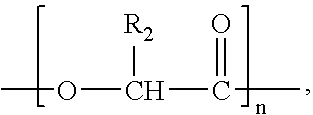
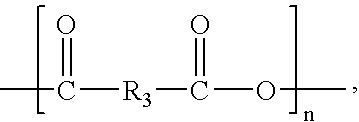
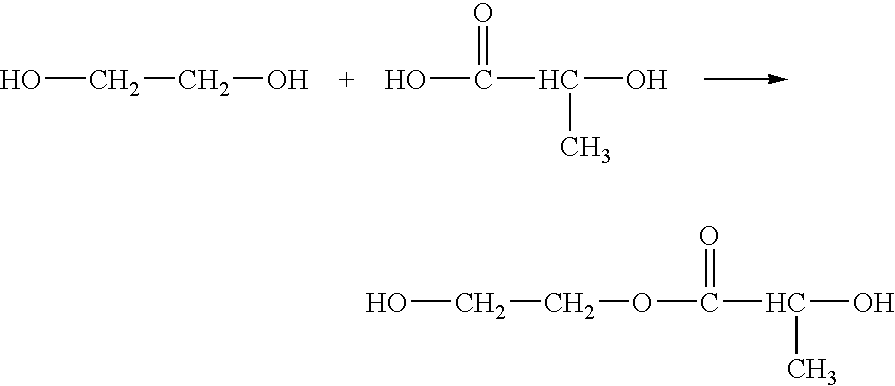
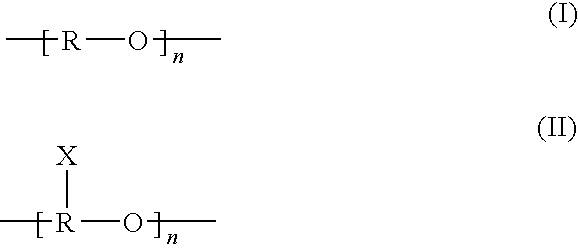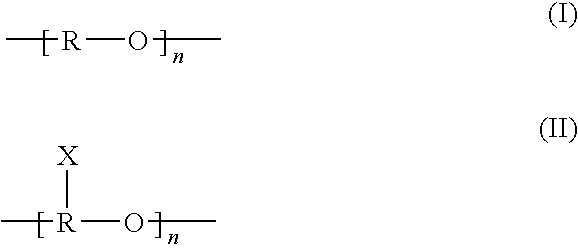Patents
Literature
1546 results about "Alkyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
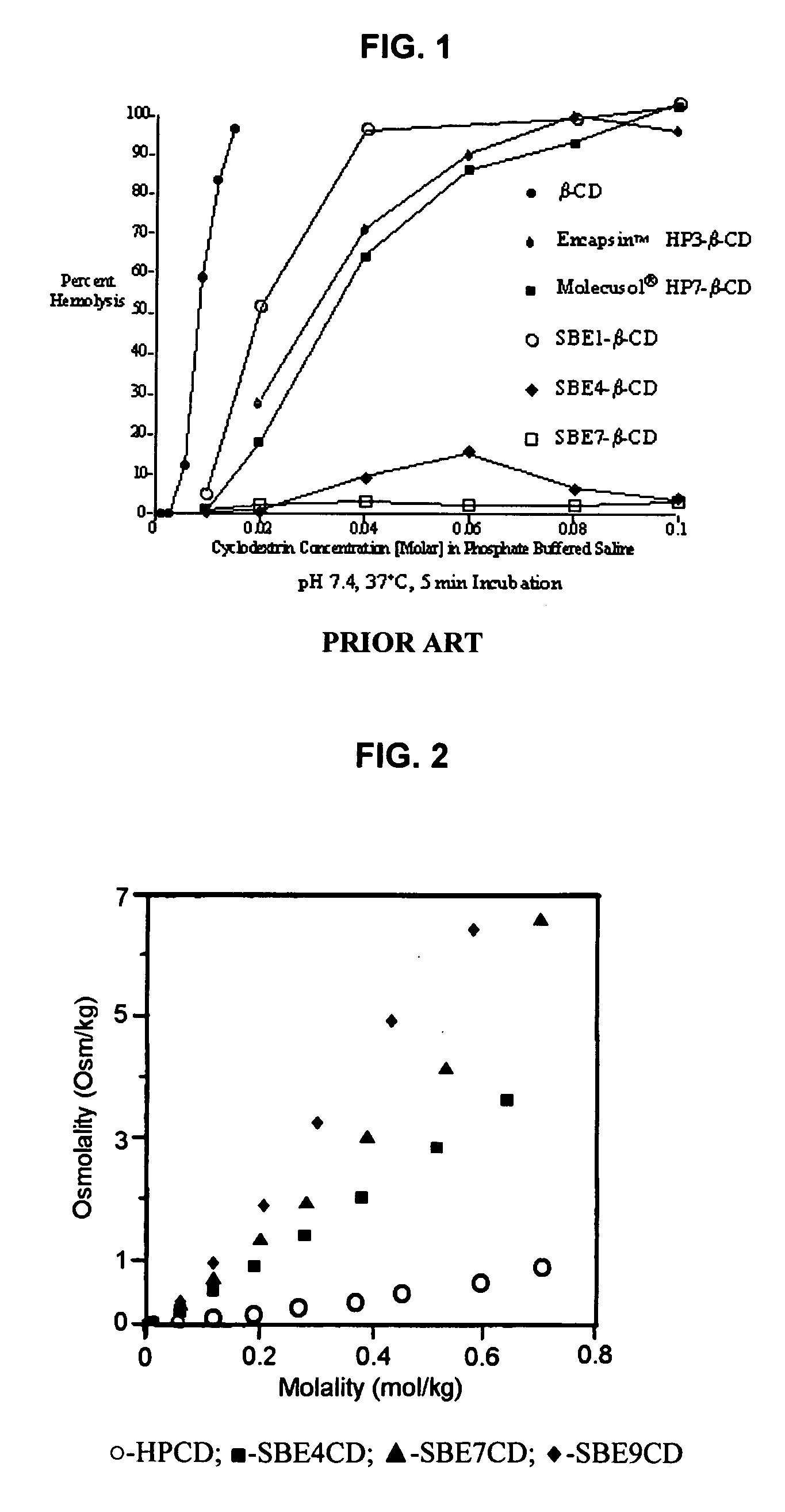
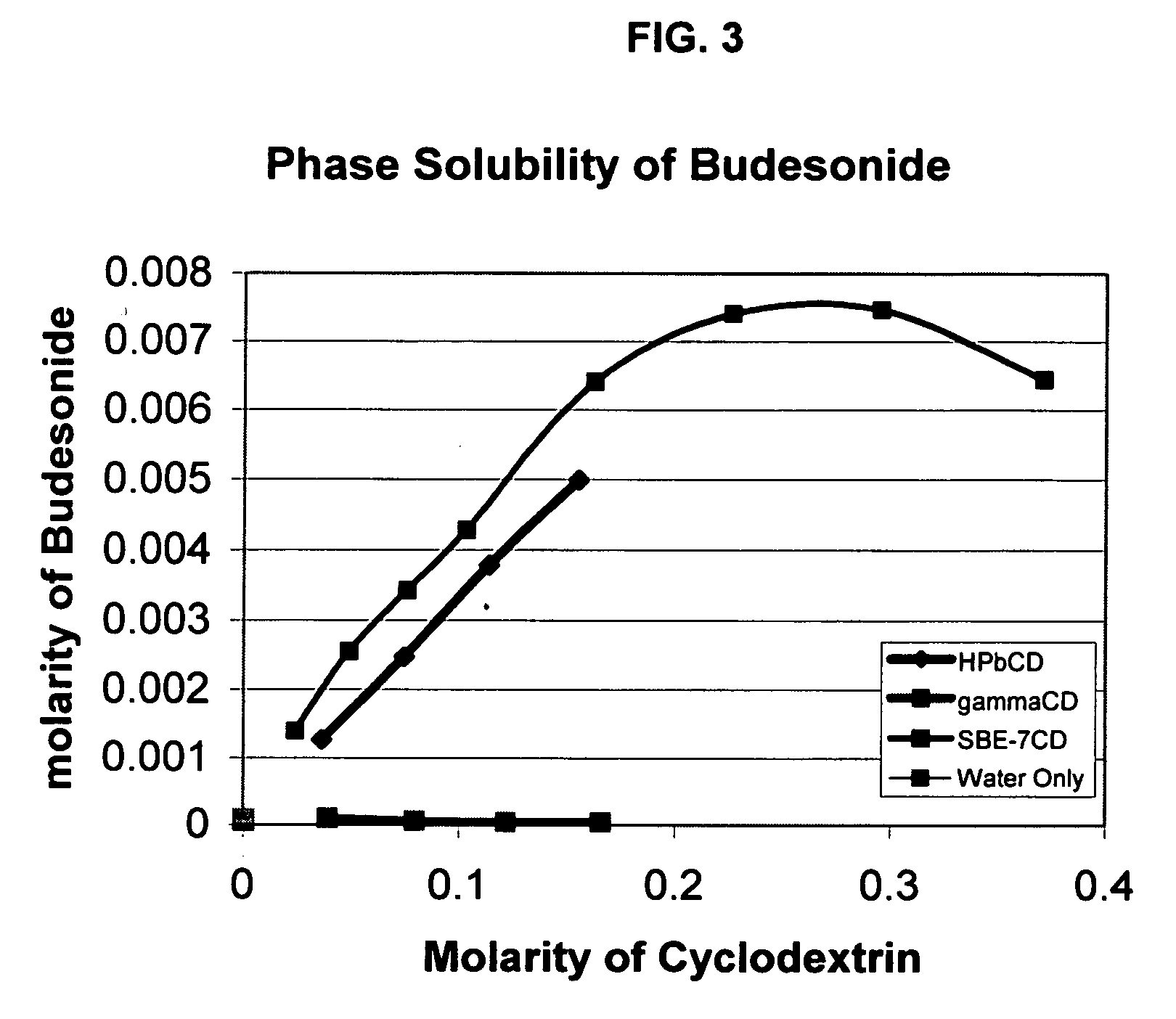
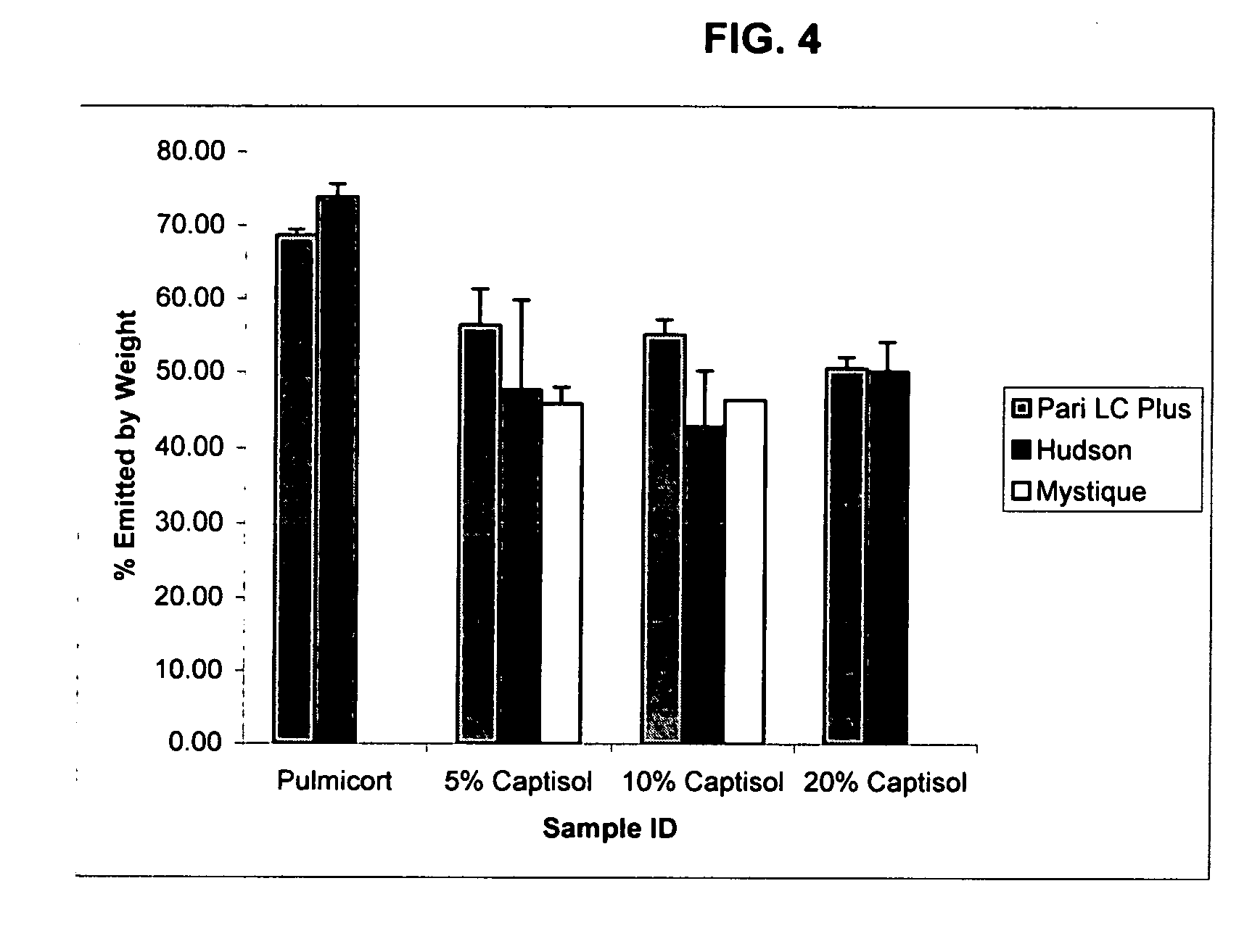
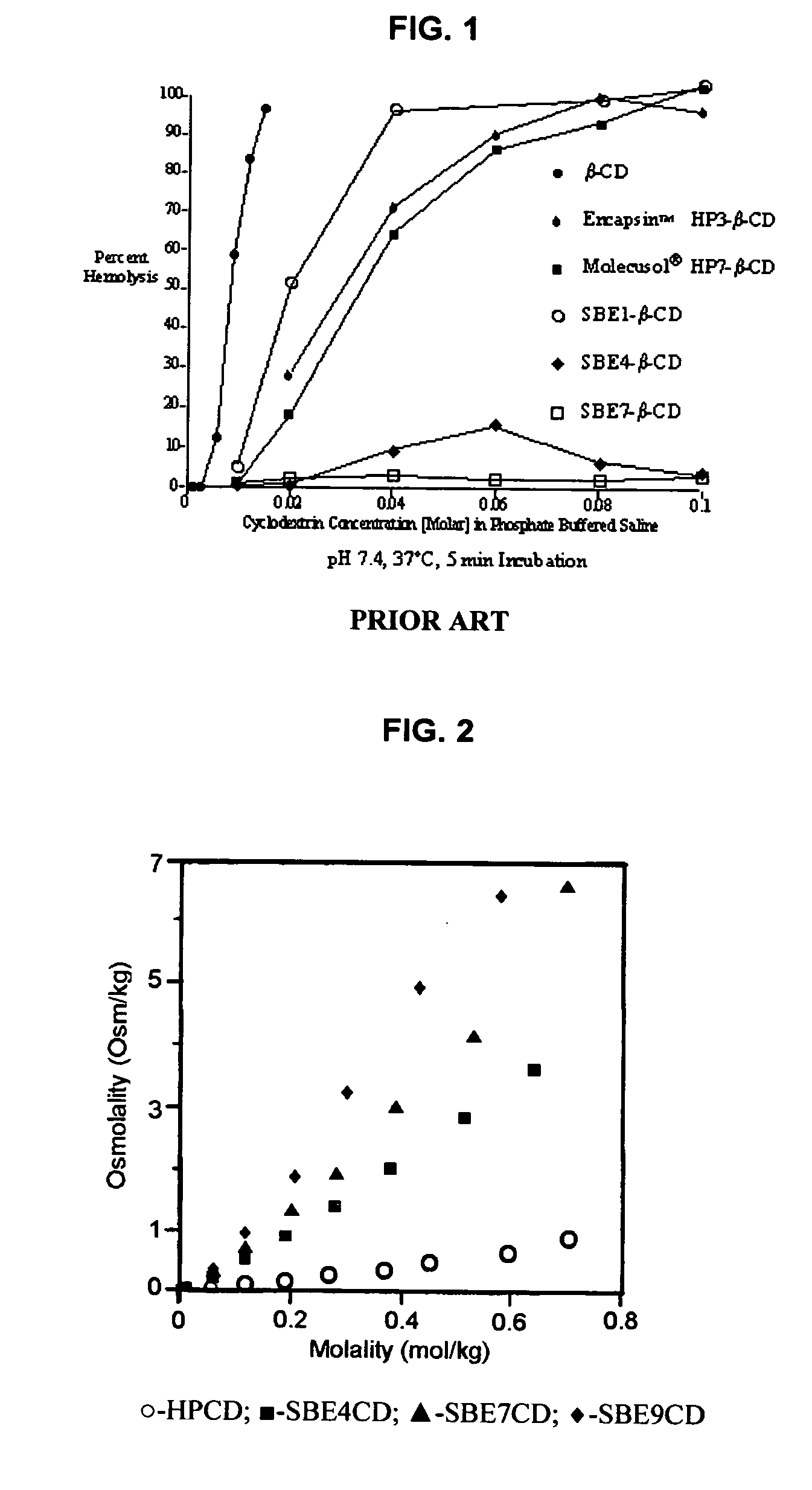
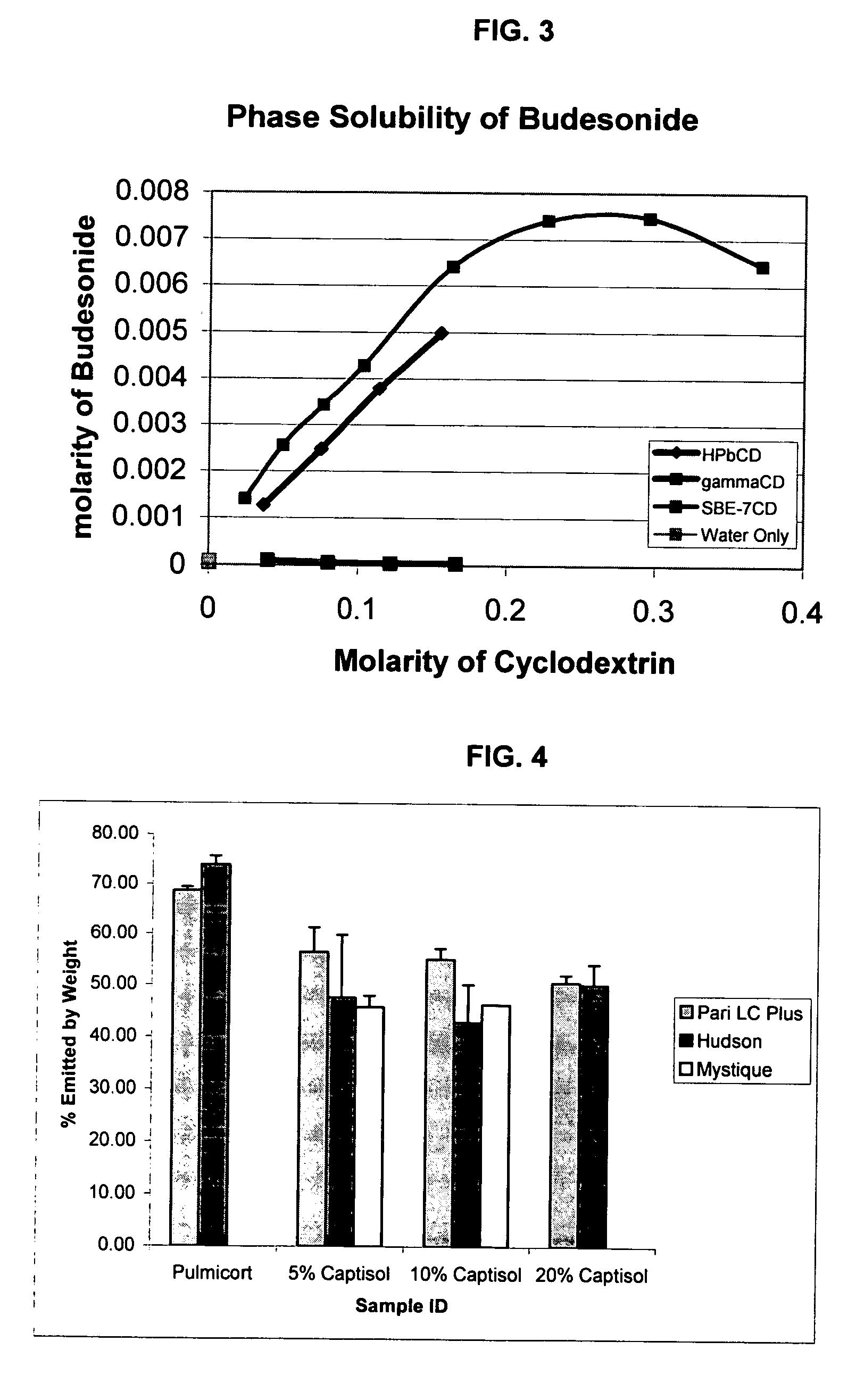
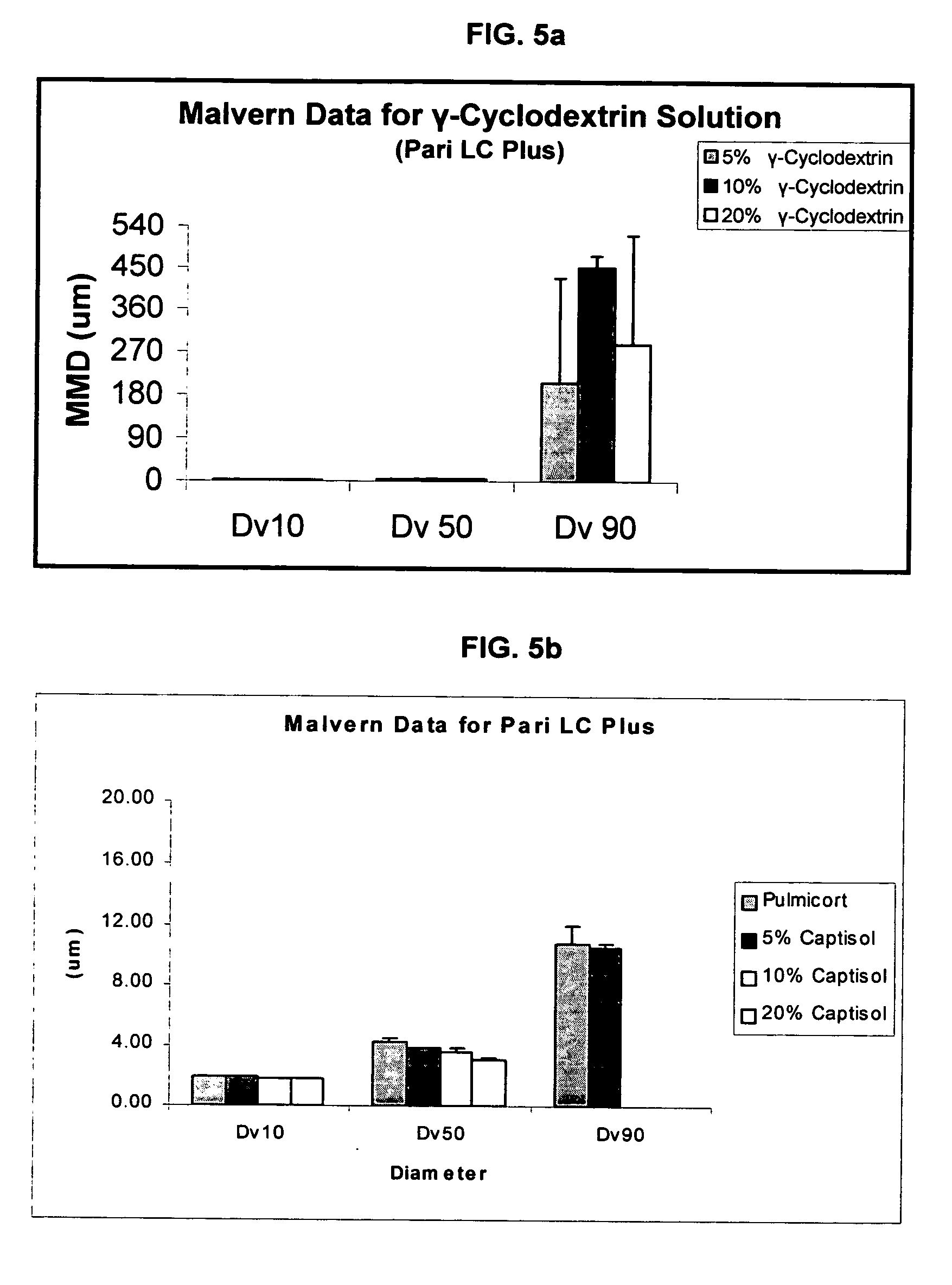
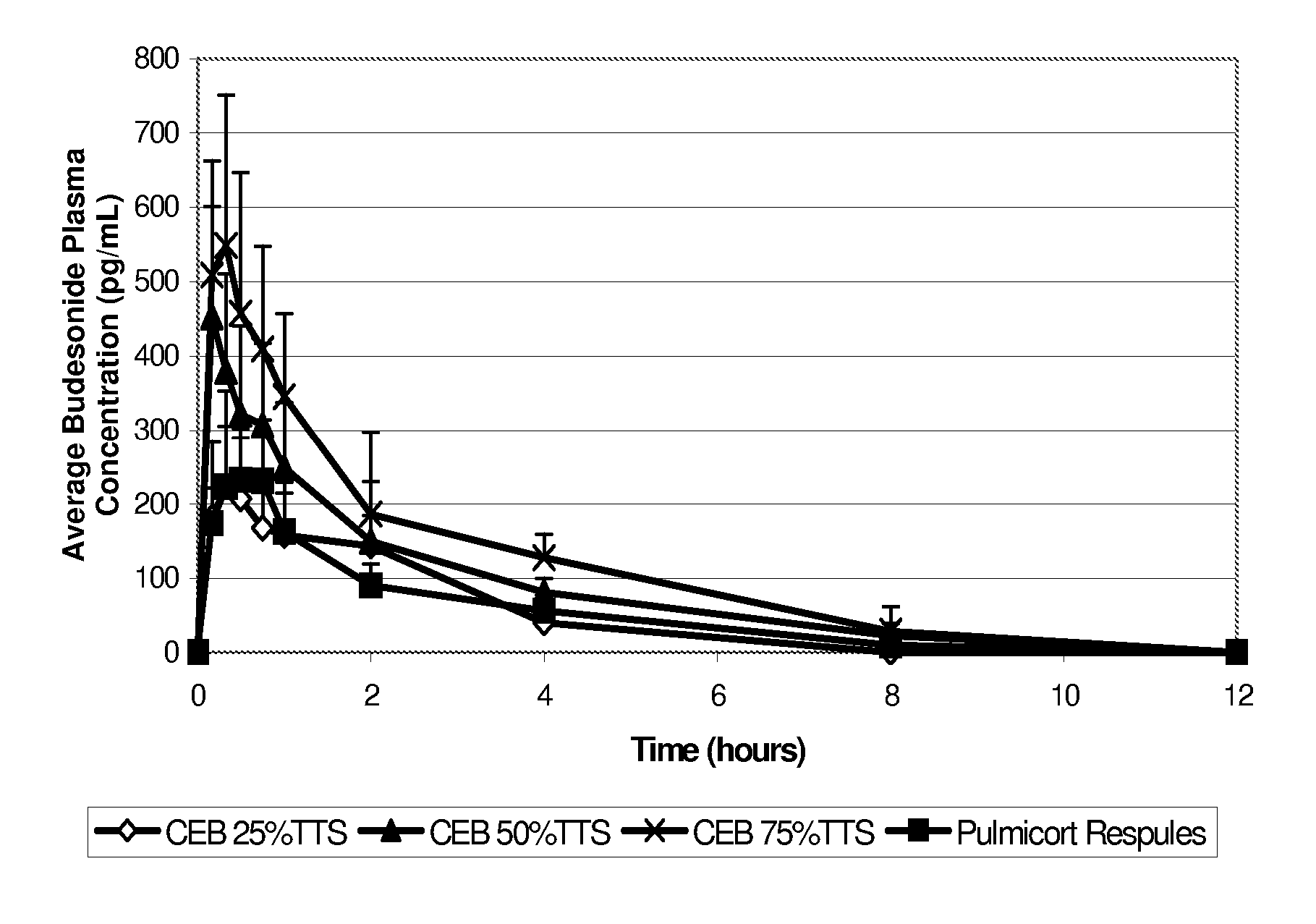
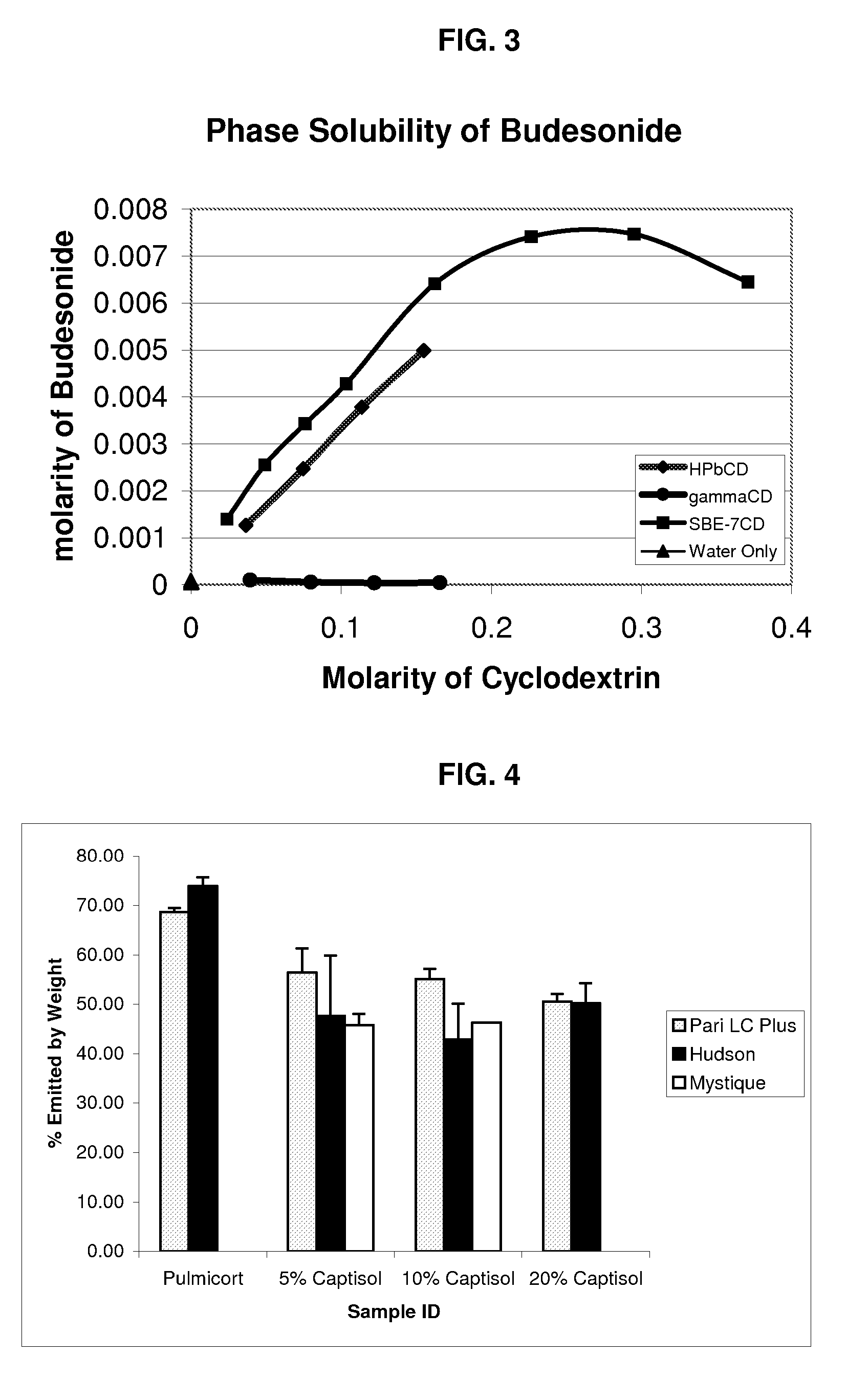
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Polypropylene glycol foamable vehicle and pharmaceutical compositions thereof
The present invention teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent water and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof. The present invention further teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent, and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Oxygen scavenging plastic compositions
The present invention relates to a composition comprising a polyester, a copolyester ether and an oxidation catalyst, wherein the copolyester ether comprises a polyether segment comprising poly(tetramethylene-co-alkylene ether). The composition has oxygen scavenging properties and produces articles having low haze. Other embodiments of the present invention disclosed herein are articles made from the composition and methods to make such articles.
Owner:INVISTA NORTH AMERICA R L
Resilient personal care composition comprising polyalkyl ether containing siloxane elastomers
InactiveUS20100092408A1Improve stabilityGood flexibilityCosmetic preparationsToilet preparationsPersonal careEther
A stable personal care composition in the form of a water-in-oil emulsion may comprise a silicone elastomer comprising a polyalkyl ether pendant or a polyalkyl ether crosslink, wherein the alkyl group contains three or more carbon atoms; a non-emulsifying silicone elastomer; an emulsifier; a polar oil; and water. The personal care composition allows for previously unattainable levels of polar oils and / or aqueous phase within a water-in-oil emulsion.
Owner:THE PROCTER & GAMBLE COMPANY
Polypropylene glycol foamable vehicle and pharmaceutical compositions thereof
InactiveUS20080152596A1Easy to useCosmetic preparationsBiocidePolypropylene glycolSurface-active agents
The present invention teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent water and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.The present invention further teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent, and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Removal of cured silicone adhesive for reworking electronic components
InactiveUS20020000239A1Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsOrganic baseSolvent
Owner:IBM CORP
Process for carbonylation of alkyl ethers
ActiveUS20070238897A1Organic compound preparationPreparation from carboxylic acid esters/lactonesCarboxylic acidCarbonylation
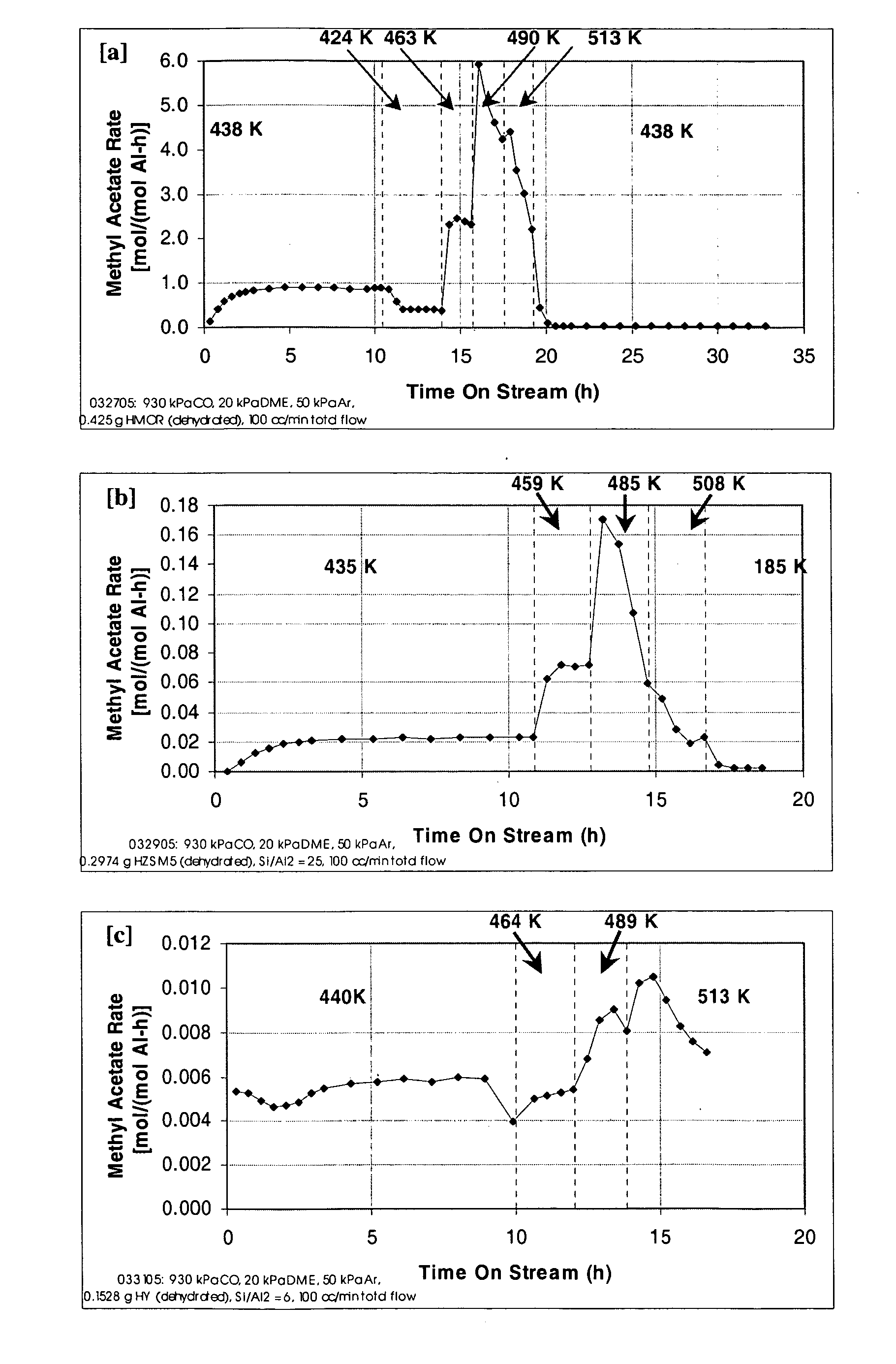
A product comprising a lower alkyl ester of a lower aliphatic carboxylic acid is produced by a process comprising reacting under substantially anhydrous conditions a lower alkyl ether with carbon monoxide in the presence of a zeolite catalyst having an 8-member ring channel which is interconnected with a channel defined by a ring with greater than or equal to 8 members, the 8-member ring having a window size of at least 2.5 Angstroms×at least 3.6 Angstroms and at least one Brønsted acid site and the zeolite having a silica:X2O3 ratio of at least 5, wherein X is selected from aluminum, boron, iron, gallium and mixtures thereof.
Owner:THE BRITISH PETROLEUM CO LTD +1
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Taste-masked formulations containing sertraline and sulfoalkyl ether cyclodextrin
InactiveUS20050250738A1High photochemical stabilityReduce probabilityBiocideOrganic active ingredientsSertralineFruit juice
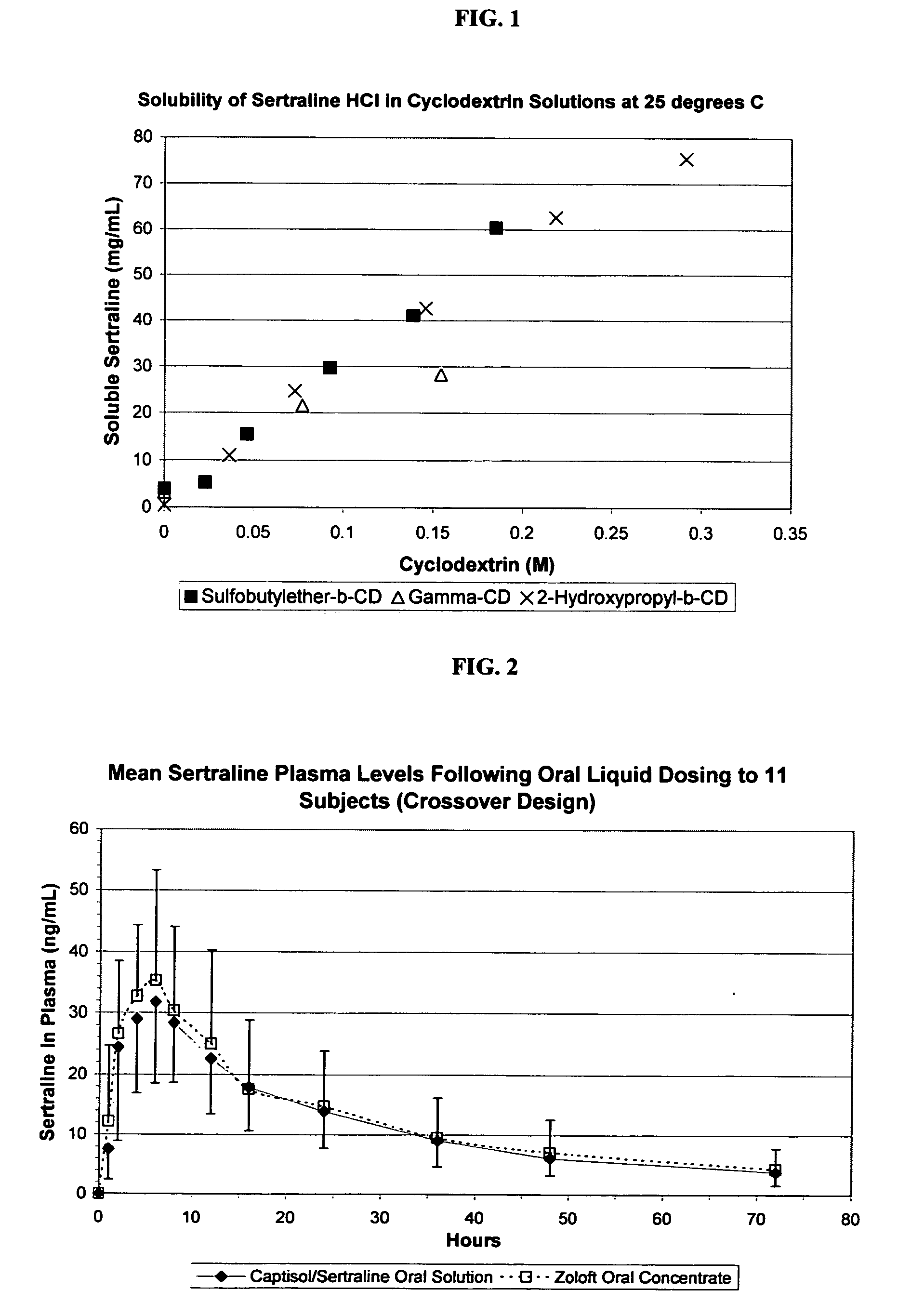
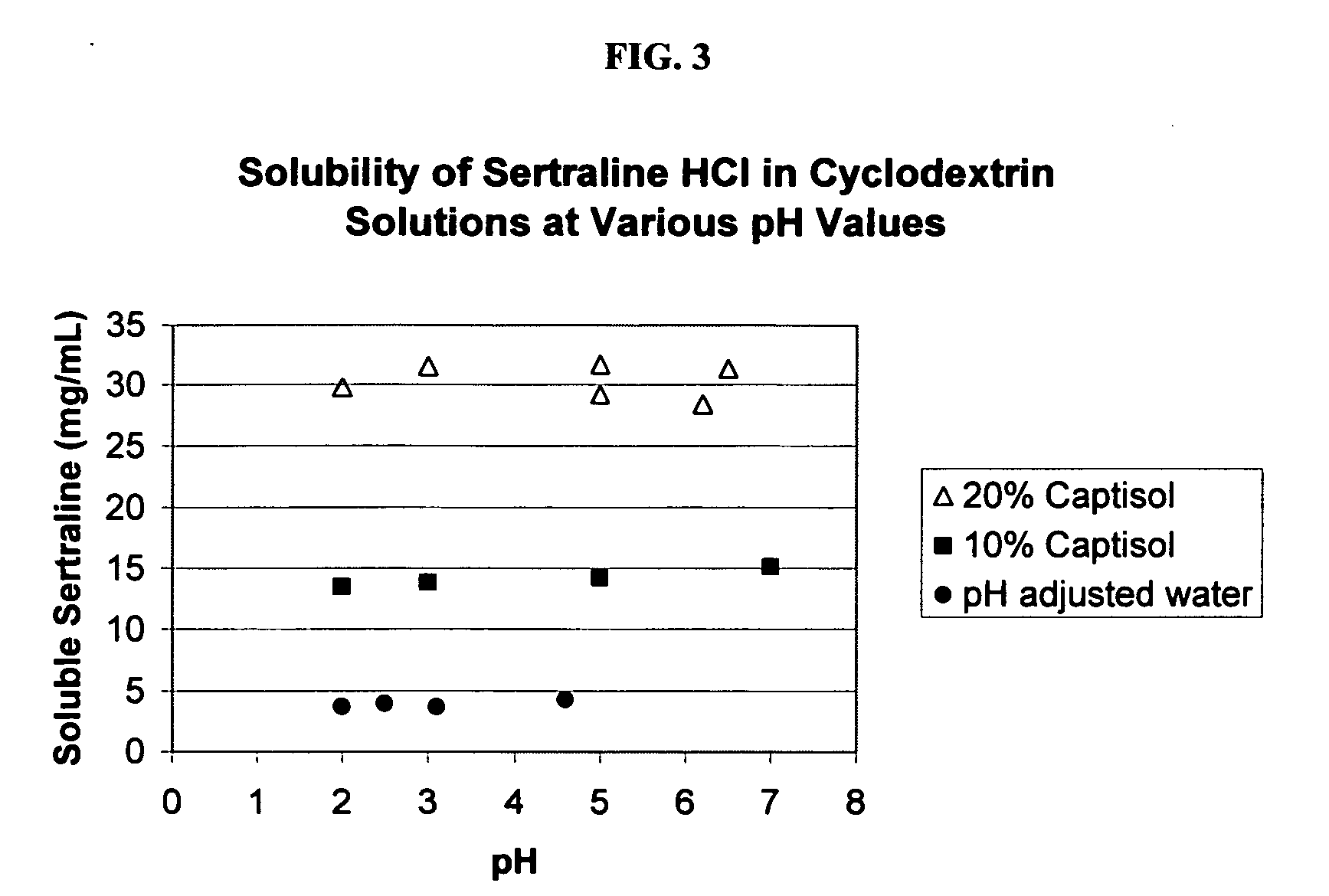
The present invention provides aqueous oral formulations containing sertraline, or a pharmaceutically acceptable salt thereof, and a sulfoalkyl ether cyclodextrin. The liquid formulations are pleasant tasting, convenient to use, and chemically and physically stable. The liquid formulations can be administered directly or diluted before administration. Unlike the commercially available ZOLOFT™ formulation, the liquid formulations herein do not precipitate upon dilution with water, fruit juices, sodas or other pharmaceutically acceptable oral liquid carriers. The sulfoalkyl ether cyclodextrin-containing formulation provides significant advantages over the marketed non-aqueous formulation and other cyclodextrin-containing formulations of sertraline. The formulation can be self-preserved against microbial growth. The SAE-CD-containing formulation of sertraline can be provided in liquid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid formulations can be prepared. The formulation is available as a clear solution or a suspension.
Owner:CYDEX PHARMACEUTICALS INC
Injectable biodegradable particles
According to an aspect of the invention, injectable polymeric particles are provided that contain a copolymer that contains a hydroxy-acid-based repeat unit selected from a mono(hydroxy acid) unit and a poly(hydroxy acid) unit, an alkyl-ether-based repeat unit selected from a mono(alkyl ether) unit and a poly(alkyl ether) unit, and an acid-based repeat unit selected from a unit comprising multiple carboxylic acid groups and a derivative thereof Other aspects of the invention pertain to methods of making such particles. Still other aspects of the invention pertain to injectable compositions that comprise such particles and to methods of treatment that employ such injectable compositions.
Owner:BOSTON SCI SCIMED INC
Fuel additive composition and method for treatment of middle distillate fuels and gasoline
InactiveUS6923838B2Increase and improvement of performanceIncrease and improvement of and lubricityLiquid carbonaceous fuelsFuel additivesVegetable oilAntioxidant
A fuel additive for middle distillate fuels is a mixture of at least one methyl, ethyl, propyl or butyl ester of a vegetable oil or a C16-C18 fatty acid, at least one alkyl ether of propylene glycol, a surfactant, and an antioxidant. A fuel additive for gasoline is a mixture of ligroin or toluene / xylene, at least one alkyl ether of propylene glycol, a surfactant, and tertiary amyl methyl ether.
Owner:ADVANCED COMBUSTION TECH
Aerosol formulations for buccal and pulmonary application
InactiveUS6436367B1Antibacterial agentsOrganic active ingredientsChamomile extractOleic Acid Triglyceride
A mixed micellar aerosol pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal lauryl sulphate, at least three micelle forming compounds, a phenol and a propellant. The micelle forming compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linoleic acid, linolenic acid, monoolein, monooleates, monolaurates, borage oil, evening of primrose oil, menthol, trihydroxy oxo cholanyl glycine and pharmaceutically acceptable salts thereof, glycerin, polyglycerin, lysine, polylysine, triolein, polyoxyethylene ethers and analogues thereof, polidocanol alkyl ethers and analogues thereof. The amount of each micelle forming compound is present in a concentration of from 1 to 20 wt. / wt. % of the total formulation, and the total concentration of micelle forming compounds are less than 50 wt. / wt. % of the formulation. The propellant, e.g. a fluorocarbon propellant, provides enhanced absorption of the pharmaceutical agent.
Owner:GENEREX PHARMA
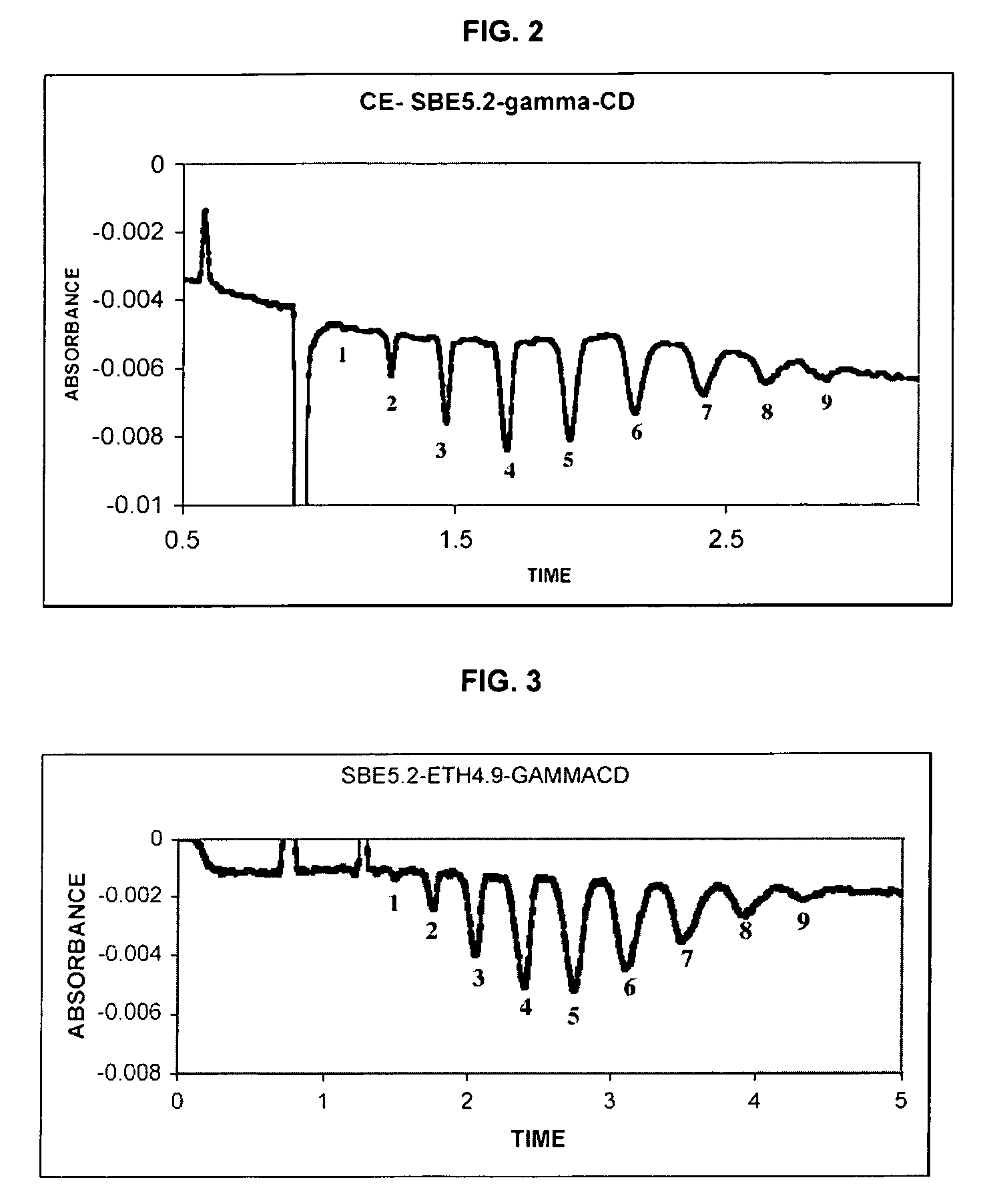
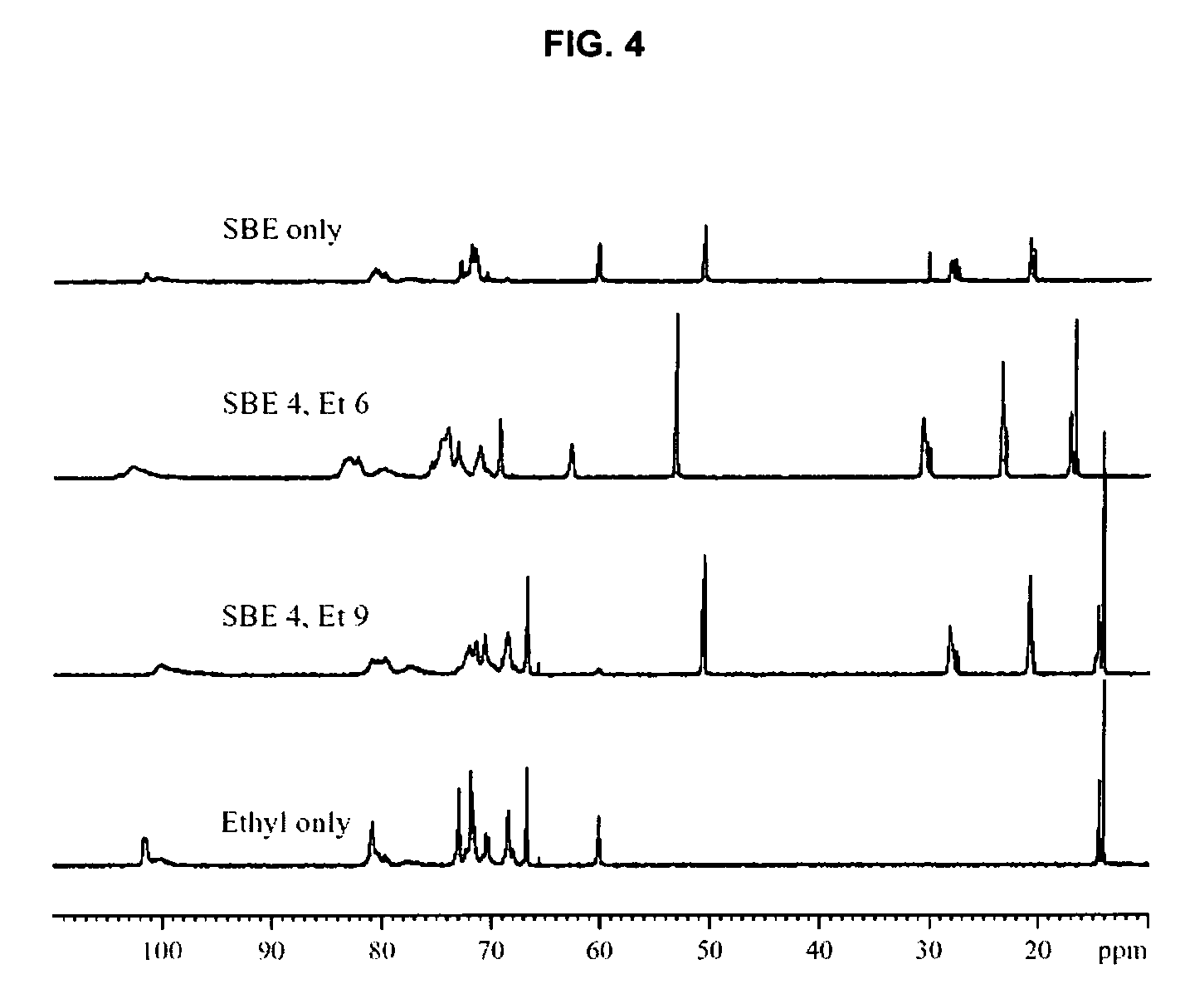
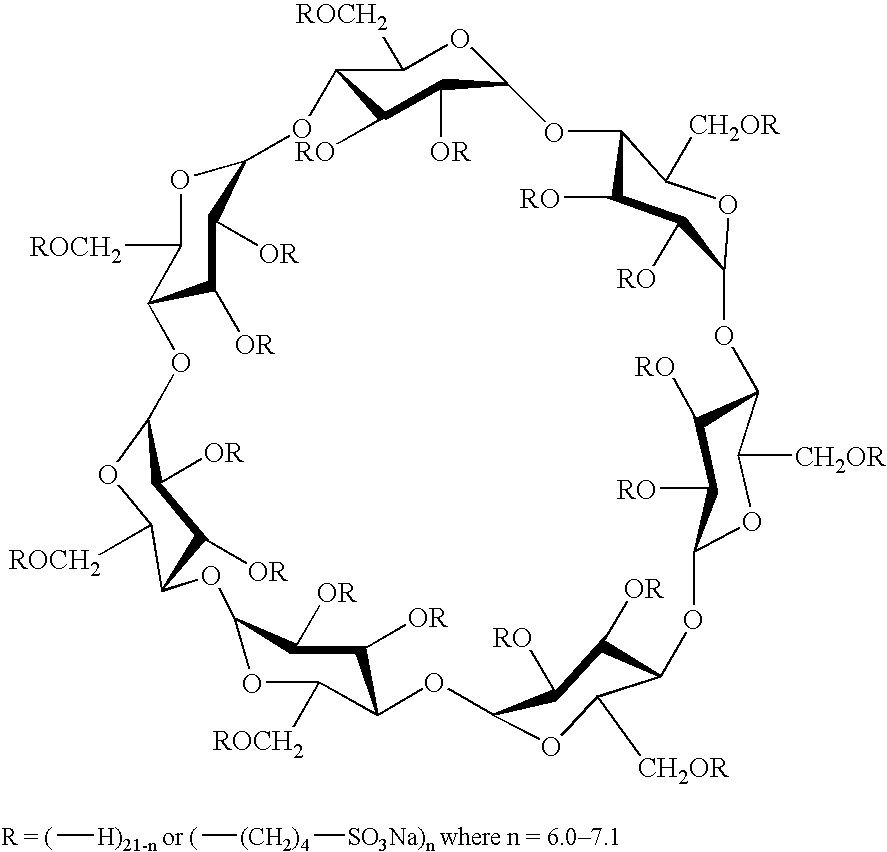
Sulfoalkyl ether-alkyl ether cyclodextrin derivatives
ActiveUS7625878B2Improve propertiesHigh degree of substitutionBiocideOrganic active ingredientsSolubilityCyclodextrin derivative
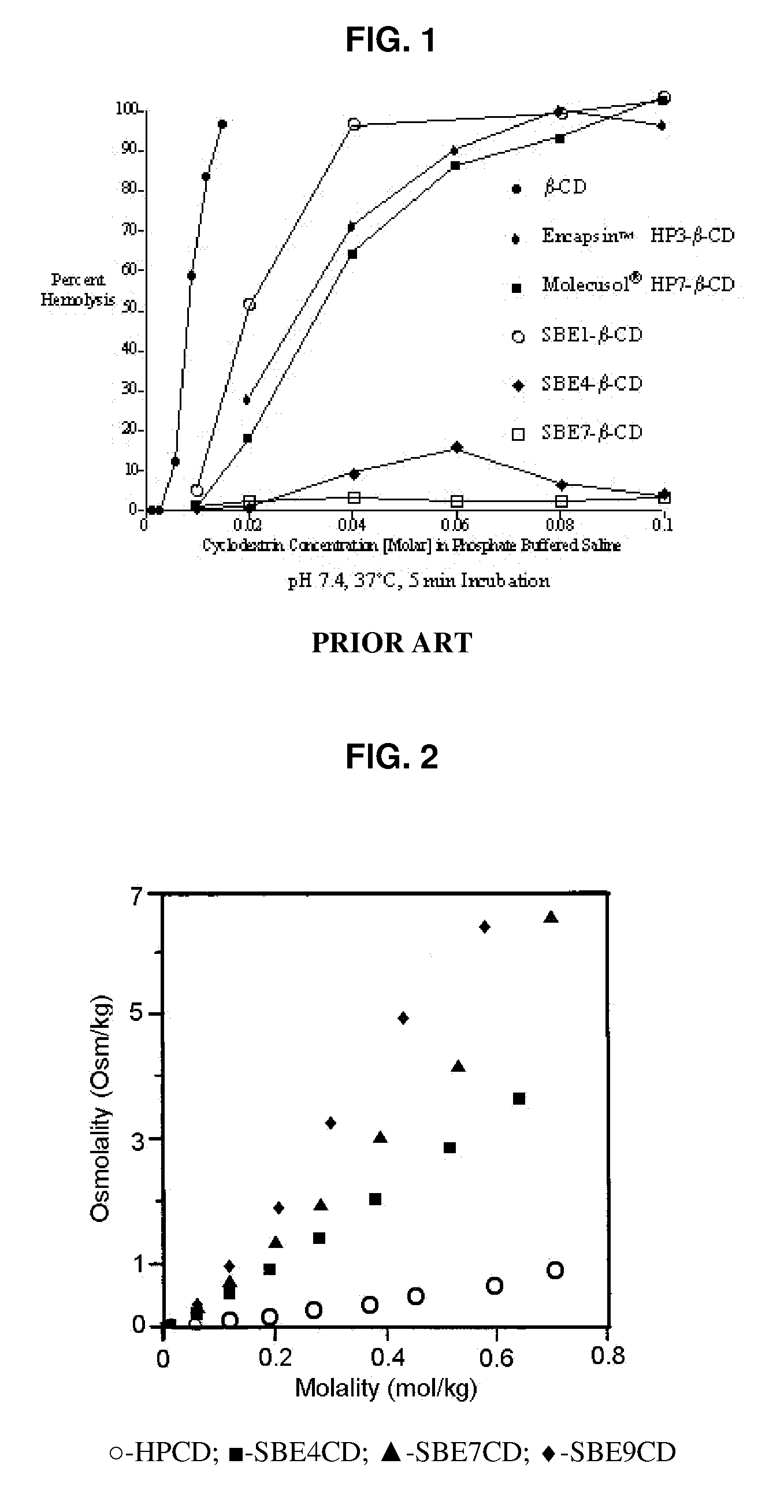
A sulfoalkyl ether-alkyl ether cyclodextrin (SAE-AE-CD) derivative is provided. The SAE-AE-CD possesses advantages over known SAE-CD and AE-CD derivatives as well as over the parent cyclodextrin by being more water soluble and less membrane disturbing. The SAE-AE-CD includes at least one sulfoalkyl ether group and at least one alkyl ether group even though the degree of substitution for the functional groups can be different. The SAE functional group can be present in molar excess over the AE functional group and vice versa. The total degree of substitution of the cyclodextrin, with respect to both functional groups, can be varied such that a minority or a majority of the hydroxyl moieties of the CD are derivatized. The SAE-AE-CD derivative can be used to solubilize compounds with insufficient water solubility. In some cases, they also stabilize compounds in solution against degradation or to solubilize degradation products formed during degradation. In addition, SAE-AE-CD can also be used for other purposes such as osmotic agents, agents used to mask the taste of problematic drugs. Surprisingly, while AE-CDs are known to be toxic by being membrane disturbing, SAE-AE-CDs are less membrane disturbing and therefore have greater safety.
Owner:UNIVERSITY OF KANSAS
Polyurethane power transmission belt
ActiveUS20090227406A1Increase load capacityIncrease resistanceV-beltsRopes and cables for vehicles/pulleyElastomerFiber
A power transmission belt having a main belt body portion of elastomeric material, a tensile reinforcement such as carbon fiber cord, disposed in said body portion, and a sheave contact portion integral with said main body portion. The elastomeric material includes the reaction product of a polyisocyanate prepolymer composition and a chain extender. The polyisocyanate prepolymer composition is prepared by reacting a diisocyanate and a polyol substantially free of moieties oxidative at less than about 150° C. and at least one triol crosslinker selected from the same group of polyols. Either before or after reacting the prepolymer, a plasticizer selected from the group consisting of alkyl-ether di-alkylesters such as polyethylene glycol di-alkylester is added thereto. The chain extender is an aromatic symmetric primary or secondary diamine chain extenders.
Owner:THE GATES CORP
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
Dpi formulation containing sulfoalkyl ether cyclodextrin
ActiveUS20070175472A1Increase pressure dropSuitable for useOrganic active ingredientsPowder deliveryThroatActive agent
An inhalable dry powder formulation containing SAE-CD and an active agent is provided. The formulation is adapted for administration by DPI. The SAE-CD serves as a carrier rather than as an absorption enhancer. The average particle size of the SAE-CD is large enough to preclude (for the most part) pulmonary deposition thereof. Following release from the DPI device, the SAE-CD-containing particles dissociate from the active agent-containing particles in the buccal cavity or throat, after which the active agent-containing particles continue deeper into the respiratory tract. The physicochemical and morphological properties of the SAE-CD are easily modified to permit optimization of active agent and carrier interactions. Drugs having a positive, neutral or negative electrostatic charge can be delivered by DPI when SAE-CD is used as a carrier.
Owner:CYDEX PHARMACEUTICALS INC
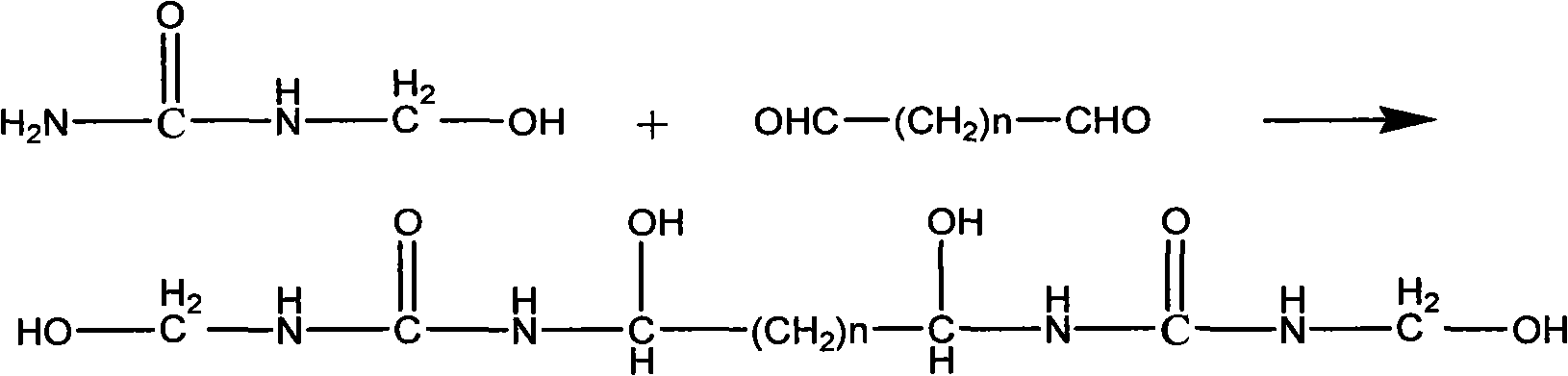
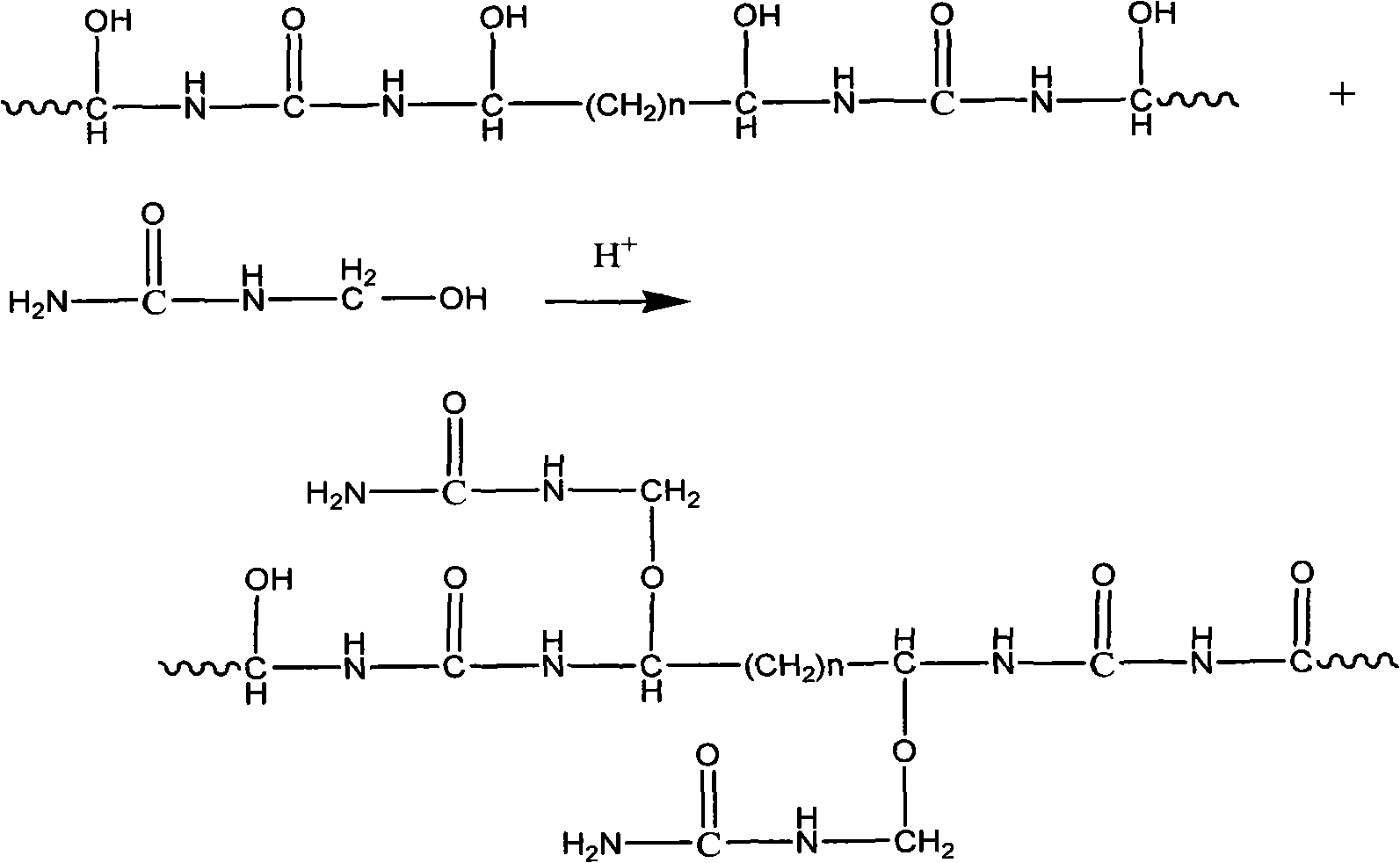
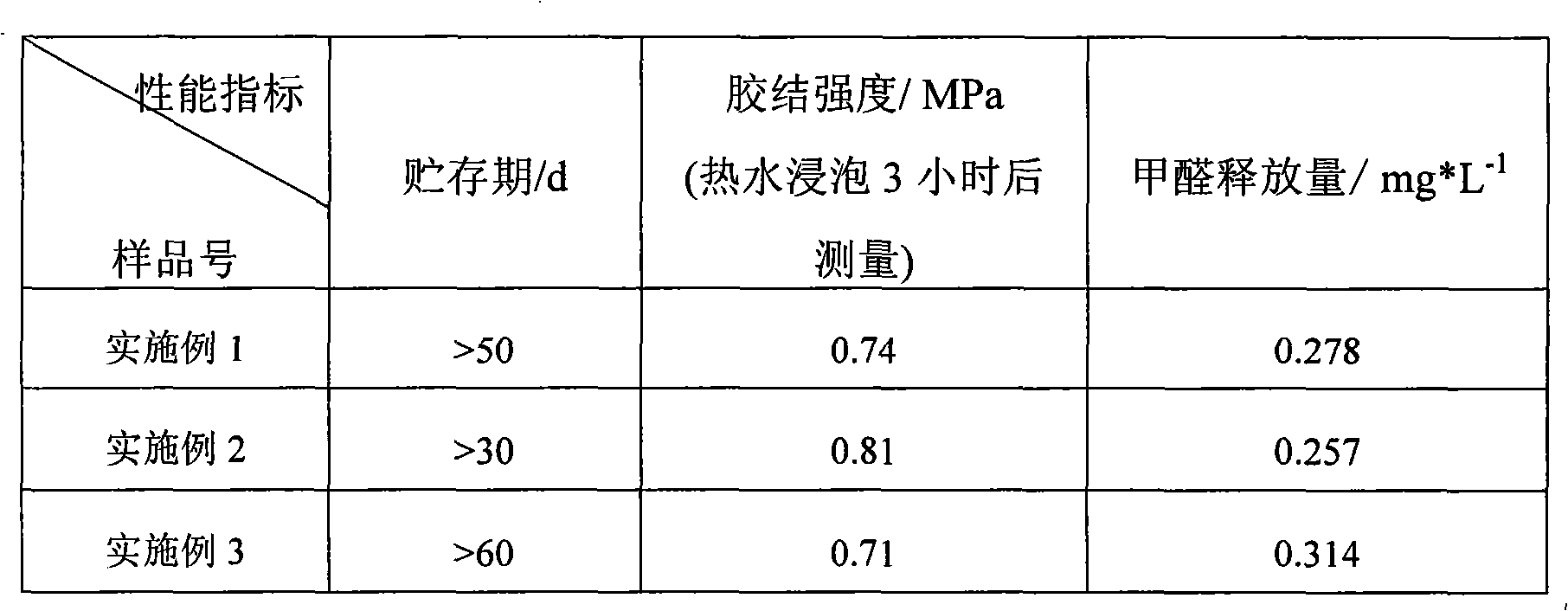
Environmental protection urea-formaldehyde resin and preparation method thereof
InactiveCN101265314ALow free formaldehyde contentExtended shelf lifeAldehyde/ketone condensation polymer adhesivesThermal waterWeak base
A novel environmental protective urea-formaldehyde resin and a preparation method belong to the field of wood processing adhesives. The urea-formaldehyde resin is formed by the reaction of urea, formaldehyde, aldehyde, one or more stabilizers and modifier according to the route of weak base-weak acid-weak base. The method firstly controls the F / U feed ratio, the pH value and the temperature during the reaction process to reduce the content of free formaldehyde in the resin; then the aldehyde is introduced to allow the resin to generate the stable alkyl ether (-(CH2)n-O-(CH2)n-) structure, thus reducing the content of methylene-ether bond (-CH2-O-CH2-) in the resin structure, simultaneously reducing the using amount of the formaldehyde and further greatly reducing the release amount of the formaldehyde during the using process of a plate from the two aspects; in addition, the introduction of a long chain and polyaldehyde can ensure the resin to have great bonding strength and water resistance. The urea-formaldehyde resin which is prepared by the invention has the advantages of low content of free formaldehyde, simple process, low cost, etc., the release amount of the formaldehyde of the plate which is prepared by using the adhesive achieves the E0 level standard, and the high bonding strength can be maintained after being boiled in hot water.
Owner:BEIJING UNIV OF CHEM TECH
Sulfoalkyl ether-alkyl ether cyclodextrin derivatives
ActiveUS20060258537A1Improve propertiesHigh degree of substitutionOrganic active ingredientsBiocideSolubilityCyclodextrin Derivatives
A sulfoalkyl ether-alkyl ether cyclodextrin (SAE-AE-CD) derivative is provided. The SAE-AE-CD possesses advantages over known SAE-CD and AE-CD derivatives as well as over the parent cyclodextrin by being more water soluble and less membrane disturbing. The SAE-AE-CD includes at least one sulfoalkyl ether group and at least one alkyl ether group even though the degree of substitution for the functional groups can be different. The SAE functional group can be present in molar excess over the AE functional group and vice versa. The total degree of substitution of the cyclodextrin, with respect to both functional groups, can be varied such that a minority or a majority of the hydroxyl moieties of the CD are derivatized. The SAE-AE-CD derivative can be used to solubilize compounds with insufficient water solubility. In some cases, they also stabilize compounds in solution against degradation or to solubilize degradation products formed during degradation. In addition, SAE-AE-CD can also be used for other purposes such as osmotic agents, agents used to mask the taste of problematic drugs. Surprisingly, while AE-CDs are known to be toxic by being membrane disturbing, SAE-AE-CDs are less membrane disturbing and therefore have greater safety.
Owner:UNIVERSITY OF KANSAS
Process for carbonylation of alkyl ethers
ActiveUS7465822B2Organic compound preparationPreparation from carboxylic acid esters/lactonesCarboxylic acidCarbonylation
A product comprising a lower alkyl ester of a lower aliphatic carboxylic acid is produced by a process comprising reacting under substantially anhydrous conditions a lower alkyl ether with carbon monoxide in the presence of a zeolite catalyst having an 8-member ring channel which is interconnected with a channel defined by a ring with greater than or equal to 8 members, the 8-member ring having a window size of at least 2.5 Angstroms×at least 3.6 Angstroms and at least one Brønsted acid site and the zeolite having a silica:X2O3 ratio of at least 5, wherein X is selected from aluminum, boron, iron, gallium and mixtures thereof.
Owner:BP CHEM LTD +1
Process for applying fluoropolymer powder coating as a primer layer and an overcoat
InactiveUS20060110601A1Superior and durable adhesionIncrease temperatureSynthetic resin layered productsPackagingTetrafluoroethylenePolymer science
The invention relates to use of a tetrafluoroethylene / perfluoroolefin copolymer applied as a primer powder in conjunction with a powder overcoat of tetrafluoroethylene / perfluoro (vinyl alkyl ether) copolymer, also known as perfluoroalkoxy polymer (PFA), which when baked onto a substrate gives superior and more durable adhesion of the coating system to the substrate. The quality of the adhesion is measured by a boiling water peel test.
Owner:THE CHEMOURS CO FC LLC
Sulfonated copolyetherester compositions from hydroxyalkanoic acids and shaped articles produced therefrom
InactiveUS7193029B2Optimized balance of physical propertyOptimized balanceWrappers shrinkageMonocomponent polyetheresters artificial filamentEtherDicarboxylic acid
The present invention provides certain sulfonated copolyetherester compositions containing hydroxyalkanoic acids and processes for producing such sulfonated aromatic copolyetheresters. The invention further provides shaped articles, preferably in the shape of films, coatings and laminates, having improved thermal properties, wherein the shaped articles are produced from the certain sulfonated copolyetherester compositions. Some of these materials are also biocompostable. The sulfonated copolyetheresters are produced from a mixture of an aromatic dicarboxylic acid component, hydroxyalkanoic acid component, a sulfonate component, a poly(alkylene ether) glycol component, a glycol component, an optional other glycol component, an optional branching agent, and an optional color reducing agent.
Owner:EI DU PONT DE NEMOURS & CO
Processes for recovering oligomers of glycols and polymerization catalyst from waste streams
Processes for recovering and recycling oligomers and polymerization catalyst from aqueous waste streams generated during the production of polyalkylene ether glycols are provided. The processes utilize membranes; preferably reverse osmosis or nanofilter membranes. The processes can reduce waste and costs associated with the polymerization of polyalkylene ether glycols by recovery and recycling of glycols and catalysts
Owner:SUNKARA HARI BABU +2
Liquid Compositions Containing Urease Inhibitors and Glycol Alkyl Ethers and Methods of Making a Use Thereof
ActiveUS20140047883A1Agriculture gas emission reductionUrea compound fertilisersSolubilityNitrification inhibitors
The present invention provides improved solvent systems for the preparation of liquid formulations of urease or nitrification inhibitors, specifically NBPT, comprising alkylene glycol alkyl ethers. The solvent systems provided good solubility of the urease inhibitor as well as at least one of improved stability, lower flammability, lower toxicity, improved cold temperature storage, improved handling, improved adsorption onto and / or solubility with solid media such as UFP or urea. Methods of making and using the compositions are also provided.
Owner:KOCH AGRONOMIC SERVICES LLC
Process for carbonylation of alkyl ethers
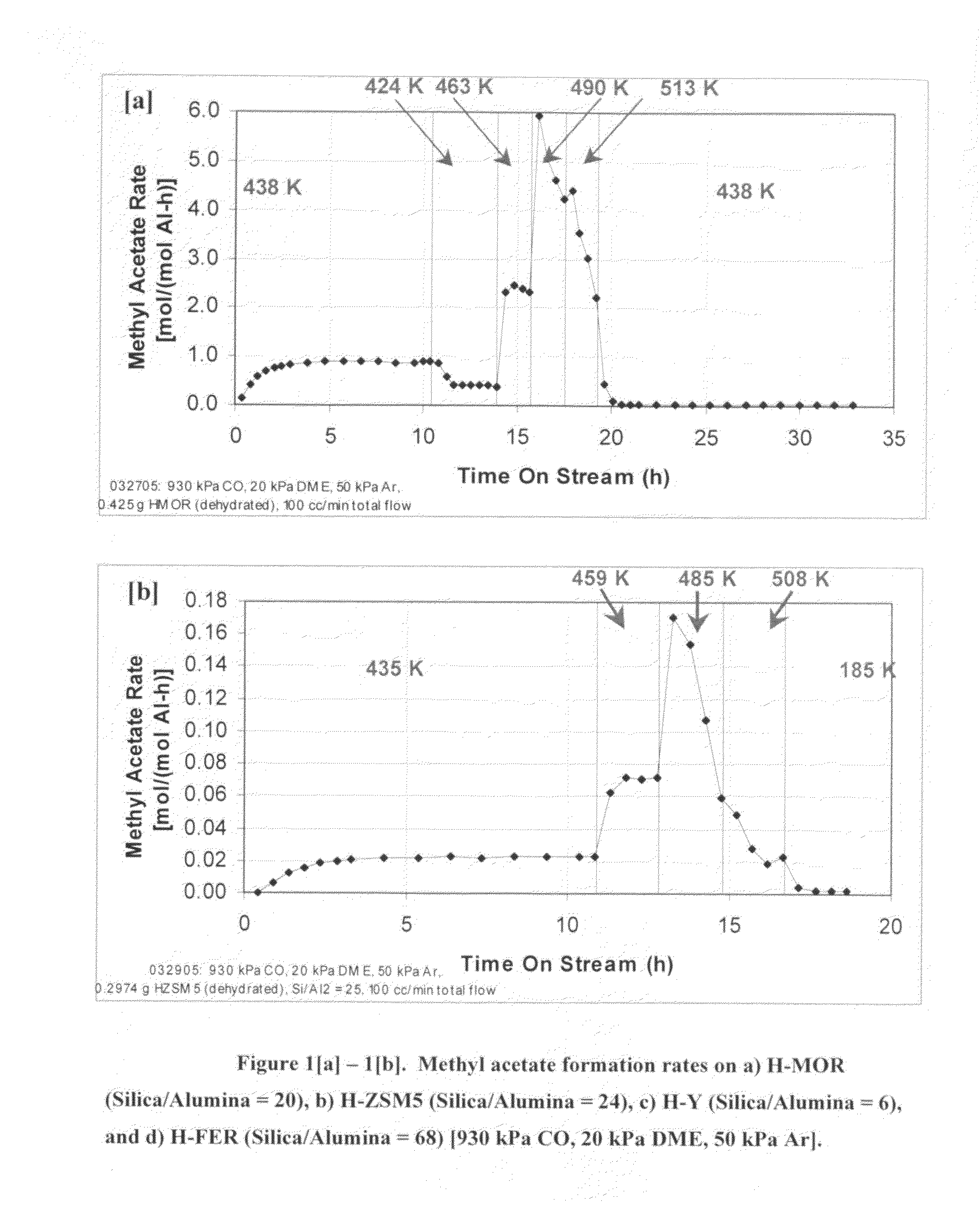
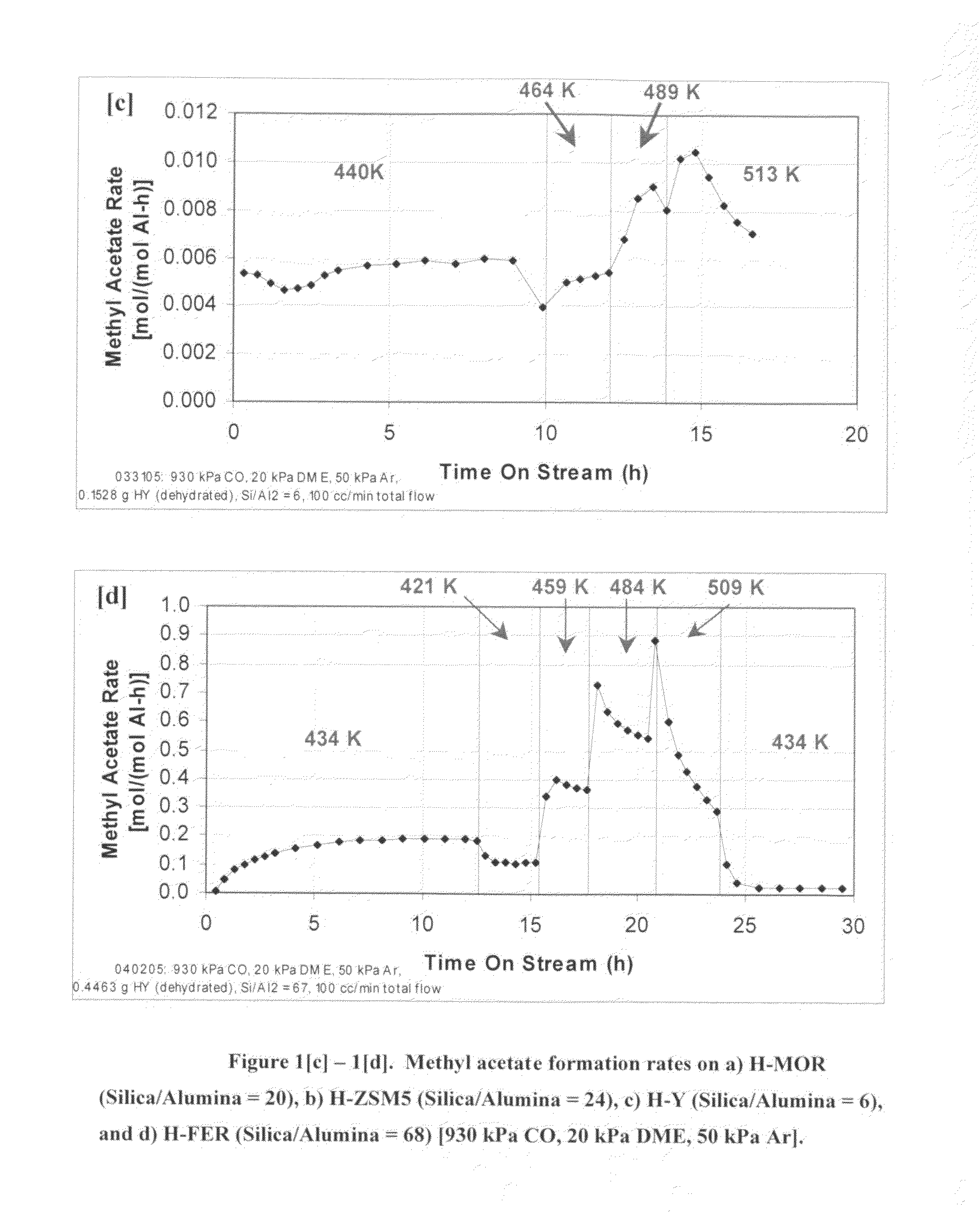
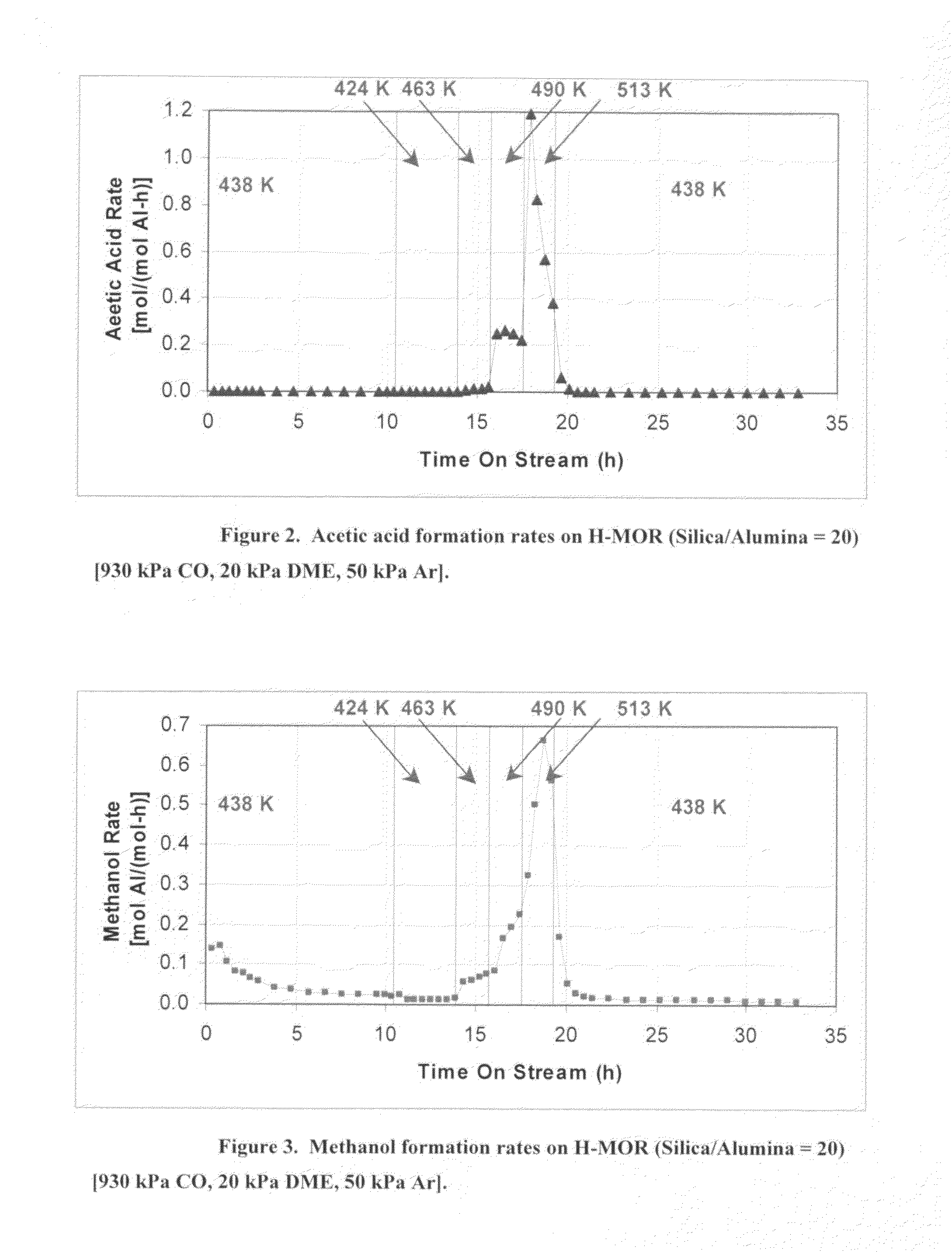
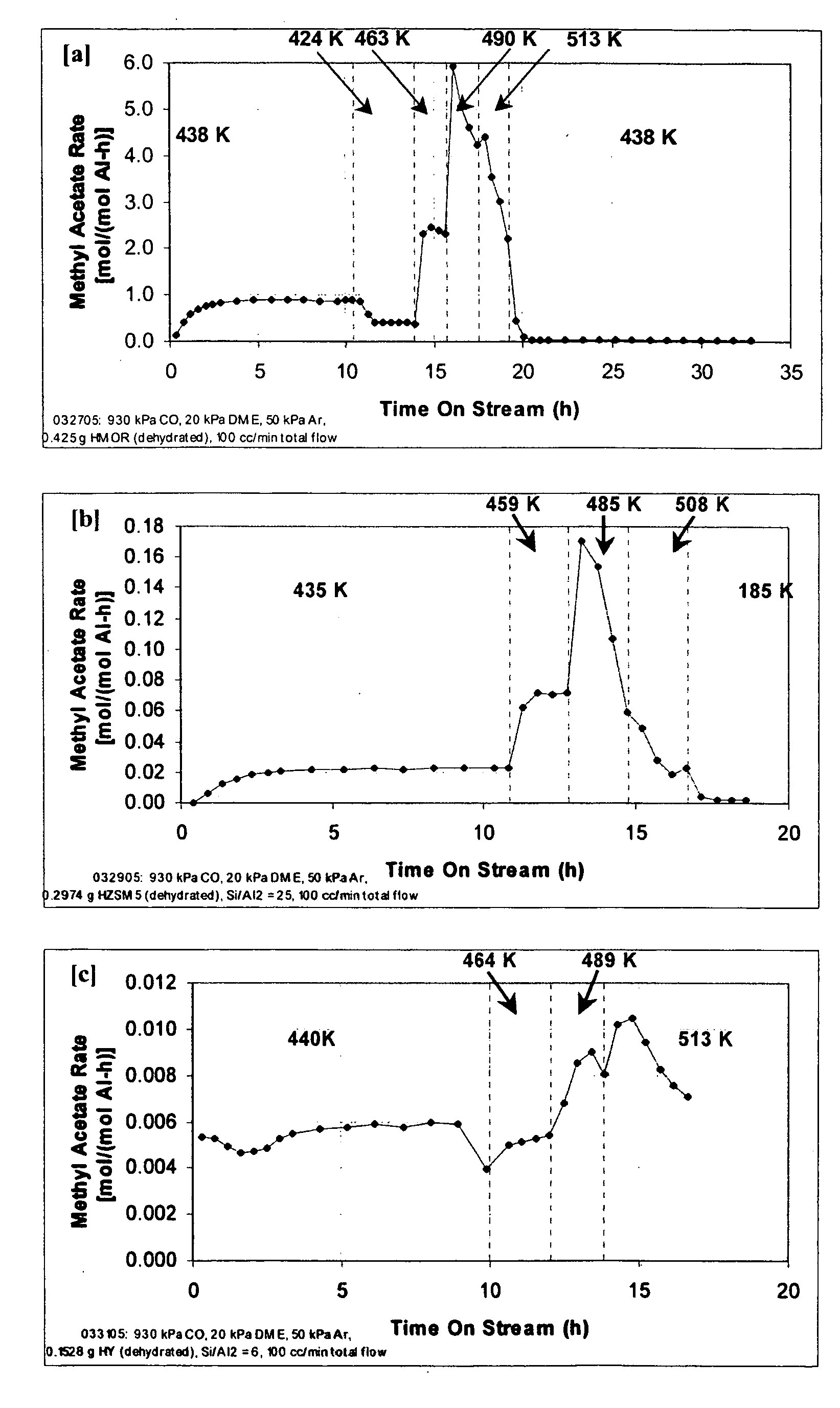
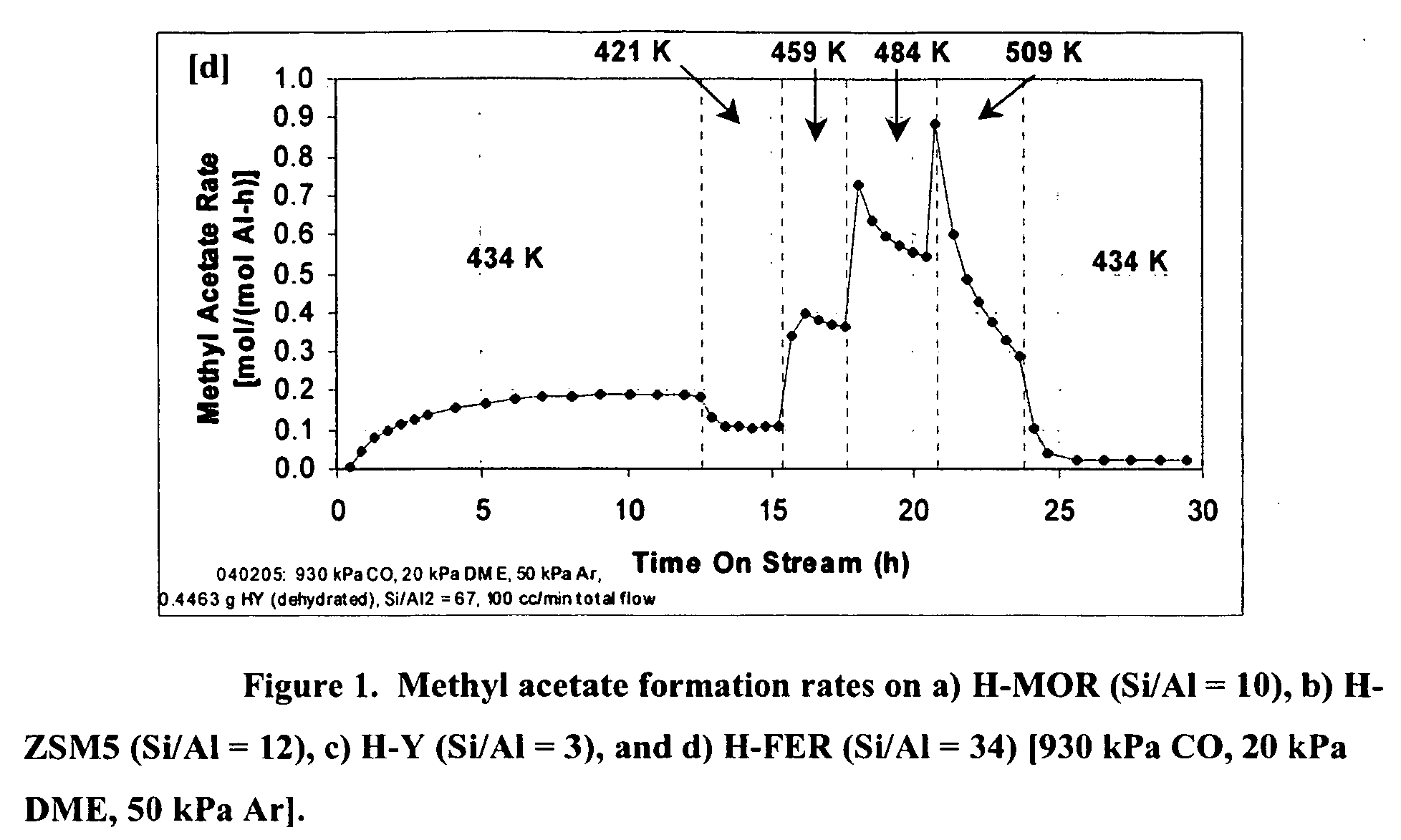
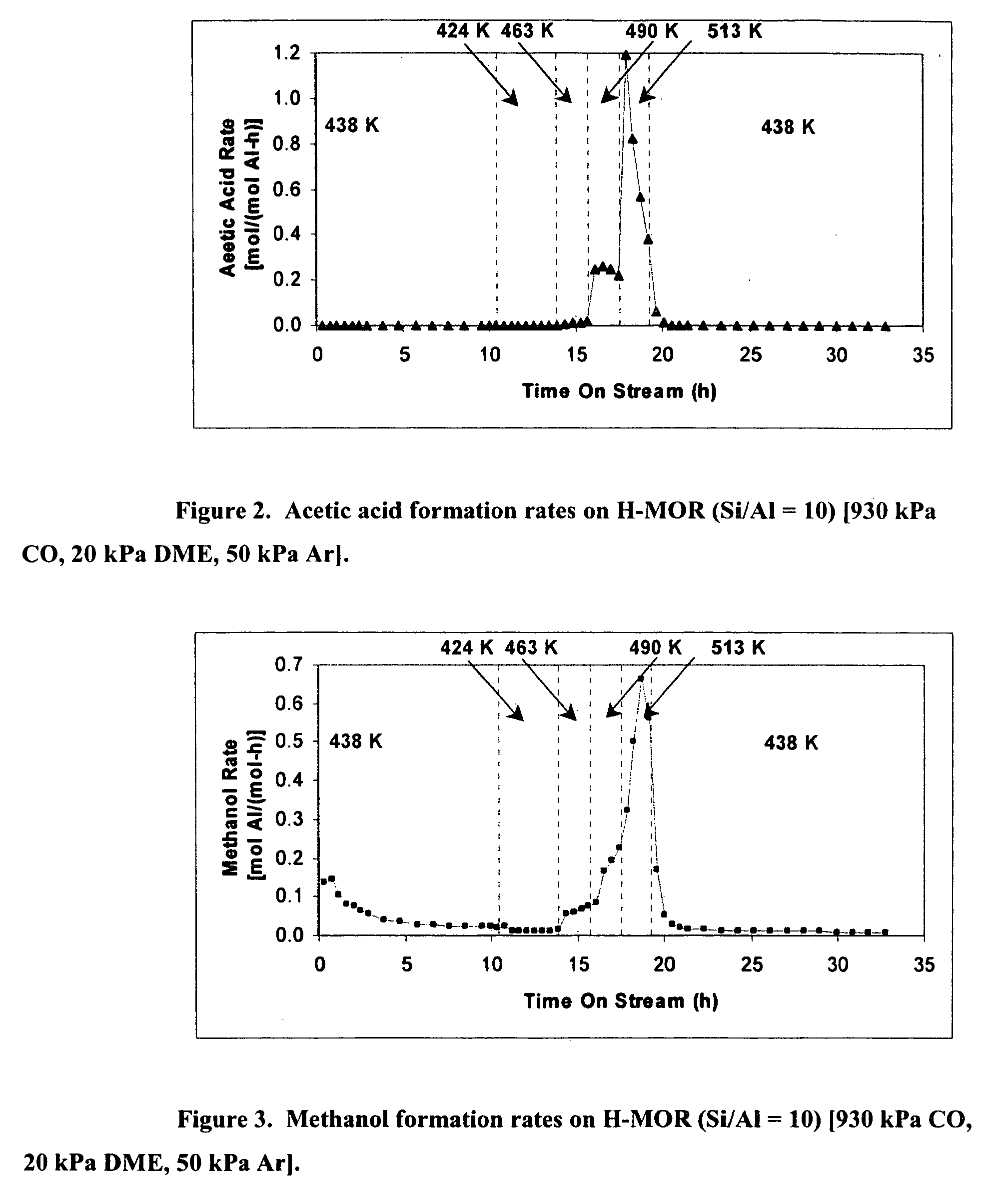
InactiveUS20060252959A1Organic compound preparationPreparation from carboxylic acid esters/lactonesMordeniteMethyl acetate
A product comprising a lower alkyl ester of a lower aliphatic carboxylic acid is produced by a process comprising reacting a lower alkyl ether with carbon monoxide in the presence of a catalyst comprising mordenite and / or ferrierite, under substantially anhydrous conditions. More specifically, methyl acetate is selectively produced by reaction of dimethyl ether with carbon monoxide in the presence of a catalyst comprising mordenite or ferrierite, under substantially anhydrous conditions.
Owner:RGT UNIV OF CALIFORNIA +1
Gel laundry detergent composition
InactiveUS7297674B2Improve clarityHigh transparencySoap detergents with organic compounding agentsNon-ionic surface-active compoundsActive agentPolypropylene glycol
The present invention provides a shear thinning, transparent gel laundry detergent composition, comprising a surfactant system containing surfactant material selected from an anionic surfactant, a nonionic surfactant or a mixture thereof, and from 0.1 to 10% by weight of a clarity improving agent being a glycol dialkyl ether selected froma mono-or polyethylene glycol dialkyl ether having the formula(CpH2p+1)O—(CH2CH2O)n—(CqH2q+1), (I)a mono- or polypropylene glycol dialkyl ether having the formula(CpH2p+1)O—(CH2CH (CH3)O)n—(CqH2q+1), (II) and mixtures thereof,wherein p and q independently are integers in the range of from 1 to 5, and n is an integer in the range of from 1 to 50, preferably 1 to 10. It has been found that this gel laundry composition is highly transparent, such that particles can be suspended therein for improving visual appearance.
Owner:HENKEL IP & HOLDING GMBH
Process for the purification of gases
InactiveUS6277345B1Improve efficiencyIncreased gas throughputHydrogenOrganic chemistryPoly(ethylene glycol) dimethyl etherHydrogen
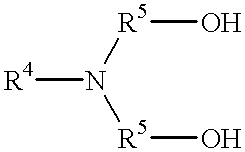
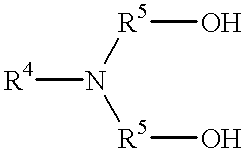
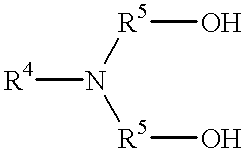
The present invention relates to the use of an absorption liquid for purifying a gas by removal of gaseous, acidic impurities. The gas to be purified can be any gas, such as synthesis gas or natural gas, which contains gaseous, acidic impurities such as CO2, H2S, SO2, CS2, HCN, COS or mercaptans. The absorption liquid comprises:A) from 0.01 to 4% by weight of at least one compound of the formulaB) from 0.001 to 8.0% by weight of water, andC) at least one polyalkylene glycol alkyl ether of the formulato 100% by weight,whereR1 is C1-C4-alkyl,R2 is ethylene or 2-methylethylene,R3 is hydrogen or C1-C4-alkyl,R4 is hydrogen or C1-C4-alkyl,R5 is C1-C4-alkylene andX is an integer from 1 to 10. The amine may be N-methyldiethanolamine and the ether may be polyethylene glycol dimethyl ether.
Owner:CLARIANT PROD DEUT GMBH
Lithographic printing plate developing compositions
InactiveUS20100216067A1Solve the real problemDiluted working strength developersSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusCarboxylic acidFatty alcohol
A concentrated developer can be prepared with less than 60 weight % water and still remain in a single phase with little or no haze or precipitation. This developer concentrate also includes a water-soluble or water-miscible organic solvent, one or more alkyl ether carboxylic acid, coconut oil alkanolamine, coconut fatty alcohol polyglycol ether, β-naphtholethoxylate, and block propylene oxide-ethylene oxide in an amount of at least 0.1 and up to 50 weight % solids, and optionally an alkyl naphthalene sulfonate in an amount of up to 40 weight % solids. The developer concentrate can be diluted up to 80:1 or greater with water and used to process imaged lithographic printing plate precursors.
Owner:EASTMAN KODAK CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com