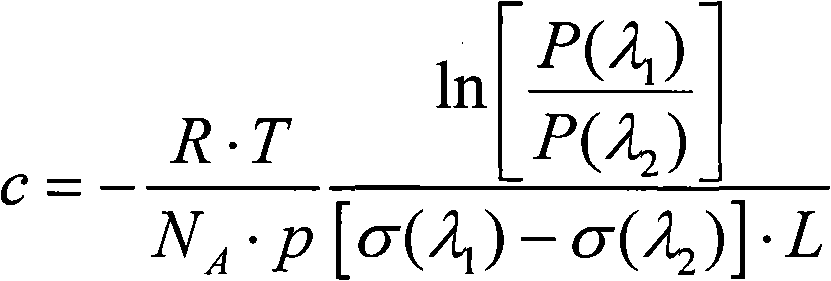Patents
Literature
98results about How to "Eliminate cross-contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
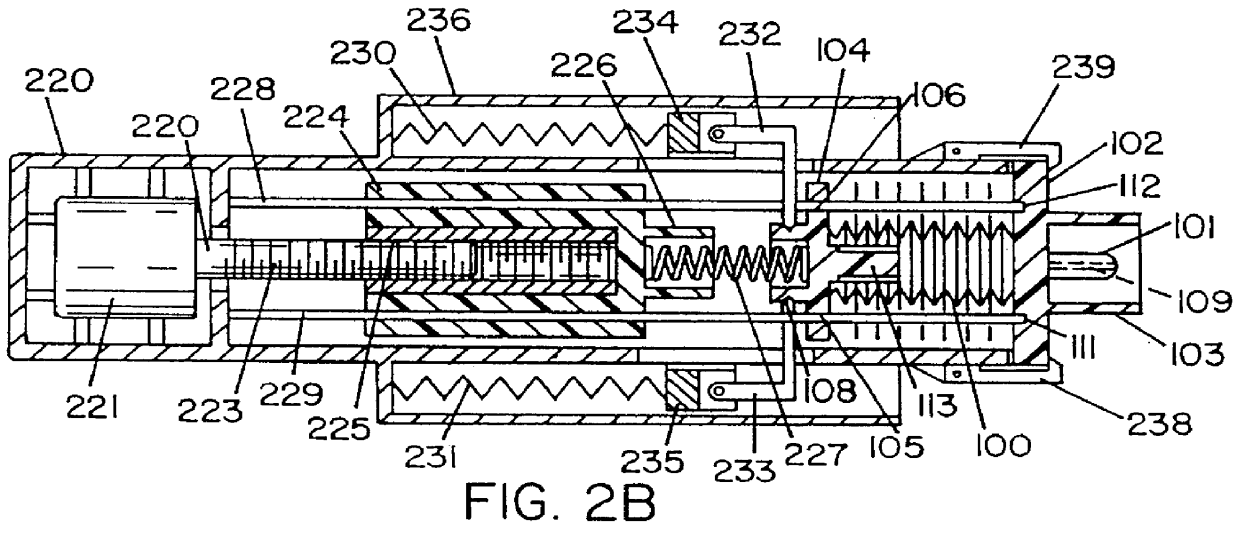
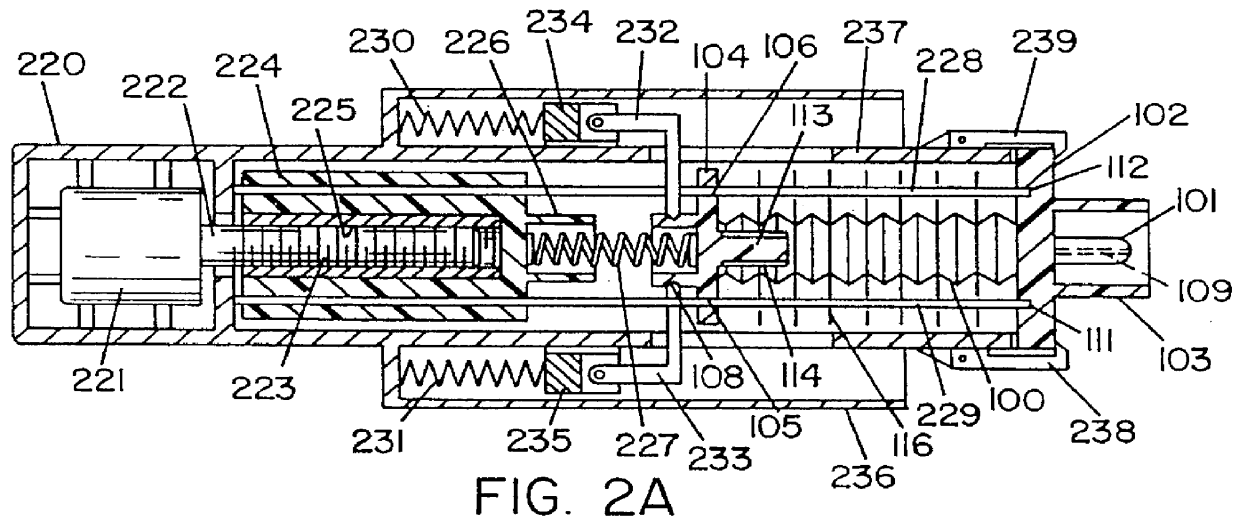
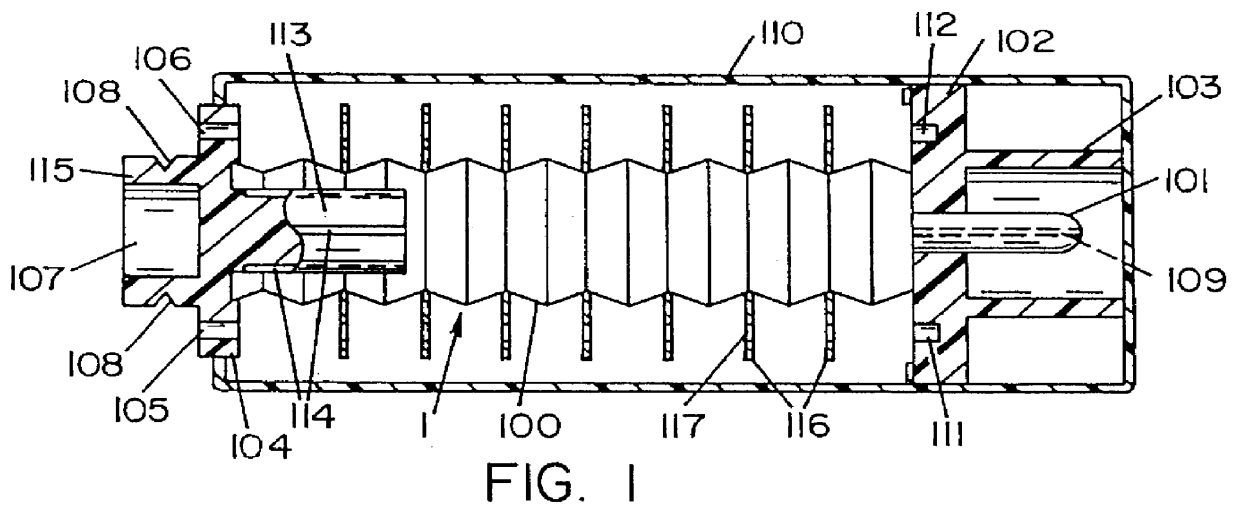
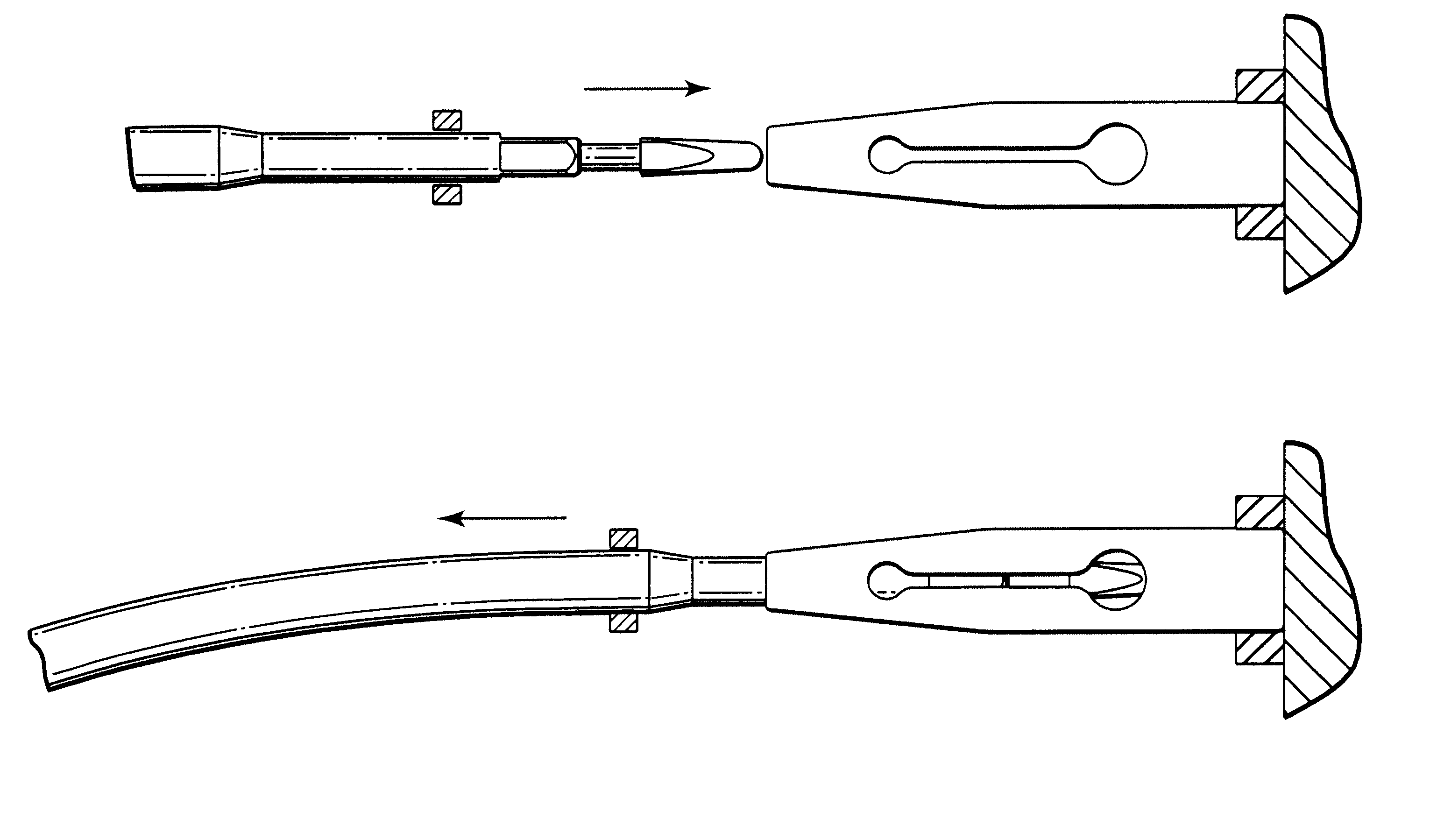
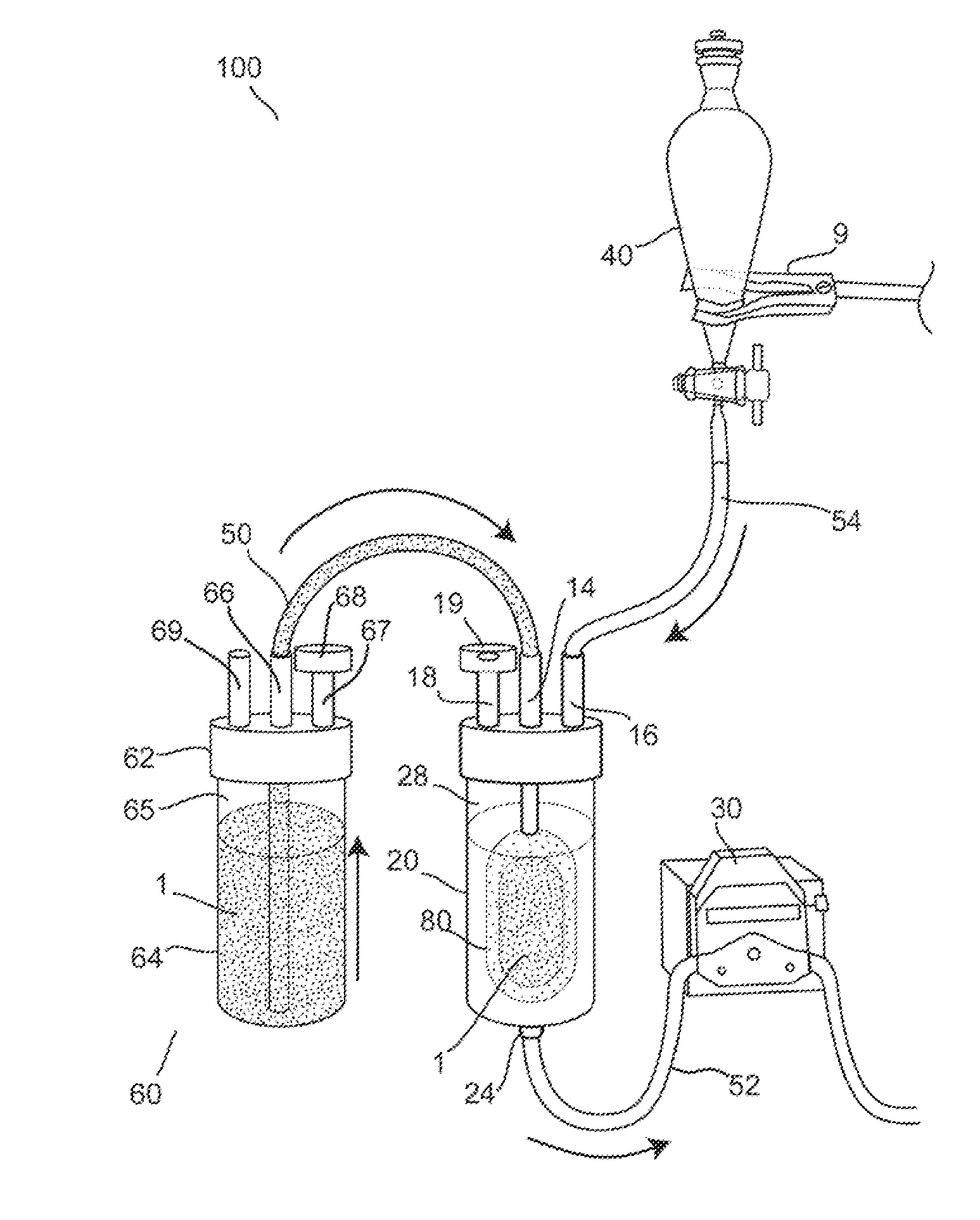
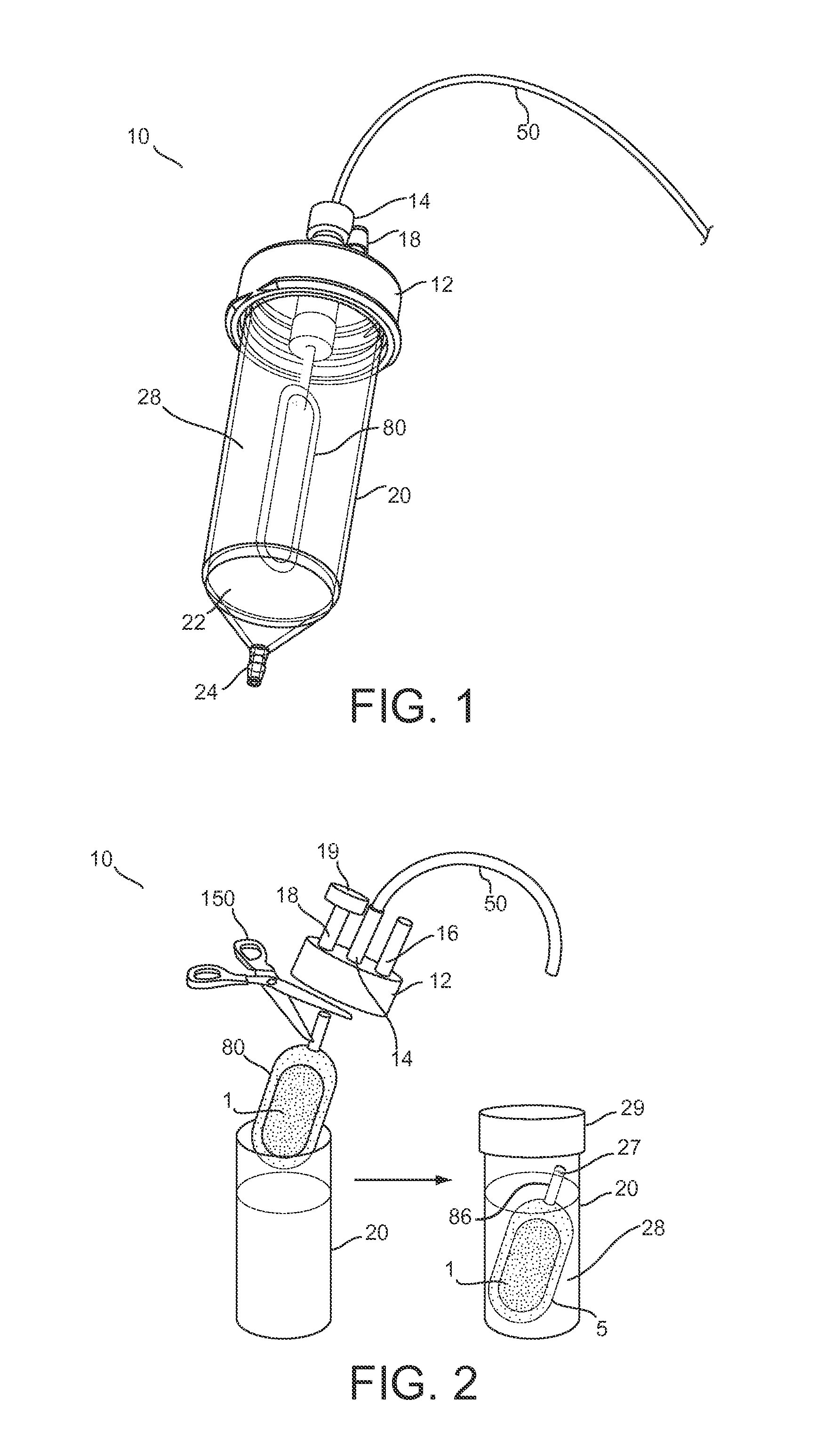
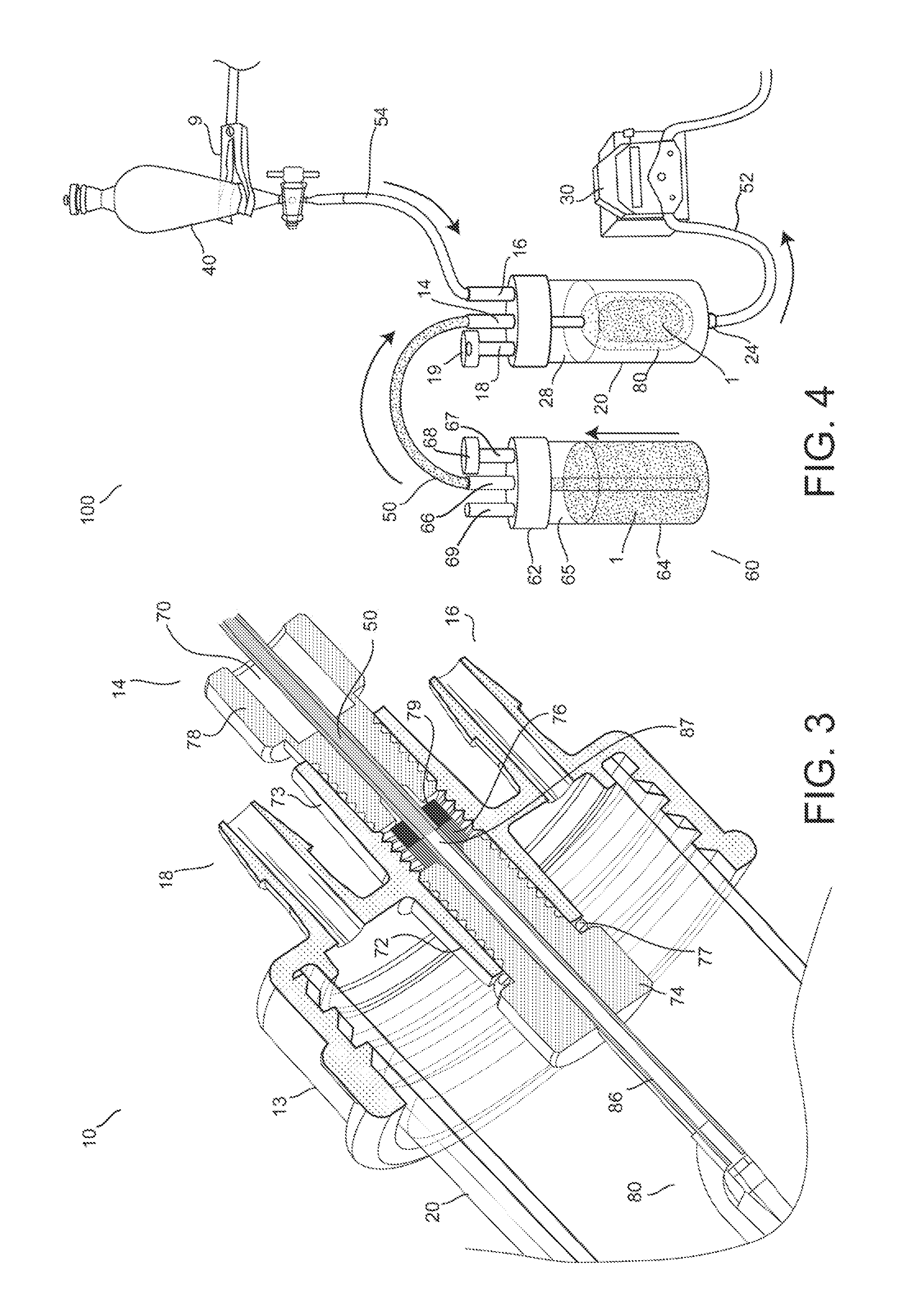
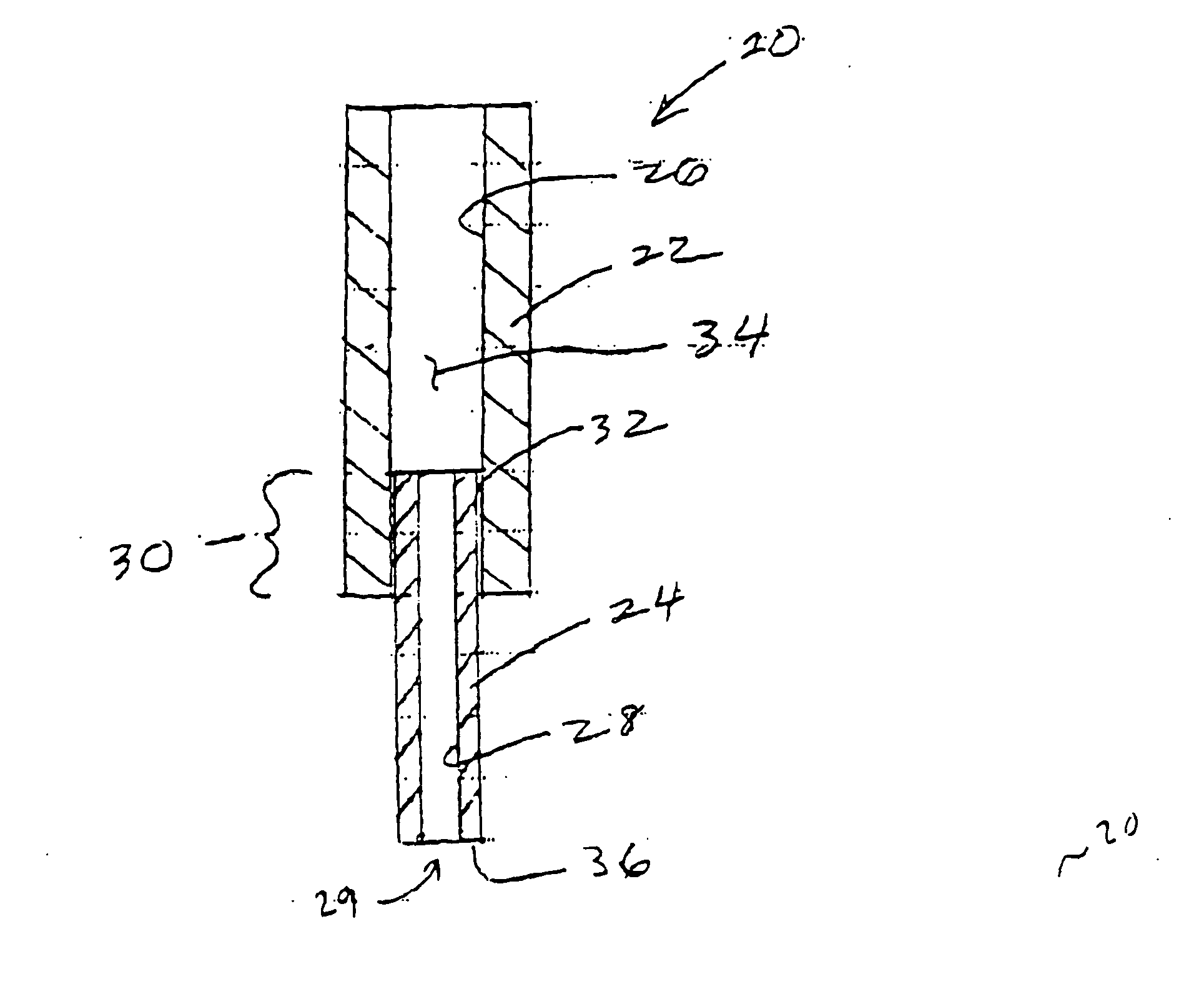
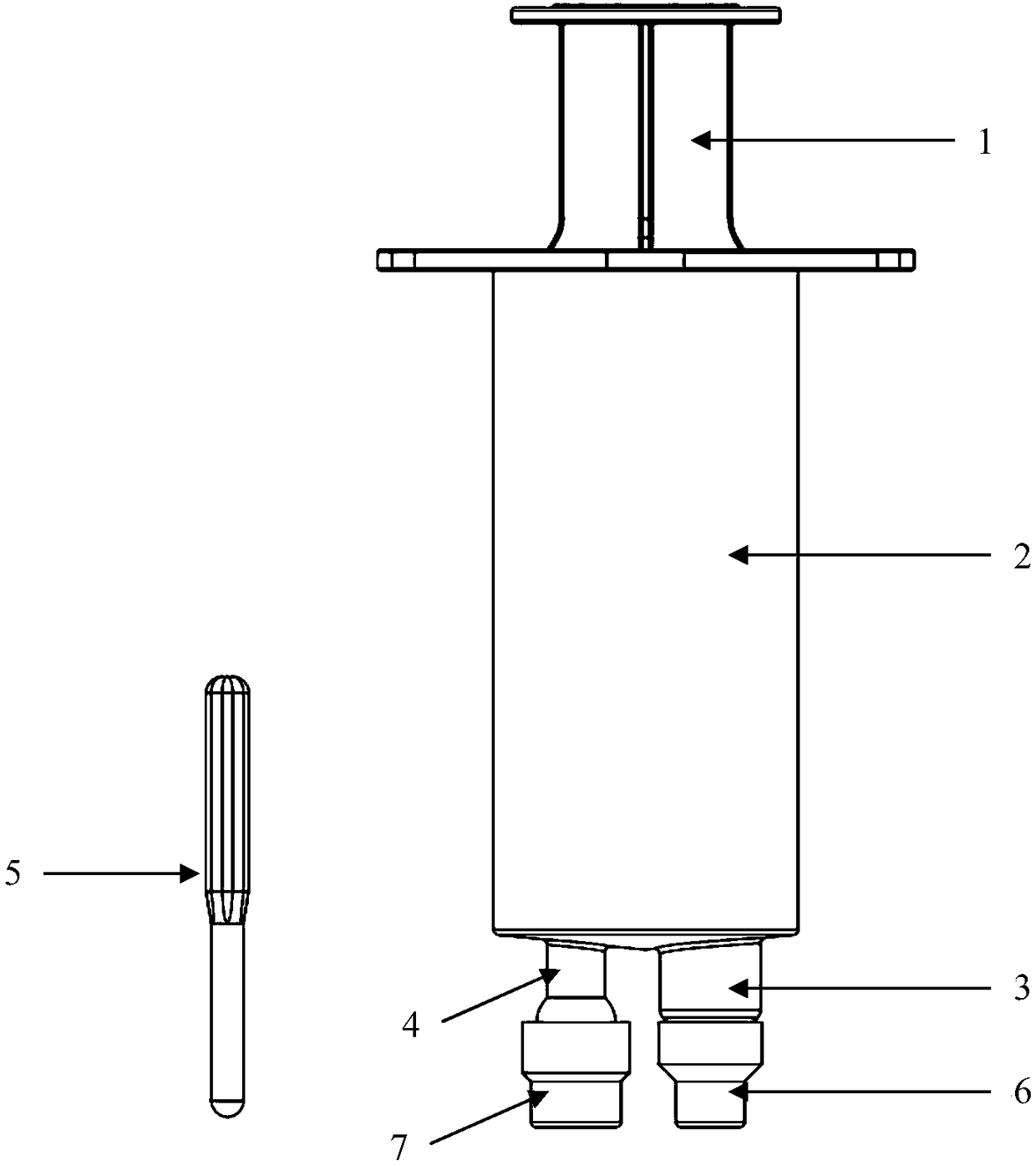
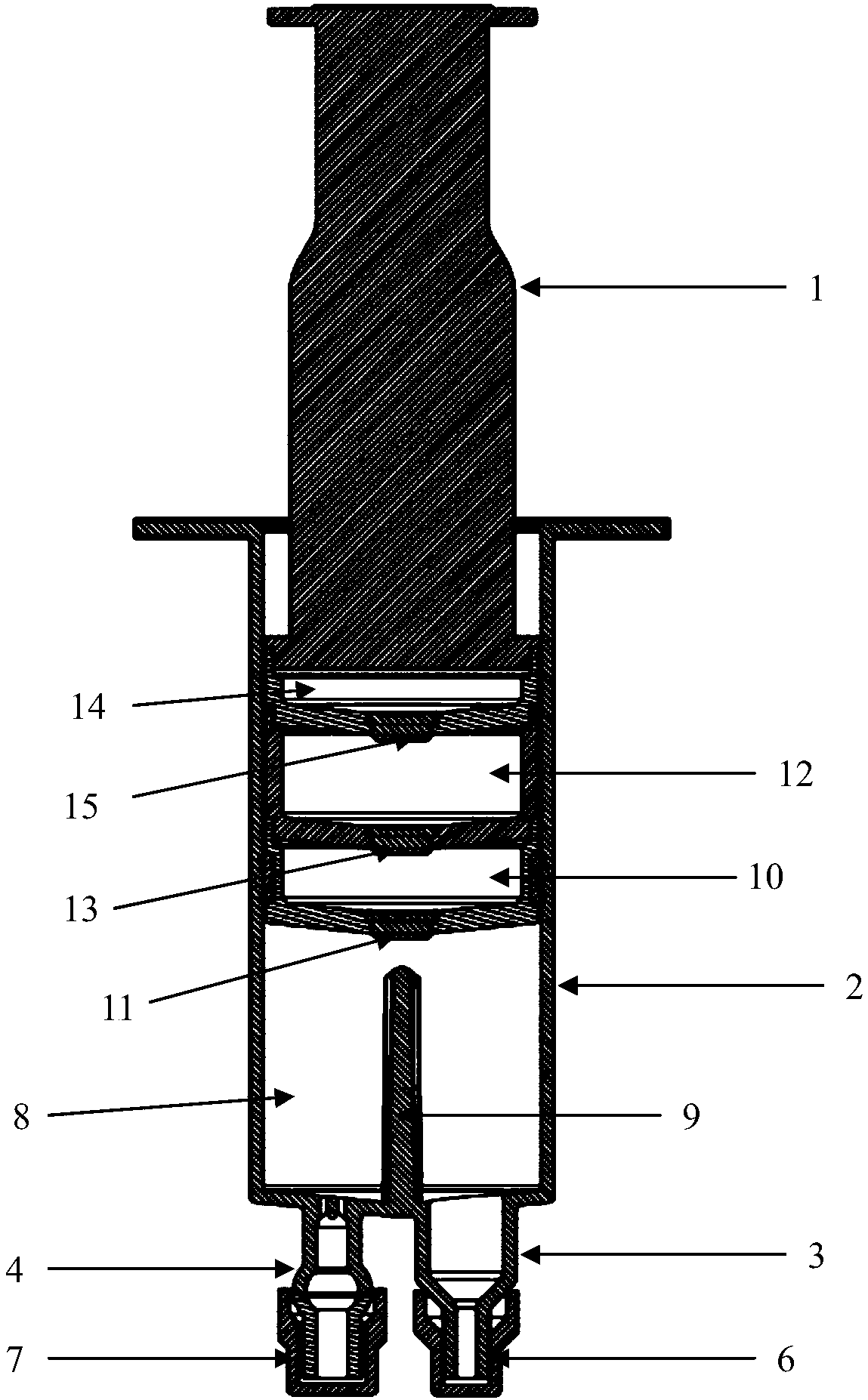
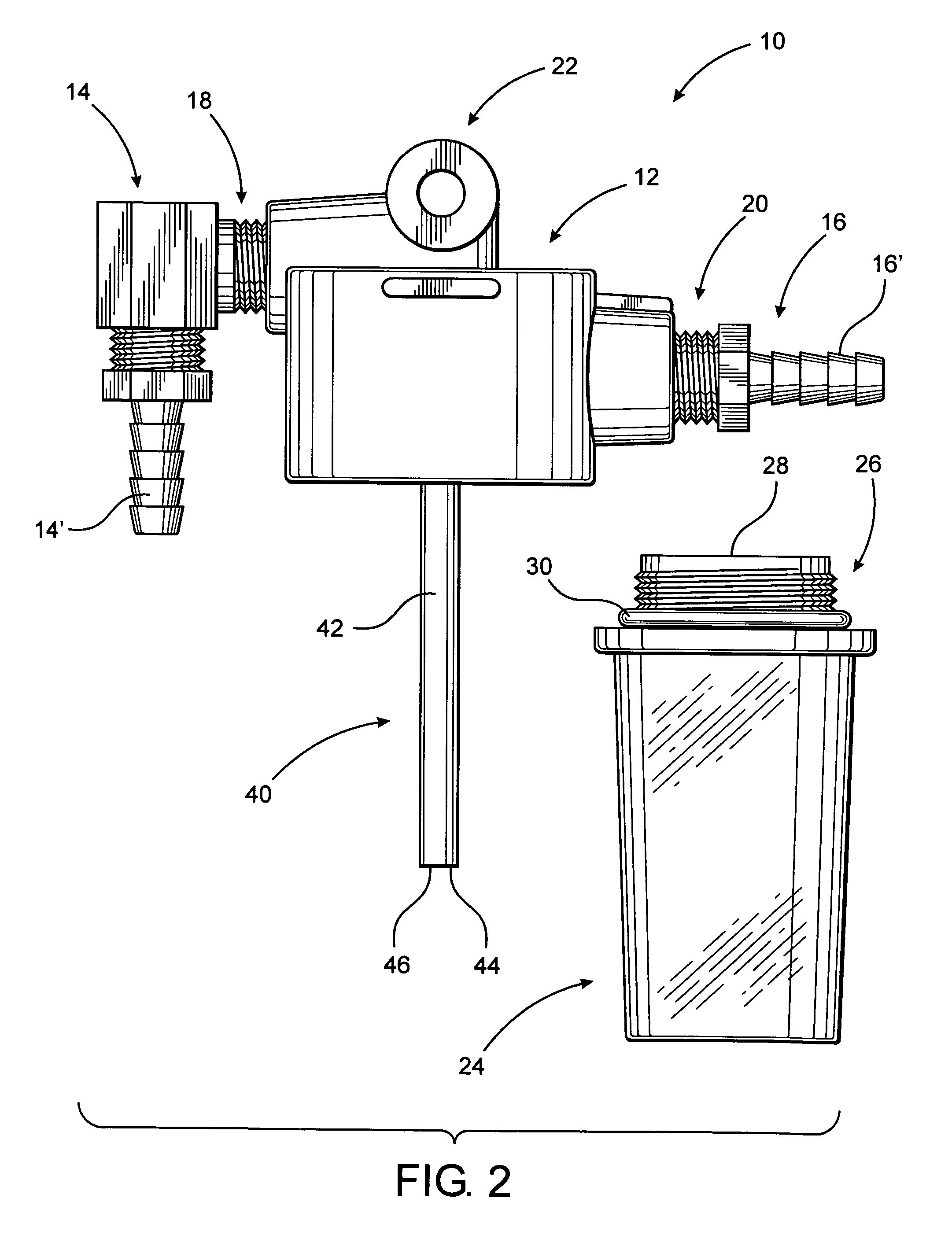
Hypodermic fluid dispenser
InactiveUS6056716AEfficient injectionEliminate the problemAmpoule syringesJet injection syringesHypodermoclysisJet flow
A jet injector system for injecting fluid into a body. The jet injection system includes capsules for holding the material to be injected, apparatus for applying force to the capsule(s) to eject the injection material(s) and a perforator for directing the jet stream for the respective materials into the body. A flyweight system is described for developing jet injection pressures, and latching devices control the flyweight system. An injector system for injecting more than one fluid is described.
Owner:DANTONIO CONSULTANTS INT INC
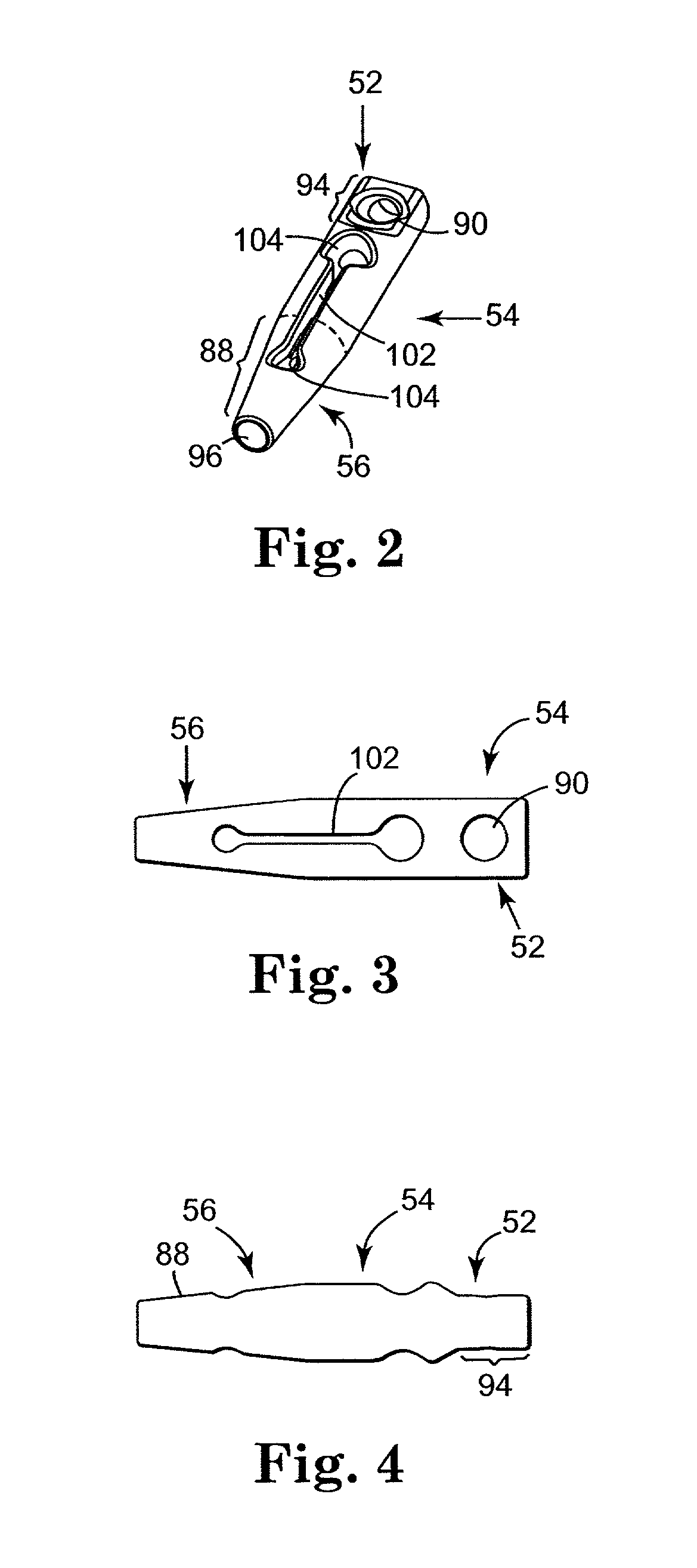
Sling assembly with secure and convenient attachment
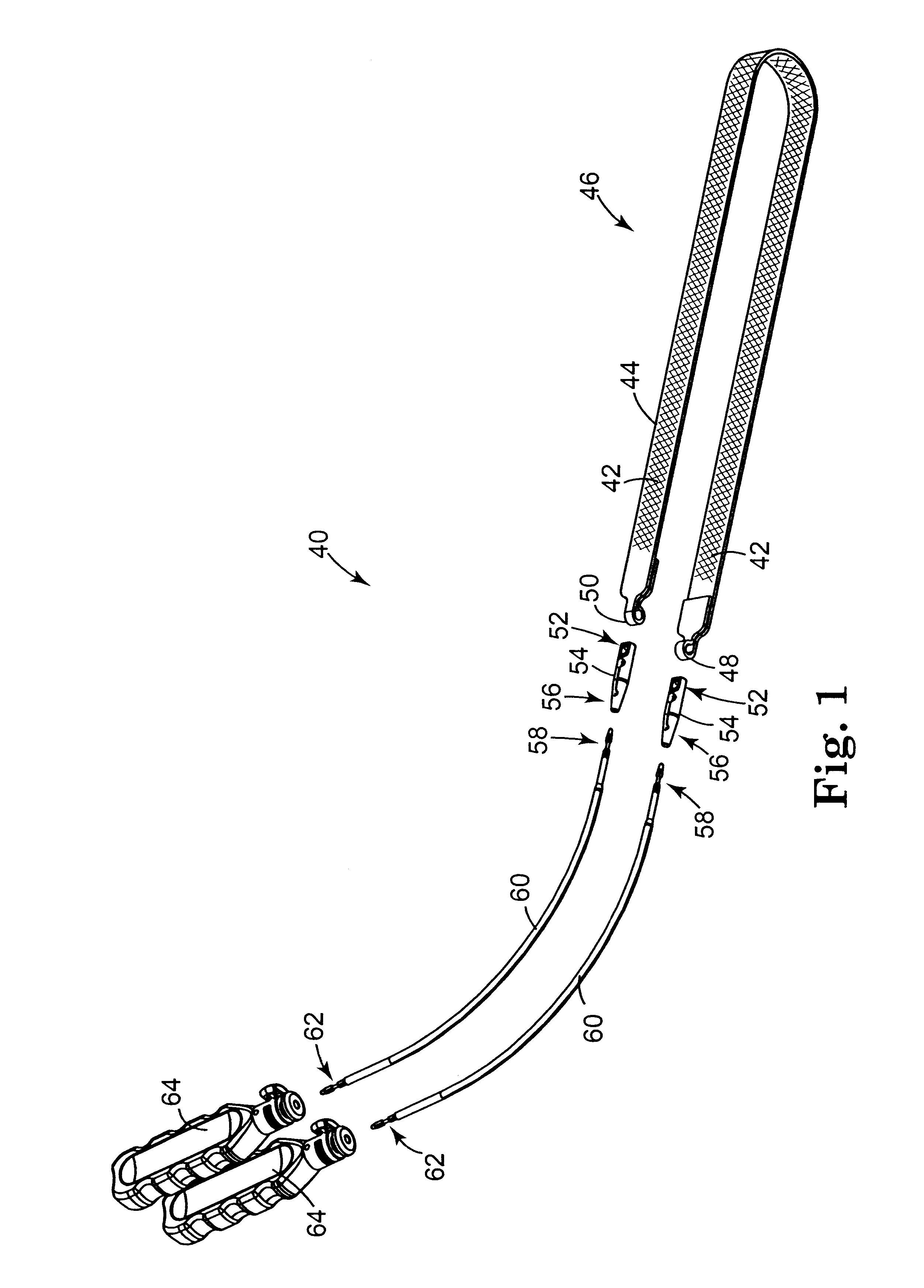
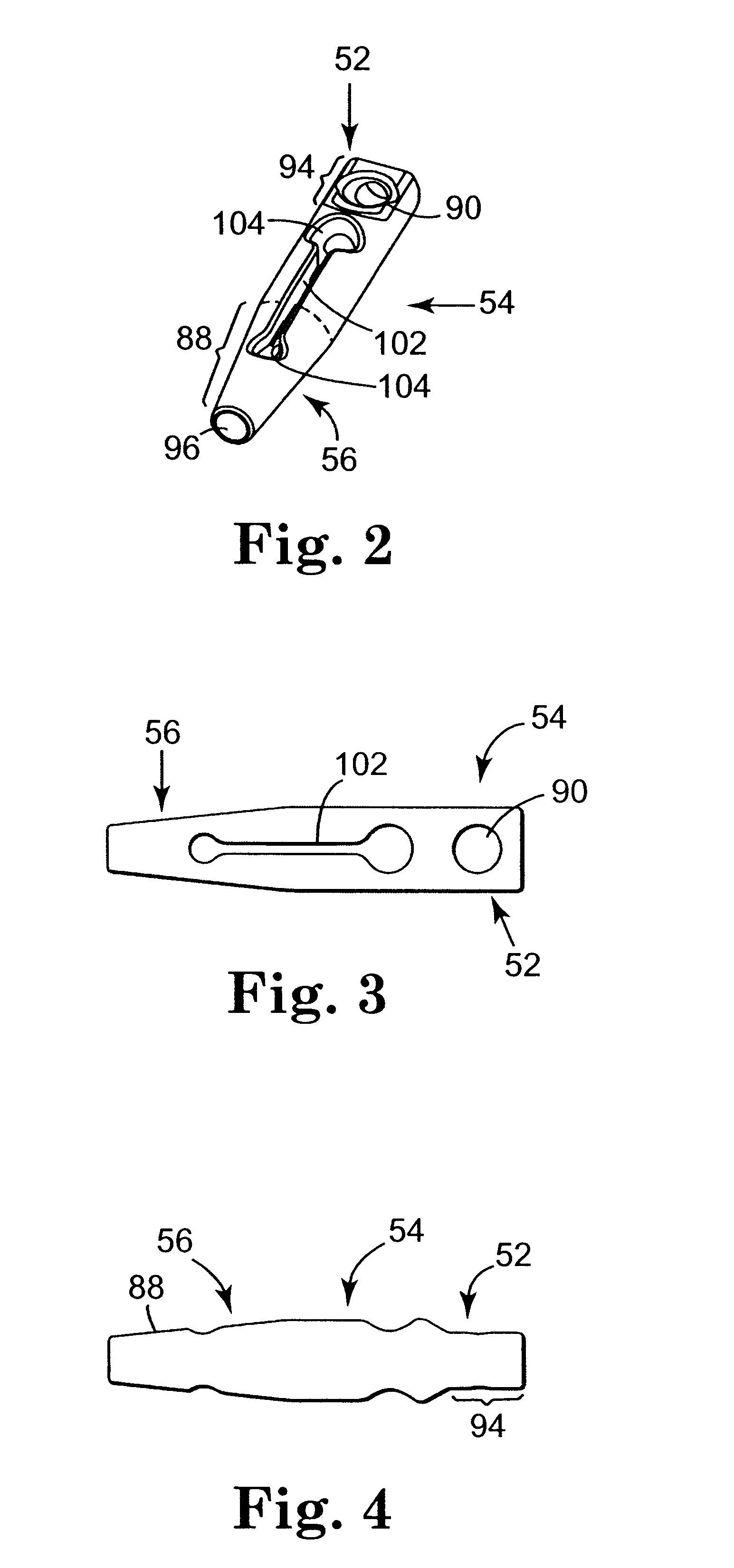
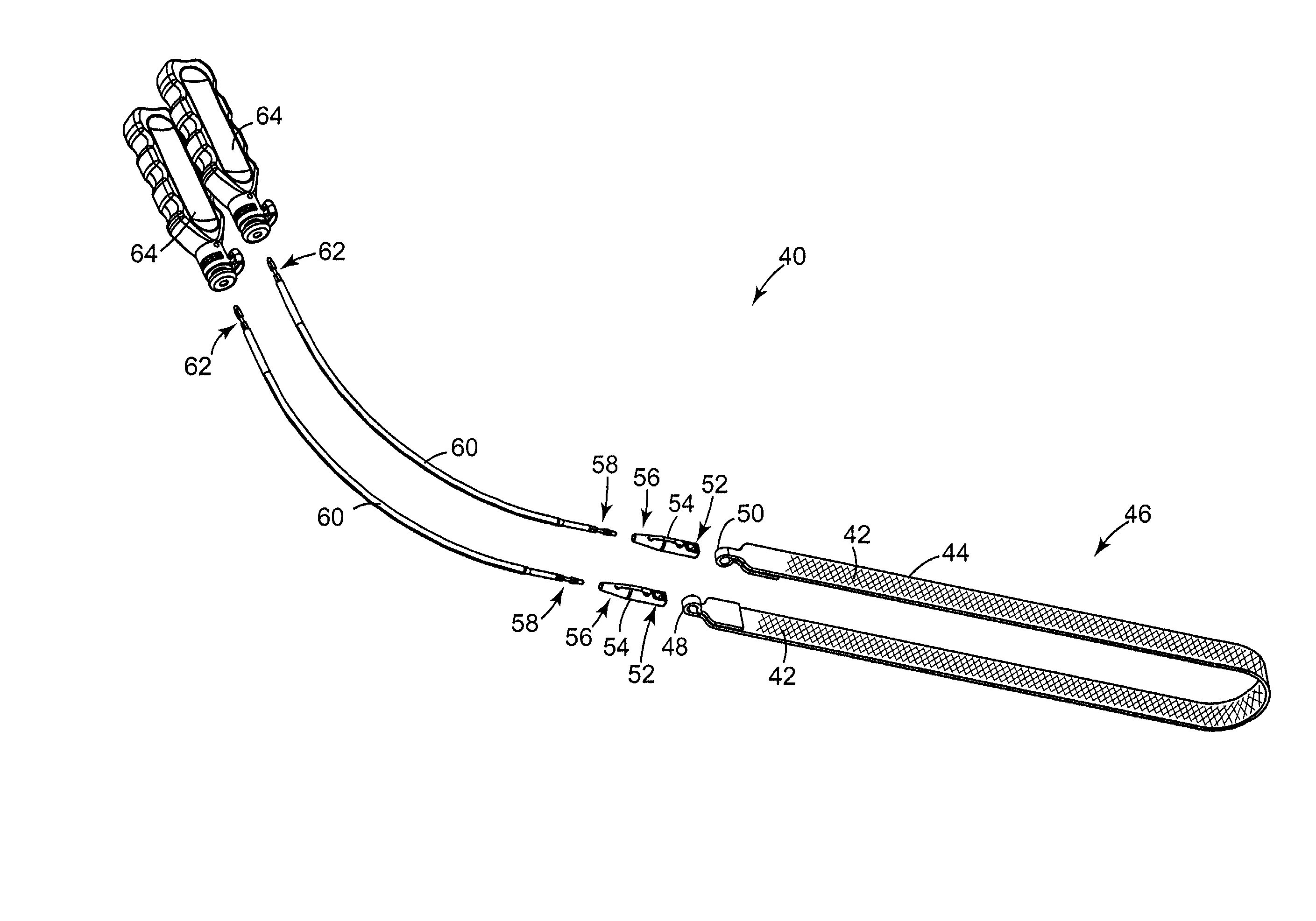
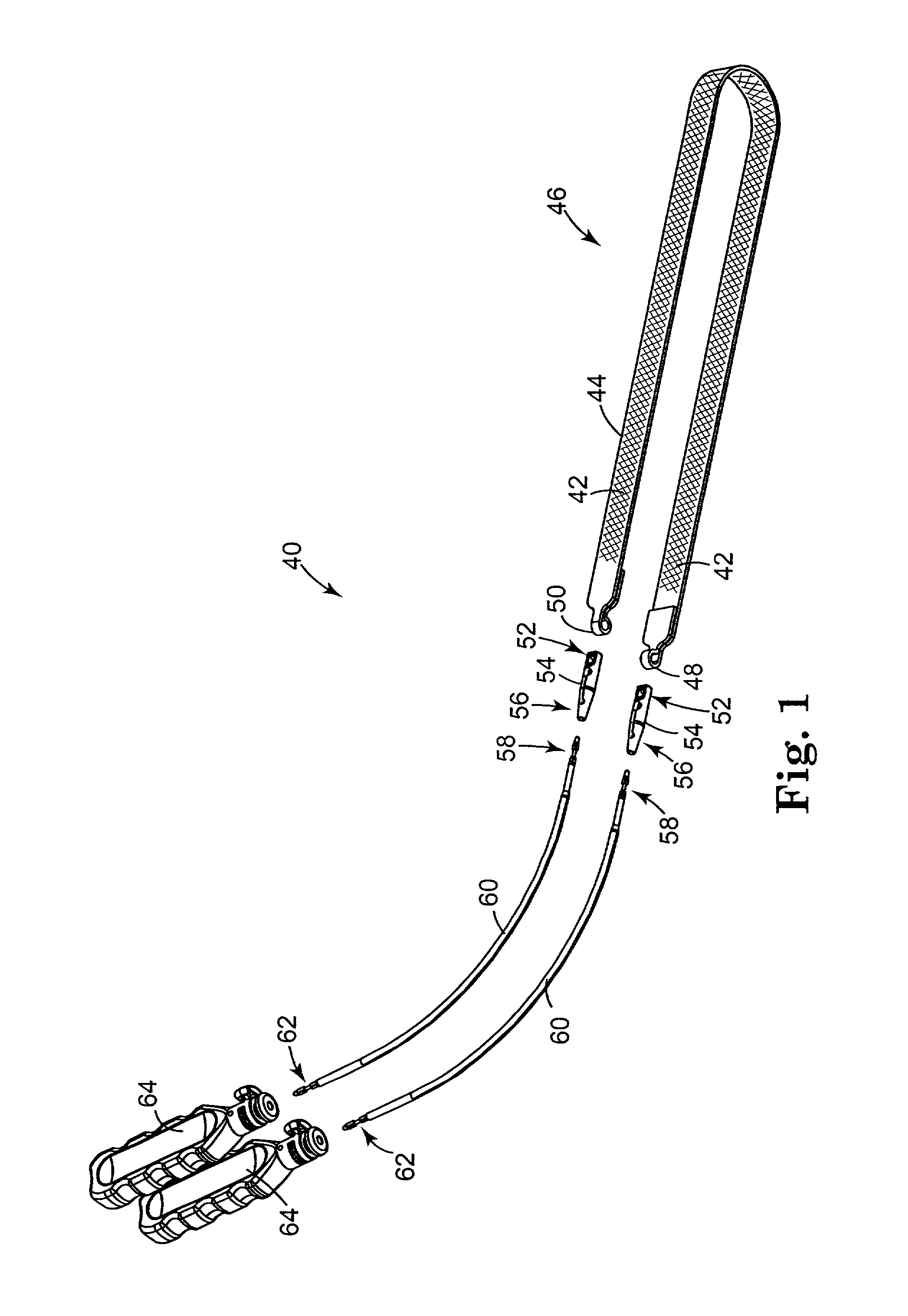
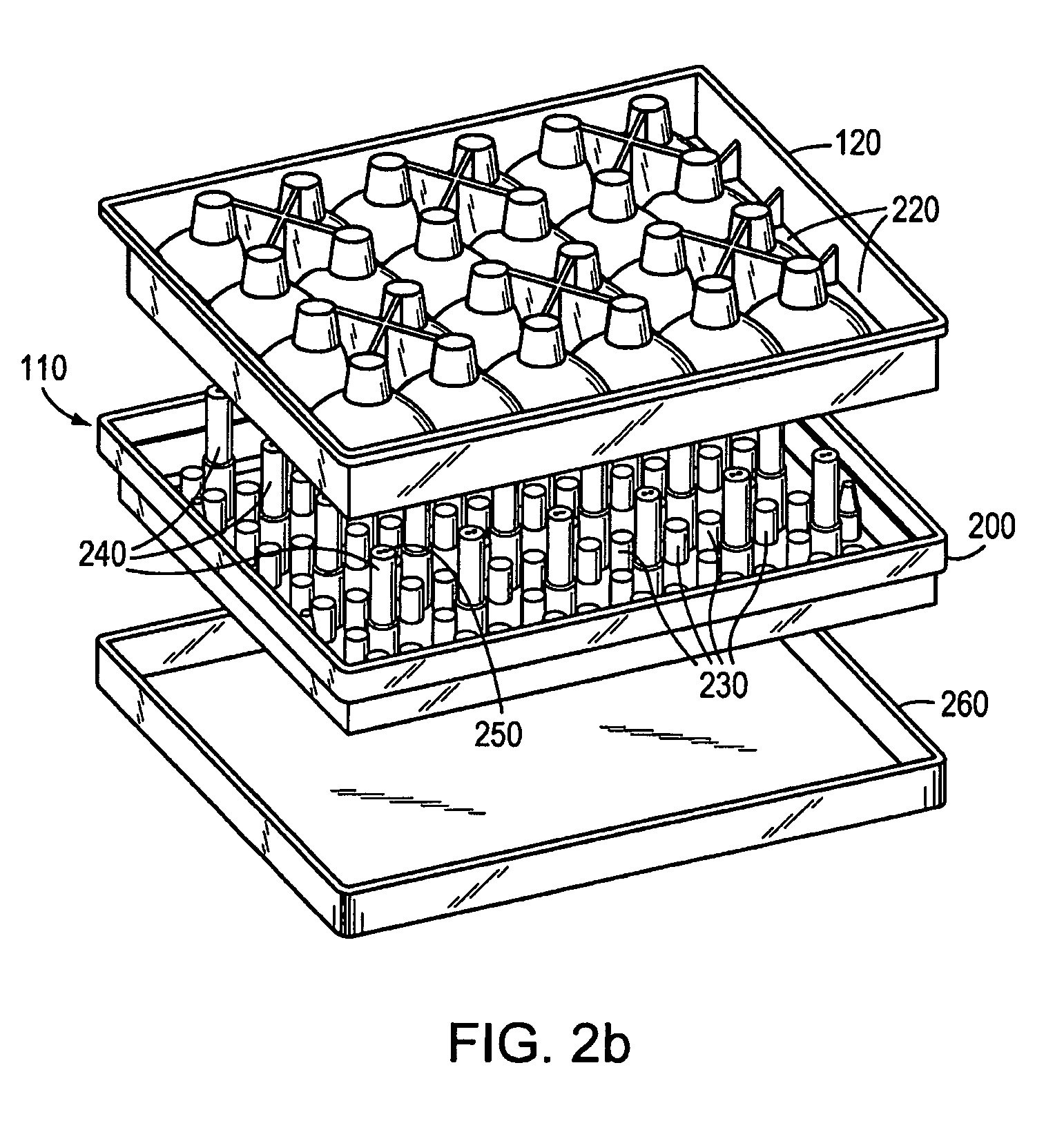
InactiveUS6641525B2Eliminate cross-contaminationAvoid pollutionSuture equipmentsSurgical furnitureOperative Surgical ProceduresSurgical department
Surgical articles that are conveniently and securely coupled are disclosed. Improved surgical procedures are also disclosed.
Owner:STASKIN DAVID MD DR
Sling assembly with secure and convenient attachment
InactiveUS20020151762A1Decrease rigidity and resistanceReduce amountSuture equipmentsSurgical furnitureSurgical departmentSurgical procedures
Surgical articles that are conveniently and securely coupled are disclosed. Improved surgical procedures are also disclosed.
Owner:STASKIN DAVID MD DR
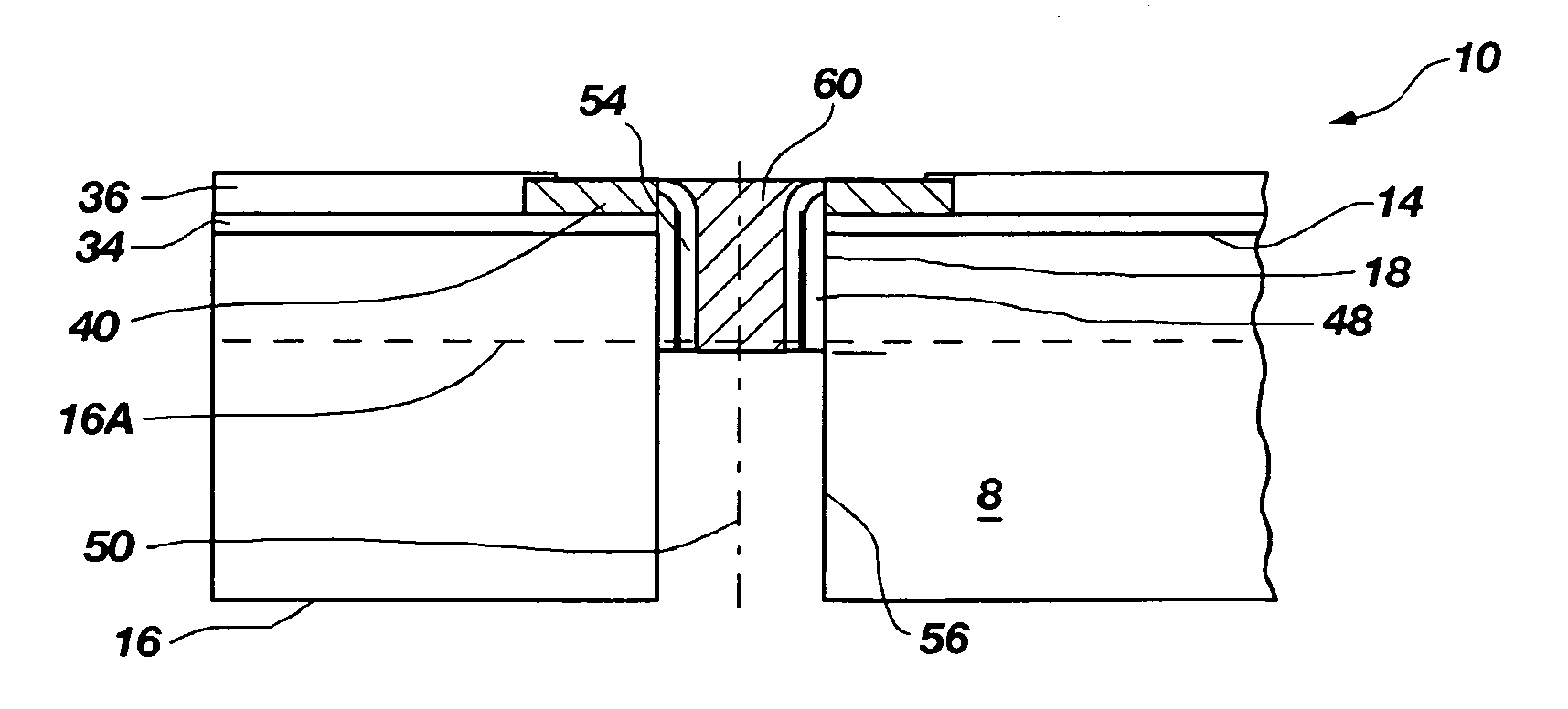
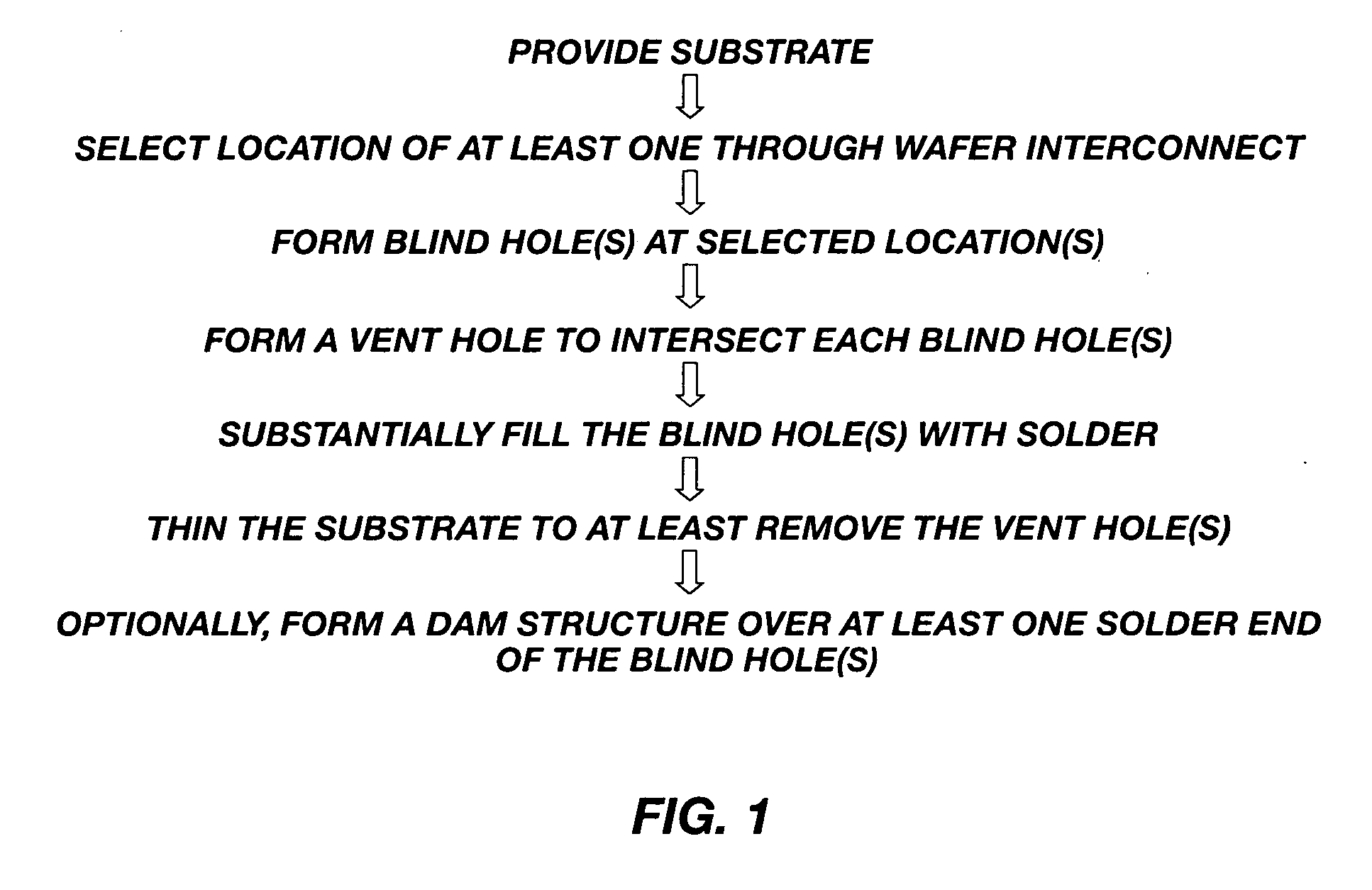
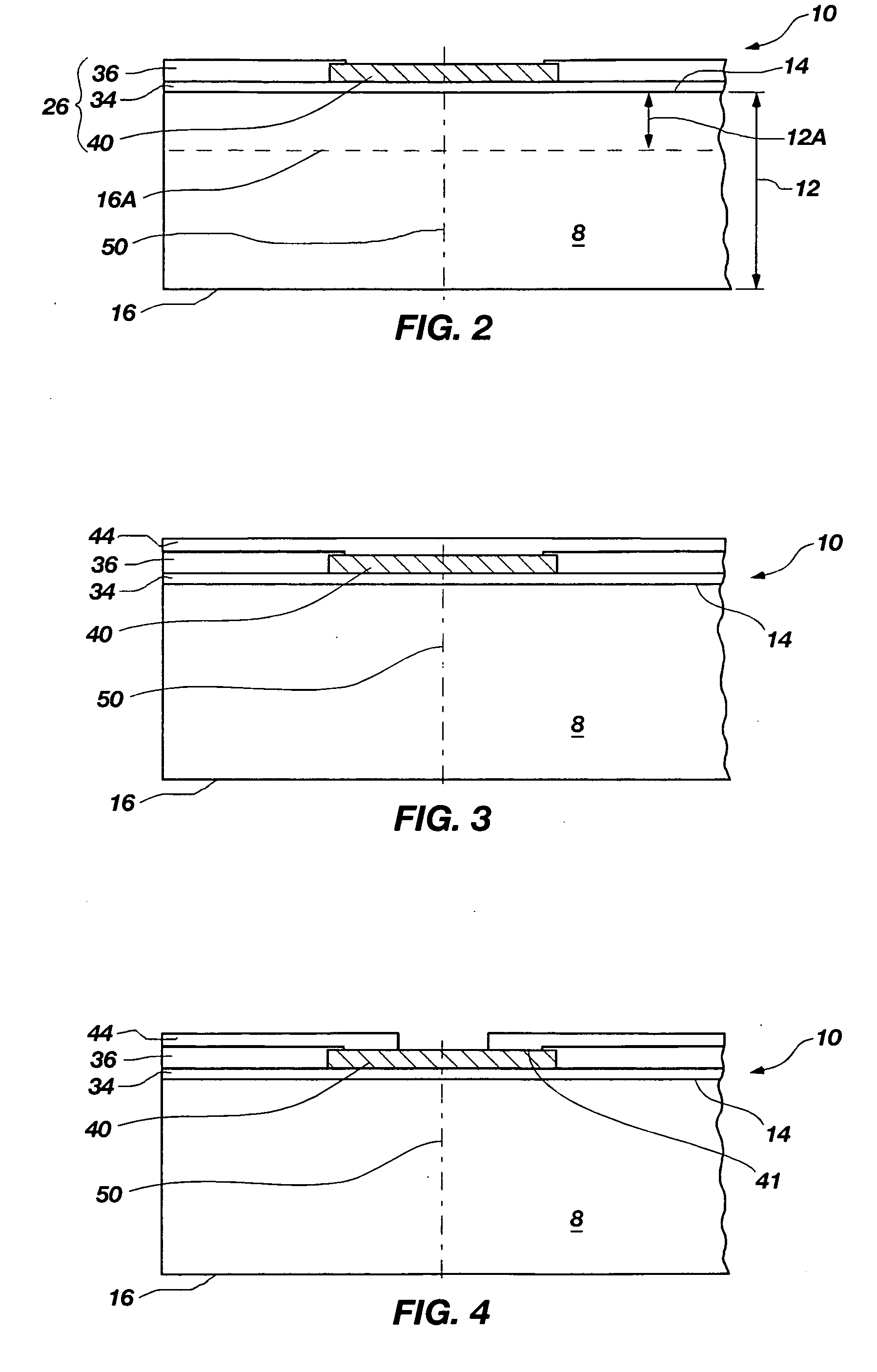
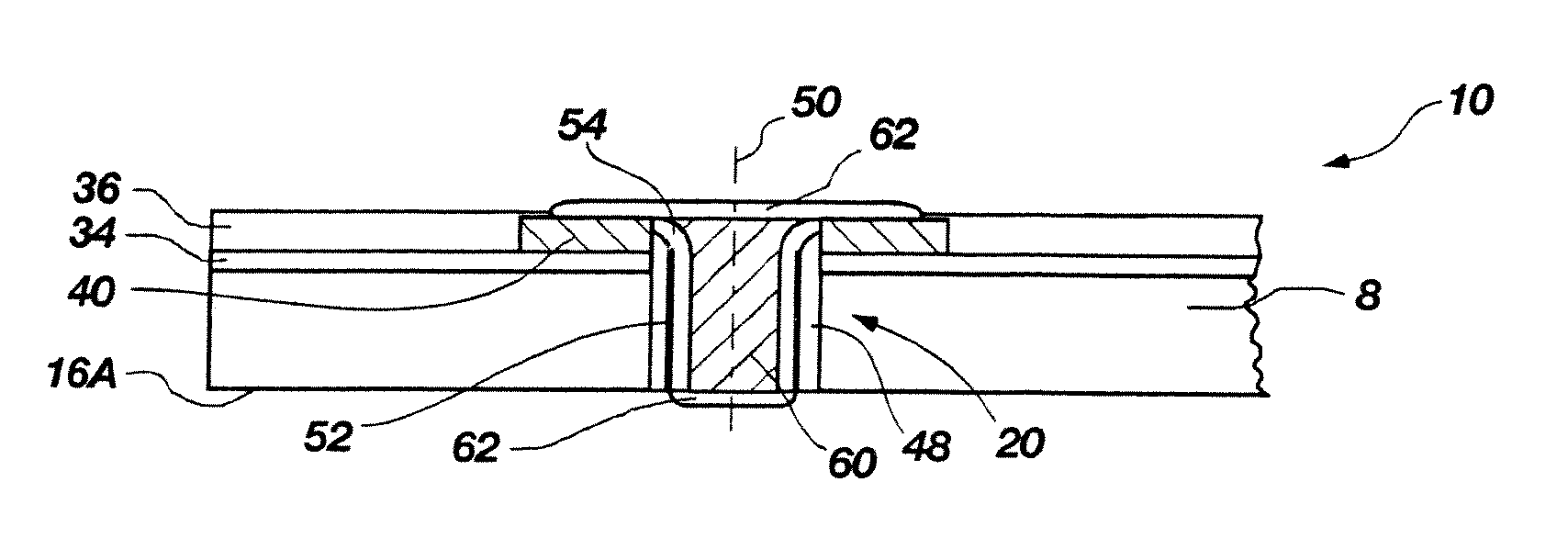
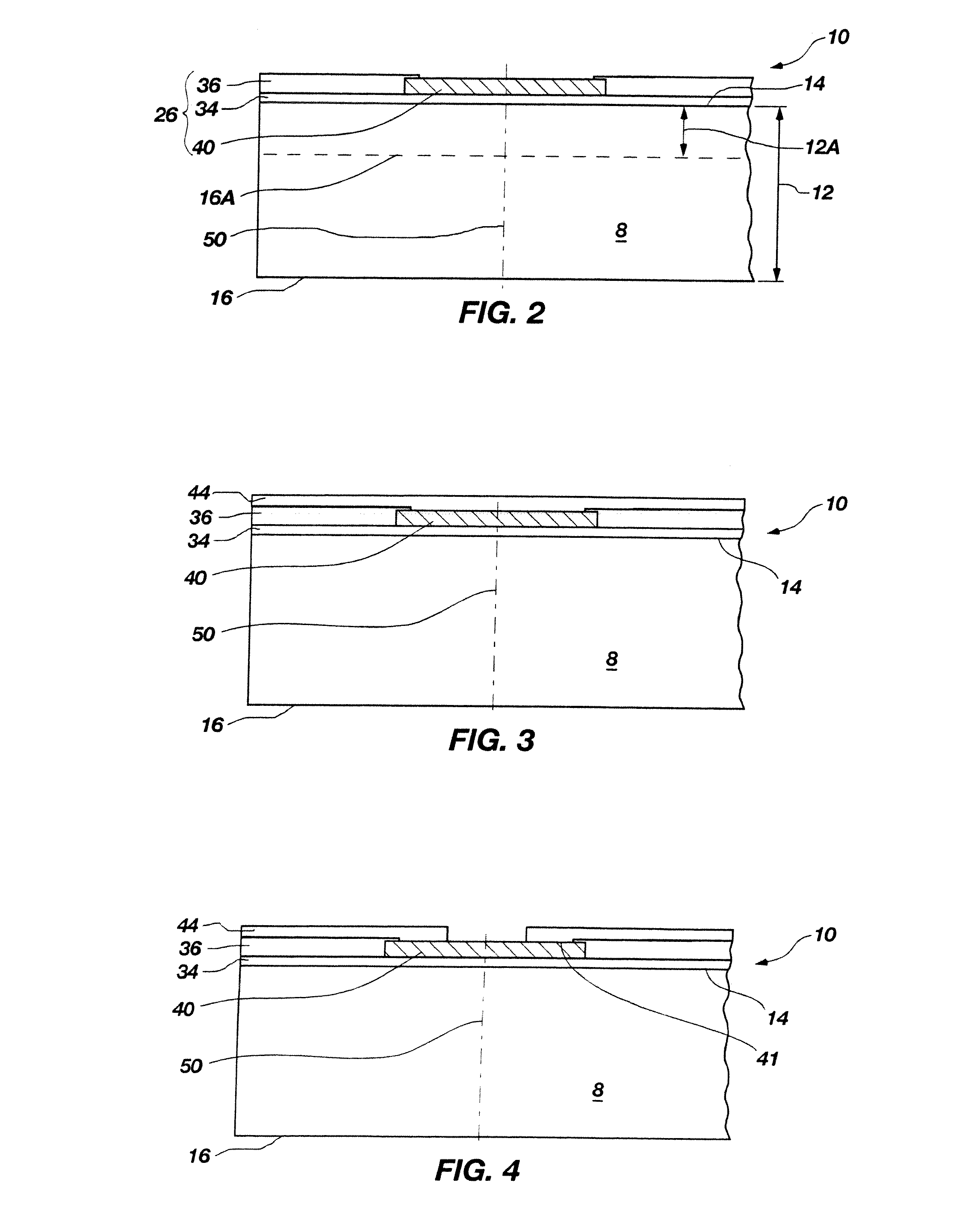
Methods for forming through-wafer interconnects, intermediate structures so formed, and devices and systems having at least one solder dam structure
ActiveUS20070045779A1Conveniently attachedEliminate cross-contaminationSemiconductor/solid-state device detailsSolid-state devicesVitrificationDevice material
A method for forming through-wafer interconnects (TWI) in a substrate of a thickness in excess of that of a semiconductor die such as a semiconductor wafer. Blind holes are formed from the active surface, sidewalls thereof passivated and coated with a solder-wetting material. A vent hole is then formed from the opposite surface (e.g., wafer back side) to intersect the blind hole. The blind hole is solder filled, followed by back thinning of the vent hole portion of the wafer to a final substrate thickness to expose the solder and solder-wetting material at both the active surface and the thinned back side. A metal layer such as nickel, having a glass transition temperature greater than that of the solder, may be plated to form a dam structure covering one or both ends of the TWI including the solder and solder-wetting material to prevent leakage of molten solder from the TWI during high temperature excursions. Intermediate structures of semiconductor devices, semiconductor devices and systems are also disclosed.
Owner:MICRON TECH INC
Methods for forming through-wafer interconnects and devices and systems having at least one dam structure
ActiveUS20070262424A1Conveniently attachedEliminate cross-contaminationSemiconductor/solid-state device detailsSolid-state devicesVitrificationDevice material
A method for forming through-wafer interconnects (TWI) in a substrate. Blind holes are formed from a surface, sidewalls thereof passivated and coated with a conductive material. A vent hole is then formed from the opposite surface to intersect the blind hole. The blind hole is solder filled, followed by back thinning of the vent hole portion of the wafer to a final substrate thickness to expose the solder and conductive material at both the active surface and the thinned back side. A metal layer having a glass transition temperature greater than that of the solder may be plated to form a dam structure covering one or both ends of the TWI. Intermediate structures of semiconductor devices, semiconductor devices and systems are also disclosed.
Owner:MICRON TECH INC
Reagent Delivery System, Dispensing Device and Container for a Biological Staining Apparatus
ActiveUS20090325309A1Allocation is accurateEliminate cross-contaminationAnalysis using chemical indicatorsTesting/calibration apparatusMicroscope slideStaining
The invention concerns reagent delivery system for an apparatus for processing of biological samples arranged on microscope slides, comprising a reagent section having one or more reagent containers; a slide section in which at least one microscope slide is arranged; a probe for dispensing a portion of reagent onto a predetermined microscope slide, and means for handling the probe. The probe comprises a continuous prove tubing element extending through a rigid probe member and connecting the probe tip to a pneumatic pressure regulation device. The reagent containers are adapted for cooperation with the probe tip. In this manner a high though-put and a very low carry over of fluid residues is achieved since there is no assembled parts making up the inside volume of the probe in which the fluid may be retained.
Owner:AGILENT TECH INC
Cell analysis apparatus and method
ActiveUS20080014571A1Low costShorten the timeBioreactor/fermenter combinationsBiological substance pretreatmentsLiquid mediumDrug compound
Devices and methods that measure one or more properties of a living cell culture that is contained in liquid media within a vessel, and typically analyzes plural cell cultures contained in plural vessels such as the wells of a multiwell microplate substantially in parallel. The devices incorporate a sensor that remains in equilibrium with, e.g., remains submerged within, the liquid cell media during the performance of a measurement and during addition of one or more cell affecting fluids such as solutions of potential drug compounds.
Owner:AGILENT TECH INC
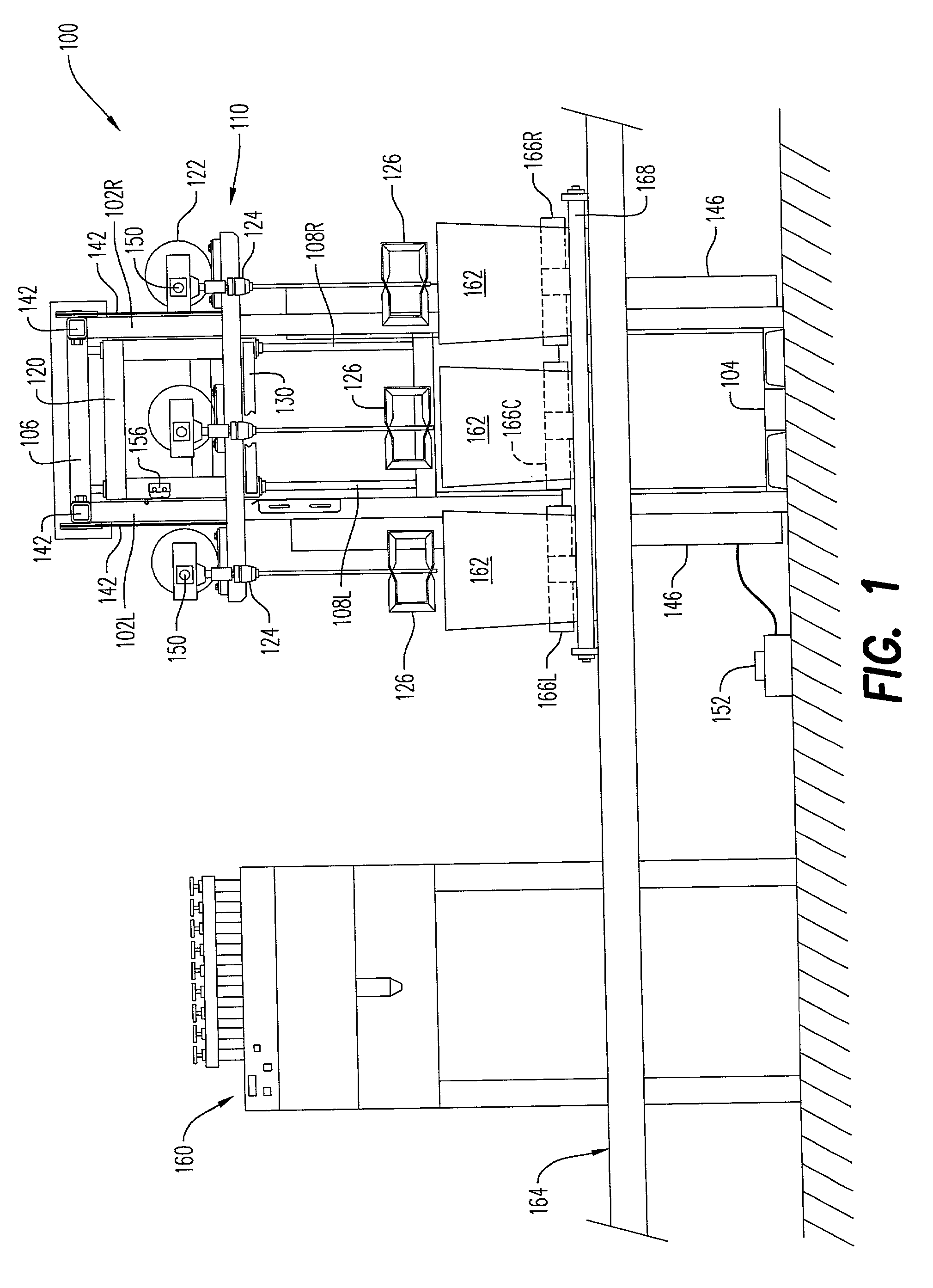
Driven load-bearing system
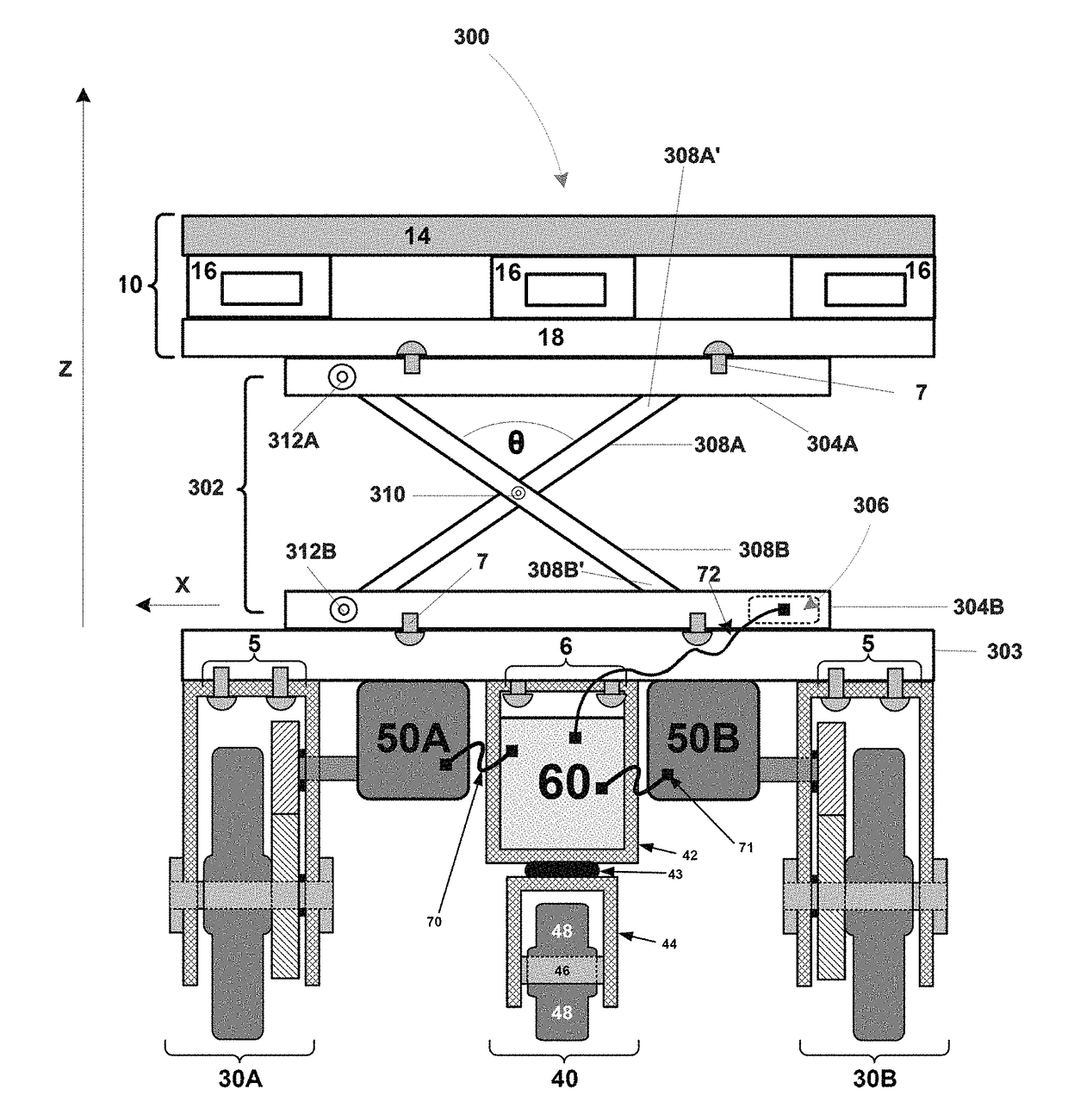
ActiveUS20170253283A1Avoid damageEliminate cross-contaminationNon-electrical signal transmission systemsLifting devicesElectric cablesBraking system
A device for supporting and moving a load within an area may include a motive construct configured to support the load and move the load to a ground location within the area; a lift mechanism coupled to the motive construct, the lift mechanism being configured to effect movement of the motive construct to a height relative to the ground location; and a remote controller operatively coupled to the device via at least one of a cable connection and a wireless connection, the remote controller being configured to steer the motive construct and / or to control the lift mechanism. The device may further include at least one sensor to detect a characteristic of the load. The device may further include a braking system.
Owner:EIDELSON ARTHUR
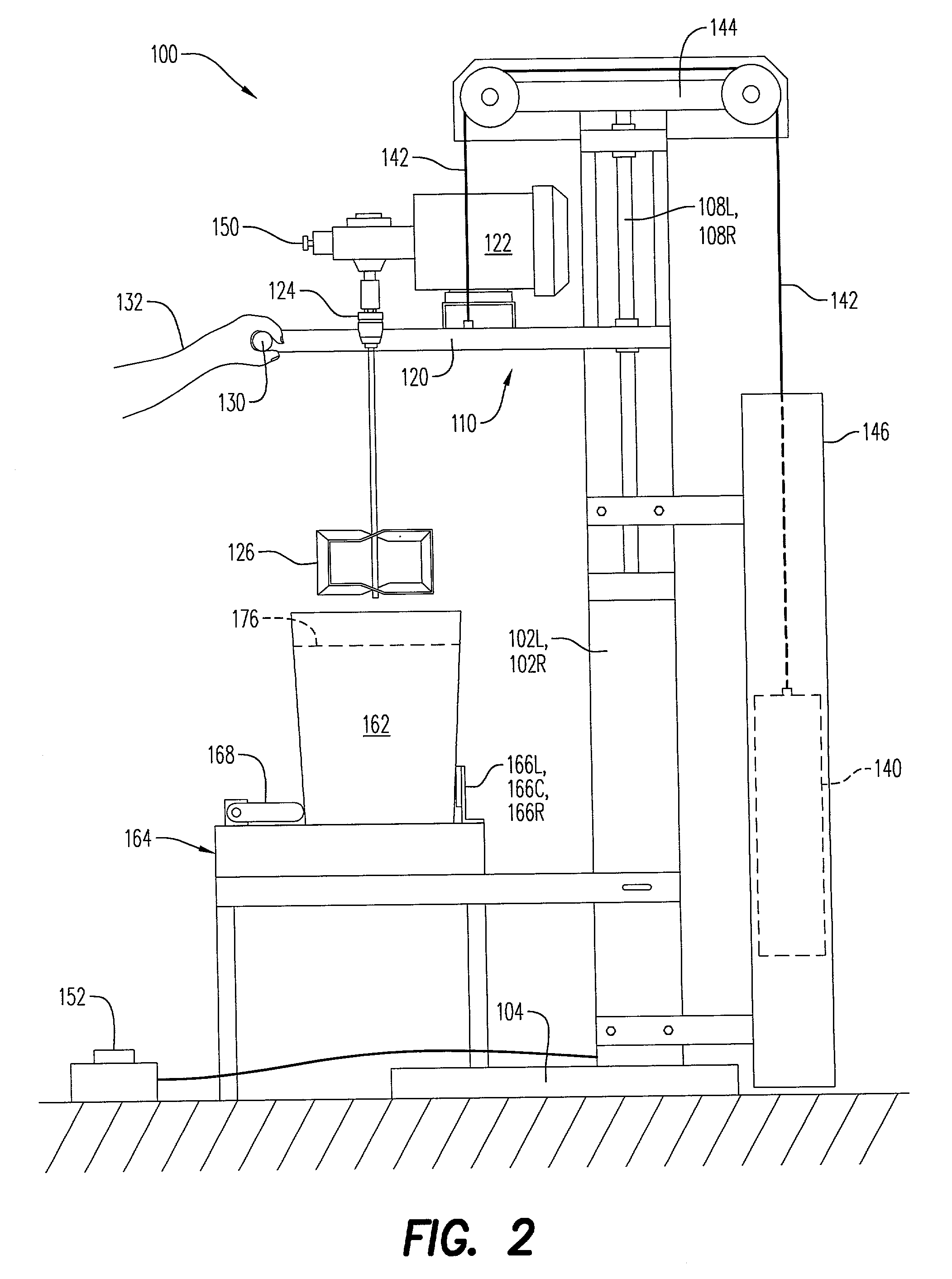
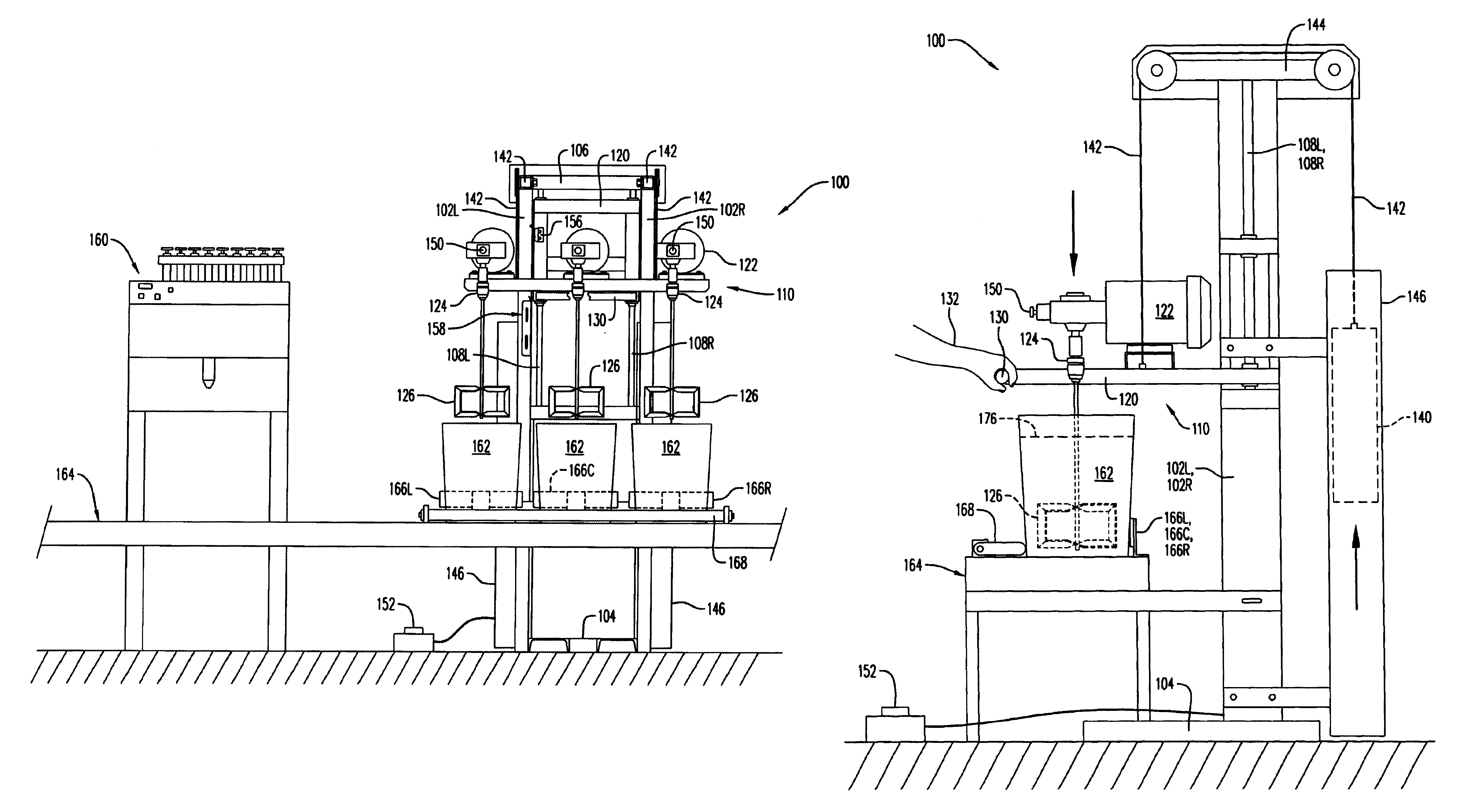
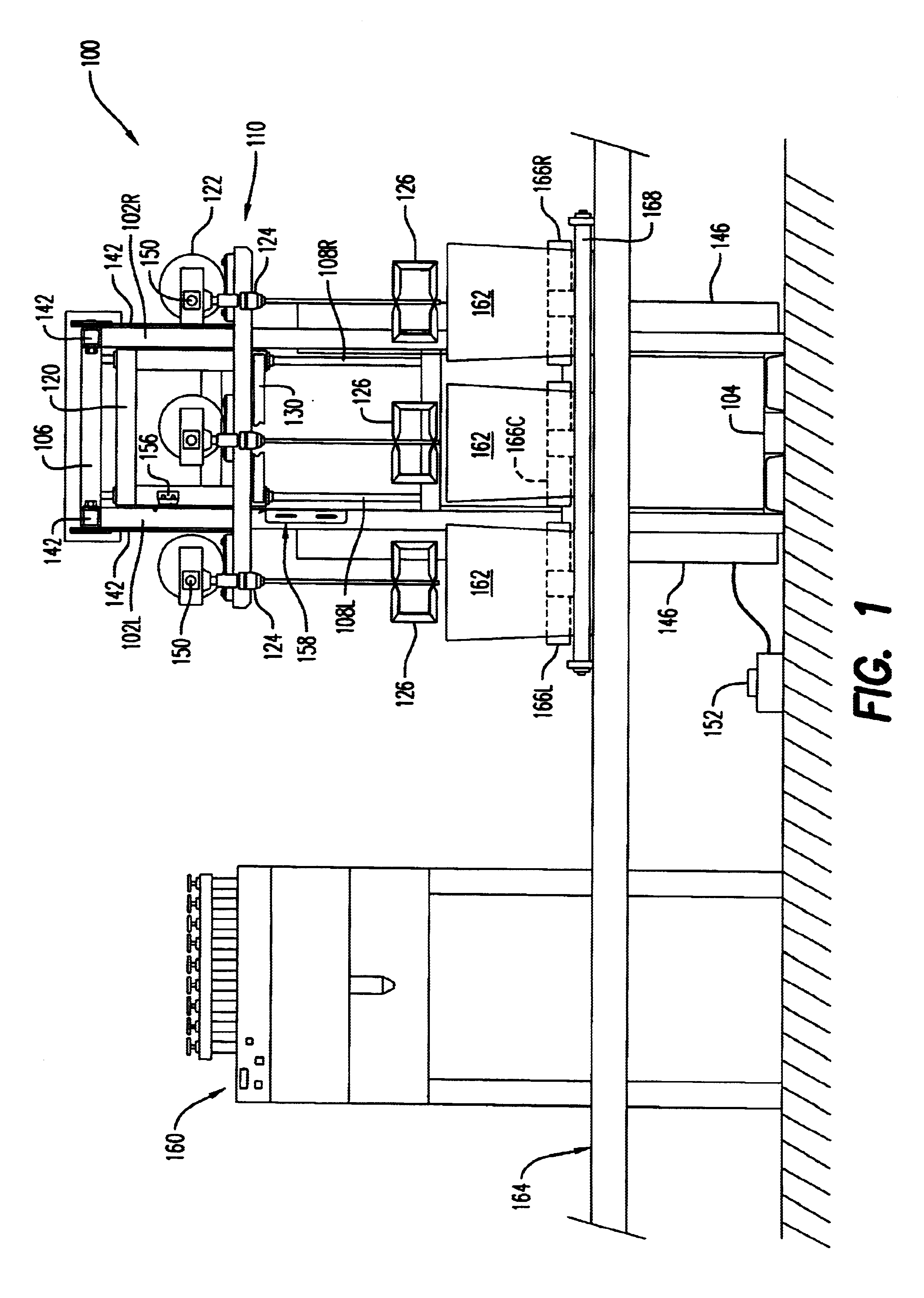
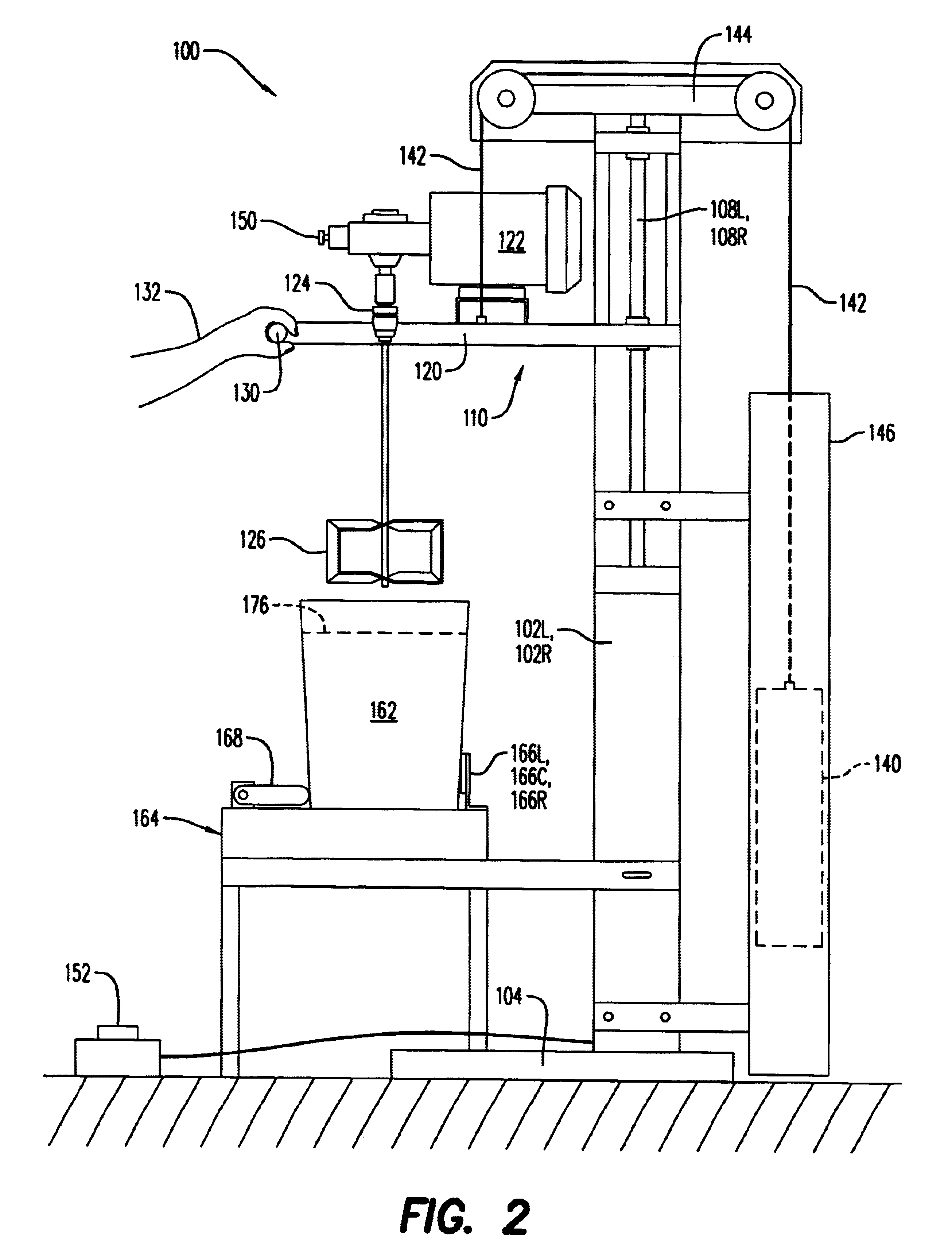
Stirring apparatus for large containers
InactiveUS20030058734A1Safe handlingMinimize messShaking/oscillating/vibrating mixersOther chemical processesAdditive ingredientContamination
An apparatus for safely handling and securely holding a multitude of large and heavy open topped pails, for loading ingredients into these pails, and for stirring the ingredients within these pails into a fully homogenized state. The apparatus is adapted for simple removal and easy cleaning of the stirring components, and to minimize mess and eliminate cross contamination.
Owner:I C T C HLDG
Cell analysis apparatus and method
ActiveUS8658349B2Low costShorten the timeBioreactor/fermenter combinationsBiological substance pretreatmentsLiquid mediumDrug compound
Devices and methods that measure one or more properties of a living cell culture that is contained in liquid media within a vessel, and typically analyzes plural cell cultures contained in plural vessels such as the wells of a multiwell microplate substantially in parallel. The devices incorporate a sensor that remains in equilibrium with, e.g., remains submerged within, the liquid cell media during the performance of a measurement and during addition of one or more cell affecting fluids such as solutions of potential drug compounds.
Owner:AGILENT TECH INC
Device and microchip for sorting particles
InactiveCN102317755AEliminate cross-contaminationEliminate biohazardsBioreactor/fermenter combinationsBiological substance pretreatmentsFlow cellHigh velocity
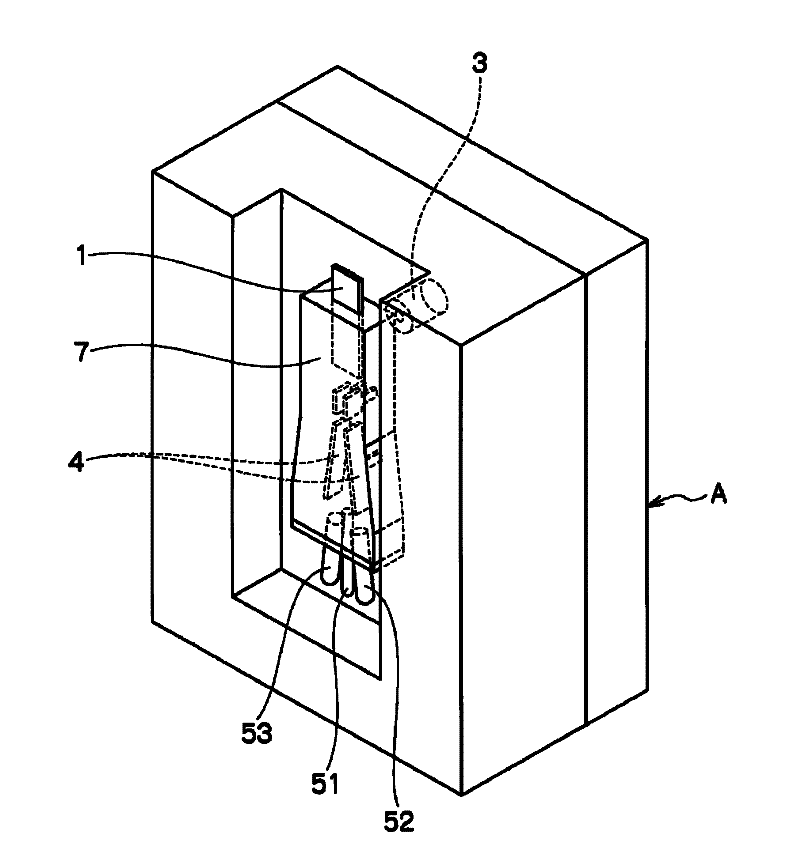
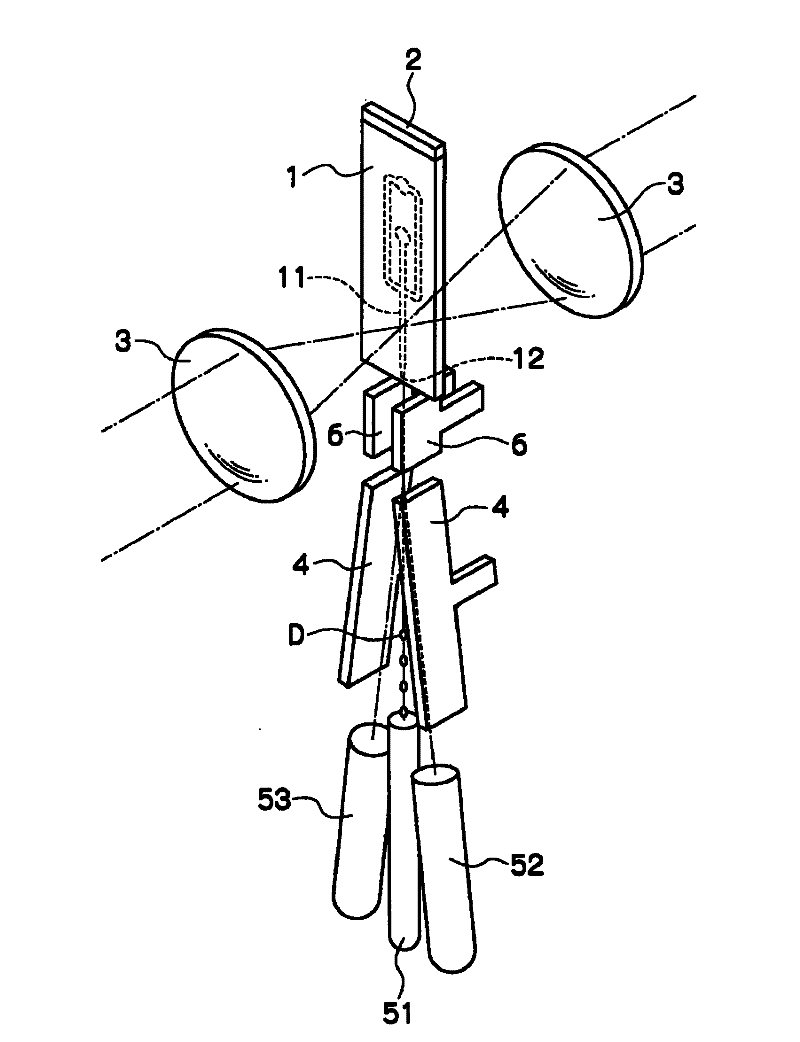
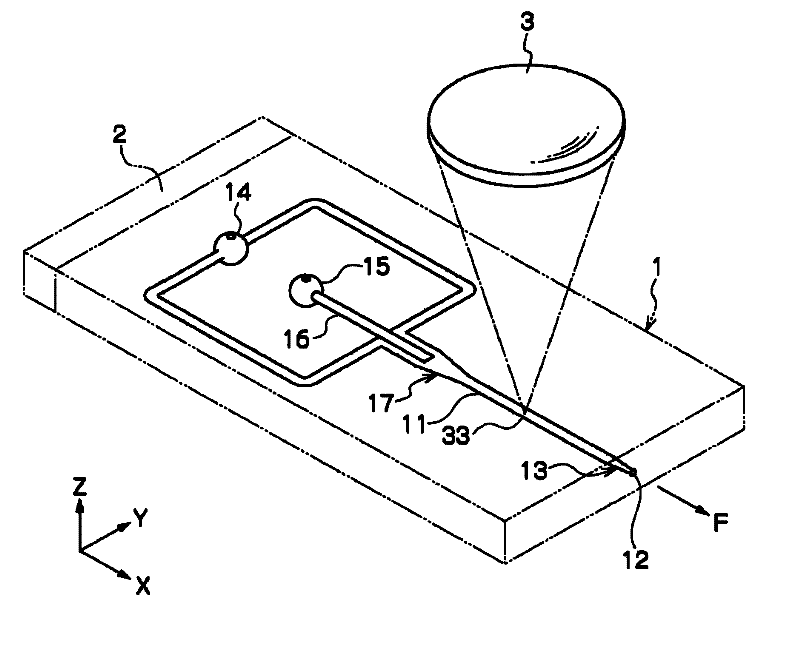
Disclosed is a sorting device capable of high-speed analysis and sorting with safety, high speed, and low cost by preventing cross-contamination between samples, contamination of samples, biohazard to the users, obviating the needs for an expensive flow cell and an expensive orifice part and for fine adjustment of the flow cell and the orifice. The device (A) for sorting particles is provided with a microchip (1) in which a flow channel (11) through which liquid containing particles is flown and an orifice (12) for discharging the liquid flowing through the flow channel (11) to a space outside the chip are provided, a vibratory element (2) for making and ejecting a droplet of the liquid at the orifice (12), a charging means for giving charge to the ejected droplet (D), an optical detecting means (3) for detecting an optical characteristic of the particles flowing through the flow channel (11), paired electrodes (4,4) arranged along the direction of movement of the ejected droplet (D) and opposed with the moving droplet (D) therebetween, and two or more vessels for collecting the droplet (D) passing between the paired electrodes (4, 4).
Owner:SONY CORP
Loading system for an encapsulation device
InactiveUS9433557B2Avoid cross contaminationOvercome disadvantagesImmobilised enzymesBioreactor/fermenter combinationsElectrical batteryBiomedical engineering
The present invention provides, in at least one embodiment, a loading device, system and method that loads aggregate cells into an encapsulation device for implanting into a patient. The loading system uses negative pressure from a low pressure pump in a closed system to improve safety and cell viability while allowing for even loading of the encapsulation device.
Owner:VIACYTE INC
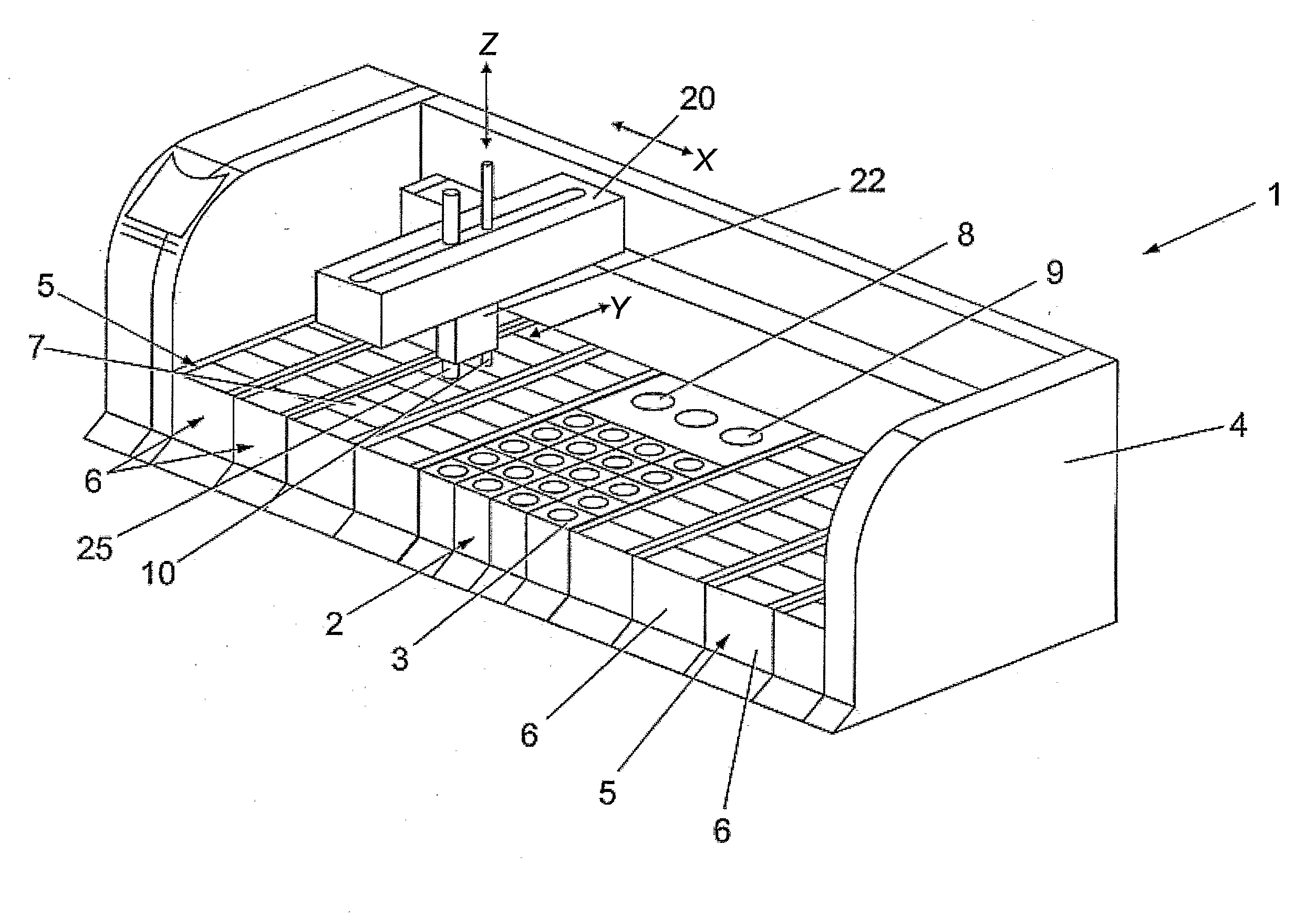
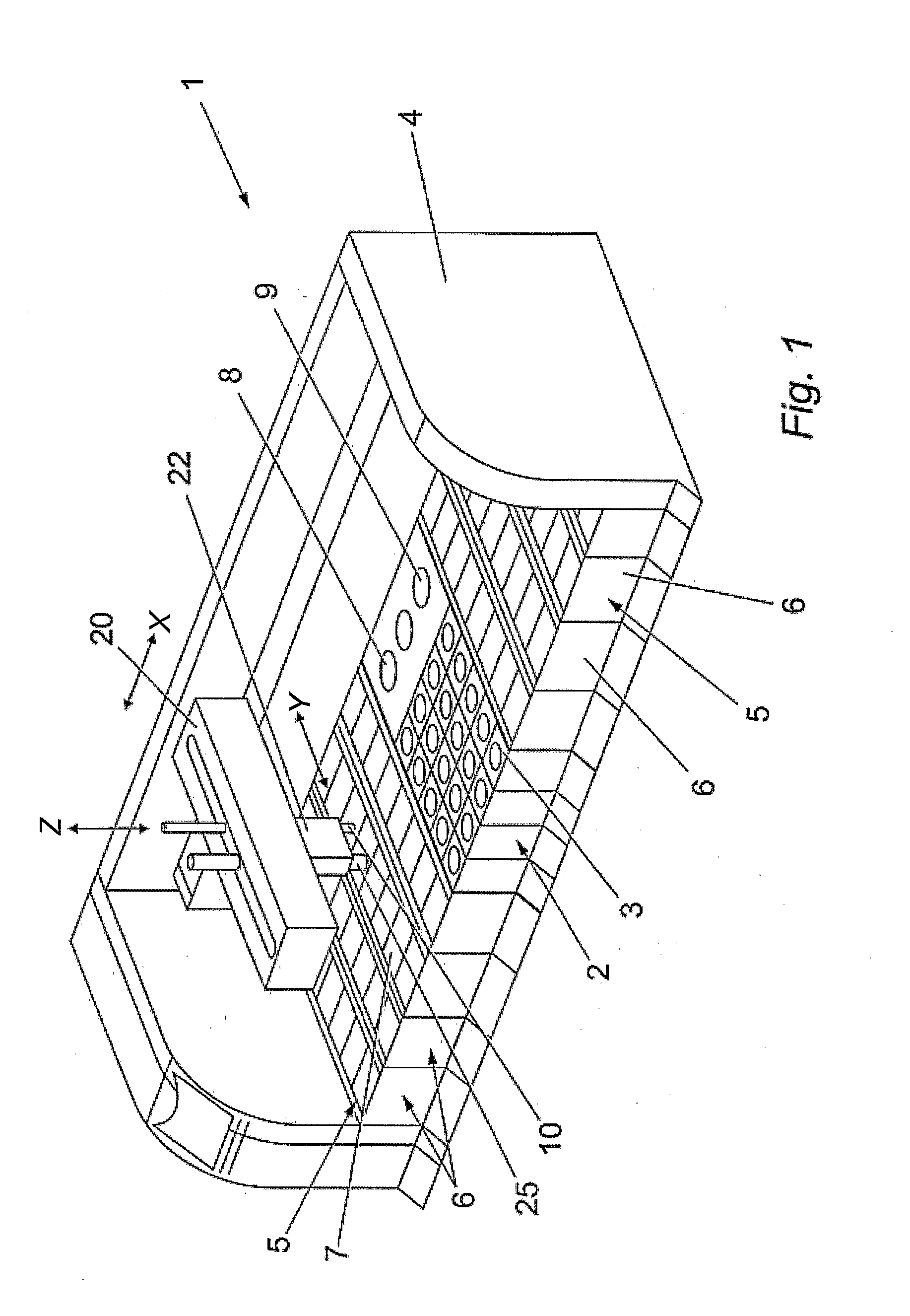
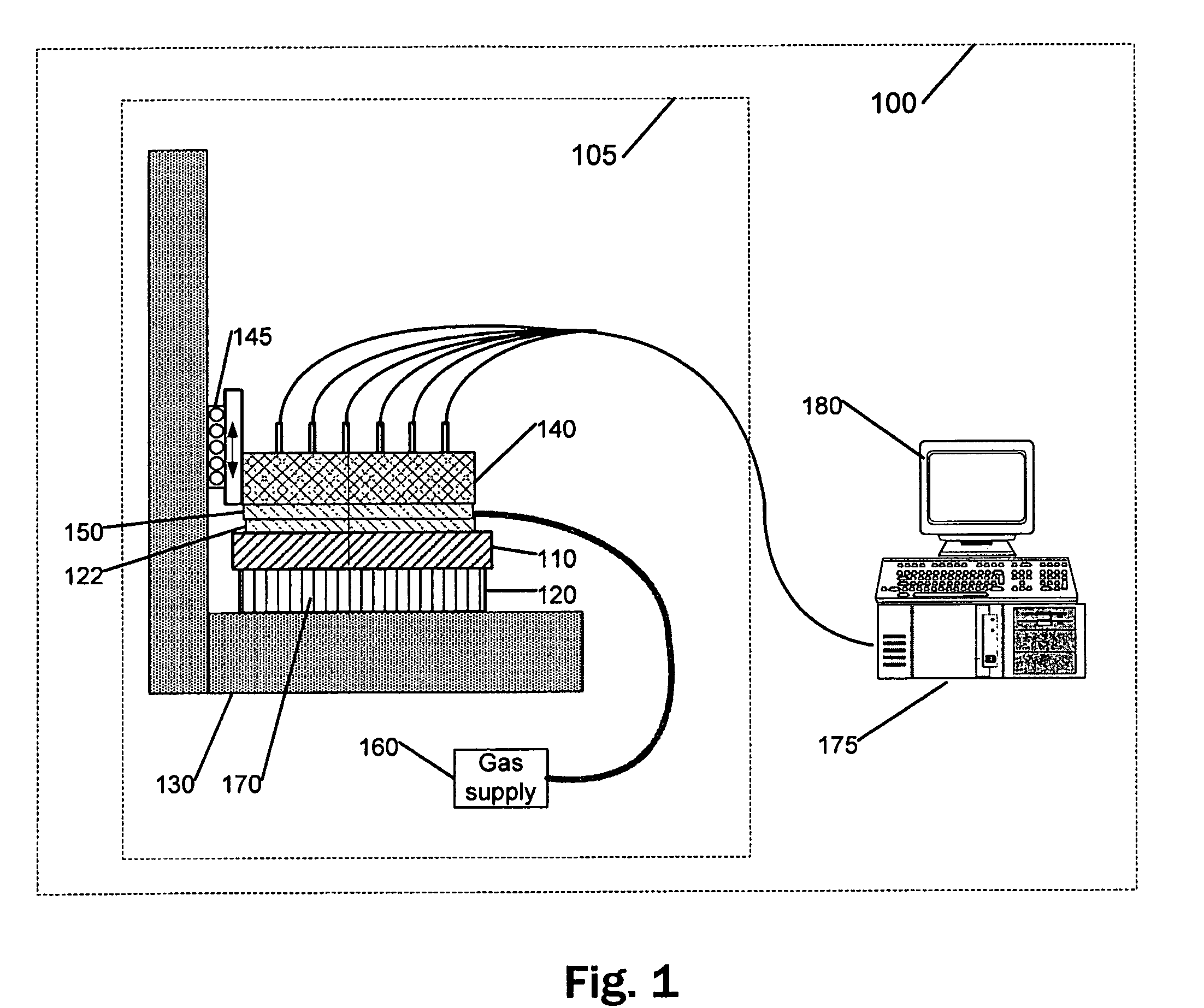
Capillary tube printing tips for microarray printing
InactiveUS20060056904A1Simple printing tipEasy to modifyAnalysis using chemical indicatorsSequential/parallel process reactionsEngineering
A microarray contact printing is formed from at least one capillary tube. The tip has concentric reservoir and printing capillary tubes, with a first capillary tube (24) and a second capillary tube (22) having an inner bore (26) with an inner diameter larger than an outer diameter of the first capillar tube (24) so that the second capillary tube (22) partially overlaps a proximal end of the first capillary tube (24). The first capillary tube (24) has a contact surface (36) at a distal end. The inner bore of the first capillary tube (24) is adapted for drawing the printing solution retained in the second capillary tube (22) and depositing a drop of a solution on a printing substrate when the contact surface (36) is moved proximate the substrate.
Owner:VANDERBILT UNIV
Method for amplifying and genotyping nucleic acid genes of human papilloma virus and assay kit for same
InactiveCN102251056AQuantitatively effectiveEliminate cross-contaminationMicrobiological testing/measurementMicroorganism based processesConserved sequenceAssay
The invention belongs to the technical field of diagnostic reagents, and particularly provides a method for amplifying and genotyping nucleic acid genes of HPV (Human Papilloma Virus) and an assay kit for the same. The method comprises the following steps of: carrying out multiple real-time quantitative fluorescence gene amplifications (QPCR) by using a hybrid primer; quantitatively determining high-risk subtype HPVs by using a quantitative probe; and differentiating HPV 16 and 18 subtypes in types by using a typing probe. The hybrid primer is designed by conservative sequences at two sides of a special area of an E1 gene encoded by the HPV; and the genotyping probe is designed according to a type-specific sequence at the center of the area. Gradient dilutions of recombinant plasmids containing 16 and 18 subtype E1-area genes are taken as standard reference. The kit comprises a cervical brush, a sample storage tube / liquid, a primer, a probe, a Taq enzyme & reaction buffer solution, and a standard contrast reference. The system can be used for quantitatively detecting 13 high-risk subtype HPVs (HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, HPV-68) and differentiating two subtypes HPV-16 and HPV-18 in types.
Owner:JIANGYIN TAIKANG BIOLOGICAL TECH
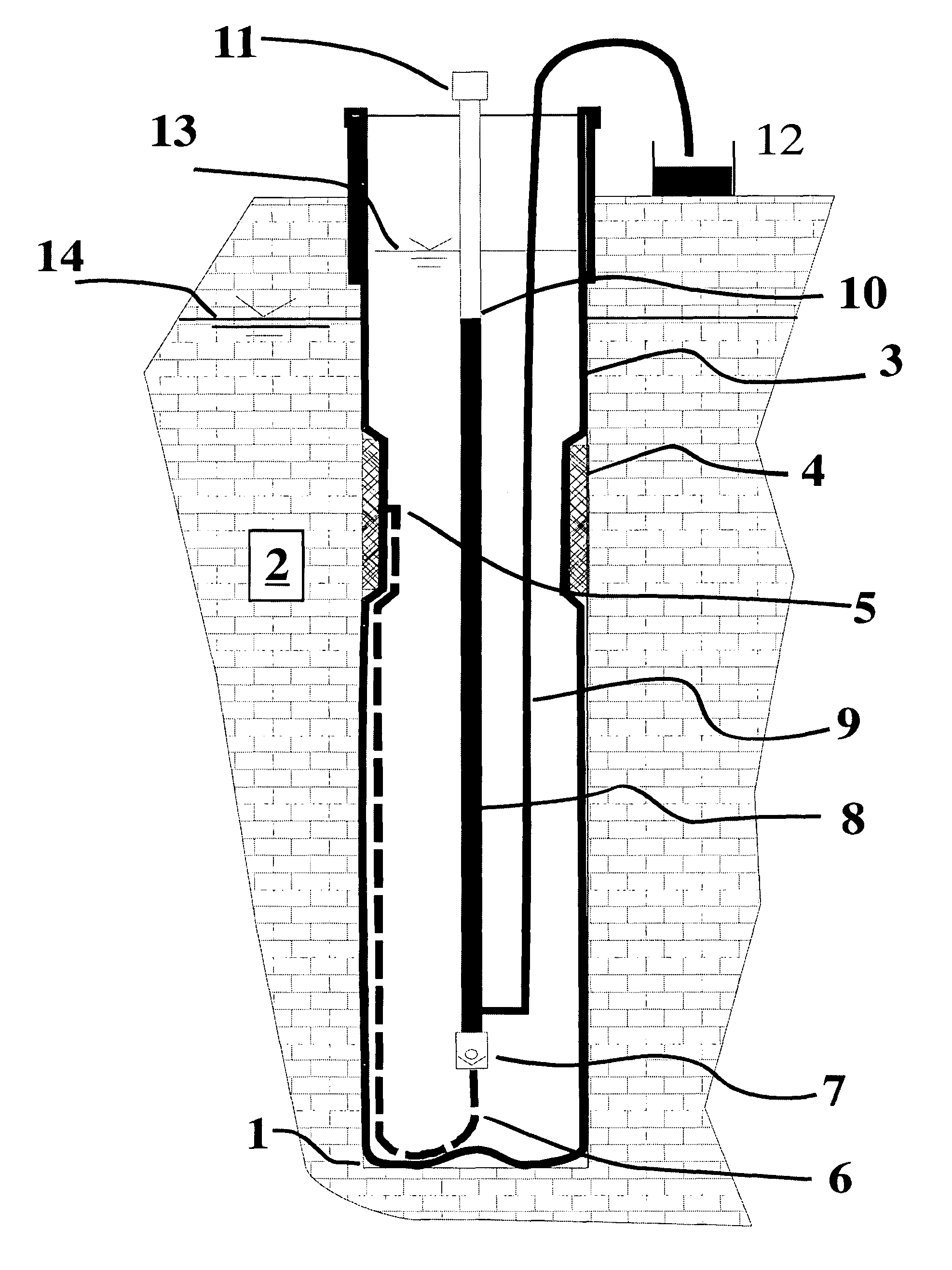
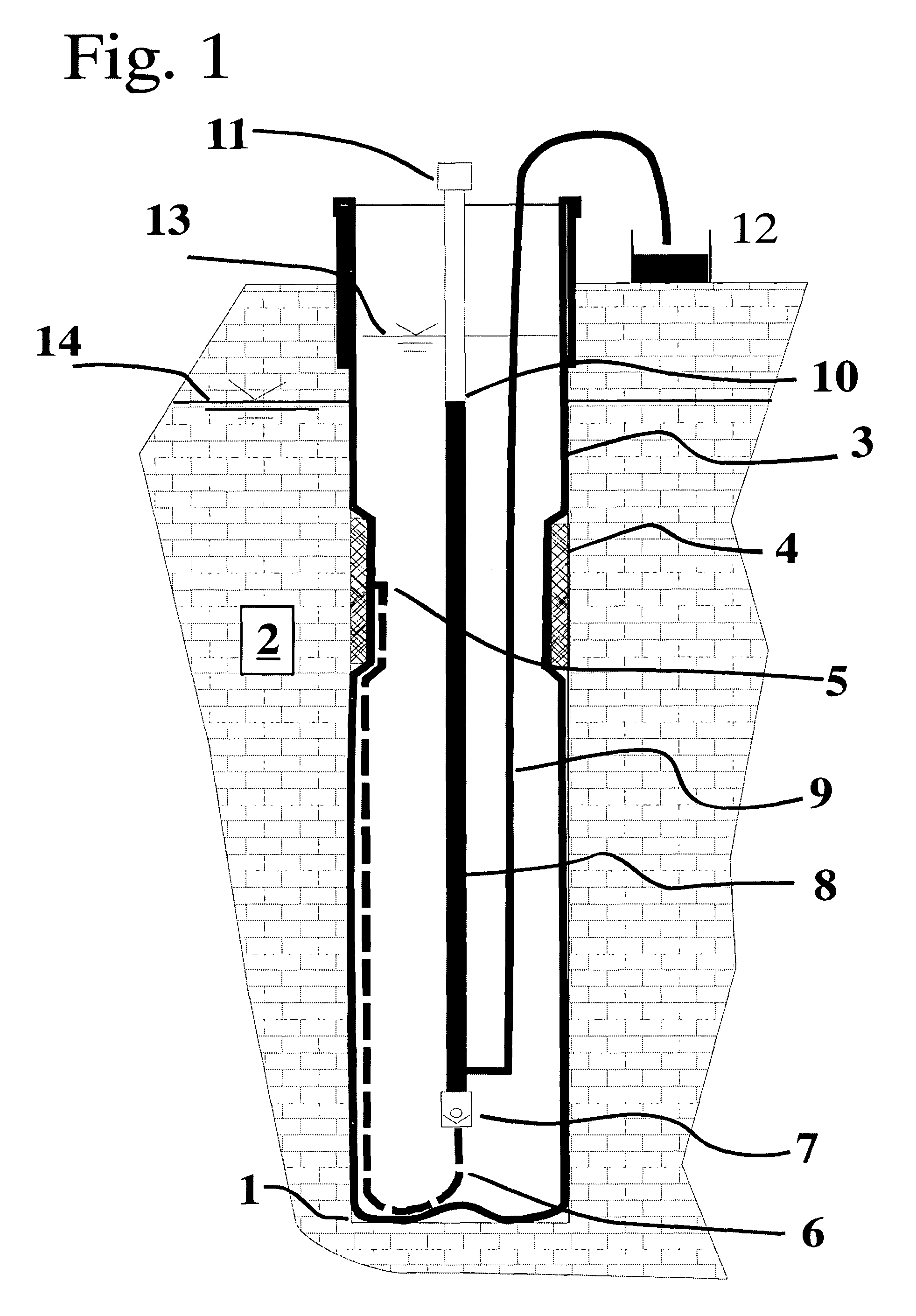
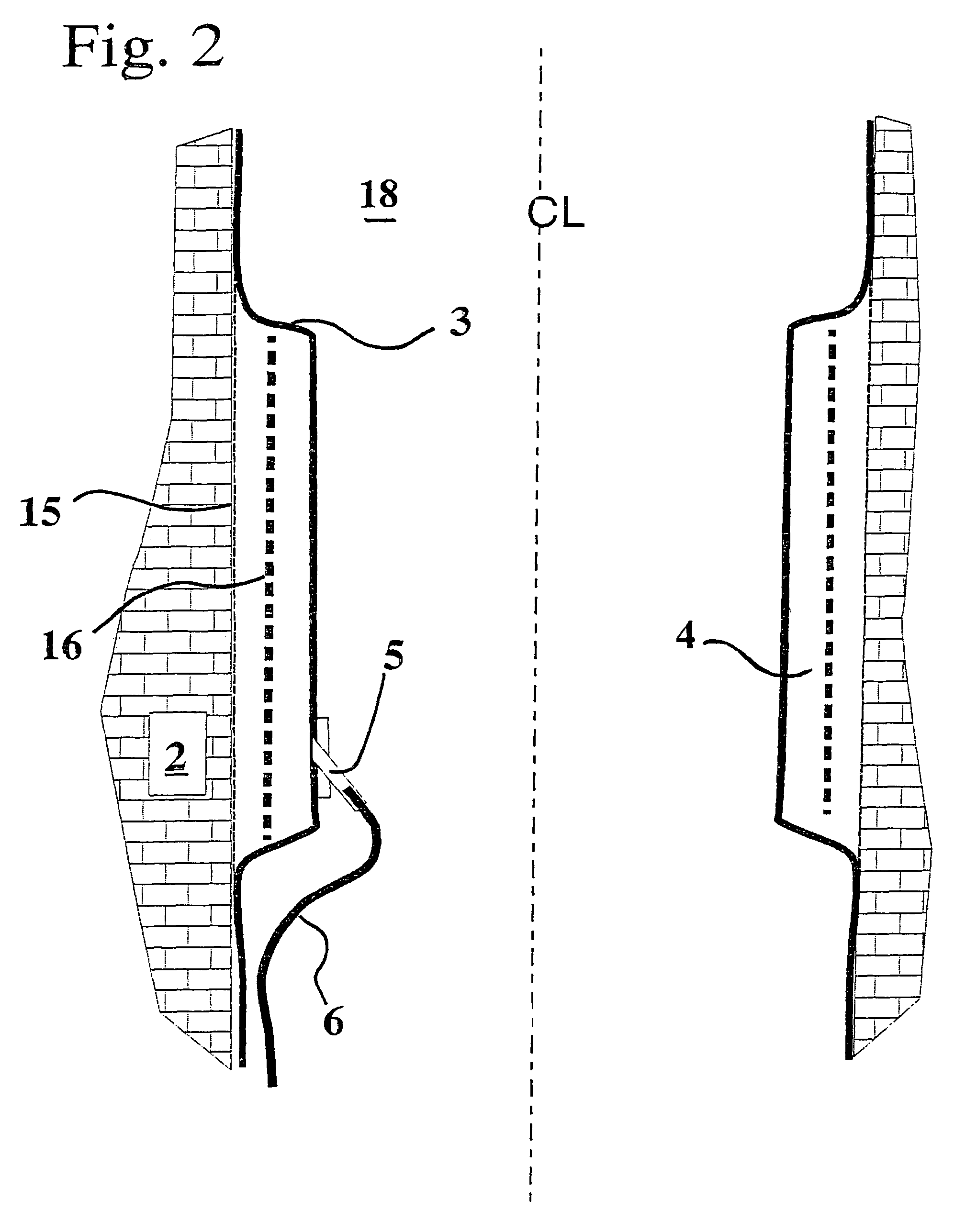
Flexible borehole liner with diffusion barrier and method of use thereof
InactiveUS7841405B2Eliminate cross-contaminationEliminate concernsSurveyDrilling rodsDiffusionDiffusion transport
Owner:KELLER CARL
High performance composite membrane
InactiveCN1304329ALittle change in thicknessImprove effectivenessSemi-permeable membranesLiquid surface applicatorsPorous substrateCross-link
A process for producing high quality reverse osmosis, nanofiltration, and ultrafiltration membranes in a high speed process provides membranes which have excellent rejection characteristics coupled with high flux capabilities. The process employs tandem coating techniques to coat a microporous substrate with a thin membrane on the order of 25 ANGSTROM to 1.0 microns. The tandem coating process comprises tandem offset gravure and subsequent slot die coating applicators, or alternatively comprises tandem slot die coating applicators. For reverse osmosis and nanofiltration membranes, a wet-on-wet coating process is used to coat a porous substrate first with an aqueous solution, and then with an organic solution to produce a cross-linked, interfacially polymerized composite membrane. Single slot coating applicators are utilized to produce ultrafiltration membranes.
Owner:科克梅姆布莱尼系统公司
Cell analysis apparatus and method
ActiveUS20140186876A1Low costShorten the timeBioreactor/fermenter combinationsBiological substance pretreatmentsLiquid mediumDrug compound
Devices and methods that measure one or more properties of a living cell culture that is contained in liquid media within a vessel, and typically analyzes plural cell cultures contained in plural vessels such as the wells of a multiwell microplate substantially in parallel. The devices incorporate a sensor that remains in equilibrium with, e.g., remains submerged within, the liquid cell media during the performance of a measurement and during addition of one or more cell affecting fluids such as solutions of potential drug compounds.
Owner:AGILENT TECH INC
Quick nucleic acid extracting device
ActiveCN108220125AReduce investmentQuick extractionBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acidChemistry
The invention discloses a quick nucleic acid extracting device. The quick nucleic acid extracting device comprises a pushing rod, a hollow tube body and a liquid storage chamber, wherein a bulged structure fixedly arranged on the bottom is arranged in the tube body; the liquid storage chamber is arranged inside the tube body; a liquid release mechanism is arranged on the bottom of the liquid storage chamber; the bulged structure is matched with the liquid release mechanism for releasing liquid inside the liquid storage chamber; and the lower part of the tube body is connected with a nucleic acid adsorption device; and the liquid storage chamber and the pushing rod are arranged, so that the liquid storage chamber and the pushing rod can move up and down inside the tube body.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Multi-well manifold assembly system for oligonucleotide synthesis
InactiveUS20140274809A1Increase pressureReduce cross contaminationLaboratory glasswaresLibrary creationOligonucleotide synthesisEngineering
A multi-well manifold assembly and method for reducing cross-contamination in continuous synthesis reactions in channels of microfluidic devices, for example oligonucleotide synthesis.
Owner:INTEGRATED DNA TECHNOLOGIES
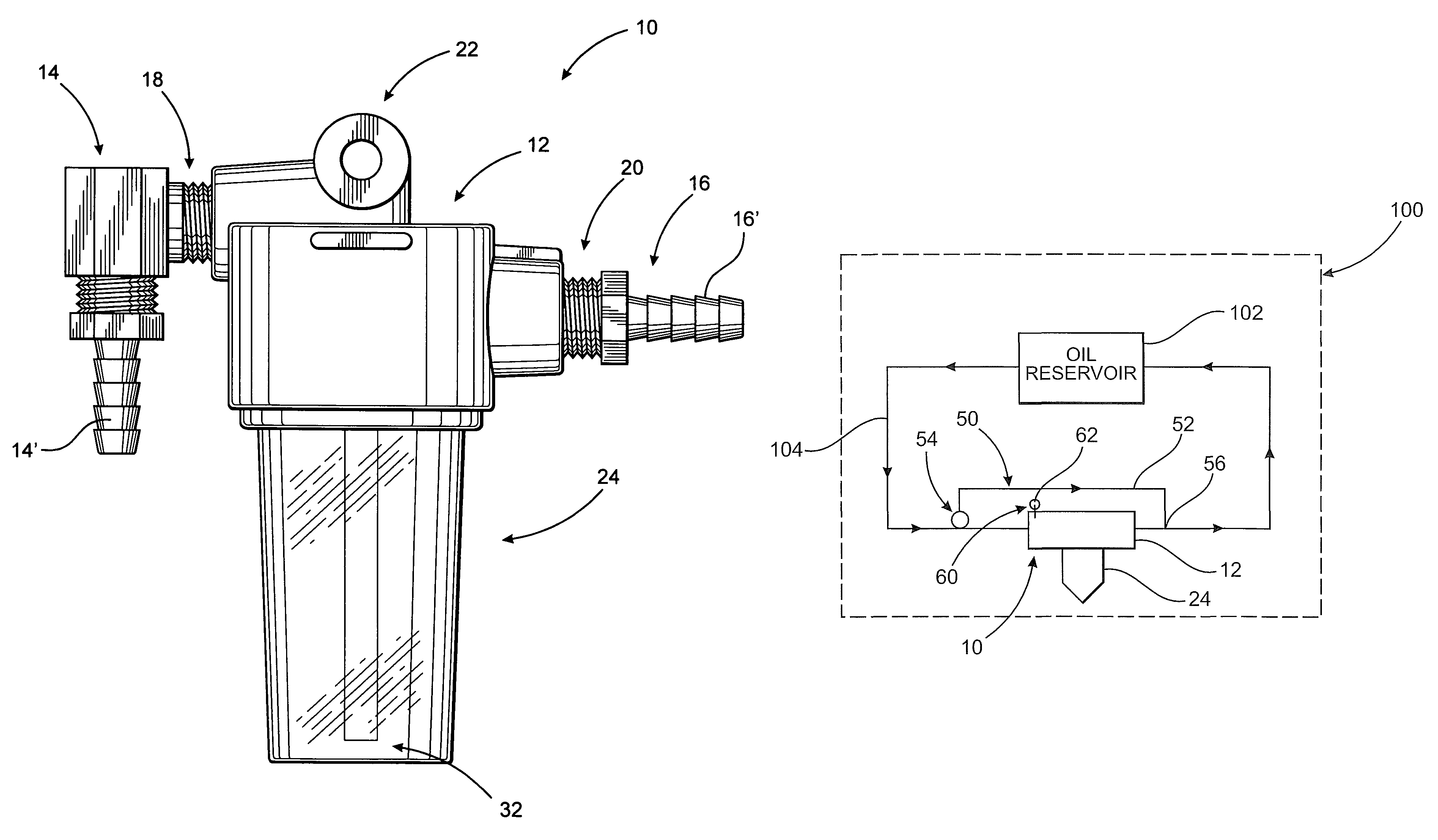
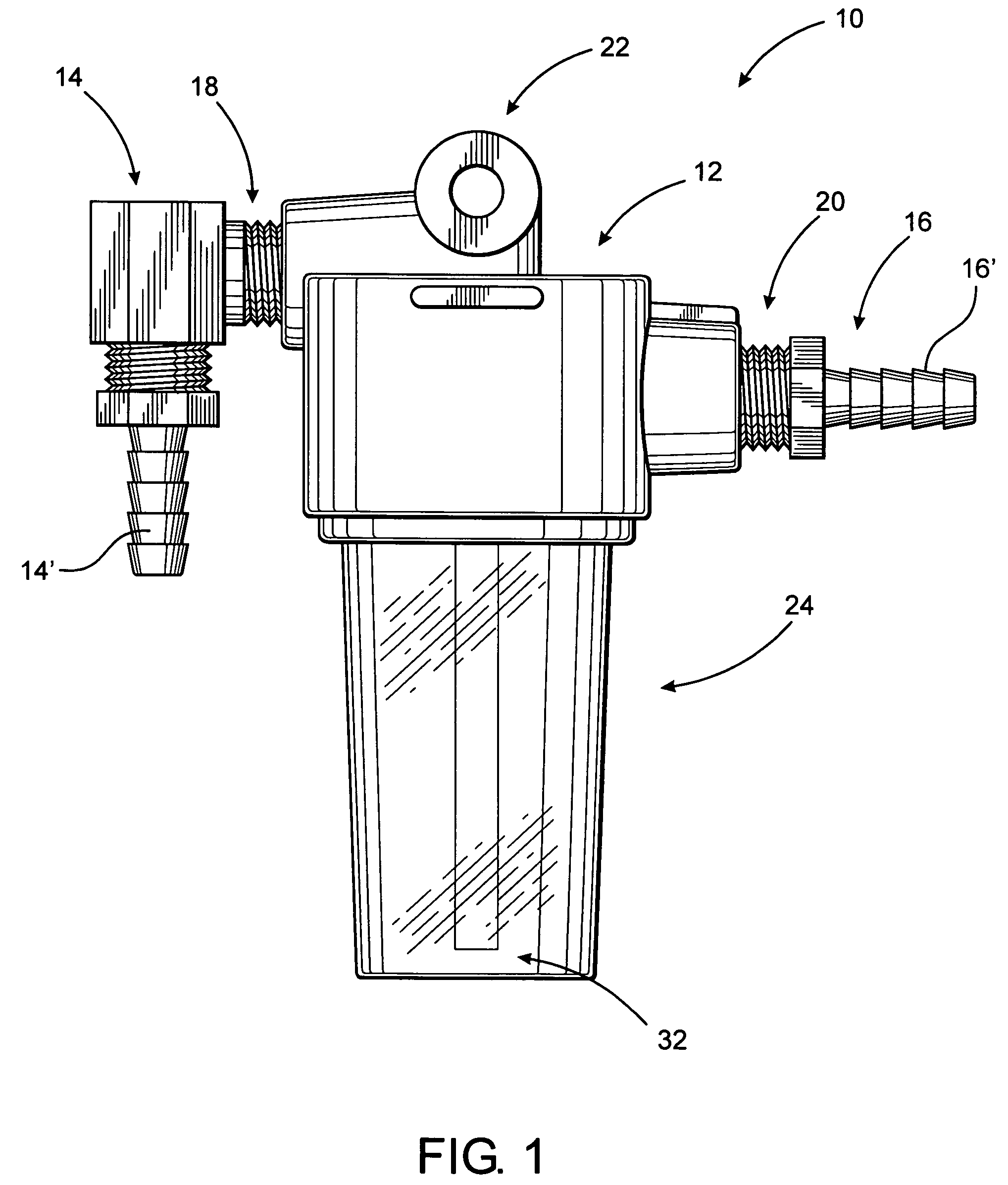
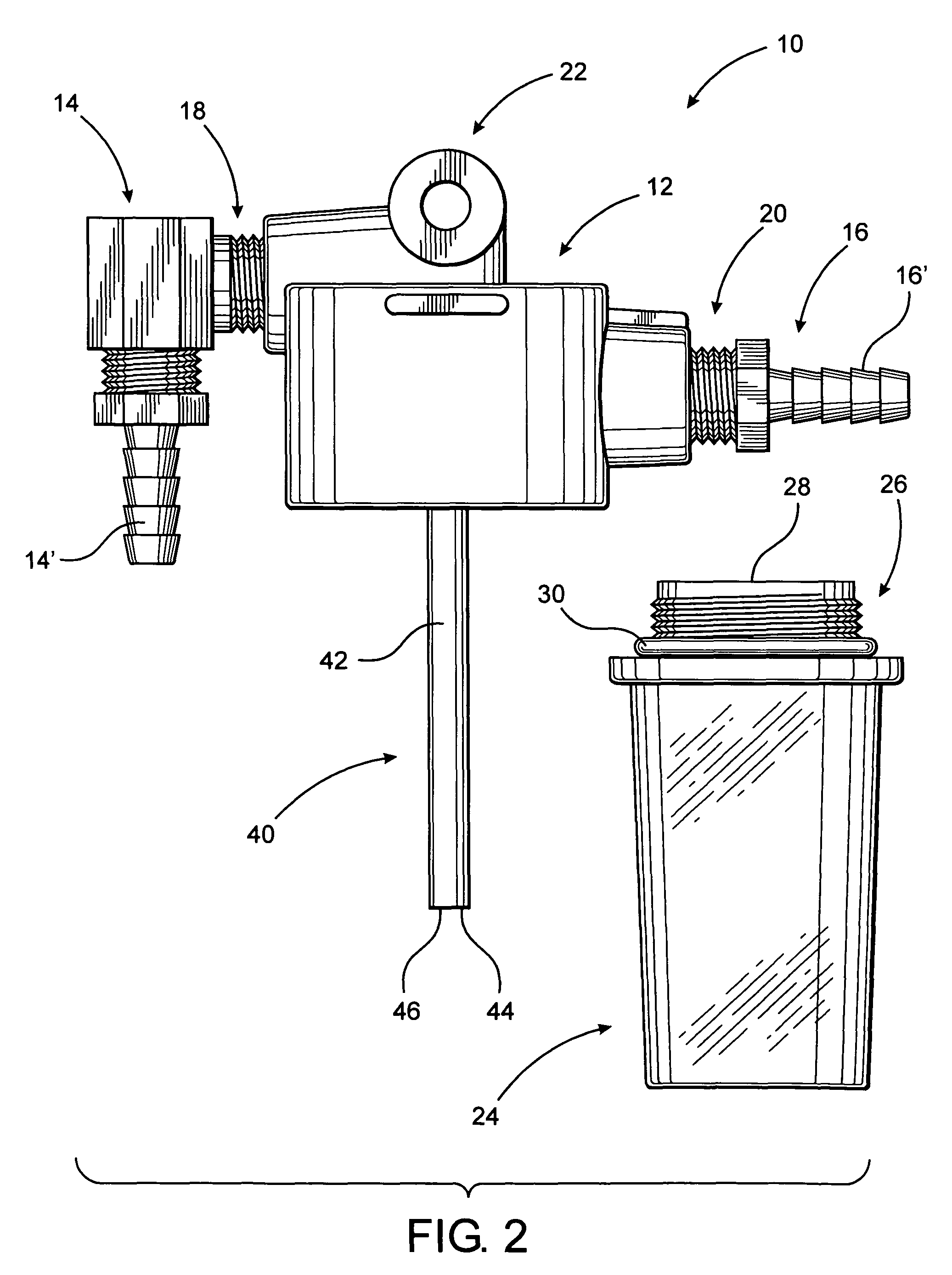
Simplified oil sampling assembly
InactiveUS7938029B2Easy to disassembleEliminate and significantly reduce possibilityWithdrawing sample devicesMaterial testing goodsContinuous flowAssembly structure
Owner:CAMPBELL D MICHAEL +1
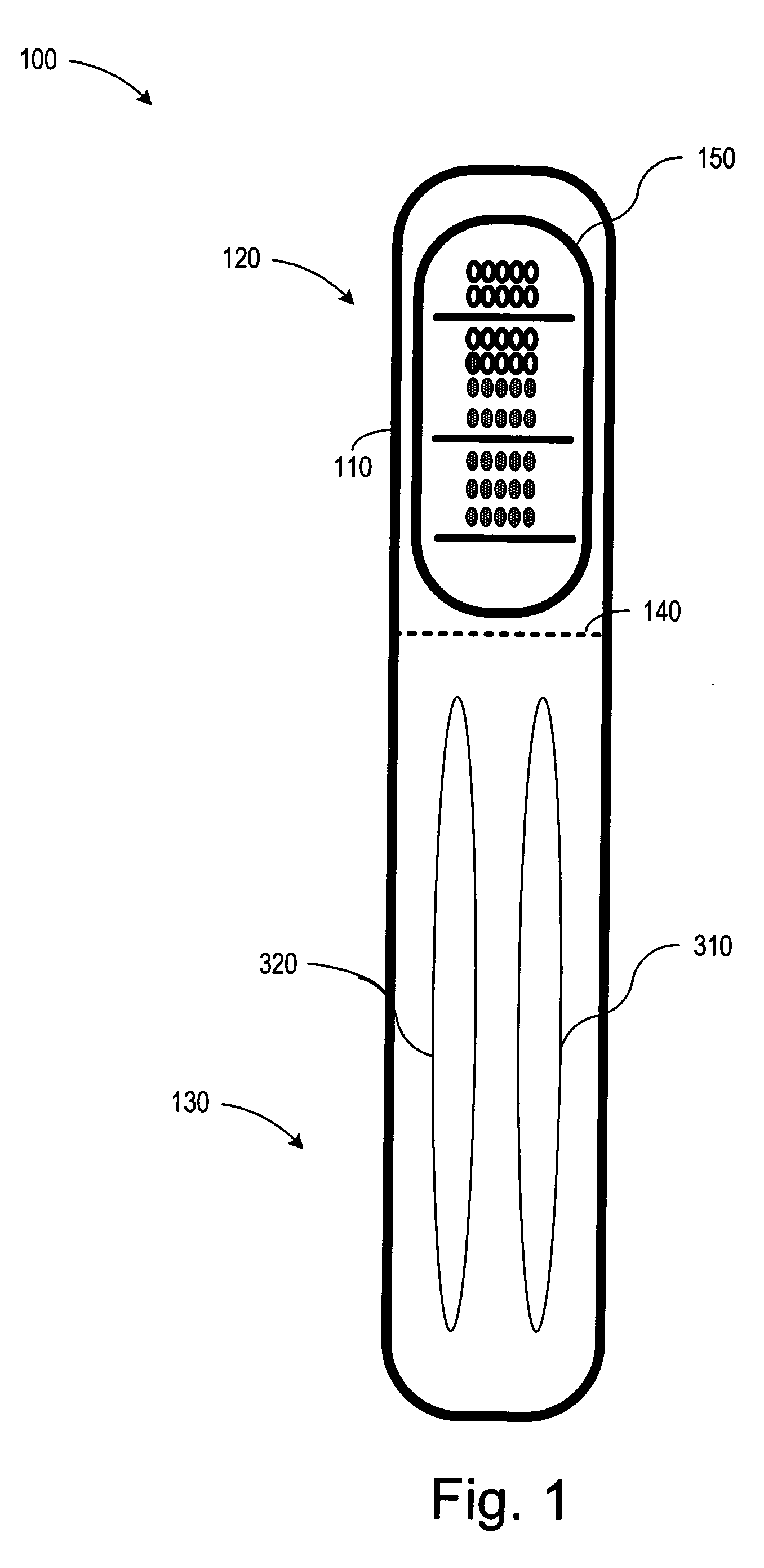
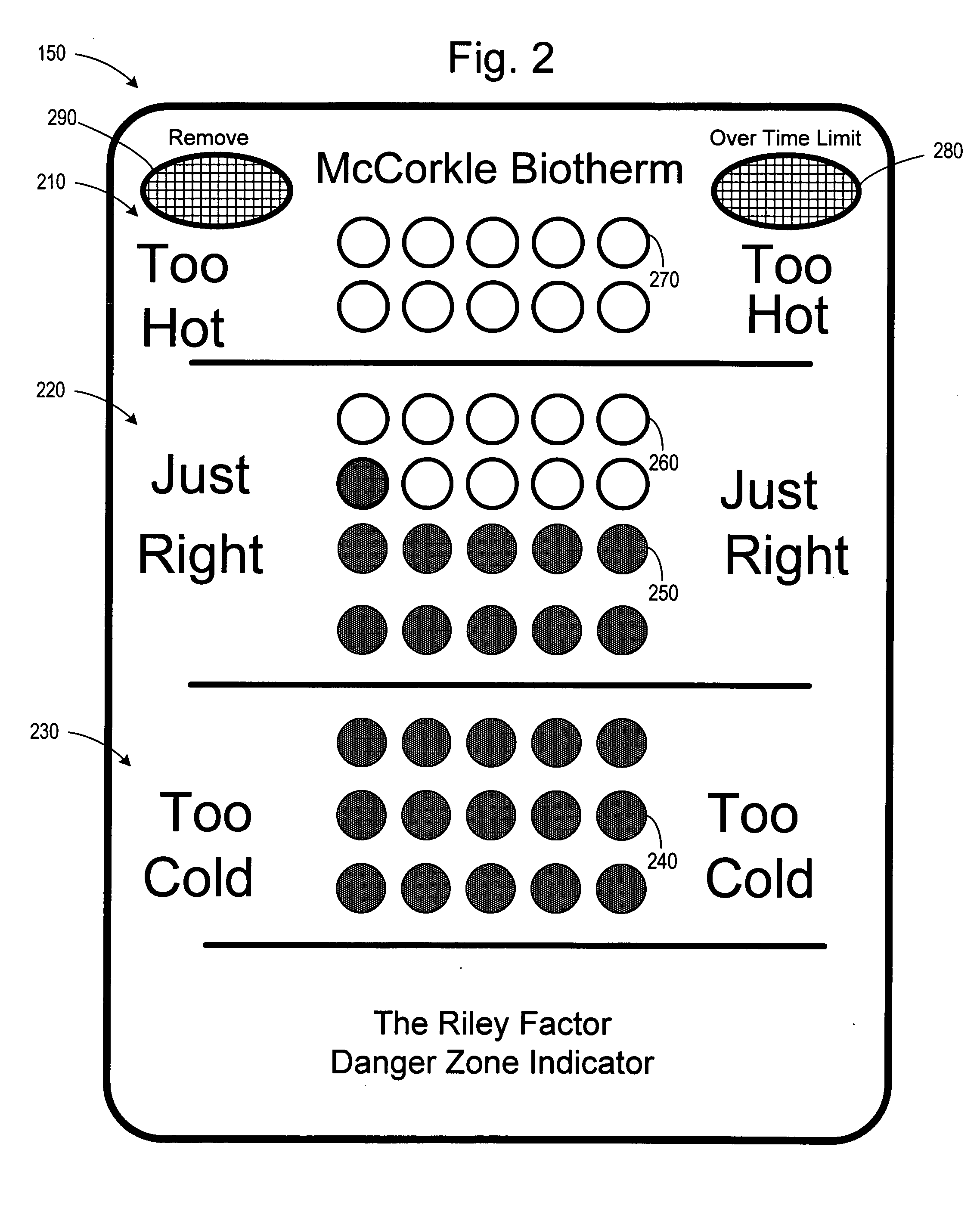
Single-use biotherm for reducing foodborne illnesses
InactiveUS20050188910A1Preventing food cross-contaminationReducing food borne illnessThermometer detailsThermometers using physical/chemical changesFoodborne IllnessesEngineering
A single use disposable thermometer / biotherm including devices and methods for preventing food cross-contamination and reducing foodborne illnesses. A single-use biotherm includes a biotherm body having a proximal end and a distal end, with a frangible portion fracturably attached to the biotherm body. A temperature sensitive arrangement is located in or on the biotherm body. A first temperature indicating arrangement is located in or on the frangible portion and coupled to the temperature sensitive arrangement. Fracturing the frangible portion from the biotherm body captures the temperature indicated by the temperature indicating arrangement. A method of reducing foodborne disease involves providing a single use biotherm; inserting the biotherm into a food product; and receiving an indication of the temperature of the food from the biotherm. The biotherm is then disposed after receiving the temperature indication from the food product.
Owner:MCCORKLE POLLY DEE
Manufacture of biologic cellular products
InactiveUS20060246546A1Avoid cross contaminationEliminate cross-contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsFluoropolymerFermentation
The manufacture of cellular product such as a protein by expression from a cell line in a bioreactor is carried out wherein the bioreactor is a container made of flexible film, at least the interior surface of the container being fluoropolymer, and in addition, the nutrient medium and other agents used in the fermentation or cell culture process can be prepared in a separate container of the same film.
Owner:EI DU PONT DE NEMOURS & CO
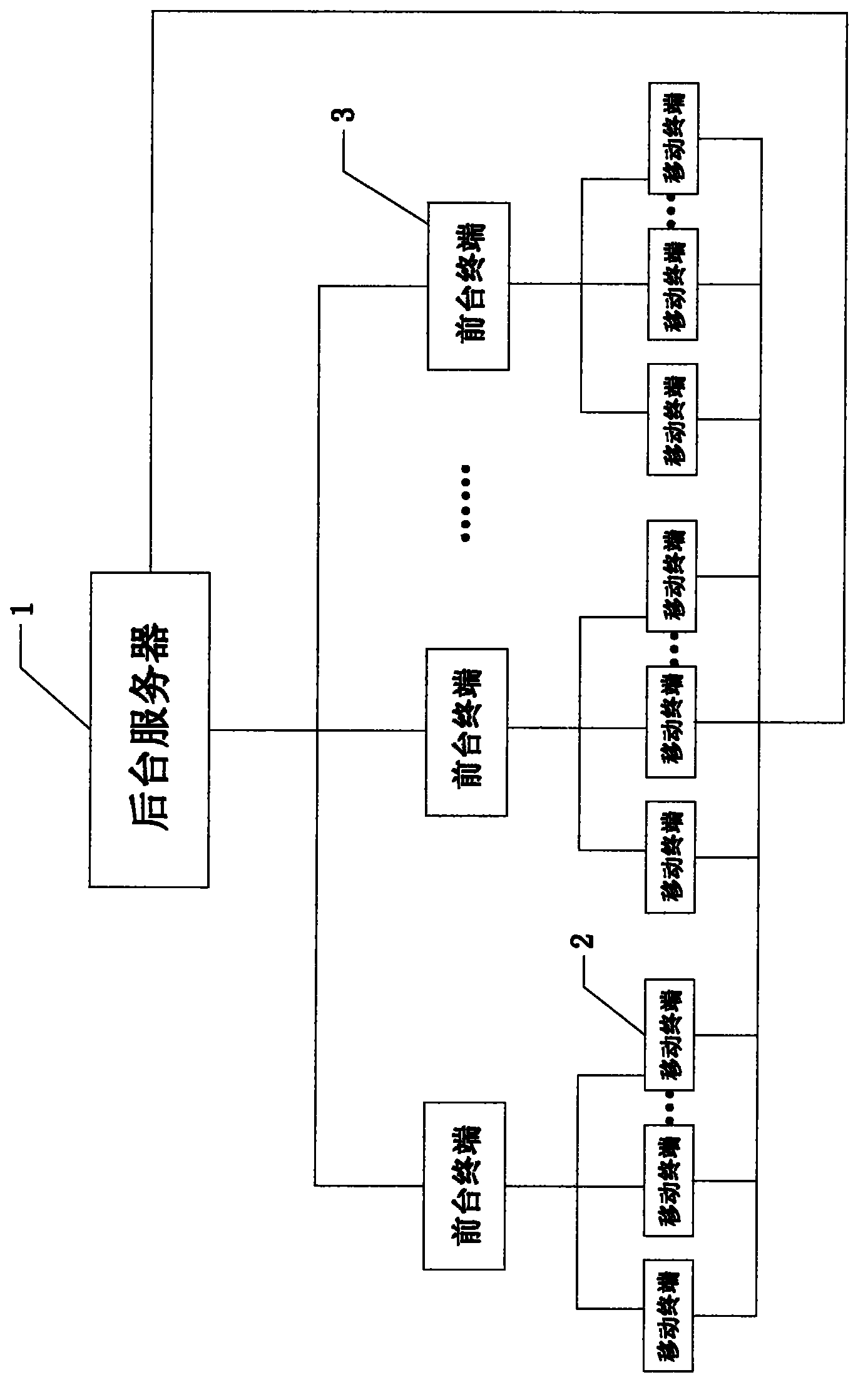
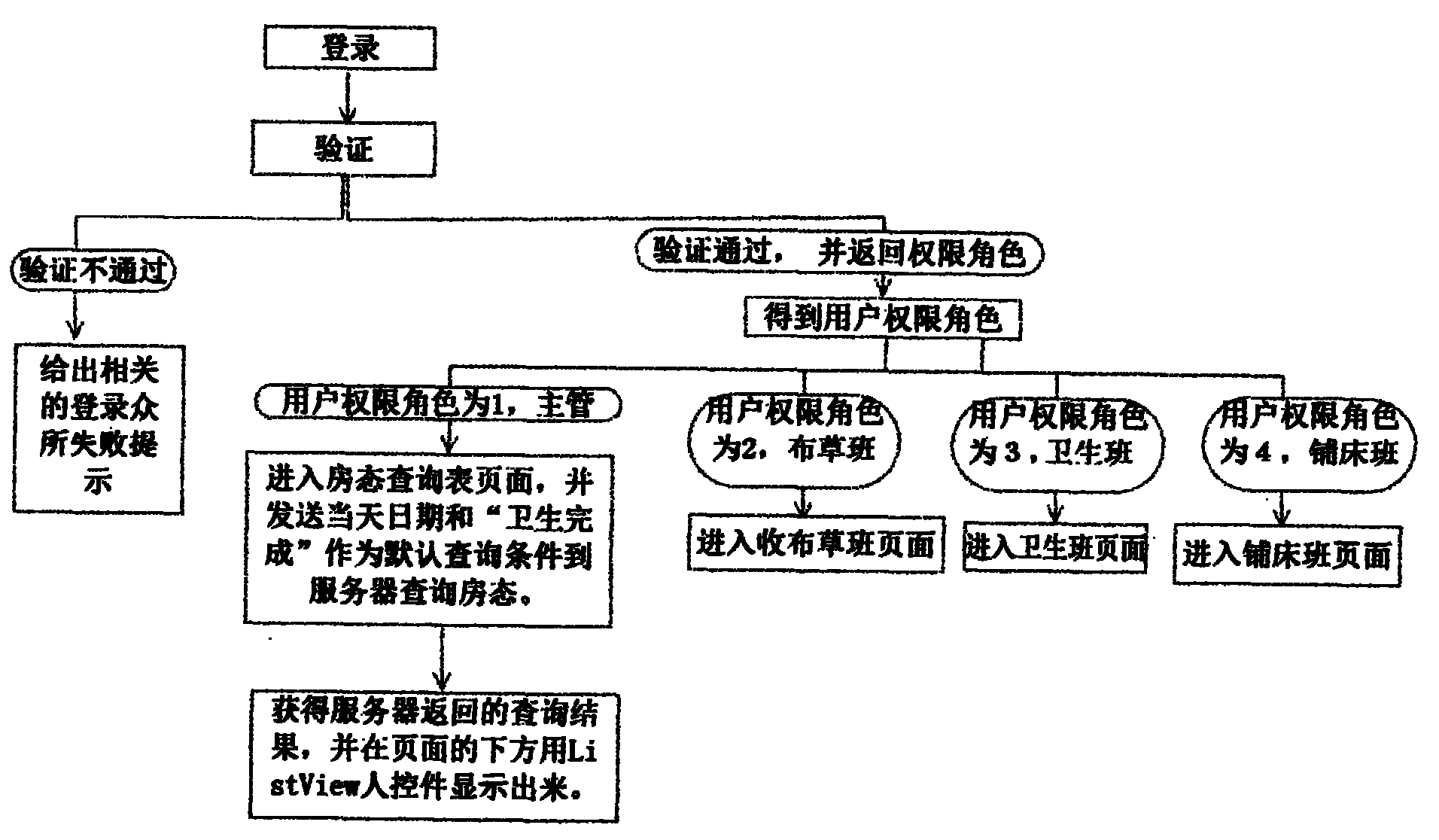
Intelligent hotel workflow management system, process management method and check-in registering method
ActiveCN102938110AAchieve fairnessEliminate the disadvantages of subjective judgmentResourcesWorkflow management systemComputer science
The invention discloses an intelligent hotel workflow management system, a process management method and a check-in registering method. The management system comprises a mobile terminal, a backstage server and a reception terminal, wherein the mobile terminal is respectively connected with the backstage server and the reception terminal, and the backstage server is connected with the reception terminal. The process management method comprises the steps of: logging in a system platform on a mobile terminal, judging a logged privilege role after verification and then carrying out corresponding operation. The check-in registering method comprises the process of: generating an order in the reception terminal, prearranging rooms, finishing check-in registering preparation, handling check-in procedures, serving customers during the process the customers are in the hotel, carrying out check-out procedures by the customers, clearing up check-in records of the customers and confirming the correctness of the check-in records with the customers. According to the management system, the working achievements of staff can be recorded objectively and accurately, the shortage of subjective judgment by supervisors is overcome, and the fairness of the management can be realized to the maximum extent.
Owner:广州市赢商住电子商务有限公司
Reagent disk
The invention provides a reagent disk comprising a substrate, a first rotating shaft rotatablely arranged on the substrate, a reagent rack assembly used for placing a reagent bottle, and a driving element used for driving the first rotating shaft to rotate, wherein the reagent rack assembly comprises a connecting shaft connected with the first rotating shaft. According to the reagent disk provided by the invention, the driving element is used for providing motive power to drive the first rotating shaft and the reagent rack assembly to rotate so as to uniformly mix magnetic bead reagents while placing the reagents; since the uniformly mixed reagent is directly selected in the reagent bottle when needing to select the reagent to perform experimental analysis, the magnetic bead reagents liable to generate settlement are not specially mixed, thereby omitting the time of singly performing the uniform mixing operation, and further shortening the time sequence period of the whole system and improving the integral efficiency of a full-automatic instrument. Moreover, no object directly touches the reagent in the reagent bottle in a working process of the reagent disk, thereby reducing the cleaning liquid consumption and completely eliminating the cross contamination of different reagents.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
Homogeneous florfenicol particles and preparation method thereof
InactiveCN105168148AImprove uniformityFlat surfaceOrganic active ingredientsGranular deliveryLipid formationPolyethylene glycol
The invention provides homogeneous florfenicol particles and a preparation method thereof. The homogeneous florfenicol particles comprises, in percentage by weight, 2%-50% a florfenicol raw material and 50%-98% of an auxiliary material, wherein the auxiliary material is selected from one or more of hydrogenated castor oil, hydrogenated soybean oil, stearic acid, solid lipid wax and solid-state polyethylene glycol. Firstly, the auxiliary material is heated and melted at the temperature of 70 DEG C-90 DEG C and then uniformly stirred, the florfenicol raw material and the melted auxiliary material are mixed, sheared and uniformly stirred, a mixed solution is obtained, sprayed, condensed and granulated, and the homogeneous florfenicol particles are obtained. According to the homogeneous florfenicol particles and the preparation method, a main chemical in the homogeneous florfenicol particles is uniformly distributed, the homogeneous florfenicol particles have smooth surfaces, the flowability and the dispersibility of the particles are improved, the particles are easily mixed during use, and the problems about uniformity and wall adhering of florfenicol preparaitons as well as chemical residues, animal poisoning and the like due to non-uniform mixing during use are solved.
Owner:FOSHAN STANDARD BIO TECH
Simplified oil sampling assembly
InactiveUS20080098827A1Easy to disassembleEliminate and significantly reduce possibilityWithdrawing sample devicesMaterial testing goodsEngineeringContinuous flow
The present invention is directed to an oil sampling assembly structured to collect an oil sample from the oil circulating system of an engine or other device and including a housing connected in fluid communication with the oil circulating system and having a container removably connected thereto. During operation of the oil circulating system a continuous flow of oil into and out of the container will occur. When the oil circulating system is not operating, oil and any contaminants contained therein will collect within the container. The container and oil sample is detached from the housing, replaced by another container, and connected to a closure for transport to a testing facility.
Owner:CAMPBELL D MICHAEL +1
Stirring apparatus for large containers
InactiveUS6652135B2Safe handlingGood removal effectShaking/oscillating/vibrating mixersOther chemical processesAdditive ingredientContamination
An apparatus for safely handling and securely holding a multitude of large and heavy open topped pails, for loading ingredients into these pails, and for stirring the ingredients within these pails into a fully homogenized state. The apparatus is adapted for simple removal and easy cleaning of the stirring components, and to minimize mess and eliminate cross contamination.
Owner:I C T C HLDG
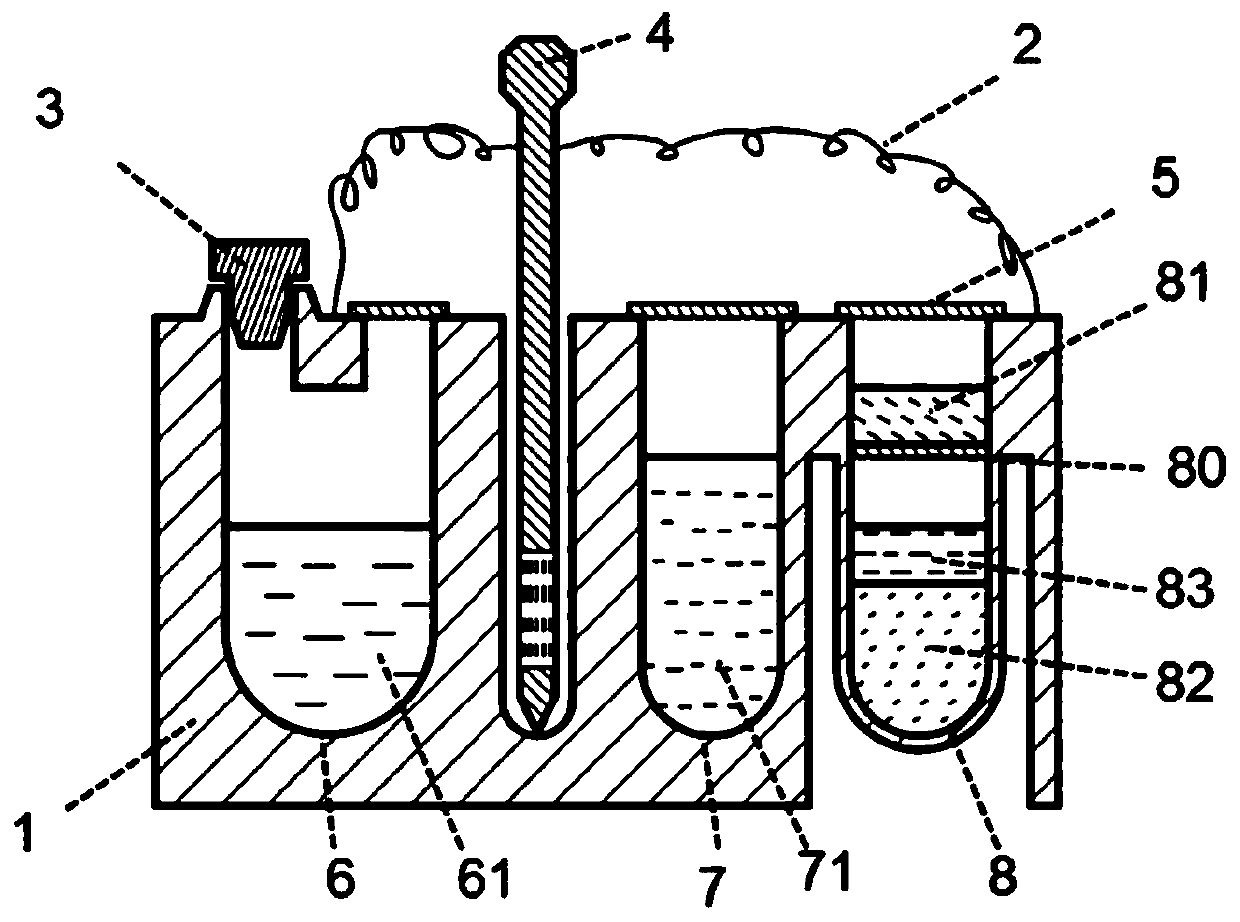
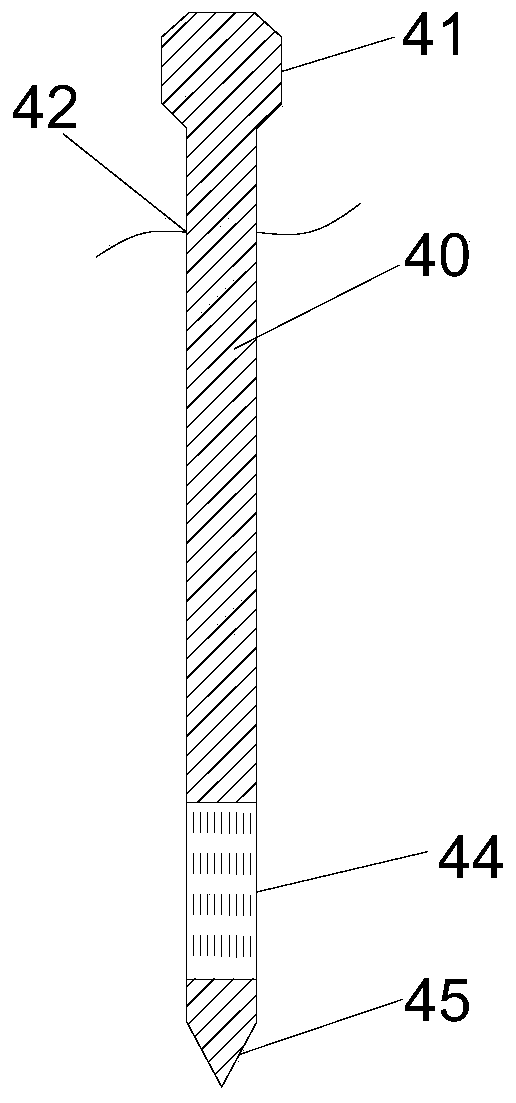
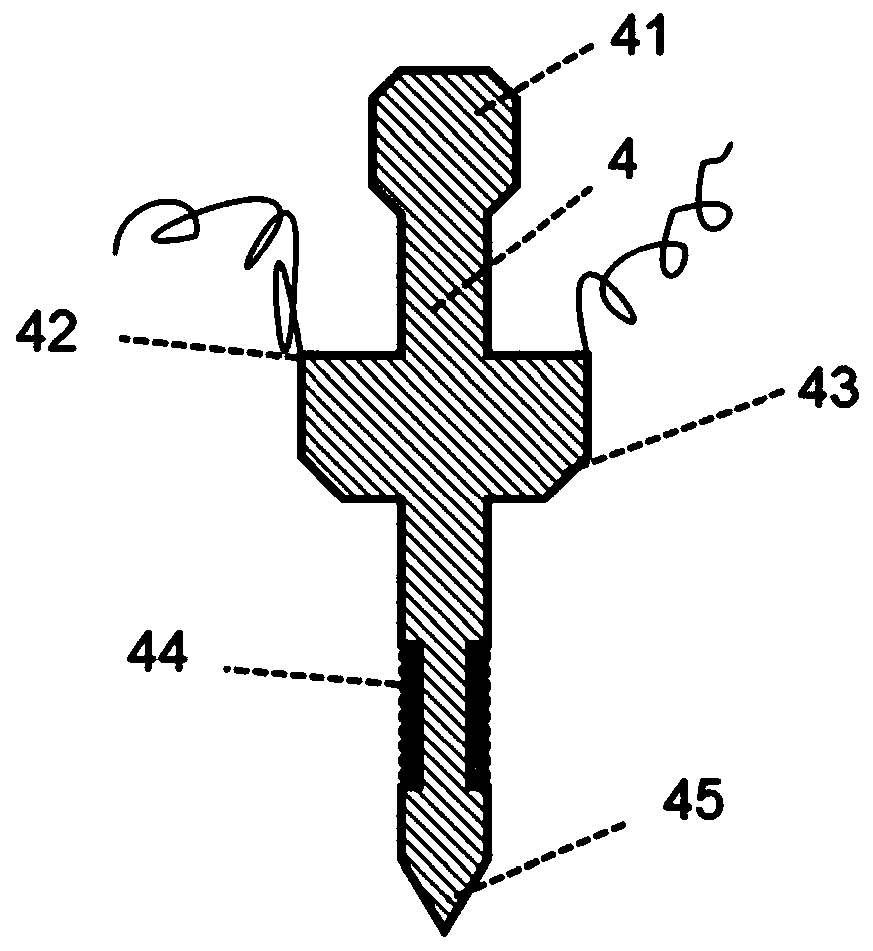
Kit for nucleic acid analysis under totally-enclosed condition, and device and analysis method for nucleic acid analysis
ActiveCN111500408AEliminate cross-contaminationImprove reliabilityBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismDisease
The invention discloses a kit capable of carrying out nucleic acid analysis under a totally-enclosed condition. The kit comprises a box body provided with a sample inlet and a sealing cover, a flexible upper cover in sealed butt joint with the box body to form a sealed cavity, one or more probes, and one or more functional containers. The sample inlet is positioned outside the sealed cavity; the sampling ends of the one or more probes are arranged in the sealed cavity, and the peripheral sides of the probes are connected with the flexible upper cover in a sealed mode; and at least one functional container is communicated with the sample inlet; and corresponding functional reagents are contained in one or more containers. The invention also provides a device and a method for nucleic acid analysis by using the kit. The nucleic acid extraction probe, the analysis reagent, the corresponding functional container and the like are sealed in independent space, so that a problem of cross contamination in the processes of sample pretreatment, transfer, amplification reaction and the like can be effectively solved, and the kit is suitable for multiple application fields of disease diagnosis,water quality detection, air monitoring, gene analysis, microbial research, pathogen detection and the like.
Owner:ZHEJIANG UNIV
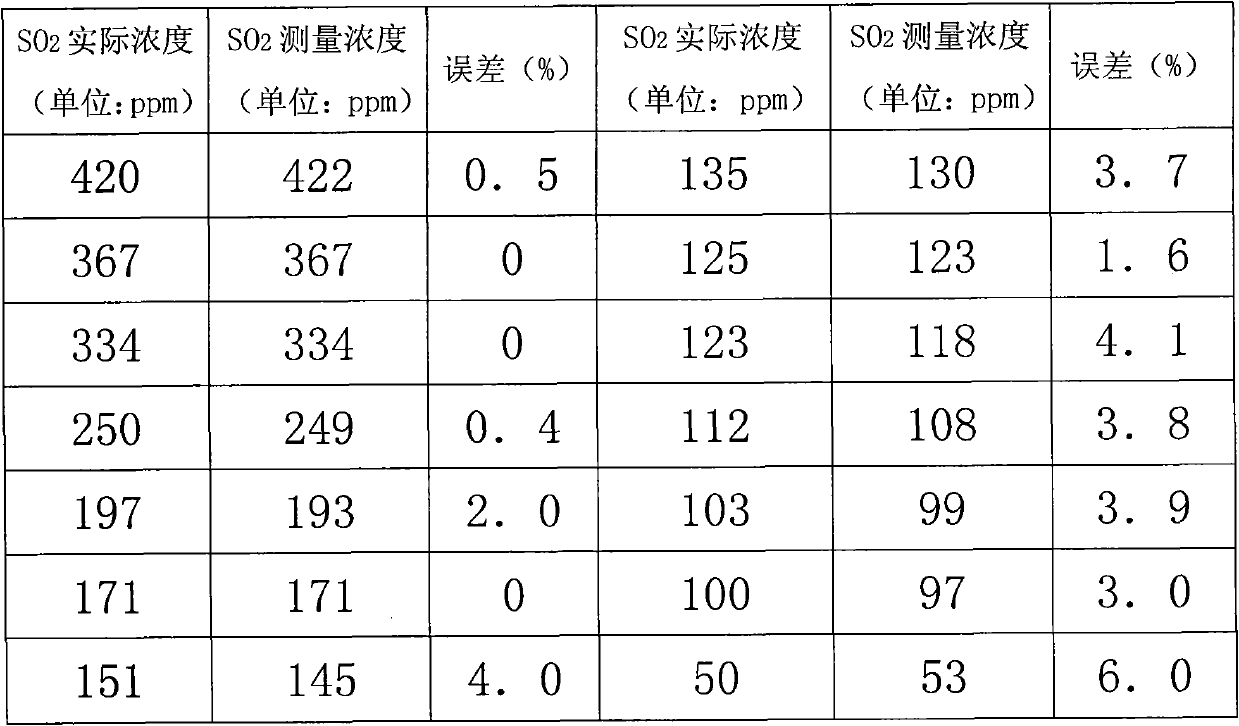
Sulfur dioxide analyzer and analyzing method based on ultra-violet light-emitting diode
ActiveCN101907563AEliminate cross-contaminationAvoid errorsColor/spectral properties measurementsOptical pathChemistry
The invention discloses a sulfur dioxide analyzer based on an ultra-violet light-emitting diode, comprising the ultra-violet light-emitting diode, an absorption cell, a spectrometer and a computer connected on the spectrometer, wherein the two sides of the absorption cell are respectively provided with an incident window and an emergent window which correspond to each other and are transparent to ultra-violet rays, and the absorption cell is also provided with an air inlet and an air outlet; an incident optical fiber is connected between the ultra-violet light-emitting diode and the incident window of the absorption cell, and the two ends of the incident optical fiber are respectively provide with a collimator; an emergent optical fiber is connected between the emergent window of the absorption cell and the spectrometer, and the two ends of the emergent optical fiber are respectively provided with a collimator; and the included angle between the incident window and the ultra-violet light path and the included angle between the emergent window and the transmitted ultra-violet light path are acute angles or obtuse angles close to right angles. The invention also discloses a sulfur dioxide analyzing method based on the ultra-violet light-emitting diode. The invention has the advantages of accurate measurement, large measurement range and short measurement time.
Owner:广东盈峰科技有限公司
Driven loadable construct system and method for using the same
ActiveUS20140277955A1Avoid damageEliminate cross-contaminationNon-electrical signal transmission systemsAnalogue computers for trafficRotational energyAutomotive engineering
An apparatus, system, and method for a driven loadable construct, the apparatus, system and method utilizing a construct, at least one rotatable wheel mounted for enabling the construct to roll along a surface, at least one motor and power source coupled to the construct to be used to impart rotational energy to the at least one rotatable wheel, and a controller to control the signals for operation of the at least one rotatable wheel which moves the construct.
Owner:EIDELSON ARTHUR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com