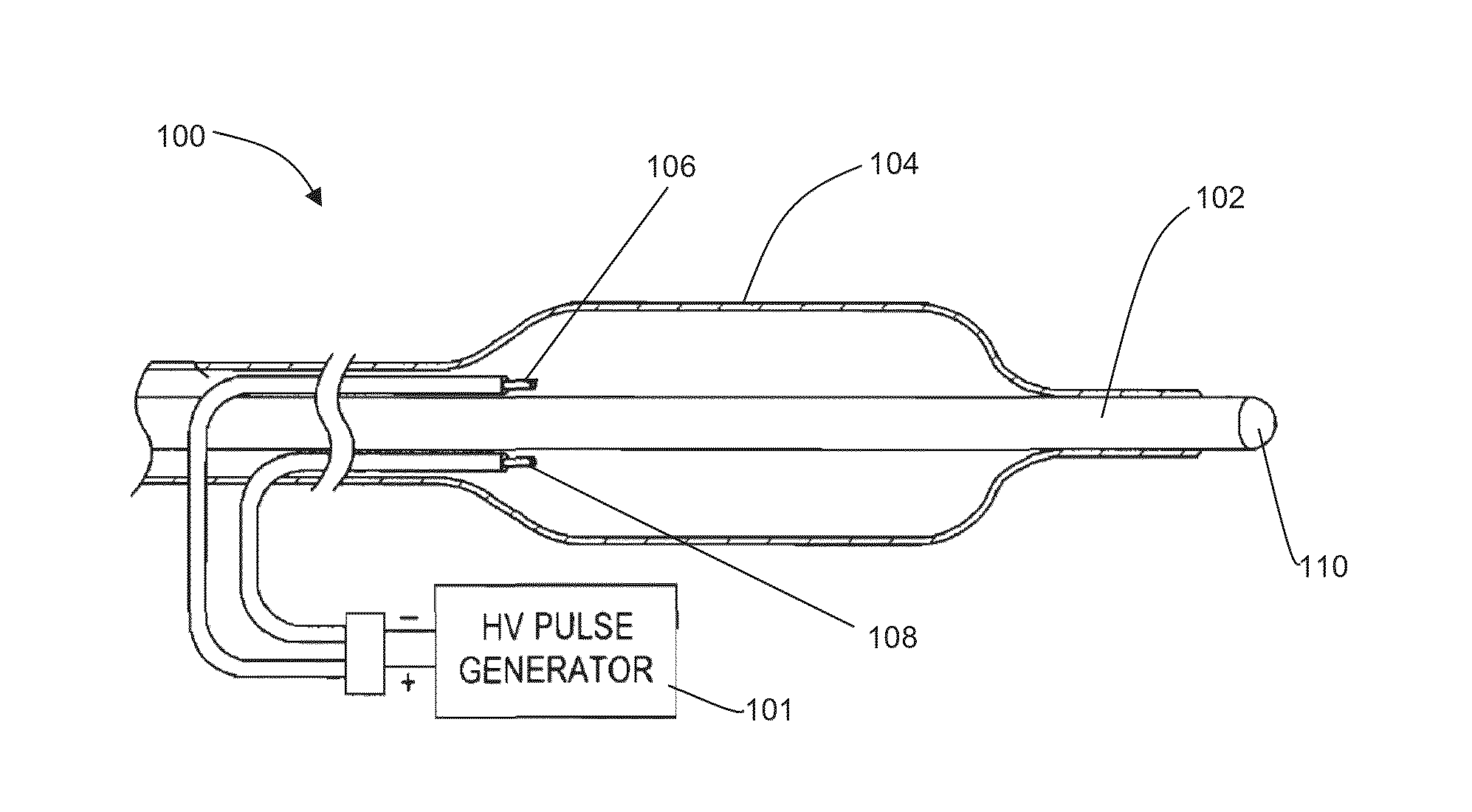
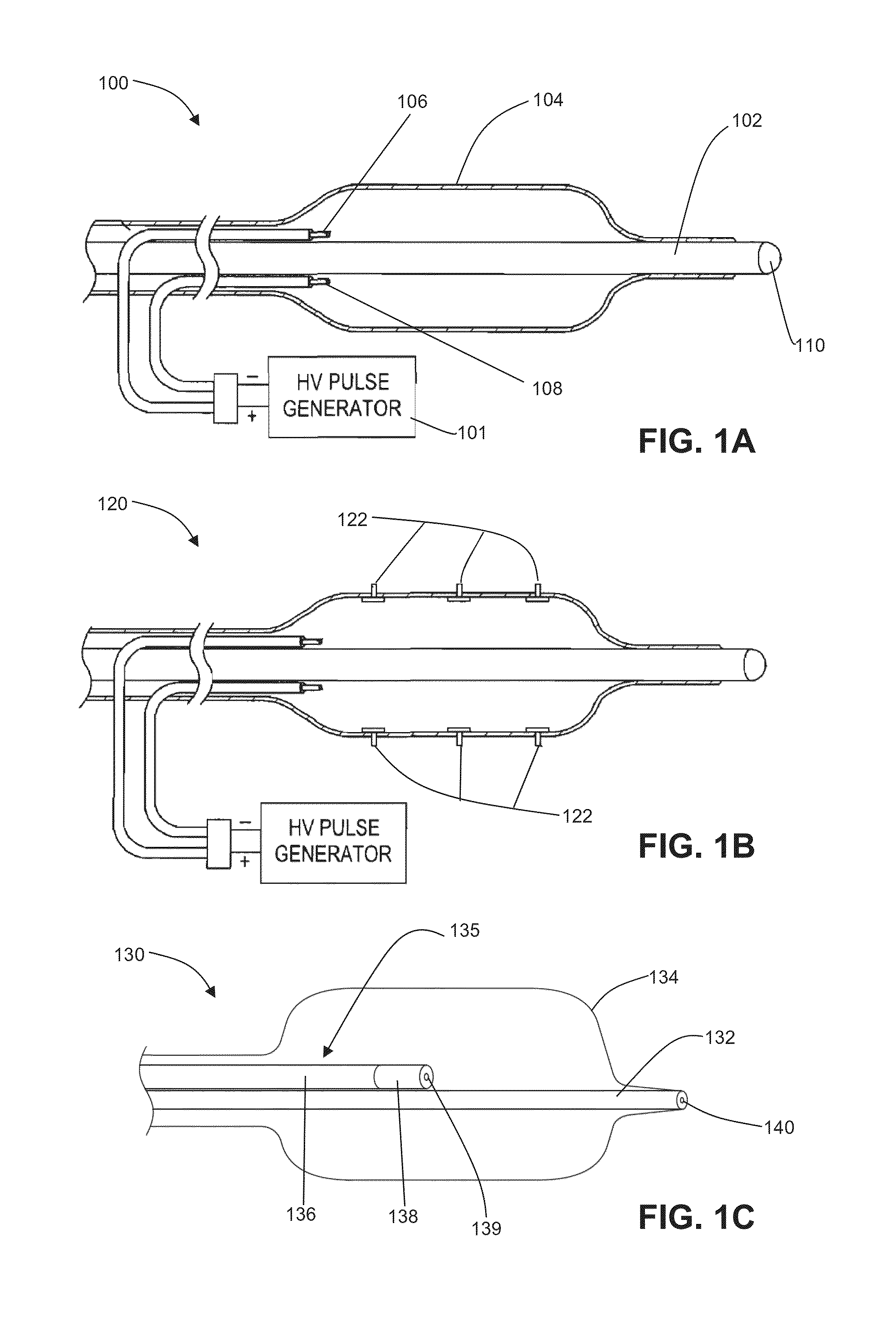
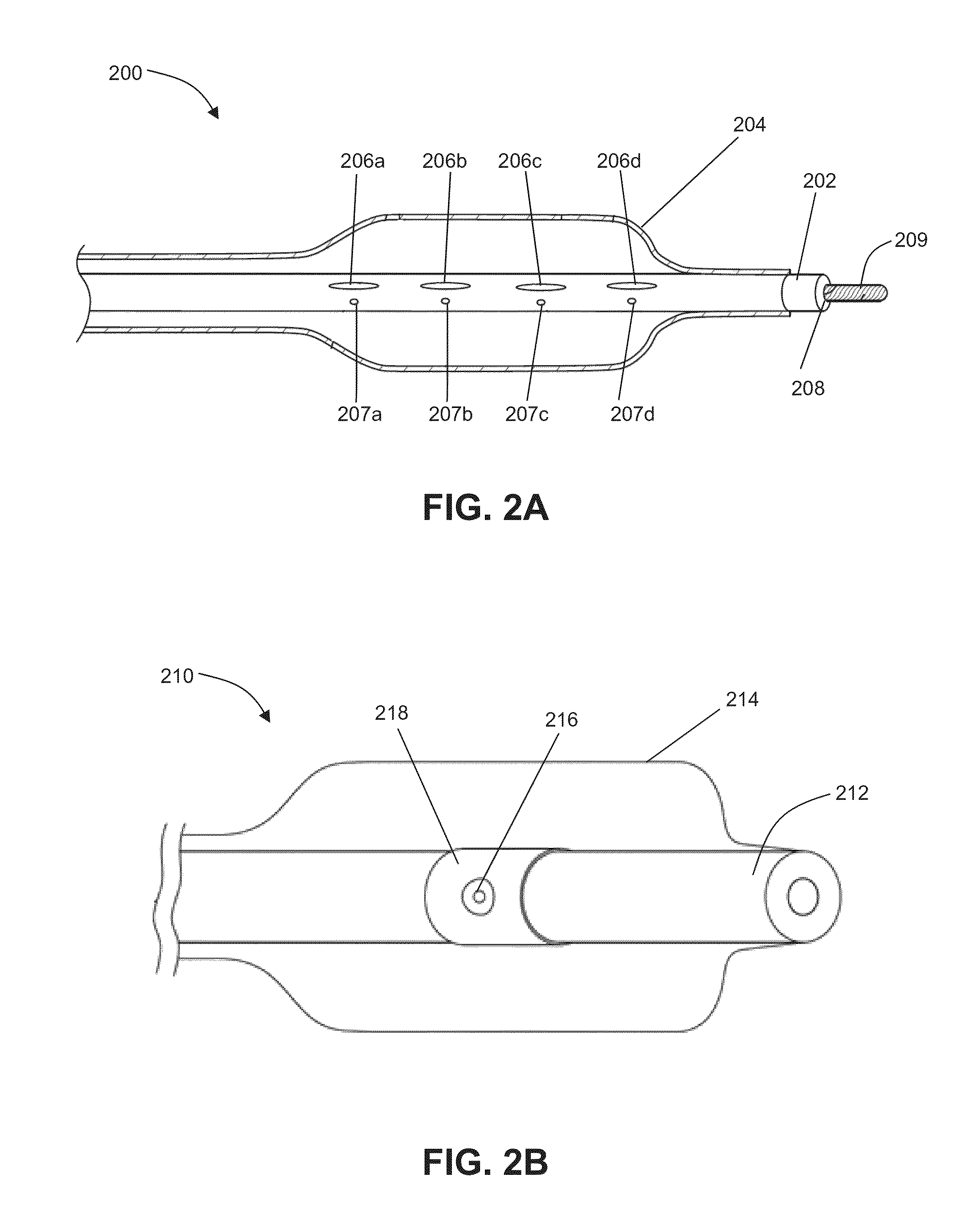
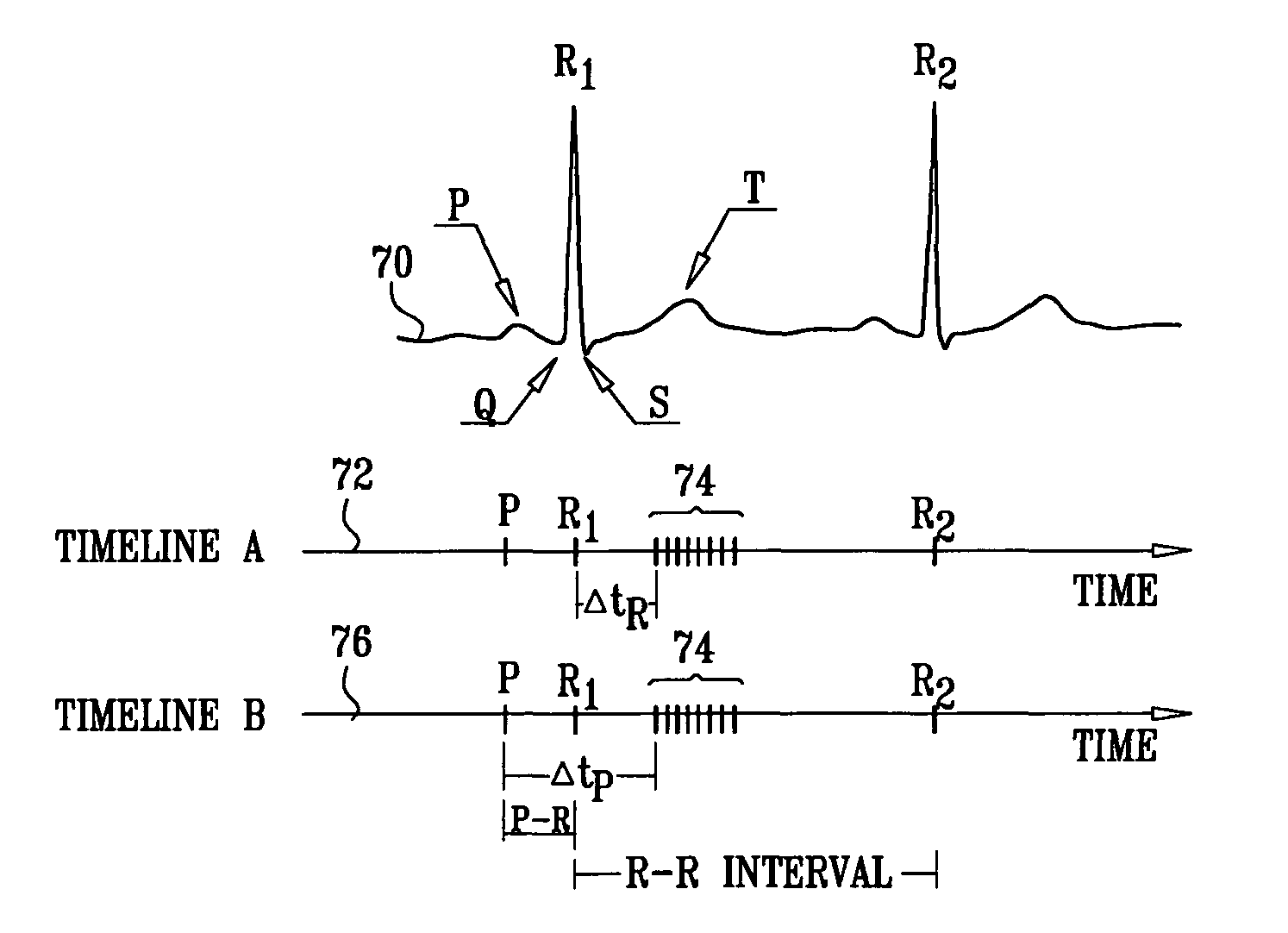
Patents
Literature
40 results about "Carotid sinus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
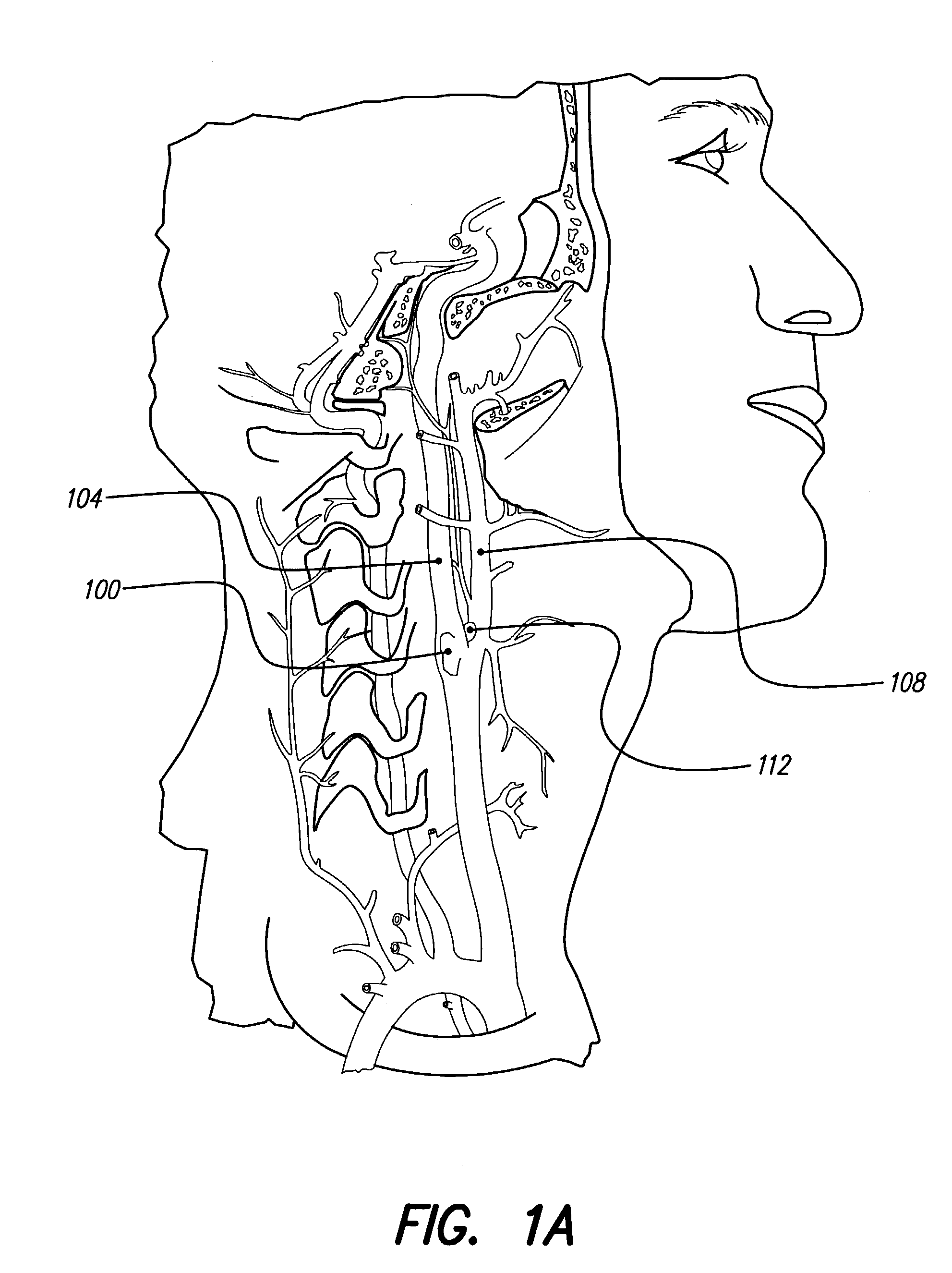
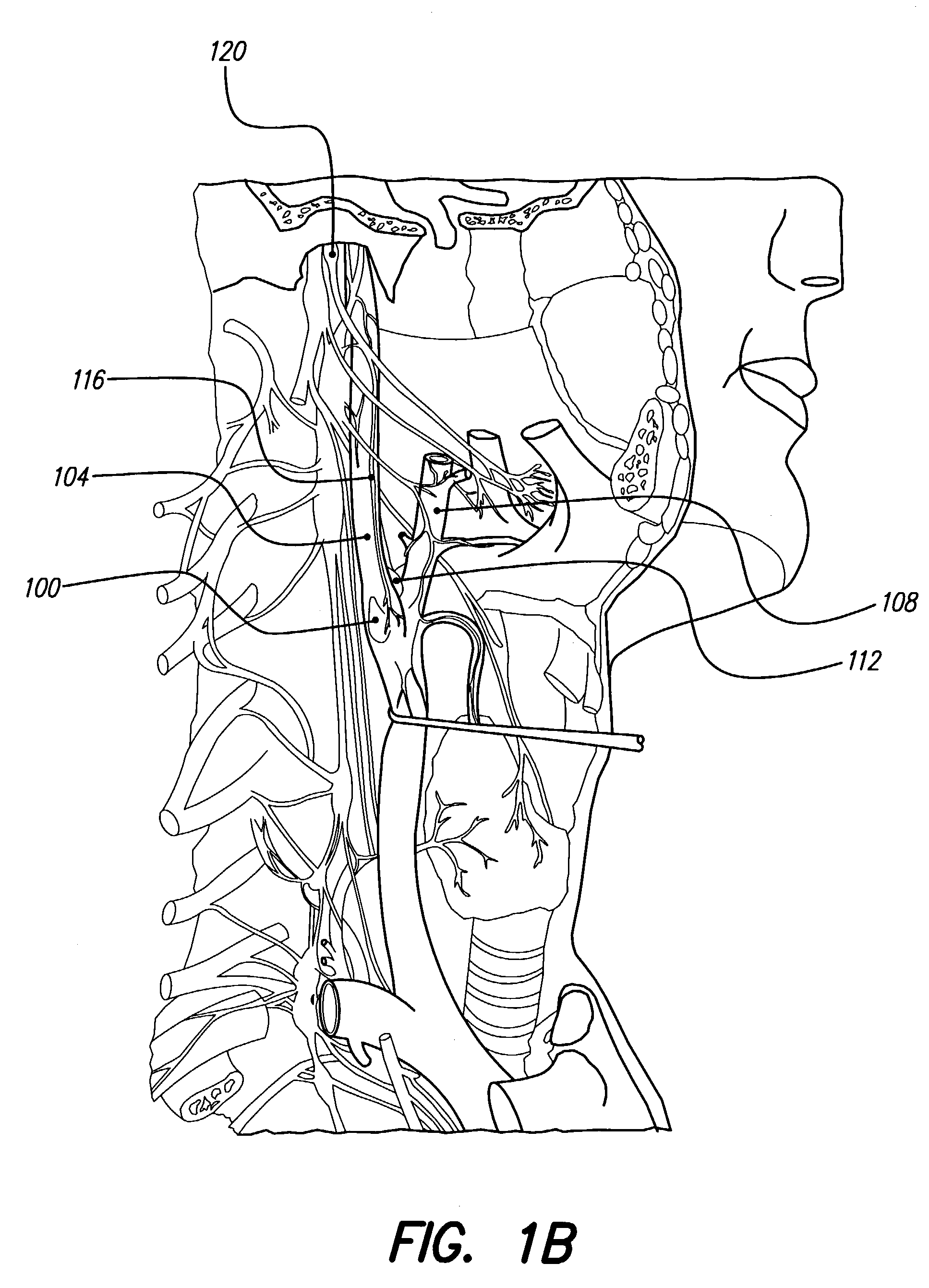
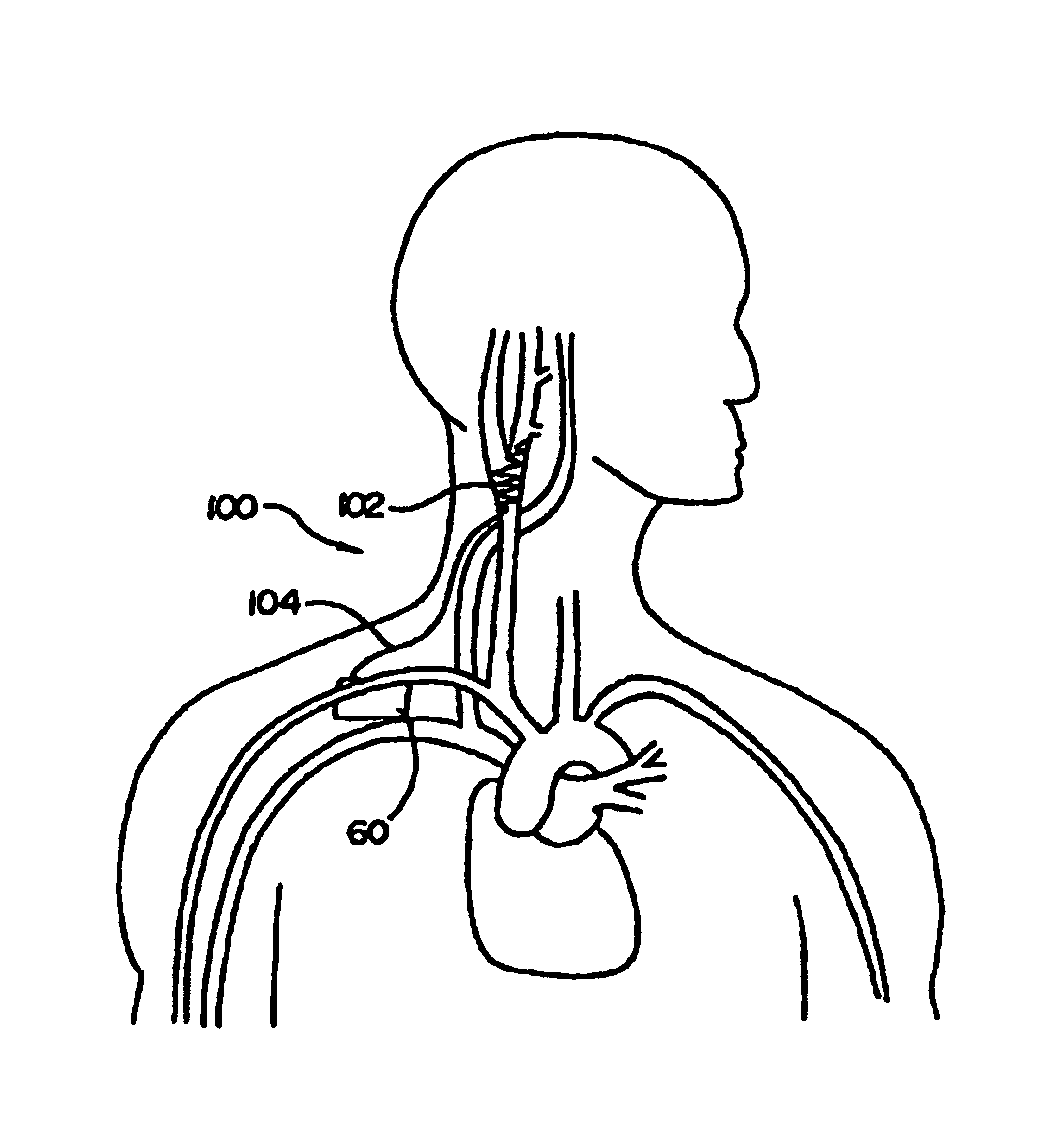
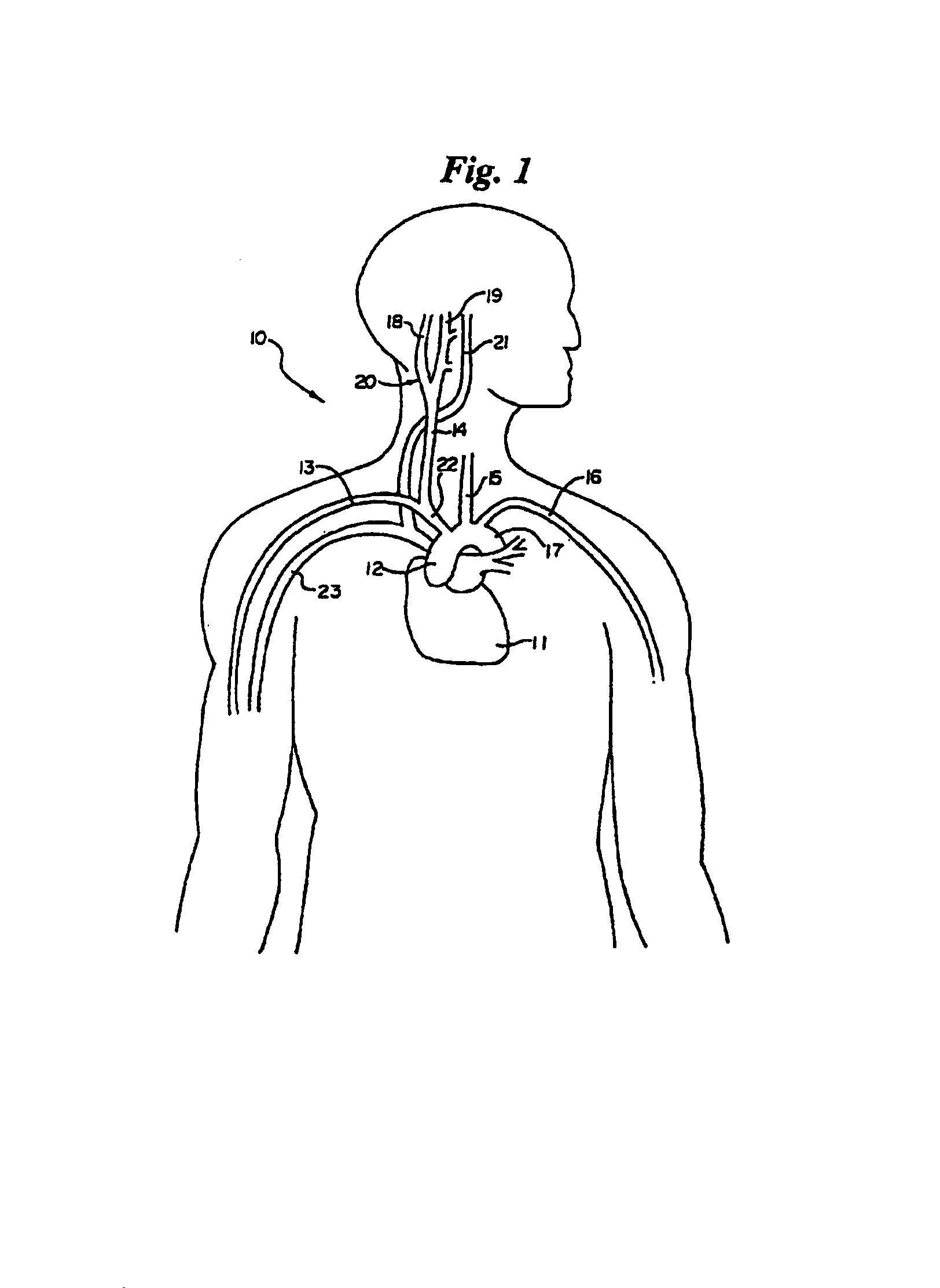
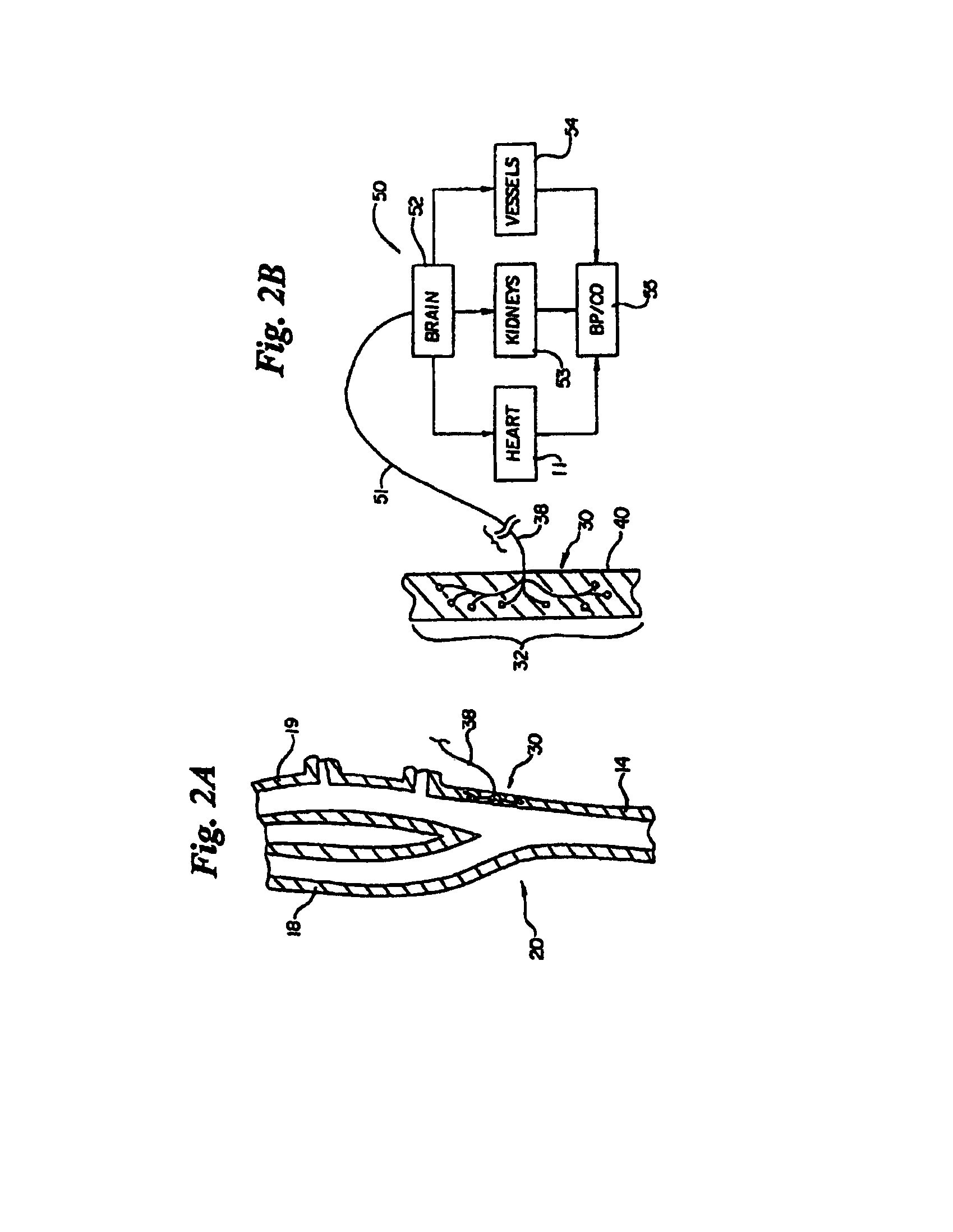
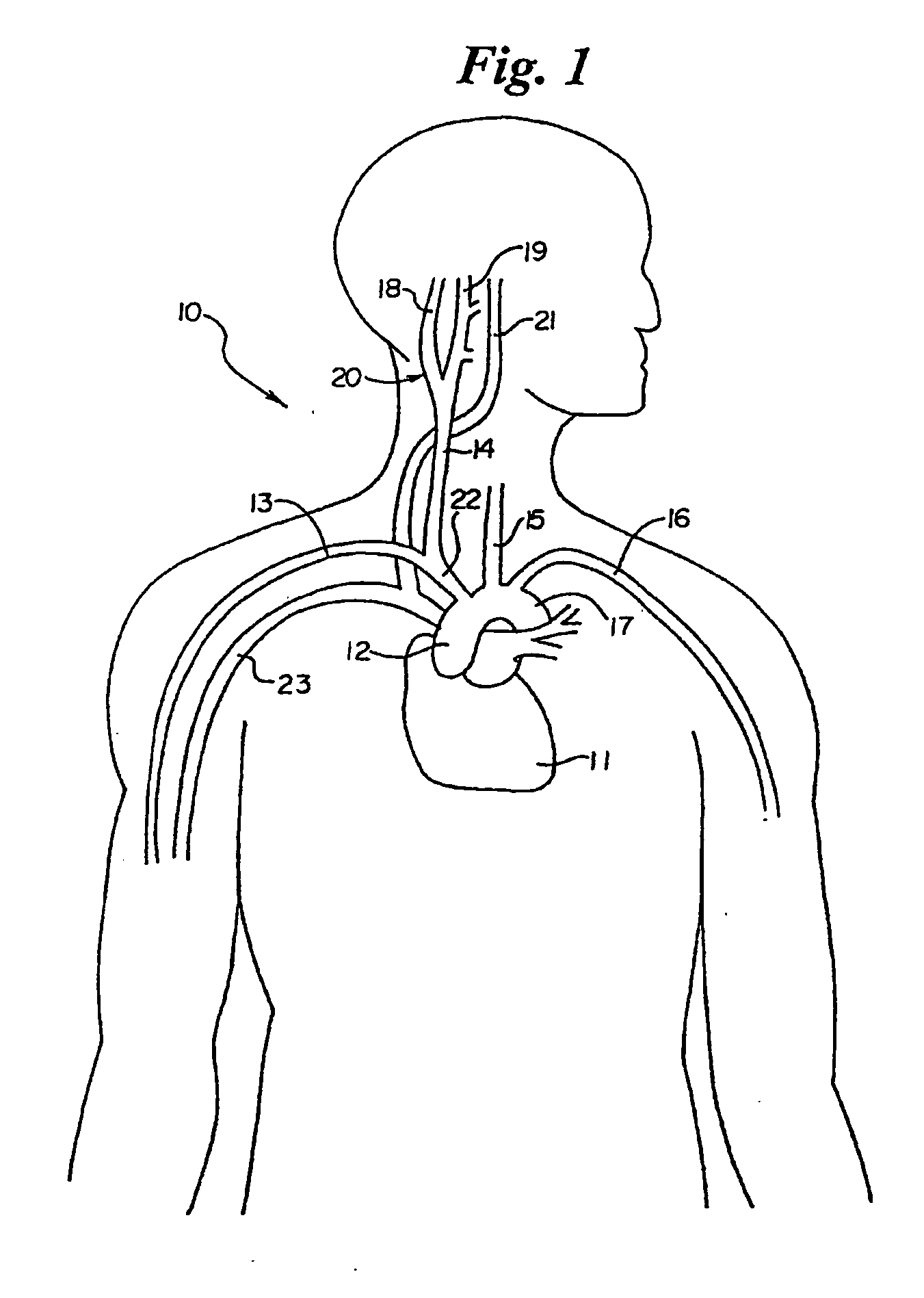
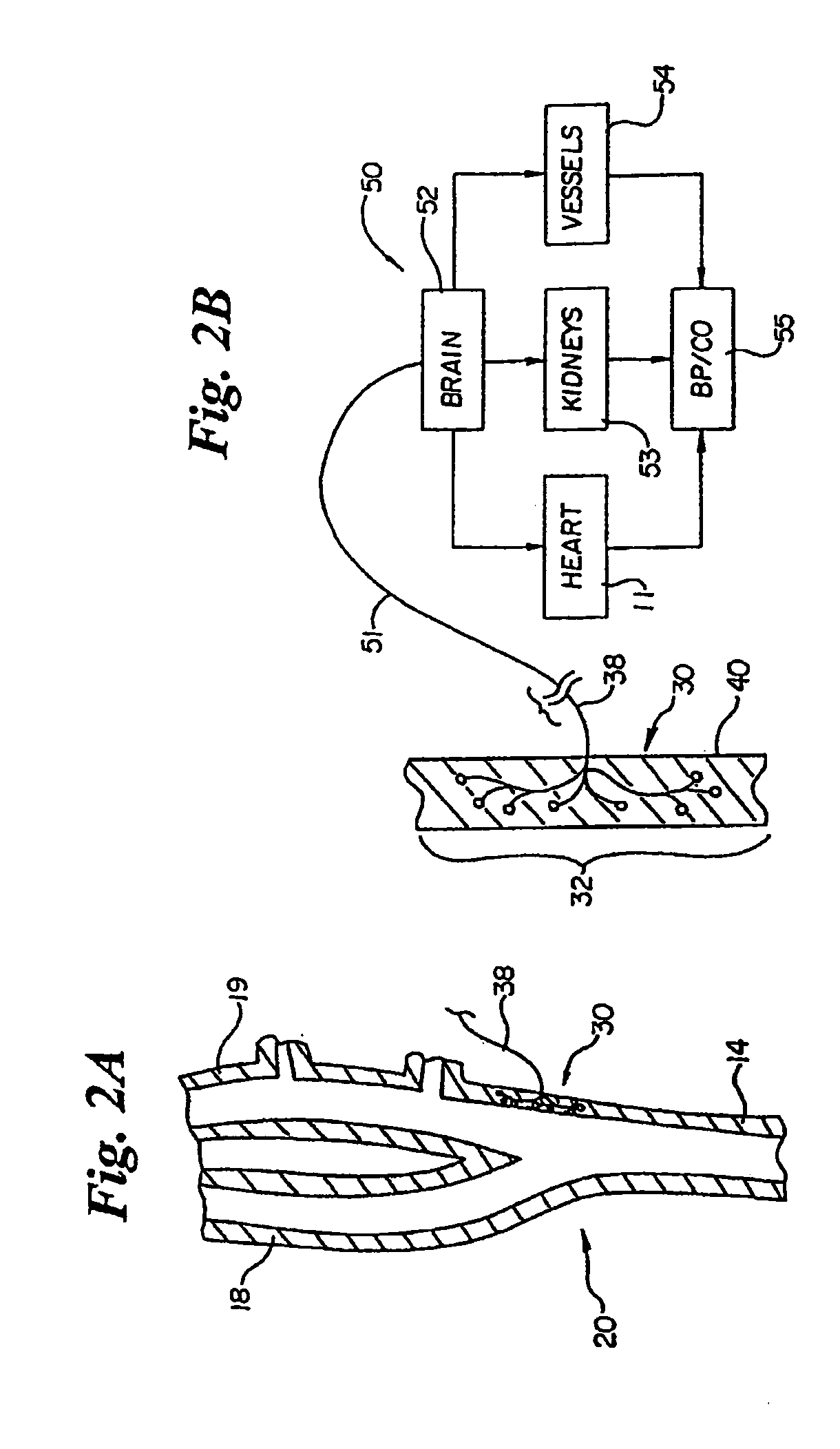
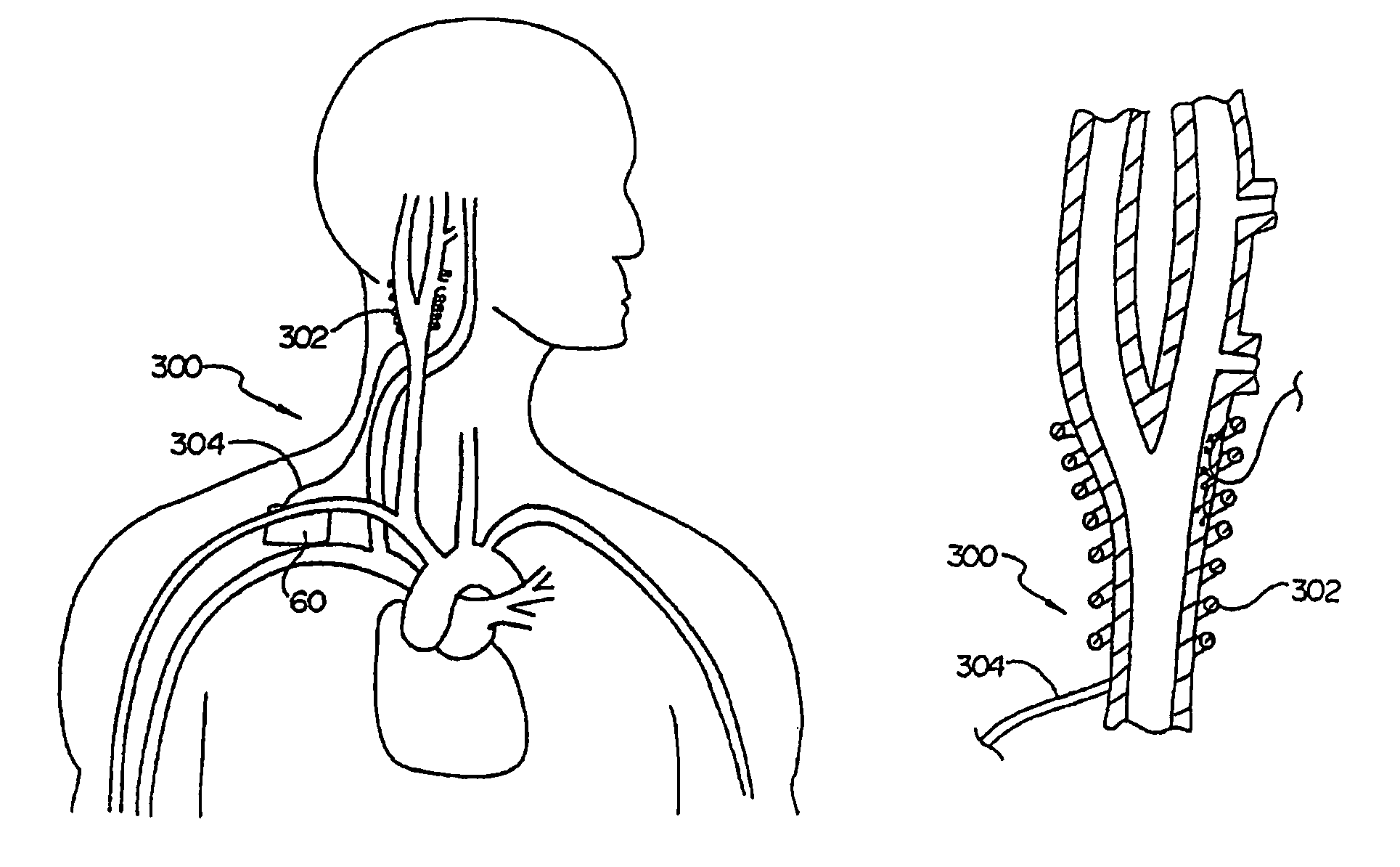
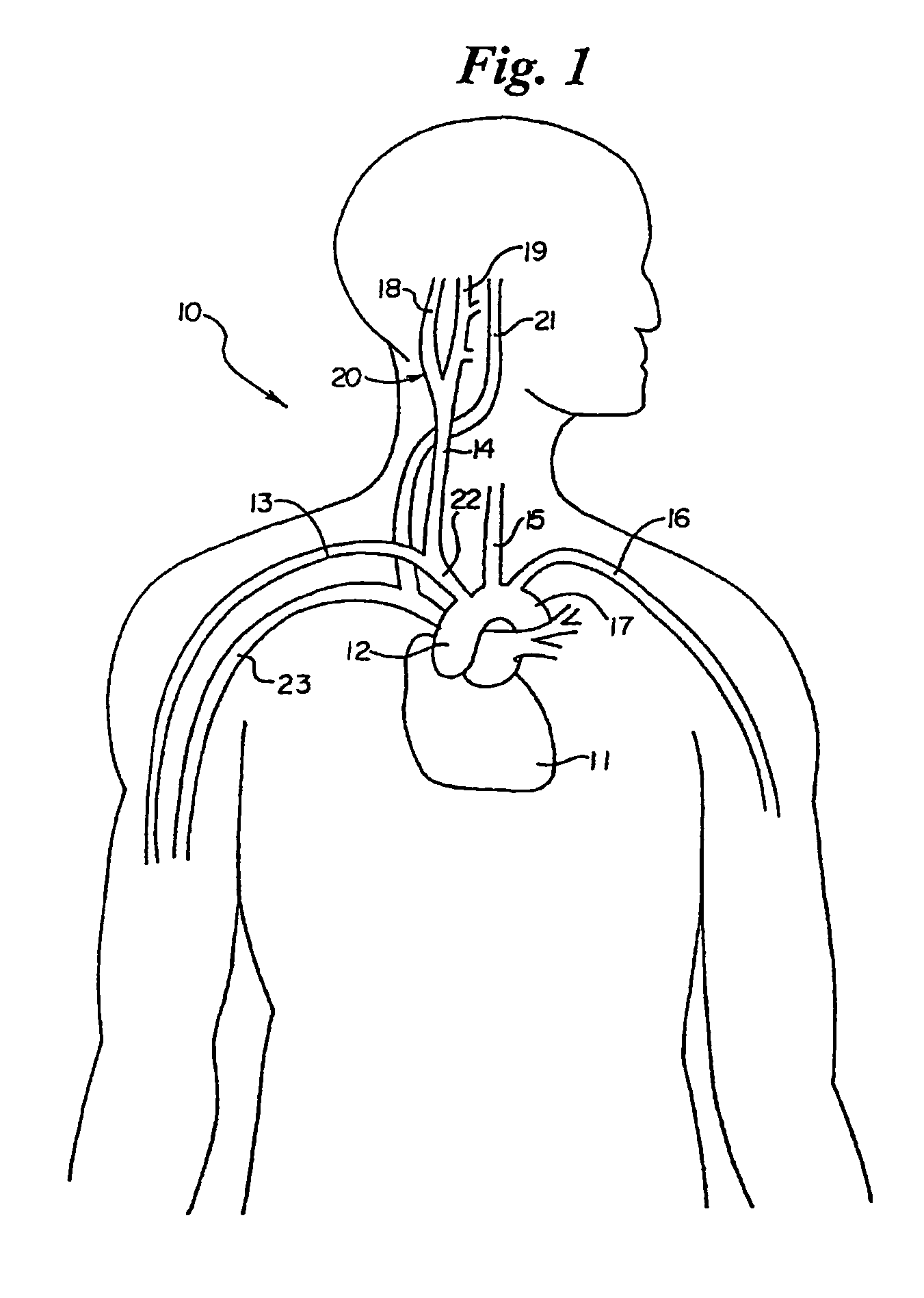
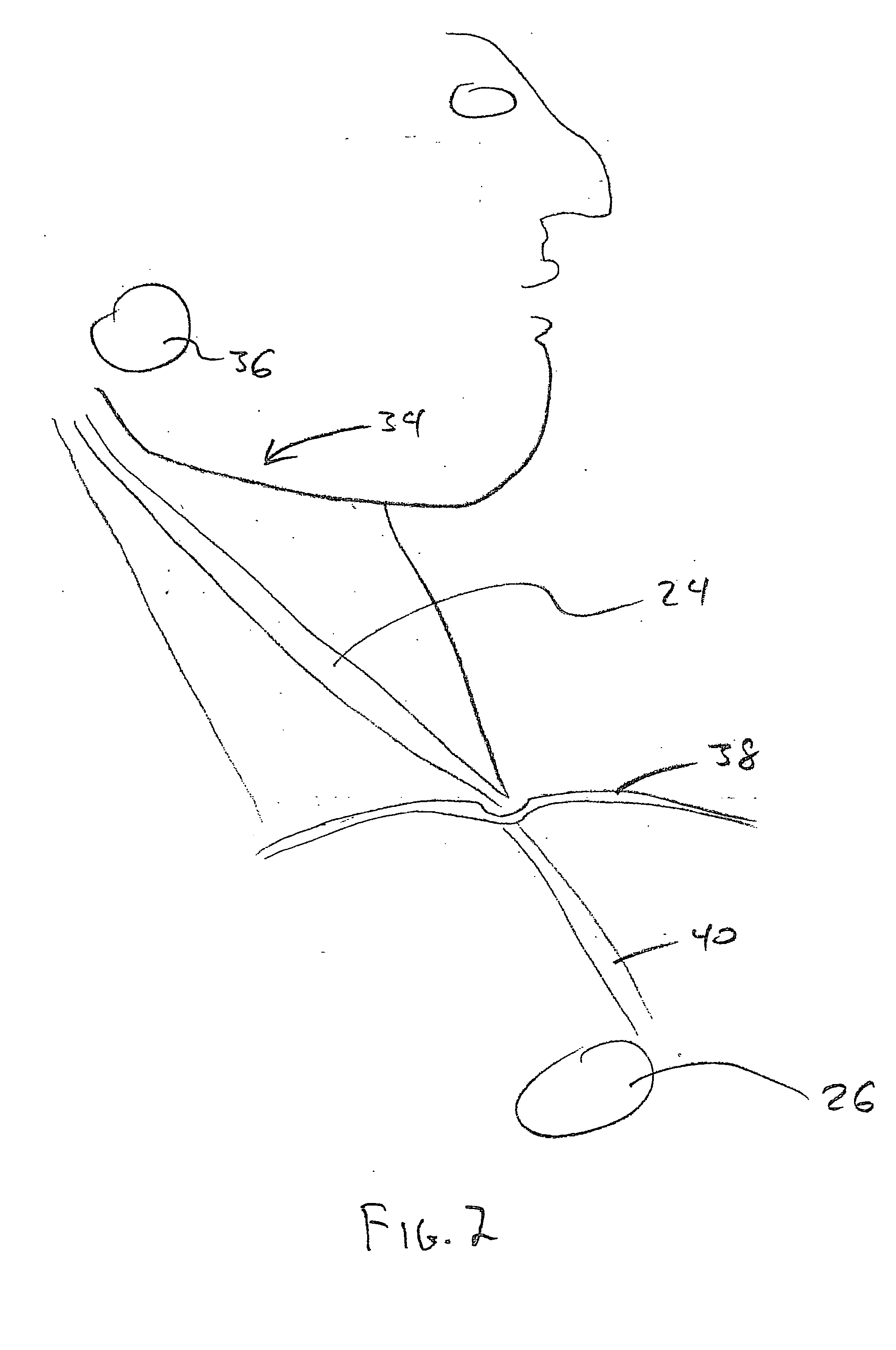
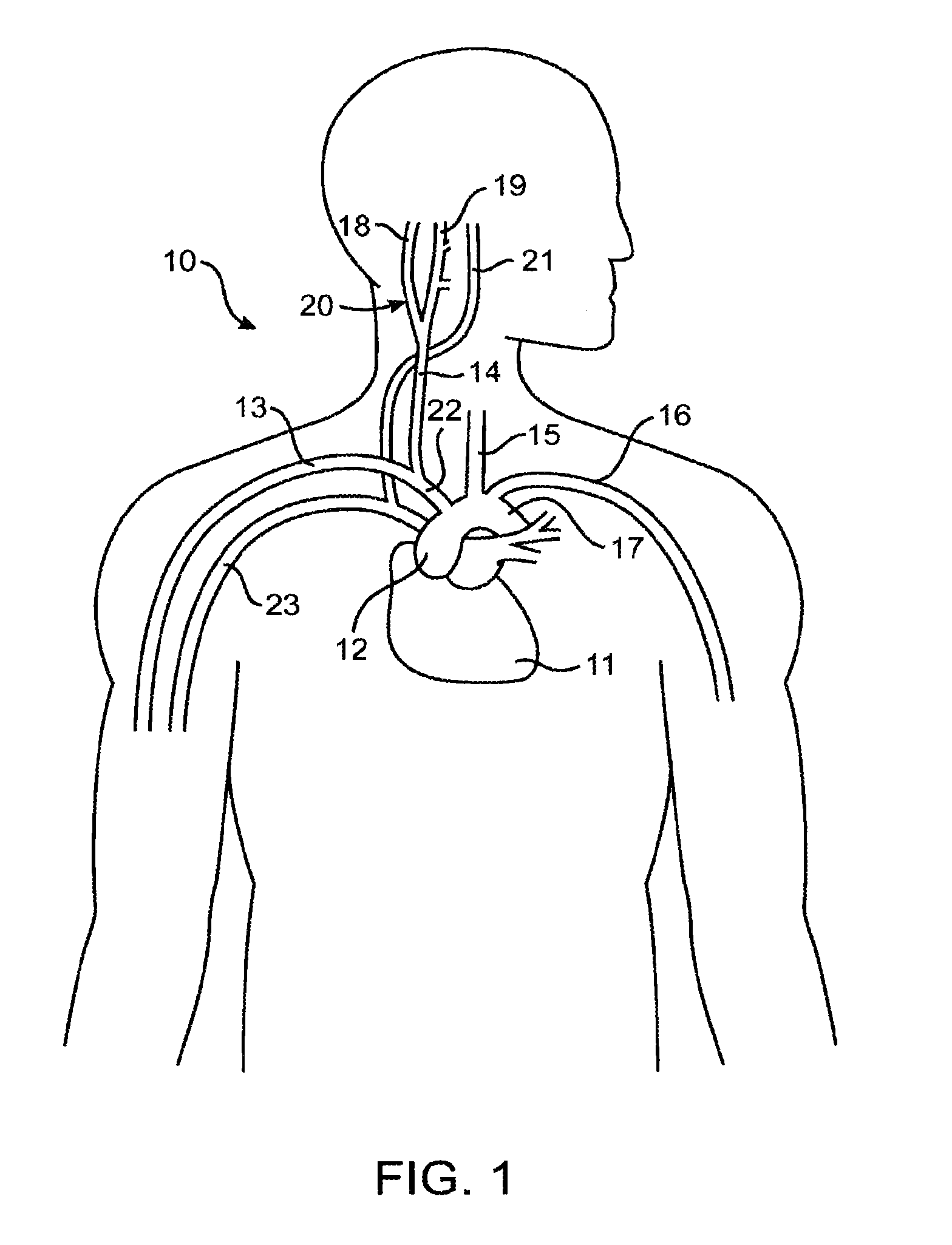
In human anatomy, the carotid sinus is a dilated area at the base of the internal carotid artery just superior to the bifurcation of the internal carotid and external carotid at the level of the superior border of thyroid cartilage. The carotid sinus extends from the bifurcation to the "true" internal carotid artery. The carotid sinus is sensitive to pressure changes in the arterial blood at this level. It is the major baroreception site in humans and most mammals.
Treatment of hypertension
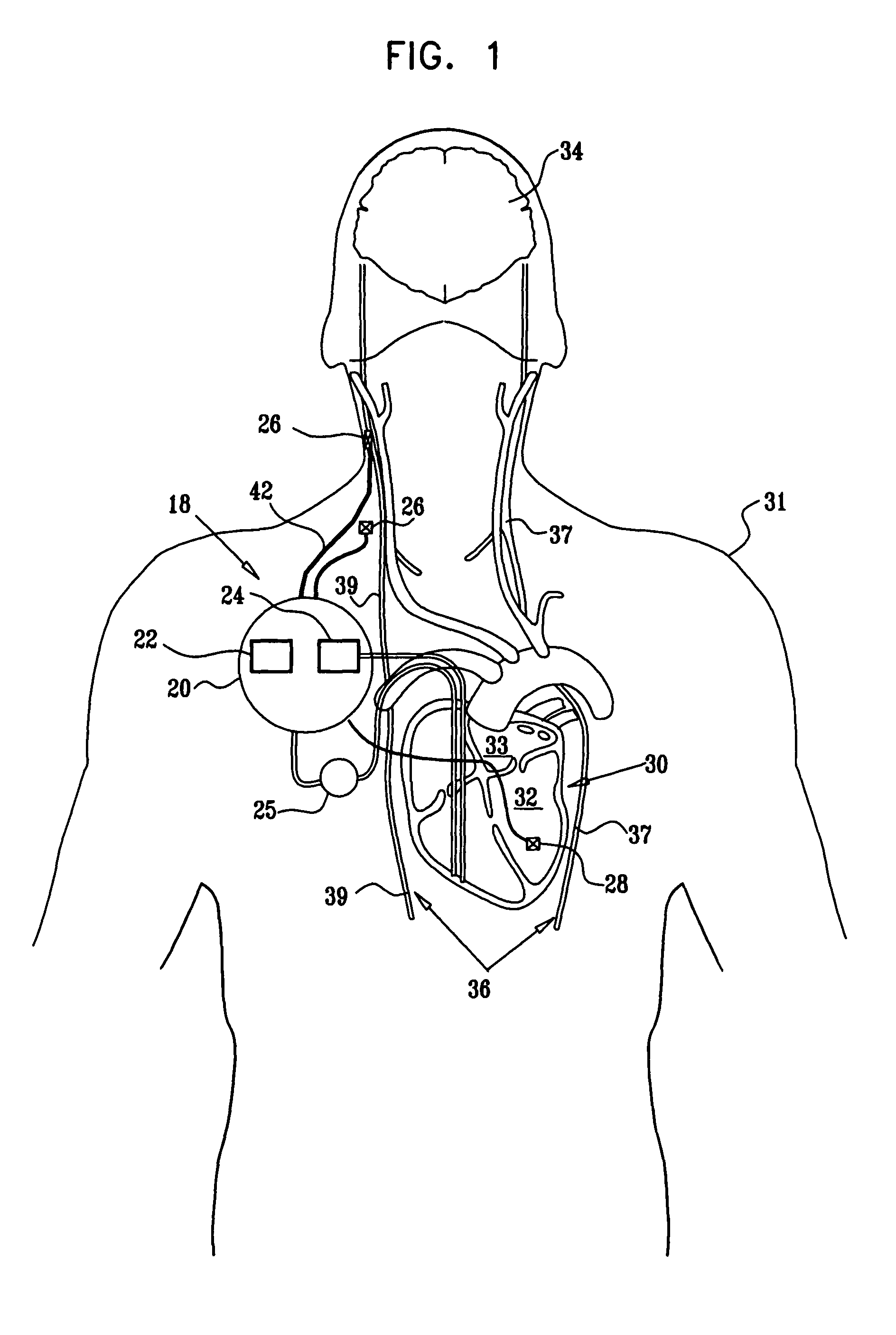
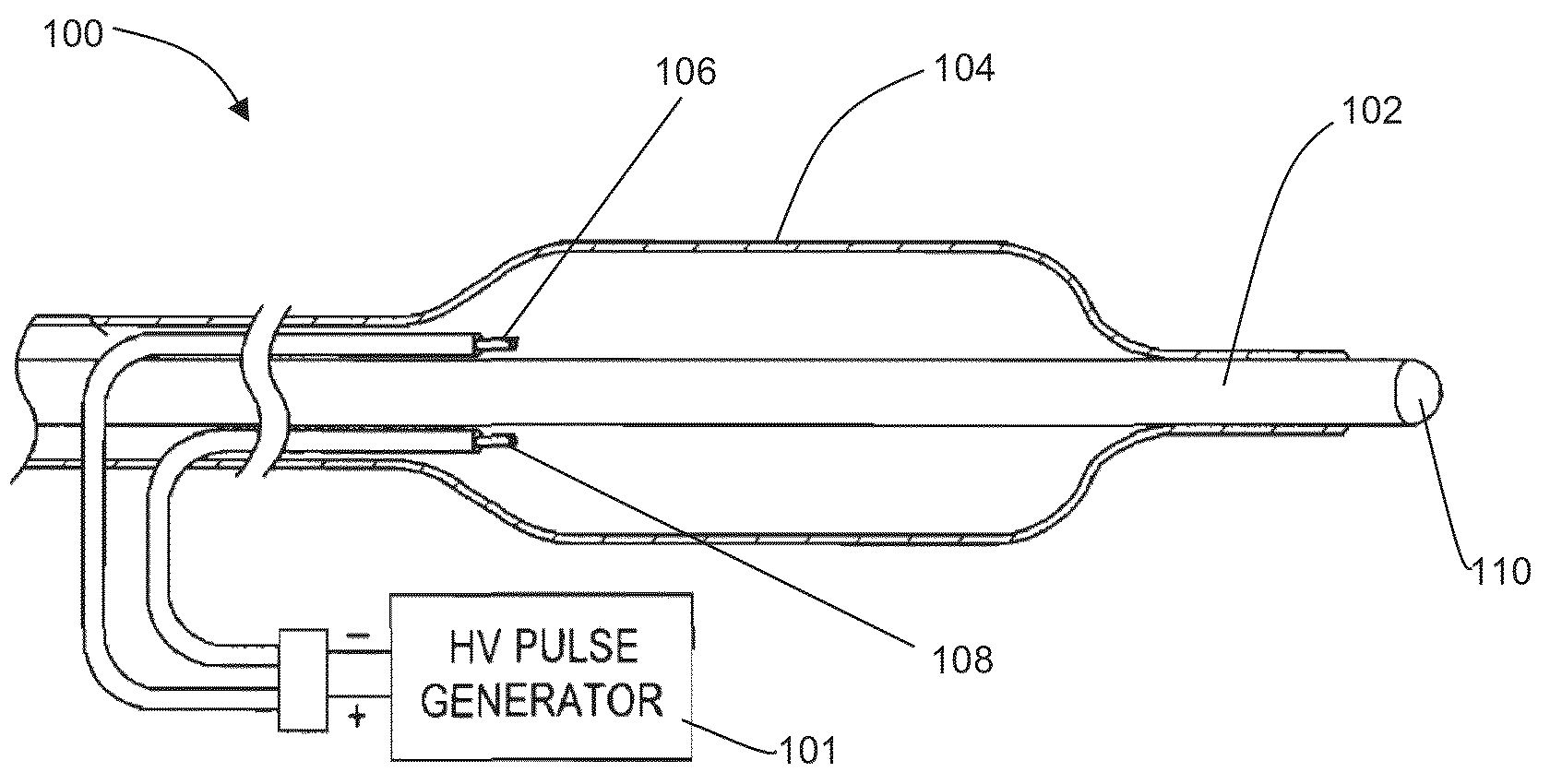
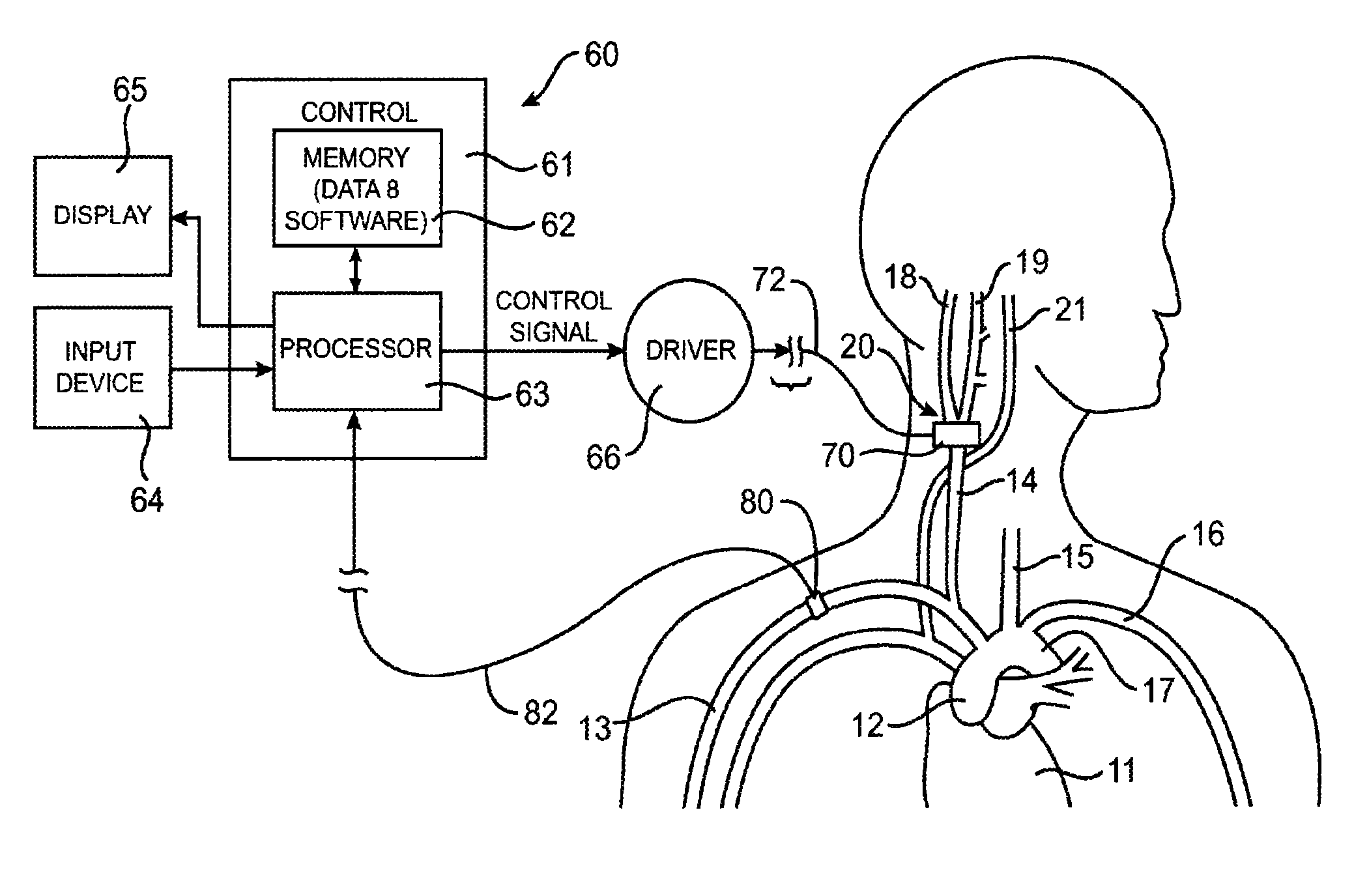
InactiveUS7155284B1Sufficient amountAvoid the needPharmaceutical delivery mechanismImplantable neurostimulatorsCarotid bodyElectrical stimulations
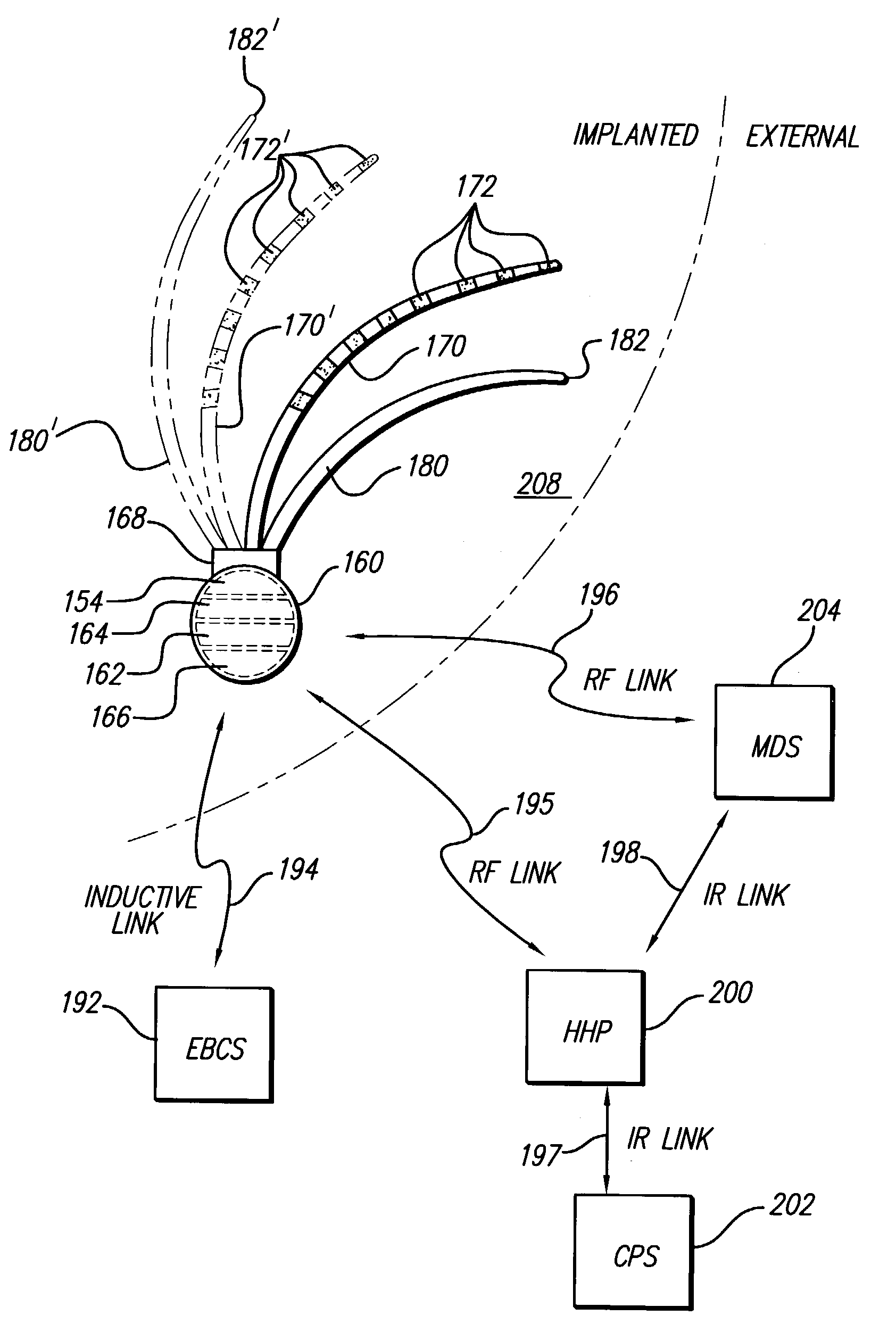
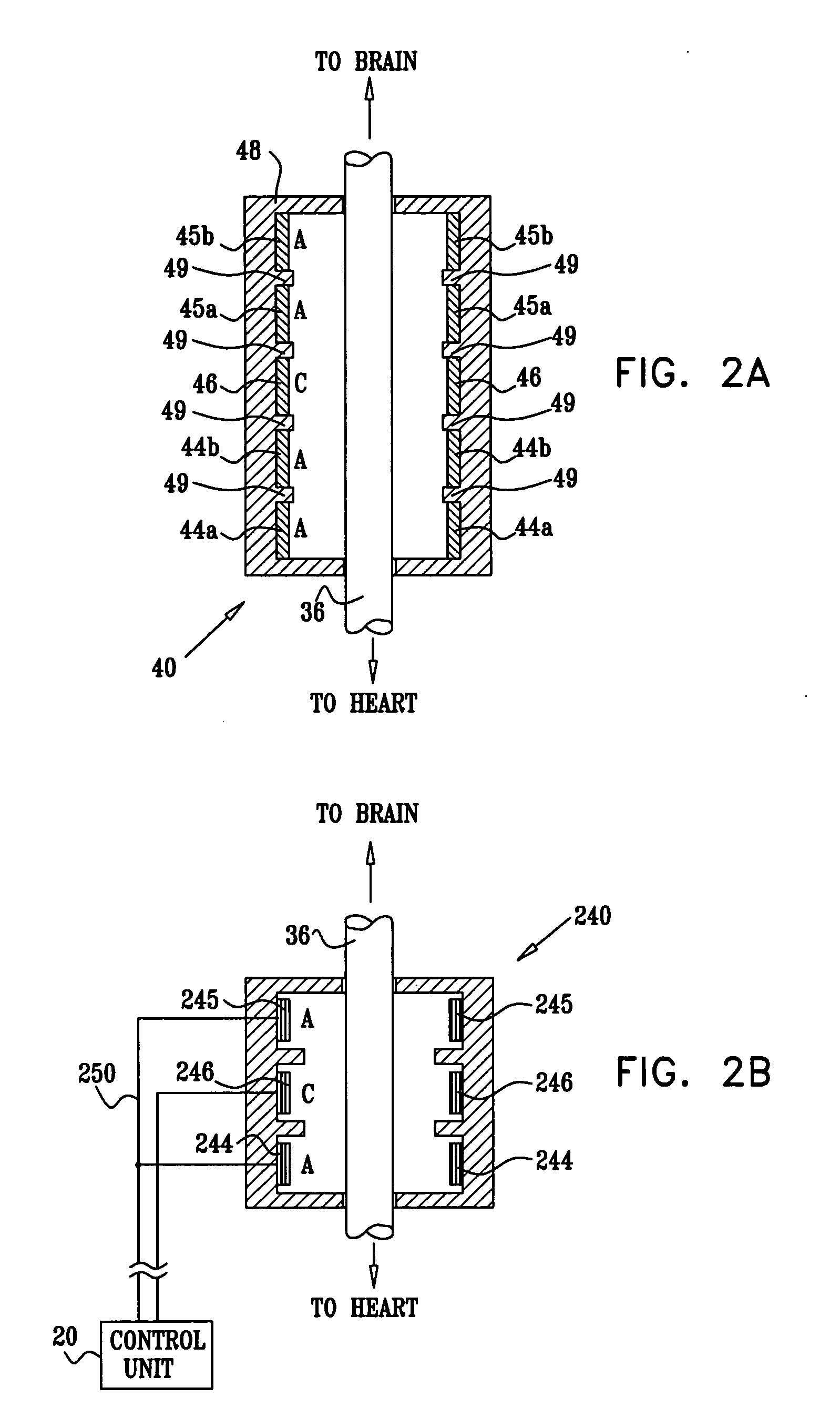
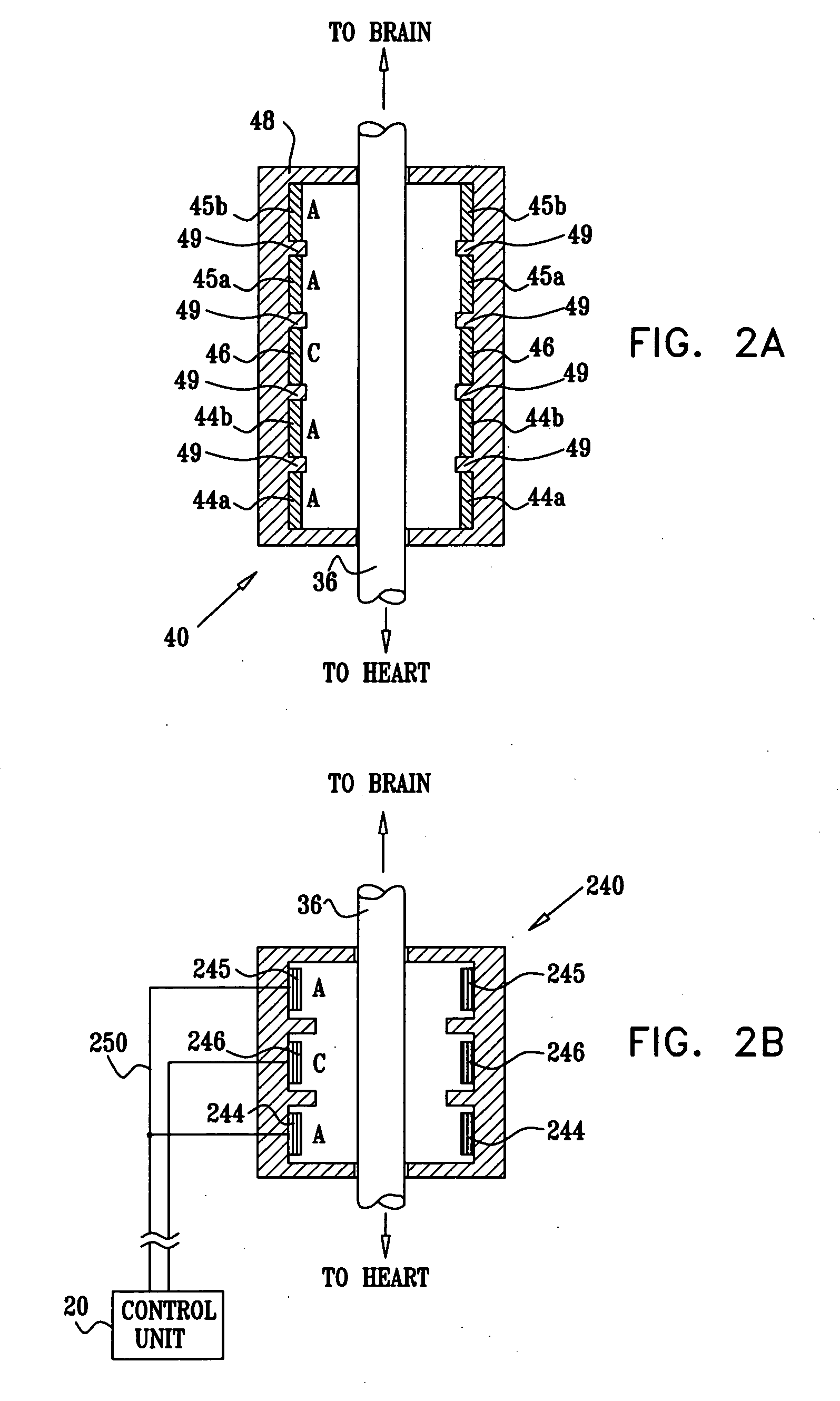
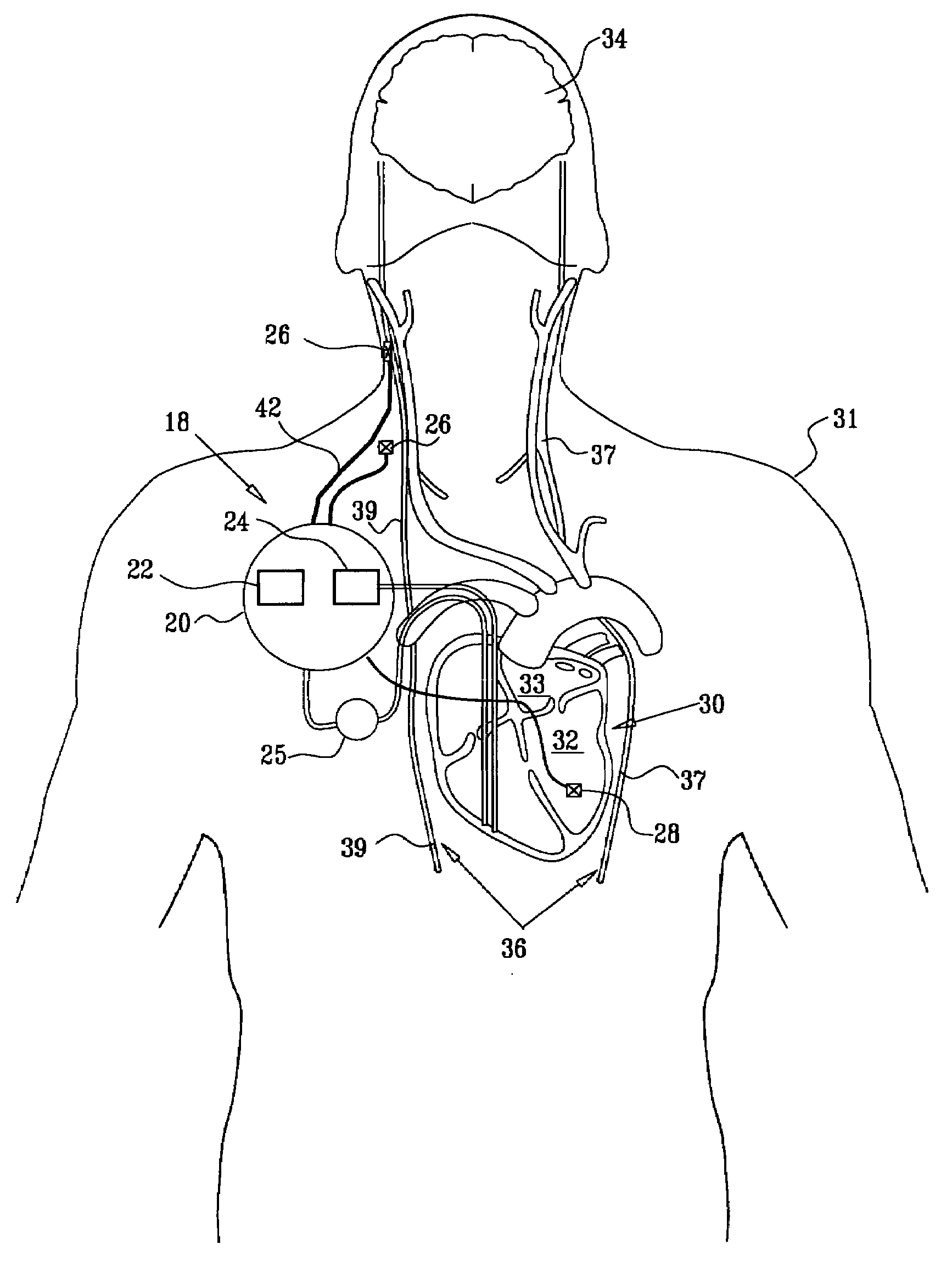
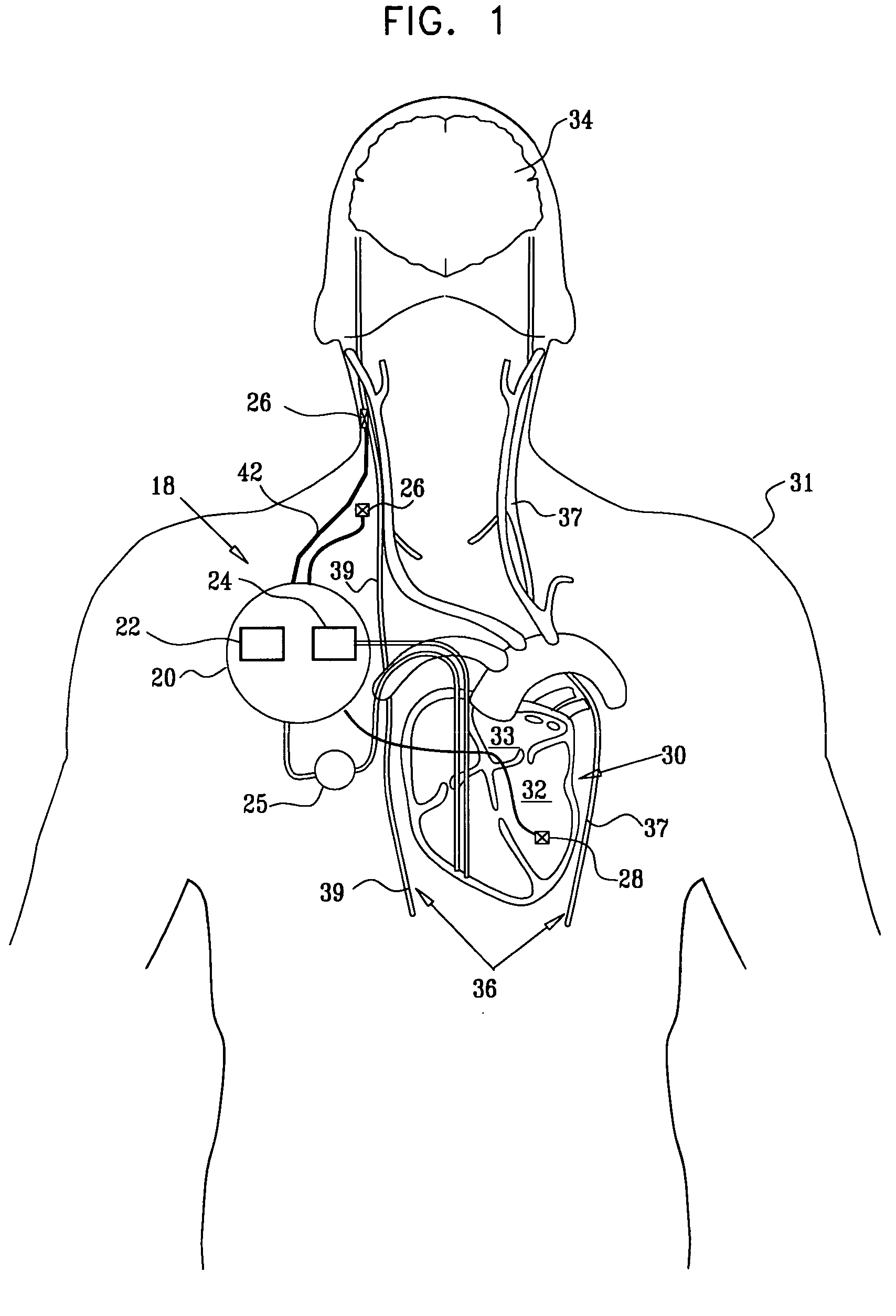
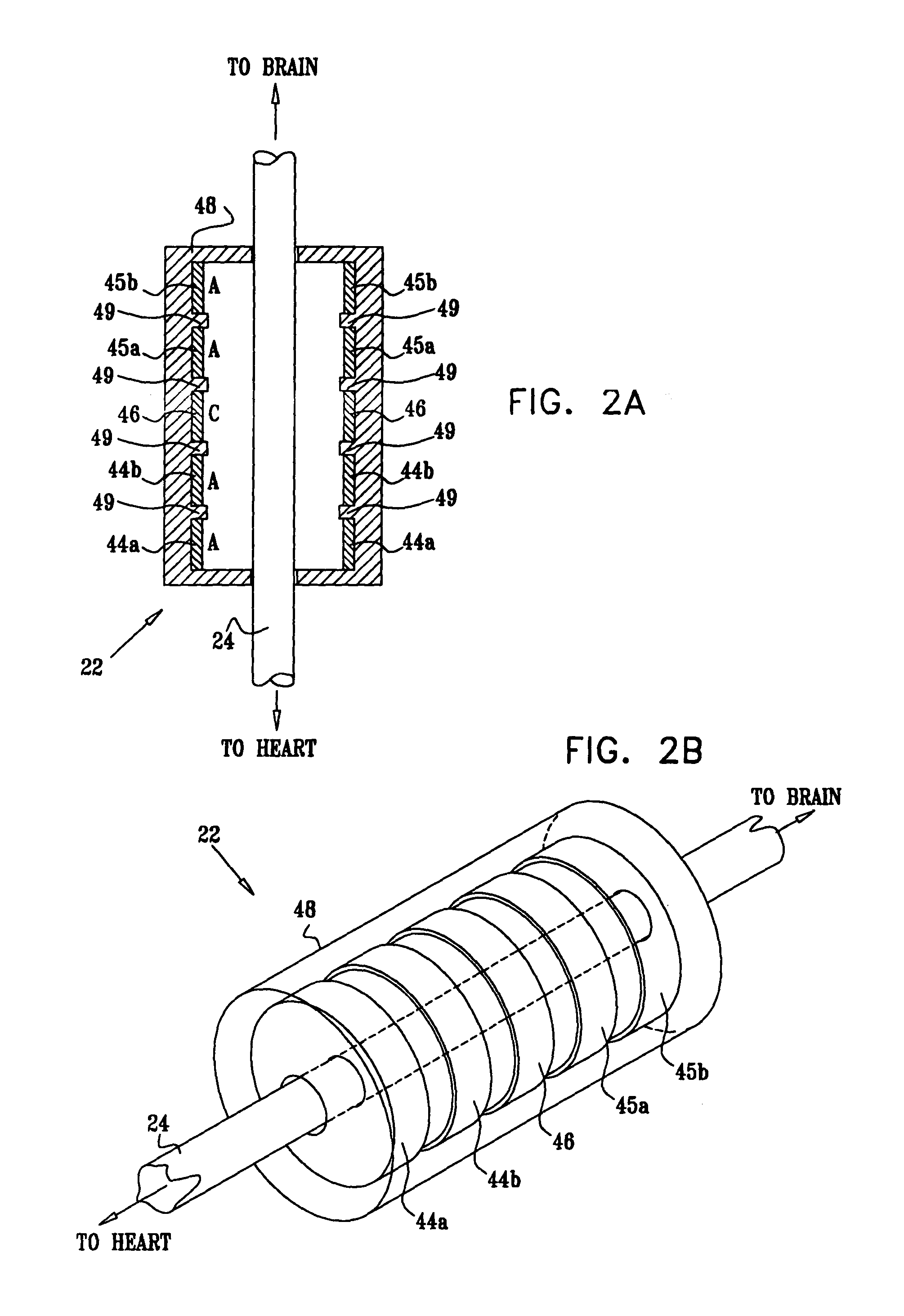
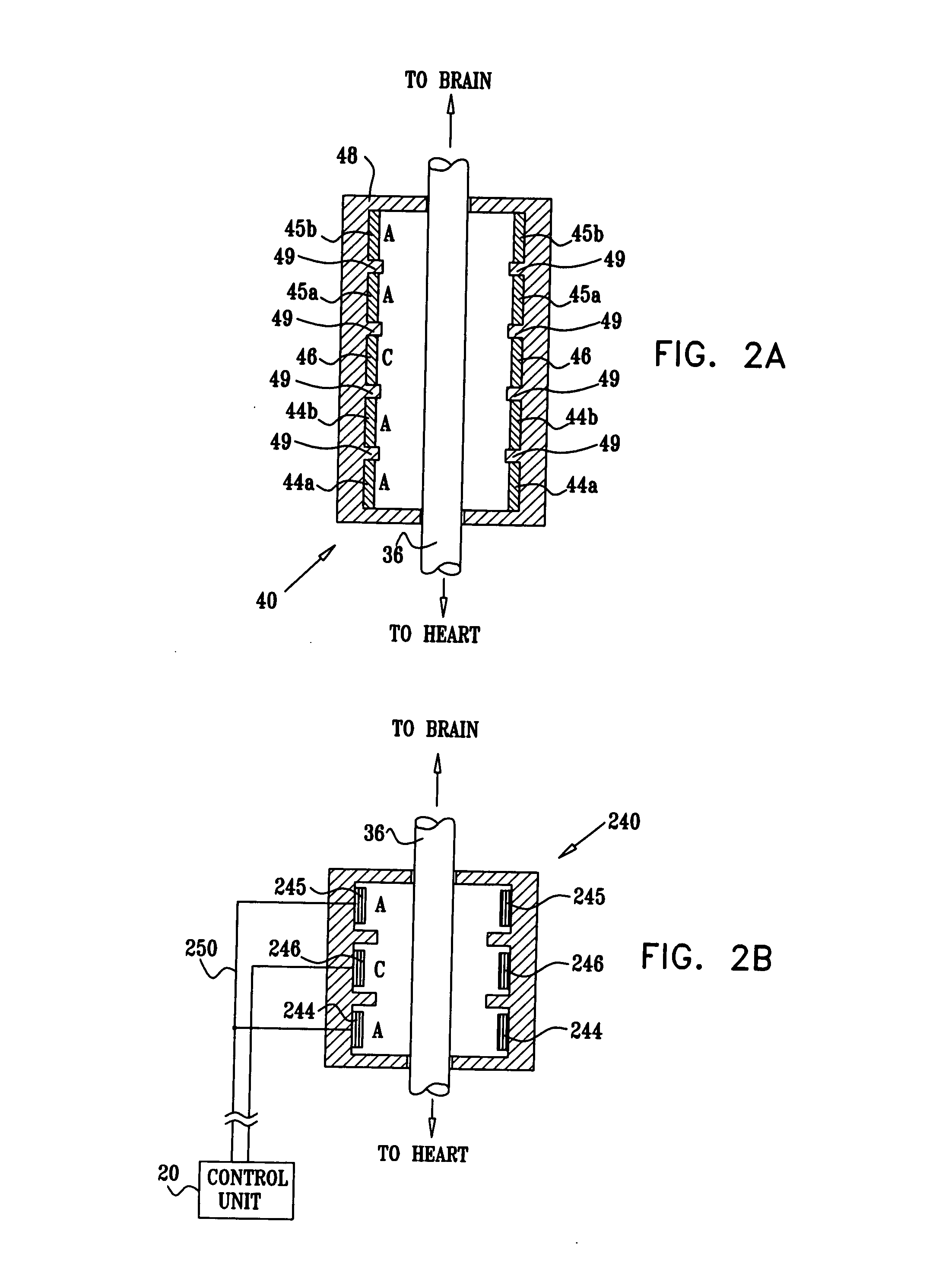
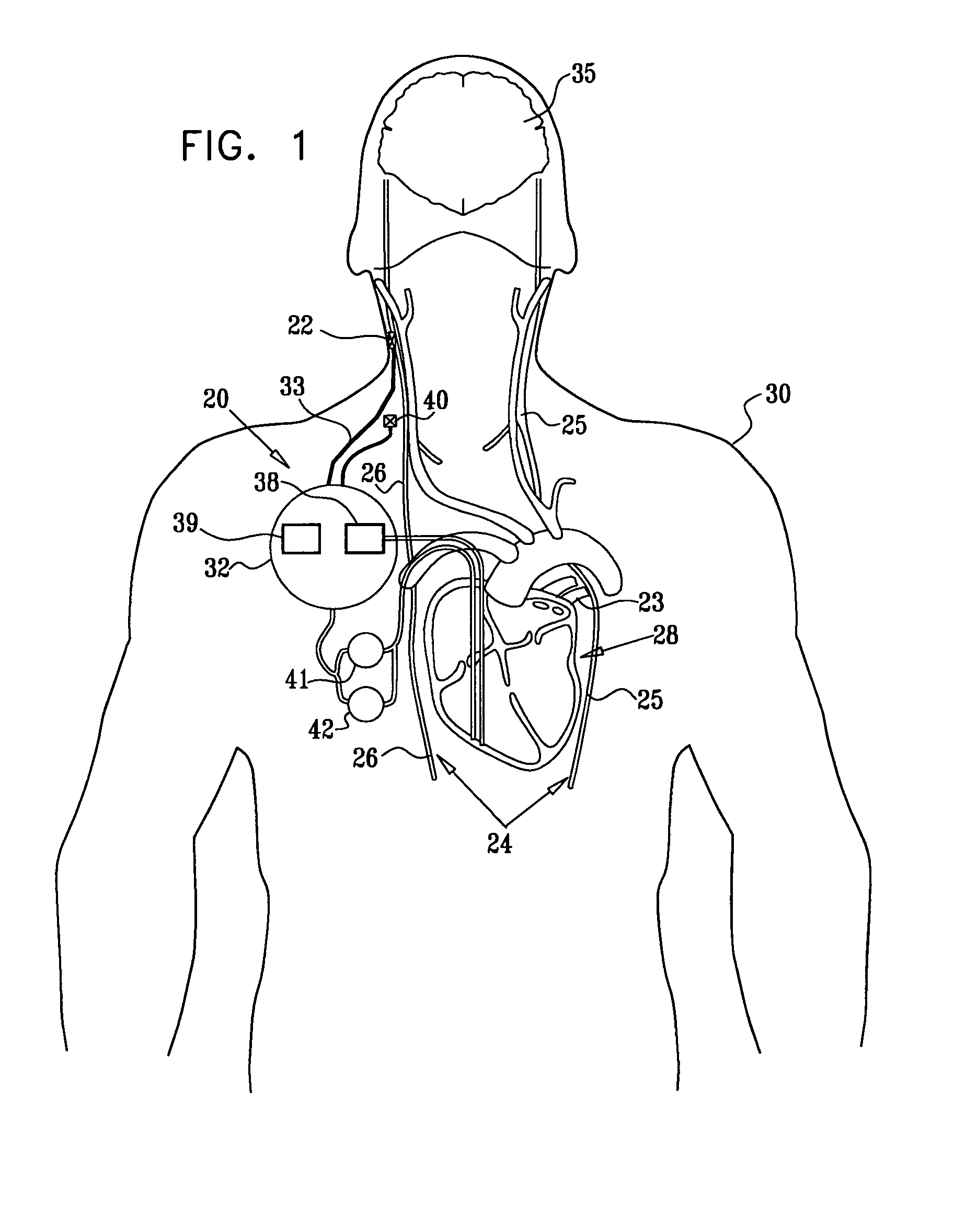
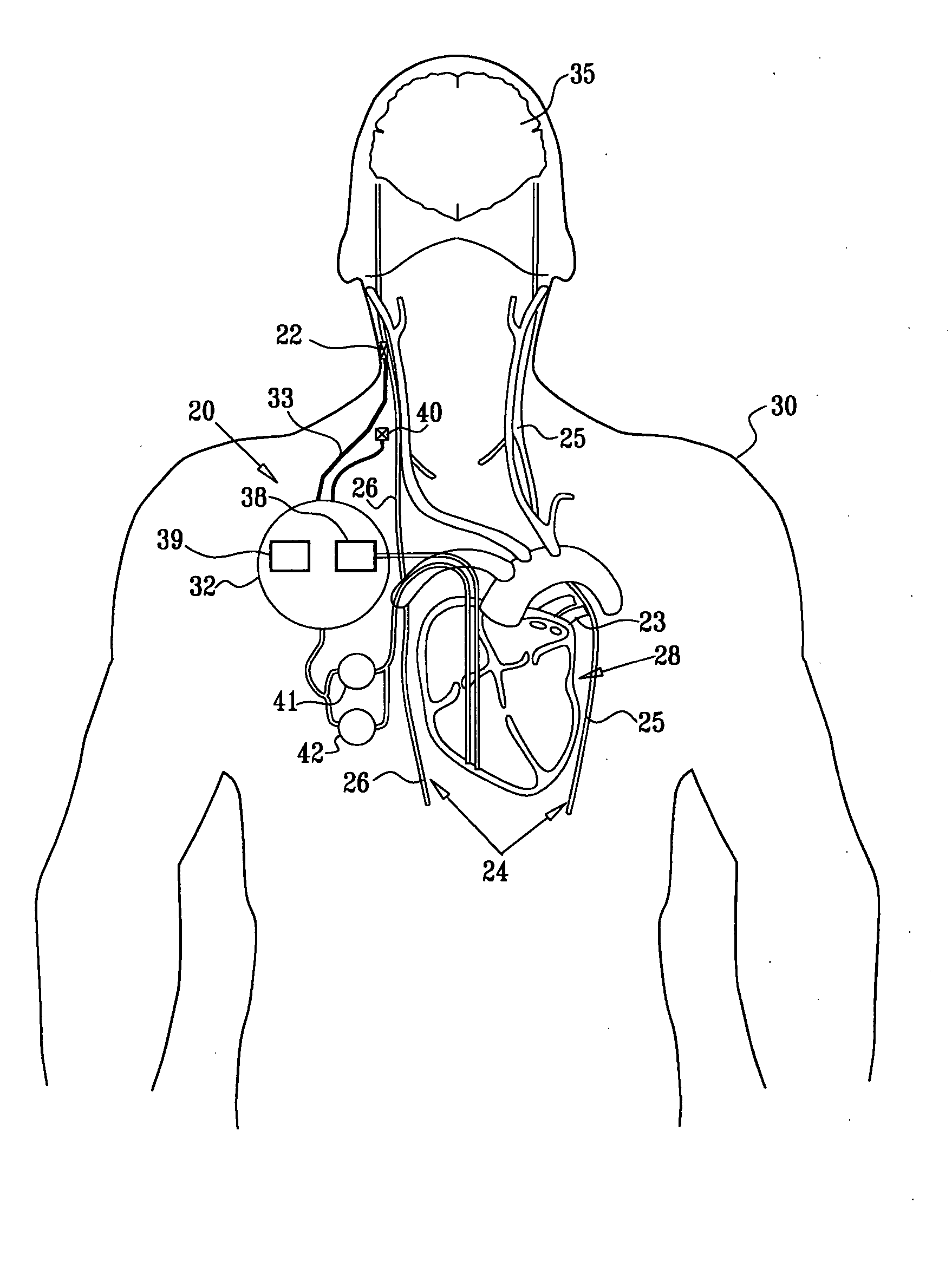
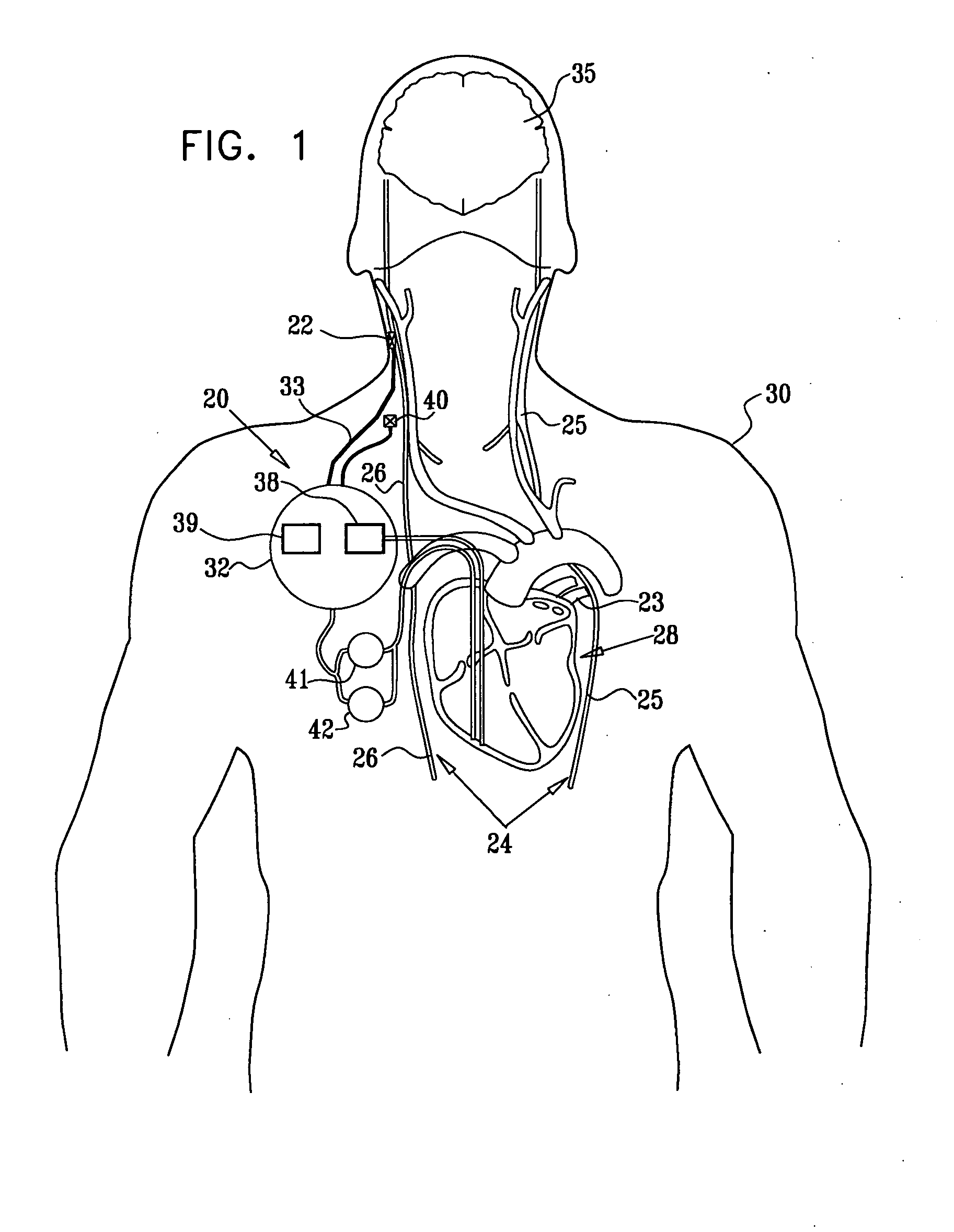
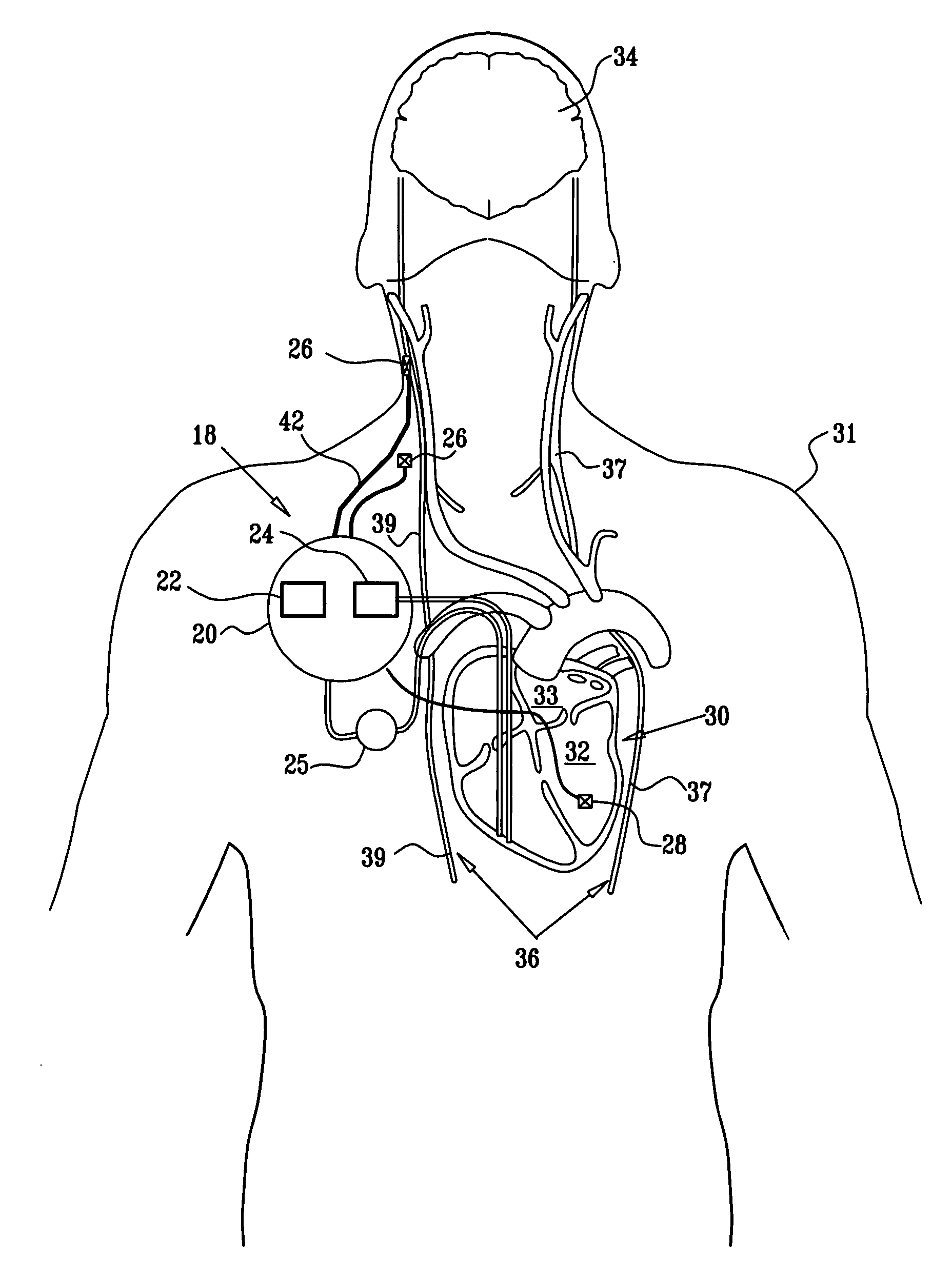
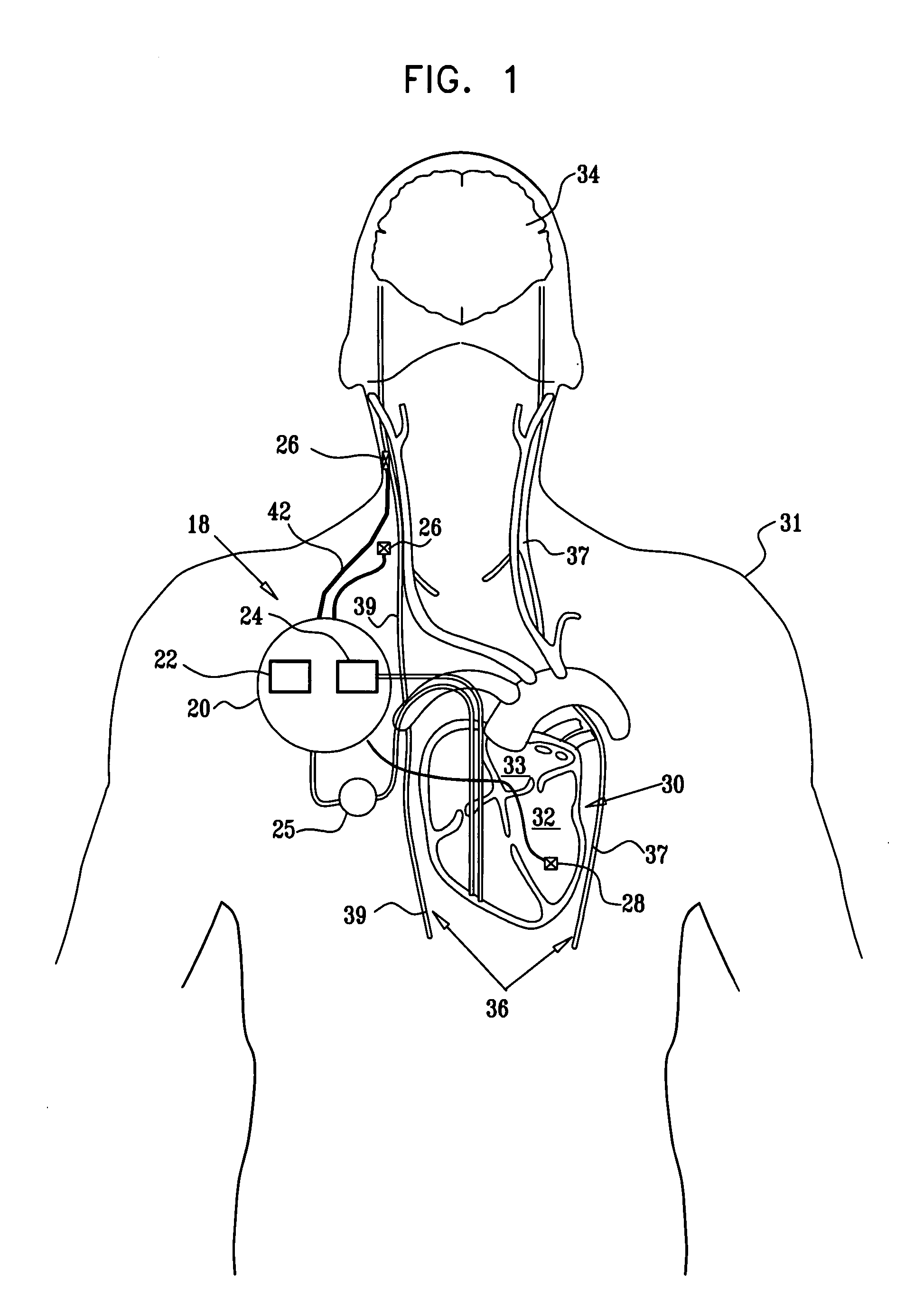
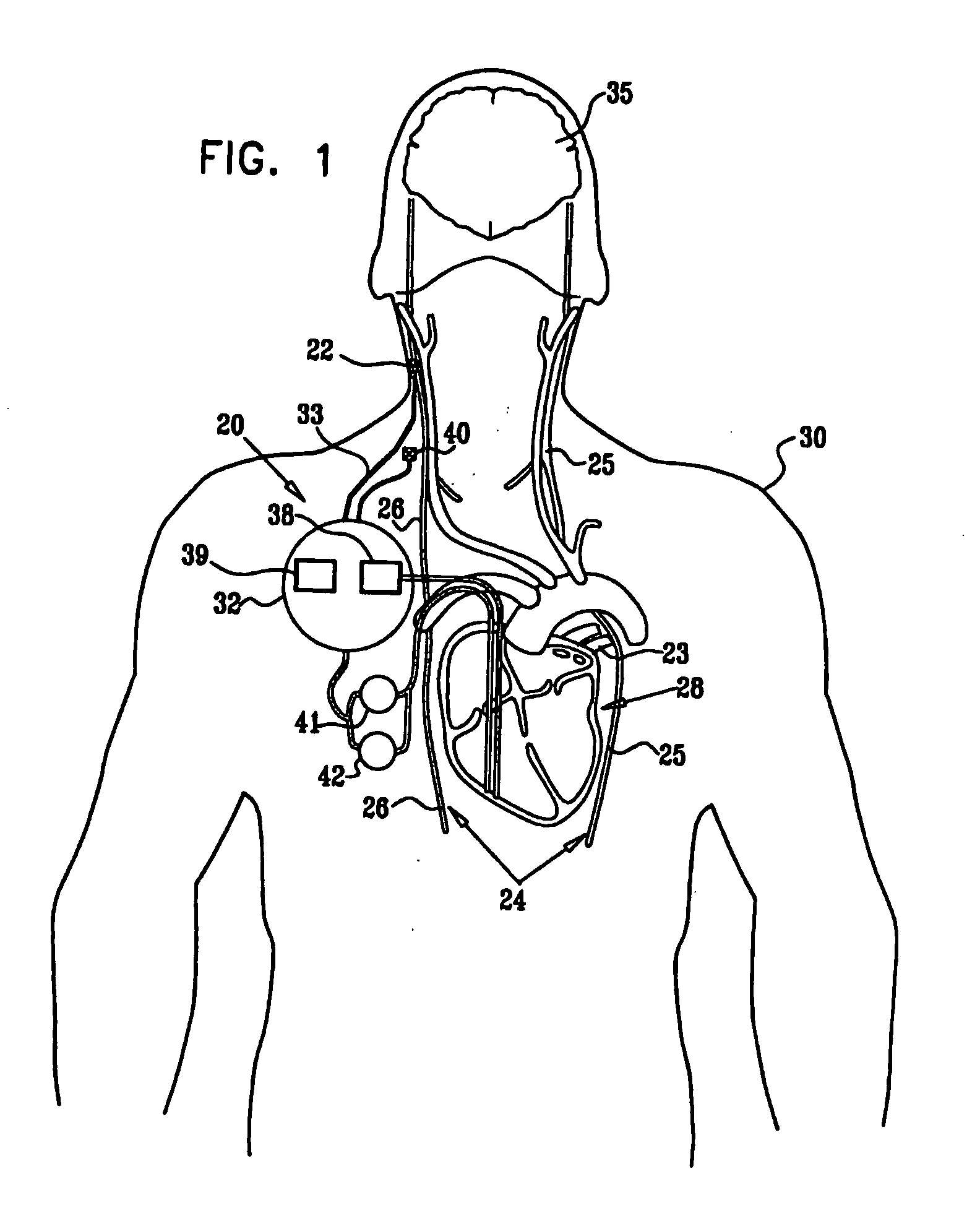
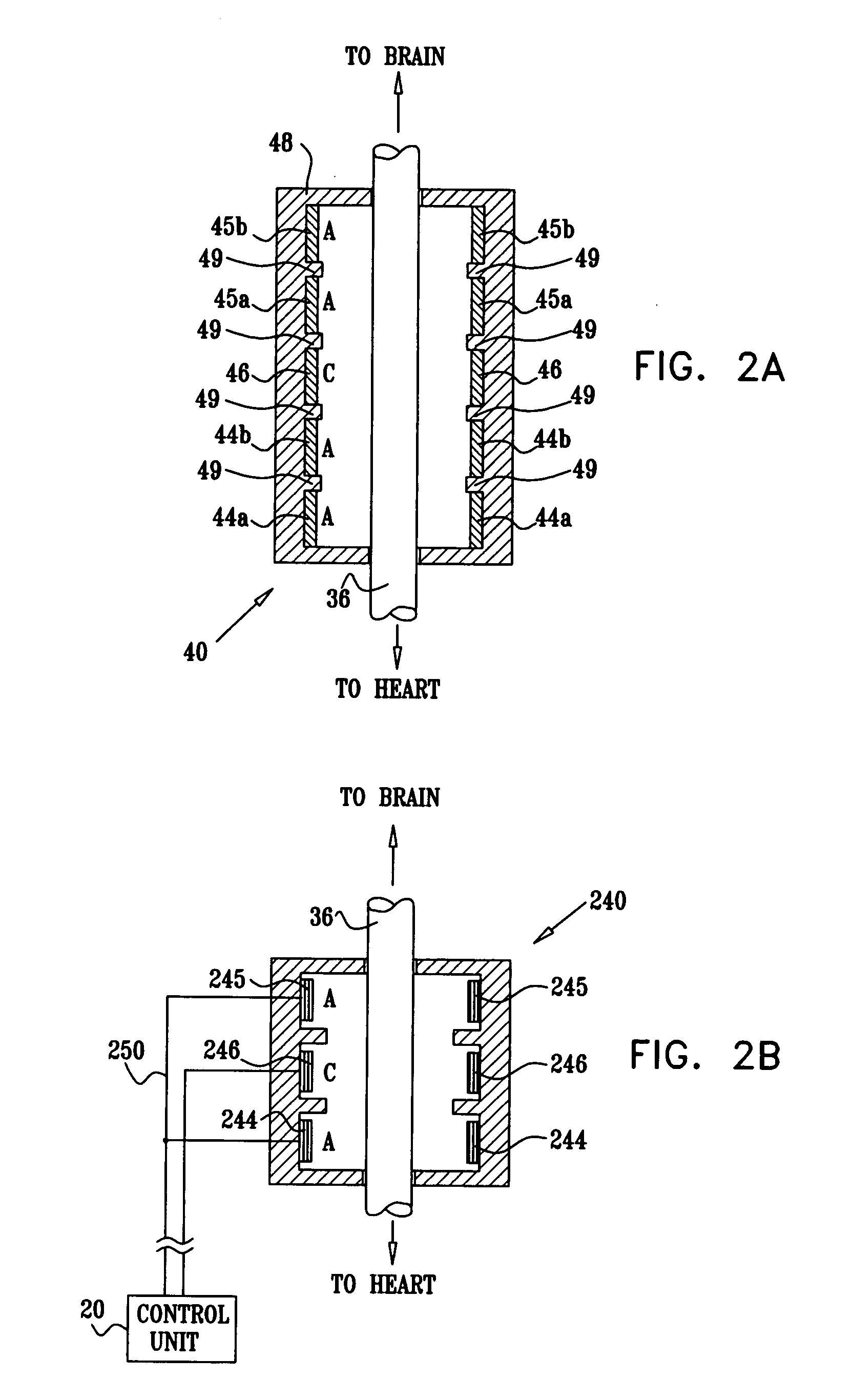
Treatment of hypertension includes implantation of the discharge portion(s) of a catheter and / or electrical stimulation electrode(s) adjacent the tissue(s) to be stimulated. Stimulation pulses, i.e., drug infusion pulses and / or electrical pulses, are supplied by one or more implanted stimulators, through the catheter and possibly also a lead, tunneled subcutaneously between the stimulator and stimulation site. A microstimulator(s) may also / instead deliver electrical stimulation pulses. Stimulation sites include the carotid sinus and carotid body, among other locations. Treatments include drugs used for acute and / or chronic treatment of hypertension. In a number of embodiments, a need for or response to treatment is sensed, and the electrical and / or infusion pulses adjusted accordingly.
Owner:BOSTON SCI NEUROMODULATION CORP
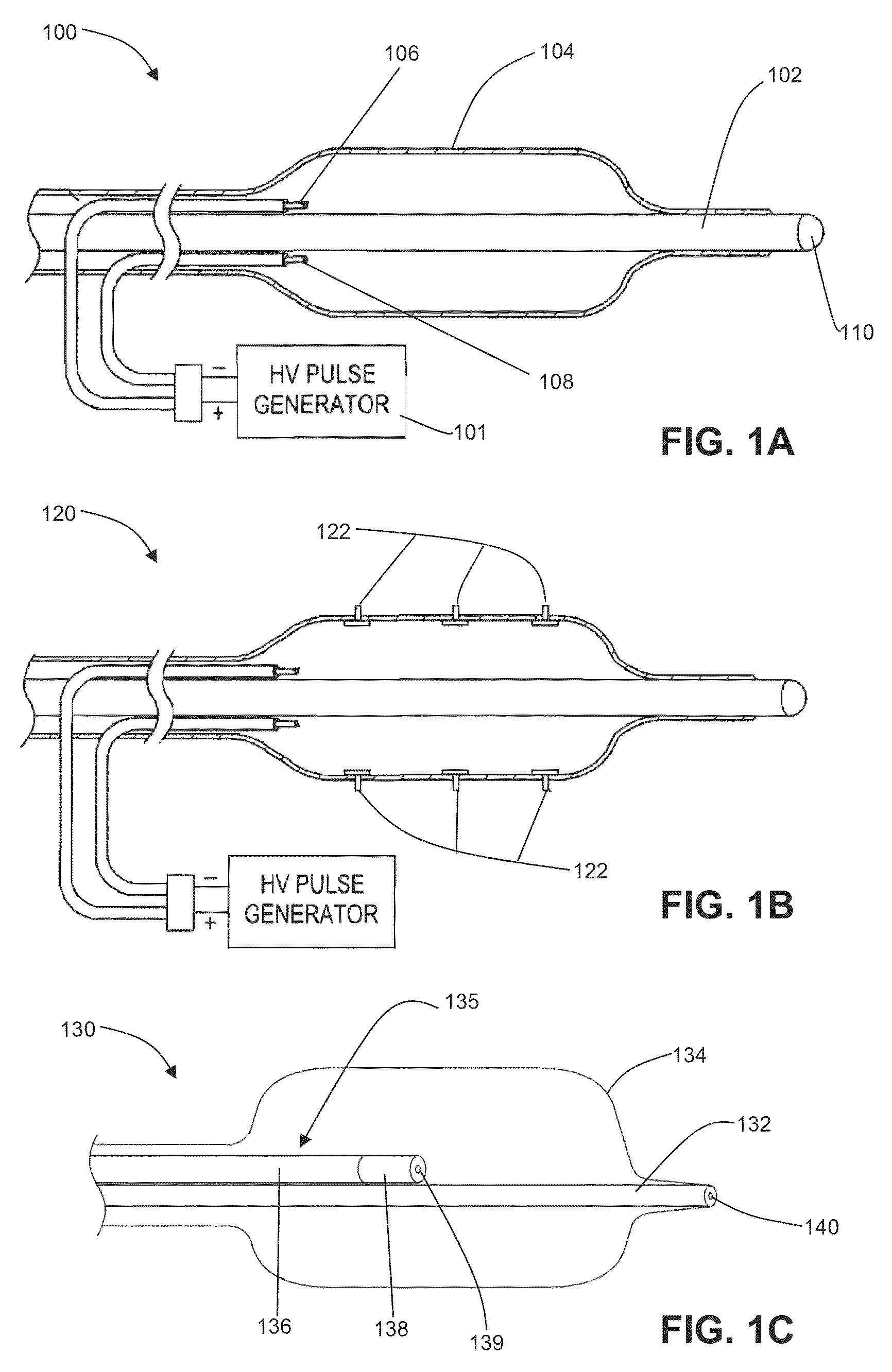
Electrode designs and methods of use for cardiovascular reflex control devices
InactiveUS7158832B2RemissionReduce pressureInternal electrodesExternal electrodesNervous systemBaroreceptor function
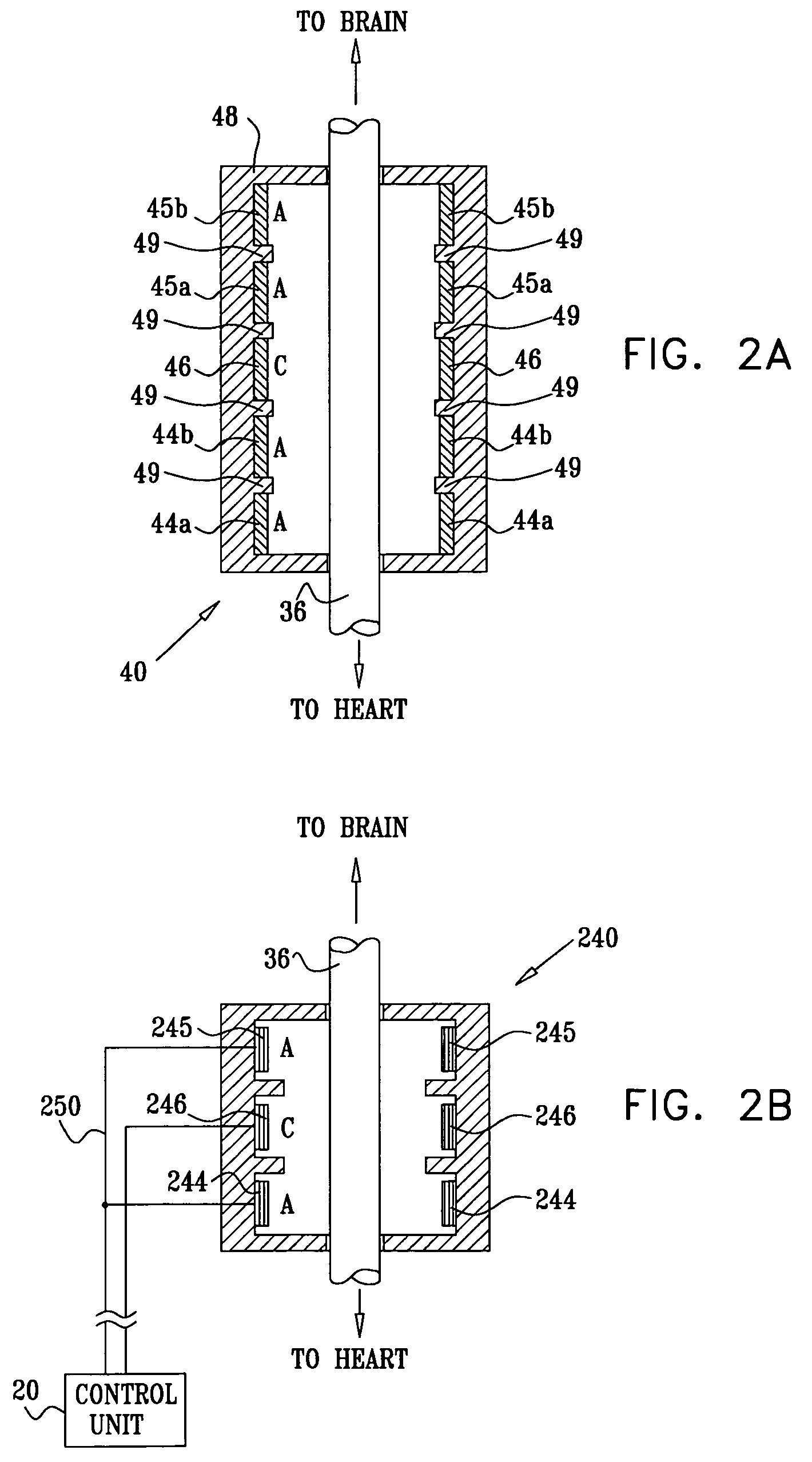
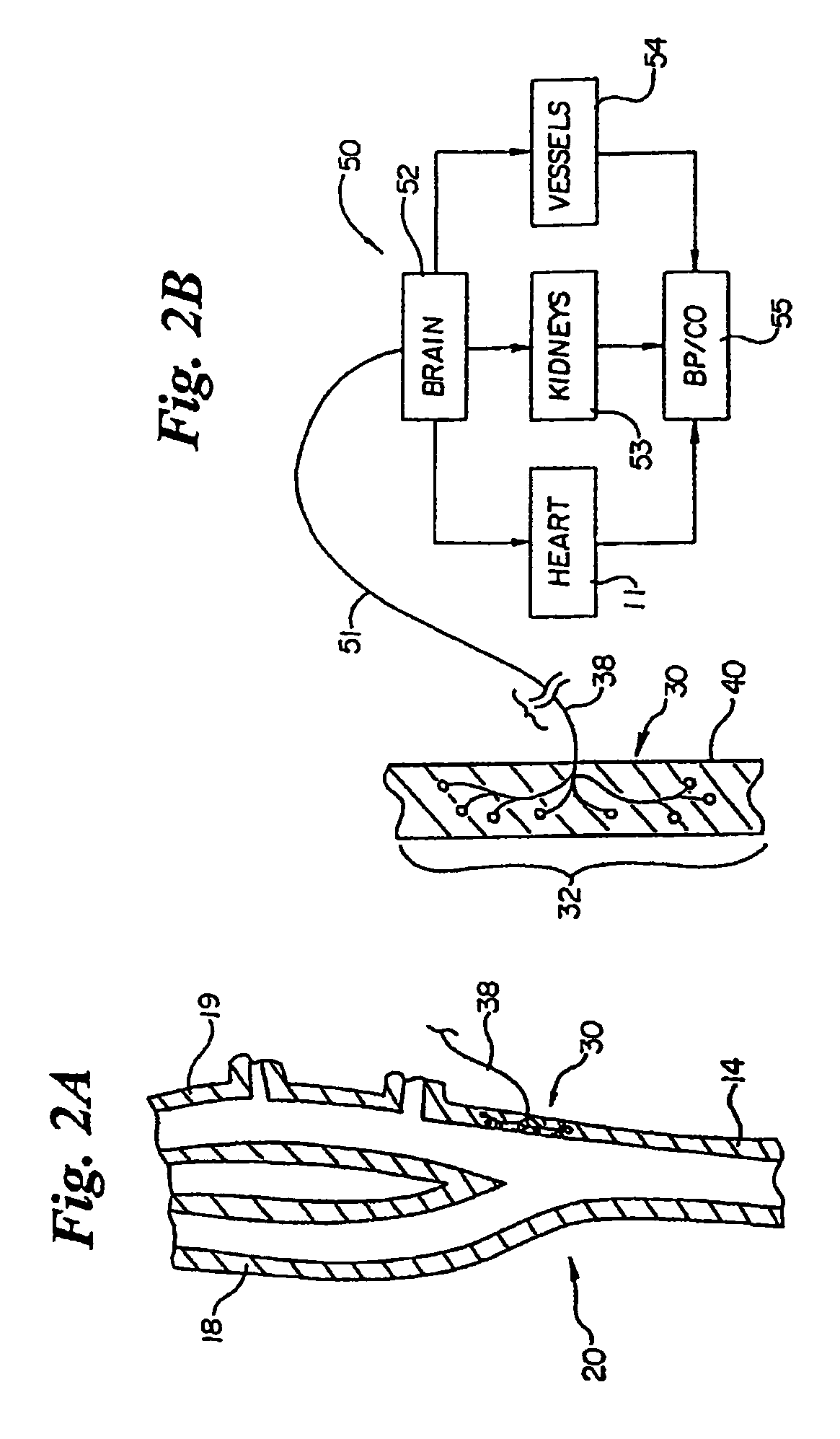
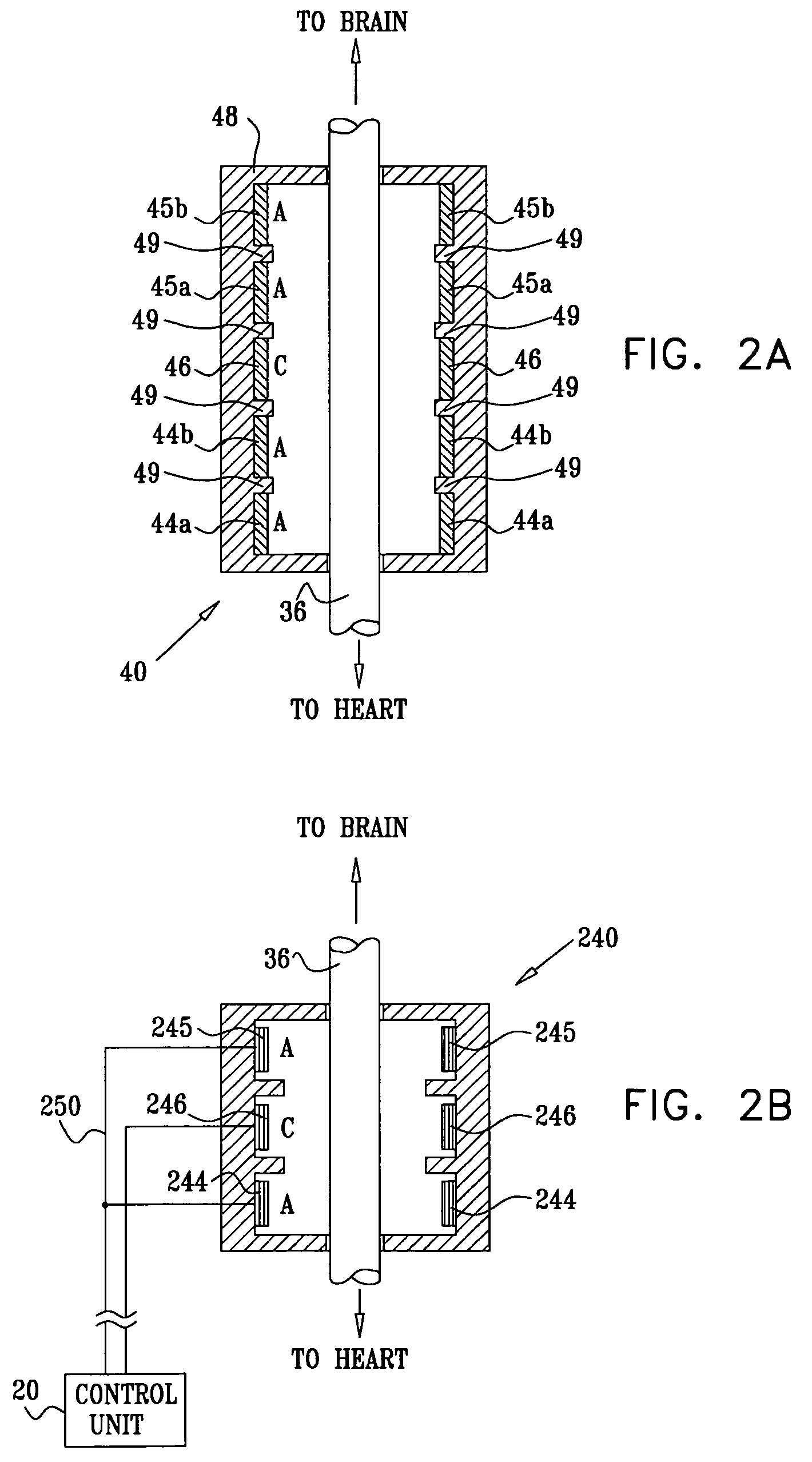
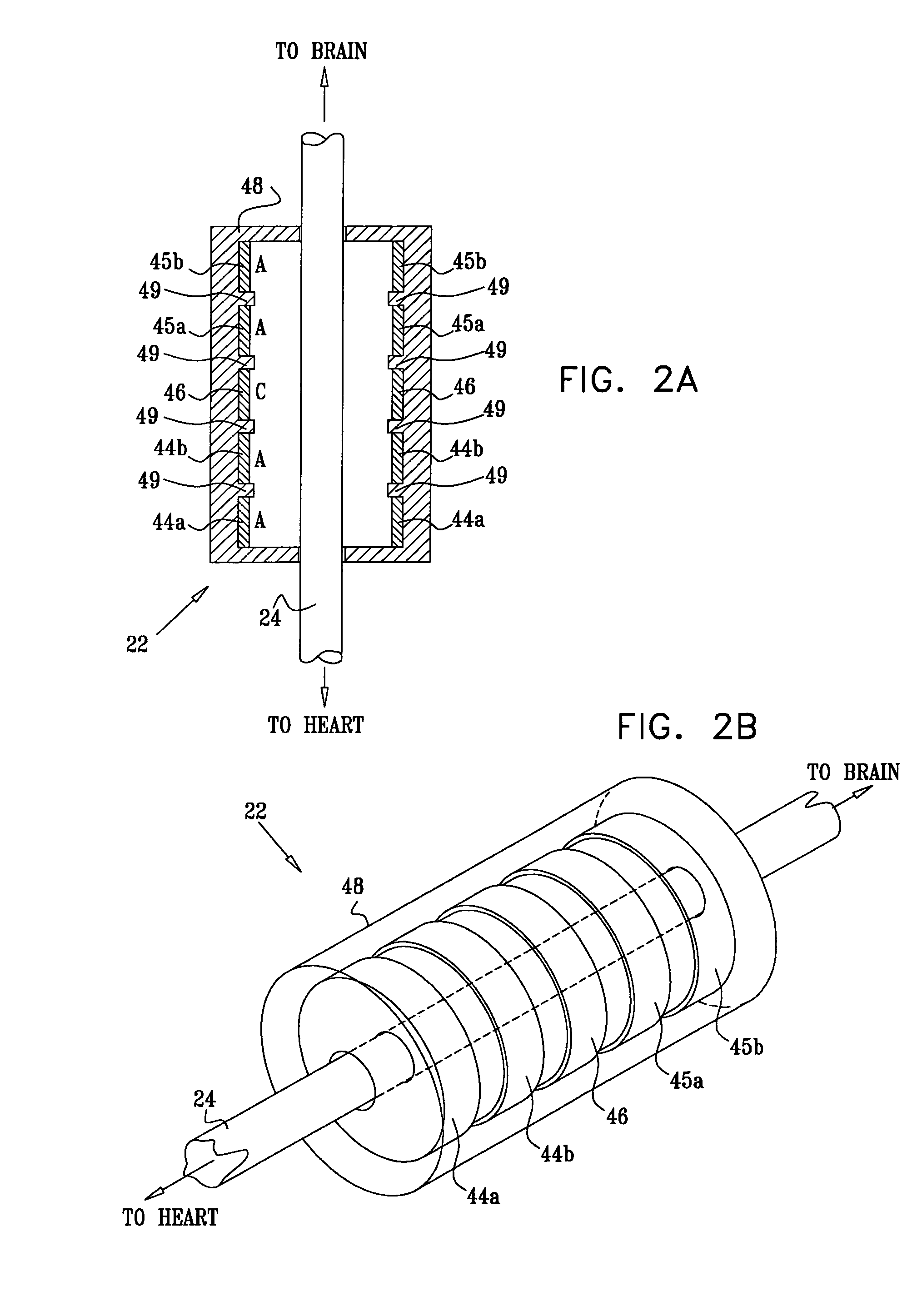
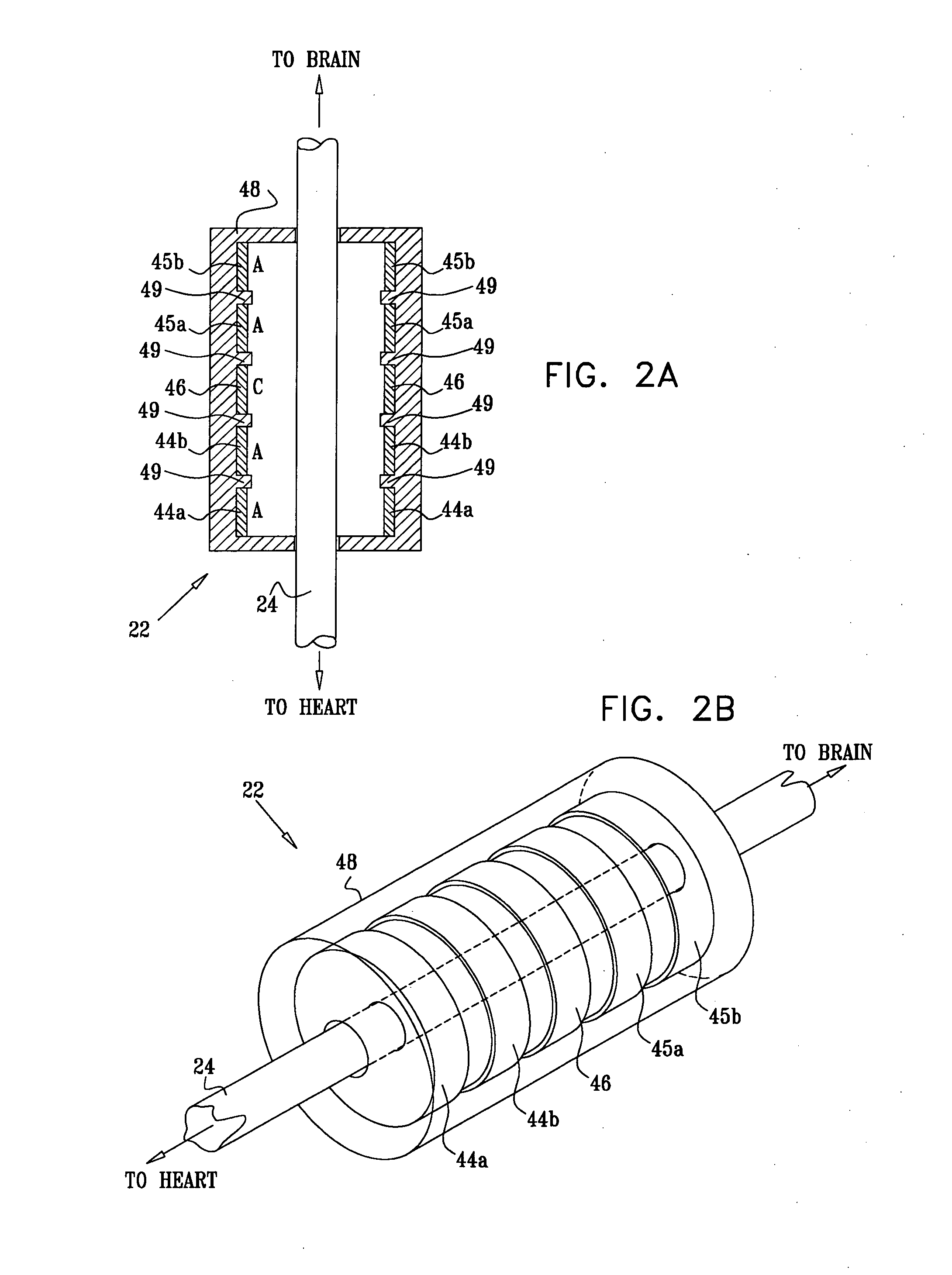
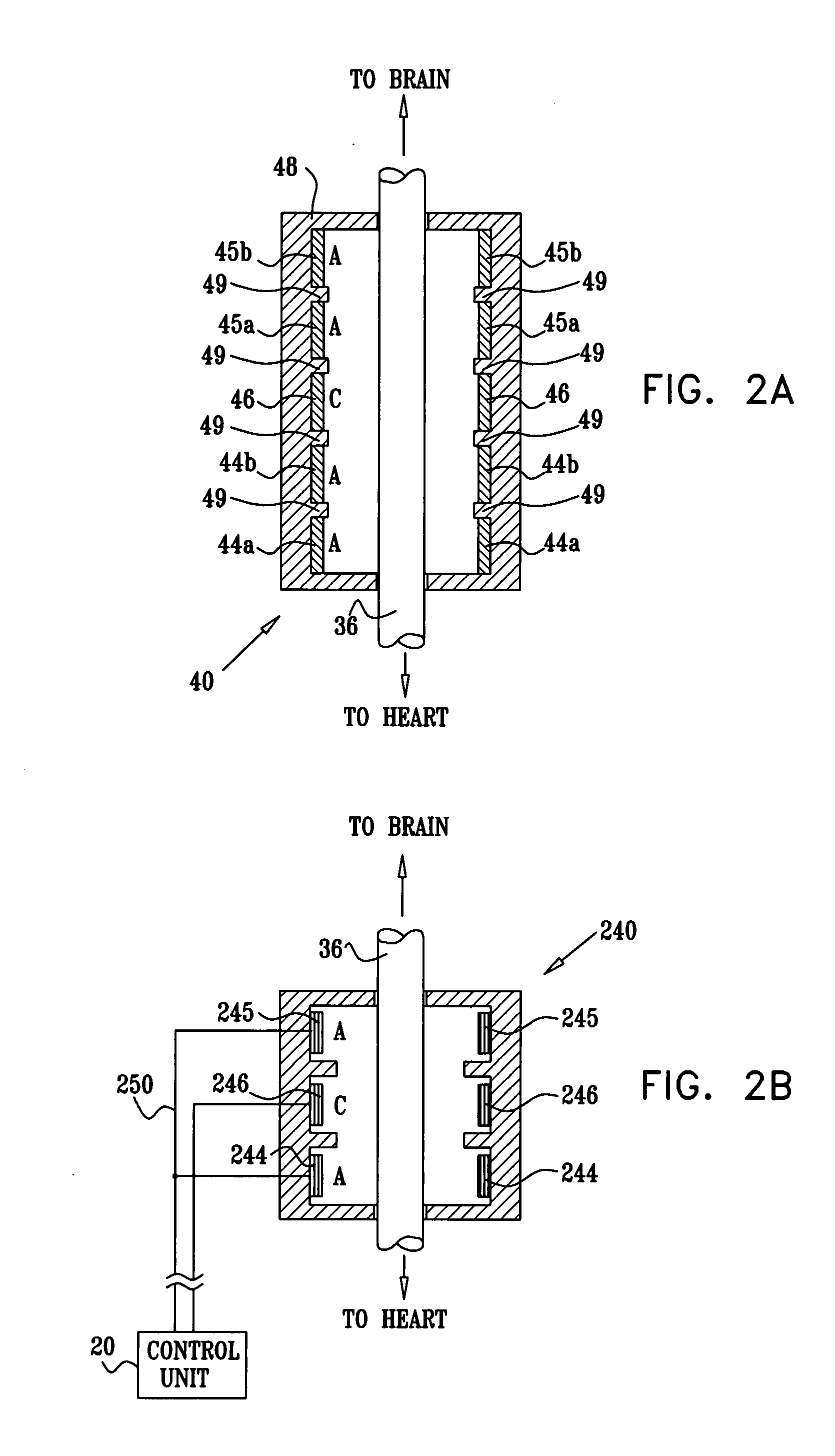
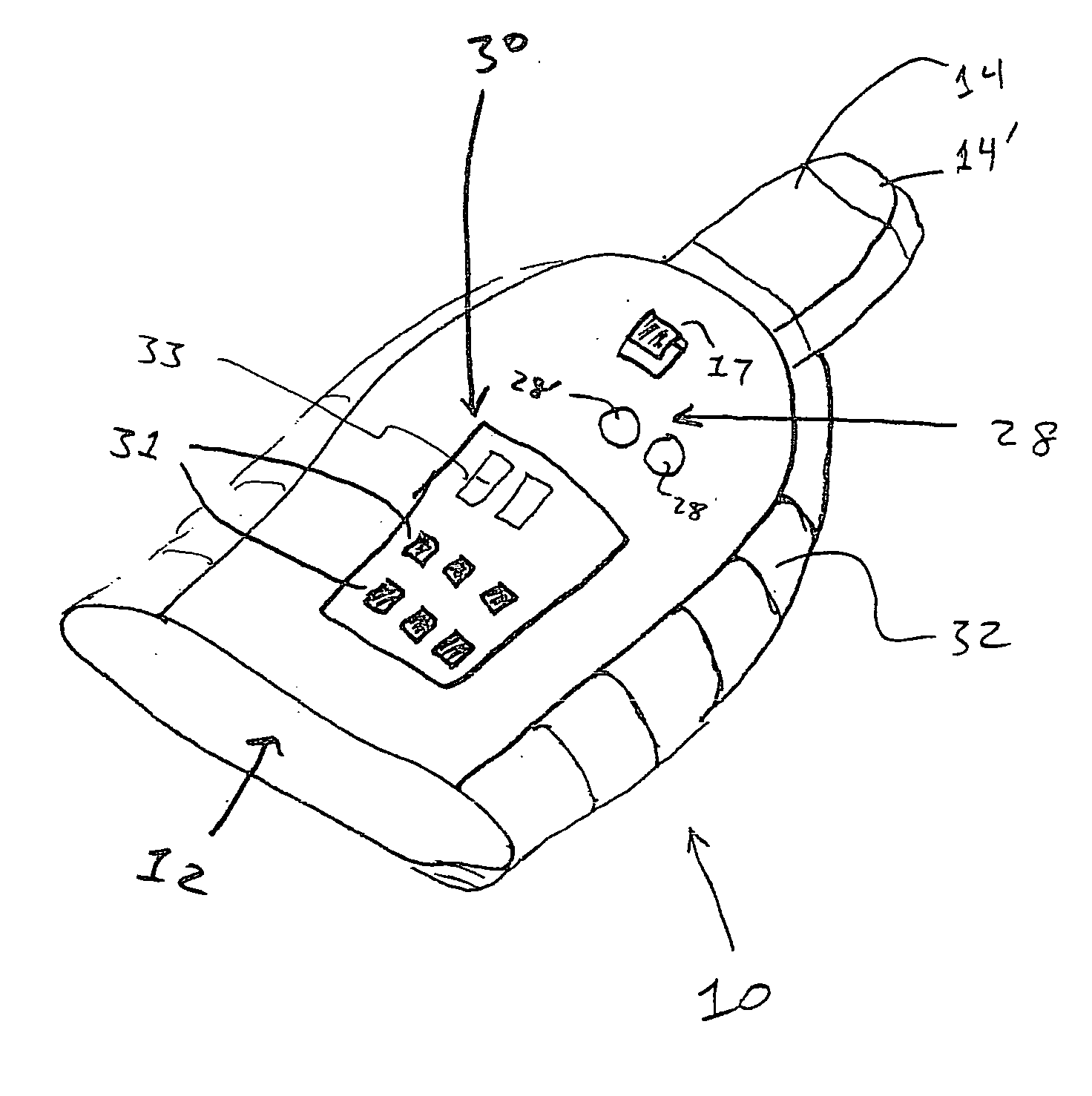
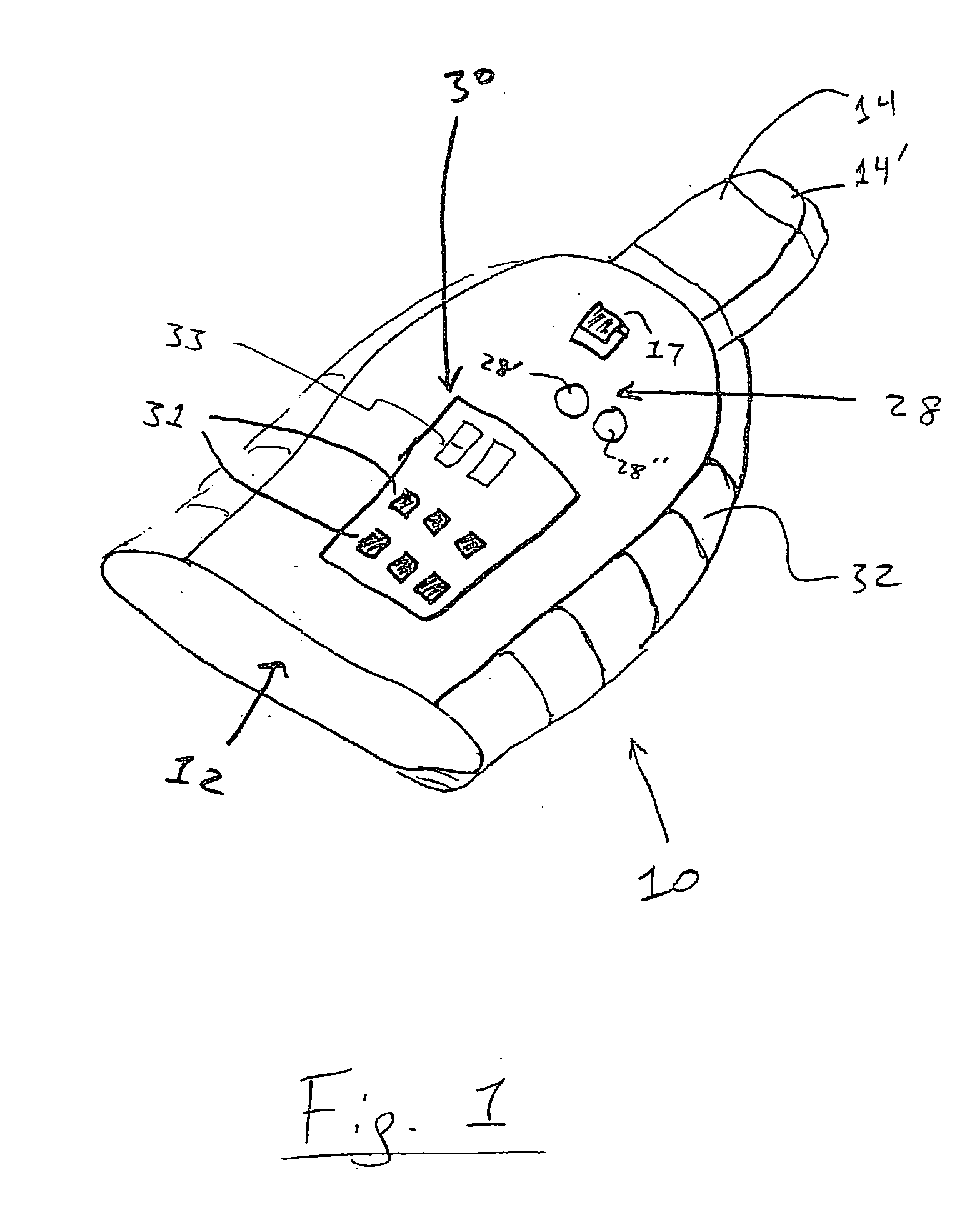
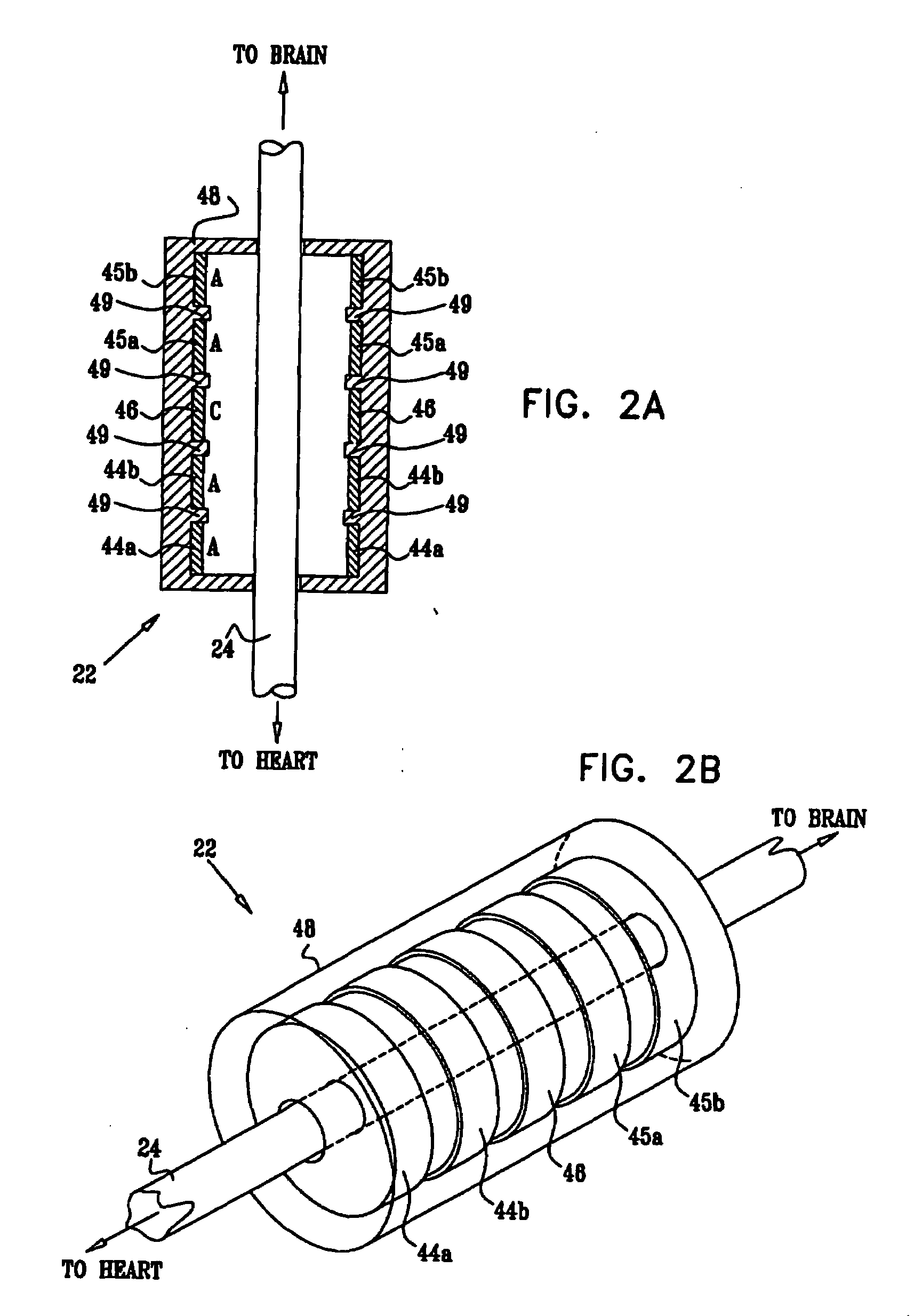
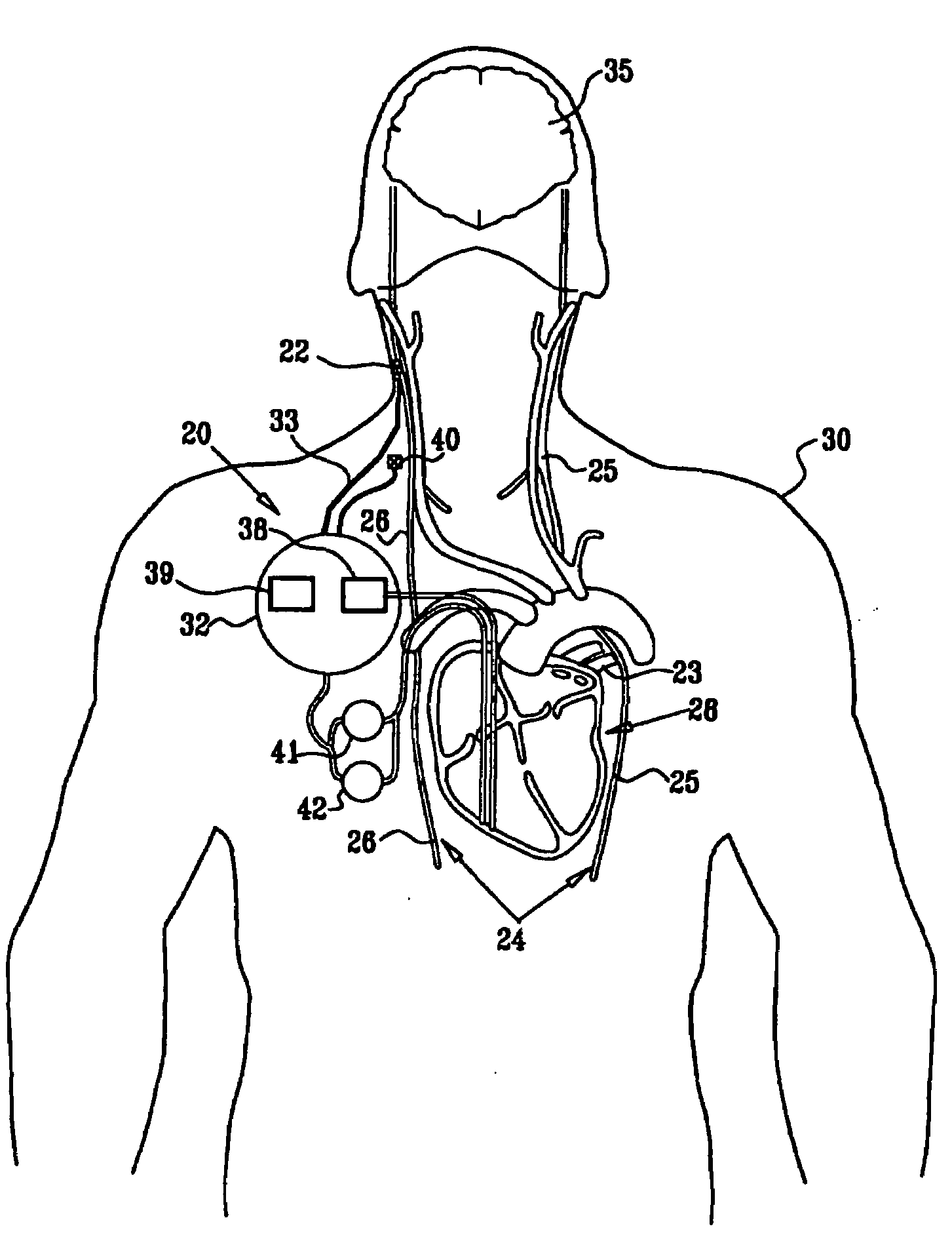
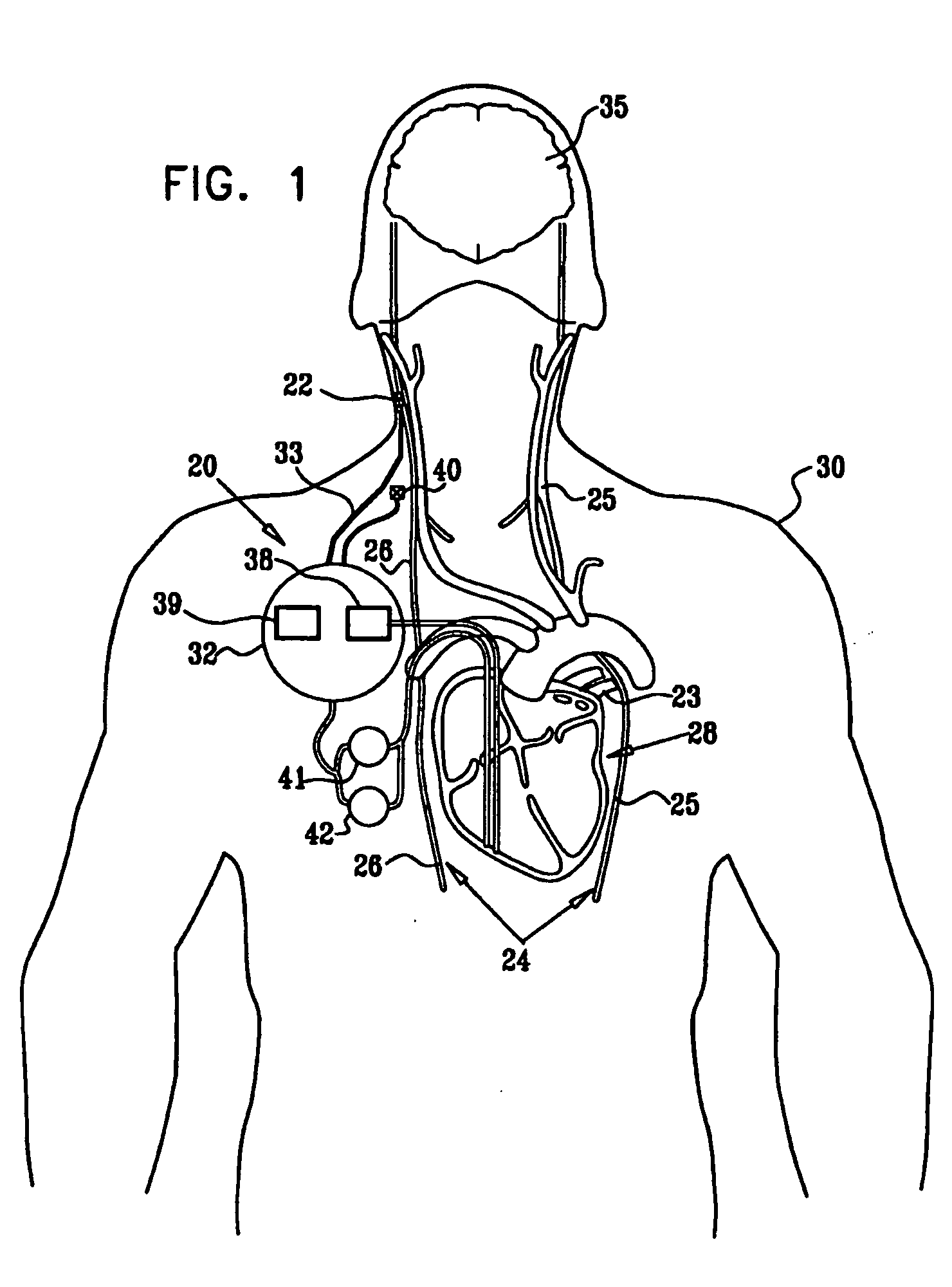
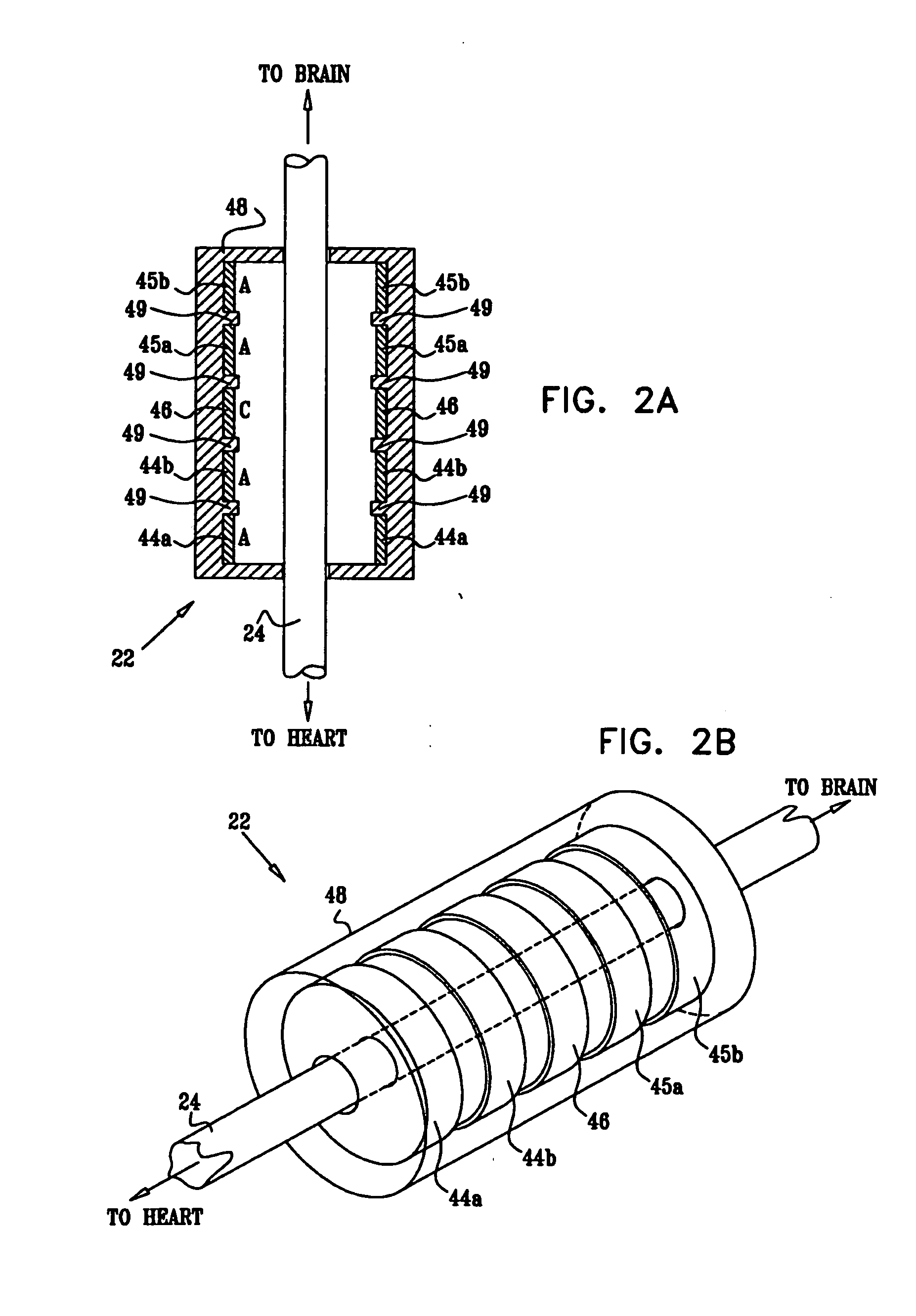
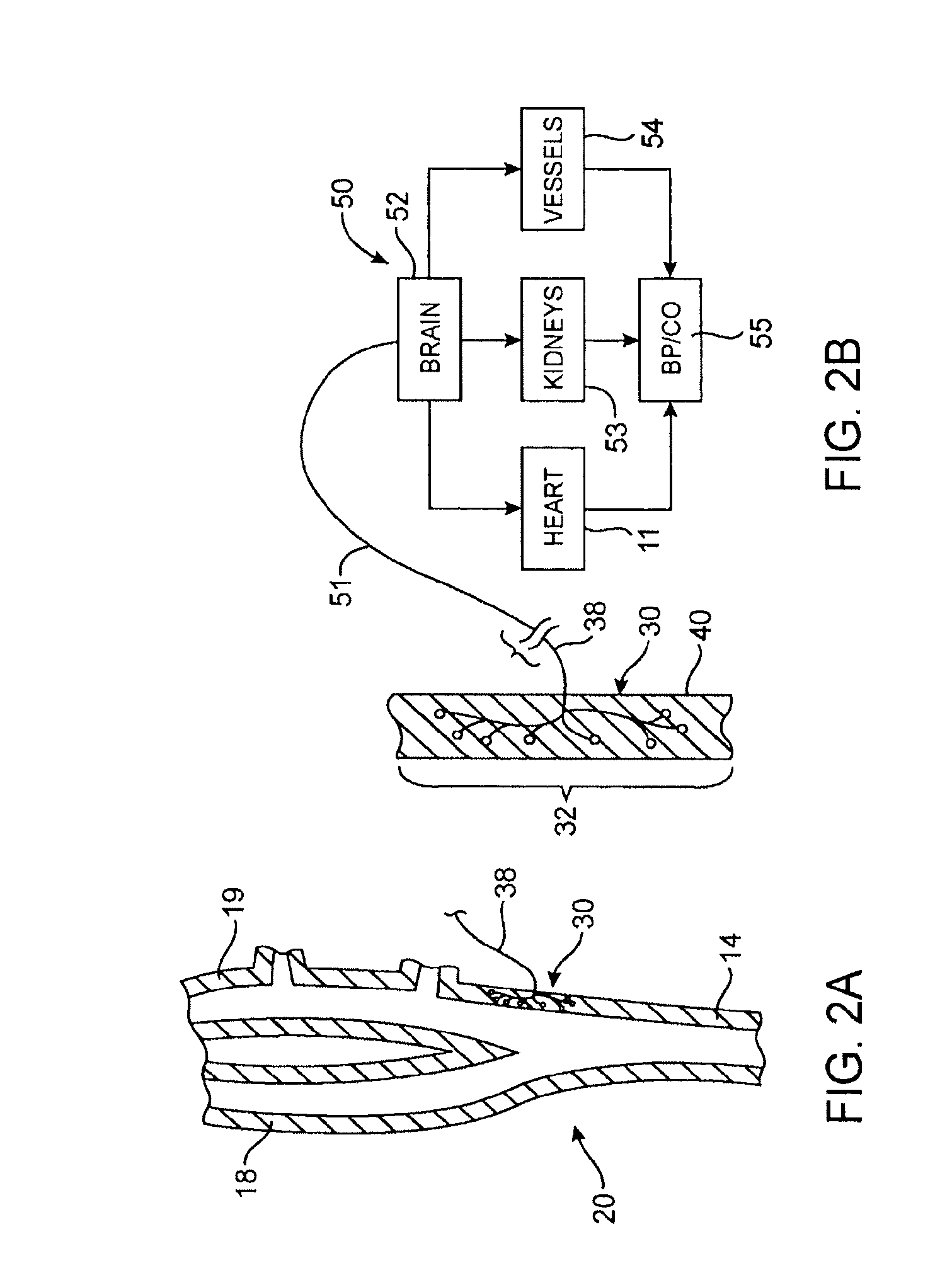
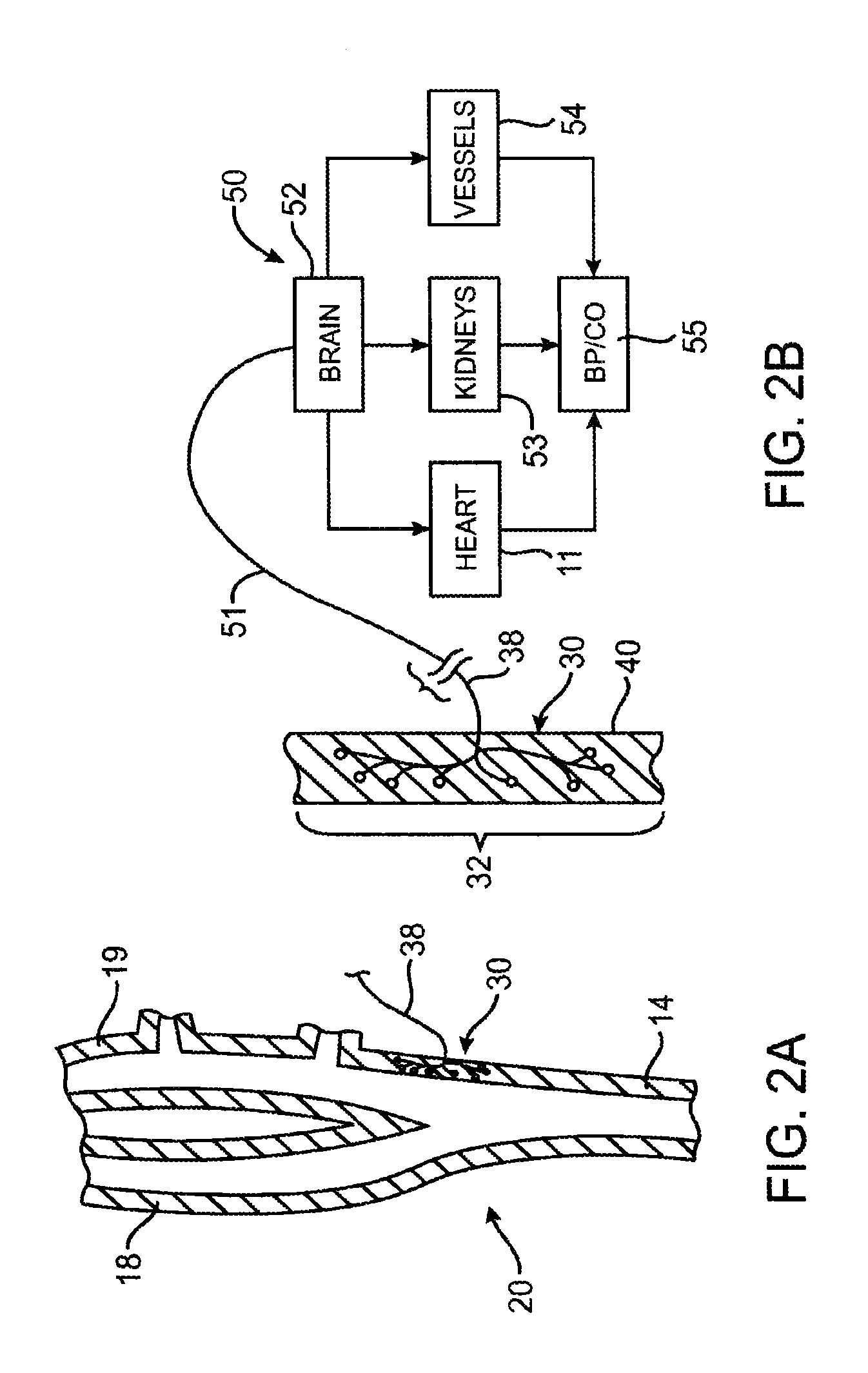
Devices, systems and methods by which the blood pressure, nervous system activity, and neurohornonal activity may be selectively and controllably reduced by activating baroreceptors. A baroreceptor activation device is positioned near a baroreceptor, preferably in the carotid sinus. The baroreceptor activation device may utilize electrodes to activate the baroreceptors. The electrodes may be adapted for connection to the carotid arteries at or near the carotid sinus, and may be designed to minimize extraneous tissue stimulation.
Owner:CVRX
Techniques for applying, configuring, and coordinating nerve fiber stimulation
ActiveUS20050267542A1Decreased heart rateEliminate side effectsSpinal electrodesHeart stimulatorsCardiac arrhythmiaCarotid sinus
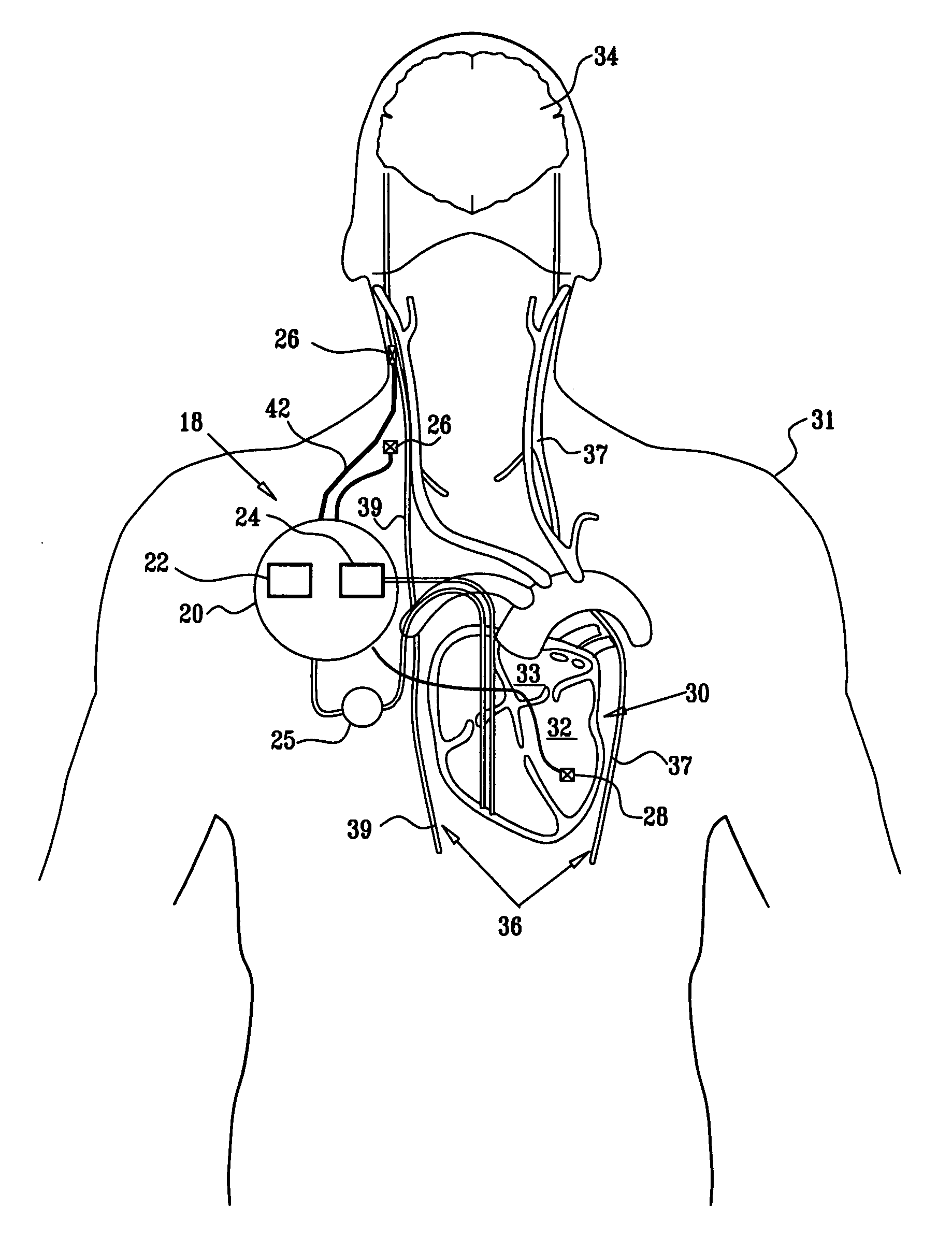
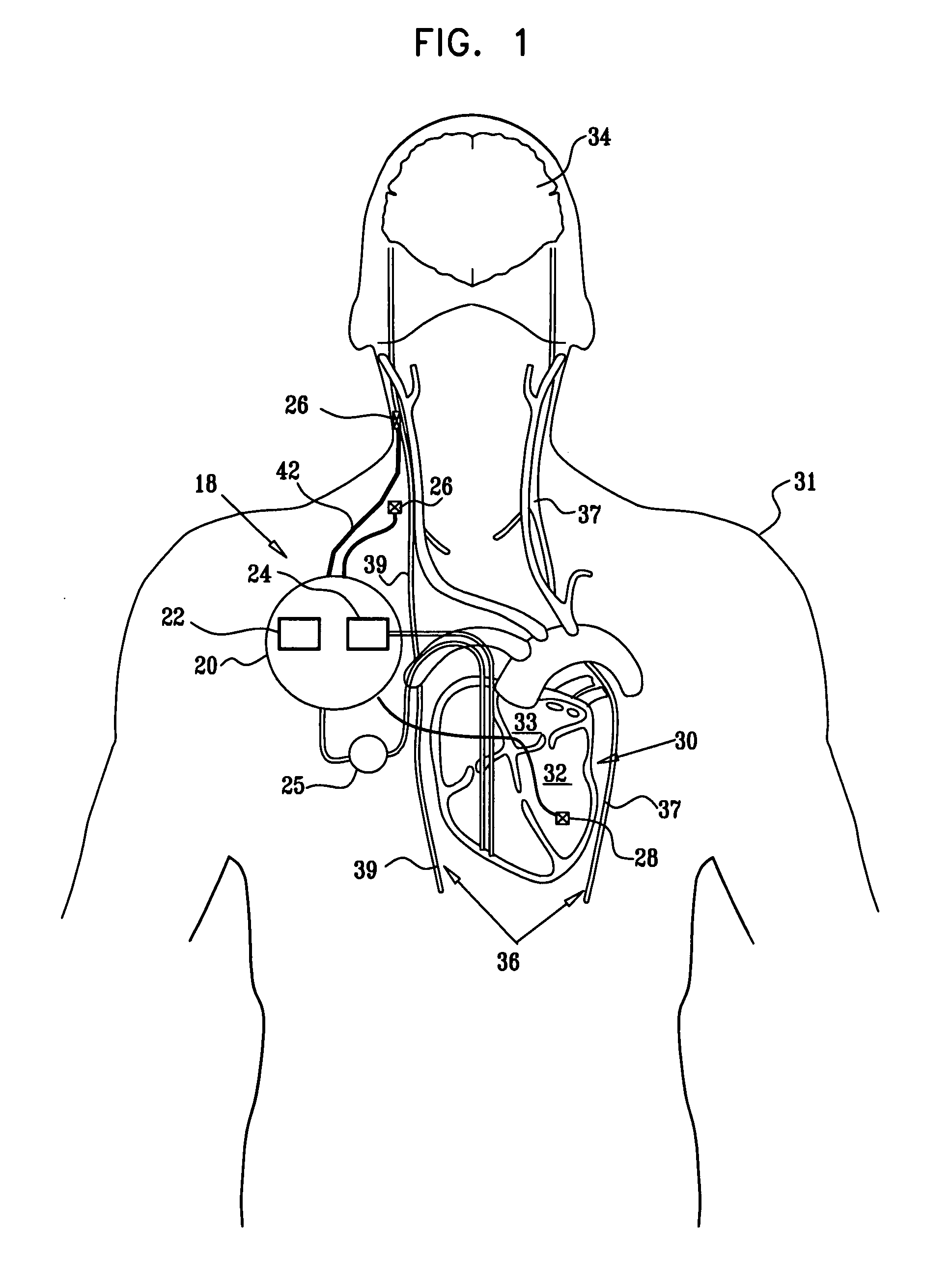
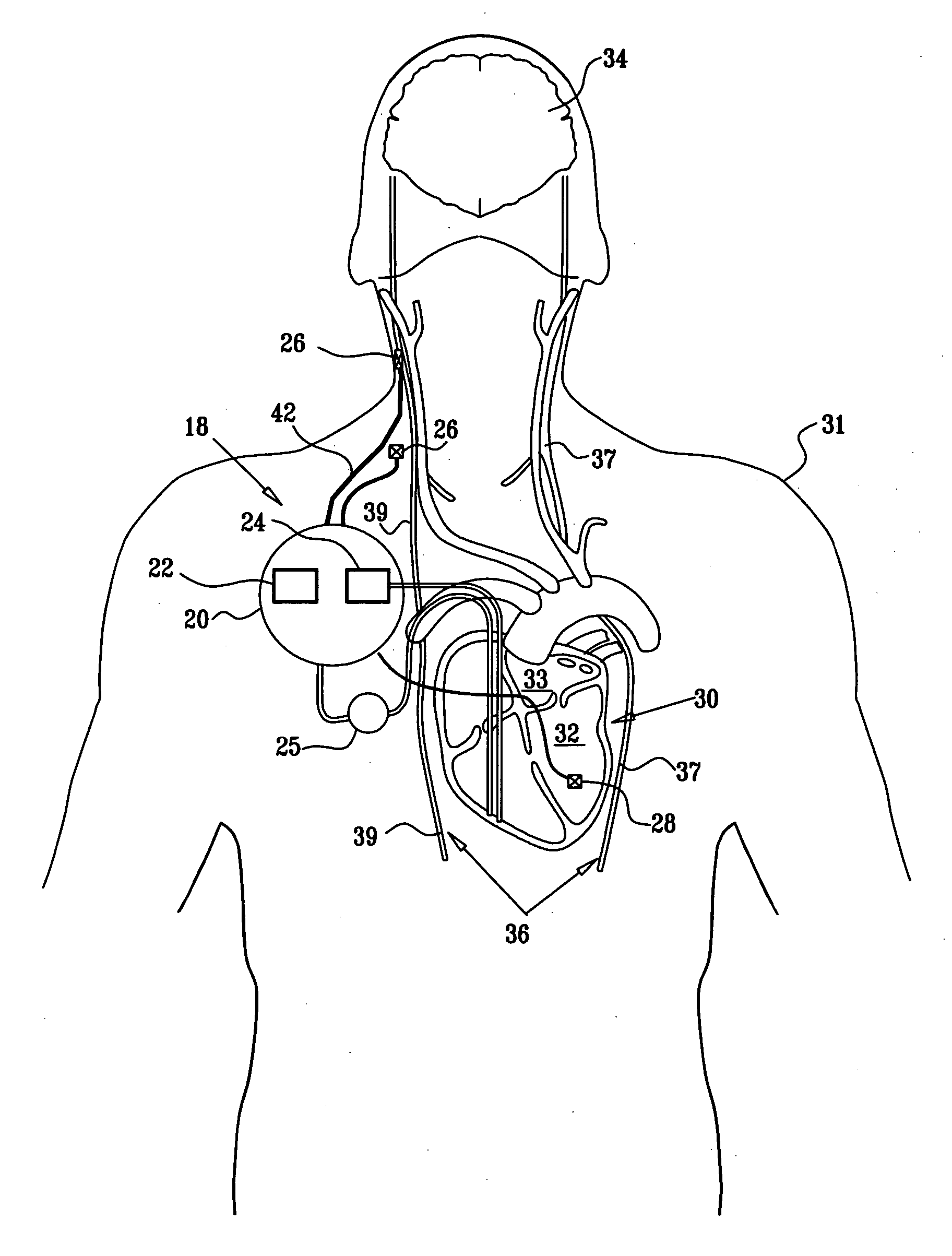
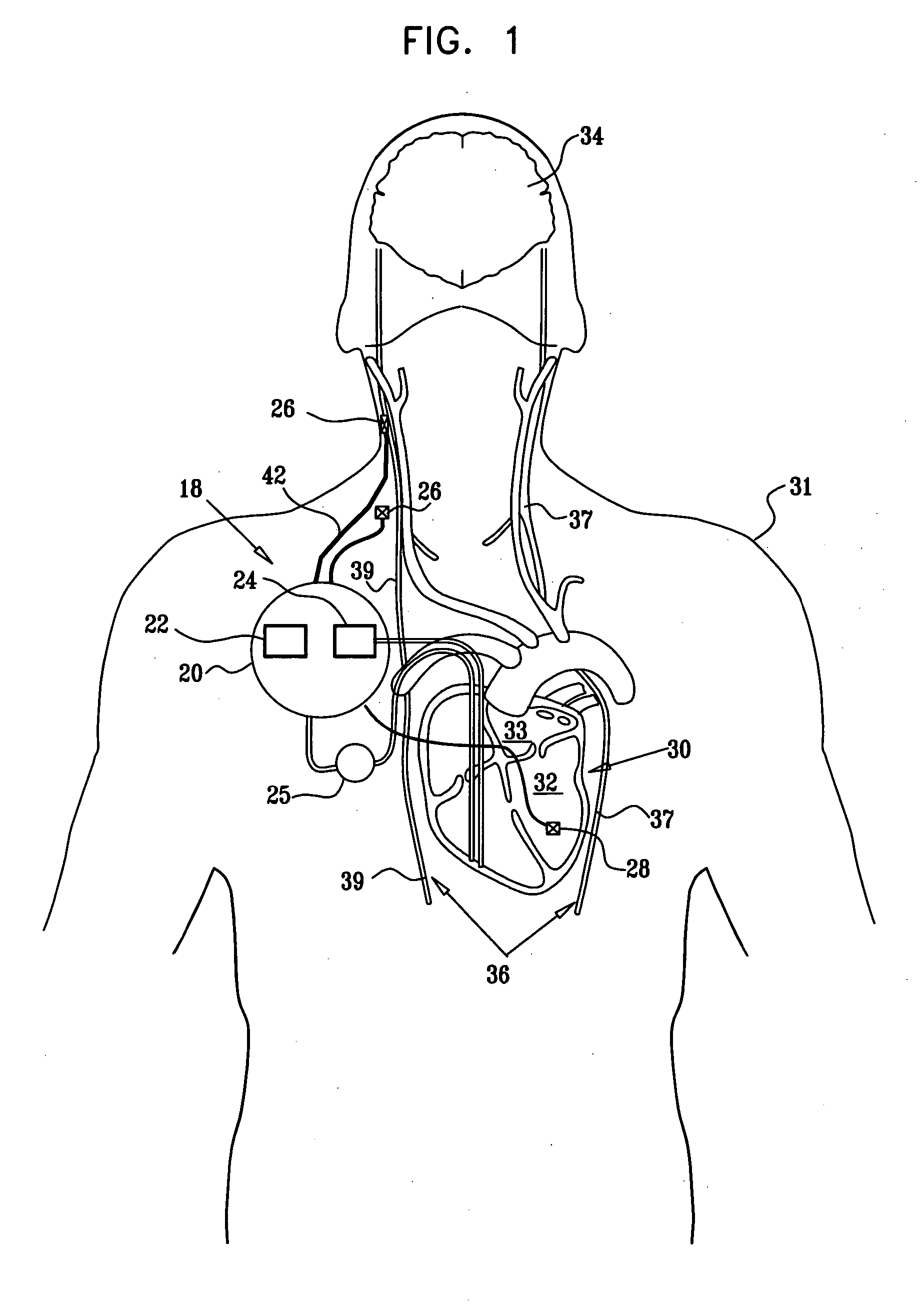
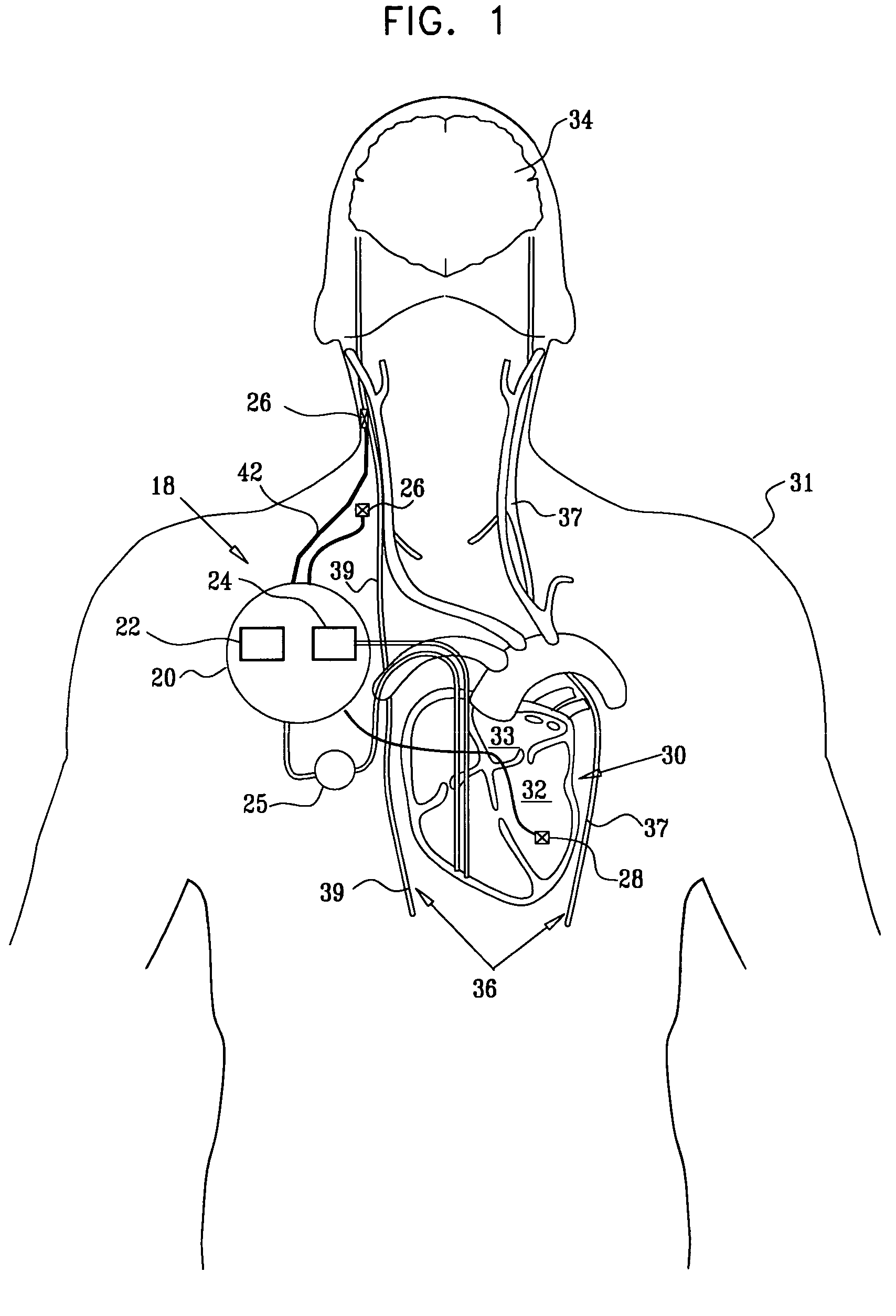
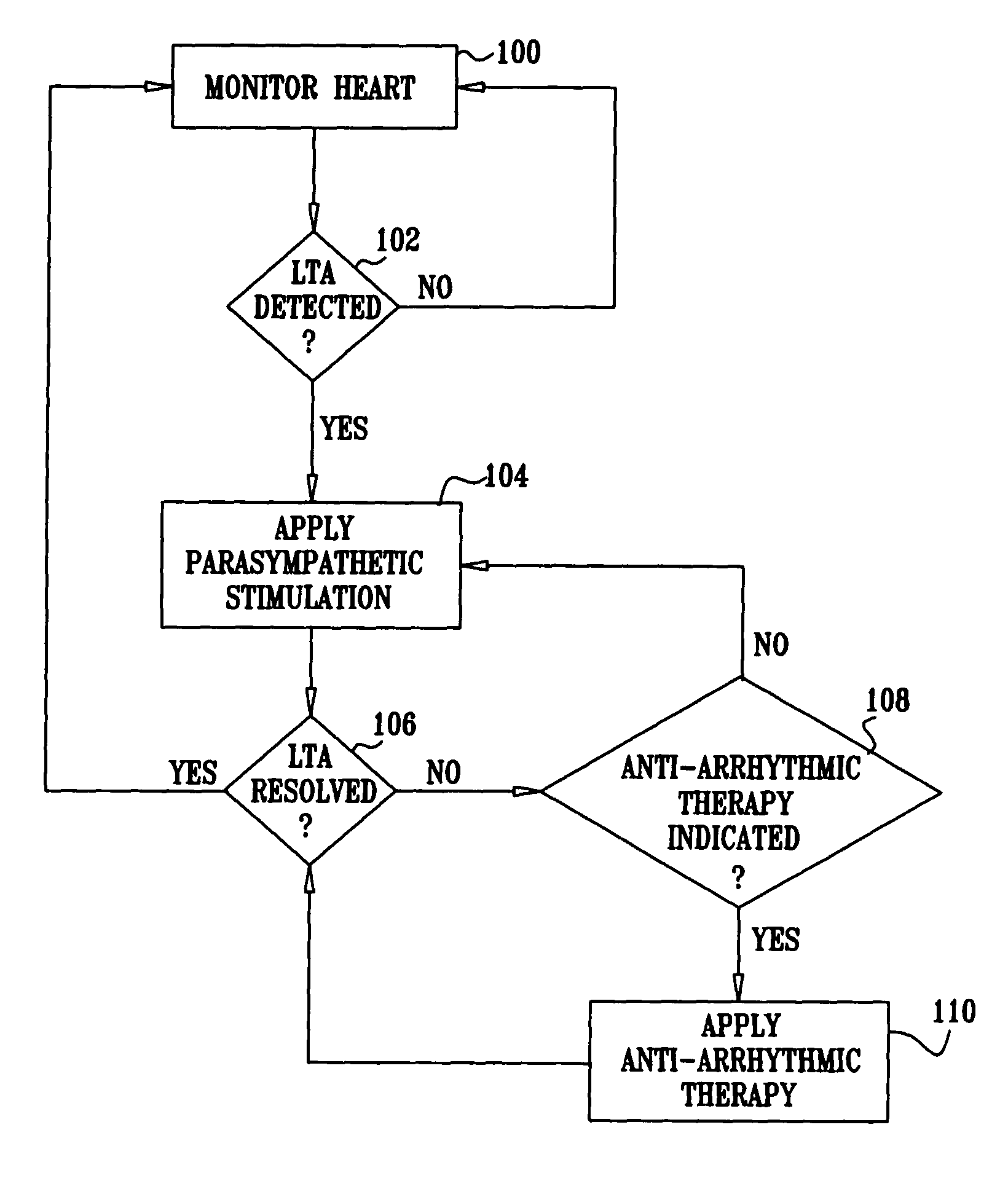
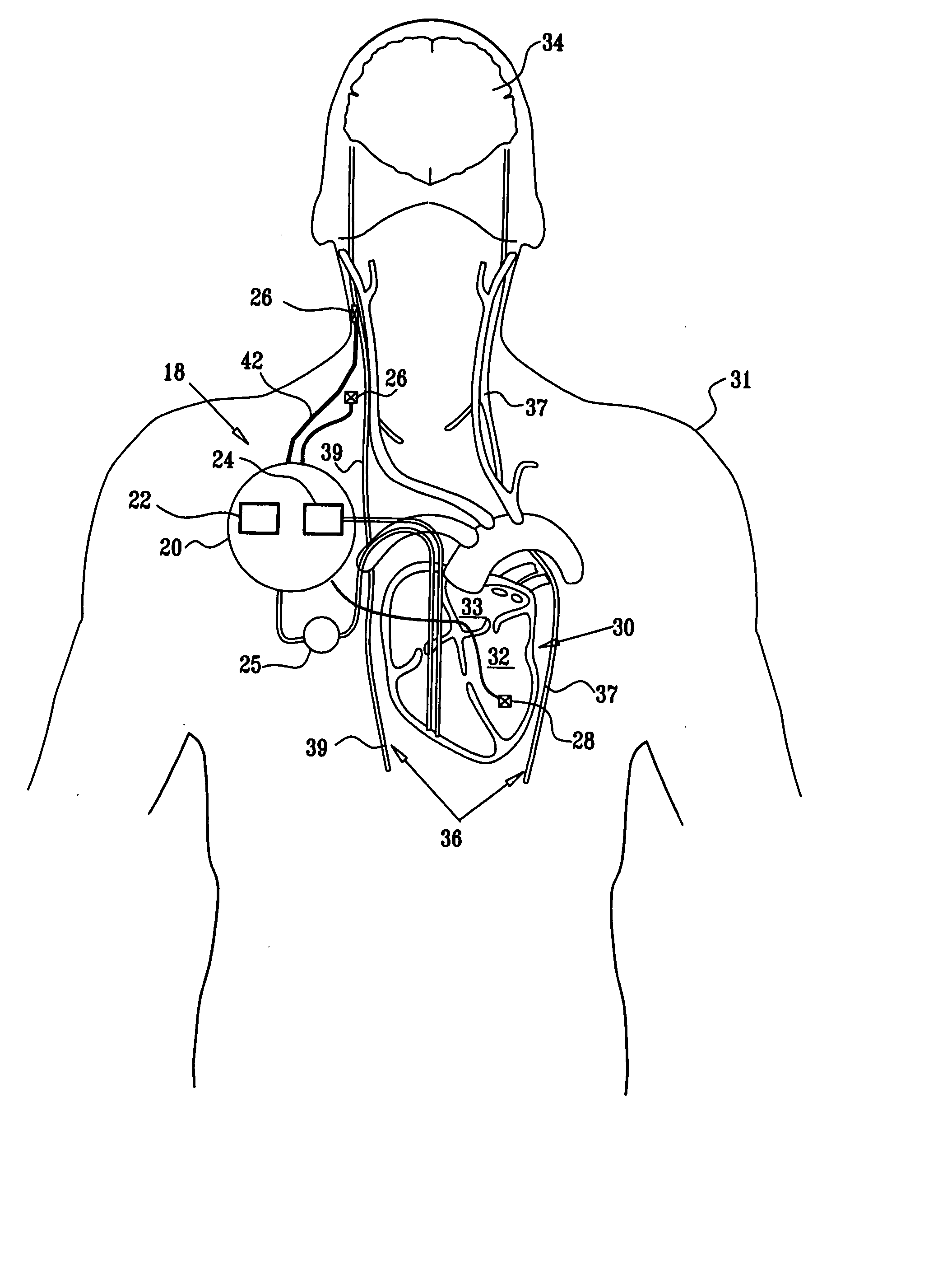
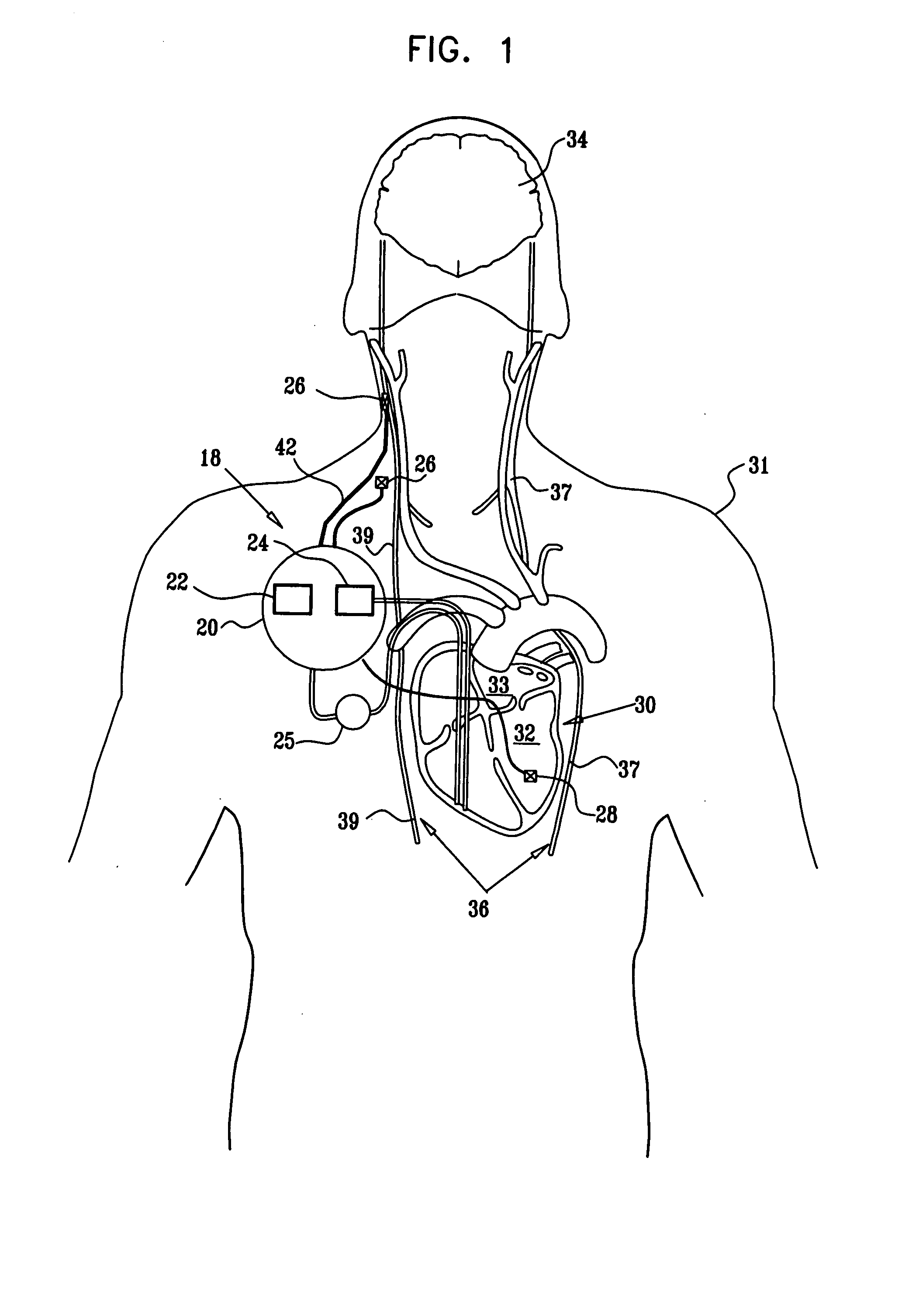
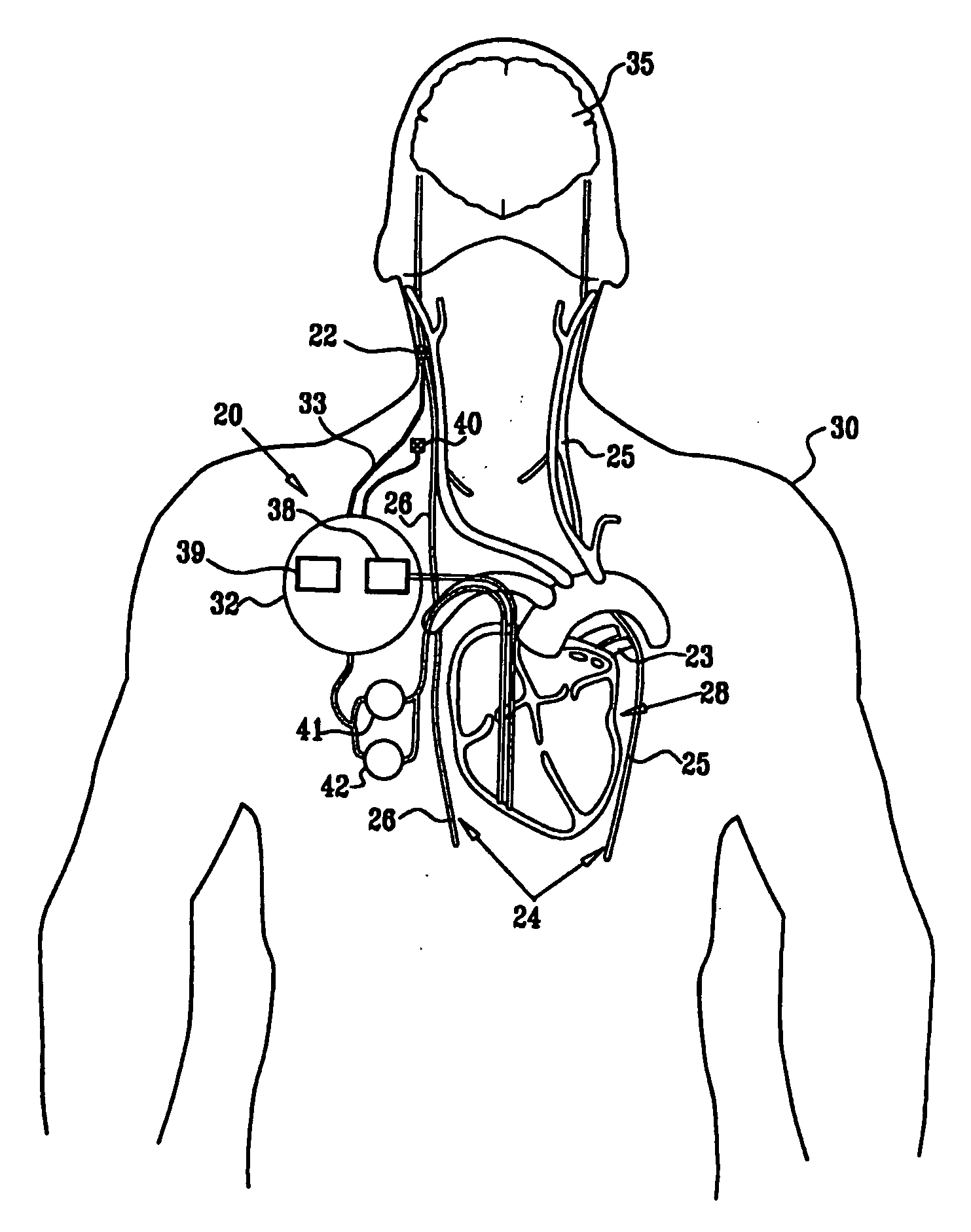
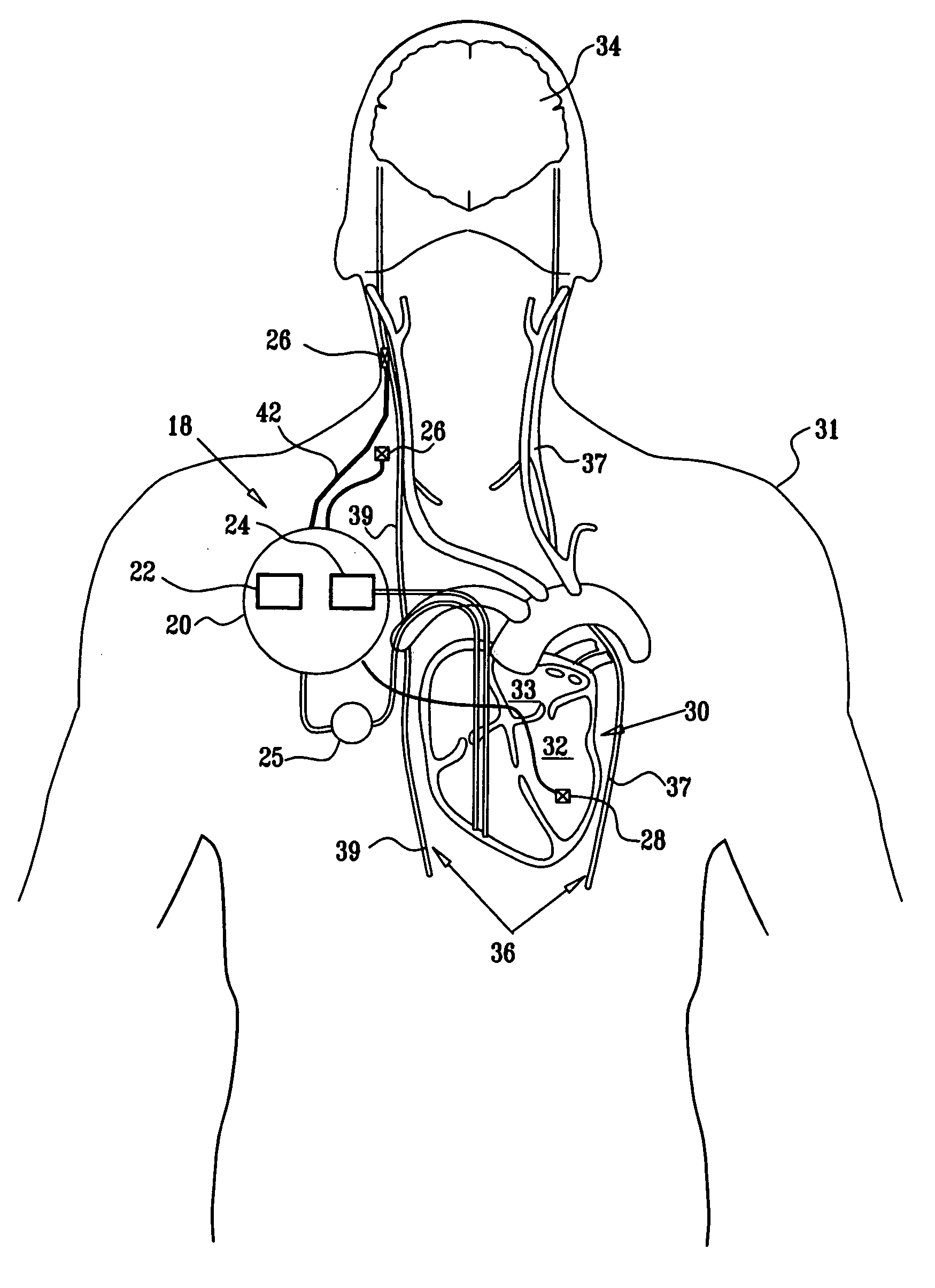
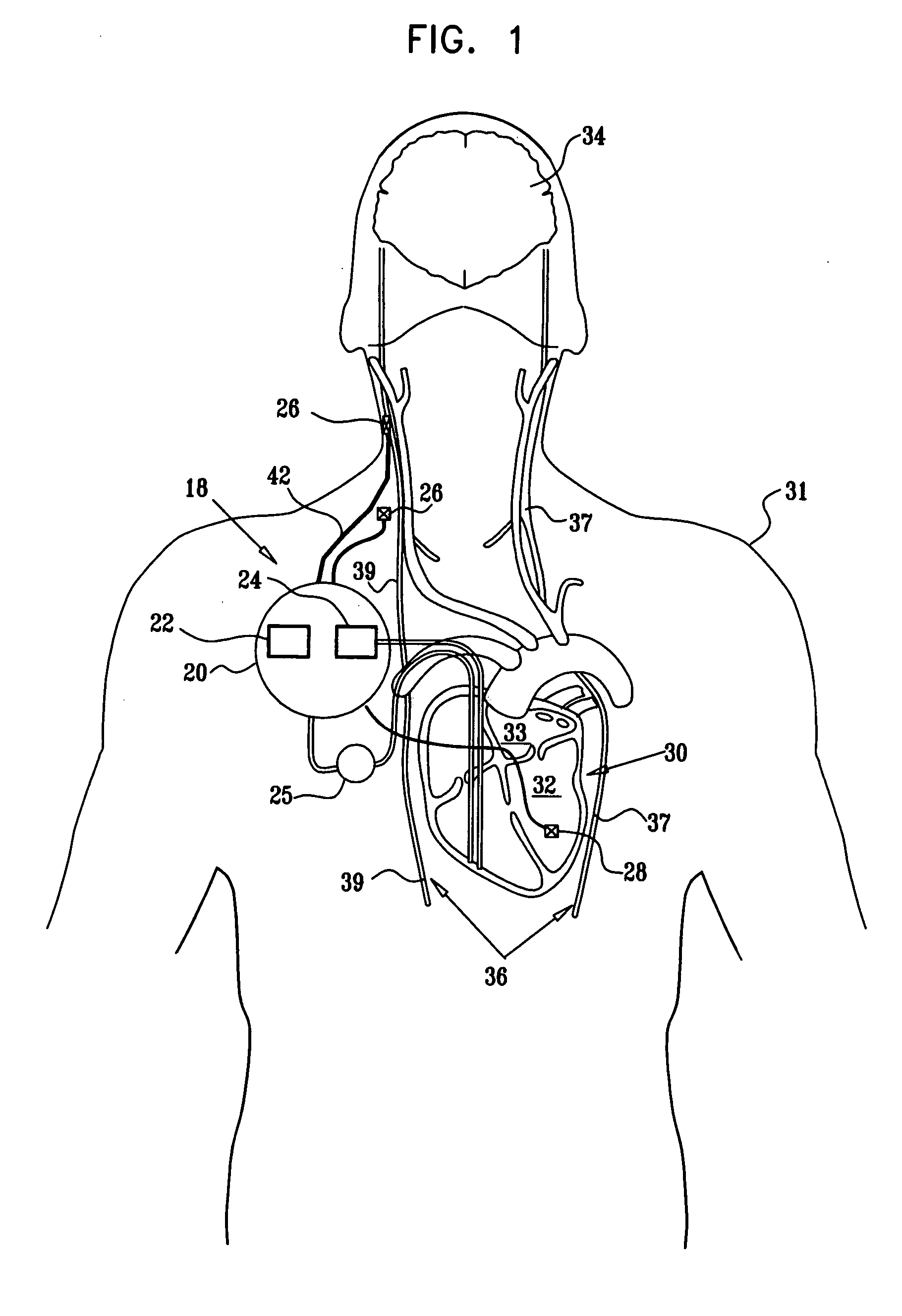
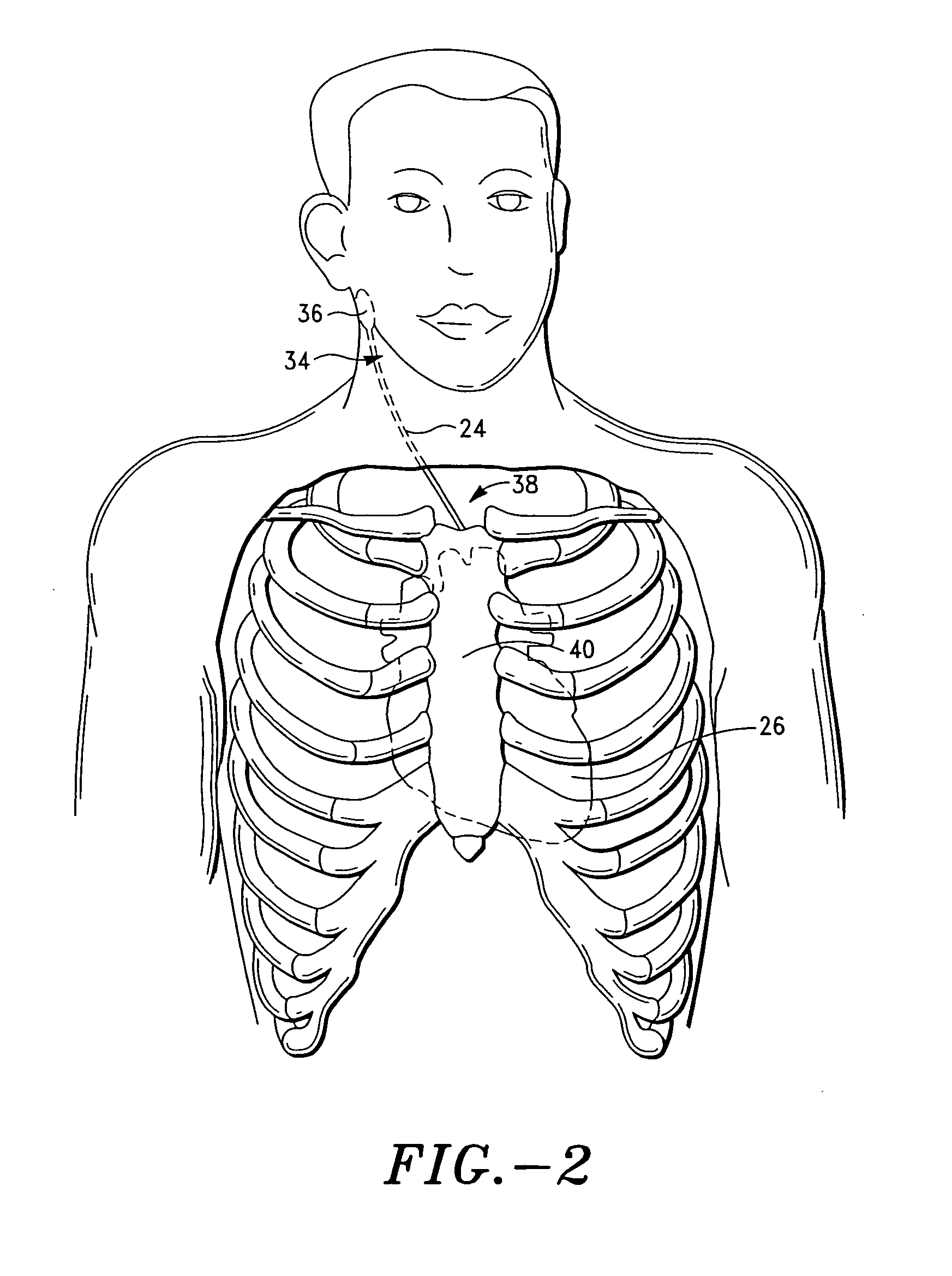
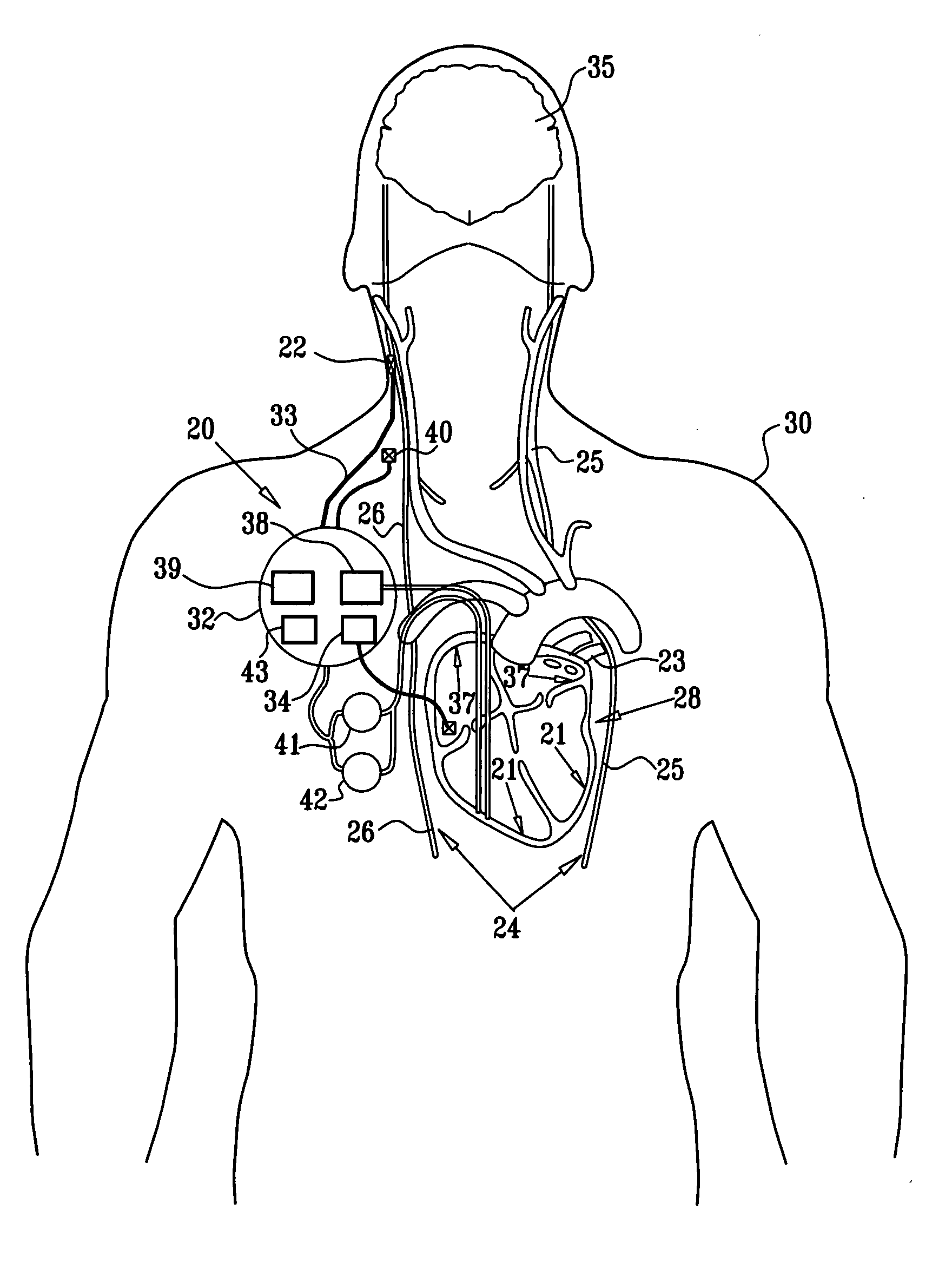
Apparatus is provided including an implantable sensor, adapted to sense an electrical parameter of a heart of a subject, and a first control unit, adapted to apply pulses to the heart responsively to the sensed parameter, the pulses selected from the list consisting of: pacing pulses and anti-arrhythmic energy. The apparatus further includes an electrode device, adapted to be coupled to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a right ventricle of the subject, and a jugular vein of the subject; and a second control unit, adapted to drive the electrode device to apply to the site a current that increases parasympathetic tone of the subject and affects a heart rate of the subject. The first and second control units are not under common control. At least one of the control units is adapted to coordinate an aspect of its operation with an aspect of operation of the other control unit. Other embodiments are also described.
Owner:MEDTRONIC INC
Parasympathetic pacing therapy during and following a medical procedure, clinical trauma or pathology
ActiveUS20060206155A1Increase parasympathetic toneReduced responseSpinal electrodesHeart stimulatorsParasympathetic ganglionPathology diagnosis
A treatment method is provided, including identifying a subject as one who is selected to undergo an interventional medical procedure, and, in response to the identifying, reducing a likelihood of a potential adverse effect of the procedure by applying an electrical current to a parasympathetic site of the subject selected from the group consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a jugular vein of the subject, a right ventricle of the subject, a parasympathetic ganglion of the subject, and a parasympathetic nerve of the subject.
Owner:MEDTRONIC INC
Techniques for reducing pain associated with nerve stimulation
Apparatus is provided including an electrode device and a control unit. The electrode device is configured to be coupled to a site of a subject selected from the group consisting of: a vagus nerve, an epicardial fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a right ventricle, a right atrium, and a jugular vein. The control unit is configured to drive the electrode device to apply to the site a current in at least first and second bursts, the first burst including a plurality of pulses, and the second burst including at least one pulse, and set (a) a pulse repetition interval (PRI) of the first burst to be on average at least 20 ms, (b) an interburst interval between initiation of the first burst and initiation of the second burst to be less than 10 seconds, (c) an interburst gap between a conclusion of the first burst and the initiation of the second burst to have a duration greater than the average PRI, and (d) a burst duration of the first burst to be less than a percentage of the interburst interval between, the percentage being less than 67%. Other embodiments are also described.
Owner:MEDTRONIC INC
Stimulus regimens for cardiovascular reflex control
ActiveUS20050251212A1Reduces excessive blood pressureDeleterious effectElectrotherapyAngle modulation detailsNervous systemRegimen
Devices, systems and methods by which the blood pressure, nervous system activity, and neurohormonal activity may be selectively and controllably reduced by activating baroreceptors. A baroreceptor activation device is positioned near a baroreceptor, for example a baroreceptor in the carotid sinus. A control system may be used to modulate the baroreceptor activation device. The control system may utilize an algorithm defining a stimulus regimen which promotes long term efficacy and reduces power requirements / consumption.
Owner:CVRX
Stimulus regimens for cardiovascular reflex control
InactiveUS7840271B2Reduce pressureDeleterious effectElectrotherapyAngle modulation detailsNervous systemRegimen
Devices, systems and methods by which the blood pressure, nervous system activity, and neurohormonal activity may be selectively and controllably reduced by activating baroreceptors. A baroreceptor activation device is positioned near a baroreceptor, for example a baroreceptor in the carotid sinus. A control system may be used to modulate the baroreceptor activation device. The control system may utilize an algorithm defining a stimulus regimen which promotes long term efficacy and reduces power requirements / consumption.
Owner:CVRX
Techniques for reducing pain associated with nerve stimulation
Apparatus is provided including an electrode device and a control unit. The electrode device is configured to be coupled to a site of a subject selected from the group consisting of: a vagus nerve, an epicardial fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a right ventricle, a right atrium, and a jugular vein. The control unit is configured to drive the electrode device to apply to the site a current in at least first and second bursts, the first burst including a plurality of pulses, and the second burst including at least one pulse, and set (a) a pulse repetition interval (PRI) of the first burst to be on average at least 20 ms, (b) an interburst interval between initiation of the first burst and initiation of the second burst to be less than 10 seconds, (c) an interburst gap between a conclusion of the first burst and the initiation of the second burst to have a duration greater than the average PRI, and (d) a burst duration of the first burst to be less than a percentage of the interburst interval between, the percentage being less than 67%. Other embodiments are also described.
Owner:MEDTRONIC INC
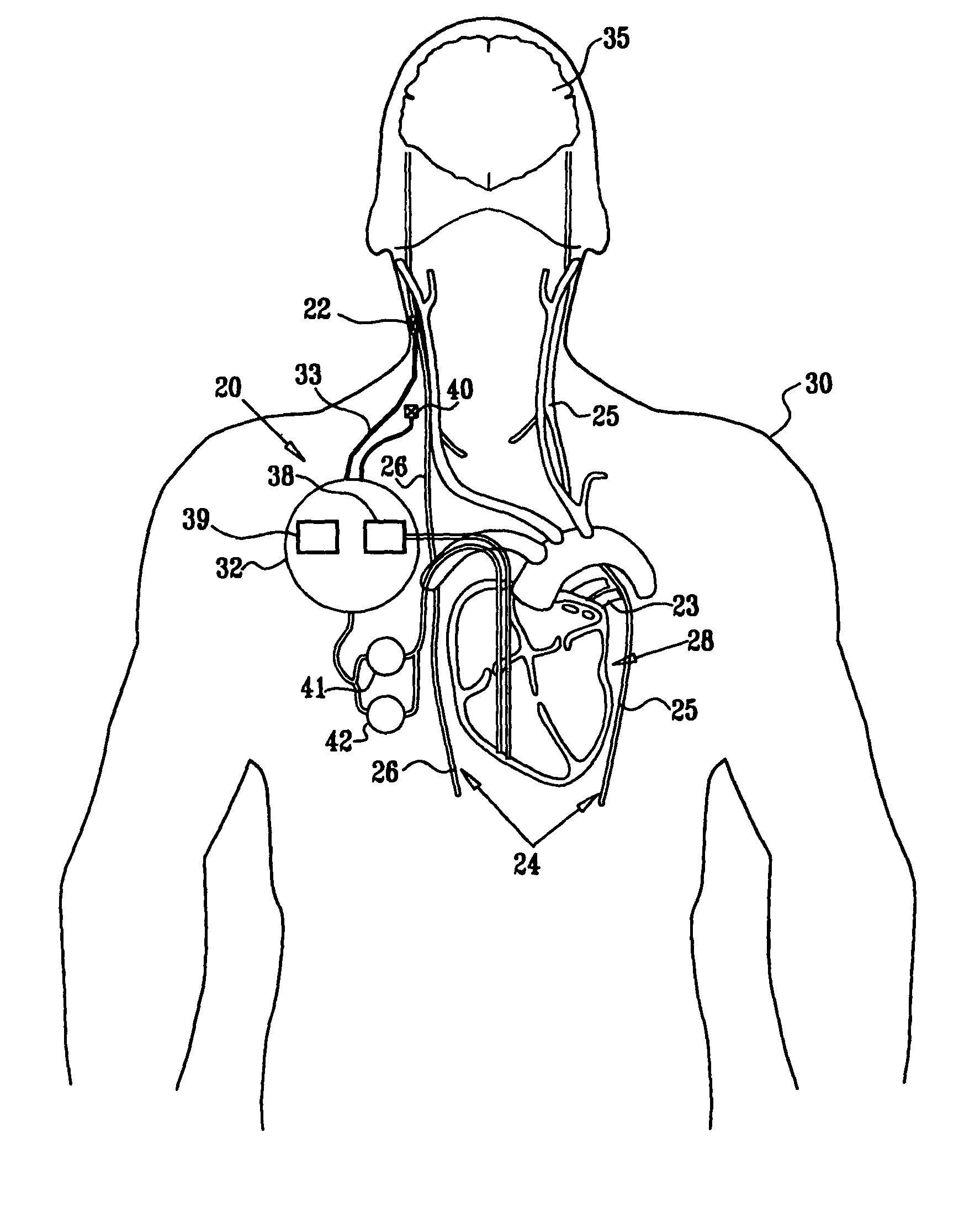
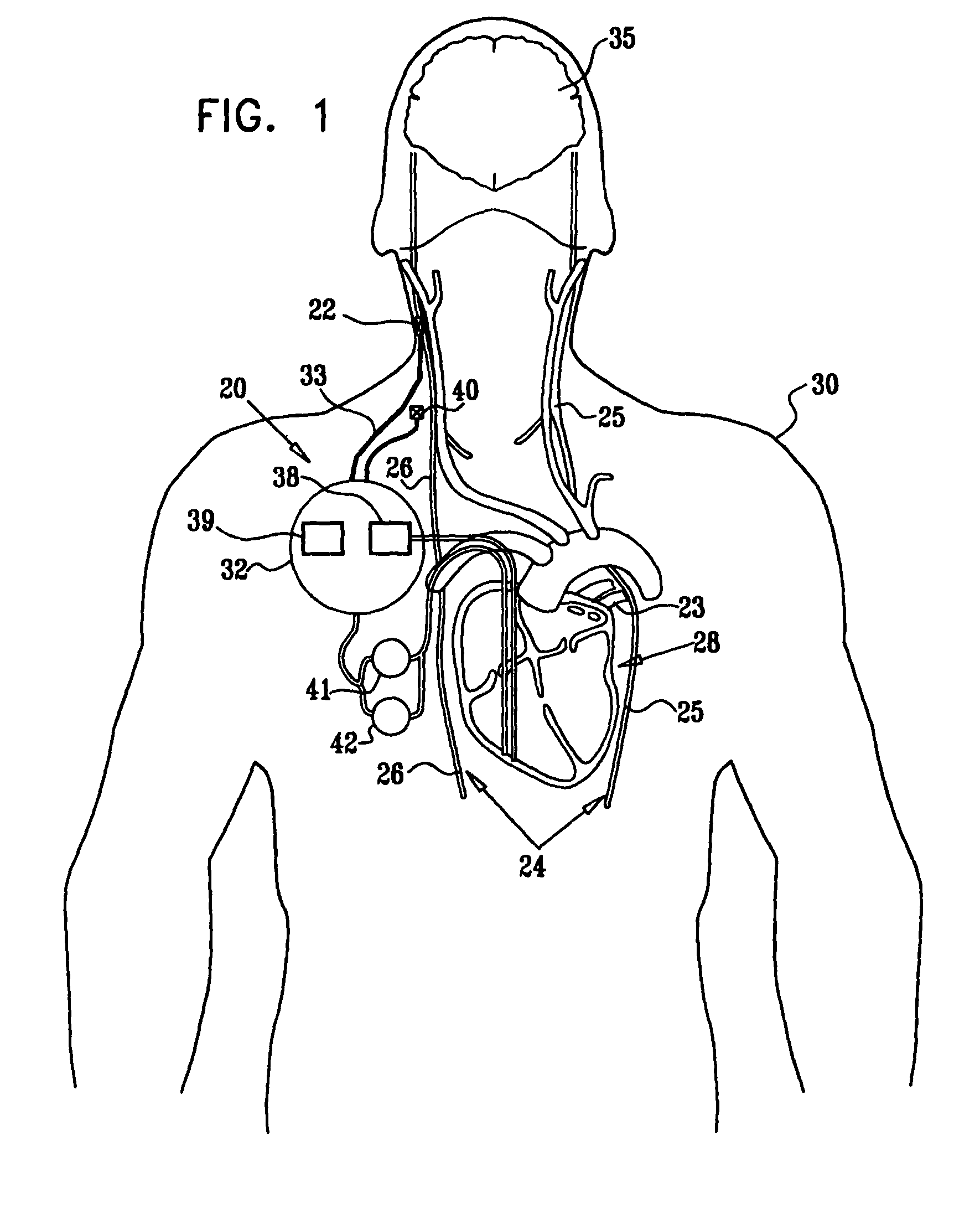
Vagal stimulation for anti-embolic therapy
InactiveUS20060271115A1Increase volumeIncrease stimulationSpinal electrodesHeart defibrillatorsEmbolization TherapyBlood flow
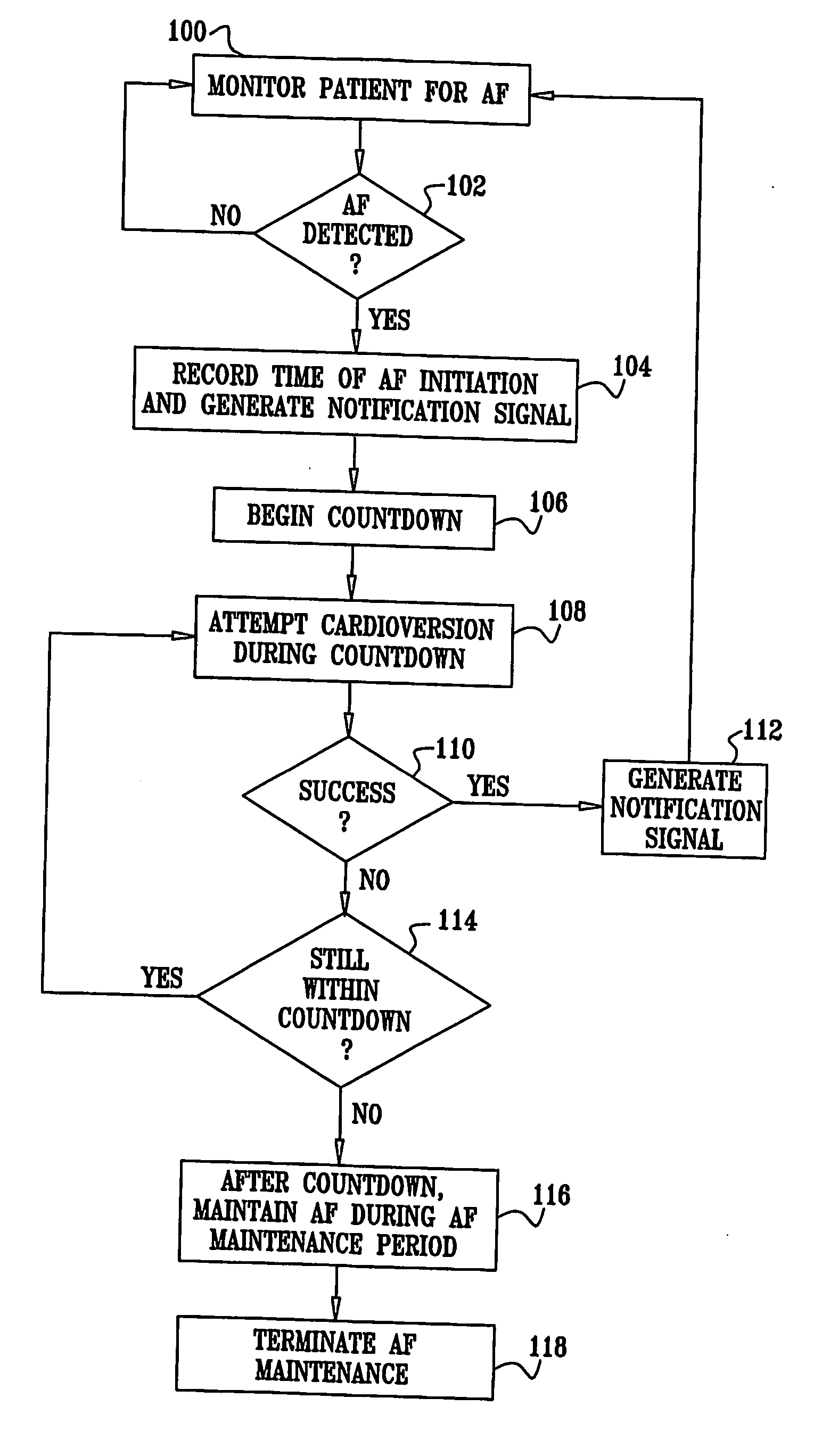
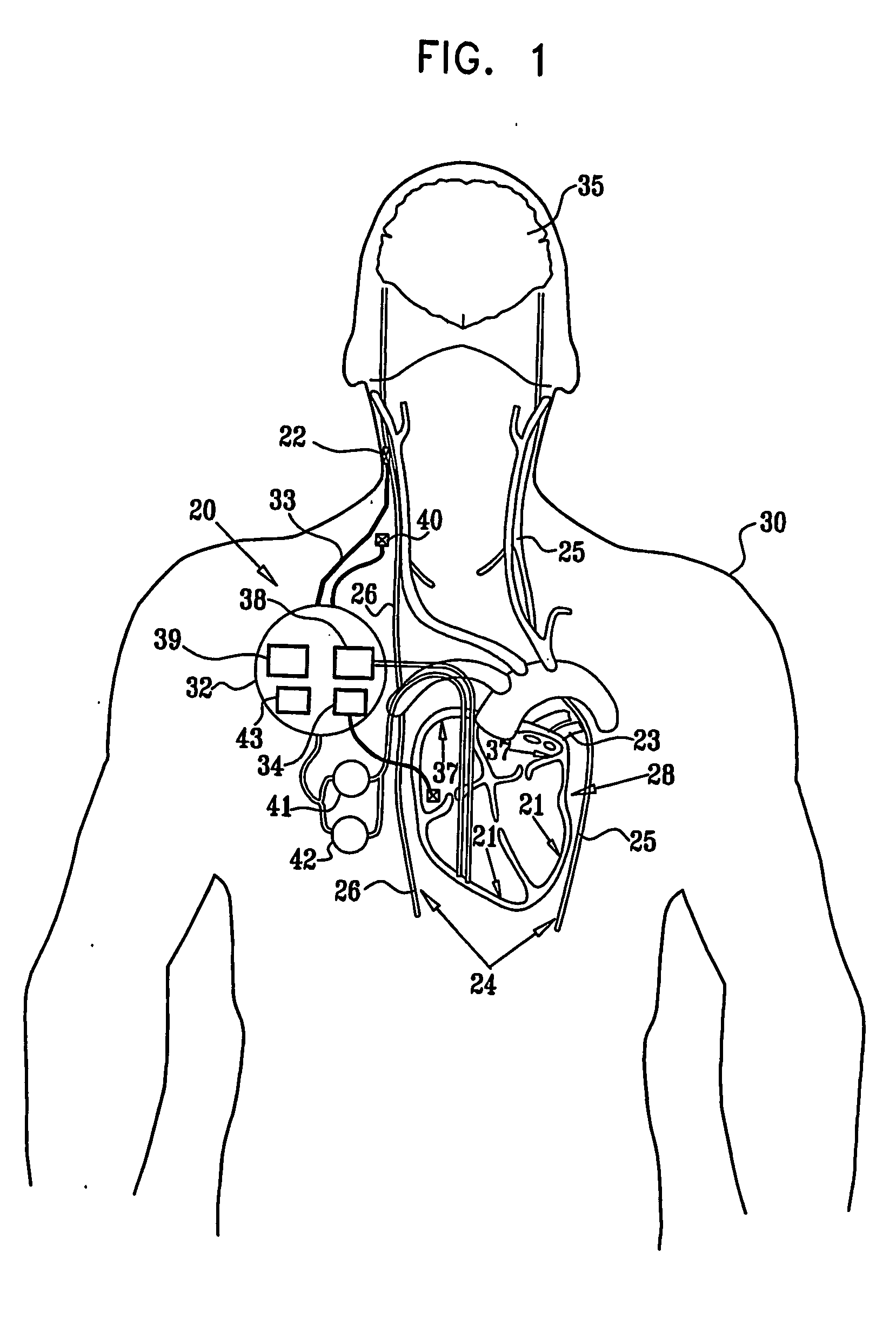
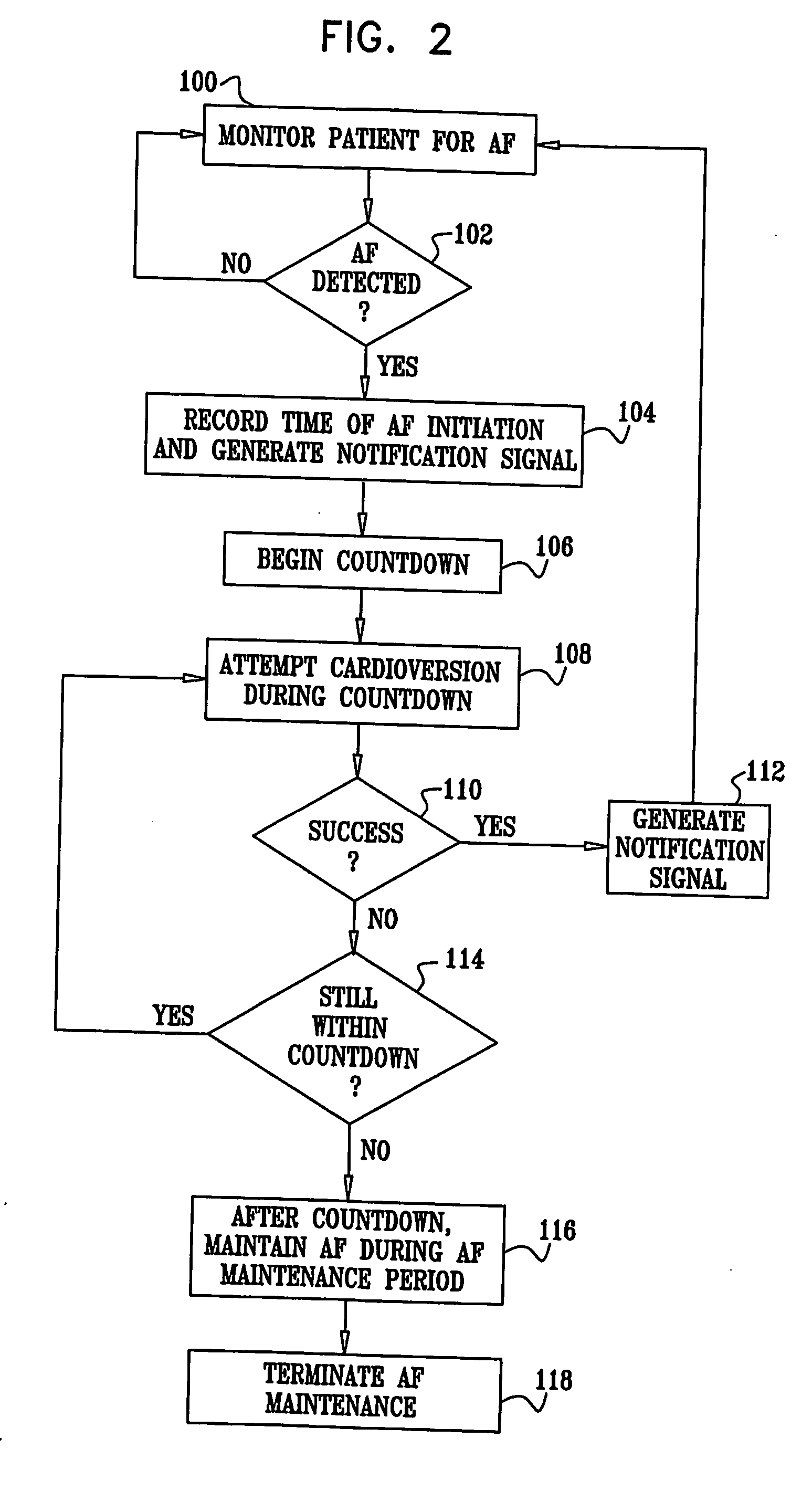
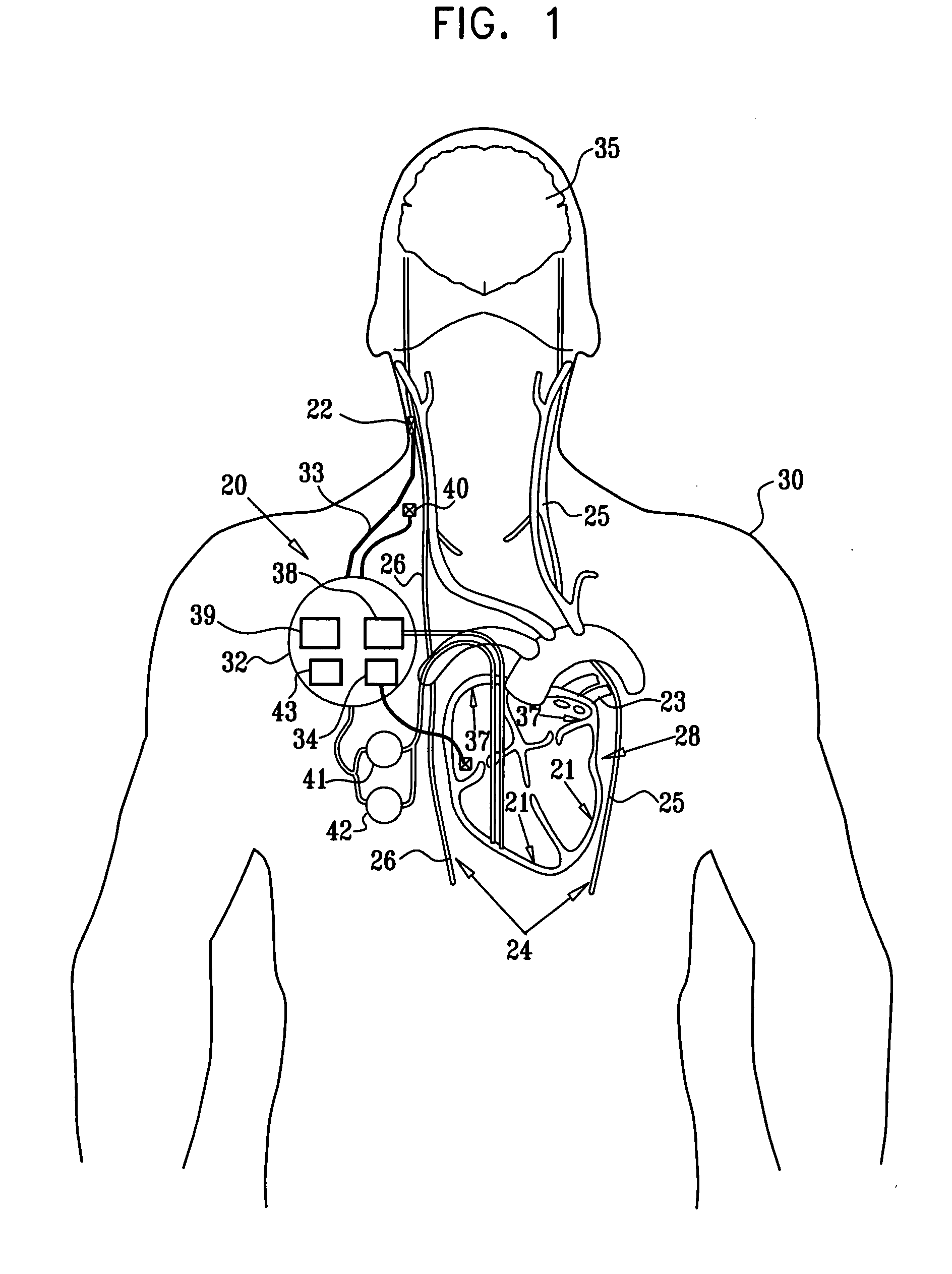
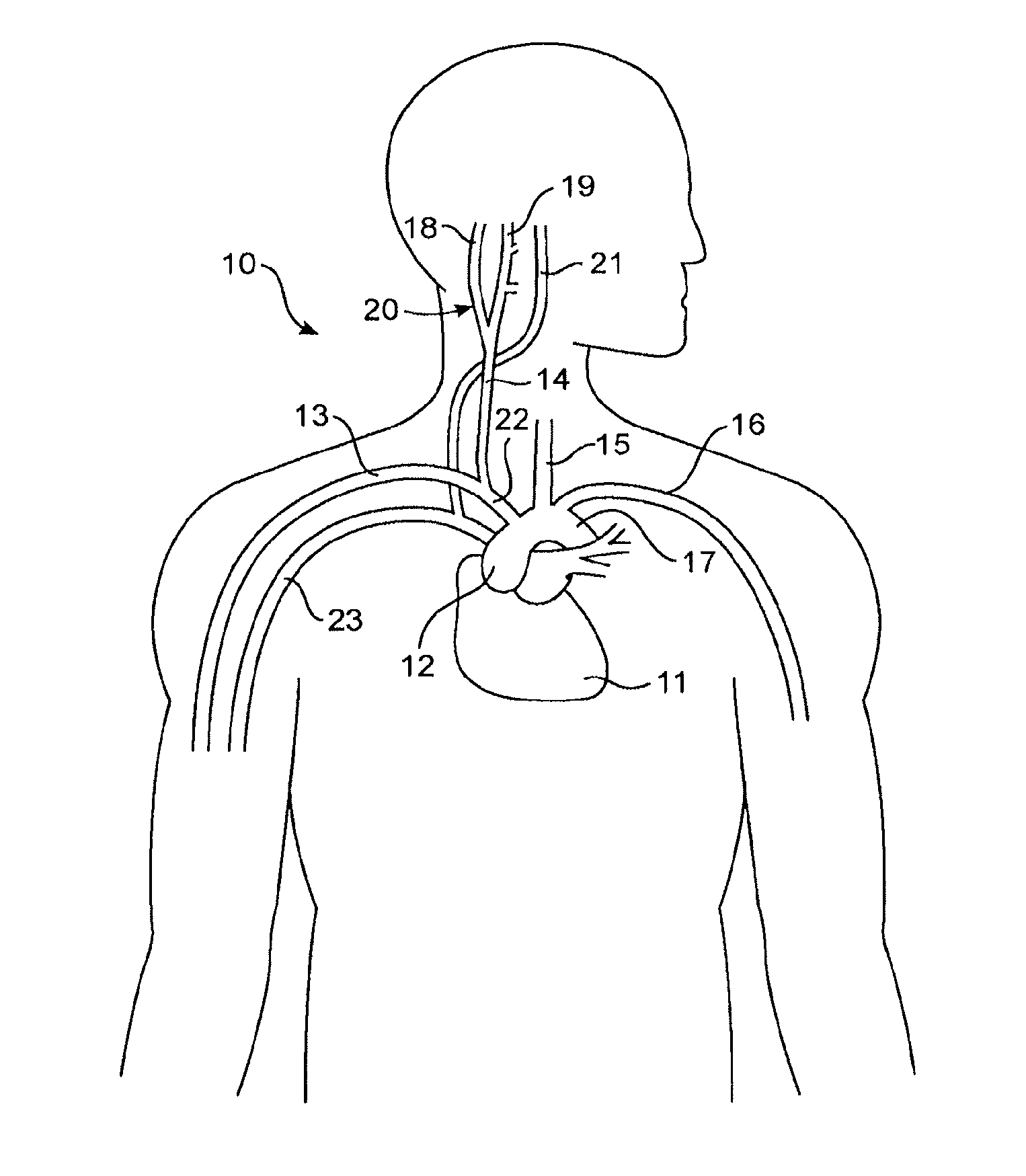
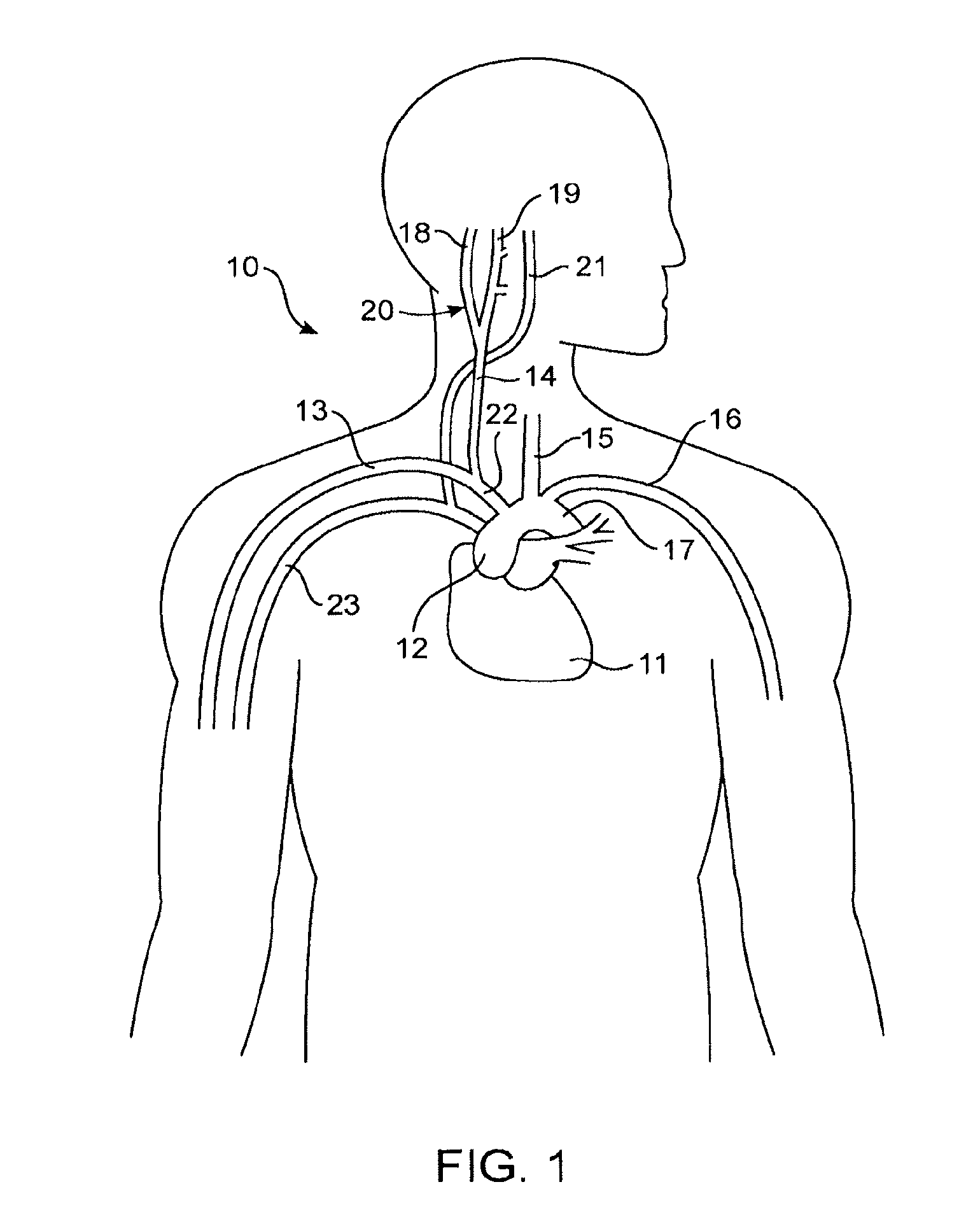
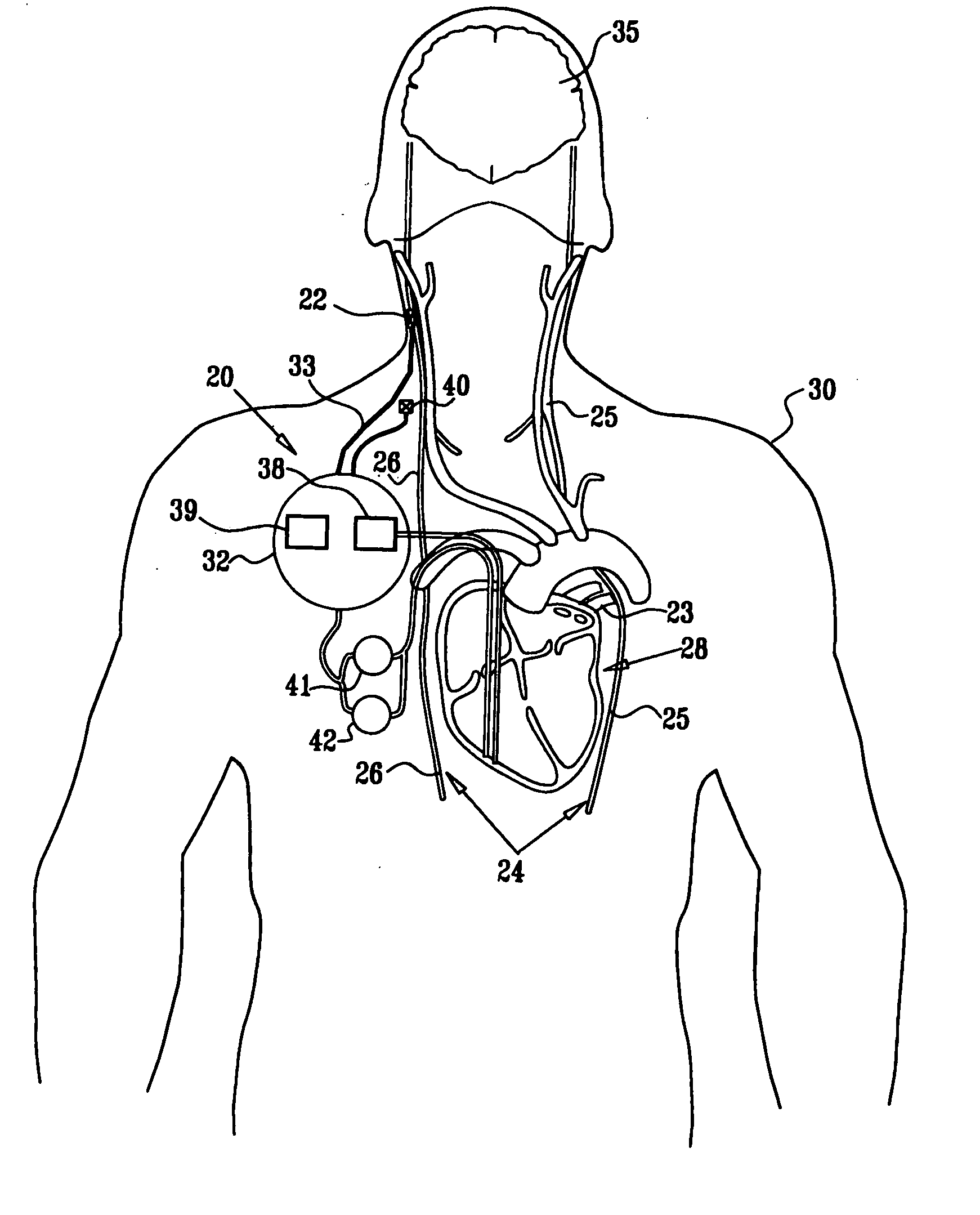
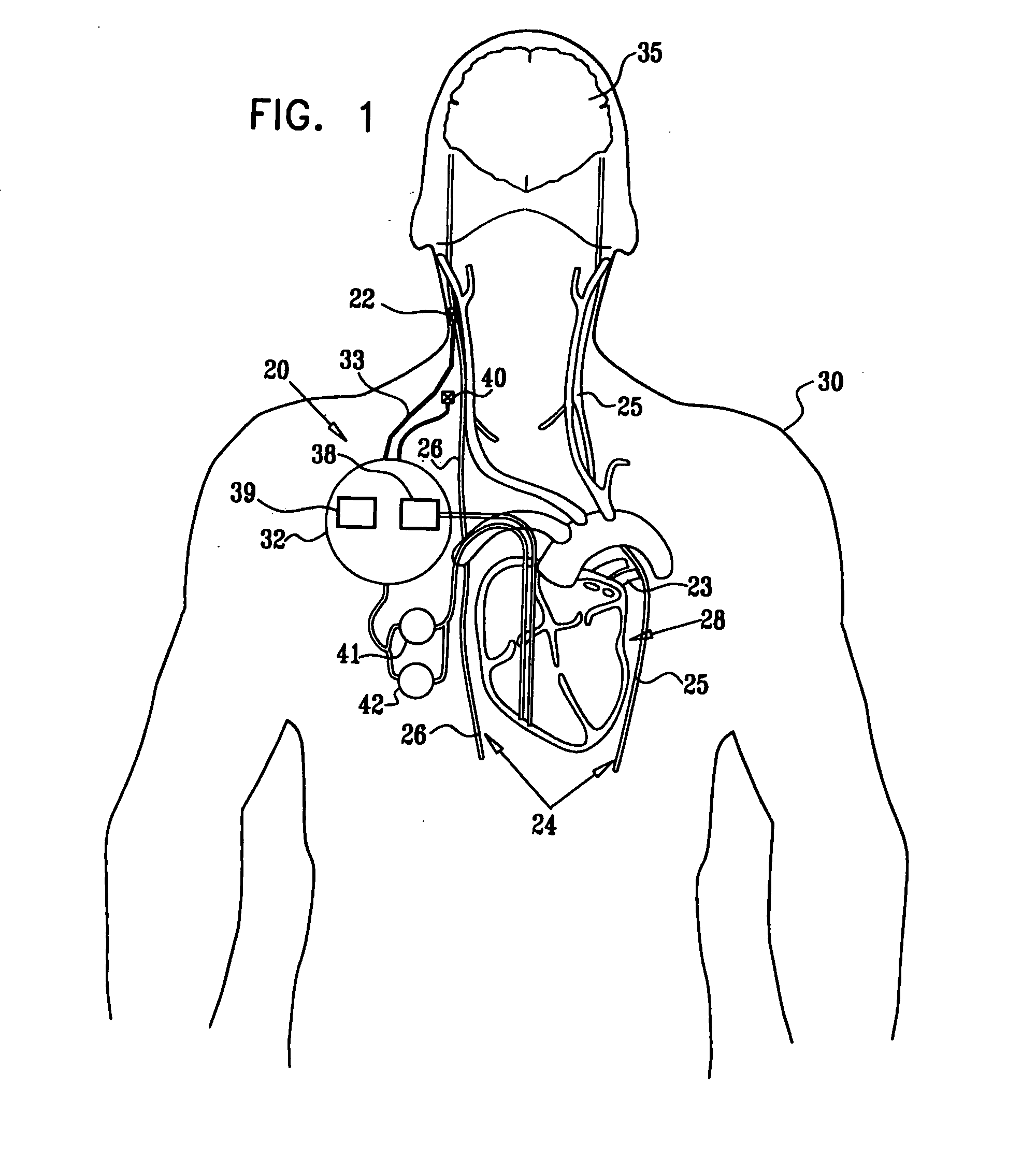
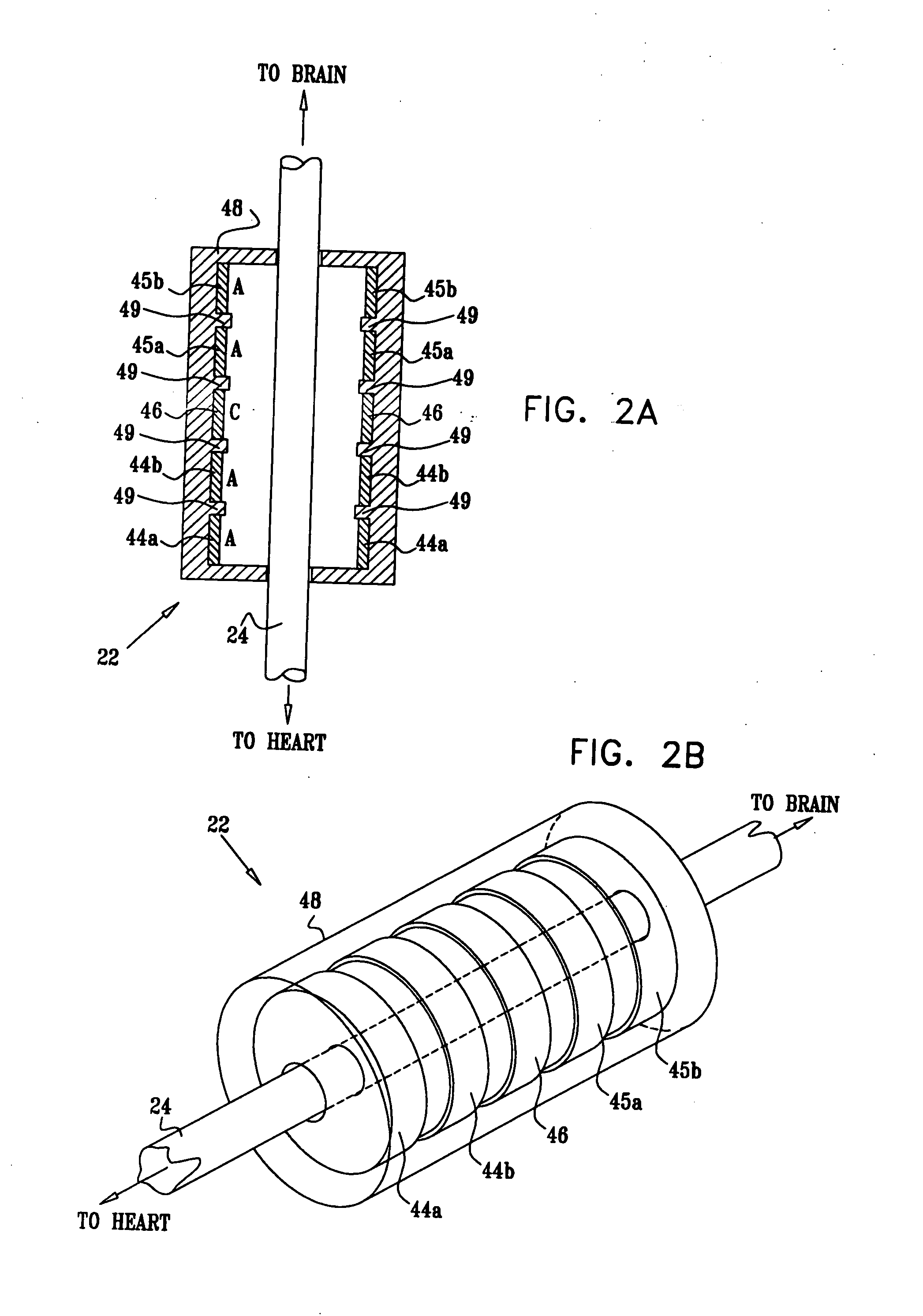
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
Parasympathetic stimulation for treating ventricular arrhythmia
InactiveUS7904151B2Reduce riskFew potential side effectHeart defibrillatorsCardiac arrhythmiaCarotid sinus
Apparatus is provided including an implantable sensor, adapted to sense an electrical parameter of a heart of a subject, and a first control unit, adapted to apply pulses to the heart responsively to the sensed parameter, the pulses selected from the list consisting of: pacing pulses and anti-arrhythmic energy. The apparatus further includes an electrode device, adapted to be coupled to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a right ventricle of the subject, and a jugular vein of the subject; and a second control unit, adapted to drive the electrode device to apply to the site a current that increases parasympathetic tone of the subject and affects a heart rate of the subject. The first and second control units are not under common control. At least one of the control units is adapted to coordinate an aspect of its operation with an aspect of operation of the other control unit. Other embodiments are also described.
Owner:MEDTRONIC INC
Parasympathetic stimulation for prevention and treatment of atrial fibrillation
A method is provided, including identifying that a subject is at risk of suffering from atrial fibrillation (AF). Responsively to the identifying, a risk of an occurrence of an episode of the AF is reduced by applying an electrical current to a site of the subject selected from the group consisting of: a vagus nerve, a sinoatrial (SA) node fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a jugular vein, an azygos vein, an innominate vein, and a subclavian vein, and configuring the current to stimulate autonomic nervous tissue in the site. Other embodiments are also described.
Owner:MEDTRONIC INC
Parasympathetic stimulation for treating ventricular arrhythmia
InactiveUS20080091240A1Decreased heart rateEliminate side effectsHeart stimulatorsCardiac arrhythmiaCarotid sinus
Apparatus is provided including an implantable sensor, adapted to sense an electrical parameter of a heart of a subject, and a first control unit, adapted to apply pulses to the heart responsively to the sensed parameter, the pulses selected from the list consisting of: pacing pulses and anti-arrhythmic energy. The apparatus further includes an electrode device, adapted to be coupled to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a right ventricle of the subject, and a jugular vein of the subject; and a second control unit, adapted to drive the electrode device to apply to the site a current that increases parasympathetic tone of the subject and affects a heart rate of the subject. The first and second control units are not under common control. At least one of the control units is adapted to coordinate an aspect of its operation with an aspect of operation of the other control unit. Other embodiments are also described.
Owner:MEDTRONIC INC
Shockwave nerve therapy system and method
ActiveUS20140046229A1Reduce and block activationModulate neural activitySurgeryChiropractic devicesBaroreceptor functionBiological activation
Disclosed herein are intravascular systems and methods for modulating the activation of neural activity using shock wave devices. Such systems and methods may be used to modulate the activity of the renal plexus and / or baroreceptors of the carotid sinus for the treatment of hypertension.
Owner:SHOCKWAVE MEDICAL
Techniques for prevention of atrial fibrillation
A method is provided, including identifying that a subject is at risk of suffering from atrial fibrillation (AF). Responsively to the identifying, a risk of an occurrence of an episode of the AF is reduced by applying an electrical current to a site of the subject selected from the group consisting of: a vagus nerve, a sinoatrial (SA) node fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a jugular vein, an azygos vein, an innominate vein, and a subclavian vein, and configuring the current to stimulate autonomic nervous tissue in the site. Other embodiments are also described.
Owner:MEDTRONIC INC
Techniques for prevention of atrial fibrillation
ActiveUS20070179543A1Prevent electrical remodelingReduce riskSpinal electrodesHeart defibrillatorsInnominate veinBiology
A method is provided, including identifying that a subject is at risk of suffering from atrial fibrillation (AF). Responsively to the identifying, a risk of an occurrence of an episode of the AF is reduced by applying an electrical current to a site of the subject selected from the group consisting of: a vagus nerve, a sinoatrial (SA) node fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a jugular vein, an azygos vein, an innominate vein, and a subclavian vein, and configuring the current to stimulate autonomic nervous tissue in the site. Other embodiments are also described.
Owner:MEDTRONIC INC
Method for surgically implanting an electrode device
InactiveUS20080161894A1Reduce riskFew potential side effectSpinal electrodesSurgeryCarotid sinusRight ventricles
Apparatus is provided including an implantable sensor, adapted to sense an electrical parameter of a heart of a subject, and a first control unit, adapted to apply pulses to the heart responsively to the sensed parameter, the pulses selected from the list consisting of: pacing pulses and anti-arrhythmic energy. The apparatus further includes an electrode device, adapted to be coupled to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a right ventricle of the subject, and a jugular vein of the subject; and a second control unit, adapted to drive the electrode device to apply to the site a current that increases parasympathetic tone of the subject and affects a heart rate of the subject. The first and second control units are not under common control. At least one of the control units is adapted to coordinate an aspect of its operation with an aspect of operation of the other control unit. Other embodiments are also described.
Owner:MEDTRONIC INC
Device and procedure to treat cardiac atrial arrhythmias
A non-invasive vagal stimulation device and method. The device comprises a body having a vibration member. The stimulation is created by the vibration member which has a vibratory rate that can be adjusted from being off to a preferred operating range. The non-invasive stimulation method consists of placing the non-invasive stimulation device in the vicinity of the carotid artery bifrication where arises a carotid sinus and body which contain afferent sensory nerves that travel to medulla oblongata of brain, and either applying pressure in place, or moving the device along the target arm. The method can be accomplished either with the vibration feature of the device turned on or off.
Owner:NEUROSIGNAL TECH
Combined parasympathetic stimulation and drug therapy
InactiveUS20080125843A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsMedical devicesNervous systemMyelitis
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Treatment for disorders by parasympathetic stimulation
InactiveUS20080086182A1Increase parasympathetic toneReduced responseSpinal electrodesHeart defibrillatorsDiseaseParasympathetic ganglion
Owner:MEDTRONIC INC
Device and procedure to treat cardiac atrial arrhythmias
A non-invasive vagal stimulation device and method. The device comprises a body having a vibration member. The stimulation is created by the vibration member which has a vibratory rate that can be adjusted from being off to a preferred operating range. The non-invasive stimulation method consists of placing the non-invasive stimulation device in the vicinity of the carotid artery bifrication where arises a carotid sinus and body which contain afferent sensory nerves that travel to medulla oblongata of brain, and either applying pressure in place, or moving the device along the target arm. The method can be accomplished either with the vibration feature of the device turned on or off.
Owner:ORTIZ & LOPEZ PLLC
Parasympathetic stimulation for heart conditions
InactiveUS20080125819A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsHeart stimulatorsMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Vagal stimulation for cardioversion of atrial fibrillation
InactiveUS20080091241A1Reduce frequencyIncreased riskSpinal electrodesHeart defibrillatorsBlood flowThrombus
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
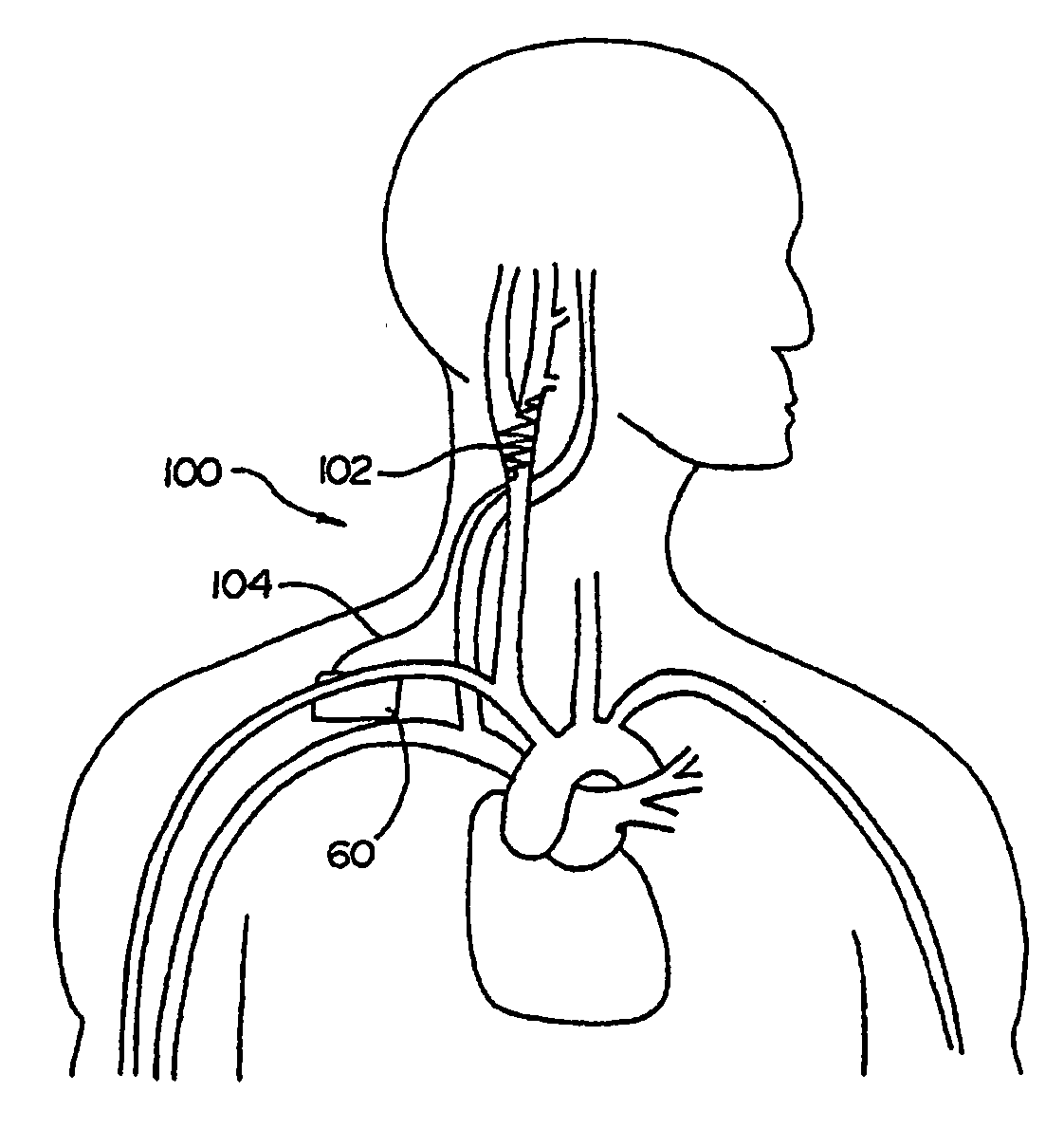
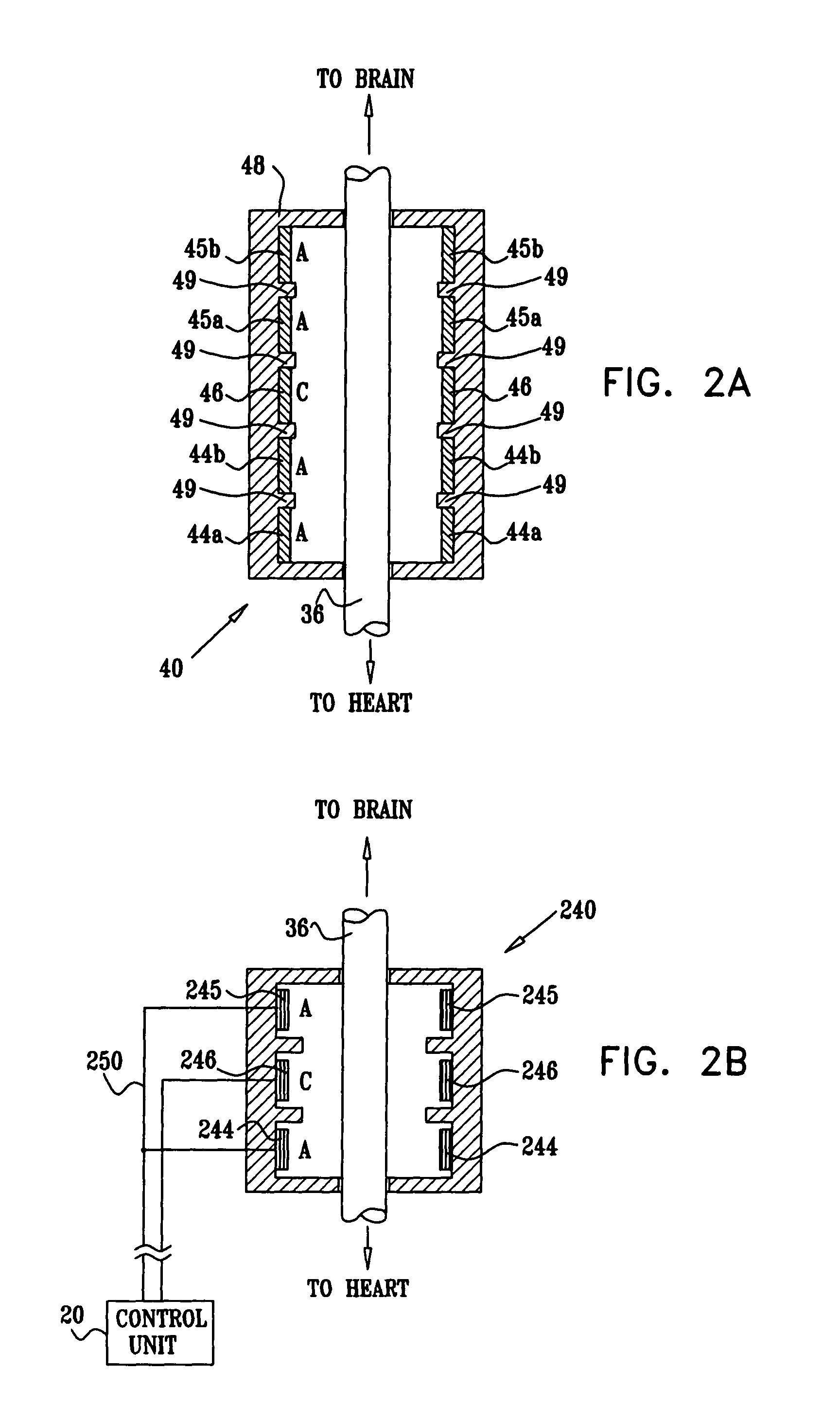
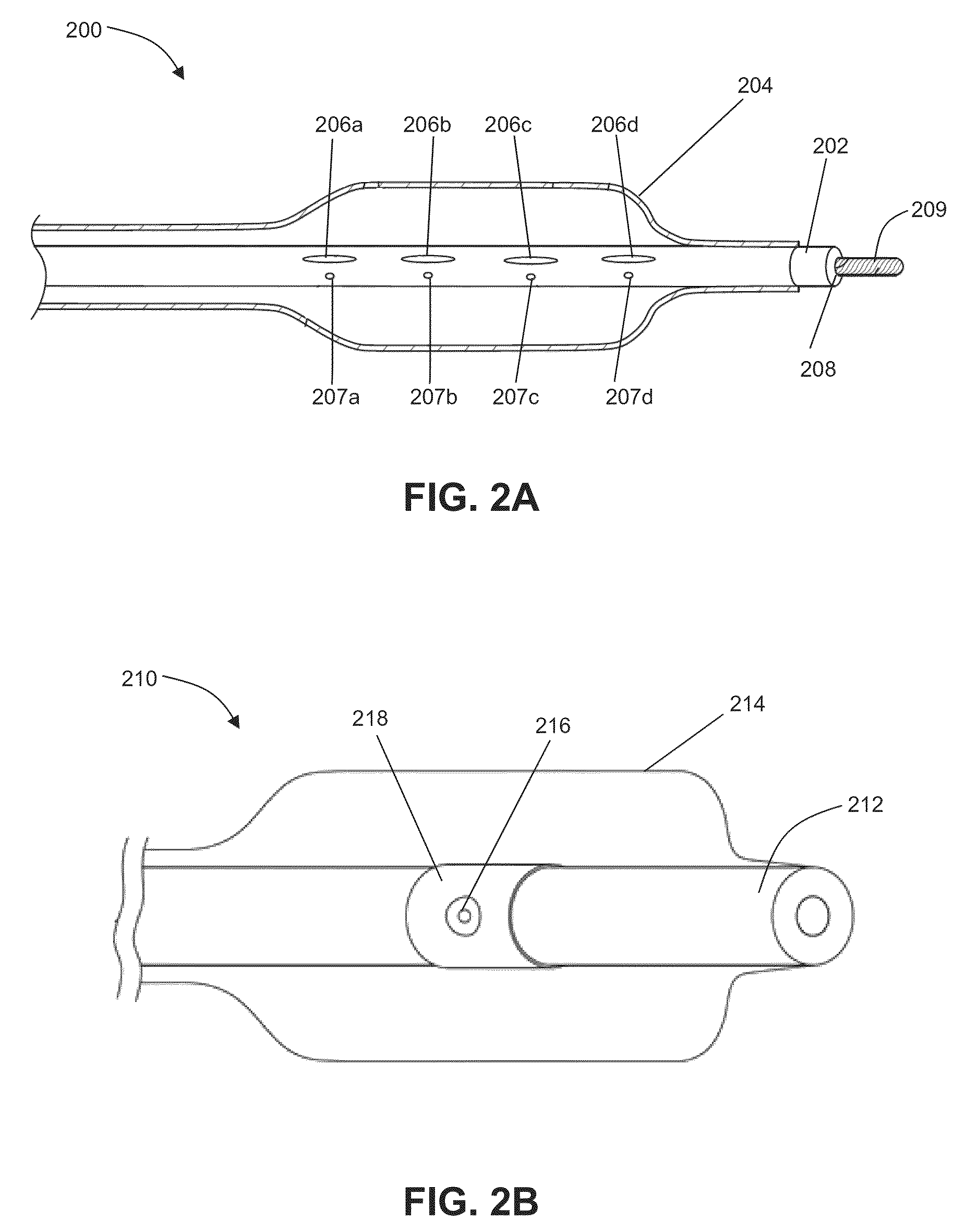
ECG input to implantable pulse generator using carotid sinus leads
InactiveUS20080097540A1Reduce pressureDeleterious effectSpinal electrodesTransvascular endocardial electrodesElectricityControl system
A monitoring device is provided to monitor a patient. The monitoring device includes at least one lead having an electrode. The lead is positioned proximate a location within the patient's body, and the lead is adapted to sense cardiac electrical activity. The monitoring device also includes a control system coupled to the at least one lead to receive a signal representative of the cardiac electrical activity.
Owner:CVRX
Techniques for prevention of atrial fibrillation
ActiveUS20080177338A1Prevent electrical remodelingReduce riskSpinal electrodesExternal electrodesInnominate veinAtrial cavity
A method is provided, including identifying that a subject is at risk of suffering from atrial fibrillation (AF). Responsively to the identifying, a risk of an occurrence of an episode of the AF is reduced by applying an electrical current to a site of the subject selected from the group consisting of: a vagus nerve, a sinoatrial (SA) node fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a jugular vein, an azygos vein, an innominate vein, and a subclavian vein, and configuring the current to stimulate autonomic nervous tissue in the site. Other embodiments are also described.
Owner:MEDTRONIC INC
Shockwave nerve therapy system and method
ActiveUS9237984B2Reduce and block activationModulate neural activitySurgeryVibration massageBaroreceptor functionBiological activation
Disclosed herein are intravascular systems and methods for modulating the activation of neural activity using shock wave devices. In one embodiment, the system is used to treat the nerves in the renal plexus. In a second embodiment, the system is used to treat the baroreceptors in the carotid sinus. In a preferred embodiment, the shock wave generator is in the form of electrodes mounted within an inflatable balloon.
Owner:SHOCKWAVE MEDICAL
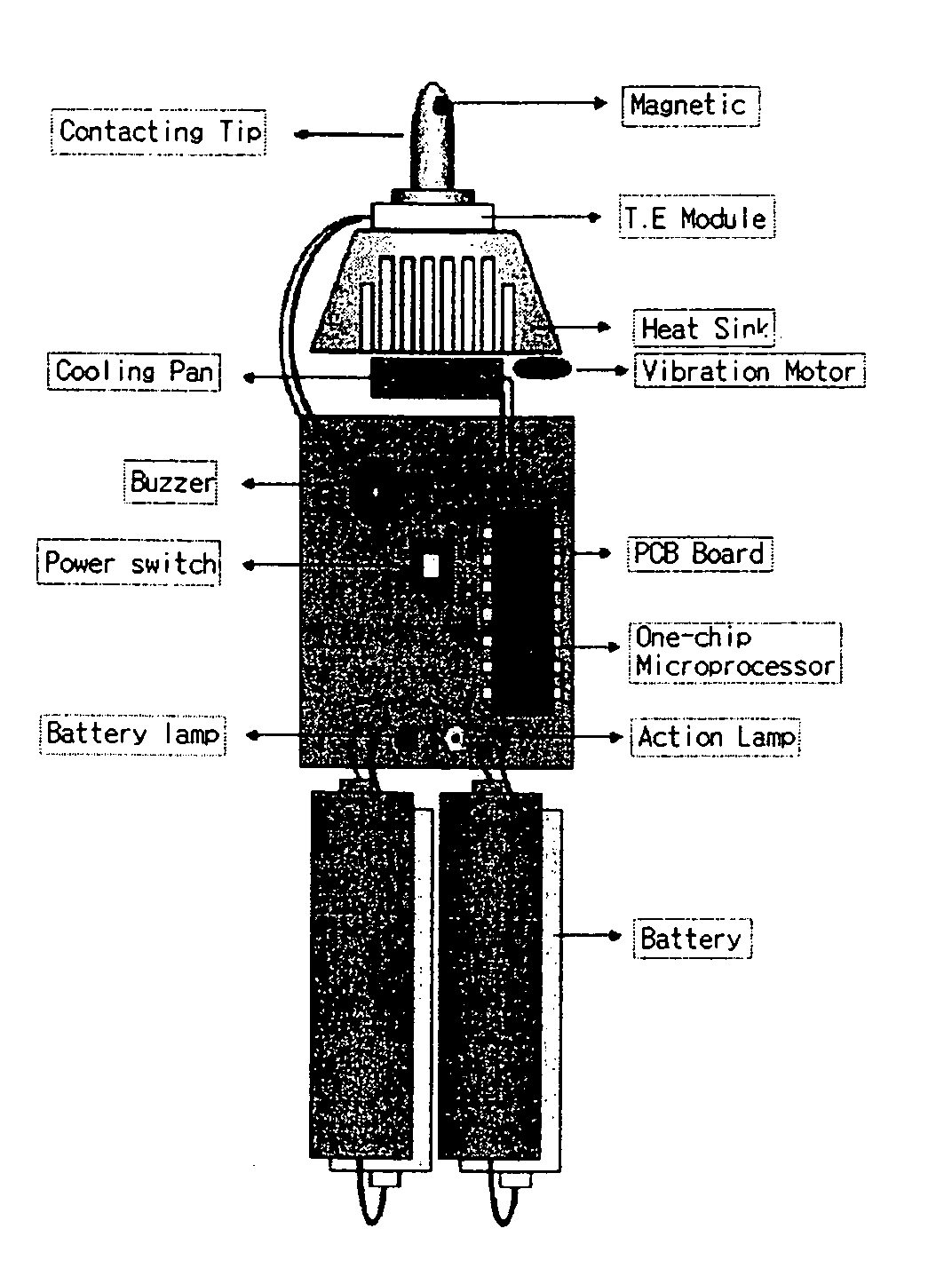
Hypertension descending device
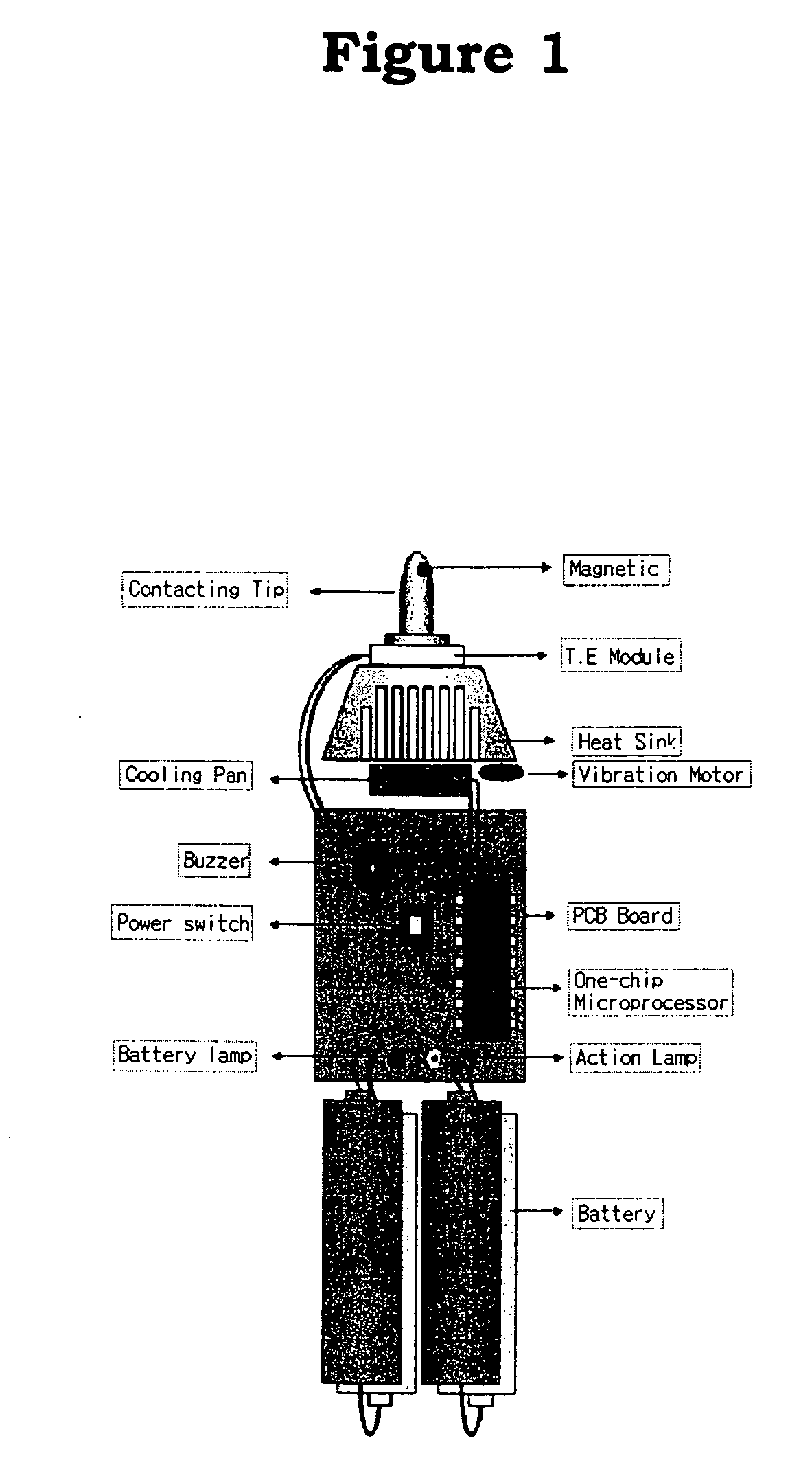
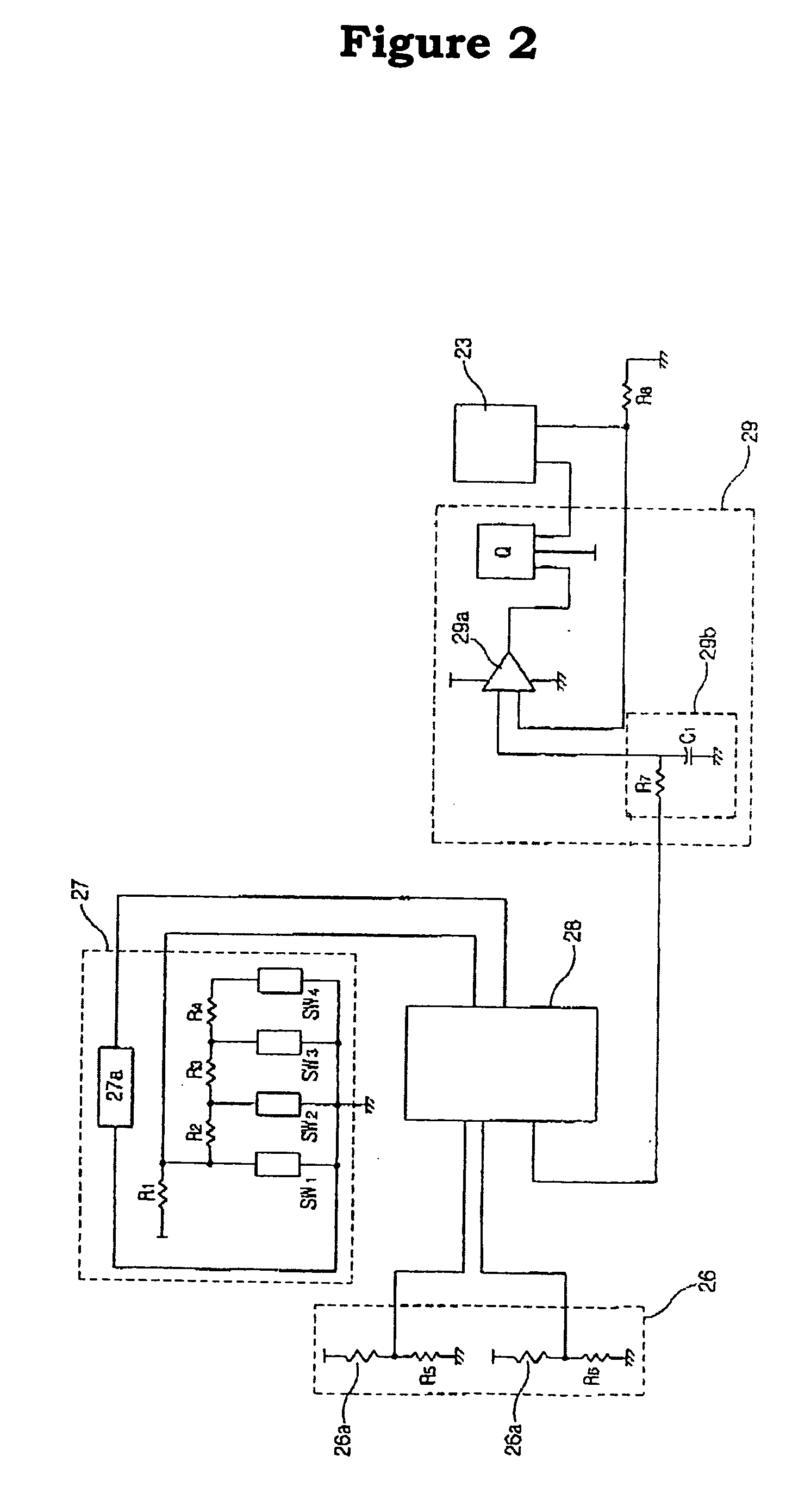
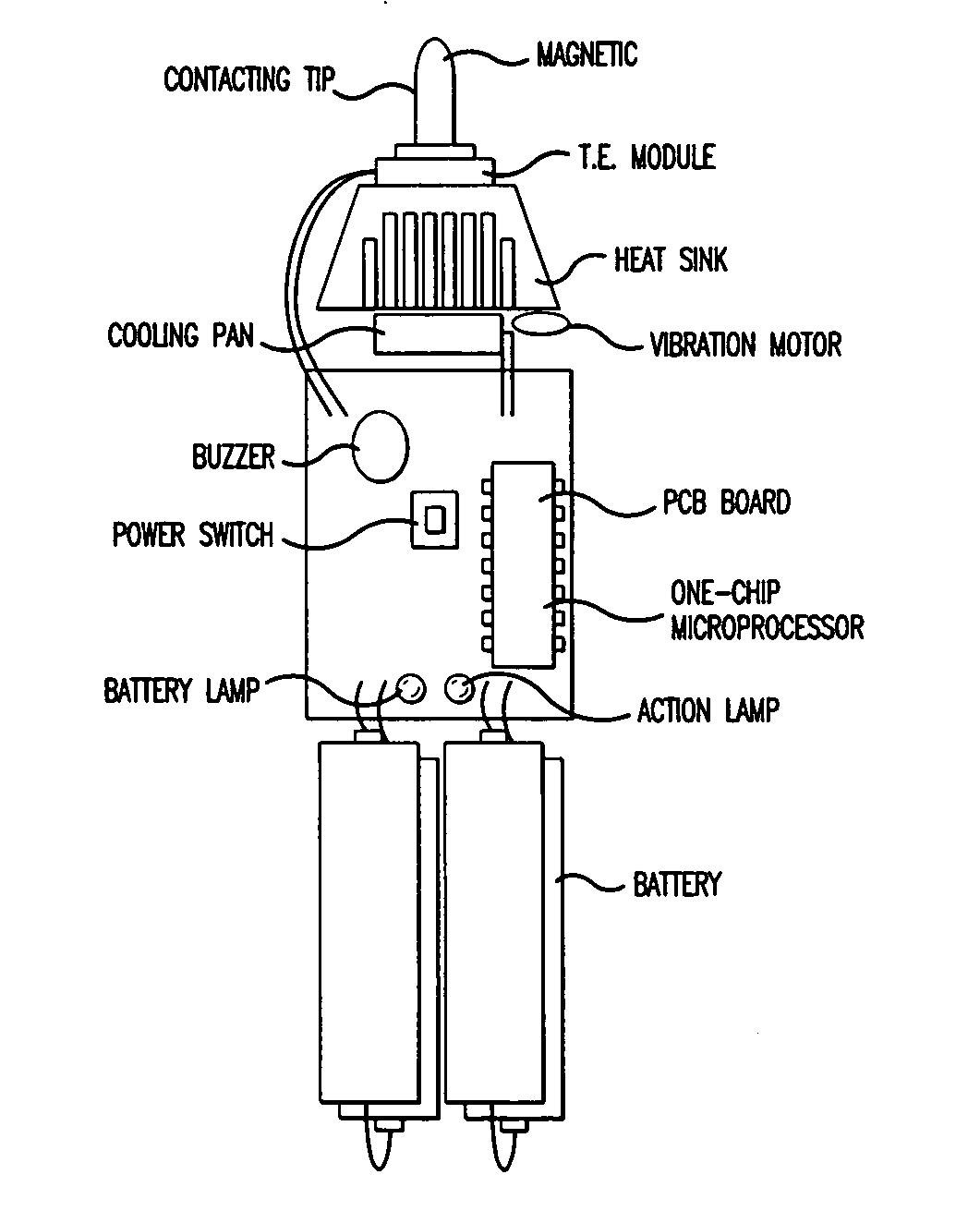
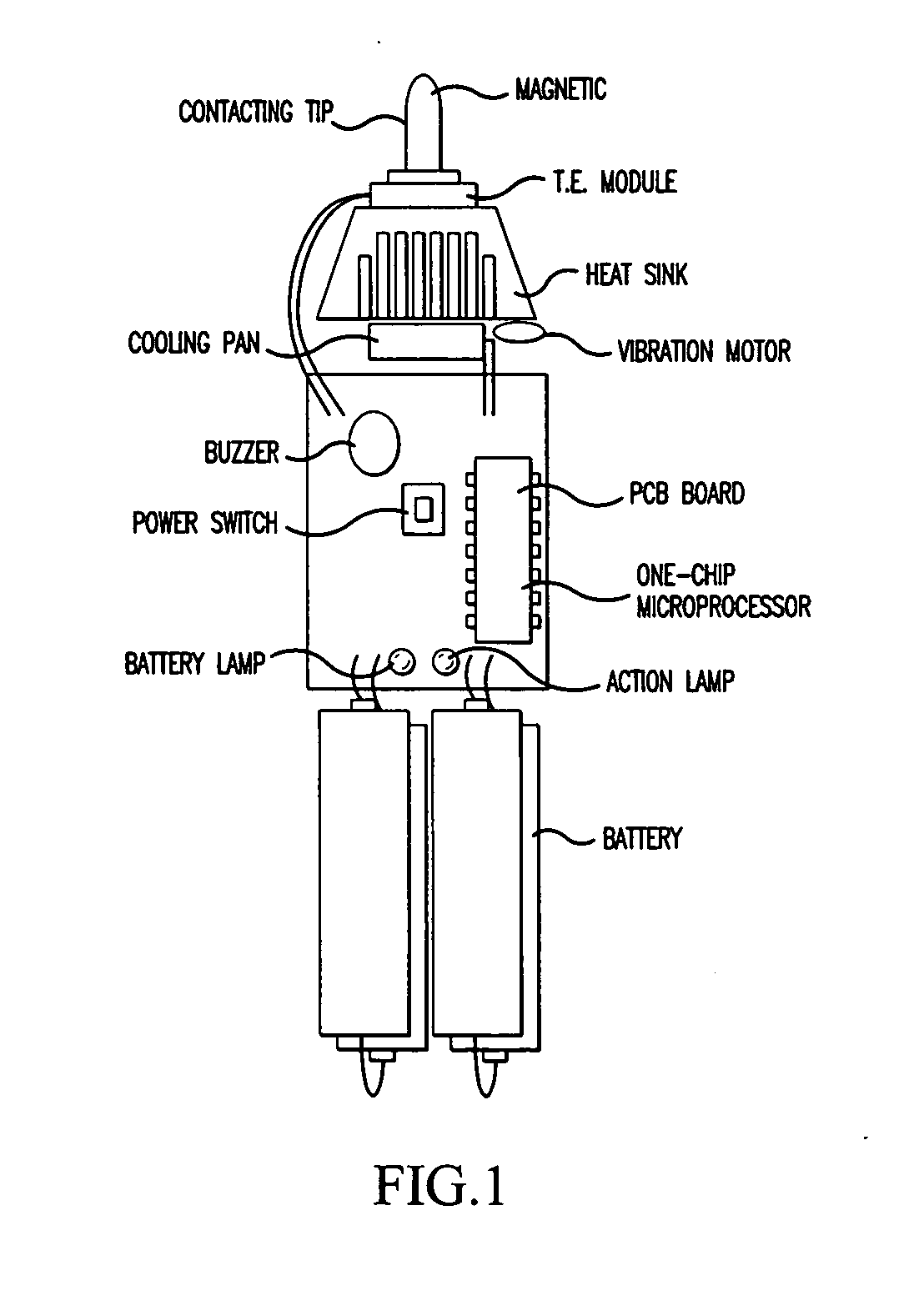
InactiveUS20050085879A1Improve cooling efficiencyDevices for heating/cooling reflex pointsDevices for pressing relfex pointsCarotid sinusCooling temperature
Disclosed is a hypertension descending device capable of curing a hypertension disease by stimulating a patient's Acupuncture point Renying (or referred to as “carotid sinus”) by repeated cold pressure vibration. The hypertension descending device is designed to allow common users to use it easily, and provides a good hypertension descent effect by stimulating the patient's carotid sinus (Acupuncture point Renying) portion while maintaining a uniform cooling temperature of the contact tip, which is in contact with the patient's carotid sinus (Acupuncture point Renying) portion.
Owner:AHN MOON HWI +1
Hypertension descending device
InactiveUS20060178715A1Improve cooling efficiencyDevices for heating/cooling reflex pointsDevices for pressing relfex pointsAnesthesiaCarotid sinus
Disclosed is a hypertension descending device capable of curing a hypertension disease by stimulating a patient's Acupuncture point Renying (or referred to as “carotid sinus”) by repeated cold pressure vibration. The hypertension descending device is designed to allow common users to use it easily, and provides a good hypertension descent effect by stimulating the patient's carotid sinus(Acupuncture point Renying) portion while maintaining a uniform cooling temperature of the contact tip, which is in contact with the patient's carotid sinus(Acupuncture point Renying) portion.
Owner:PHYSIOCUE
Skin-outer carotid sinus massage blood-pressure-reducing device
InactiveCN105943348ADrop in timeRealize individualized and intelligent blood pressure reductionVibration massageEvaluation of blood vesselsMassageCarotid sinus
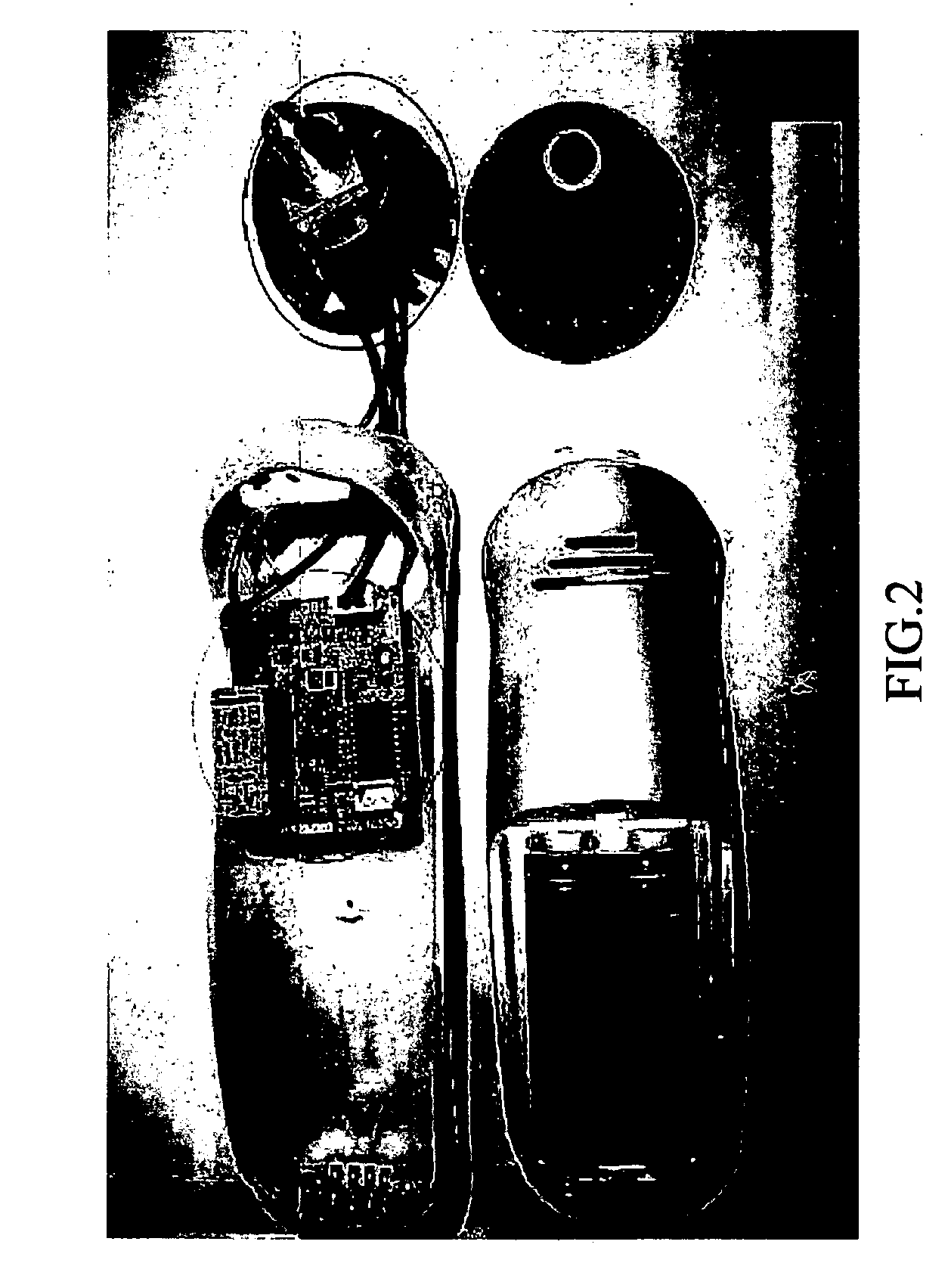
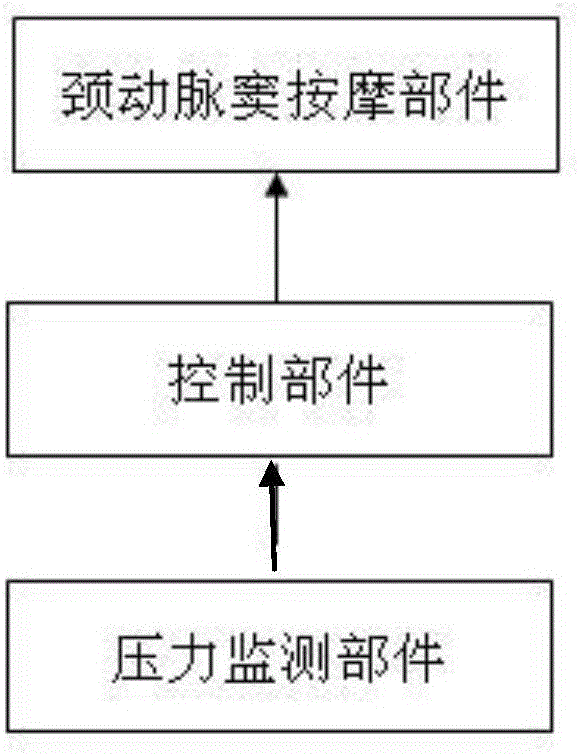
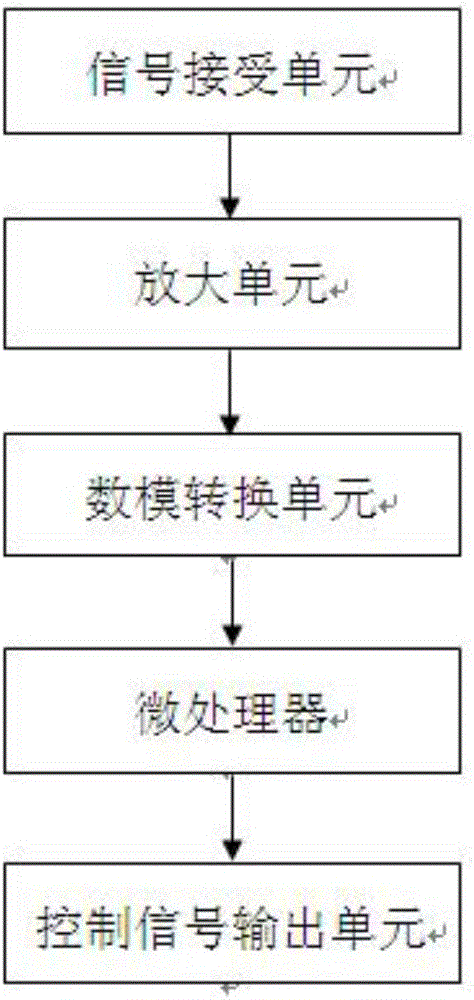
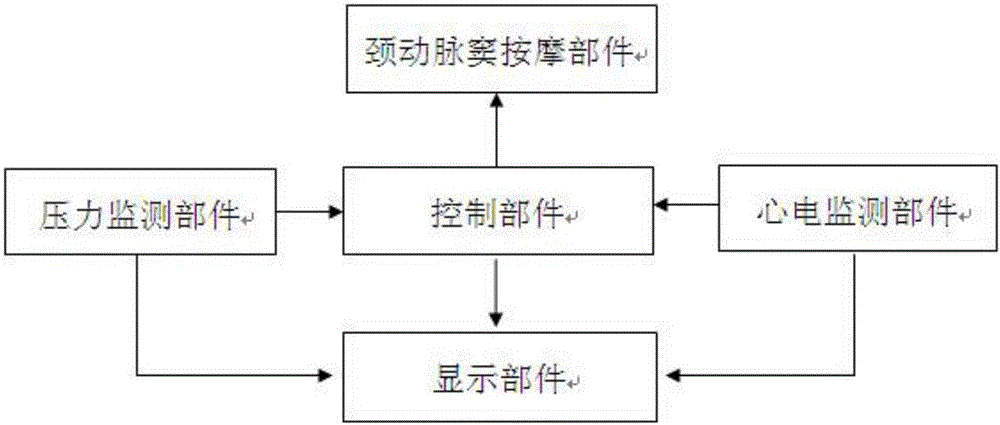
The invention belongs to the field of medical apparatus and instruments, and in particular relates to a skin-outer carotid sinus massage blood-pressure-reducing device. The skin-outer carotid sinus massage blood-pressure-reducing device comprises a carotid sinus massage part, a pressure monitoring part, a control part, a display part, an electrocardiogram monitoring part and a fixing part, wherein a microprocessor of the control part analyzes a pressure signal from the pressure monitoring part, so that the massage mode of the carotid sinus massage part fixed to the local skin-outer part of the carotid sinus is adjusted, and thus the feedback blood pressure reduction is realized. The skin-outer carotid sinus massage blood-pressure-reducing device is used for treating high blood pressure, is a noninvasive blood pressure reducing device, is convenient to use, is safe and effective, and has a great clinical application value.
Owner:张健
Electrode structures and methods for their use in cardiovascular reflex control
InactiveUS20100222831A1Reduce pressureDeleterious effectSpinal electrodesTransvascular endocardial electrodesNervous systemRegimen
Devices, systems and methods are described by which the blood pressure, nervous system activity, and neurohormonal activity may be selectively and controllably reduced by activating baroreceptors. A baroreceptor activation device is positioned near a baroreceptor, preferably a baroreceptor located in the carotid sinus. A control system may be used to modulate the baroreceptor activation device. The control system may utilize an algorithm defining a stimulus regimen which promotes long term efficacy and reduces power requirements / consumption. The baroreceptor activation device may utilize electrodes to activate the baroreceptors. The electrodes may be adapted for connection to the carotid arteries at or near the carotid sinus, and may be designed to minimize extraneous tissue stimulation.
Owner:CVRX
Magnetic stimulation physiotherapy device functioning through carotid sinus
ActiveCN107551403AThere will be no security risksMagnetotherapy using coils/electromagnetsElectrical stimulationsCarotid sinus
The present invention provides a magnetic stimulation physiotherapy device functioning through carotid sinus. The device comprises a pulse generator, a circulating water cooling box, a handle, magnetic stimulation coils, isolation pads, a cable, a U-shaped clamp and a water-cooled tube. The magnetic stimulation physiotherapy device functioning through carotid sinus can employ a principle of a carotid sinus being sensitive to physical stimulation to treat hypertension. Compared to a traditional carotid sinus electrical stimulation technology, the magnetic stimulation physiotherapy device functioning through carotid sinus can generate magnetic fields with high, middle and low frequencies through a time-varying current, the magnetic fields with high, middle and low frequencies penetrate tissue to be focused at the carotid sinus, and electromagnetic induction generates a treatment eddy current. The magnetic stimulation coils are not in direct contact with tissue, there are no potential risks caused by a similar electrical stimulation technology, and invasive surgery is not needed.
Owner:徐格林 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com