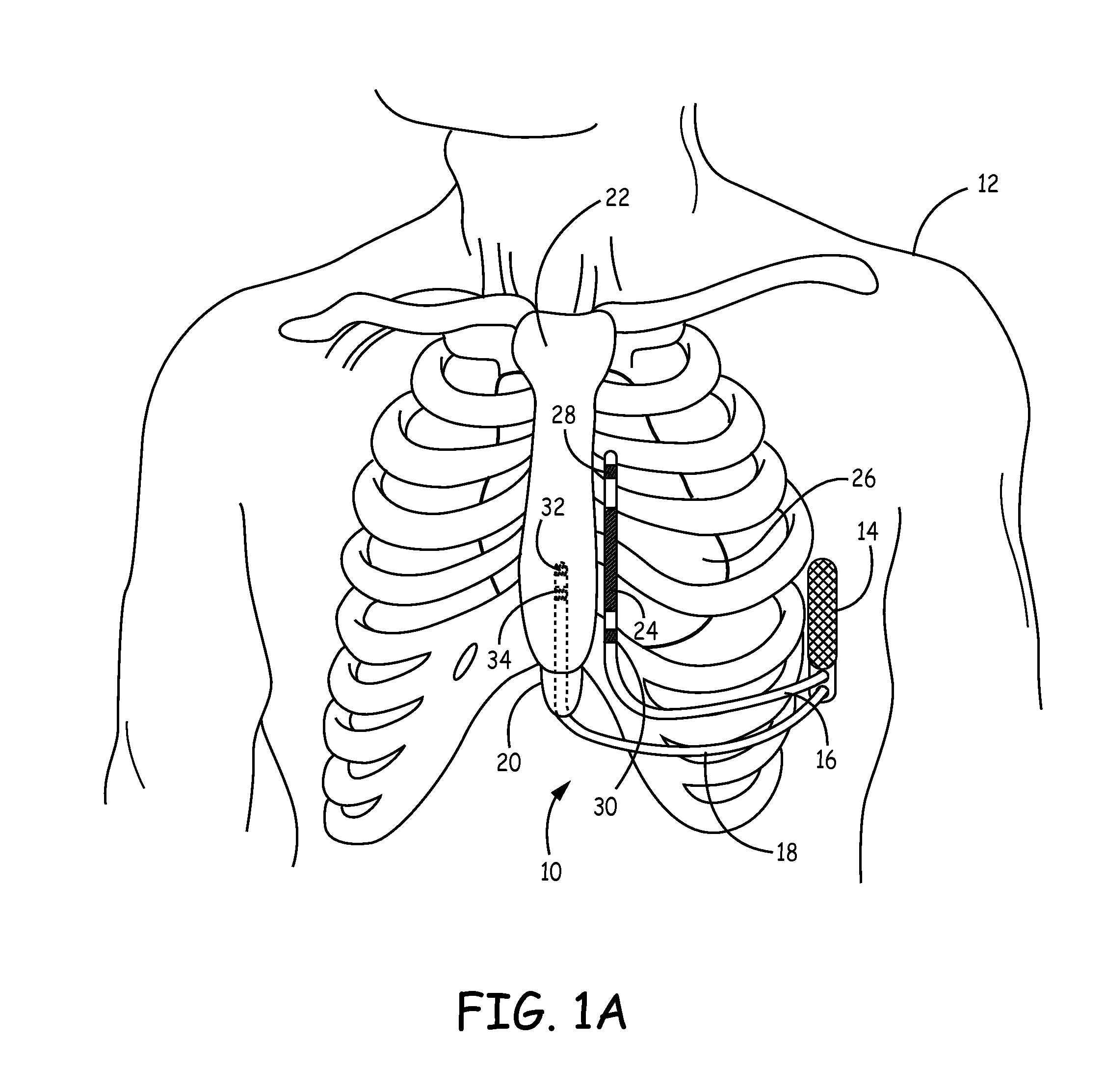
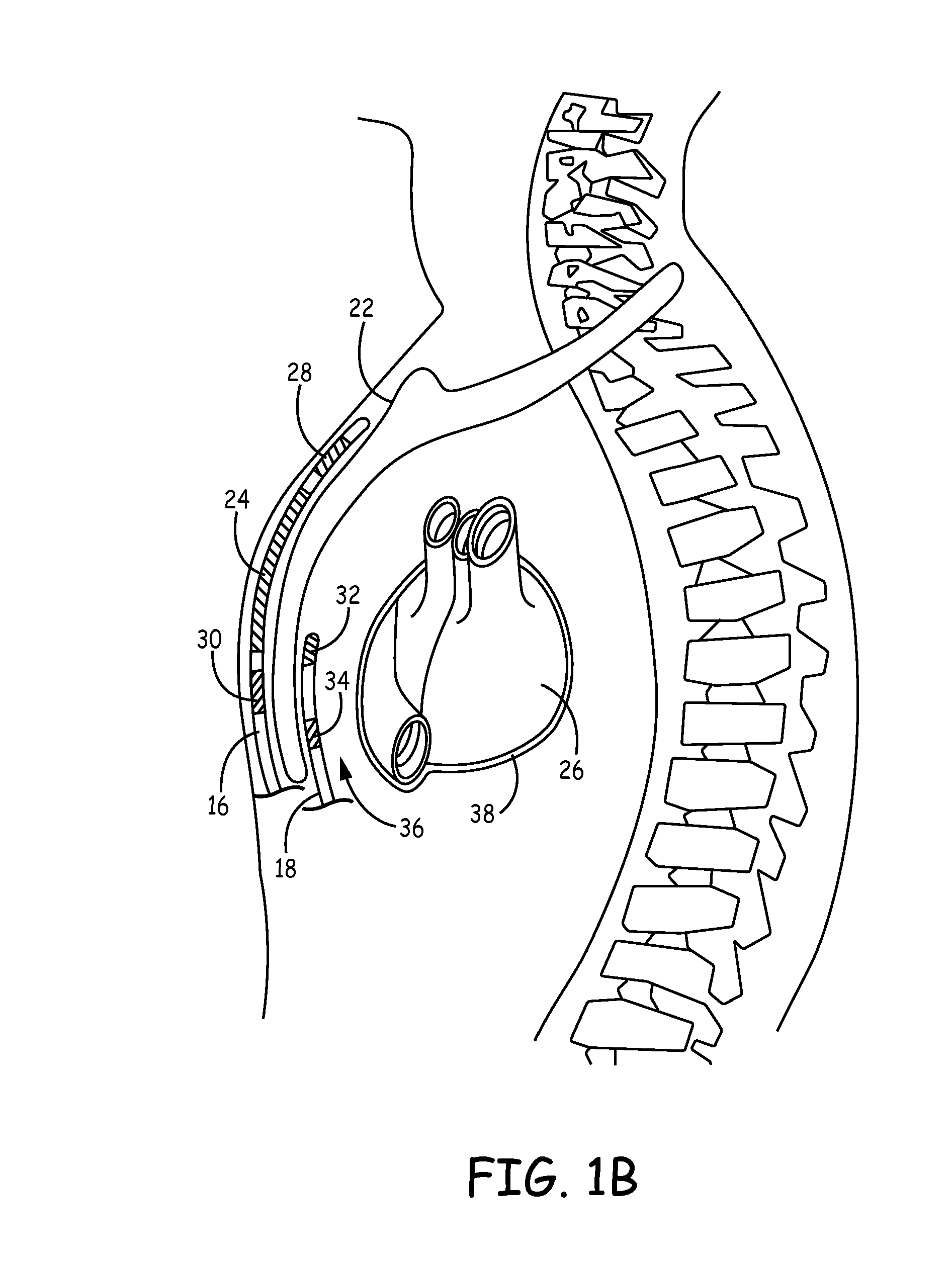
Patents
Literature
193 results about "Implantable cardioverter-defibrillator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
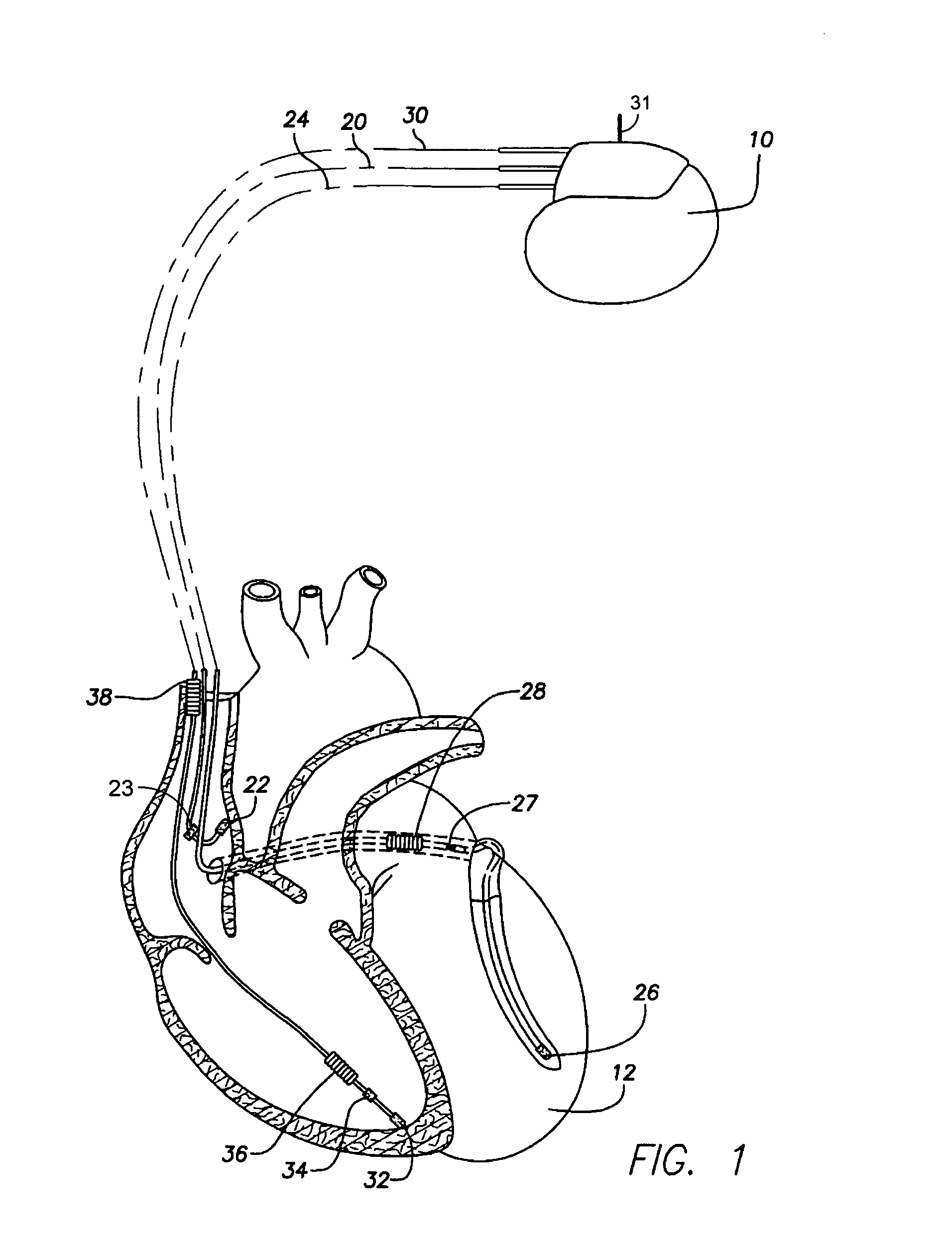
An implantable cardioverter-defibrillator (ICD) or automated implantable cardioverter defibrillator (AICD) is a device implantable inside the body, able to perform cardioversion, defibrillation, and (in modern versions) pacing of the heart. The device is therefore capable of correcting most life-threatening cardiac arrhythmias. The ICD is the first-line treatment and prophylactic therapy for patients at risk for sudden cardiac death due to ventricular fibrillation and ventricular tachycardia. Current devices can be programmed to detect abnormal heart rhythms and deliver therapy via programmable antitachycardia pacing in addition to low-energy and high-energy shocks.
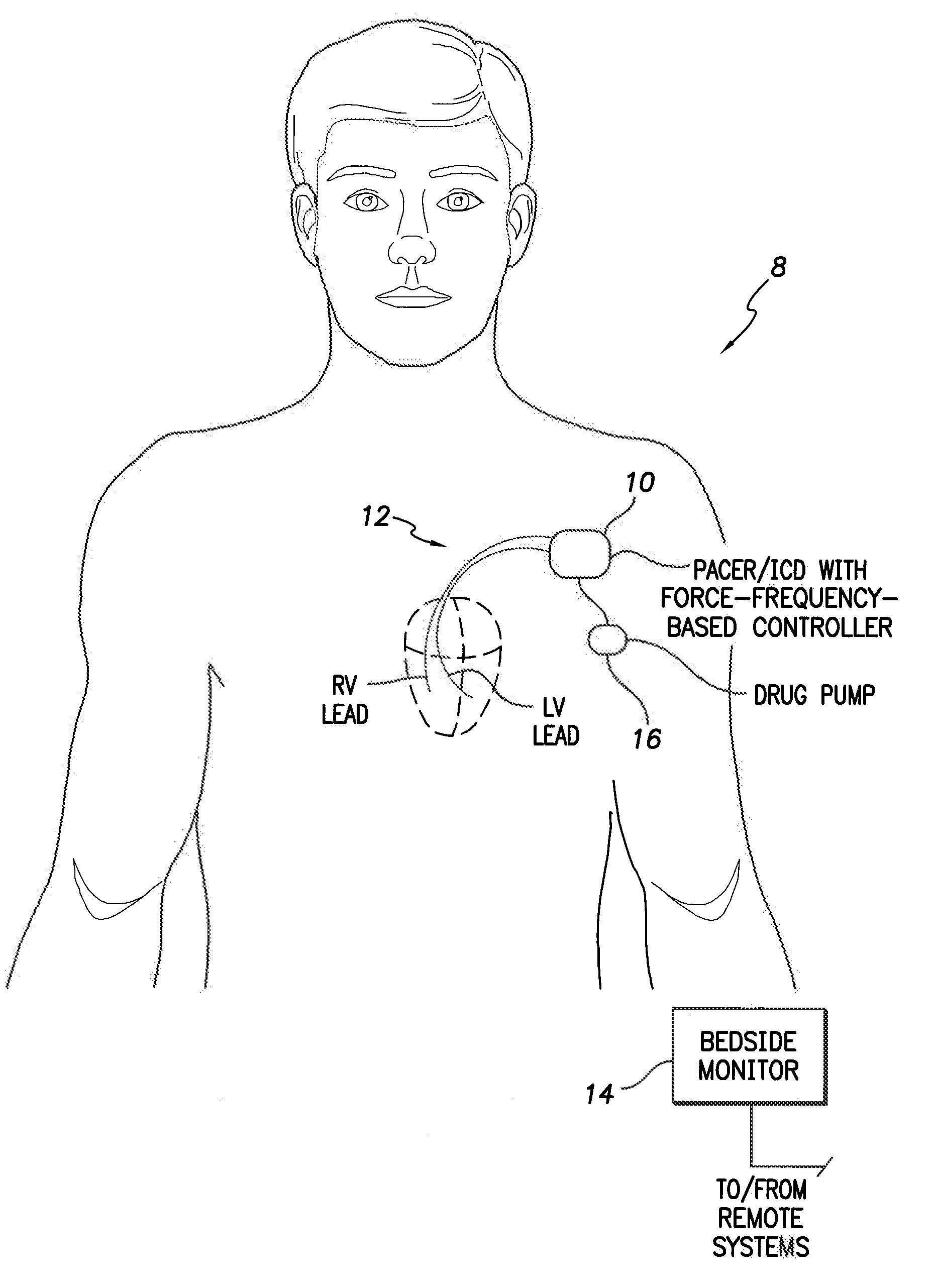
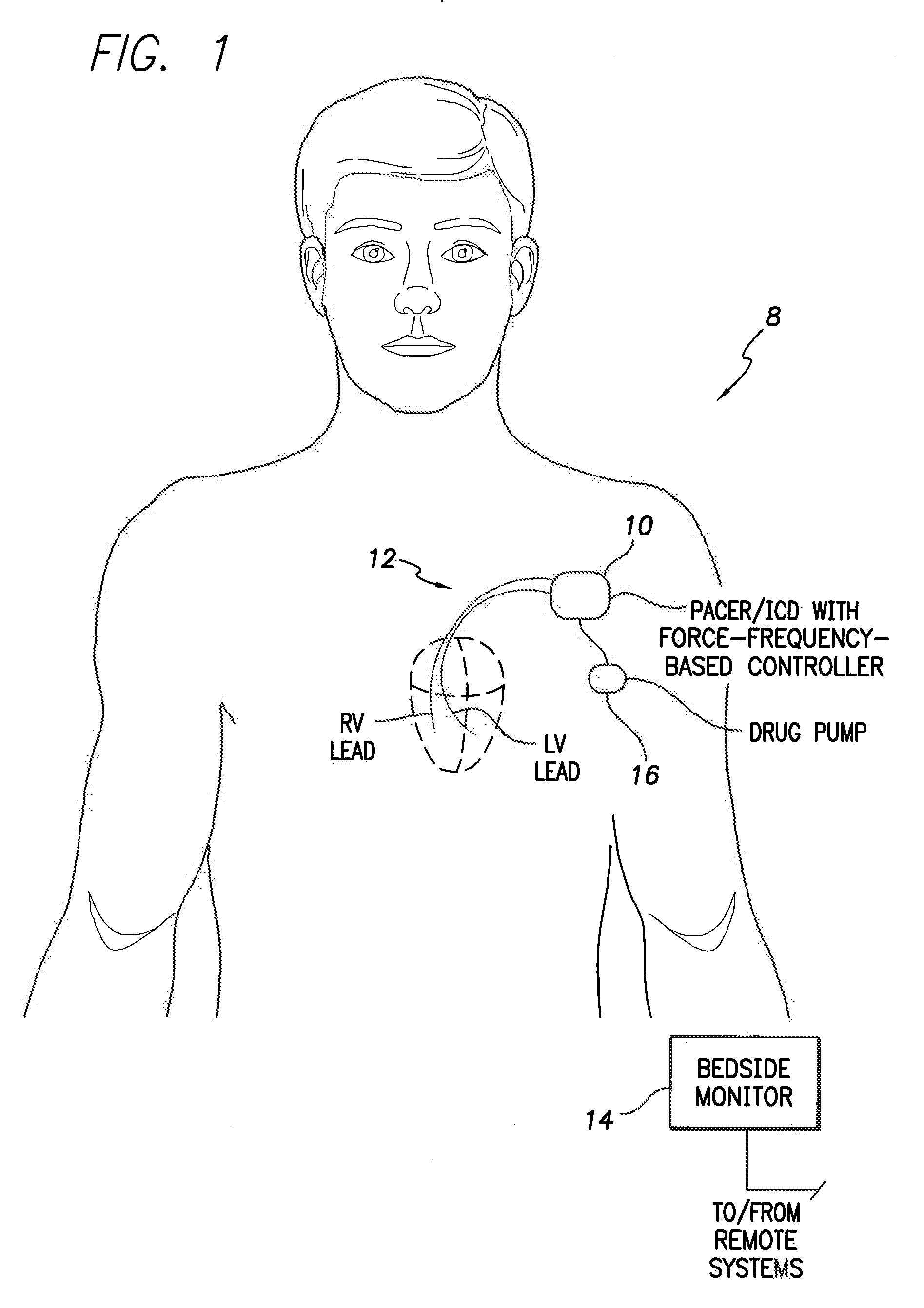
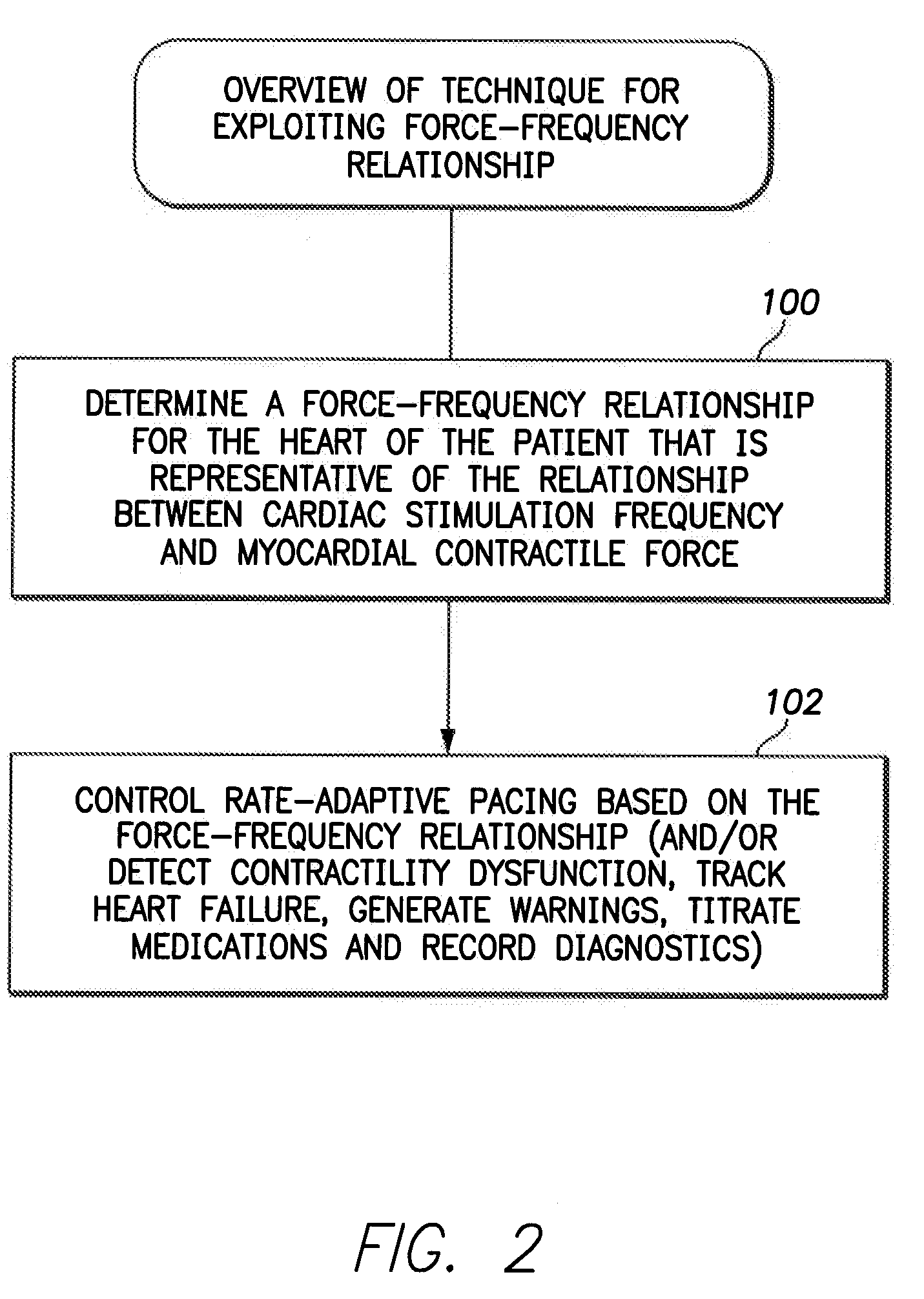
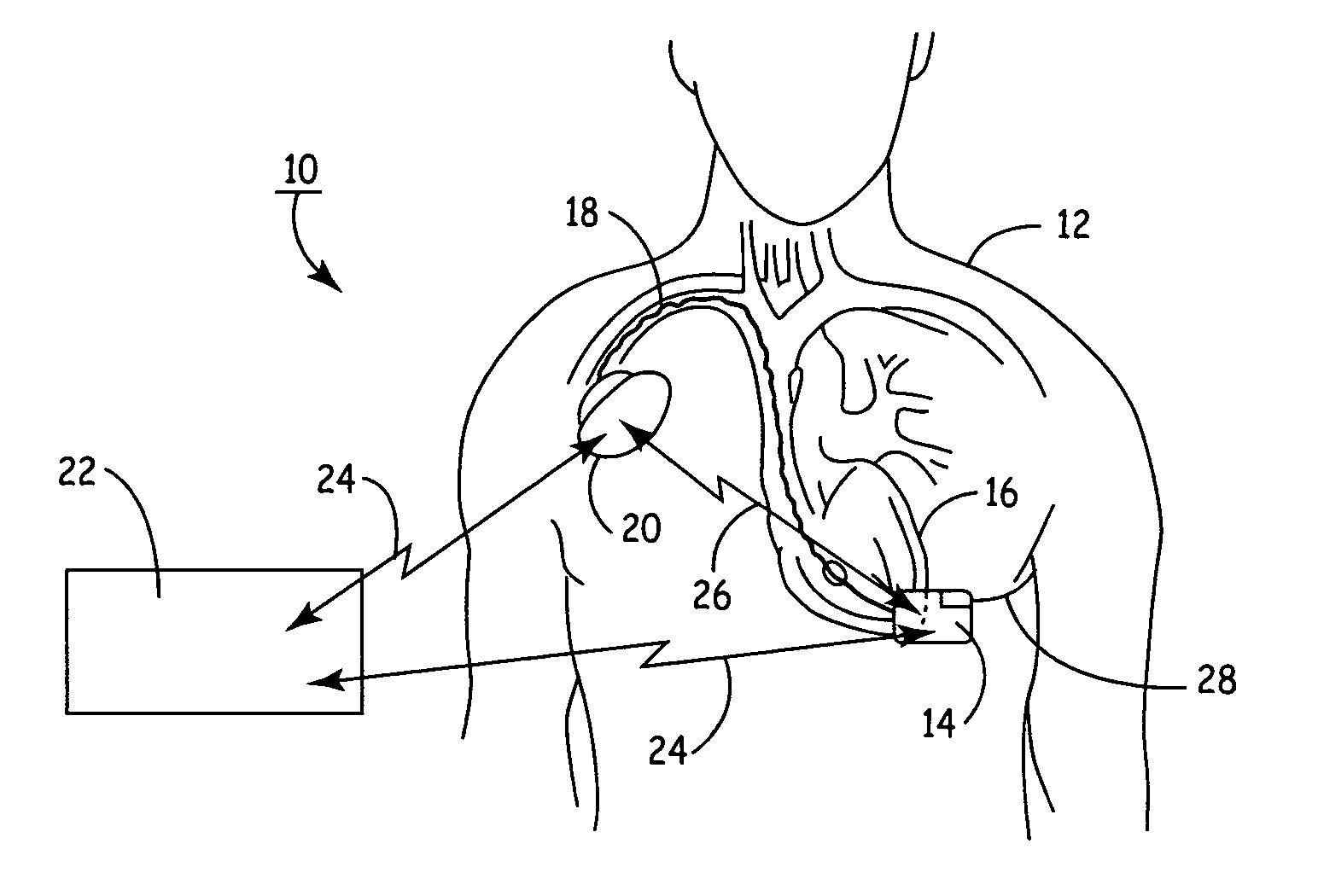
System and method for controlling rate-adaptive pacing based on a cardiac force-frequency relation detected by an implantable medical device
InactiveUS20100234906A1Reduce slopeDecrease in abscissaCatheterHeart stimulatorsCardiac pacemaker electrodeImplantable cardioverter-defibrillator
Techniques are provided for use in controlling rate-adaptive pacing within implantable medical devices such as pacemakers or implantable cardioverter-defibrillators (ICDs). In one example, a force-frequency relationship is determined for the heart of the patient, which is representative of the relationship between cardiac stimulation frequency and myocardial contractile force. To this end, various parameters are detected for use as surrogates for contractile force, including selected systolic pressure parameters and cardiogenic impedance parameters. Rate-adaptive pacing is then controlled based on the detected force-frequency relationship to, for example, deactivate rate-adaptive pacing if the slope and / or abscissa of the force-frequency relationship indicates significant contractility dysfunction within the patient. In other examples, rather than deactivating rate-adaptive pacing, control parameters are adjusted to render the rate-adaptive pacing less aggressive. In still other examples, trends in the slope and / or abscissa of the force-frequency relationship are monitored to detect contractility dysfunction and / or heart failure and titrate medications accordingly.
Owner:PACESETTER INC
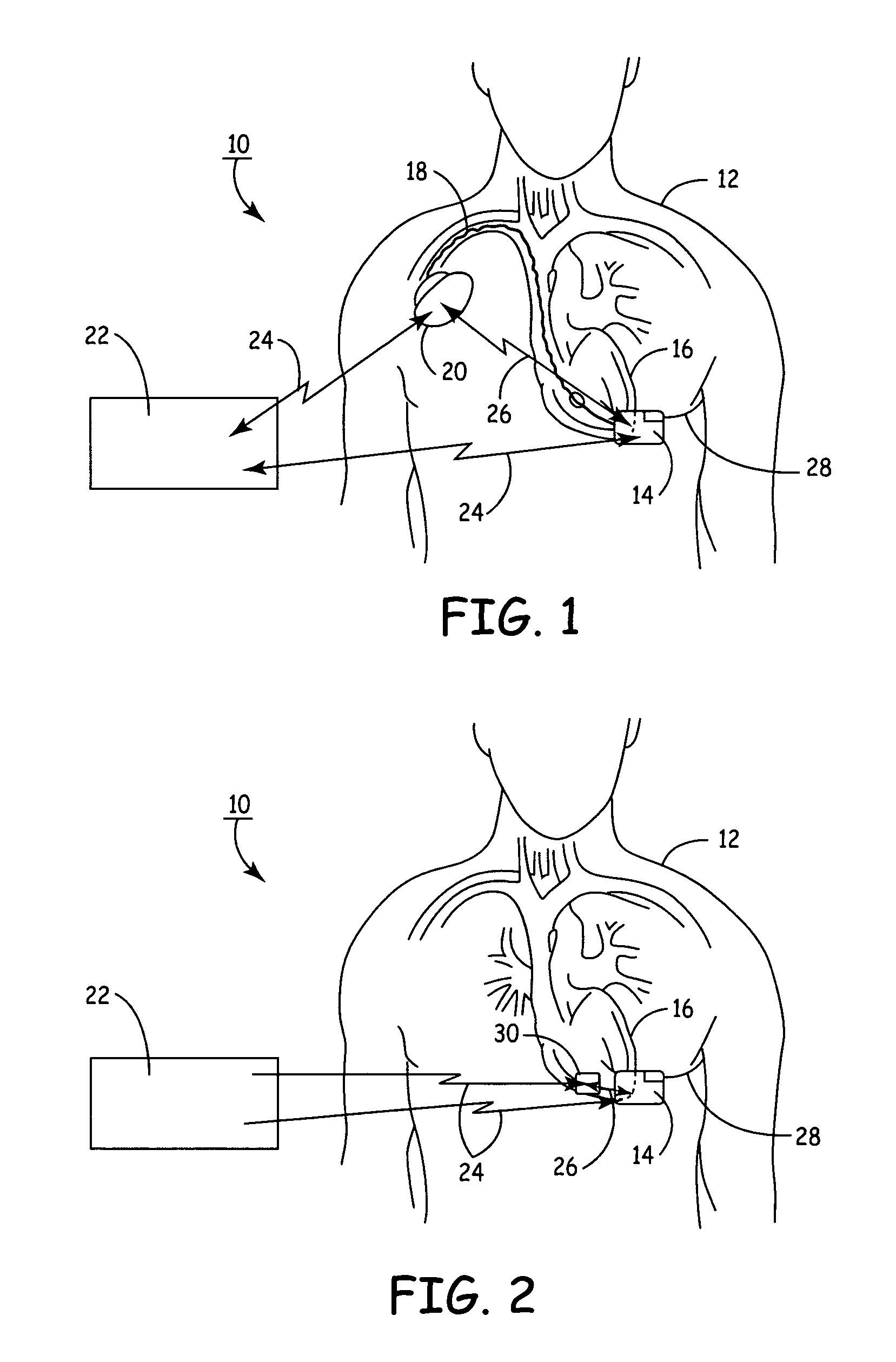
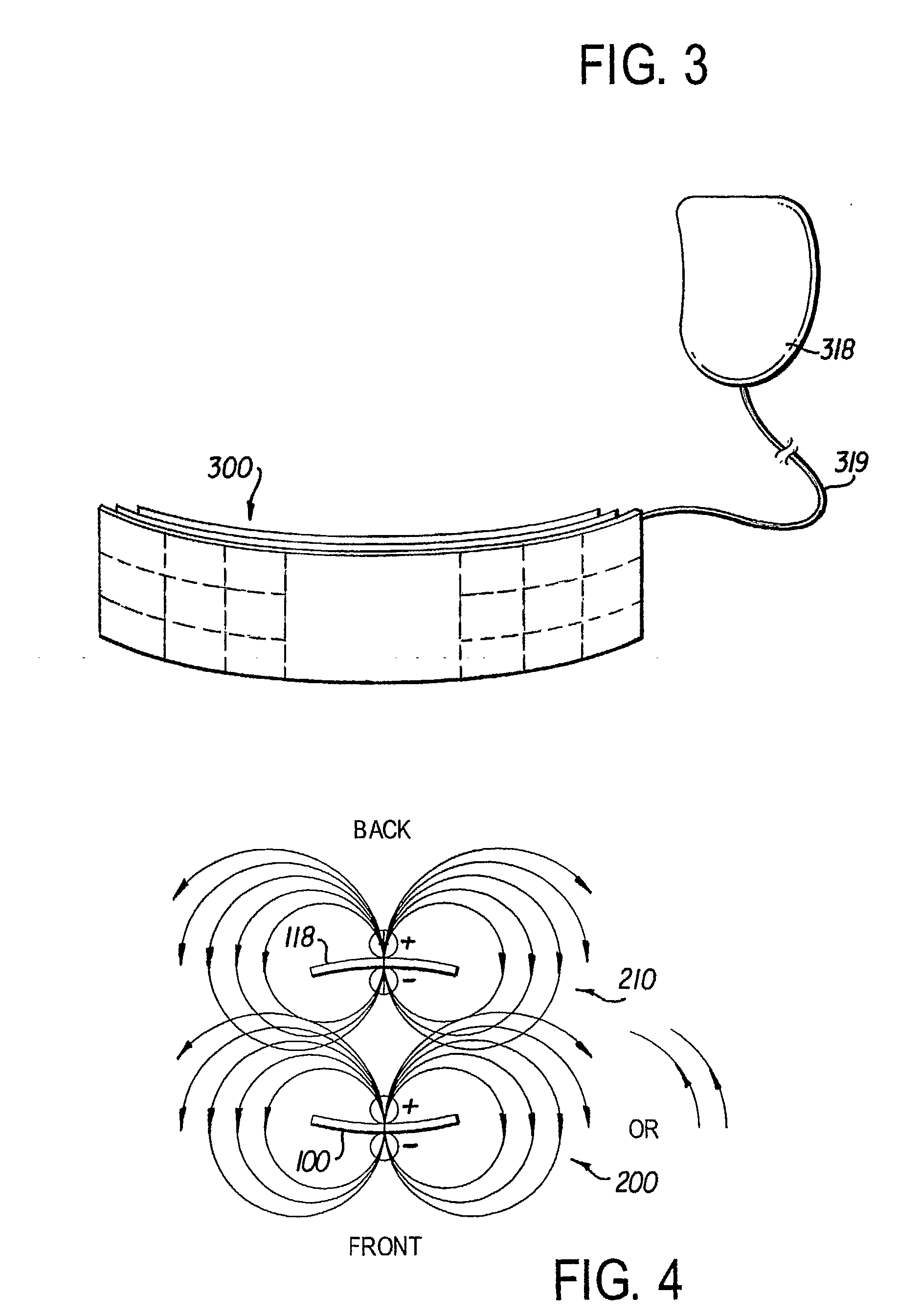
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS20060241701A1Relieve painSafe and effective operationHeart defibrillatorsSubcutaneous implantationCardiac pacemaker electrode
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDS) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
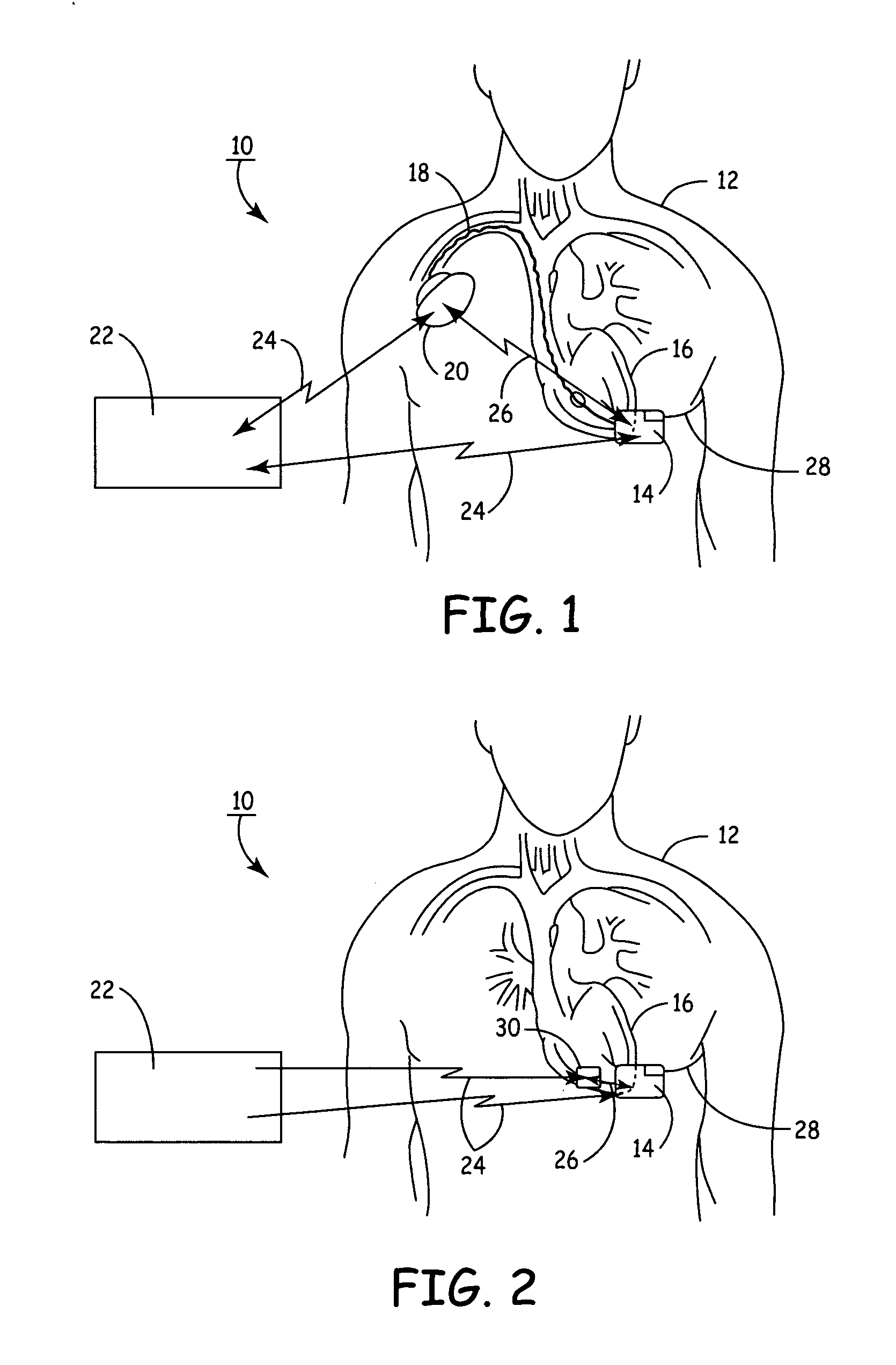
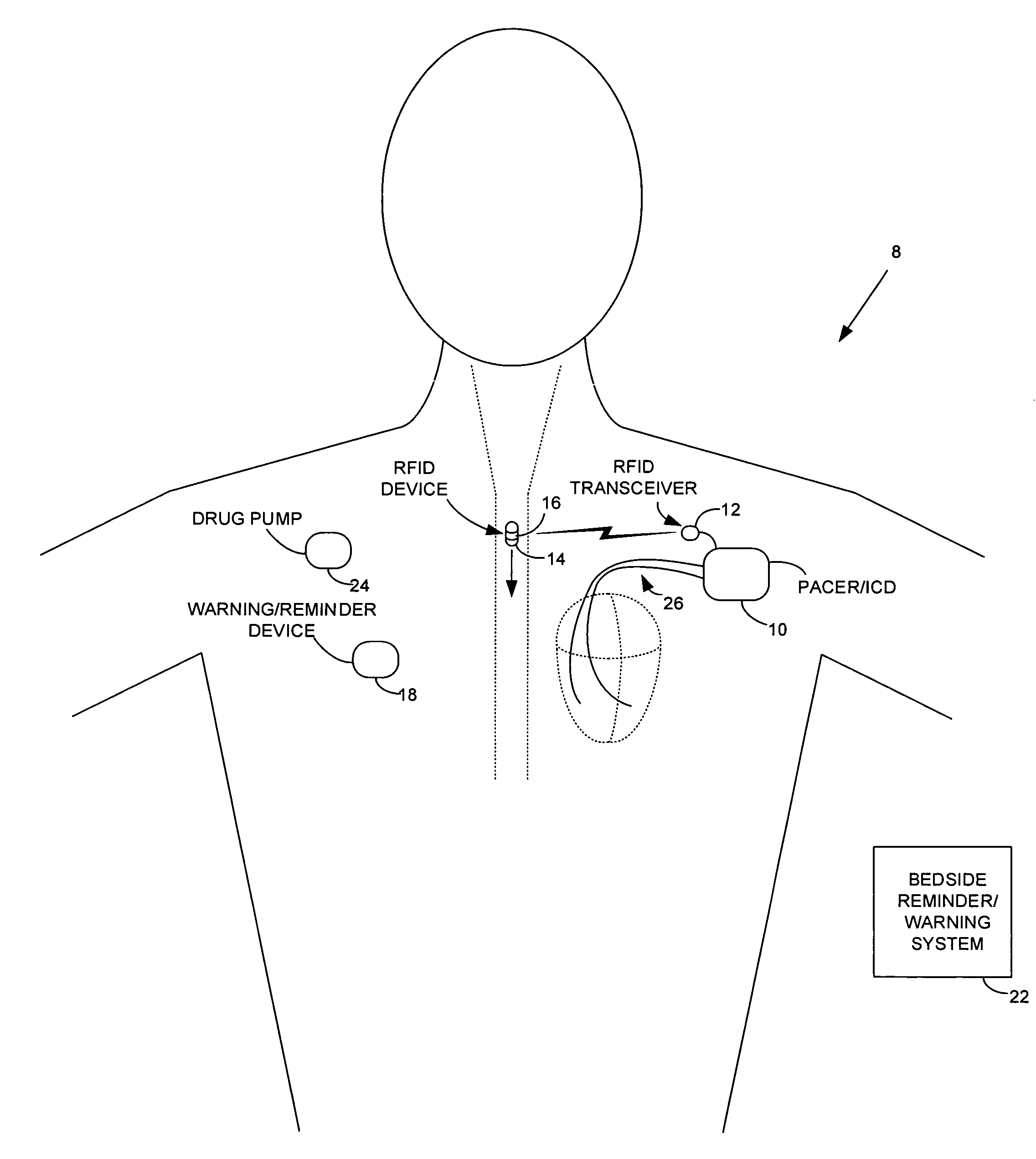
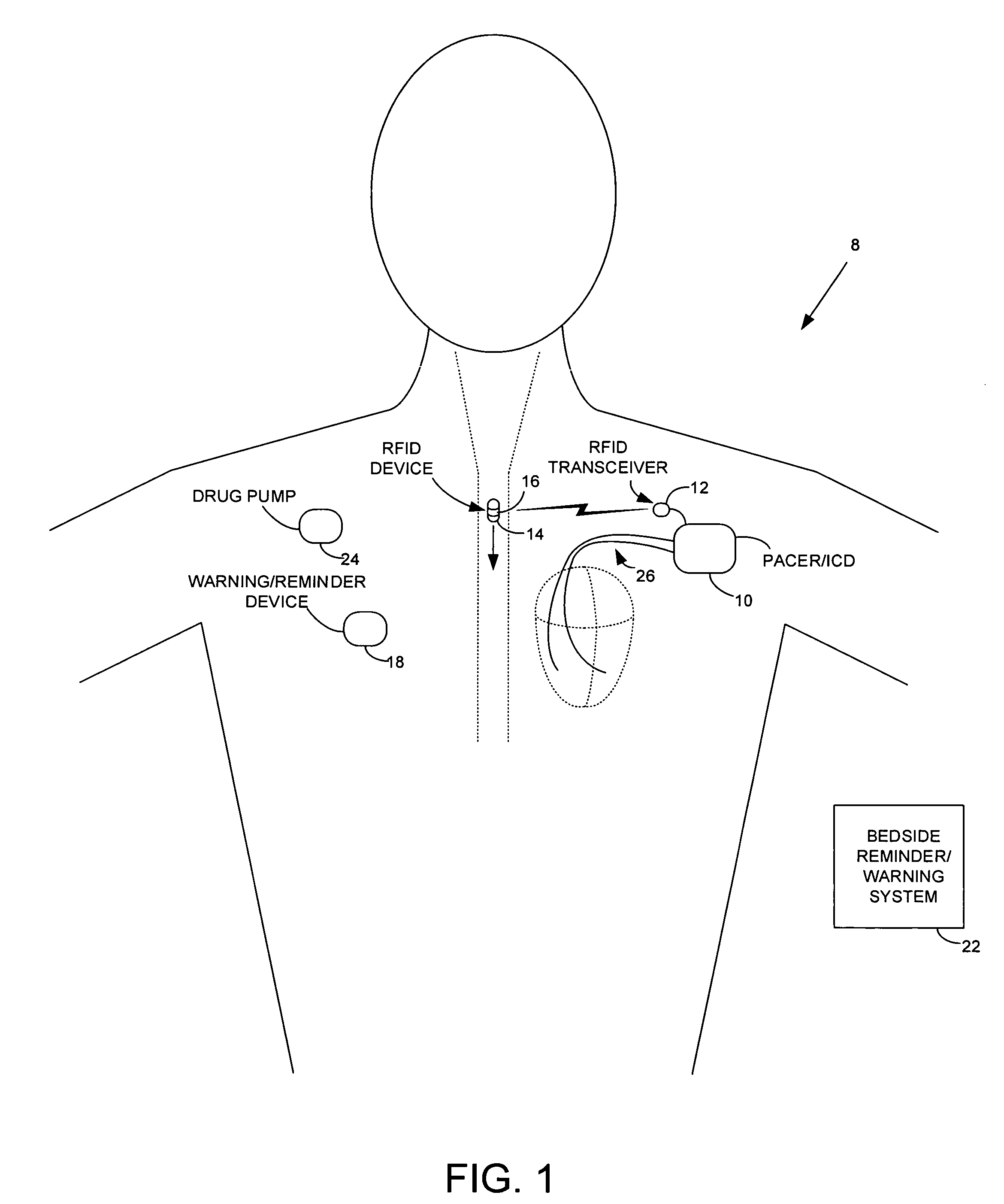
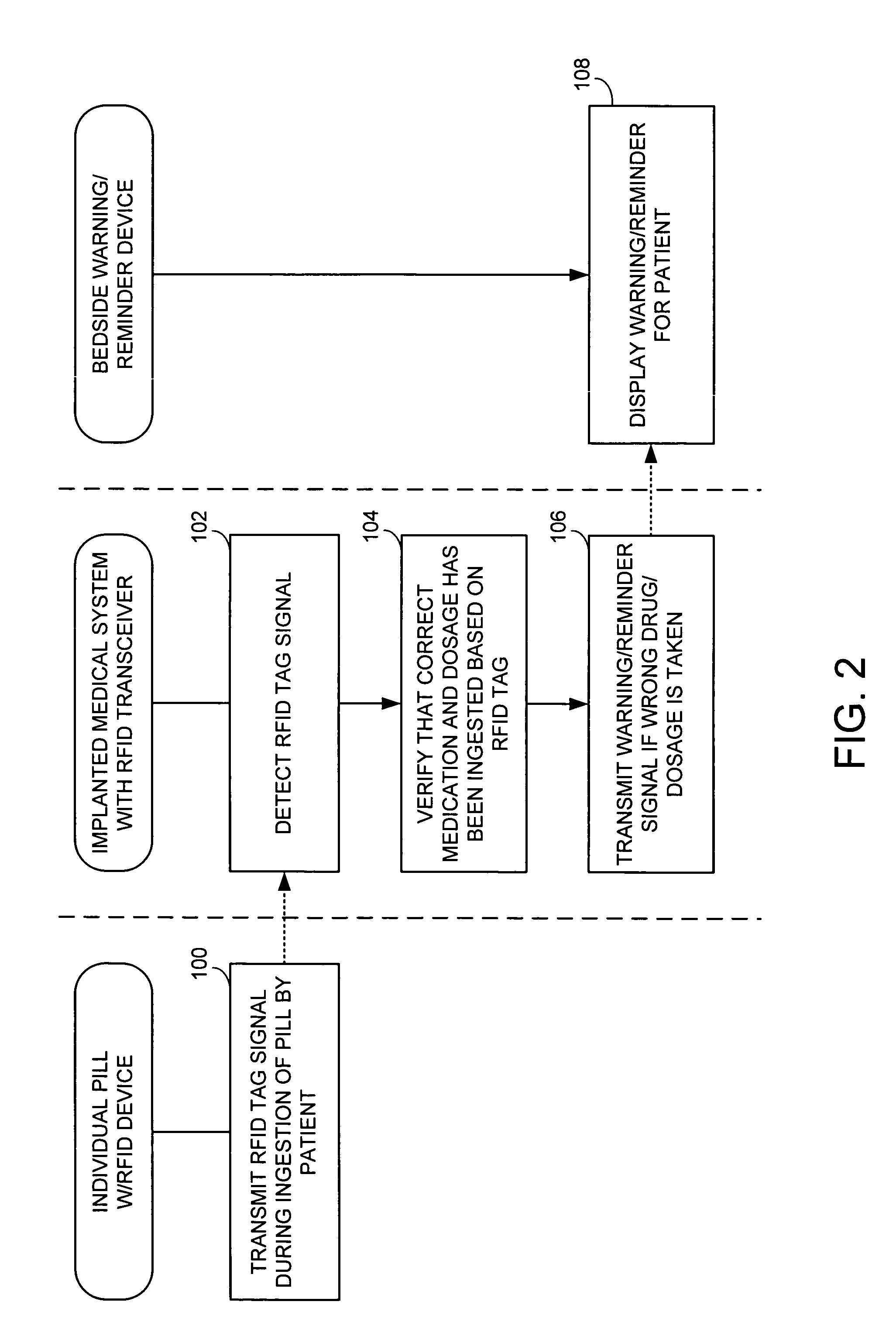
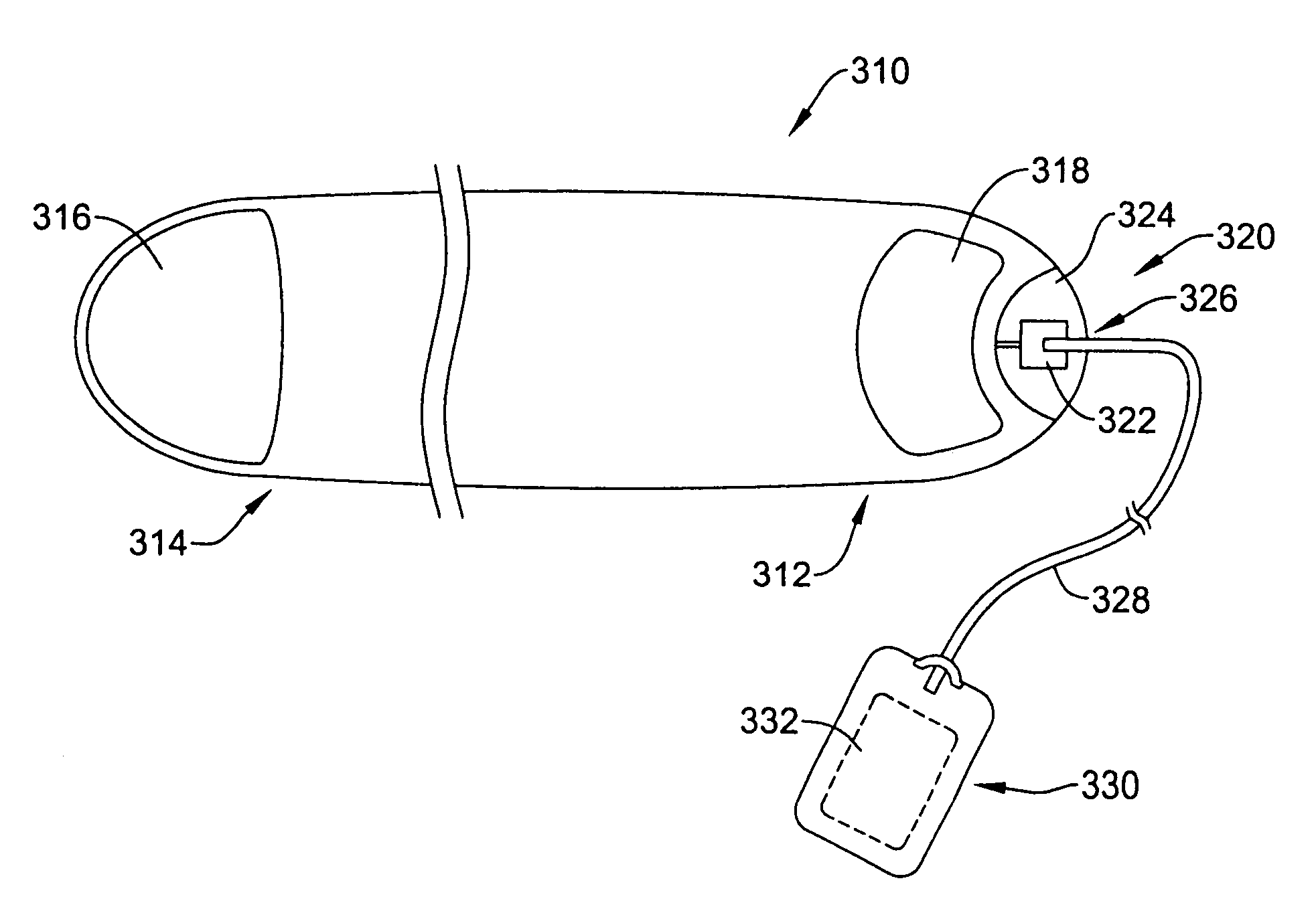
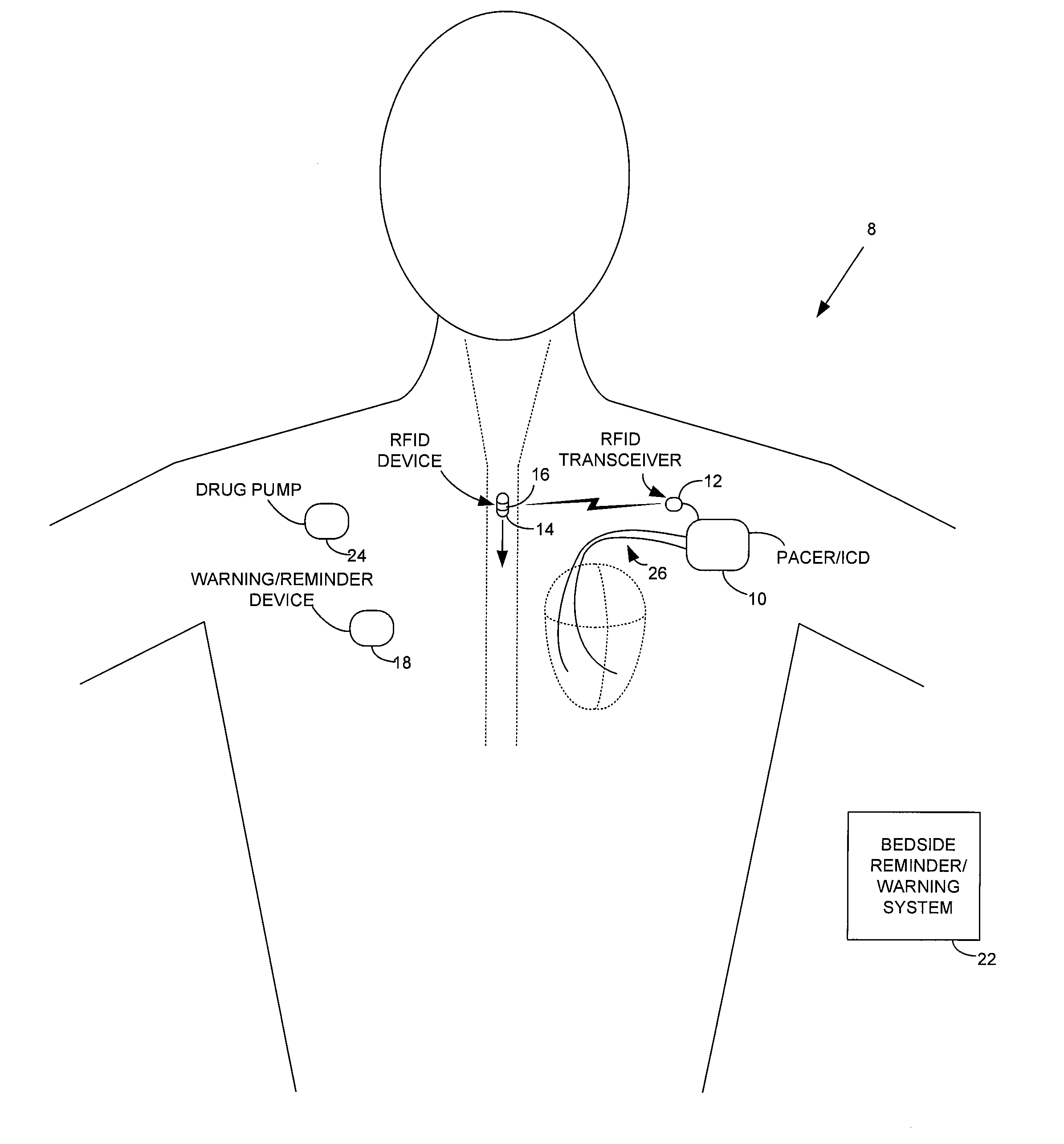
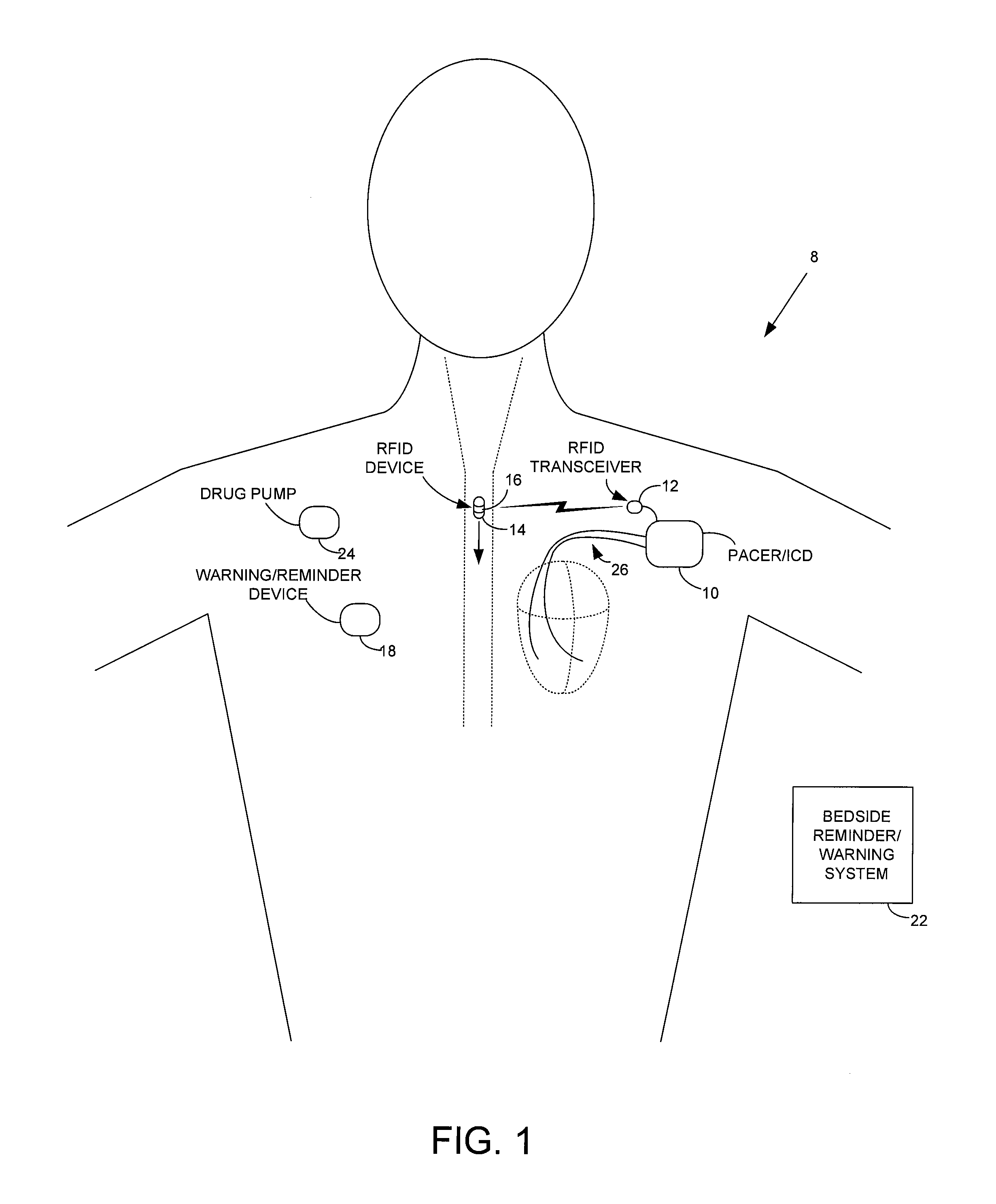
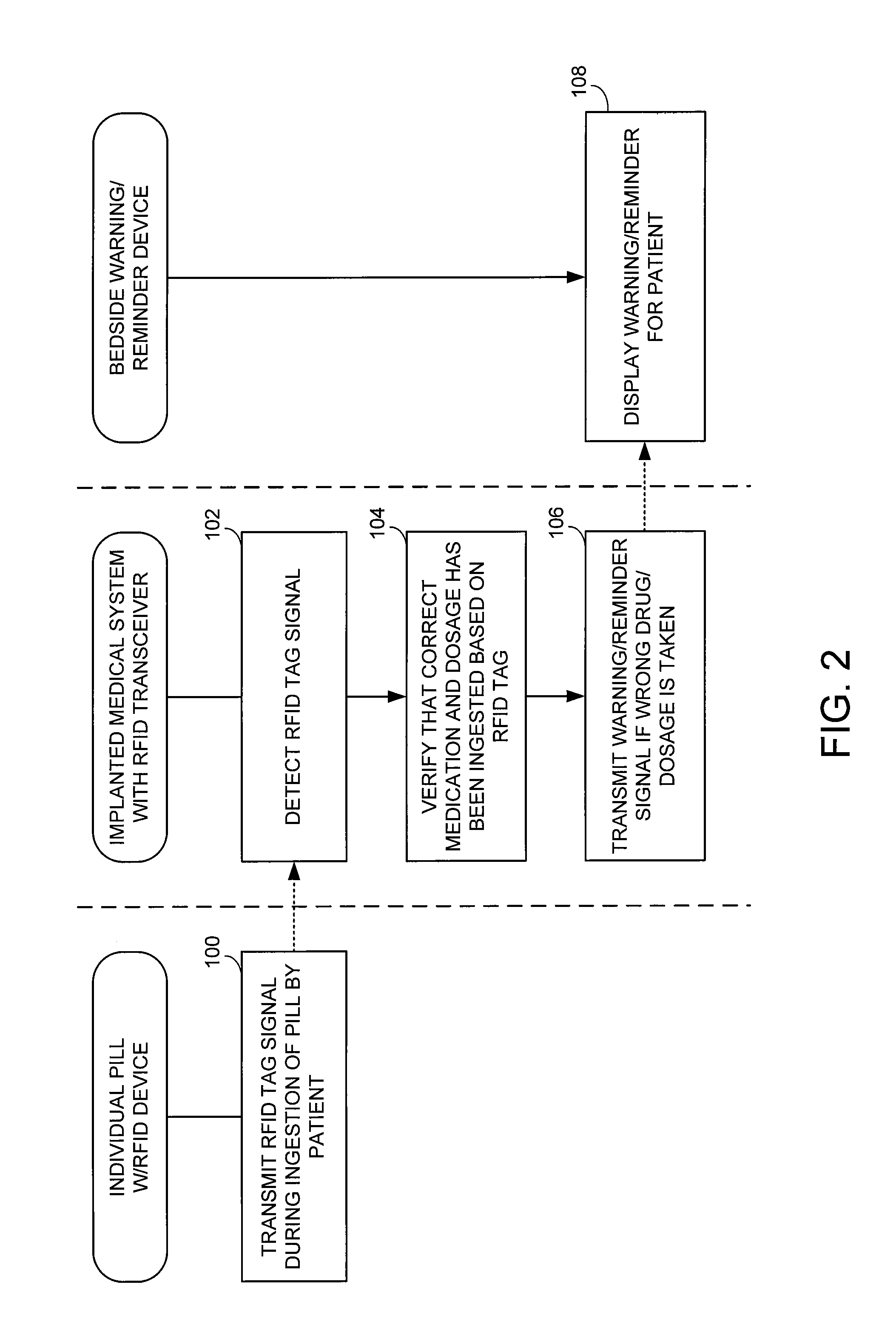
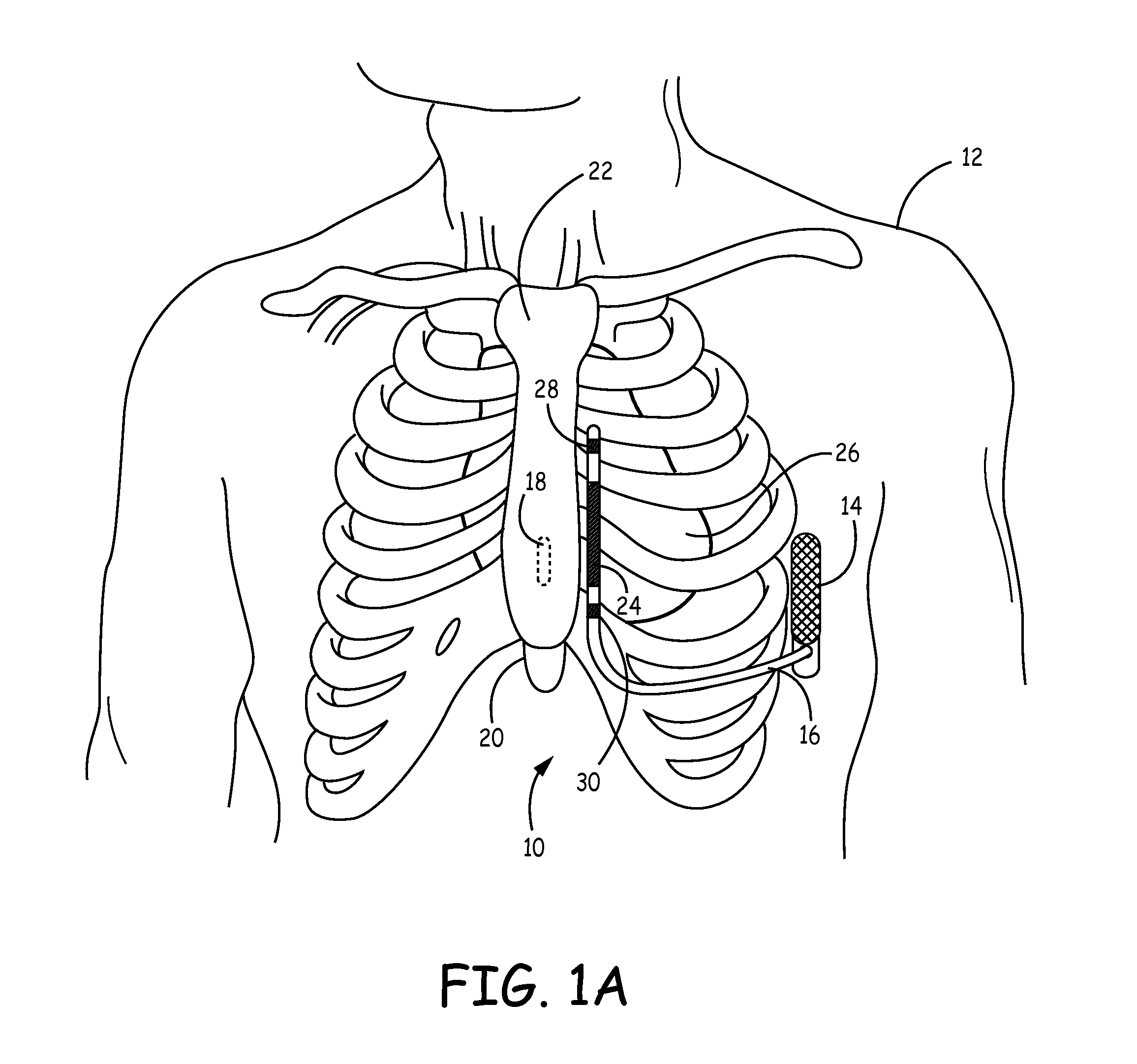
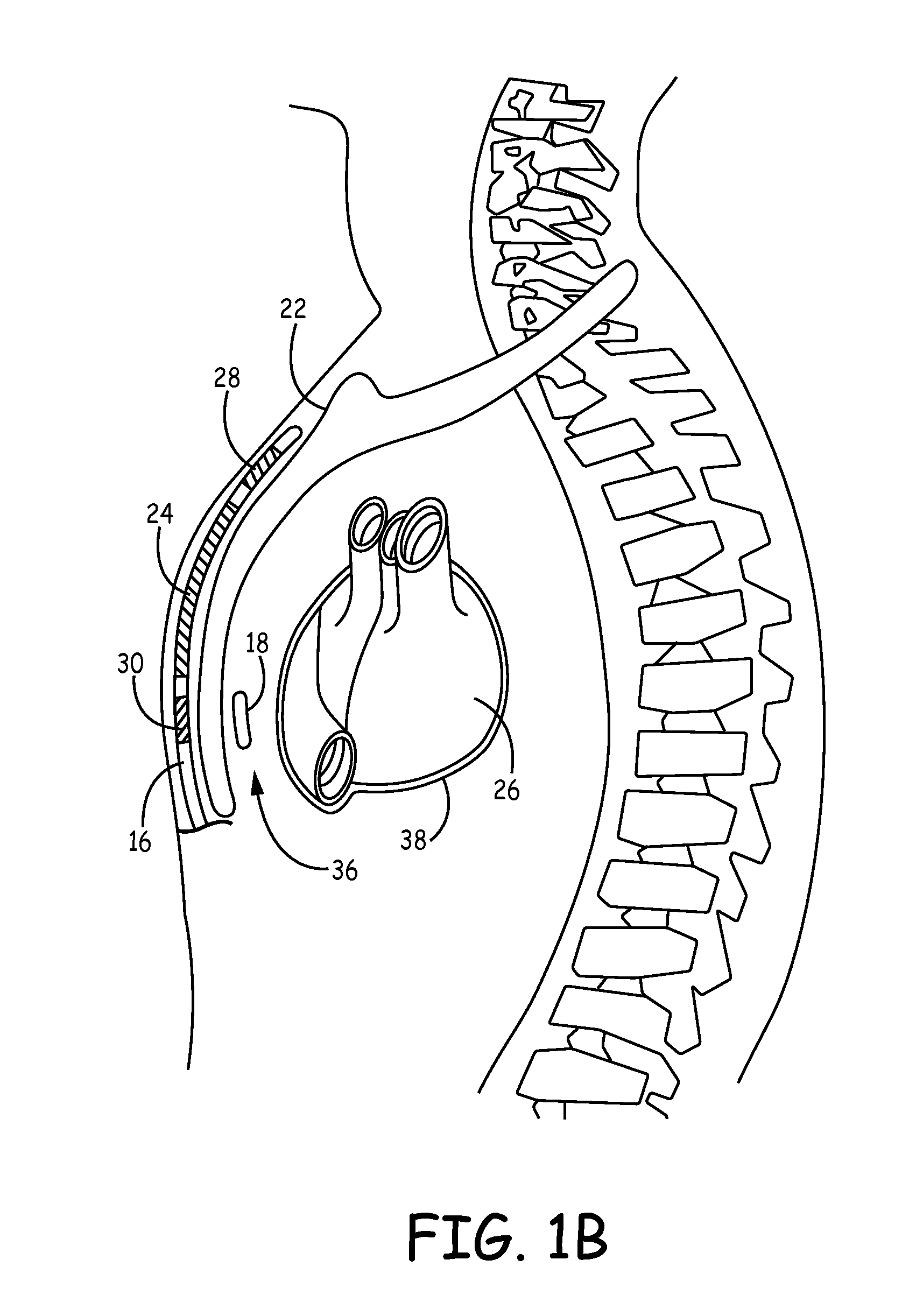
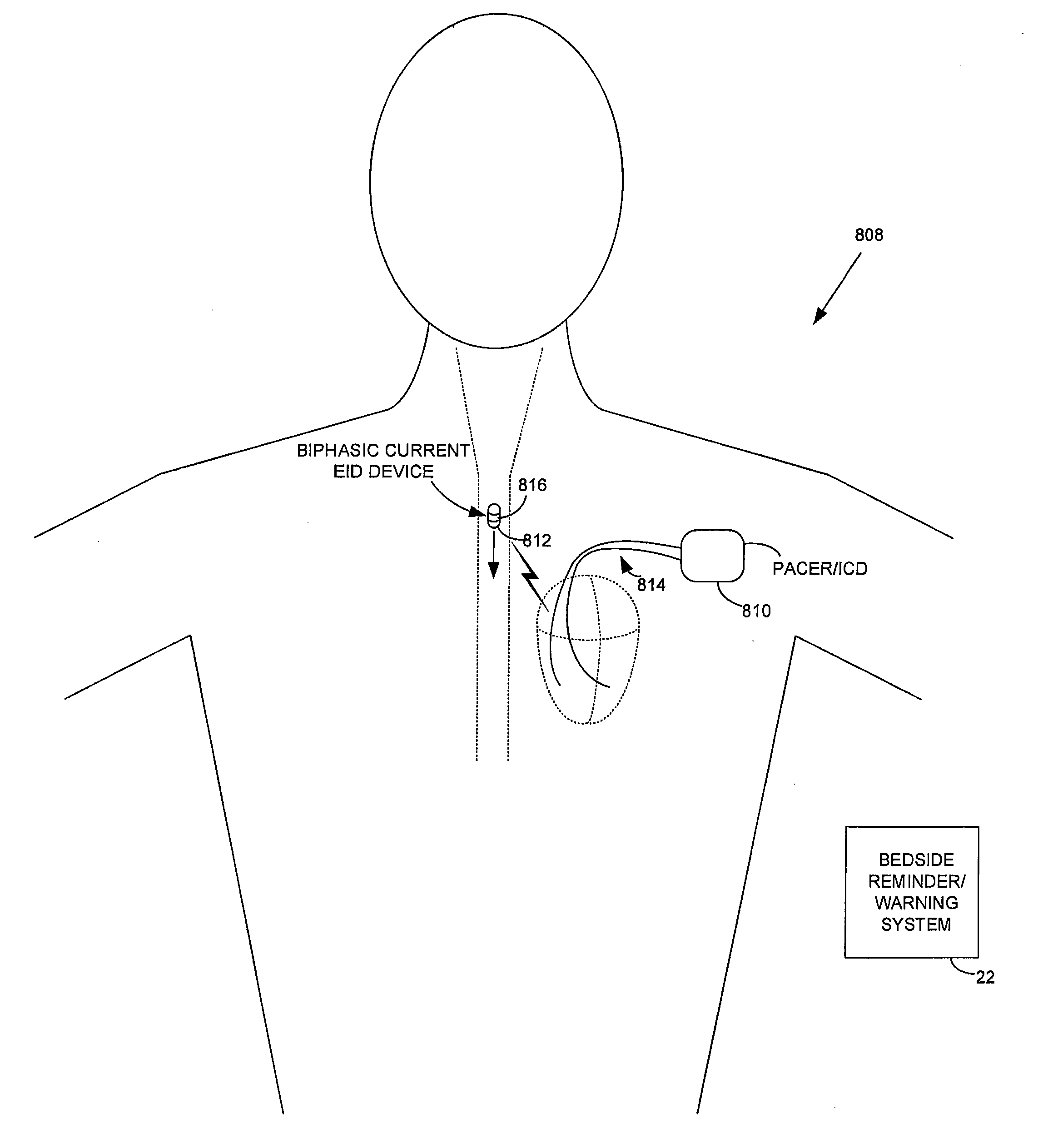
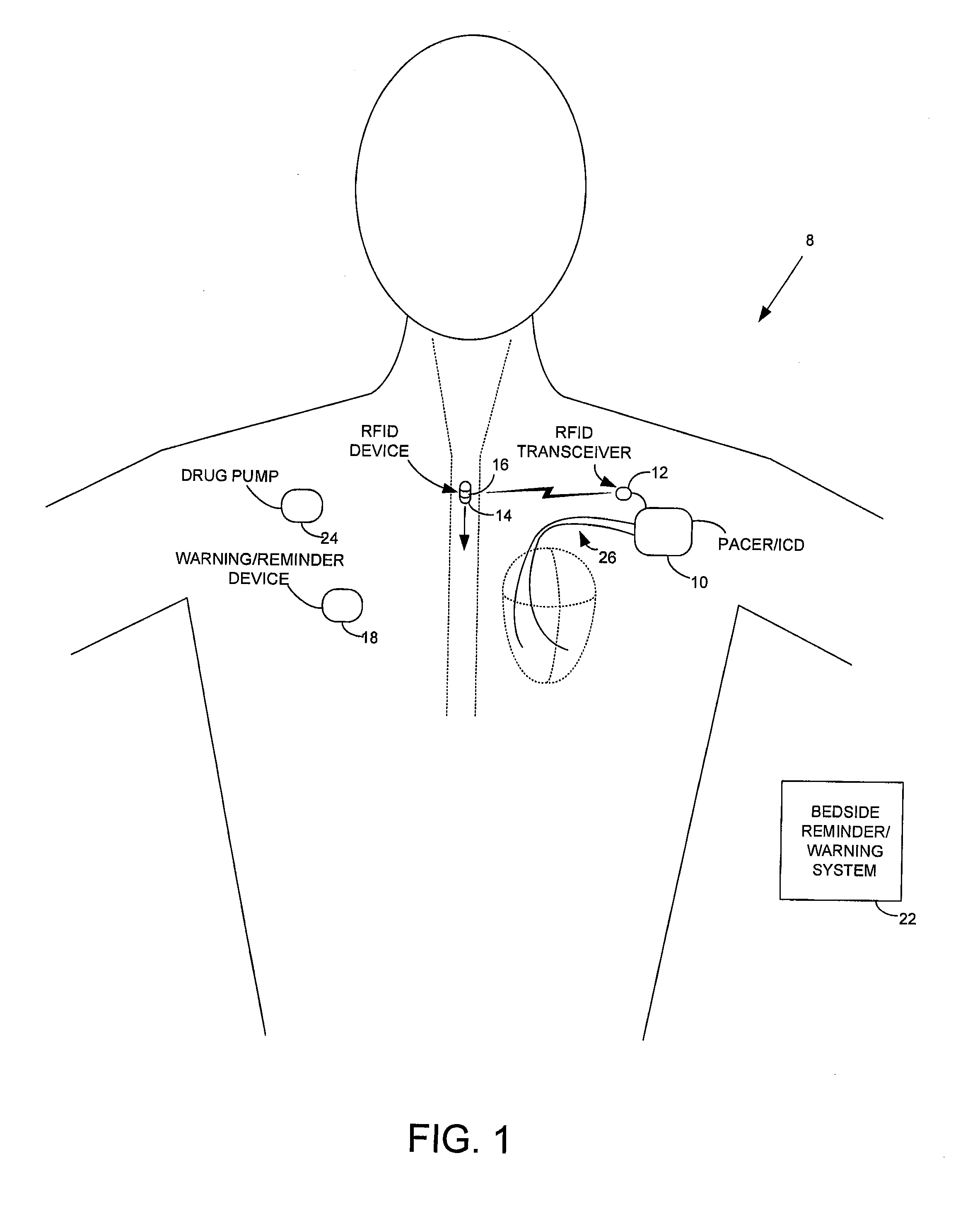
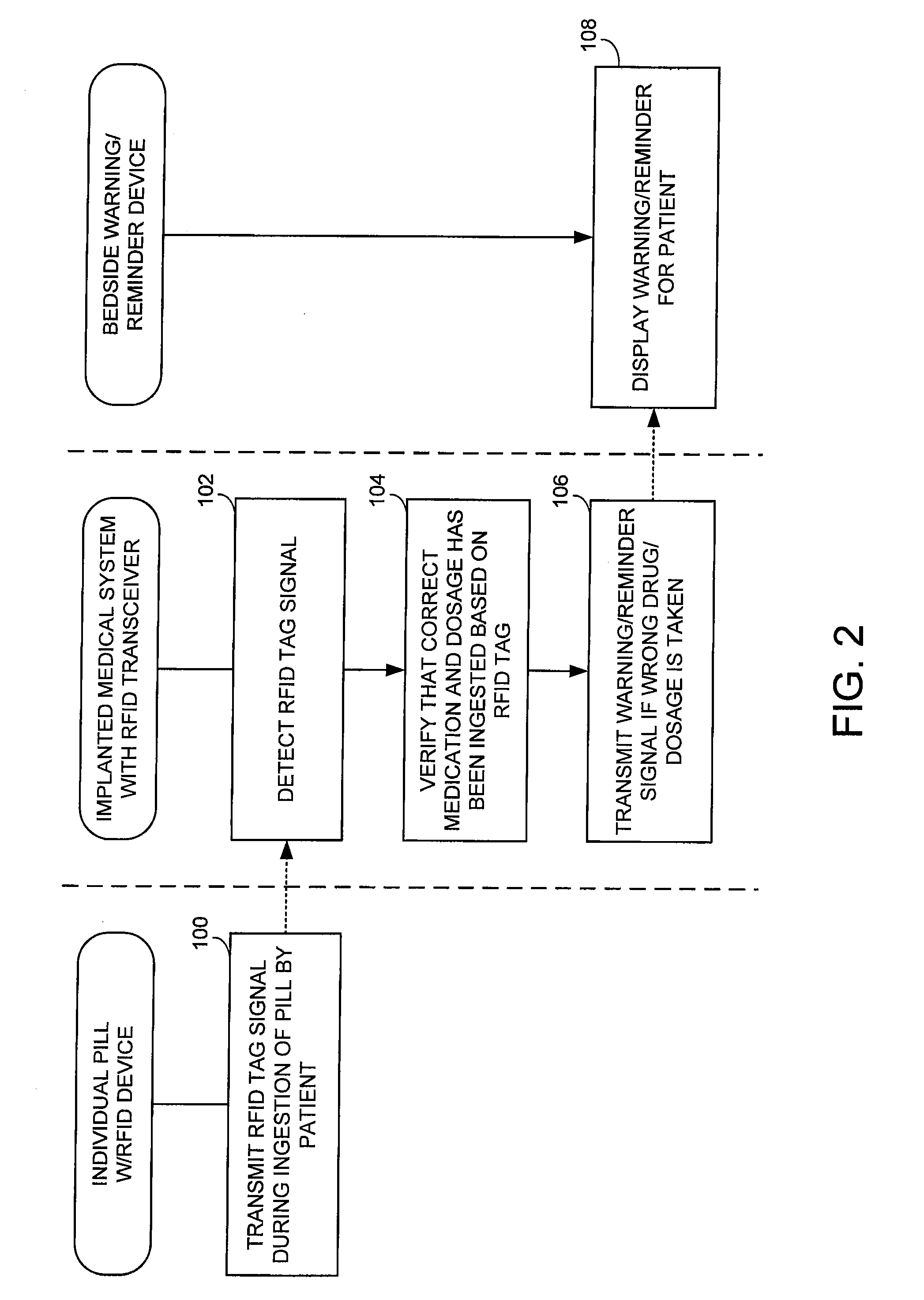
Method and apparatus for monitoring ingestion of medications using an implantable medical device
ActiveUS7414534B1Reduce the possibilityIncrease chances of survivalDrug and medicationsSurgeryTransceiverCardiac pacemaker electrode
An implantable medical device, such as a pacemaker or implantable cardioverter defibrillator (ICD), is configured to automatically detect ingestion of medications to verify that prescribed medications are taken in a timely manner and at the correct dosage. Briefly, individual pills are provided with miniature radio frequency identification (RFID) devices capable of transmitting RFID tag signals, which identify the medication contained within the pill and its dosage. The implanted device is equipped with an RFID transceiver for receiving tag signals from a pill as it is being ingested. The implanted system decodes the tag to identify the medication and its dosage, then accesses an onboard database to verify that the medication being ingested was in fact prescribed to the patient and to verify that the correct dosage was taken. Warning signals are generated if the wrong medication or the wrong dosage was taken. Therapy may also be automatically adjusted. Non-RF-based ID devices are also described, which instead transmit ID data via biphasic current pulses.
Owner:PACESETTER INC
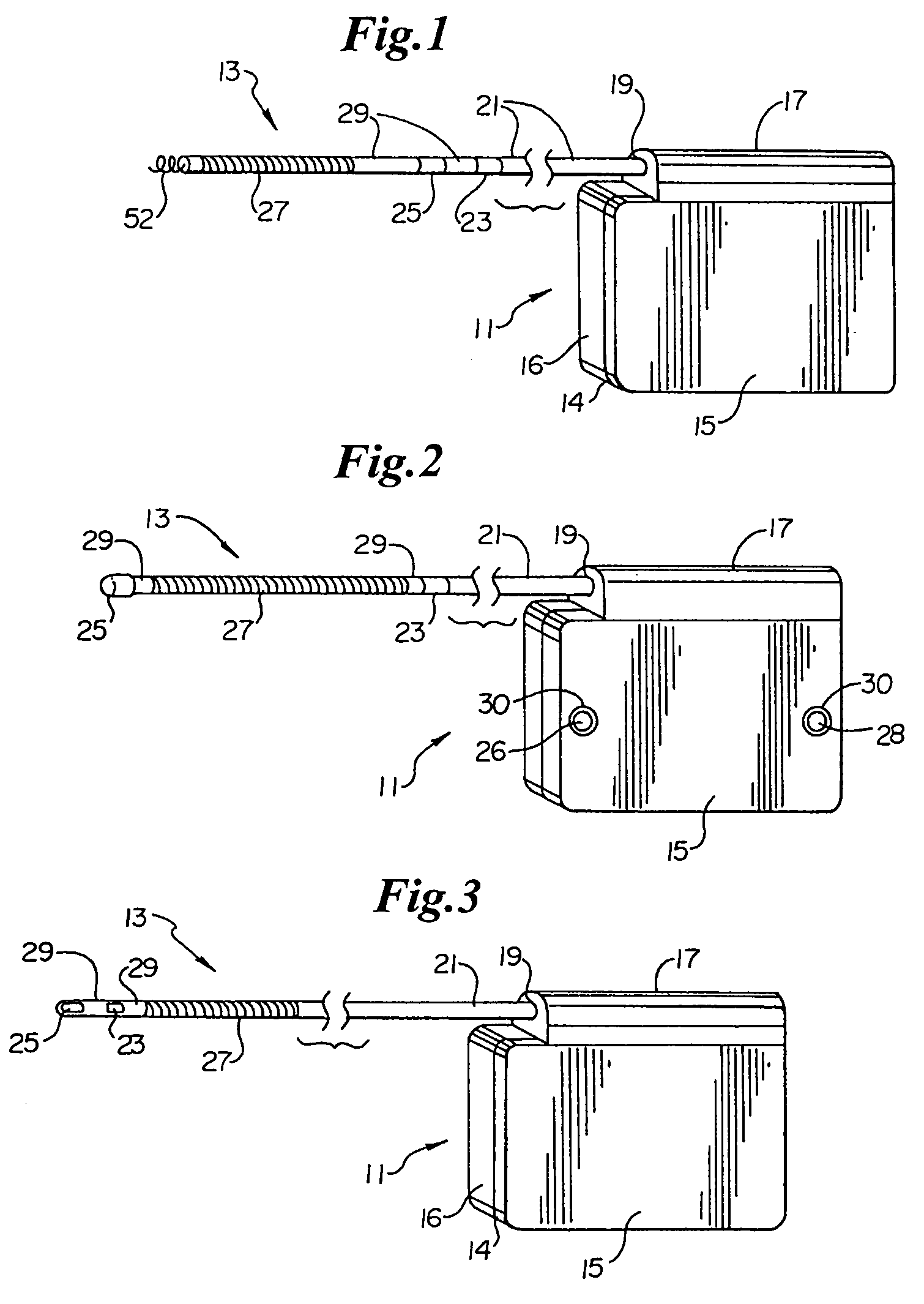
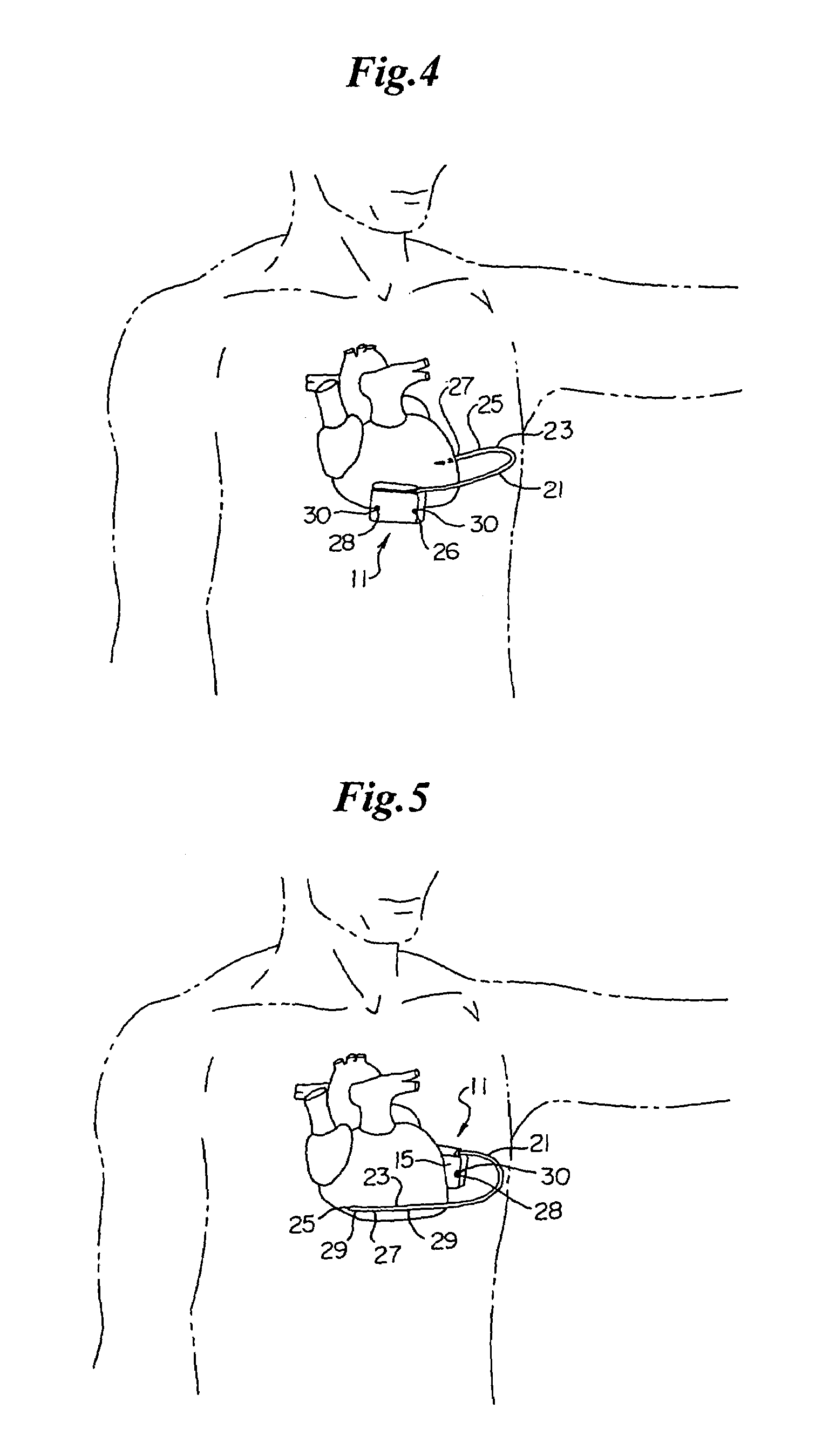
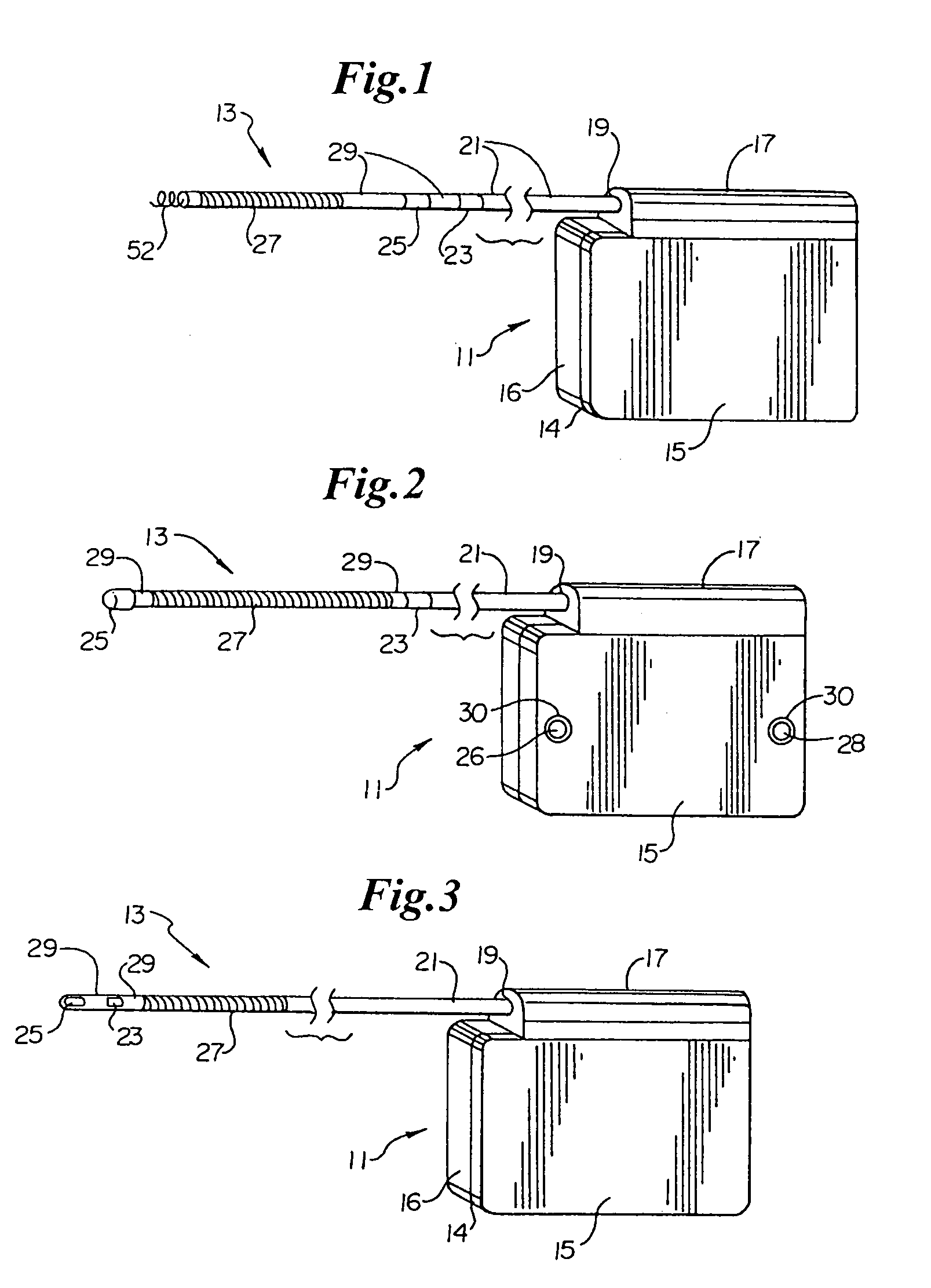
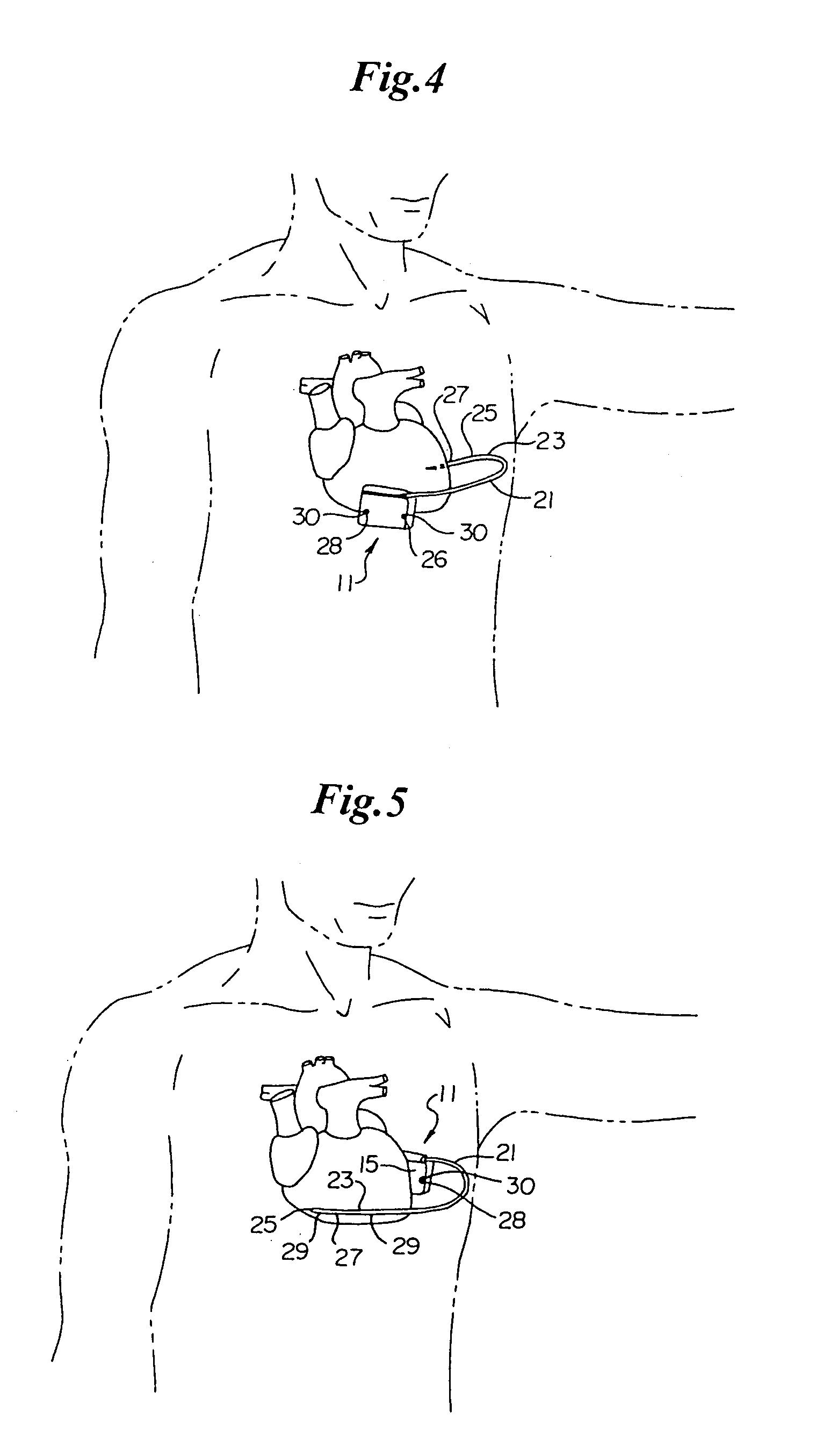
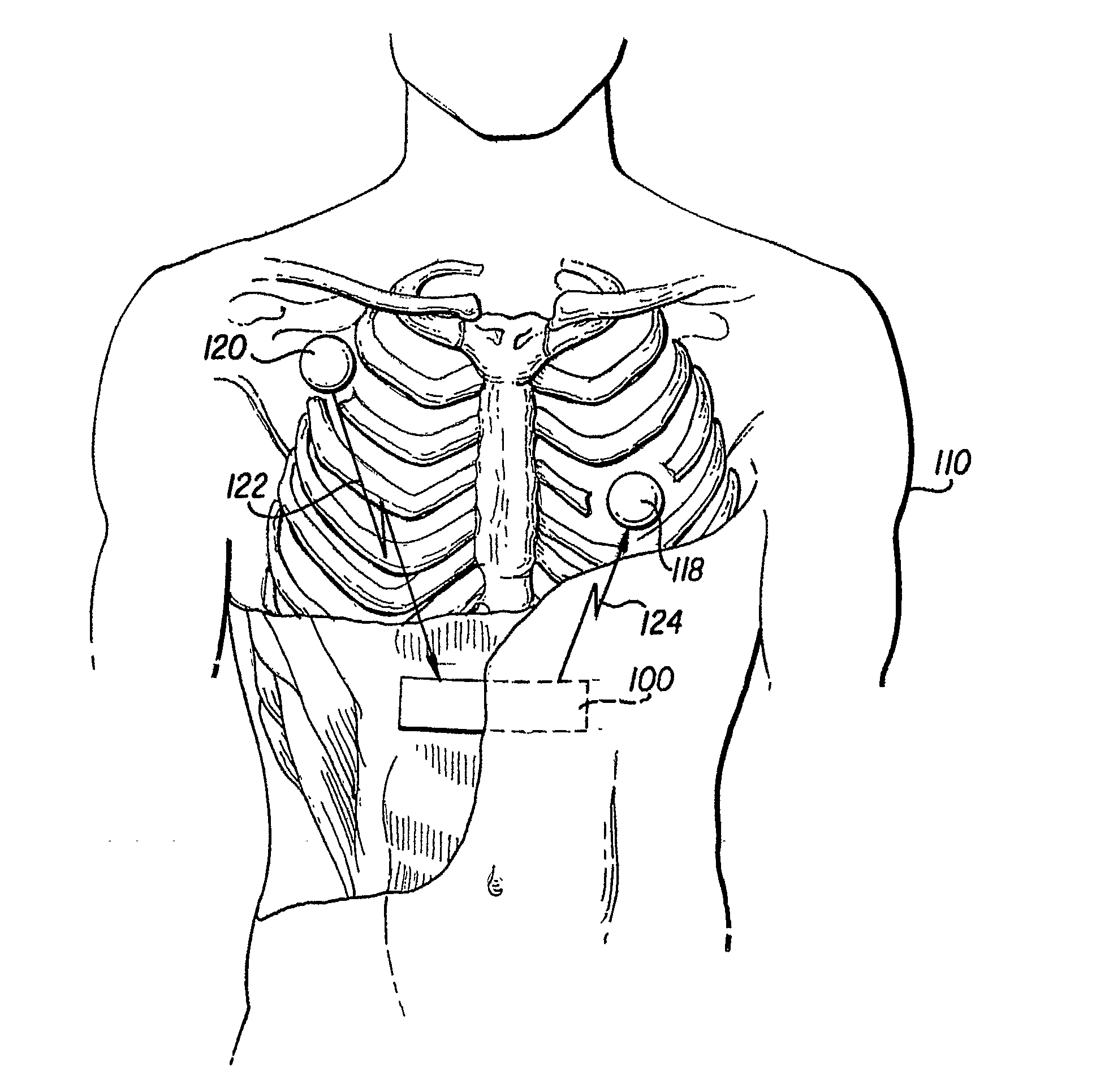
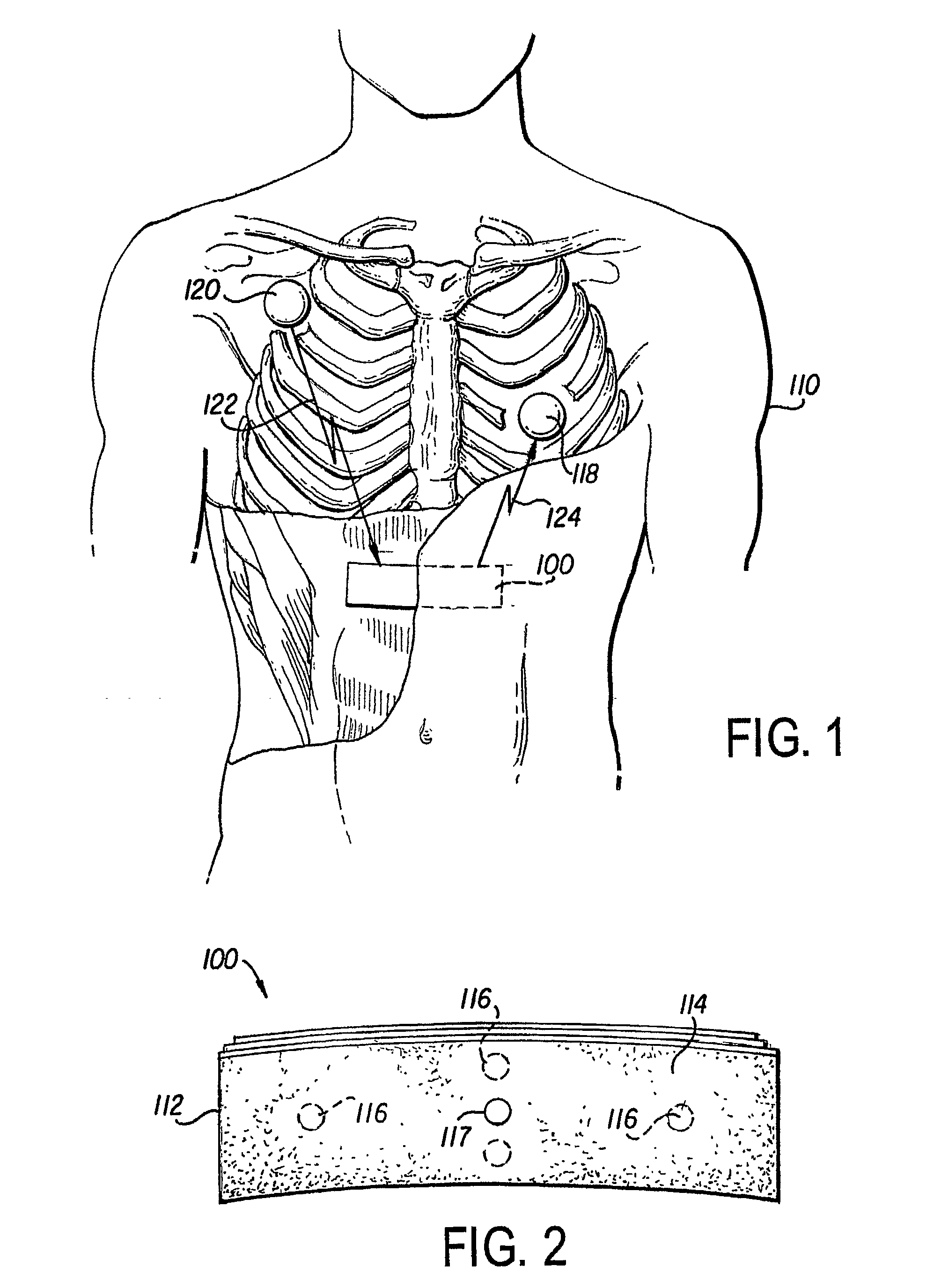
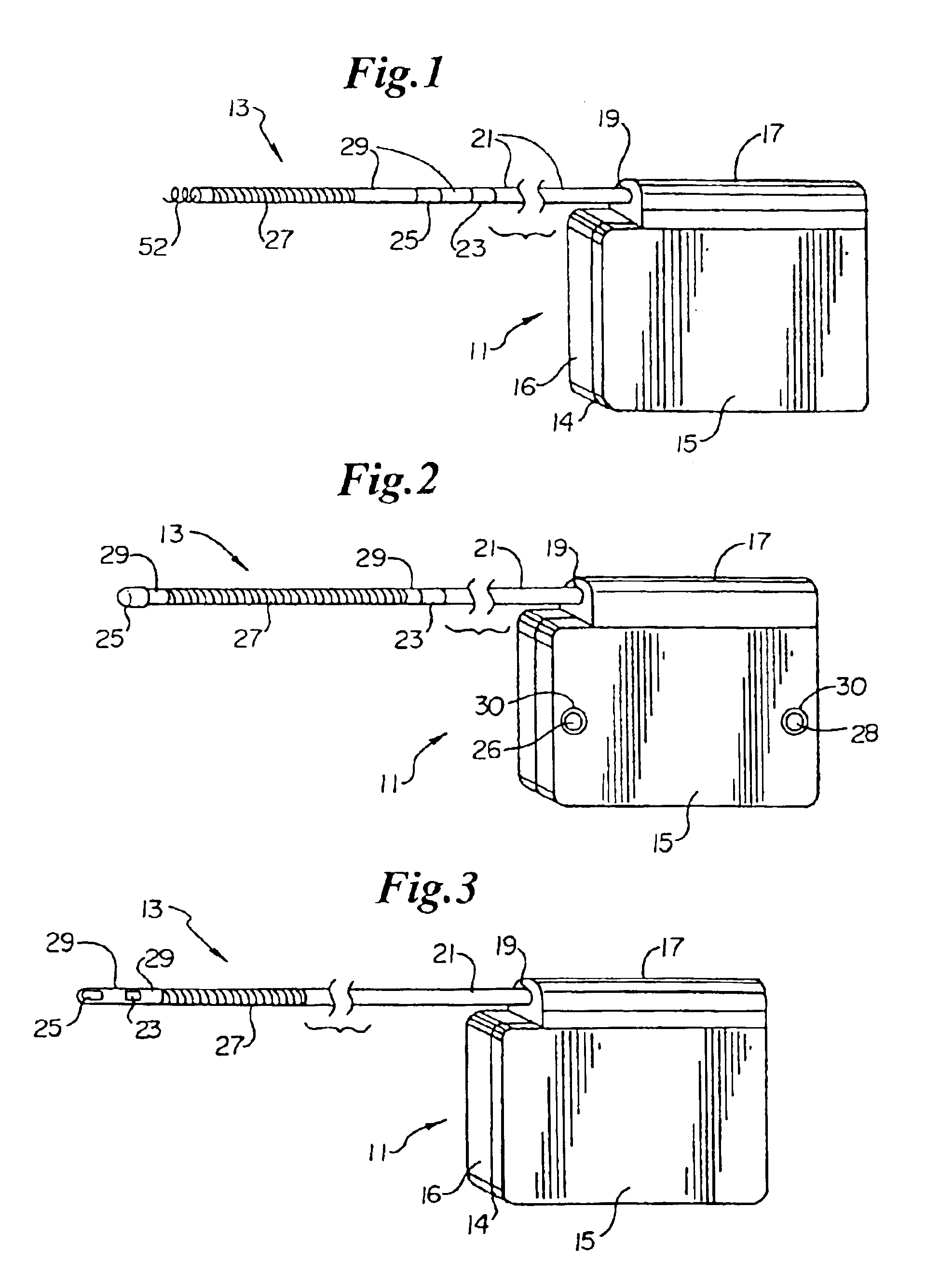
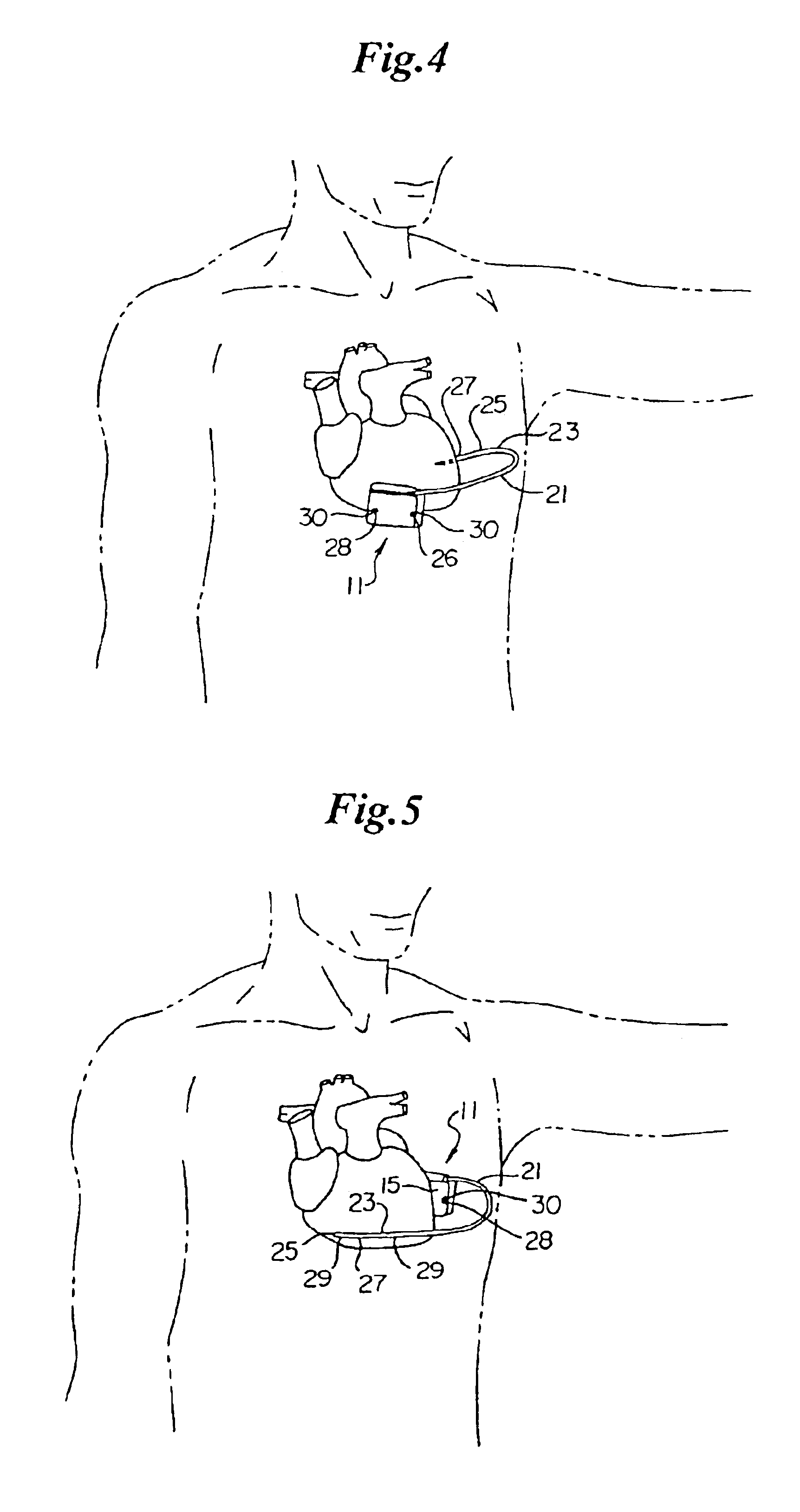
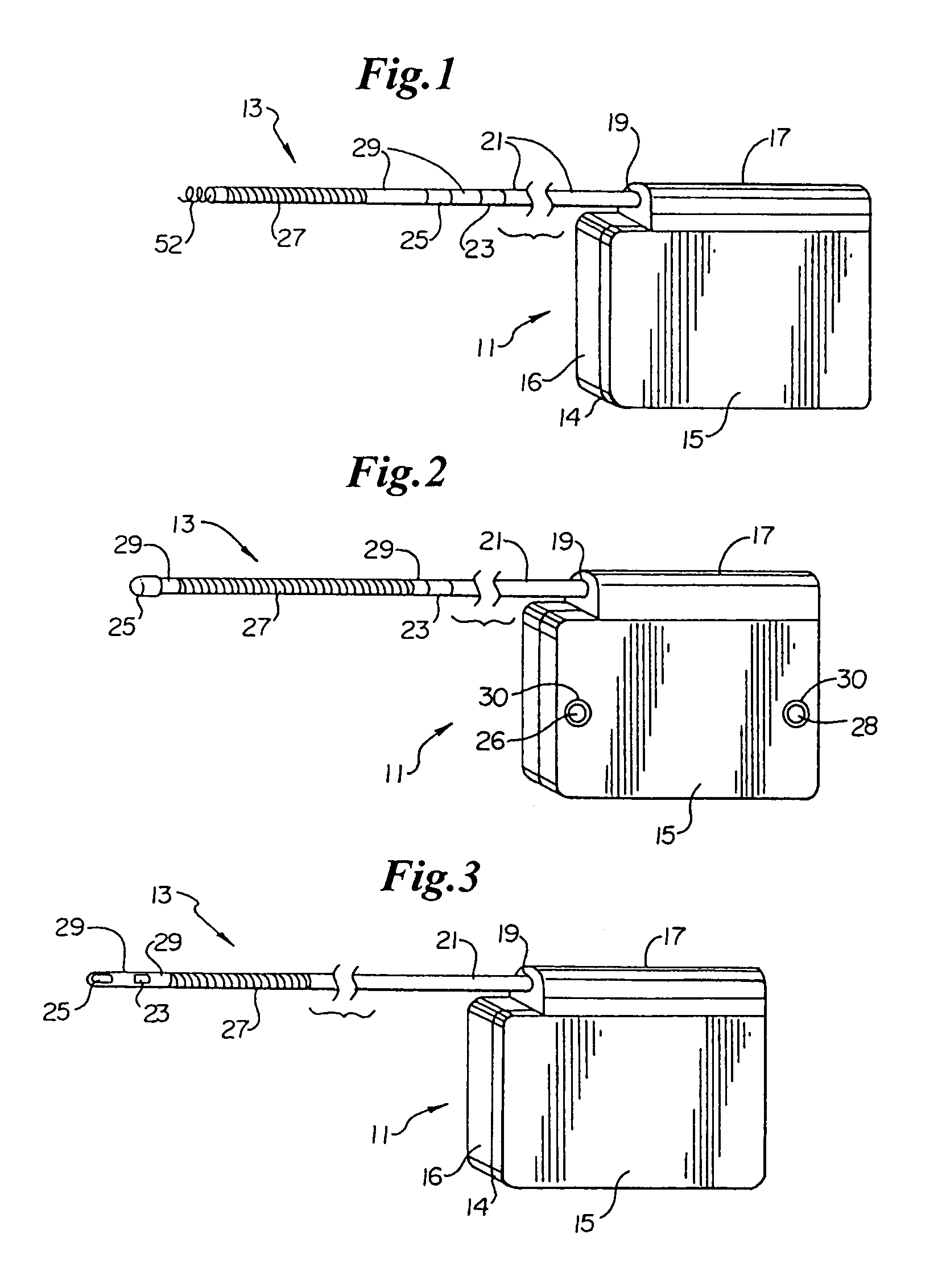
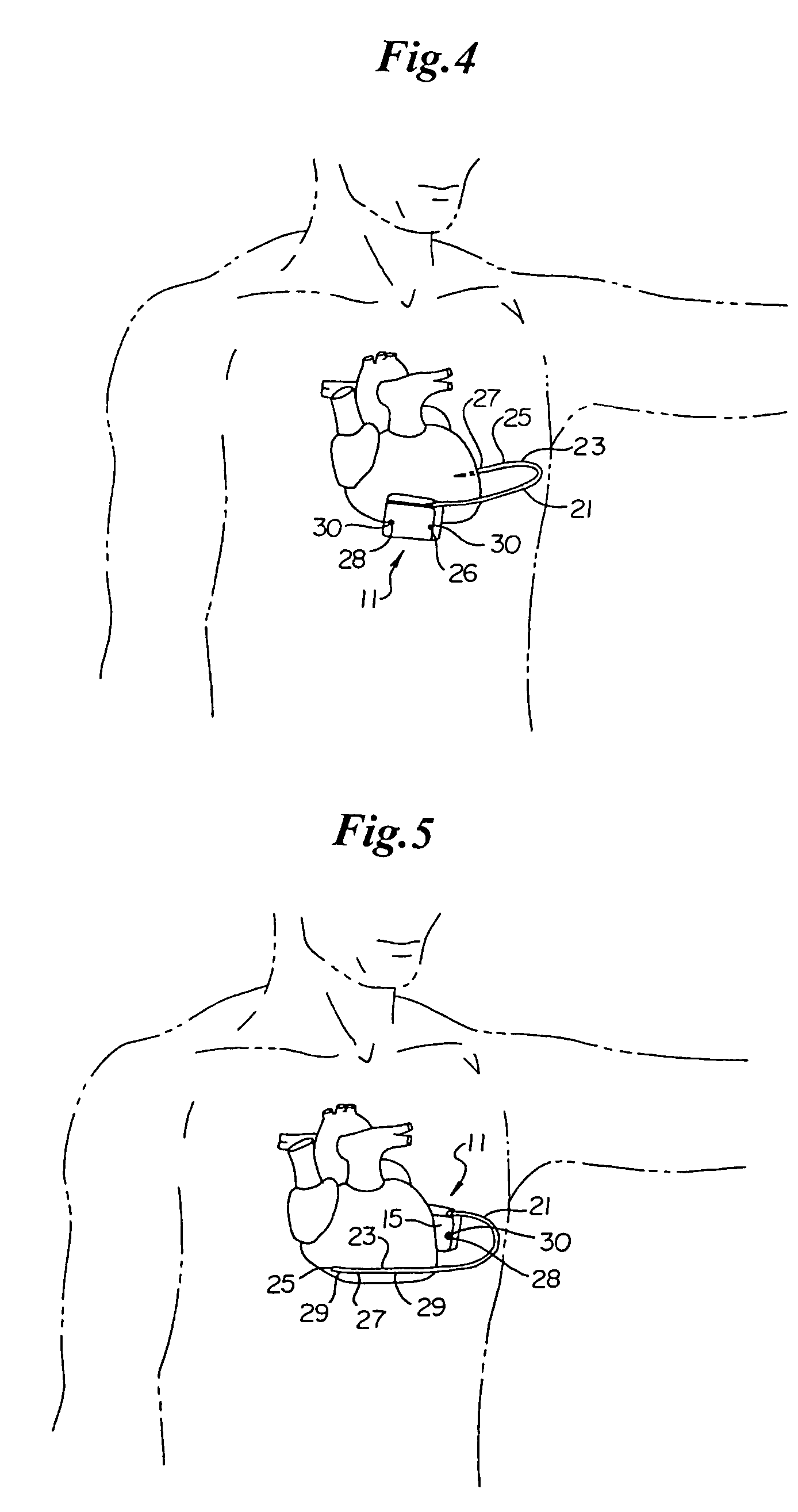
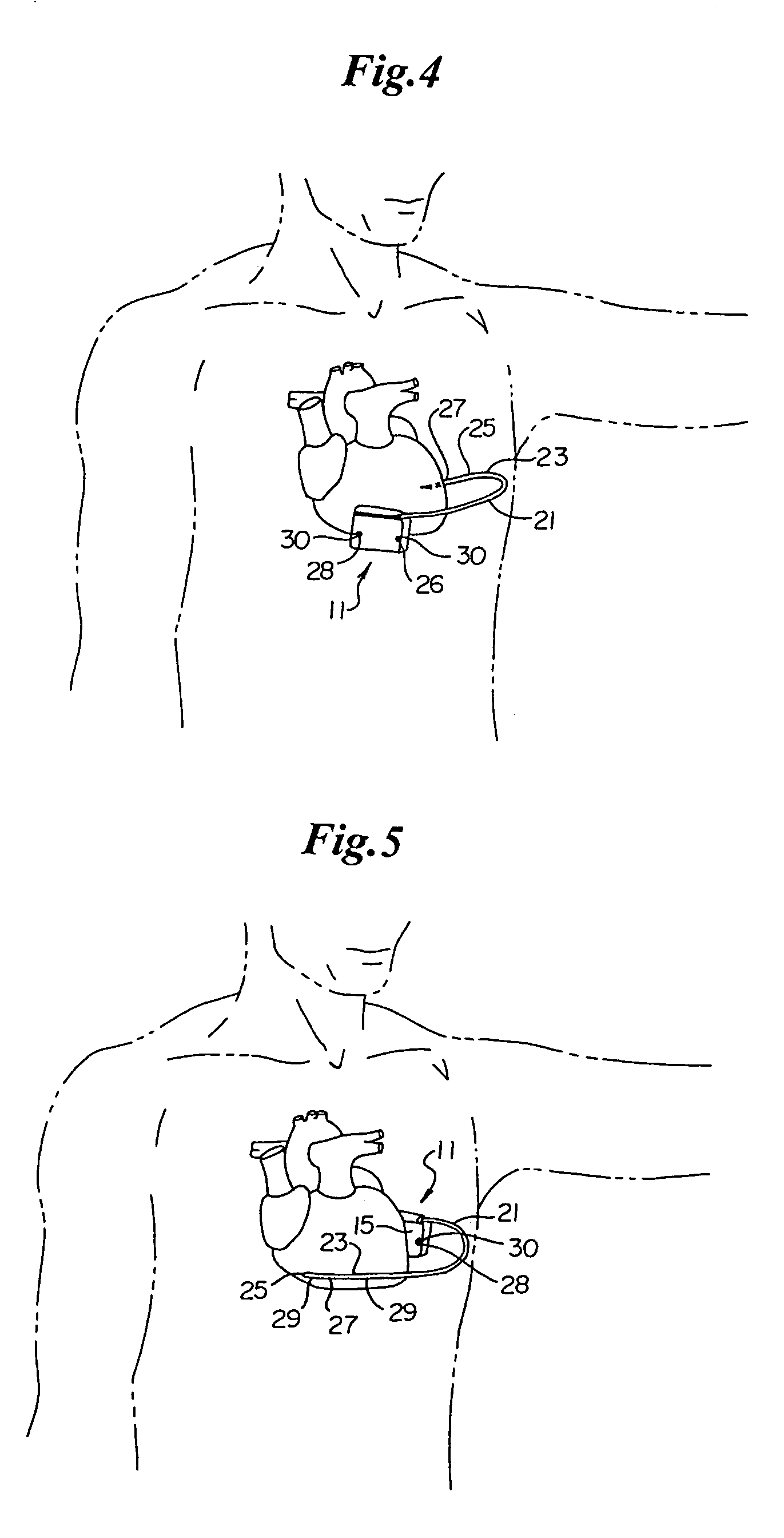
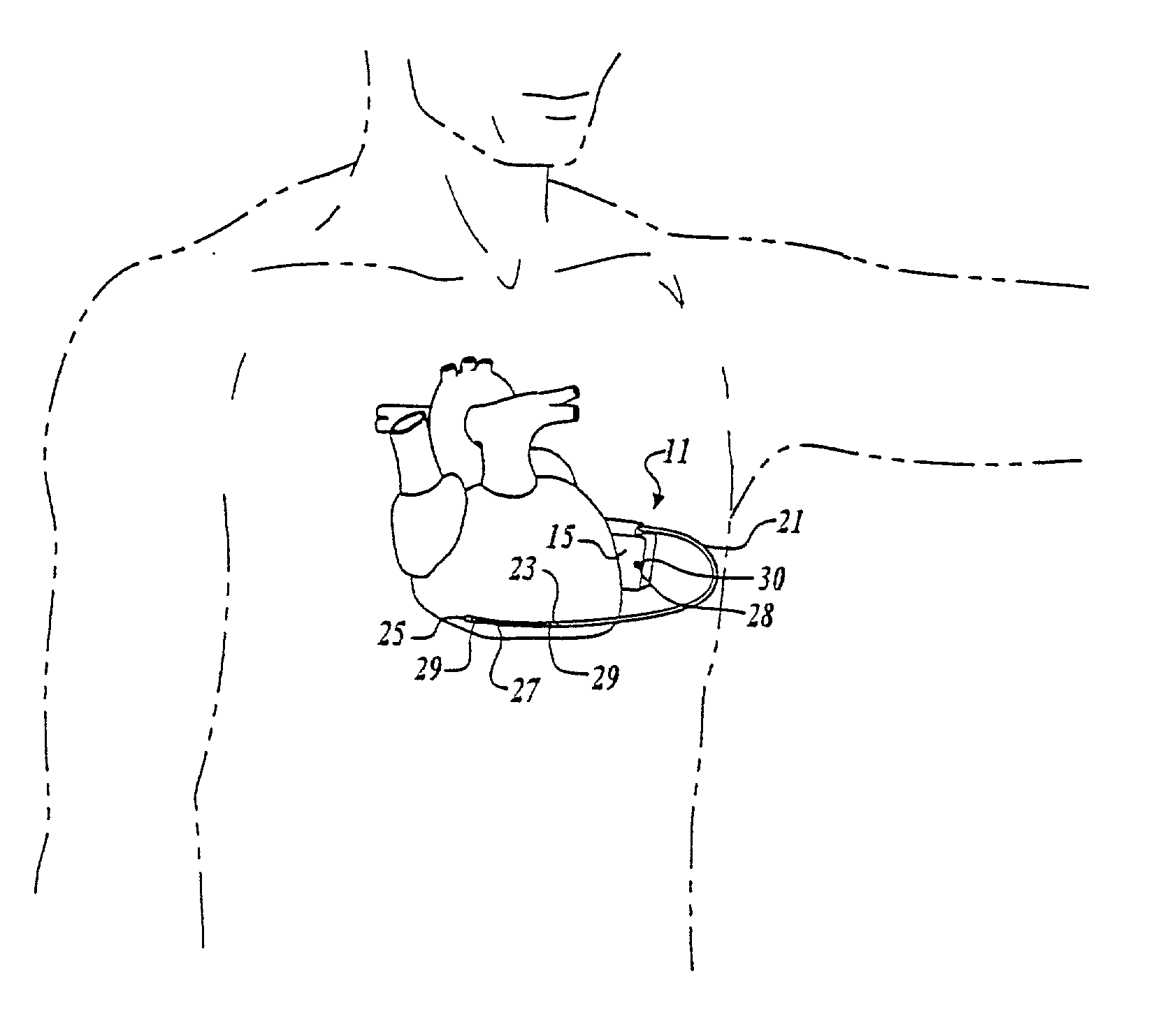
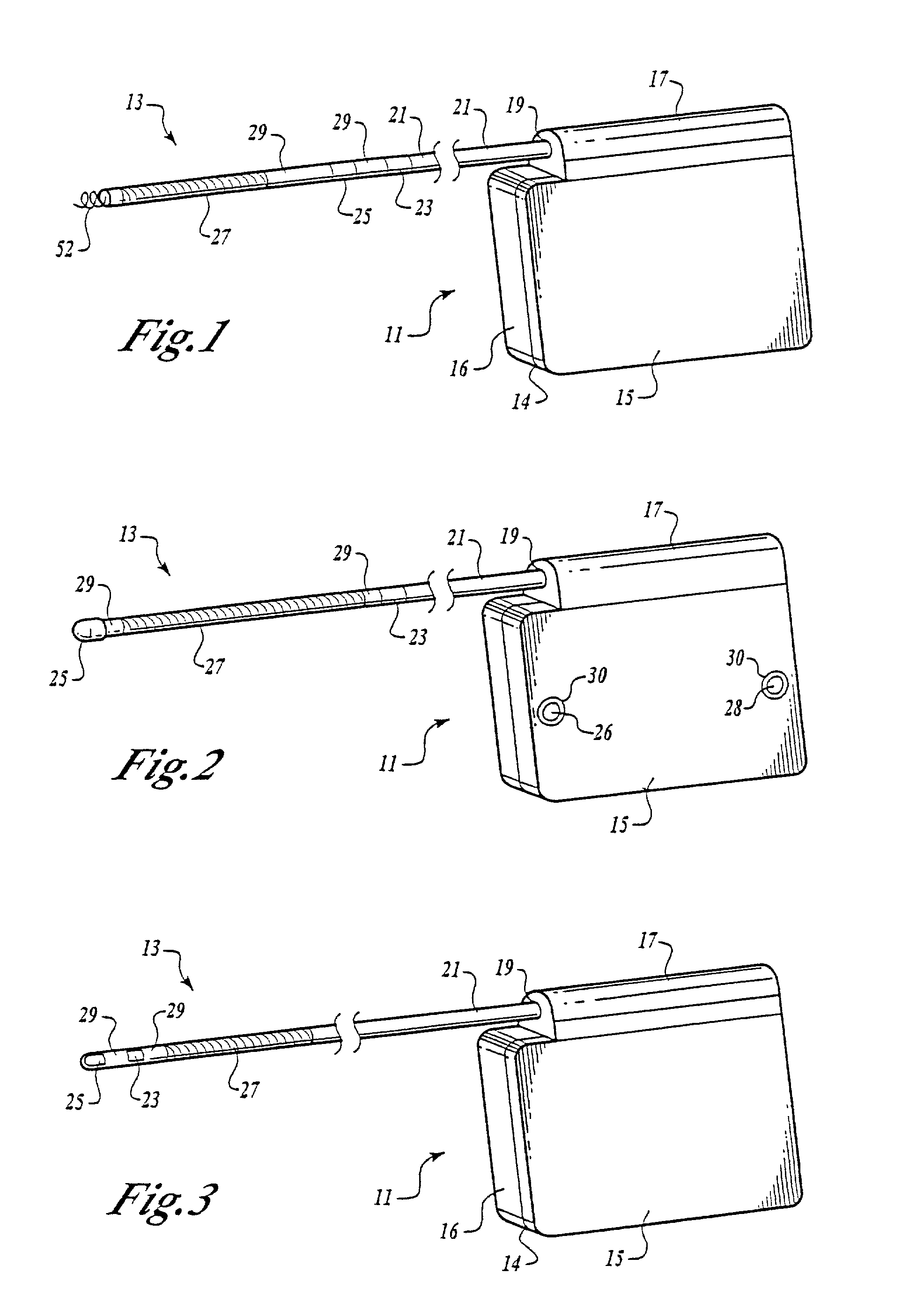
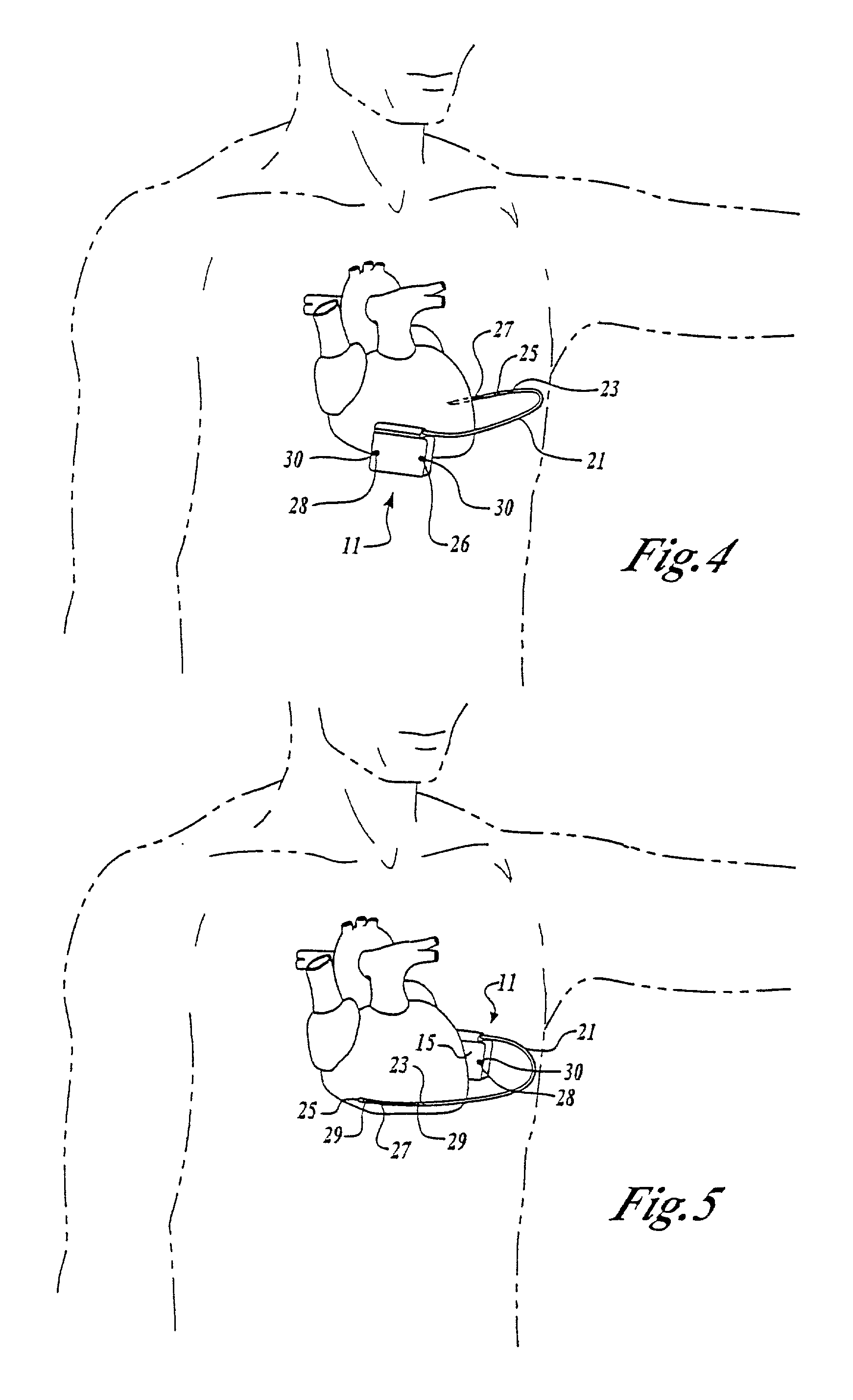
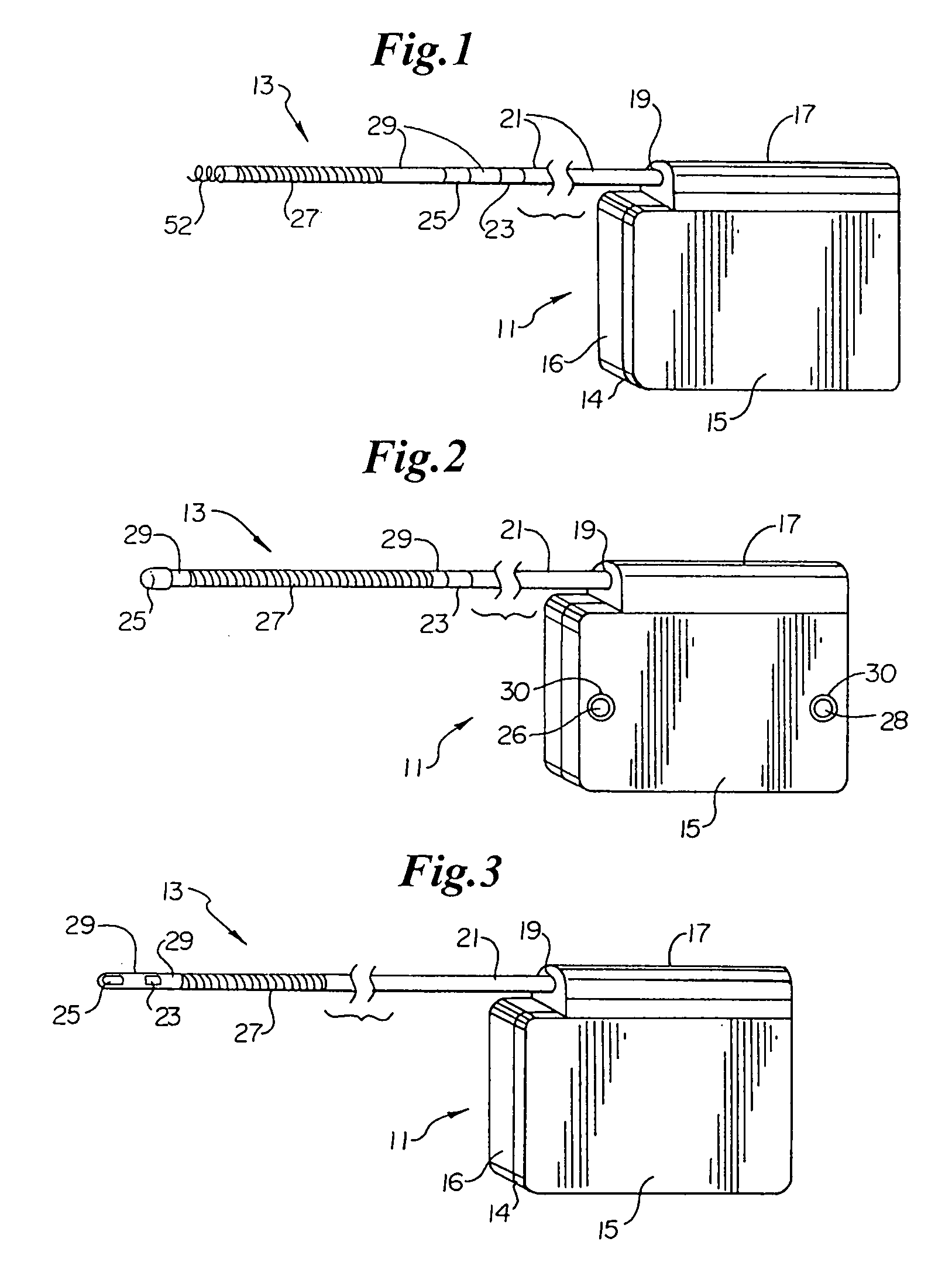
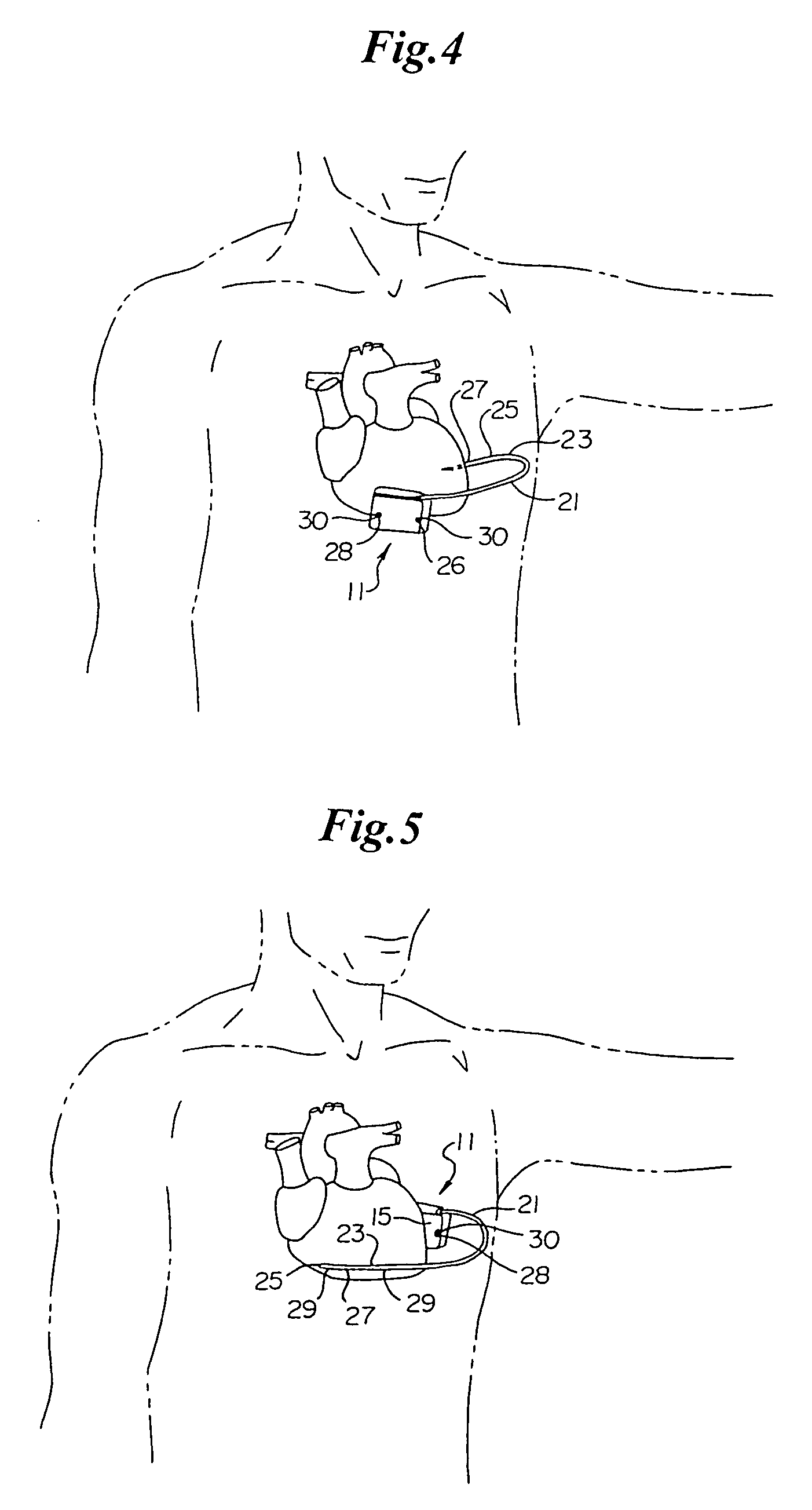
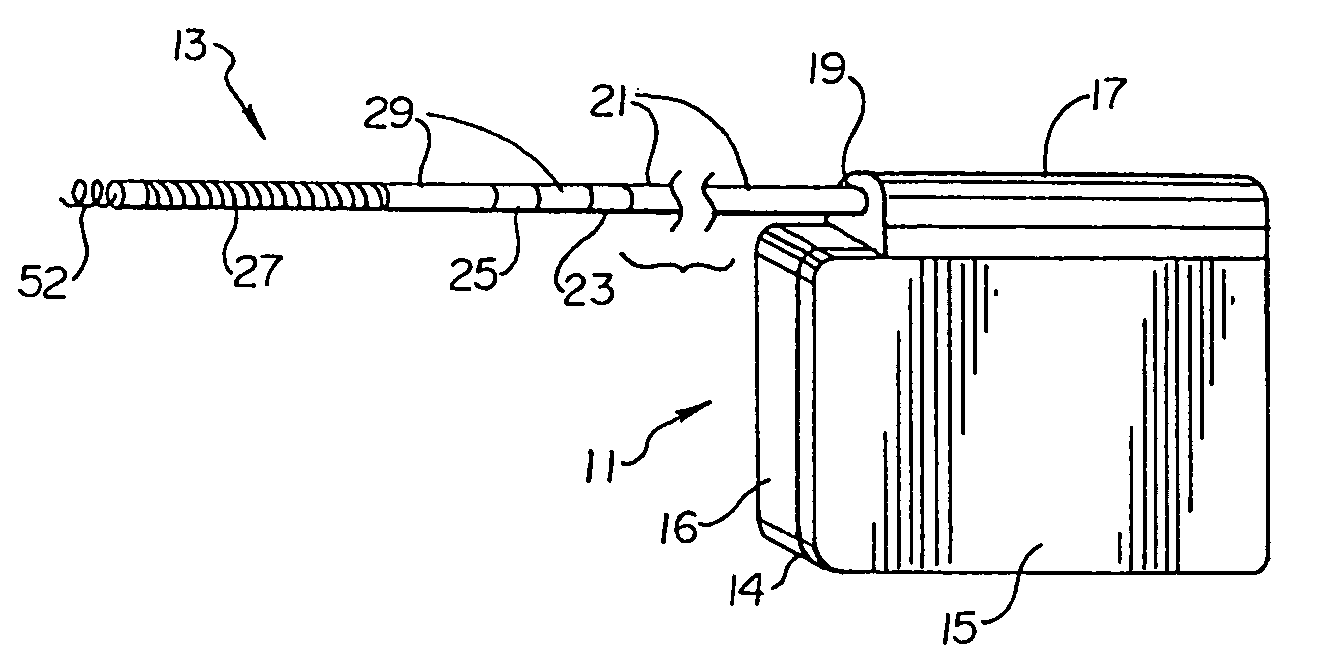
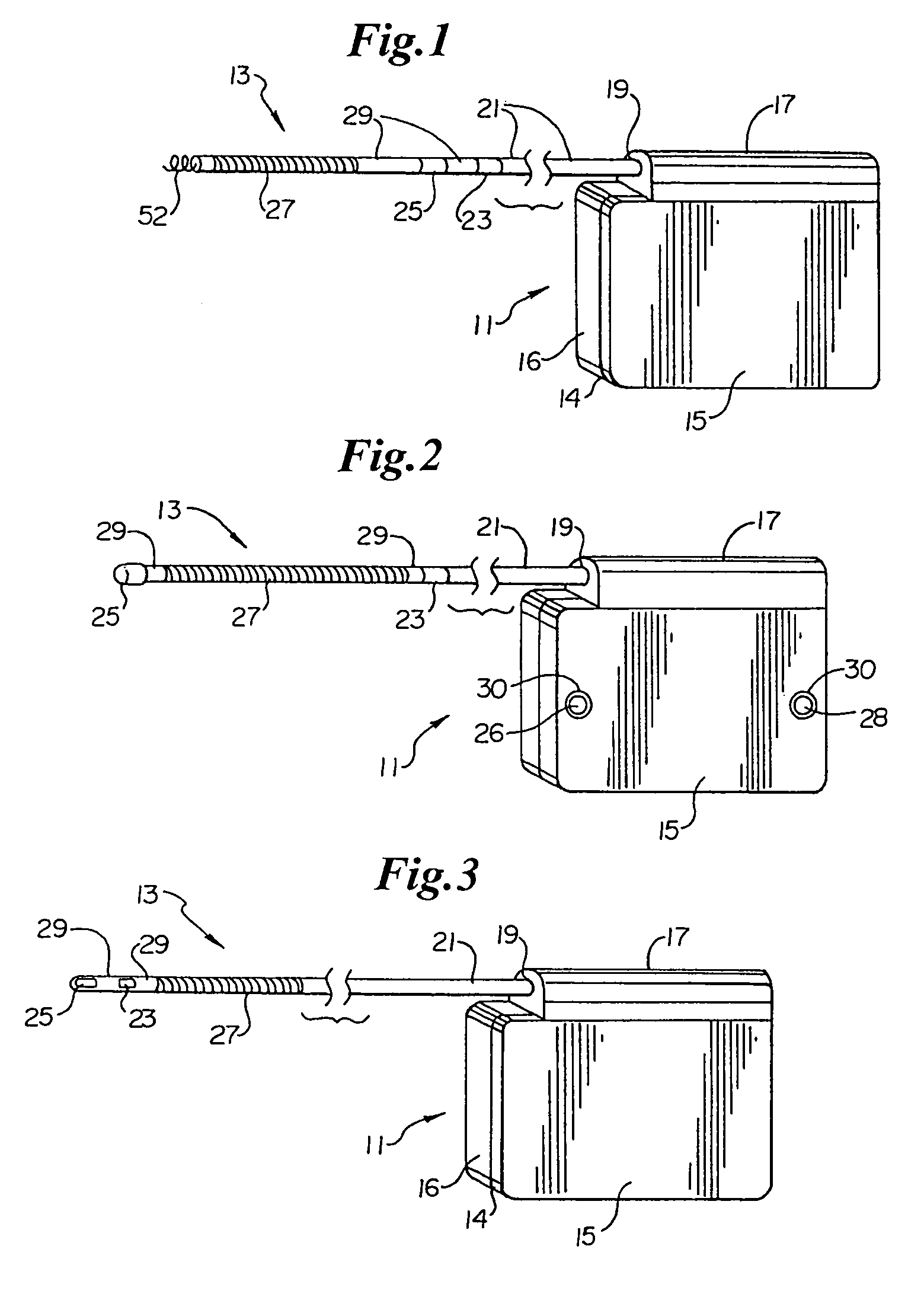
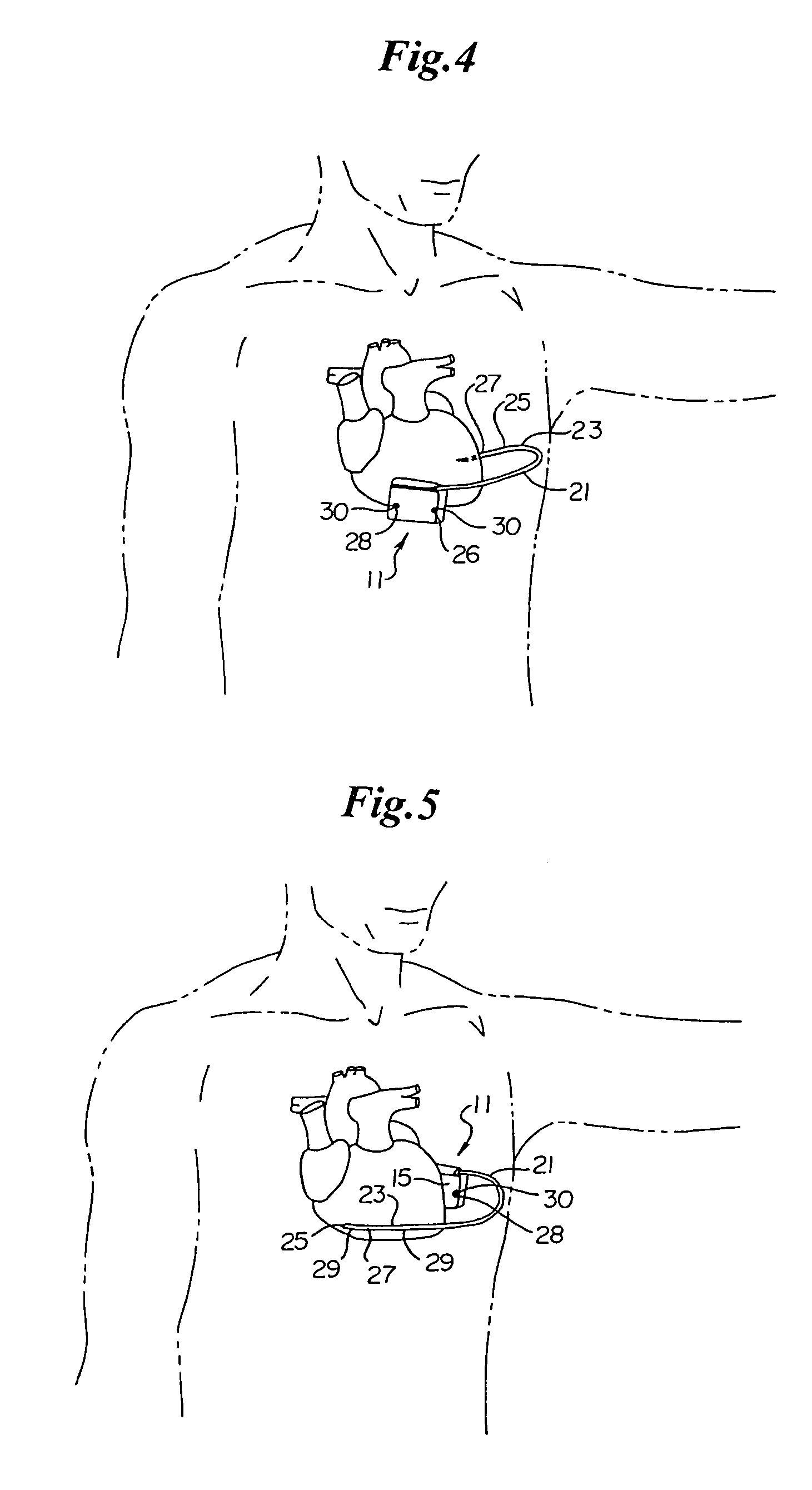
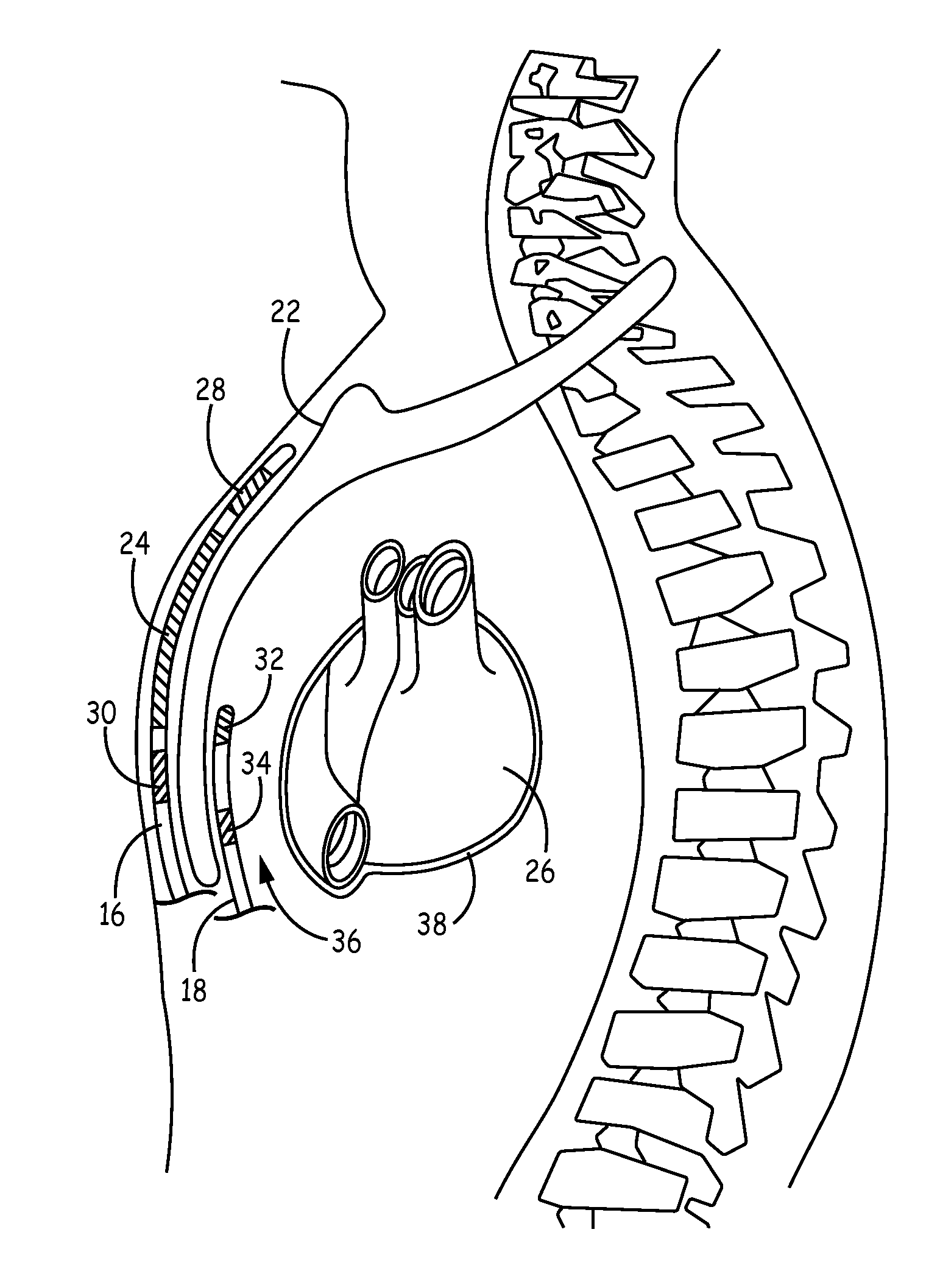
Method of insertion and implantation of implantable cardioverter-defibrillator canisters
InactiveUS6866044B2DiagnosticsHeart defibrillatorsThoracic cavityImplantable cardioverter-defibrillator
One embodiment of the present invention provides a method of inserting an implantable cardioverter-defibrillator within a patient, the method including the steps of providing a cardioverter-defibrillator canister having at least a portion of the cardioverter-defibrillator canister being non planar to maintain the cardioverter-defibrillator canister in a predetermined relationship with respect to a patient's heart, subcutaneously over a patient's ribcage; making a single incision into the patient; and advancing the cardioverter-defibrillator canister through the single incision and subcutaneously over the patient's ribcage.
Owner:CAMERON HEALTH
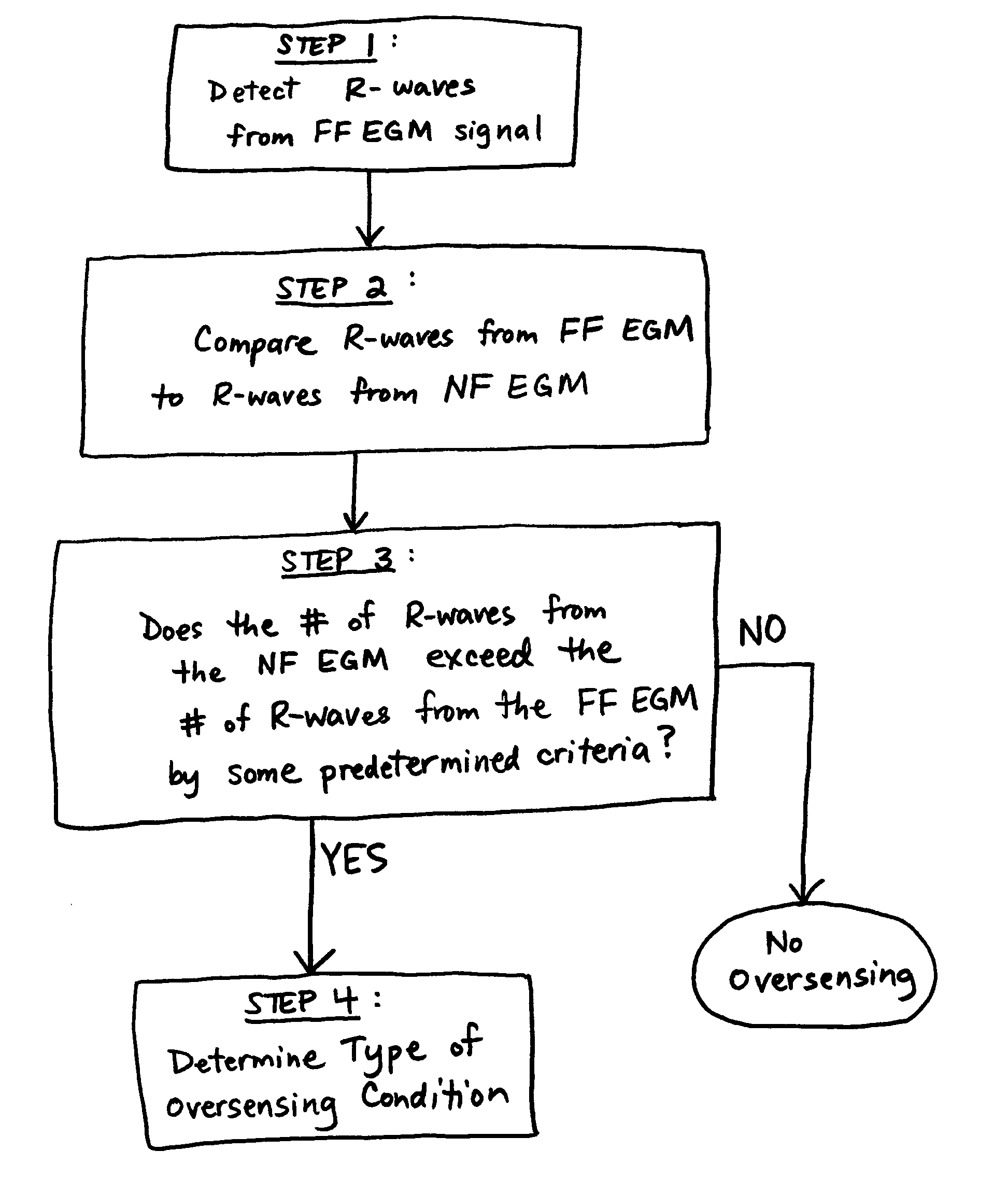
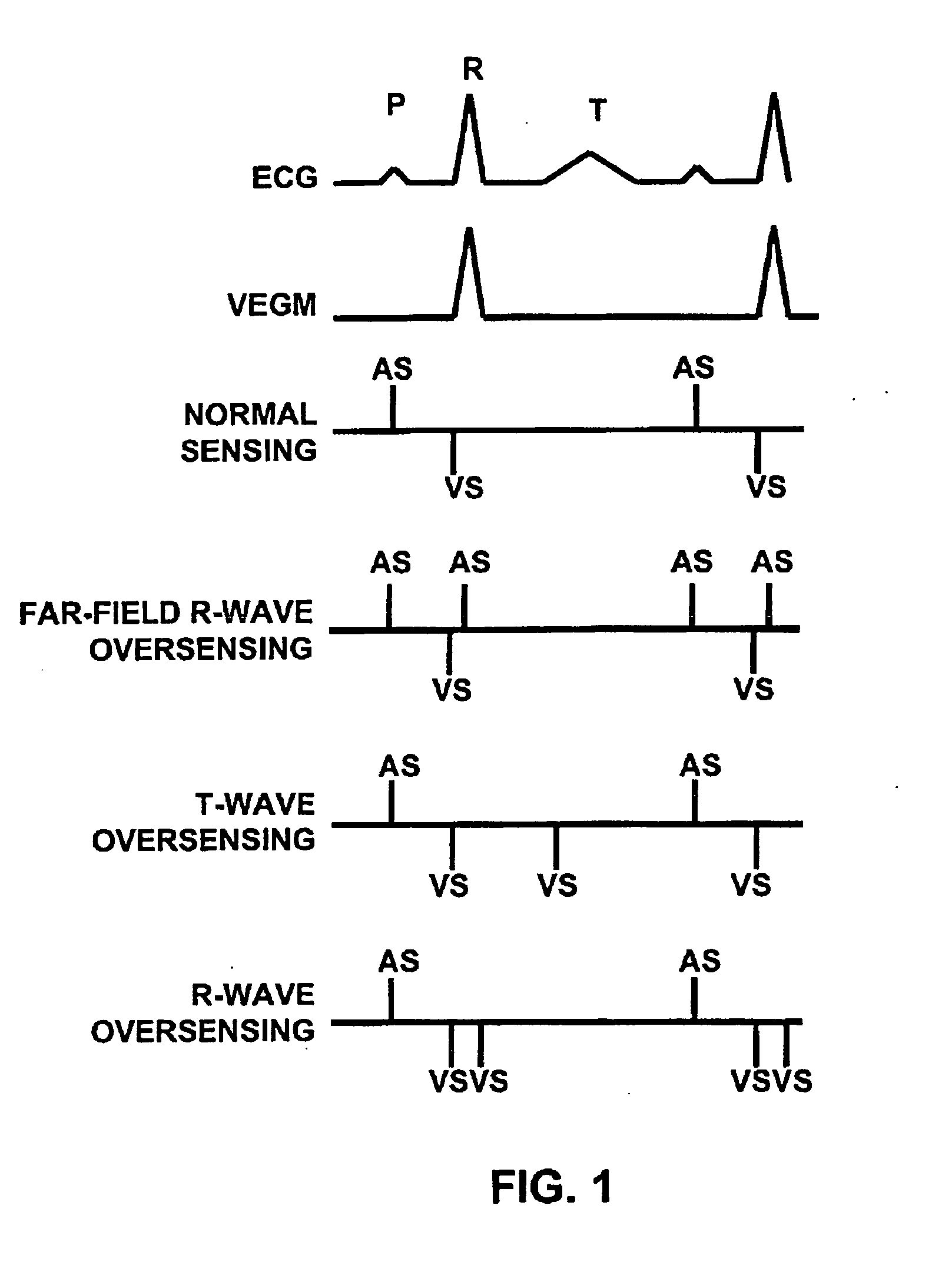
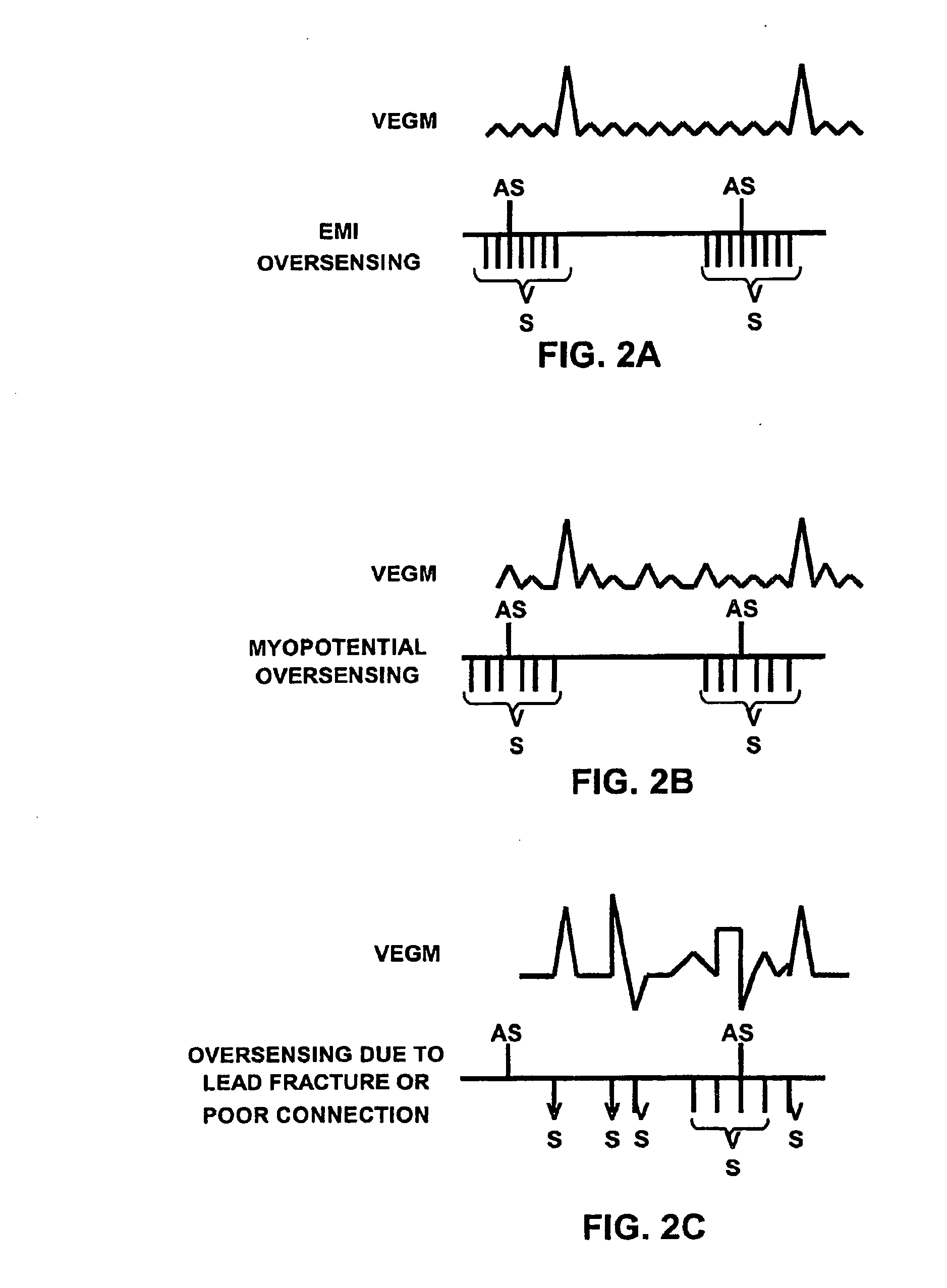
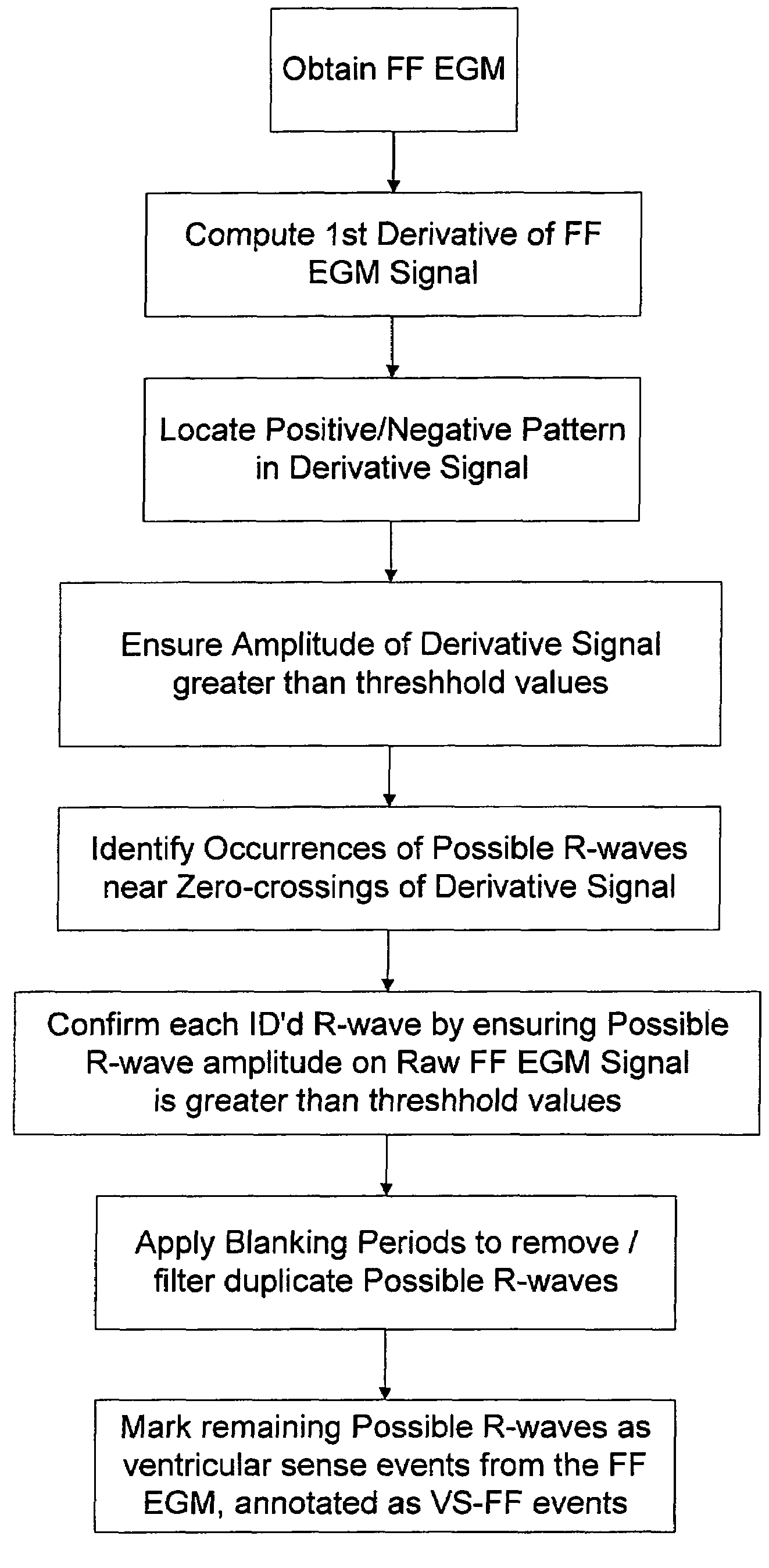
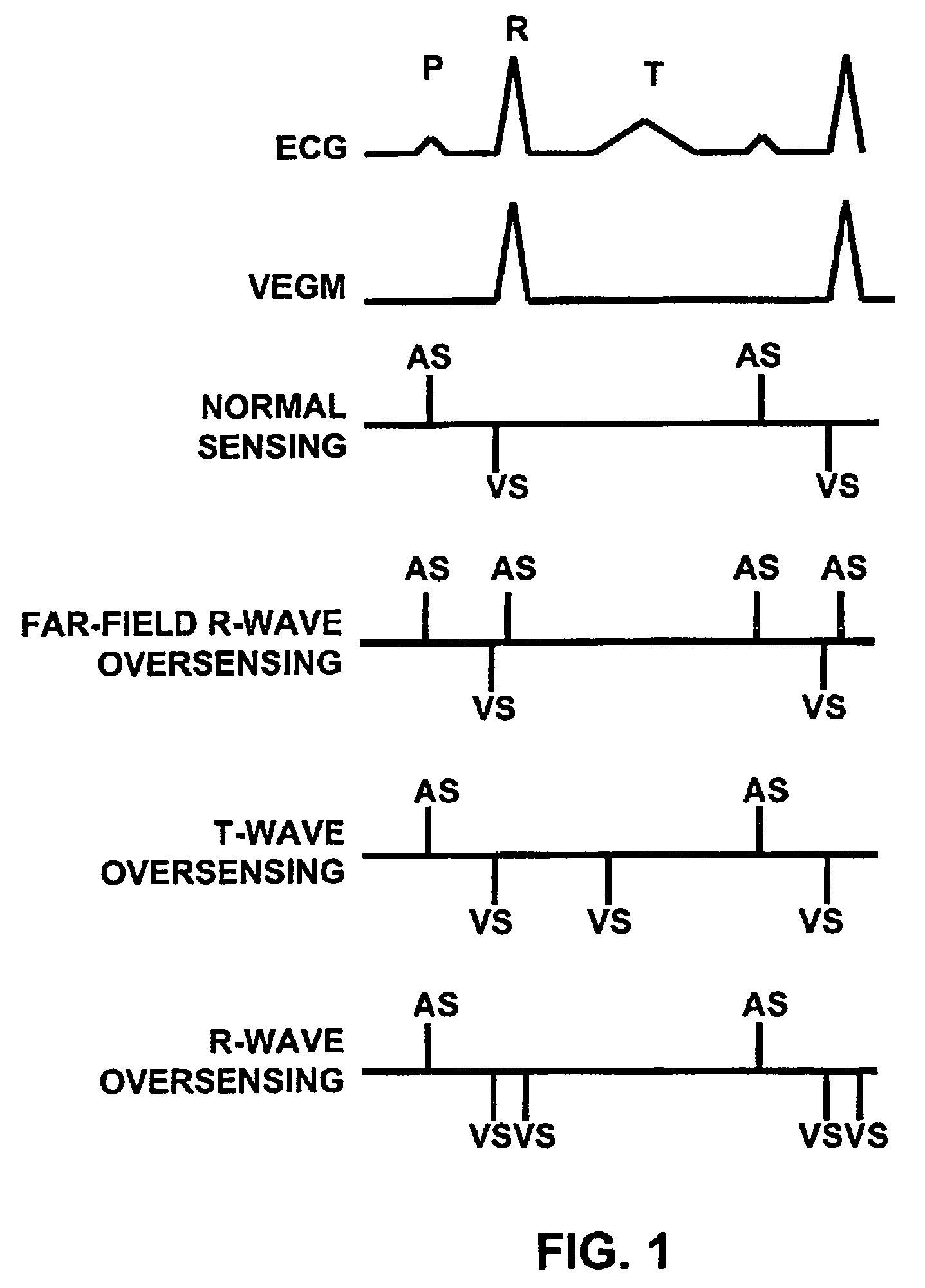
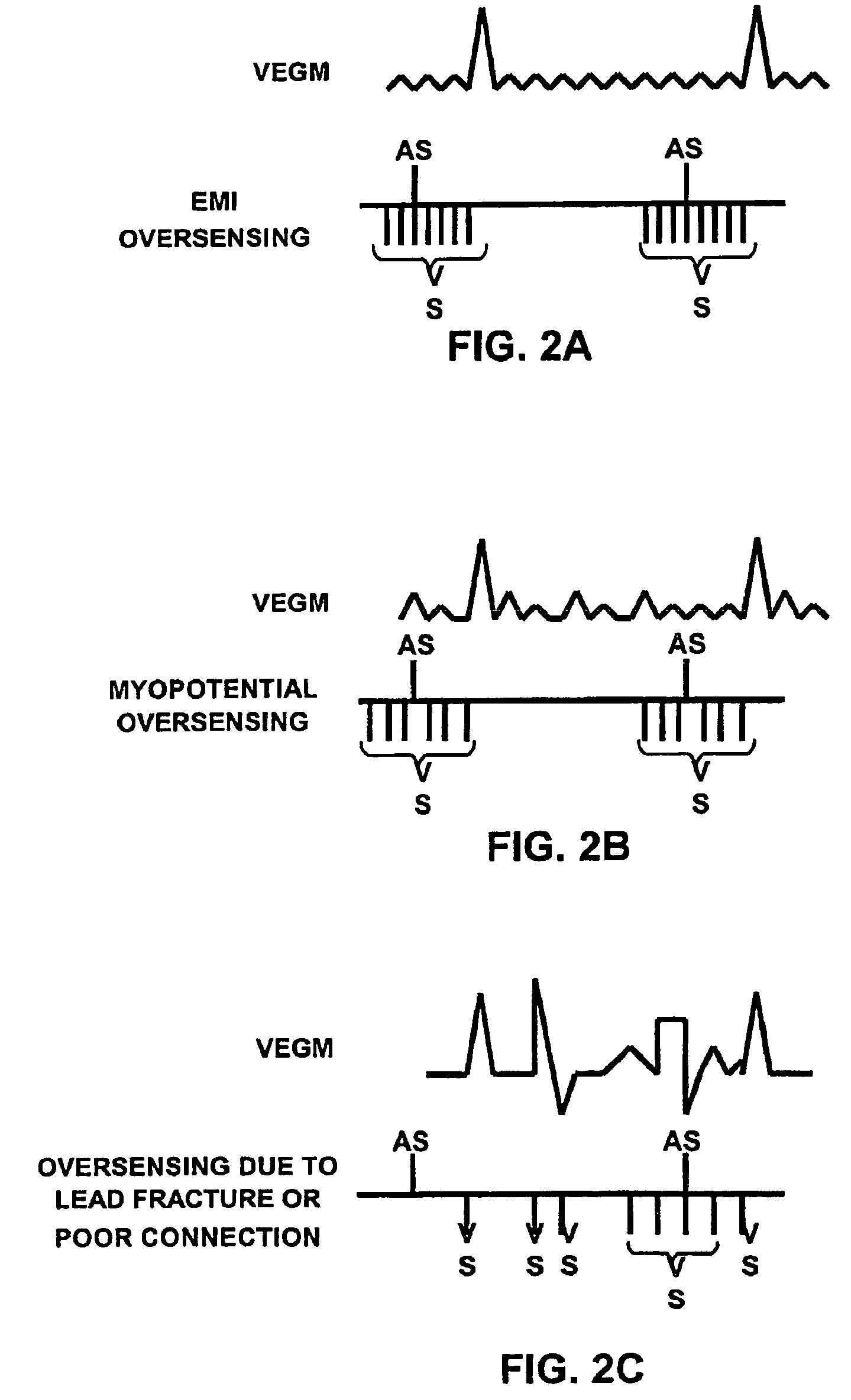
Method and apparatus for identifying oversensing using far-field intracardiac electrograms and marker channels
A method for identifying and classifying various types of oversensing in implantable medical devices (IMDs), such as implantable cardioverter defibrillators (ICDs), to assist a physician in choosing corrective action to reduce the likelihood of oversensing and inappropriate therapy delivery. Far-field electrogram (EGM) signals are analyzed to detect the occurrence of R-waves, and the result is compared to the number and pattern of R-waves sensed by the IMD and indicated on the marker channel. A marker channel with more sensed R-waves than indicated by analysis of the far-field EGM indicates the presence of oversensing, including double-counting of R-waves, T-wave oversensing, lead malfunction or failure, poor lead connections, noise associated with electromagnetic interference, non-cardiac myopotentials, etc. Identification of the type of oversensing may be determined by analysis of the number and pattern of marker channel sensed R-waves with respect to the timing of the R-waves detected from the far-field EGM.
Owner:MEDTRONIC INC
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS7991467B2Relieve painSafe and effective operationCatheterHeart stimulatorsCardiac pacemaker electrodeImplantable cardioverter-defibrillator
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDs) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
Subcutaneous ICD with motion artifact noise suppression
A subcutaneous implantable cardioverter defibrillator (SubQ ICD) includes a housing carrying electrodes for sensing ECG signals and delivering therapy. A sensor detects local motion in the area of the housing and produces a noise signal related to motion artifact noise contained in ECG signals derived from the electrode array. An adaptive noise cancellation circuit enhances ECG signals based on the local motion noise signal. A therapy delivery circuit delivers cardioversion and defibrillation pulses based upon the enhanced ECG signals.
Owner:MEDTRONIC INC
Subcutaneous electrode with improved contact shape for transthorasic conduction
One embodiment of the present invention provides a lead electrode assembly for use with an implantable cardioverter-defibrillator subcutaneously implanted outside the ribcage between the third and twelfth ribs comprising the electrode.
Owner:CAMERON HEALTH
Implantable cardioverter-defibrillator having two spaced apart shocking electrodes on housing
InactiveUS6988003B2Heart defibrillatorsHeart stimulatorsThoracic cavityImplantable cardioverter-defibrillator
One embodiment of the present invention provides an implantable cardioverter-defibrillator for subcutaneous positioning over a patient's ribcage, the implantable cardioverter-defibrillator includes a housing having a first end and a second end; a first electrode disposed upon the first end of the housing; a second electrode disposed upon the second end of the housing; an electrical circuit located within the housing, wherein the electrical circuit is electrically coupled to the first electrode and the second electrode; and a lead electrode electrically coupled to the electrical circuit located within the housing.
Owner:CAMERON HEALTH
Method and apparatus for monitoring ingestion of medications using an implantable medical device
InactiveUS20080288027A1Reduce the possibilityIncrease chances of survivalDrug and medicationsSurgeryTransceiverCardiac pacemaker electrode
An implantable medical device, such as a pacemaker or implantable cardioverter defibrillator (ICD), is configured to automatically detect ingestion of medications to verify that prescribed medications are taken in a timely manner and at the correct dosage. Briefly, individual pills are provided with miniature radio frequency identification (RFID) devices capable of transmitting RFID tag signals, which identify the medication contained within the pill and its dosage. The implanted device is equipped with an RFID transceiver for receiving tag signals from a pill as it is being ingested. The implanted system decodes the tag to identify the medication and its dosage, then accesses an onboard database to verify that the medication being ingested was in fact prescribed to the patient and to verify that the correct dosage was taken. Warning signals are generated if the wrong medication or the wrong dosage was taken. Therapy may also be automatically adjusted. Non-RF-based ID devices are also described, which instead transmit ID data via biphasic current pulses.
Owner:PACESETTER INC
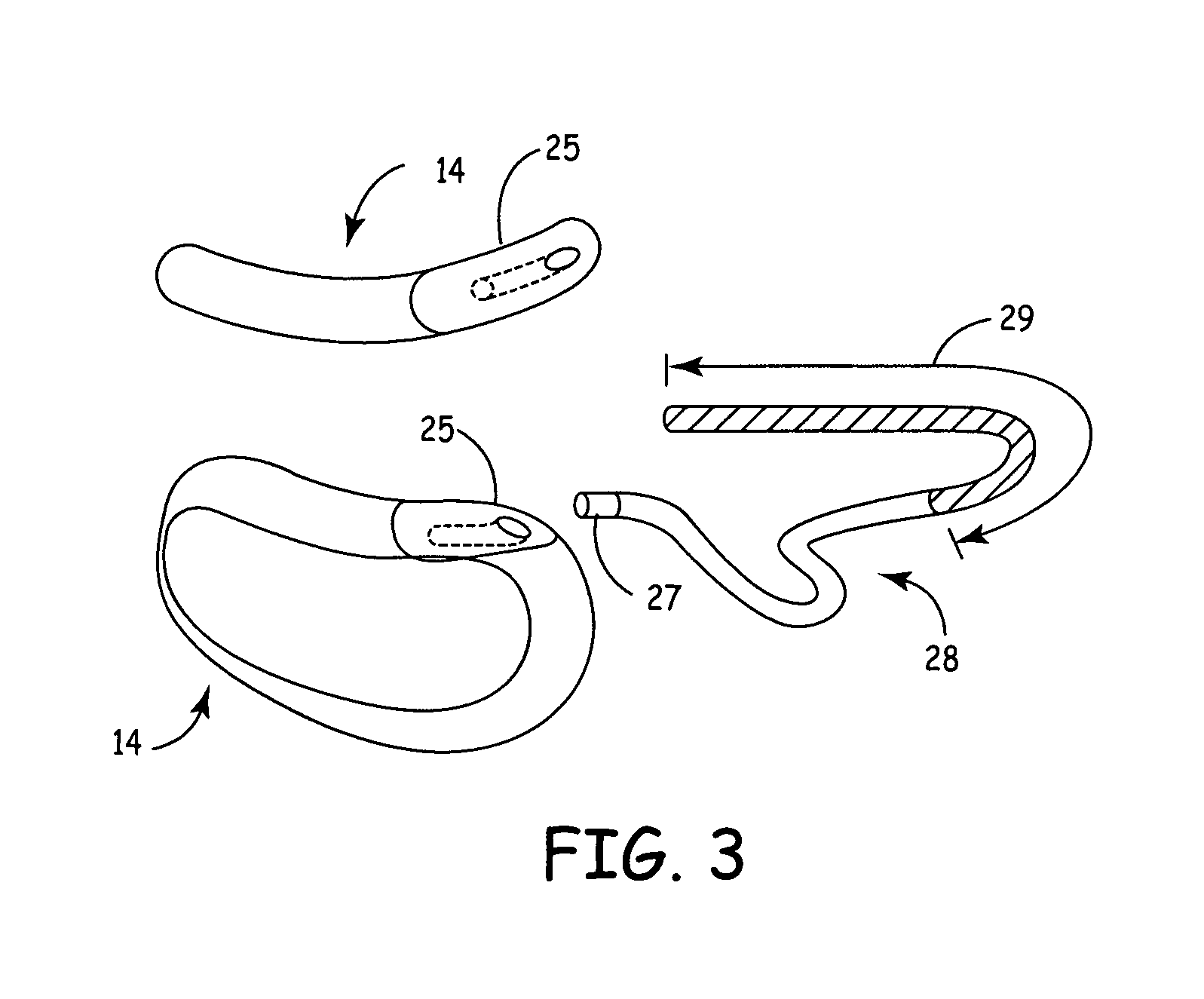
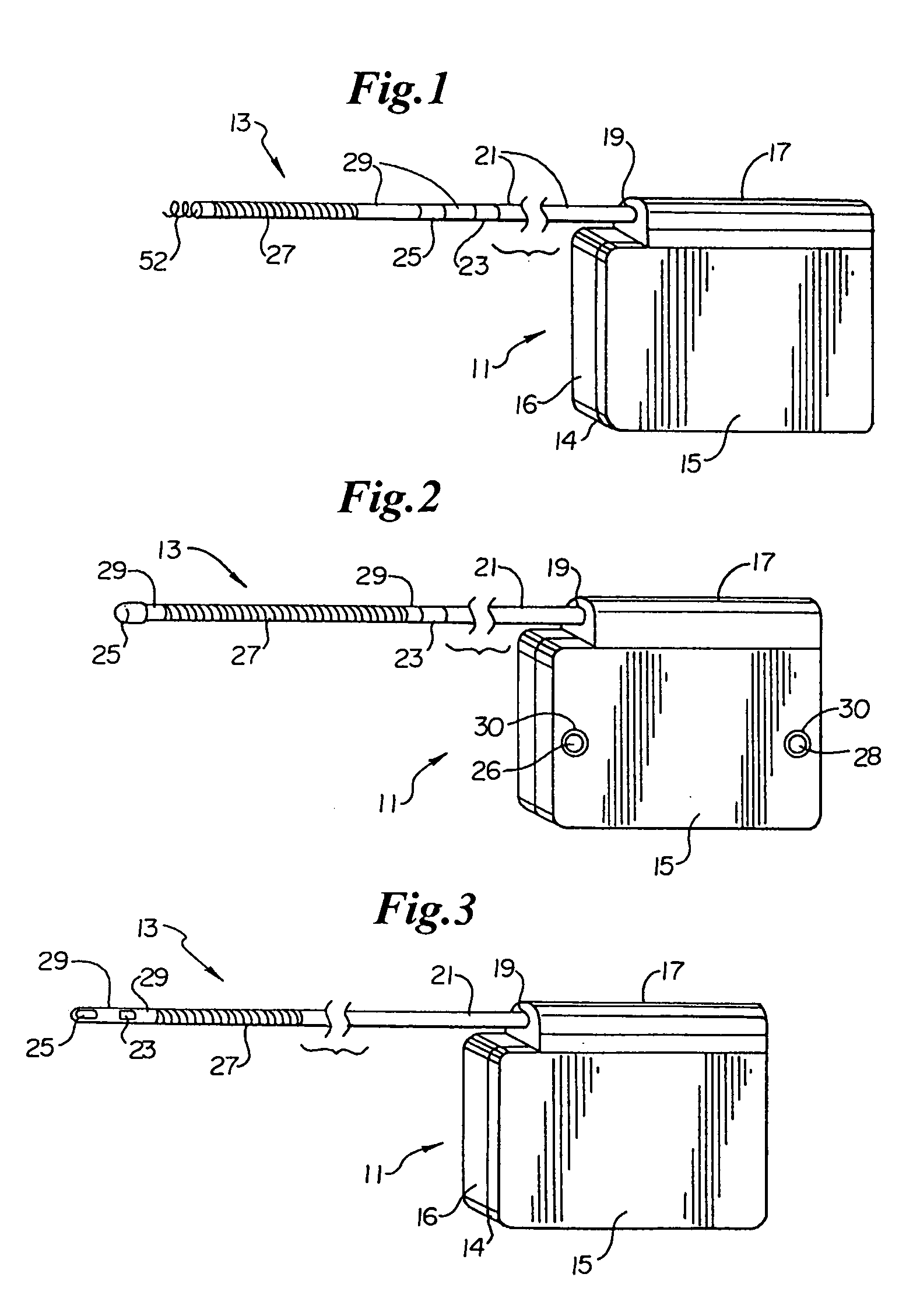
Subcutaneous implantable cardioverter-defibrillator employing a telescoping lead
One embodiment of the present invention provides an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing including a first electrode; a telescoping lead assembly including a second electrode, wherein the telescoping lead assembly is electrically and mechanically coupled to the housing; and an electrical circuit located within and electrically coupled to the housing.
Owner:CAMERON HEALTH
Method and apparatus for identifying oversensing using far-field intracardiac electrograms and marker channels
A method for identifying and classifying various types of oversensing in implantable medical devices (IMDs), such as implantable cardioverter defibrillators (ICDs), to assist a physician in choosing corrective action to reduce the likelihood of oversensing and inappropriate therapy delivery. Far-field electrogram (EGM) signals are analyzed to detect the occurrence of R-waves, and the result is compared to the number and pattern of R-waves sensed by the IMD and indicated on the marker channel. A marker channel with more sensed R-waves than indicated by analysis of the far-field EGM indicates the presence of oversensing, including double-counting of R-waves, T-wave oversensing, lead malfunction or failure, poor lead connections, noise associated with electromagnetic interference, non-cardiac myopotentials, etc. Identification of the type of oversensing may be determined by analysis of the number and pattern of marker channel sensed R-waves with respect to the timing of the R-waves detected from the far-field EGM.
Owner:MEDTRONIC INC
Leadless Implantable Cardioverter Defibrillator
A leadless implantable cardioverter defibrillator (5) for treatment of sudden cardiac death includes a controller and at least one remote module. The defibrillator does not require transvenous / vascular access for intracardiac lead placement. The controller is leadless and uses subcutaneous tissue in proximity of the chest and abdomen for both sensing and defibrillation. The controller and one or more remote sensors sense a need for defibrillation and wireless communicate with the controller. The controller and one of the sensors discharge a synchronized defibrillation pulse to the surrounding subcutaneous tissue in proximity to the heart.
Owner:UNIVERSITY OF ROCHESTER
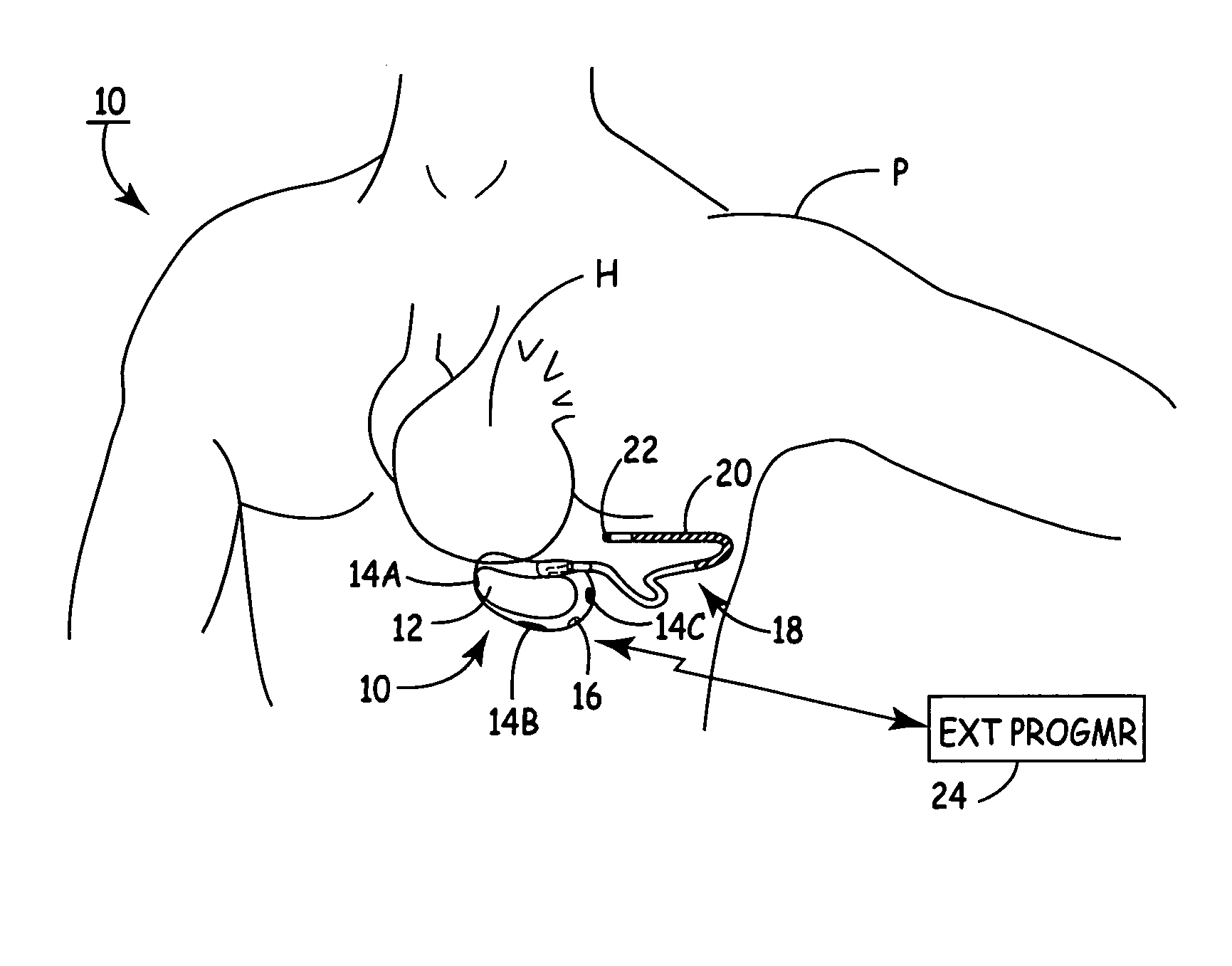
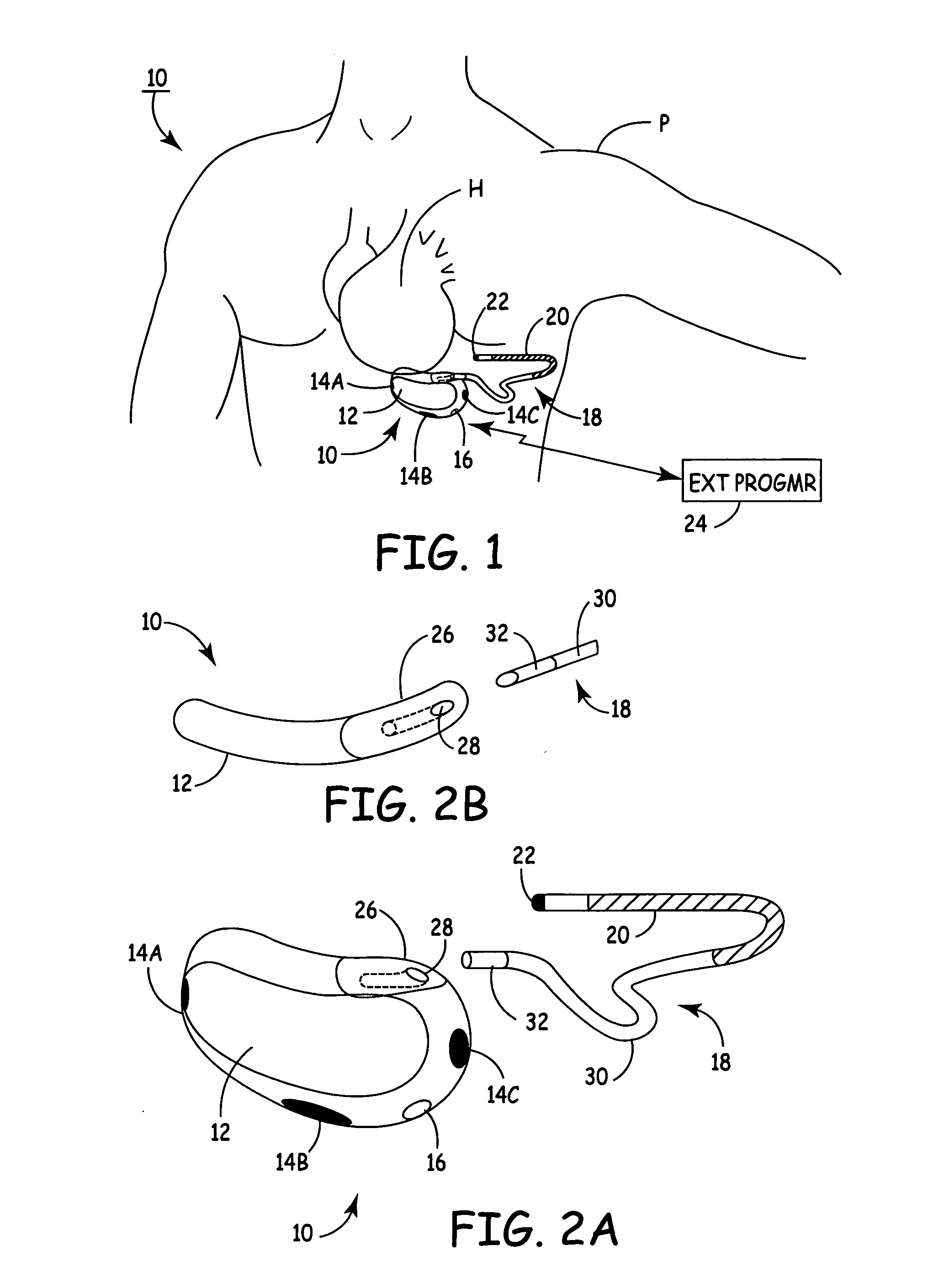
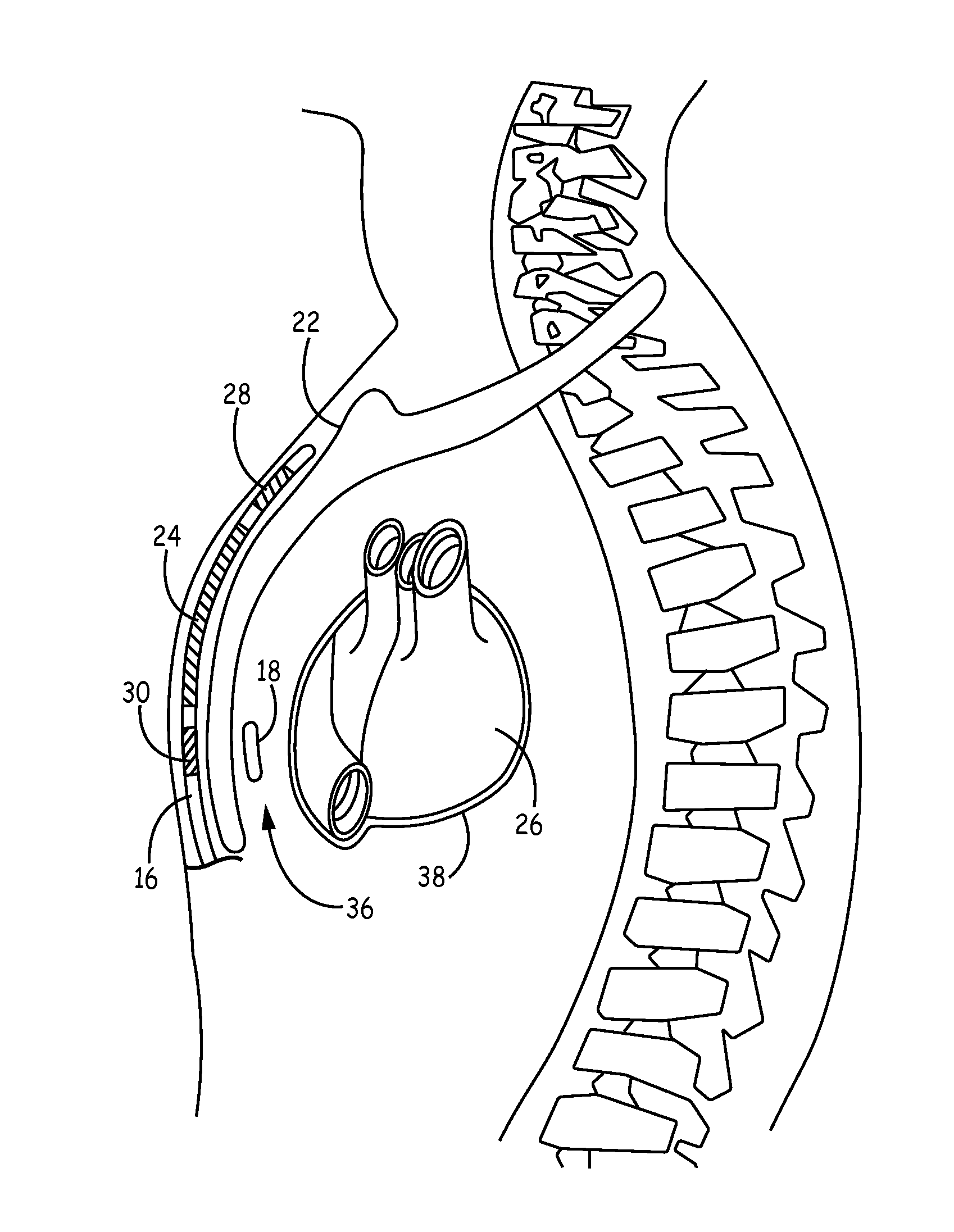
Implantable medical device system having implantable cardioverter-defibrillator (ICD) system and substernal leadless pacing device
Implantable cardiac systems and methods for providing substernal pacing in an ICD system are described. In one example, an implantable cardiac system comprises an ICD system and an implantable leadless pacing device (LPD) communicatively coupled to the ICD system. The ICD system includes an ICD and an implantable defibrillation lead having a proximal portion coupled to the ICD and a distal portion having a defibrillation electrode configured to deliver a defibrillation shock to a heart of the patient. The LPD includes a housing, a first electrode on the housing, a second electrode on the housing, and a pulse generator within the housing and electrically coupled to the first electrode and the second electrode. The housing of the LPD is implanted substantially within an anterior mediastinum of the patient and the pulse generator is configured to deliver pacing pulses to a heart via the first and second electrodes.
Owner:MEDTRONIC INC
Method and apparatus for monitoring ingestion of medications using an implantable medical device
InactiveUS20100139672A1Reduce the possibilityIncrease chances of survivalDrug and medicationsSurgeryTransceiverCardiac pacemaker electrode
Owner:PACESETTER INC
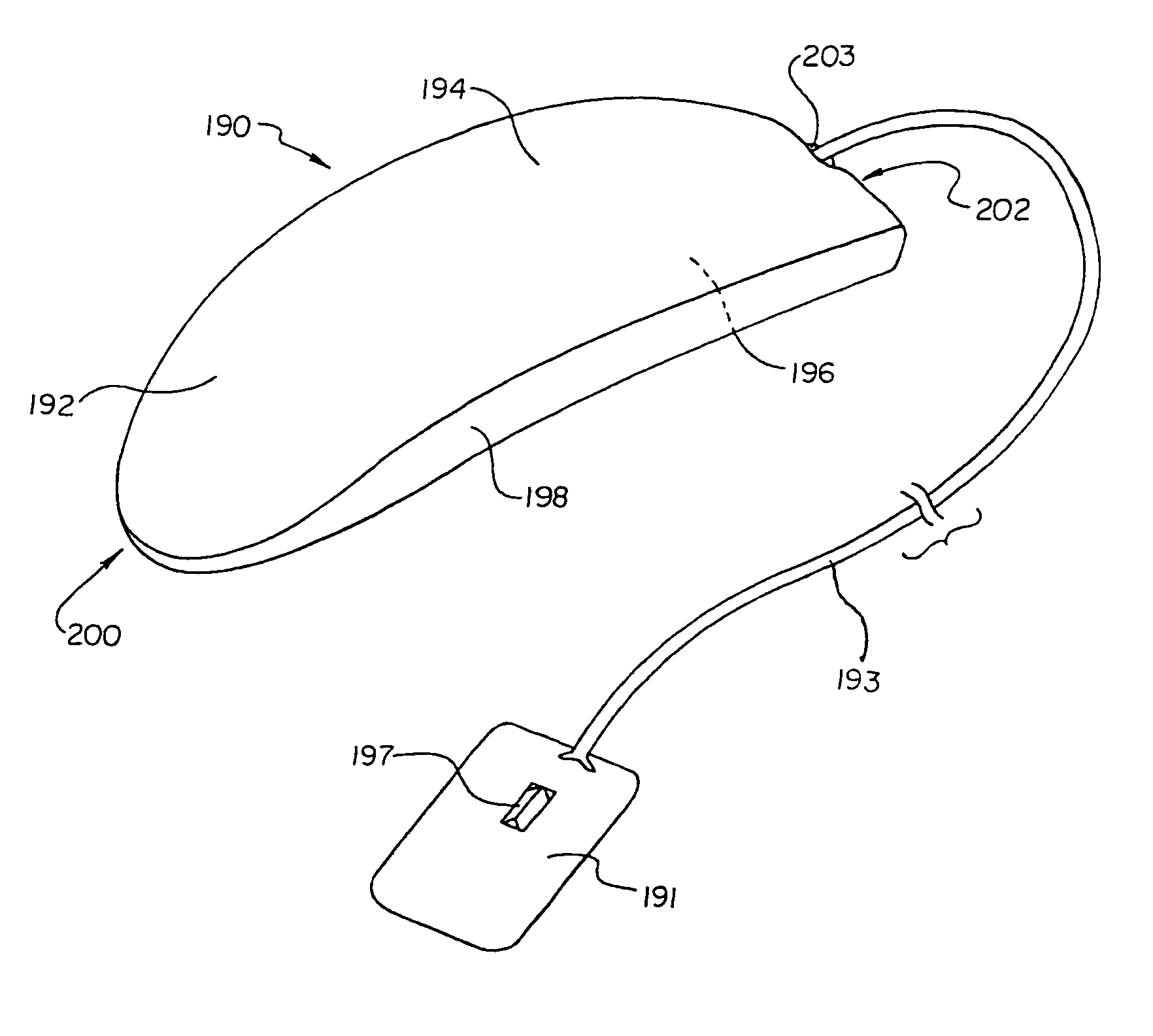
Implantable leadless cardiac device with flexible flaps for sensing
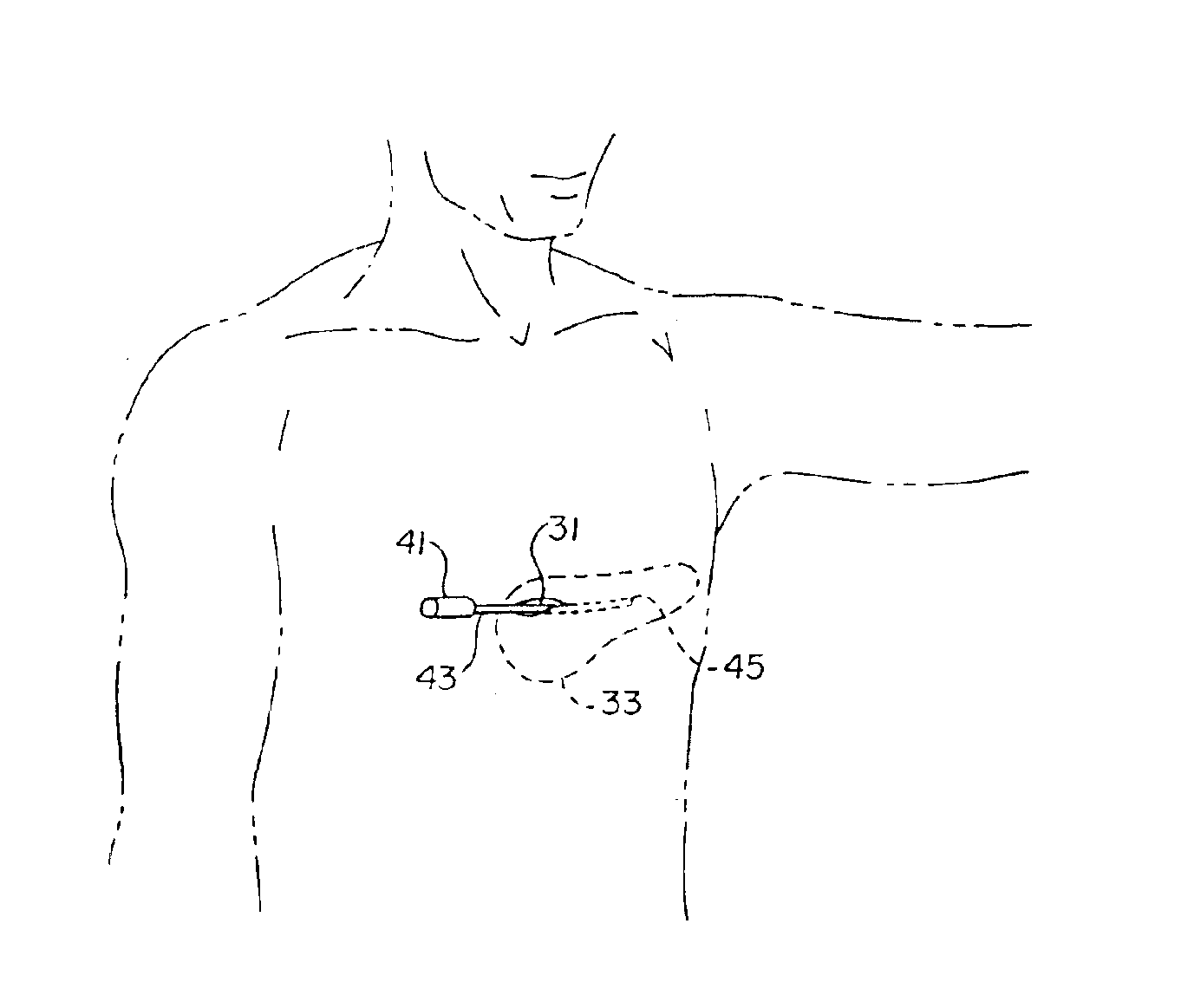
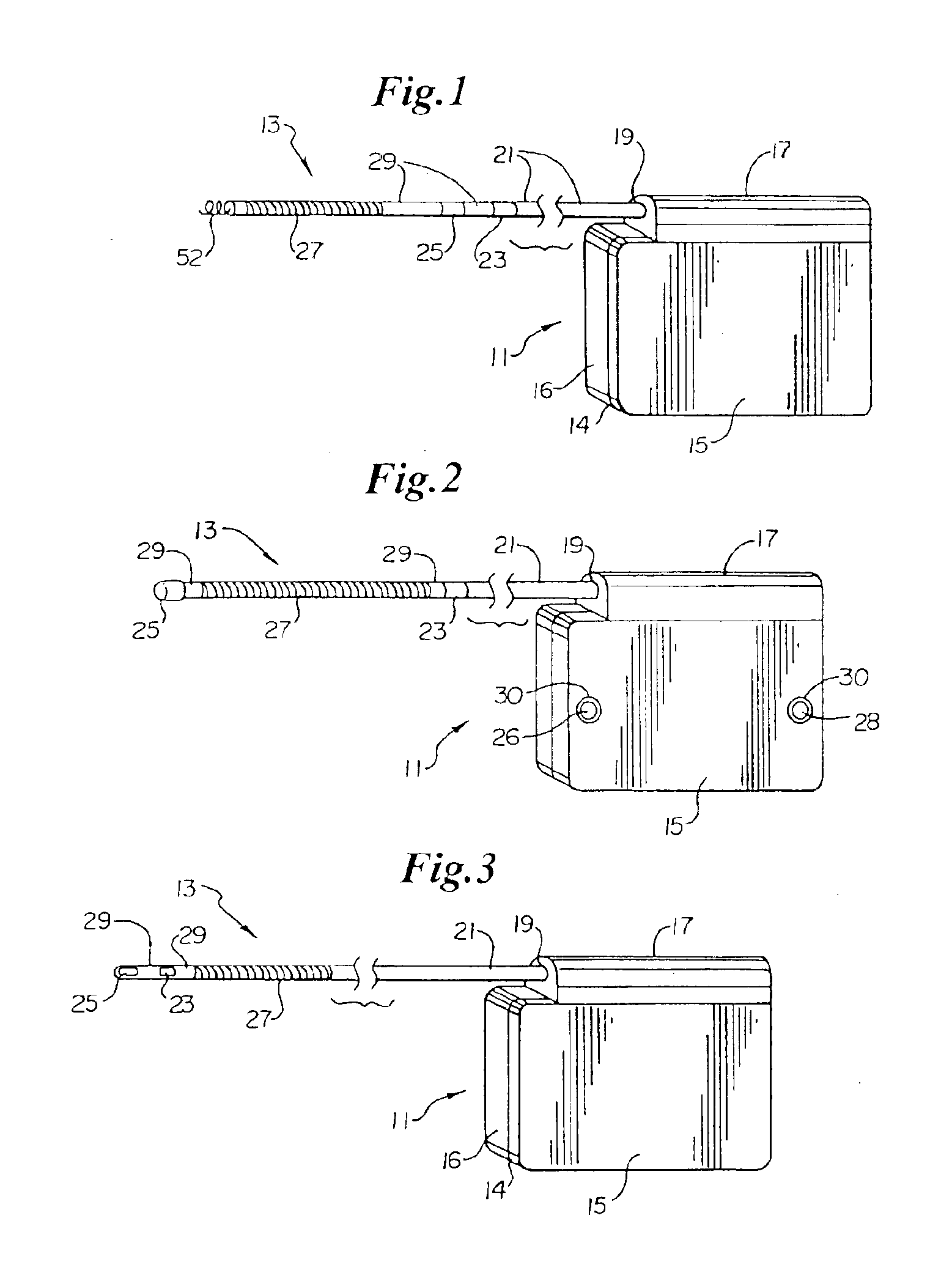
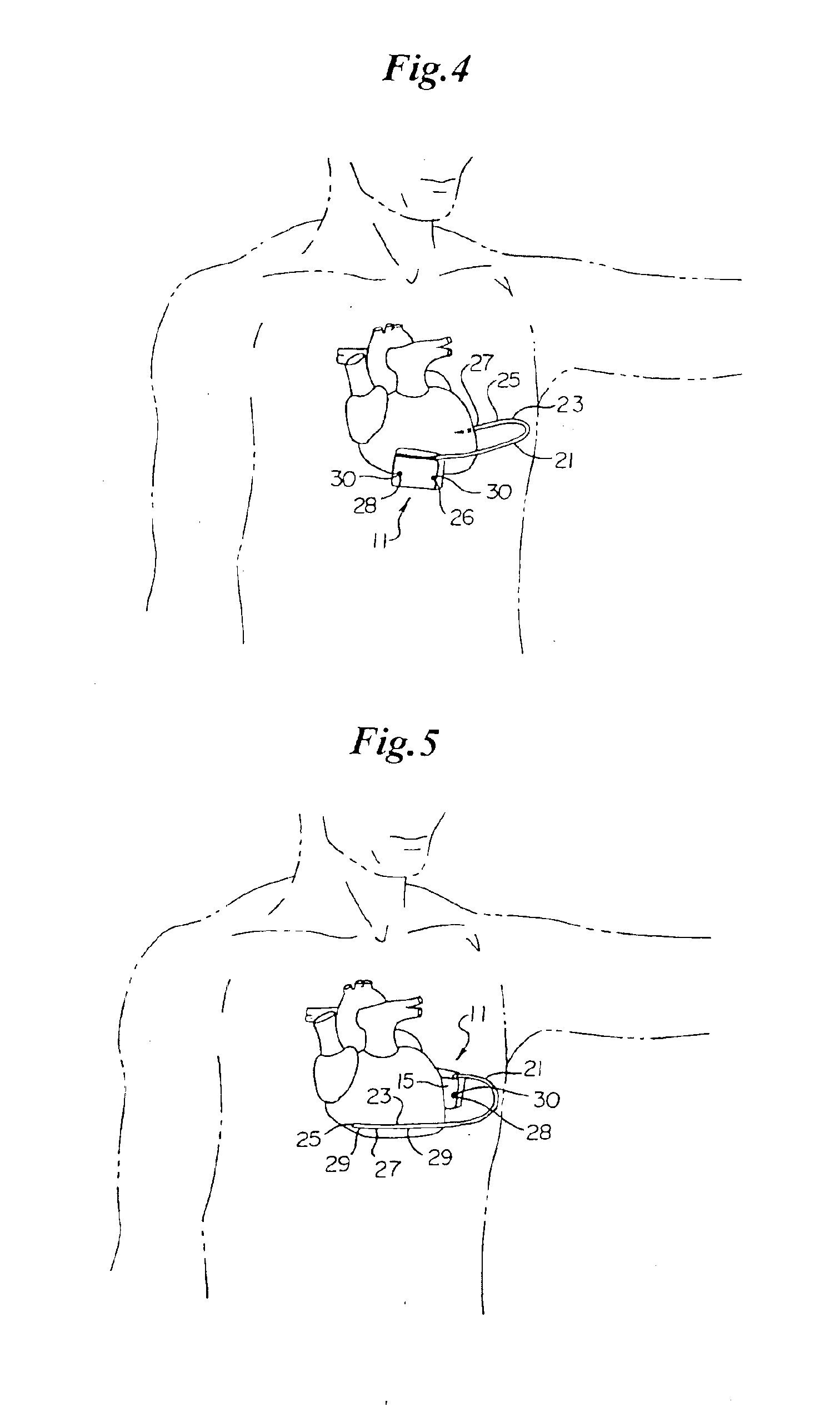
A technique for performing sensing operations in the body subcutaneously employs, in combination, the housing of an electrical stimulator such as a pacemaker or an implantable cardioverter defibrillator and an integral flap member of flexible insulative sheet material which has at least one arm projecting away therefrom to an extreme tip end. Each arm is rolled about itself from the tip end on a lateral axis transverse of the longitudinal axis of the arm into a compact unit adjacent the housing. An incision is made through the skin and a region beneath the skin enlarged to form a cavity. The compact unit is then inserted into the cavity and unrolled about the lateral axis of each arm so as to be laid flat under the skin. When positioned into a desired orientation, each flap member is sutured to the skin to maintain the desired orientation.
Owner:PACESETTER INC
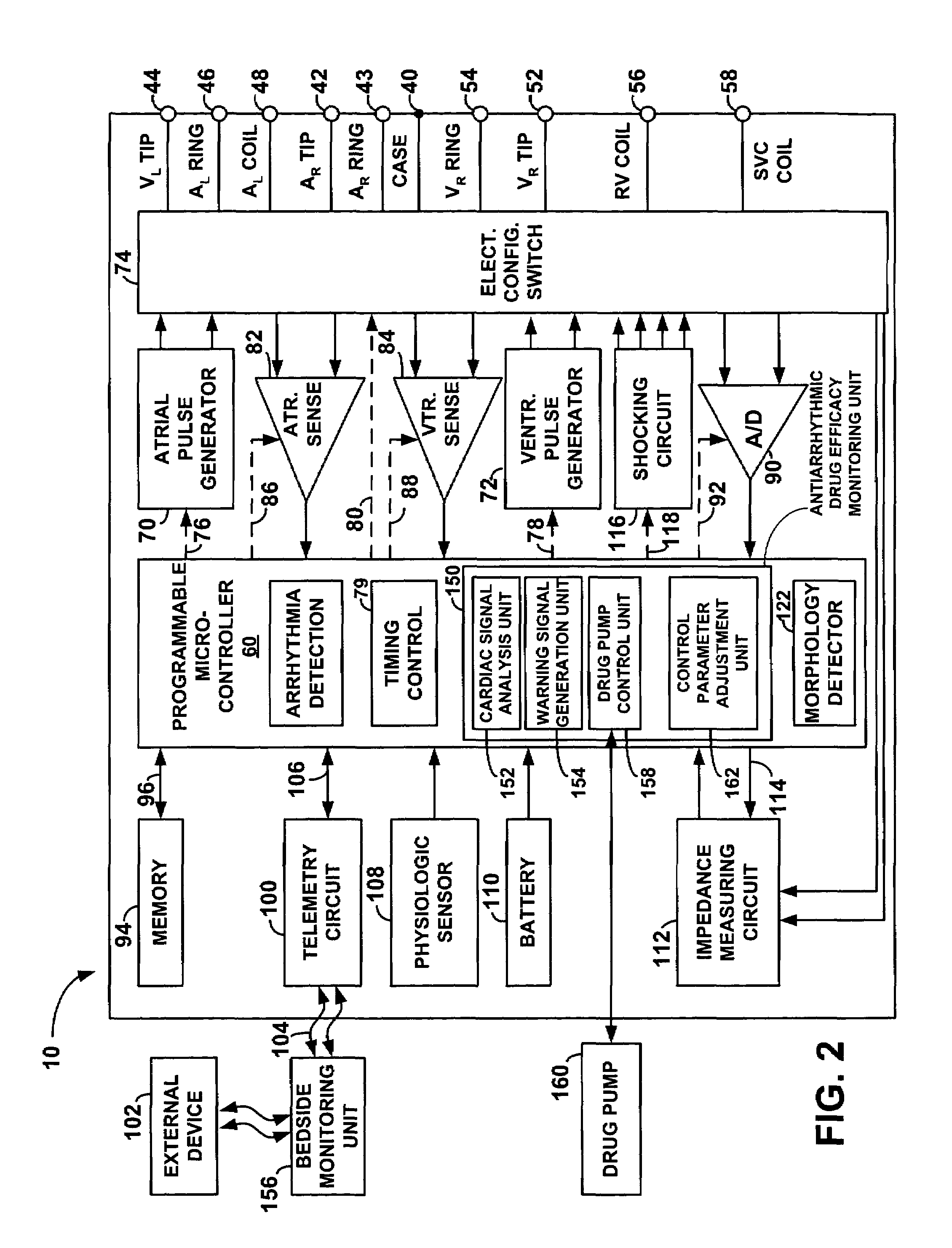
Method and apparatus for monitoring drug effects on cardiac electrical signals using an implantable cardiac stimulation device
InactiveUS7142911B2Increase aggressivenessIncrease dosePhysical therapies and activitiesDrug and medicationsEcg signalCardiac pacemaker electrode
An implantable cardiac stimulation device, such as a pacemaker or Implantable Cardioverter Defibrillator, is configured to automatically monitor the effects of antiarrhythmic drugs on cardiac electrical signals within a patient to verify the efficacy of the drugs taken. In one example, an analysis of patient cardiac electrical signals is performed by comparing the cardiac electrical signals with values representative of the effects of different classes of antiarrhythmic drugs. If the implantable device determines that the prescribed antiarrhythmic drugs have not been effective, a warning signal is generated. The warning signal is conveyed directly to the patient via a bedside monitor and to the patient's physician via remote connection to an external programmer device so that both are notified of the drug efficacy problems. Additionally, the implantable device may be configured to automatically adjust pacing and defibrillation control parameters in an attempt to compensate for any lack of efficacy in the drugs. For example, the aggressiveness of overdrive pacing may be increased. Alternatively, a drug pump is controlled to adjust the dosage of antiarrhythmic drugs if an initial dosage is found to be ineffective.
Owner:PACESETTER INC
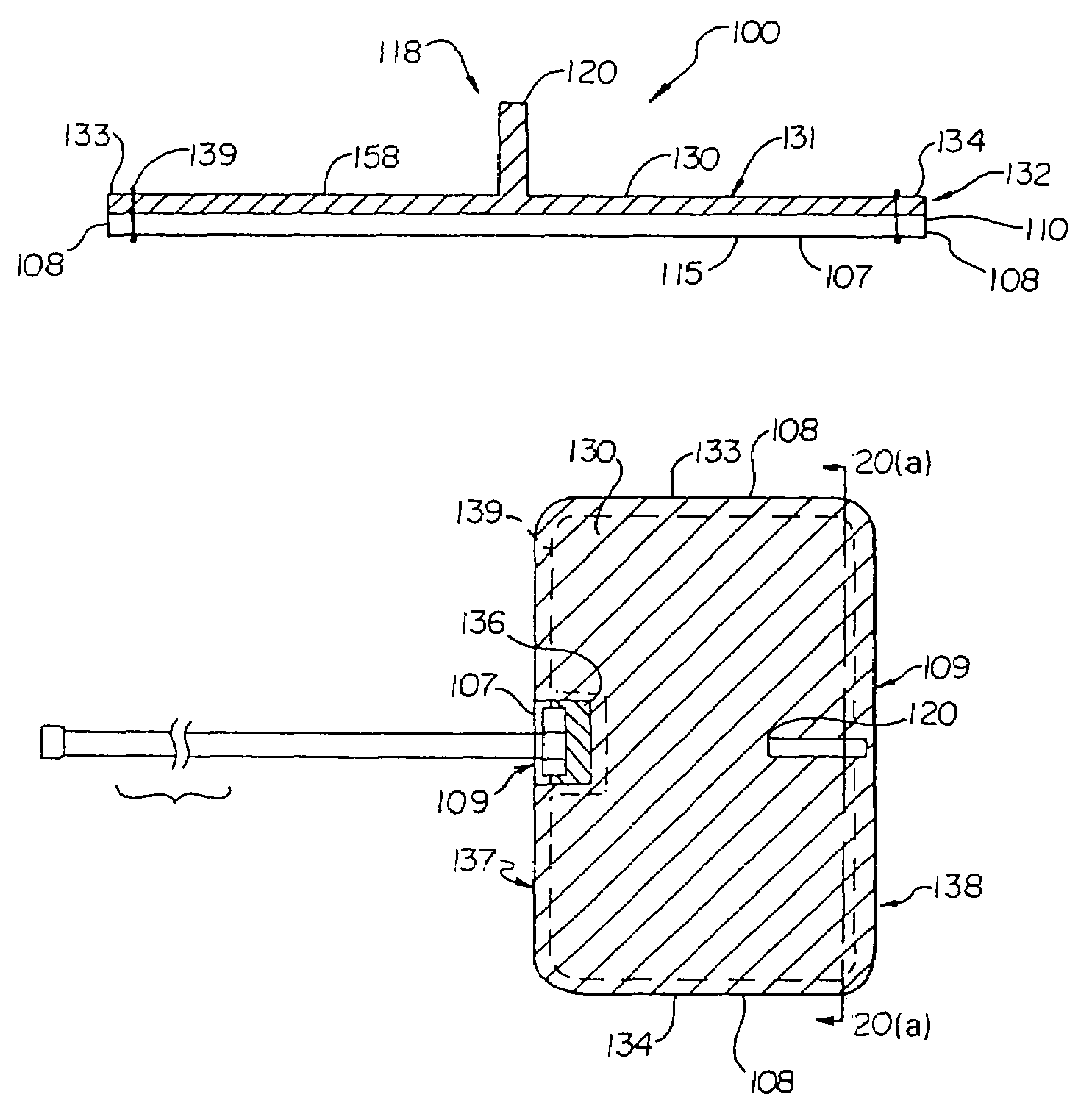
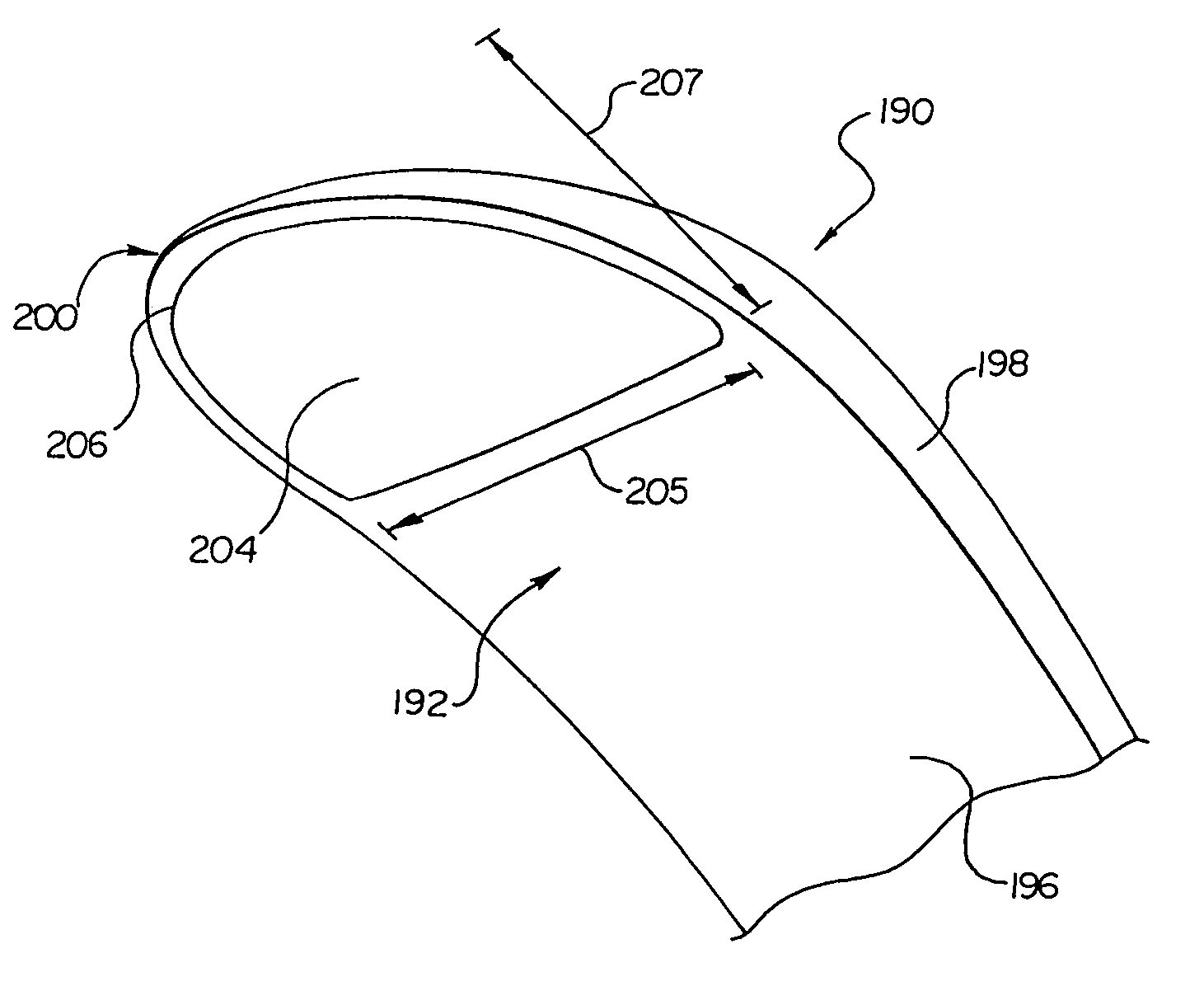
Canister designs for implantable cardioverter-defibrillators
One embodiment of the present invention provides an implantable cardioverter defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing; having a first surface and a second surface, wherein the first surface comprises an electrically insulated material and the second surface comprises an electrically conductive material; and an electrical circuit located within the housing, wherein the electrical circuit is electrically coupled to the second surface of the housing.
Owner:CAMERON HEALTH
Flexible subcutaneous implantable cardioverter-defibrillator
One embodiment of the present invention provides an implantable cardioverter-defibrillator for subcutaneous positioning over a patient's ribcage, the implantable cardioverter-defibrillator including a housing, wherein the housing conforms to the patient's ribcage when subcutaneously positioned; an electrode disposed upon a portion of the housing; and an electrical circuit located within the housing, wherein the electrical circuit is electrically coupled to the electrode.
Owner:CAMERON HEALTH
Cardioverter-defibrillator having a focused shocking area and orientation thereof
One embodiment of the present invention provides an implantable cardioverter defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing; an electrical circuit located within the housing; a first electrode coupled to the electrical circuit and located on the housing; and a second electrode coupled to the electrical circuit.
Owner:CAMERON HEALTH
Monophasic waveform for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator
Owner:CAMERON HEALTH
Duckbill-shaped implantable cardioverter-defibrillator canister and method of use
An implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and twelfth rib within a patient includes a duck-bill shaped housing. The housing includes a proximal end and distal end, where the width of the proximal end is less than the width of the distal end, and an electrode located on the housing.
Owner:CAMERON HEALTH
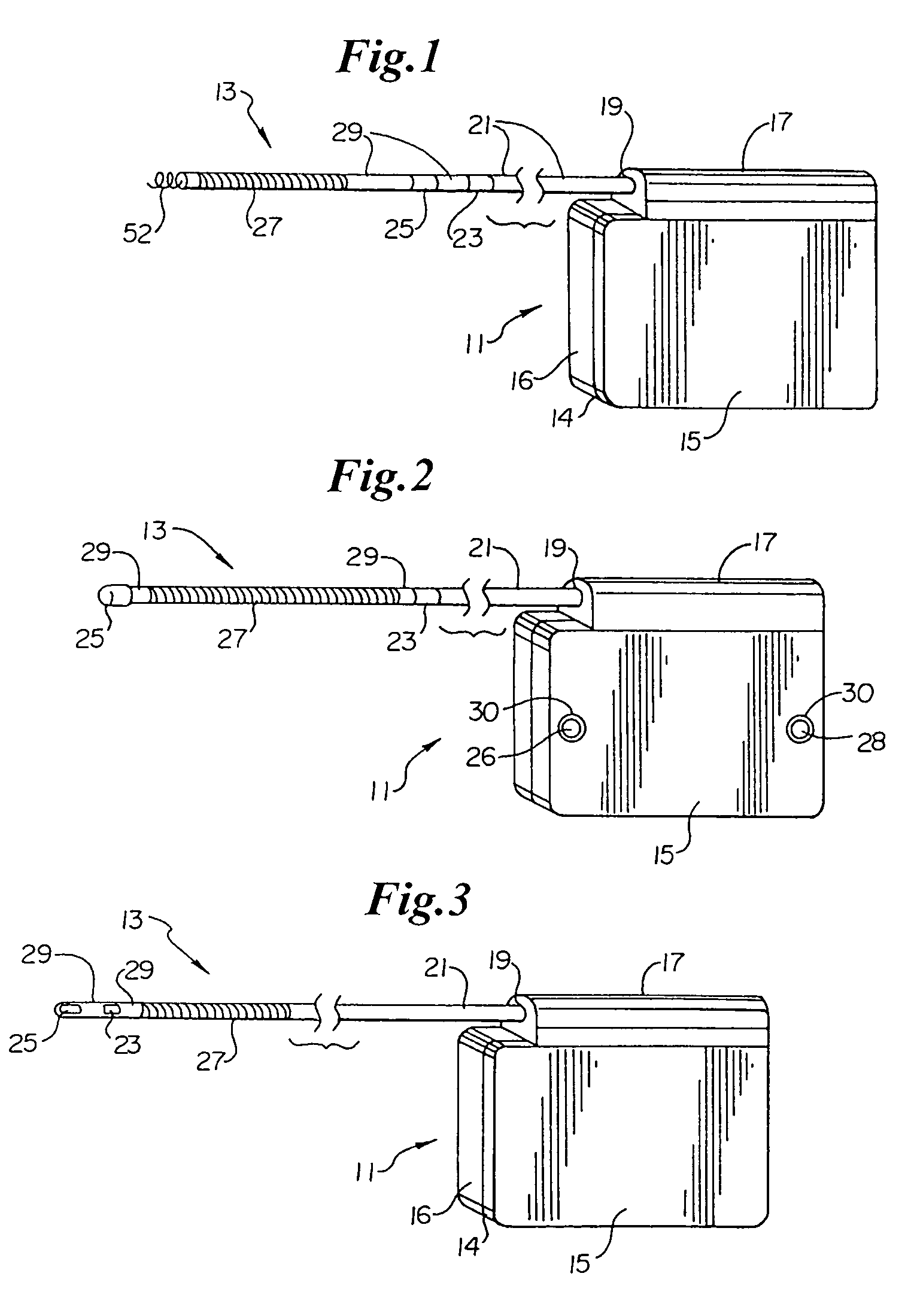
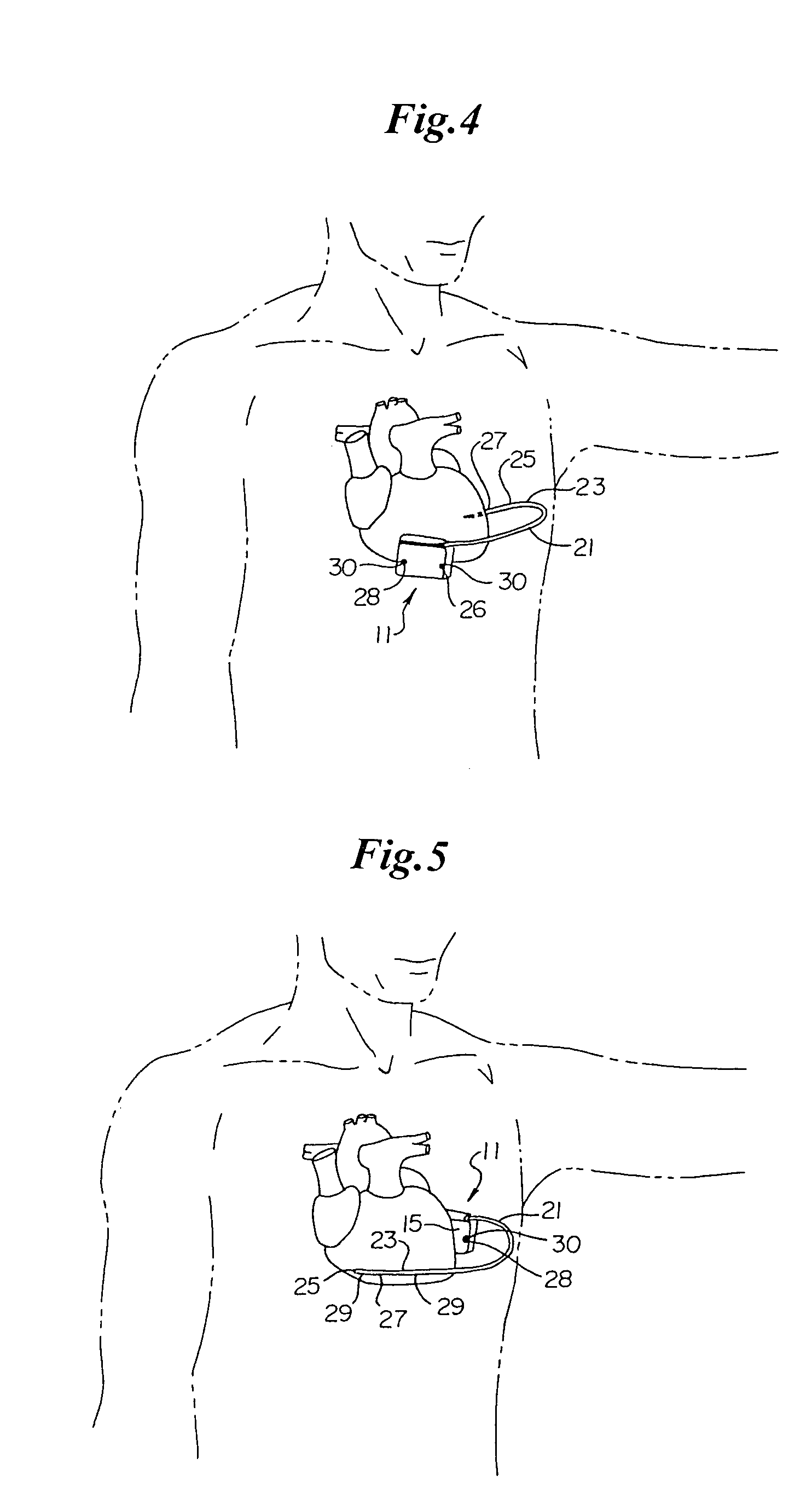
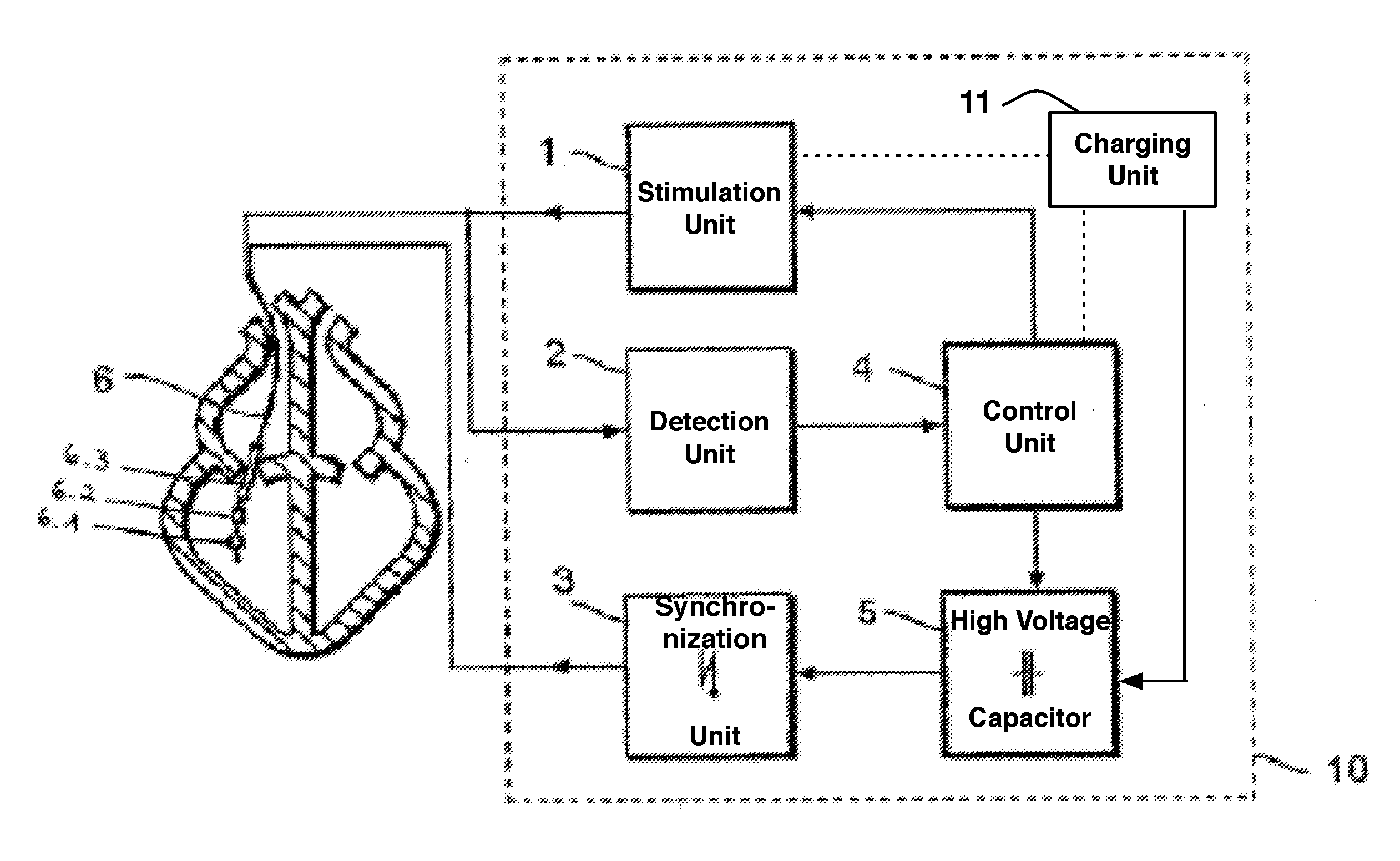
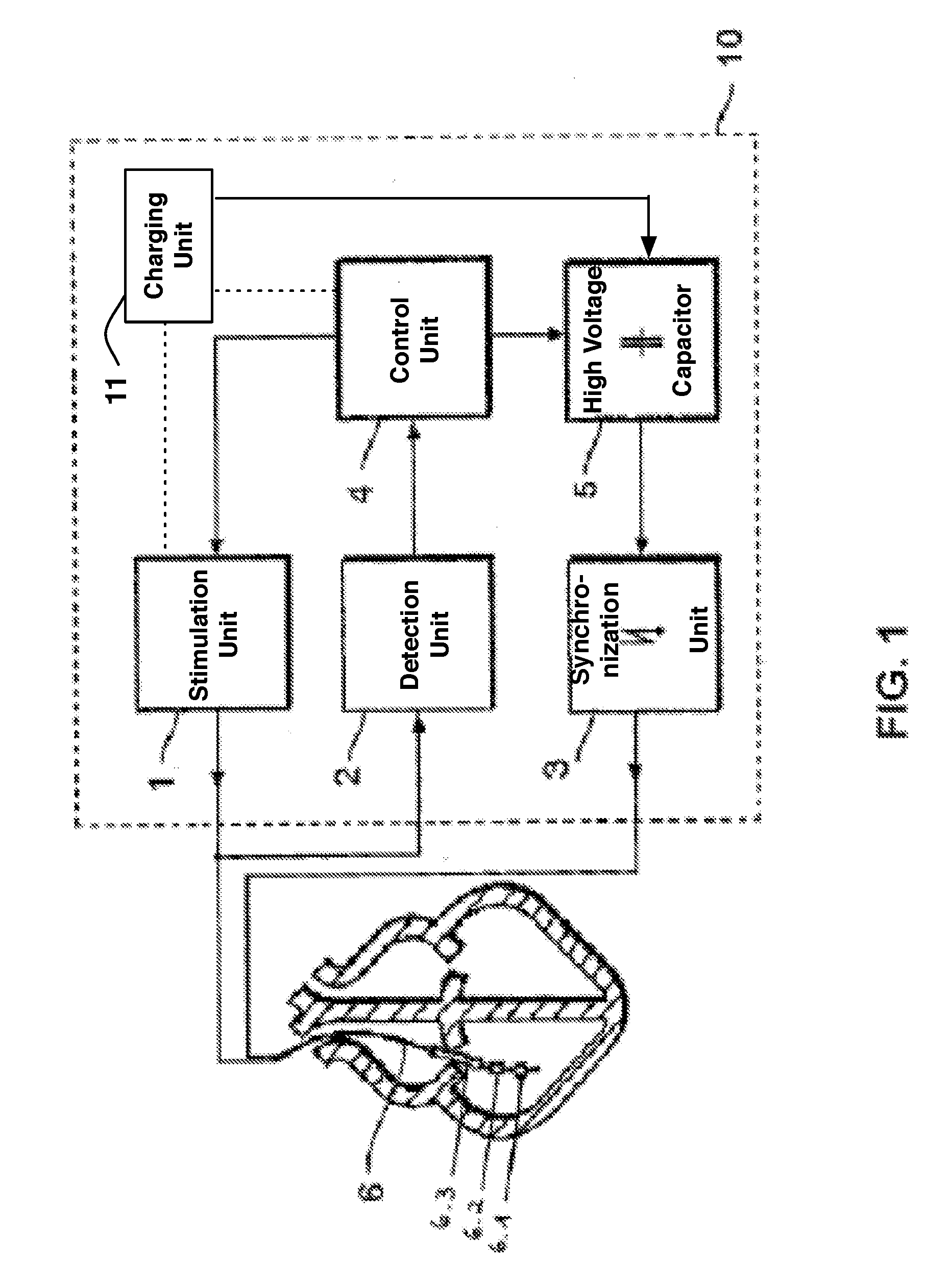
Implantable cardioverter-defibrillator including arrhythmia detection criteria
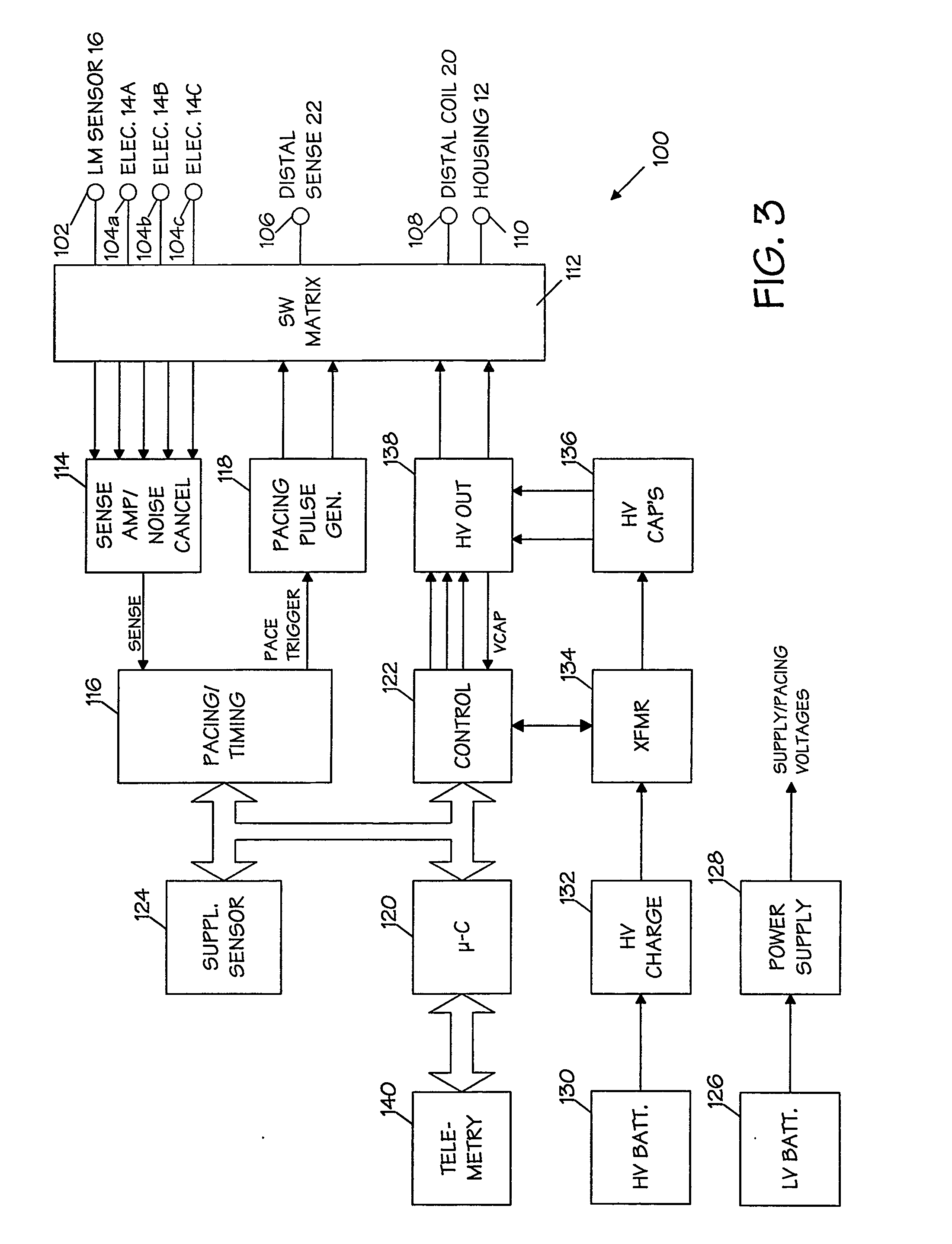
ActiveUS9393436B2Improve reliabilityLower success rateHeart defibrillatorsMedicineHigh voltage capacitors
An implantable cardioverter-defibrillator system that includes at least one or more stimulation units, one or more detection units, one or more control units, two or more electrode poles and one or more high voltage capacitors. The at least one control unit is connected with the at least one stimulation unit, and the at least one control unit is connected with at the least one detection unit. The two or more electrode poles are in contact with body tissue, and the one or more high voltage capacitors are charged by at least one charging unit, wherein the at least one charging unit is connected to the at least one control unit.
Owner:BIOTRONIK SE & CO KG
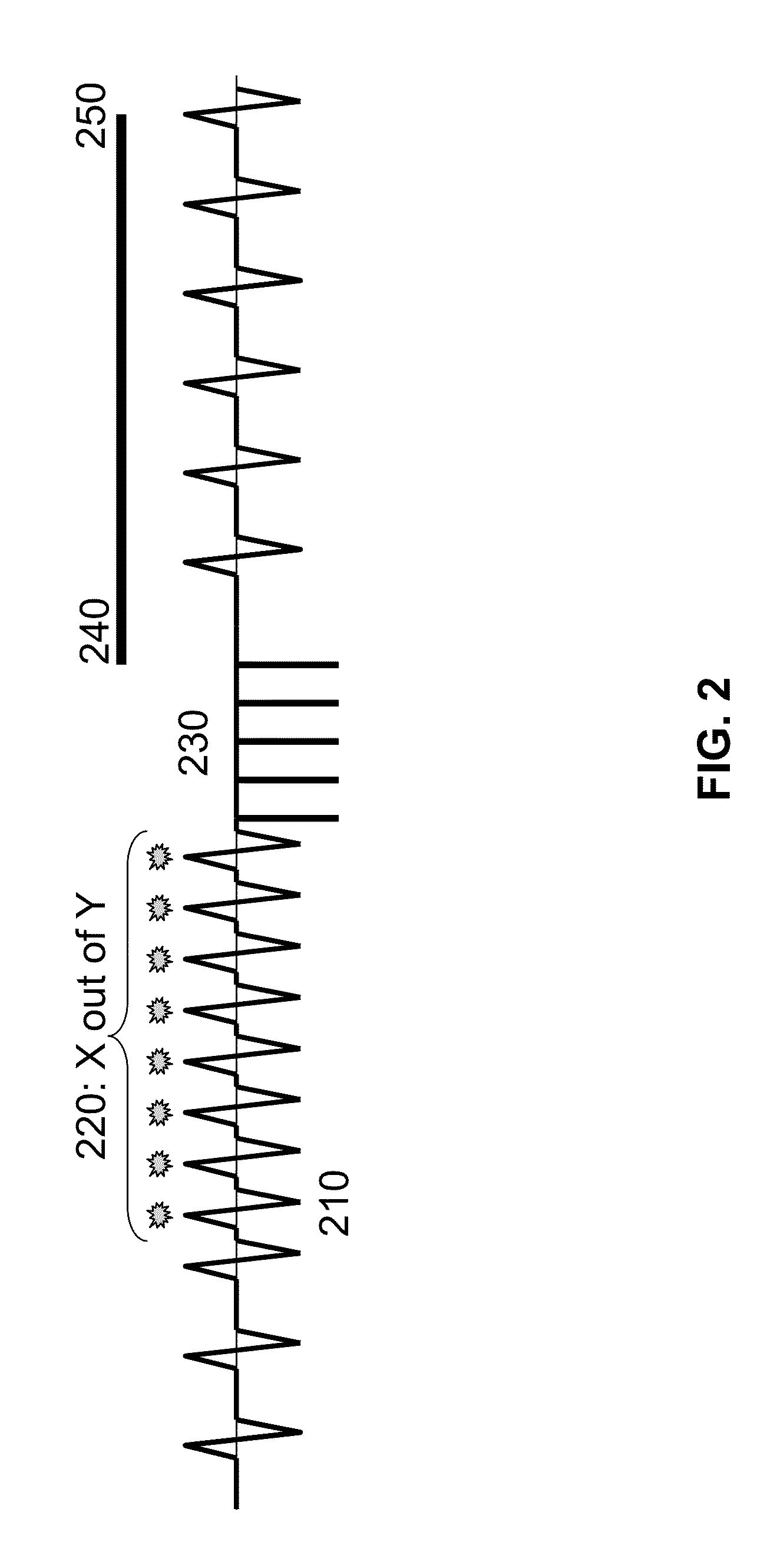
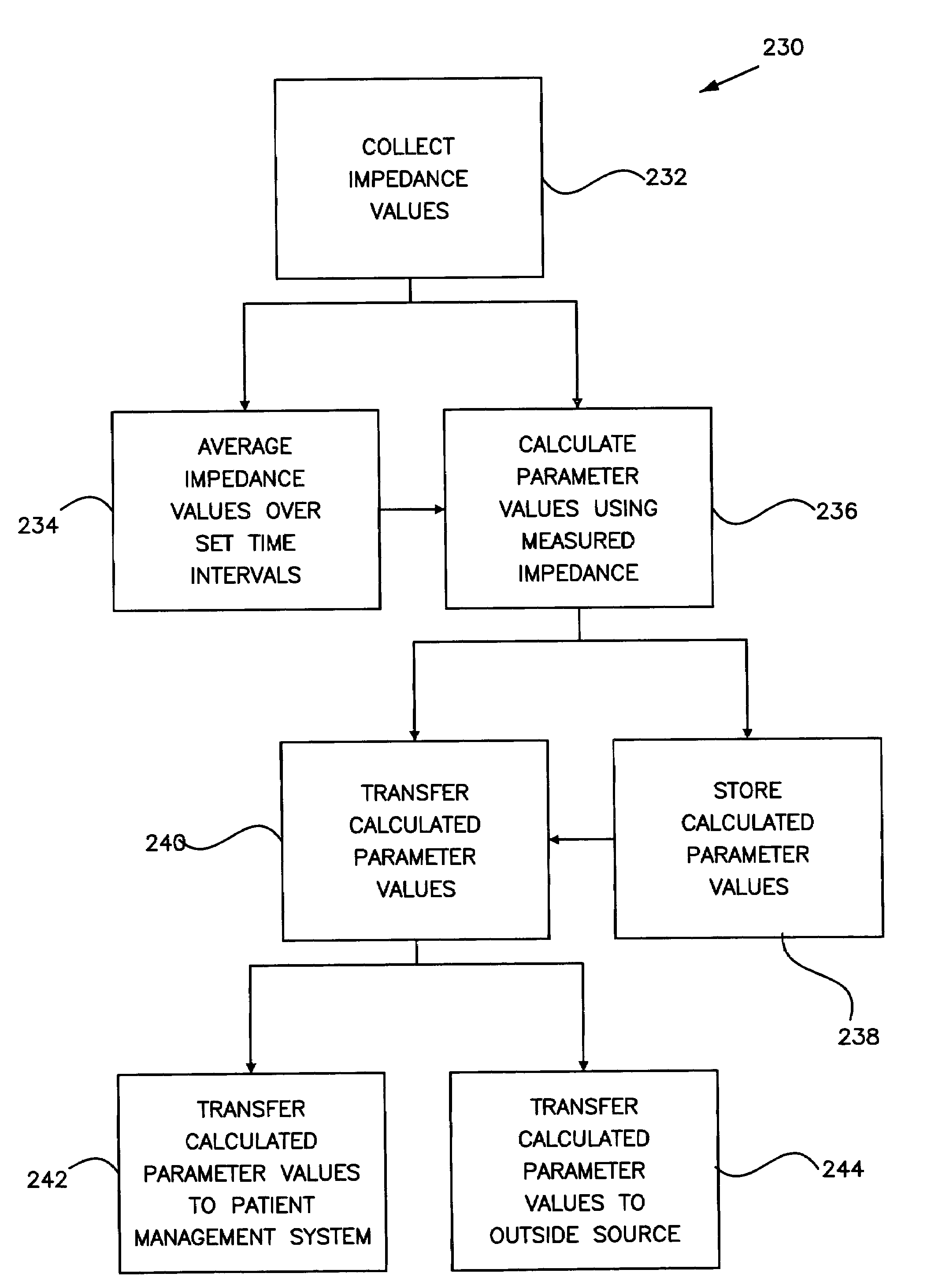
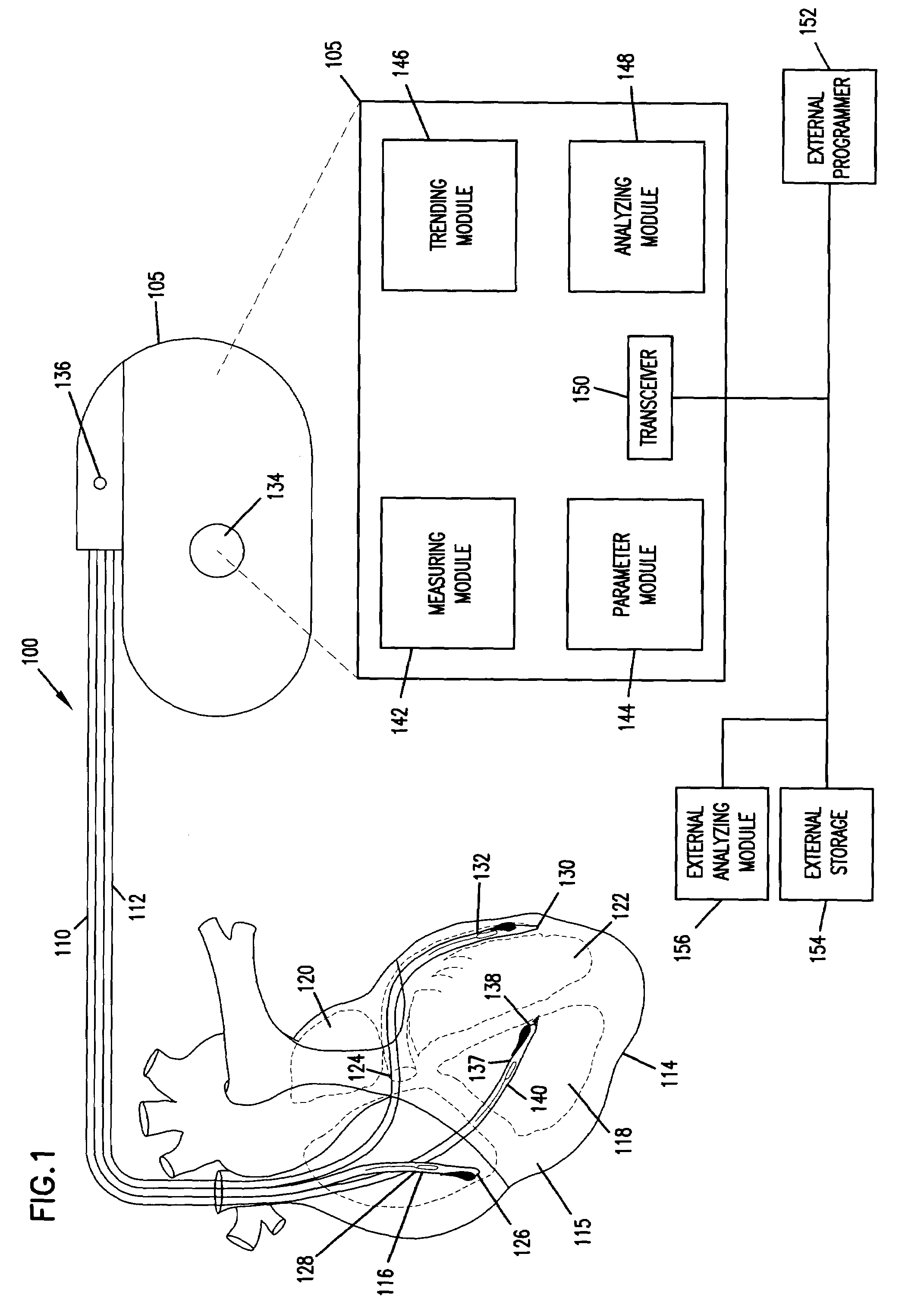
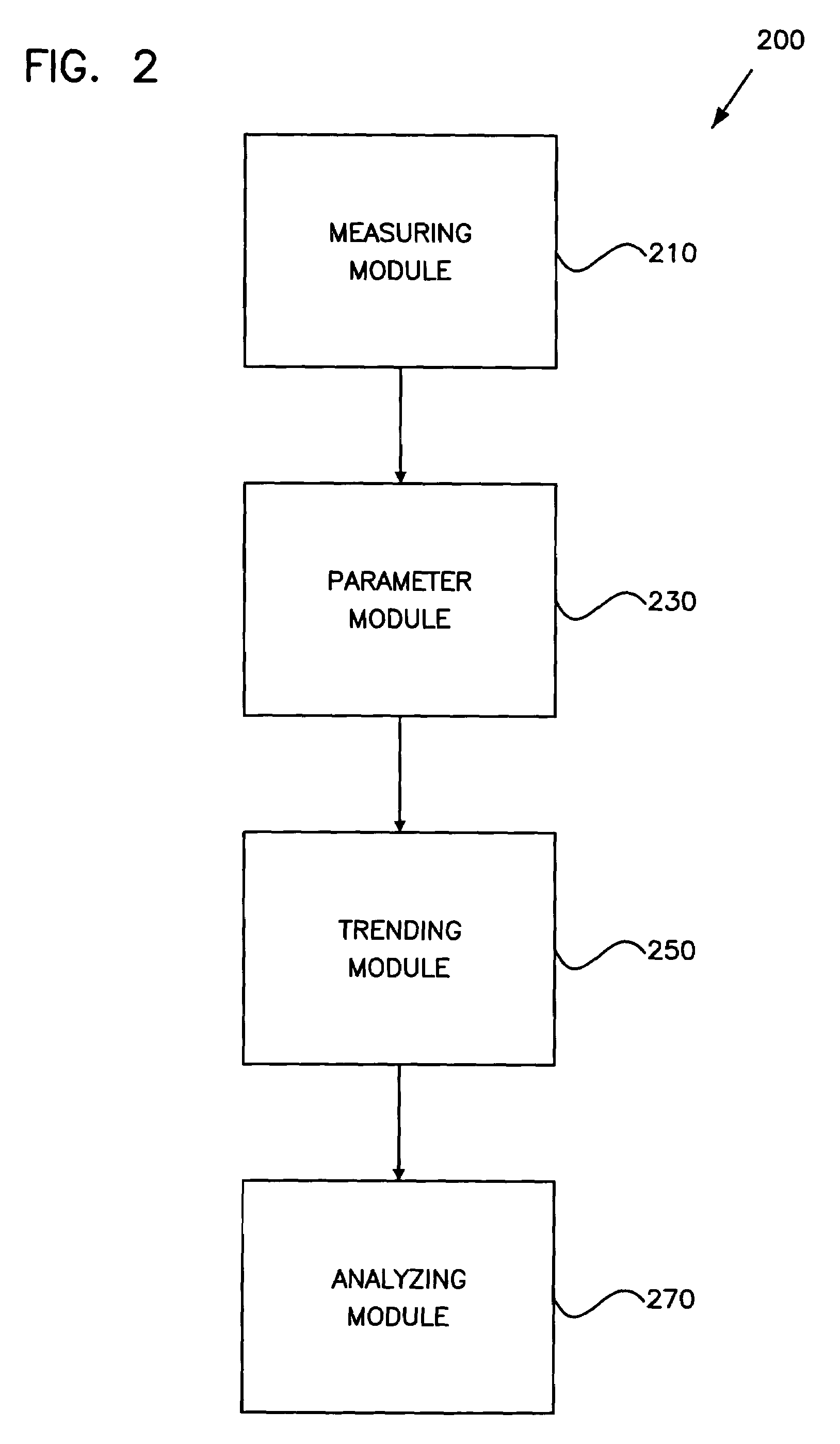
Method and apparatus for trending a physiological cardiac parameter
ActiveUS7171258B2Prediction of efficacyMonitor progressElectrotherapySensorsCardiac pacemaker electrodeSympathetic tone
The present invention relates to an implantable cardioverter-defibrillator or pacemaker whose standard circuitry is used to trend a physiological cardiac parameter using intra-cardiac impedance measurements. The trend information may be used to predict the onset of a sudden cardiac death (SCD) event. By being able to predict the onset of an SCD event, patients and their physicians may be forewarned of a life-threatening event allowing them to respond accordingly. The trend information may also be used to predict the efficacy of cardiac-related medications, monitor progress of congestive heart failure, detect the occurrence of myocardial infarction, or simply track changes in sympathetic tone.
Owner:CARDIAC PACEMAKERS INC
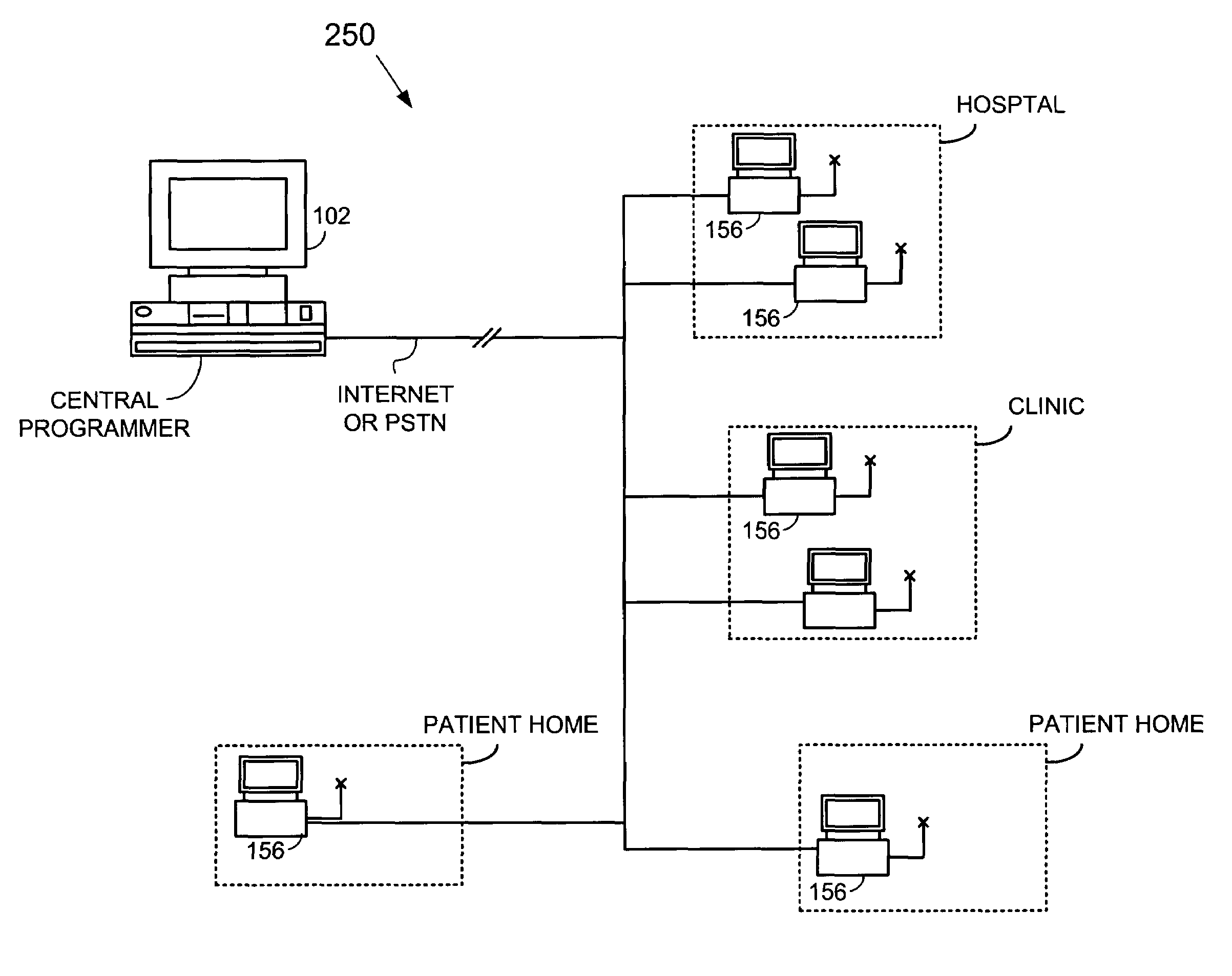
Method and apparatus to backup, update and share data among implantable cardiac stimulation device programmers
InactiveUS6961617B1Easy access to dataEasy accessElectrotherapyCardiac pacemaker electrodePatient data
A system is provided for backing up and synchronizing data stored within a set of programmers used for programming implantable cardiac stimulation devices such as pacemakers or implantable cardioverter defibrillators (ICDs). The data from the programmers that is backed up and synchronized includes programmer software, set up and configuration data, programming parameters, patient personal data, implantable device diagnostic data, and patient diagnostic data received from implanted devices. The implanted devices are classified into one or more groups and the programmer backup system merges and synchronizes data received from all programmers within a particular group. In other words, the backup system operates to ensure that each programmer within a particular group shares the same set up and configuration data, programmer software, patient contact data, and device diagnostic information. Programmers within different groups may have different data stored therein, particularly different set up and configuration data, patient data and the like. The merging and synchronization of data may depend upon the particular type of data. For example, all devices connected to the backup system may be synchronized to have the same programmer software, whereas only programmers within a particular group may be synchronized to have the same set up and configuration data, patient data, and the like.
Owner:PACESETTER INC
Anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator
A power supply for an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib and using a lead system that does not directly contact a patient's heart or reside in the intrathoracic blood vessels and far providing anti-bradycardia pacing energy to the heart, comprising a capacitor subsystem for storing the anti-bradycardia pacing energy for delivery to the patient's heart; and a battery subsystem electrically coupled to the capacitor subsystem for providing the anti-bradycardia pacing energy to the capacitor subsystem.
Owner:CAMERON HEALTH
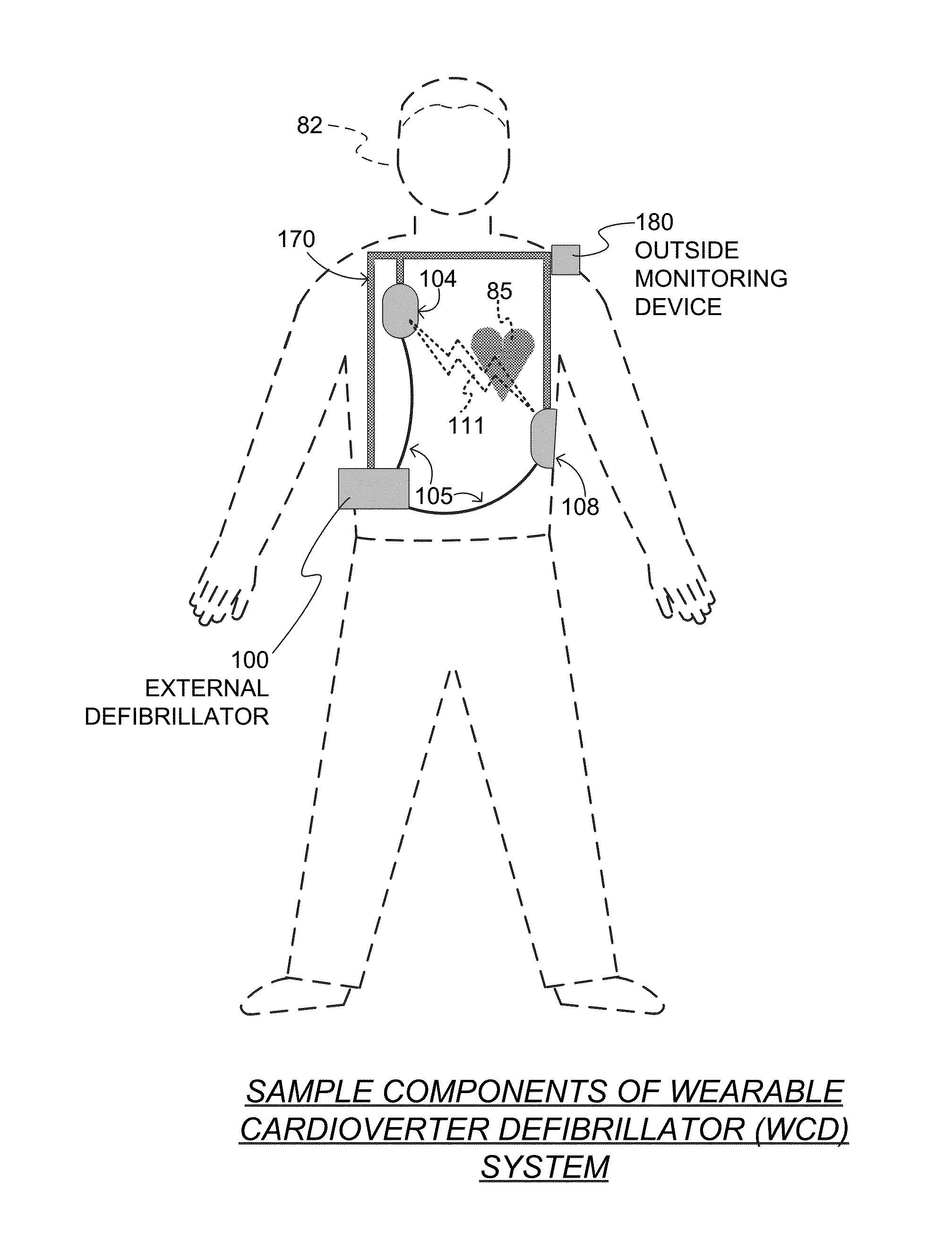
Wearable cardioverter defibrillator (WCD) system informing patient that it is validating just-detected cardiac arrhythmia
ActiveUS20160082277A1Reduce morbidityHeart defibrillatorsDiagnostic recording/measuringVentricular tachycardiaWearable cardioverter defibrillator
In some embodiments, a wearable cardioverter defibrillator (“WCD”) system may output an opening human-perceptible indication, after detecting a shockable cardiac arrhythmia but before validating it. This may succeed in informing the patient that the WCD system is working, and in particular analyzing a just-detected cardiac arrhythmia. The information may give comfort and confidence to the patient who may be conscious, and be experiencing only ventricular tachycardia but not ventricular fibrillation.
Owner:WEST AFFUM HLDG DAC
Implantable cardioverter defibrillator with automatically adaptable sensing configuration
Owner:MEDTRONIC INC
Ceramics and/or other material insulated shell for active and non-active S-ICD can
An implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing, wherein at least a portion of the housing is curved; an electrical circuit; and at least one electrically conductive surface integrally positioned on at least one portion of the house, wherein at least one electrically conductive surface is coupled to the electrical circuit.
Owner:CAMERON HEALTH
Implantable cardioverter-defibrillator (ICD) system including substernal pacing lead
An implantable cardiac defibrillator (ICD) system includes an ICD implanted subcutaneously in a patient, a defibrillation lead having a proximal portion coupled to the ICD and a distal portion having a defibrillation electrode configured to deliver a defibrillation or cardioversion shock to a heart of the patient, and a pacing lead that includes a distal portion having one or more electrodes and a proximal portion coupled to the ICD. The distal portion of the pacing lead is implanted at least partially along a posterior side of a sternum of the patient within the anterior mediastinum. The ICD is configured to provide pacing pulses to the heart of the patient via the pacing lead and provide defibrillation shocks to the patient via the defibrillation lead. As such, the implantable cardiac system provides pacing from the substernal space for an extravascular ICD system.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com