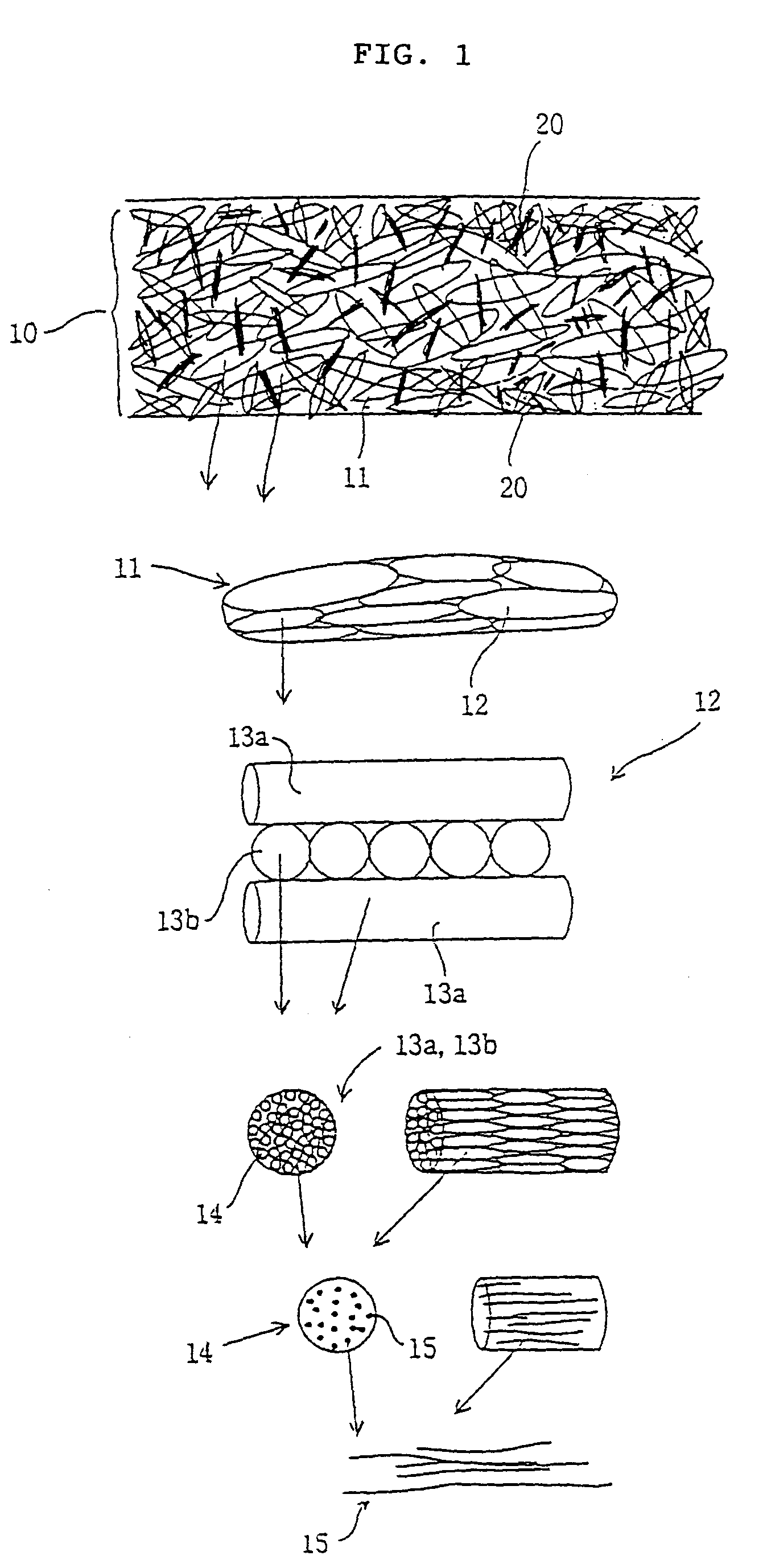
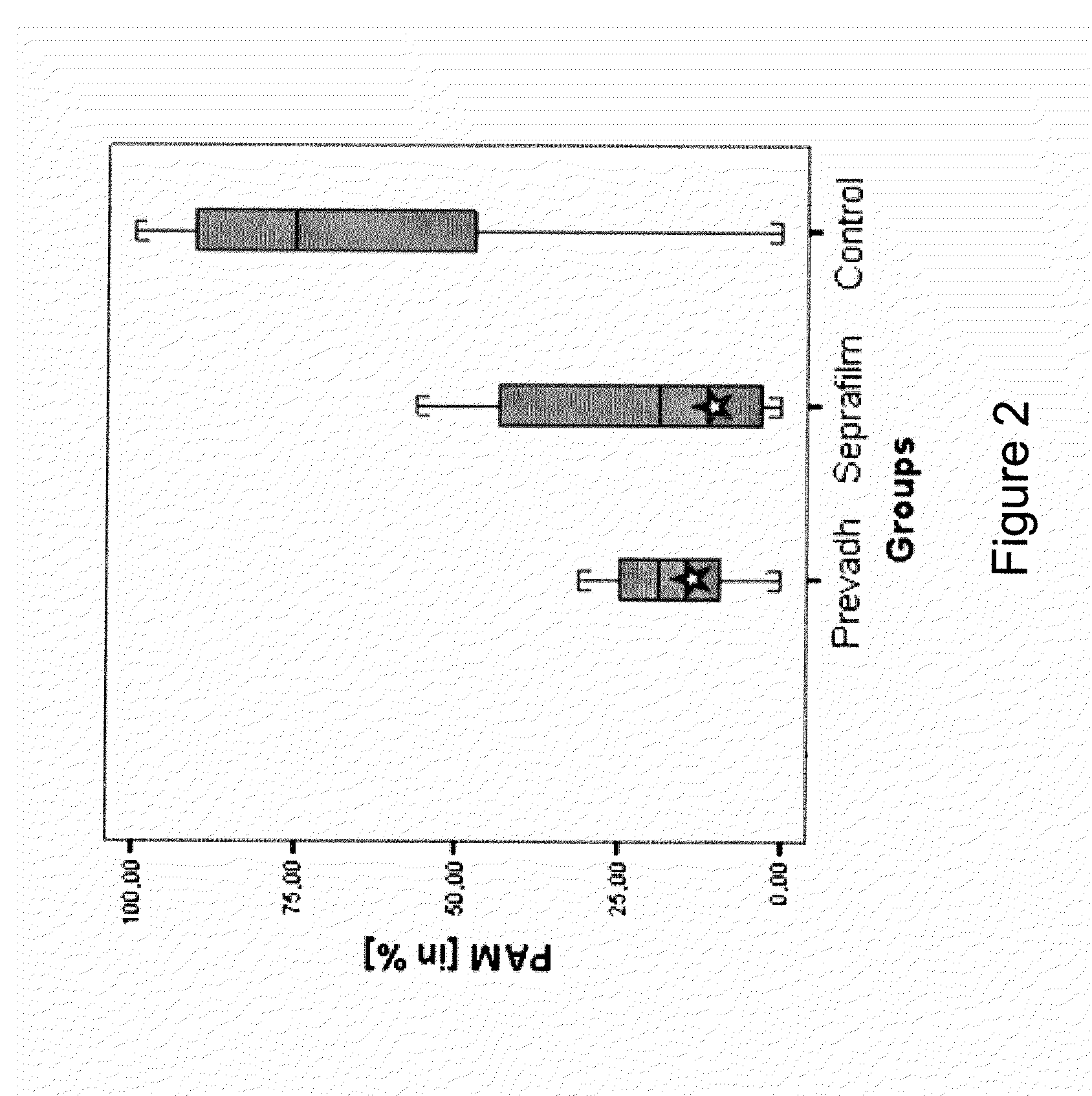
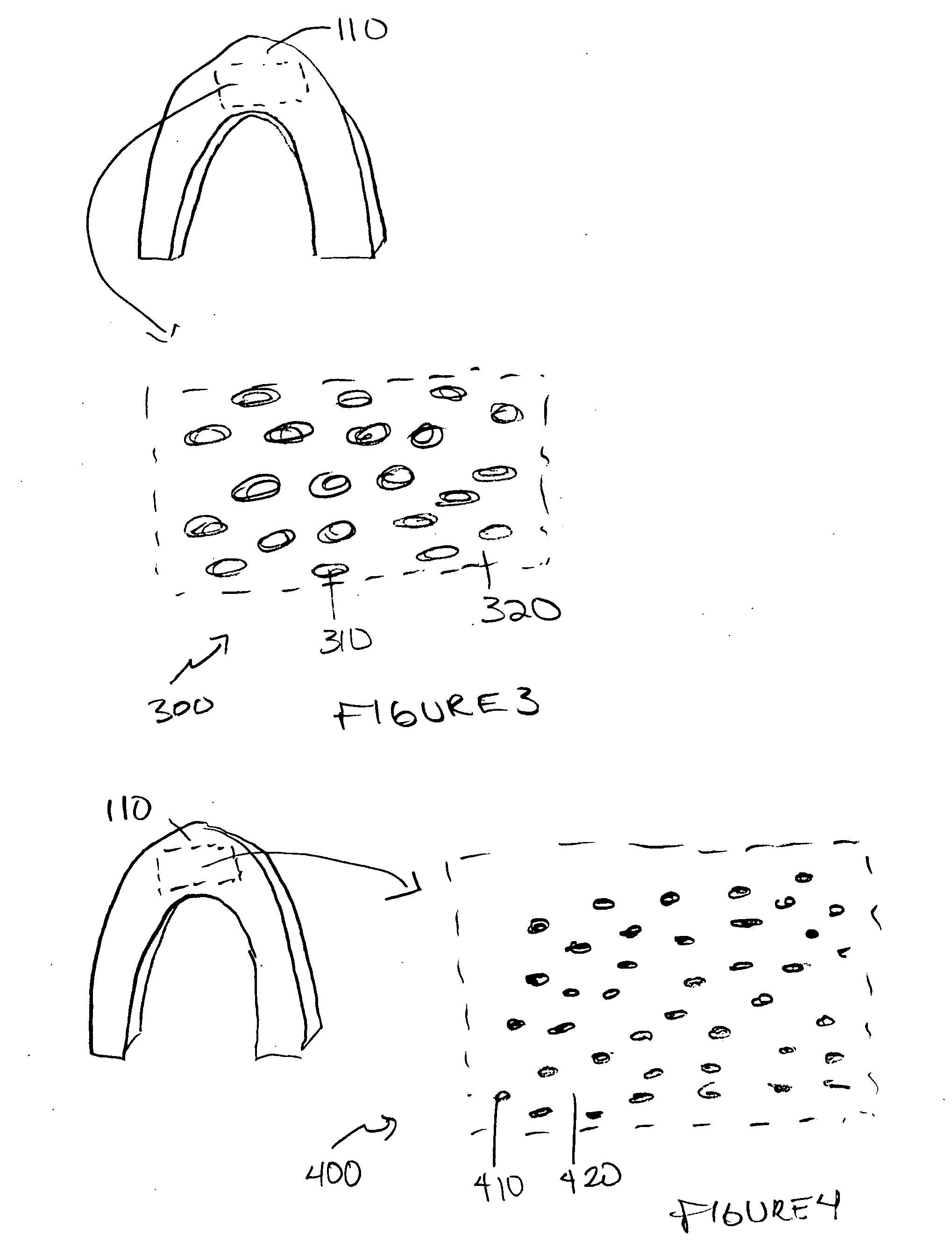
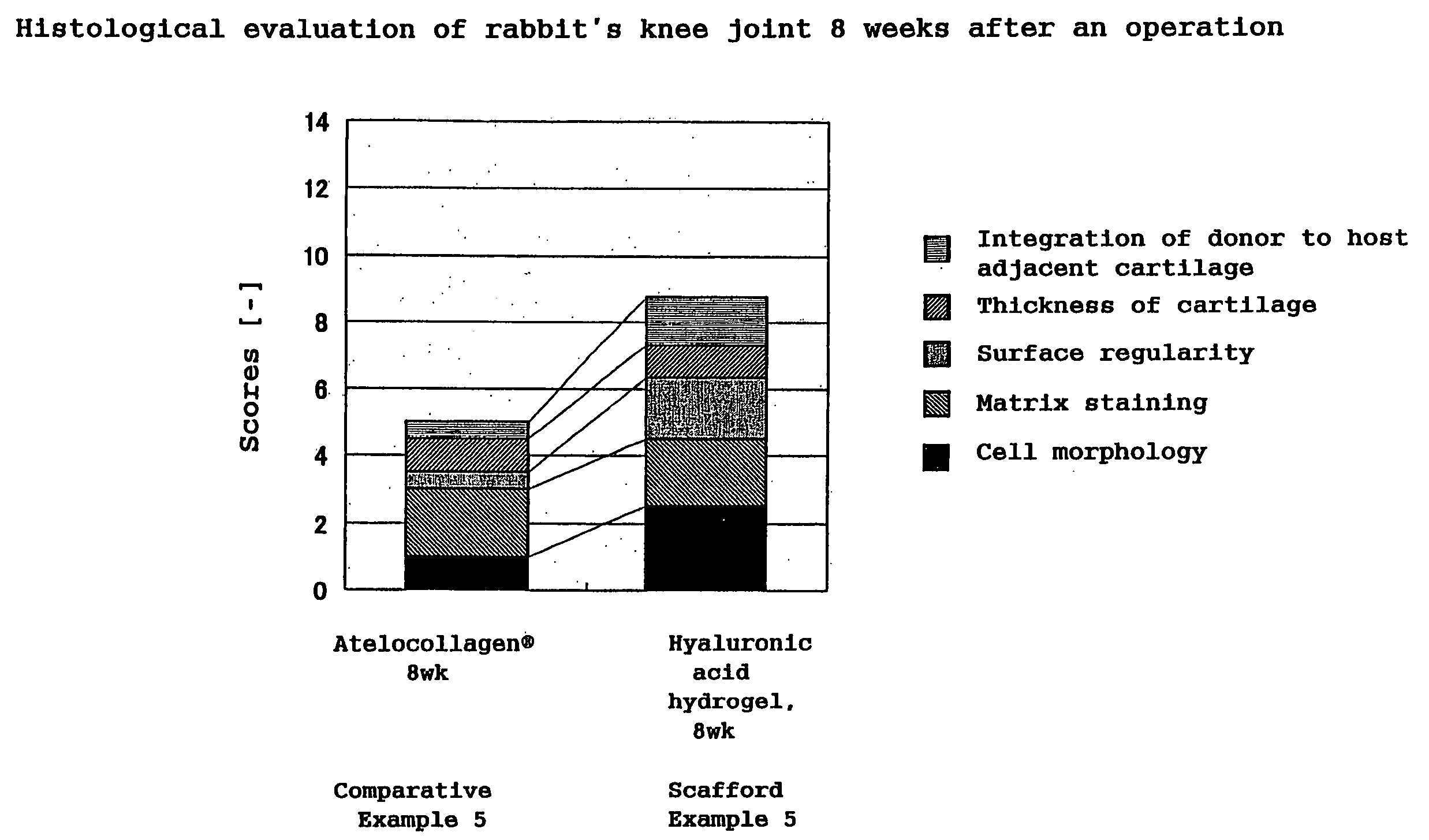
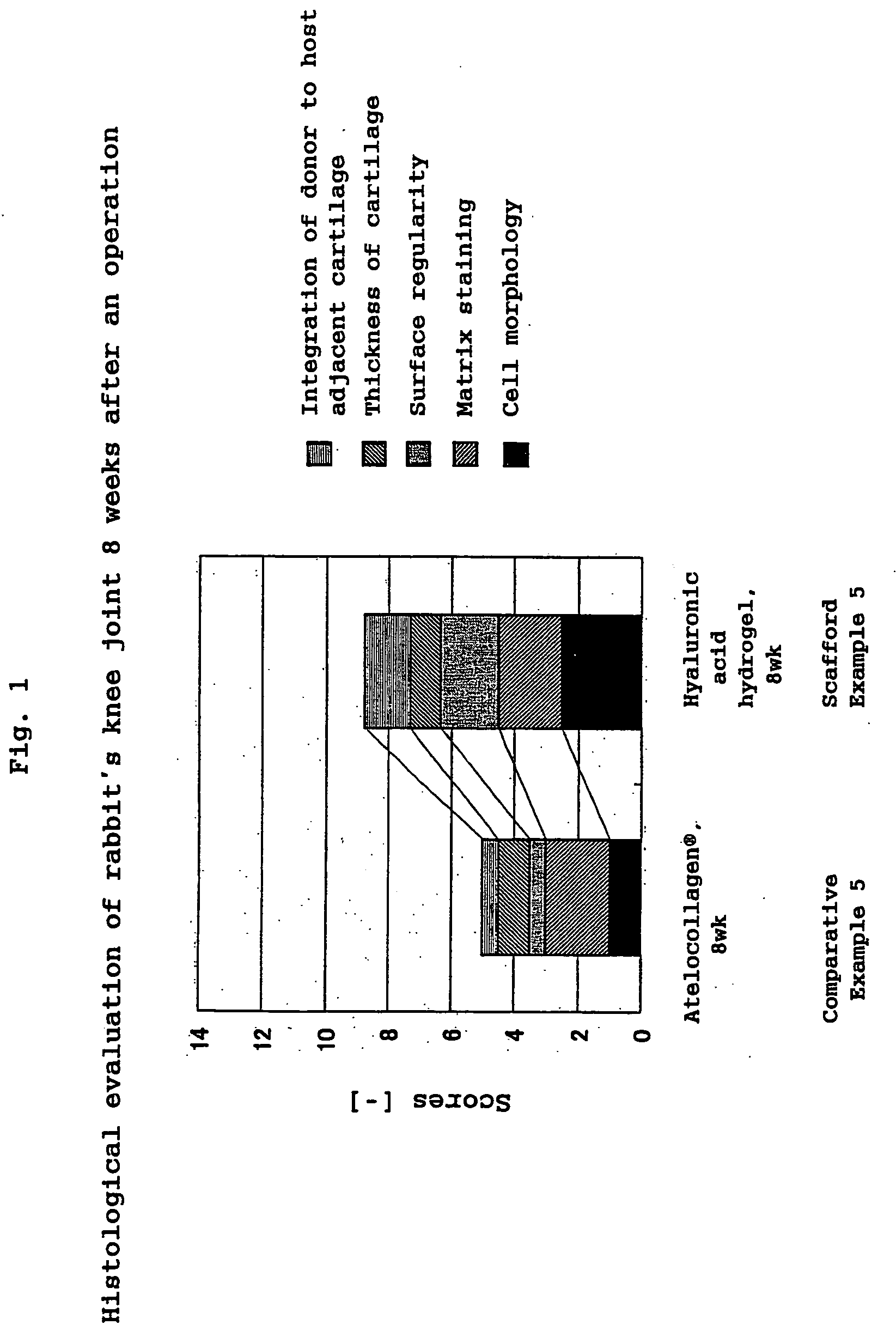
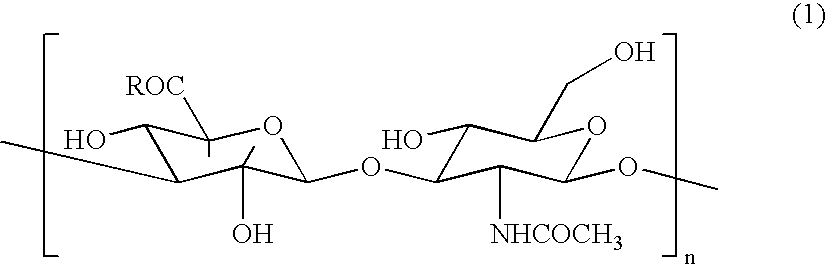
Patents
Literature
11493 results about "Biocompatibility Testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sometimes one hears of biocompatibility testing that is a large battery of in vitro test that is used in accordance with ISO 10993 (or other similar standards) to determine if a certain material (or rather biomedical product) is biocompatible.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
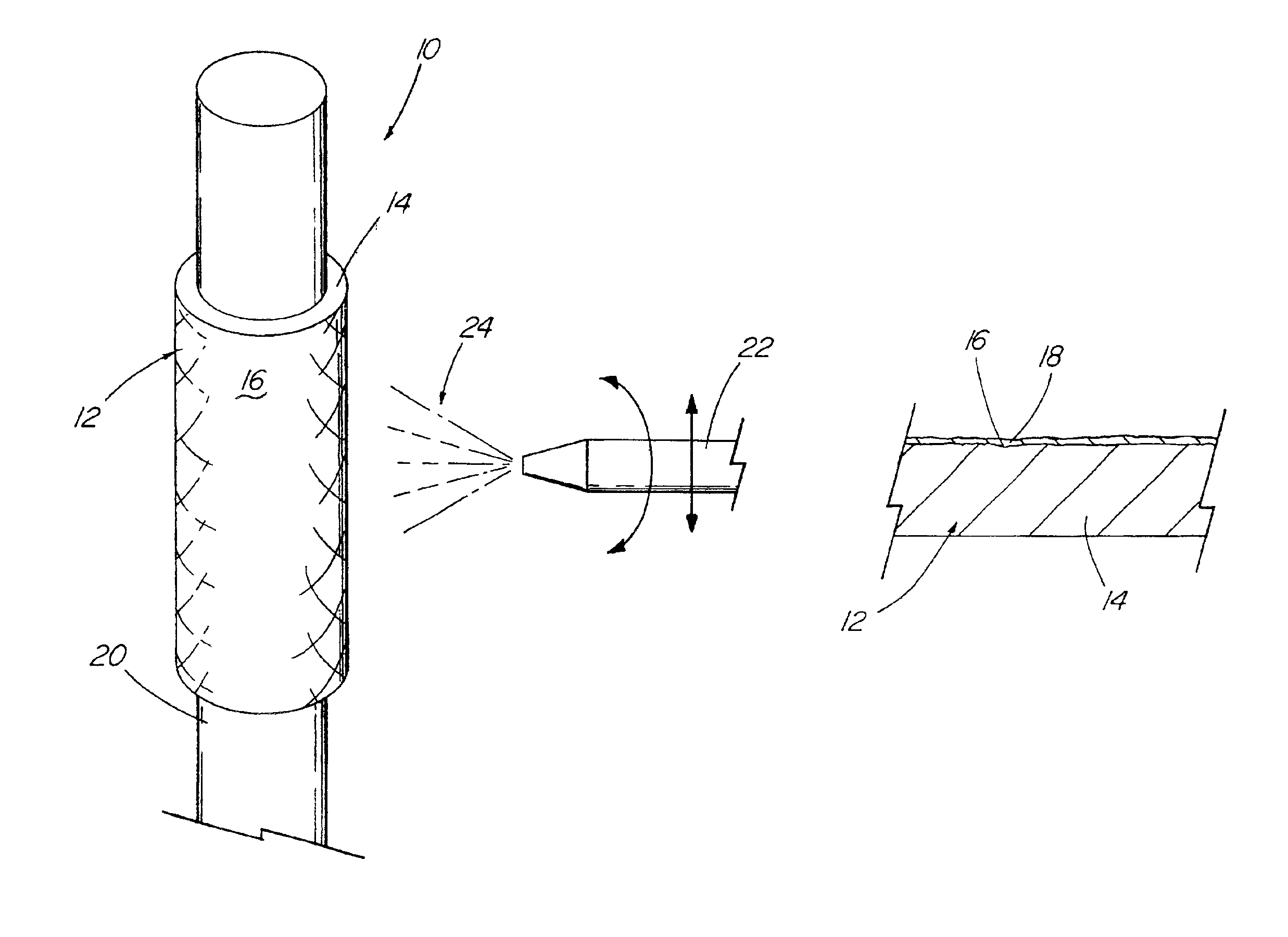
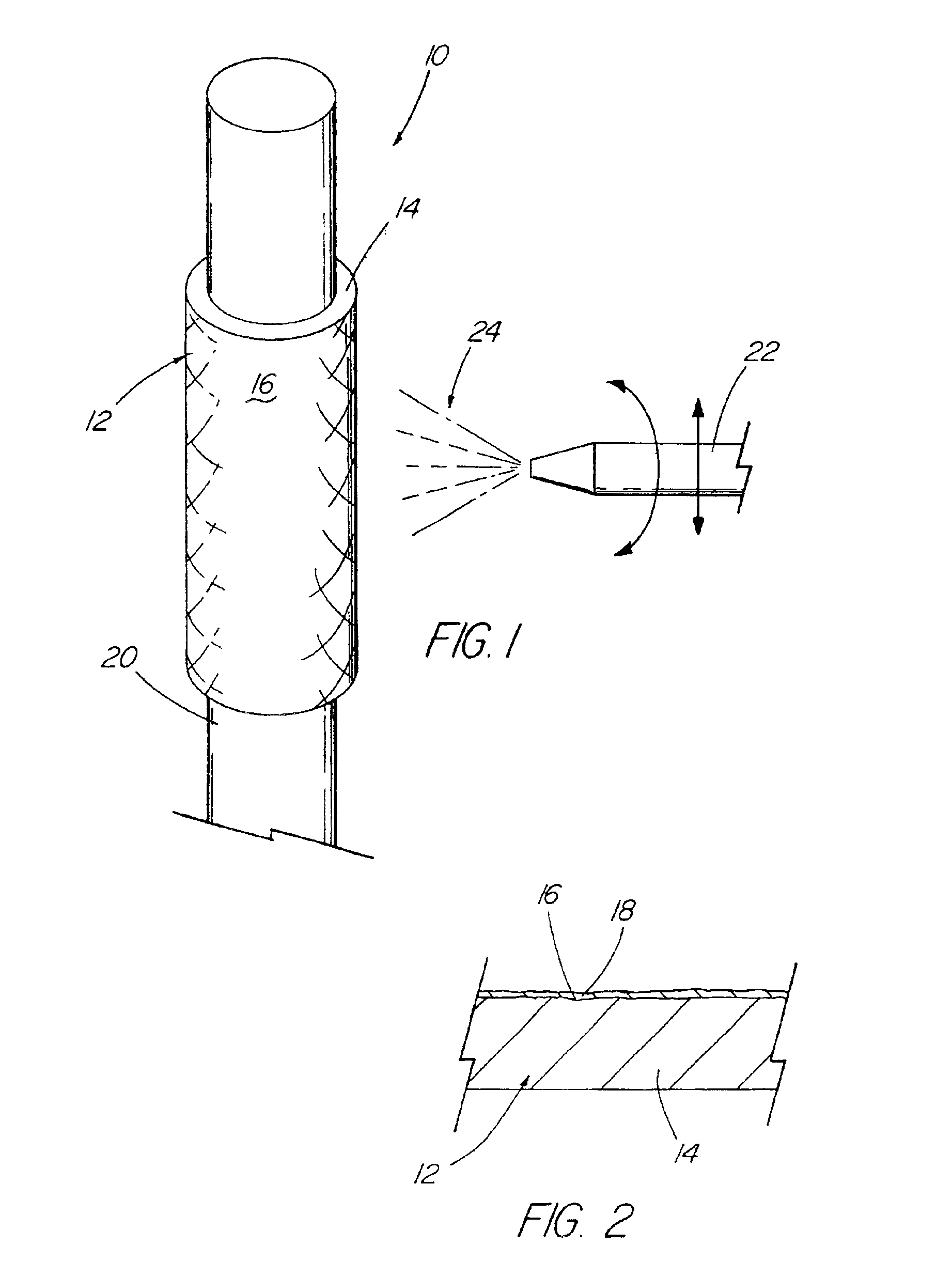
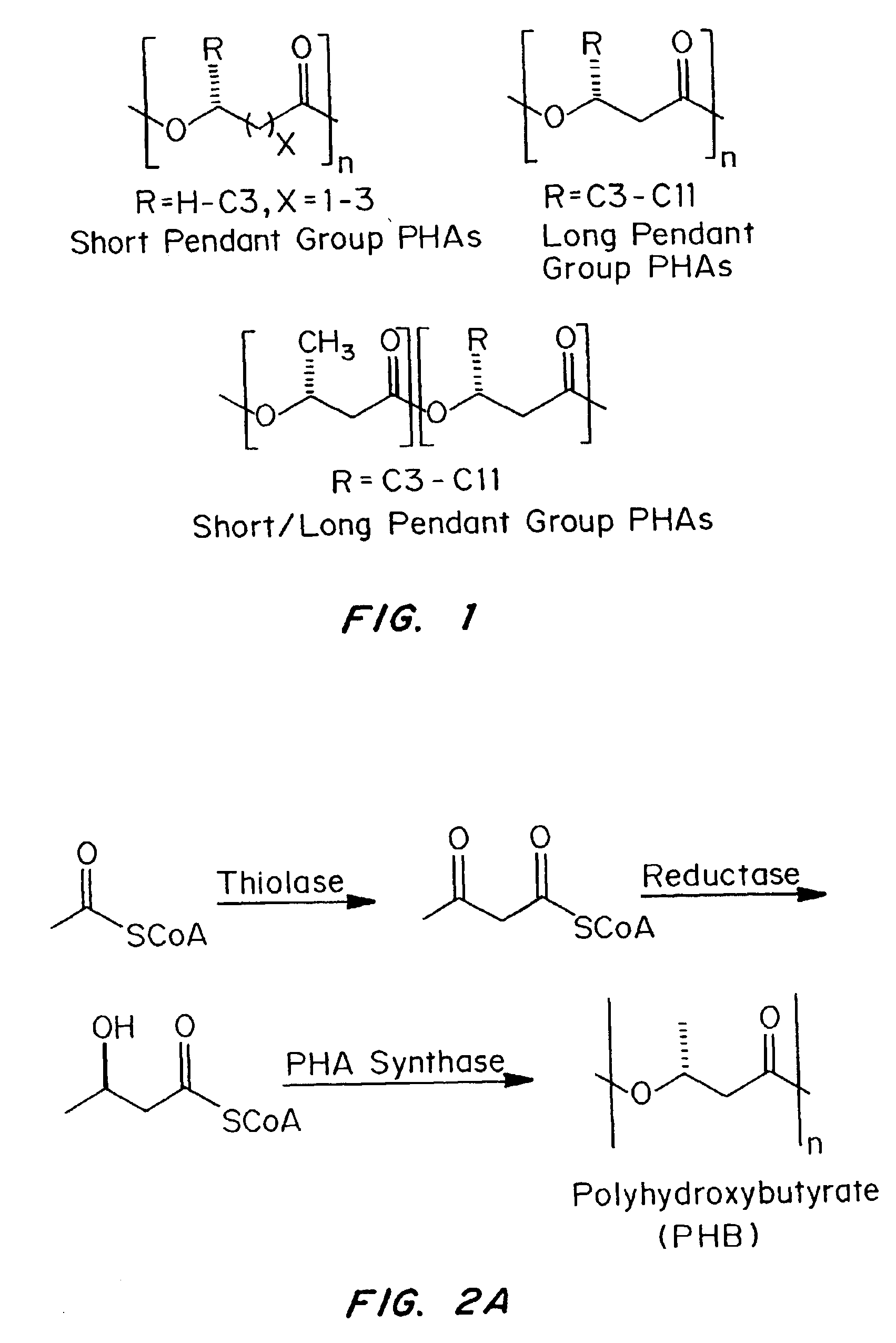
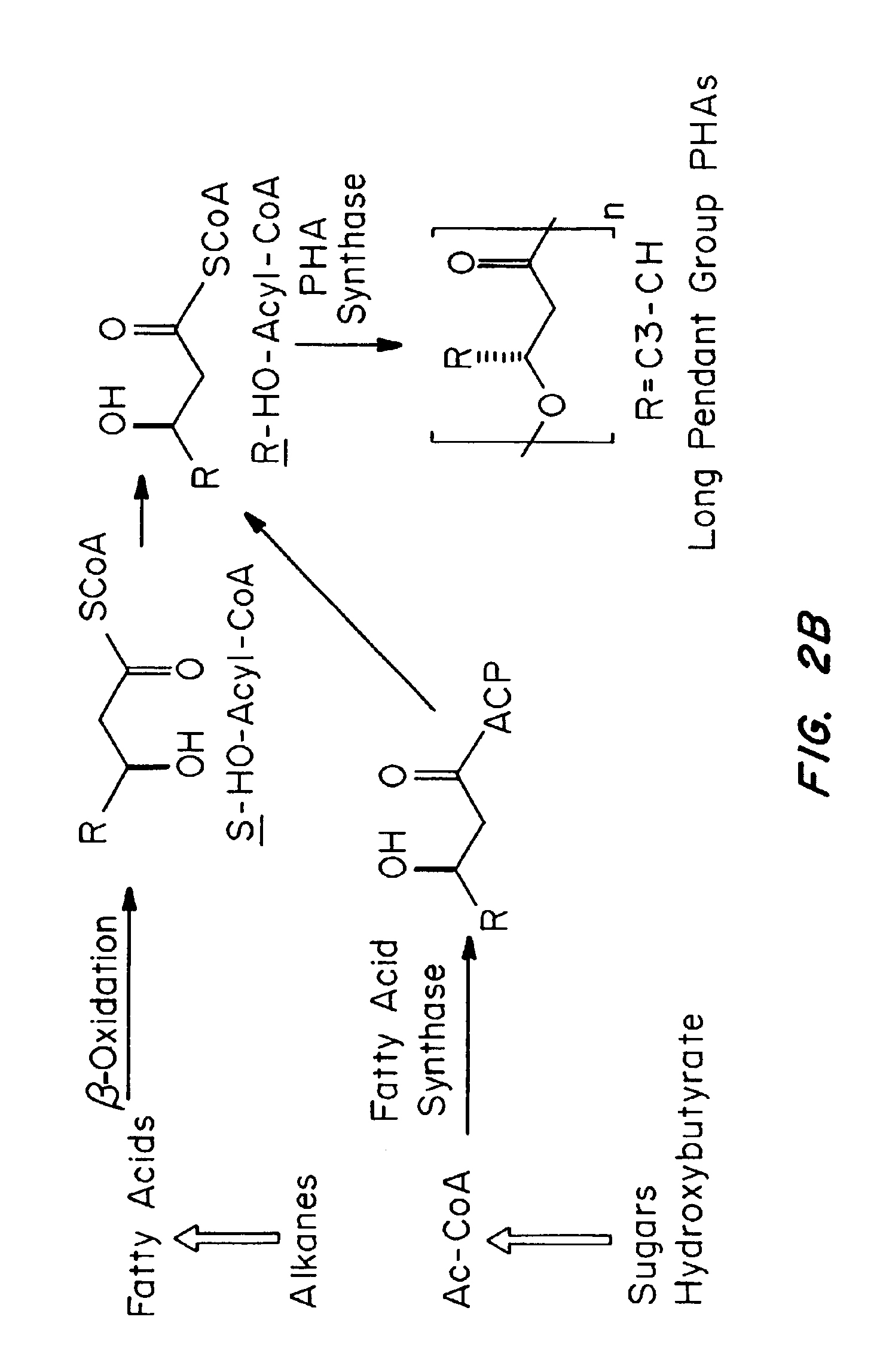
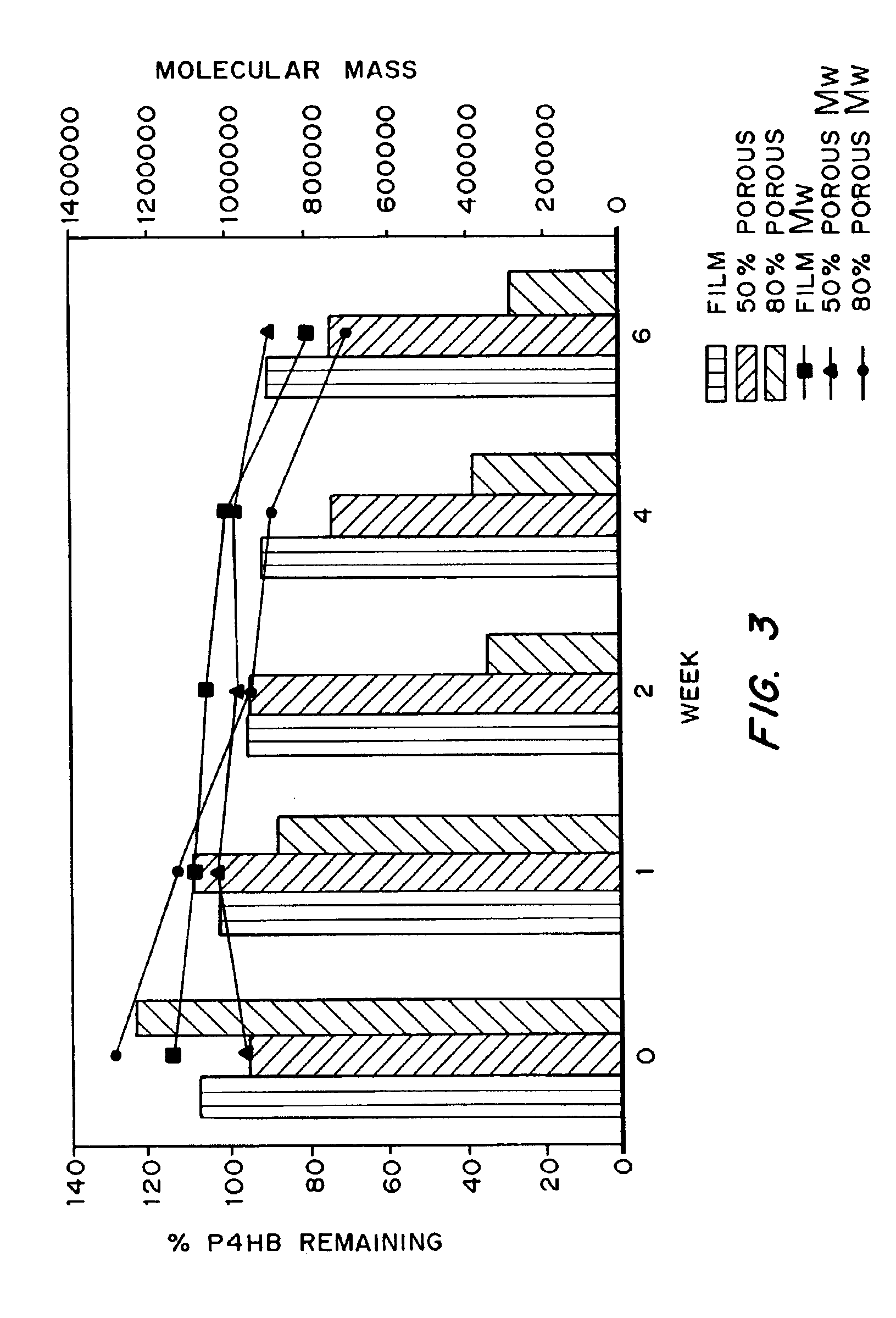
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Coated implantable medical device
InactiveUS6918927B2Good worker safetyLow costOrganic active ingredientsSurgerySodium bicarbonateMedicine
A medical device (10) includes a structure (12) adapted for introduction into a patient, the structure (12) being formed of a preferably non-porous base material (14) having a roughened or textured surface (16). The structure (12) is conveniently configured as a vascular stent with a base material (14) of stainless steel, nitinol or another suitable material. The medical device (10) also includes a layer (18) of a bioactive material posited directly upon the roughened or textured surface (16) of the base material (14) of the structure (12). The surface (16) of the base material (14) is roughened or textured by etching or by abrasion with sodium bicarbonate or another suitable grit. A preferred roughened or textured surface (16) is thought to have a mean surface roughness of about 10 μin. (about 250 nm) and a surface roughness range between about 1 μin. and about 100 μin. (about 25 nm and about 2.5 μm). The particularly preferred use of sodium bicarbonate as the abrasive to provide roughness or texture to the surface (16) of the base material (14) of the structure (12) is additionally advantageous in the low toxicity of the sodium bicarbonate to production workers, the ease of product and waste cleanup, and the biocompatibility of any residual sodium bicarbonate.
Owner:COOK MEDICAL TECH LLC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Thermoplastic copolymer of tetrafluoroethylene and perfluoromethyl vinyl ether and medical devices employing the copolymer
An improved elastomeric material is described comprising an essentially noncross-linkable amorphous copolymer of tetrafluoroethylene (TFE) and perfluoromethyl vinyl ether (PMVE) which is both a thermoplastic and exhibits exceptional mechanical properties. This material is particularly suitable for use in ultra-clean environments, and particularly for use in an implantable device, since it does not contain contaminants that previous thermoset TFE / PMVE copolymers have required. Among the improved properties of the present invention are excellent biocompatibility, high matrix tensile strength, high clarity, high abrasion resistance, high purity, adequate elasticity, and ease of processing due to the thermoplastic, and noncross-linkable structure of the copolymer. The material of the present invention is also a high strength bonding agent particularly suited for bonding porous PTFE to itself or to other porous substances at room or elevated temperatures.
Owner:WL GORE & ASSOC INC
Implant with composite coating
InactiveUS6261322B1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:SHALBY ADVANCED TECH INC
High strength suture with collagen fibers
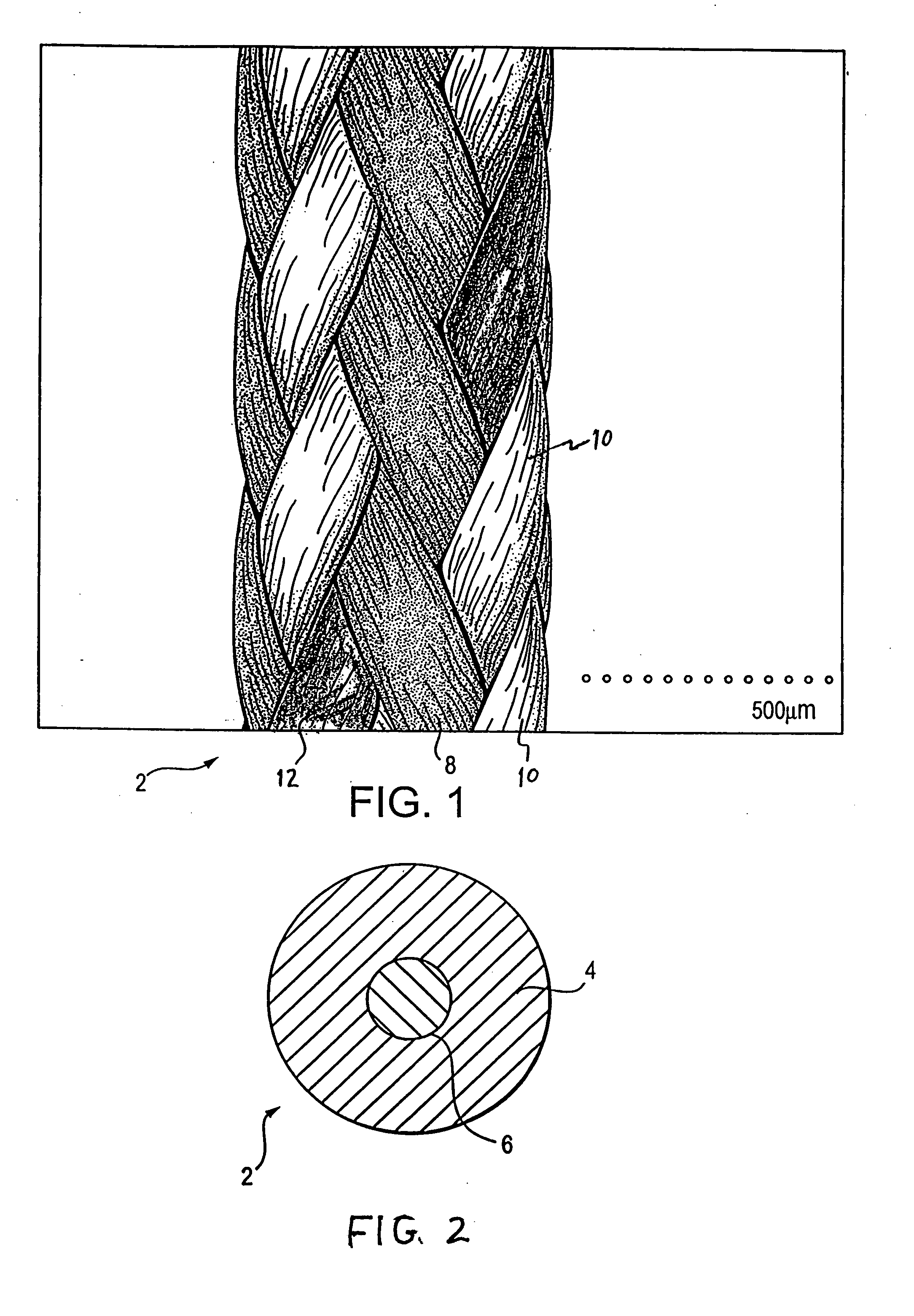
InactiveUS20050033362A1High strengthGood tissue compatibilitySuture equipmentsSurgical needlesPolyesterTissue remodeling
Owner:ARTHREX
Bone Cement And Methods Of Use Thereof
InactiveUS20070032567A1High unit weightImprove wettabilityImpression capsSurgical adhesivesPolymer scienceBiocompatibility Testing
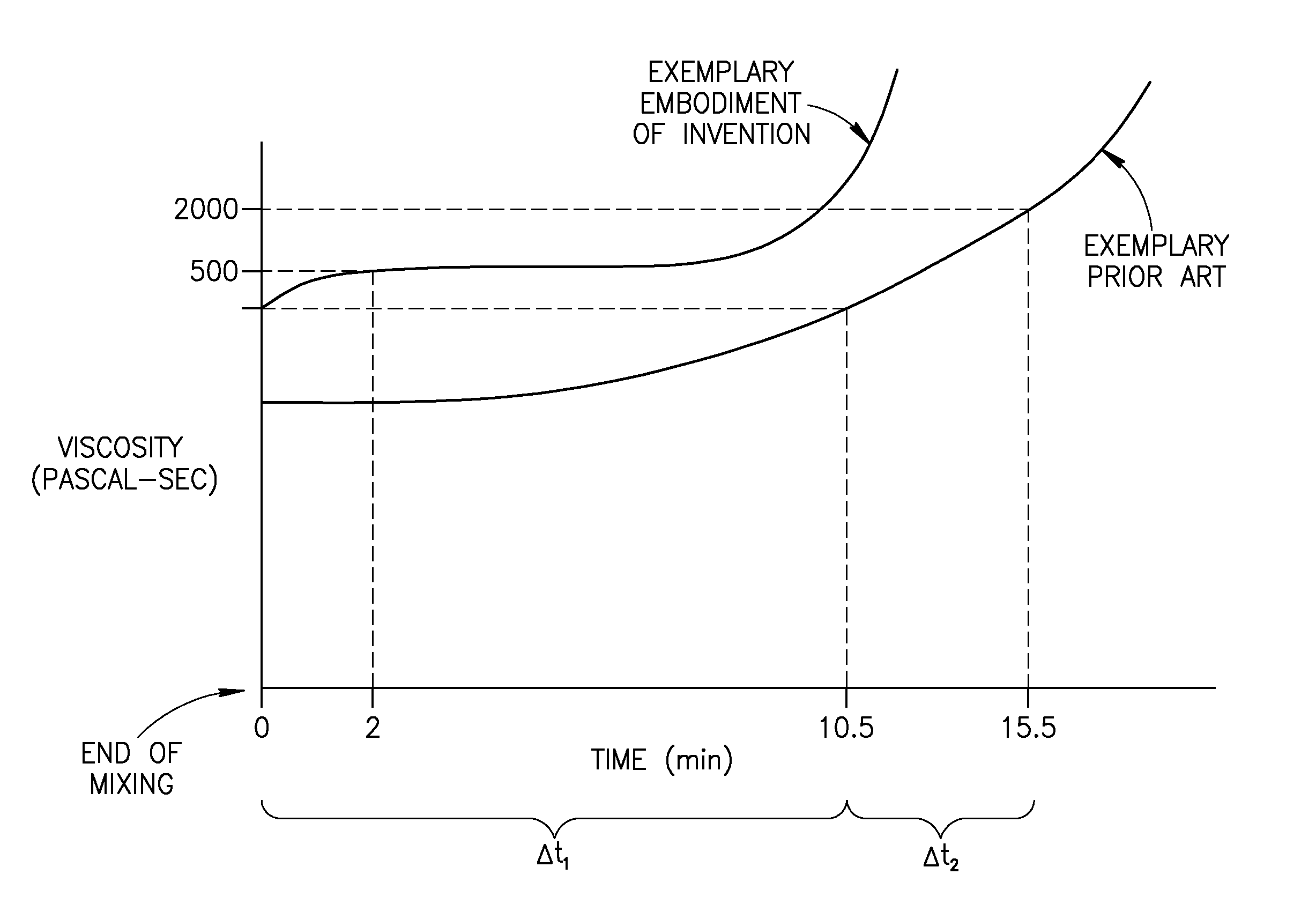
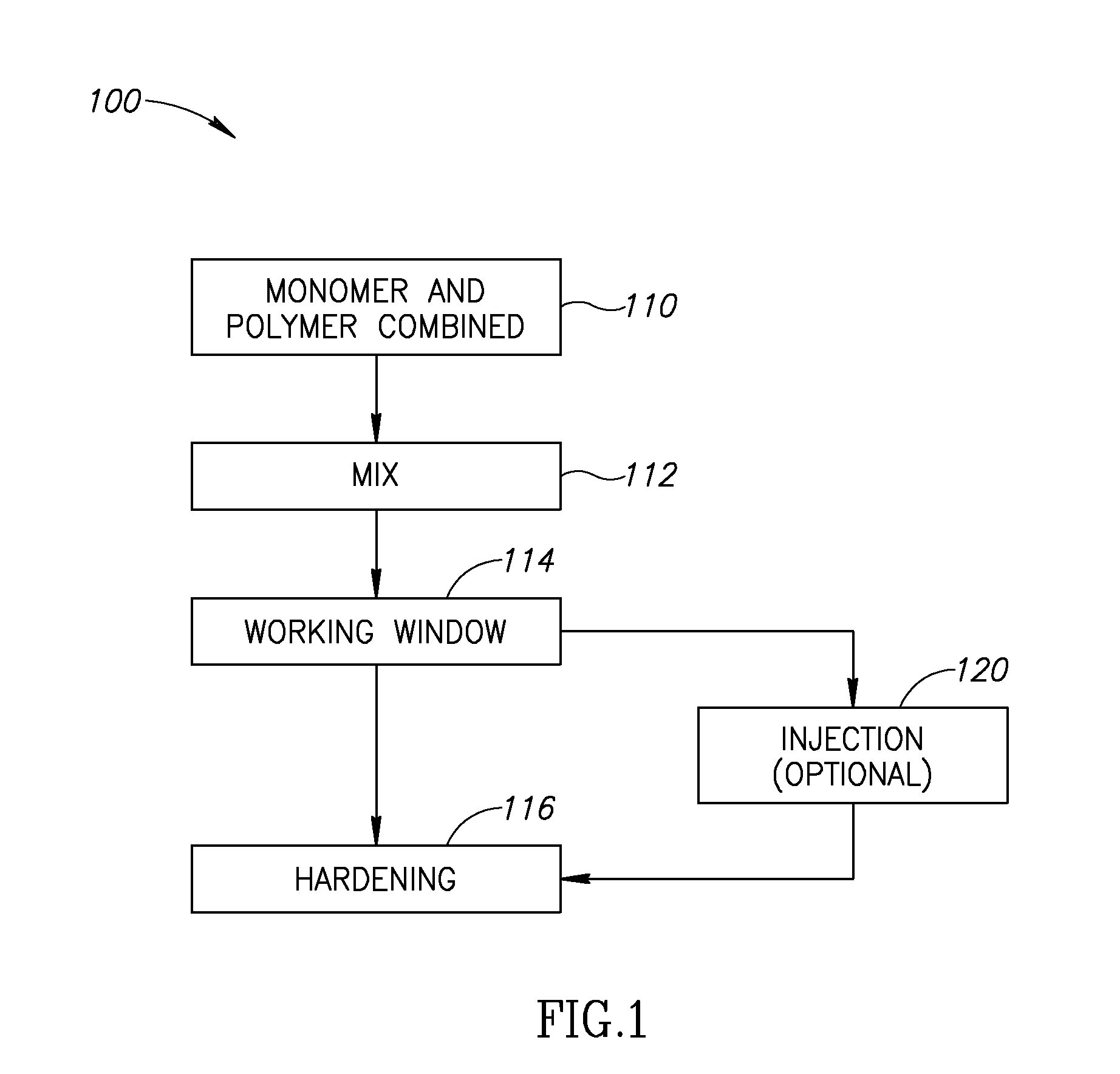
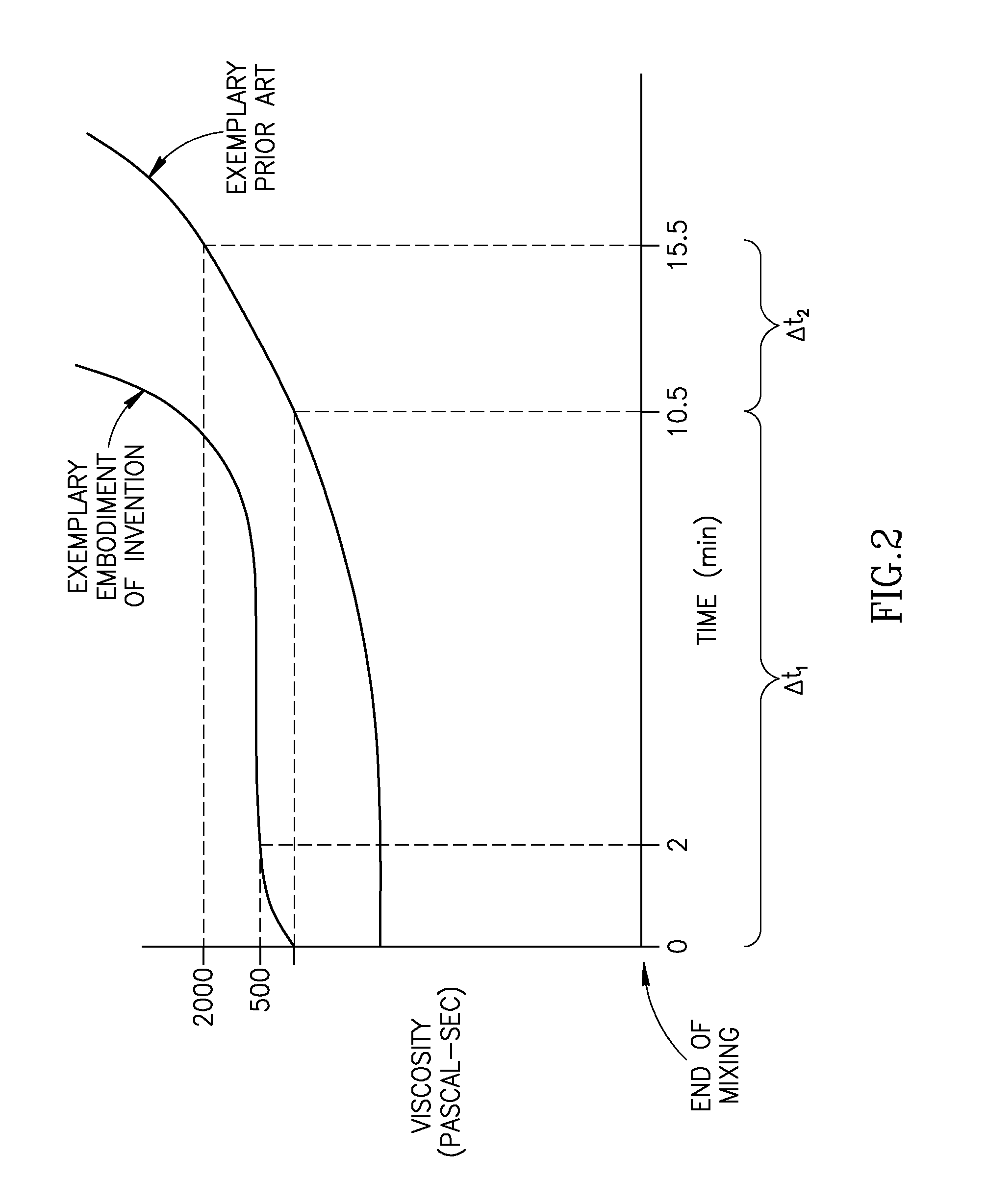
A bone cement comprising an acrylic polymer mixture. The cement is characterized in that it achieves a viscosity of at least 500 Pascal-second within 180 seconds following initiation of mixing of a monomer component and a polymer component and characterized by sufficient biocompatibility to permit in-vivo use.
Owner:DEPUY SYNTHES PROD INC
Multifunctional medical articles
ActiveUS20070003588A1Minimize timeMaximum flexibilityOrganic active ingredientsPowder deliveryMedicineBiocompatibility Testing
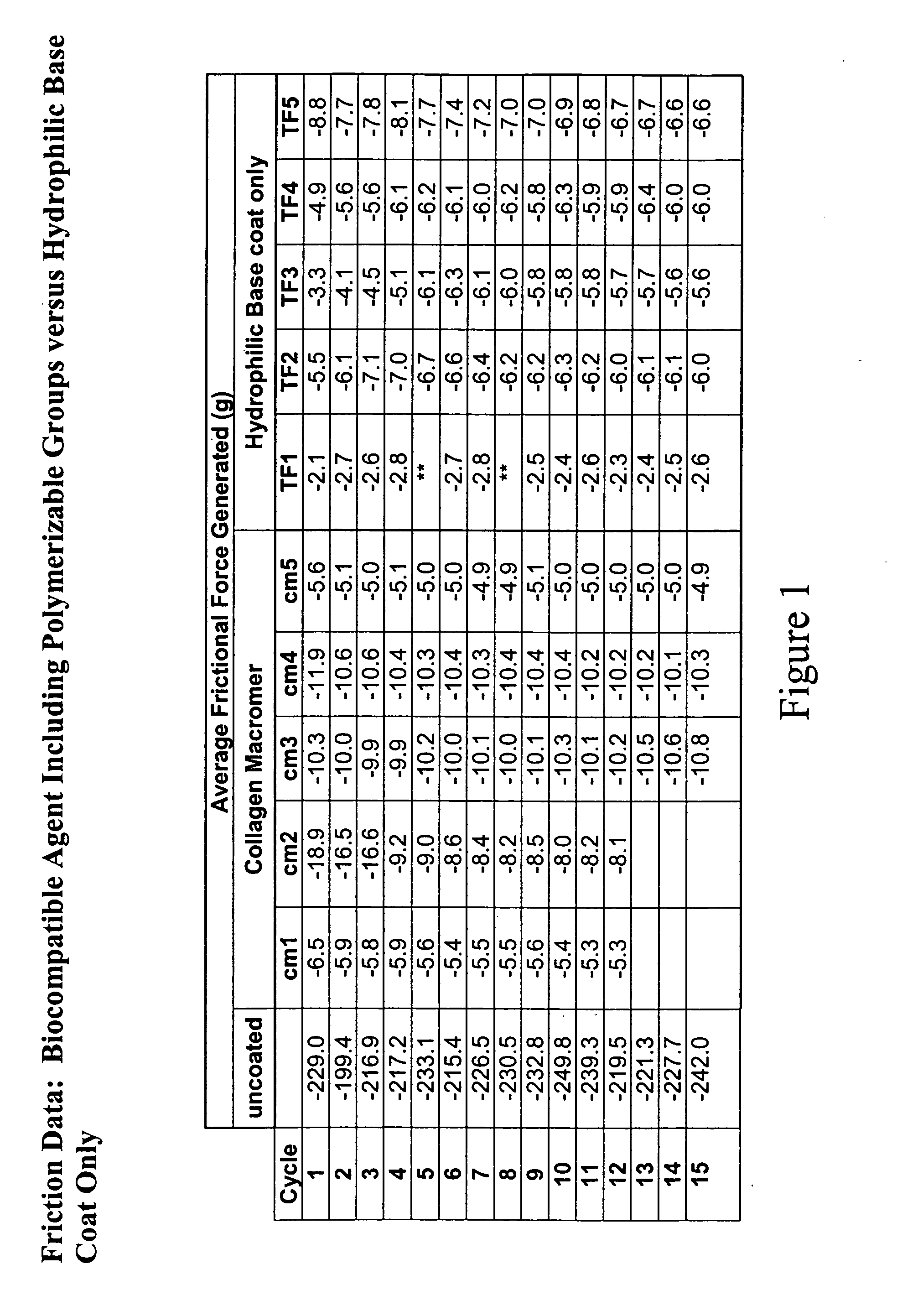
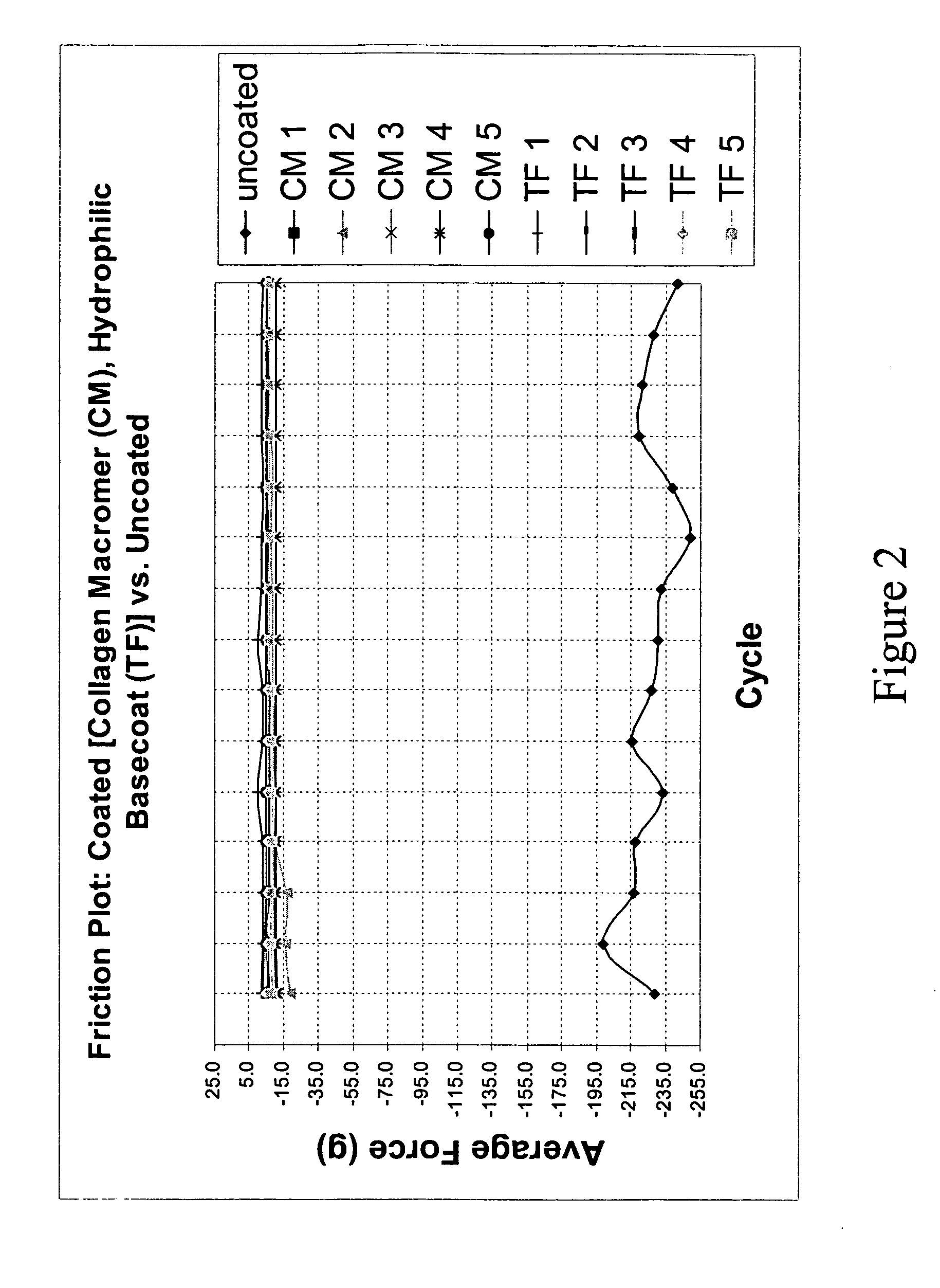
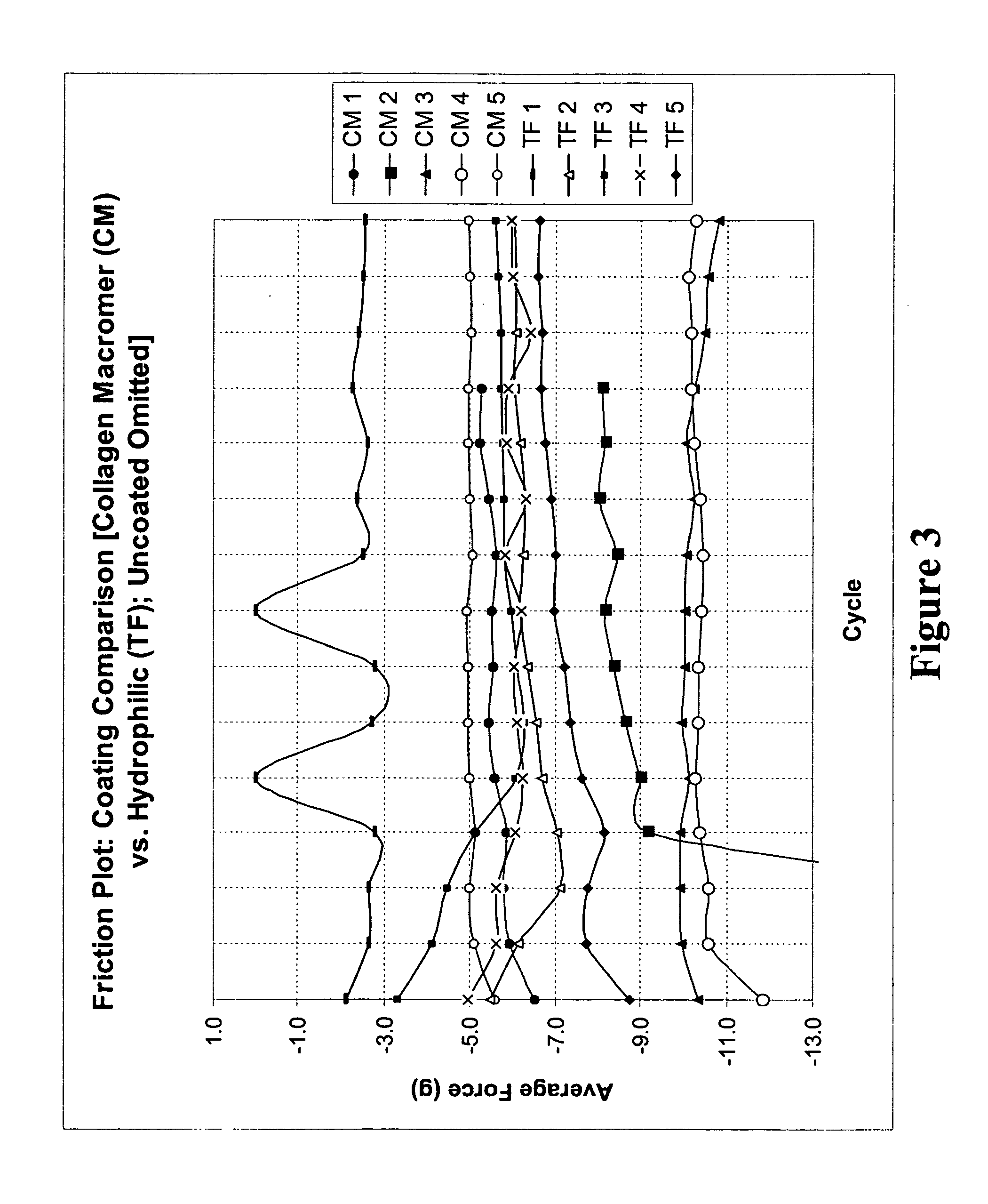
The invention provides medical articles that include more than one biocompatibility-promoting function. In one aspect, the invention provides a medical articles that include a polymeric matrix including more than one biocompatible agent, wherein each biocompatible agent is provided at a distinct portion of the medical article surface. Methods of making medical articles, as well as methods of using the same, are also described.
Owner:SURMODICS INC
Intervertebral implant
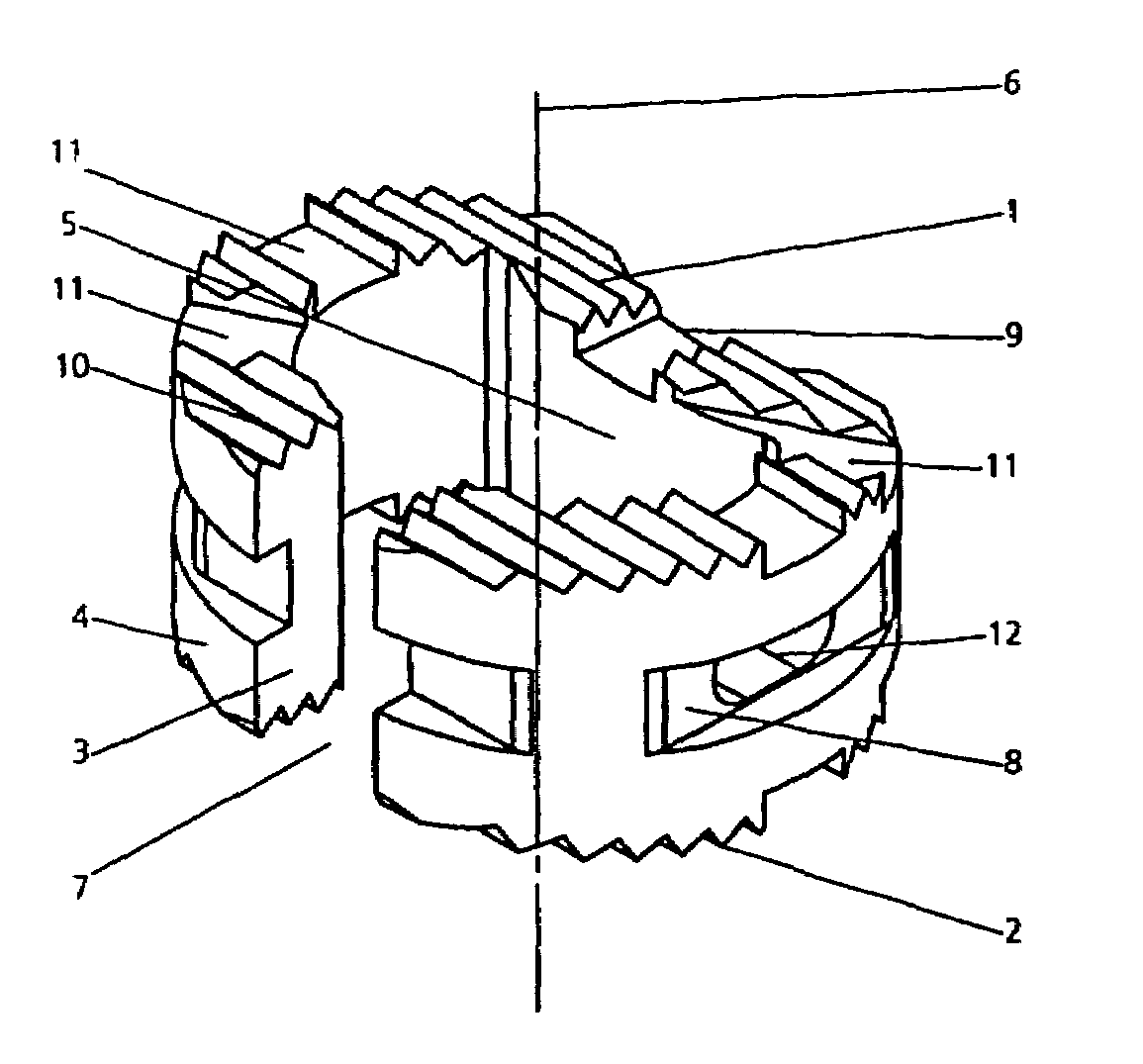
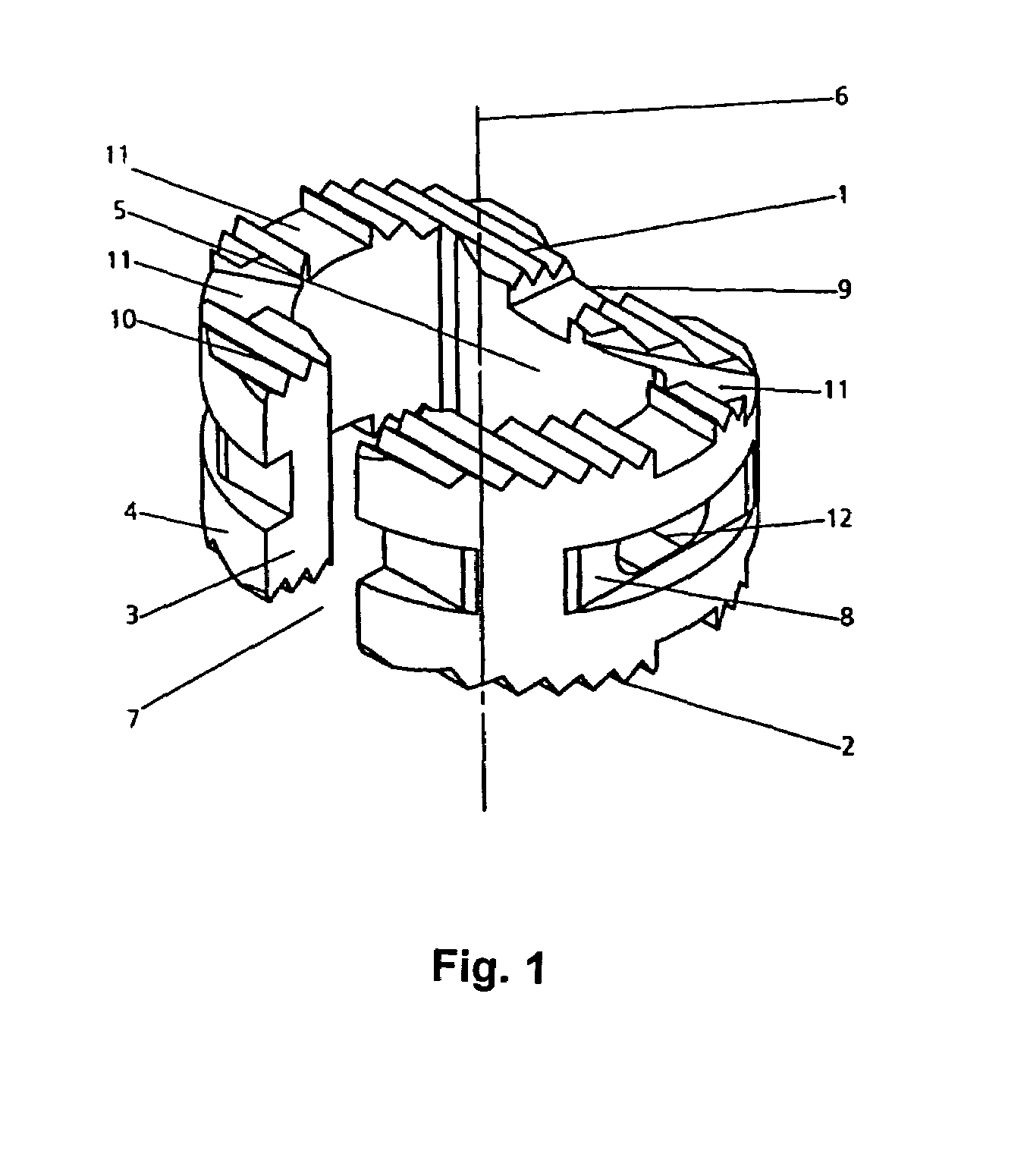
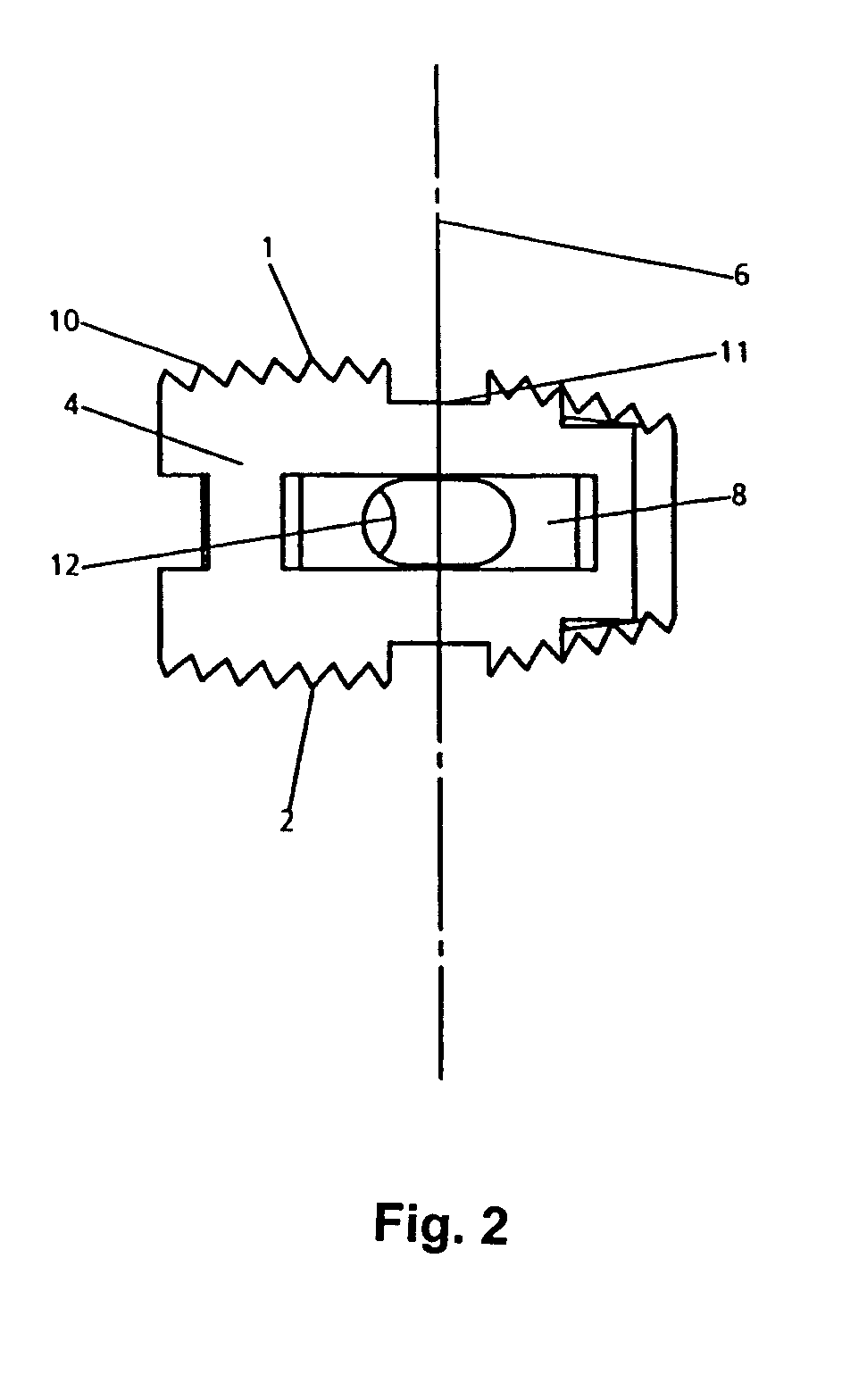
InactiveUS7303583B1High degreePrevent implantationSurgeryDomestic plumbingLateral regionBiocompatibility Testing
Owner:SYNTHES USA
Implantable Stimulation Electrode with a Coating for Increasing Tissue Compatibility
InactiveUS20080234790A1Avoid tissue irritationGood biocompatibilitySurgeryInternal electrodesImplantable Stimulation ElectrodesIrritation
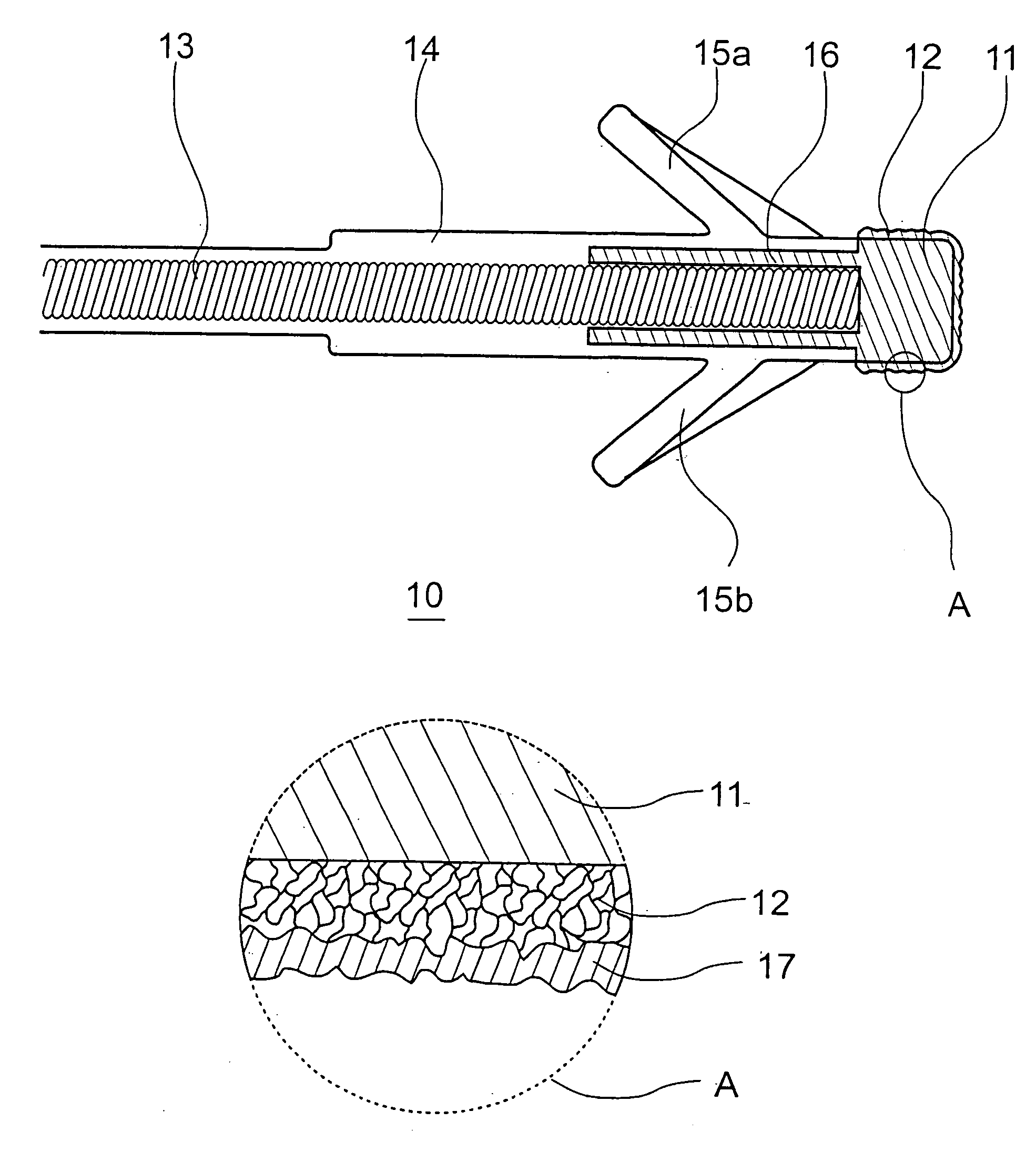
An implantable stimulation electrode for use with an implantable tissue stimulator, especially a pacemaker, a defibrillator, a bone stimulator or a neurostimulator includes a metal base body, optionally one or more intermediate layers disposed on the base body and a coating covering the base body and, optionally, intermediate layers in order to increase tissue compatibility. The coating should prevent tissue irritations after implantation and more particularly increase the stimulus threshold associated therewith, have very high biocompatibility and also has an anti-inflammatory effect. An increase in tissue compatibility is achieved by virtue of the fact that the coating has a polysaccharide layer made of hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Reduced pressure treatment system having a dual porosity pad
A wound closure apparatus having a porous pad adapted to be fluidly connected to a vacuum pump. The pad is placed over or within a tissue site, and the vacuum pump may be activated to draw air and exudates from the tissue site. The pad contains multiple pore sizes to prevent granulation tissue from migrating into the pad as reduced pressure treatment is applied. The pad has an outer surface adjacent the wound with pore sizes of a diameter of approximately 100 microns or less to prevent tissue from growing into the pad and is treated for biocompatibility.
Owner:KCI LICENSING INC
Bioprosthetic Heart Valve With Polyphosphazene
This disclosure encompasses a bioprosthetic heart valve having a polyphosphazene polymer such as poly[bis(trifluoroethoxy)phosphazene], which exhibits improved antithrombogenic, biocompatibility, and hemocompatibility properties. A method of manufacturing a bioprosthetic heart valve having a polyphosphazene polymer is also described.
Owner:CELONOVA BIOSCIENCES INC
Wound dressing material containing silk fibroin and sericin as main component and method for preparing same
There is provided a novel wound dressing material which has biocompatibility and infection controllability as essential properties required for such a material, especially excellent flexibility and water absorption properties, thereby accelerating smooth regeneration of a skin defect without stripping off the regenerating skin while removing the material from the skin. A healing agent is added to the wound dressing material which comprises an amorphous film of a crystallinity below 10% and contains fibroin and sericin as a main component.
Owner:NAT INST OF AGROBIOLOGICAL SCI +1
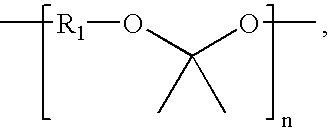
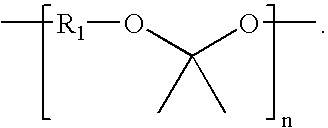
Biodegradable triblock copolymers for implantable devices
InactiveUS20090004243A1Reduce adverse effectsImprove mechanical propertiesBiocidePeptide/protein ingredientsDevice formBiocompatibility Testing
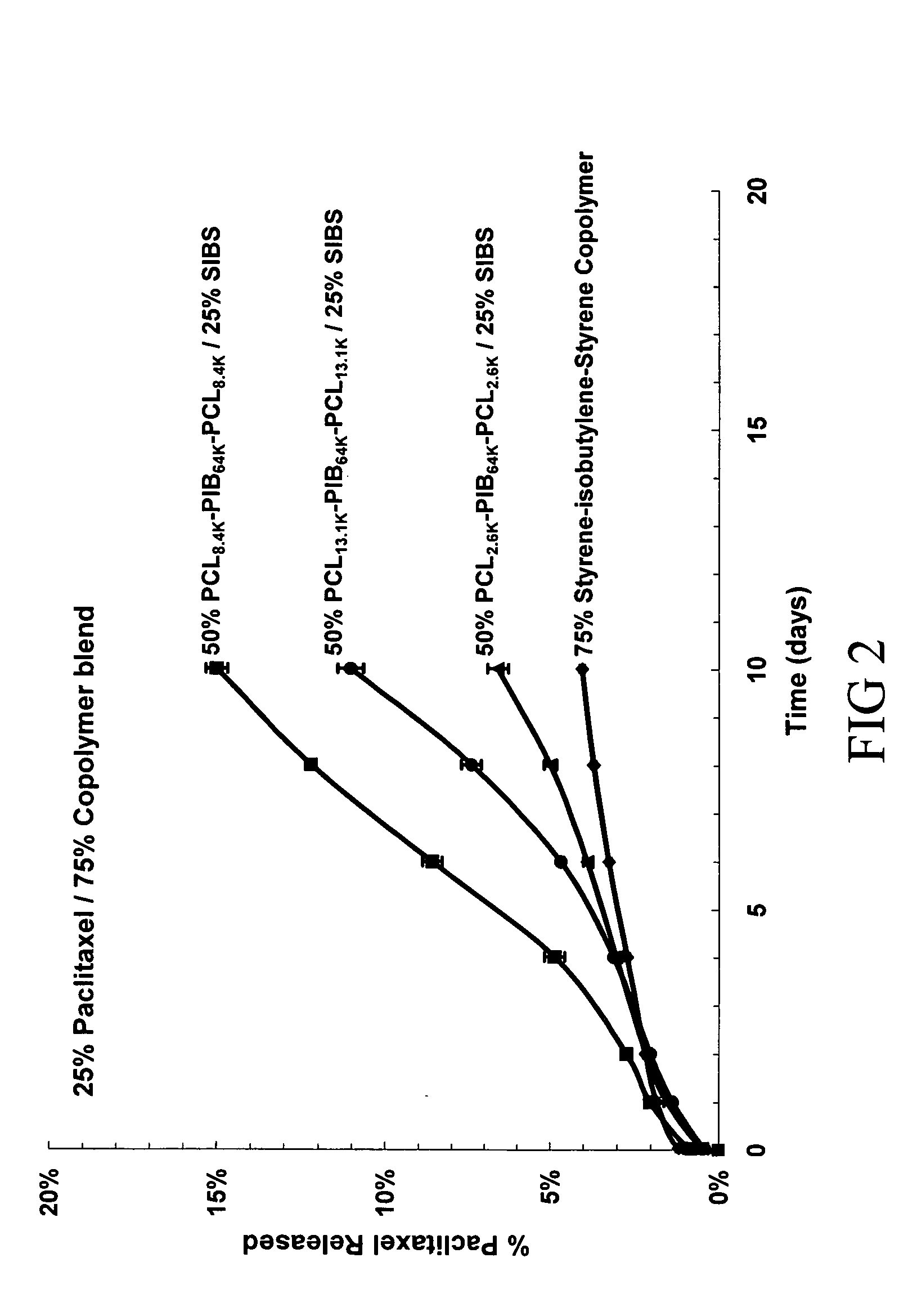
The present invention is directed to polymeric materials made of biodegradable, bioabsorbable triblock copolymers and implantable devices (e.g., drug-delivery stents) containing such polymeric materials. The polymeric materials may also contain at least one therapeutic substance. The polymeric materials are formulated so as to improve the mechanical and adhesion properties, degradation, biocompatibility and drug permeability of such materials and, thus, implantable devices formed of such materials.
Owner:ABBOTT CARDIOVASCULAR
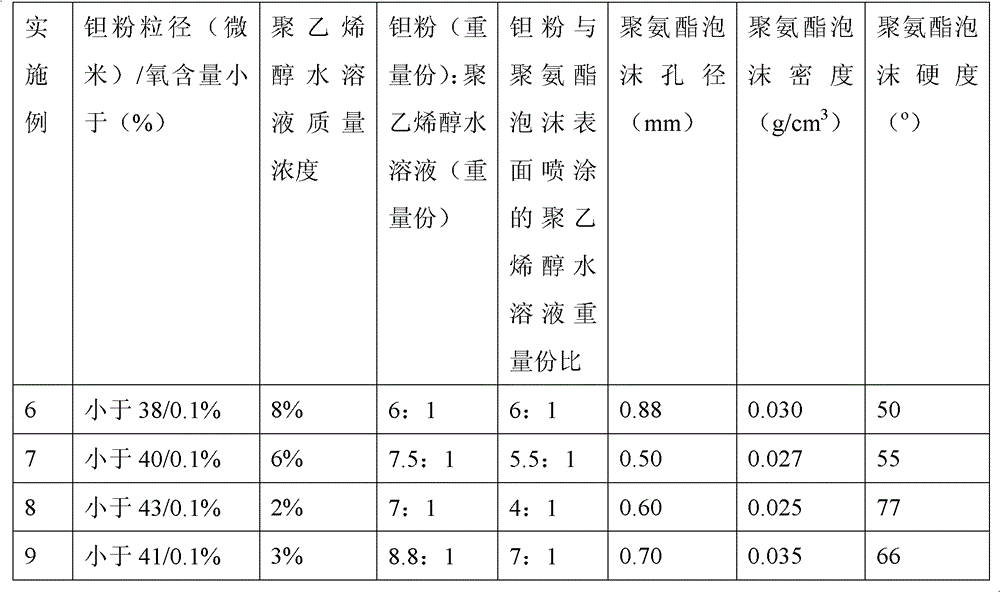
Method for preparing medical porous tantalum implant material
ActiveCN102796894AReduce contentImprove mechanical propertiesProsthesisPolyvinyl alcoholBiocompatibility Testing
The invention discloses a method for preparing a medical porous tantalum material. The method comprises the following steps of: mixing a poly ethanol aqueous solution and tantalum powder to obtain slurry, wherein the mass concentration of the poly ethanol aqueous solution is 2 to 8 percent; injecting the slurry into an organic foam by vibrating and pressurizing, wherein the vibrating frequency is 20 to 80 times / min; drying; degreasing; sintering, namely raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3>Pa, preserving heat for 120 to 240 minutes, cooling to 200 to 300 DEG C along with a furnace, raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min again, preserving heat for 180 to 240 minutes, raising temperature to 2,000 to 2,200 DEG C at the speed of 5 to 10 DEG C / min, and preserving heat for 120 to 360 minutes; cooling; and performing thermal treatment, namely raising temperature to 800 to 900 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3> Pa, preserving heat for 240 to 480 minutes, cooling to 400 DGE C at the speed of 2 to 5 DGE C / min, preserving heat for 120 to 300 minutes, and cooling to room temperature along with the furnace. The porous tantalum prepared by the method is very suitable to be used for the medical implant material for replacing bearing bone tissues, and biocompatibility and the mechanical property can be guaranteed simultaneously.
Owner:CHONGQING RUNZE PHARM CO LTD
Coating compositions for bioactive agents
InactiveUS20050244459A1Effectively to surfaceEasy to removeStentsPowder deliveryActive agentInsertion stent
A coating composition and related method for use in applying a bioactive agent to a surface in a manner that will permit the bioactive agent to be released from the coating in vivo. The composition is particularly well suited for coating the surface of implantable medical device, such as a stent or catheter, in order to permit the device to release bioactive agent to the surrounding tissue over time. The composition includes a plurality of compatible polymers having different properties that can permit them to be combined together to provide an optimal combination of such properties as durability, biocompatibility, and release kinetics.
Owner:SURMODICS INC
Implant with composite coating
InactiveUS20020016635A1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:CONSENSUS ORTHOPEDICS
Metal alloy for medical devices and implants
InactiveUS20080312740A1Maintain good propertiesImprove uniformityStentsHeart valvesNiobiumBiocompatibility Testing
Owner:ABBOTT IRELAND
Medical implant or device
The present invention relates to a medical device or implant made at least in part of a high strength, low modulus metal alloy comprising Niobium, Tantalum, and at least one element selected from the group consisting of Zirconium, Tungsten and Molybdenum. The medical devices according to the present invention provide superior characteristics with regard to biocompatibility, radio-opacity and MRI compatibility.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Suturable adhesion-preventing membrane
InactiveUS6977231B1High strengthGood biocompatibilitySuture equipmentsSynthetic resin layered productsCross-linkDecomposition
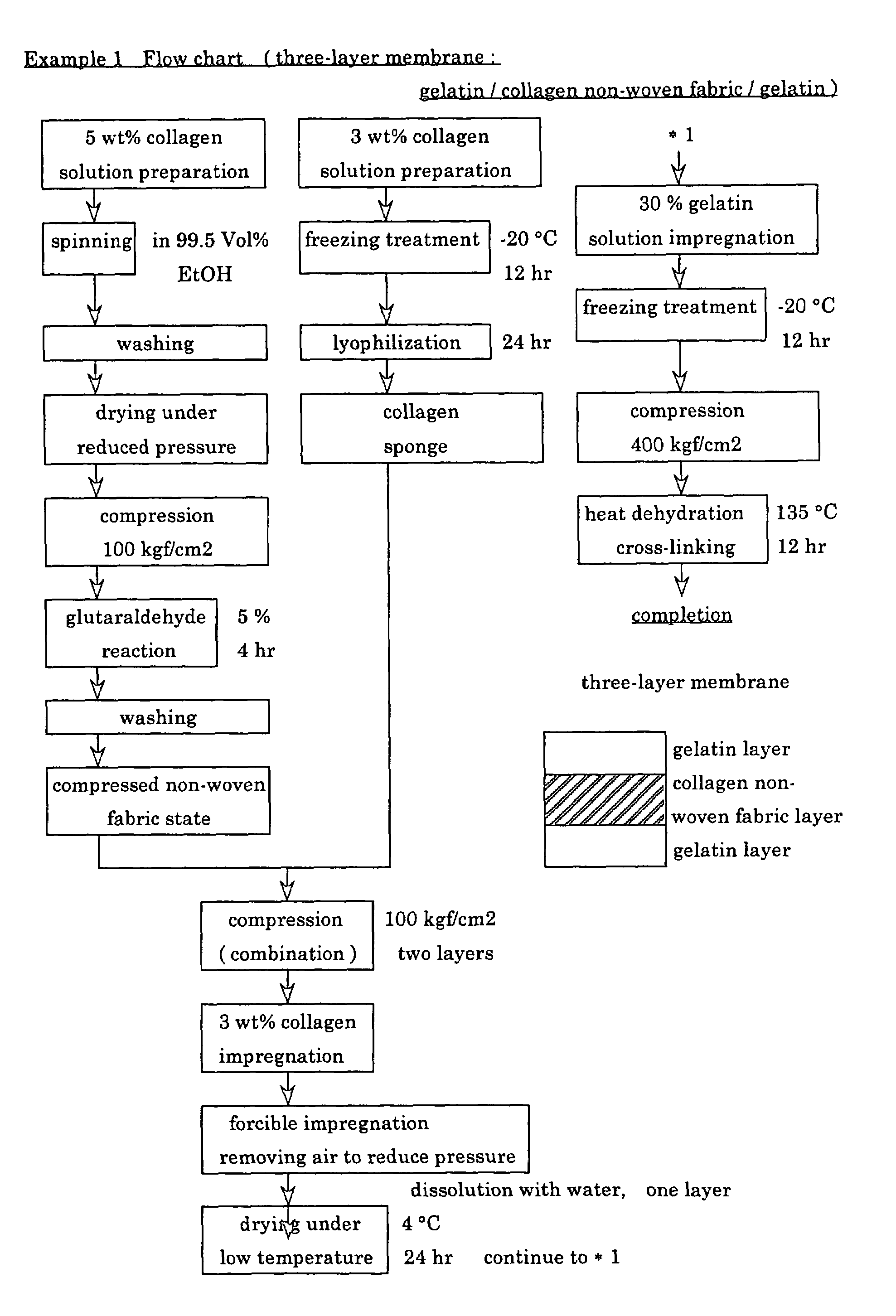
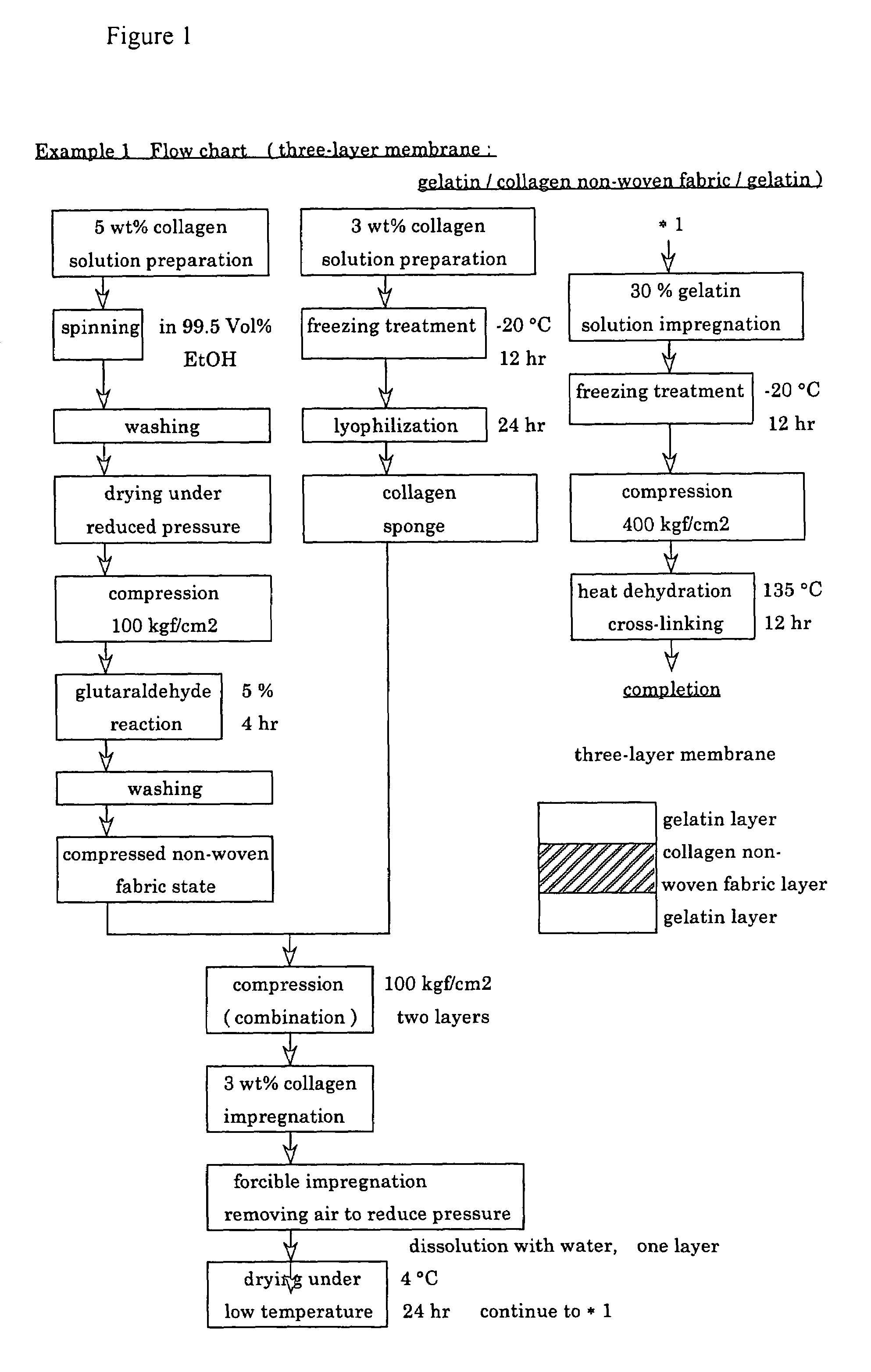
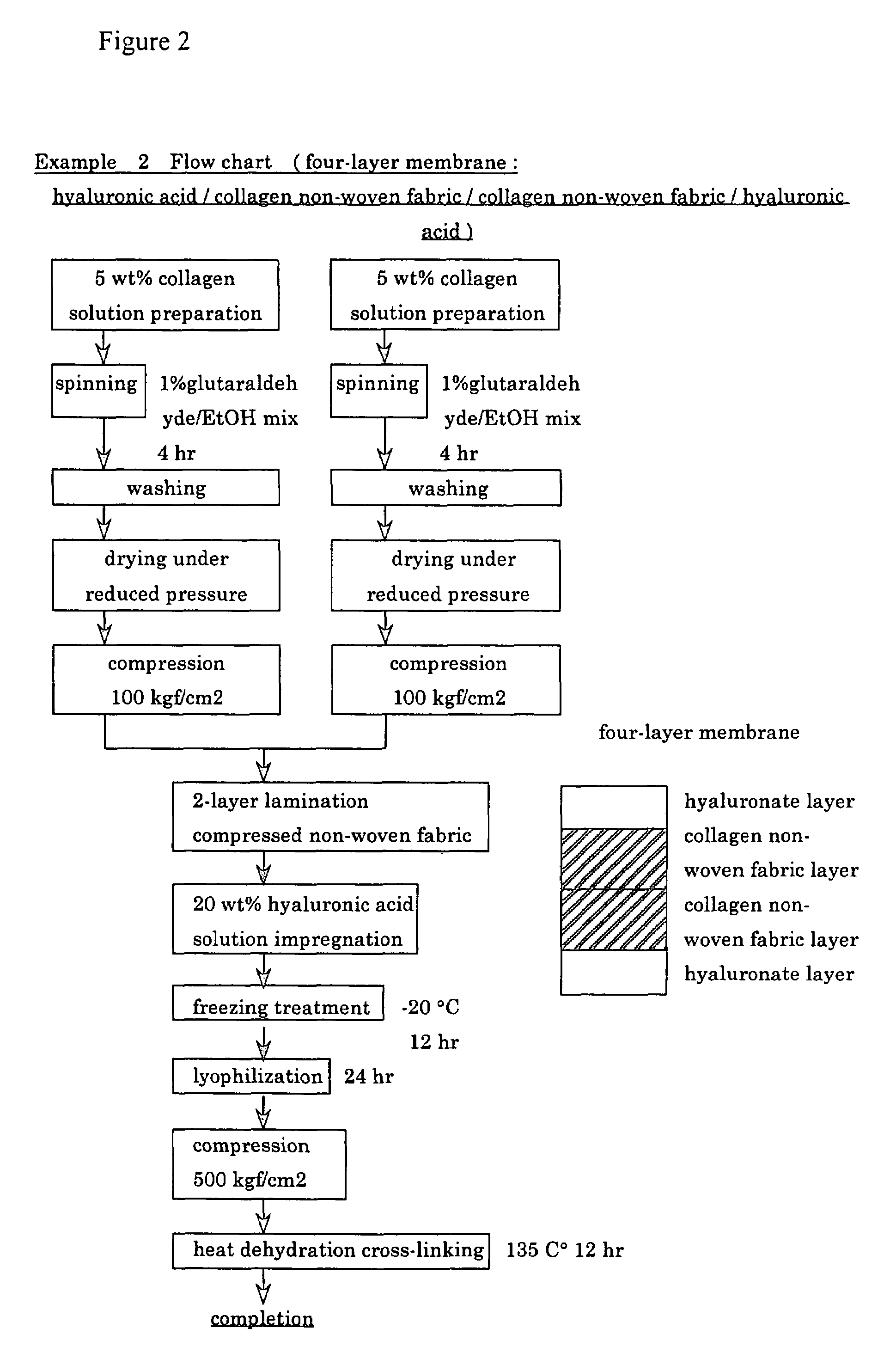
A suturable adhesion-preventing membrane has high suture strength, good biocompatibility, decomposition and absorption in a living body, sufficient adhesion-preventing effect, and desirable guided tissue regeneration. The membrane is composed of at least one non-woven fabric layer made of collagen fibers, or a laminated membranous substance consisting of at least one non-woven fabric layer made of collagen fibers and at least one sponge layer made of collagen, and a coating layer of gelatin or hyaluronic acid on the surface or surfaces of the above membrane. Preferably, the membrane comprises one to six compressed cross-linked collagen non-woven fabric layers wherein a layer has a fibers having a fiber diameter of 0.05 mm to 1.0 mm, a bulk density of 5.0×10−4 to 5 g / cm3 and a thickness of 0.1 mm to 50 mm, and a coating layer containing gelatin or hyaluronic acid and having a thickness of 0.05 mm to 20 mm, wherein the coating layer covers one or both sides or a part or whole of the surface of the membrane.
Owner:NIPRO CORP
Medical devices having porous polymeric regions for controlled drug delivery and regulated biocompatibility
The present invention relates to phase separated polymeric regions and to their use in conjunction with implantable or insertable medical devices. In some aspects of the invention, phase separated polymeric regions are provided that include (a) at least one biostable polymeric phase and (b) at least one biodisintegrable polymeric phase, which is of nanoscale dimensions and which undergoes biodisintegration such that the phase separated polymeric region becomes a nanoporous polymeric region in vivo. Other aspects of the invention are directed to methods of making implantable or insertable medical devices having at least one nanoporous polymeric region. These methods include (a) providing a phase separated polymeric region comprising a stable polymeric phase and a disintegrable polymeric phase of nanoscale dimensions, (b) selectively removing the disintegrable polymeric phase thereby producing the nanoporous polymeric region. In still other aspects, implantable or insertable medical devices are provided which have phase separated polymeric regions that include (a) at least one block copolymer having at least one biostable polymer block and at least one biodisintegrable polymer block and (b) at least one therapeutic agent which is released in vivo upon implantation or insertion of the medical device.
Owner:BOSTON SCI SCIMED INC
Nanofibrous nonwoven membrane of silk fibroin for guided bone tissue regeneration and manufacturing method thereof
InactiveUS20060095137A1Easy to controlSimple processMaterial nanotechnologyElectric discharge heatingBone tissueBiocompatibility Testing
The present invention relates to a membrane for guided bone tissue regeneration and, more particularly, to a membrane for guided bone tissue regeneration having a structure that silk fibroin nanofibers obtained by removing sericin from silk fibers are formed as a nonwoven, and a manufacturing method thereof. A membrane for guided bone tissue regeneration according to the present invention has a predetermined strength, biocompatibility, and biodegradability, and may maintain a sustained drug release system, when drugs are added in the manufacturing process. Additionally, a membrane for guided bone tissue regeneration according to the present invention may be modified corresponding to the condition of usage, because a thickness of the membrane may be adjusted by controlling fineness of nanofibers, compactness of nanofibers, and pore size of a multiporous structure may be adjusted, in a nonwoven manufacturing process. A nanofibrous membrane for guided bone tissue regeneration according to the present invention is manufactured by freezing rapidly, drying a silk fibroin solution obtained by removing sericin from silk fibers, and by electrospinning after dissolving the dried silk fibroin in an electrospinning solvent. The membrane according to the present invention has excellent adhesion and air permeability, and is thereby effective in regeneration of damaged periodontal tissues.
Owner:SEOUL NAT UNIV R&DB FOUND
Nano-silver antibacterial hydrogel and preparation method thereof
InactiveCN102698313AAvoid Residual ToxicityAct as a sterilizerSurgeryAbsorbent padsEscherichia coliPorosity
The invention discloses nano-silver antibacterial hydrogel and a preparation method thereof, wherein the antibacterial hydrogel does not contain a chemical cross-linking agent, a cross-linking sensitizing agent and a cross-linking initiator; the porosity is above 90%; the cross-linking degree is above 50%; by weight, the nano-silver antibacterial hydrogel comprises 3-20% of natural polymer or a derivative thereof, 0-20% of synthetic polymer and 0..005-0.2% of nano-silver; and the nano-silver antibacterial hydrogel is prepared by blending the natural polymer or the derivative thereof, the synthetic polymer, a compound containing silver ions and water and then adopting a radiation cross-linking method. The nano-silver antibacterial hydrogel disclosed by the utility model has good biocompatibility and uniform nano-silver distribution and slow-release capability and is capable of effectively inhibiting bacteria, such as escherichia coli.
Owner:北京同威典石技术有限公司
Injectable bone-replacement mixture
InactiveUS20060041033A1Optimal radio-opacityGood biocompatibilityImpression capsSurgical adhesivesInjectable boneRadiographic contrast media
An injectable mixture for substituting bone tissue in situ comprises: A) a two-component powder / liquid bone cement which upon mixing forms a self-hardening cement paste; and B) a third component consisting of a liquid which essentially is non-miscible with the cement paste and which is suitable to be washed out after hardening of said mixture in situ, resulting in a porous bone substituting material; and C) an X-ray contrast agent which is an organic substance. The injectable bone substitute material for bone augmentation has adaptable mechanical properties, an optimal radio-opacity without any inorganic X-ray contrast agent and therefore good biocompatibility.
Owner:SYNTHES USA
Collagen material and its production process
InactiveUS7084082B1Avoid stickingPromote regenerationSuture equipmentsSynthetic resin layered productsEndocraniumBiocompatibility Testing
The objective of the present invention is to provide a collagen material that possesses physical properties to an extent that allows suturing while still maintaining the biochemical properties inherently possessed by collagen, and retains its shape for a certain amount of time even after application to the body; its production process; and, a medical material on which it is based, examples of which include a artificial tube for nerve, artificial tube for spinal cord, artificial esophagus, artificial trachea, artificial blood vessel, artificial valve or alternative medical membranes such as artificial endocranium, artificial ligaments, artificial tendons, surgical sutures, surgical prostheses, surgical reinforcement, wound protecting materials, artificial skin and artificial cornea, characterized by filling or having inside a substance having biocompatibility that can be degraded and absorbed in the body into a matrix of a non-woven fabric-like multi-element structure of collagen fibers having ultra-fine fibers of collagen as its basic unit.
Owner:TAPIC INT
Stent Coating For Eluting Medication
InactiveUS20060200231A1Good biocompatibilityPrevention and therapyStentsSurgeryPorosityDiamond-like carbon
A vascular stent comprising a drug-eluting outer layer of a porous sputtered columnar metal having each column capped with a biocompatible carbon-containing material is described. This is done by placing the stent over a close-fitting mandrel and rotating the assembly in a sputter flux. The result is a coating that is evenly distributed over the outward-facing side of the stent's wire mesh while preventing the sputtered columnar coating from reaching the inward facing side where a smooth hemocompatible surface is required. The stent is then removed from the mandrel, exposing all surfaces, and finally coated with a layer of carbon such as amorphous carbon or diamond-like carbon. The carbonaceous coating enhances biocompatibility without preventing elutriation of a therapeutic drug provided in the porosity formed between the columnar structures. The result is a stent that is adapted to both the hemodynamic and the immune response requirements of its vascular environment.
Owner:WILSON GREATBATCH LTD
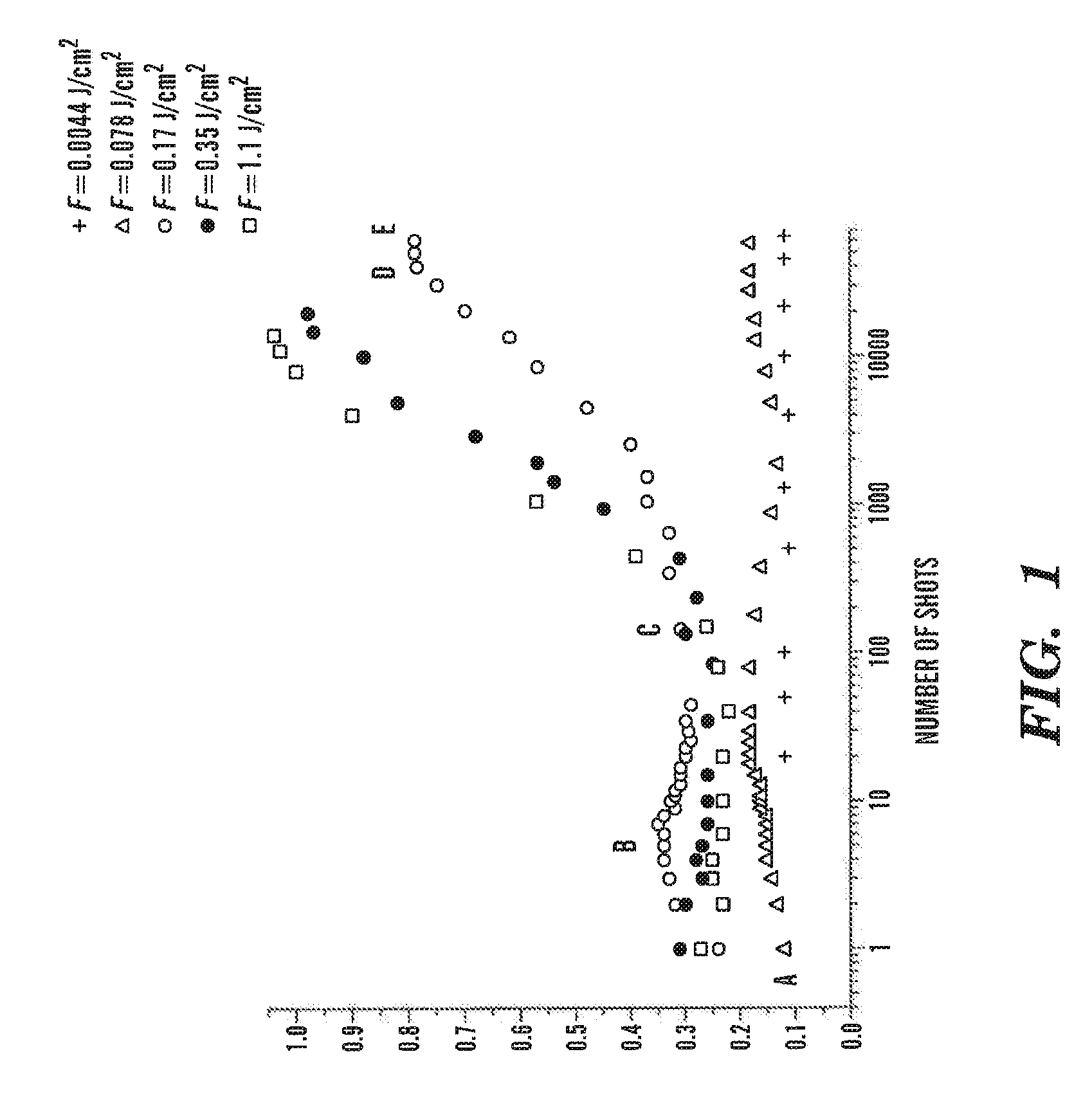
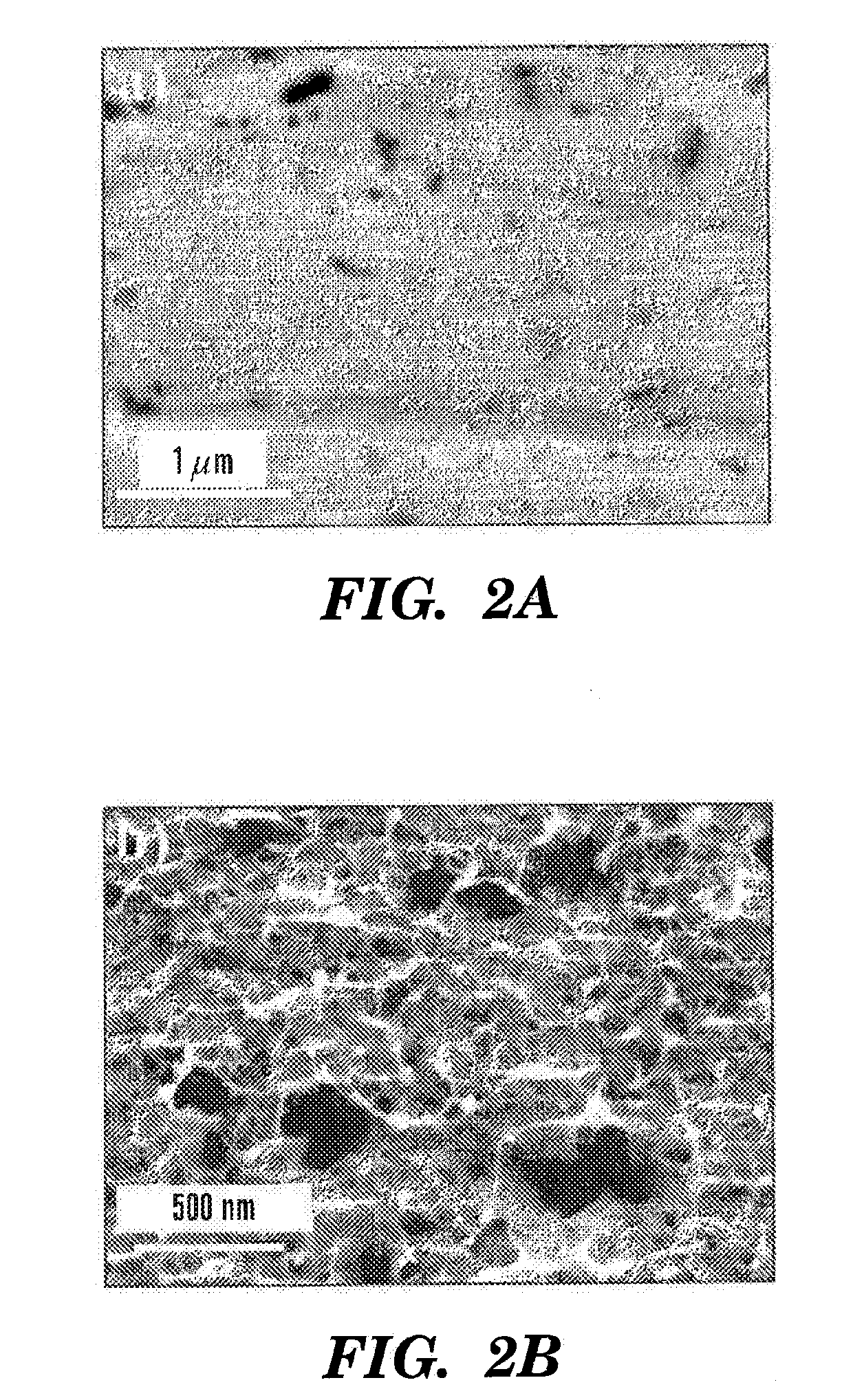
Femtosecond Laser Pulse Surface Structuring Methods and Materials Resulting Therefrom
InactiveUS20080299408A1Material nanotechnologyEngine sealsBiocompatibility TestingMaterials processing
Embodiments of the present invention are generally directed to materials processing methods using femtosecond duration laser pulses, and to the altered materials obtained by such methods. The resulting nanostructured (with or without macro- and micro-structuring) materials have a variety of applications, including, for example, aesthetic applications for jewelry or ornamentation; biomedical applications related to biocompatibility; catalysis applications; and modification of, for example, the optical and hydrophilic properties of materials including selective coloring.
Owner:UNIVERSITY OF ROCHESTER
Method of fabricating an implantable medical device to reduce chance of late inflammatory response
The invention provides a method for fabricating an implantable medical device to increase biocompatibility of the device, the method comprising: heat setting a polymer construct, wherein the polymer construct is at a temperature range of from about Tg to about 0.6(Tm−Tg)+Tg such that the set polymer construct comprises a crystalline structure having crystals at a size less than about 2 microns; and fabricating an implantable medical device from the heat set polymer construct.
Owner:ABBOTT CARDIOVASCULAR
Hyaluronic Acid Compound, Hydrogel Thereof and Joint Treating Material
ActiveUS20080193538A1Good biological adaptabilityPowder deliveryBiocideBiocompatibility TestingHyaluronic acid
A hyaluronic acid compound which is a reaction product between hyaluronic acid and a phosphatidyl ethanolamine. This compound has biocompatibility and bio-safety, and can be formed into a hydrogel or molded form having a certain shape. Making use of these properties, it is used to treat a knee joint, prevent the accretion of a tissue after an operation and keep a skin wet.
Owner:TEIJIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com