Patents
Literature
79results about How to "Pain minimization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
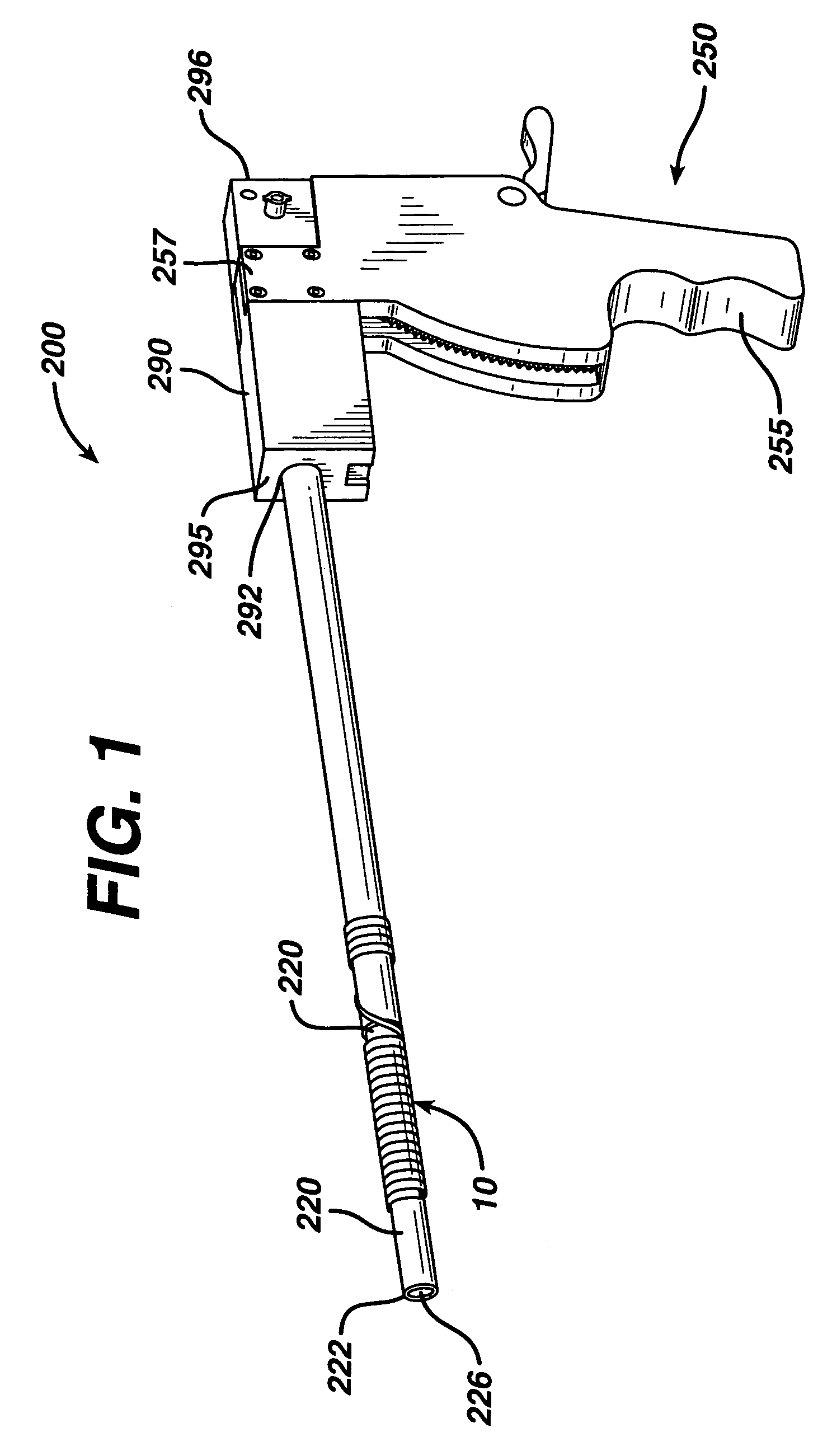
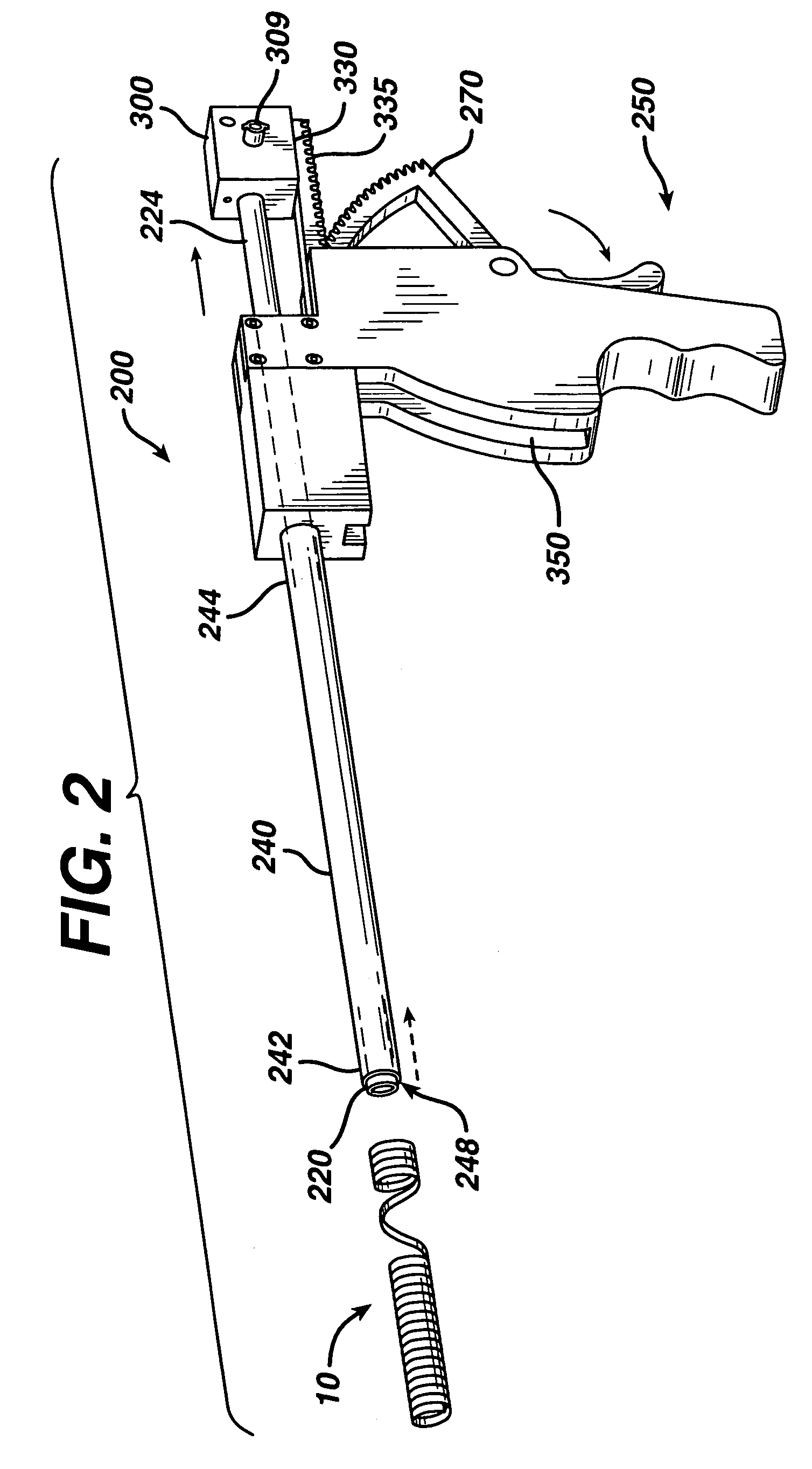
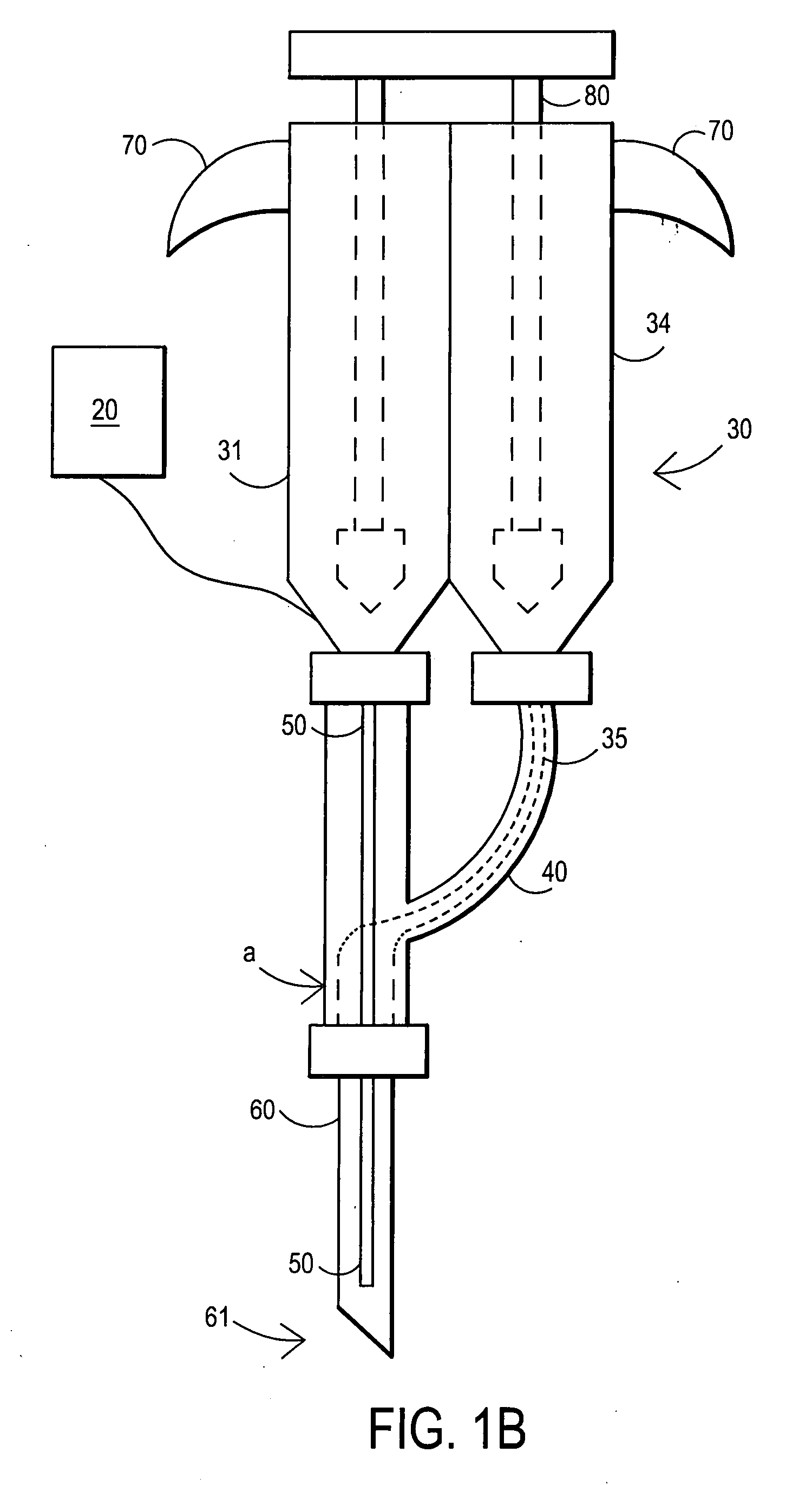
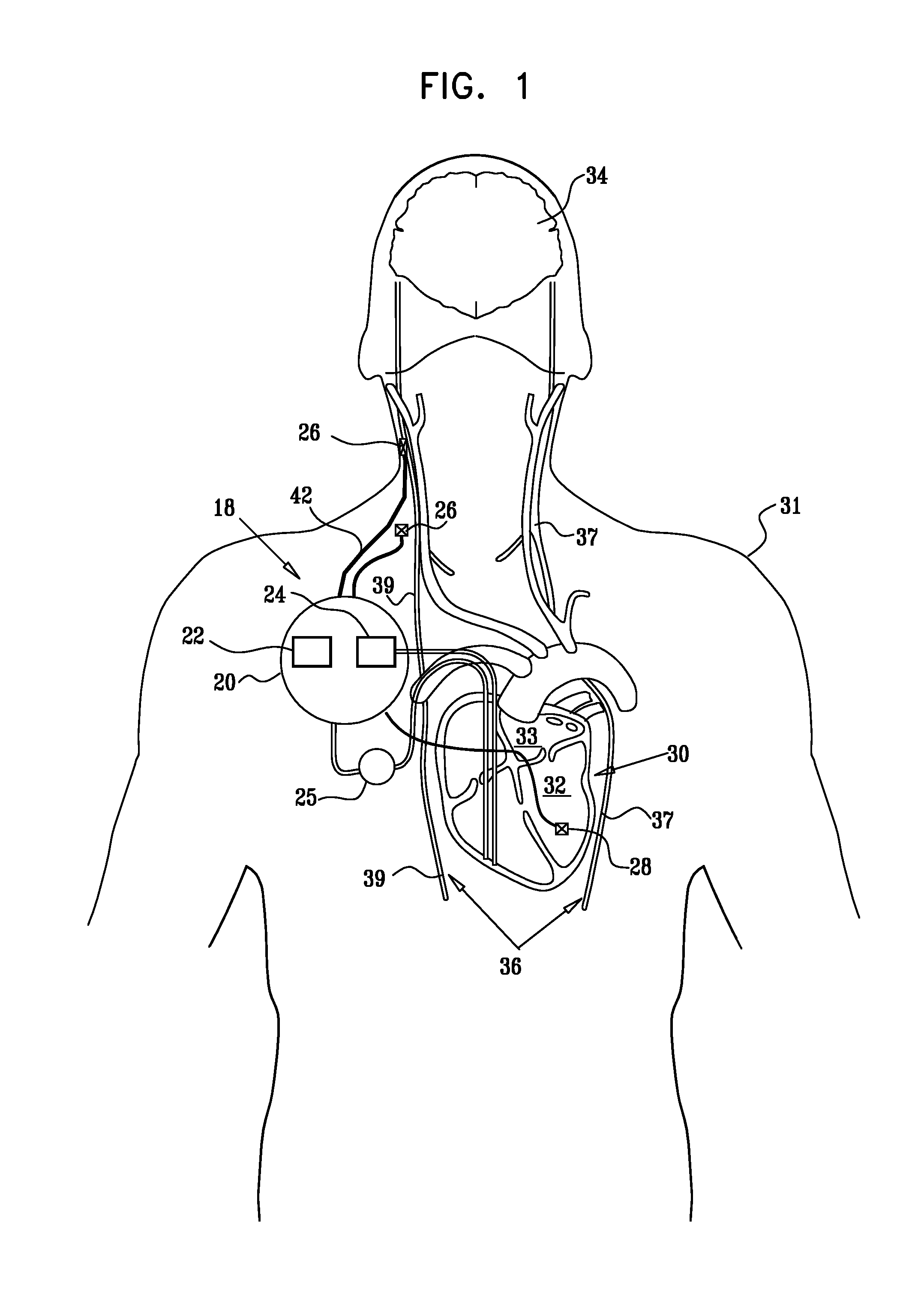
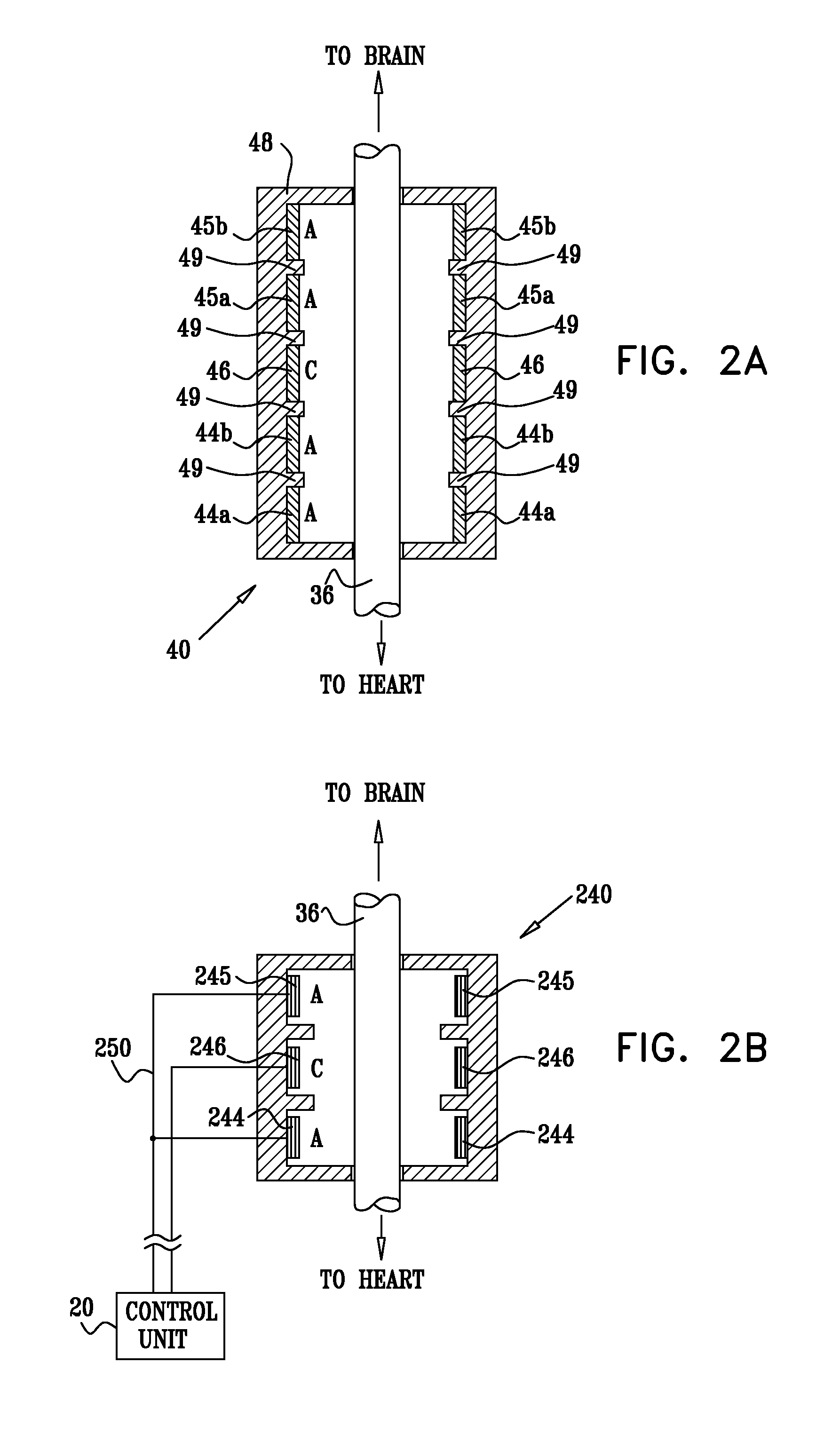
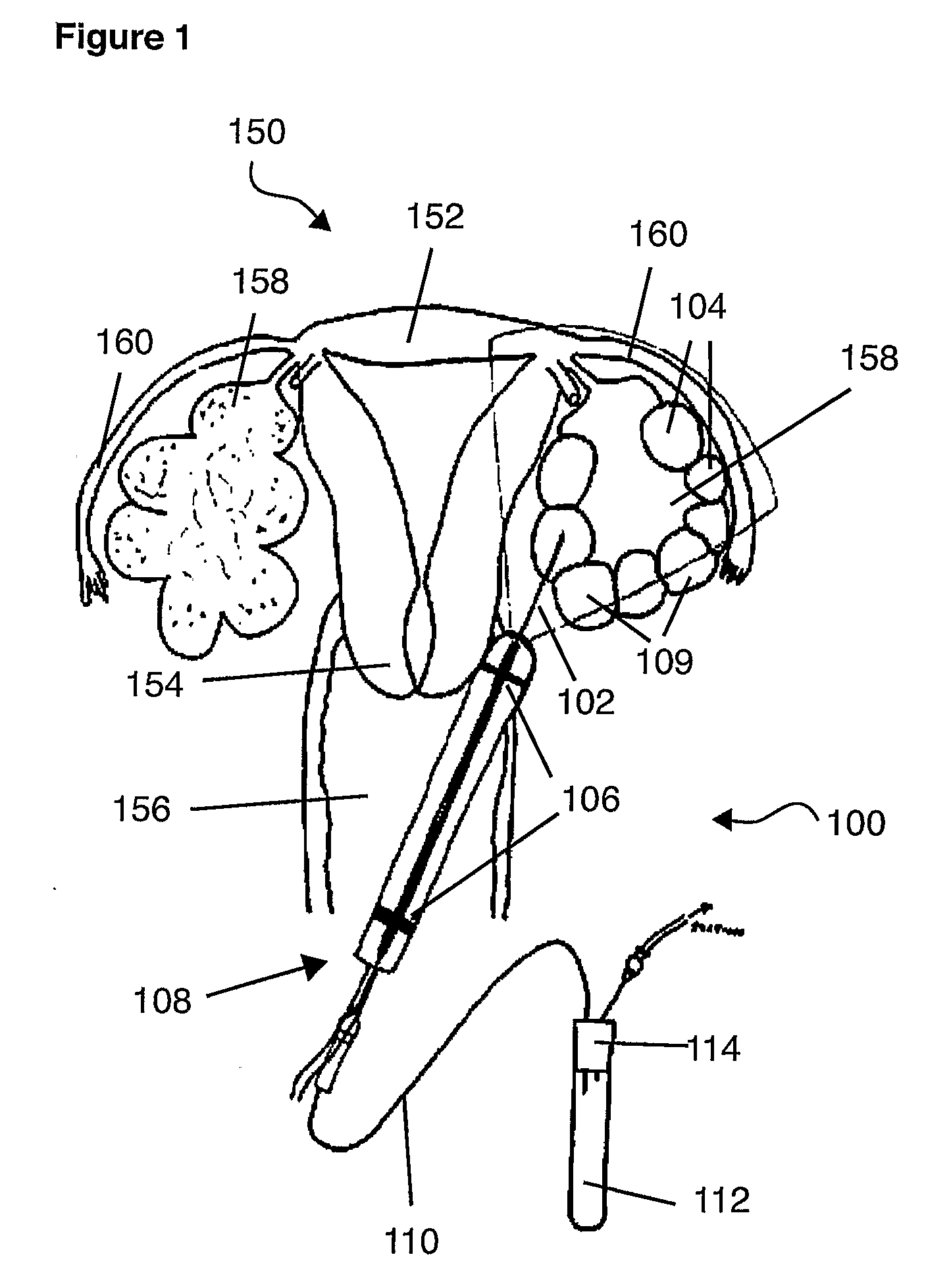
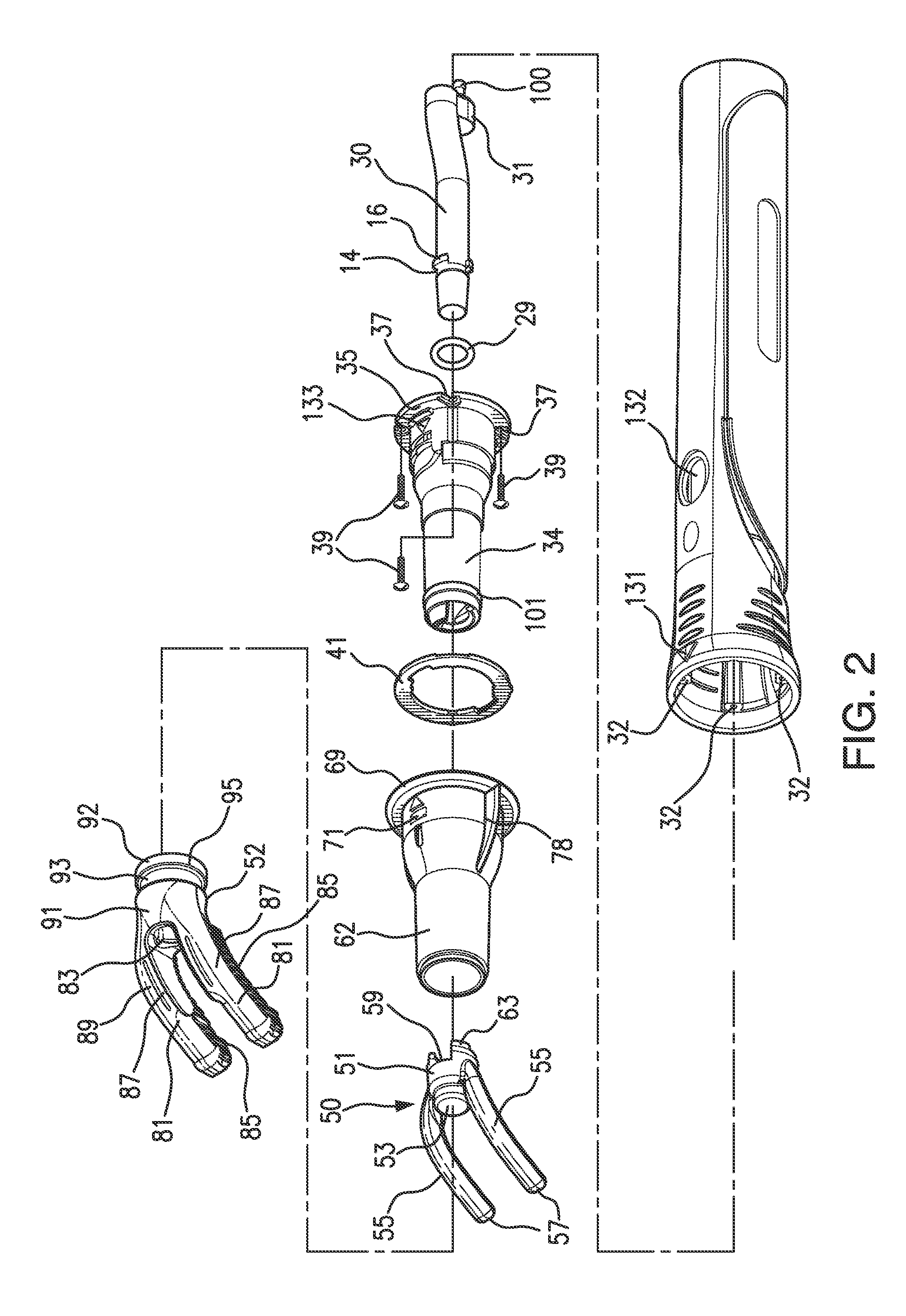
Techniques for reducing pain associated with nerve stimulation
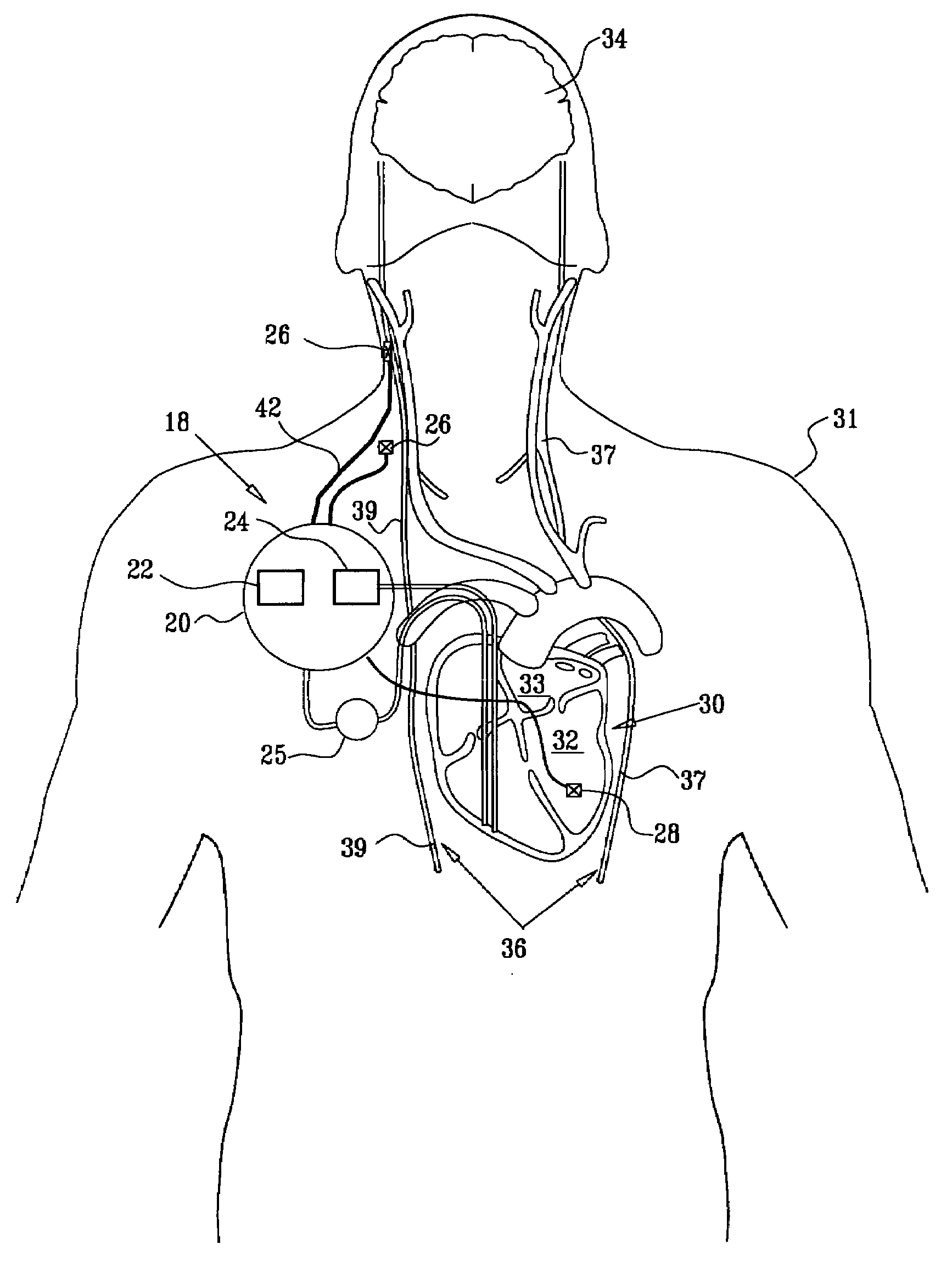
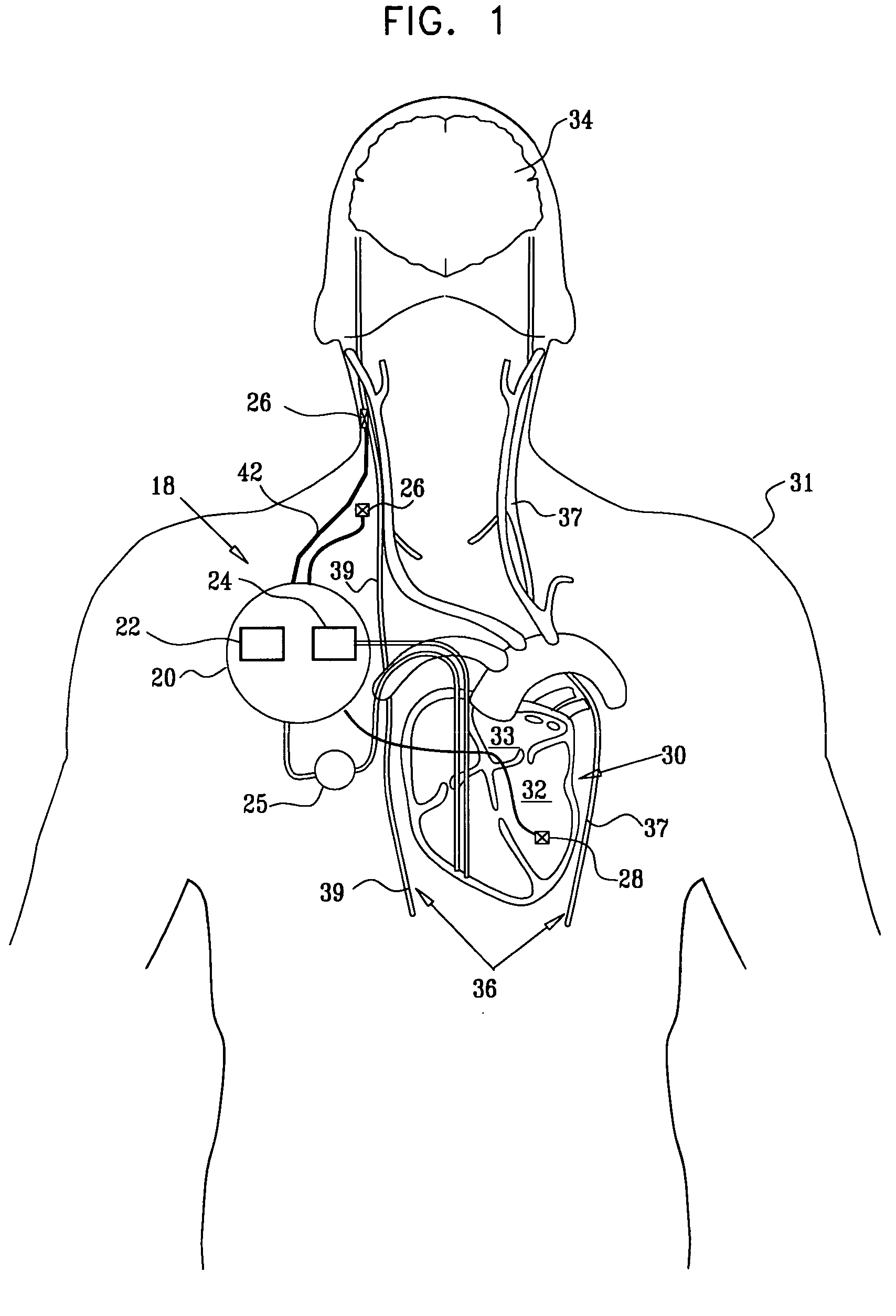
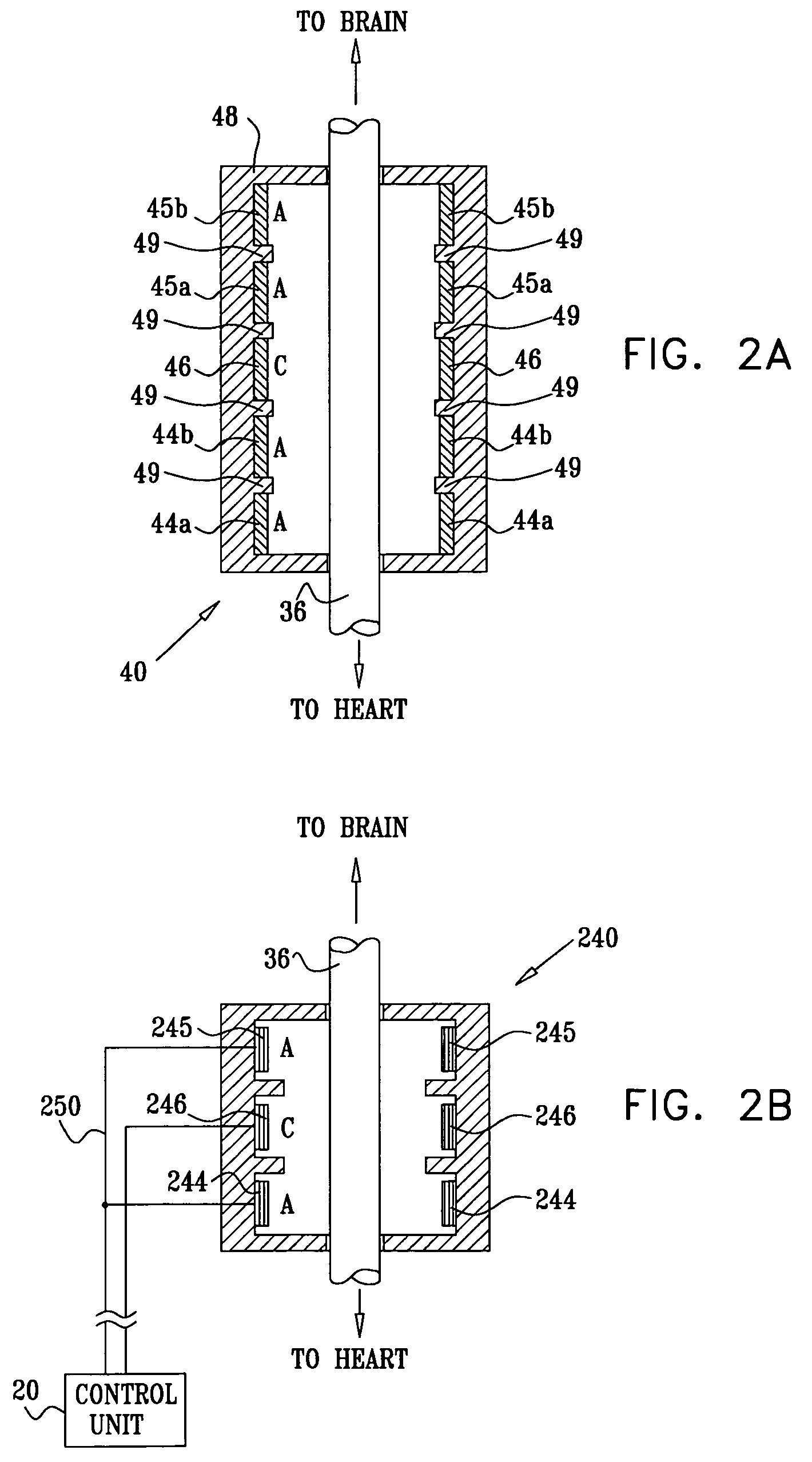
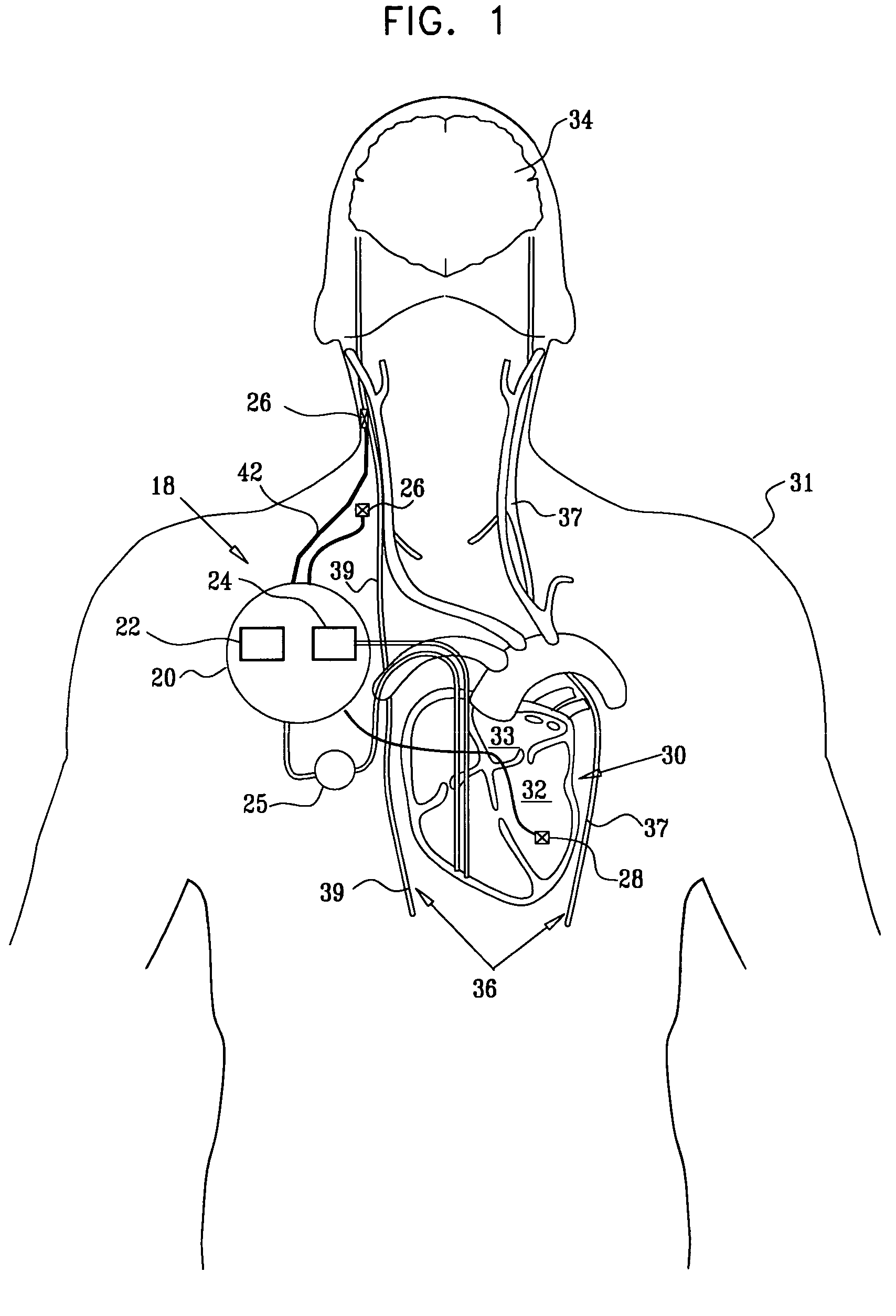
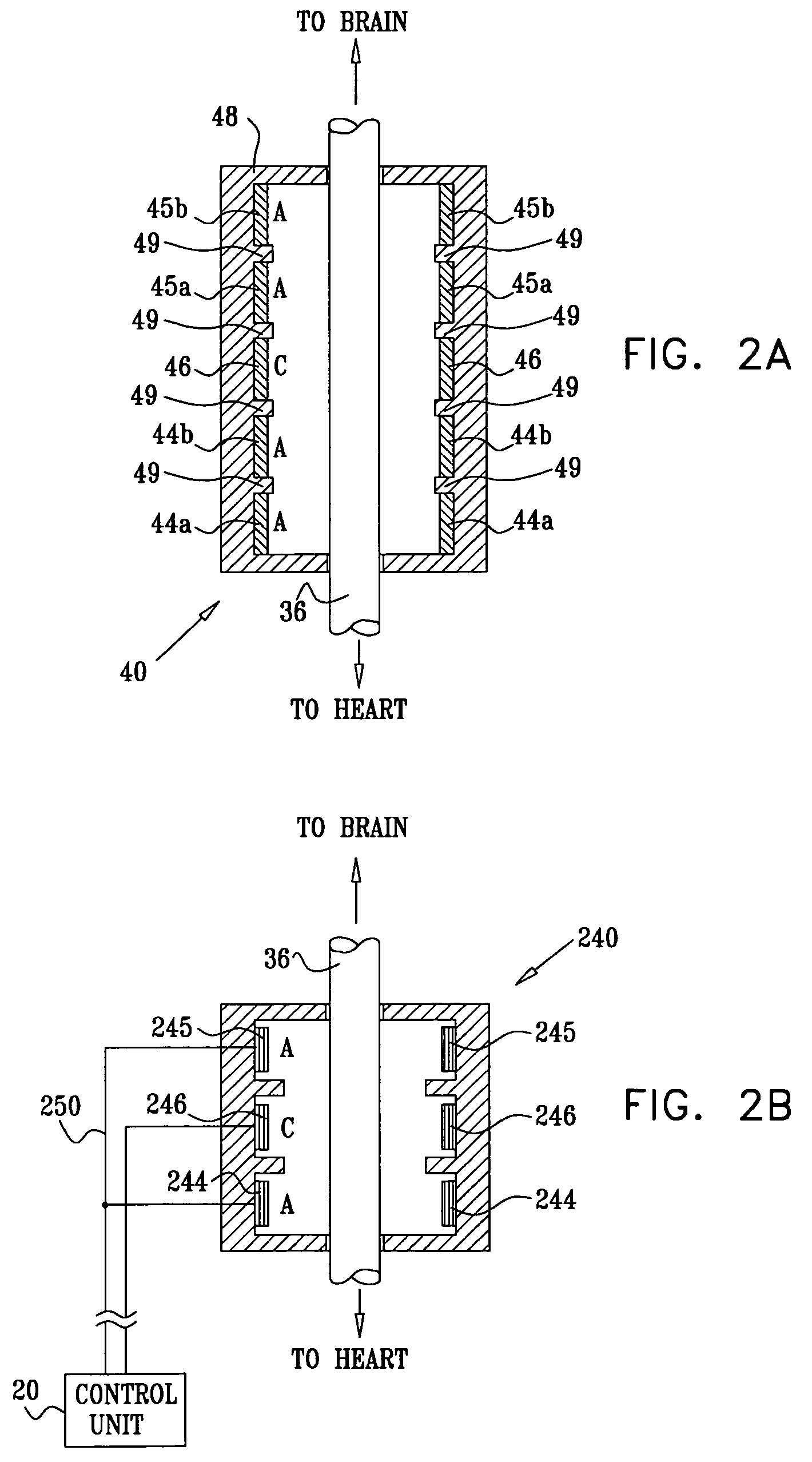
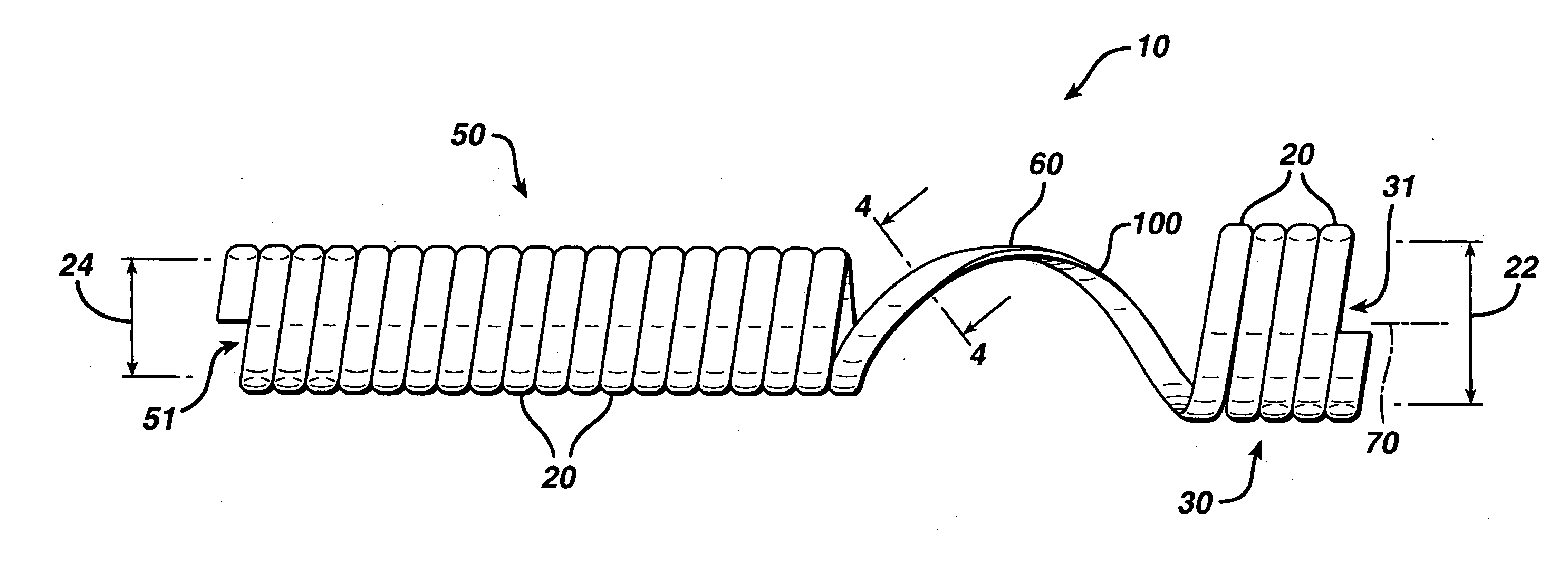
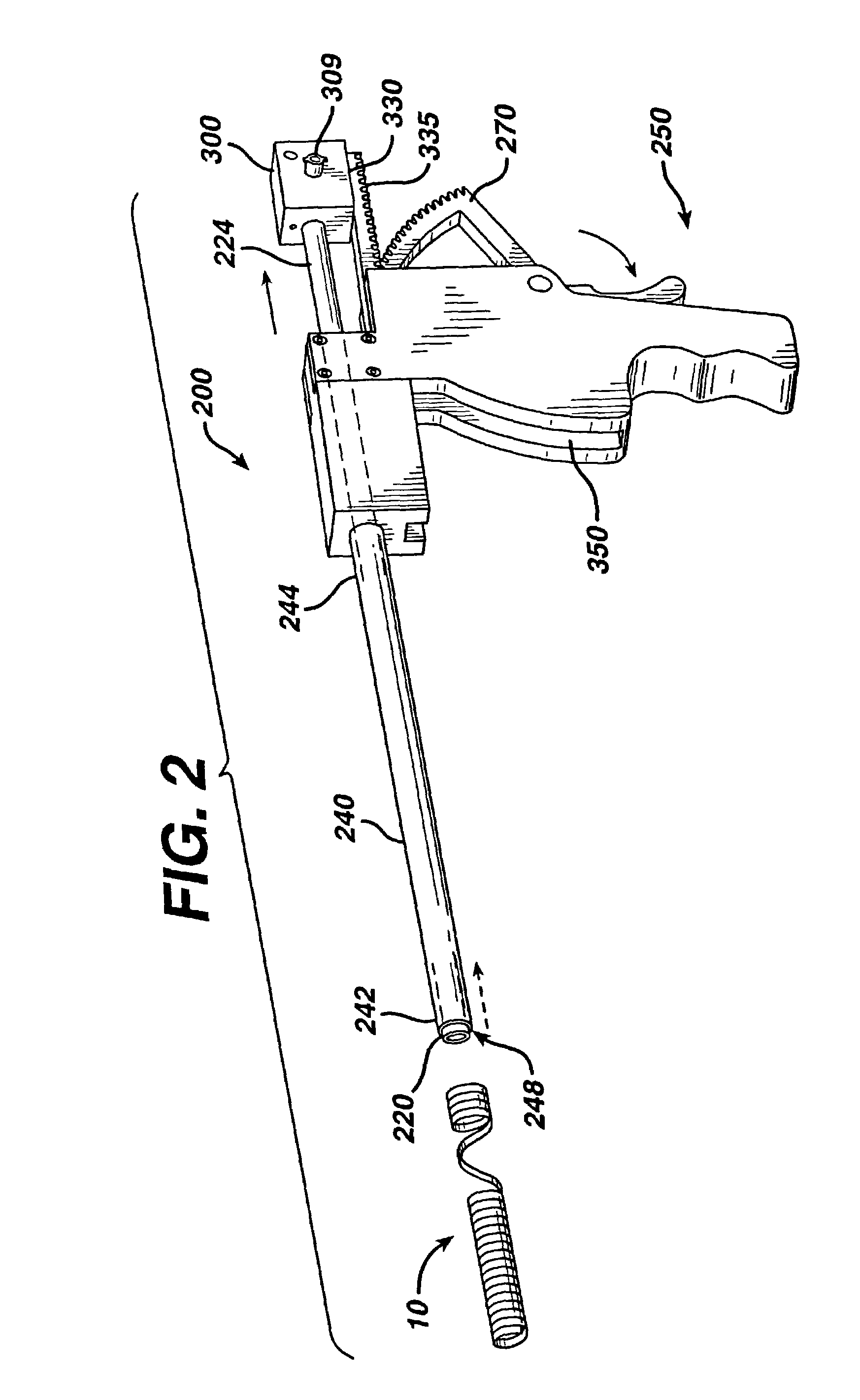
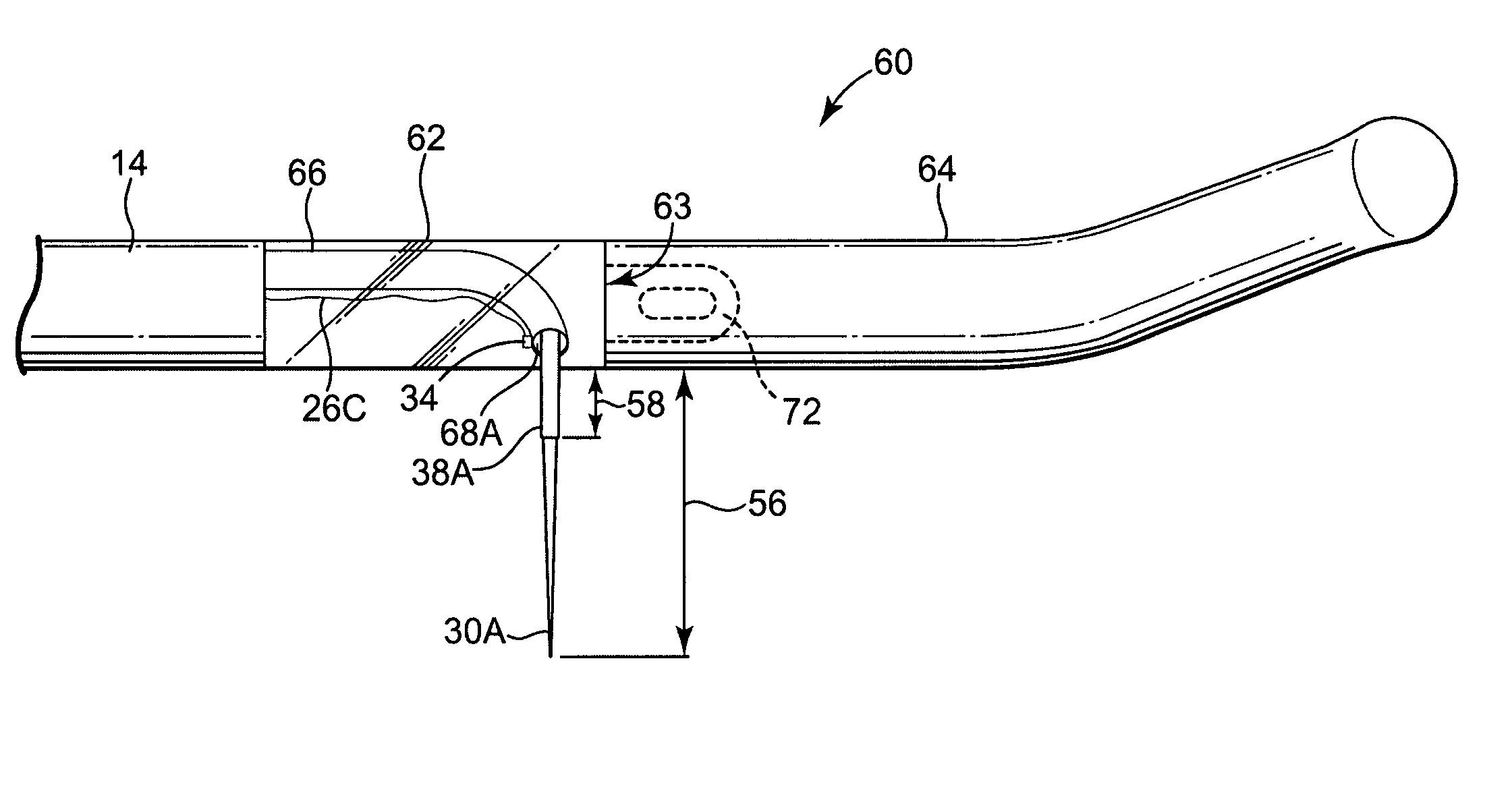
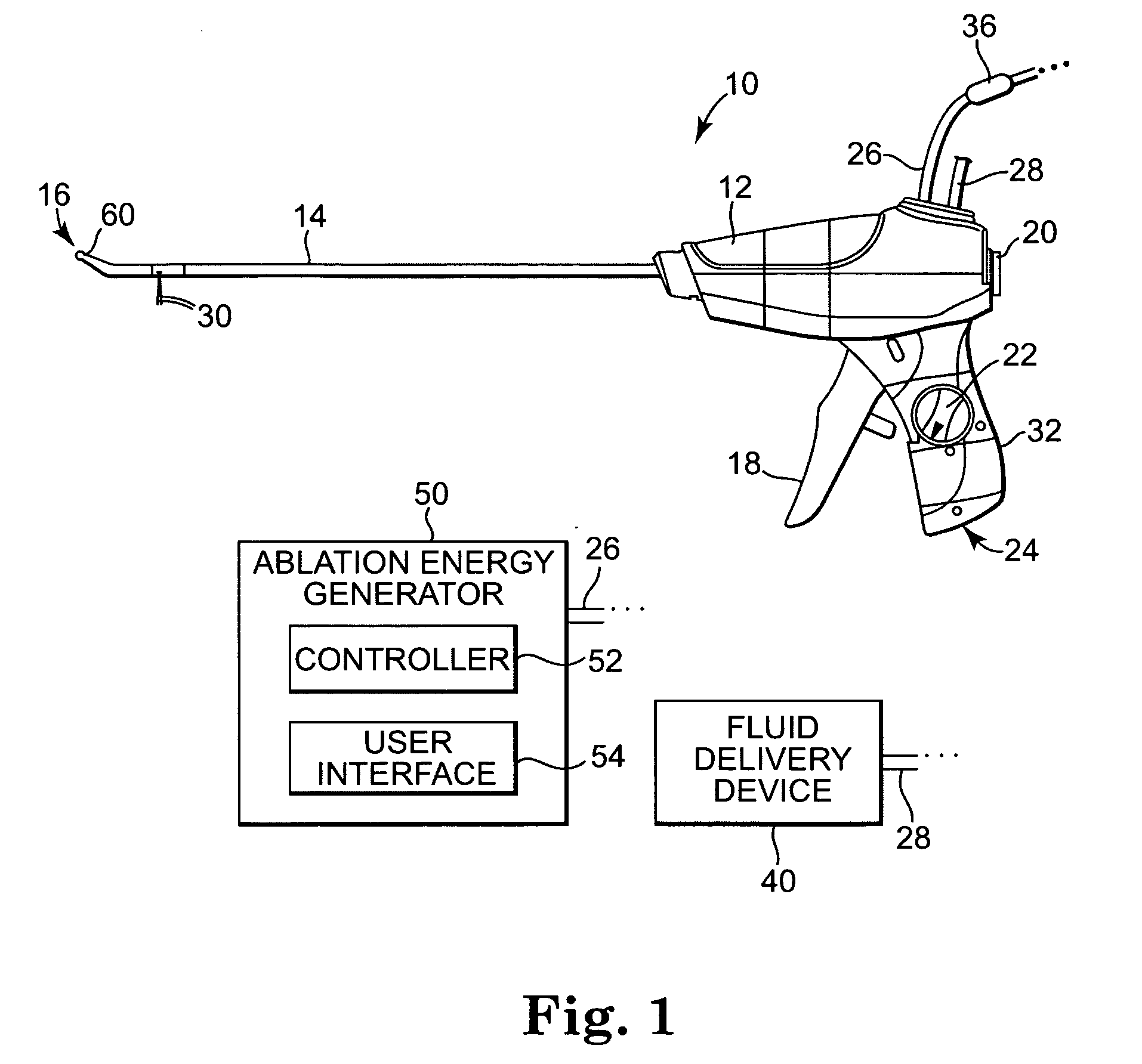
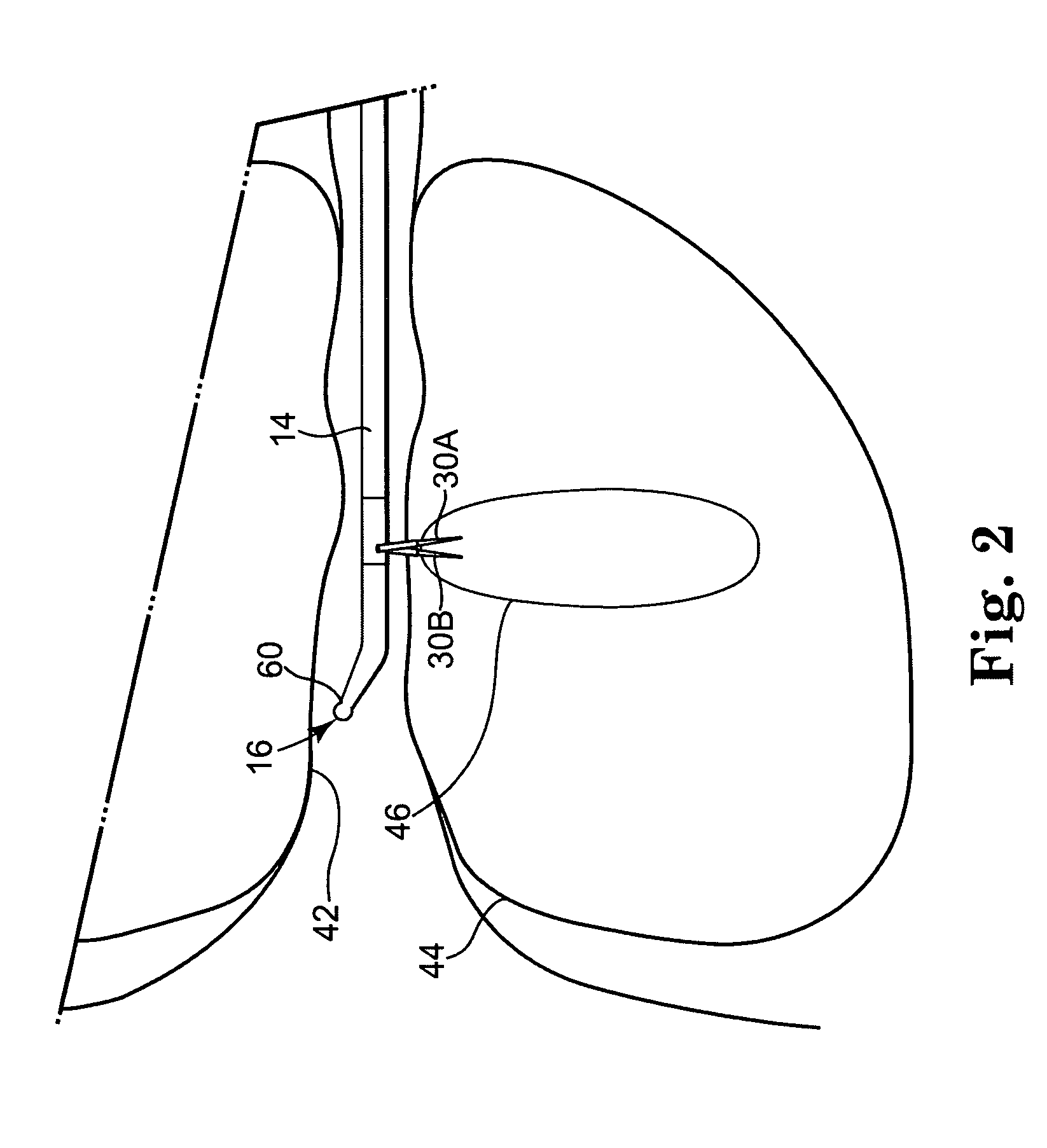
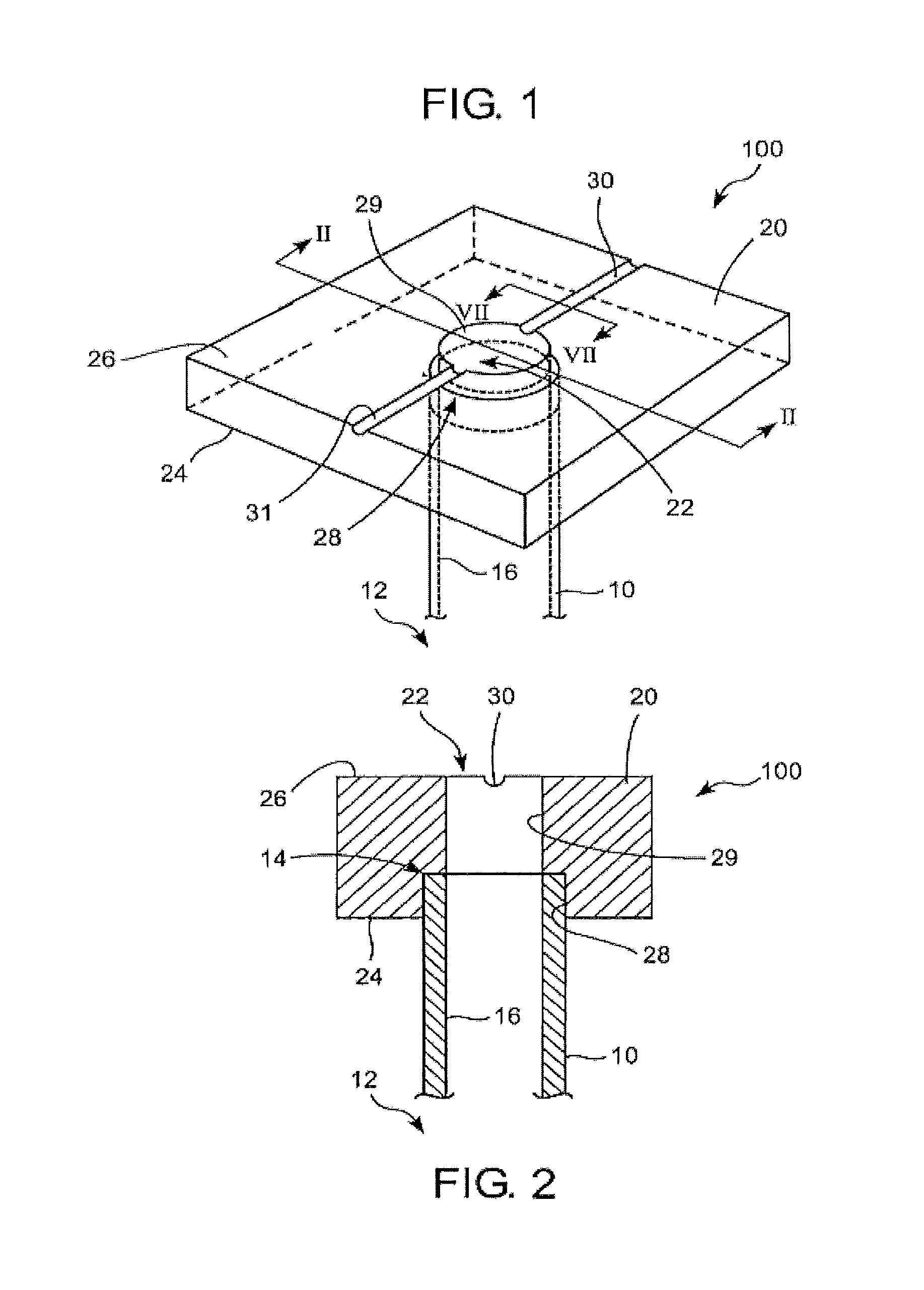
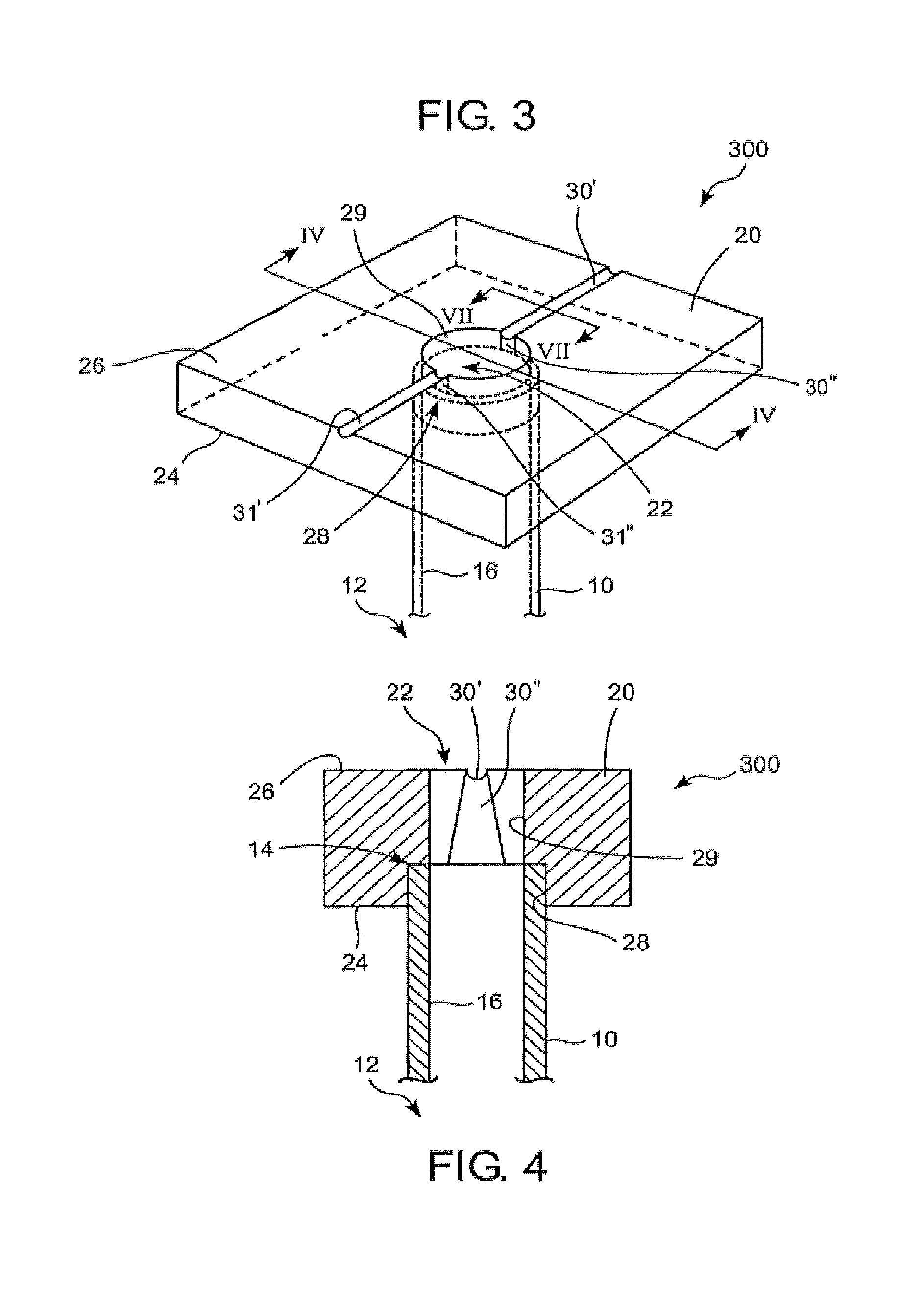
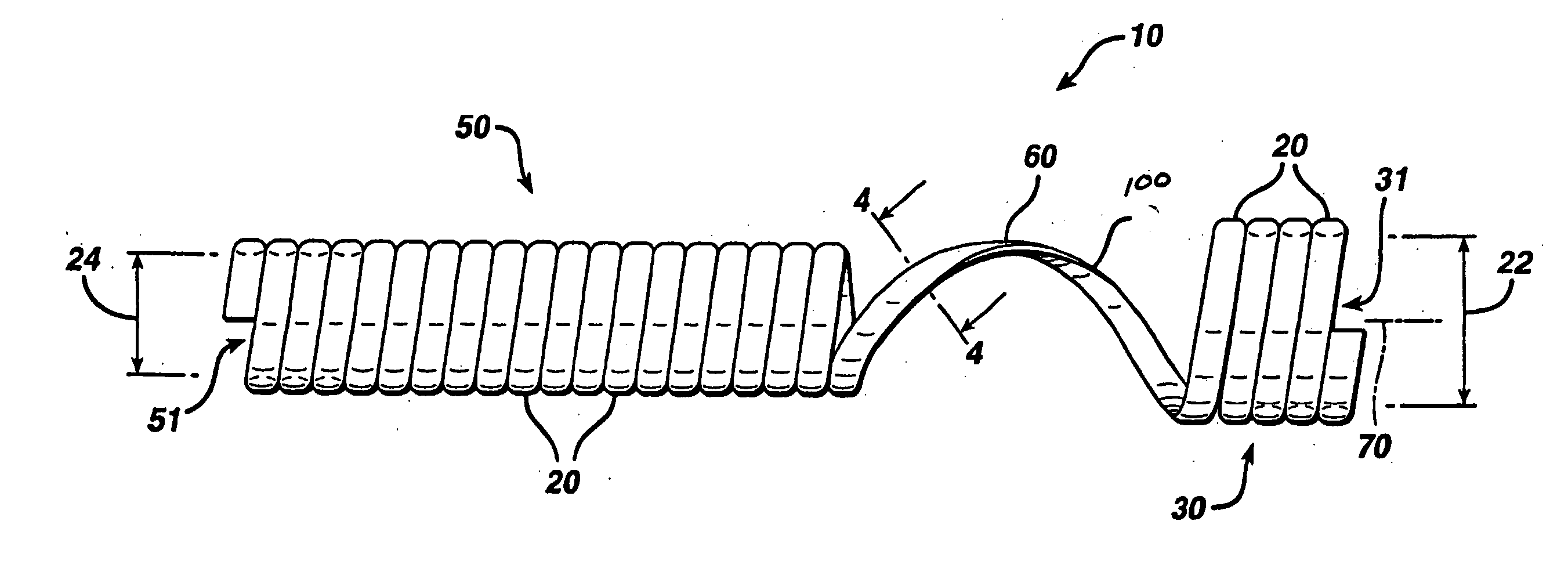
Apparatus is provided including an electrode device and a control unit. The electrode device is configured to be coupled to a site of a subject selected from the group consisting of: a vagus nerve, an epicardial fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a right ventricle, a right atrium, and a jugular vein. The control unit is configured to drive the electrode device to apply to the site a current in at least first and second bursts, the first burst including a plurality of pulses, and the second burst including at least one pulse, and set (a) a pulse repetition interval (PRI) of the first burst to be on average at least 20 ms, (b) an interburst interval between initiation of the first burst and initiation of the second burst to be less than 10 seconds, (c) an interburst gap between a conclusion of the first burst and the initiation of the second burst to have a duration greater than the average PRI, and (d) a burst duration of the first burst to be less than a percentage of the interburst interval between, the percentage being less than 67%. Other embodiments are also described.
Owner:MEDTRONIC INC
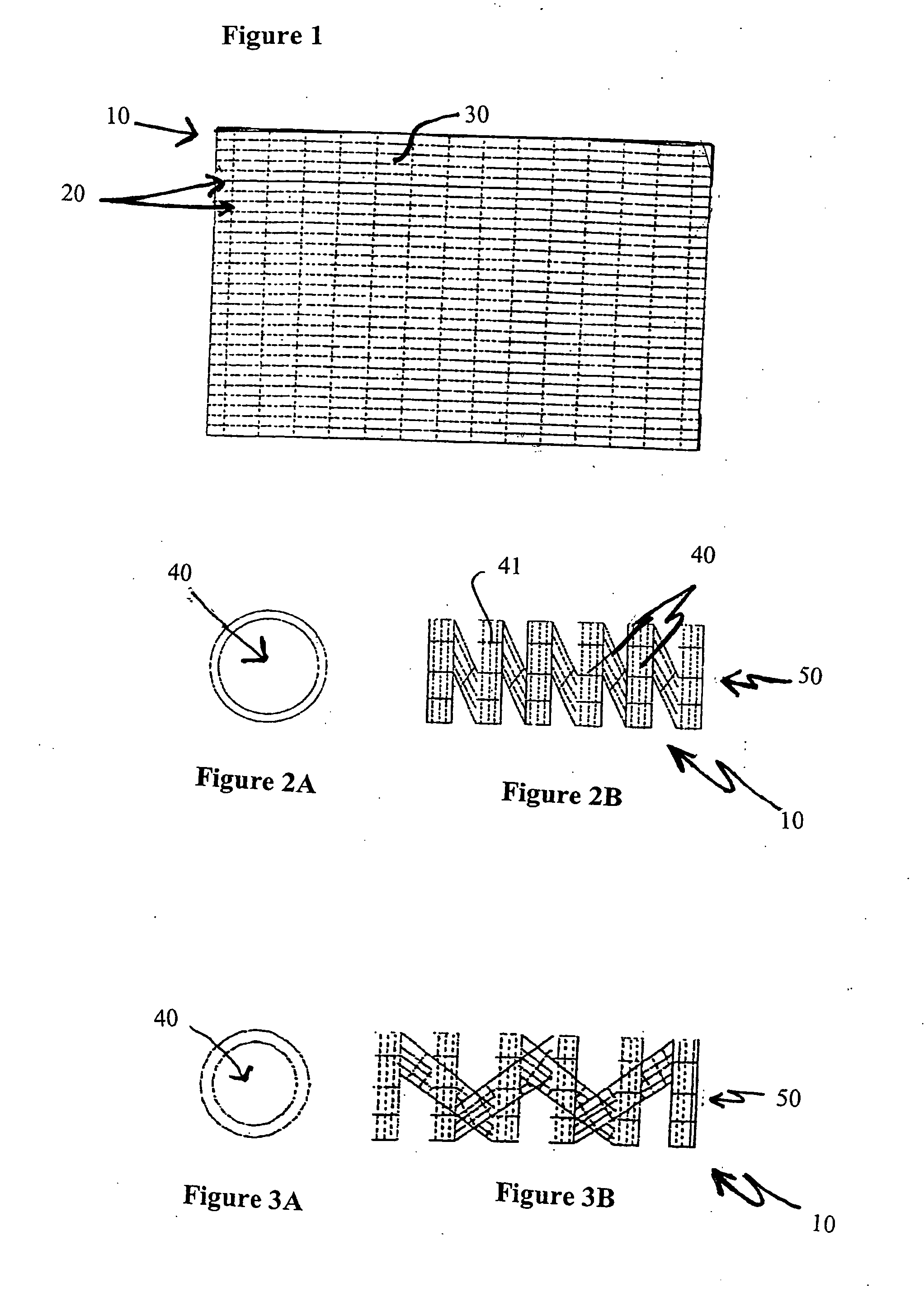
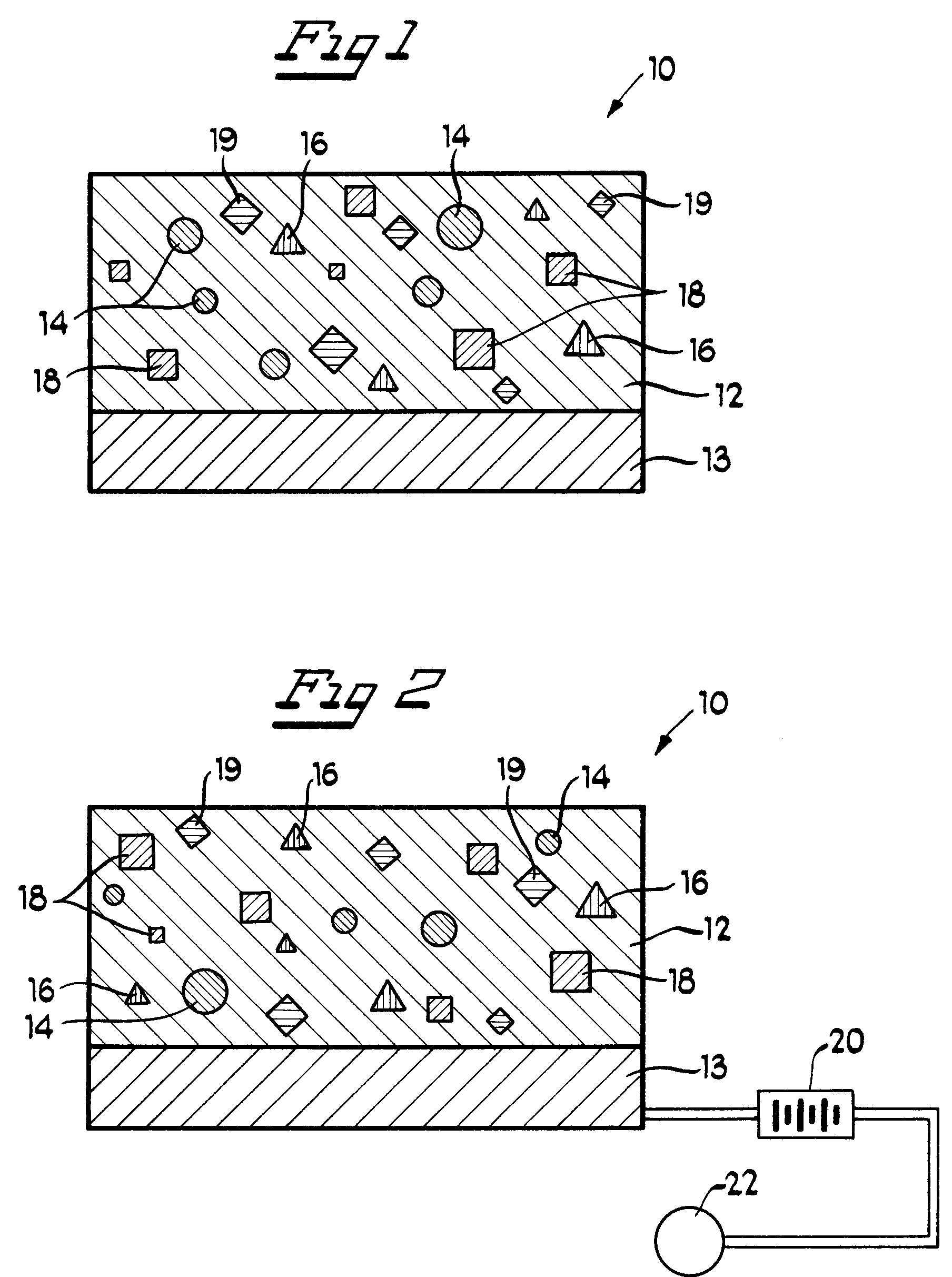
Biodegradable stent
Owner:ETHICON INC
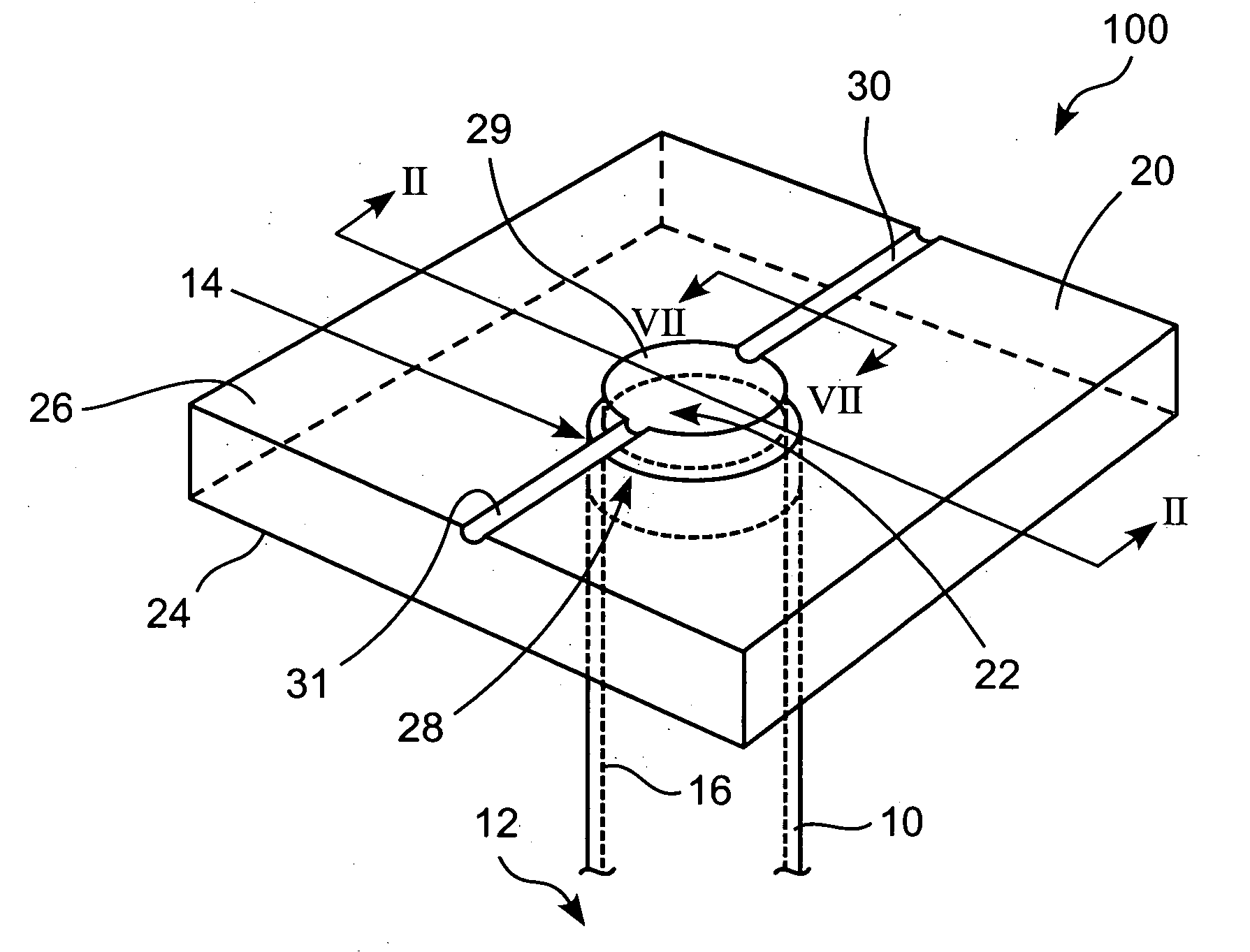
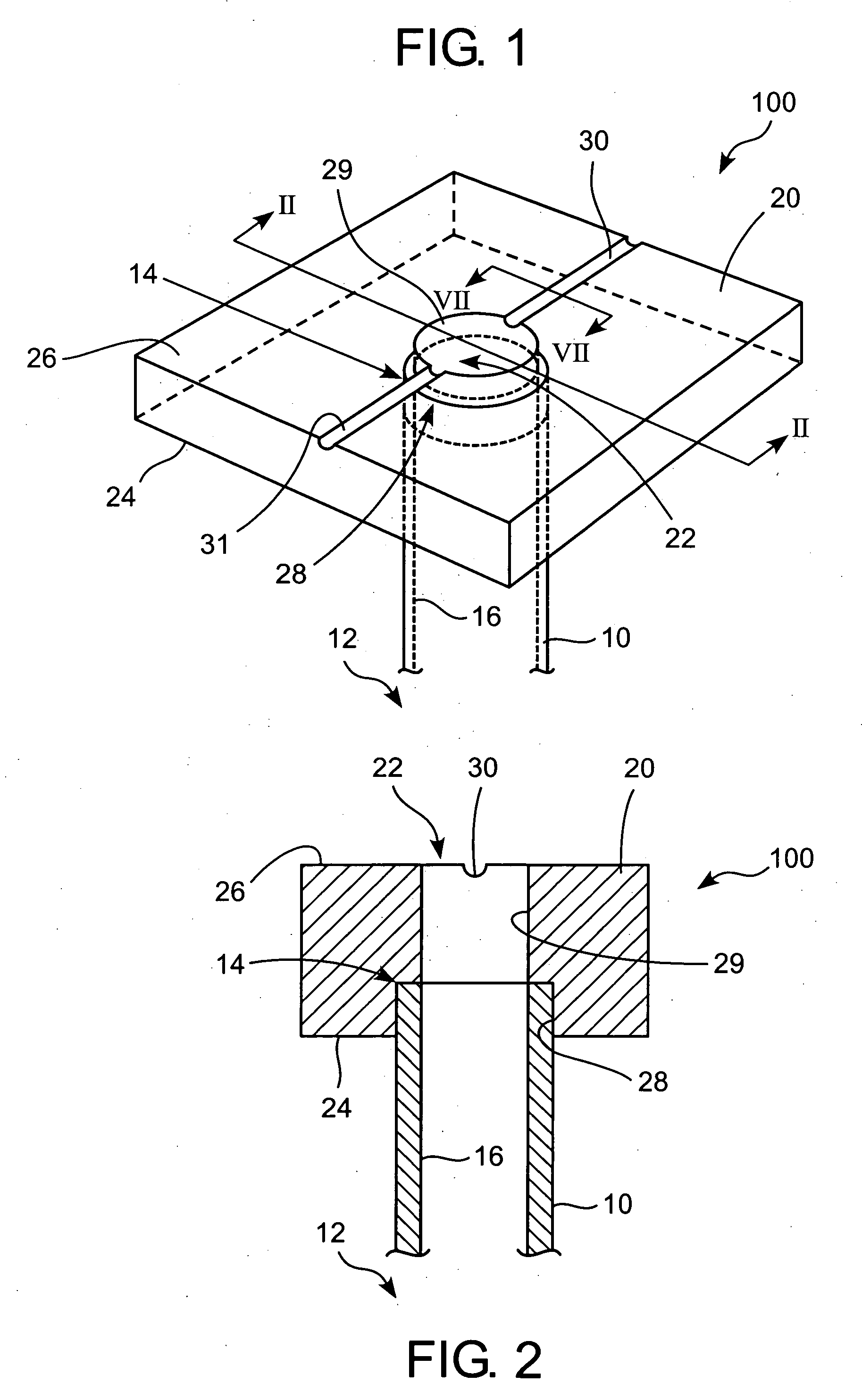
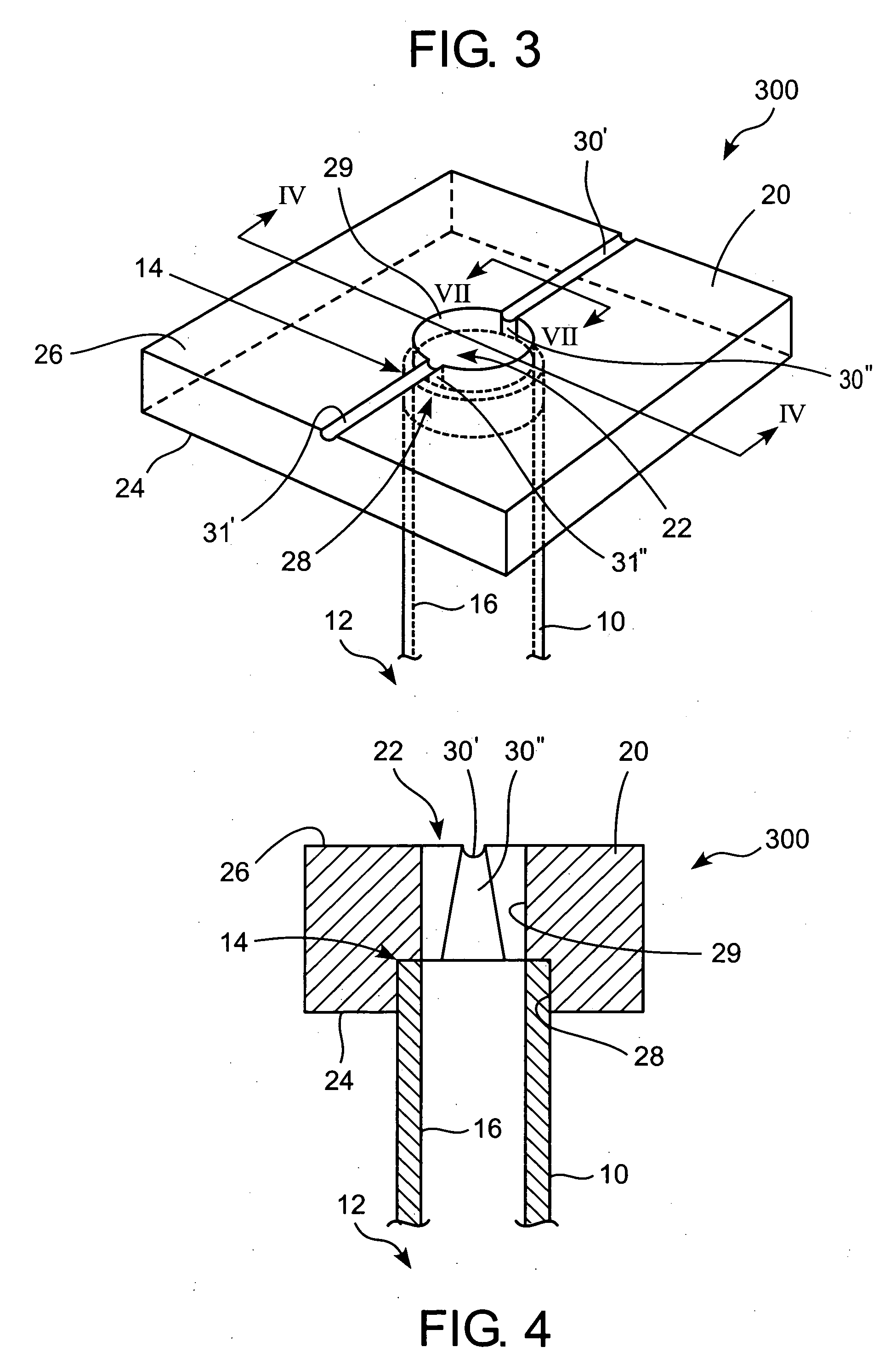
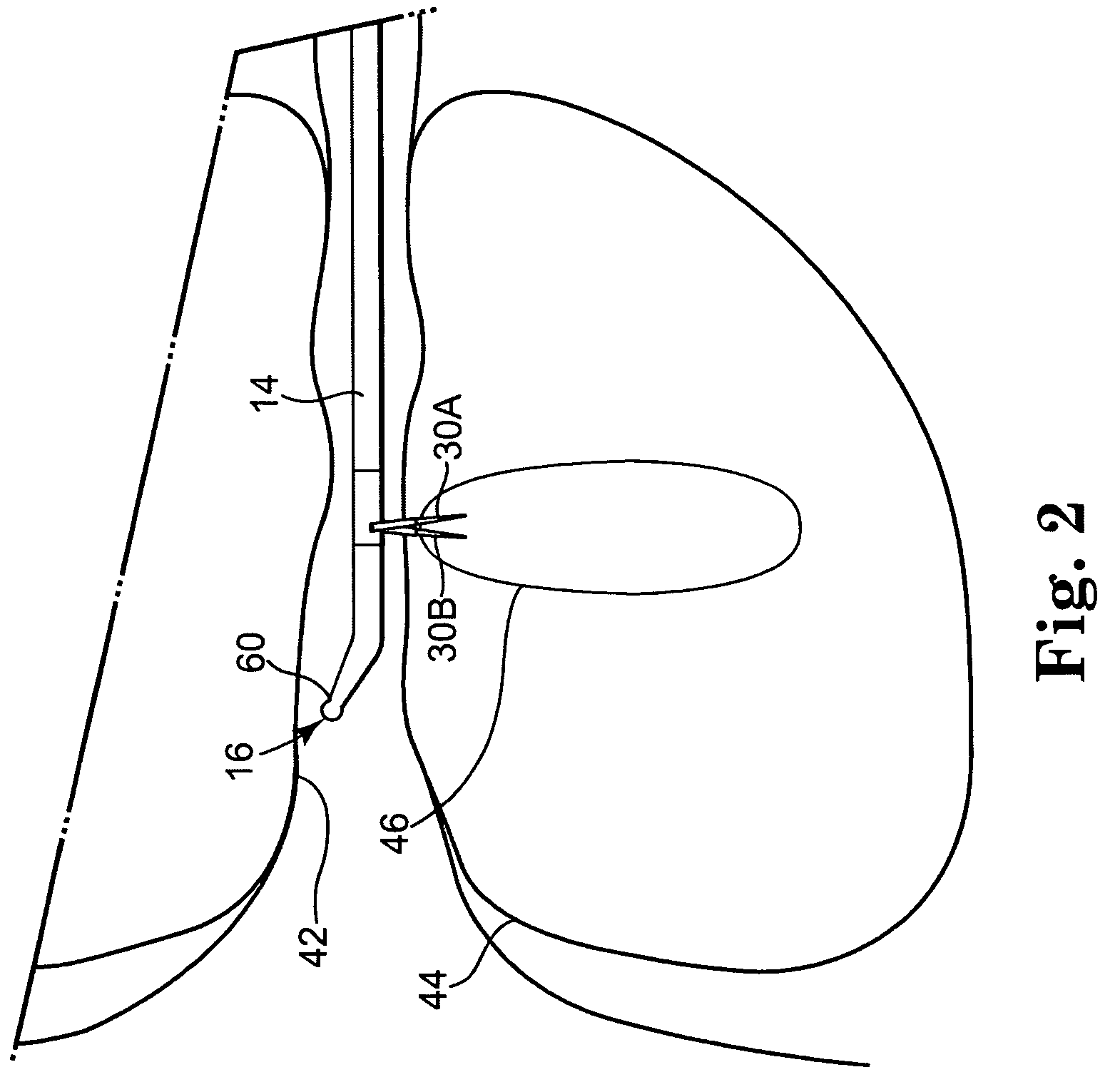
Method for repairing intervertebral discs
ActiveUS20080103564A1Increase pressureHalt leakageDiagnosticsPharmaceutical delivery mechanismIntervertebral discBiomedical engineering
A method of repairing a defect in an annulus fibrosus of an intervertebral disc, without excising the entire nucleus pulposus of the disc. The method includes inserting an introducer needle through the annulus fibrosus by puncturing the annulus fibrosus with the introducer needle, injecting an in situ curable, bio-compatible polymerizable or polymeric material composition into the disc through the introducer needle directly or indirectly so that the in situ curable composition contacts a defect in the annulus fibrosus; and curing said material in situ.
Owner:PAUZA KEVIN
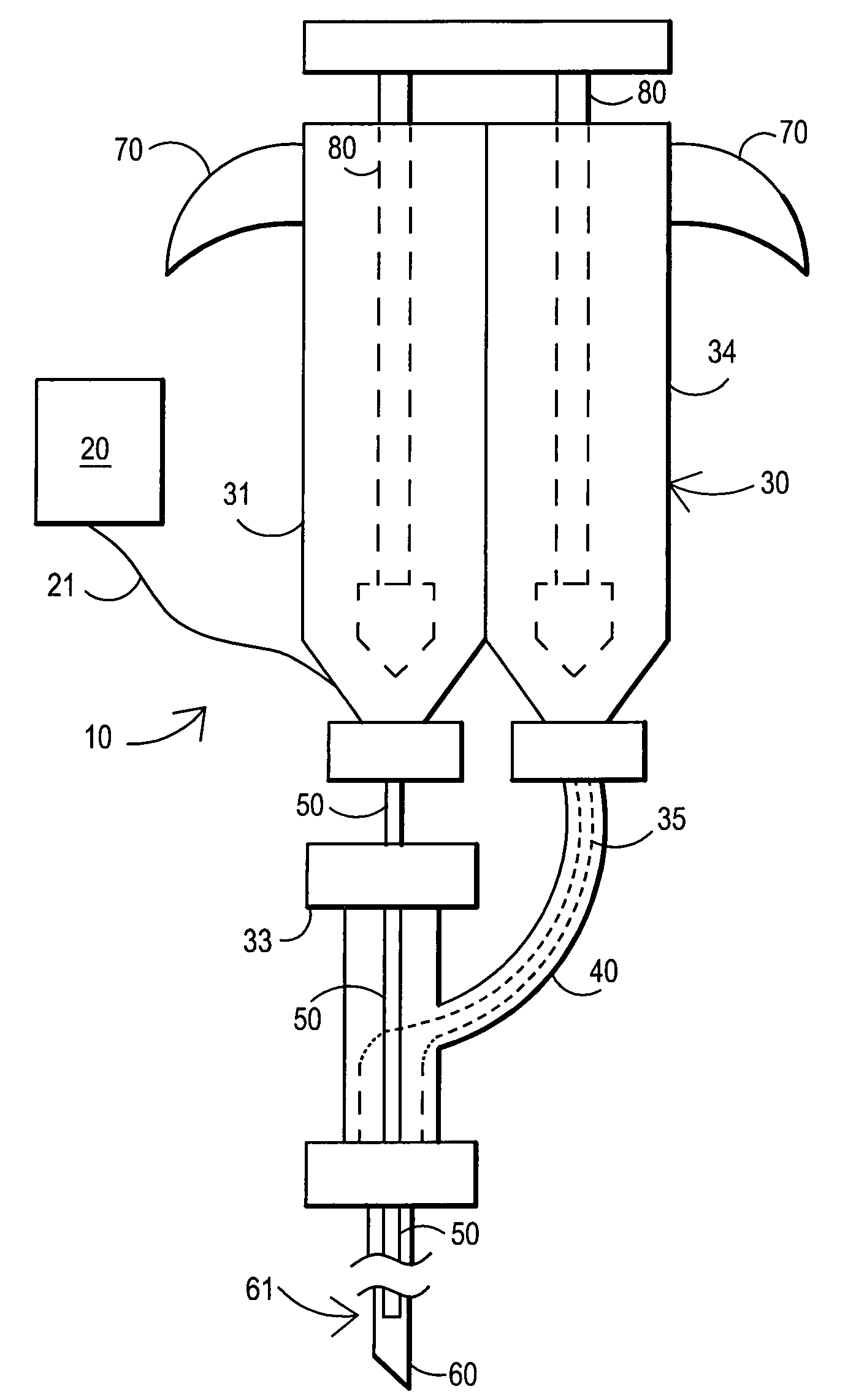
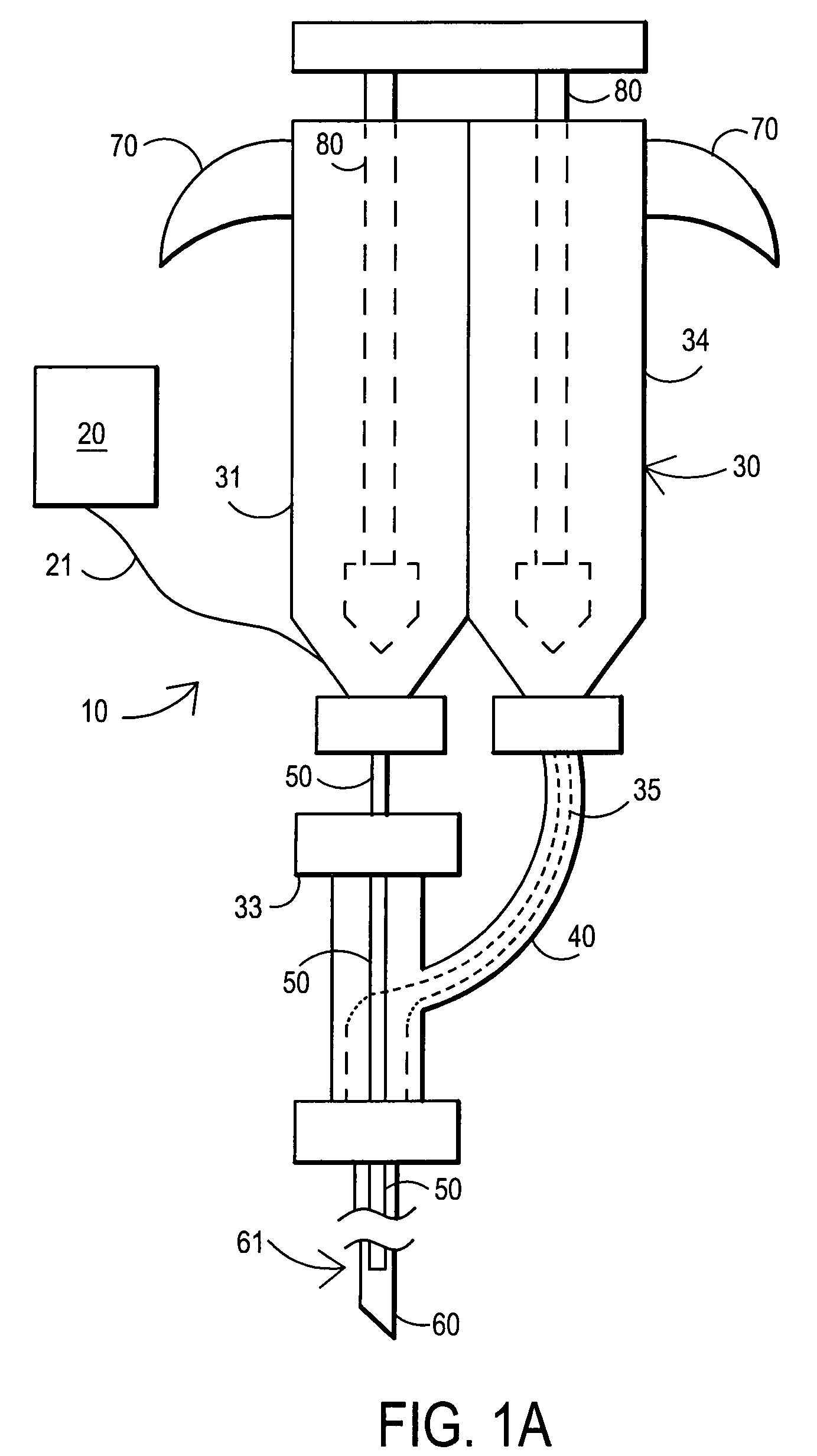
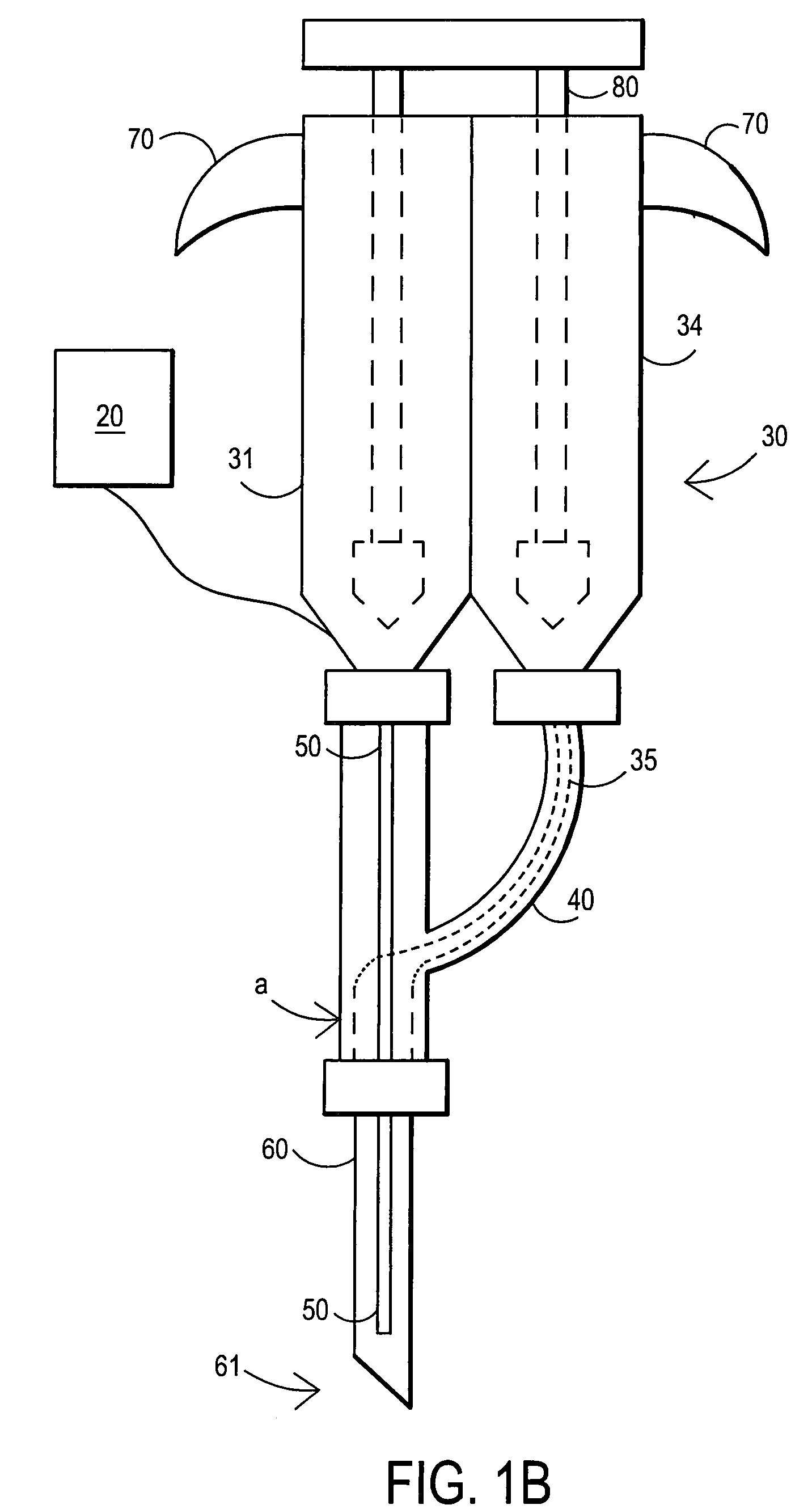
Devices and methods for facilitating fluid transport
ActiveUS20070078358A1Easy to transportShorten the timeDiagnostic recording/measuringSensorsFluid transportEngineering
Arrangements are provided including a base having a bore disposed therein extending from a first surface of the base through a second surface of the base, a fluid transport tube having a first end, a second end opposite the first end, and a lumen having an inner diameter, at least the second end of the tube being received within the bore of the base, and at least one fluid transport enhancing groove having at least a first section disposed in the second surface of the base and in fluid communication with the bore.
Owner:INTUITY MEDICAL INC
Methods and devices for renal nerve blocking
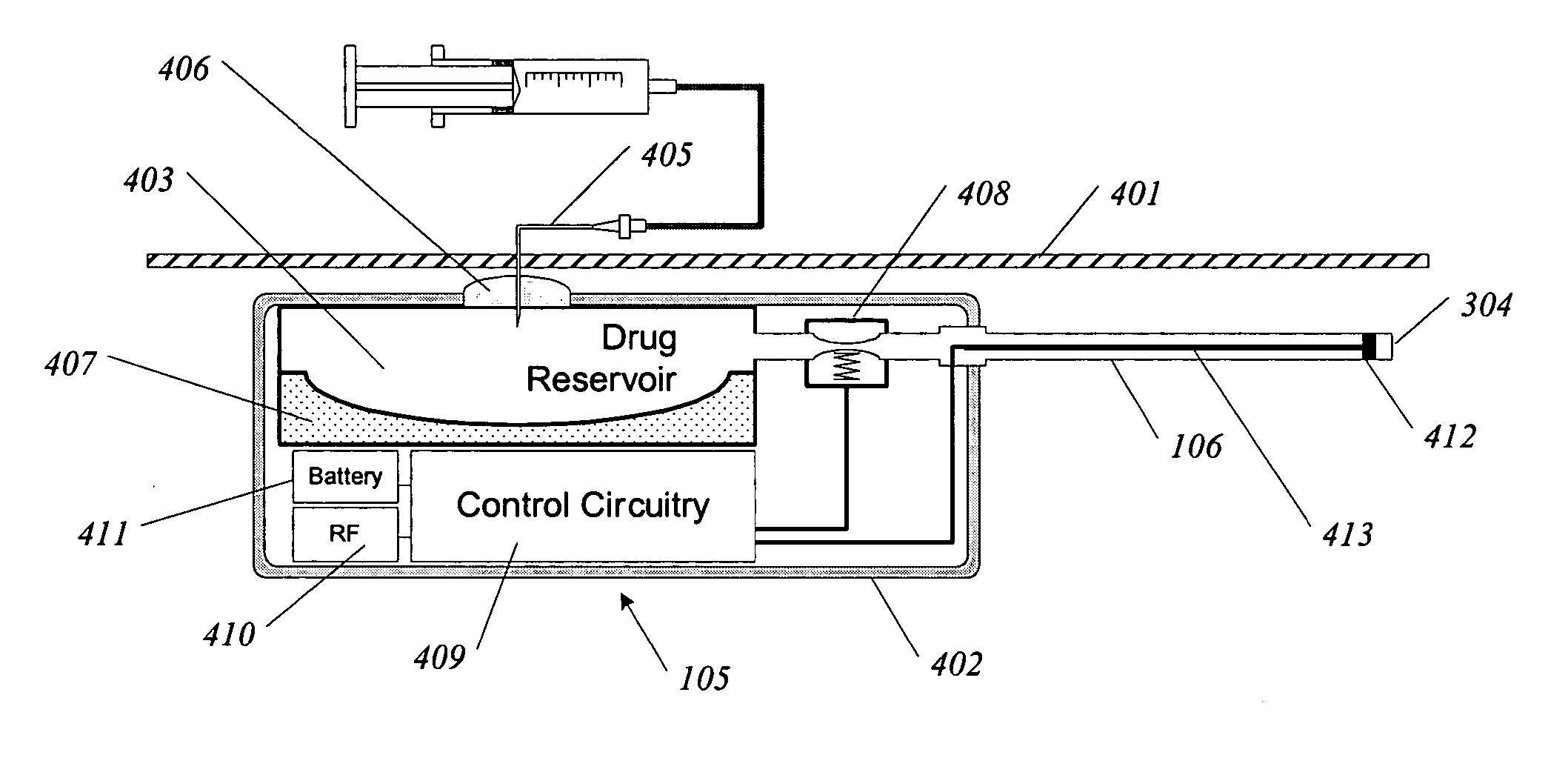
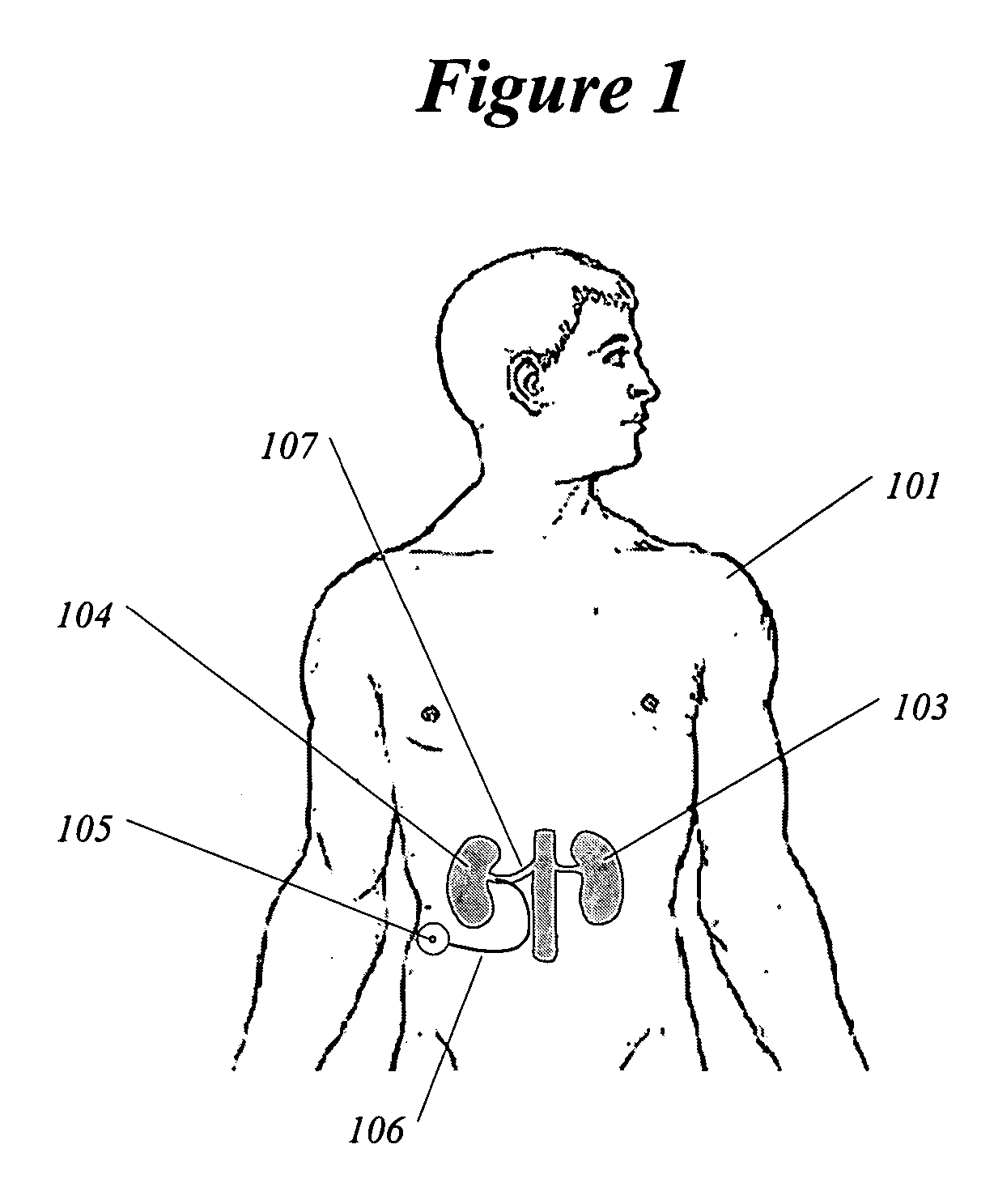
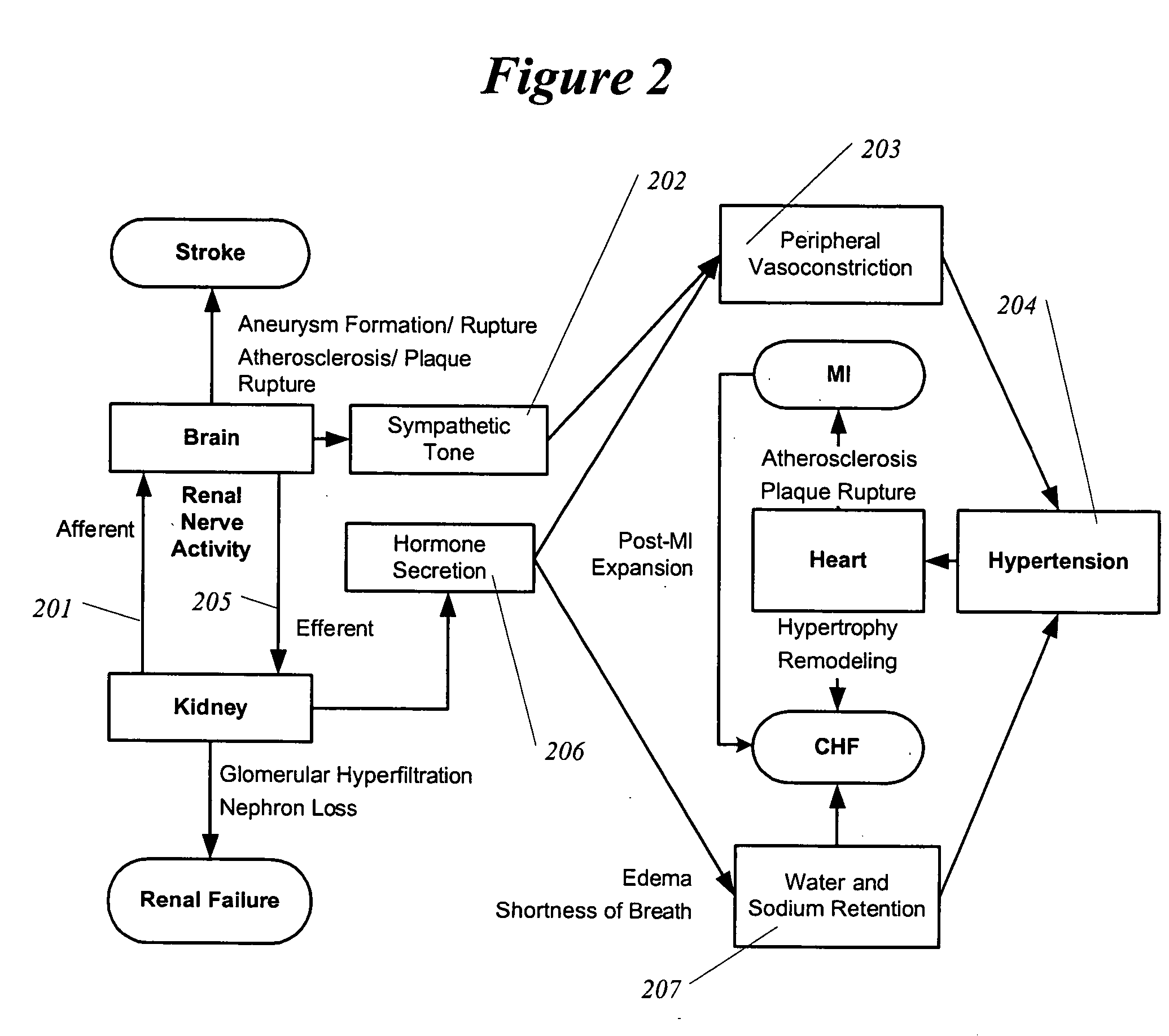
InactiveUS20050192638A1Shorten the progressResolution of overloadPharmaceutical delivery mechanismImplantable neurostimulatorsRenal nerveImplanted device
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump or a drug eluding implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
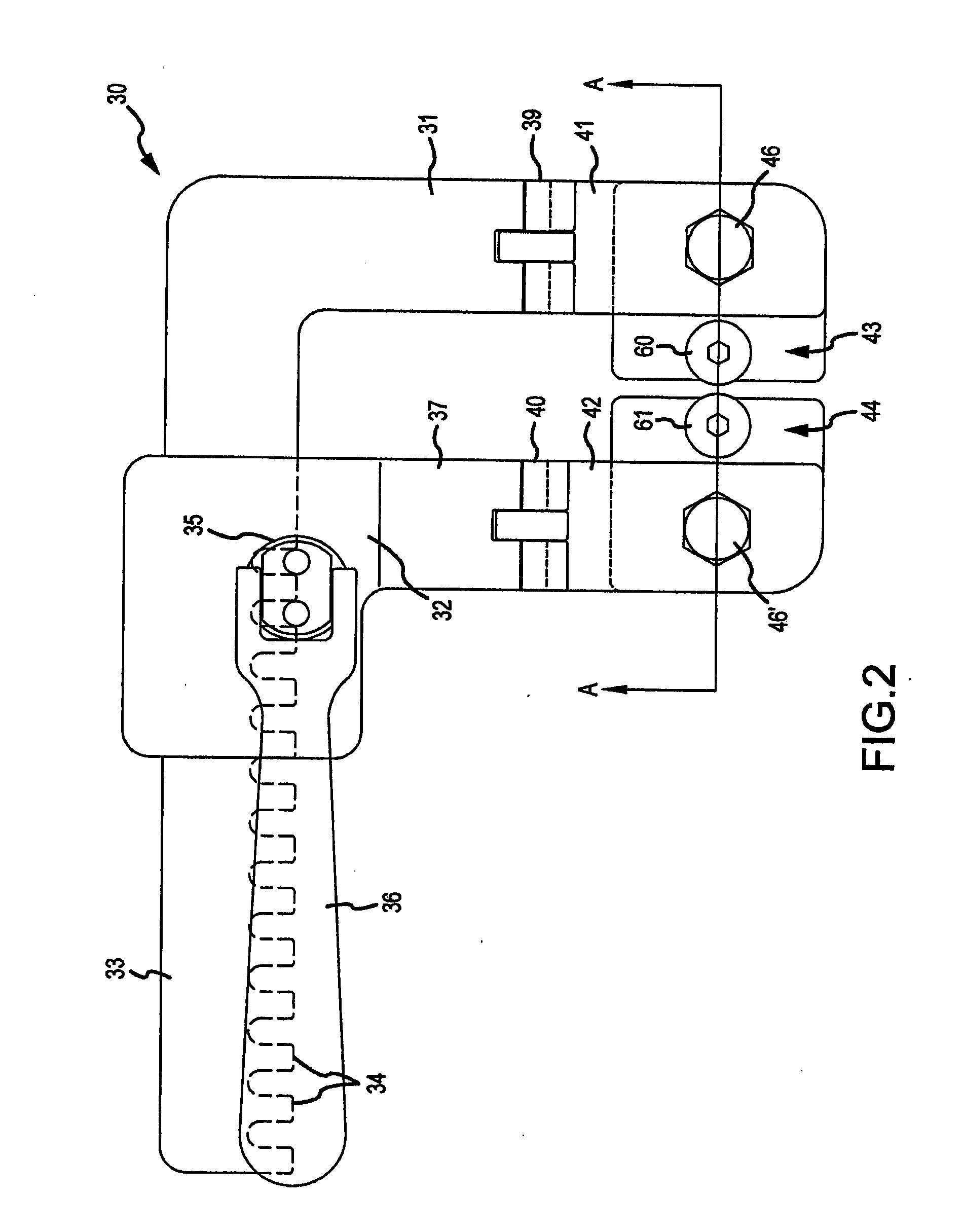
Techniques for reducing pain associated with nerve stimulation
Apparatus is provided including an electrode device and a control unit. The electrode device is configured to be coupled to a site of a subject selected from the group consisting of: a vagus nerve, an epicardial fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a right ventricle, a right atrium, and a jugular vein. The control unit is configured to drive the electrode device to apply to the site a current in at least first and second bursts, the first burst including a plurality of pulses, and the second burst including at least one pulse, and set (a) a pulse repetition interval (PRI) of the first burst to be on average at least 20 ms, (b) an interburst interval between initiation of the first burst and initiation of the second burst to be less than 10 seconds, (c) an interburst gap between a conclusion of the first burst and the initiation of the second burst to have a duration greater than the average PRI, and (d) a burst duration of the first burst to be less than a percentage of the interburst interval between, the percentage being less than 67%. Other embodiments are also described.
Owner:MEDTRONIC INC
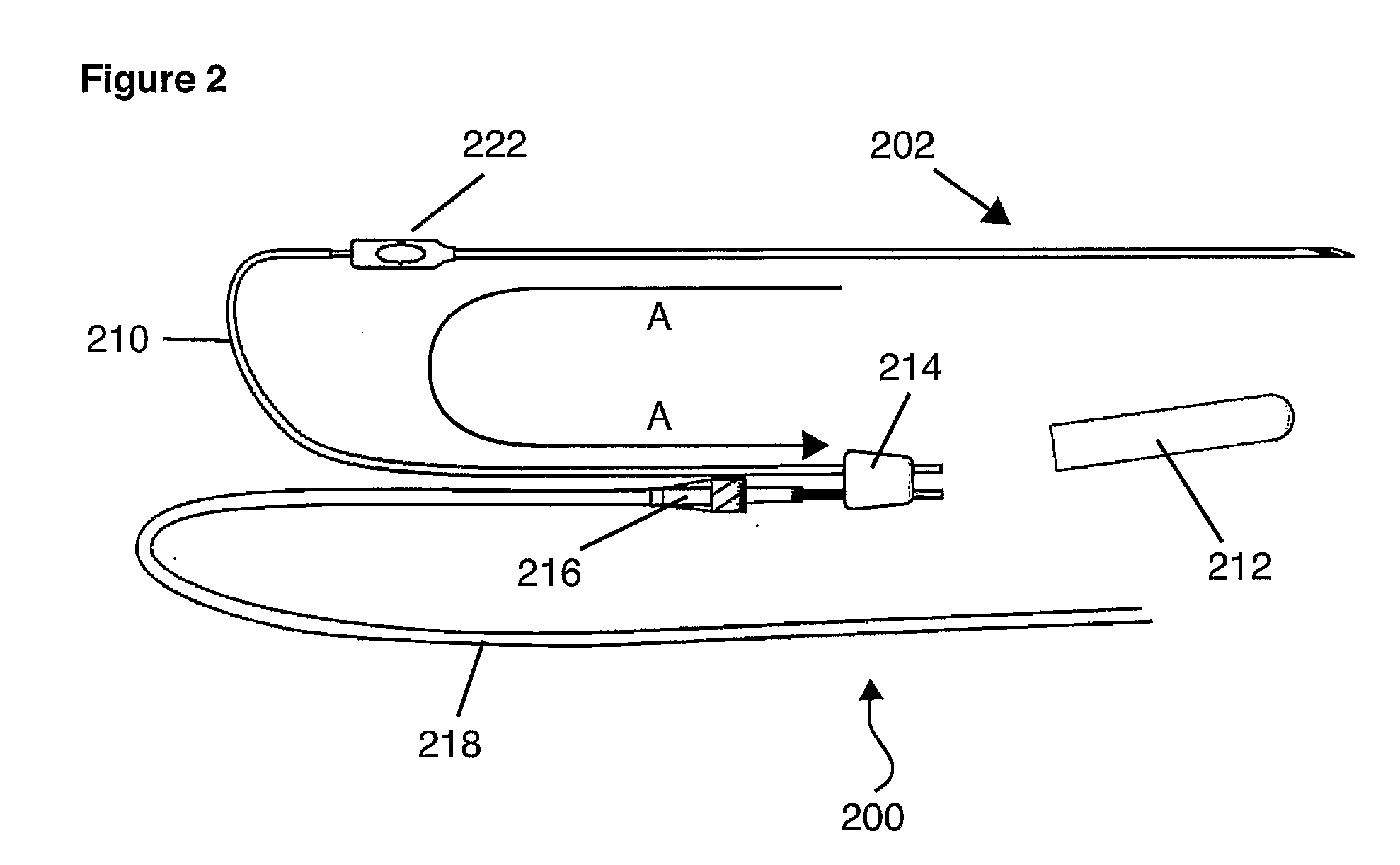
Transurethral needle ablation system
ActiveUS7261710B2Minimize preparation timeImprove throughputSurgical needlesSurgical instruments for heatingUrethraRigid core
A one-piece, single-use disposable device for transurethral needle ablation (TUNA) of prostate tissue to alleviate BPH is disclosed. The device may include a flexible catheter tip including a rigid core and a flexible tip. The device may also include a single use lockout to help ensure that the device is used to perform only one ablation procedure on a single patient. The device may further include a simplified needle deployment mechanism and / or an automatic needle retraction mechanism.
Owner:MEDTRONIC INC
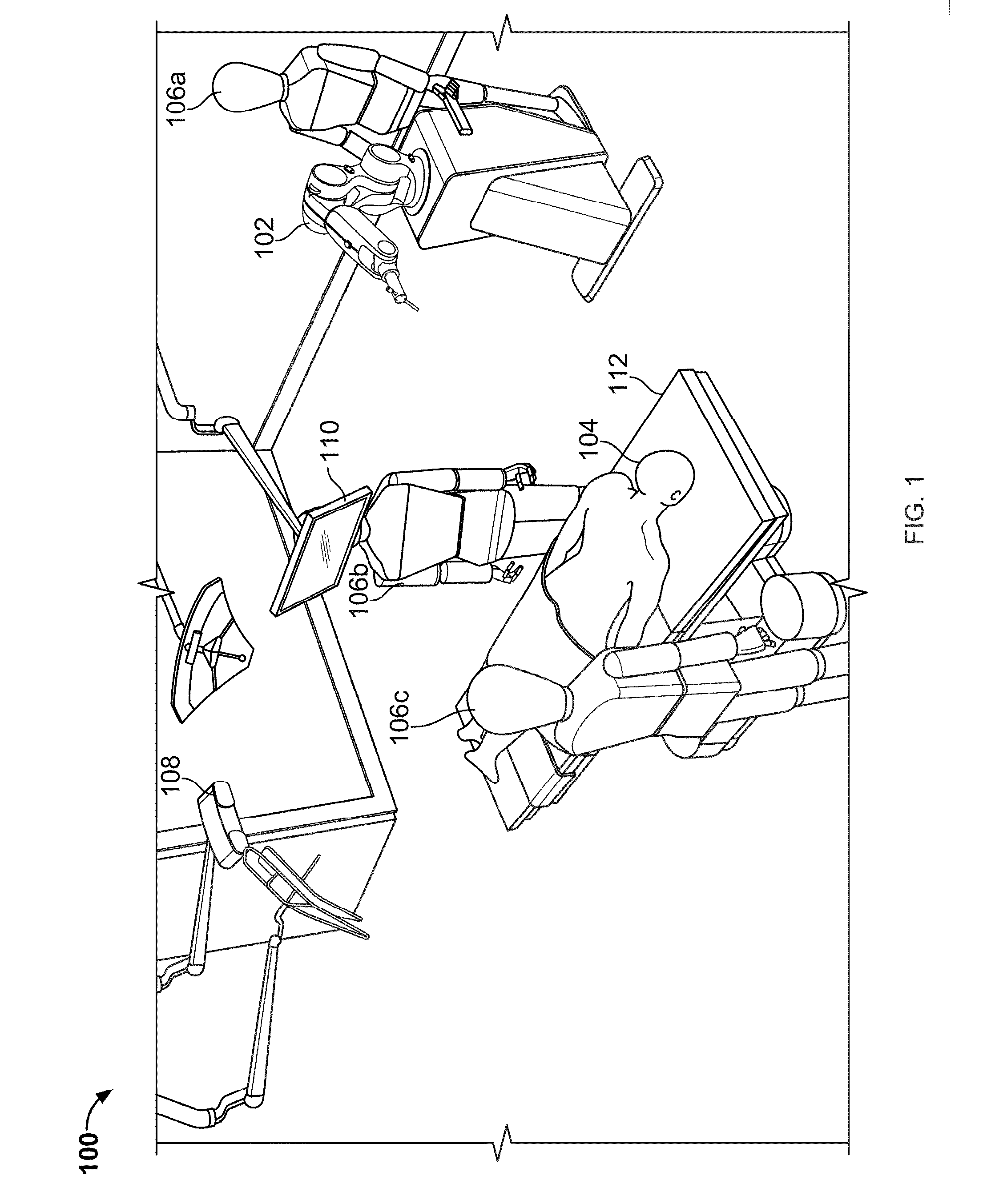
Systems and methods for performing minimally invasive spinal surgery with a robotic surgical system using a percutaneous technique
ActiveUS20160235492A1Improve precisionImprove usabilityProgramme controlProgramme-controlled manipulatorLess invasive surgerySkin opening
Described herein are systems, apparatus, and methods for precise placement and guidance of tools during surgery, particularly spinal surgery, using minimally invasive surgical techniques. Several minimally invasive approaches to spinal surgeries were conceived, percutaneous technique being one of them. This procedures looks to establish a skin opening as small as possible by accessing inner organs via needle-puncture of the skin. The percutaneous technique is used in conjunction with a robotic surgical system to further enhance advantages of manual percutaneous techniques by improving precision, usability and / or shortening surgery time by removal of redundant steps.
Owner:KB MEDICAL SA
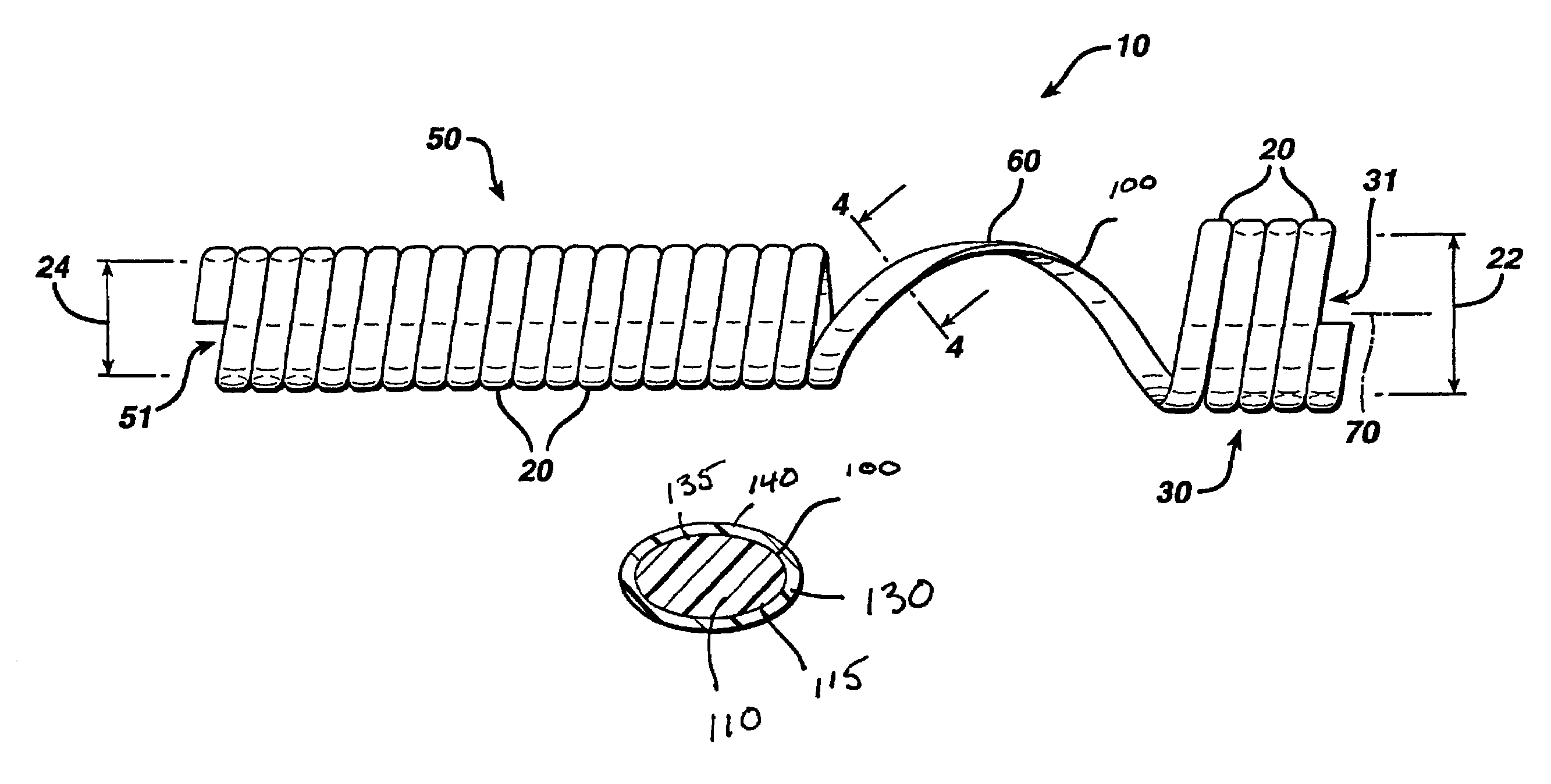
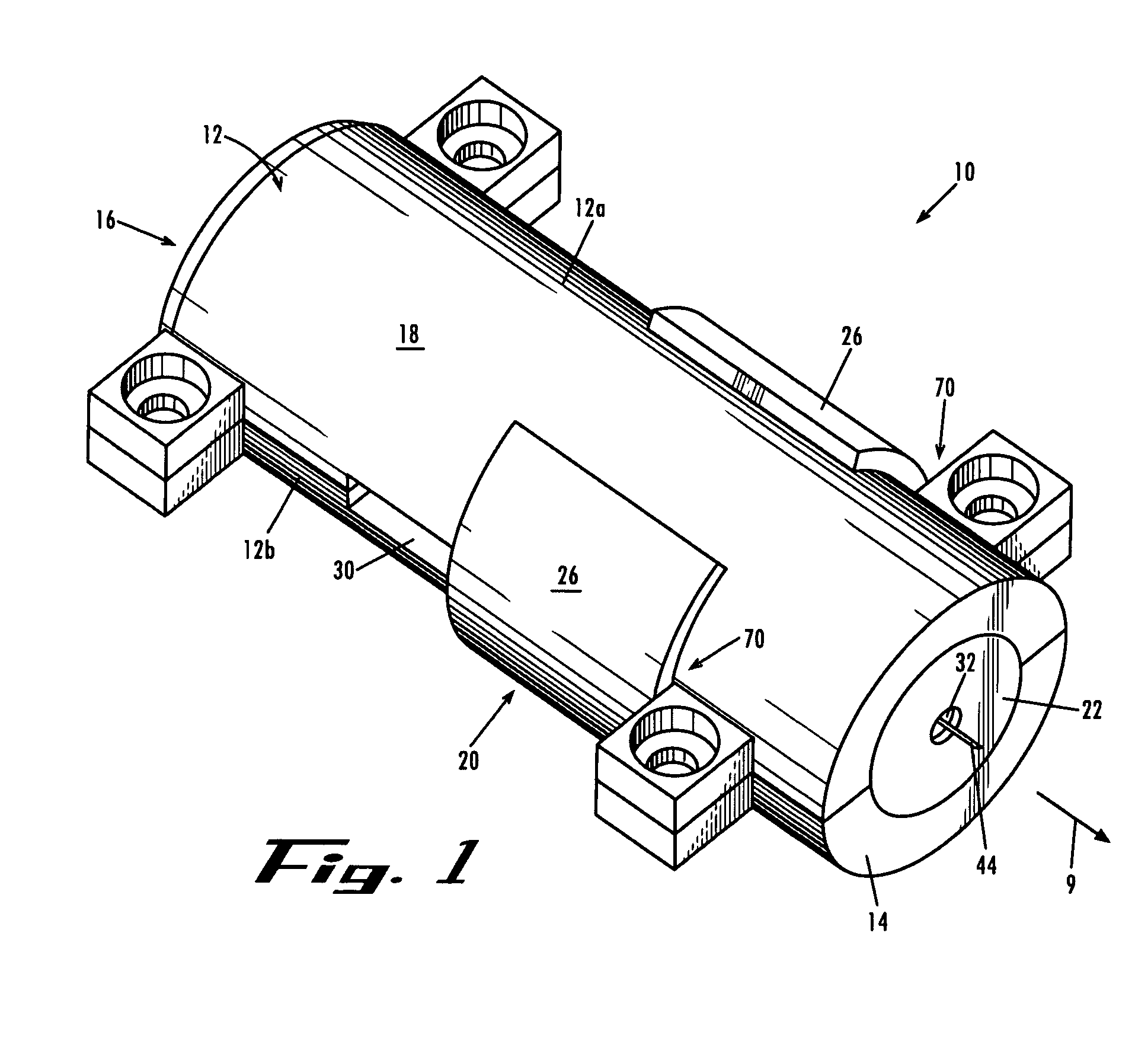
Biodegradable stent
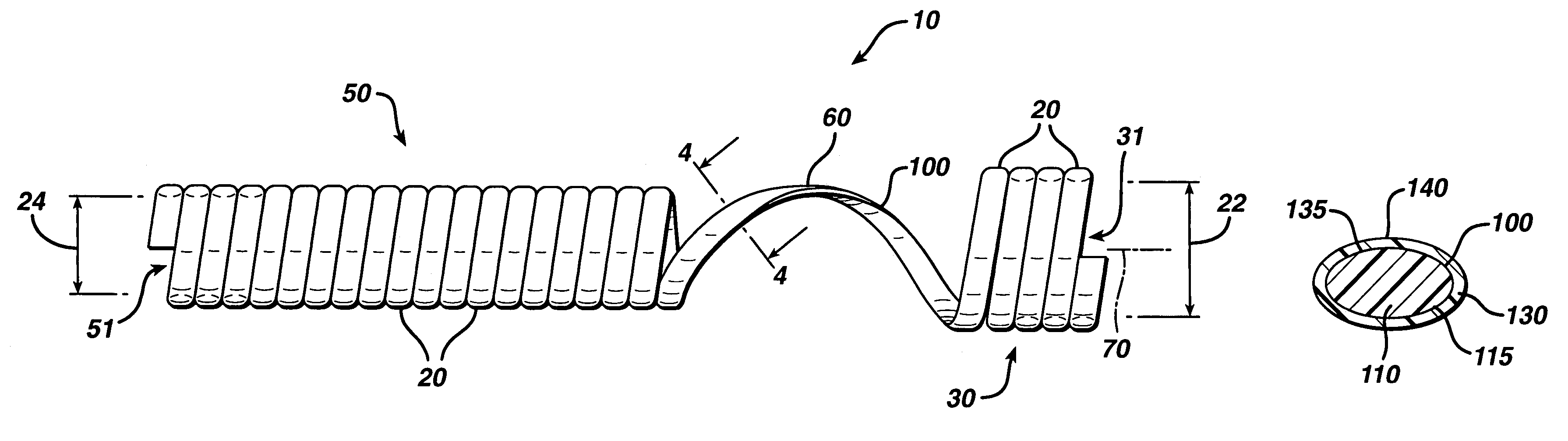
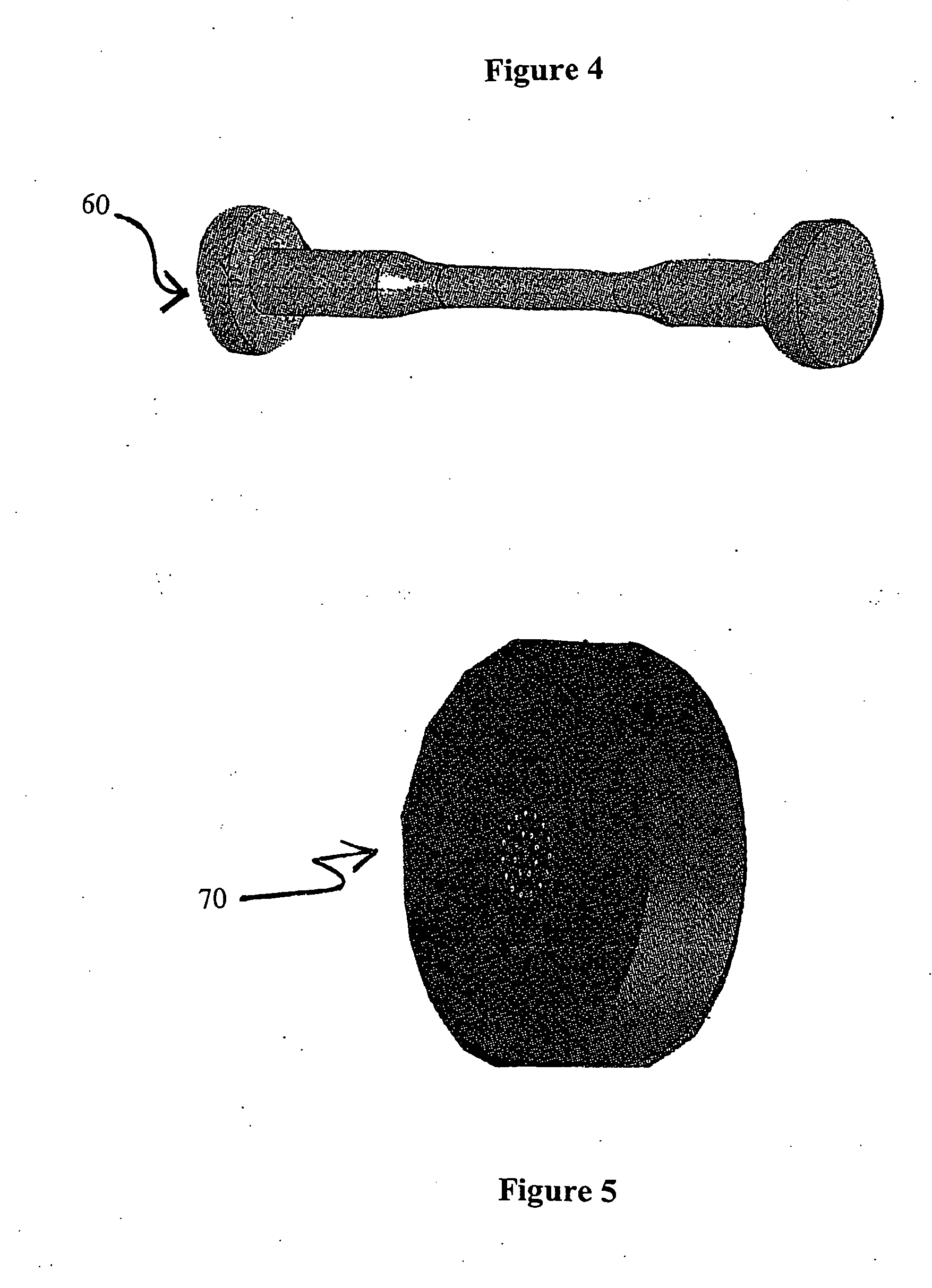
The present invention is directed to stents that are formed into a helical structure having a plurality of coils, a longitudinal axis, an internal longitudinal passage, a distal section and a proximal section having different diameters; where the structure is made from a fiber having a cross-section and including an inner core having an exterior surface made from a biodegradable polymer having a first degradation rate and an outer section made from a blend of a first biodegradable polymer component and a second biodegradable polymer component covering the exterior surface of the inner core and having a second degradation rate; where the second degradation rate is lower than the first degradation rate.
Owner:ETHICON INC
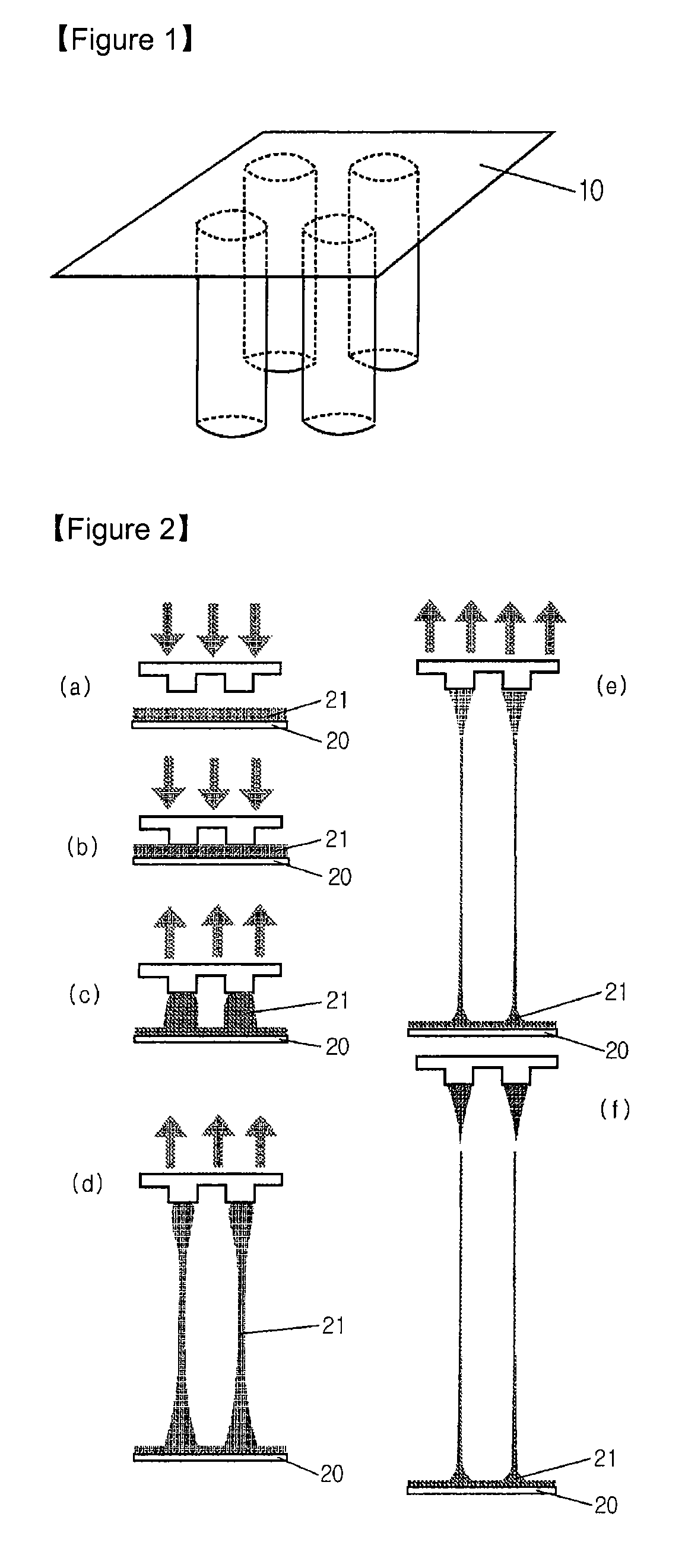
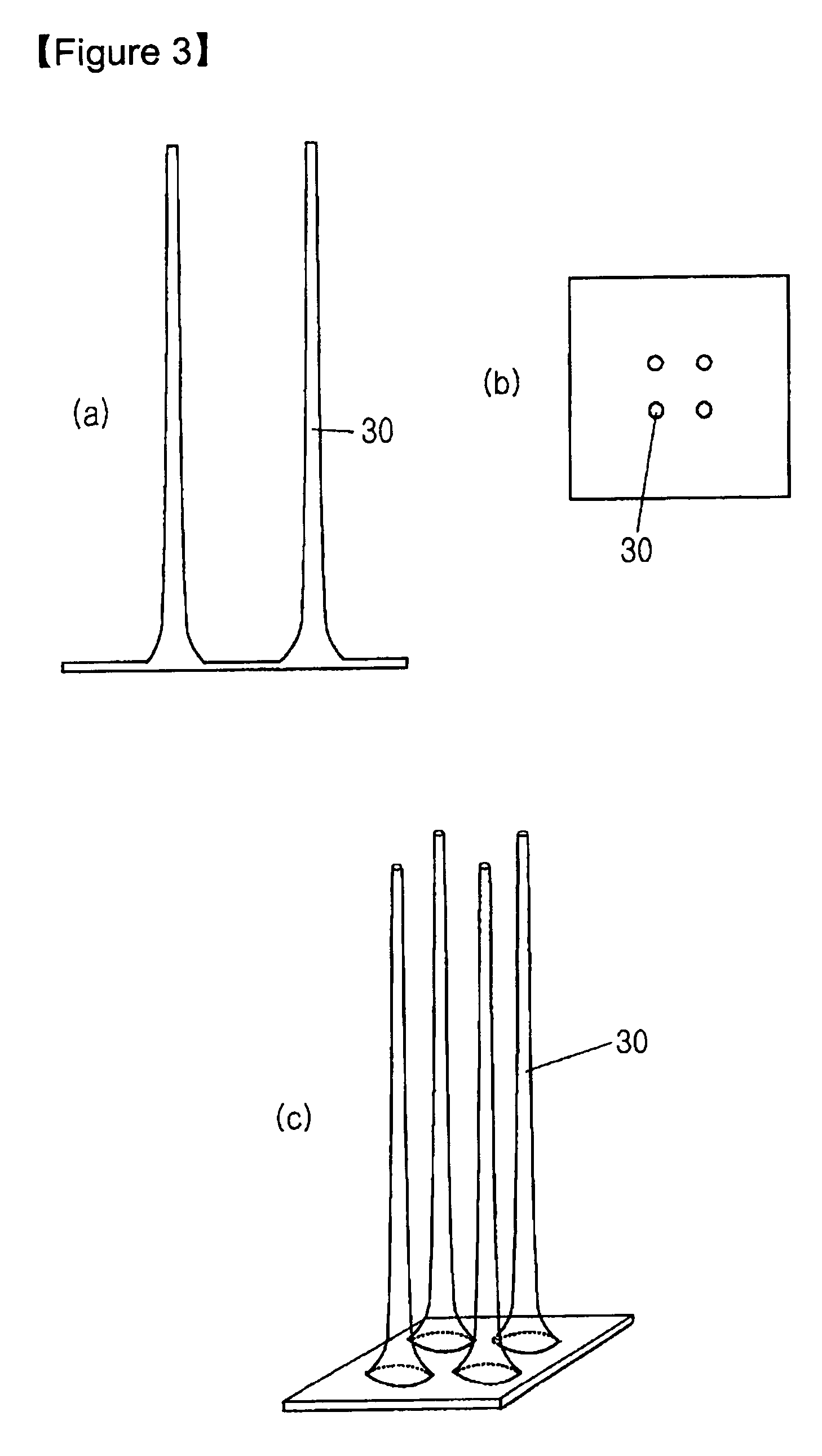
Solid type microneedle and methods for preparing it
Disclosed herein are biodegradable solid microneedles and a fabrication method thereof. The microneedles are small in diameter and are long and hard enough to pass through the stratum corneum. Thus, the biodegradable solid microneedles can be used for painless transdermal drug delivery, the detection of biological samples such as blood, and biopsy.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Biodegradable stent
A biodegradable stent for implantation into a lumen in a human body. The stent in one embodiment is made from a biodegradable fiber having an inner core and an outer layer. The outer layer is a blend of two polymer components. The inner core has a first degradation rate, and the outer layer has a second degradation rate. The second degradation rate is slower than the first degradation rate. The fiber softens in vivo such that the stent is readily passed from the lumen as a softened fragment or filament after a pre-determined period of time through normal flow of body fluids passing through the lumen.
Owner:ETHICON INC
Transurethral needle ablation system with flexible catheter tip
ActiveUS20060079880A1Minimize preparation timeHigh patient throughputSurgical needlesSurgical instruments for heatingUrethraRigid core
A one-piece, single-use disposable device for transurethral needle ablation (TUNA) of prostate tissue to alleviate BPH is disclosed. The device may include a flexible catheter tip including a rigid core and a flexible tip. The device may also include a single use lockout to help ensure that the device is used to perform only one ablation procedure on a single patient. The device may further include a simplified needle deployment mechanism and / or an automatic needle retraction mechanism.
Owner:MEDTRONIC INC
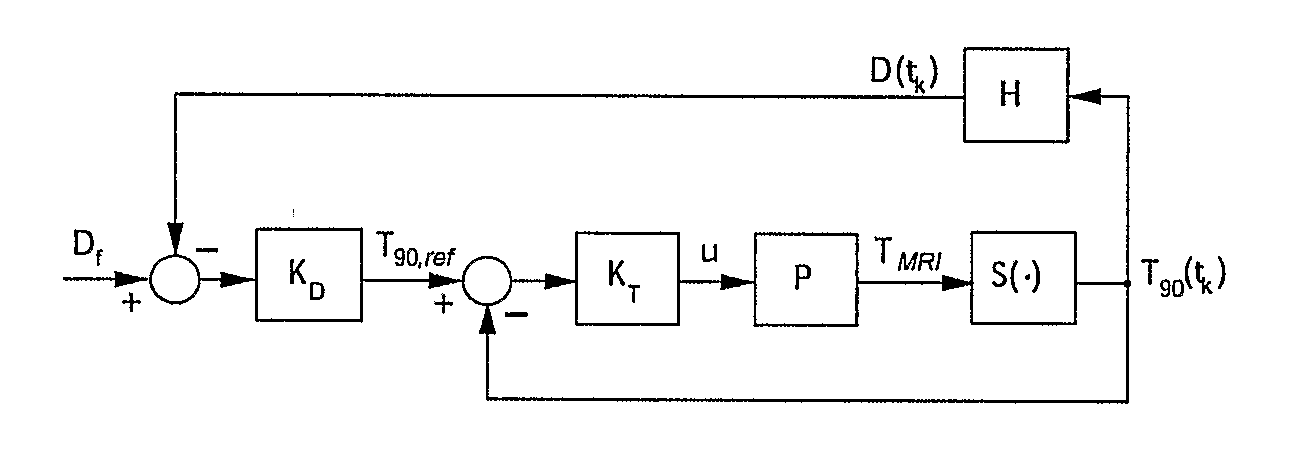
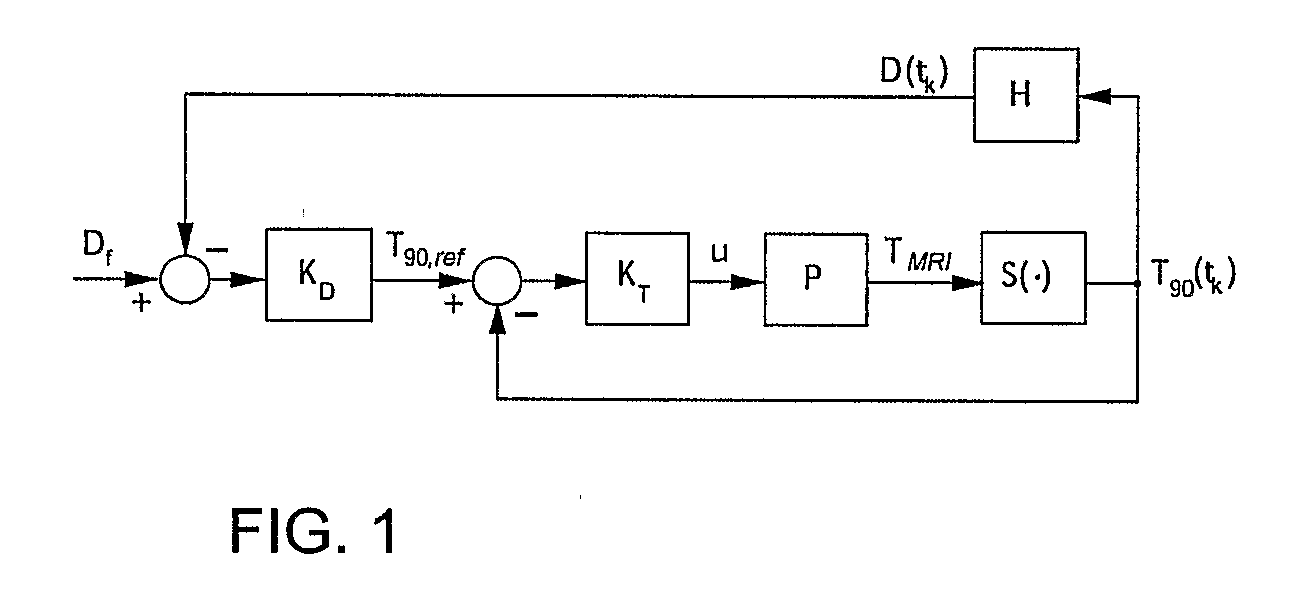
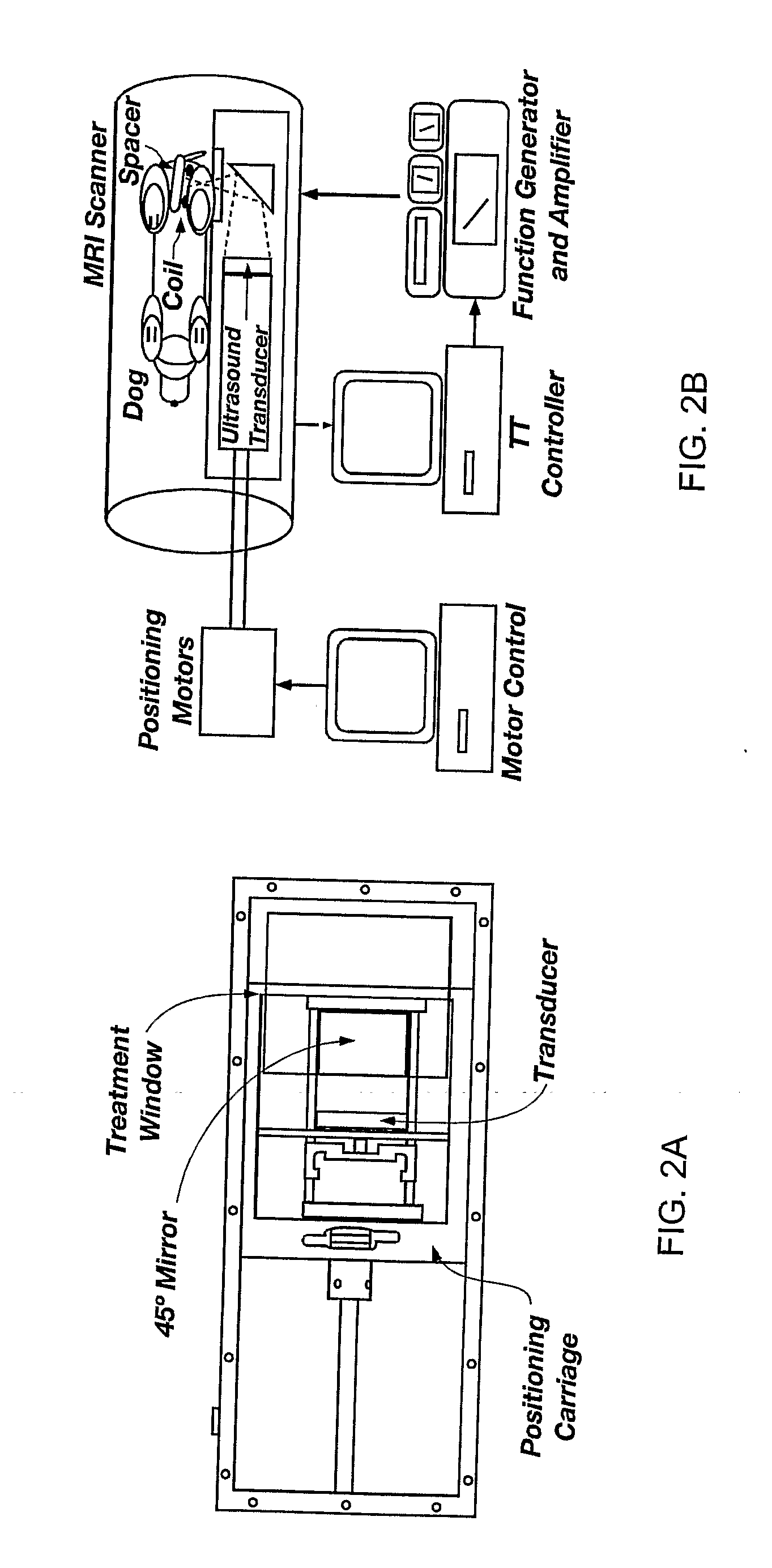
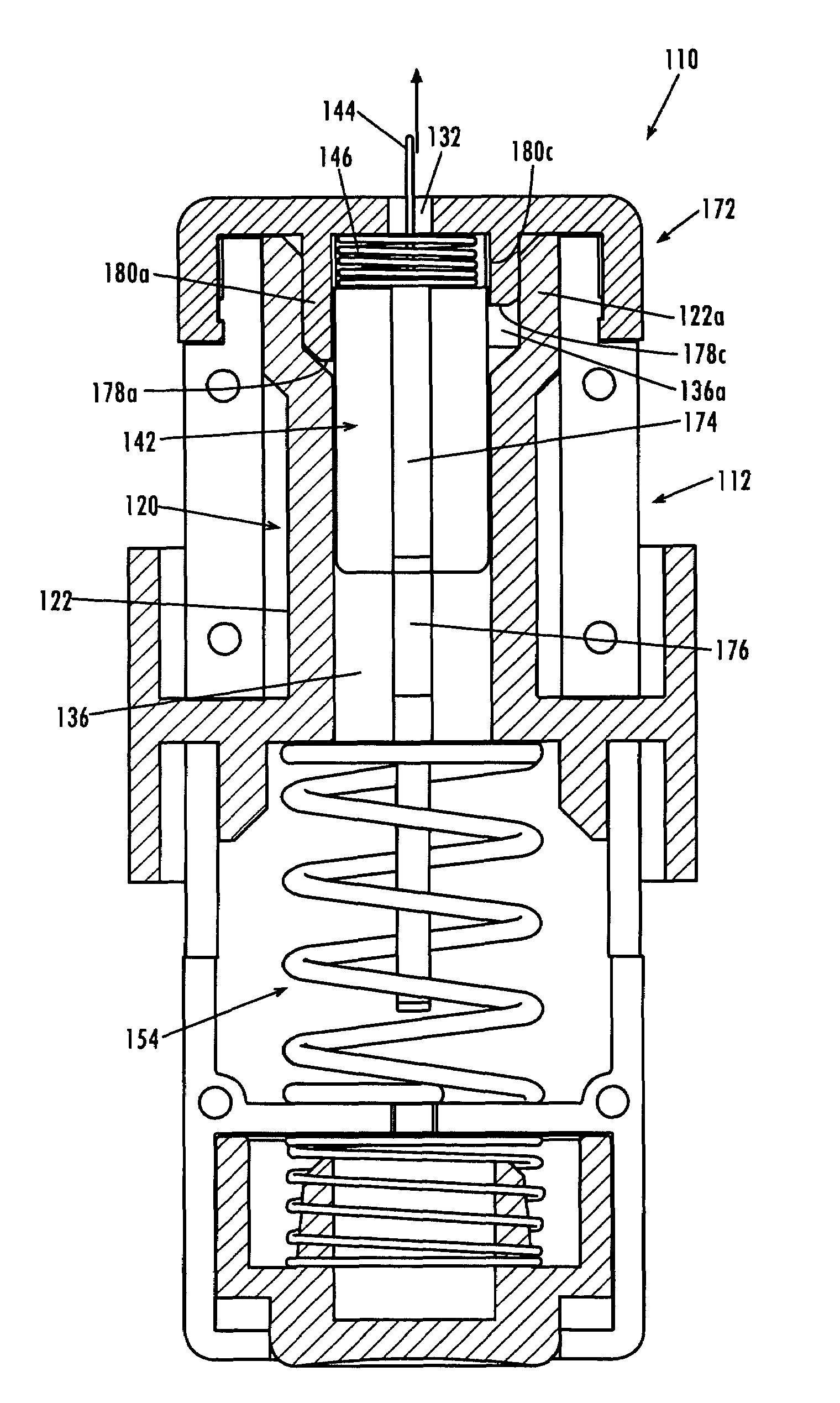
Minimum time feedback control of efficacy and safety of thermal therapies
InactiveUS20110137147A1Robustness with respectShorten treatment timeUltrasound therapySurgical instrument detailsMedicineFeedback controller
A thermal treatment control system including an imaging device for specifying the geometry and / or location of the treatment target, a thermal energy element for applying a thermal treatment for the heating or cooling of a target tissue for therapeutic purposes, a thermal energy detecting element for detecting a measured tissue response to the thermal treatment and a feedback controller for a real-time modification of the intensity and spatial distribution of the thermal dose in order to achieve therapeutic efficacy over a minimum or reduced treatment time while satisfying treatment constraints imposed to limit damage to normal tissues.
Owner:UNIV OF UTAH RES FOUND
Biodegradable stents
InactiveUS20070050018A1Faster rate of degradationFaster rateStentsBlood vesselsInsertion stentGenetic Materials
A stent comprising a matrix and a fiber reinforcement about which the matrix is chemically or mechanically attached. The matrix is provided with heavier loads of pharmaceutically active ingredients or genetic materials as a result of the increased strength and mechanical characteristics provided to the stent by the fiber reinforcement. The fiber reinforcement can be comprised of a plurality of mono-filament fibers spaced and oriented in a flat weave pattern to which the matrix is chemically or mechanically attached. Degradation rates of the materials that comprise the matrix and the fiber reinforcement can be varied to vary the time period in which the stent maintains its mechanical characteristics or releases the pharmaceutically active ingredients or genetic materials therefrom. Multiple stage release profiles can be provided by providing multiple layers of matrices and fiber reinforcements, whereby different pharmaceutically active ingredients or genetic materials or different concentrations thereof, can be released according to the degradation profiles of the matrix and fiber reinforcment.
Owner:WAINWRIGHT JOHN
Techniques for reducing pain associated with nerve stimulation
ActiveUS20110137365A1Minimize damagePain minimizationHeart stimulatorsArtificial respirationVeinCoronary sinus
Owner:MEDTRONIC INC
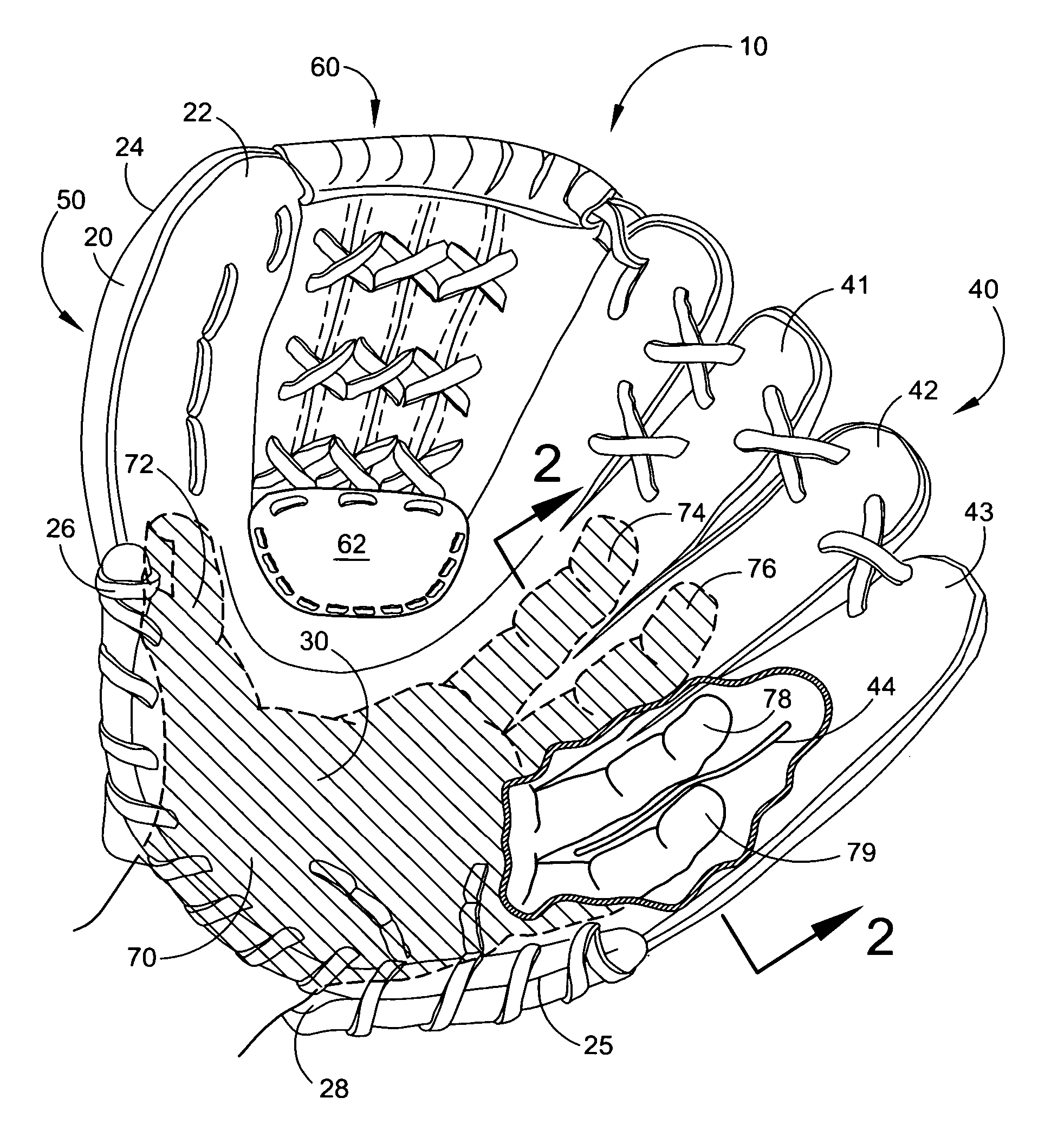
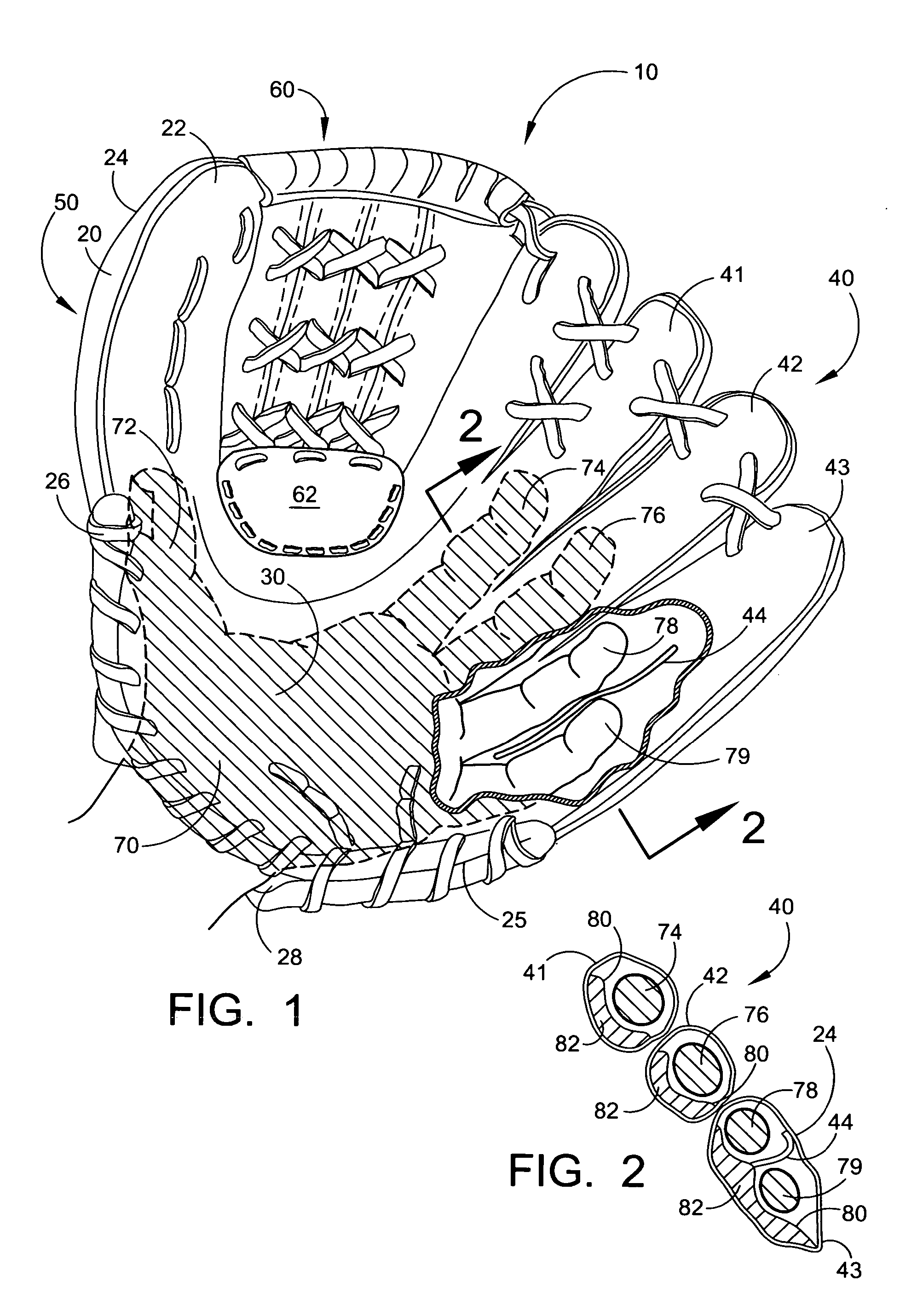
Baseball glove
InactiveUS20060218689A1Pain minimizationMinimize discomfortSport apparatusProtective garmentEngineeringMechanical engineering
The present invention is directed to a new and improved baseball glove. The glove includes an outer shell that contains a finger portion, a thumb portion, and a palm portion. The finger portion comprises two or three finger holsters, the outermost holster accommodates two or three fingers, the remaining finger holster(s) accommodates one or two fingers. One or more finger separator(s) reside within the outermost finger holster to prevent discomfort of the fingers. A web portion is attached between the finger portion and the thumb portion. A protective padding strip is located within the surrounding area immediately adjacent to the web portion. The finger portion and thumb portion are spaced apart to minimize the impact of a caught ball with the user's hand. A web-reinforcement patch is located over a variable region surrounding the intersection of the lower region of the web portion and the outer shell.
Owner:BROWN TIMOTHY E
Biodegradable stent
A biodegradable stent for implantation into a lumen in a human body. The stent in one embodiment is made from a biodegradable fiber having an inner core and an outer layer. The outer layer is a blend of two polymer components. The inner core has a first degradation rate, and the outer layer has a second degradation rate. The second degradation rate is slower than the first degradation rate. The fiber softens in vivo such that the stent is readily passed from the lumen as a softened fragment or filament after a pre-determined period of time through normal flow of body fluids passing through the lumen.
Owner:ETHICON INC
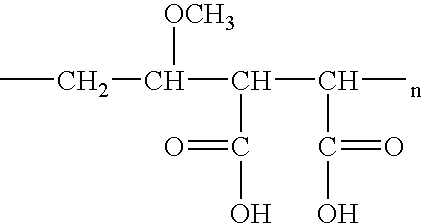
Improved buffer gel for iontophoresis electrodes
InactiveUS20050197618A1Efficient deliveryImprove buffering effectBiocideOrganic active ingredientsBuffering agentBiomedical engineering
An iontophoretic delivery device utilizing a polymeric gel matrix to ameliorate pH deviation within the device wherein the pH of the polymeric gel matrix is buffered at select pH ranges in order to reduce skin irritation. The polymeric gel matrix is housed within the iontophoretic delivery device as a buffering agent wherein the matrix provides a suitable physiological pH level above 4.0 and particularly the pH level deviates between approximately 4.1 to approximately 4.9, and preferably at the pH 4.5 for an effective operation of the iontophoretic delivery of a medicament to treat affected areas of a living subject's body.
Owner:ENCORE MEDICAL ASSET CORP
Thoracic retractors and methods
InactiveUS20120330106A1Pain minimizationAvoid contactSuture equipmentsSurgical needlesIntercostal nervesLongissimus Thoracis
A surgical thoracic retractor has retraction members that grip the anterior and posterior surfaces of the ribs between vice-like jaws to prevent a crushing or other force being applied to the intercostal nerves, thus minimizing the patient's post-operative pain.
Owner:GENESEE BIOMEDICAL
Method for repairing intervertebral discs
ActiveUS8357147B2Reduce and eliminate migrationAvoid contactDiagnosticsMedical devicesIntervertebral diskBiomedical engineering
A method of repairing a defect in an annulus fibrosus of an intervertebral disc, without excising the entire nucleus pulposus of the disc. The method includes inserting an introducer needle through the annulus fibrosus by puncturing the annulus fibrosus with the introducer needle, injecting an in situ curable, bio-compatible polymerizable or polymeric material composition into the disc through the introducer needle directly or indirectly so that the in situ curable composition contacts a defect in the annulus fibrosus; and curing said material in situ.
Owner:PAUZA KEVIN
Portable, adjustable disposable medical suction system and method of use
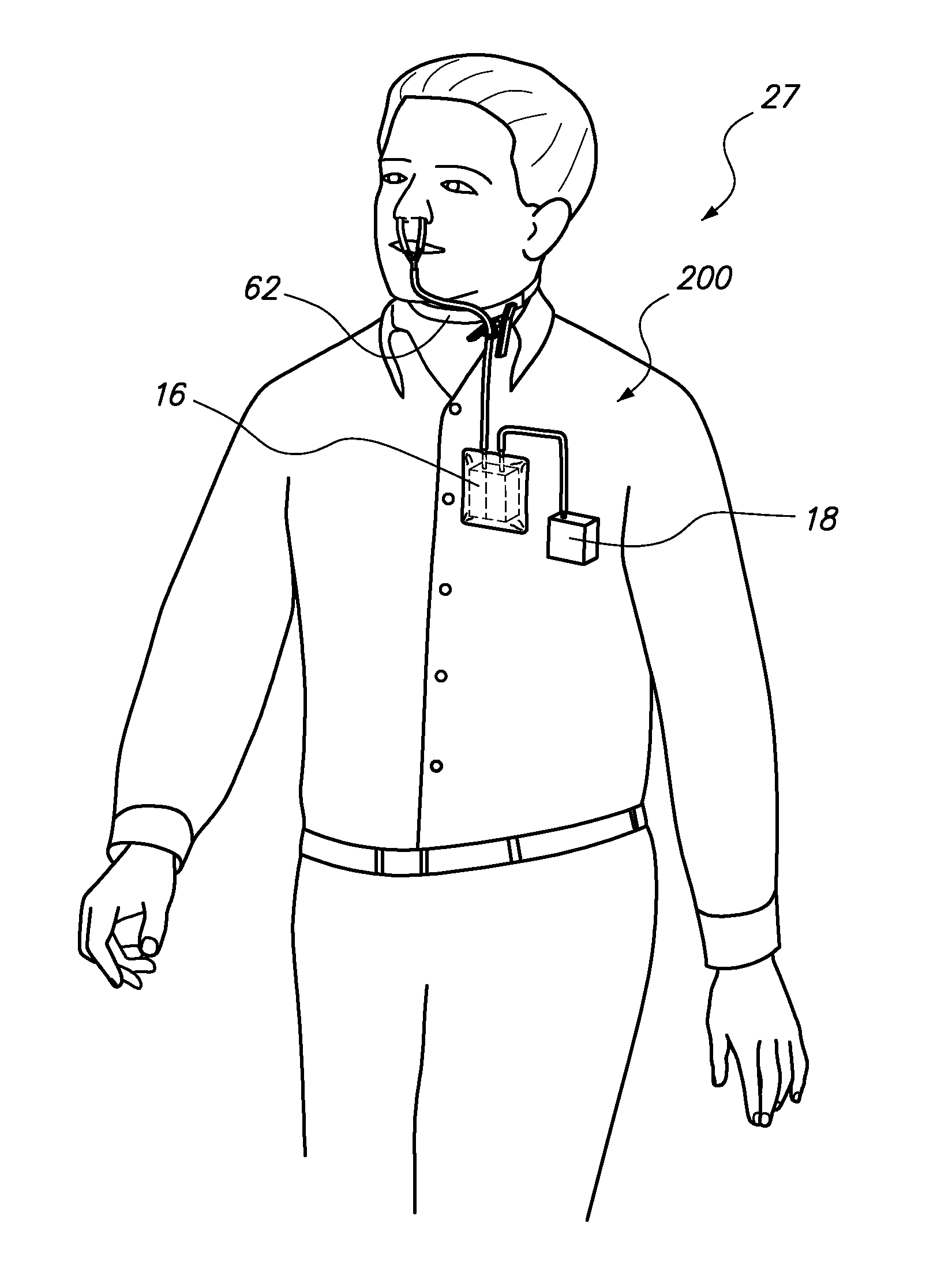
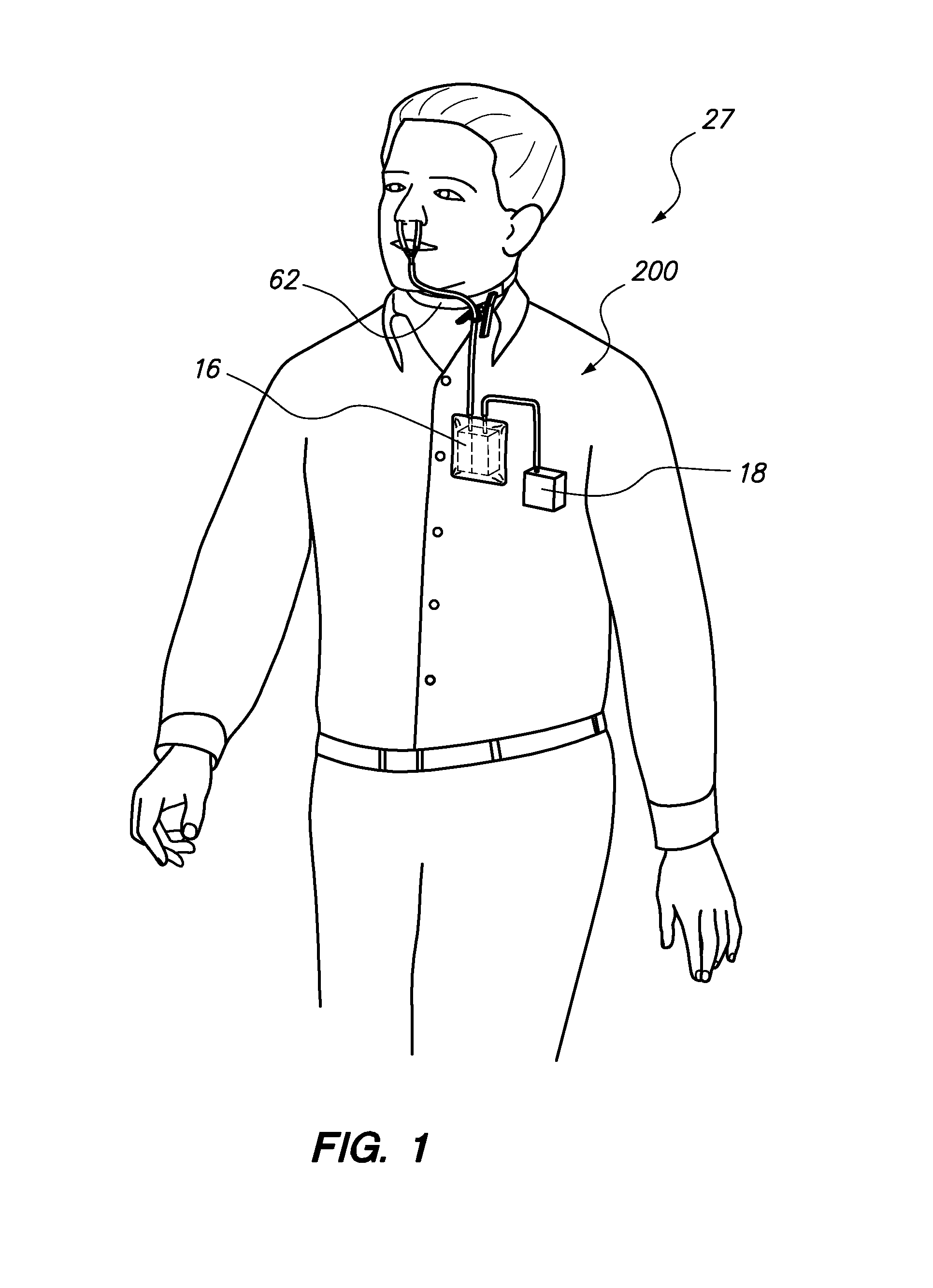
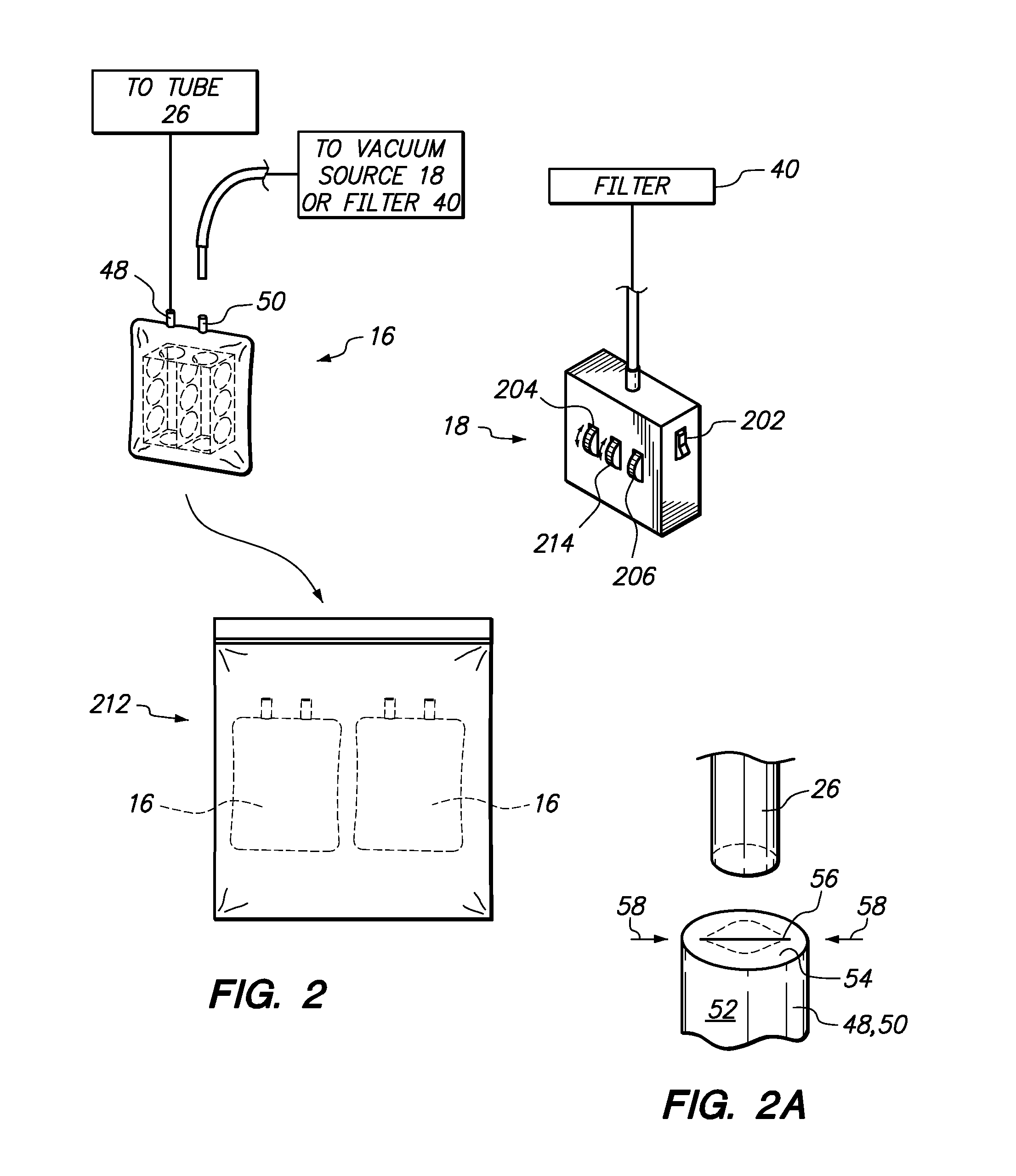
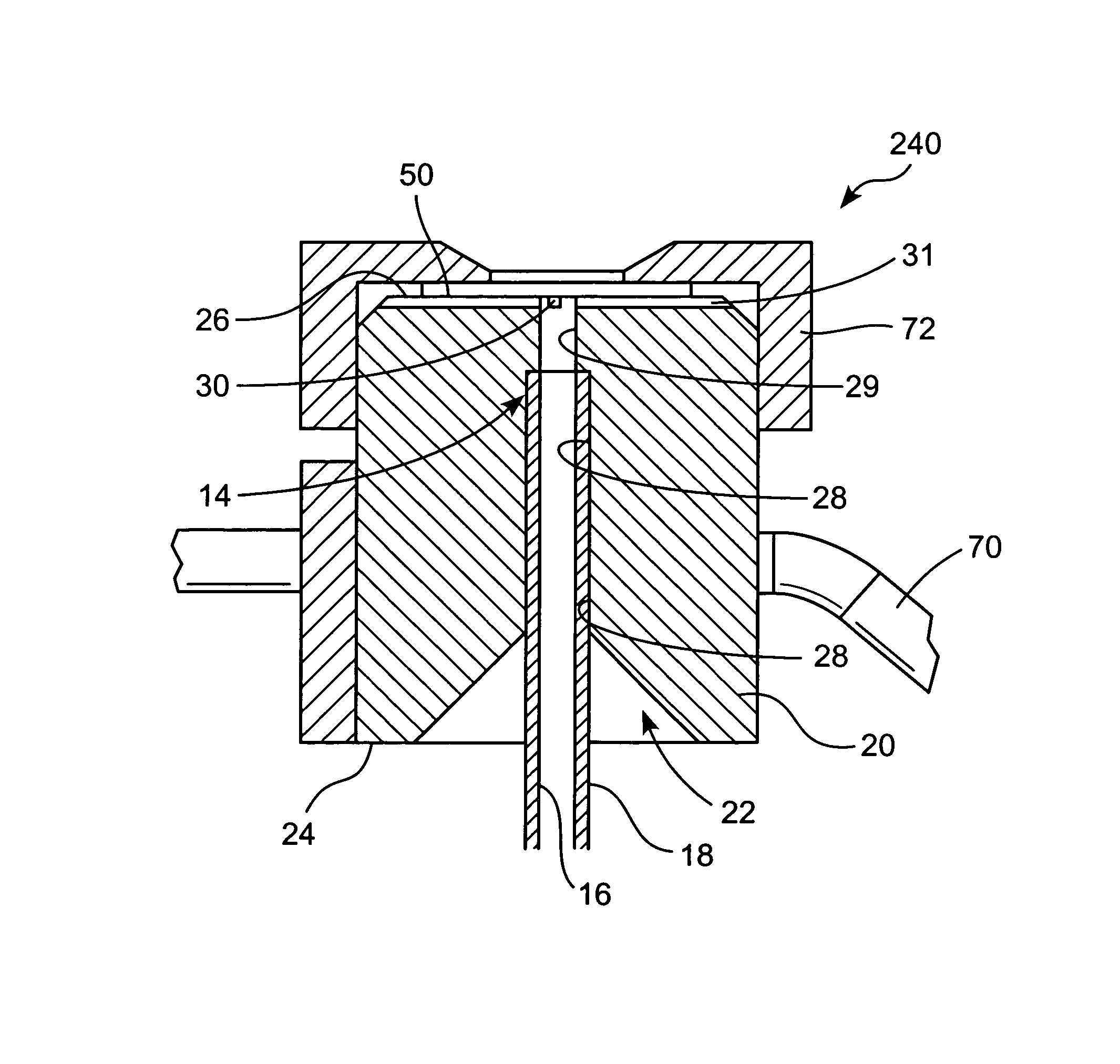
ActiveUS20130327326A1Pain minimizationMinimize discomfortRespiratorsMedical devicesSurgical siteEngineering
A portable, adjustable disposable medical suction system and method of use is disclosed. The system includes a delivery device to provide a conduit from a surgical site on a patient to the portable, adjustable medical suction system. The suction system may be a battery powered vacuum pump with one or more different adjustments directed to a strength of the vacuum, duration of the vacuum and interval between suction. The delivery device may be an intranasal tampon device or a tube inserted into the surgical site. During surgery and recovery at a surgical center or hospital, the vacuum source attached to the delivery system may be an industrial type vacuum source. Upon discharge, the suction system can be connected to the delivery system so that bleeding can be resolved by the patient in the comfort of his / her own home. The system and method of use is safer and mitigates body fluid borne contamination.
Owner:BRENNAN GEORGE
Devices and methods for facilitating fluid transport
Arrangements are provided including a base having a bore disposed therein extending from a first surface of the base through a second surface of the base, a fluid transport tube having a first end, a second end opposite the first end, and a lumen having an inner diameter, at least the second end of the tube being received within the bore of the base, and at least one fluid transport enhancing groove having at least a first section disposed in the second surface of the base and in fluid communication with the bore.
Owner:INTUITY MEDICAL INC
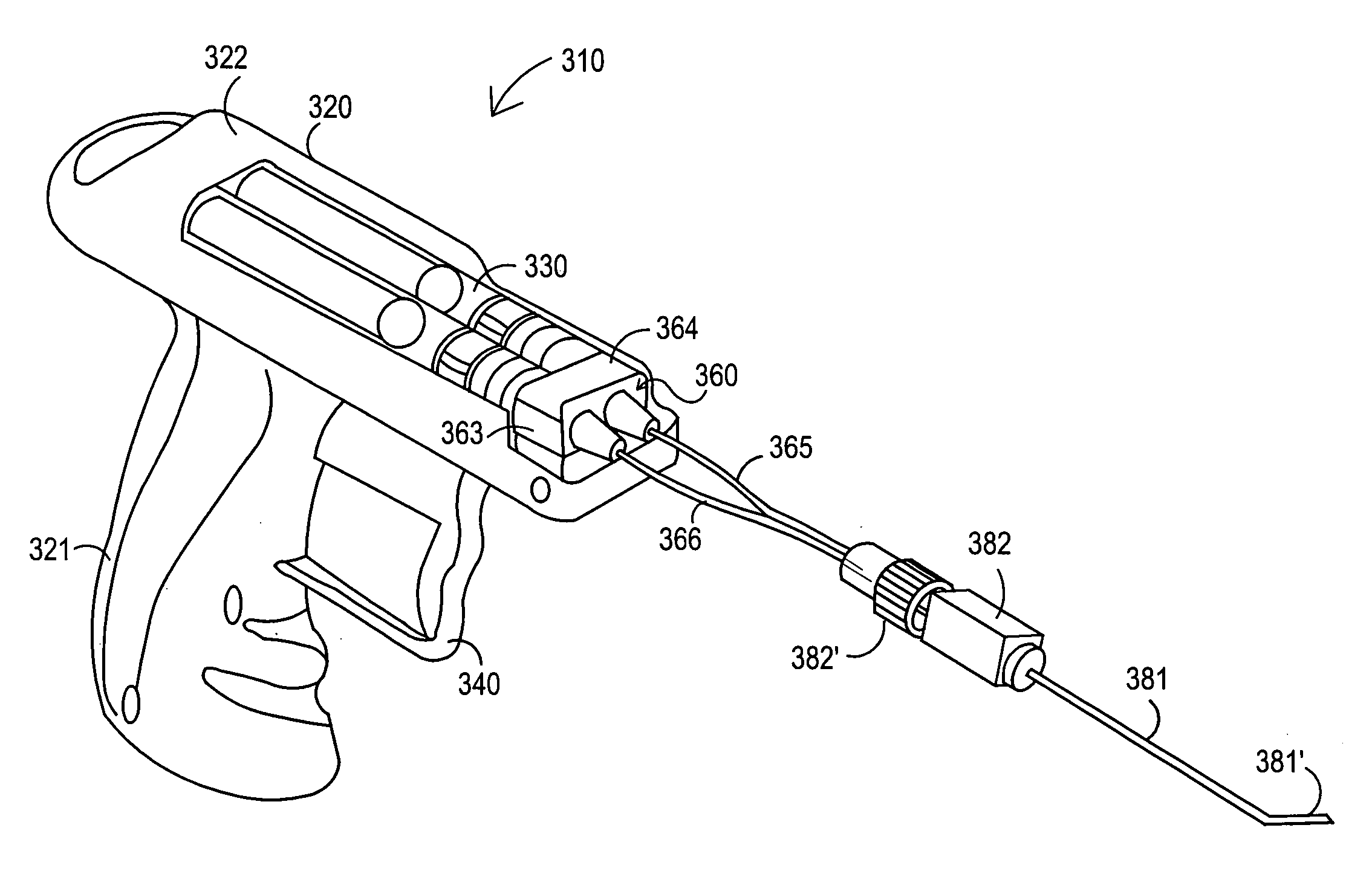
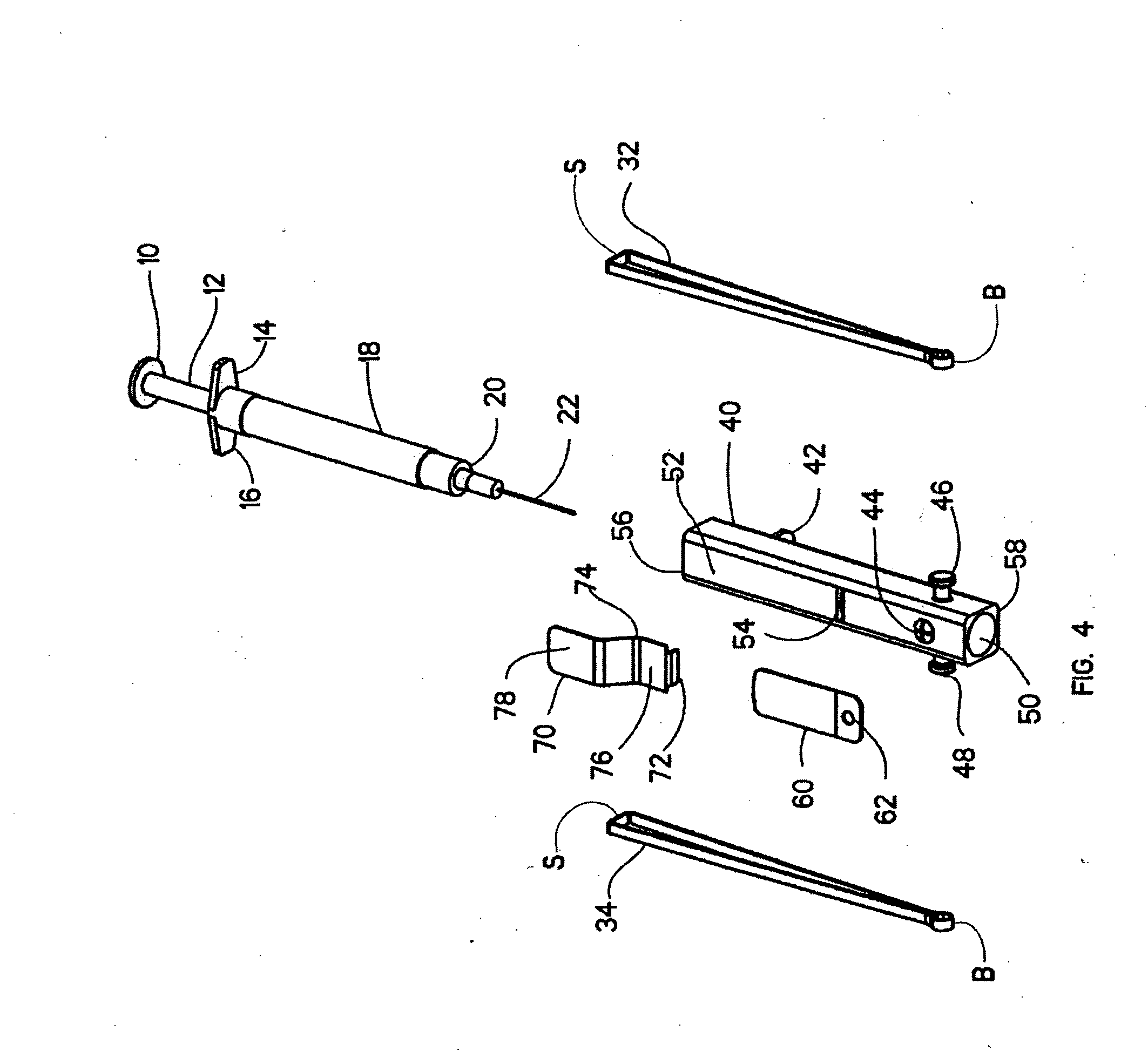
Injection applicator for a hypodermic syringe
InactiveUS20060106342A1Inexpensive and simple applicatorEasy to useInfusion syringesIntravenous devicesAxial forceBiomedical engineering
There is provided an injection device comprising a barrel adapted for receiving a syringe assembly, a trigger assembly, tension spring means and stopping means. The trigger assembly comprises retractable blocking means for blocking the syringe assembly at a first axial point within the barrel and a trigger for retracting said blocking means from blocking the syringe along said first axial point along the barrel. The tension spring means has a barrel end mountable on the barrel and a syringe end mountable on the syringe assembly, whereby said tension spring means imparts an axial force to the syringe assembly when the syringe assembly is in a cocked position. The stopping means are for retaining the syringe assembly in an injected position within the barrel upon release of the syringe assembly from the cocked position followed by axial travel of the syringe assembly within the barrel under the influence of the axial force.
Owner:COX MICHAEL
Multi injection microneedle theraphy system
InactiveCN101557848AImprove absorption rateReduce processing timeAutomatic syringesMicroneedlesMulti injectionHuman skin
Owner:株式会社艾莫克尔
Biodegradable stent
A biodegradable stent for implantation into a lumen in a human body. The stent in one embodiment is made from a biodegradable fiber having an inner core and an outer layer. The outer layer is a blend of two polymer components. The inner core has a first degradation rate, and the outer layer has a second degradation rate. The second degradation rate is slower than the first degradation rate. The fiber softens in vivo such that the stent is readily passed from the lumen as a softened fragment or filament after a predetermined period of time through normal flow of body fluids passing through the lumen.
Owner:DATTA ARINDAM +3
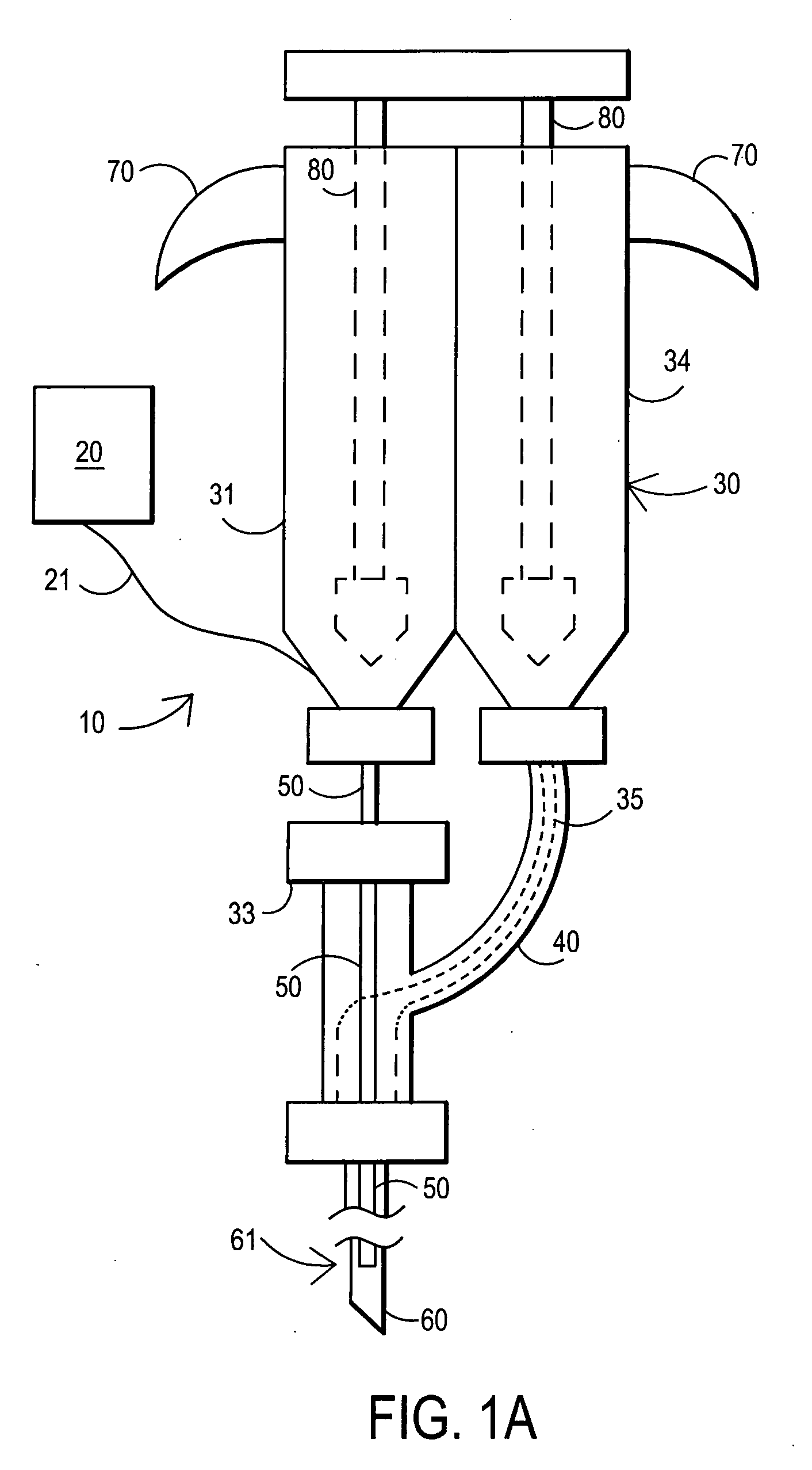
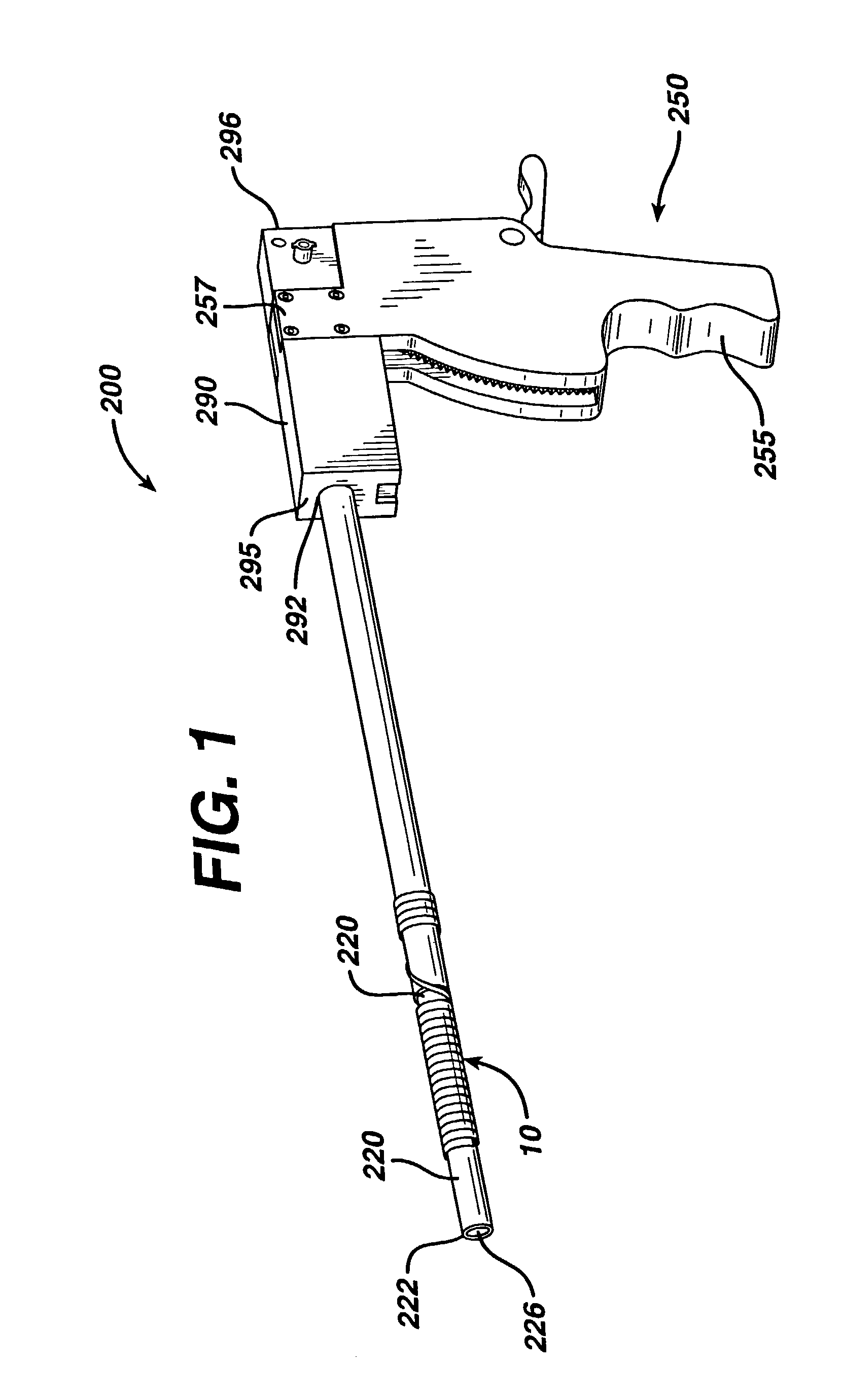
Sampling needle
ActiveUS20100179377A1Pain minimizationMinimize discomfortAnimal reproductionSurgical needlesAnimal subjectBiomedical engineering
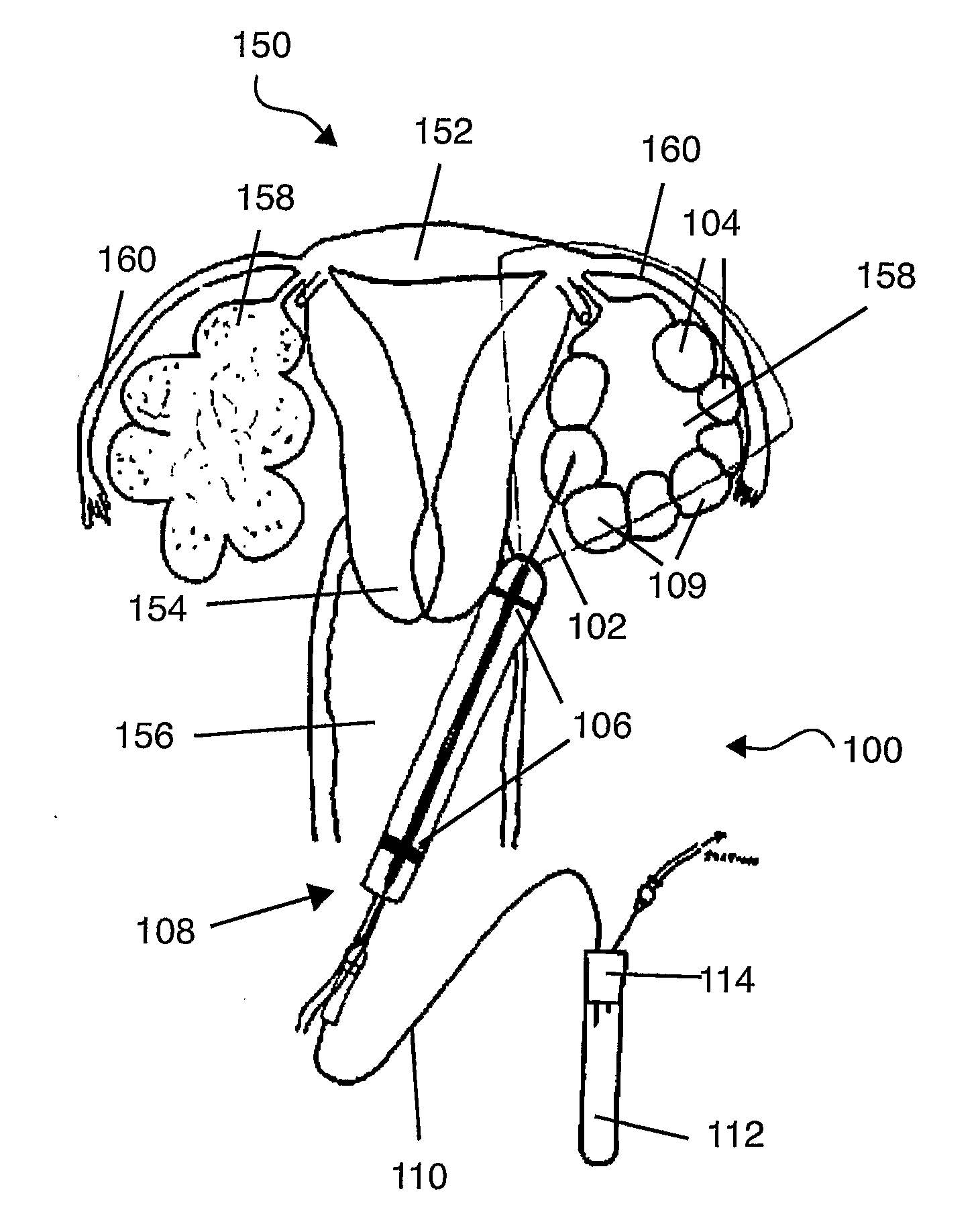
The invention relates to a sampling needle, in particular to a needle suitable for oocyte retrieval from a human or animal subject, said needle comprising a first tubular region in fluid communication with a second tubular region, the first tubular region comprising a leading end for insertion into a subject and the second tubular region comprising a trailing end for fluid communication with a means for receiving a fluid, in which the first tubular region has an outer diameter which is less than the outer diameter of the second tubular region and the first tubular region has an inner diameter which is less than the inner diameter of the second tubular region. The invention also relates to a method of retrieving a sample, in particular an oocyte, from a human or animal subject.
Owner:VITROLIFE SWEDEN AB
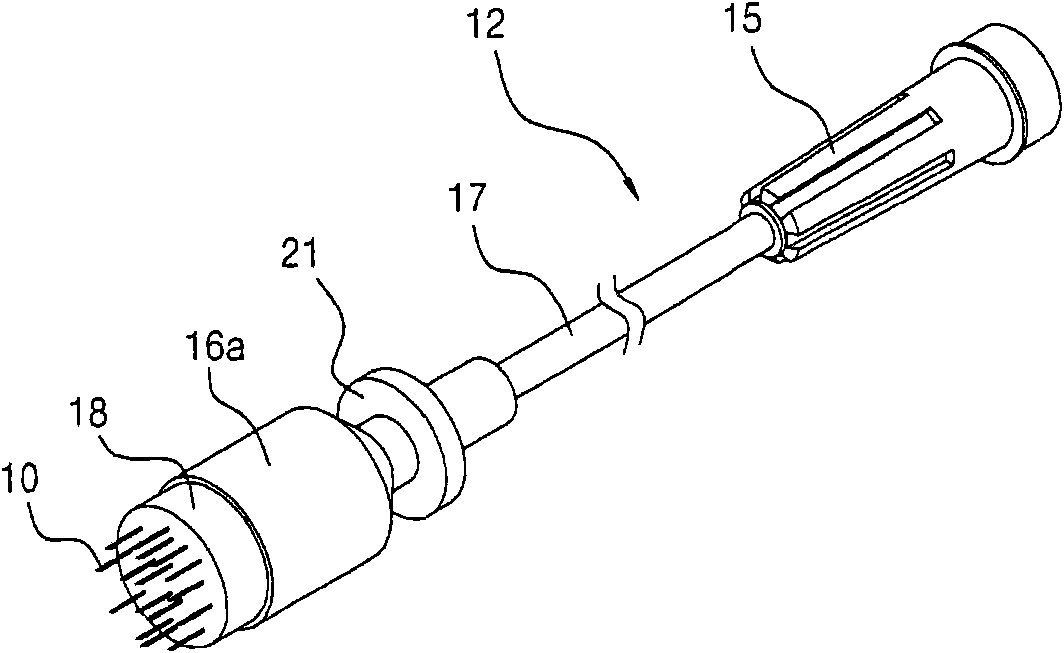
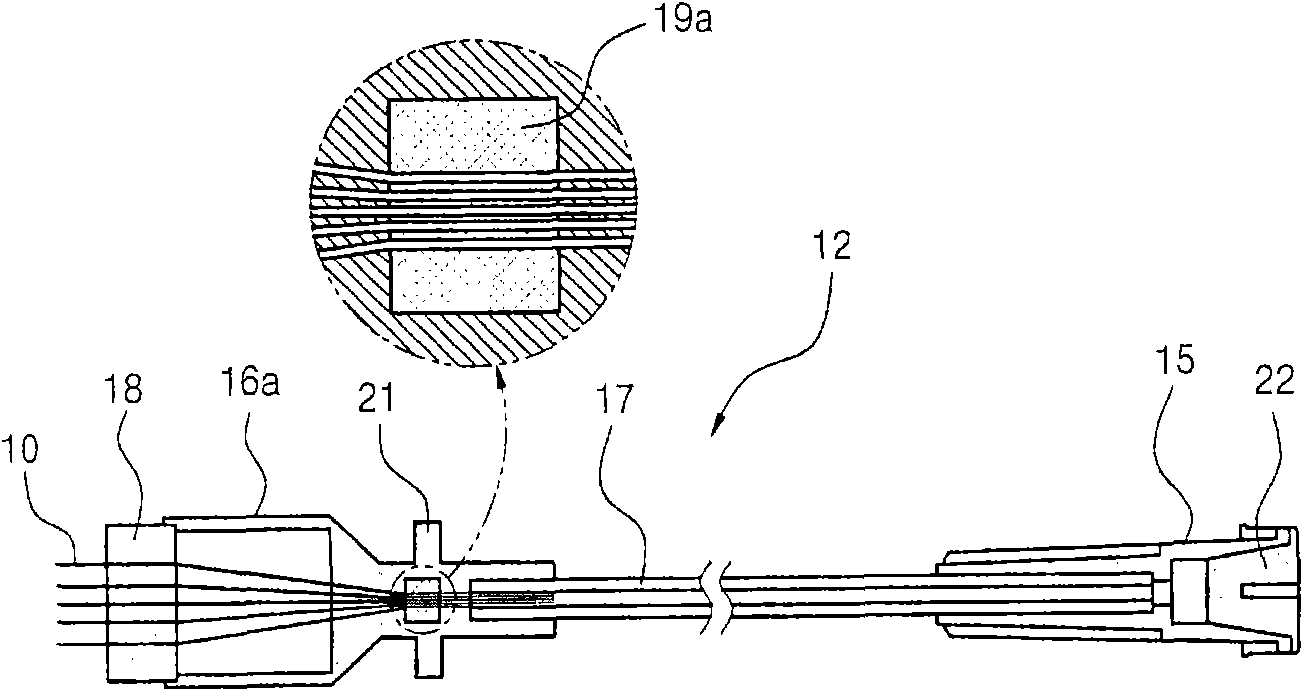
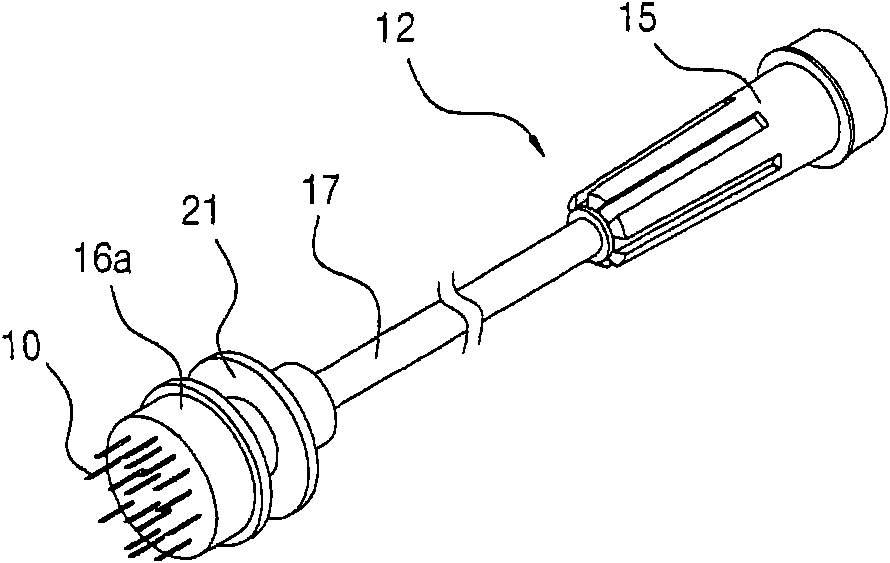
System and method for pain reduction during skin puncture and breakable tip therefor
ActiveUS20110319812A1Easily and inexpensively utilizedFree from painSurgeryMedical devicesInjection siteEngineering
An instrument, article and method are provided for minimizing pain during administration by injection of a liquid, such as, an anesthetic. The instrument has a forward end. A lightpipe mounted freely for vibration projects out of the forward end. The article, a single use tip, is composed of a tip sleeve removably mounted on the forward end of the instrument and a tip member removably mounted on the projecting lightpipe to vibrate a preselected injection site on a human or animal. The tip sleeve and tip member are covered by an elastic overmold that enables the tip member to vibrate freely with respect to the tip sleeve and light from the lightpipe to illuminate the injection site. The overmold of the single use tip is torn during removal of the single use tip from the instrument.
Owner:BING INNOVATIONS
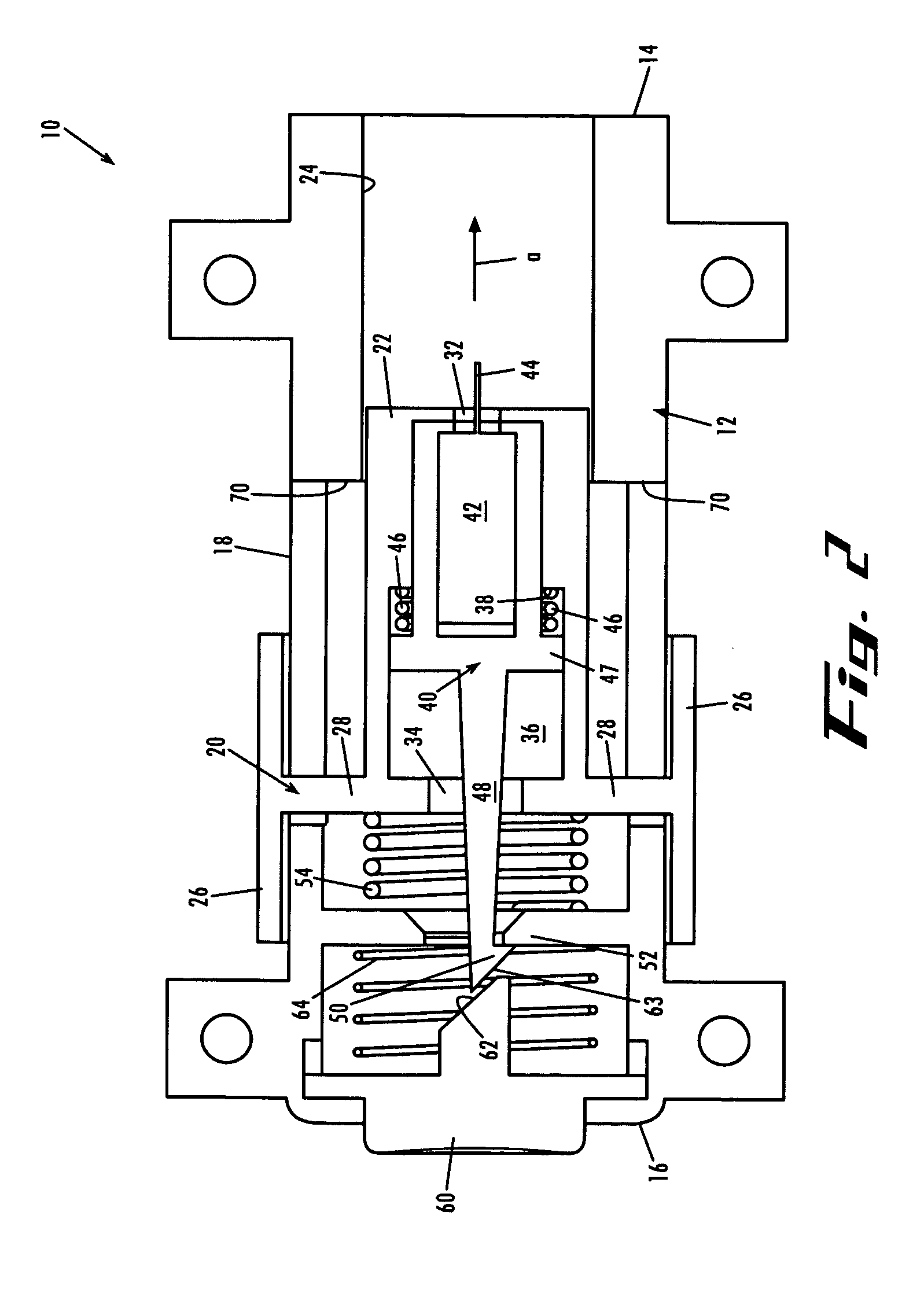
Lancing device with floating lancet
ActiveUS7494498B2Minimize rockingMinimize lateral movementSurgical needlesCatheterEngineeringRisk stroke
A lancing device including a drive mechanism with a drive spring and a lancet carrier engaged and driven by the drive spring, and further including a lancet with a sharp lancing tip, wherein the lancet floats relative to the carrier and is decoupled from the drive mechanism during at least a portion of a lancing stroke. In one example embodiment the lancet is held in a sled that floats in the carrier, and in another example embodiment the lancet by itself floats in the carrier.
Owner:FACET TECH LLC
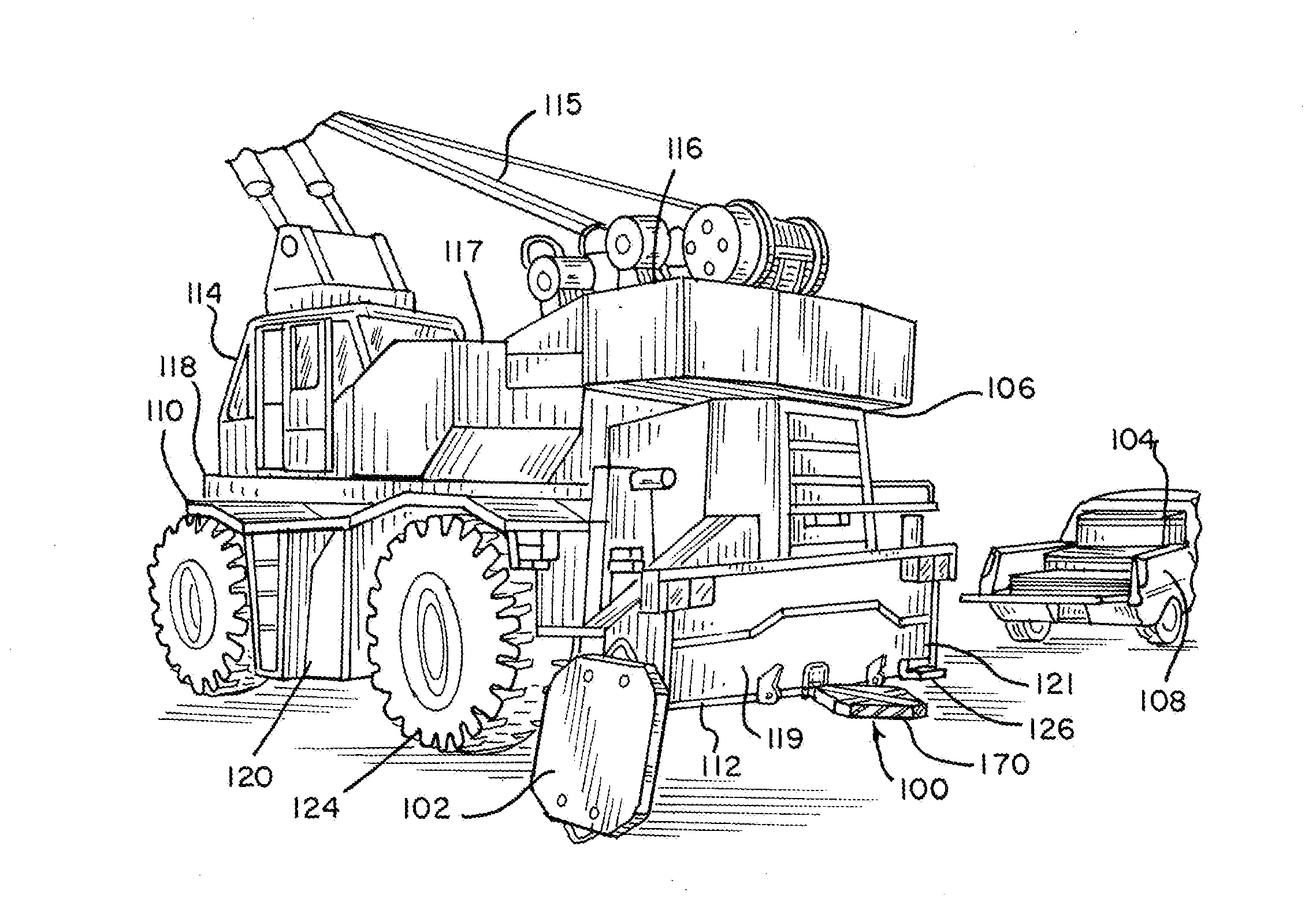
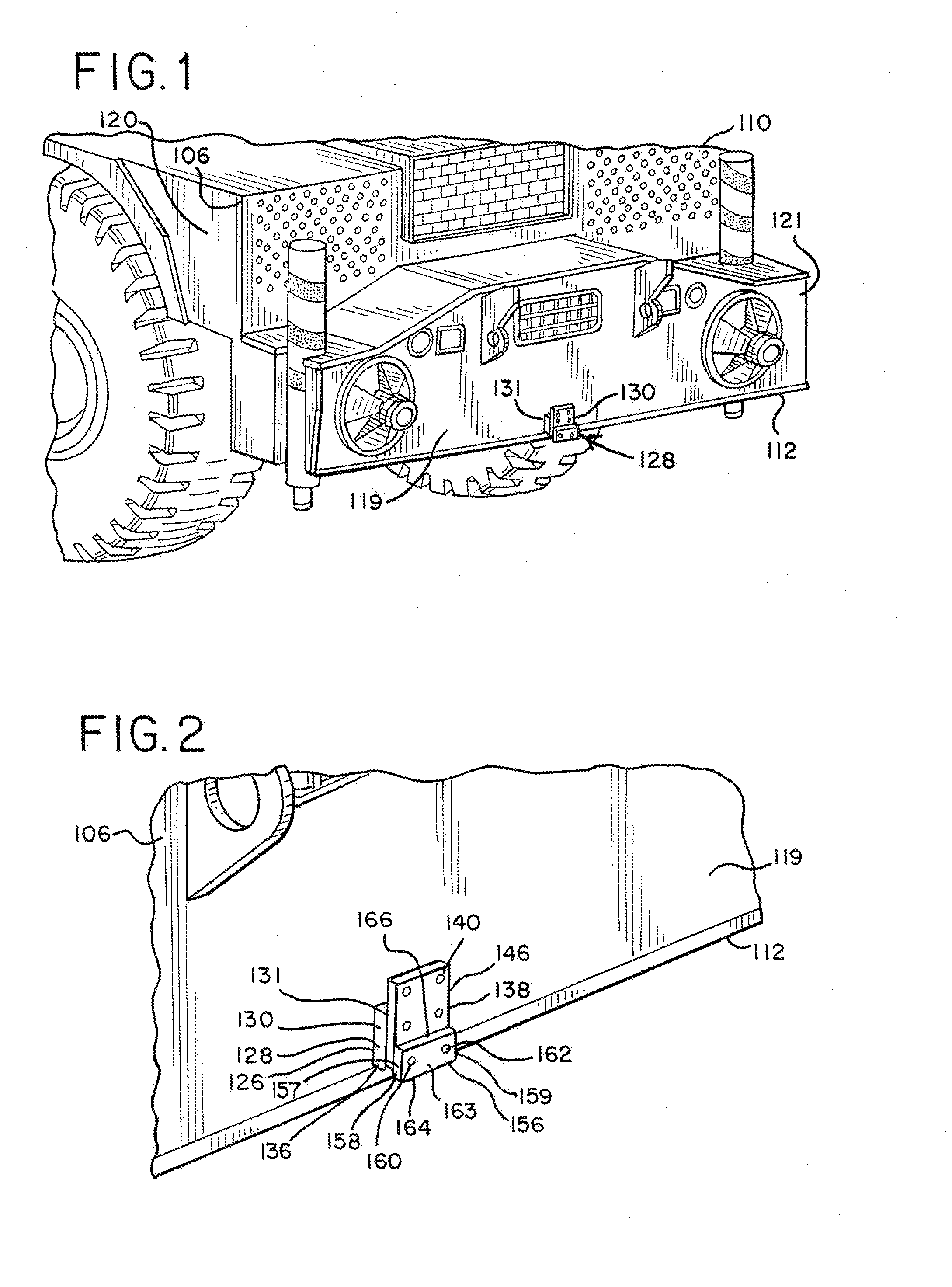
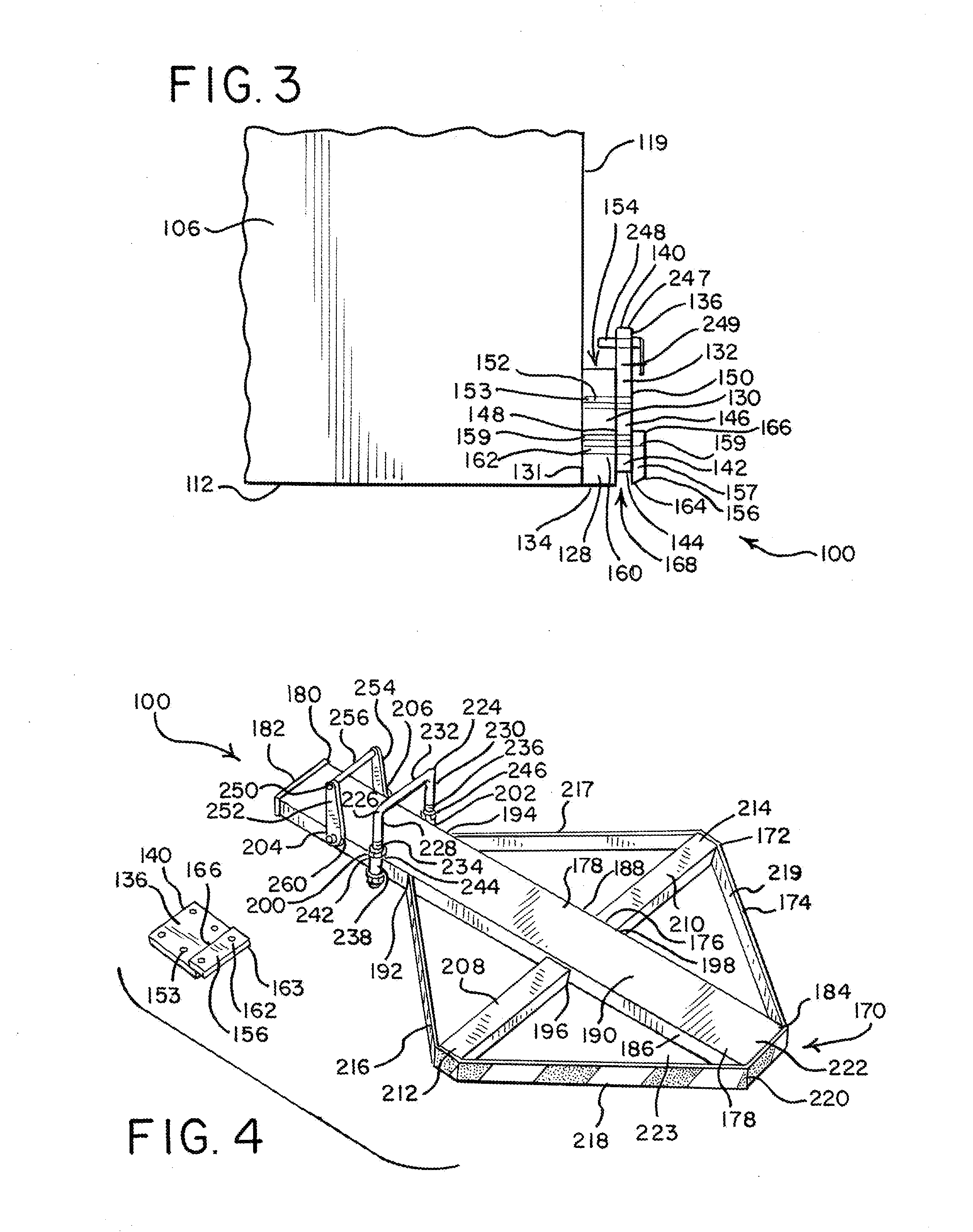
Crane Mat Carrier
ActiveUS20130213920A1Easy to transportEasy to useSupplementary fittingsStowing appliancesEngineeringLocking plate
A user-friendly crane mat carrier is provided for conveniently transporting, storing and supporting crane mats, pads and other items. The economical light weight crane mat assembly can comprise a frame assembly with a main load-supporting bar, crossbars and perimeter bars. The special crane mat carrier can be easily connected to a crane, such as with a drawbar fixture, securing plate and locking plate. A hanger assembly with a suspension bar can be provided to hang the crane mat carrier. A latch mechanism can also be provided to securely lock the crane mat carrier.
Owner:TOTAL SUPPORT
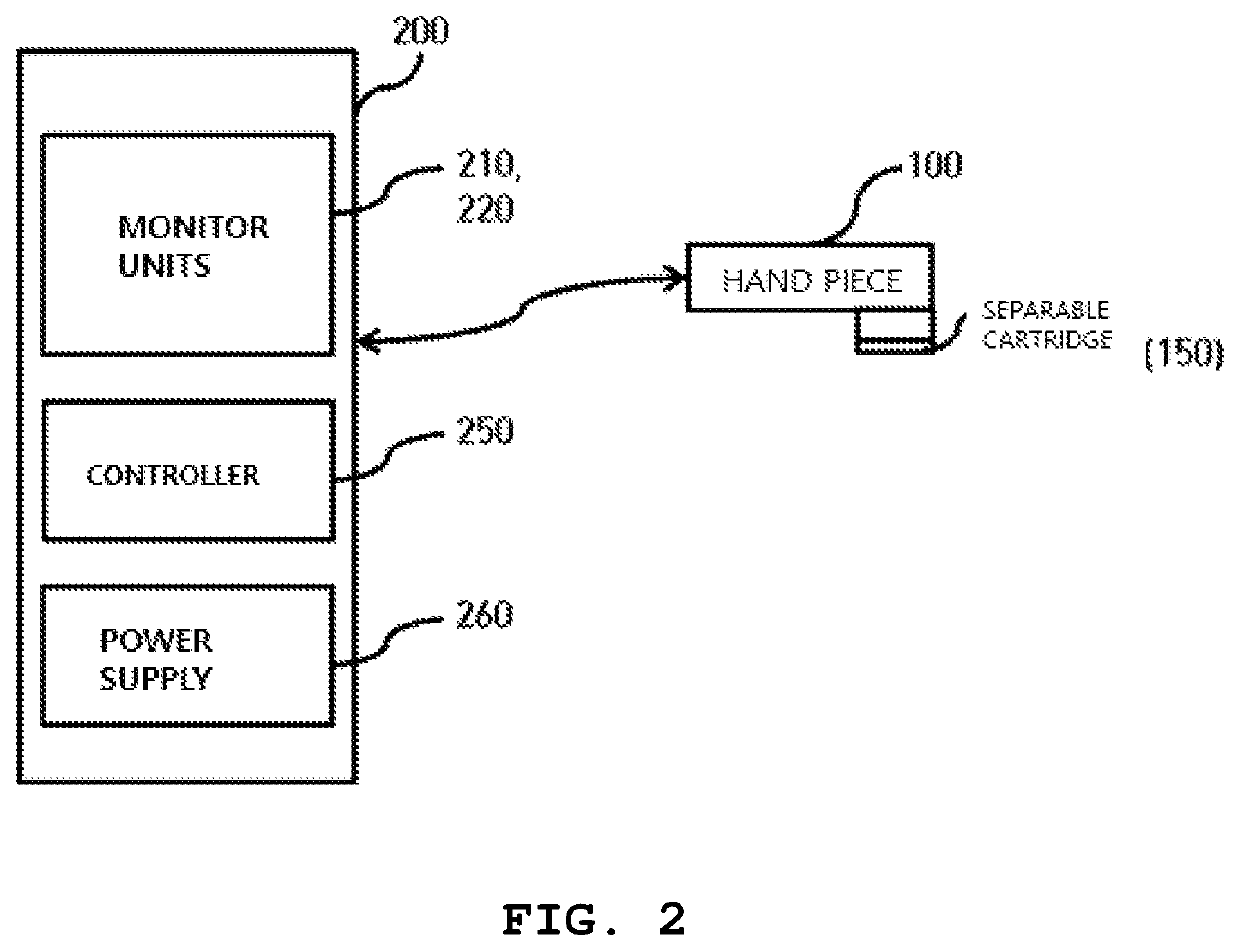
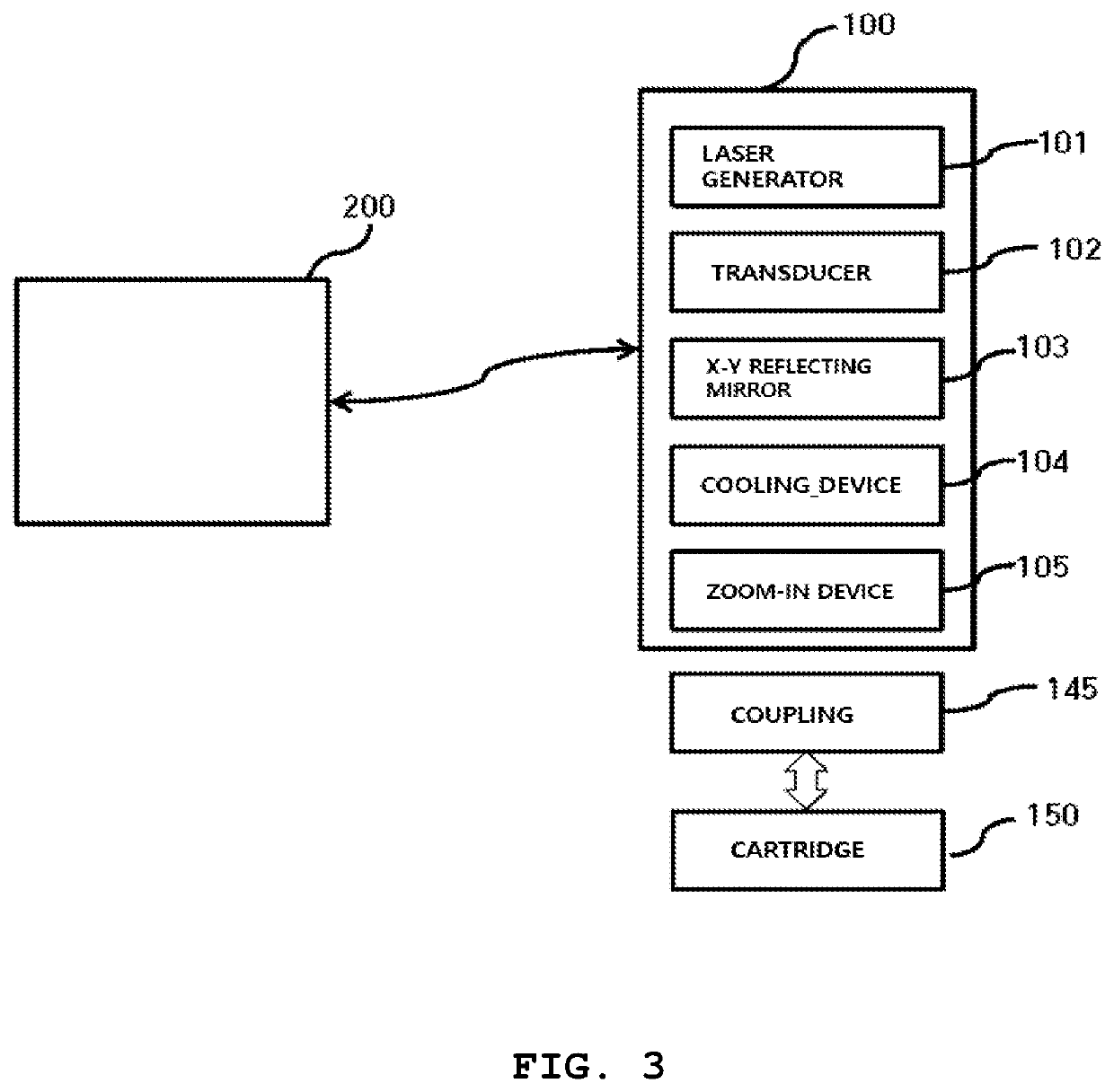
High-intensity focused ultrasound device
ActiveUS10639504B2Extended service lifeReduce maintenance costsUltrasound therapySurgical navigation systemsUltrasound deviceHigh intensity
A high-intensity focused ultrasound is configured such that a disposable separable cartridge is attached to and detached from the ultrasound device, so that a practitioner, i.e. a doctor, can obtain coordinates of a skin tissue of a subject using a scanner of an ultrasonic transducer and locate an accurate procedure point in real time. A procedure can be performed on the accurate procedure point of the skin tissue without repeated procedures.
Owner:KIM YOU IN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com