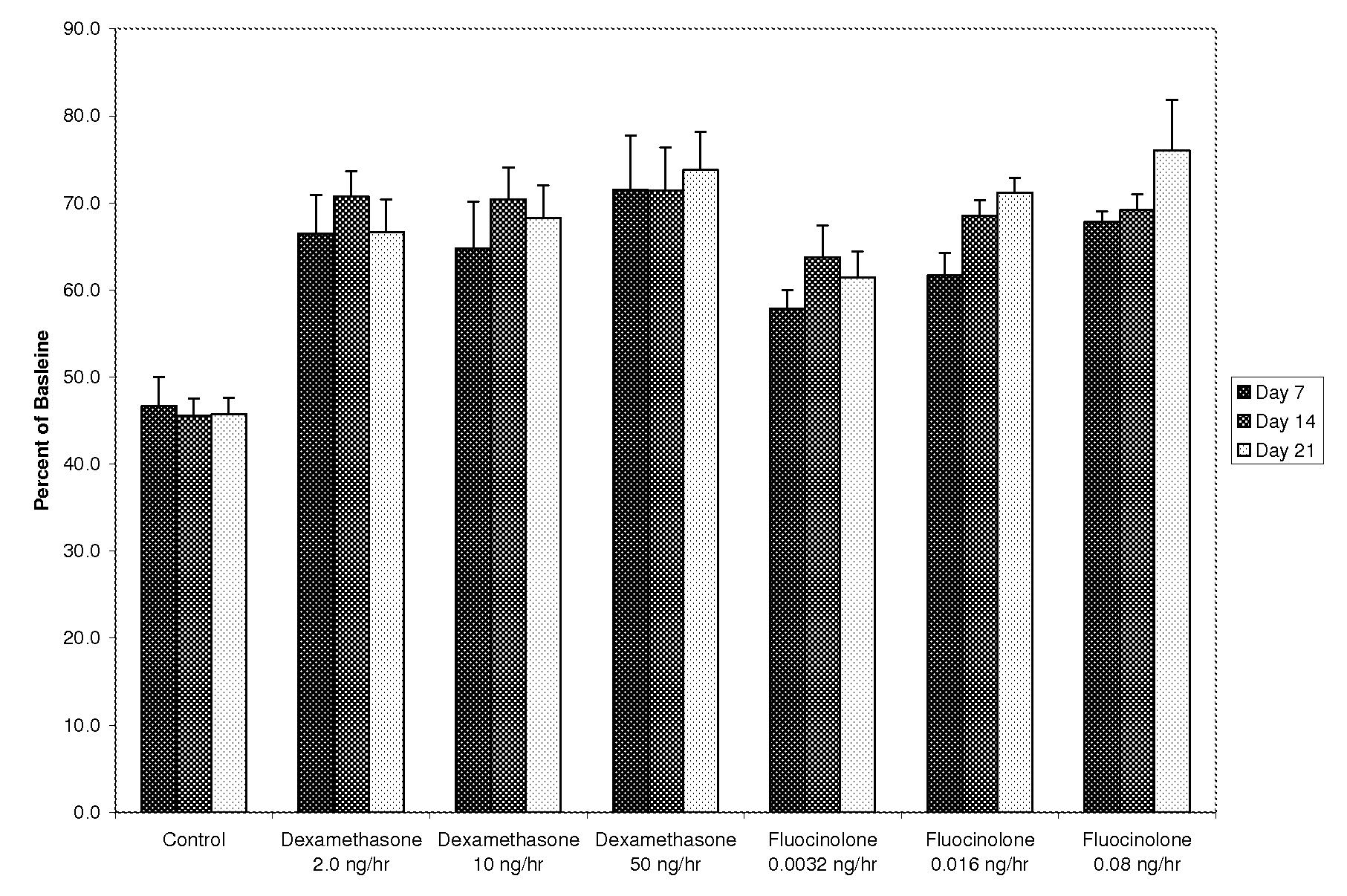
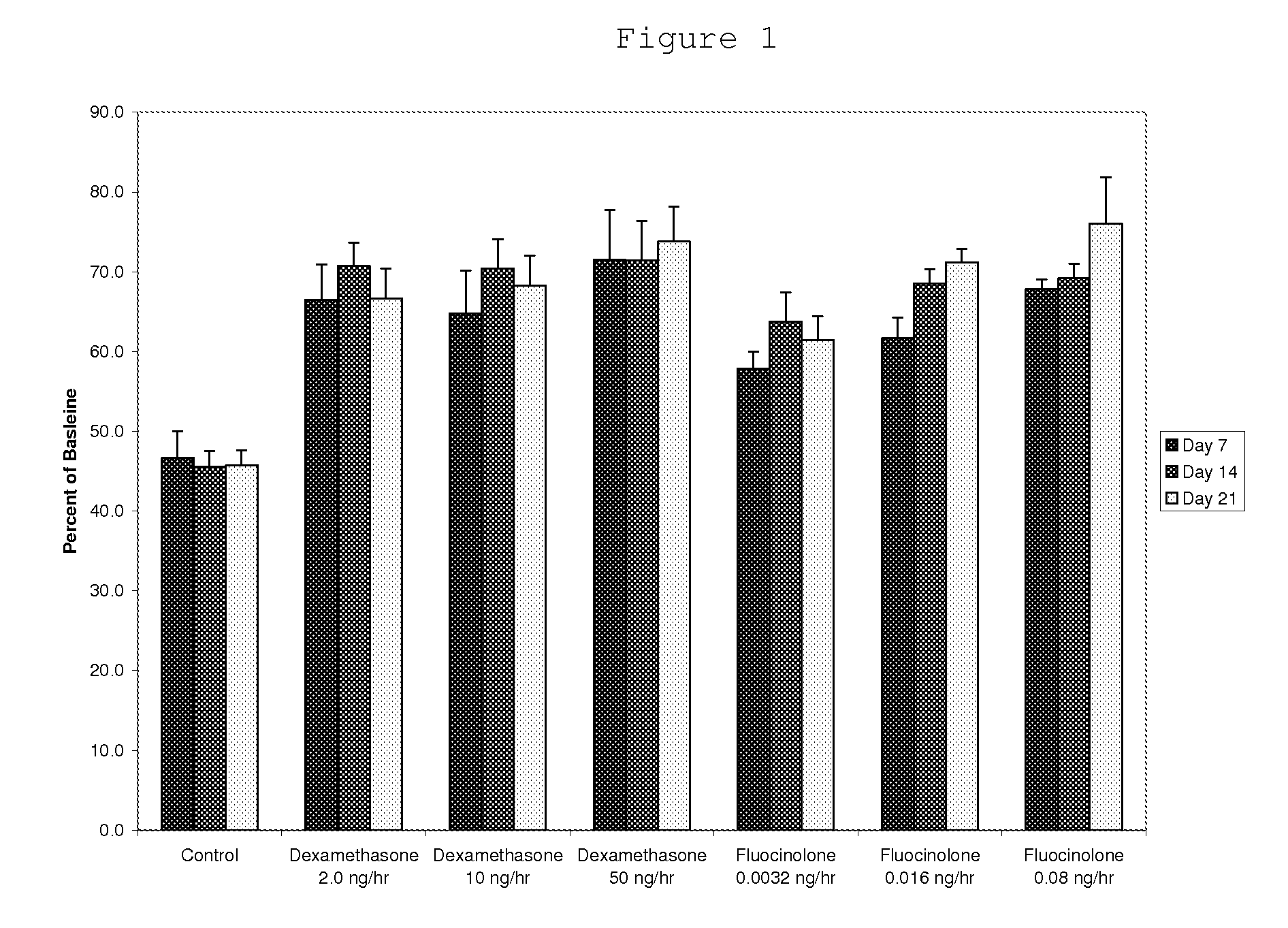
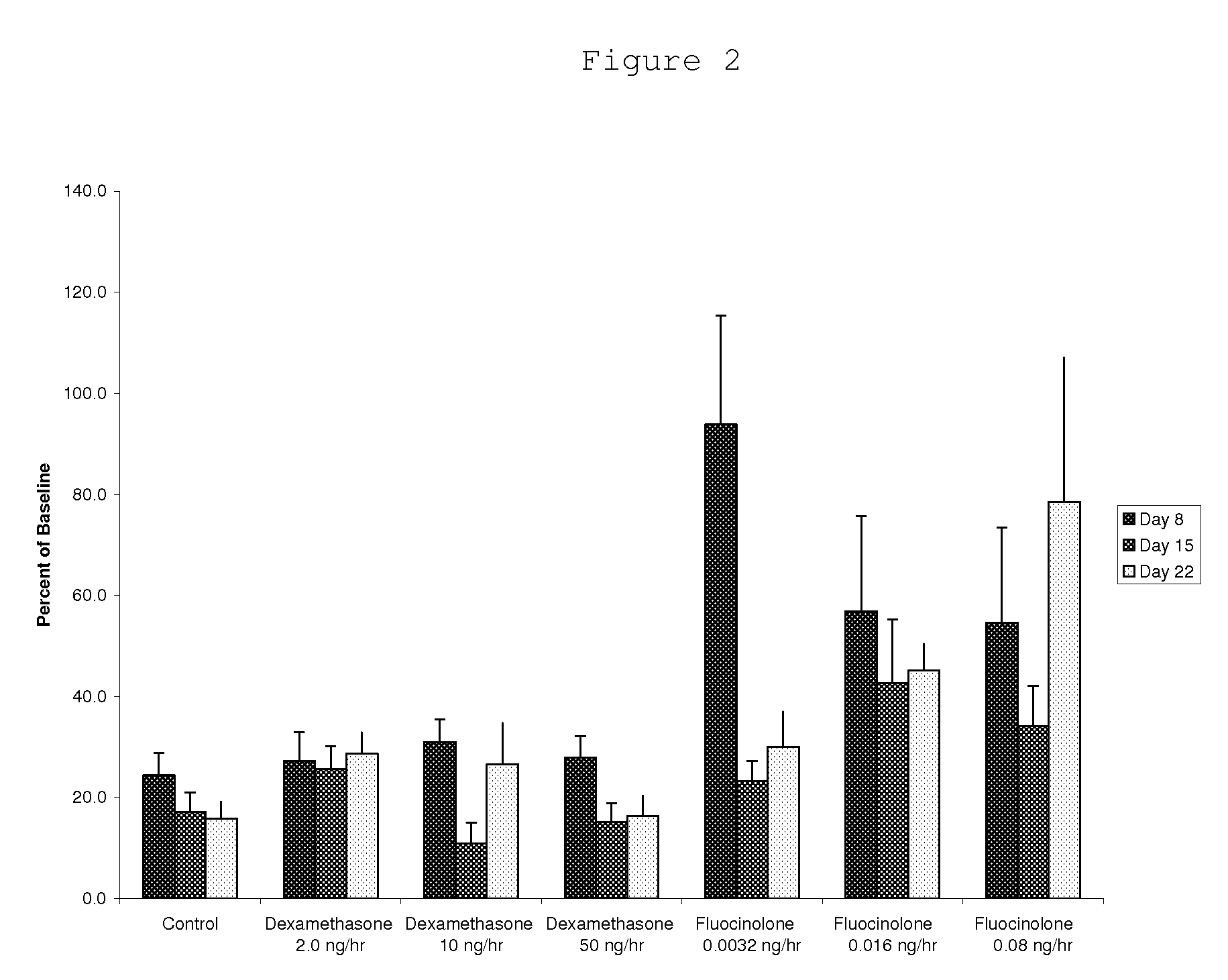
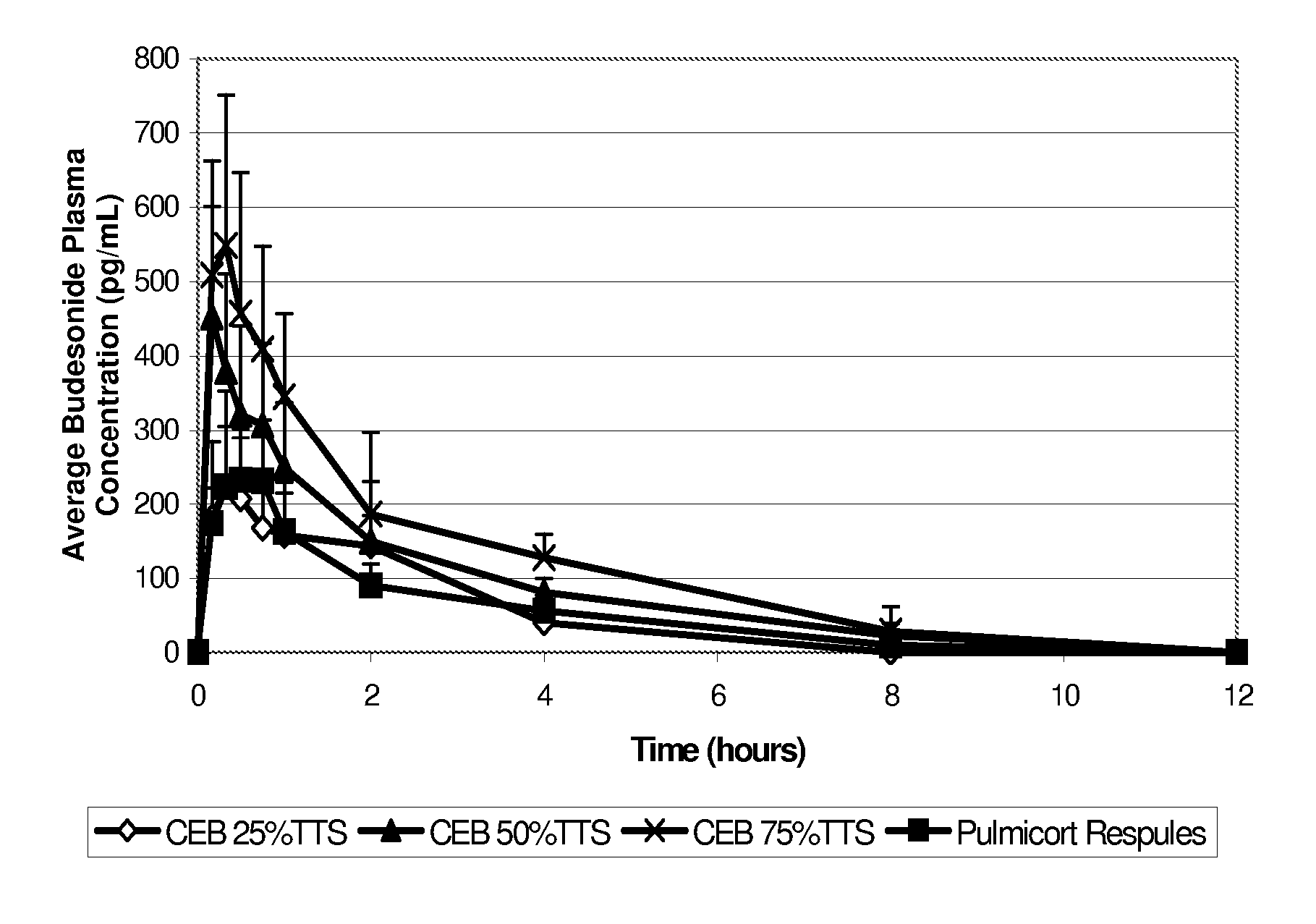
Patents
Literature
520 results about "Corticosteroid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
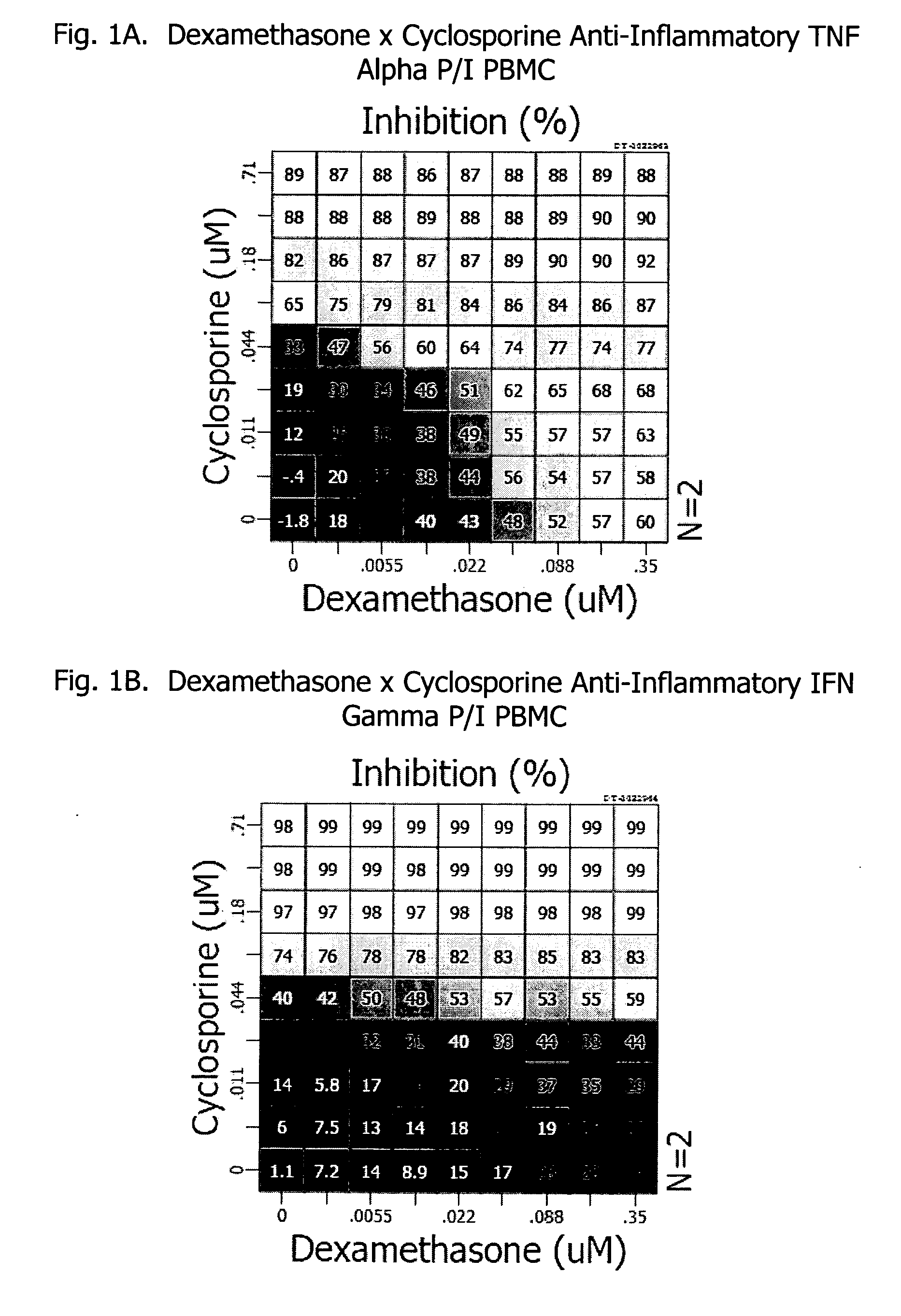
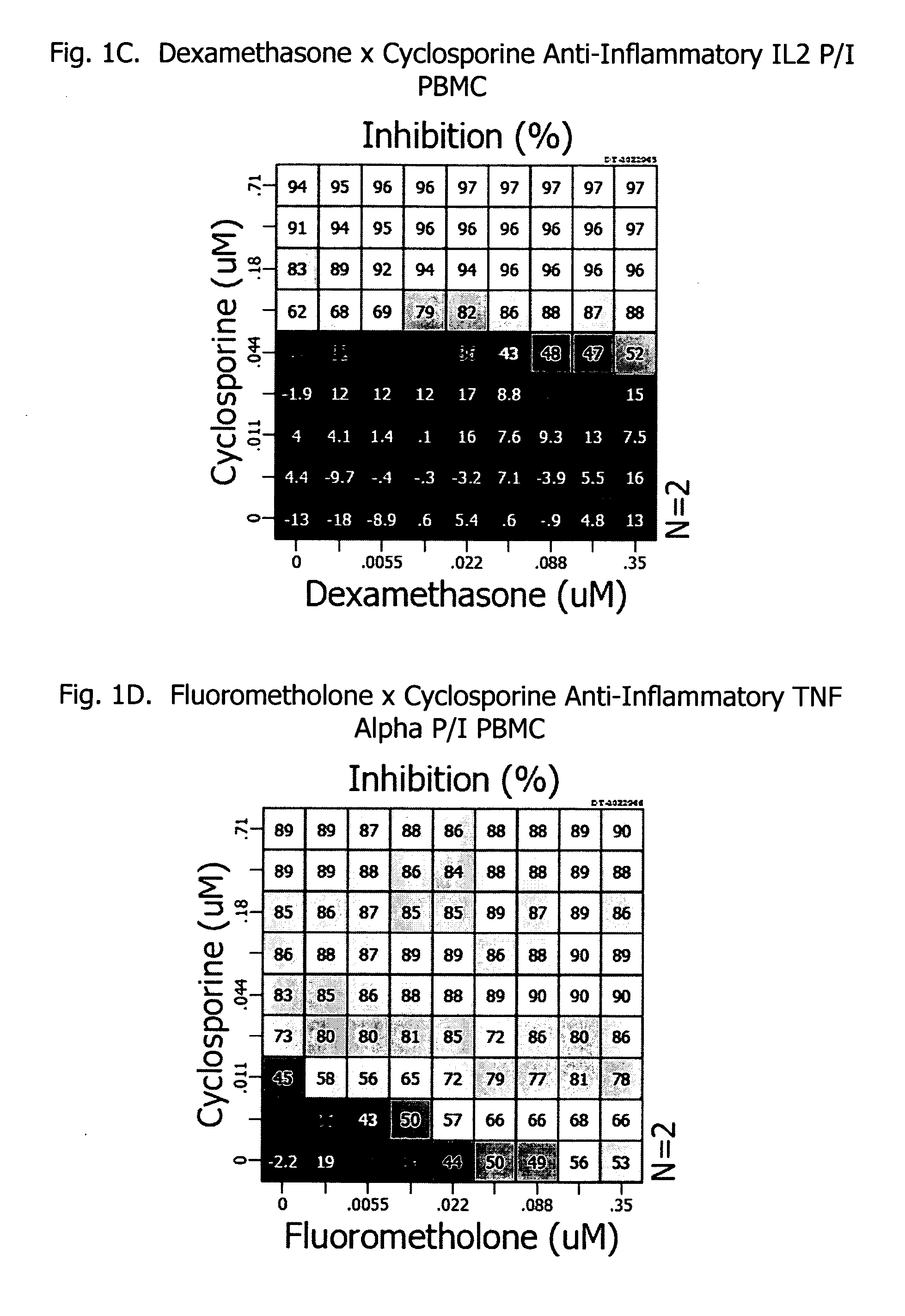
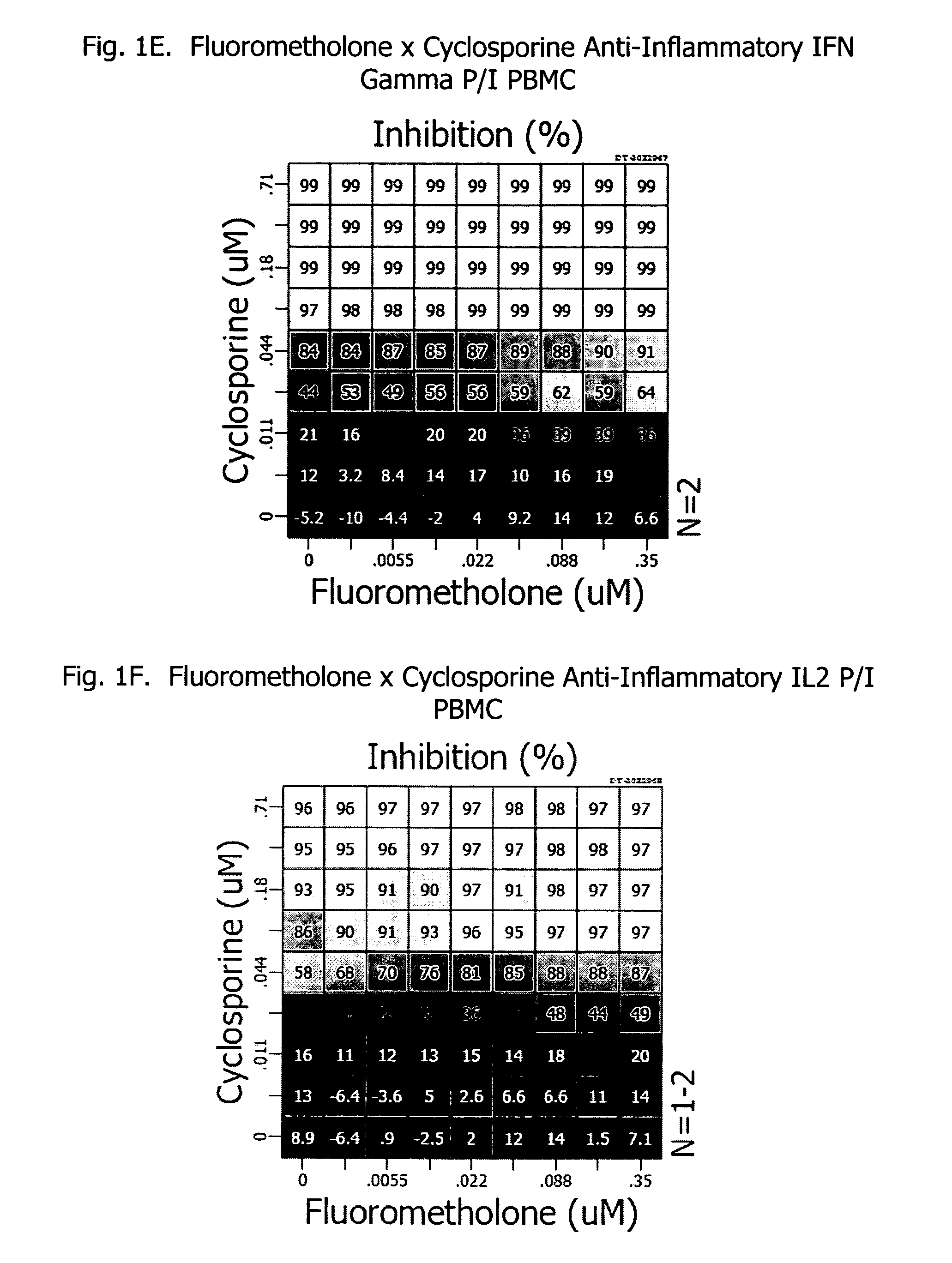
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.
Compositions and methods for treating a posterior segment of an eye
InactiveUS20050101582A1Improve abilitiesIncrease viscosity of compositionAntibacterial agentsOrganic active ingredientsShear ratePharmacology
Compositions, and methods of using such compositions, useful for injection into the posterior segments of human or animal eyes are provided. Such compositions include corticosteroid component-containing particles present in a therapeutically effective amount, a viscosity inducing component, and an aqueous carrier component. The compositions have viscosities of at least about 10 cps or about 100 cps at a shear rate of 0.1 / second. In a preferred embodiment, the viscosity is in the range of from about 140,000 cps to about 300,000 cps. The compositions advantageously suspend the particles for prolonged periods of time.
Owner:ALLERGAN INC
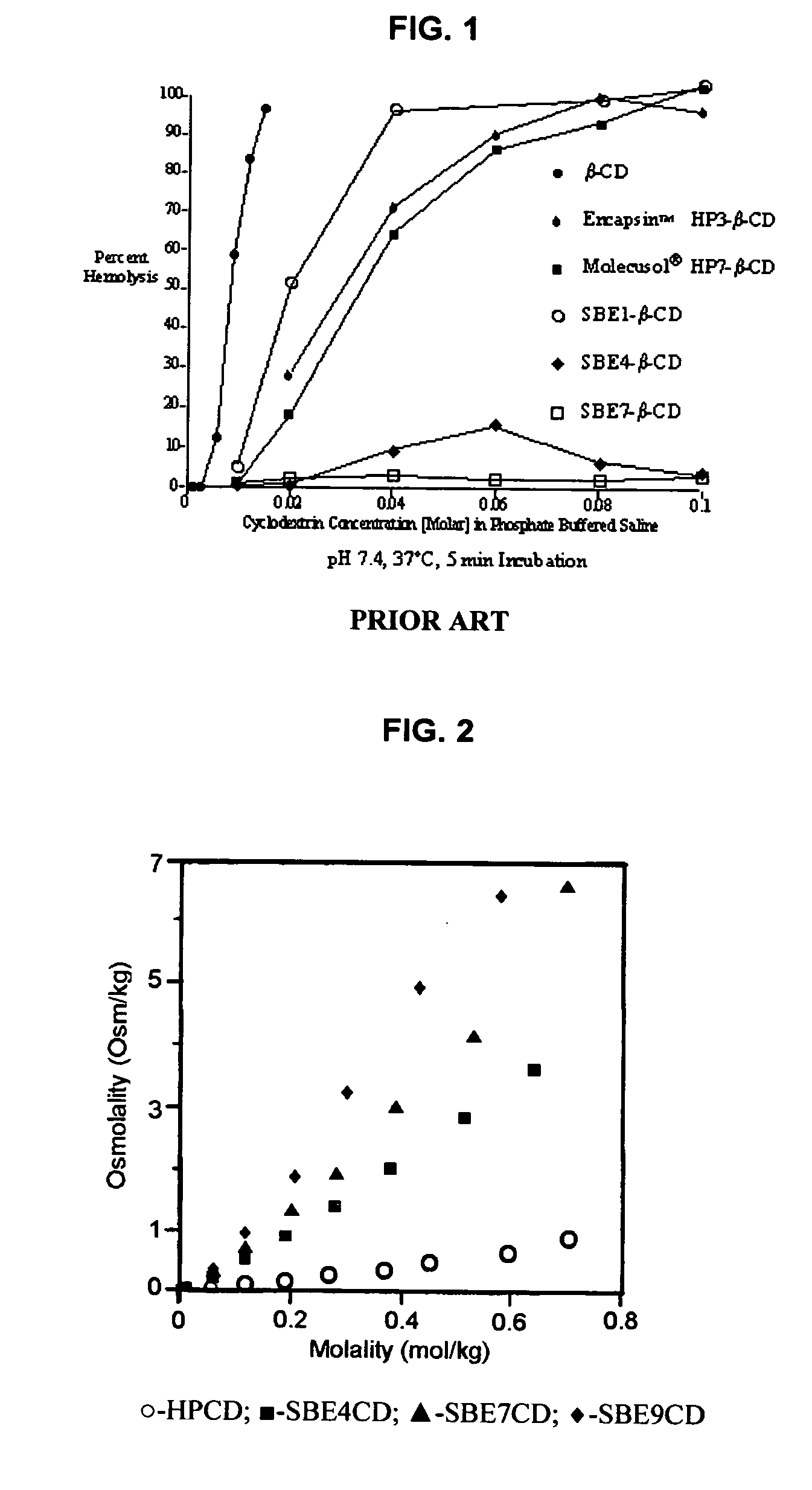
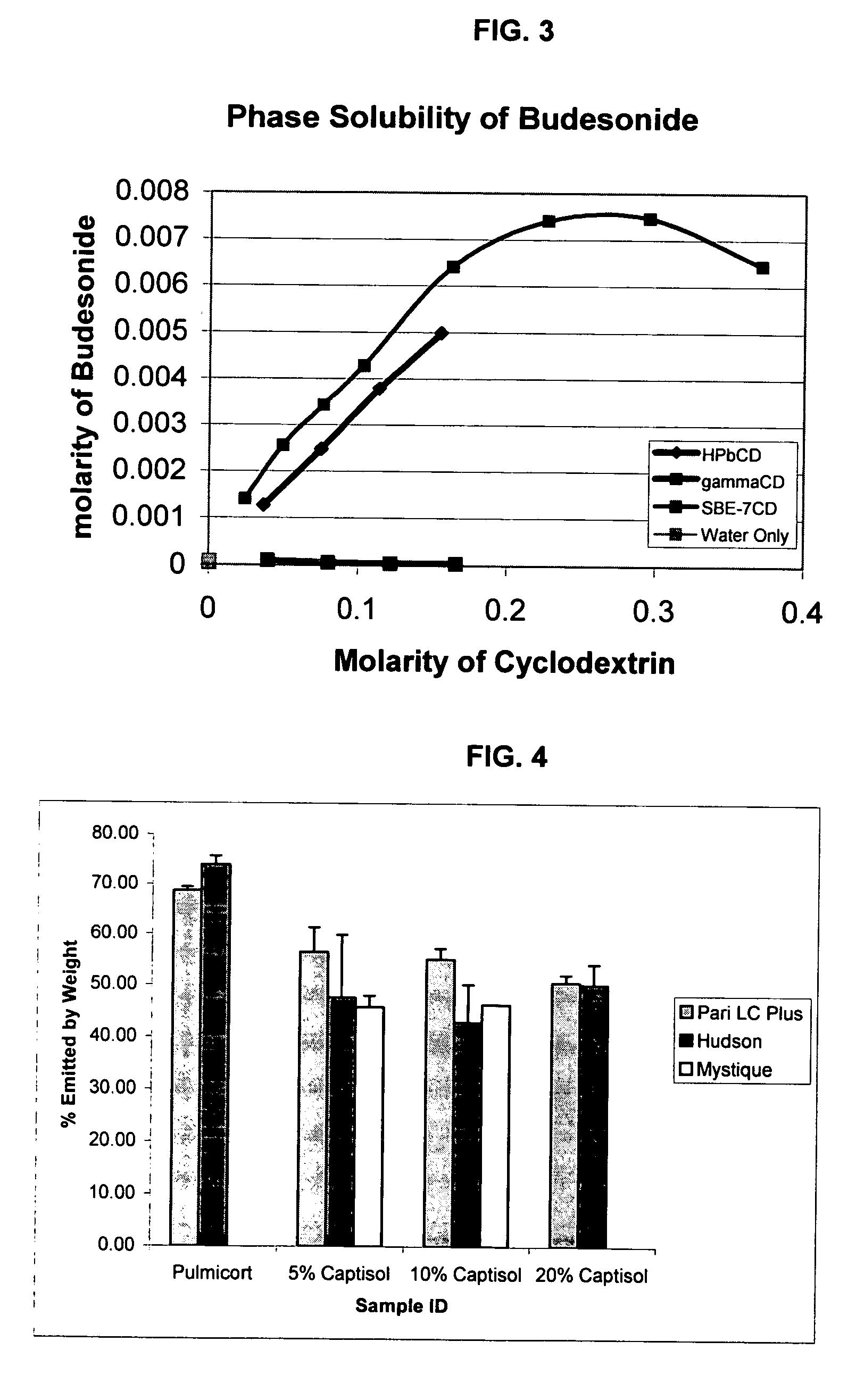
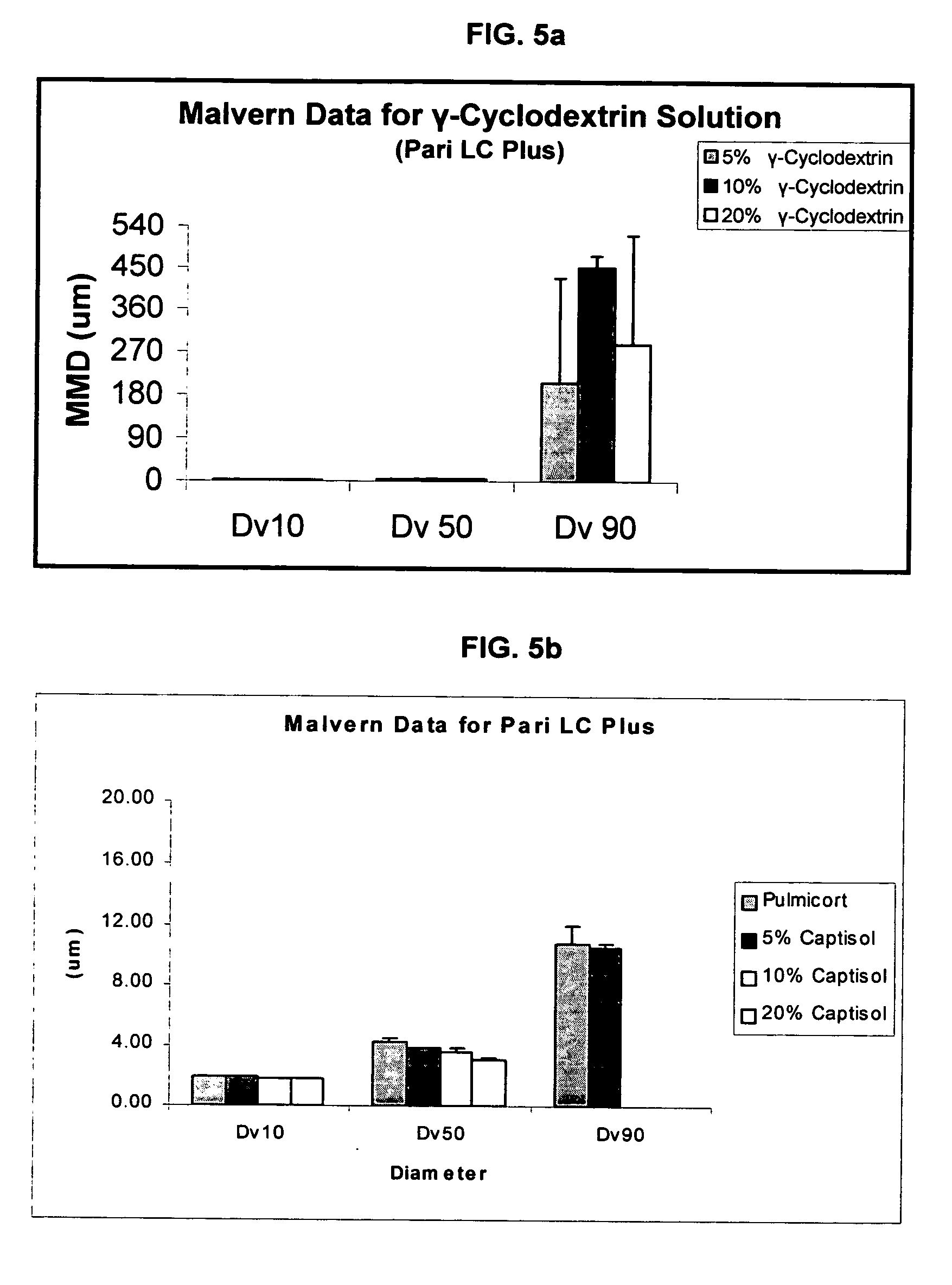
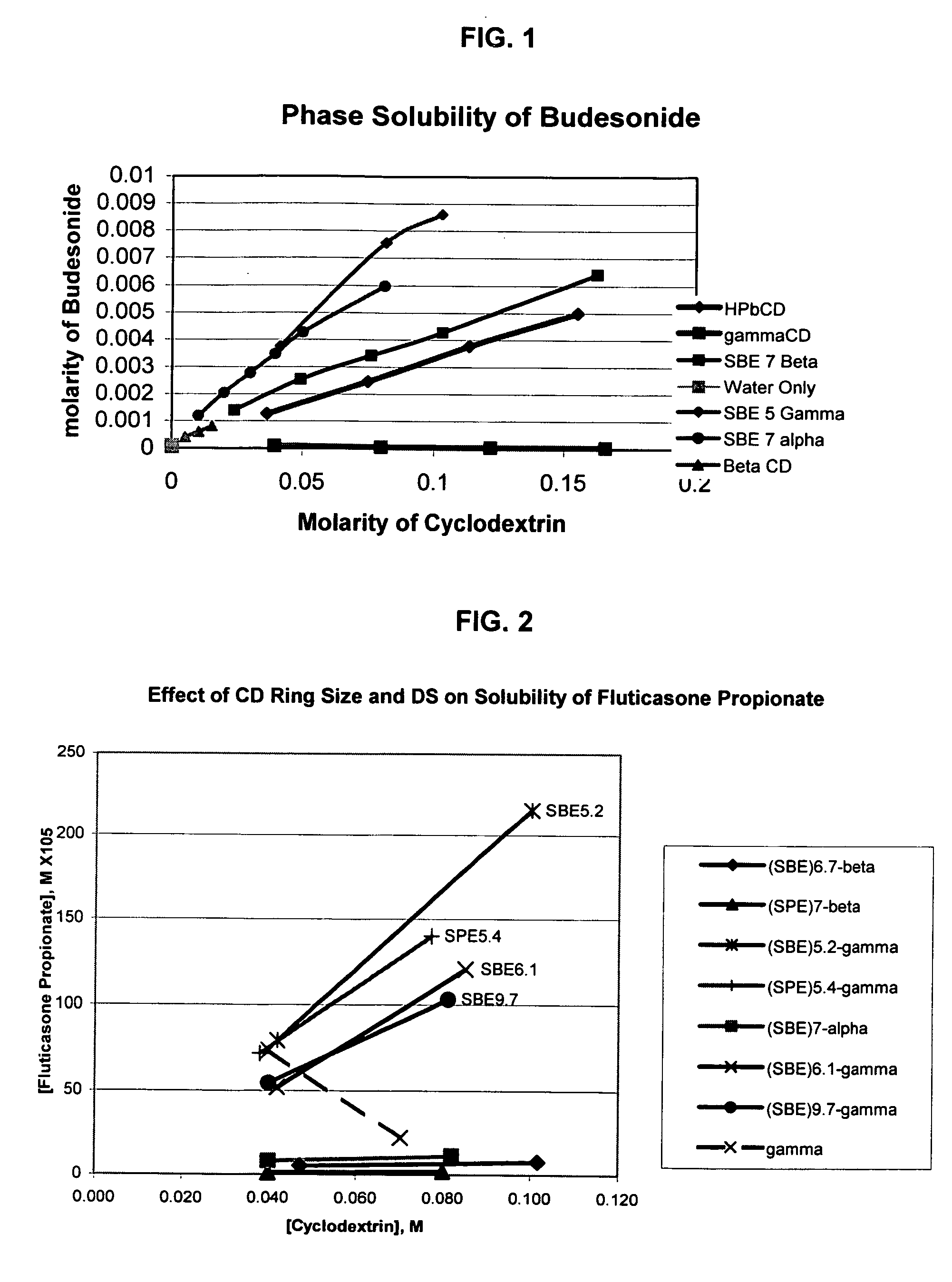
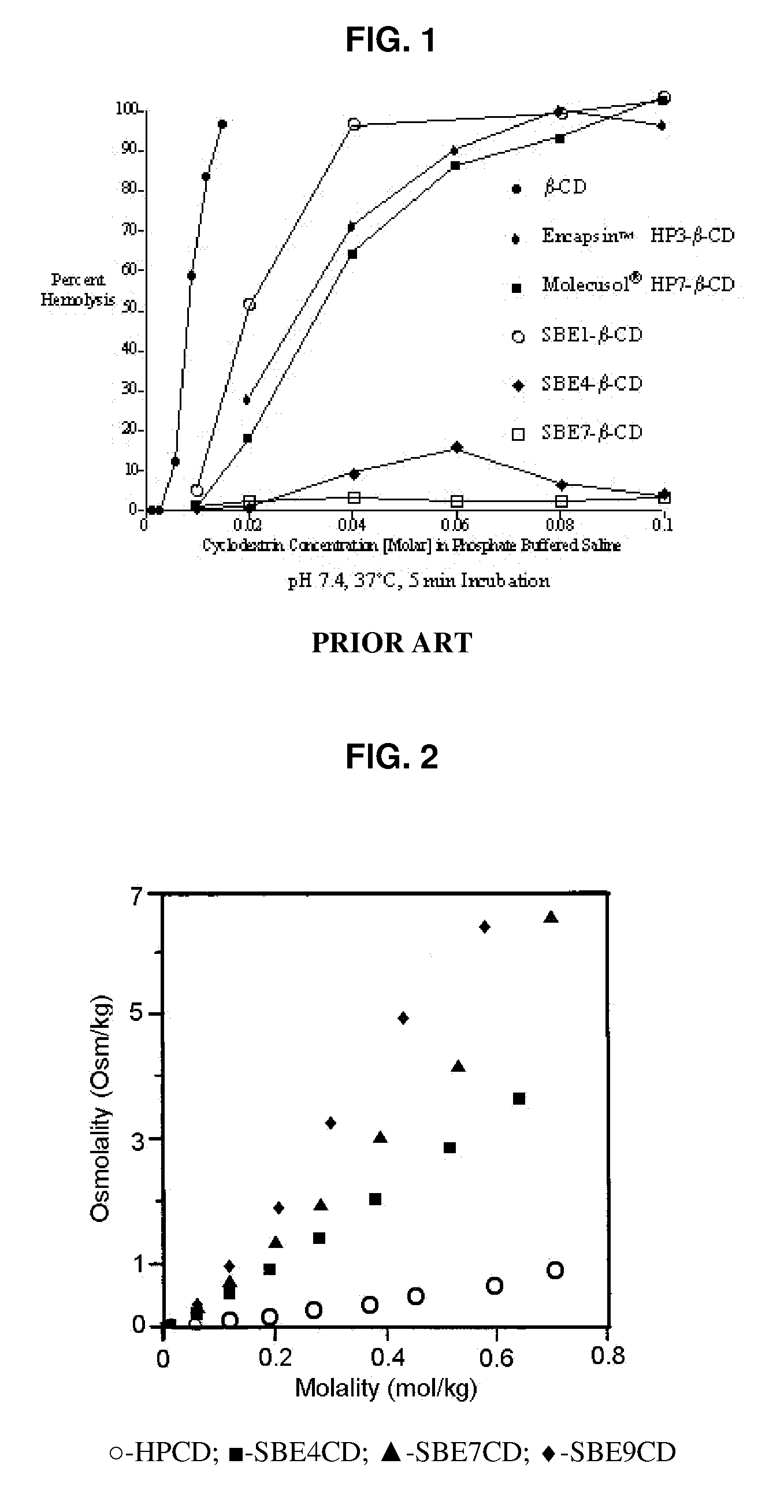
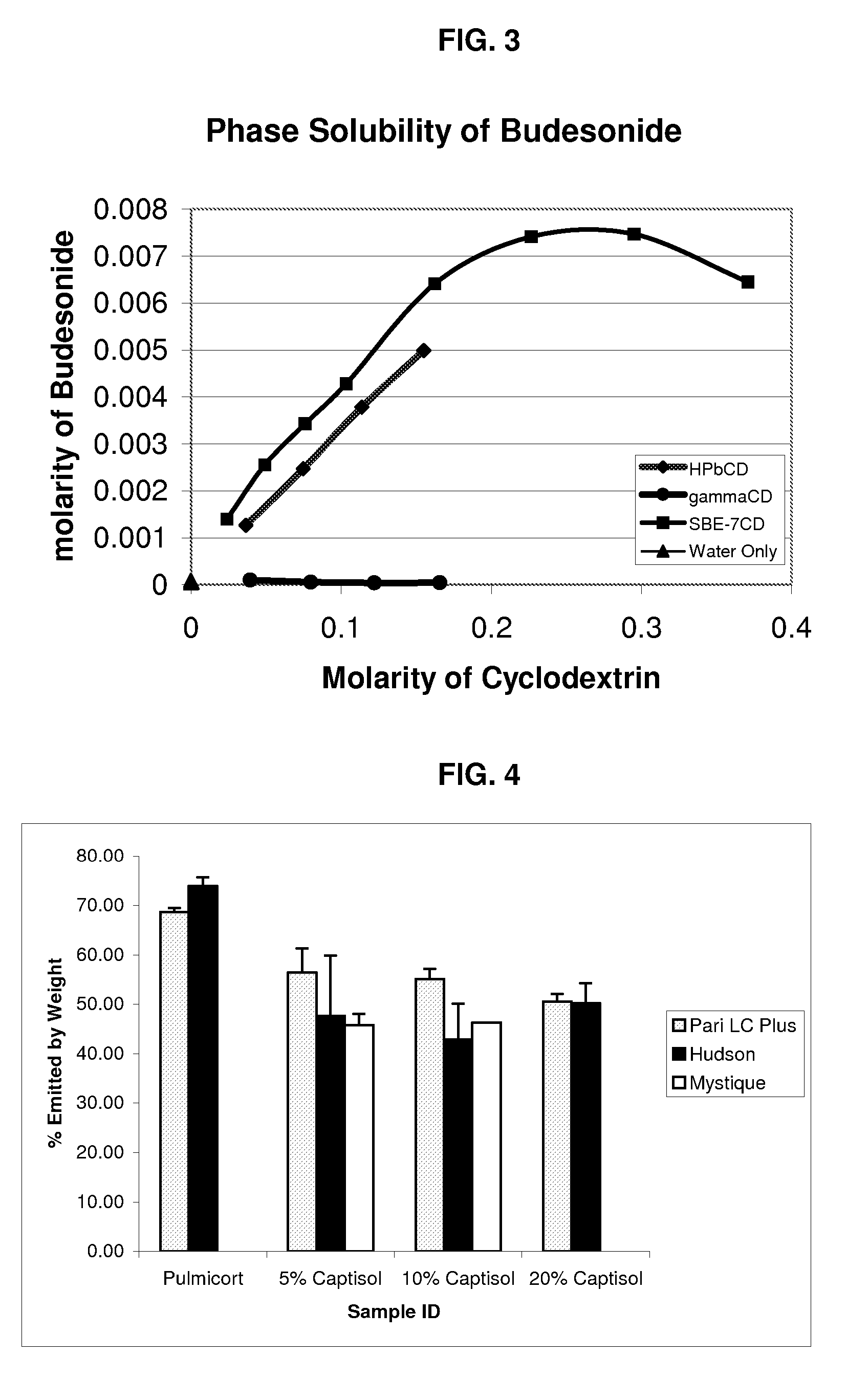
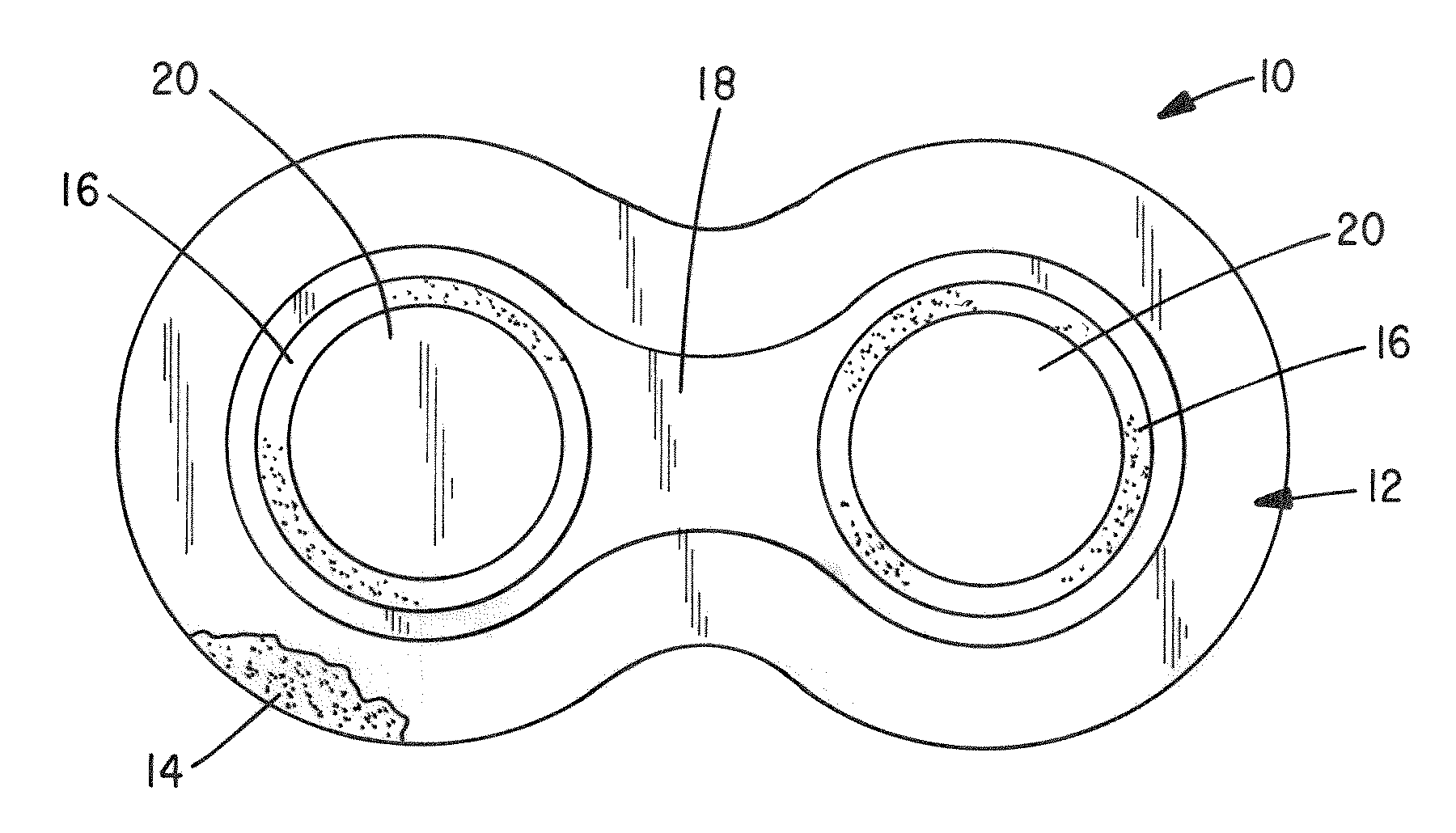
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
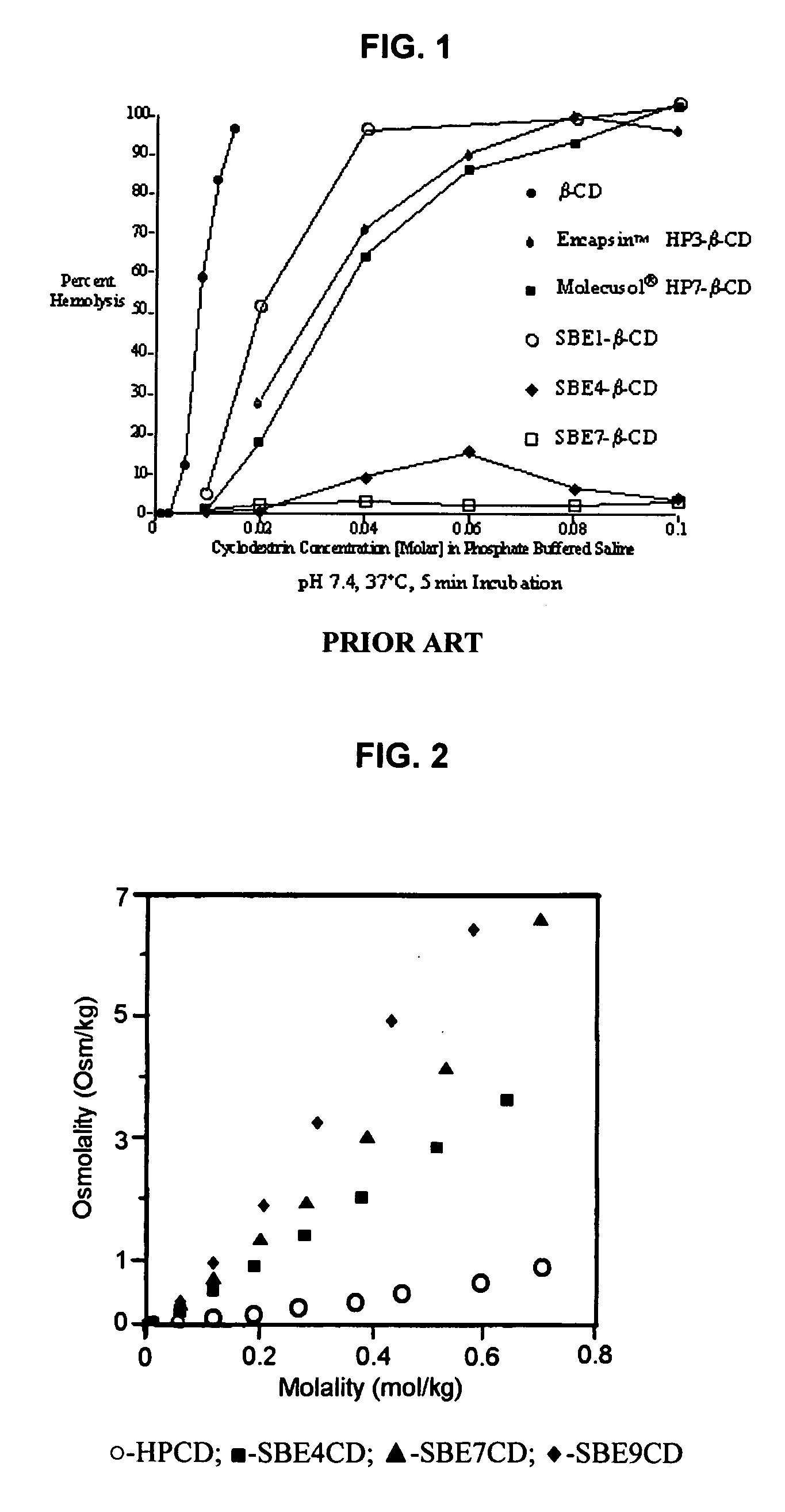
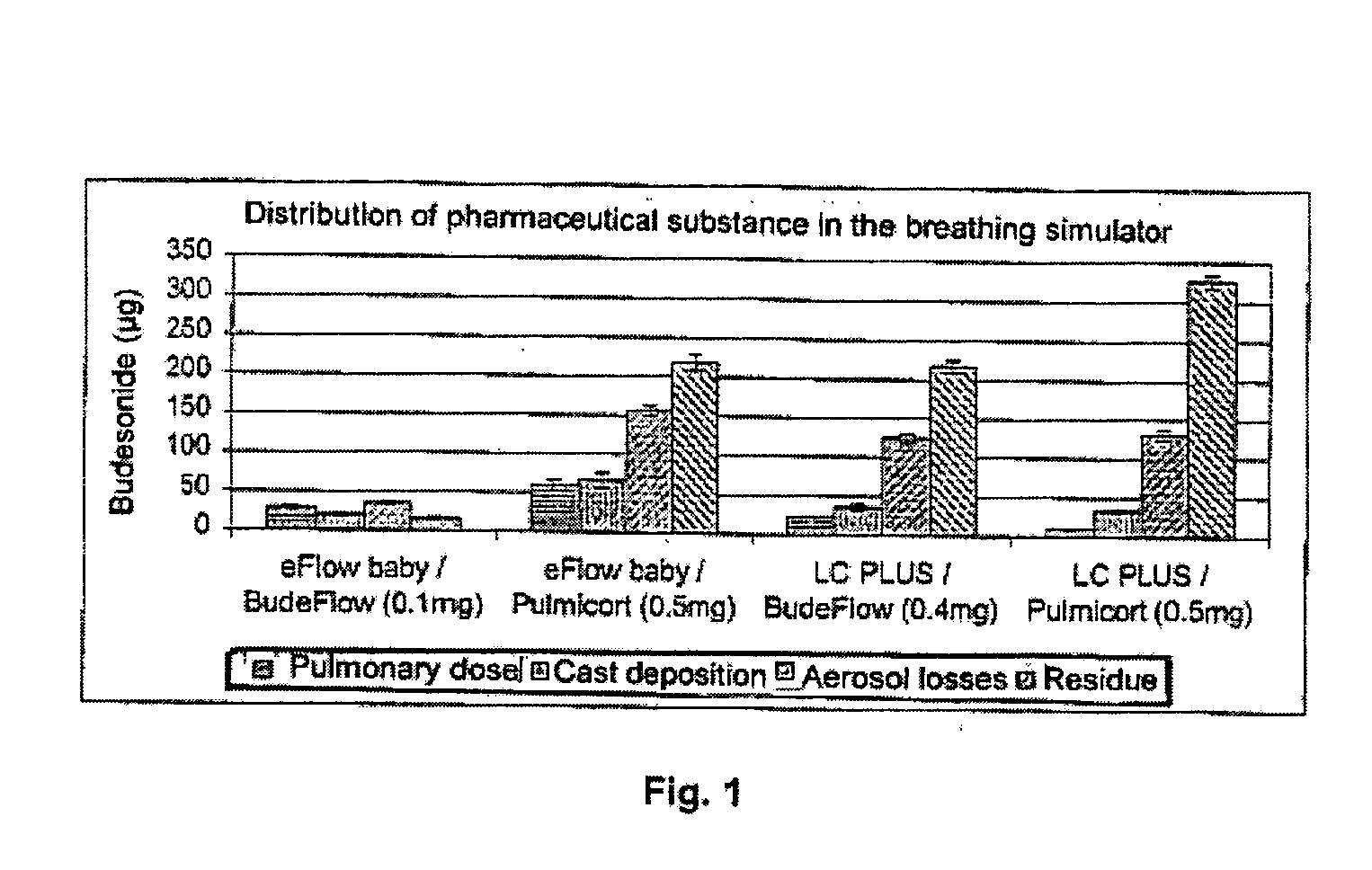
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Ophthalmic compositions and methods for treating ophthalmic conditions
InactiveUS20060009498A1Delayed extended releaseEnhance and improve visionBiocideSenses disorderFluid compositionSolvent
Compositions, and methods of using such compositions, useful for placement, for example injection, into the interior of human or animal eyes are provided. Such compositions include a therapeutic component, such as one or more corticosteroids, a biocompatible polymeric component, and a solvent component. The composition is in a fluid form before placement in the interior of an eye, and becomes less fluid after the composition is placed in the eye to form an extended or delayed release drug delivery element or system. The drug delivery element is formed by the dissipation of the solvent from the composition when the composition is placed in the interior of an eye. One example of a composition includes triamcinolone acetonide as a therapeutic agent. A method of treating an ophthalmic condition, or otherwise improving or enhancing vision of a patient, comprises placing the fluid composition in the interior of the eye. The method may be practiced by injecting the fluid composition into the interior of the eye.
Owner:ALLERGAN INC
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
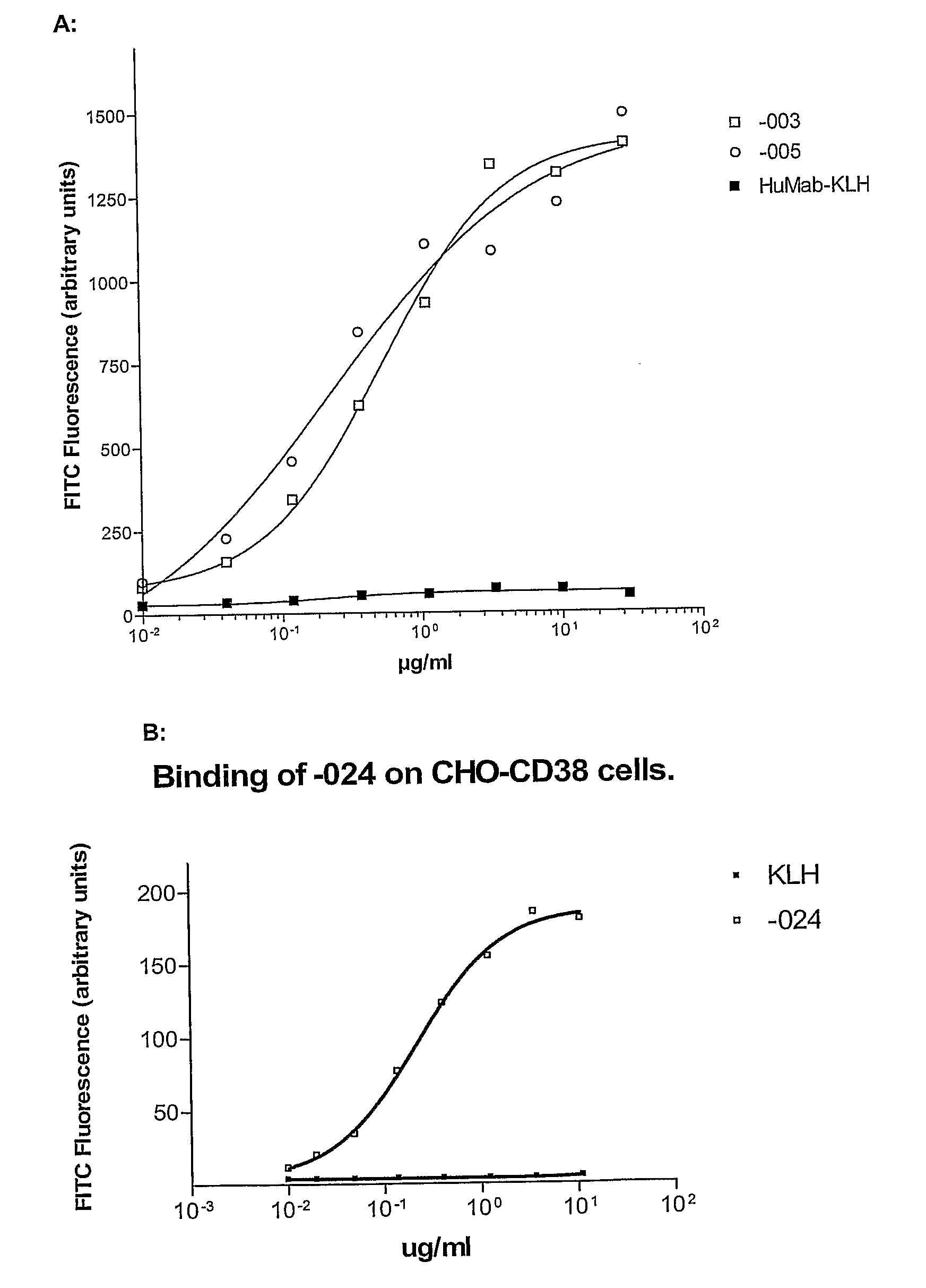
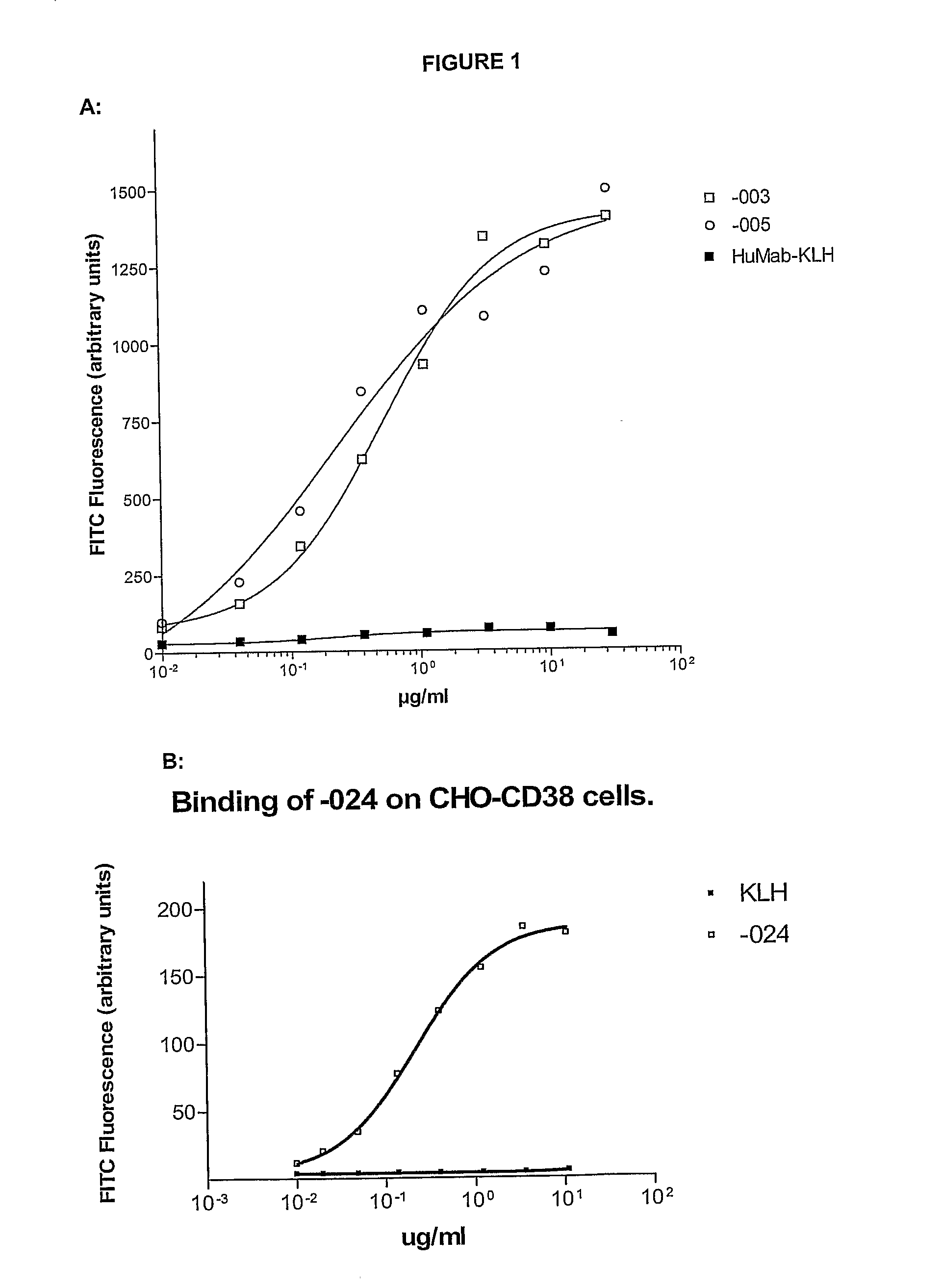
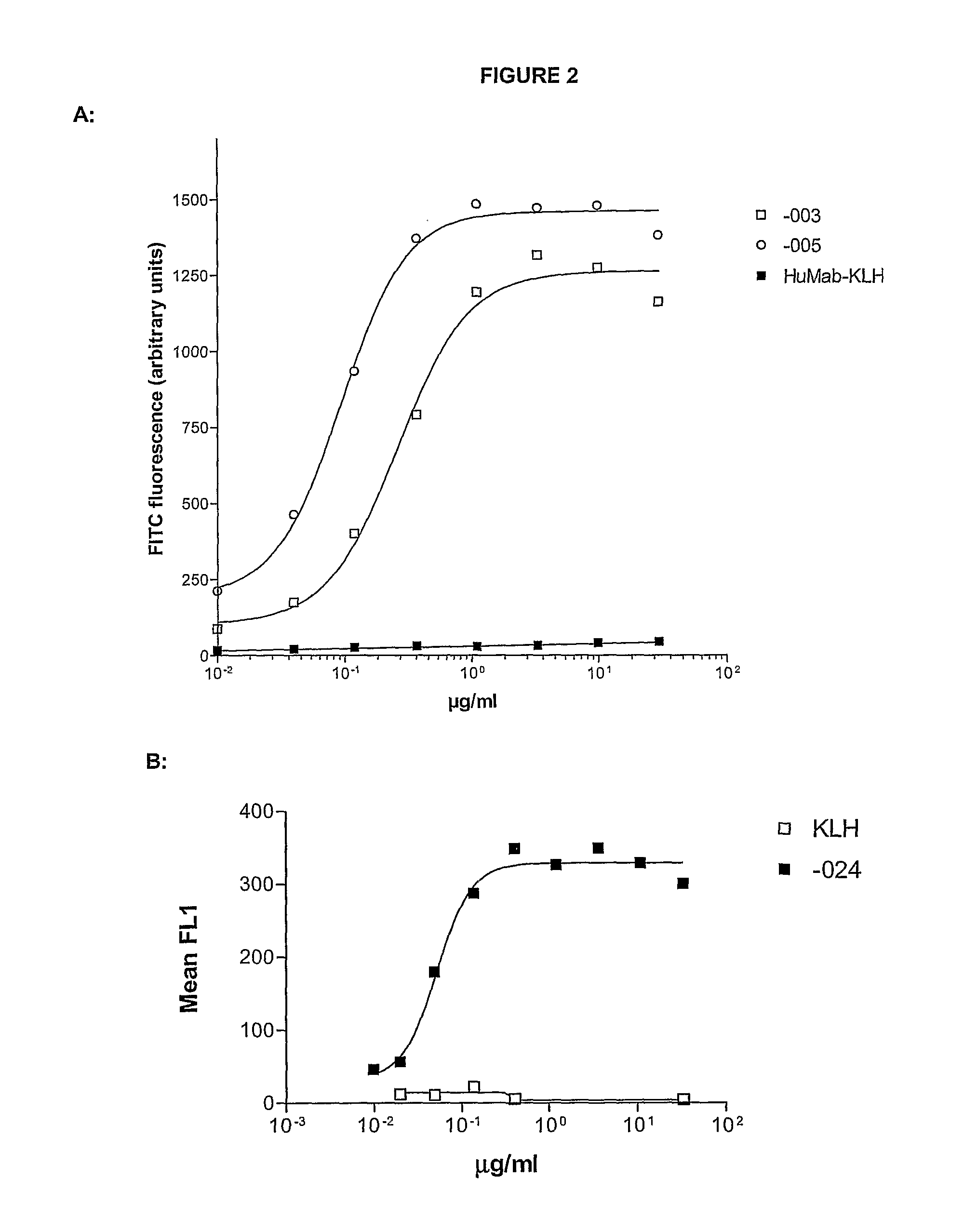
Combination treatment of cd38-expressing tumors
ActiveUS20100092489A1Good curative effectImprove survivalBoron compound active ingredientsSkeletal disorderAntiendomysial antibodiesOncology
The invention relates to novel method for the treatment of cancer using a combination therapy comprising an antibody that binds CD38, a corticosteroid and a non- corticosteroid chemotherapeutic agent.
Owner:GENMAB AS
Corticosteroid-containing pharmaceutical composition
InactiveUS7078058B2Improve permeabilityEasy to handleOrganic active ingredientsBiocideScalp psoriasisDisease
A foamable pharmaceutical composition comprising a corticosteroid, a quick-break foaming agent, a propellant and a buffering agent, sufficient to buffer the composition to within the range of pH 3.0 to 6.0 is disclosed. The quick-break foaming agent typically comprises an aliphatic alcohol, water, a fatty alcohol and a surface active agent. Due to the nature of the compositions of the invention, they are especially well-suited for use in the treatment of various skin diseases, and in particular, in the treatment of scalp psoriasis.
Owner:STIEFEL WEST COAST
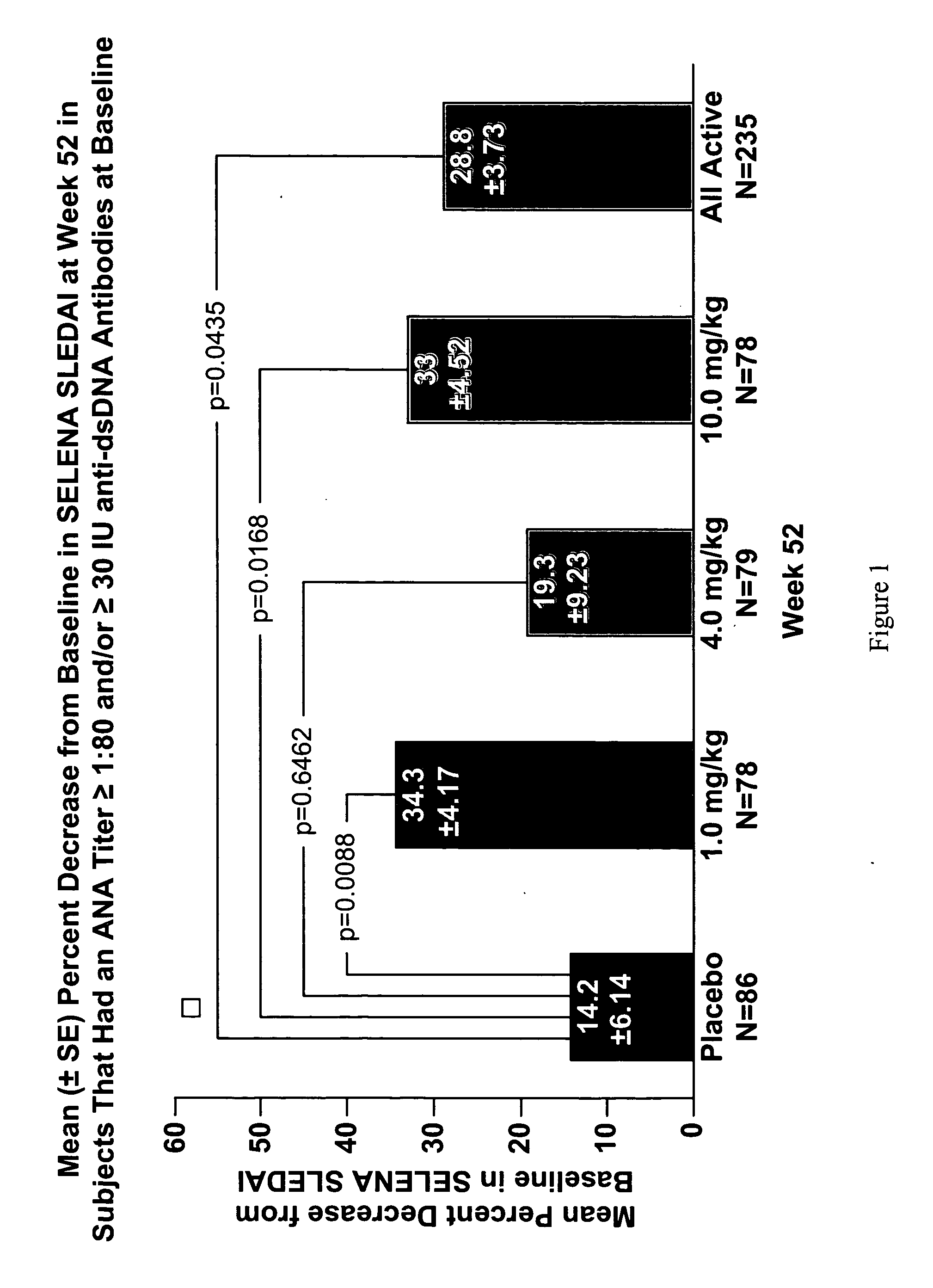
Methods and compositions for use in treatment of patients with autoantibody positive disease
InactiveUS20070086979A1Reduce frequencyReduce in quantityPeptide/protein ingredientsAntipyreticAnti-dsDNA antibodiesSystemic lupus erythematosus
The present invention relates to methods and compositions for use in treatment of patients with autoantibody positive disease. In a specific embodiment, the present invention relates to a method of treating a patient that has an ANA titer of 1:80 or greater and / or greater than or equal to 30 IU / ml of anti-dsDNA antibodies in his / her blood plasma or serum comprising administering a therapeutically effective amount of an immunomodulatory agent, such as an antagonist of Neutrokine-alpha. Additionally provided is a method of reducing the frequency and / or quantity of corticosteroid administration to patients. In preferred embodiments, the patient has systemic lupus erythematosus. Methods for determining if a lupus patient is responding to medical treatment are also provided.
Owner:HUMAN GENOME SCI INC
Therapeutic and prophylactic methods for neuromuscular disorders
InactiveUS20050014733A1Improve muscle massHigh strengthOrganic active ingredientsBiocideDiseaseNeuromuscular disease
The disclosure provides methods for treating neuromuscular disorders in mammals. The disclosed methods include administering therapeutically effective amounts of a GDF-8 inhibitor and a corticosteroid to a subject susceptible to, or having, a neuromuscular disorder, so as to maintain desirable levels of muscle function.
Owner:WYETH LLC
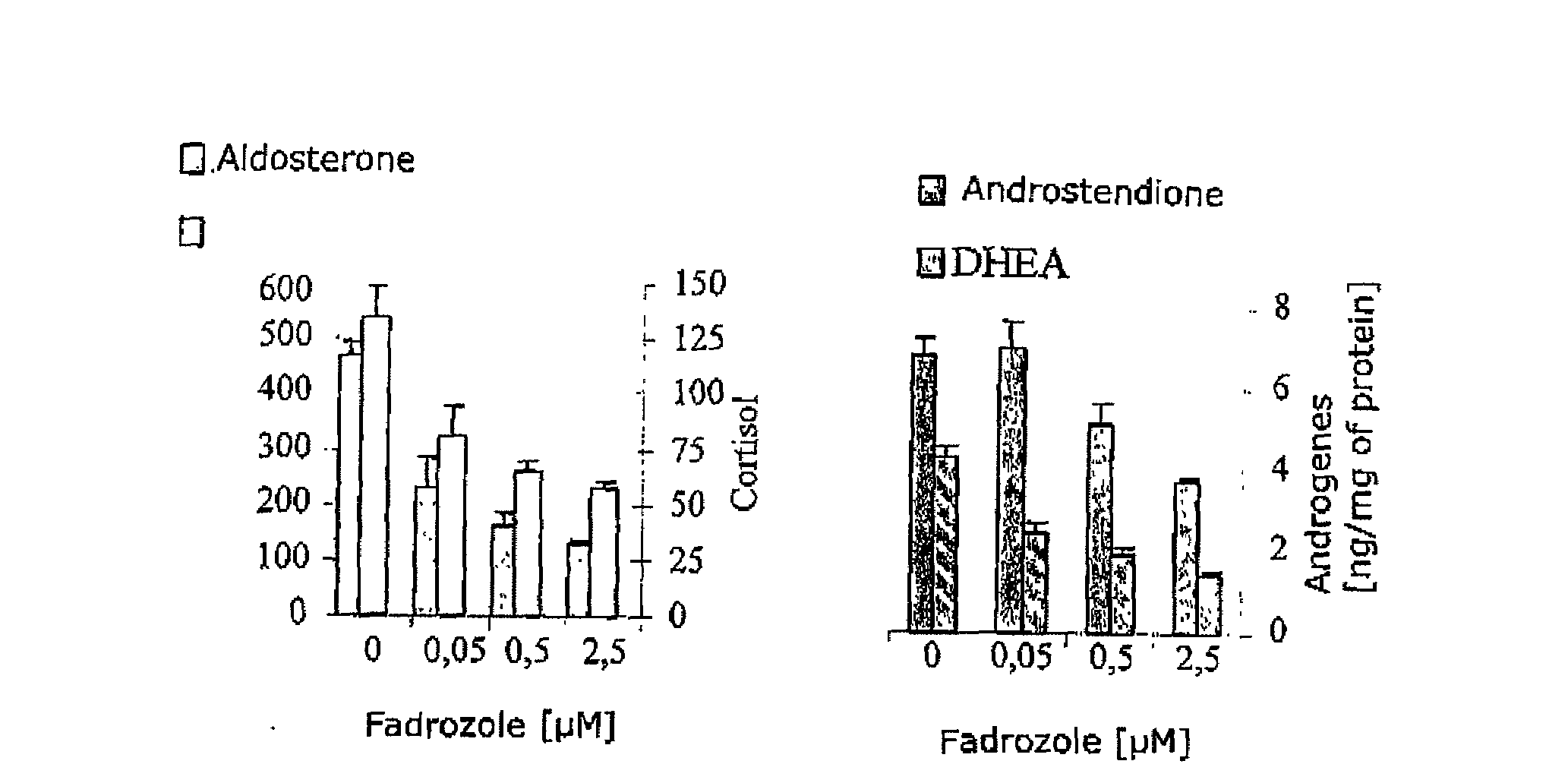
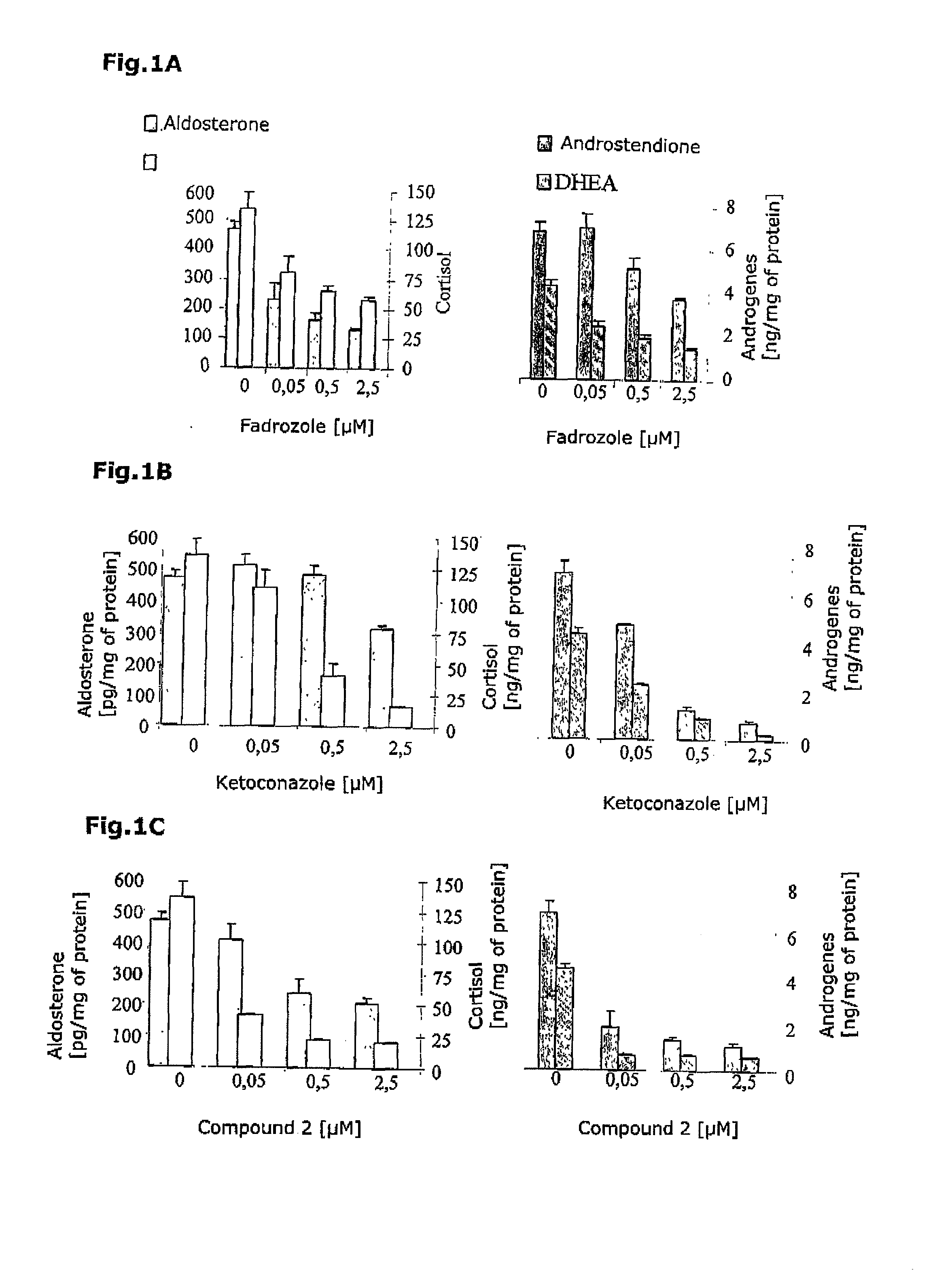
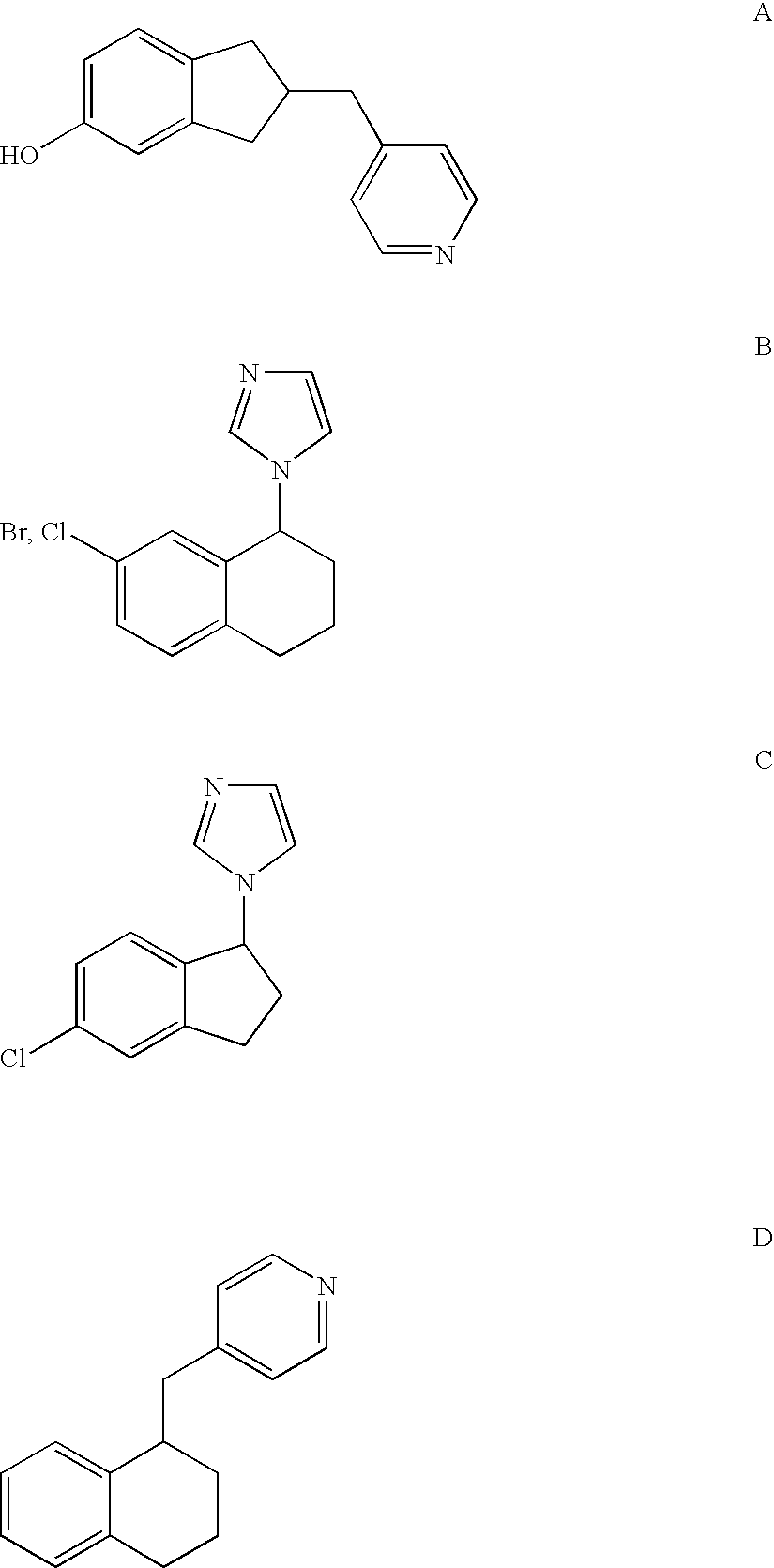
Selective Inhibitors of Human Corticosteroid Synthases
The invention relates to compounds for selectively inhibiting human corticosteroid synthases CYP1 1 B1 and CYP1 1 B2, and to the production and use thereof for treating hypercortisolism, diabetes mellitus, hyperaldosteronism, cardiac insufficiency, myocardial fibrosis, depression, age-related cognitive decline and metabolic syndrome.
Owner:UNIV DES SAARLANDES
Combination products
Owner:SIGMOID PHARM LIMITED
Combinations for the treatment of inflammatory disorders
InactiveUS6897206B2Suppression of TNFα levelBiocideCompound screeningTricyclic antidepressantTricyclic anti-depressants
The invention features a method for treating a patient having an inflammatory disorder, by administering to the patient (i) a tricyclic antidepressant (e.g., amoxapine); and (ii) a corticosteroid (e.g., prednisolone) simultaneously or within 14 days of each other in amounts sufficient to reduce or inhibit inflammation.
Owner:ZALICUS INC
Corticosteroid compositions
ActiveUS20090123550A1Good dispersionEasily resuspendedBiocideOrganic active ingredientsDiseaseInflammation
Provided herein are methods for treating, preventing or alleviating the symptoms of and inflammation associated with inflammatory diseases and conditions of the gastrointestinal tract, for example, those involving the esophagus. Also provided herein are pharmaceutical compositions useful for the methods of the present invention.
Owner:VIROPHARMA HLDG
Oral delayed immediate release formulation and method for preparing the same
InactiveUS6183780B1Simple and inexpensive formulation methodSimple and inexpensive methodNervous disorderPeptide/protein ingredientsAnti-emeticTranquilizing Agents
The invention relates to an Oral Delayed Immediate Release formulation comprising a compressed core containing one or more active substances surrounded with a coating, wherein release of active substance from the core is caused by rupture of the coating after a definite lag-time, said core comprising one or more immediate release carriers and having no substantial swelling properties upon exposure to gastrointestinal fluids. The invention further relates to formulations containing an Immediate Release formulation combined with one or more Oral Delayed Immediate Release formulations with different lag-times and to a method of preparing an Oral Delayed Immediate Release formulation.The Oral Delayed Immediate Release formulation may be used for the application of active substances whenever a certain lag-time before release is advantageous, such as in be the case of anti-asthmatics, anti-emetics, cardiotonics, vasodilators, anti-vertigo and anti-meniere compounds, anti-hypertensives, sedatives, anti-depressants, anti-anxiety compounds, cortico-steroids, general anti-inflammatory compounds, anti-inflammatory compounds for gastrointestinal use, anti-ulceratives, analgetics, anti-aritmics, anti-rheumatics, anti-arthritic compounds and anti-angina compounds.The Oral Delayed Immediate Release formulation may also be used for the application of biological active compounds such as proteins, peptides, enzymes, vaccines and oligonucleotides.
Owner:ABBOTT PROD OPERATION AG
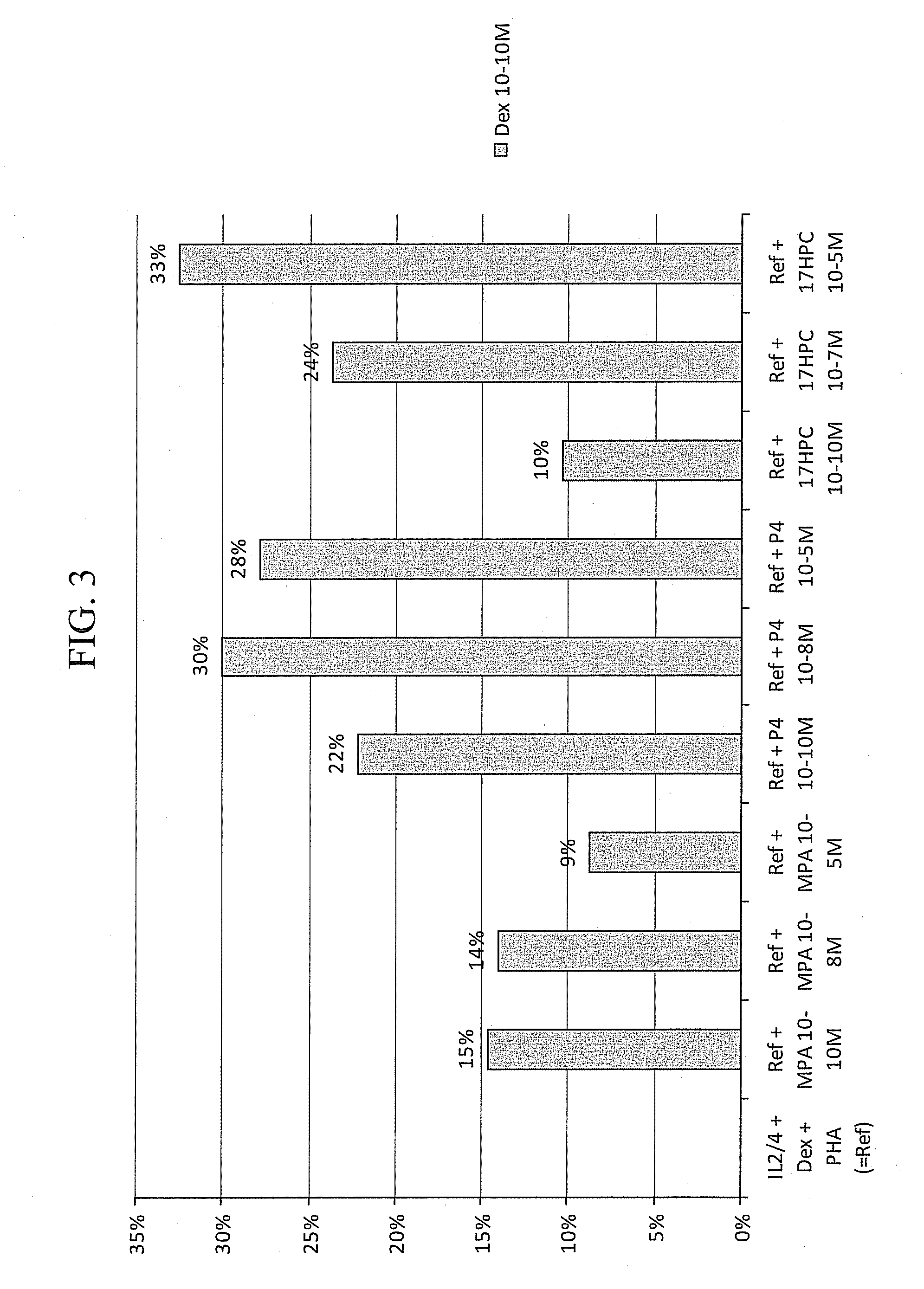
Methods for the use of progestogen as a glucocorticoid sensitizer
ActiveUS20110195031A1Improve responsivenessImprove toleranceBiocideSenses disorderSterolGlucocorticoid Sensitivity
Provided are methods and kits for administering progestogen as a glucocorticoid sensitizer to restore corticosteroid sensitivity or reverse the glucocorticoid insensitivity or enhance glucocorticoid sensitivity, in order to treat one or more glucocorticoid insensitivity related diseases or conditions. For example, these include methods for reversing the glucocorticoid insensitivity in a subject having no history of menstrual cycle-related exacerbation or allergy to self-hormones, particularly progesterone, such as premenstrual or perimenstrual deterioration in the symptoms, e.g., premenstrual worsening of atopic dermatitis or premenstrual exacerbations of asthma, and exhibiting relatively or totally refractory responses to glucocorticoid therapy, e.g., glucocorticoid resistance. The methods and kits provide for the administration of a sex hormone to the subject who is corticosteroid dependent or corticoid resistant or unresponsive or intolerant to corticosteroids.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
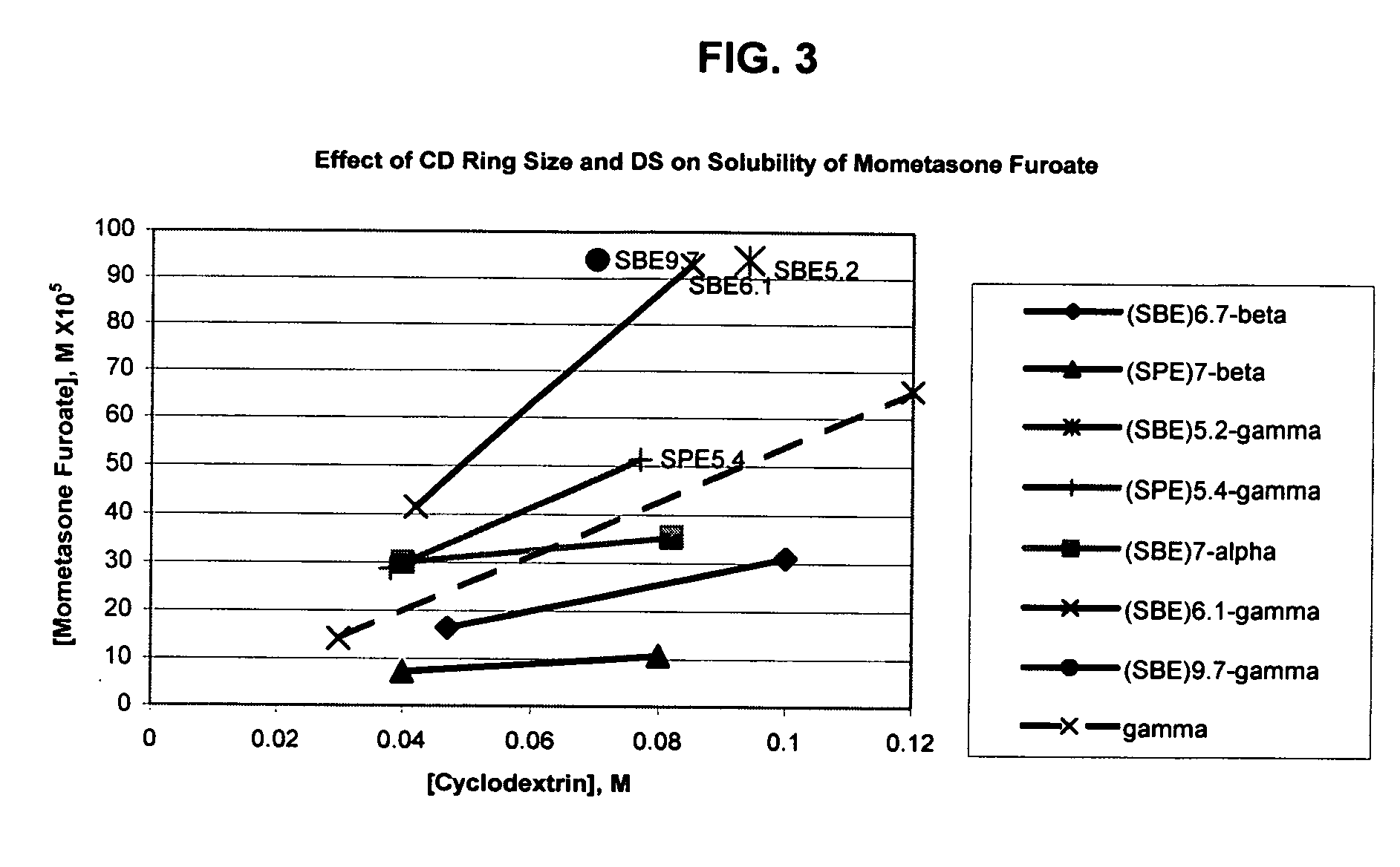
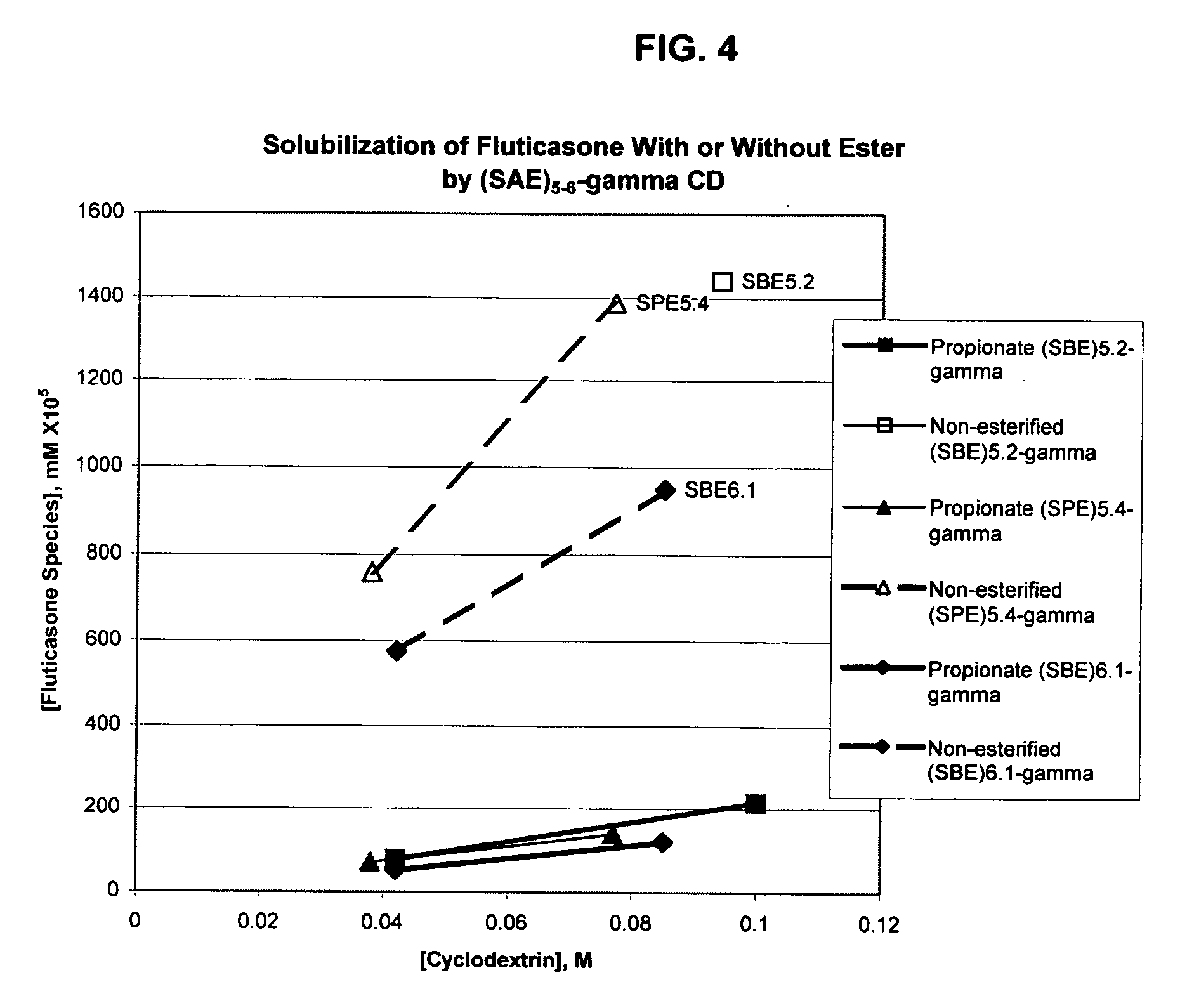
Inhalant formulation containing sulfoalkyl ether gamma-cyclodextrin and corticosteroid
InactiveUS20070020298A1Reduce the degradation rateIncrease productivityOrganic active ingredientsBiocideNasal cavityNebulizer
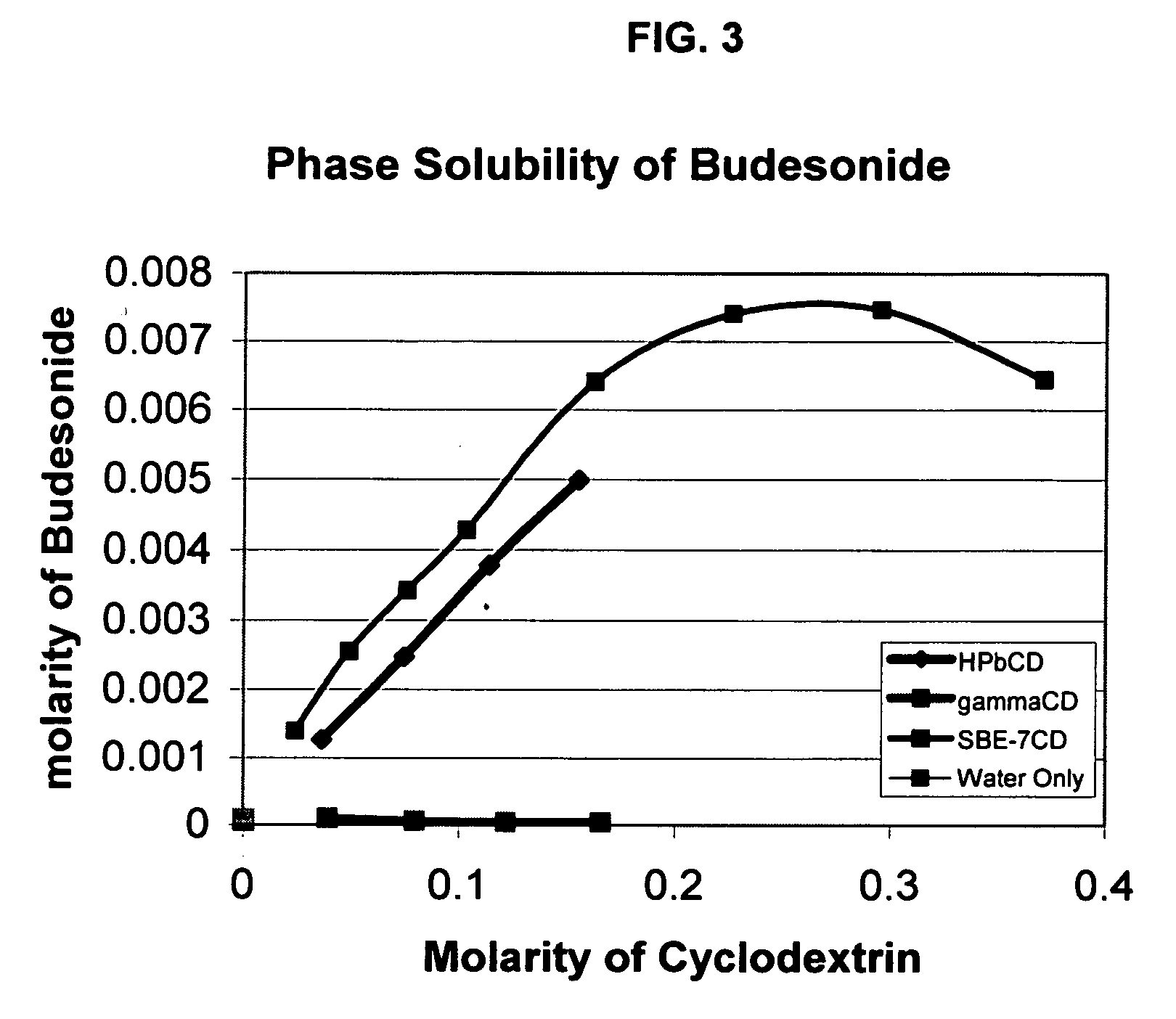
An inhalable formulation containing SAE-γ-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-γ-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation can include one or more additional therapeutic agents for use in combination with the corticosteroid. SAE-γ-CD is especially useful for solubilizing esterified corticosteroids.
Owner:CYDEX PHARMACEUTICALS INC
Locally administrated low doses of corticosteroids
This invention provides for using a locally delivered low dose of a corticosteroid to treat pain caused by any inflammatory disease including sciatica, herniated disc, stenosis, mylopathy, low back pain, facet pain, osteoarthritis, rheumatoid arthritis, osteolysis, tendonitis, carpal tunnel syndrome, or tarsal tunnel syndrome. More specifically, a locally delivered low dose of a corticosteroid can be released into the epidural space, perineural space, or the foramenal space at or near the site of a patient's pain by a drug pump or a biodegradable drug depot.
Owner:MEDTRONIC INC +1
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
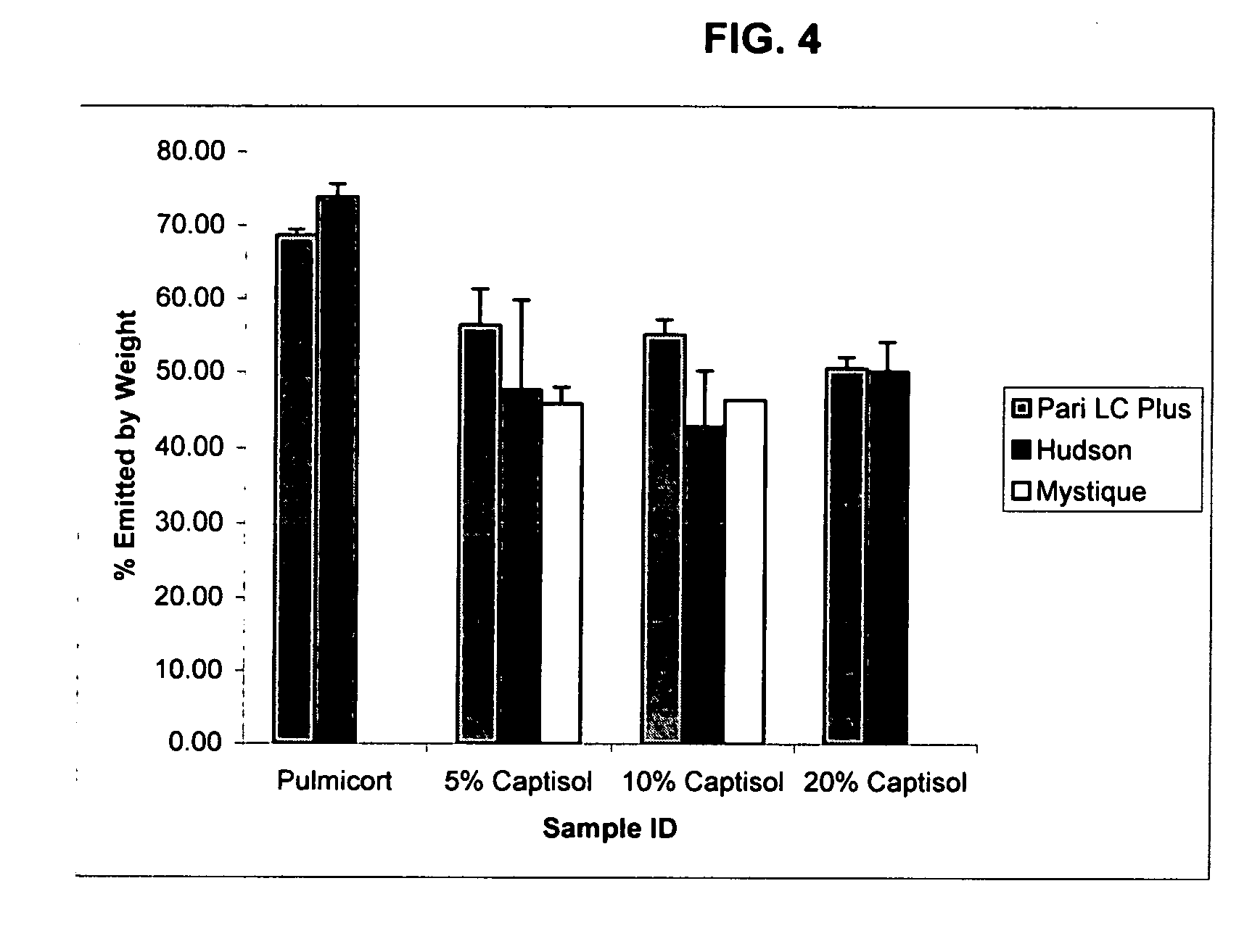
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
Transdermal Methods and Systems for the Delivery of Corticosteroid Compounds
An integrated iontophoresis skin-worn patch and method for delivering a therapeutically effective amount of a corticosteroid drug compound in a systemically-safe and skin-safe manner for site-specific treatment of inflammation pain is disclosed.
Owner:TEIKOKU PHARMA USA INC
Topical wound therapeutic compositions
There is provided a composition for healing burns and wounds in mammals, which contains a cromolyn compound or the combination of a cromolyn compound and hyaluronic acid a corticosteroid. Advantageously, elements found in amniotic fluid are also included.
Owner:ALPHAMED PHARMA CORP
Topical wound therapeutic compositions
There is provided a composition for healing burns and wounds in mammals, which contains a cromolyn compound or the combination of a cromolyn compound and hyaluronic acid a corticosteroid.Advantageously, elements found in amniotic fluid are also included.
Owner:ALPHAMED PHARMA CORP
Orally Administered Corticosteroid Compositions
ActiveUS20110081411A1Antibacterial agentsOrganic active ingredientsGastrointestinal inflammationInflammation
The present invention is directed to orally administered corticosteroid compositions. The present invention also provides a method for treating a condition associated with inflammation of the gastrointestinal tract in an individual. The method comprises administering to an individual in need thereof a pharmaceutical composition of the present invention.
Owner:ELLODI PHARM LP
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
High oil-content emollient aerosol foam compositions
Described herein are high oil-content emulsions and compositions for the treatment of inflammatory skin disorders. The emulsions may be formulated as aerosol compositions. The aerosol propellant may be a hydrofluoroalkane propellant. The emulsions or compositions may comprise active agents, such as corticosteroids. Also described are methods of treating inflammatory skin disorders, comprising the step of applying to an affected area of a subject in need thereof a therapeutically-effective amount of an inventive emulsion or aerosol composition.
Owner:PRECISION DERMATOLOGY
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveUS20070065374A1Useful in prophylaxis and chronic treatment of asthmaGood curative effectPowder deliveryBiocidePediatric patientPatient compliance
Nanoparticulate compositions comprising a corticosteroid and a leukotriene receptor antagonist are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining a leukotriene receptor antagonist with a corticosteroid in a particle size ranges of less than 2000 nm in a single formulation results in improved efficacy. In addition, patient compliance is enhanced since only one dosage form is needed. Furthermore, local administration of the leukotriene receptor antagonist results in less liver toxicity since the liver will be exposed to lower amounts of drug than happens following oral administration. The drug compositions according to the invention can be formulated into inhalation, nasal, or ocular formulations.
Owner:ELAN PHRMA INT LTD
Combination therapy of respiratory diseases using antibodies and anti-inflammatory agents
InactiveUS7208162B2Useful in treatmentAntibacterial agentsImmunoglobulins against virusesInterleukin 6Disease
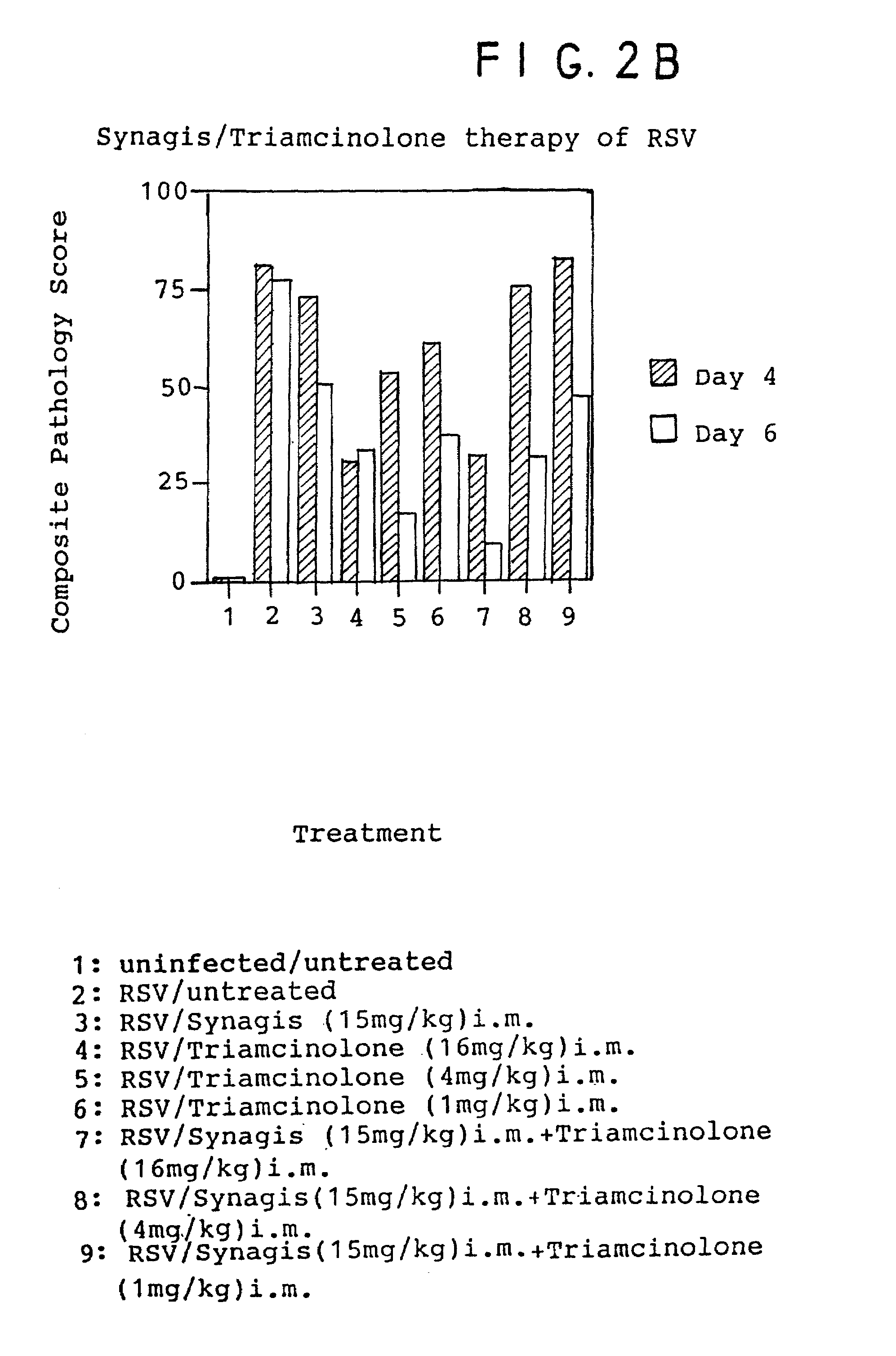
Therapeutically effective anti-viral compositions, useful especially against respiratory diseases caused or mediated by respiratory syncytial virus (RSV) are disclosed, wherein said compositions comprise at least one anti-RSV antibody, including high affinity antibodies, and an additional anti-inflammatory agent, especially corticosteroids, as well as anti-inflammatory antibodies, especially anti-interleukin-6. Also disclosed are methods of using such compositions to treat and / or prevent respiratory diseases. Such compositions may optionally contain other non-antibody anti-viral agents.
Owner:AREXIS +1
Method of wound healing and scar modulation
InactiveUS20110009374A1Effective controlNatural functional and aesthetic characteristicOrganic active ingredientsAntipyreticWound healingBone healing
The invention relates to methods of promoting wound healing and reducing scar formation by administration of corticosteroids, and pharmaceutical compositions comprising corticosteroids.
Owner:MOKO THERAPEUTICS
Corticosteroid Topical Dispersion with Low Content of Surfactant
ActiveUS20070299044A1Improved pharmacological profileReduce systemic absorptionOrganic active ingredientsAntipyreticSide effectAdditive ingredient
The invention provides novel compositions of water-insoluble corticosteroid drug in combination with antimicrobial agents and very low concentrations of polymers and surfactants for topical, otic and ophthalmic treatment. The invention provides stable aqueous suspension where the ingredients remain in such a state so as to allow for immediate re-suspension, when desired, even after extended periods of settling. The invention provides also a method for treating inflammation with low systemic absorption and side-effects of the corticosteroid.
Owner:BAYER HEALTHCARE ANIMAL HEALTH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com