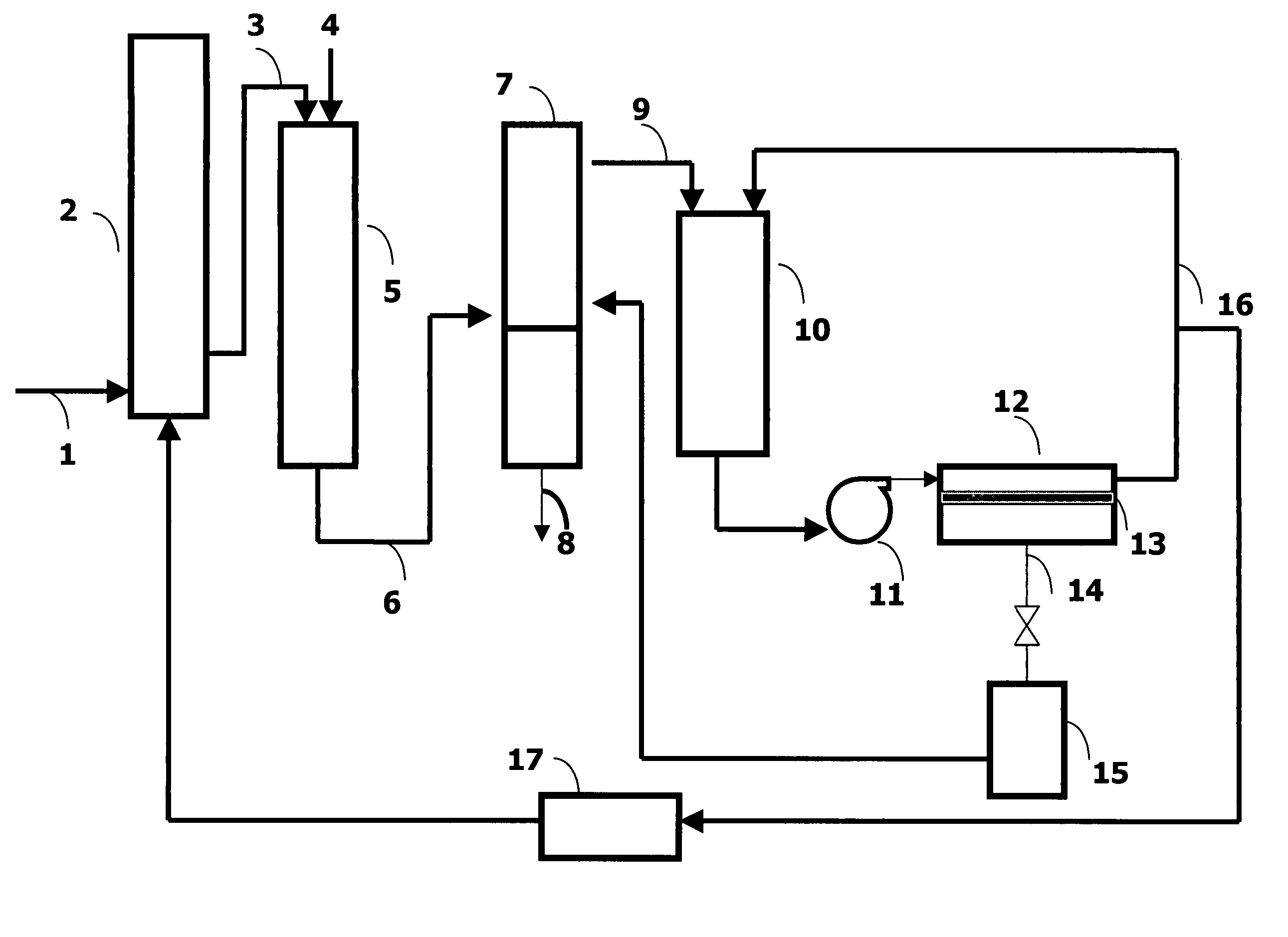
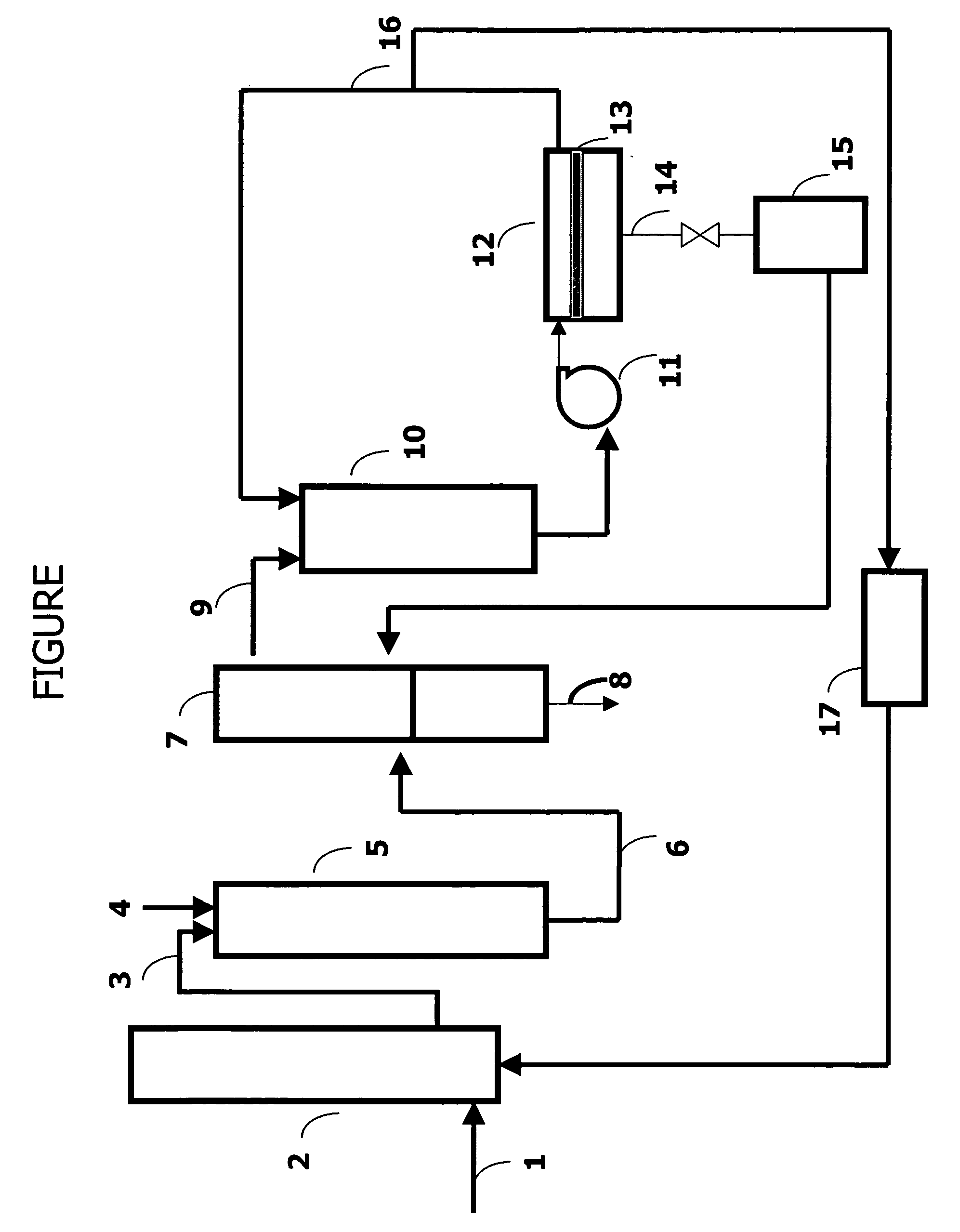
Processes for recovering oligomers of glycols and polymerization catalyst from waste streams
a polymerization catalyst and glycol technology, applied in the direction of reverse osmosis, organic chemistry, ether preparation, etc., can solve the problems of high maintenance cost, high capital cost, and reduced polymer yield,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Feed Preparation
[0060] 1,3-Propanediol, 13.9 kg, and 139 g concentrated sulfuric acid were added to a 22-L glass reactor and the contents polymerized at 160° C. under nitrogen until the number average molecular weight was approximated 1700. A portion (1.9 kg) of the crude polymer was transferred to a 5-L glass reactor with 1.9 kg of distilled water and the reaction mixture stirred slowly under a nitrogen blanket while heated to 100° C. After 4 hours, the heater and the stirrer were turned off and the mixture was allowed to separate into two phases by gravity. The aqueous phase was separated from the polyether phase by decantation. Distilled water (1.9 kg) was again added to the polymer phase and the reaction mixture was heated to 50° C. and then cooled. After separation into clear phases, the aqueous phase was removed from the polymer phase. The above procedure was repeated four more times and all of the collected aqueous phases were combined. A total of 5.5 gallons (20.8 L) of aq...
example 2
[0063] A FILM TEC SW30-2521 Reverse Osmosis membrane element was used and characterized as in Example 1 prior to testing, using sodium chloride solution and the procedure of Test Method 2, above. The goal was a rejection of greater than 98.5%. The 8 L feed solution was prepared as described in Example 1 except the number of water washing steps were less, resulting in higher oligomer content. This feed solution was added to the feed tank, and circulated and concentrated in the same way. For this test the material was concentrated only to (1.31×) factor and the operating pressure was 64 psig (545 kPa). The membrane separation conditions and the results are summarized and compared in Tables 2 and 3.
example 3
[0064] A DESAL DK-2540-1072 nanofilter membrane element was used and characterized as in Example 1 prior to testing, but using magnesium sulfate solution instead of the sodium chloride solution as in Test Method 2, above. The goal was a rejection of greater than 99%. The procedure was similar to Example 1, with 15.7 L of feed solution containing crude prepared as in Example 1 added to the feed tank, and circulated and concentrated in the same way. For this test the material was concentrated to (8.07×) factor and the operating pressure was 400 psig (2860 kPa). The membrane separation conditions and the results are summarized and compared in Tables 2 and 3.
TABLE 2Membrane Separation Summary Results from Examples 1-3Membrane typeSWHR-2540ASW30-2521-ADK-2540-1072Example123Pressure, psig (kPa)400 (2860)64 (543)400 (2860)Total volume5.5 gal (20.8 L)8.0 L15.7 LCollected permeate4.5 gal (17.0 L)1.9 L13.8 LConcentration factor5.5 X1.31 X8.0 XTotal acid in feed, g57.520.8837.6Total acid in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com