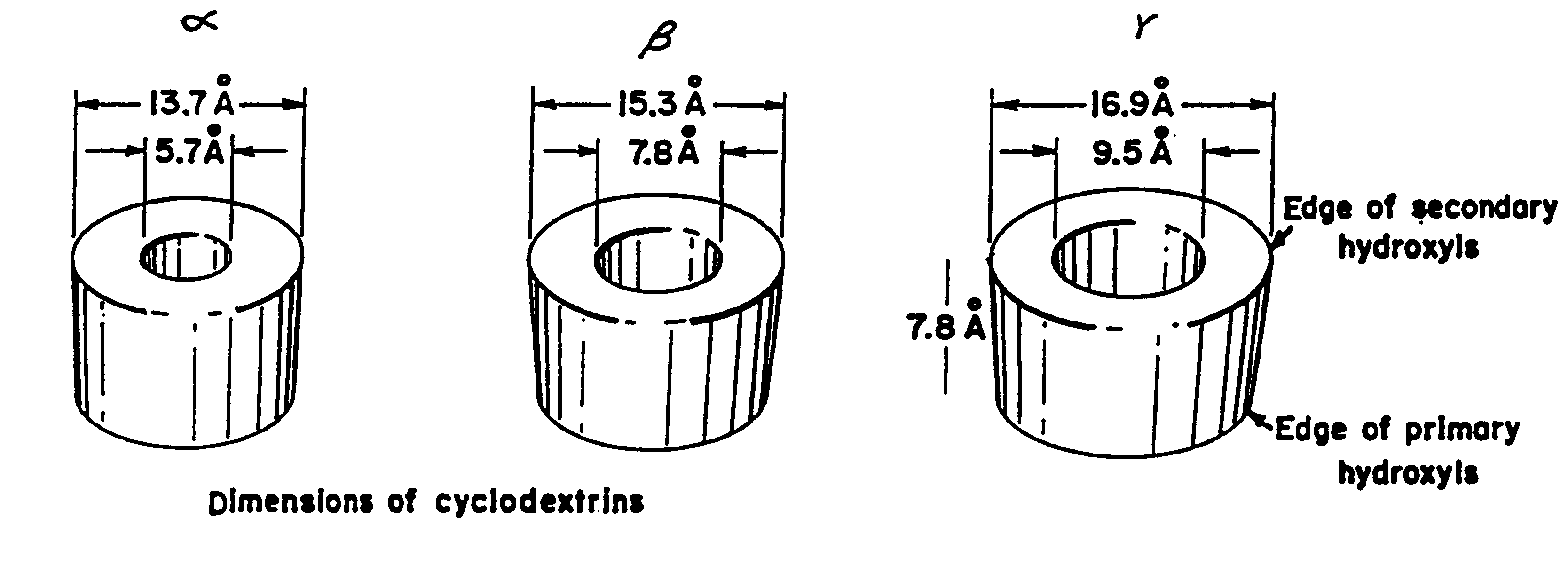
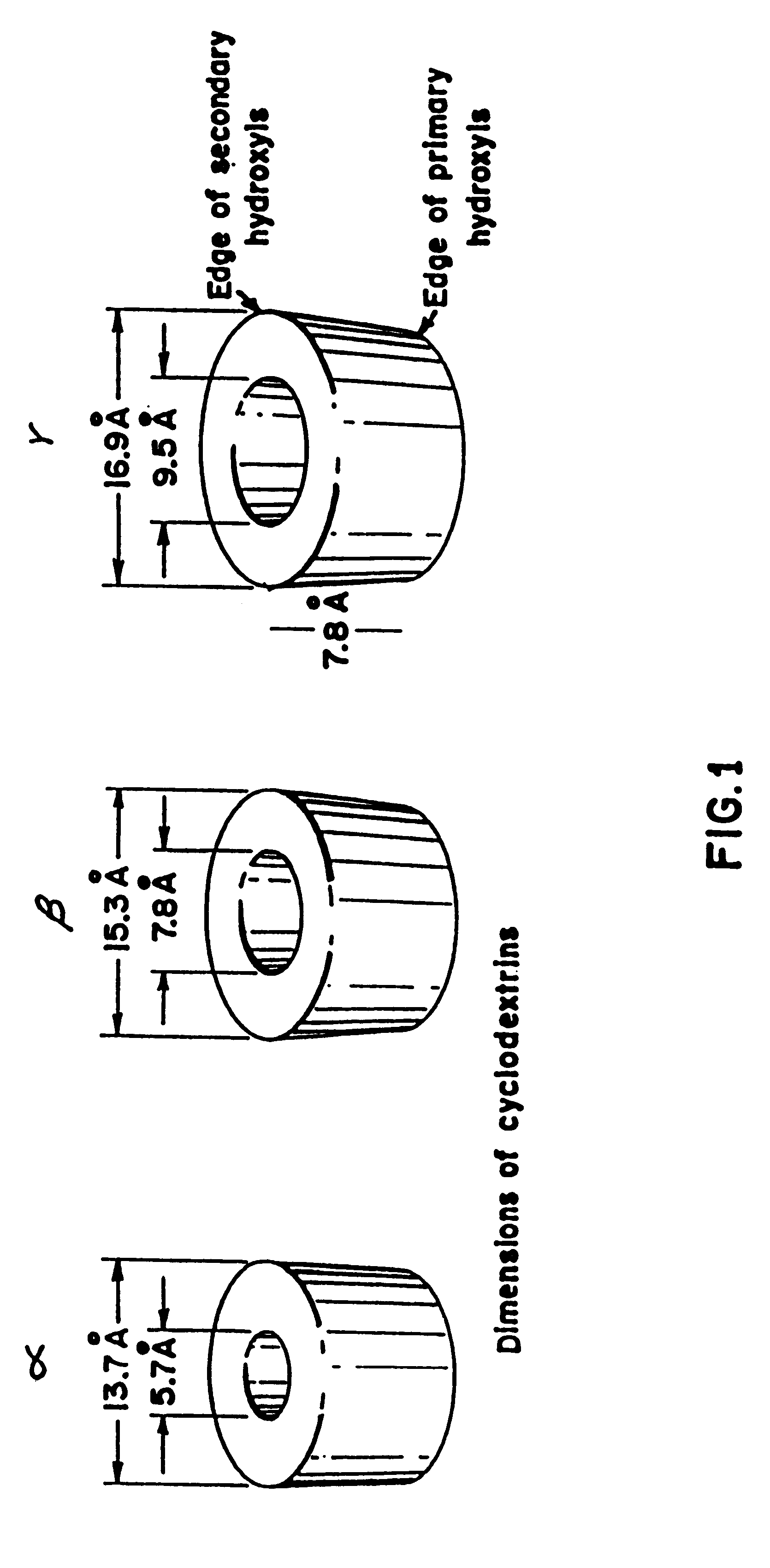
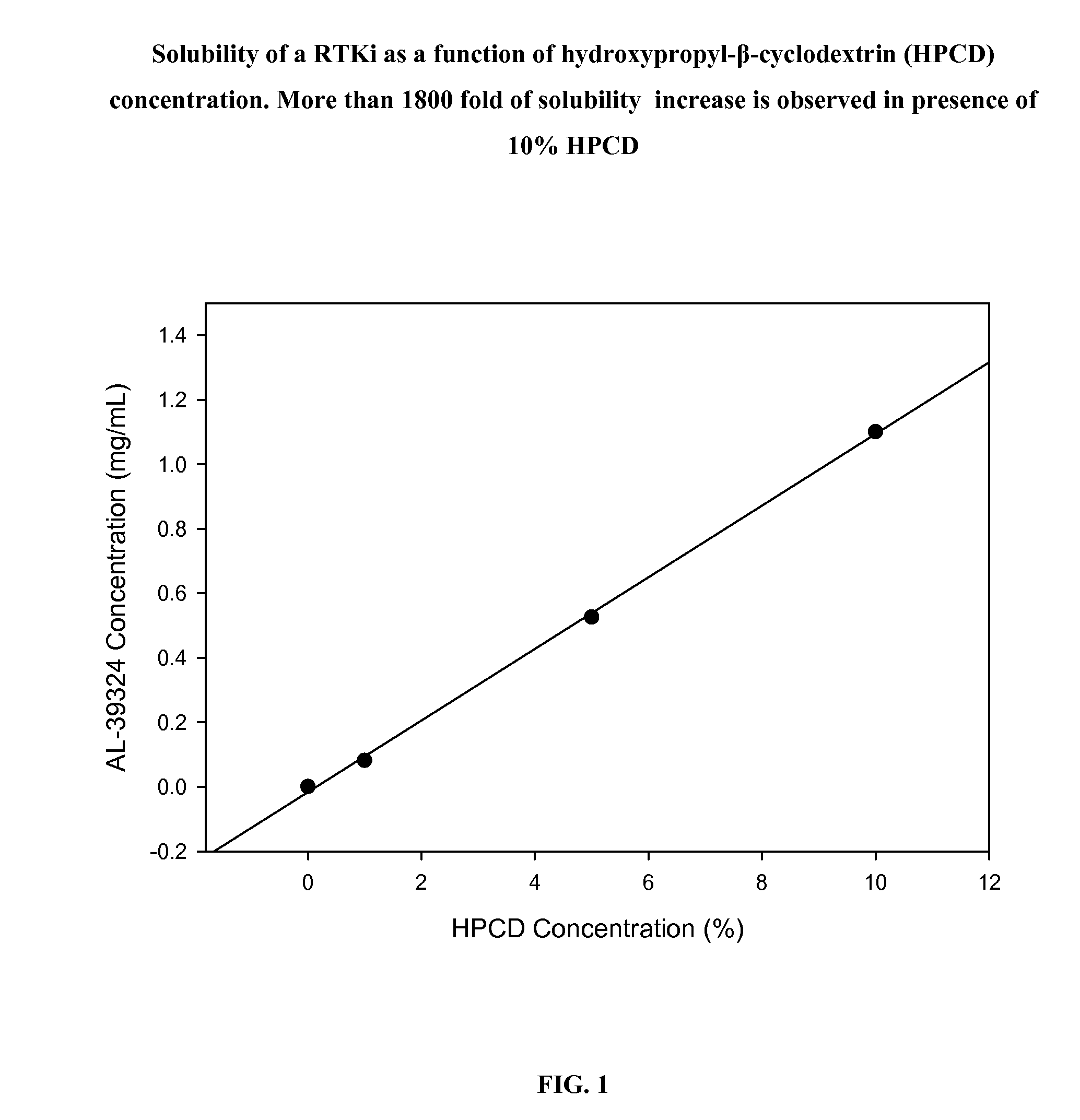
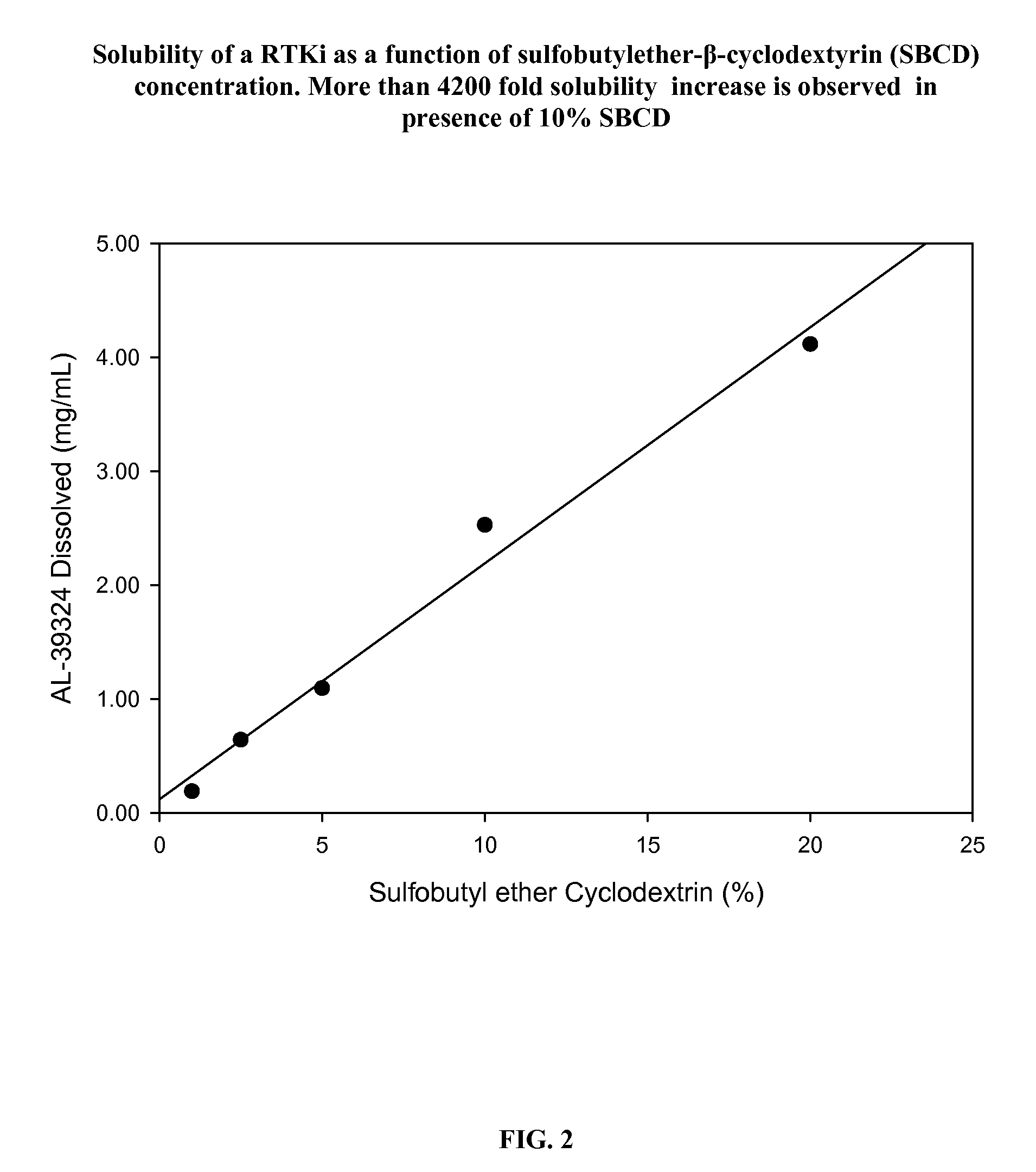
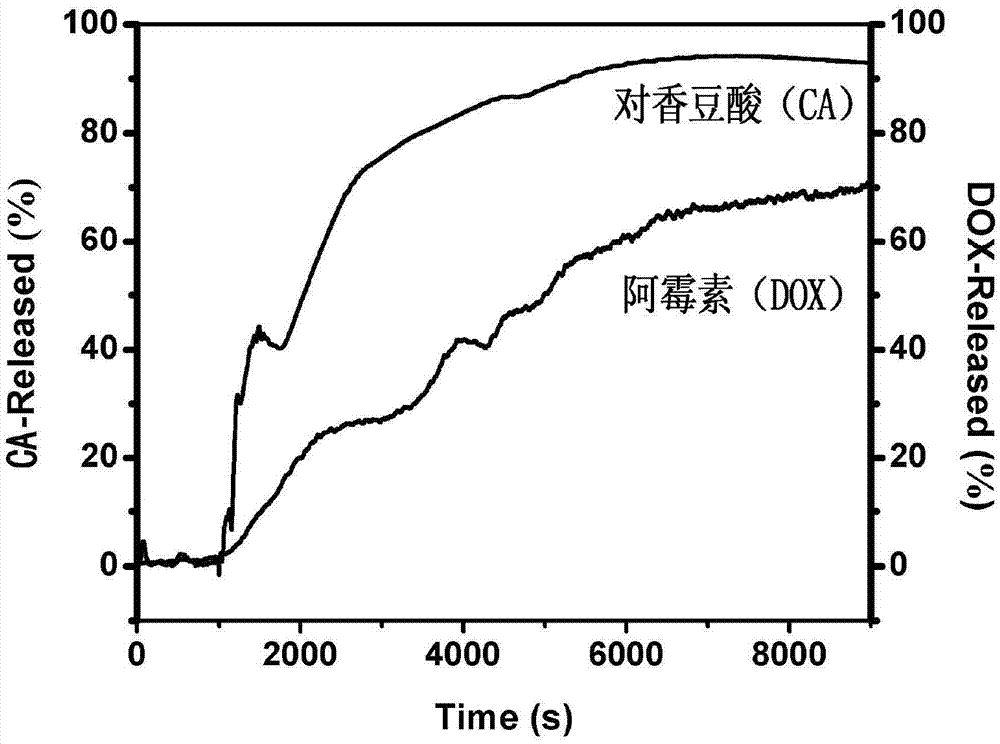
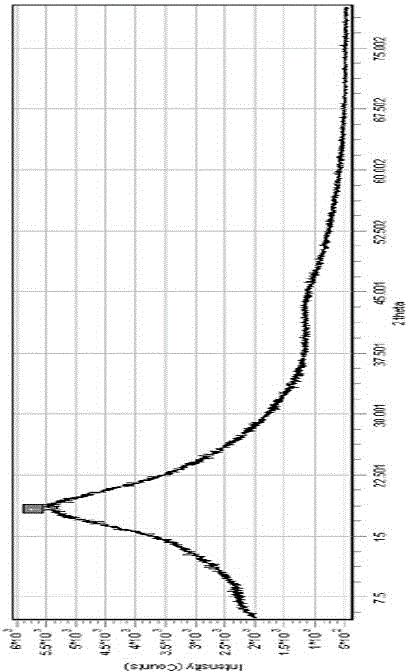
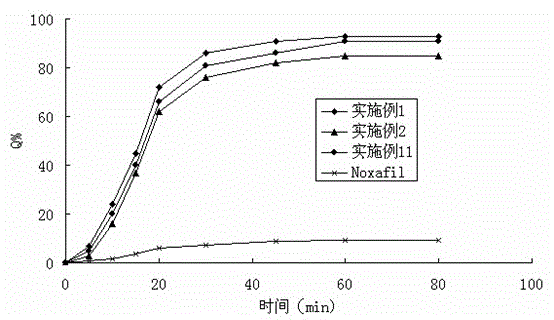
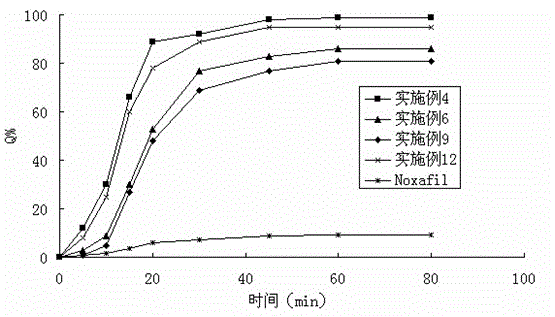
Patents
Literature
325 results about "Cyclodextrin Derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclodextrin solubilizers for liquid and semi-solid formulations
InactiveUS20060105045A1Reduce capacityImprove solubilityPowder deliveryBiocideSolubilityOrganic solvent
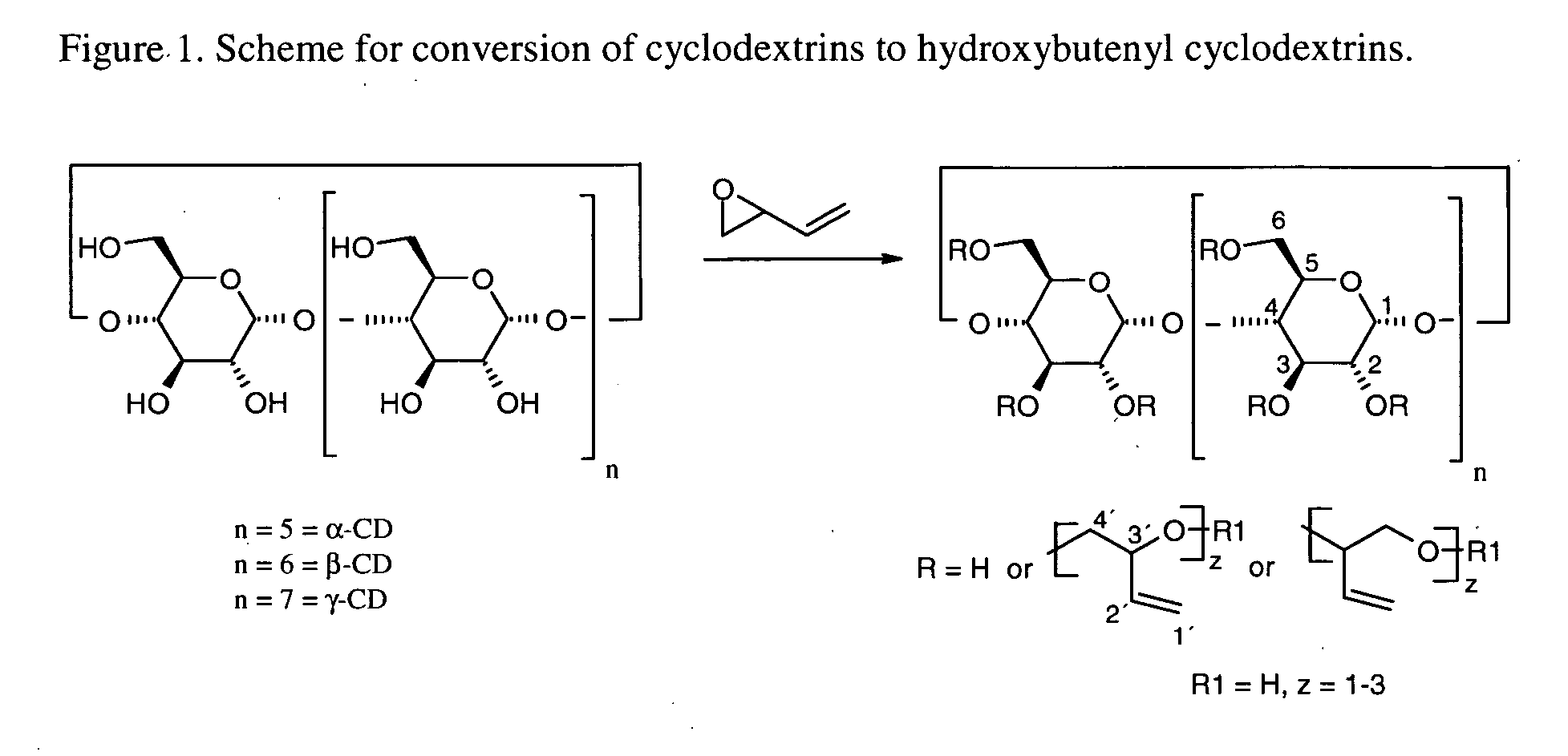
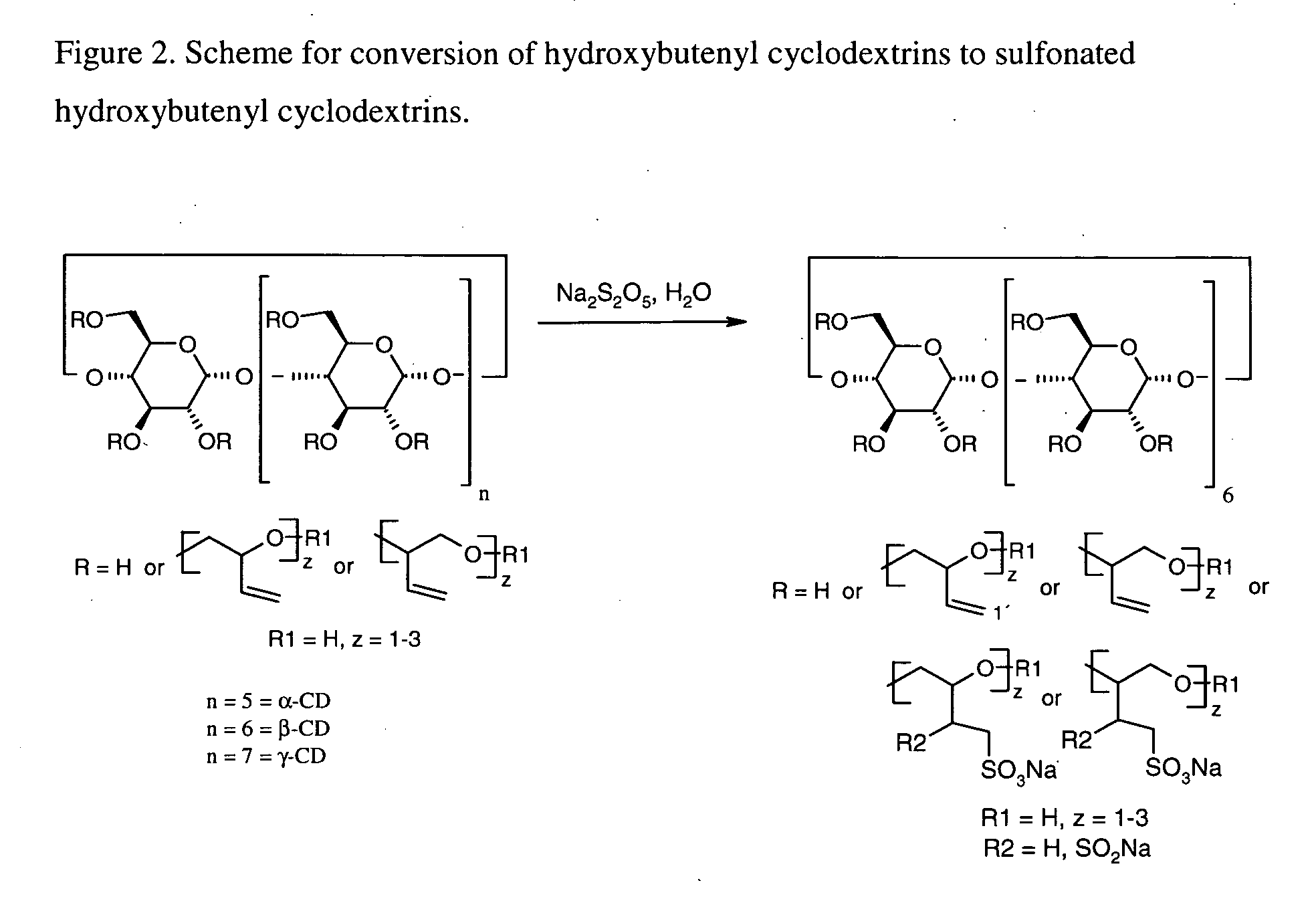
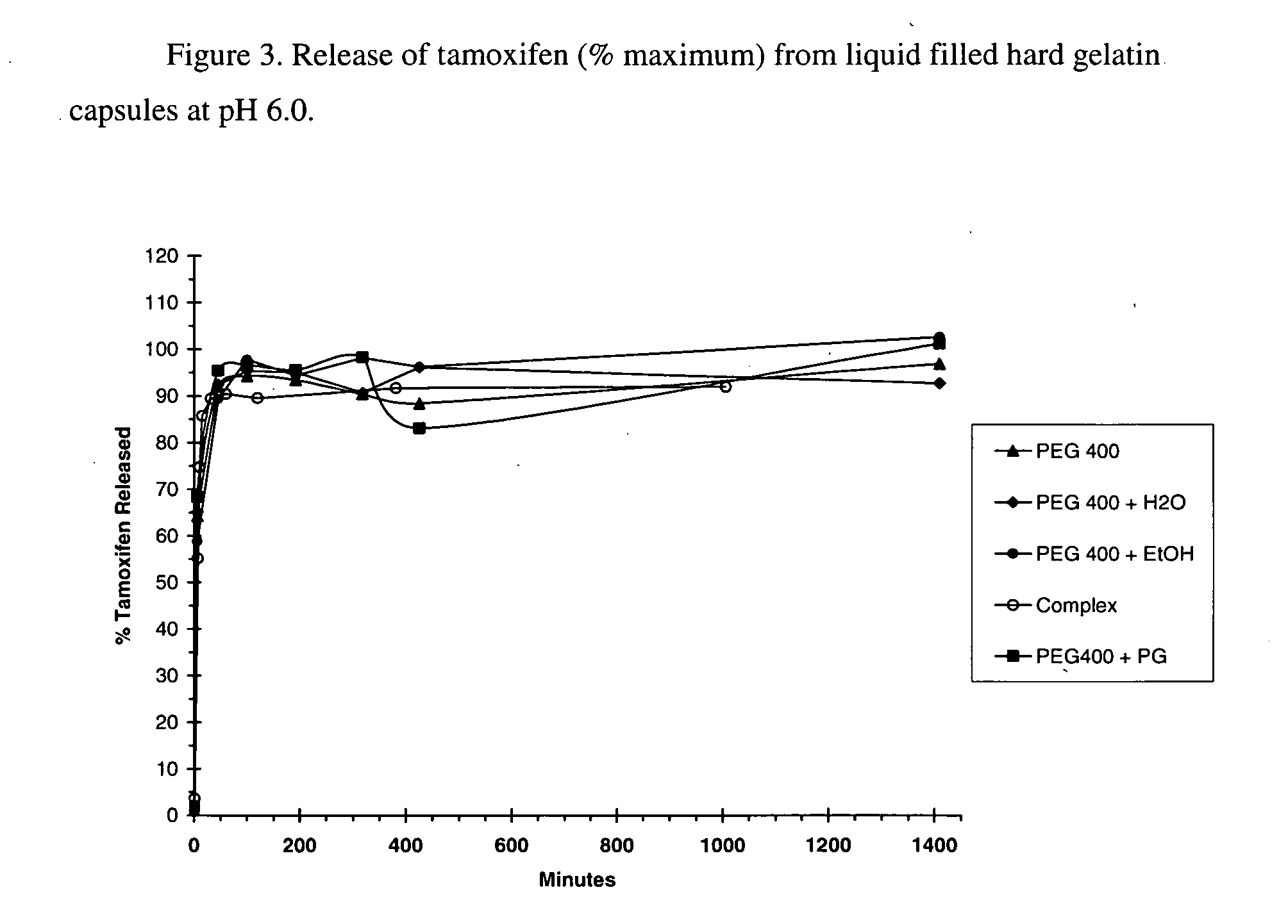
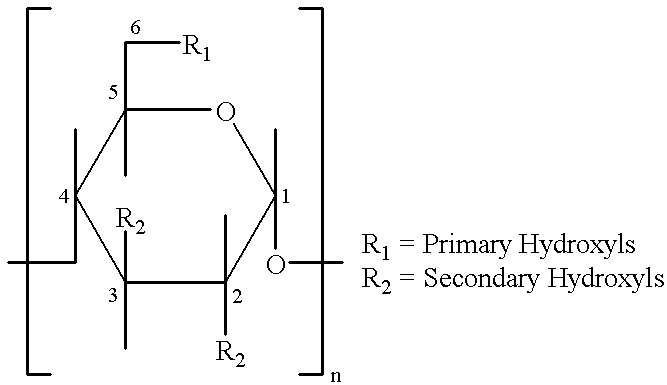
This invention is directed to compositions comprising a water-miscible organic solvent or solvent mixture and one or more cyclodextrin derivatives soluble in the organic water-miscible organic solvent or solvent mixture. In some embodiments, the composition comprises one or more compounds. The invention is further directed to capsules containing the compositions of the present invention and the administration of pharmaceutically active compounds or molecules to subjects. The invention also includes methods of enhancing the solubility of a compound comprising forming a complex or mixture of a compound with a hydroxybutenyl cyclodextrin and a water-miscible solvent.
Owner:EASTMAN CHEM CO
Barrier material comprising a thermoplastic and a compatible cyclodextrin derivative
InactiveUS6218013B1Easy to derivatizeImprove surface propertiesSemi-permeable membranesFlexible coversThermoplasticMoisture
A barrier film composition can comprise a thermoplastic web comprising a thermoplastic polymer and a dispersed cyclodextrin composition having substituents that compatibilize the cyclodextrin in the film. The thermoplastic / cyclodextrin film obtains substantial barrier properties from the interaction between the substituted cyclodextrin in the film material with a permeant. The substituents on the cyclodextrin molecule causes the cyclodextrin to be dispersible and stable in the film material resulting in an extrudable thermoplastic. Such materials can be used as a single layer film material, a multilayer film material which can be coated or uncoated and can be used in structural materials wherein the thermoplastic is of substantial thickness resulting in structural stiffness. The cooperation between the cyclodextrin and the thermoplastic polymer provides barrier properties to a web wherein a permeant can be complexed or entrapped by the cyclodextrin compound and held within the film preventing the permeant from passing through the film into the interior of a film, an enclosure or container. The permeant can comprise a variety of well known materials such as moisture, aliphatic or aromatic hydrocarbons, monomer materials, off flavors, toxic compounds etc.
Owner:CELLRESIN TECH
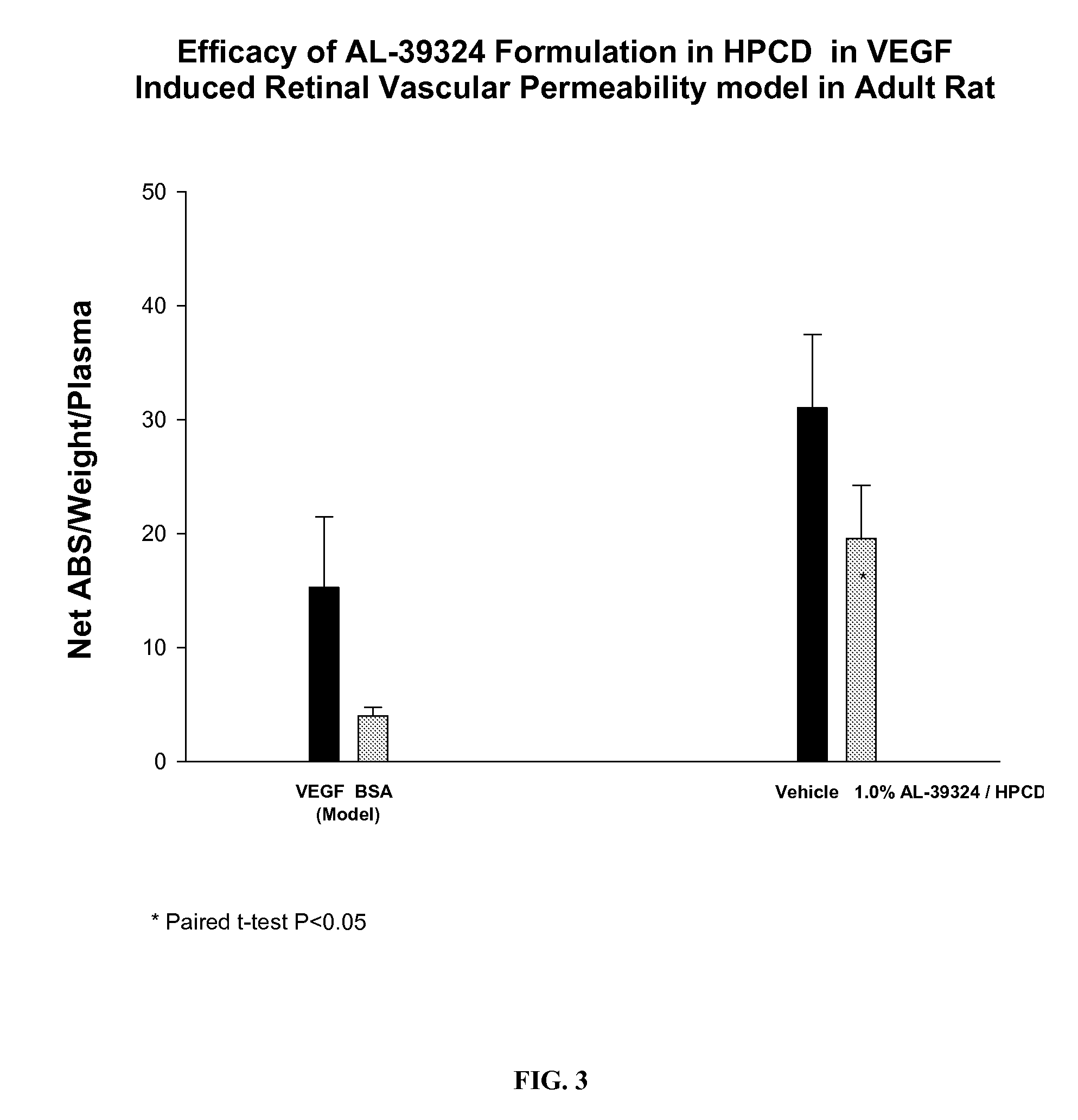
PHARMACEUTICAL COMPOSITION FOR DELIVERY OF RECEPTOR TYROSINE KINASE INHIBITING (RTKi) COMPOUNDS TO THE EYE
InactiveUS20070149480A1Successfully solubilizeBio availability of the drug can be modulatedBiocideNanomedicineCompound (substance)Cyclodextrin derivative
The present invention relates to development of efficacious pharmaceutical compositions comprising an anti-angiogenic compound in a therapeutically effective amount complexed with or encapsulated in a cyclodextrin derivative.
Owner:ALCON INC
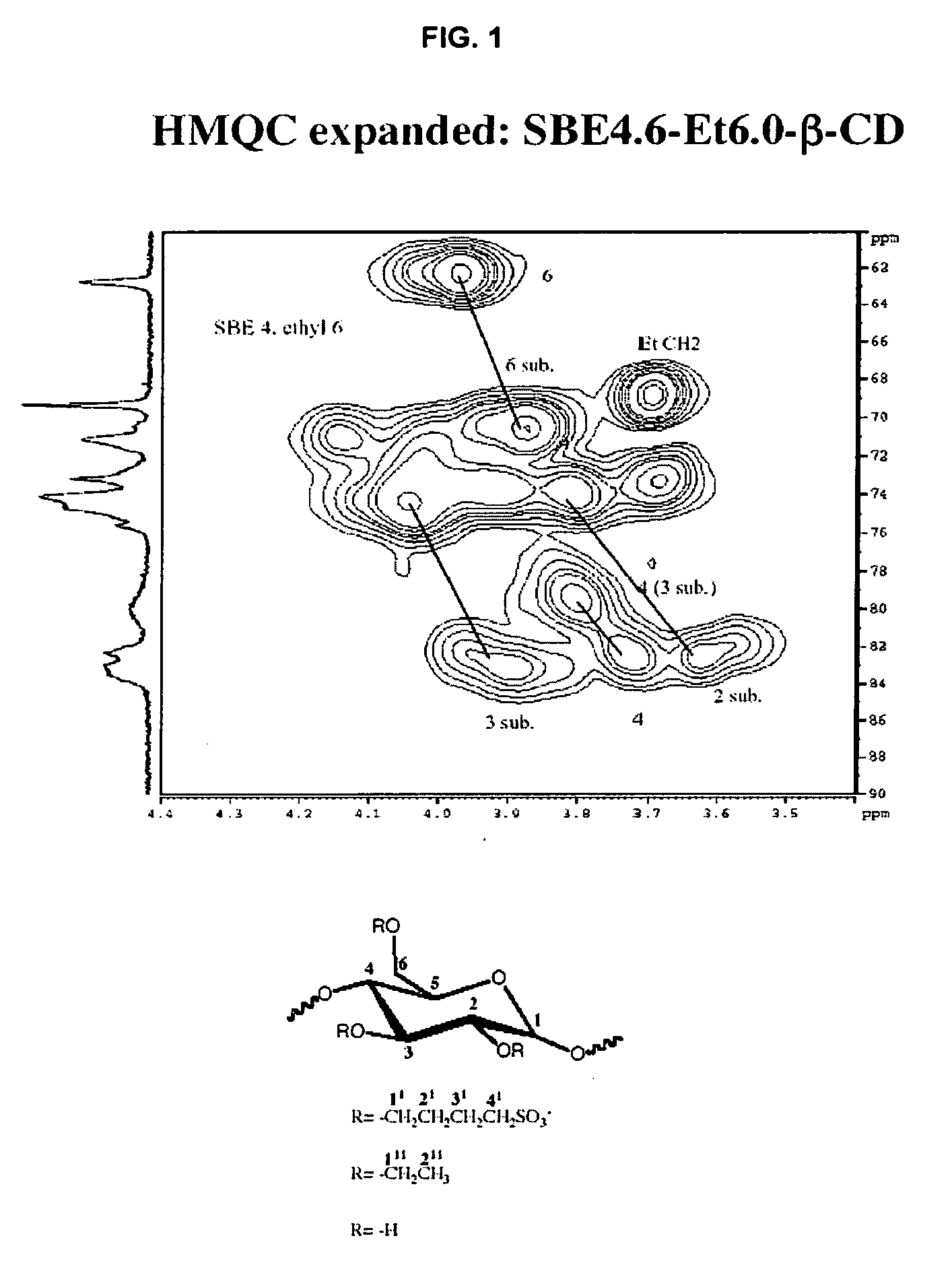
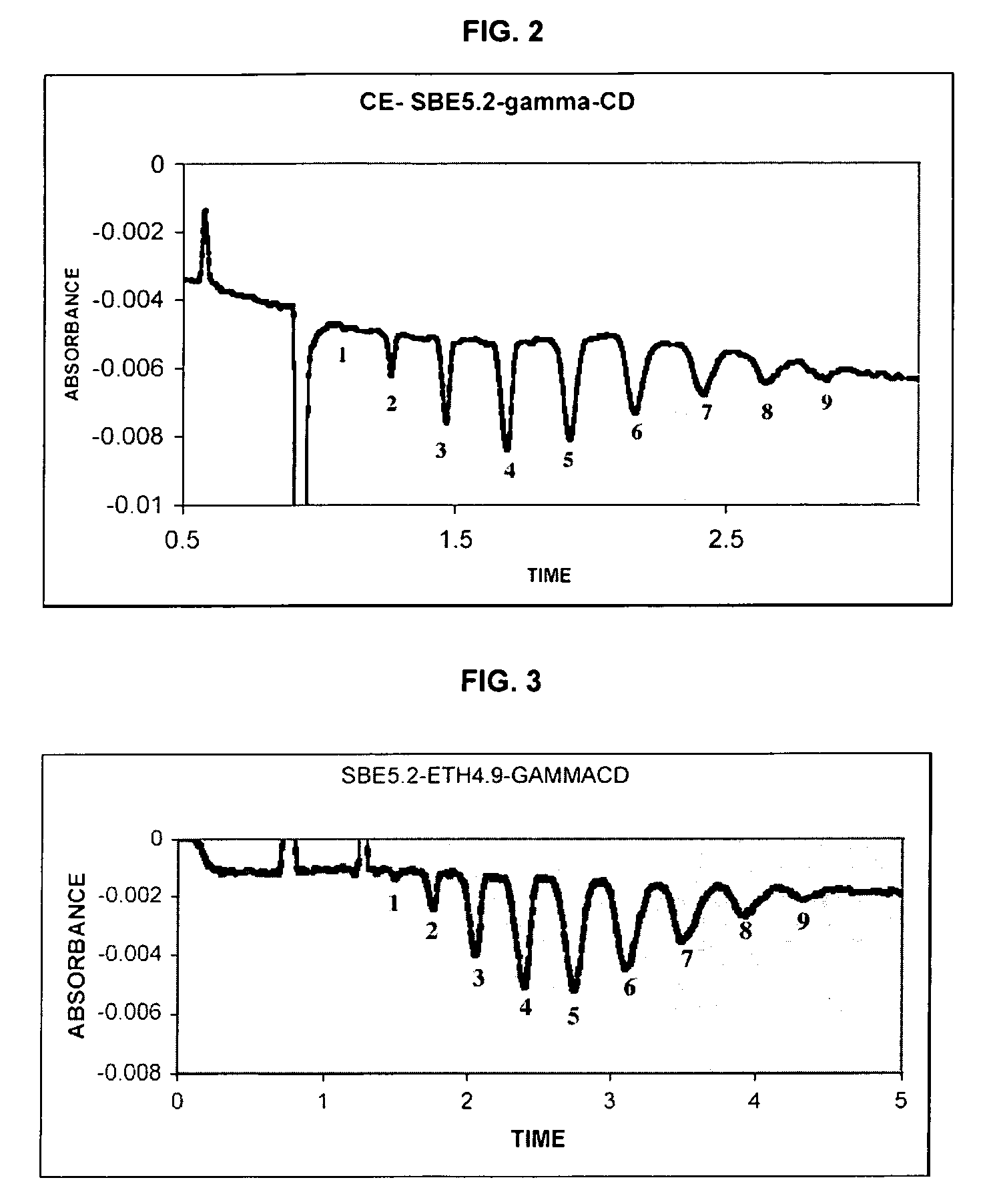
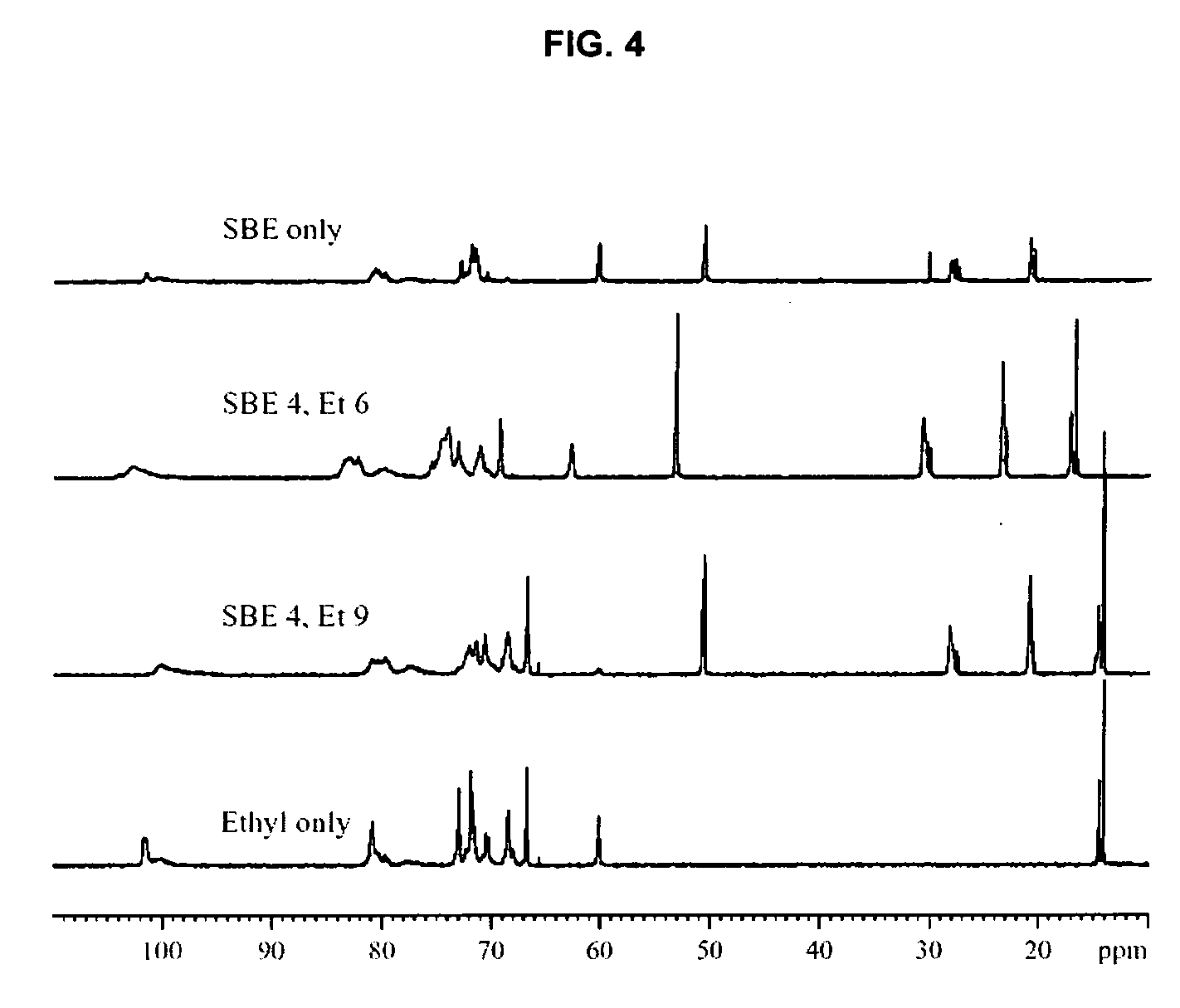
Sulfoalkyl ether-alkyl ether cyclodextrin derivatives
ActiveUS20060258537A1Improve propertiesHigh degree of substitutionOrganic active ingredientsBiocideSolubilityCyclodextrin Derivatives
A sulfoalkyl ether-alkyl ether cyclodextrin (SAE-AE-CD) derivative is provided. The SAE-AE-CD possesses advantages over known SAE-CD and AE-CD derivatives as well as over the parent cyclodextrin by being more water soluble and less membrane disturbing. The SAE-AE-CD includes at least one sulfoalkyl ether group and at least one alkyl ether group even though the degree of substitution for the functional groups can be different. The SAE functional group can be present in molar excess over the AE functional group and vice versa. The total degree of substitution of the cyclodextrin, with respect to both functional groups, can be varied such that a minority or a majority of the hydroxyl moieties of the CD are derivatized. The SAE-AE-CD derivative can be used to solubilize compounds with insufficient water solubility. In some cases, they also stabilize compounds in solution against degradation or to solubilize degradation products formed during degradation. In addition, SAE-AE-CD can also be used for other purposes such as osmotic agents, agents used to mask the taste of problematic drugs. Surprisingly, while AE-CDs are known to be toxic by being membrane disturbing, SAE-AE-CDs are less membrane disturbing and therefore have greater safety.
Owner:UNIVERSITY OF KANSAS
Drug delivery to the back of the eye
InactiveUS20050234018A1Reduce deliveryAntibacterial agentsBiocideActive agentCyclodextrin derivative
Disclosed herein are methods of delivering drugs or therapeutically active agents to the back of the eye via topical administration of compositions comprising cyclodextrin derivatives. Compositions related thereto are also disclosed herein.
Owner:ALLERGAN INC
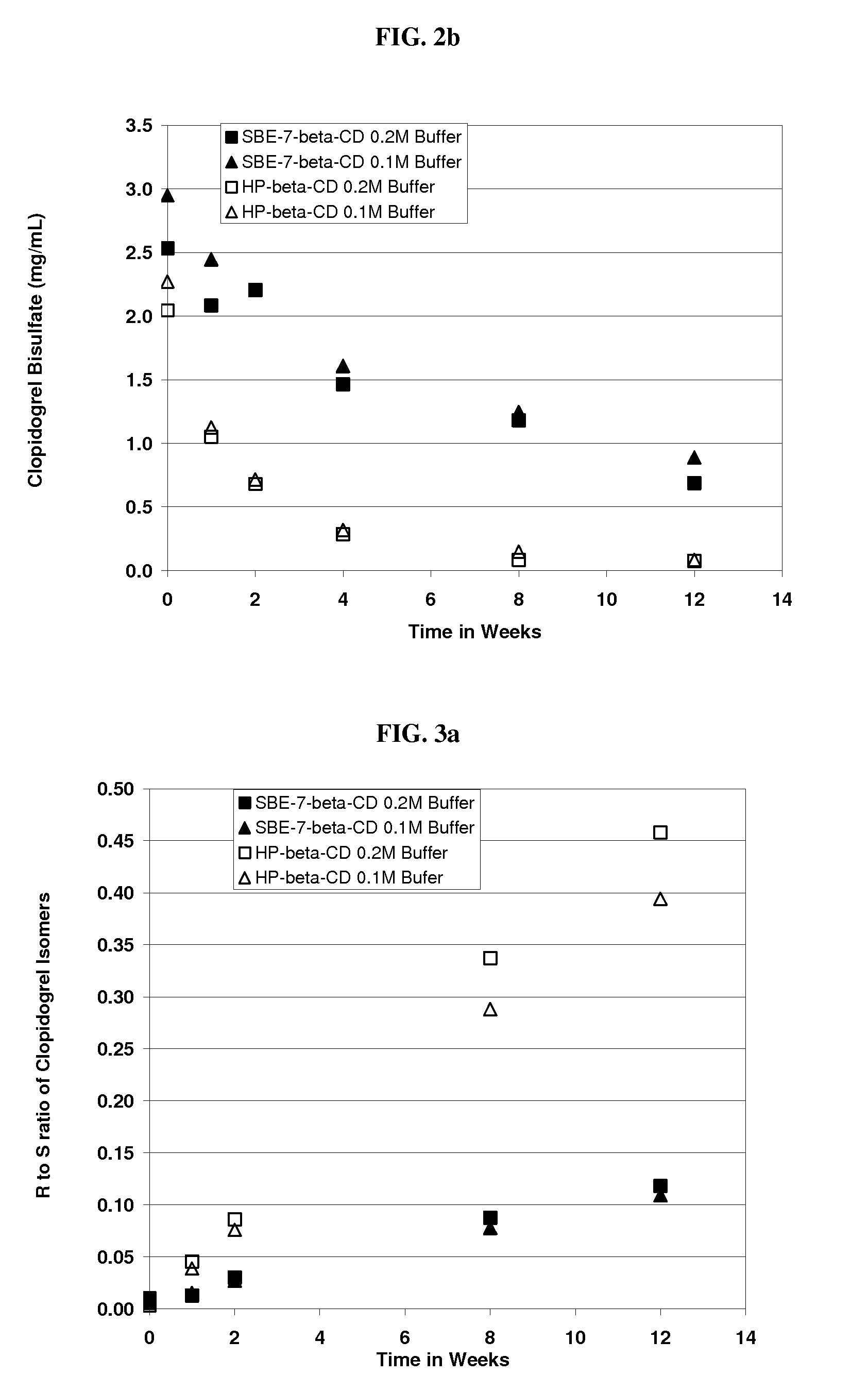
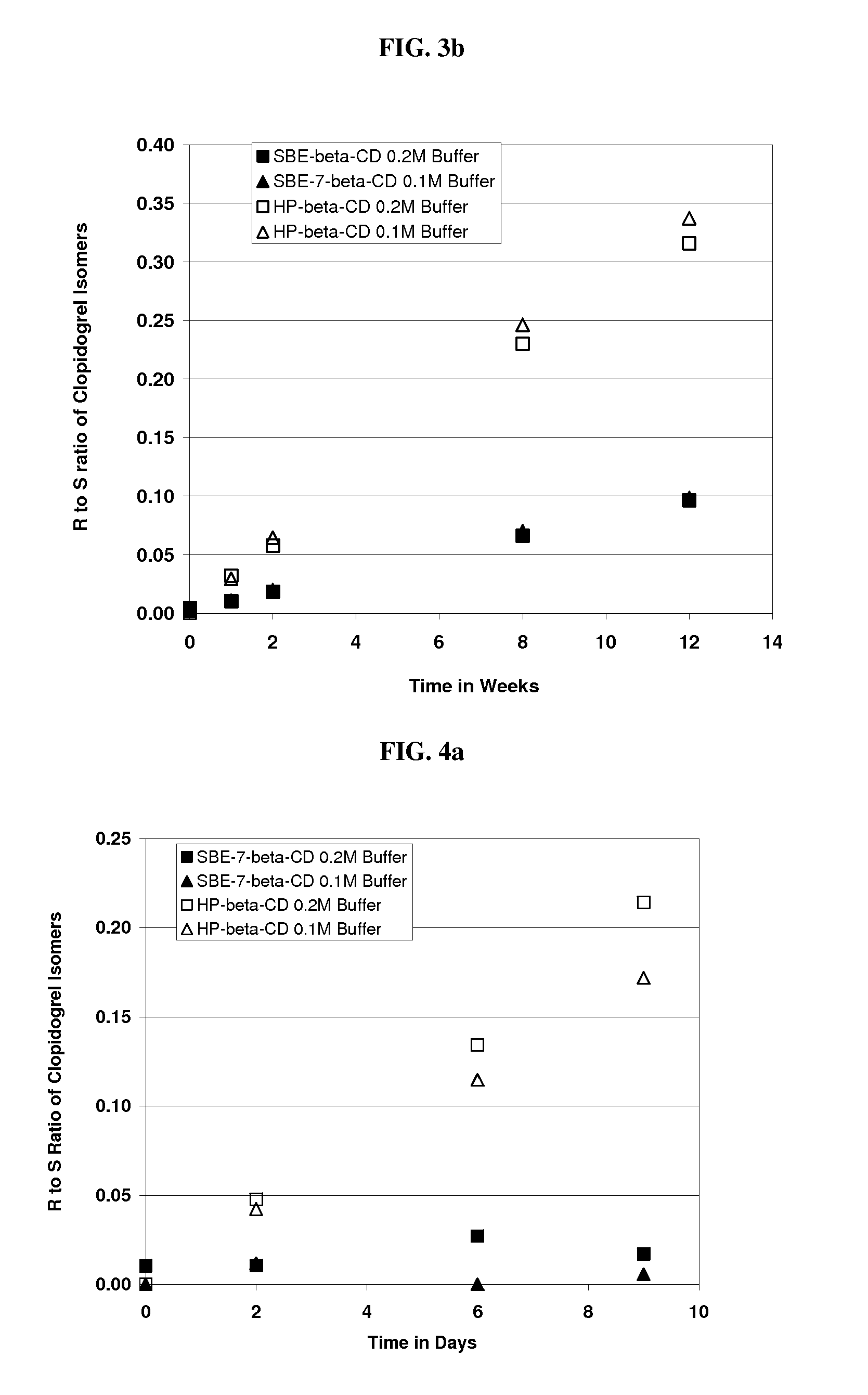
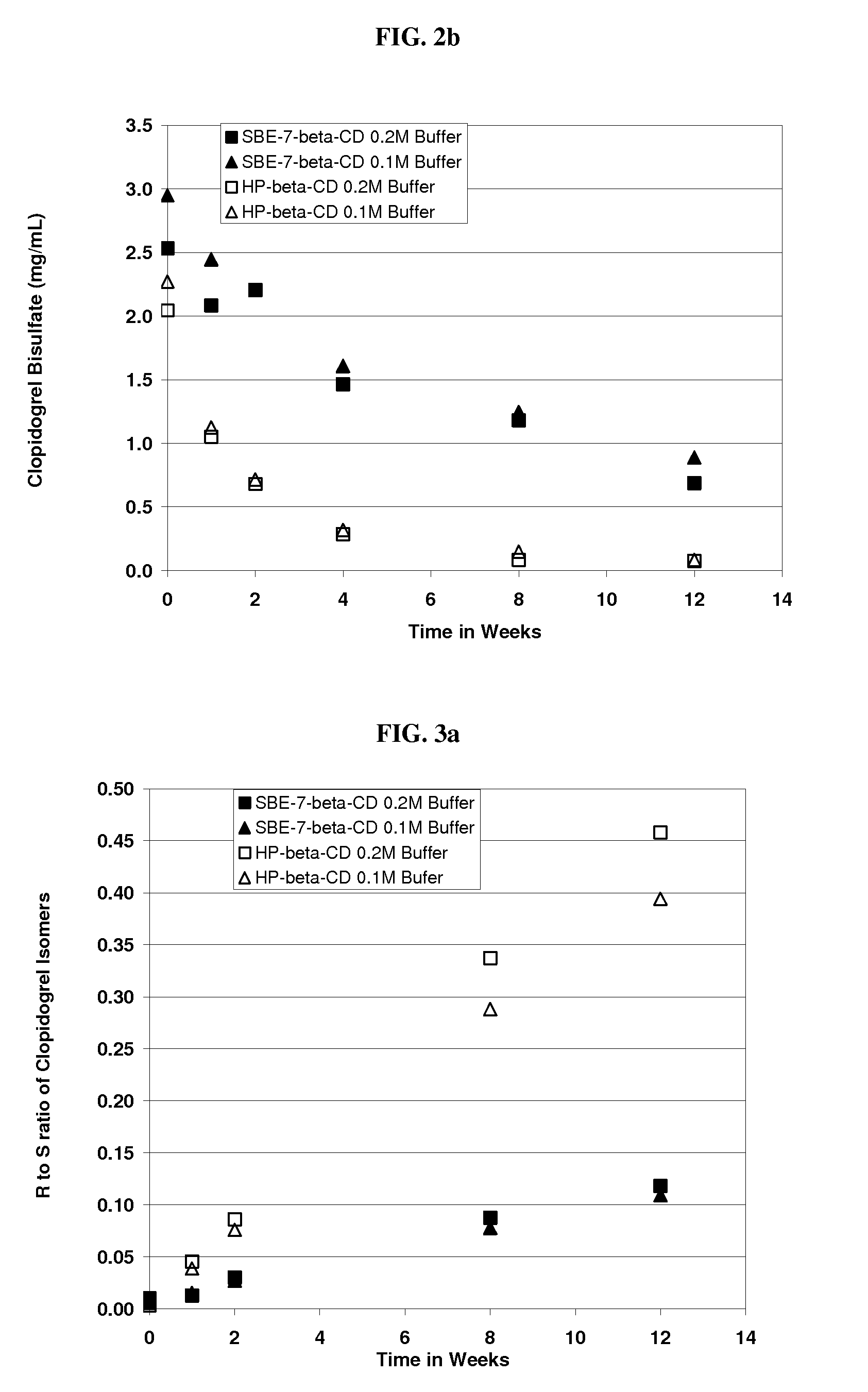
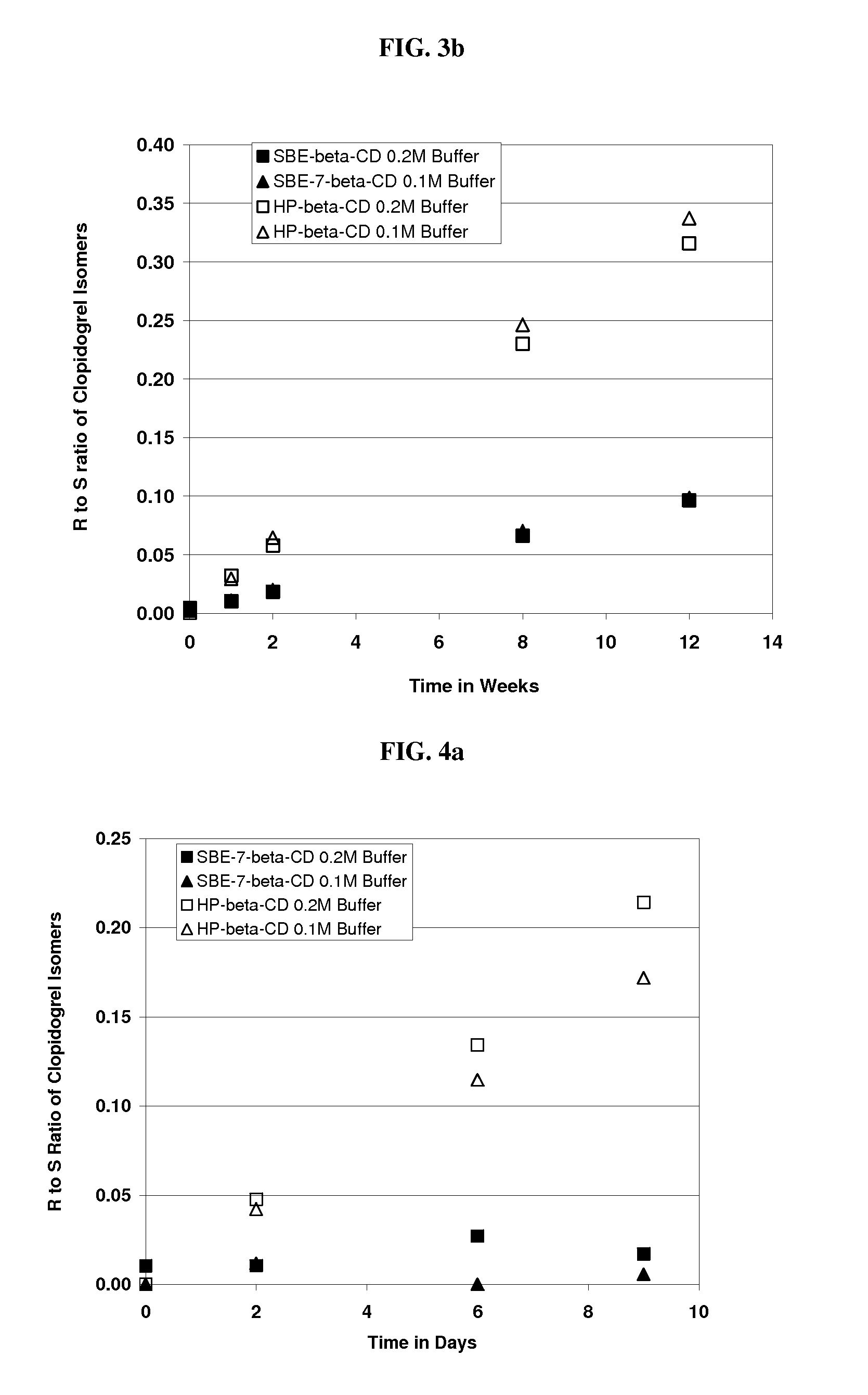
Formulations Containing Clopidogrel and Sulfoalkyl Ether Cyclodextrin and Methods of Use
ActiveUS20100292268A1Reduce chemical degradationReduce probabilityBiocideAntipyreticEtherCyclodextrin derivative
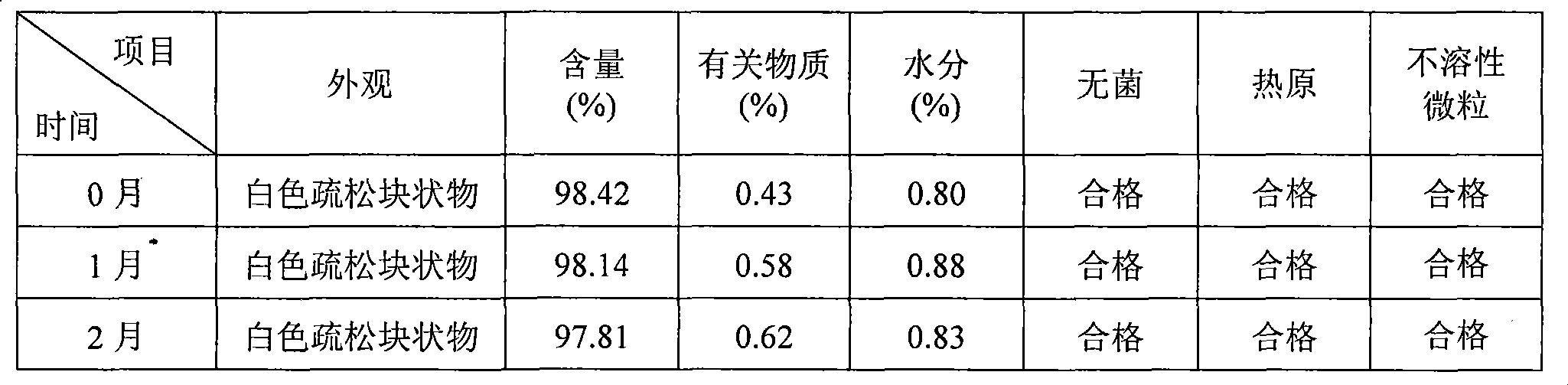
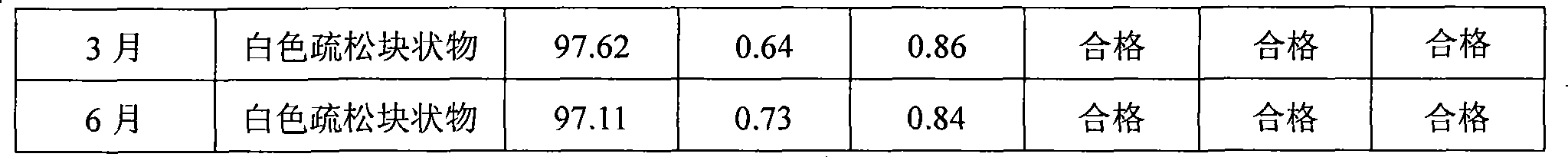
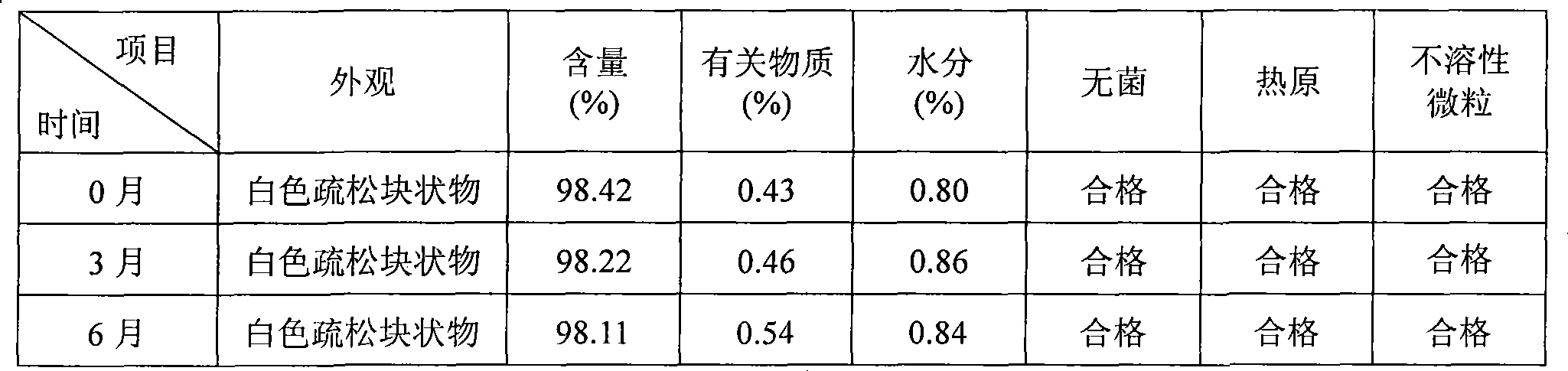
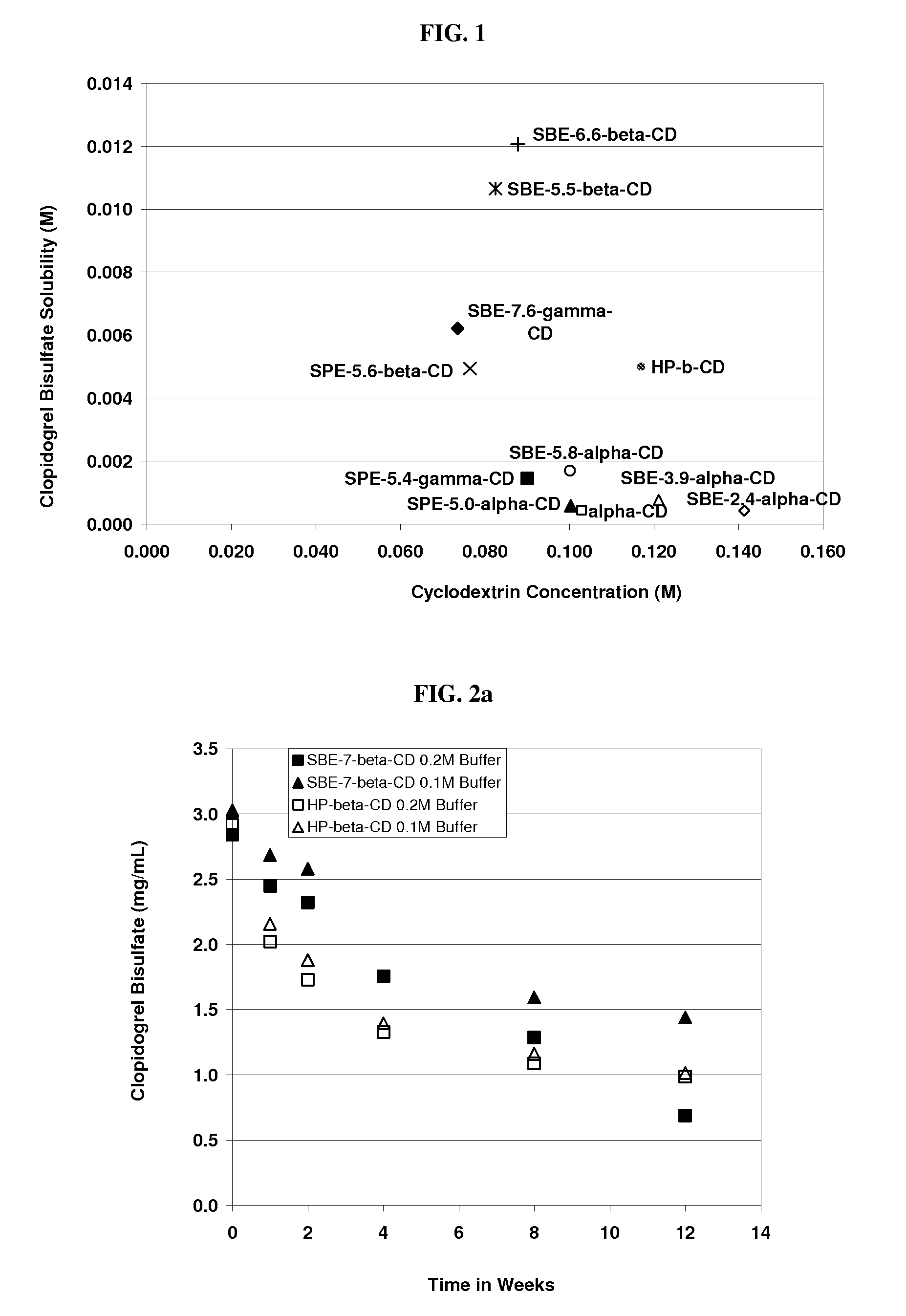
The present invention provides compositions containing clopidogrel, present as a free base or a pharmaceutically acceptable salt thereof, and sulfoalkyl ether cyclodextrin (SAE-CD). The compositions can be liquid, suspension or solid compositions. They can be adapted for oral, peroral or parenteral administration. The SAE-CD serves to aid in dissolution and stabilization of the clopidogrel in aqueous media. The stability of clopidogrel against hydrolytic degradation, thermal degradation, and photolytic degradation are improved. SAE-CD provides improved results over other cyclodextrin derivatives. The SAE-CD-containing composition of clopidogrel can be provided in liquid form, solid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid compositions can be prepared. The liquid composition is optionally available as a clear solution. The compositions herein can be administered perorally or parenterally and provide substantial pharmacokinetic, pharmacodynamic and / or therapeutic advantages over a tablet composition administered perorally and excluding SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
Hydroxypropyl- sulfobutyl-beta- cyclodextrin and its preparation method, analytical method and pharmaceutical uses
ActiveCN1800221ALow hemolyticHemolyticOrganic active ingredientsComponent separationPharmaceutical drugCyclodextrin Derivatives
The invention relates to a hydroxypropyl -sulfo butyl -ª‰-cyclodextrin and its preparing method, analysis method and the applying in medicine, wherein the hydroxypropyl -sulfo butyl -ª‰-cyclodextrin is the cyclodextrin derivation displaced by hydroxypropyl and sulfo butyl. The n-(2, 3, 6-O-2-hydroxypropyl )-m-(2, 3, 6-O-sulfo butyl)-ª‰-cyclodextrin, each mole cyclodextrin displaced group number is n hydroxypropyl and m sulfo butyl, wherein n is the average displaced degree of the hydroxypropyl displaced group; m is the average displaced degree of the sulfo butyl displaced group; n+mú¢Z is the total average displaced degree; nú¢any of the 1,2,3,4,5,6, 7,8,9; m=any of the 1,2,3,4,5,6, 7,8,9; z=any of the 1,2,3,4,5,6, 7,8,9,10.
Owner:BIKA BIOTECH GUANGZHOU CO LTD
Complex of organic medicines and beta-cyclodextrin derivatives and its preparing process
InactiveUS20050215520A1Fast dissolutionImprove stabilityBiocideNervous disorderSimple Organic CompoundsOrganic solvent
The present invention relates to a process of preparing a water-soluble complex of water-insoluble or sparingly-soluble organic medicines and beta-cyclodextrin derivatives: a. dissolving successively or simultaneously the organic medicines and the beta-cyclodextrin derivatives in organic solvent(s) at certain mole ratio or mass ratio in the presence of suitable amount of water; b. removing the organic solvent from the solution of the step a to obtain aqueous solution of the organic medicine and beta-cyclodextrin derivative; and c. removing water from the aqueous solution to obtain the complex of the organic medicine and beta-cyclodextrin derivative. The invention also provides a sterile water-soluble complex of water-insoluble or sparingly-soluble organic medicines and beta-cyclodextrin derivatives. A fully-water-soluble complex can be prepared from any water-insoluble or sparingly-soluble organic medicines or other organic compounds according to the present invention. Thus, appreciably convenient means are provided for industrial uses of such organic medicine preparations and of other organic compounds.
Owner:ADVANCED LIUS PHARMATECH LLC
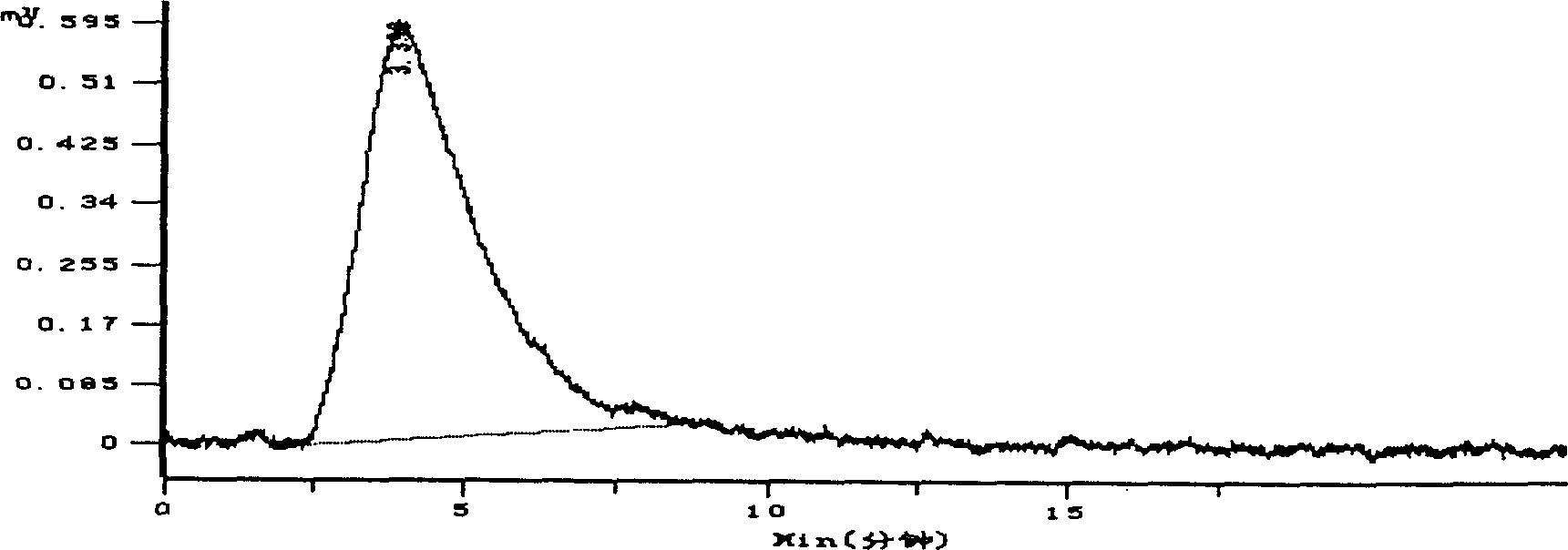
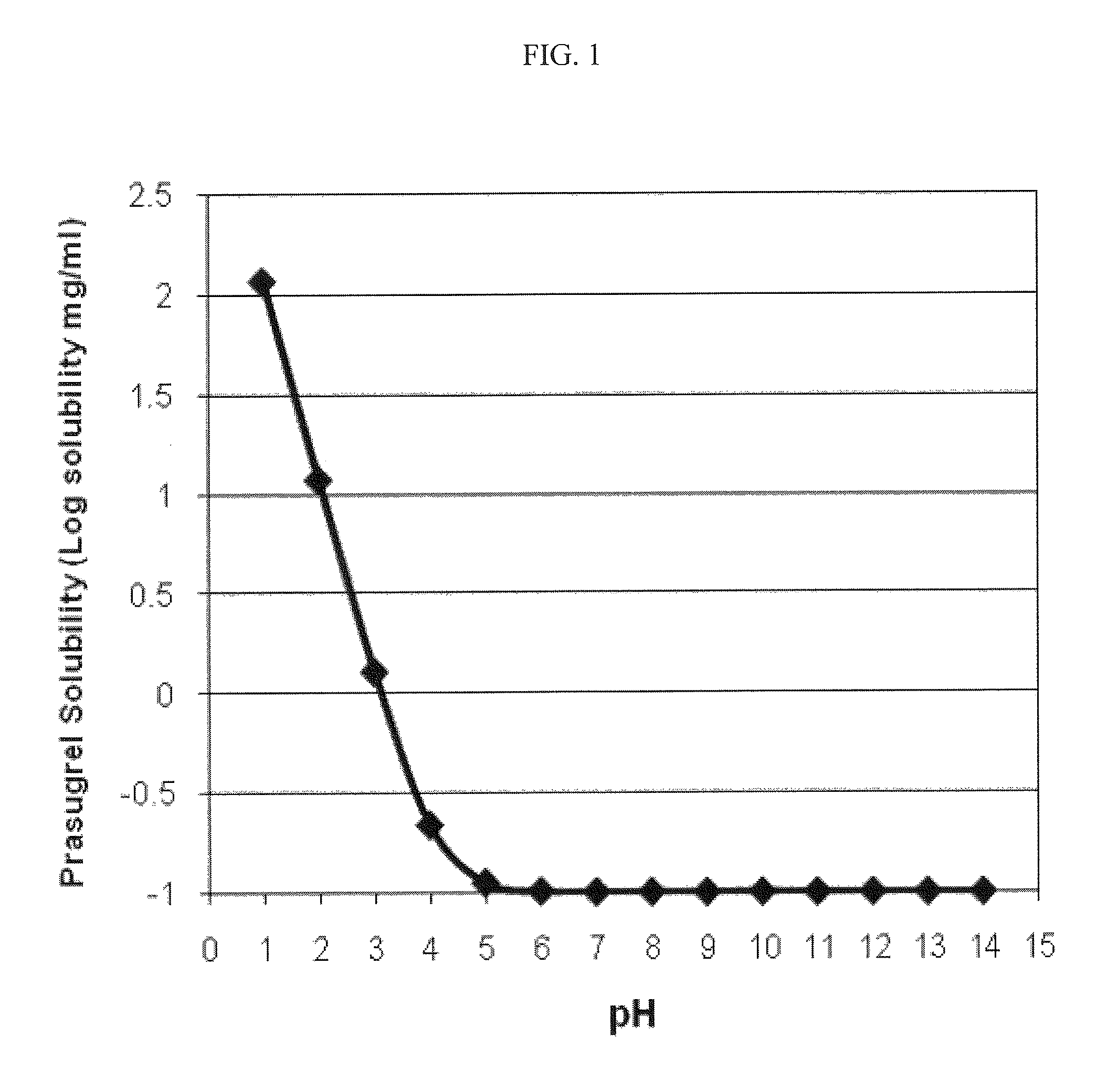
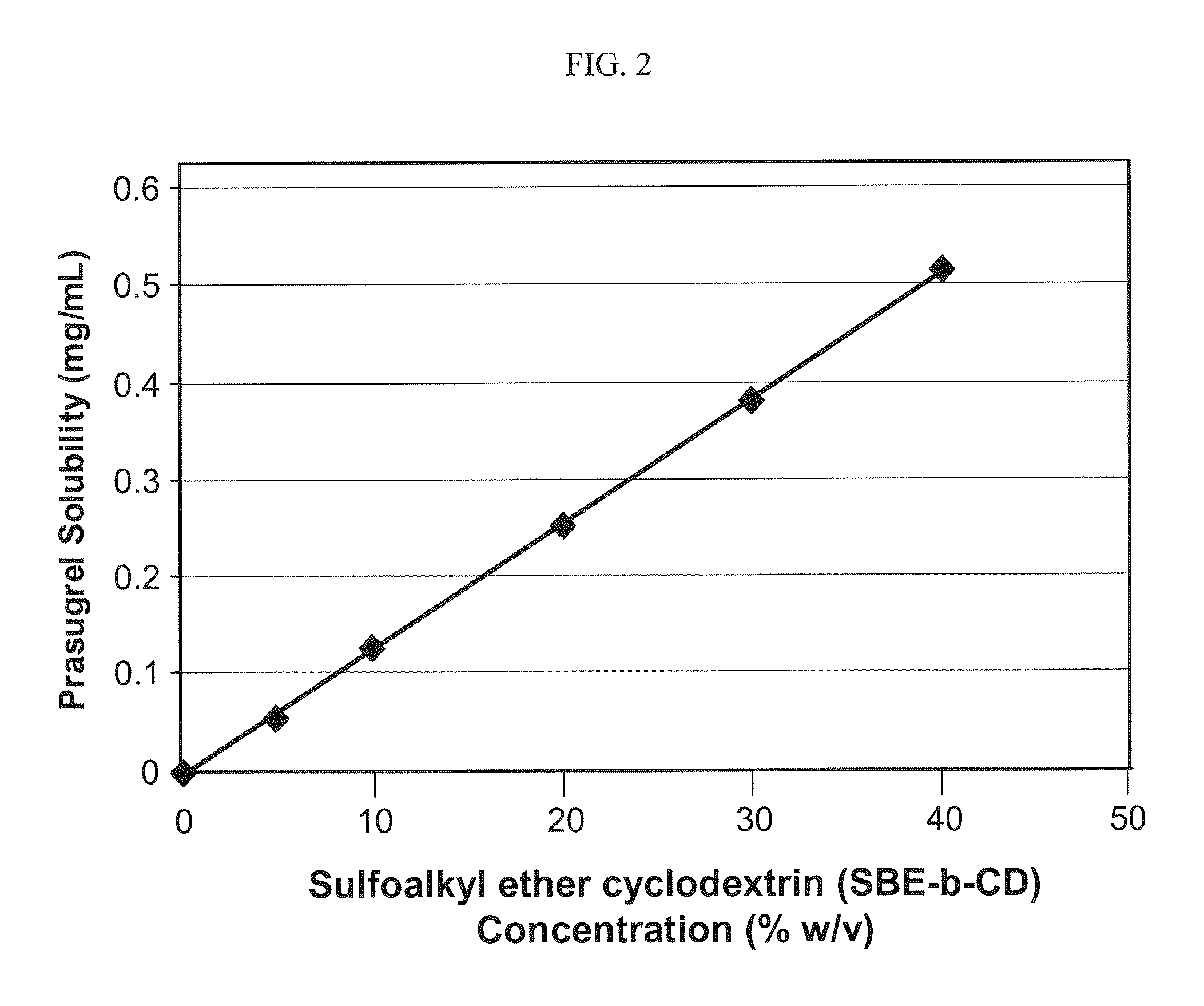
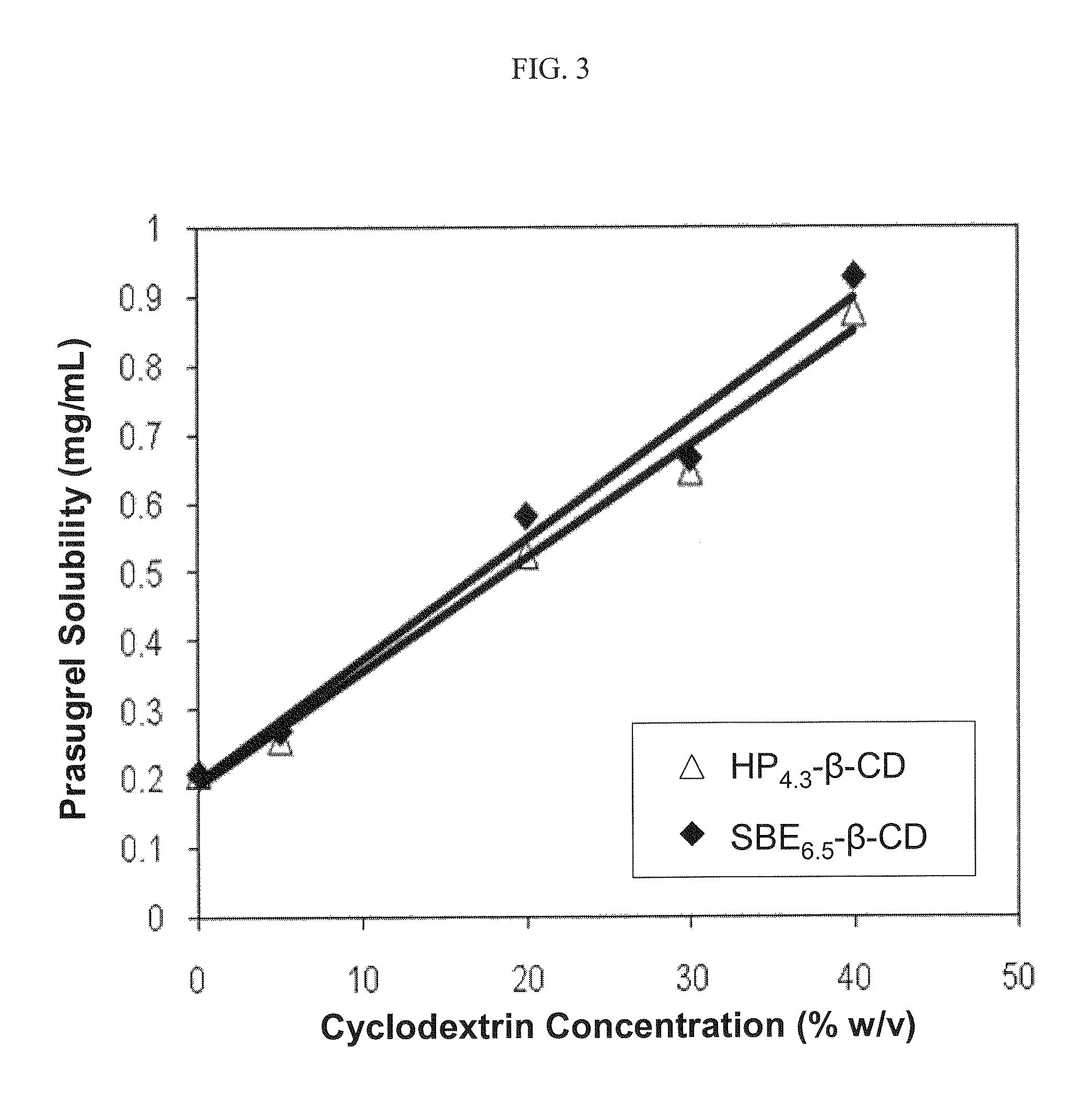
Pharmaceutical compositions comprising prasugrel and cyclodextrin derivatives and methods of making and using the same
ActiveUS8236782B2Minimize toxicology and side-effect profileTitrated more safely and/or easilyBiocidePeptide/protein ingredientsCyclodextrin DerivativesPrasugrel
The present invention is directed to pharmaceutical compositions comprising prasugrel and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMACEUTICALS INC
High-COD special non-phosphorus corrosion and scale inhibitor and preparation method thereof
ActiveCN104261575AGood anti-scaling effectGood corrosion inhibition effectSpecific water treatment objectivesTreatment using complexing/solubilising chemicalsTungstateWater quality
The invention provides a high-COD (Carbon Oxide Demand) special non-phosphorus corrosion and scale inhibitor and preparation method thereof. The corrosion and scale inhibitor mainly comprises cyclodextrin or cyclodextrin derivatives, a soluble metal salt, a copolymer containing a sulfonic acid group, a homopolymer containing carboxylic acid, an acrylic acid / ethyl acrylate / 2-acrylamide-2-methallylsulphonate copolymer, tetrahydroglyoxaline matters, sodium gluconate, and quaternary ammonium salt. The non-phosphorus corrosion and scale inhibitor is suitable for an open circulated cooling water system, and suitable for surface water high in COD and free of treatment, and the non-phosphorus corrosion and scale inhibitor is added into the circulated cooling water system by an automatic reagent feeding device. The non-phosphorus corrosion and scale inhibitor is environmentally-friendly, avoids using phosphorous compounds or non-renewable molybdate or tungstate, has the characteristic of being free from phosphorus and nitrogen, meets the requirement of environmental protection, reduces the environmental burden, has a sterilizing ingredient at the same time, and effectively reduces the problems of bacterial corrosion and algae growth in a circulated water system.
Owner:SHANDONG TIANQING TECH DEV
Medicament composition for water-soluble injection of paclitaxel, preparation method and uses thereof
ActiveCN101366696AOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingHydroxystearic Acid
The invention discloses a water-soluble drug composition of taxol for injection. The drug composition comprises taxol as an active component, polyethyleneglycol 15- hydroxy stearic acid ester as a solubilizer, alcohol as a latent solvent and injection water as a solvent. Polysorbate, cyclodextrin derivatives, poloxamer, polyethylene glycol (PEG) and reducing sugar are also added and are favorable for improving the solubility and stability of the taxol in the composition. The composition is prepared to freeze-dried powder injection for injection, can not produce serious anaphylactic reaction, has small simulation, remarkably improves the clinical compliance of patients and has good water solubility and high convenience for medication by clinicians. The powder injection has good stability, can be stored at room temperature and is favorable for storage and transportation. The powder injection is simple in preparation process, easy to control quality, low in production cost and convenient for industrialized production and also greatly reduces the economic burden of the patients for medication. The invention also discloses a method for preparing the drug composition and clinical application thereof.
Owner:姚定全
Silybin injection containing cyclo dextrin or its derivatives
InactiveCN1391894ASolve the problem of insolubleOrganic active ingredientsDigestive systemWater insolubleCyclodextrin
The present invention features that silybin is included by cyclodextrin or its derivative so as to dissolve in water to prepare injection. The present invention solves the problem of water insoluble silybin for being suitable for intravenous injection.
Owner:广州瑞济生物技术有限公司
Formulations containing clopidogrel and sulfoalkyl ether cyclodextrin and methods of use
The present invention provides compositions containing clopidogrel, present as a free base or a pharmaceutically acceptable salt thereof, and sulfoalkyl ether cyclodextrin (SAE-CD). The compositions can be liquid, suspension or solid compositions. They can be adapted for oral, peroral or parenteral administration. The SAE-CD serves to aid in dissolution and stabilization of the clopidogrel in aqueous media. The stability of clopidogrel against hydrolytic degradation, thermal degradation, and photolytic degradation are improved. SAE-CD provides improved results over other cyclodextrin derivatives. The SAE-CD-containing composition of clopidogrel can be provided in liquid form, solid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid compositions can be prepared. The liquid composition is optionally available as a clear solution. The compositions herein can be administered perorally or parenterally and provide substantial pharmacokinetic, pharmacodynamic and / or therapeutic advantages over a tablet composition administered perorally and excluding SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
Pharmaceutical composition containing arctigenin and preparation method
InactiveCN101036644AGood water solubilityOvercome the defect of poor solubilityOrganic active ingredientsAntipyreticCyclodextrin DerivativesCyclodextrin derivative
A composition for medicine having arctigenin and a method for preparing the same, in particular the arctigenin is involved in a cyclodextrin derivative, which mixture ratio is in a range of 1g:3g-30g, and the composition can effectively increase solubility and bioavailability of the arctigenin.
Owner:海南盛科天然药物研究院有限公司
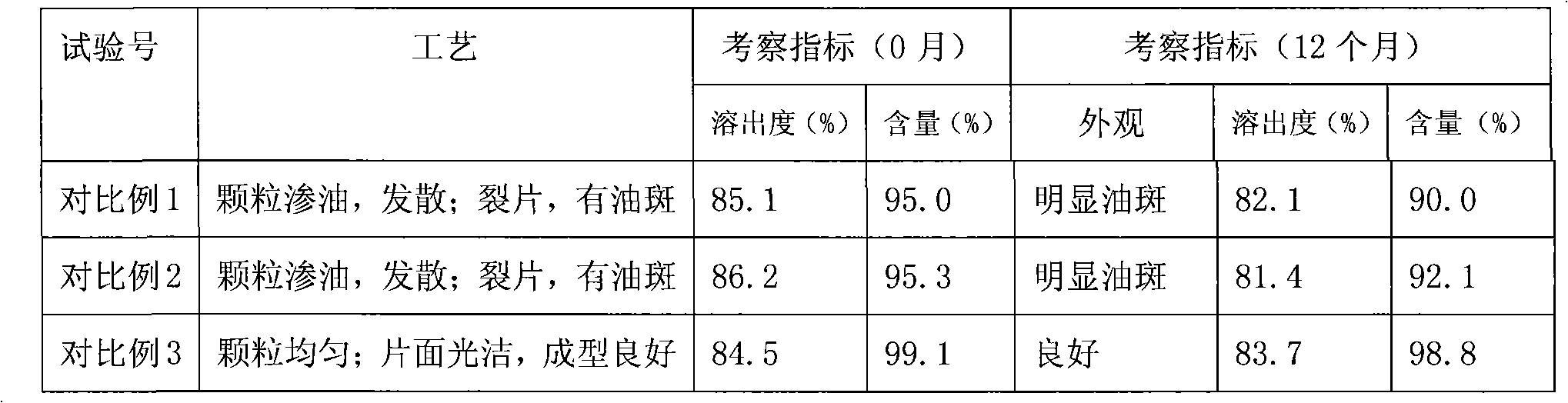
Butylbenzene phthalein tablet and preparation method thereof
ActiveCN101342152AStable outputHigh dissolution rateOrganic active ingredientsMacromolecular non-active ingredientsCyclodextrinDissolution
The invention relates to a butyl phthalide tablet and a preparation method thereof. The butyl phthalide tablet is composed of butyl phthalide solid powders and other medical troche auxiliary materials; wherein, the butyl phthalide solid powders are composed of the components with the following weight percentage: 9 percent to 30 percent of butyl phthalide, 0.01 percent to 1.2 percent of emulsifiers and 70 percent to 90 percent of cyclodextrin or cyclodextrin ramification. The troche which is made with the invention has favorable forming, stable product quality and high dissolution rate.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Insoluble drug delivery system based on water-soluble cyclodextrin
InactiveCN1879887AMild reaction conditionsThe purification process is simplePill deliveryCapsule deliveryChemistryWater soluble
The invention relates to an insoluble medicine feeding system based on soluble cyclodextrin polymer, with high yield and the application in batch production, wherein said medicine compound is formed by insoluble medicine and cyclodextrin derivant, whose mass ratio is 0.1-100:1; and said insoluble medicine is the one whose solubility is less than 1% in room temperature; the soluble cyclodextrin polymer uses cyclodextrin as crosslink agent to be concentrated with cyclodextrin monomer. The inventive product is not shaped powder, whose average molecular weight can reach 10000.
Owner:SHENYANG PHARMA UNIVERSITY
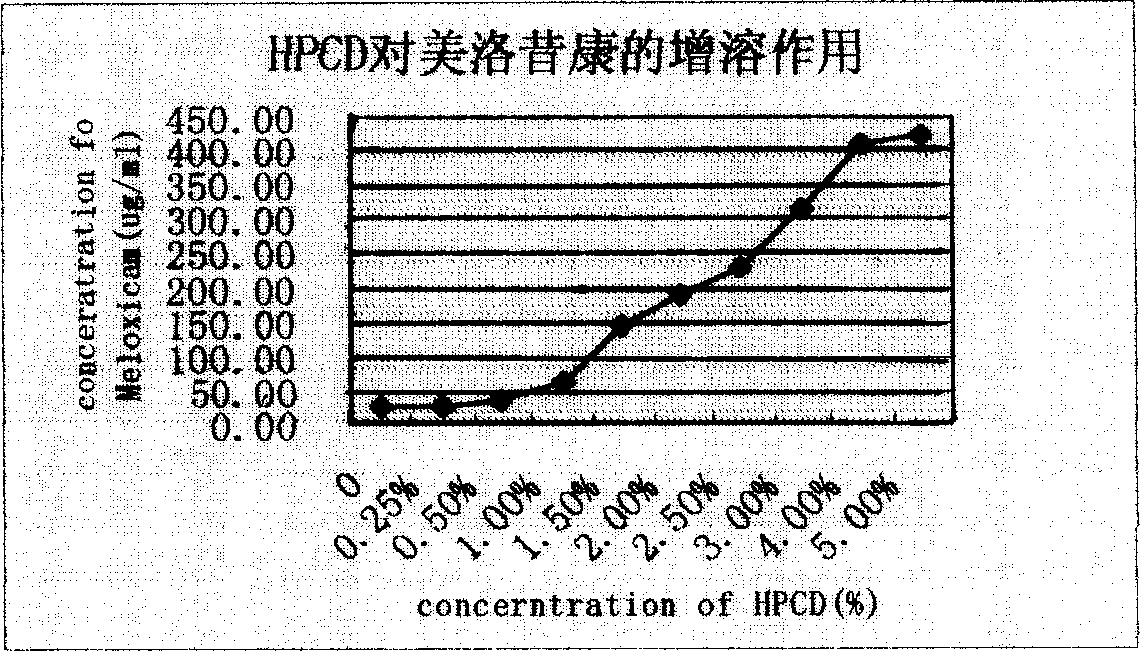
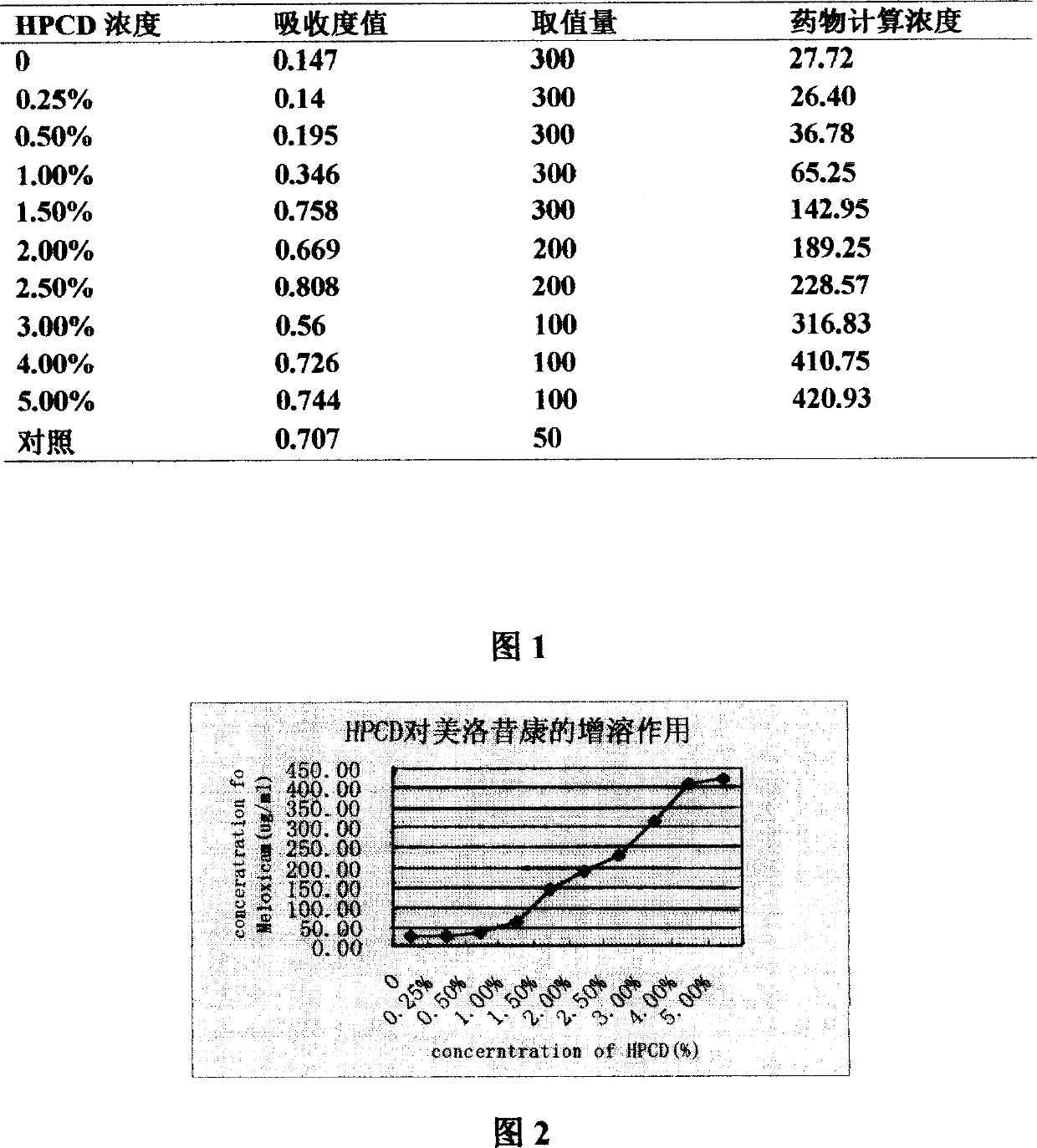
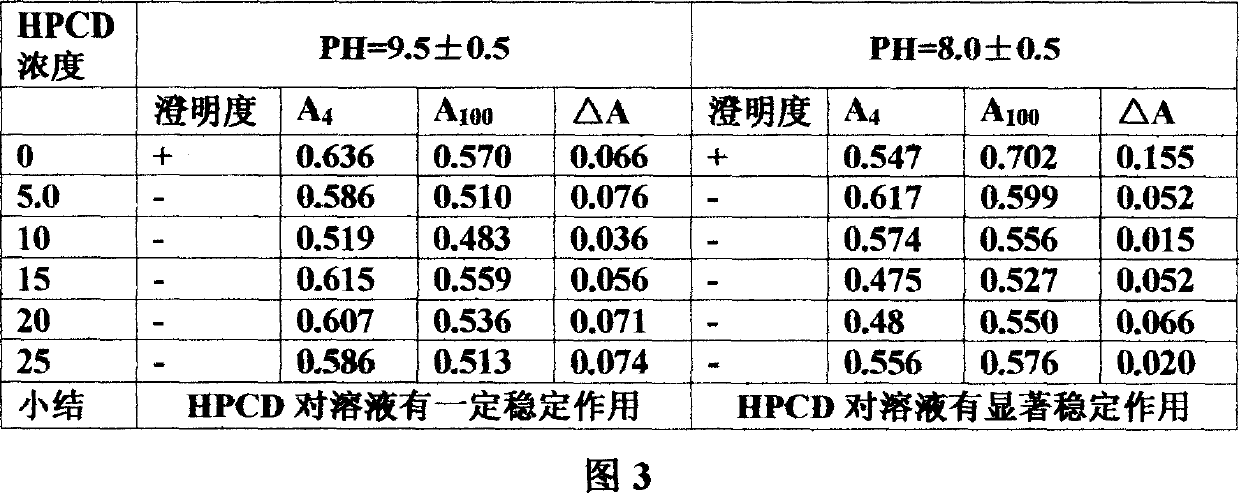
Mezloxicon liquid preparation using HPCD as solubilizing and stabilizing agent and its preparation method
InactiveCN1493292ASmall individual differencesAvoid gastrointestinal side effectsOrganic active ingredientsAntipyreticMeloxicamAdditive ingredient
A liquid preparation of meloxican is prepared from the meloxicam or its medical salt and water-soluble cyclodextrin derivative HP-beta-CD used as solubilizer and stabilizer, proportionally mixing, adding cosolvent, pH regulator and water for injection, heating and membrane filtering. It has long storage time at low temp.
Owner:SHENYANG PHARMA UNIVERSITY
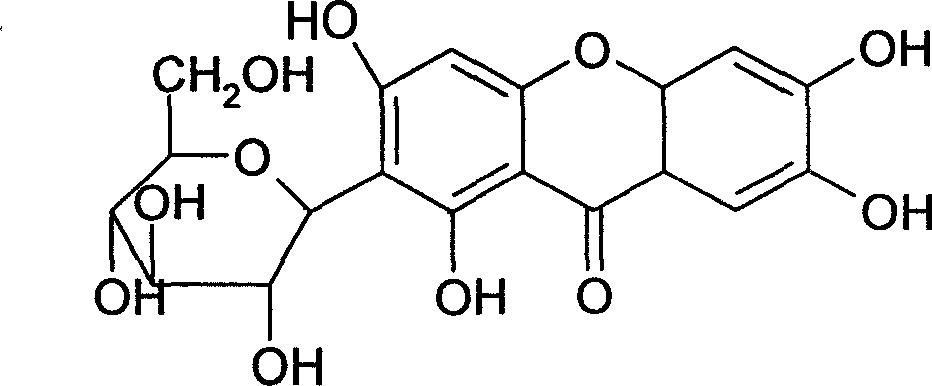
Medicinal composition containing mangiferin and its preparing method
InactiveCN1977855AGood water solubilityOvercome the defect of poor solubilityAntibacterial agentsOrganic active ingredientsCyclodextrin derivativeChemistry
The present invention relates to a medicine composition containing mangiferin and its preparation method. It is characterized by that the mangiferin is contained in the cyclodextrin derivative, and the mixing ratio of both them is as follows: mangiferin 1g and cyclodextrin derivative 3g-30g. It can effectively raise solubility and bioavailability of mangiferin.
Owner:海南盛科天然药物研究院有限公司
Beta-cyclodextrin derivatives as antibacterial agents
The invention provides a new class of antibiotics to which pathogenic bacteria have not been exposed, and thus should not have developed resistance. This new class of antibiotics are derivatives of β-cyclodextrin (β-CD), which is a cyclic molecule comprising seven D-glucose units.
Owner:PINNACLE PHARMA INC
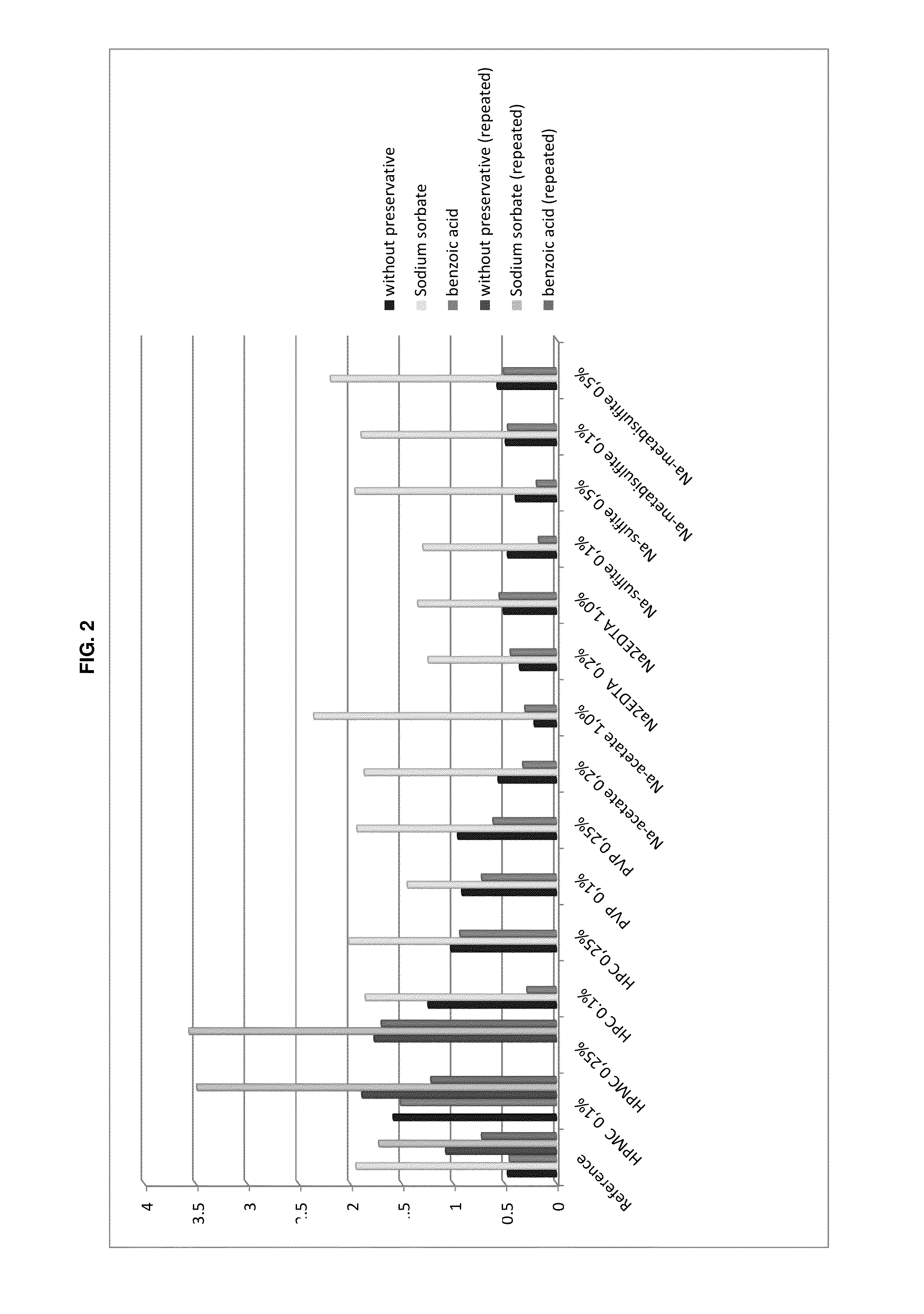
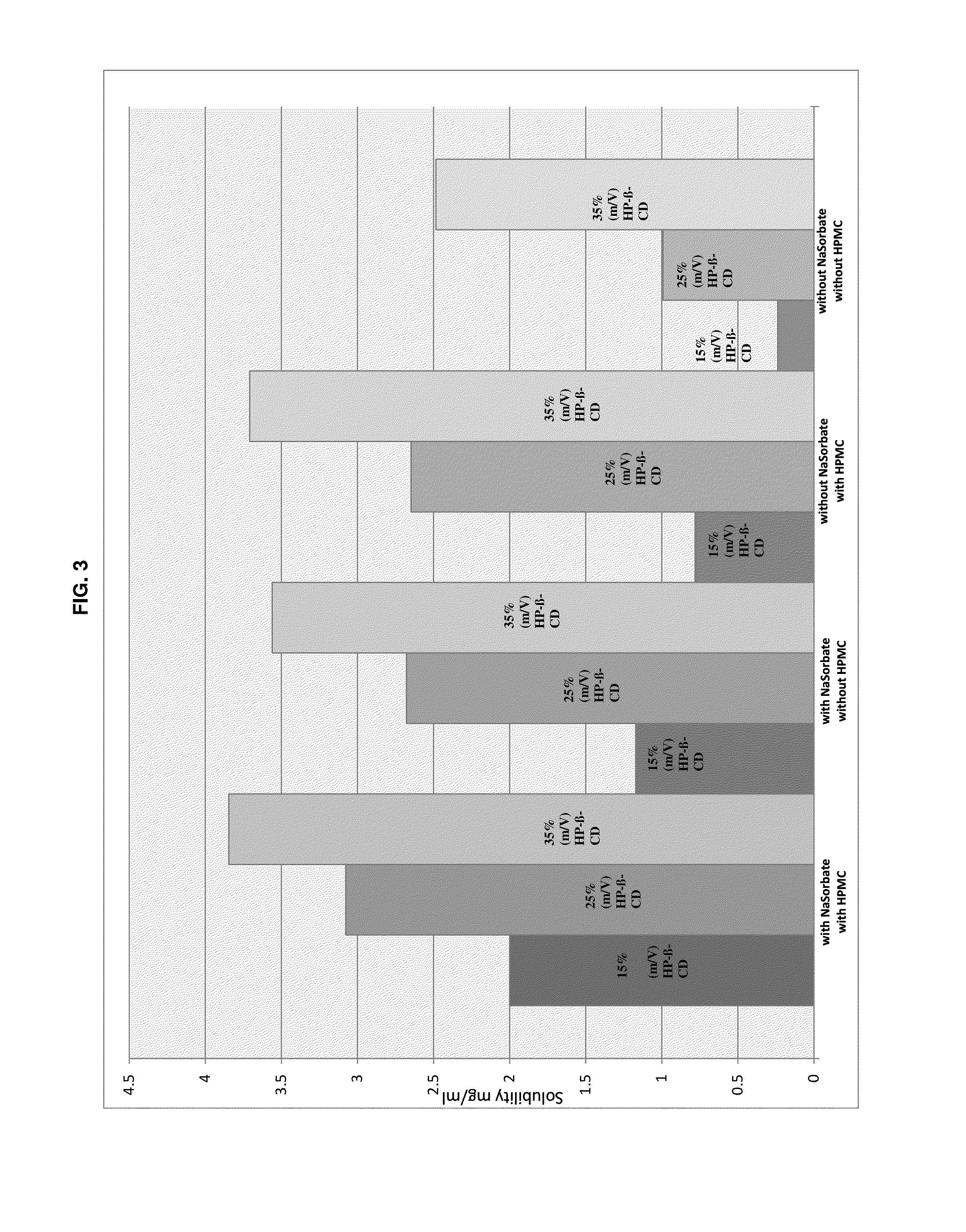
Preserved etherified cyclodextrin derivatives containing liquid aqueous pharmaceutical composition
ActiveUS20150025082A1Inhibit microbial growthRetaining its effectivenessAntibacterial agentsNervous disorderSodium acetrizoateSodium acetate
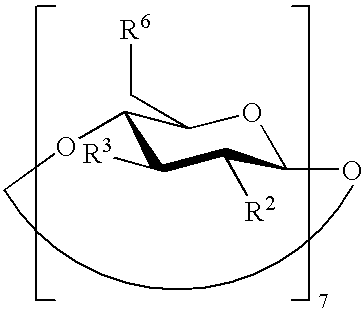
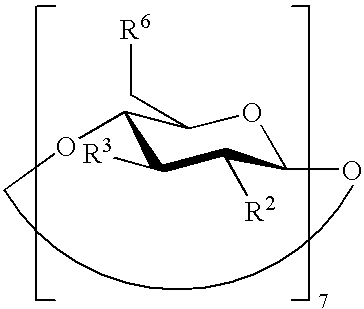
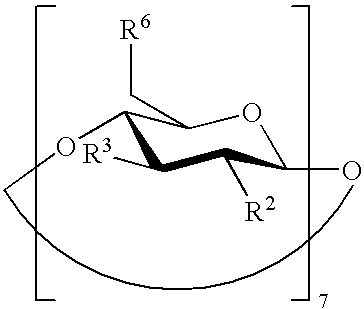
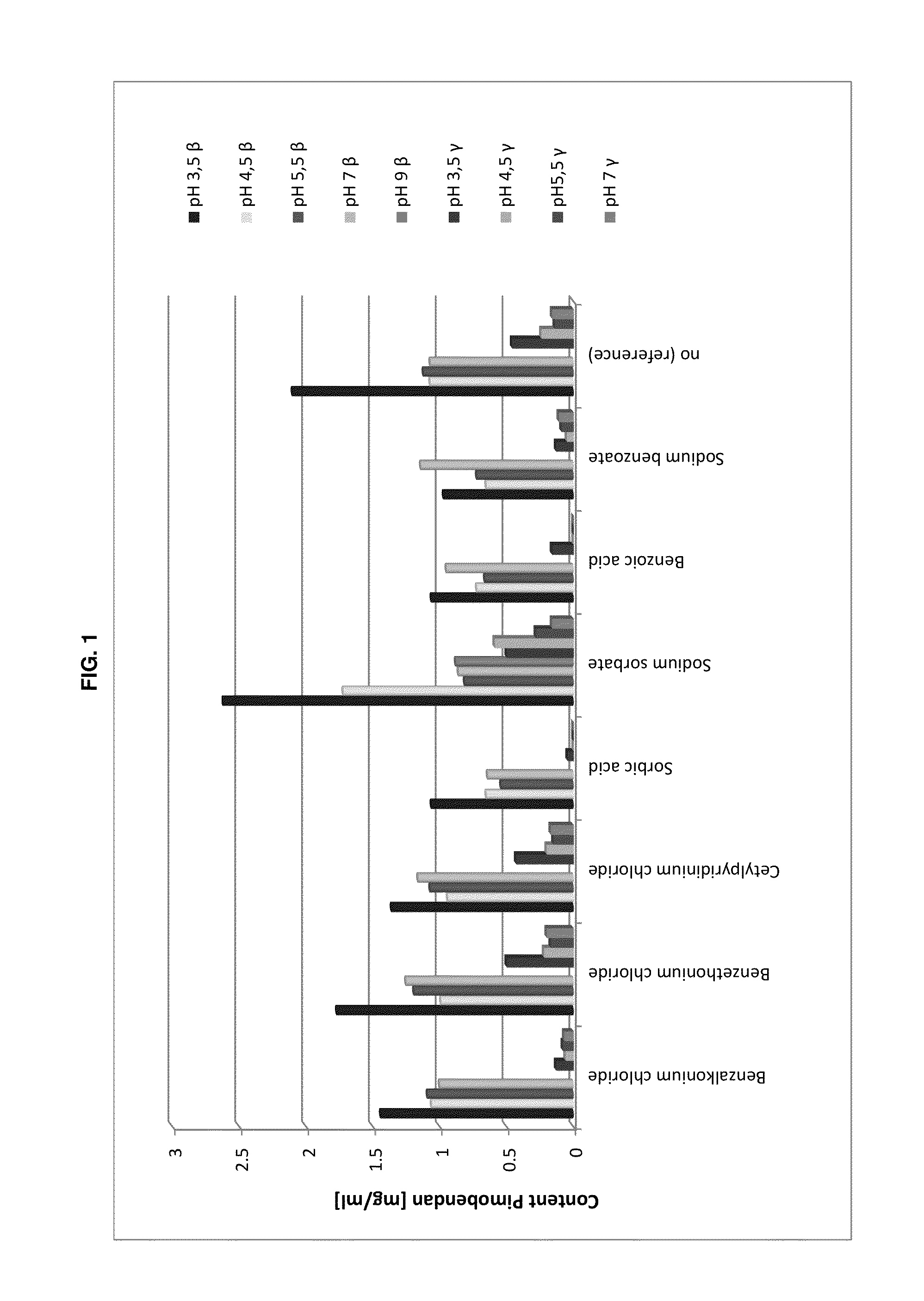
The present invention is directed to a preserved liquid aqueous pharmaceutical composition comprising one or more etherified cyclodextrin derivatives; one or more water-soluble preservatives; preferably selected from the group consisting of sorbic acid or salts thereof, preferably sodium sorbate, potassium sorbate, calcium sorbate; benzoic acid or salts thereof, preferably sodium benzoate; benzalkonium chloride; benzethonium chloride; cetylpyridinium chloride; sodium metabisulfite; sodium acetate; parabenes and salts thereof, preferably methylparabene, ethylparabene, propylparabene, butylparabene, butylparabene sodium; or combinations thereof; and at least one pharmaceutically active compound which is poorly water-soluble, very poorly water-soluble or water-insoluble. The liquid aqueous pharmaceutical composition provides an acceptable solubility of the pharmaceutically active compound, such as pimobendan, in aqueous solution whereby the water-soluble preservatives retain their effectiveness in the presence of the etherified cyclodextrin derivatives allowing the use in an oral administration form.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Pinocembrin and cyclodextrin or cyclodextrin derivative inclusion compound
ActiveCN101537188ALess irritating to blood vesselsDisintegrates quicklyOrganic active ingredientsAntimycoticsSolubilityDisease
The invention discloses a pinocembrin and cyclodextrin or cyclodextrin derivative inclusion compound, wherein cyclodextrin or a cyclodextrin derivative is used to include the pinocembrin. The inclusion compound contains the pinocembrin and the cyclodextrin or cyclodextrin derivative in a molar ratio of 1:1-100. The inclusion compound can improve the water solubility of the pinocembrin and give good play of the therapeutic action of the pinocembrin. The inclusion compound is suitable for the preparation of sold dosage forms and liquid dosage forms required by clinics, including infusion solution, water injection, powder injection, oral solution, syrup, tablets, capsules, granules and dispersible tablets. The inclusion compound can be used for preparing drugs for preventing and\or treating cardiovascular and cerebrovascular diseases, particularly brain stroke, as well as drugs for preventing and\or treating bacterial and\or fungal infections.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Tablet and process for producing the same
InactiveUS20060172005A1Oral cavity can be acceleratedGood disintegrationOrganic active ingredientsBiocideCyclodextrin derivativeCyclodextrin Derivatives
An object of the present invention relates to a tablet which comprises an active ingredient and a cyclodextrin or a cyclodextrin derivative and rapidly disintegrates in the oral cavity, etc. The present invention provides a tablet in which 70% by mass or more of the components of the tablet is a cyclodextrin or a cyclodextrin derivative and a method for manufacturing a tablet comprising the steps of: mixing constituent components of the tablet which comprises as constituent components an active ingredient and a cyclodextrin or a cyclodextrin derivative and in which 70% by mass or more of the total constituent components of the tablet is the cyclodextrin or the cyclodextrin derivative; and subsequently tableting the resultant mixture.
Owner:KYOWA HAKKO KOGYO CO LTD
Amorphous taclolimus solid dispersion having an enhanced solubility and pharmaceutical composition comprising same
InactiveCN101115758AGood water solubilityImprove bioavailabilityOrganic active ingredientsSenses disorderSolubilityOrganic acid
An amorphous solid dispersion of tacrolimus comprising tacrolimus, a substituted cyclodextrin derivative and an organic acid, the solid dispersion having high thermodynamic stability and solubility, providing enhanced release rate and bioavailability.
Owner:HANMI PHARMA
Lyophilized liposome composition encapsulating a water-soluble drug and preparation process thereof
InactiveUS20130315987A1Improve concentrationLow toxicityBiocideCarbohydrate active ingredientsCholesterolMedicine
Disclosed is a lyophilized liposome composition encapsulating a water-soluble drug and a preparation process thereof. The lyophilized liposome composition comprises a water-soluble drug, a phospholipid, a polyethylene glycol-derivatized phospholipid, cholesterol and a lyoprotectant, wherein the lyoprotectant comprises a saccharide and a cyclodextrin or cyclodextrin derivative. The encapsulation rate of the lyophilized liposome composition encapsulating the water-soluble drug is ≧90%.
Owner:REGENEX PHARMA LTD
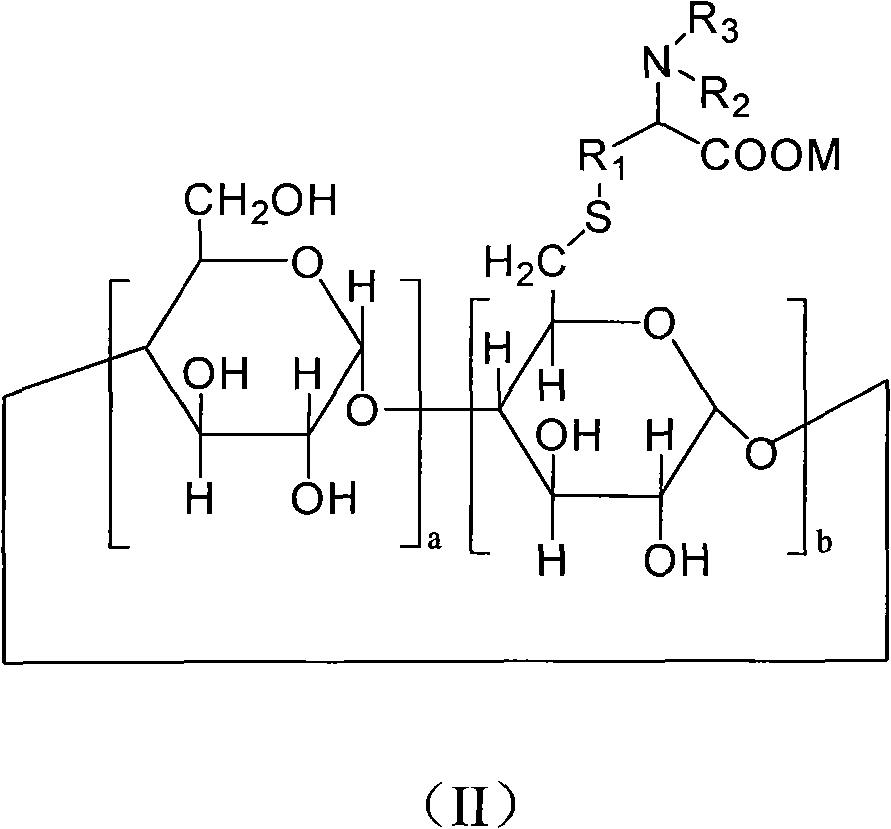
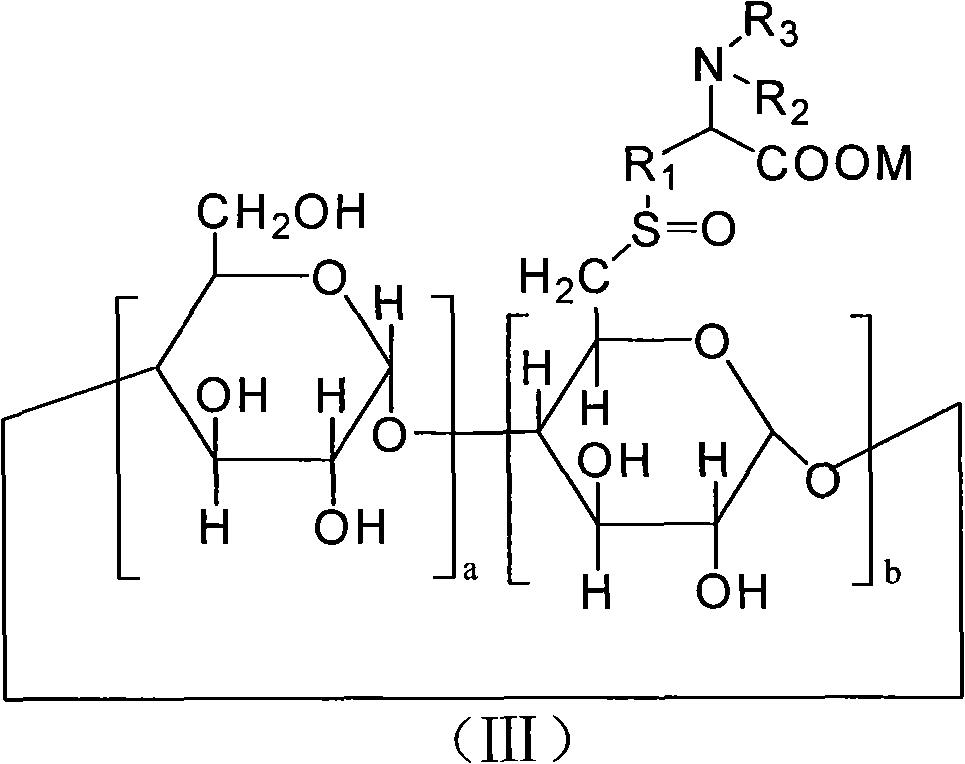
6-deoxy alpha-amino acid derivative cyclodextrin, preparation and application thereof
ActiveCN102060941AThe preparation process is stableHigh yieldOrganic active ingredientsMuscular disorderCyclodextrin derivativeThioether
The invention provides 6-deoxy alpha-amino acid derivative cyclodextrin which is a target substance obtained by the condensation of an amino acid derivative and halogenated cyclodextrin in the presence of alkali. The 6-deoxy alpha-amino acid cyclodextrin derivatives comprise a 6-deoxy thioether alpha-amino acid derivative cyclodextrin, a 6-deoxy sulfoxide alpha-amino acid derivative cyclodextrin and a 6-deoxy sulfonyl alpha-amino acid derivative cyclodextrin. The compounds provided by the invention can be used for reversing the phenomenon of nerve muscle relaxation induced by human or animal muscle relaxants, have rapid reversing and antagonizing effects on muscle relaxation induced by the muscle relaxants, can be used for preparing medicaments with muscle relaxation antagonizing effect, have the characteristics of low production cost and wide muscle relaxation reversing and antagonizing range and have effectively increased clinical application range. More importantly, the safe dose of the compounds provided by the invention is increased by at least one time compared with the known compounds. The general formula (I) of the compounds is shown in the specifications.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Dual-response multiple-medicine delivery system based on cyclodextrin
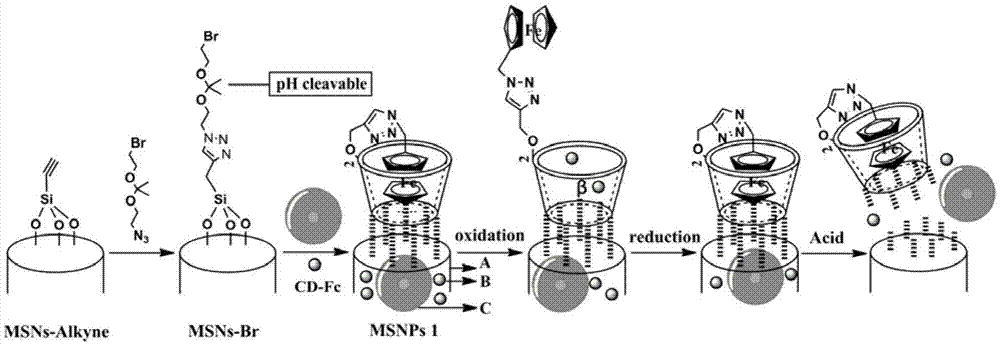
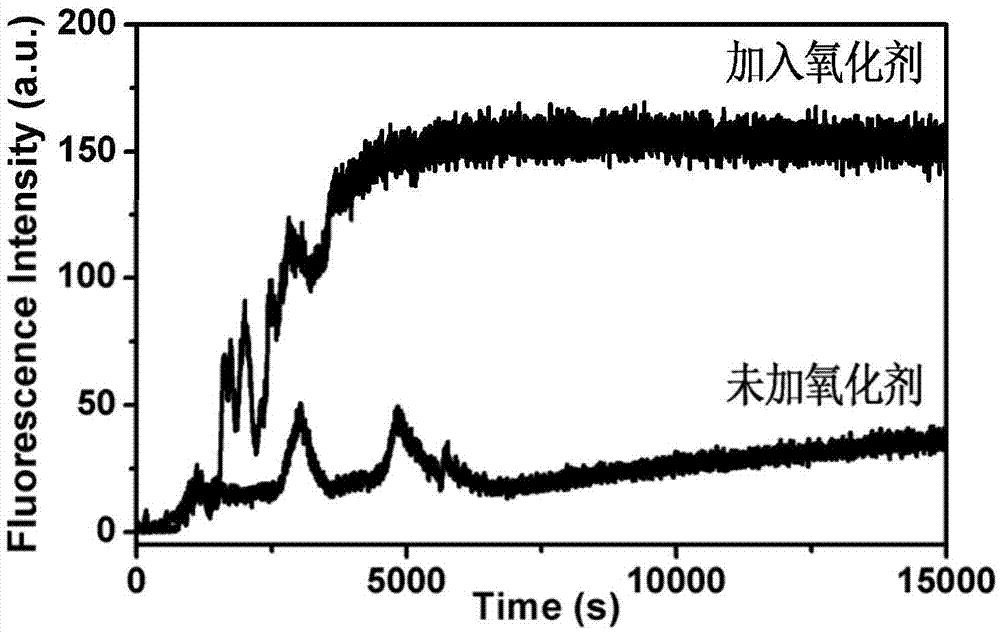
InactiveCN106902356ADual stimulus responseImmediate response to stimuliPowder deliveryOrganic active ingredientsDual responseMicrosphere
The invention discloses a dual-response multiple-medicine delivery system based on cyclodextrin. Firstly, through the action of a donor and a receptor, a macrocyclic molecule cyclodextrin serving as a host and a molecule ferrocene group serving as a guest form a self-inclusion complex; the dissociation and the inclusion of the host to the guest can be realized by utilizing redox regulation and control; the effect that a micromolecular medicine is subjected to adsorption-release through a cavity of the cyclodextrin is realized; secondly, a carbon chain containing an acetal group is introduced to connect a derivative of the cyclodextrin and a silicon dioxide microsphere; in an acid environment, an acetal bond is ruptured; the derivative of the cyclodextrin is quitted; a macromolecule is further released. The medicine delivery system provided by the invention has the characteristics of redox and dual response in the acid environment, moreover, can be used for seriatim or synchronously releasing medicine molecules in different sizes, can be used for controlling administration time, a position and a dosage, and has a favorable application prospect.
Owner:NANJING UNIV OF SCI & TECH
Solid dispersion of antifungal agent
InactiveCN104546724AImprove solubilityPromote absorptionOrganic active ingredientsPowder deliveryAntifungalSmall intestine
The invention belongs to the field of a medicinal preparation and specifically relates to a posaconazole solid dispersion and a preparation method of the solid dispersion and drug use. The invention provides a posaconazole solid dispersion and its drug use. The posaconazole solid dispersion contains posaconazole, a cyclodextrin derivative and a water-soluble or enteric carrier material, wherein mass ratio of posaconazole to the cyclodextrin derivative is 1:1-4 or mass ratio of posaconazole to the cyclodextrin derivative to the water-soluble or enteric carrier material is 1:1-4:0.001-3. The posaconazole solid dispersion can remarkably raise dissolution rate of the difficultly-soluble antifungal agent posaconazole in the small intestine and also can significantly enhance bioavailability of posaconazole. By the use of a dosage form prepared from the solid dispersion, the preparation technology is simple, and quality of the dosage form is stable.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Cyclodextrin grafted copolymer type ceramic water reducer and preparation method thereof
The invention belongs to the technical field of preparation of ceramic water reducers and discloses a cyclodextrin grafted copolymer type ceramic water reducer and a preparation method thereof. The water reducer is prepared from cyclodextrin or a cyclodextrin derivative, 3-(2-methylacryloyl ethoxyl dimethylamine) propanesulfonate, a chain transfer agent, an initiator, a chain terminator and deionized water. Due to a special molecular structure of cyclodextrin, the water reducer prepared by the preparation method has very good water-reducing and dispersing effects and meanwhile the water reducer which is amphoteric ionic polyelectrolyte is good in chemical property and thermal stability, strong in hydratability and insusceptible to the pH value of a solution. In addition, the water reducer prepared by the preparation method provided by the invention further has an enhancing effect on ceramic.
Owner:国科广化(南雄)新材料研究院有限公司 +1
Method for induding andrographolide by cyclodextrin and medicinal preparation
InactiveCN1437939ADoes not change chemical propertiesGood water solubilityOrganic active ingredientsAntipyreticCyclodextrinUltrasonic generator
The present invention discloses a method for including andrographolide by using cyclodextrin and its medicinal preparation. The andrographolide is an importent active extract of medicinal plant andrographis. Its inclusion method includes: adding 10-60g of cyclodextrin or its derivative into distilled water and dissolving it; adding 6g of andrographolide extract into 5-10 ml of medicinal methyl alcohol, heating and dissolving it; pouring the cyclodextrin aqueous solution into andrographolide ethyl alcohol solution; ultrasonic vibrating mixed solution in ultrasonic generator for 20-30 min., adopting thin-layer chromatography to identify the cyclodextrin-andrographolide inclusion quality. It does not change chemical property and effective activity of the andrographolide, can be prepared intovarious dosage forms.
Owner:侯文阁
Composition for preparing nanoporous material
InactiveUS20060040509A1Improve mechanical propertiesImprove stabilityMaterial nanotechnologySemiconductor/solid-state device manufacturingCyclodextrin derivativeCyclodextrin Derivatives
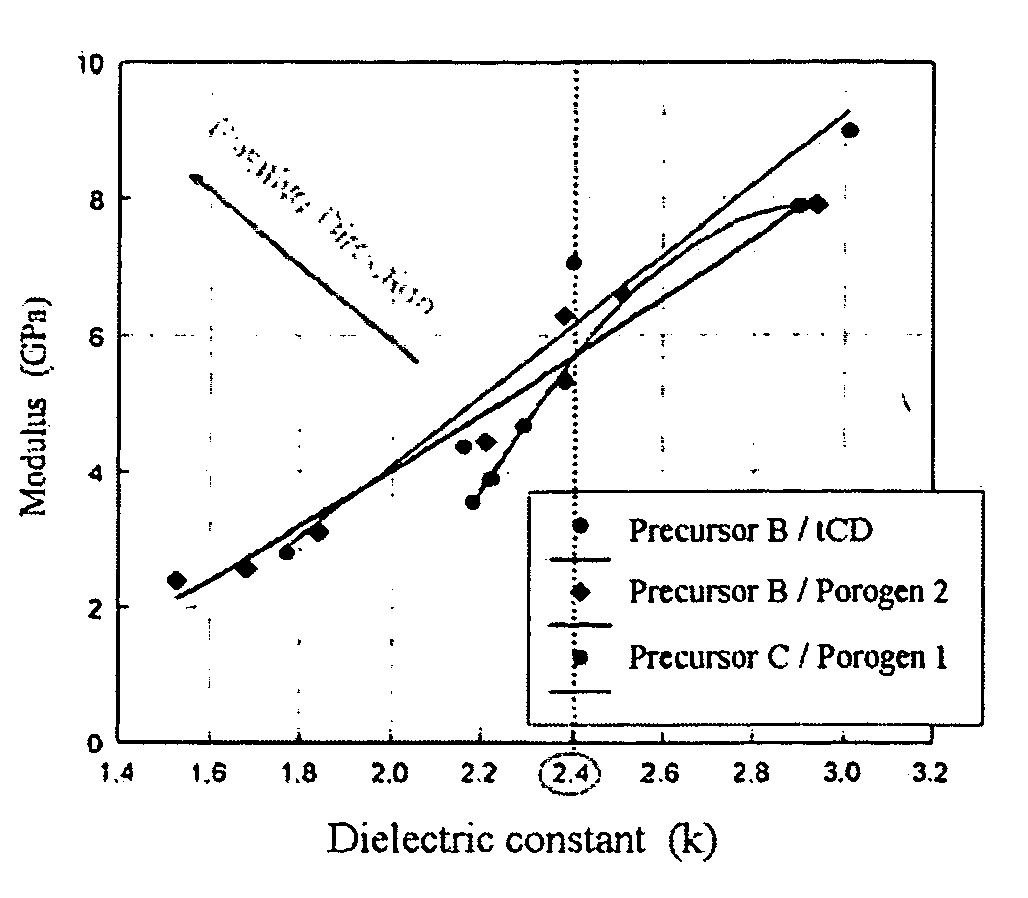
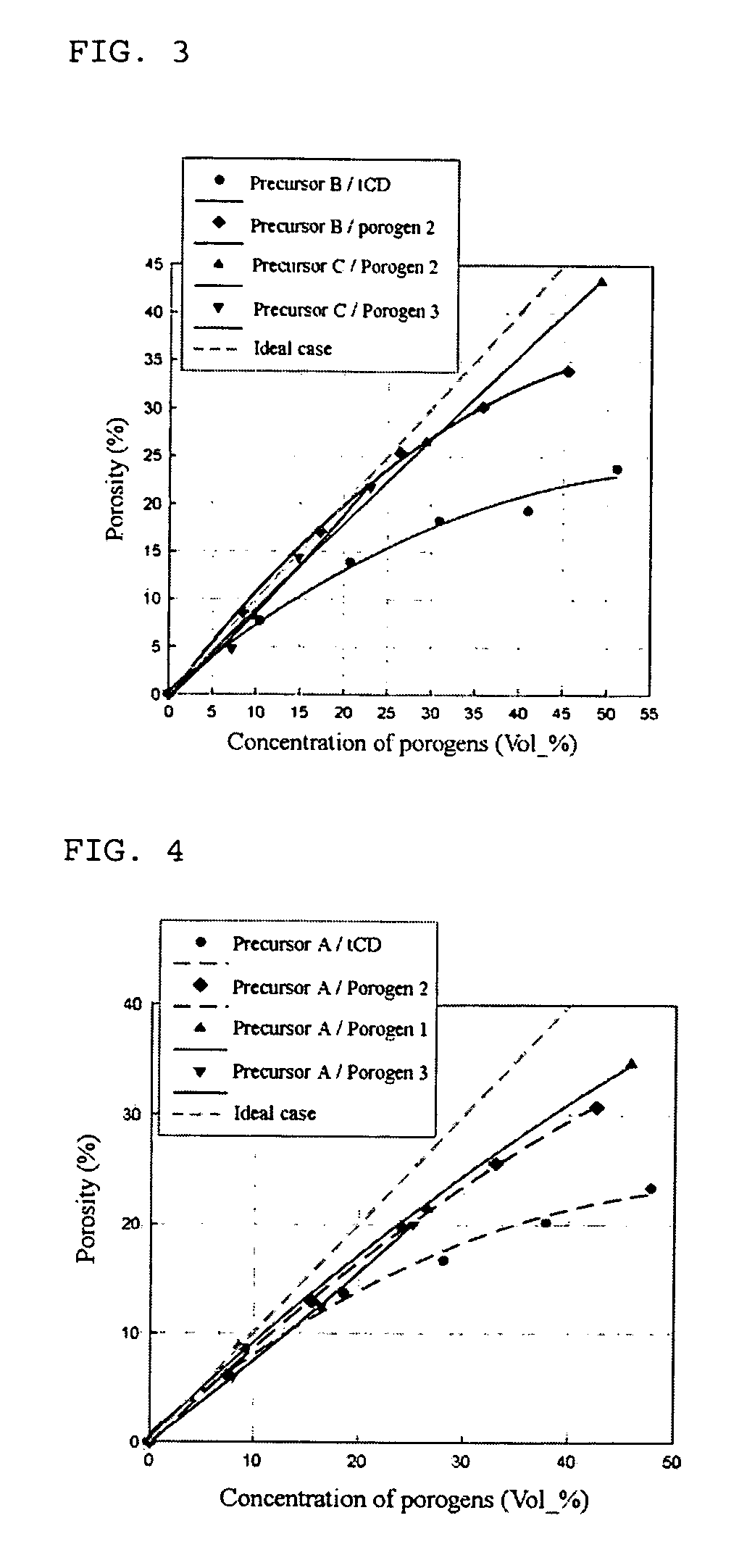
Disclosed herein is a composition for preparing a nanoporous material. The composition comprises i) a cyclodextrin derivative, ii) a thermostable matrix precursor, and iii) a solvent for dissolving the components i) and ii). The composition enables the preparation of a low dielectric constant film in which nanopores with a size of 20 Å or less are uniformly distributed.
Owner:SAMSUNG CORNING PRECISION MATERIALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com