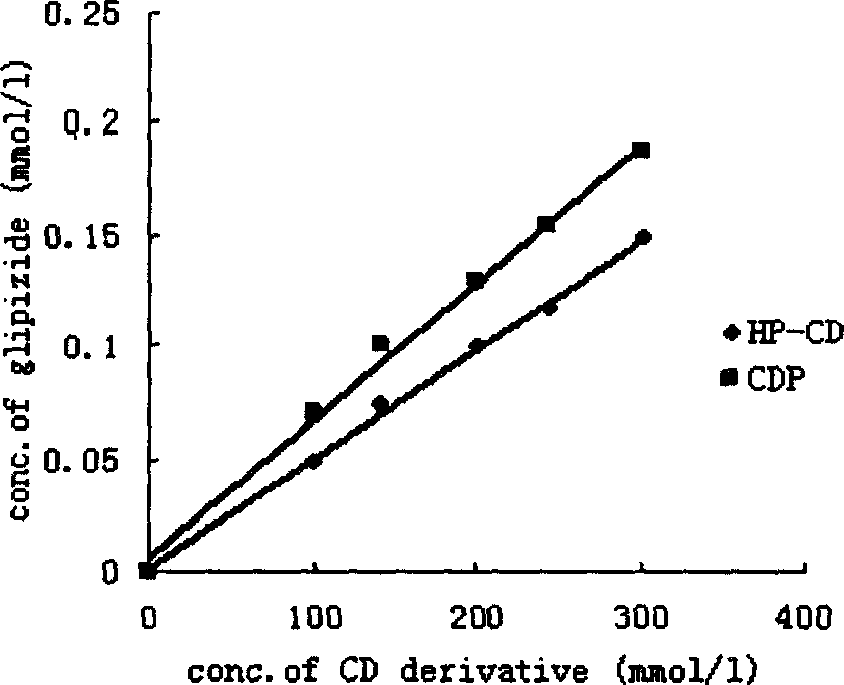
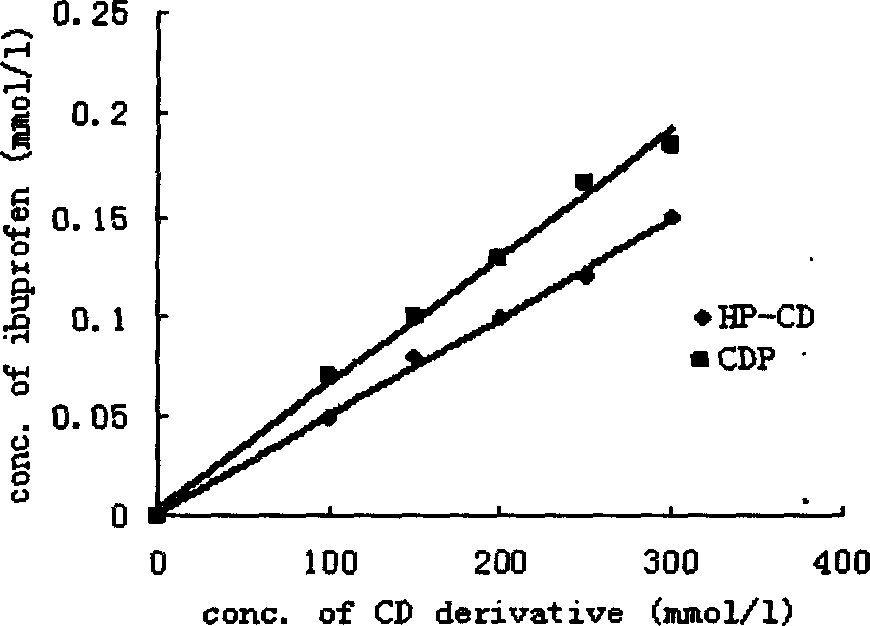
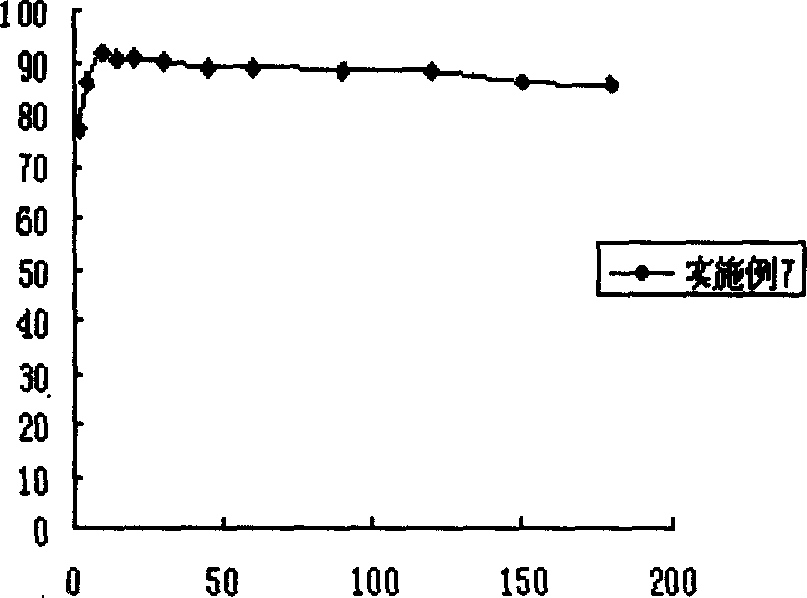
Insoluble drug delivery system based on water-soluble cyclodextrin
A cyclodextrin polymer, insoluble drug technology, applied in the directions of inactive components of polymer compounds, pill delivery, capsule delivery, etc., can solve problems such as general application of non-ionic water-soluble cyclodextrin polymers that have not yet been suggested , to achieve the effect of high yield, mild reaction conditions, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Synthesis of water-soluble β-cyclodextrin polymer
[0024] 22.7 g (0.02 moles) of β-cyclodextrin were dissolved in 80 g (56 ml, 0.6 moles) of 30% NaOH at room temperature and stirred for 24 hours. The reaction solution was heated to 30° C., and 29.38 ml (34.84 g, 0.2 moles) of ethylene glycol diglycidyl ether was slowly added under stirring for 1 hour, and reacted at 30° C. for 4 hours, and a small amount of acetone was added to terminate the reaction. The reactant was cooled to room temperature, and the reaction solution was neutralized with 6 mol / l hydrochloric acid until the pH value was neutral, then the reaction solution was dialyzed to remove salt and unreacted components, concentrated under reduced pressure, finally precipitated with absolute ethanol, and vacuum-dried. 17.5 g of product are obtained. The cyclodextrin content was determined to be 26.4% by the iodine titration method (Acta Chim. Hung., 100, 265 (1979)).
Embodiment 2
[0025] Example 2 Synthesis of water-soluble γ-cyclodextrin polymer
[0026] 27.1 g (0.02 moles) of γ-cyclodextrin were dissolved in 60 g (40 ml, 0.5 moles) of 33% NaOH at room temperature and stirred for 24 hours. The reaction solution was heated to 30° C., and 44.1 ml (52.26 g, 0.3 moles) of ethylene glycol diglycidyl ether was slowly added for 1 hour under stirring, and reacted at 30° C. for 6 hours, and a small amount of acetone was added to terminate the reaction. The reactant was cooled to room temperature, and the reaction solution was neutralized with 6 mol / l hydrochloric acid until the pH value was neutral, then the reaction solution was dialyzed to remove salt and unreacted components, concentrated under reduced pressure, finally precipitated with absolute ethanol, and vacuum-dried. 14.2 g of product are obtained. The content of cyclodextrin was determined to be 22.3% by titration iodine method.
Embodiment 3
[0027] Example 3 Synthesis of water-soluble γ-cyclodextrin polymer
[0028]27.1 g (0.02 moles) of γ-cyclodextrin were dissolved in 112 g (56 ml, 1.4 moles) of 50% NaOH at room temperature and stirred for 24 hours. The reaction solution was heated to 65° C., and then 23.52 ml (27.76 g, 0.3 moles) of epichlorohydrin was added rapidly, and reacted at 65° C. for 4 hours, and a small amount of acetone was added to terminate the reaction. The reactant was cooled to room temperature, and the reaction solution was neutralized with 6 mol / l hydrochloric acid until the pH value was neutral, then the reaction solution was dialyzed to remove salt and unreacted components, concentrated under reduced pressure, finally precipitated with absolute ethanol, and vacuum-dried. 10.5 g of product are obtained. The content of cyclodextrin was determined to be 33.4% by titration iodine method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com