Process for carbonylation of alkyl ethers
a technology of alkyl ether and carbonylation process, which is applied in the preparation of carboxylic acid esters/lactones, chemistry apparatus and processes, and organic chemistry, etc., can solve the problems of low production of iodine-containing byproducts, and none of them have been commercialized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
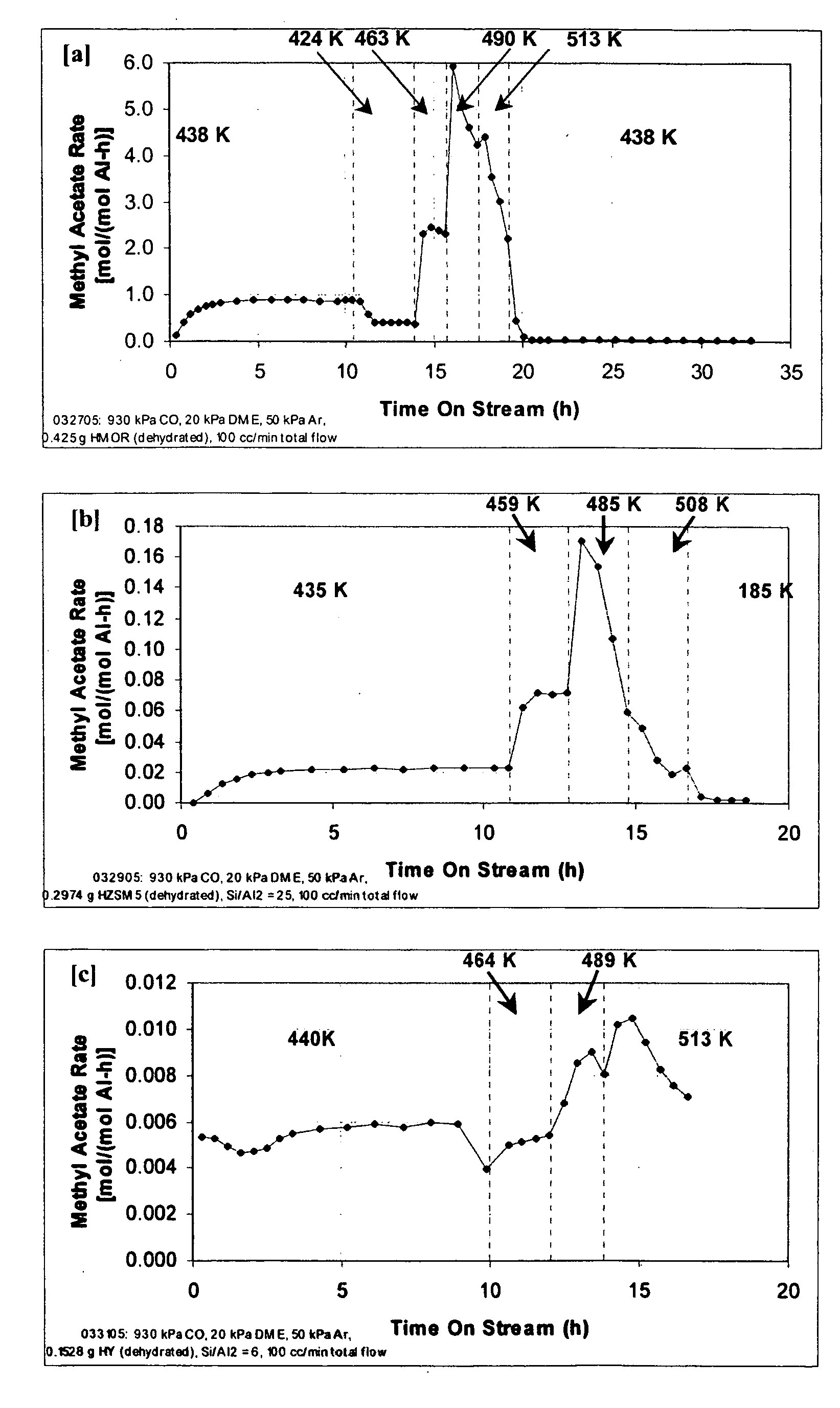
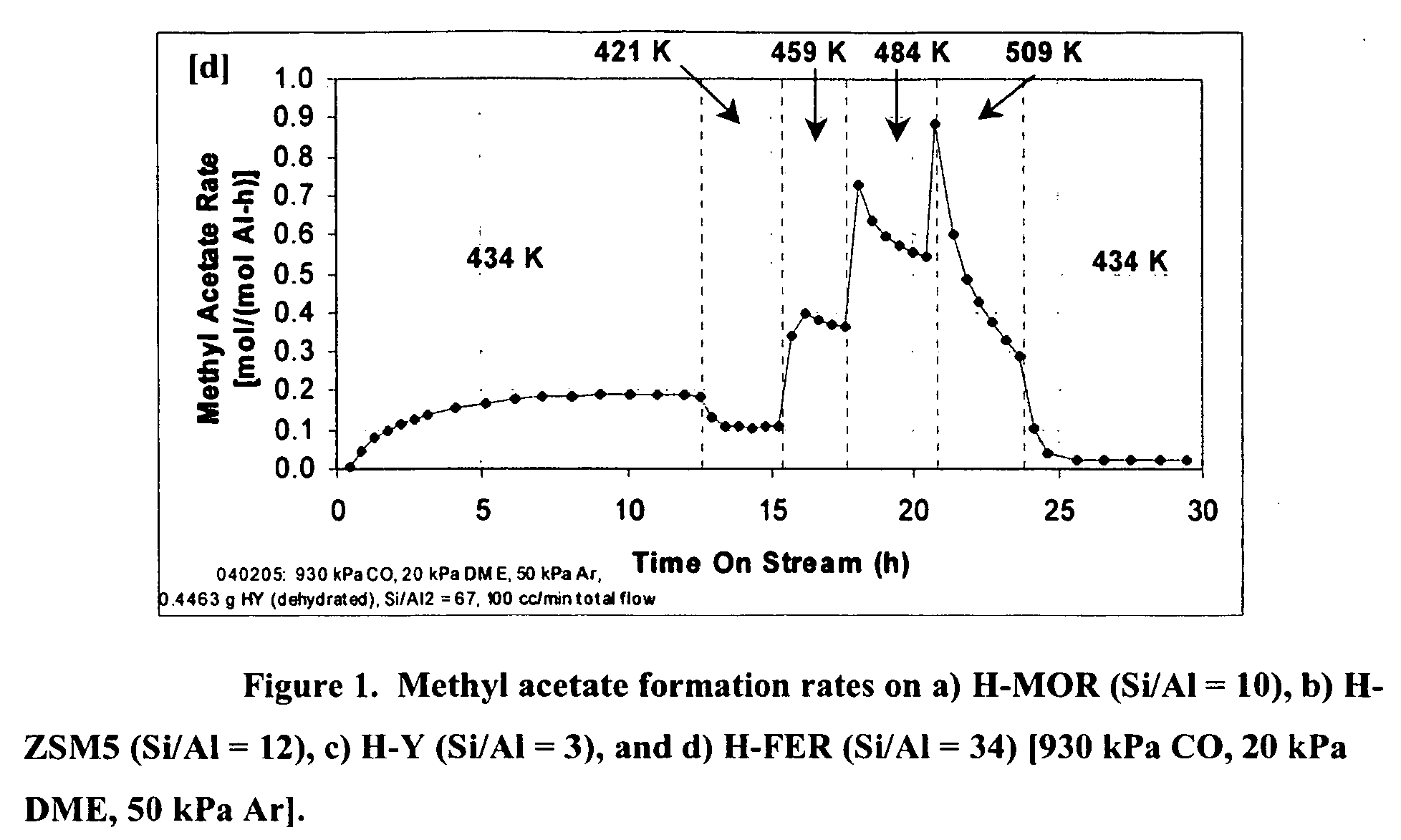
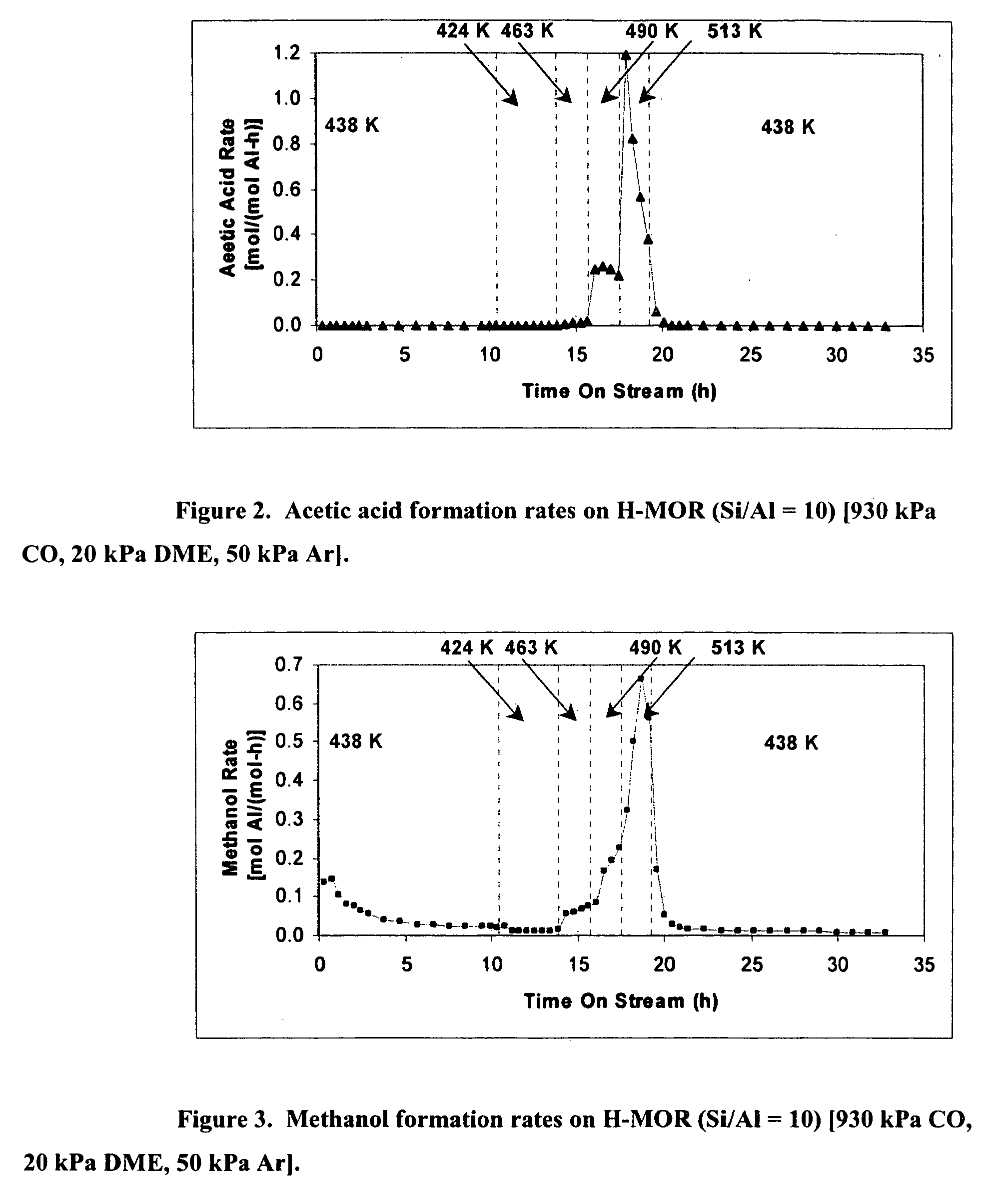
[0018] In brief, this invention comprises a process for producing a product comprising a lower alkyl ester of a lower aliphatic carboxylic acid comprising reacting a lower alkyl ether with carbon monoxide in the presence of a catalyst comprising mordenite or ferrierite, under substantially anhydrous conditions.
[0019] More specifically, the invention herein comprises a process for producing methyl acetate by reaction of dimethyl ether with carbon monoxide in the presence of a catalyst comprising mordenite or ferrierite, under substantially anhydrous conditions.
[0020] One component of the feed to the process comprises (primarily) a lower alkyl ether, that is, a compound having the formula
R1—O—R2
in which R1 and R2 are independently C1-C6 alkyl groups or R1+R2 together form a C2-C6 alkylene group. The total number of carbon atoms in groups R1 and R2, if R1 and R2 are alkyl groups, is from 2 to 12, preferably from 2 to 8, most preferably from 2 to 6. Preferably, R1 and R2 are straig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com