Alpha helix cationic polypeptide as well as preparation method and application of alpha helix cationic polypeptide
A cationic polymerization and α-helix technology, applied in the field of polymer material technology and pharmacy, can solve the problems of inability to maintain α-helix, poor membrane penetration ability, and poor gene transfection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
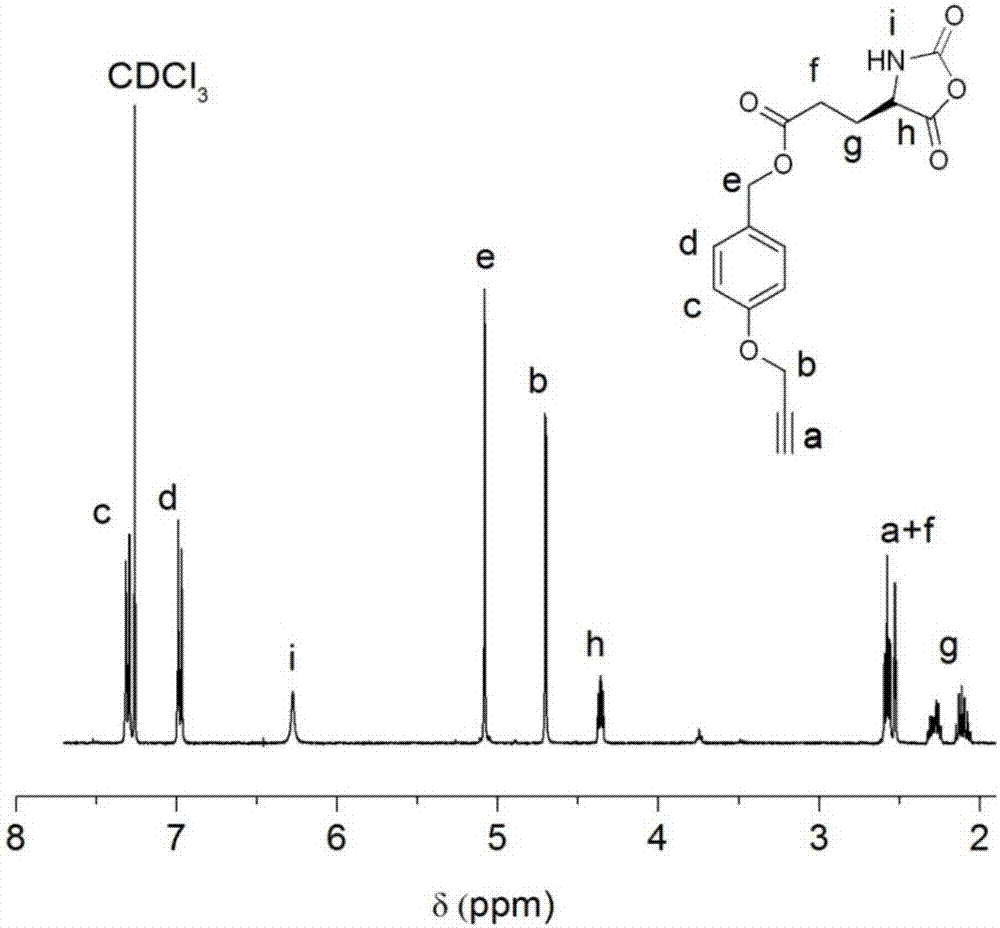
[0076] (1) Potassium carbonate (15.2 g, 0.11 mol) and p-hydroxybenzyl alcohol (9.3 g, 0.075 mol) were dissolved in 150 mL of acetone, and propyne bromide (10 mL, 0.09 mol) and 18-crown-6 (0.1 mL). The solution was refluxed in an oil bath at 75°C, and the acetone was removed by rotary evaporation after 12 h. Add 200 mL of water to the crude product, extract 3 times with 30 mL of dichloromethane (DCM), and remove the lower organic phase. The organic phase was washed with 15% sodium hydroxide (200 mL) and saturated brine (200 mL), and washed with Na 2 SO 4 Dry, filter, and remove the solvent to obtain compound 1;
[0077] (2) Compound 1 (8.5 g, 52 mmol) was dissolved in dichloromethane, and thionyl chloride (5 mL, 68 mmol) was added dropwise in an ice bath. Stir the reaction at room temperature for 3.5 h, add 100 mL of water to quench thionyl chloride, extract the organic phase three times with 50 mL of water, and wash with MgSO 4 Dry, filter, and remove the solvent to obtai...
Embodiment 2
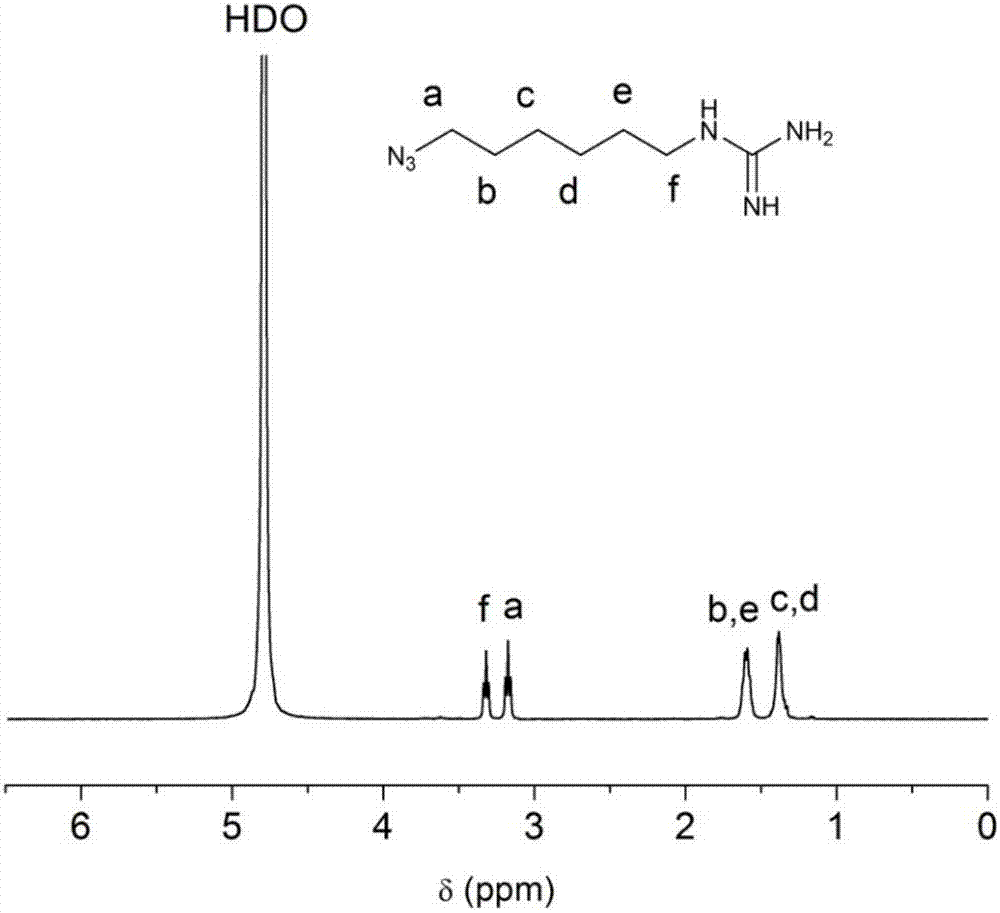
[0081] (1) Add 1,6-dibromohexane (1.26mL, 8 mmol) and sodium azide (1.6g, 24 mmol) into 19 mL DMF and react at 60°C for 24h. (There will be insoluble matter in the reaction) After adding about 150 mL of water to dissolve it, extract it with 20 mL of ether three times, collect the organic phase, dry it with sodium sulfate, filter, and rotary evaporate to obtain compound 5;
[0082] (2) Take compound 5 (3.33 g, 20 mmol), Et2O (15 mL), EtOAc (15 mL), 5% HCl (30 mL, 5 mL concentrated HCl + 25 mL H 2O), slowly add triphenylphosphine (5.51 g, 22 mmol) under ice-bath conditions (0°C) to ensure two-phase separation, ensure that the reaction is longer than 1 h under ice-water bath, and react at room temperature for 24 h; add 30 mL of 1M HCl (2.5 mL concentrated hydrochloric acid + 27.5 mL water), discard the upper organic phase after stratification occurs, and take the lower aqueous phase; extract the aqueous phase with 20 mL DCM for 3 times, collect the lower aqueous phase; then adjus...
Embodiment 3
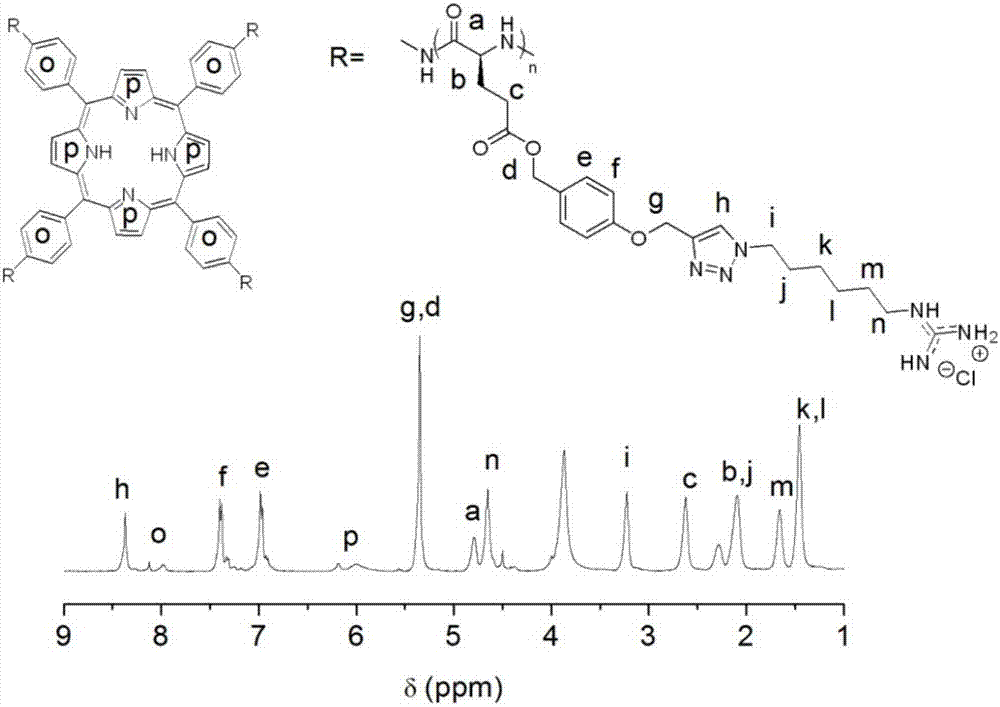
[0085] (1) In the glove box, the five-membered ring N-carboxylic acid anhydride of Example 1 was dissolved in N,N-dimethylformamide, and then 5,10,15,20-tetrakis(4-aminobenzene)-porphyrin was added phenoline (TAPP) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene, and precipitated with ice methanol after stirring at room temperature for 72 h; 5,10,15,20-tetra The molar ratio of (4-aminobenzene)-porphyrin (TAPP) to the five-membered ring N-carboxylic acid anhydride of Example 1 is 1:60;
[0086] (2) In the glove box, the intermediate product (20 mg, 0.072 mmol alkynyl) was dissolved in DMF, and the azide terminal compound (0.144 mmol) and pentamethyldiethylenetriamine (15 μL , 0.072 mmol), then copper bromide (2 mg, 0.0144 mmol) was added, and the reaction was stirred at room temperature in the glove box for 48 hours; after the reaction was completed, it was taken out from the glove box, opened and stirred for 20 min, then added 1 mL of 1M hydrochloric acid, dialyzed with water for 3 d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com