Patents
Literature
130 results about "Alpha helix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
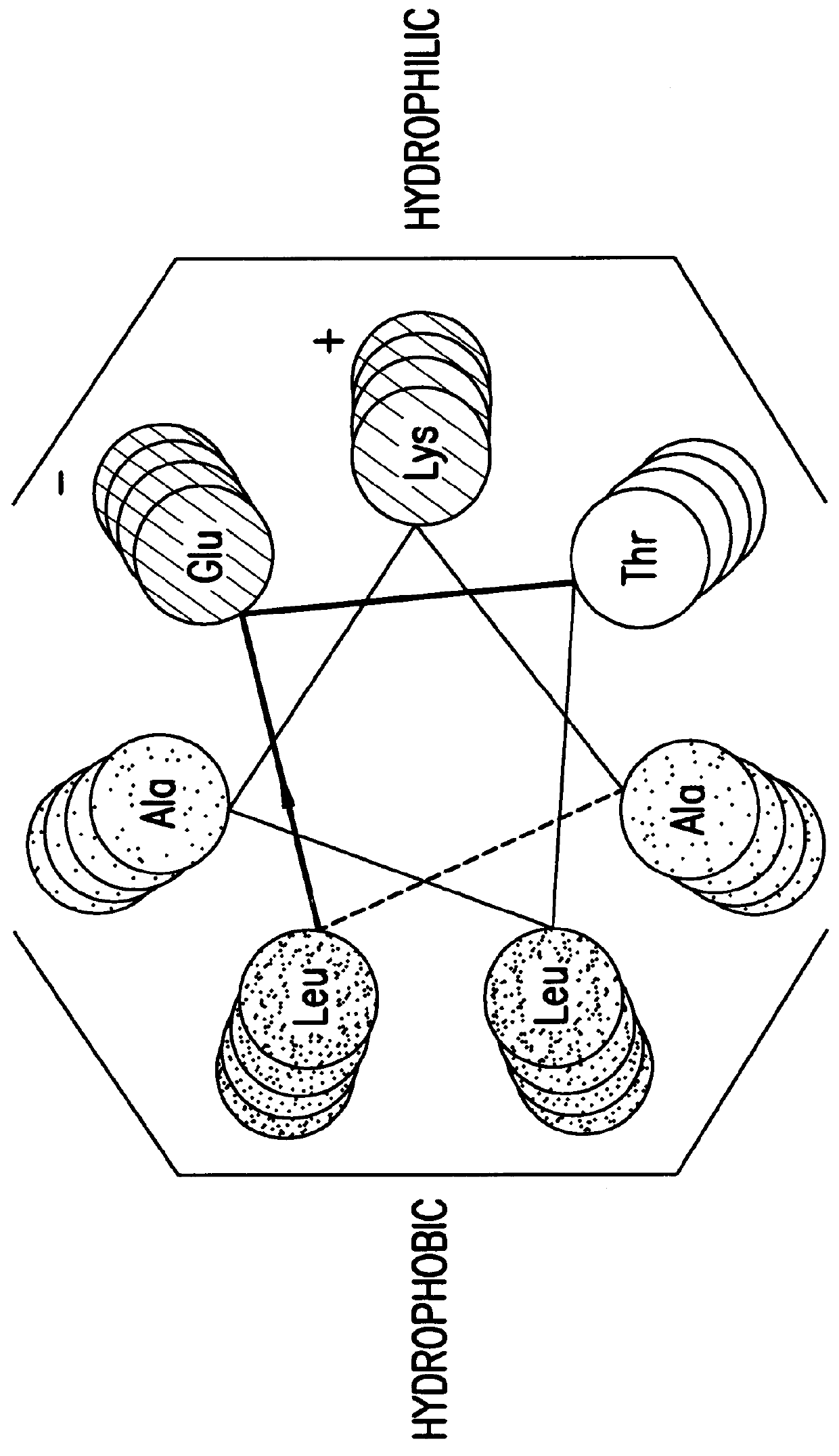
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located three or four residues earlier along the protein sequence.
CRY1C polypeptides having improved toxicity to lepidopteran insects
Disclosed are novel synthetically-modified B. thuringiensis nucleic acid segments encoding delta -endotoxins having insecticidal activity against lepidopteran insects. Also disclosed are synthetic crystal proteins encoded by these novel nucleic acid sequences. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention. Also disclosed are methods for modifying, altering, and mutagenizing specific loop regions between the alpha helices in domain 1 of these crystal proteins, including Cry1C, to produce genetically-engineered recombinant cry* genes, and the proteins they encode which have improved insecticidal activity. In preferred embodiments, novel Cry1C* amino acid segments and the modified cry1C* nucleic acid sequences which encode them are disclosed.
Owner:MONSANTO CO (MONSANTO CY)
Polypeptide compositions toxic to lepidopteran insects and methods for making same
Disclosed are novel synthetically-modified B. thuringiensis nucleic acid segments encoding delta -endotoxins having insecticidal activity against lepidopteran insects. Also disclosed are synthetic crystal proteins encoded by these novel nucleic acid sequences. Methods of making and using these genes and proteins are disclosed as well as methods for the recombinant expression, and transformation of suitable host cells. Transformed host cells and transgenic plants expressing the modified endotoxin are also aspects of the invention. Also disclosed are methods for modifying, altering, and mutagenizing specific loop regions between the alpha helices in domain 1 of these crystal proteins, including Cry1C, to produce genetically-engineered recombinant cry* genes, and the proteins they encode which have improved insecticidal activity. In preferred embodiments, novel Cry1C* amino acid segments and the modified cry1C* nucleic acid sequences which encode them are disclosed.
Owner:MONSANTO TECH LLC
Methods for treating acromegaly and giantism with growth hormone antagonists
The present invention relates to antagonists of vertebrate growth hormones obtained by mutation of the third alpha helix of such proteins (especially bovine or human GHs). These mutants-have growth-inhibitory or other GH-antagonizing effects. These novel hormones may be administered exogenously to animals, or transgenic animals may be made that express the antagonist. Animals have been made which exhibited a reduced growth phenotype. The invention also describes methods of treating acromegaly, gigantism, cancer, diabetes, vascular eye diseases (diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, retinopathy of sickle-cell anemia, etc.) as well as nephropathy and other diseases, by administering an effective amount of a growth hormone antagonist. The invention also provides pharmaceutical formulations comprising one or more growth hormone antagonists.
Owner:OHIO UNIV EDISON ANIMAL BIOTECH INST
Dideoxynucleotide-triphosphate utilization by the hyper-thermophilic DNA polymerase from the archaeon Pyrococcus furiosus
InactiveUS6333183B1High sensitivityImprove thermal stabilityBacteriaSugar derivativesDideoxynucleotide TriphosphatesBinding site
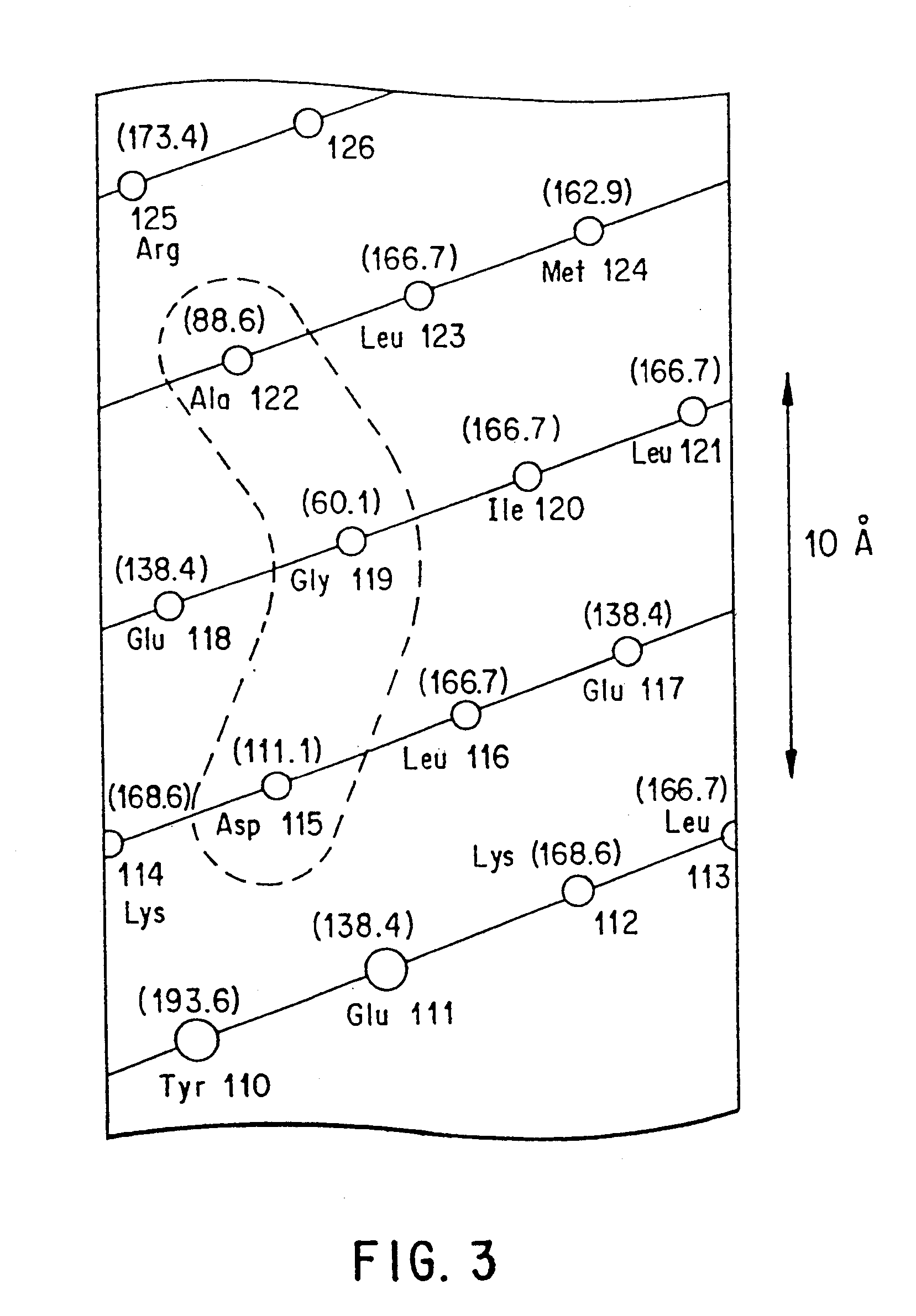
Polymerases from the Pol I family which are able to efficiently use ddNTPs have demonstrated a much improved performance when used to sequence DNA. A number of mutations have been made to the gene coding for the Pol II family DNA polymerase from the archaeon Pyrococcus furiosus with the aim of improving ddNTP utilisation. "Rational" alterations to amino acids likely to be near the dNTP binding site (based on sequence homologies and structural information) did not yield the desired level of selectivity for ddNTPs. However, alteration at four positions (Q472, A486, L490 and Y497) gave rise to variants which incorporated ddNTPs better than the wild type, allowing sequencing reactions to be carried out at lowered ddNTP:dNTP ratios. Wild type Pfu-Pol required a ddNTP:dNTP ratio of 30:1; values of 5:1 (Q472H), 1:3 (L490Y), 1:5 (A486Y) and 5:1 (Y497A) were found with the four mutants; A486Y representing a 150-fold improvement over the wild type. A486, L490 and Y407 are on an alpha-helix that lines the dNTP binding groove, but the side chains of the three amino acids point away from this groove; Q472 is in a loop that connects this alpha-helix to a second long helix. None of the four amino acids can contact the dNTP directly. Therefore, the increased selectivity for ddNTPs is likely to arise from two factors: 1) Small overall changes in conformation that subtly alter the nucleotide triphosphate binding site such that ddNTPs become favoured; 2) interference with a conformational change that may be critical both for the polymerisation step and discrimination between different nucleotide triphosphates.
Owner:GE HEALTHCARE BIO SCI CORP
Material for activating epidermal cell multiplication
InactiveUS6440740B1Improve propertiesEpidermal cells/skin cellsArtificial cell constructsAdhesion processCell adhesion
The present invention provides a novel material for activating epidermal cell multiplication on which not only effective adhesion and multiplication of human and mammalian epidermal cells is accomplished also intercellular adhesion is promoted to form a plain cellular layer. The present invention provides a material for activating epidermal cell multiplication which contains silk fibroin of alpha-type or alpha-helix X-ray diffraction pattern as a principal constituent. The present material fulfills the principal requirements for activating epidermal cells of greatly improved cellular adhesion rate, multiplication, intercellular adhesion and growth of cell layers.
Owner:NAT INST OF AGROBIOLOGICAL SCI
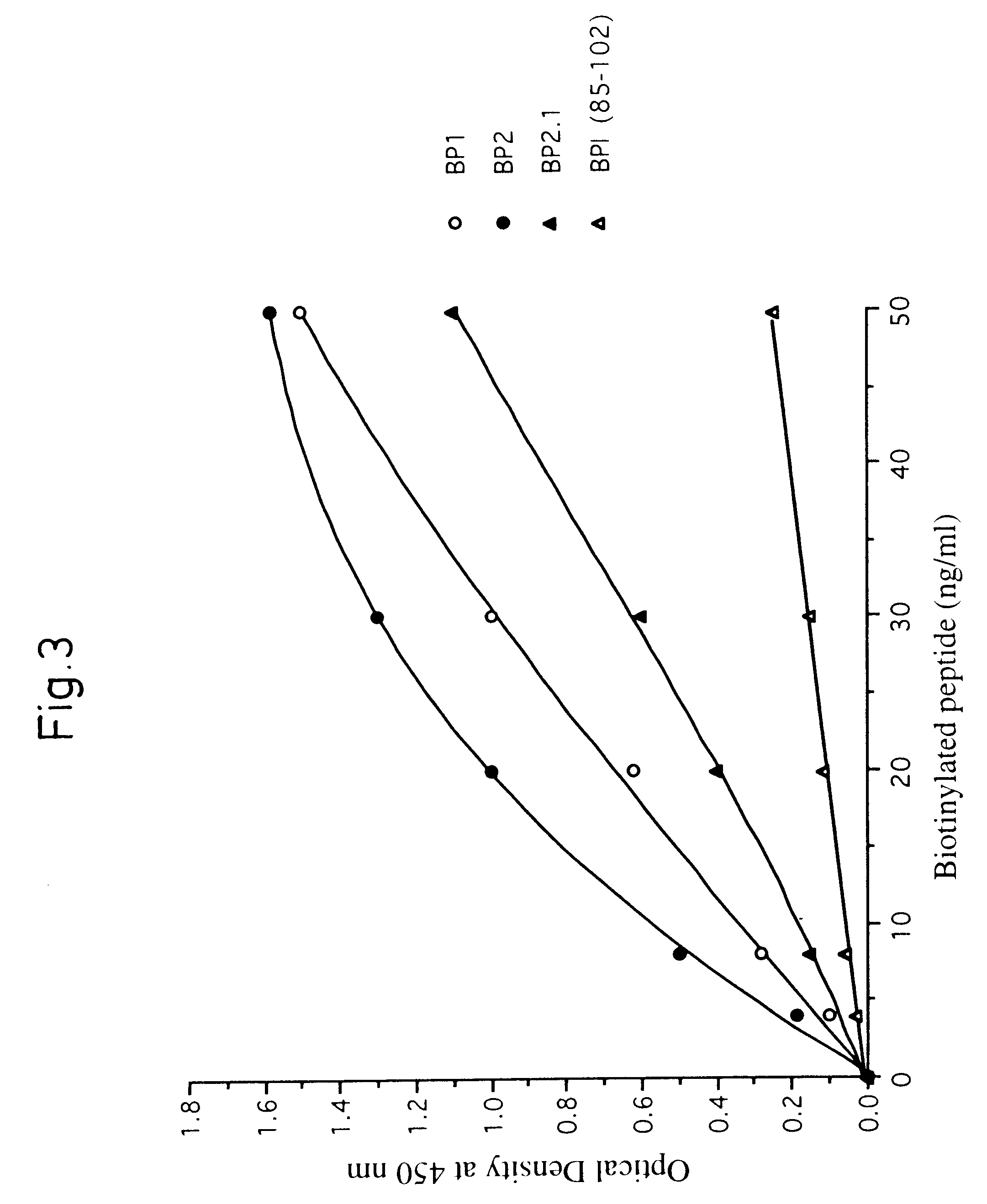
Proline-locked stapled peptides and uses thereof
ActiveUS20150239937A1Oral bioavailabilityImprove stabilitySenses disorderNervous disorderCross-linkSide chain
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome
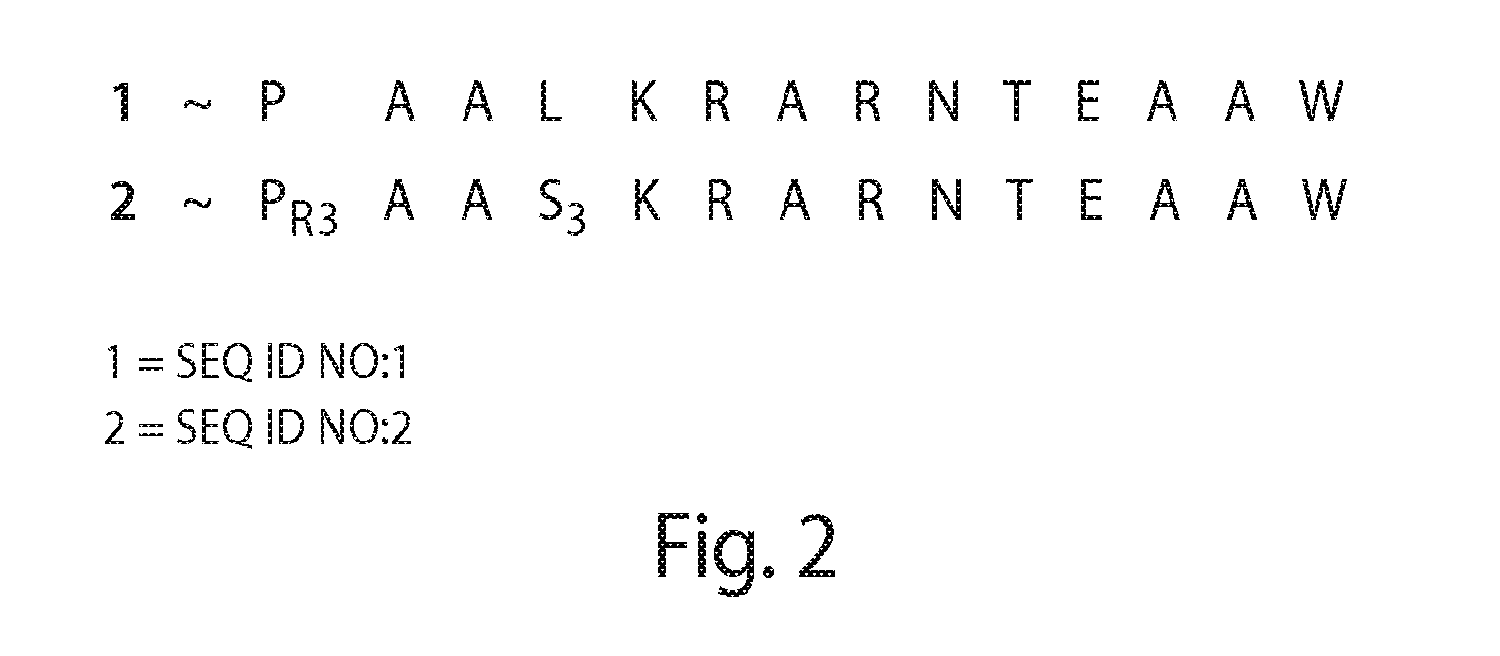
A peptide with an amino acid composition such that the peptide is amphipathic, cationic and forms a stable alpha-helix and has the following structure comprising at least 12 amino acidsA=an amino acid selected from the basic amino acids Lys,Arg or HisB=an amino acid selected from the aromatic amino acids Phe, Trp or TyrC=an amino acid selected from the group comprising the hydrophobic amino acids Leu, Ile, Val or Ala, andsaid peptide has either the orientation according to the formula or the retro orientation thereof, wherein at least 0-m of the repetitive sequence motifs (A2-B2-C1-A3) have the retro orientation and the remaining repetitive motifs (A2-B2-C1-A3) have the orientation as presented in the formula and wherein,R1-R2- and R3 are a number of amino acids, and whereinm=1-10, preferably 2-8, more preferably 2-5 andn=1-3, a pharmaceutical composition comprising such a peptide application thereof in treatment or diagnosis related to i.a. parasite infection topical and systemic tumors and septic shock.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT +1
Proline-locked stapled peptides and uses thereof
ActiveUS9617309B2Oral bioavailabilityImprove stabilitySenses disorderNervous disorderCross-linkSide chain
The present invention provides a new type of alpha-helix nucleating cross-link (“staple”) formed by olefin metathesis of a proline derivative with an alkenyl side chain and another amino acid derivative with an alkenyl side chain. The proline derivatives as described herein have been found to be strong nucleators of alpha-helix formation. The invention also provides moieties for shielding the free amide N—H's at the N-terminus of an alpha-helix, thereby further stabilizing the helix. The proline derivatives, precursors prior to cross-linking, and the cross-linked peptides are provided as well as methods of using and preparing these compounds and peptides.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
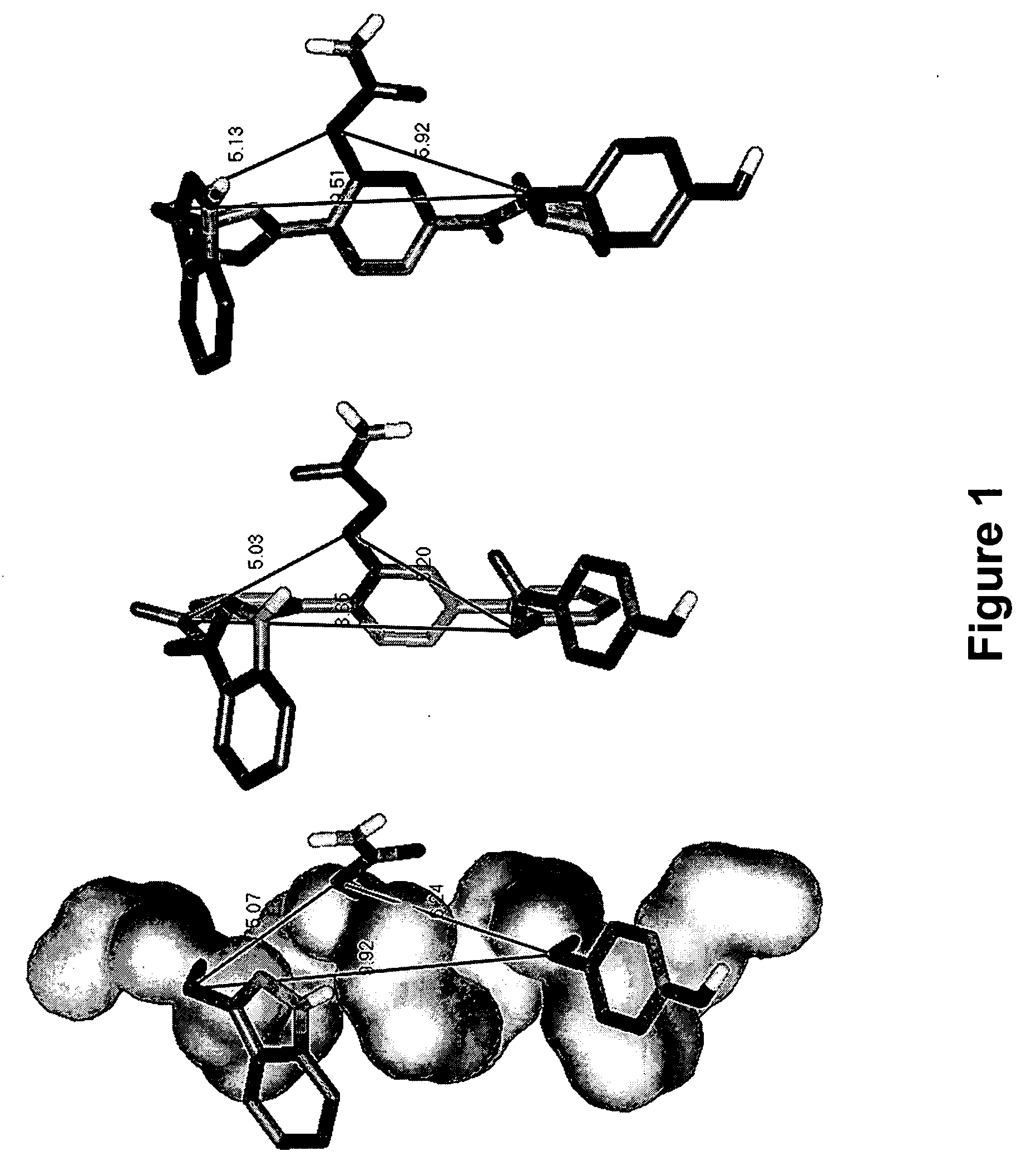
Novel scaffolds for beta-helix mimicry
Functionalized pyridazine derivatives having a low molecular weight and pharmaceutical compositions thereof are useful as alpha-helix mimetics and for treating conditions and / or disorders mediated by alpha-helix-binding receptors and proteins.
Owner:THE SCRIPPS RES INST
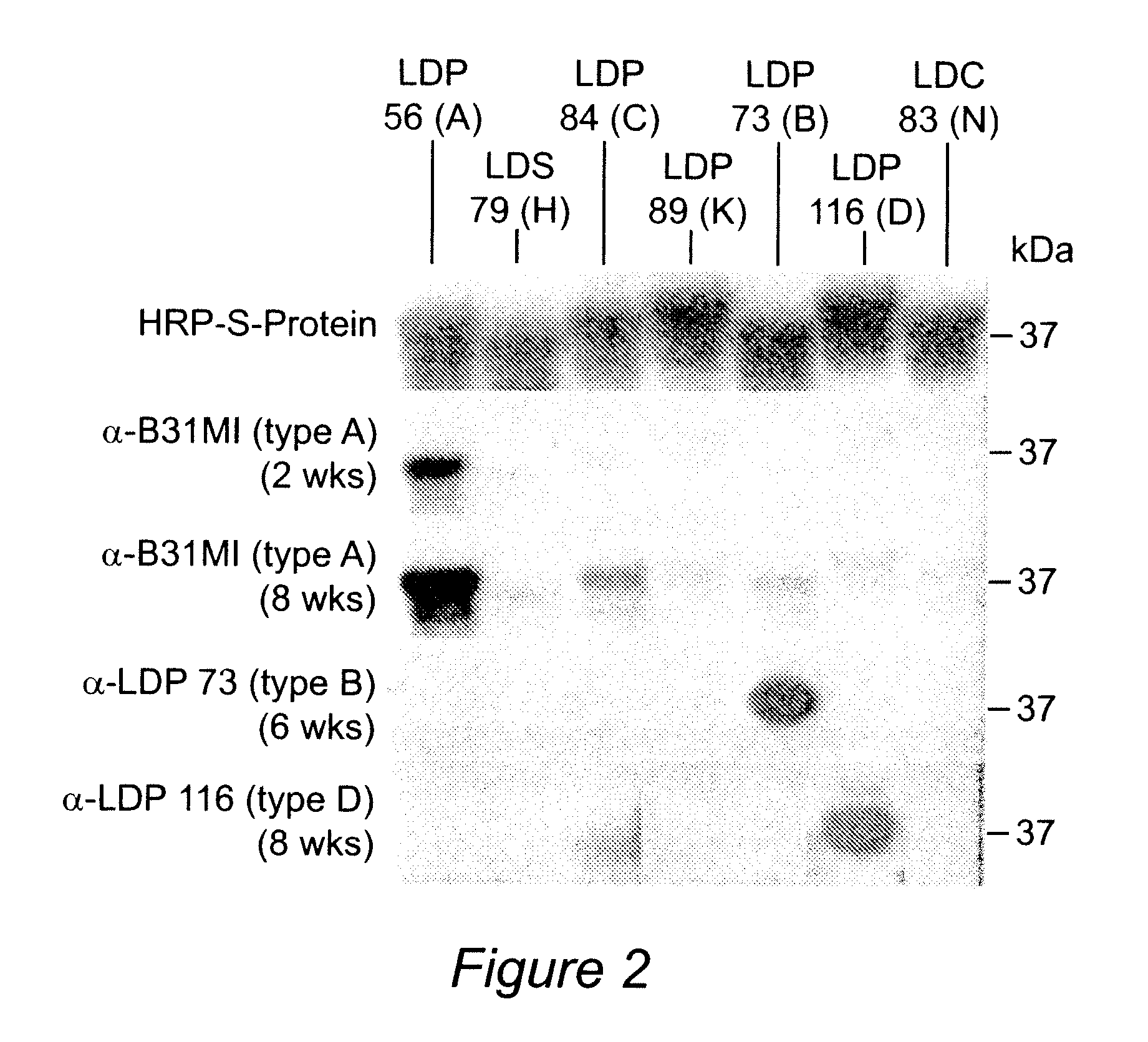
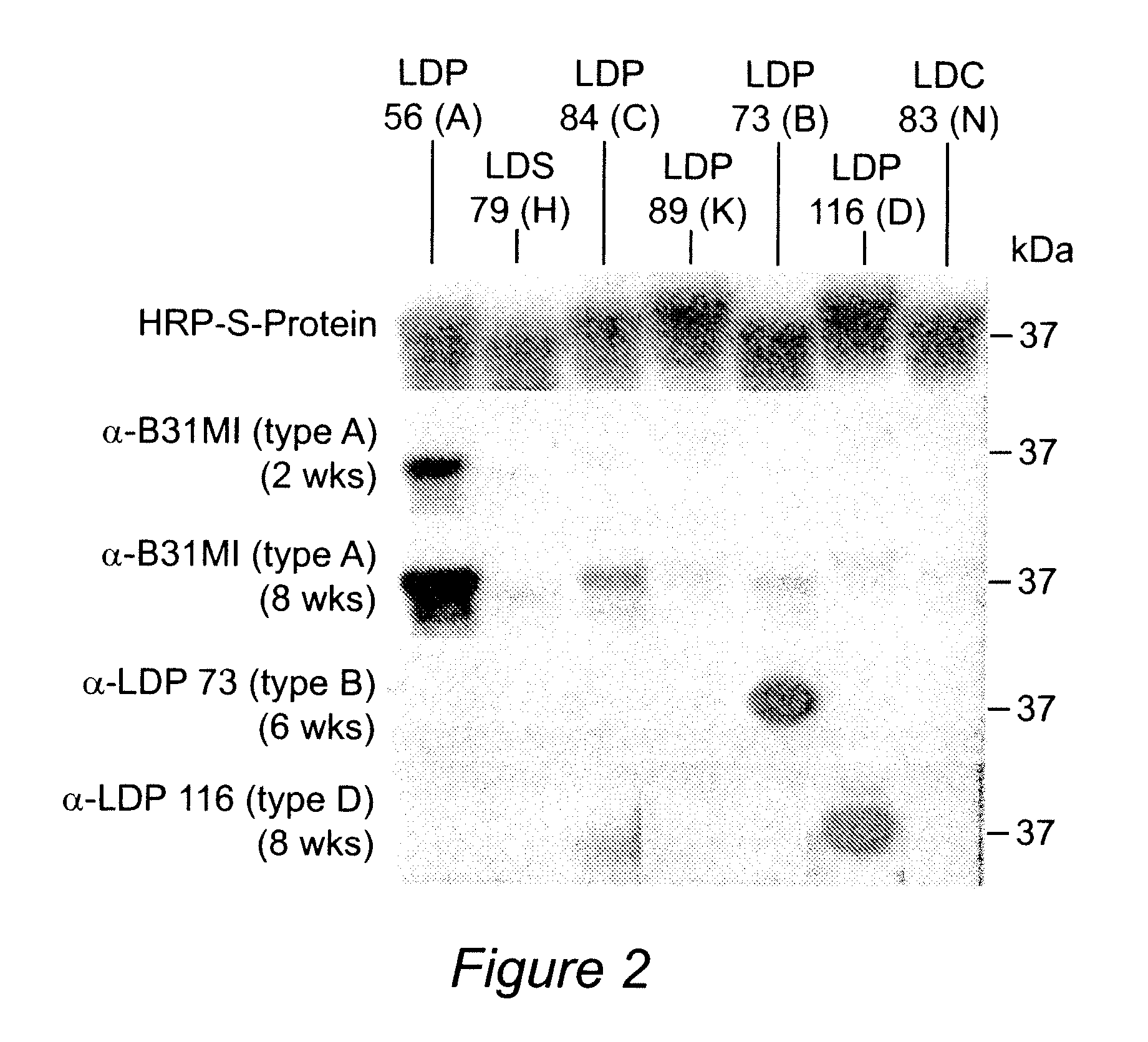
Polyvalent Chimeric OspC Vaccinogen and Diagnostic Antigen
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
PH (Potential of Hydrogen) response antibacterial peptide as well as preparation method and application thereof
ActiveCN109232719AMaintain biological activityGood biocompatibilityAntibacterial agentsPeptide/protein ingredientsAlpha helixBiocompatibility Testing
The invention discloses pH (Potential of Hydrogen) response antibacterial peptide as well as a preparation method and application thereof. The invention firstly provides the antibacterial peptide witha simple amino acid sequence and the amino acid sequence only comprises hydrophilic lysine and hydrophobic leucine, but has key factors with an antibacterial effect of natural antibacterial peptide:positive charge, a hydrophobic domain, amphipathy, alpha helix secondary conformation and the like; the end part of the antibacterial peptide is simply modified and the bioactivity of the antibacterial peptide can be maintained; and meanwhile, the biocompatibility is improved. According to the pH response antibacterial peptide provided by the invention, an acid response molecule is modified on anantibacterial polypeptide chain, and the polypeptide can have a sterilization effect in a slightly-acidic environment of a bacterial infection part and also has the effect of reducing the toxicity ofthe antibacterial peptide on mammalian cells in a physiological environment.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Polypeptide nucleic acid vector, preparation method and uses thereof
ActiveCN105463002AHigh transfection efficiencySmall toxicityGenetic material ingredientsPharmaceutical non-active ingredientsAlpha helixCytotoxicity
The present invention relates to a nucleic acid vector, particularly to a peptide nucleic acid vector containing a cell-penetrating peptide and an antibacterial peptide having an alpha-helix amphiphilic structure. The present invention further relates to a complex containing the nucleic acid vector and nucleic acid molecules. The present invention further relates to preparation methods and uses of the nucleic acid vector and the nucleic acid vector / nucleic acid molecule complex. According to the present invention, the nucleic acid vector has characteristics of high transfection efficiency, simple preparation method, low cytotoxicity and the like, and provides effective treatment way for gene therapy.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
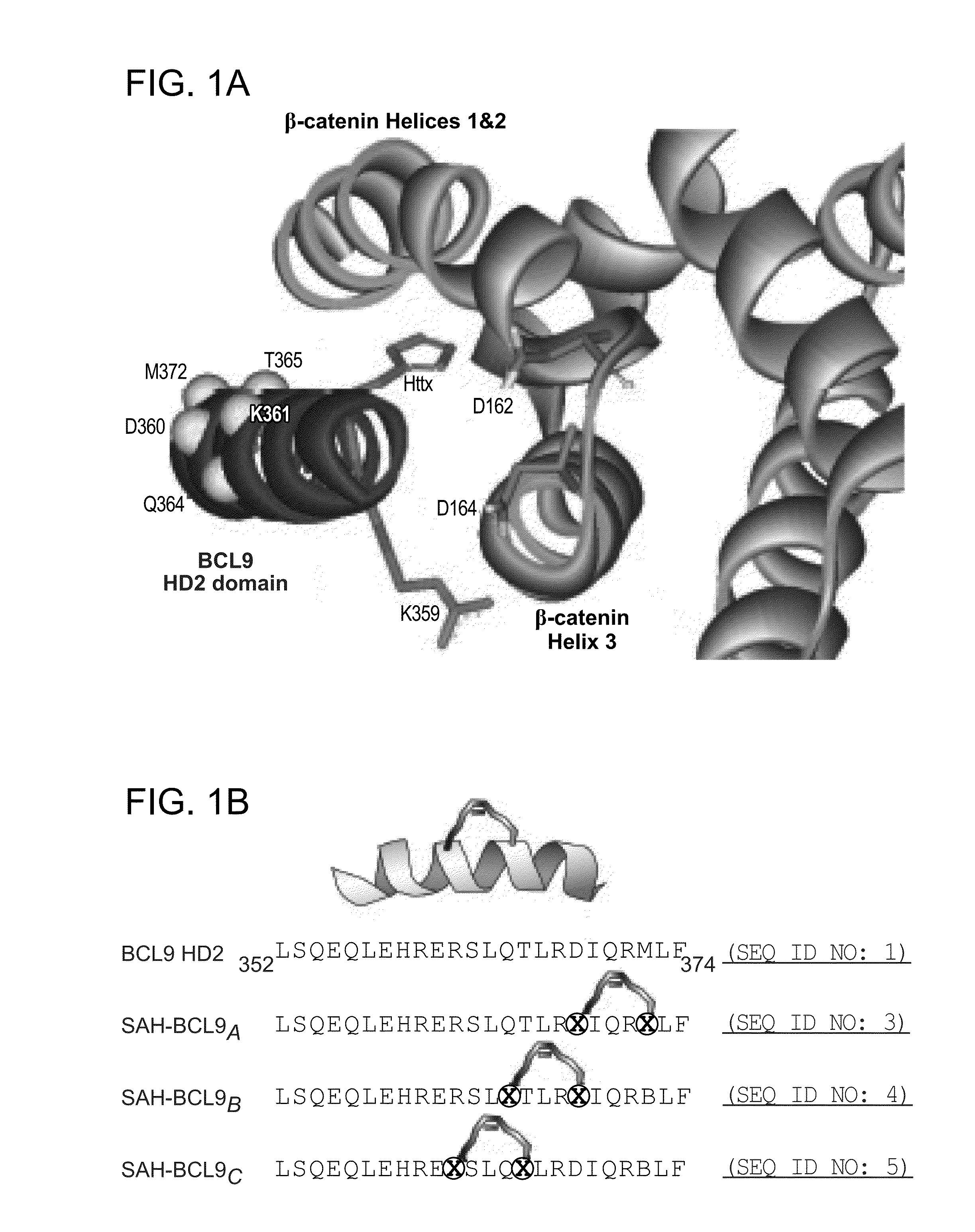
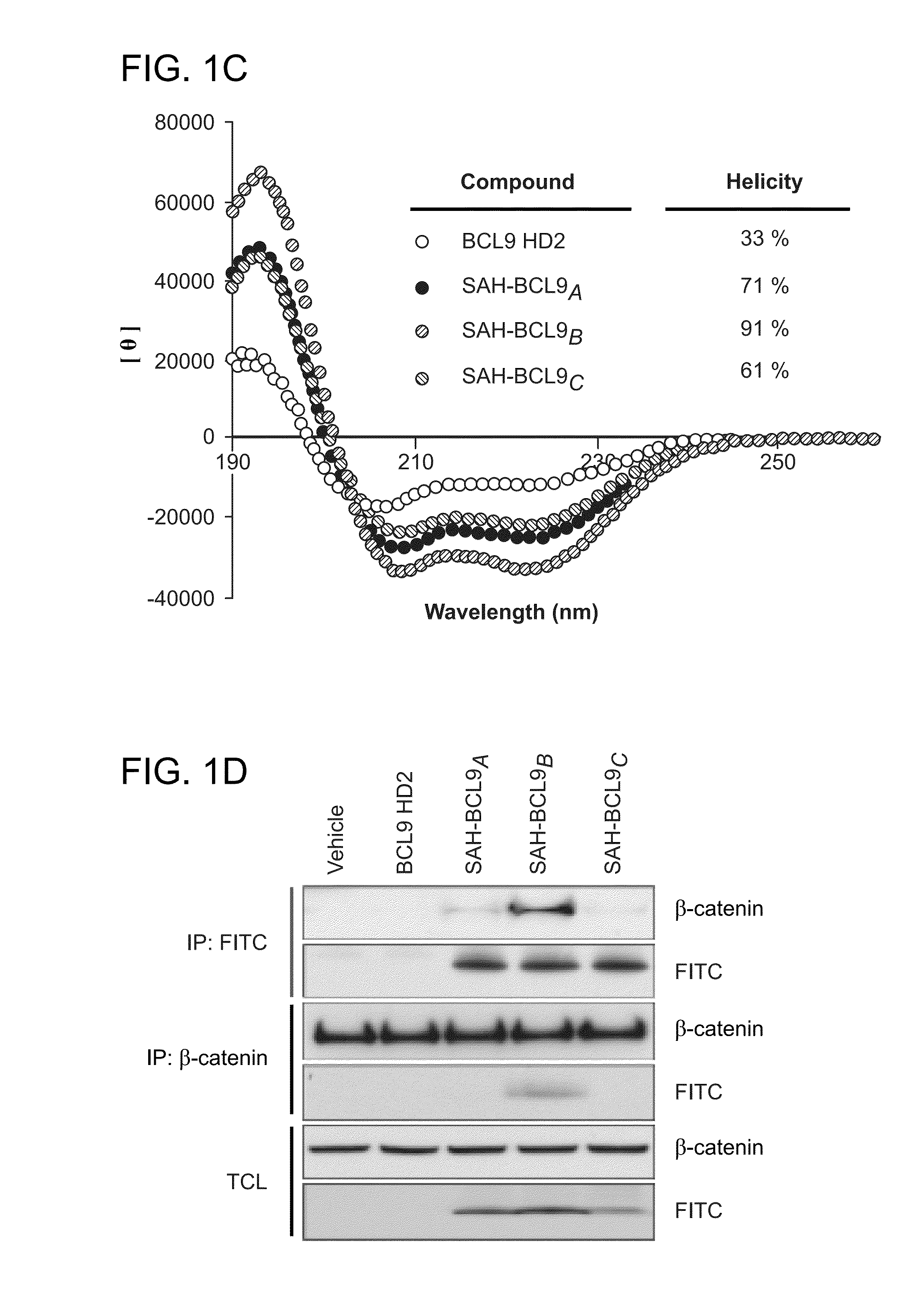
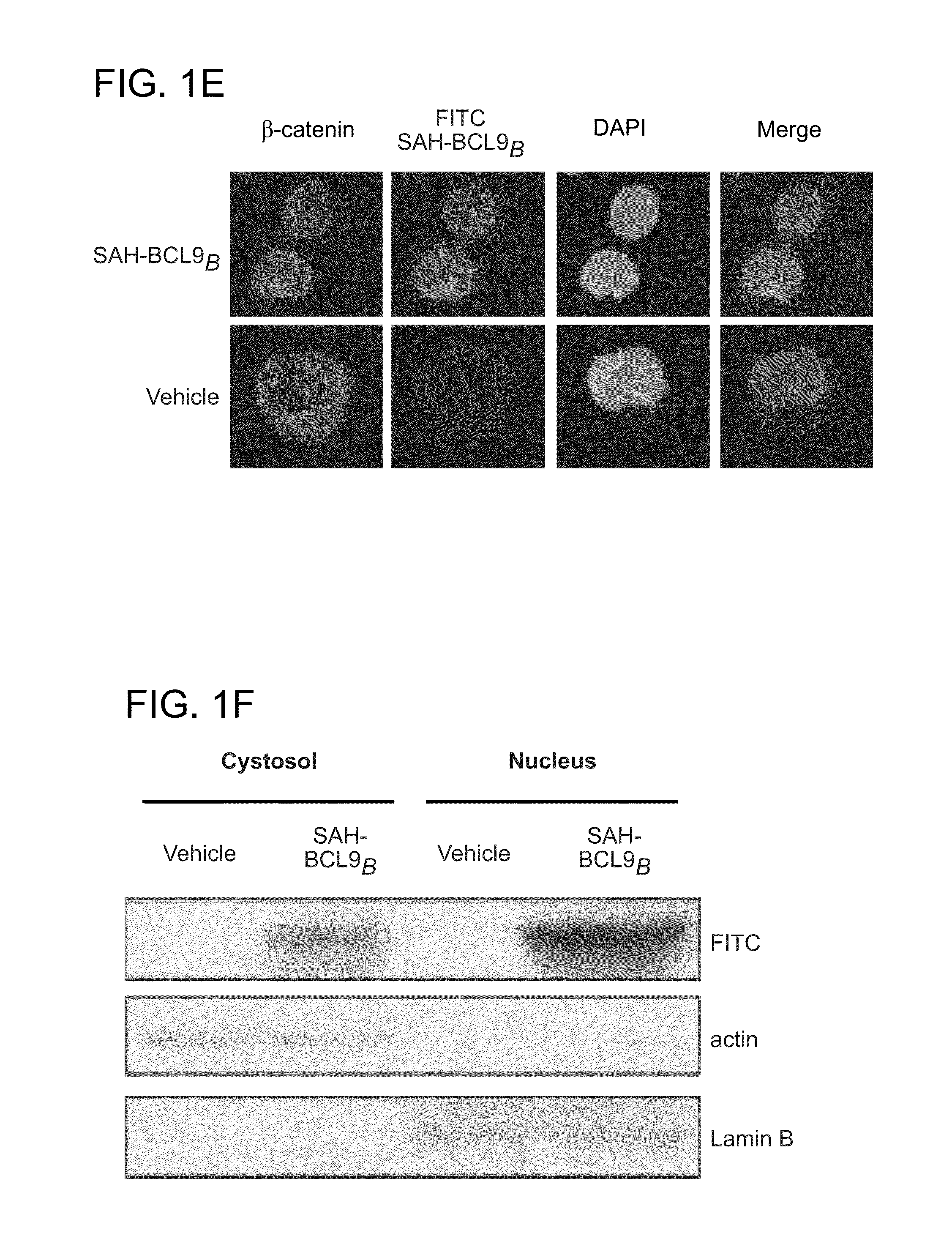
Targeting deregulated wnt signaling in cancer using stabilized alpha-helices of bcl-9
ActiveUS20140113857A1Improved pharmacokinetic propertiesGood acid stabilityPeptide/protein ingredientsImmunoglobulinsHuman cancerΒ catenin signaling
The invention provides structurally-constrained peptides by hydrocarbon stapling of a BCL9 HD2 helix for use as a therapeutic agent. The invention further provides methods and kits for use of the structurally-constrained peptide of the instant invention. The invention is based, at least in part, on the results provided herein demonstrating that hydrocarbon stapled helical peptides display excellent proteolytic, acid, and thermal stability, restore the native helical structure of the peptide, possess superior pharmacokinetic properties compared to the corresponding unmodified peptides, and are highly effective in binding to β-catenin in vitro, in cellulo, and in vivo, disrupting the BCL-9 / β-catenin interaction, and thereby interfering with deregulated Wnt / β-catenin signaling for therapeutic benefit in a variety of human diseases including human cancer.
Owner:DANA FARBER CANCER INST INC
Affinity chromatography matrix
ActiveUS8859726B2Increase capacityGuaranteed cost-effective operationPeptide/protein ingredientsSerum immunoglobulinsAlpha helixAntibody Affinity Chromatography
The present invention relates to a method of separating one or more immunoglobulin containing proteins from a liquid. The method includes first contacting the liquid with a separation matrix comprising ligands immobilised to a support; allowing the immunoglobulin containing proteins to adsorb to the matrix by interaction with the ligands; followed by an optional step of washing the adsorbed immunoglobulin containing proteins; and recovering said immunoglobulin containing proteins by contacting the matrix with an eluent which releases the proteins. The method improves upon previous separation methods in that each of the ligands comprises one or more of a protein A domain (E, D, A, B, C), or protein Z, or a functional variant thereof, with at least one of the monomers having a substitution of the C-terminal most proline residue after the third alpha-helix.
Owner:CYTIVA BIOPROCESS R&D AB
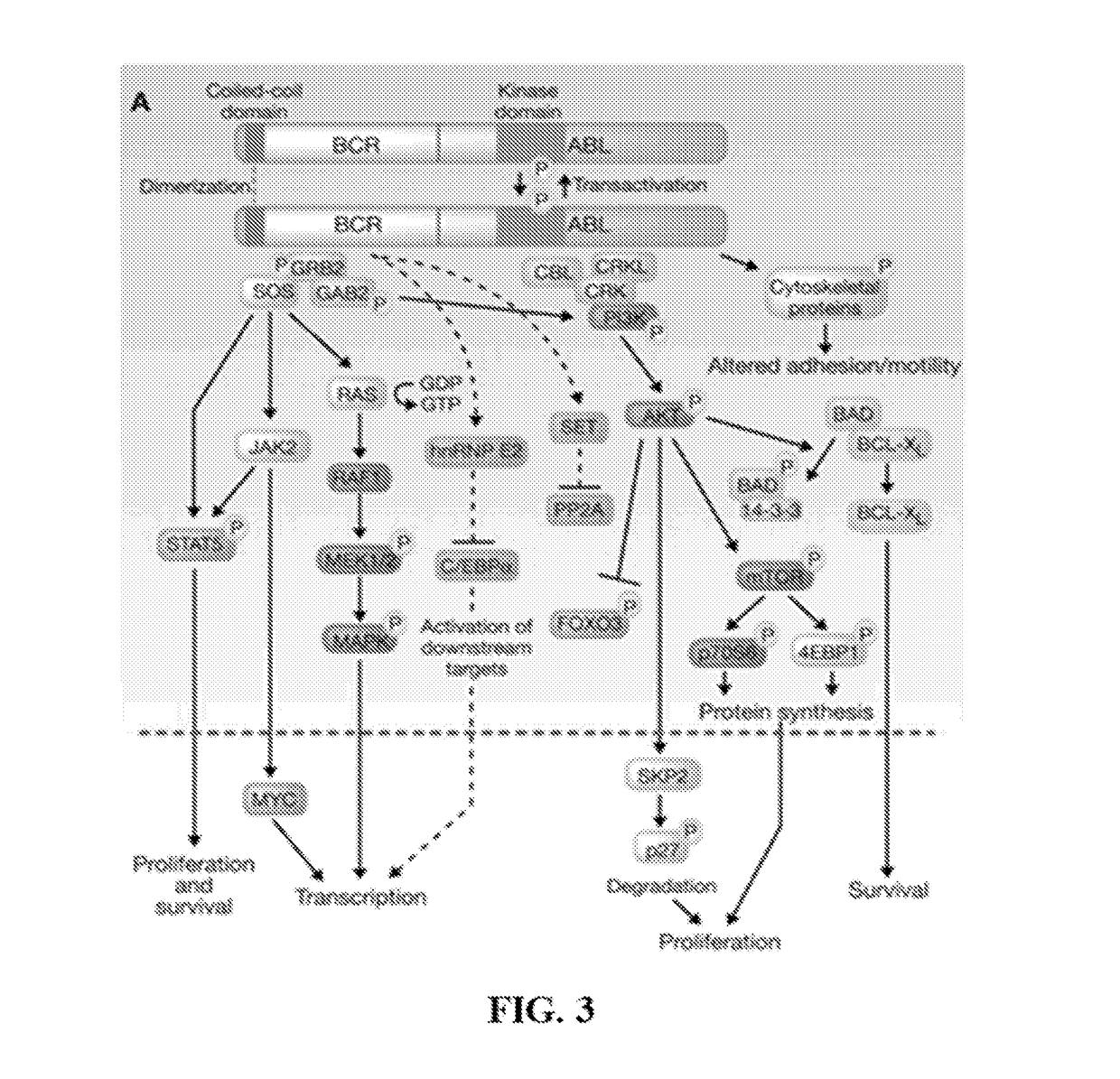
Peptide Inhibitors of BCR-ABL Oligomerization
In one aspect, the invention relates to peptides comprising the Bcr-Abl coiled-coil oligomerization domain and an alpha helix stabilizing moiety, mutant forms thereof, truncated forms thereof, derivatives thereof, and related peptides, which are useful as inhibitors of the Bcr-Abl chimeric protein; pharmaceutical compositions comprising the compounds; and methods of treating hyperproliferative disorders associated with Bcr-Abl using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:UNIV OF UTAH RES FOUND
Polypeptide and nanometer particles thereof for promoting dendritic cells to take in antigen peptides and applications thereof
ActiveCN103910802AUniform particle sizeGood dispersionPharmaceutical non-active ingredientsAntibody medical ingredientsTreatment effectEpidermal Dendritic Cells
The invention discloses a polypeptide and nanometer particles thereof for carrying antigen peptides, preparation methods and applications thereof, and belongs to the field of biological science and drug carriers. The polypeptide is formed by series connection of an alpha-helix polypeptide, a connecting sequence and an antigen peptide in a covalent bond manner, wherein the amino acid sequence of the alpha-helix polypeptide is FAEKFKEAVKDYFAKFWD, the amino acid sequence of the connecting sequence is GSG, and the connecting sequence of the antigen peptide is KVPRNQDWL. The polypeptide and the nanometer particles prepared from the polypeptides are capable of effectively improving the tumor antigen peptide uptake efficiency of the dendritic cells and promoting the prevention and treatment effect of tumors after the DC (dendritic cell) takes in the antigen peptides.
Owner:HUAZHONG UNIV OF SCI & TECH
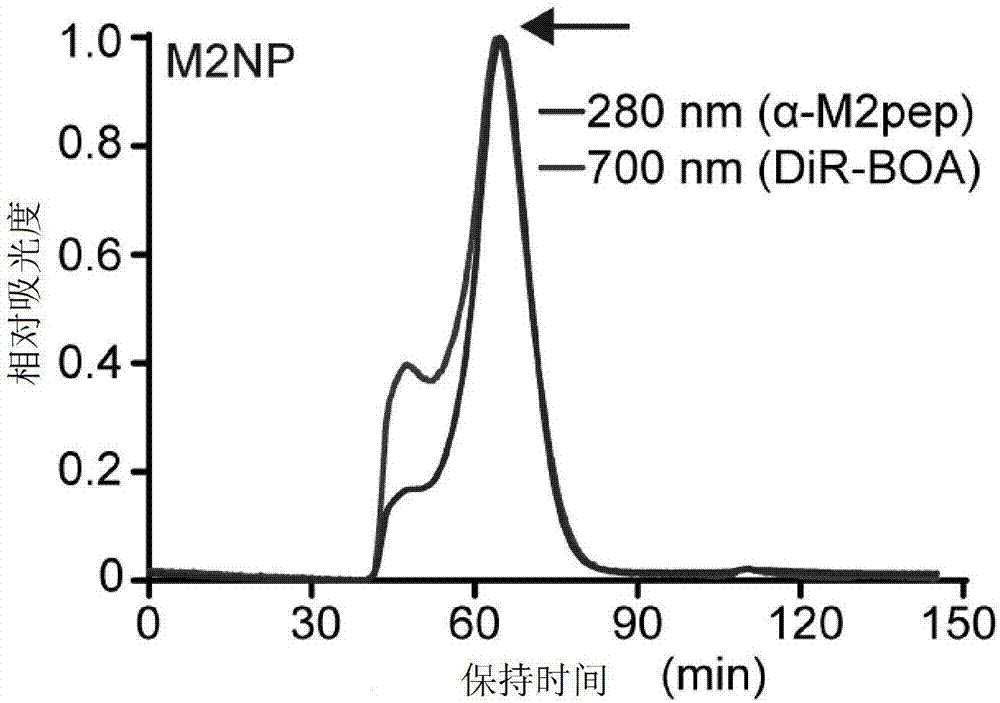
Double-target polypeptide of tumor-associated macrophage, nano particle, preparation and application
ActiveCN107573418AGrowth inhibitionGood physical and chemical propertiesMacromolecular non-active ingredientsHybrid peptidesSide effectAlpha helix
The invention discloses double-target polypeptide of a tumor-associated macrophage, a nano particle, preparation and an application and relates to the technical field of bioscience and drug carriers.The double-target polypeptide is prepared by series connection of alpha helix polypeptide, a catenation sequence and M2 macrophage target peptide in a covalent bond form. The nano particle prepared bythe double-target polypeptide can perform efficient targeted transportation of drugs to the tumor-associated macrophage, so that the tumor-associated macrophage is specifically eliminated and tumor growth is inhibited; a preparation technology of the nano particle targeting to the tumor-associated macrophage is simple; large-scale production is facilitated; most raw materials for preparing the nano particle are used clinically or used in clinical experiments; and a detection result shows that no toxic or side effects are caused on physiologic and biochemical indexes of a mouse.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Alpha-helix mimetics and methods relating to the treatment of fibrotic disorders
The invention provides α-mimetic structures and a chemical library relating thereto. Additionally, the invention provides methods wherein α-mimetic compounds are used to treat fibrotic disorders.
Owner:INST FOR CHEM GENOMICS
Amphiphilic alpha helix self-assembling peptide and application thereof
The invention relates to an amphiphilic alpha helix self-assembling peptide derived from SEQ ID NO:1 and an application of the amphiphilic alpha helix self-assembling peptide in protein production and purification. When the amphiphilic alpha helix self-assembling peptide and target protein are subjected to fusion expression, the amphiphilic alpha helix self-assembling peptide can induce the fusion protein to form active aggregate in cell, by comparing with polypeptide in SEQ ID NO: 1, the spatial distribution of the formed active aggregate in the cell is changed, a host cell growth state is improved, and fusion protein output is increased.
Owner:TSINGHUA UNIV
Modified avian pancreatic polypeptide miniature binding proteins
The present invention provides a protein scaffold, such as an avian pancreatic polypeptide, that can be modified by substitution of two or more amino acid residues that are exposed on the alpha helix domain of the polypeptide when the polypeptide is in a tertiary form.
Owner:YALE UNIV
Polymer solid electrolyte material, solid electrolyte membrane and method for preparing same
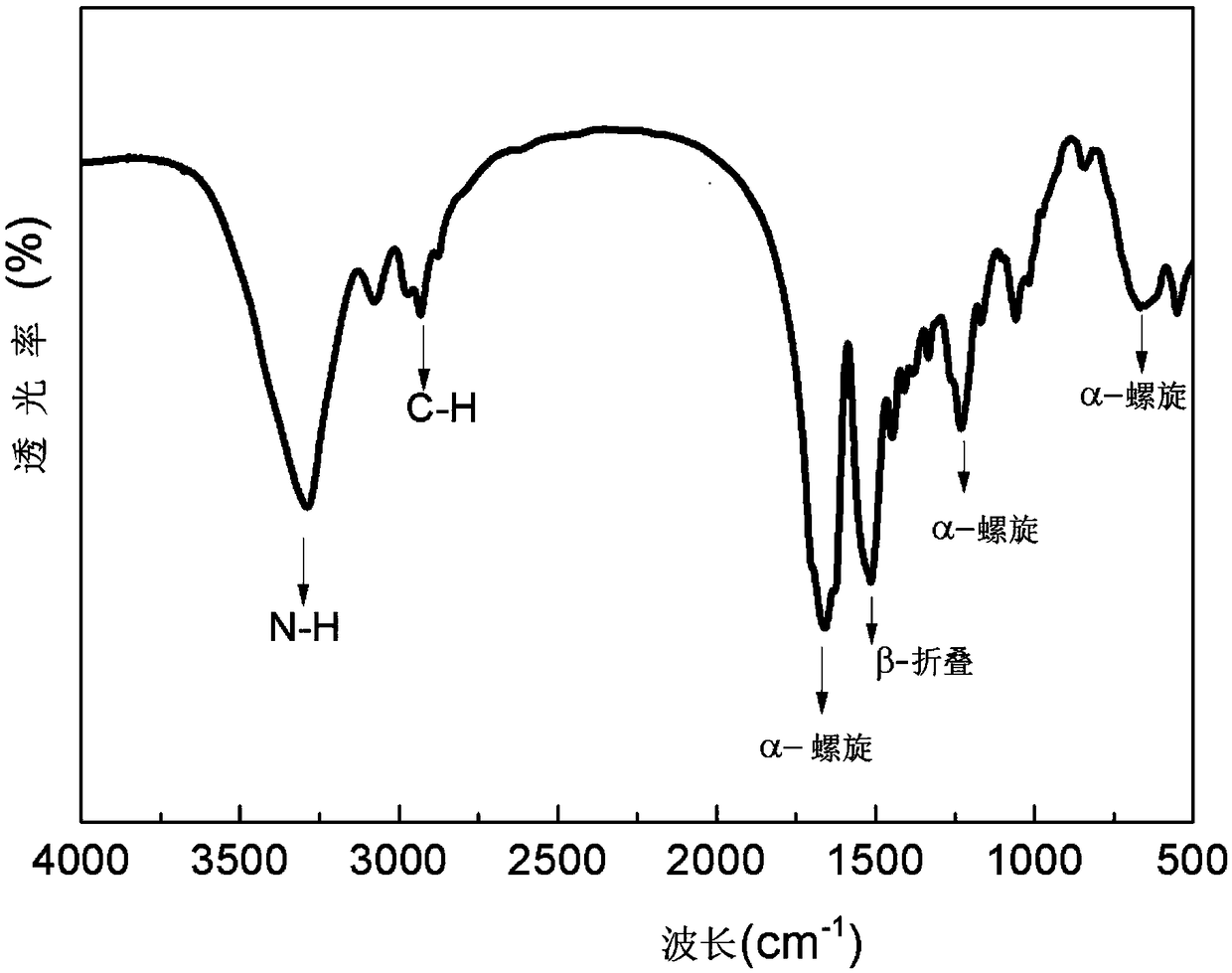
ActiveCN108417890AIncrease room temperature ionic conductivity valuesImprove high temperature resistanceSecondary cellsSolid state electrolyteAlpha helix
The invention discloses a polymer solid electrolyte material, a solid electrolyte membrane and a method for preparing the same. The polymer solid electrolyte material comprises, by weight, 1-20 wt% oflithium salt, 3-60 wt% of polymer matrix materials and 20-96 wt% of silk fibroin. The polymer solid electrolyte material, the solid electrolyte membrane and the method have the advantages that preparation processes are simple, the polymer solid electrolyte material, the solid electrolyte membrane and the method are low in cost, the silk fibroin and the polymer matrix materials contain large quantities of oxygen-containing functional groups and are beneficial to quickly conducting lithium ions, and accordingly the room-temperature ion conductivity of the polymer solid electrolyte material canbe improved; the high-temperature resistance and the mechanical strength of the polymer solid electrolyte material can be enhanced by alpha-helix and beta-pleated sheet structures in the silk fibroin,growth of lithium dendrites can be inhibited, and the polymer solid electrolyte material and the solid electrolyte membrane can be prevented from being pierced by the lithium dendrites.
Owner:ETRUST POWER ETP GRP LTD
Polyvalent chimeric OspC vaccinogen and diagnostic antigen
InactiveUS7794727B2Broad protectionAntibacterial agentsBacterial antigen ingredientsEpitopeAlpha helix
A chimeric polyvalent recombinant protein for use as a vaccine and diagnostic for Lyme disease is provided. The chimeric protein comprises epitopes of the loop 5 region and / or the alpha helix 5 region of outer surface protein C (OspC) types. The OspC types may be associated with mammalian Borrelia infections.
Owner:VIRGINIA COMMONWEALTH UNIV
Novel synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome
InactiveUS20040049011A1Prevent septic shockPeptide/protein ingredientsPeptide preparation methodsAlpha helixSaxitoxin
A peptide with an amino acid composition such that the peptide is amphipathic, cationic and forms a stable alpha-helix and has the following structure comprising at least 12 amino acids R1-R2-A1-B1-(A2-B2-C1-A3)m-(C2)n-R3, wherein A=an amino acid selected from the basic amino acids Lys,Arg or His B=an amino acid selected from the aromatic amino acids Phe, Trp or Tyr C=an amino acid selected from the group comprising the hydrophobic amino acids Leu, Ile, Val or Ala, and said peptide has either the orientation according to the formula or the retro orientation thereof, wherein at least 0-n of the repetitive sequence motifs (A2-B2-C1-A3) have the retro orientation and the remaining repetitive motifs (A2-B2-C1-A3) have the orientation as presented in the formula and wherein, R1-R2- and R3 are a number of amino acids, and wherein m=1-10, preferably 2-8, more preferably 2-5 and n=1-3, a pharmaceutical composition comprising such a peptide application thereof in treatment or diagnosis related to i.a. parasite infection topical and systemic tumors and septic shock.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Efficient alpha-helix antibacterial peptide GV and preparation method and application thereof
InactiveCN106366162ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsChemical synthesisAlpha helix
The invention relates to an efficient alpha-helix antibacterial peptide GV and a preparation method and application thereof. The antibacterial peptide GV is an antibacterial peptide with high cell selectivity and achieves an optimal point of junction of the antibacterial activity and cell toxicity of the antibacterial peptide. A sequence of the antibacterial peptide GV is represented by a sequence table SEQ ID No. 1. The preparation method comprises the following steps: (1) carrying out designing in accordance with alpha-helix peptide GRX2RX3RX2RG serves as a template, so as to obtain a brand-new antibacterial peptide GV, wherein X= V; (2) synthesizing a polypeptide crude product through a polypeptide synthesizer by a solid-phase chemical synthesis method; purifying the synthesized polypeptide by using out-phase high-performance liquid chromatography, and carrying out identification on the synthesized polypeptide by using electrospray mass spectrography, thereby preparing the polypeptide. The antibacterial peptide GV provided by the invention has relatively high bacteriostatic activity and relatively low hemolytic activity and is the highest in therapeutic index, thereby having great development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Polypeptide, lipoprotein-like nano particle and application thereof
ActiveCN106831997AAchieve multi-functionalityStable formationApolipeptidesAntibody mimetics/scaffoldsCancer cellAlpha helix
The invention discloses a polypeptide, a lipoprotein-like nano particle (LNP) and application thereof. The LNP contains at least one phospholipid and at least one polypeptide. A polypeptide series helix-ring-helix structure polypeptide contains at least two amphiphilic alpha helixes, and the two amphiphilic alpha helixes are connected through at least one connecting peptide containing a cyclic structure. The helix part of the polypeptide is imbedded into a lipoprotein-like nano particle surface layer, stable formation of lipid nano particles with uniform size and relatively small particle diameters can be promoted, meanwhile cyclic sequences stretch out of the surfaces of the lipoprotein-like nano particles, and functional sequence insertion is further facilitated, so that multi-functionalization of the LNP is realized. Moreover, the application of the LNP can be further expanded by using an LNP packaged lyophobically functional substance, and for example, the LNP can be used as a drug delivery system; the drug delivery system has the characteristics of being low in toxicity and high in stability, facilitating functionalization and the like, and a simple, convenient and effective means is provided for target killing of cancer cells and cell and tissue imaging.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Designer bioactive proteins
InactiveUS20150337294A1Increase productionSimple compositionSugar derivativesOther foreign material introduction processesAlpha helixProtein methods
Methods and compositions are provided for binding a target molecule comprising expressing a recombinant binding protein, such as an anticalin, an OB-domain protein, or an alpha-helix coiled-coil forming polypeptide in a plant. Methods for producing such a recombinant binding protein that binds to a target molecule are also provided. Further provided are plant lipocalin, OB-domain, and alpha-helix coiled-coil forming polypeptide coding sequences and recombinant polypeptides derived therefrom.
Owner:MONSANTO TECH LLC
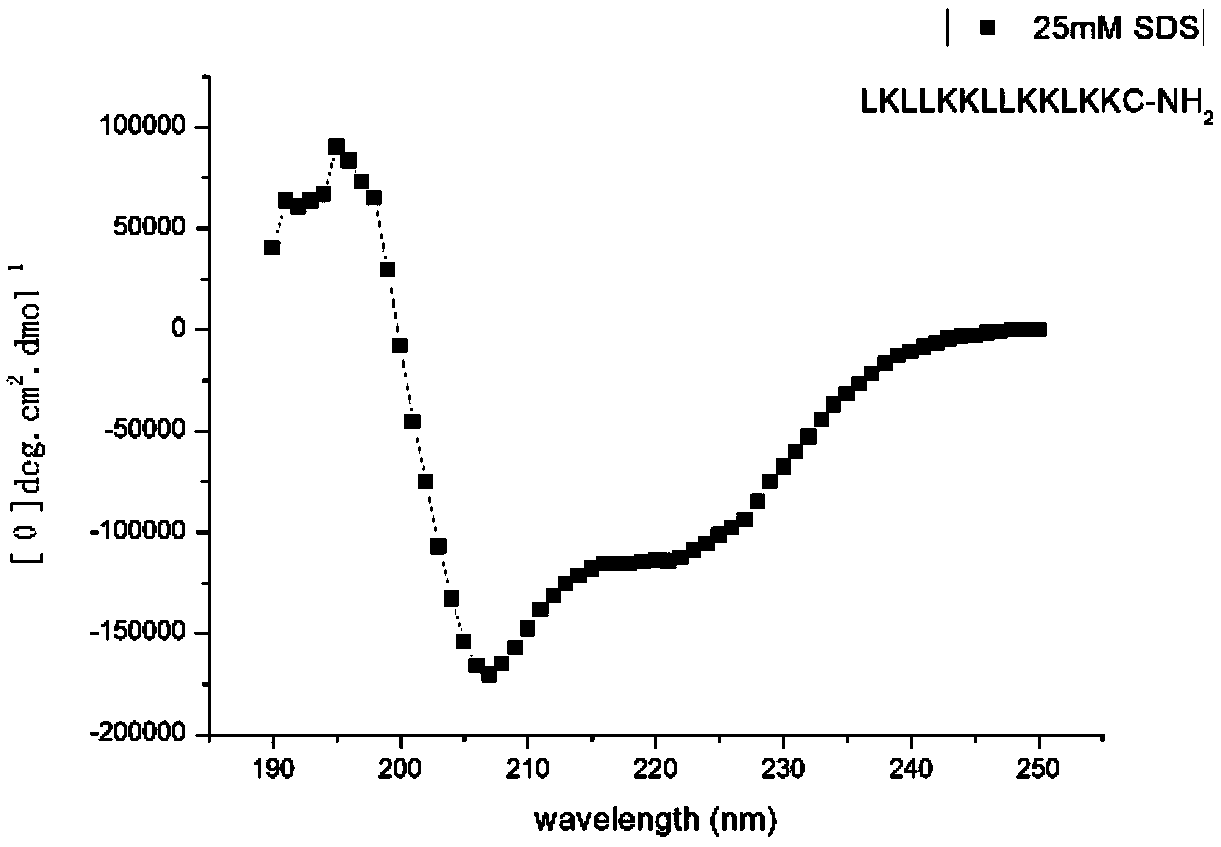
Alpha helix cationic polypeptide as well as preparation method and application of alpha helix cationic polypeptide
ActiveCN108003343AEndocytosis into goodExcellent membrane penetrating activityPowder deliveryEnergy modified materialsDiseaseCancer cell
The invention provides an alpha helix cationic polypeptide as well as a preparation method and application of the alpha helix cationic polypeptide. The alpha helix cationic polypeptide takes a photosensitizer porphyrin as a core and alpha helix polypeptides as four arms, and a nucleotide drug prepared from the polymer can be applied to a nucleotide drug delivery system. The polymer provided by theinvention has good biocompatibility, low cytotoxicity and light activation ability. The polymer provided by the invention is self-assembled in a water solution to form nanoparticles which has good stability, biocompatibility and low cytotoxicity; the preparation method is simple and high in repeatability, and the polymer serving as a carrier is not only capable of protecting nucleic acid such asDNA from being degraded, but also capable of being combined with the scale effect of the nanoparticles so as to be used for treating diseases; and in addition, the transfection promoting effect for different cancer cells can be achieved by virtue of the light activation ability.
Owner:SUZHOU UNIV
Alpha helix cell-penetrating peptide multimer, preparation method therefor and use thereof
The present invention relates to an alpha helix cell-penetrating peptide multimer, a preparation method therefor and a use thereof and, particularly, to a peptide multimer comprising a plurality of double-sided peptides, a preparation method of the peptide multimer, and a composition comprising the peptide multimer as an effective ingredient for preventing or treating HIV or a composition comprising the peptide multimer and a biological active substance for delivering an active substance into a cell.
Owner:SEOUL NAT UNIV R&DB FOUND
Genetically engineered alpha helix polypeptide
A polypeptide having alpha -helix structure, which has a hydrophilic surface and a hydrophobic surface, and which is produced by genetic engineering techniques is disclosed. According to the present invention, stable polypetide molecules having alpha -helix structure, which have a constant molecular weight and which can be used in various applications represented by materials for medical applications, can be provided.
Owner:MIURA KIN ICHIRO +5
Hydrocarbon stapled stabilized alpha-helices of the HIV-1 GP41 membrane proximal external region
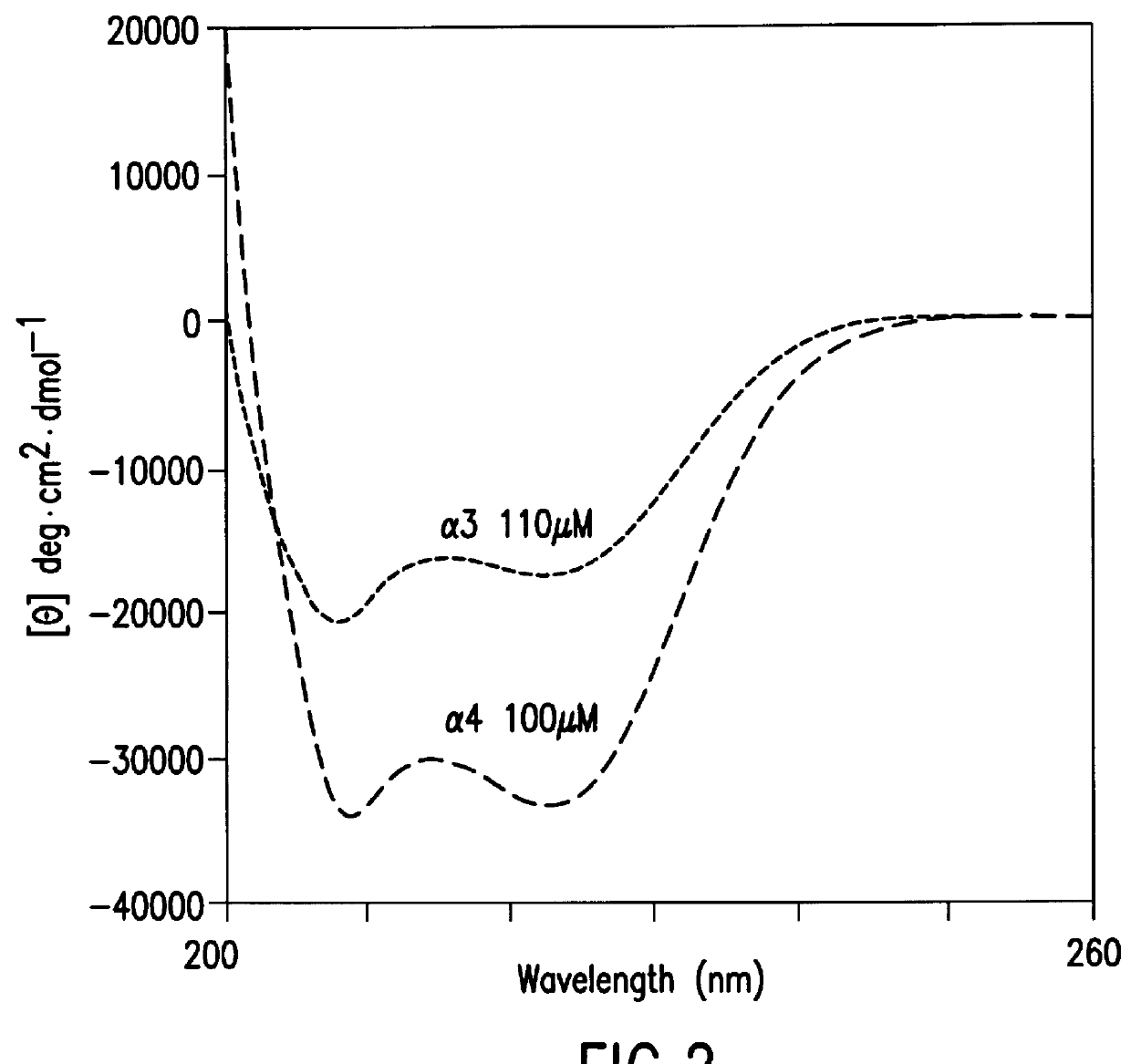
The invention provides structurally constrained viral peptides for use as therapeutic and vaccination agents, and for the production of antibodies for use in a number of applications including as therapeutic agents. The invention further provides methods and kits for use of the structurally constrained peptides and antibodies of the instant invention. The invention is based, at least in part, on the result provided herein demonstrating that viral hydrocarbon stapled helical peptides display excellent proteolytic, acid, and thermal stability, restore the native helical structure of the peptide, are highly effective in interfering with the viral fusogenic process, and possess superior pharmacokinetic properties compared to the corresponding unmodified peptides.
Owner:DANA FARBER CANCER INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com