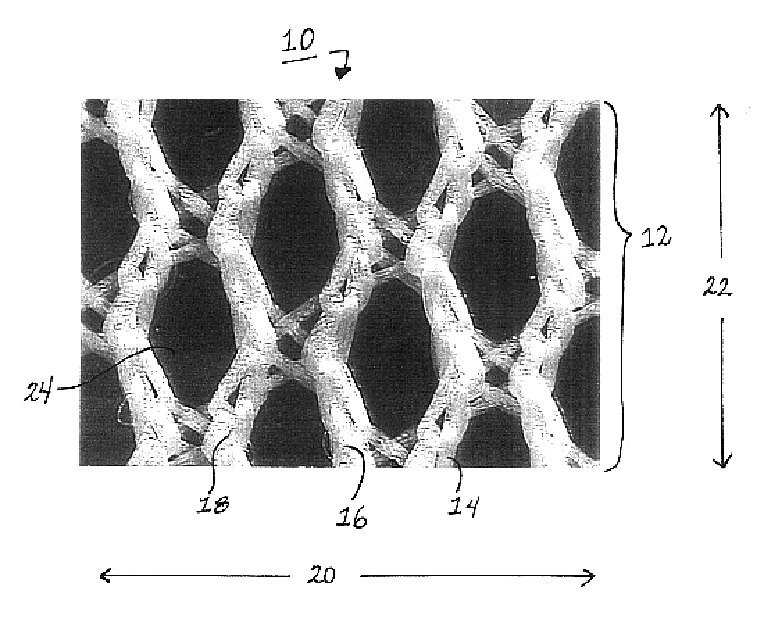
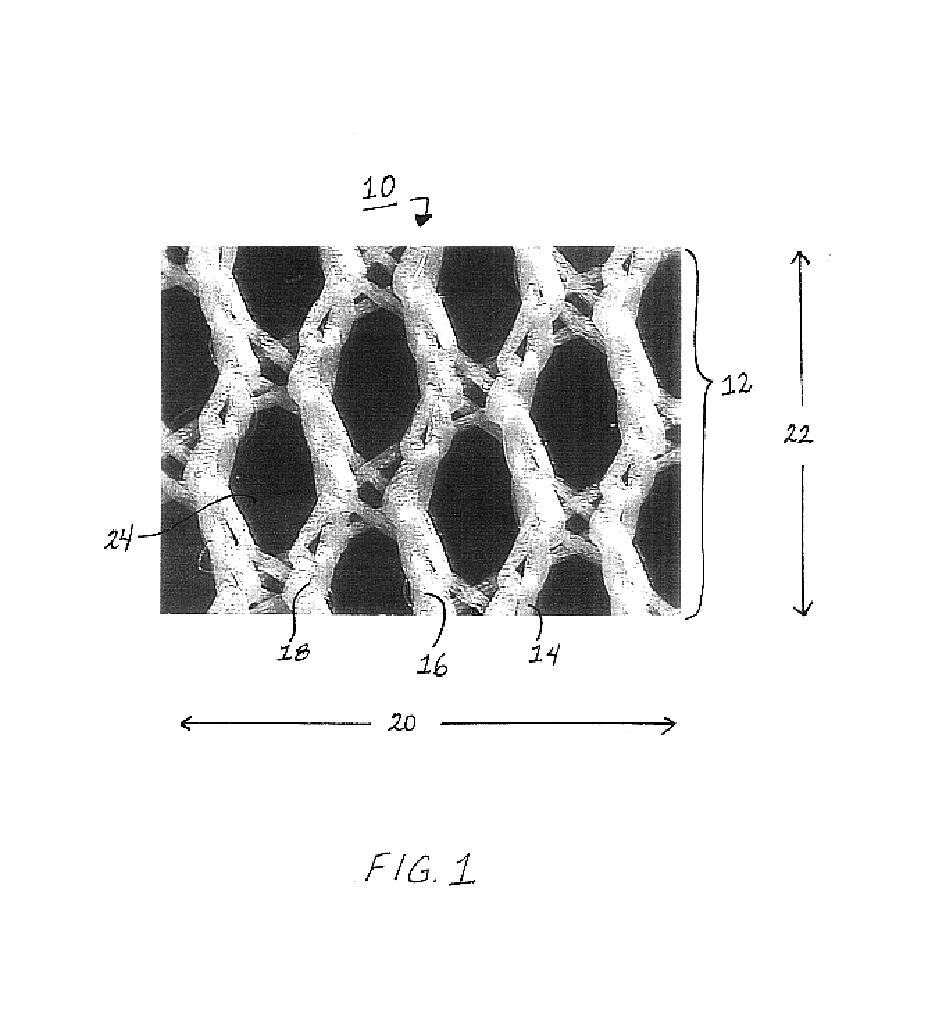
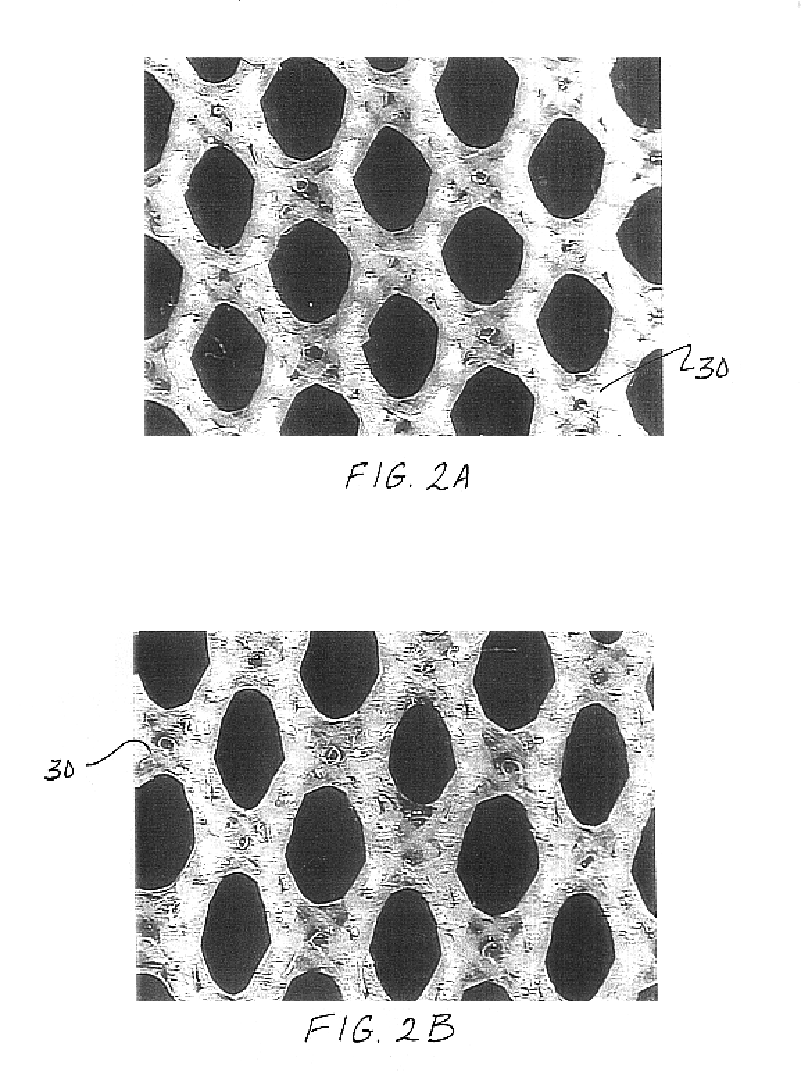
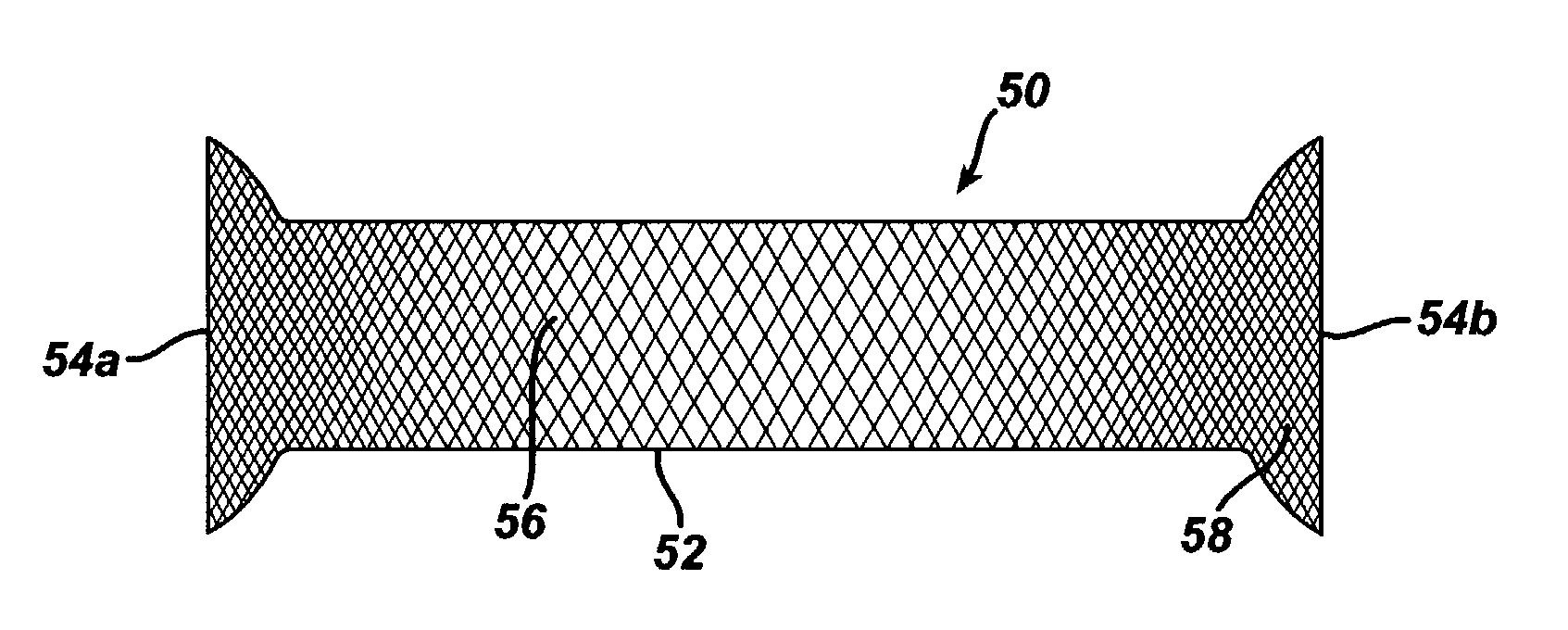
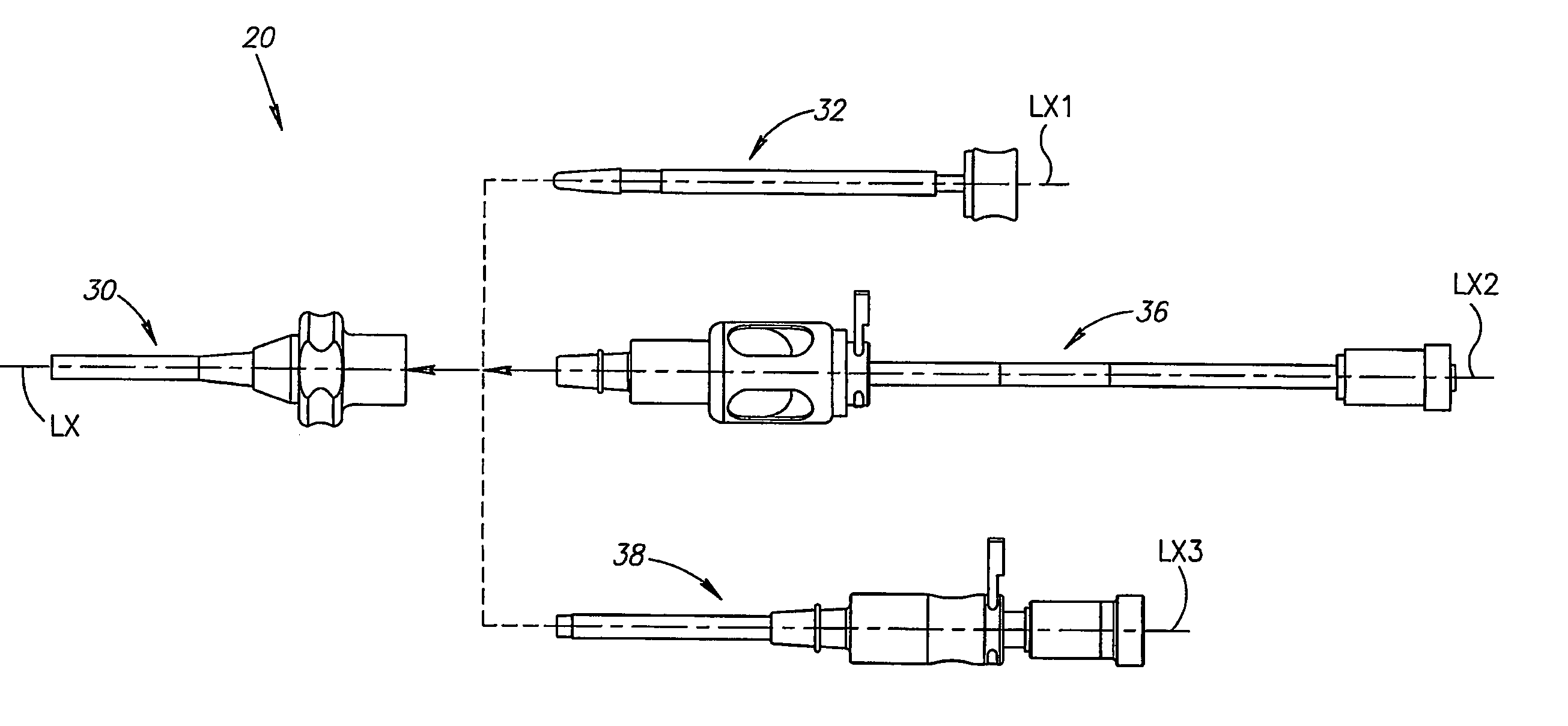
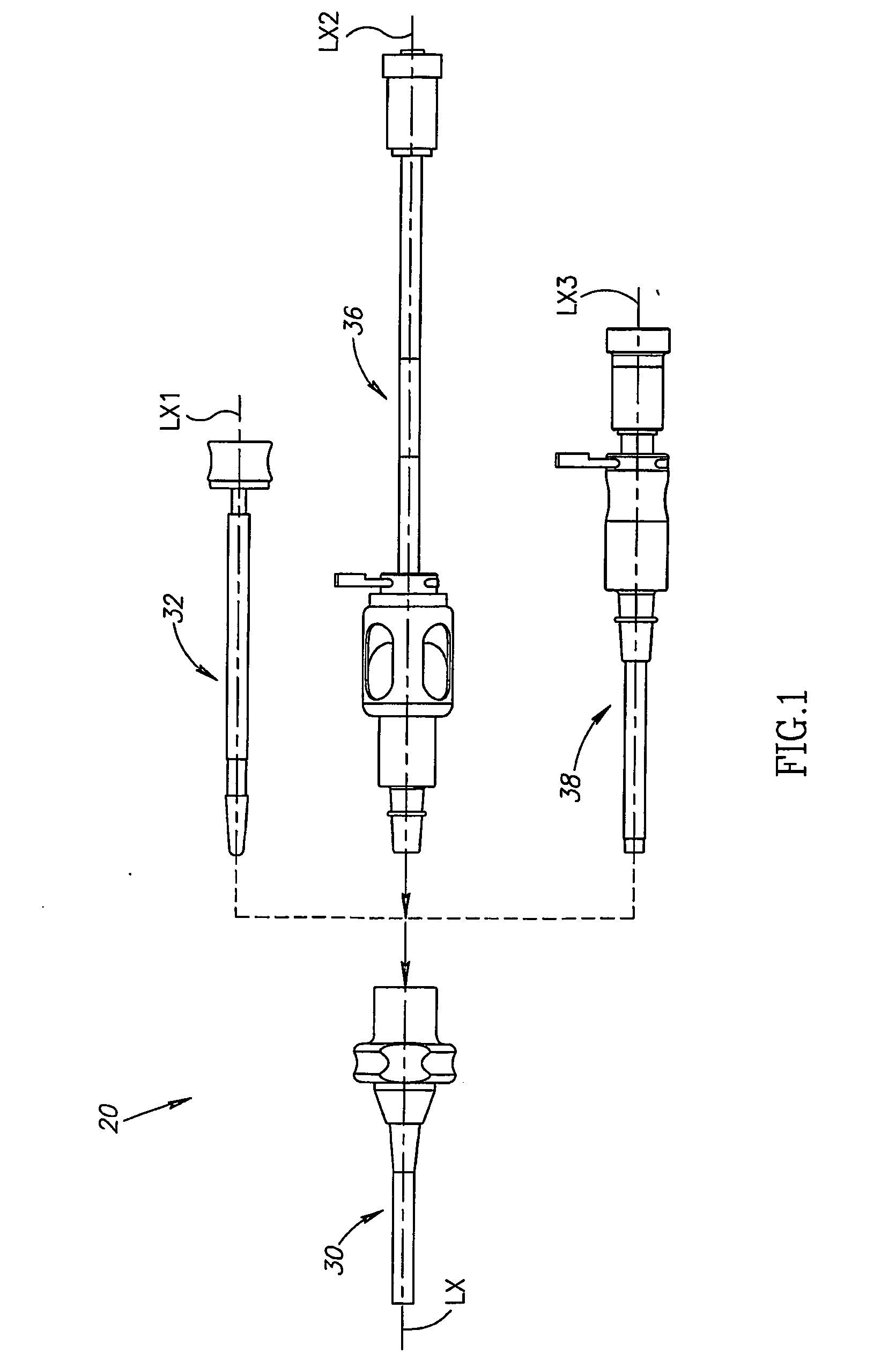
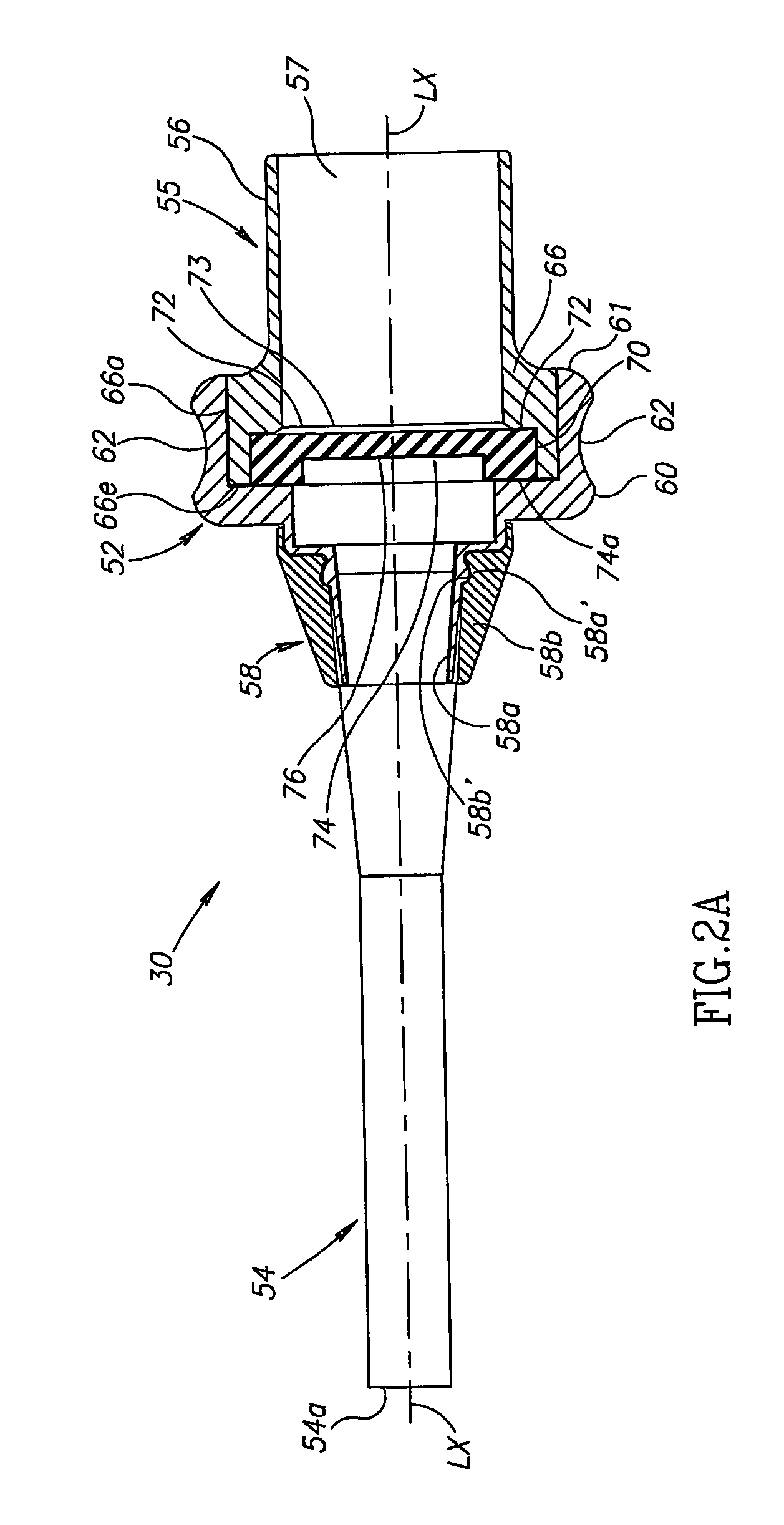
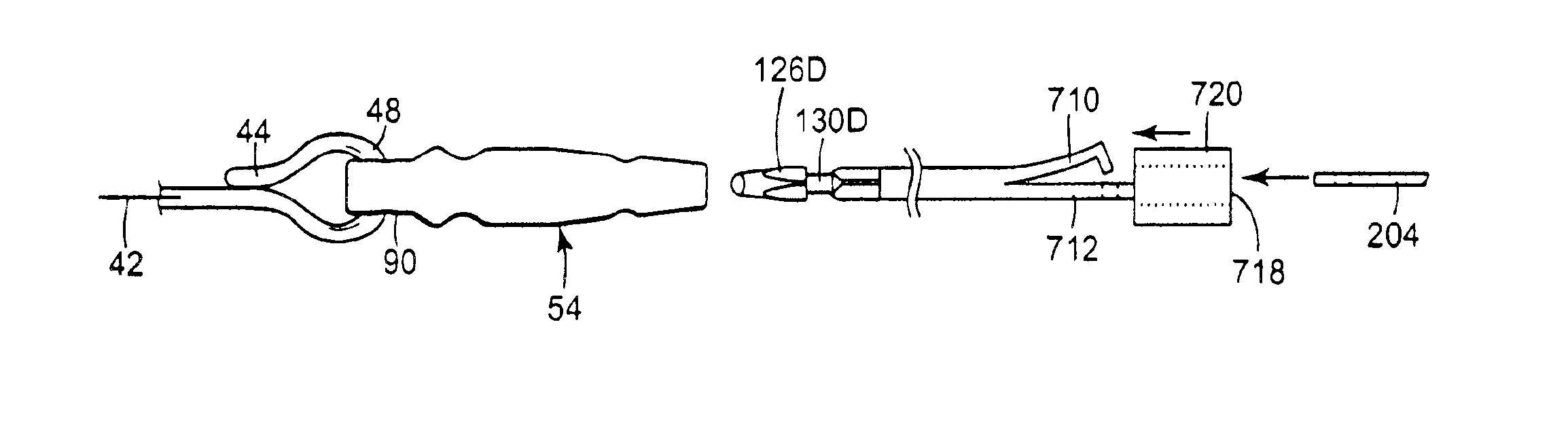
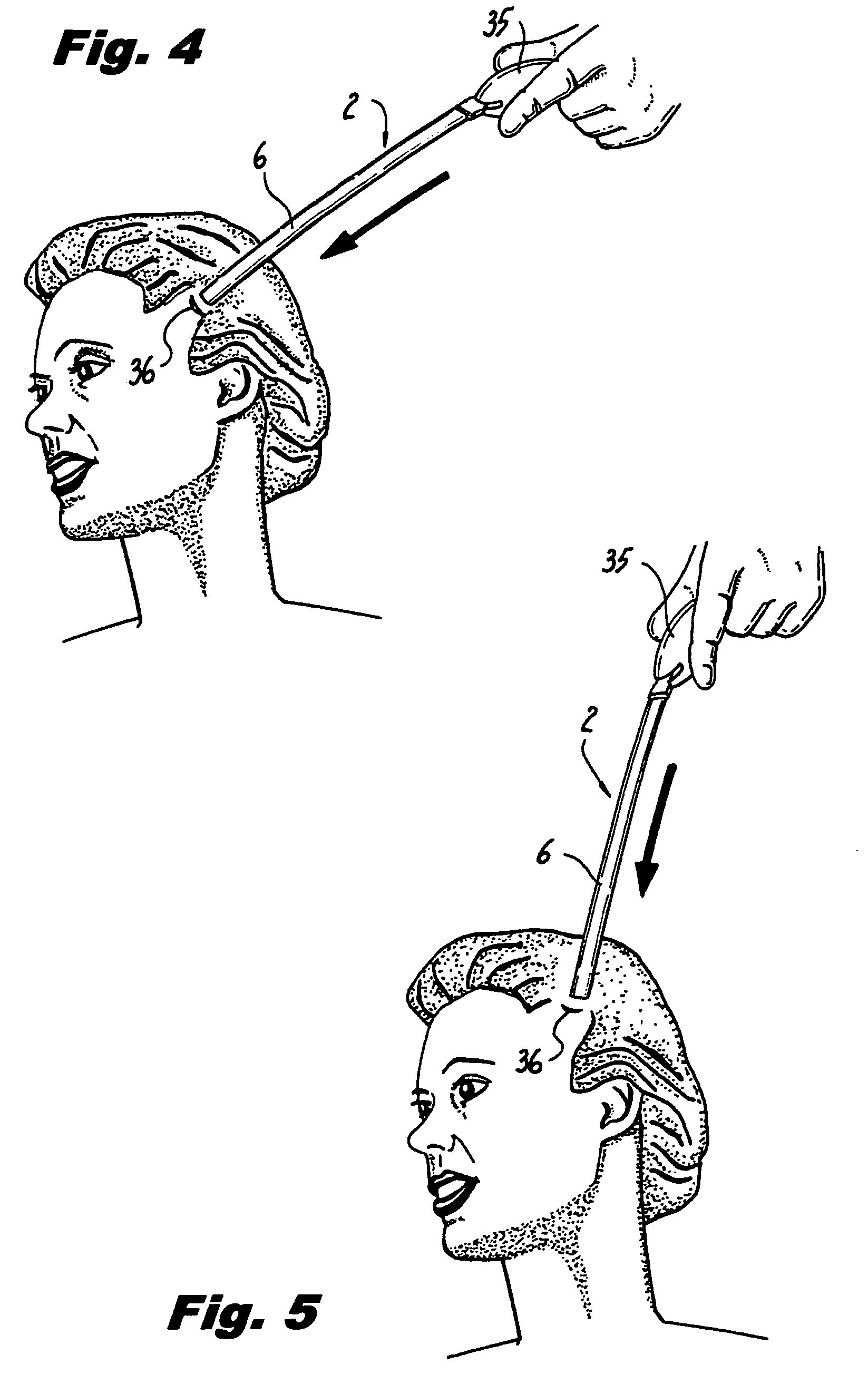Patents
Literature
1462results about "Anti-incontinence devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
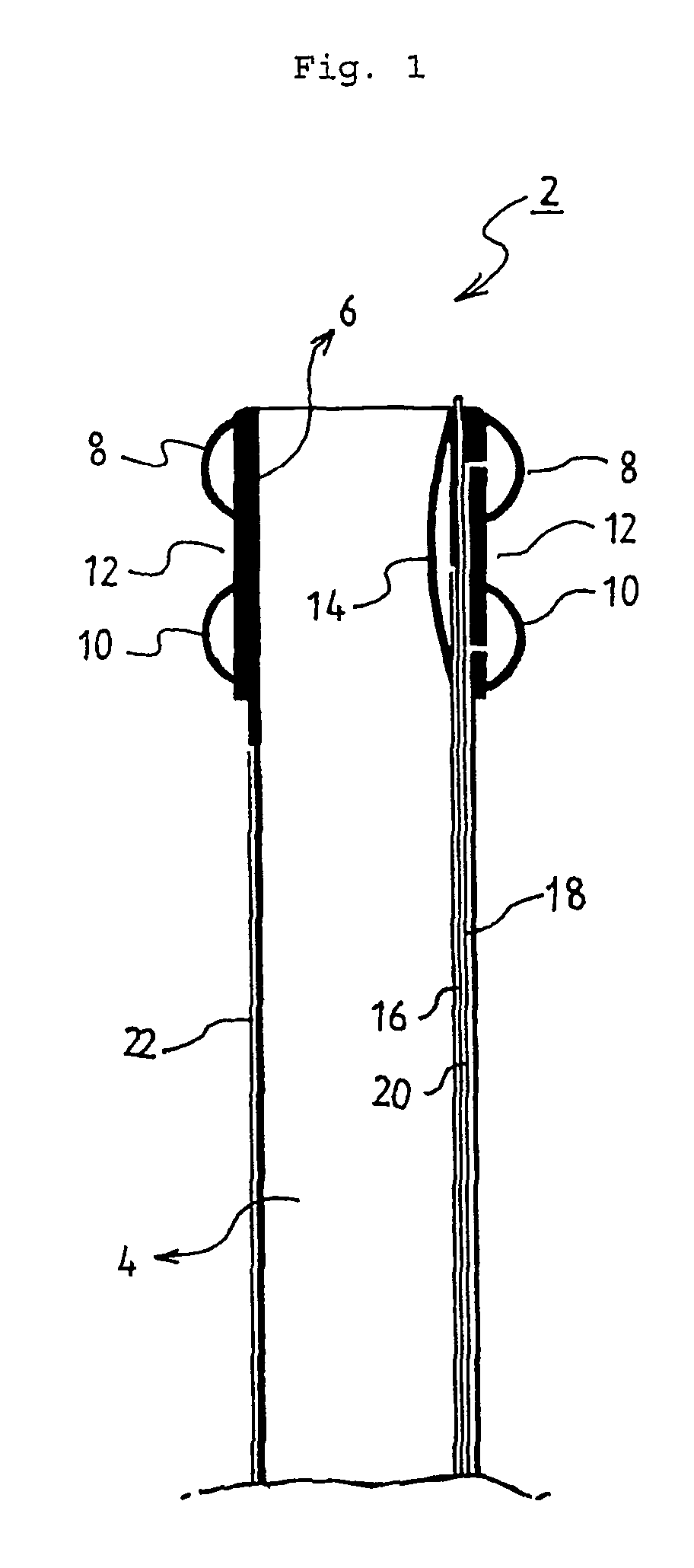
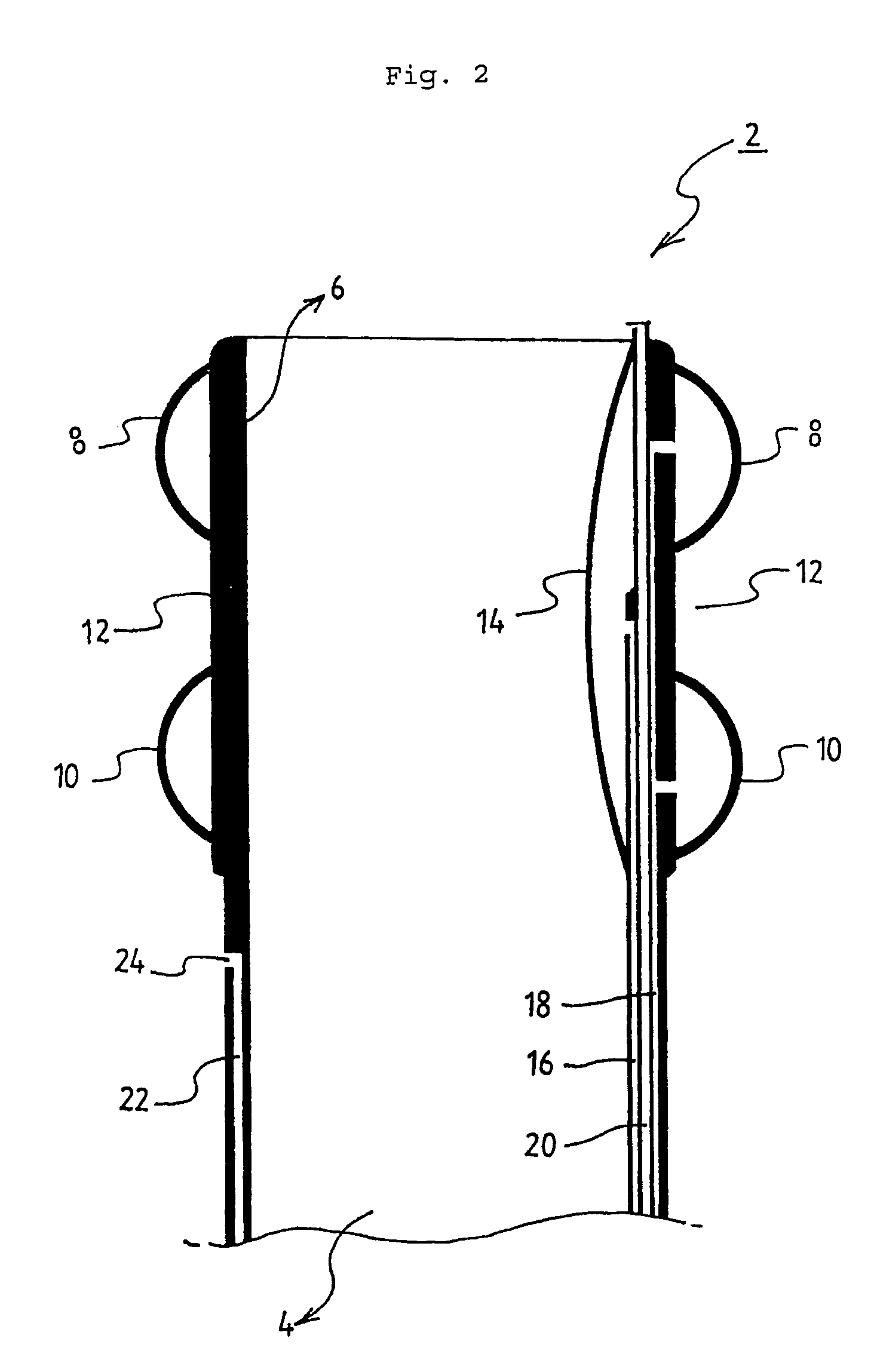
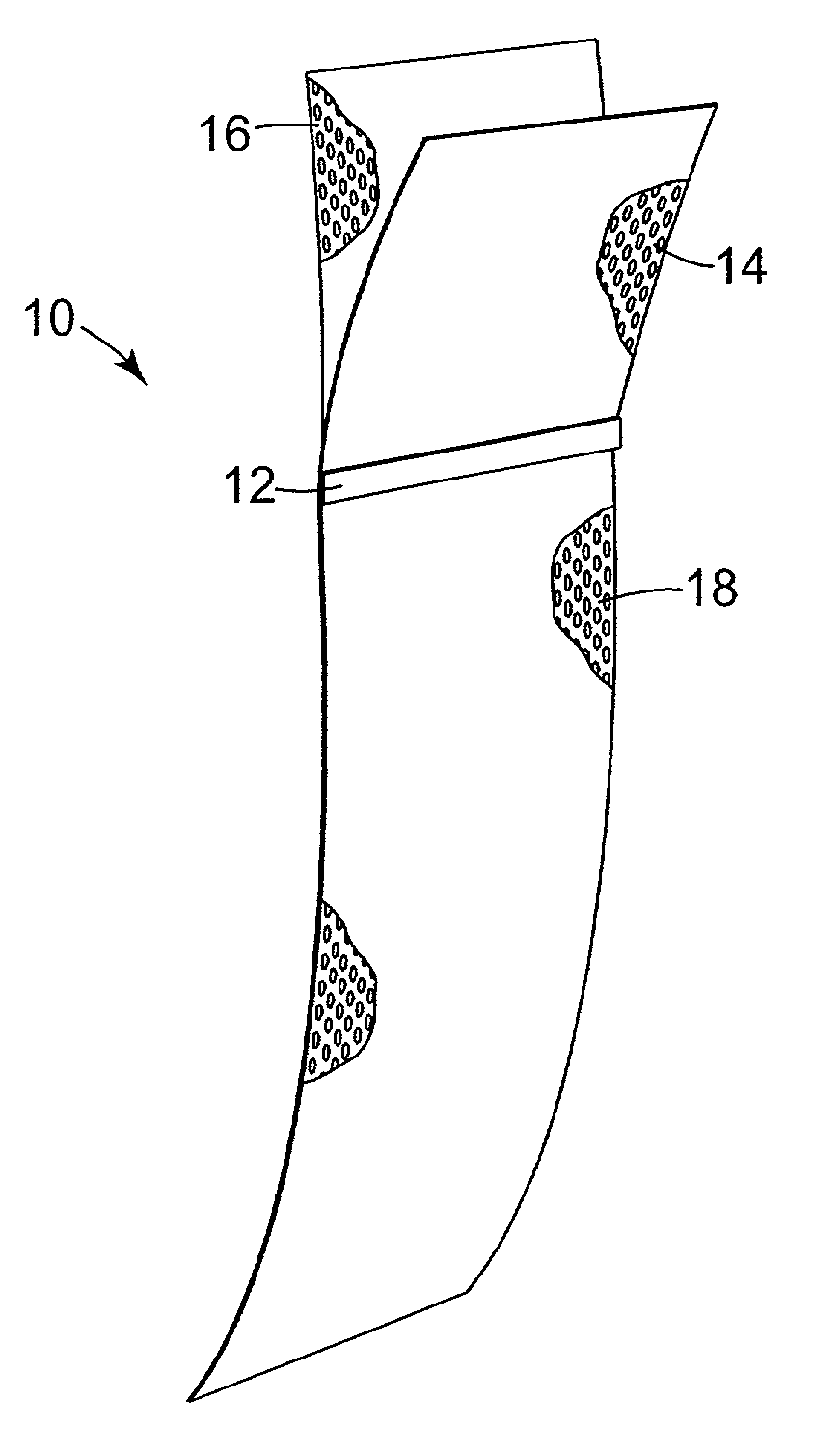
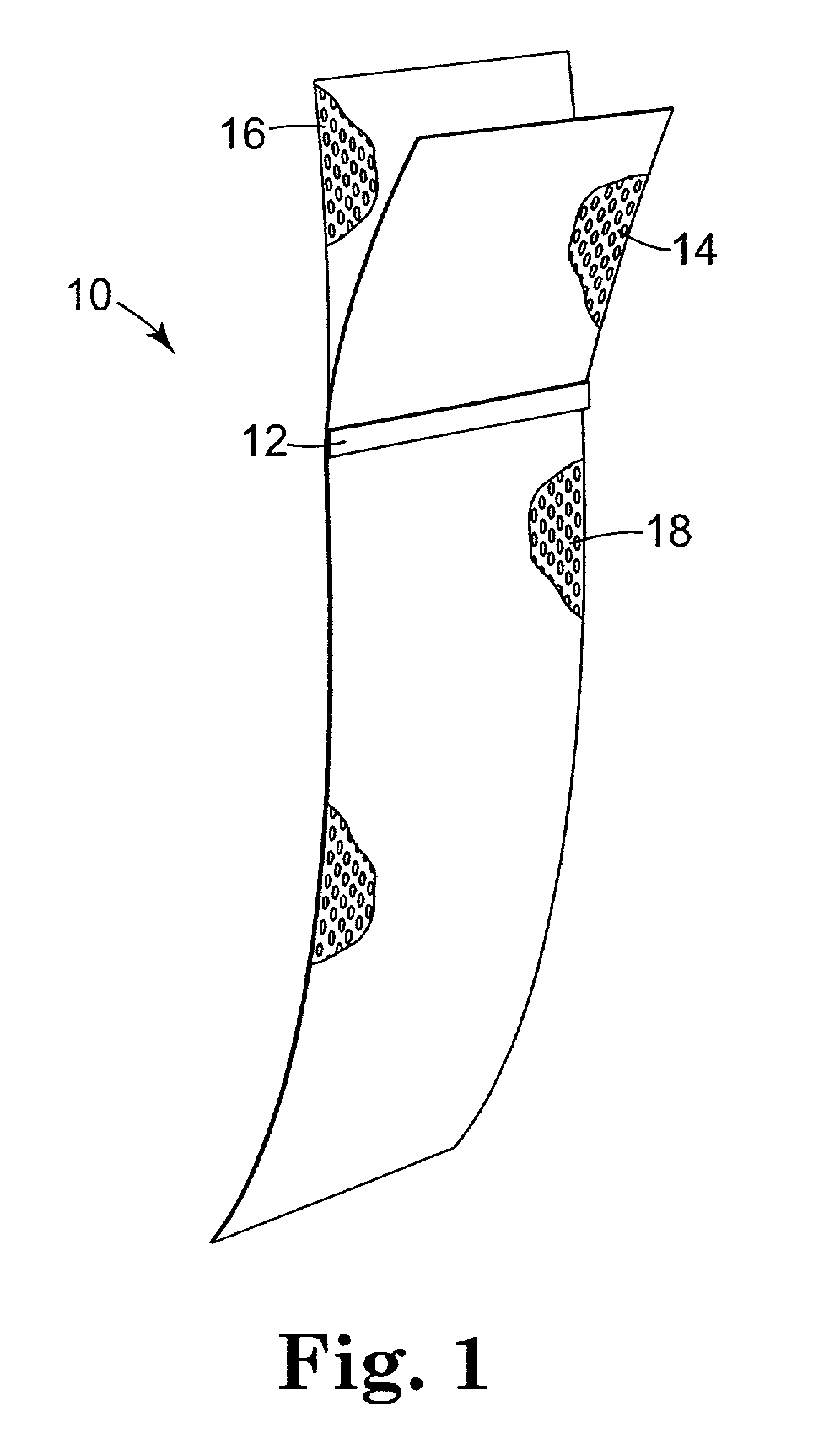
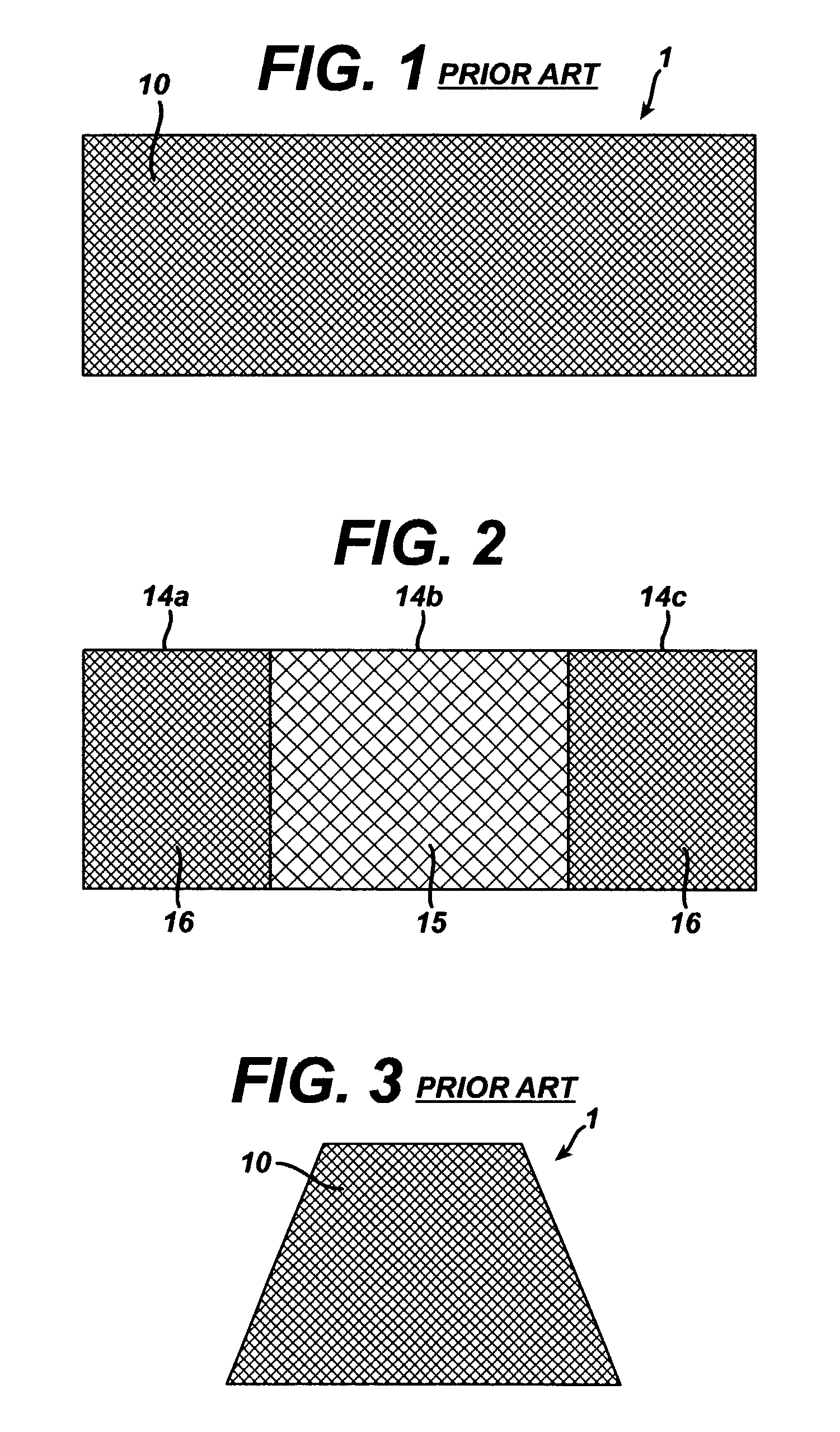
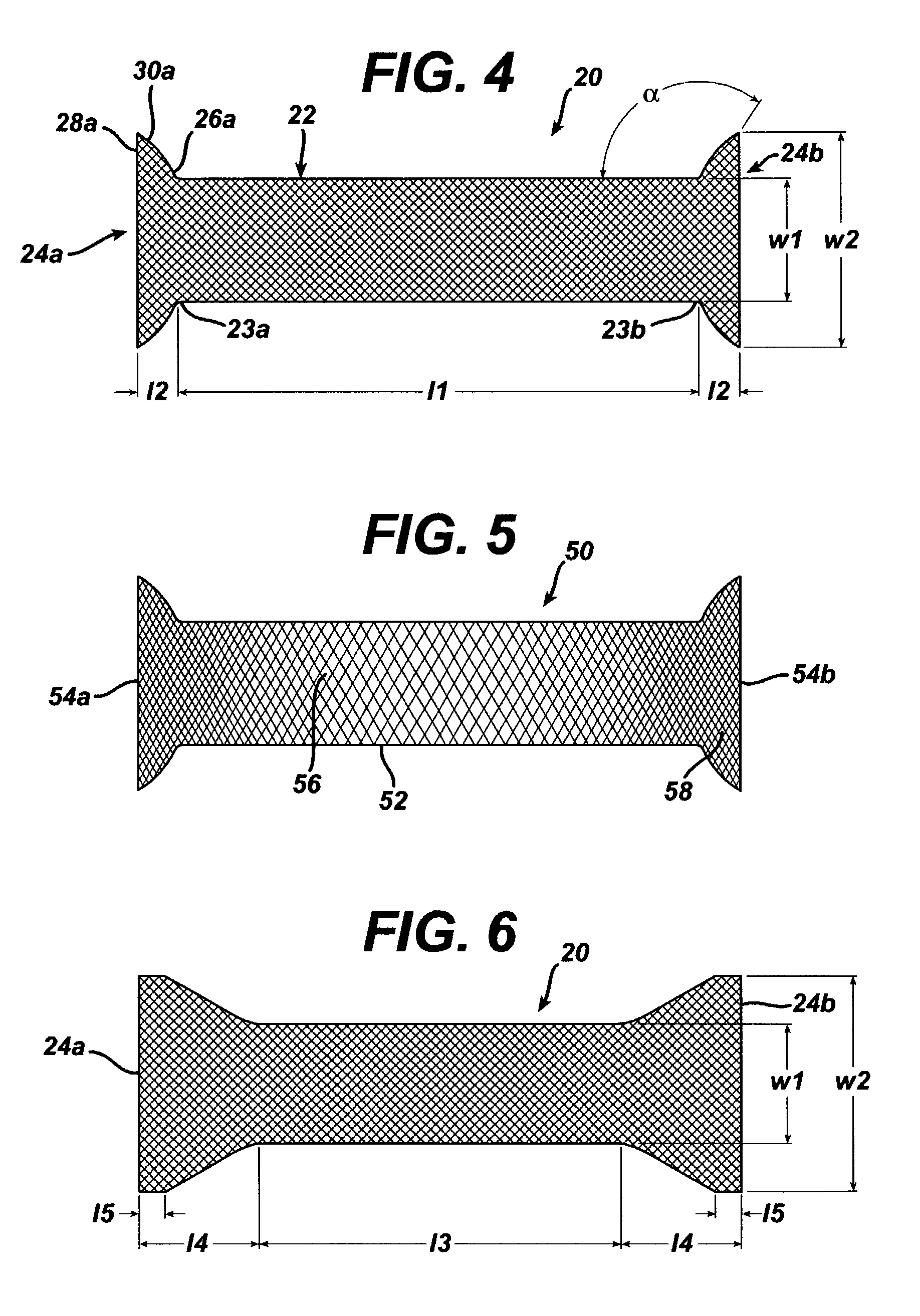
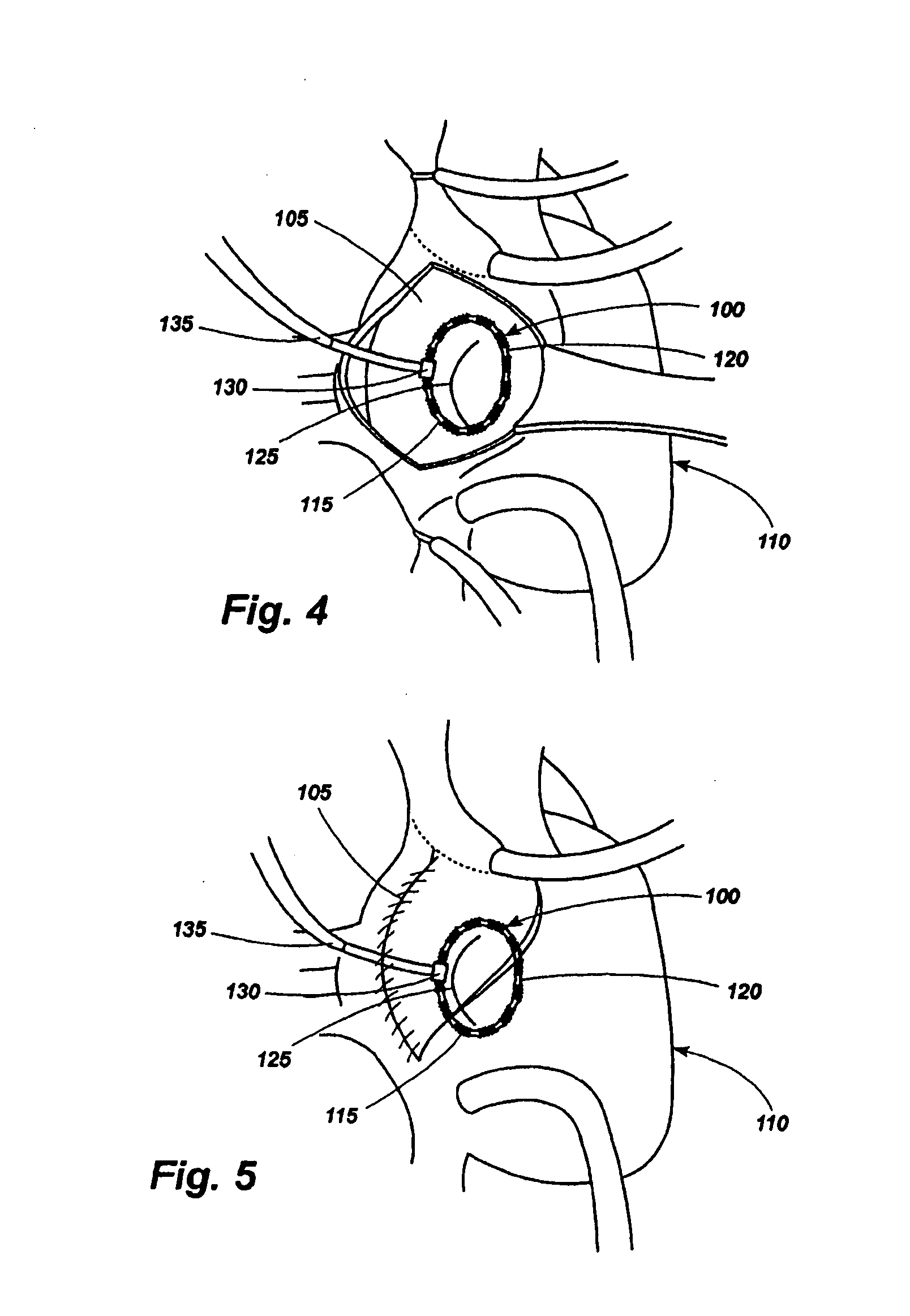
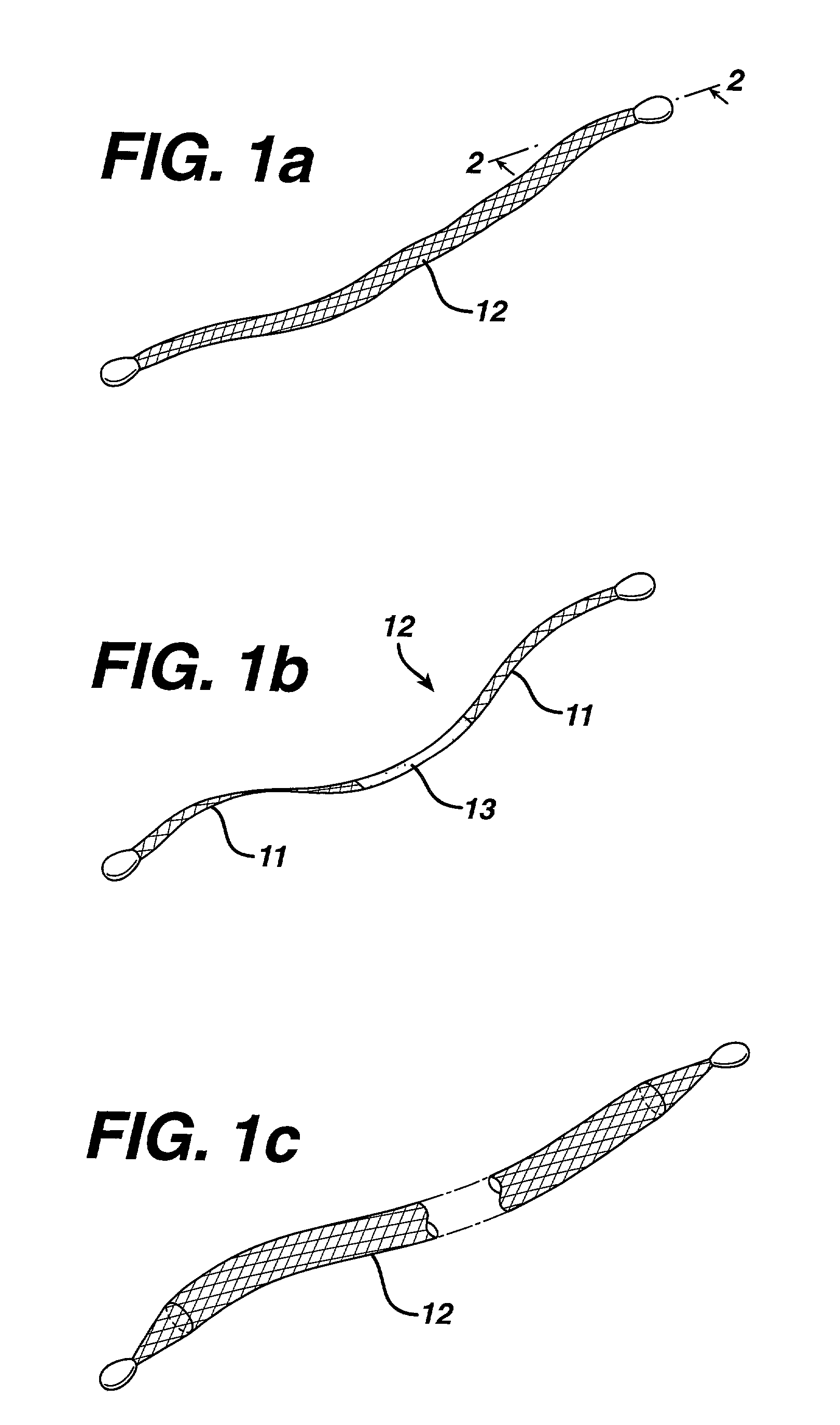
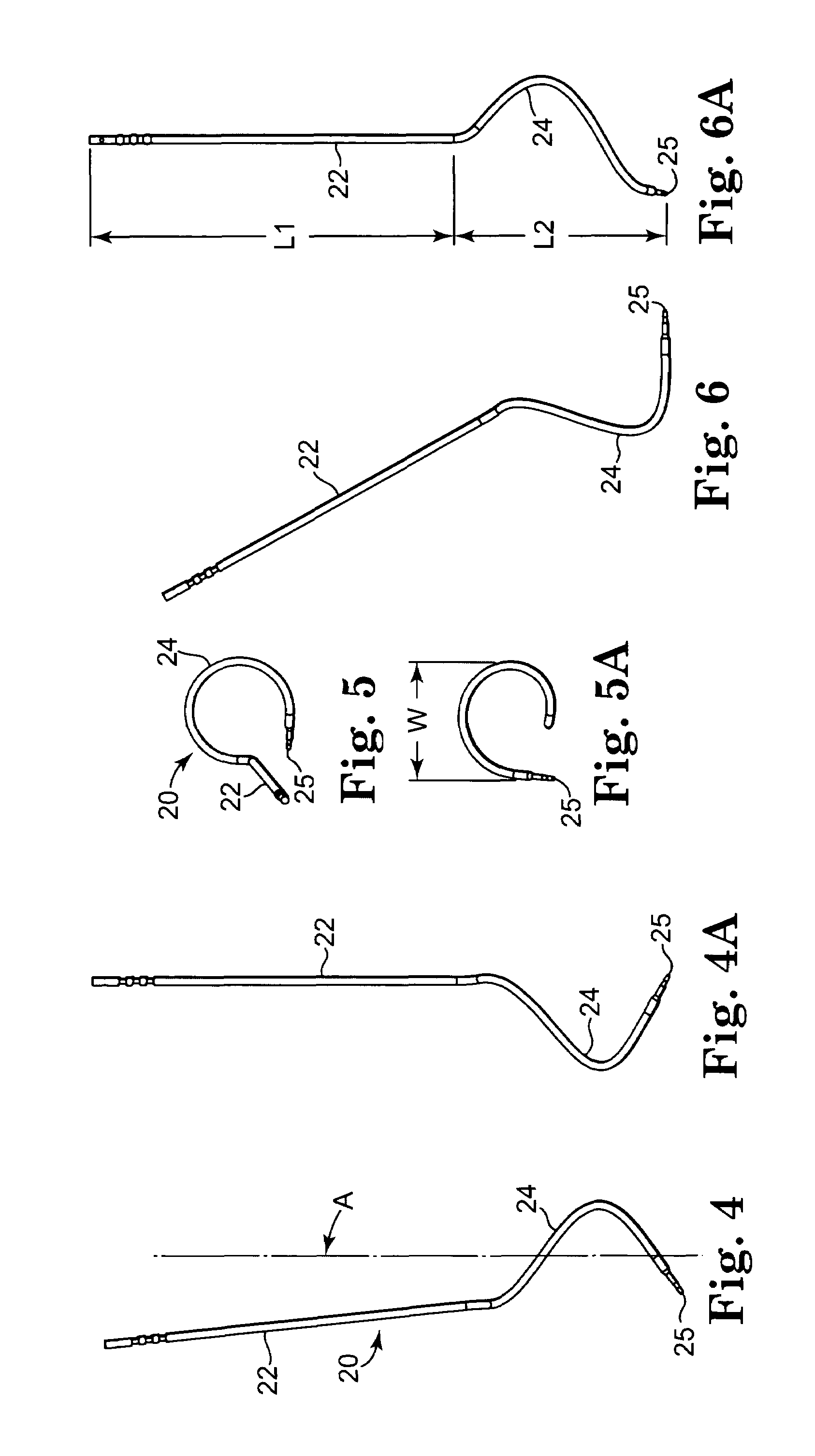
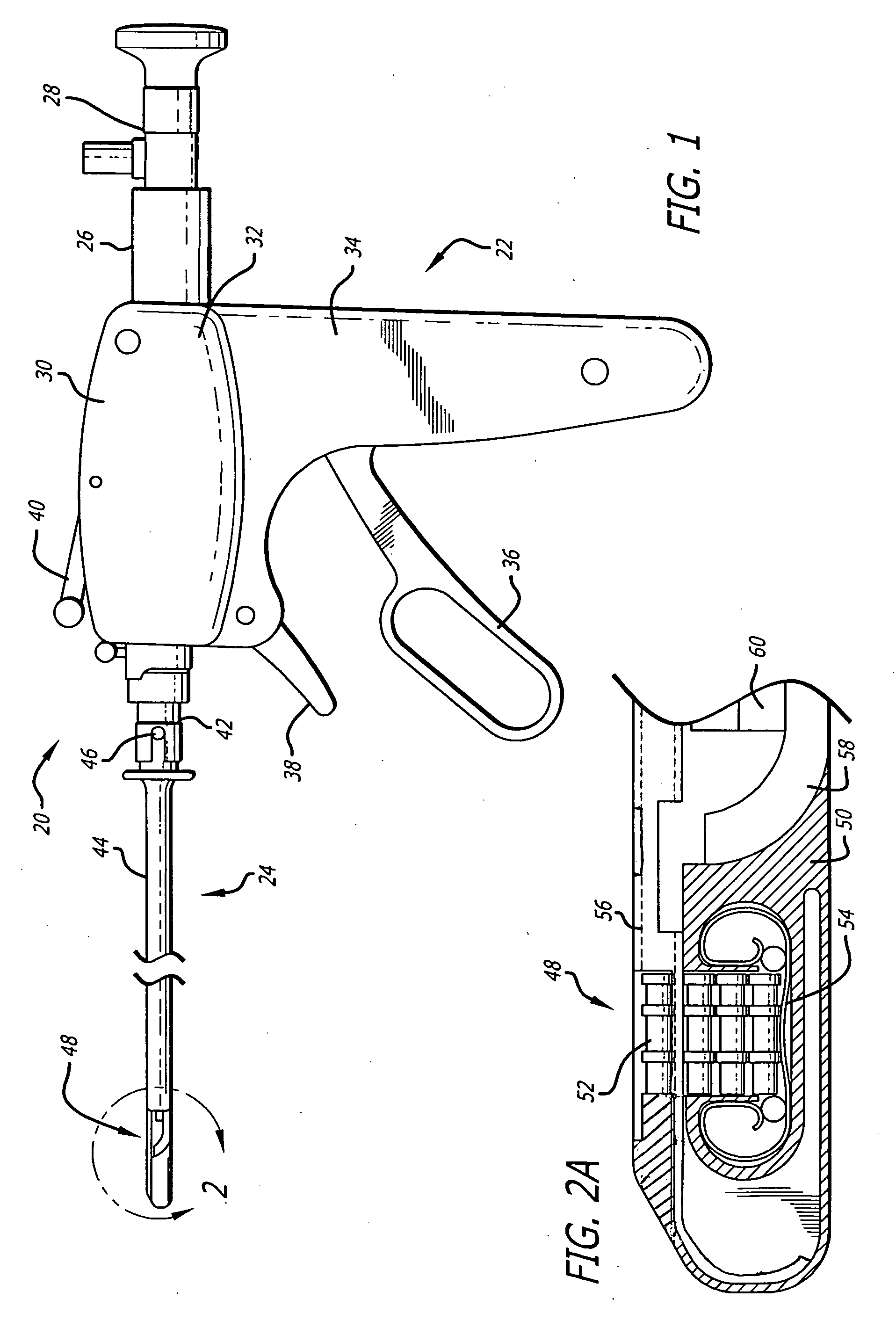
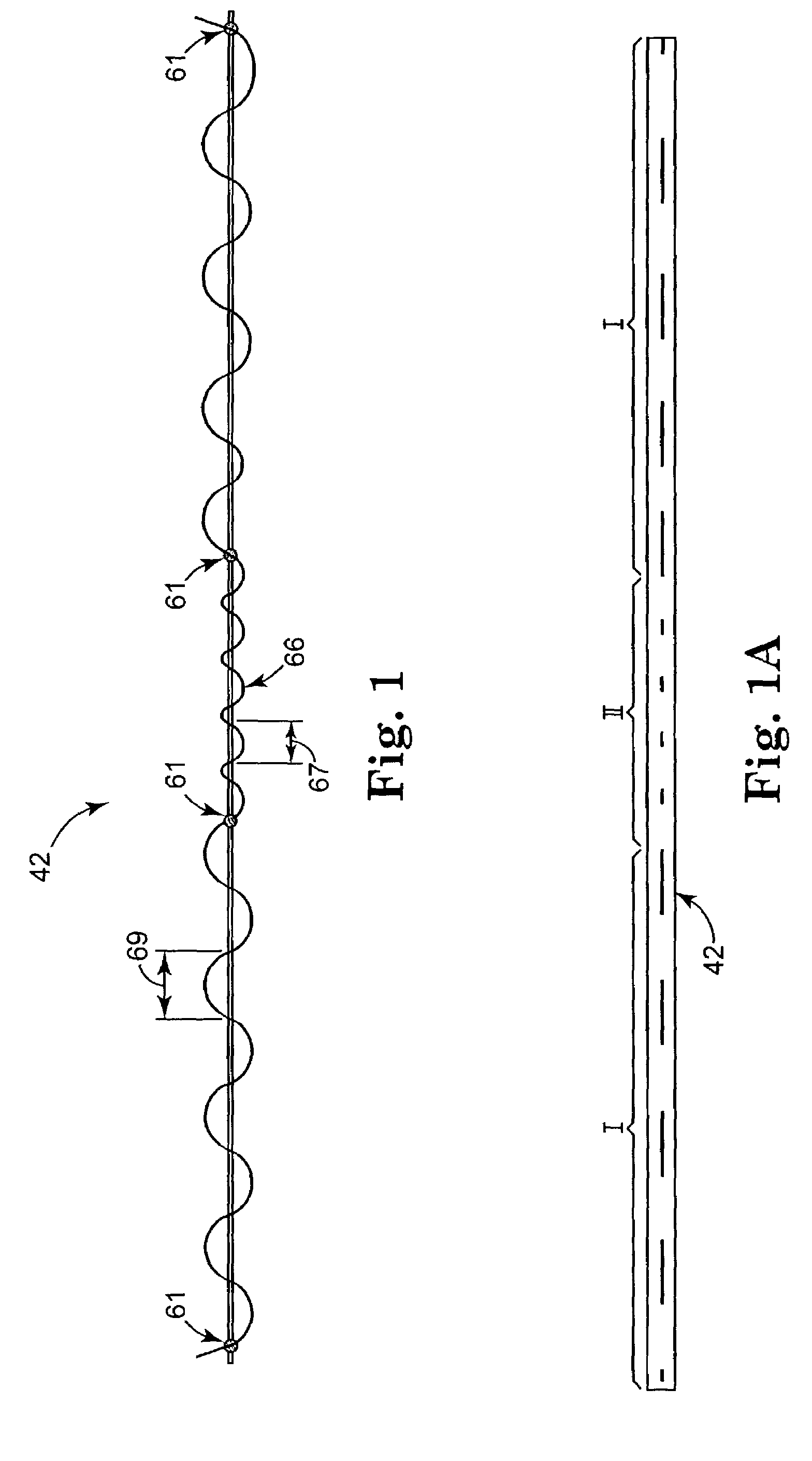
Method of facial reconstructive surgery using a self-anchoring tissue lifting device
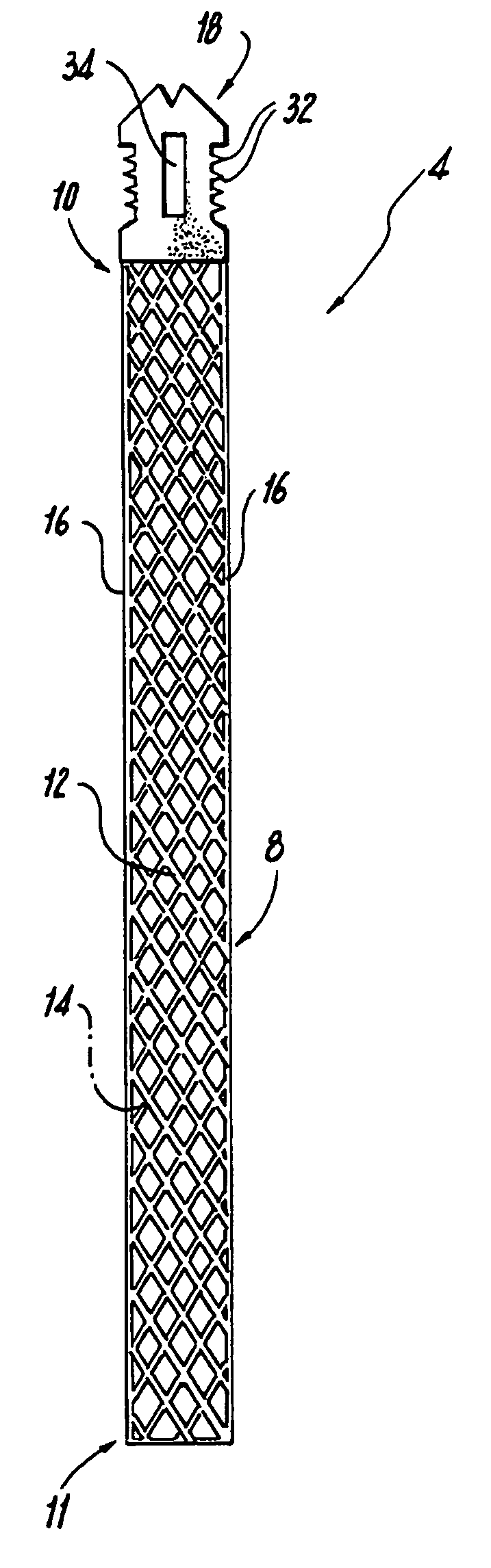
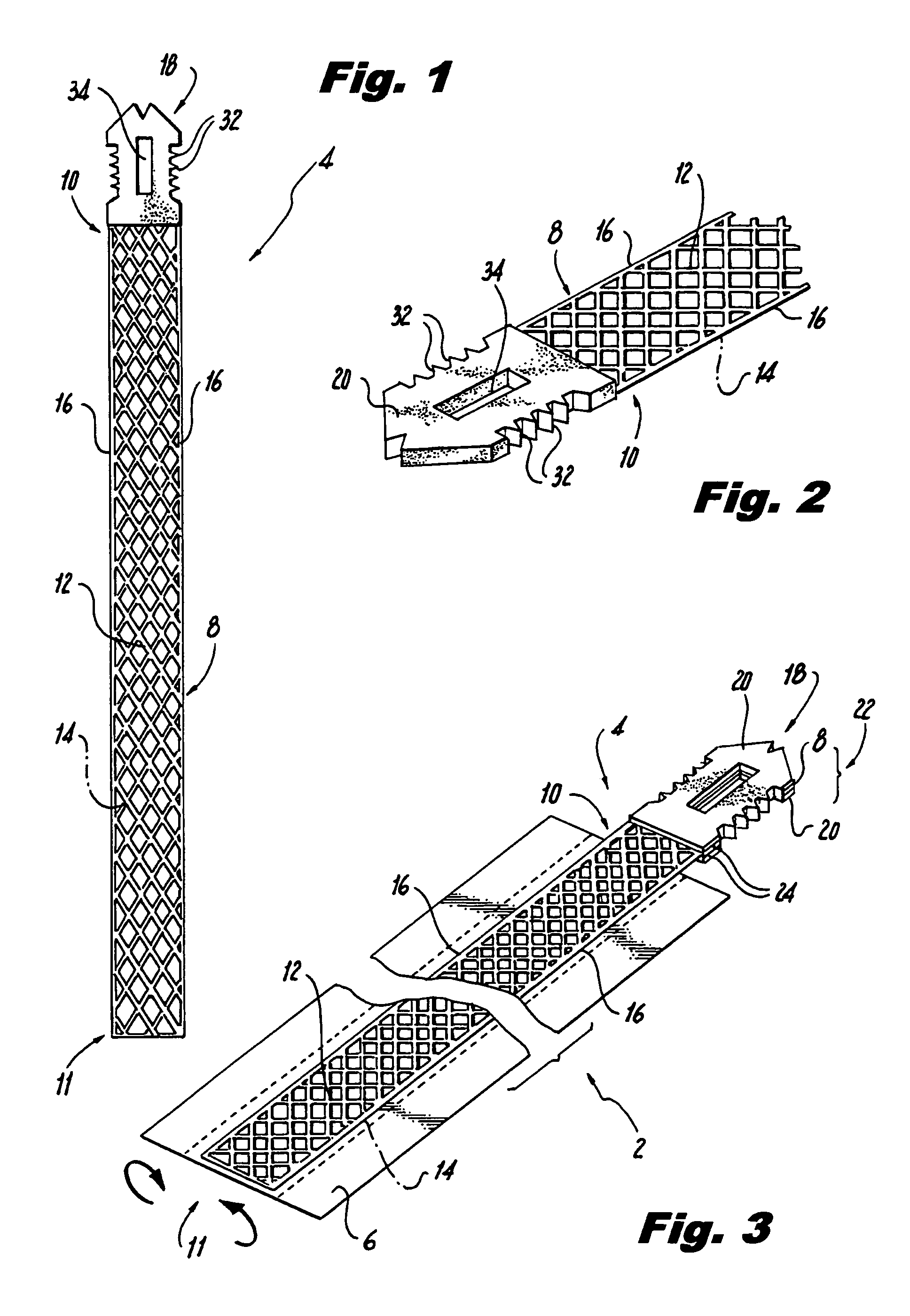
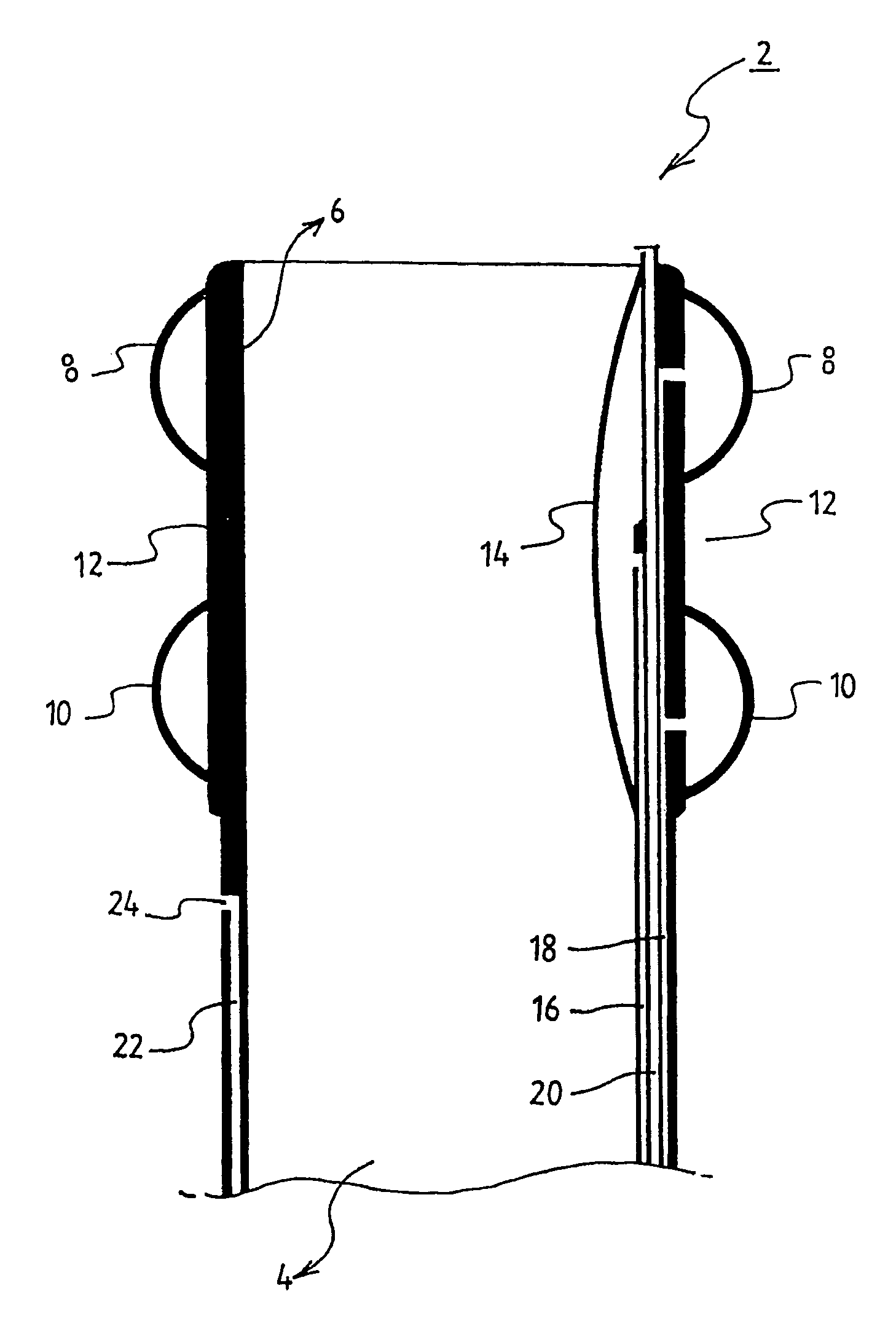
ActiveUS8721666B2Good cosmetic effectSimplify facial reconstructive surgeriesSuture equipmentsCosmetic implantsEyelidCheek
A self-anchoring tissue lifting device for use with facial cosmetic reconstructive surgery includes an implant and a removable foil cover disposed on the implant. The implant includes an elongated mesh strip having a distal end on which is situated a tissue anchoring fleece material. Opposite lateral edges of the mesh material are preferably laser cut during the manufacturing process of the implant to provide a plurality of tissue engaging prickles along the longitudinal length of the implant. For treating the mid face and jowl, a stab incision is made within the hairline of the temple region of the patient and the device is applied from the temporal area to the peak of the ipsilateral cheek to capture the malar fat pad to correct midface abnormalities or the ptotic tissue causing the jowl.
Owner:ETHICON INC
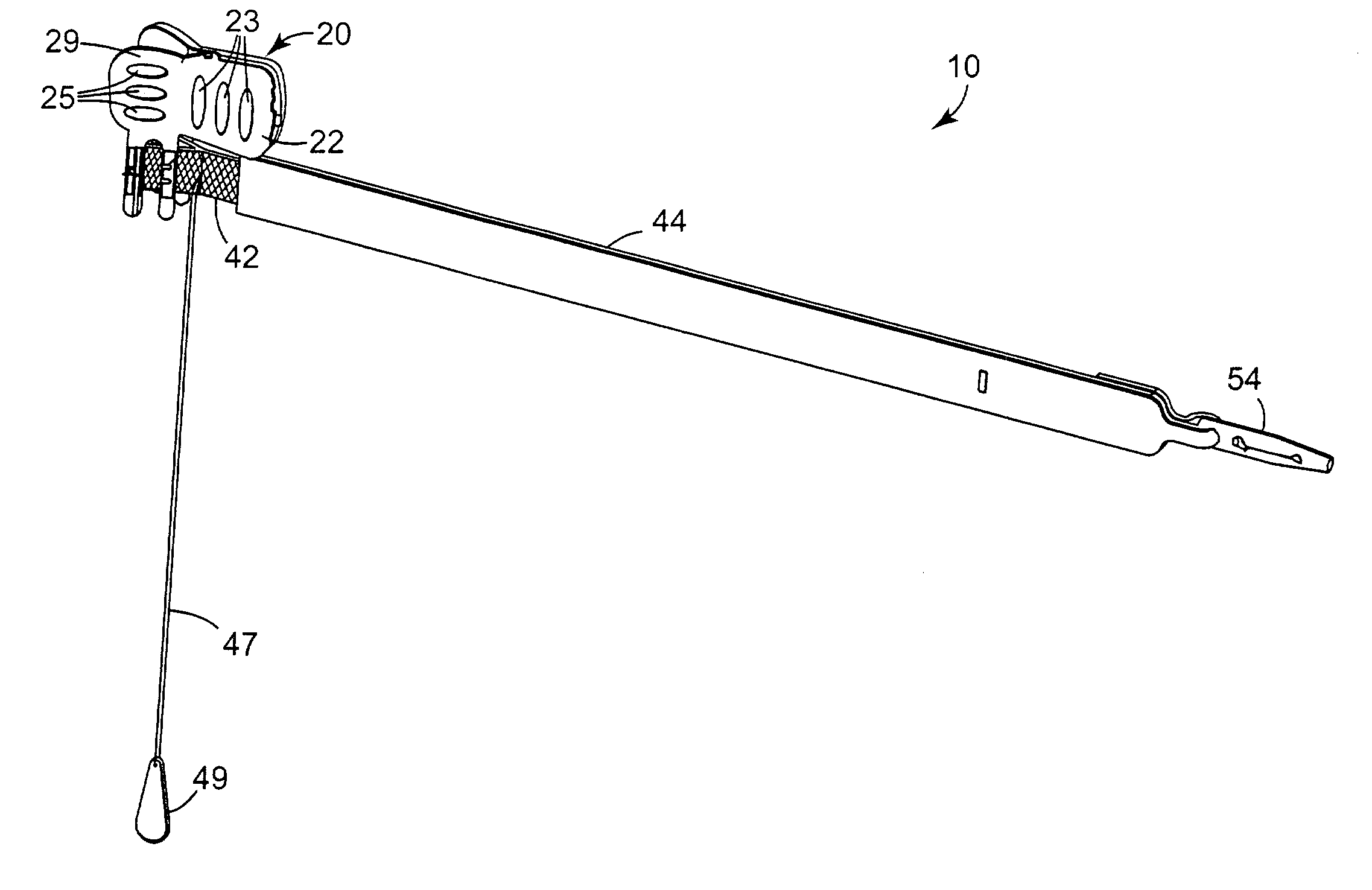
Indwelling fecal diverting device
ActiveUS8398669B2Improve securityEasy to installBalloon catheterAnti-incontinence devicesFecesSurgery
Disclosed is an indwelling fecal diverting device. The device comprises an elongate tube formed, at an upper end thereof, with a tubular body part; a pair of fixing balloons attached up and down to an outer surface of the tubular body part such that a clamping portion is defined between the fixing balloons; and a tube opening and closing balloon attached to an inner surface of the tubular body part. An injection passage is defined in the tube so that a remedial liquid can be injected through the injection passage to the outside of the tube to medically treat an anastomosed portion of an intestinal tract of a patient. The indwelling fecal diverting device is fitted into the intestinal tract of the patient, air is supplied into the fixing balloons to inflate them, and the intestinal tract is clamped around the clamping portion using a clamping band.
Owner:YUSHIN MEDICAL +1
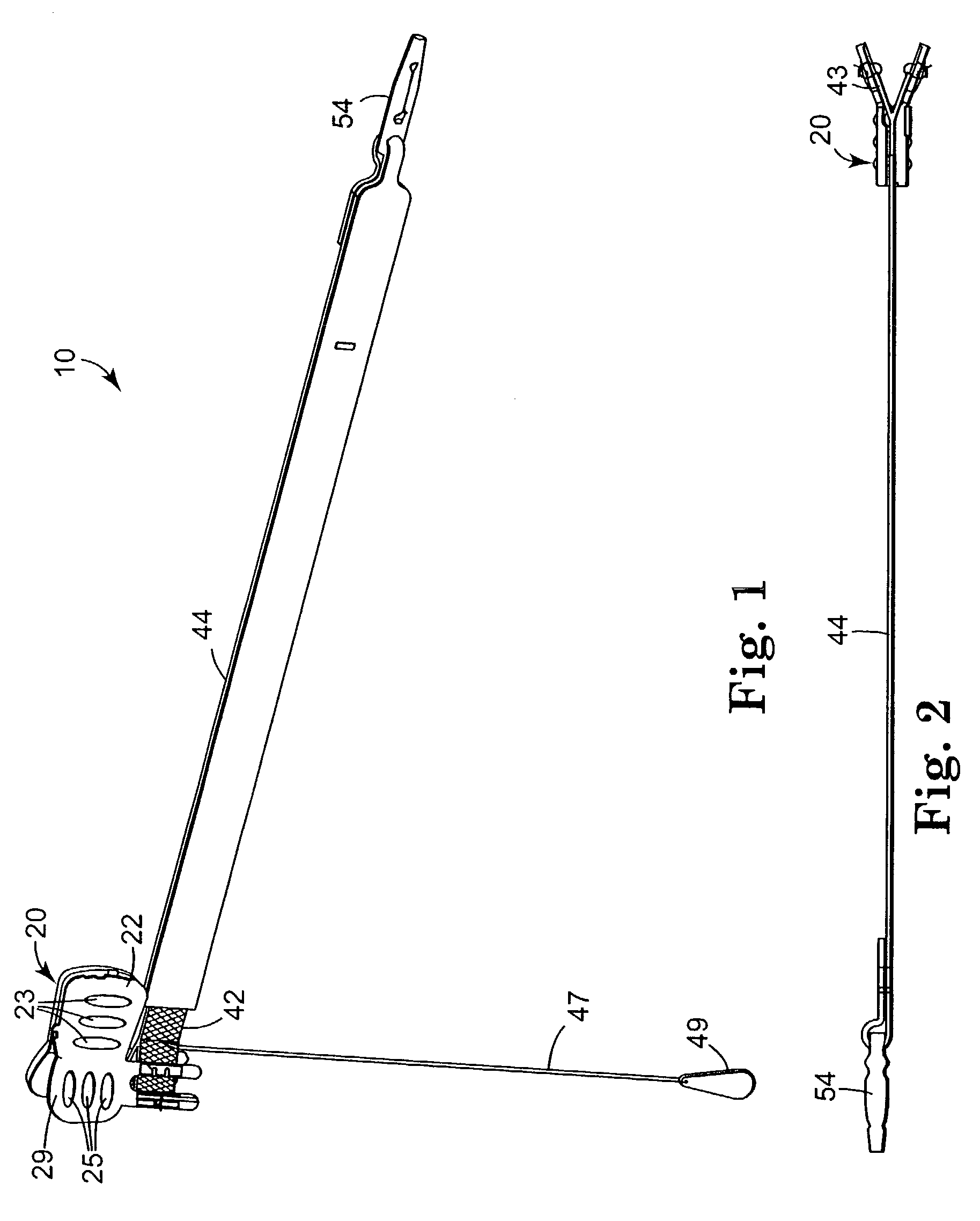
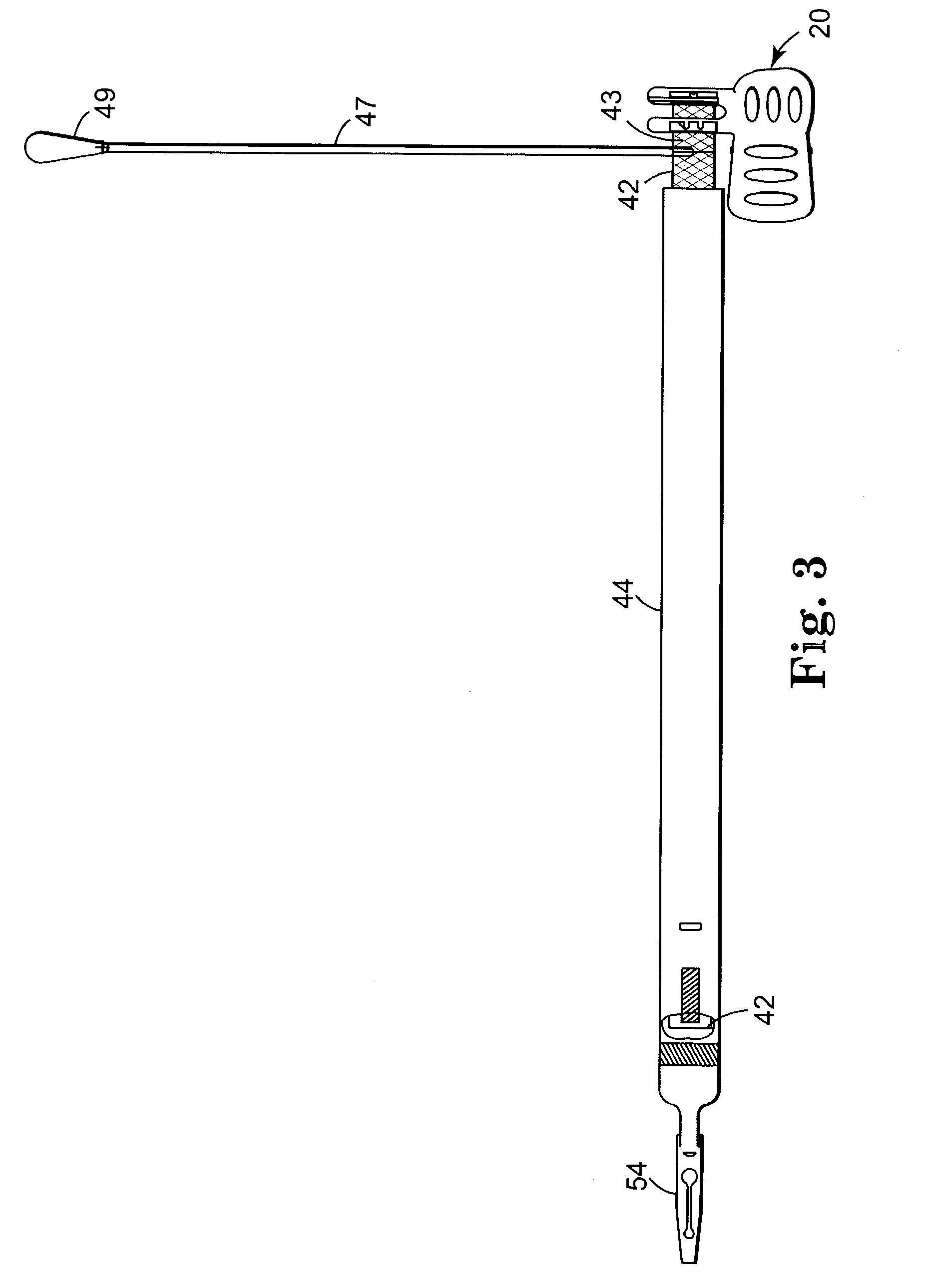
Implantable article and method for treating urinary incontinence using means for repositioning the implantable article
InactiveUS6652450B2Adversely therapeutic effectPrevent permanent deformationSuture equipmentsIncision instrumentsDiseaseUrological Disorders
An implantable article and method of use are disclosed to treat urological disorders. The biocompatible device includes a sling assembly configured to be minimally invasive and provide sufficient support to the target site. In addition, the configuration of the sling assembly also allows the position of the sling to be permanently changed during and / or after implantation.
Owner:STASKIN DAVID MD DR
Transobturator surgical articles and methods
Owner:ASTORA WOMENS HEALTH
Implantable article and method
InactiveUS20020028980A1Good treatment effectEncourage tissue ingrowthSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
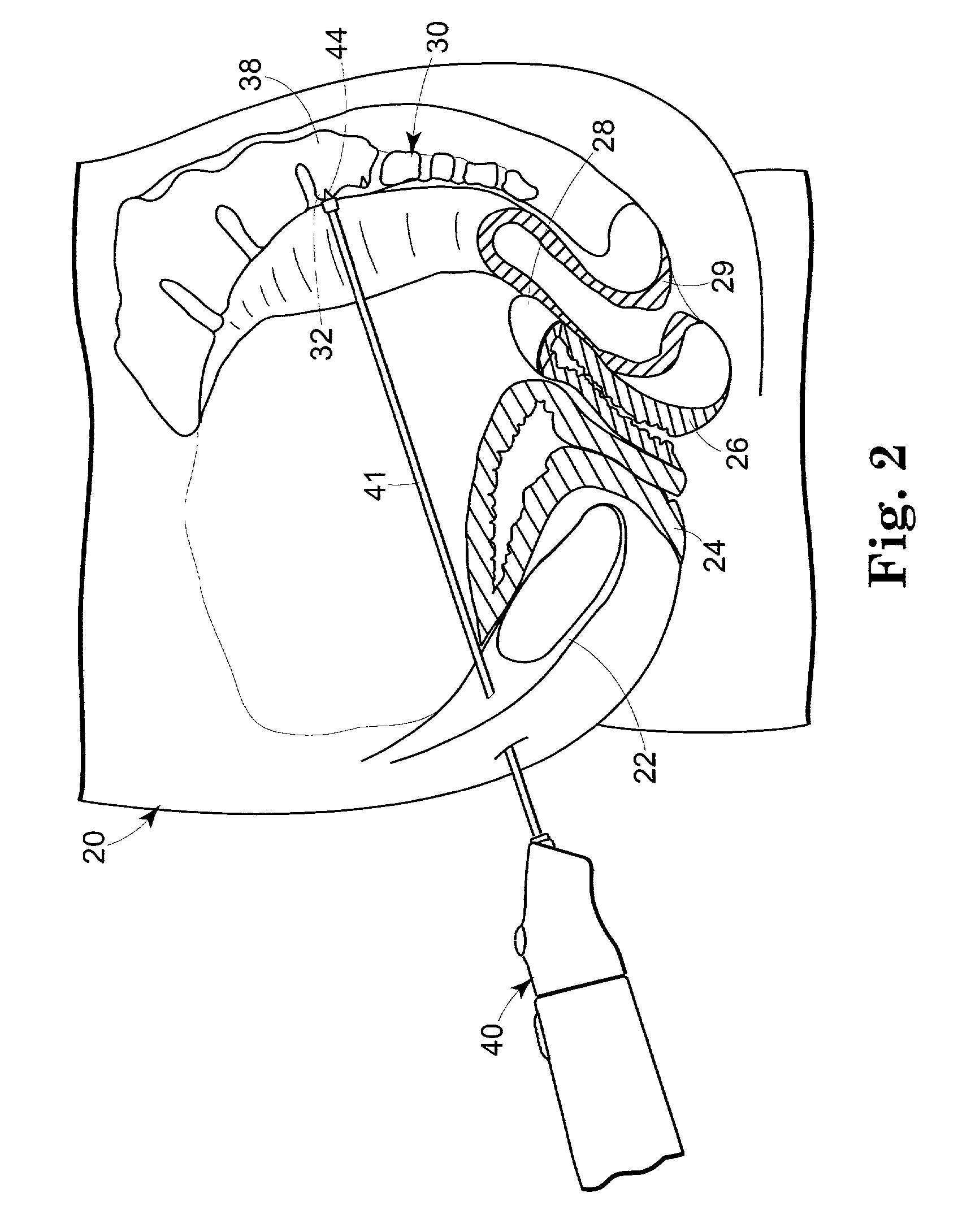
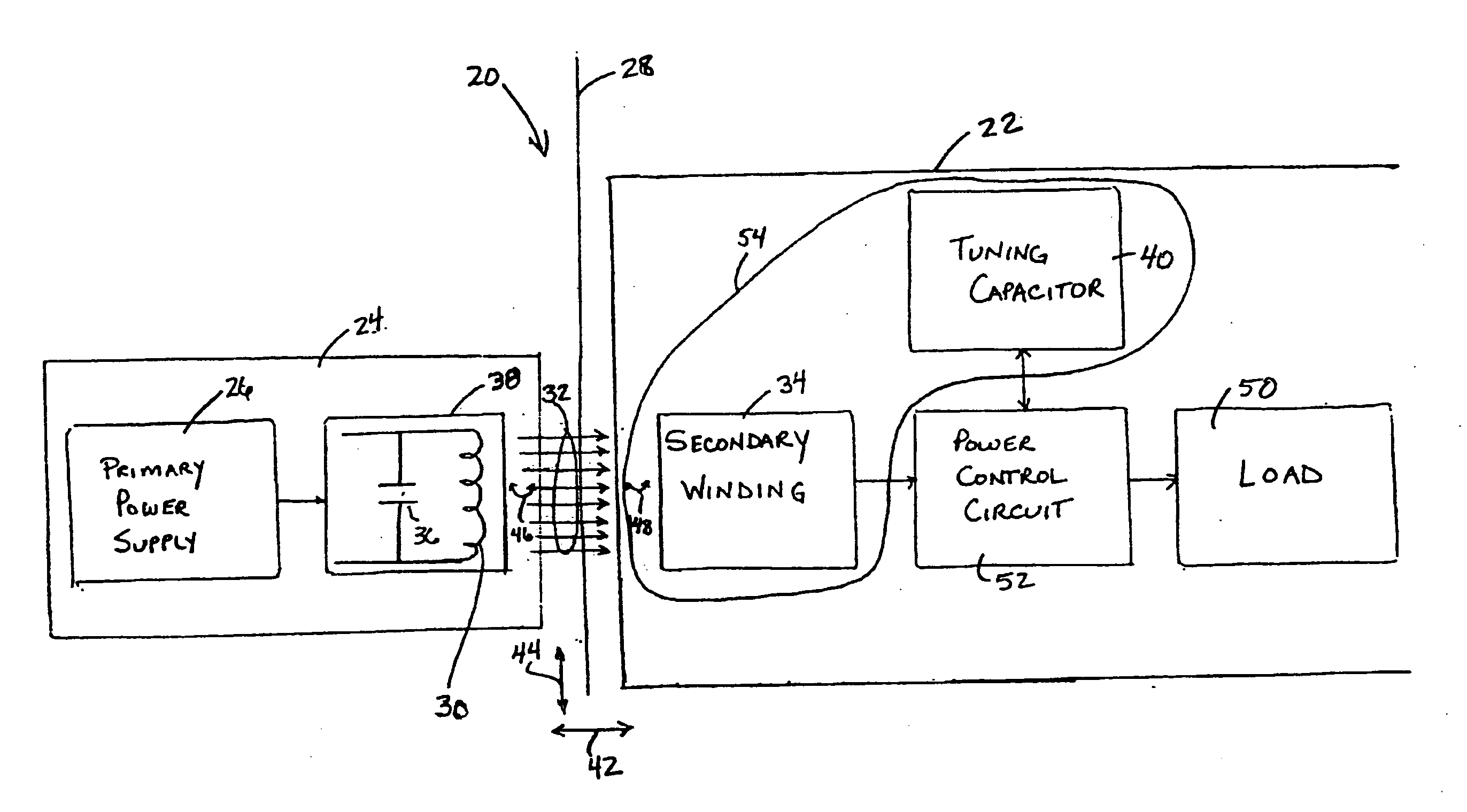
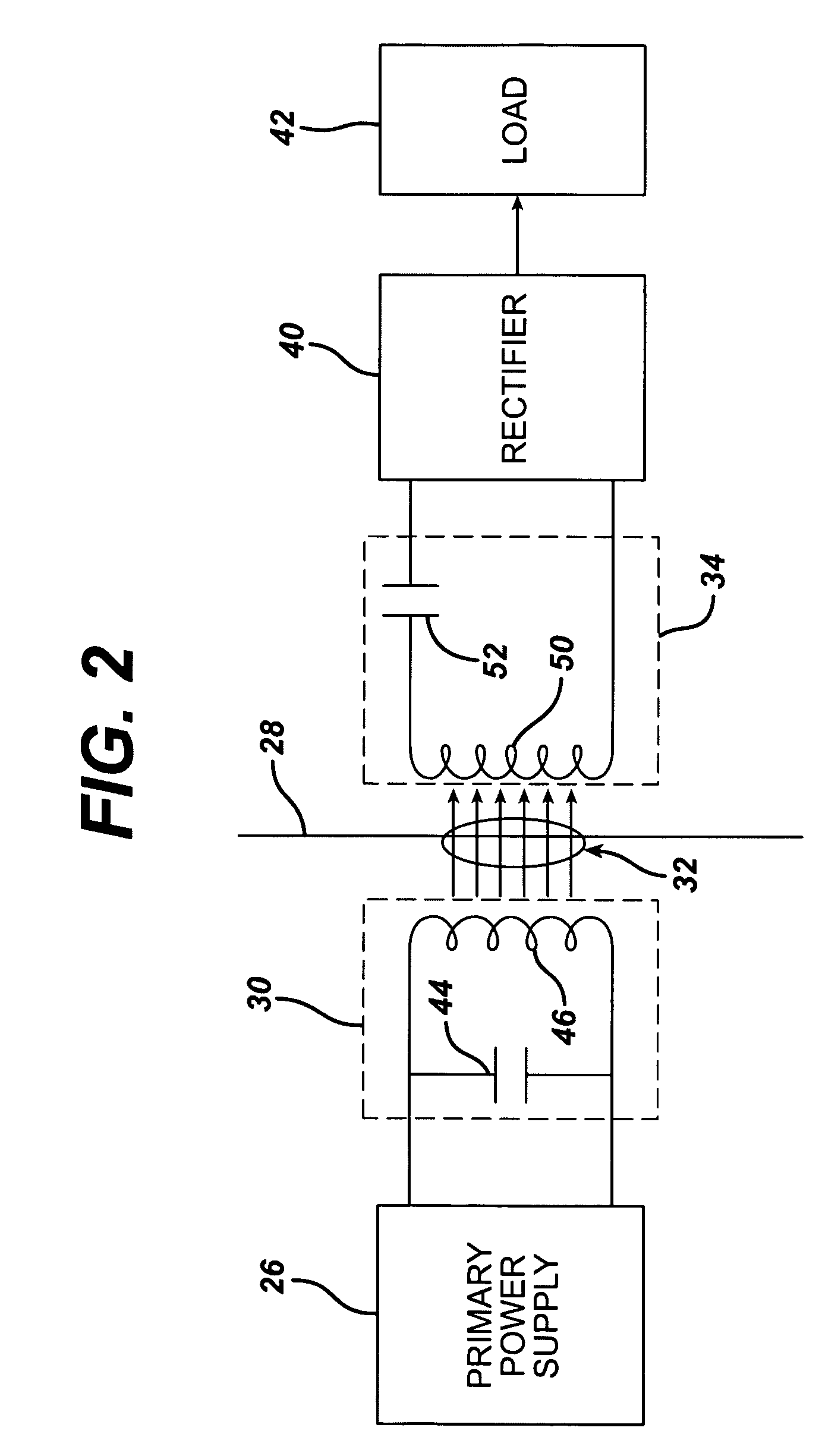
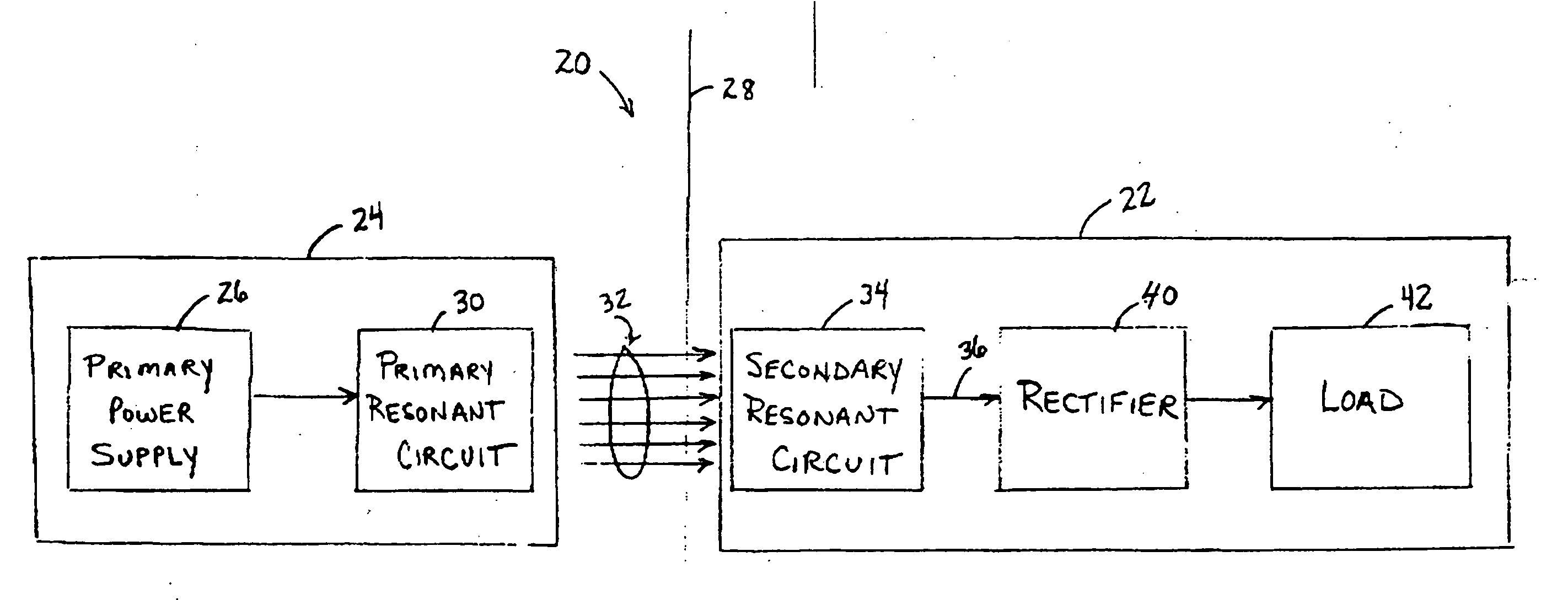
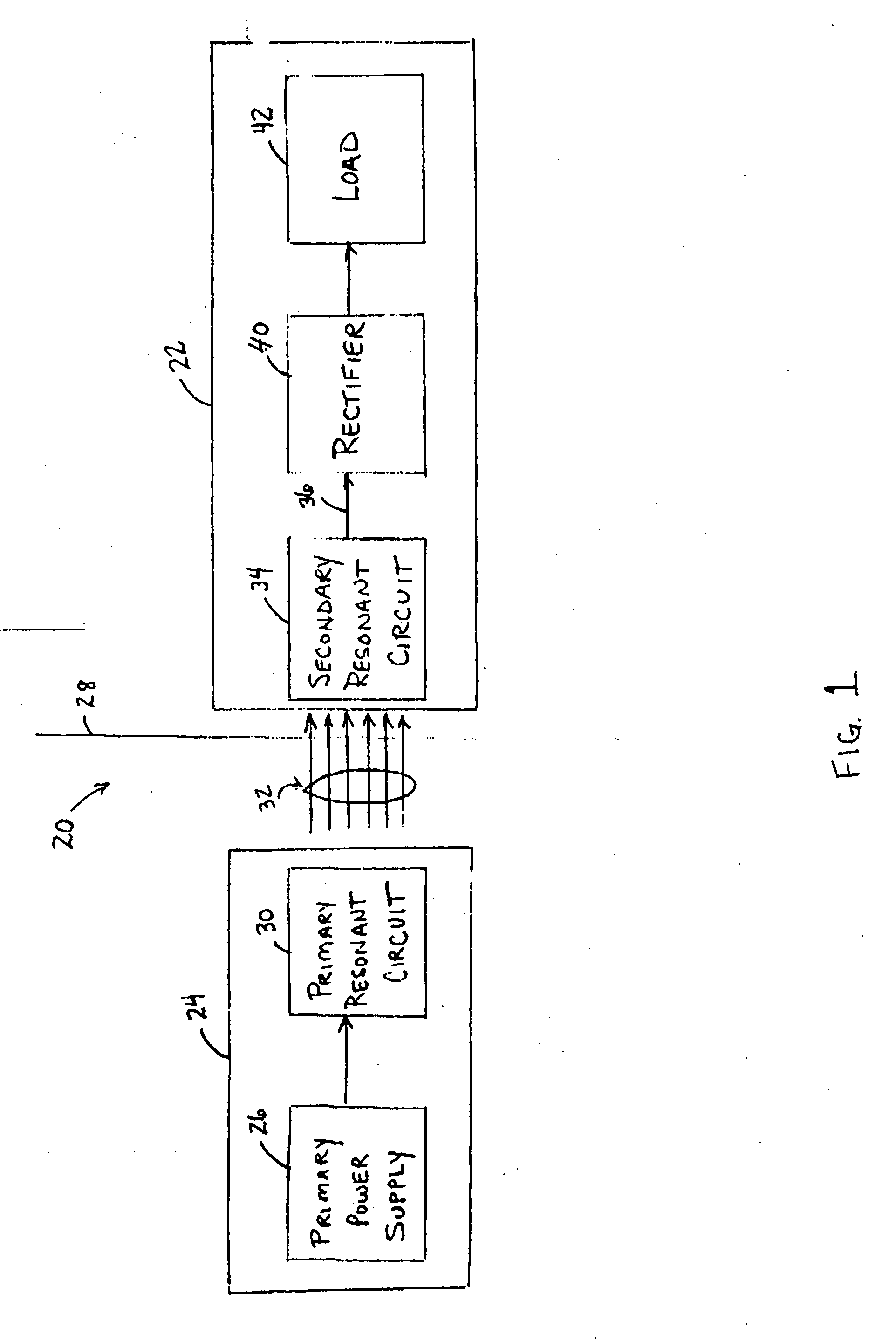
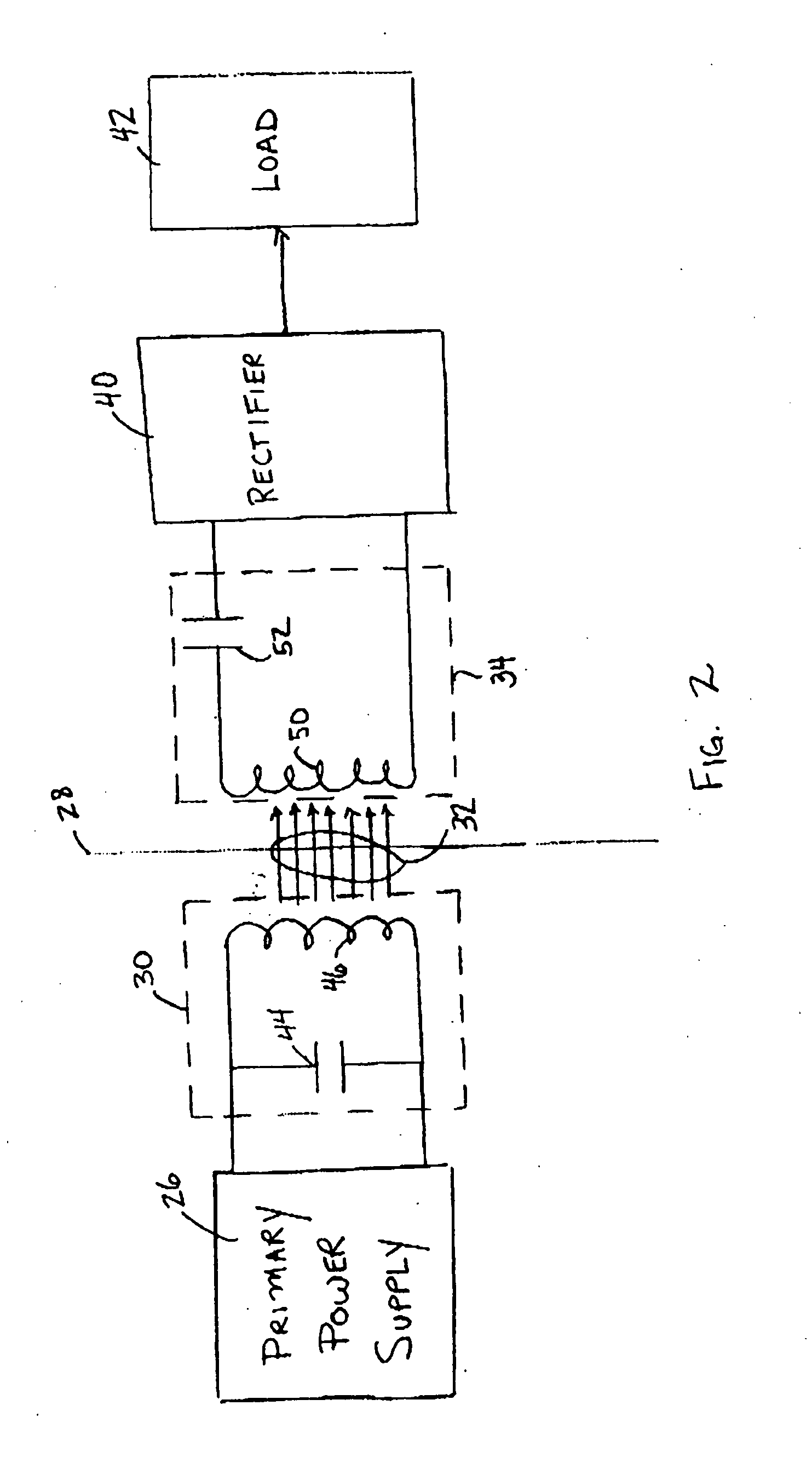
Medical implant having closed loop transcutaneous energy transfer (TET) power transfer regulation circuitry
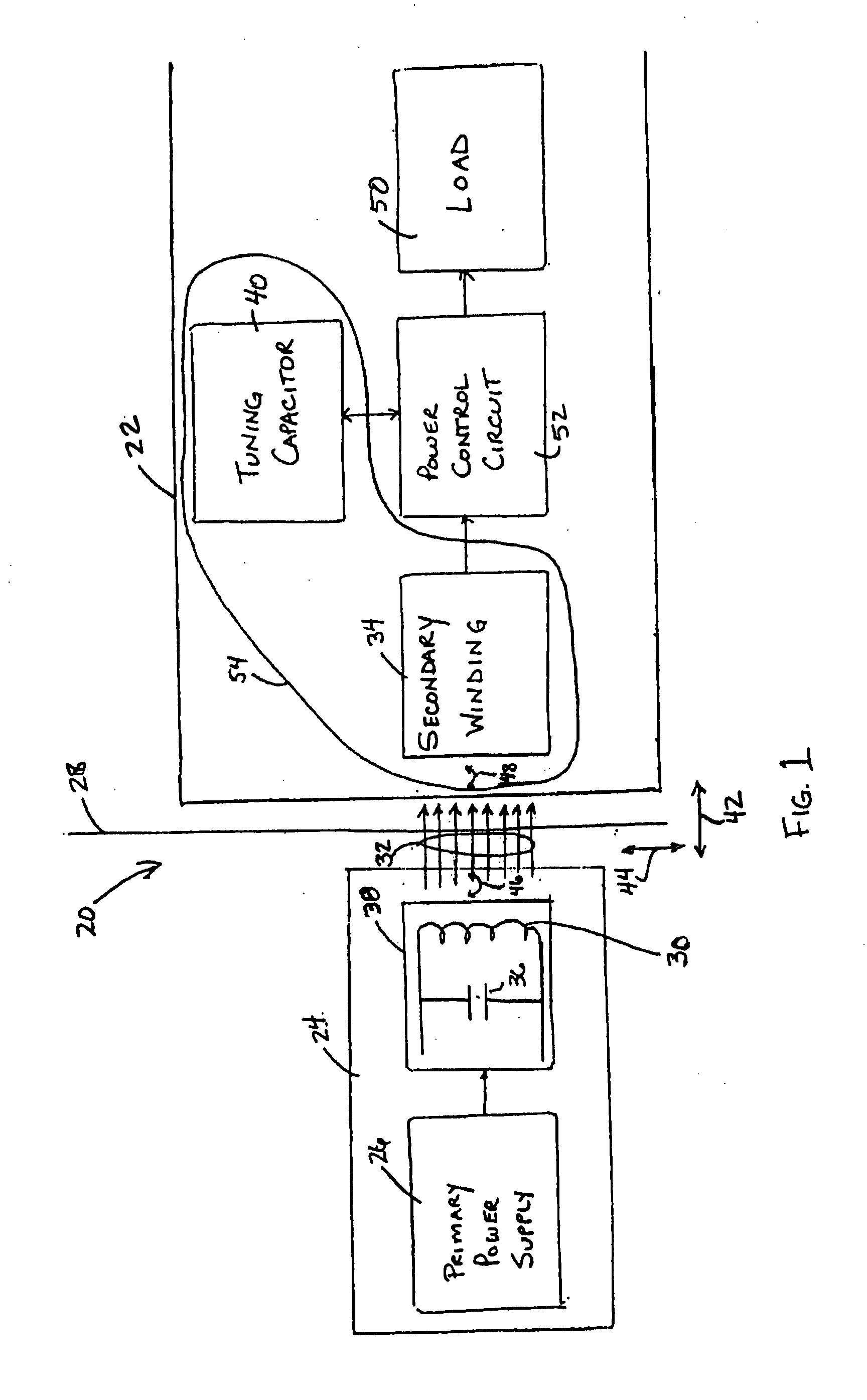
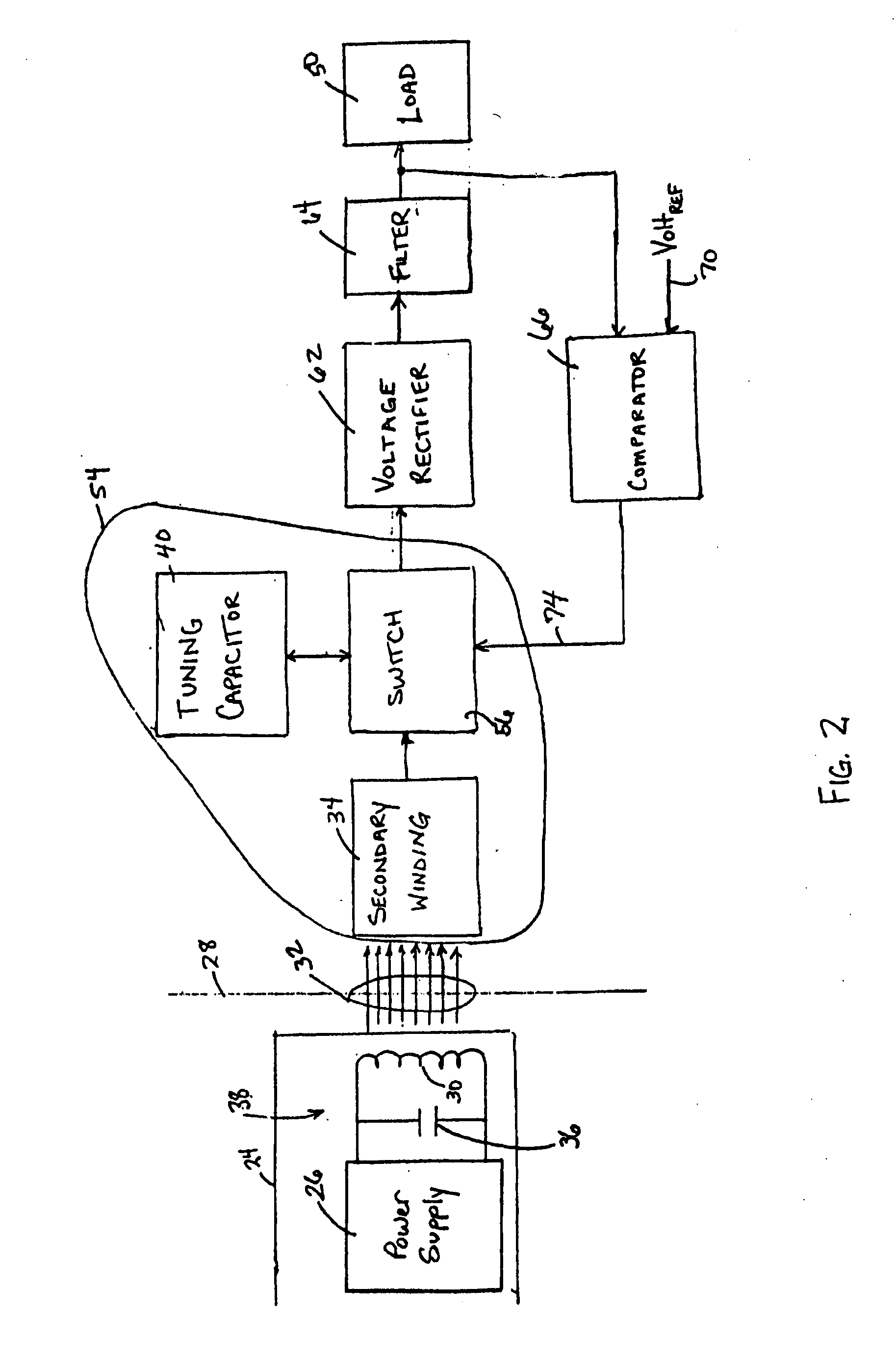
InactiveUS20050288739A1Not easy to damageLess susceptible to inoperabilityElectrotherapyAnti-incontinence devicesElectrical batteryClosed loop
An implantable medical device, such as a bi-directional infuser device for hydraulically controlling an artificial sphincter (e.g., adjustable gastric band) benefits from being remotely powered by transcutaneous energy transfer (TET), obviating the need for batteries. In order for active components in the medical device to operate, a sinusoidal power signal received by a secondary coil is rectified and filtered. An amount of power transferred is modulated. In one version, a voltage comparison is made of a resulting power supply voltage as referenced to a threshold to control pulse width modulation (PWM) of the received sinusoidal power signal, achieving voltage regulation. Versions incorporate detuning or uncoupling of the secondary coil to achieve PWM control without causing excessive heating of the medical device.
Owner:ETHICON ENDO SURGERY INC
Low frequency transcutaneous energy transfer to implanted medical device
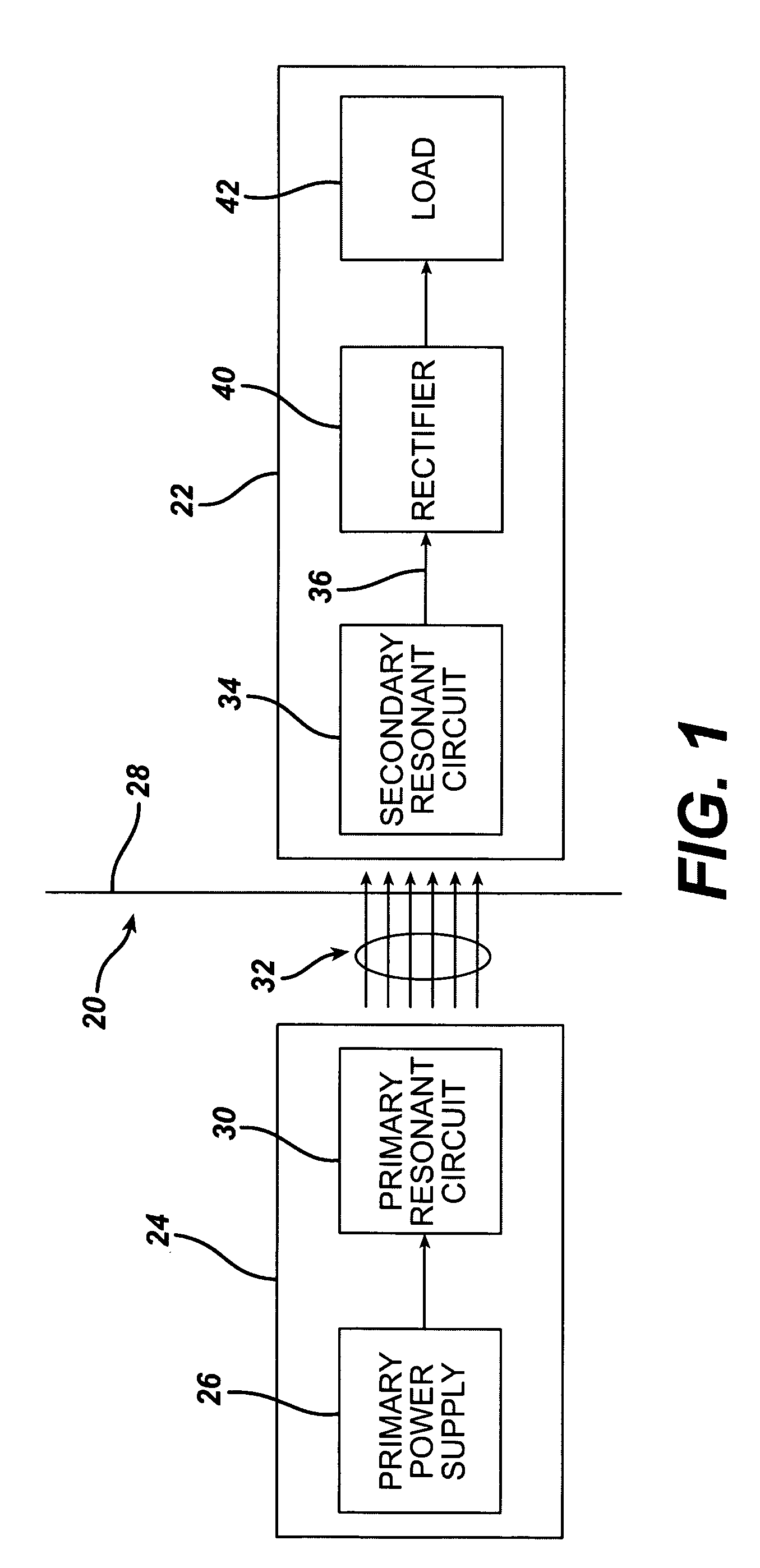
InactiveUS7599743B2Efficiently penetrate physical boundaryMore powerElectrotherapyAnti-incontinence devicesEnergy transferComputer module
An implantable medical device system advantageously utilizes low frequency (e.g., about 1-100 kHz) transcutaneous energy transfer (TET) for supplying power from an external control module to an implantable medical device, avoiding power dissipation through eddy currents in a metallic case of an implant and / or in human tissue, thereby enabling smaller implants using a metallic case such as titanium and / or allowing TET signals of greater strength thereby allowing placement more deeply within a patient without excessive power transfer inefficiencies.
Owner:ETHICON ENDO SURGERY INC
Method and apparatus for correction for gynecological pathologies including treatment of female cystocele
Owner:BOSTON SCI SCIMED INC
Coated sling material
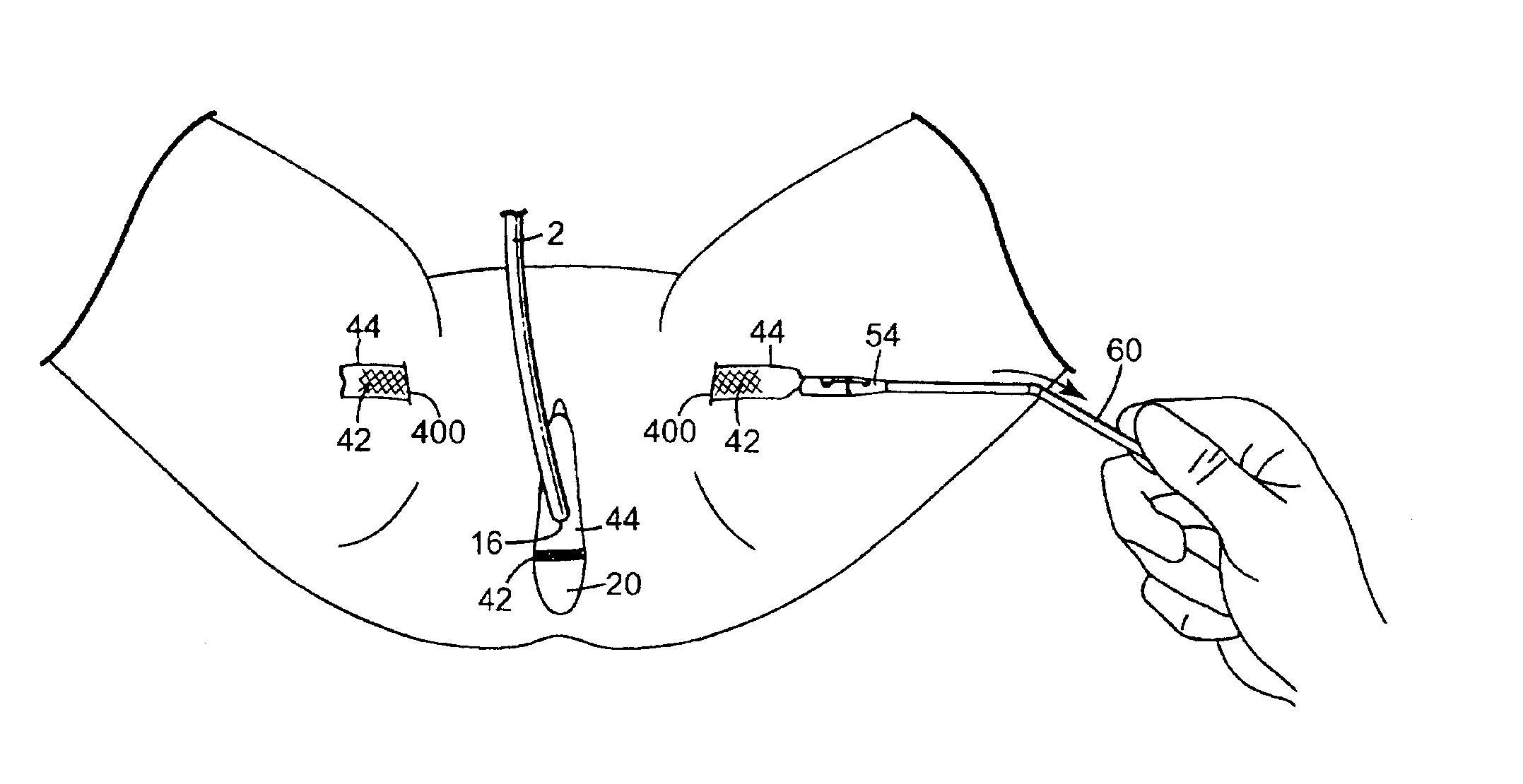
InactiveUS7025063B2Minimize complexityGood biocompatibilitySuture equipmentsAnti-incontinence devicesUrethraShort urethra
The present invention relates to a sling, methods of making and using a sling, and kits comprising a sling for treating urinary incontinence. The sling has multiple elongation properties that serve to improve the support of the urethra. The sling may comprise a coated material adapted for urethral suspension. The coated sling has properties that appear to enhance the sling elongation characteristics. The coated sling further includes properties that reduce its susceptibility to bacterial infections. The sling further includes properties to enhance the proper tensioning of the sling.
Owner:BOSTON SCI SCIMED INC
Mesh for pelvic floor repair
InactiveUS7087065B2Reduce vaginal prolapseIncrease heightSuture equipmentsAnti-incontinence devicesPelvisPelvic floor repair
A woven mesh is provided for supporting tissue or an organ within a female patient's pelvis, and a method for using the same. The mesh includes a central portion having a width, a length, first and second ends, and first and second side edges, and first and second wing portions extending from the first and second ends of the central portion respectively. The first and second wing portions each have a width, a length, and first and second peripheral edges. The width of the wing portions are each greater than the width of the central portion, and when positioned within the female patient, the central portion is positioned below and supports the tissue or organ. A mesh is also provided having woven fibers with voids therebetween, and having a central portion and first and second ends. The average size of the voids at least in the central portion is at least 25–50 mm2, and when positioned within the patient, the central portion is positioned below and supports the tissue or organ.
Owner:ETHICON INC
Devices and methods for selective surgical removal of tissue
ActiveUS7738969B2Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalVascular structure
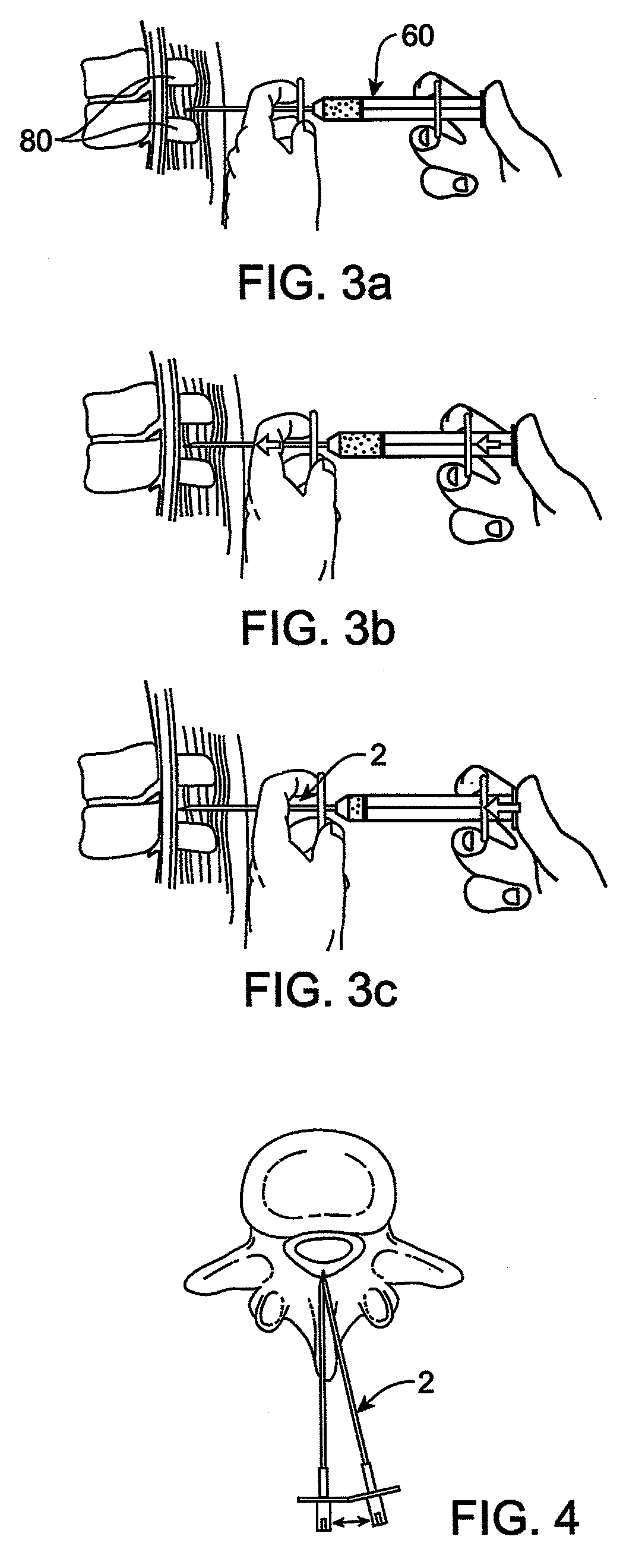
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
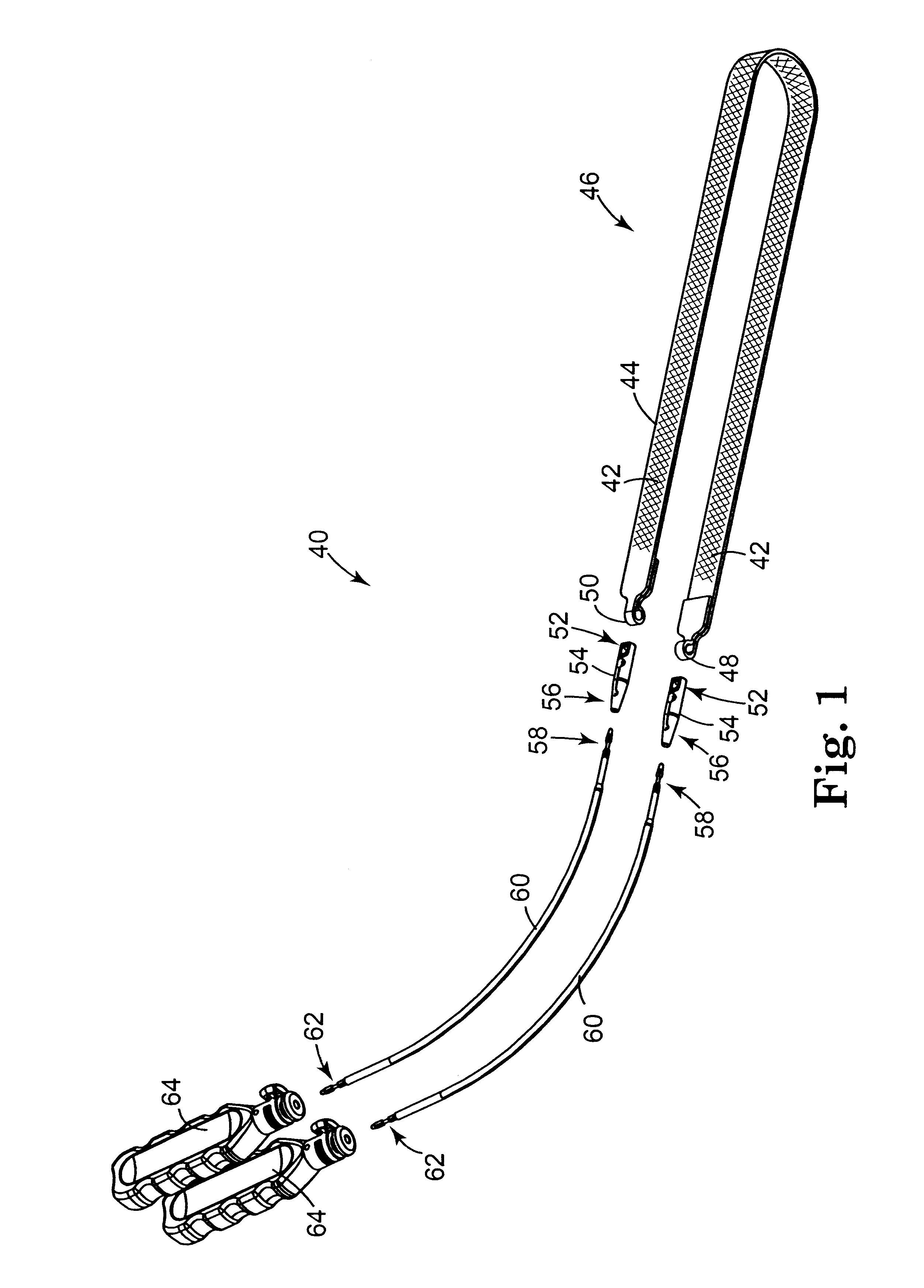
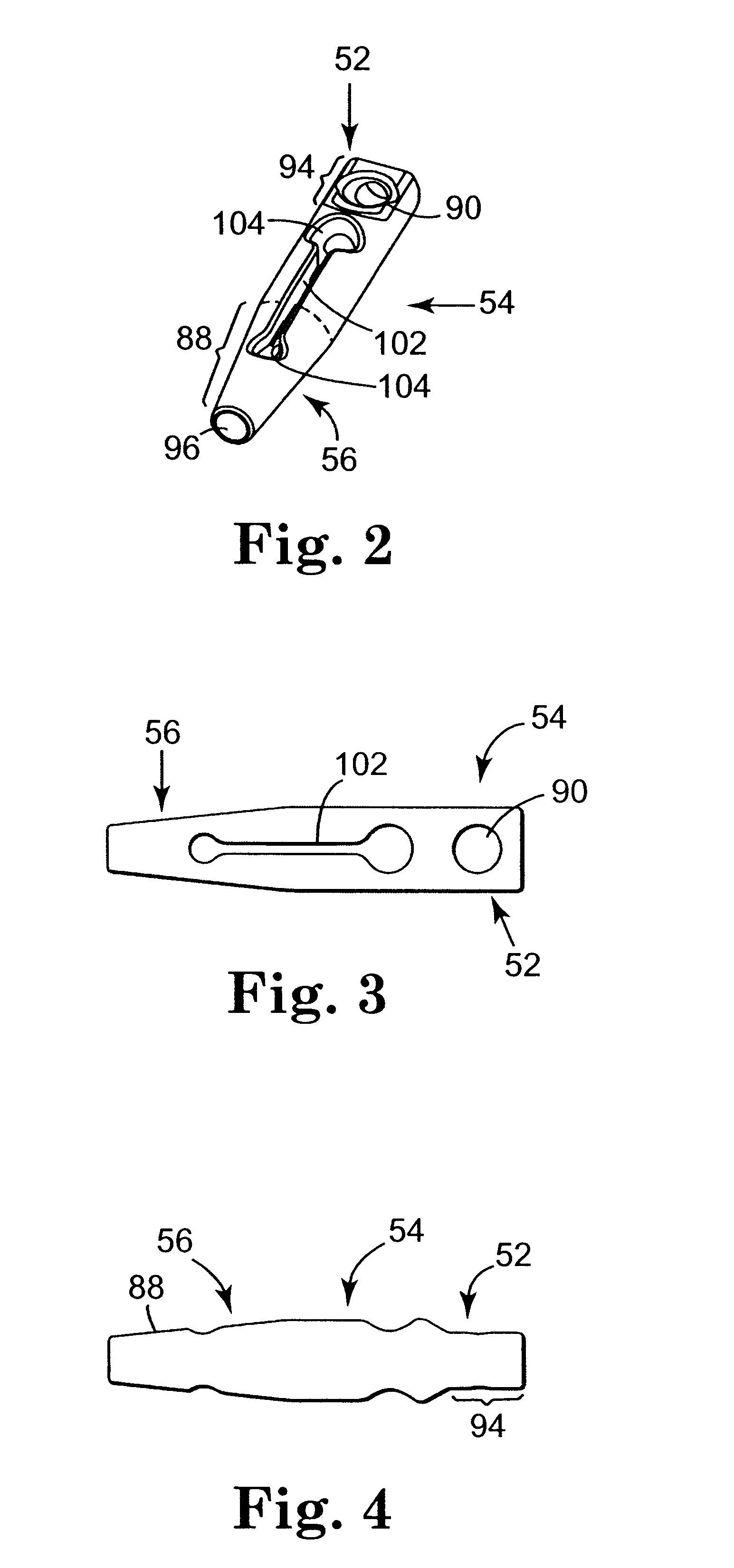
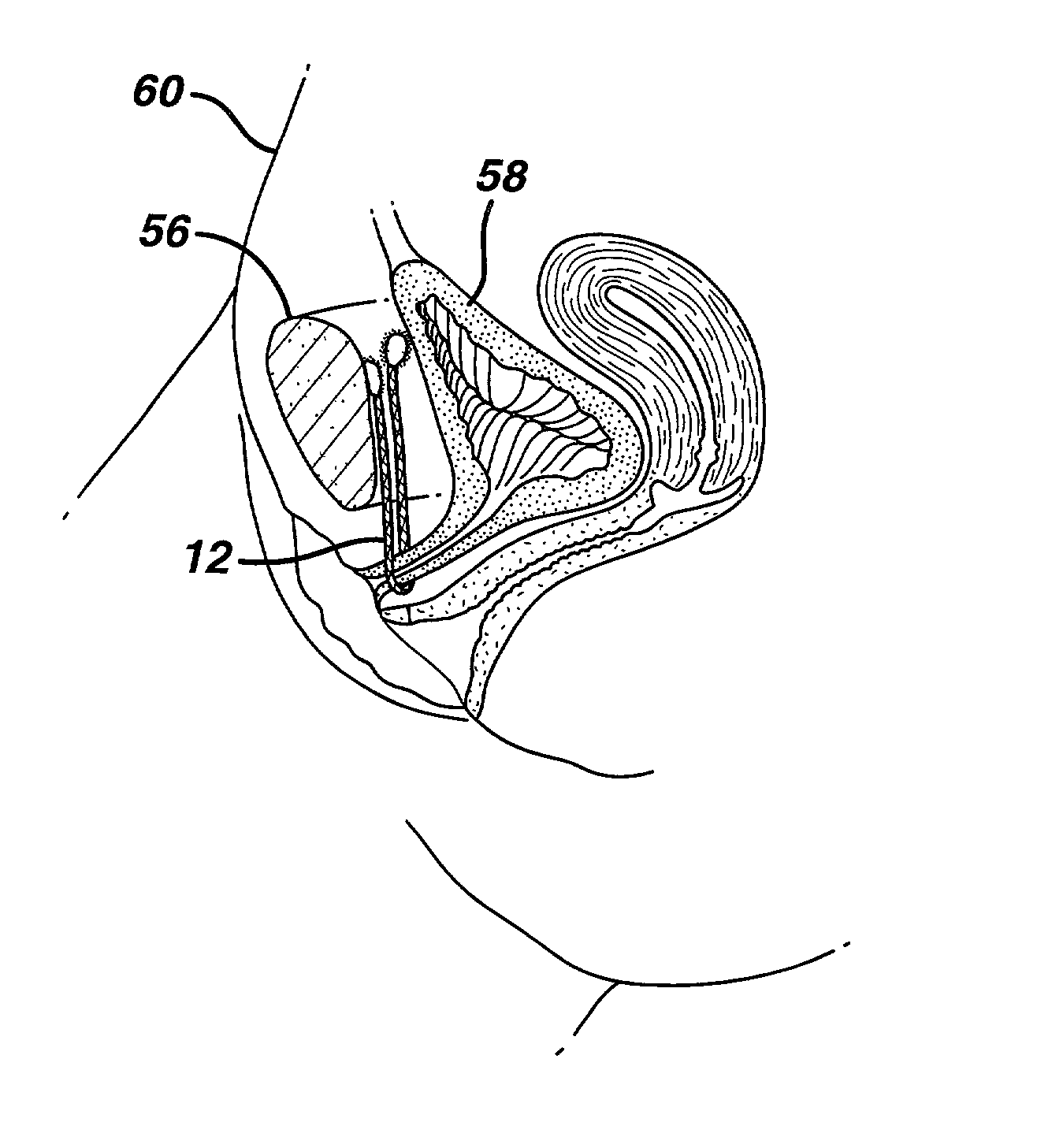
Method and apparatus for treating pelvic organ prolapse
ActiveUS20050245787A1Convenient for operatorsEasy to operateSuture equipmentsAnti-incontinence devicesFirst pathwayIschial spine
A method of treating pelvic organ prolapse is provided. The method generally includes the steps of establishing a first pathway between the external perirectal region of the patient to the region of the ischial spine in tissue on one side of the prolapsed organ, followed by establishing a second pathway in tissue on the contralateral side of the prolapsed organ. A support member, which includes a central support portion and two end portions, is positioned in a position to reposition said prolapsed organ in said organ's anatomically correct location. The end portions of the support member are introduced through the respective tissue pathways, followed by adjustment of the end portions so that the support member is located in a therapeutic relationship to the prolapsed organ that is to be supported. An apparatus and kit for said treatment is further provided.
Owner:ASTORA WOMENS HEALTH
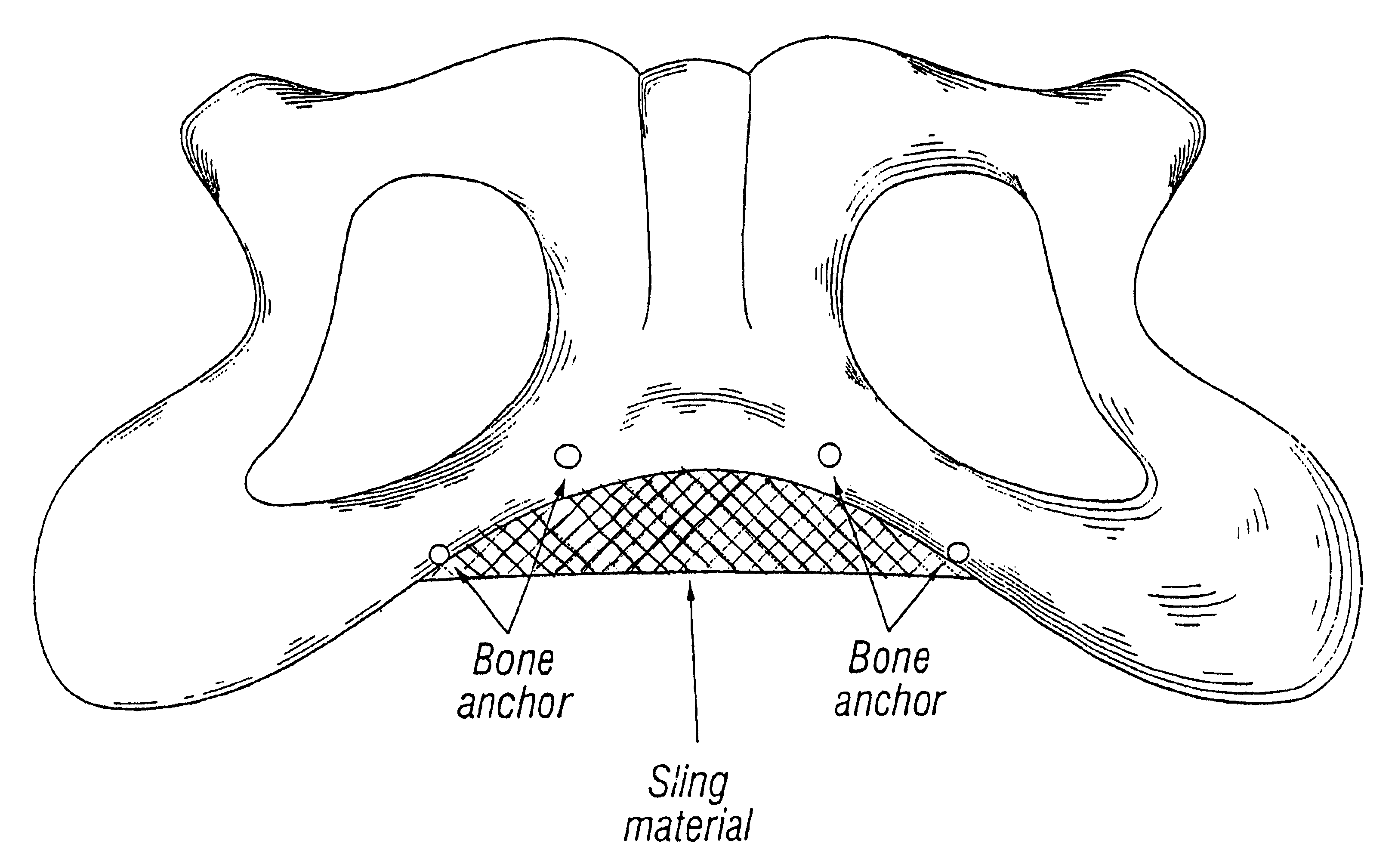
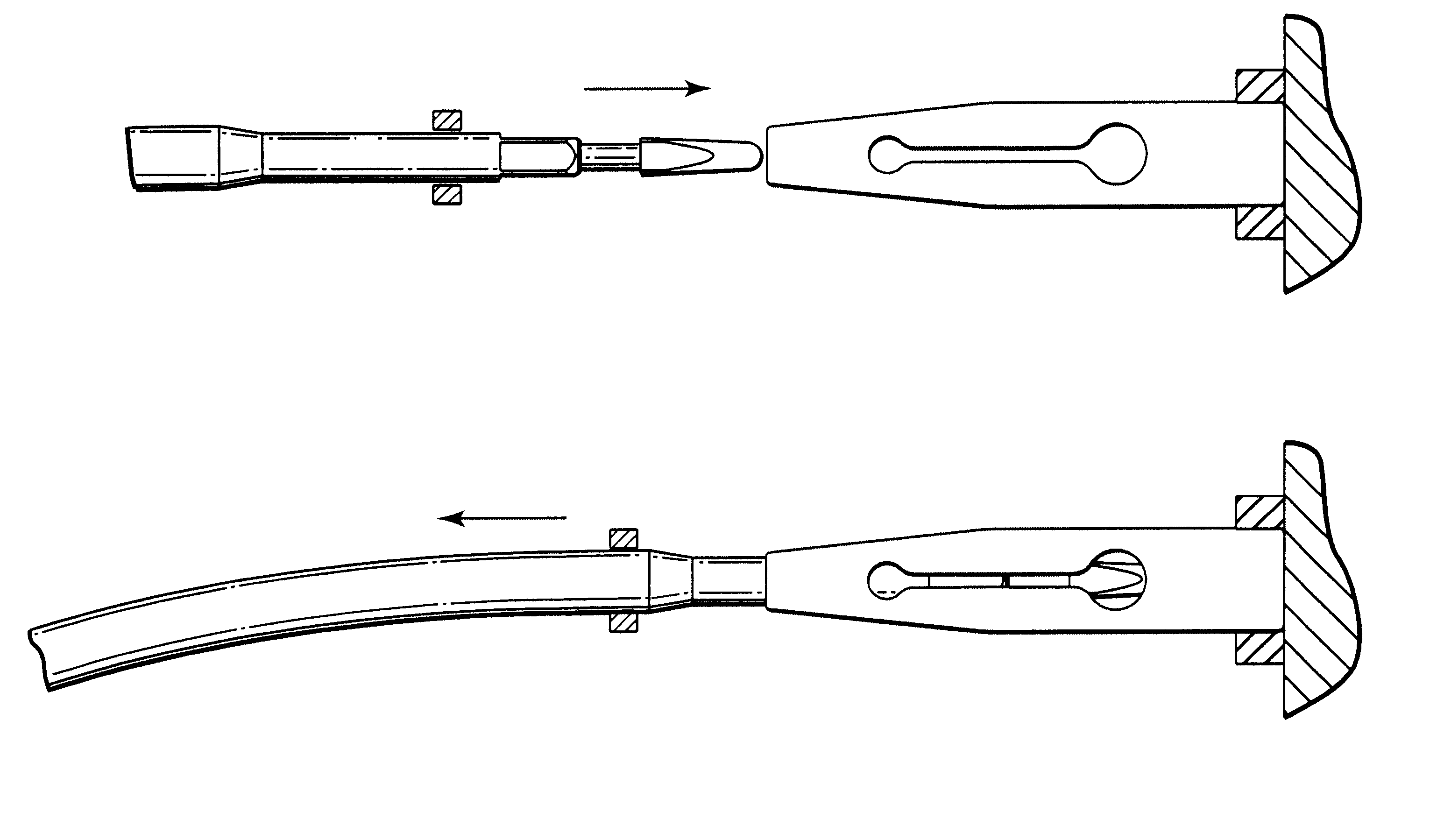
System for securing sutures, grafts and soft tissue to bone and periosteum
InactiveUS20050004576A1Easy constructionEasy to useSuture equipmentsAnti-incontinence devicesEngineeringPeriosteum
Self-anchoring slings and deployment mechanisms for use therewith in selectively positioning a sling into position within the body. According to a preferred embodiment, the sling comprises an elongate sling portion having opposed ends. Formed upon each respective opposed end is an anchor member operative to be percutaneously advanced through soft tissue at a selected target site in a first direction but resist movement in an opposed direction. Such anchor members are operative to extend in opposed directions to thus enable a sling to be securely affixed into position and resist sag or otherwise lose its ability to support a given structure.
Owner:SPRINGBOARD MEDICAL VENTURES
Implantable article and method
InactiveUS6592515B2Alleviate challengeGood treatment effectSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
Mechanical and electrical sensing for incontinence treatment
InactiveUS6896651B2Inhibit involuntary urine flowFunction increaseElectrotherapyAnti-incontinence devicesUrethraStress incontinence
A device and method for treatment of urinary stress incontinence. At least one electrode is implanted in a pelvic muscle of a patient. A control unit receives signals indicative of abdominal stress in the patient and responsive thereto applies an electrical waveform to the electrode which stimulates the muscle to contract, so as to inhibit involuntary urine flow through the patient's urethra due to the stress.
Owner:ASTORA WOMENS HEALTH +1
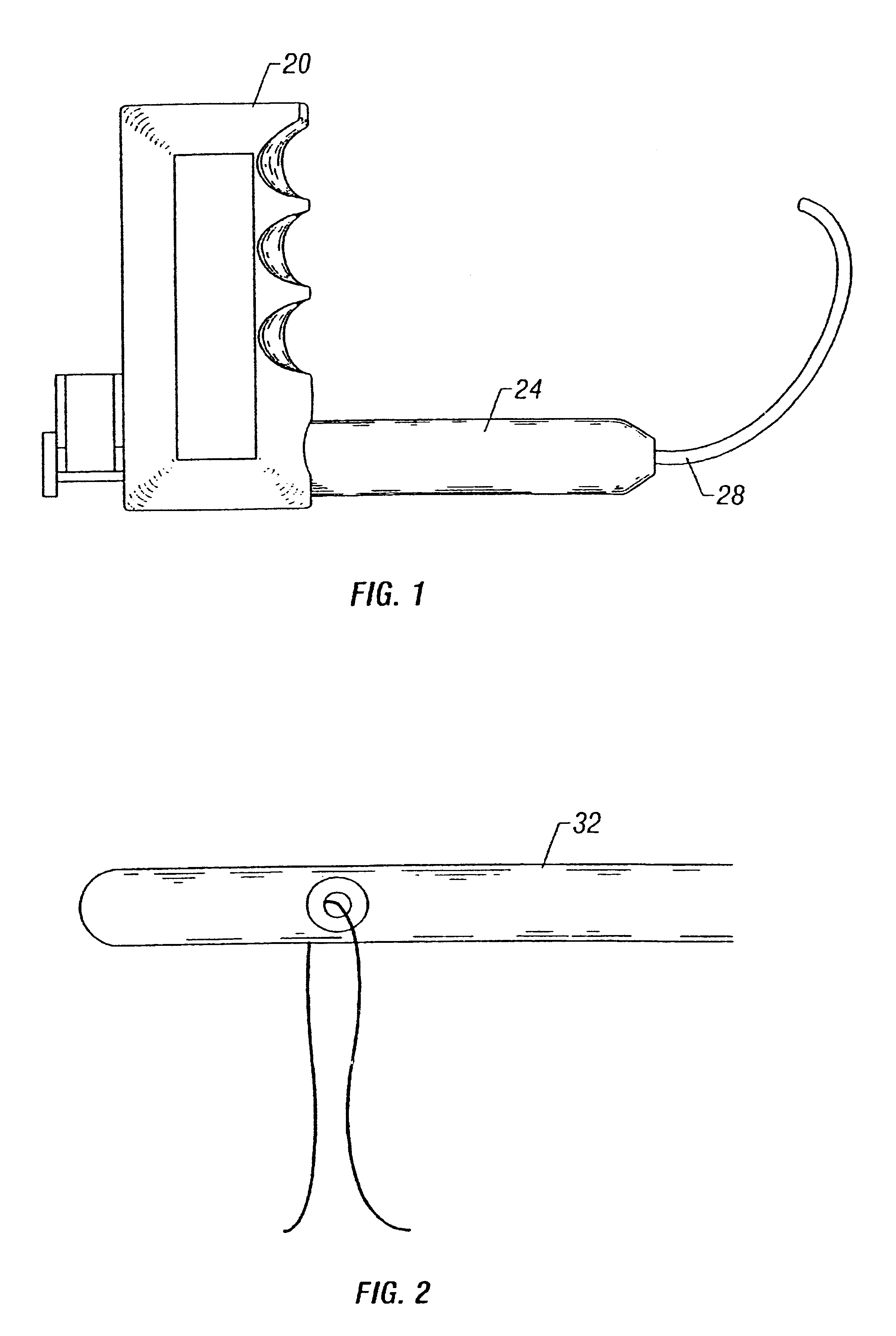
Bone anchor-insertion tool and surgical method employing same
InactiveUS6042583APrevent saggingSufficient pressureSuture equipmentsDiagnosticsEngineeringSurgical template
An anchor-insertion tool for securing an anchor having a suture extending therefrom to a structure within a patient's body is provided. The anchor-insertion tool comprises an elongate shaft having distal and proximal ends, an anchor-receiving tip at the distal end, at least one groove extending from the tip at least partially along the length of the shaft, and at least one depression extending about the circumference of the shaft and intersecting the groove. A loaded anchor-insertion tool is provided, along with a surgical method for employing the anchor-insertion tool. A surgical template and suture retriever are also provided.
Owner:R J & J A FAMILY LLP +1
Low frequency transcutaneous energy transfer to implanted medical device
InactiveUS20050288741A1Efficiently penetrate physical boundaryWithout excessive power lossElectrotherapyAnti-incontinence devicesEnergy transferComputer module
An implantable medical device system advantageously utilizes low frequency (e.g., about 1-100 kHz) transcutaneous energy transfer (TET) for supplying power from an external control module to an implantable medical device, avoiding power dissipation through eddy currents in a metallic case of an implant and / or in human tissue, thereby enabling smaller implants using a metallic case such as titanium and / or allowing TET signals of greater strength thereby allowing placement more deeply within a patient without excessive power transfer inefficiencies.
Owner:ETHICON ENDO SURGERY INC
Method and apparatus for treating pelvic organ prolapse
ActiveUS7500945B2Less riskEasy to operateSuture equipmentsAnti-incontinence devicesFirst pathwayIschial spine
A method of treating pelvic organ prolapse is provided. The method generally includes the steps of establishing a first pathway between the external perirectal region of the patient to the region of the ischial spine in tissue on one side of the prolapsed organ, followed by establishing a second pathway in tissue on the contralateral side of the prolapsed organ. A support member, which includes a central support portion and two end portions, is positioned in a position to reposition said prolapsed organ in said organ's anatomically correct location. The end portions of the support member are introduced through the respective tissue pathways, followed by adjustment of the end portions so that the support member is located in a therapeutic relationship to the prolapsed organ that is to be supported. An apparatus and kit for said treatment is further provided.
Owner:ASTORA WOMENS HEALTH
Implantable devices for controlling the size and shape of an anatomical structure or lumen
ActiveUS20080027483A1Suture equipmentsAnti-incontinence devicesAnatomical structuresMinimally invasive procedures
An implantable device system for controlling the dimensions of internal anatomic passages corrects physiologic dysfunctions resulting from a structural lumen which is either too large or too small. Implantable devices are disclosed which employ various mechanisms for adjusting and maintaining the size of an orifice to which they are attached. Systems permit the implants to be implanted using minimally invasive procedures and permit final adjustments to the dimensions of the implants after the resumption of normal flow of anatomic fluids in situ.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Method and apparatus for cystocele repair
A method for cystocele repair comprising the steps of: establishing four pathways in tissue around a bladder of a patient, introducing a strap into each of said pathways, and positioning beneath said bladder of said patient a support member having each said strap connected thereto such that said bladder of said patient is supported by said support member and a bulge of said bladder into a vagina of said patient is reduced.
Owner:ASTORA WOMENS HEALTH
Sling assembly with secure and convenient attachment
InactiveUS6641525B2Eliminate cross-contaminationAvoid pollutionSuture equipmentsSurgical furnitureOperative Surgical ProceduresSurgical department
Surgical articles that are conveniently and securely coupled are disclosed. Improved surgical procedures are also disclosed.
Owner:STASKIN DAVID MD DR
Surgical instrument and method for treating female urinary incontinence
InactiveUS20020128670A1Reduces risk of perforationReduce riskSuture equipmentsAnti-incontinence devicesUrethraVaginal walls
The invention relates to a surgical instrument and a method for treating female urinary incontinence. A tape or mesh is permanently implanted into the body as a support for the urethra. In one embodiment, portions of the tape comprise tissue growth factors and adhesive bonding means for attaching portions of the tape to the pubic bone. In a further embodiment, portions of the tape comprise attachment means for fastening portions of the tape to fascia within the pelvic cavity. In both embodiments the tape is implanted with a single incision through the vaginal wall.
Owner:ULMSTEN ULF +1
Methods and devices for the treatment of neurological and physiological disorders
InactiveUS20050015129A1Easy to optimizeOvercome pacing defectStentsElectrotherapyPhysical therapyCardiac arrhythmia
Novel devices and methods that affect the neurologic and biological electrical conduction systems for the treatment various neurological and physiological disorders. Localized mechanical forces imparted by the inventive devices and methods modify or alter the mechanoelectric and or electrochemical properties of the affected tissues and biologic systems. Combinations of various technologies can be incorporated into the devices and methodologies for specific treatments. The devices and methods can be used to treat a number of neurologic and physiologic disorders such as Parkinson's, epilepsy, atrial fibrillation, cardiac arrhythmia, obesity, and others.
Owner:MISCHE HANS A
Incontinence sling
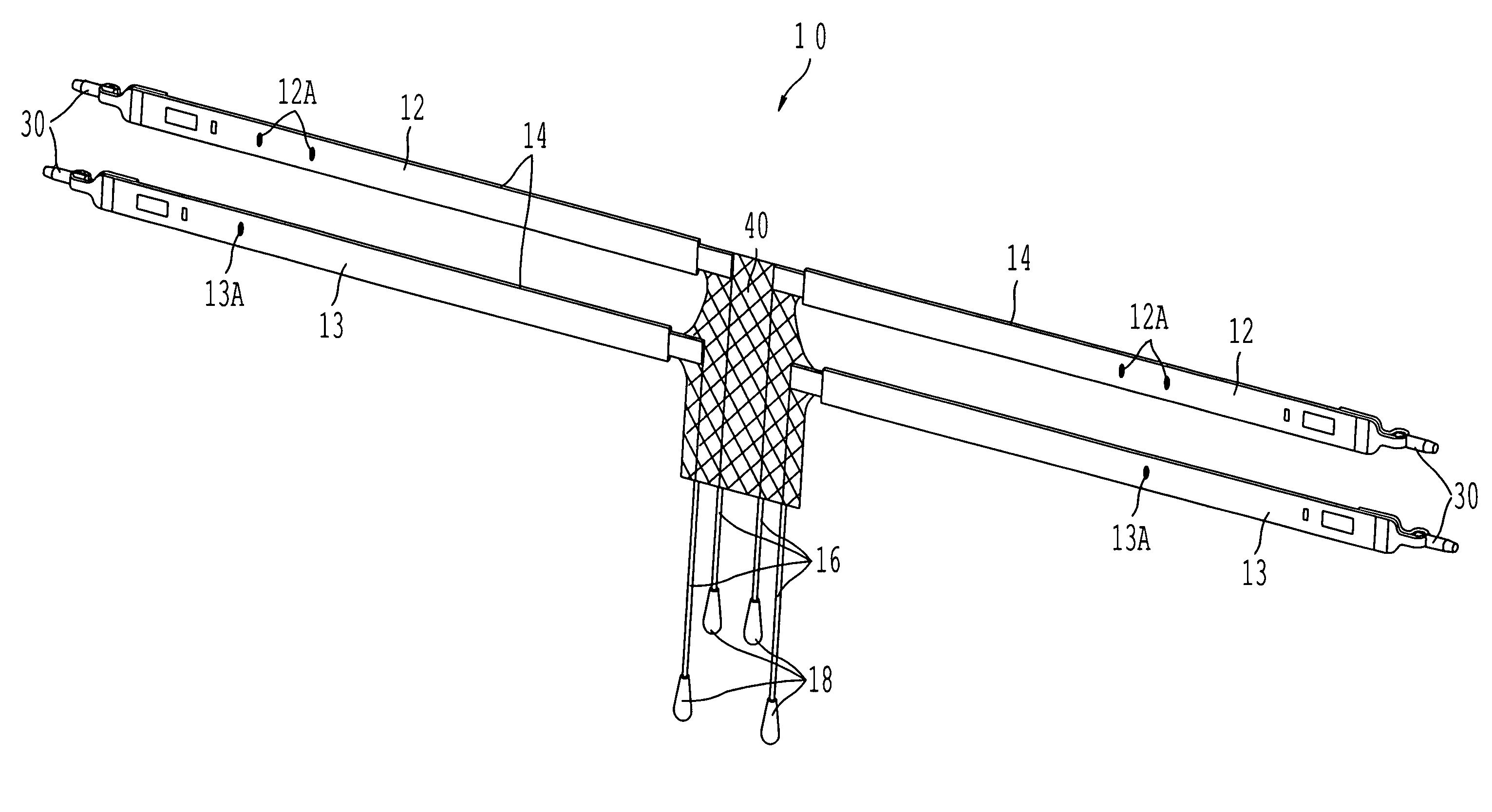
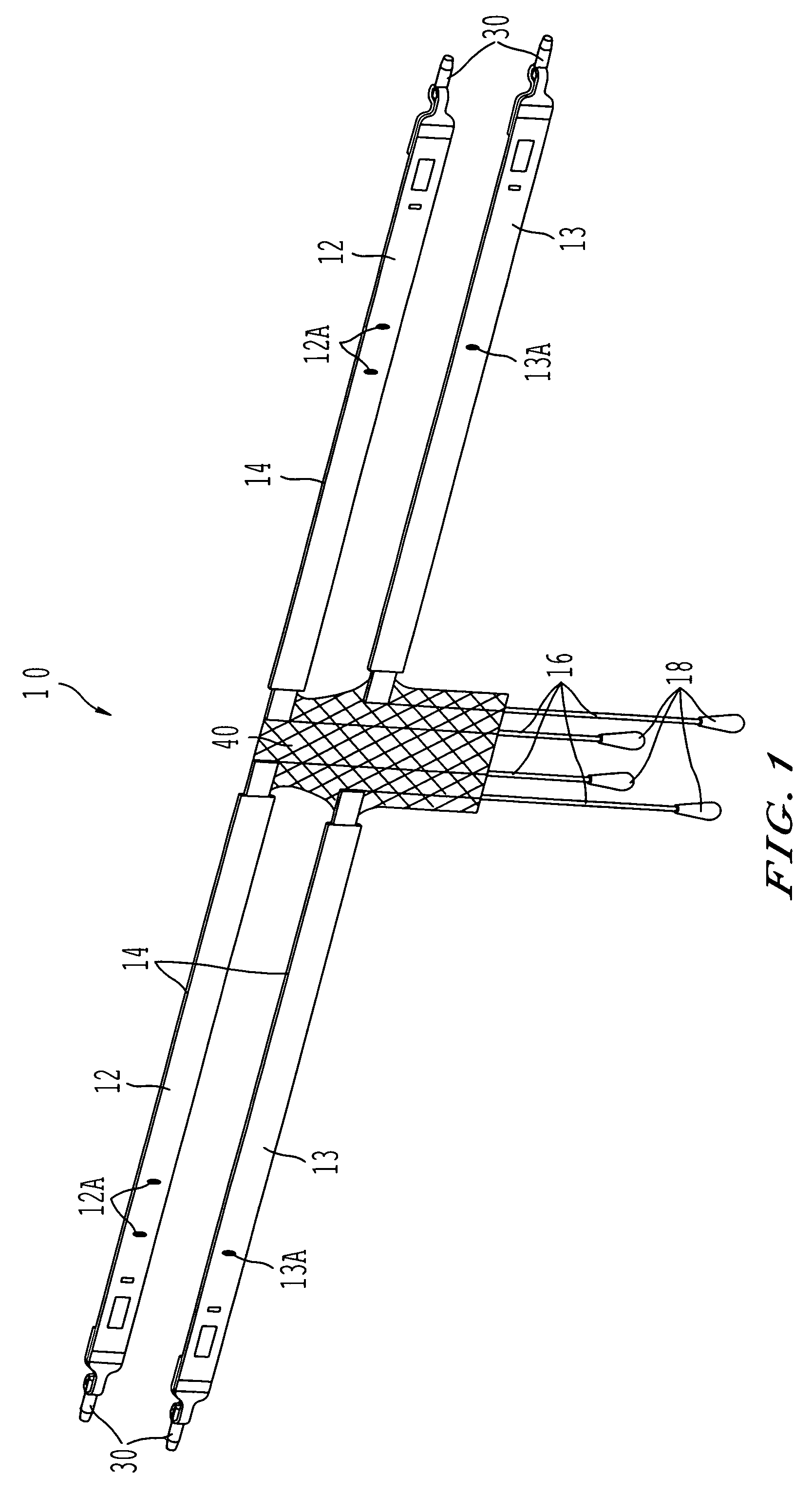
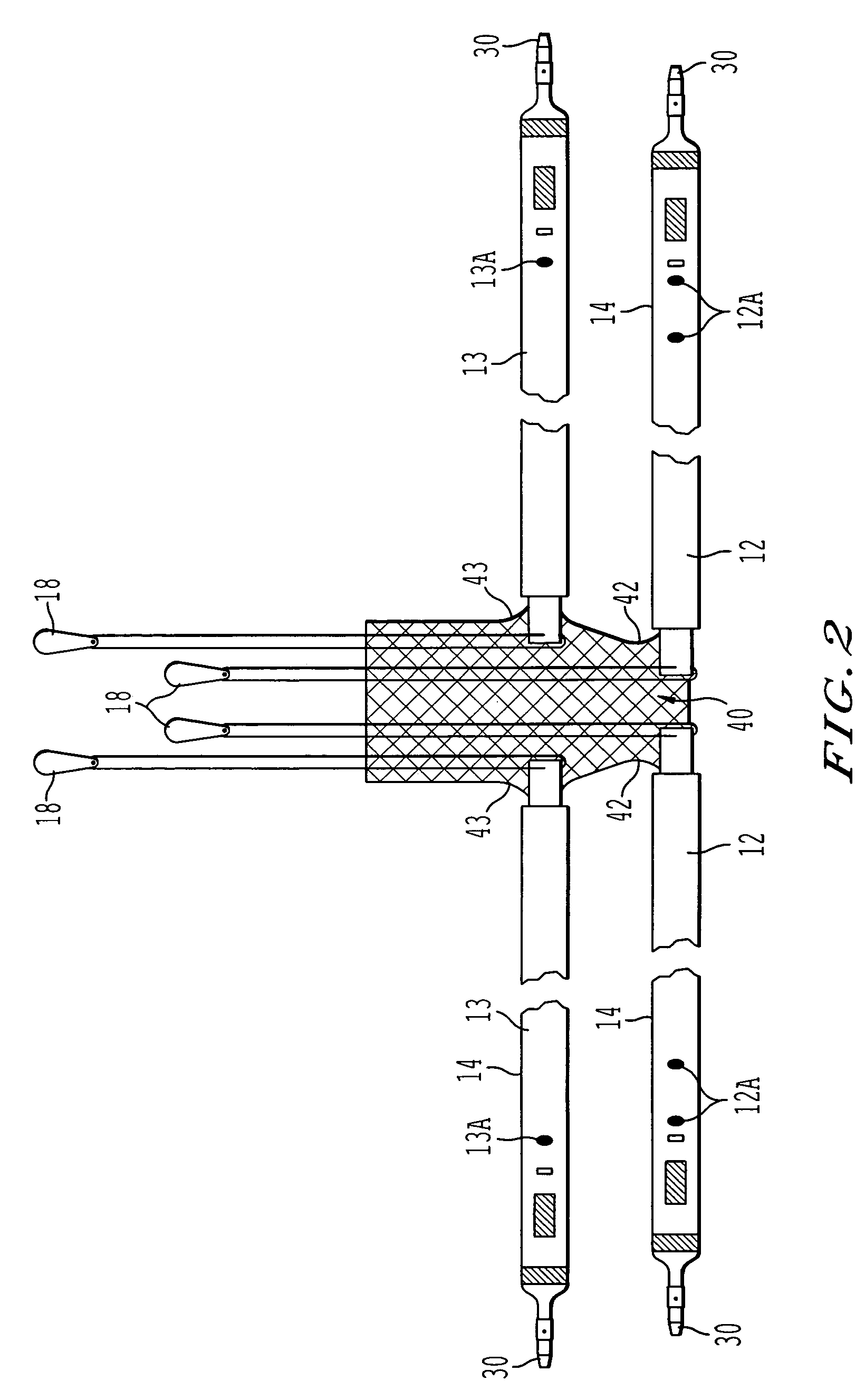
A sling for controlling urinary incontinence is disclosed. The sling is formed from a tube having substantially lengthwise inextensible end portions and an elastically lengthwise extensible intermediate portion between the end portions. The intermediate portion has a U-shape which cradles the urethra. The end portions extend through the abdominal tissue to anchor the intermediate portion in position. The sling places the urethra under a transverse compressive load to hold it closed and prevent inadvertent urination. The tube is formed from interlaced filamentary members. Interlacing may be by warp knitting, weaving using a leno weave or braiding using a tri-axial braid structure. The end portions have a rough texture to facilitate anchoring in the tissue of the abdominal wall. The intermediate portion is smooth and soft to prevent tissue erosion.
Owner:STOUT MEDICAL GROUP
Insertion and retrieval system for inflatable devices
InactiveUS20050131442A1Reduce chanceEliminate chanceAnti-incontinence devicesSurgical needlesUrethraCoupling
A system for deploying and retrieving implantable articles, such as balloons, typically for treating incontinence, from the bladder includes a urethral module, this deployed in the urethra and bladder with the coupling of a mandrel. The deployed urethral module, with the mandrel removed therefrom, provides a channel to the bladder for a magazine unit, that deploys the balloon, and a retriever, that retrieves the balloon, and is ultimately removed from the body with the balloon in a deflated or contracted state.
Owner:INNOVENTIONS INC
Surgical articles and methods
InactiveUS7048682B2Improve convenienceProlong surgery timeSuture equipmentsIncision instrumentsMedical disorderSurgical department
Surgical instruments, implantable articles and surgical procedures disclosed for treating medical disorders, particularly incontinence. Improved surgical sling procedures are disclosed. Novel surgical instruments and kits for use in sling procedures are also disclosed. The present invention affords options for surgeons with concomitant advantages to the patient and the healthcare provider.
Owner:STASKIN DAVID MD DR
Prolapse repair
Surgical instruments for prolapse repair are disclosed. The surgical instruments have straight portions and helical portions.
Owner:ASTORA WOMENS HEALTH
Pelvic floor implant system and method of assembly
Owner:ASTORA WOMENS HEALTH
Apparatus and method for manipulating or retracting tissue and anatomical structure
ActiveUS20070049929A1Easy to testPromote healingSuture equipmentsAnti-incontinence devicesAnatomical structuresBiomedical engineering
Intergrated systems and associated method for manipulating tissues and anatomical or other structures in medical applications for the purpose of treating diseases or disorders or other purposes. In one aspect, the system includes a delivery device configured to deploy and implant anchoring devices for such purposes.
Owner:TELEFLEX LIFE SCI LTD
Sling delivery system and method of use
InactiveUS6971986B2Structural damageReduce the amount requiredSuture equipmentsIncision instrumentsDiseaseSide effect
An apparatus and method of use are disclosed to treat urological disorders. The biocompatible device includes a handle, needle, dilator and sling assembly configured to be minimally invasive and provide sufficient support to the target site. In addition, the configuration of the sling assembly also allows the sling to be adjusted during and / or after implantation. The device and treatment procedure are highly effective and produce little to no side effects or complications. Further, operative risks, pain, infections and post operative stays are reduced, thereby improving patient quality of life.
Owner:STASKIN DAVID MD DR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com