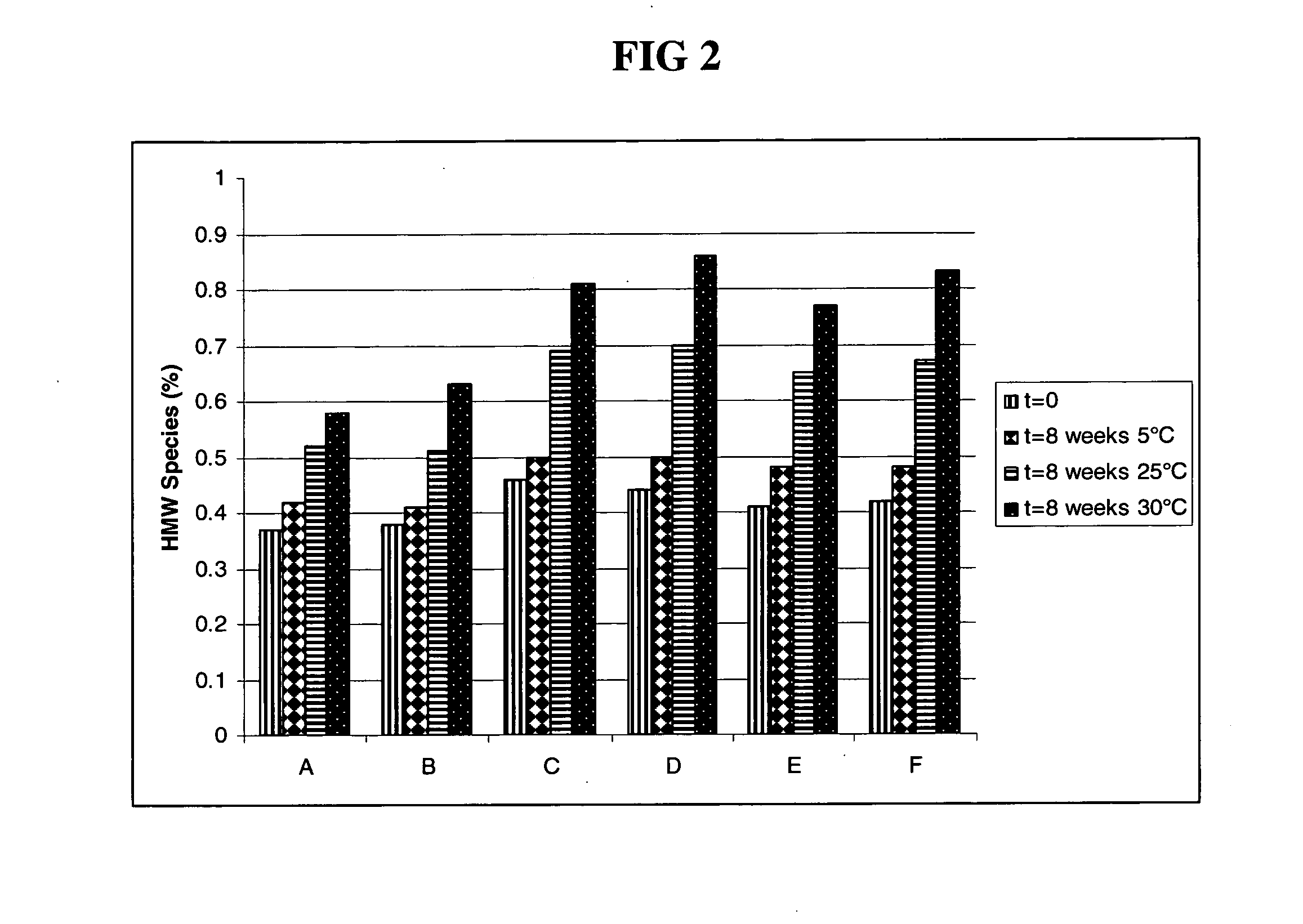
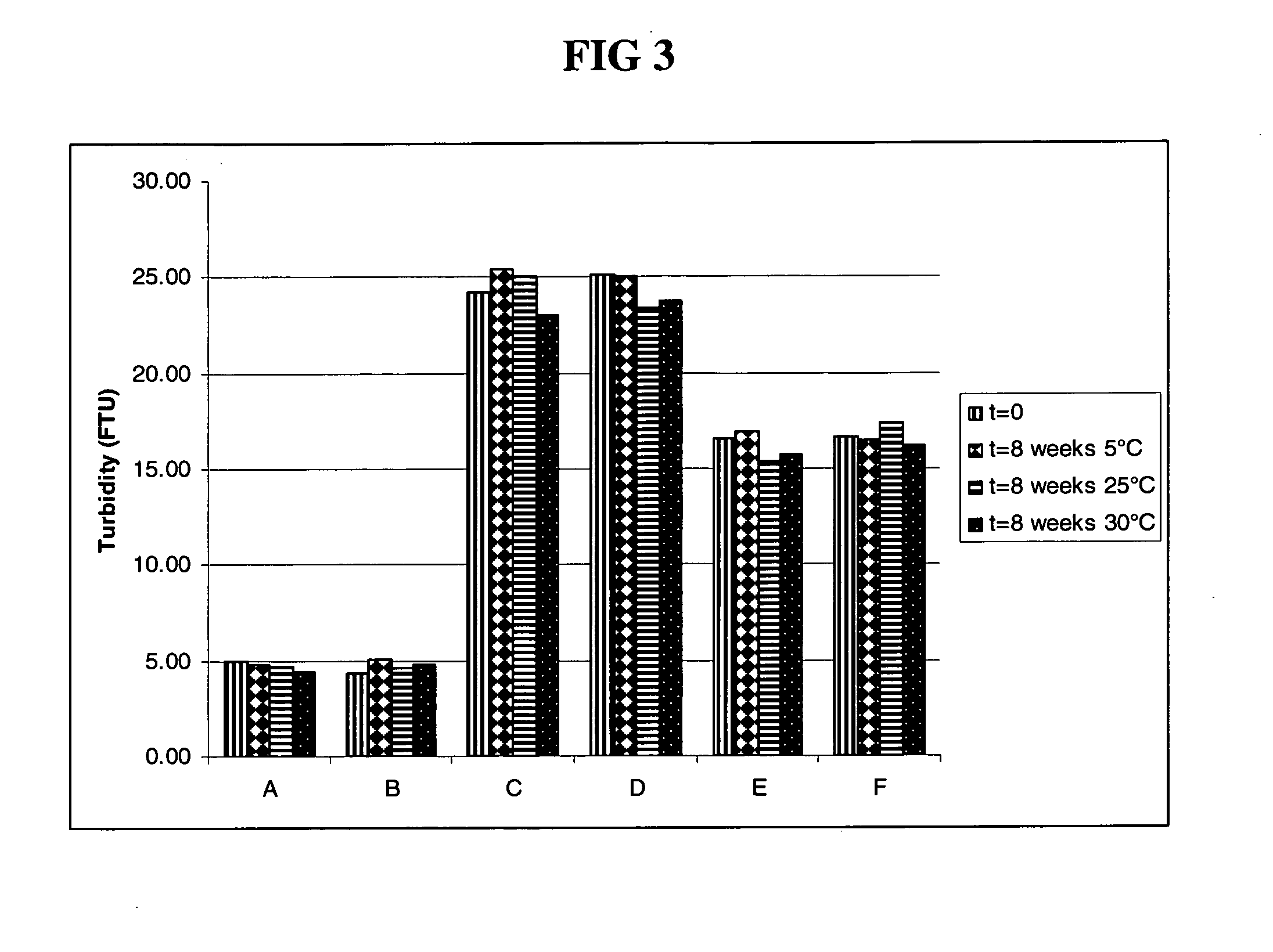
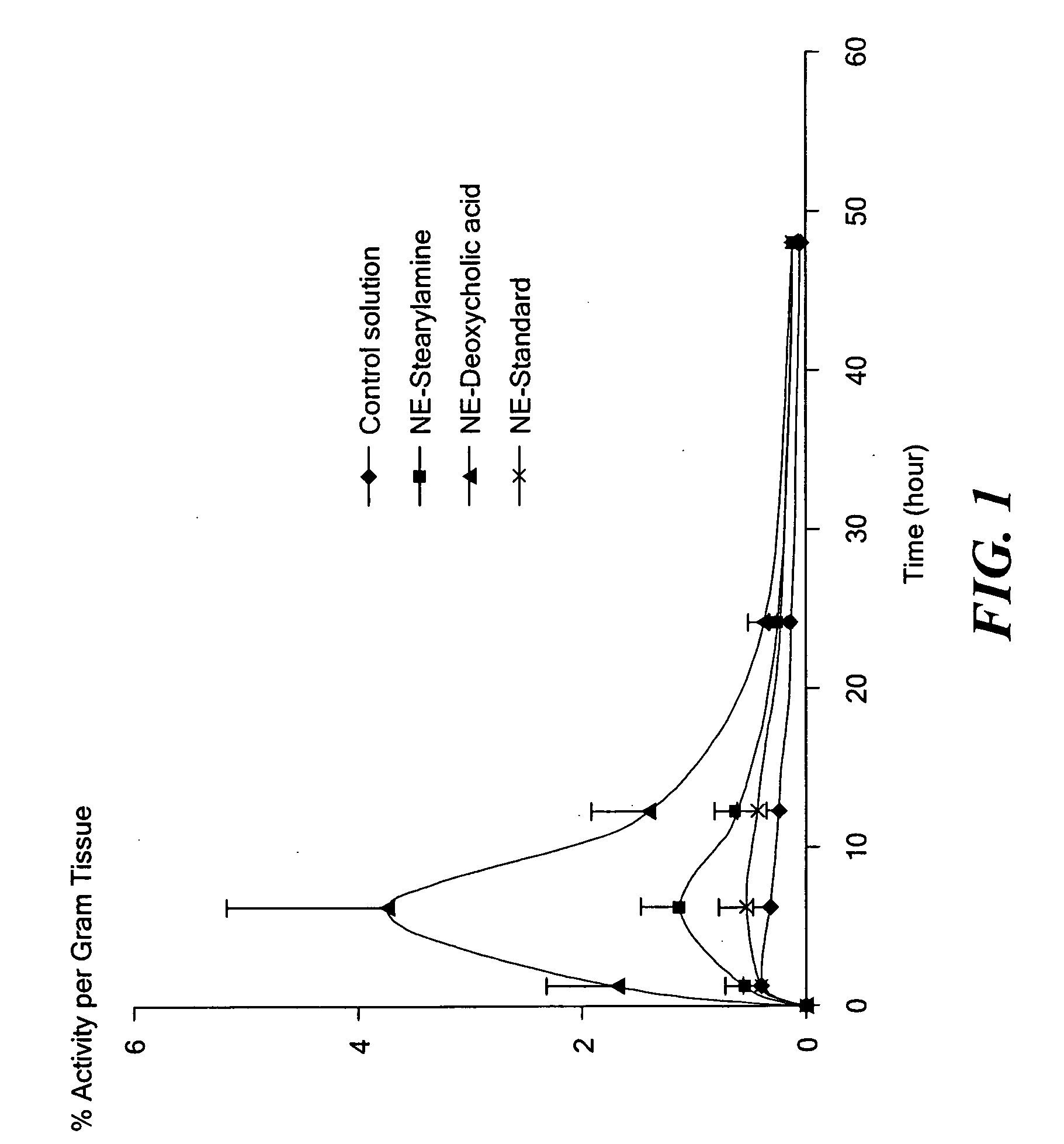
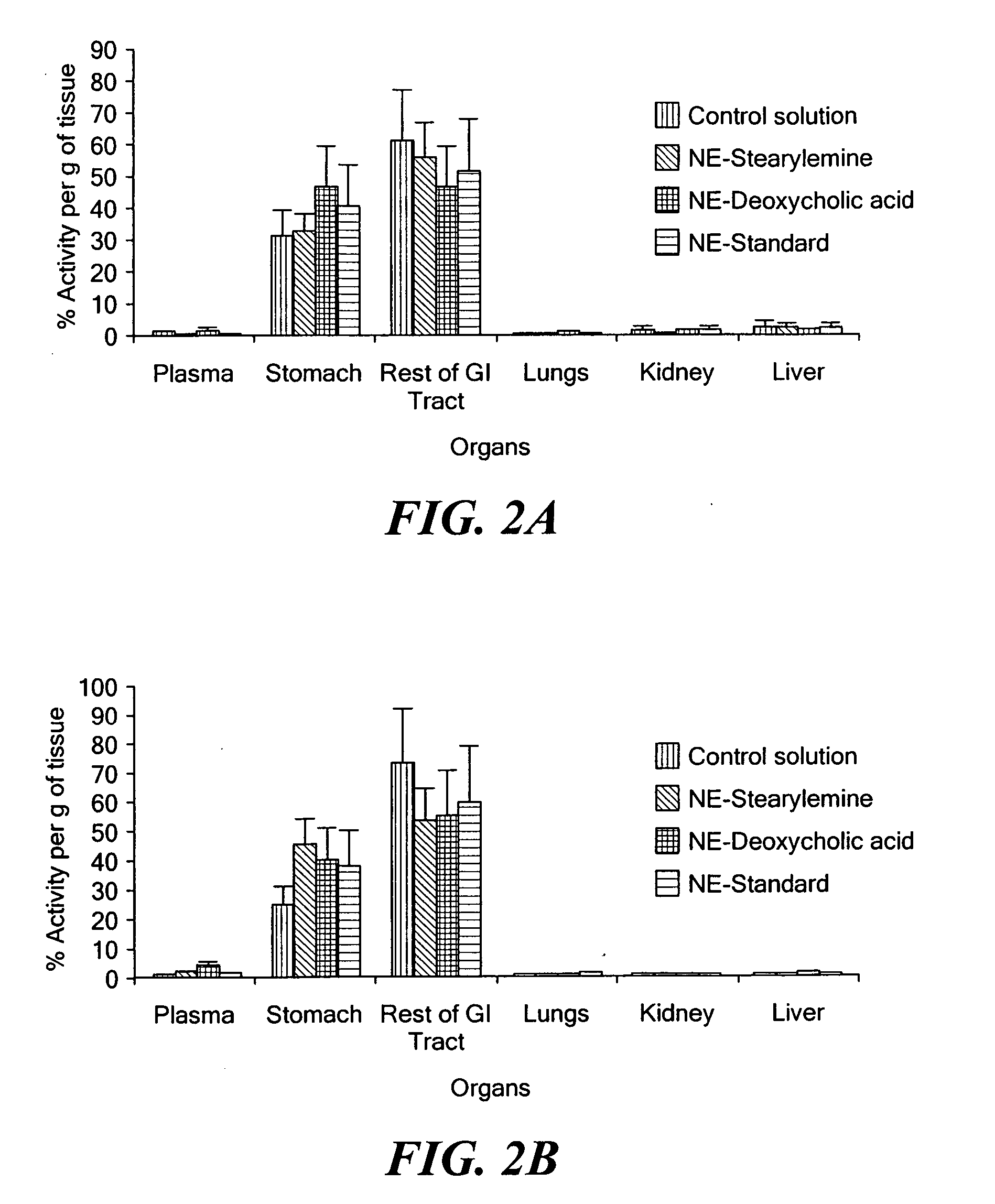
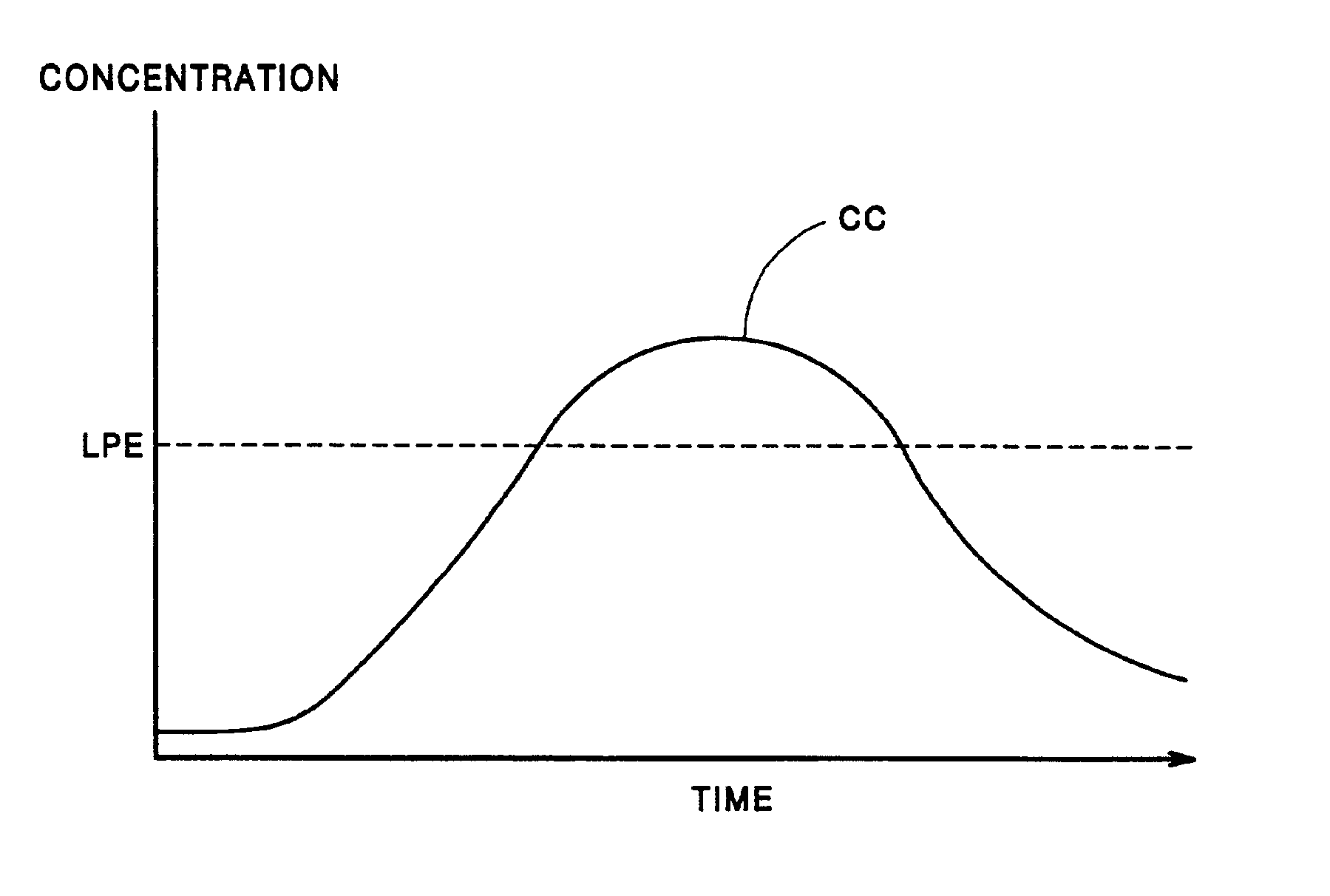
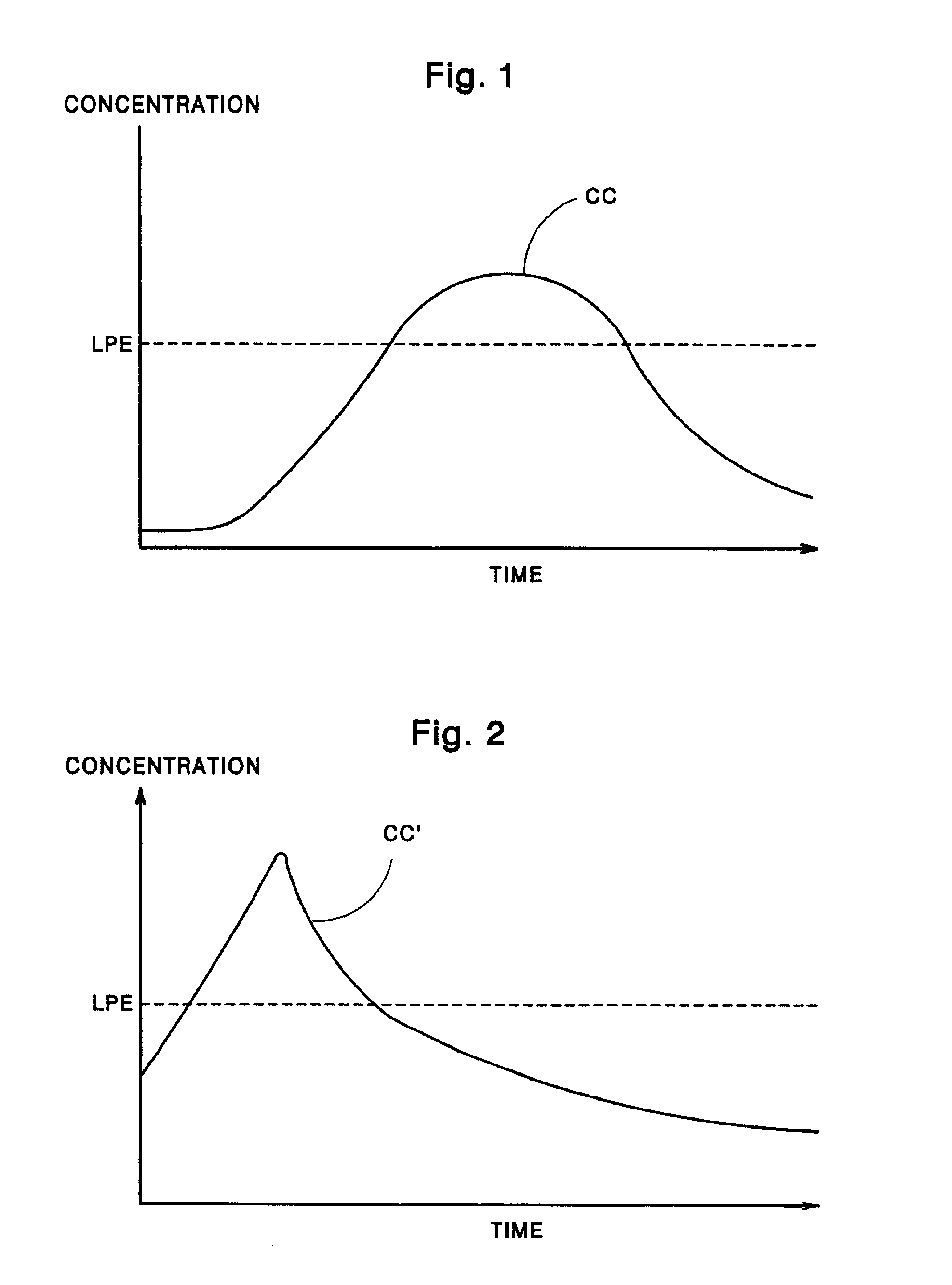
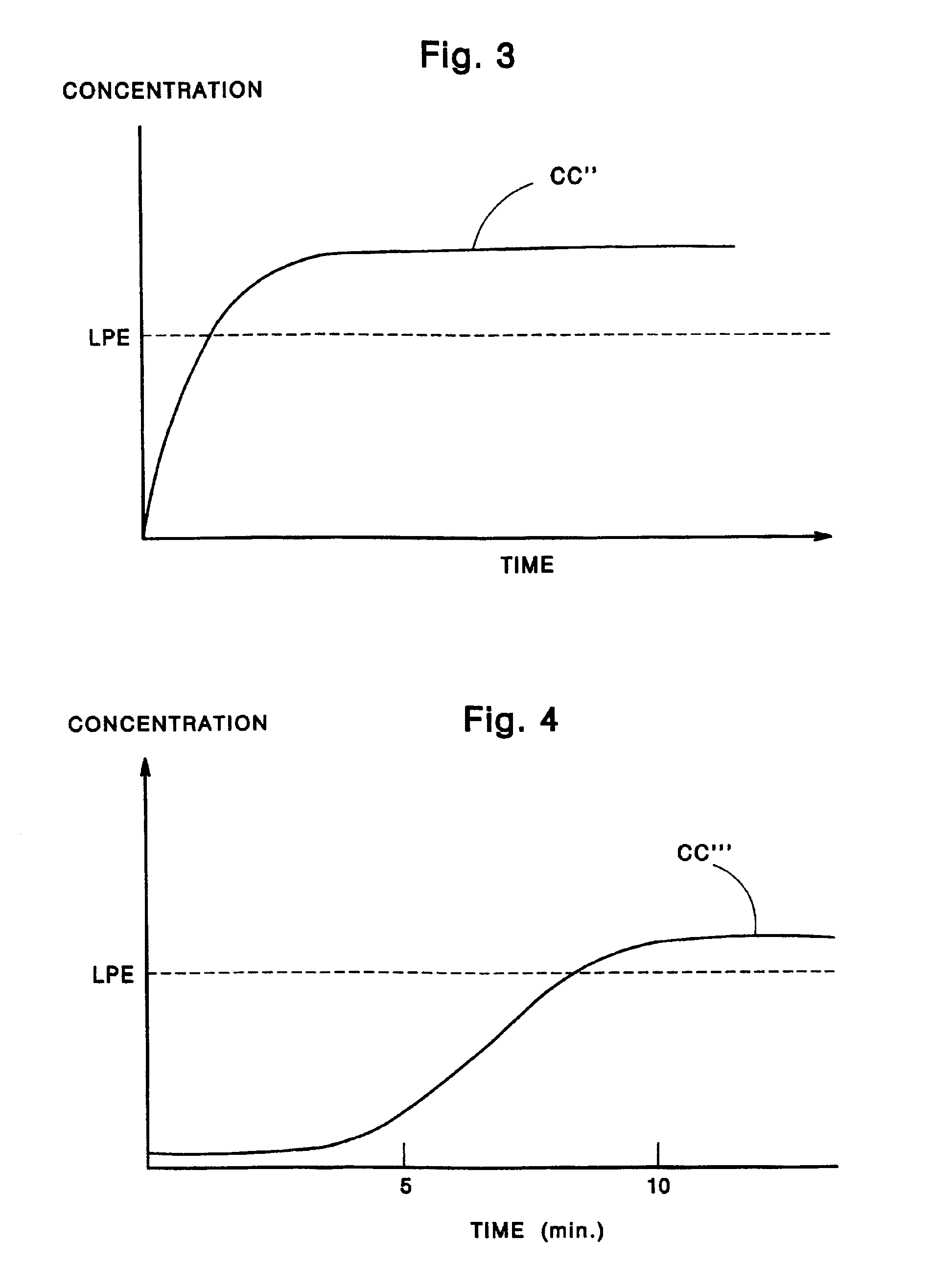
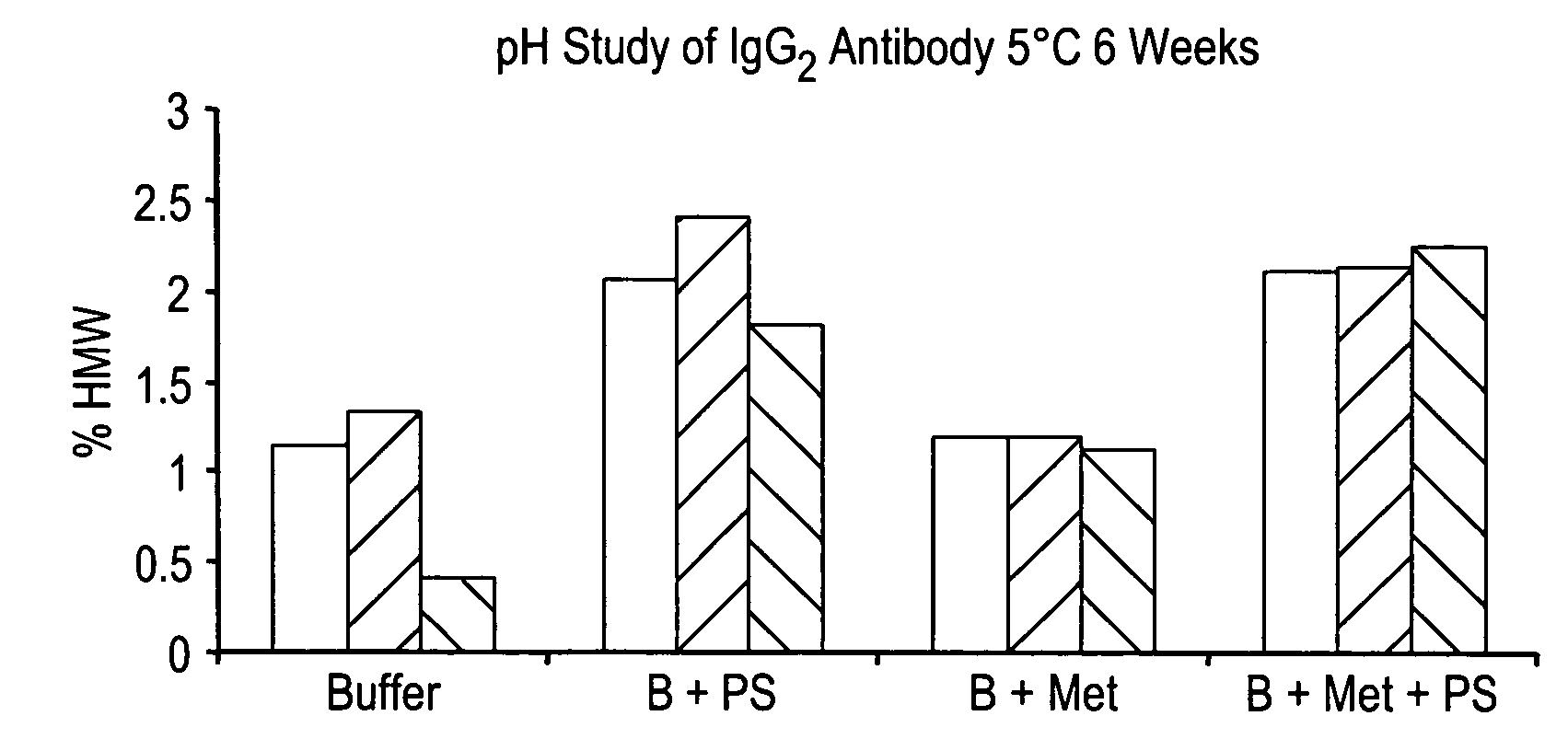
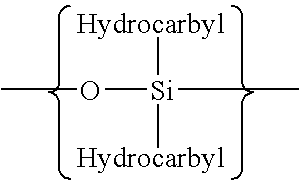
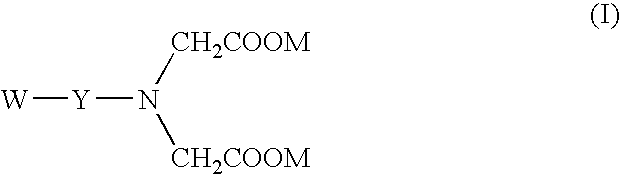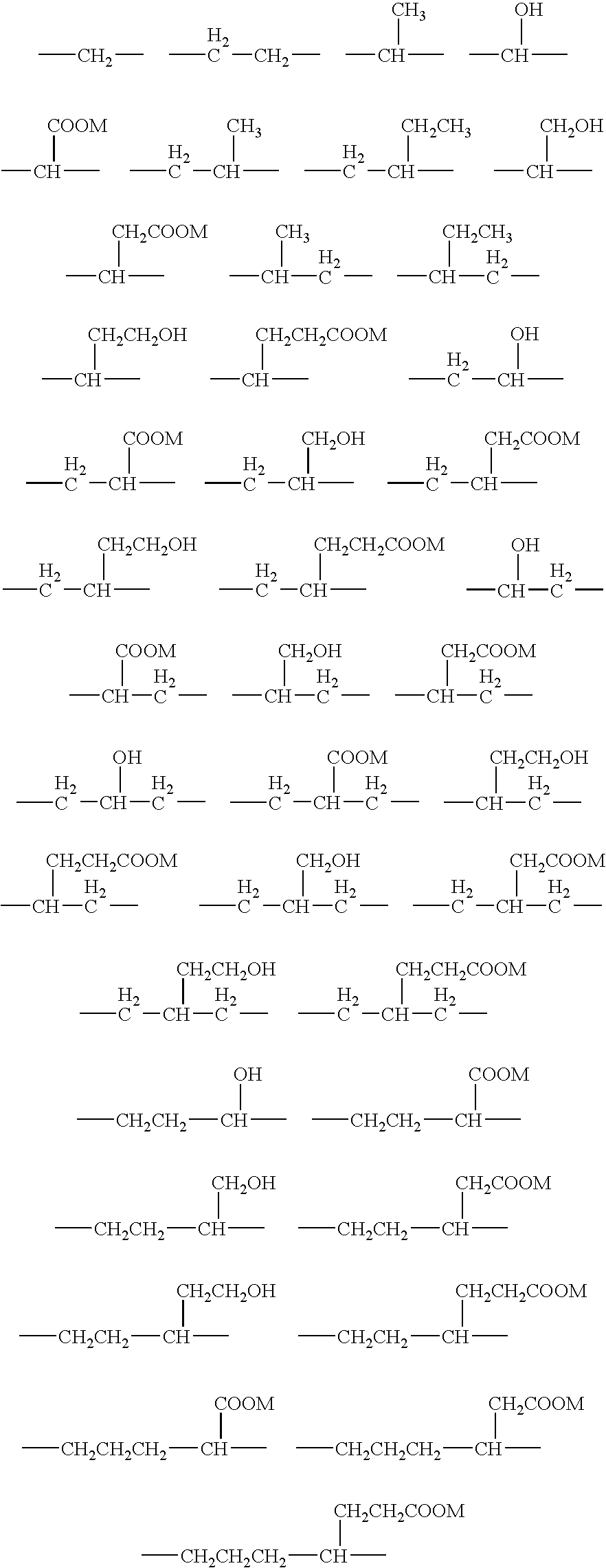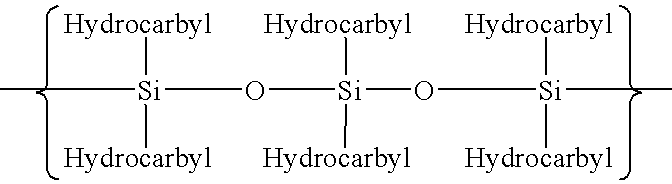Patents
Literature
3200 results about "Buffering agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
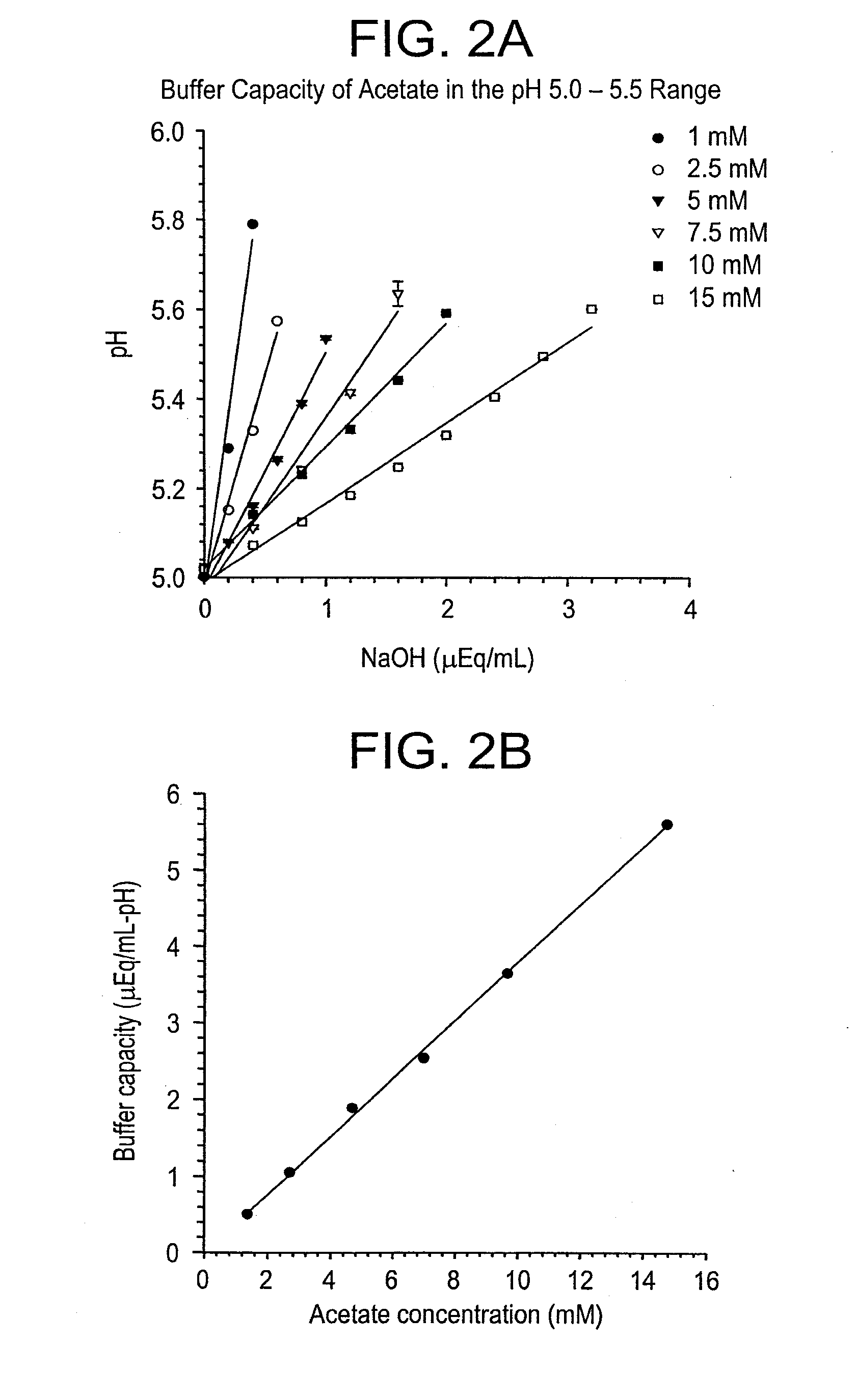
A buffering agent is a weak acid or base used to maintain the acidity (pH) of a solution near a chosen value after the addition of another acid or base. That is, the function of a buffering agent is to prevent a rapid change in pH when acids or bases are added to the solution. Buffering agents have variable properties—some are more soluble than others; some are acidic while others are basic. As pH managers, they are important in many chemical applications, including agriculture, food processing, biochemistry, medicine and photography.
Water-absorbing agent and production process therefor, and sanitary material
InactiveUS20040106745A1Improve balanceMaintain stable propertiesAbsorbent padsBandagesWater solubleMethods of production
There is provided: a production process for a water-absorbing agent having stable properties in a short time; and a water-absorbing agent. The production process comprises the step of blending an acid-group-containing water-absorbent resin powder with a noncrosslinkable water-soluble inorganic base and / or an irreducible alkaline-metal-salt pH buffer and further with a dehydratable crosslinking agent reactable with the acid group, thereby subjecting the resin powder to crosslinking treatment, or comprises the step of blending an acid-group-containing water-absorbent resin powder with the above base and / or pH buffer and further with a crosslinking agent reactable with the acid group, thereby subjecting the resin powder to crosslinking treatment, wherein the resin powder has a weight-average particle diameter of 300 to 600 mum wherein the ratio of fine powders having particle diameters of not larger than 150 mum in the resin powder is not more than 10 weight %.
Owner:NIPPON SHOKUBAI CO LTD
Polymerization of halogen-containing monomers using siloxane surfactant
Halogenated polymers are prepared by a process comprising polymerizing at least one halogen-containing monomer in an aqueous medium containing monomer, radical initiator, and siloxane surfactant. The medium may optionally contain one or more of an antifoulant, a buffering agent and a chain-transfer agent.
Owner:ARKEMA INC
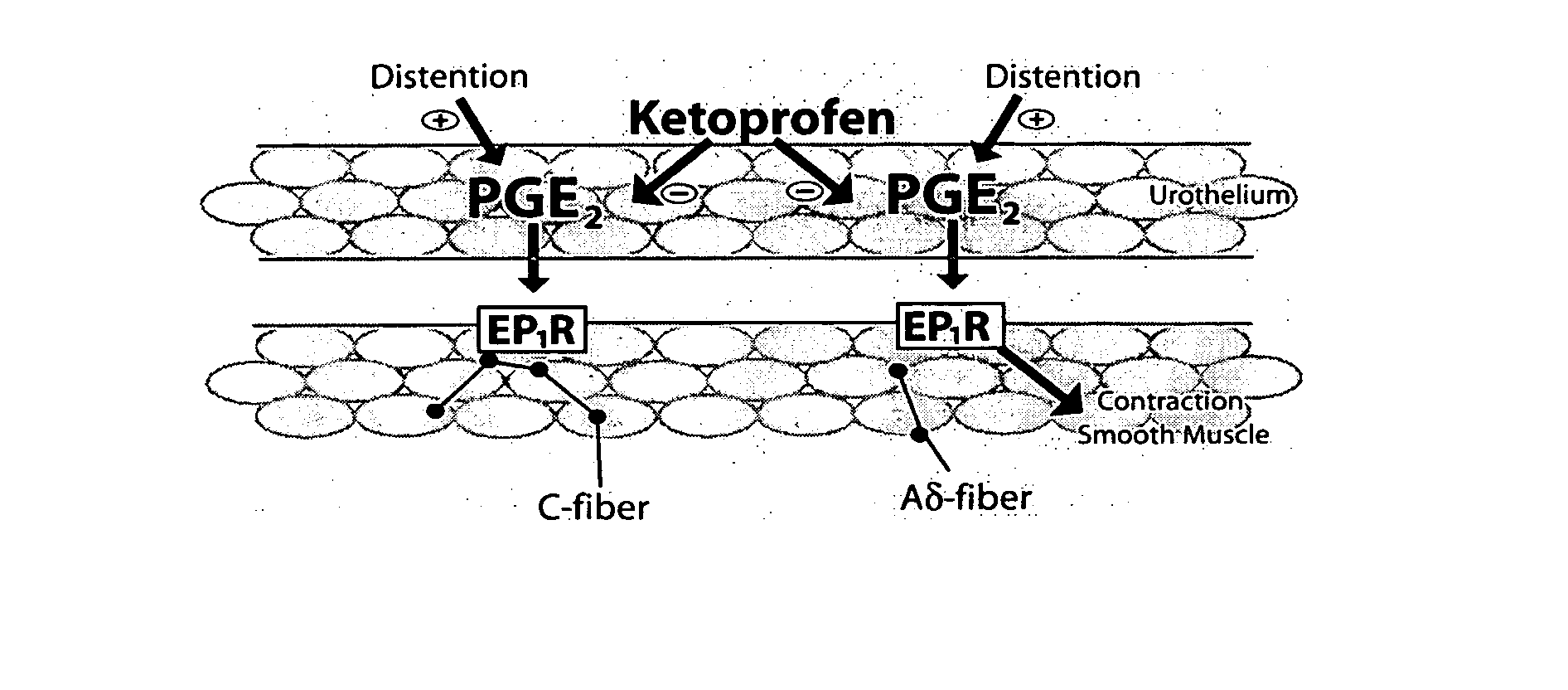
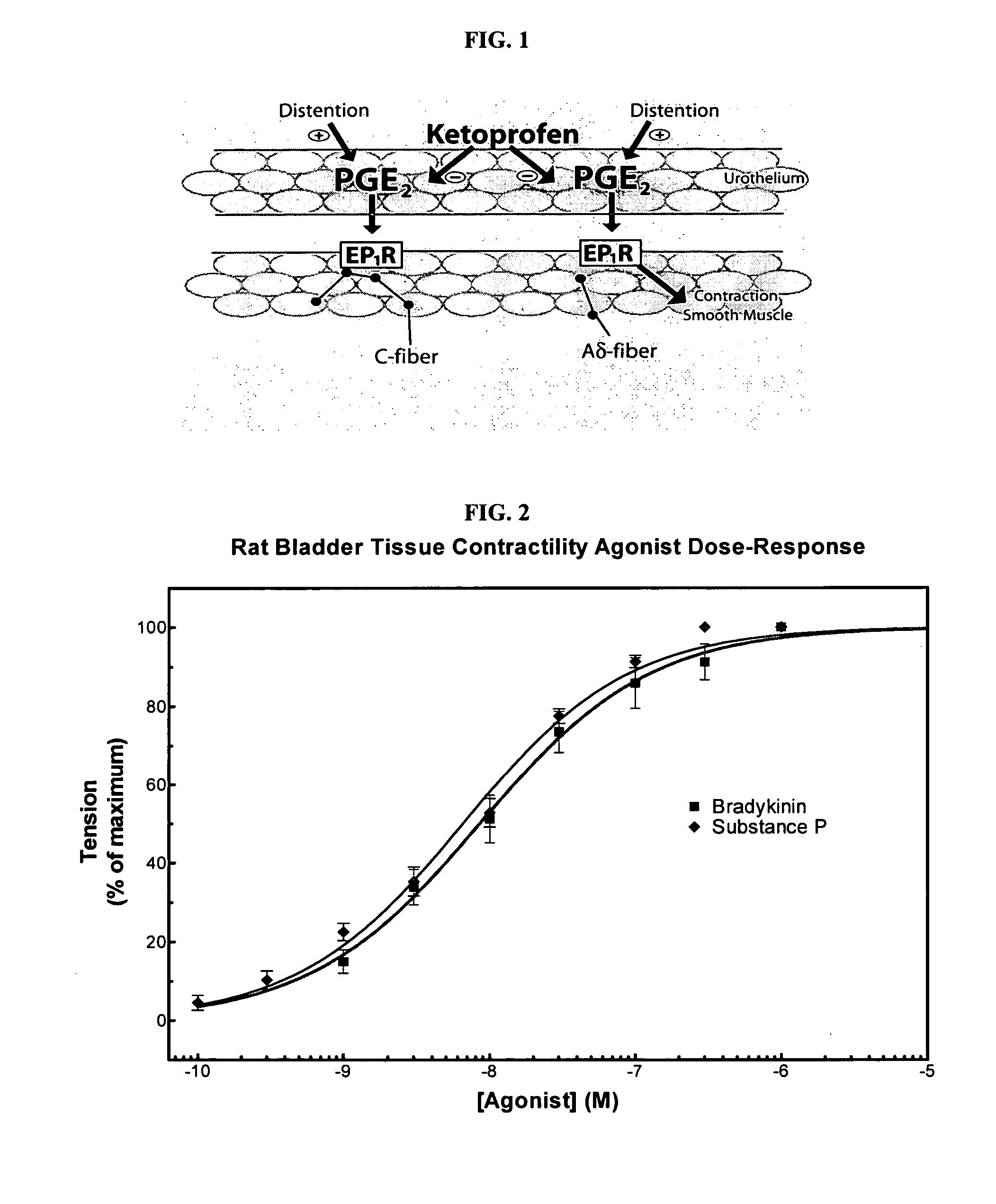
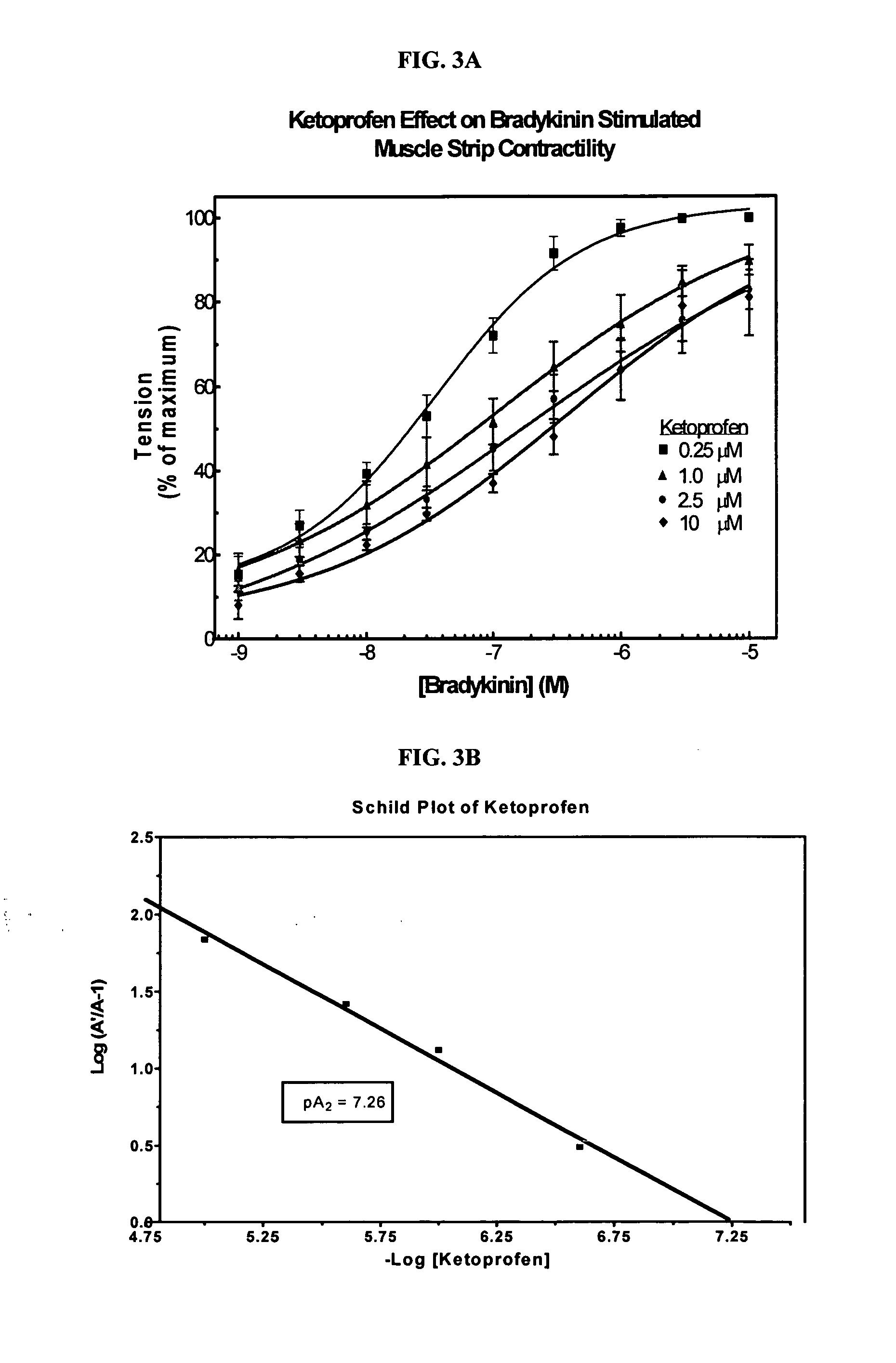
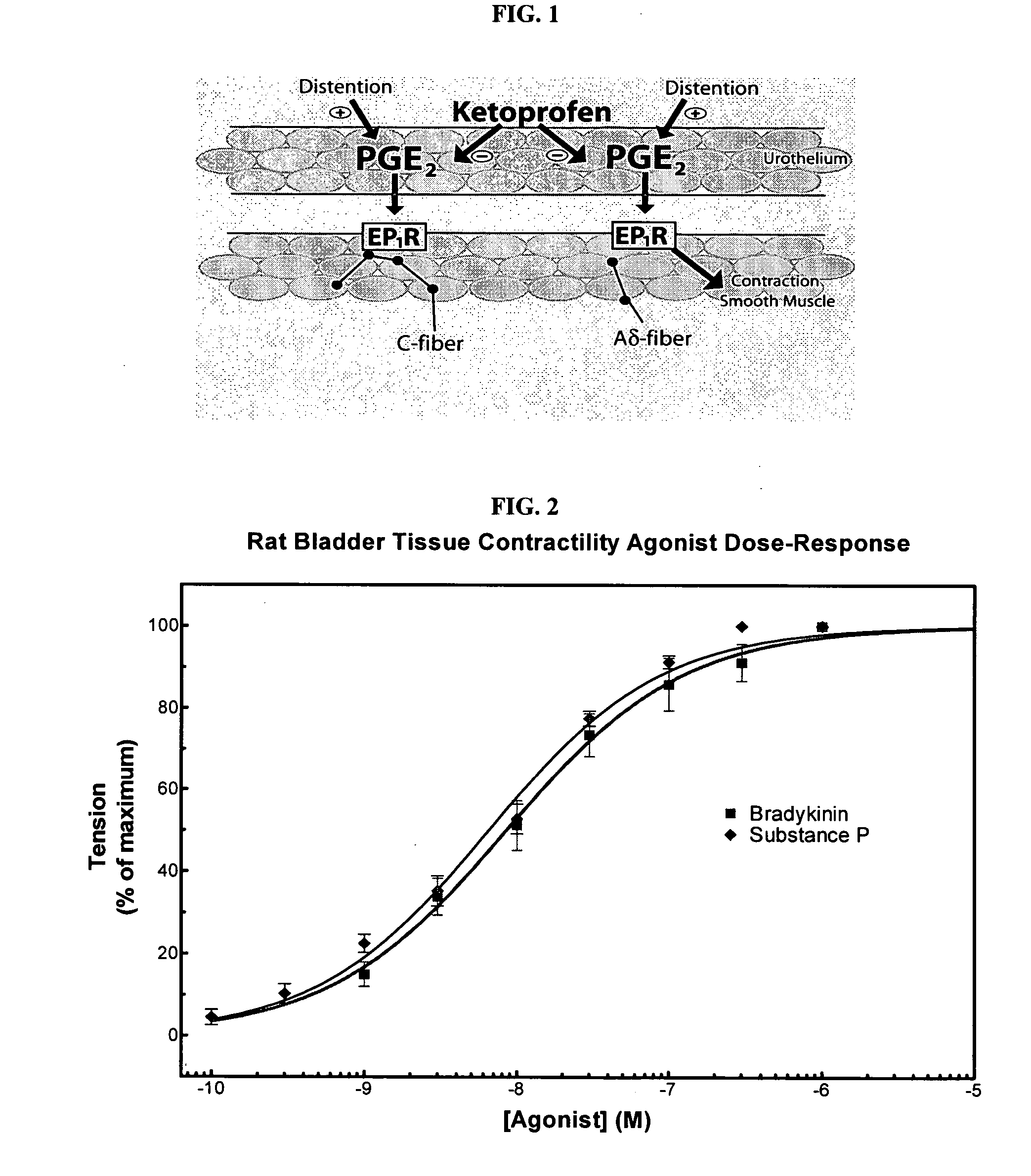
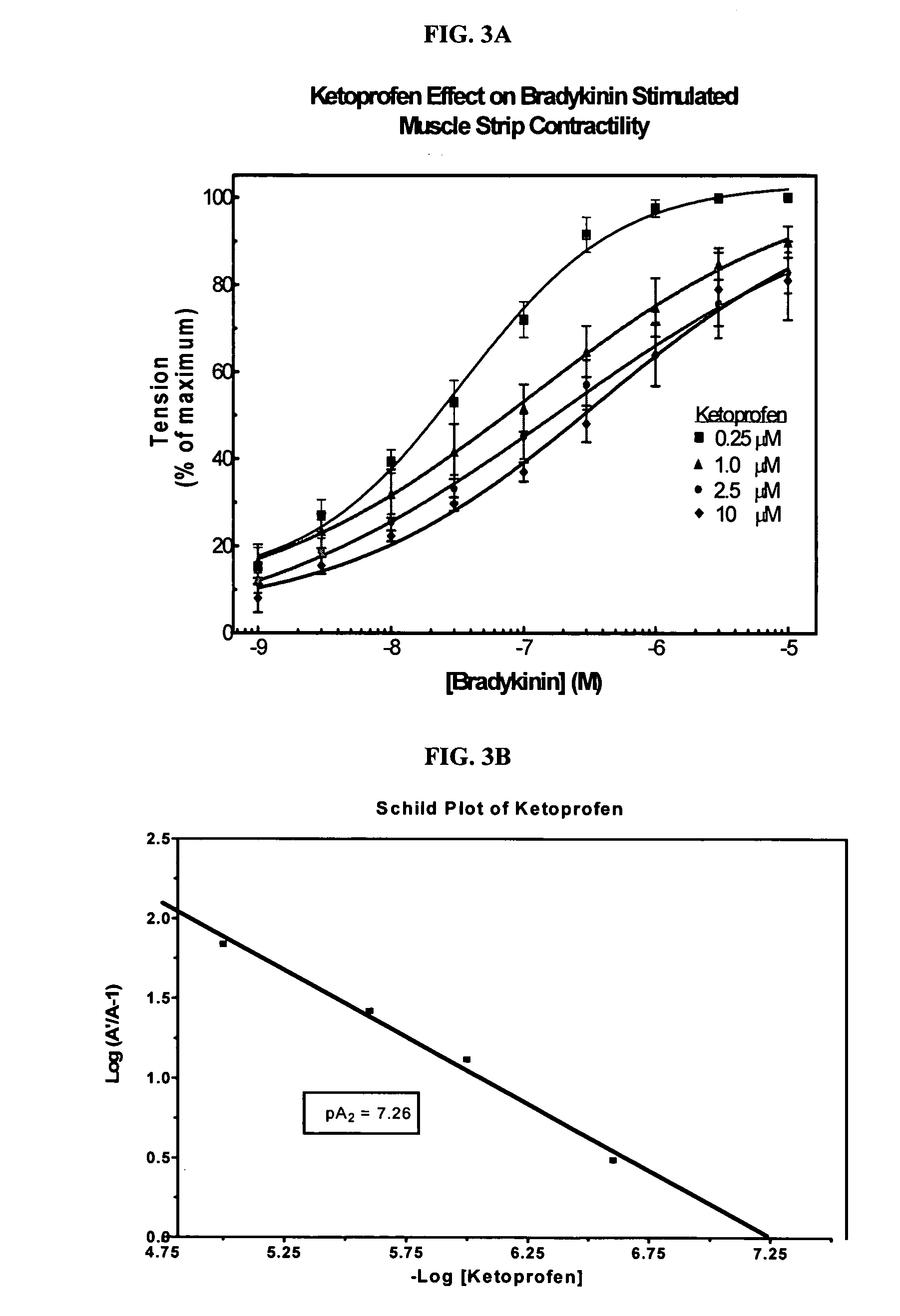
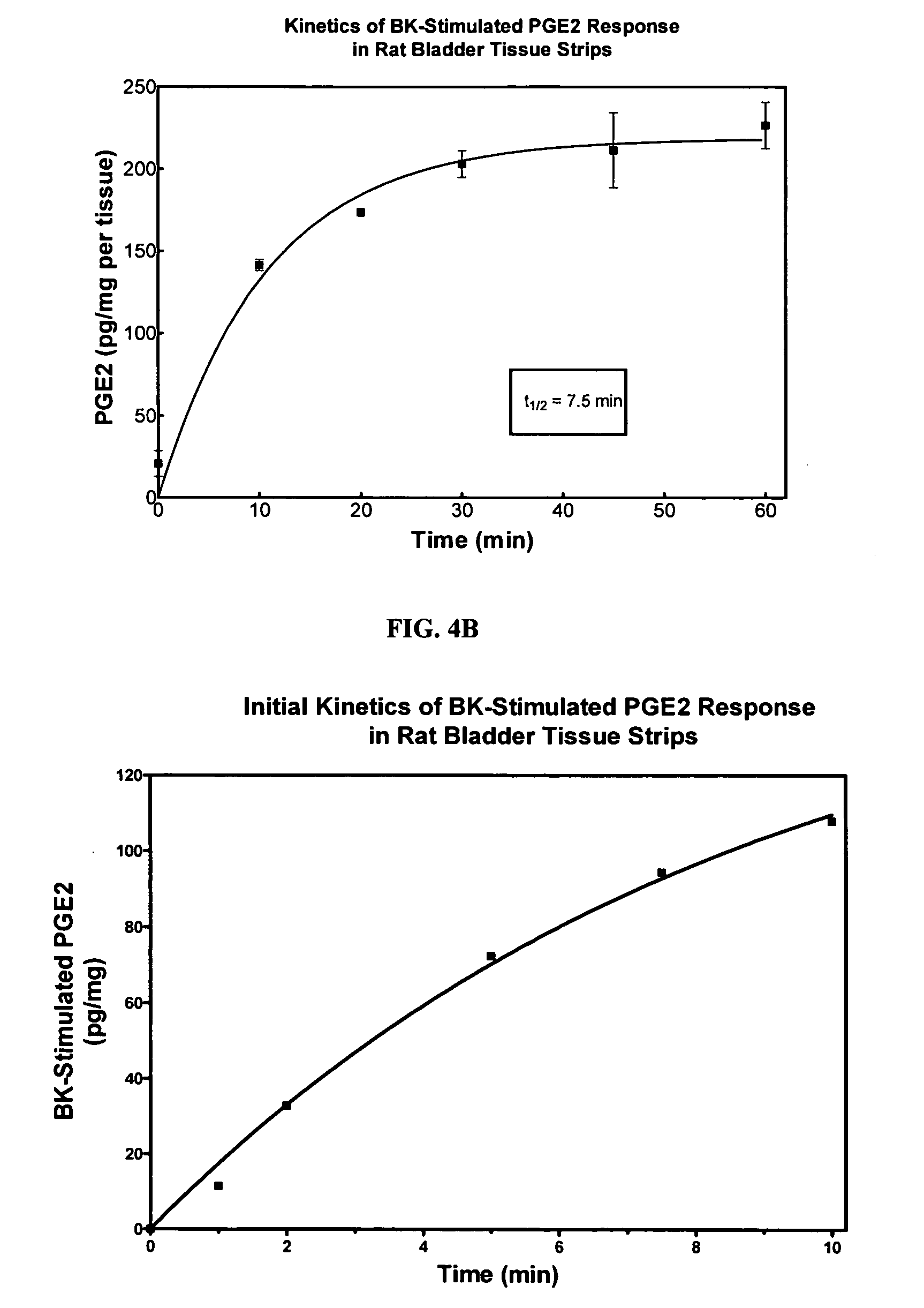
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
InactiveUS20070248639A1Inhibiting pain/inflammationPrevent spasmsBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
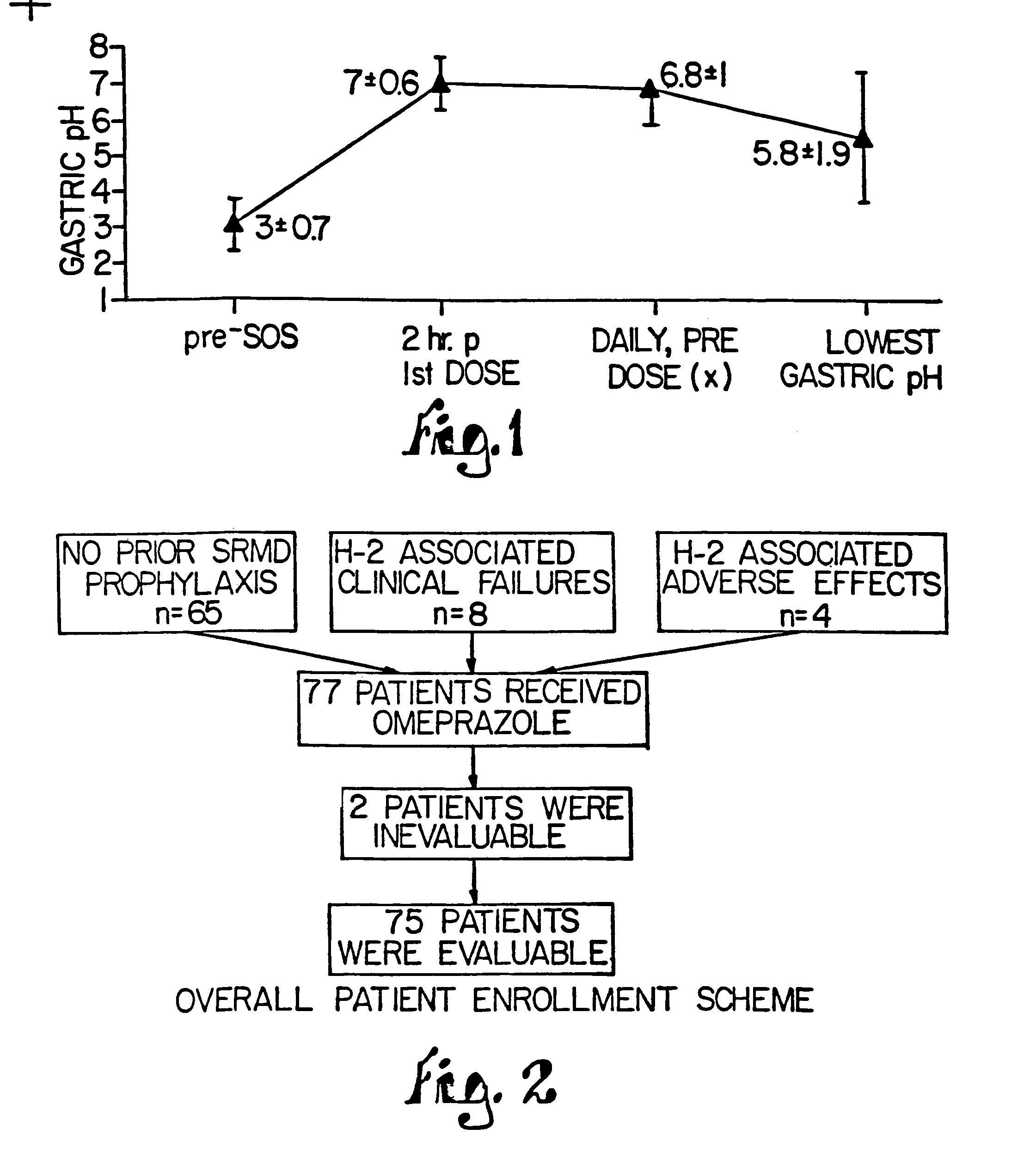
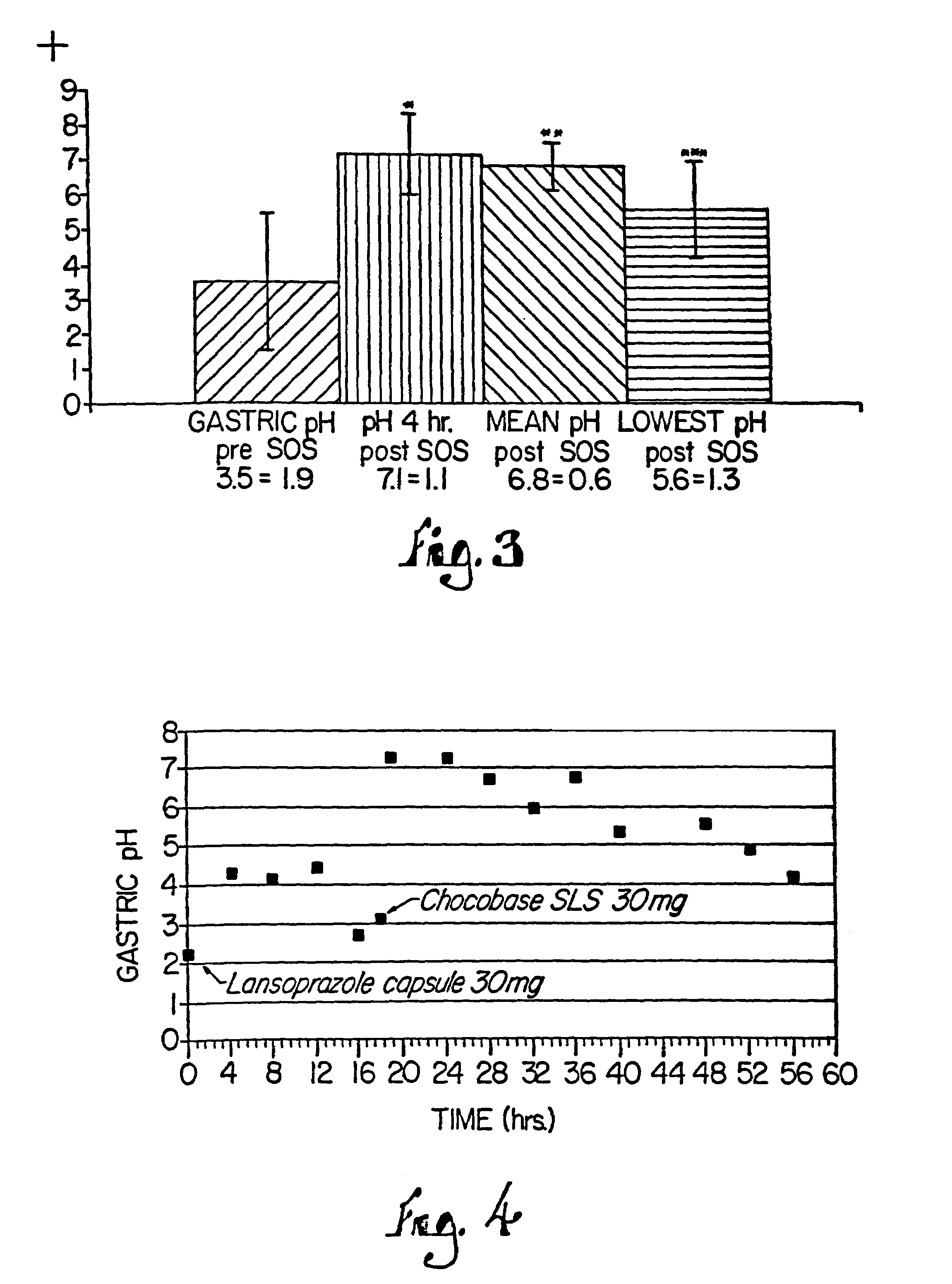
Combination of proton pump inhibitor, buffering agent, and nonsteroidal anti-inflammatory drug
InactiveUS20050249806A1Preventing gastric acid related disorderReduce riskBiocideSenses disorderNonsteroidal Antiinflammatory Drugs/NSAIDsBuffering agent
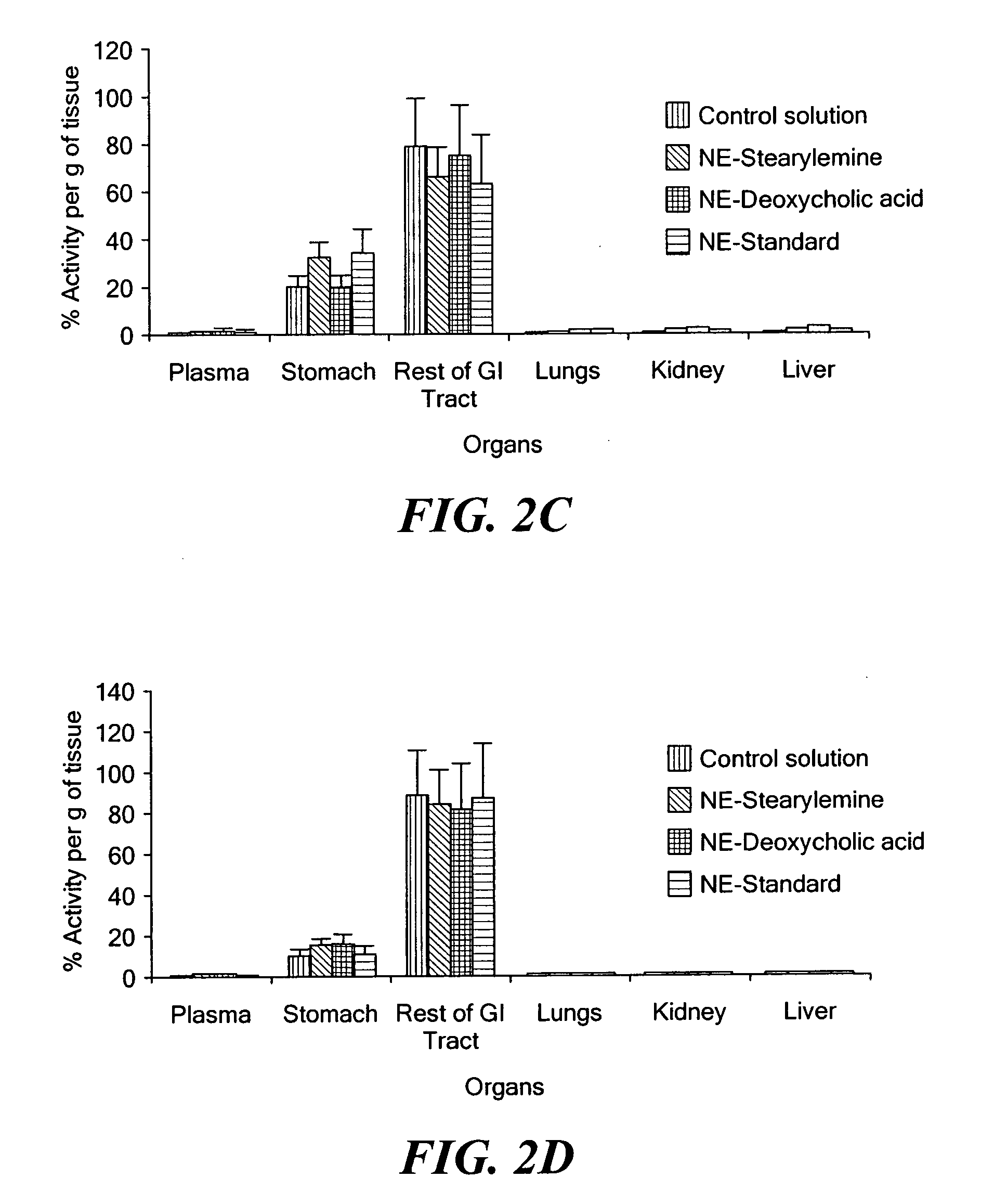
Pharmaceutical compositions comprising a proton pump inhibitor, one or more buffering agent and a nonsteroidal anti-inflammatory drug are described. Methods are described for treating gastric acid related disorders and treating inflammatory disorders, using pharmaceutical compositions comprising a proton pump inhibitor, a buffering agent, and a nonsteroidal anti-inflammatory drug.
Owner:SANTARUS
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
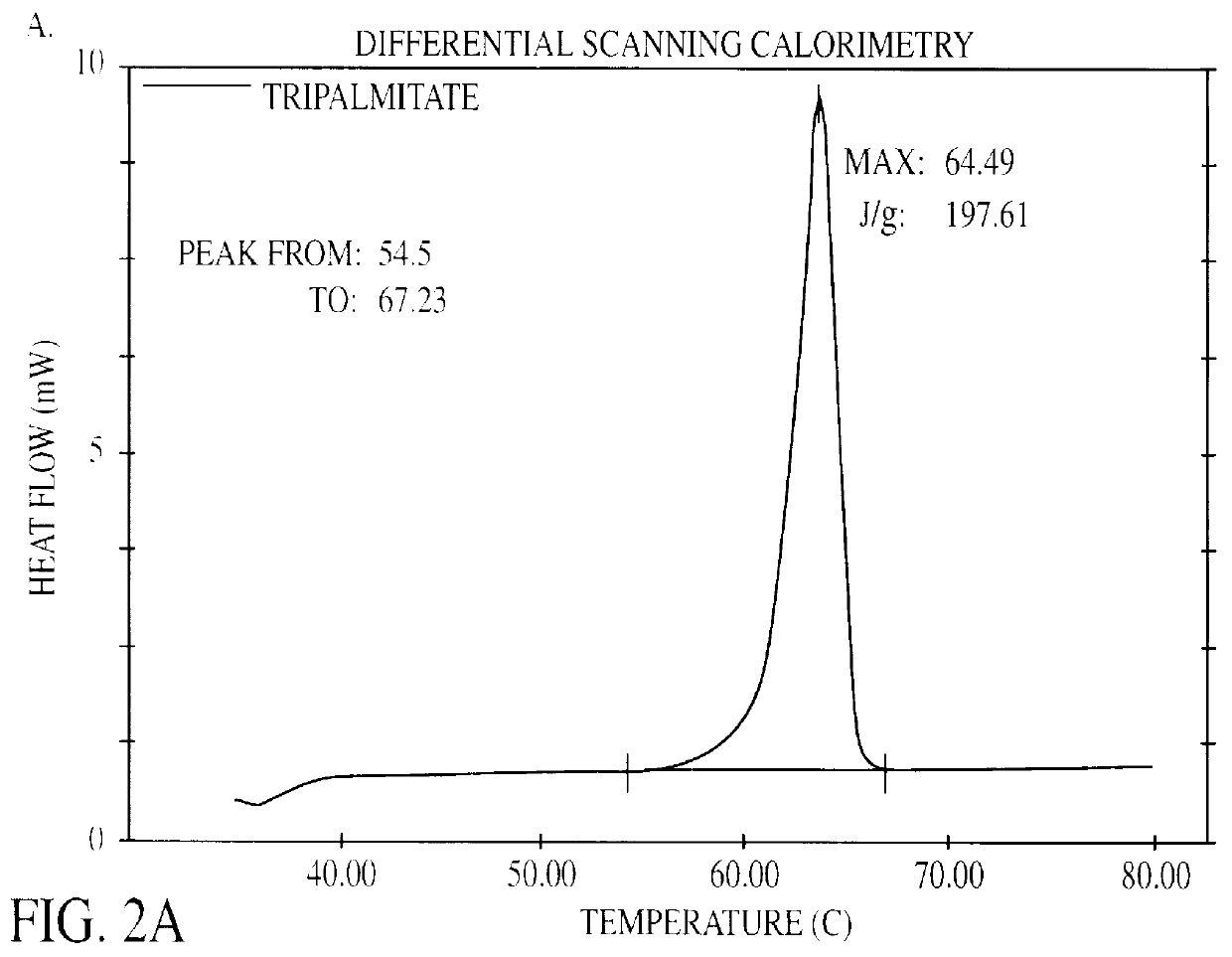
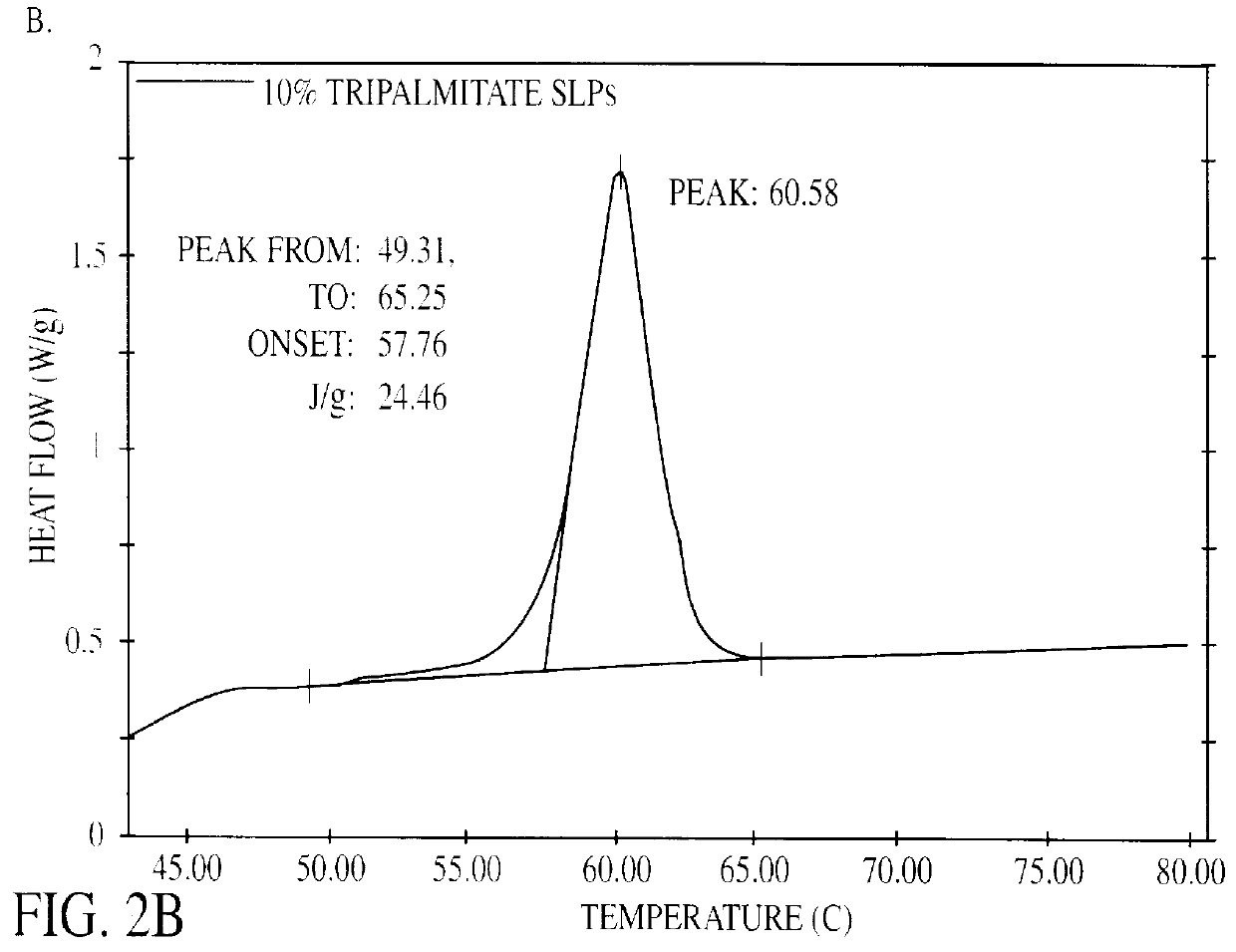
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
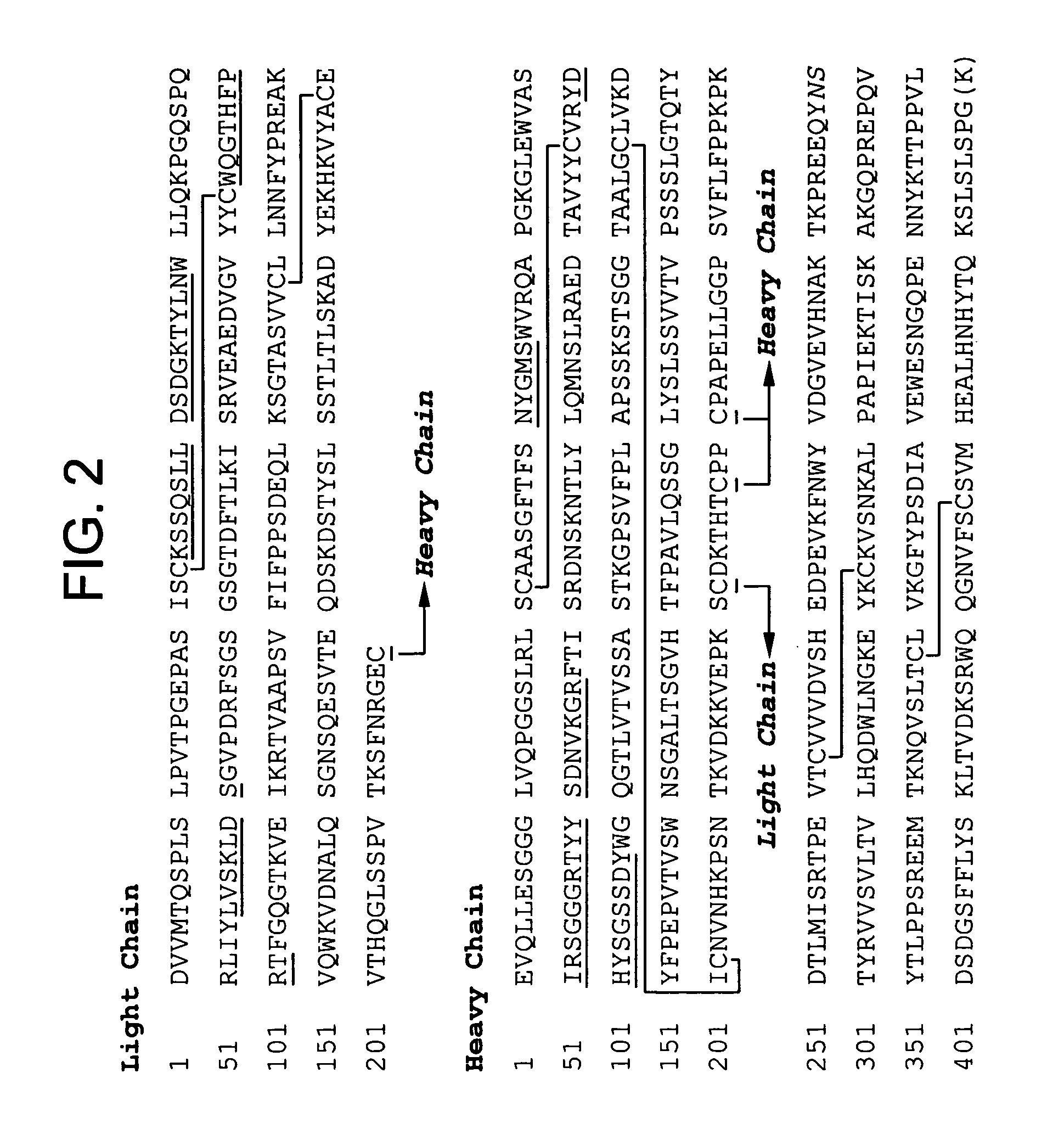
Subcutaneous anti-HER2 antibody formulations and uses thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Novel nanoemulsion formulations
InactiveUS20070148194A1Reducing surfactant side-effectsImprove oral bioavailabilityDispersion deliveryEmulsion deliveryBuffering agentAntioxidant
An oil-in-water nanoemulsion delivery system that includes at least one oil having a concentration of greater than or equal to 2% (w / w) of at least one polyunsaturated fatty acid, preferably of the omega-3 or omega-6 family, is disclosed. The delivery system further includes at least one emulsifier and also an aqueous phase. Preferably, one or more hydrophobic therapeutic, monitoring and / or diagnostic agents are dispersed in the oil phase. The nanoemulsions may optionally contain other conventional pharmaceutical aids such as stabilizers, preservatives, buffering agents, antioxidants, polymers, proteins and charge inducing agents. The invention also relates to a process for preparing the nanoemulsions and to their use in the oral, parenteral, opthalmic, nasal, rectal or topical delivery of hydrophobic therapeutic, monitoring or diagnostic agents.
Owner:NORTHEASTERN UNIV +1
Pharmaceutical compositions comprising substituted benzimidazoles and methods of using same
InactiveUS20050054682A1Improve stabilityDecreased time to therapeutic effectBiocideDispersion deliveryBuffering agentPharmacology
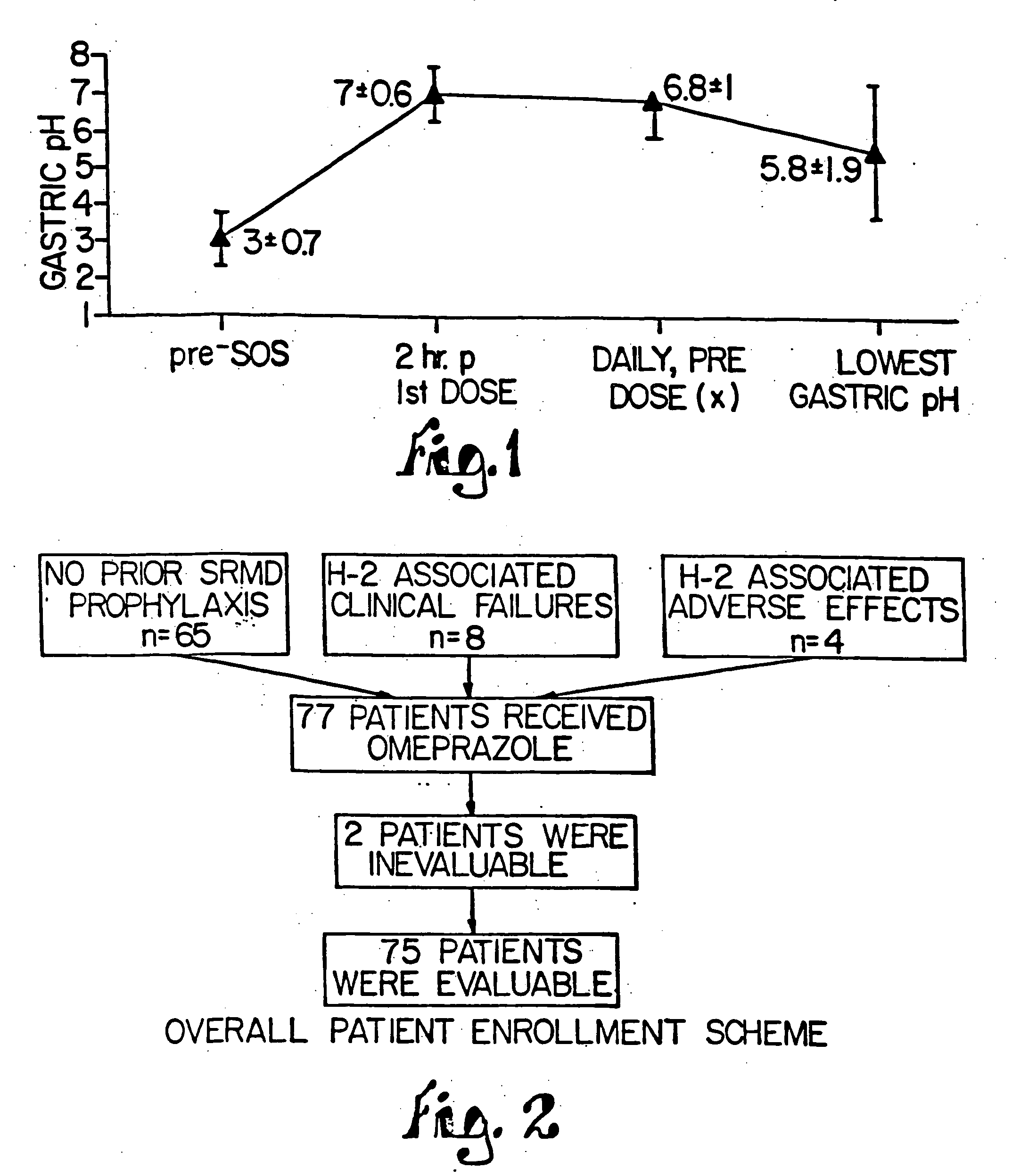
The present invention is directed to, inter alia, pharmaceutical compositions comprising at least one proton pump inhibitor and at least one buffering agent. Compositions of the invention are useful in treating, inter alia, gastric acid related disorders.
Owner:UNIVERSITY OF MISSOURI
Cyclooxygenase inhibitor and calcium channel antagonist compositions and methods for use in urological procedures
ActiveUS20060263393A1Inhibits pain/inflammation and spasmInhibiting pain/inflammationBiocideNervous disorderNifedipineCyclooxygenase
Compositions of a cyclooxygenase inhibitor and a calcium channel antagonist in a liquid carrier. The composition may be administered the the urinary tract during urological diagnostic, interventional, surgical and other medical procedures. One disclosed composition comprises ketoprofen and nifedipine in a liquid irrigation carrier, and includes a solubilizing agent, stabilizing agents and a buffering agent.
Owner:OMEROS CORP
Breaker and displacement fluid and method of use
ActiveUS20080200354A1Sufficient hydrostatic controlEasy to placeFlushingDrilling compositionWater basedEmulsion
A method of cleaning a wellbore prior to the production of oil or gas is disclosed, wherein the wellbore has been drilled with an invert emulsion drilling mud that forms an invert emulsion filter cake. The method may include the steps of circulating a breaker fluid into the wellbore, where the breaker fluid includes an aqueous fluid, and imino diacetic acid or salt thereof. Optionally an acid buffering agent, and a weighting age are also included. The breaker fluid is formulated such that after a predetermined period of time and the filter cake present in the wellbore or on the wellbore face is substantially degraded. Other methods may also include drilling the wellbore with a water-based drilling mud that forms a water-based filter cake, wherein the method may include the steps of circulating a breaker fluid into the wellbore, where the breaker fluid may include an aqueous fluid, and an iminodiacetic acid or a salt thereof.
Owner:MI
Corticosteroid-containing pharmaceutical composition
InactiveUS7078058B2Improve permeabilityEasy to handleOrganic active ingredientsBiocideScalp psoriasisDisease
A foamable pharmaceutical composition comprising a corticosteroid, a quick-break foaming agent, a propellant and a buffering agent, sufficient to buffer the composition to within the range of pH 3.0 to 6.0 is disclosed. The quick-break foaming agent typically comprises an aliphatic alcohol, water, a fatty alcohol and a surface active agent. Due to the nature of the compositions of the invention, they are especially well-suited for use in the treatment of various skin diseases, and in particular, in the treatment of scalp psoriasis.
Owner:STIEFEL WEST COAST
Enhanced Delivery of Nicotine, THC, Tobacco, Cannabidiol or Base Alkaloid from an Electronic Cigarette or Other Vapor or Smoke Producing Device Through Use of an Absorption Conditioning Unit
A method for the administration of nicotine, THC, tobacco, cannabidiol or a base alkaloid includes administering in the oral or nasal cavity an absorption conditioning unit having at least two agents selected from the group consisting of (a) a buffer agent, (b) a capturing agent, (c) a penetration agent, and (d) a thermal agent, to the mammal, and then administering by inhalation a bioactive agent selected from the group consisting of nicotine, THC, cannabidiol and a base alkaloid. The absorption conditioning unit may be in a dosage form not containing a drug. The absorption conditioning unit may create a pH in the oral cavity or nasal cavity of 7.8-10 for a period of ten minutes or more after administration, the dosage form not containing an acid and not containing a drug.
Owner:FUISZ RICHARD C +1
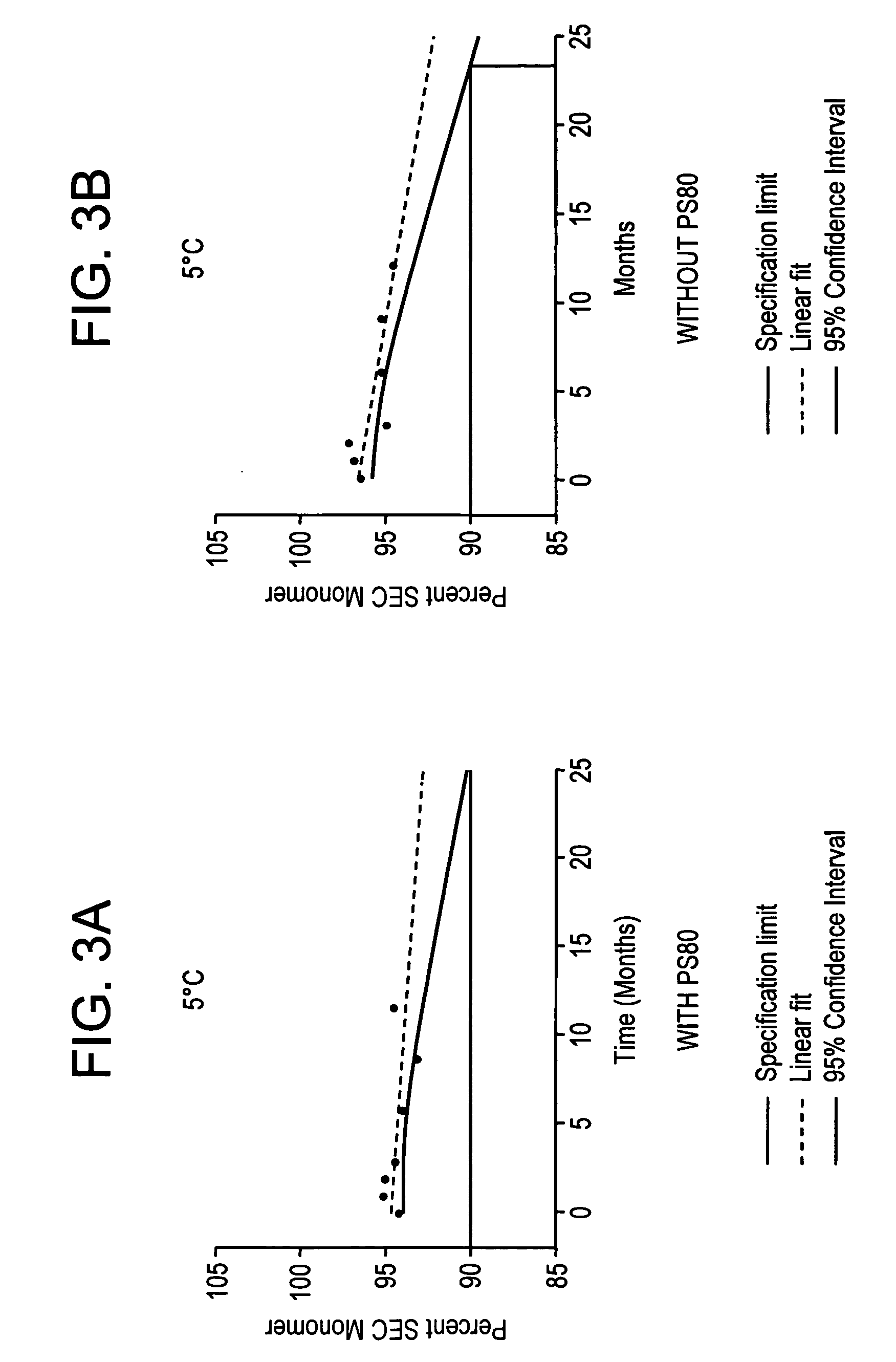
Self-Buffering Protein Formulations
InactiveUS20080311078A1Peptide/protein ingredientsInorganic non-active ingredientsDiseaseHuman medicine
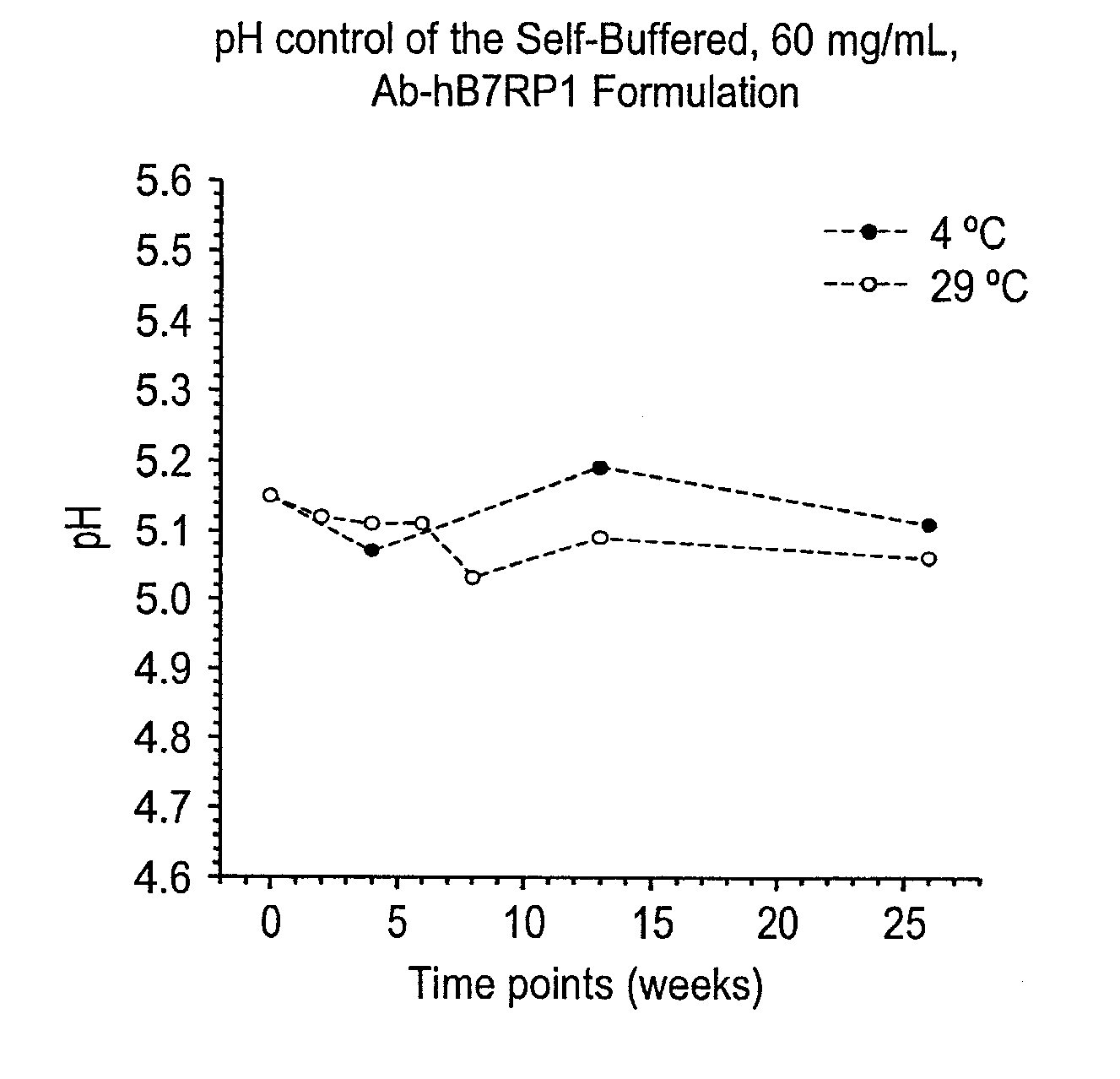
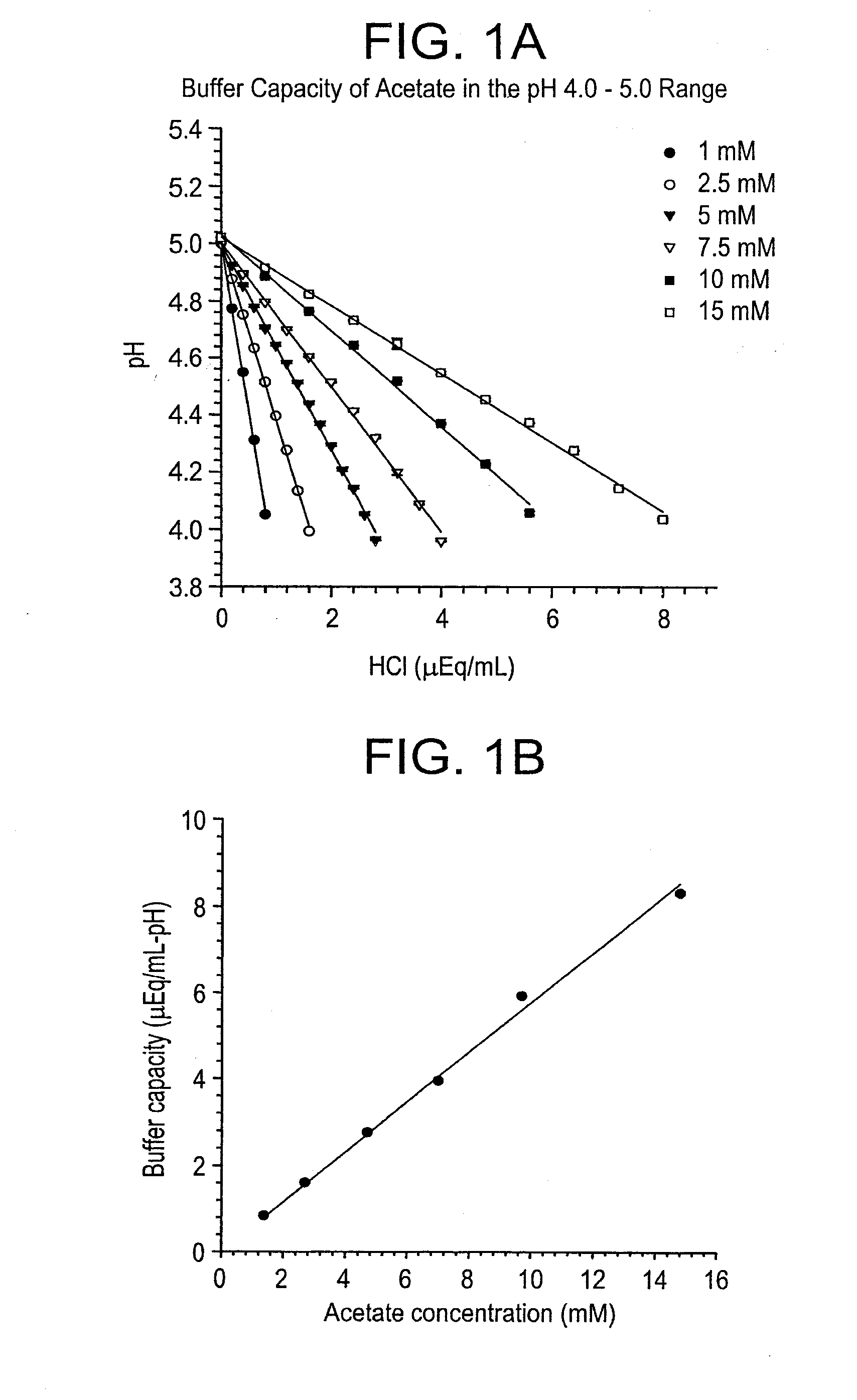
The invention herein described, provides, among other things, self-buffering protein formulations. Particularly, the invention provides self-buffering pharmaceutical protein formulations that are suitable for veterinary and human medical use. The self-buffering protein formulations are substantially free of other buffering agents, stably maintain pH for the extended time periods involved in the distribution and storage of pharmaceutical proteins for veterinary and human medical use. The invention further provides methods for designing, making, and using the formulation. In addition to other advantages, the formulations avoid the disadvantages associated with the buffering agents conventionally used in current formulations of proteins for pharmaceutical use. The invention in these and other respects can be productively applied to a wide variety of proteins and is particularly useful for making and using self-buffering formulations of pharmaceutical proteins for veterinary and medical use, especially, in particular, for the treatment of diseases in human subjects.
Owner:AMGEN INC
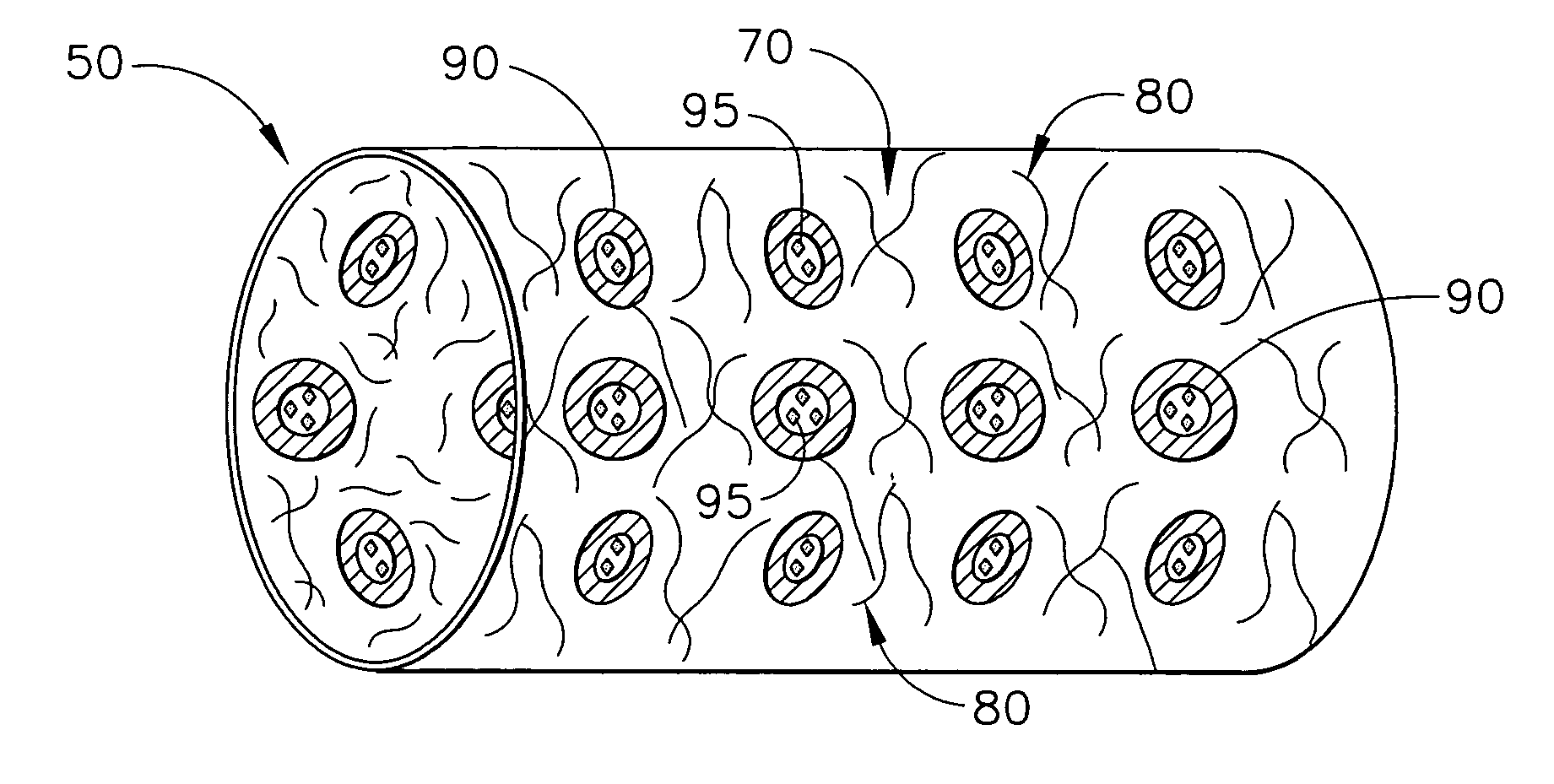
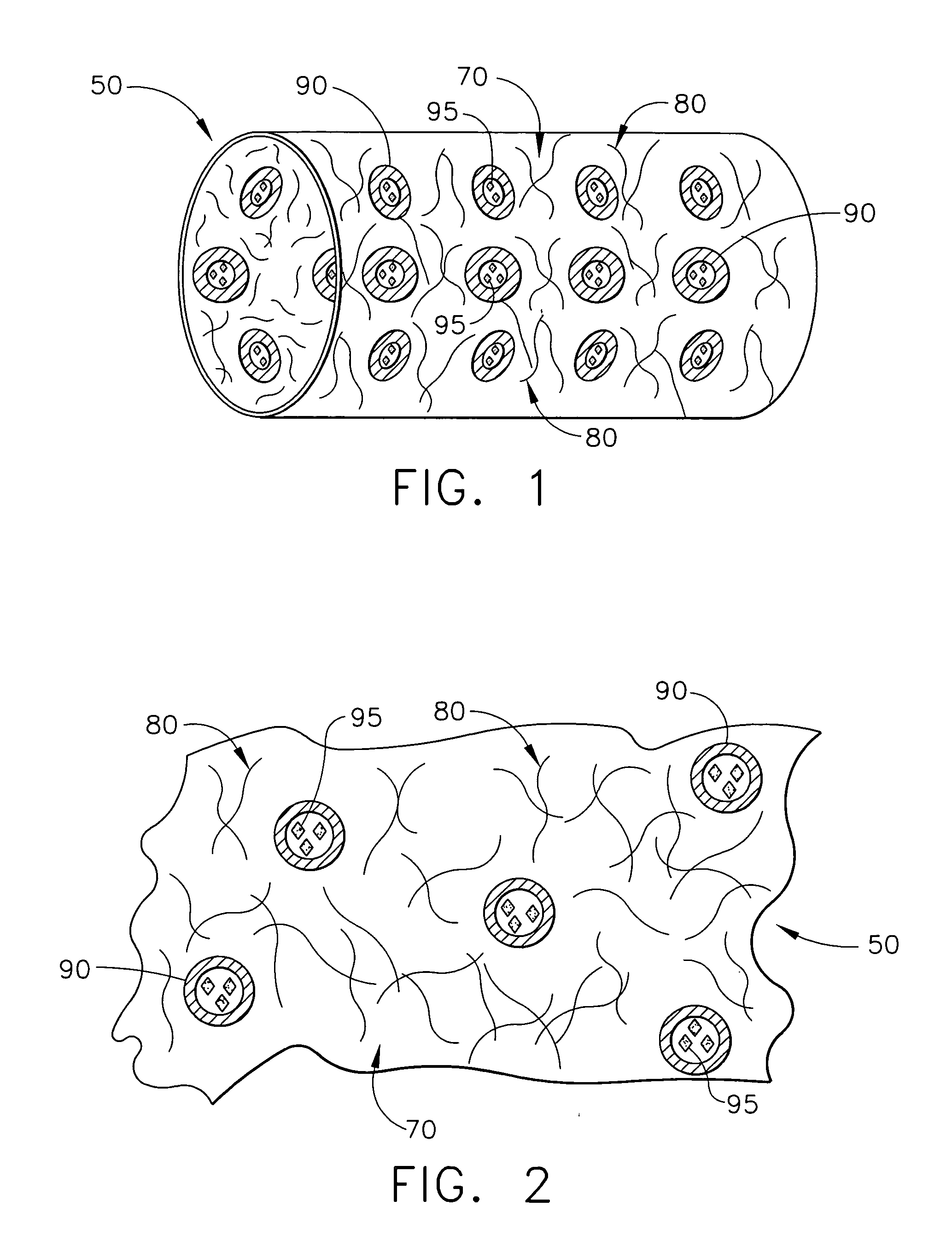
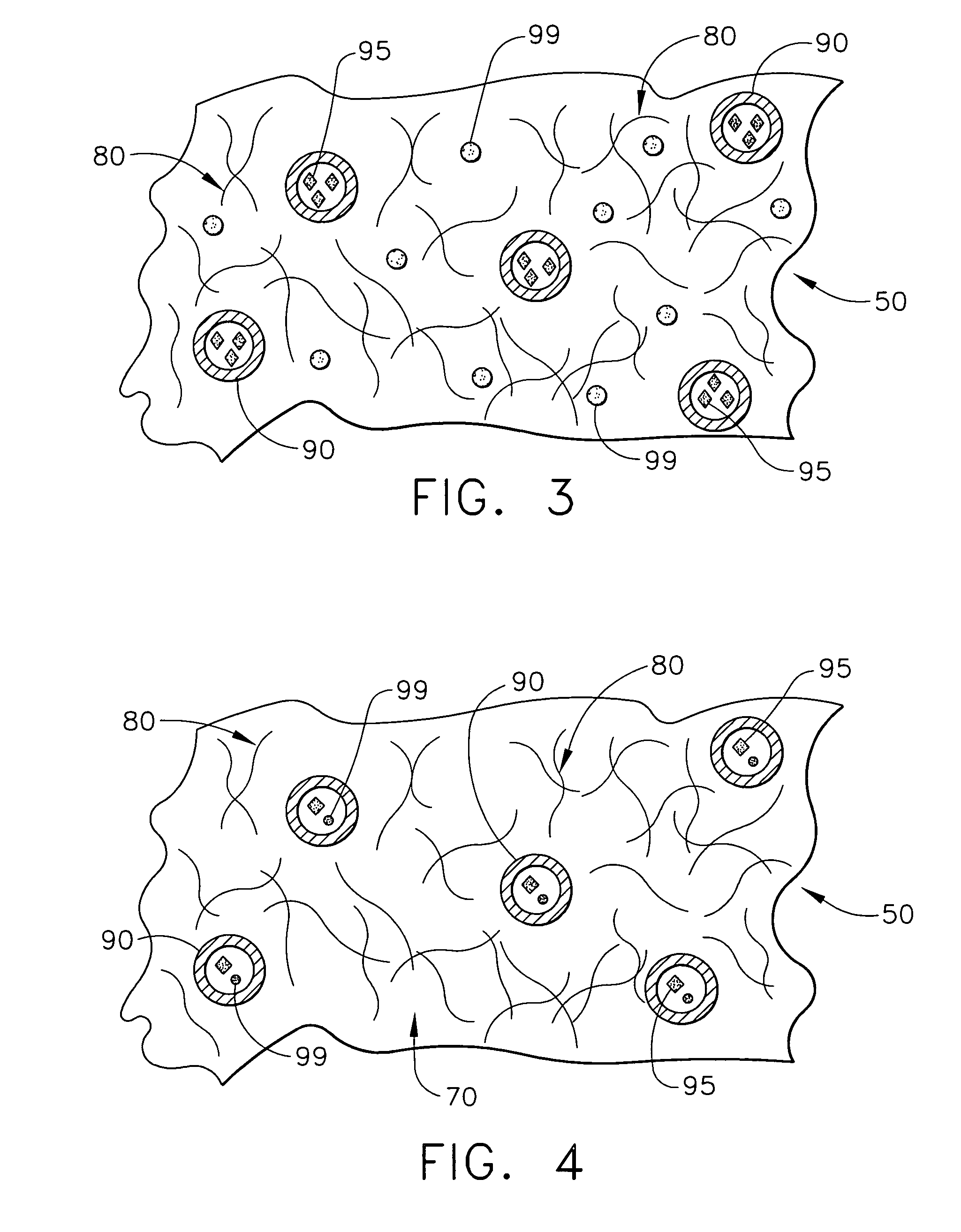
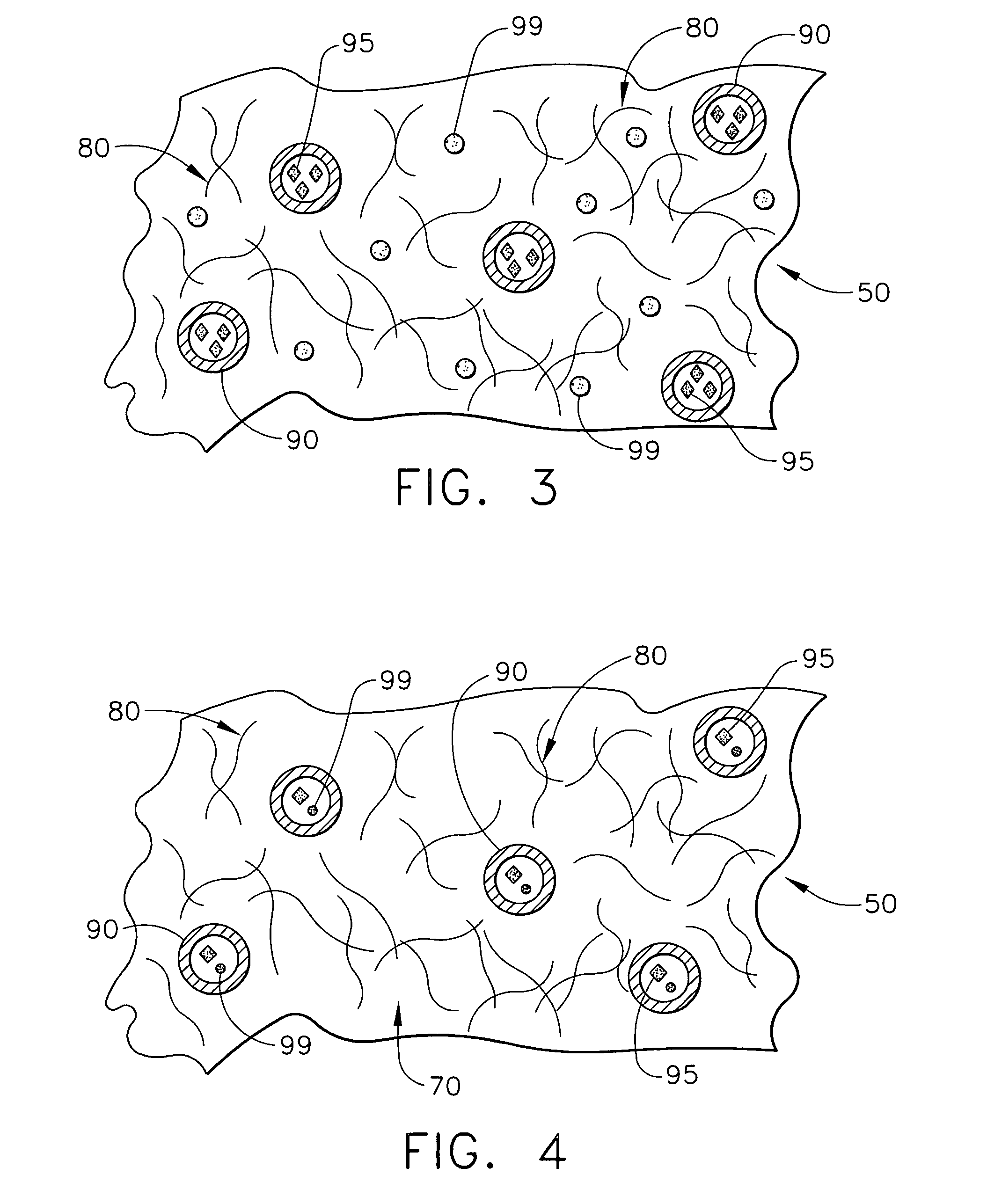
Biodegradable medical implant with encapsulated buffering agent
ActiveUS20050267565A1Control acidic effectEliminate the effects ofStentsBiocideBuffering agentMedical device
A medical device for placement at a site in a patient's body and for controlling pH levels at the site in the patient's body includes one or more structural components made of a first biodegradable and / or bioabsorbable material or, alternatively, one or more structural components having a coating thereon made of a first biodegradable and / or bioabsorbable material. The device also includes a buffering agent and at least one second biodegradable and / or bioabsorbable material on or in the one or more structural components, or alternatively, on or in the coating on the one or more structural components. The at least one second biodegradable and / or bioabsorbable material encapsulates the buffering agent and the buffering agent is dispersed from the at least one second biodegradable and / or bioabsorbable material in response to hydrolysis of the first biodegradable and / or bioabsorbable material. Additionally, the device can include a drug that is either also encapsulated by the at least one second biodegradable and / or bioabsorbable material or is included with the first biodegradable and / or bioabsorbable material.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS6893654B2Convenient and reliable and practicalReduce cravingsBiocidePowder deliveryOpiateInitial dose
A two-stage medicine delivery system provides an initial dose of medicine and a second dose of medicine. The initial and second doses are capable of achieving a rapid pharmacological effect and a prolonged pharmacological effect, respectively. The two-stage medicine delivery system preferably delivers a craving reduction substance, in which case, the rapid and prolonged pharmacological effects include a rapid and prolonged craving reduction. Preferably, the delivery system is a nicotine delivery system which is provided in chewing gum form or lozenge form and which provides the nicotine in a transmucosally absorbable form. The two-stage medicine delivery system preferably releases a buffering agent which increases a pH level in a user's mouth to facilitate absorption of the medicine when the delivery system is placed in the user's mouth. A method of making the medicine delivery system also is provided. The system and apparatus can be adapted to reduce cravings for alcohol, food, drugs (e.g., cocaine, opiates and the like) and tobacco products, especially tobacco products containing nicotine.
Owner:JSR NTI
Anti a beta antibody formulation
ActiveUS20060193850A1Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
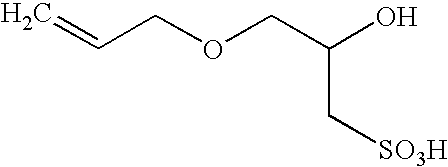
Polymerization of fluoromonomers using a 3-allyloxy-2-hydroxy-1-propanesulfonic acid salt as surfactant
InactiveUS6869997B2Reduce the binding forceOrganic chemistryFibre treatmentFluoropolymerBuffering agent
Fluoropolymers are prepared by a process comprising polymerizing at least one fluoromonomer in an aqueous reaction medium containing monomer, a radical initiator and a 3-allyloxy-2-hydroxy-1-propanesulfonic acid salt as surfactant. The medium may optionally contain one or more of an antifoulant, a buffering agent and a chain-transfer agent.
Owner:ARKEMA INC
Immunoglobulin formulation and method of preparation thereof
InactiveUS20050053598A1Fixed volumeNervous disorderAntipyreticPharmaceutical formulationVariable weight
A stable aqueous pharmaceutical formulation comprising a therapeutically effective amount of an antibody, polysorbate 80, a buffer which inhibits polysorbate oxidation is described along with methods of making the preparation. Also described are formulations with high antibody concentrations which maintain fixed volumes and which may be used on patients of variable weight.
Owner:BIOGEN MA INC
Stable aqueous spore-containing formulation
Stable aqueous spore-containing chemical formulations having a low to medium viscosity profile are provided. The formulations comprise at least one spore in a mixture of water and at least one water miscible solvent, optionally at least one surfactant, optionally at least one stabilizer such as a metal salt, optionally at least one biocide, optionally at least one buffer, and optionally at least one chemical insecticide or fungicide or a mixture thereof. The formulations are particularly suitable as a seed coating and foliar spray. Methods of preparation as well as methods of treating a plant are also provided.
Owner:BASF CORP
Buffered coated nicotine containing products
InactiveUS20080286341A1Rapid sufficient uptakePleasant tasteBiocideNervous disorderBuffering agentAmino acid
Coated oral dosage forms for the delivery of nicotine in any form to a subject by rapid intraoral delivery of nicotine comprising at least one core, nicotine in any form and / or a nicotine mimicking agent, at least one coating layer and optionally at least one or more other additives, wherein said at last one coating layer is buffered, whereby is used at least one amino acid as buffering agent. Also contemplated are a method for the delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as a method for producing said coated product and use of the same for obtaining a rapid intraoral uptake of nicotine.
Owner:ANDERSSON SVEN BORJE +5
Biodegradable vascular device with buffering agent
ActiveUS20050278015A1Control acidic effectEliminate the effects ofStentsHeart valvesVascular deviceBuffering agent
A vascular or cardiovascular medical device for placement at a site in a patient's body and for controlling pH levels at the site in the patient's body includes one or more structural components made of a biodegradable and / or bioabsorbable material, or alternatively, a coating thereon made of a biodegradable and / or bioabsorbable material. A buffering agent is provided on or in the biodegradable and / or bioabsorbable material and the buffering agent is dispersed from the biodegradable and / or bioabsorbable material in response to hydrolysis of the biodegradable and / or bioabsorbable material. Additionally, the vascular or cardiovascular medical device can include a drug that is included with the biodegradable and / or bioabsorbable material. The vascular or cardiovascular medical device can also be a stent or a valve.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
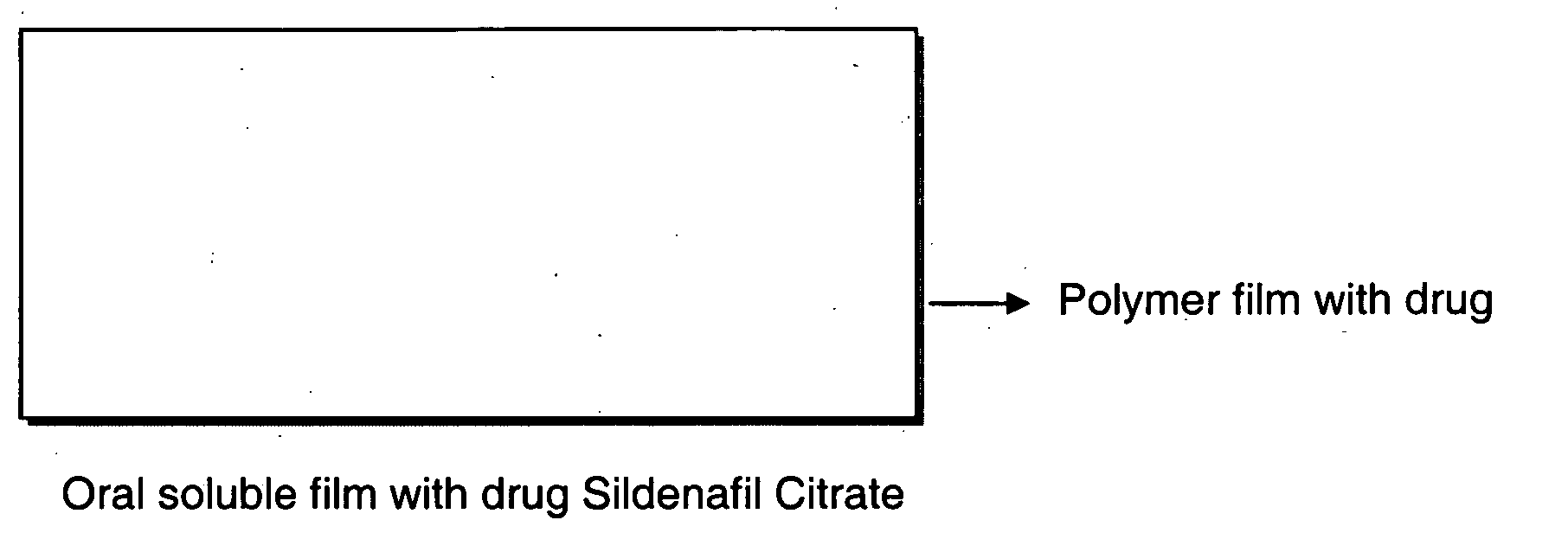
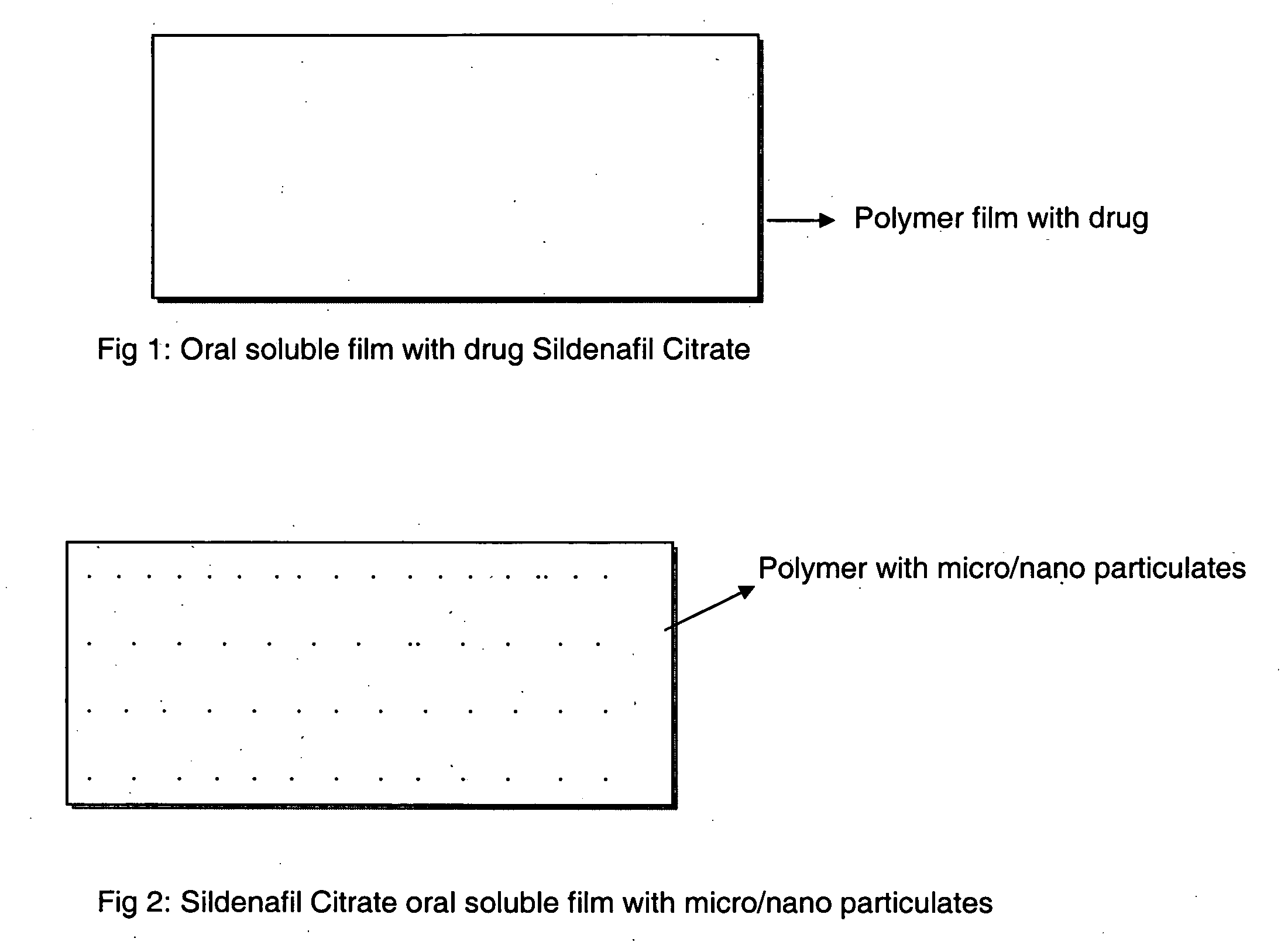
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Compositions containing pipercillin and tazobactam useful for injection
InactiveUS6900184B2Reduce formationMaintain stabilityBiocidePeptide/protein ingredientsParticulatesDepressant
The invention pertains to pharmaceutical compositions of Zosyn® piperacillin with tazobactam in the presence of a buffer, preferably citrate, a particulate formation inhibitor, preferably EDTA optionally an aminoglycoside which when frozen and thawed or lyophilized and reconstituted reform a solution which has decreased particulate formation.
Owner:MEDICAL SAFETY PROD
Stabilized liquid polypeptide formulations
InactiveUS20060210557A1Provide stabilityMaintain biological activityBiocideOrganic active ingredientsMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of polypeptides, in particular, therapeutic antigen-binding polypeptides such as antibodies and the like, for example, anti-Aβ antibodies. The formulations generally include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine, and thus, the formulations are suitable for several different routes of administration.
Owner:WYETH LLC
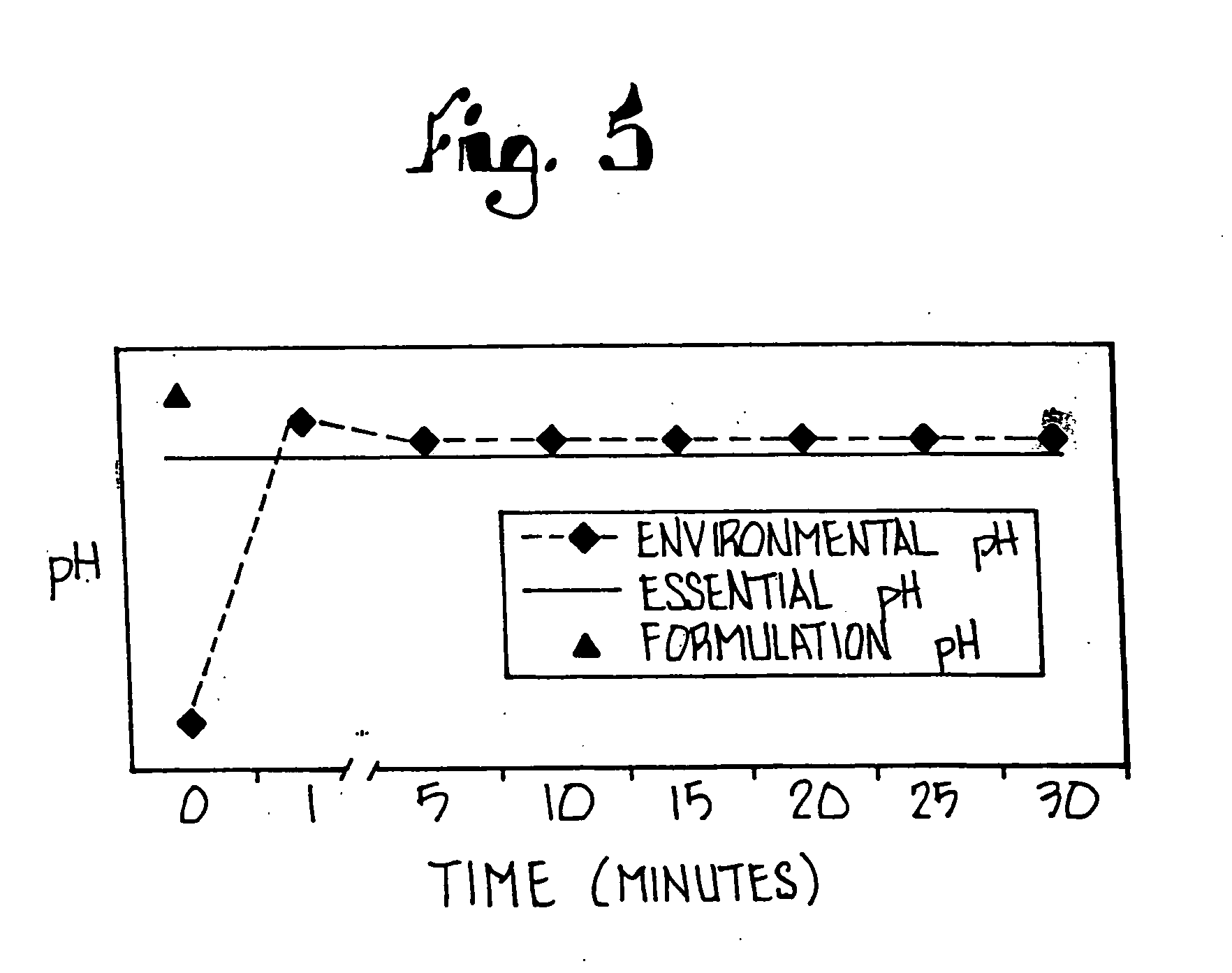
Methods for buffer stabilized aqueous deacylation
The present invention relates to novel methods for stabilizing aqueous deacylation, via use of buffers in the production of sucralose. The present invention provides a process for producing sucralose from an acyl-sucralose compound whereby the acyl-sucralose compound is deacylated in the presence of a buffering agent, which stabilizes the pH of the feed mixture and decreases the accumulation of undesired anhydro compounds. Further, the present invention provides a process whereby the acyl-sucralose compound is deacylated directly either prior to or after removal of the tertiary amide reaction vehicle from the neutralized chlorination feed mixture. An aqueous solution of sucralose including salts and other compounds is produced, from which sucralose is recovered by extraction and purified by crystallization.
Owner:TATE & LYLE TECH LTD
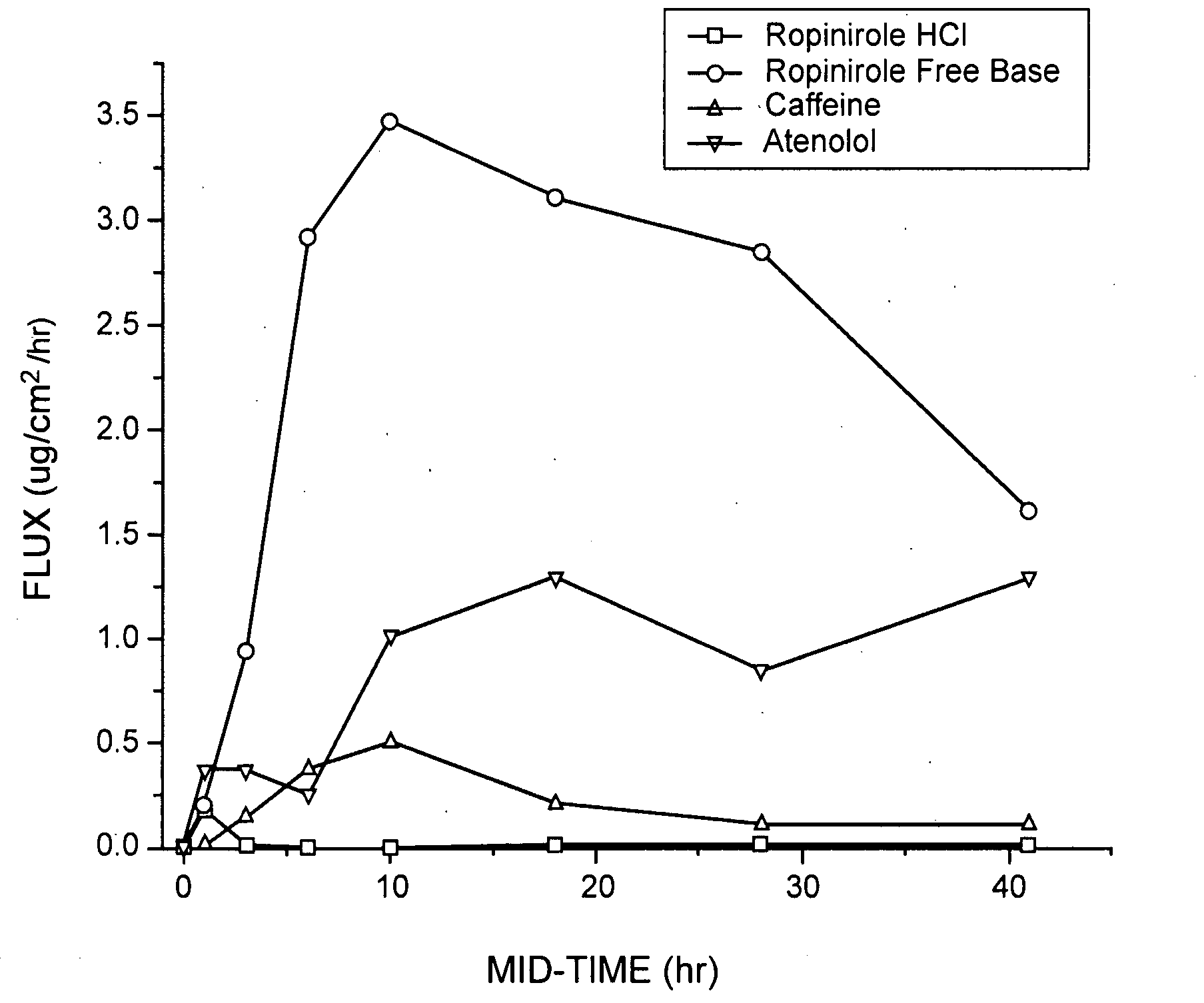
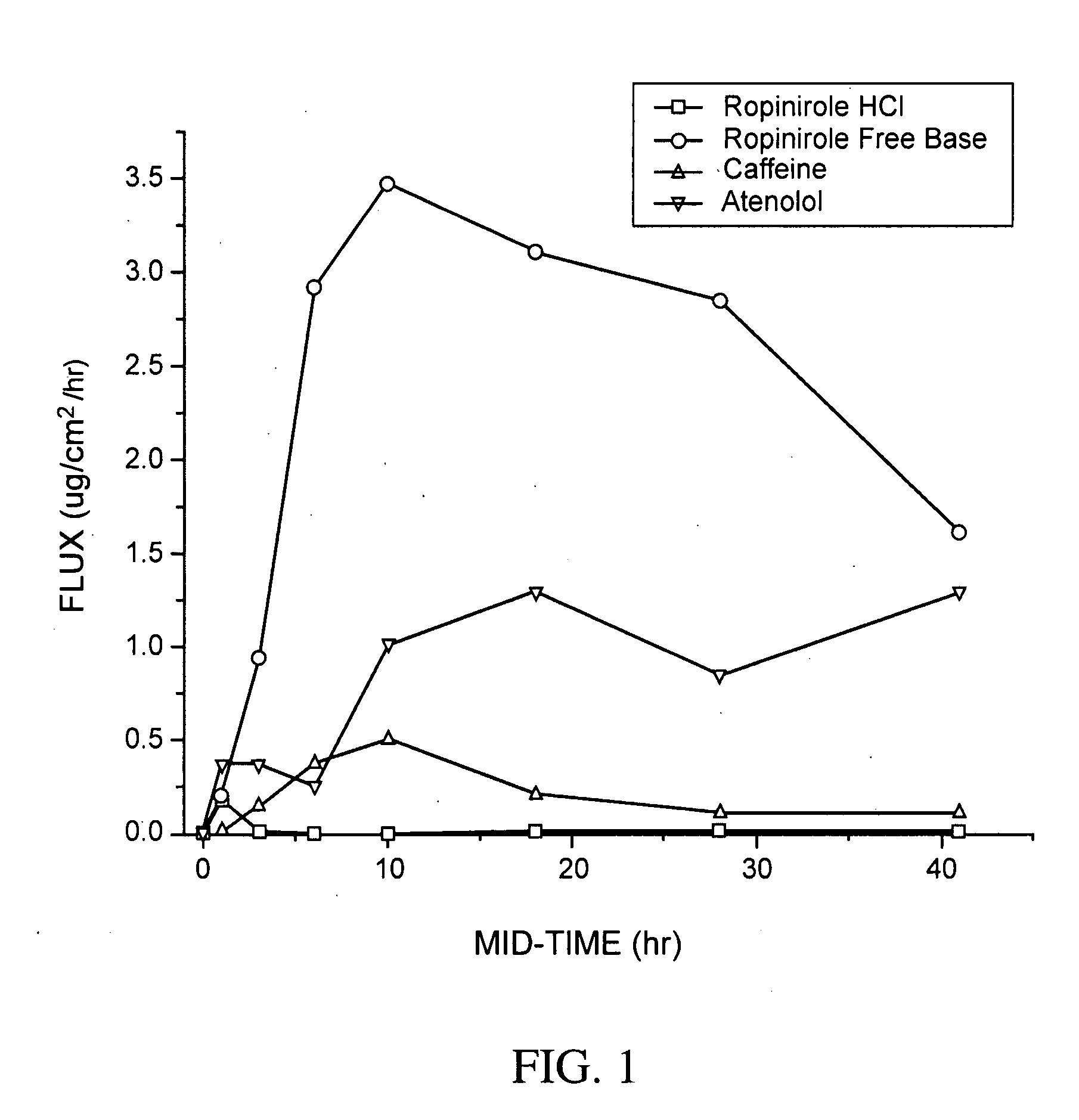
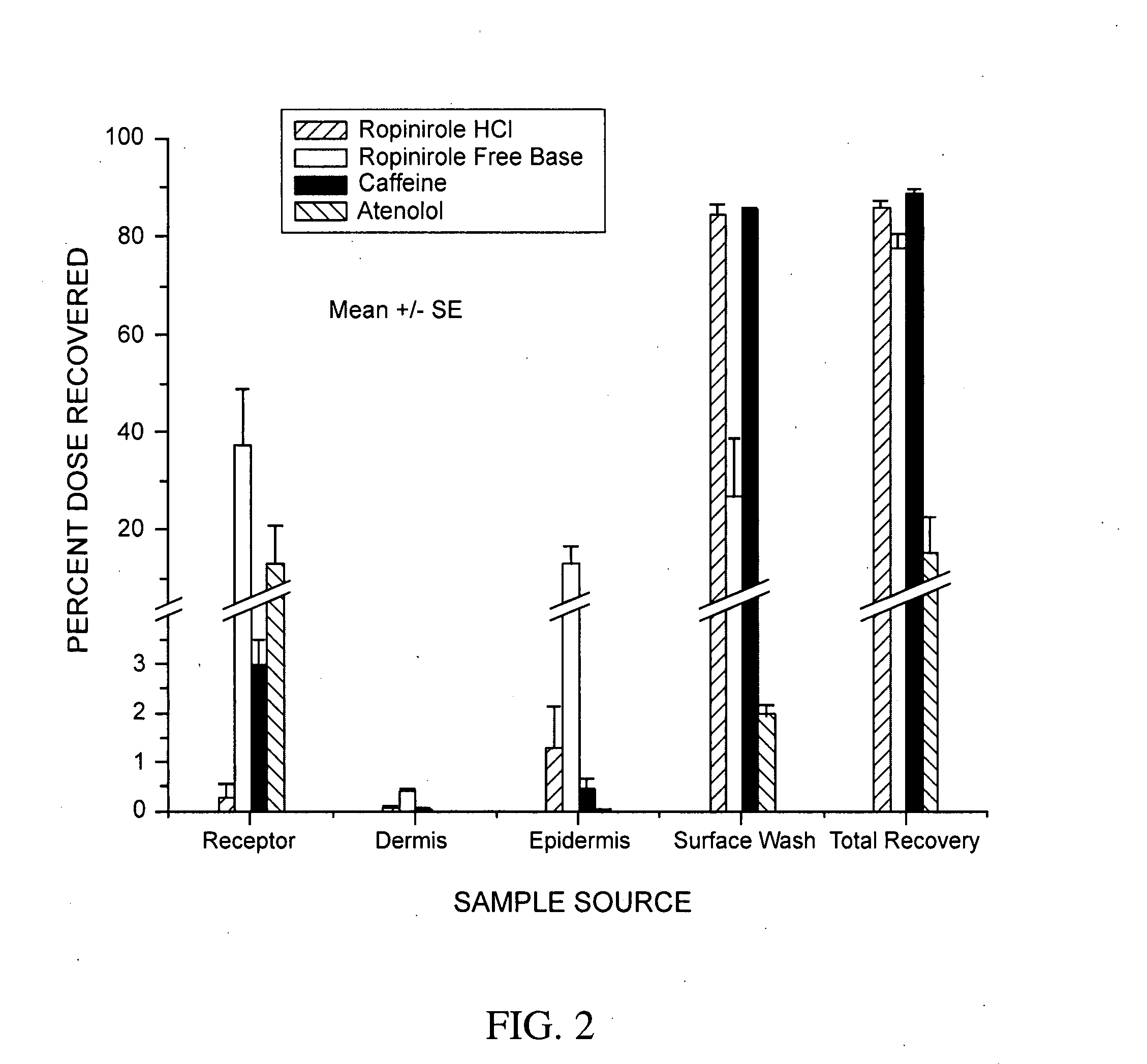
Pharmaceutical compositions of ropinirole and methods of use thereof
InactiveUS20080004329A1Easy to produceBiocideNervous disorderPharmaceutical drugPharmaceutical medicine
The present invention comprises compositions for pharmaceutical drug delivery of an indolone (e.g., ropinirole), or a pharmaceutically acceptable salt thereof. The composition may, for example, be a gel suitable for transdermal application. The compositions of the present invention typically comprise a hydroalcoholic vehicle, one or more antioxidant, and one or more buffering agent, wherein the pH of the gel is usually between about pH 7 and about pH 9. The compositions may include further components, for example, the hydroalcoholic vehicle may further comprise additional solvent(s), antioxidant(s), cosolvent(s), penetration enhancer(s), buffering agent(s), and / or gelling agent(s). The compositions may be used for the treatment of a variety of neurological disorders.
Owner:JAZZ PHARMA
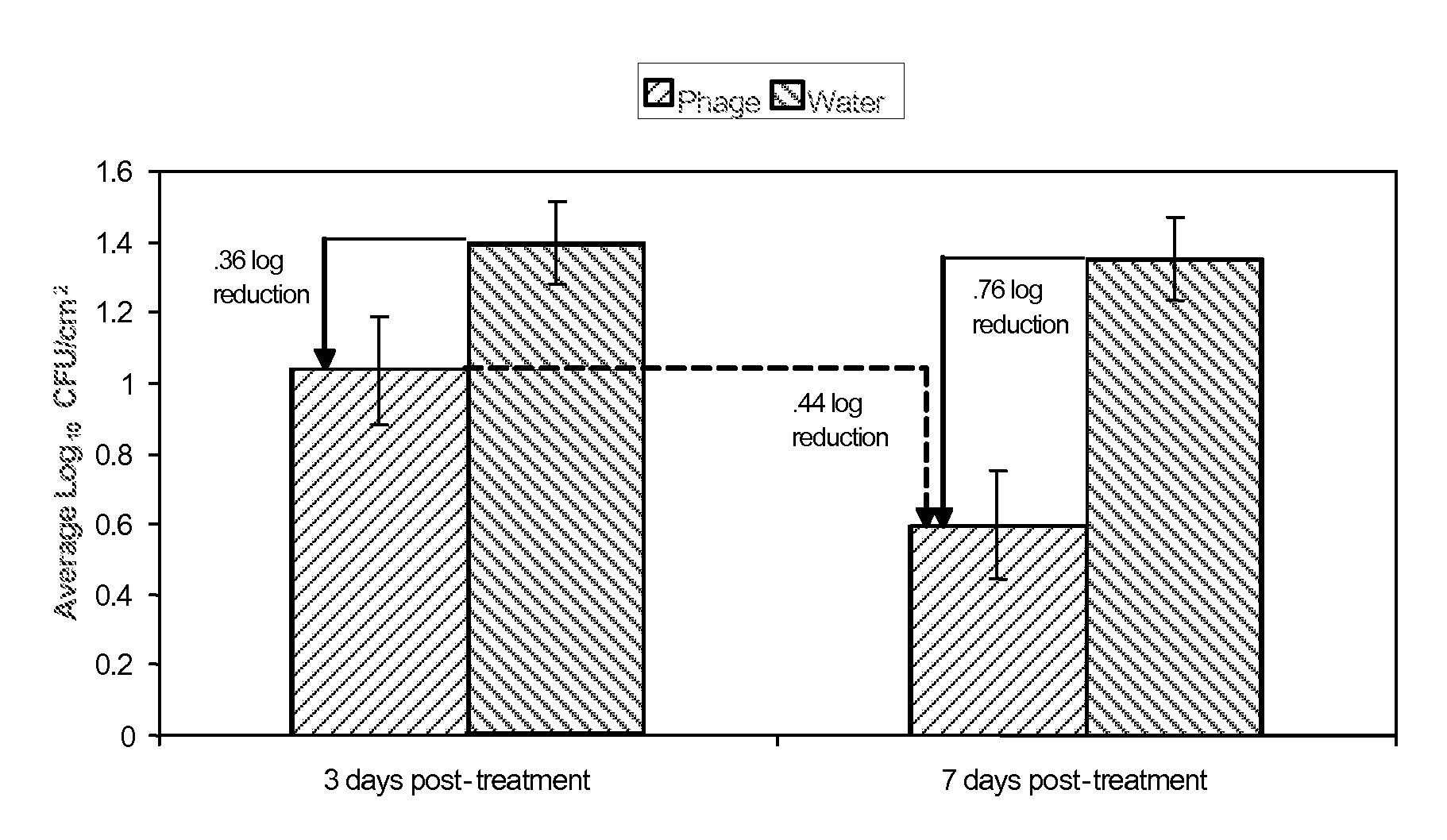
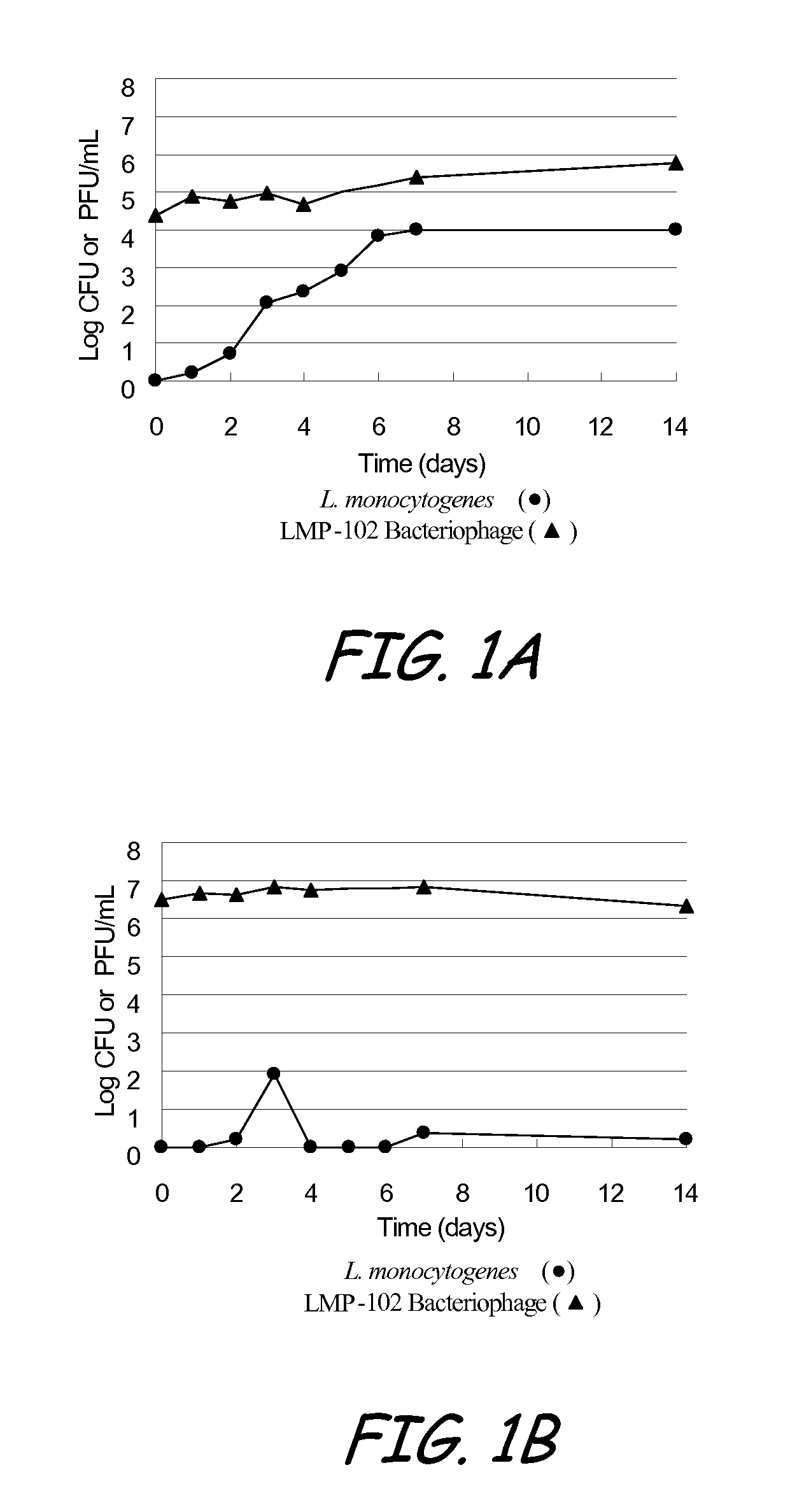
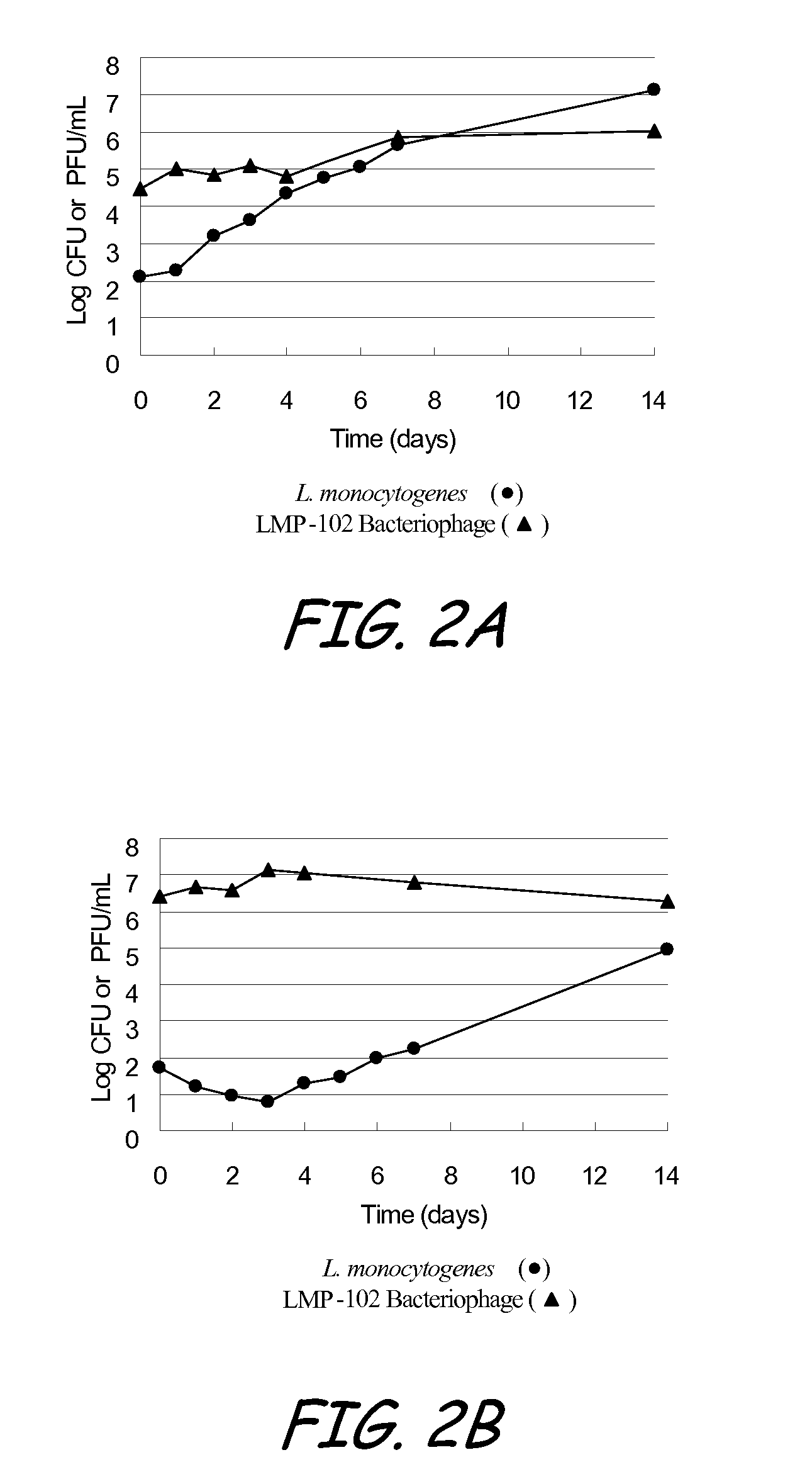
Bacteriophage treatment for reducing and preventing bacterial contamination
ActiveUS20090246336A1Process stabilityImprove performanceBiocideMilk preservationBacteroidesAdjuvant
A system and method for reducing or preventing bacterial contamination in food includes application of a bacteriophage treatment to any type of food product at any stage of processing the food product. The bacteriophage treatment may also be applied to non-food surfaces and water systems, which may be susceptible to bacterial contamination and subsequent spread of bacteria. The bacteriophage treatment comprises at least one bacteriophage in a concentration sufficient to reduce or prevent bacterial contamination from pathogenic bacteria and / or spoilage bacteria. In some embodiments, the bacteriophage is able to reduce or eliminate bacteria introduced to a food product after the bacteriophage treatment was applied to the food product. In some embodiments, the bacteriophage treatment includes a buffering agent to maintain the bacteriophage at a pH level that sustains the bacteriophage. In some embodiments, the bacteriophage treatment includes a surfactant and / or a thickener to aid in applying the bacteriophage. Additional adjuvants and enhancers may be used in some embodiments to stabilize the bacteriophage or enhance its performance as an antibacterial agent.
Owner:ECOLAB USA INC
High capacity electrode and methods for its fabrication and use
InactiveUS20070099084A1Improve cycle lifeMinimised mechanical strainElectrode manufacturing processesSecondary cellsAlloyBuffering agent
A battery electrode comprises an electrically conductive substrate having an electrochemically active electrode composition supported thereupon. The composition includes an active material capable of reversibly alloying with lithium, which material shows a volume change upon such reversible alloying. The composition includes a buffering agent which accommodates the volume change in the active material and minimizes mechanical strain in the composition. The active composition may further include materials such as carbon. The active material may comprise silicon, aluminum, antimony, antimony oxides, bismuth, bismuth oxides, tin, tin oxides, chromium, chromium oxides, tungsten, and tungsten oxides or lithium alloys of the foregoing. The buffering agent may comprise a metal or a metal oxide or lithium alloys of the foregoing. Also disclosed are batteries which incorporate these electrodes, methods for the fabrication of the electrodes and methods for the fabrication and operation of the batteries.
Owner:A123 SYSTEMS LLC
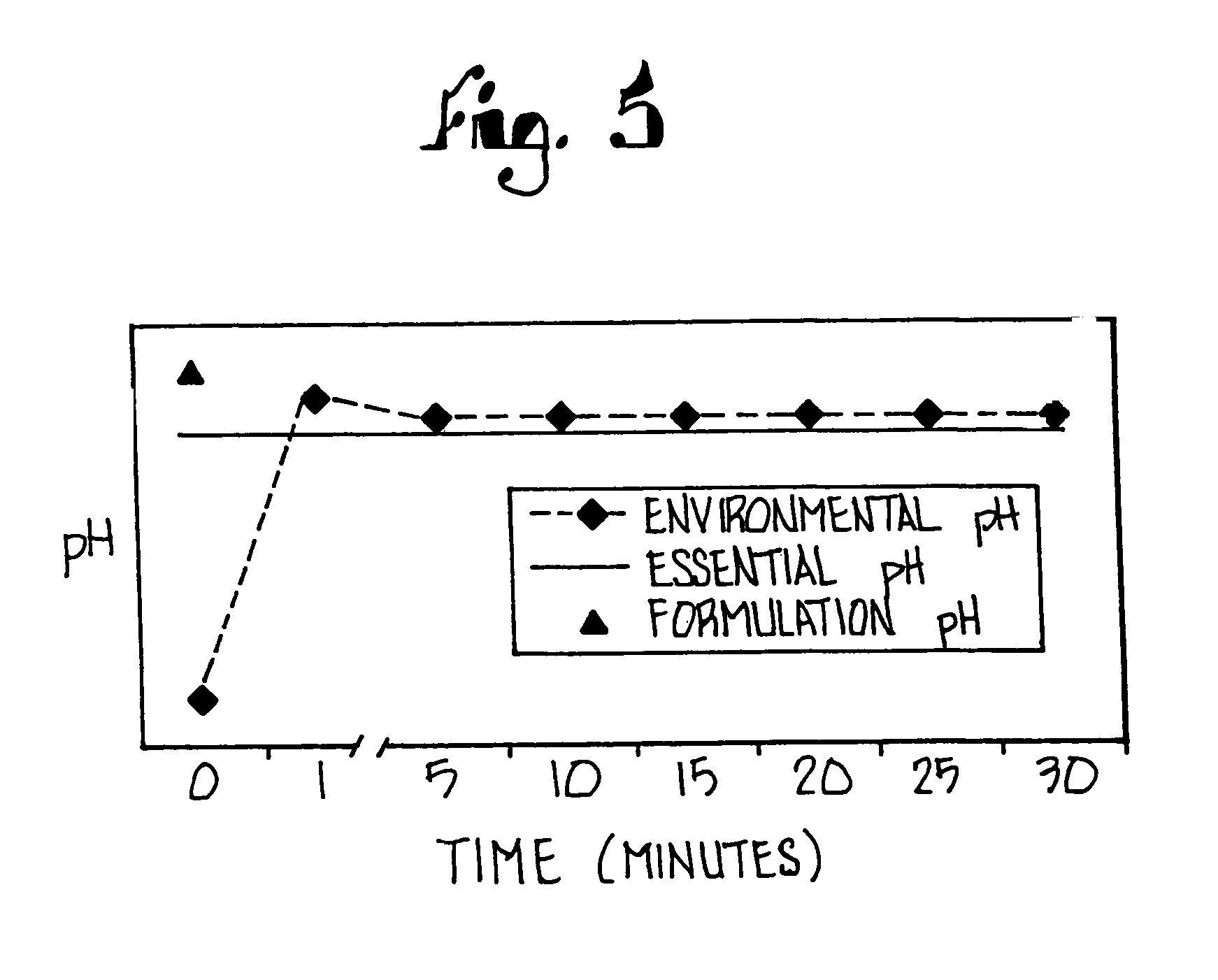
Substituted benzimidazole dosage forms and method of using same
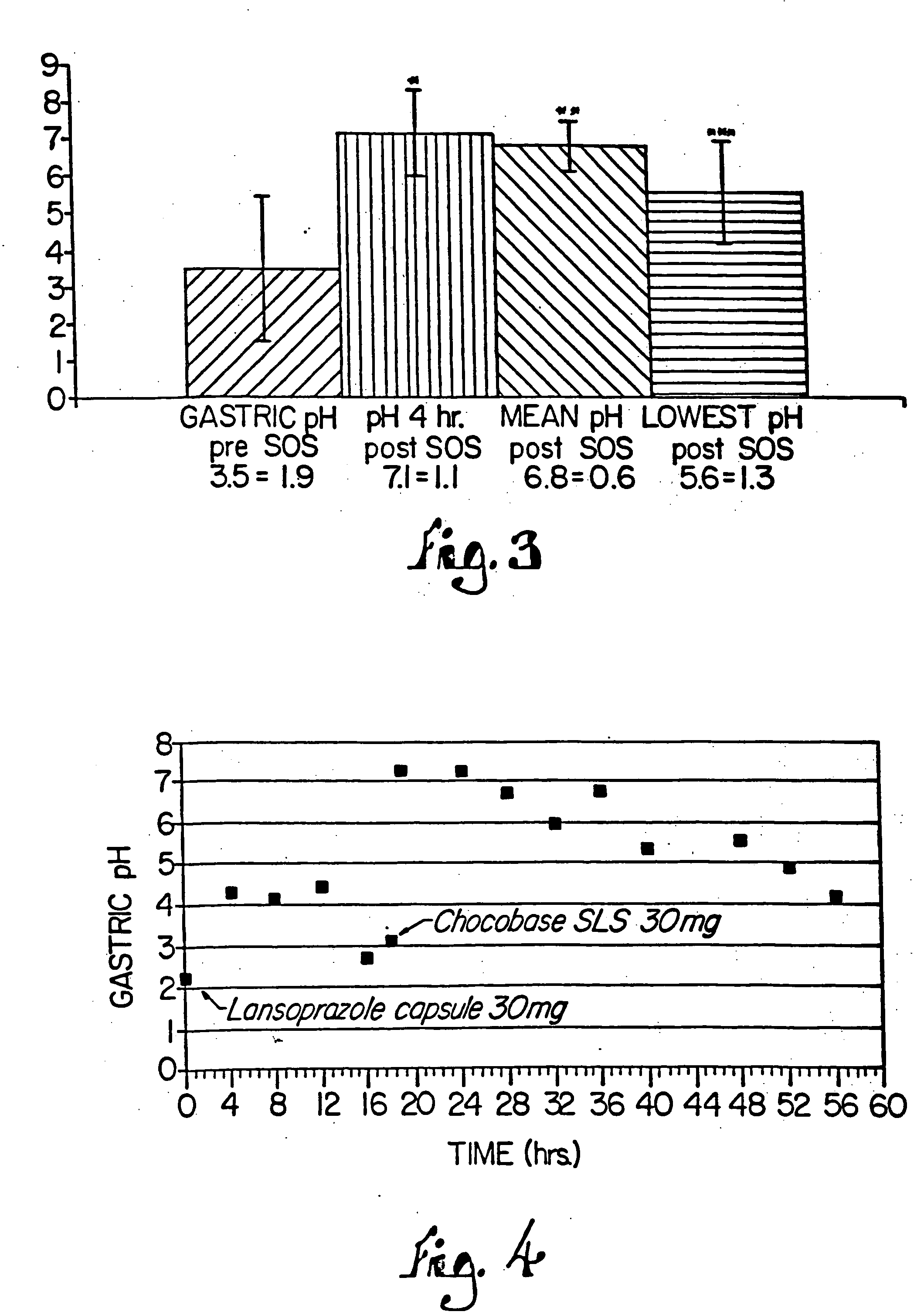
InactiveUS7399772B2Easy to prepareImprove pharmacological activityBiocidePowder deliveryEffervescent PowderPharmaceutical formulation
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor and at least one buffering agent. Also provided is a pharmaceutical composition further comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and, a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules.
Owner:UNIVERSITY OF MISSOURI
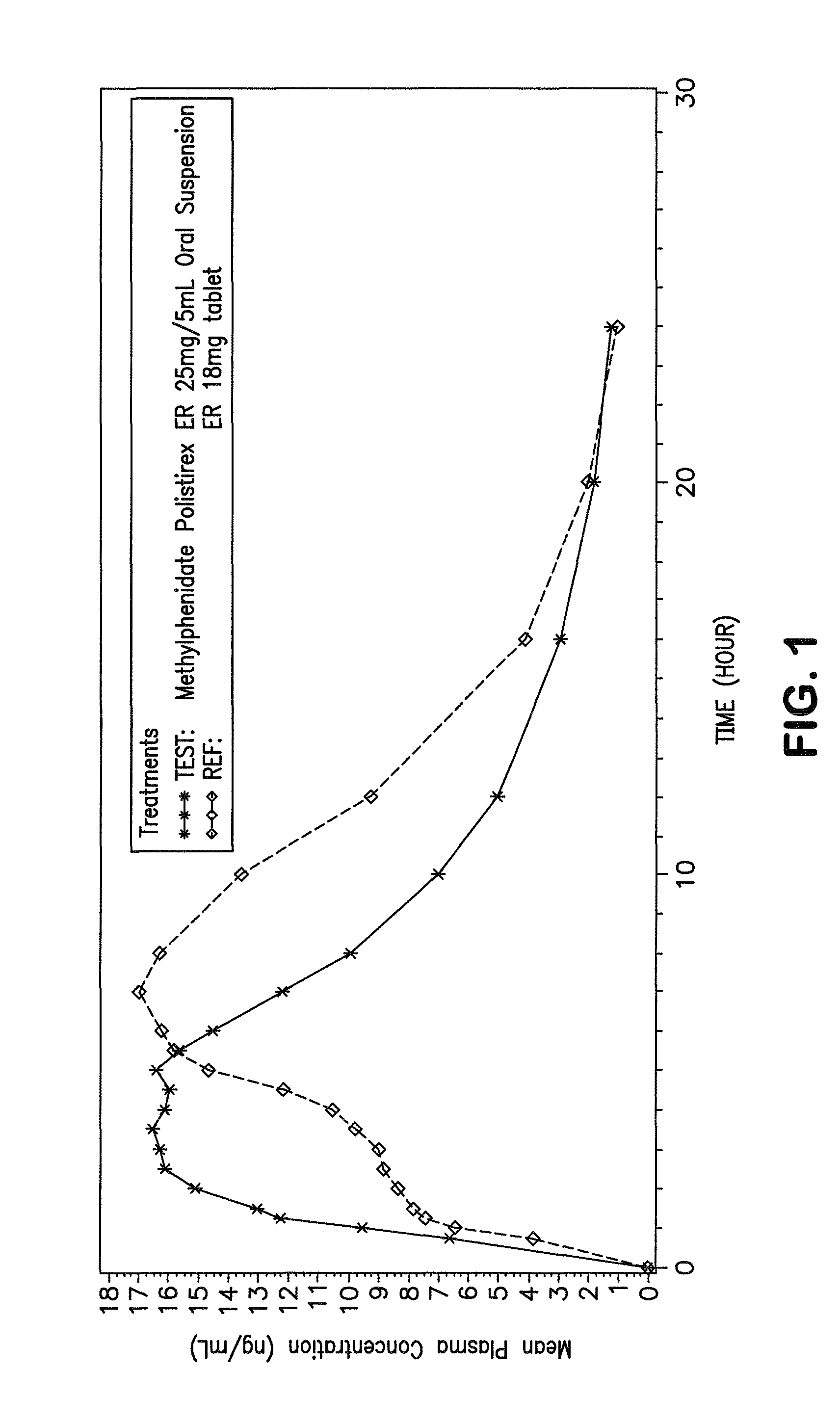
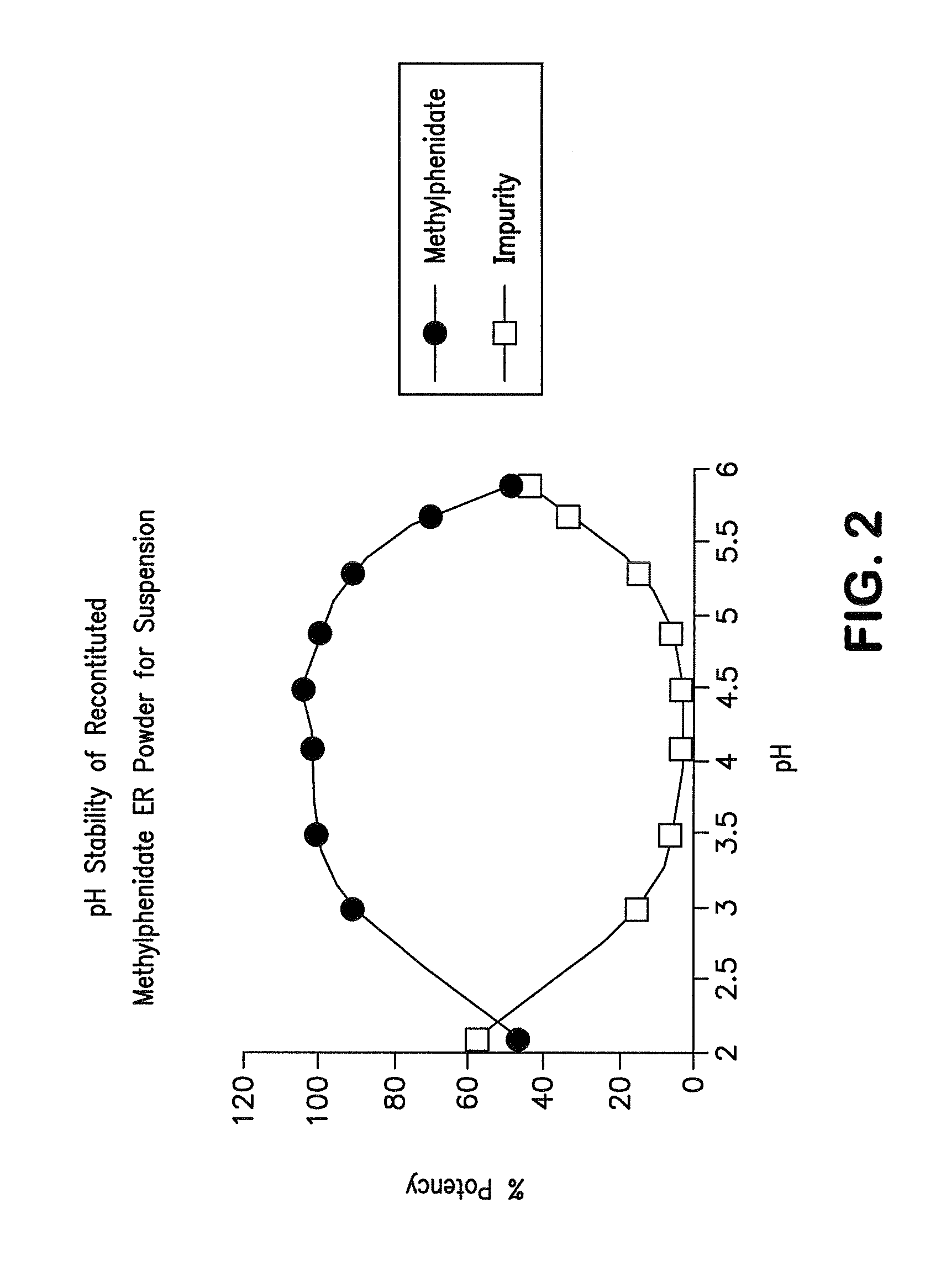
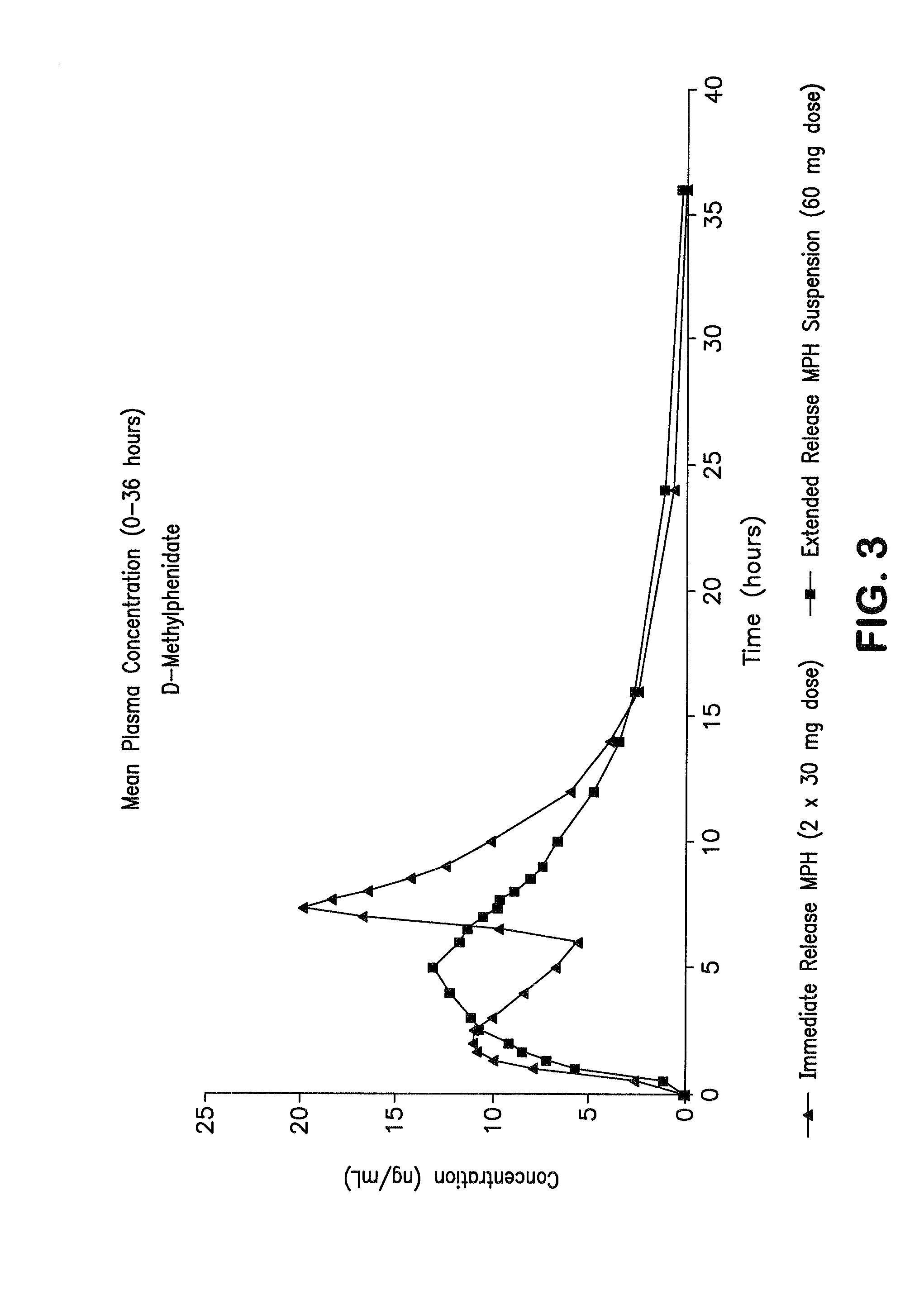
Orally effective methylphenidate extended release powder and aqueous suspension product
An oral methylphenidate powder which is reconstitutable into a final oral aqueous sustained release formulation containing at least about 50%, or at least about 80% by weight water based on the total weight of the suspension, is provided. The powder is a blend containing a combination of an uncoated methylphenidate-ion exchange resin complex, a barrier coated methylphenidate-ion exchange resin complex-matrix, and a water soluble buffering agent such that upon formed into an aqueous liquid formulation, the formulation has a pH in the range of about 3.5 to about 5, or about 4 to about 4.5. Following administration of a single dose of the oral aqueous methylphenidate suspension, a therapeutically effective amount of methylphenidate is reached in less than one hour and the composition provides a twelve-hour extended release profile.
Owner:TRIS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com