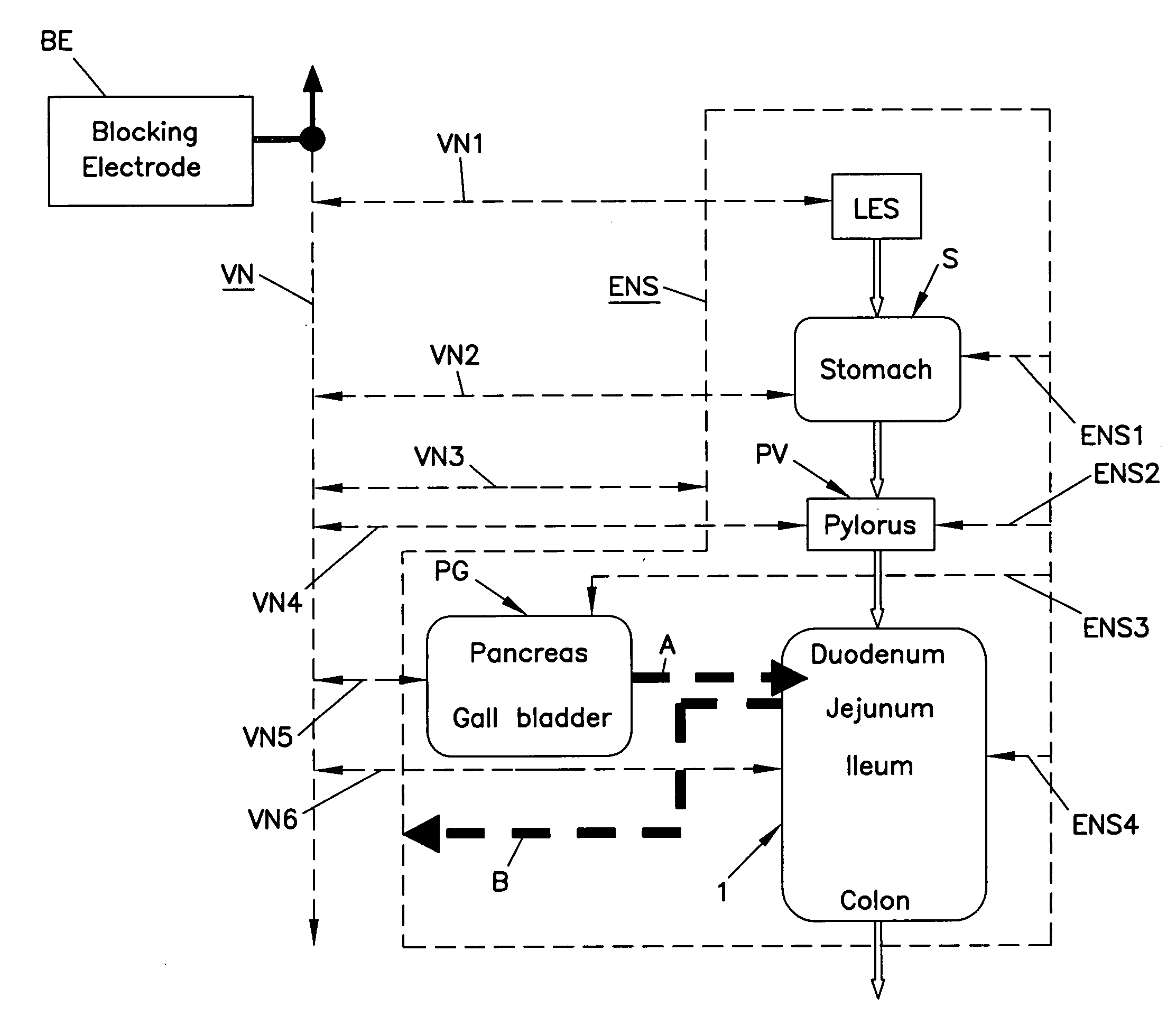
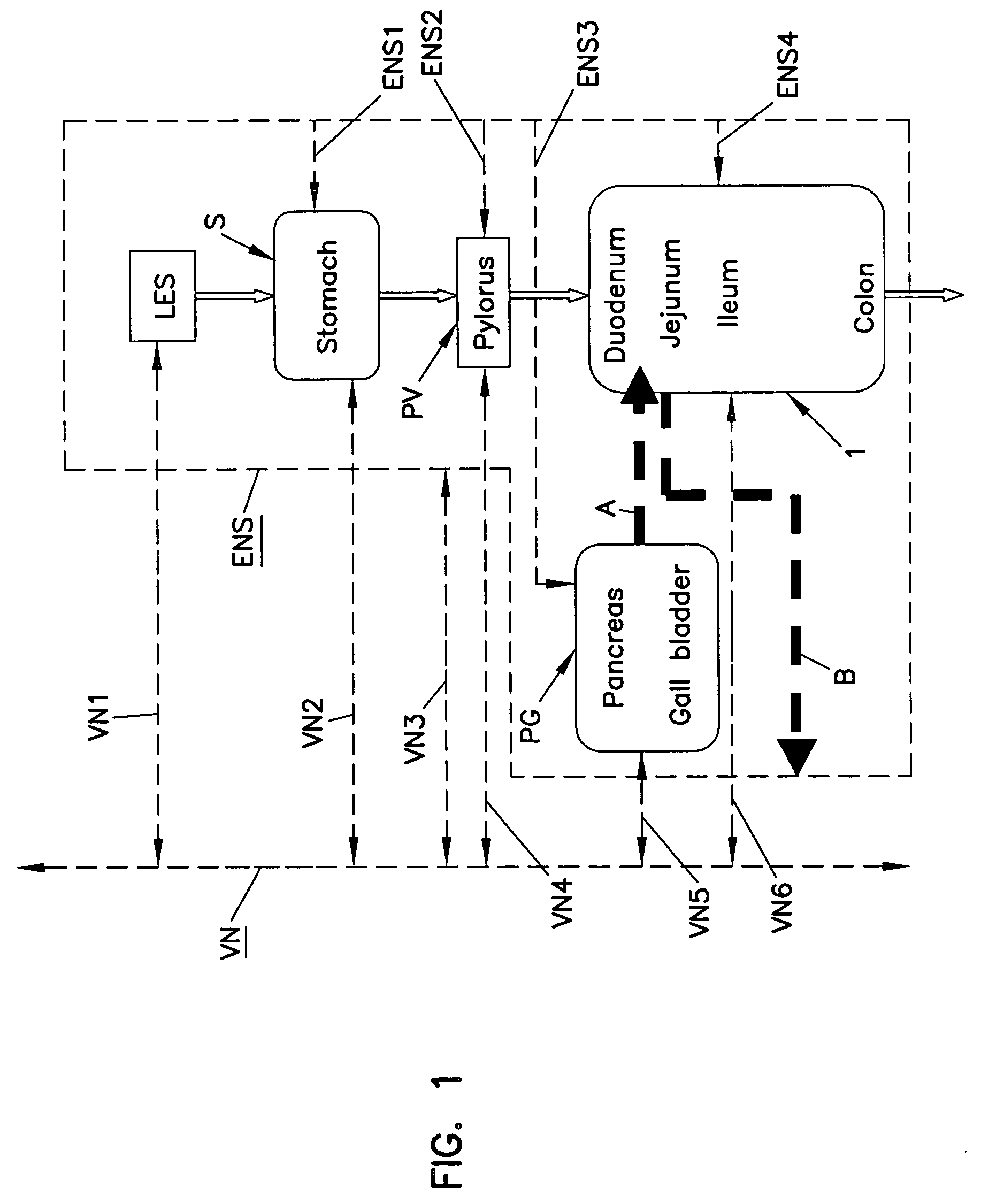
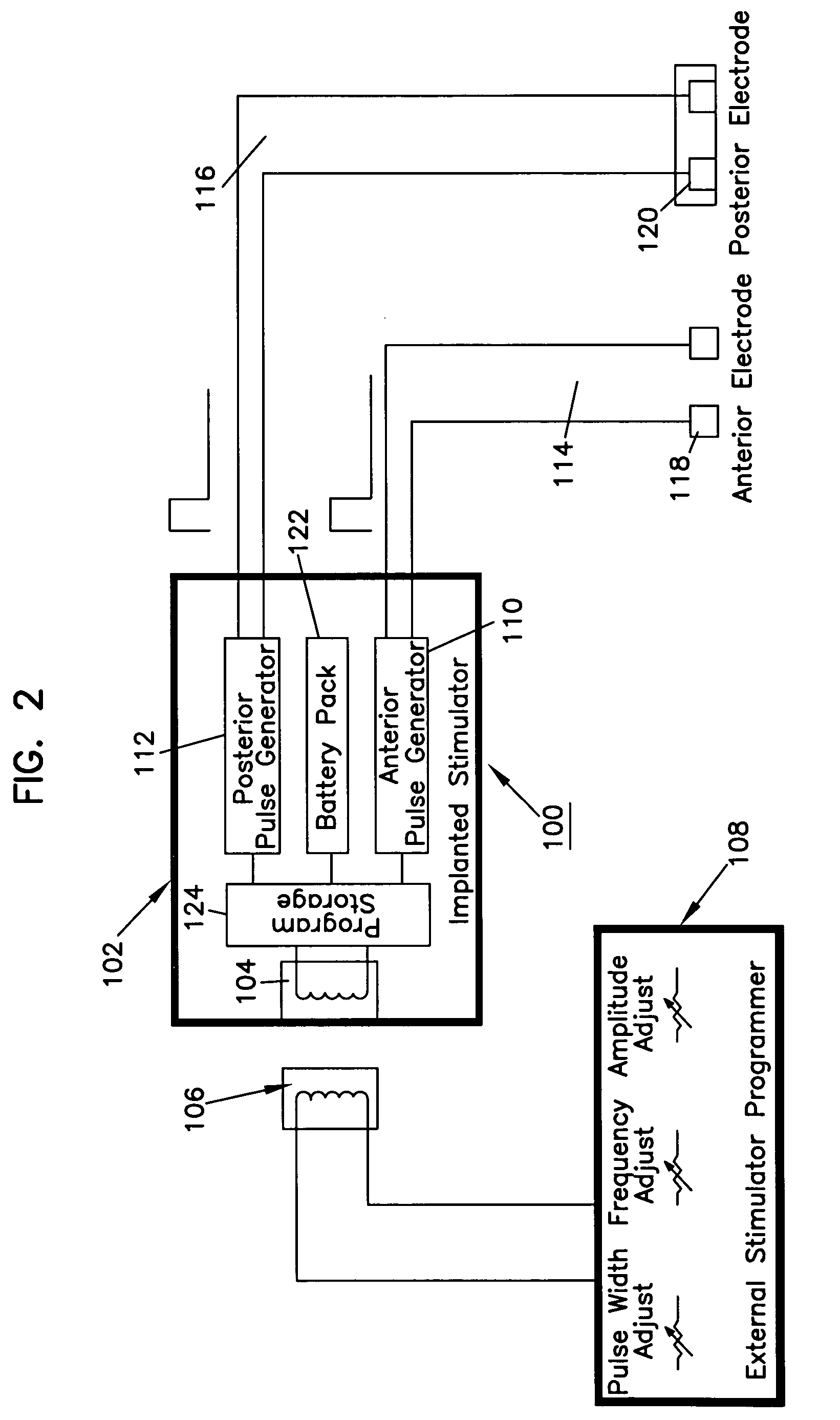
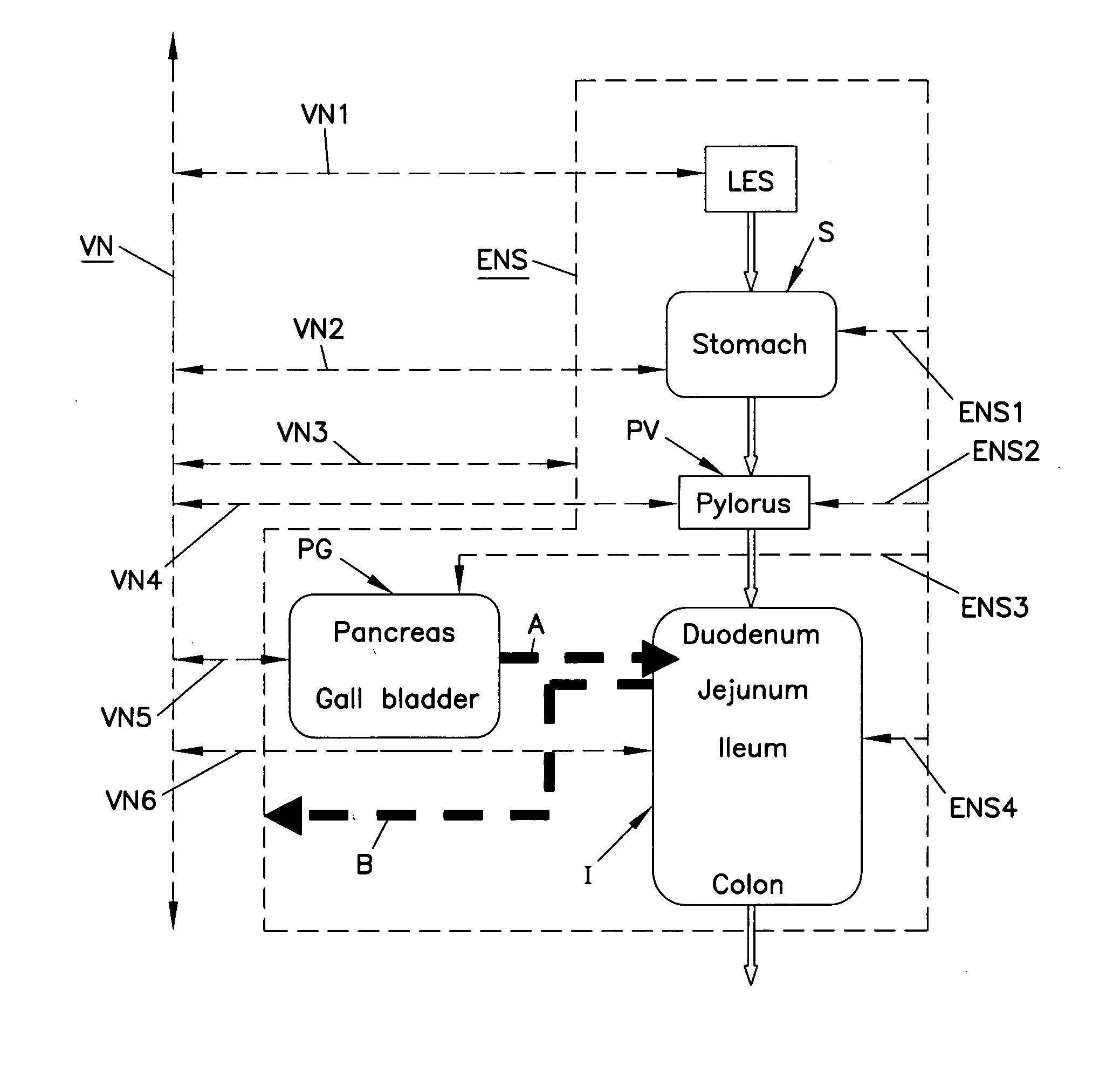
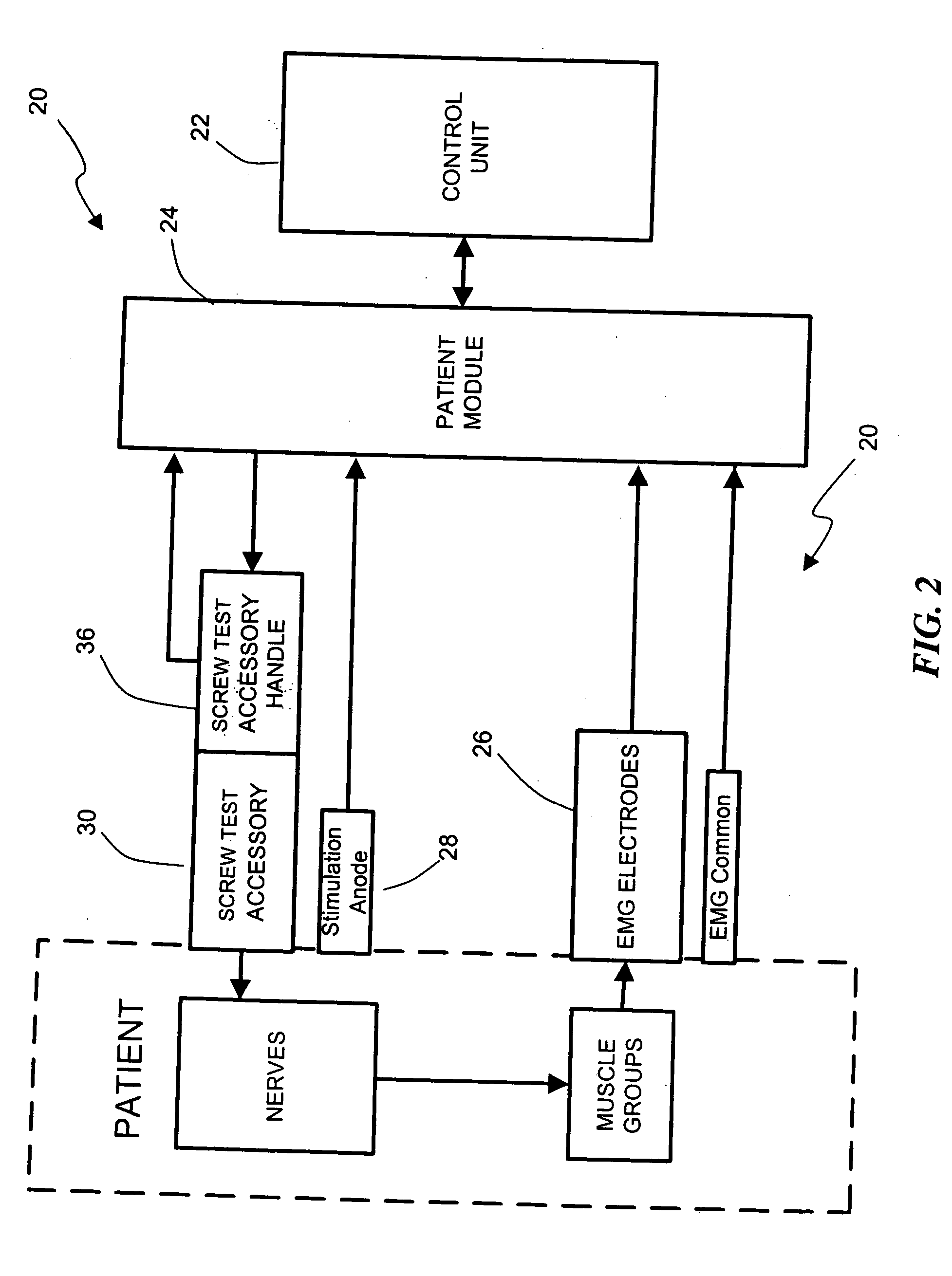
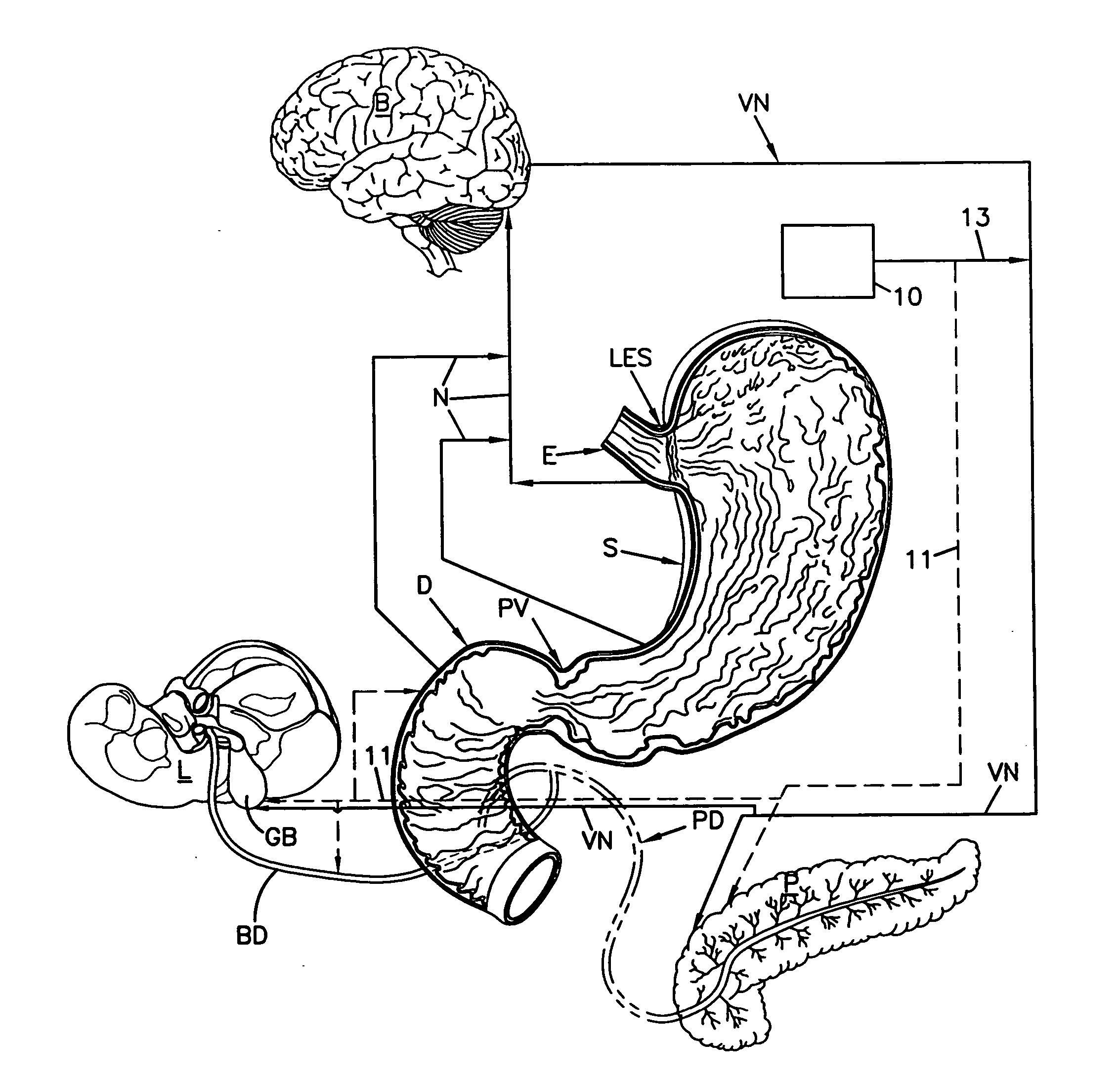
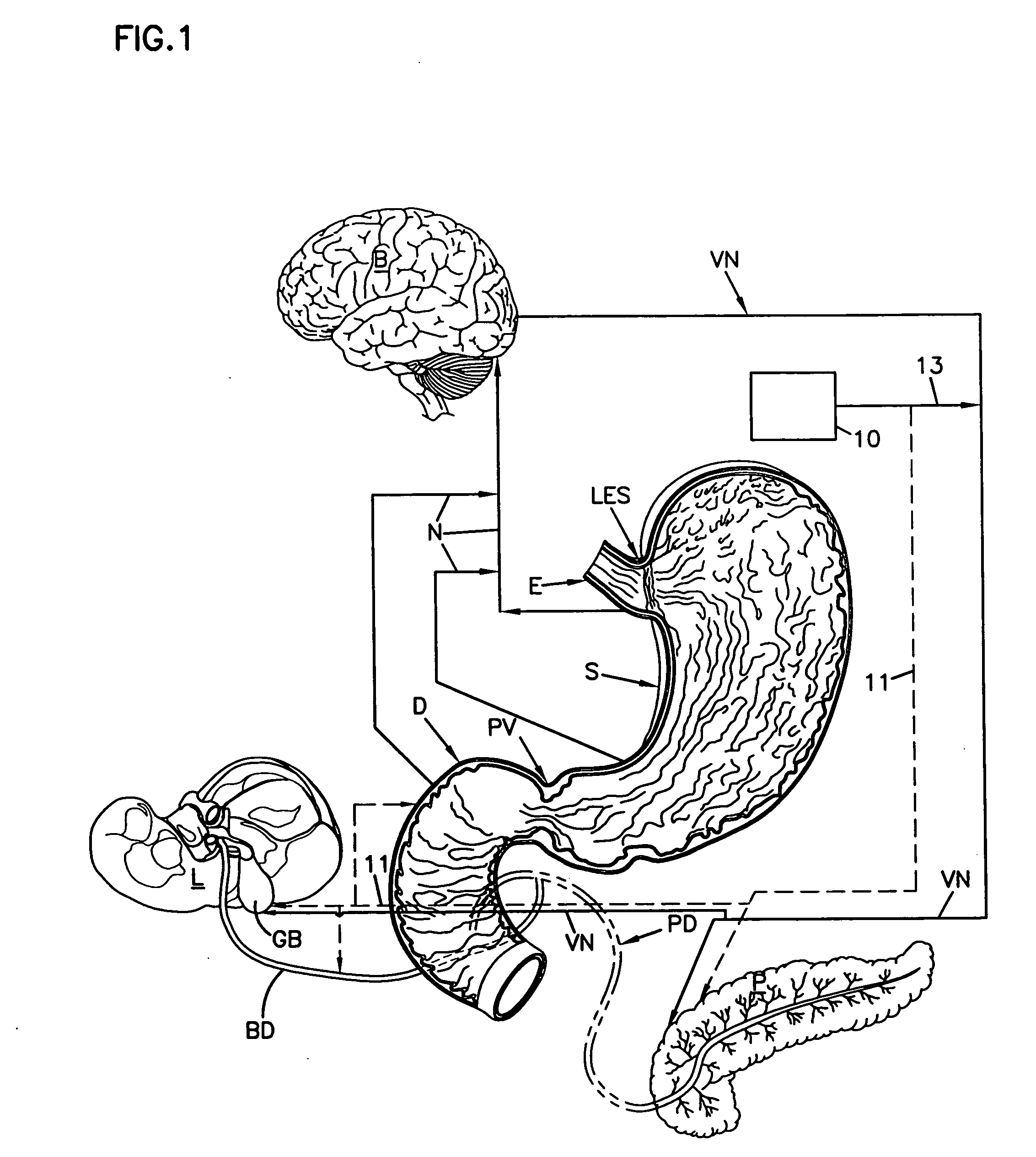
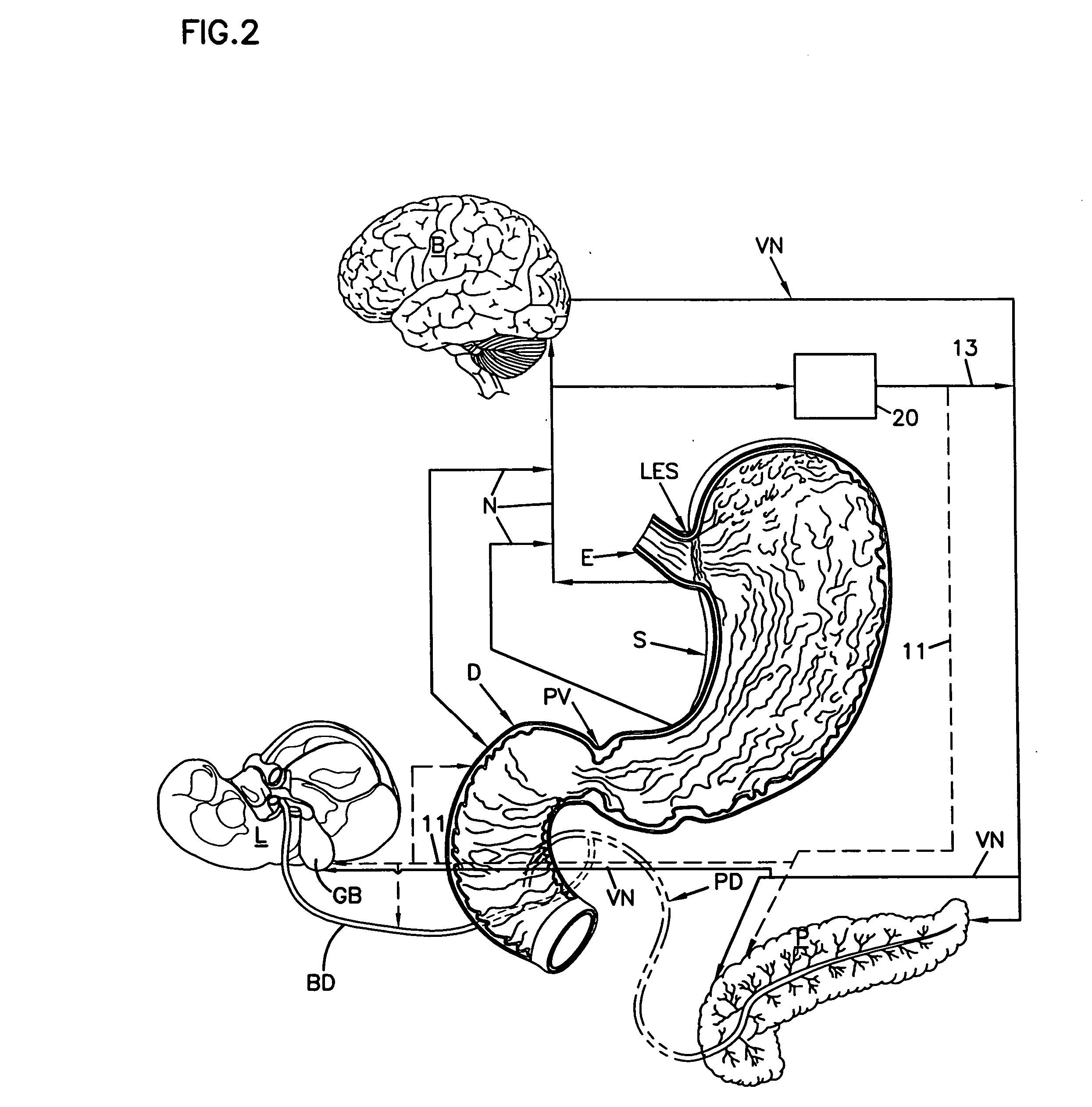
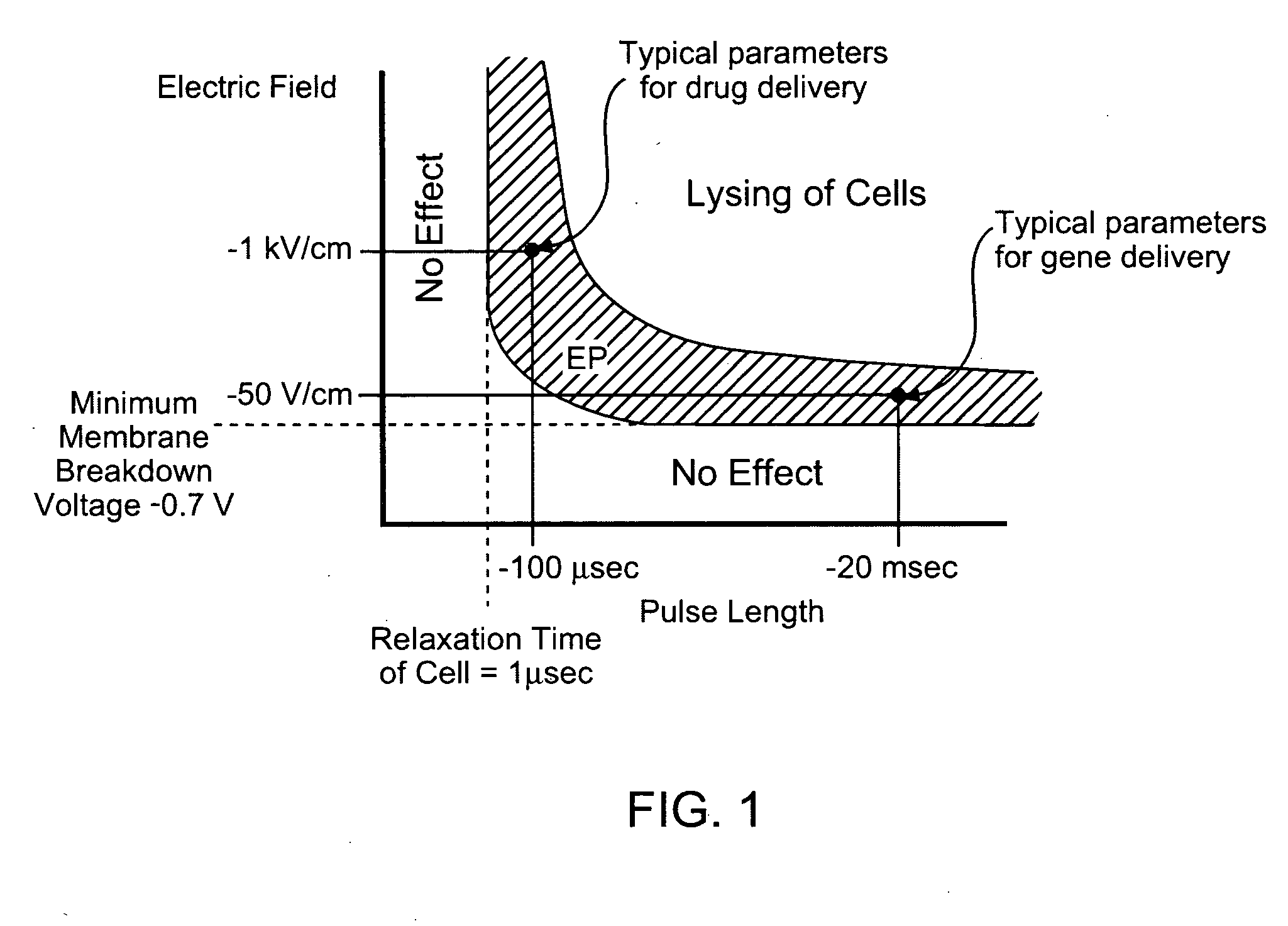
Patents
Literature
1812results about "Internal electrodes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
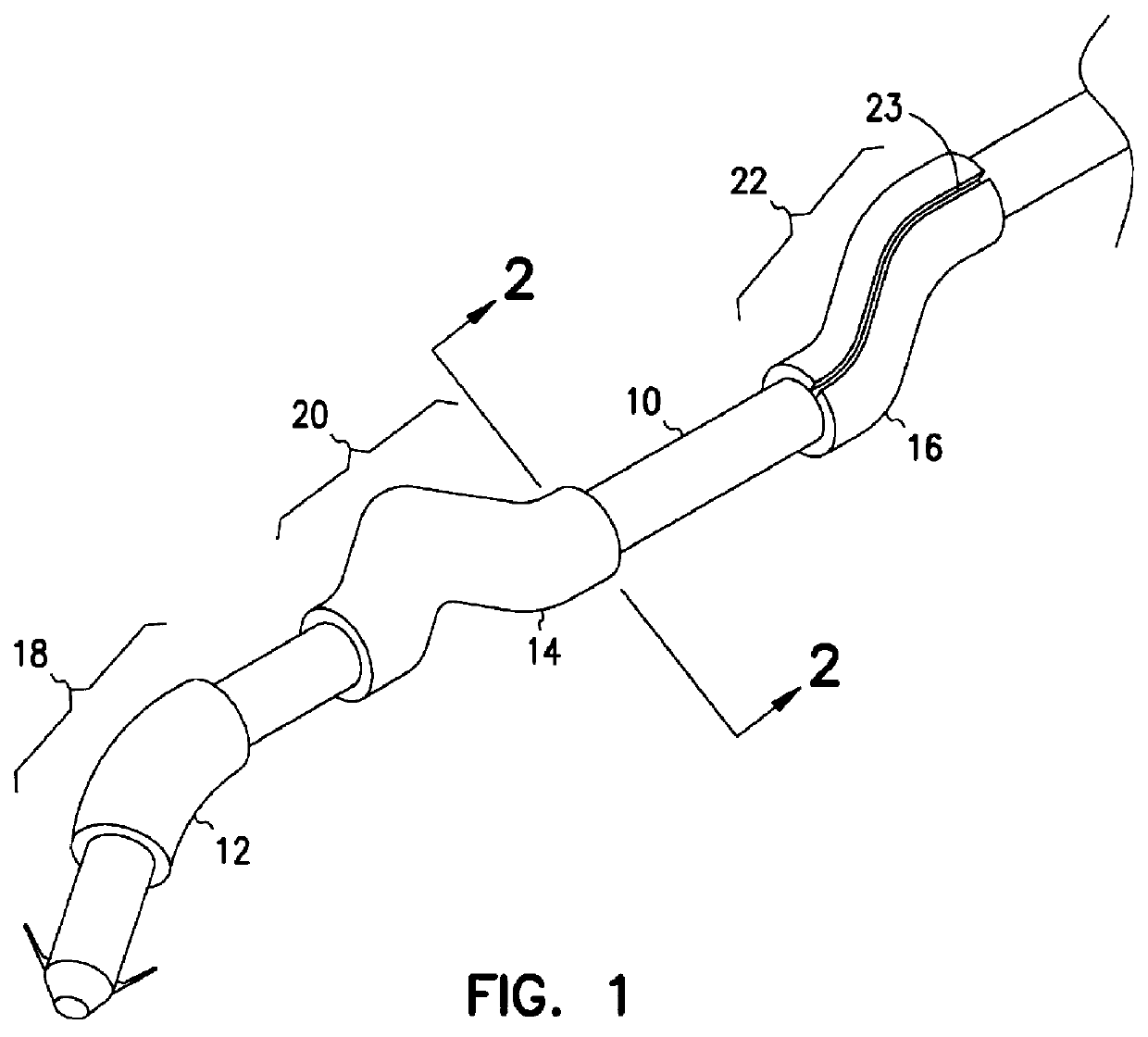
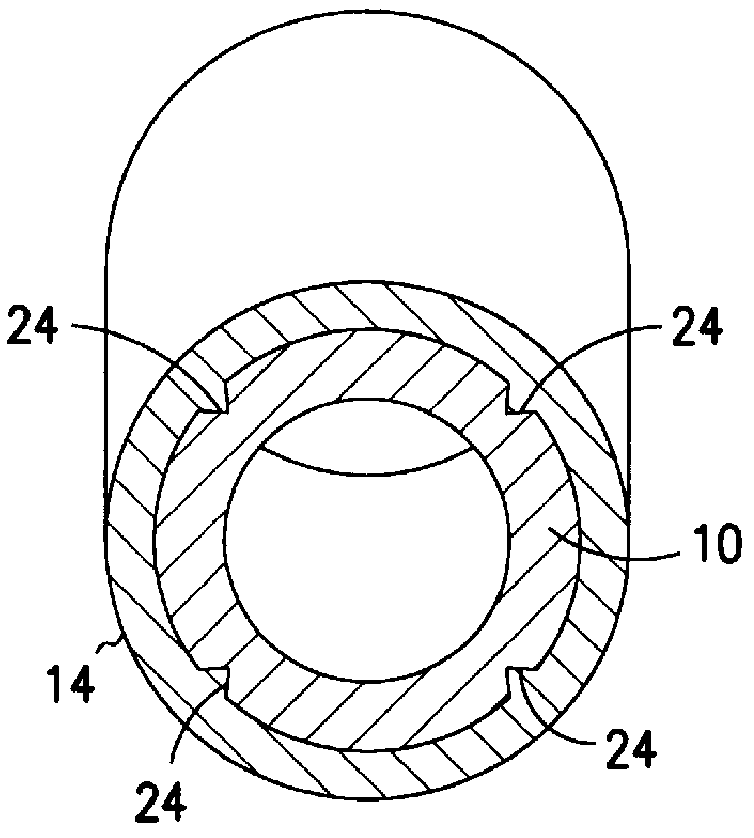
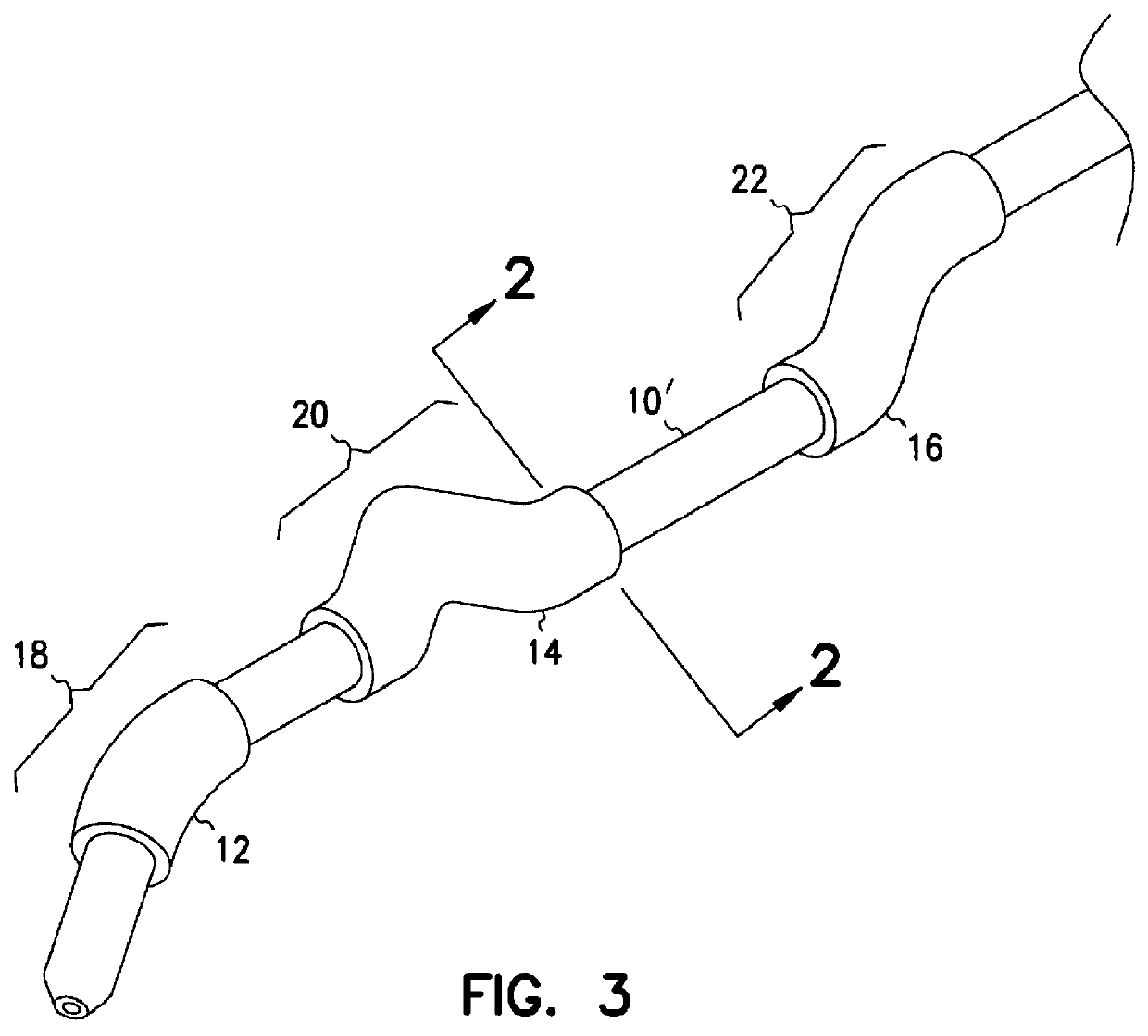
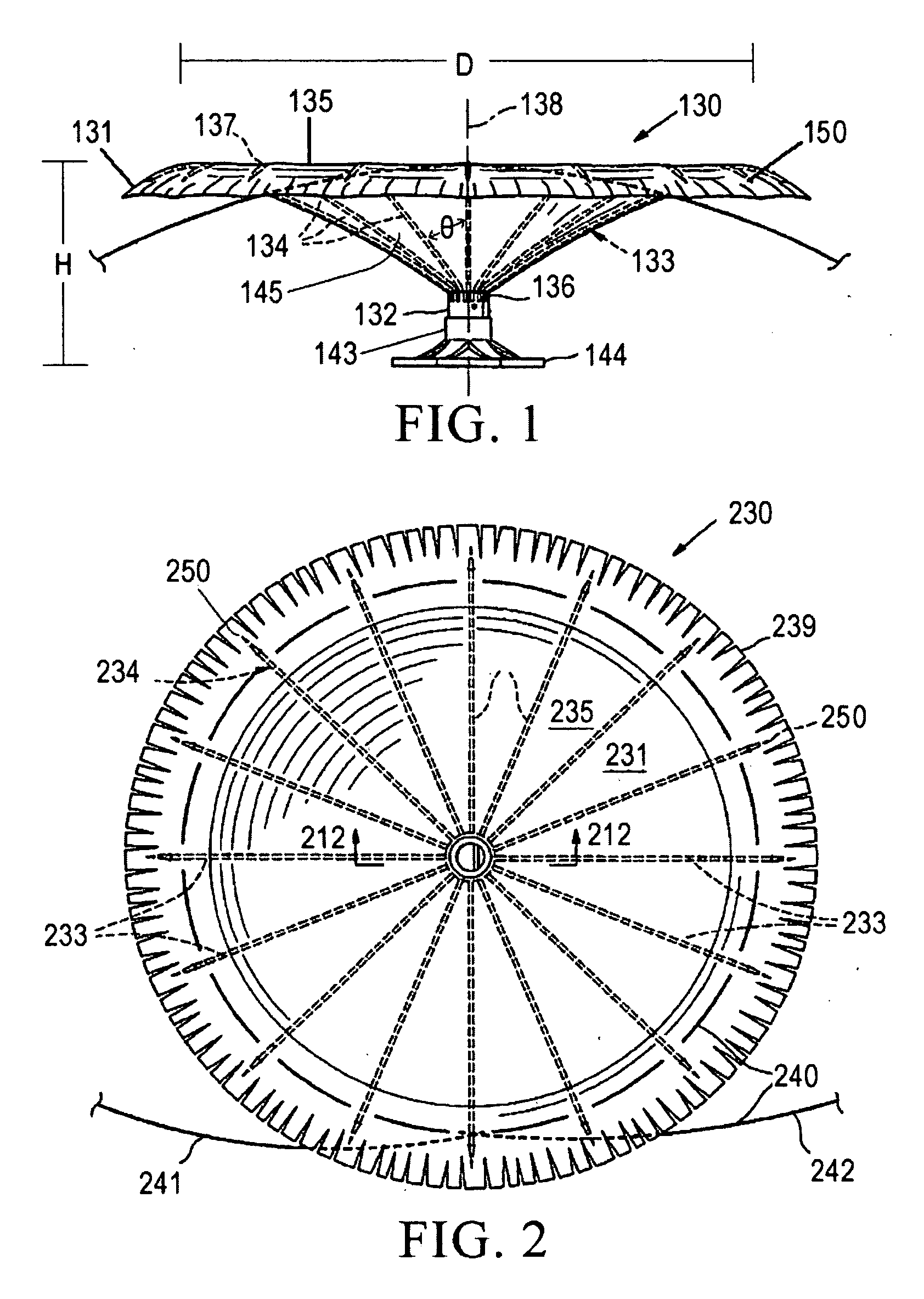
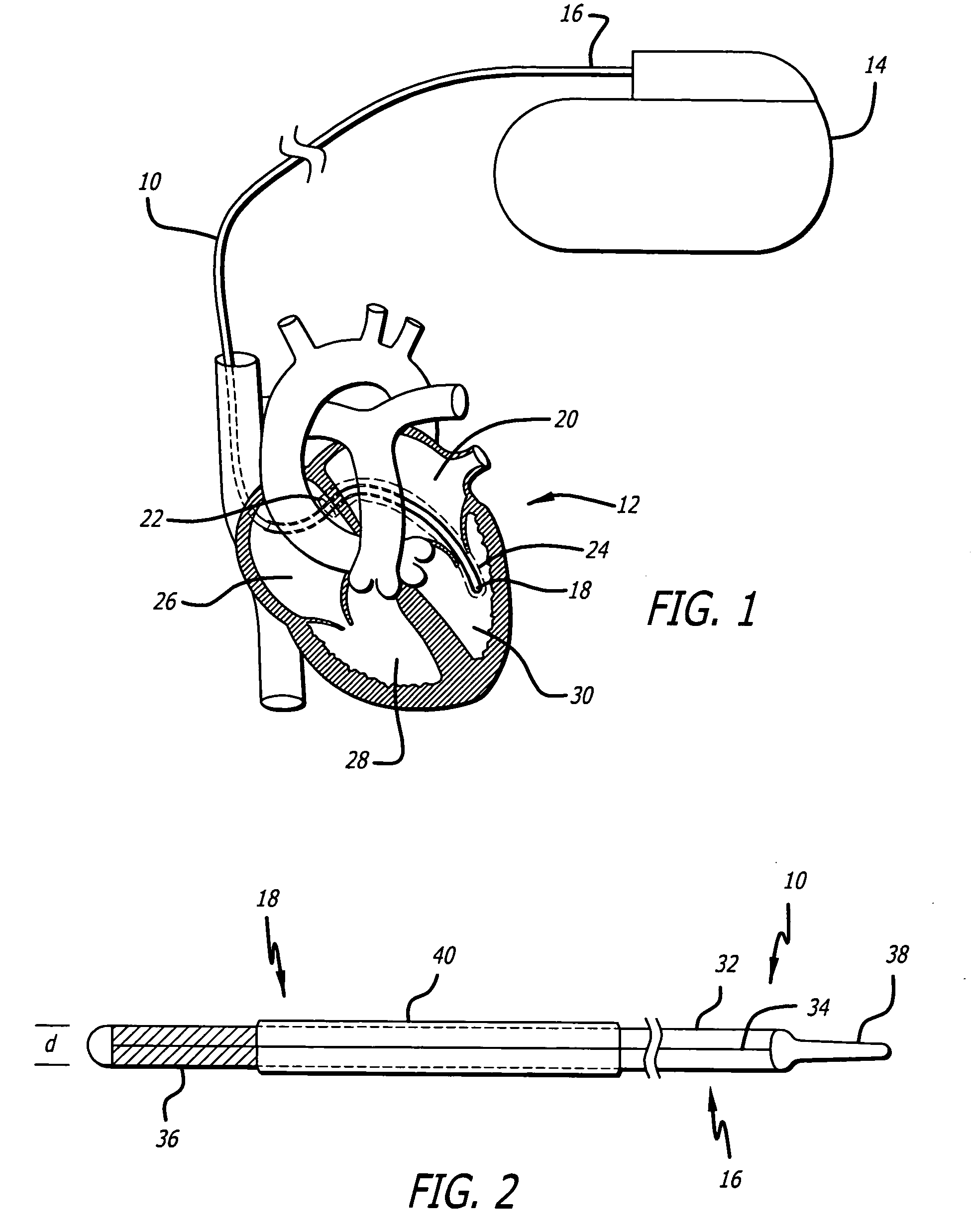
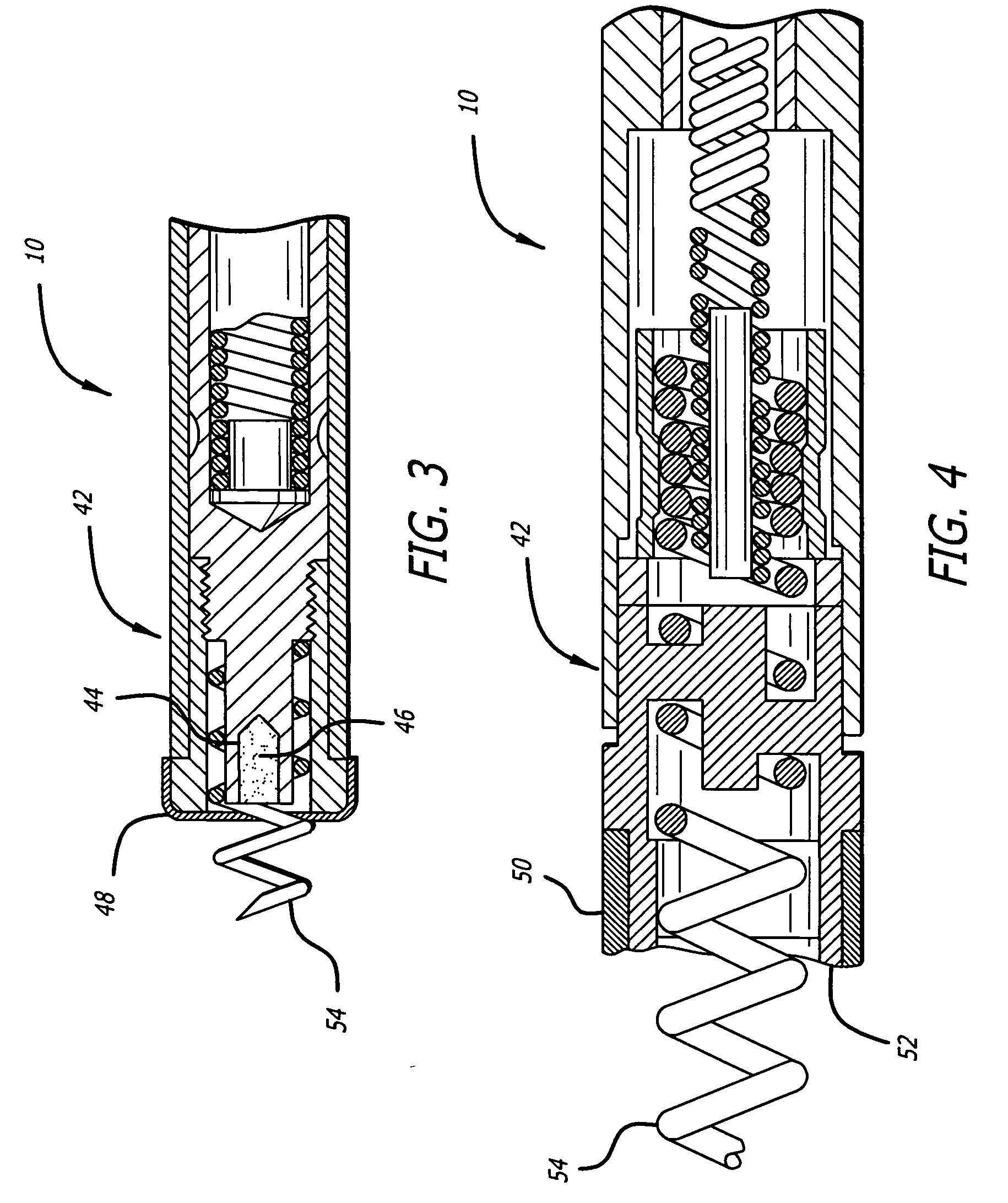
Apparatus for imparting physician-determined shapes to implantable tubular devices
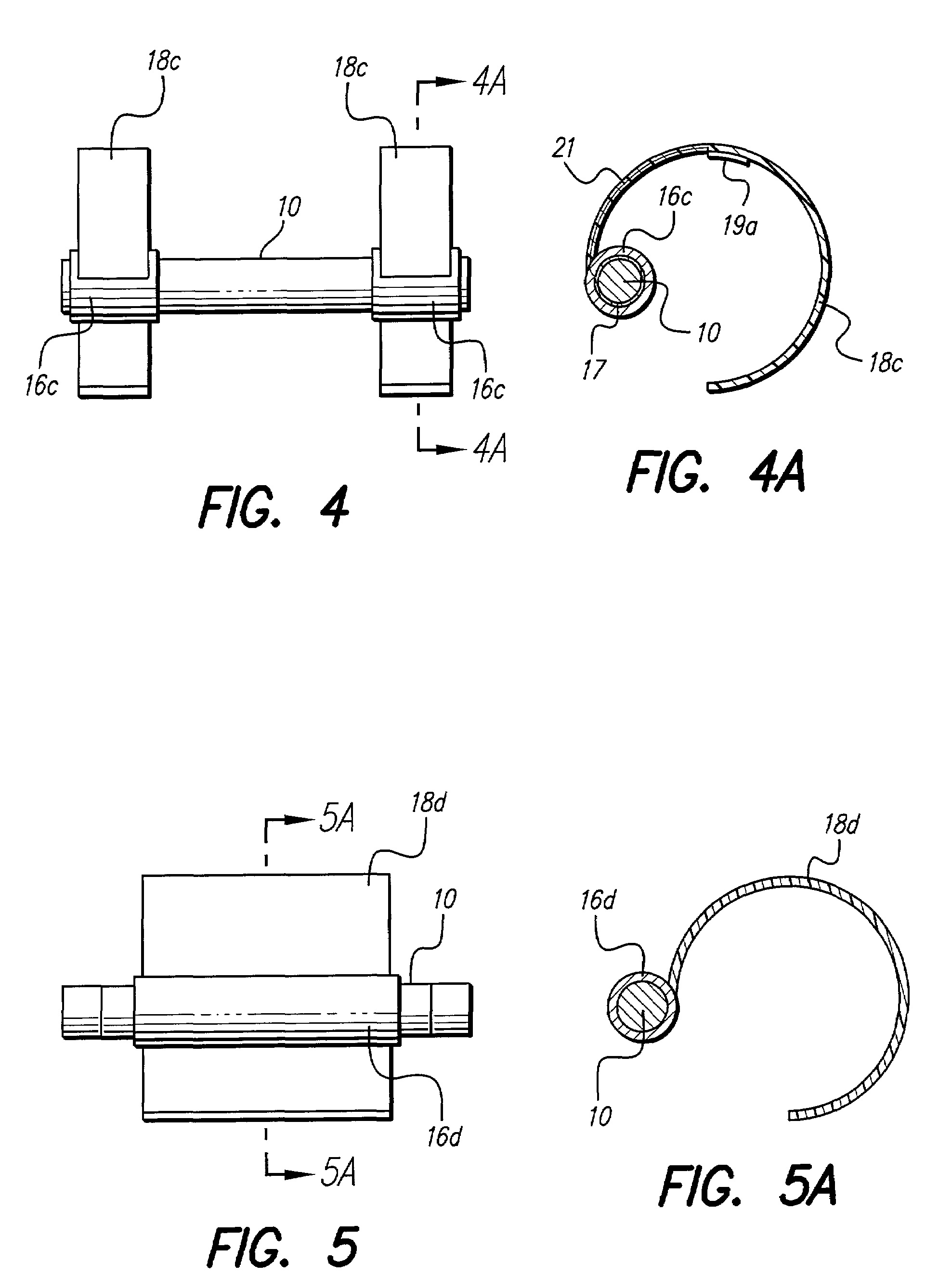
A tubular sleeve is provided for enabling a physician to impart a preselected shape in an implantable tubular device, such as a cardiac lead, a catheter, or some other tubular structure. The sleeve may be deformed by the surgeon before or at the time of implantation to customize the shape of the tubular device to the particular anatomical structures to be encountered by the device. To retain the deformation imparted by the physician, the sleeve may be composed of a heat-sensitive shape-memory material or an elastomeric material provided with a plastically deformable rib.
Owner:INTERMEDICS
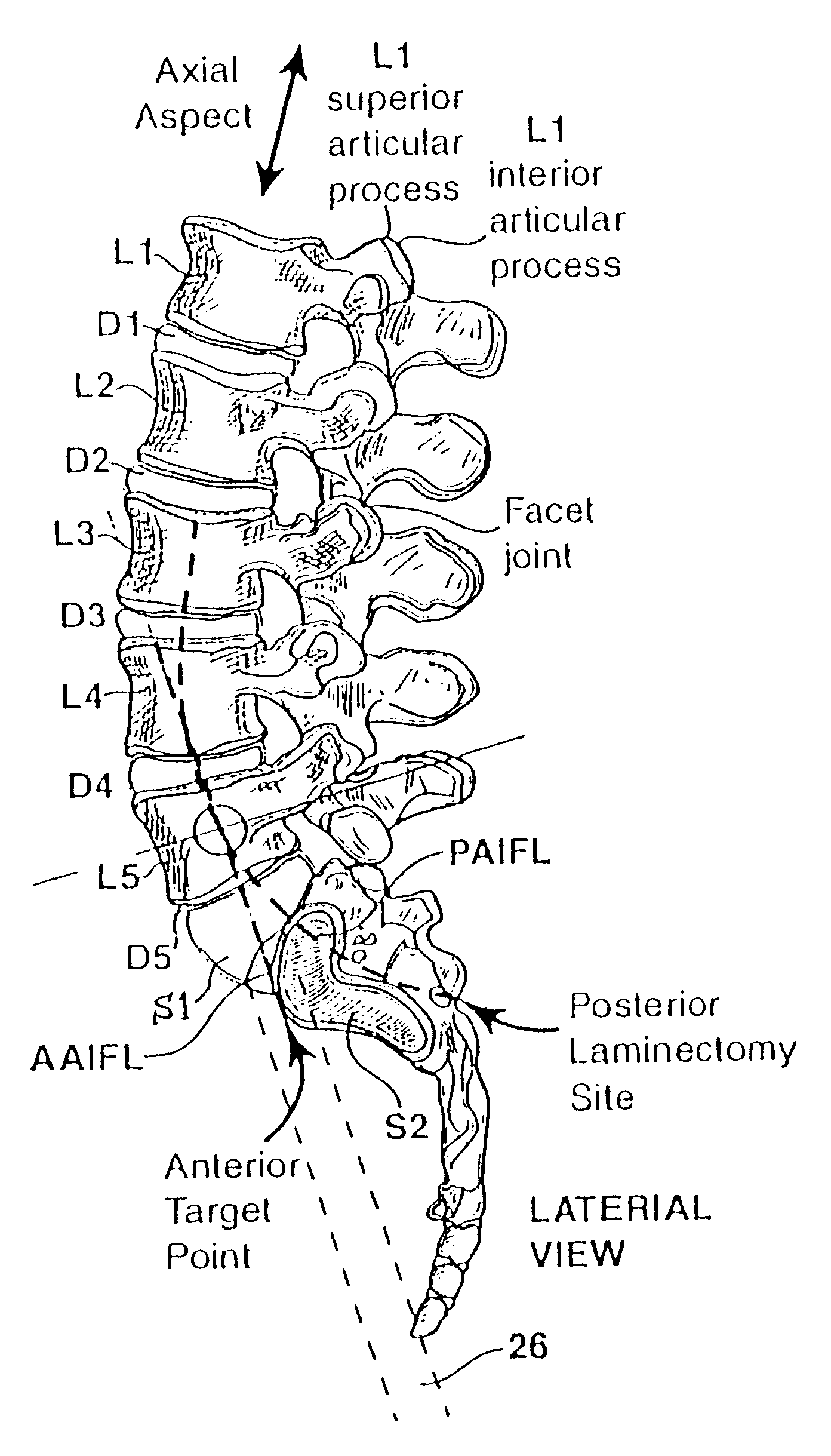
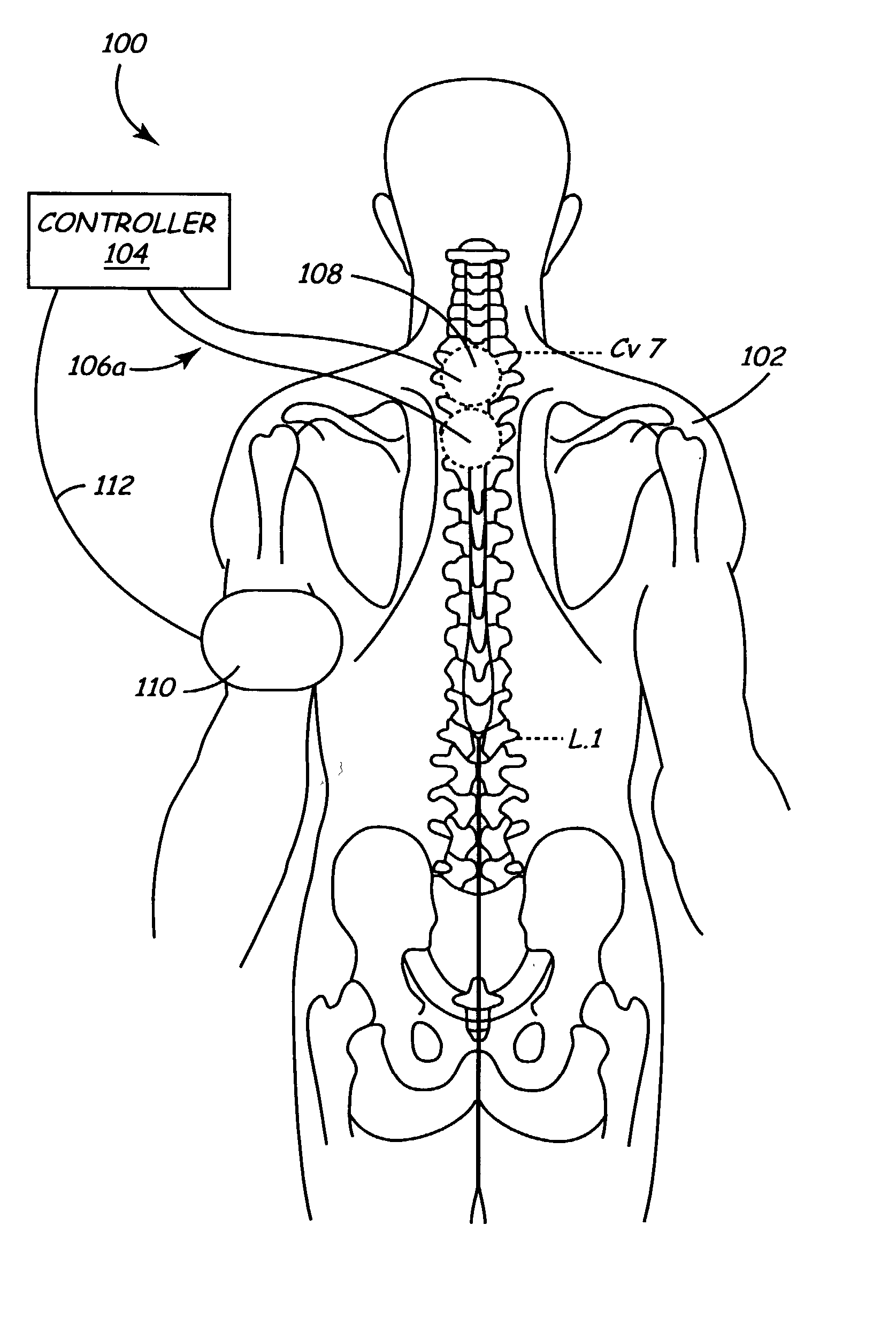
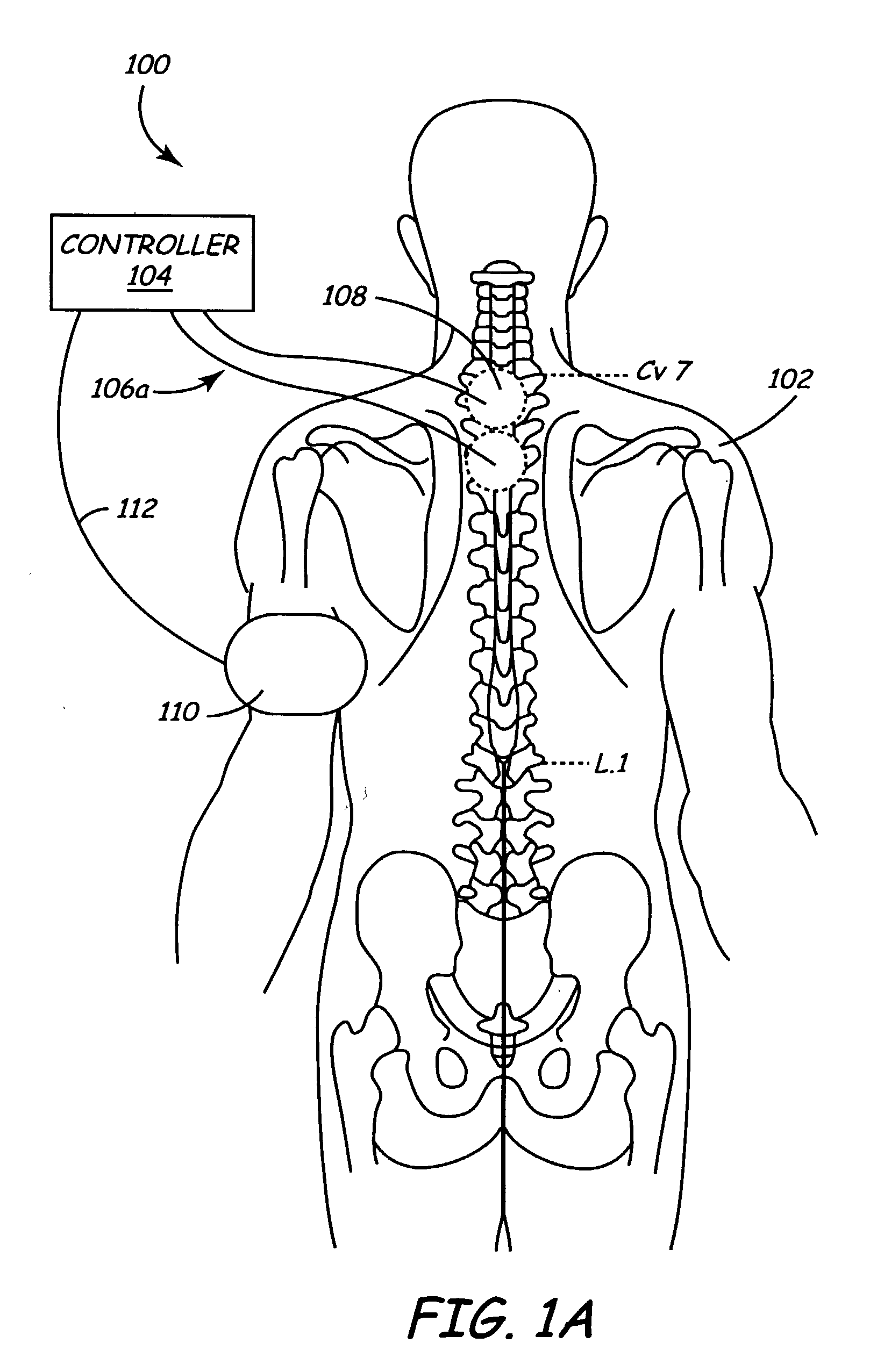
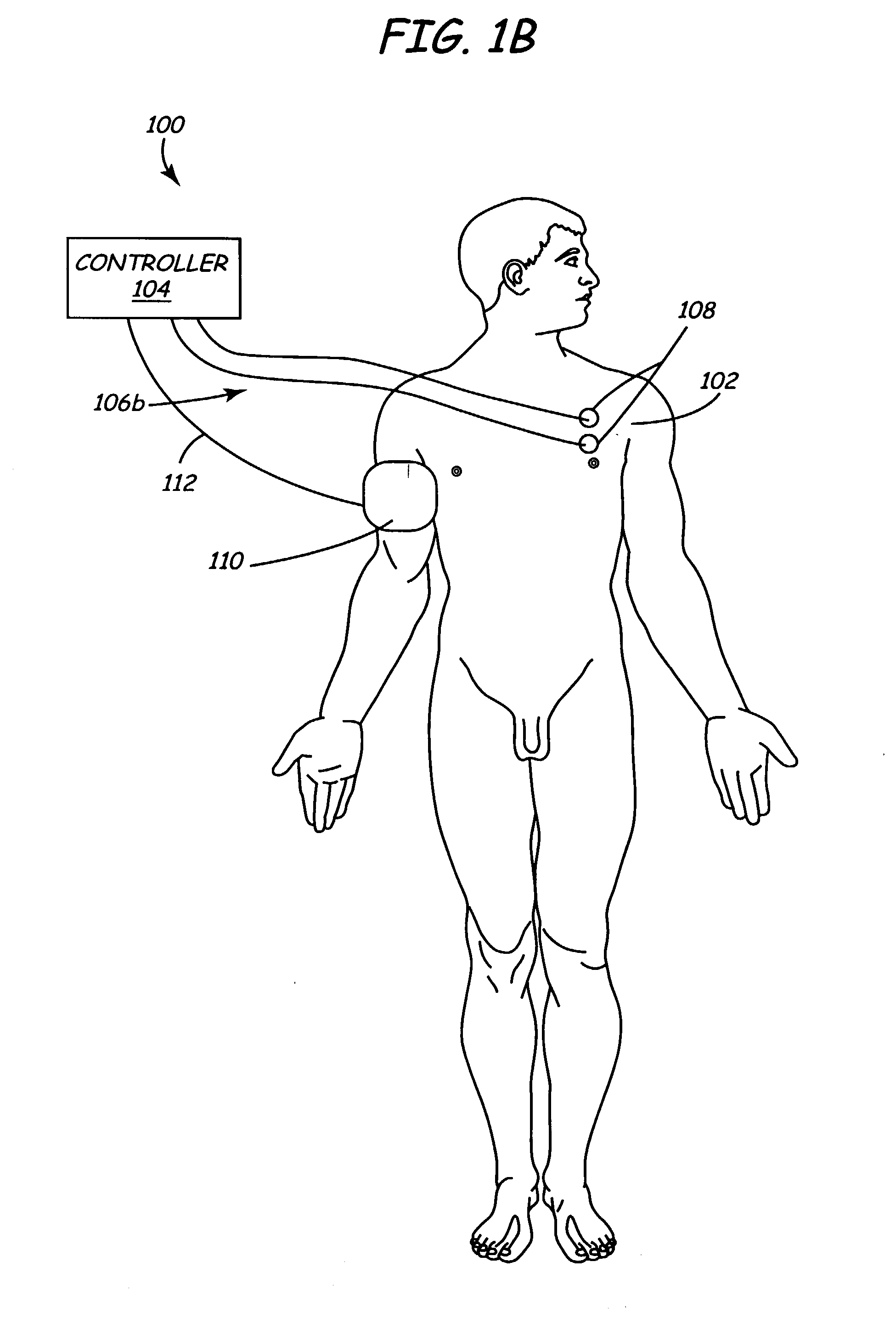
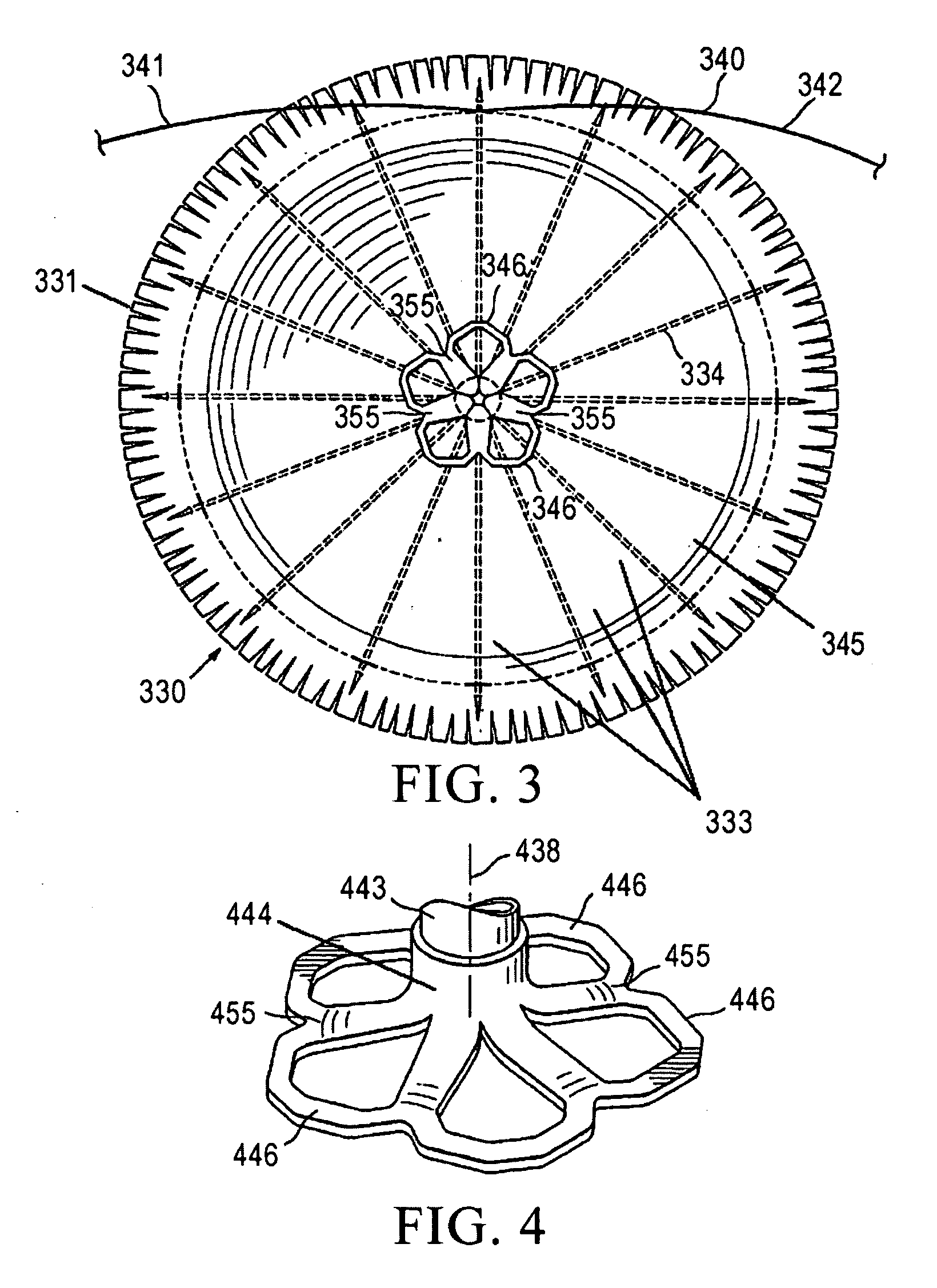
Methods and apparatus for performing therapeutic procedures in the spine
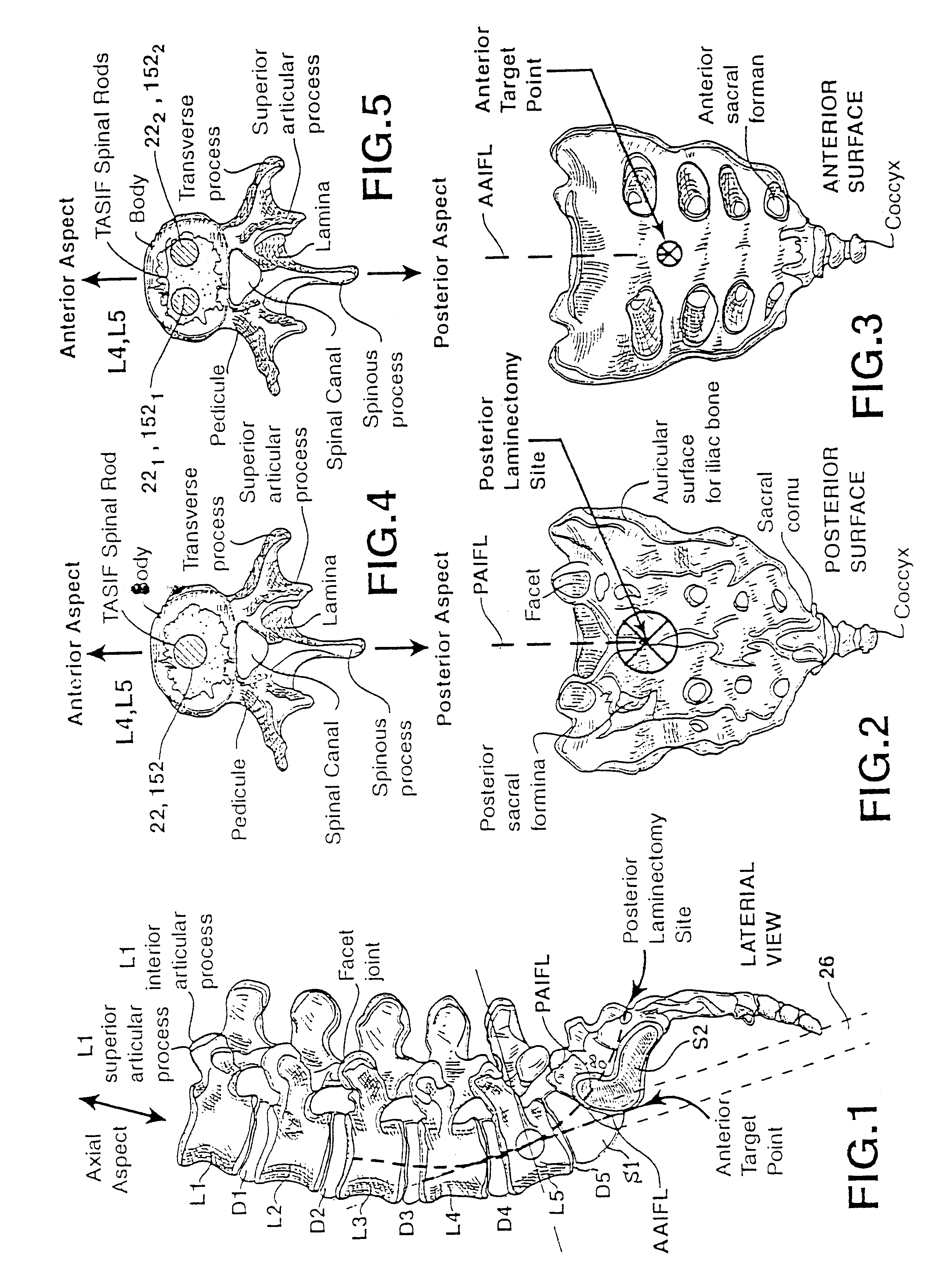
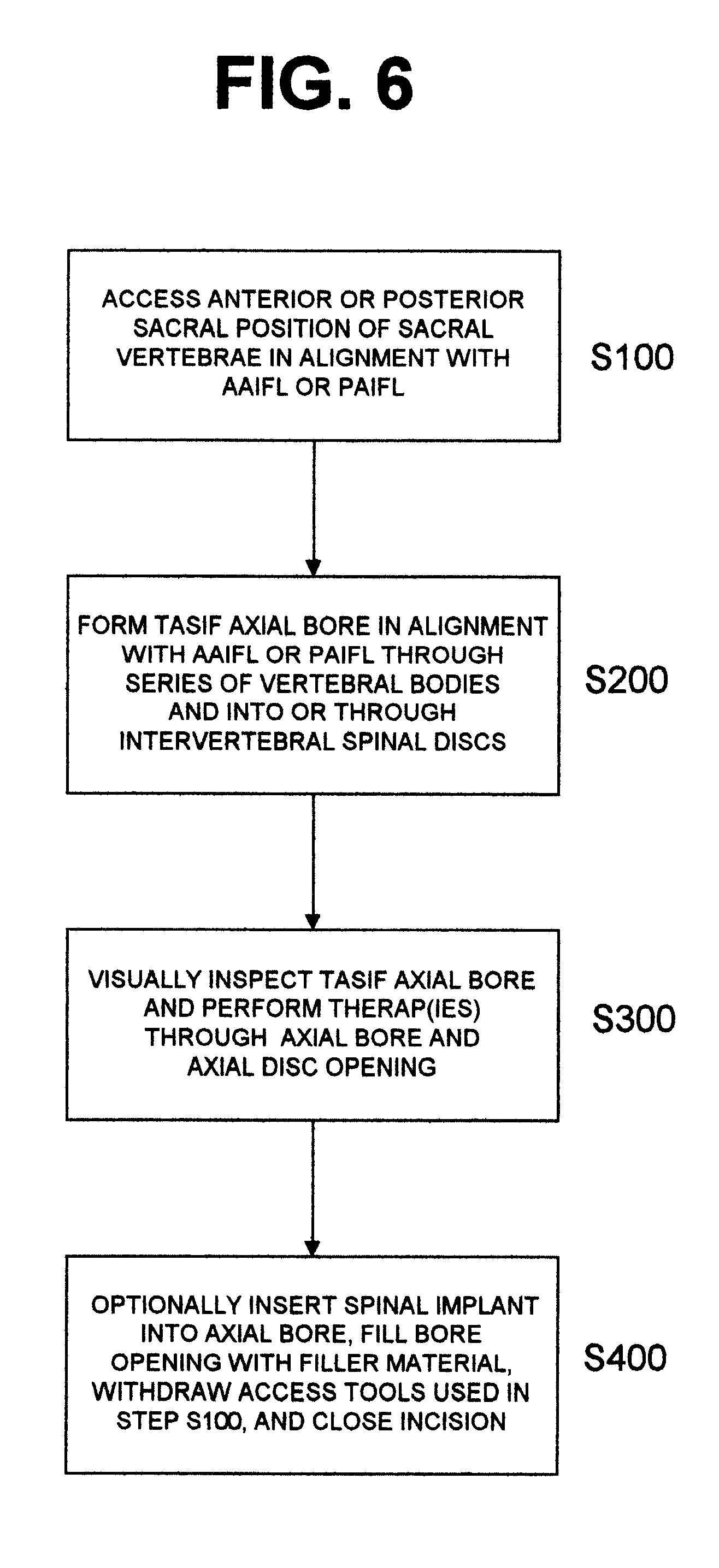
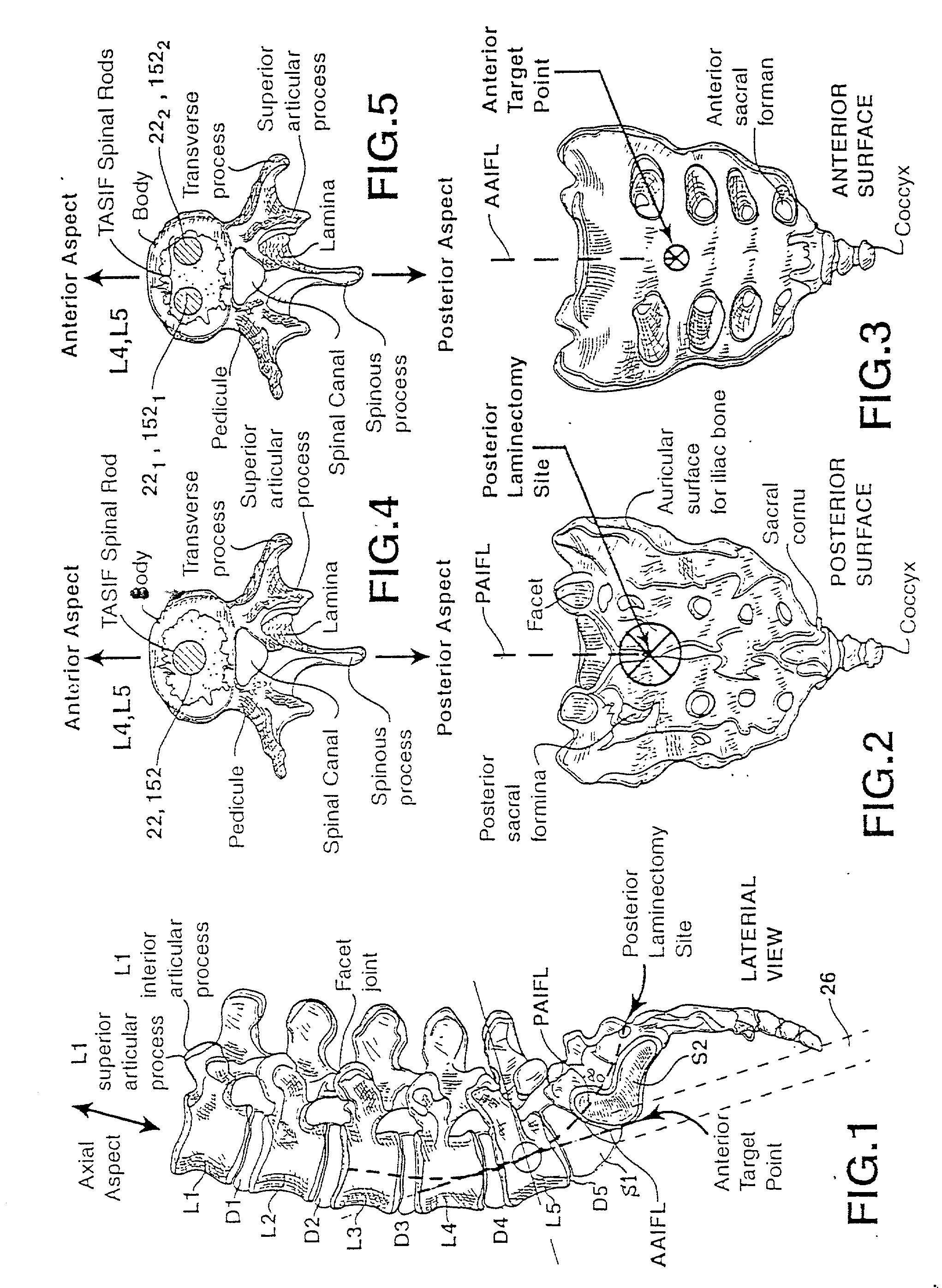
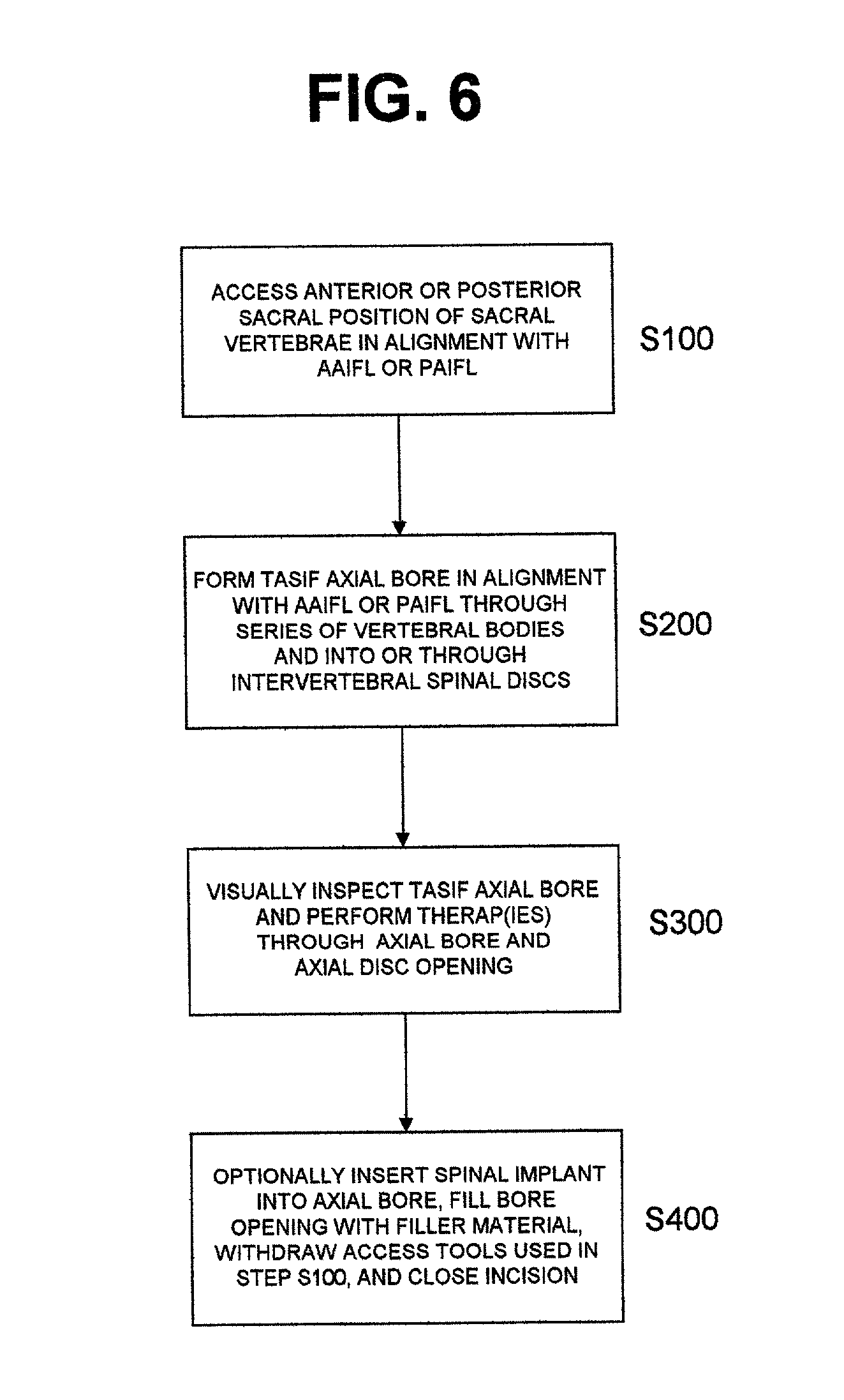
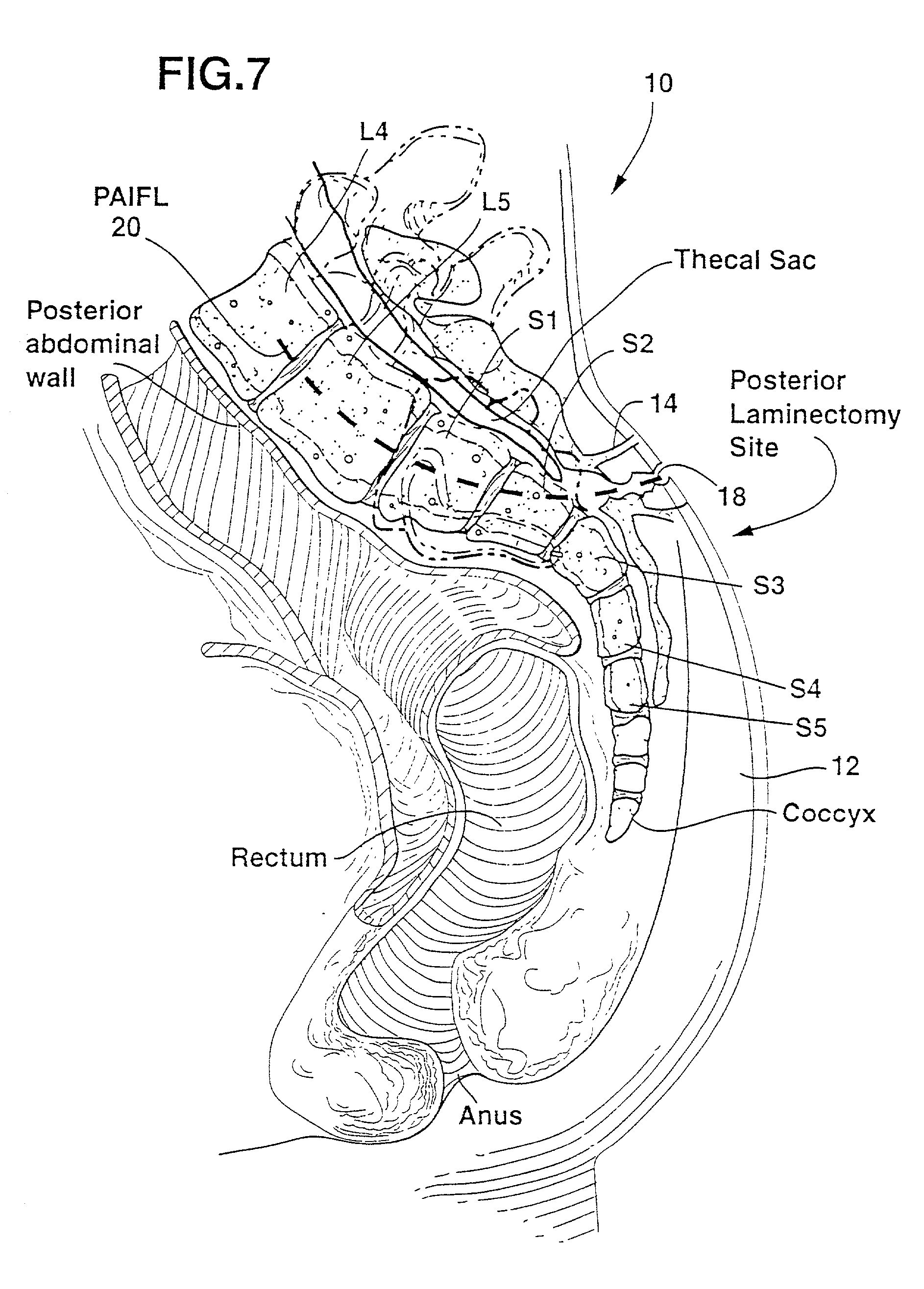
Methods and apparatus for forming one or more trans-sacral axial instrumentation / fusion (TASIF) axial bore through vertebral bodies in general alignment with a visualized, anterior or posterior axial instrumentation / fusion line (AAIFL or PAIFL) in a minimally invasive, low trauma, manner and providing a therapy to the spine employing the axial bore. Anterior or posterior starting positions aligned with the AAIFL or PAIFL are accessed through respective anterior and posterior tracts. Curved or relatively straight anterior and curved posterior TASIF axial bores are formed from the anterior and posterior starting positions. The therapies performed through the TASIF axial bores include discoscopy, full and partial discectomy, vertebroplasty, balloon-assisted vertebroplasty, drug delivery, electrical stimulation and various forms of spinal disc cavity augmentation, spinal disc replacement, fusion of spinal motion segments and implantation of radioactive seeds. Axial spinal implants and bone growth materials can be placed into single or multiple parallel or diverging TASIF axial bores to fuse two or more vertebrae, or distract or shock absorb two or more vertebrae.
Owner:MIS IP HLDG LLC
Medical devices having electrodes mounted thereon and methods of manufacturing therefor
InactiveUS20120130217A1For automatic positioningContact member manufacturingElectrocardiographyMedical deviceBiomedical engineering
Medical devices, methods of manufacturing medical devices, and systems comprising medical devices are provided. The device comprises a shaft having a major lumen sized to receive a second medical device and an electrode mounted thereon. The shaft includes an inner liner and outer layer. The method of manufacture includes forming a shaft by forming an inner liner and an outer layer by covering the inner liner with a polymeric material. The method further includes mounting an electrode onto the shaft of the device, and then heating and cooling the shaft. The system comprises a medical device having a shaft and an electrode mounted thereon. The shaft has a major lumen sized to receive another device. The system further comprises an electronic control unit configured to receive signals from the electrode and to determine a position of the electrode and / or monitor electrophysiological data.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Devices, methods, and systems for shrinking tissues
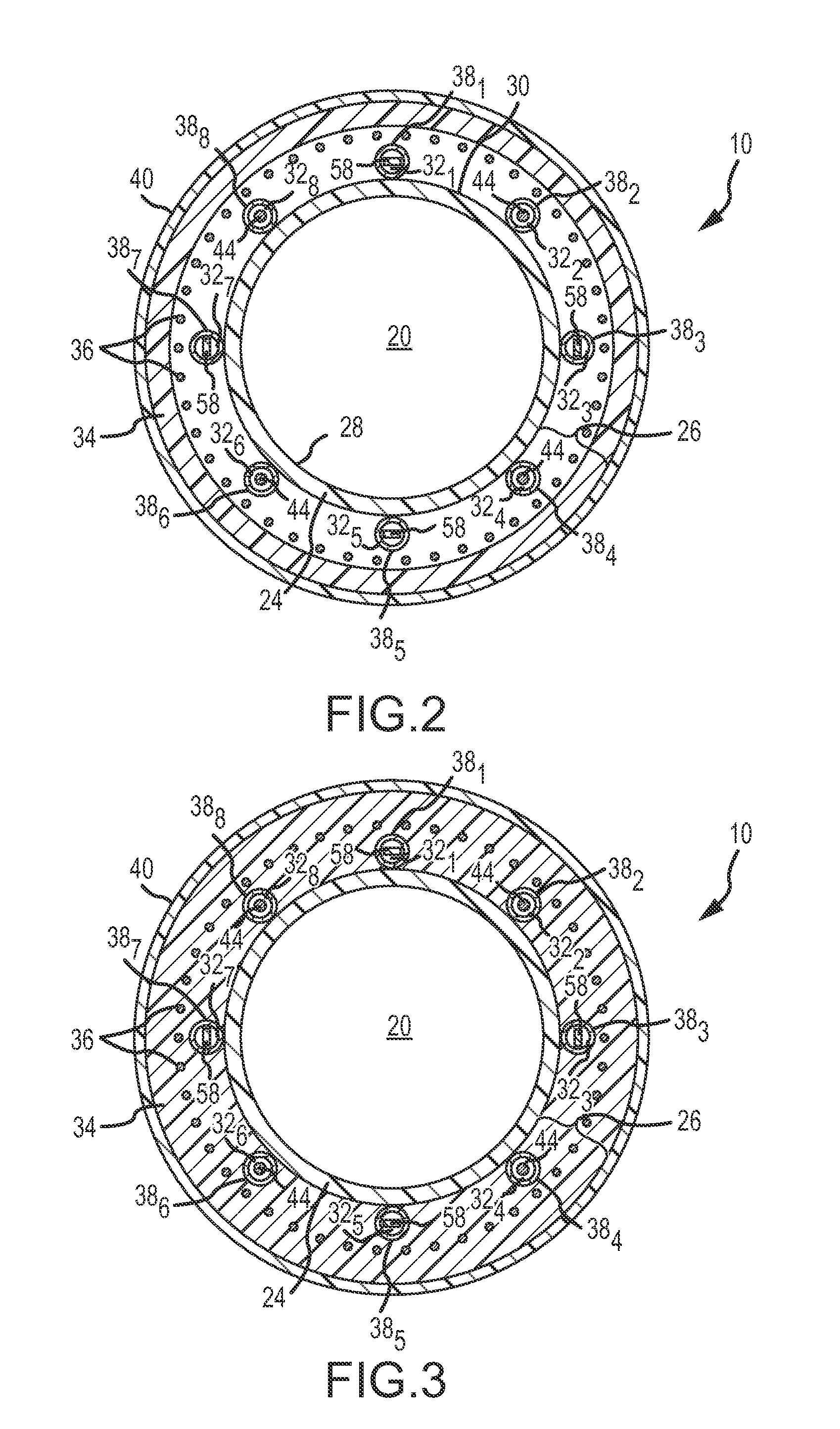
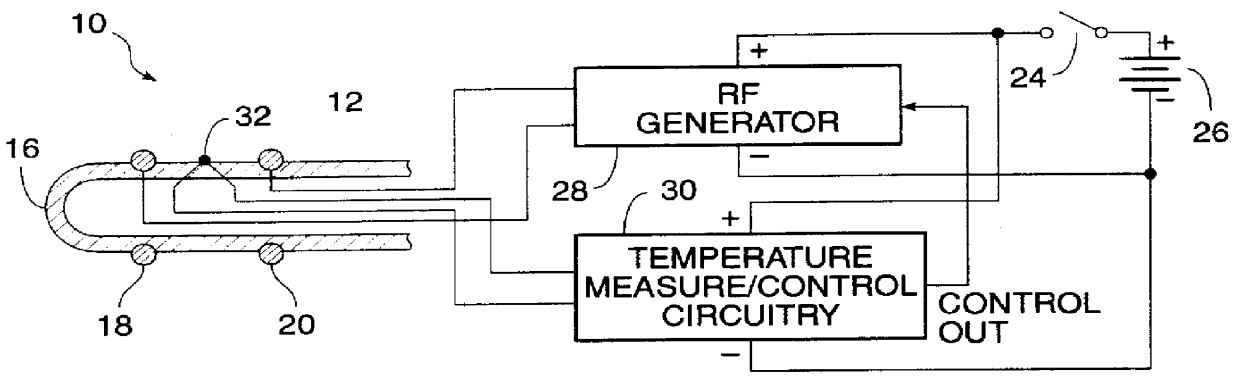
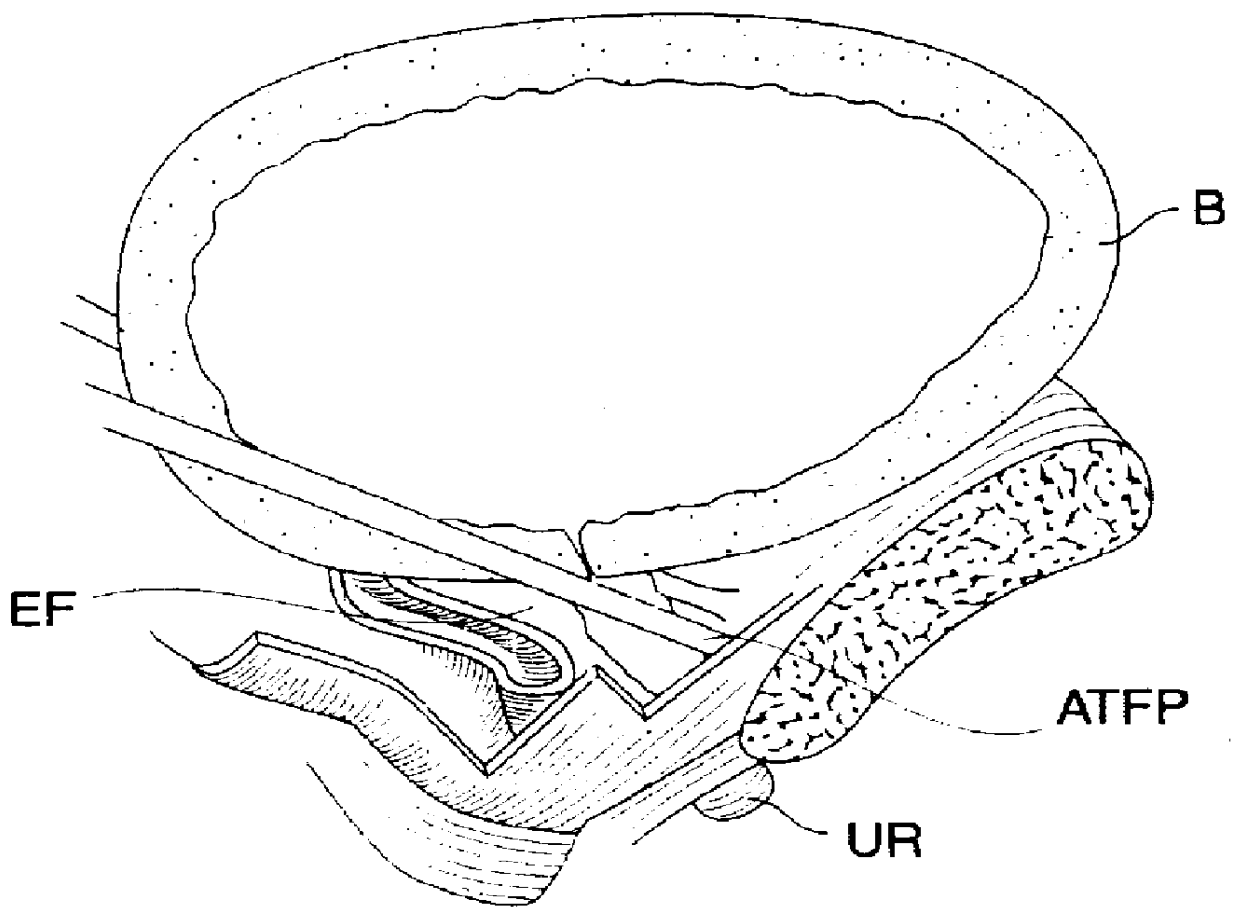
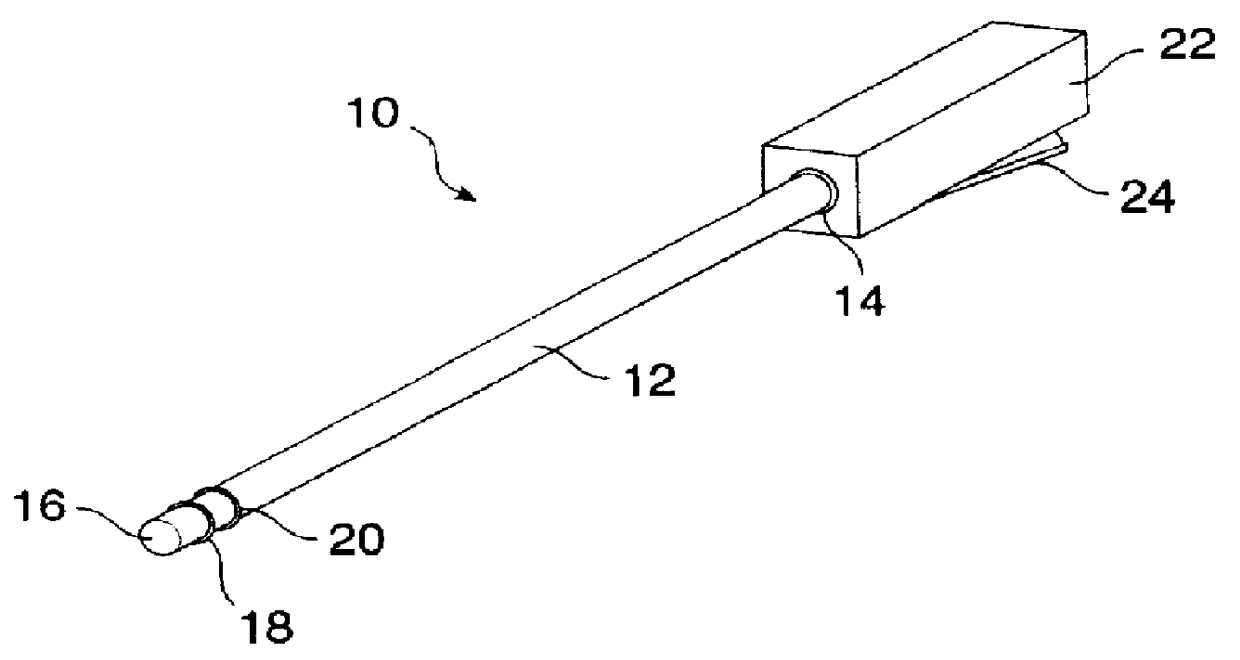
InactiveUS6091995AReduce power levelControl depthSurgical needlesInternal electrodesSphincterPelvic supports
Devices, systems, and method for treating urinary incontinence generally rely on energy delivered to a patient's own pelvic support tissue to selectively contract or shrink at least a portion of that pelvic support tissue so as to reposition the bladder. The energy will preferably be applied to the endopelvic fascia and / or an arcus tendineus fascia pelvis. The invention provides a variety of devices and methods for applying gentle resistive heating of these and other tissues to cause them to contract without imposing significant injury on the surrounding tissue structures. Alternatively, heat-applying probes are configured to heat tissue structures which comprise or support a patient's urethra. By applying sufficient energy over a predetermined time, the tissue can be raised to a temperature which results in contraction without significant necrosis or other tissue damage. By selectively contracting the support tissues, the bladder neck, sphincter, and other components of the urinary tract responsible for the control of urinary flow can be reconfigured or supported in a manner which reduces urinary leakage.
Owner:VERATHON
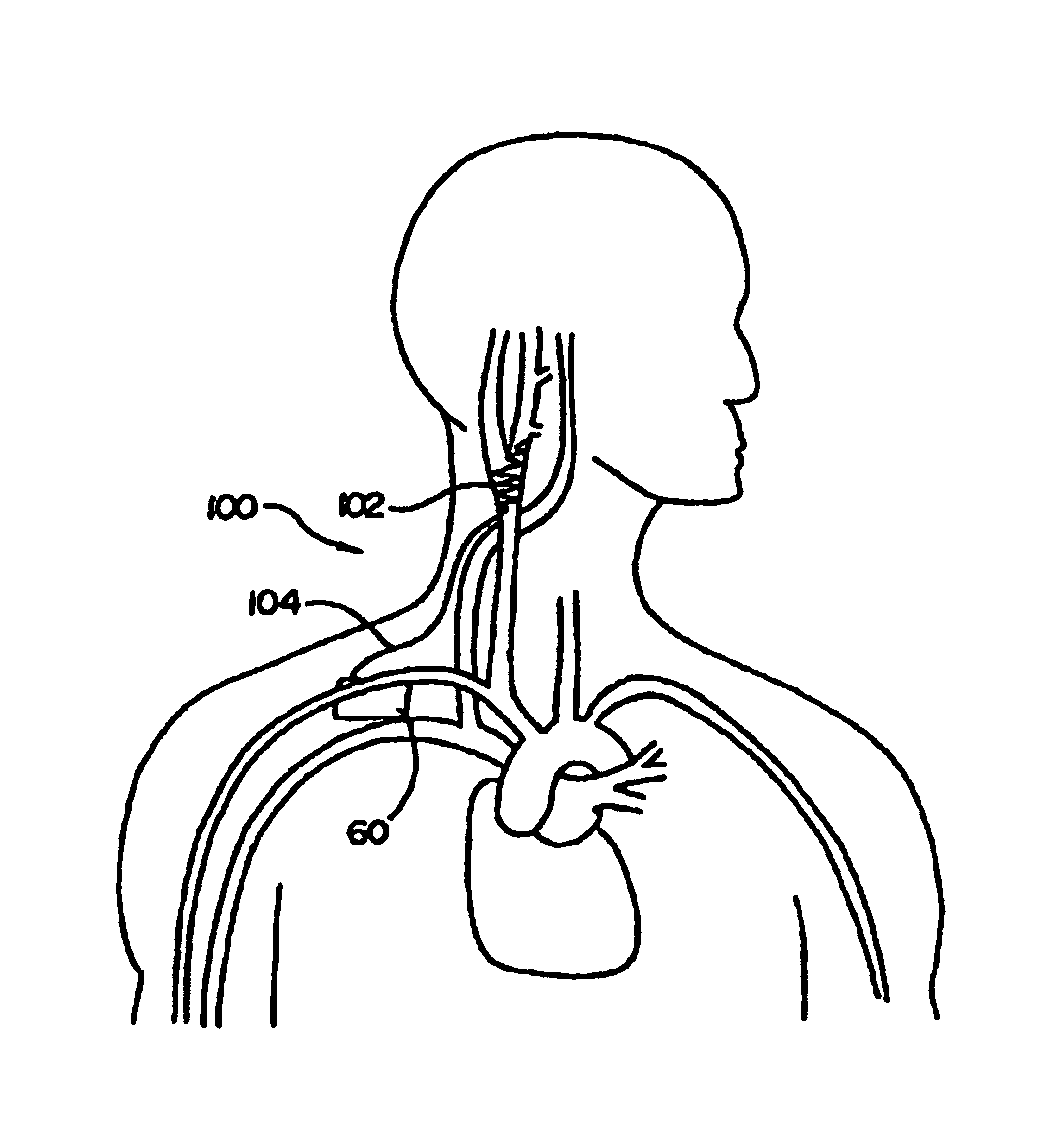
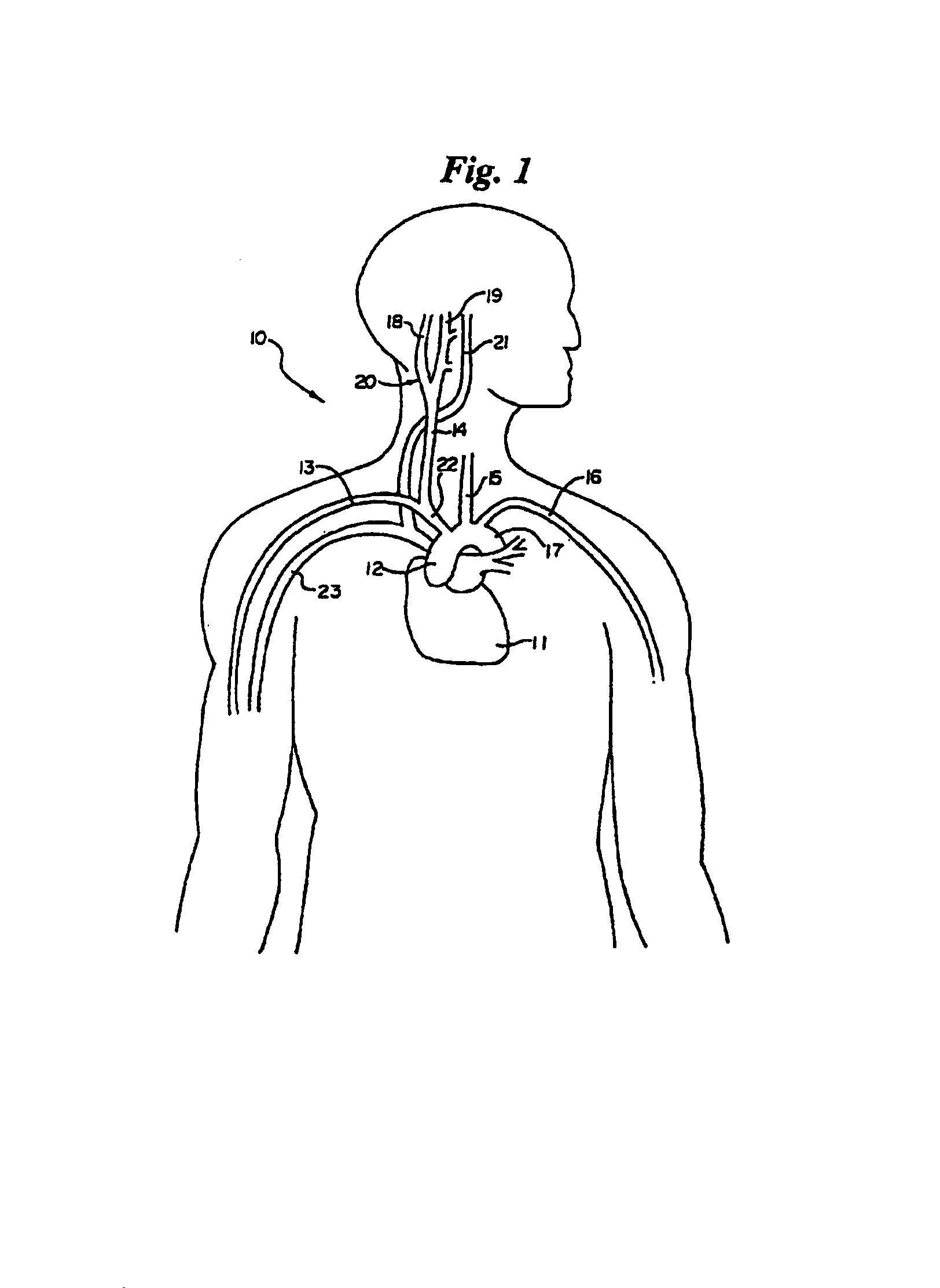
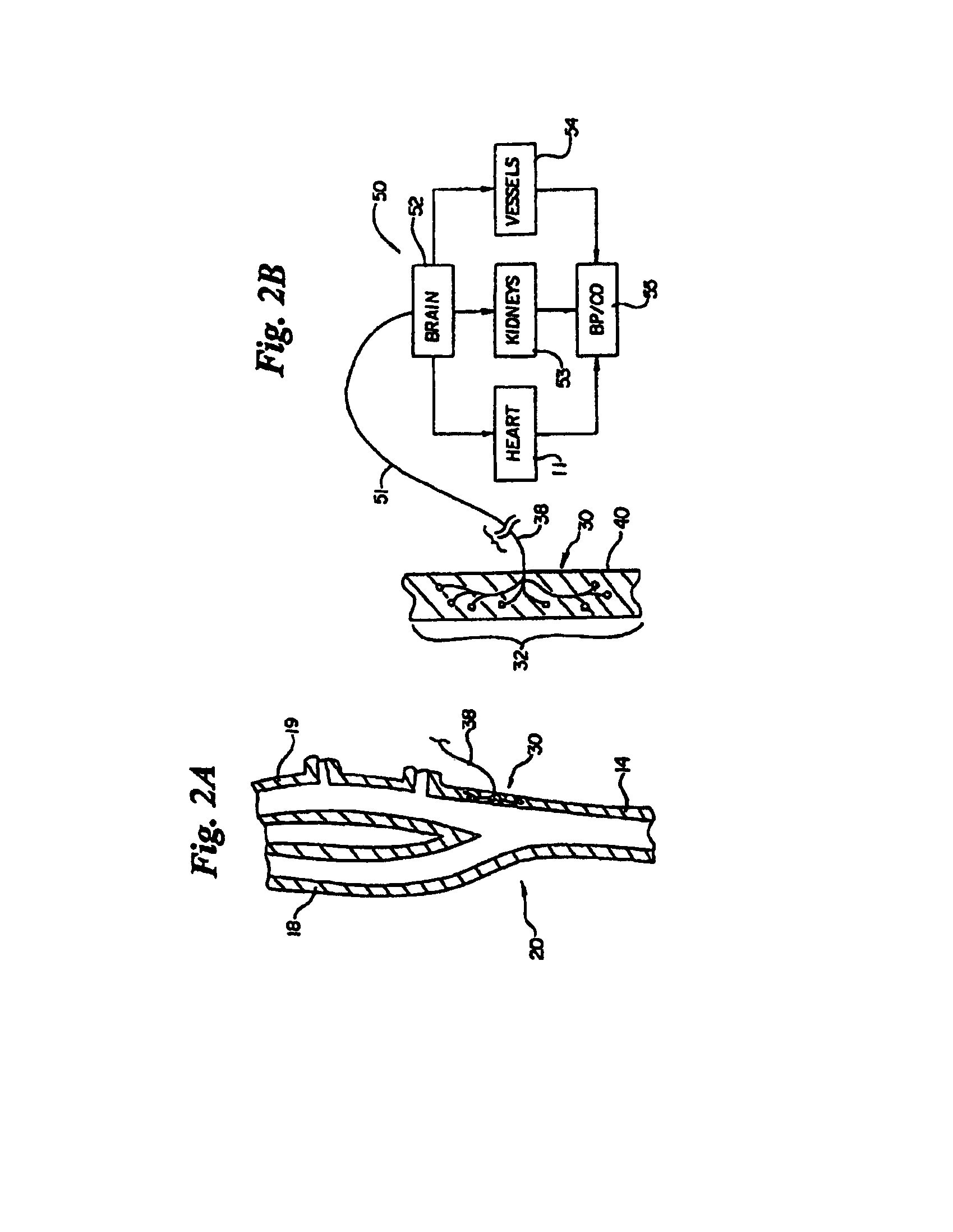
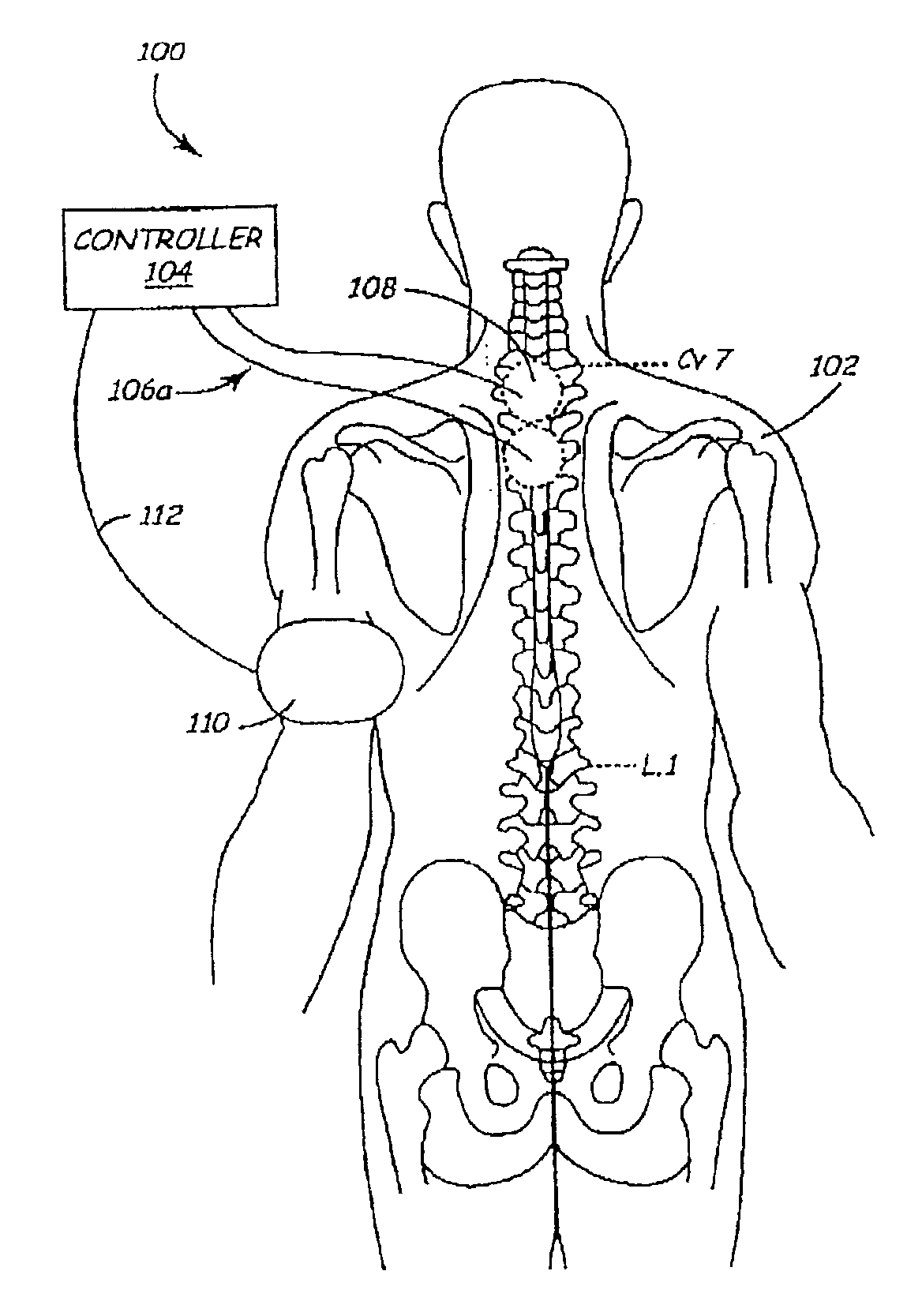
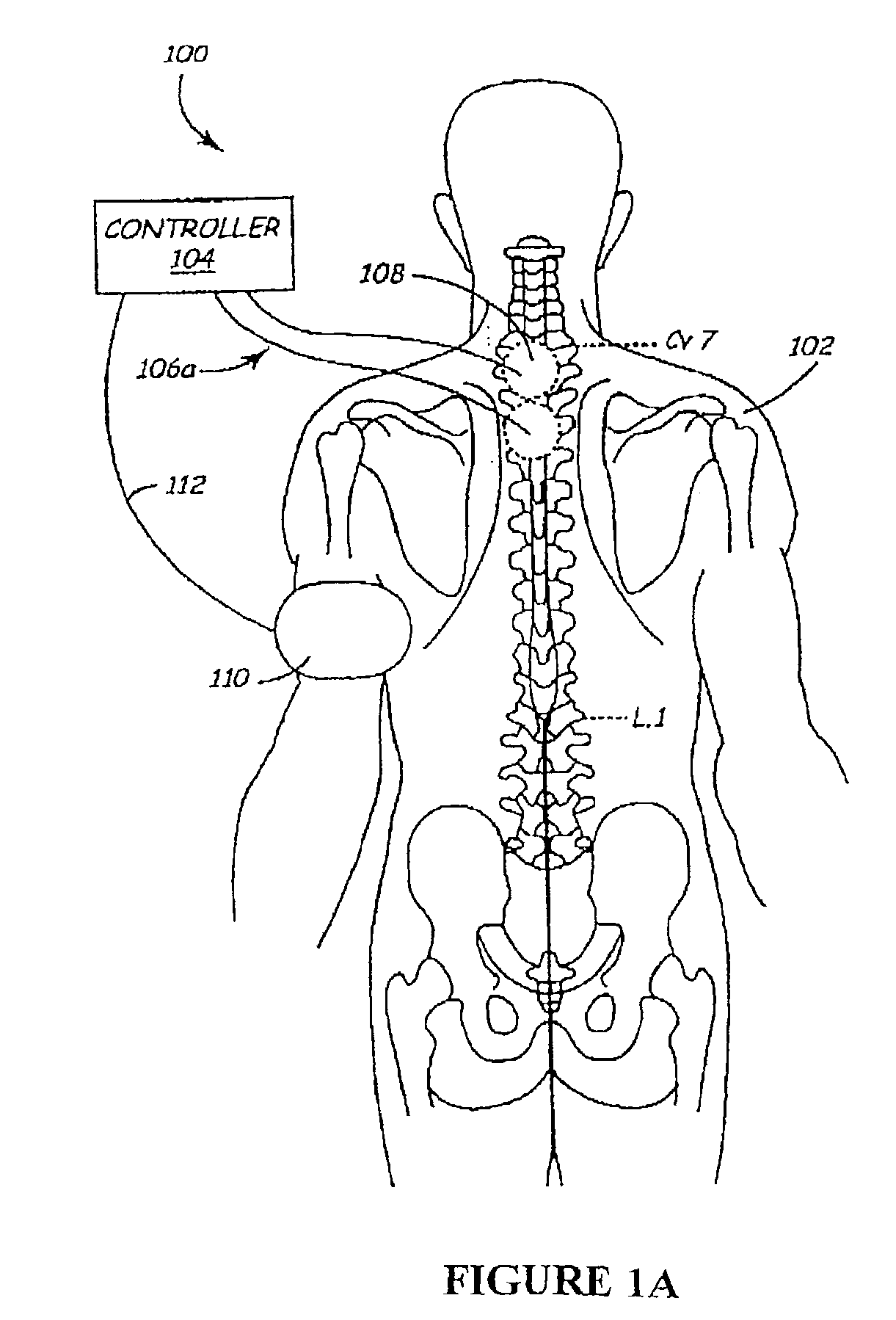
Method and apparatus for electrically stimulating the nervous system to improve ventricular dysfunction, heart failure, and other cardiac conditions
A method and apparatus are used to provide therapy to a patient experiencing ventricular dysfunction or heart failure. At least one electrode is located in a region associated with nervous tissue, such as nerve bundles T1-T4, in a patient's body. Electrical stimulation is applied to the at least one electrode to improve the cardiac efficiency of the patient's heart. One or more predetermined physiologic parameters of the patient are monitored, and the electrical stimulation is adjusted based on the one or more predetermined physiologic parameters.
Owner:MEDTRONIC INC
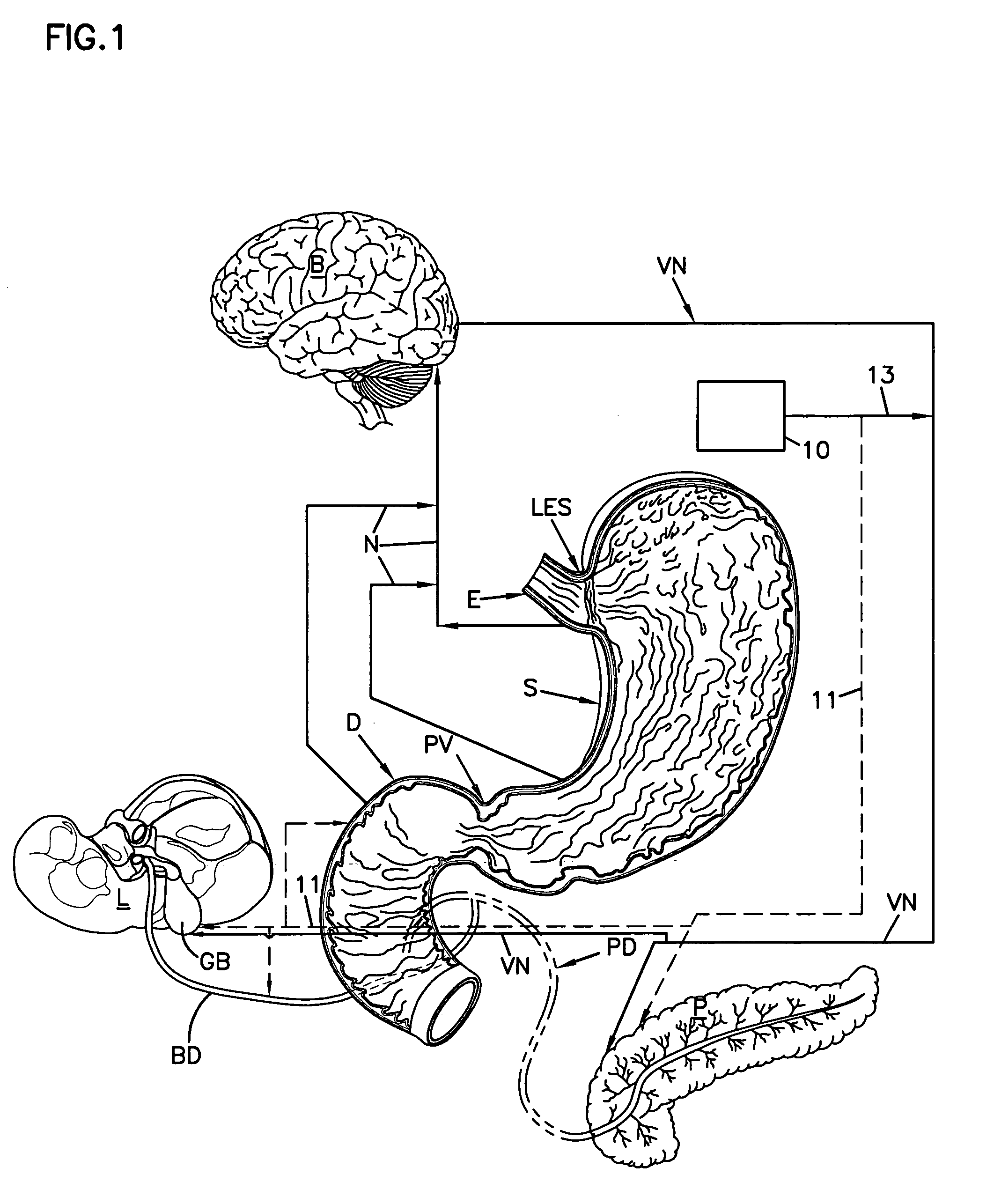
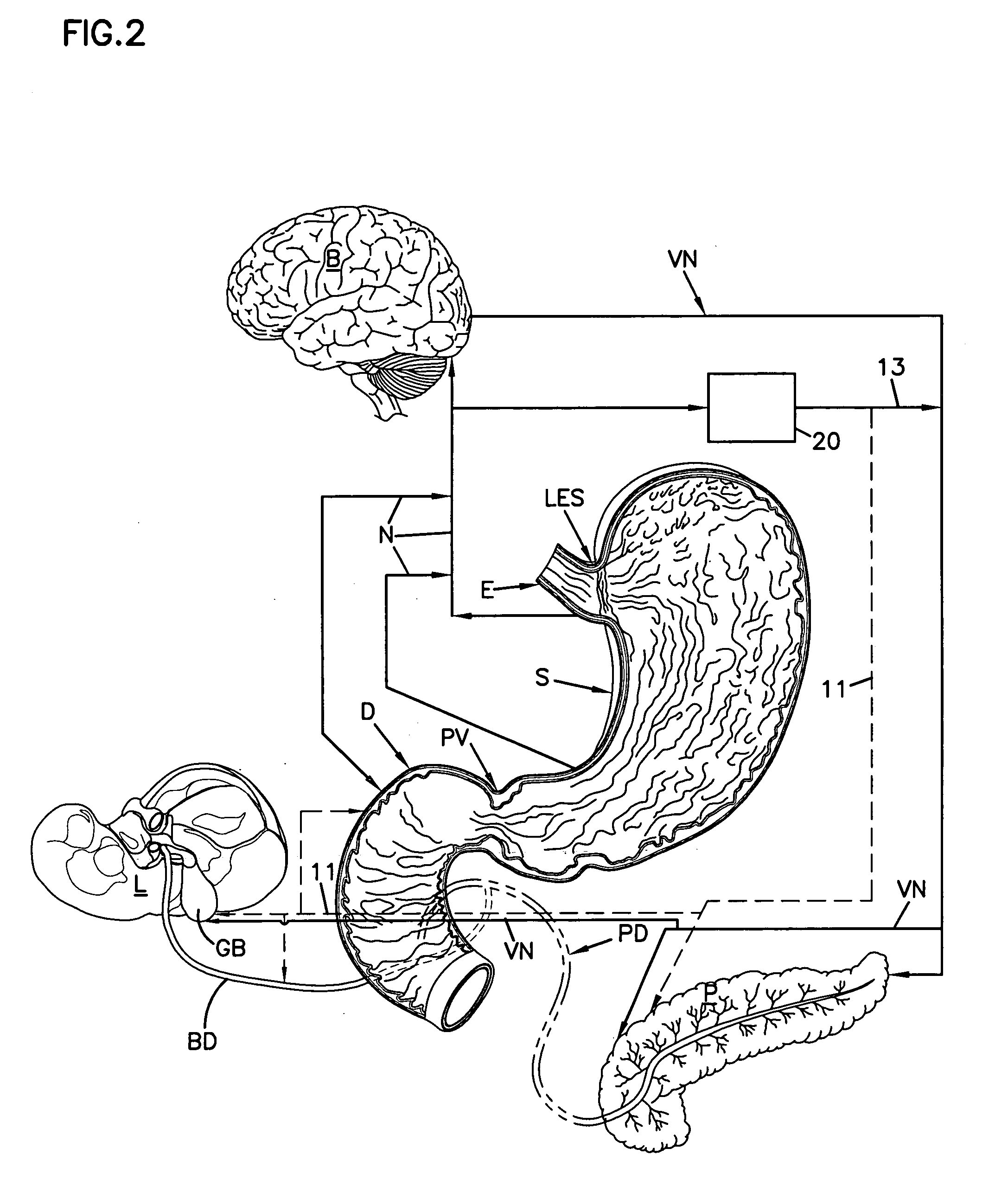
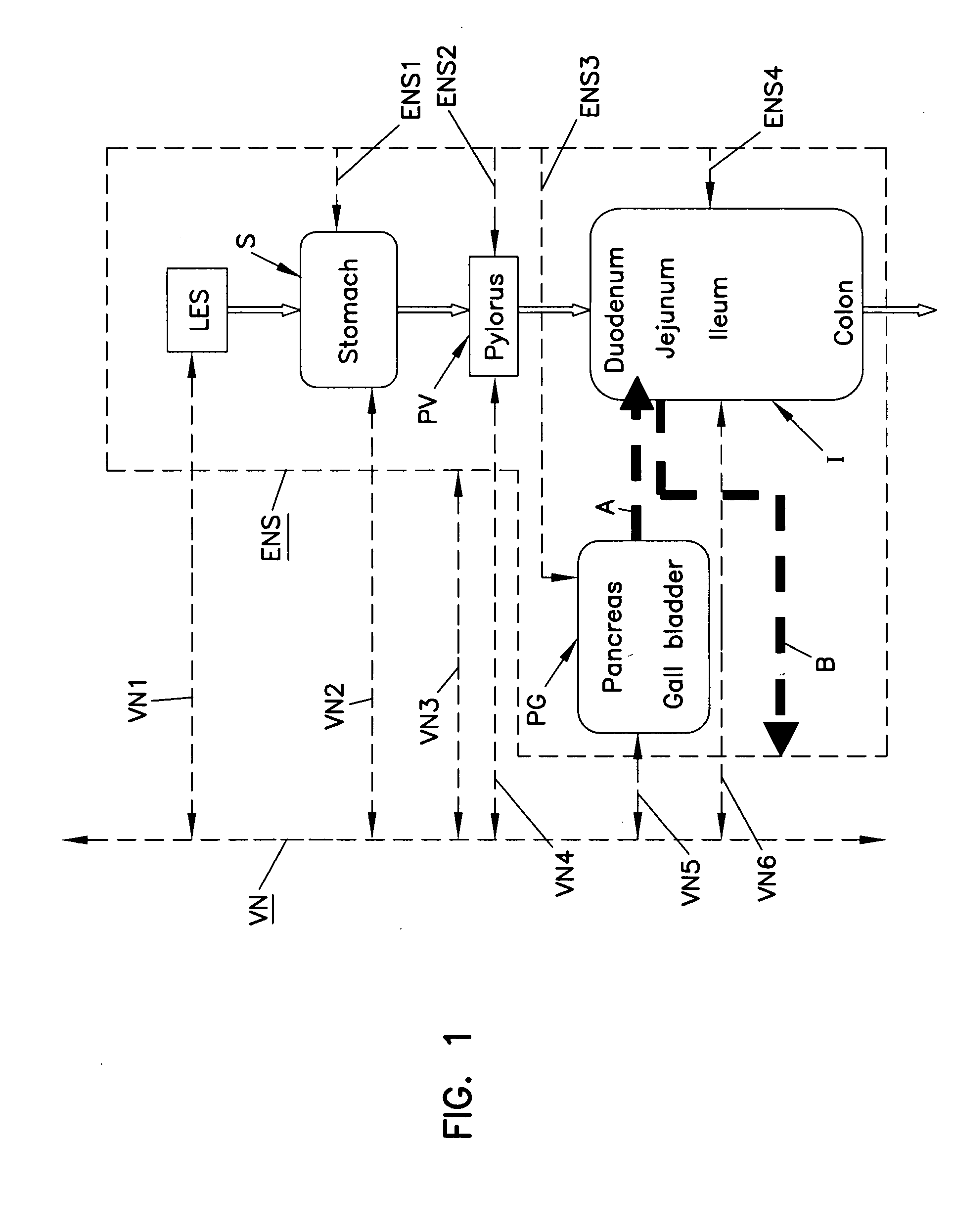
Obesity treatment with electrically induced vagal down regulation
At least one of a plurality of disorders of a patient characterized at least in part by vagal activity innervating at least one of a plurality of organs of the patient is treated by a method that includes positioning a neurostimulator carrier around a body organ of the patient where the organ is innervated by at least a vagal trunk. An electrode is disposed on the carrier and positioned at the vagal trunk. An electrical signal is applied to the electrode to modulate vagal activity by an amount selected to treat the disorder. The signal may be a blocking or a stimulation signal.
Owner:RESHAPE LIFESCIENCES INC
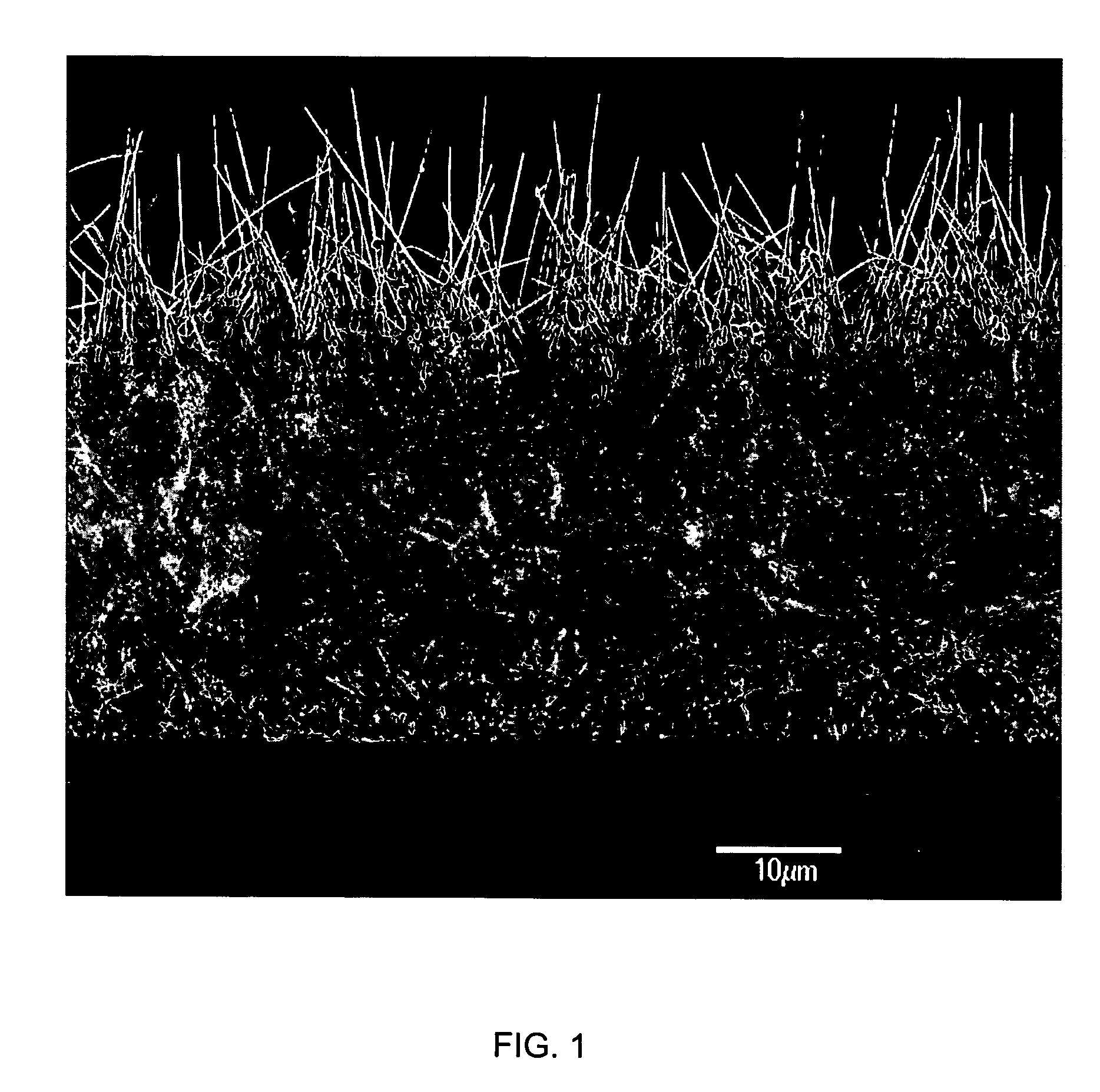
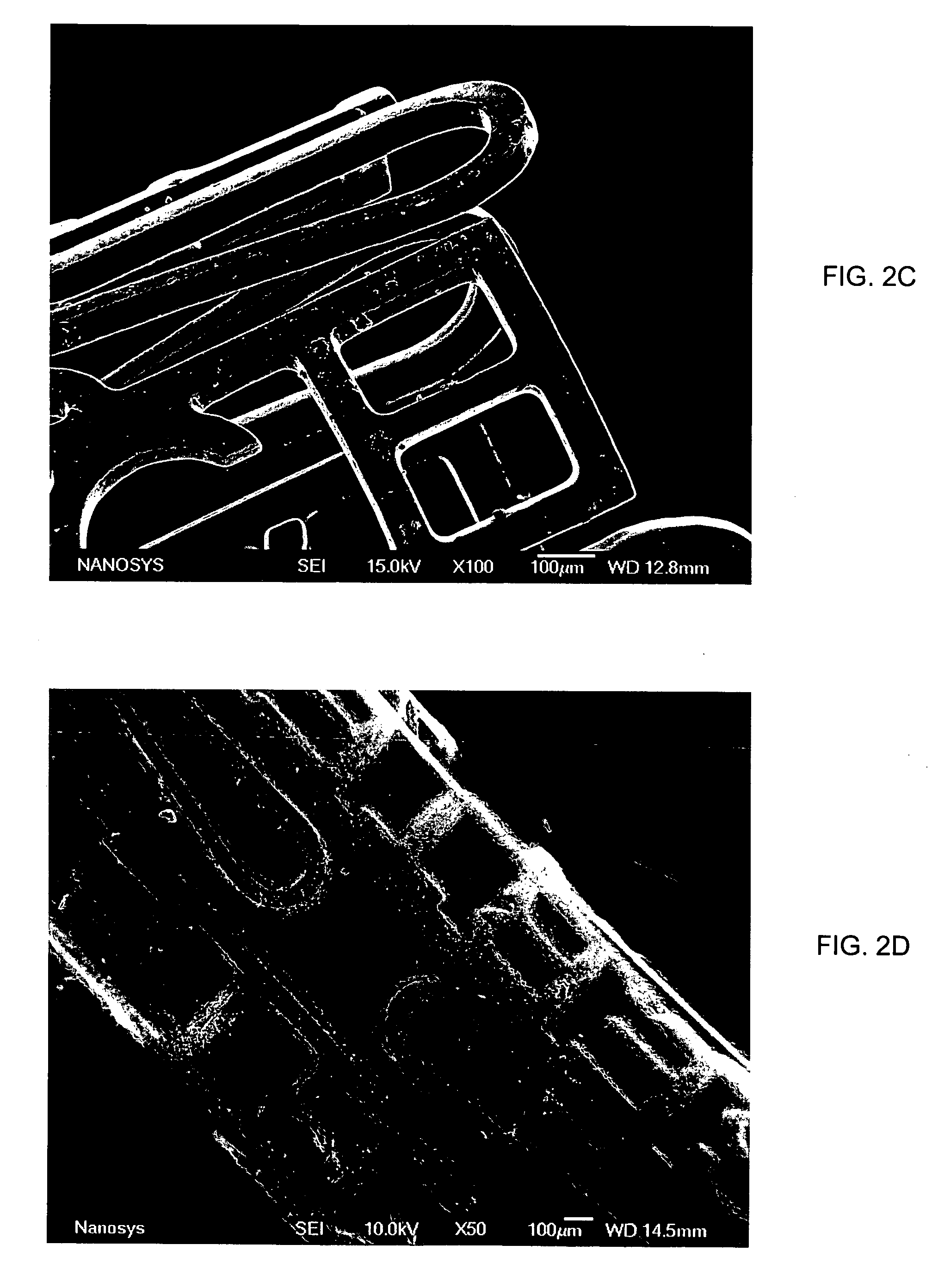
Medical device applications of nanostructured surfaces
InactiveUS20060204738A1Improve adhesionIncrease frictionBiocideMaterial nanotechnologyOsteoblastNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:GLO TECH LLC
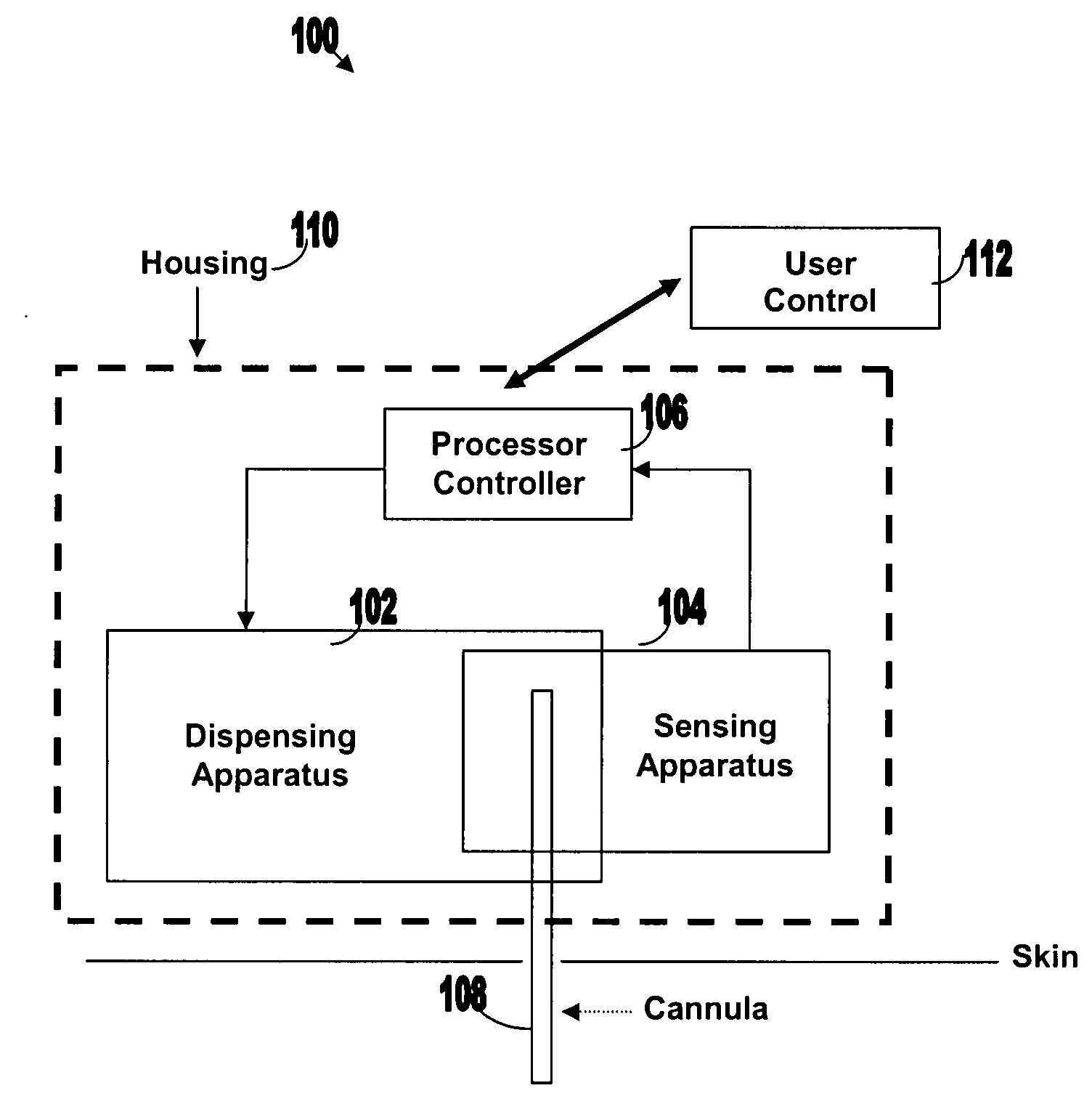
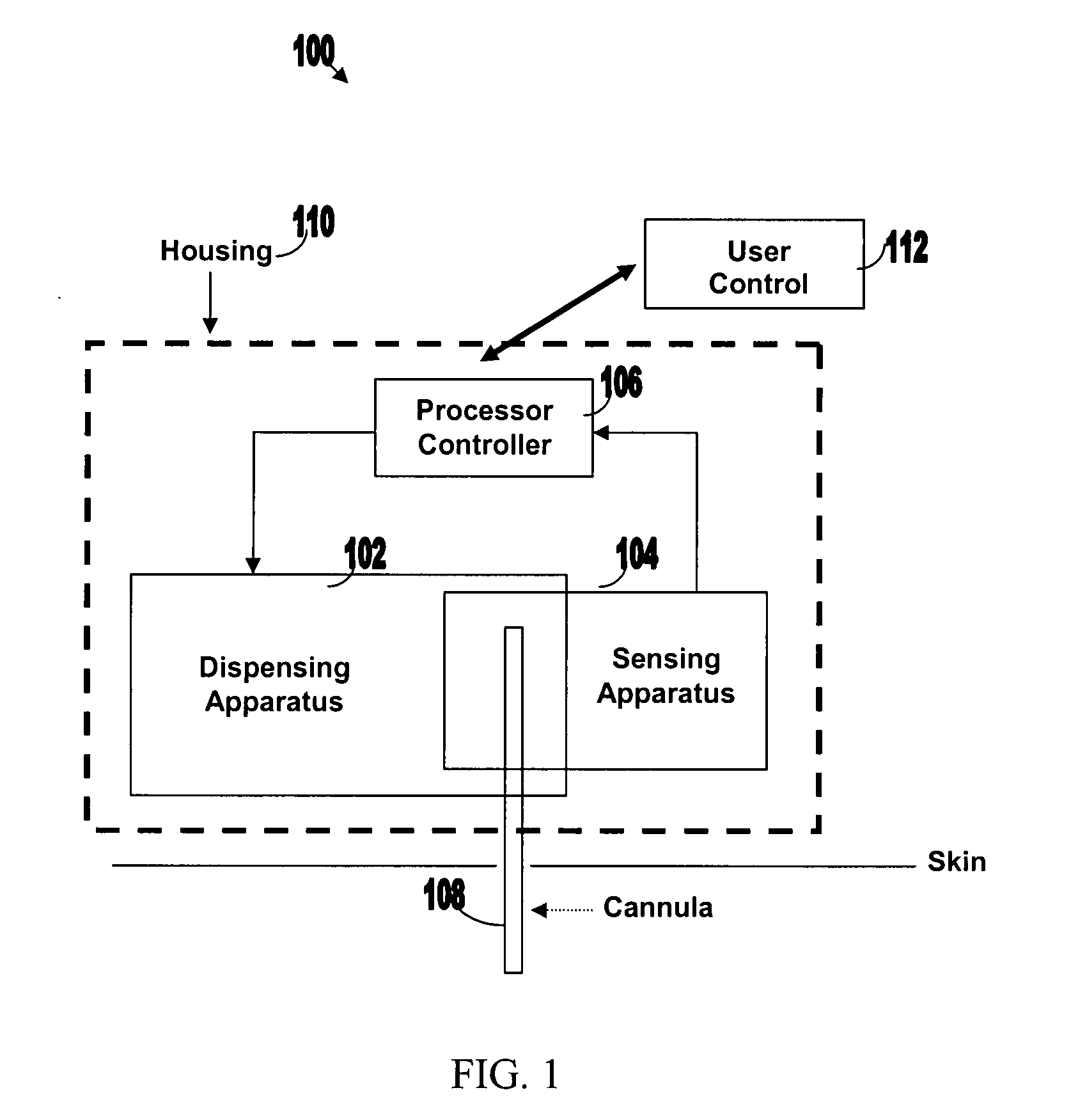
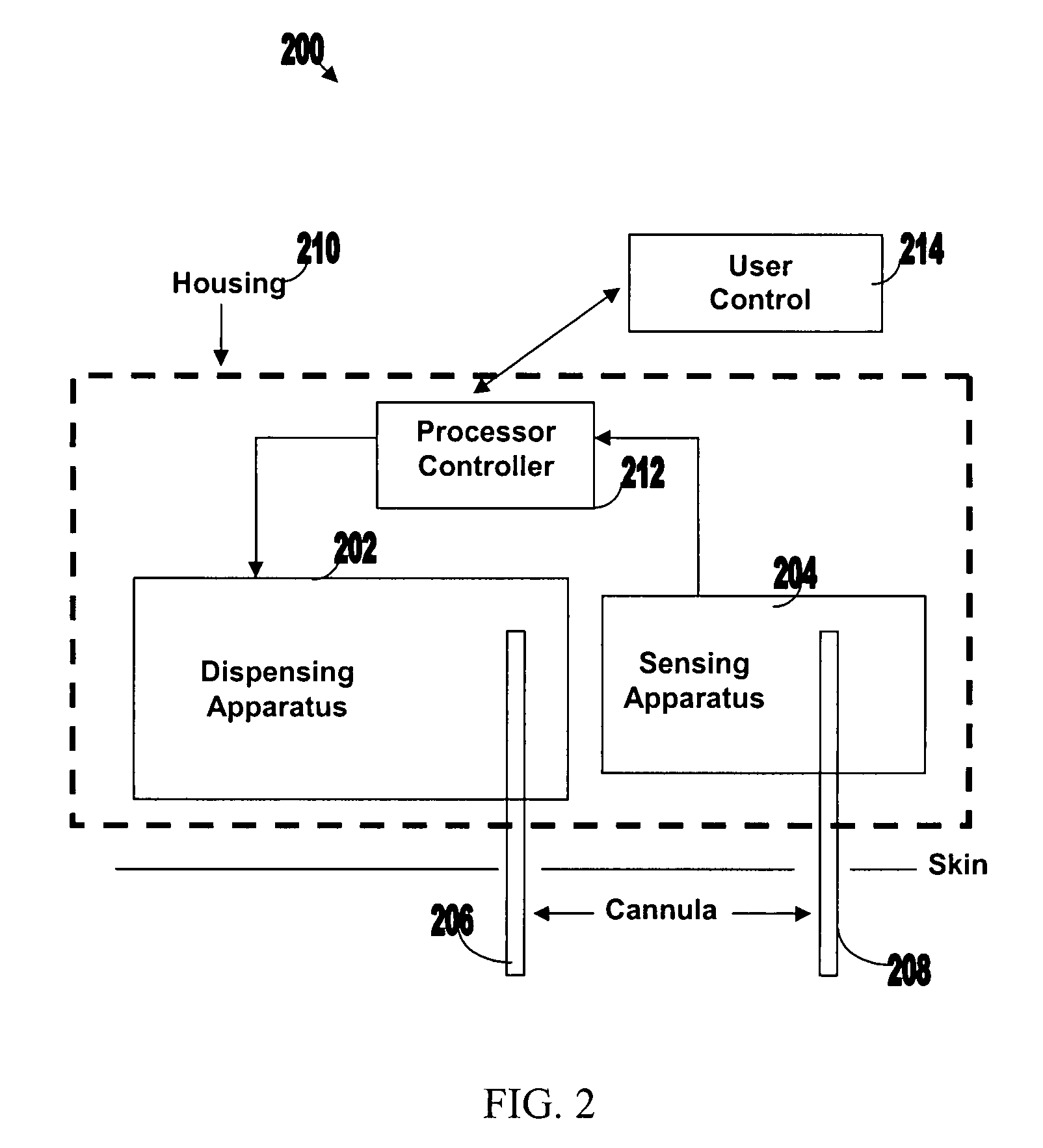
Systems and methods for sensing analyte and dispensing therapeutic fluid
Systems and methods are provided for sensing analyte (e.g., glucose) and / or dispensing therapeutic fluid (e.g., insulin). The systems and methods are based on transporting the therapeutic fluid through a cannula at least a portion of which is permeable to molecule of the analyte. Sensing and detection of the concentration level of the analyte can be carried out by optical sensing, electrochemical sensing, acoustical sensing etc. Sensing and dispensing can be carried out by sensing and dispensing device operating in either closed or semi-closed loop.
Owner:ROCHE DIABETES CARE INC
Methods of affecting hypothalamic-related conditions
The present invention relates to a method of affecting a hypothalamic-related condition by electrically and / or chemically stimulating the hypothalamus. Also provided are methods of stimulating a hypothalamic-related target site by responding to a sensor signal relating to a physiological activity of the body associated with the hypothalamic-related condition desired to be affected. The present invention also describes a method of directly or indirectly modulating hormones synthesized or released by the hypothalamus to affect hypothalamic-related conditions involving hormonal function, dysfunction or imbalance.
Owner:THE CLEVELAND CLINIC FOUND
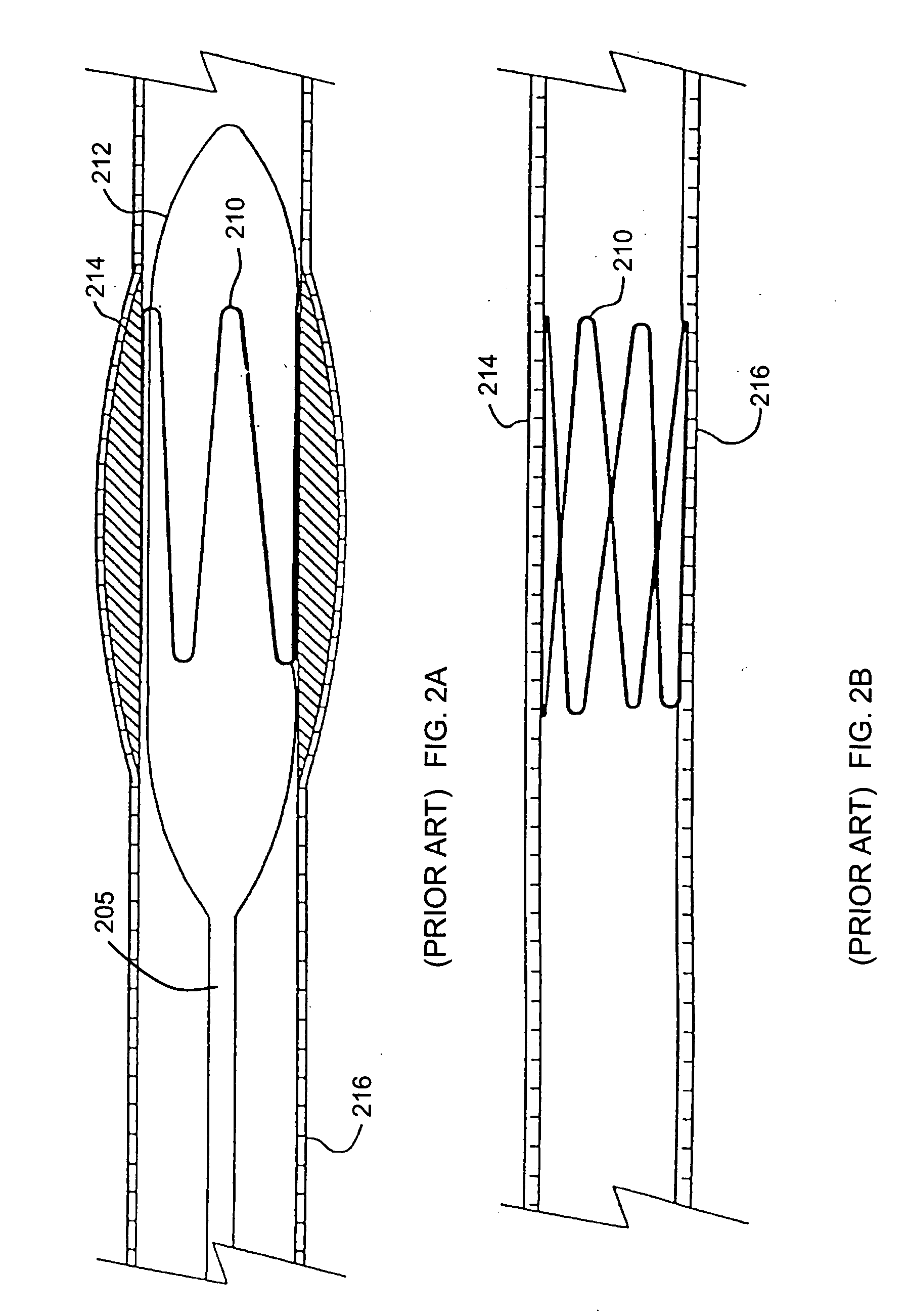
Methods and apparatus for fabricating leads with conductors and related flexible lead configurations
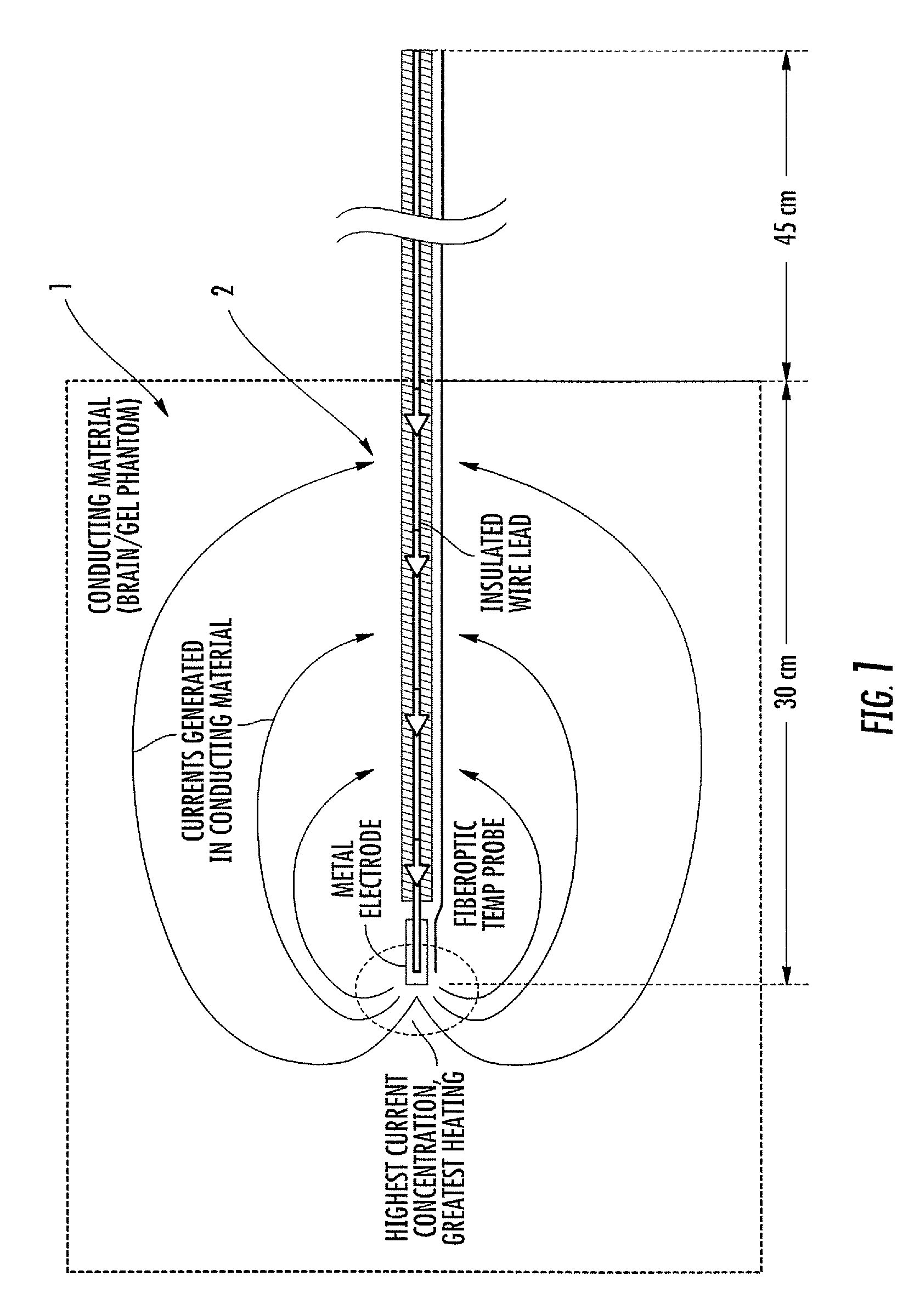
ActiveUS20080262584A1Prevent undesired heatingEasy to useInternal electrodesMaterial strength using steady bending forcesElectrical conductorDegree Celsius
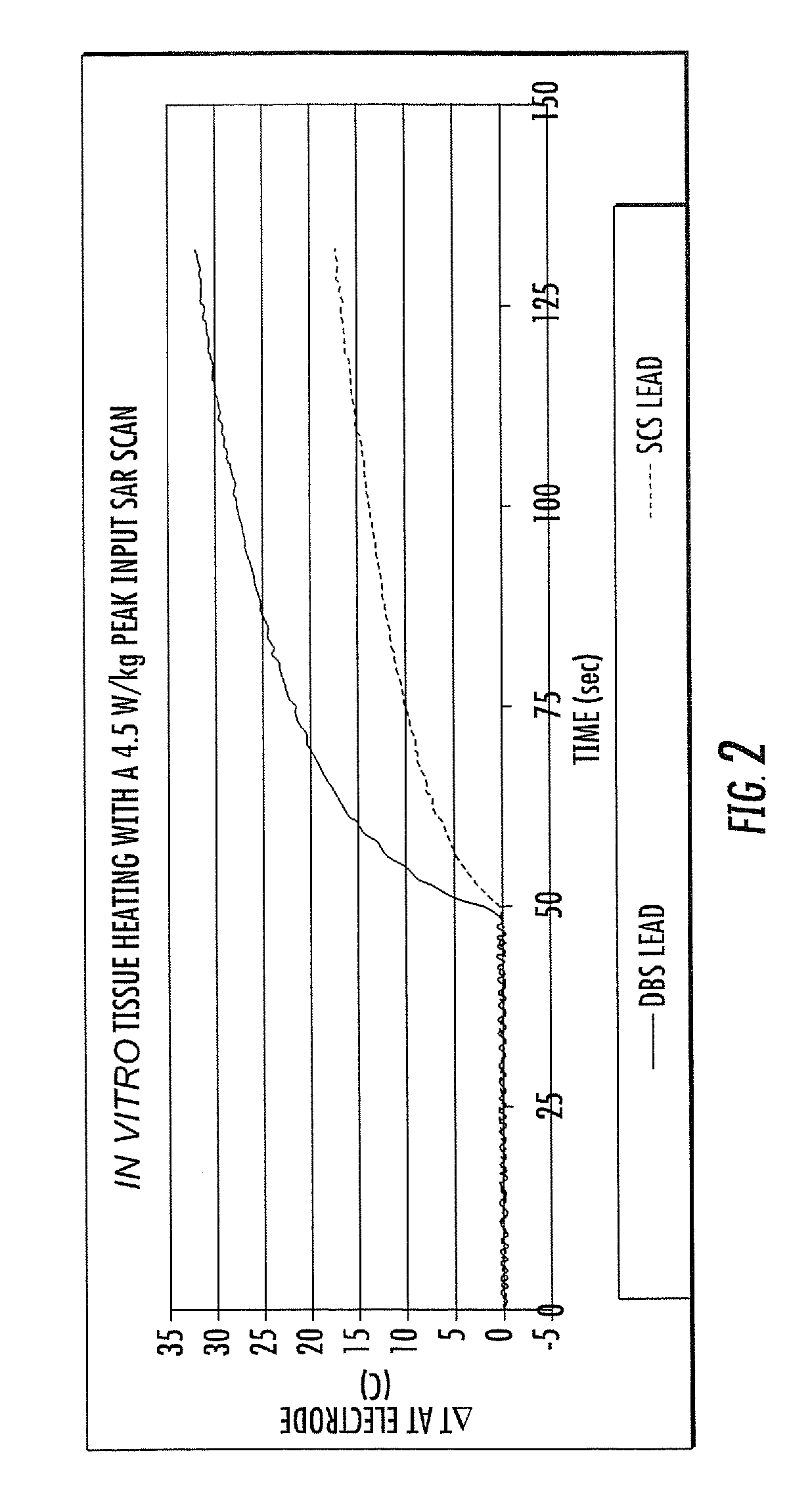
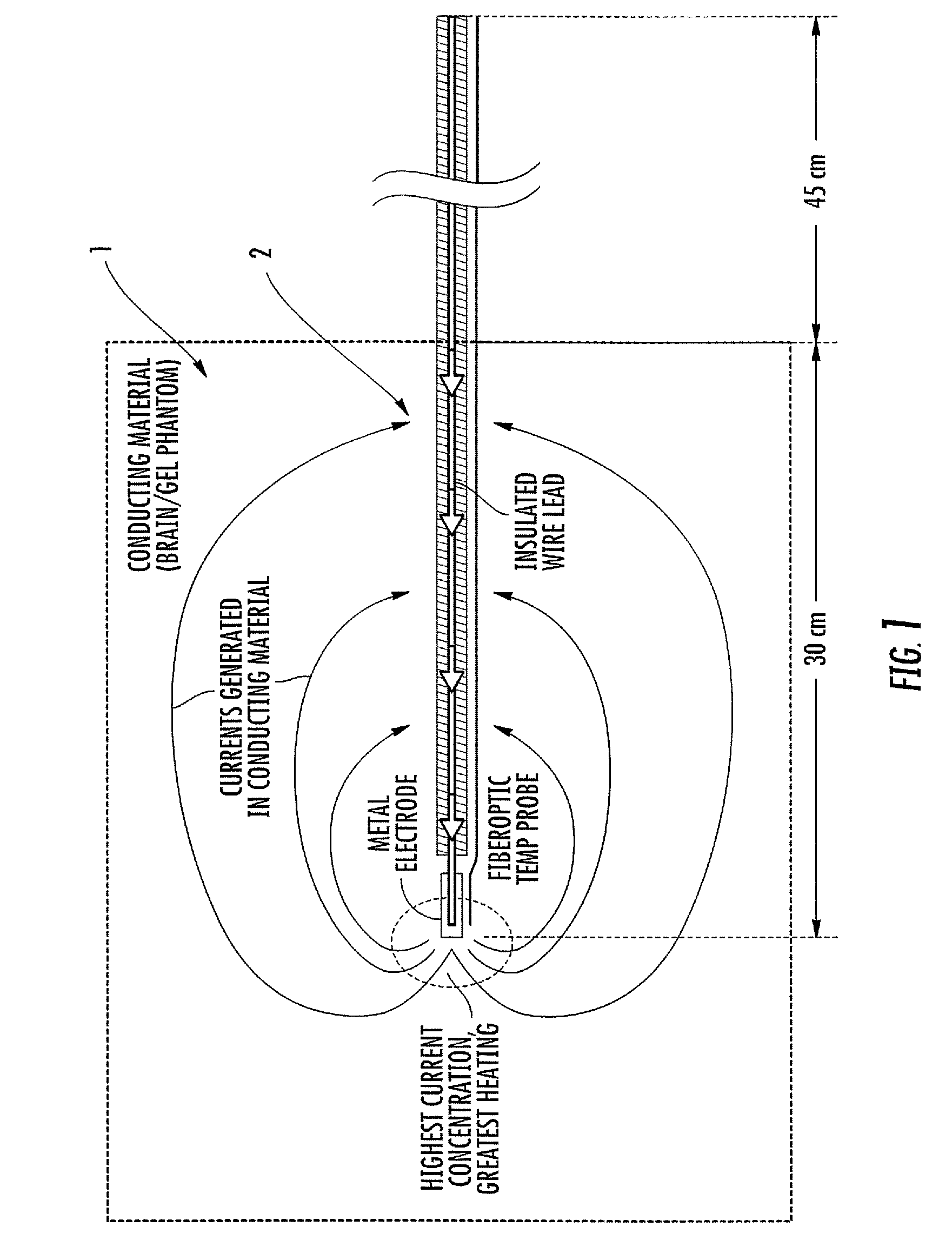
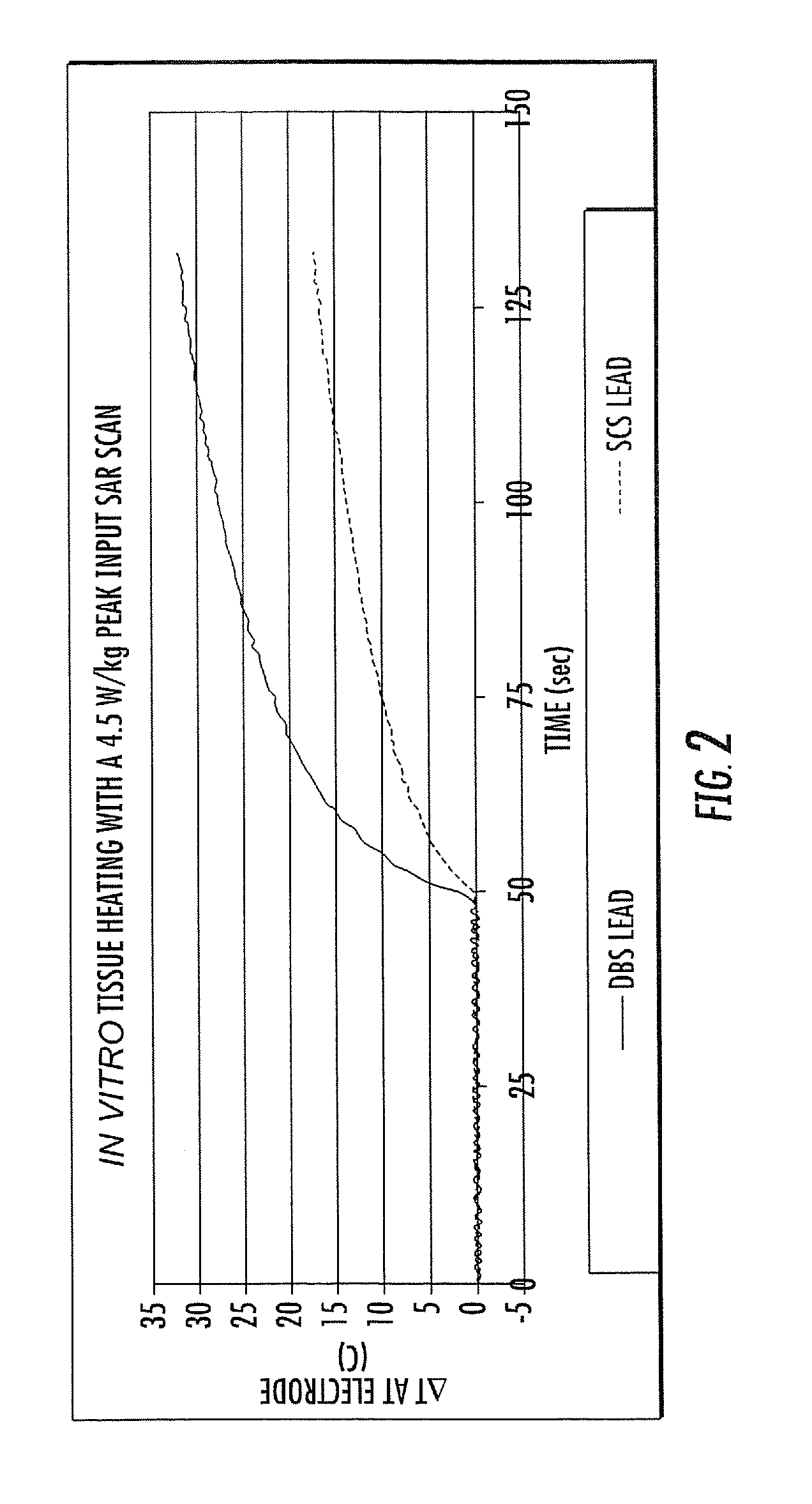
MRI / RF compatible leads include at least one conductor, a respective conductor having at least one segment with a multi-layer stacked coil configuration. The lead can be configured so that the lead heats local tissue less than about 10 degrees Celsius (typically about 5 degrees Celsius or less) or does not heat local tissue when a patient is exposed to target RF frequencies at a peak input SAR of at least about 4 W / kg and / or a whole body average SAR of at least about 2 W / kg. Related leads and methods of fabricating leads are also described.
Owner:MRI INTERVENTIONS INC +1
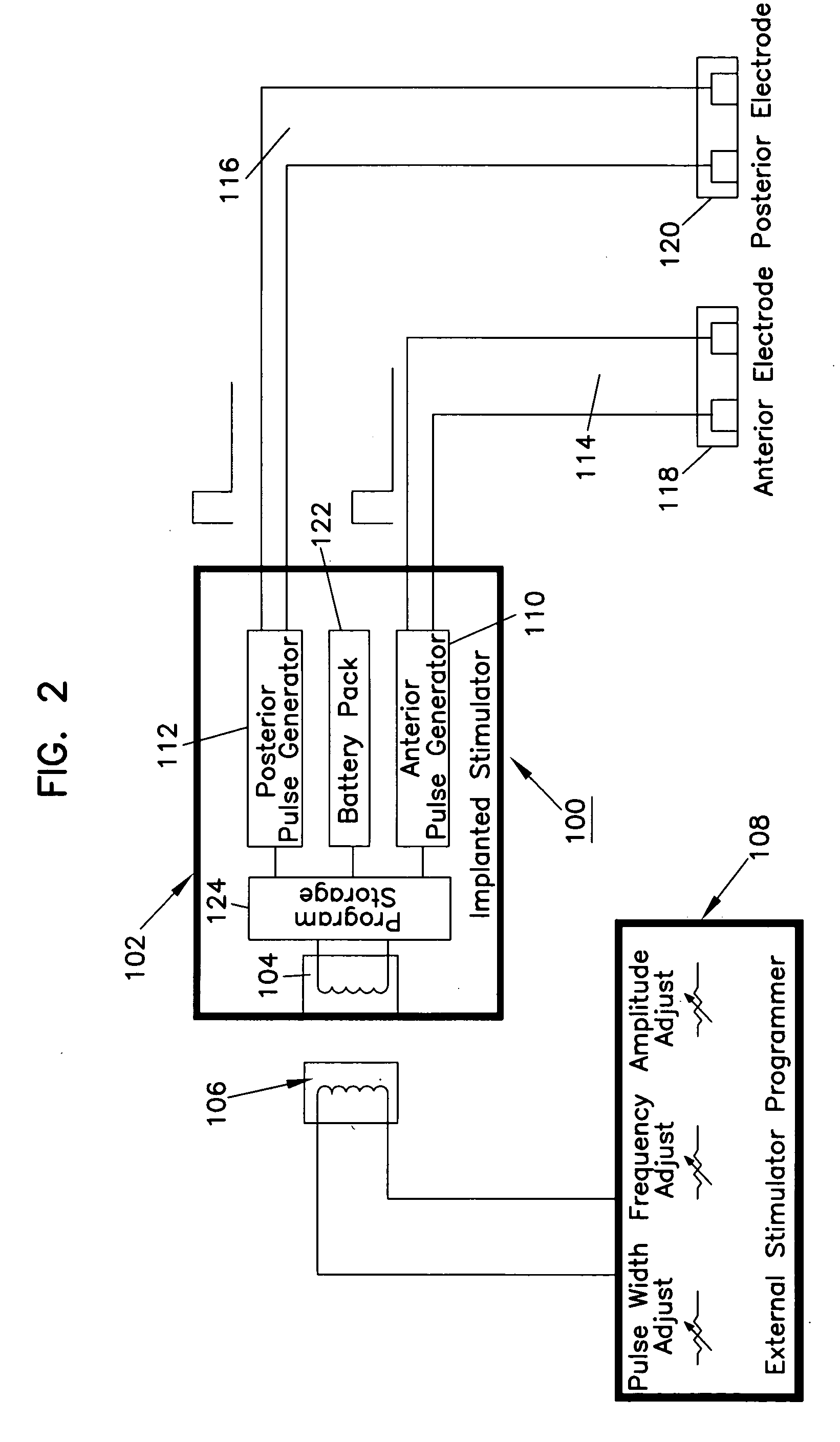
Apparatus and method for treatment of neurological and neuropsychiatric disorders using programmerless implantable pulse generator system
InactiveUS6760626B1Internal electrodesImplantable neurostimulatorsCranial nervesElectrical stimulations
System and method for neuromodulation therapy of neurological and neuropsychiatric disorders comprises an implantable lead and pulse generator system for providing the appropriate electrical stimulation to a cranial nerve such as the vagus nerve. The implantable pulse generator having prepackaged / predetermined programs stored in the memory of the pulse generator, and means for accessing these with an external magnet. The pulse generator adapted to selectively activate predetermined programs with the external magnet, thereby eliminating the need for an external programmer. The elimination of the external programmer resulting in significant cost reduction with essentially the same functionality.
Owner:NEURO & CARDIAC TECH
Electrode designs and methods of use for cardiovascular reflex control devices
InactiveUS7158832B2RemissionReduce pressureInternal electrodesExternal electrodesNervous systemBaroreceptor function
Devices, systems and methods by which the blood pressure, nervous system activity, and neurohornonal activity may be selectively and controllably reduced by activating baroreceptors. A baroreceptor activation device is positioned near a baroreceptor, preferably in the carotid sinus. The baroreceptor activation device may utilize electrodes to activate the baroreceptors. The electrodes may be adapted for connection to the carotid arteries at or near the carotid sinus, and may be designed to minimize extraneous tissue stimulation.
Owner:CVRX
Medical device with flexible printed circuit
A catheter or lead having a flexible printed circuit for conveying signals and / or energy. Each trace may be in electrical connection with one or more external electrical contacts. More specifically, each trace is typically electrically connected to a single contact. The traces and contacts may assist in diagnosis and / or detection of bio-electrical signals emitted by organs, and may transmit such signals to a connector or diagnostic device affixed to the catheter. The external electrical contacts may detect bioelectric energy or may deliver electrical or thermal energy to a target site.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Fixation device for implantable microdevices
InactiveUS7054692B1Inhibit migrationPrevent retreatInternal electrodesDiagnostic recording/measuringMedicineBiomedical engineering
A fixation device fixes the position of an implantable microminiature device residing proximally to a target site such as a nerve or a muscle. In one embodiment, the device comprises a sheath and a means for attaching the device to adjacent tissue. The means for attaching may be any one of several embodiments including one or more grasping members, a combination of grasping members and one or more helices, or an extension adapted to accept a suture. In another embodiment, the fixation device comprises an assembly residing in the implantation pathway, behind the stimulation device, thus preventing retreat of the stimulation device in the pathway. In a preferred use, the fixation device fixes the position of a microstimulator component of a Peripheral Nerve Stimulation (PNS) system.
Owner:BOSTON SCI NEUROMODULATION CORP
Intravascular implant system
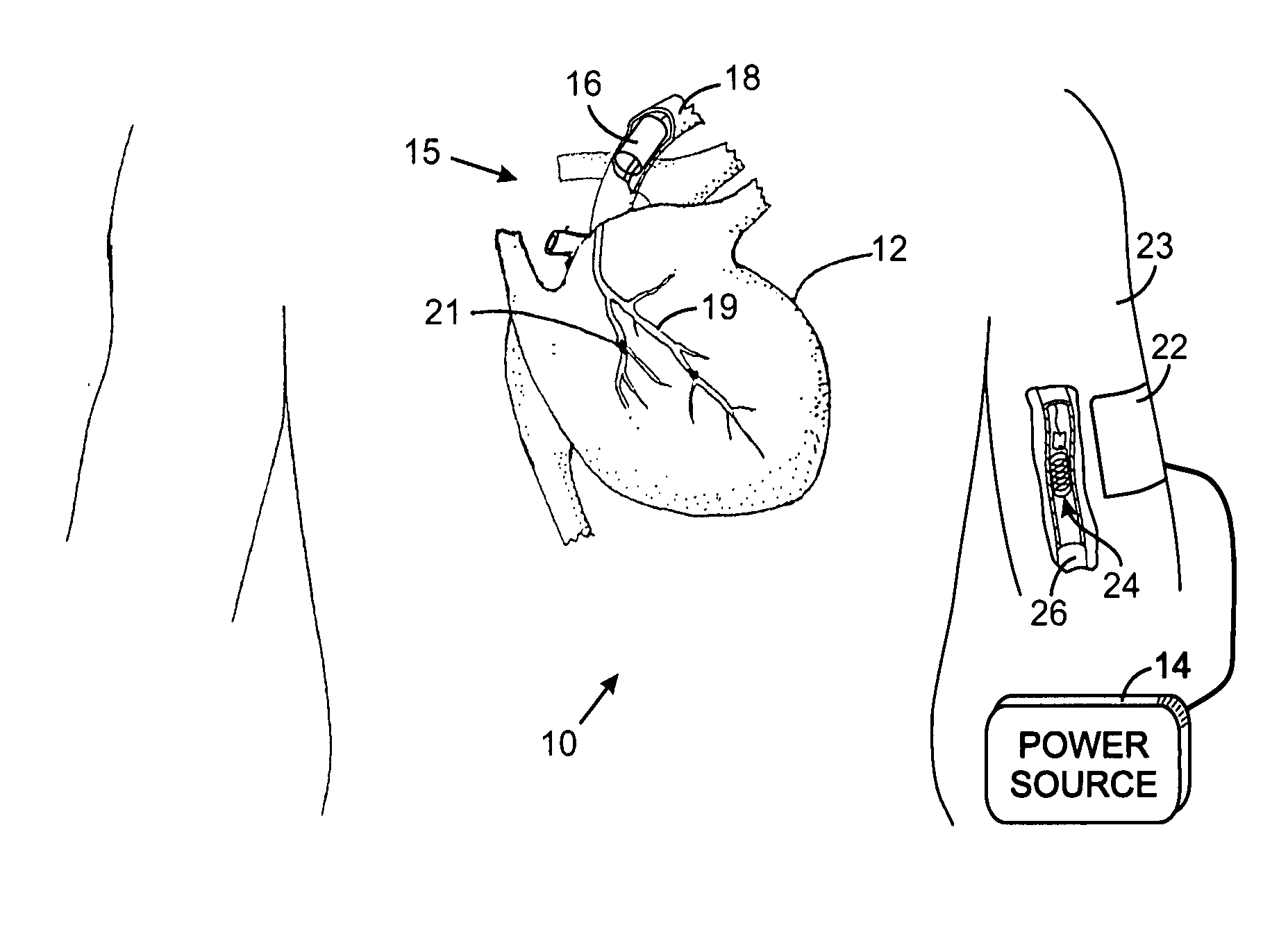
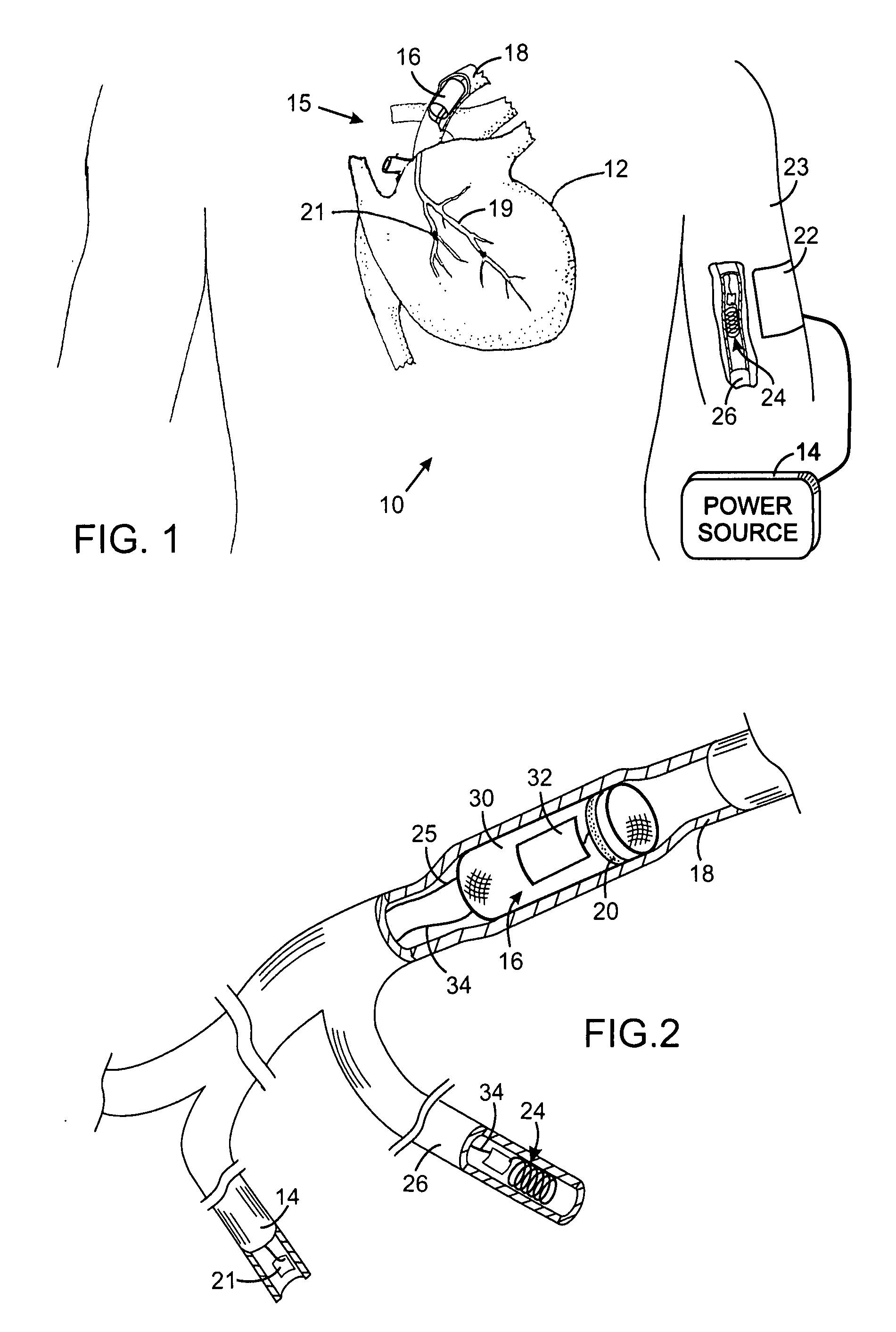
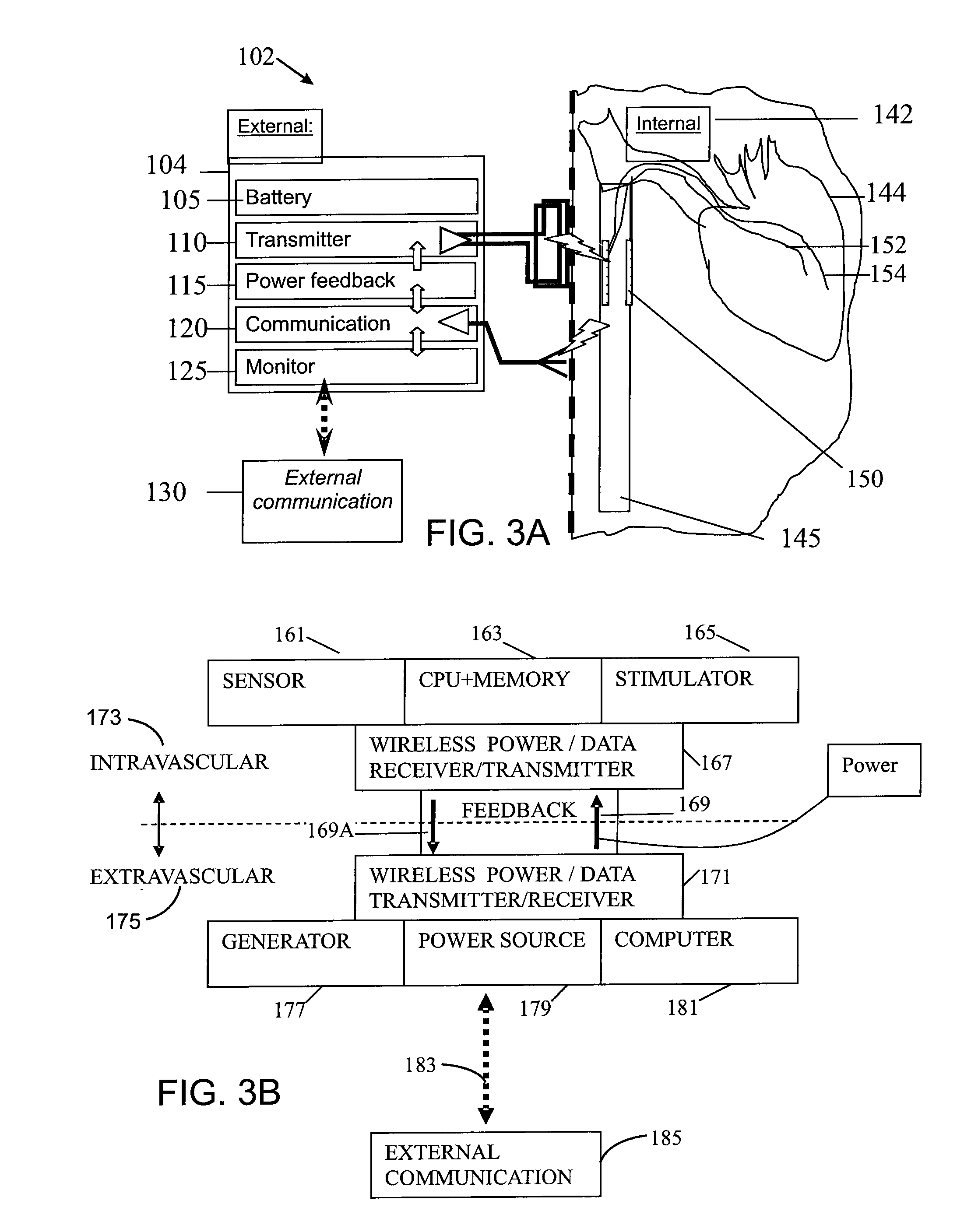
An intravascular implantable system for providing electrical stimulation of tissue inside an animal to deal with a clinical condition is described. The system comprises a power supply module supplying energy to the implantable system, an implanted control module controlling operation of the implantable system and producing desired digital waveforms. Each desired digital waveform has an envelope with a predetermined attribute. An implanted intravascular sensing module sensing at least one parameter of interest for the purpose of dealing with the clinical condition. An intravascular stimulation module is provided to electrically stimulate the tissue with an output waveform that is substantially similar to the desired digital waveform produced by the control module.
Owner:KENERGY INC
Controlled vagal blockage therapy
A method for treating at least one of a plurality of disorders characterized at least in part by vagal activity includes positioning an electrode around a body organ innervated by the vagus. An electrical signal is applied to the electrode to modulate vagal activity. The electrical signal is applied at a frequency selected for the signal to create a neural conduction block to the vagus with the neural conduction block selected to at least partially block nerve impulses on the vagus. The application of the electrical signal is discontinued. The application of the signal and the discontinuing of the signal are repeated with durations of the discontinuing and the application selected to treat the disorder.
Owner:RESHAPE LIFESCIENCES INC
High frequency vagal blockage therapy
A method for treating at least one of a plurality of disorders characterized at least in part by vagal activity includes positioning an electrode at a body organ innervated by the vagus. An electrical signal is applied to the electrode to modulate vagal activity. The electrical signal is applied at a frequency in excess of 3,000 Hz for the signal to create a neural conduction block to the vagus with the neural conduction block selected to at least partially block nerve impulses on the vagus.
Owner:RESHAPE LIFESCIENCES INC
System and methods for performing dynamic pedicle integrity assessments
ActiveUS20060025703A1Overcomes drawbackSurgical needlesInternal electrodesIntegrity assessmentEngineering
The present invention involves systems and related methods for performing dynamic pedicle integrity assessments involving the use of neurophysiology.
Owner:NUVASIVE
Intraluminal electrode apparatus and method
At least one of a plurality of disorders of a patient associated with vagal activity innervating at least one of a plurality of organs of the patient at an innervation site are treated by positioning a neurostimulator carrier within a body lumen of the patient. An electrode disposed on the carrier is positioned at a mucosal layer of the lumen. An electrical signal is applied to the electrode to modulate vagal activity by an amount selected to treat the disorder. The signal may be a blocking or a stimulation signal.
Owner:RESHAPE LIFESCIENCES INC
MRI and RF compatible leads and related methods of operating and fabricating leads
ActiveUS20080243218A1Prevent undesired heatingEasy to useLine/current collector detailsInternal electrodesElectricityCelsius Degree
RF / MRI compatible leads include at least one conductor that turns back on itself at least twice in a lengthwise direction, and can turn back on itself at least twice at multiple locations along its length. The at least one electrical lead can be configured so that the lead heats local tissue less than about 10 degrees Celsius (typically about 5 degrees Celsius or less) or does not heat local tissue when a patient is exposed to target RF frequencies at a peak input SAR of at least about 4 W / kg and / or a whole body average SAR of at least about 2 W / kg. Related devices and methods of fabricating leads are also described.
Owner:MRI INTERVENTIONS INC +1
Cardiac device and methods of use thereof
InactiveUS20070161846A1Reduced elastic recoilShorten speedHeart valvesInternal electrodesCardiac deviceDiastolic function
Devices and methods are described herein which are directed to the treatment of a patient's heart having, or one which is susceptible to heart failure, to improve diastolic function.
Owner:EDWARDS LIFESCIENCES CORP
Methods and apparatus for the regulation of hormone release
ActiveUS7221979B2Modulate hormone levelPrevent hormone imbalancesInternal electrodesExternal electrodesCatecholamineElectrical stimulations
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7–L2 and the associated neural fibers within a region of the T7–L2 dermatomes
Owner:MEDTRONIC INC
Lead assembly for implantable microstimulator
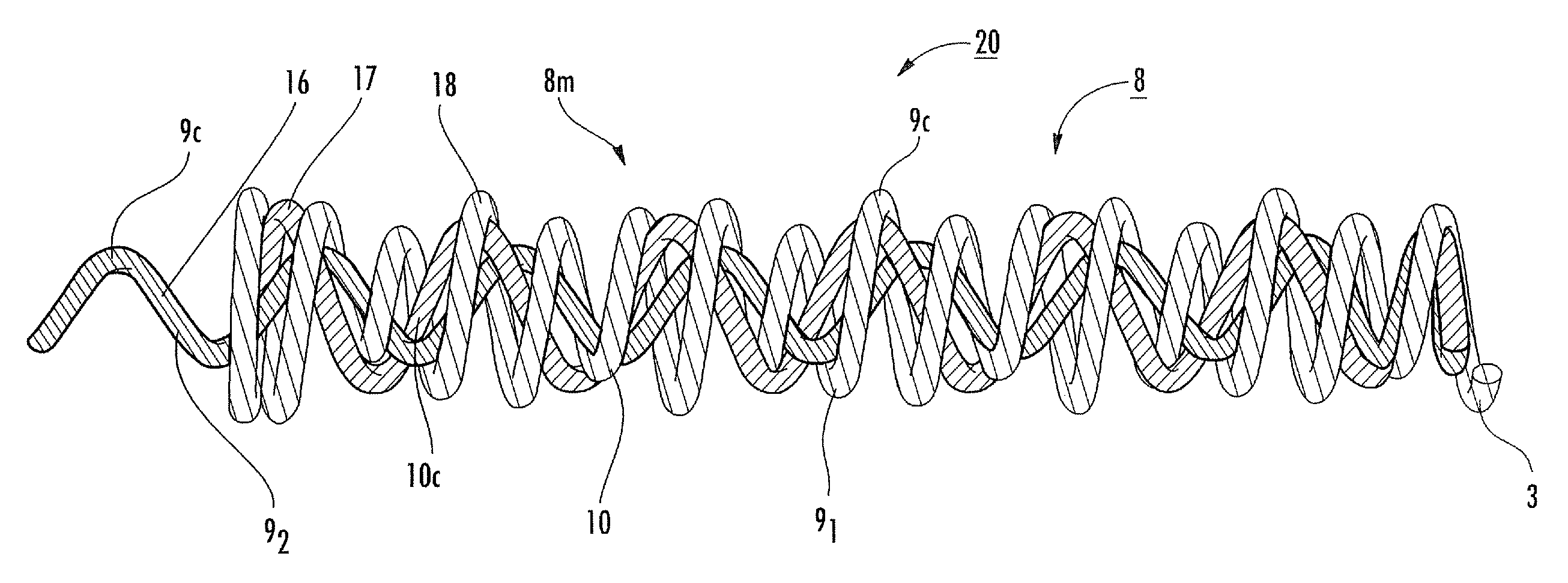
InactiveUS20050165465A1Contact member manufacturingCoupling device detailsElectrical conductorMicro devices
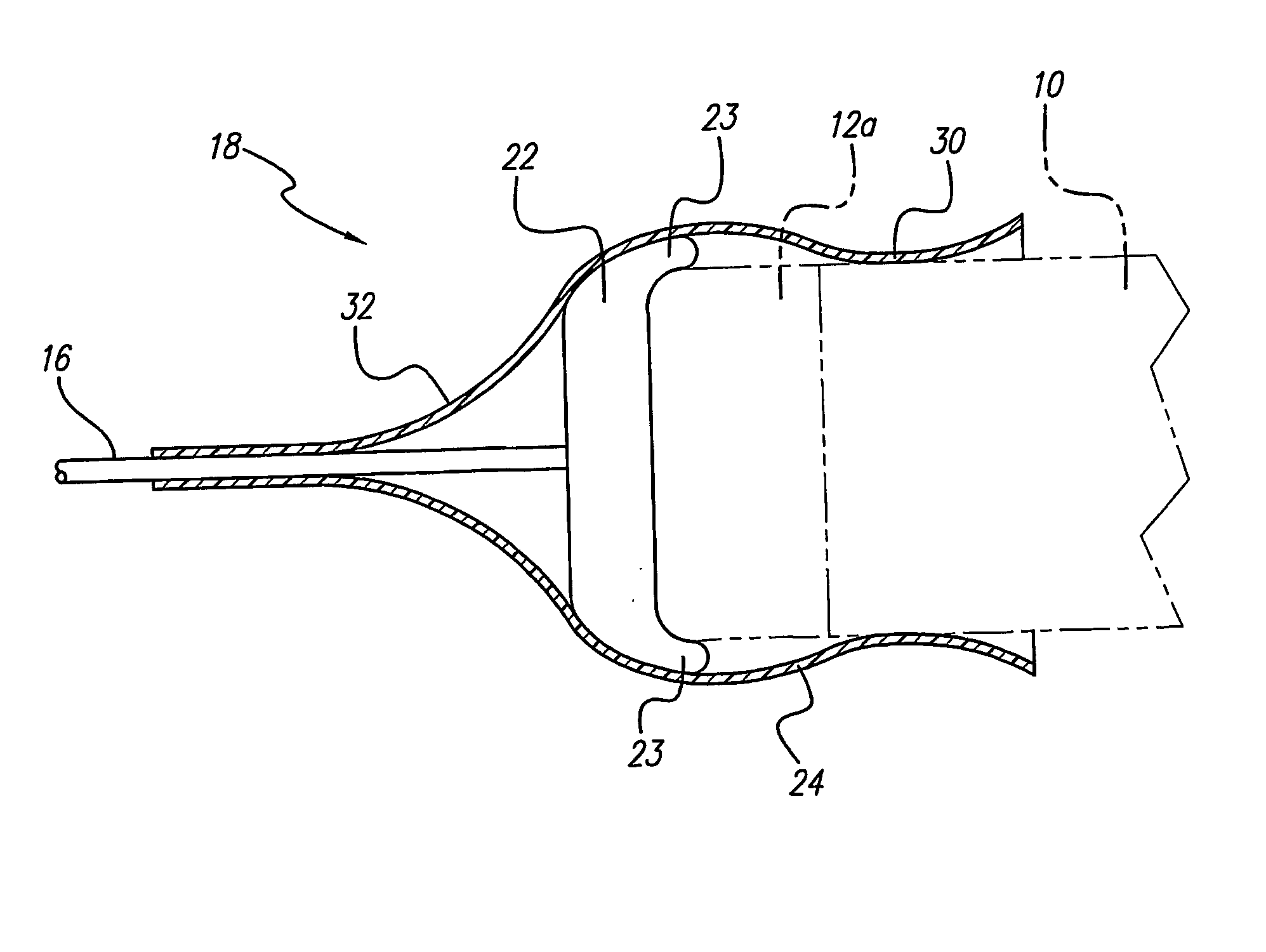
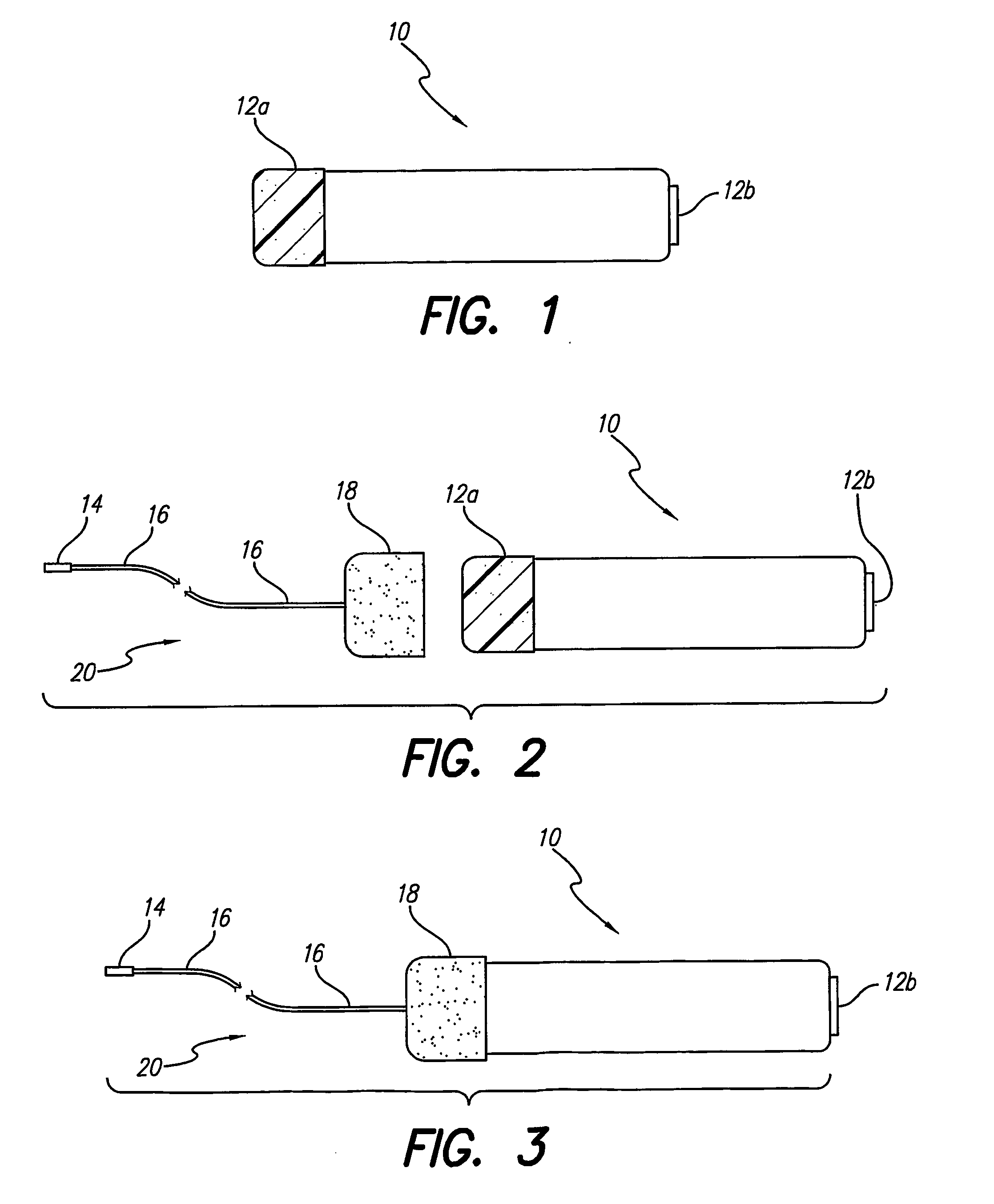
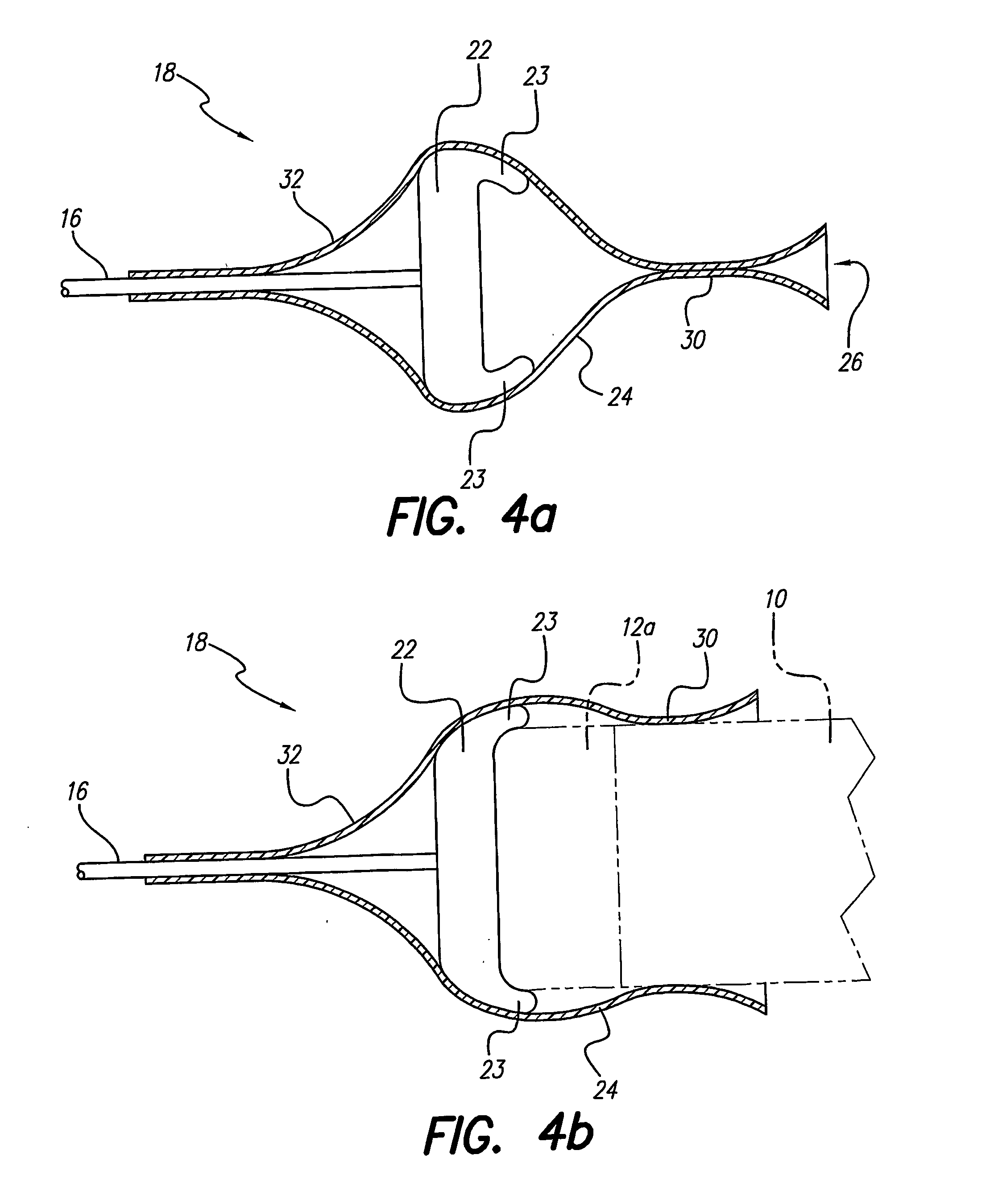
A lead assembly (20) for a small implantable medical device (10) (a.k.a., microdevice (10) provides means to attach a remote electrode (14) to microdevice (10), which means inhibit fluid ingress when microdevice (10) is not attached to lead assembly (20). Microdevices may provide either or both tissue stimulation and sensing. Known microdevices (10) include spaced apart electrodes (12a, 12b) on the outer surface of the microdevice. Lead assembly (20) includes an insulated lead (16) including a proximal end and a distal end, with at least one conductor therebetween; at least one electrode (14) at the distal end of the lead and electrically connected to the at least one conductor, and a connector (18) attached to the proximal end of the lead and adapted to be removably connectable to microdevice (10). Connector (18) includes at least one contact (22) to electrically connect at least one device electrode (12a) on microdevice (10) to at least one conductor, thereby electrically connecting at least electrode (12a) and the at least one electrode (14) at the distal end of lead (16). Lead assembly (20) is configured to inhibit fluid ingress into the connector (18). A number of embodiments of the invention, capable of inhibiting fluid ingress into connector (18), are taught.
Owner:BOSTON SCI NEUROMODULATION CORP
Electroporation to interrupt blood flow
InactiveUS20050171574A1High level of controlRestricted blood flowSurgical needlesInternal electrodesAbnormal tissue growthBlood flow
A method for disrupting blood flow to undesirable tissue such as cells of a cancerous or non-cancerous tumor is disclosed. It involves the placement of electrodes into or near the vicinity of vessels supplying blood to the undesirable tissue and through the application of electrical pulses causing blood flow disruption. The electric pulses irreversibly permeate the cell membranes, thereby invoking cell death. The irreversibly permeabilized cells are left in situ and are removed by the body immune system. The process may further comprise monitoring blood flow and / or infusion of a material such as a chemotherapeutic agent or marker into the blood.
Owner:RGT UNIV OF CALIFORNIA
Methods and apparatus for performing therapeutic procedures in the spine
Methods and apparatus for forming one or more trans-sacral axial instrumentation / fusion (TASIF) axial bore through vertebral bodies in general alignment with a visualized, anterior or posterior axial instrumentation / fusion line (AAIFL or PAIFL) in a minimally invasive, low trauma, manner and providing a therapy to the spine employing the axial bore. Anterior or posterior starting positions aligned with the AAIFL or PAIFL are accessed through respective anterior and posterior tracts. Curved or relatively straight anterior and curved posterior TASIF axial bores are formed from the anterior and posterior starting positions. The therapies performed through the TASIF axial bores include discoscopy, full and partial discectomy, vertebroplasty, balloon-assisted vertebroplasty, drug delivery, electrical stimulation and various forms of spinal disc cavity augmentation, spinal disc replacement, fusion of spinal motion segments and implantation of radioactive seeds. Axial spinal implants and bone growth materials can be placed into single or multiple parallel or diverging TASIF axial bores to fuse two or more vertebrae, or distract or shock absorb two or more vertebrae.
Owner:MIS IP HLDG LLC
MRI compatible implanted electronic medical device and lead
An implantable biocompatible lead that is also compatible with a magnetic resonance imaging scanner for the purpose of diagnostic quality imaging is described. The implantable electrical lead comprises a plurality of coiled insulated conducting wires wound in a first direction forming a first structure of an outer layer of conductors of a first total length with a first number of turns per unit length and a plurality of coiled insulated conducting wires wound in a second direction forming a second structure of an inner layer of conductors of a second total length with a second number of turns per unit length. The first and the second structures are separated by a distance with a layer of dielectric material. The distance and dielectric material are chosen based on the field strength of the MRI scanner. The lead may further comprise a conducting layer formed by coating a material consisting of medium conducting particles in physical contact with each other and a mechanically flexible, biocompatible layer forming an external layer of lead and in contact with body tissue or body fluids.
Owner:KENERGY INC
Lead electrode for use in an MRI-safe implantable medical device
A lead configured to be implanted into a patient's body comprises a lead body and a conductive filer positioned within the lead body and having a distal portion. An electrode is electrically coupled to the lead body and comprises a stimulation portion, a bobbin, and at least one coil of wire wound on the bobbin and electrically coupled between the stimulation portion and the distal end region to form an inductor between the distal end region and the stimulation portion.
Owner:MEDTRONIC INC
Method for improving cardiac function
A method and a device for improving cardiac function are provided. The device is packaged in a collapsed state in an end of a catheter. Portions of a frame construction of the device spring outwardly when the catheter is withdrawn from the device. Anchoring formations on the frame construction secure the frame construction to a myocardium of the heart. A membrane secured to the frame construction then forms a division between volumes of an endocardial cavity of the heart on opposing sides of the membrane.
Owner:EDWARDS LIFESCIENCES CORP
Drug eluting implants to prevent cardiac apoptosis
Implantable devices are configured to be positioned in or near the heart and to carry and deliver an anti-apoptotic drug to a treatment site in or near the heart. The implantable devices include, but are not limited to, leads, stents, heart valves, atrial septal defect devices, cardiac patches and ventricular restraint devices. Depending on the composition of the device, the drug may be carried by the device through a coating applied to the device, or may be included in the device during the device manufacturing process. The drug may also be included in microparticles, such a microspheres, that are delivered locally through a conduit, such as a catheter.
Owner:CARDIAC PACEMAKERS INC
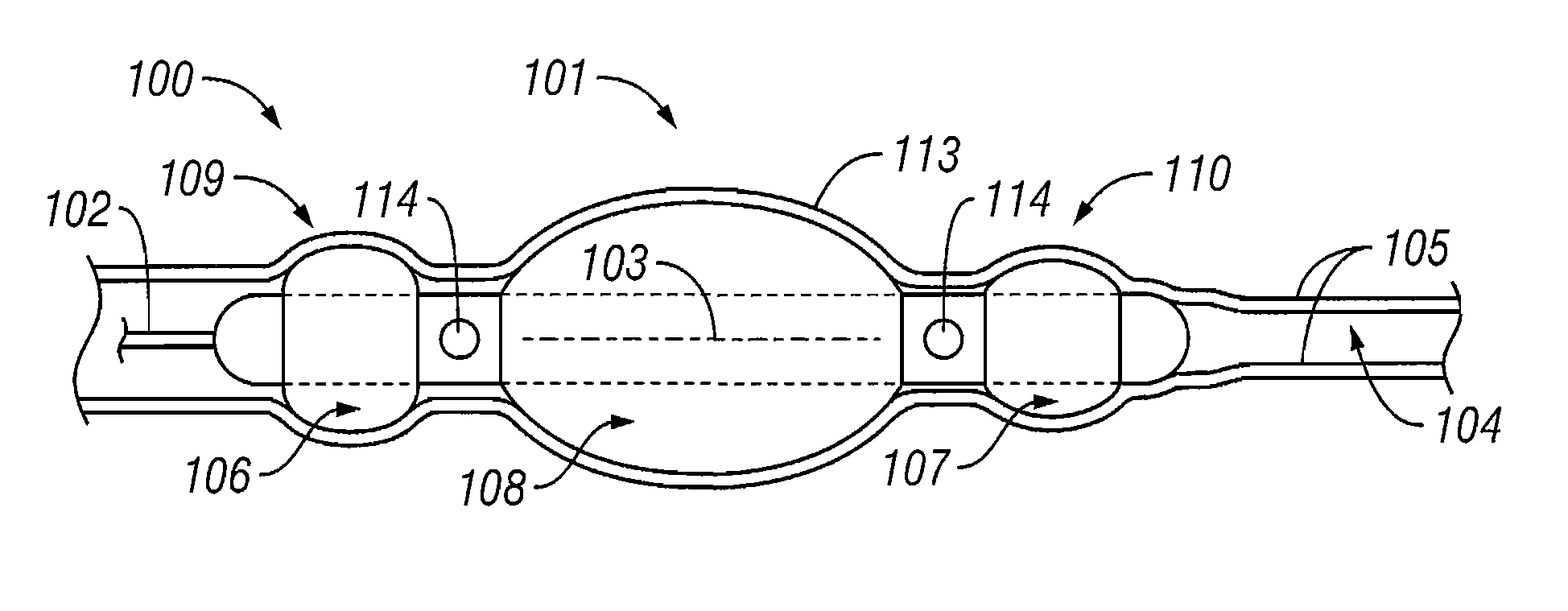
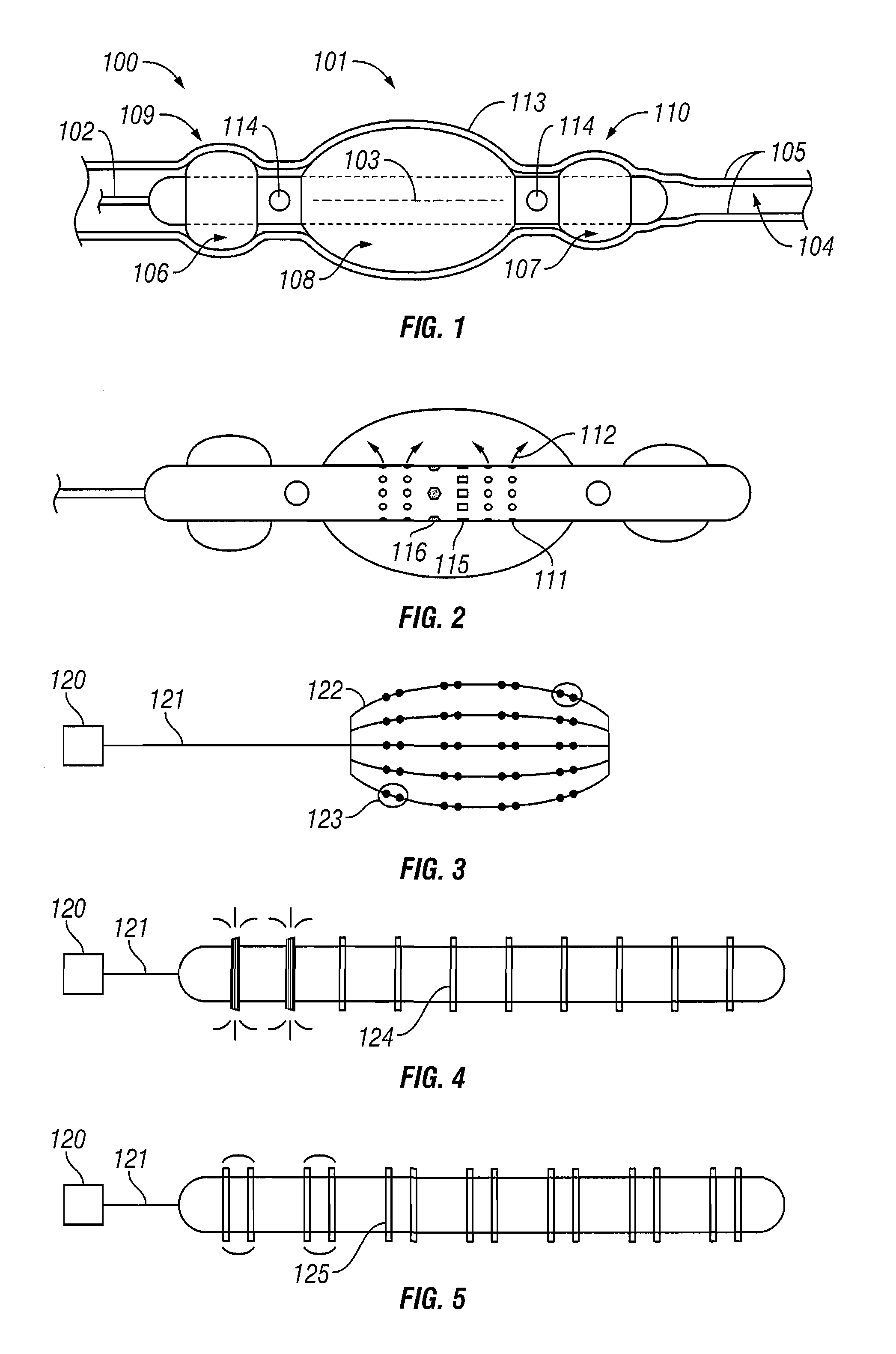
Shrinkage of dilatations in the body
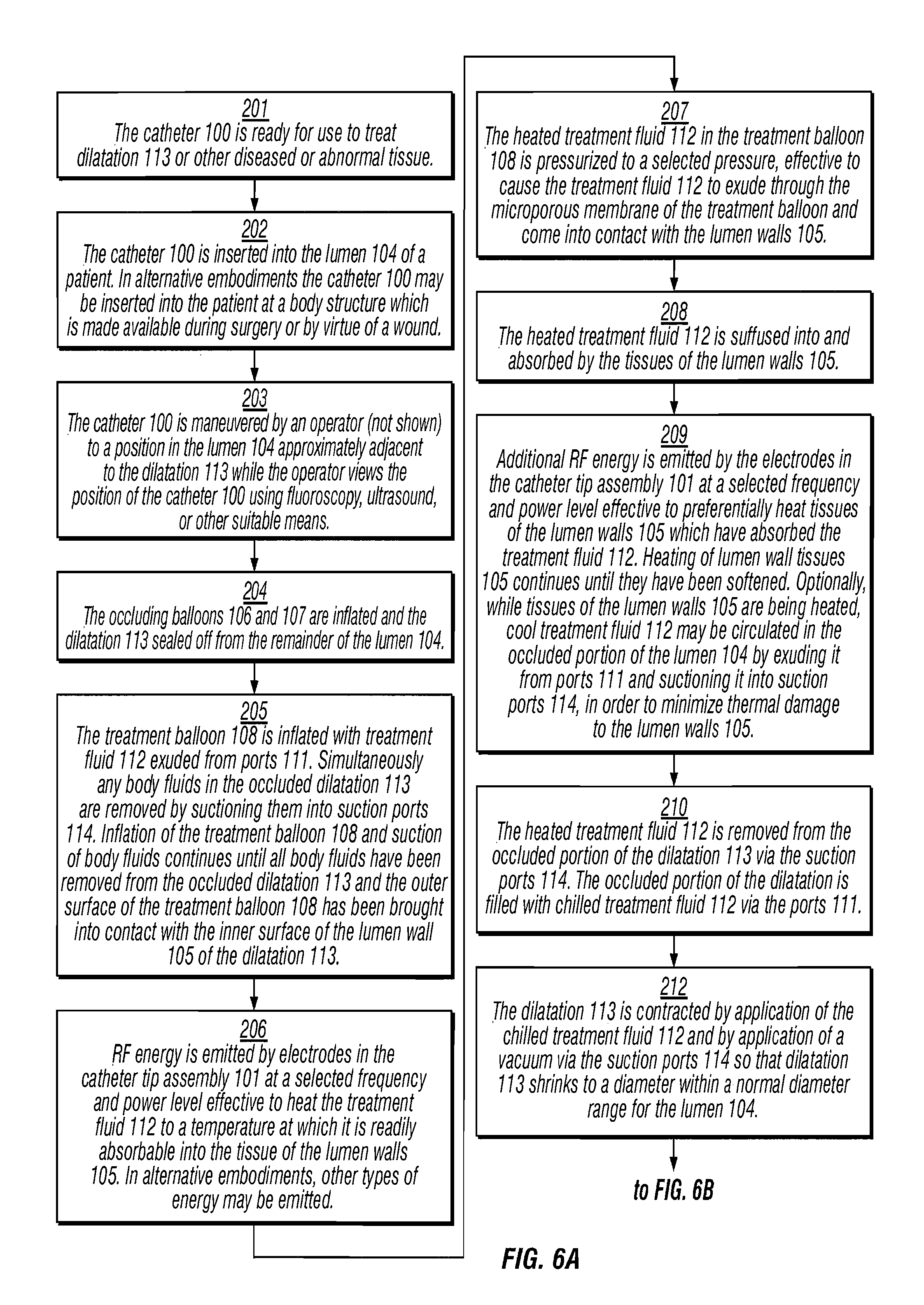
InactiveUS7184827B1Reduce the overall diameterEnergy preciseStentsBalloon catheterSaline solutionsBody fluid
A method and system for shrinking dilatations of a body, removing excess, weak or diseased tissue, and strengthening remaining tissues of the lumen walls. A catheter is disposed near the dilatation and fixed in position by inflatable occlusion balloons. Body fluids present in the occluded dilatation are evacuated and treatment fluid is exuded under pressure into the dilatation. Pressure is maintained by the treatment fluid while energy is applied by the catheter to heat the treatment fluid, causing the lumen walls to absorb the treatment fluid. Additional energy is then applied so as to preferentially heat the lumen wall tissues which have absorbed the treatment fluid, while at the same time treatment fluid is circulated to cool the inner surface of the lumen walls. The dilatation is occluded, a saline solution is introduced and absorbed into the lumen-wall tissue in the occluded region of the dilatation and then heated by application of radio frequency (“RF”) or other energy in order to soften only the lumen-wall tissue of the dilatation, the dilatation is shrunk by application of a chilled saline solution and a vacuum, and additional RF or other energy is emitted to ablate, further shrink, and harden only the lumen-wall tissue of the dilatation, without destroying the inner surface of the lumen or other tissues of the body beyond the lumen walls, thereby promoting growth of epithelial cells.
Owner:EDWARDS STUART D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com