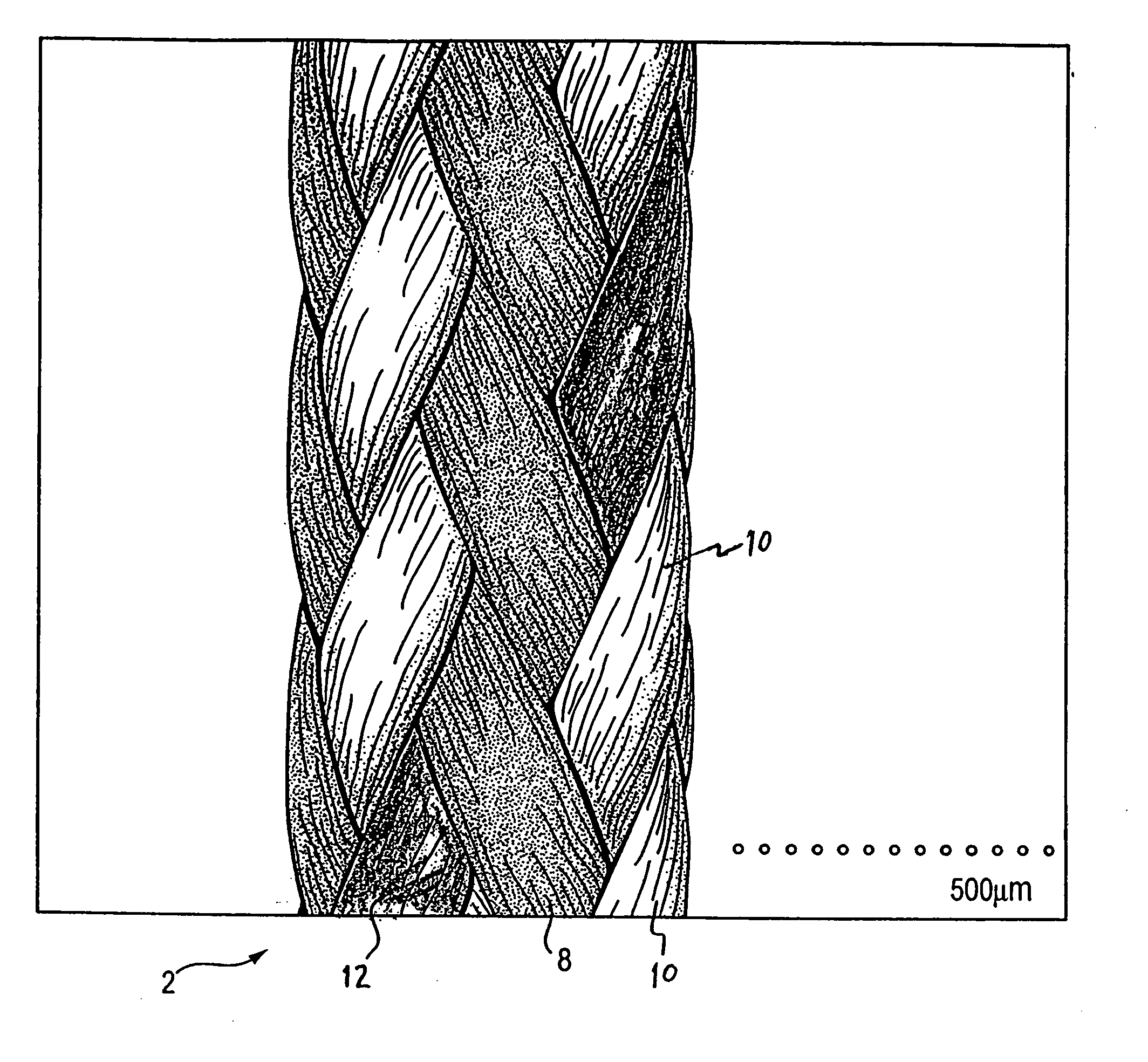
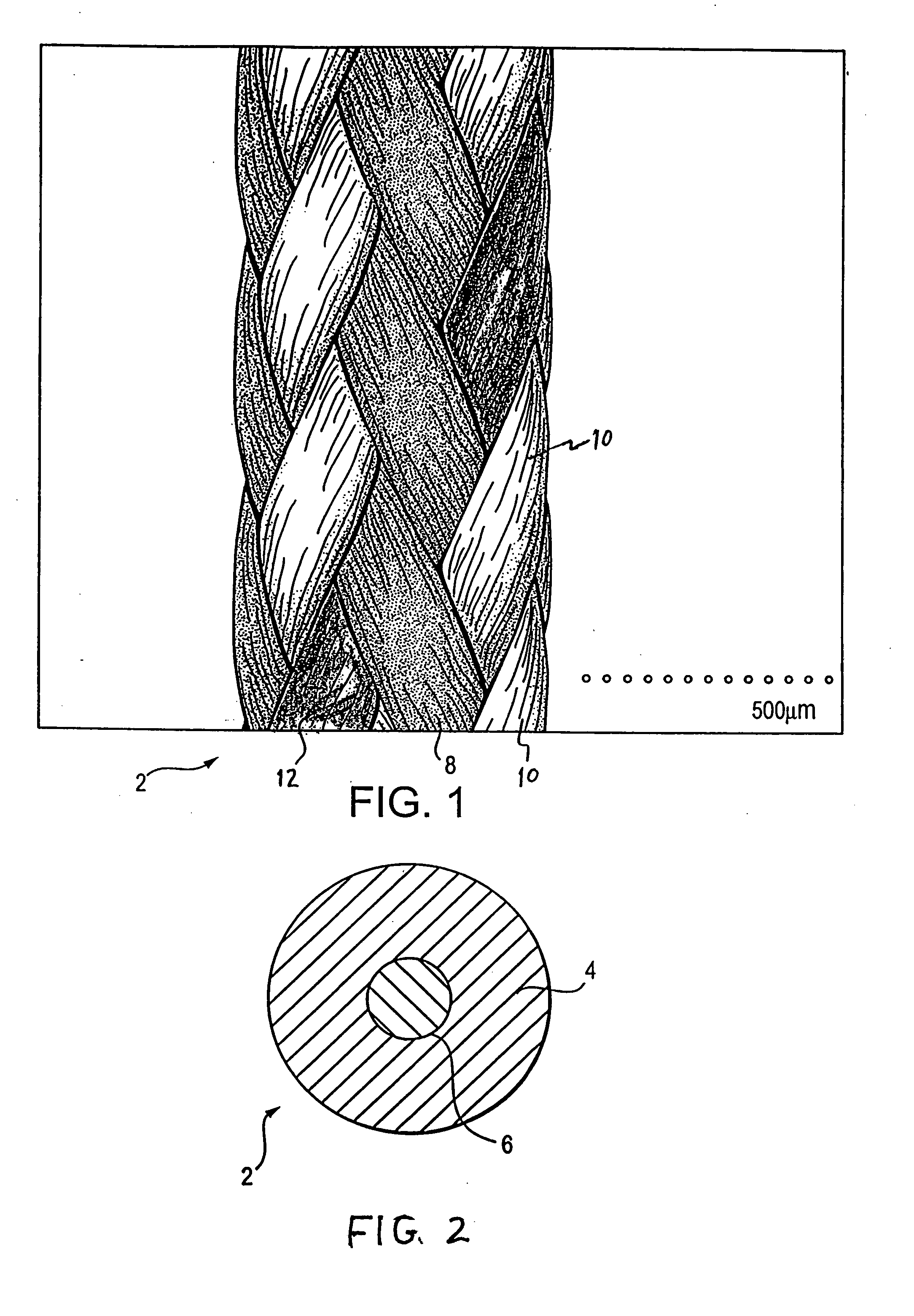
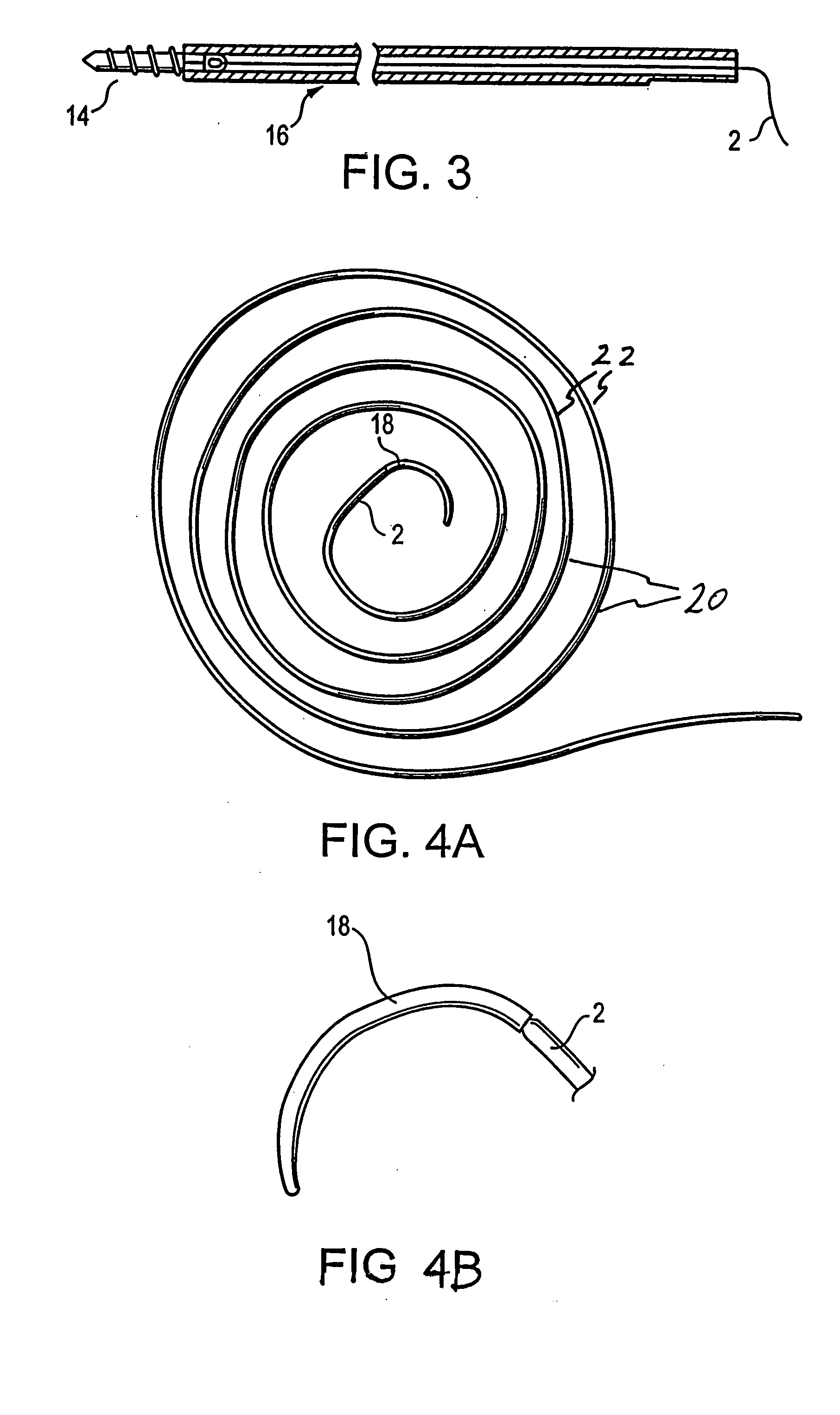
Patents
Literature
93 results about "Tissue remodeling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tissue remodeling is the reorganization or renovation of existing tissues. Tissue remodeling can be either physiological or pathological. The process can either change the characteristics of a tissue such as in blood vessel remodeling, or result in the dynamic equilibrium of a tissue such as in bone remodeling. Macrophages repair wounds and remodel tissue by producing extracellular matrix and proteases to modify that specific matrix.
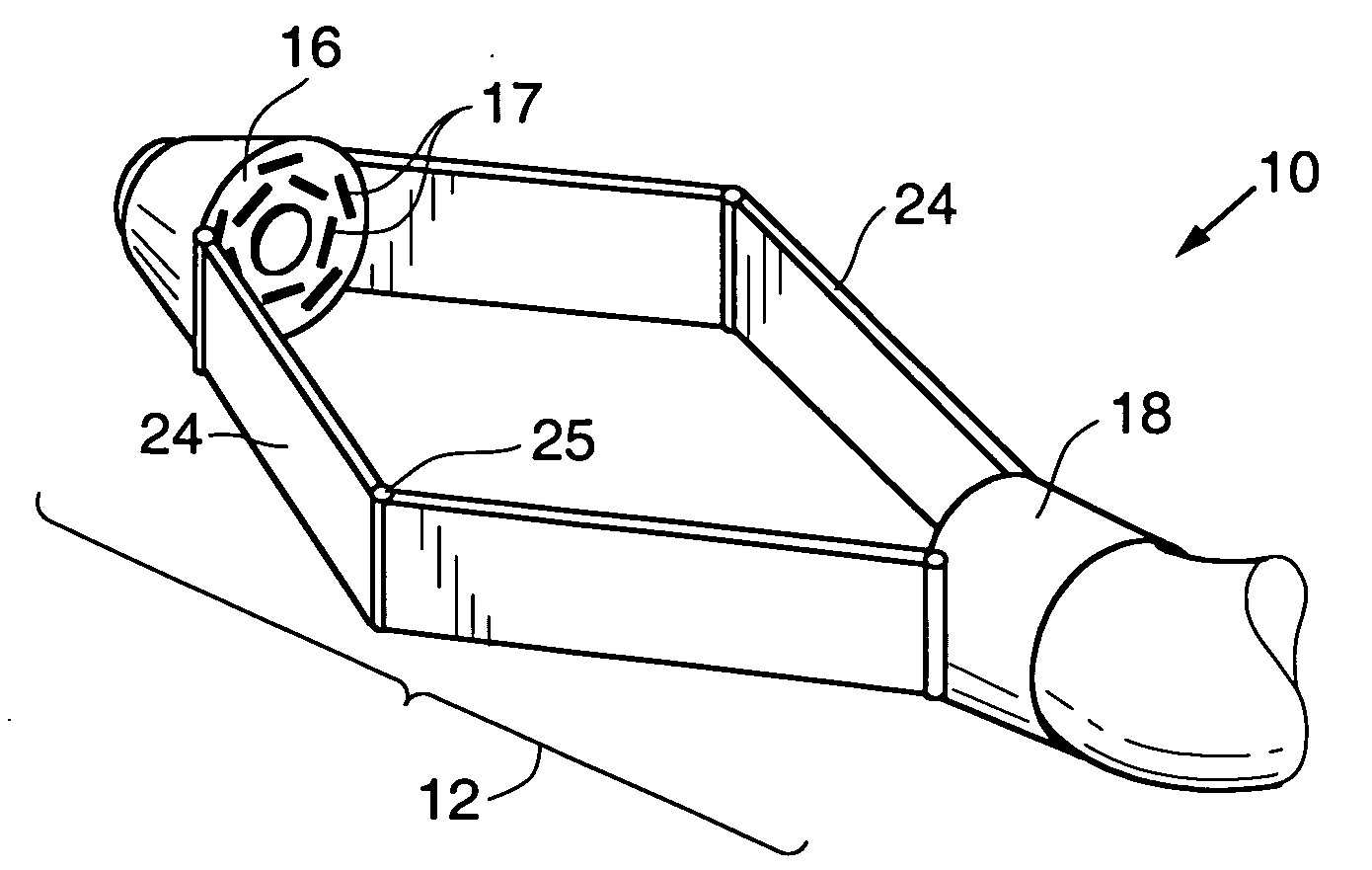
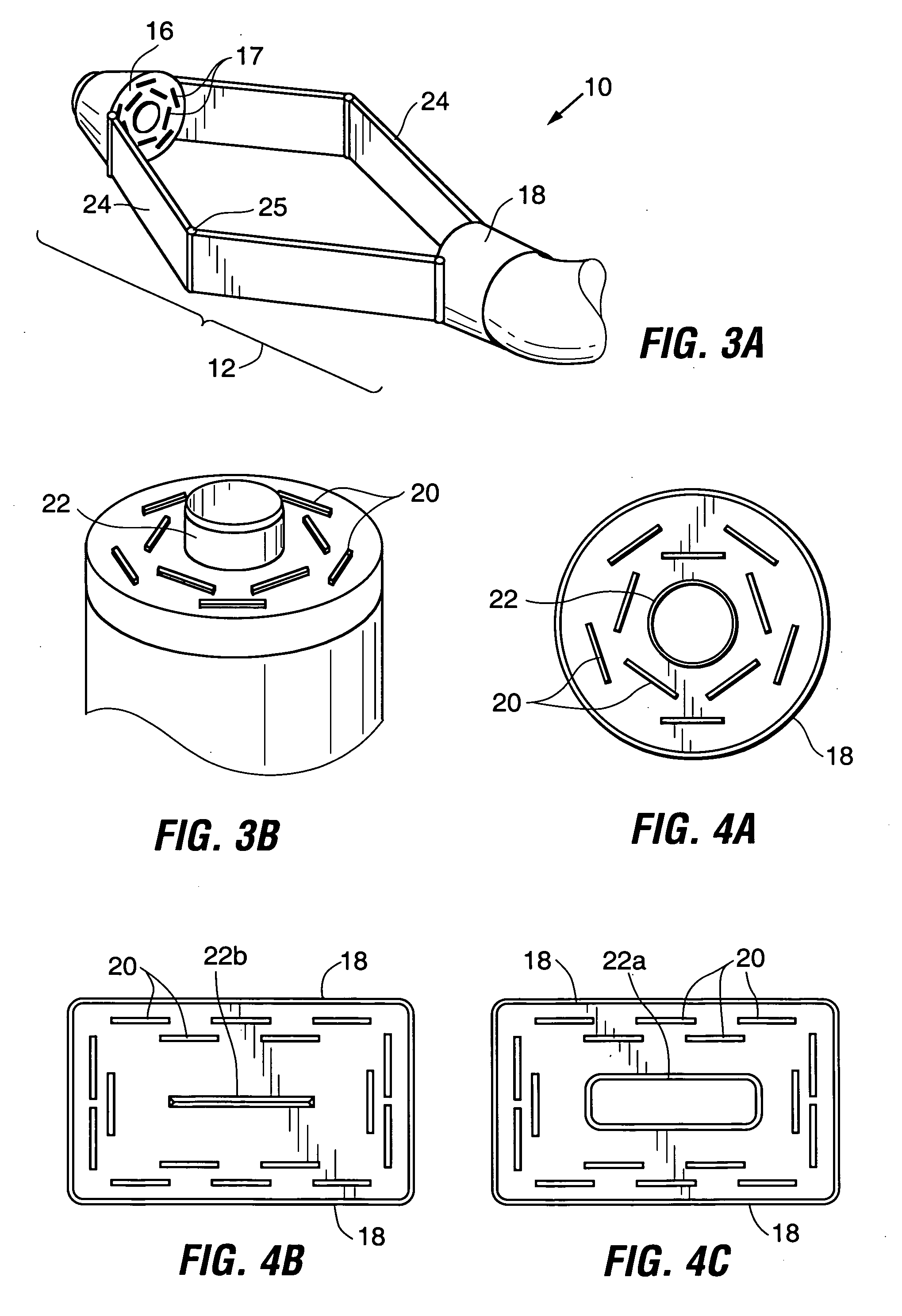
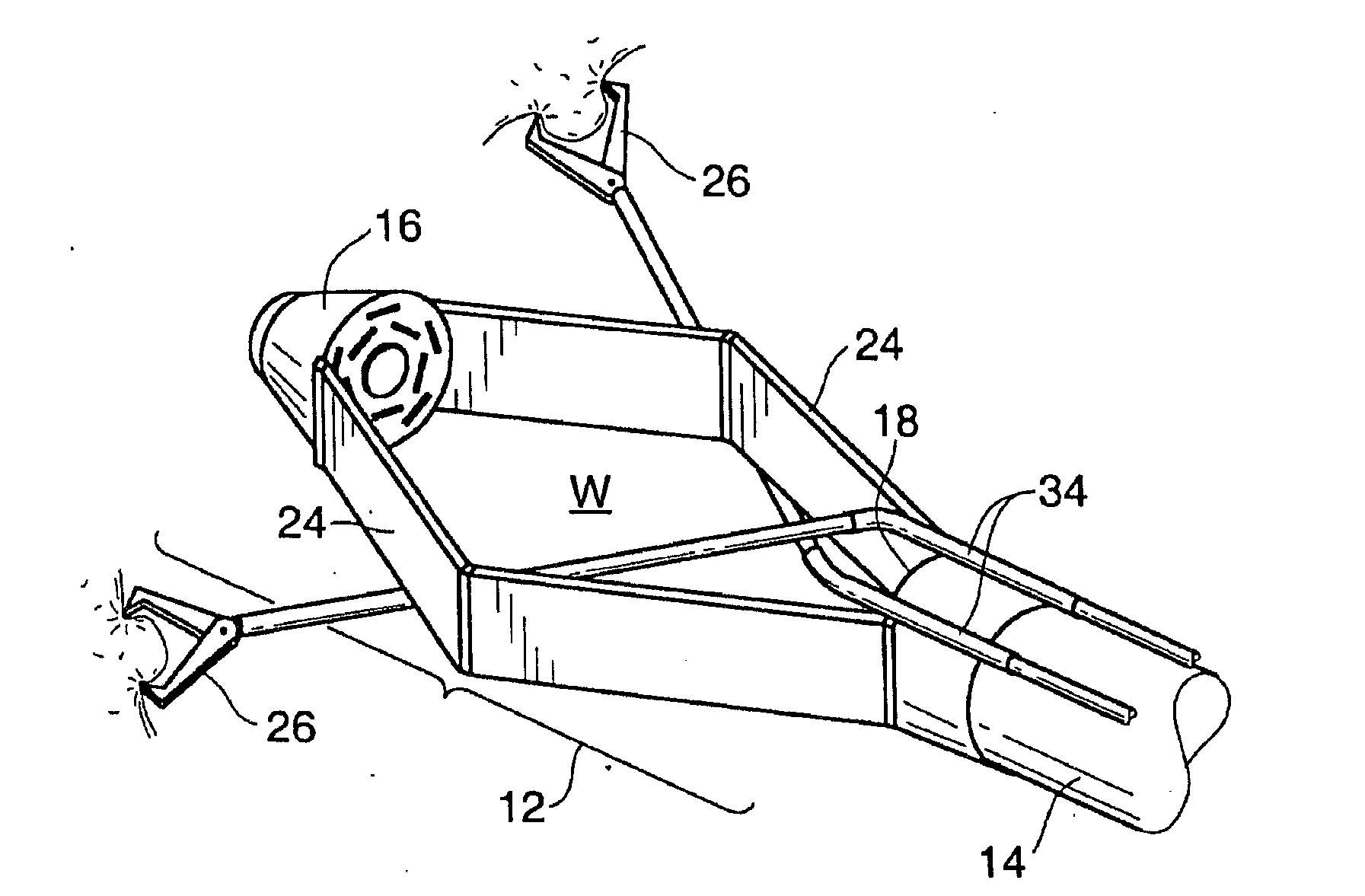
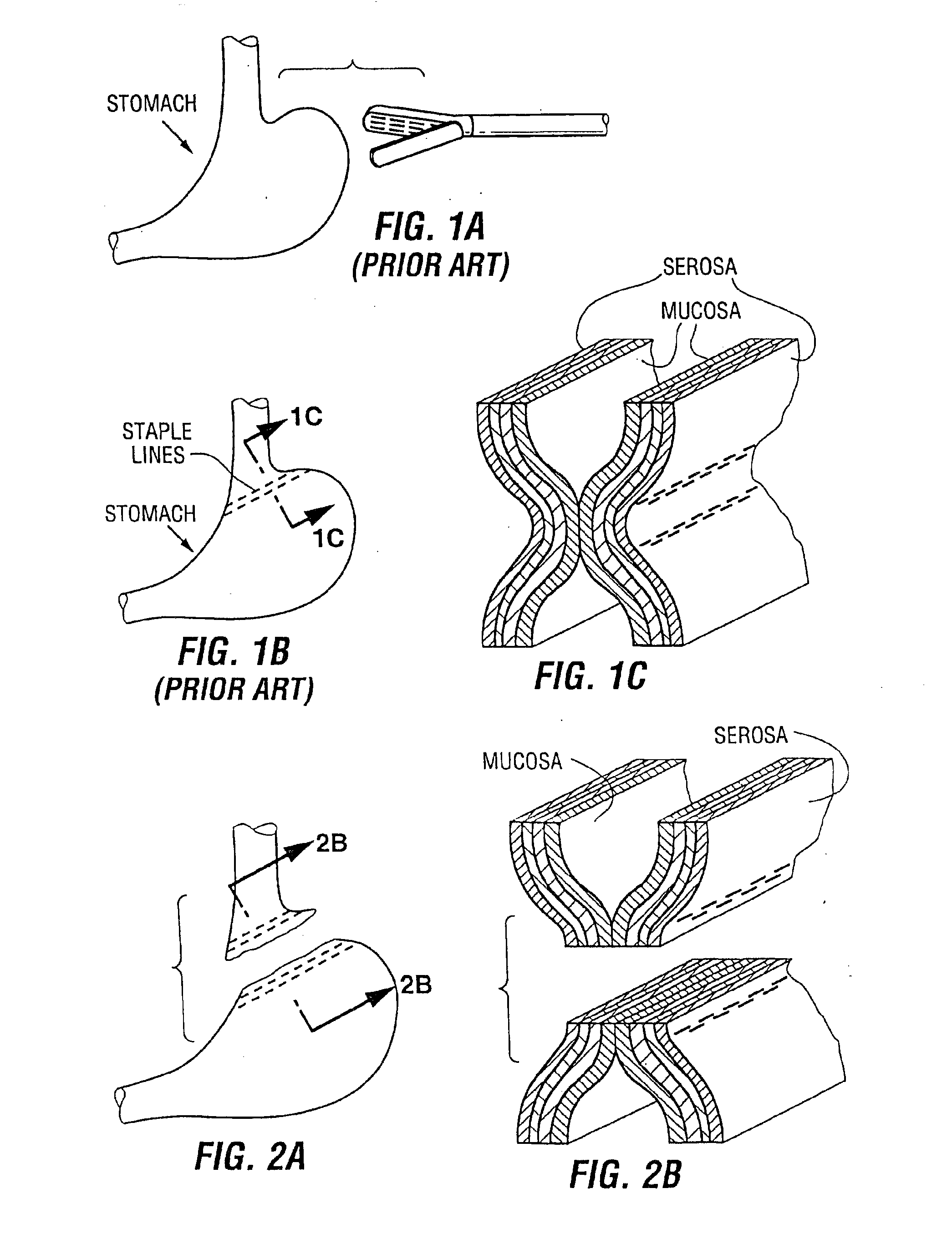
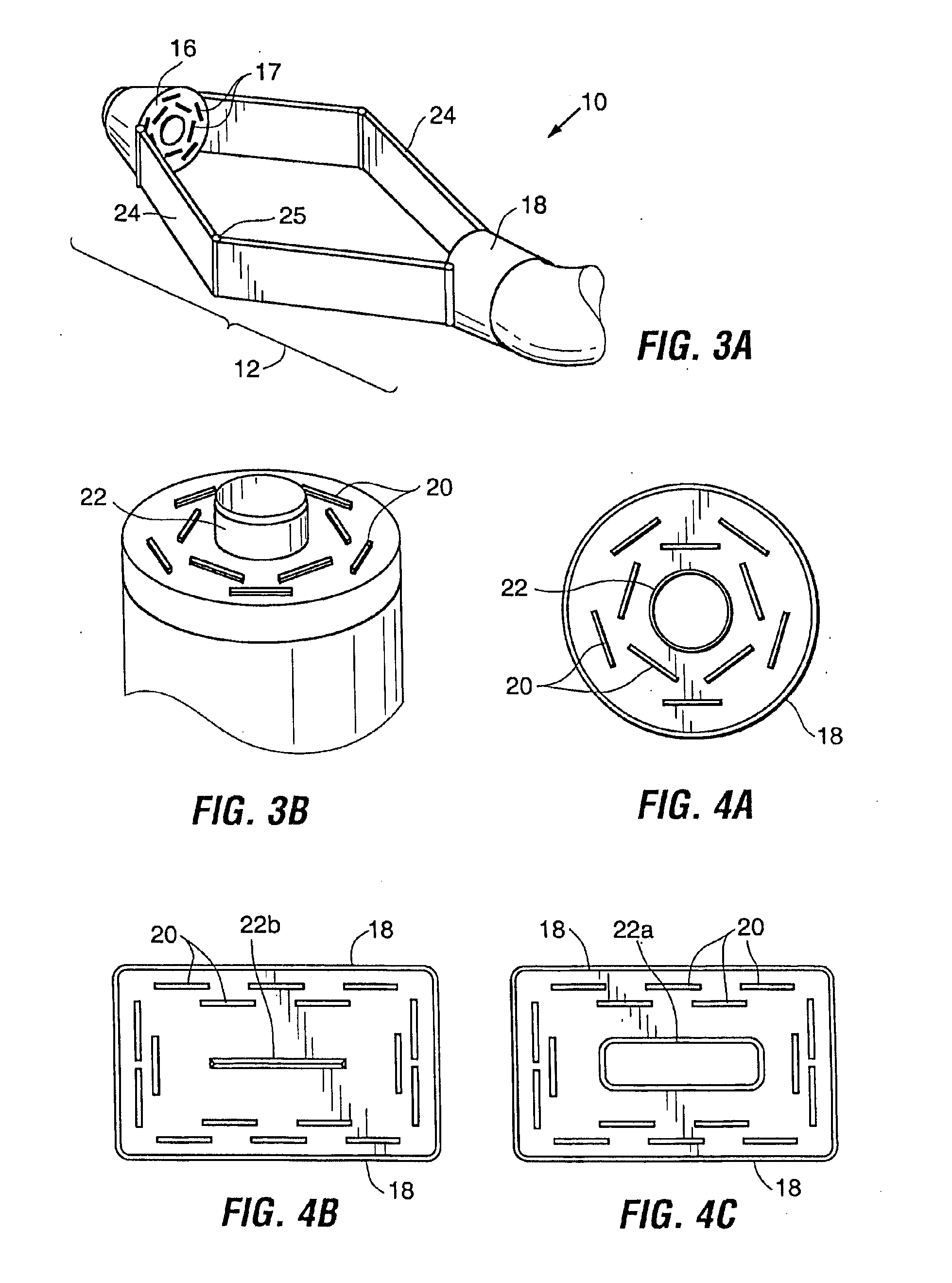
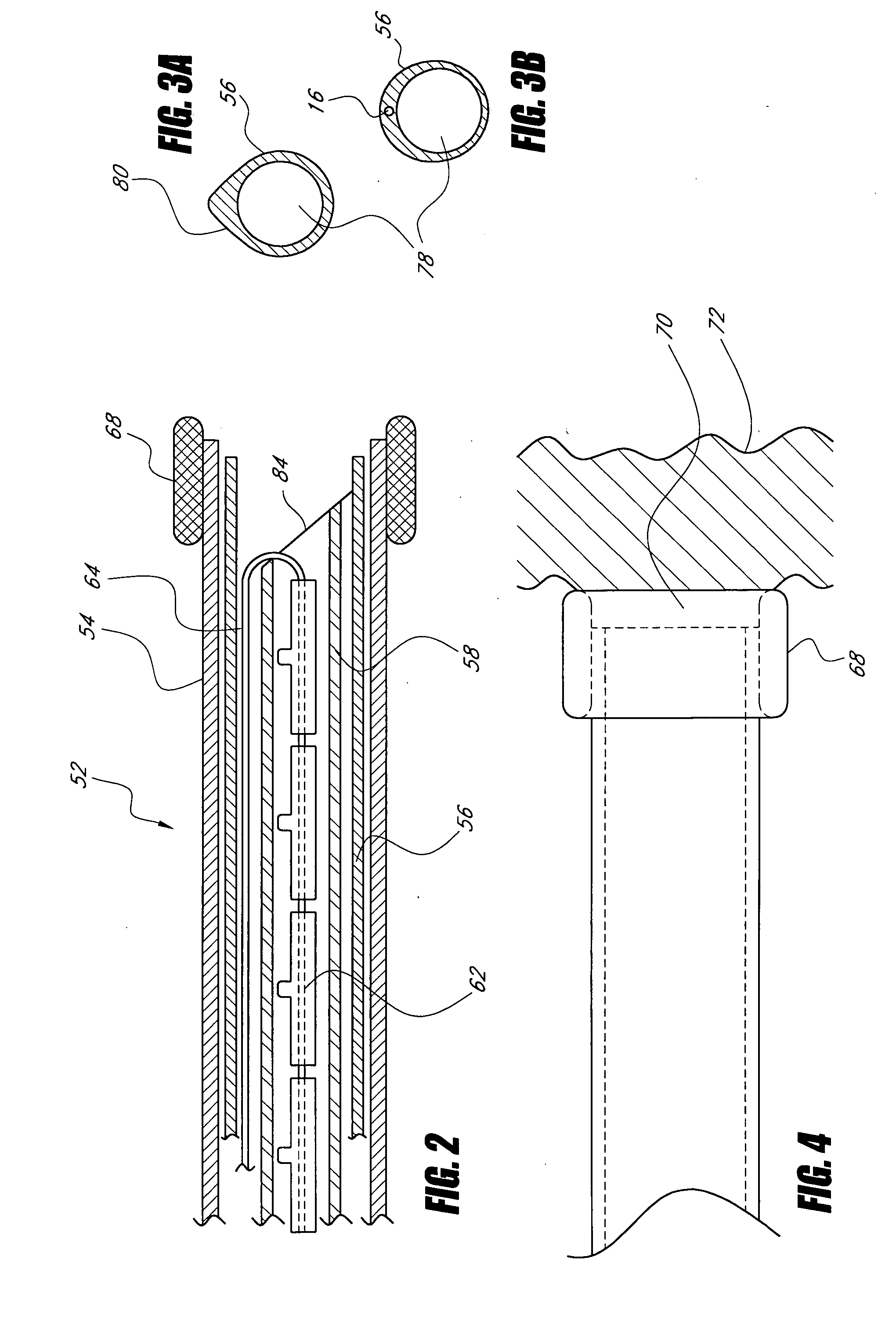
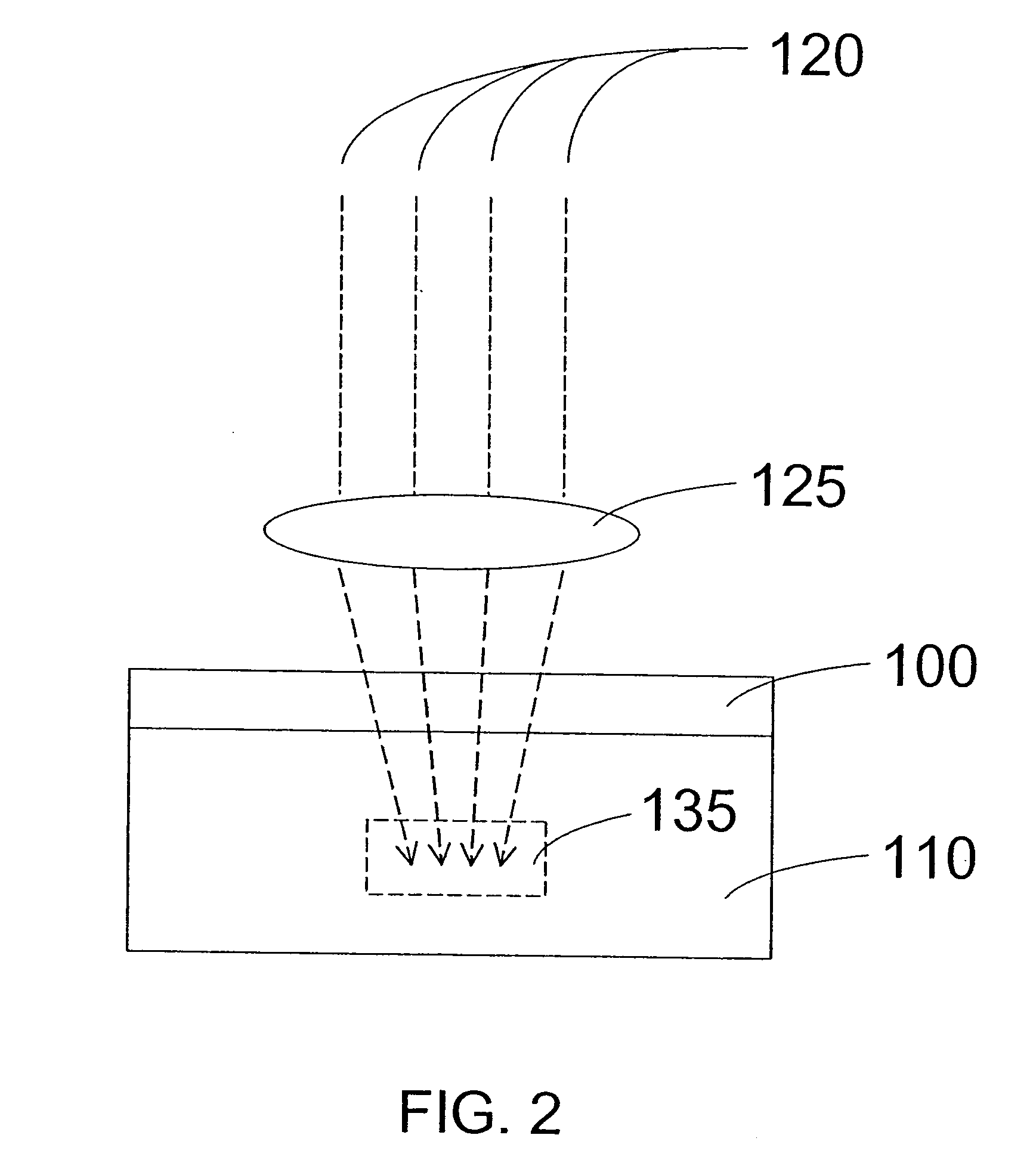
Devices and methods for stomach partitioning
A device and method for remodeling or partitioning a body cavity, hollow organ or tissue tract includes graspers operable to engage two or more sections of tissue within a body cavity and to draw the engaged tissue between a first and second members of a tissue remodeling tool. The two or more pinches of tissue are held in complete or partial alignment with one another as staples or other fasteners are driven through the pinches, thus forming a four-layer tissue plication. Over time, adhesions formed between the opposed serosal layers create strong bonds that can facilitate retention of the plication over extended durations, despite the forces imparted on them by stomach movement. A cut or cut-out may be formed in the plication during or separate from the stapling step to promote edge-to-edge healing effects that will enhance tissue knitting / adhesion.
Owner:BAROSENSE
Devices and methods for stomach partitioning
A device and method for remodeling or partitioning a body cavity, hollow organ or tissue tract includes graspers operable to engage two or more sections of tissue within a body cavity and to draw the engaged tissue between a first and second members of a tissue remodeling tool. The two or more pinches of tissue are held in complete or partial alignment with one another as staples or other fasteners are driven t9hrough the pinches, thus forming a four-layer tissue plication. Over time, adhesions formed between the opposed serosal layers create strong bonds that can facilitate retention of the plication over extended durations, despite the forces imparted on them by stomach movement. A cut or cut-out may be formed in the plication during or separate from the stapling step to promote edge-to-edge healing effects that will enhance tissue knitting / adhesion.
Owner:BOSTON SCI SCIMED INC
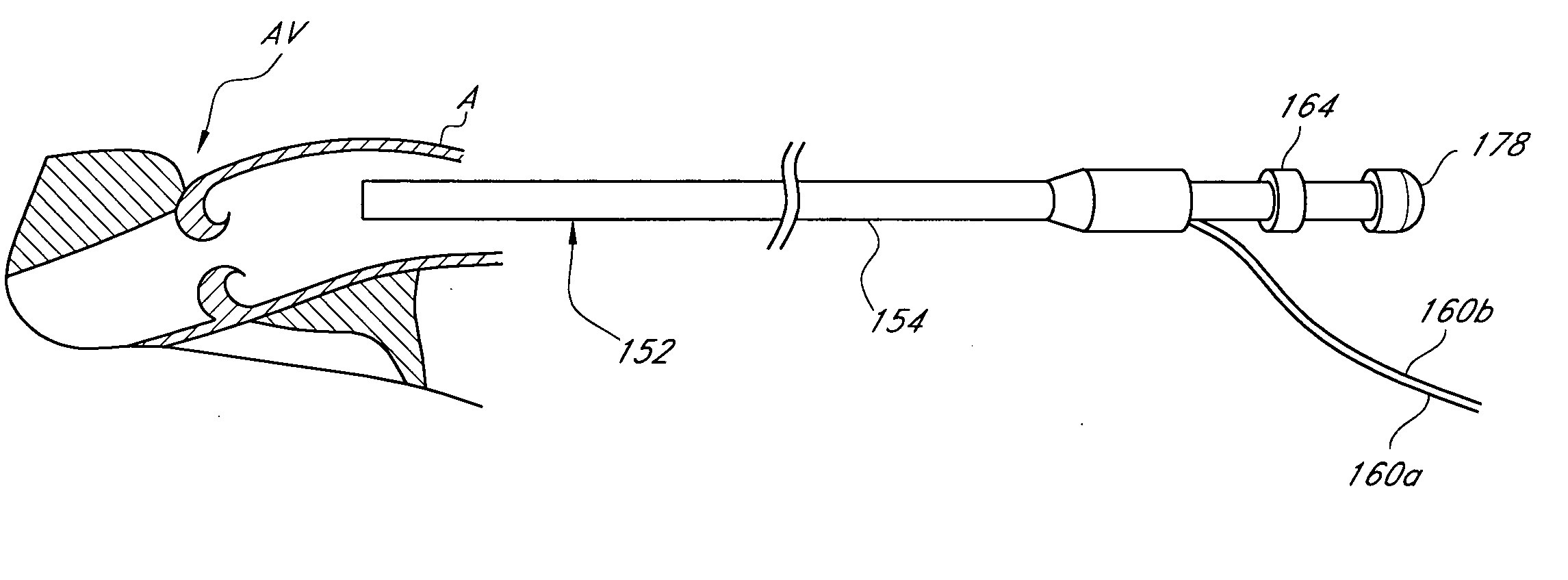
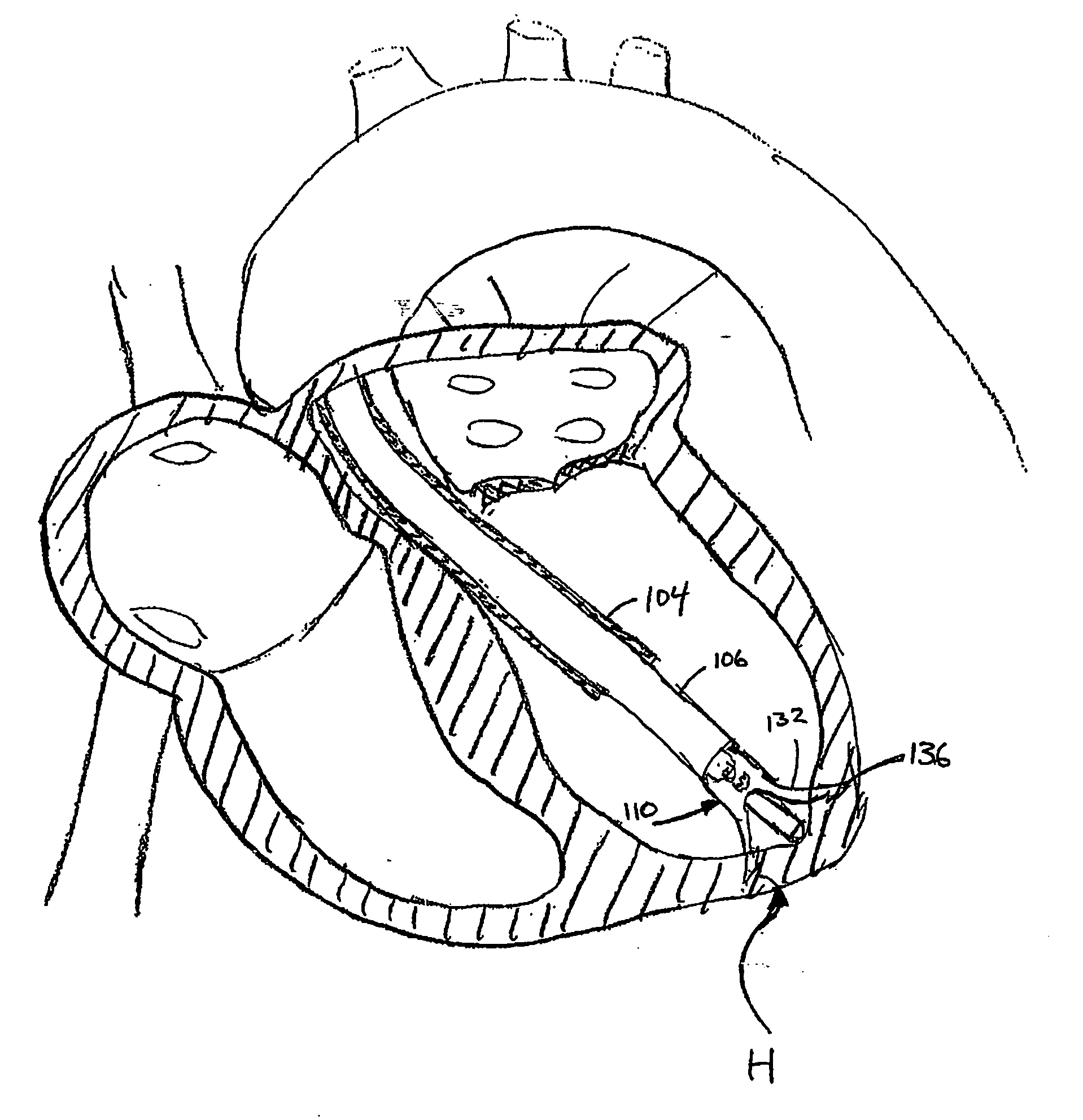
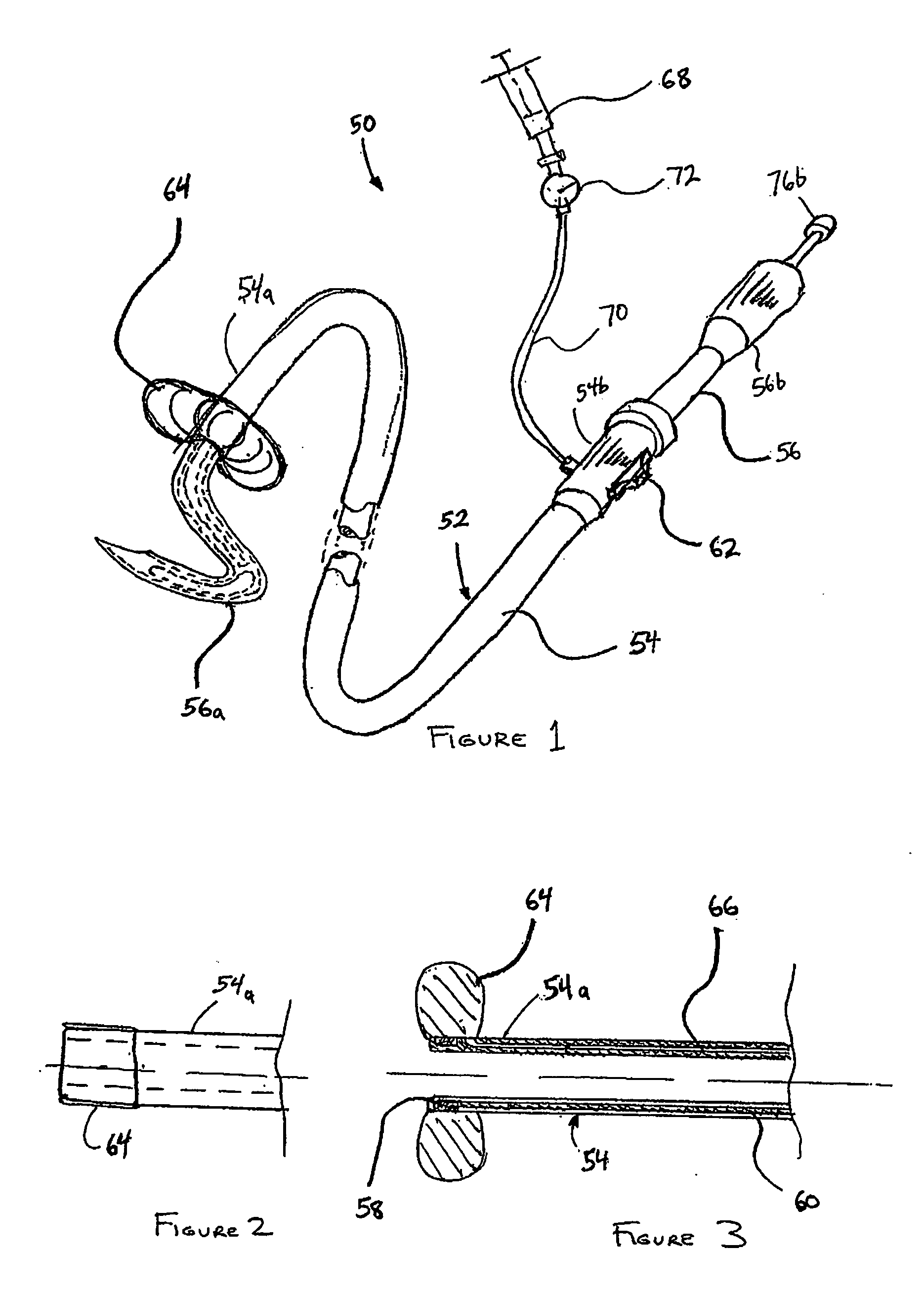
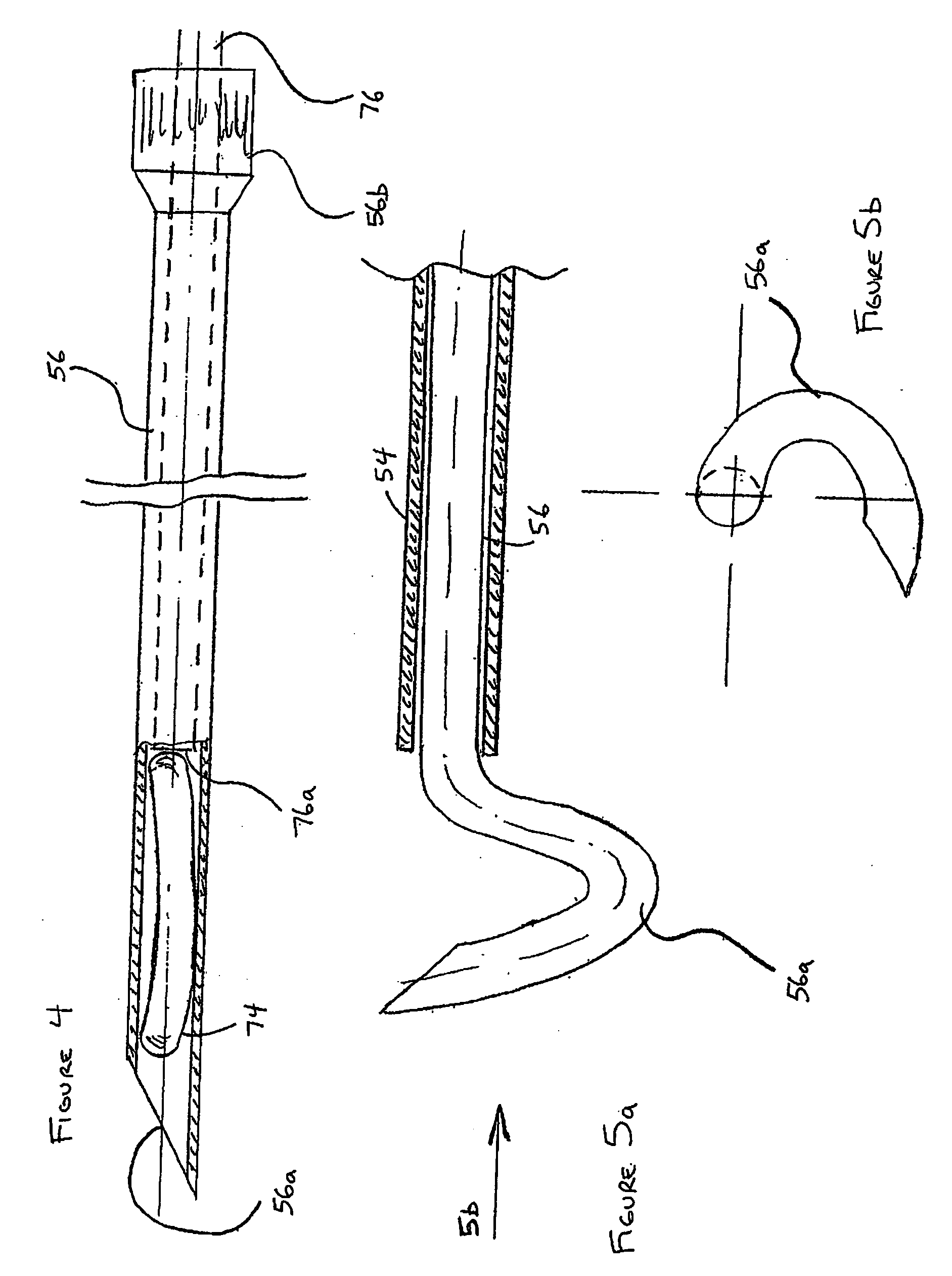
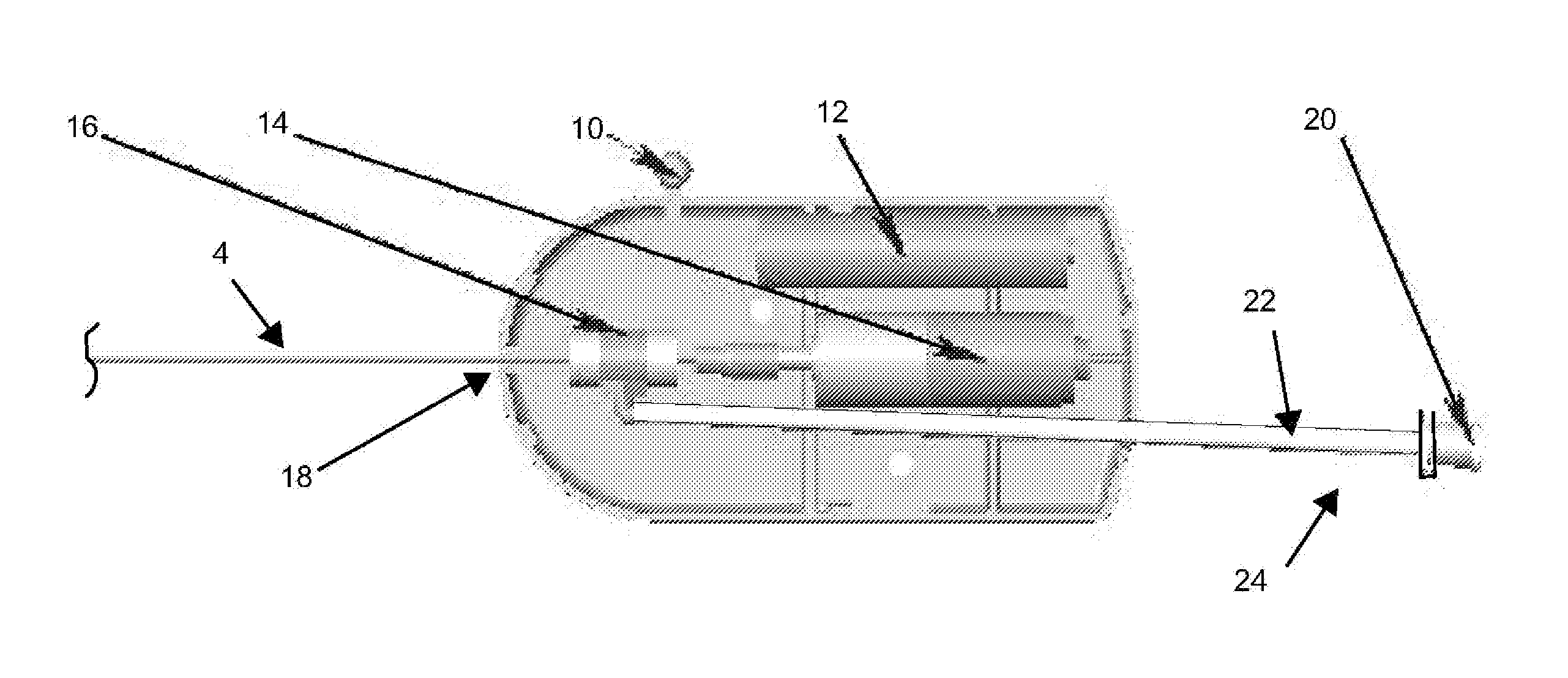
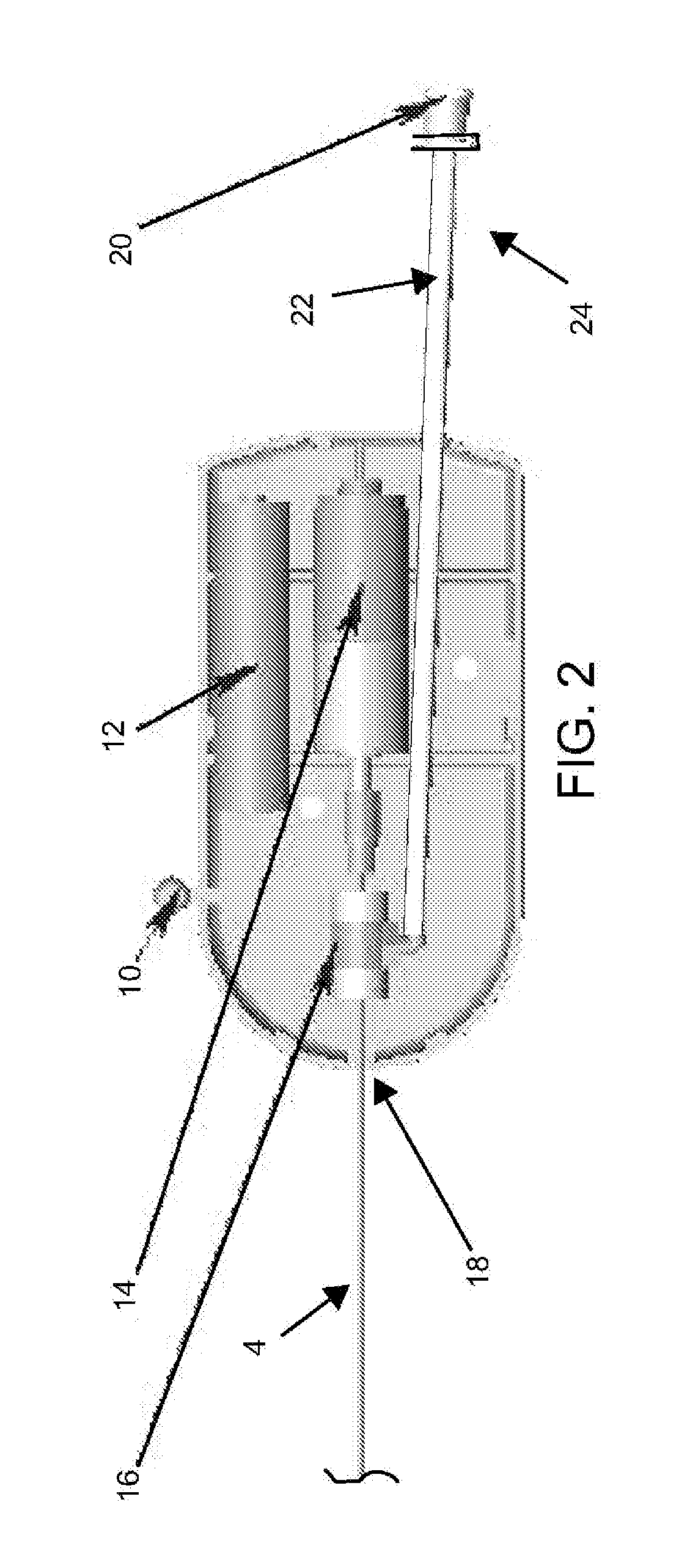
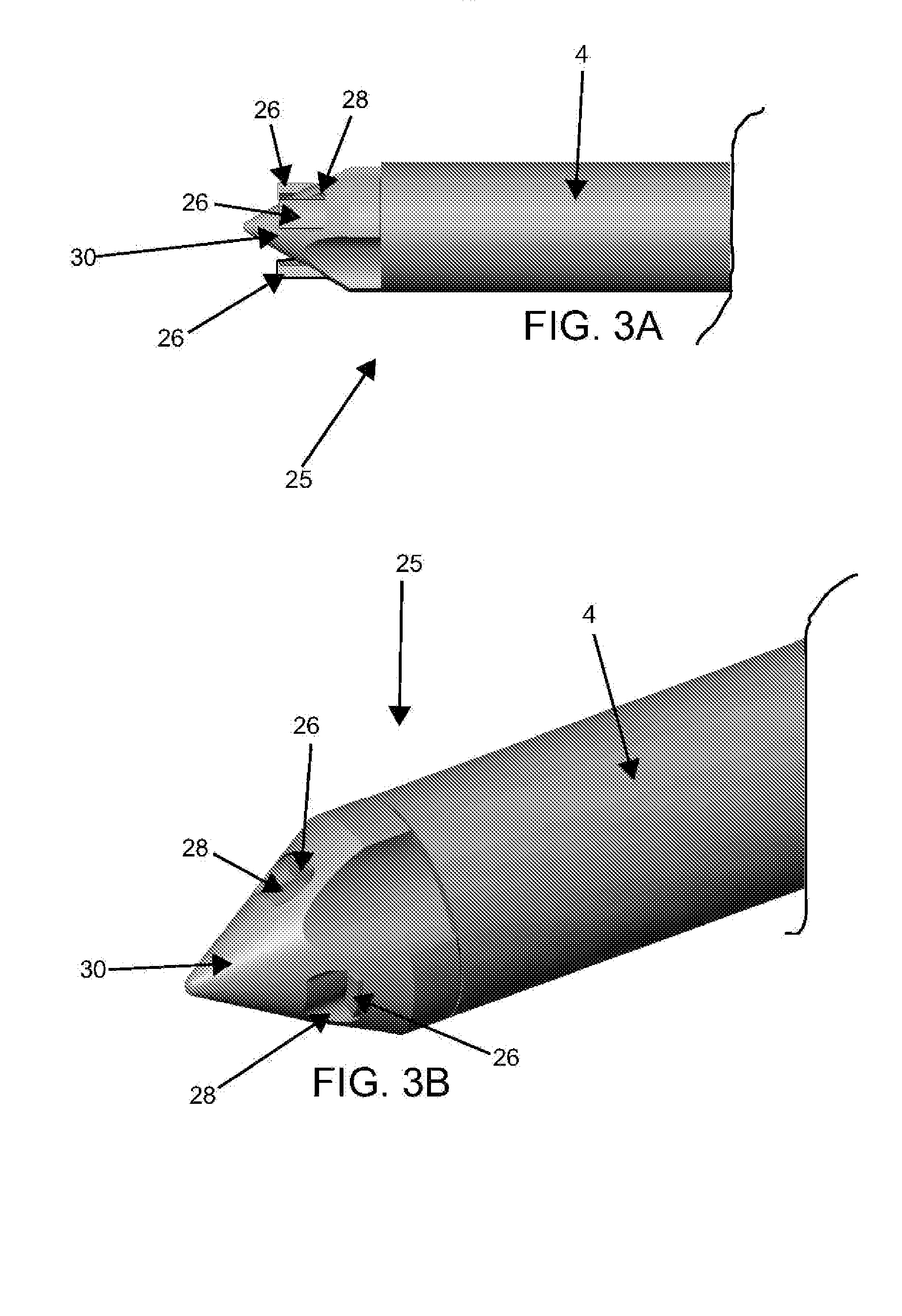
Catheter-based tissue remodeling devices and methods
ActiveUS20070112425A1Improve valve functionFunction increaseSuture equipmentsSurgical needlesLeft Ventricle RemodelingTissue remodeling
Methods and systems for closing an opening or defect in tissue, closing a lumen or tubular structure, cinching or remodeling a cavity or repairing a valve preferably utilizing a purse string or elastic device. The preferred devices and methods are directed toward catheter-based percutaneous, transvascular techniques used to facilitate placement of the devices within lumens, such as blood vessels, or on or within the heart to perform structural defect repair, such as valvular or ventricular remodeling. In some methods, the catheter is positioned within the right ventricle, wherein the myocardial wall or left ventricle may be accessed through the septal wall to position a device configured to permit reshaping of the ventricle. The device may include a line or a plurality of anchors interconnected by a line. In one arrangement, the line is a coiled member.
Owner:EDWARDS LIFESCIENCES CORP
Method and apparatus for dermatological treatment and tissue reshaping
ActiveUS20050222565A1Treatment safetyMinor side effectsElectrotherapySurgical needlesWound healingTissue remodeling
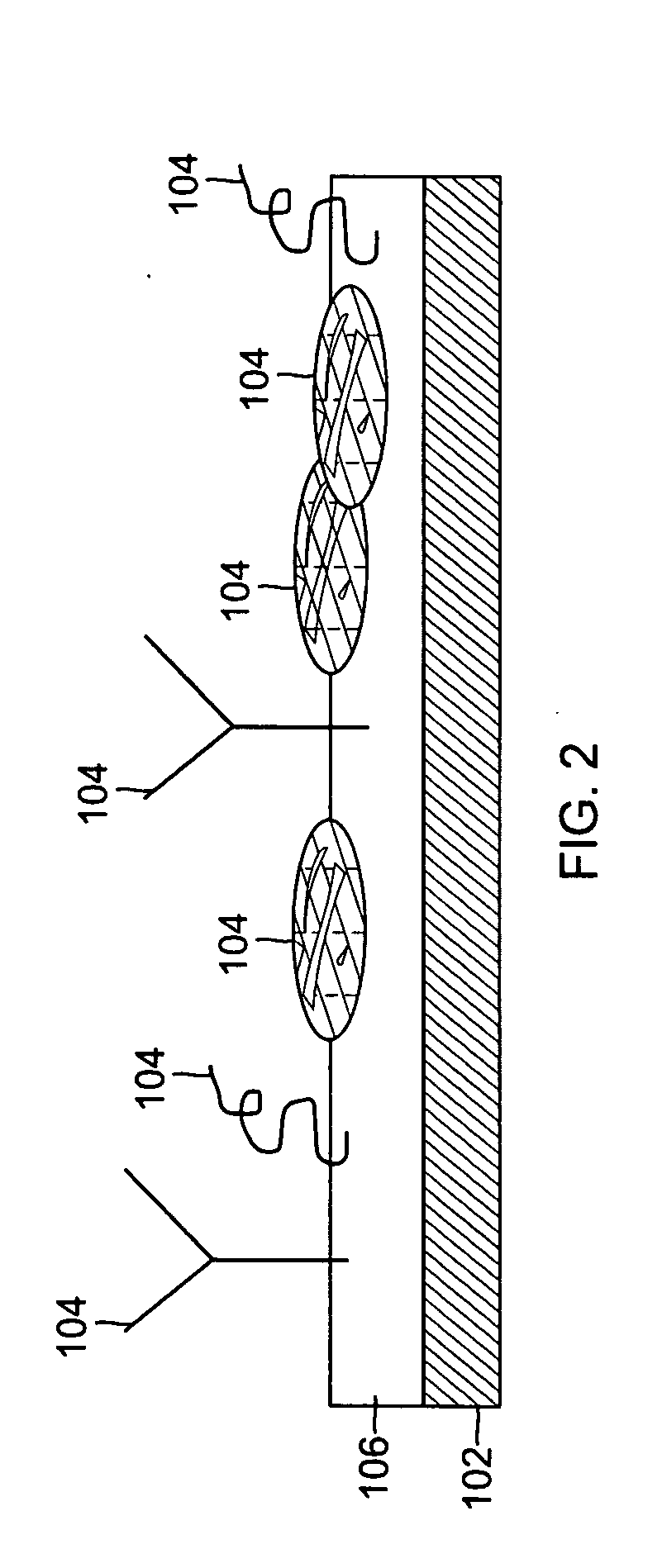
The present invention provides improved methods and apparatus for skin treatment and tissue remodeling. The apparatus includes an array of needles that penetrate the skin and serve as electrodes to deliver radio frequency current or other electrical or optical energy into the tissue being treated, causing thermal damage in controlled patterns. The damaged regions promote beneficial results such as uniform skin tightening by stimulation of wound healing and collagen growth.
Owner:THE GENERAL HOSPITAL CORP
Catheter with cryogenic and heating ablation
InactiveUS7097641B1Improve versatilityEnhancing speed and placement lesionCatheterSurgical instruments for heatingTissue remodelingCelsius Degree
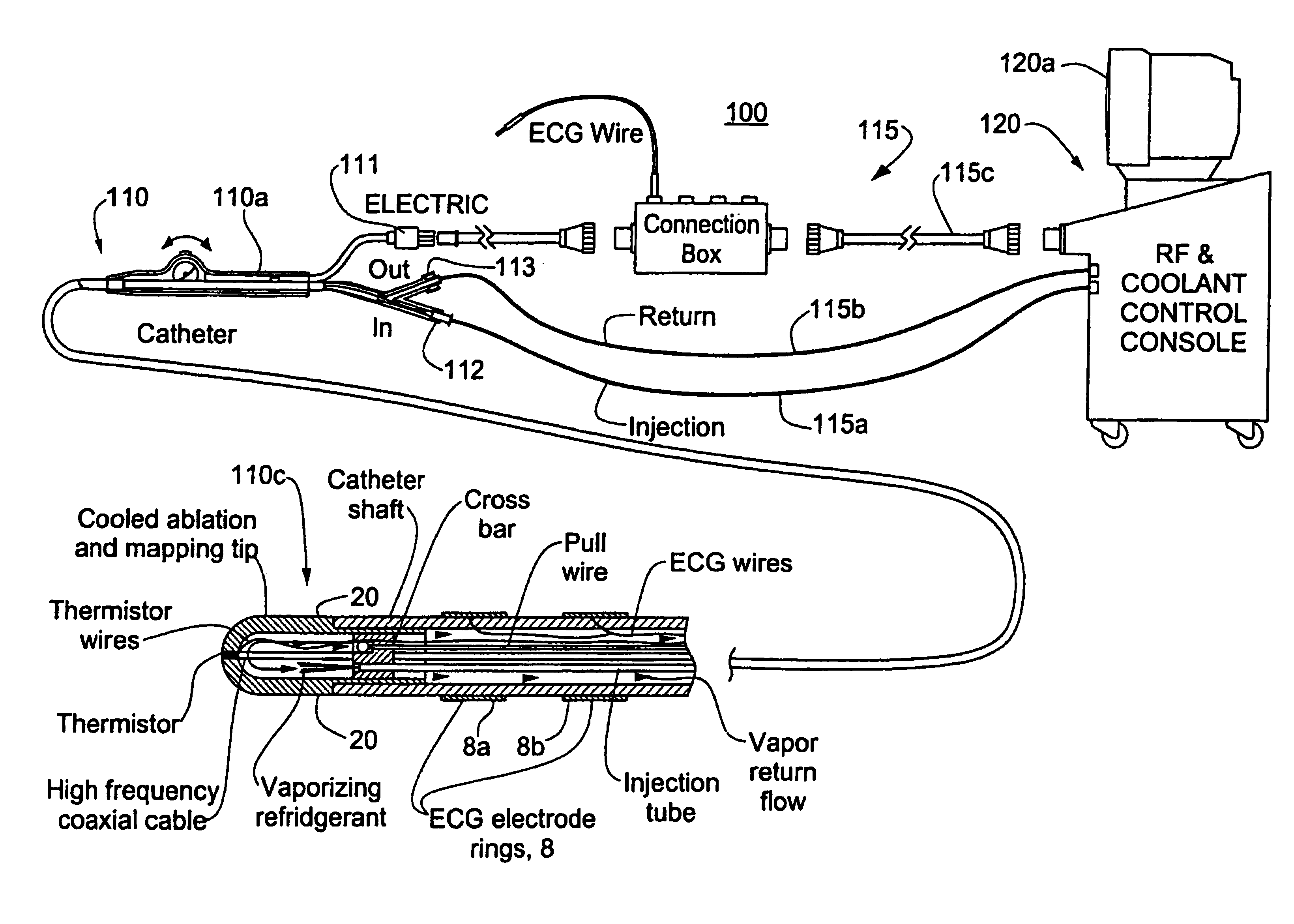
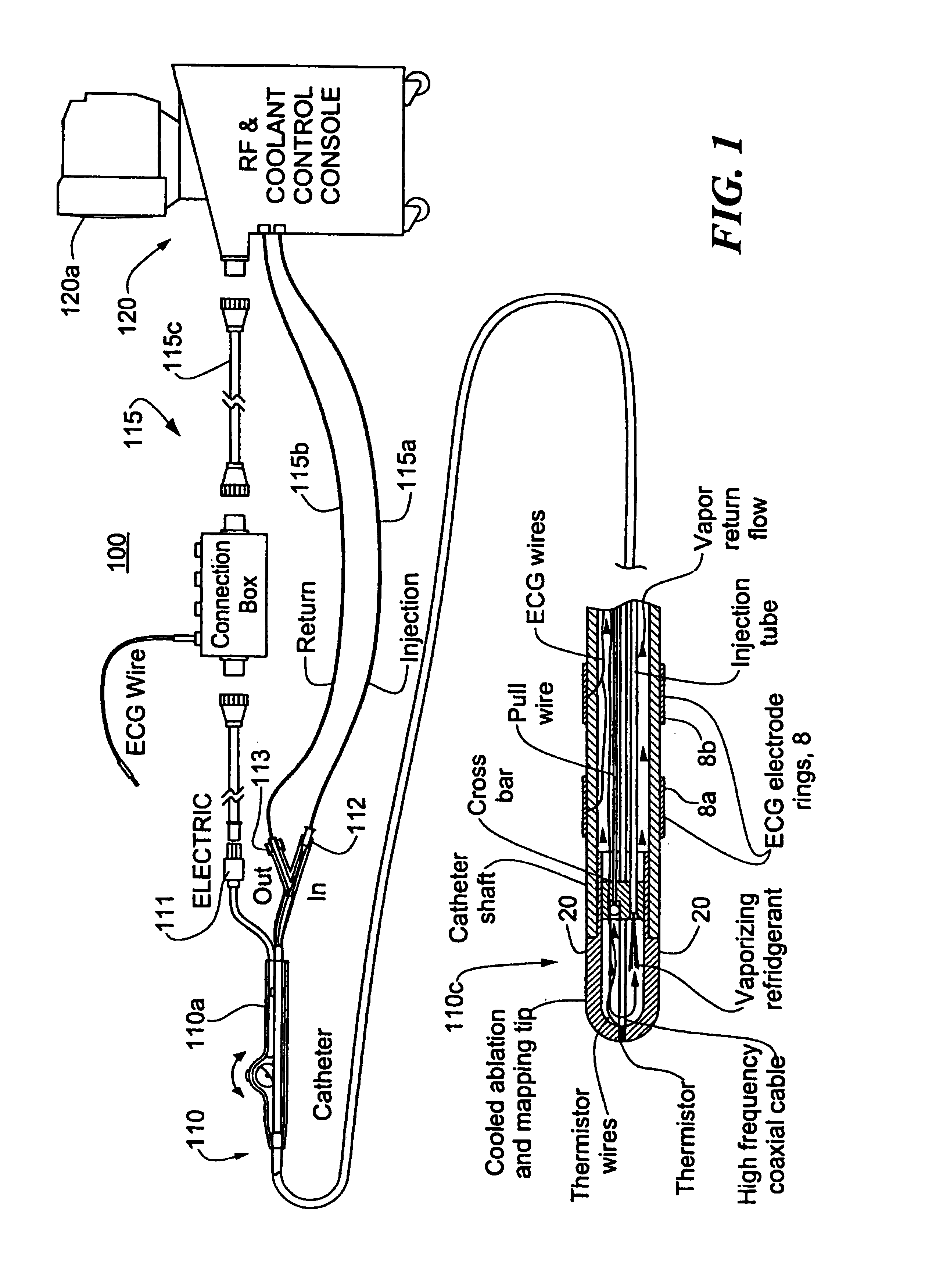
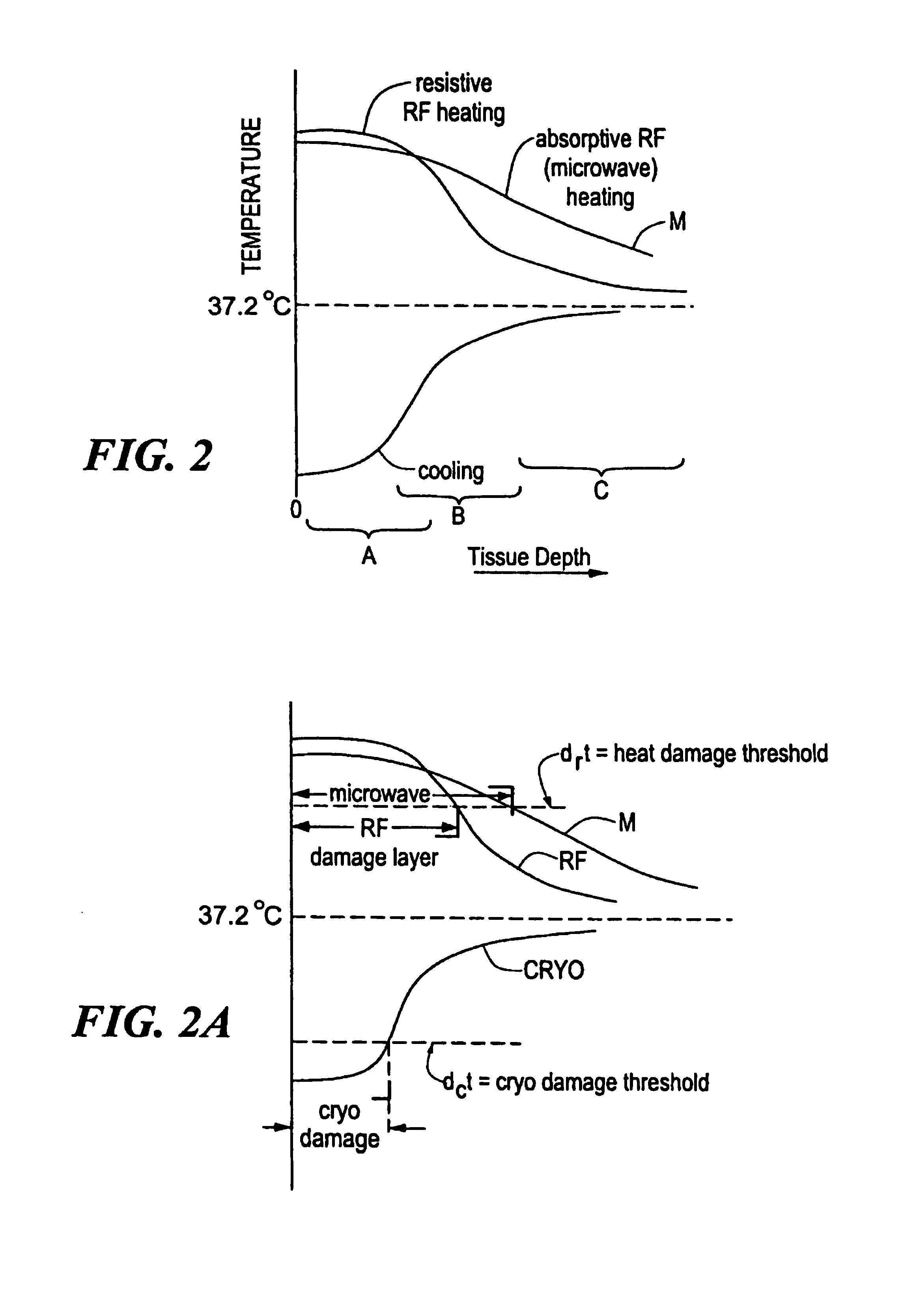
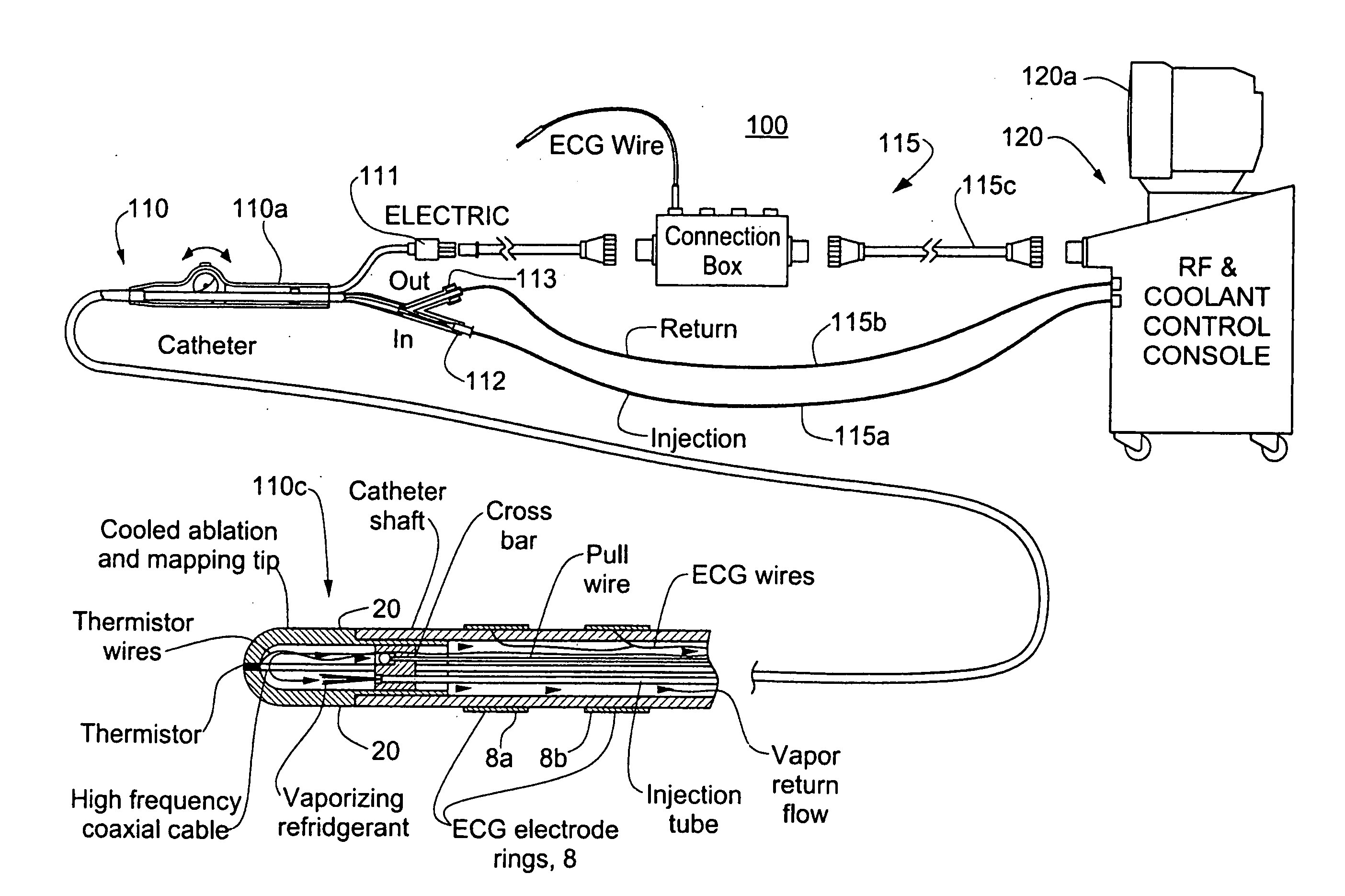
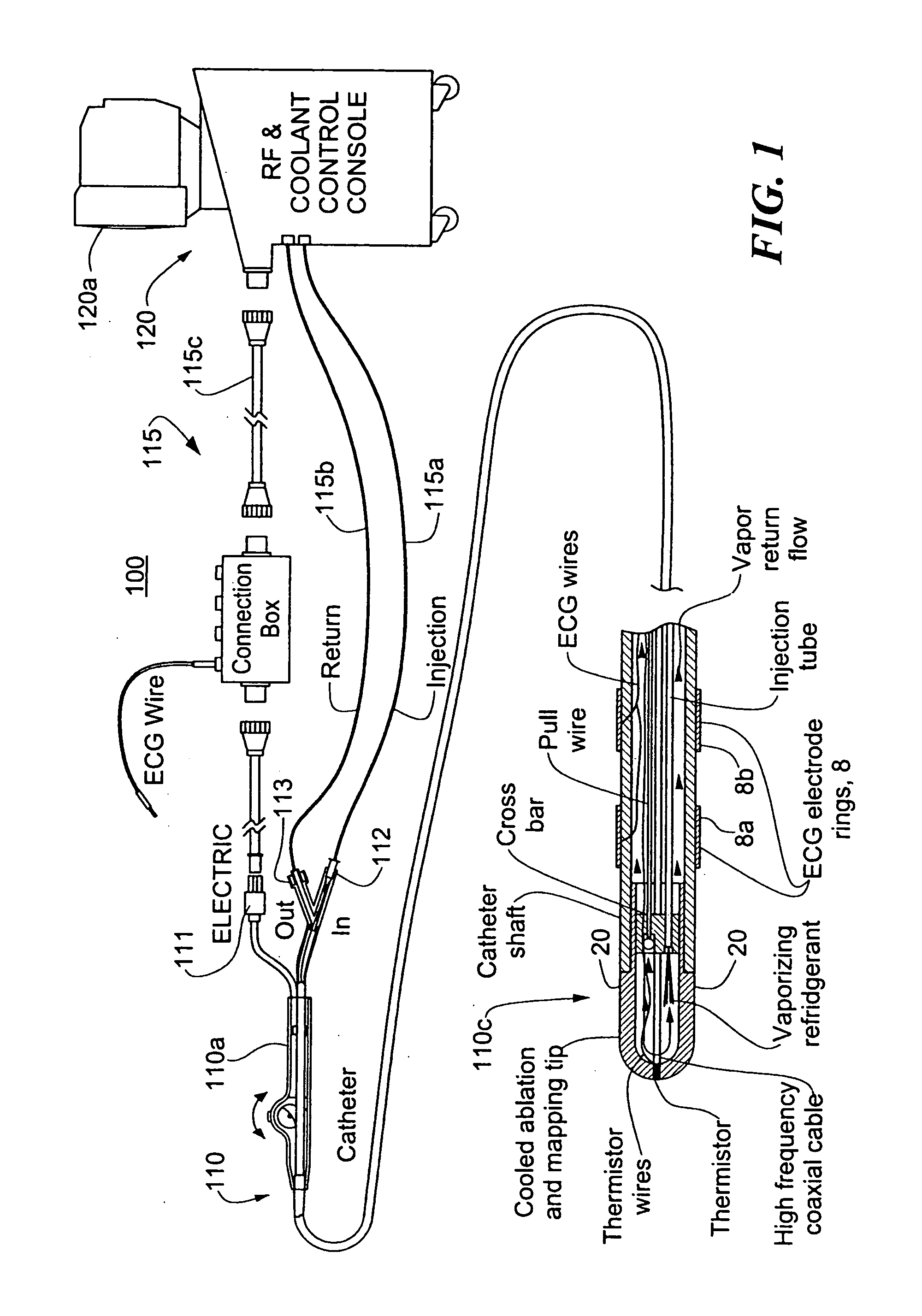
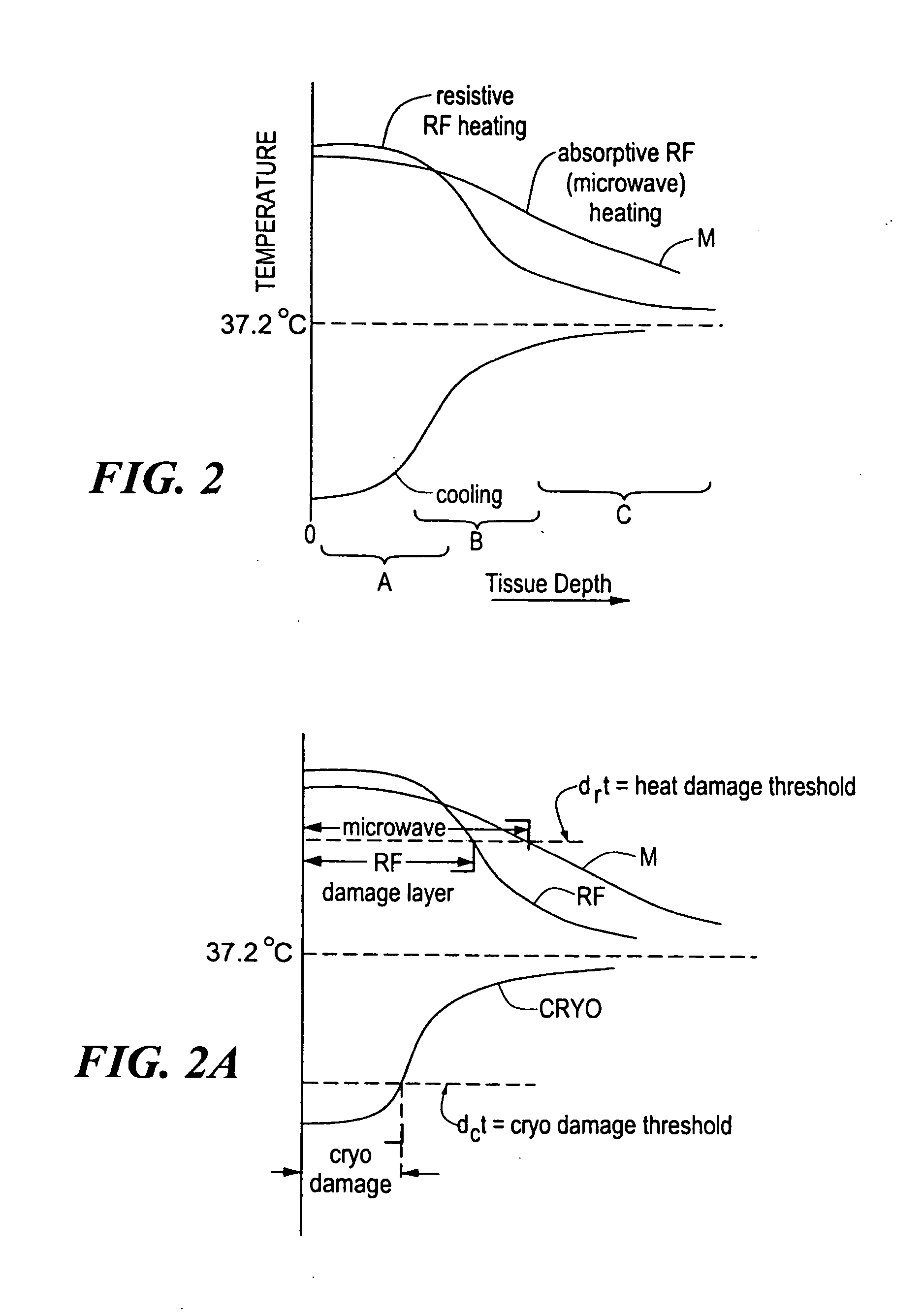
A catheter includes a cryoablation tip with an electrically-driven ablation assembly for heating tissue. The cryoablation tip may be implemented with a cooling chamber through which a controllably injected coolant circulates to lower the tip temperature, and having an RF electrode at its distal end. The RF electrode may be operated to warm cryogenically-cooled tissue, or the coolant may be controlled to conductively cool the tissue in coordination with an RF treatment regimen, allowing greater versatility of operation and enhancing the lesion size, speed or placement of multi-lesion treatment or single lesion re-treatment cycles. In one embodiment a microwave energy source operates at a frequency to extend beyond the thermal conduction depth, or to penetrate the cryogenic ice ball and be absorbed in tissue beyond an ice boundary, thus extending the depth and / or width of a single treatment locus. In another embodiment, the cooling and the application of RF energy are both controlled to position the ablation region away from the surface contacted by the electrode, for example to leave surface tissue unharmed while ablating at depth or to provide an ablation band of greater uniformity with increasing depth. The driver or RF energy source may supply microwave energy at a frequency effective to penetrate the ice ball which develops on a cryocatheter, and different frequencies may be selected for preferential absorption in a layer of defined thickness at depth in the nearby tissue. The catheter may operate between 70 and minus 70 degrees Celsius for different tissue applications, such as angioplasty, cardiac ablation and tissue remodeling, and may preset the temperature of the tip or adjacent tissue, and otherwise overlay or delay the two different profiles to tailor the shape or position where ablation occurs or to speed up a treatment cycle.
Owner:MEDTRONIC CRYOCATH LP
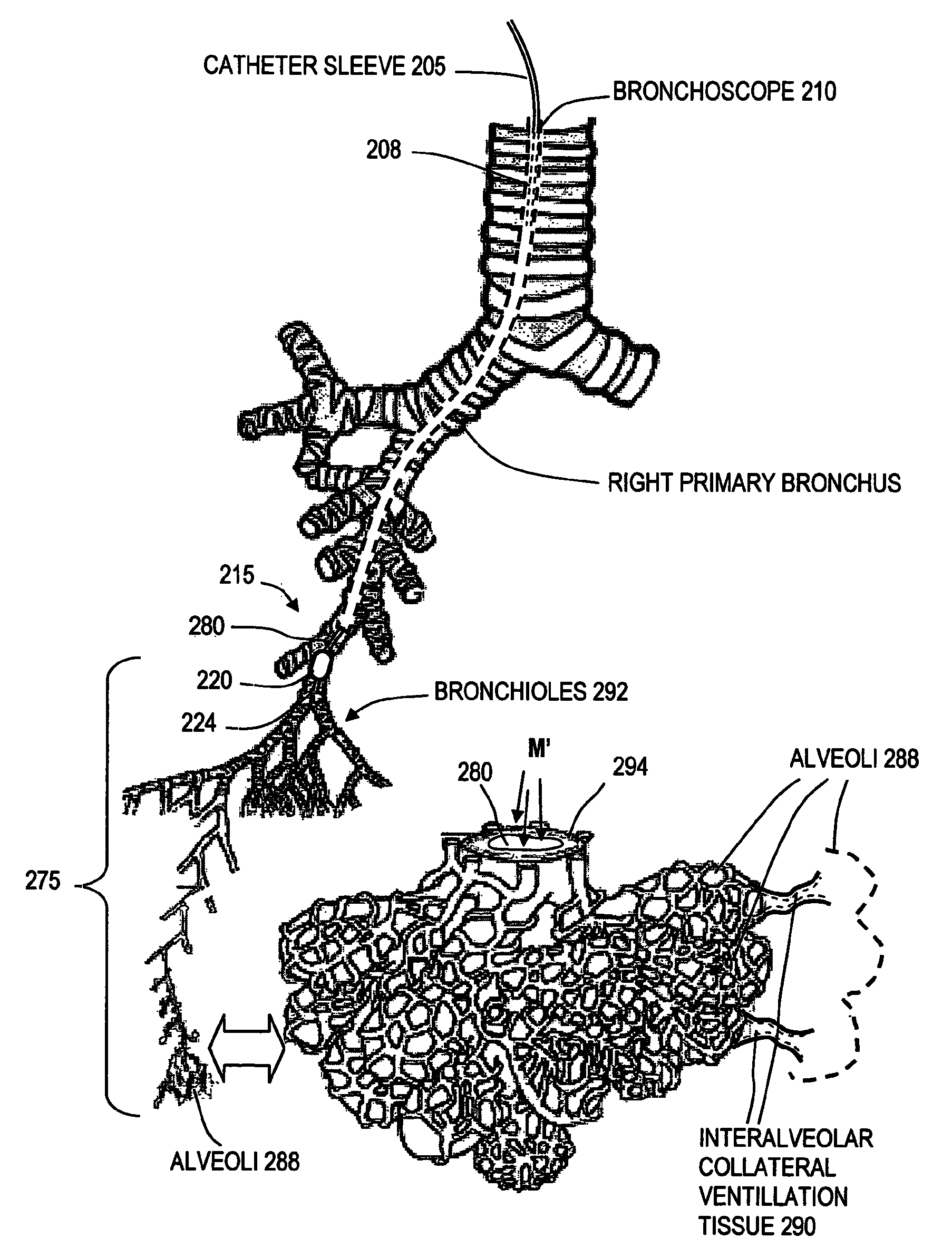
Medical instruments and techniques for treating pulmonary disorders
ActiveUS20080132826A1Enhance tissue remodelingReducing lung volumeMedical devicesFluid jet surgical cuttersThermal energyDisease
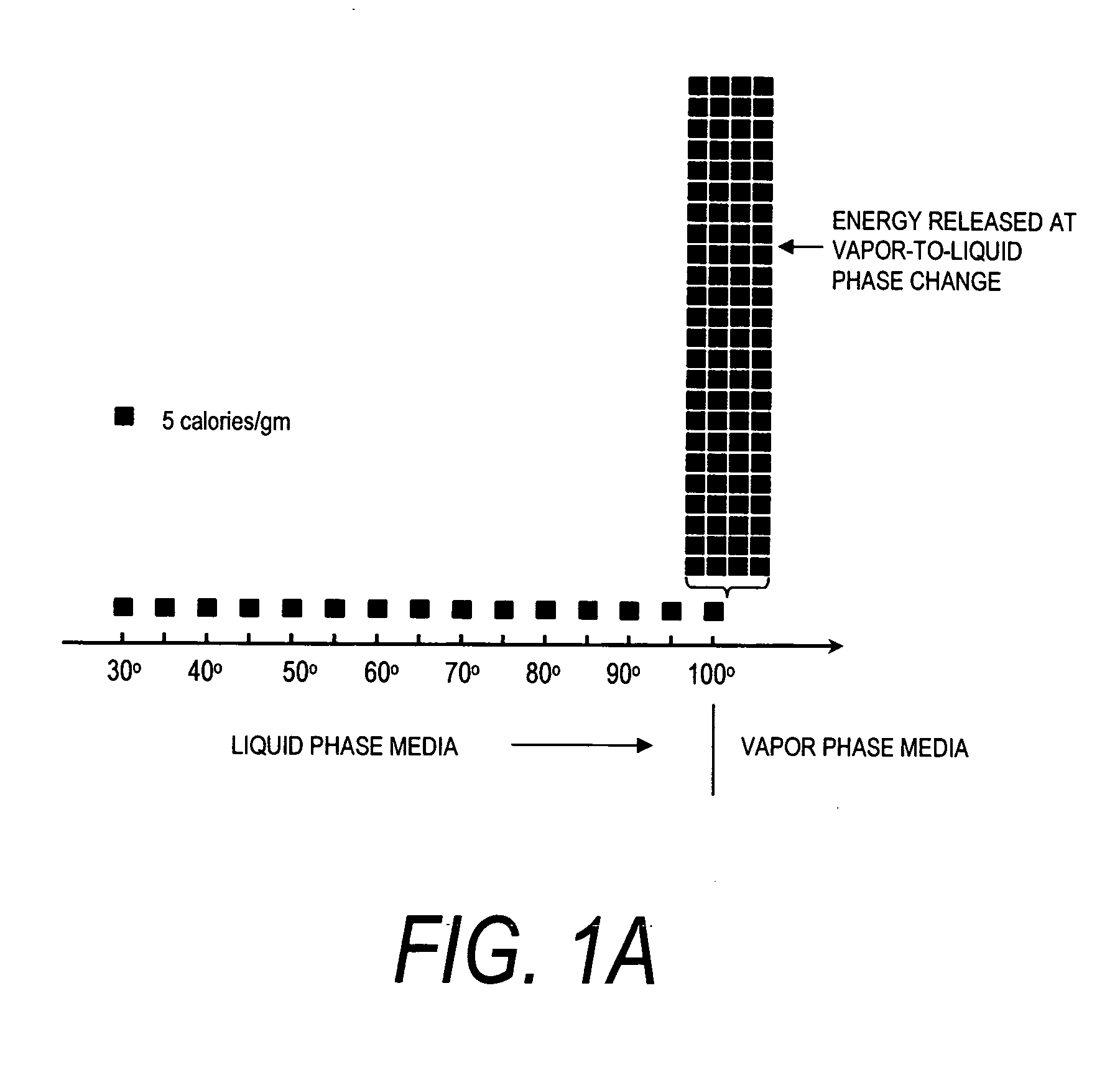
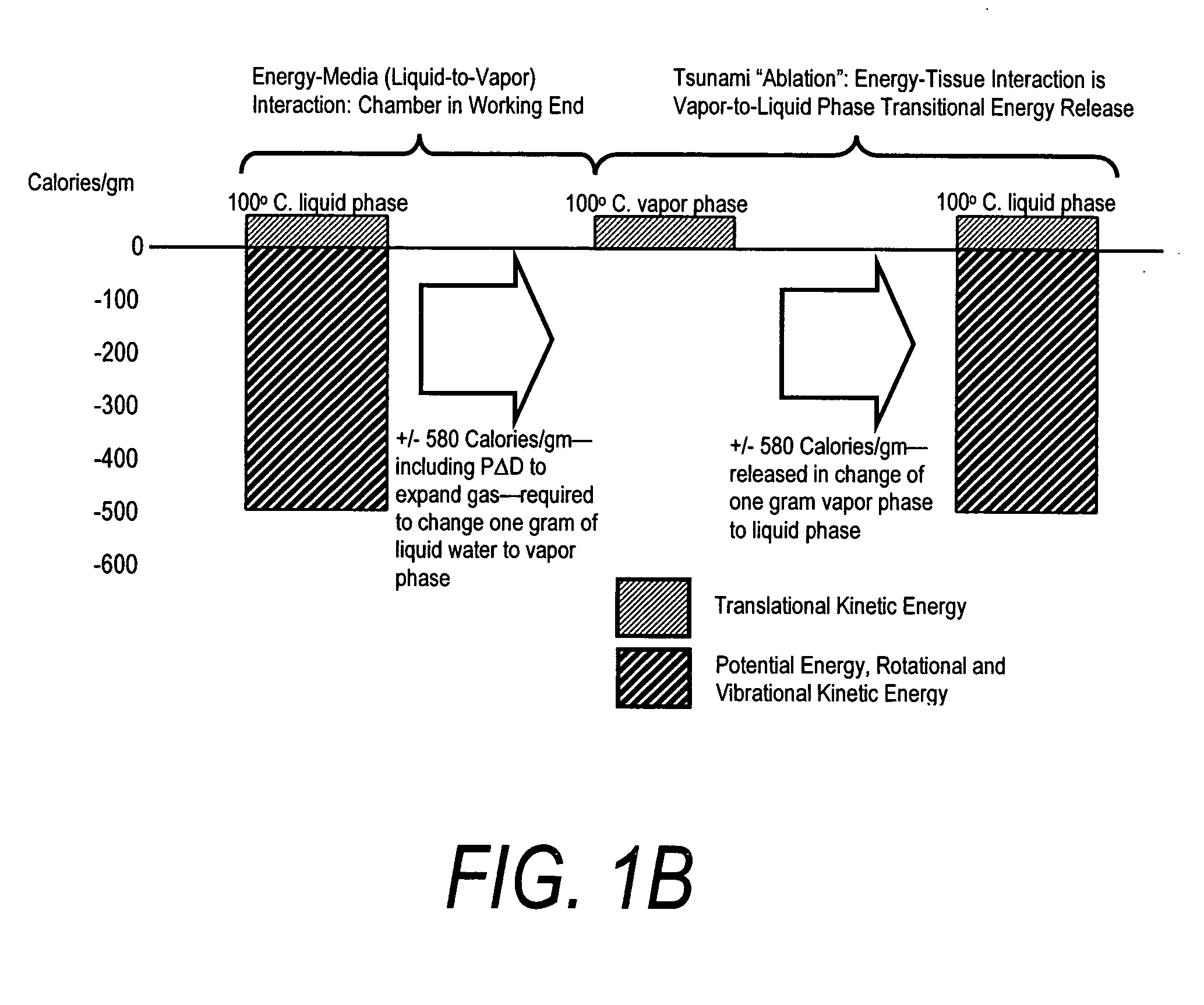
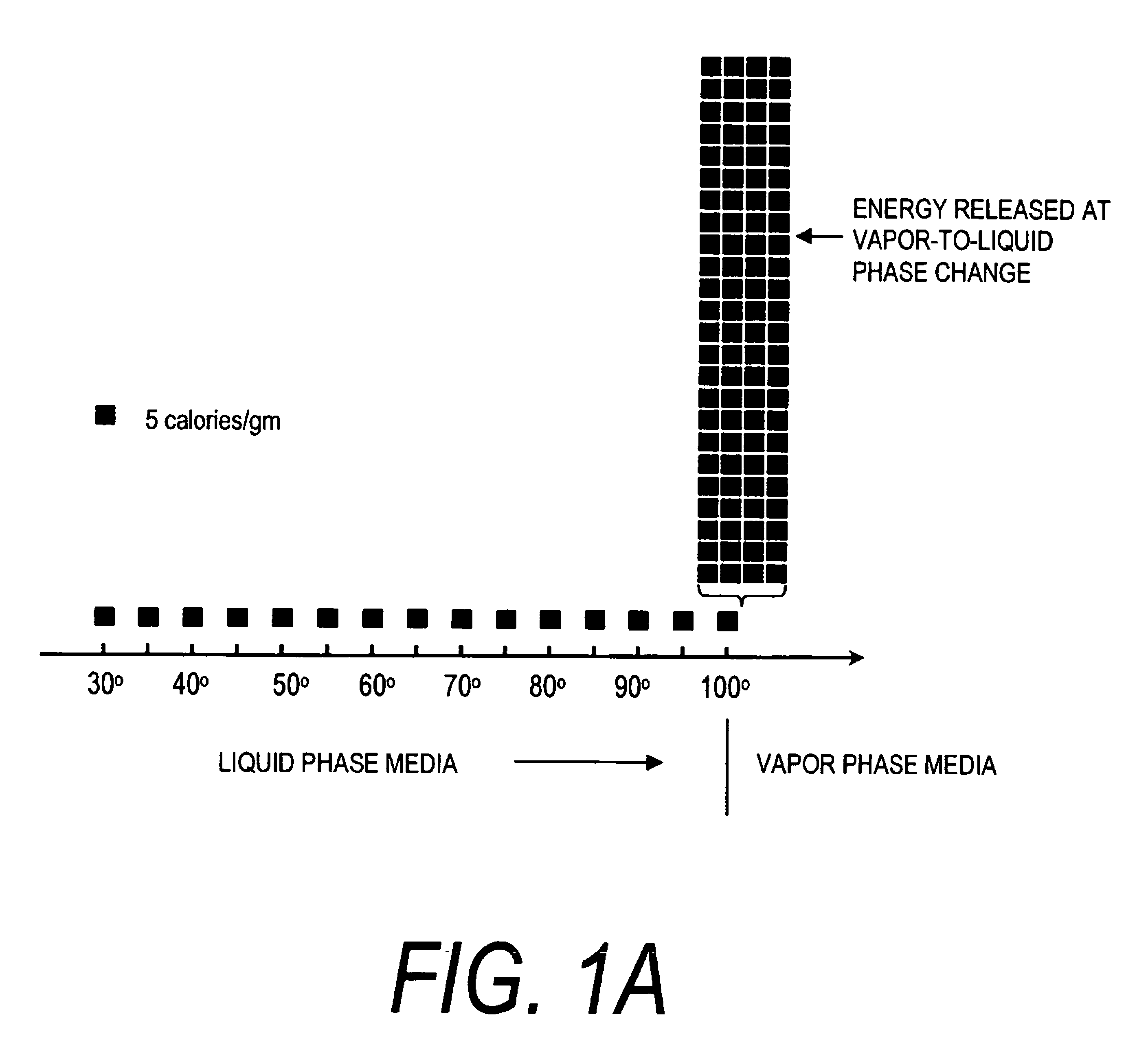
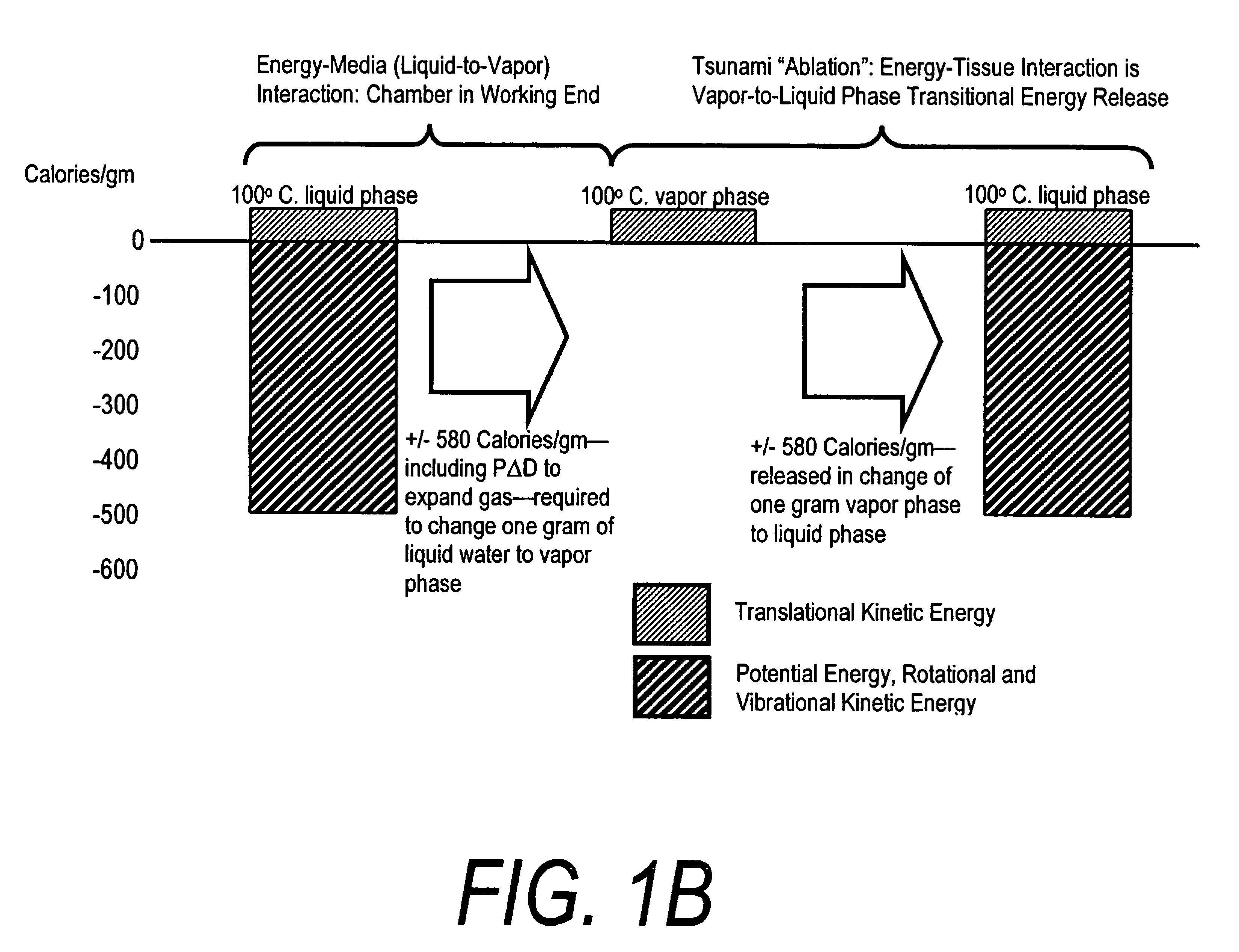
A surgical instrument for delivering energy to lung tissue, for example to cause lung volume reduction. In one embodiment, an elongated catheter has a handle portion that includes an interior chamber that is supplied with a biocompatible liquid media under pressure. An energy source delivers energy to the media to cause a liquid-to-vapor phase change within the interior chamber and ejects a flow of vapor media from the working end of the catheter. The delivery of energy and the flow of vapor are controlled by a computer controller to cause a selected pressure and selected volume of vapor to propagate to the extremities of the airways. Contemporaneously, the vapor undergoes a vapor-to-liquid phase transition which delivers a large amount of energy to airway tissue. The thermal energy delivered is equivalent to the heat of vaporization of the fluid media, which shrinks and collapses the treated airways. The treated tissue is the maintained in a collapsed state by means of aspiration for a short interval to enhance tissue remodeling. Thereafter, the patient's wound healing response causes fibrosis and further remodeling to cause permanent lung volume reduction.
Owner:TSUNAMI MEDTECH
Devices and methods for tissue access
ActiveUS20060089633A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
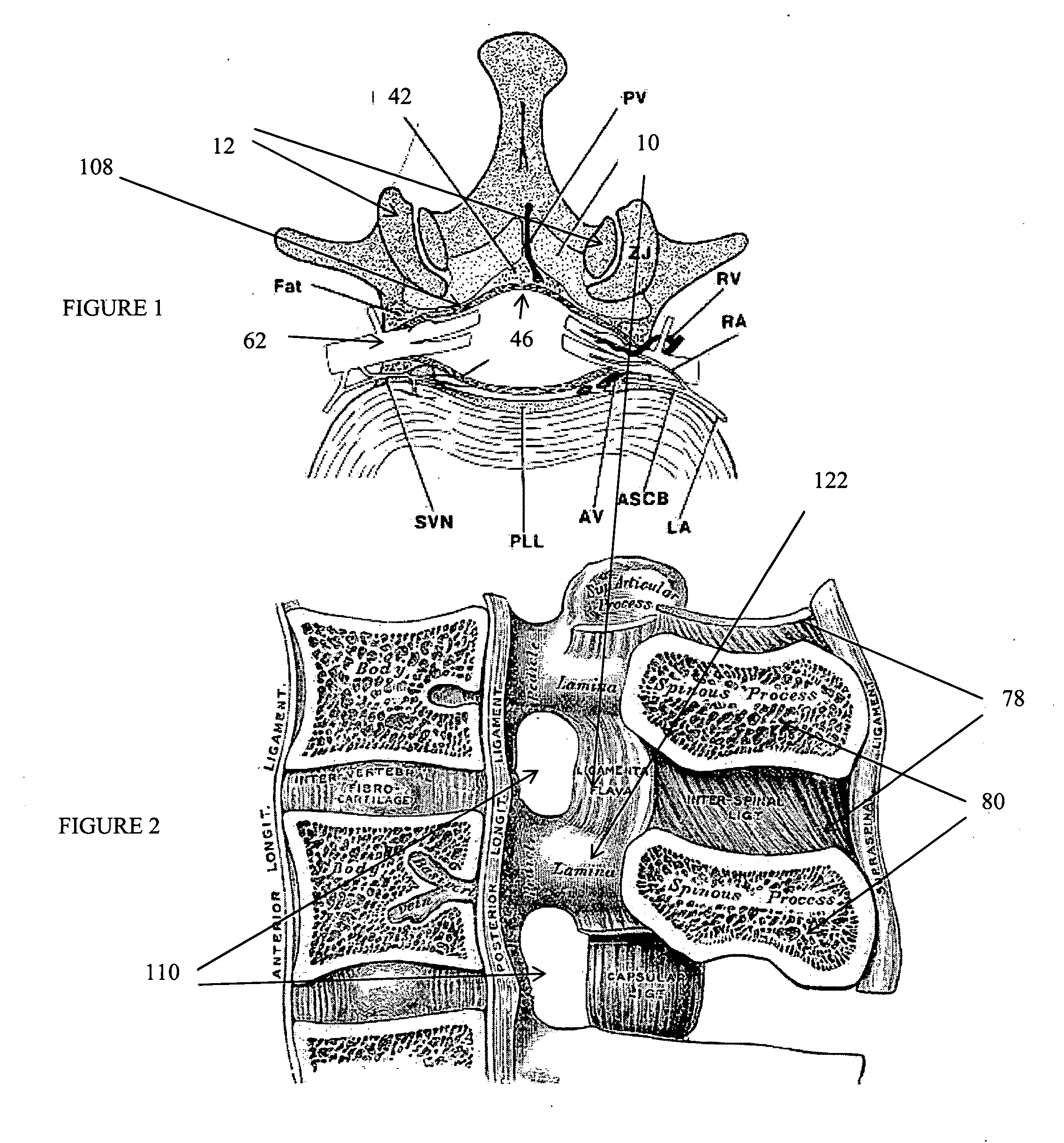
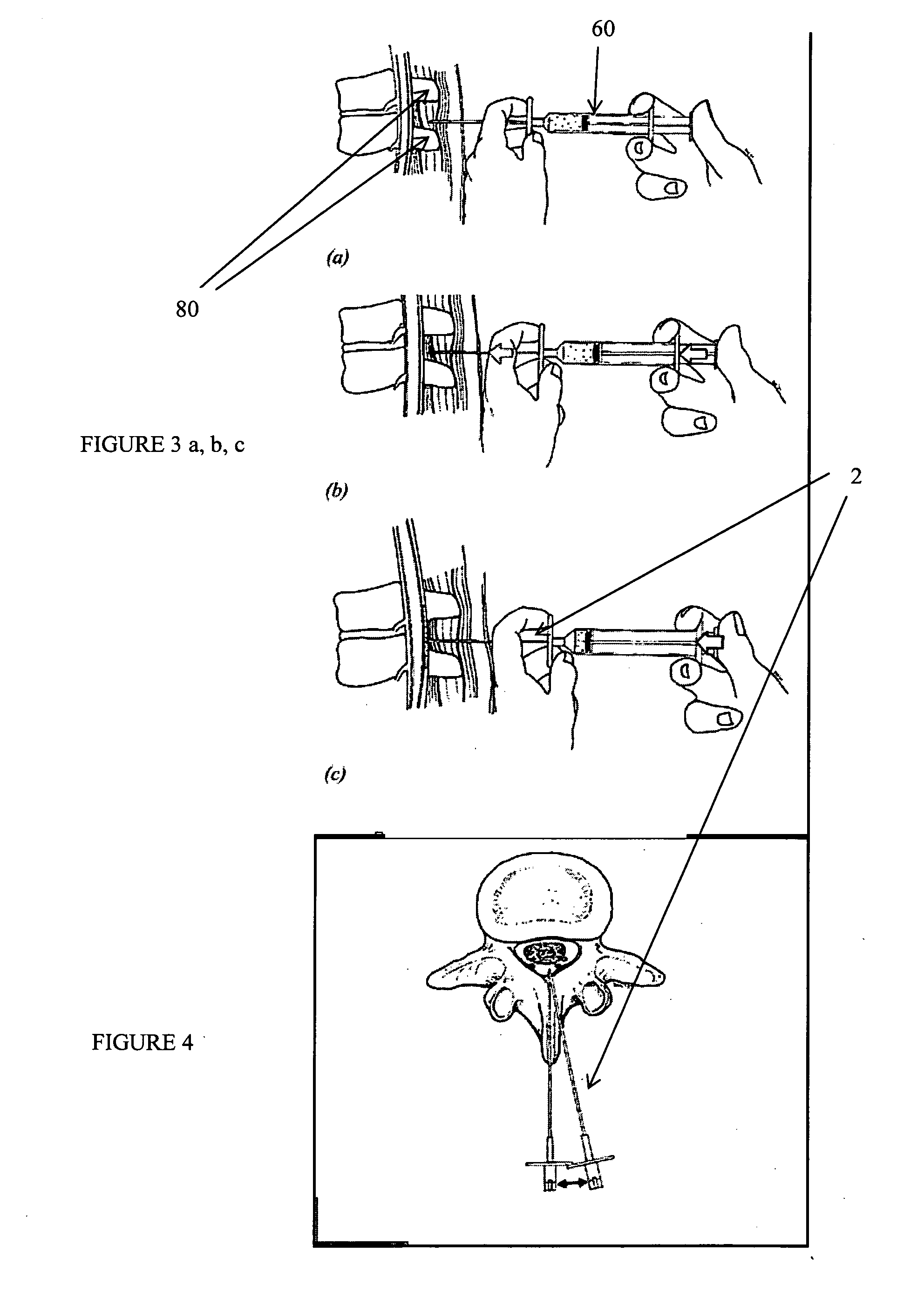
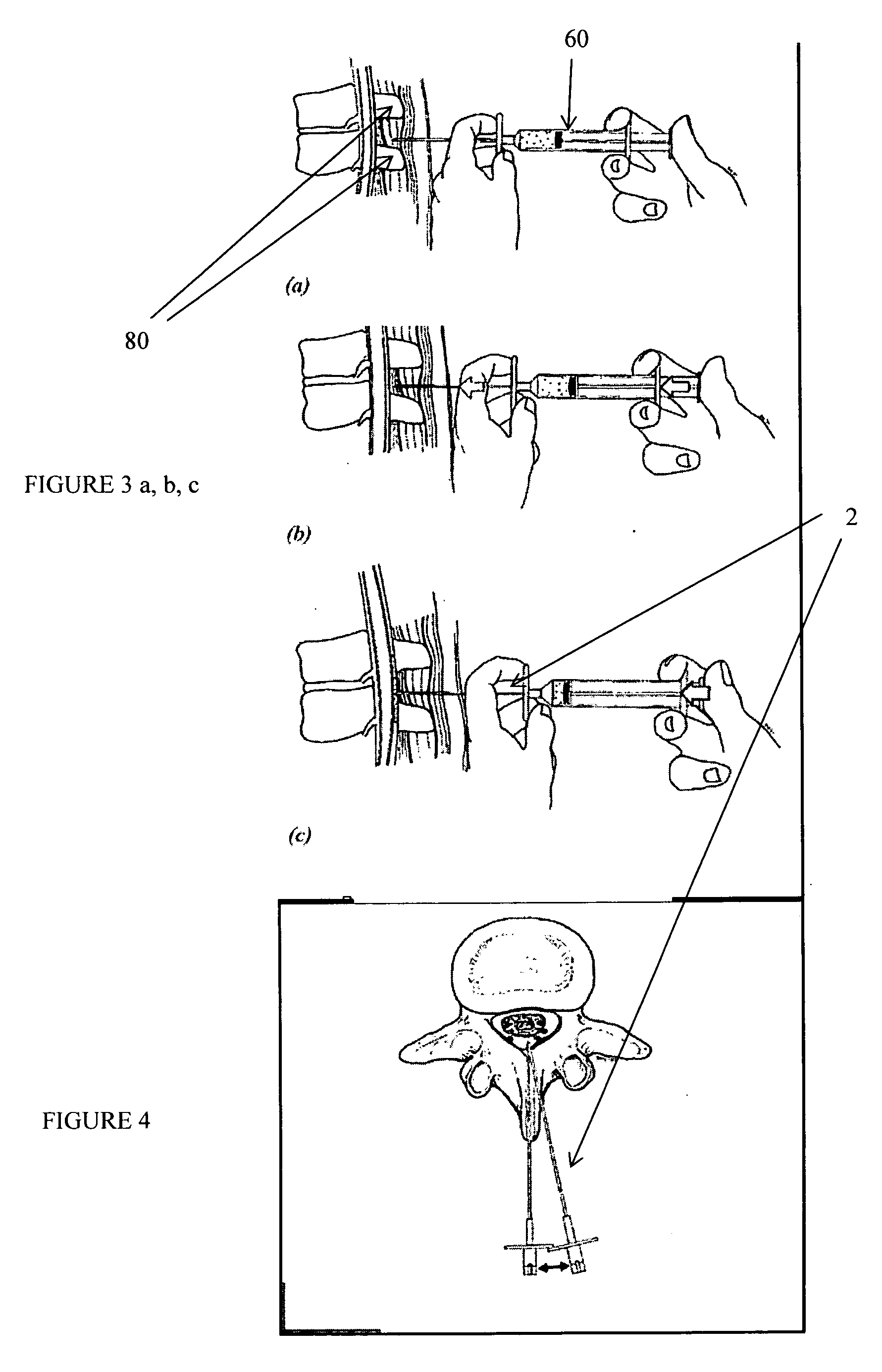
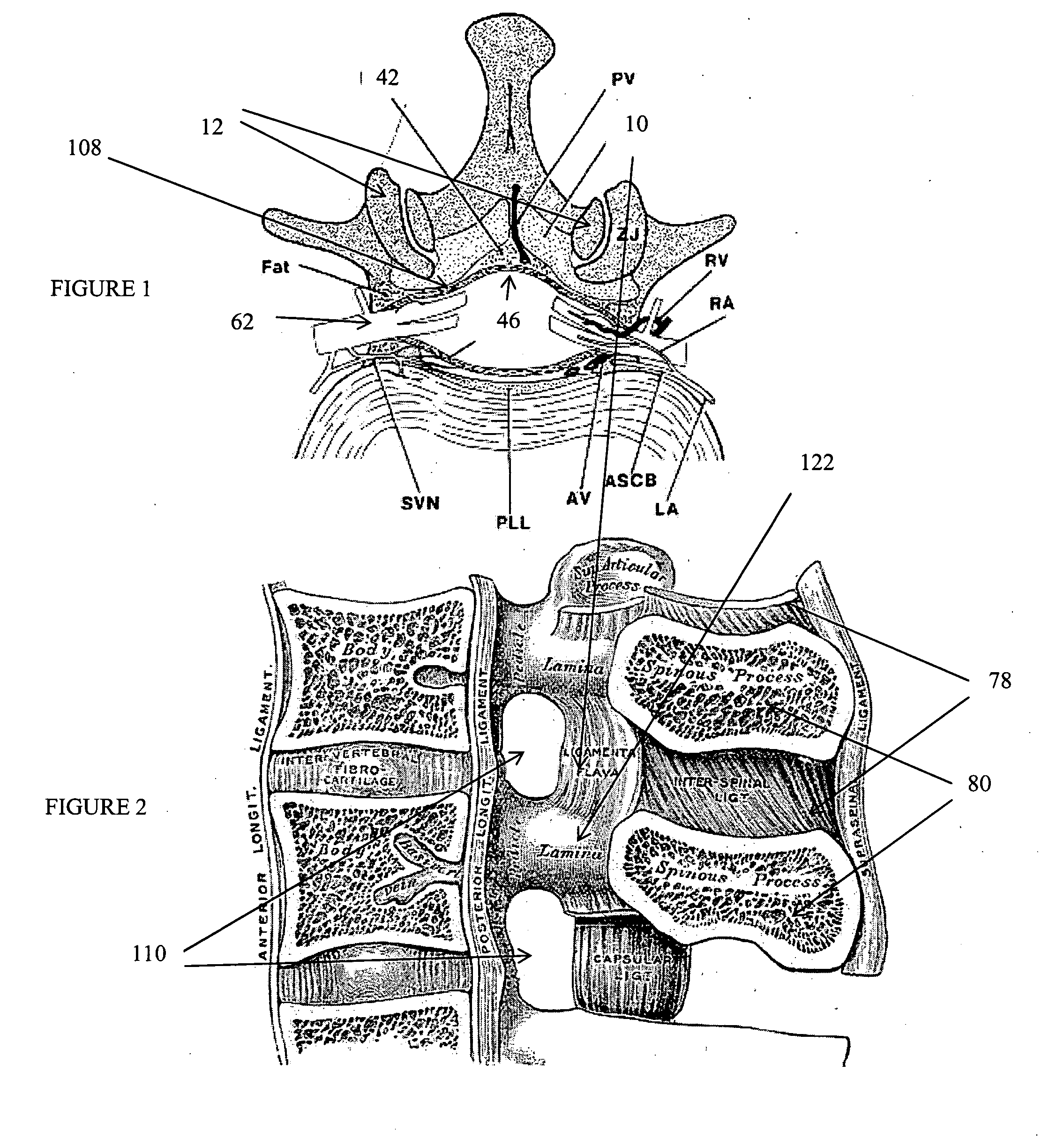
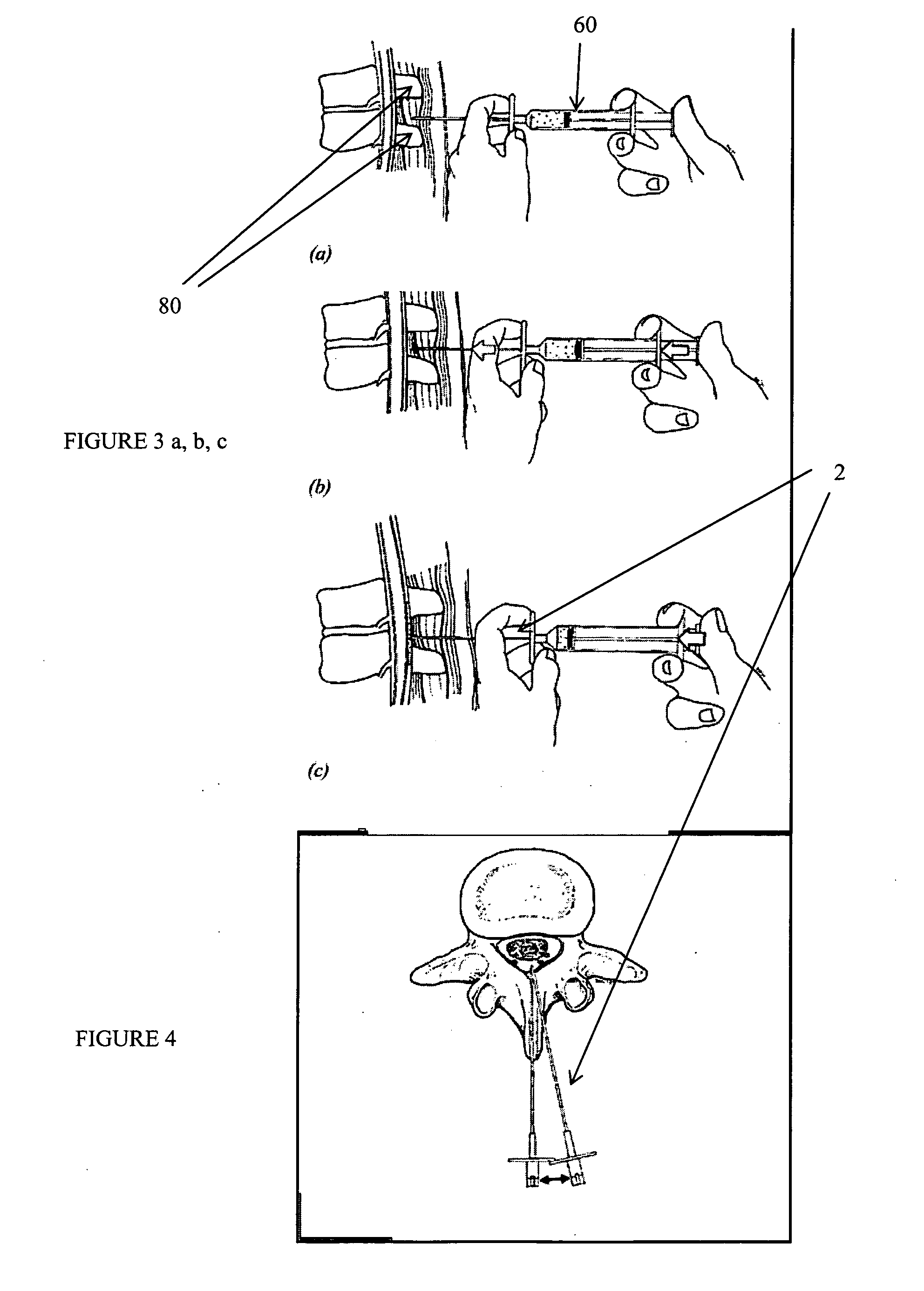
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue abrasion device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the abrasive surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for tissue access
InactiveUS20060122458A1Enabling symptomatic reliefApproach can be quite invasiveCannulasDiagnosticsSurgical departmentNerve stimulation
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:BAXANO
High strength suture with collagen fibers
InactiveUS20050033362A1High strengthGood tissue compatibilitySuture equipmentsSurgical needlesPolyesterTissue remodeling
Owner:ARTHREX
Devices and methods for tissue modification
ActiveUS20060095059A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
Owner:MIS IP HLDG LLC +1
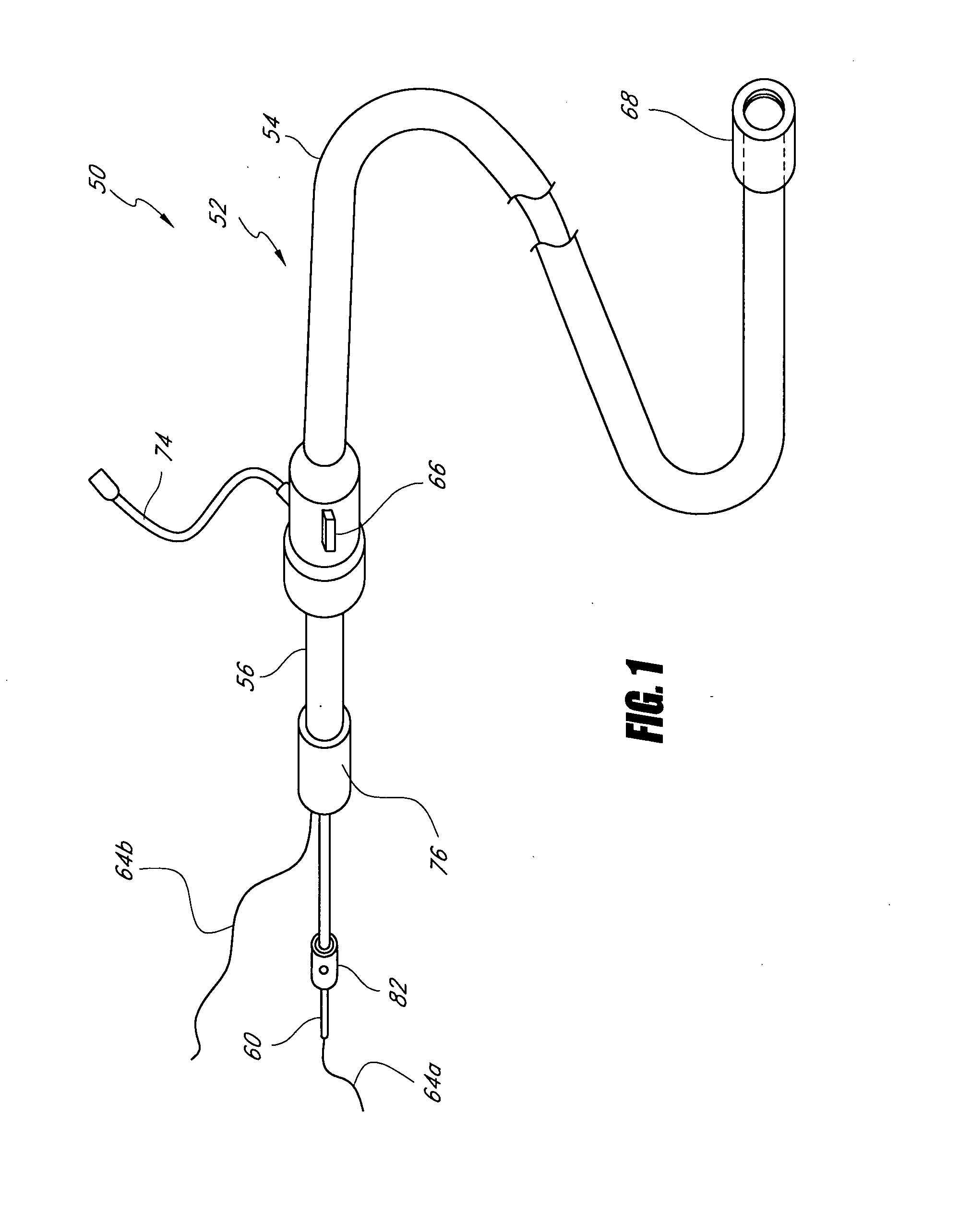
Catheter-based tissue remodeling devices and methods
Methods and systems for closing an opening or defect in tissue, closing a lumen or tubular structure, cinching or remodeling a cavity or repairing a valve preferably utilizing a purse string or elastic device. The preferred devices and methods are directed toward catheter-based percutaneous, transvascular techniques used to facilitate placement of the devices within lumens, such as blood vessels, or on or within the heart to perform structural defect repair, such as valvular or ventricular remodeling. In some methods, the catheter is positioned within the right ventricle, wherein the myocardial wall or left ventricle may be accessed through the septal wall to position a device configured to permit reshaping of the ventricle. The device may include a line or a plurality of anchors interconnected by a line. In one arrangement, the line is a coiled member.
Owner:EDWARDS LIFESCIENCES CORP
Method and apparatus for dermatological treatment
ActiveUS7331953B2Easy to processSimple to useSurgical instrument detailsLight therapyWound healingTissue remodeling
Owner:THE GENERAL HOSPITAL CORP
Catheter-based tissue remodeling devices and methods
InactiveUS20060135970A1Reduce volumeLower the volumeSuture equipmentsSurgical instrument detailsTissue remodelingLeft ventricle wall
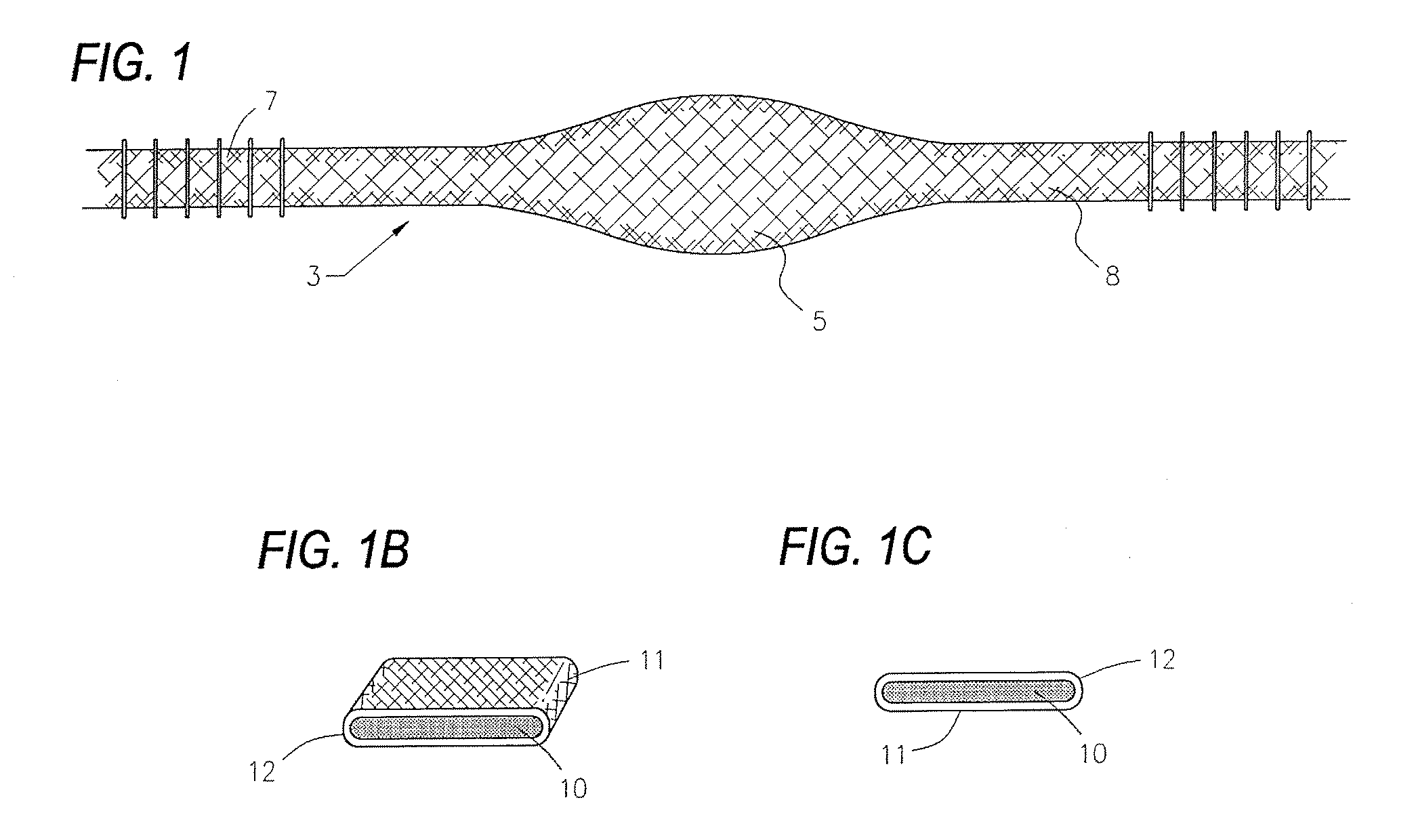
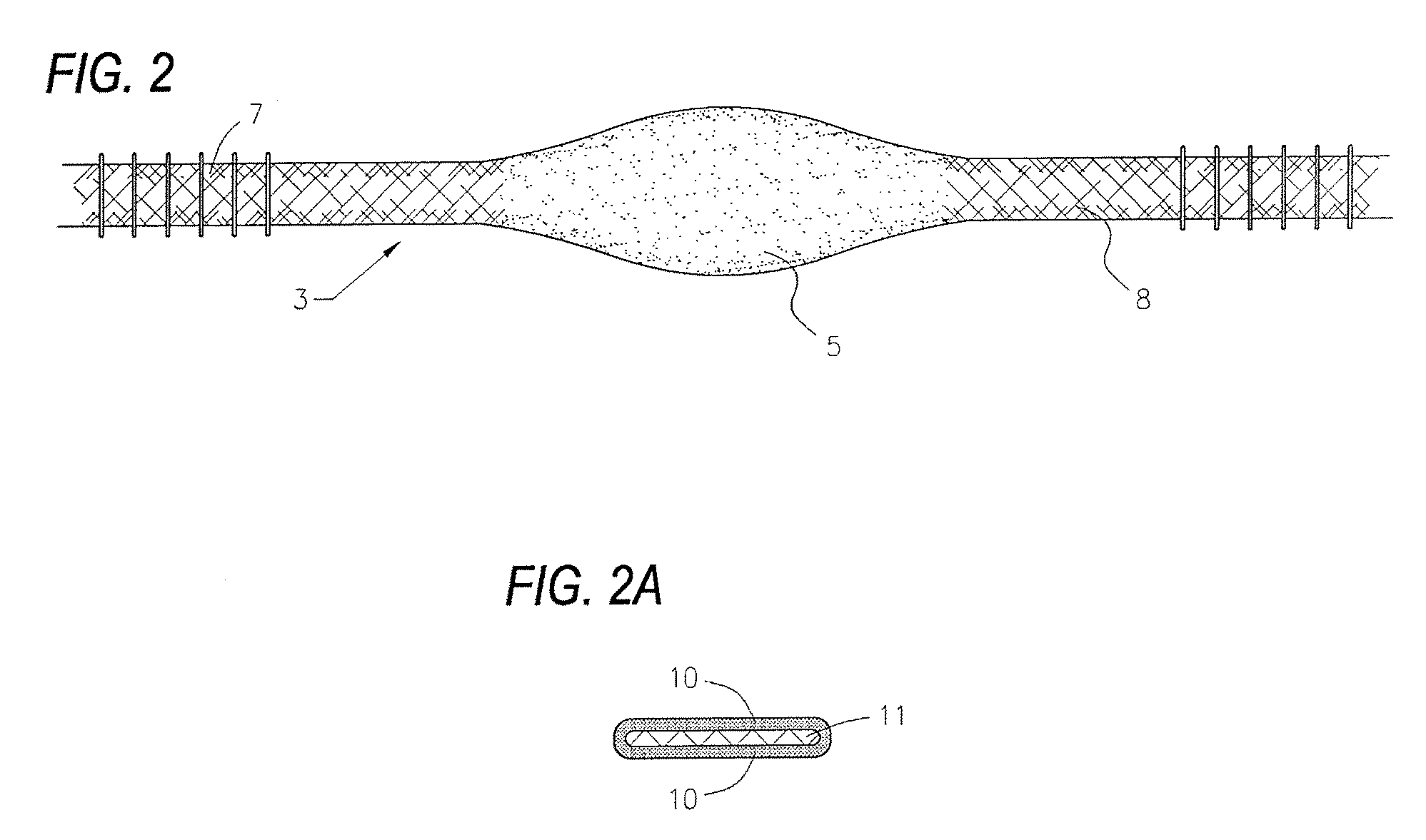
Devices and methods utilizing a catheter to remodel soft tissue of a patient and, in a preferred embodiment, to reduce the volume of the left ventricle of a heart. In one embodiment, one or more sutures are passed through a wall of the ventricle. The ends of the one suture and, more preferably, the multiples sutures are drawn together to draw tissue portions towards one another. In another embodiment, tissue remodeling clip is implanted into a wall of the ventricle. Ends of the clip are resiliently biased to move relative to one another to draw tissue portions towards one another. In yet another embodiment, a tissue remodeling anchor includes a base and a plurality of legs attached to the base. The legs of the tissue anchor are implanted into a wall of the ventricle and moved toward one another to draw tissue portions toward one another. A retaining member is positioned on the tissue anchor to prevent the legs from moving apart.
Owner:BENVENUE MEDICAL
Medical instruments and techniques for treating pulmonary disorders
ActiveUS7892229B2Enhance tissue remodelingReduce volumeMedical devicesFluid jet surgical cuttersTissue remodelingDisease
A surgical instrument for delivering energy to lung tissue, for example to cause lung volume reduction. In one embodiment, an elongated catheter has a handle portion that includes an interior chamber that is supplied with a biocompatible liquid media under pressure. An energy source delivers energy to the media to cause a liquid-to-vapor phase change within the interior chamber and ejects a flow of vapor media from the working end of the catheter. The delivery of energy and the flow of vapor are controlled by a computer controller to cause a selected pressure and selected volume of vapor to propagate to the extremities of the airways. Contemporaneously, the vapor undergoes a vapor-to-liquid phase transition which delivers a large amount of energy to airway tissue. The thermal energy delivered is equivalent to the heat of vaporization of the fluid media, which shrinks and collapses the treated airways. The treated tissue is the maintained in a collapsed state by means of aspiration for a short interval to enhance tissue remodeling. Thereafter, the patient's wound healing response causes fibrosis and further remodeling to cause permanent lung volume reduction.
Owner:TSUNAMI MEDTECH
Catheter-based tissue remodeling devices and methods
InactiveUS20060135968A1Reduce volumeLower the volumeSuture equipmentsSurgical instrument detailsTissue remodelingLeft ventricle wall
Devices and methods utilizing a catheter to remodel soft tissue of a patient and, in a preferred embodiment, to reduce the volume of the left ventricle of a heart. In one embodiment, one or more sutures are passed through a wall of the ventricle. The ends of the one suture and, more preferably, the multiples sutures are drawn together to draw tissue portions towards one another. In another embodiment, tissue remodeling clip is implanted into a wall of the ventricle. Ends of the clip are resiliently biased to move relative to one another to draw tissue portions towards one another. In yet another embodiment, a tissue remodeling anchor includes a base and a plurality of legs attached to the base. The legs of the tissue anchor are implanted into a wall of the ventricle and moved toward one another to draw tissue portions toward one another. A retaining member is positioned on the tissue anchor to prevent the legs from moving apart.
Owner:BENVENUE MEDICAL
Devices and methods for selective surgical removal of tissue
ActiveUS20060094976A1Improve securityAvoid injuryCannulasAnti-incontinence devicesTissue remodelingLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Catheter with cryogenic and electrical heating ablation
InactiveUS20060004351A1Reduce tip temperatureReduce movement sequenceCatheterDiagnostic recording/measuringTissue remodelingCelsius Degree
A catheter includes a cryoablation tip with an electrically-driven ablation assembly for heating tissue. The cryoablation tip may be implemented with a cooling chamber through which a controllably injected coolant circulates to lower the tip temperature, and having an RF electrode at its distal end. The RF electrode may be operated to warm cryogenically-cooled tissue, or the coolant may be controlled to conductively cool the tissue in coordination with an RF treatment regimen, allowing greater versatility of operation and enhancing the lesion size, speed or placement of multi-lesion treatment or single lesion re-treatment cycles. In one embodiment a microwave energy source operates at a frequency to extend beyond the thermal conduction depth, or to penetrate the cryogenic ice ball and be absorbed in tissue beyond an ice boundary, thus extending the depth and / or width of a single treatment locus. In another embodiment, the cooling and the application of RF energy are both controlled to position the ablation region away from the surface contacted by the electrode, for example to leave surface tissue unharmed while ablating at depth or to provide an ablation band of greater uniformity with increasing depth. The driver or RF energy source may supply microwave energy at a frequency effective to penetrate the ice ball which develops on a cryocatheter, and different frequencies may be selected for preferential absorption in a layer of defined thickness at depth in the nearby tissue. The catheter may operate between 70 and minus 70 degrees Celsius for different tissue applications, such as angioplasty, cardiac ablation and tissue remodeling, and may preset the temperature of the tip or adjacent tissue, and otherwise overlay or delay the two different profiles to tailor the shape or position where ablation occurs or to speed up a treatment cycle.
Owner:MEDTRONIC CRYOCATH LP
Method and apparatus for dermatological treatment
ActiveUS20050222555A1Treatment safetyRepair and alleviates skin defectSurgical instrument detailsLight therapyWound healingTissue remodeling
The present invention provides improved methods and apparatus for skin treatment. The apparatus includes multiple sources of optical energy or several blades that are scanned along a region of skin to form micro-line patterns of damaged tissue. The micro-lines are small in at least one dimension, having a width of less than about 1 mm, and the wounded regions promote beneficial results by stimulation of wound healing and tissue remodeling.
Owner:THE GENERAL HOSPITAL CORP
Method and apparatus for dermatological treatment
ActiveUS20080058784A1Easy to processSimple to useSurgical instrument detailsLight therapyWound healingTissue remodeling
The present invention provides improved methods and apparatus for skin treatment. The apparatus includes multiple sources of optical energy or several blades that are scanned along a region of skin to form micro-line patterns of damaged tissue. The micro-lines are small in at least one dimension, having a width of less than about 1 mm, and the wounded regions promote beneficial results by stimulation of wound healing and tissue remodeling.
Owner:THE GENERAL HOSPITAL CORP
Expandable rotating device and method for tissue aspiration
InactiveUS20080208230A1Relieve pressureVaccination/ovulation diagnosticsExcision instrumentsTissue remodelingControl system
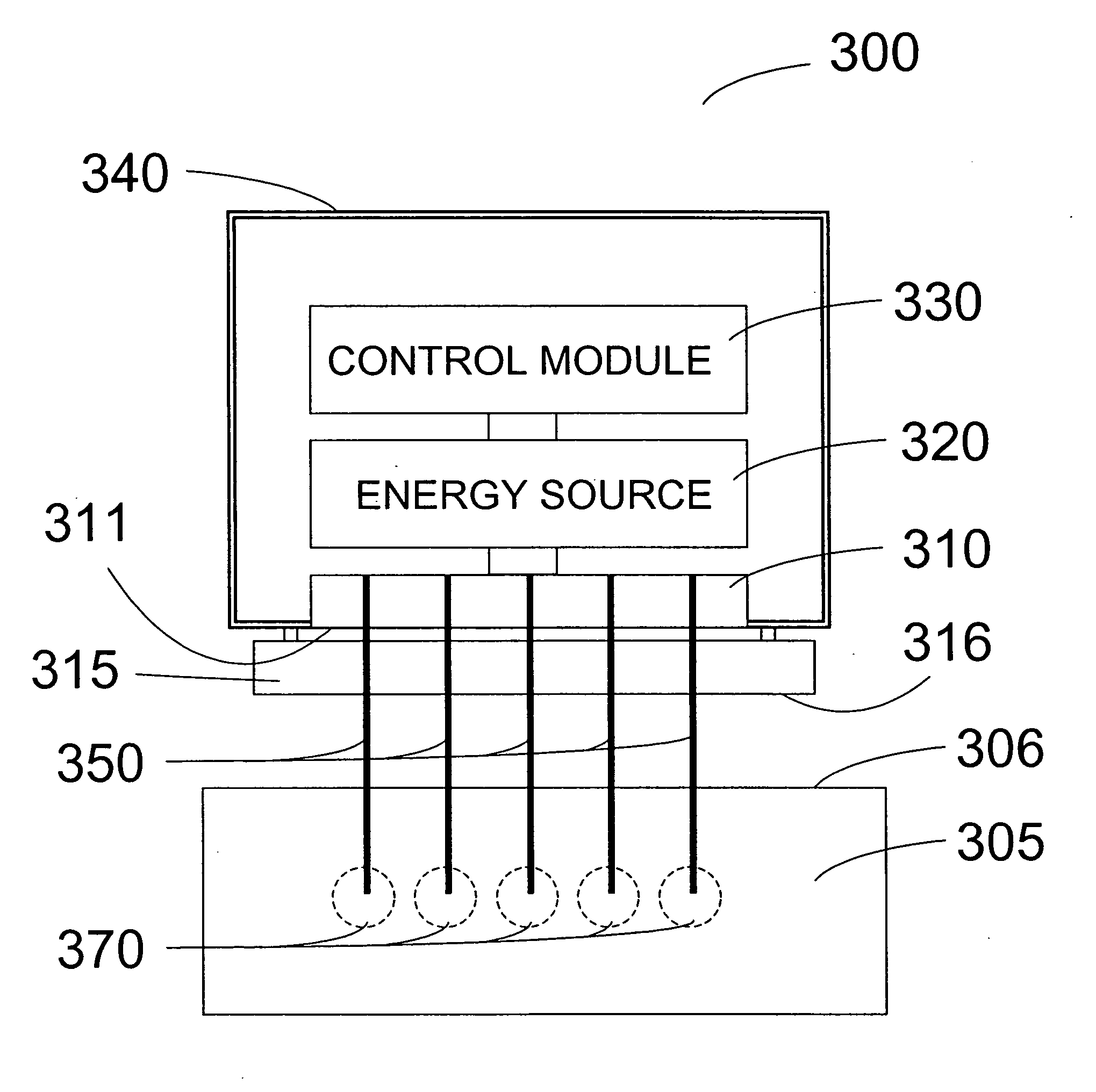
An apparatus and method for removing tissue and / or other material from a patient includes a shaft and a tissue disrupting mechanism operatively coupled to the shaft. The shaft may be coupled to a handpiece or a robotic or remote-controlled system. The mechanism may comprise a rotatable or other movable element having a distal portion with fixed or adjustable radial dimensions. The mechanism may have one or more tissue cutting, chopping, grinding, emulsifying or disrupting features with an adjustable outer diameter for removing substantial tissues. The apparatus may be configured to urge or draw substantial material into the device upon rotation or other movement of the shaft and / or tissue, and may optionally be coupled to sources of suction or aspiration. A radiofrequency or other energy source is optionally included for tissue ablation or other tissue remodeling effects, and / or to enhance coagulation.
Owner:SPINE VIEW INC
Devices and methods for tissue access
InactiveUS20060100651A1Improve securityAvoid injuryEar treatmentCannulasTissue remodelingSpinal column
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Surgical instrument for treating female urinary stress incontinence
InactiveUS20100198002A1High tensile strengthPrevents voiding dysfunctionAnti-incontinence devicesTissue remodelingUrethra
A suburethral sling device and method for treating female urinary stress incontinence which is anatomically configured to implant into the lower abdomen of a female in a manner providing support to mid-urethral and bladder neck sphincteric continence sites with the sling defining in part, mesh and tissue remodeling portions. The sling is deployed via a sling transfer instrument having distal and proximal ends with the instrument comprising in part a progressively curved shaft portion positioned between the distal and proximal ends. An insertion handle of the transfer instrument is secured to the curved metal shaft section guiding the shaft tip through the tissues of the abdomen in an anterior / posterior direction as well as a cephalad / caudad direction.
Owner:ODONNELL PAT D
Programmed-release, nanostructured biological construct
InactiveUS20080311172A1Good biocompatibilityEnhanced interactionSuture equipmentsPowder deliveryTissue remodelingBio engineering
A biologically engineered construct comprising of a polymeric biomatrix, designed with a nanophase texture, and a therapeutic agent for the purpose of tissue regeneration and / or controlled delivery of regenerative factors and therapeutic substances after it is implanted into tissues, vessels, or luminal structures within the body. The therapeutic agent may be a therapeutic substance or a biological agent, such as antibodies, ligands, or living cells. The nanophase construct is designed to maximize lumen size, promote tissue remodeling, and ultimately make the implant more biologically compatible. The nano-textured polymeric biomatrix may comprise one or more layers containing therapeutic substances and / or beneficial biological agents for the purpose of controlled, differential substance / drug delivery into the luminal and abluminal surfaces of the vessel or lumen, and the attraction of target molecules / cells that will regenerate functional tissue. The topographic and biocompatible features of this layered biological construct provides an optimal environment for tissue regeneration along with a programmed-release, drug delivery system to improve physiological tolerance of the implant, and to maximize the cellular survival, migration, and integration within the implanted tissues.
Owner:SCHAPIRA JAY N +1
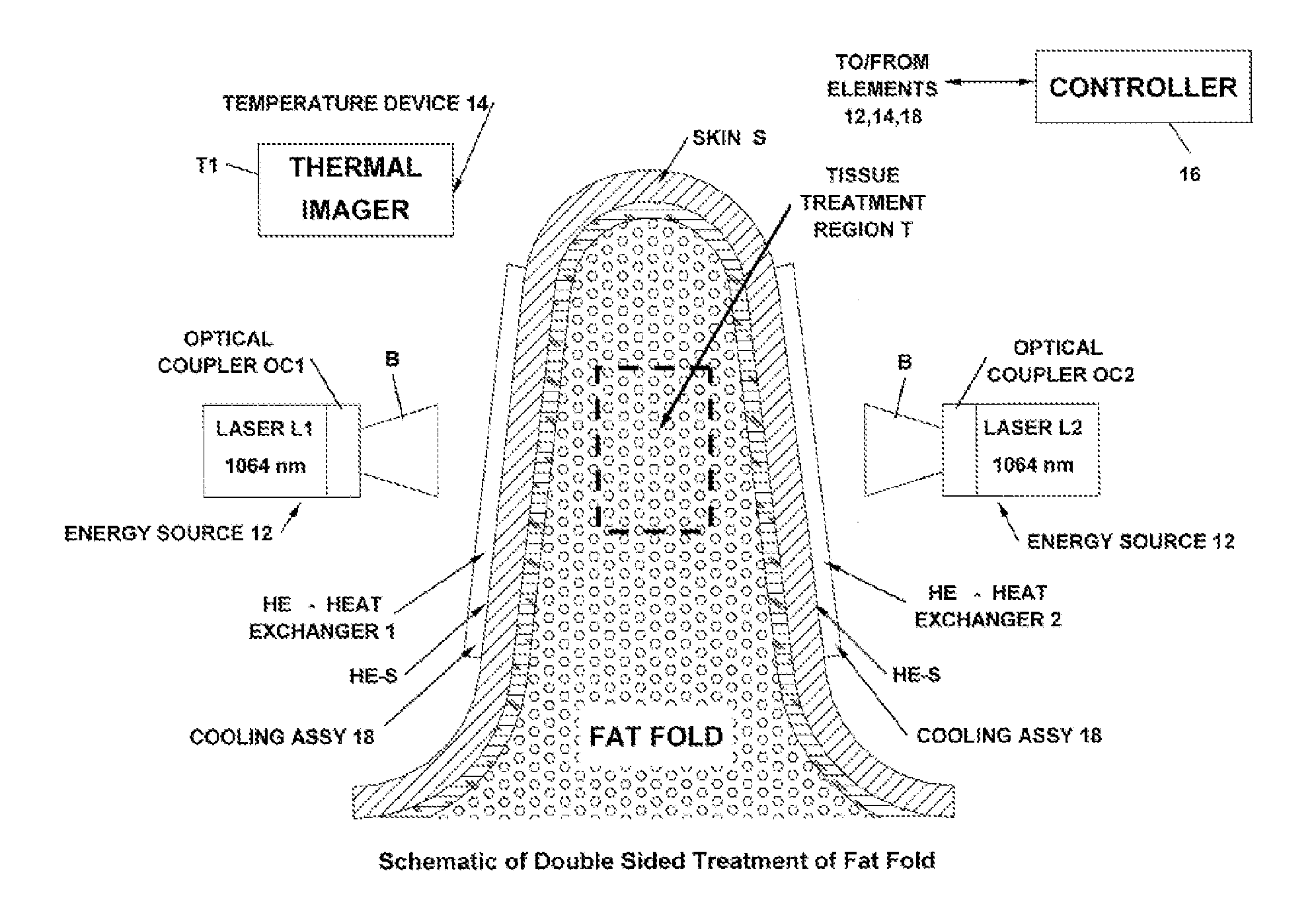
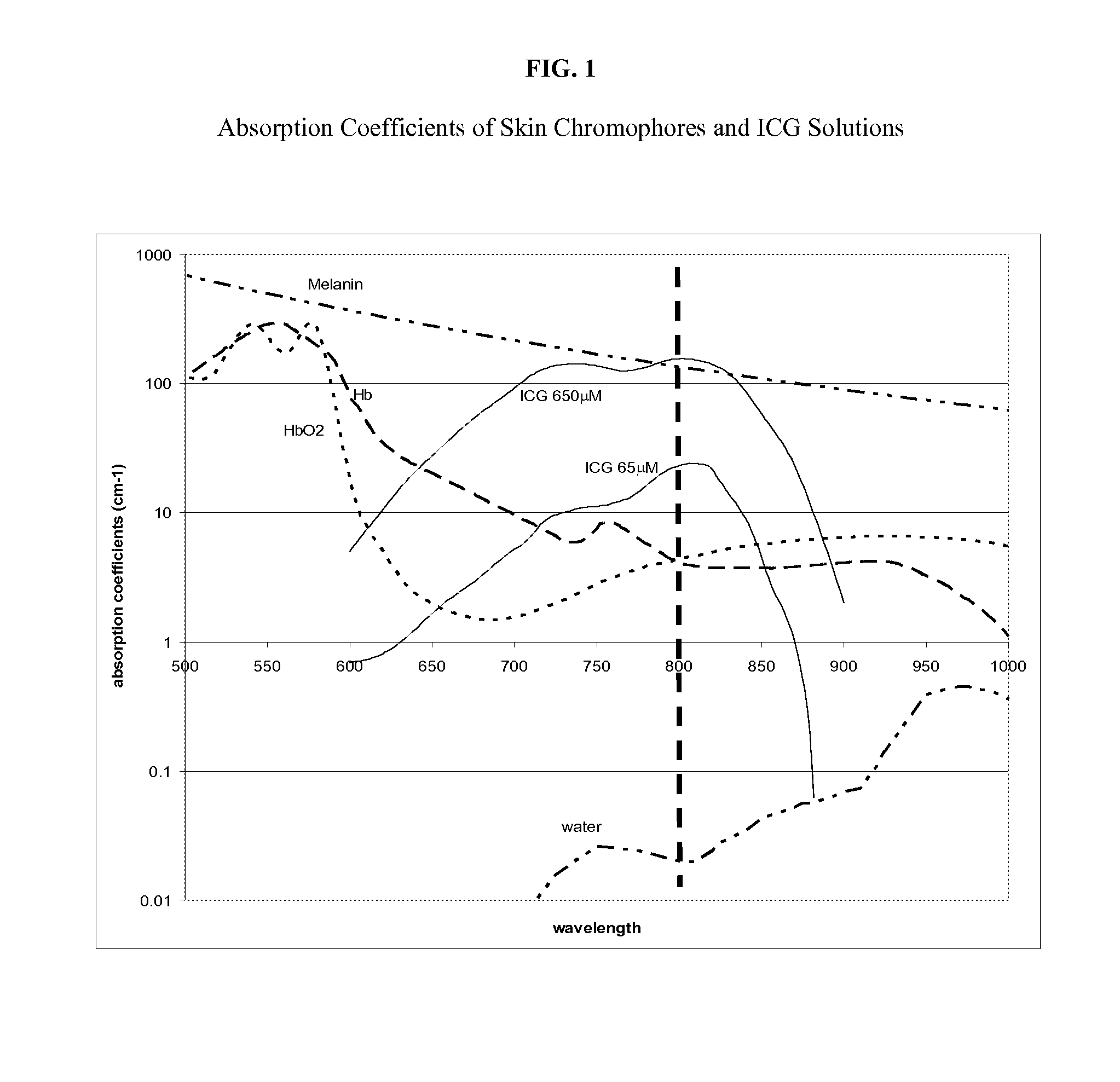
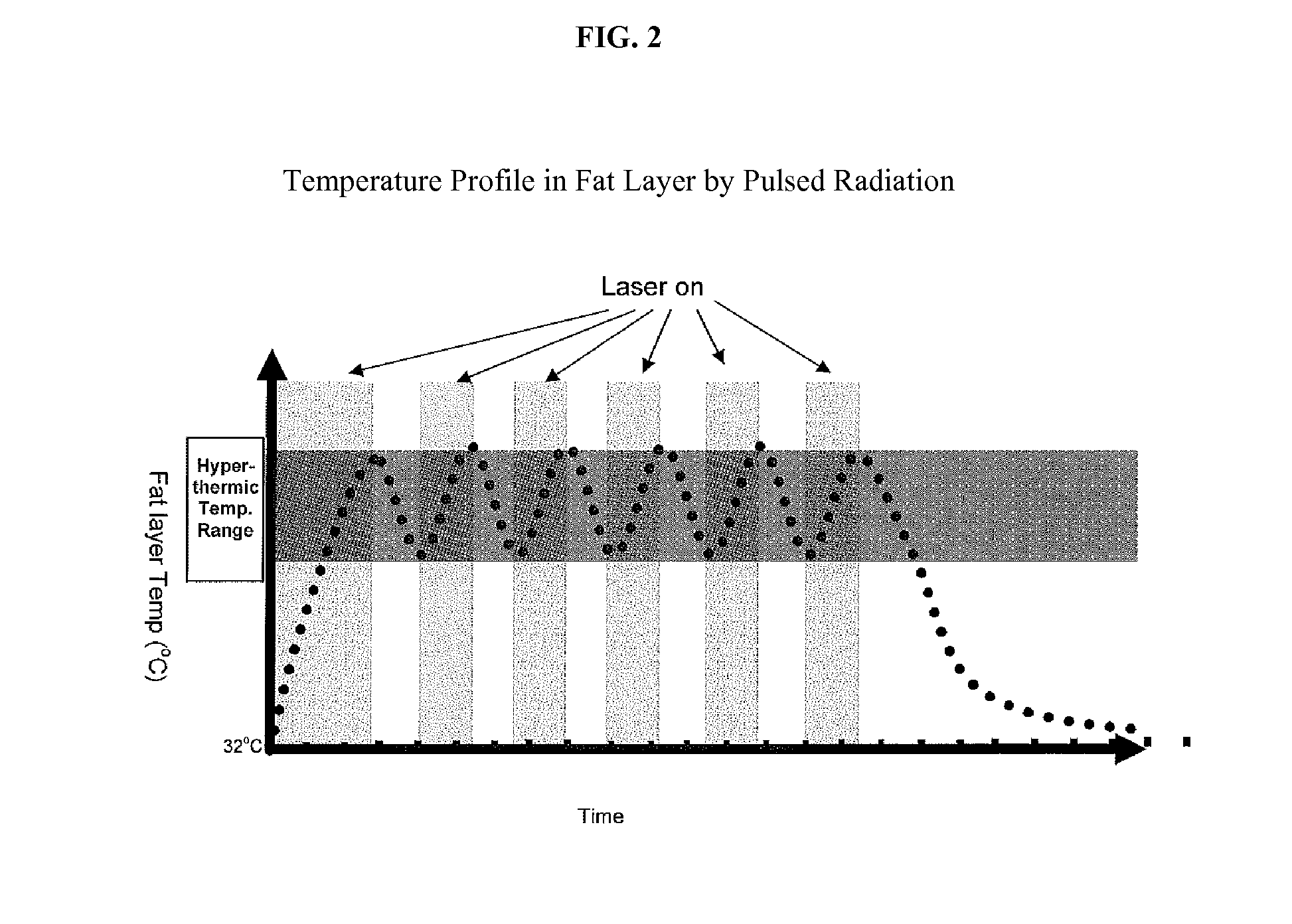
Non-Invasive Fat Reduction by Hyperthermic Treatment
InactiveUS20140025033A1Extended temperature rangeSuitable for applicationMedical devicesSurgical instruments for heatingTissue remodelingNon invasive
The present disclosure relates systems and methods for tissue remodeling, that ameliorate fat deposits by disrupting adipocytes through low-temperature extended treatment time approaches, in conjunction with selective treatment and / or localized cooling of the treatment site to prevent or minimize damage to non-target tissues.
Owner:CYNOSURE
Multi-layer porous scaffold and preparation method thereof
InactiveCN101874751AAvoid weakening separationGood biocompatibilityProsthesisTissue remodelingTissue repair
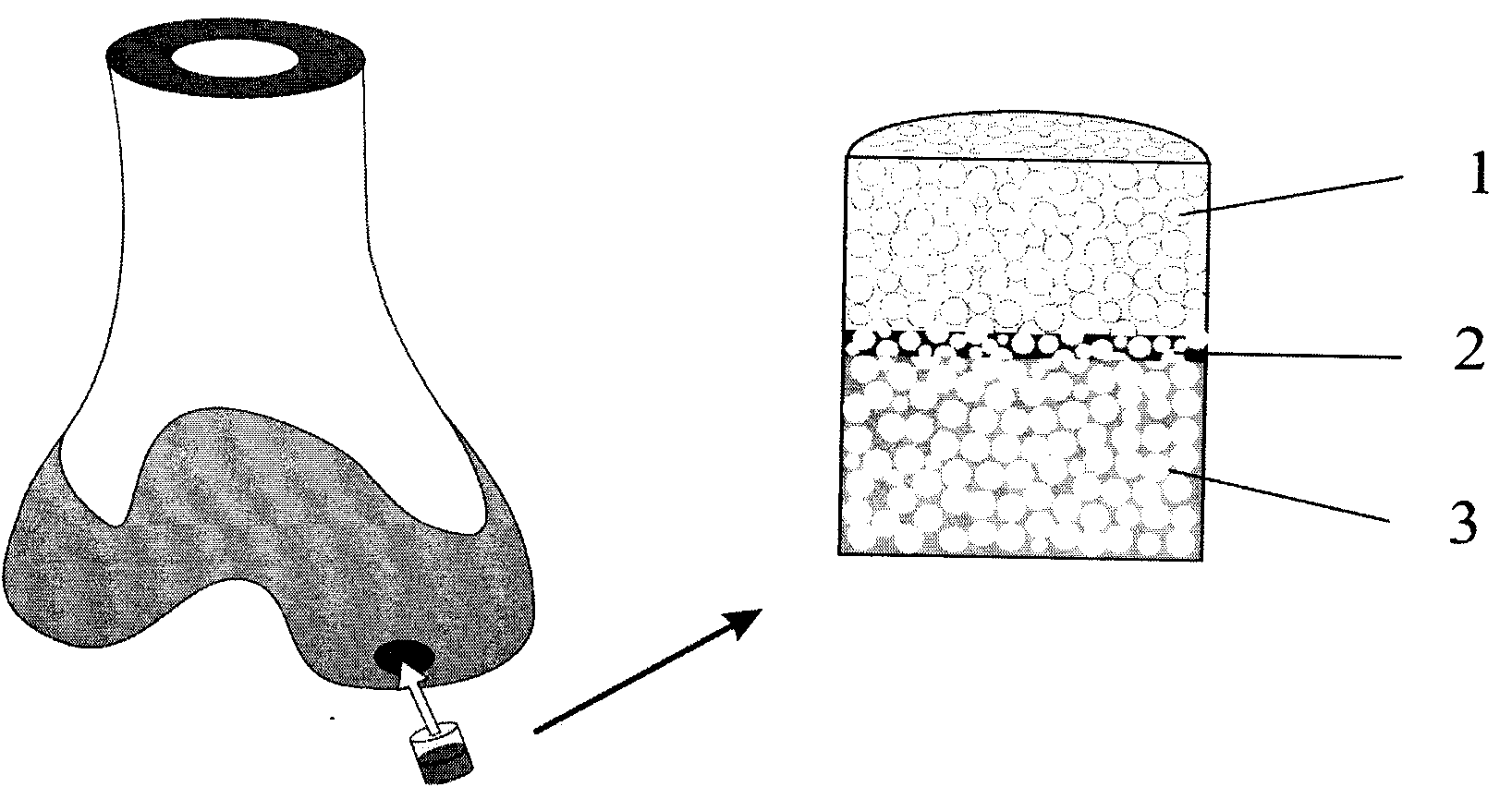
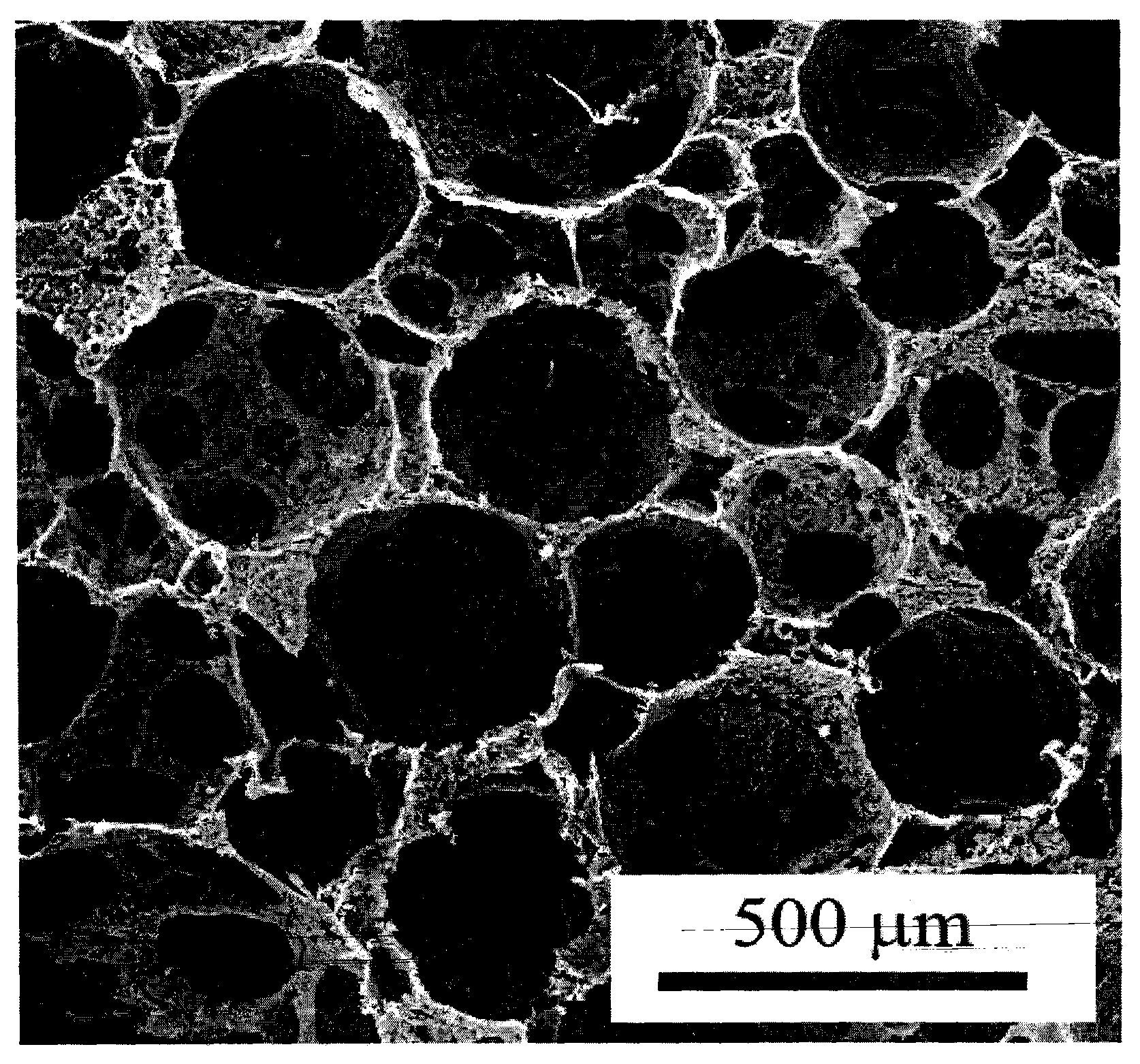
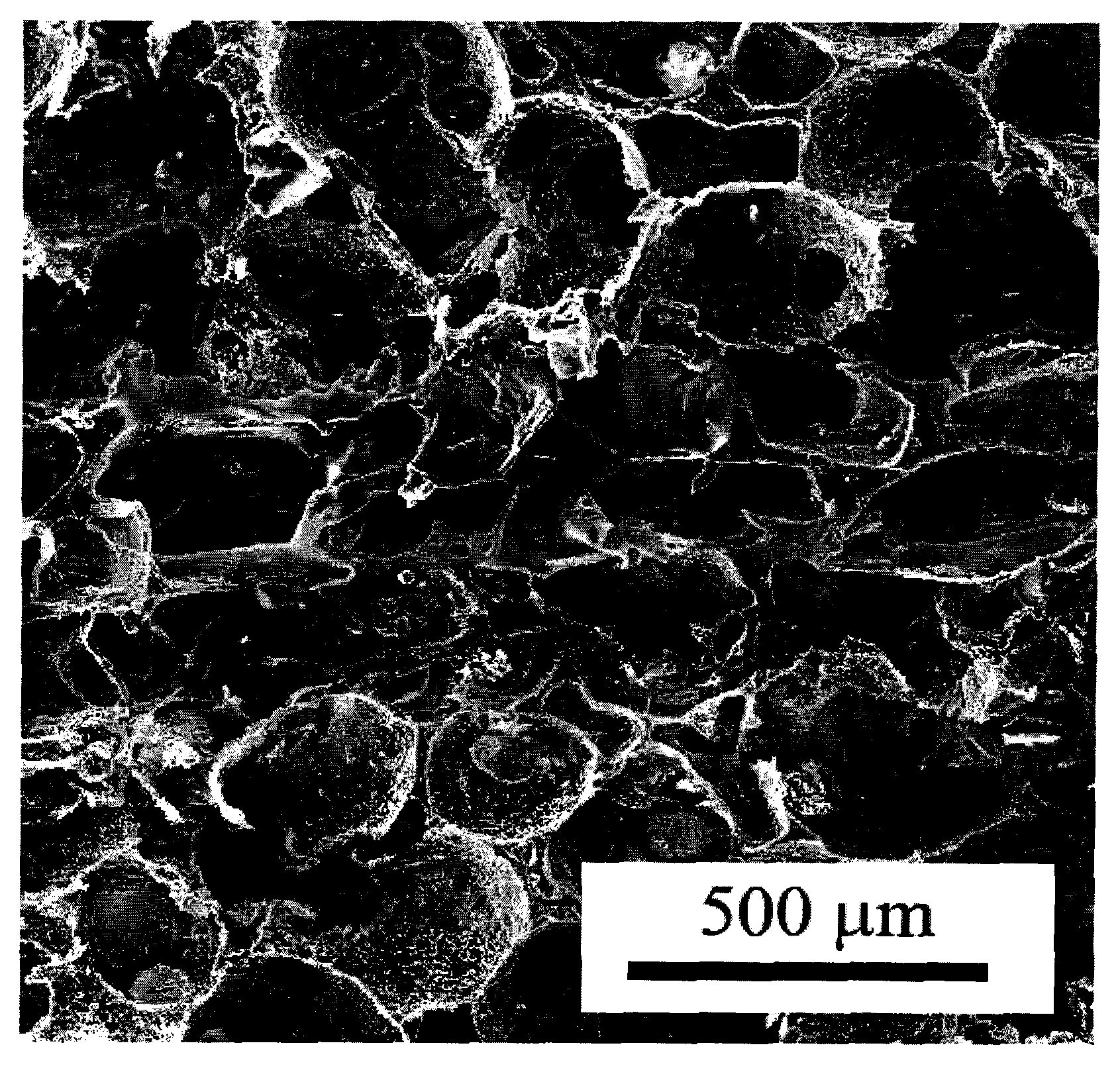
The invention belongs to the technical field of biological materials and tissue repair and particularly relates to a multi-layer porous scaffold and a preparation method thereof. A suitable porous material is selected, after being clipped according to set size and requirements, the material is bonded by pore-forming adhesive which contains mixture of components of polymer / pore-forming particles / solvent, and pore-forming agent is removed after solidification and bonding, thus preparing the porous scaffold which has multi-layer structures communicated with each other. The scaffold has multi-layer structures, porous transition layers are among the layers of the scaffold, and the pores in an upper layer and a lower layer of the scaffold are communicated with each other; and the scaffold is suitable for transition of different cells and fusion of tissues in a process of tissue remodeling, and weakened separation between layers of the remolding tissue is avoided. The scaffold is suitable for a bionic three-dimensional cell scaffold which repairs a multi-layer tissue and other application fields.
Owner:FUDAN UNIV
Modulation of immune responses in blood-borne mesenchymal cells
The present invention relates to a population of blood borne mammalian cells that express a unique profile of surface markers that includes certain markers typical of connective tissue fibroblasts, and are referred to herein as "blood-borne mesenchymal cells." In particular, it relates to the isolation, characterization and uses of such blood-borne mesenchymal cells. The cells of the present invention can be distinguished from peripheral blood leukocytes by their distinct size, morphology, cell surface phenotype and biologic activities, and are likewise distinguishable from connective tissue fibroblasts by other surface phenotypic markers. These cells proliferate in culture, and in vivo, as demonstrated in animal models, are capable of migrating into wound sites from the blood. Therefore, such blood-borne mesenchymal cells may have a wide range of applications, including, but not limited to, the promotion of wound healing, tissue remodeling, and for gene therapy.
Owner:FERRING BV
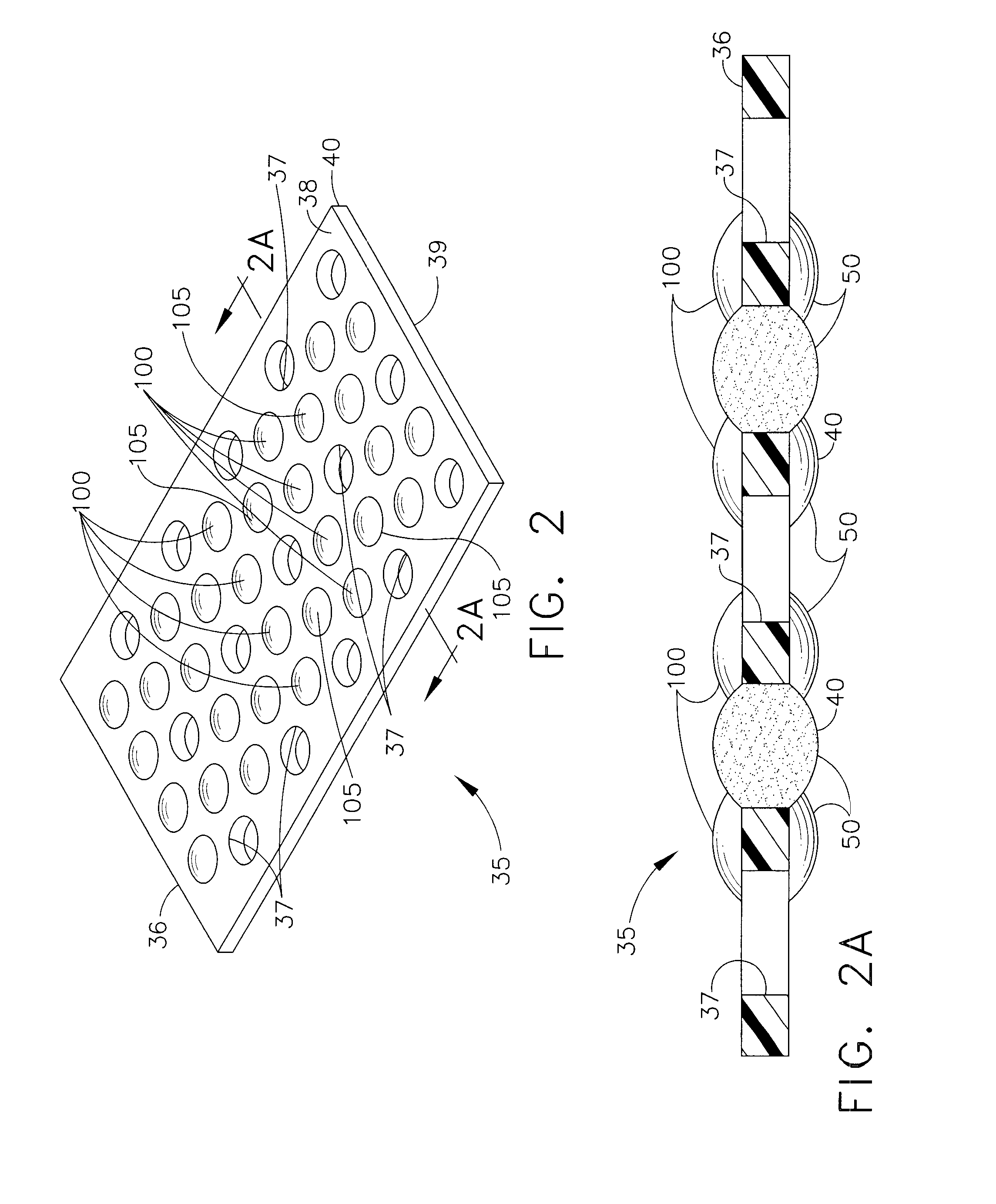
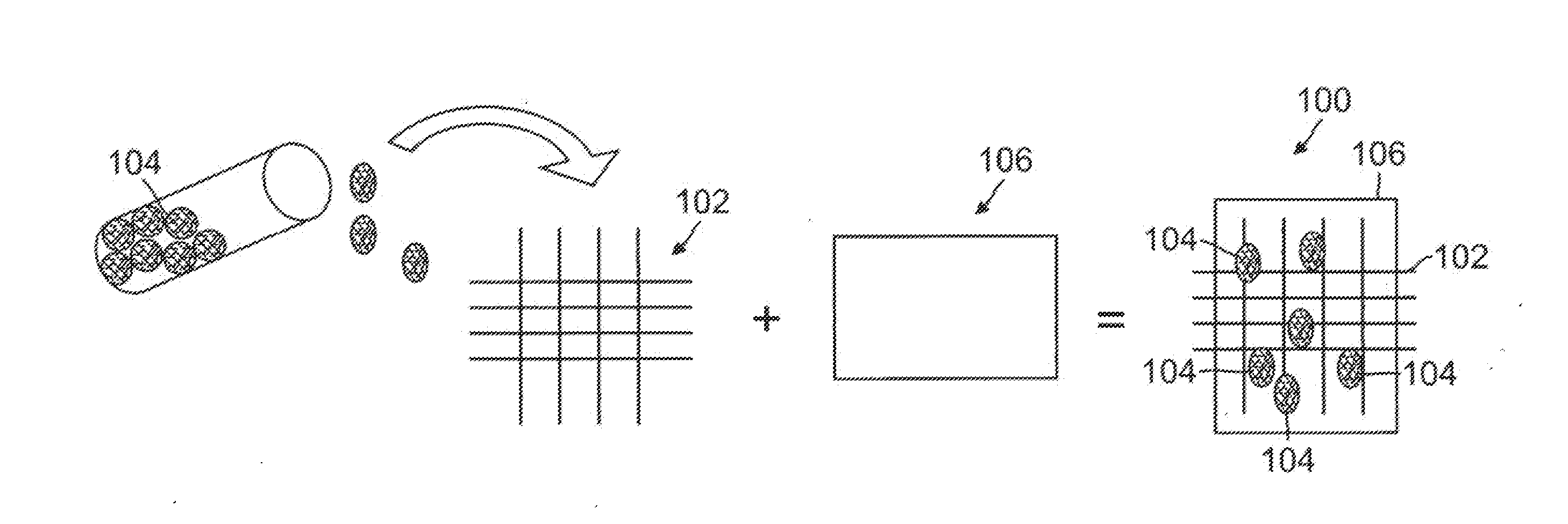
Controlled Adhesive Locations Facilitating Tissue Remodeling
A surgical implant for adhering two portions of tissue together with a polymer adhesive is disclosed. The surgical implant has a matrix structure with one or more layers and a plurality of holes for tissue growth therethrough. The matrix structure controls placement of the adhesive to minimize adhesive area and maximize tissue regrowth areas. In addition, the surgical implant can include drugs and adhesive initiators, and can include multiple layers of structure with any combination of holes, drugs, adhesives and adhesive initiators within. Additionally, the surgical implant can be surrounded by a rapidly dissolving pouch to prevent unwanted polymerization of the adhesive prior to placement in the body.
Owner:ETHICON ENDO SURGERY INC
Blood-borne mesenchymal cells
InactiveUS6174526B1Promote wound healingBiocideMammal material medical ingredientsTissue remodelingSurface marker
The present invention relates to a population of blood borne mammalian cells that express a unique profile of surface markers that includes certain markers typical of connective tissue fibroblasts, and are referred to herein as "blood-borne mesenchymal cells." In particular, it relates to the isolation, characterization and uses of such blood-borne mesenchymal cells. The cells of the present invention can be distinguished from peripheral blood leukocytes by their distinct size, morphology, cell surface phenotype and biologic activities, and are likewise distinguishable from connective tissue fibroblasts by other surface phenotypic markers. These cells proliferate in culture, and in vivo, as demonstrated in animal models, are capable of migrating into wound sites from the blood. Therefore, such blood-borne mesenchymal cells may have a wide range of applications, including, but not limited to, the promotion of wound healing, tissue remodeling, and for gene therapy.
Owner:FERRING BV
Programmed-release, nanostructured biological construct for stimulating cellular engraftment for tissue regeneration
InactiveUS20110268776A1Good biocompatibilityEnhanced interactionNanomedicinePharmaceutical delivery mechanismTissue remodelingBiological agent
A biologically engineered construct comprising of a polymeric biomatrix, designed with a nanophase texture, and a therapeutic agent for the purpose of tissue regeneration and / or controlled delivery of regenerative factors and therapeutic substances after it is implanted into tissues, vessels, or luminal structures within the body. The therapeutic agent may be a therapeutic substance or a biological agent, such as antibodies, ligands, or living cells. The nanophase construct is designed to maximize lumen size, promote tissue remodeling, and ultimately make the implant more biologically compatible. The nano-textured polymeric biomatrix may comprise one or more layers containing therapeutic substances and / or beneficial biological agents for the purpose of controlled, physiological, differential substance / drug delivery into the luminal and abluminal surfaces of the vessel or lumen, and the attraction of target molecules / cells that will regenerate functional tissue. The topographic and biocompatible features of this layered biological construct provides an optimal environment for tissue regeneration along with a programmed-release, drug delivery system to improve physiological tolerance of the implant, and to maximize the cellular survival, migration, and integration within the implanted tissues.
Owner:SCHAPIRA JAY N +1
Surgical instrument for treating female urinary stress incontinence
InactiveUS7771345B1High tensile strengthPrevents voiding dysfunctionAnti-incontinence devicesTissue remodelingUrethra
A suburethral sling device and method for treating female urinary stress incontinence which is anatomically configured to implant into the lower abdomen of a female in a manner providing support to mid-urethral and bladder neck sphincteric continence sites with the sling defining in part, mesh and tissue remodeling portions. The sling is deployed via a sling transfer instrument having distal and proximal ends with the instrument comprising in part a progressively curved shaft portion positioned between the distal and proximal ends. An insertion handle of the transfer instrument is secured to the curved metal shaft section guiding the shaft tip through the tissues of the abdomen in an anterior / posterior direction as well as a cephalad / caudad direction.
Owner:ODONNELL PAT D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com