Patents
Literature
50914 results about "Electric cables" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
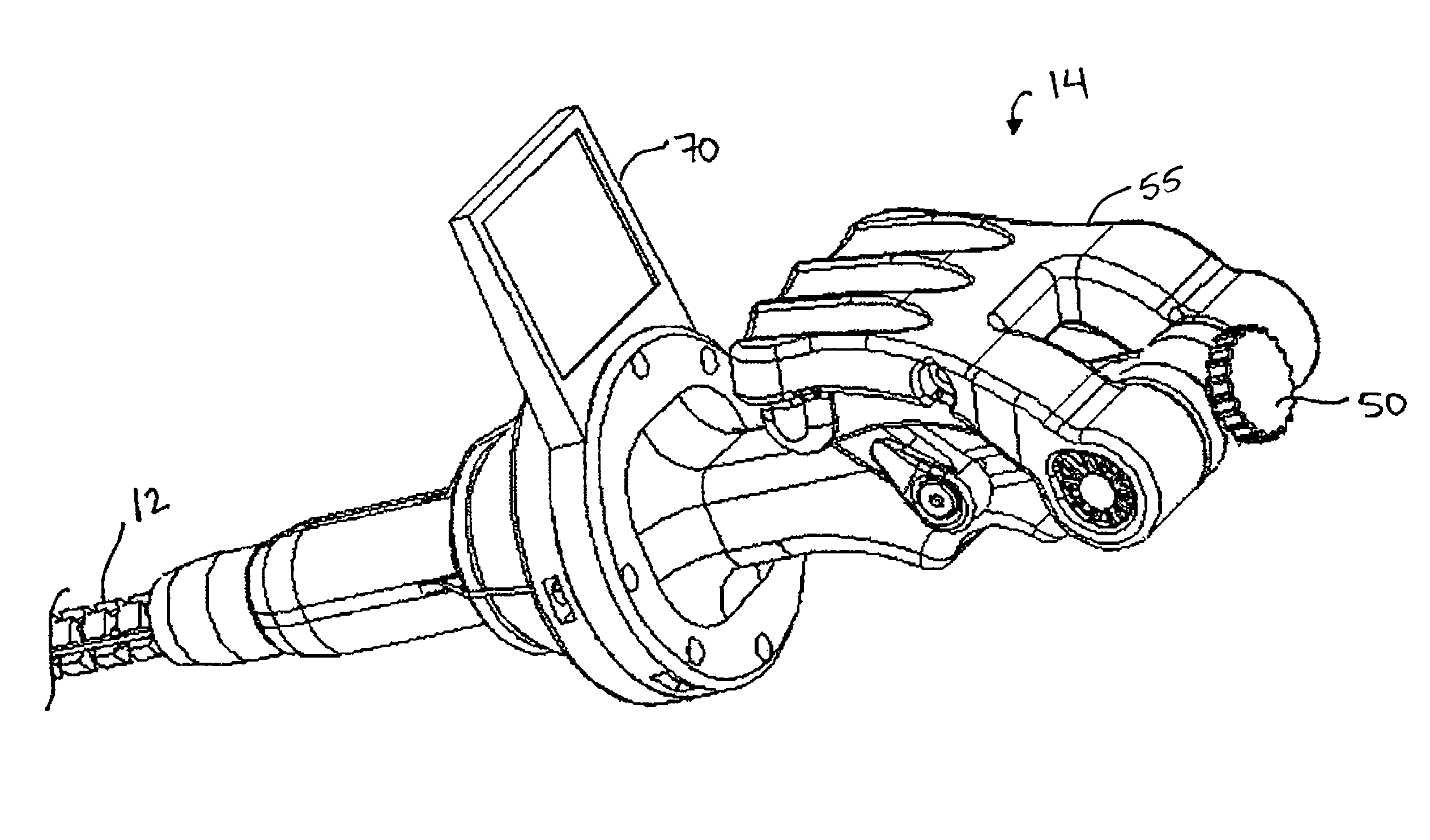
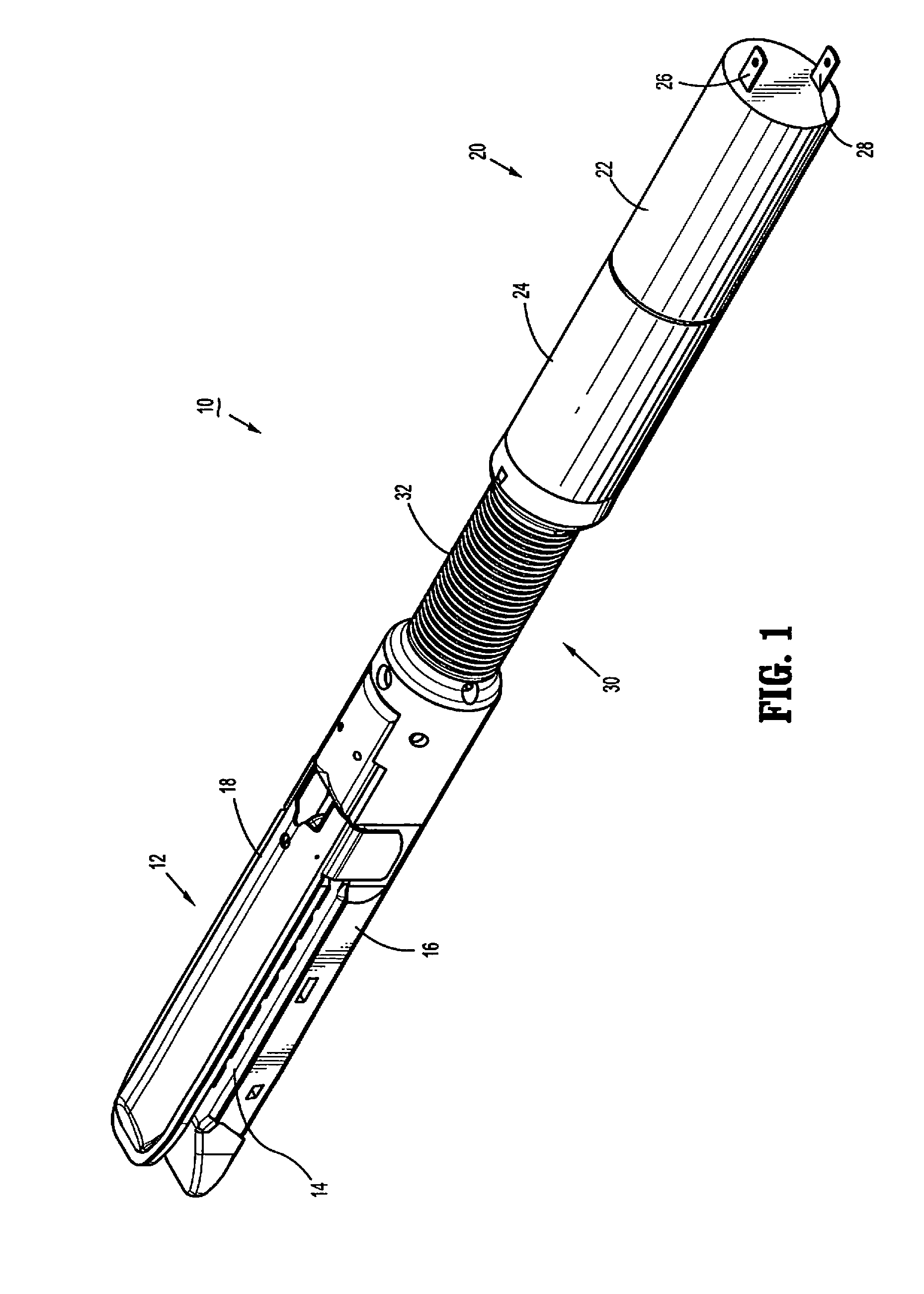
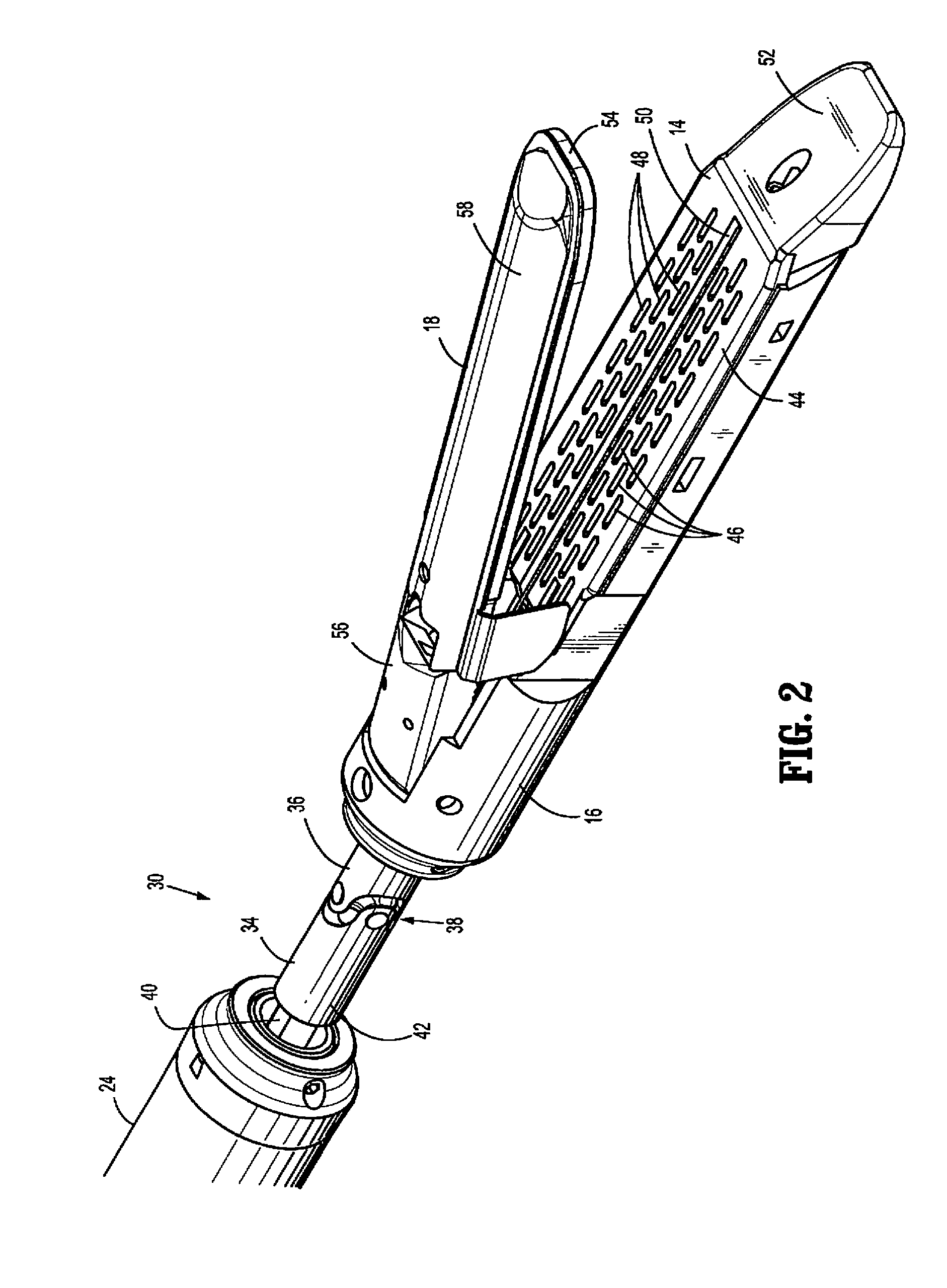
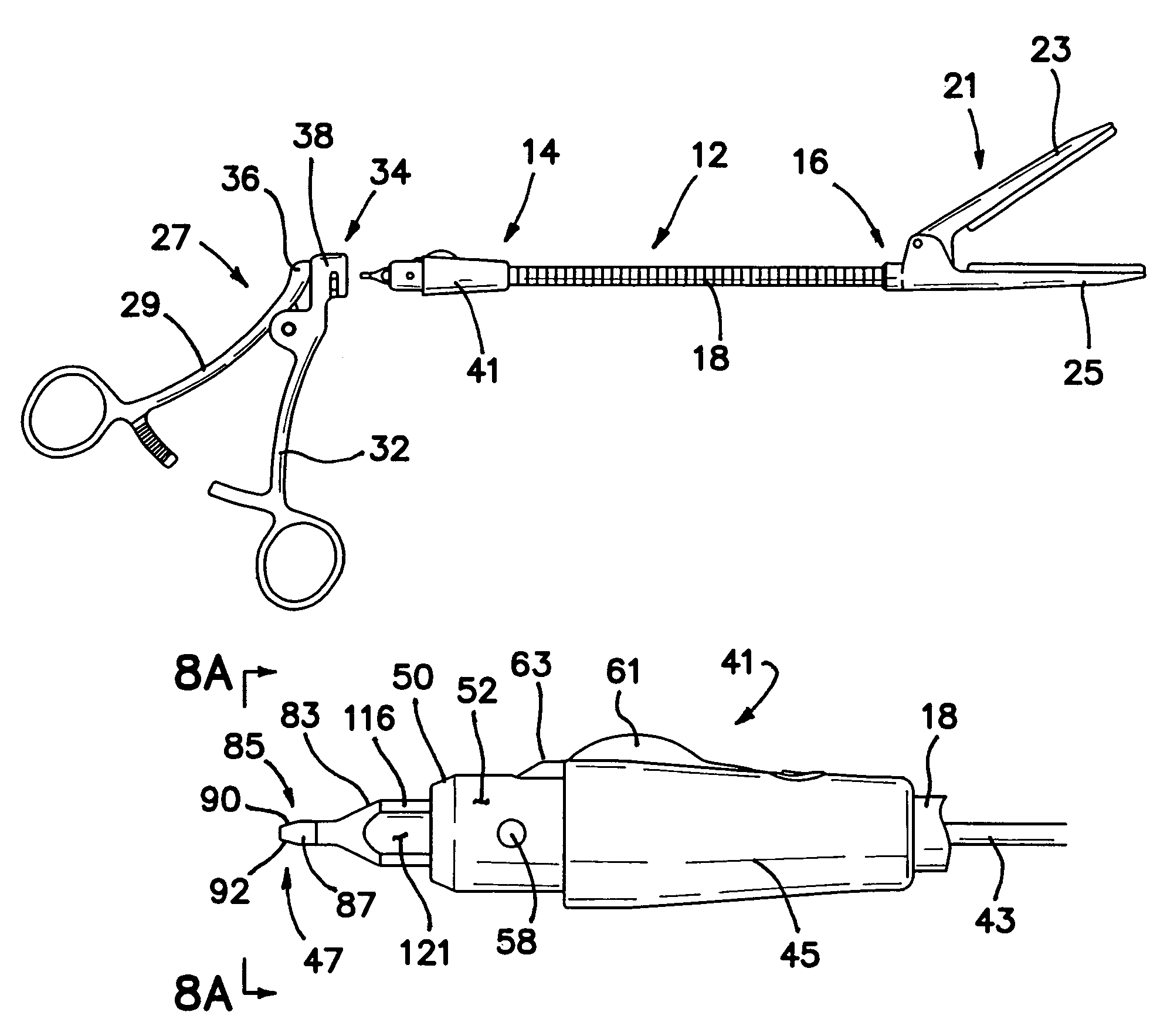
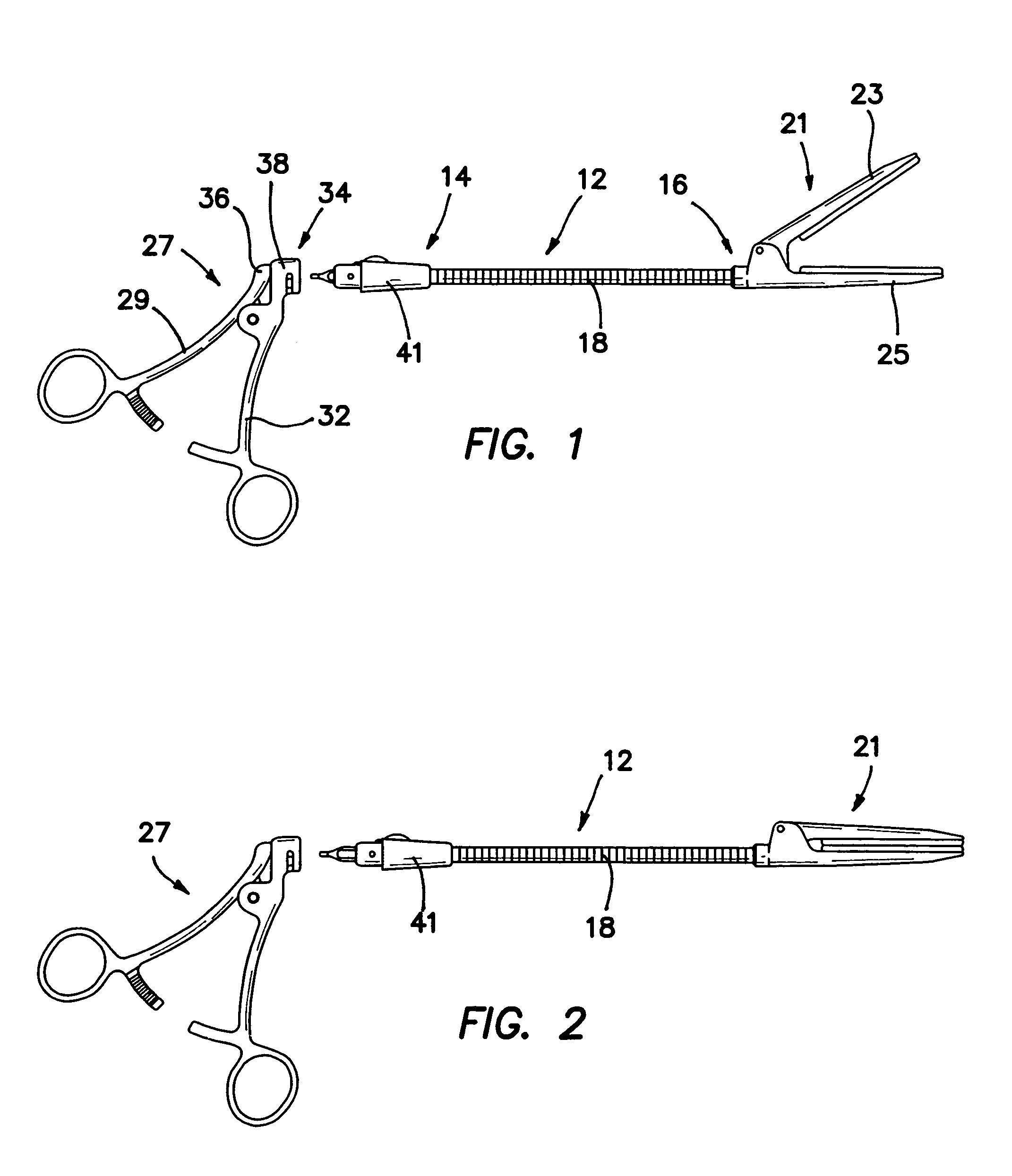
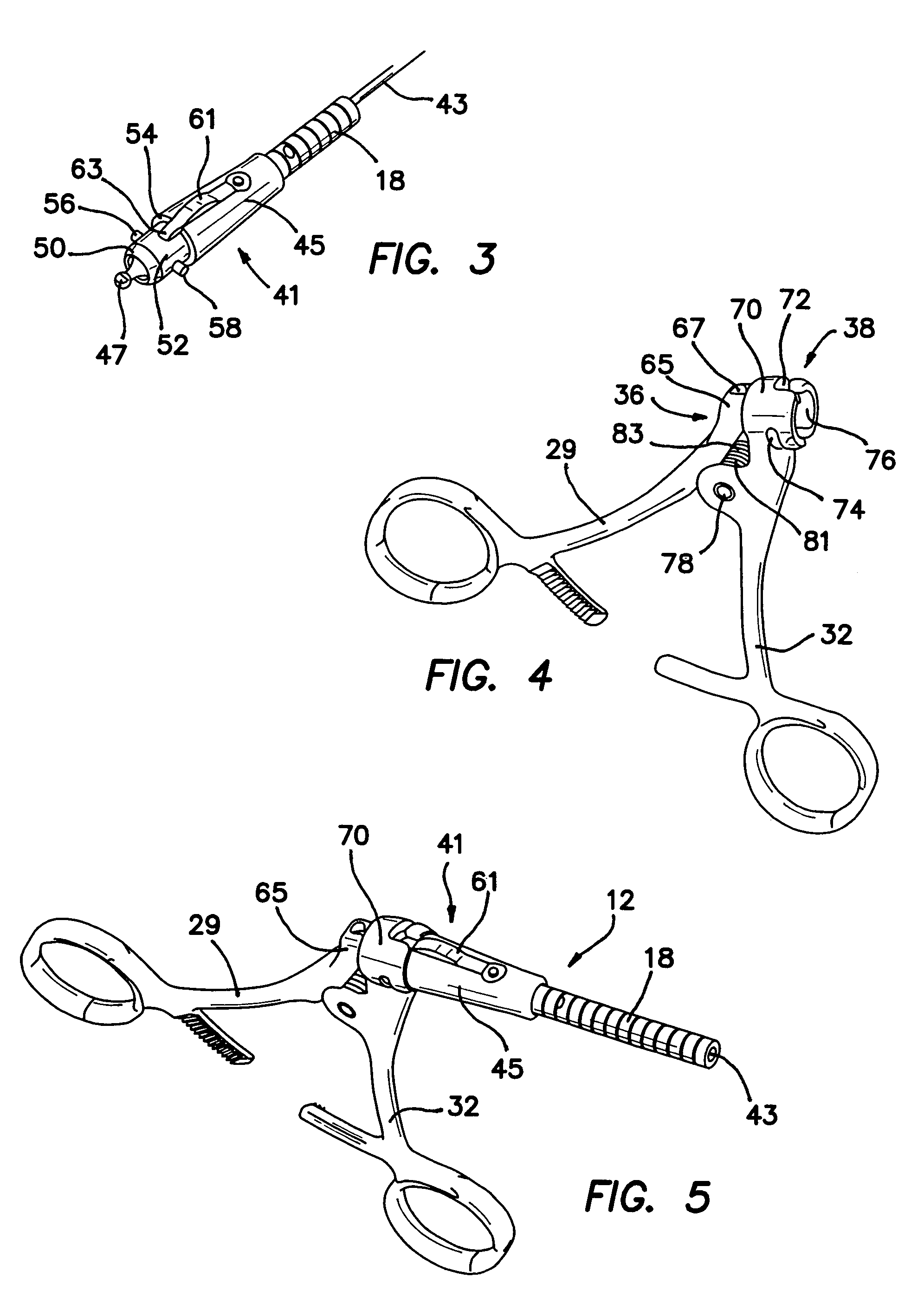
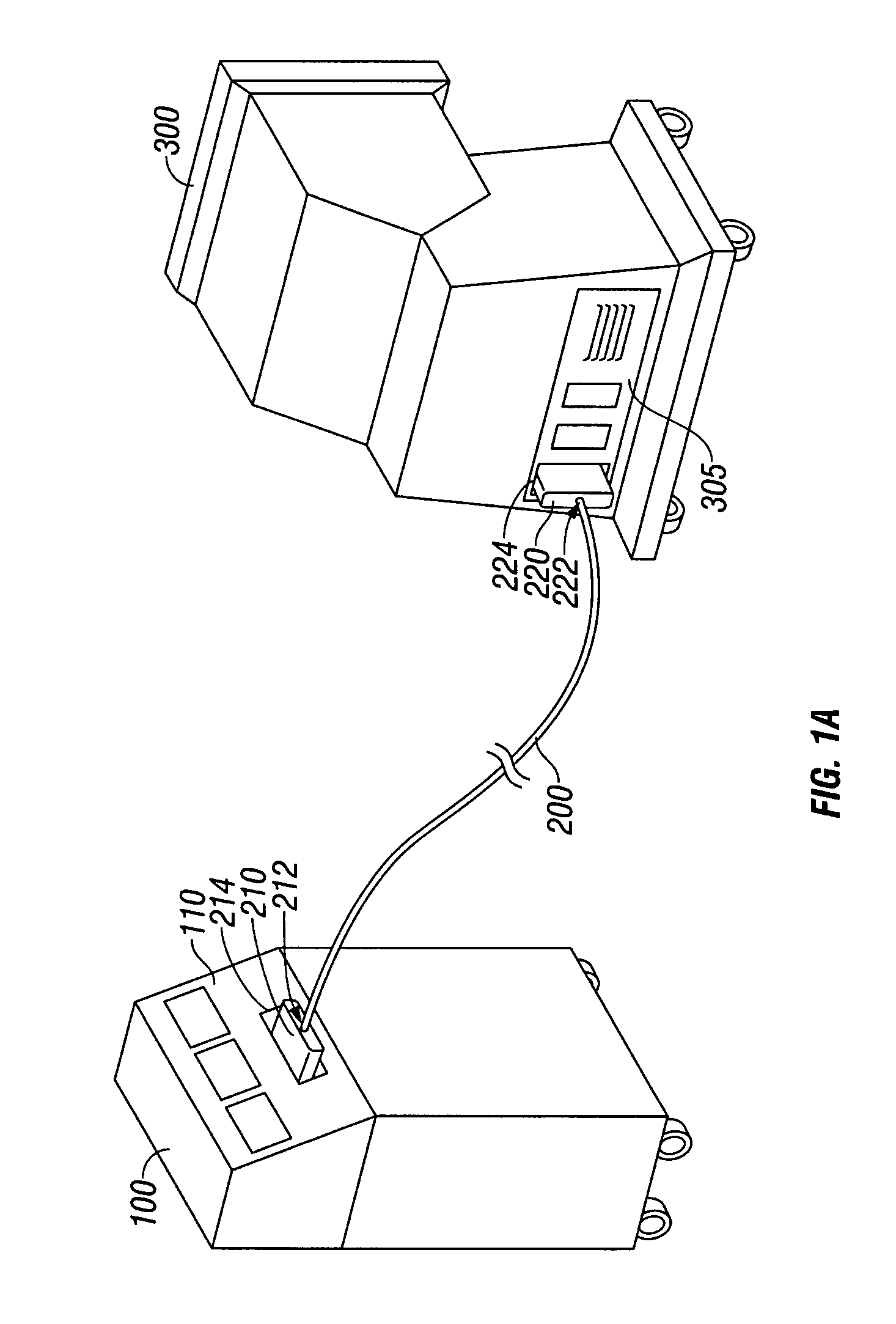
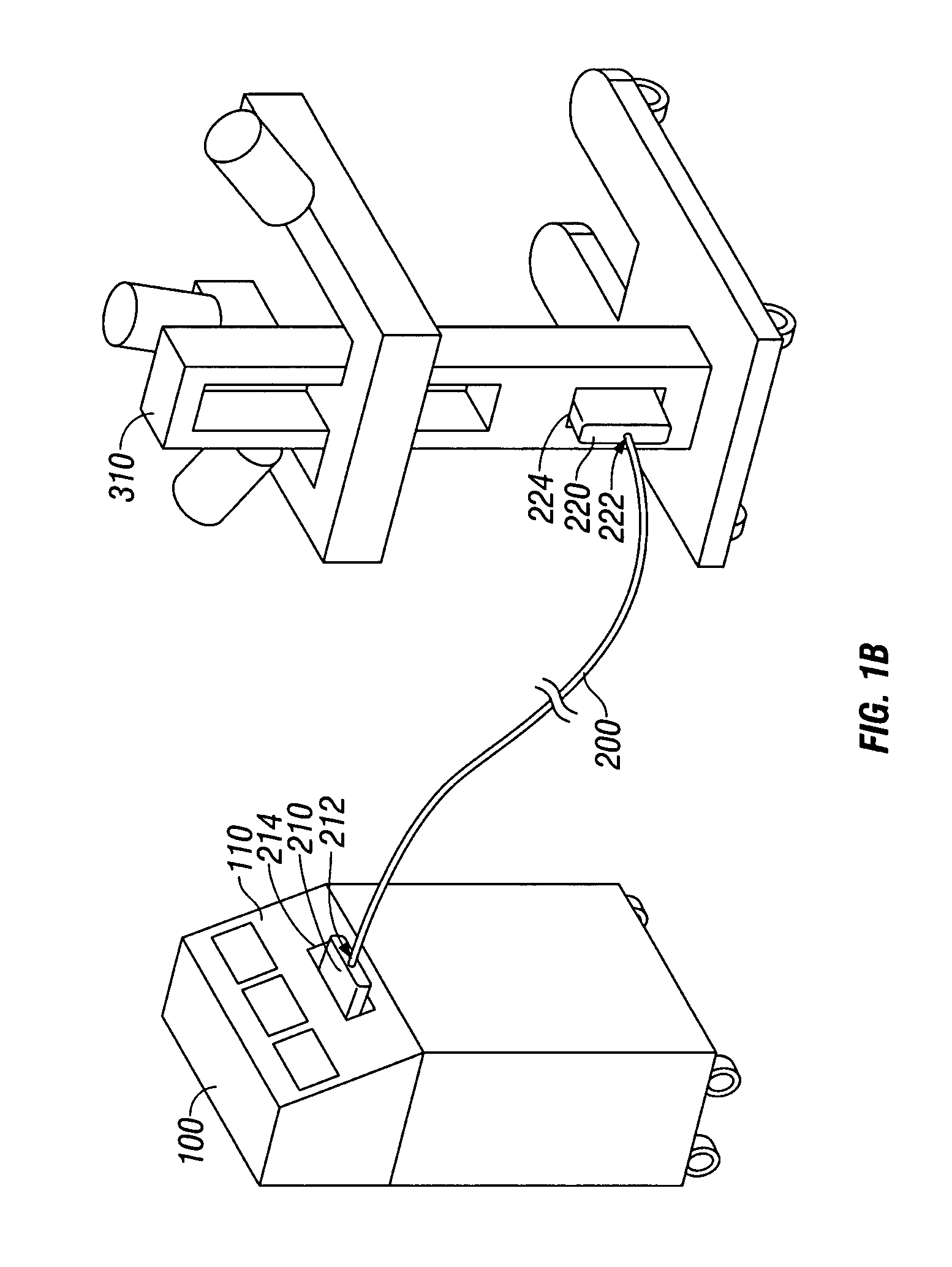
Surgical fastener and cutter with single cable actuator
ActiveUS7575144B2Effective rotationEnergy efficiencySuture equipmentsStapling toolsEngineeringActuator
Methods and devices are provided for controlling rotation and actuation of an end effector on a surgical fastening device. In an exemplary embodiment, a single cable actuator is provided and is movable between a first position, in which it is effective to rotate an end effector without actuating (i.e., closing and firing) the end effector, and a second position, in which it is effective to actuate the end effector without rotating the end effector. The single cable can also be effective to close opposed jaws of the end effector.
Owner:ETHICON ENDO SURGERY INC
Flexible surgical stapler with motor in the head
A surgical stapler having a remote motorized staple head is provided and generally includes a handle having a control button and a highly flexible cable extending distally from the handle. A housing incorporating a staple assembly is affixed to a distal end of the flexible cable. The housing may incorporate articulating structure to position the staple assembly relative to the remainder of the housing.
Owner:COVIDIEN LP
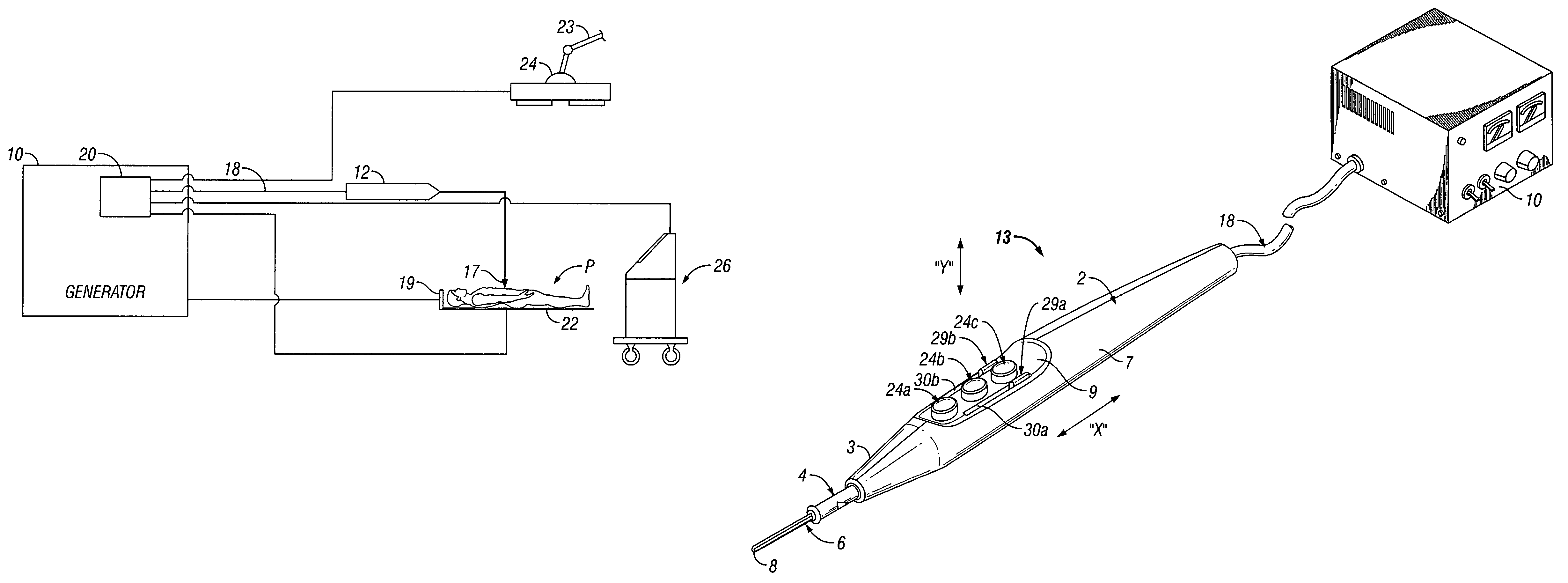
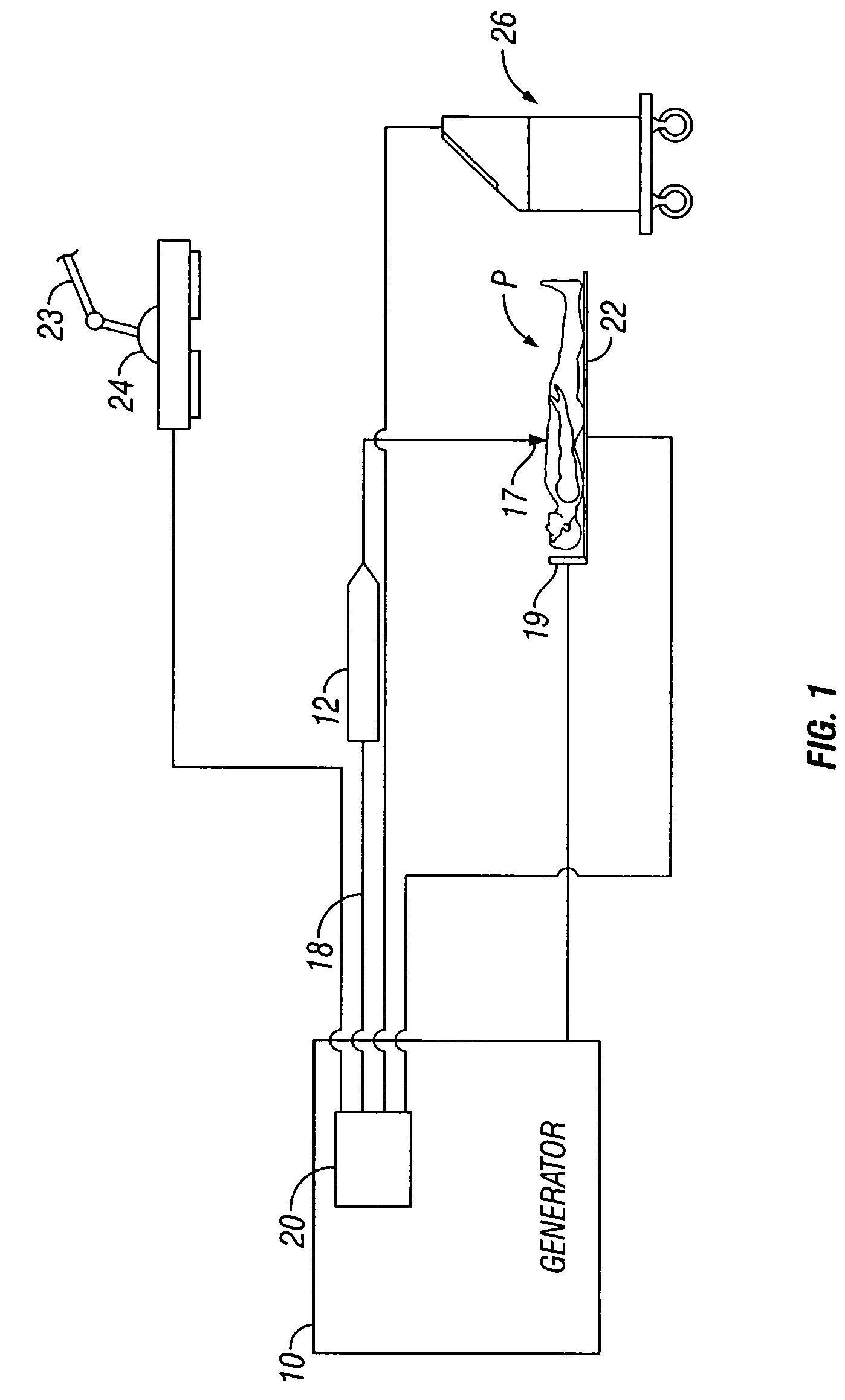
Handheld electrosurgical apparatus for controlling operating room equipment
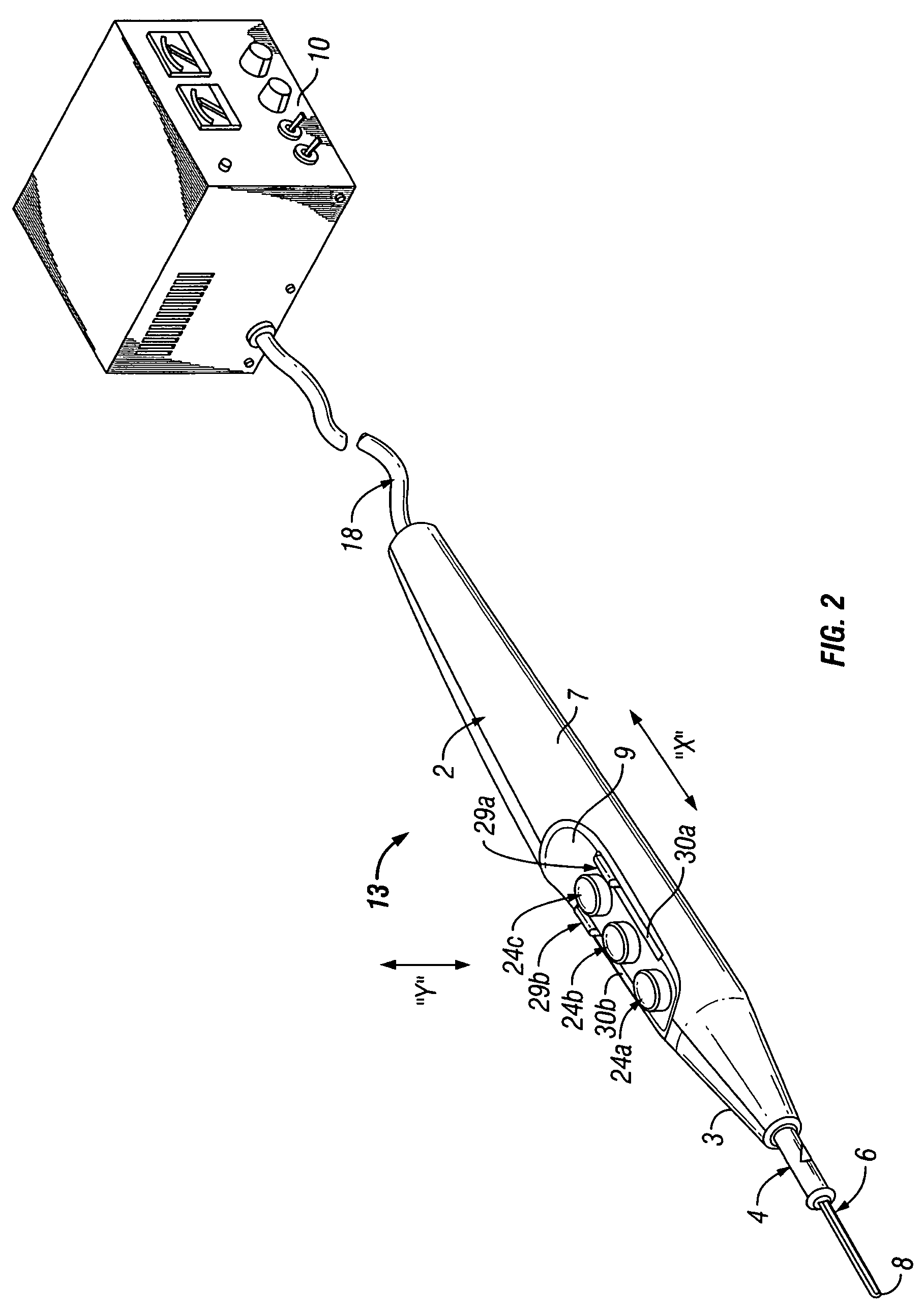
A system and apparatus for controlling operating room equipment during an electrosurgical procedure is disclosed. The system includes an electrosurgical generator, a controller in electrical communication with and configured to control the electrosurgical generator and at least one operating room device, and a handpiece having a housing and a cable extending proximally from the housing providing electrical connection to the controller, the handpiece further includes first controls for controlling the generator and second controls for controlling at least one operating room device.
Owner:COVIDIEN AG
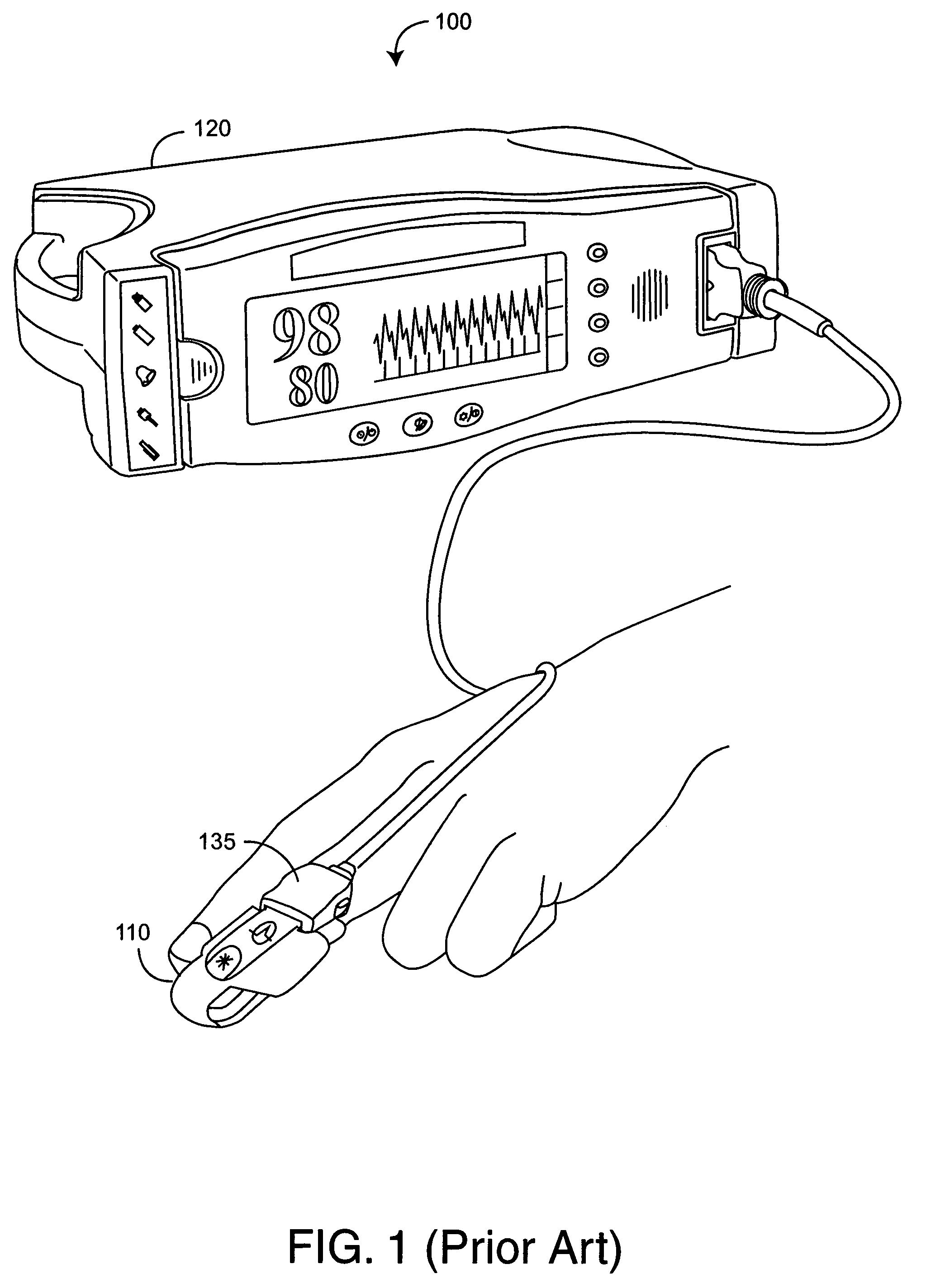
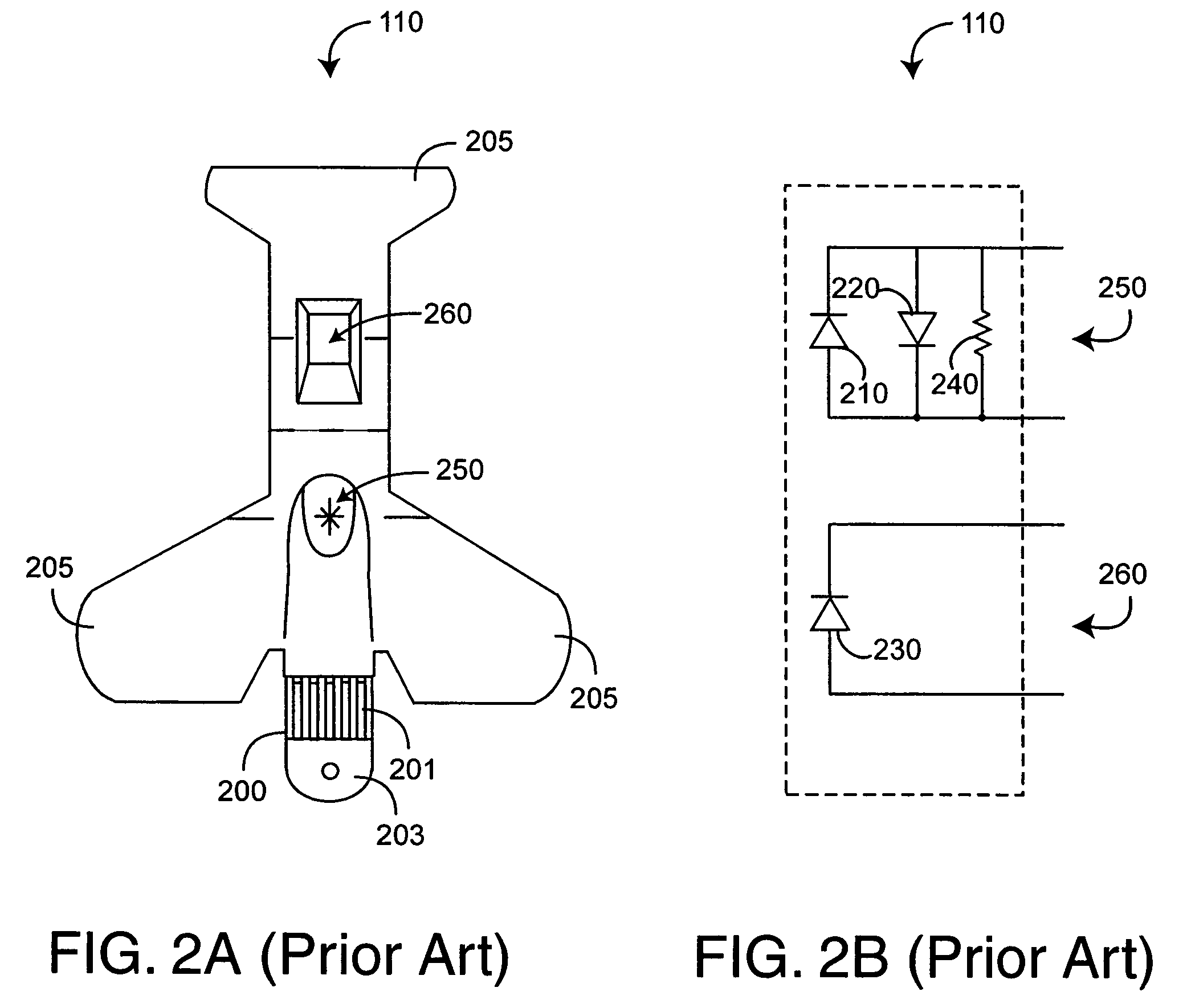
Pulse oximetry sensor adaptor
InactiveUS6993371B2Avoid complex processCost of very criticalDiagnostic recording/measuringSensorsAudio power amplifierPulse oximetry
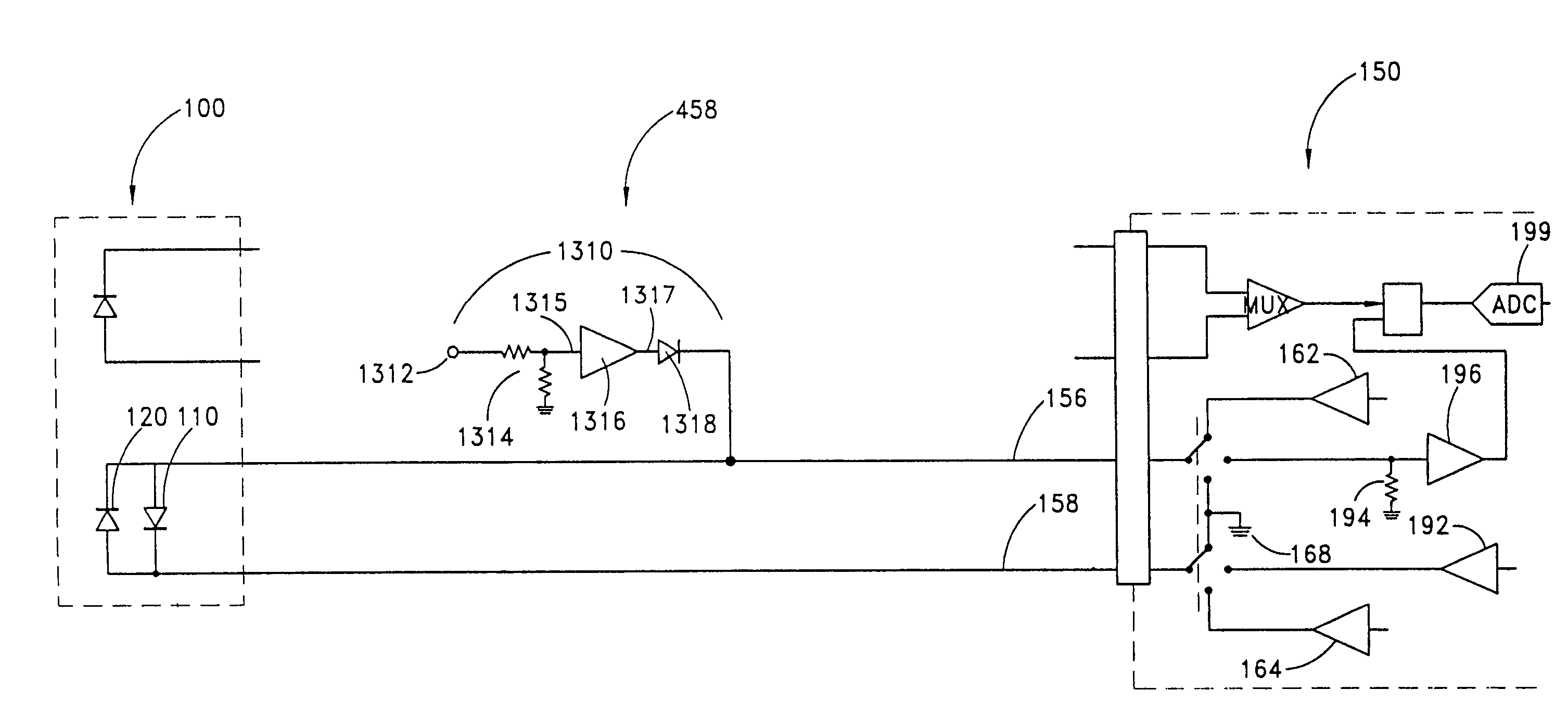
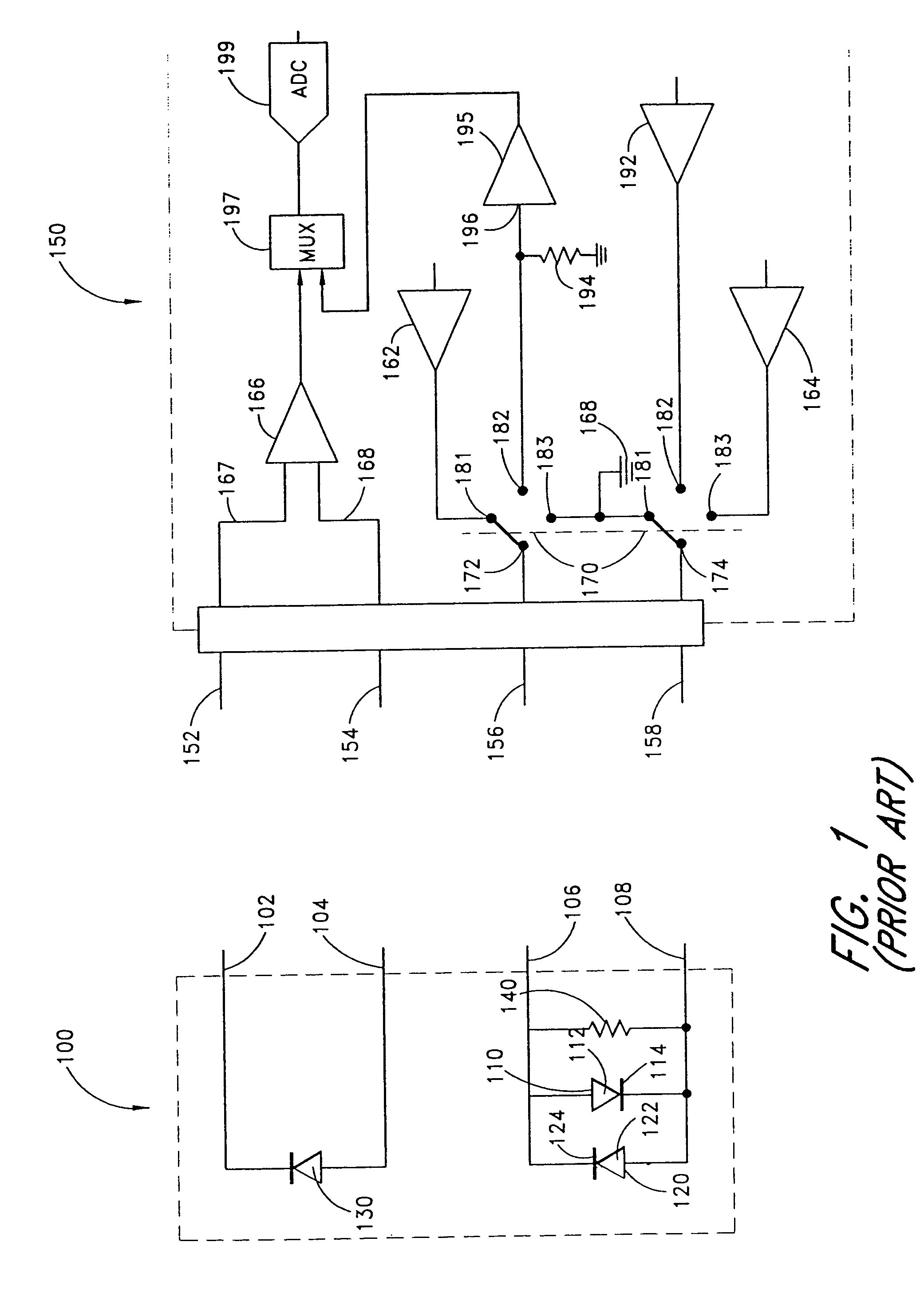
An adapter allows the interconnection of a sensor originating from one manufacturer to be coupled with conventionally incompatible monitors originating from other manufacturers to form a properly functioning pulse oximetry system. The adapter matches a sensor driver in a monitor to the current requirements and light source configuration of a sensor. The adapter also matches a sensor's light detector signal level to the dynamic range requirements of a monitor preamplifier. Further, the adapter provides compatible sensor calibration, sensor type and security information to a monitor. The adapter may have a self-contained power source or it may derive power from the monitor, allowing both passive and active adapter components. The adapter is particular suited as an adapter cable, replacing a conventional patient cable or sensor cable as the interconnection between a sensor to a monitor in a pulse oximetry system.
Owner:JPMORGAN CHASE BANK NA
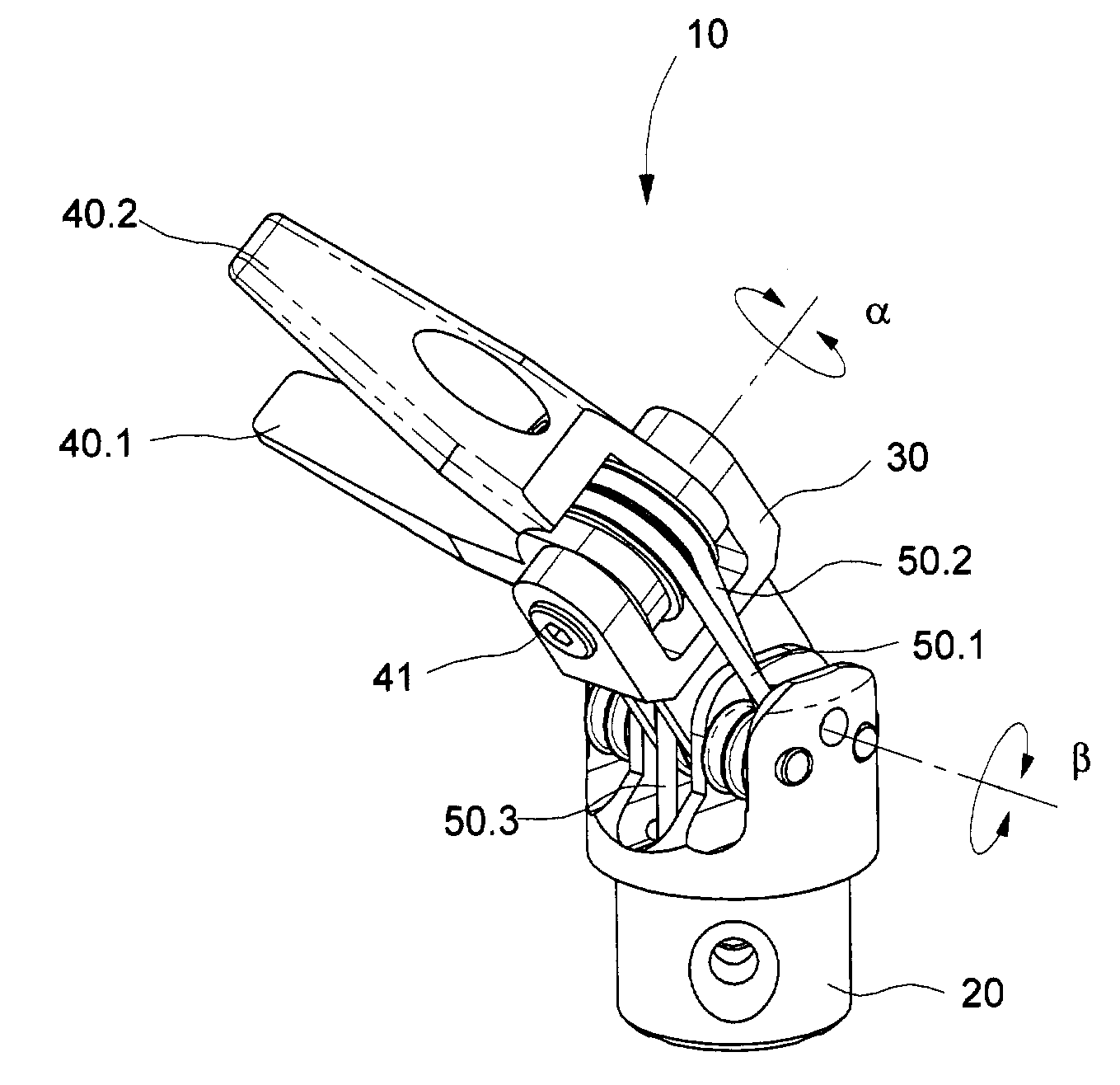
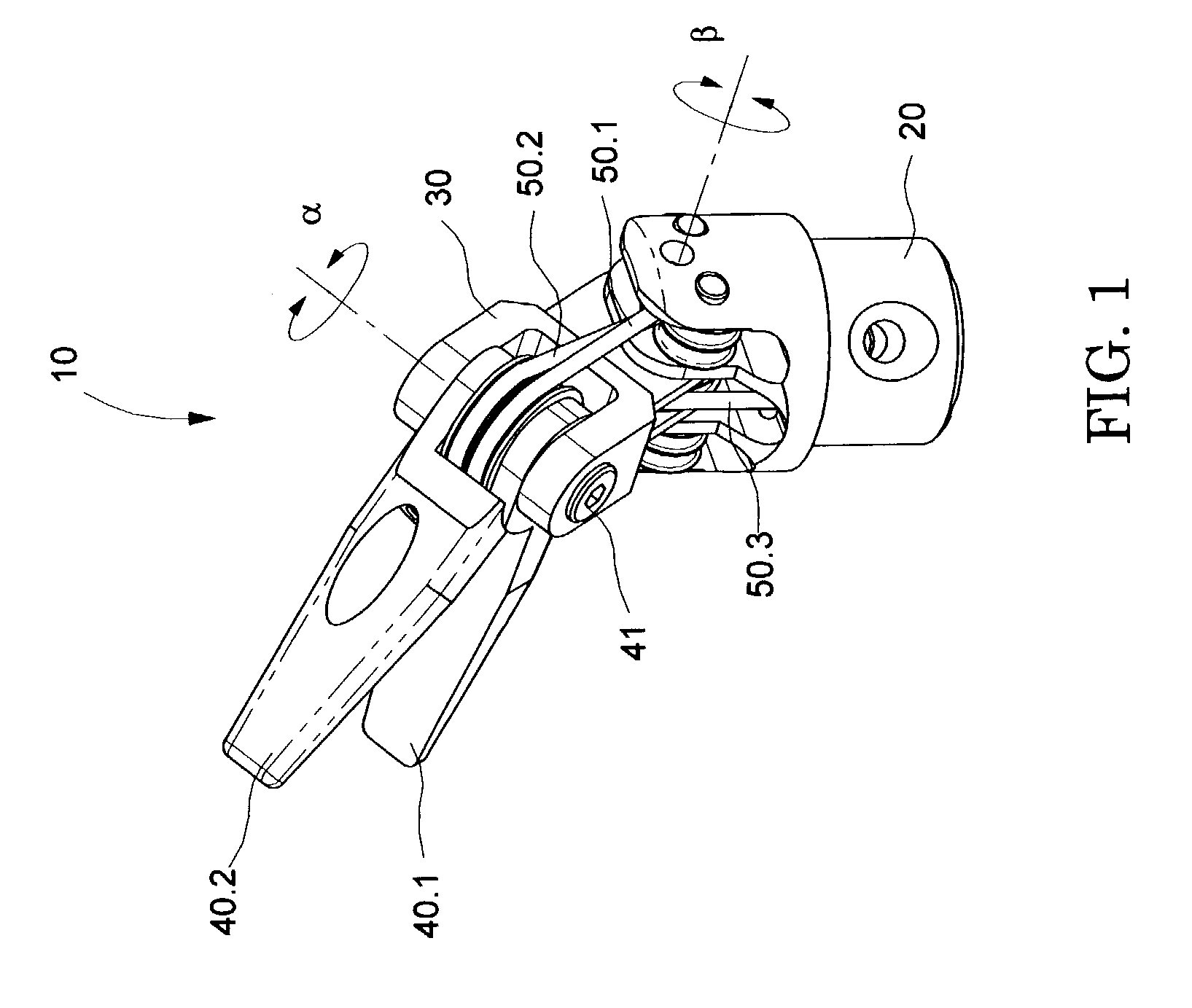
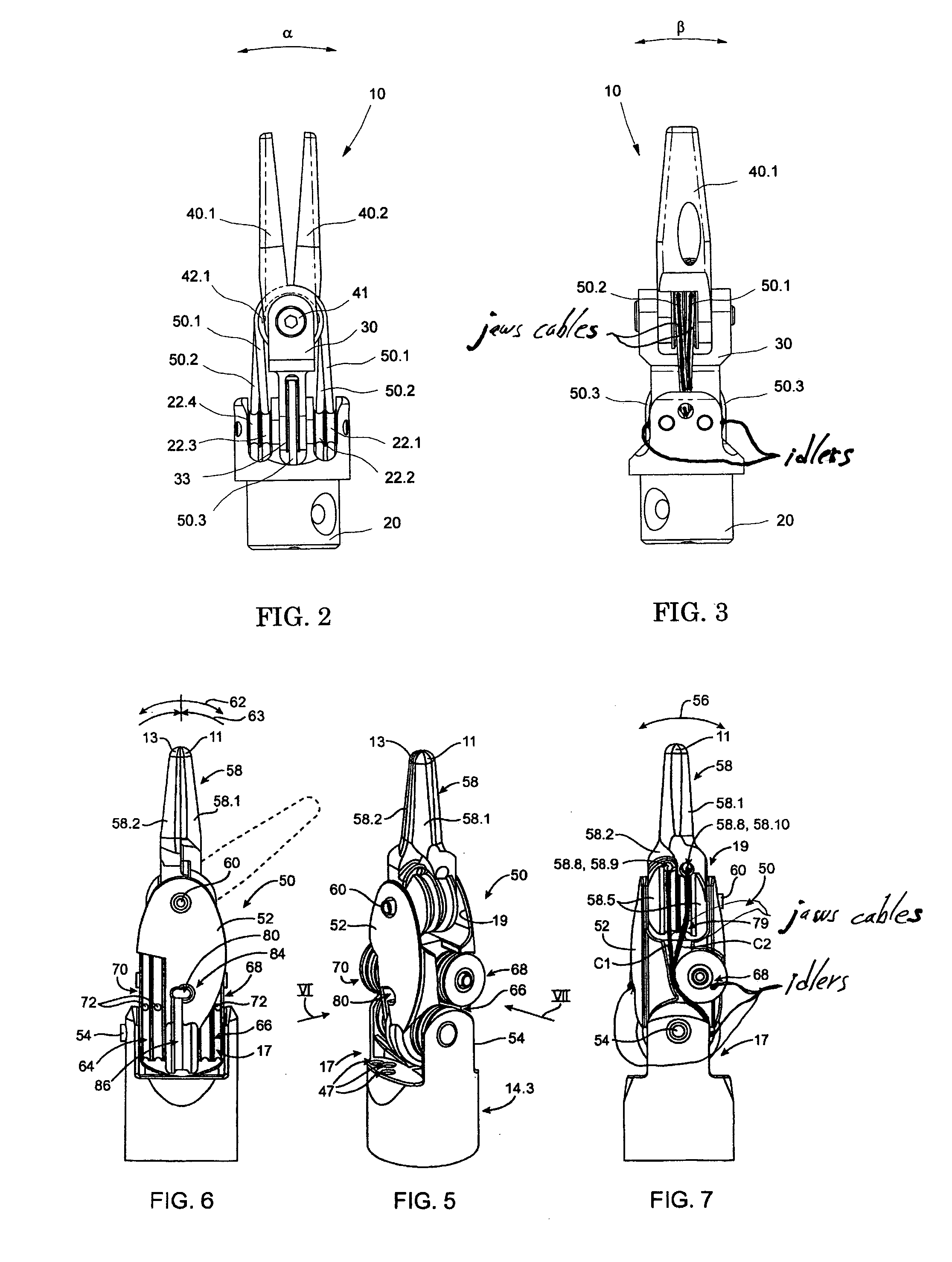
Wrist with decoupled motion transmission
InactiveUS6969385B2Reduce inertiaImprove device performanceMechanical apparatusJointsEngineeringActuator
The present invention is a wrist mechanism and a method for making robotic devices in which the transmission of motion, force and / or torque around a revolute joint can be accomplished without coupling. This construction allows mounting the actuators on the base or lower elements of a mechanism, so that only linkage elements move the end-effector. Thus reducing inertia of the moving elements and increasing performance of the device. The decoupled motion of the end-effector or links is achieved by routing their transmission cables around idler pulleys placed parallel to the joint rotation axis on an optimal position such any stretch on the transmission cable is minimized. In particular, this construction can be use for robotic surgical tools that have two independently driven jaws, decoupled and orthogonal from its articulating wrist. This device may be used in grasping, cutting, suturing or alike operations.
Owner:MOREYRA MANUEL RICARDO
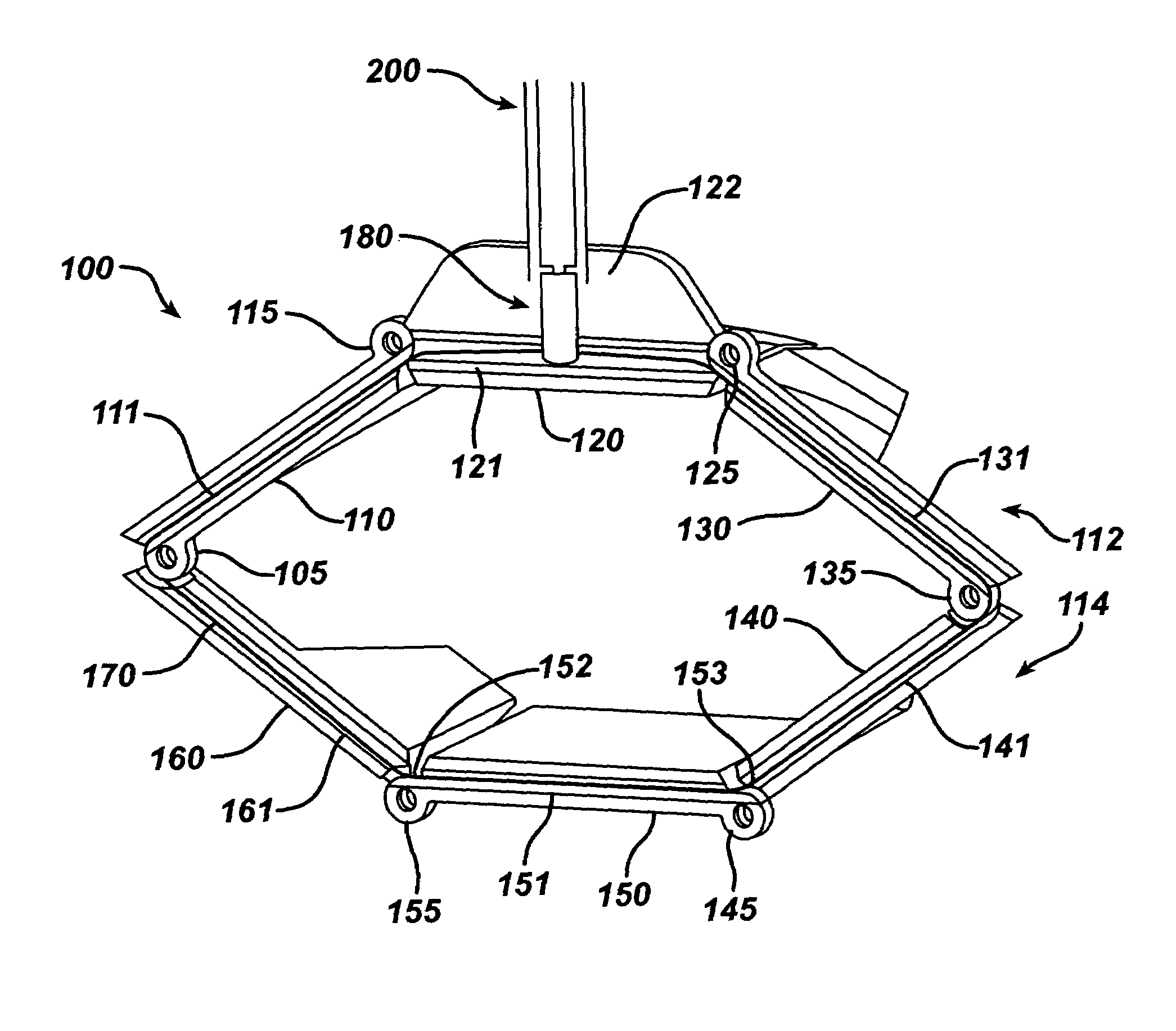
Low profile surgical retractor
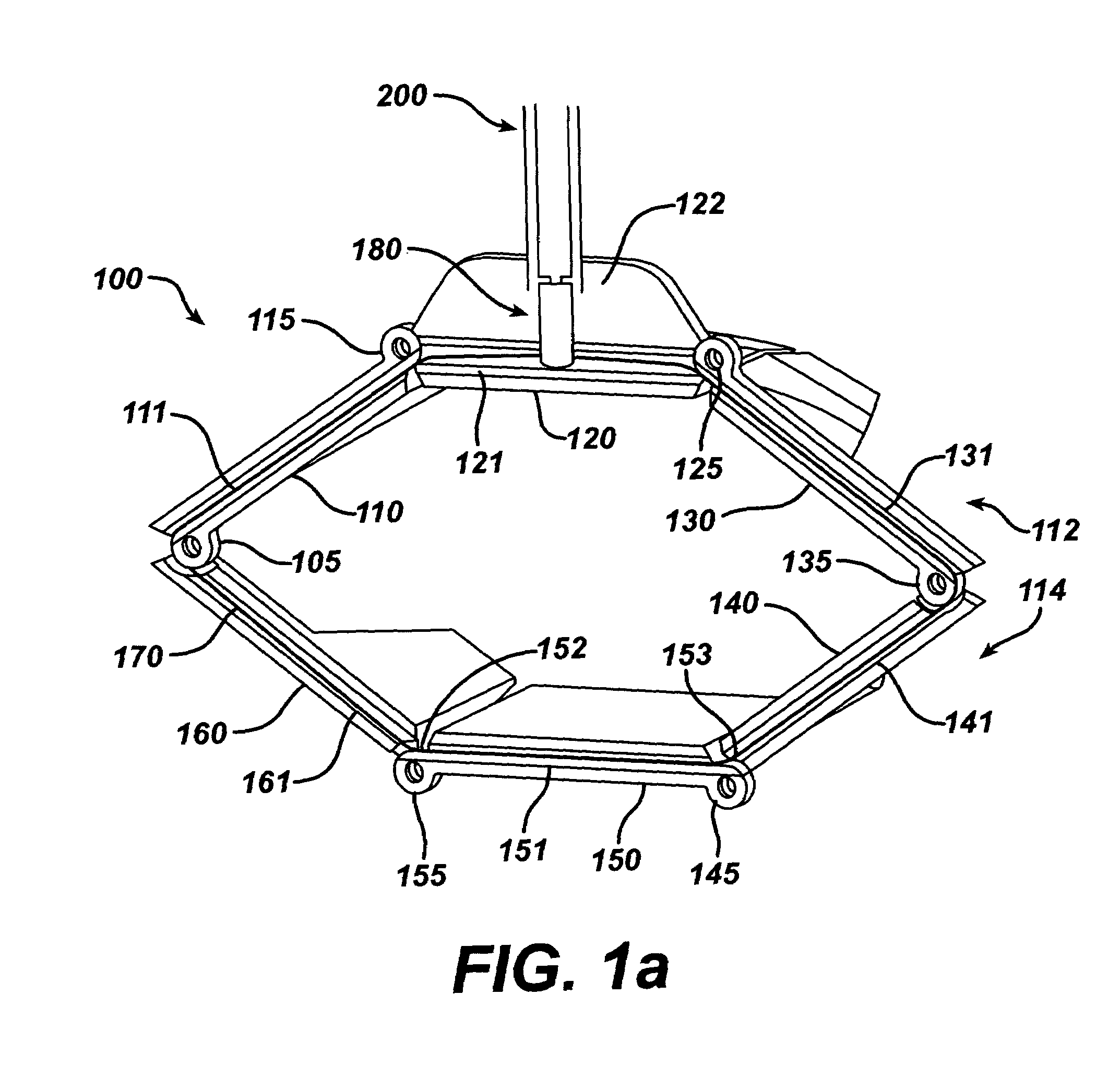
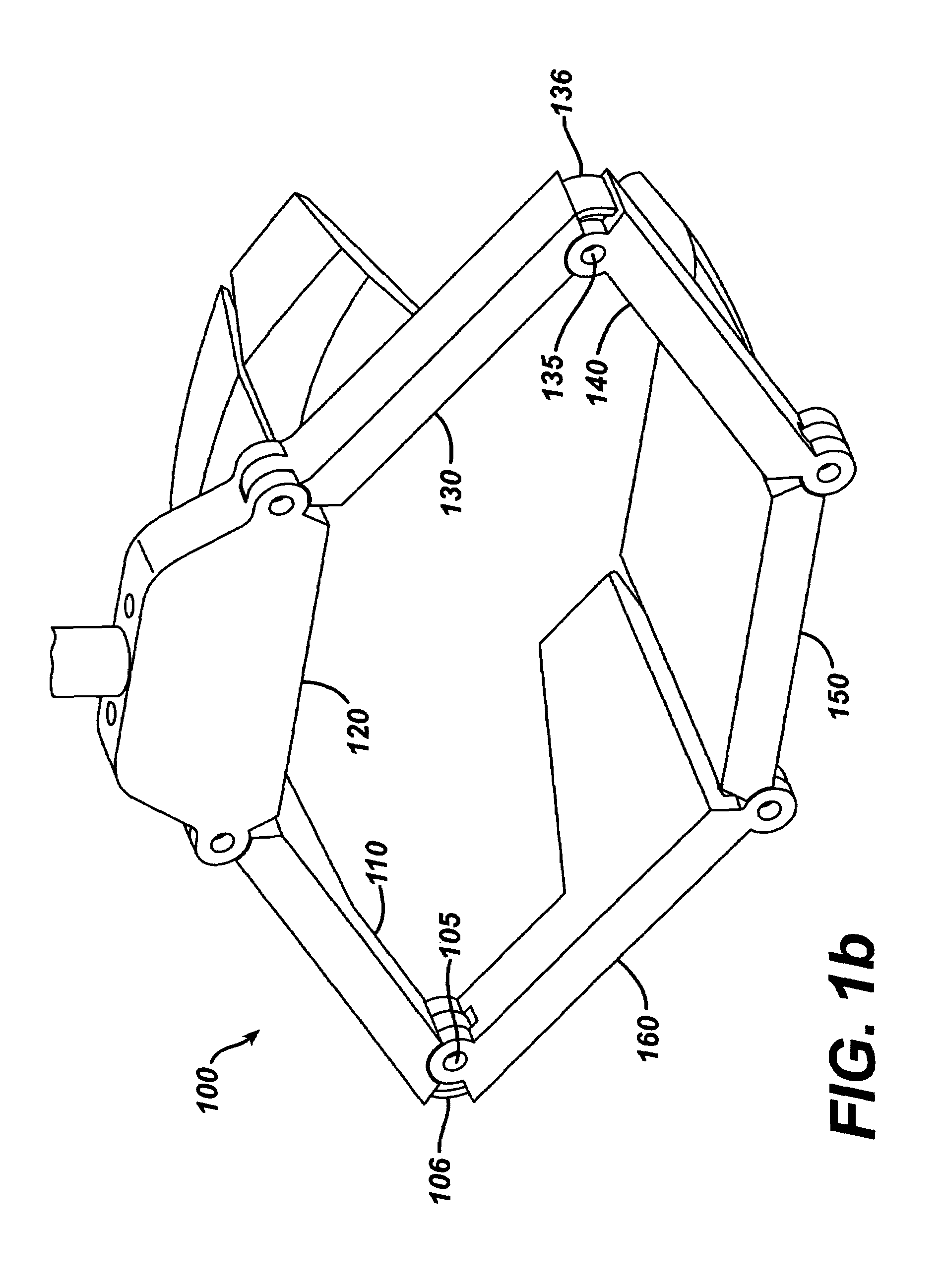
A compact, low-profile surgical retractor (100) avoids the need for a bulky frame. The retractor includes retractor blade components (110, 120, 130, 140, 150, 160) joined pivotally. A cable (170) is guided by each retractor blade component. A winding mechanism (180), such as a spool, is carried by one of the retractor blade components (120) for winding up the cable to cause the retractor blade components to transition from a closed position to an open position. The winding mechanism may be actuated by a detachable handle (200). The retractor blade components may be unitary, injection-molded pieces. The retractor blade components may be fixed pivotally, such as by pins, or pivotally joined by living hinges. A less-invasive surgical method is also provided in which the retractor is inserted into the body through an incision.
Owner:EDWARDS LIFESCIENCES LLC
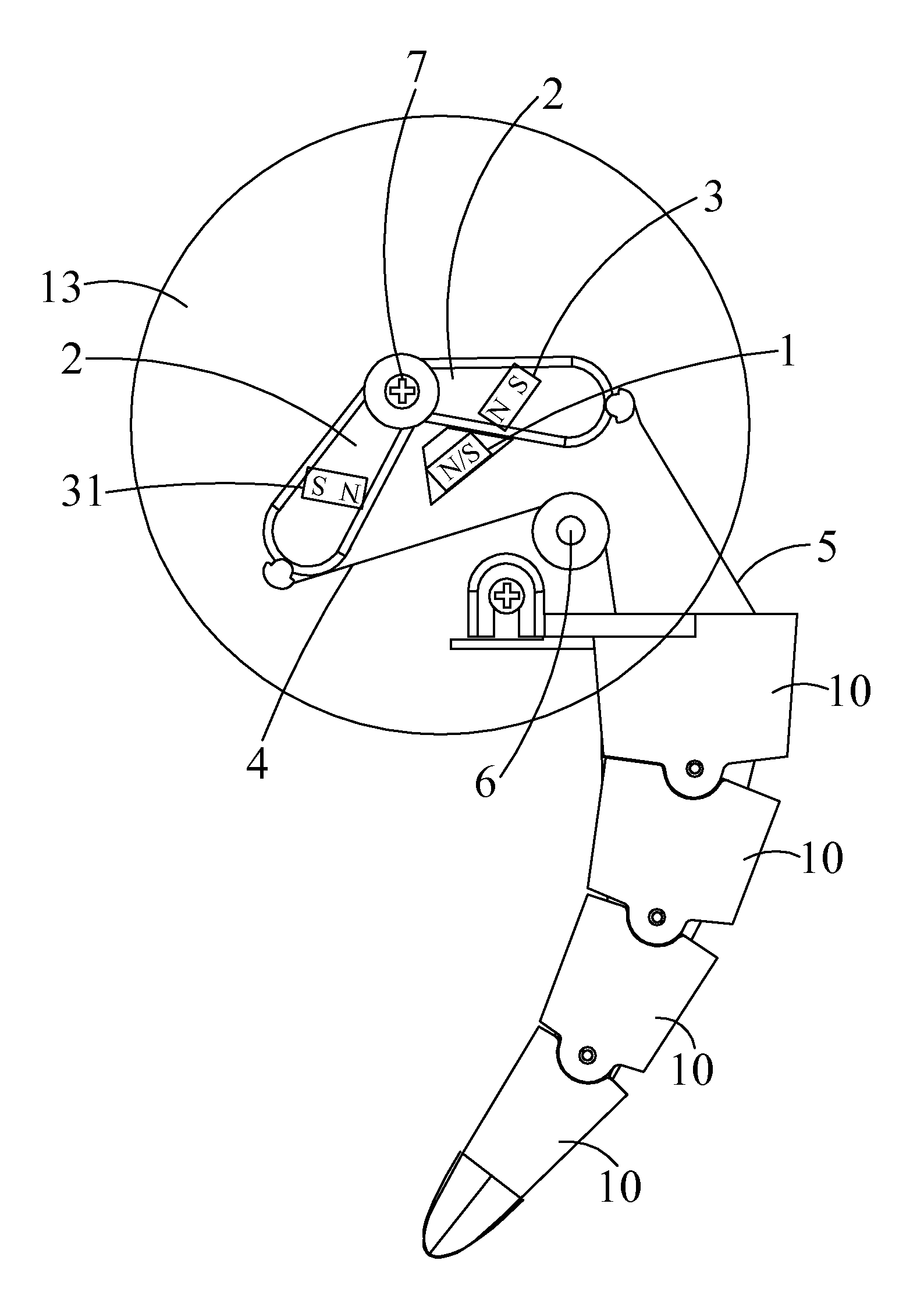
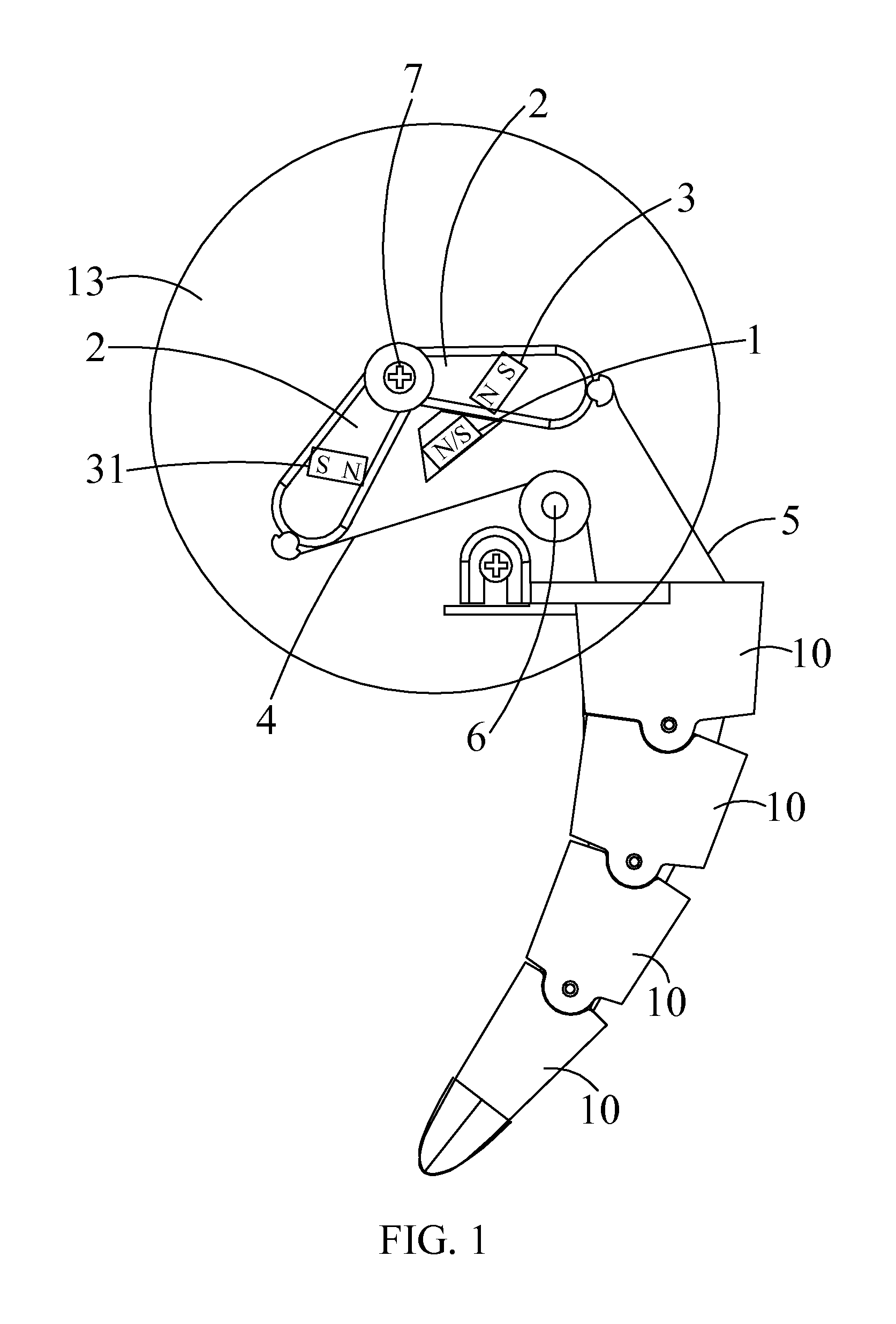
Simulation dog tail swinging installment
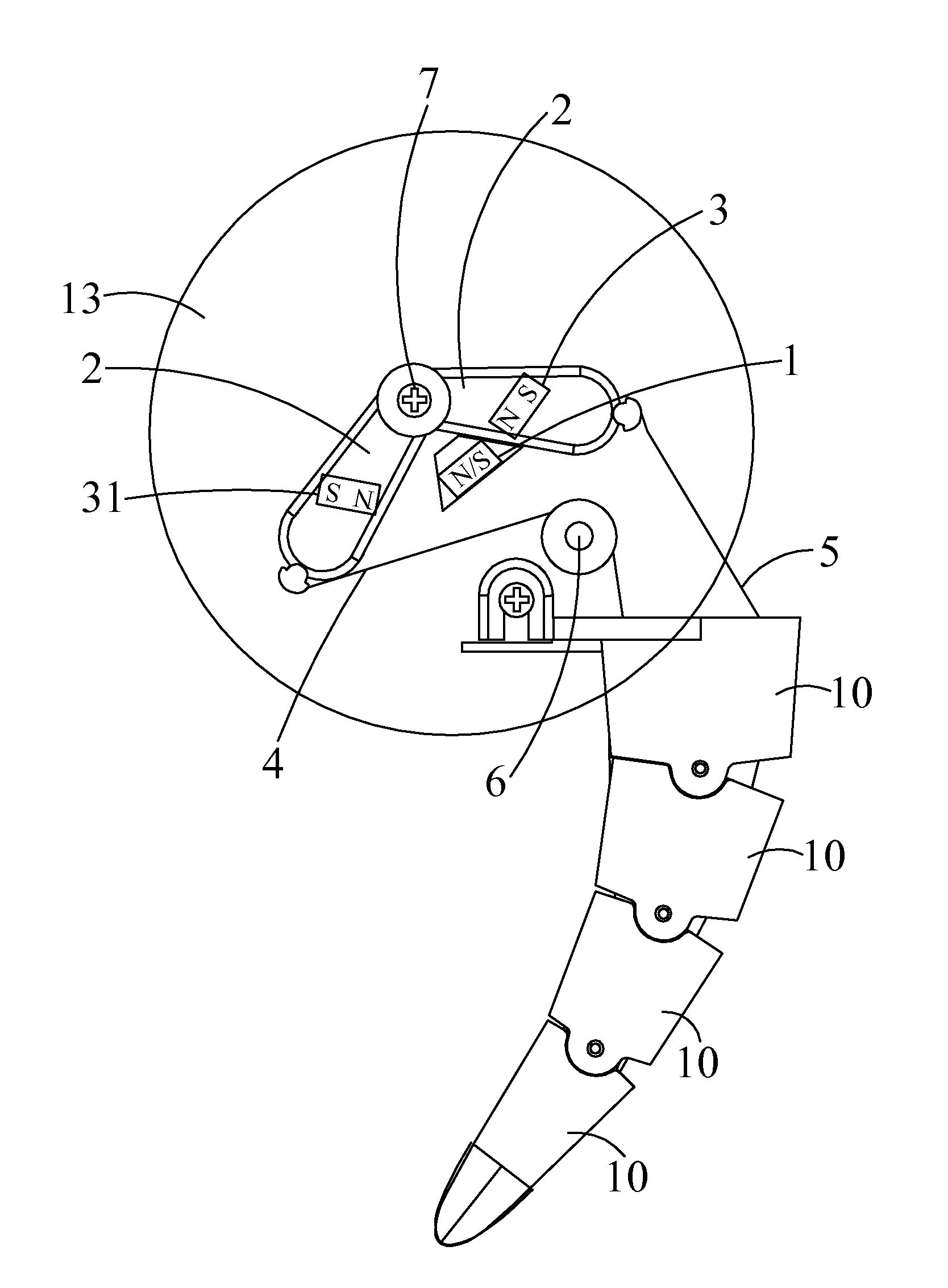
A simulation tail swinging installment includes a base plate, an electromagnetic coil disposed on the base plate, a battery module configured for supplying power to the electromagnetic coil, and a control circuit coupled to the battery module. The simulation tail swinging installment further includes a furcated component having two arms disposed with respect to the electromagnetic coil, with the two arms located on opposite sides of the electromagnetic coil, respectively. Each of the arms includes a permanent magnet positioned in correspondence with the electromagnetic coil. The furcated component is mounted to the base plate through a pivot connected to the base plate. A first driving cable and a second driving cable are attached to the two arms, respectively. The first driving cable and the second driving cable extend through and along two side portions of a simulation tail and secured to a distal end of the simulation tail.
Owner:TSUI KING LAM
Shielded optical probe having an electrical connector
InactiveUS7132641B2Eliminate needEasy to adaptRotary current collectorInvestigating moving sheetsElectrical connectionEngineering
A noninvasive optical probe has an electrical connector for connecting the optical probe to a cable connector. According to one embodiment, the electrical connector includes a durable flexible tab suspended between the housing of the optical probe and a protective cover. The electrical connector also advantageously forms, according to various embodiments, a flexible, plugable, lockable, removable, and sealable electrical connection.
Owner:JPMORGAN CHASE BANK NA
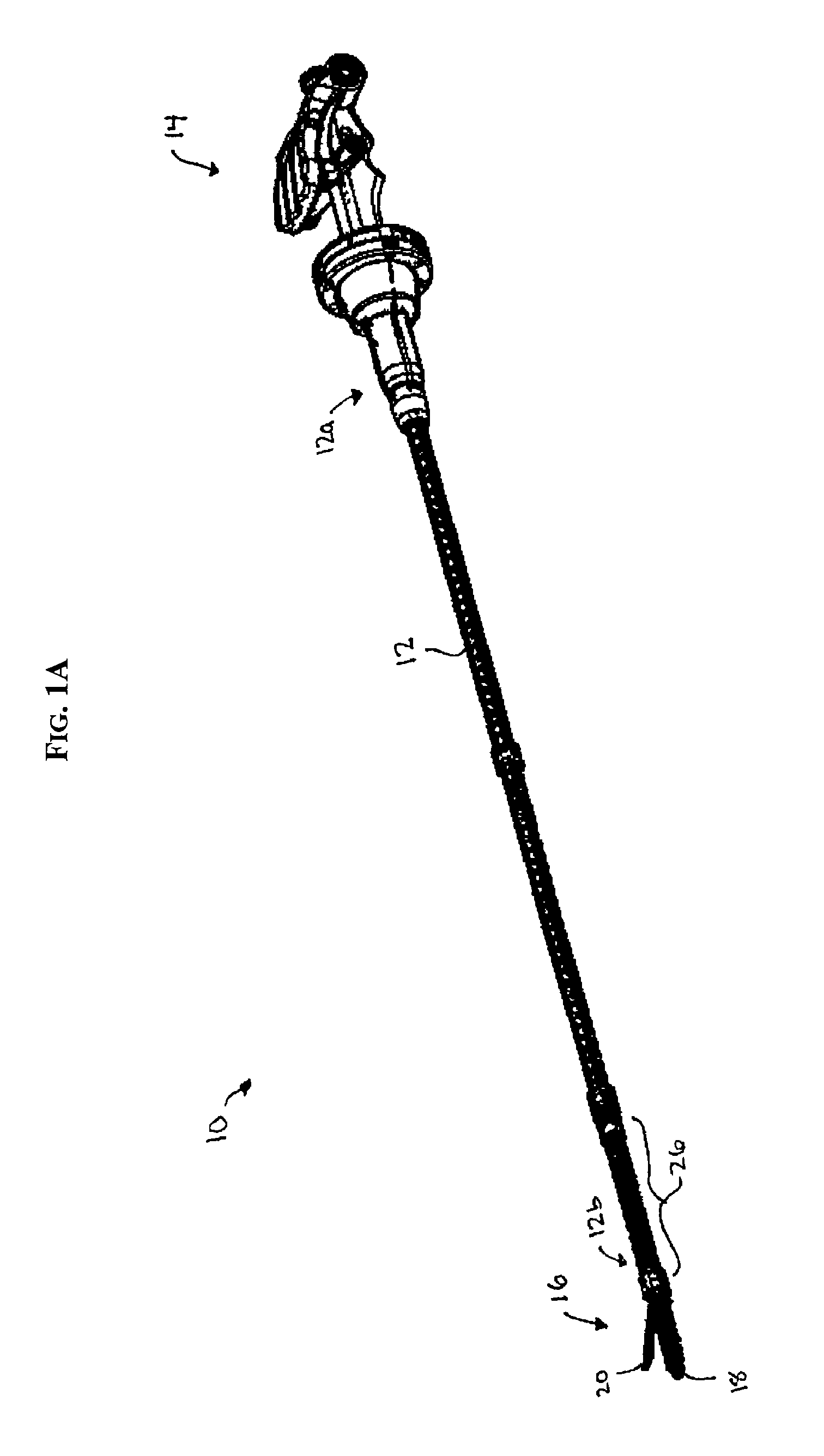
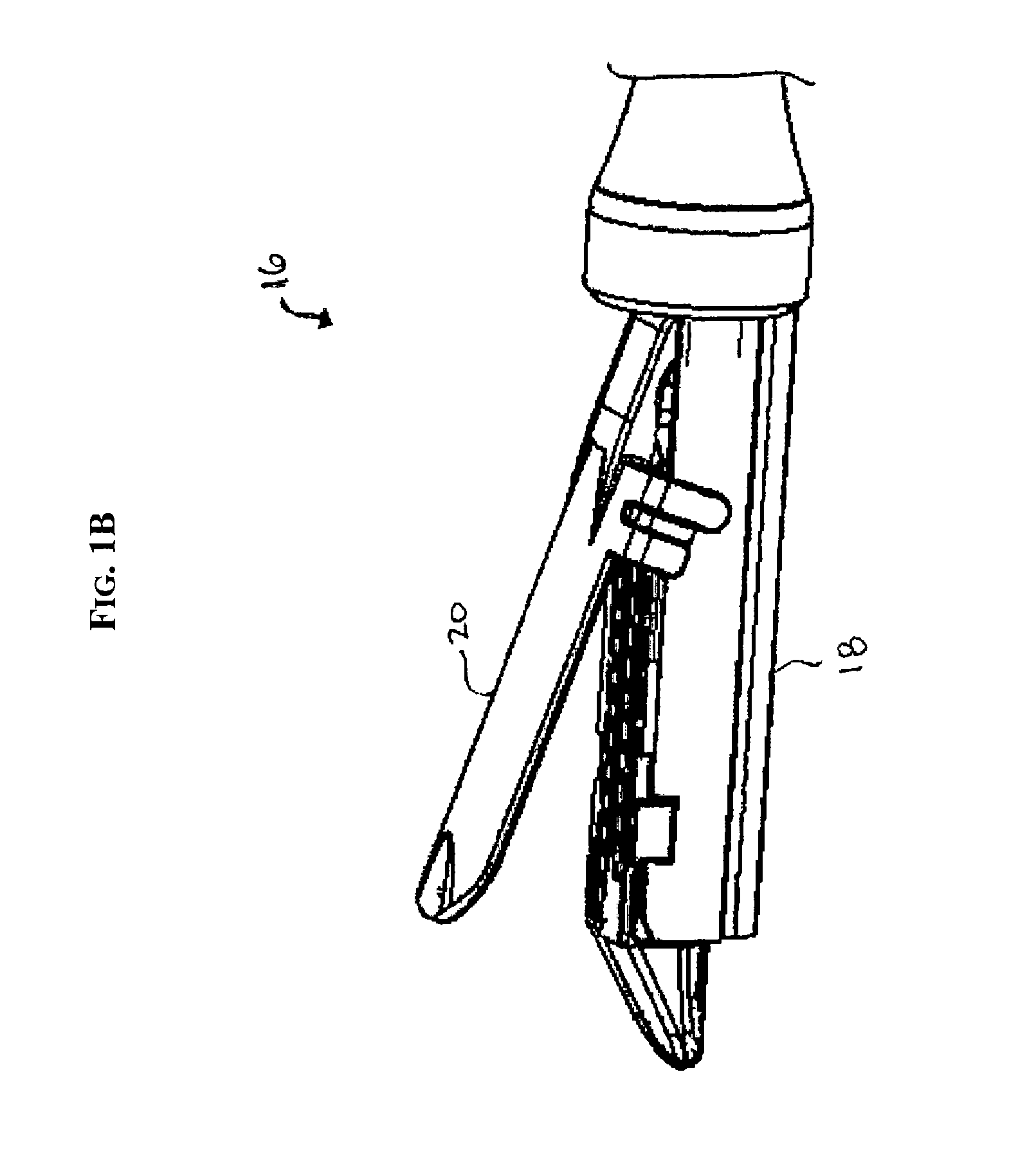
Surgical instrument with removable shaft apparatus and method
ActiveUS7025775B2Easily and repeatedly sterilizedSimple designDiagnosticsSurgical forcepsEngineeringBearing surface
A surgical instrument includes a shaft having a proximal end and a distal end with an operating device disposed at the distal end. A cable assembly is carried by the shaft and extends proximally to a terminus. A handle assembly coupled to the cable assembly concludes a first handle and a second handle. The first handle includes portions configured to receive the terminus and the proximal end of the shaft while portions of the second handle are configured to receive the terminus of the cable assembly. The proximal end of the shaft and the terminus are simultaneously rotatable to cover the proximal end of the shaft to the portions of the first handle and to couple the terminus to the portions of the second handle. The handle assembly can be made sterilizable with a minimum of non-bearing surfaces, while the shaft assembly can be made disposable and interchangeable with various operating devices. An associated method includes the step of releasably locking the shaft assembly to the handle assembly.
Owner:APPL MEDICAL RESOURCES CORP
Connector switch
An interconnection between a sensor and a monitor has a cable, an information element and a switch. The cable has conductors providing electrical communication between a sensor connector and a monitor connector. The information element is readable by the monitor and mounted in the sensor connector, the monitor connector or the cable. A switch is mounted in the sensor connector and is responsive to the sensor connecting to and disconnecting from the sensor connector so as to alter the readability of said information element.
Owner:JPMORGAN CHASE BANK NA
Body implantable lead including one or more conductive polymer electrodes and methods for fabricating same
InactiveUS6999821B2Transvascular endocardial electrodesDiagnostic recording/measuringElectrical conductorCoronary sinus
A body implantable lead comprises a lead body including a conductive polymer electrode disposed along a distal end portion of the lead body for performing one or more of the functions consisting of pacing, sensing, cardioversion and defibrillation. An electrical conductor, preferably in the form of a multistrand cable conductor, couples the conductive polymer electrode with a proximal end of the lead body. The conductive polymer electrode encapsulates the conductor and is in electrical contact therewith along the length, and preferably along substantially the entire length, of the conductive polymer electrode. The lead body may comprise a multilumen polymer housing, the conductor being contained within one of the lumens of the housing. The conductive polymer electrode may be disposed within a window formed in the lead body. Alternatively, the conductive polymer electrode may comprise multiple electrode sections within a corresponding number of windows formed in the lead body and spaced apart along the length thereof. Further, the window and the conductive polymer electrode disposed therein may extend helically about the lead body. Because of its flexibility and because it can have a small diameter, the lead of the invention is particularly advantageous for implantation in the small, tortuous vessels of the coronary sinus region of the heart for left side stimulation and / or sensing.Methods of fabricating lead bodies incorporating conductive polymer electrodes are also disclosed.
Owner:PACESETTER INC
Manual and automatic probe calibration
InactiveUS7496391B2Complicates designIncreased expenseRadiation pyrometrySpectrum investigationElectronic componentProbe calibration
Embodiments of the present disclosure include an oximeter sensor system including a reusable portion including a substantially rigid connector connected to an end of a cable. The substantially rigid connector includes an electronic element housing at least one electronic component of a probe. The system also includes a disposable portion including a flexible wrap comprising a substantially rigid connection port shaped to receive the substantially rigid connector in a releasably securable manner.
Owner:JPMORGAN CHASE BANK NA
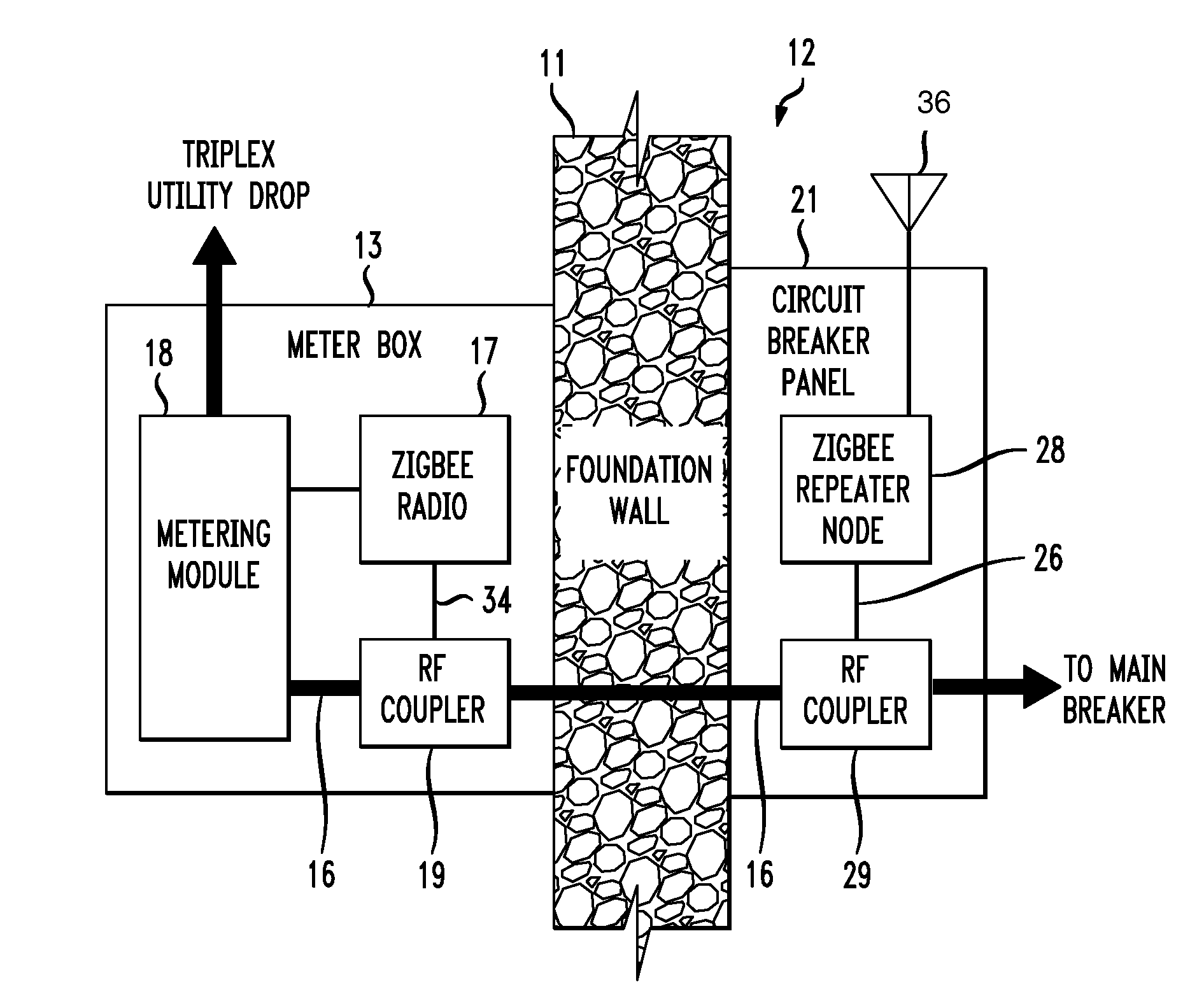
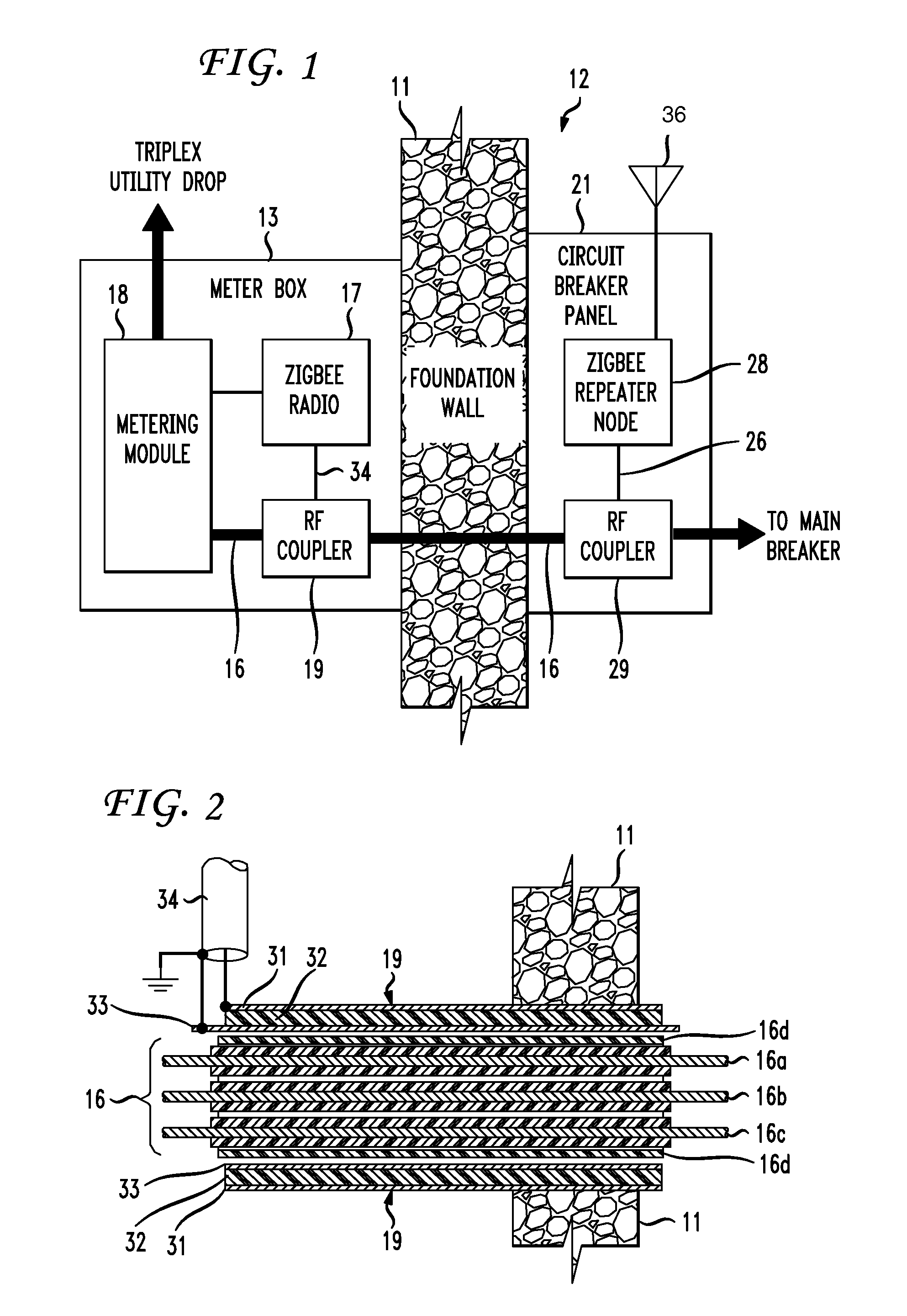
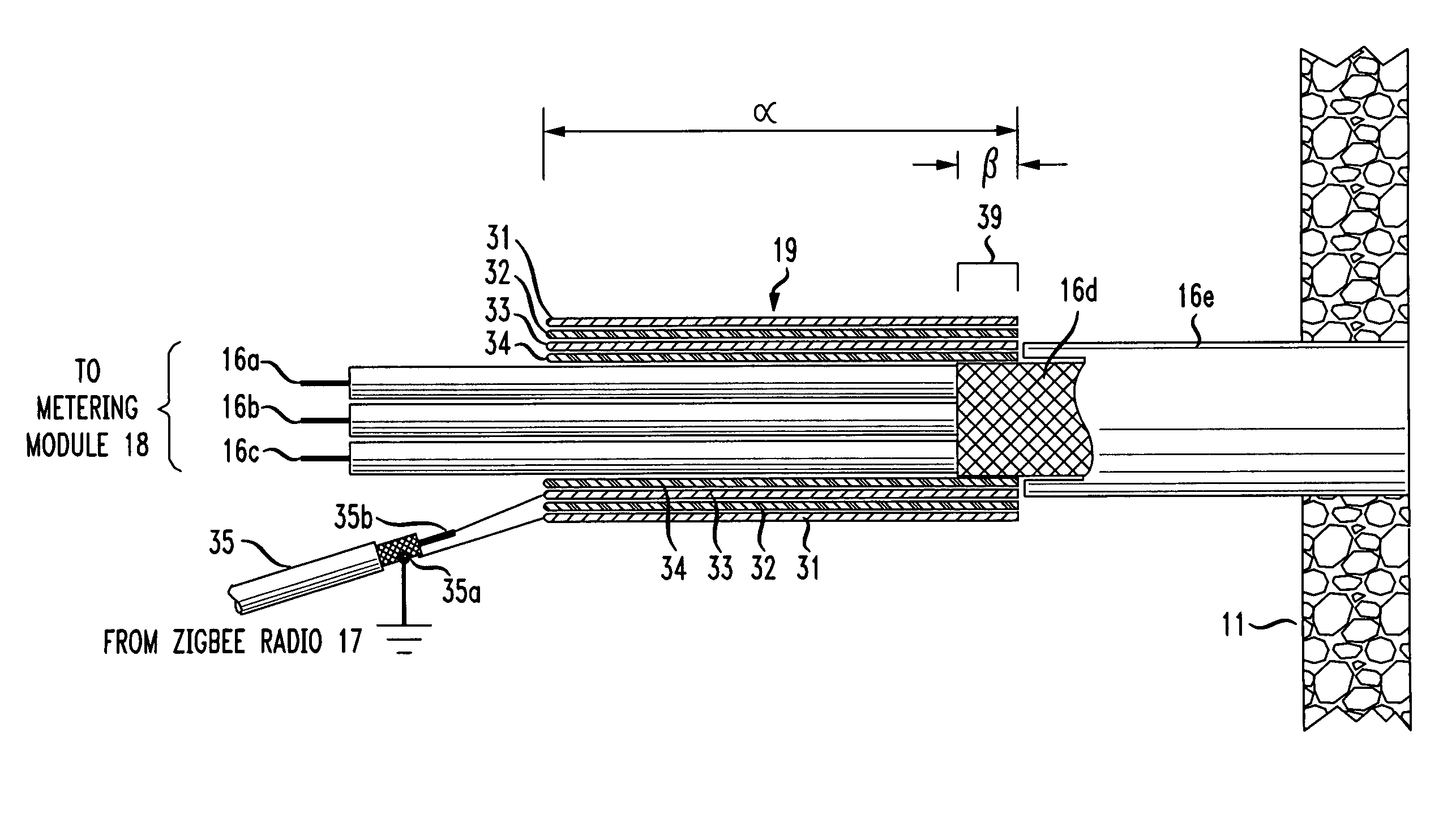
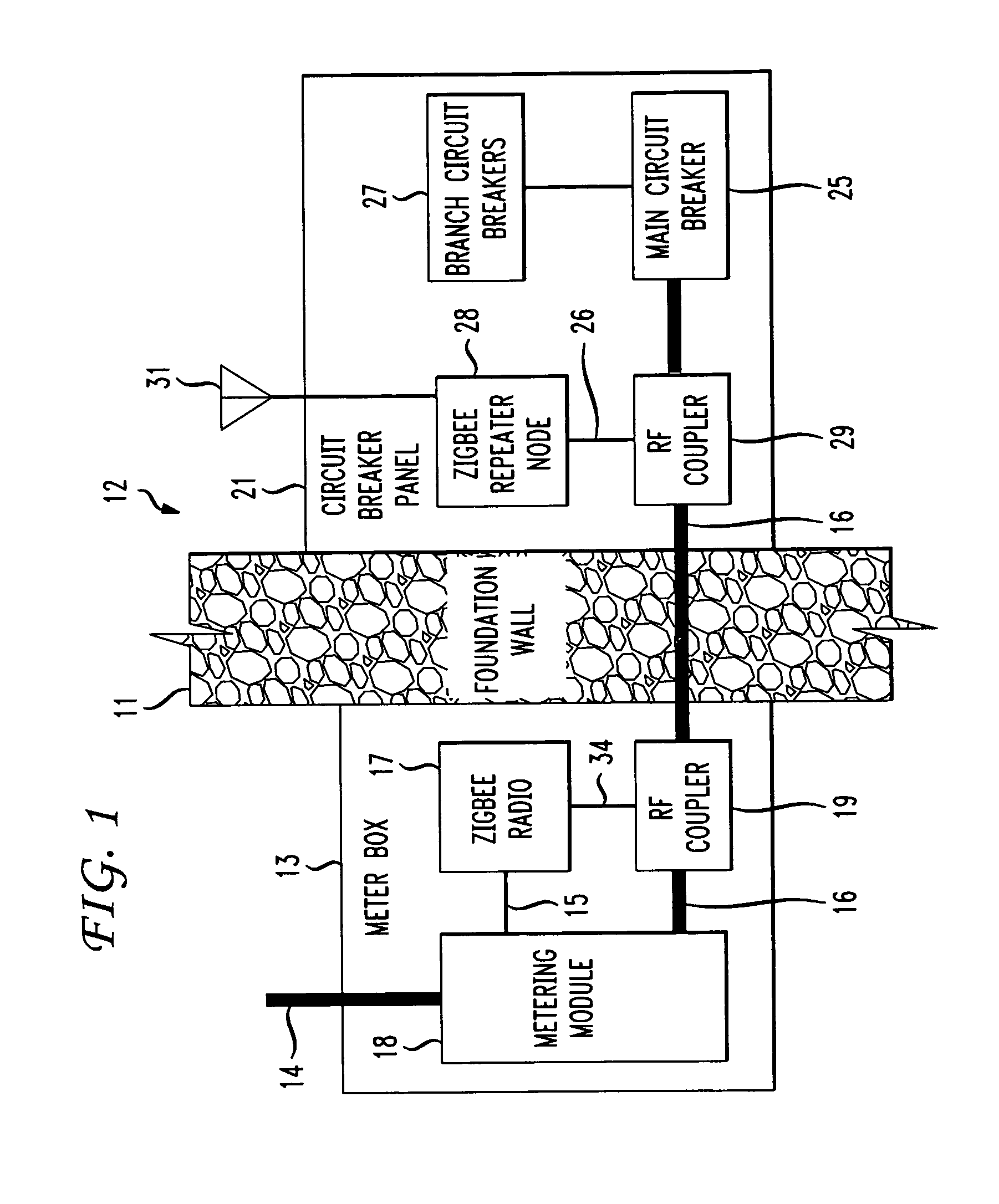
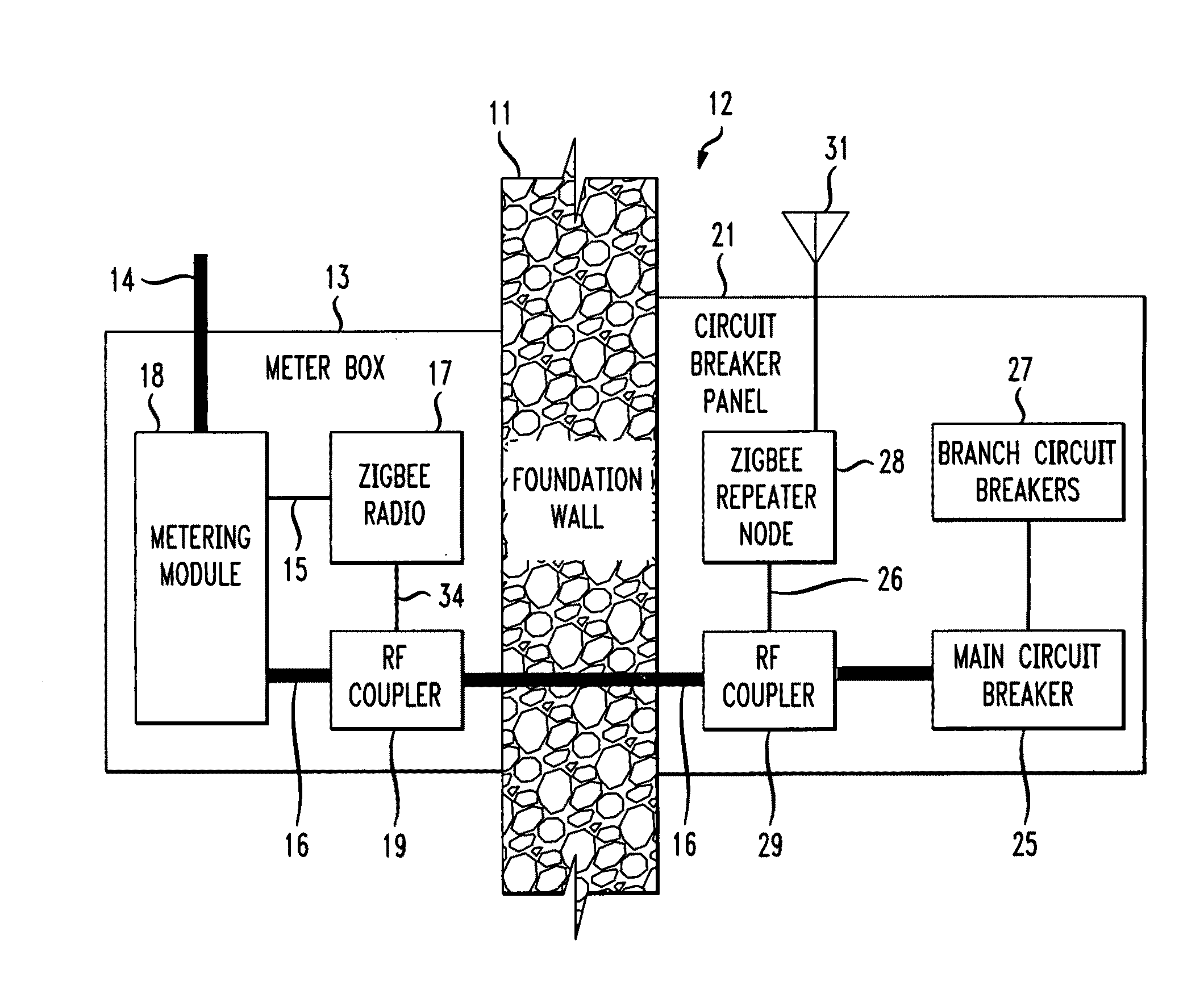
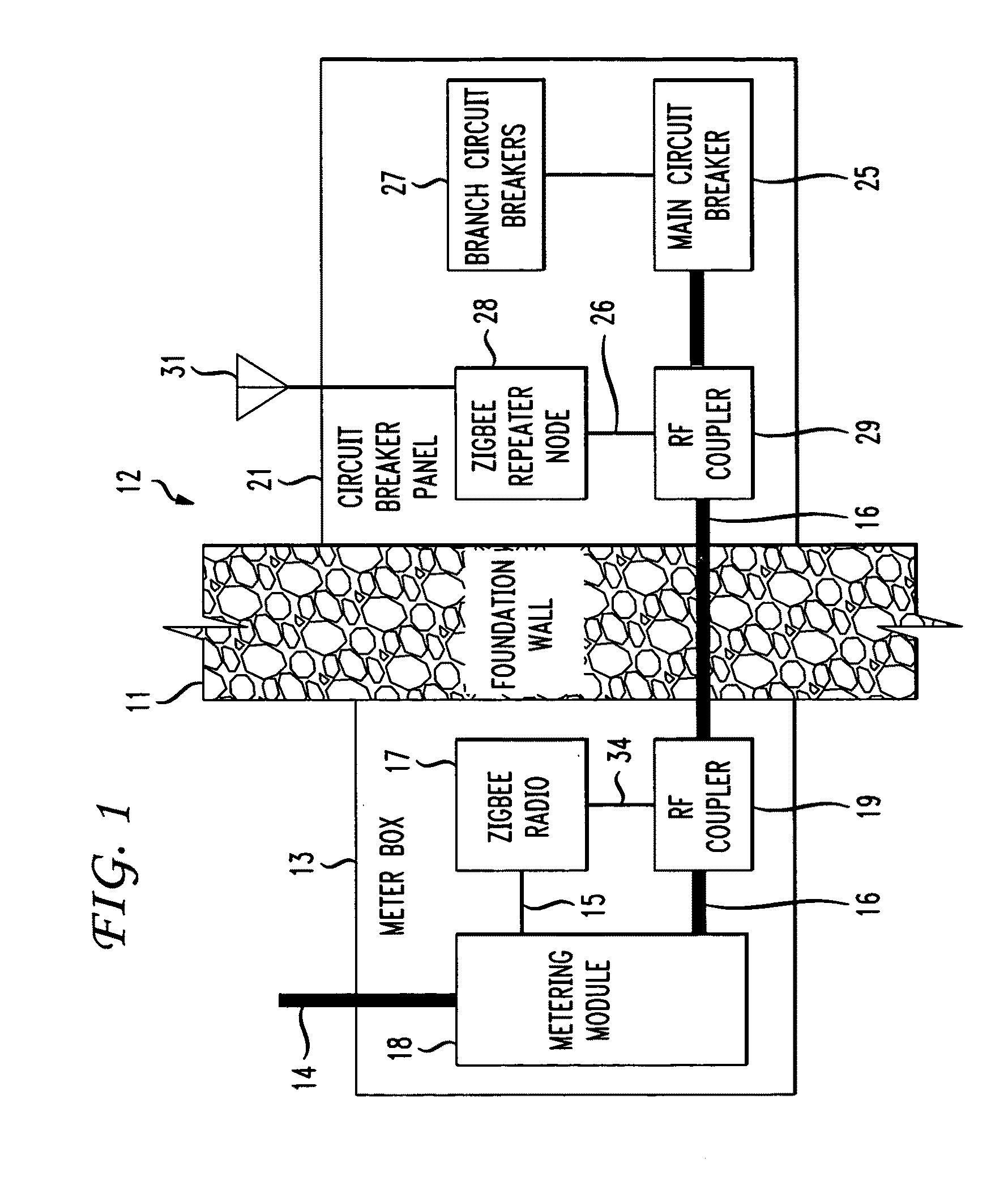
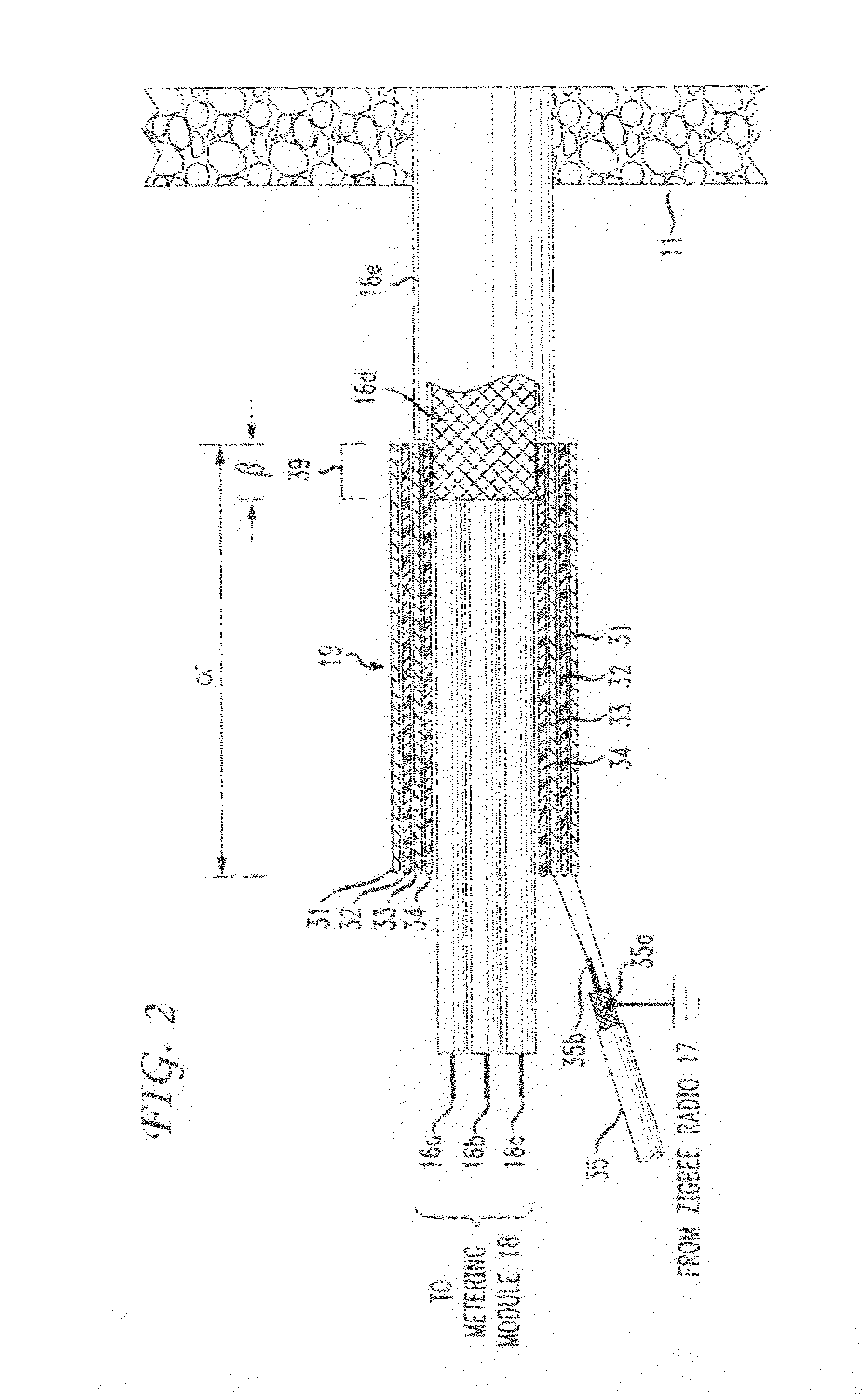
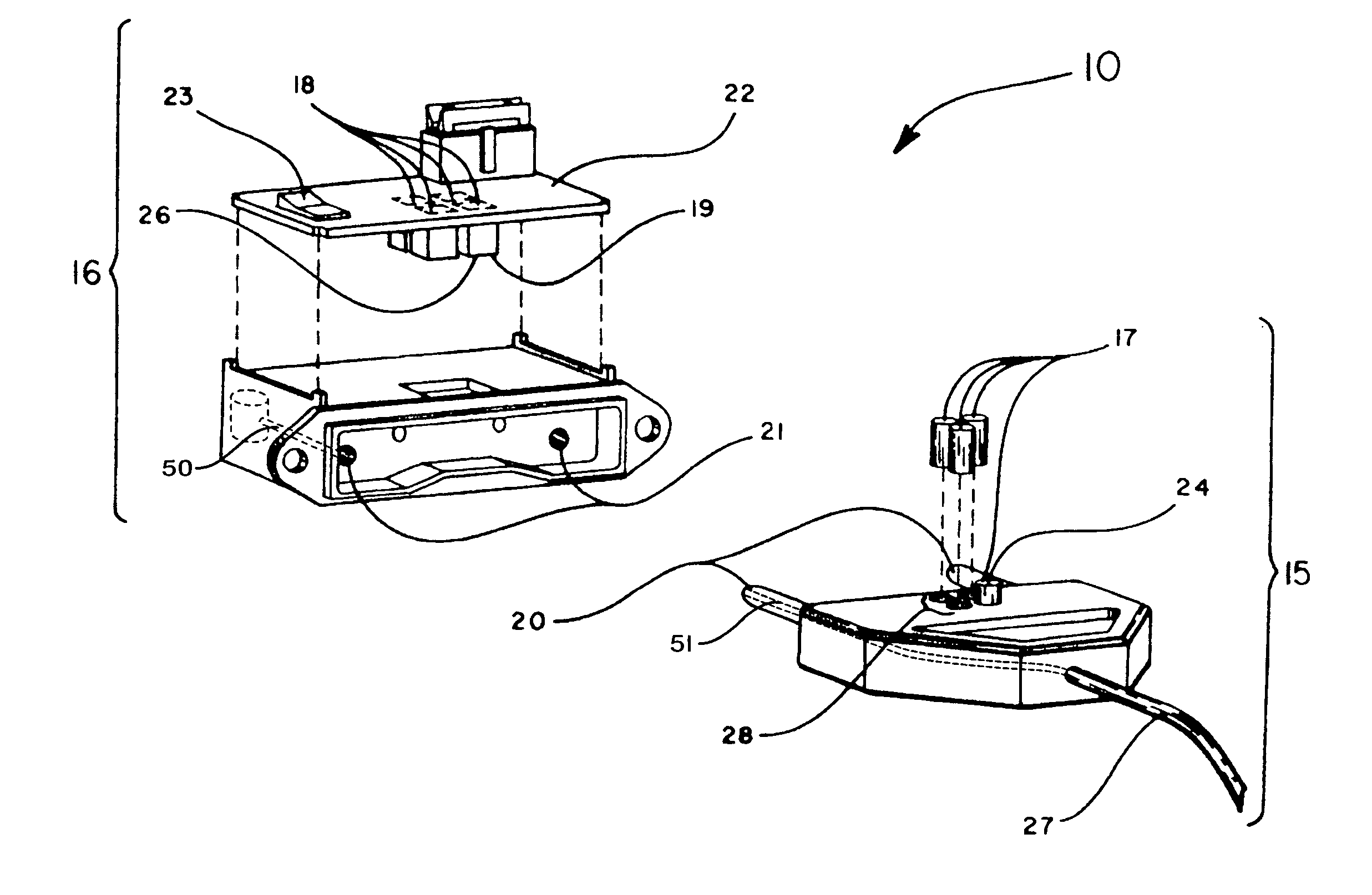
Technique for conveying a wireless-standard signal through a barrier
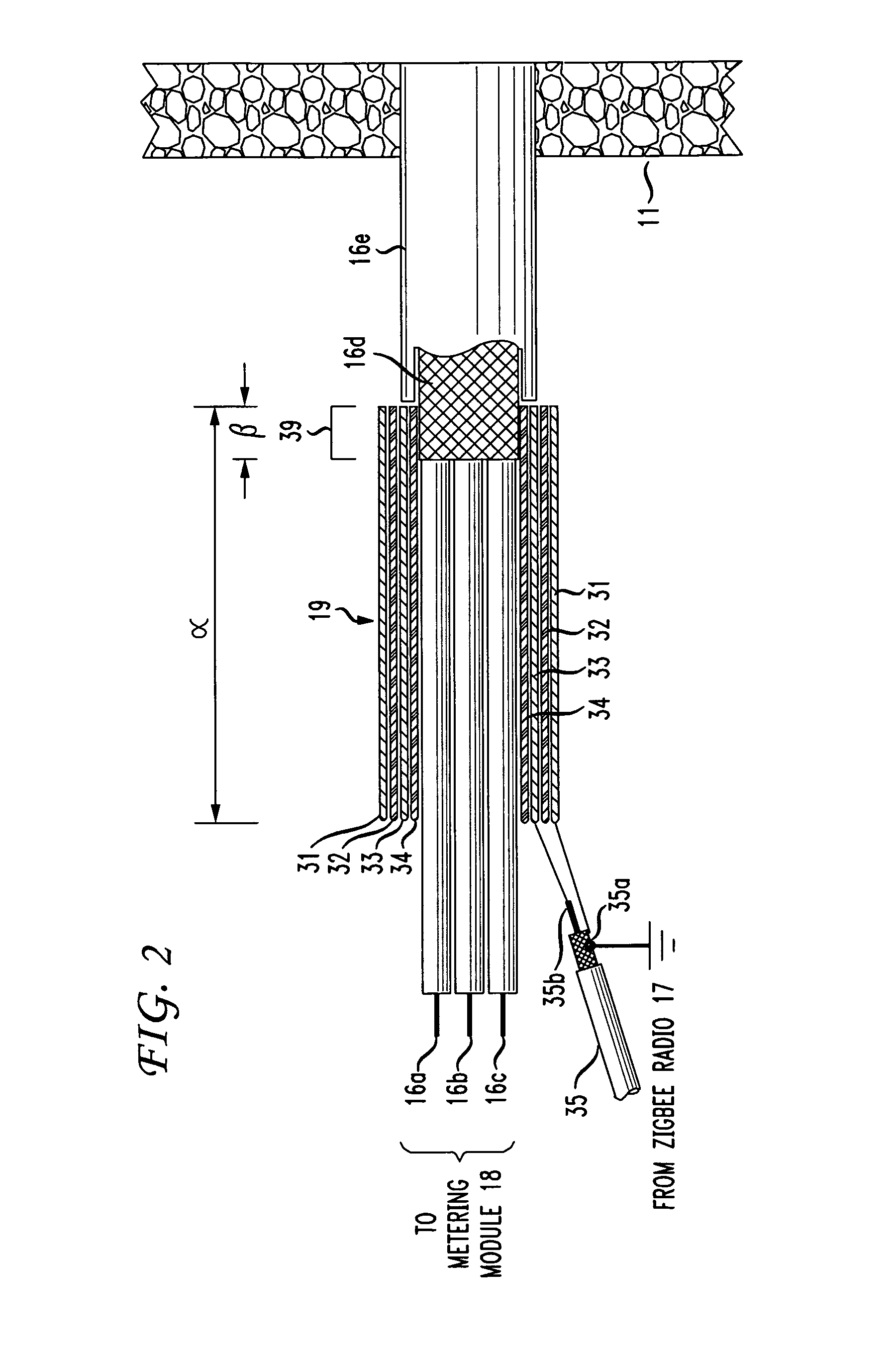
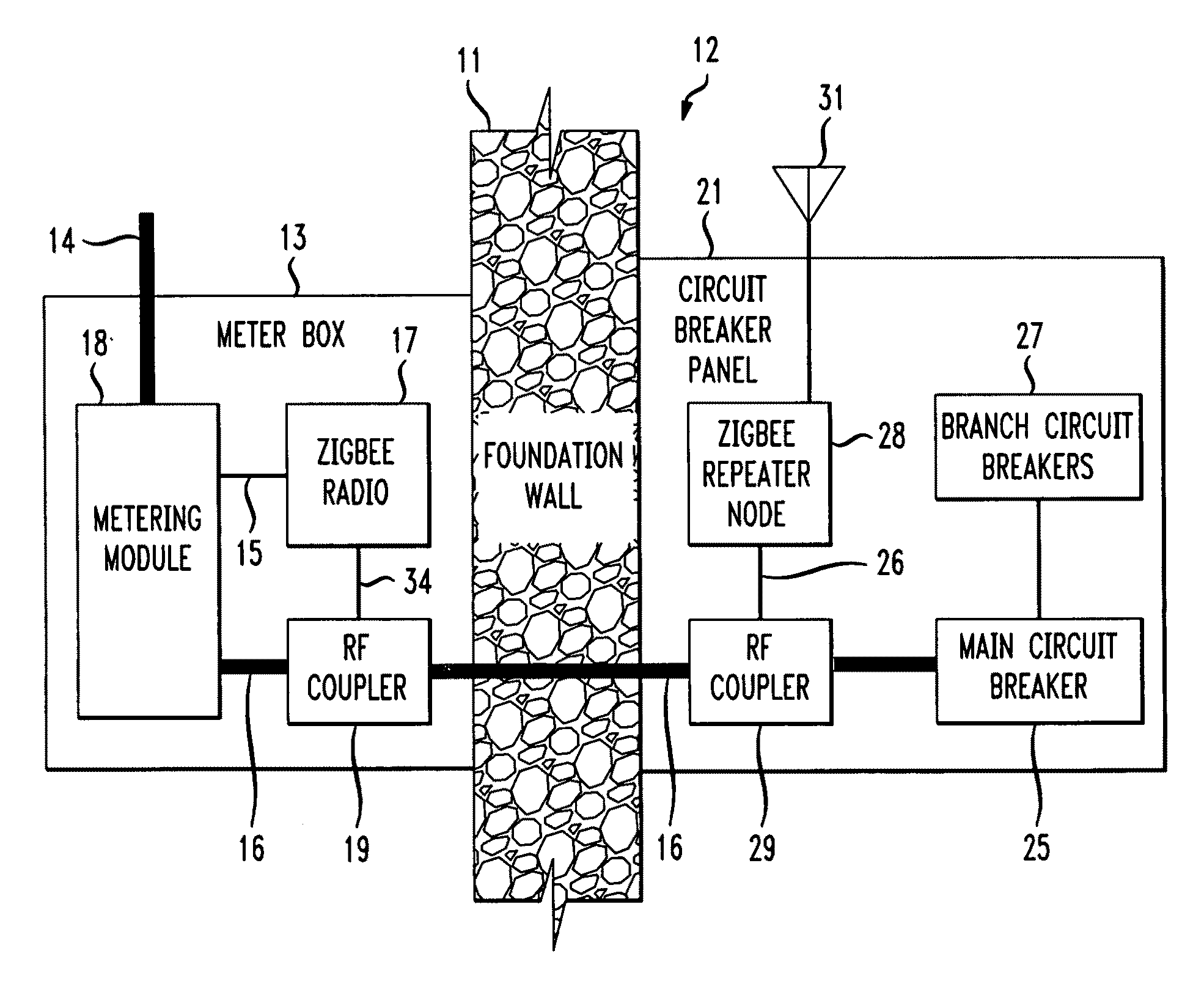
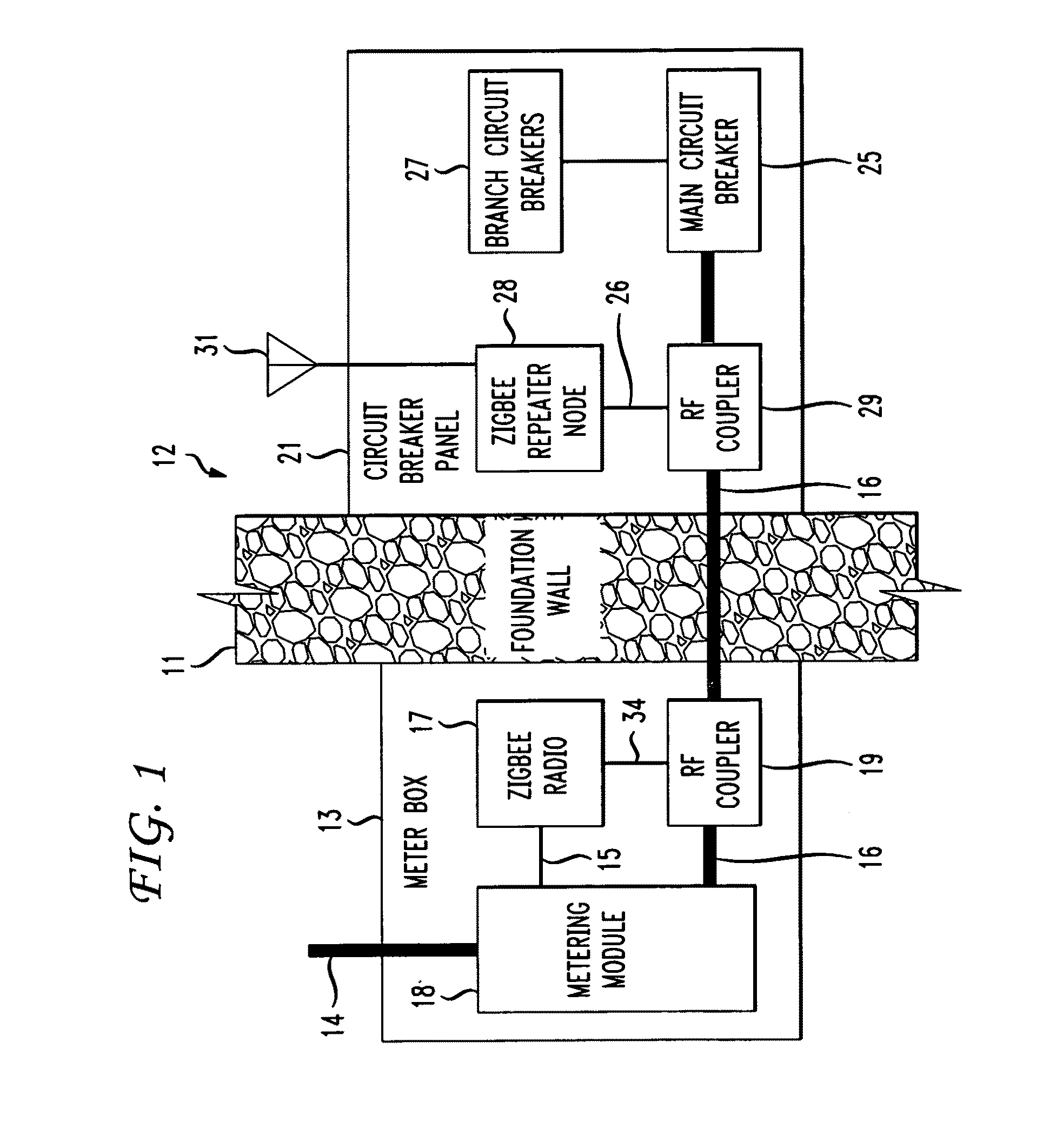
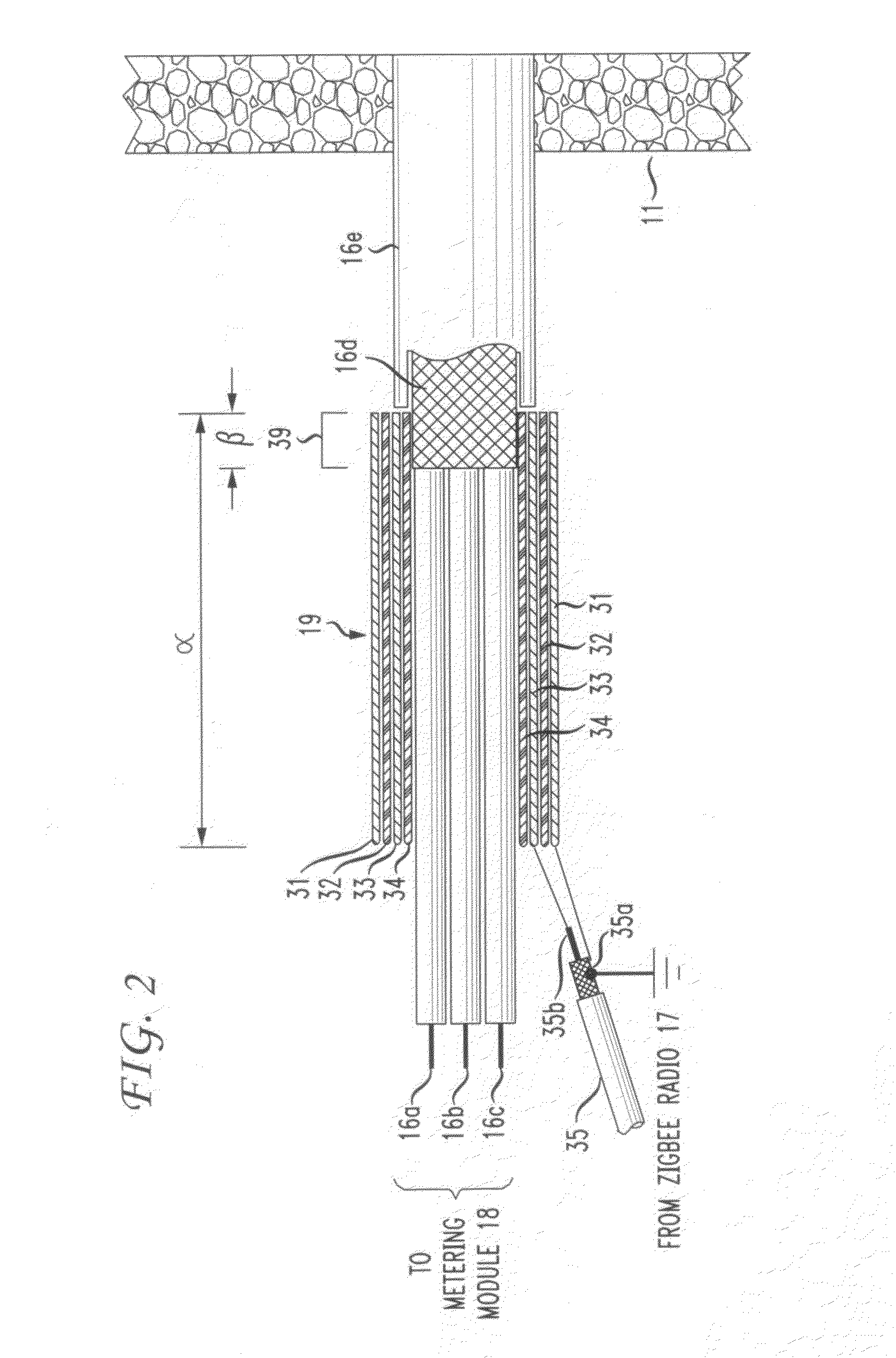
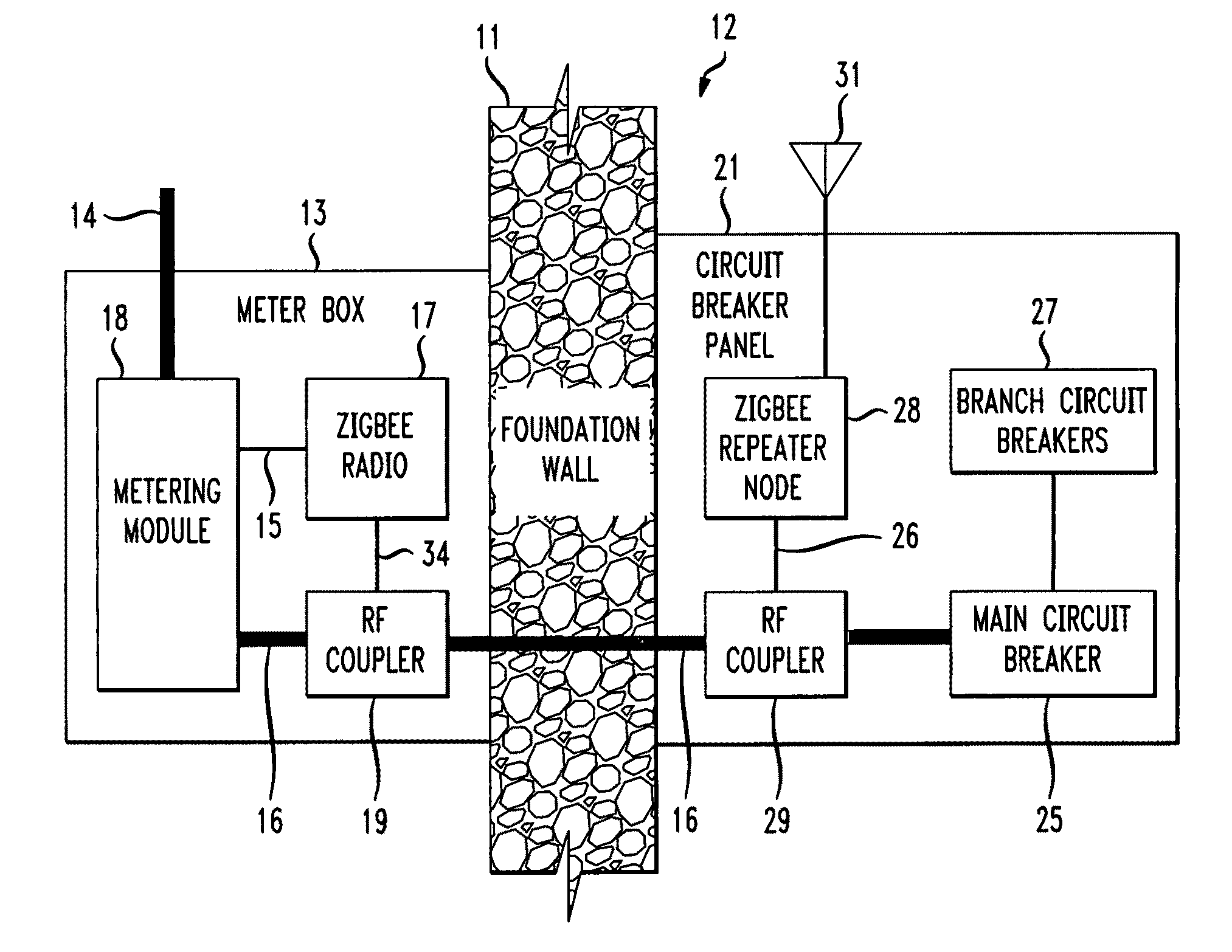
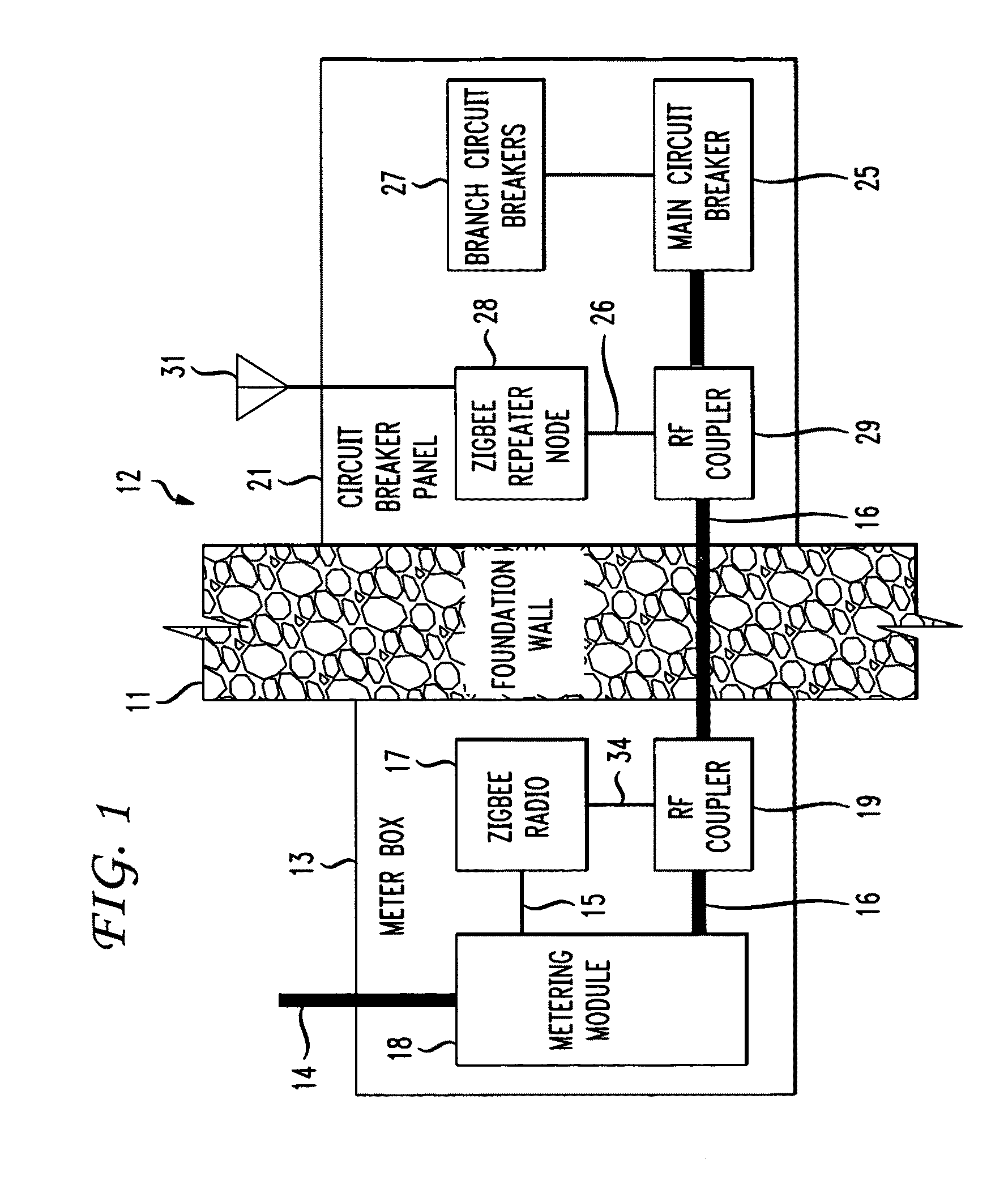
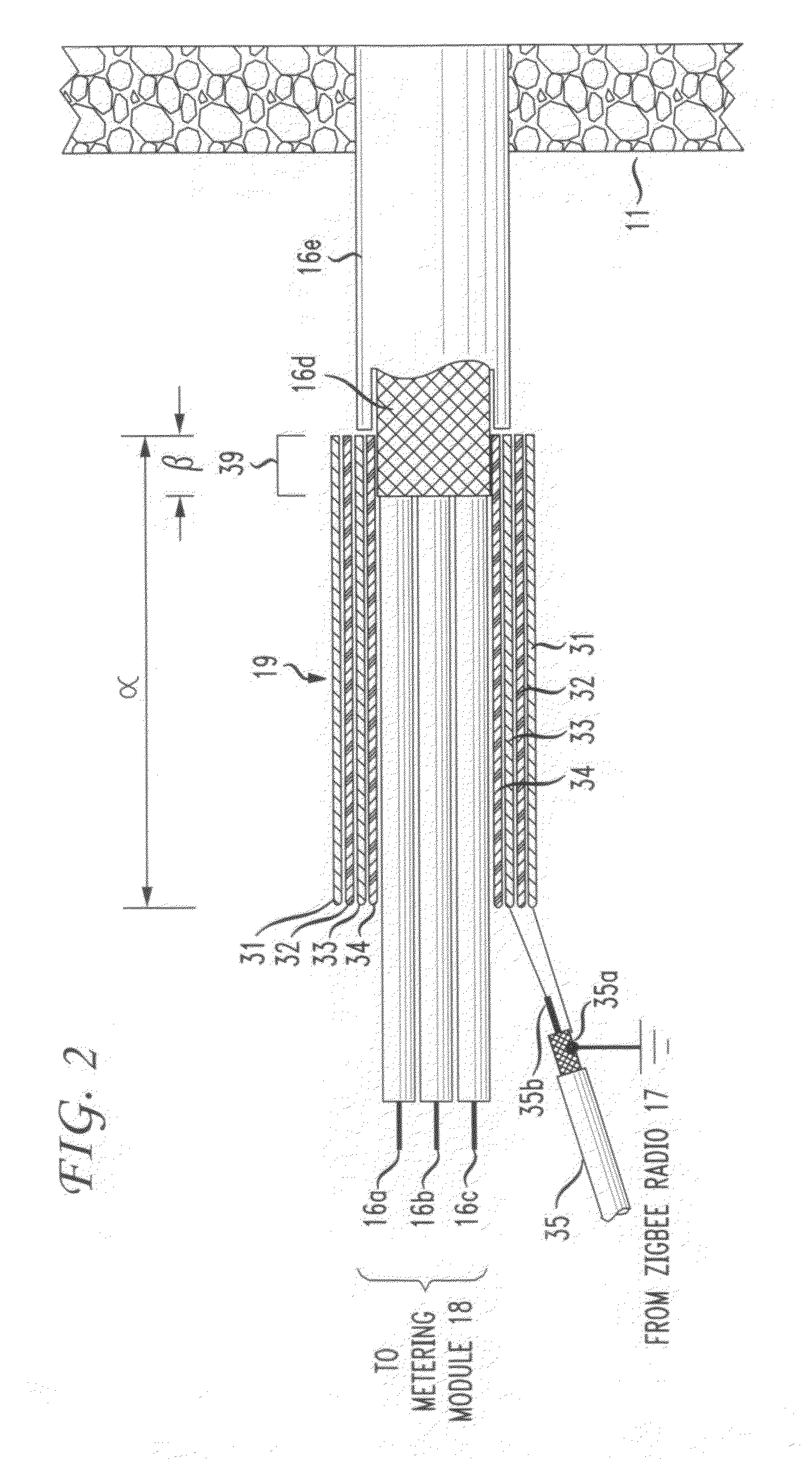
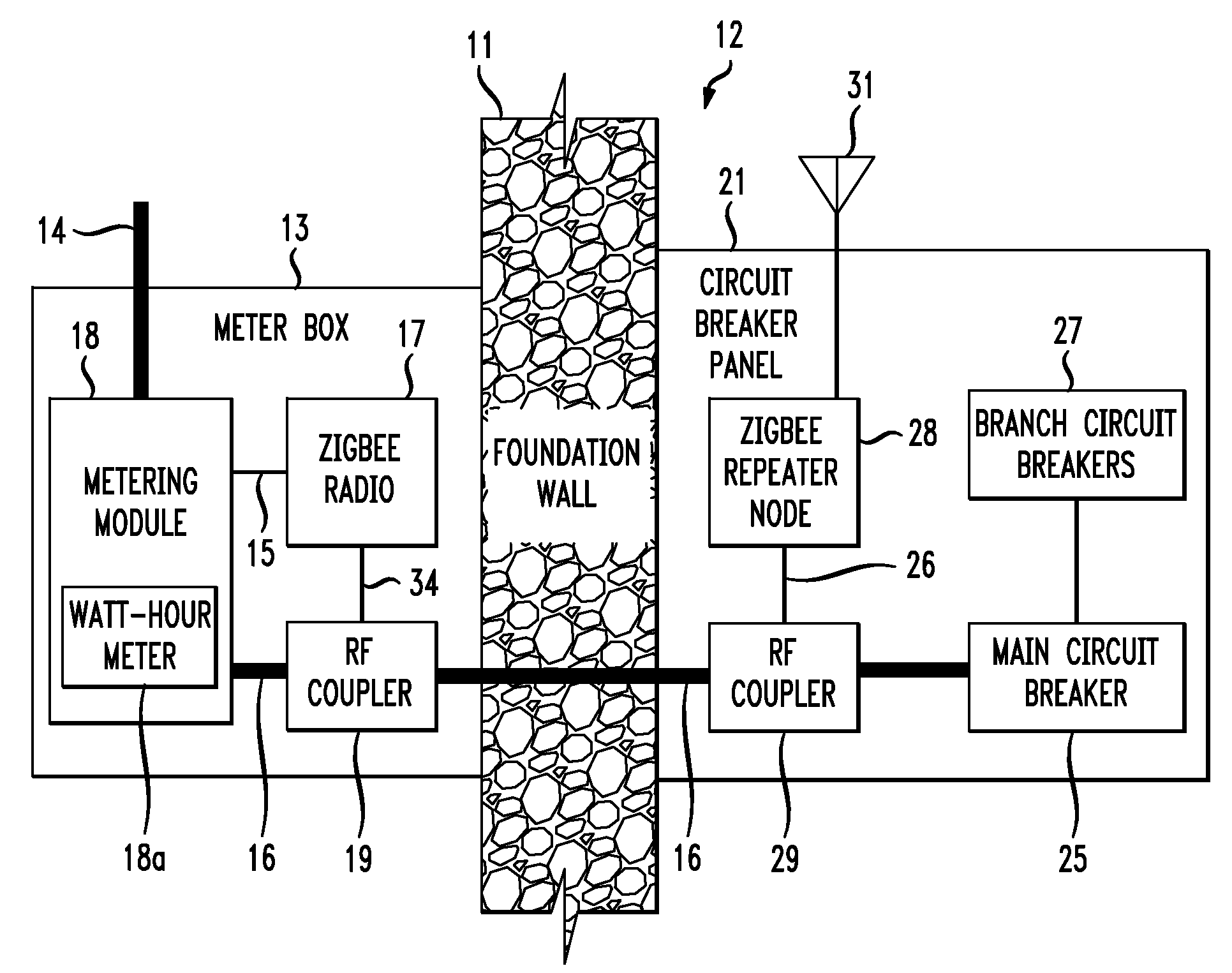
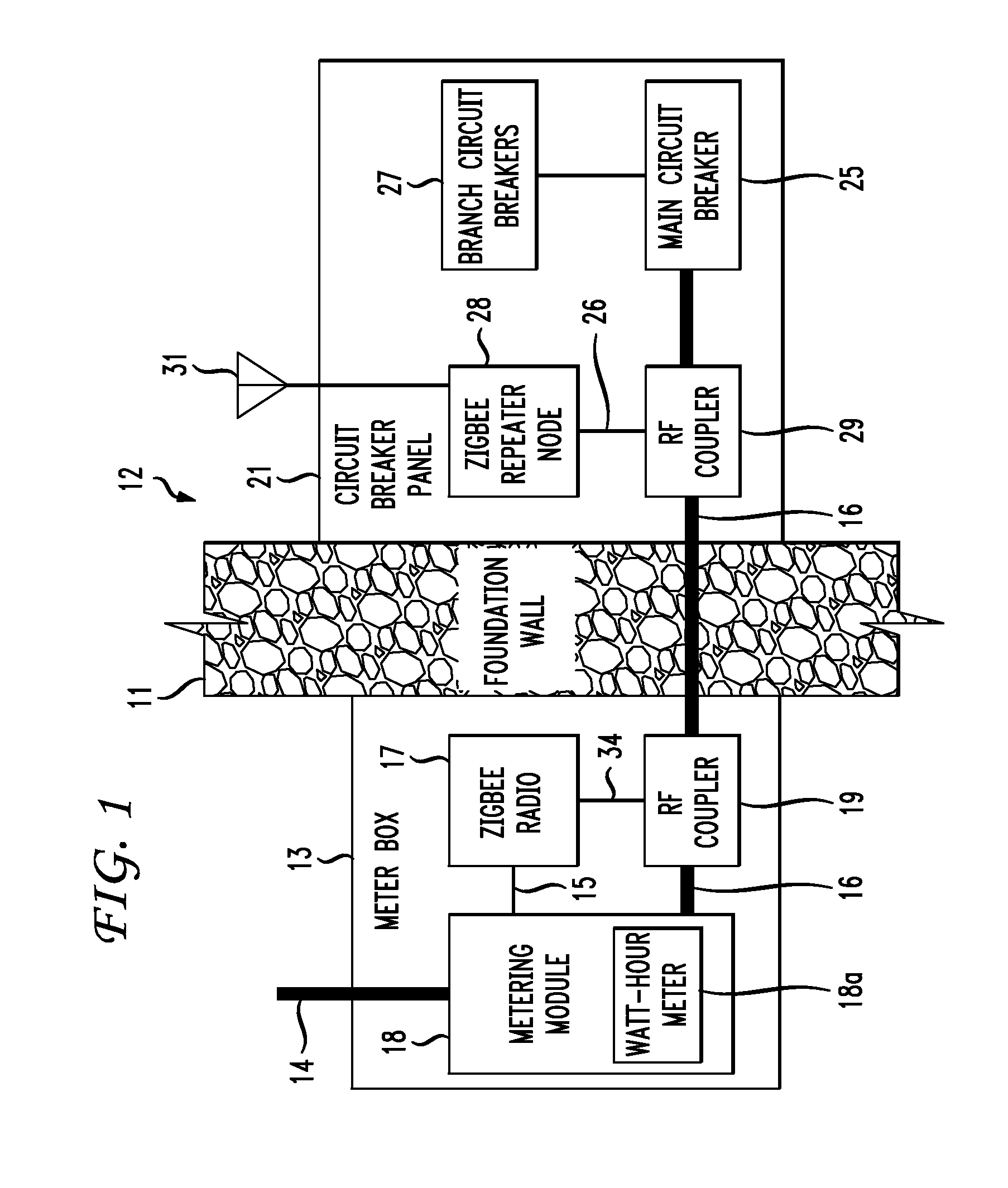
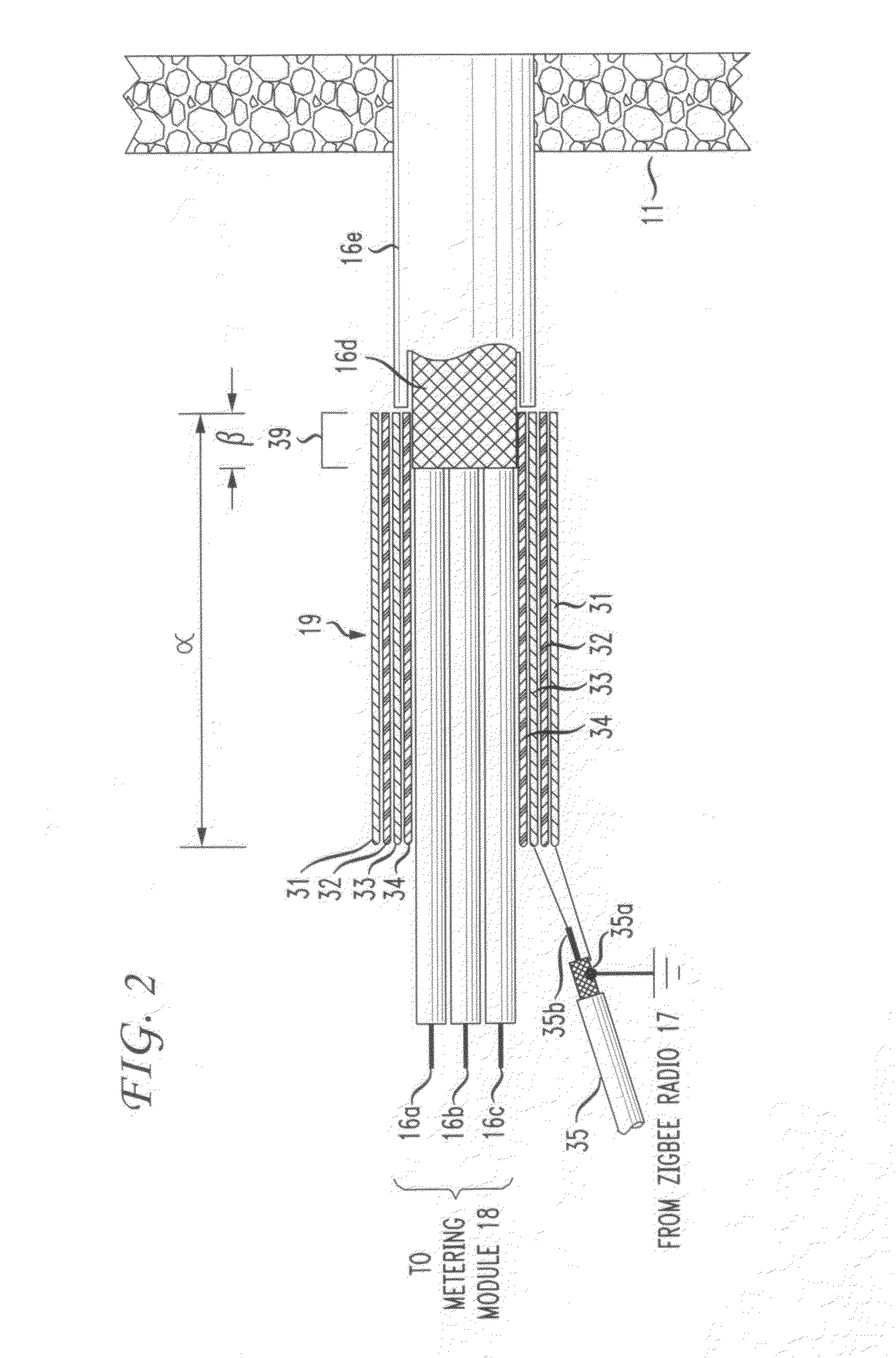
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I L P
Surface wave coupler
ActiveUS8212635B2Frequency-division multiplex detailsPower distribution line transmissionElectrical conductorEngineering
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I LP
Flexible instrument
A robotic medical system comprises a catheter having an elongated shaft with a plurality of flexible segments spaced apart along the catheter, and a drive unit coupled to the catheter. The catheter may have a plurality of cables respectively terminating at the flexible segments, in which case, the drive unit will be coupled to the plurality of cables. The robotic medical system further comprises a user interface remote from the drive unit and configured for generating at least one command, and an electric controller configured, in response to the command(s), for directing the drive unit to independently bend the flexible segments (e.g., three flexible segments).
Owner:AURIS HEALTH INC
Using surface wave propagation to communicate an information-bearing signal through a barrier
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I L P
Using an electric power cable as the vehicle for communicating an information-bearing signal through a barrier
ActiveUS8253516B2Frequency-division multiplex detailsOne-port networksPower cableElectrical conductor
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it as a surface wave along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I L P
Using surface wave propagation to communicate an information-bearing signal through a barrier
ActiveUS8269583B2Frequency-division multiplex detailsTelephonic communicationElectrical conductorRadio frequency signal
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I L P
Flexible surgical stapler with motor in the head
A surgical stapler having a remote motorized staple head is provided and generally includes a handle having a control button and a highly flexible cable extending distally from the handle. A housing incorporating a staple assembly is affixed to a distal end of the flexible cable. The housing may incorporate articulating structure to position the staple assembly relative to the remainder of the housing.
Owner:TYCO HEALTHCARE GRP LP
Using an electric power cable as the vehicle for communicating an information-bearing signal through a barrier
ActiveUS20110136432A1Frequency-division multiplex detailsPower distribution line transmissionElectrical conductorPower cable
The RF signal generated by a ZigBee radio on the outside of a building structure is conveyed to the interior of the building by guiding it as a surface wave along an electric cable bundle that passes through the building's wall to supply domestic electric power to the interior of the structure. The RF signal is launched by a unique coupler comprising a pair of insulated foil conductors.
Owner:AT&T INTPROP I L P
Smart recognition apparatus and method
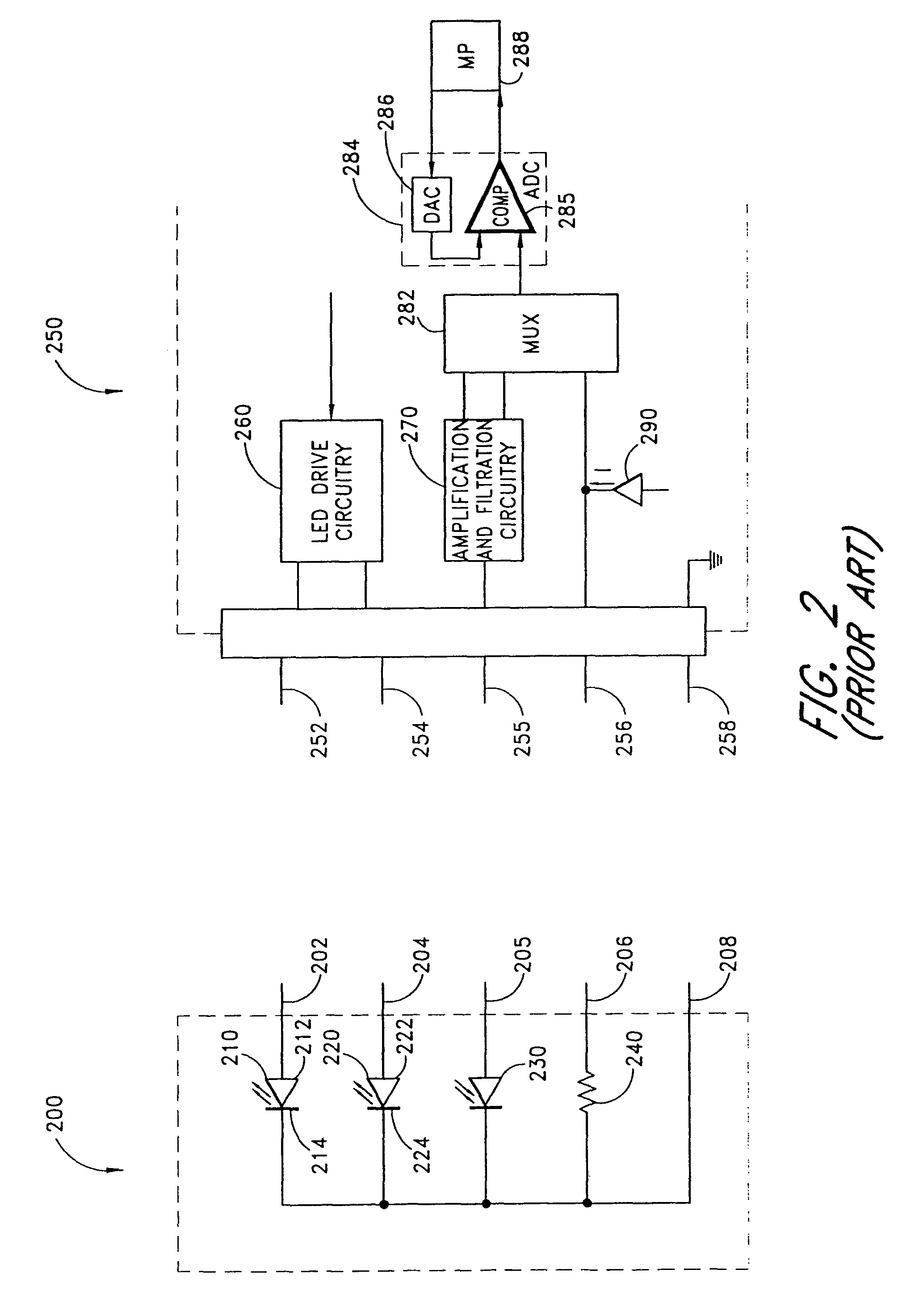
InactiveUS7044949B2Avoid problemsTight optical and mechanical toleranceDiagnosticsClose-range type systemsProximateElectrosurgery
A qualifying connection for an instrument attaches to a source of electrosurgery energy and to the instrument and has first and second parts coupled to the instrument and the source, respectively. Optical couplings on the connection transmit invisible energy to identify the instrument and are proximate on the first and second parts. A light modifier on the first part is proximal to the second part for modification of radiation in the infrared wavelengths so infrared transmitters encode signals and non-contact coded proximity detectors on the second part are the coupled detectors. Mechanical attachments include conjugating male and female portions which physically extend between the parts and matingly engage. An identifying circuit couples to the second part and responds to invisible light optically communicated across the couplings for verifying the type of instrument connected by the cable to the source. A method of using the connection has steps including juxtaposing and conjugating the parts with attachments and couplings for transmitting invisible optical energy to identify the instrument. The method includes modifying the invisible optical energy with geographically disposed proximate couplings of the parts when the attachments engage and the couplings are proximate. Passing and assessing signals of the modified energy are transmitted through the connection and to an identifying circuit in the source.
Owner:COVIDIEN AG
Real time monitoring of cable patch panel
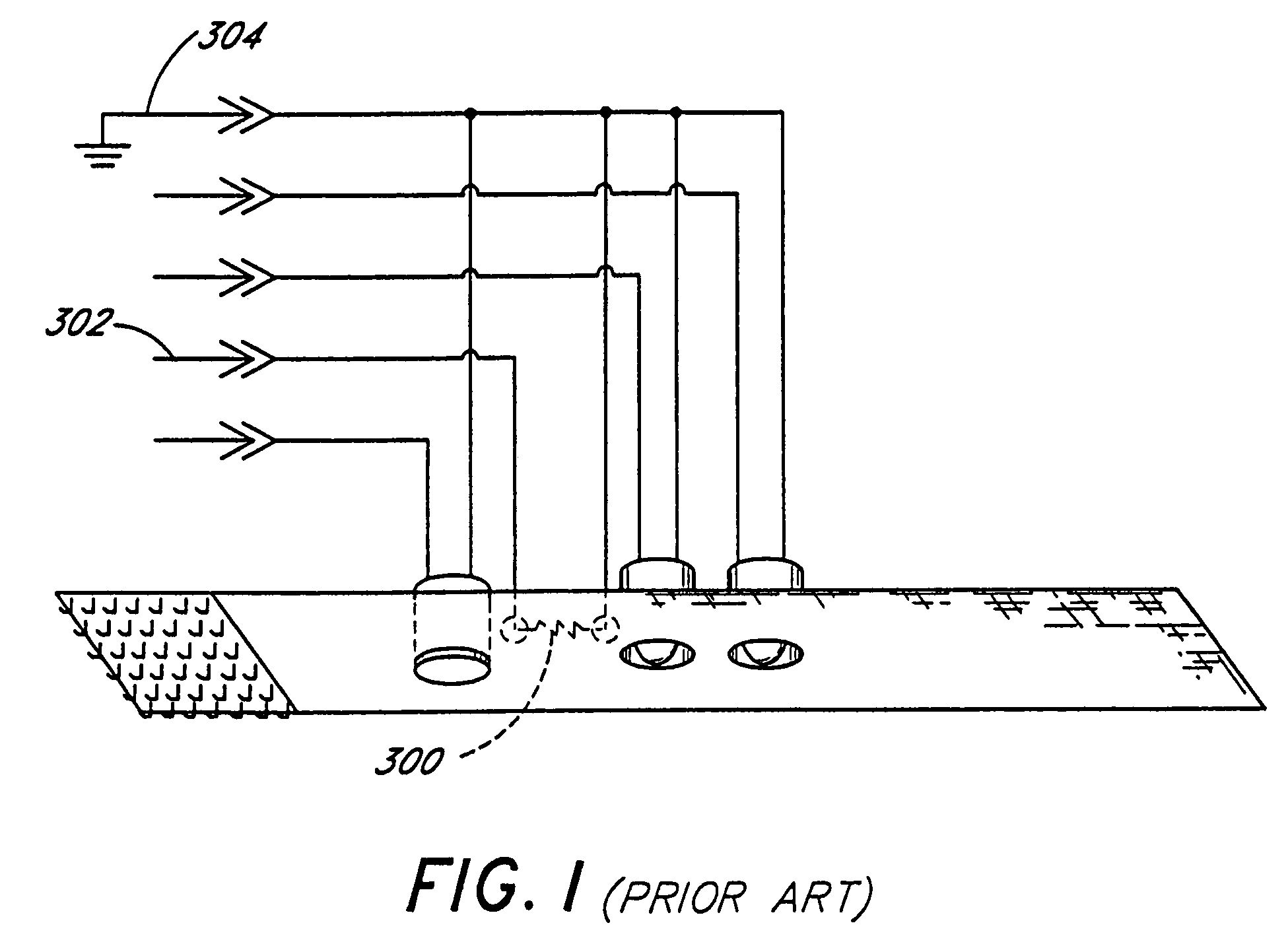
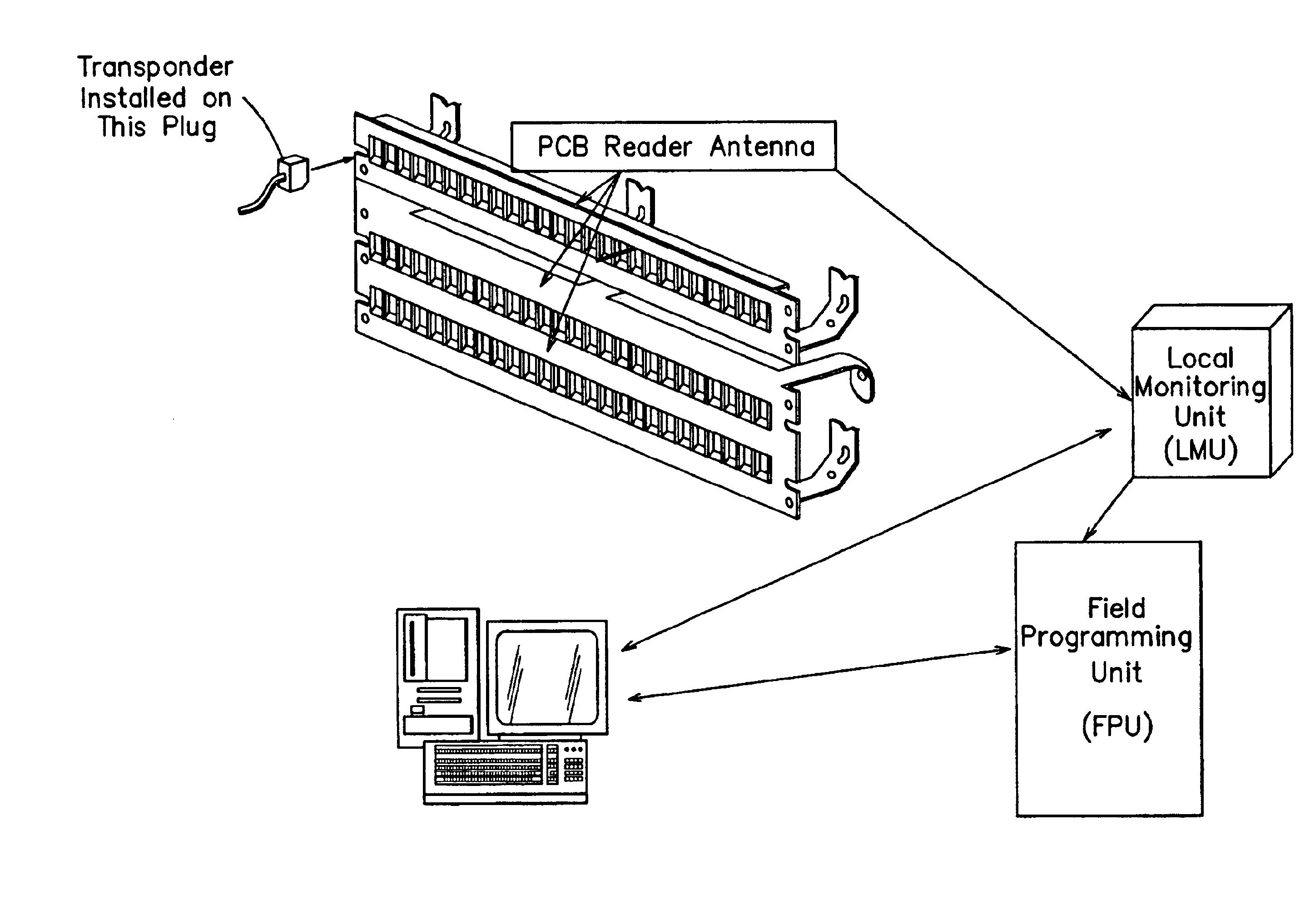
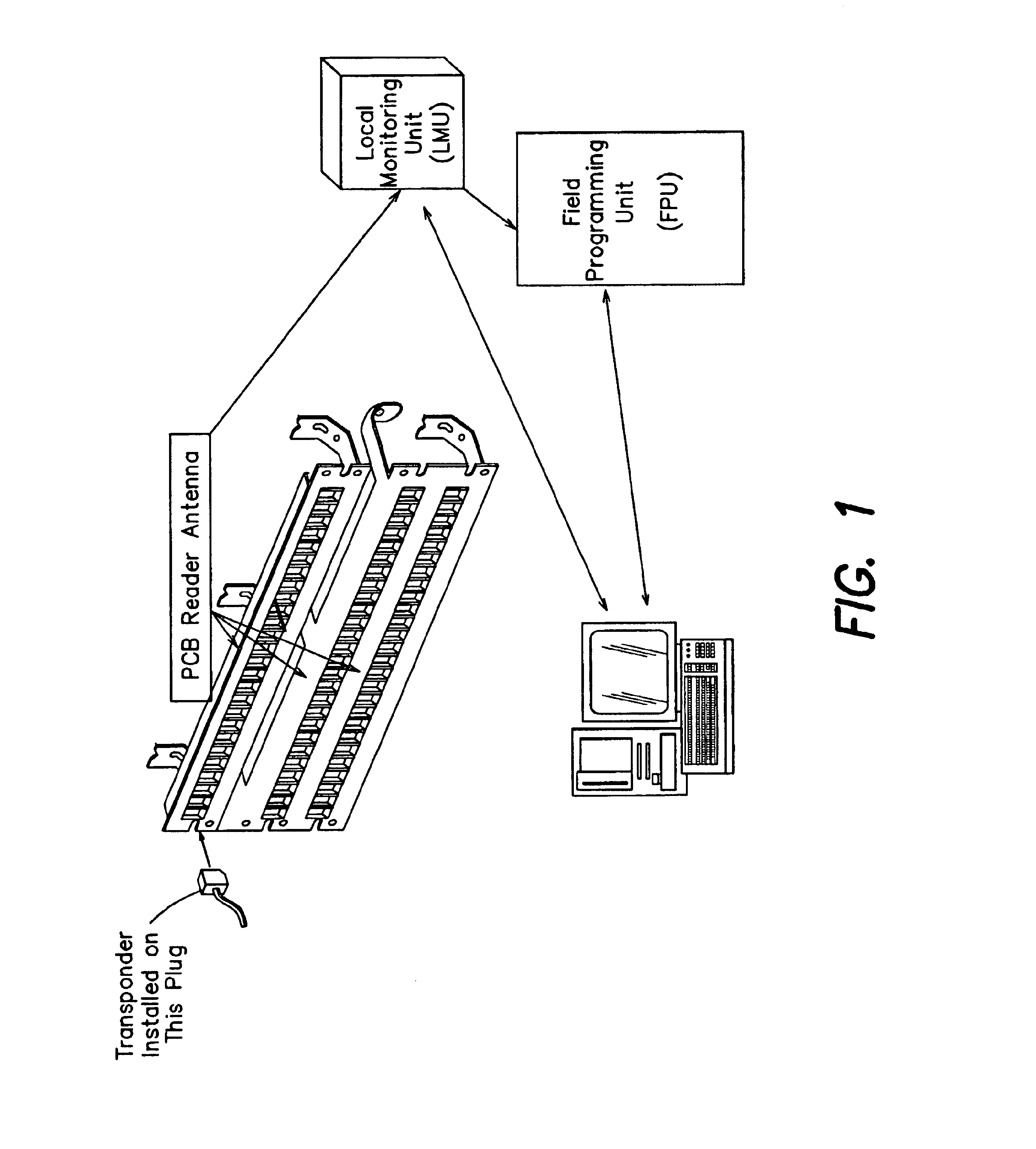
InactiveUS6784802B1Electrically conductive connectionsElectric connection testingPatch panelMonitoring system
A system and method for monitoring connectivity in a cable system includes radio frequency identification (RFID) transponders on cable ends and RFID sensors at connection points. The RFID sensors are connected to a central monitoring system. Presence of a particular cable end at a particular connection point is detected and recorded by the central monitoring system.
Owner:NORDXCDT
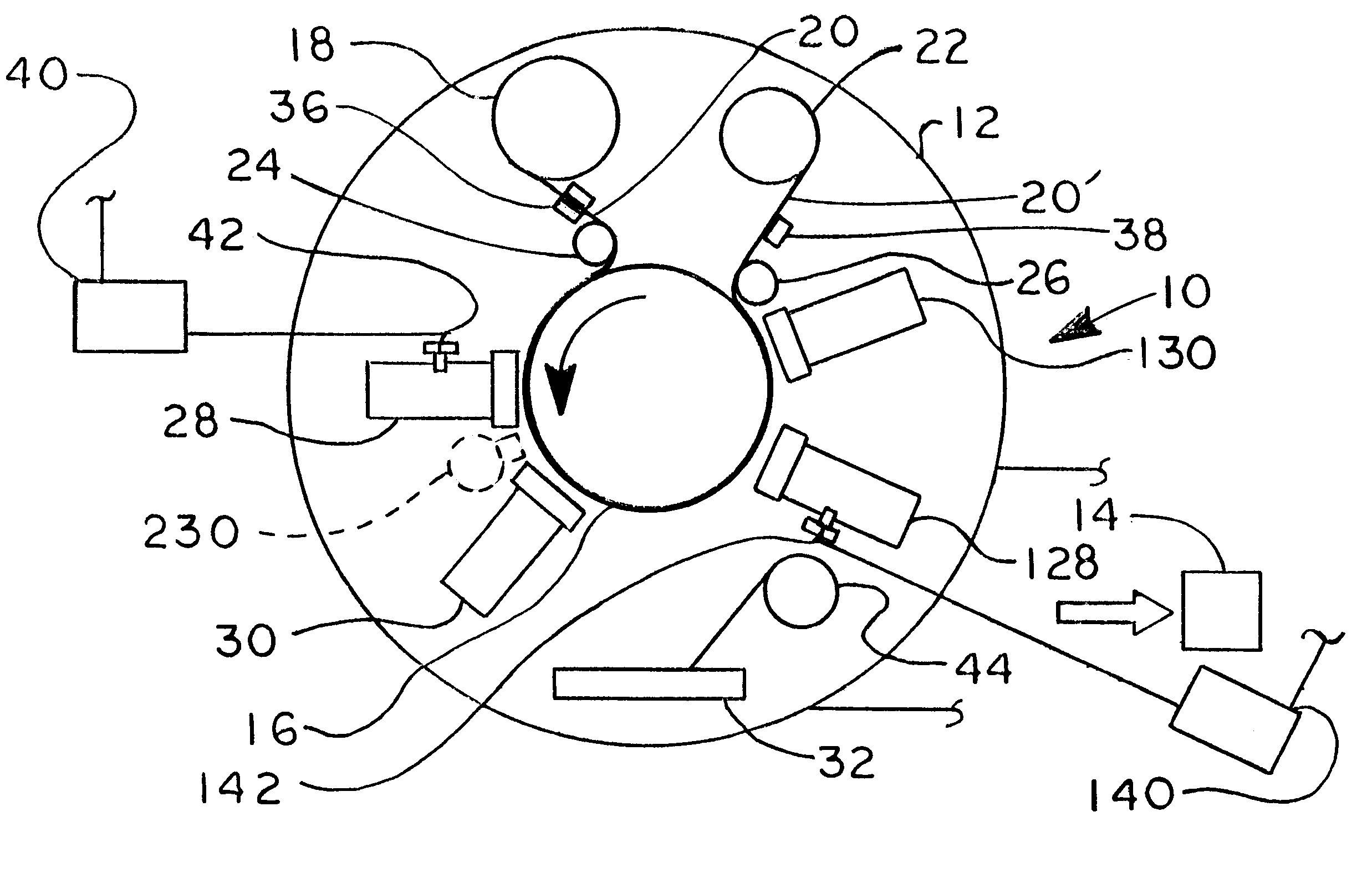
Articulating Surgical Device
An articulation mechanism for a surgical instrument includes an articulation assembly, a plurality of cables, and a trigger. The cables are coupled to the articulation assembly at a proximal end thereof and extend distally therefrom. The cables are configured to engage an end effector assembly of the surgical instrument at a distal end thereof. The trigger is coupled to the articulation assembly and is selectively moveable from a shipping position to a use position. In the shipping position, the cables are substantially un-tensioned. In the use position, the cables are disposed in an initial tensioned position. In the use position, the trigger is moveable between an unlocked position and a locked position. In the unlocked position, the cables are selectively tensionable to articulate the end effector assembly. In the locked position, the tensions on the cables are maintained to lock the end effector assembly in position.
Owner:TYCO HEALTHCARE GRP LP
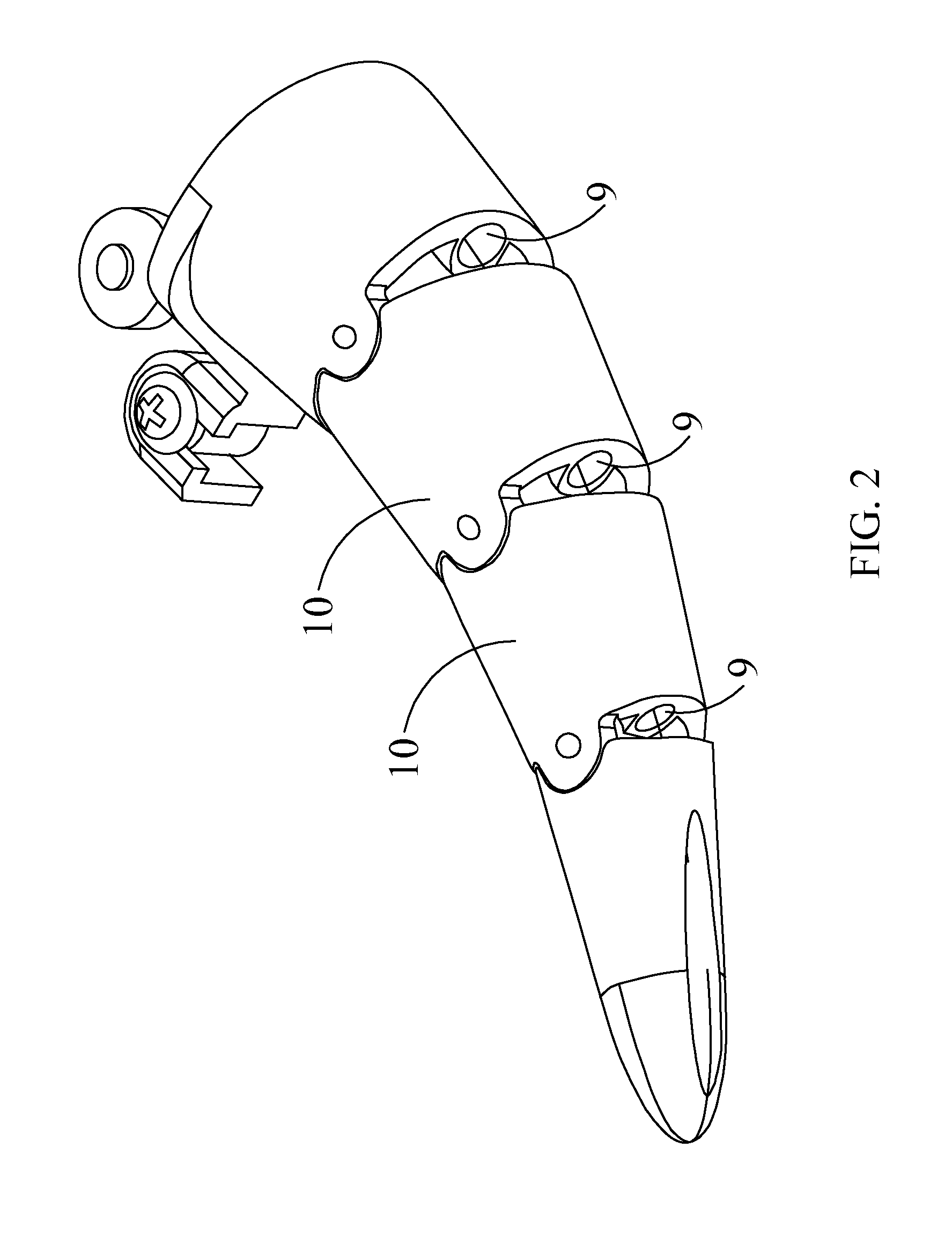
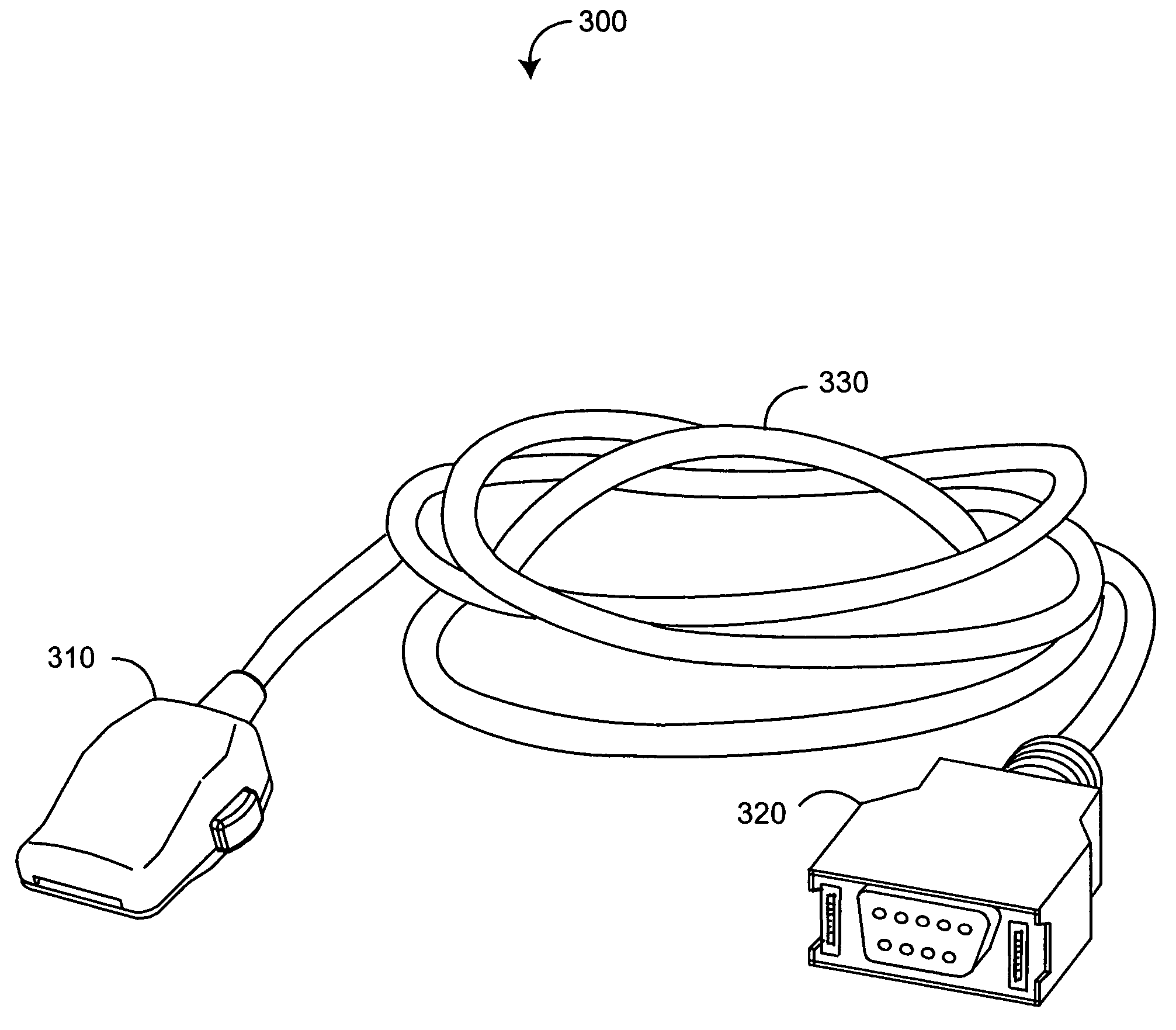
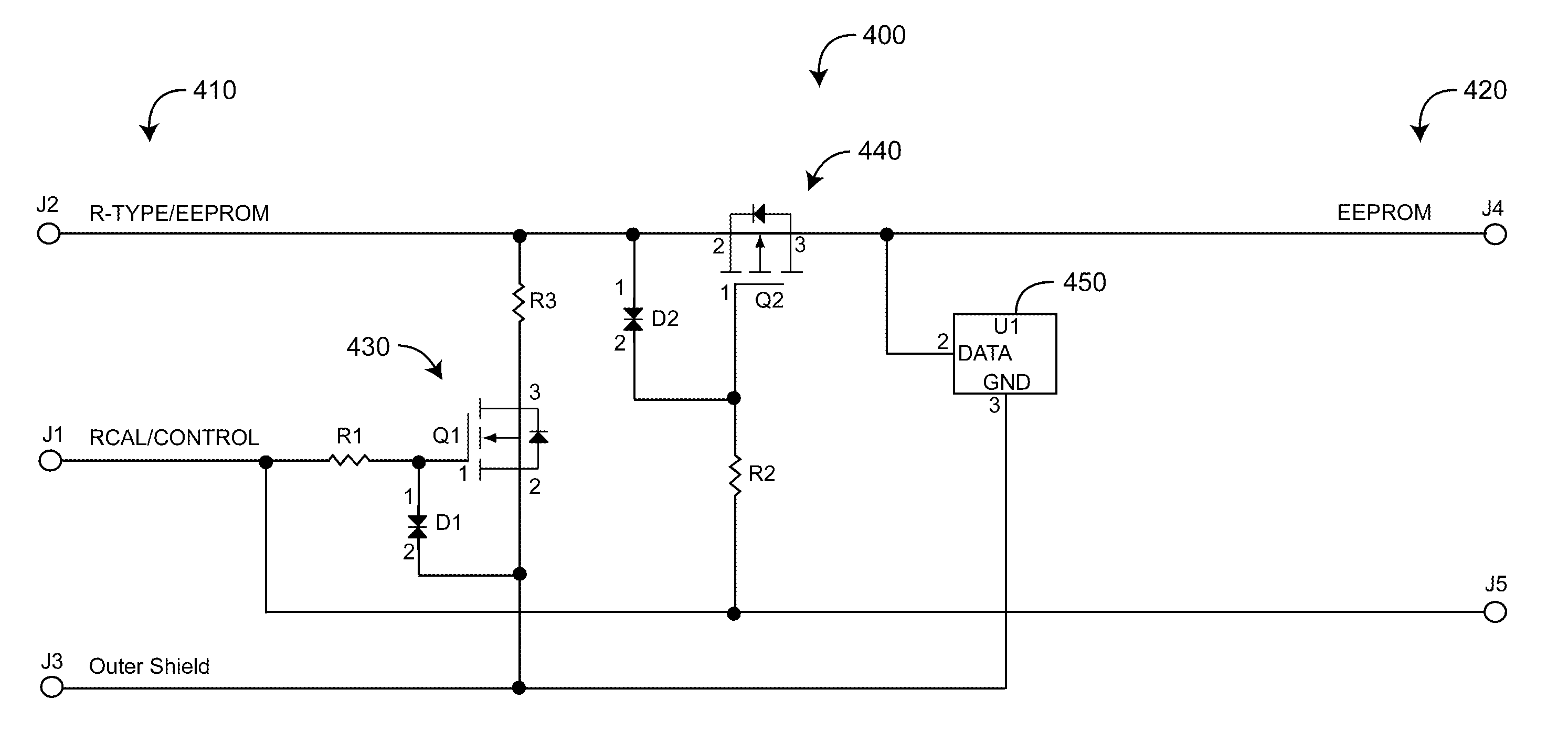
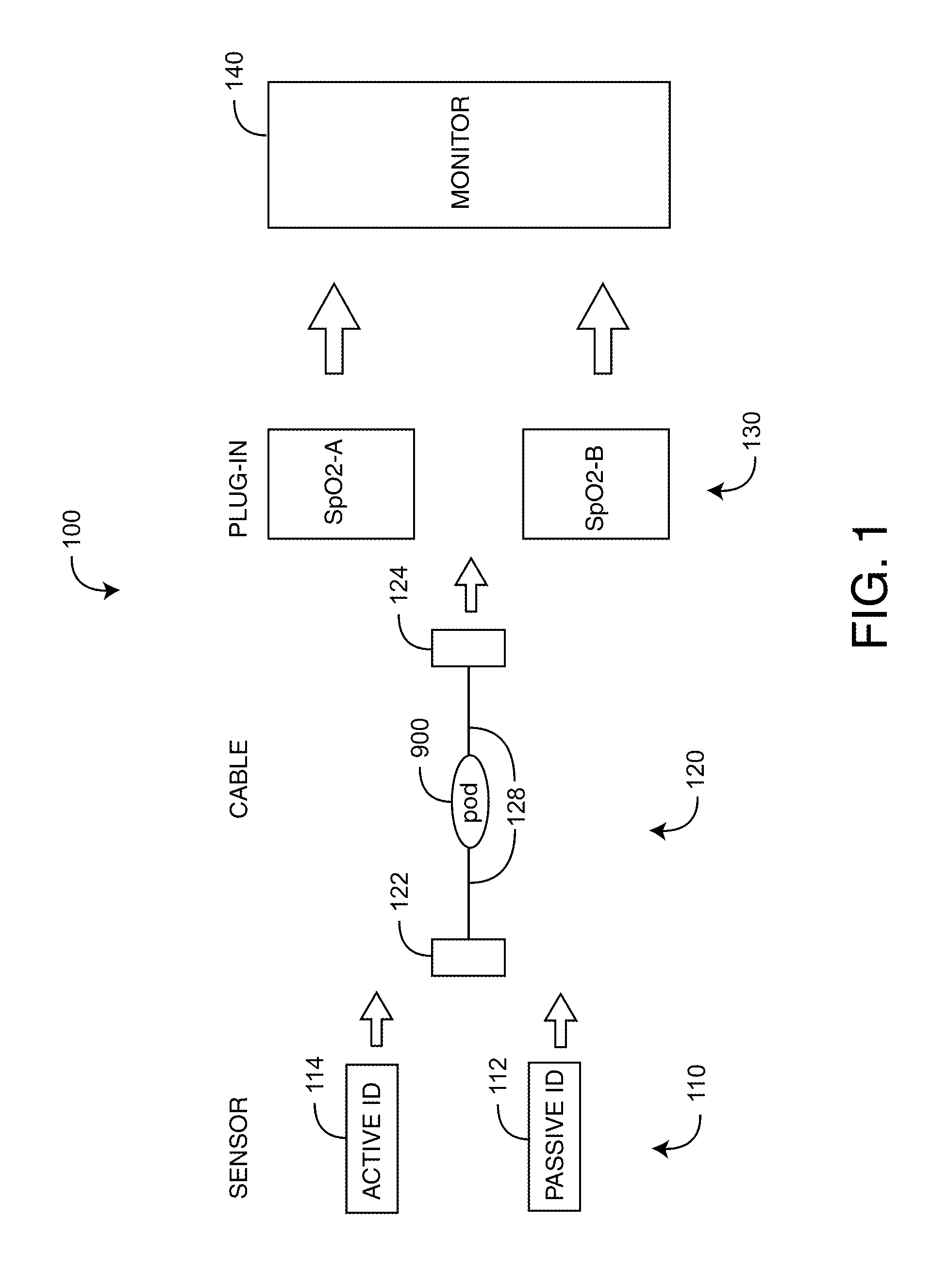
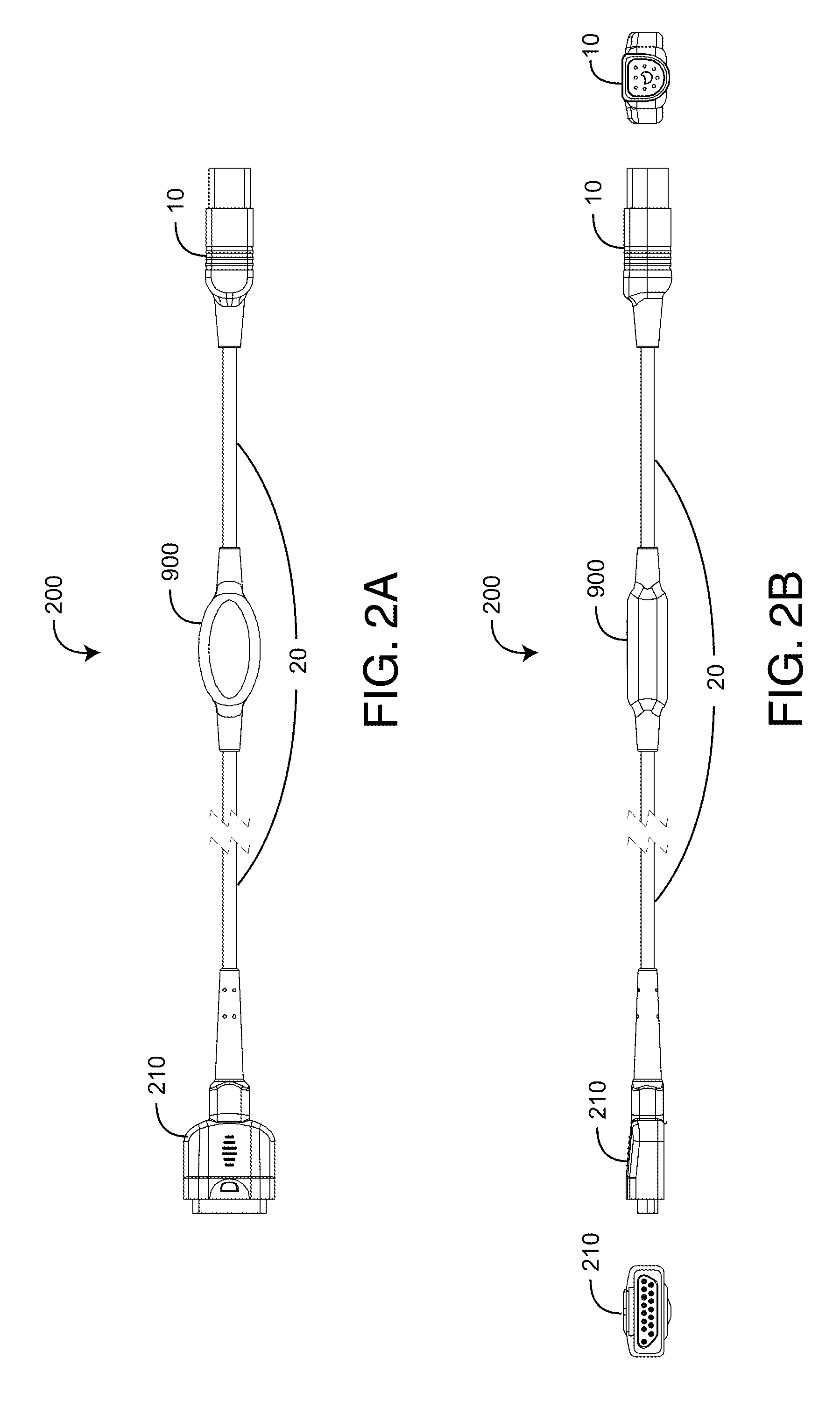
Sensor adapter cable
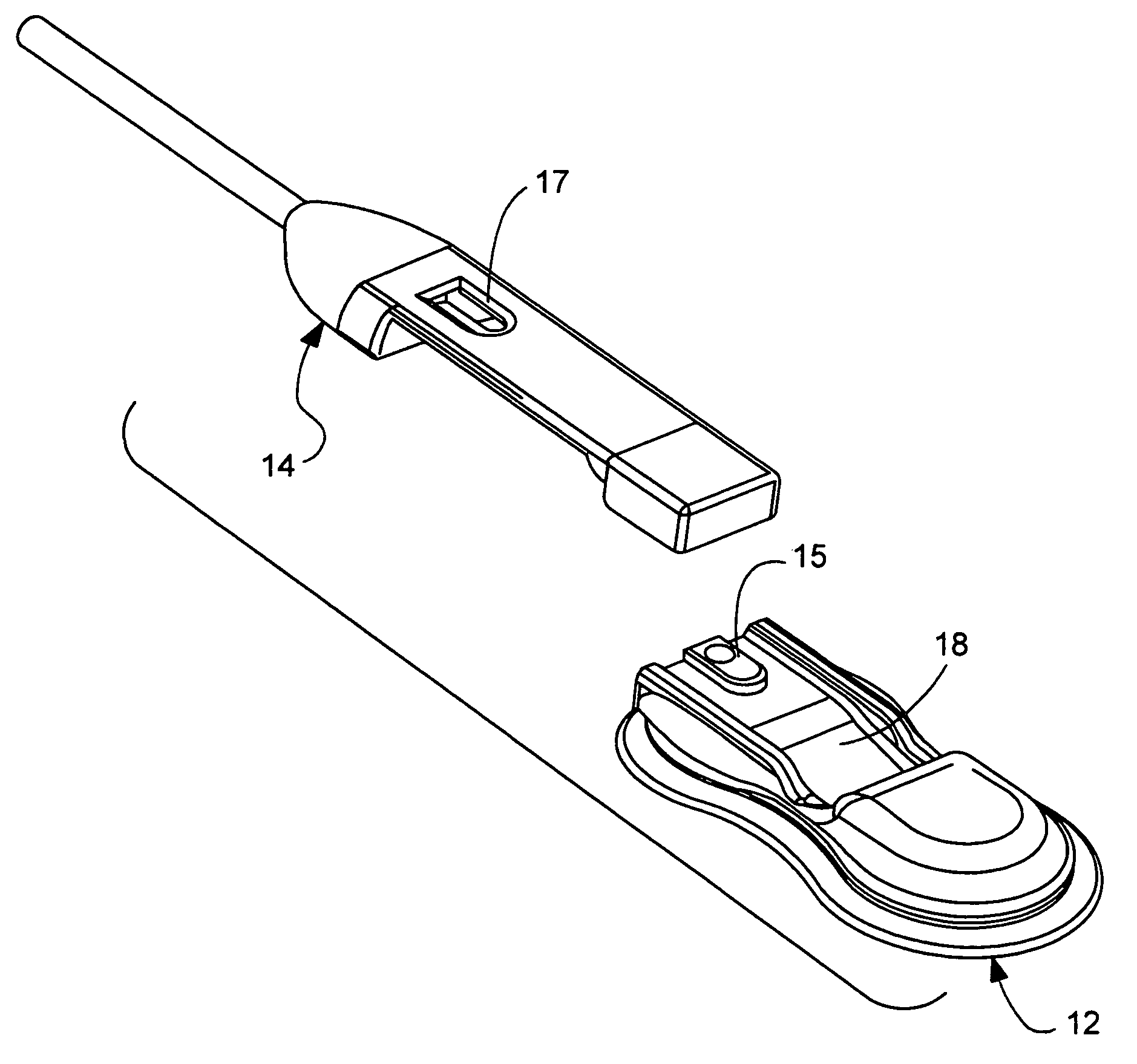
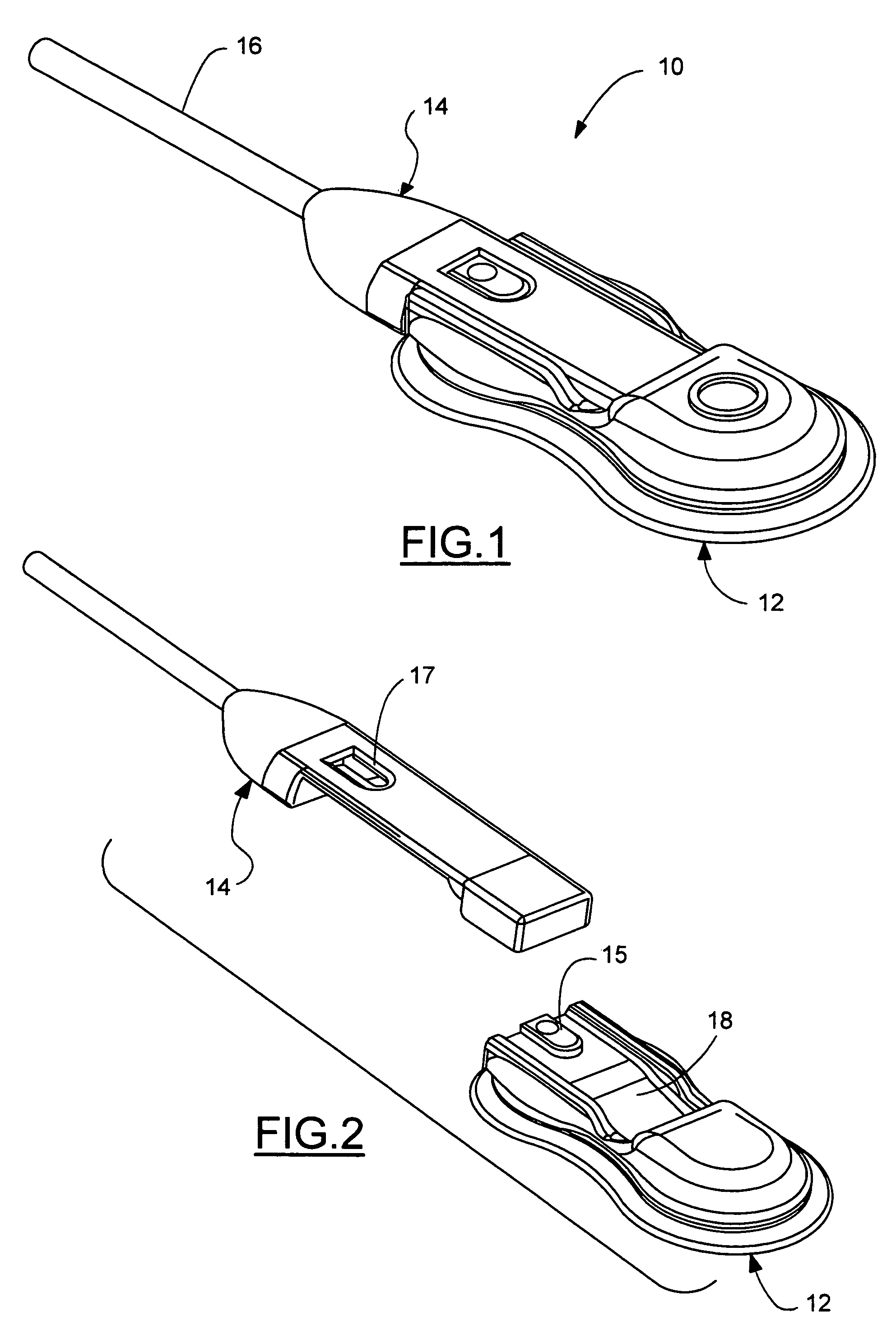
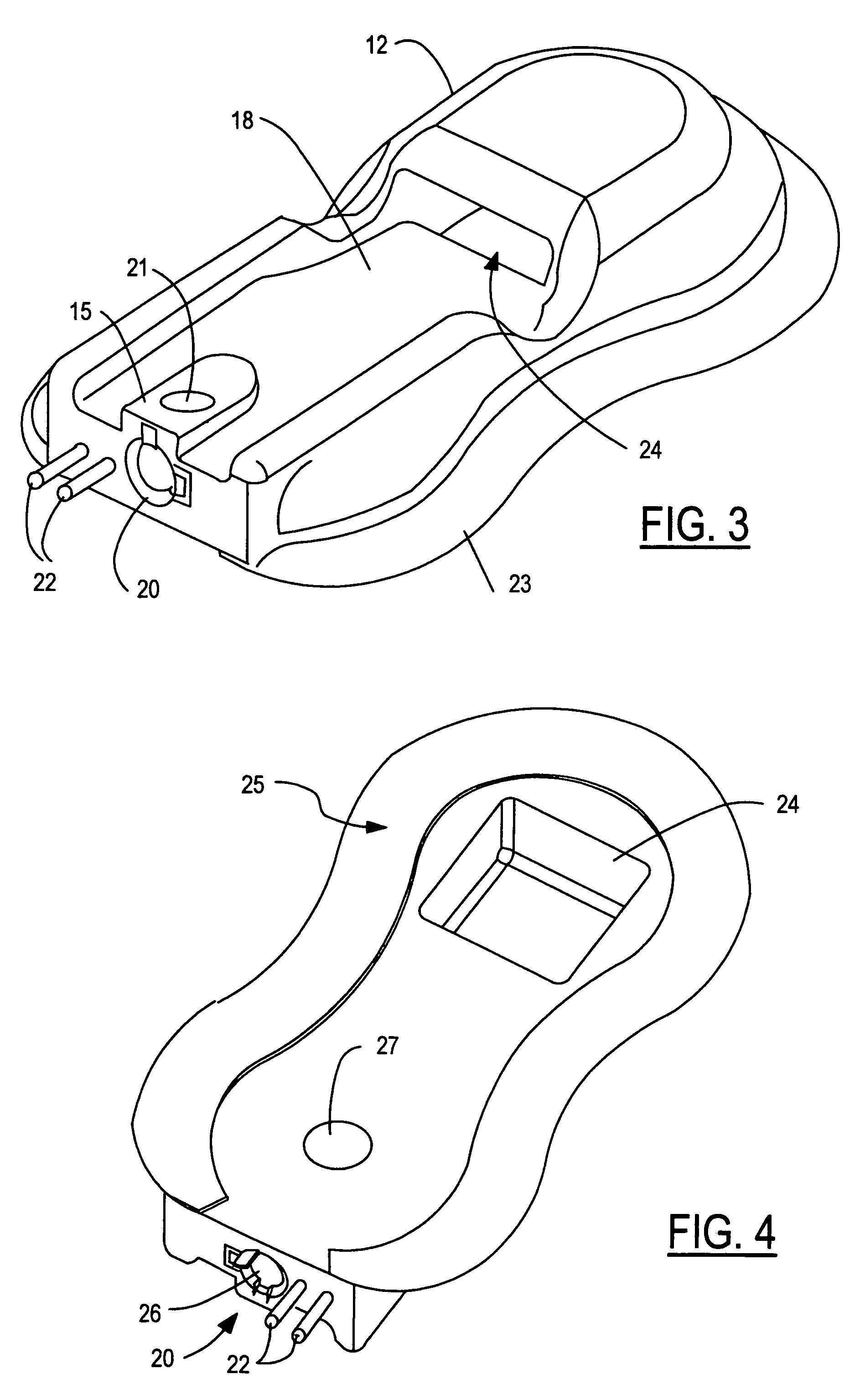
ActiveUS9138180B1Easy interconnectionCoupling device detailsTwo-part coupling devicesEngineeringElectric cables
Owner:MASIMO CORP
Method of forming a hybrid polymer film
InactiveUS6214422B1Fine surfaceLow costFixed capacitor dielectricSynthetic resin layered productsThermoplasticCross-link
A hybrid film, comprising a first polymer film having a plasma-treated surface and a second polymer film having first and second surfaces, with the first surface of the second polymer film being disposed along the first plasma-treated surface of the first polymer film, has superior thermal and mechanical properties that improve performance in a number of applications, including food packaging, thin film metallized and foil capacitors, metal evaporated magnetic tapes, flexible electrical cables, and decorative and optically variable films. One or more metal layers may be deposited on either the plasma-treated surface of the substrate and / or the radiation-cured acrylate polymer A ceramic layer may be deposited on the radiation-cured acrylate polymer to provide an oxygen and moisture barrier film. The hybrid film is produced using a high speed, vacuum polymer deposition process that is capable of forming thin, uniform, high temperature, cross-liked acrylate polymers on specific thermoplastic or thermoset films. Radiation curing is employed to cross-link the acrylate monomer. The hybrid film can be produced in-line with the metallization or ceramic coating process, in the same vacuum chamber and with minimal additional cost.
Owner:SIGMA LAB OF ARIZONA
Mid-line connector and method for pipe-in-pipe electrical heating
ActiveUS20050054228A1Increase axial loadAvoid local accumulationPipe heating/coolingPipe-jointsElectricityElectrical conductor
For heating a pipe-in-pipe pipeline with power provided through an electric cable, mid-line connector is provided including: a connector housing joinable to the outer pipe of the pipeline; a blank pipe positioned within the connector housing and joinable to the inner pipe of the pipeline; at least one pocket mounted in the connector housing, wherein the cable is mateable with the at least one pocket; a blank pipe conductor electrically coupled between the at least one pocket and the blank pipe; and an outer pipe conductor electrically coupled between the at least one pocket and the outer pipe.
Owner:SHELL OIL CO
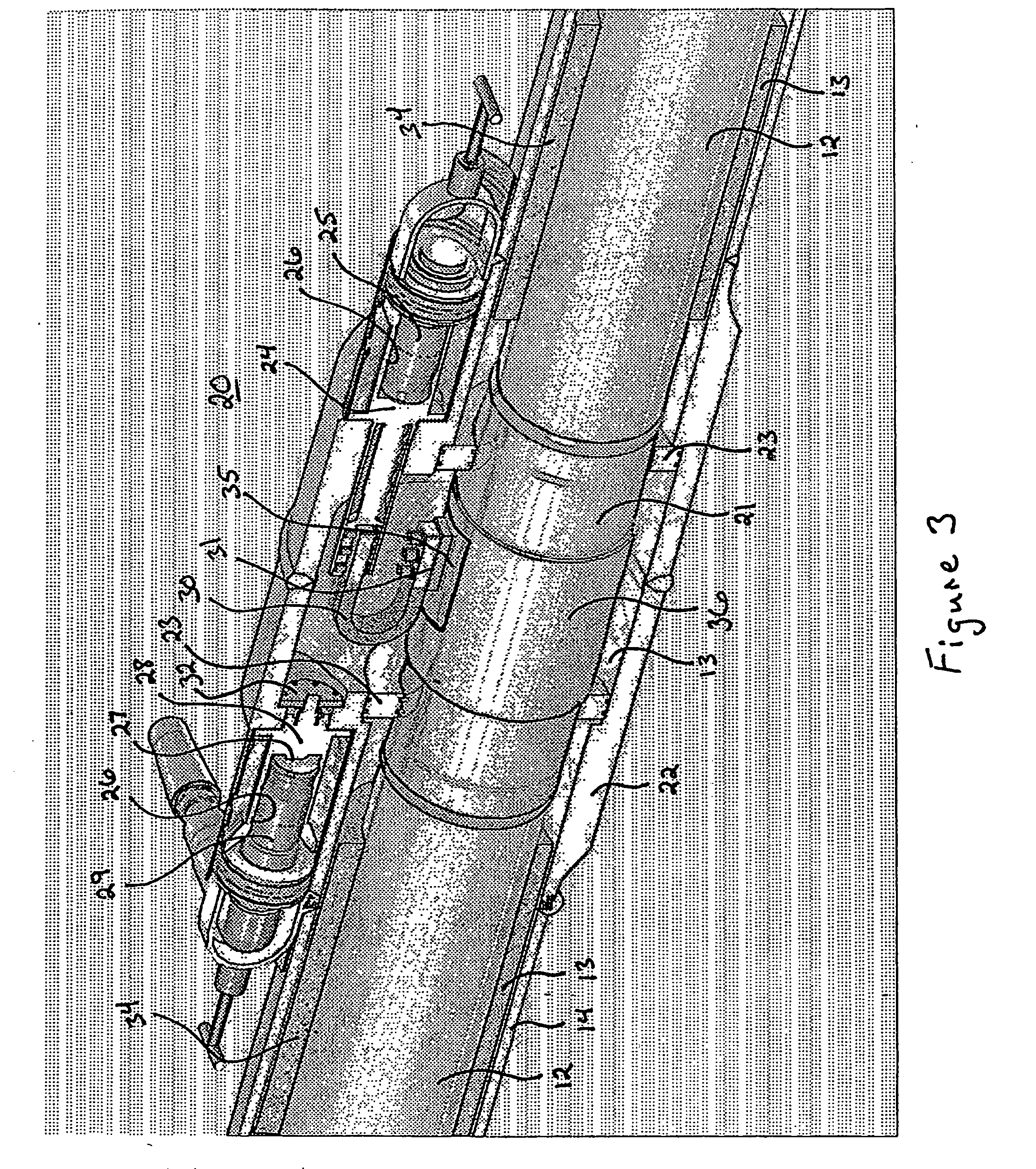
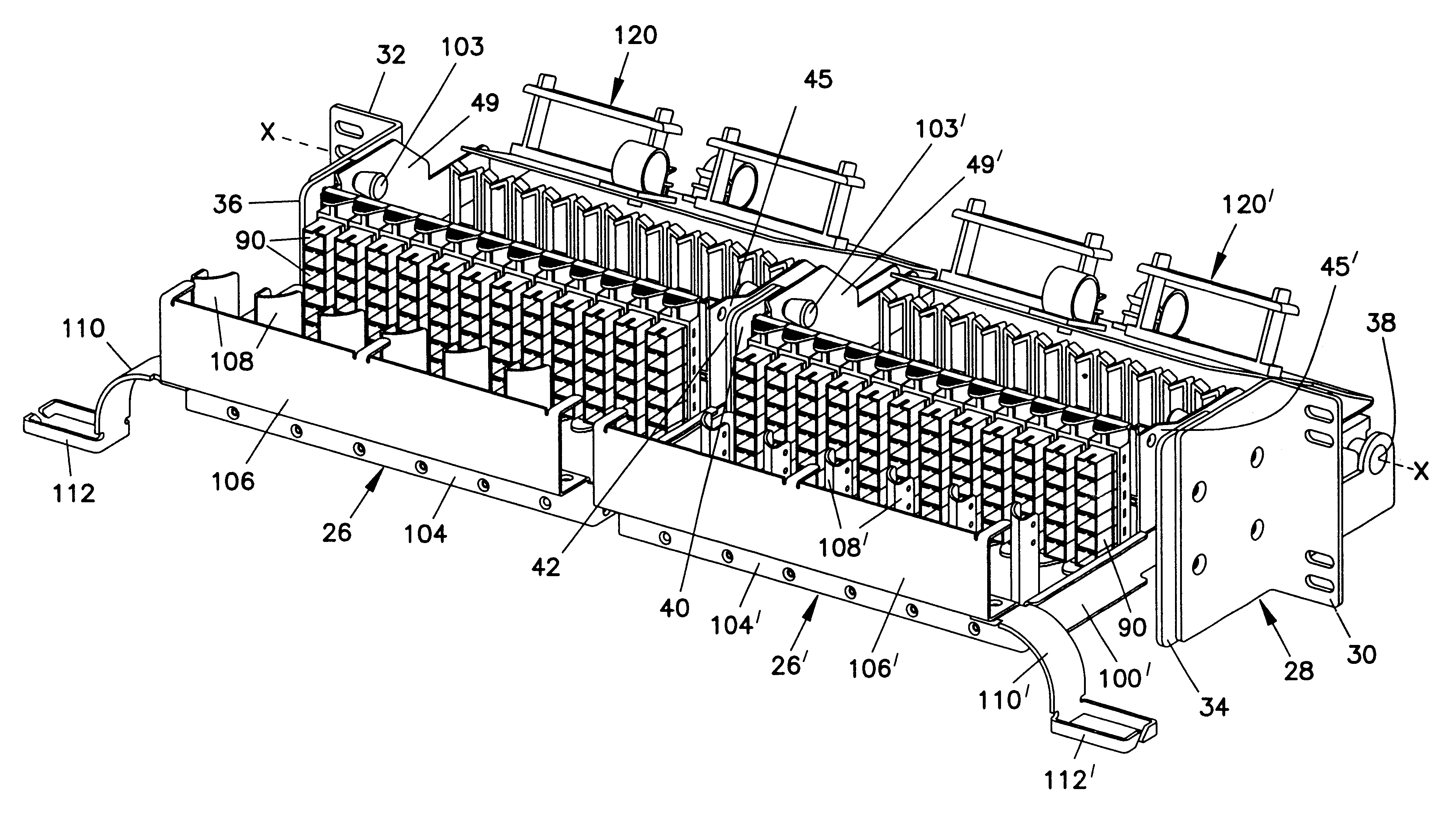
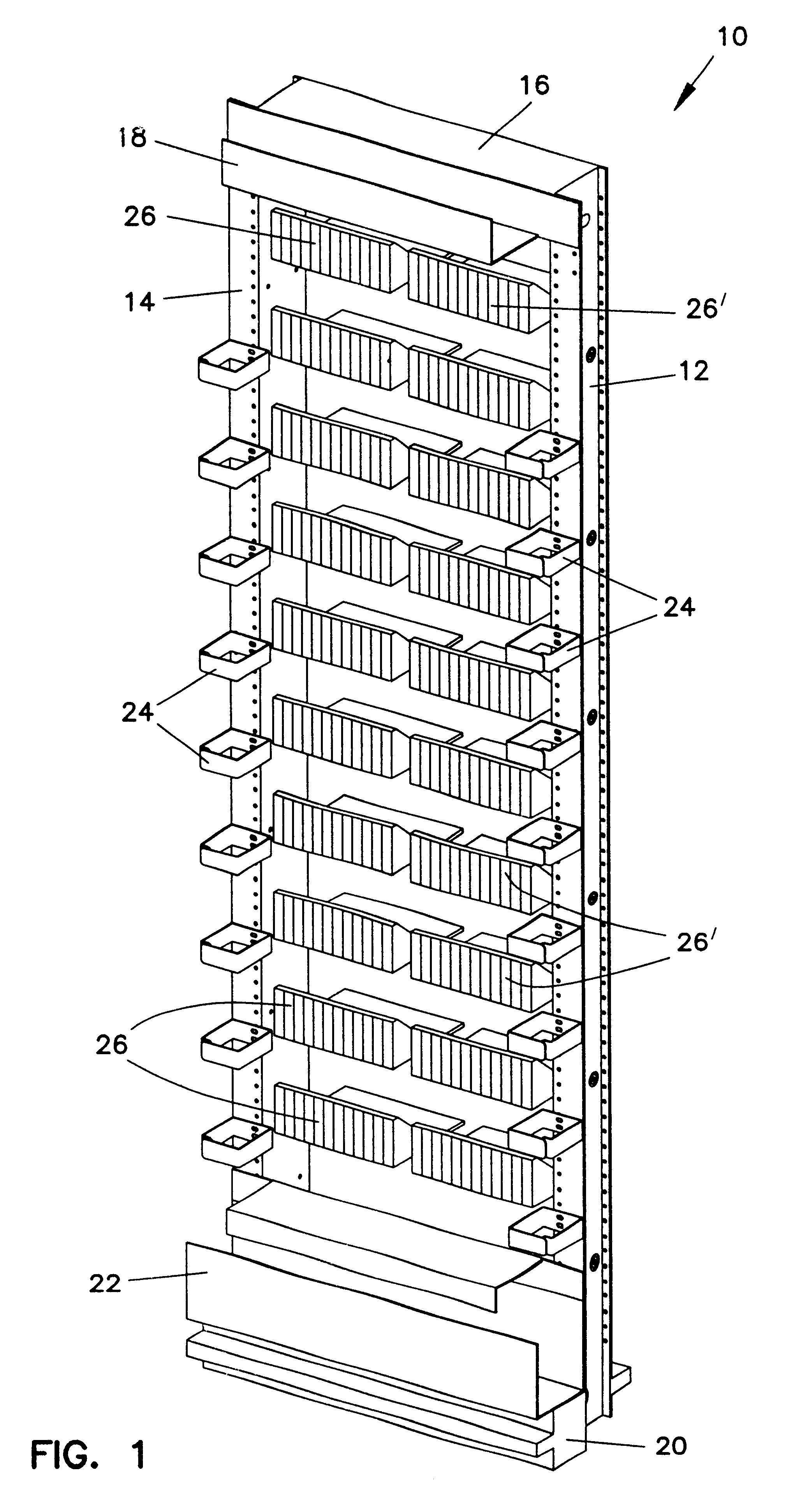
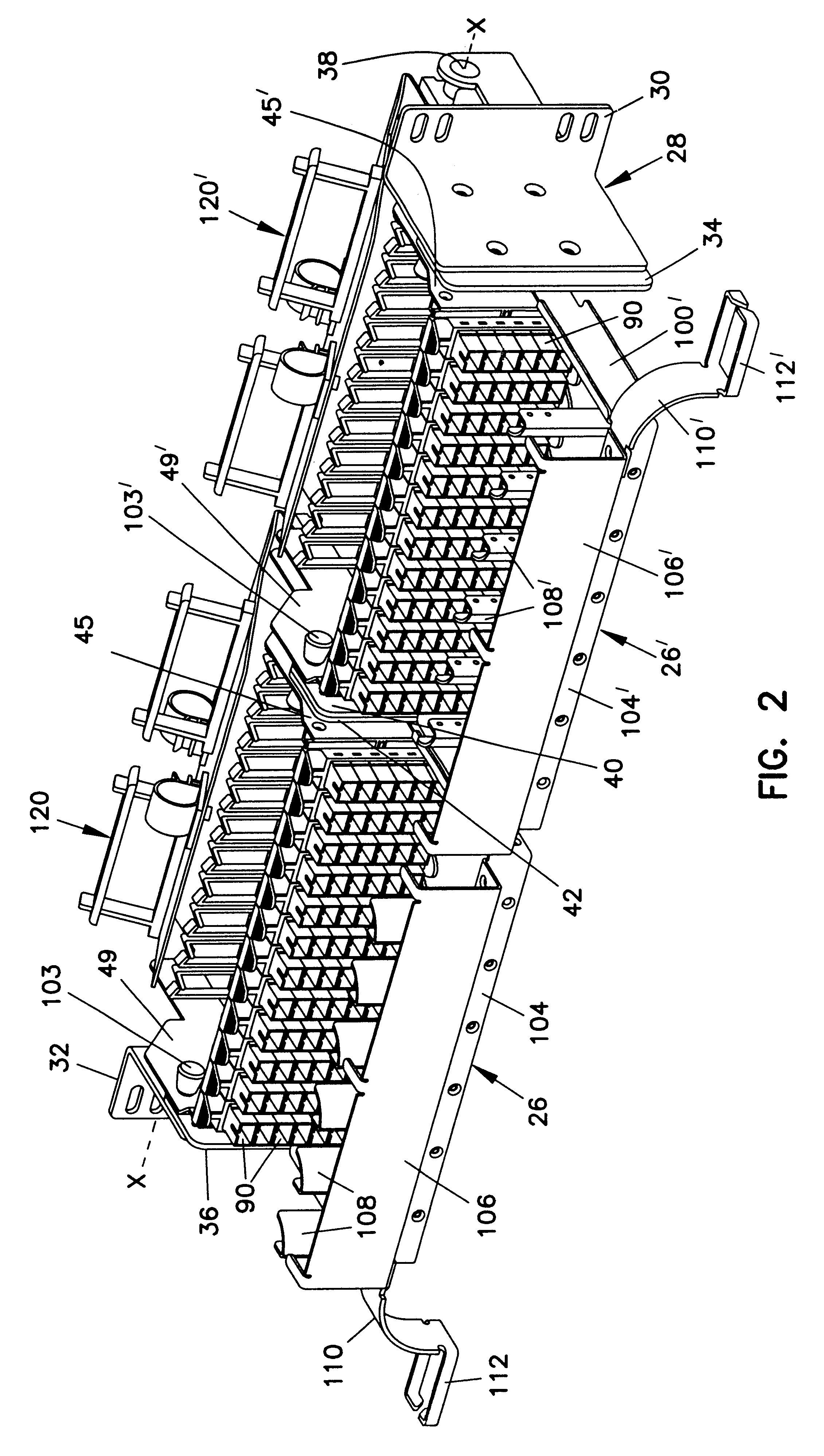
High-density cable distribution frame
InactiveUSRE38311E1Control displacementSmall movementCoupling light guidesFibre mechanical structuresDistribution frameFiber
A fiber distribution frame (10) includes a fixture (26) having a plurality of modules (58) mounted side-by-side within said fixture with each of the modules being individually mounted in a line of travel. Each of the modules (58) can be locked in any one of a plurality of discrete positions within the line of travel. Each of the modules (58) contains a plurality of adapters (90) for receiving and retaining fiber optic connectors. Further, the fixture (26) may be tilted downwardly to provide access to the rear of the fixture.
Owner:ADC TELECOMMUNICATIONS +1
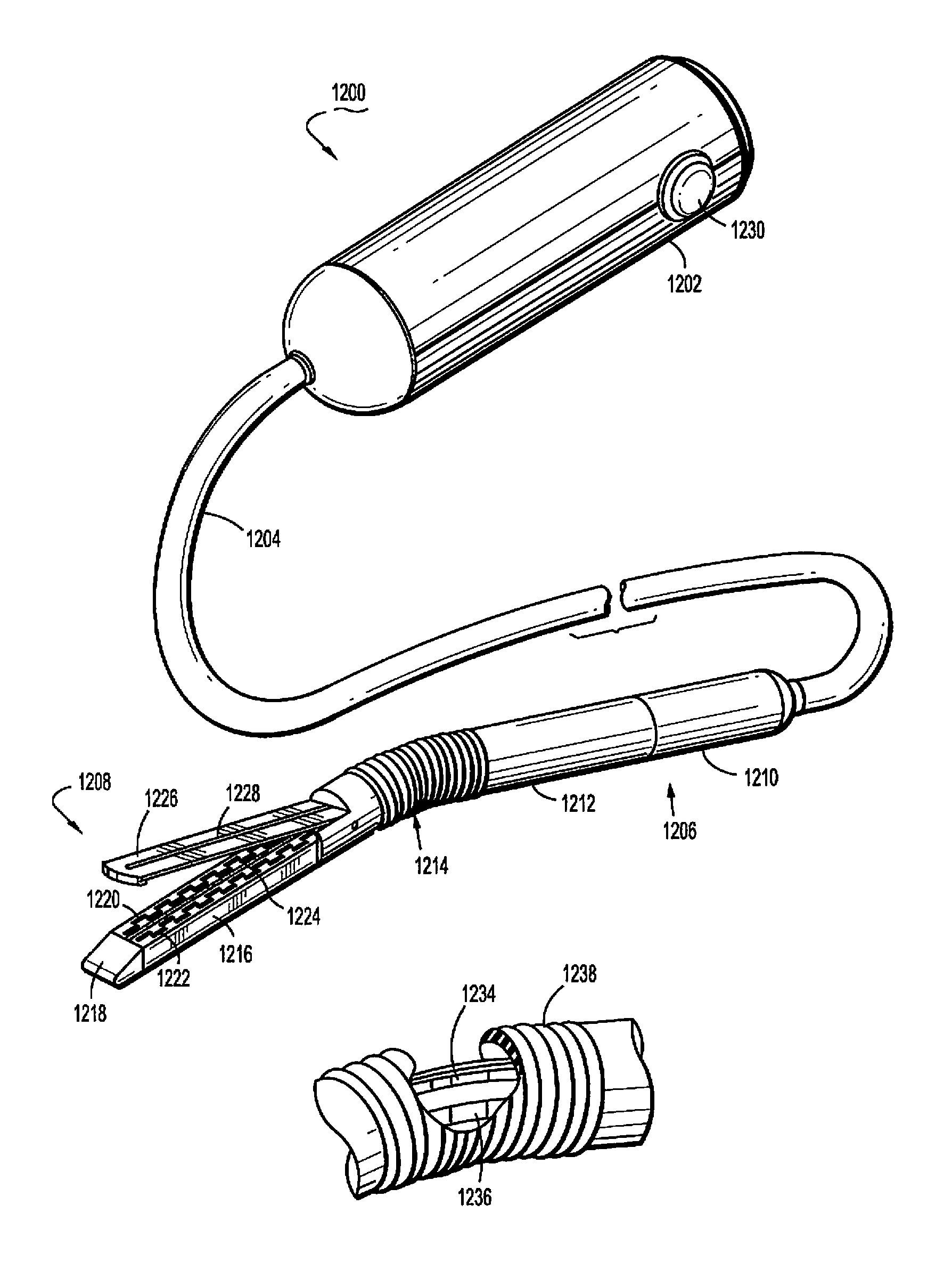
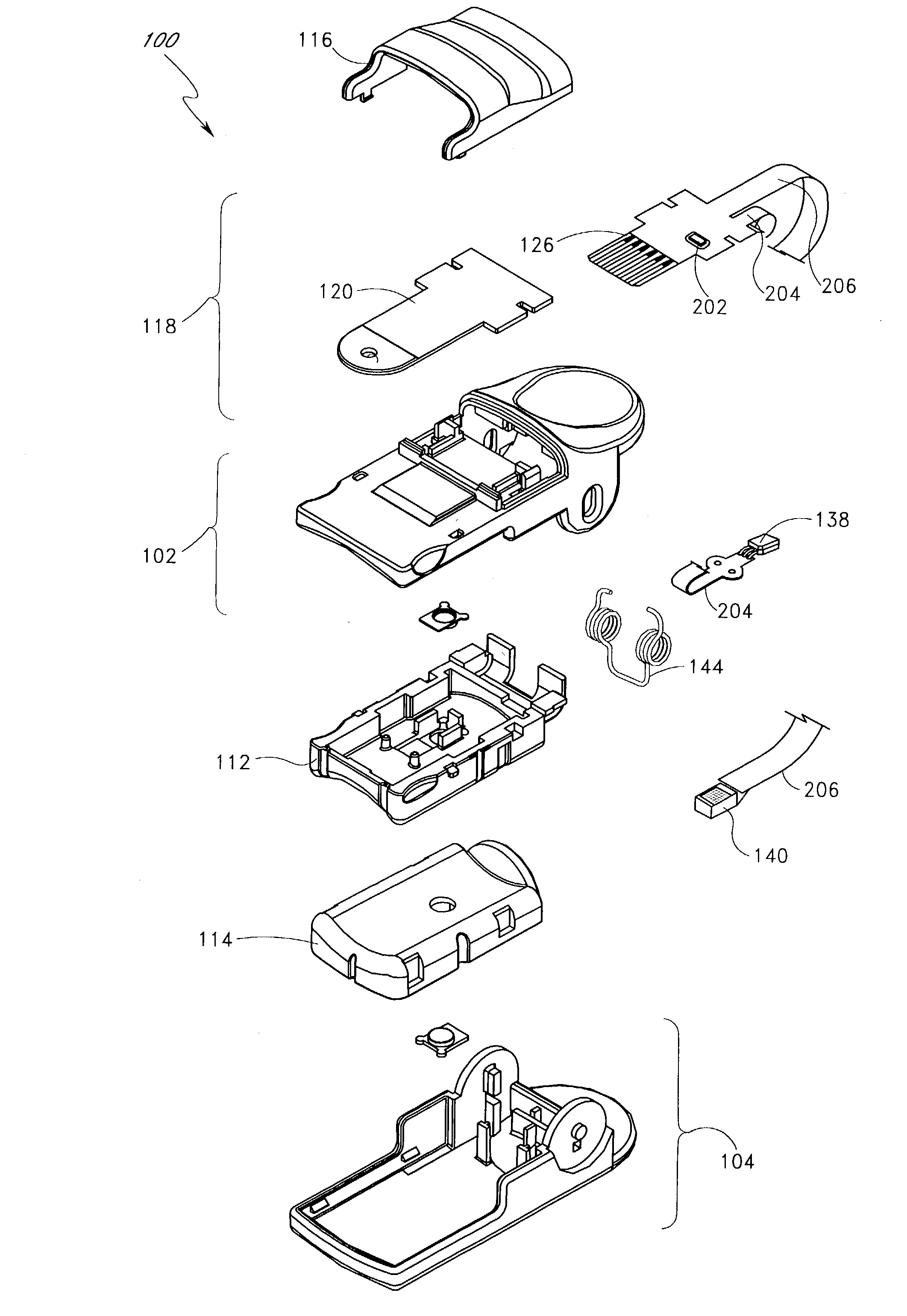
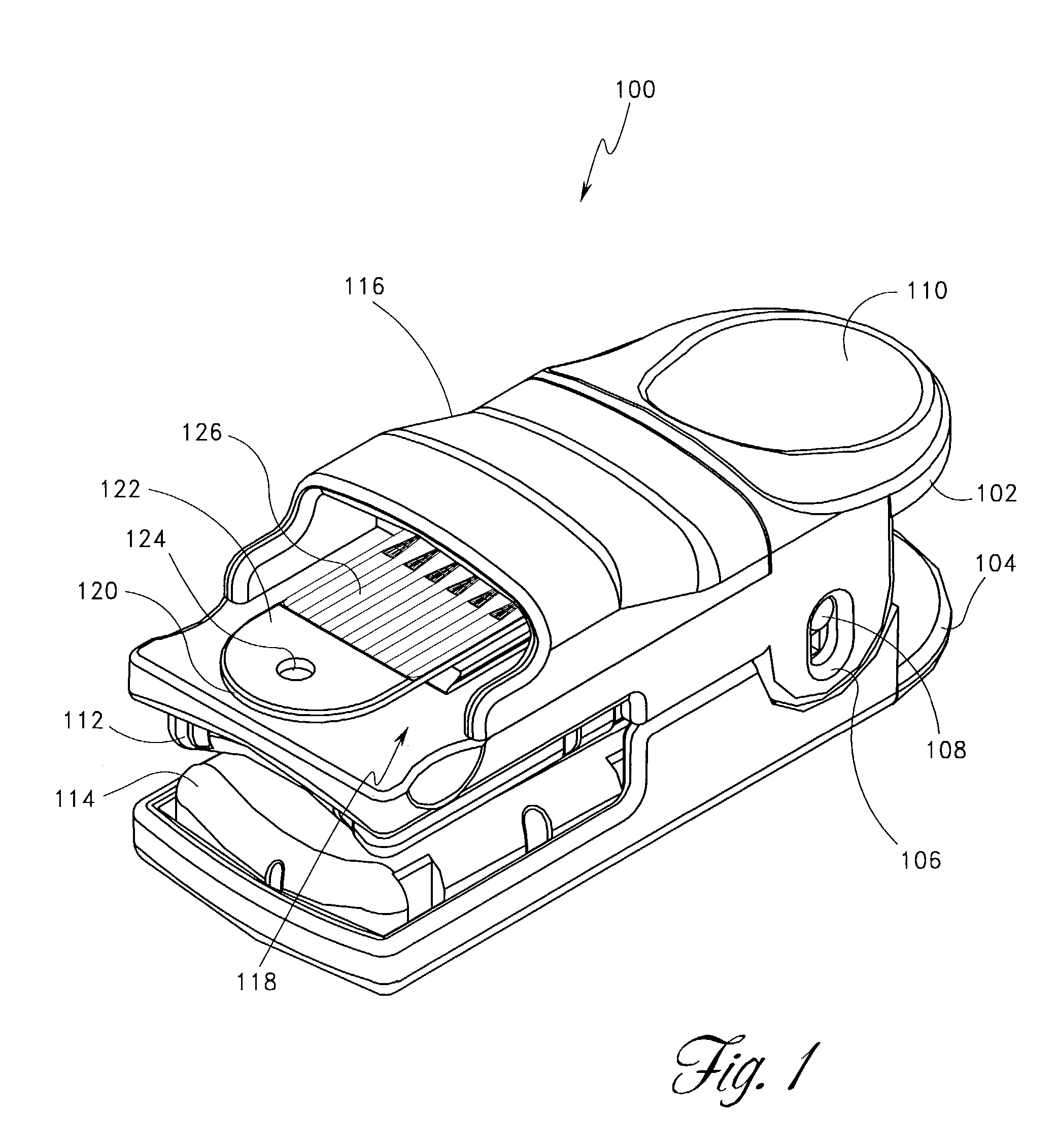
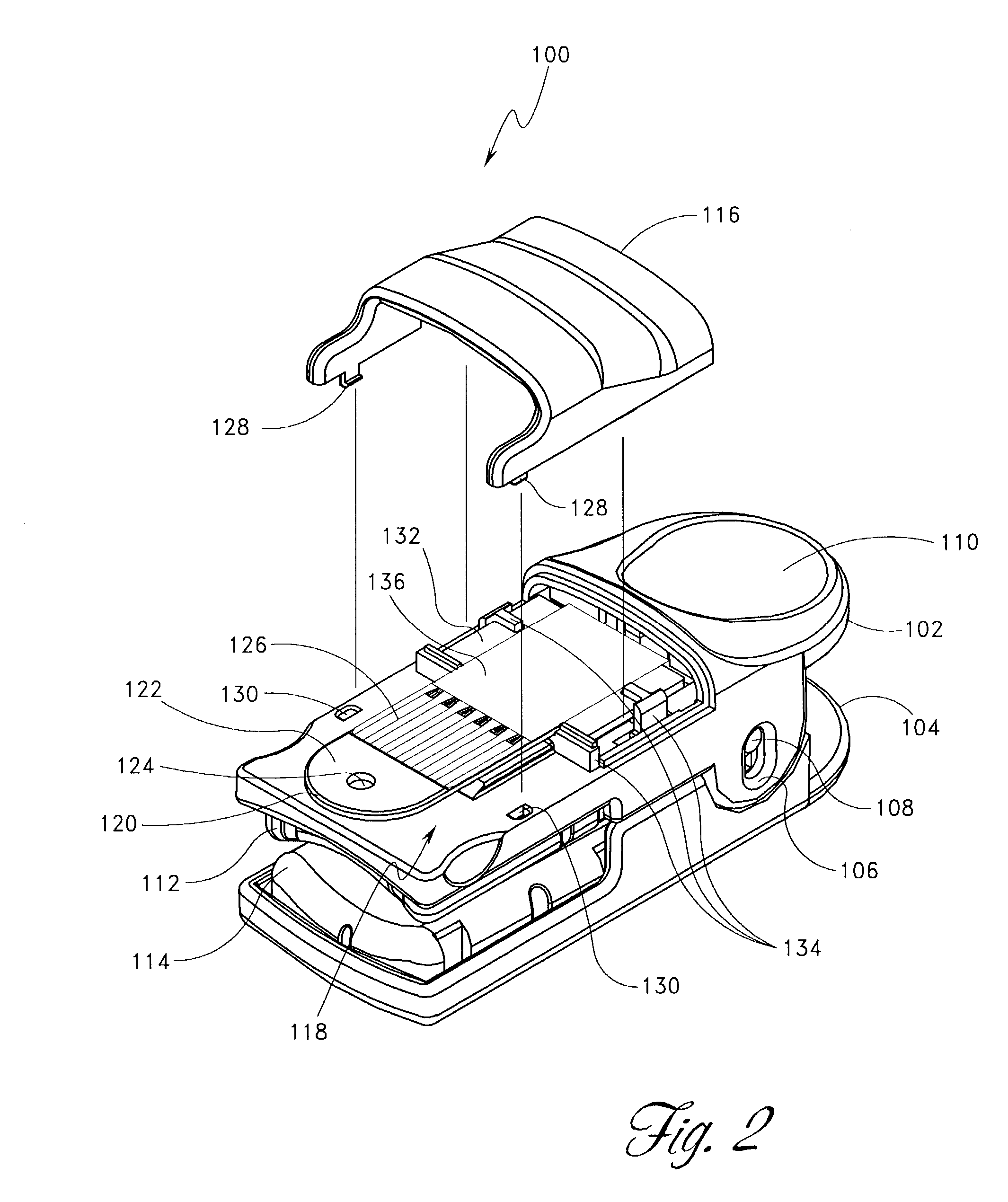
Near infrared spectroscopy device with reusable portion
InactiveUS7706853B2Small sizeFirmly attachedMaterial analysis by optical meansDiagnostic recording/measuringSurgical operationHigh energy
A NIRS sensor device for brain monitoring is small in size, provides reliable attachment to a patient, blocks ambient light, is easy to use, is hygienic, and supports data integration with surgical and monitoring systems. The sensor device is coupled to a remote near infrared light source via a hybrid cable. Since the light source is remotely located, a source adapted for providing high energy, short pulses can easily be used so that there is less chance of interference by superficial non-brain tissues and less interference from ambient light. In addition, the remote location avoids changes in output of local light sources experienced in the prior art during hypothermia procedures (e.g., bandwidth shifts in LEDs as a result of lowered temperature). The higher energy may be achieved by the use of laser diodes as opposed to locally-mounted LEDs typically used in the prior art. The sensor device is a two-piece design comprising a reusable portion containing the photodetector(s) and a disposable portion that receives the light from the reusable portion and bends it to direct the light into the brain.
Owner:TERUMO CARDIOVASCULAR SYST CORP
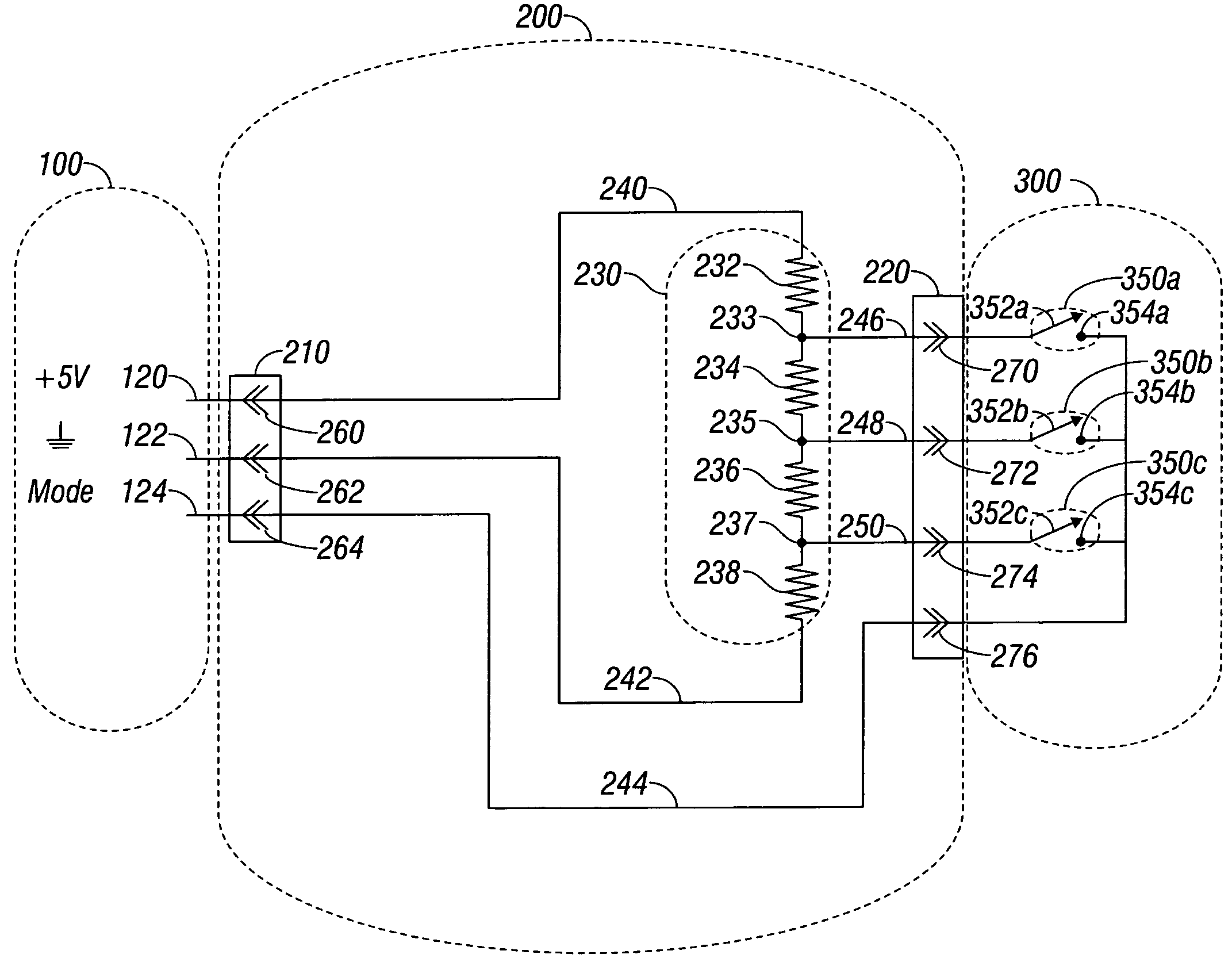
Connection cable and method for activating a voltage-controlled generator
ActiveUS7834484B2Batteries circuit arrangementsIncorrect coupling preventionSurgical operationElectrosurgery
A connection cable is disclosed for controlling a voltage-controlled generator such as an electrosurgery generator from a controlling device such as a robotic surgery system. The cable includes a first connector adapted to connect to a voltage-controlled generator and a second connector adapted to connect to a controlling device. Within the cable is a voltage divider interdisposed between the first connector and the second connector. The voltage divider is configured to divide a reference voltage provided by the voltage-controlled generator into at least one control voltage which is selectable by the controlling device. The cable additionally includes a plurality of electrical wires which operatively connect the first connector, the second connector and the voltage divider. During robotic electrosurgery, said operating parameters can be actuated by a surgeon operating at the robotic surgical system console, which causes a corresponding control voltage to be switched to a control voltage input on an electrosurgery generator, which, in turn, generates a corresponding electrosurgical signal in response thereto.
Owner:COVIDIEN LP
Simulation dog tail swinging installment
InactiveUS20110003528A1Low costReduce frictionDollsSelf-moving toy figuresEngineeringControl circuit
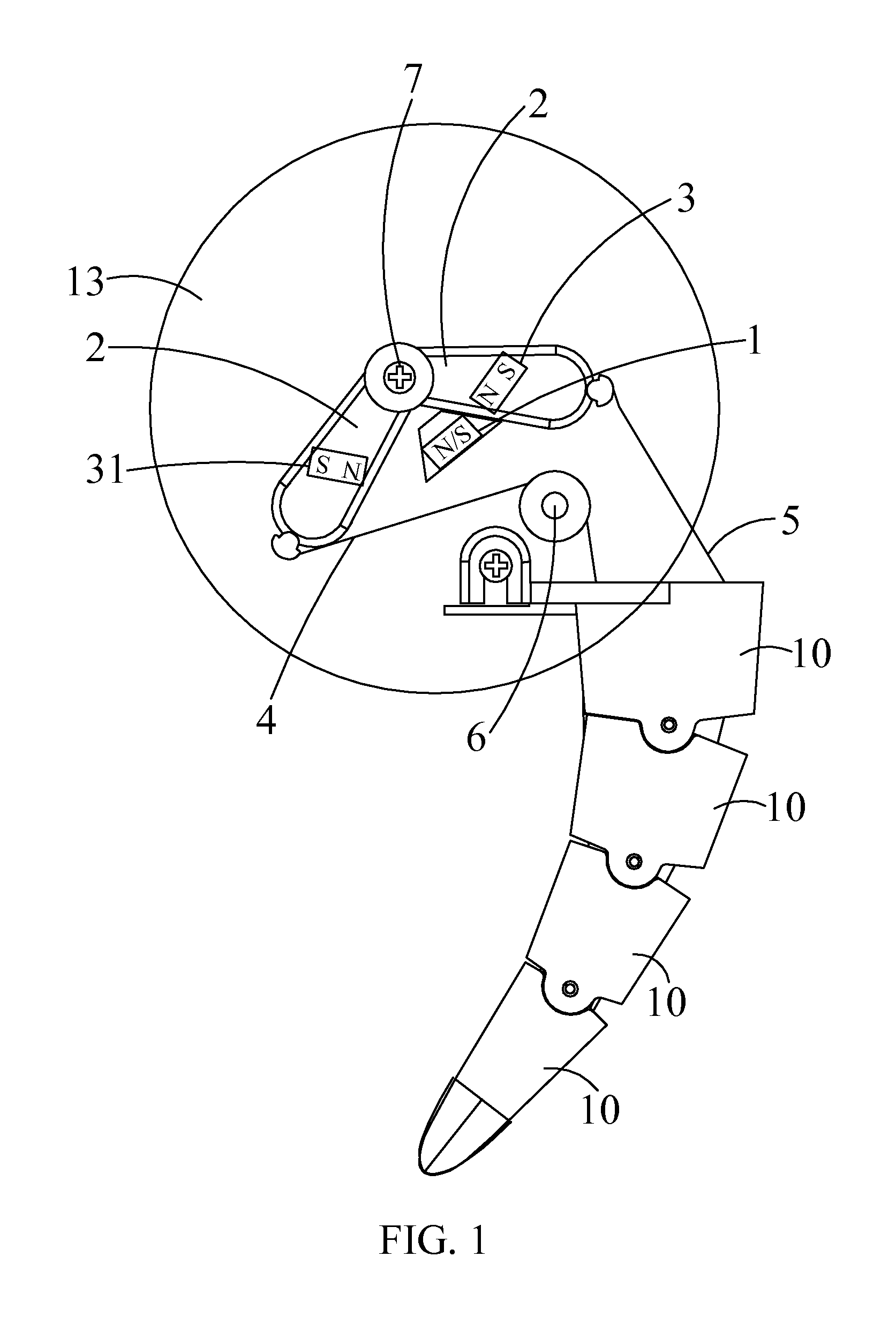
A simulation tail swinging installment includes a base plate, an electromagnetic coil disposed on the base plate, a battery module configured for supplying power to the electromagnetic coil, and a control circuit coupled to the battery module. The simulation tail swinging installment further includes a furcated component having two arms disposed with respect to the electromagnetic coil, with the two arms located on opposite sides of the electromagnetic coil, respectively. Each of the arms includes a permanent magnet positioned in correspondence with the electromagnetic coil. The furcated component is mounted to the base plate through a pivot connected to the base plate. A first driving cable and a second driving cable are attached to the two arms, respectively. The first driving cable and the second driving cable extend through and along two side portions of a simulation tail and secured to a distal end of the simulation tail.
Owner:TSUI KING LAM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com