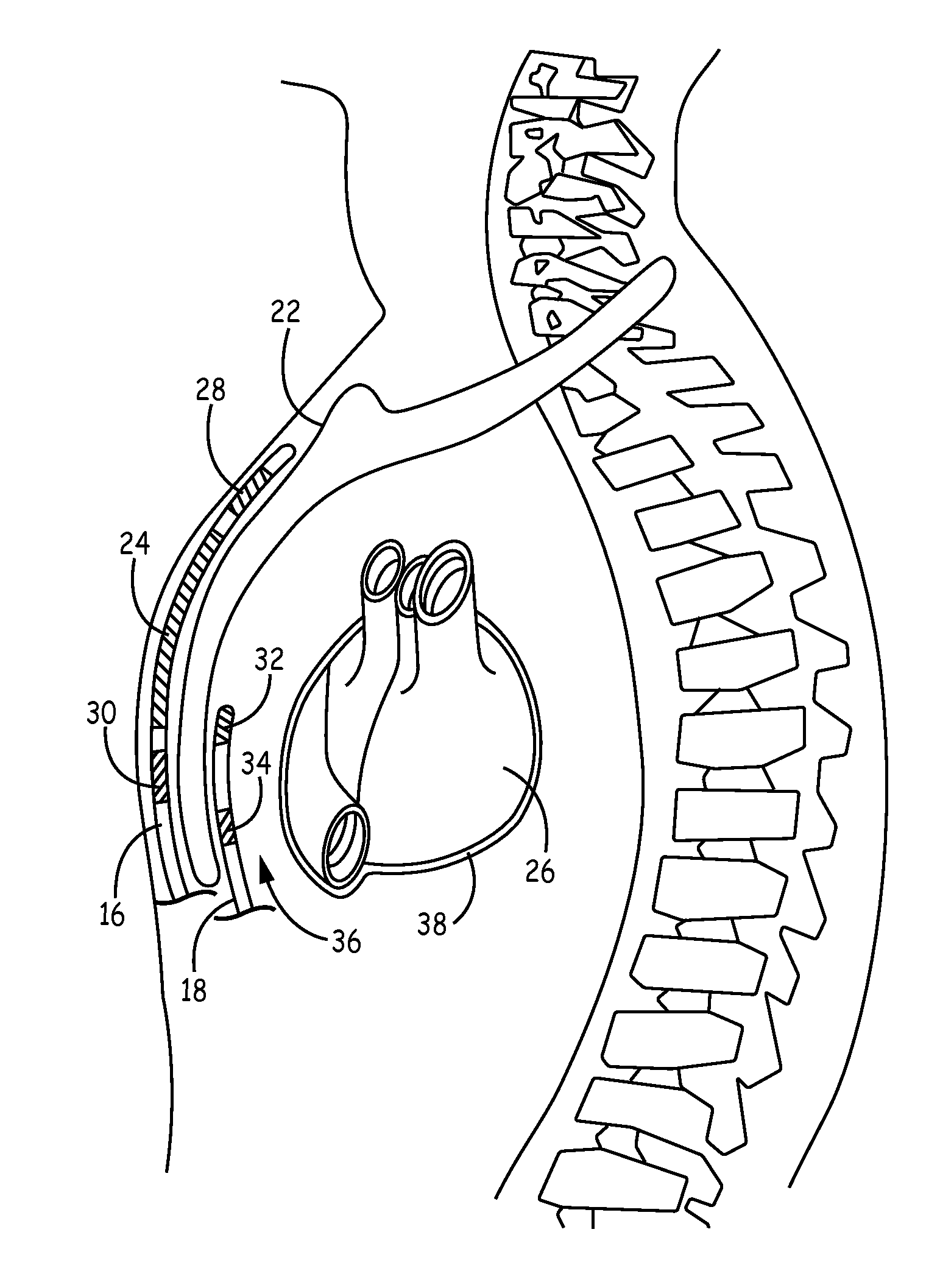
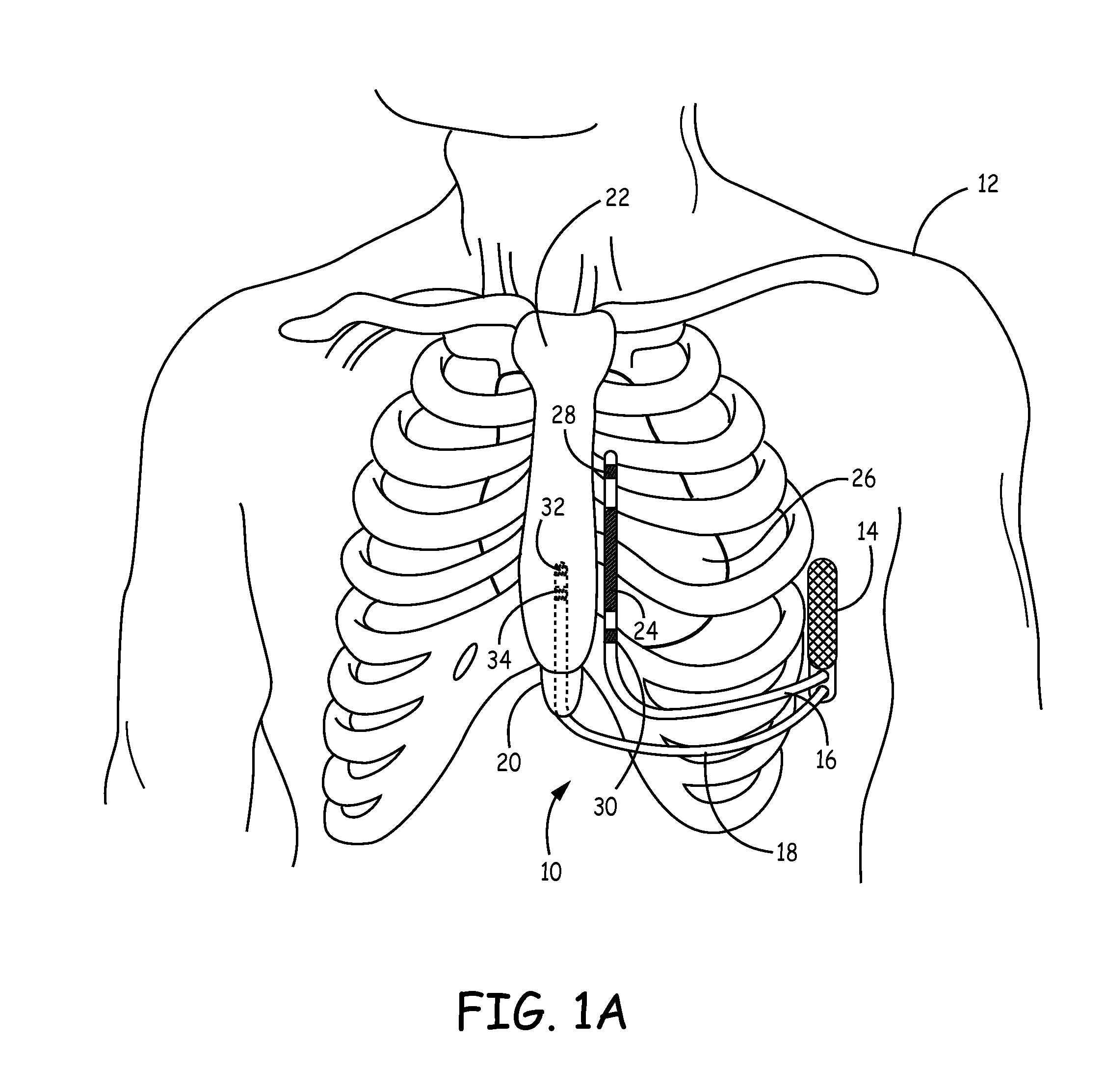
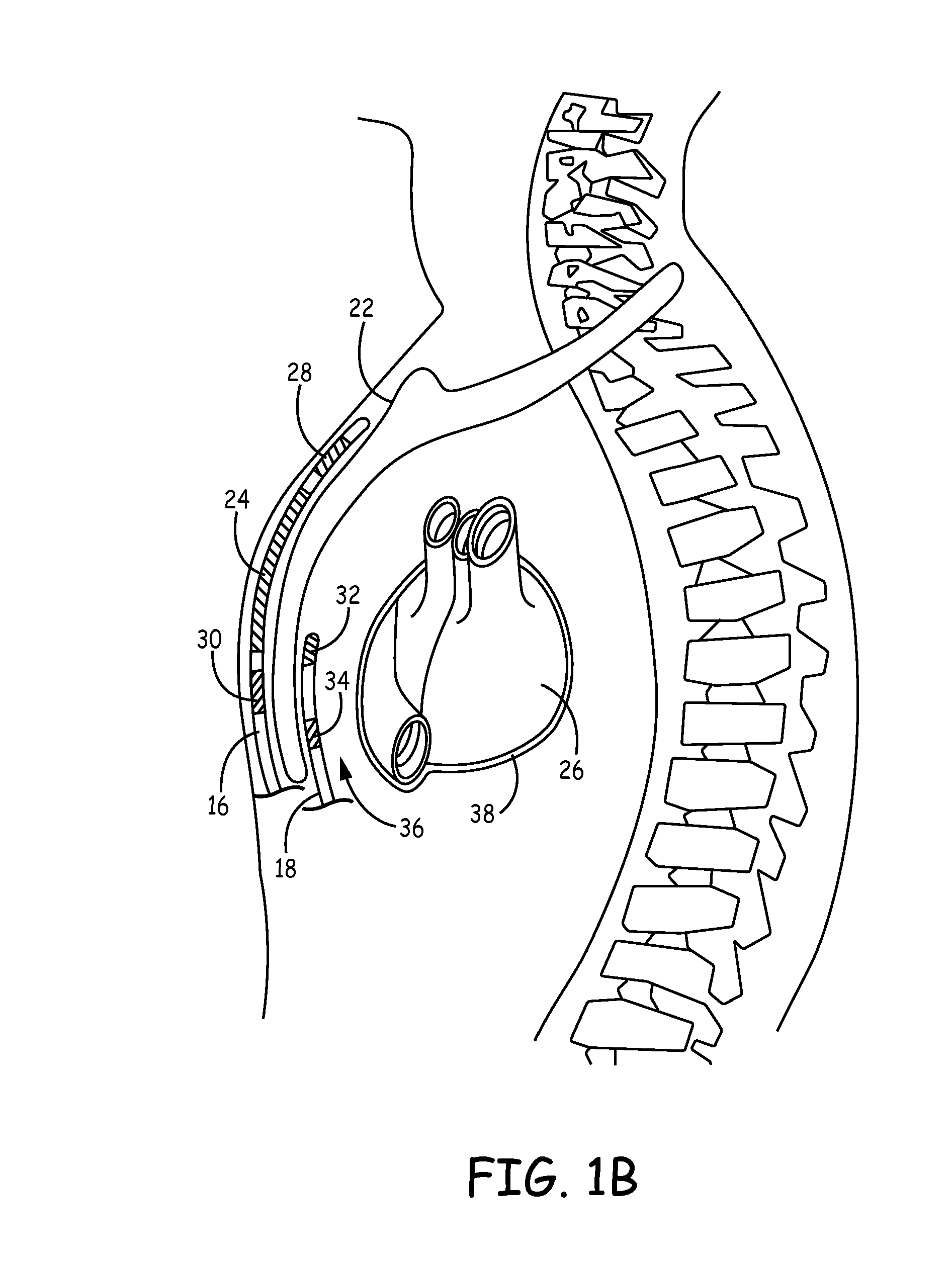
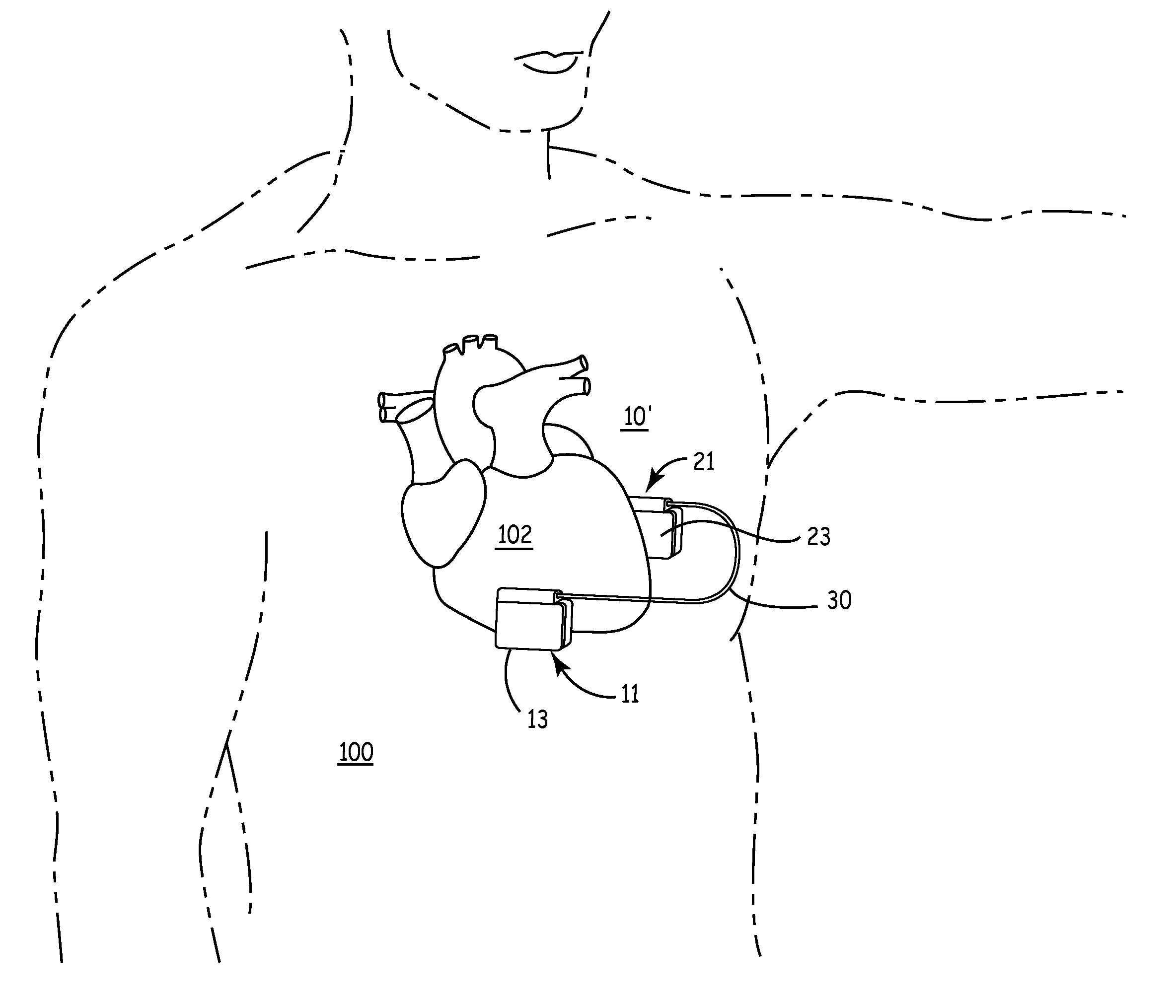
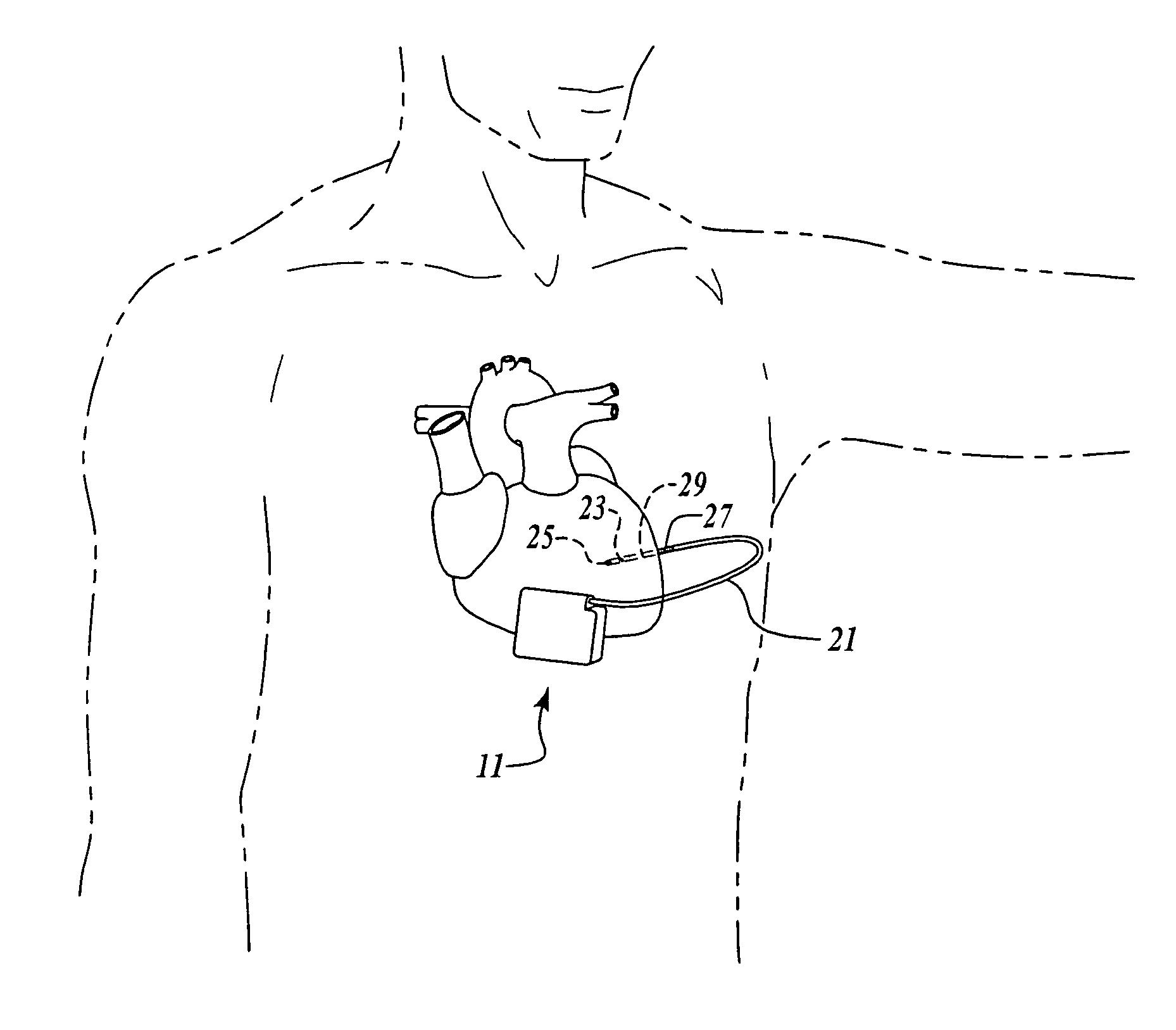
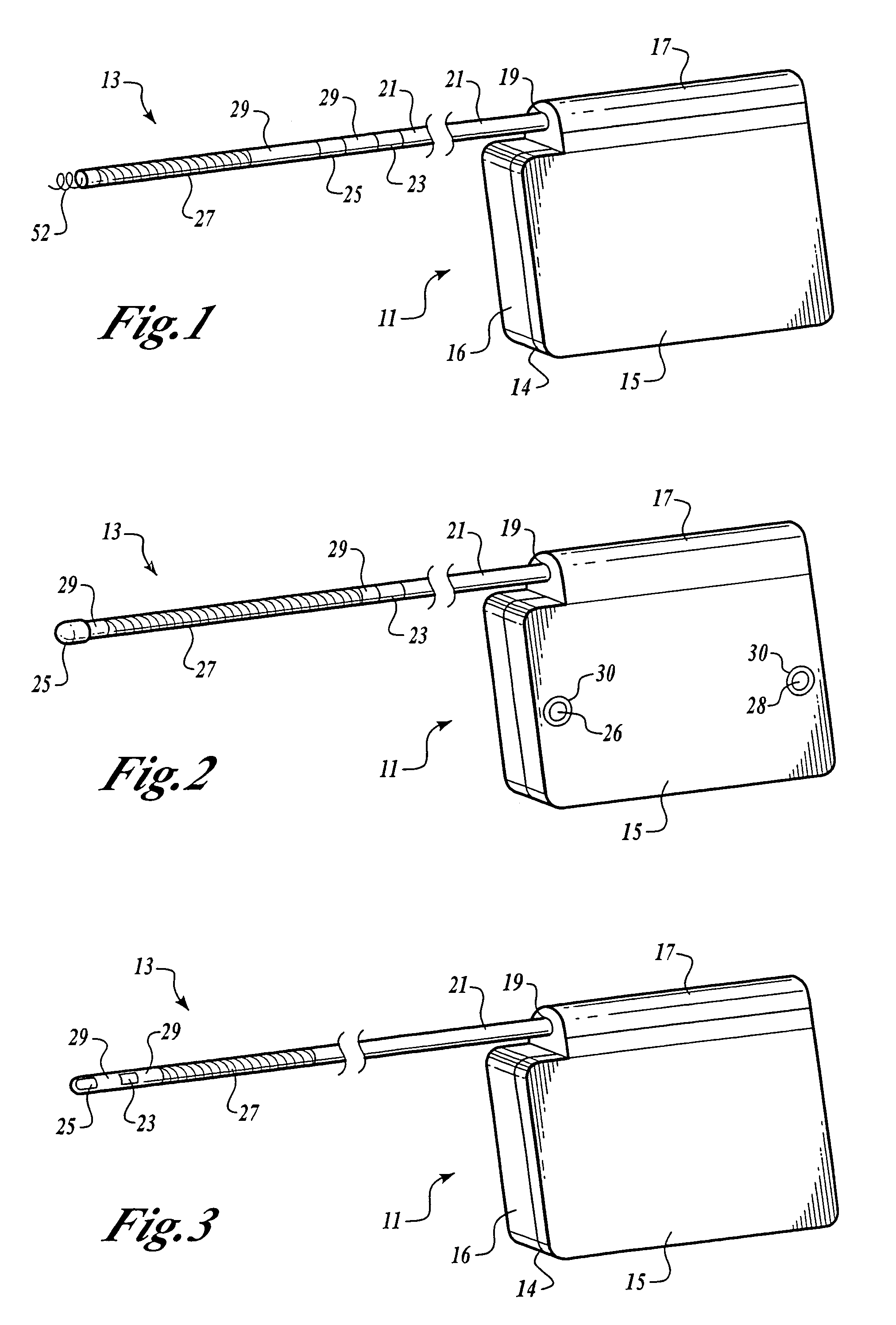
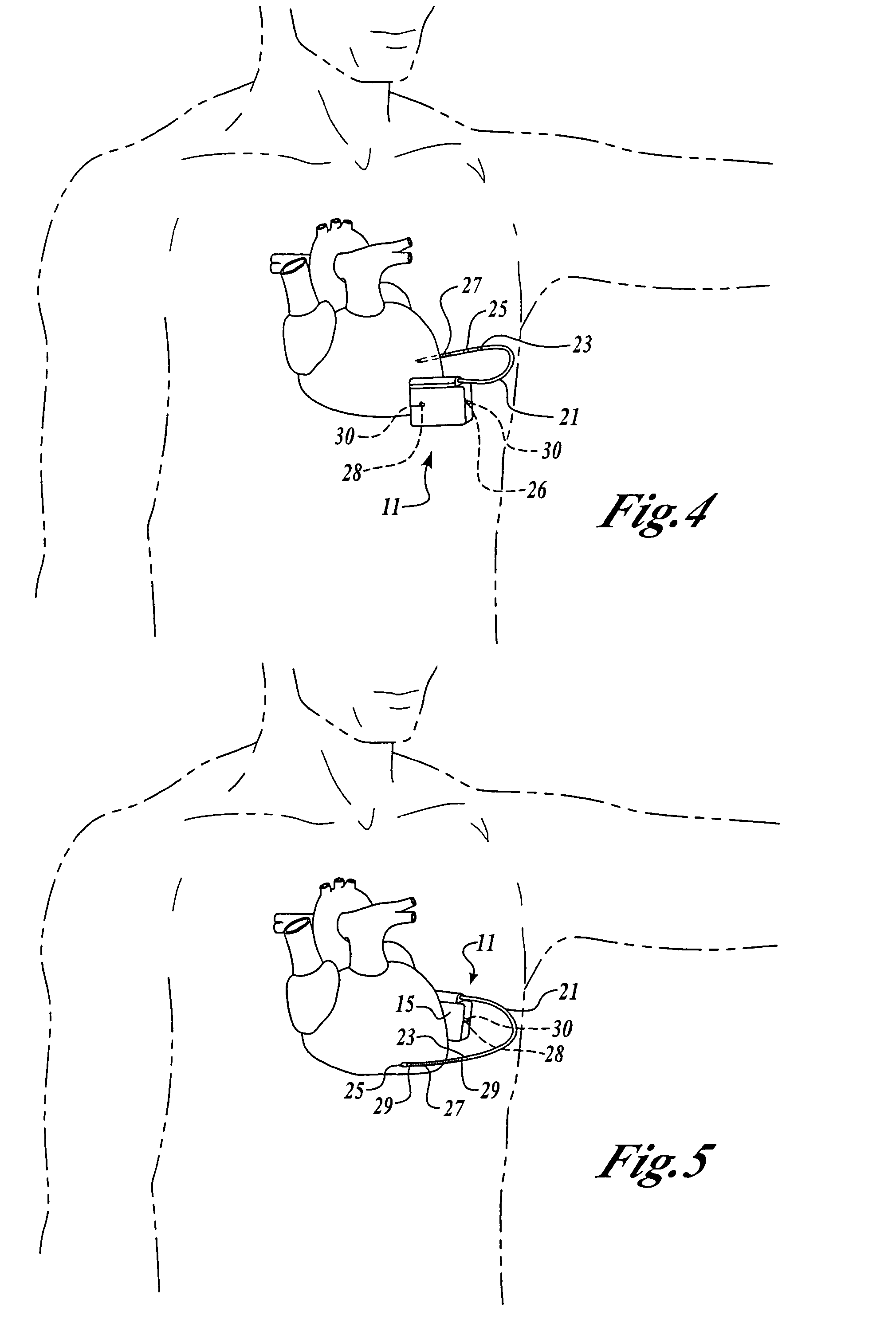
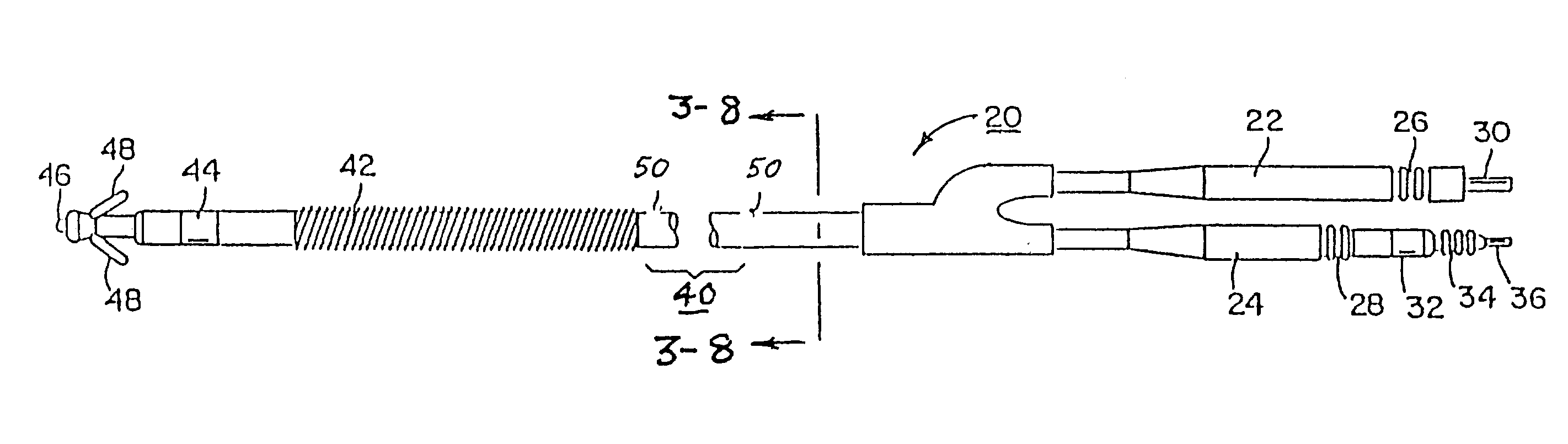
Patents
Literature
130 results about "Cardioversion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
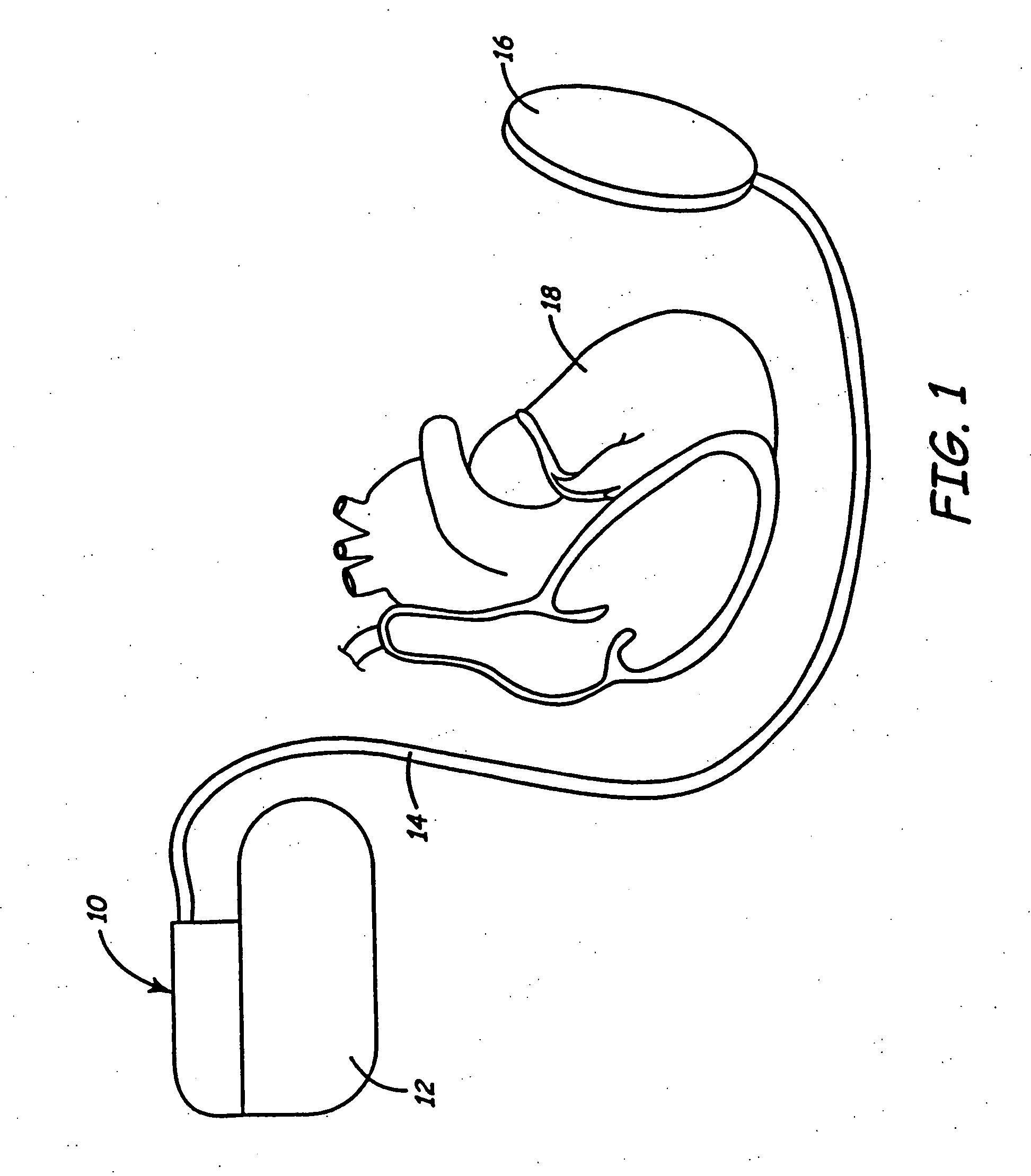
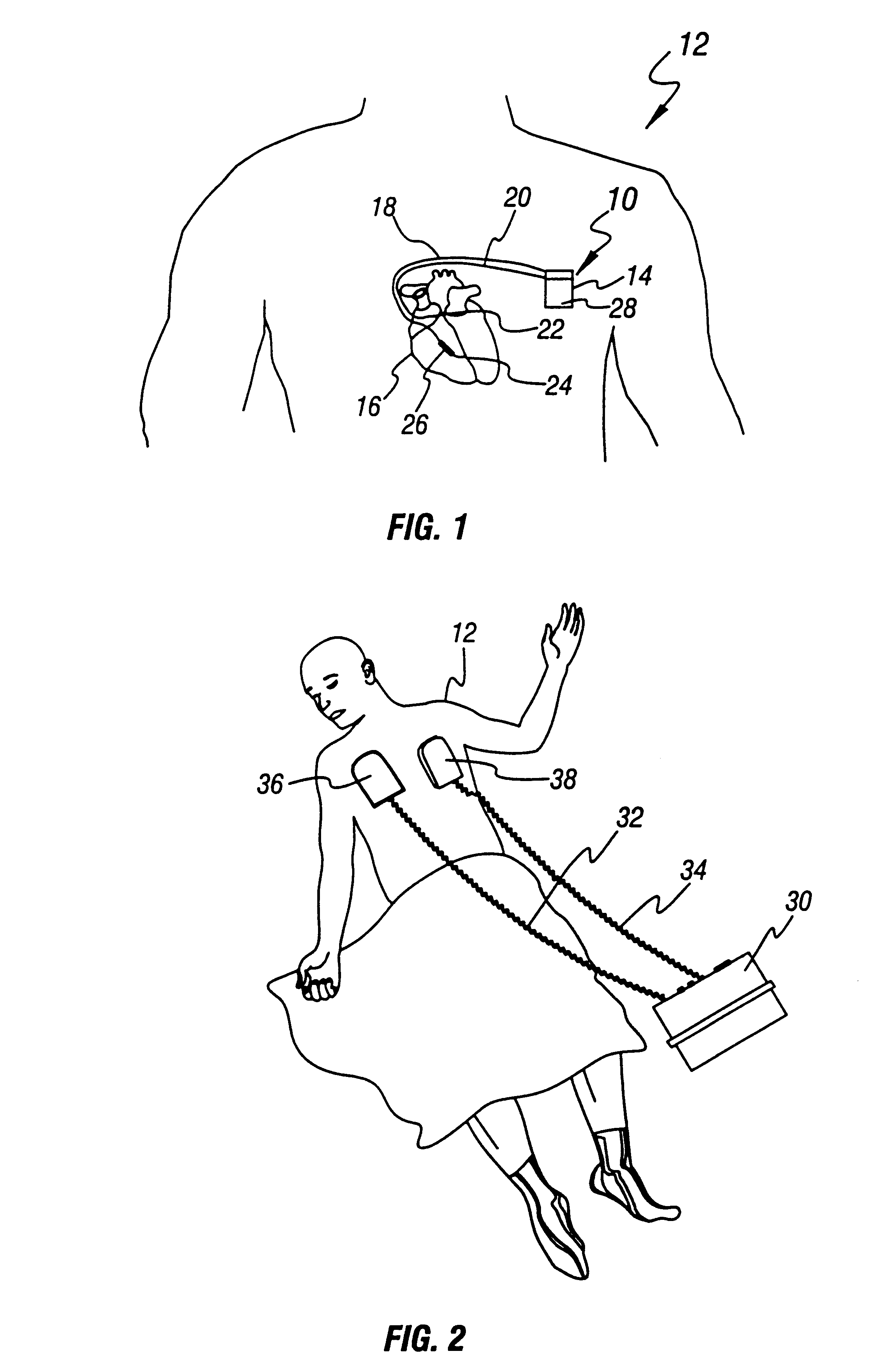
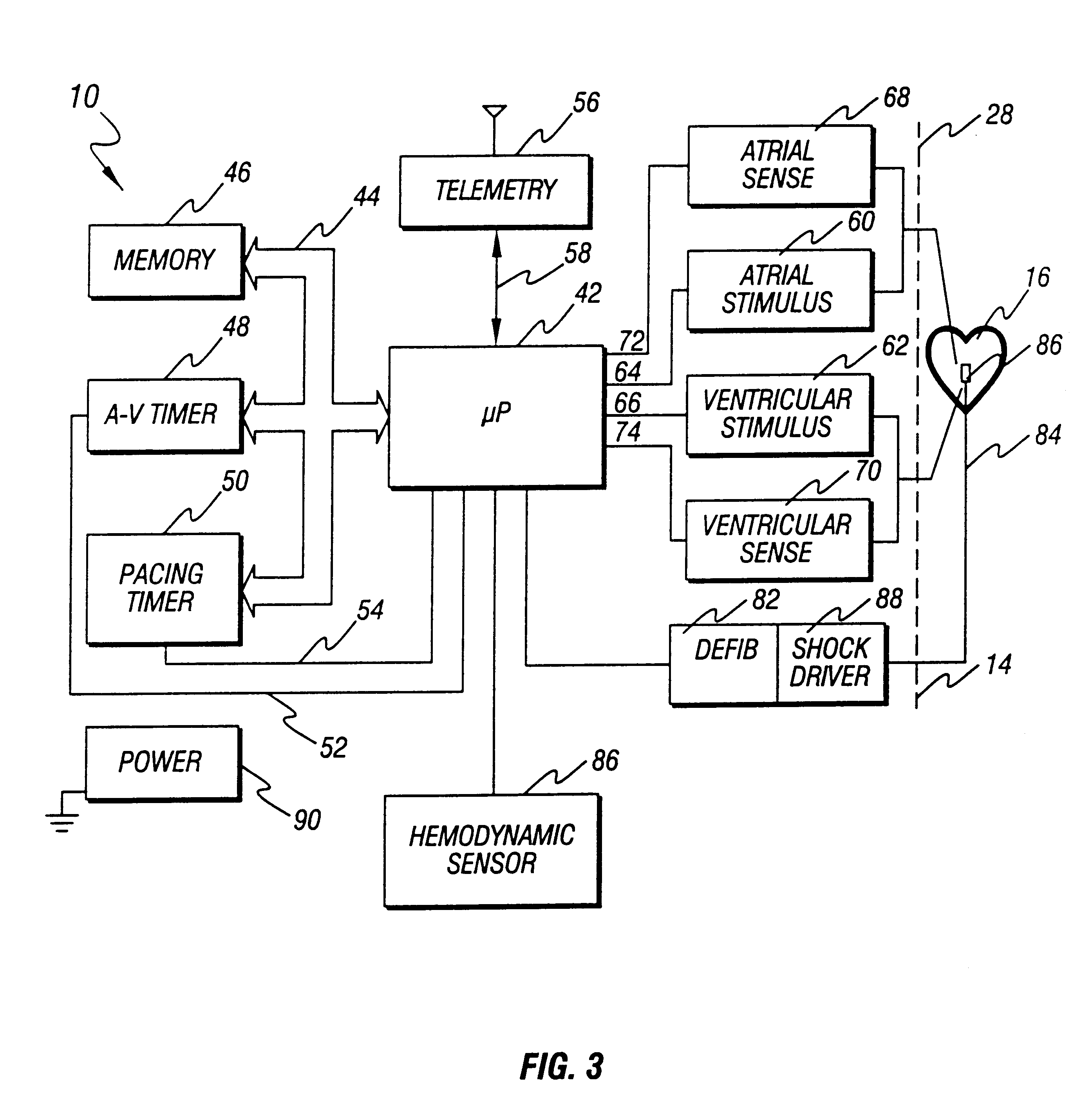
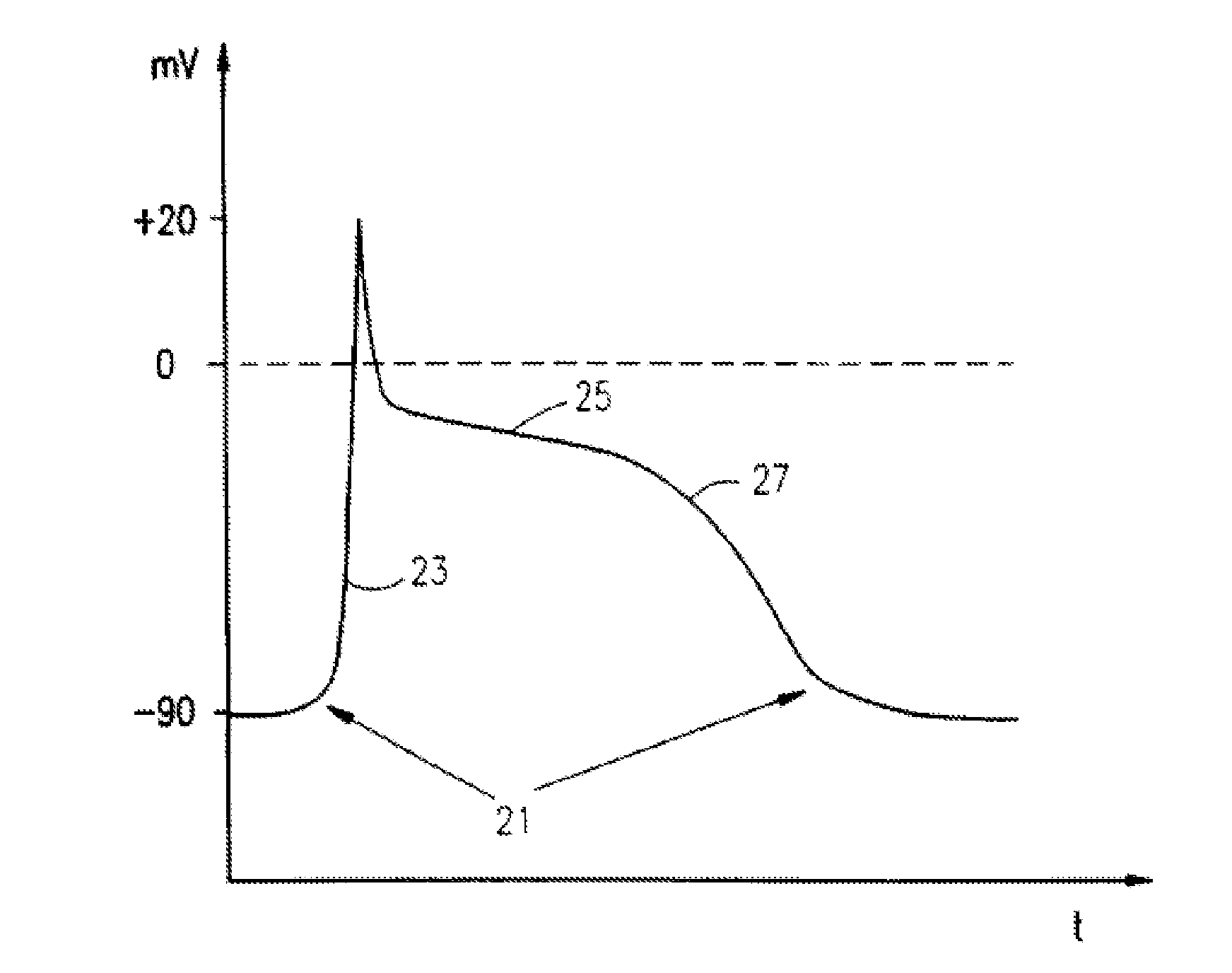
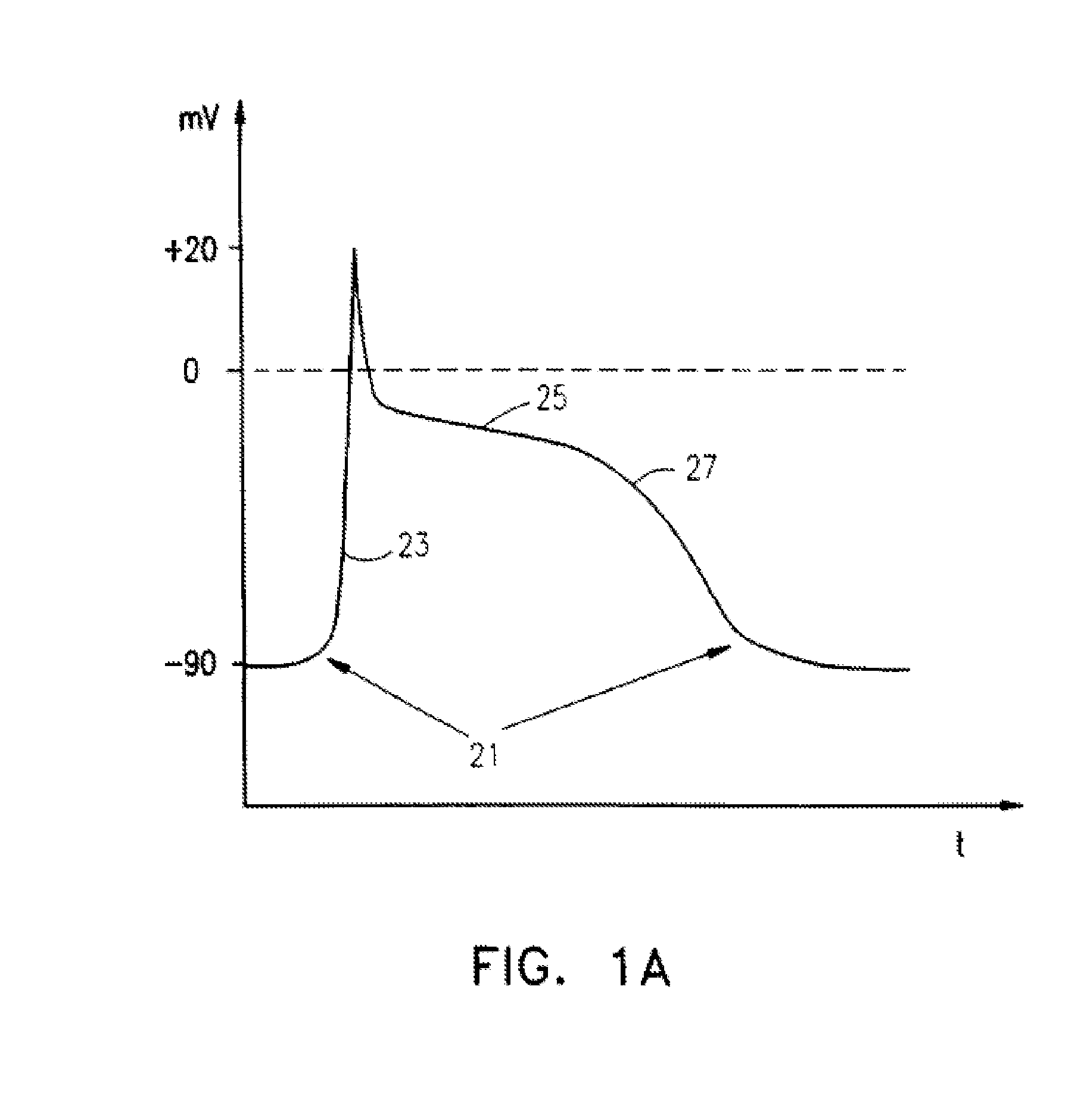
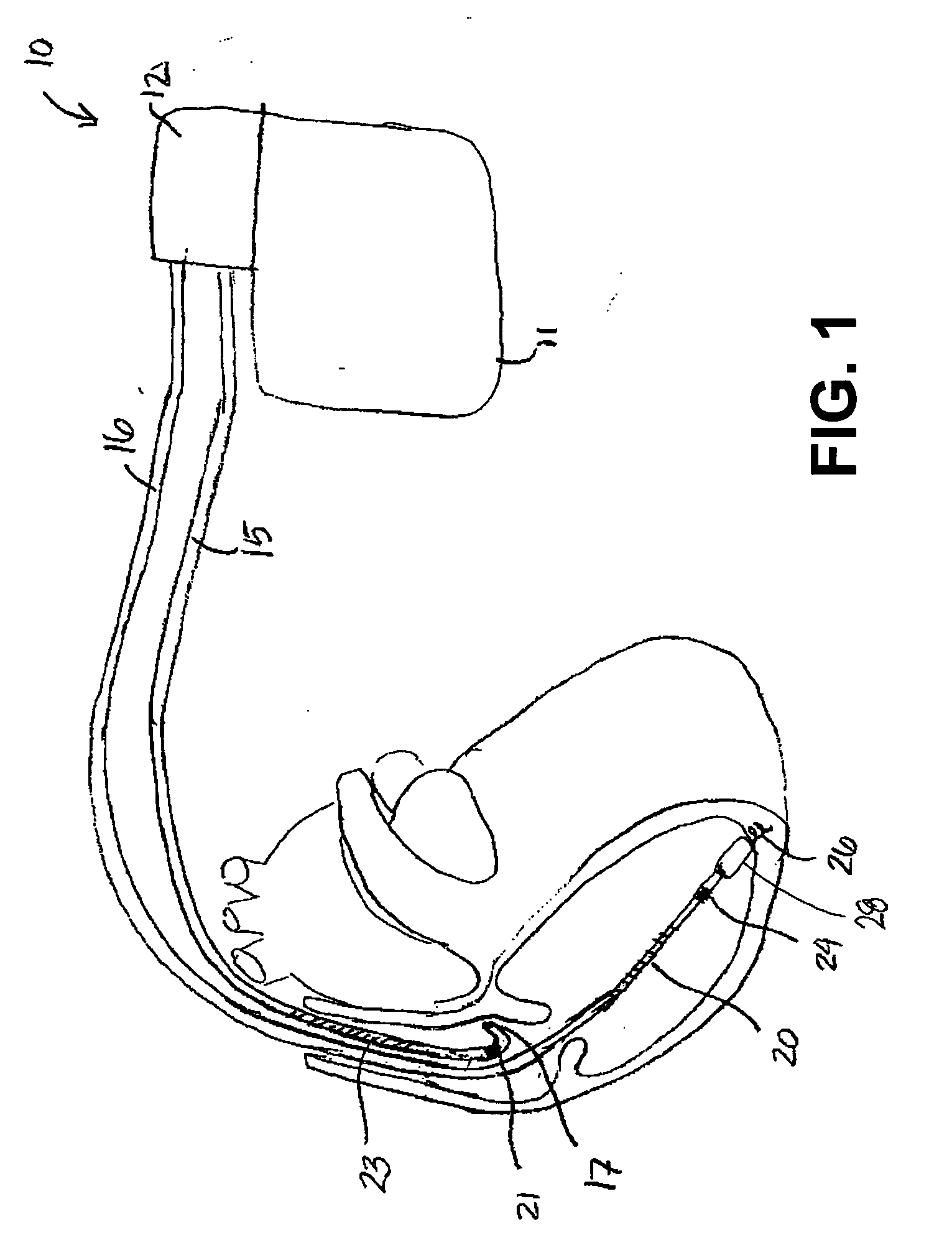
Cardioversion is a medical procedure by which an abnormally fast heart rate (tachycardia) or other cardiac arrhythmia is converted to a normal rhythm using electricity or drugs. Synchronized electrical cardioversion uses a therapeutic dose of electric current to the heart at a specific moment in the cardiac cycle, restoring the activity of the electrical conduction system of the heart. (Defibrillation uses a therapeutic dose of electric current to the heart at a random moment in the cardiac cycle, and is the most effective resuscitation measure for cardiac arrest associated with ventricular fibrillation and pulseless ventricular tachycardia.) Pharmacologic cardioversion, also called chemical cardioversion, uses antiarrhythmia medication instead of an electrical shock.
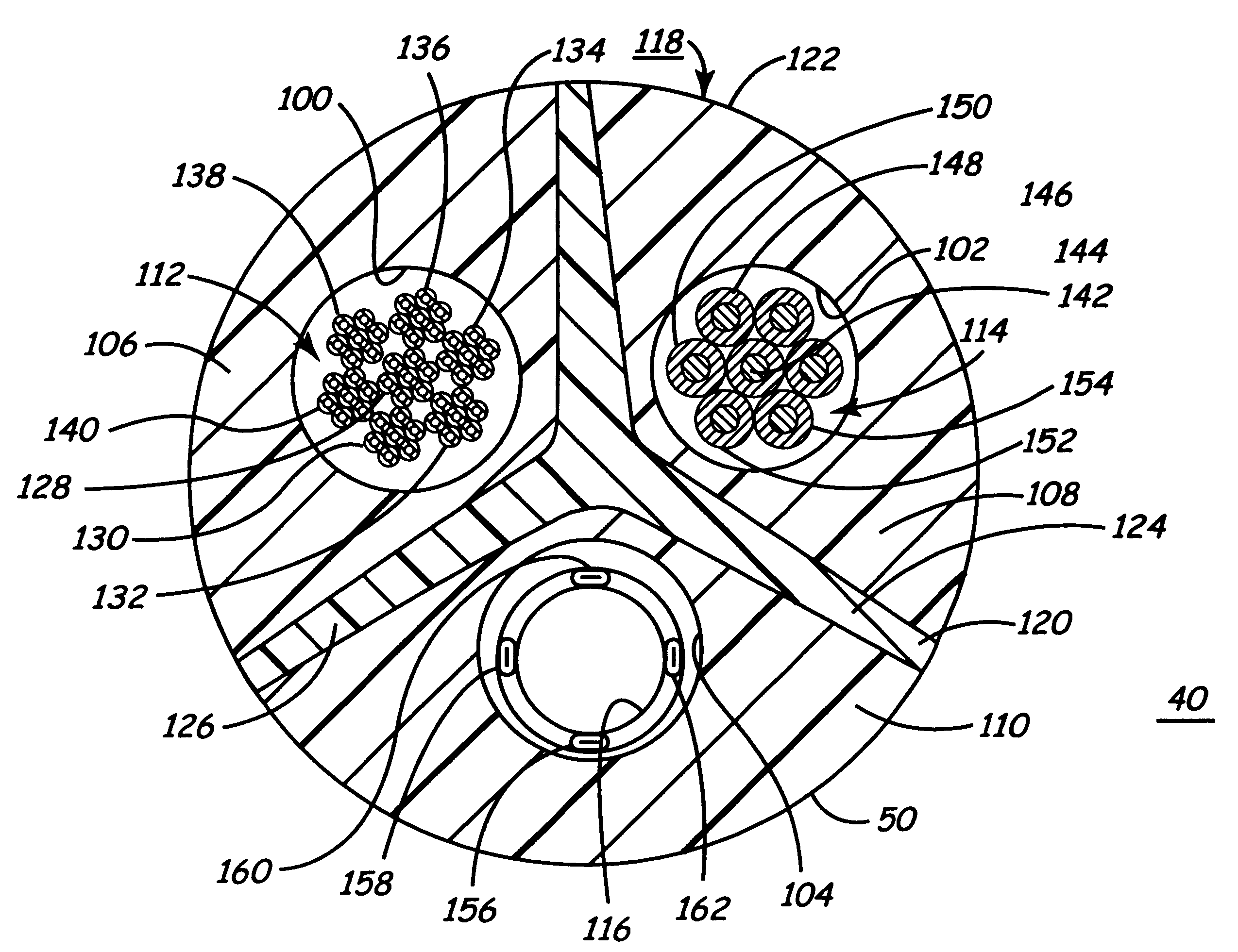
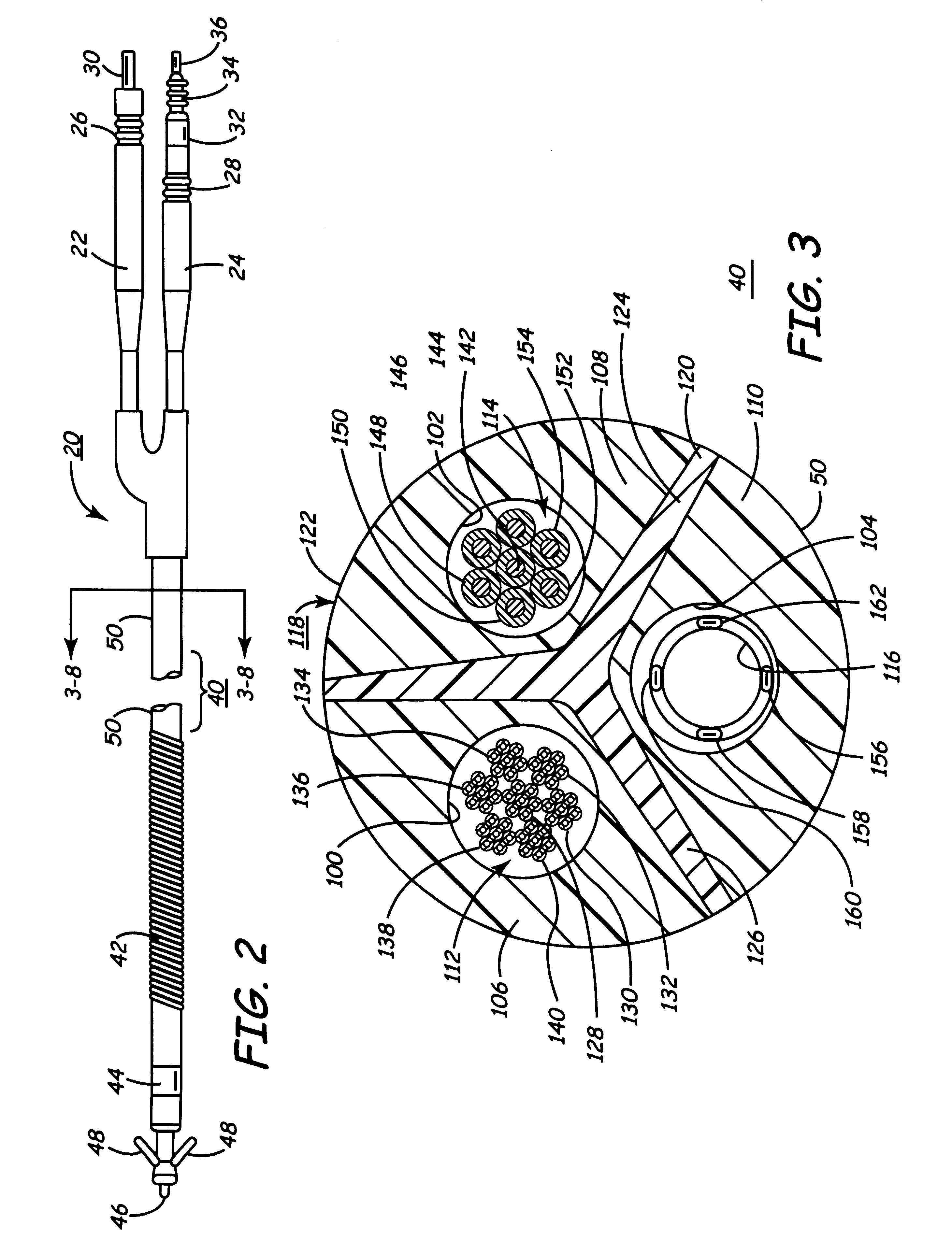
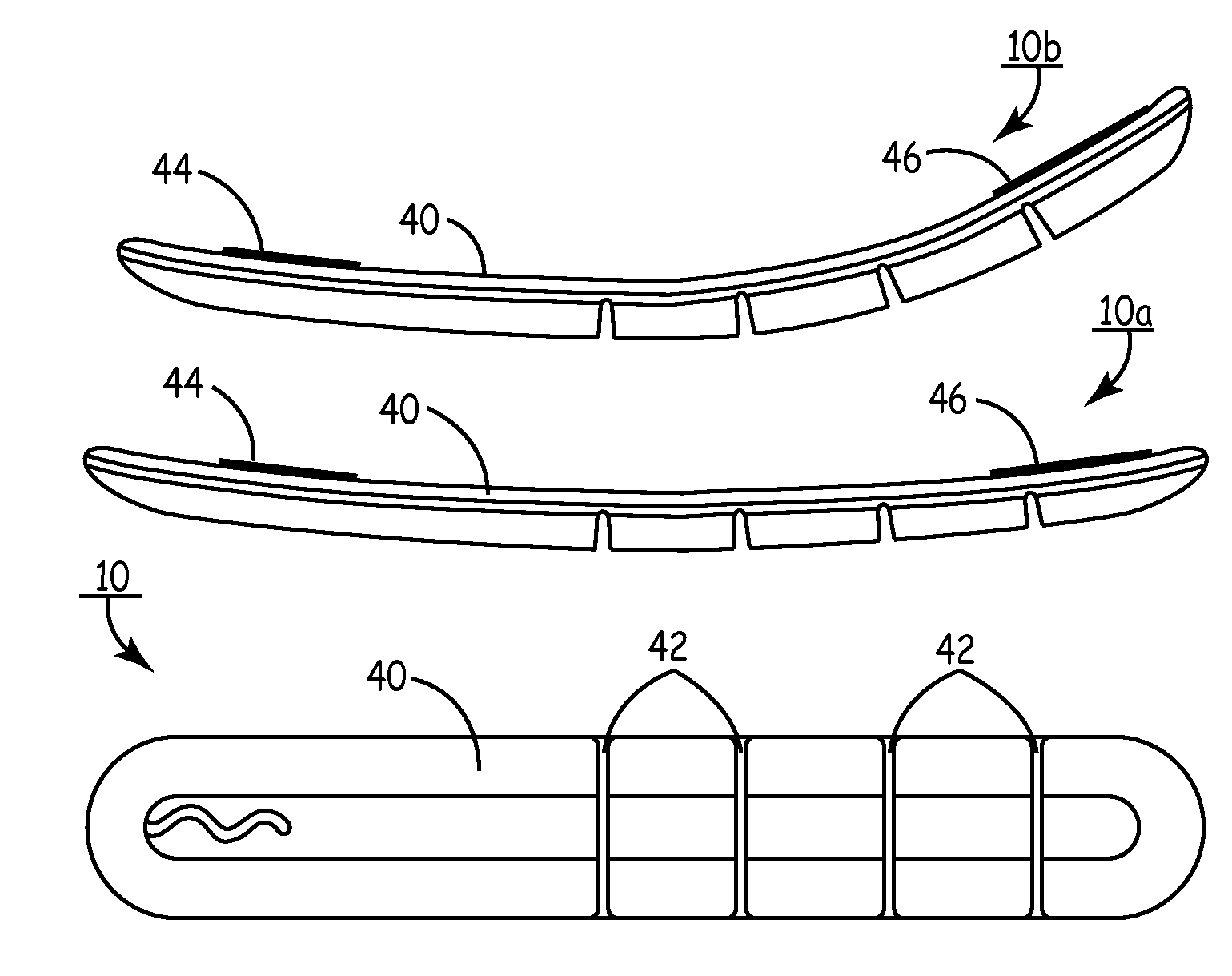
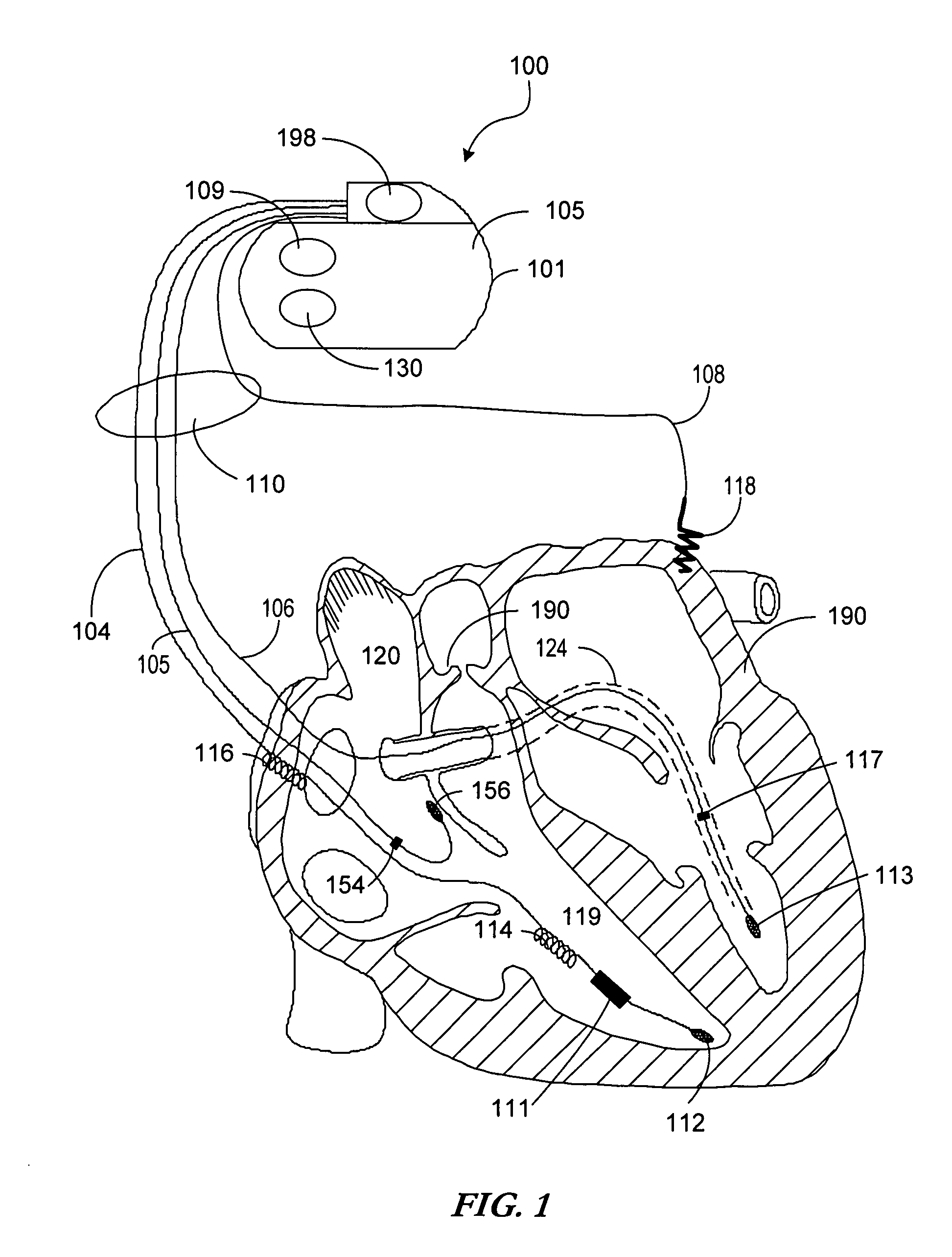
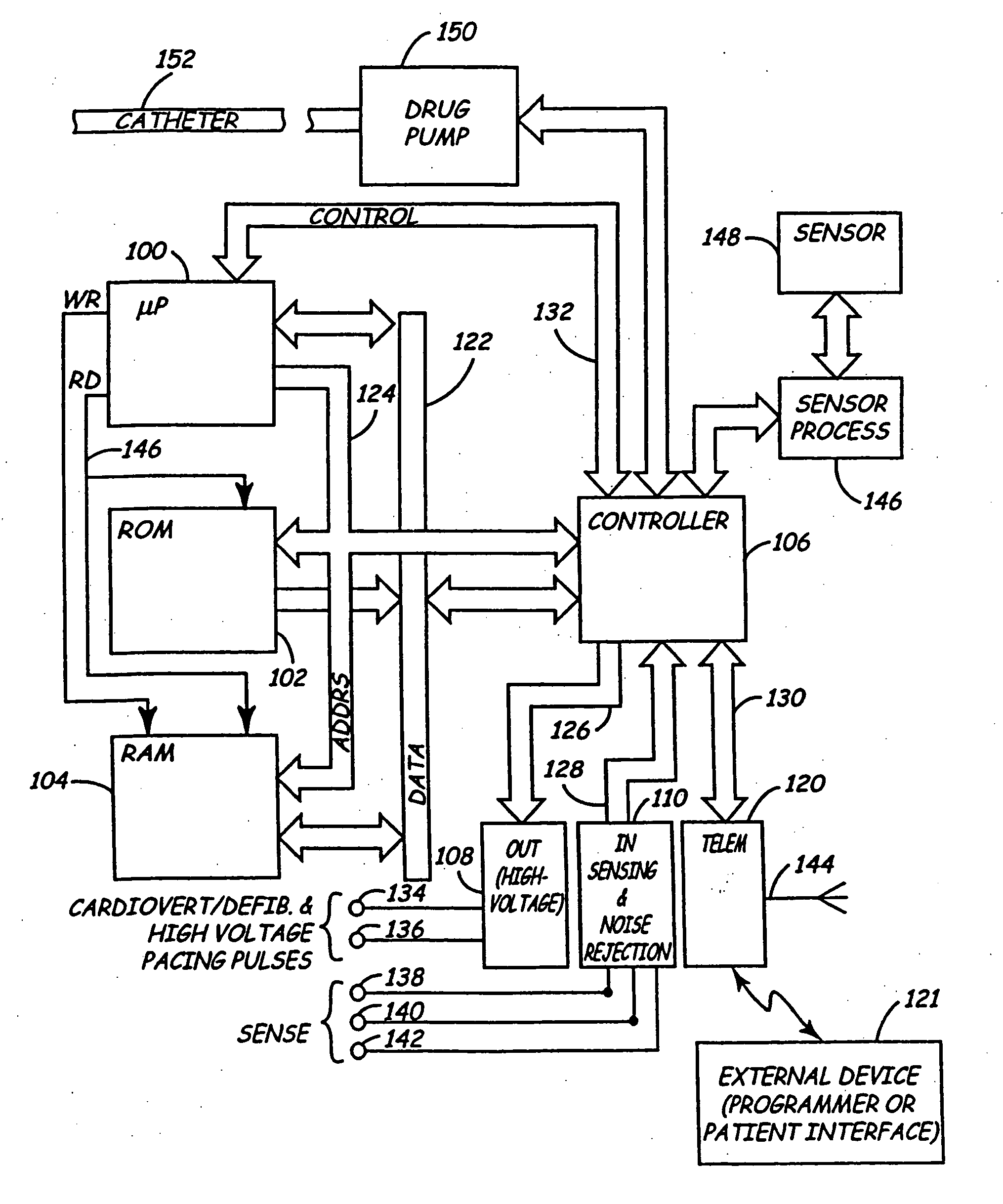
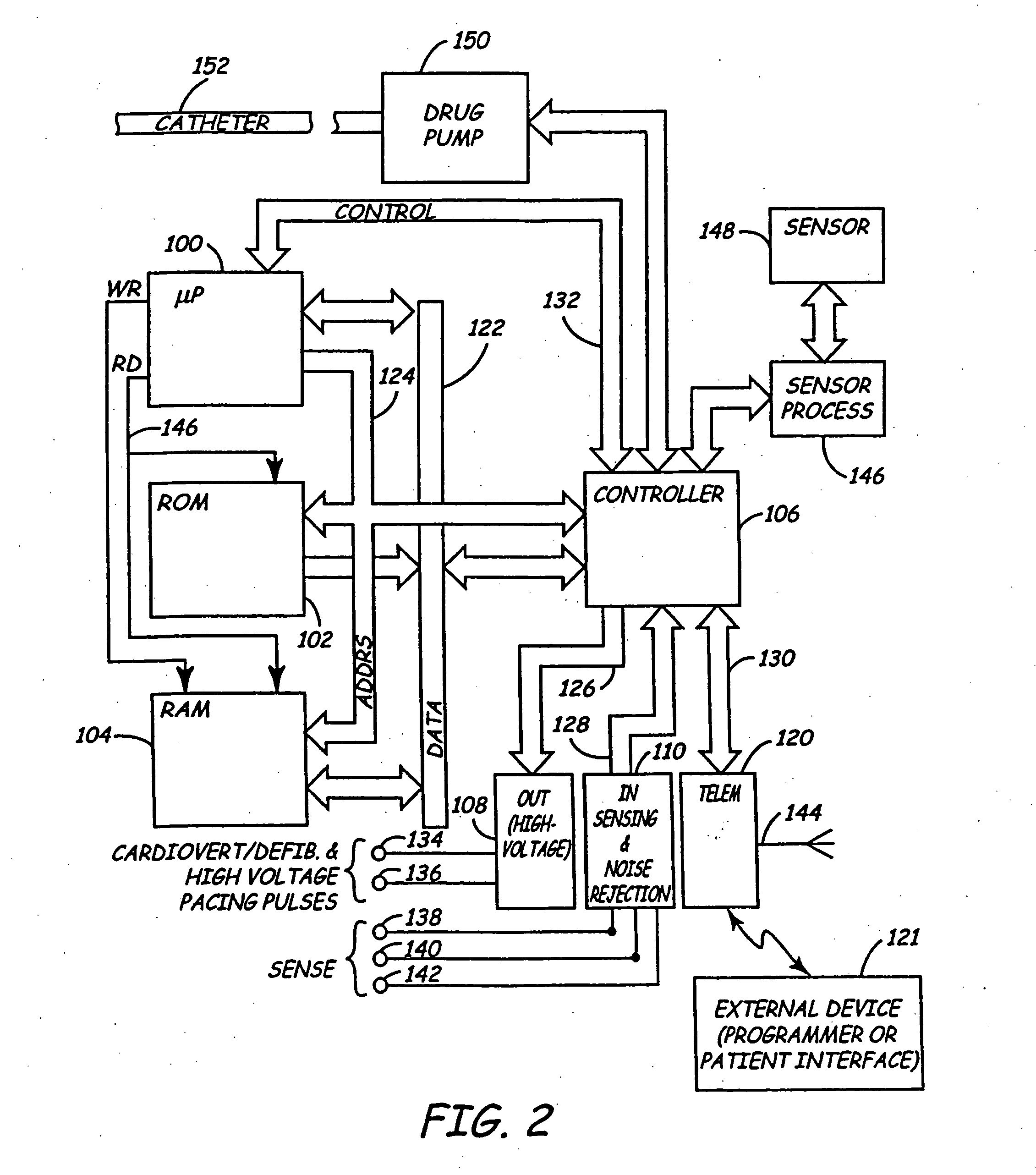
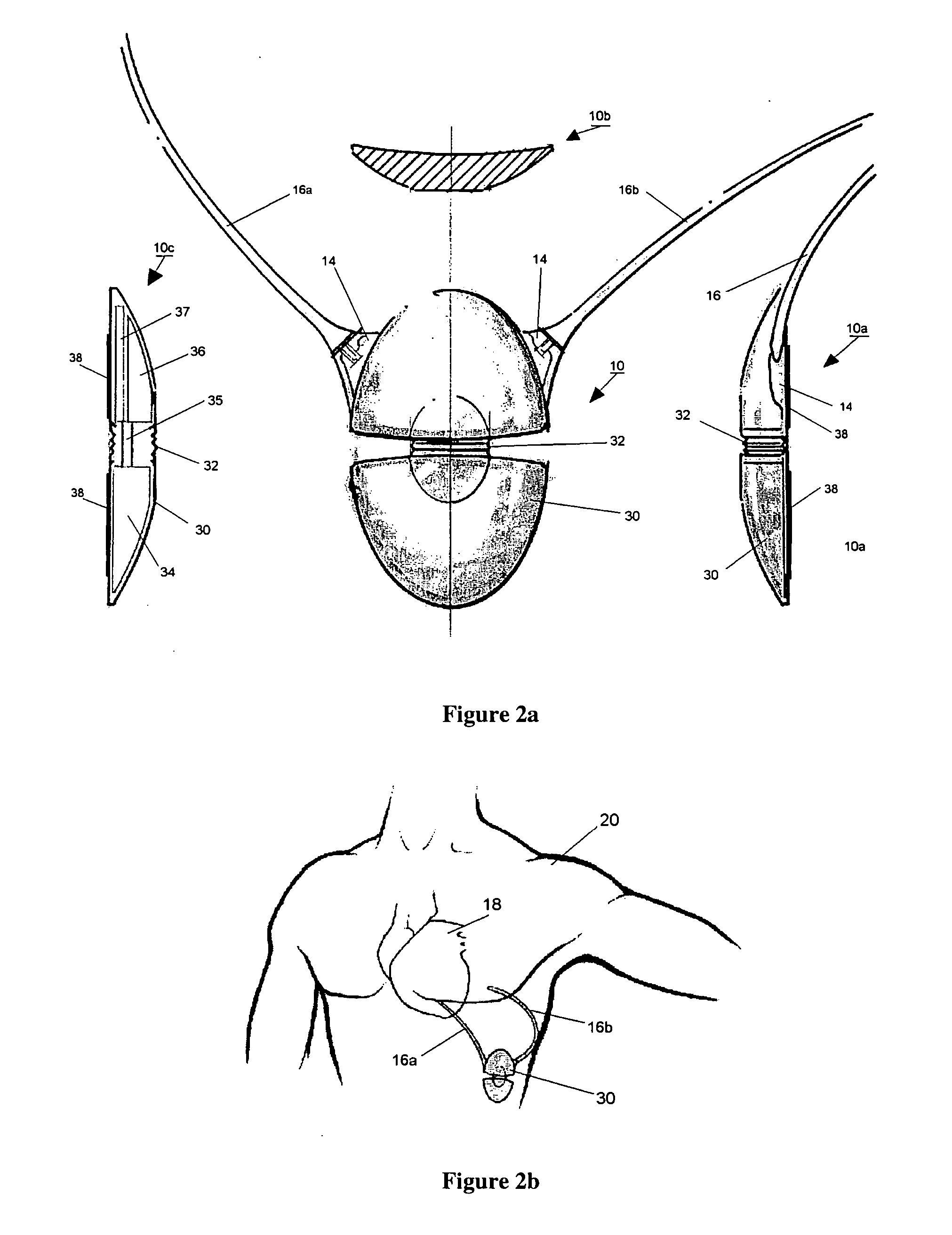
Body implantable lead including one or more conductive polymer electrodes and methods for fabricating same
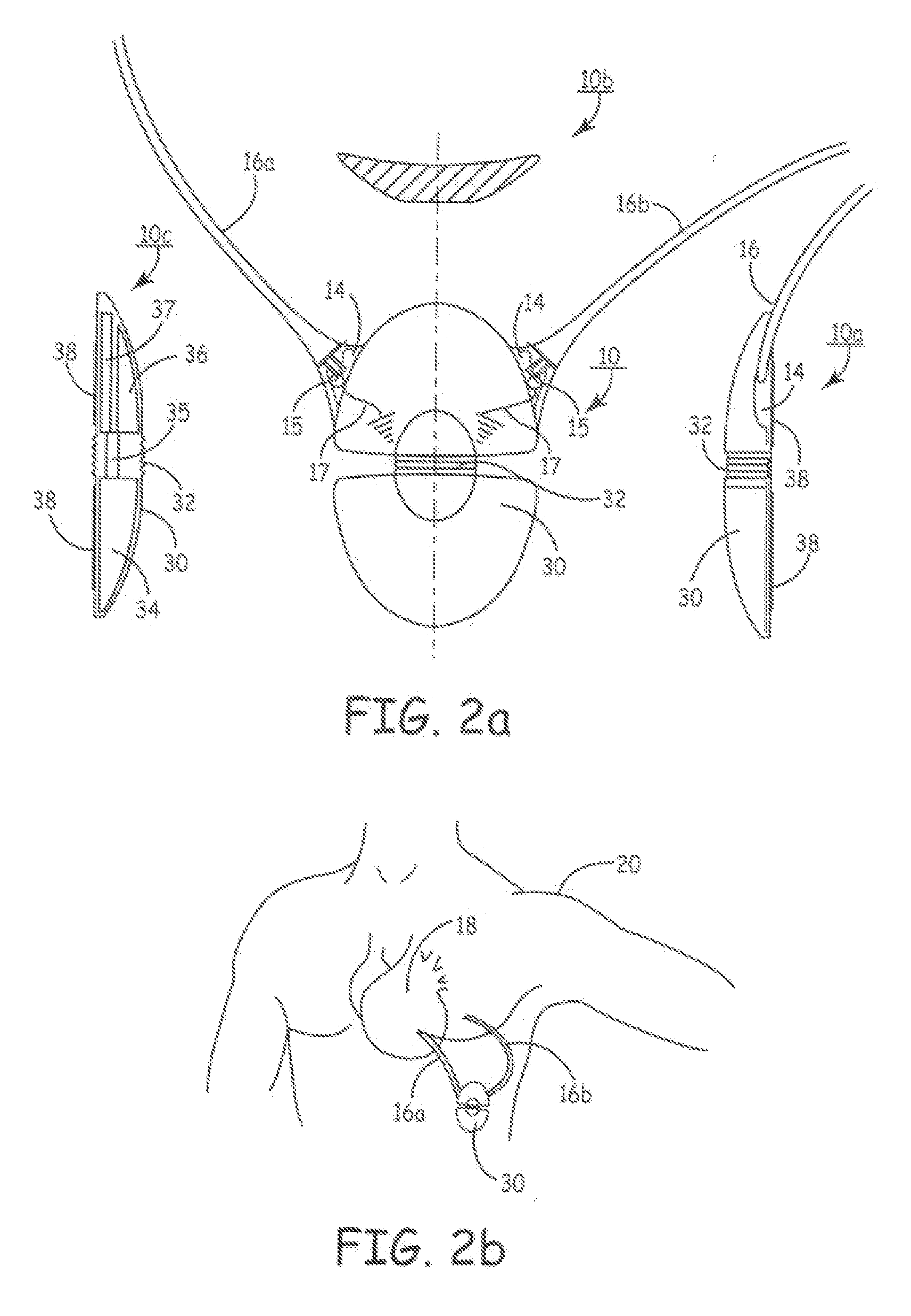
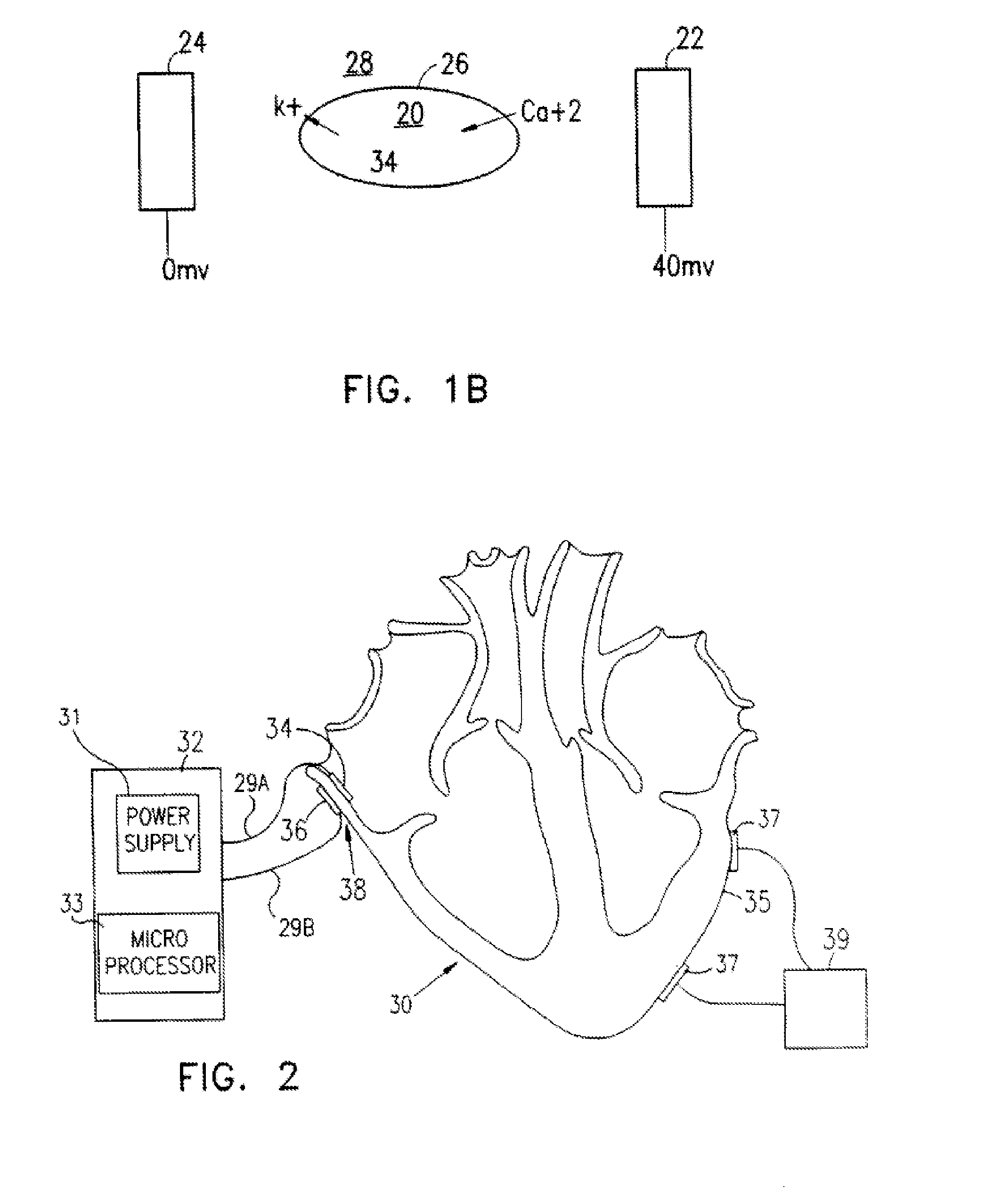
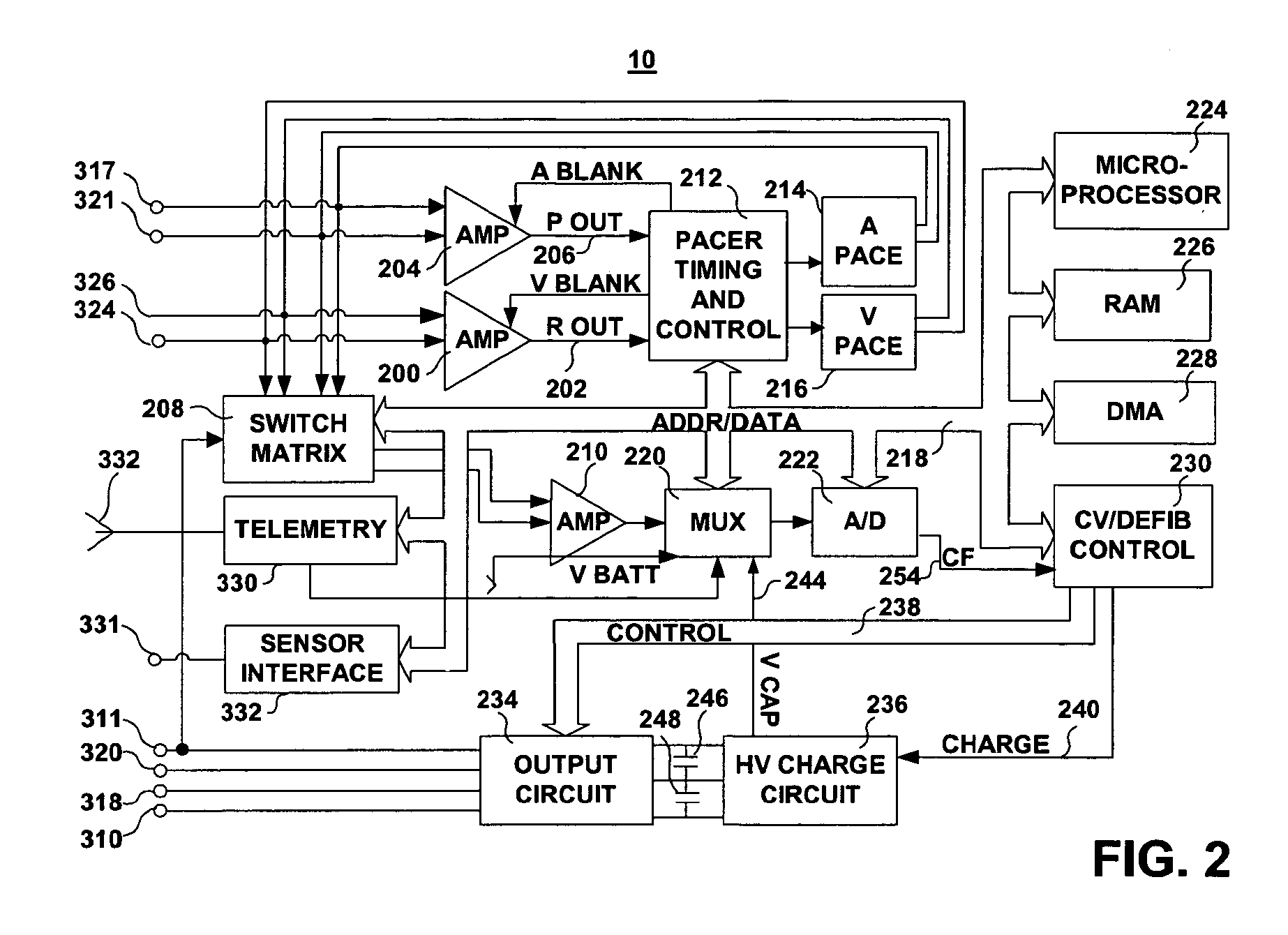
InactiveUS6999821B2Transvascular endocardial electrodesDiagnostic recording/measuringElectrical conductorCoronary sinus
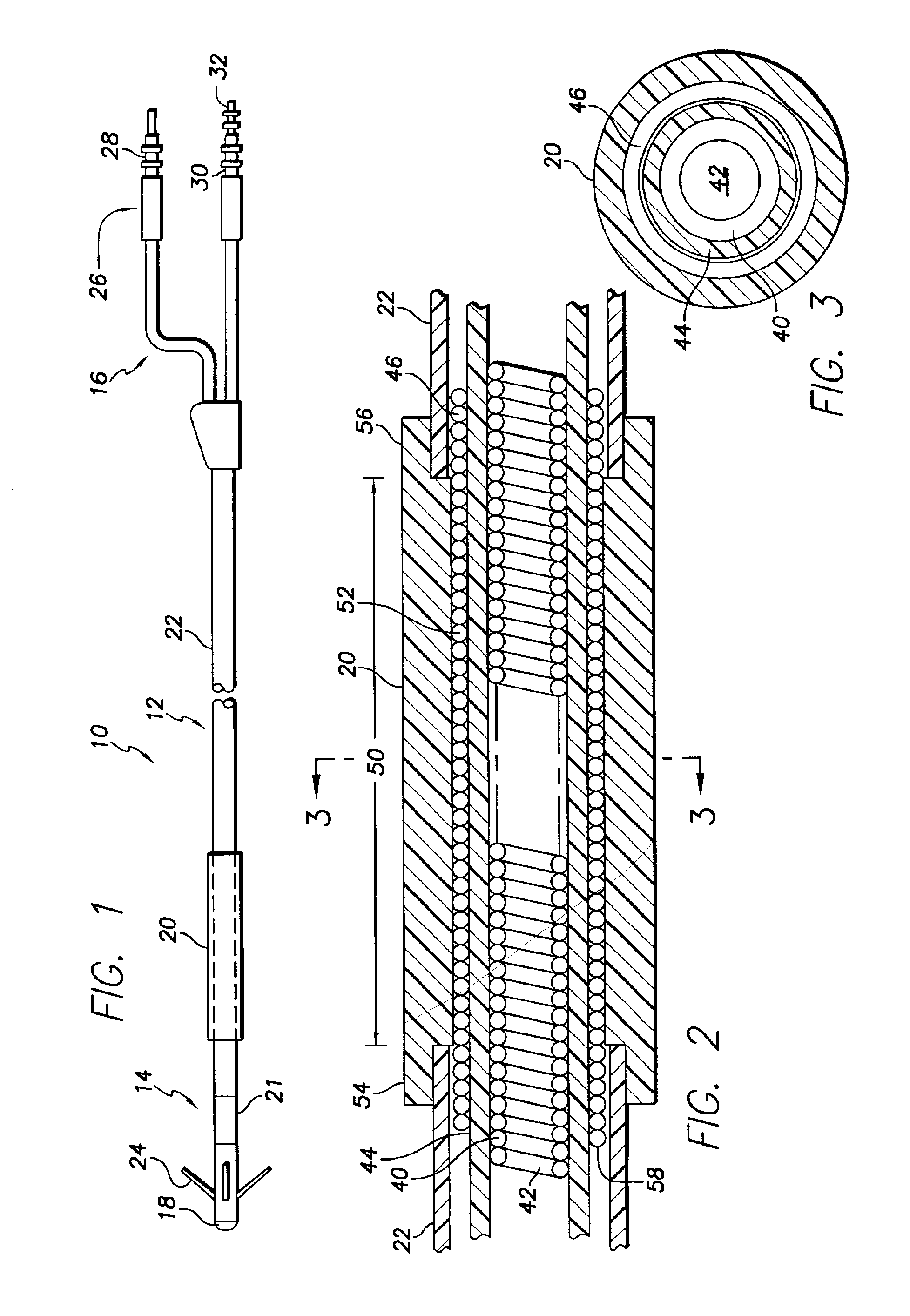
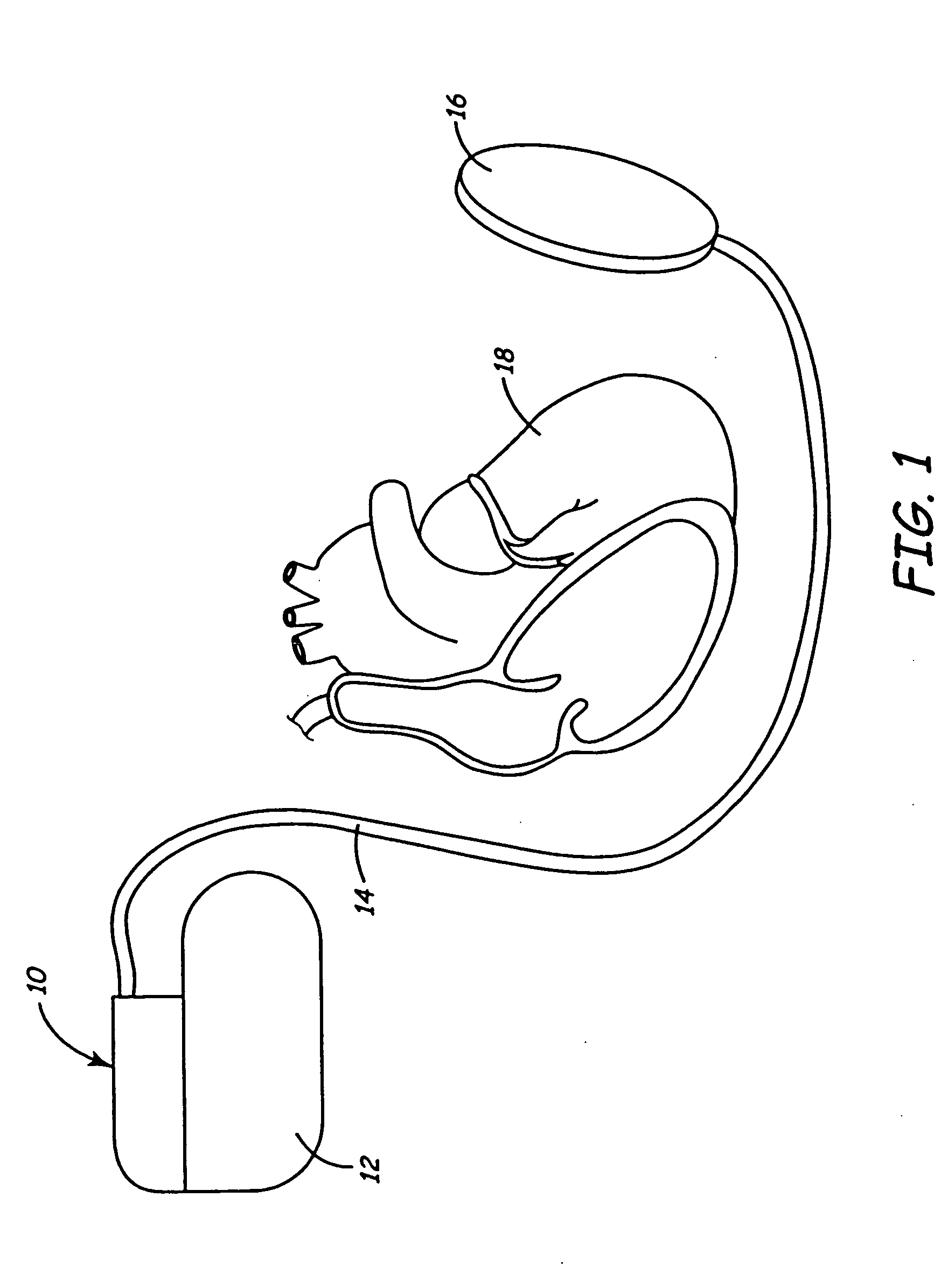
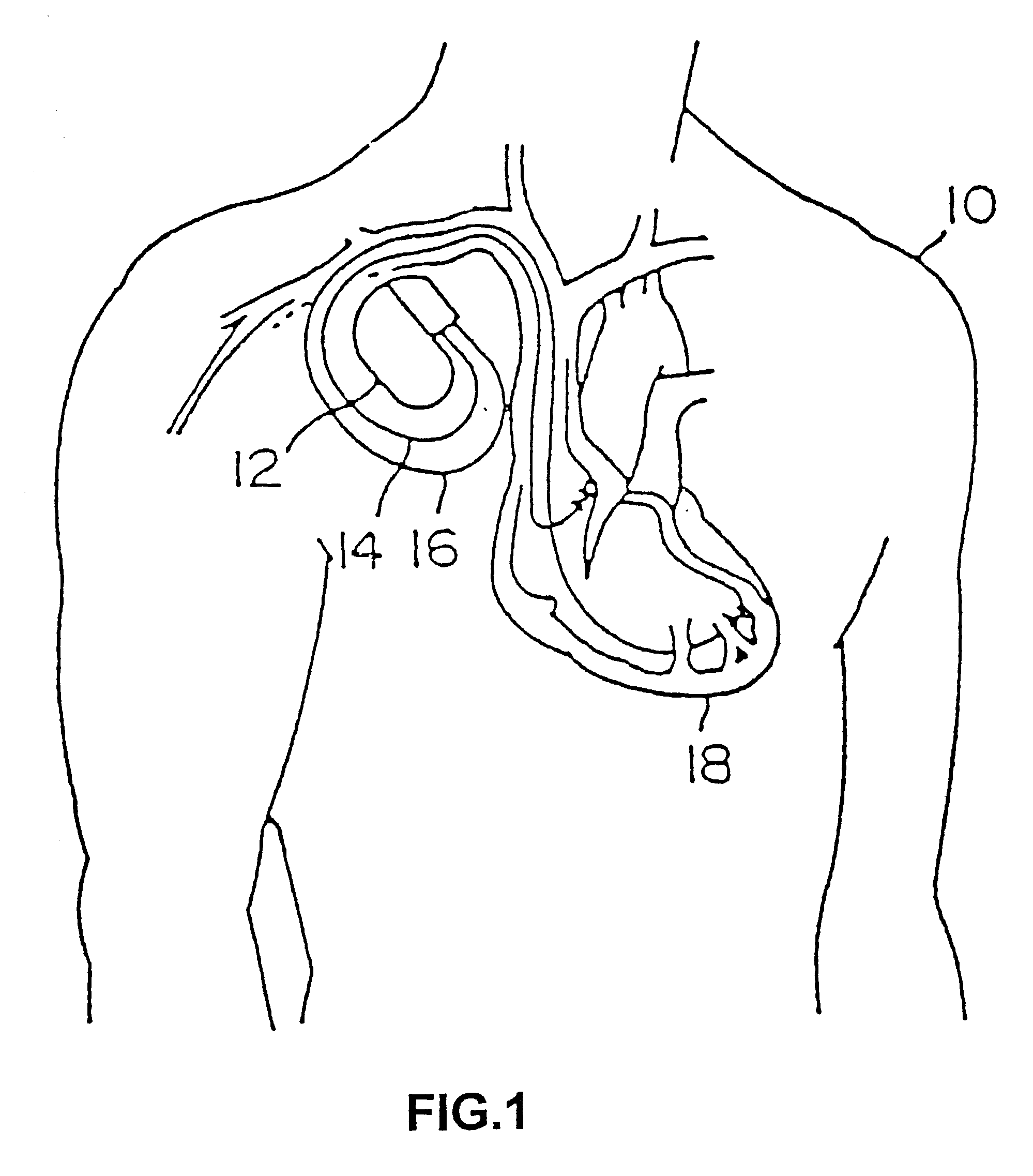
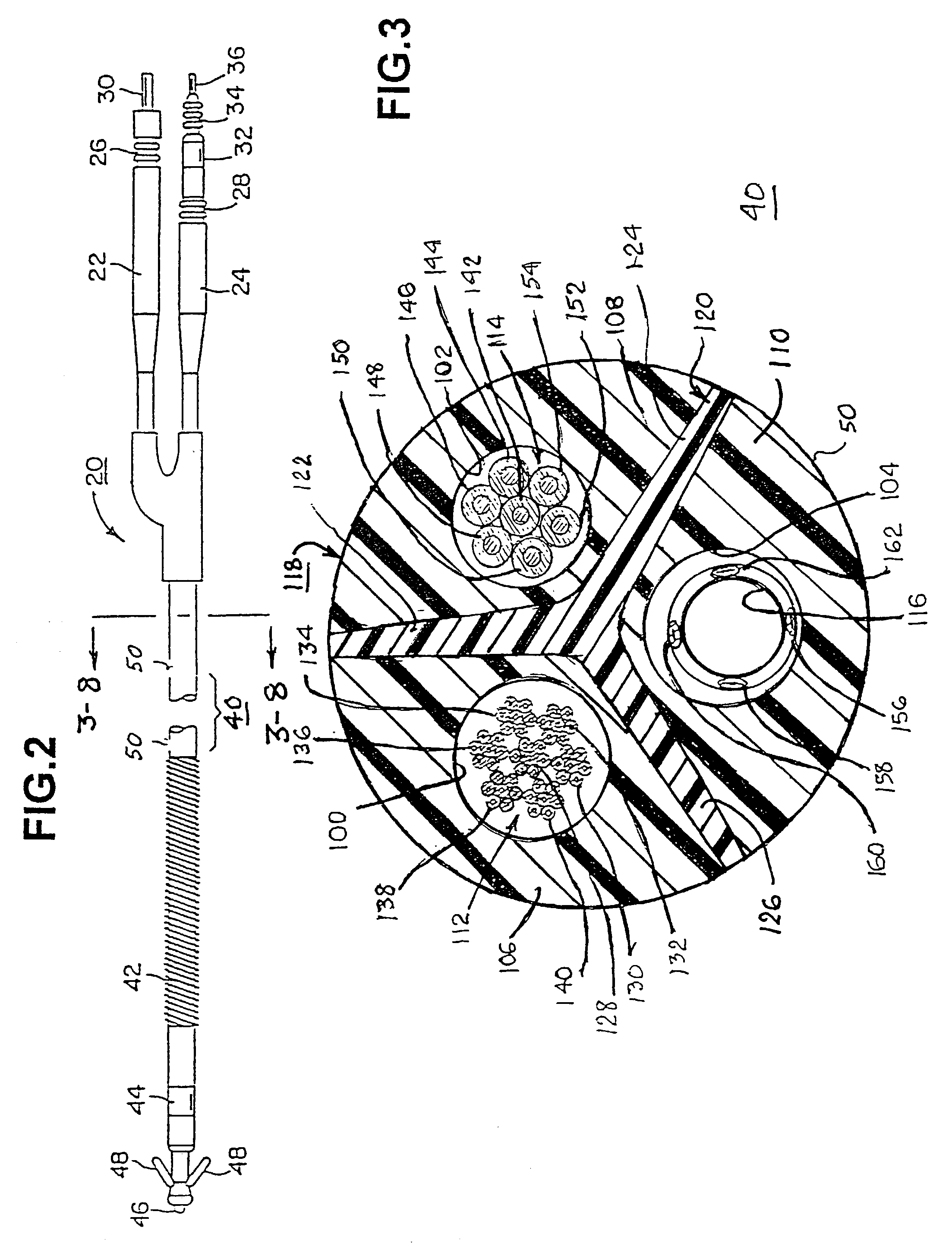
A body implantable lead comprises a lead body including a conductive polymer electrode disposed along a distal end portion of the lead body for performing one or more of the functions consisting of pacing, sensing, cardioversion and defibrillation. An electrical conductor, preferably in the form of a multistrand cable conductor, couples the conductive polymer electrode with a proximal end of the lead body. The conductive polymer electrode encapsulates the conductor and is in electrical contact therewith along the length, and preferably along substantially the entire length, of the conductive polymer electrode. The lead body may comprise a multilumen polymer housing, the conductor being contained within one of the lumens of the housing. The conductive polymer electrode may be disposed within a window formed in the lead body. Alternatively, the conductive polymer electrode may comprise multiple electrode sections within a corresponding number of windows formed in the lead body and spaced apart along the length thereof. Further, the window and the conductive polymer electrode disposed therein may extend helically about the lead body. Because of its flexibility and because it can have a small diameter, the lead of the invention is particularly advantageous for implantation in the small, tortuous vessels of the coronary sinus region of the heart for left side stimulation and / or sensing.Methods of fabricating lead bodies incorporating conductive polymer electrodes are also disclosed.
Owner:PACESETTER INC
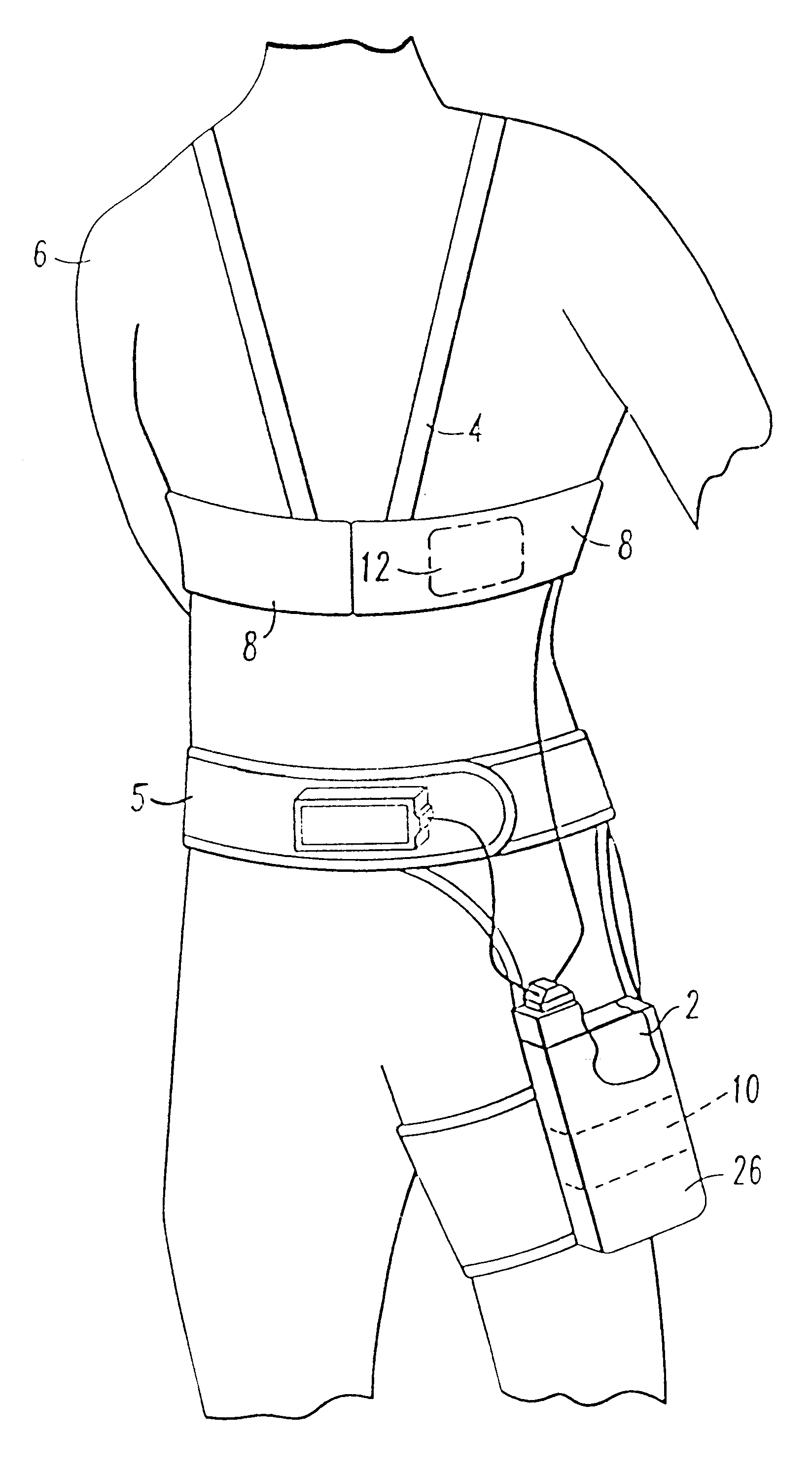
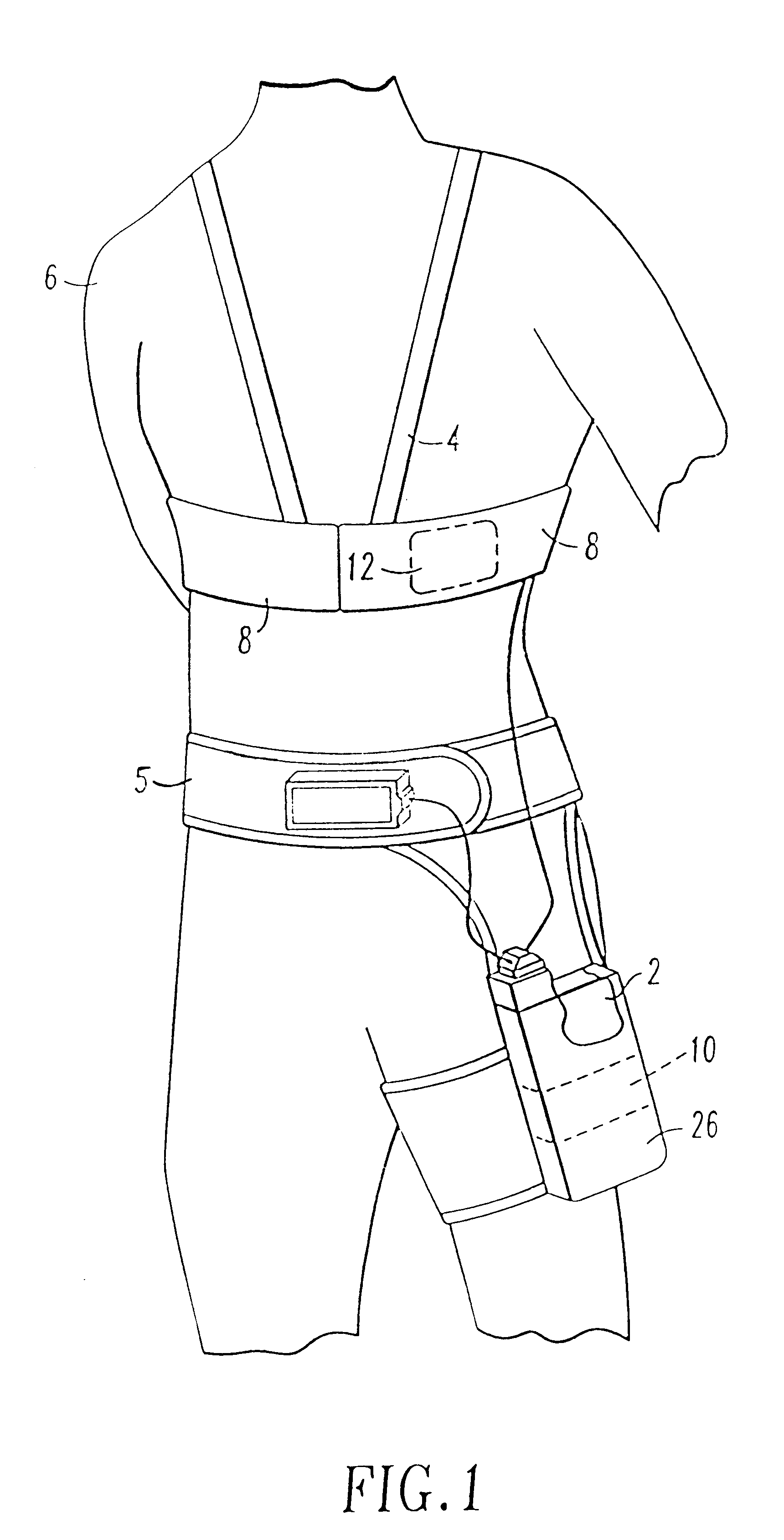
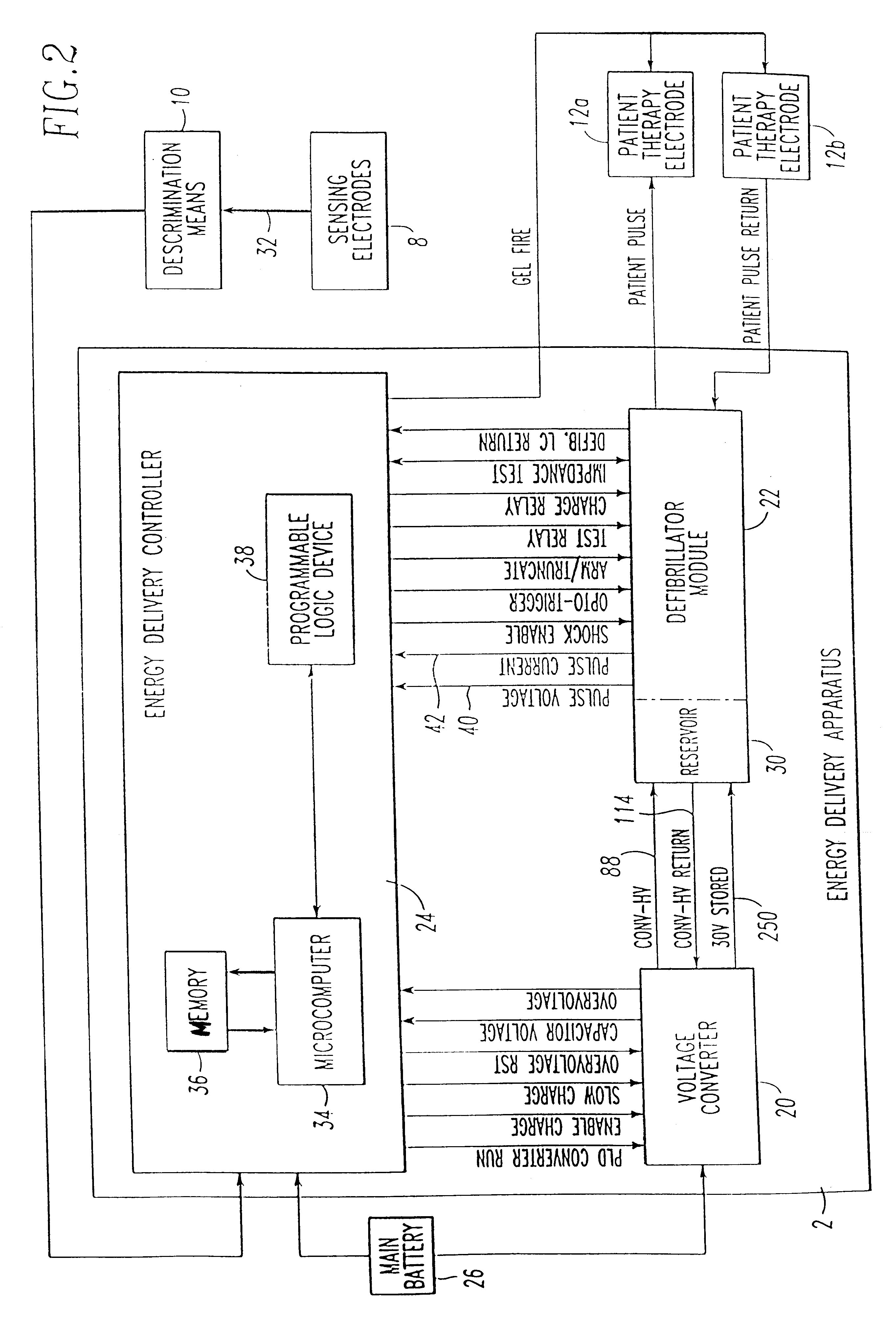
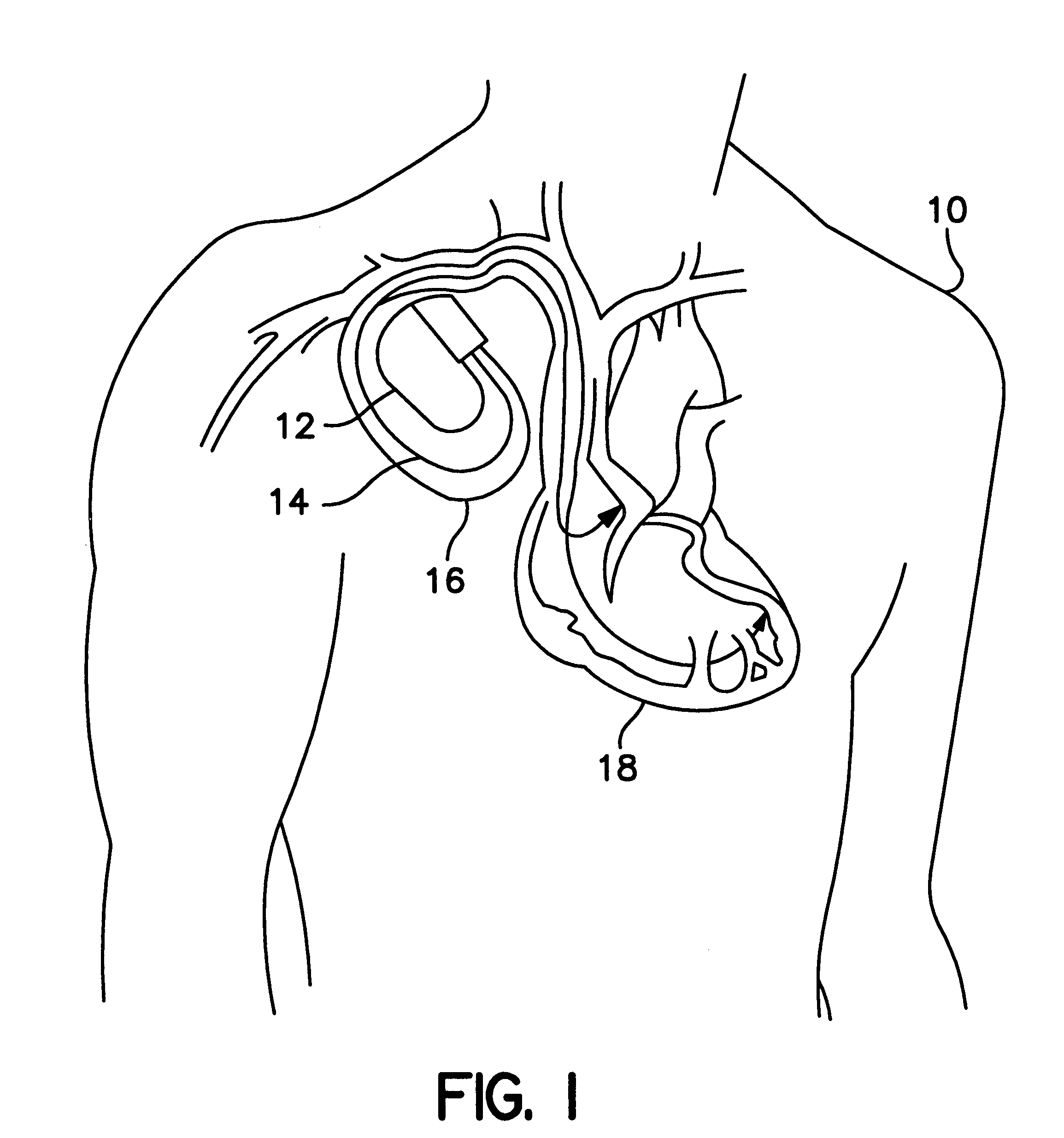
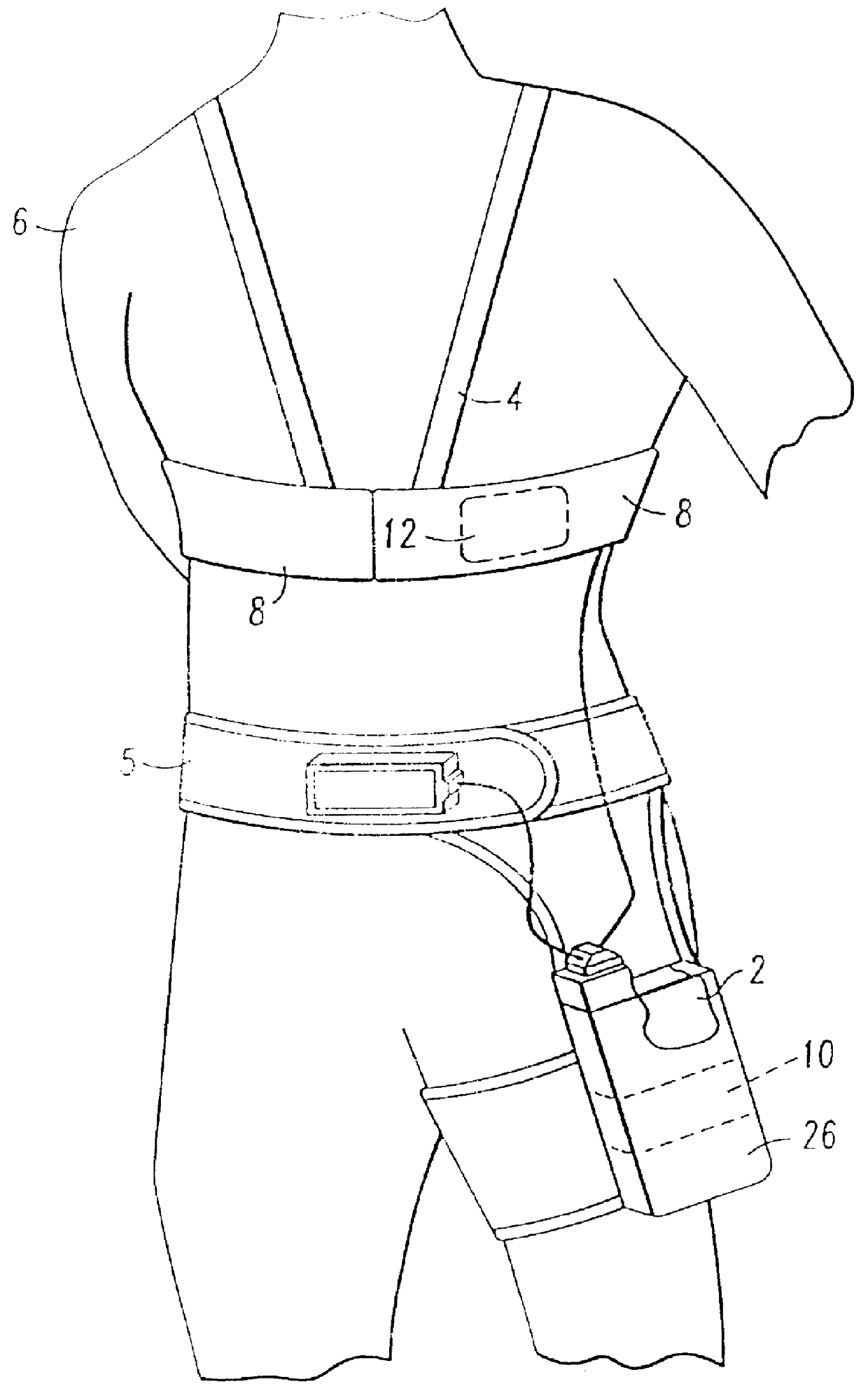
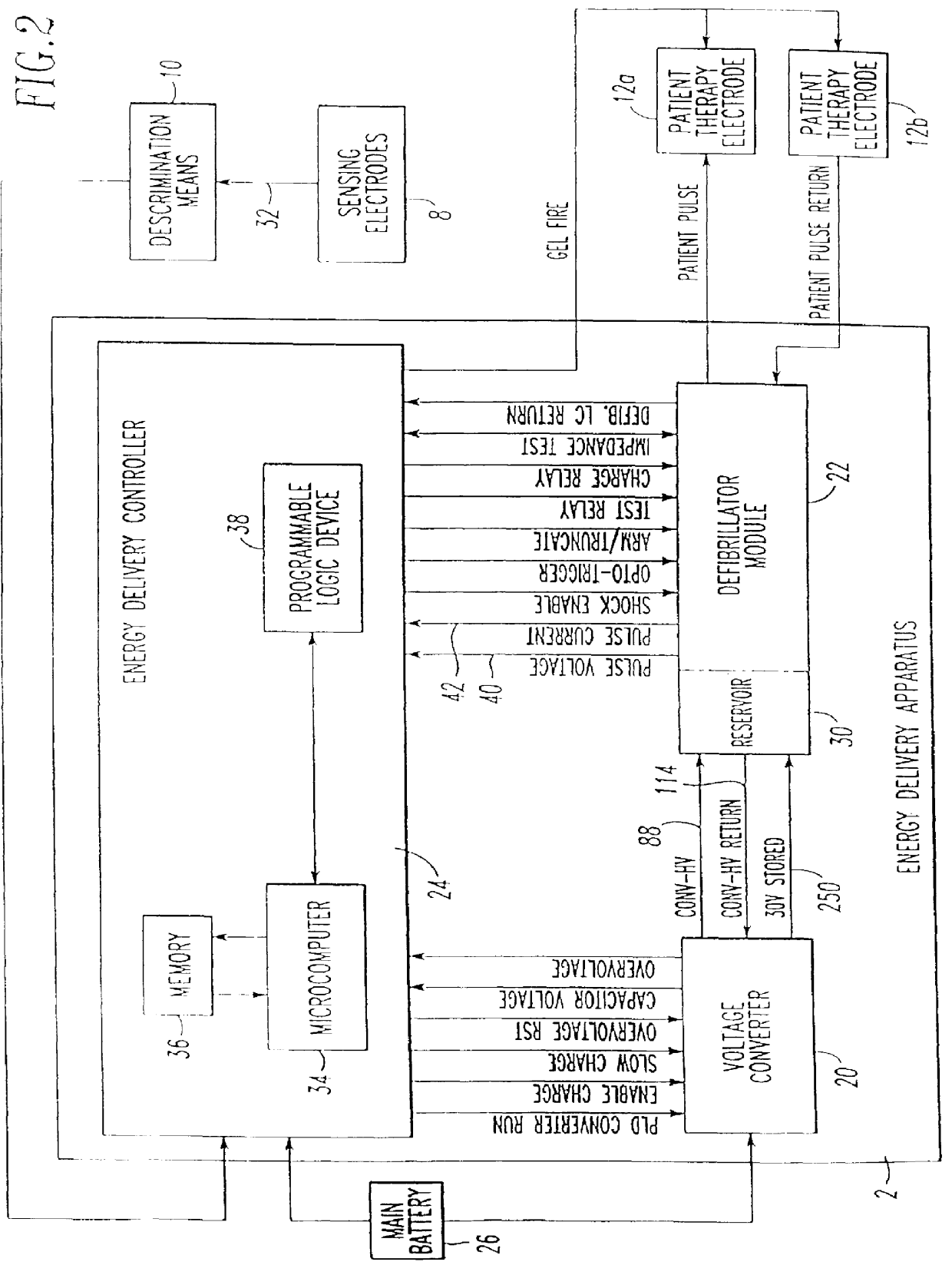
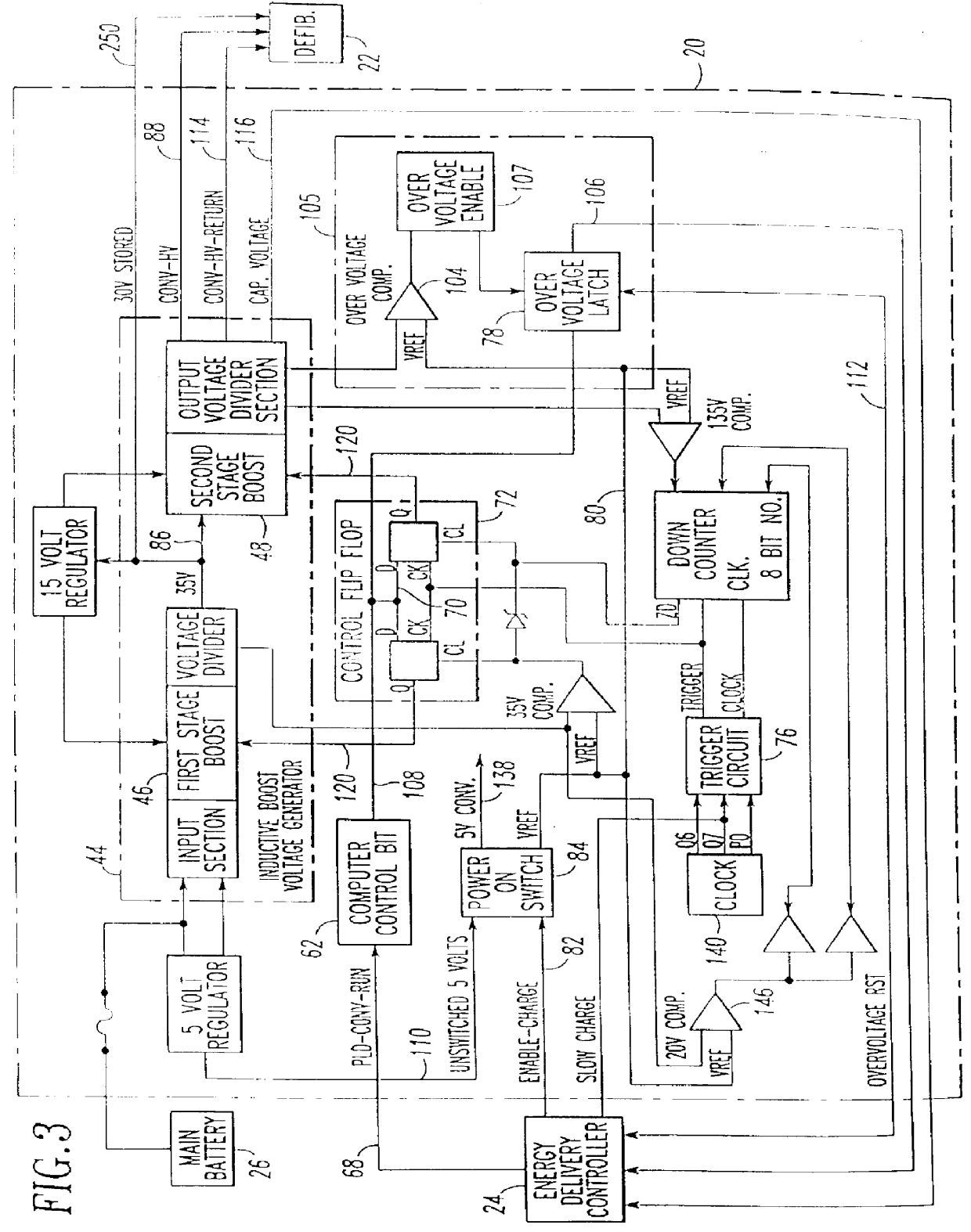
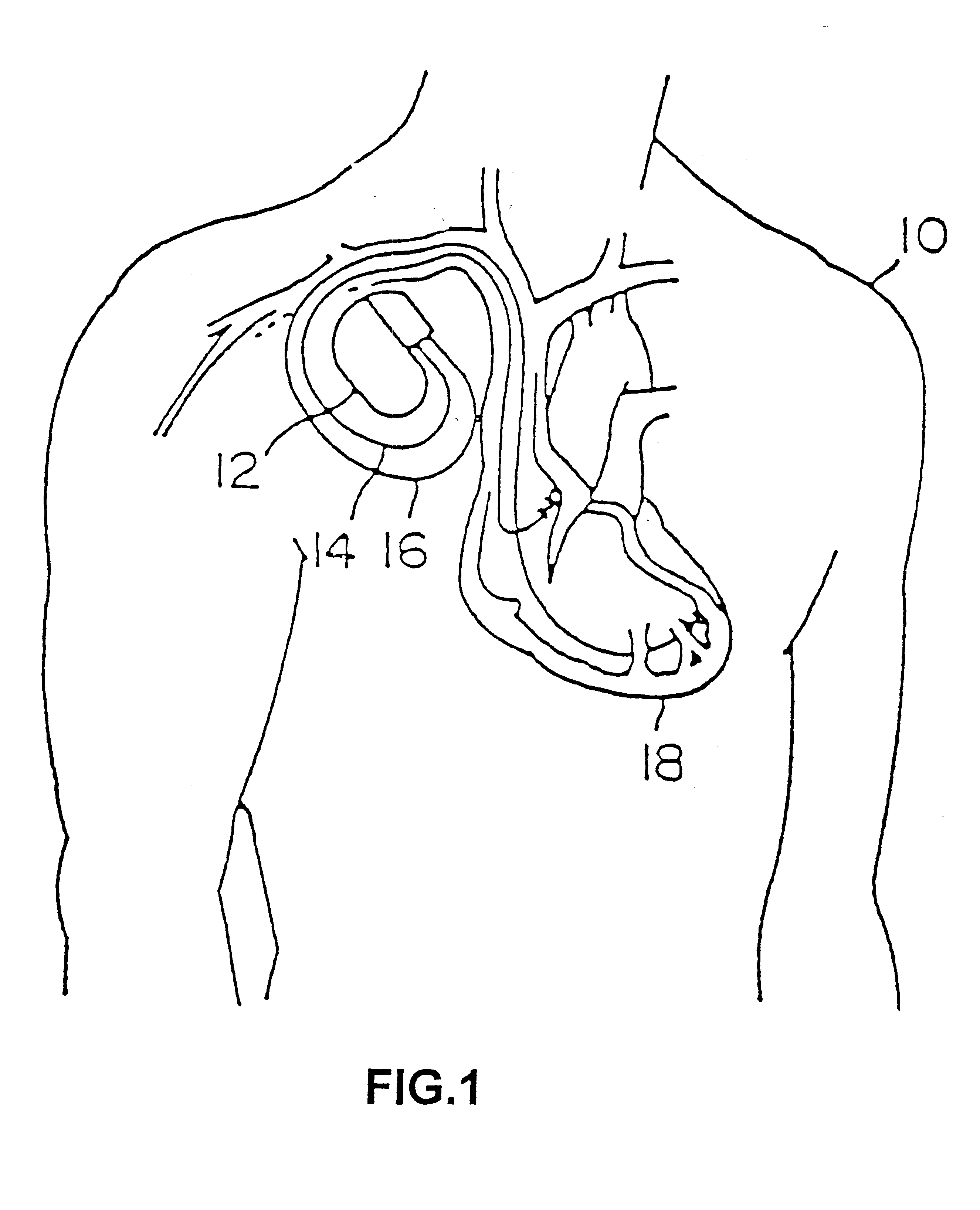
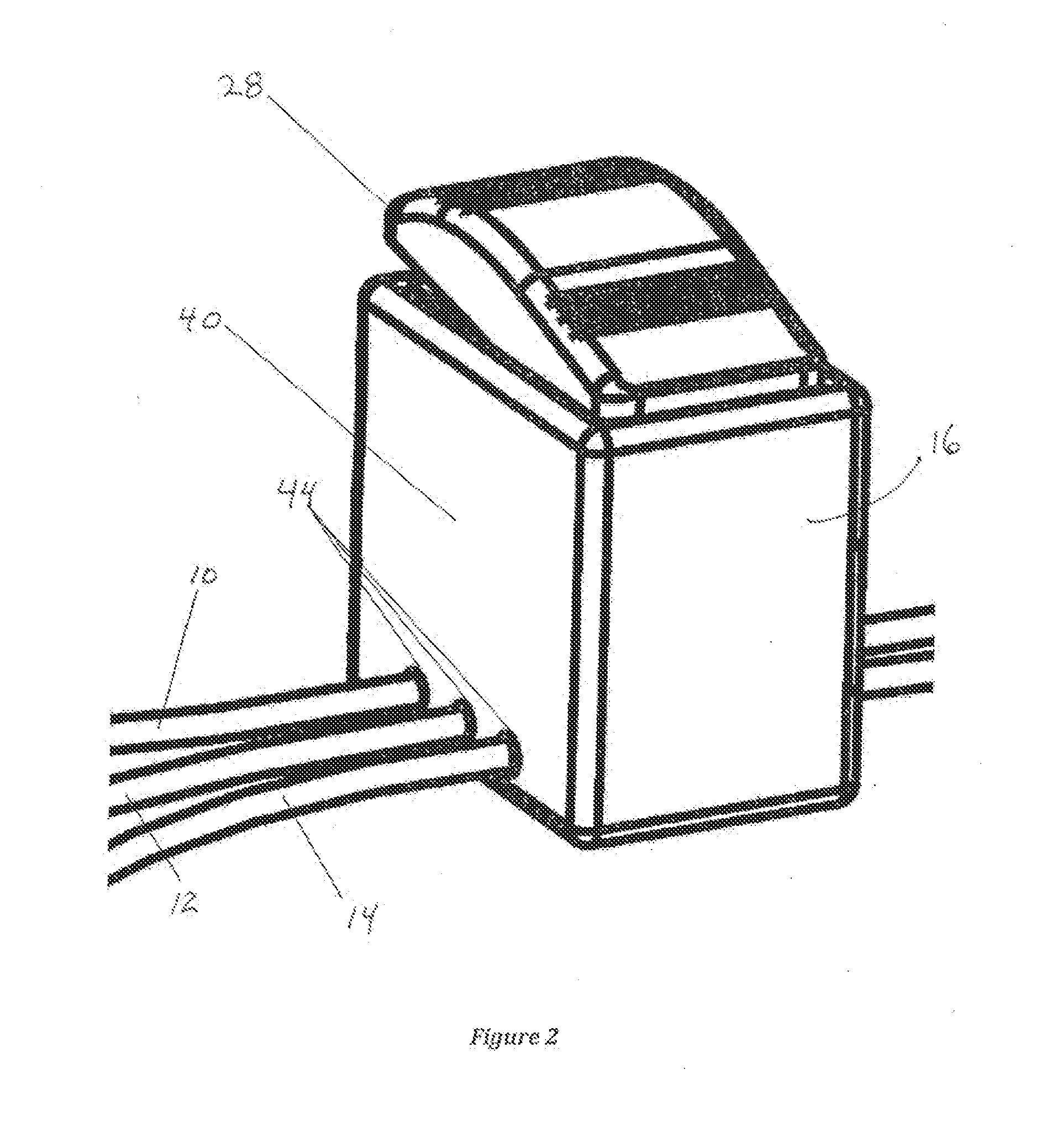
Patient-worn energy delivery apparatus
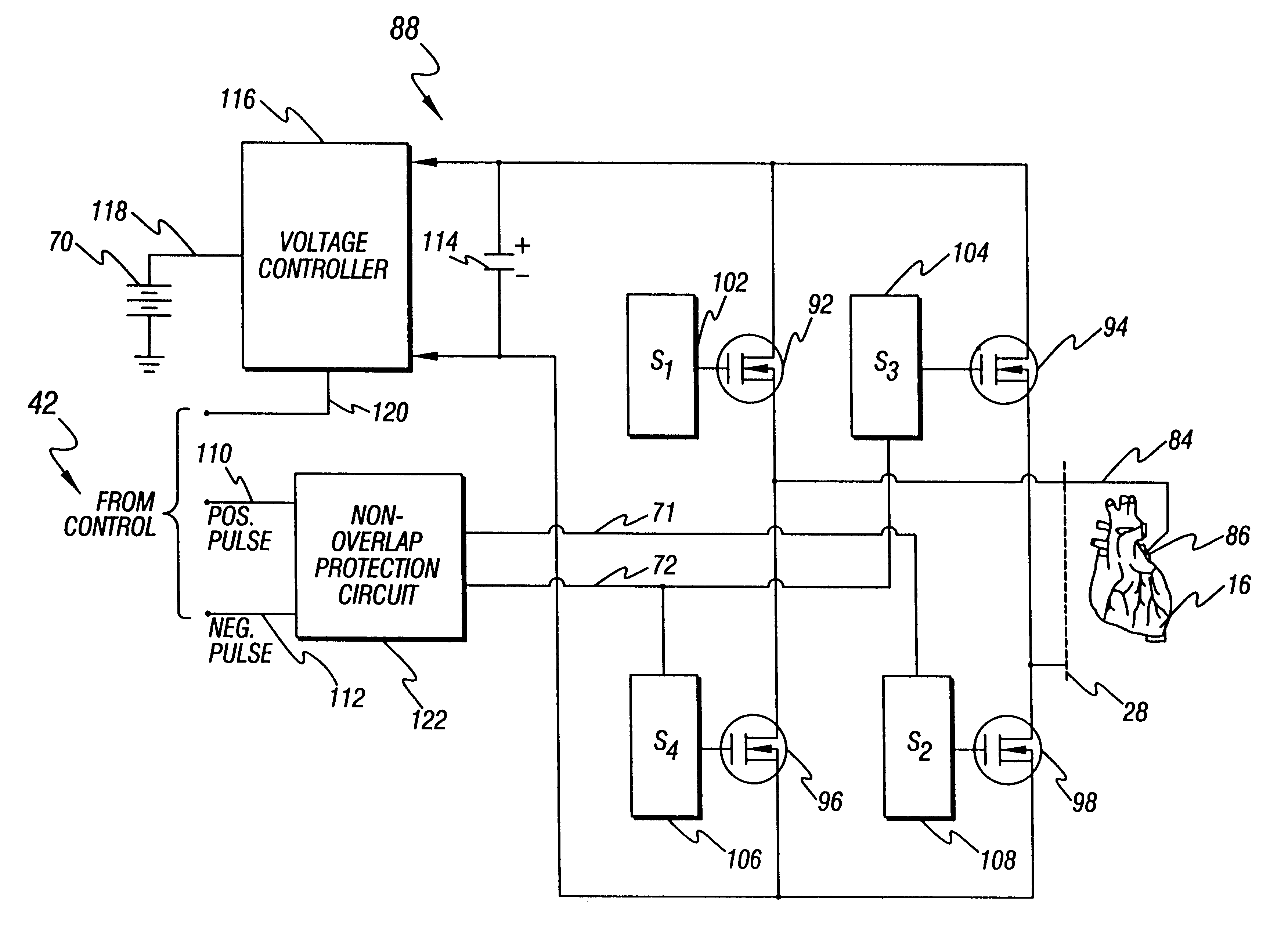
A patient-worn energy delivery apparatus for imparting electrical therapy to the body of a patient responsive to an occurrence of a treatable condition includes a voltage converter for converting electrical energy from an initial voltage to a final voltage, and a defibrillator electrically coupled between the converter and the patient and having an energy reservoir for receiving the electrical energy. The defibrillator produces preshaped electrical pulses such as defibrillation pulses and cardioversion pulses. The apparatus additionally includes an energy delivery controller electrically coupled to the patient and the converter and the defibrillator. The controller causes the converter to provide the electrical energy to the defibrillator at a specific charging rate in response to an energy level in the reservoir. The apparatus may include a plurality of electrodes interposed between the defibrillator and the patient and each electrode preferably has an impedance reducing means contained therein. One embodiment of the apparatus may include a H-bridge to produce a positive-going pulse segment and the negative-going pulse segment within the biphasic exponential signals. The apparatus periodically measures the energy as it is being delivered to the patient and can pre-emptively stop or truncate the pulse in the event an error condition is detected, such as an overvoltage condition or if the energy level approaches a predetermined level. The electrical components which store and release the energy minimize the size and expense of the apparatus, while isolating the microcomputer from the high energy levels as the therapeutic pulse is delivered.
Owner:ZOLL MEDICAL CORPORATION
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS20060241701A1Relieve painSafe and effective operationHeart defibrillatorsSubcutaneous implantationCardiac pacemaker electrode
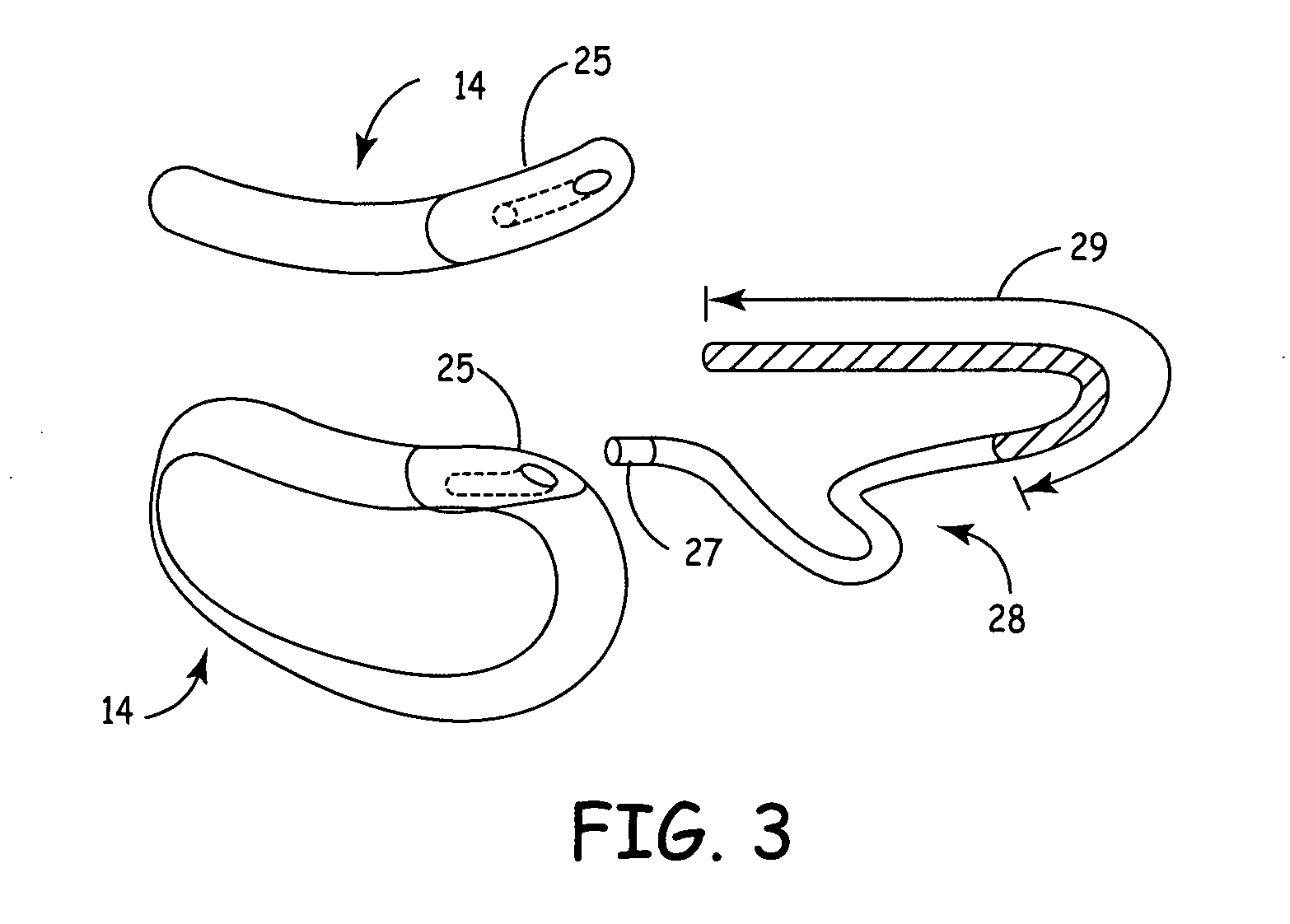
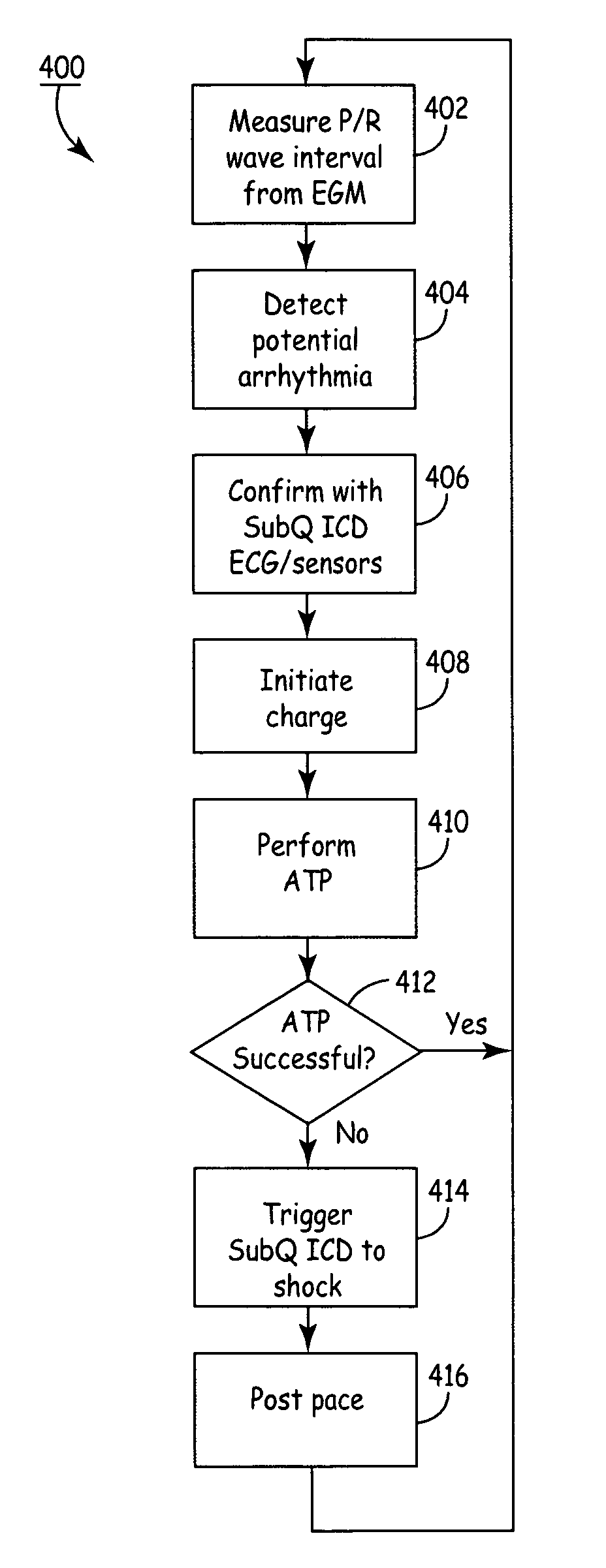
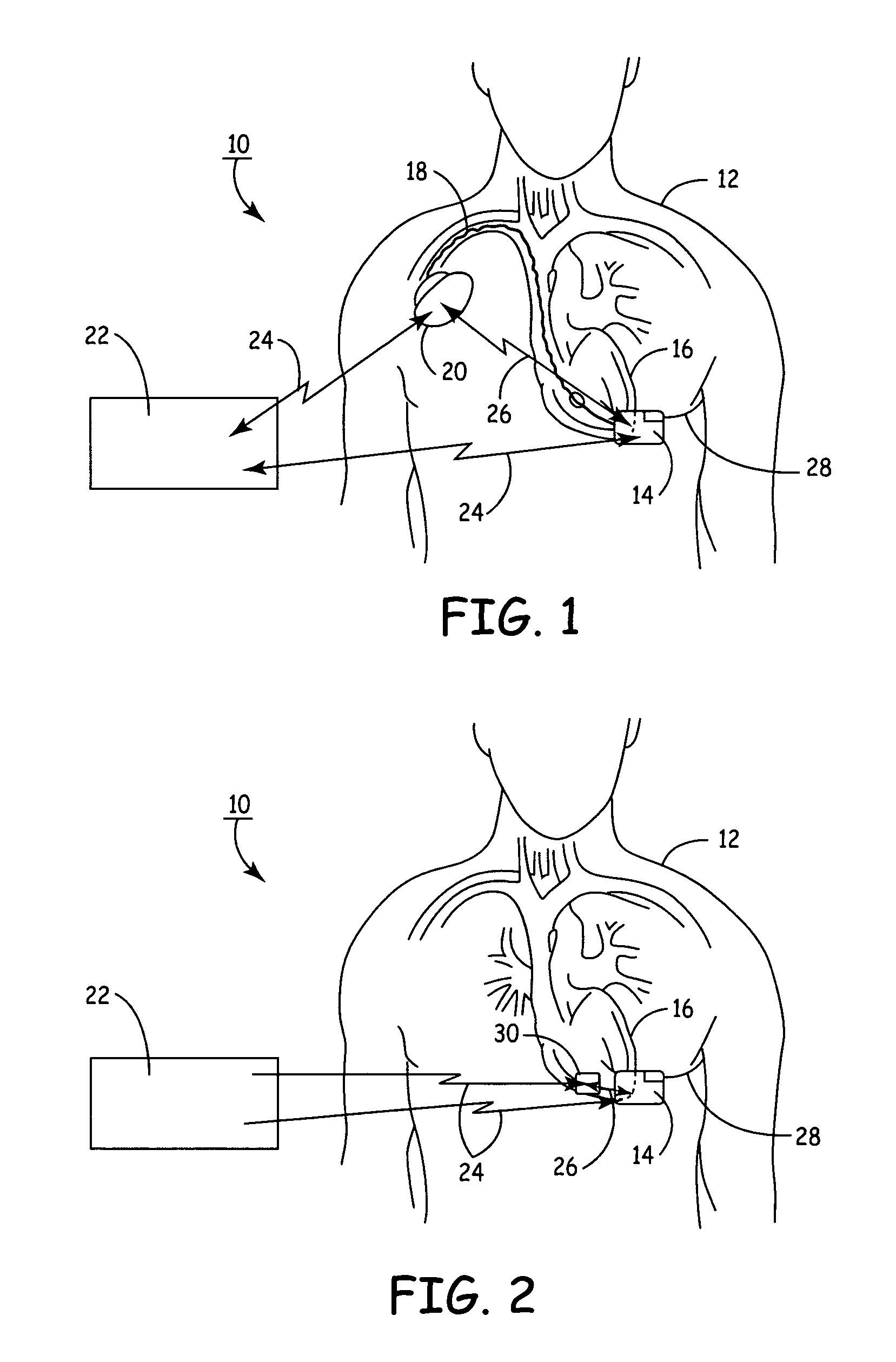
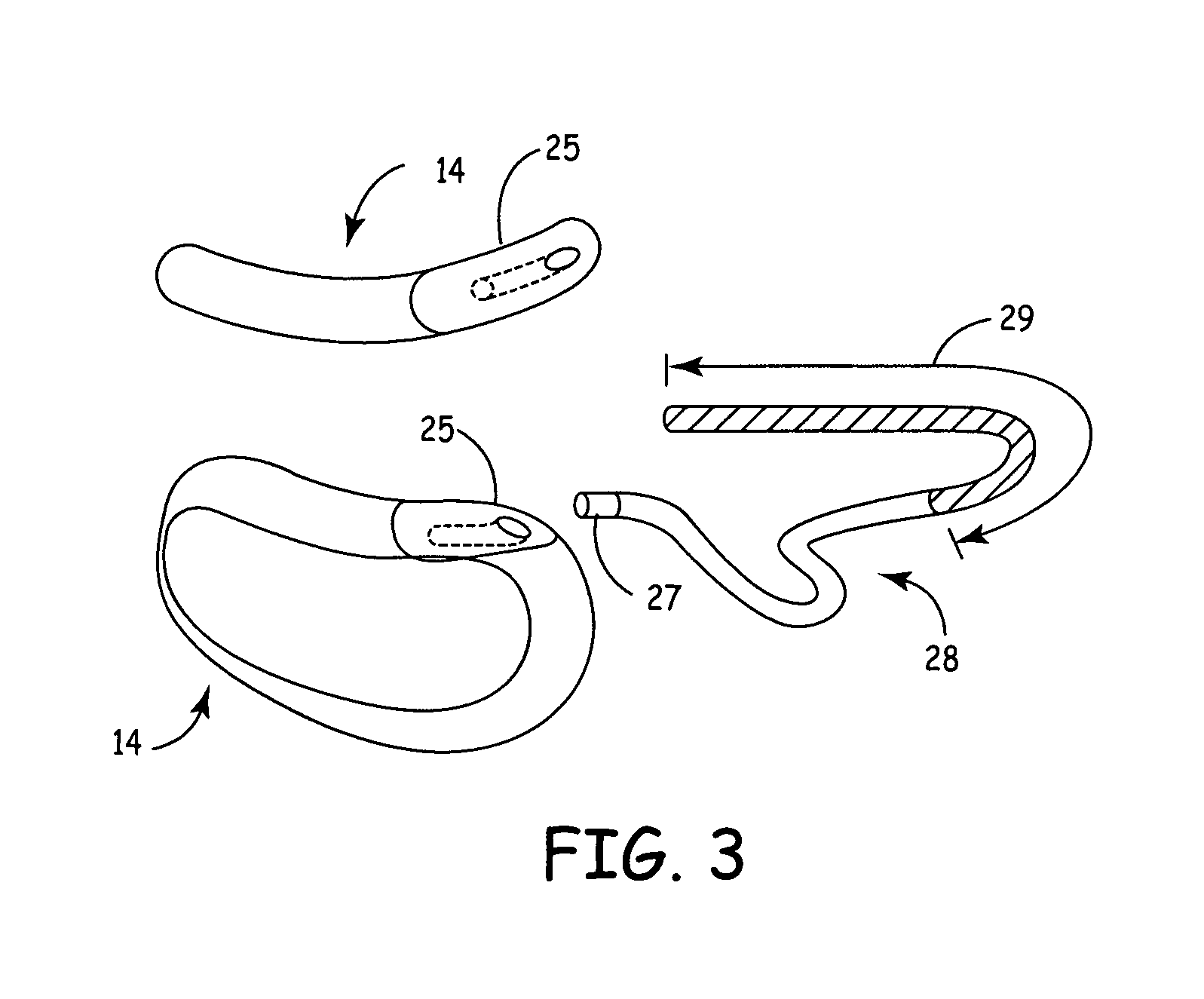
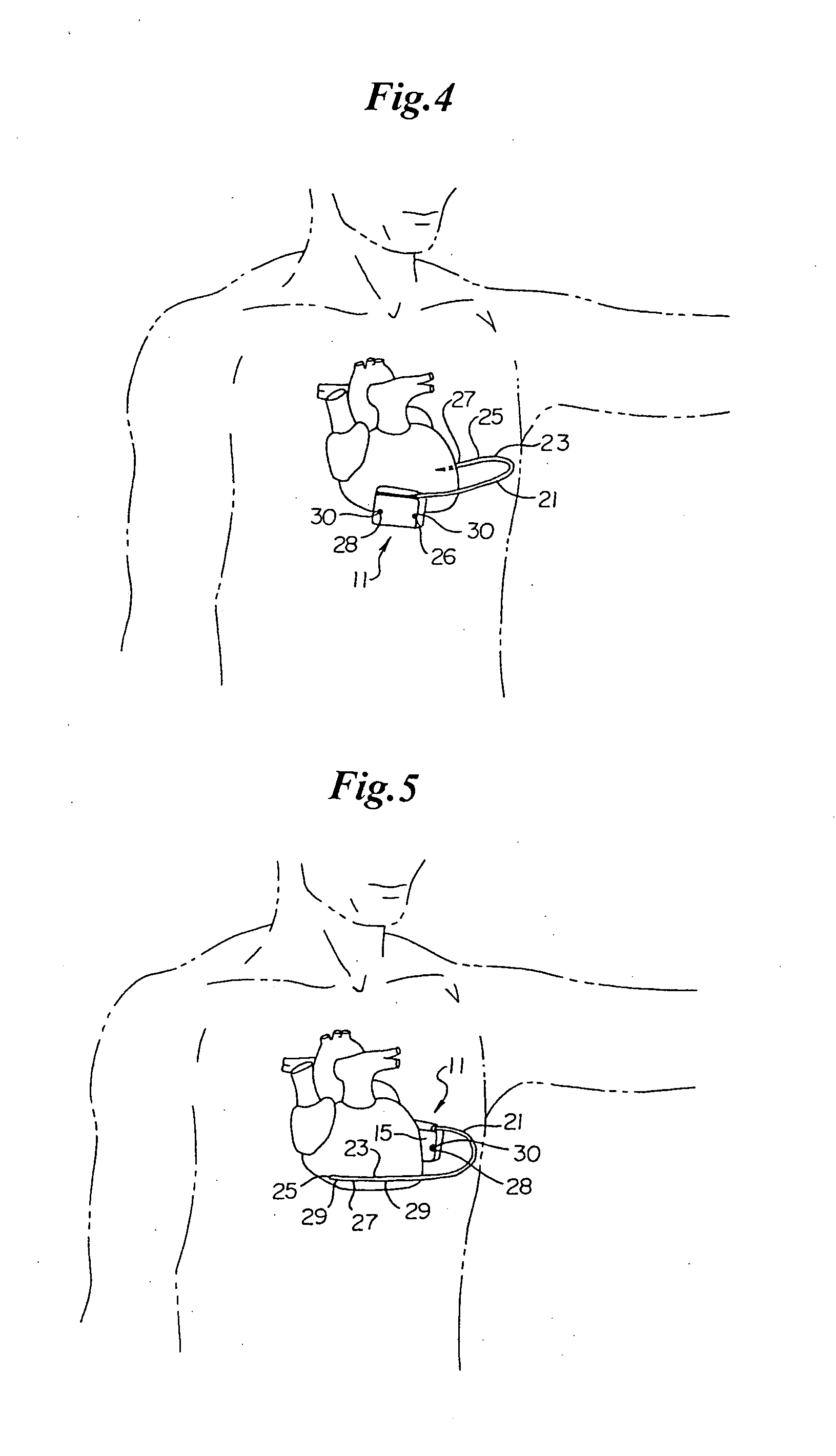
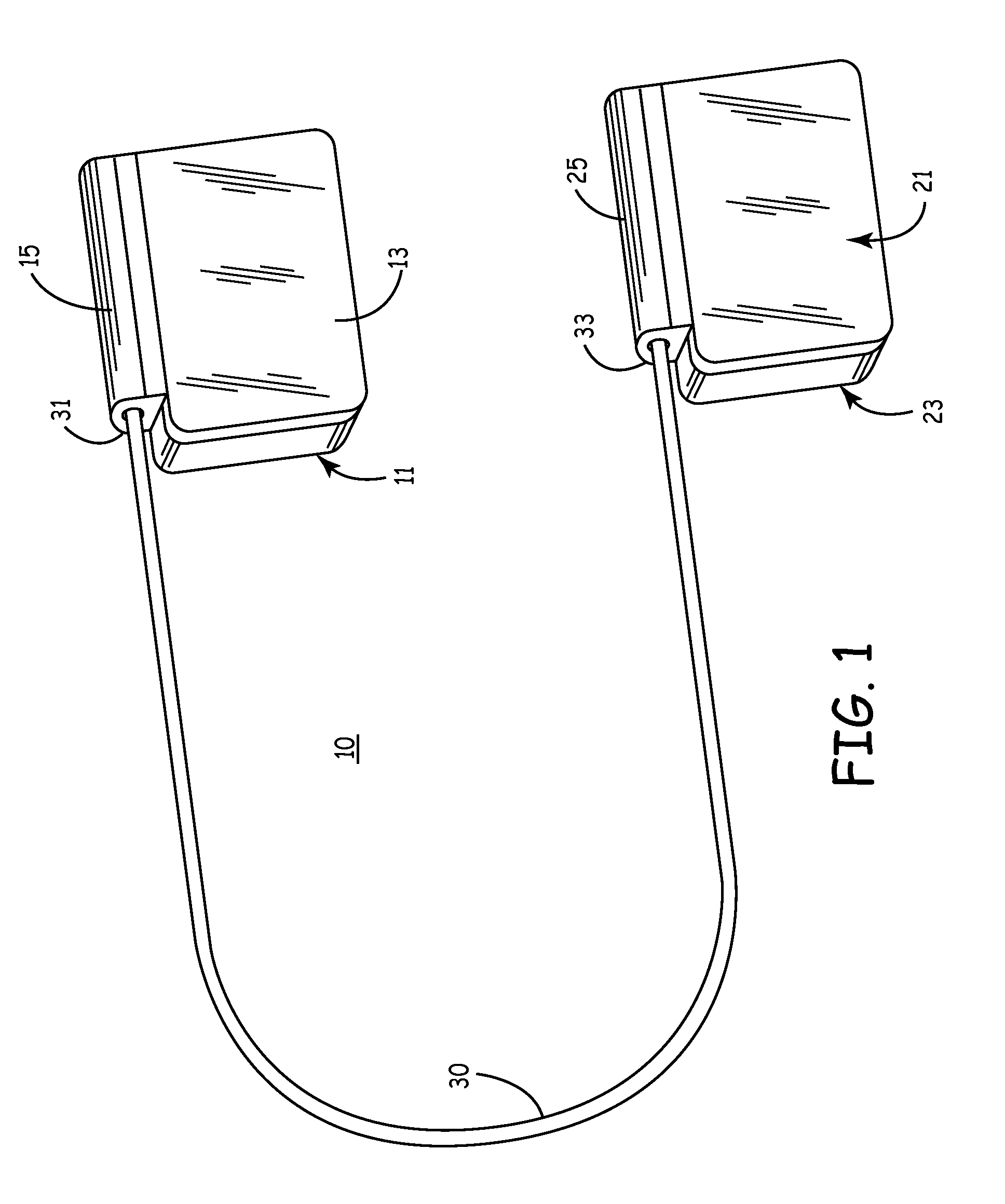
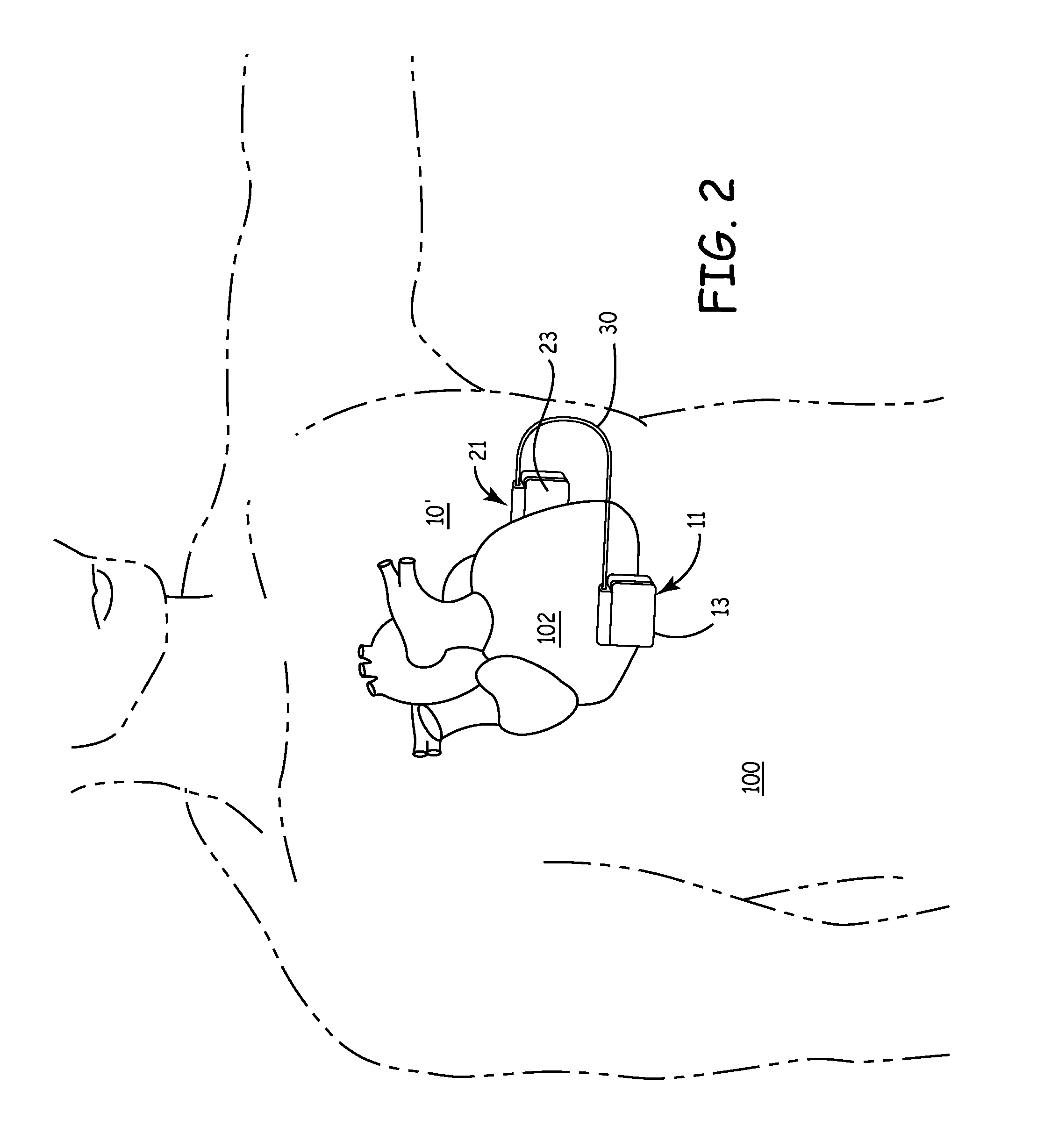
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDS) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS7991467B2Relieve painSafe and effective operationCatheterHeart stimulatorsCardiac pacemaker electrodeImplantable cardioverter-defibrillator
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDs) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
Subcutaneous ICD with motion artifact noise suppression
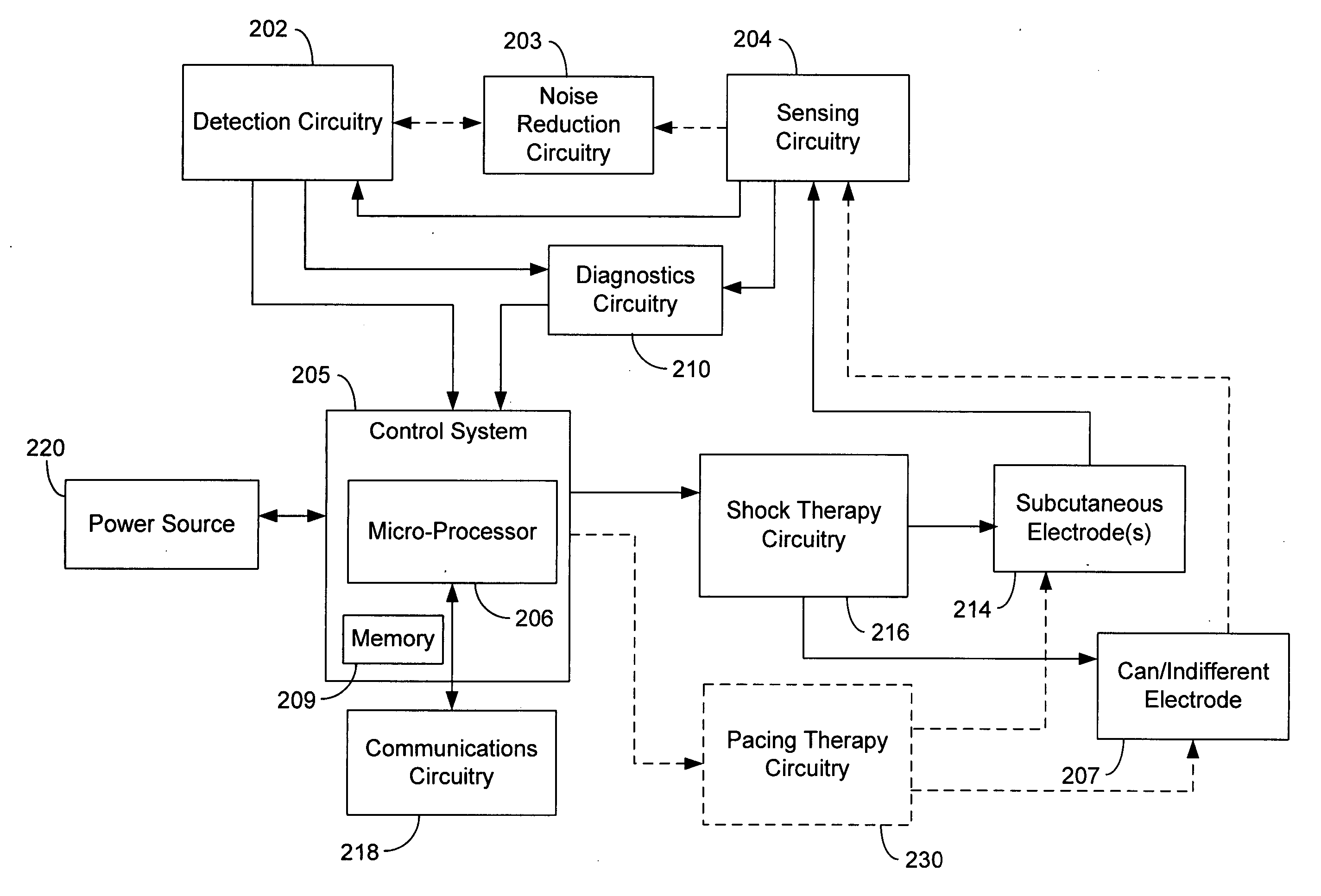
A subcutaneous implantable cardioverter defibrillator (SubQ ICD) includes a housing carrying electrodes for sensing ECG signals and delivering therapy. A sensor detects local motion in the area of the housing and produces a noise signal related to motion artifact noise contained in ECG signals derived from the electrode array. An adaptive noise cancellation circuit enhances ECG signals based on the local motion noise signal. A therapy delivery circuit delivers cardioversion and defibrillation pulses based upon the enhanced ECG signals.
Owner:MEDTRONIC INC
Medical lead conductor fracture visualization method and apparatus
InactiveUS6295476B1Overcome difficultiesEasy to understandTransvascular endocardial electrodesDiagnostic recording/measuringBody organsElectricity
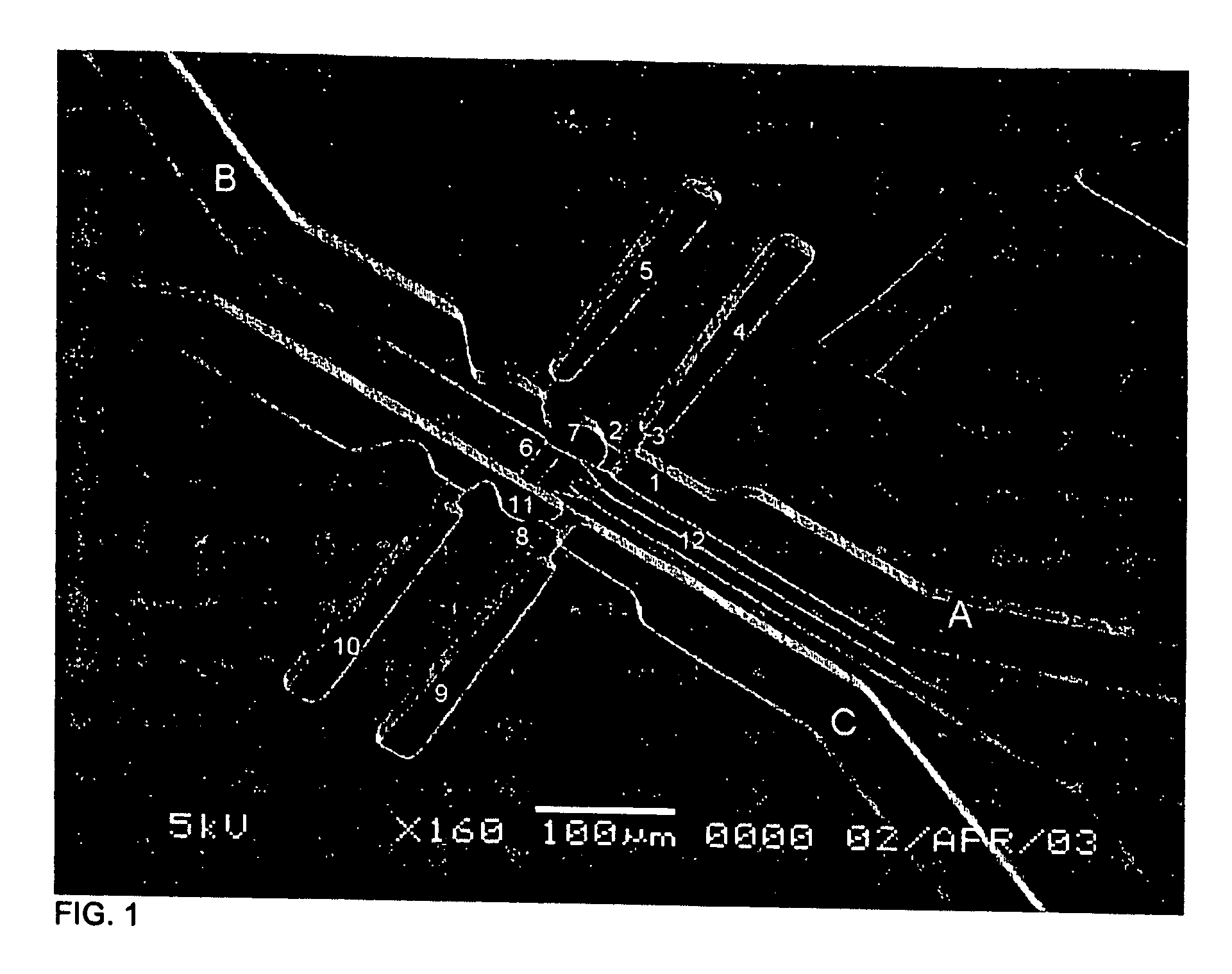
Methods for sensing or electrical stimulation of body organs or tissues are disclosed wherein a lead conductor wire or filament of a stranded lead conductor generates a radioactive emission when it is fractured sufficiently or is completely broken. The conductor wire or filament is formed of an inner core and an outer sheath surrounding the inner core, wherein the inner core is irradiated or is formed of a radioactive isotope in an alloy that provides an enhanced radiopaque aura when the sheath is fractured and the inner core is exposed. When the conductor wire or filament is intact, the radioactive inner core is fully encased within the outer sheath, and the outer sheath blocks or reduces radioactive emission along its length to a constant, relatively low level. In use, the emission is detected externally to the body, and the detection signifies that a fracture or break has occurred. Such leads preferably comprise cardiac leads for delivering electrical stimulation to the heart, e.g., pacing pulses and cardioversion / defibrillation shocks, and / or sensing the cardiac electrogram, having multiple lead conductors encased in a lead body subject to fracture under stress. The lead conductors can comprise mono-filar or multi-filar, parallel wound, coiled wires that arranged in a co-axial manner or in a side-by-side arrangement within the lead body. Or the straight or coiled lead conductors can be formed of a strand comprising a plurality of outer filaments wound helically about a central core filament or of a cable comprising a plurality of such peripheral strands wound helically about a central core strand. At least the outer filaments of a stranded conductor and peripheral strands of a conductor cable are formed with the radioactive core.
Owner:MEDTRONIC INC
Patient-worn energy delivery apparatus
A patient-worn energy delivery apparatus for imparting electrical therapy to the body of a patient responsive to an occurrence of a treatable condition. The apparatus includes a voltage converter for converting electrical energy from an initial voltage to a final voltage at a plurality of charging rates, and a defibrillator electrically coupled between the converter and the patient and having an energy reservoir for receiving the electrical energy. The defibrillator produces preshaped electrical pulses such as defibrillation pulses and cardioversion pulses. The apparatus additionally includes an energy delivery controller electrically coupled to the patient and the converter and the defibrillator. The controller causes the converter to provide the electrical energy to the defibrillator at a specific charging rate in response to an energy level in the reservoir. The apparatus may include a plurality of electrodes interposed between the defibrillator and the patient and each electrode preferably has an impedance reducing means contained therein. One embodiment of the apparatus may include a H-bridge to produce a positive-going pulse segment and the negative-going pulse segment within the biphasic exponential signals.
Owner:ZOLL MEDICAL CORPORATION
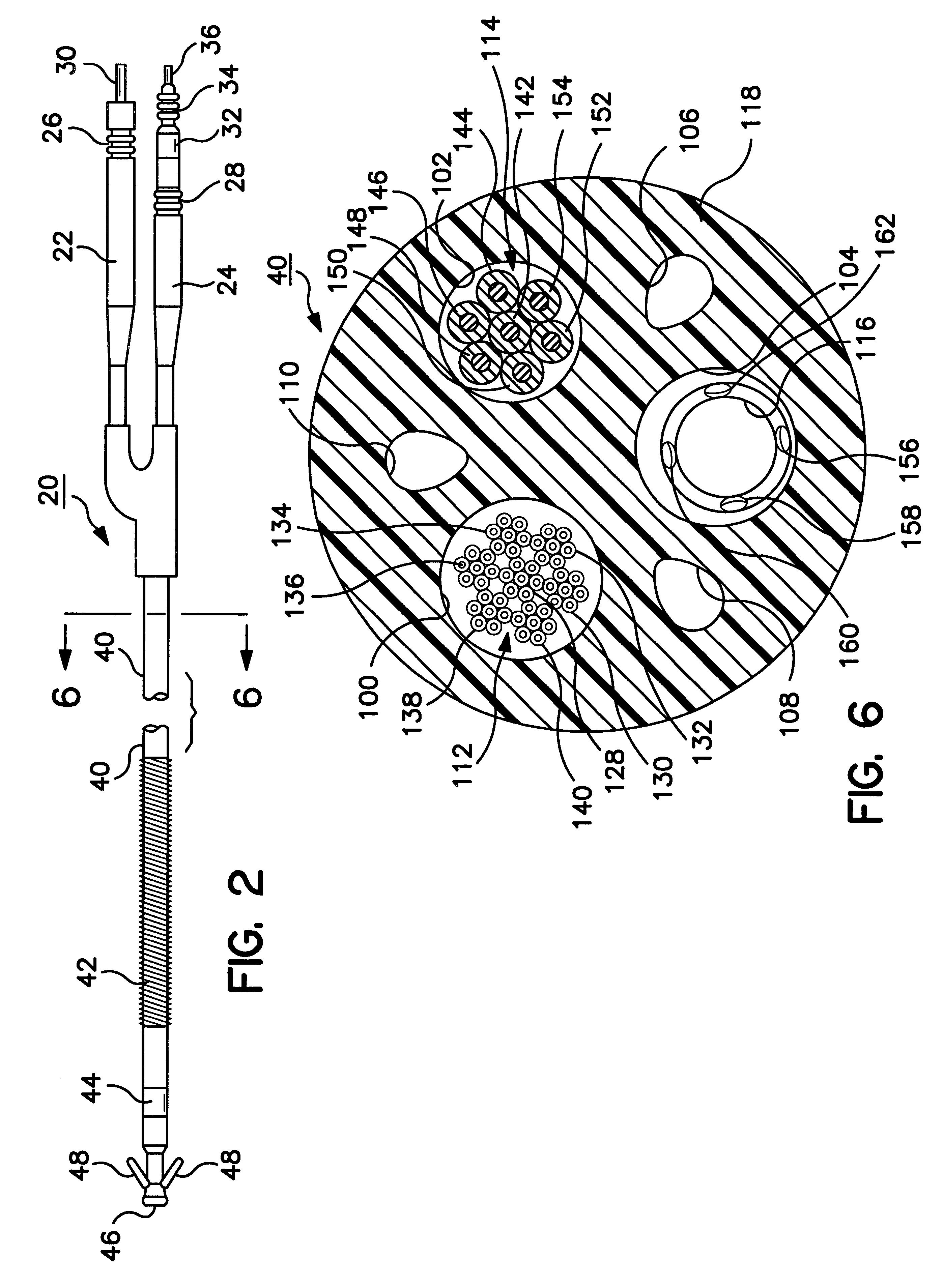
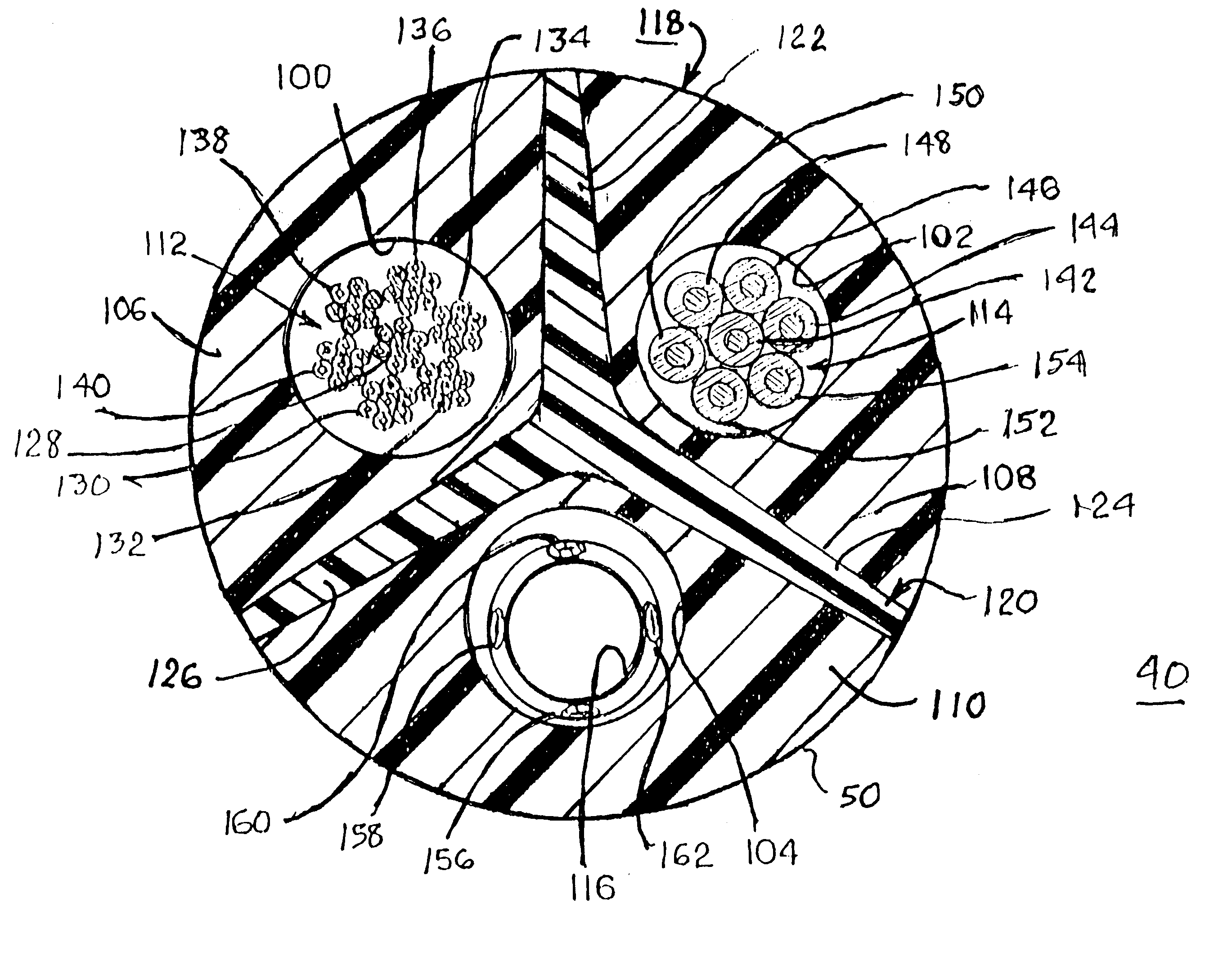
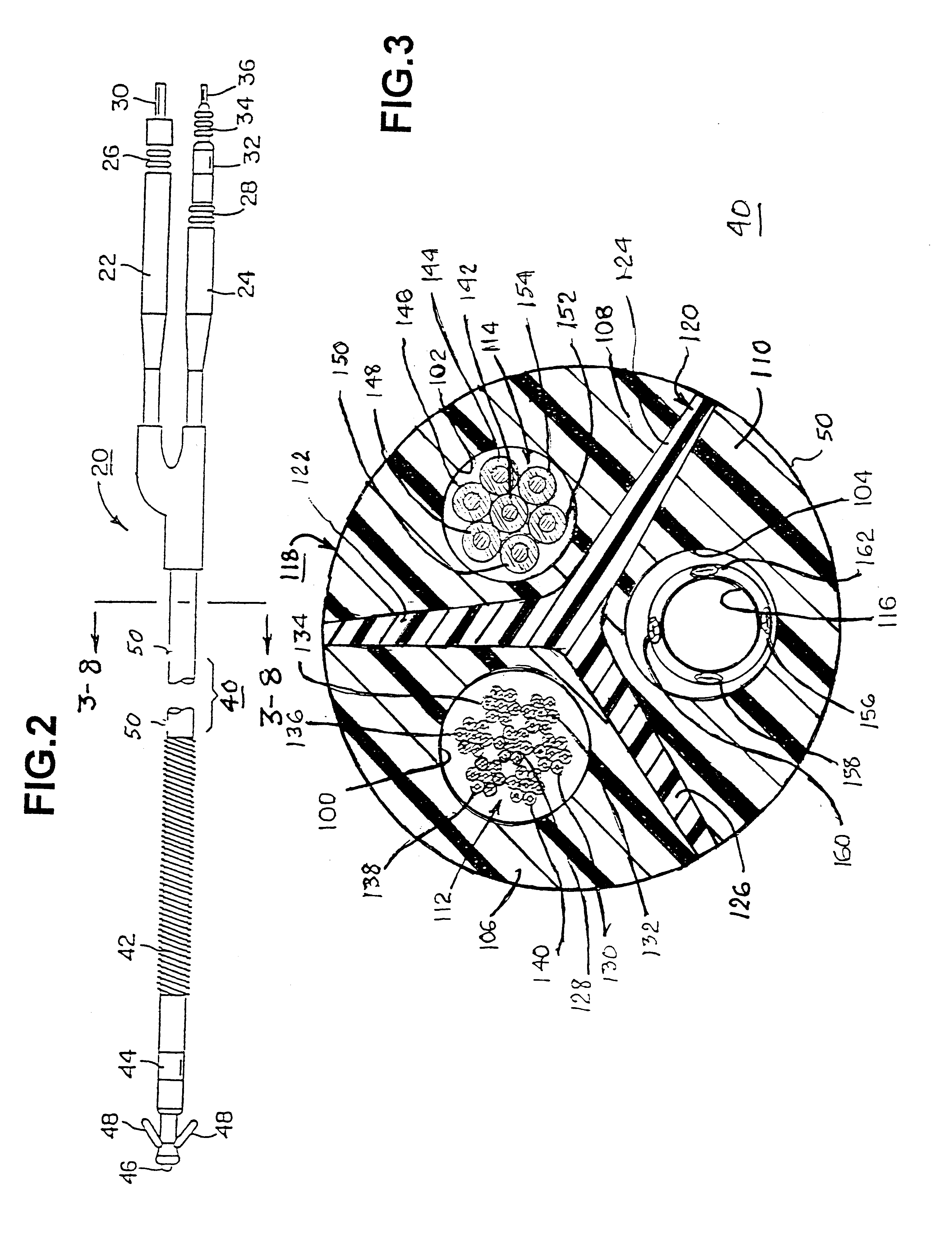
Co-extruded, multi-lumen medical lead
InactiveUS6434430B2Transvascular endocardial electrodesDiagnostic recording/measuringElectrical stimulationsBending stiffness
Owner:MEDTRONIC INC
Co-extruded, multi-lumen medical lead
InactiveUS6400992B1Transvascular endocardial electrodesExternal electrodesElectrical stimulationsBending stiffness
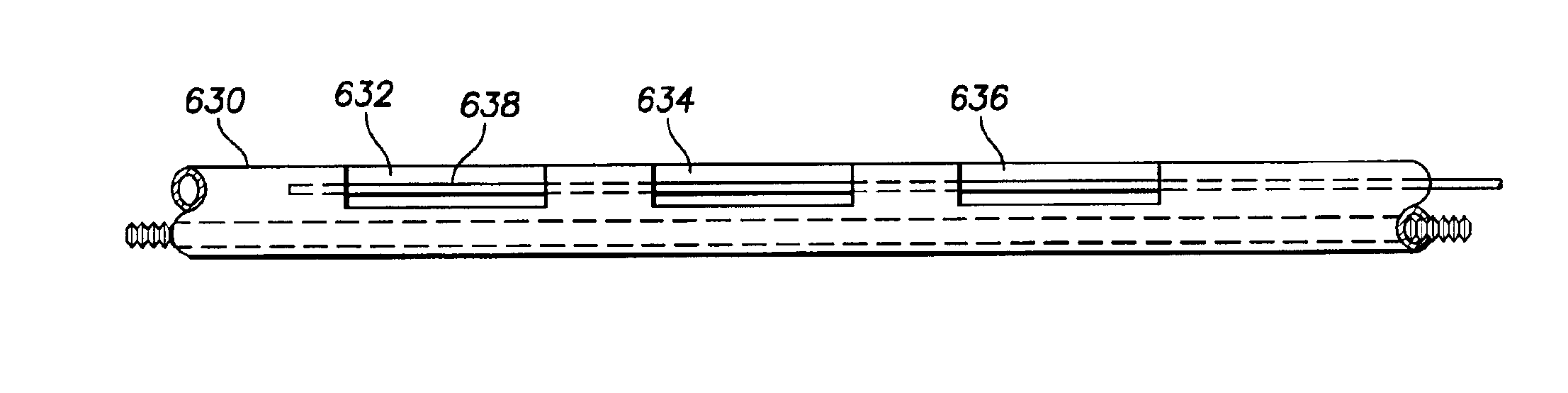
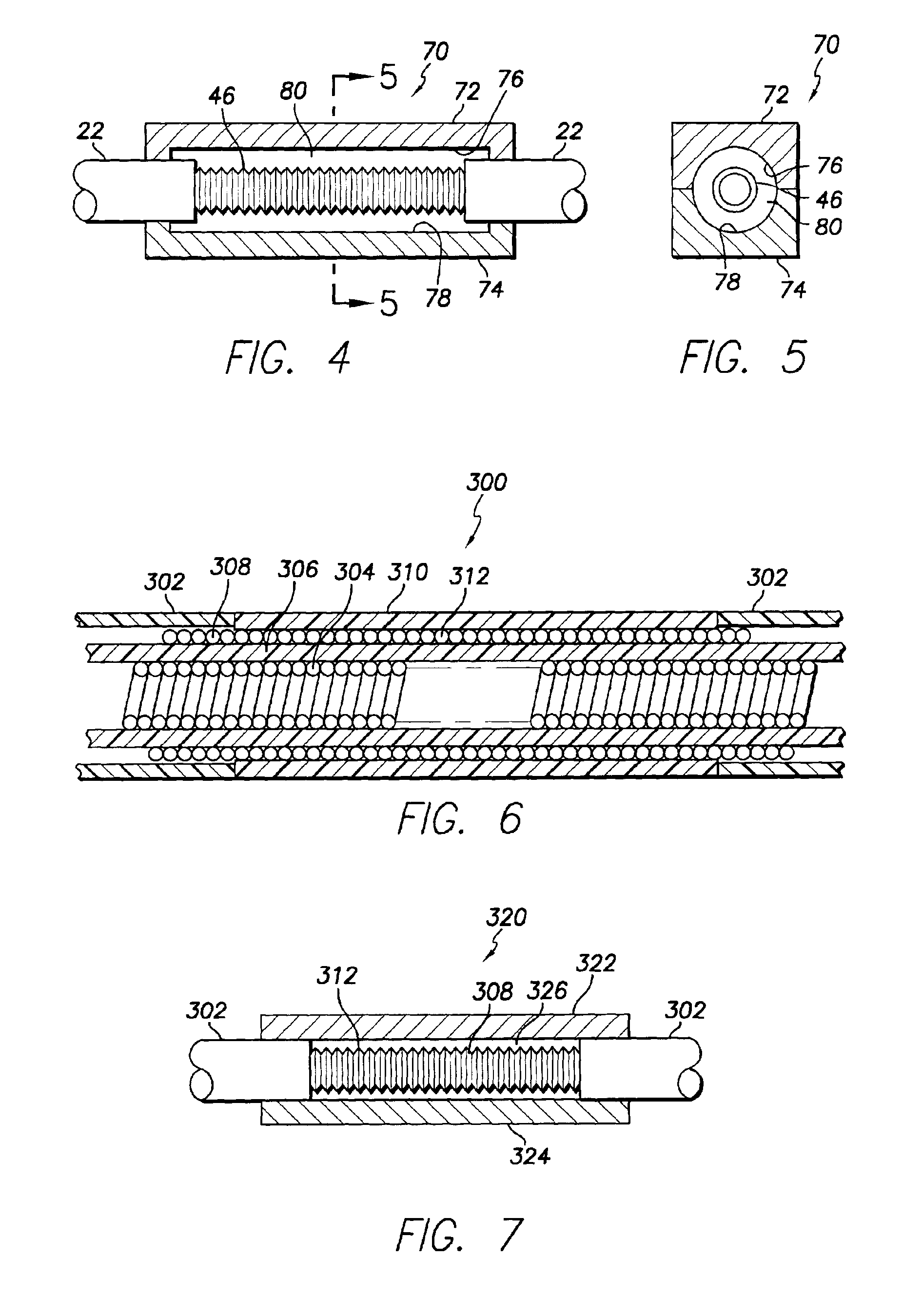
Medical electrical leads for sensing or electrical stimulation of body organs or tissues, particularly implantable cardiac leads for delivering pacing pulses and cardioversion / defibrillation shocks, and / or sensing the cardiac electrogram (EGM) or other physiologic data and their methods of fabrication are disclosed. A lead body sheath is co-extruded in a co-extrusion process using bio-compatible, electrically insulating, materials of differing durometers in differing axial sections thereof, resulting in a unitary lead body sheath having differing stiffness sections including axial segments or webs or lumen encircling rings or other structures in its cross-section. The lead body sheath is co-extruded to have an outer surface adapted to be exposed to the environment or to be enclosed within an outer sheath and to have a plurality of lead conductor lumens for receiving and enclosing a like plurality of lead conductors of the same or differing types. The lead body sheath can be co-extruded of a plurality of sheath segments containing a lead conductor lumen and formed of a first durometer material or of differing durometer materials. A web of a further durometer material can be co-extruded extending between the adjoining boundaries of the axial sheath segments and bonding the adjacent segments together. The lead body sheath can be tailored to exhibit differing bending stiffnesses away from the lead body sheath axis in selected polar directions around 360° circumference of the sheath body.
Owner:MEDTRONIC INC
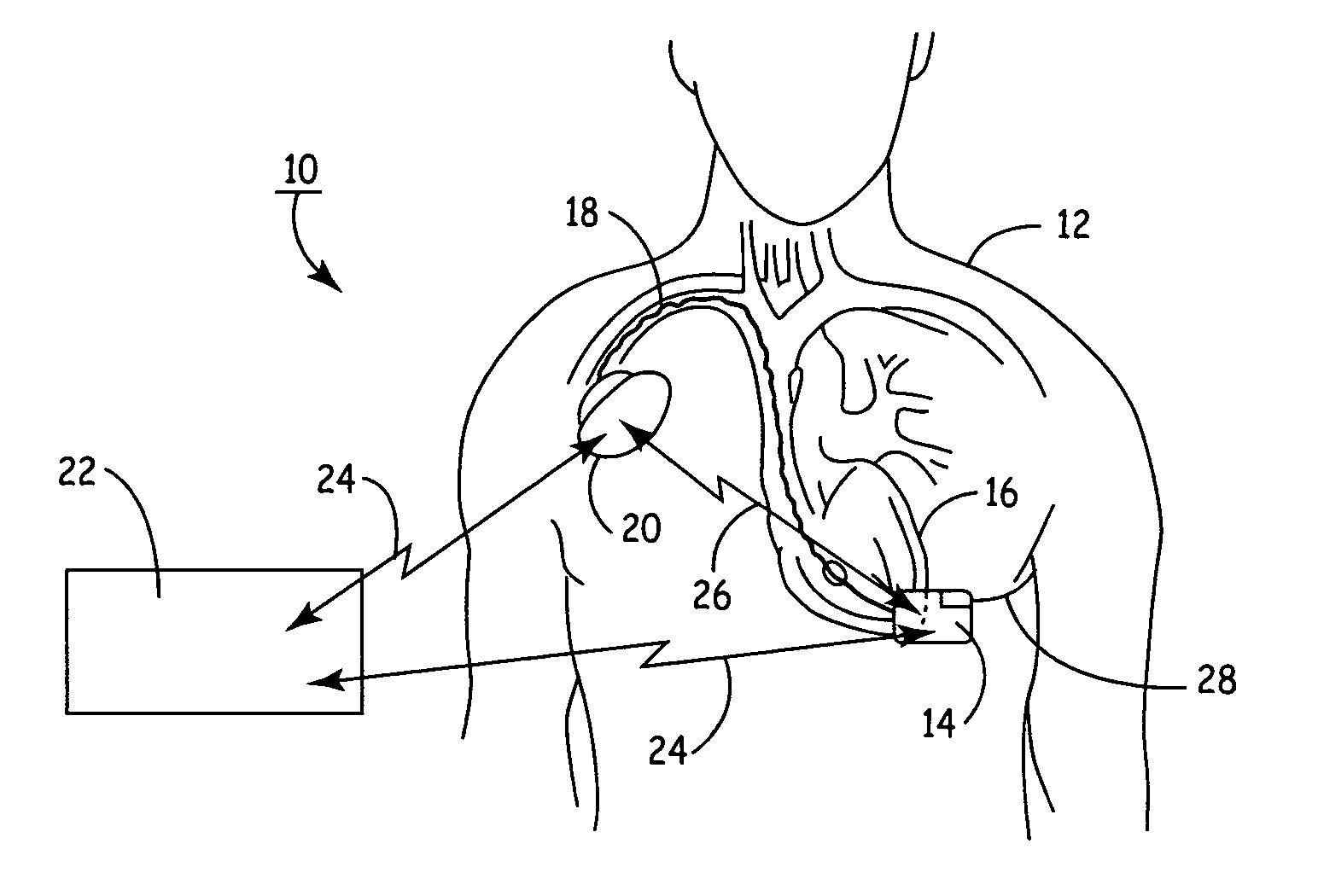
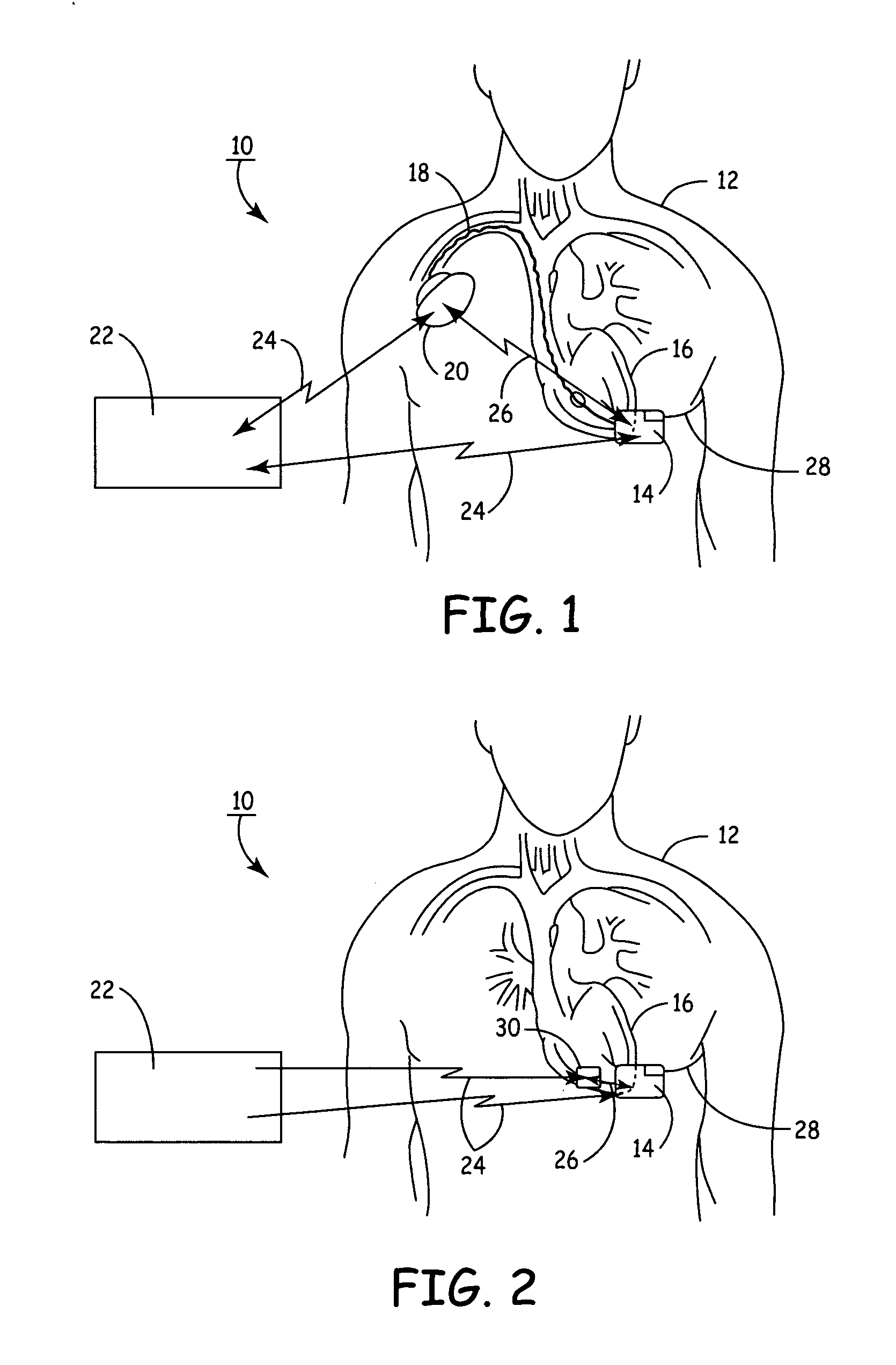
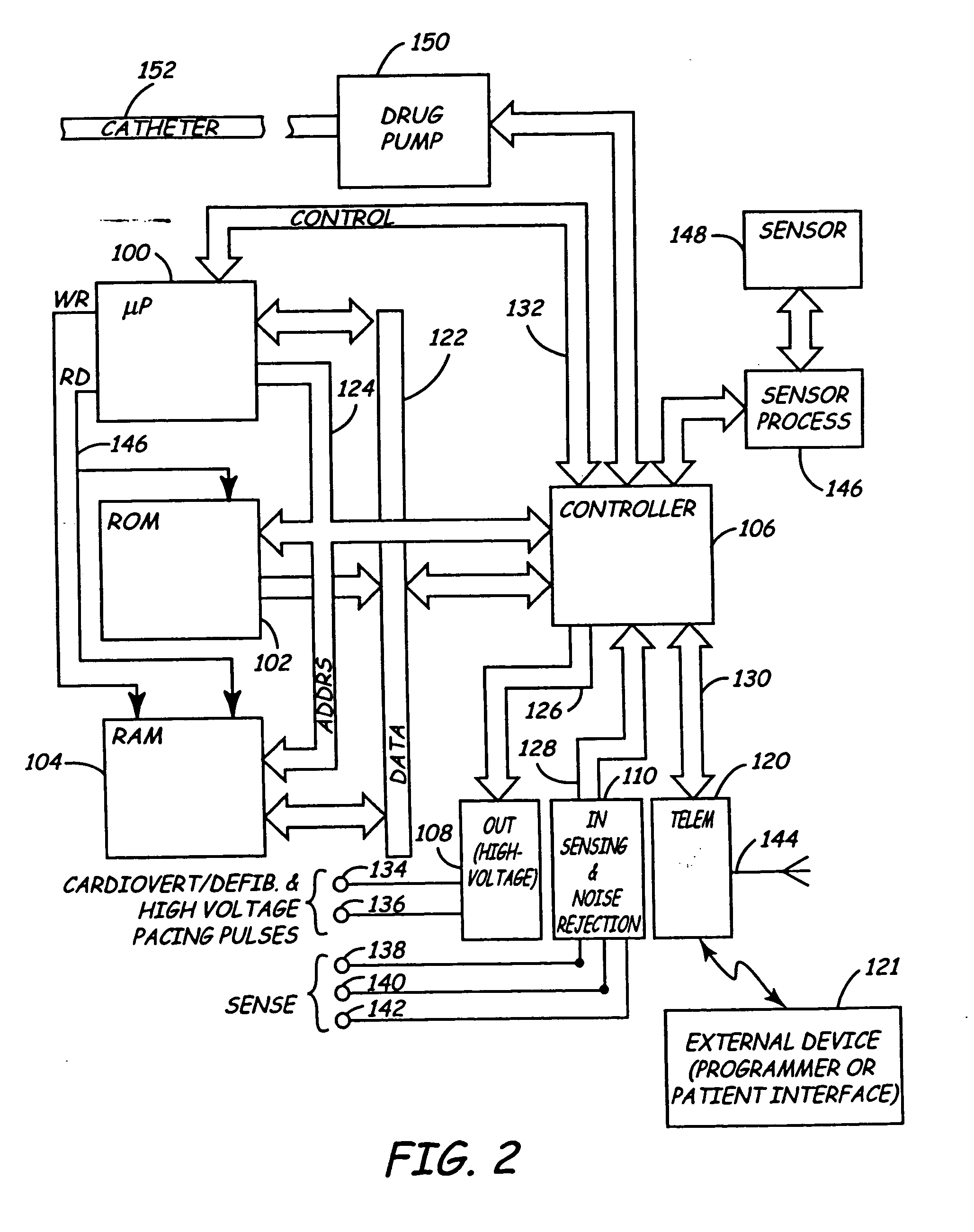
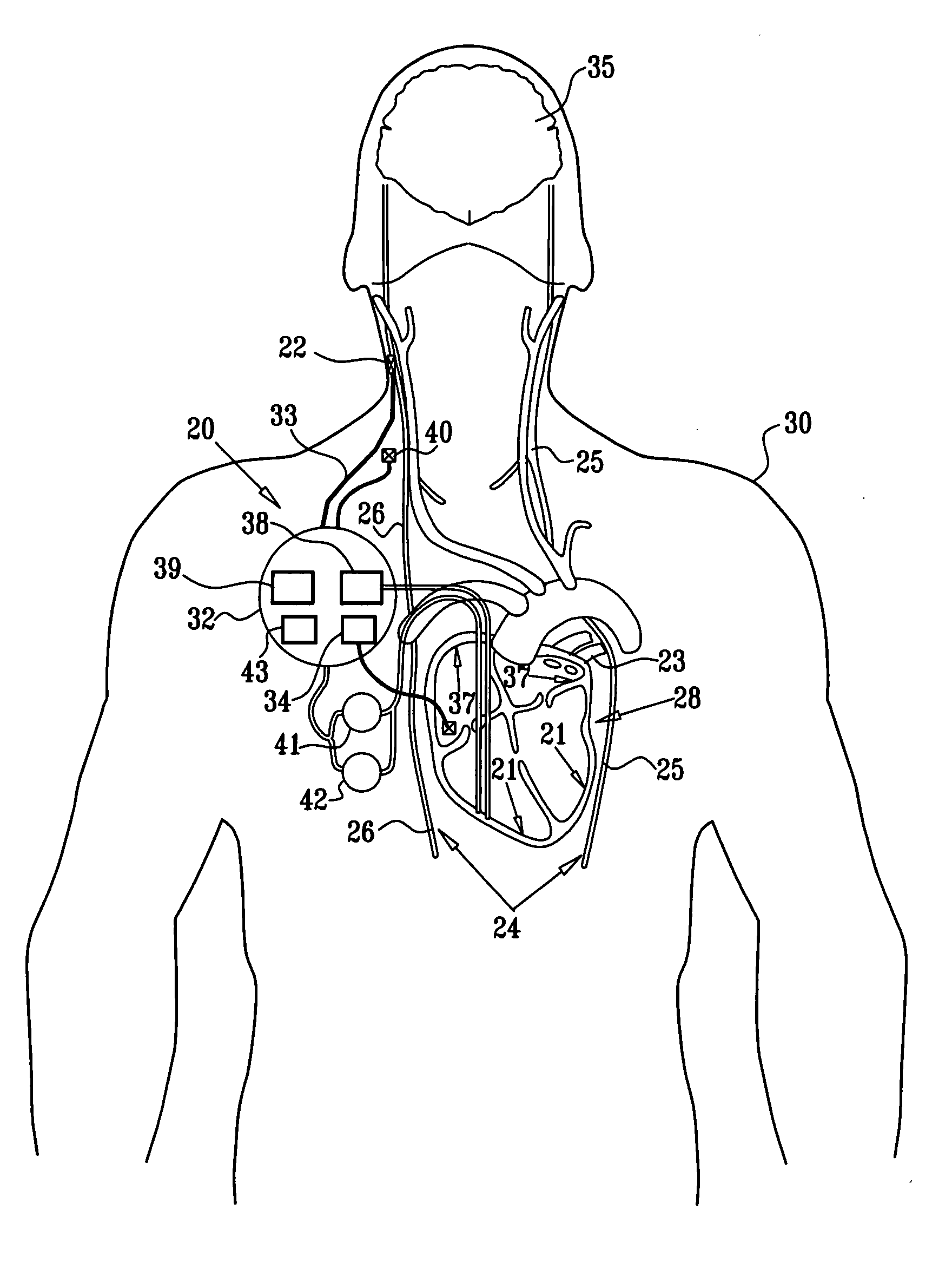
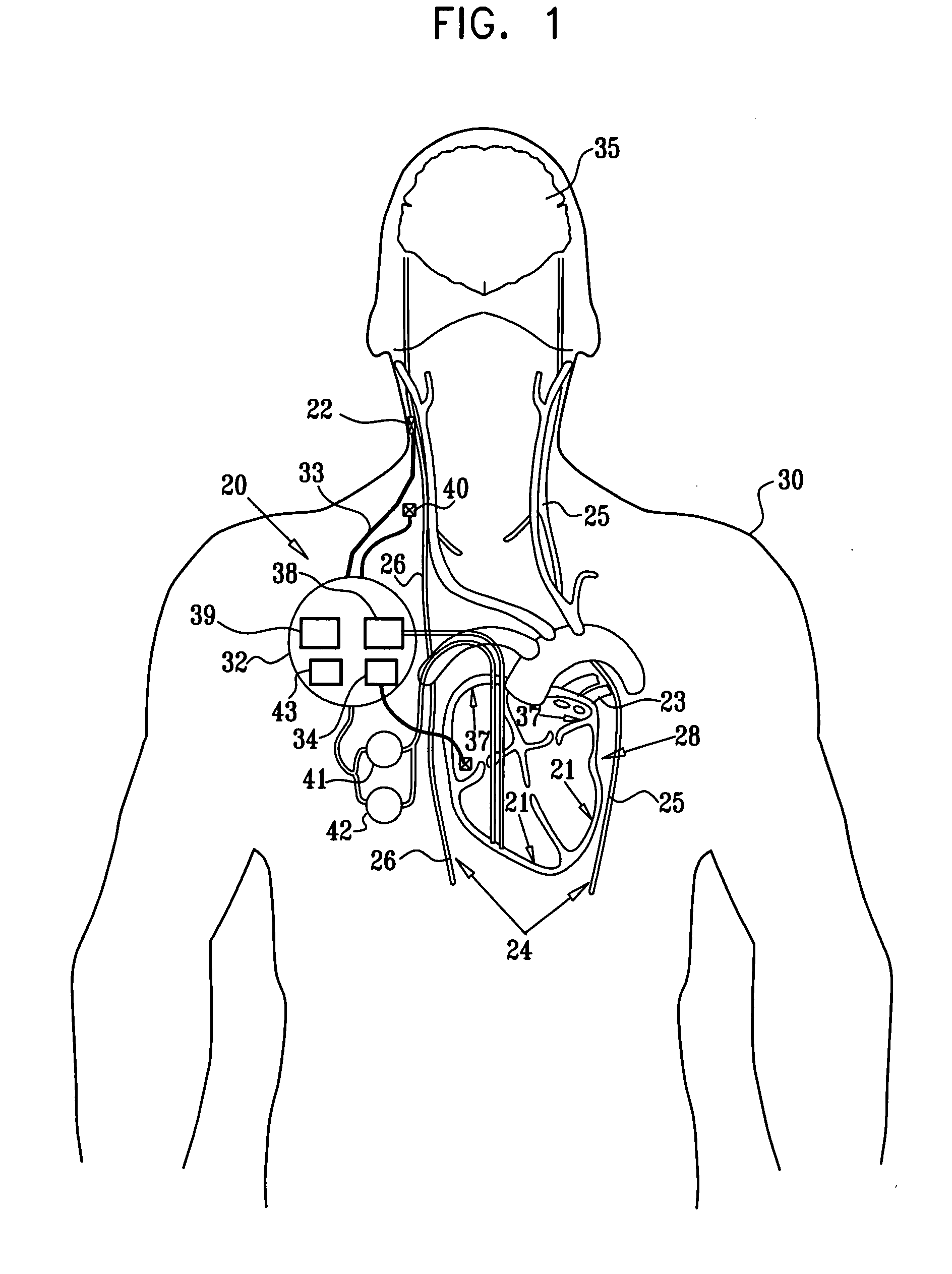
Apparatus for detecting and treating ventricular arrhythmia
A system and method for long-term monitoring of cardiac conditions such as arrhythmias is disclosed. The invention includes a pulse generator including means for sensing an arrhythmia. The pulse generator is coupled to at least one subcutaneous electrode or electrode array for providing electrical stimulation such as cardioversion / defibrillation shocks and / or pacing pulses. The electrical stimulation may be provided between multiple subcutaneous electrodes, or between one or more such electrodes and the housing of the pulse generator. In one embodiment, the pulse generator includes one or more electrodes that are isolated from the can. These electrodes may be used to sense cardiac signals.
Owner:HEINRICH STEPHEN D +1
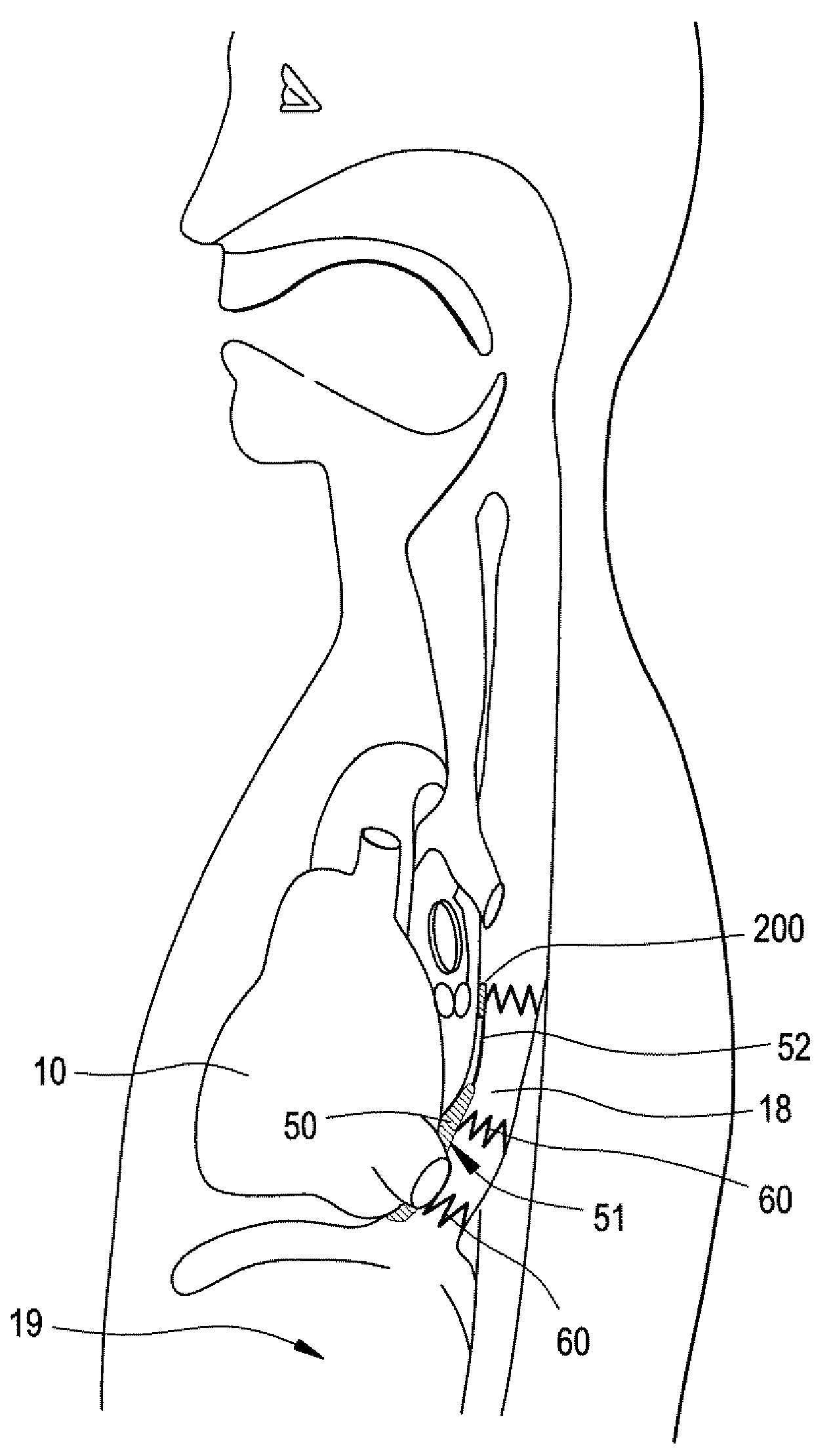
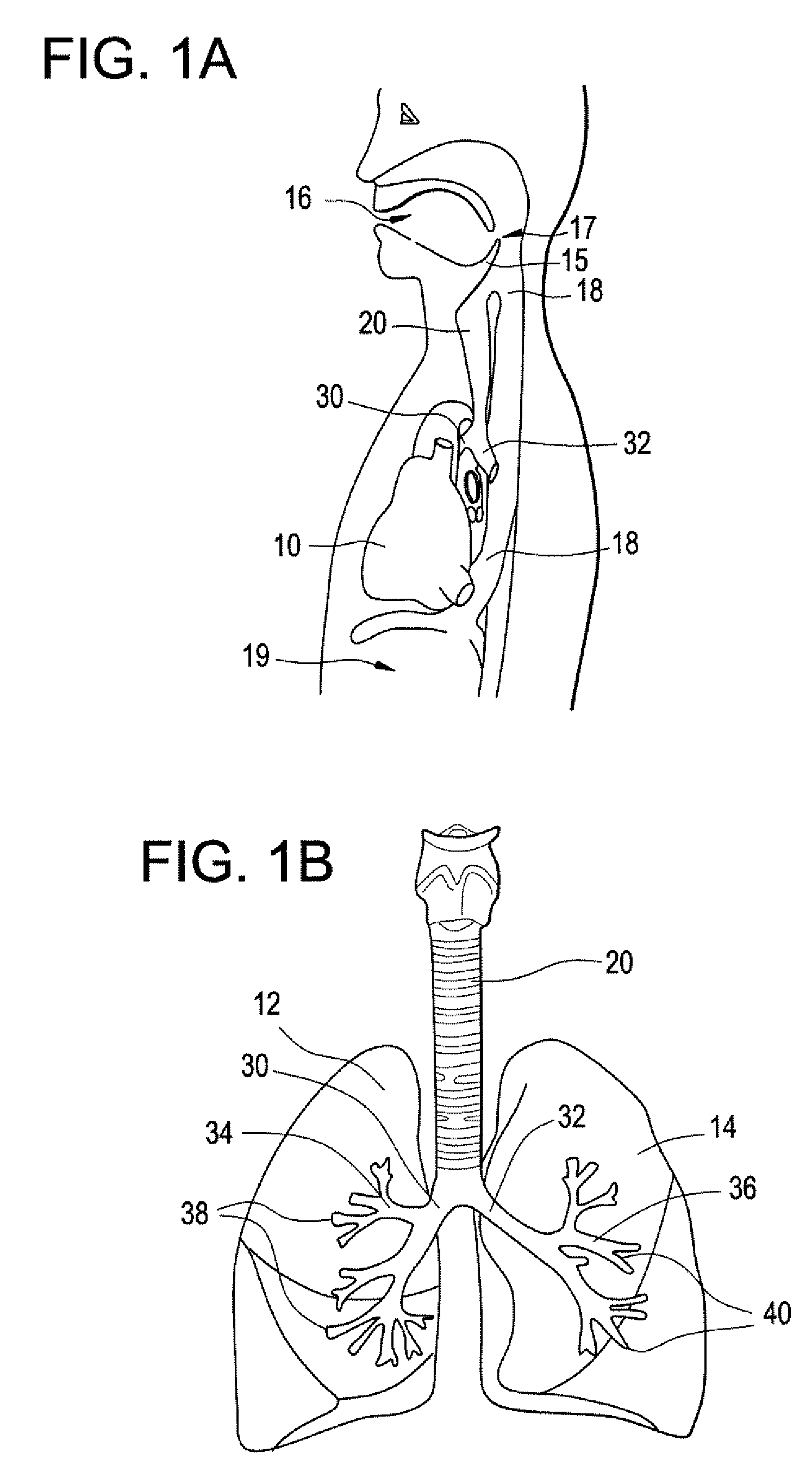
Implantable Devices and Methods for Stimulation of Cardiac or Other Tissues
An implantable stimulation system is provided for stimulation of the heart, phrenic nerve, gastric system, or other tissue structures accessible via a patient's upper gastrointestinal system or airway. The stimulation system includes an implantable controller housing including a pulse generator; at least one electrical lead attachable to the pulse generator; and at least one electrode carried by the electrical lead that is positionable and fixable within the upper gastrointestinal tract or airway. The controller housing may be adaptable for subcutaneous implantation, or within the upper gastrointestinal tract or airway, wherein the controller housing is proportioned to substantially permit fluid and solid flow through the upper gastrointestinal tract or airway about the controller housing. The pulse generator may be operable to deliver one or more electrical pulses effective in cardiac pacing, cardiac defibrillation, cardioversion, cardiac resynchronization therapy, diaphragm pacing, phrenic nerve stimulation, gastric electrical stimulation, or a combination thereof.
Owner:E PACING
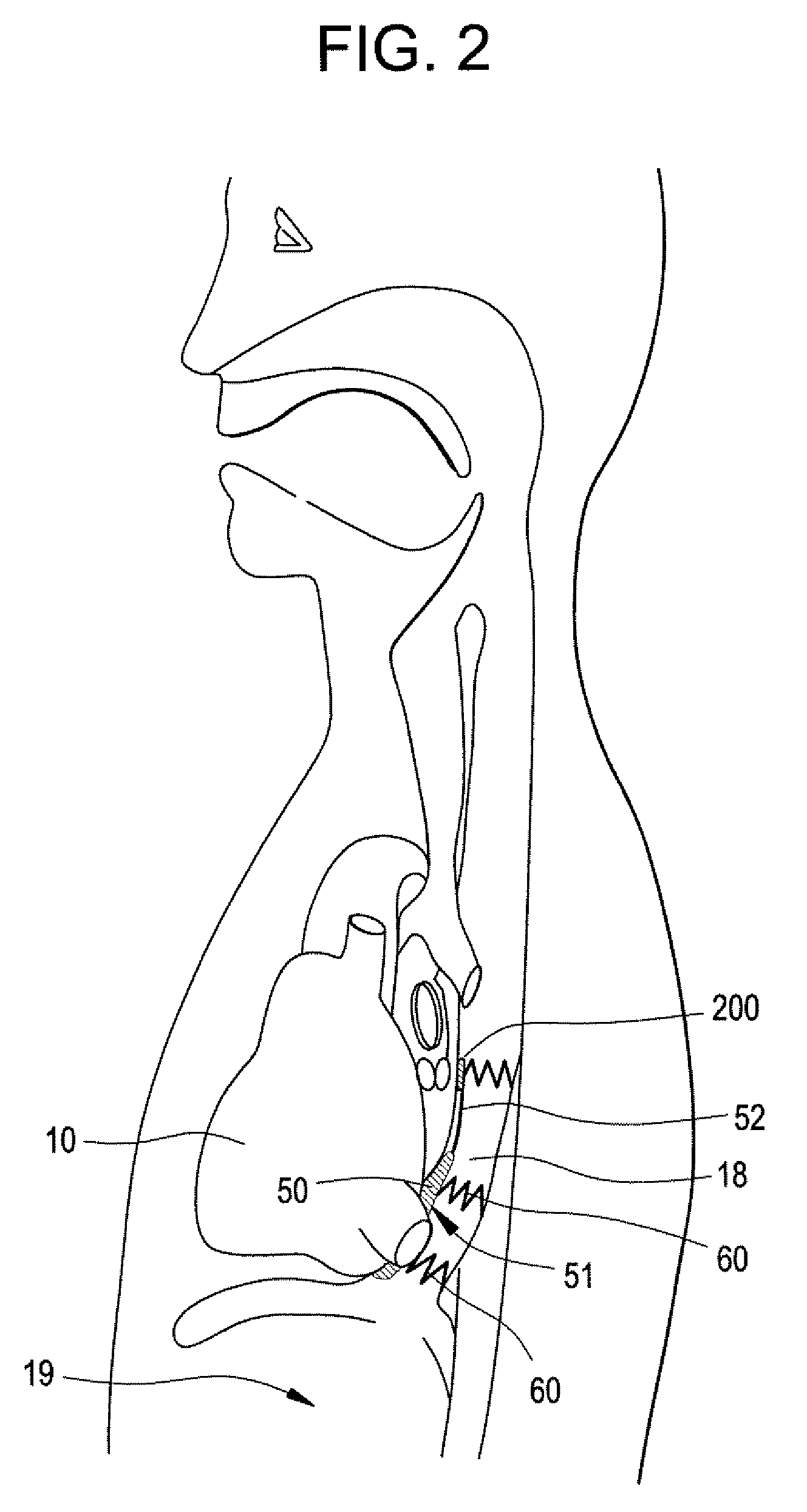
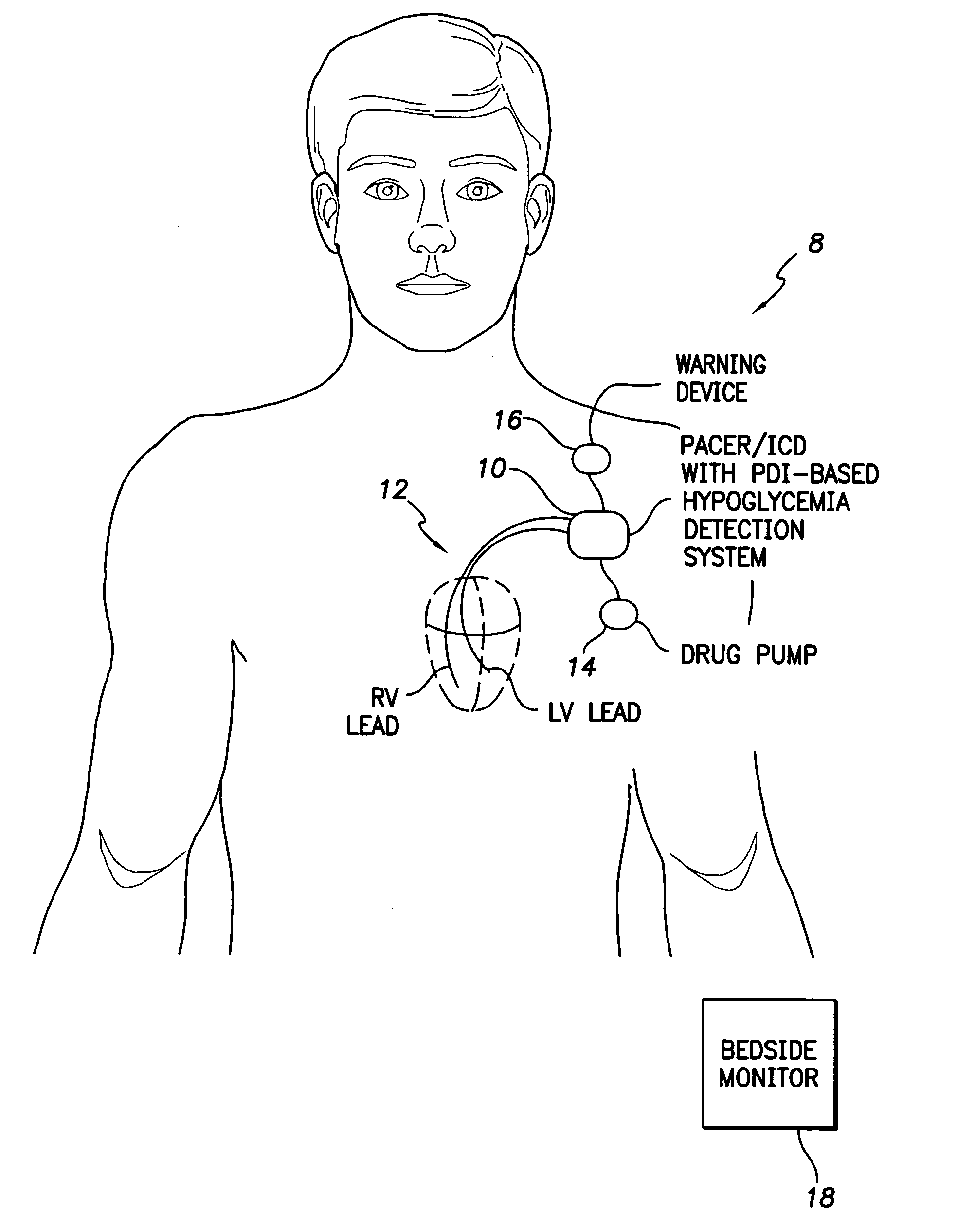
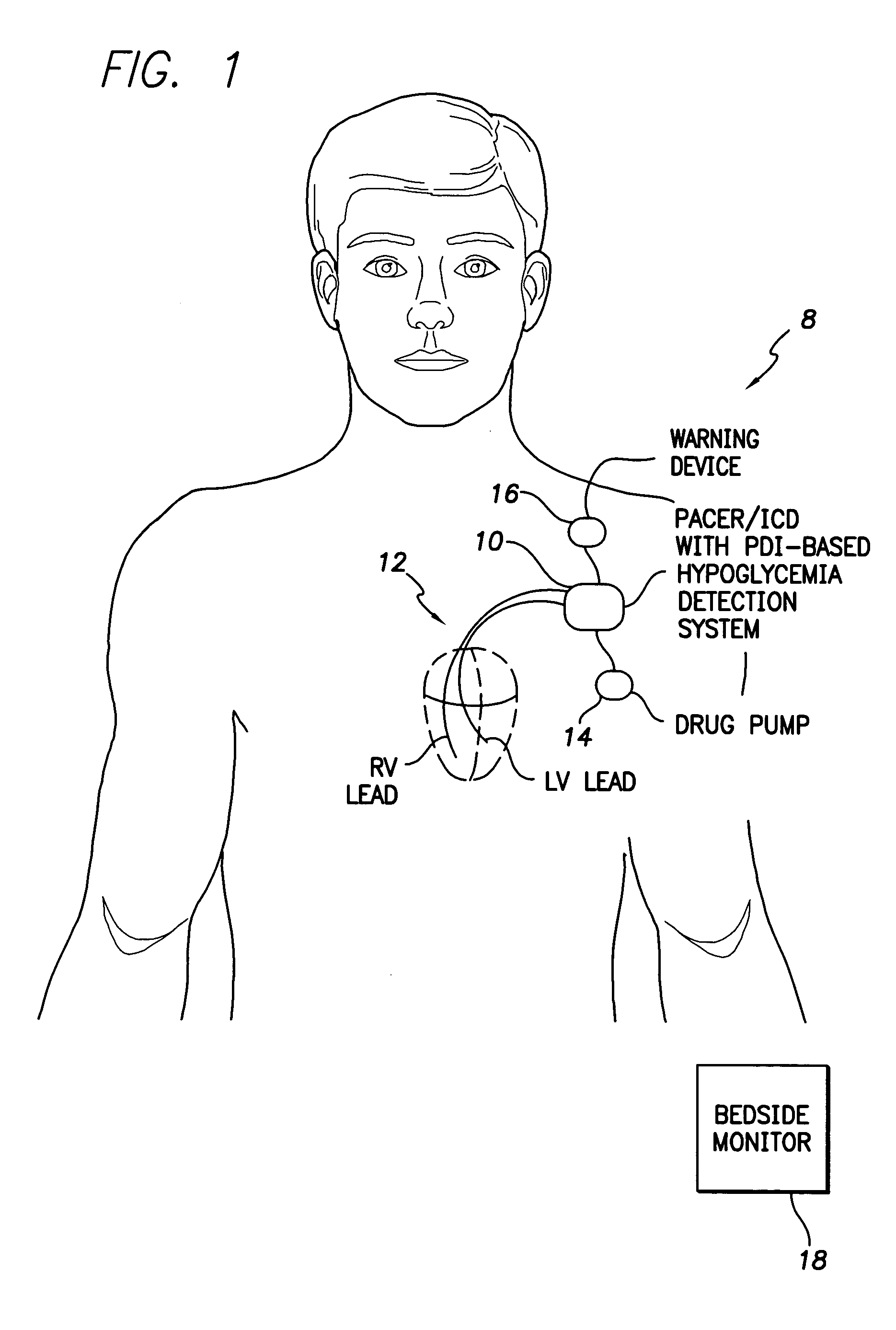
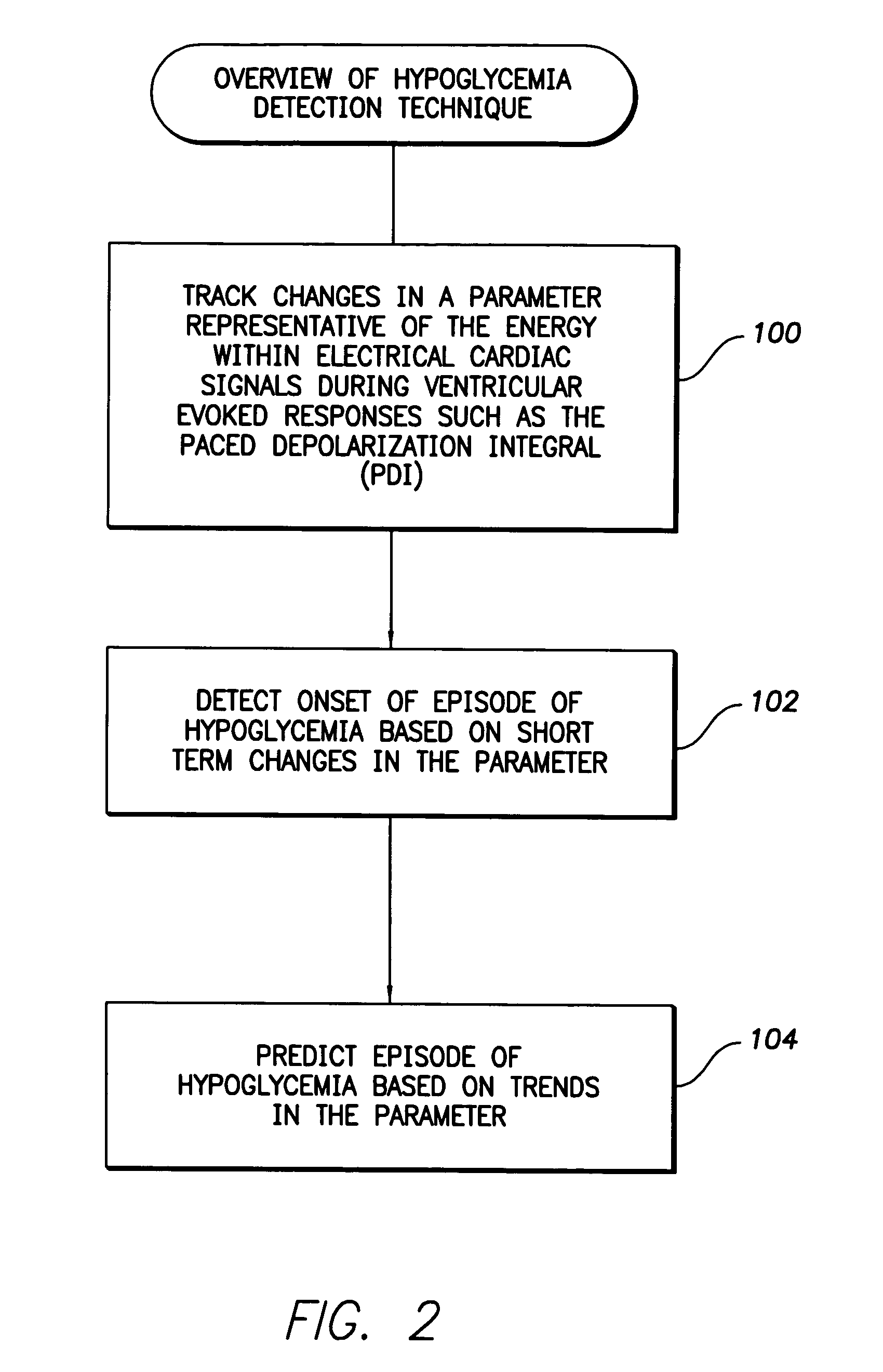
System and method for detecting hypoglycemia based on a paced depolarization integral using an implantable medical device
ActiveUS20060247685A1Improve blood sugar controlReduce deliveryElectrocardiographyMedical devicesCardiac pacemaker electrodeInsulin dependent
Techniques are provided for use with an implantable medical device such as a pacemaker or implantable cardioverter / defibrillator (ICD) for predicting and detecting hypoglycemia. In one example, the device tracks changes in a paced depolarization integral (PDI). A significant increase in PDI over a relatively short period of time indicates the onset of hypoglycemia (this can also be confirmed with QT changes). Upon detection of hypoglycemia, appropriate warning signals are generated to alert the patient. Certain therapies automatically provided by the implantable device may also be controlled in response to hypoglycemia. For example, if the patient is an insulin-dependent diabetic and the implantable device is equipped with an insulin pump capable of delivering insulin directly into the bloodstream, insulin delivery is automatically suspended until blood glucose levels return to acceptable levels. If the device is an ICD, it may be controlled to begin charging defibrillation capacitors upon detection of hypoglycemia so as to permit prompt delivery of a defibrillation shock, which may be needed if hypoglycemia triggers ventricular fibrillation. The detection techniques may be used in conjunction with other hypoglycemia detection techniques to improve detection specificity.
Owner:PACESETTER INC
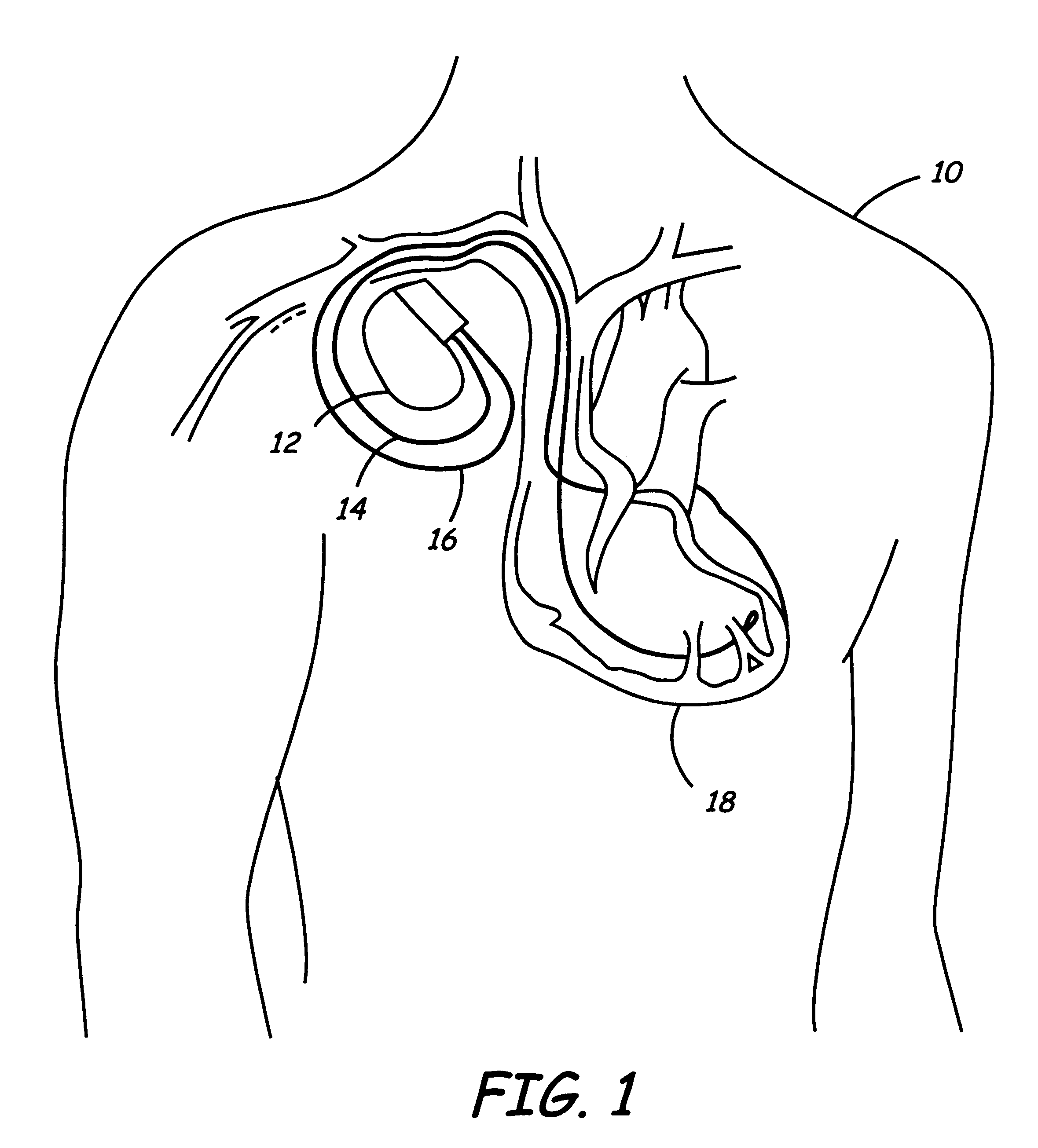
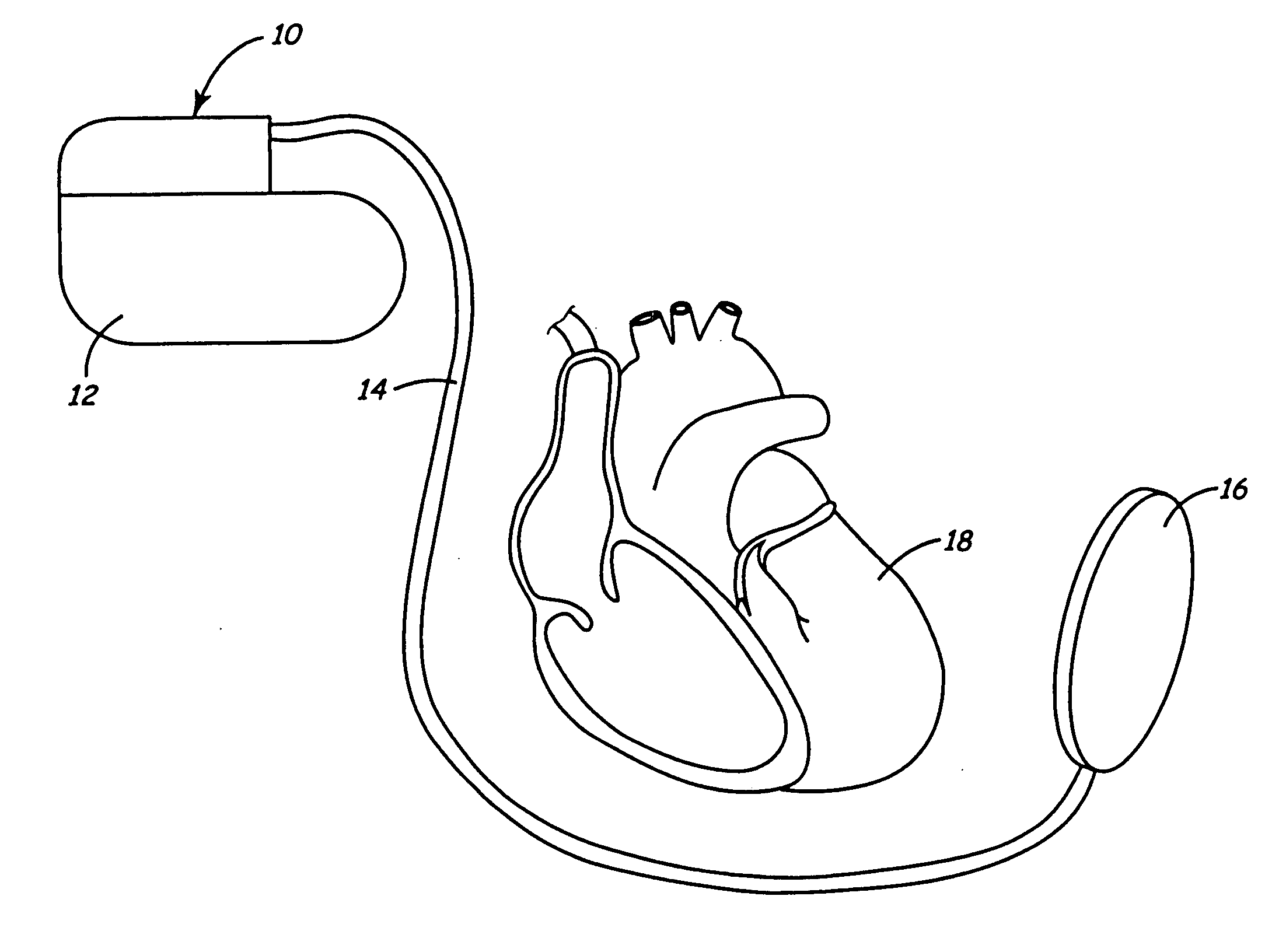
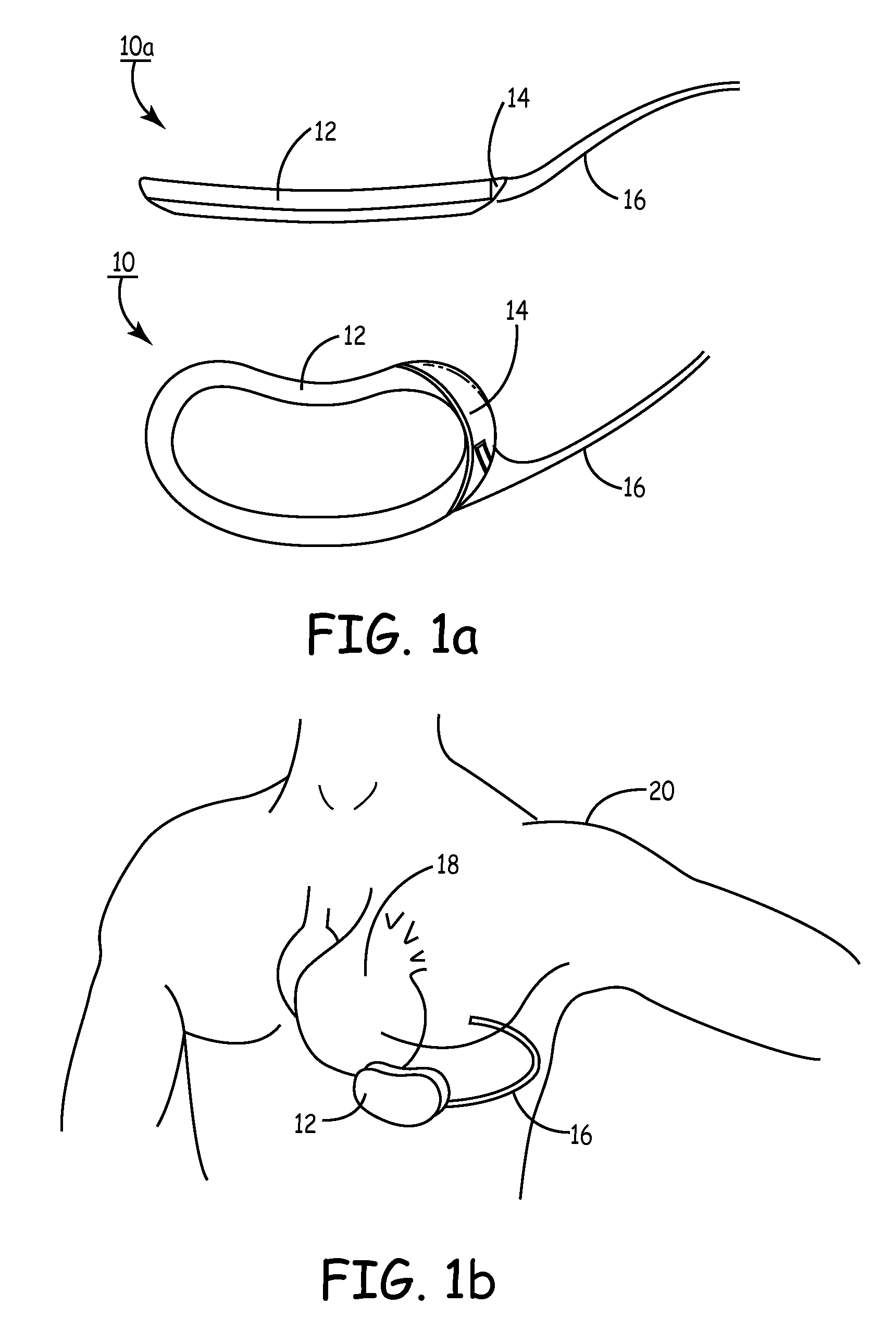
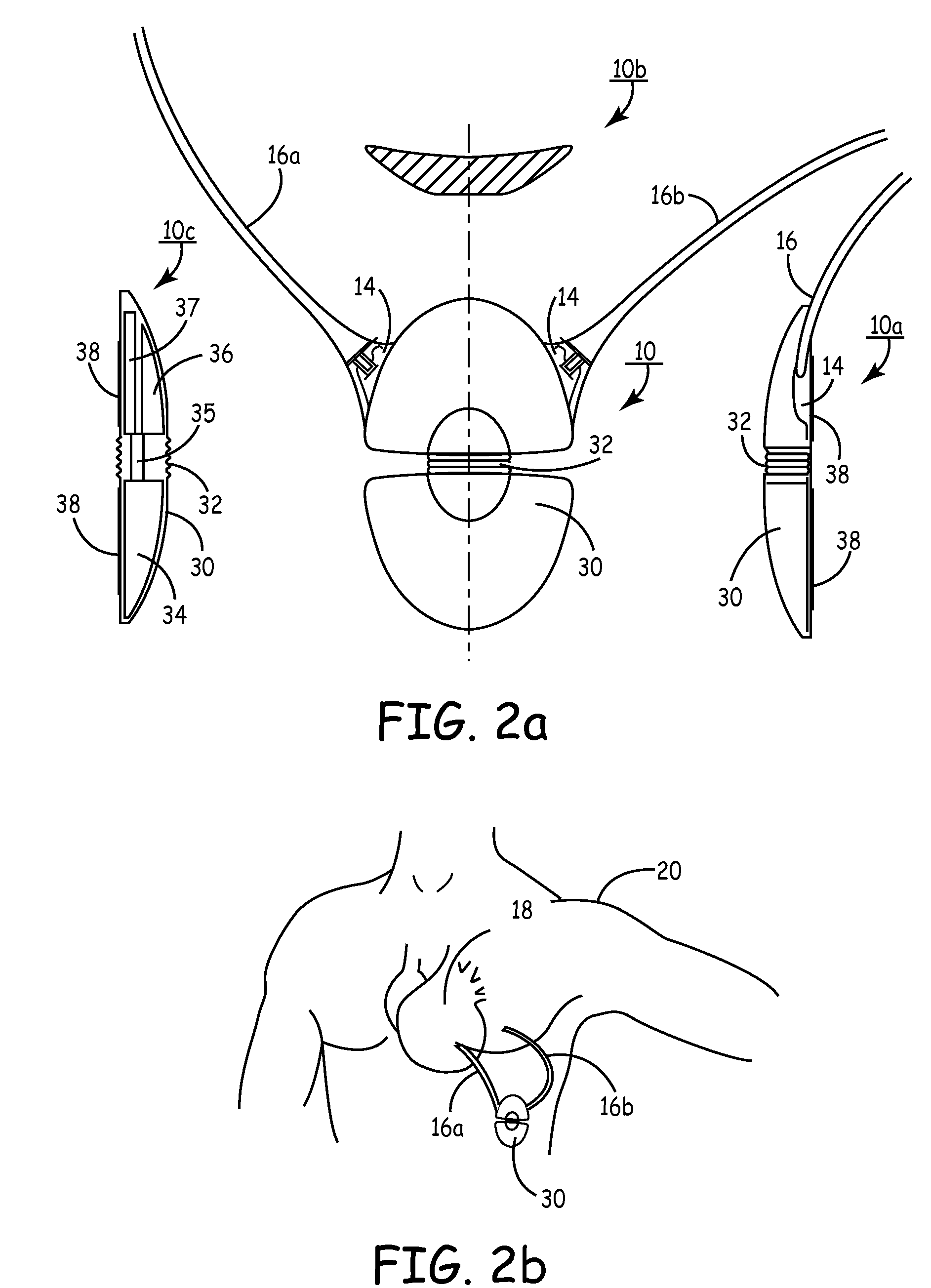
Subcutaneous cardioverter-defibrillator
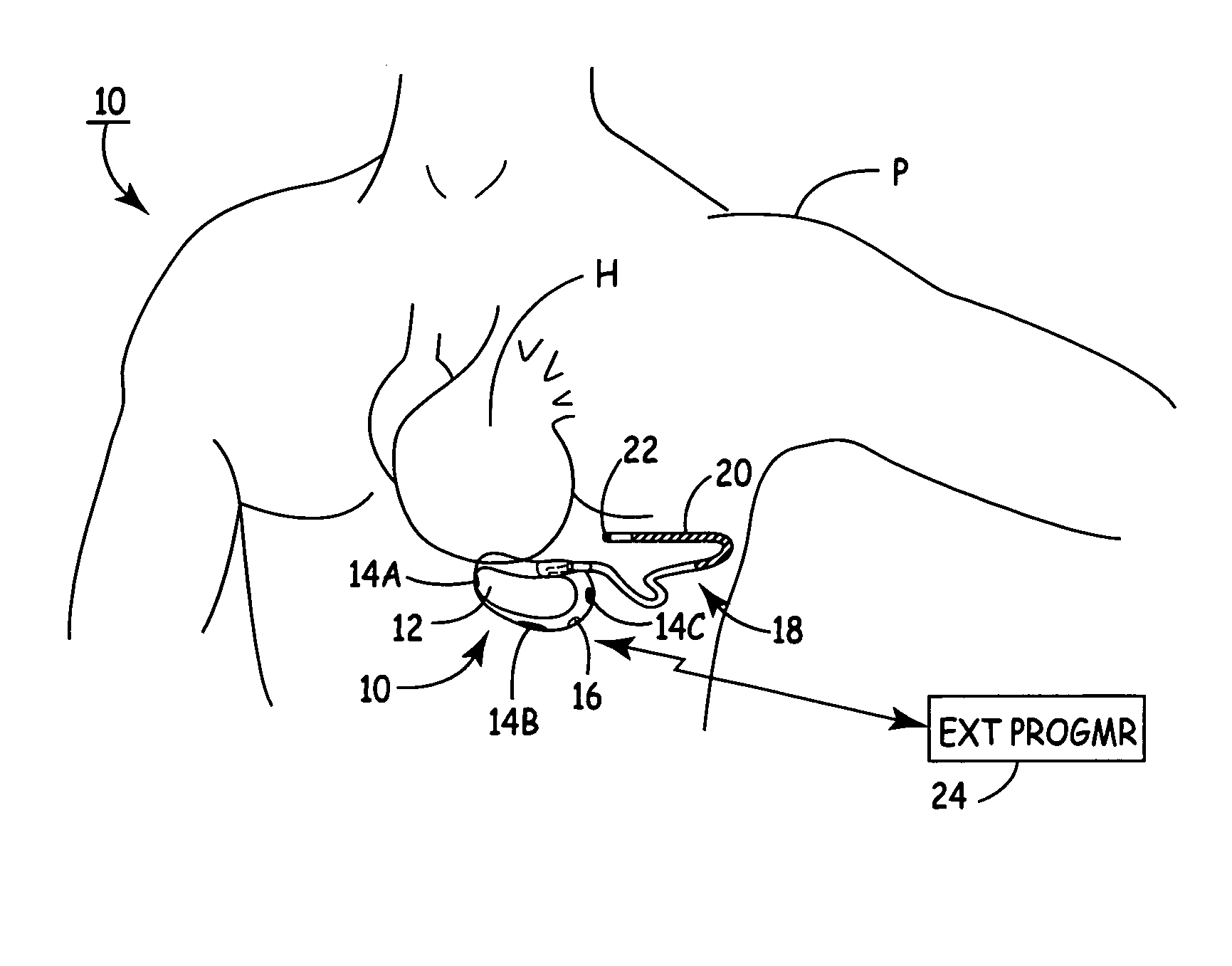
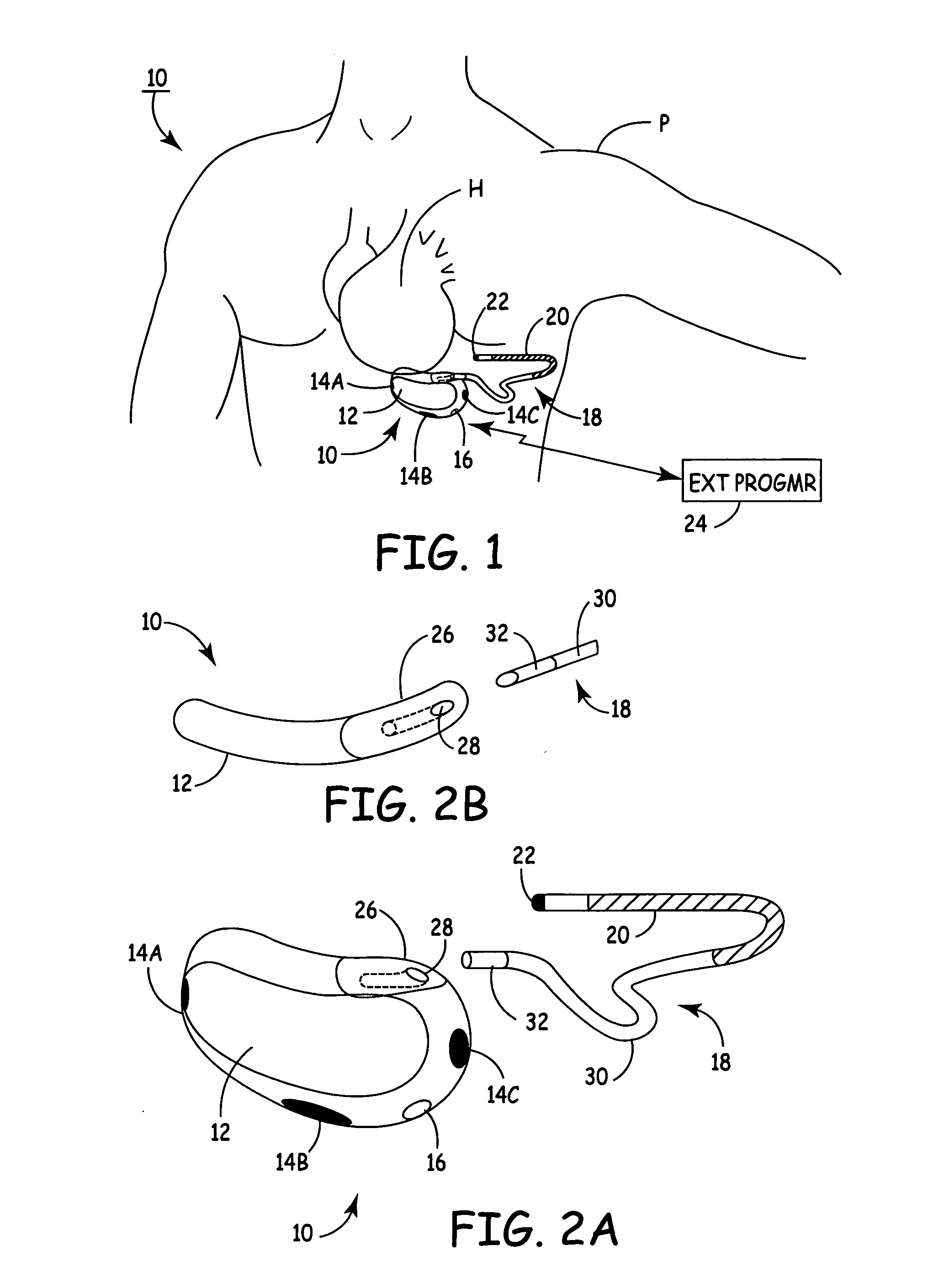
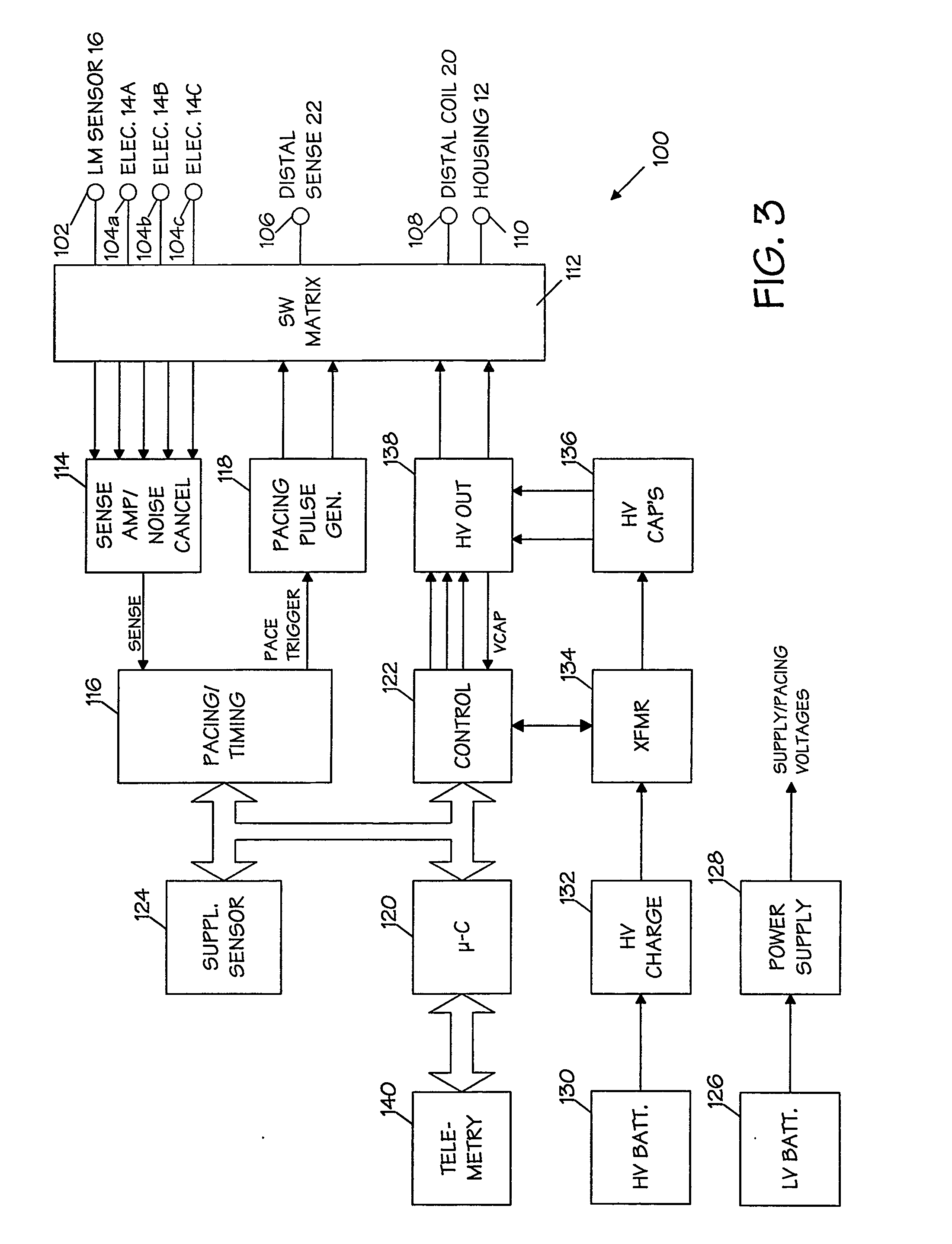
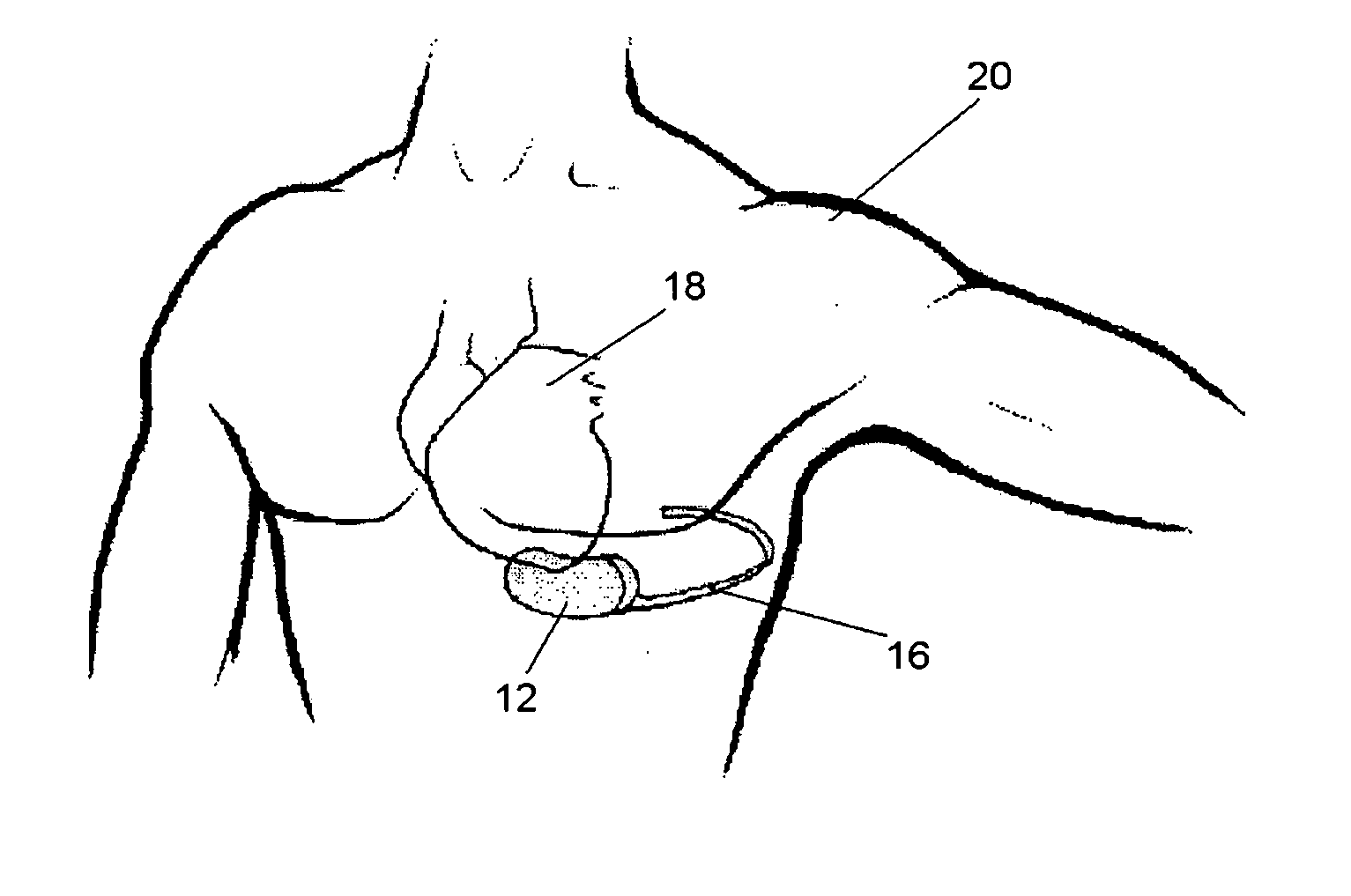
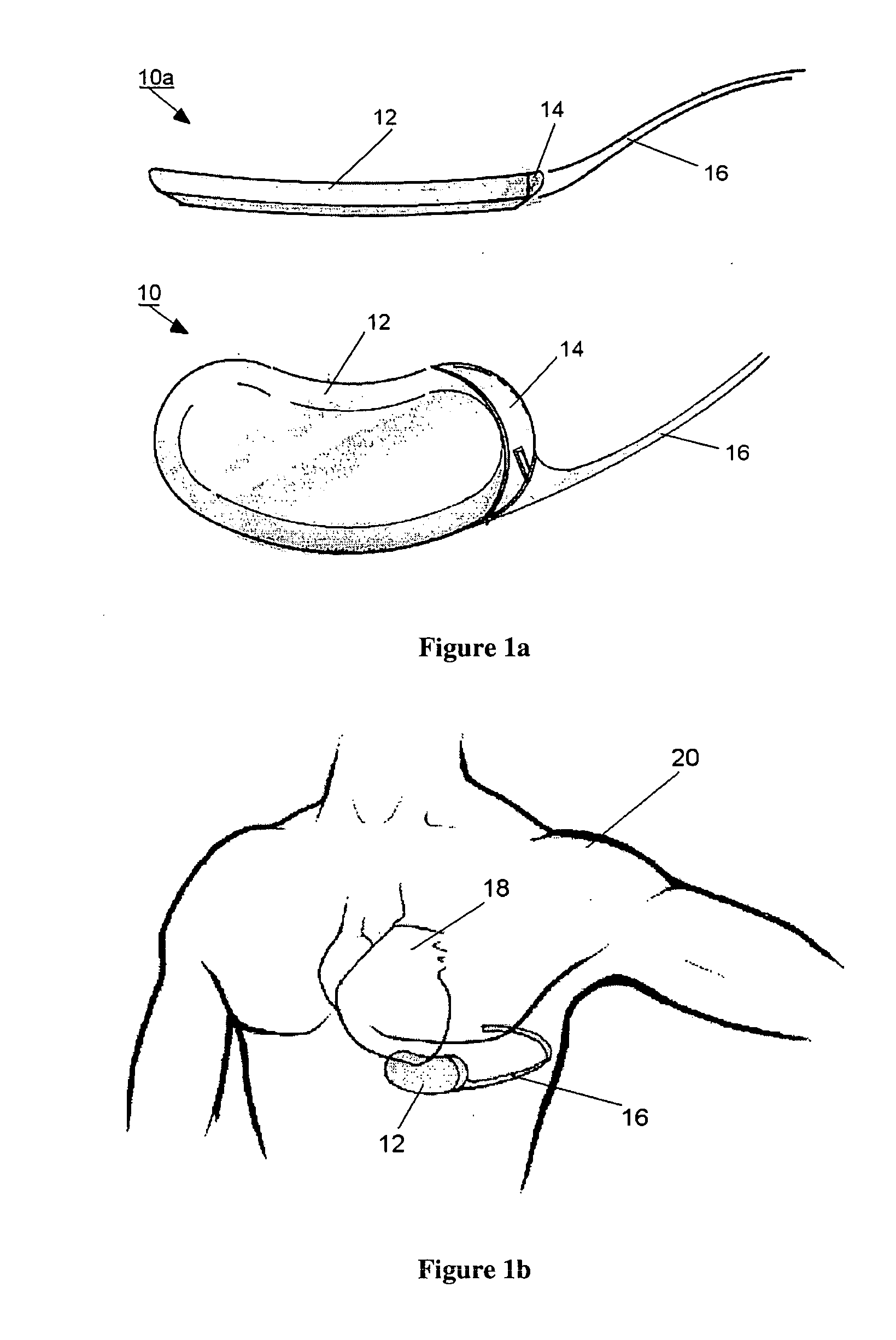
SubQ ICDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with 1 or, optionally, 2 subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, 2 hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort.
Owner:MEDTRONIC INC
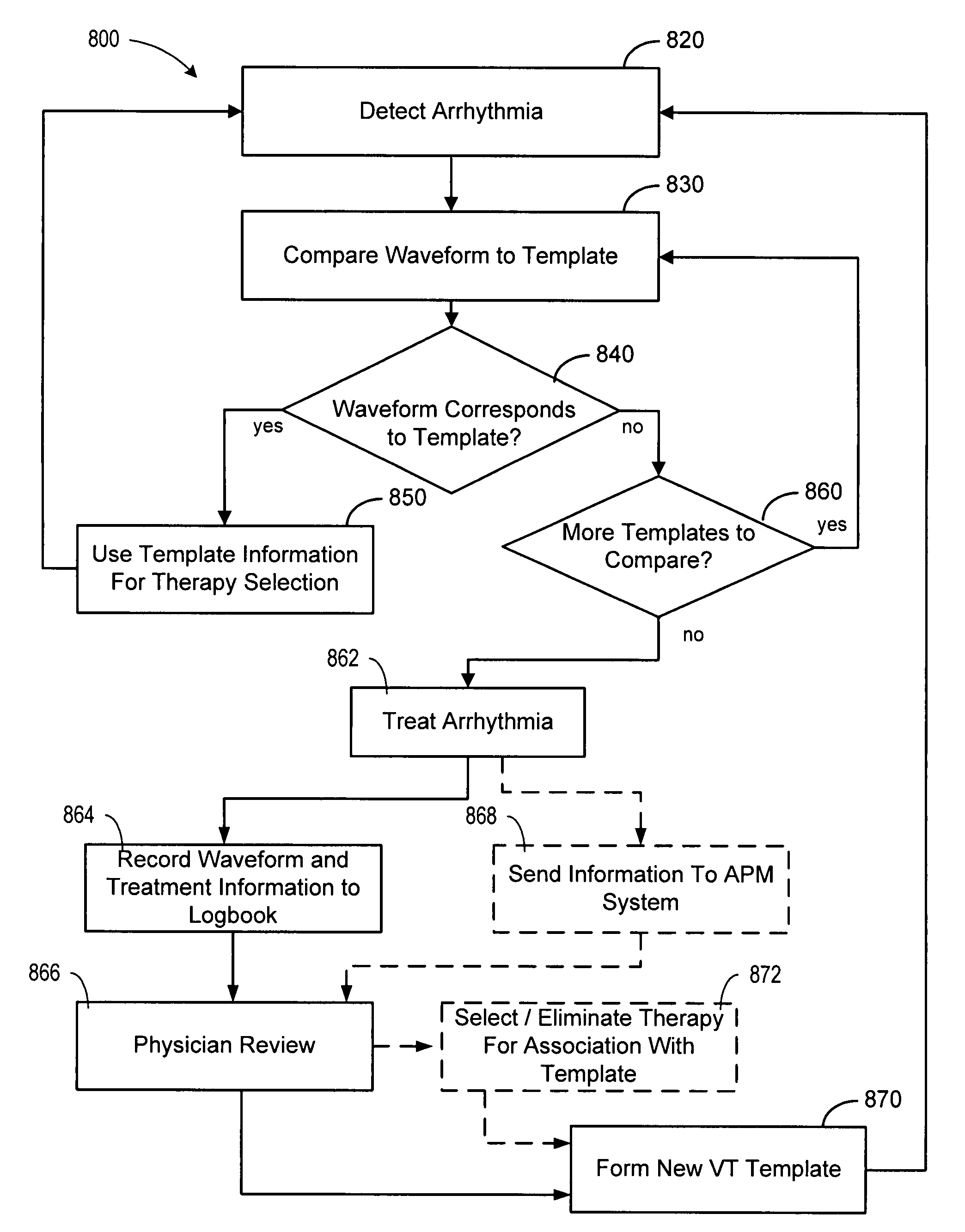
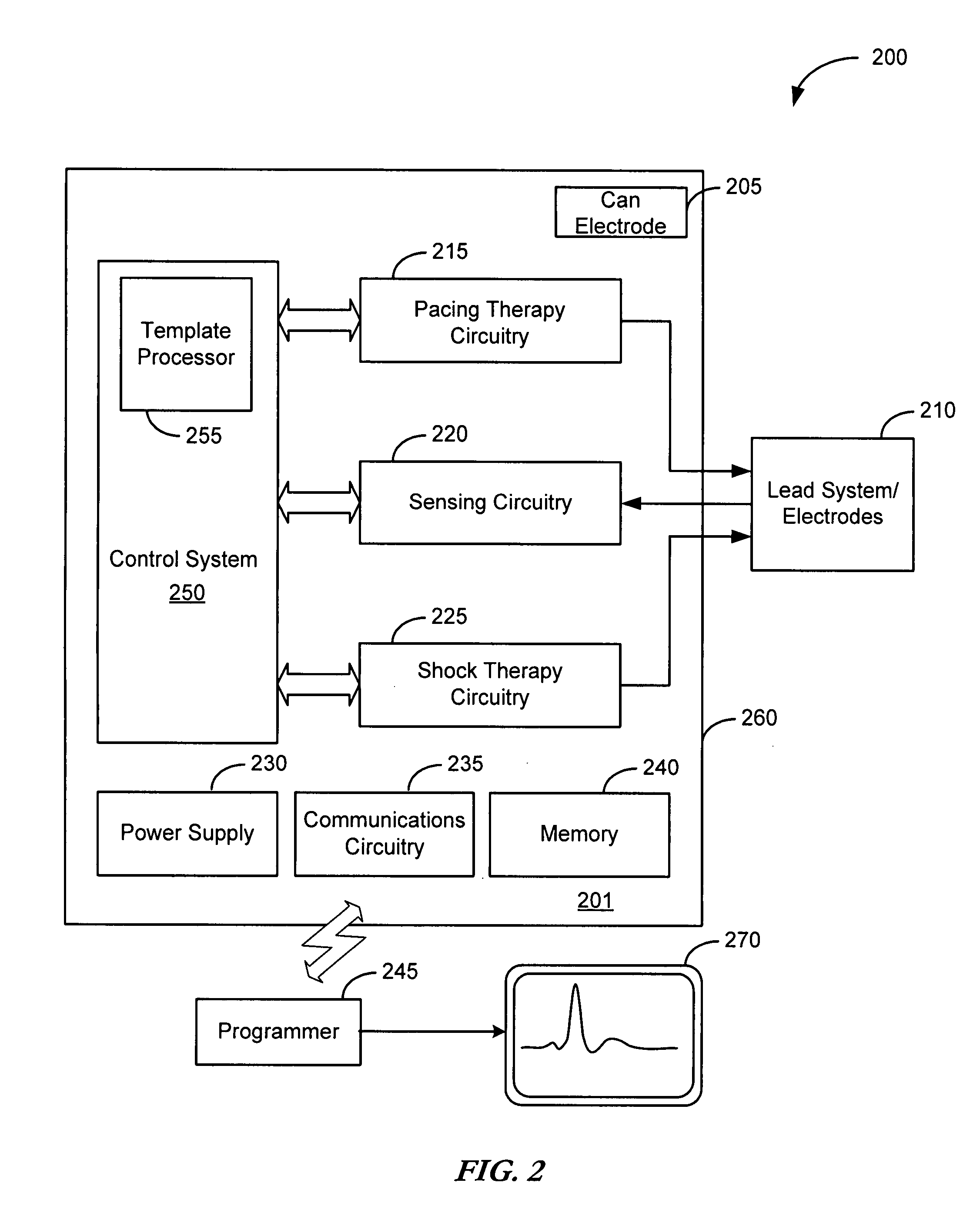
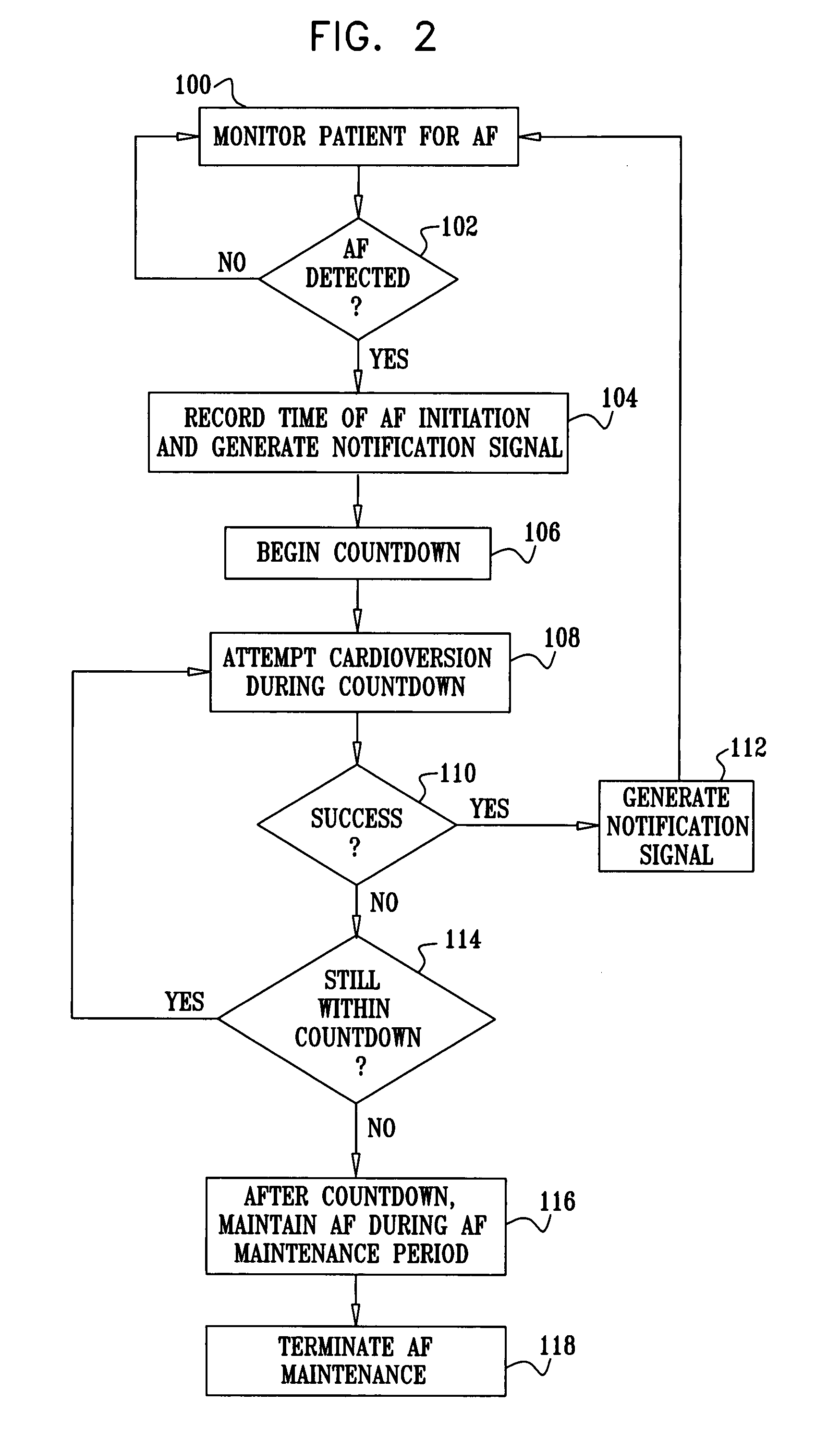
Cardiac tachyarrhythmia therapy selection based on patient response information
Cardiac treatment methods and devices providing templates representative of past tachyarrythmia events, each template associated with a therapy. A cardiac waveform is detected, and if it corresponds to a particular template associated with a previous therapy that was satisfactory in terminating a past event, the previous therapy is delivered again. If unsatisfactory, the previous therapy is eliminated as an option. If, for example, the previous therapy was an antitachycardia pacing therapy unsatisfactory in terminating the past tachyarrythmia event, delivery of the antitachycardia pacing therapy is eliminated as an option. Instead of ATP therapy, one or more of a cardioversion, defibrillation, or alternate anti-tachycardia pacing therapy may be associated with the particular template. Cardiac waveforms and templates may correspond in terms of one or more of morphology, timing, drug regimen, medication, neural activity, patient activity, hemodynamic status, cardiac tissue impedance, transthoracic impedance, or other information corresponding to the episode.
Owner:CARDIAC PACEMAKERS INC
Packaging technology for non-transvenous cardioverter/defibrillator devices
Packaging techniques for a non-transvenous implantable cardioverter / defibrillator include a housing and a frame within the housing for holding electronic components. A header is disposed on the housing and includes at least one feedthrough extending through the housing for providing electrical communication to and from the electronic components within the housing.
Owner:CAMERON HEALTH
Positionally adaptable implantable cardiac device
InactiveUS20060167502A1Facilitates controller selectionFacilitate controller selectionHeart defibrillatorsInternal electrodesEngineeringCardiac activity
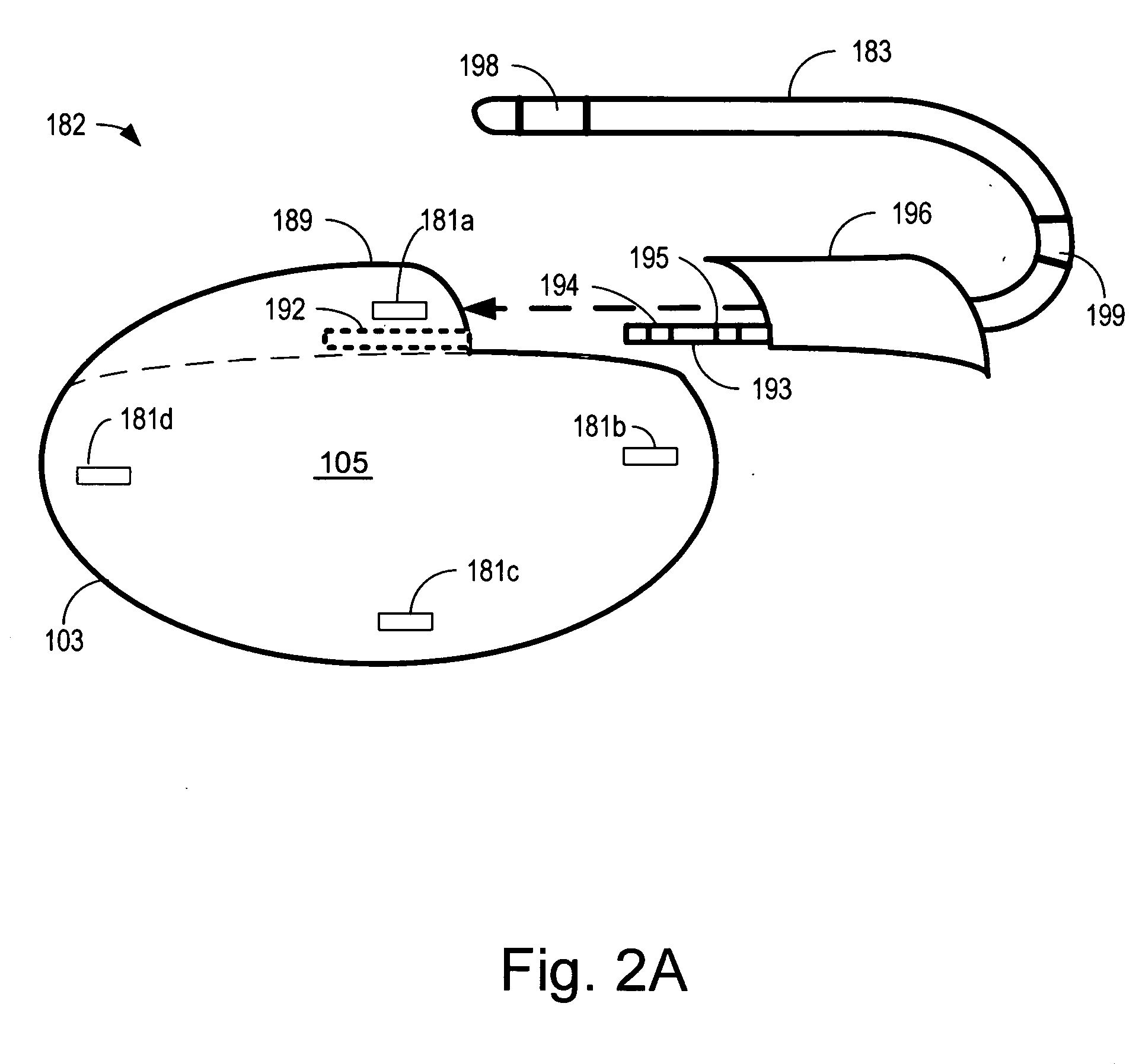
Cardiac sensing and / or stimulation devices and methods that adapt to implant location and positioning, and may employ automated vector selection from multiple electrodes. Devices include a housing having a first face opposing a second face, and an edge extending around the perimeter. A pulse generator and controller are coupled to three or more electrodes. Electrode arrangement facilitates selection of the particular electrodes that sense cardiac activity irrespective of one or more of positioning of the device, rotation of the housing, and which of the first and second faces of the housing is orientated toward the patient's skin. A first vector may be selected that provides for sensing cardiac activity, and a second vector may sense skeletal muscle activity. The vectors may be selected based on amplitude or signal-to-noise ratio exceeding a predetermined threshold. Methods may involve delivering defibrillation or cardioversion energy and / or determining cardiac rhythm states using selected vectors.
Owner:CARDIAC PACEMAKERS INC
Apparatus for detecting and treating ventricular arrhythmia
A system and method for long-term monitoring of cardiac conditions such as arrhythmias is disclosed. The invention includes a pulse generator including means for sensing an arrhythmia. The pulse generator is coupled to at least one subcutaneous electrode or electrode array for providing electrical stimulation such as cardioversion / defibrillation shocks and / or pacing pulses. The electrical stimulation may be provided between multiple subcutaneous electrodes, or between one or more such electrodes and the housing of the pulse generator. In one embodiment, the pulse generator includes one or more electrodes that are isolated from the can. These electrodes may be used to sense cardiac signals.
Owner:BOSTON SCI SCIMED INC
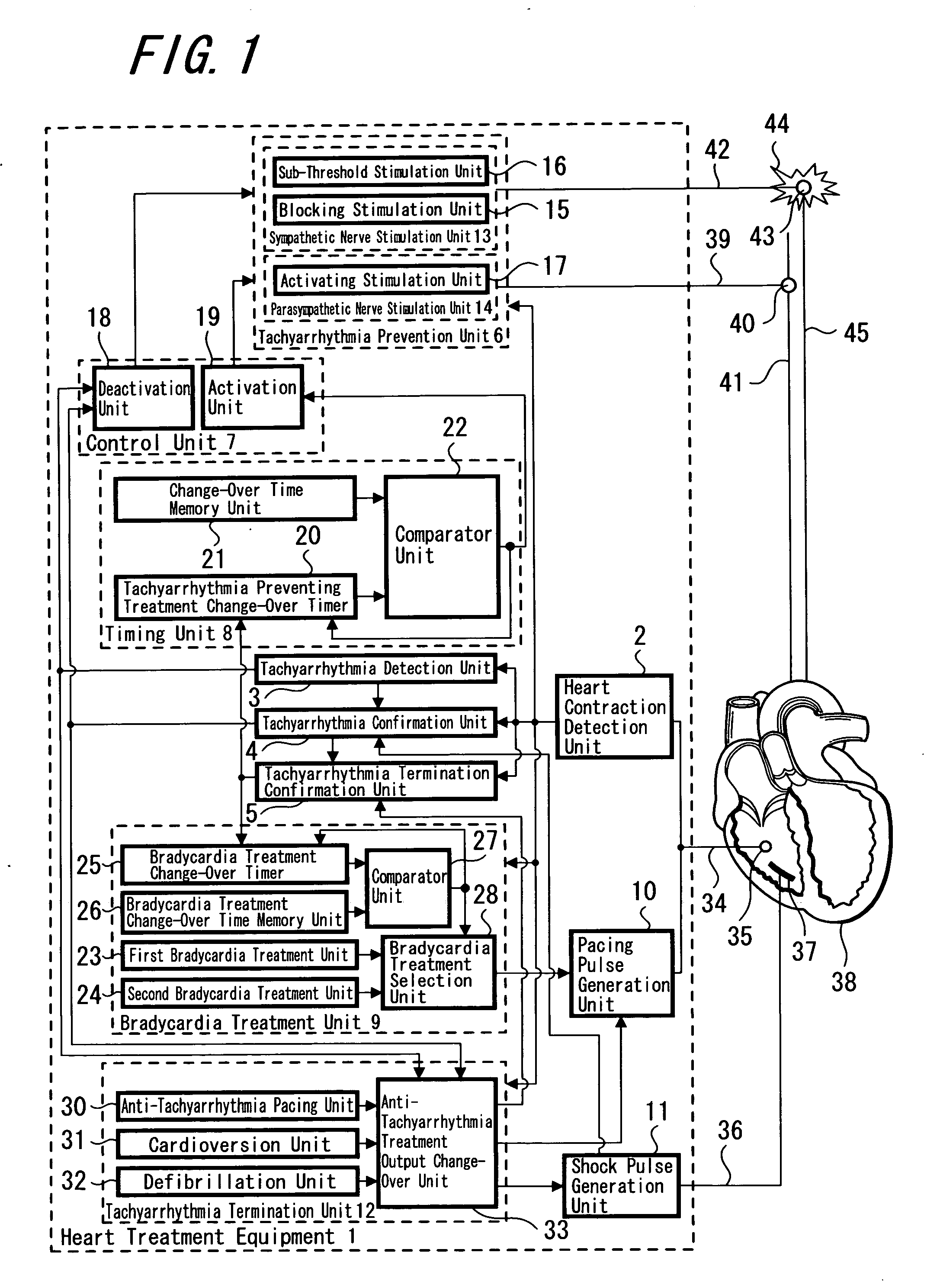
Heart treatment equipment and heart treatment method
InactiveUS20070100380A1Prevent deterioration of hemodynamicsReducing and stopping currentHeart defibrillatorsHeart stimulatorsTherapeutic DevicesSympathetic nerve
There is disclosed heart treatment equipment in which it is possible to carry out controlling of a preventive treatment after an anti-tachyarrhthmia treatment in order to execute prevention and treatment of a fatal arrhythmia and the anti-tachyarrhythmia treatment is carried out by controlling tachyarrhythmia prevention means and tachyarrhythmia treatment means (tachyarrhythmia termination means) according to the result of detecting occurrence of tachyarrhythmia. In the anti-tachyarrhythmia treatment, there is provided with a structure for reducing or stopping the activation current with respect to the vagus nerve or a repression current with respect to the sympathetic nerve after a supply of an electroshock to the heart such as cardioversion, defibrillation or the like, so that it is possible to prevent deterioration of hemodynamics, recurrence of fatal arrhythmia and supraventicular arrhythmia such as atrial fibrillation or the like.
Owner:TERUMO KK
Method and system for switching shock vectors and decreasing transthoracic impedance for cardioversion and defibrillation
InactiveUS20140163663A1Lower impedanceIncrease currentExternal electrodesShock waveBiomedical engineering
A method and system for improving the effectiveness of cardioversion or defibrillation through the ability to switch shock vectors and to reduce transthoracic impedance. A “shock vector” or “shocking vector” is herein defined as the path and direction which electrical current follows in traversing a patient body cavity between two external adhesive electrode patches. An external multiple patch system comprises at least two options for a shocking vector once external patches are applied and adhered to desired locations on a patient's body. A manual switching mechanism in the system provides the ability to direct current from a defibrillator to either of two or more specified shocking vectors. A method and system of decreasing transthoracic impedance comprises wrapping material around a patient's body to apply pressure to adhered patches. This mechanism further reduces transthoracic impedance by increasing effective pressure on the patches through use of pressure-focusing mechanisms located between a patch and a strap. An integrated mechanism would provide qualitative and / or quantitative feedback on the force being applied to the desired patches.
Owner:NEXUS CONTROL SYST
Method and apparatus for treatment of cardiac electromechanical dissociation
An apparatus and method for treating post-defibrillation electromechanical dissociation ("EMD"). A first embodiment comprises an implantable defibrillator, which may include cardioversion and pacemaker capabilities, which has the capability of detecting and treating post defibrillation EMD. The stimulator / defibrillator has one or more leads with electrodes. At least one electrode for defibrillation may be an endocardial or epicardial electrode or other suitable defibrillation electrode. A sense circuit senses the electrical condition of the heart of the patient. A hemodynamic sensor senses a parameter correlated to the state of blood flow. The cardiac stimulator / defibrillator detects ventricular tachyarrhythmia including fibrillation and terminates ventricular tachyarrhythmia. After termination of the ventricular tachyarrhythmia, the stimulator / defibrillator can detect the presence of electrical rhythm in the heart correlated, however, with inadequate blood flow to sustain life. Under such conditions, the device provides an output to stimulate the heart to overcome electromechanical dissociation and restore adequate blood flow. The device may also be an external therapy device, as part of, or in conjunction with an external defibrillator. The method for treating the heart to restore blood flow where electromechanical dissociation occurs after termination of a ventricular tachyarrhythmia or ventricular fibrillation comprises identifying electromechanical disassociation after termination of a ventricular tachyarrhythmia or a fibrillation and providing electrical therapy, the therapy comprising a series of packets of electrical pulses.
Owner:INTERMEDICS
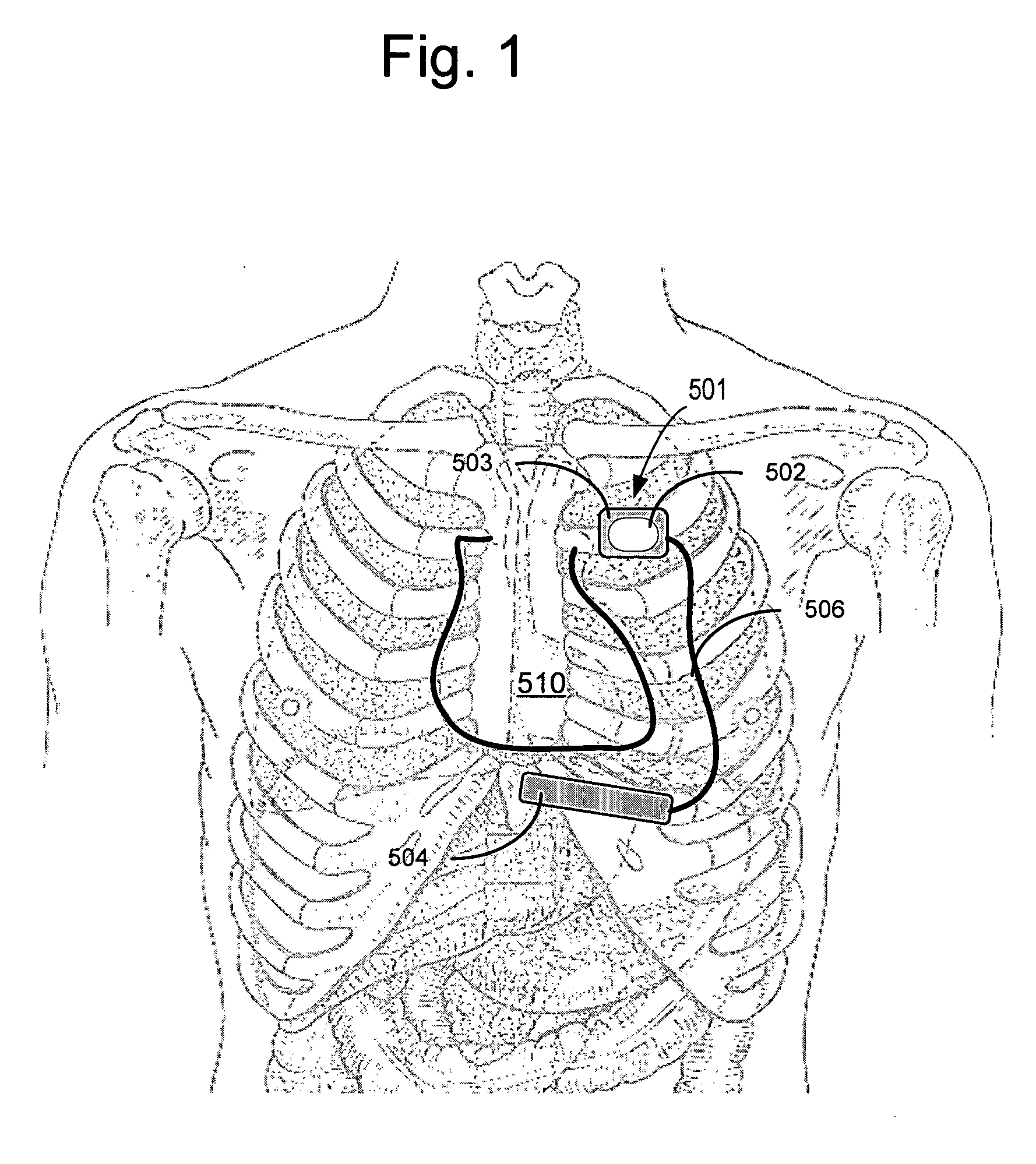
Implantable cardioverter-defibrillator (ICD) system including substernal pacing lead
An implantable cardiac defibrillator (ICD) system includes an ICD implanted subcutaneously in a patient, a defibrillation lead having a proximal portion coupled to the ICD and a distal portion having a defibrillation electrode configured to deliver a defibrillation or cardioversion shock to a heart of the patient, and a pacing lead that includes a distal portion having one or more electrodes and a proximal portion coupled to the ICD. The distal portion of the pacing lead is implanted at least partially along a posterior side of a sternum of the patient within the anterior mediastinum. The ICD is configured to provide pacing pulses to the heart of the patient via the pacing lead and provide defibrillation shocks to the patient via the defibrillation lead. As such, the implantable cardiac system provides pacing from the substernal space for an extravascular ICD system.
Owner:MEDTRONIC INC
Subcutaneous implantable cardioverter/defibrillator
InactiveUS7069075B2Inhibit migrationHeart defibrillatorsHeart stimulatorsLow voltageHigh voltage capacitors
Owner:MEDTRONIC INC
Methods and Apparatus for Selectively Shunting Energy in an Implantable Extra-Cardiac Defibrillation Device
InactiveUS20080183230A1Sufficient massEasy to implantSubcutaneous electrodesExternal electrodesPatient comfortThoracic cavity
The disclosure provides methods and apparatus for simultaneously providing protection to an implantable medical device, such as an extra-cardiac implantable defibrillator (EID), while allowing efficacious therapy delivery via an external defibrillator (e.g., an automated external defibrillator, or AED). Due to the orientation of the electrodes upon application of therapy via, for example, via an AED the structure of the EID essentially blocks therapy delivery. In addition, but for the teaching of this disclosure sensitive circuitry of an EID can be damaged during application of external high voltage therapy thus rendering the EID inoperable. EIDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with one or, optionally, two subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, two hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort. One aspect includes partially insulating a surface of an EID that faces away from a heart while maintaining a major conductive surface facing the heart.
Owner:MEDTRONIC INC
Electrical muscle controller and pacing with hemodynamic enhancement
InactiveUS20080140142A1Improve usabilityStrength of electric field is changedEpicardial electrodesHeart chamberHemodynamics
A method of modifying the force of contraction of at least a portion of a heart chamber is provided. A non-excitatory electric field of given duration is applied, at a delay after an activation of the heart, which increase the force of contraction by at least 5%. Apparatus is also provided for pacing with hemodynamic improvement. Circuitry applies an extended pacing signal to electrodes to pace the heart, the extended pacing signal having an overall duration greater than 8 ms from a time of initiation of application of that portion of the signal that initiates action potential propagation. The signal includes a train of biphasic pulses having pulse durations of at least 1 ms, and the signal has an amplitude that is at least three times as great as a threshold for pacing the heart and that is sufficient neither for cardioversion nor for defibrillation. Other embodiments are also described.
Owner:IMPULSE DYNAMICS NV
Method of implanting ICD and subcutaneous lead
InactiveUS7076294B2Easy to identifyElectrocardiographyHeart defibrillatorsVeinSubcutaneous implantation
A subcutaneous implantable cardioverter-defibrillator which has an electrically active canister which houses a source of electrical energy, a capacitor, and operational circuitry that senses the presence of potentially fatal heart rhythms delivers cardioversion defibrillation energy between at least one subcutaneous lead and the electrically active canister. There are no transvenous, intracardiac, or epicardial electrodes. The electrically active canister and at least one lead is implanted utilizing a curved introducer to implant the electrically active canister subcutaneously at various locations around the thorax.
Owner:CAMERON HEALTH
Co-extruded, multi-lumen medical lead
InactiveUS20020183824A1Transvascular endocardial electrodesExternal electrodesElectrical stimulationsBending stiffness
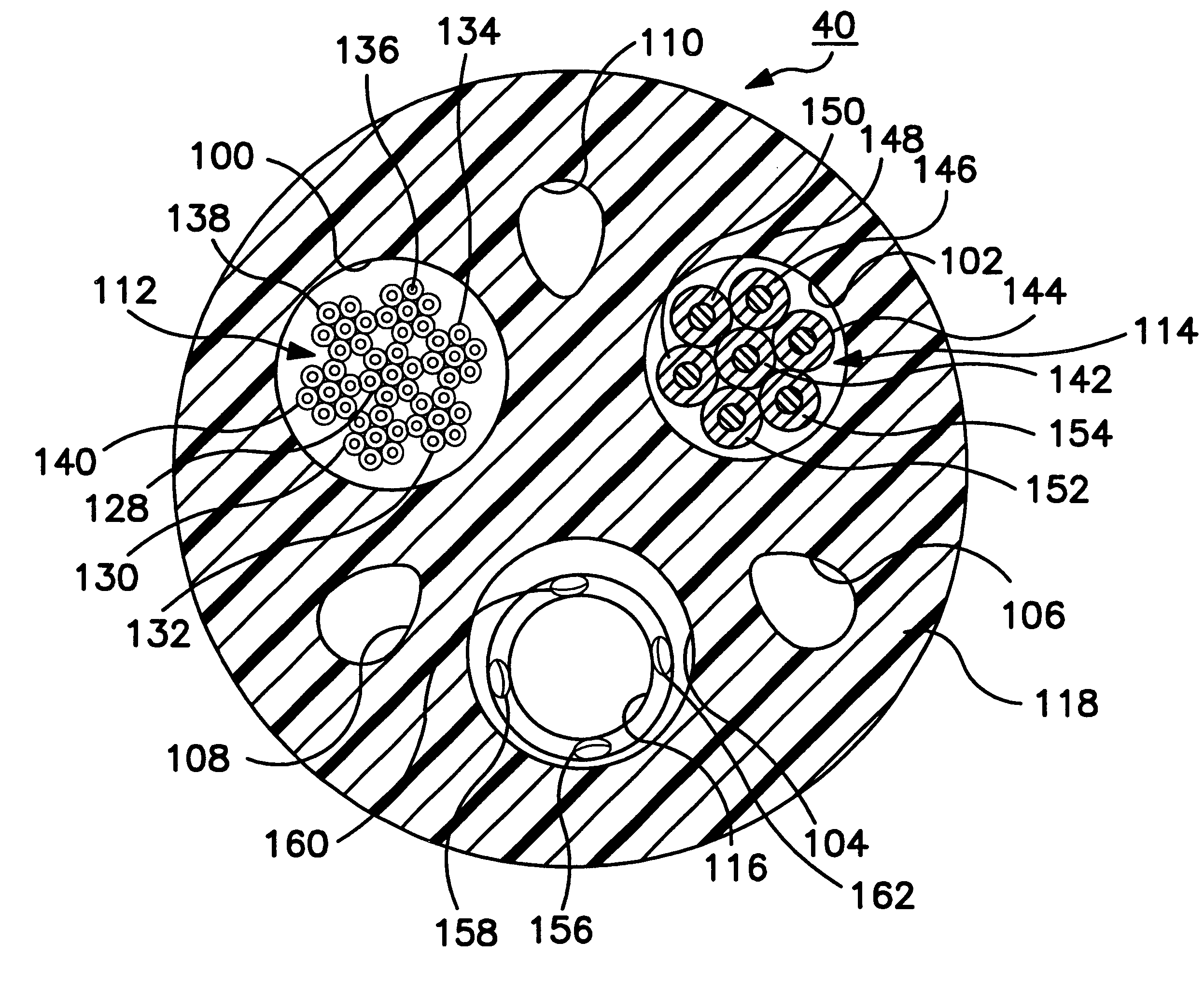
Medical electrical leads for sensing or electrical stimulation of body organs or tissues, particularly implantable cardiac leads for delivering pacing pulses and cardioversion / defibrillation shocks, and / or sensing the cardiac electrogram (EGM) or other physiologic data and their methods of fabrication are disclosed. A lead body sheath is co-extruded in a co-extrusion process using bio-compatible, electrically insulating, materials of differing durometers in differing axial sections thereof, resulting in a unitary lead body sheath having differing stiffness sections including axial segments or webs or lumen encircling rings or other structures in its cross-section. The lead body sheath is co-extruded to have an outer surface adapted to be exposed to the environment or to be enclosed within an outer sheath and to have a plurality of lead conductor lumens for receiving and enclosing a like plurality of lead conductors of the same or differing types. The lead body sheath can be co-extruded of a plurality of sheath segments containing a lead conductor lumen and formed of a first durometer material or of differing durometer materials. A web of a further durometer material can be co-extruded extending between the adjoining boundaries of the axial sheath segments and bonding the adjacent segments together. The lead body sheath can be tailored to exhibit differing bending stiffnesses away from the lead body sheath axis in selected polar directions around the 360° circumference of the sheath body.
Owner:MEDTRONIC INC
Subcutaneous cardioverter-defibrillator
ActiveUS20060247688A1Convenient to implantHeart defibrillatorsSubcutaneous electrodesThoracic structureThoracic cavity
SubQ ICDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with 1 or, optionally, 2 subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, 2 hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort.
Owner:MEDTRONIC INC
Vagal stimulation for cardioversion of atrial fibrillation
InactiveUS20080091241A1Reduce frequencyIncreased riskSpinal electrodesHeart defibrillatorsBlood flowThrombus
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
System and method for treating an adverse cardiac condition using combined pacing and drug delivery
InactiveUS6941168B2Reduce riskImprove pumping efficiencyHeart defibrillatorsHeart stimulatorsRegimenCardiac arrhythmia
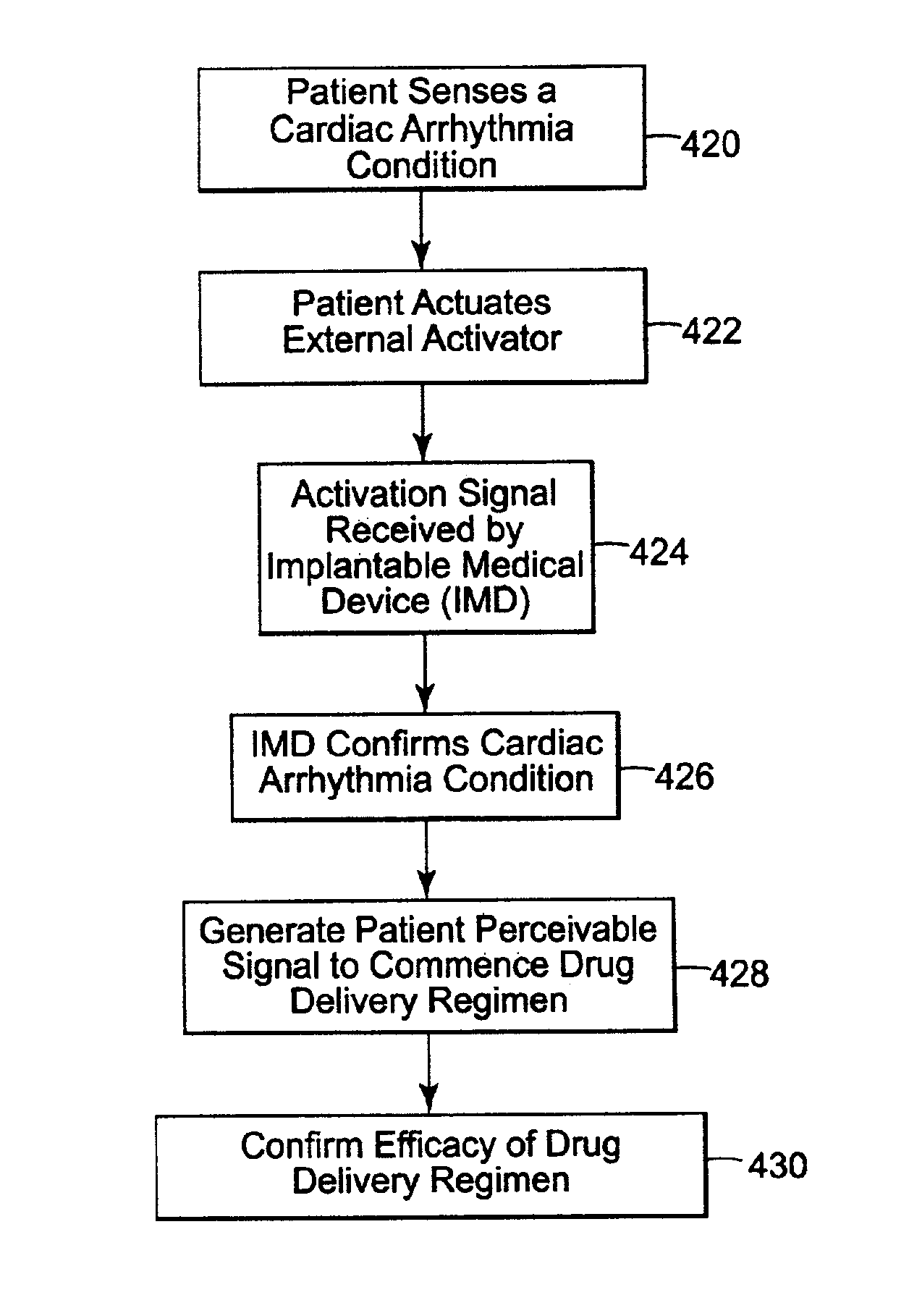
A system and method of treating an adverse cardiac condition, such as cardiac arrhythmia or a non-arrhythmic event, involves producing, by use of a patient actuatable non-implanted activator, an activation signal in response to a patient sensing a perceived adverse cardiac condition. The method further involves confirming, by an implantable medical device provided within the patient, that the patient is experiencing an actual adverse cardiac condition. A perceivable initiating signal instructing the patient or a physician to commence with a drug delivery regimen to treat the actual cardiac adverse condition is generated by the non-implanted activator. In one approach, the implantable medical device operates in a safe mode of pacing during drug treatment of the actual adverse cardiac condition. In another approach, an appropriate pacing, cardioversion or defibrillation regimen is initiated to treat the actual adverse cardiac condition.
Owner:CARDIAC PACEMAKERS INC
Suppression of high rate pacing for reducing myocardial ischemic irritability
InactiveUS20060247700A1Increased myocardial irritabilityIncrease irritabilityElectrocardiographyHeart stimulatorsHigh rateCardiac pacemaker electrode
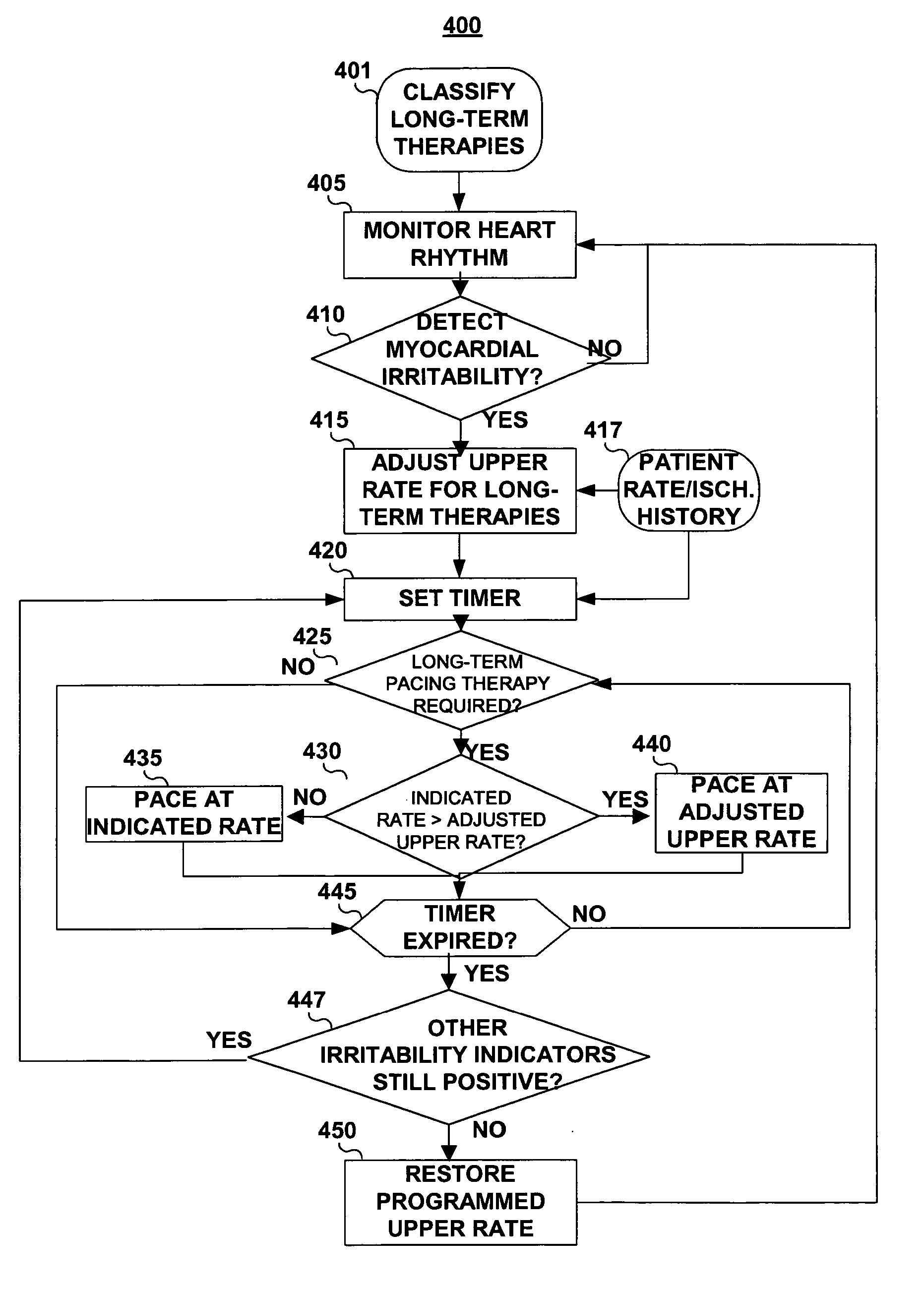
A medical device capable of delivering automatic rate-adjusting pacing therapies is provided having an adjustable upper rate limit responsive to an indication of myocardial irritability. The device, which may be embodied as a pacemaker, a pacemaker / cardioverter / defibrillator, or the like, responds to the detection of an arrhythmia as an indicator of myocardial irritability by adjusting an upper rate limit. The adjusted upper rate limit is applied as the maximum allowable pacing rate during delivery of any pacing therapies previously defined as “long-term” pacing therapies.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com