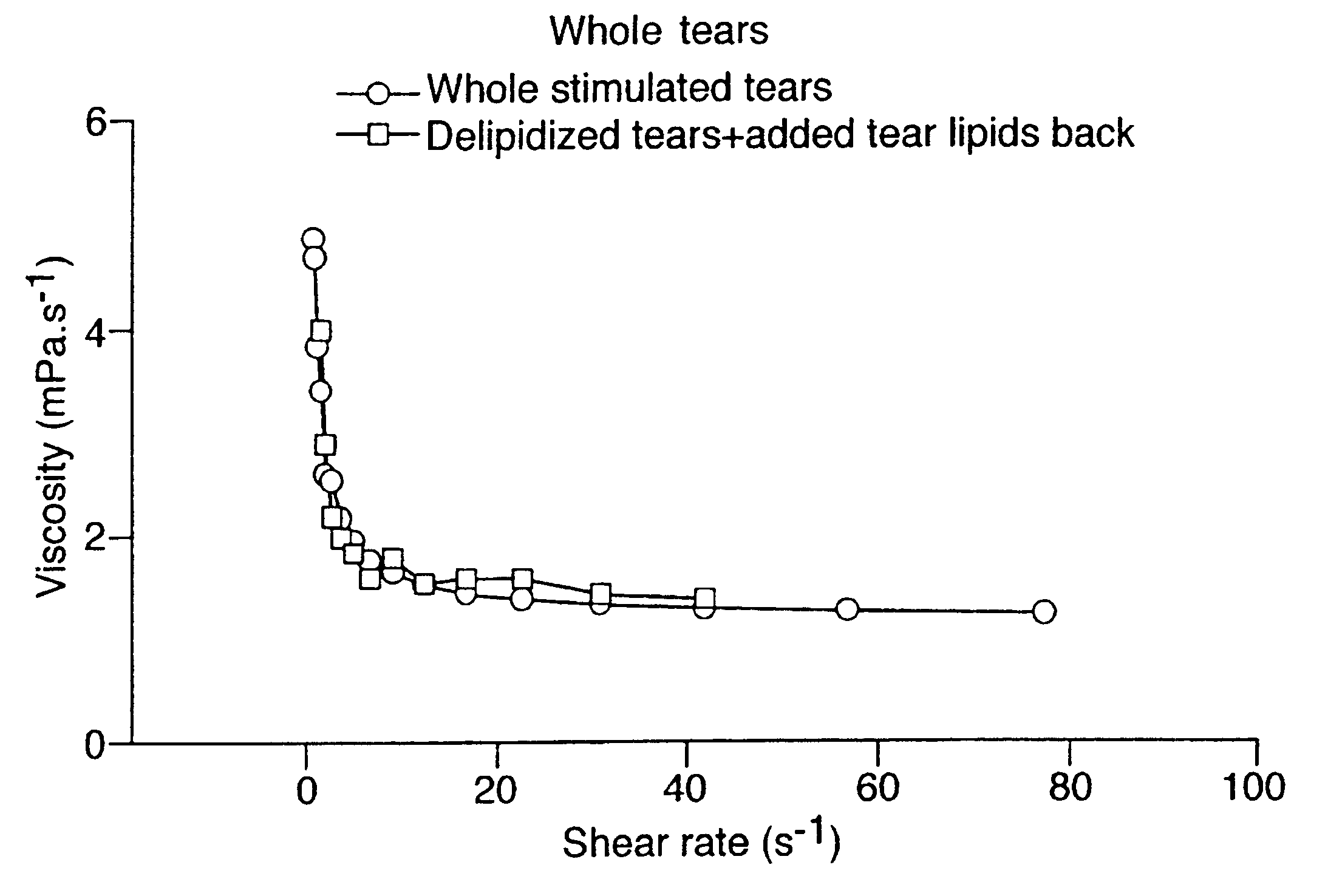
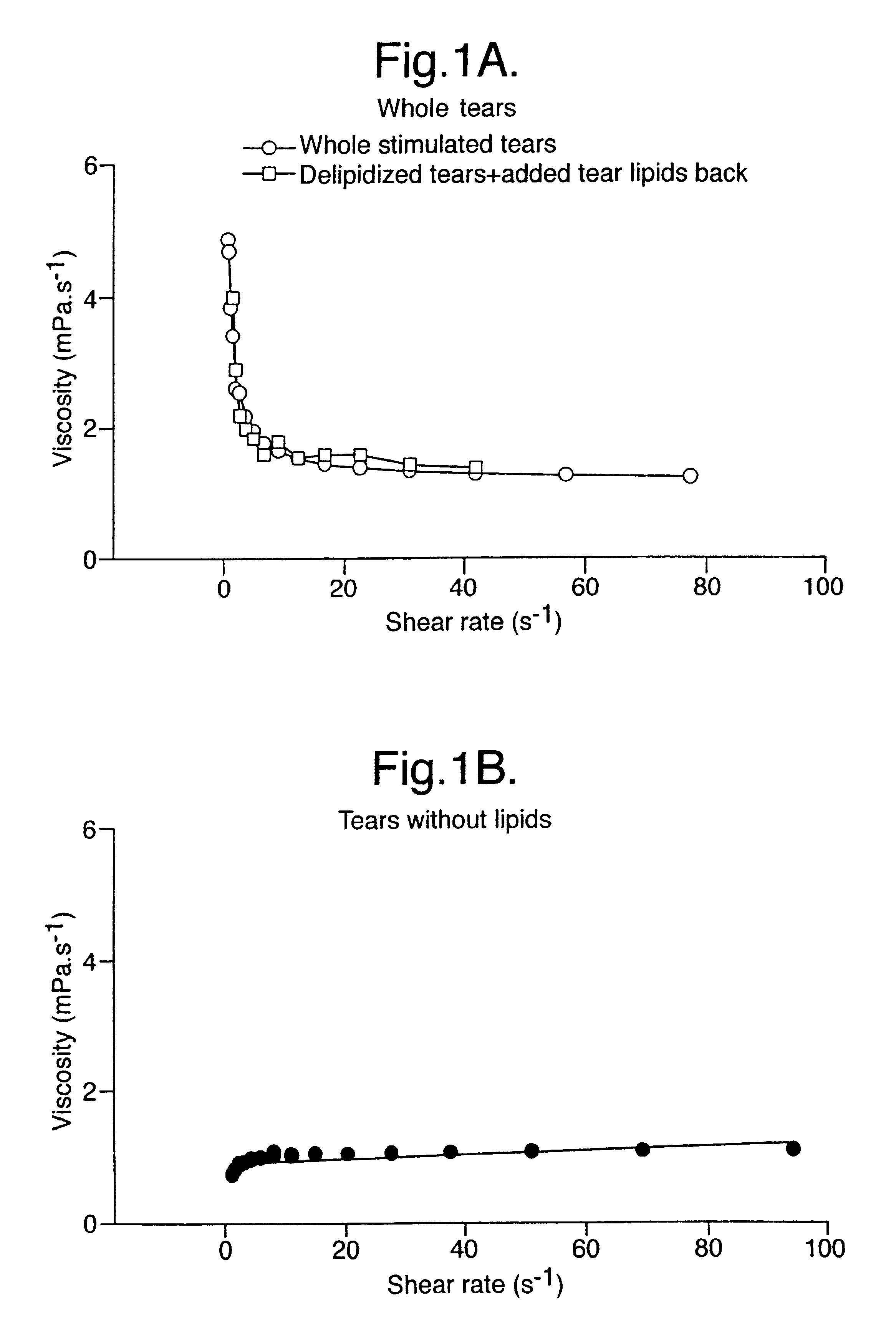
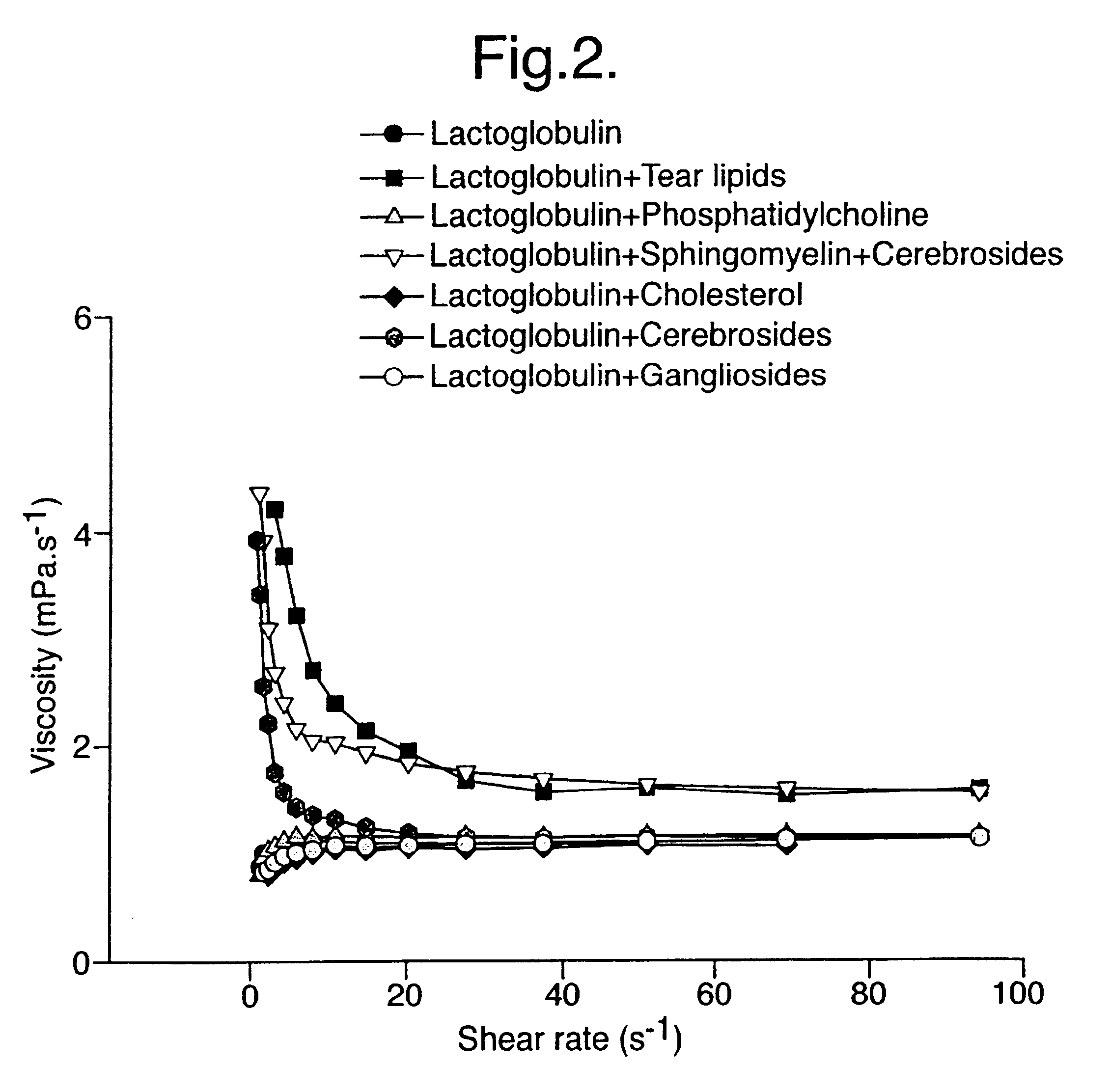
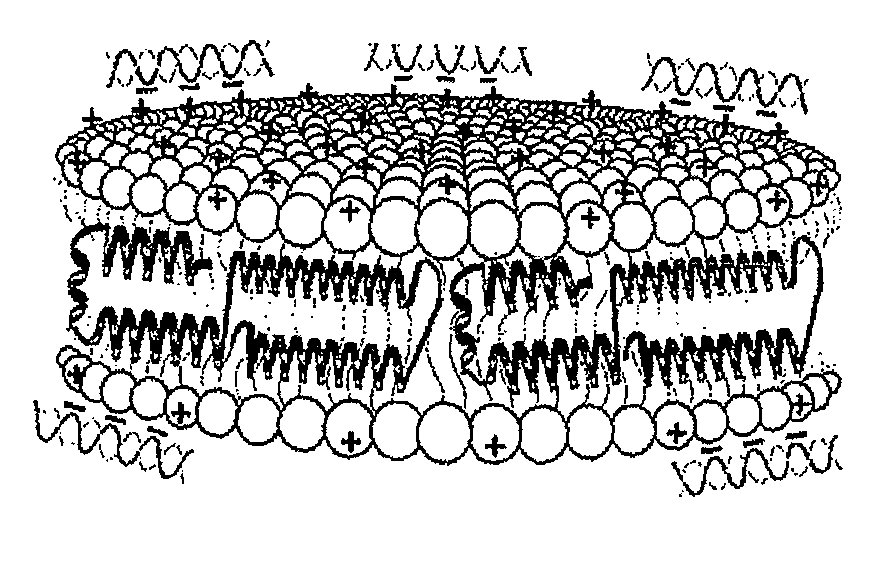
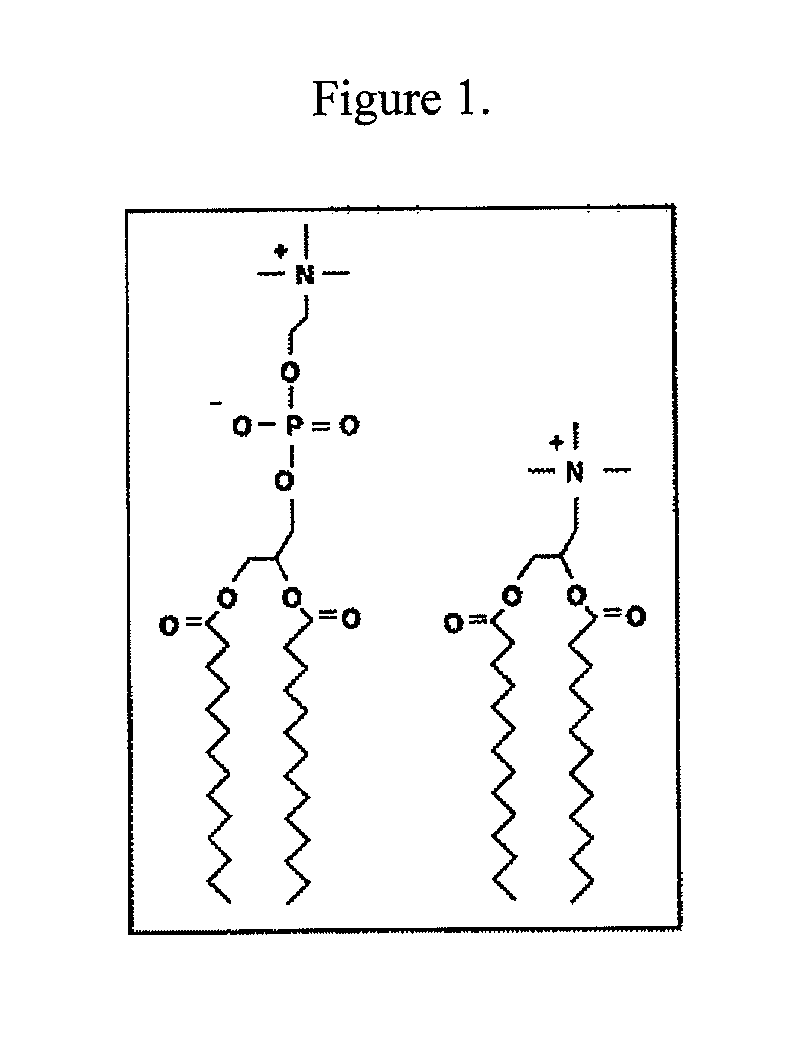
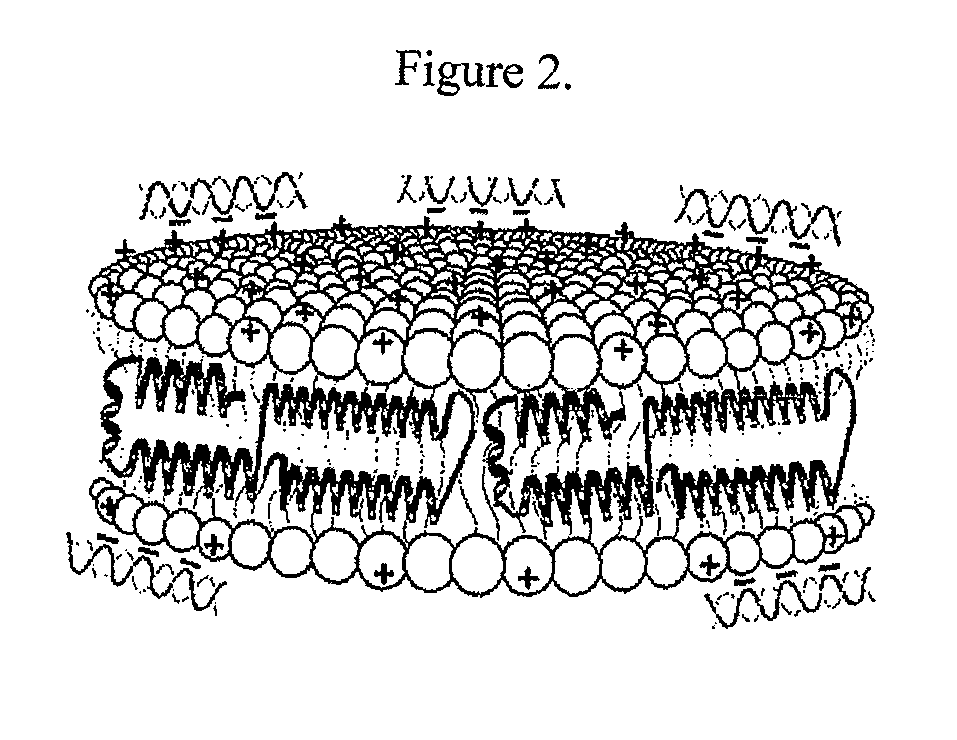
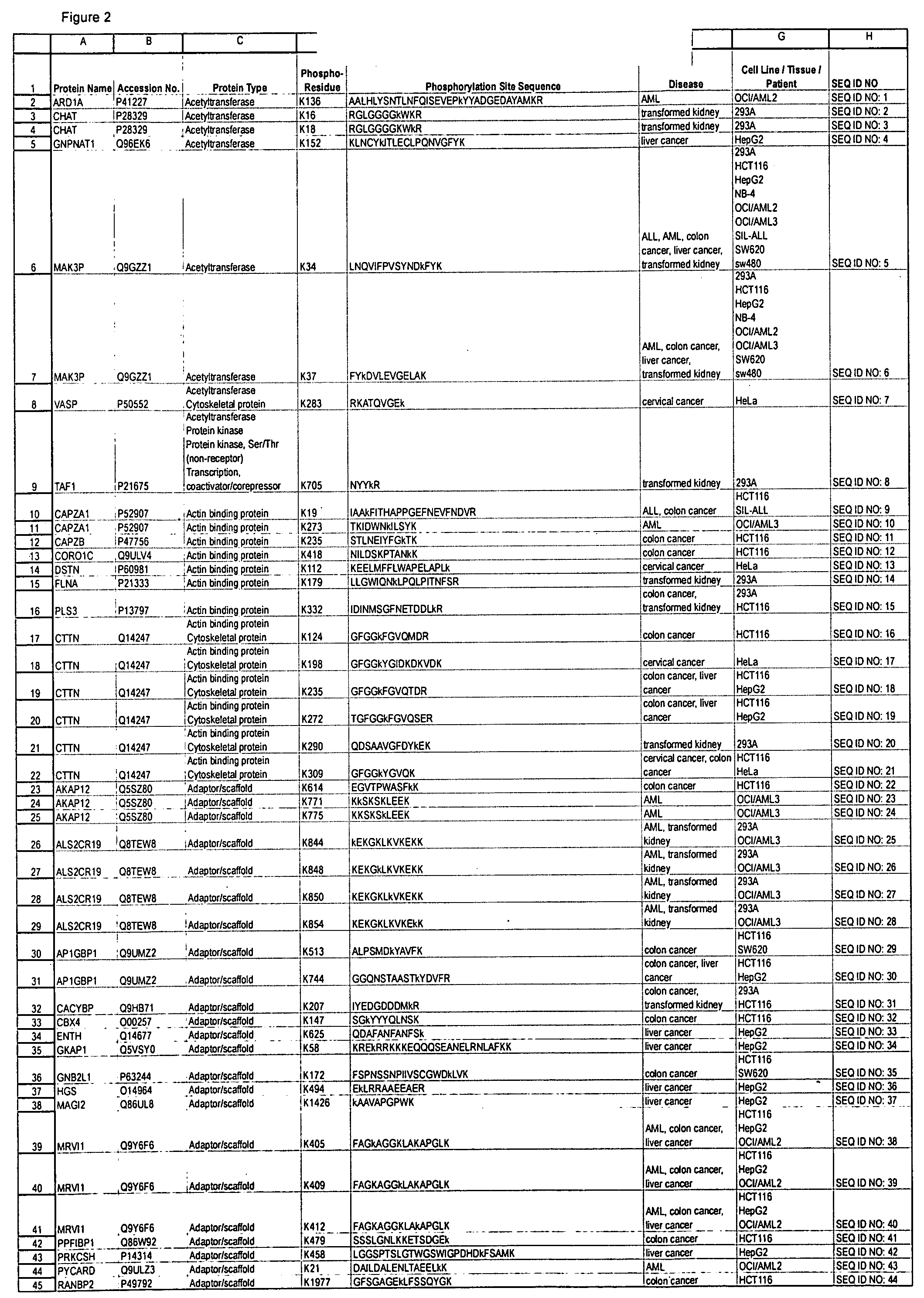
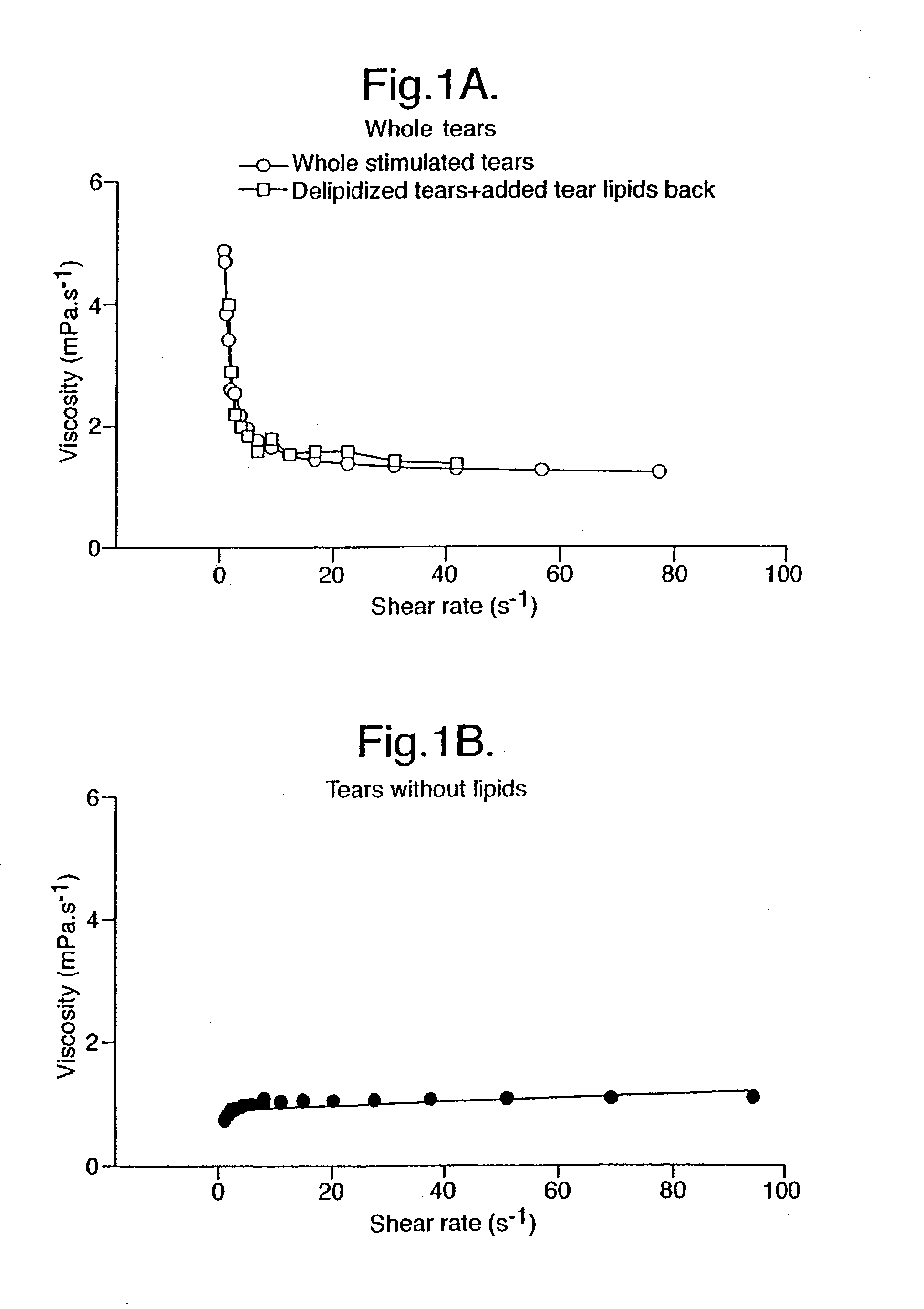
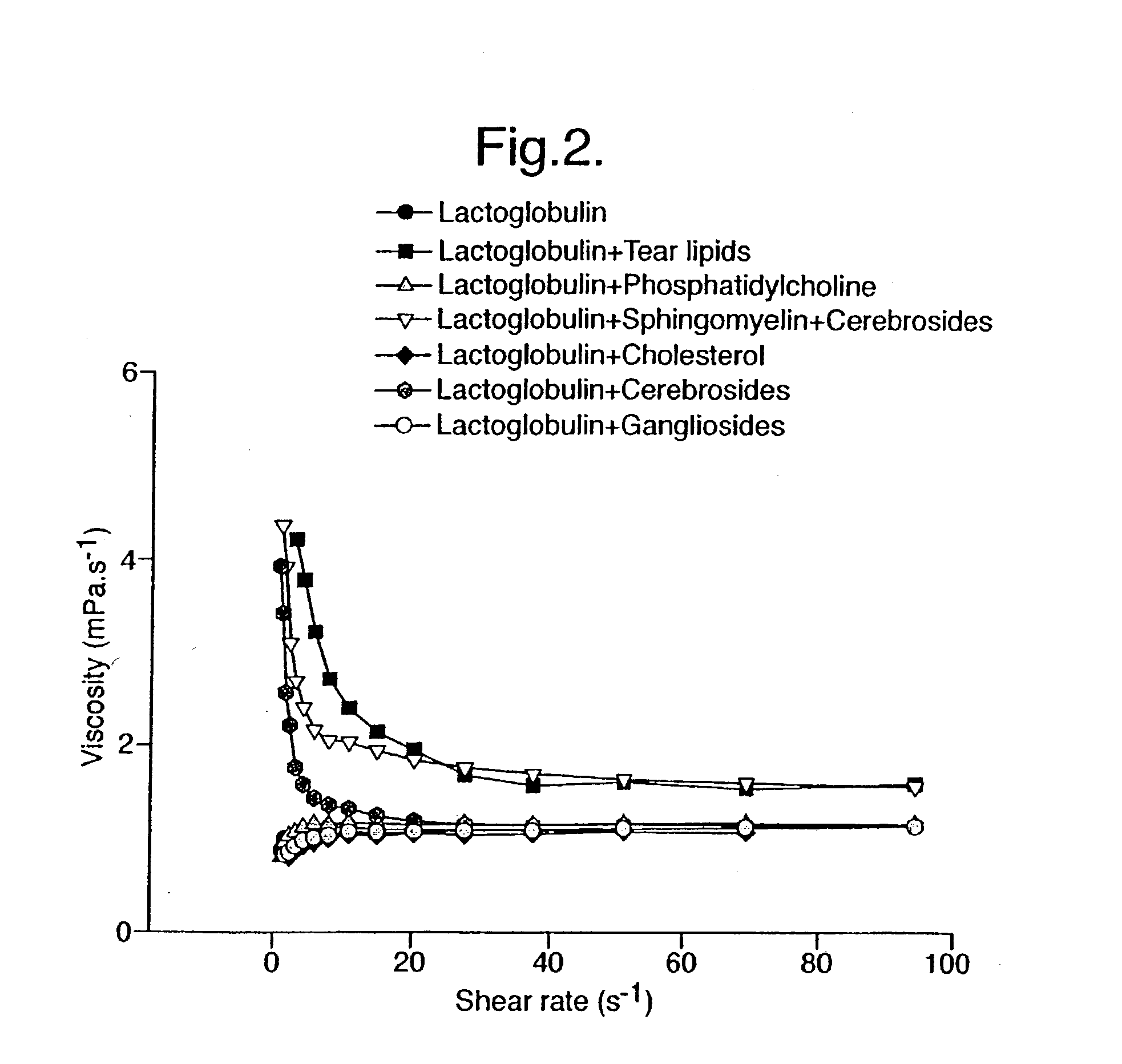
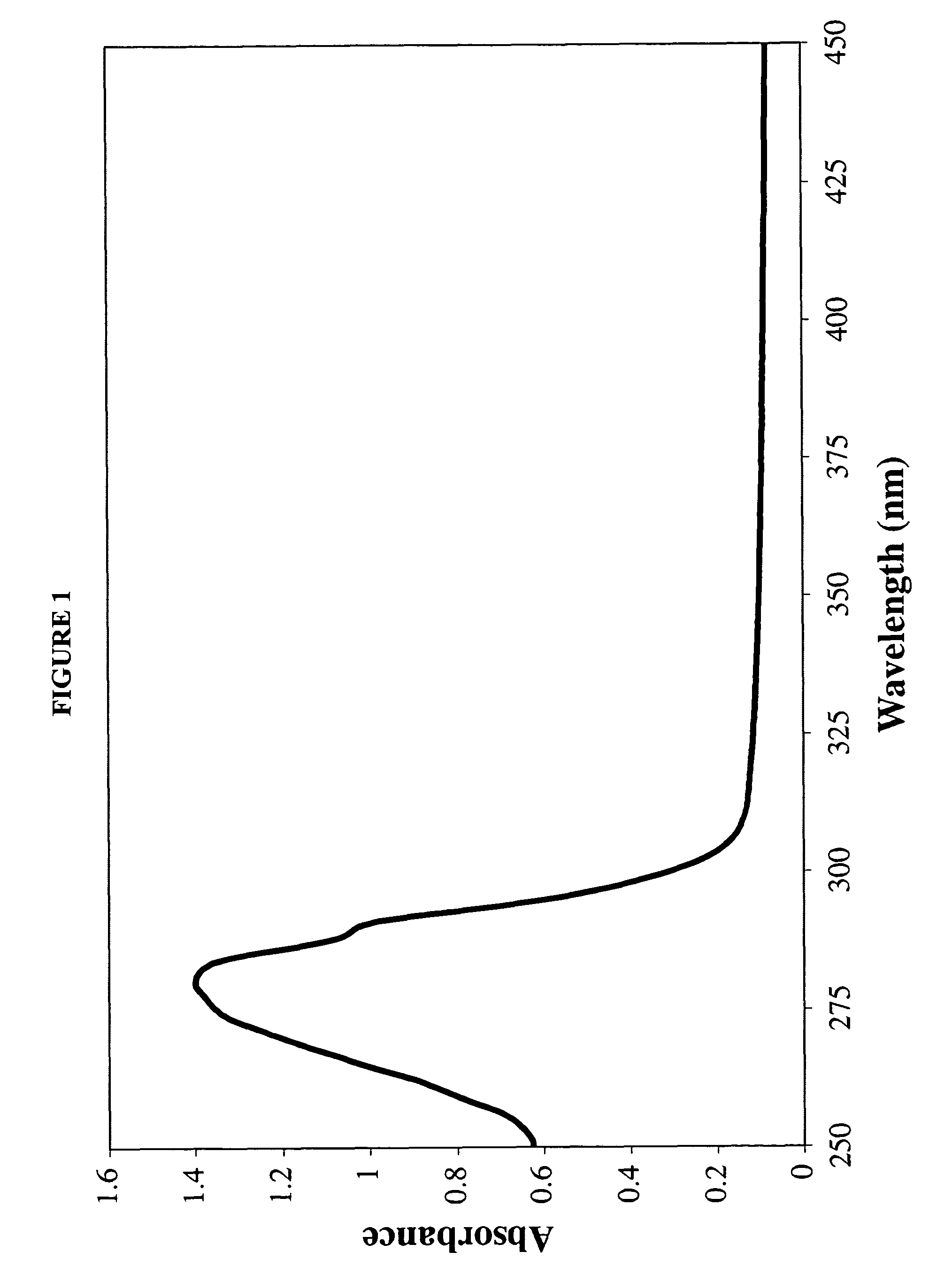
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Lipophilic nucleic acid delivery vehicle and methods of use thereof
ActiveUS20110237435A1Increase stability of particleImprove stabilityOrganic active ingredientsBiocideLipid formationDelivery vehicle
Provided herein are compositions and methods for delivery of nucleic acids to individuals and to cells, including nucleic acid delivery particles that comprising a lipid-binding polypeptide, a lipid bilayer comprising one or more cationic lipids, and a nucleic acid.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Lipophilic drug delivery vehicle and methods of use thereof
ActiveUS7824709B2Increase stability of particleAntibacterial agentsBiocideActive agentDelivery vehicle
The invention provides compositions and methods for delivery of a bioactive agent to an individual. Delivery vehicles are provided that include a bioactive agent in disc shaped particles that include one or more lipid binding polypeptides circumscribing the perimeter of a lipid bilayer in which the bioactive agent is localized. Chimeric lipid binding polypeptides are also provided and may be used to add additional functional properties to the delivery particles.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Method of screening for drugs that block ligand binding to a lipid binding protein
Methods are disclosed to screen for drugs that interfere with the binding between a specific lipid-binding protein and a selected ligand. Successful candidates have potential in disease treatments, particularly diabetes and some forms of cancer.
Owner:FFA SCI
Isolated phospholipid-protein particles
InactiveUS20080248565A1Speed up the processImprove solubilityPeptide preparation methodsDepsipeptidesADAMTS ProteinsApolipoproteins E
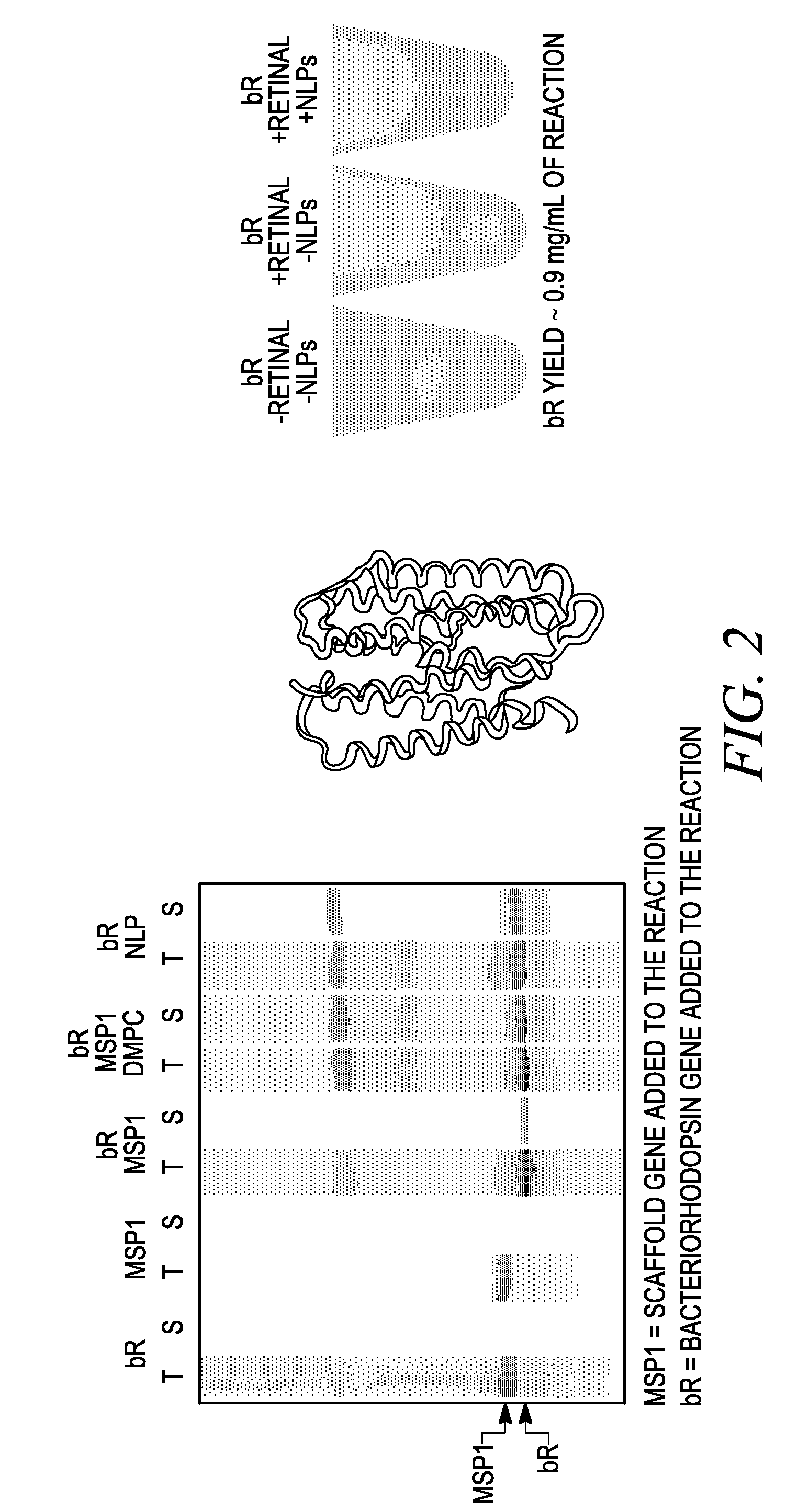
Systems and methods are provided for producing a protein of interest that is typically not amenable to expression in soluble form in in vitro expression systems. In some aspects, the invention provides methods of synthesizing proteins using in vitro protein synthesis systems that include a scaffold protein such as apolipoprotein or an amphipathic alpha helix containing (“AAHC”) protein, in which higher yields of soluble protein are produced than in the absence of the scaffold protein. The scaffold proteins may be provided in an in vitro protein synthesis system associated with lipid or not associated with lipid. The scaffold protein may be provided as a protein per se or may be encoded by a nucleic acid template and co-expressed with the protein of interest. The invention also provides compositions and kits for synthesis of proteins in soluble form, in which the compositions and kits include cell extracts for protein expression and isolation.
Owner:LIFE TECH CORP
Treatment of conditions related to cecal ligation shock
InactiveUS8541371B2Improve survival rateRelieve symptomsOrganic active ingredientsNervous disorderDiseaseCytotoxicity
Techniques, methods and lavages are disclosed for prevention or treatment of shock, particularly cecal ligation or cecal inoculation shock, by administering a specific therapeutic agent, which is able to use smaller volumes of reagent to achieve partial to complete inhibition, than other previously described techniques. The agent includes a combination of enzyme inhibitor, cytotoxic lipid binding protein, and antibiotic.
Owner:RGT UNIV OF CALIFORNIA
Glycoconjugation of polypeptides using oligosaccharyltransferases
The current invention provides polypeptides and polypeptide conjugates that include an exogenous N-linked glycosylation sequence. The N-linked glycosylation sequence is preferably a substrate for an oligosaccharyltransferase (e.g., bacterial PgIB), which can catalyze the transfer of a glycosyl moiety from a lipid-bound glycosyl donor molecule (e.g., a lipid-pyrophosphate-linked glycosyl moiety) to an asparagine (N) residue of the glycosylation sequence. In one example, the asparagine residue is part of an exogenous N-linked glycosylation sequence of the invention. The invention further provides methods of making the polypeptide conjugates that include contacting a polypeptide having an N-linked glycosylation sequence of the invention and a lipid-pyrophosphate-linked glycosyl moiety (or phospholipid-linked glycosyl moiety) in the presence of an oligosaccharyltransferase under conditions sufficient for the enzyme to transfer the glycosyl moiety to an asparagine residue of the N-linked glycosylation sequence. Exemplary glycosyl moieties that can be conjugated to the glycosylation sequence include GlcNAc, GlcNH, bacillosamine, 6-hydroybacillosamine, GalNAc, GaINH, GlcNAc-GlcNAc, GlcNAc-GlcNH, GlcNAc-Gal, GlcNAc-GlcNAc-Gal-Sia, GlcNAc-Gal-Sia, GlcNAc-GlcNAc-Man, and GlcNAc-GlcNAc-Man(Man)2. The transferred glycosyl moiety is optionally modified with a modifying group, such as a polymer (e.g., PEG). In one example, the modified glycosyl moiety is a GIcNAc or a sialic acid moiety.
Owner:蔚所番有限公司
Detection methods employing hcv core lipid and DNA binding domain monoclonal antibodies
ActiveUS20170052184A1Easy to adaptImmunoglobulins against virusesDisease diagnosisEpitopeDNA-binding domain
The present disclosure provides detection methods employing HCV core lipid binding domain and DNA binding domain monoclonal antibodies or antibody fragments. In certain embodiments, the lipid binding domain monoclonal antibody or antibody fragment recognizes an epitope in amino acids 141 to 161 of HCV core protein and the DNA binding domain antibody or antibody fragment recognizes an epitope in amino acids 95-123 (e.g., in amino acids 99-117) of HCV core protein.
Owner:ABBOTT LAB INC +1
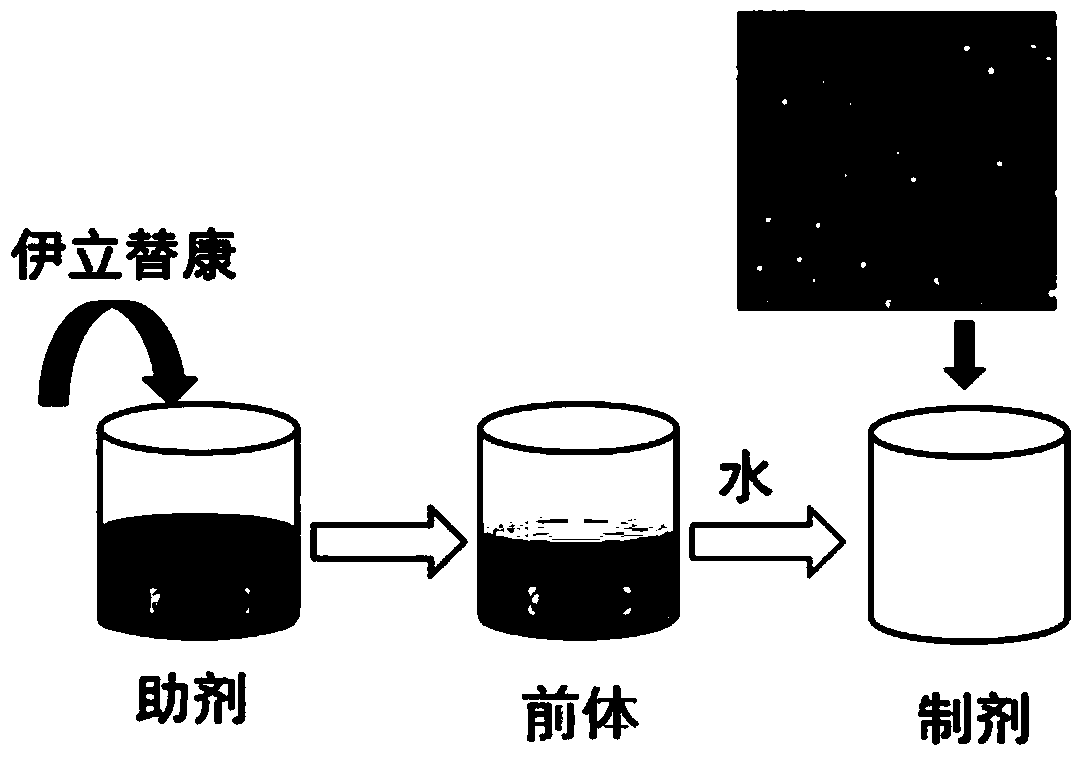
Irinotecan nano lipid binding preparation and preparation method thereof
ActiveCN103735504AUniform particle size distributionLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetTumor targeting
The invention relates to an irinotecan nano lipid binding preparation and a preparation method thereof. The irinotecan nano lipid binding preparation contains phospholipid, polyethylene glycol-dodecahydroxycy stearate, glycerol, ethanol and irinotecan, as well as an aqueous solvent for injection. The irinotecan nano lipid binding preparation is even in grain size, and as the lipid binding preparation, capable of reducing the toxicity of the irinotecan with long circulation effect and enhancing the tumor targeted enrichment effect and improving the compliance of a patient, and the preparation method is simple in process, high in repeatability and applicable to industrial production.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Biosensor device for sensing amphipathic analytes
Owner:BECTON DICKINSON & CO
Lipophilic drug delivery vehicle and methods of use thereof
The invention provides compositions and methods for delivery of a bioactive agent to an individual. Delivery vehicles are provided that include a bioactive agent in disc shaped particles that include one or more lipid binding polypeptides circumscribing the perimeter of a lipid bilayer in which the bioactive agent is localized. Chimeric lipid binding polypeptides are also provided and may be used to add additional functional properties to the delivery particles.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Method capable of promoting migration of dendritic cells to lymph nodes and achieving multi-mode imaging simultaneously
ActiveCN105477630APromote migrationGood biocompatibilityPowder deliveryPeptide/protein ingredientsTreatment effectDendritic cell
The invention discloses a method capable of promoting migration of dendritic cells to lymph nodes and achieving multi-mode imaging simultaneously. The method is implemented by mDCs (mature dendritic cells) carrying magnetic fluorescent nanoparticles in an externally applied magnetic field. The magnetic fluorescent nanoparticles are formed by organically combining oleate-magnetic iron oxide particles, two phospholipids, near-infrared probes and an antigen fusion peptide with lipid binding capability through self-assembly. The method has the advantages that efficiency of DC living migration to a lymphoid tissue can be improved effectively, tumor prevention and treatment effects after DCs absorb antigen peptides are promoted, and multi-mode living imaging detection can be performed.
Owner:HUAZHONG UNIV OF SCI & TECH
Lipophilic nucleic acid delivery vehicle and methods of use thereof
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Reelin deficiency or dysfunction and methods related thereto
A method of measuring Reelin as a biomarker, to non-destructively assess or predict DHA levels in the brain and in other, currently inaccessible or difficult-to-access, key components of the central nervous system (CNS) is described. Also described is a method to prevent, delay the onset of, or treat Reelin deficiency or dysfunction and / or a disease or condition associated with Reelin deficiency or dysfunction, comprising administering to a patient diagnosed with or suspected of having a Reelin deficiency or dysfunction an amount of a PUFA, and particularly an omega-3 PUFA, and more particularly, docosahexaenoic acid (DHA) or a precursor or source thereof, to compensate for the effects of Reelin deficiency or dysfunction in the patient. Also described is a method to prevent or reduce development defects or disorders associated with Reelin dysfunction or deficiency through the supplemental use of polyunsaturated fatty acids (PUFAs- unsaturated fatty acids having two or more double bonds), and particularly highly unsaturated fatty acids (HUFAs- unsaturated fatty acids having three or more double bonds), and more particularly a HUFA selected from arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), and even more particularly omega-3 HUFAs, and more particularly DHA, to: compensate for reduced fatty acid binding protein or function thereof in the patient; compensate for reduced brain lipid binding protein or function thereof in the patient; improve the activity of fatty acid binding proteins in the patient.
Owner:MARTEK BIOSCIENCES CORP (N D GES D STAATES DELAWARE) COLUMBIA
Reagens for the Detection of Protein Acetylation Signaling Pathways
InactiveUS20090124023A1Immunoglobulins against animals/humansBiological testingCell Surface ProteinsMetabolic enzymes
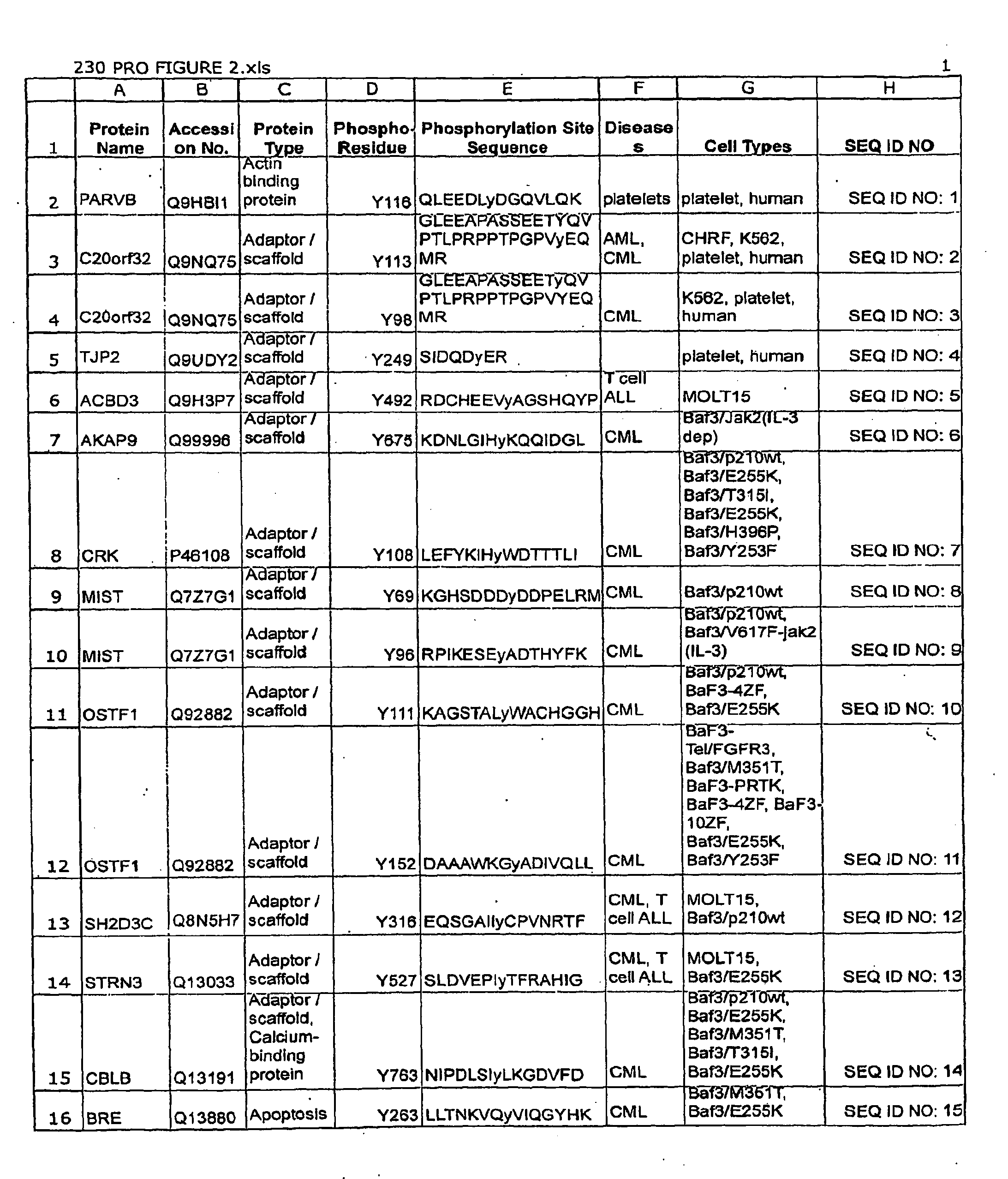
The invention discloses 432 novel acetylation sites identified in signal transduction proteins and pathways underlying human protein acetylation signaling pathways, and provides acetylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these acetylated sites / proteins, as well as methods of using the reagents for such purpose. Among the acetylation sites identified are sites occurring in the following protein types: Acetyltransferases, Adaptor / Scaffold proteins, Actin binding proteins, Adhesion proteins, Apoptosis proteins, Calcium-binding proteins, Cell Cycle Regulation proteins, Cell Surface proteins, DNA binding proteins, DNA replication proteins, Channel proteins, Chaperone proteins, Cellular Metabolism enzymes, Cytoskeletal proteins, DNA repair proteins, Endoplasmic reticulum proteins, Enzyme proteins, G protein and GTPase Activating proteins, Guanine Nucleotide Exchange Factors, Helicase proteins, Isomerase proteins, Extracelluar matrix proteins, Hydrolases, Ligase proteins, Lipid kinases, Inhibtor proteins, Lipid Binding proteins and Lyases.
Owner:CELL SIGNALING TECHNOLOGY
Artificial tear formulation
InactiveUS20040126419A1Reproduce viscosityReproduce surface tension propertyAntibacterial agentsBiocideLipid formationKERATOCONJUNCTIVITIS SICCA
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Processes for the preparation of lipophilic drug delivery vehicles
The invention provides compositions and methods for delivery of a bioactive agent to an individual. Delivery vehicles are provided that include a bioactive agent in disc shaped particles that include one or more lipid binding polypeptides circumscribing the perimeter of a lipid bilayer in which the bioactive agent is localized. Chimeric lipid binding polypeptides are also provided and may be used to add additional functional properties to the delivery particles.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
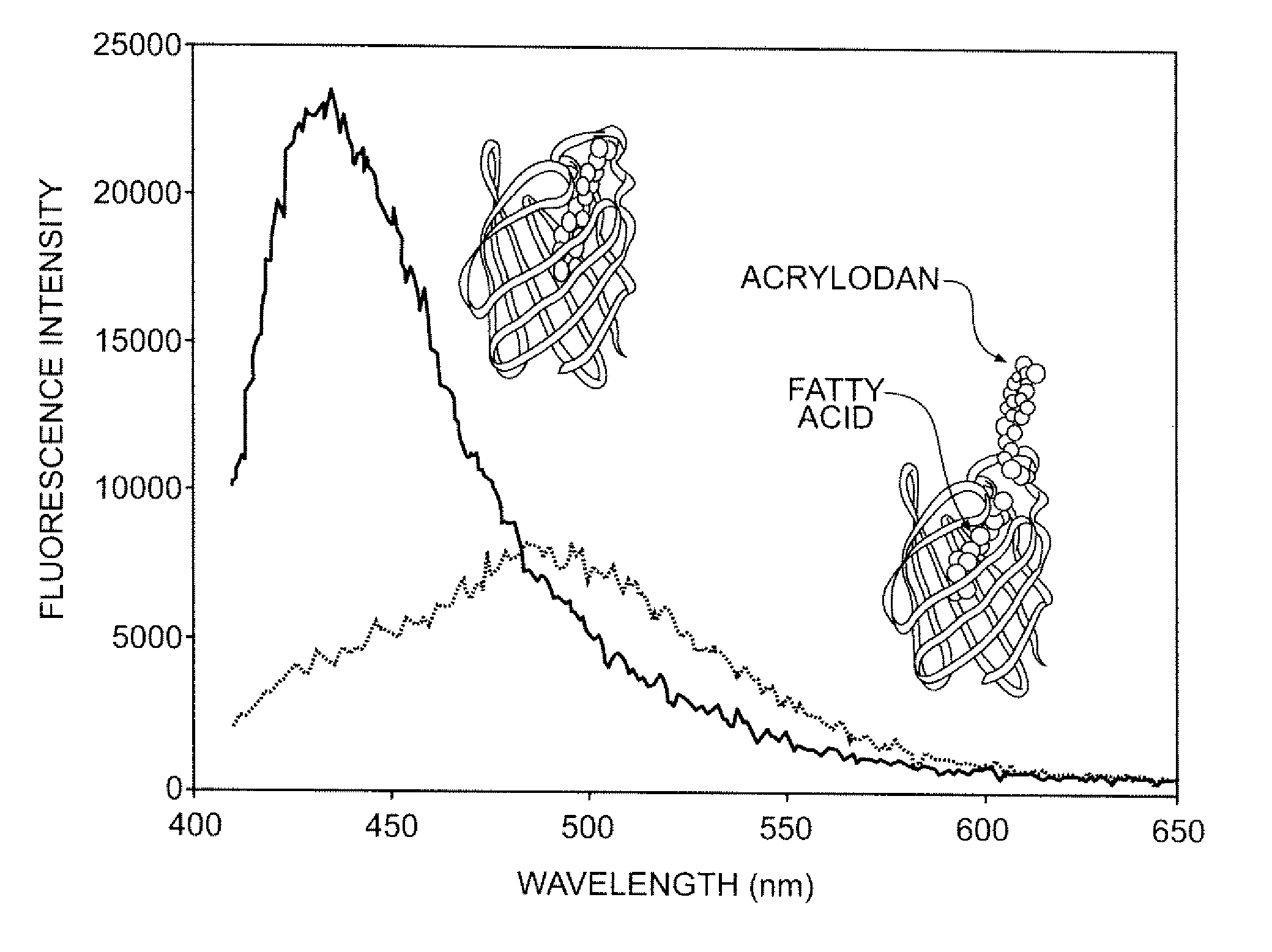
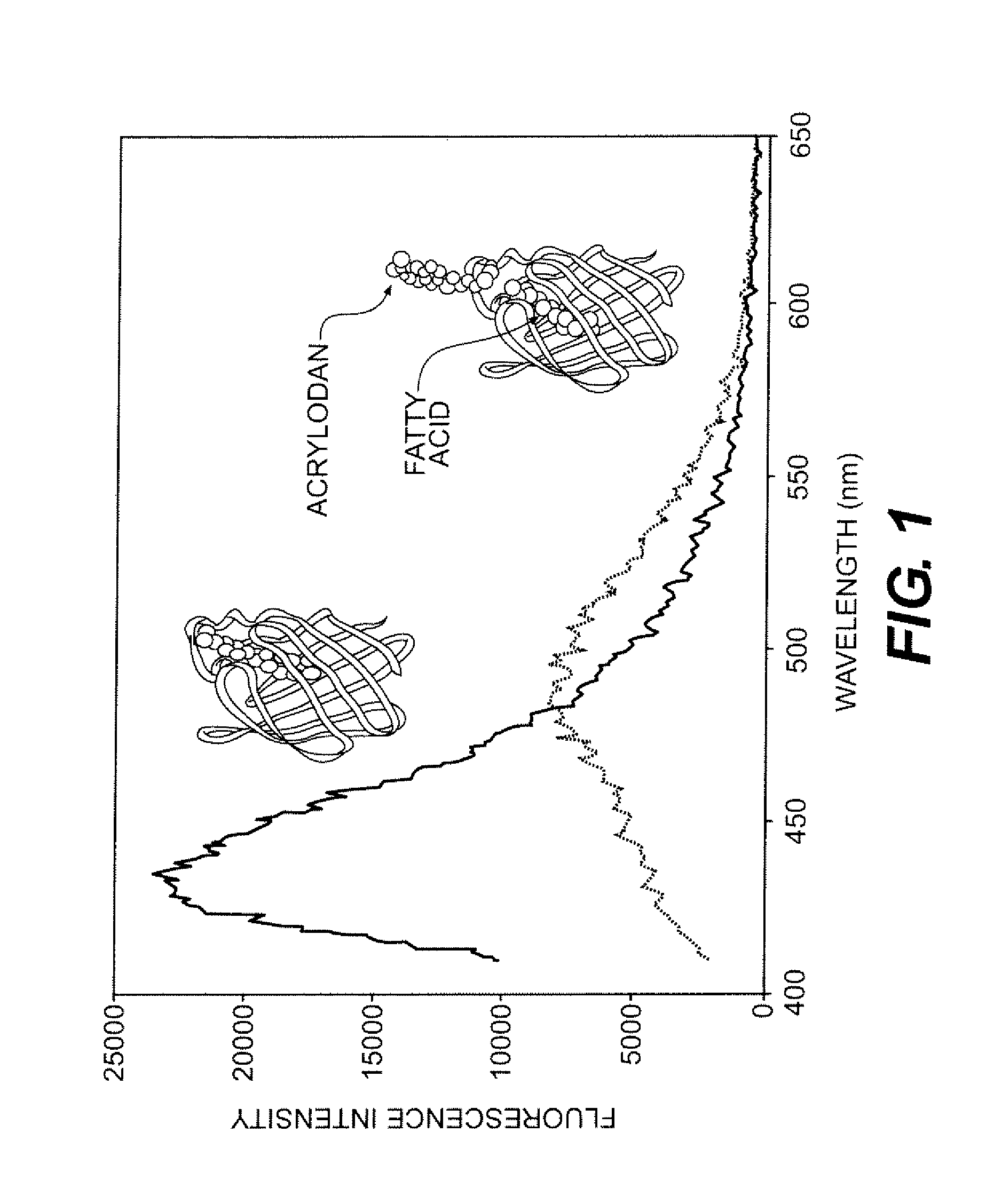
Compositions and methods for quantitatively monitoring lipids
InactiveUS8686115B2Microbiological testing/measurementEnzyme stabilisationPhospholipaseBruton's tyrosine kinase
Provided herein are fluorescent lipid binding proteins (FLBPs). The FLBPs comprise a lipid binding domain linked to a fluorophore, whrereby the fluorophore's fluorescence emission undergoes a spectral change upon lipid binding. the fluorophore is selected from the group consisting of 2-dimethylamino-6-acyl-naphthalene (DAN) and RED fluorophore and the lipid binding protein is selected from the group consisting of ENTH domain of epsin 1, C2 domain of bovine lactadherin, C 1B domain of protein kinase C-gamma, C2 domain of cytosolic phospholipase A2-beta, and PH domain of Bruton's tyrosine kinase PH.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Colorimetric and fluorescent proteins
ActiveUS20130157284A1High affinityBioreactor/fermenter combinationsBiological substance pretreatmentsLipid formationRetinoid
The invention relates to intracellular lipid binding proteins that bind retinoids and / or dye ligands and that are modified to transmit or emit light at a variety of different wavelengths.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
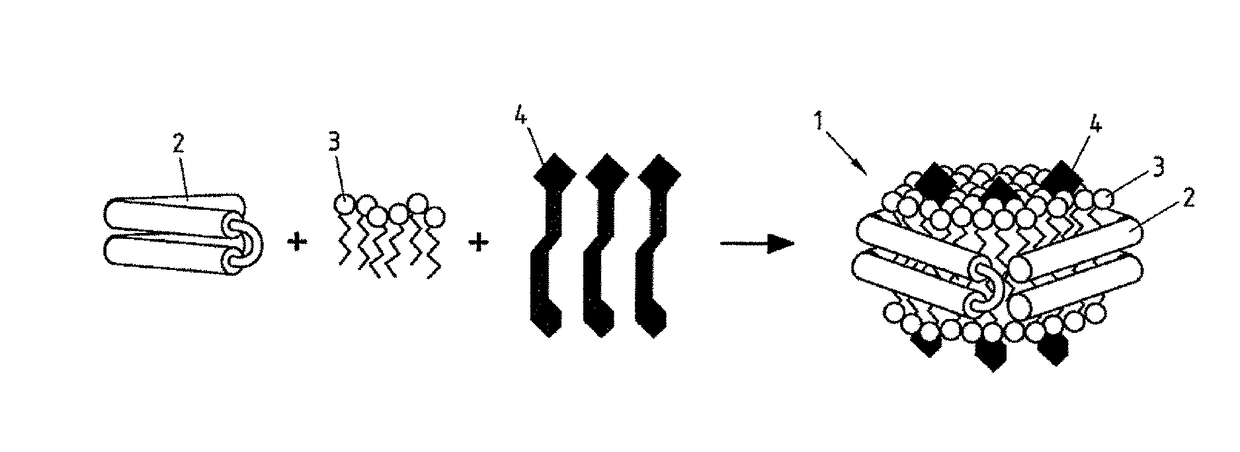
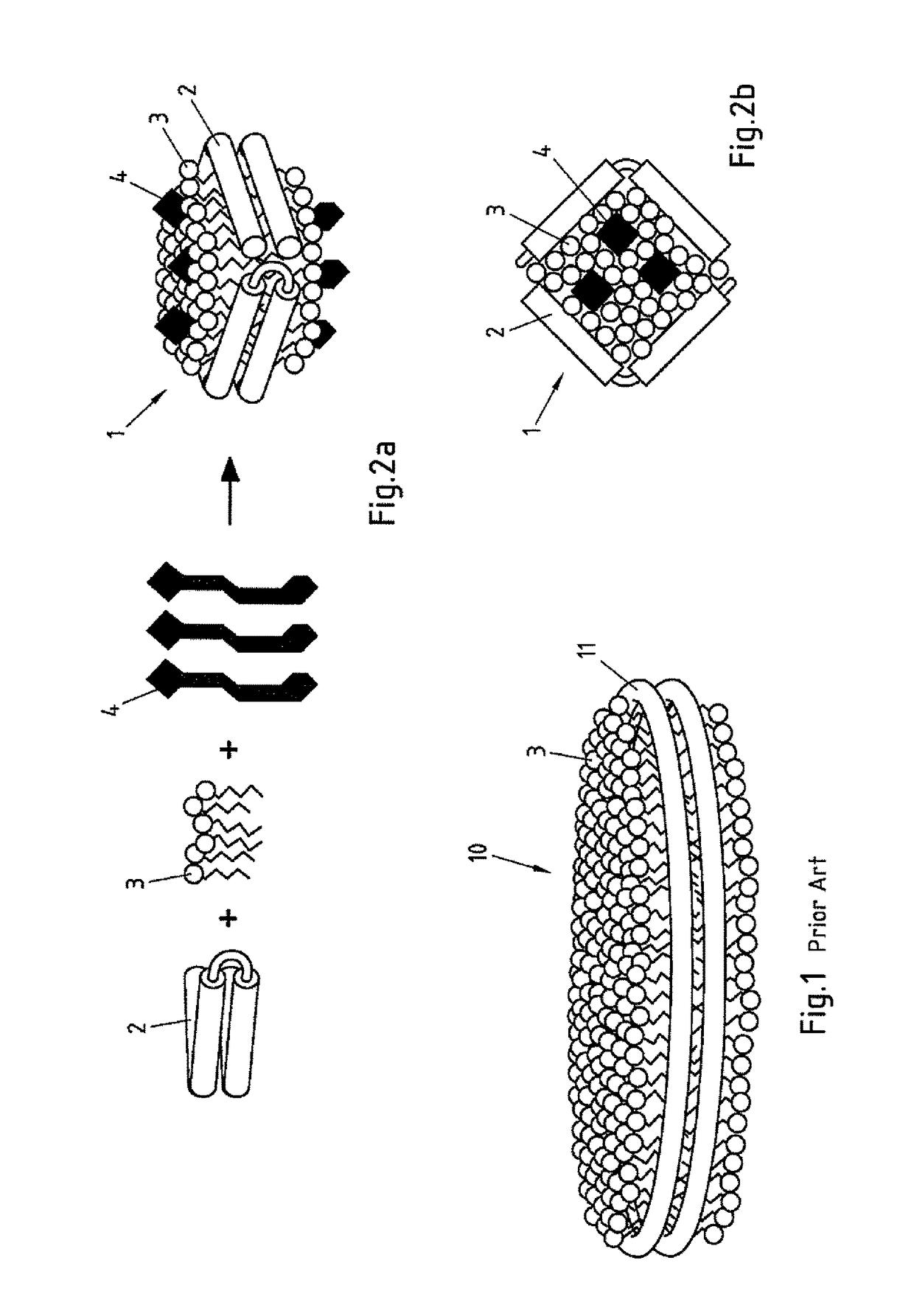
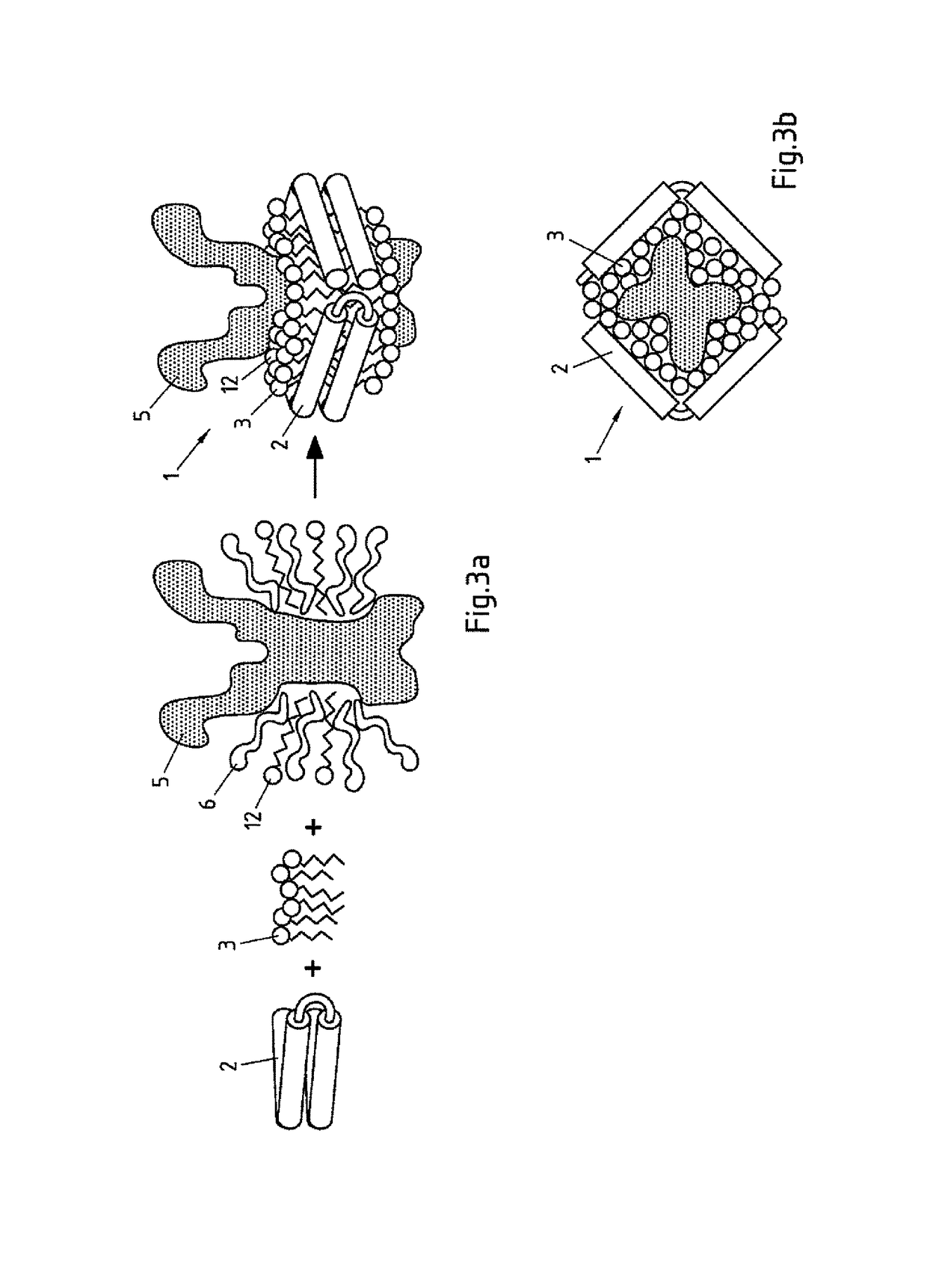
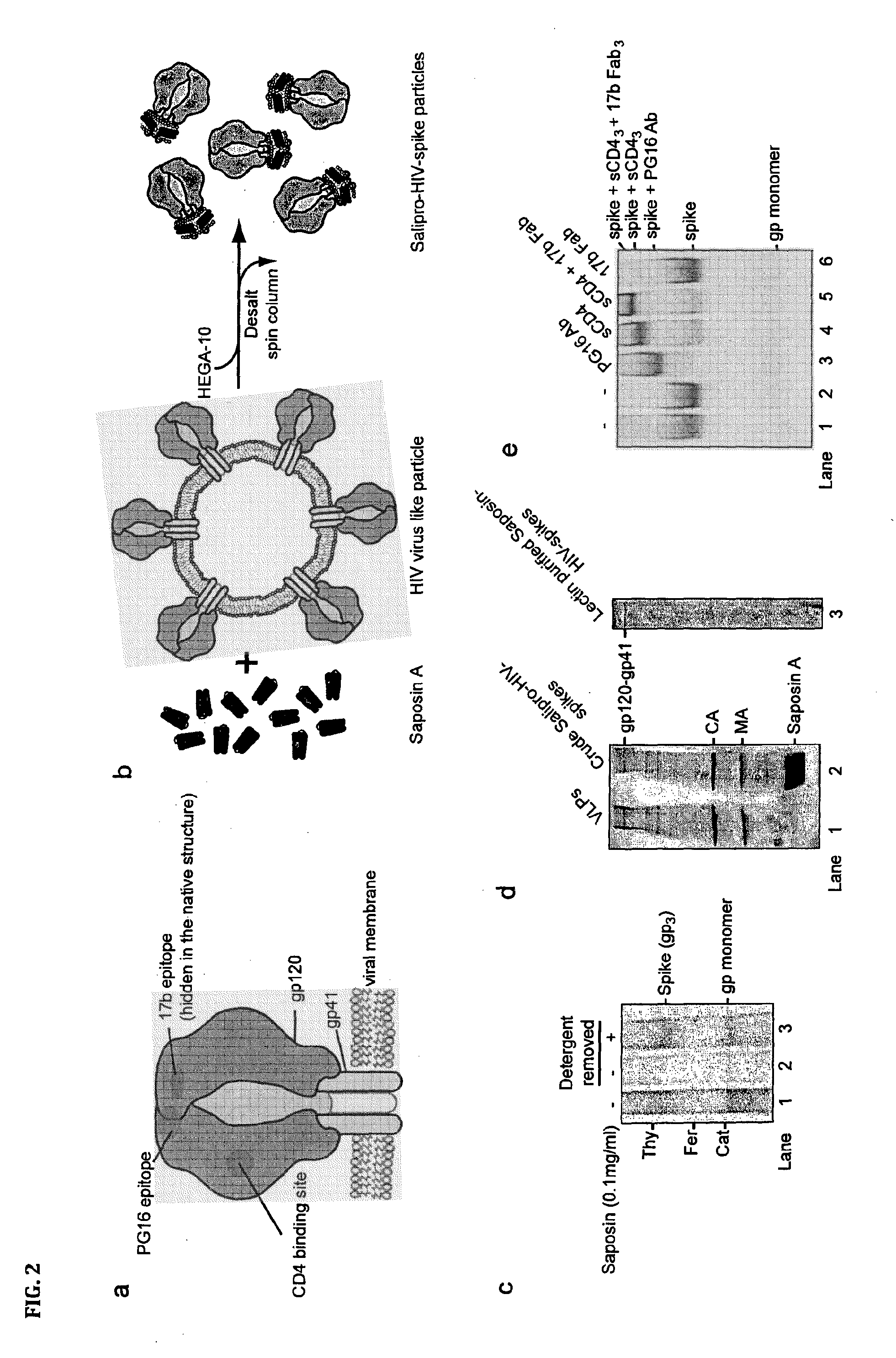
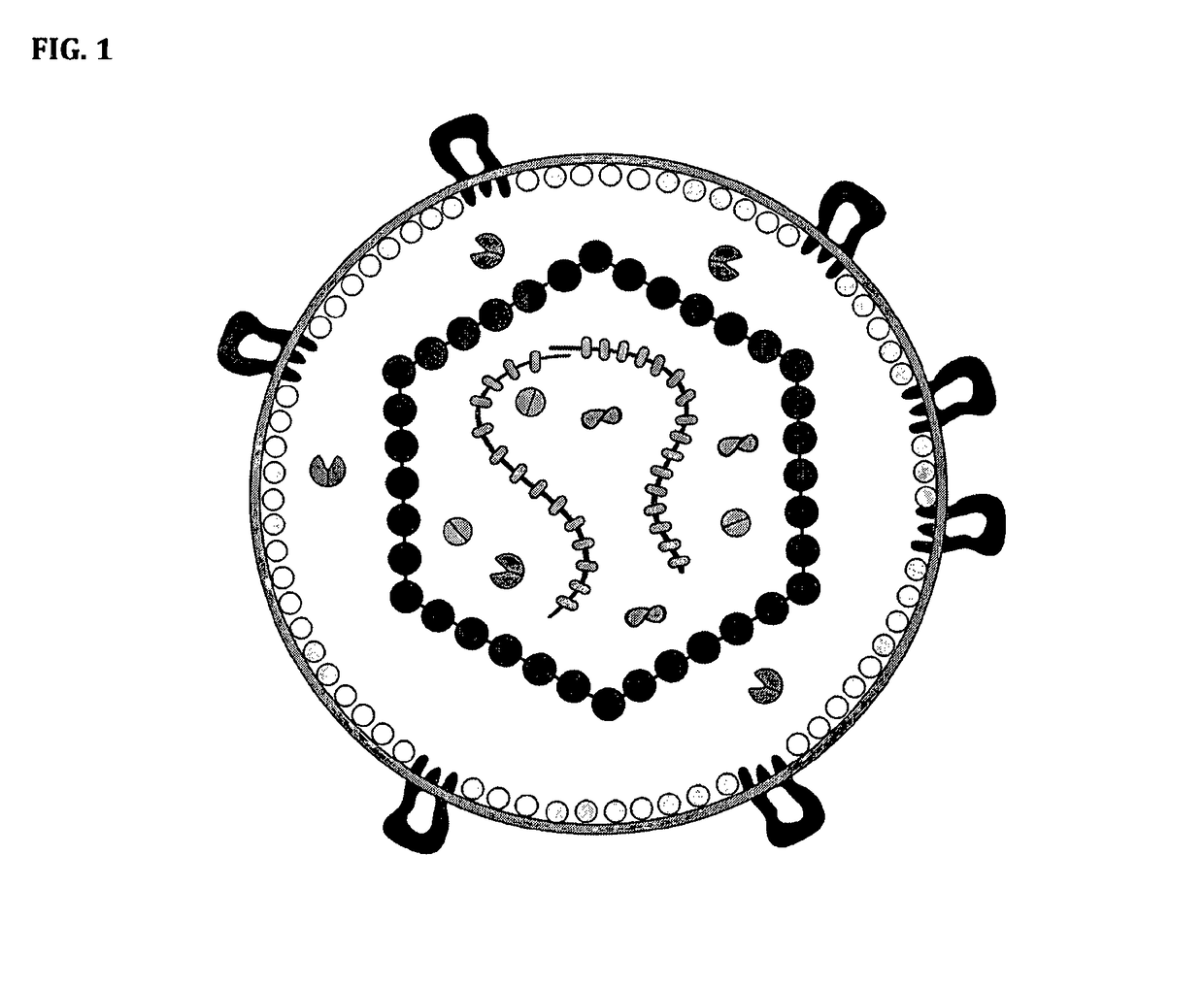
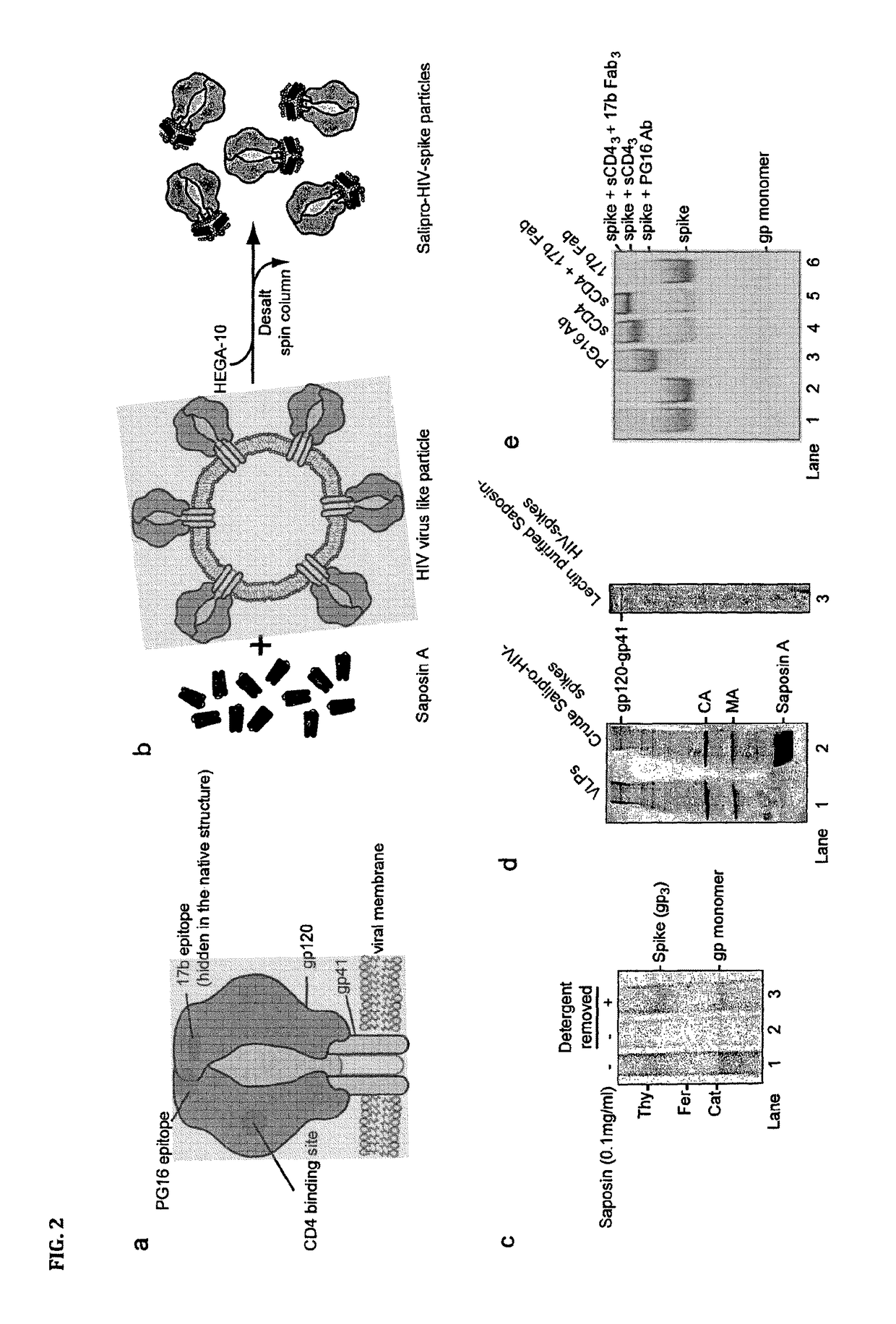
Salipro particles
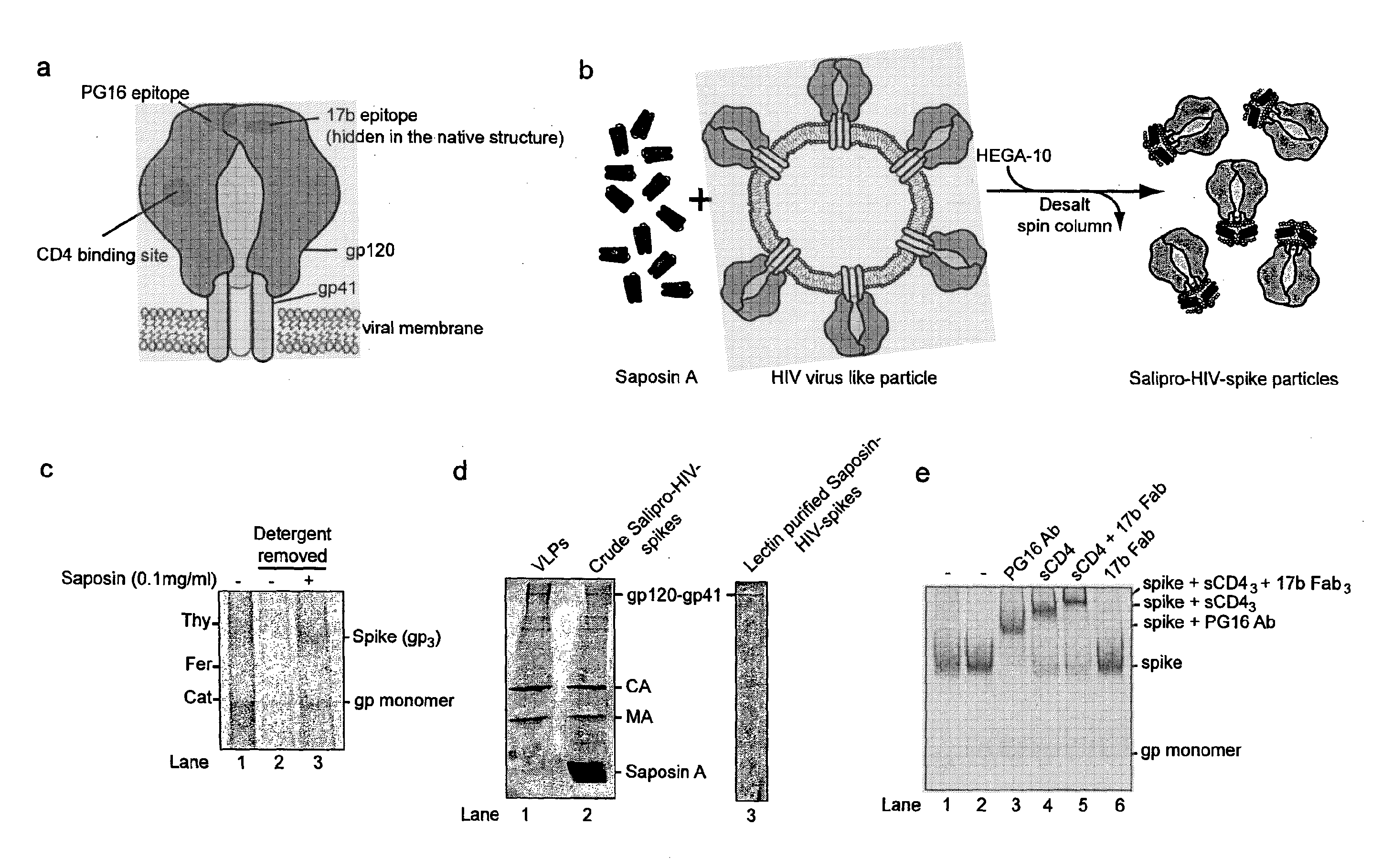
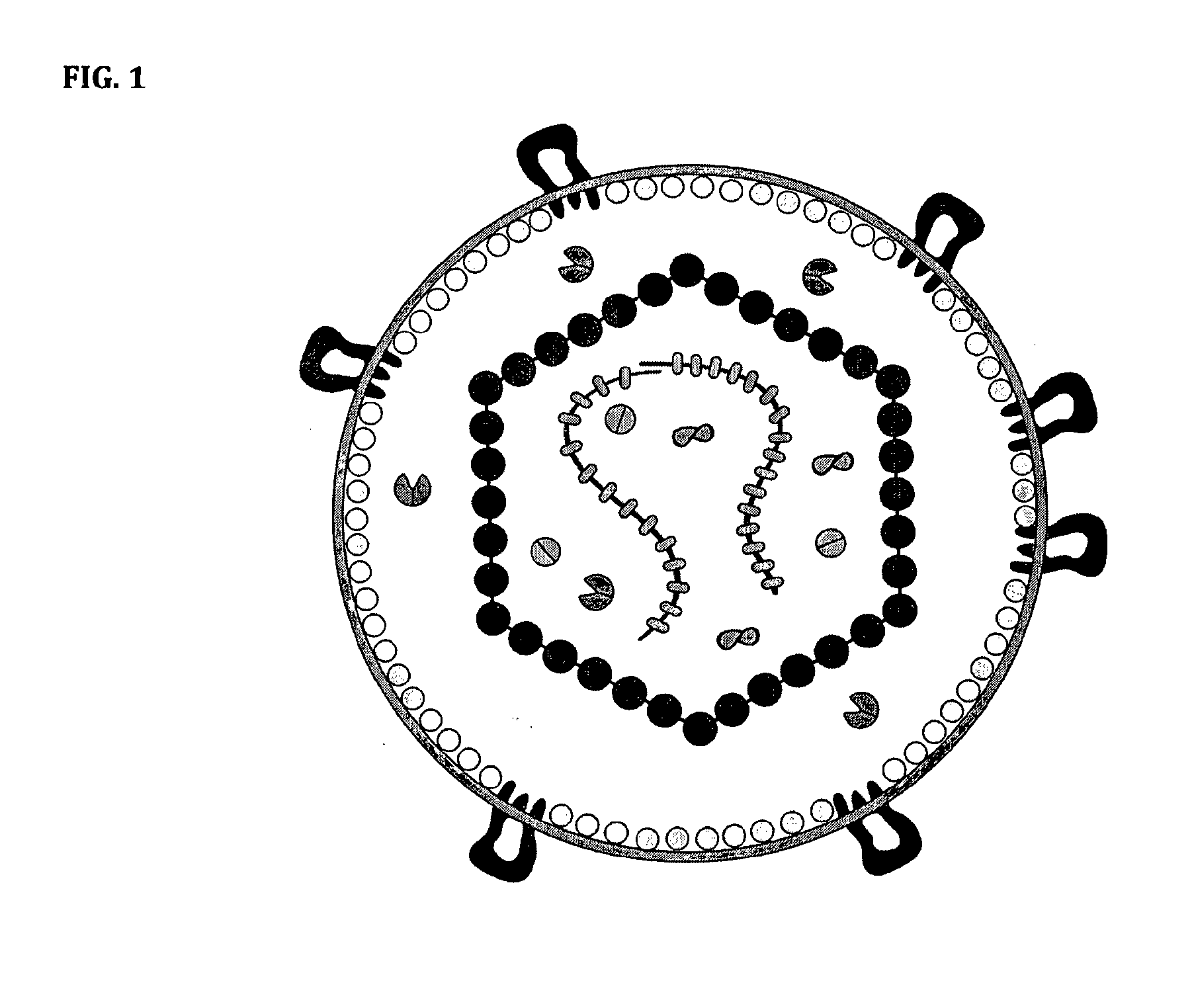
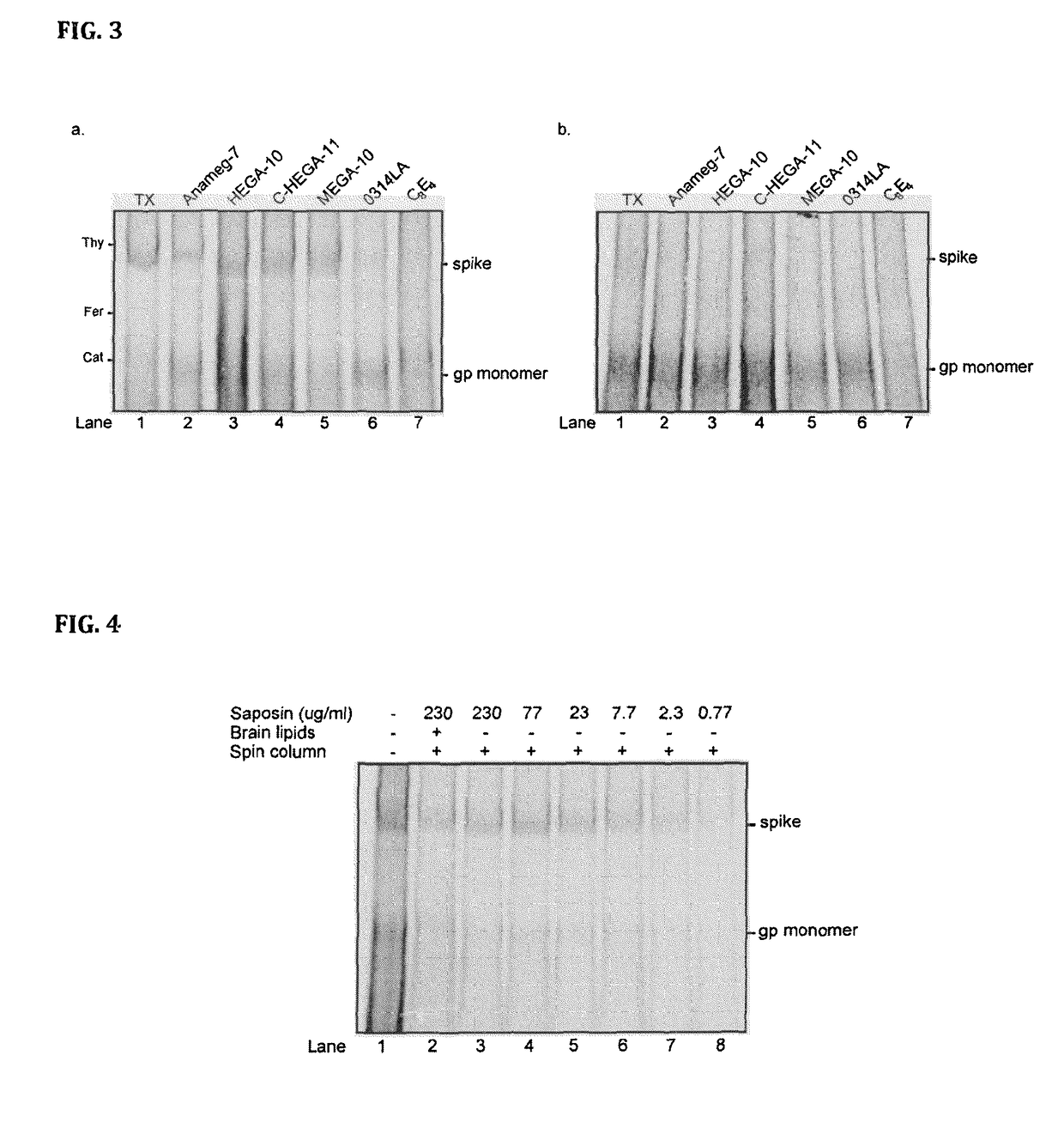
The invention provides a nanoscale particle comprising a lipid binding polypeptide, lipids and a hydrophobic agent, wherein the hydrophobic agent is different from the lipids, and wherein the lipid binding polypeptide is a saposin-like protein or a derivative or truncated form thereof. The invention further provides a process for preparing a particle comprising a saposin-like protein or a derivative or truncated form thereof and lipids comprising the step of (a) contacting the saposin-like protein or a derivative or truncated form thereof with solubilized lipids in a liquid environment and (b) allowing for the self-assembly of the particle at a pH of from 5.0 to 10.0. In addition, the invention provides a pharmaceutical composition comprising the particle of the invention for delivering a hydrophobic agent to an individual in need thereof and includes the use of the particle of the invention in preventing, treating or lessening the severity of a disease or its use in a diagnostic method, a cosmetic treatment, as hydrophobic agent delivery particle, as a tool for drug development, drug screening, membrane protein research or as vaccination formulation.
Owner:SALIPRO BIOTECH AB
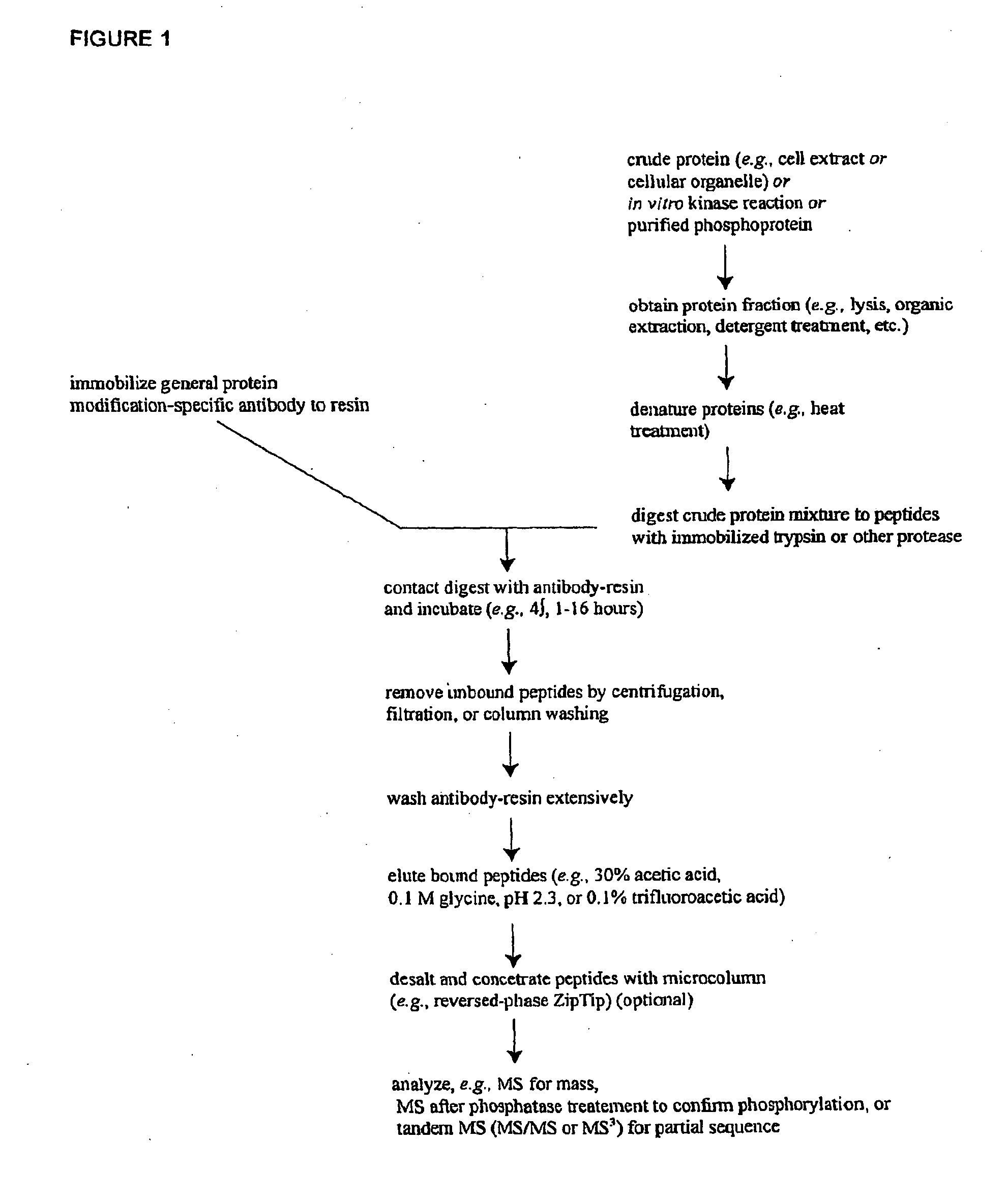
Reagents for the Detection of Protein Phosphorylation in Leukemia Signaling Pathways
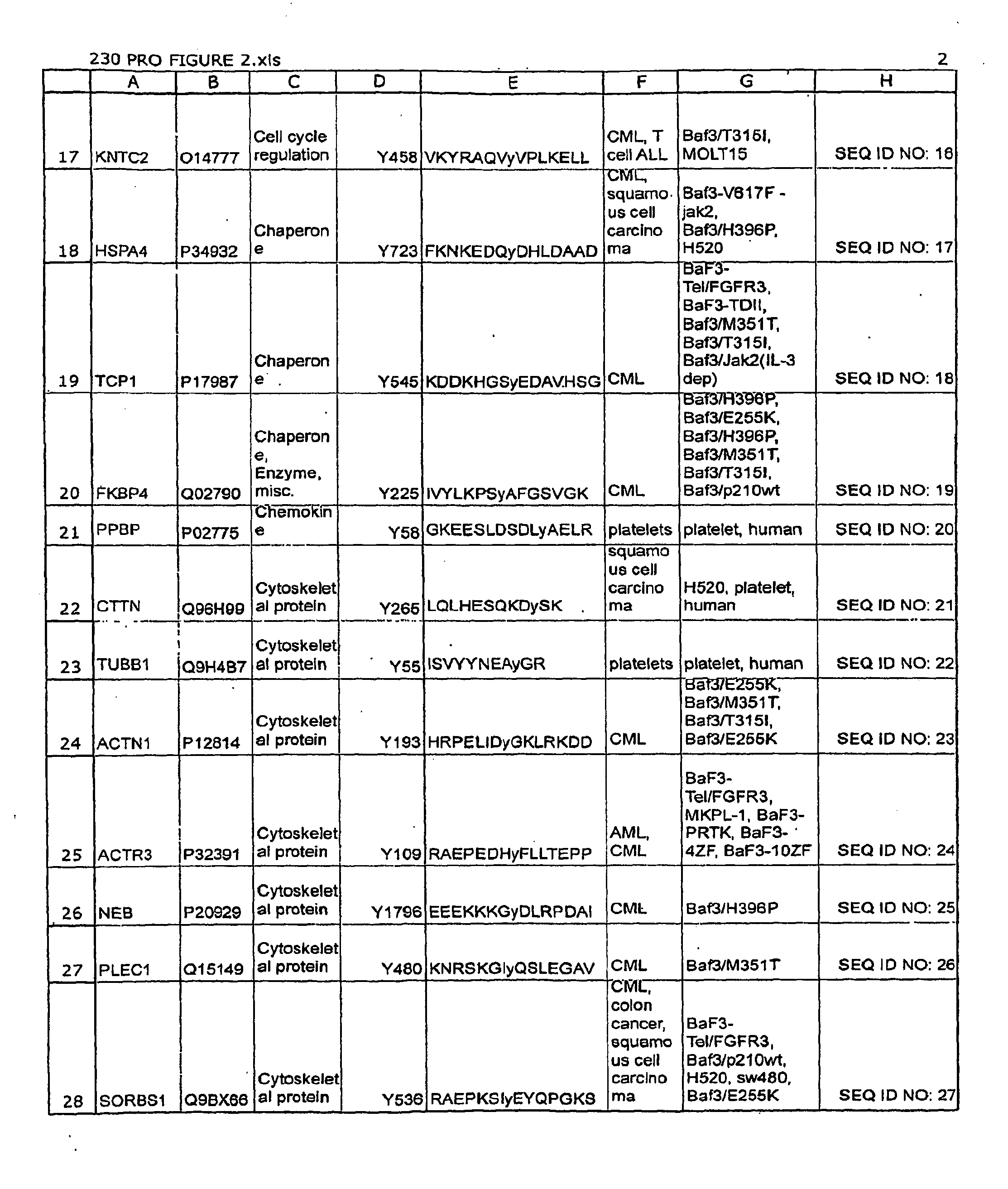
The invention discloses nearly 123 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human Leukemia, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: protein kinases, adaptor / scaffold proteins, phosphatase / phospholipases, G proteins / GTPase activating proteins / guanine nucleotide exchange factors, cellular metabolism enzymes, DNA binding proteins, cytoskeletal proteins, cell cycle regulation proteins, proteases, RNA binding proteins, transcription proteins, translation initiation complex proteins, transferases, ubiquitin conjugating system proteins, vesicle proteins, actin binding proteins, apoptosis proteins, chemokine proteins, enzyme proteins extra cellular matrix proteins, helicases, hydrolases, immunoglobin superfamily proteins, inhibitor proteins, isomerases, ligases, lipid binding proteins, methyltransferases, motor proteins, receptor proteins, and chaperone proteins.
Owner:CELL SIGNALING TECHNOLOGY
Antigen and method for production thereof
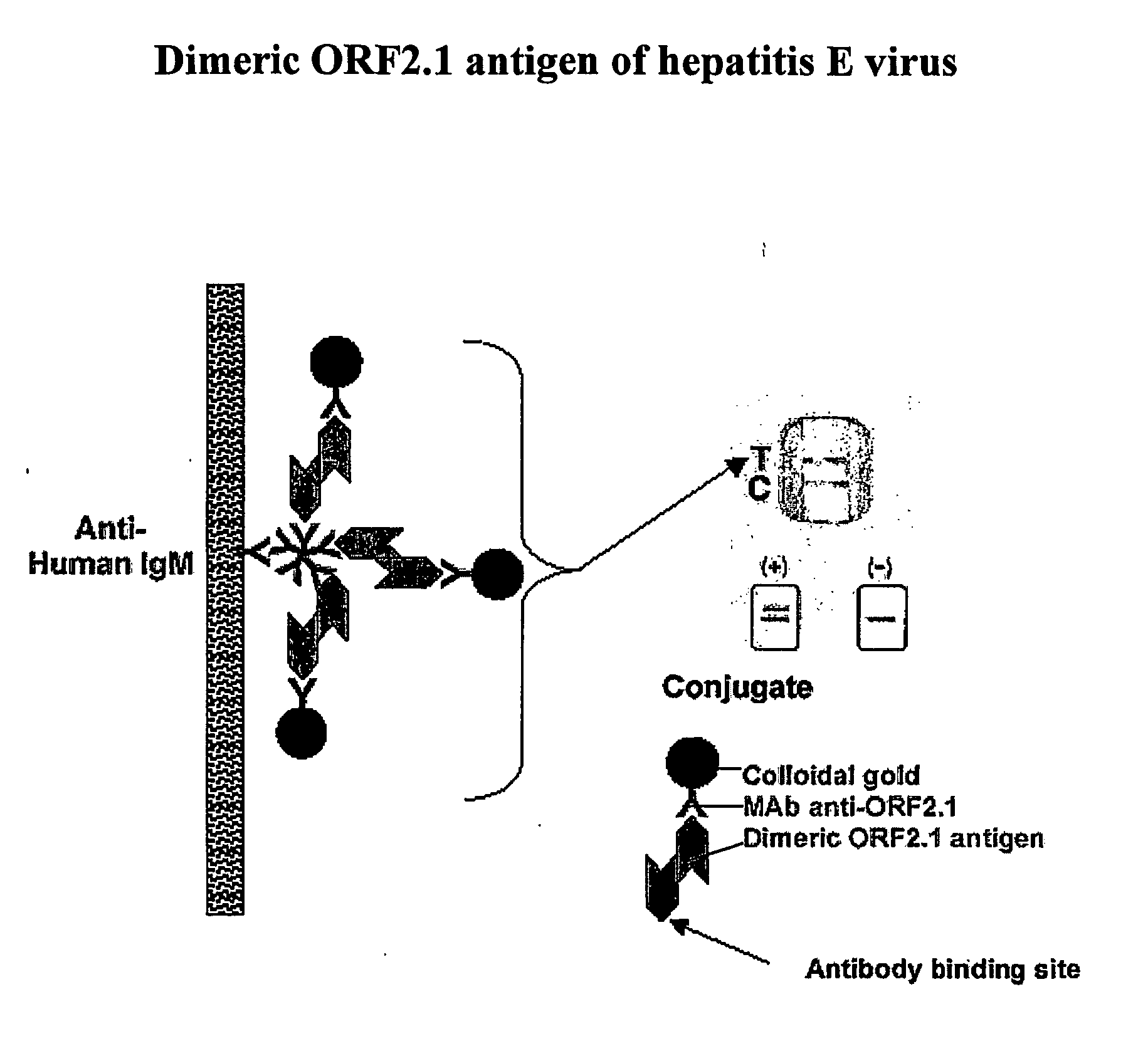
ActiveUS20160220664A1Accurately presentedDegree of thermostabilityViral antigen ingredientsReverse transcribing RNA virusesAntigenLipid binding
The invention refers to a method for producing an antigen comprising at least one hydrophobic or partially hydrophobic antigen molecule from a virus, a bacterium, fungus, protozoan, parasite, a human neoplastic cell or an animal neoplastic, tumour or 5 cancer cell, the method comprising the steps of providing a virus, or cell comprising an antigen molecule, purifying the cell comprising the antigen molecule, solubilizing the antigen molecule in a solubilizing agent that preserves an intact antigen molecule upon solubilisation and reconstituting the antigen molecule in a lipid-binding polypeptide that provides a lipid membrane mimicking environment and a reconstituted antigen particle 10 obtained by this method.
Owner:SALIPRO BIOTECH AB
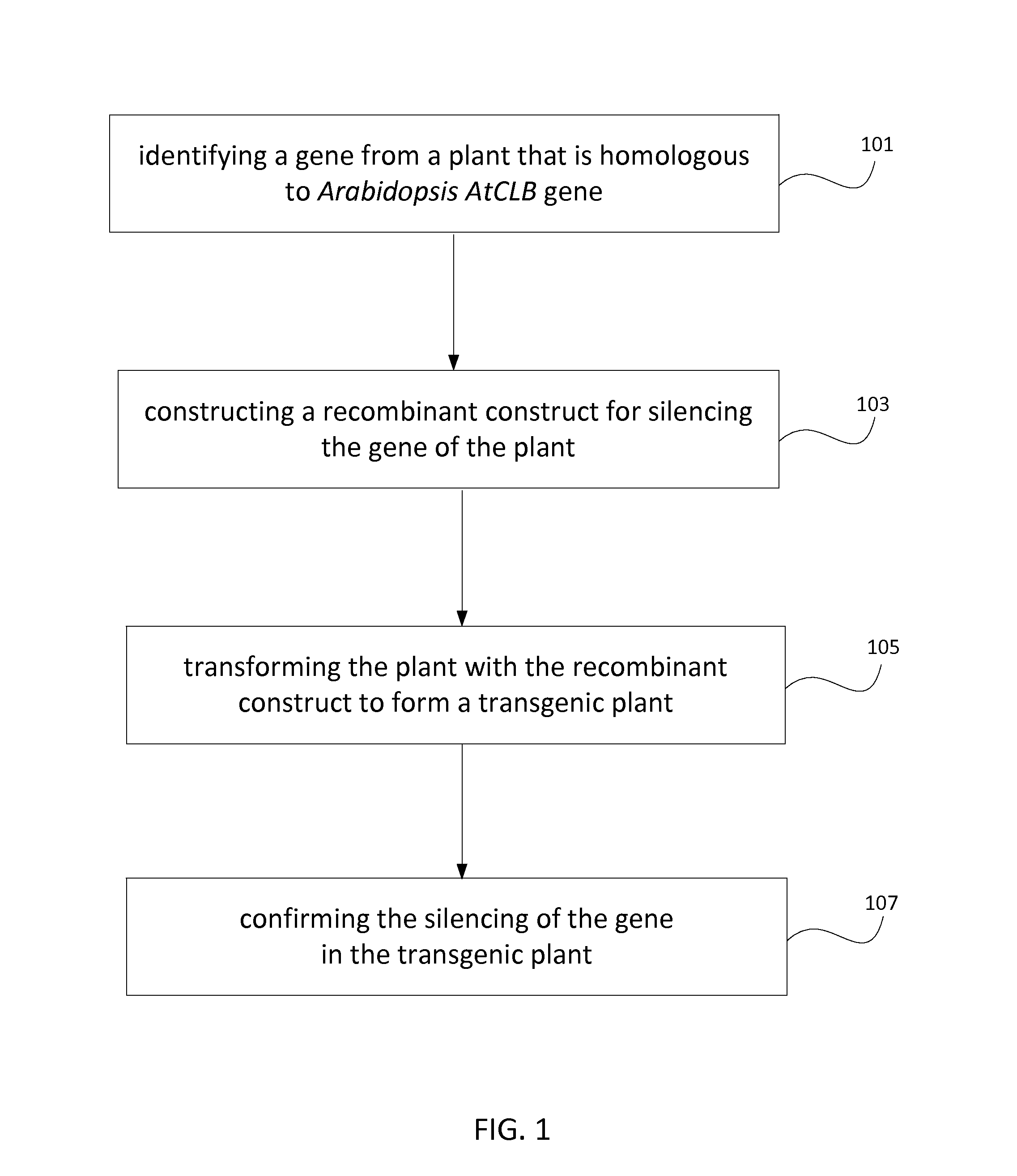
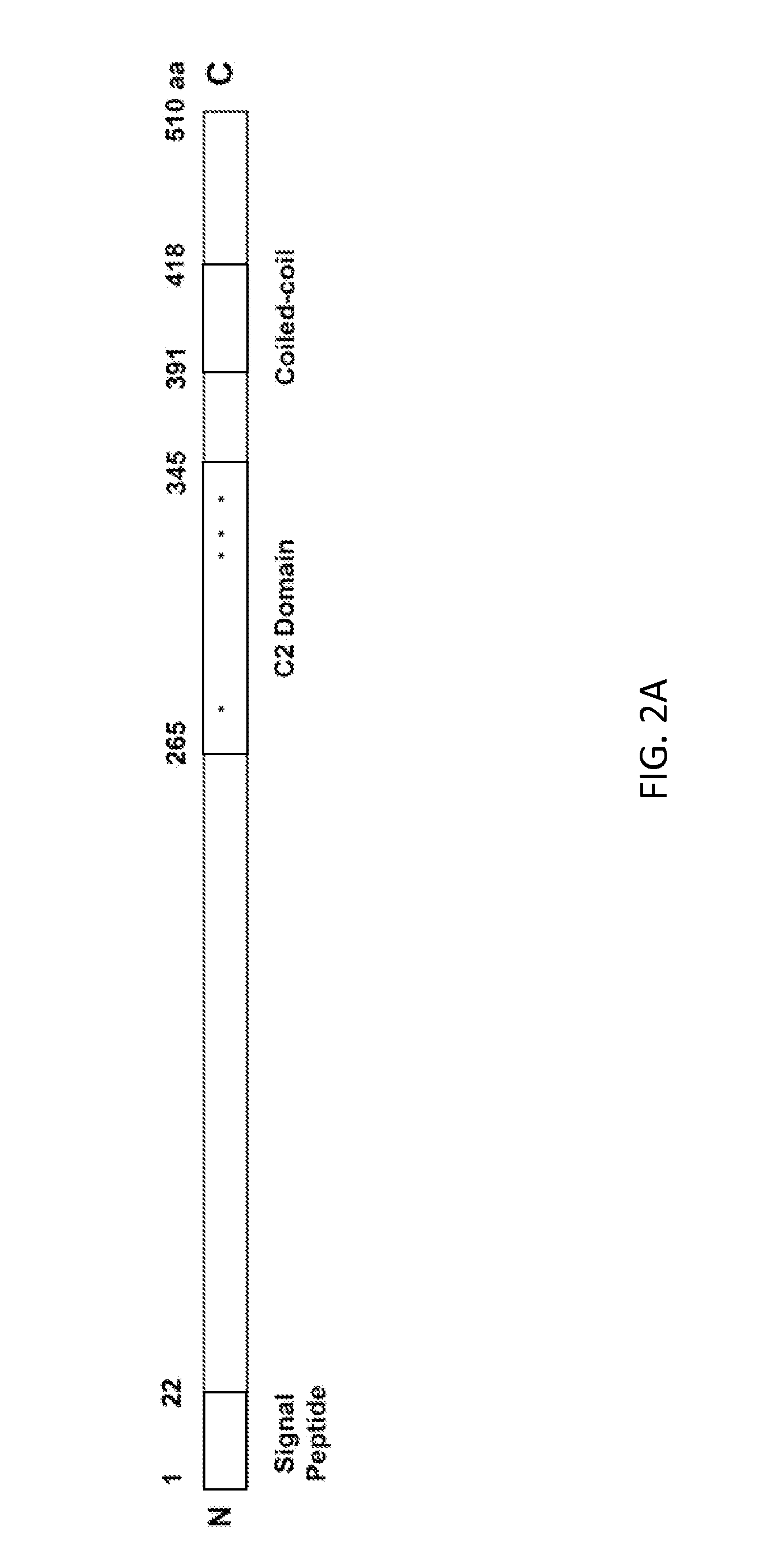
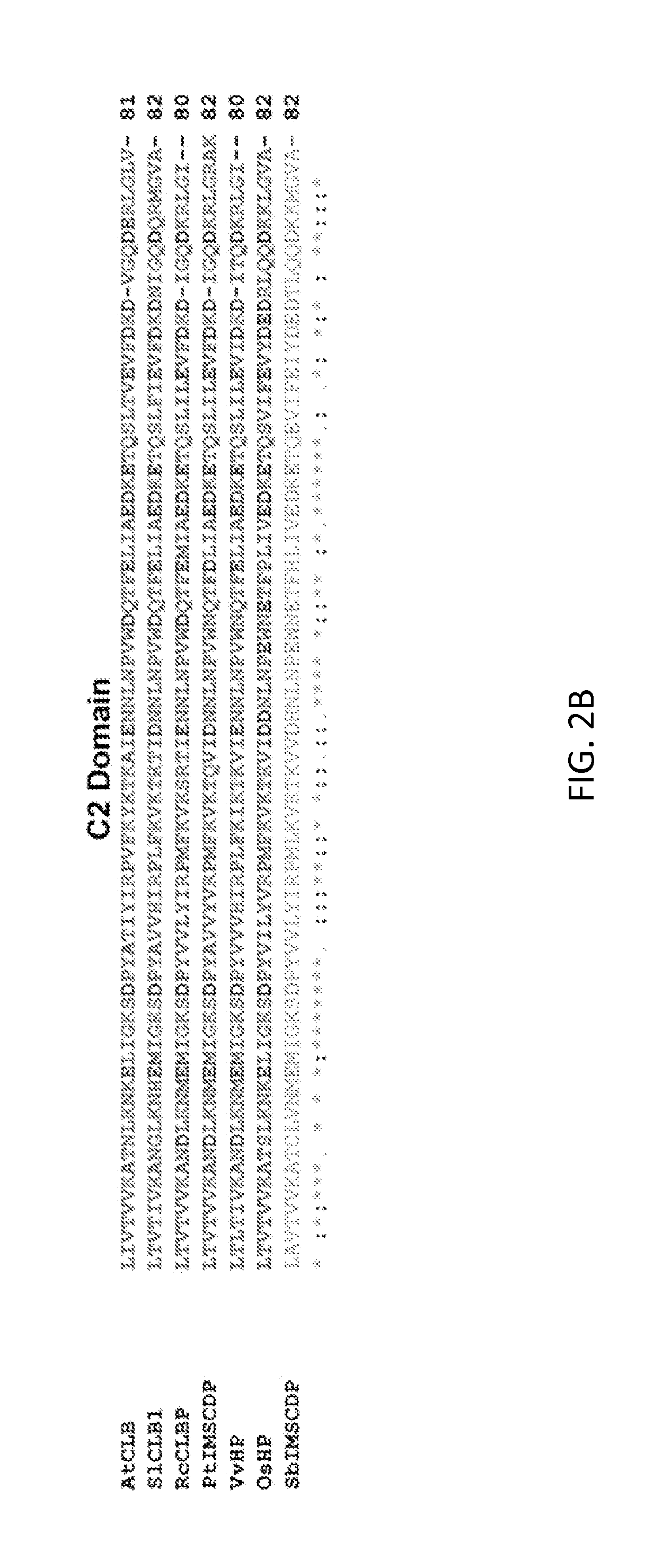
Method for producing stress tolerant transgenic plant by silencing a gene encoding calcium-dependent lipid-binding protein with c2 domain and applications of the same
InactiveUS20140082767A1Other foreign material introduction processesFermentationNucleotideLipid binding
A transgenic plant includes a first gene having a first polynucleotide and a recombinant construct having a second polynucleotide. The first polynucleotide is homologous to a Atclb gene fragment represented by nucleotide acids 963-1205 of SEQ ID NO:1. The first polynucleotide encodes a C2-like domain, and the Atclb gene fragment encodes a C2 domain represented by amino acids 265-345 of SEQ ID NO:2. The C2-like domain is homologous to the C2 domain. The second polynucleotide has at least 90% identity to the first polynucleotide or a sequence complimentary to the first polynucleotide. The recombinant construct is configured to silence the first gene such that the transgenic plant is tolerant to abiotic stress. A method to produce the transgenic plants.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Artificial cell membrane comprising supported lipid bilayer connected with probes having controllable mobility and method for analyzing interaction between molecules using the same
ActiveUS20170115286A1High resolutionMicrobiological testing/measurementArtificial cell constructsLipid formationMaterial scattering
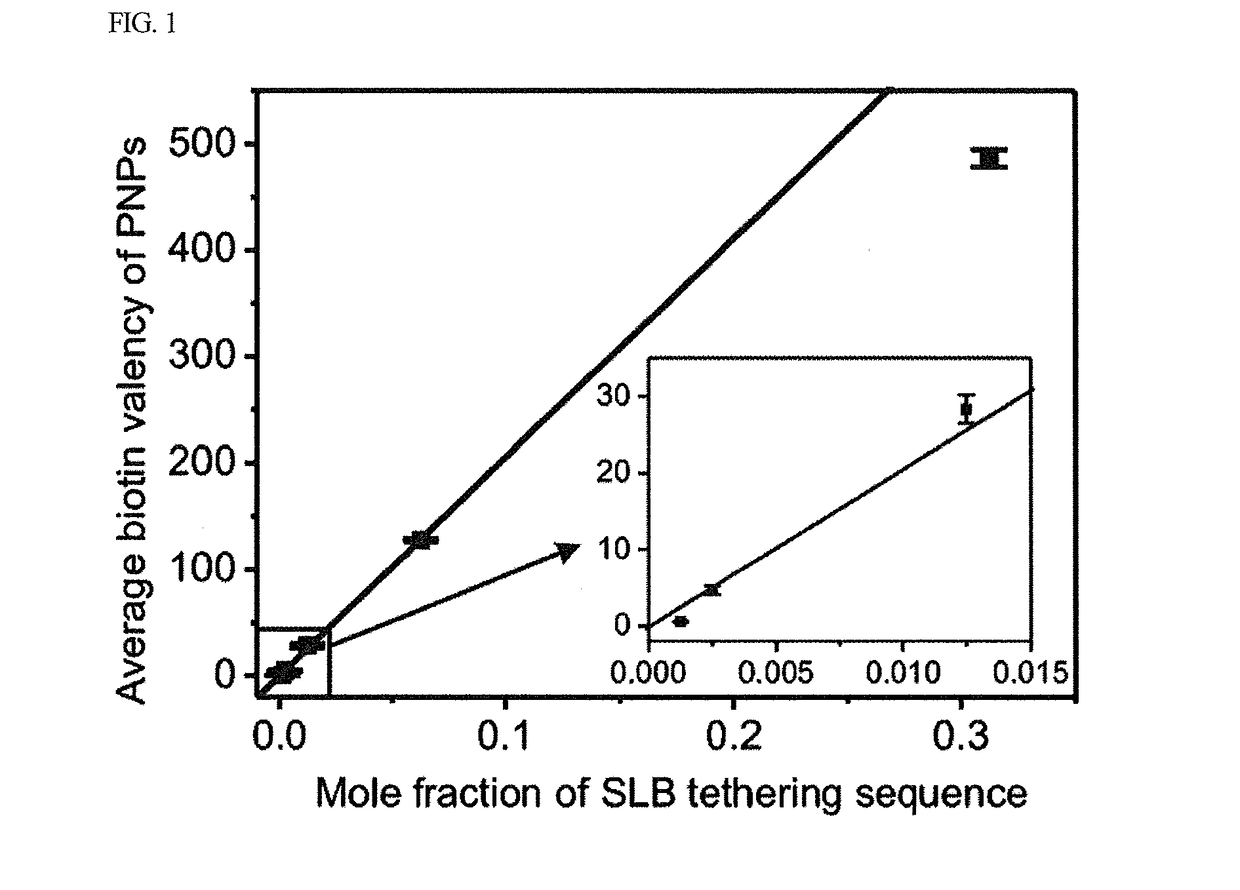
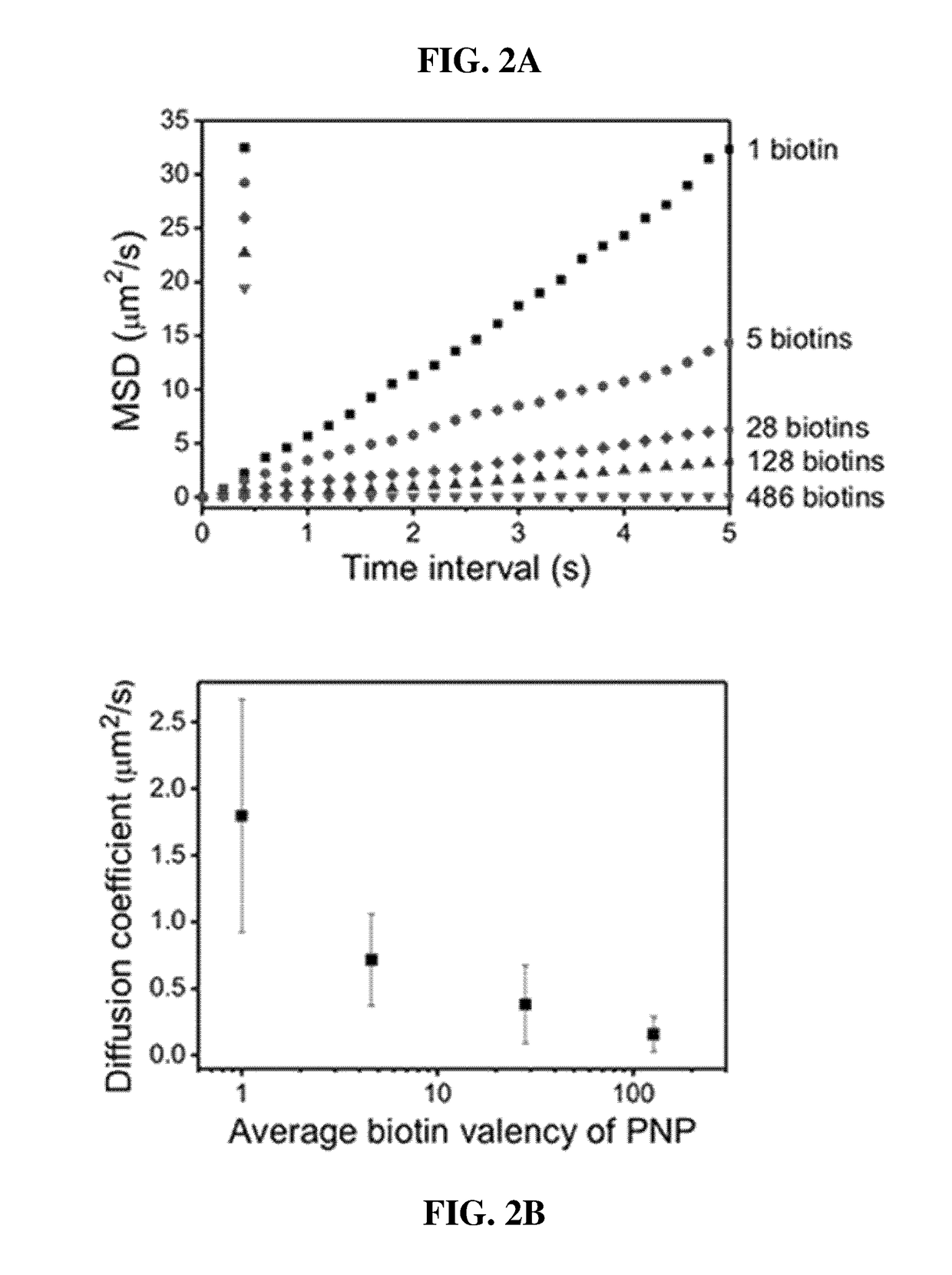
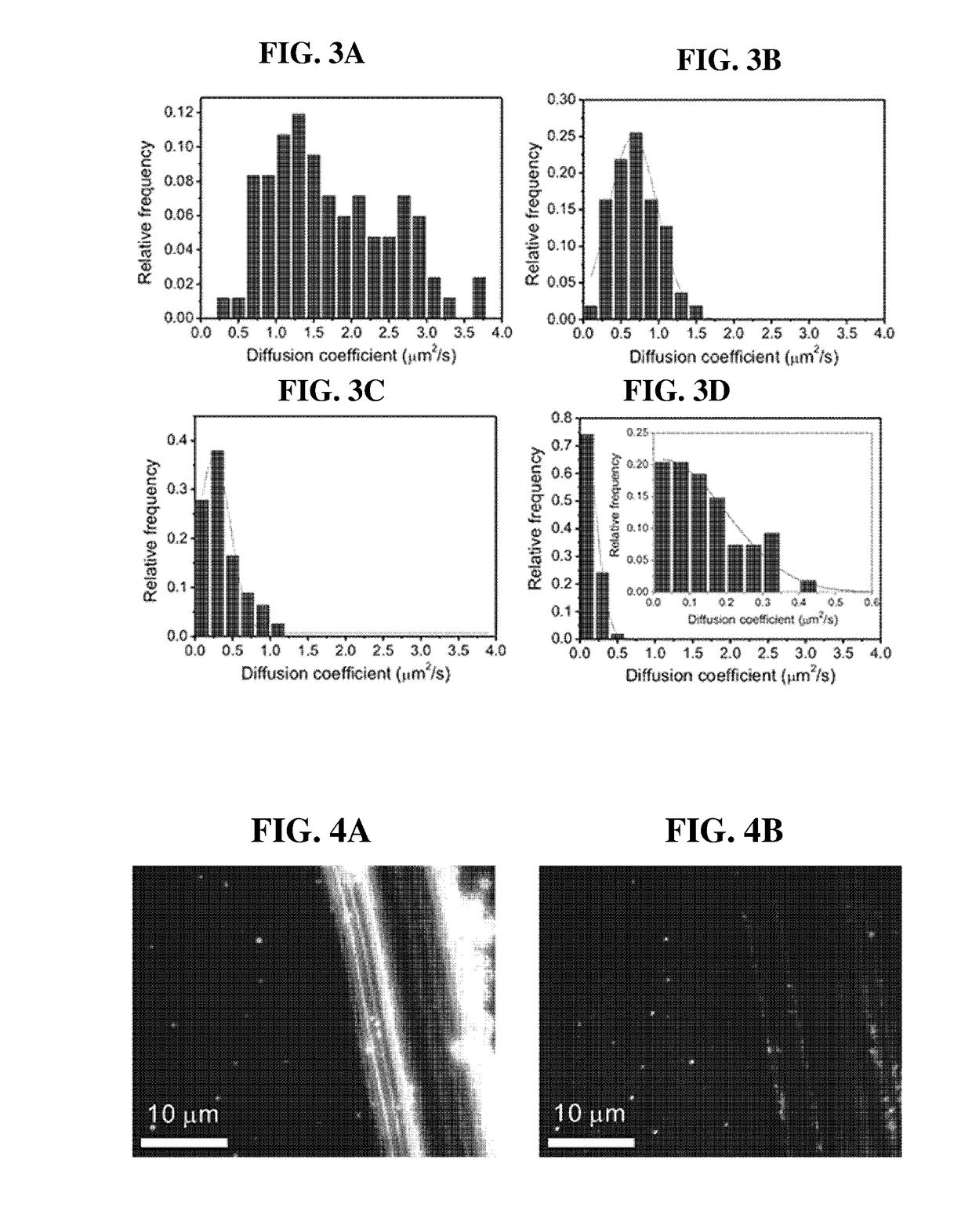
Disclosed herein are an artificial cell membrane including a supported lipid bilayer (SLB) including a substrate and mobility-decreased metal particles bonded onto the substrate; an analysis device or kit including the artificial cell membrane and examining the interactions between molecules, in which one molecule is bonded to the surface of a mobility-decreased metal particle bonded to the artificial cell membrane and the other molecule is bonded to the surface of a mobility-increased metal particle bonded to a lipid at a low valency; a method of examining the interactions between molecules using the analysis device; a kit for quantitative or qualitative analysis of a target material including the artificial cell membrane by plasmonic scattering measurements; and a multiple analysis kit capable of detecting a plurality of target materials using a plurality of metal particles having different plasmonic scattering wavelengths and / or having mobility on a supported lipid bilayer.According to the artificial cell membrane including a supported lipid bilayer containing metal particles attached thereto, the fluidity of the metal particles on the lipid can be controlled by adjusting the number of ligands bonded to the metal particles. Therefore, target molecules for analyzing the interactions therebetween on two types of metal particles having different fluidity are introduced onto the artificial cell membrane, thereby monitoring the movements of the metal particles through plasmonic scattering so as to analyze the interactions between the target molecules. In this case, multiple analysis of simultaneously detecting and quantifying a plurality of target materials using the artificial cell membrane of the present invention, plasmonic scattering wavelengths, and a plurality of particles having different fluidity can be performed.
Owner:SEOUL NAT UNIV R&DB FOUND +1
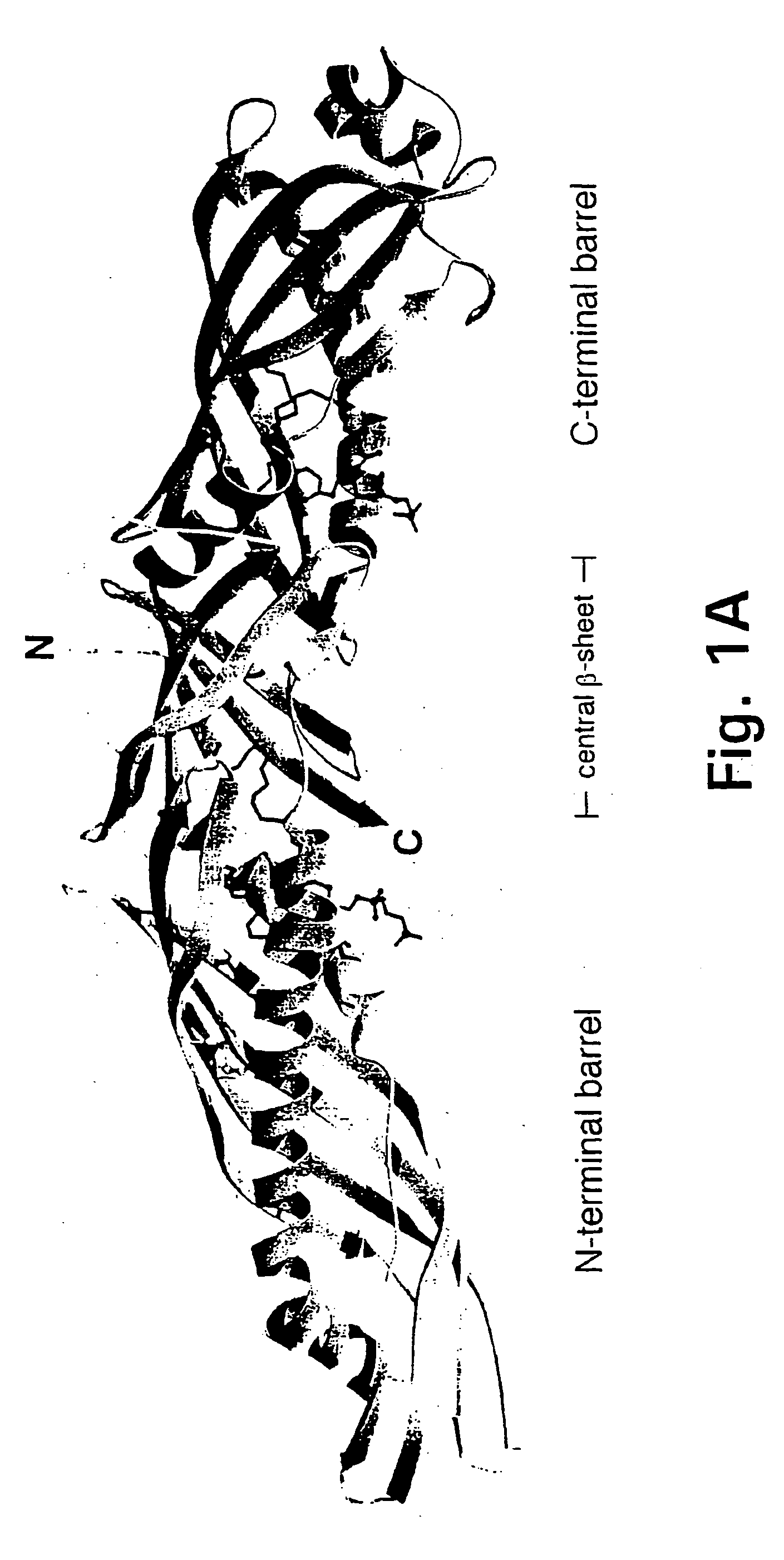
Bactericidal/permeability-increasing protein: crystallization, x-ray diffraction, three-dimensional structure determination, rational drug design and molecular modeling or related proteins
InactiveUS20050209837A1Change surface chargeAltered lipid binding pocketsAnalogue computers for chemical processesBactericidal/permeability-increasing proteinLipid formationLipid Transport
The present invention solves the three-dimensional structure of BPI and thereby provides atomic coordinates of BPI from the analysis of x-ray diffraction patterns of sufficiently high resolution for three-dimensional structure determination of the protein, as well as methods for rational drug design, based on using amino acid sequence data and / or x-ray diffraction data provided on computer readable media, as analyzed on a computer system having suitable computer algorithms; and atomic coordinates are provided yielding structural information on related proteins, including the lipid binding and lipid transport protein family that includes BPI, LBP, CETP and PLTP.
Owner:BEAMER LESA +2
Compositions and methods for measuring nuclear receptor ligands
Disclosed herein are compositions and methods used for detecting and measuring ligands for nuclear receptors and intracellular lipid binding proteins in both in vitro and in vivo samples.
Owner:CORNELL RES FOUNDATION INC
Antigen and method for production thereof
ActiveUS10159729B2Degree of thermostabilityEnhance immune responseViral antigen ingredientsReverse transcribing RNA virusesAntigenCancer cell
The invention refers to a method for producing an antigen comprising at least one hydrophobic or partially hydrophobic antigen molecule from a virus, a bacterium, fungus, protozoan, parasite, a human neoplastic cell or an animal neoplastic, tumor or 5 cancer cell, the method comprising the steps of providing a virus, or cell comprising an antigen molecule, purifying the cell comprising the antigen molecule, solubilizing the antigen molecule in a solubilizing agent that preserves an intact antigen molecule upon solubilization and reconstituting the antigen molecule in a lipid-binding polypeptide that provides a lipid membrane mimicking environment and a reconstituted antigen particle 10 obtained by this method.
Owner:SALIPRO BIOTECH AB
Binding Assay Components
InactiveUS20070298432A1Maximize availabilityPreserve and enhance availabilityMicrobiological testing/measurementNanoinformaticsLipid formationAnalyte
In one embodiment, the present invention provides a detection complex which is useful for detecting a specific analyte of interest in a sample. The complex comprises a detection marker indirectly connected to an analyte binding partner by a bridging complex. This arrangement serves to preserve or enhance the availability of analyte binding sites on the analyte binding partner and consequently enhances detection of the analyte. In some embodiments, the present invention provides a detection complex useful for detecting a specific antibody of interest in a sample. In accordance with one aspect of the present invention, methods are provided to detect one or more antibodies using a bridging complex comprising multimeric, dimeric, or chimeric molecules or particles each comprising an antigen and coupled to detection markers through the use of antibodies or a protein binding molecule, nucleic acid binding molecule, carbohydrate binding molecule or lipid binding molecule.
Owner:HEPGENICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com